User login
Evaluation of 3 Fixation Devices for Tibial-Sided Anterior Cruciate Ligament Graft Backup Fixation
Restoration of stability with return to activity is generally expected after anterior cruciate ligament (ACL) reconstruction; long-term success rates range from 75% to 95%.1 However, graft failure occurs most frequently with soft-tissue grafts fixated only with interference screws.2,3 Fixation failure also occurs more frequently at the tibial site.2 This failure has been attributed to extensive graft slippage in cases of soft-tissue fixation with interference screws.2 Interference screw fixation alone, with a double-looped hamstring tendon graft, fails at 350 N in young human tibiae.4,5 However, failure is limited with use of a bone–tendon–bone graft or with backup fixation, particularly at the tibial site.3 The superiority of bicortical fixation has also been proven.5-7
In addition, as shown in a goat model, ACL graft fixation is a major cause of failure in the immediate postoperative period, before biological incorporation of the graft.8 Fixation techniques for soft-tissue grafts must withstand stresses during the healing period (grafts may take up to 12 weeks to incorporate).9 Failures may result from forces exerted on the graft—forces that may be as high as 450 to 700 N during daily activities.10,11 Within the tibial tunnel, various fixation devices are used, including interference screws, staples, pins, buttons, and interference screw/sheath constructs.12,13 Primary fixation is commonly achieved with interference screws because of their ease of insertion and greater stiffness. However, fixation of the soft-tissue graft is influenced by several variables, including bone density, insertion torque, thread geometry, and interference screw material.14-16 Many of these variables, which are a source of inconsistency and concern during the immediate postoperative period, have led surgeons to seek alternative methods of backup fixation at the tibial site. Nevertheless, good clinical and subjective results have been found after ACL reconstruction with a 4-stranded semitendinosus tendon at 10-year follow-up.17
An anchor used in rotator cuff repair is the SwiveLock system (Arthrex). Major advantages of this system include ease and speed of insertion, good strength, and reduced need for later hardware removal.
We conducted a study to biomechanically evaluate 3 methods of tibial-sided fixation for ACL reconstruction: fully threaded interference screw only, interference screw backed with 4.75-mm SwiveLock anchor, and fully threaded bio-interference screw backed with 4.5-mm bicortical screw. We hypothesized that a fully threaded bio-interference screw backed with a 4.75-mm SwiveLock anchor would provide mechanical strength no different from that provided by backup fixation with a bicortical post at the tibial site. We further hypothesized that SwiveLock backup fixation would provide more strength than fixation with bio-interference screw alone.
Materials and Methods
The design of this study was adapted from one used by Walsh and colleagues,3 who compared 3 fixation methods: retrograde interference screw, suture button, and combined fixation. Tibiae inspected before selection showed no signs of injury or abnormality. Bovine extensor tendons, which lacked any defects along their entire length, were stored in saline 0.9% solution. Both the tibiae and the extensor tendons were stored at –20°C before completion of the tibial-sided ACL reconstruction. Thirty fresh-frozen, skeletally mature porcine proximal tibiae were selected and thawed at 4°C before preparation. Specimens were prepared by potting the diaphysis in fiberglass resin, and a tunnel 9 mm in diameter was drilled through the anteromedial aspect of the tibia.
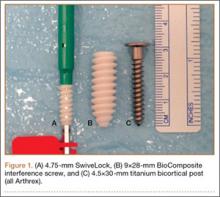
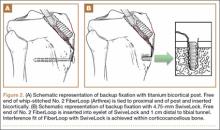
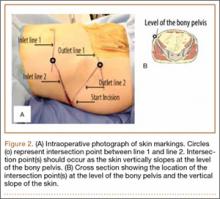
For consistency, one author (CAV) prepared all 30 specimens. Both tails of all 30 bovine extensor tendons were whip-stitched with No. 2 FiberLoop (Arthrex) 9 mm in diameter. Grafts and tibiae were randomly divided into 3 sample groups. The first group was prepared by antegrade graft fixation within the tibial tunnel using a fully threaded 9×28-mm BioComposite interference screw (Arthrex). The second and third groups used the same primary fixation within the tibial tunnel along with 2 types of secondary fixation. These backup fixation groups included a 4.5-mm titanium bicortical post (Arthrex) and a 4.75-mm BioComposite SwiveLock C anchor (Arthrex) (Figure 1). The FiberLoop at the ends of the distal graft tails for backup groups were fixated 1 cm distal to the tibial tunnel and tapped before insertion of backup devices (Figures 2A, 2B). Insertion was completed after 4.5-mm bicortical and 4.75-mm unicortical drilling and tapping of the anteromedial cortices for the titanium posts and SwiveLocks, respectively. The free ends of the whip-stitched No. 2 FiberLoop were tied to the proximal end of the titanium post with a single surgical knot followed by 5 square knots.3 The free ends of the No. 2 FiberLoop were inserted into the eyelet of the 4.75-mm SwiveLock and 1 cm directly inferior to the tibial tunnel. Interference fit of FiberLoop with SwiveLock was achieved within the corticocancellous bone of the tibiae. All samples retained a 30-mm tendon loop superior to the tibial plateau to simulate intra-articular ACL length. Specimens were then stored at –20°C and thawed at 4°C before biomechanical testing.
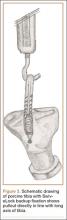
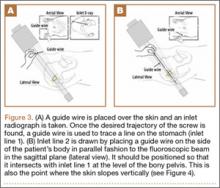
Each of the 30 tibiae was tested once. Each testing group consisted of 10 porcine tibiae. The tendons were kept moist during the entire testing procedure by spraying them thoroughly with saline 0.9% solution. Mechanical testing was performed with an Instron 8871 system with a 5-kN load cell secured to the crosshead. A fixed-angle aperture, attached to the testing surface, was adjusted so that the tendon would be pulled in line with the tibial tunnel. A hook fixture suspended from clevis and dowel was used to secure the tendon to the crosshead (Figure 3). A small preload of 5 N manually applied to each sample was followed by a precycling regimen of 10 cycles between 10 N and 50 N at 1 Hz. Precycling was performed to remove slack from the system. Mechanical testing consisted of 500 cycles between 50 N and 250 N at 1 Hz followed by pull to failure at 20 mm per minute. Load and displacement data were recorded at 500 Hz.
An a priori power analysis was not performed because 6 specimens per group in the study from which the testing protocol was adapted demonstrated sufficient power among 3 testing categories.3 In addition, other studies have demonstrated similar testing protocols using 10 specimens per testing group.7,12,13,18 The data for each sample were analyzed with OriginPro 8.0 software (OriginLab). Ultimate load, yield load, stiffness, and cyclic displacement of the 3 sample groups were compared with 1-way analysis of variance (α = 0.05). Holm-Sidak tests were used for post hoc analysis.19P < .05 was statistically significant.
Results
None of the 30 specimens failed during preloading. Modes of failure were consistent among groups. All 10 specimens in the interference-screw-only group failed by graft slippage past the screw in the tibial tunnel. Nineteen of the 20 specimens in the backup-fixation groups failed by graft slippage past the screw and suture cutout through the distal graft tail. In the bicortical-post backup group, 1 failure was attributed to tendon tearing proximal to whip-stitching. There were no instances of hardware breakage or failure of either titanium screw or SwiveLock anchor.
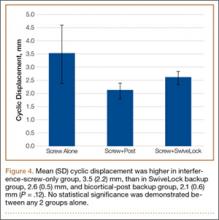
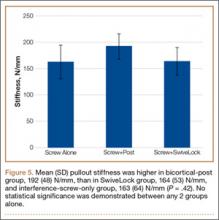
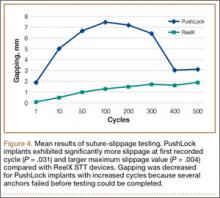
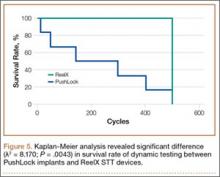
Mean (SD) cyclic displacement was higher in the interference-screw-only group, 3.5 (2.2) mm, than in the SwiveLock backup group, 2.6 (0.5) mm, and the bicortical-post backup group, 2.1 (0.6) mm; no statistical significance was demonstrated between any 2 of these groups alone (P = .12) (Figure 4). Mean (SD) pullout stiffness was higher in the bicortical-post backup group, 192 (48) N/mm, than in the SwiveLock backup group, 164 (53) N/mm, and the screw-only group, 163 (64) N/mm (P = .42) (Figure 5). Mean (SD) initial load at 5 mm of displacement was higher in the bicortical-post backup group, 482 (156) N, and the SwiveLock backup group, 423 (94) N, than in the screw-only group, 381 (169) N (P = .30).
Mean (SD) yield load was higher in the bicortical-post backup group, 829 (253) N, than in the SwiveLock backup group, 642 (172) N, and the interference-screw-only group, 496 (133) N (P = .003). Statistical significance was demonstrated between the screw-only and bicortical-post groups (P = .002) and between the screw-only and SwiveLock groups (P = .048). There was no statistical difference between the bicortical-post and SwiveLock groups (P = .07).
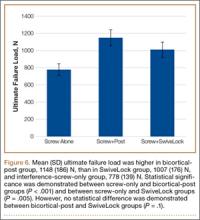
Mean (SD) ultimate load to failure was higher in the bicortical-post backup group, 1148 (186) N, than in the SwiveLock backup group, 1007 (176) N, and the interference-screw-only group, 778 (139) N (Figure 6). The difference was statistically significant, whereby the screw-only group failed at a lower load compared with the bicortical-post group (P < .001) and the SwiveLock group (P = .005). The 2 backup groups were not statistically different (P = .1).
Discussion
We investigated whether a fully threaded bio-interference screw backed with a 4.75-mm SwiveLock anchor would provide mechanically equivalent pullout strength within the tibial tunnel during ACL reconstruction with soft-tissue allografts in comparison either with a fully threaded bio-interference screw backed with a bicortical post or with a fully threaded bio-interference screw without backup fixation. The results of the study support this hypothesis. With SwiveLock used for backup fixation, there was no significant difference in stiffness or cyclic load displacement between the screw-only, SwiveLock, and bicortical-post groups. However, adding backup fixation could particularly help improve fixation consistency. Specifically, although after only 500 cycles there was no statistically significant difference in cyclic displacement, continued cycling may be clinically relevant if graft slippage exceeded limits to allow for healing within the tibial tunnel. Conversely, a significantly larger difference was found between the SwiveLock, bicortical-post, and screw-only groups in yield load and ultimate load to failure. However, there was no significant difference between the SwiveLock and bicortical-post groups.
In this study, interference screw with SwiveLock backup demonstrated a mean (SD) ultimate load to failure of 1007 (176) N, comparable to that found by Walsh and colleagues3 for retrograde bio-interference screw with suture button, 1027 (157.11) N. In a study comparing quadrupled hamstring tibial graft fixation, Intrafix (DePuy Mitek) and an 8×25-mm Bioscrew (Linvatec) demonstrated mean (SD) single-cycle yield loads of 1332 (304) N and 612 (176) N, respectively.13 These results are similar to the ultimate yield loads in the present study: bicortical-post group, 1148 (186) N; SwiveLock group, 1007 (176) N; screw-only group, 778 (139) N. Differences may be attributed to hamstring tendons used in a quadrupled manner in the aforementioned study.12,13 Last, mean (SD) ultimate load to failure in a study that used only a retrograde bio-interference screw (9×20 mm) was 679.00 (109.44) N,3 similar to the 778 (139) N found for interference-screw-only in the present study. The difference is likely attributable to the longer screw (9×28 mm) in our study. Using SwiveLock C in cortical bone, Barber and colleagues18 found mean (SD) loads to failure up to 711.9 (89.1) N.
Clinically, it has been shown that a statistically significant increase in anterior laxity occurred between 4 months and 2 years in 10.7% of patients who underwent hamstring ACL reconstruction.20 The knees were clinically categorized as unstable or severely abnormal. The authors concluded that the clinical outcome was more likely influenced by the methods used to fix the free ends of the graft, specifically with 2 staples or a washer. To simulate early postoperative rehabilitation in the present study, cyclic loading of the graft was performed. Ultimate load to failure was then determined in order to evaluate catastrophic failure strength of the backup fixation devices in comparison with the interference-screw-only group without supplementary fixation.
It has been shown in autologous hamstring ACL reconstruction that a centrally placed polyethylene screw with sheath (Intrafix) performed as well as a standard, eccentrically placed metal interference screw with staple.10 It is therefore logical that backup fixation with use of a similar device (eg, SwiveLock, bicortical post) is necessary to ensure comparable clinical outcomes in relation to a screw/sheath device that has been shown to endure the highest yield loads.2,9,12,13,21-23 Potential benefits of using SwiveLock anchors for backup fixation include a statistically significant increased mean (SD) ultimate yield load of 229 (176) N over interference screw only. These results are similar to those in comparable studies: 218.3 (59.7) N24 and 165 (24.15) N25 in healthy bone with a reported bone mineral density (BMD) of 1.39 g/cm2, similar to that of skeletally mature porcine tibia (1.220-1.675 g/cm²).3 In addition, ease of insertion of this device over a bicortical post was demonstrated. The titanium post required bicortical drilling as well as measurement with a depth gauge to ensure adequate screw length. This process appeared to require more time during specimen preparation and theoretically could prove to be more dangerous clinically.7 However, caution in using a SwiveLock anchor in osteoporotic bone is advised because of reduced pullout strength.26 In this case, bicortical-post backup fixation may be more suitable. Moreover, although not demonstrated in this study, hardware prominence and irritation with a post may cause postoperative morbidity necessitating future removal.20 Hardware removal was the most common reason for additional surgery using hamstring tendons as graft.20 A second surgery for hardware removal was required in 21% of these patients.20 This is unlikely to occur with a SwiveLock, as the anchor is buried within cortical bone.
Limitations
Regarding use of nonhuman tissues in a biomechanical model, porcine tibiae and bovine extensor tendons were used because of availability, consistency among specimens, and cost-effectiveness. However, bovine extensor tendons have been shown to exhibit stiffness and viscoelastic properties similar to those of a human hamstring graft.27 In addition, the BMD of the porcine tibiae used in this study was not tested because of time involved and cost-efficiency. However, it has been shown that average BMD of porcine tibiae, 1.220-1.675 g/cm², is similar to that in a young athletic population, 1.24-1.62 g/cm2.3,28-31 We therefore assumed similarity to a young athletic population and uniformity of BMD of the porcine tibiae used in this study.
In addition, the biomechanical testing protocol did not simulate physiologic loading within the tibial tunnel. Moreover, the testing protocol used loads of only 250 N during cyclic testing for 500 cycles. This simulates only the early rehabilitation period and not the healing period, which may last up to 12 weeks.9 In addition, as previously mentioned, forces on the graft may be as high as 450 to 700 N.11,32 Pullout testing in line with the long axis of the tibia was performed in order to compare mechanical testing results with those of similar studies.3,12,13 Last, the P of .07 for the comparison of ultimate load to failure between the 2 backup fixation groups suggests that this study may have been underpowered.
Conclusion
This study demonstrated an effective, alternative, and equivalent backup fixation device that can help prevent graft slippage within the tibial tunnel during soft-tissue ACL reconstruction. Potential benefits of using SwiveLock anchors for backup fixation include a significantly increased ultimate yield load (229 N) when supplementing an interference screw, ease of insertion compared with a bicortical post, and the improbable need for future hardware removal. We support using SwiveLock for supplementary fixation at the tibial tunnel site when using soft-tissue grafts in ACL reconstruction.
1. Wetzler MJ, Bartolozzi AR, Gillespie MJ, Rubenstein DL, Ciccotti MG, Miller LS. Revision anterior cruciate ligament reconstruction. Oper Tech Orthop. 1996;6(3):181-189.
2. Scheffler SU, Südkamp NP, Göckenjan A, Hoffmann RF, Weiler A. Biomechanical comparison of hamstring and patellar tendon graft anterior cruciate ligament reconstruction techniques: the impact of fixation level and fixation method under cyclic loading. Arthroscopy. 2002;18(3):304-315.
3. Walsh MP, Wijdicks CA, Parker JB, Hapa O, LaPrade RF. A comparison between a retrograde interference screw, suture button, and combined fixation on the tibial side in an all-inside anterior cruciate ligament reconstruction: a biomechanical study in a porcine model. Am J Sports Med. 2009;37(1):160-167.
4. Howell SM, Hull ML. Aggressive rehabilitation using hamstring tendons: graft construct, tibial tunnel placement, fixation properties, and clinical outcome. Am J Knee Surg. 1998;11(2):120-127.
5. Magen HE, Howell SM, Hull ML. Structural properties of six tibial fixation methods for anterior cruciate ligament soft tissue grafts. Am J Sports Med. 1999;27(1):35-43.
6. Beynnon BD, Meriam CM, Ryder SH, Fleming BC, Johnson RJ. The effect of screw insertion torque on tendons fixed with spiked washers. Am J Sports Med. 1998;26(4):536-539.
7. Post WR, King SS. Neurovascular risk of bicortical tibial drilling for screw and spiked washer fixation of soft-tissue anterior cruciate ligament graft. Arthroscopy. 2001;17(3):244-247.
8. Holden JP, Grood ES, Butler DL, et al. Biomechanics of fascia lata ligament replacements: early postoperative changes in the goat. J Orthop Res. 1988;6(5):639-647.
9. Rodeo SA, Arnoczky SP, Torzilli PA, Hidaka C, Warren RF. Tendon-healing in a bone tunnel. A biomechanical and histological study in the dog. J Bone Joint Surg Am. 1993;75(12):1795-1803.
10. Frank CB, Jackson DW. The science of reconstruction of the anterior cruciate ligament. J Bone Joint Surg Am. 1997;79(10):1556-1576.
11. Markolf KL, Willems MJ, Jackson SR, Finerman GA. In situ calibration of miniature sensors implanted into the anterior cruciate ligament. Part I: strain measurements. J Orthop Res. 1998;16(4):455-463.
12. Kousa P, Teppo LN, Jarvinen TL, Vihavainen M, Kannus P, Jarvinen M. The fixation strength of six hamstring tendon graft fixation devices in anterior cruciate ligament reconstruction: I. Femoral site. Am J Sports Med. 2003;3 (2)1:174-181.
13. Kousa P, Jarvinen TL, Vihavainen M, Kannus P, Jarvinen M. The fixation strength of six hamstring tendon graft fixation devices in anterior cruciate ligament reconstruction: II. Tibial site. Am J Sports Med. 2003;31(2):182-188.
14. Brand JC Jr, Pienkowski D, Steenlage E, Hamilton D, Johnson DL, Caborn DN. Interference screw fixation strength of a quadrupled hamstring tendon graft is directly related to bone mineral density and insertion torque. Am J Sports Med. 2000;28(5):705-710.
15. Weiler A, Hoffmann RF, Siepe CJ, Kolbeck SF, Südkamp NP. The influence of screw geometry on hamstring tendon interference fit fixation. Am J Sports Med. 2000;28(3):356-359.
16. Weiler A, Hoffmann RF, Stähelin AC, Bail HJ, Siepe CJ, Südkamp NP. Hamstring tendon fixation using interference screws: a biomechanical study in calf tibial bone. Arthroscopy. 1998;14(1):29-37.
17. Streich NA, Reichenbacher S, Barié A, Buchner M, Schmitt H. Long-term outcome of anterior cruciate ligament reconstruction with an autologous four-strand semitendinosus tendon autograft. Int Orthop. 2013;37(2):279-284.
18. Barber FA, Herbert MA, Beavis C, Barrera Oro F. Suture anchor materials, eyelets, and designs: update 2008. Arthroscopy. 2008;24(8):859-867.
19. Aickin M, Gensler H. Adjusting for multiple testing when reporting research results: the Bonferroni vs Holm methods. Am J Public Health. 1996;86(5):726-728.
20. Howell SM, Deutsch ML. Comparison of endoscopic and two-incision techniques for reconstructing a torn anterior cruciate ligament using hamstring tendons. Arthroscopy. 1999;15(6):594-606.
21. Gwynne-Jones DP, Draffin J, Vane A, Craig R, McMahon S. Failure strengths of concentric and eccentric implants for hamstring graft fixation. ANZ J Surg. 2008;78(3):177-181.
22. Hayes DA, Watts MC, Tevelen GA, Crawford RW. Central versus peripheral tibial screw placement in hamstring anterior cruciate ligament reconstruction: in vitro biomechanics. Arthroscopy. 2005;21(6):703-706.
23. Shino K, Pflaster DS. Comparison of eccentric and concentric screw placement for hamstring graft fixation in the tibial tunnel. Knee Surg Sports Traumatol Arthrosc. 2000;8(2):73-75.
24. Prevrhal S, Fuerst T, Fan B, et al. Quantitative ultrasound of the tibia depends on both cortical density and thickness. Osteoporosis Int. 2001;12(1):28-34.
25. Pietschmann MF, Gülecyüz MF, Fieseler S, et al. Biomechanical stability of knotless suture anchors used in rotator cuff repair in healthy and osteopenic bone. Arthroscopy. 2010;26(8):1035-1044.
26. Burns JP, Snyder SJ, Albritton M. Arthroscopic rotator cuff repair using triple-loaded anchors, suture shuttles, and suture savers. J Am Acad Orthop Surg. 2007;15(7):432-444.
27. Tetsumura S, Fujita A, Nakajima M, Abe M. Biomechanical comparison of different fixation methods on the tibial side in anterior cruciate ligament reconstruction: a biomechanical study in porcine tibial bone. J Orthop Sci. 2006;11(3):278-282.
28. Alfredson H, Nordstrom P, Lorentzon R. Total and regional bone mass in female soccer players. Calcif Tissue Int. 1996;59(6):438-442.
29. Nevill AM, Holder RL, Stewart AD. Modeling elite male athletes’ peripheral bone mass, assessed using regional dual x-ray absorptiometry. Bone. 2003;32(1):62-68.
30. Nordström P, Lorentzon R. Site-specific bone mass differences of the lower extremities in 17-year-old ice hockey players. Calcif Tissue Int. 1996;59(6):4443-4448.
31. Patzer T, Santo G, Olender GD, Wellmann M, Hurschler C, Schofer MD. Suprapectoral or subpectoral position for biceps tenodesis: biomechanical comparison of four different techniques in both positions. J Shoulder Elbow Surg. 2012;21(1):116-125.
32. De Wall M, Scholes CJ, Patel S, Coolican MR, Parker DA. Tibial fixation in anterior cruciate ligament reconstruction: a prospective randomized study comparing metal interference screw and staples with a centrally placed polyethylene screw and sheath. Am J Sports Med. 2011;39(9):1858-1864.
Restoration of stability with return to activity is generally expected after anterior cruciate ligament (ACL) reconstruction; long-term success rates range from 75% to 95%.1 However, graft failure occurs most frequently with soft-tissue grafts fixated only with interference screws.2,3 Fixation failure also occurs more frequently at the tibial site.2 This failure has been attributed to extensive graft slippage in cases of soft-tissue fixation with interference screws.2 Interference screw fixation alone, with a double-looped hamstring tendon graft, fails at 350 N in young human tibiae.4,5 However, failure is limited with use of a bone–tendon–bone graft or with backup fixation, particularly at the tibial site.3 The superiority of bicortical fixation has also been proven.5-7
In addition, as shown in a goat model, ACL graft fixation is a major cause of failure in the immediate postoperative period, before biological incorporation of the graft.8 Fixation techniques for soft-tissue grafts must withstand stresses during the healing period (grafts may take up to 12 weeks to incorporate).9 Failures may result from forces exerted on the graft—forces that may be as high as 450 to 700 N during daily activities.10,11 Within the tibial tunnel, various fixation devices are used, including interference screws, staples, pins, buttons, and interference screw/sheath constructs.12,13 Primary fixation is commonly achieved with interference screws because of their ease of insertion and greater stiffness. However, fixation of the soft-tissue graft is influenced by several variables, including bone density, insertion torque, thread geometry, and interference screw material.14-16 Many of these variables, which are a source of inconsistency and concern during the immediate postoperative period, have led surgeons to seek alternative methods of backup fixation at the tibial site. Nevertheless, good clinical and subjective results have been found after ACL reconstruction with a 4-stranded semitendinosus tendon at 10-year follow-up.17
An anchor used in rotator cuff repair is the SwiveLock system (Arthrex). Major advantages of this system include ease and speed of insertion, good strength, and reduced need for later hardware removal.
We conducted a study to biomechanically evaluate 3 methods of tibial-sided fixation for ACL reconstruction: fully threaded interference screw only, interference screw backed with 4.75-mm SwiveLock anchor, and fully threaded bio-interference screw backed with 4.5-mm bicortical screw. We hypothesized that a fully threaded bio-interference screw backed with a 4.75-mm SwiveLock anchor would provide mechanical strength no different from that provided by backup fixation with a bicortical post at the tibial site. We further hypothesized that SwiveLock backup fixation would provide more strength than fixation with bio-interference screw alone.
Materials and Methods
The design of this study was adapted from one used by Walsh and colleagues,3 who compared 3 fixation methods: retrograde interference screw, suture button, and combined fixation. Tibiae inspected before selection showed no signs of injury or abnormality. Bovine extensor tendons, which lacked any defects along their entire length, were stored in saline 0.9% solution. Both the tibiae and the extensor tendons were stored at –20°C before completion of the tibial-sided ACL reconstruction. Thirty fresh-frozen, skeletally mature porcine proximal tibiae were selected and thawed at 4°C before preparation. Specimens were prepared by potting the diaphysis in fiberglass resin, and a tunnel 9 mm in diameter was drilled through the anteromedial aspect of the tibia.
For consistency, one author (CAV) prepared all 30 specimens. Both tails of all 30 bovine extensor tendons were whip-stitched with No. 2 FiberLoop (Arthrex) 9 mm in diameter. Grafts and tibiae were randomly divided into 3 sample groups. The first group was prepared by antegrade graft fixation within the tibial tunnel using a fully threaded 9×28-mm BioComposite interference screw (Arthrex). The second and third groups used the same primary fixation within the tibial tunnel along with 2 types of secondary fixation. These backup fixation groups included a 4.5-mm titanium bicortical post (Arthrex) and a 4.75-mm BioComposite SwiveLock C anchor (Arthrex) (Figure 1). The FiberLoop at the ends of the distal graft tails for backup groups were fixated 1 cm distal to the tibial tunnel and tapped before insertion of backup devices (Figures 2A, 2B). Insertion was completed after 4.5-mm bicortical and 4.75-mm unicortical drilling and tapping of the anteromedial cortices for the titanium posts and SwiveLocks, respectively. The free ends of the whip-stitched No. 2 FiberLoop were tied to the proximal end of the titanium post with a single surgical knot followed by 5 square knots.3 The free ends of the No. 2 FiberLoop were inserted into the eyelet of the 4.75-mm SwiveLock and 1 cm directly inferior to the tibial tunnel. Interference fit of FiberLoop with SwiveLock was achieved within the corticocancellous bone of the tibiae. All samples retained a 30-mm tendon loop superior to the tibial plateau to simulate intra-articular ACL length. Specimens were then stored at –20°C and thawed at 4°C before biomechanical testing.
Each of the 30 tibiae was tested once. Each testing group consisted of 10 porcine tibiae. The tendons were kept moist during the entire testing procedure by spraying them thoroughly with saline 0.9% solution. Mechanical testing was performed with an Instron 8871 system with a 5-kN load cell secured to the crosshead. A fixed-angle aperture, attached to the testing surface, was adjusted so that the tendon would be pulled in line with the tibial tunnel. A hook fixture suspended from clevis and dowel was used to secure the tendon to the crosshead (Figure 3). A small preload of 5 N manually applied to each sample was followed by a precycling regimen of 10 cycles between 10 N and 50 N at 1 Hz. Precycling was performed to remove slack from the system. Mechanical testing consisted of 500 cycles between 50 N and 250 N at 1 Hz followed by pull to failure at 20 mm per minute. Load and displacement data were recorded at 500 Hz.
An a priori power analysis was not performed because 6 specimens per group in the study from which the testing protocol was adapted demonstrated sufficient power among 3 testing categories.3 In addition, other studies have demonstrated similar testing protocols using 10 specimens per testing group.7,12,13,18 The data for each sample were analyzed with OriginPro 8.0 software (OriginLab). Ultimate load, yield load, stiffness, and cyclic displacement of the 3 sample groups were compared with 1-way analysis of variance (α = 0.05). Holm-Sidak tests were used for post hoc analysis.19P < .05 was statistically significant.
Results
None of the 30 specimens failed during preloading. Modes of failure were consistent among groups. All 10 specimens in the interference-screw-only group failed by graft slippage past the screw in the tibial tunnel. Nineteen of the 20 specimens in the backup-fixation groups failed by graft slippage past the screw and suture cutout through the distal graft tail. In the bicortical-post backup group, 1 failure was attributed to tendon tearing proximal to whip-stitching. There were no instances of hardware breakage or failure of either titanium screw or SwiveLock anchor.
Mean (SD) cyclic displacement was higher in the interference-screw-only group, 3.5 (2.2) mm, than in the SwiveLock backup group, 2.6 (0.5) mm, and the bicortical-post backup group, 2.1 (0.6) mm; no statistical significance was demonstrated between any 2 of these groups alone (P = .12) (Figure 4). Mean (SD) pullout stiffness was higher in the bicortical-post backup group, 192 (48) N/mm, than in the SwiveLock backup group, 164 (53) N/mm, and the screw-only group, 163 (64) N/mm (P = .42) (Figure 5). Mean (SD) initial load at 5 mm of displacement was higher in the bicortical-post backup group, 482 (156) N, and the SwiveLock backup group, 423 (94) N, than in the screw-only group, 381 (169) N (P = .30).
Mean (SD) yield load was higher in the bicortical-post backup group, 829 (253) N, than in the SwiveLock backup group, 642 (172) N, and the interference-screw-only group, 496 (133) N (P = .003). Statistical significance was demonstrated between the screw-only and bicortical-post groups (P = .002) and between the screw-only and SwiveLock groups (P = .048). There was no statistical difference between the bicortical-post and SwiveLock groups (P = .07).
Mean (SD) ultimate load to failure was higher in the bicortical-post backup group, 1148 (186) N, than in the SwiveLock backup group, 1007 (176) N, and the interference-screw-only group, 778 (139) N (Figure 6). The difference was statistically significant, whereby the screw-only group failed at a lower load compared with the bicortical-post group (P < .001) and the SwiveLock group (P = .005). The 2 backup groups were not statistically different (P = .1).
Discussion
We investigated whether a fully threaded bio-interference screw backed with a 4.75-mm SwiveLock anchor would provide mechanically equivalent pullout strength within the tibial tunnel during ACL reconstruction with soft-tissue allografts in comparison either with a fully threaded bio-interference screw backed with a bicortical post or with a fully threaded bio-interference screw without backup fixation. The results of the study support this hypothesis. With SwiveLock used for backup fixation, there was no significant difference in stiffness or cyclic load displacement between the screw-only, SwiveLock, and bicortical-post groups. However, adding backup fixation could particularly help improve fixation consistency. Specifically, although after only 500 cycles there was no statistically significant difference in cyclic displacement, continued cycling may be clinically relevant if graft slippage exceeded limits to allow for healing within the tibial tunnel. Conversely, a significantly larger difference was found between the SwiveLock, bicortical-post, and screw-only groups in yield load and ultimate load to failure. However, there was no significant difference between the SwiveLock and bicortical-post groups.
In this study, interference screw with SwiveLock backup demonstrated a mean (SD) ultimate load to failure of 1007 (176) N, comparable to that found by Walsh and colleagues3 for retrograde bio-interference screw with suture button, 1027 (157.11) N. In a study comparing quadrupled hamstring tibial graft fixation, Intrafix (DePuy Mitek) and an 8×25-mm Bioscrew (Linvatec) demonstrated mean (SD) single-cycle yield loads of 1332 (304) N and 612 (176) N, respectively.13 These results are similar to the ultimate yield loads in the present study: bicortical-post group, 1148 (186) N; SwiveLock group, 1007 (176) N; screw-only group, 778 (139) N. Differences may be attributed to hamstring tendons used in a quadrupled manner in the aforementioned study.12,13 Last, mean (SD) ultimate load to failure in a study that used only a retrograde bio-interference screw (9×20 mm) was 679.00 (109.44) N,3 similar to the 778 (139) N found for interference-screw-only in the present study. The difference is likely attributable to the longer screw (9×28 mm) in our study. Using SwiveLock C in cortical bone, Barber and colleagues18 found mean (SD) loads to failure up to 711.9 (89.1) N.
Clinically, it has been shown that a statistically significant increase in anterior laxity occurred between 4 months and 2 years in 10.7% of patients who underwent hamstring ACL reconstruction.20 The knees were clinically categorized as unstable or severely abnormal. The authors concluded that the clinical outcome was more likely influenced by the methods used to fix the free ends of the graft, specifically with 2 staples or a washer. To simulate early postoperative rehabilitation in the present study, cyclic loading of the graft was performed. Ultimate load to failure was then determined in order to evaluate catastrophic failure strength of the backup fixation devices in comparison with the interference-screw-only group without supplementary fixation.
It has been shown in autologous hamstring ACL reconstruction that a centrally placed polyethylene screw with sheath (Intrafix) performed as well as a standard, eccentrically placed metal interference screw with staple.10 It is therefore logical that backup fixation with use of a similar device (eg, SwiveLock, bicortical post) is necessary to ensure comparable clinical outcomes in relation to a screw/sheath device that has been shown to endure the highest yield loads.2,9,12,13,21-23 Potential benefits of using SwiveLock anchors for backup fixation include a statistically significant increased mean (SD) ultimate yield load of 229 (176) N over interference screw only. These results are similar to those in comparable studies: 218.3 (59.7) N24 and 165 (24.15) N25 in healthy bone with a reported bone mineral density (BMD) of 1.39 g/cm2, similar to that of skeletally mature porcine tibia (1.220-1.675 g/cm²).3 In addition, ease of insertion of this device over a bicortical post was demonstrated. The titanium post required bicortical drilling as well as measurement with a depth gauge to ensure adequate screw length. This process appeared to require more time during specimen preparation and theoretically could prove to be more dangerous clinically.7 However, caution in using a SwiveLock anchor in osteoporotic bone is advised because of reduced pullout strength.26 In this case, bicortical-post backup fixation may be more suitable. Moreover, although not demonstrated in this study, hardware prominence and irritation with a post may cause postoperative morbidity necessitating future removal.20 Hardware removal was the most common reason for additional surgery using hamstring tendons as graft.20 A second surgery for hardware removal was required in 21% of these patients.20 This is unlikely to occur with a SwiveLock, as the anchor is buried within cortical bone.
Limitations
Regarding use of nonhuman tissues in a biomechanical model, porcine tibiae and bovine extensor tendons were used because of availability, consistency among specimens, and cost-effectiveness. However, bovine extensor tendons have been shown to exhibit stiffness and viscoelastic properties similar to those of a human hamstring graft.27 In addition, the BMD of the porcine tibiae used in this study was not tested because of time involved and cost-efficiency. However, it has been shown that average BMD of porcine tibiae, 1.220-1.675 g/cm², is similar to that in a young athletic population, 1.24-1.62 g/cm2.3,28-31 We therefore assumed similarity to a young athletic population and uniformity of BMD of the porcine tibiae used in this study.
In addition, the biomechanical testing protocol did not simulate physiologic loading within the tibial tunnel. Moreover, the testing protocol used loads of only 250 N during cyclic testing for 500 cycles. This simulates only the early rehabilitation period and not the healing period, which may last up to 12 weeks.9 In addition, as previously mentioned, forces on the graft may be as high as 450 to 700 N.11,32 Pullout testing in line with the long axis of the tibia was performed in order to compare mechanical testing results with those of similar studies.3,12,13 Last, the P of .07 for the comparison of ultimate load to failure between the 2 backup fixation groups suggests that this study may have been underpowered.
Conclusion
This study demonstrated an effective, alternative, and equivalent backup fixation device that can help prevent graft slippage within the tibial tunnel during soft-tissue ACL reconstruction. Potential benefits of using SwiveLock anchors for backup fixation include a significantly increased ultimate yield load (229 N) when supplementing an interference screw, ease of insertion compared with a bicortical post, and the improbable need for future hardware removal. We support using SwiveLock for supplementary fixation at the tibial tunnel site when using soft-tissue grafts in ACL reconstruction.
Restoration of stability with return to activity is generally expected after anterior cruciate ligament (ACL) reconstruction; long-term success rates range from 75% to 95%.1 However, graft failure occurs most frequently with soft-tissue grafts fixated only with interference screws.2,3 Fixation failure also occurs more frequently at the tibial site.2 This failure has been attributed to extensive graft slippage in cases of soft-tissue fixation with interference screws.2 Interference screw fixation alone, with a double-looped hamstring tendon graft, fails at 350 N in young human tibiae.4,5 However, failure is limited with use of a bone–tendon–bone graft or with backup fixation, particularly at the tibial site.3 The superiority of bicortical fixation has also been proven.5-7
In addition, as shown in a goat model, ACL graft fixation is a major cause of failure in the immediate postoperative period, before biological incorporation of the graft.8 Fixation techniques for soft-tissue grafts must withstand stresses during the healing period (grafts may take up to 12 weeks to incorporate).9 Failures may result from forces exerted on the graft—forces that may be as high as 450 to 700 N during daily activities.10,11 Within the tibial tunnel, various fixation devices are used, including interference screws, staples, pins, buttons, and interference screw/sheath constructs.12,13 Primary fixation is commonly achieved with interference screws because of their ease of insertion and greater stiffness. However, fixation of the soft-tissue graft is influenced by several variables, including bone density, insertion torque, thread geometry, and interference screw material.14-16 Many of these variables, which are a source of inconsistency and concern during the immediate postoperative period, have led surgeons to seek alternative methods of backup fixation at the tibial site. Nevertheless, good clinical and subjective results have been found after ACL reconstruction with a 4-stranded semitendinosus tendon at 10-year follow-up.17
An anchor used in rotator cuff repair is the SwiveLock system (Arthrex). Major advantages of this system include ease and speed of insertion, good strength, and reduced need for later hardware removal.
We conducted a study to biomechanically evaluate 3 methods of tibial-sided fixation for ACL reconstruction: fully threaded interference screw only, interference screw backed with 4.75-mm SwiveLock anchor, and fully threaded bio-interference screw backed with 4.5-mm bicortical screw. We hypothesized that a fully threaded bio-interference screw backed with a 4.75-mm SwiveLock anchor would provide mechanical strength no different from that provided by backup fixation with a bicortical post at the tibial site. We further hypothesized that SwiveLock backup fixation would provide more strength than fixation with bio-interference screw alone.
Materials and Methods
The design of this study was adapted from one used by Walsh and colleagues,3 who compared 3 fixation methods: retrograde interference screw, suture button, and combined fixation. Tibiae inspected before selection showed no signs of injury or abnormality. Bovine extensor tendons, which lacked any defects along their entire length, were stored in saline 0.9% solution. Both the tibiae and the extensor tendons were stored at –20°C before completion of the tibial-sided ACL reconstruction. Thirty fresh-frozen, skeletally mature porcine proximal tibiae were selected and thawed at 4°C before preparation. Specimens were prepared by potting the diaphysis in fiberglass resin, and a tunnel 9 mm in diameter was drilled through the anteromedial aspect of the tibia.
For consistency, one author (CAV) prepared all 30 specimens. Both tails of all 30 bovine extensor tendons were whip-stitched with No. 2 FiberLoop (Arthrex) 9 mm in diameter. Grafts and tibiae were randomly divided into 3 sample groups. The first group was prepared by antegrade graft fixation within the tibial tunnel using a fully threaded 9×28-mm BioComposite interference screw (Arthrex). The second and third groups used the same primary fixation within the tibial tunnel along with 2 types of secondary fixation. These backup fixation groups included a 4.5-mm titanium bicortical post (Arthrex) and a 4.75-mm BioComposite SwiveLock C anchor (Arthrex) (Figure 1). The FiberLoop at the ends of the distal graft tails for backup groups were fixated 1 cm distal to the tibial tunnel and tapped before insertion of backup devices (Figures 2A, 2B). Insertion was completed after 4.5-mm bicortical and 4.75-mm unicortical drilling and tapping of the anteromedial cortices for the titanium posts and SwiveLocks, respectively. The free ends of the whip-stitched No. 2 FiberLoop were tied to the proximal end of the titanium post with a single surgical knot followed by 5 square knots.3 The free ends of the No. 2 FiberLoop were inserted into the eyelet of the 4.75-mm SwiveLock and 1 cm directly inferior to the tibial tunnel. Interference fit of FiberLoop with SwiveLock was achieved within the corticocancellous bone of the tibiae. All samples retained a 30-mm tendon loop superior to the tibial plateau to simulate intra-articular ACL length. Specimens were then stored at –20°C and thawed at 4°C before biomechanical testing.
Each of the 30 tibiae was tested once. Each testing group consisted of 10 porcine tibiae. The tendons were kept moist during the entire testing procedure by spraying them thoroughly with saline 0.9% solution. Mechanical testing was performed with an Instron 8871 system with a 5-kN load cell secured to the crosshead. A fixed-angle aperture, attached to the testing surface, was adjusted so that the tendon would be pulled in line with the tibial tunnel. A hook fixture suspended from clevis and dowel was used to secure the tendon to the crosshead (Figure 3). A small preload of 5 N manually applied to each sample was followed by a precycling regimen of 10 cycles between 10 N and 50 N at 1 Hz. Precycling was performed to remove slack from the system. Mechanical testing consisted of 500 cycles between 50 N and 250 N at 1 Hz followed by pull to failure at 20 mm per minute. Load and displacement data were recorded at 500 Hz.
An a priori power analysis was not performed because 6 specimens per group in the study from which the testing protocol was adapted demonstrated sufficient power among 3 testing categories.3 In addition, other studies have demonstrated similar testing protocols using 10 specimens per testing group.7,12,13,18 The data for each sample were analyzed with OriginPro 8.0 software (OriginLab). Ultimate load, yield load, stiffness, and cyclic displacement of the 3 sample groups were compared with 1-way analysis of variance (α = 0.05). Holm-Sidak tests were used for post hoc analysis.19P < .05 was statistically significant.
Results
None of the 30 specimens failed during preloading. Modes of failure were consistent among groups. All 10 specimens in the interference-screw-only group failed by graft slippage past the screw in the tibial tunnel. Nineteen of the 20 specimens in the backup-fixation groups failed by graft slippage past the screw and suture cutout through the distal graft tail. In the bicortical-post backup group, 1 failure was attributed to tendon tearing proximal to whip-stitching. There were no instances of hardware breakage or failure of either titanium screw or SwiveLock anchor.
Mean (SD) cyclic displacement was higher in the interference-screw-only group, 3.5 (2.2) mm, than in the SwiveLock backup group, 2.6 (0.5) mm, and the bicortical-post backup group, 2.1 (0.6) mm; no statistical significance was demonstrated between any 2 of these groups alone (P = .12) (Figure 4). Mean (SD) pullout stiffness was higher in the bicortical-post backup group, 192 (48) N/mm, than in the SwiveLock backup group, 164 (53) N/mm, and the screw-only group, 163 (64) N/mm (P = .42) (Figure 5). Mean (SD) initial load at 5 mm of displacement was higher in the bicortical-post backup group, 482 (156) N, and the SwiveLock backup group, 423 (94) N, than in the screw-only group, 381 (169) N (P = .30).
Mean (SD) yield load was higher in the bicortical-post backup group, 829 (253) N, than in the SwiveLock backup group, 642 (172) N, and the interference-screw-only group, 496 (133) N (P = .003). Statistical significance was demonstrated between the screw-only and bicortical-post groups (P = .002) and between the screw-only and SwiveLock groups (P = .048). There was no statistical difference between the bicortical-post and SwiveLock groups (P = .07).
Mean (SD) ultimate load to failure was higher in the bicortical-post backup group, 1148 (186) N, than in the SwiveLock backup group, 1007 (176) N, and the interference-screw-only group, 778 (139) N (Figure 6). The difference was statistically significant, whereby the screw-only group failed at a lower load compared with the bicortical-post group (P < .001) and the SwiveLock group (P = .005). The 2 backup groups were not statistically different (P = .1).
Discussion
We investigated whether a fully threaded bio-interference screw backed with a 4.75-mm SwiveLock anchor would provide mechanically equivalent pullout strength within the tibial tunnel during ACL reconstruction with soft-tissue allografts in comparison either with a fully threaded bio-interference screw backed with a bicortical post or with a fully threaded bio-interference screw without backup fixation. The results of the study support this hypothesis. With SwiveLock used for backup fixation, there was no significant difference in stiffness or cyclic load displacement between the screw-only, SwiveLock, and bicortical-post groups. However, adding backup fixation could particularly help improve fixation consistency. Specifically, although after only 500 cycles there was no statistically significant difference in cyclic displacement, continued cycling may be clinically relevant if graft slippage exceeded limits to allow for healing within the tibial tunnel. Conversely, a significantly larger difference was found between the SwiveLock, bicortical-post, and screw-only groups in yield load and ultimate load to failure. However, there was no significant difference between the SwiveLock and bicortical-post groups.
In this study, interference screw with SwiveLock backup demonstrated a mean (SD) ultimate load to failure of 1007 (176) N, comparable to that found by Walsh and colleagues3 for retrograde bio-interference screw with suture button, 1027 (157.11) N. In a study comparing quadrupled hamstring tibial graft fixation, Intrafix (DePuy Mitek) and an 8×25-mm Bioscrew (Linvatec) demonstrated mean (SD) single-cycle yield loads of 1332 (304) N and 612 (176) N, respectively.13 These results are similar to the ultimate yield loads in the present study: bicortical-post group, 1148 (186) N; SwiveLock group, 1007 (176) N; screw-only group, 778 (139) N. Differences may be attributed to hamstring tendons used in a quadrupled manner in the aforementioned study.12,13 Last, mean (SD) ultimate load to failure in a study that used only a retrograde bio-interference screw (9×20 mm) was 679.00 (109.44) N,3 similar to the 778 (139) N found for interference-screw-only in the present study. The difference is likely attributable to the longer screw (9×28 mm) in our study. Using SwiveLock C in cortical bone, Barber and colleagues18 found mean (SD) loads to failure up to 711.9 (89.1) N.
Clinically, it has been shown that a statistically significant increase in anterior laxity occurred between 4 months and 2 years in 10.7% of patients who underwent hamstring ACL reconstruction.20 The knees were clinically categorized as unstable or severely abnormal. The authors concluded that the clinical outcome was more likely influenced by the methods used to fix the free ends of the graft, specifically with 2 staples or a washer. To simulate early postoperative rehabilitation in the present study, cyclic loading of the graft was performed. Ultimate load to failure was then determined in order to evaluate catastrophic failure strength of the backup fixation devices in comparison with the interference-screw-only group without supplementary fixation.
It has been shown in autologous hamstring ACL reconstruction that a centrally placed polyethylene screw with sheath (Intrafix) performed as well as a standard, eccentrically placed metal interference screw with staple.10 It is therefore logical that backup fixation with use of a similar device (eg, SwiveLock, bicortical post) is necessary to ensure comparable clinical outcomes in relation to a screw/sheath device that has been shown to endure the highest yield loads.2,9,12,13,21-23 Potential benefits of using SwiveLock anchors for backup fixation include a statistically significant increased mean (SD) ultimate yield load of 229 (176) N over interference screw only. These results are similar to those in comparable studies: 218.3 (59.7) N24 and 165 (24.15) N25 in healthy bone with a reported bone mineral density (BMD) of 1.39 g/cm2, similar to that of skeletally mature porcine tibia (1.220-1.675 g/cm²).3 In addition, ease of insertion of this device over a bicortical post was demonstrated. The titanium post required bicortical drilling as well as measurement with a depth gauge to ensure adequate screw length. This process appeared to require more time during specimen preparation and theoretically could prove to be more dangerous clinically.7 However, caution in using a SwiveLock anchor in osteoporotic bone is advised because of reduced pullout strength.26 In this case, bicortical-post backup fixation may be more suitable. Moreover, although not demonstrated in this study, hardware prominence and irritation with a post may cause postoperative morbidity necessitating future removal.20 Hardware removal was the most common reason for additional surgery using hamstring tendons as graft.20 A second surgery for hardware removal was required in 21% of these patients.20 This is unlikely to occur with a SwiveLock, as the anchor is buried within cortical bone.
Limitations
Regarding use of nonhuman tissues in a biomechanical model, porcine tibiae and bovine extensor tendons were used because of availability, consistency among specimens, and cost-effectiveness. However, bovine extensor tendons have been shown to exhibit stiffness and viscoelastic properties similar to those of a human hamstring graft.27 In addition, the BMD of the porcine tibiae used in this study was not tested because of time involved and cost-efficiency. However, it has been shown that average BMD of porcine tibiae, 1.220-1.675 g/cm², is similar to that in a young athletic population, 1.24-1.62 g/cm2.3,28-31 We therefore assumed similarity to a young athletic population and uniformity of BMD of the porcine tibiae used in this study.
In addition, the biomechanical testing protocol did not simulate physiologic loading within the tibial tunnel. Moreover, the testing protocol used loads of only 250 N during cyclic testing for 500 cycles. This simulates only the early rehabilitation period and not the healing period, which may last up to 12 weeks.9 In addition, as previously mentioned, forces on the graft may be as high as 450 to 700 N.11,32 Pullout testing in line with the long axis of the tibia was performed in order to compare mechanical testing results with those of similar studies.3,12,13 Last, the P of .07 for the comparison of ultimate load to failure between the 2 backup fixation groups suggests that this study may have been underpowered.
Conclusion
This study demonstrated an effective, alternative, and equivalent backup fixation device that can help prevent graft slippage within the tibial tunnel during soft-tissue ACL reconstruction. Potential benefits of using SwiveLock anchors for backup fixation include a significantly increased ultimate yield load (229 N) when supplementing an interference screw, ease of insertion compared with a bicortical post, and the improbable need for future hardware removal. We support using SwiveLock for supplementary fixation at the tibial tunnel site when using soft-tissue grafts in ACL reconstruction.
1. Wetzler MJ, Bartolozzi AR, Gillespie MJ, Rubenstein DL, Ciccotti MG, Miller LS. Revision anterior cruciate ligament reconstruction. Oper Tech Orthop. 1996;6(3):181-189.
2. Scheffler SU, Südkamp NP, Göckenjan A, Hoffmann RF, Weiler A. Biomechanical comparison of hamstring and patellar tendon graft anterior cruciate ligament reconstruction techniques: the impact of fixation level and fixation method under cyclic loading. Arthroscopy. 2002;18(3):304-315.
3. Walsh MP, Wijdicks CA, Parker JB, Hapa O, LaPrade RF. A comparison between a retrograde interference screw, suture button, and combined fixation on the tibial side in an all-inside anterior cruciate ligament reconstruction: a biomechanical study in a porcine model. Am J Sports Med. 2009;37(1):160-167.
4. Howell SM, Hull ML. Aggressive rehabilitation using hamstring tendons: graft construct, tibial tunnel placement, fixation properties, and clinical outcome. Am J Knee Surg. 1998;11(2):120-127.
5. Magen HE, Howell SM, Hull ML. Structural properties of six tibial fixation methods for anterior cruciate ligament soft tissue grafts. Am J Sports Med. 1999;27(1):35-43.
6. Beynnon BD, Meriam CM, Ryder SH, Fleming BC, Johnson RJ. The effect of screw insertion torque on tendons fixed with spiked washers. Am J Sports Med. 1998;26(4):536-539.
7. Post WR, King SS. Neurovascular risk of bicortical tibial drilling for screw and spiked washer fixation of soft-tissue anterior cruciate ligament graft. Arthroscopy. 2001;17(3):244-247.
8. Holden JP, Grood ES, Butler DL, et al. Biomechanics of fascia lata ligament replacements: early postoperative changes in the goat. J Orthop Res. 1988;6(5):639-647.
9. Rodeo SA, Arnoczky SP, Torzilli PA, Hidaka C, Warren RF. Tendon-healing in a bone tunnel. A biomechanical and histological study in the dog. J Bone Joint Surg Am. 1993;75(12):1795-1803.
10. Frank CB, Jackson DW. The science of reconstruction of the anterior cruciate ligament. J Bone Joint Surg Am. 1997;79(10):1556-1576.
11. Markolf KL, Willems MJ, Jackson SR, Finerman GA. In situ calibration of miniature sensors implanted into the anterior cruciate ligament. Part I: strain measurements. J Orthop Res. 1998;16(4):455-463.
12. Kousa P, Teppo LN, Jarvinen TL, Vihavainen M, Kannus P, Jarvinen M. The fixation strength of six hamstring tendon graft fixation devices in anterior cruciate ligament reconstruction: I. Femoral site. Am J Sports Med. 2003;3 (2)1:174-181.
13. Kousa P, Jarvinen TL, Vihavainen M, Kannus P, Jarvinen M. The fixation strength of six hamstring tendon graft fixation devices in anterior cruciate ligament reconstruction: II. Tibial site. Am J Sports Med. 2003;31(2):182-188.
14. Brand JC Jr, Pienkowski D, Steenlage E, Hamilton D, Johnson DL, Caborn DN. Interference screw fixation strength of a quadrupled hamstring tendon graft is directly related to bone mineral density and insertion torque. Am J Sports Med. 2000;28(5):705-710.
15. Weiler A, Hoffmann RF, Siepe CJ, Kolbeck SF, Südkamp NP. The influence of screw geometry on hamstring tendon interference fit fixation. Am J Sports Med. 2000;28(3):356-359.
16. Weiler A, Hoffmann RF, Stähelin AC, Bail HJ, Siepe CJ, Südkamp NP. Hamstring tendon fixation using interference screws: a biomechanical study in calf tibial bone. Arthroscopy. 1998;14(1):29-37.
17. Streich NA, Reichenbacher S, Barié A, Buchner M, Schmitt H. Long-term outcome of anterior cruciate ligament reconstruction with an autologous four-strand semitendinosus tendon autograft. Int Orthop. 2013;37(2):279-284.
18. Barber FA, Herbert MA, Beavis C, Barrera Oro F. Suture anchor materials, eyelets, and designs: update 2008. Arthroscopy. 2008;24(8):859-867.
19. Aickin M, Gensler H. Adjusting for multiple testing when reporting research results: the Bonferroni vs Holm methods. Am J Public Health. 1996;86(5):726-728.
20. Howell SM, Deutsch ML. Comparison of endoscopic and two-incision techniques for reconstructing a torn anterior cruciate ligament using hamstring tendons. Arthroscopy. 1999;15(6):594-606.
21. Gwynne-Jones DP, Draffin J, Vane A, Craig R, McMahon S. Failure strengths of concentric and eccentric implants for hamstring graft fixation. ANZ J Surg. 2008;78(3):177-181.
22. Hayes DA, Watts MC, Tevelen GA, Crawford RW. Central versus peripheral tibial screw placement in hamstring anterior cruciate ligament reconstruction: in vitro biomechanics. Arthroscopy. 2005;21(6):703-706.
23. Shino K, Pflaster DS. Comparison of eccentric and concentric screw placement for hamstring graft fixation in the tibial tunnel. Knee Surg Sports Traumatol Arthrosc. 2000;8(2):73-75.
24. Prevrhal S, Fuerst T, Fan B, et al. Quantitative ultrasound of the tibia depends on both cortical density and thickness. Osteoporosis Int. 2001;12(1):28-34.
25. Pietschmann MF, Gülecyüz MF, Fieseler S, et al. Biomechanical stability of knotless suture anchors used in rotator cuff repair in healthy and osteopenic bone. Arthroscopy. 2010;26(8):1035-1044.
26. Burns JP, Snyder SJ, Albritton M. Arthroscopic rotator cuff repair using triple-loaded anchors, suture shuttles, and suture savers. J Am Acad Orthop Surg. 2007;15(7):432-444.
27. Tetsumura S, Fujita A, Nakajima M, Abe M. Biomechanical comparison of different fixation methods on the tibial side in anterior cruciate ligament reconstruction: a biomechanical study in porcine tibial bone. J Orthop Sci. 2006;11(3):278-282.
28. Alfredson H, Nordstrom P, Lorentzon R. Total and regional bone mass in female soccer players. Calcif Tissue Int. 1996;59(6):438-442.
29. Nevill AM, Holder RL, Stewart AD. Modeling elite male athletes’ peripheral bone mass, assessed using regional dual x-ray absorptiometry. Bone. 2003;32(1):62-68.
30. Nordström P, Lorentzon R. Site-specific bone mass differences of the lower extremities in 17-year-old ice hockey players. Calcif Tissue Int. 1996;59(6):4443-4448.
31. Patzer T, Santo G, Olender GD, Wellmann M, Hurschler C, Schofer MD. Suprapectoral or subpectoral position for biceps tenodesis: biomechanical comparison of four different techniques in both positions. J Shoulder Elbow Surg. 2012;21(1):116-125.
32. De Wall M, Scholes CJ, Patel S, Coolican MR, Parker DA. Tibial fixation in anterior cruciate ligament reconstruction: a prospective randomized study comparing metal interference screw and staples with a centrally placed polyethylene screw and sheath. Am J Sports Med. 2011;39(9):1858-1864.
1. Wetzler MJ, Bartolozzi AR, Gillespie MJ, Rubenstein DL, Ciccotti MG, Miller LS. Revision anterior cruciate ligament reconstruction. Oper Tech Orthop. 1996;6(3):181-189.
2. Scheffler SU, Südkamp NP, Göckenjan A, Hoffmann RF, Weiler A. Biomechanical comparison of hamstring and patellar tendon graft anterior cruciate ligament reconstruction techniques: the impact of fixation level and fixation method under cyclic loading. Arthroscopy. 2002;18(3):304-315.
3. Walsh MP, Wijdicks CA, Parker JB, Hapa O, LaPrade RF. A comparison between a retrograde interference screw, suture button, and combined fixation on the tibial side in an all-inside anterior cruciate ligament reconstruction: a biomechanical study in a porcine model. Am J Sports Med. 2009;37(1):160-167.
4. Howell SM, Hull ML. Aggressive rehabilitation using hamstring tendons: graft construct, tibial tunnel placement, fixation properties, and clinical outcome. Am J Knee Surg. 1998;11(2):120-127.
5. Magen HE, Howell SM, Hull ML. Structural properties of six tibial fixation methods for anterior cruciate ligament soft tissue grafts. Am J Sports Med. 1999;27(1):35-43.
6. Beynnon BD, Meriam CM, Ryder SH, Fleming BC, Johnson RJ. The effect of screw insertion torque on tendons fixed with spiked washers. Am J Sports Med. 1998;26(4):536-539.
7. Post WR, King SS. Neurovascular risk of bicortical tibial drilling for screw and spiked washer fixation of soft-tissue anterior cruciate ligament graft. Arthroscopy. 2001;17(3):244-247.
8. Holden JP, Grood ES, Butler DL, et al. Biomechanics of fascia lata ligament replacements: early postoperative changes in the goat. J Orthop Res. 1988;6(5):639-647.
9. Rodeo SA, Arnoczky SP, Torzilli PA, Hidaka C, Warren RF. Tendon-healing in a bone tunnel. A biomechanical and histological study in the dog. J Bone Joint Surg Am. 1993;75(12):1795-1803.
10. Frank CB, Jackson DW. The science of reconstruction of the anterior cruciate ligament. J Bone Joint Surg Am. 1997;79(10):1556-1576.
11. Markolf KL, Willems MJ, Jackson SR, Finerman GA. In situ calibration of miniature sensors implanted into the anterior cruciate ligament. Part I: strain measurements. J Orthop Res. 1998;16(4):455-463.
12. Kousa P, Teppo LN, Jarvinen TL, Vihavainen M, Kannus P, Jarvinen M. The fixation strength of six hamstring tendon graft fixation devices in anterior cruciate ligament reconstruction: I. Femoral site. Am J Sports Med. 2003;3 (2)1:174-181.
13. Kousa P, Jarvinen TL, Vihavainen M, Kannus P, Jarvinen M. The fixation strength of six hamstring tendon graft fixation devices in anterior cruciate ligament reconstruction: II. Tibial site. Am J Sports Med. 2003;31(2):182-188.
14. Brand JC Jr, Pienkowski D, Steenlage E, Hamilton D, Johnson DL, Caborn DN. Interference screw fixation strength of a quadrupled hamstring tendon graft is directly related to bone mineral density and insertion torque. Am J Sports Med. 2000;28(5):705-710.
15. Weiler A, Hoffmann RF, Siepe CJ, Kolbeck SF, Südkamp NP. The influence of screw geometry on hamstring tendon interference fit fixation. Am J Sports Med. 2000;28(3):356-359.
16. Weiler A, Hoffmann RF, Stähelin AC, Bail HJ, Siepe CJ, Südkamp NP. Hamstring tendon fixation using interference screws: a biomechanical study in calf tibial bone. Arthroscopy. 1998;14(1):29-37.
17. Streich NA, Reichenbacher S, Barié A, Buchner M, Schmitt H. Long-term outcome of anterior cruciate ligament reconstruction with an autologous four-strand semitendinosus tendon autograft. Int Orthop. 2013;37(2):279-284.
18. Barber FA, Herbert MA, Beavis C, Barrera Oro F. Suture anchor materials, eyelets, and designs: update 2008. Arthroscopy. 2008;24(8):859-867.
19. Aickin M, Gensler H. Adjusting for multiple testing when reporting research results: the Bonferroni vs Holm methods. Am J Public Health. 1996;86(5):726-728.
20. Howell SM, Deutsch ML. Comparison of endoscopic and two-incision techniques for reconstructing a torn anterior cruciate ligament using hamstring tendons. Arthroscopy. 1999;15(6):594-606.
21. Gwynne-Jones DP, Draffin J, Vane A, Craig R, McMahon S. Failure strengths of concentric and eccentric implants for hamstring graft fixation. ANZ J Surg. 2008;78(3):177-181.
22. Hayes DA, Watts MC, Tevelen GA, Crawford RW. Central versus peripheral tibial screw placement in hamstring anterior cruciate ligament reconstruction: in vitro biomechanics. Arthroscopy. 2005;21(6):703-706.
23. Shino K, Pflaster DS. Comparison of eccentric and concentric screw placement for hamstring graft fixation in the tibial tunnel. Knee Surg Sports Traumatol Arthrosc. 2000;8(2):73-75.
24. Prevrhal S, Fuerst T, Fan B, et al. Quantitative ultrasound of the tibia depends on both cortical density and thickness. Osteoporosis Int. 2001;12(1):28-34.
25. Pietschmann MF, Gülecyüz MF, Fieseler S, et al. Biomechanical stability of knotless suture anchors used in rotator cuff repair in healthy and osteopenic bone. Arthroscopy. 2010;26(8):1035-1044.
26. Burns JP, Snyder SJ, Albritton M. Arthroscopic rotator cuff repair using triple-loaded anchors, suture shuttles, and suture savers. J Am Acad Orthop Surg. 2007;15(7):432-444.
27. Tetsumura S, Fujita A, Nakajima M, Abe M. Biomechanical comparison of different fixation methods on the tibial side in anterior cruciate ligament reconstruction: a biomechanical study in porcine tibial bone. J Orthop Sci. 2006;11(3):278-282.
28. Alfredson H, Nordstrom P, Lorentzon R. Total and regional bone mass in female soccer players. Calcif Tissue Int. 1996;59(6):438-442.
29. Nevill AM, Holder RL, Stewart AD. Modeling elite male athletes’ peripheral bone mass, assessed using regional dual x-ray absorptiometry. Bone. 2003;32(1):62-68.
30. Nordström P, Lorentzon R. Site-specific bone mass differences of the lower extremities in 17-year-old ice hockey players. Calcif Tissue Int. 1996;59(6):4443-4448.
31. Patzer T, Santo G, Olender GD, Wellmann M, Hurschler C, Schofer MD. Suprapectoral or subpectoral position for biceps tenodesis: biomechanical comparison of four different techniques in both positions. J Shoulder Elbow Surg. 2012;21(1):116-125.
32. De Wall M, Scholes CJ, Patel S, Coolican MR, Parker DA. Tibial fixation in anterior cruciate ligament reconstruction: a prospective randomized study comparing metal interference screw and staples with a centrally placed polyethylene screw and sheath. Am J Sports Med. 2011;39(9):1858-1864.
Risk Factors for Thromboembolic Events After Surgery for Ankle Fractures
Venous thromboembolic events (VTEs), encompassing both deep vein thrombosis (DVT) and pulmonary embolism (PE), are potentially fatal events that can occur after orthopedic surgery.1 In patients who do not receive prophylaxis, VTE incidence can be as high as 70% for total hip arthroplasty,2 26% for hip fracture,3 and 5% for ankle fracture.4 Based on the relatively low incidence of VTE after ankle fractures and insufficient evidence for VTE prophylaxis in this population, the American Orthopaedic Foot and Ankle Society and the American College of Chest Physicians do not recommend routine screening or prophylaxis for VTE in patients with ankle fractures.1,5 Nevertheless, certain patients may be at increased risk for VTE after open reduction and internal fixation (ORIF) of an ankle fracture. In such cases, further consideration for prophylaxis may be warranted.
Other studies of VTEs have identified general risk factors of increased age, obesity, prior thromboembolic disease, oral contraceptive use, multitrauma, varicose veins, and prolonged immobilization, among others.1,6,7 In orthopedics, most of this research comes from total joint arthroplasty and hip fracture studies. However, there is relatively limited data for ankle fracture. The best studies directly addressing VTE after ORIF of ankle fractures have had important limitations, including missing patient data and suboptimal capture of VTE occurrences,8-10 possibly leading to underestimates of the incidence of VTEs.
Given the limited data available, we conducted a retrospective national-cohort study to determine the incidence of and independent risk factors for VTEs after ankle fracture ORIF. If patients who are at higher risk for VTE can be identified, they can and should be carefully monitored and be considered for VTE prophylaxis. This information is needed for patient counseling and clinical decision-making.
Materials and Methods
This retrospective study used the American College of Surgeons National Surgical Quality Improvement Program (ACS-NSQIP) database, which captures data from more than 370 participating US hospitals.11 In ACS-NSQIP, 150 patient variables are collected from operative reports, medical records, and patient interviews by trained clinical reviewers.11,12 Patients are identified prospectively and randomly sampled at participating hospitals. Routine auditing is performed to ensure high-quality data. Clinical data are collected for the entire 30-day postoperative period, regardless of discharge status during this time.
Patients who underwent ankle fracture ORIF between 2005 and 2012 were identified in the ACS-NSQIP database. They were initially selected by the postoperative diagnosis of ankle fracture (International Classification of Diseases, Ninth Revision codes 824.0-824.9). Of these patients, only those with primary Current Procedural Terminology codes 27766 (ORIF of medial malleolus fracture), 27769 (ORIF of posterior malleolus fracture), 27792 (ORIF of lateral malleolus fracture), 27814 (ORIF of bimalleollar fracture), and 27822/27823 (ORIF of trimalleollar fracture) were included in the analysis. Patients with incomplete perioperative data were excluded, leaving 4412 patients (out of the initial 4785) for analysis.
Patient characteristics, including sex, age, height, weight, and history of smoking, were collected from the ACS-NSQIP database. Body mass index (BMI) was calculated from each patient’s height and weight. Age was divided into approximately 20-year increments, beginning with age 18 years, in order to compare younger, middle-aged, and elderly groups of patients with ankle fractures. BMI was divided into categories based on the World Health Organization definitions of obesity: under 25 kg/m2 (normal weight), 25 to 30 kg/m2 (overweight), 30 to 35 kg/m2 (class I obesity), and 35 kg/m2 or over (class II and class III obesity).13
Information about medical comorbidities is also available in the ACS-NSQIP database. History of pulmonary disease was defined as a history of dyspnea, severe chronic obstructive pulmonary disease, ventilator-assisted respiration within 48 hours before surgery, or current pneumonia. History of heart disease was defined as a history of congestive heart failure (CHF) or angina within 1 month before admission, myocardial infarction within 6 months before admission, cardiac surgery, or percutaneous coronary intervention. American Society of Anesthesiologists (ASA) classes 3 and above signify severe systemic disease. Steroid use was defined as requiring regular administration of corticosteroid medications within 1 month before surgery. Disseminated cancer was defined as a malignancy that has spread to 1 or more sites besides the primary site.
Functional status was defined as the ability to perform activities of daily living (ADLs) within 30 days before surgery. Best functional status during this period was recorded. ACS-NSQIP defines ADLs as the “activities usually performed in the course of a normal day in a person’s life,” including bathing, feeding, dressing, toileting, and mobility. An independent patient does not require assistance for any ADLs; a partially dependent patient requires assistance for some ADLs; and a totally dependent patient requires assistance in all ADLs. Partially and totally dependent patients were grouped for analysis. Anesthesia type was separated into general and nongeneral, which includes monitored anesthesia care, spinal anesthesia, and regional anesthesia.
ACS-NSQIP also records the occurrence of multiple events up to 30 days after surgery. For our study, VTE was defined as the occurrence of a DVT or a PE during this period. ACS-NSQIP defines DVT as a new blood clot or thrombus identified within a vein—with confirmation by duplex ultrasonography, venogram, or computed tomography (CT)—that required therapy (anticoagulation, placement of vena cava filter, and/or clipping of vena cava). PE is recorded if ventilation/perfusion (VQ) scan, CT examination, transesophageal echocardiogram, pulmonary arteriogram, CT angiogram, or any other definitive modality is positive.
Statistical analyses were performed with Stata Version 11.2 (StataCorp). Demographic and comorbidity variables were tested for association with occurrence of VTE using bivariate and multivariate logistic regression.
Final multivariate models were constructed with a backward stepwise process that initially included all potential variables and sequentially excluded variables with the highest P value until only those with P < .200 remained. Variables with .050 < P < .200 were left in the model to control for potential confounding but are not considered significantly associated with the outcome. Statistical significance was established at a 2-sided α of 0.050 (P < .050). The fitness of the final logistic regression model was assessed with the C statistic and the Hosmer-Lemeshow goodness-of-fit test.
Results
For the 4412 ankle fracture patients who met the inclusion criteria, mean (SD) age was 50.9 (18.2) years, and mean (SD) BMI was 30.4 (7.6) kg/m2. The cohort was 40.4% male. Surgery was performed on 235 patients (5.3%) with medial malleolus fracture, 1143 patients (25.9%) with lateral malleolus fracture, 1705 patients (38.6%) with bimalleollar fracture, and 1329 patients (30.1%) with trimalleollar fracture. Table 1 summarizes the patient characteristics.
Of the 33 patients (0.8%) with a VTE recorded within the first 30 postoperative days, 16 (0.4% of all patients) had a DVT recorded, 14 (0.3% of all patients) had a PE recorded, and 3 (0.1% of all patients) had both a DVT and a PE recorded. In 13 (39.4%) of the 33 patients with a VTE, the event occurred after discharge. VTEs were reported a mean (SD) of 11.5 (9.6) days after surgery. No patient in this study died of VTE.
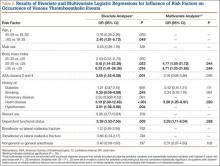
Bivariate logistic regressions were performed to test the association of each patient variable with the occurrence of a VTE. Results are listed in Table 2. The bivariate analyses revealed significant associations between VTE after ankle fracture ORIF and the patient variables of age 60 years or older (odds ratio [OR], 2.40; 95% confidence interval [CI], 1.01-5.72), class I obesity (BMI, 30-35 kg/m2: OR, 5.15, 95% CI, 1.14-23.28), class II and class III obesity (BMI, ≥35 kg/m2: OR, 6.33, 95% CI, 1.41-28.38), ASA classes 3 and 4 (OR, 3.05; 95% CI, 1.53-6.08), history of heart disease (OR, 5.10; 95% CI, 2.08-12.49), history of hypertension (OR, 2.81; 95% CI, 1.39-5.66), and dependent functional status (OR, 3.39; 95% CI, 1.52-7.56).
Multivariate logistic regression was used to control for potential confounding variables and determine which factors were independently associated with VTEs. Results of this analysis are listed in Table 2 as well. The multivariate analysis revealed that the patient variables of class I obesity (BMI, 30-35 kg/m2: OR, 4.77; 95% CI, 1.05-21.72; P = .044), class II and class III obesity (BMI, ≥35 kg/m2: OR, 4.71; 95% CI, 1.03-21.68; P = .046), history of heart disease (OR, 3.28; 95% CI, 1.20-8.97; P = .020), and dependent functional status (OR, 2.59; 95% CI, 1.11-6.04; P = .028) were independently associated with an increased rate of VTEs. Of note, anesthesia type was not significantly associated with occurrence of VTE on bivariate or multivariate analysis.
The C statistic of the final multivariate model was 0.76, indicating very good distinguishing ability. The Hosmer-Lemeshow goodness-of-fit test showed no evidence of lack of fit.
Discussion
Citing the lack of conclusive evidence and the low incidence of VTE after ankle fracture surgery, current recommendations are to avoid routine VTE prophylaxis in the postoperative management of patients who undergo this surgery.1,5 However, it is important to identify patients who are at increased risk, as some may benefit from VTE prophylaxis. In the present study, we used the large, high-quality ACS-NSQIP database collecting information from multiple US hospitals to examine risk factors for VTE after ankle fracture ORIF. We identified 4412 patients who underwent ankle fracture ORIF between 2005 and 2012, and found an overall VTE incidence of 0.8%. Multivariate analysis identified obesity, history of heart disease, and dependent functional status as independent risk factors for VTE after ankle fracture ORIF.
This study’s 0.8% incidence of VTE after ankle fracture ORIF is consistent with the range (0.29%-5%) reported in other ankle fracture studies.4,8-10,14-18 We found that VTEs occurred a mean of about 11 days after surgery, and no patient died of VTE.
Obesity (BMI, ≥30 kg/m2) had the strongest association with VTEs in this study. Obesity, which is a growing public health concern, can make postoperative care and mobilization more difficult.19 Obesity has previously been associated with VTEs after ankle fractures, and BMI of over 25 kg/m2 is one of the Caprini criteria for thrombosis risk factor assessment.6,10 In our study, however, BMI of 25 to 30 kg/m2 was not associated with an increased VTE rate, indicating that moderately overweight patients may not be at significantly higher risk for VTE (compared with patients with normal BMI) and may not need VTE prophylaxis. VTE prophylaxis after ankle fracture surgery may be considered in patients with BMI over 30 kg/m2.
History of heart disease was also associated with VTEs in this study. Patients with a history of heart disease were at 3 times the risk for VTE within 30 days of ankle fracture surgery. This association is also consistent with the Caprini criteria, which include acute myocardial infarction and CHF as risk factors for venous thrombosis.6 Other studies have found associations between CHF and VTE and between cardiovascular risk factors and VTE.7,20 The association between cardiovascular disease and VTE may derive from the decreased venous flow rate associated with CHF or an overall vascular disease state. These patients may benefit from heightened surveillance and postoperative prophylaxis for VTE.
Dependent functional status was the final risk factor found to be associated with VTE after ankle fracture ORIF. This association likely derives from an inability to mobilize independently, leading to increased venous stasis. Immobilization has been previously associated with increased risk for VTE after ankle surgery.7,14,16,20 Caretakers should be aware of this increased risk during the postoperative period and diligently monitor these patients for signs and symptoms of VTE. Prophylaxis may also be considered in this patient population.
Several risk factors that were significant on bivariate analysis (increased age; increased ASA class; history of diabetes, pulmonary disease, hypertension) were not significant in the final multivariate model. This finding suggests covariance between these factors and those that were significant in the final multivariate model. In particular, age and increased overall comorbidity (represented by increased ASA class) were not significant in our multivariate model—contrary to findings of other studies.8-10 It is possible that history of heart disease alone was responsible for the association between overall comorbidity and VTE in those studies. In the present study, separating and controlling for individual comorbidities could have allowed this association to be more precisely characterized.
The characteristics of the ACS-NSQIP database limited our study in several ways. First, although ACS-NSQIP makes significant efforts to collect as many patient variables as possible, some information is not captured. Data about additional factors that may affect VTE risk (eg, history of previous VTE, hypercoagulable state, history of malignancy other than disseminated cancer, tourniquet time, patient position in operating room) were not available. Second, data are collected only on those postoperative adverse events that occur within 30 days after surgery; data on VTEs that occur later are not captured. However, it has been shown that the majority of VTEs occur within the first 30 days after lower extremity trauma and surgery,21,22 so this follow-up interval was deemed adequate for capture of VTE data. Third, the database does not include information on the prophylactic regimens used for these patients—which may have weakened the associations between predictor variables and VTE risk and led to an underestimated effect size. VTE incidence, as well as the odds of developing a VTE with one of the identified risk factors, may actually be higher than reported in this study.
Conclusion
VTEs are serious complications that can occur after ORIF of ankle fractures. In this study, the overall incidence of VTE after ankle fracture ORIF was 0.8%. Although the American Orthopaedic Foot and Ankle Society and the American College of Chest Physicians do not recommend routine screening or prophylaxis for VTE in patients with ankle fractures,1,5 the results of this study showed there may be a benefit in emphasizing VTE prophylaxis after ankle fracture ORIF in patients with obesity, history of heart disease, or dependent functional status. At minimum, these patients should be more carefully monitored for development of VTEs.
1. American Orthopaedic Foot and Ankle Society. Position statement: the use of VTED prophylaxis in foot and ankle surgery. http://www.aofas.org/medical-community/health-policy/Documents/VTED-Position-Statement-Approv-7-9-13-FINAL.pdf. Updated 2013. Accessed May 10, 2015.
2. Grady-Benson JC, Oishi CS, Hanson PB, Colwell CW Jr, Otis SM, Walker RH. Routine postoperative duplex ultrasonography screening and monitoring for the detection of deep vein thrombosis. A survey of 110 total hip arthroplasties. Clin Orthop Relat Res. 1994;(307):130-141.
3. Salzman EW, Harris WH, DeSanctis RW. Anticoagulation for prevention of thromboembolism following fractures of the hip. New Engl J Med. 1966;275(3):122-130.
4. Patil S, Gandhi J, Curzon I, Hui AC. Incidence of deep-vein thrombosis in patients with fractures of the ankle treated in a plaster cast. J Bone Joint Surg Br. 2007;89(10):1340-1343.
5. Falck-Ytter Y, Francis CW, Johanson NA, et al; American College of Chest Physicians. Prevention of VTE in orthopedic surgery patients: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(2 suppl):e278S-e325S.
6. Caprini JA. Thrombosis risk assessment as a guide to quality patient care. Dis Mon. 2005;51(2-3):70-78.
7. Mayle RE Jr, DiGiovanni CW, Lin SS, Tabrizi P, Chou LB. Current concepts review: venous thromboembolic disease in foot and ankle surgery. Foot Ankle Int. 2007;28(11):1207-1216.
8. Jameson SS, Augustine A, James P, et al. Venous thromboembolic events following foot and ankle surgery in the English National Health Service. J Bone Joint Surg Br. 2011;93(4):490-497.
9. SooHoo NF, Eagan M, Krenek L, Zingmond DS. Incidence and factors predicting pulmonary embolism and deep venous thrombosis following surgical treatment of ankle fractures. Foot Ankle Surg. 2011;17(4):259-262.
10. Shibuya N, Frost CH, Campbell JD, Davis ML, Jupiter DC. Incidence of acute deep vein thrombosis and pulmonary embolism in foot and ankle trauma: analysis of the National Trauma Data Bank. J Foot Ankle Surg. 2012;51(1):63-68.
11. American College of Surgeons National Surgical Quality Improvement Program. User Guide for the 2012 ACS NSQIP Participant Use Data File. http://site.acsnsqip.org/wp-content/uploads/2013/10/ACSNSQIP.PUF_.UserGuide.2012.pdf. Published October 2013. Accessed May 10, 2015.
12. Khuri SF, Henderson WG, Daley J, et al; Principal Investigators of Patient Safety in Surgery Study. Successful implementation of the Department of Veterans Affairs’ National Surgical Quality Improvement Program in the private sector: the Patient Safety in Surgery study. Ann Surg. 2008;248(2):329-336.
13. Must A, Spadano J, Coakley EH, Field AE, Colditz G, Dietz WH. The disease burden associated with overweight and obesity. JAMA. 1999;282(16):1523-1529.
14. Mizel MS, Temple HT, Michelson JD, et al. Thromboembolism after foot and ankle surgery. A multicenter study. Clin Orthop Relat Res. 1998;(348):180-185.
15. Solis G, Saxby T. Incidence of DVT following surgery of the foot and ankle. Foot Ankle Int. 2002;23(5):411-414.
16. Hanslow SS, Grujic L, Slater HK, Chen D. Thromboembolic disease after foot and ankle surgery. Foot Ankle Int. 2006;27(9):693-695.
17. Pelet S, Roger ME, Belzile EL, Bouchard M. The incidence of thromboembolic events in surgically treated ankle fracture. J Bone Joint Surg Am. 2012;94(6):502-506.
18. Manafi Rasi A, Kazemian G, Emami Moghadam M, et al. Deep vein thrombosis following below knee immobilization: the need for chemoprophylaxis. Trauma Mon. 2013;17(4):367-369.
19. Sabharwal S, Root MZ. Impact of obesity on orthopaedics. J Bone Joint Surg Am. 2012;94(11):1045-1052.
20. Kadous A, Abdelgawad AA, Kanlic E. Deep venous thrombosis and pulmonary embolism after surgical treatment of ankle fractures: a case report and review of literature. J Foot Ankle Surg. 2012;51(4):457-463.
21. Forsythe RM, Peitzman AB, DeCato T, et al. Early lower extremity fracture fixation and the risk of early pulmonary embolus: filter before fixation? J Trauma. 2011;70(6):1381-1388.
22. Bjørnarå BT, Gudmundsen TE, Dahl OE. Frequency and timing of clinical venous thromboembolism after major joint surgery. J Bone Joint Surg Br. 2006;88(3):386-391.
Venous thromboembolic events (VTEs), encompassing both deep vein thrombosis (DVT) and pulmonary embolism (PE), are potentially fatal events that can occur after orthopedic surgery.1 In patients who do not receive prophylaxis, VTE incidence can be as high as 70% for total hip arthroplasty,2 26% for hip fracture,3 and 5% for ankle fracture.4 Based on the relatively low incidence of VTE after ankle fractures and insufficient evidence for VTE prophylaxis in this population, the American Orthopaedic Foot and Ankle Society and the American College of Chest Physicians do not recommend routine screening or prophylaxis for VTE in patients with ankle fractures.1,5 Nevertheless, certain patients may be at increased risk for VTE after open reduction and internal fixation (ORIF) of an ankle fracture. In such cases, further consideration for prophylaxis may be warranted.
Other studies of VTEs have identified general risk factors of increased age, obesity, prior thromboembolic disease, oral contraceptive use, multitrauma, varicose veins, and prolonged immobilization, among others.1,6,7 In orthopedics, most of this research comes from total joint arthroplasty and hip fracture studies. However, there is relatively limited data for ankle fracture. The best studies directly addressing VTE after ORIF of ankle fractures have had important limitations, including missing patient data and suboptimal capture of VTE occurrences,8-10 possibly leading to underestimates of the incidence of VTEs.
Given the limited data available, we conducted a retrospective national-cohort study to determine the incidence of and independent risk factors for VTEs after ankle fracture ORIF. If patients who are at higher risk for VTE can be identified, they can and should be carefully monitored and be considered for VTE prophylaxis. This information is needed for patient counseling and clinical decision-making.
Materials and Methods
This retrospective study used the American College of Surgeons National Surgical Quality Improvement Program (ACS-NSQIP) database, which captures data from more than 370 participating US hospitals.11 In ACS-NSQIP, 150 patient variables are collected from operative reports, medical records, and patient interviews by trained clinical reviewers.11,12 Patients are identified prospectively and randomly sampled at participating hospitals. Routine auditing is performed to ensure high-quality data. Clinical data are collected for the entire 30-day postoperative period, regardless of discharge status during this time.
Patients who underwent ankle fracture ORIF between 2005 and 2012 were identified in the ACS-NSQIP database. They were initially selected by the postoperative diagnosis of ankle fracture (International Classification of Diseases, Ninth Revision codes 824.0-824.9). Of these patients, only those with primary Current Procedural Terminology codes 27766 (ORIF of medial malleolus fracture), 27769 (ORIF of posterior malleolus fracture), 27792 (ORIF of lateral malleolus fracture), 27814 (ORIF of bimalleollar fracture), and 27822/27823 (ORIF of trimalleollar fracture) were included in the analysis. Patients with incomplete perioperative data were excluded, leaving 4412 patients (out of the initial 4785) for analysis.
Patient characteristics, including sex, age, height, weight, and history of smoking, were collected from the ACS-NSQIP database. Body mass index (BMI) was calculated from each patient’s height and weight. Age was divided into approximately 20-year increments, beginning with age 18 years, in order to compare younger, middle-aged, and elderly groups of patients with ankle fractures. BMI was divided into categories based on the World Health Organization definitions of obesity: under 25 kg/m2 (normal weight), 25 to 30 kg/m2 (overweight), 30 to 35 kg/m2 (class I obesity), and 35 kg/m2 or over (class II and class III obesity).13
Information about medical comorbidities is also available in the ACS-NSQIP database. History of pulmonary disease was defined as a history of dyspnea, severe chronic obstructive pulmonary disease, ventilator-assisted respiration within 48 hours before surgery, or current pneumonia. History of heart disease was defined as a history of congestive heart failure (CHF) or angina within 1 month before admission, myocardial infarction within 6 months before admission, cardiac surgery, or percutaneous coronary intervention. American Society of Anesthesiologists (ASA) classes 3 and above signify severe systemic disease. Steroid use was defined as requiring regular administration of corticosteroid medications within 1 month before surgery. Disseminated cancer was defined as a malignancy that has spread to 1 or more sites besides the primary site.
Functional status was defined as the ability to perform activities of daily living (ADLs) within 30 days before surgery. Best functional status during this period was recorded. ACS-NSQIP defines ADLs as the “activities usually performed in the course of a normal day in a person’s life,” including bathing, feeding, dressing, toileting, and mobility. An independent patient does not require assistance for any ADLs; a partially dependent patient requires assistance for some ADLs; and a totally dependent patient requires assistance in all ADLs. Partially and totally dependent patients were grouped for analysis. Anesthesia type was separated into general and nongeneral, which includes monitored anesthesia care, spinal anesthesia, and regional anesthesia.
ACS-NSQIP also records the occurrence of multiple events up to 30 days after surgery. For our study, VTE was defined as the occurrence of a DVT or a PE during this period. ACS-NSQIP defines DVT as a new blood clot or thrombus identified within a vein—with confirmation by duplex ultrasonography, venogram, or computed tomography (CT)—that required therapy (anticoagulation, placement of vena cava filter, and/or clipping of vena cava). PE is recorded if ventilation/perfusion (VQ) scan, CT examination, transesophageal echocardiogram, pulmonary arteriogram, CT angiogram, or any other definitive modality is positive.
Statistical analyses were performed with Stata Version 11.2 (StataCorp). Demographic and comorbidity variables were tested for association with occurrence of VTE using bivariate and multivariate logistic regression.
Final multivariate models were constructed with a backward stepwise process that initially included all potential variables and sequentially excluded variables with the highest P value until only those with P < .200 remained. Variables with .050 < P < .200 were left in the model to control for potential confounding but are not considered significantly associated with the outcome. Statistical significance was established at a 2-sided α of 0.050 (P < .050). The fitness of the final logistic regression model was assessed with the C statistic and the Hosmer-Lemeshow goodness-of-fit test.
Results
For the 4412 ankle fracture patients who met the inclusion criteria, mean (SD) age was 50.9 (18.2) years, and mean (SD) BMI was 30.4 (7.6) kg/m2. The cohort was 40.4% male. Surgery was performed on 235 patients (5.3%) with medial malleolus fracture, 1143 patients (25.9%) with lateral malleolus fracture, 1705 patients (38.6%) with bimalleollar fracture, and 1329 patients (30.1%) with trimalleollar fracture. Table 1 summarizes the patient characteristics.
Of the 33 patients (0.8%) with a VTE recorded within the first 30 postoperative days, 16 (0.4% of all patients) had a DVT recorded, 14 (0.3% of all patients) had a PE recorded, and 3 (0.1% of all patients) had both a DVT and a PE recorded. In 13 (39.4%) of the 33 patients with a VTE, the event occurred after discharge. VTEs were reported a mean (SD) of 11.5 (9.6) days after surgery. No patient in this study died of VTE.
Bivariate logistic regressions were performed to test the association of each patient variable with the occurrence of a VTE. Results are listed in Table 2. The bivariate analyses revealed significant associations between VTE after ankle fracture ORIF and the patient variables of age 60 years or older (odds ratio [OR], 2.40; 95% confidence interval [CI], 1.01-5.72), class I obesity (BMI, 30-35 kg/m2: OR, 5.15, 95% CI, 1.14-23.28), class II and class III obesity (BMI, ≥35 kg/m2: OR, 6.33, 95% CI, 1.41-28.38), ASA classes 3 and 4 (OR, 3.05; 95% CI, 1.53-6.08), history of heart disease (OR, 5.10; 95% CI, 2.08-12.49), history of hypertension (OR, 2.81; 95% CI, 1.39-5.66), and dependent functional status (OR, 3.39; 95% CI, 1.52-7.56).
Multivariate logistic regression was used to control for potential confounding variables and determine which factors were independently associated with VTEs. Results of this analysis are listed in Table 2 as well. The multivariate analysis revealed that the patient variables of class I obesity (BMI, 30-35 kg/m2: OR, 4.77; 95% CI, 1.05-21.72; P = .044), class II and class III obesity (BMI, ≥35 kg/m2: OR, 4.71; 95% CI, 1.03-21.68; P = .046), history of heart disease (OR, 3.28; 95% CI, 1.20-8.97; P = .020), and dependent functional status (OR, 2.59; 95% CI, 1.11-6.04; P = .028) were independently associated with an increased rate of VTEs. Of note, anesthesia type was not significantly associated with occurrence of VTE on bivariate or multivariate analysis.
The C statistic of the final multivariate model was 0.76, indicating very good distinguishing ability. The Hosmer-Lemeshow goodness-of-fit test showed no evidence of lack of fit.
Discussion
Citing the lack of conclusive evidence and the low incidence of VTE after ankle fracture surgery, current recommendations are to avoid routine VTE prophylaxis in the postoperative management of patients who undergo this surgery.1,5 However, it is important to identify patients who are at increased risk, as some may benefit from VTE prophylaxis. In the present study, we used the large, high-quality ACS-NSQIP database collecting information from multiple US hospitals to examine risk factors for VTE after ankle fracture ORIF. We identified 4412 patients who underwent ankle fracture ORIF between 2005 and 2012, and found an overall VTE incidence of 0.8%. Multivariate analysis identified obesity, history of heart disease, and dependent functional status as independent risk factors for VTE after ankle fracture ORIF.
This study’s 0.8% incidence of VTE after ankle fracture ORIF is consistent with the range (0.29%-5%) reported in other ankle fracture studies.4,8-10,14-18 We found that VTEs occurred a mean of about 11 days after surgery, and no patient died of VTE.
Obesity (BMI, ≥30 kg/m2) had the strongest association with VTEs in this study. Obesity, which is a growing public health concern, can make postoperative care and mobilization more difficult.19 Obesity has previously been associated with VTEs after ankle fractures, and BMI of over 25 kg/m2 is one of the Caprini criteria for thrombosis risk factor assessment.6,10 In our study, however, BMI of 25 to 30 kg/m2 was not associated with an increased VTE rate, indicating that moderately overweight patients may not be at significantly higher risk for VTE (compared with patients with normal BMI) and may not need VTE prophylaxis. VTE prophylaxis after ankle fracture surgery may be considered in patients with BMI over 30 kg/m2.
History of heart disease was also associated with VTEs in this study. Patients with a history of heart disease were at 3 times the risk for VTE within 30 days of ankle fracture surgery. This association is also consistent with the Caprini criteria, which include acute myocardial infarction and CHF as risk factors for venous thrombosis.6 Other studies have found associations between CHF and VTE and between cardiovascular risk factors and VTE.7,20 The association between cardiovascular disease and VTE may derive from the decreased venous flow rate associated with CHF or an overall vascular disease state. These patients may benefit from heightened surveillance and postoperative prophylaxis for VTE.
Dependent functional status was the final risk factor found to be associated with VTE after ankle fracture ORIF. This association likely derives from an inability to mobilize independently, leading to increased venous stasis. Immobilization has been previously associated with increased risk for VTE after ankle surgery.7,14,16,20 Caretakers should be aware of this increased risk during the postoperative period and diligently monitor these patients for signs and symptoms of VTE. Prophylaxis may also be considered in this patient population.
Several risk factors that were significant on bivariate analysis (increased age; increased ASA class; history of diabetes, pulmonary disease, hypertension) were not significant in the final multivariate model. This finding suggests covariance between these factors and those that were significant in the final multivariate model. In particular, age and increased overall comorbidity (represented by increased ASA class) were not significant in our multivariate model—contrary to findings of other studies.8-10 It is possible that history of heart disease alone was responsible for the association between overall comorbidity and VTE in those studies. In the present study, separating and controlling for individual comorbidities could have allowed this association to be more precisely characterized.
The characteristics of the ACS-NSQIP database limited our study in several ways. First, although ACS-NSQIP makes significant efforts to collect as many patient variables as possible, some information is not captured. Data about additional factors that may affect VTE risk (eg, history of previous VTE, hypercoagulable state, history of malignancy other than disseminated cancer, tourniquet time, patient position in operating room) were not available. Second, data are collected only on those postoperative adverse events that occur within 30 days after surgery; data on VTEs that occur later are not captured. However, it has been shown that the majority of VTEs occur within the first 30 days after lower extremity trauma and surgery,21,22 so this follow-up interval was deemed adequate for capture of VTE data. Third, the database does not include information on the prophylactic regimens used for these patients—which may have weakened the associations between predictor variables and VTE risk and led to an underestimated effect size. VTE incidence, as well as the odds of developing a VTE with one of the identified risk factors, may actually be higher than reported in this study.
Conclusion
VTEs are serious complications that can occur after ORIF of ankle fractures. In this study, the overall incidence of VTE after ankle fracture ORIF was 0.8%. Although the American Orthopaedic Foot and Ankle Society and the American College of Chest Physicians do not recommend routine screening or prophylaxis for VTE in patients with ankle fractures,1,5 the results of this study showed there may be a benefit in emphasizing VTE prophylaxis after ankle fracture ORIF in patients with obesity, history of heart disease, or dependent functional status. At minimum, these patients should be more carefully monitored for development of VTEs.
Venous thromboembolic events (VTEs), encompassing both deep vein thrombosis (DVT) and pulmonary embolism (PE), are potentially fatal events that can occur after orthopedic surgery.1 In patients who do not receive prophylaxis, VTE incidence can be as high as 70% for total hip arthroplasty,2 26% for hip fracture,3 and 5% for ankle fracture.4 Based on the relatively low incidence of VTE after ankle fractures and insufficient evidence for VTE prophylaxis in this population, the American Orthopaedic Foot and Ankle Society and the American College of Chest Physicians do not recommend routine screening or prophylaxis for VTE in patients with ankle fractures.1,5 Nevertheless, certain patients may be at increased risk for VTE after open reduction and internal fixation (ORIF) of an ankle fracture. In such cases, further consideration for prophylaxis may be warranted.
Other studies of VTEs have identified general risk factors of increased age, obesity, prior thromboembolic disease, oral contraceptive use, multitrauma, varicose veins, and prolonged immobilization, among others.1,6,7 In orthopedics, most of this research comes from total joint arthroplasty and hip fracture studies. However, there is relatively limited data for ankle fracture. The best studies directly addressing VTE after ORIF of ankle fractures have had important limitations, including missing patient data and suboptimal capture of VTE occurrences,8-10 possibly leading to underestimates of the incidence of VTEs.
Given the limited data available, we conducted a retrospective national-cohort study to determine the incidence of and independent risk factors for VTEs after ankle fracture ORIF. If patients who are at higher risk for VTE can be identified, they can and should be carefully monitored and be considered for VTE prophylaxis. This information is needed for patient counseling and clinical decision-making.
Materials and Methods
This retrospective study used the American College of Surgeons National Surgical Quality Improvement Program (ACS-NSQIP) database, which captures data from more than 370 participating US hospitals.11 In ACS-NSQIP, 150 patient variables are collected from operative reports, medical records, and patient interviews by trained clinical reviewers.11,12 Patients are identified prospectively and randomly sampled at participating hospitals. Routine auditing is performed to ensure high-quality data. Clinical data are collected for the entire 30-day postoperative period, regardless of discharge status during this time.
Patients who underwent ankle fracture ORIF between 2005 and 2012 were identified in the ACS-NSQIP database. They were initially selected by the postoperative diagnosis of ankle fracture (International Classification of Diseases, Ninth Revision codes 824.0-824.9). Of these patients, only those with primary Current Procedural Terminology codes 27766 (ORIF of medial malleolus fracture), 27769 (ORIF of posterior malleolus fracture), 27792 (ORIF of lateral malleolus fracture), 27814 (ORIF of bimalleollar fracture), and 27822/27823 (ORIF of trimalleollar fracture) were included in the analysis. Patients with incomplete perioperative data were excluded, leaving 4412 patients (out of the initial 4785) for analysis.
Patient characteristics, including sex, age, height, weight, and history of smoking, were collected from the ACS-NSQIP database. Body mass index (BMI) was calculated from each patient’s height and weight. Age was divided into approximately 20-year increments, beginning with age 18 years, in order to compare younger, middle-aged, and elderly groups of patients with ankle fractures. BMI was divided into categories based on the World Health Organization definitions of obesity: under 25 kg/m2 (normal weight), 25 to 30 kg/m2 (overweight), 30 to 35 kg/m2 (class I obesity), and 35 kg/m2 or over (class II and class III obesity).13
Information about medical comorbidities is also available in the ACS-NSQIP database. History of pulmonary disease was defined as a history of dyspnea, severe chronic obstructive pulmonary disease, ventilator-assisted respiration within 48 hours before surgery, or current pneumonia. History of heart disease was defined as a history of congestive heart failure (CHF) or angina within 1 month before admission, myocardial infarction within 6 months before admission, cardiac surgery, or percutaneous coronary intervention. American Society of Anesthesiologists (ASA) classes 3 and above signify severe systemic disease. Steroid use was defined as requiring regular administration of corticosteroid medications within 1 month before surgery. Disseminated cancer was defined as a malignancy that has spread to 1 or more sites besides the primary site.
Functional status was defined as the ability to perform activities of daily living (ADLs) within 30 days before surgery. Best functional status during this period was recorded. ACS-NSQIP defines ADLs as the “activities usually performed in the course of a normal day in a person’s life,” including bathing, feeding, dressing, toileting, and mobility. An independent patient does not require assistance for any ADLs; a partially dependent patient requires assistance for some ADLs; and a totally dependent patient requires assistance in all ADLs. Partially and totally dependent patients were grouped for analysis. Anesthesia type was separated into general and nongeneral, which includes monitored anesthesia care, spinal anesthesia, and regional anesthesia.
ACS-NSQIP also records the occurrence of multiple events up to 30 days after surgery. For our study, VTE was defined as the occurrence of a DVT or a PE during this period. ACS-NSQIP defines DVT as a new blood clot or thrombus identified within a vein—with confirmation by duplex ultrasonography, venogram, or computed tomography (CT)—that required therapy (anticoagulation, placement of vena cava filter, and/or clipping of vena cava). PE is recorded if ventilation/perfusion (VQ) scan, CT examination, transesophageal echocardiogram, pulmonary arteriogram, CT angiogram, or any other definitive modality is positive.
Statistical analyses were performed with Stata Version 11.2 (StataCorp). Demographic and comorbidity variables were tested for association with occurrence of VTE using bivariate and multivariate logistic regression.
Final multivariate models were constructed with a backward stepwise process that initially included all potential variables and sequentially excluded variables with the highest P value until only those with P < .200 remained. Variables with .050 < P < .200 were left in the model to control for potential confounding but are not considered significantly associated with the outcome. Statistical significance was established at a 2-sided α of 0.050 (P < .050). The fitness of the final logistic regression model was assessed with the C statistic and the Hosmer-Lemeshow goodness-of-fit test.
Results
For the 4412 ankle fracture patients who met the inclusion criteria, mean (SD) age was 50.9 (18.2) years, and mean (SD) BMI was 30.4 (7.6) kg/m2. The cohort was 40.4% male. Surgery was performed on 235 patients (5.3%) with medial malleolus fracture, 1143 patients (25.9%) with lateral malleolus fracture, 1705 patients (38.6%) with bimalleollar fracture, and 1329 patients (30.1%) with trimalleollar fracture. Table 1 summarizes the patient characteristics.
Of the 33 patients (0.8%) with a VTE recorded within the first 30 postoperative days, 16 (0.4% of all patients) had a DVT recorded, 14 (0.3% of all patients) had a PE recorded, and 3 (0.1% of all patients) had both a DVT and a PE recorded. In 13 (39.4%) of the 33 patients with a VTE, the event occurred after discharge. VTEs were reported a mean (SD) of 11.5 (9.6) days after surgery. No patient in this study died of VTE.
Bivariate logistic regressions were performed to test the association of each patient variable with the occurrence of a VTE. Results are listed in Table 2. The bivariate analyses revealed significant associations between VTE after ankle fracture ORIF and the patient variables of age 60 years or older (odds ratio [OR], 2.40; 95% confidence interval [CI], 1.01-5.72), class I obesity (BMI, 30-35 kg/m2: OR, 5.15, 95% CI, 1.14-23.28), class II and class III obesity (BMI, ≥35 kg/m2: OR, 6.33, 95% CI, 1.41-28.38), ASA classes 3 and 4 (OR, 3.05; 95% CI, 1.53-6.08), history of heart disease (OR, 5.10; 95% CI, 2.08-12.49), history of hypertension (OR, 2.81; 95% CI, 1.39-5.66), and dependent functional status (OR, 3.39; 95% CI, 1.52-7.56).
Multivariate logistic regression was used to control for potential confounding variables and determine which factors were independently associated with VTEs. Results of this analysis are listed in Table 2 as well. The multivariate analysis revealed that the patient variables of class I obesity (BMI, 30-35 kg/m2: OR, 4.77; 95% CI, 1.05-21.72; P = .044), class II and class III obesity (BMI, ≥35 kg/m2: OR, 4.71; 95% CI, 1.03-21.68; P = .046), history of heart disease (OR, 3.28; 95% CI, 1.20-8.97; P = .020), and dependent functional status (OR, 2.59; 95% CI, 1.11-6.04; P = .028) were independently associated with an increased rate of VTEs. Of note, anesthesia type was not significantly associated with occurrence of VTE on bivariate or multivariate analysis.
The C statistic of the final multivariate model was 0.76, indicating very good distinguishing ability. The Hosmer-Lemeshow goodness-of-fit test showed no evidence of lack of fit.
Discussion
Citing the lack of conclusive evidence and the low incidence of VTE after ankle fracture surgery, current recommendations are to avoid routine VTE prophylaxis in the postoperative management of patients who undergo this surgery.1,5 However, it is important to identify patients who are at increased risk, as some may benefit from VTE prophylaxis. In the present study, we used the large, high-quality ACS-NSQIP database collecting information from multiple US hospitals to examine risk factors for VTE after ankle fracture ORIF. We identified 4412 patients who underwent ankle fracture ORIF between 2005 and 2012, and found an overall VTE incidence of 0.8%. Multivariate analysis identified obesity, history of heart disease, and dependent functional status as independent risk factors for VTE after ankle fracture ORIF.
This study’s 0.8% incidence of VTE after ankle fracture ORIF is consistent with the range (0.29%-5%) reported in other ankle fracture studies.4,8-10,14-18 We found that VTEs occurred a mean of about 11 days after surgery, and no patient died of VTE.
Obesity (BMI, ≥30 kg/m2) had the strongest association with VTEs in this study. Obesity, which is a growing public health concern, can make postoperative care and mobilization more difficult.19 Obesity has previously been associated with VTEs after ankle fractures, and BMI of over 25 kg/m2 is one of the Caprini criteria for thrombosis risk factor assessment.6,10 In our study, however, BMI of 25 to 30 kg/m2 was not associated with an increased VTE rate, indicating that moderately overweight patients may not be at significantly higher risk for VTE (compared with patients with normal BMI) and may not need VTE prophylaxis. VTE prophylaxis after ankle fracture surgery may be considered in patients with BMI over 30 kg/m2.
History of heart disease was also associated with VTEs in this study. Patients with a history of heart disease were at 3 times the risk for VTE within 30 days of ankle fracture surgery. This association is also consistent with the Caprini criteria, which include acute myocardial infarction and CHF as risk factors for venous thrombosis.6 Other studies have found associations between CHF and VTE and between cardiovascular risk factors and VTE.7,20 The association between cardiovascular disease and VTE may derive from the decreased venous flow rate associated with CHF or an overall vascular disease state. These patients may benefit from heightened surveillance and postoperative prophylaxis for VTE.
Dependent functional status was the final risk factor found to be associated with VTE after ankle fracture ORIF. This association likely derives from an inability to mobilize independently, leading to increased venous stasis. Immobilization has been previously associated with increased risk for VTE after ankle surgery.7,14,16,20 Caretakers should be aware of this increased risk during the postoperative period and diligently monitor these patients for signs and symptoms of VTE. Prophylaxis may also be considered in this patient population.
Several risk factors that were significant on bivariate analysis (increased age; increased ASA class; history of diabetes, pulmonary disease, hypertension) were not significant in the final multivariate model. This finding suggests covariance between these factors and those that were significant in the final multivariate model. In particular, age and increased overall comorbidity (represented by increased ASA class) were not significant in our multivariate model—contrary to findings of other studies.8-10 It is possible that history of heart disease alone was responsible for the association between overall comorbidity and VTE in those studies. In the present study, separating and controlling for individual comorbidities could have allowed this association to be more precisely characterized.
The characteristics of the ACS-NSQIP database limited our study in several ways. First, although ACS-NSQIP makes significant efforts to collect as many patient variables as possible, some information is not captured. Data about additional factors that may affect VTE risk (eg, history of previous VTE, hypercoagulable state, history of malignancy other than disseminated cancer, tourniquet time, patient position in operating room) were not available. Second, data are collected only on those postoperative adverse events that occur within 30 days after surgery; data on VTEs that occur later are not captured. However, it has been shown that the majority of VTEs occur within the first 30 days after lower extremity trauma and surgery,21,22 so this follow-up interval was deemed adequate for capture of VTE data. Third, the database does not include information on the prophylactic regimens used for these patients—which may have weakened the associations between predictor variables and VTE risk and led to an underestimated effect size. VTE incidence, as well as the odds of developing a VTE with one of the identified risk factors, may actually be higher than reported in this study.
Conclusion
VTEs are serious complications that can occur after ORIF of ankle fractures. In this study, the overall incidence of VTE after ankle fracture ORIF was 0.8%. Although the American Orthopaedic Foot and Ankle Society and the American College of Chest Physicians do not recommend routine screening or prophylaxis for VTE in patients with ankle fractures,1,5 the results of this study showed there may be a benefit in emphasizing VTE prophylaxis after ankle fracture ORIF in patients with obesity, history of heart disease, or dependent functional status. At minimum, these patients should be more carefully monitored for development of VTEs.
1. American Orthopaedic Foot and Ankle Society. Position statement: the use of VTED prophylaxis in foot and ankle surgery. http://www.aofas.org/medical-community/health-policy/Documents/VTED-Position-Statement-Approv-7-9-13-FINAL.pdf. Updated 2013. Accessed May 10, 2015.
2. Grady-Benson JC, Oishi CS, Hanson PB, Colwell CW Jr, Otis SM, Walker RH. Routine postoperative duplex ultrasonography screening and monitoring for the detection of deep vein thrombosis. A survey of 110 total hip arthroplasties. Clin Orthop Relat Res. 1994;(307):130-141.
3. Salzman EW, Harris WH, DeSanctis RW. Anticoagulation for prevention of thromboembolism following fractures of the hip. New Engl J Med. 1966;275(3):122-130.
4. Patil S, Gandhi J, Curzon I, Hui AC. Incidence of deep-vein thrombosis in patients with fractures of the ankle treated in a plaster cast. J Bone Joint Surg Br. 2007;89(10):1340-1343.
5. Falck-Ytter Y, Francis CW, Johanson NA, et al; American College of Chest Physicians. Prevention of VTE in orthopedic surgery patients: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(2 suppl):e278S-e325S.
6. Caprini JA. Thrombosis risk assessment as a guide to quality patient care. Dis Mon. 2005;51(2-3):70-78.
7. Mayle RE Jr, DiGiovanni CW, Lin SS, Tabrizi P, Chou LB. Current concepts review: venous thromboembolic disease in foot and ankle surgery. Foot Ankle Int. 2007;28(11):1207-1216.
8. Jameson SS, Augustine A, James P, et al. Venous thromboembolic events following foot and ankle surgery in the English National Health Service. J Bone Joint Surg Br. 2011;93(4):490-497.
9. SooHoo NF, Eagan M, Krenek L, Zingmond DS. Incidence and factors predicting pulmonary embolism and deep venous thrombosis following surgical treatment of ankle fractures. Foot Ankle Surg. 2011;17(4):259-262.
10. Shibuya N, Frost CH, Campbell JD, Davis ML, Jupiter DC. Incidence of acute deep vein thrombosis and pulmonary embolism in foot and ankle trauma: analysis of the National Trauma Data Bank. J Foot Ankle Surg. 2012;51(1):63-68.
11. American College of Surgeons National Surgical Quality Improvement Program. User Guide for the 2012 ACS NSQIP Participant Use Data File. http://site.acsnsqip.org/wp-content/uploads/2013/10/ACSNSQIP.PUF_.UserGuide.2012.pdf. Published October 2013. Accessed May 10, 2015.
12. Khuri SF, Henderson WG, Daley J, et al; Principal Investigators of Patient Safety in Surgery Study. Successful implementation of the Department of Veterans Affairs’ National Surgical Quality Improvement Program in the private sector: the Patient Safety in Surgery study. Ann Surg. 2008;248(2):329-336.
13. Must A, Spadano J, Coakley EH, Field AE, Colditz G, Dietz WH. The disease burden associated with overweight and obesity. JAMA. 1999;282(16):1523-1529.
14. Mizel MS, Temple HT, Michelson JD, et al. Thromboembolism after foot and ankle surgery. A multicenter study. Clin Orthop Relat Res. 1998;(348):180-185.
15. Solis G, Saxby T. Incidence of DVT following surgery of the foot and ankle. Foot Ankle Int. 2002;23(5):411-414.
16. Hanslow SS, Grujic L, Slater HK, Chen D. Thromboembolic disease after foot and ankle surgery. Foot Ankle Int. 2006;27(9):693-695.
17. Pelet S, Roger ME, Belzile EL, Bouchard M. The incidence of thromboembolic events in surgically treated ankle fracture. J Bone Joint Surg Am. 2012;94(6):502-506.
18. Manafi Rasi A, Kazemian G, Emami Moghadam M, et al. Deep vein thrombosis following below knee immobilization: the need for chemoprophylaxis. Trauma Mon. 2013;17(4):367-369.
19. Sabharwal S, Root MZ. Impact of obesity on orthopaedics. J Bone Joint Surg Am. 2012;94(11):1045-1052.
20. Kadous A, Abdelgawad AA, Kanlic E. Deep venous thrombosis and pulmonary embolism after surgical treatment of ankle fractures: a case report and review of literature. J Foot Ankle Surg. 2012;51(4):457-463.
21. Forsythe RM, Peitzman AB, DeCato T, et al. Early lower extremity fracture fixation and the risk of early pulmonary embolus: filter before fixation? J Trauma. 2011;70(6):1381-1388.
22. Bjørnarå BT, Gudmundsen TE, Dahl OE. Frequency and timing of clinical venous thromboembolism after major joint surgery. J Bone Joint Surg Br. 2006;88(3):386-391.
1. American Orthopaedic Foot and Ankle Society. Position statement: the use of VTED prophylaxis in foot and ankle surgery. http://www.aofas.org/medical-community/health-policy/Documents/VTED-Position-Statement-Approv-7-9-13-FINAL.pdf. Updated 2013. Accessed May 10, 2015.
2. Grady-Benson JC, Oishi CS, Hanson PB, Colwell CW Jr, Otis SM, Walker RH. Routine postoperative duplex ultrasonography screening and monitoring for the detection of deep vein thrombosis. A survey of 110 total hip arthroplasties. Clin Orthop Relat Res. 1994;(307):130-141.
3. Salzman EW, Harris WH, DeSanctis RW. Anticoagulation for prevention of thromboembolism following fractures of the hip. New Engl J Med. 1966;275(3):122-130.
4. Patil S, Gandhi J, Curzon I, Hui AC. Incidence of deep-vein thrombosis in patients with fractures of the ankle treated in a plaster cast. J Bone Joint Surg Br. 2007;89(10):1340-1343.
5. Falck-Ytter Y, Francis CW, Johanson NA, et al; American College of Chest Physicians. Prevention of VTE in orthopedic surgery patients: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(2 suppl):e278S-e325S.
6. Caprini JA. Thrombosis risk assessment as a guide to quality patient care. Dis Mon. 2005;51(2-3):70-78.
7. Mayle RE Jr, DiGiovanni CW, Lin SS, Tabrizi P, Chou LB. Current concepts review: venous thromboembolic disease in foot and ankle surgery. Foot Ankle Int. 2007;28(11):1207-1216.
8. Jameson SS, Augustine A, James P, et al. Venous thromboembolic events following foot and ankle surgery in the English National Health Service. J Bone Joint Surg Br. 2011;93(4):490-497.
9. SooHoo NF, Eagan M, Krenek L, Zingmond DS. Incidence and factors predicting pulmonary embolism and deep venous thrombosis following surgical treatment of ankle fractures. Foot Ankle Surg. 2011;17(4):259-262.
10. Shibuya N, Frost CH, Campbell JD, Davis ML, Jupiter DC. Incidence of acute deep vein thrombosis and pulmonary embolism in foot and ankle trauma: analysis of the National Trauma Data Bank. J Foot Ankle Surg. 2012;51(1):63-68.
11. American College of Surgeons National Surgical Quality Improvement Program. User Guide for the 2012 ACS NSQIP Participant Use Data File. http://site.acsnsqip.org/wp-content/uploads/2013/10/ACSNSQIP.PUF_.UserGuide.2012.pdf. Published October 2013. Accessed May 10, 2015.
12. Khuri SF, Henderson WG, Daley J, et al; Principal Investigators of Patient Safety in Surgery Study. Successful implementation of the Department of Veterans Affairs’ National Surgical Quality Improvement Program in the private sector: the Patient Safety in Surgery study. Ann Surg. 2008;248(2):329-336.
13. Must A, Spadano J, Coakley EH, Field AE, Colditz G, Dietz WH. The disease burden associated with overweight and obesity. JAMA. 1999;282(16):1523-1529.
14. Mizel MS, Temple HT, Michelson JD, et al. Thromboembolism after foot and ankle surgery. A multicenter study. Clin Orthop Relat Res. 1998;(348):180-185.
15. Solis G, Saxby T. Incidence of DVT following surgery of the foot and ankle. Foot Ankle Int. 2002;23(5):411-414.
16. Hanslow SS, Grujic L, Slater HK, Chen D. Thromboembolic disease after foot and ankle surgery. Foot Ankle Int. 2006;27(9):693-695.
17. Pelet S, Roger ME, Belzile EL, Bouchard M. The incidence of thromboembolic events in surgically treated ankle fracture. J Bone Joint Surg Am. 2012;94(6):502-506.
18. Manafi Rasi A, Kazemian G, Emami Moghadam M, et al. Deep vein thrombosis following below knee immobilization: the need for chemoprophylaxis. Trauma Mon. 2013;17(4):367-369.
19. Sabharwal S, Root MZ. Impact of obesity on orthopaedics. J Bone Joint Surg Am. 2012;94(11):1045-1052.
20. Kadous A, Abdelgawad AA, Kanlic E. Deep venous thrombosis and pulmonary embolism after surgical treatment of ankle fractures: a case report and review of literature. J Foot Ankle Surg. 2012;51(4):457-463.
21. Forsythe RM, Peitzman AB, DeCato T, et al. Early lower extremity fracture fixation and the risk of early pulmonary embolus: filter before fixation? J Trauma. 2011;70(6):1381-1388.
22. Bjørnarå BT, Gudmundsen TE, Dahl OE. Frequency and timing of clinical venous thromboembolism after major joint surgery. J Bone Joint Surg Br. 2006;88(3):386-391.
Isolating Suture Slippage During Cadaveric Testing of Knotless Anchors
Knotless suture anchor fixation techniques continue to evolve as efficient, low-profile options for arthroscopic rotator cuff repair (RCR).1,2 Excellent outcomes have been reported for constructs that use knotless fixation laterally, typically in suture bridge-type configurations.2-4 Early comparative biomechanical and clinical studies have also demonstrated equivalent results for all-knotless versus conventional constructs for arthroscopic RCR.5-10 Given the increased use and availability of multiple implant designs, it is important to supplement our clinical knowledge of these devices with laboratory studies delineating the biomechanical properties of the anchors that are used to help guide appropriate clinical use of the implants in specific patient populations.
Several biomechanical studies have shown suture slippage to be the weak but crucial link in the design of knotless anchors and the most likely mode of in vivo failure.11,12 Other studies have demonstrated frequent anchor dislodgement from bone, but these analyses involved use of elderly cadaveric specimens and relatively high-force testing protocols.12,13 Because suture-retention force may have exceeded anchor resistance to pullout (imparted by weak cadaveric bone in such biomechanical settings), the focus on suture-retention properties was limited.11 It is thought that, in clinical practice, the majority of patients who undergo RCR tend not to generate the high forces (relative to resistance to bone pullout) used to cause the anchor pullouts observed in biomechanical studies, particularly in the early postoperative setting.11-15 Cadaveric testing, however, often involves use of specimens with diminished bone mineral density (BMD), relative to age, because of the illness and other factors leading to death and donation.
Using a novel testing apparatus, we isolated, analyzed, and compared suture slippage in 2 anchor designs, one with entirely press-fit suture clamping and the other reliant on an intrinsic suture-locking mechanism.
Materials and Methods
Six human cadaveric proximal humeri specimens were used for this biomechanical study. Mean (SD) age was 53.3 (5.7) years (range, 46-59 years). Middle-aged specimens were used in order to best represent the quality of bone typically encountered in RCR surgery. To approximate tissue in clinical use, we used fresh-frozen cadaver tissue. Specimens were maintained at –20°C until about 24 hours before use and then were thawed to room temperature for testing. Specimens were included only if they had a completely intact humeral head and no prior surgery or hardware placement. Before instrumentation, dual-energy x-ray absorptiometry with a QDR-1000 scanner (Hologic) was used to determine BMD of all proximal humeri.
Two knotless suture anchors were compared: PushLock (4.5×18.5 mm; Arthrex) and ReelX STT (5.5×19.4 mm; Stryker). These anchors have multiple surgical indications (including RCR), allow patient-specific tissue tensioning, and use polyetheretherketone eyelets. The clamping force for PushLock depends entirely on the interference fit achieved for the suture between the outside of the anchor and the surrounding trabecular/cortical bone after device insertion, whereas the suture in ReelX is secured within the anchor shaft entirely by an internal ratchet-locking mechanism.
For anchor insertion, shoulders were dissected down to the greater tuberosity of the proximal humerus, and all implants were inserted (by a fellowship-trained surgeon in accordance with manufacturer guidelines) at a 25° insertion angle with manufacturer-supplied instruments. One anchor of each type (Figure 1) was inserted into the center of the rotator cuff footprint on the greater tuberosity of each specimen. Anterior and posterior positions were randomized, and an anchor from the other group was inserted into the matching location on the contralateral matched-pair specimen. In all instances, distance between the anterior and posterior anchors was 2 cm, and anchors were placed midway between the articular margin and the lateral edge of the greater tuberosity (Figure 2). Two strands of size 2 ultrahigh-molecular-weight–polyethylene Force Fiber (Stryker) were loaded into all anchors.
A custom urethane fixture was secured over the center of each anchor to allow testing to focus on suture slippage by minimizing anchor migration (Figure 3). The small aperture of this device allowed suture tails to pass freely through the center of the fixture but prevented disengagement and proximal migration of the suture anchor from the underlying bone through contact of the urethane fixture with the anchor perimeter. Any system deformation observed during testing was restricted to the suture and/or the anchor’s suture-locking mechanism. Testing fixtures also oriented the suture anchor coaxial with the axis of tension, creating a worst-case loading scenario (Figure 3).
PushLock implants were inserted with 5 pounds of tension, as indicated, using a manufacturer-supplied suture tensioner, and ReelX devices were inserted and locked with 2 full rotations, as specified by the manufacturer. After one end of each suture was cut, as would be done in vivo, the 2 other suture ends, which would have been part of the RCR in vivo, were tied together to form an 8-cm circumference loop that was brought through the urethane fixture. Humeri were then mounted in a materials testing system (MTS 810; MTS Systems) servohydraulic load frame, and the suture loop was passed around a cross-bar on the actuator of the testing device. A mechanical testing protocol consisting of modest repetitive forces was carefully chosen to simulate expected activity during rehabilitation after RCR.15 In this protocol, a 60-second preload of 10 N was followed by tensile loading between 10 N and 90 N at a frequency of 0.5 Hz for 500 cycles.15 Cycle duration at 3 mm and 5 mm of suture slippage (threshold for clinical failure) was recorded.12,16,17 In addition, suture slippage was measured after 1, 10, 50, 100, 200, 300, 400, and 500 cycles. The first 5 test cycles were not counted in the analysis to control for initial knot slippage. Finally, after completion of dynamic testing, samples were loaded at a displacement rate of 0.5 mm/s for tension-to-failure testing in the custom fixtures. Maximum failure load, stiffness, and failure mode were recorded. Ultimate failure was defined as suture breakage or gross suture slippage.
Paired Student t test was used to determine significant differences in suture slippage distance between the 2 groups at various cycle durations. In addition, Kaplan-Meier survival test was used to determine statistical differences in sample survival during the dynamic loading test.
Results
Mean (SD) BMD of the cadaveric shoulder specimens was 0.55 (0.13) g/cm2 (range, 0.29-0.68 g/cm2). The testing fixtures isolated suture slippage from anchor–bone disengagement. All 6 PushLock implants demonstrated slippage of more than 3 mm, and 5 of the 6 demonstrated slippage of more than 5 mm. All 6 ReelX devices exhibited slippage of less than 3 mm. In addition, PushLock demonstrated more suture slippage at cycles 1, 10, and 100 (P < .05) and more maximum slippage after 500 cycles (mean, 11.2 mm; SD, 4.7 mm) compared with ReelX (mean, 1.9 mm; SD, 0.5 mm) (P = .004). Figure 4 shows mean suture slippage at each cycle.
Kaplan-Meier analysis revealed significantly (λ2 = 8.170; P = .0043) decreased survival after dynamic testing for PushLock versus ReelX (Figure 5). Survival was defined as suture slippage of less than 5 mm after completion of dynamic testing. Only 1 of the 6 PushLock anchors completed dynamic testing; the other 5 failed via complete suture slippage from the anchor before testing could be completed. All 6 ReelX devices survived dynamic testing.
Therefore, 1 PushLock implant and all 6 ReelX devices were available for subsequent load-to-failure testing. Failure in this setting was defined as suture slippage of more than 10 mm or suture breakage. The PushLock implant failed at a maximum force of 171.8 N with a stiffness of 74.4 N/mm and eventually exhibited gross suture slippage. All 6 ReelX devices failed at a mean (SD) maximum of 273.5 (20.2) N, with a mean (SD) stiffness of 74.1 (17) N/mm. Mechanism of failure for all ReelX devices was suture breakage during the tensile load-to-failure test.
Discussion
We evaluated a new technique designed to isolate suture slippage in knotless anchors used for RCR. The impetus for developing this new method was to provide a means for better analyzing the ability of a knotless anchor to resist suture slippage in the cadaveric biomechanical testing setting. Suture slippage is an important mode of failure during such analyses.11,12 Significant slippage occurred in a range of implants before half the anchor–bone pullout strength was reached in a study using young bovine femoral heads.11 In another study, using young, high-BMD cadaveric humeral heads, initial slippage and maximum failure loads were equivalent among numerous devices using various suture-retention mechanisms, and suture slippage was the most common failure mode.12 Nevertheless, other biomechanical studies have demonstrated frequent failure caused by anchor pullout in elderly human cadaveric specimens with diminished BMD, often with high-force testing protocols.12,13 In the more modest-force, in vivo rehabilitative environment, suture slippage rather than anchor dislodgement may be the main failure mode.11-15
We compared the PushLock implant and its entirely press-fit suture clamping design with the ReelX device, which relies on an intrinsic suture-locking mechanism. Middle-aged (mean, 53.3 years; SD, 5.7 years) cadaveric humeri were tested under physiologically relevant biomechanical conditions to begin to help identify how relatively osteopenic bone may affect suture-retention properties for a given implant. The results showed that the study methodology prevented implant failure via anchor–bone pullout. To our knowledge, this was the first study to exclusively analyze suture slippage in knotless anchors. The findings indicated that implants that rely heavily on a tight interference fit of the suture between the anchor and the surrounding bone may exhibit early slippage and failure after RCR in middle-aged patients with relative osteopenia.11,12 However, this study also demonstrated that devices with intrinsic clamping mechanisms that do not depend on the quality of surrounding bone may better resist suture slippage. It is not clear that all knotless anchors with intrinsic locking mechanisms function equivalently. For instance, Pietschmann and colleagues12 found that 2 of 10 implants with a different internal clamping device were unable to resist failure via suture slippage, even in healthy bone. Similarly, in a study comparing ReelX devices with implants having a different internal suture-retention mechanism, ReelX failed at higher ultimate loads, and typically via anchor dislodgement, versus suture slippage in the other implants.18
It is important to note that, in the present study, the loads at which sutures broke in the intrinsic clamping anchors approached the maximum contractile force of the supraspinatus muscle (302 N).19,20 In addition, these loads were above the resistance of the rotator cuff tendon to cut out with modern suture material.21
This study’s limitations include use of an in vitro human cadaveric model that precluded analysis of the effects of postoperative healing. Biomechanical testing was also performed in a single row-type suture configuration with the rotator cuff tendon removed. Fixtures used during testing oriented the load coaxially with the axis of tension, creating a worst-case loading scenario. Although this form of testing may limit its clinical applicability, its purpose was to critically isolate how well a knotless anchor could resist suture slippage. The methods we used were also limited because the stability of the bone–anchor interface was not assessed. For patients with osteopenia, anchor pullout rather than suture slippage could be the most limiting factor for knotless anchor construct failure, and therefore further testing of both failure modes is needed. Future biomechanical studies should compare various knotless anchors’ suture-slippage characteristics in other constructs in physiologic testing orientations, including double-row and suture-bridge configurations, as well as with intact rotator cuff tendons. In addition, use of labral tape as a substitute for polyblend suture has been suggested to limit suture slippage, and this technique theoretically could have changed the results of this study.22
Conclusion
An implant with an internal ratcheting mechanism for suture retention demonstrated significantly less suture slippage in an axial tension evaluation protocol than a device reliant on interference fit of the suture between the anchor and surrounding bone. In the clinical setting, this may allow for less gap formation during the healing phase following RCR with a knotless anchor. There was also increased maximum load to failure, demonstrating an increased load until catastrophic failure using a device with a ratcheting internal locking mechanism.
1. Thal R. A knotless suture anchor. Design, function, and biomechanical testing. Am J Sports Med. 2001;29(5):646-649.
2. Cole BJ, ElAttrache NS, Anbari A. Arthroscopic rotator cuff repairs: an anatomic and biomechanical rationale for different suture-anchor repair configurations. Arthroscopy. 2007;23(6):662-669.
3. Kim KC, Shin HD, Cha SM, Lee WY. Comparison of repair integrity and functional outcomes for 3 arthroscopic suture bridge rotator cuff repair techniques. Am J Sports Med. 2013;41(2):271-277.
4. Choi CH, Kim SK, Cho MR, et al. Functional outcomes and structural integrity after double-pulley suture bridge rotator cuff repair using serial ultrasonographic examination. J Shoulder Elbow Surg. 2012;21(12):1753-1763.
5. Brown BS, Cooper AD, McIff TE, Key VH, Toby EB. Initial fixation and cyclic loading stability of knotless suture anchors for rotator cuff repair. J Shoulder Elbow Surg. 2008;17(2):313-318.
6. Burkhart SS, Adams CR, Burkhart SS, Schoolfield JD. A biomechanical comparison of 2 techniques of footprint reconstruction for rotator cuff repair: the SwiveLock-FiberChain construct versus standard double-row repair. Arthroscopy. 2009;25(3):274-281.
7. Hepp P, Osterhoff G, Engel T, Marquass B, Klink T, Josten C. Biomechanical evaluation of knotless anatomical double-layer double-row rotator cuff repair: a comparative ex vivo study. Am J Sports Med. 2009;37(7):1363-1369.
8. Maguire M, Goldberg J, Bokor D, et al. Biomechanical evaluation of four different transosseous-equivalent/suture bridge rotator cuff repairs. Knee Surg Sports Traumatol Arthrosc. 2011;19(9):1582-1587.
9. Millar NL, Wu X, Tantau R, Silverstone E, Murrell GA. Open versus two forms of arthroscopic rotator cuff repair. Clin Orthop Relat Res. 2009;467(4):966-978.
10. Rhee YG, Cho NS, Parke CS. Arthroscopic rotator cuff repair using modified Mason-Allen medial row stitch: knotless versus knot-tying suture bridge technique. Am J Sports Med. 2012;40(11):2440-2447.
11. Wieser K, Farshad M, Vlachopoulos L, Ruffieux K, Gerber C, Meyer DC. Suture slippage in knotless suture anchors as a potential failure mechanism in rotator cuff repair. Arthroscopy. 2012;28(11):1622-1627.
12. Pietschmann MF, Gülecyüz MF, Fieseler S, et al. Biomechanical stability of knotless suture anchors used in rotator cuff repair in healthy and osteopenic bone. Arthroscopy. 2010;26(8):1035-1044.
13. Barber FA, Hapa O, Bynum JA. Comparative testing by cyclic loading of rotator cuff suture anchors containing multiple high-strength sutures. Arthroscopy. 2010;26(9 suppl):S134-S141.
14. Barber FA, Coons DA, Ruiz-Suarez M. Cyclic load testing of biodegradable suture anchors containing 2 high-strength sutures. Arthroscopy. 2007;23(4):355-360.
15. Bynum CK, Lee S, Mahar A, Tasto J, Pedowitz R. Failure mode of suture anchors as a function of insertion depth. Am J Sports Med. 2005;33(7):1030-1034.
16. Gerber C, Schneeberger AG, Beck M, Schlegel U. Mechanical strength of repairs of the rotator cuff. J Bone Joint Surg Br. 1994;76(3):371-380.
17. Schneeberger AG, von Roll A, Kalberer F, Jacob HA, Gerber C. Mechanical strength of arthroscopic rotator cuff repair techniques: an in vitro study. J Bone Joint Surg Am. 2002;84(12):2152-2160.
18. Efird C, Traub S, Baldini T, et al. Knotless single-row rotator cuff repair: a comparative biomechanical study of 2 knotless suture anchors. Orthopedics. 2013;36(8):e1033-e1037.
19. Wright PB, Budoff JE, Yeh ML, Kelm ZS, Luo ZP. Strength of damaged suture: an in vitro study. Arthroscopy. 2006;22(12):1270-1275.
20. Burkhart SS. A stepwise approach to arthroscopic rotator cuff repair based on biomechanical principles. Arthroscopy. 2000;16(1):82-90.
21. Bisson LJ, Manohar LM. A biomechanical comparison of the pullout strength of No. 2 FiberWire suture and 2-mm FiberWire tape in bovine rotator cuff tendons. Arthroscopy. 2010;26(11):1463-1468.
22. Burkhart SS, Denard PJ, Konicek J, Hanypsiak BT. Biomechanical validation of load-sharing rip-stop fixation for the repair of tissue-deficient rotator cuff tears. Am J Sports Med. 2014;42(2):457-462.
Knotless suture anchor fixation techniques continue to evolve as efficient, low-profile options for arthroscopic rotator cuff repair (RCR).1,2 Excellent outcomes have been reported for constructs that use knotless fixation laterally, typically in suture bridge-type configurations.2-4 Early comparative biomechanical and clinical studies have also demonstrated equivalent results for all-knotless versus conventional constructs for arthroscopic RCR.5-10 Given the increased use and availability of multiple implant designs, it is important to supplement our clinical knowledge of these devices with laboratory studies delineating the biomechanical properties of the anchors that are used to help guide appropriate clinical use of the implants in specific patient populations.
Several biomechanical studies have shown suture slippage to be the weak but crucial link in the design of knotless anchors and the most likely mode of in vivo failure.11,12 Other studies have demonstrated frequent anchor dislodgement from bone, but these analyses involved use of elderly cadaveric specimens and relatively high-force testing protocols.12,13 Because suture-retention force may have exceeded anchor resistance to pullout (imparted by weak cadaveric bone in such biomechanical settings), the focus on suture-retention properties was limited.11 It is thought that, in clinical practice, the majority of patients who undergo RCR tend not to generate the high forces (relative to resistance to bone pullout) used to cause the anchor pullouts observed in biomechanical studies, particularly in the early postoperative setting.11-15 Cadaveric testing, however, often involves use of specimens with diminished bone mineral density (BMD), relative to age, because of the illness and other factors leading to death and donation.
Using a novel testing apparatus, we isolated, analyzed, and compared suture slippage in 2 anchor designs, one with entirely press-fit suture clamping and the other reliant on an intrinsic suture-locking mechanism.
Materials and Methods
Six human cadaveric proximal humeri specimens were used for this biomechanical study. Mean (SD) age was 53.3 (5.7) years (range, 46-59 years). Middle-aged specimens were used in order to best represent the quality of bone typically encountered in RCR surgery. To approximate tissue in clinical use, we used fresh-frozen cadaver tissue. Specimens were maintained at –20°C until about 24 hours before use and then were thawed to room temperature for testing. Specimens were included only if they had a completely intact humeral head and no prior surgery or hardware placement. Before instrumentation, dual-energy x-ray absorptiometry with a QDR-1000 scanner (Hologic) was used to determine BMD of all proximal humeri.
Two knotless suture anchors were compared: PushLock (4.5×18.5 mm; Arthrex) and ReelX STT (5.5×19.4 mm; Stryker). These anchors have multiple surgical indications (including RCR), allow patient-specific tissue tensioning, and use polyetheretherketone eyelets. The clamping force for PushLock depends entirely on the interference fit achieved for the suture between the outside of the anchor and the surrounding trabecular/cortical bone after device insertion, whereas the suture in ReelX is secured within the anchor shaft entirely by an internal ratchet-locking mechanism.
For anchor insertion, shoulders were dissected down to the greater tuberosity of the proximal humerus, and all implants were inserted (by a fellowship-trained surgeon in accordance with manufacturer guidelines) at a 25° insertion angle with manufacturer-supplied instruments. One anchor of each type (Figure 1) was inserted into the center of the rotator cuff footprint on the greater tuberosity of each specimen. Anterior and posterior positions were randomized, and an anchor from the other group was inserted into the matching location on the contralateral matched-pair specimen. In all instances, distance between the anterior and posterior anchors was 2 cm, and anchors were placed midway between the articular margin and the lateral edge of the greater tuberosity (Figure 2). Two strands of size 2 ultrahigh-molecular-weight–polyethylene Force Fiber (Stryker) were loaded into all anchors.
A custom urethane fixture was secured over the center of each anchor to allow testing to focus on suture slippage by minimizing anchor migration (Figure 3). The small aperture of this device allowed suture tails to pass freely through the center of the fixture but prevented disengagement and proximal migration of the suture anchor from the underlying bone through contact of the urethane fixture with the anchor perimeter. Any system deformation observed during testing was restricted to the suture and/or the anchor’s suture-locking mechanism. Testing fixtures also oriented the suture anchor coaxial with the axis of tension, creating a worst-case loading scenario (Figure 3).
PushLock implants were inserted with 5 pounds of tension, as indicated, using a manufacturer-supplied suture tensioner, and ReelX devices were inserted and locked with 2 full rotations, as specified by the manufacturer. After one end of each suture was cut, as would be done in vivo, the 2 other suture ends, which would have been part of the RCR in vivo, were tied together to form an 8-cm circumference loop that was brought through the urethane fixture. Humeri were then mounted in a materials testing system (MTS 810; MTS Systems) servohydraulic load frame, and the suture loop was passed around a cross-bar on the actuator of the testing device. A mechanical testing protocol consisting of modest repetitive forces was carefully chosen to simulate expected activity during rehabilitation after RCR.15 In this protocol, a 60-second preload of 10 N was followed by tensile loading between 10 N and 90 N at a frequency of 0.5 Hz for 500 cycles.15 Cycle duration at 3 mm and 5 mm of suture slippage (threshold for clinical failure) was recorded.12,16,17 In addition, suture slippage was measured after 1, 10, 50, 100, 200, 300, 400, and 500 cycles. The first 5 test cycles were not counted in the analysis to control for initial knot slippage. Finally, after completion of dynamic testing, samples were loaded at a displacement rate of 0.5 mm/s for tension-to-failure testing in the custom fixtures. Maximum failure load, stiffness, and failure mode were recorded. Ultimate failure was defined as suture breakage or gross suture slippage.
Paired Student t test was used to determine significant differences in suture slippage distance between the 2 groups at various cycle durations. In addition, Kaplan-Meier survival test was used to determine statistical differences in sample survival during the dynamic loading test.
Results
Mean (SD) BMD of the cadaveric shoulder specimens was 0.55 (0.13) g/cm2 (range, 0.29-0.68 g/cm2). The testing fixtures isolated suture slippage from anchor–bone disengagement. All 6 PushLock implants demonstrated slippage of more than 3 mm, and 5 of the 6 demonstrated slippage of more than 5 mm. All 6 ReelX devices exhibited slippage of less than 3 mm. In addition, PushLock demonstrated more suture slippage at cycles 1, 10, and 100 (P < .05) and more maximum slippage after 500 cycles (mean, 11.2 mm; SD, 4.7 mm) compared with ReelX (mean, 1.9 mm; SD, 0.5 mm) (P = .004). Figure 4 shows mean suture slippage at each cycle.
Kaplan-Meier analysis revealed significantly (λ2 = 8.170; P = .0043) decreased survival after dynamic testing for PushLock versus ReelX (Figure 5). Survival was defined as suture slippage of less than 5 mm after completion of dynamic testing. Only 1 of the 6 PushLock anchors completed dynamic testing; the other 5 failed via complete suture slippage from the anchor before testing could be completed. All 6 ReelX devices survived dynamic testing.
Therefore, 1 PushLock implant and all 6 ReelX devices were available for subsequent load-to-failure testing. Failure in this setting was defined as suture slippage of more than 10 mm or suture breakage. The PushLock implant failed at a maximum force of 171.8 N with a stiffness of 74.4 N/mm and eventually exhibited gross suture slippage. All 6 ReelX devices failed at a mean (SD) maximum of 273.5 (20.2) N, with a mean (SD) stiffness of 74.1 (17) N/mm. Mechanism of failure for all ReelX devices was suture breakage during the tensile load-to-failure test.
Discussion
We evaluated a new technique designed to isolate suture slippage in knotless anchors used for RCR. The impetus for developing this new method was to provide a means for better analyzing the ability of a knotless anchor to resist suture slippage in the cadaveric biomechanical testing setting. Suture slippage is an important mode of failure during such analyses.11,12 Significant slippage occurred in a range of implants before half the anchor–bone pullout strength was reached in a study using young bovine femoral heads.11 In another study, using young, high-BMD cadaveric humeral heads, initial slippage and maximum failure loads were equivalent among numerous devices using various suture-retention mechanisms, and suture slippage was the most common failure mode.12 Nevertheless, other biomechanical studies have demonstrated frequent failure caused by anchor pullout in elderly human cadaveric specimens with diminished BMD, often with high-force testing protocols.12,13 In the more modest-force, in vivo rehabilitative environment, suture slippage rather than anchor dislodgement may be the main failure mode.11-15
We compared the PushLock implant and its entirely press-fit suture clamping design with the ReelX device, which relies on an intrinsic suture-locking mechanism. Middle-aged (mean, 53.3 years; SD, 5.7 years) cadaveric humeri were tested under physiologically relevant biomechanical conditions to begin to help identify how relatively osteopenic bone may affect suture-retention properties for a given implant. The results showed that the study methodology prevented implant failure via anchor–bone pullout. To our knowledge, this was the first study to exclusively analyze suture slippage in knotless anchors. The findings indicated that implants that rely heavily on a tight interference fit of the suture between the anchor and the surrounding bone may exhibit early slippage and failure after RCR in middle-aged patients with relative osteopenia.11,12 However, this study also demonstrated that devices with intrinsic clamping mechanisms that do not depend on the quality of surrounding bone may better resist suture slippage. It is not clear that all knotless anchors with intrinsic locking mechanisms function equivalently. For instance, Pietschmann and colleagues12 found that 2 of 10 implants with a different internal clamping device were unable to resist failure via suture slippage, even in healthy bone. Similarly, in a study comparing ReelX devices with implants having a different internal suture-retention mechanism, ReelX failed at higher ultimate loads, and typically via anchor dislodgement, versus suture slippage in the other implants.18
It is important to note that, in the present study, the loads at which sutures broke in the intrinsic clamping anchors approached the maximum contractile force of the supraspinatus muscle (302 N).19,20 In addition, these loads were above the resistance of the rotator cuff tendon to cut out with modern suture material.21
This study’s limitations include use of an in vitro human cadaveric model that precluded analysis of the effects of postoperative healing. Biomechanical testing was also performed in a single row-type suture configuration with the rotator cuff tendon removed. Fixtures used during testing oriented the load coaxially with the axis of tension, creating a worst-case loading scenario. Although this form of testing may limit its clinical applicability, its purpose was to critically isolate how well a knotless anchor could resist suture slippage. The methods we used were also limited because the stability of the bone–anchor interface was not assessed. For patients with osteopenia, anchor pullout rather than suture slippage could be the most limiting factor for knotless anchor construct failure, and therefore further testing of both failure modes is needed. Future biomechanical studies should compare various knotless anchors’ suture-slippage characteristics in other constructs in physiologic testing orientations, including double-row and suture-bridge configurations, as well as with intact rotator cuff tendons. In addition, use of labral tape as a substitute for polyblend suture has been suggested to limit suture slippage, and this technique theoretically could have changed the results of this study.22
Conclusion
An implant with an internal ratcheting mechanism for suture retention demonstrated significantly less suture slippage in an axial tension evaluation protocol than a device reliant on interference fit of the suture between the anchor and surrounding bone. In the clinical setting, this may allow for less gap formation during the healing phase following RCR with a knotless anchor. There was also increased maximum load to failure, demonstrating an increased load until catastrophic failure using a device with a ratcheting internal locking mechanism.
Knotless suture anchor fixation techniques continue to evolve as efficient, low-profile options for arthroscopic rotator cuff repair (RCR).1,2 Excellent outcomes have been reported for constructs that use knotless fixation laterally, typically in suture bridge-type configurations.2-4 Early comparative biomechanical and clinical studies have also demonstrated equivalent results for all-knotless versus conventional constructs for arthroscopic RCR.5-10 Given the increased use and availability of multiple implant designs, it is important to supplement our clinical knowledge of these devices with laboratory studies delineating the biomechanical properties of the anchors that are used to help guide appropriate clinical use of the implants in specific patient populations.
Several biomechanical studies have shown suture slippage to be the weak but crucial link in the design of knotless anchors and the most likely mode of in vivo failure.11,12 Other studies have demonstrated frequent anchor dislodgement from bone, but these analyses involved use of elderly cadaveric specimens and relatively high-force testing protocols.12,13 Because suture-retention force may have exceeded anchor resistance to pullout (imparted by weak cadaveric bone in such biomechanical settings), the focus on suture-retention properties was limited.11 It is thought that, in clinical practice, the majority of patients who undergo RCR tend not to generate the high forces (relative to resistance to bone pullout) used to cause the anchor pullouts observed in biomechanical studies, particularly in the early postoperative setting.11-15 Cadaveric testing, however, often involves use of specimens with diminished bone mineral density (BMD), relative to age, because of the illness and other factors leading to death and donation.
Using a novel testing apparatus, we isolated, analyzed, and compared suture slippage in 2 anchor designs, one with entirely press-fit suture clamping and the other reliant on an intrinsic suture-locking mechanism.
Materials and Methods
Six human cadaveric proximal humeri specimens were used for this biomechanical study. Mean (SD) age was 53.3 (5.7) years (range, 46-59 years). Middle-aged specimens were used in order to best represent the quality of bone typically encountered in RCR surgery. To approximate tissue in clinical use, we used fresh-frozen cadaver tissue. Specimens were maintained at –20°C until about 24 hours before use and then were thawed to room temperature for testing. Specimens were included only if they had a completely intact humeral head and no prior surgery or hardware placement. Before instrumentation, dual-energy x-ray absorptiometry with a QDR-1000 scanner (Hologic) was used to determine BMD of all proximal humeri.
Two knotless suture anchors were compared: PushLock (4.5×18.5 mm; Arthrex) and ReelX STT (5.5×19.4 mm; Stryker). These anchors have multiple surgical indications (including RCR), allow patient-specific tissue tensioning, and use polyetheretherketone eyelets. The clamping force for PushLock depends entirely on the interference fit achieved for the suture between the outside of the anchor and the surrounding trabecular/cortical bone after device insertion, whereas the suture in ReelX is secured within the anchor shaft entirely by an internal ratchet-locking mechanism.
For anchor insertion, shoulders were dissected down to the greater tuberosity of the proximal humerus, and all implants were inserted (by a fellowship-trained surgeon in accordance with manufacturer guidelines) at a 25° insertion angle with manufacturer-supplied instruments. One anchor of each type (Figure 1) was inserted into the center of the rotator cuff footprint on the greater tuberosity of each specimen. Anterior and posterior positions were randomized, and an anchor from the other group was inserted into the matching location on the contralateral matched-pair specimen. In all instances, distance between the anterior and posterior anchors was 2 cm, and anchors were placed midway between the articular margin and the lateral edge of the greater tuberosity (Figure 2). Two strands of size 2 ultrahigh-molecular-weight–polyethylene Force Fiber (Stryker) were loaded into all anchors.
A custom urethane fixture was secured over the center of each anchor to allow testing to focus on suture slippage by minimizing anchor migration (Figure 3). The small aperture of this device allowed suture tails to pass freely through the center of the fixture but prevented disengagement and proximal migration of the suture anchor from the underlying bone through contact of the urethane fixture with the anchor perimeter. Any system deformation observed during testing was restricted to the suture and/or the anchor’s suture-locking mechanism. Testing fixtures also oriented the suture anchor coaxial with the axis of tension, creating a worst-case loading scenario (Figure 3).
PushLock implants were inserted with 5 pounds of tension, as indicated, using a manufacturer-supplied suture tensioner, and ReelX devices were inserted and locked with 2 full rotations, as specified by the manufacturer. After one end of each suture was cut, as would be done in vivo, the 2 other suture ends, which would have been part of the RCR in vivo, were tied together to form an 8-cm circumference loop that was brought through the urethane fixture. Humeri were then mounted in a materials testing system (MTS 810; MTS Systems) servohydraulic load frame, and the suture loop was passed around a cross-bar on the actuator of the testing device. A mechanical testing protocol consisting of modest repetitive forces was carefully chosen to simulate expected activity during rehabilitation after RCR.15 In this protocol, a 60-second preload of 10 N was followed by tensile loading between 10 N and 90 N at a frequency of 0.5 Hz for 500 cycles.15 Cycle duration at 3 mm and 5 mm of suture slippage (threshold for clinical failure) was recorded.12,16,17 In addition, suture slippage was measured after 1, 10, 50, 100, 200, 300, 400, and 500 cycles. The first 5 test cycles were not counted in the analysis to control for initial knot slippage. Finally, after completion of dynamic testing, samples were loaded at a displacement rate of 0.5 mm/s for tension-to-failure testing in the custom fixtures. Maximum failure load, stiffness, and failure mode were recorded. Ultimate failure was defined as suture breakage or gross suture slippage.
Paired Student t test was used to determine significant differences in suture slippage distance between the 2 groups at various cycle durations. In addition, Kaplan-Meier survival test was used to determine statistical differences in sample survival during the dynamic loading test.
Results
Mean (SD) BMD of the cadaveric shoulder specimens was 0.55 (0.13) g/cm2 (range, 0.29-0.68 g/cm2). The testing fixtures isolated suture slippage from anchor–bone disengagement. All 6 PushLock implants demonstrated slippage of more than 3 mm, and 5 of the 6 demonstrated slippage of more than 5 mm. All 6 ReelX devices exhibited slippage of less than 3 mm. In addition, PushLock demonstrated more suture slippage at cycles 1, 10, and 100 (P < .05) and more maximum slippage after 500 cycles (mean, 11.2 mm; SD, 4.7 mm) compared with ReelX (mean, 1.9 mm; SD, 0.5 mm) (P = .004). Figure 4 shows mean suture slippage at each cycle.
Kaplan-Meier analysis revealed significantly (λ2 = 8.170; P = .0043) decreased survival after dynamic testing for PushLock versus ReelX (Figure 5). Survival was defined as suture slippage of less than 5 mm after completion of dynamic testing. Only 1 of the 6 PushLock anchors completed dynamic testing; the other 5 failed via complete suture slippage from the anchor before testing could be completed. All 6 ReelX devices survived dynamic testing.
Therefore, 1 PushLock implant and all 6 ReelX devices were available for subsequent load-to-failure testing. Failure in this setting was defined as suture slippage of more than 10 mm or suture breakage. The PushLock implant failed at a maximum force of 171.8 N with a stiffness of 74.4 N/mm and eventually exhibited gross suture slippage. All 6 ReelX devices failed at a mean (SD) maximum of 273.5 (20.2) N, with a mean (SD) stiffness of 74.1 (17) N/mm. Mechanism of failure for all ReelX devices was suture breakage during the tensile load-to-failure test.
Discussion
We evaluated a new technique designed to isolate suture slippage in knotless anchors used for RCR. The impetus for developing this new method was to provide a means for better analyzing the ability of a knotless anchor to resist suture slippage in the cadaveric biomechanical testing setting. Suture slippage is an important mode of failure during such analyses.11,12 Significant slippage occurred in a range of implants before half the anchor–bone pullout strength was reached in a study using young bovine femoral heads.11 In another study, using young, high-BMD cadaveric humeral heads, initial slippage and maximum failure loads were equivalent among numerous devices using various suture-retention mechanisms, and suture slippage was the most common failure mode.12 Nevertheless, other biomechanical studies have demonstrated frequent failure caused by anchor pullout in elderly human cadaveric specimens with diminished BMD, often with high-force testing protocols.12,13 In the more modest-force, in vivo rehabilitative environment, suture slippage rather than anchor dislodgement may be the main failure mode.11-15
We compared the PushLock implant and its entirely press-fit suture clamping design with the ReelX device, which relies on an intrinsic suture-locking mechanism. Middle-aged (mean, 53.3 years; SD, 5.7 years) cadaveric humeri were tested under physiologically relevant biomechanical conditions to begin to help identify how relatively osteopenic bone may affect suture-retention properties for a given implant. The results showed that the study methodology prevented implant failure via anchor–bone pullout. To our knowledge, this was the first study to exclusively analyze suture slippage in knotless anchors. The findings indicated that implants that rely heavily on a tight interference fit of the suture between the anchor and the surrounding bone may exhibit early slippage and failure after RCR in middle-aged patients with relative osteopenia.11,12 However, this study also demonstrated that devices with intrinsic clamping mechanisms that do not depend on the quality of surrounding bone may better resist suture slippage. It is not clear that all knotless anchors with intrinsic locking mechanisms function equivalently. For instance, Pietschmann and colleagues12 found that 2 of 10 implants with a different internal clamping device were unable to resist failure via suture slippage, even in healthy bone. Similarly, in a study comparing ReelX devices with implants having a different internal suture-retention mechanism, ReelX failed at higher ultimate loads, and typically via anchor dislodgement, versus suture slippage in the other implants.18
It is important to note that, in the present study, the loads at which sutures broke in the intrinsic clamping anchors approached the maximum contractile force of the supraspinatus muscle (302 N).19,20 In addition, these loads were above the resistance of the rotator cuff tendon to cut out with modern suture material.21
This study’s limitations include use of an in vitro human cadaveric model that precluded analysis of the effects of postoperative healing. Biomechanical testing was also performed in a single row-type suture configuration with the rotator cuff tendon removed. Fixtures used during testing oriented the load coaxially with the axis of tension, creating a worst-case loading scenario. Although this form of testing may limit its clinical applicability, its purpose was to critically isolate how well a knotless anchor could resist suture slippage. The methods we used were also limited because the stability of the bone–anchor interface was not assessed. For patients with osteopenia, anchor pullout rather than suture slippage could be the most limiting factor for knotless anchor construct failure, and therefore further testing of both failure modes is needed. Future biomechanical studies should compare various knotless anchors’ suture-slippage characteristics in other constructs in physiologic testing orientations, including double-row and suture-bridge configurations, as well as with intact rotator cuff tendons. In addition, use of labral tape as a substitute for polyblend suture has been suggested to limit suture slippage, and this technique theoretically could have changed the results of this study.22
Conclusion
An implant with an internal ratcheting mechanism for suture retention demonstrated significantly less suture slippage in an axial tension evaluation protocol than a device reliant on interference fit of the suture between the anchor and surrounding bone. In the clinical setting, this may allow for less gap formation during the healing phase following RCR with a knotless anchor. There was also increased maximum load to failure, demonstrating an increased load until catastrophic failure using a device with a ratcheting internal locking mechanism.
1. Thal R. A knotless suture anchor. Design, function, and biomechanical testing. Am J Sports Med. 2001;29(5):646-649.
2. Cole BJ, ElAttrache NS, Anbari A. Arthroscopic rotator cuff repairs: an anatomic and biomechanical rationale for different suture-anchor repair configurations. Arthroscopy. 2007;23(6):662-669.
3. Kim KC, Shin HD, Cha SM, Lee WY. Comparison of repair integrity and functional outcomes for 3 arthroscopic suture bridge rotator cuff repair techniques. Am J Sports Med. 2013;41(2):271-277.
4. Choi CH, Kim SK, Cho MR, et al. Functional outcomes and structural integrity after double-pulley suture bridge rotator cuff repair using serial ultrasonographic examination. J Shoulder Elbow Surg. 2012;21(12):1753-1763.
5. Brown BS, Cooper AD, McIff TE, Key VH, Toby EB. Initial fixation and cyclic loading stability of knotless suture anchors for rotator cuff repair. J Shoulder Elbow Surg. 2008;17(2):313-318.
6. Burkhart SS, Adams CR, Burkhart SS, Schoolfield JD. A biomechanical comparison of 2 techniques of footprint reconstruction for rotator cuff repair: the SwiveLock-FiberChain construct versus standard double-row repair. Arthroscopy. 2009;25(3):274-281.
7. Hepp P, Osterhoff G, Engel T, Marquass B, Klink T, Josten C. Biomechanical evaluation of knotless anatomical double-layer double-row rotator cuff repair: a comparative ex vivo study. Am J Sports Med. 2009;37(7):1363-1369.
8. Maguire M, Goldberg J, Bokor D, et al. Biomechanical evaluation of four different transosseous-equivalent/suture bridge rotator cuff repairs. Knee Surg Sports Traumatol Arthrosc. 2011;19(9):1582-1587.
9. Millar NL, Wu X, Tantau R, Silverstone E, Murrell GA. Open versus two forms of arthroscopic rotator cuff repair. Clin Orthop Relat Res. 2009;467(4):966-978.
10. Rhee YG, Cho NS, Parke CS. Arthroscopic rotator cuff repair using modified Mason-Allen medial row stitch: knotless versus knot-tying suture bridge technique. Am J Sports Med. 2012;40(11):2440-2447.
11. Wieser K, Farshad M, Vlachopoulos L, Ruffieux K, Gerber C, Meyer DC. Suture slippage in knotless suture anchors as a potential failure mechanism in rotator cuff repair. Arthroscopy. 2012;28(11):1622-1627.
12. Pietschmann MF, Gülecyüz MF, Fieseler S, et al. Biomechanical stability of knotless suture anchors used in rotator cuff repair in healthy and osteopenic bone. Arthroscopy. 2010;26(8):1035-1044.
13. Barber FA, Hapa O, Bynum JA. Comparative testing by cyclic loading of rotator cuff suture anchors containing multiple high-strength sutures. Arthroscopy. 2010;26(9 suppl):S134-S141.
14. Barber FA, Coons DA, Ruiz-Suarez M. Cyclic load testing of biodegradable suture anchors containing 2 high-strength sutures. Arthroscopy. 2007;23(4):355-360.
15. Bynum CK, Lee S, Mahar A, Tasto J, Pedowitz R. Failure mode of suture anchors as a function of insertion depth. Am J Sports Med. 2005;33(7):1030-1034.
16. Gerber C, Schneeberger AG, Beck M, Schlegel U. Mechanical strength of repairs of the rotator cuff. J Bone Joint Surg Br. 1994;76(3):371-380.
17. Schneeberger AG, von Roll A, Kalberer F, Jacob HA, Gerber C. Mechanical strength of arthroscopic rotator cuff repair techniques: an in vitro study. J Bone Joint Surg Am. 2002;84(12):2152-2160.
18. Efird C, Traub S, Baldini T, et al. Knotless single-row rotator cuff repair: a comparative biomechanical study of 2 knotless suture anchors. Orthopedics. 2013;36(8):e1033-e1037.
19. Wright PB, Budoff JE, Yeh ML, Kelm ZS, Luo ZP. Strength of damaged suture: an in vitro study. Arthroscopy. 2006;22(12):1270-1275.
20. Burkhart SS. A stepwise approach to arthroscopic rotator cuff repair based on biomechanical principles. Arthroscopy. 2000;16(1):82-90.
21. Bisson LJ, Manohar LM. A biomechanical comparison of the pullout strength of No. 2 FiberWire suture and 2-mm FiberWire tape in bovine rotator cuff tendons. Arthroscopy. 2010;26(11):1463-1468.
22. Burkhart SS, Denard PJ, Konicek J, Hanypsiak BT. Biomechanical validation of load-sharing rip-stop fixation for the repair of tissue-deficient rotator cuff tears. Am J Sports Med. 2014;42(2):457-462.
1. Thal R. A knotless suture anchor. Design, function, and biomechanical testing. Am J Sports Med. 2001;29(5):646-649.
2. Cole BJ, ElAttrache NS, Anbari A. Arthroscopic rotator cuff repairs: an anatomic and biomechanical rationale for different suture-anchor repair configurations. Arthroscopy. 2007;23(6):662-669.
3. Kim KC, Shin HD, Cha SM, Lee WY. Comparison of repair integrity and functional outcomes for 3 arthroscopic suture bridge rotator cuff repair techniques. Am J Sports Med. 2013;41(2):271-277.
4. Choi CH, Kim SK, Cho MR, et al. Functional outcomes and structural integrity after double-pulley suture bridge rotator cuff repair using serial ultrasonographic examination. J Shoulder Elbow Surg. 2012;21(12):1753-1763.
5. Brown BS, Cooper AD, McIff TE, Key VH, Toby EB. Initial fixation and cyclic loading stability of knotless suture anchors for rotator cuff repair. J Shoulder Elbow Surg. 2008;17(2):313-318.
6. Burkhart SS, Adams CR, Burkhart SS, Schoolfield JD. A biomechanical comparison of 2 techniques of footprint reconstruction for rotator cuff repair: the SwiveLock-FiberChain construct versus standard double-row repair. Arthroscopy. 2009;25(3):274-281.
7. Hepp P, Osterhoff G, Engel T, Marquass B, Klink T, Josten C. Biomechanical evaluation of knotless anatomical double-layer double-row rotator cuff repair: a comparative ex vivo study. Am J Sports Med. 2009;37(7):1363-1369.
8. Maguire M, Goldberg J, Bokor D, et al. Biomechanical evaluation of four different transosseous-equivalent/suture bridge rotator cuff repairs. Knee Surg Sports Traumatol Arthrosc. 2011;19(9):1582-1587.
9. Millar NL, Wu X, Tantau R, Silverstone E, Murrell GA. Open versus two forms of arthroscopic rotator cuff repair. Clin Orthop Relat Res. 2009;467(4):966-978.
10. Rhee YG, Cho NS, Parke CS. Arthroscopic rotator cuff repair using modified Mason-Allen medial row stitch: knotless versus knot-tying suture bridge technique. Am J Sports Med. 2012;40(11):2440-2447.
11. Wieser K, Farshad M, Vlachopoulos L, Ruffieux K, Gerber C, Meyer DC. Suture slippage in knotless suture anchors as a potential failure mechanism in rotator cuff repair. Arthroscopy. 2012;28(11):1622-1627.
12. Pietschmann MF, Gülecyüz MF, Fieseler S, et al. Biomechanical stability of knotless suture anchors used in rotator cuff repair in healthy and osteopenic bone. Arthroscopy. 2010;26(8):1035-1044.
13. Barber FA, Hapa O, Bynum JA. Comparative testing by cyclic loading of rotator cuff suture anchors containing multiple high-strength sutures. Arthroscopy. 2010;26(9 suppl):S134-S141.
14. Barber FA, Coons DA, Ruiz-Suarez M. Cyclic load testing of biodegradable suture anchors containing 2 high-strength sutures. Arthroscopy. 2007;23(4):355-360.
15. Bynum CK, Lee S, Mahar A, Tasto J, Pedowitz R. Failure mode of suture anchors as a function of insertion depth. Am J Sports Med. 2005;33(7):1030-1034.
16. Gerber C, Schneeberger AG, Beck M, Schlegel U. Mechanical strength of repairs of the rotator cuff. J Bone Joint Surg Br. 1994;76(3):371-380.
17. Schneeberger AG, von Roll A, Kalberer F, Jacob HA, Gerber C. Mechanical strength of arthroscopic rotator cuff repair techniques: an in vitro study. J Bone Joint Surg Am. 2002;84(12):2152-2160.
18. Efird C, Traub S, Baldini T, et al. Knotless single-row rotator cuff repair: a comparative biomechanical study of 2 knotless suture anchors. Orthopedics. 2013;36(8):e1033-e1037.
19. Wright PB, Budoff JE, Yeh ML, Kelm ZS, Luo ZP. Strength of damaged suture: an in vitro study. Arthroscopy. 2006;22(12):1270-1275.
20. Burkhart SS. A stepwise approach to arthroscopic rotator cuff repair based on biomechanical principles. Arthroscopy. 2000;16(1):82-90.
21. Bisson LJ, Manohar LM. A biomechanical comparison of the pullout strength of No. 2 FiberWire suture and 2-mm FiberWire tape in bovine rotator cuff tendons. Arthroscopy. 2010;26(11):1463-1468.
22. Burkhart SS, Denard PJ, Konicek J, Hanypsiak BT. Biomechanical validation of load-sharing rip-stop fixation for the repair of tissue-deficient rotator cuff tears. Am J Sports Med. 2014;42(2):457-462.
Comparison of Outcomes and Costs of Tension-Band and Locking-Plate Osteosynthesis in Transverse Olecranon Fractures: A Matched-Cohort Study
Olecranon fractures are a common injury, representing 10% of all upper extremity fractures.1 Displaced fractures require fixation to restore anatomical alignment and minimize posttraumatic arthrosis.2,3 Multiple surgical techniques have been developed to treat these fractures, with implant choice largely dictated by fracture pattern and associated injuries. Simple, noncomminuted, transverse, proximal fractures can be treated with a tension-band construct, and fractures that are comminuted, oblique, distal to the midpoint of the sigmoid notch, or associated with complex elbow injuries generally require locking-plate fixation.4,5 Although both tension bands and locking plates have been used successfully (Figures 1A, 1B), they remain some of the most frequently removed orthopedic implants, usually because of implant prominence.6
Both fixation devices have potential advantages and disadvantages. Tension-band fixation requires relatively “low-tech” instrumentation and implants and, as a result, has less cost and potentially less operative time for application. As it is smaller than a plate-and-screw construct, a tension band may be less prone to prominence, but this has not been substantiated in the literature.7-14 Implant migration has been a reported complication of tension-band fixation.7,11,13,15
Locking-plate fixation has been shown to be biomechanically stronger,16 and some reports have shown fewer repeat operations for implant prominence than with tension-band fixation.1,8,17-22 Because of more advanced product development and manufacturing, however, it comes at a higher cost. Plate fixation also requires more steps for application, which may require more operative time, and implant prominence has remained a problem, even with modern plates with lower profiles.19
Previous studies of olecranon fixation have included complex fractures and osteotomies or did not include current-generation precontoured locking plates. We found no other study that compared the outcomes, complications, and costs of tension-band and modern locking-plate fixation of isolated transverse olecranon fractures.
To determine if there are significant differences in outcomes and costs between tension-band and locking-plate fixation of transverse olecranon fractures in adults, we retrospectively compared functional outcomes, complications, and costs in 2 matched cohorts of displaced transverse olecranon fractures. We hypothesized that there would be no differences in functional outcomes, implant prominence, posttraumatic arthrosis, complications, or operative time, but that costs would be less with tension-band fixation.
Materials and Methods
After obtaining institutional review board approval, we retrospectively reviewed the medical records of patients who had undergone fixation of an isolated, transverse, noncomminuted olecranon fracture (Orthopaedic Trauma Association 21B1) at our institution between 2004 and 2011. Inclusion criteria included use of a tension-band construct or a precontoured locking plate, skeletal maturity at time of injury, and minimum 2-year follow-up. Exclusion criteria were open fractures, osteotomies, any other ipsilateral upper extremity fracture, and fractures with comminution, obliquity, or distal location.
Although, based on fracture pattern, tension-band fixation is appropriate for olecranon osteotomies used for distal humeral exposure, we did not include osteotomies because functional outcomes would likely be different from those of true olecranon fractures, in addition to the possibility that the soft-tissue injury from a distal humeral fracture and resultant exposure could result in a different level of implant prominence. To control for demographic variables, we used a cohort design in which patients were matched on age and length of follow-up.
During the study period, we treated 287 olecranon fractures. Forty-nine patients met the inclusion criteria. The study population consisted of 20 patients, 10 in each cohort matched on age and length of follow-up. There were no statistically significant differences between groups in demographic variables, including dominant arm involved and number of worker’s compensation claims (Table 1). Mechanisms of injury were similar in the groups. In the tension-band group, 9 patients fell directly onto their elbow, and 1 fell onto her outstretched hand. In the locking-plate group, 8 patients fell directly onto the elbow, 1 fell onto her outstretched hand, and 1 was injured in a motorcycle accident.
All surgeons, regardless of implant selected, used a posterior incision that curved slightly laterally about the tip of the olecranon. Surgeon preference determined which fixation construct to use. Tension-band fixation was performed using 2 bicortical Kirschner wires and a stainless-steel wire through a distal drill hole to complete the tension band. Of the 10 locking-plate constructs used, 4 were PERI-LOC olecranon locking plates (Smith & Nephew), 3 were LCP olecranon plates (Synthes), and 3 were periarticular proximal ulna locking plates (Zimmer).
All returning patients were seen by either Dr. Amini or Mr. Wilson and underwent range of motion (ROM) measurement with a goniometer; assessment for subjective and objective implant prominence (graded none, mild, moderate, or severe/already had implant removed); and functional scoring using the Mayo Elbow Performance Score (MEPS) and the Quick Disability of the Arm, Shoulder, and Hand (QDASH). Results were classified excellent (MEPS, >90), good (75-89), fair (60-74), and poor (<60).23
Anteroposterior and lateral radiographs of the elbow were obtained at follow-up and were examined for maintenance/integrity of implants, radiographic union, and posttraumatic arthrosis. Arthrosis was graded using the Broberg and Morrey24 classification: grade 0 (normal elbow), grade 1 (slight joint-space narrowing with minimal osteophyte formation), grade 2 (moderate joint-space narrowing with moderate osteophyte formation), grade 3 (severe degenerative changes with gross destruction of joint).
Medical records were examined to determine surgery time. Billing information was examined to determine charges related to each operation, specifically the charge for the implants and the overall charge for the operation, which included anesthesia charges. Subsequent operations were included as applicable.
Student t test was used to compare differences in normative data, and Pearson χ2 test to compare differences in categorical data. Differences with P < .05 were considered significant.
Results
There were no clinically or statistically significant differences in ROM or functional outcomes (Table 2). According to MEPS, results were excellent in 8 and good in 2 patients in the tension-band group and excellent in 7 and good in 3 patients in the locking-plate group.
In patients who had implants removed, average time to subsequent procedure was 6.2 months, and all patients who underwent implant removal did so before 1-year follow-up. Implant removal was required in 4 tension-band patients and 1 locking-plate patient (P = .12). Similarly, 7 tension-band patients (including those with implants removed) and 3 locking-plate patients had implant-related symptoms, with the difference trending (P = .07) toward significance (Table 2).
Patients who elected to have their implants removed tended to be younger than those who did not (45.7 vs 56.0 years); the difference (P = .14) was not significant. Worker’s compensation status did not affect the decision to undergo implant removal. At final follow-up, there were no differences in ROM or functional outcomes between patients who had implants removed and those who did not. No variable predicted which patients had implants removed or not (Table 3).
Implant charges were $207.97 for the tension-band cohort and $6688.52 for the locking-plate cohort (P < .0001). Operative charges for the index procedures were $5171.06 for tension-band fixation and $14,160.26 for locking-plate fixation (P < .0001). Overall operative charges, including charges for subsequent operations, were $6598.36 in the tension-band cohort and $14,333.46 in the locking-plate cohort (P = .001). In a comparison of combined charges for index procedure and implant removal (excluding other repeat operations), charges were $6025.56 for the tension-band cohort and $14,333.46 for the locking-plate cohort (P = .0002). Even if all patients with tension-band fixation and no patients with locking-plate fixation had implant removal, mean charges for all operative care would still be significantly (P = .0005) less in the tension-band cohort than in the locking-plate cohort ($7307.31 vs $14,160.26) (Table 4).
Surgery time was significantly (P = .025) less for tension-band fixation than for locking-plate fixation (55.3 vs 85.4 minutes) (Table 2).
Four tension-band patients and 3 locking-plate patients had radiographic evidence of grade 1 posttraumatic arthrosis (P = .64). None required subsequent procedures. Patients with posttraumatic arthrosis had slightly less flexion, but there was no difference in overall flexion-extension arc or functional outcomes between patients with and without arthrosis (Table 5).
The locking-plate cohort had no other complications, and the tension-band cohort had 3. In 1 tension-band patient, the wire disengaged from the Kirschner wires. The fracture healed, but a subsequent procedure was required for symptomatic implant prominence (Figures 2A–2C). Another tension-band patient developed both posttraumatic arthrofibrosis and cubital tunnel syndrome, in addition to a prominent implant. She underwent capsular release, ulnar nerve transposition, and implant removal. At final follow-up, motion was improved, and ulnar nerve symptoms were resolved. There were no infections in either group. Overall, there were no statistically significant differences in complications between groups.
Discussion
We conducted this study to determine differences between tension-band and locking-plate fixation of isolated, closed, noncomminuted, transverse olecranon fractures. Few studies have directly compared tension-band and locking-plate fixation,8,10,19,25 particularly in reference to outcomes of functional scores, implant prominence, complications, operative time, and cost-effectiveness. We found no study that clinically compared these implants since the advent of precontoured locking plates, and no study that compared results in similar fracture patterns. In our study, we found no differences in functional or radiographic outcomes between groups, but significant differences in charges and overall cost of care.
Our findings suggest that patients return to high functional level an average of 4.3 years after fixation of an olecranon fracture with either a tension band or a locking plate. Both cohorts achieved QDASH scores equivalent to normative values for the general population,26 and all patients in both cohorts achieved either good or excellent results based on MEPS values.23 This is comparable to reported functional outcomes in the literature, with previous reports suggesting 86% to 92% of patients obtain good or excellent results.1,7,8,12,14,17,18,27 The rate of posttraumatic arthrosis in both cohorts was low, and, when present, arthrosis was radiographically mild (no patient had grade 2 or 3 arthrosis). Patients with and without radiographic evidence of arthrosis had similar ROM and functional outcomes.
Our findings also suggest a trend toward fewer implant-related symptoms and less need for implant removal in patients treated with locking plates. Although both implants have high rates of prominence requiring removal, most studies support our findings that tension bands are more prominent than locking plates. Fixation has been reported to cause prominence requiring removal in 42% to 82% of patients with tension bands7-14 and 0% to 47% of patients with locking plates.1,8,17,18,20-22,28 It is important to note that many earlier studies either were conducted before the advent of precontoured locking plates or were not comparative.1,7,9-14,17,18,20-22,28 In one recent study, however, Edwards and colleagues19 surveyed 138 patients and found very similar implant removal rates: 63.6% for tension bands and 62.5% for locking plates. Nevertheless, implant removal rates for fixation of olecranon fractures remain high, regardless of implant used.
Our data did not reveal any difference in ROM or functional outcomes between patients who had and did not have implants removed. This suggests, first, that QDASH and MEPS may not be sensitive in identifying patients with implant prominence, as neither questionnaire incorporates implant prominence into its scoring, and, second, that implant removal does not significantly impair ROM. As a result, surgeons should consider asking patients specifically about symptoms of prominent implants once there is convincing evidence of union and counseling them about implant removal if appropriate.
To our knowledge, the differences in cost and operative time between tension-band and locking-plate fixation have not been previously reported. Our data suggest that the financial differences resulted mainly from implant charges; overall, tension-band fixation was roughly half the cost of locking-plate fixation. In addition, in patients who eventually had implants removed, the cost of implant removal was relatively small compared with the cost of the initial fixation in both cohorts. As a result, even if all patients in the tension-band cohort and no patients in the locking-plate cohort had implants removed, tension-band fixation and subsequent implant removal would still cost half as much as locking-plate fixation without implant removal. Moreover, fixation with a tension band took roughly 30 minutes less than fixation with a plate. Less time in the operating room likely contributed to the additional cost savings realized with tension-band fixation beyond those directly resulting from implant cost.
The strength of this study lies in the homogeneity of cohorts. Each cohort was matched primarily on age and secondarily on length of follow-up. All patients had closed, proximal, transverse fractures without comminution, and we excluded olecranon osteotomies as these represent an entity different from true fractures. Fractures with comminution or distal extension may represent more severe injuries, and functional scores, complications, hardware prominence, and operative time might have been affected by inclusion of these fractures. Further, there were no infections in either group to skew the rate of implant prominence or removal.
The weaknesses of the study lie in its limited sample sizes, retrospective design, and lack of long-term follow-up. Group size was limited by our attempts to create homogenous cohorts. As a result, some patients were not included as participants because of strict exclusion criteria. Most notably, we excluded any fracture not appropriate for tension-band fixation, as well as open fractures and osteotomies. Despite the retrospective nature of the study, all patients were examined by the investigators at final follow-up (minimum, 2 years) for the purpose of this study. It is possible that these functional results may not be sustained over the long term, as the risk for posttraumatic arthrosis in articular injuries builds with time. Although some patients may want to have implants removed later, all our study patients who had implants removed had them removed within 1 year, and all 20 patients were reached at minimum 2-year follow-up. Thus, it is unlikely but possible that some of the other study patients will elect to have implants removed.
1. Buijze G, Kloen P. Clinical evaluation of locking compression plate fixation for comminuted olecranon fractures. J Bone Joint Surg Am. 2009;91(10):
2416-2420.
2. Newman SD, Mauffrey C, Krikler S. Olecranon fractures. Injury. 2009;40(6):575-581.
3. Veillette CJ, Steinmann SP. Olecranon fractures. Orthop Clin North Am. 2008;39(2):229-236.
4. Baecher N, Edwards S. Olecranon fractures. J Hand Surg Am. 2013;38(3):593-604.
5. Hak DJ, Golladay GJ. Olecranon fractures: treatment options. J Am Acad Orthop Surg. 2000;8(4):266-275.
6. Busam ML, Esther RJ, Obremskey WT. Hardware removal: indications and expectations. J Am Acad Orthop Surg. 2006;14(2):113-120.
7. Chalidis BE, Sachinis NC, Samoladas EP, Dimitriou CG, Pournaras JD. Is tension band wiring technique the “gold standard” for the treatment of olecranon fractures? A long term functional outcome study. J Orthop Surg Res. 2008;3:9.
8. Hume MC, Wiss DA. Olecranon fractures: a clinical and radiographic comparison of tension-band wiring and plate fixation. Clin Orthop Relat Res. 1992;(285):229-235.
9. Karlsson MK, Hasserius R, Besjakov J, Karlsson C, Josefsson PO. Comparison of tension-band and figure-of-eight wiring techniques for treatment of olecranon fractures. J Shoulder Elbow Surg. 2002;11(4):377-382.
10. Lindenhovius AL, Brouwer KM, Doornberg JN, Ring DC, Kloen P. Long-term outcome of operatively treated fracture-dislocations of the olecranon. J Orthop Trauma. 2008;22(5):325-331.
11. Macko D, Szabo RM. Complications of tension-band wiring of olecranon fractures. J Bone Joint Surg Am. 1985;67(9):1396-1401.
12. Romero JM, Miran A, Jensen CH. Complications and re-operation rate after tension-band wiring of olecranon fractures. J Orthop Sci. 2000;5(4):318-320.
13. Rommens PM, Schneider RU, Reuter M. Functional results after operative treatment of olecranon fractures. Acta Chir Belg. 2004;104(2):191-197.
14. Villanueva P, Osorio F, Commessatti M, Sanchez-Sotelo J. Tension-band wiring for olecranon fractures: analysis of risk factors for failure. J Shoulder Elbow Surg. 2006;15(3):351-356.
15. Sahajpal D, Wright TW. Proximal ulna fractures. J Hand Surg Am. 2009;34(2):357-362.
16. Rouleau DM, Sandman E, van Riet R, Galatz LM. Management of fractures of the proximal ulna. J Am Acad Orthop Surg. 2013;21(3):149-160.
17. Anderson ML, Larson AN, Merten SM, Steinmann SP. Congruent elbow plate fixation of olecranon fractures. J Orthop Trauma. 2007;21(6):386-393.
18. Bailey CS, MacDermid J, Patterson SD, King GJ. Outcome of plate fixation of olecranon fractures. J Orthop Trauma. 2001;15(8):542-548.
19. Edwards SG, Cohen MS, Lattanza LL, et al. Surgeon perceptions and patient outcomes regarding proximal ulna fixation: a multicenter experience. J Shoulder Elbow Surg. 2012;21(12):1637-1643.
20. Munoz-Mahamud E, Fernandez-Valencia JA, Riba J. Plate osteosynthesis for severe olecranon fractures. J Orthop Surg. 2010;18(1):80-84.
21. Simpson NS, Goodman LA, Jupiter JB. Contoured LCDC plating of the proximal ulna. Injury. 1996;27(6):411-417.
22. Tejwani NC, Garnham IR, Wolinsky PR, Kummer FJ, Koval KJ. Posterior olecranon plating: biomechanical and clinical evaluation of a new operative technique. Bull Hosp Jt Dis. 2002-2003;61(1-2):27-31.
23. Morrey BF, An KN. Functional evaluation of the elbow. In: Morrey BF, Sanchez-Sotelo J, eds. The Elbow and Its Disorders. 4th ed. Philadelphia, PA: Elsevier; 2008:87-88.
24. Broberg MA, Morrey BF. The results of delayed excision of the radial head for fracture. J Bone Joint Surg Am. 1986;68(5):669-674.
25. Horne JG, Tanzer TL. Olecranon fractures: a review of 100 cases. J Trauma. 1981;21(6):469-472.
26. Hunsaker FG, Cioffi DA, Amadio PC, Wright JG, Caughlin B. The American Academy of Orthopaedic Surgeons outcomes instruments: normative values from the general population. J Bone Joint Surg Am. 2002;84(2):208-215.
27. Ikeda M, Fukushima Y, Kobayashi Y, Oka Y. Comminuted fractures of the olecranon. Management by bone graft from the iliac crest and multiple tension-band wiring. J Bone Joint Surg Br. 2001;83(6):805-808.
28. Erturer RE, Sever C, Sonmez MM, Ozcelik IB, Akman S, Ozturk I. Results of open reduction and plate osteosynthesis in comminuted fracture of the olecranon. J Shoulder Elbow Surg. 2011;20(3):449-454.
Olecranon fractures are a common injury, representing 10% of all upper extremity fractures.1 Displaced fractures require fixation to restore anatomical alignment and minimize posttraumatic arthrosis.2,3 Multiple surgical techniques have been developed to treat these fractures, with implant choice largely dictated by fracture pattern and associated injuries. Simple, noncomminuted, transverse, proximal fractures can be treated with a tension-band construct, and fractures that are comminuted, oblique, distal to the midpoint of the sigmoid notch, or associated with complex elbow injuries generally require locking-plate fixation.4,5 Although both tension bands and locking plates have been used successfully (Figures 1A, 1B), they remain some of the most frequently removed orthopedic implants, usually because of implant prominence.6
Both fixation devices have potential advantages and disadvantages. Tension-band fixation requires relatively “low-tech” instrumentation and implants and, as a result, has less cost and potentially less operative time for application. As it is smaller than a plate-and-screw construct, a tension band may be less prone to prominence, but this has not been substantiated in the literature.7-14 Implant migration has been a reported complication of tension-band fixation.7,11,13,15
Locking-plate fixation has been shown to be biomechanically stronger,16 and some reports have shown fewer repeat operations for implant prominence than with tension-band fixation.1,8,17-22 Because of more advanced product development and manufacturing, however, it comes at a higher cost. Plate fixation also requires more steps for application, which may require more operative time, and implant prominence has remained a problem, even with modern plates with lower profiles.19
Previous studies of olecranon fixation have included complex fractures and osteotomies or did not include current-generation precontoured locking plates. We found no other study that compared the outcomes, complications, and costs of tension-band and modern locking-plate fixation of isolated transverse olecranon fractures.
To determine if there are significant differences in outcomes and costs between tension-band and locking-plate fixation of transverse olecranon fractures in adults, we retrospectively compared functional outcomes, complications, and costs in 2 matched cohorts of displaced transverse olecranon fractures. We hypothesized that there would be no differences in functional outcomes, implant prominence, posttraumatic arthrosis, complications, or operative time, but that costs would be less with tension-band fixation.
Materials and Methods
After obtaining institutional review board approval, we retrospectively reviewed the medical records of patients who had undergone fixation of an isolated, transverse, noncomminuted olecranon fracture (Orthopaedic Trauma Association 21B1) at our institution between 2004 and 2011. Inclusion criteria included use of a tension-band construct or a precontoured locking plate, skeletal maturity at time of injury, and minimum 2-year follow-up. Exclusion criteria were open fractures, osteotomies, any other ipsilateral upper extremity fracture, and fractures with comminution, obliquity, or distal location.
Although, based on fracture pattern, tension-band fixation is appropriate for olecranon osteotomies used for distal humeral exposure, we did not include osteotomies because functional outcomes would likely be different from those of true olecranon fractures, in addition to the possibility that the soft-tissue injury from a distal humeral fracture and resultant exposure could result in a different level of implant prominence. To control for demographic variables, we used a cohort design in which patients were matched on age and length of follow-up.
During the study period, we treated 287 olecranon fractures. Forty-nine patients met the inclusion criteria. The study population consisted of 20 patients, 10 in each cohort matched on age and length of follow-up. There were no statistically significant differences between groups in demographic variables, including dominant arm involved and number of worker’s compensation claims (Table 1). Mechanisms of injury were similar in the groups. In the tension-band group, 9 patients fell directly onto their elbow, and 1 fell onto her outstretched hand. In the locking-plate group, 8 patients fell directly onto the elbow, 1 fell onto her outstretched hand, and 1 was injured in a motorcycle accident.
All surgeons, regardless of implant selected, used a posterior incision that curved slightly laterally about the tip of the olecranon. Surgeon preference determined which fixation construct to use. Tension-band fixation was performed using 2 bicortical Kirschner wires and a stainless-steel wire through a distal drill hole to complete the tension band. Of the 10 locking-plate constructs used, 4 were PERI-LOC olecranon locking plates (Smith & Nephew), 3 were LCP olecranon plates (Synthes), and 3 were periarticular proximal ulna locking plates (Zimmer).
All returning patients were seen by either Dr. Amini or Mr. Wilson and underwent range of motion (ROM) measurement with a goniometer; assessment for subjective and objective implant prominence (graded none, mild, moderate, or severe/already had implant removed); and functional scoring using the Mayo Elbow Performance Score (MEPS) and the Quick Disability of the Arm, Shoulder, and Hand (QDASH). Results were classified excellent (MEPS, >90), good (75-89), fair (60-74), and poor (<60).23
Anteroposterior and lateral radiographs of the elbow were obtained at follow-up and were examined for maintenance/integrity of implants, radiographic union, and posttraumatic arthrosis. Arthrosis was graded using the Broberg and Morrey24 classification: grade 0 (normal elbow), grade 1 (slight joint-space narrowing with minimal osteophyte formation), grade 2 (moderate joint-space narrowing with moderate osteophyte formation), grade 3 (severe degenerative changes with gross destruction of joint).
Medical records were examined to determine surgery time. Billing information was examined to determine charges related to each operation, specifically the charge for the implants and the overall charge for the operation, which included anesthesia charges. Subsequent operations were included as applicable.
Student t test was used to compare differences in normative data, and Pearson χ2 test to compare differences in categorical data. Differences with P < .05 were considered significant.
Results
There were no clinically or statistically significant differences in ROM or functional outcomes (Table 2). According to MEPS, results were excellent in 8 and good in 2 patients in the tension-band group and excellent in 7 and good in 3 patients in the locking-plate group.
In patients who had implants removed, average time to subsequent procedure was 6.2 months, and all patients who underwent implant removal did so before 1-year follow-up. Implant removal was required in 4 tension-band patients and 1 locking-plate patient (P = .12). Similarly, 7 tension-band patients (including those with implants removed) and 3 locking-plate patients had implant-related symptoms, with the difference trending (P = .07) toward significance (Table 2).
Patients who elected to have their implants removed tended to be younger than those who did not (45.7 vs 56.0 years); the difference (P = .14) was not significant. Worker’s compensation status did not affect the decision to undergo implant removal. At final follow-up, there were no differences in ROM or functional outcomes between patients who had implants removed and those who did not. No variable predicted which patients had implants removed or not (Table 3).
Implant charges were $207.97 for the tension-band cohort and $6688.52 for the locking-plate cohort (P < .0001). Operative charges for the index procedures were $5171.06 for tension-band fixation and $14,160.26 for locking-plate fixation (P < .0001). Overall operative charges, including charges for subsequent operations, were $6598.36 in the tension-band cohort and $14,333.46 in the locking-plate cohort (P = .001). In a comparison of combined charges for index procedure and implant removal (excluding other repeat operations), charges were $6025.56 for the tension-band cohort and $14,333.46 for the locking-plate cohort (P = .0002). Even if all patients with tension-band fixation and no patients with locking-plate fixation had implant removal, mean charges for all operative care would still be significantly (P = .0005) less in the tension-band cohort than in the locking-plate cohort ($7307.31 vs $14,160.26) (Table 4).
Surgery time was significantly (P = .025) less for tension-band fixation than for locking-plate fixation (55.3 vs 85.4 minutes) (Table 2).
Four tension-band patients and 3 locking-plate patients had radiographic evidence of grade 1 posttraumatic arthrosis (P = .64). None required subsequent procedures. Patients with posttraumatic arthrosis had slightly less flexion, but there was no difference in overall flexion-extension arc or functional outcomes between patients with and without arthrosis (Table 5).
The locking-plate cohort had no other complications, and the tension-band cohort had 3. In 1 tension-band patient, the wire disengaged from the Kirschner wires. The fracture healed, but a subsequent procedure was required for symptomatic implant prominence (Figures 2A–2C). Another tension-band patient developed both posttraumatic arthrofibrosis and cubital tunnel syndrome, in addition to a prominent implant. She underwent capsular release, ulnar nerve transposition, and implant removal. At final follow-up, motion was improved, and ulnar nerve symptoms were resolved. There were no infections in either group. Overall, there were no statistically significant differences in complications between groups.
Discussion
We conducted this study to determine differences between tension-band and locking-plate fixation of isolated, closed, noncomminuted, transverse olecranon fractures. Few studies have directly compared tension-band and locking-plate fixation,8,10,19,25 particularly in reference to outcomes of functional scores, implant prominence, complications, operative time, and cost-effectiveness. We found no study that clinically compared these implants since the advent of precontoured locking plates, and no study that compared results in similar fracture patterns. In our study, we found no differences in functional or radiographic outcomes between groups, but significant differences in charges and overall cost of care.
Our findings suggest that patients return to high functional level an average of 4.3 years after fixation of an olecranon fracture with either a tension band or a locking plate. Both cohorts achieved QDASH scores equivalent to normative values for the general population,26 and all patients in both cohorts achieved either good or excellent results based on MEPS values.23 This is comparable to reported functional outcomes in the literature, with previous reports suggesting 86% to 92% of patients obtain good or excellent results.1,7,8,12,14,17,18,27 The rate of posttraumatic arthrosis in both cohorts was low, and, when present, arthrosis was radiographically mild (no patient had grade 2 or 3 arthrosis). Patients with and without radiographic evidence of arthrosis had similar ROM and functional outcomes.
Our findings also suggest a trend toward fewer implant-related symptoms and less need for implant removal in patients treated with locking plates. Although both implants have high rates of prominence requiring removal, most studies support our findings that tension bands are more prominent than locking plates. Fixation has been reported to cause prominence requiring removal in 42% to 82% of patients with tension bands7-14 and 0% to 47% of patients with locking plates.1,8,17,18,20-22,28 It is important to note that many earlier studies either were conducted before the advent of precontoured locking plates or were not comparative.1,7,9-14,17,18,20-22,28 In one recent study, however, Edwards and colleagues19 surveyed 138 patients and found very similar implant removal rates: 63.6% for tension bands and 62.5% for locking plates. Nevertheless, implant removal rates for fixation of olecranon fractures remain high, regardless of implant used.
Our data did not reveal any difference in ROM or functional outcomes between patients who had and did not have implants removed. This suggests, first, that QDASH and MEPS may not be sensitive in identifying patients with implant prominence, as neither questionnaire incorporates implant prominence into its scoring, and, second, that implant removal does not significantly impair ROM. As a result, surgeons should consider asking patients specifically about symptoms of prominent implants once there is convincing evidence of union and counseling them about implant removal if appropriate.
To our knowledge, the differences in cost and operative time between tension-band and locking-plate fixation have not been previously reported. Our data suggest that the financial differences resulted mainly from implant charges; overall, tension-band fixation was roughly half the cost of locking-plate fixation. In addition, in patients who eventually had implants removed, the cost of implant removal was relatively small compared with the cost of the initial fixation in both cohorts. As a result, even if all patients in the tension-band cohort and no patients in the locking-plate cohort had implants removed, tension-band fixation and subsequent implant removal would still cost half as much as locking-plate fixation without implant removal. Moreover, fixation with a tension band took roughly 30 minutes less than fixation with a plate. Less time in the operating room likely contributed to the additional cost savings realized with tension-band fixation beyond those directly resulting from implant cost.
The strength of this study lies in the homogeneity of cohorts. Each cohort was matched primarily on age and secondarily on length of follow-up. All patients had closed, proximal, transverse fractures without comminution, and we excluded olecranon osteotomies as these represent an entity different from true fractures. Fractures with comminution or distal extension may represent more severe injuries, and functional scores, complications, hardware prominence, and operative time might have been affected by inclusion of these fractures. Further, there were no infections in either group to skew the rate of implant prominence or removal.
The weaknesses of the study lie in its limited sample sizes, retrospective design, and lack of long-term follow-up. Group size was limited by our attempts to create homogenous cohorts. As a result, some patients were not included as participants because of strict exclusion criteria. Most notably, we excluded any fracture not appropriate for tension-band fixation, as well as open fractures and osteotomies. Despite the retrospective nature of the study, all patients were examined by the investigators at final follow-up (minimum, 2 years) for the purpose of this study. It is possible that these functional results may not be sustained over the long term, as the risk for posttraumatic arthrosis in articular injuries builds with time. Although some patients may want to have implants removed later, all our study patients who had implants removed had them removed within 1 year, and all 20 patients were reached at minimum 2-year follow-up. Thus, it is unlikely but possible that some of the other study patients will elect to have implants removed.
Olecranon fractures are a common injury, representing 10% of all upper extremity fractures.1 Displaced fractures require fixation to restore anatomical alignment and minimize posttraumatic arthrosis.2,3 Multiple surgical techniques have been developed to treat these fractures, with implant choice largely dictated by fracture pattern and associated injuries. Simple, noncomminuted, transverse, proximal fractures can be treated with a tension-band construct, and fractures that are comminuted, oblique, distal to the midpoint of the sigmoid notch, or associated with complex elbow injuries generally require locking-plate fixation.4,5 Although both tension bands and locking plates have been used successfully (Figures 1A, 1B), they remain some of the most frequently removed orthopedic implants, usually because of implant prominence.6
Both fixation devices have potential advantages and disadvantages. Tension-band fixation requires relatively “low-tech” instrumentation and implants and, as a result, has less cost and potentially less operative time for application. As it is smaller than a plate-and-screw construct, a tension band may be less prone to prominence, but this has not been substantiated in the literature.7-14 Implant migration has been a reported complication of tension-band fixation.7,11,13,15
Locking-plate fixation has been shown to be biomechanically stronger,16 and some reports have shown fewer repeat operations for implant prominence than with tension-band fixation.1,8,17-22 Because of more advanced product development and manufacturing, however, it comes at a higher cost. Plate fixation also requires more steps for application, which may require more operative time, and implant prominence has remained a problem, even with modern plates with lower profiles.19
Previous studies of olecranon fixation have included complex fractures and osteotomies or did not include current-generation precontoured locking plates. We found no other study that compared the outcomes, complications, and costs of tension-band and modern locking-plate fixation of isolated transverse olecranon fractures.
To determine if there are significant differences in outcomes and costs between tension-band and locking-plate fixation of transverse olecranon fractures in adults, we retrospectively compared functional outcomes, complications, and costs in 2 matched cohorts of displaced transverse olecranon fractures. We hypothesized that there would be no differences in functional outcomes, implant prominence, posttraumatic arthrosis, complications, or operative time, but that costs would be less with tension-band fixation.
Materials and Methods
After obtaining institutional review board approval, we retrospectively reviewed the medical records of patients who had undergone fixation of an isolated, transverse, noncomminuted olecranon fracture (Orthopaedic Trauma Association 21B1) at our institution between 2004 and 2011. Inclusion criteria included use of a tension-band construct or a precontoured locking plate, skeletal maturity at time of injury, and minimum 2-year follow-up. Exclusion criteria were open fractures, osteotomies, any other ipsilateral upper extremity fracture, and fractures with comminution, obliquity, or distal location.
Although, based on fracture pattern, tension-band fixation is appropriate for olecranon osteotomies used for distal humeral exposure, we did not include osteotomies because functional outcomes would likely be different from those of true olecranon fractures, in addition to the possibility that the soft-tissue injury from a distal humeral fracture and resultant exposure could result in a different level of implant prominence. To control for demographic variables, we used a cohort design in which patients were matched on age and length of follow-up.
During the study period, we treated 287 olecranon fractures. Forty-nine patients met the inclusion criteria. The study population consisted of 20 patients, 10 in each cohort matched on age and length of follow-up. There were no statistically significant differences between groups in demographic variables, including dominant arm involved and number of worker’s compensation claims (Table 1). Mechanisms of injury were similar in the groups. In the tension-band group, 9 patients fell directly onto their elbow, and 1 fell onto her outstretched hand. In the locking-plate group, 8 patients fell directly onto the elbow, 1 fell onto her outstretched hand, and 1 was injured in a motorcycle accident.
All surgeons, regardless of implant selected, used a posterior incision that curved slightly laterally about the tip of the olecranon. Surgeon preference determined which fixation construct to use. Tension-band fixation was performed using 2 bicortical Kirschner wires and a stainless-steel wire through a distal drill hole to complete the tension band. Of the 10 locking-plate constructs used, 4 were PERI-LOC olecranon locking plates (Smith & Nephew), 3 were LCP olecranon plates (Synthes), and 3 were periarticular proximal ulna locking plates (Zimmer).
All returning patients were seen by either Dr. Amini or Mr. Wilson and underwent range of motion (ROM) measurement with a goniometer; assessment for subjective and objective implant prominence (graded none, mild, moderate, or severe/already had implant removed); and functional scoring using the Mayo Elbow Performance Score (MEPS) and the Quick Disability of the Arm, Shoulder, and Hand (QDASH). Results were classified excellent (MEPS, >90), good (75-89), fair (60-74), and poor (<60).23
Anteroposterior and lateral radiographs of the elbow were obtained at follow-up and were examined for maintenance/integrity of implants, radiographic union, and posttraumatic arthrosis. Arthrosis was graded using the Broberg and Morrey24 classification: grade 0 (normal elbow), grade 1 (slight joint-space narrowing with minimal osteophyte formation), grade 2 (moderate joint-space narrowing with moderate osteophyte formation), grade 3 (severe degenerative changes with gross destruction of joint).
Medical records were examined to determine surgery time. Billing information was examined to determine charges related to each operation, specifically the charge for the implants and the overall charge for the operation, which included anesthesia charges. Subsequent operations were included as applicable.
Student t test was used to compare differences in normative data, and Pearson χ2 test to compare differences in categorical data. Differences with P < .05 were considered significant.
Results
There were no clinically or statistically significant differences in ROM or functional outcomes (Table 2). According to MEPS, results were excellent in 8 and good in 2 patients in the tension-band group and excellent in 7 and good in 3 patients in the locking-plate group.
In patients who had implants removed, average time to subsequent procedure was 6.2 months, and all patients who underwent implant removal did so before 1-year follow-up. Implant removal was required in 4 tension-band patients and 1 locking-plate patient (P = .12). Similarly, 7 tension-band patients (including those with implants removed) and 3 locking-plate patients had implant-related symptoms, with the difference trending (P = .07) toward significance (Table 2).
Patients who elected to have their implants removed tended to be younger than those who did not (45.7 vs 56.0 years); the difference (P = .14) was not significant. Worker’s compensation status did not affect the decision to undergo implant removal. At final follow-up, there were no differences in ROM or functional outcomes between patients who had implants removed and those who did not. No variable predicted which patients had implants removed or not (Table 3).
Implant charges were $207.97 for the tension-band cohort and $6688.52 for the locking-plate cohort (P < .0001). Operative charges for the index procedures were $5171.06 for tension-band fixation and $14,160.26 for locking-plate fixation (P < .0001). Overall operative charges, including charges for subsequent operations, were $6598.36 in the tension-band cohort and $14,333.46 in the locking-plate cohort (P = .001). In a comparison of combined charges for index procedure and implant removal (excluding other repeat operations), charges were $6025.56 for the tension-band cohort and $14,333.46 for the locking-plate cohort (P = .0002). Even if all patients with tension-band fixation and no patients with locking-plate fixation had implant removal, mean charges for all operative care would still be significantly (P = .0005) less in the tension-band cohort than in the locking-plate cohort ($7307.31 vs $14,160.26) (Table 4).
Surgery time was significantly (P = .025) less for tension-band fixation than for locking-plate fixation (55.3 vs 85.4 minutes) (Table 2).
Four tension-band patients and 3 locking-plate patients had radiographic evidence of grade 1 posttraumatic arthrosis (P = .64). None required subsequent procedures. Patients with posttraumatic arthrosis had slightly less flexion, but there was no difference in overall flexion-extension arc or functional outcomes between patients with and without arthrosis (Table 5).
The locking-plate cohort had no other complications, and the tension-band cohort had 3. In 1 tension-band patient, the wire disengaged from the Kirschner wires. The fracture healed, but a subsequent procedure was required for symptomatic implant prominence (Figures 2A–2C). Another tension-band patient developed both posttraumatic arthrofibrosis and cubital tunnel syndrome, in addition to a prominent implant. She underwent capsular release, ulnar nerve transposition, and implant removal. At final follow-up, motion was improved, and ulnar nerve symptoms were resolved. There were no infections in either group. Overall, there were no statistically significant differences in complications between groups.
Discussion
We conducted this study to determine differences between tension-band and locking-plate fixation of isolated, closed, noncomminuted, transverse olecranon fractures. Few studies have directly compared tension-band and locking-plate fixation,8,10,19,25 particularly in reference to outcomes of functional scores, implant prominence, complications, operative time, and cost-effectiveness. We found no study that clinically compared these implants since the advent of precontoured locking plates, and no study that compared results in similar fracture patterns. In our study, we found no differences in functional or radiographic outcomes between groups, but significant differences in charges and overall cost of care.
Our findings suggest that patients return to high functional level an average of 4.3 years after fixation of an olecranon fracture with either a tension band or a locking plate. Both cohorts achieved QDASH scores equivalent to normative values for the general population,26 and all patients in both cohorts achieved either good or excellent results based on MEPS values.23 This is comparable to reported functional outcomes in the literature, with previous reports suggesting 86% to 92% of patients obtain good or excellent results.1,7,8,12,14,17,18,27 The rate of posttraumatic arthrosis in both cohorts was low, and, when present, arthrosis was radiographically mild (no patient had grade 2 or 3 arthrosis). Patients with and without radiographic evidence of arthrosis had similar ROM and functional outcomes.
Our findings also suggest a trend toward fewer implant-related symptoms and less need for implant removal in patients treated with locking plates. Although both implants have high rates of prominence requiring removal, most studies support our findings that tension bands are more prominent than locking plates. Fixation has been reported to cause prominence requiring removal in 42% to 82% of patients with tension bands7-14 and 0% to 47% of patients with locking plates.1,8,17,18,20-22,28 It is important to note that many earlier studies either were conducted before the advent of precontoured locking plates or were not comparative.1,7,9-14,17,18,20-22,28 In one recent study, however, Edwards and colleagues19 surveyed 138 patients and found very similar implant removal rates: 63.6% for tension bands and 62.5% for locking plates. Nevertheless, implant removal rates for fixation of olecranon fractures remain high, regardless of implant used.
Our data did not reveal any difference in ROM or functional outcomes between patients who had and did not have implants removed. This suggests, first, that QDASH and MEPS may not be sensitive in identifying patients with implant prominence, as neither questionnaire incorporates implant prominence into its scoring, and, second, that implant removal does not significantly impair ROM. As a result, surgeons should consider asking patients specifically about symptoms of prominent implants once there is convincing evidence of union and counseling them about implant removal if appropriate.
To our knowledge, the differences in cost and operative time between tension-band and locking-plate fixation have not been previously reported. Our data suggest that the financial differences resulted mainly from implant charges; overall, tension-band fixation was roughly half the cost of locking-plate fixation. In addition, in patients who eventually had implants removed, the cost of implant removal was relatively small compared with the cost of the initial fixation in both cohorts. As a result, even if all patients in the tension-band cohort and no patients in the locking-plate cohort had implants removed, tension-band fixation and subsequent implant removal would still cost half as much as locking-plate fixation without implant removal. Moreover, fixation with a tension band took roughly 30 minutes less than fixation with a plate. Less time in the operating room likely contributed to the additional cost savings realized with tension-band fixation beyond those directly resulting from implant cost.
The strength of this study lies in the homogeneity of cohorts. Each cohort was matched primarily on age and secondarily on length of follow-up. All patients had closed, proximal, transverse fractures without comminution, and we excluded olecranon osteotomies as these represent an entity different from true fractures. Fractures with comminution or distal extension may represent more severe injuries, and functional scores, complications, hardware prominence, and operative time might have been affected by inclusion of these fractures. Further, there were no infections in either group to skew the rate of implant prominence or removal.
The weaknesses of the study lie in its limited sample sizes, retrospective design, and lack of long-term follow-up. Group size was limited by our attempts to create homogenous cohorts. As a result, some patients were not included as participants because of strict exclusion criteria. Most notably, we excluded any fracture not appropriate for tension-band fixation, as well as open fractures and osteotomies. Despite the retrospective nature of the study, all patients were examined by the investigators at final follow-up (minimum, 2 years) for the purpose of this study. It is possible that these functional results may not be sustained over the long term, as the risk for posttraumatic arthrosis in articular injuries builds with time. Although some patients may want to have implants removed later, all our study patients who had implants removed had them removed within 1 year, and all 20 patients were reached at minimum 2-year follow-up. Thus, it is unlikely but possible that some of the other study patients will elect to have implants removed.
1. Buijze G, Kloen P. Clinical evaluation of locking compression plate fixation for comminuted olecranon fractures. J Bone Joint Surg Am. 2009;91(10):
2416-2420.
2. Newman SD, Mauffrey C, Krikler S. Olecranon fractures. Injury. 2009;40(6):575-581.
3. Veillette CJ, Steinmann SP. Olecranon fractures. Orthop Clin North Am. 2008;39(2):229-236.
4. Baecher N, Edwards S. Olecranon fractures. J Hand Surg Am. 2013;38(3):593-604.
5. Hak DJ, Golladay GJ. Olecranon fractures: treatment options. J Am Acad Orthop Surg. 2000;8(4):266-275.
6. Busam ML, Esther RJ, Obremskey WT. Hardware removal: indications and expectations. J Am Acad Orthop Surg. 2006;14(2):113-120.
7. Chalidis BE, Sachinis NC, Samoladas EP, Dimitriou CG, Pournaras JD. Is tension band wiring technique the “gold standard” for the treatment of olecranon fractures? A long term functional outcome study. J Orthop Surg Res. 2008;3:9.
8. Hume MC, Wiss DA. Olecranon fractures: a clinical and radiographic comparison of tension-band wiring and plate fixation. Clin Orthop Relat Res. 1992;(285):229-235.
9. Karlsson MK, Hasserius R, Besjakov J, Karlsson C, Josefsson PO. Comparison of tension-band and figure-of-eight wiring techniques for treatment of olecranon fractures. J Shoulder Elbow Surg. 2002;11(4):377-382.
10. Lindenhovius AL, Brouwer KM, Doornberg JN, Ring DC, Kloen P. Long-term outcome of operatively treated fracture-dislocations of the olecranon. J Orthop Trauma. 2008;22(5):325-331.
11. Macko D, Szabo RM. Complications of tension-band wiring of olecranon fractures. J Bone Joint Surg Am. 1985;67(9):1396-1401.
12. Romero JM, Miran A, Jensen CH. Complications and re-operation rate after tension-band wiring of olecranon fractures. J Orthop Sci. 2000;5(4):318-320.
13. Rommens PM, Schneider RU, Reuter M. Functional results after operative treatment of olecranon fractures. Acta Chir Belg. 2004;104(2):191-197.
14. Villanueva P, Osorio F, Commessatti M, Sanchez-Sotelo J. Tension-band wiring for olecranon fractures: analysis of risk factors for failure. J Shoulder Elbow Surg. 2006;15(3):351-356.
15. Sahajpal D, Wright TW. Proximal ulna fractures. J Hand Surg Am. 2009;34(2):357-362.
16. Rouleau DM, Sandman E, van Riet R, Galatz LM. Management of fractures of the proximal ulna. J Am Acad Orthop Surg. 2013;21(3):149-160.
17. Anderson ML, Larson AN, Merten SM, Steinmann SP. Congruent elbow plate fixation of olecranon fractures. J Orthop Trauma. 2007;21(6):386-393.
18. Bailey CS, MacDermid J, Patterson SD, King GJ. Outcome of plate fixation of olecranon fractures. J Orthop Trauma. 2001;15(8):542-548.
19. Edwards SG, Cohen MS, Lattanza LL, et al. Surgeon perceptions and patient outcomes regarding proximal ulna fixation: a multicenter experience. J Shoulder Elbow Surg. 2012;21(12):1637-1643.
20. Munoz-Mahamud E, Fernandez-Valencia JA, Riba J. Plate osteosynthesis for severe olecranon fractures. J Orthop Surg. 2010;18(1):80-84.
21. Simpson NS, Goodman LA, Jupiter JB. Contoured LCDC plating of the proximal ulna. Injury. 1996;27(6):411-417.
22. Tejwani NC, Garnham IR, Wolinsky PR, Kummer FJ, Koval KJ. Posterior olecranon plating: biomechanical and clinical evaluation of a new operative technique. Bull Hosp Jt Dis. 2002-2003;61(1-2):27-31.
23. Morrey BF, An KN. Functional evaluation of the elbow. In: Morrey BF, Sanchez-Sotelo J, eds. The Elbow and Its Disorders. 4th ed. Philadelphia, PA: Elsevier; 2008:87-88.
24. Broberg MA, Morrey BF. The results of delayed excision of the radial head for fracture. J Bone Joint Surg Am. 1986;68(5):669-674.
25. Horne JG, Tanzer TL. Olecranon fractures: a review of 100 cases. J Trauma. 1981;21(6):469-472.
26. Hunsaker FG, Cioffi DA, Amadio PC, Wright JG, Caughlin B. The American Academy of Orthopaedic Surgeons outcomes instruments: normative values from the general population. J Bone Joint Surg Am. 2002;84(2):208-215.
27. Ikeda M, Fukushima Y, Kobayashi Y, Oka Y. Comminuted fractures of the olecranon. Management by bone graft from the iliac crest and multiple tension-band wiring. J Bone Joint Surg Br. 2001;83(6):805-808.
28. Erturer RE, Sever C, Sonmez MM, Ozcelik IB, Akman S, Ozturk I. Results of open reduction and plate osteosynthesis in comminuted fracture of the olecranon. J Shoulder Elbow Surg. 2011;20(3):449-454.
1. Buijze G, Kloen P. Clinical evaluation of locking compression plate fixation for comminuted olecranon fractures. J Bone Joint Surg Am. 2009;91(10):
2416-2420.
2. Newman SD, Mauffrey C, Krikler S. Olecranon fractures. Injury. 2009;40(6):575-581.
3. Veillette CJ, Steinmann SP. Olecranon fractures. Orthop Clin North Am. 2008;39(2):229-236.
4. Baecher N, Edwards S. Olecranon fractures. J Hand Surg Am. 2013;38(3):593-604.
5. Hak DJ, Golladay GJ. Olecranon fractures: treatment options. J Am Acad Orthop Surg. 2000;8(4):266-275.
6. Busam ML, Esther RJ, Obremskey WT. Hardware removal: indications and expectations. J Am Acad Orthop Surg. 2006;14(2):113-120.
7. Chalidis BE, Sachinis NC, Samoladas EP, Dimitriou CG, Pournaras JD. Is tension band wiring technique the “gold standard” for the treatment of olecranon fractures? A long term functional outcome study. J Orthop Surg Res. 2008;3:9.
8. Hume MC, Wiss DA. Olecranon fractures: a clinical and radiographic comparison of tension-band wiring and plate fixation. Clin Orthop Relat Res. 1992;(285):229-235.
9. Karlsson MK, Hasserius R, Besjakov J, Karlsson C, Josefsson PO. Comparison of tension-band and figure-of-eight wiring techniques for treatment of olecranon fractures. J Shoulder Elbow Surg. 2002;11(4):377-382.
10. Lindenhovius AL, Brouwer KM, Doornberg JN, Ring DC, Kloen P. Long-term outcome of operatively treated fracture-dislocations of the olecranon. J Orthop Trauma. 2008;22(5):325-331.
11. Macko D, Szabo RM. Complications of tension-band wiring of olecranon fractures. J Bone Joint Surg Am. 1985;67(9):1396-1401.
12. Romero JM, Miran A, Jensen CH. Complications and re-operation rate after tension-band wiring of olecranon fractures. J Orthop Sci. 2000;5(4):318-320.
13. Rommens PM, Schneider RU, Reuter M. Functional results after operative treatment of olecranon fractures. Acta Chir Belg. 2004;104(2):191-197.
14. Villanueva P, Osorio F, Commessatti M, Sanchez-Sotelo J. Tension-band wiring for olecranon fractures: analysis of risk factors for failure. J Shoulder Elbow Surg. 2006;15(3):351-356.
15. Sahajpal D, Wright TW. Proximal ulna fractures. J Hand Surg Am. 2009;34(2):357-362.
16. Rouleau DM, Sandman E, van Riet R, Galatz LM. Management of fractures of the proximal ulna. J Am Acad Orthop Surg. 2013;21(3):149-160.
17. Anderson ML, Larson AN, Merten SM, Steinmann SP. Congruent elbow plate fixation of olecranon fractures. J Orthop Trauma. 2007;21(6):386-393.
18. Bailey CS, MacDermid J, Patterson SD, King GJ. Outcome of plate fixation of olecranon fractures. J Orthop Trauma. 2001;15(8):542-548.
19. Edwards SG, Cohen MS, Lattanza LL, et al. Surgeon perceptions and patient outcomes regarding proximal ulna fixation: a multicenter experience. J Shoulder Elbow Surg. 2012;21(12):1637-1643.
20. Munoz-Mahamud E, Fernandez-Valencia JA, Riba J. Plate osteosynthesis for severe olecranon fractures. J Orthop Surg. 2010;18(1):80-84.
21. Simpson NS, Goodman LA, Jupiter JB. Contoured LCDC plating of the proximal ulna. Injury. 1996;27(6):411-417.
22. Tejwani NC, Garnham IR, Wolinsky PR, Kummer FJ, Koval KJ. Posterior olecranon plating: biomechanical and clinical evaluation of a new operative technique. Bull Hosp Jt Dis. 2002-2003;61(1-2):27-31.
23. Morrey BF, An KN. Functional evaluation of the elbow. In: Morrey BF, Sanchez-Sotelo J, eds. The Elbow and Its Disorders. 4th ed. Philadelphia, PA: Elsevier; 2008:87-88.
24. Broberg MA, Morrey BF. The results of delayed excision of the radial head for fracture. J Bone Joint Surg Am. 1986;68(5):669-674.
25. Horne JG, Tanzer TL. Olecranon fractures: a review of 100 cases. J Trauma. 1981;21(6):469-472.
26. Hunsaker FG, Cioffi DA, Amadio PC, Wright JG, Caughlin B. The American Academy of Orthopaedic Surgeons outcomes instruments: normative values from the general population. J Bone Joint Surg Am. 2002;84(2):208-215.
27. Ikeda M, Fukushima Y, Kobayashi Y, Oka Y. Comminuted fractures of the olecranon. Management by bone graft from the iliac crest and multiple tension-band wiring. J Bone Joint Surg Br. 2001;83(6):805-808.
28. Erturer RE, Sever C, Sonmez MM, Ozcelik IB, Akman S, Ozturk I. Results of open reduction and plate osteosynthesis in comminuted fracture of the olecranon. J Shoulder Elbow Surg. 2011;20(3):449-454.
American Academy of Orthopaedic Surgeons Disclosure Policy Fails to Accurately Inform Its Members of Potential Conflicts of Interest
The relationship and collaboration between orthopedic surgeons and the orthopedic industry are considerable. Orthopedic surgeons can provide companies with important clinical input into the design of implants, facilitate commercialization of innovations developed by clinician entrepreneurs, and help provide rapid dissemination of new technologies.1,2 However, these relationships can result in conflicts of interest, thereby influencing the physicians’ judgment and choices and ultimately patient care.3,4 Making these potential conflicts transparent through physician disclosures is an accepted way to limit the negative effects of these relationships.5 The relationship between orthopedic surgeons and industry was brought to the forefront in 2007 with a settlement between the US Department of Justice (DOJ) and the 5 largest orthopedic implant makers.6 Among other things, this settlement required that each company publicly disclose on its website, beginning in 2008, the names and locations of all surgeons and organizations it paid, and how much. The DOJ settlement was one of the impetuses that led many orthopedic societies to adopt either voluntary or mandatory disclosure policies for their members.
In 2007, the American Academy of Orthopaedic Surgeons (AAOS) developed an orthopedic disclosure program to promote transparency and confidence in its educational programs and decisions.7 One of the 2 main purposes of the disclosure program is “streamlining the disclosure process for orthopedic surgeons and others involved in organizational governance, all formats of continuing medical education [CME] and authors of enduring materials, clinical practice guidelines (CPG) and appropriate use criteria (AUC) development and editors-in-chief and editorial boards, from whom disclosure is required.”8 Disclosure is mandatory only for participants in the AAOS CME programs (including any podium or poster presentation) or authors of enduring materials; members of the AAOS Board of Directors, Board of Councilors, Board of Specialty Societies, councils, cabinets, committees, project teams, or other AAOS governance groups; editors-in-chief and editorial boards; and AAOS guideline development workgroups. Members who fail to disclose are informed they cannot participate in AAOS activities. All other members of the organization are not required to disclose any industry-related relationships, and any disclosure is completely voluntary.7 This seems contrary to the second main goal of the disclosure policy: “increase transparency throughout AAOS by making this disclosure program available to the public and to AAOS members.”8
We conducted a study to compare the disclosures posted by the top orthopedic companies with the disclosures made by their surgeon-consultants and to determine how many of these surgeons have disclosed this information on the AAOS website.
Materials and Methods
On November 26, 2012, we reviewed the websites of the top 13 orthopedic device companies by revenue (Stryker, DePuy Orthopaedics, Zimmer Holdings, Smith & Nephew, Synthes, Medtronic Spine, Biomet, DJO Global, Orthofix, NuVasive, Wright Medical Group, ArthroCare, Exactech)9 to identify their surgeon-consultants for 2011. We excluded non-US surgeons (DOJ disclosure not required), revenues under $1000, and reimbursement for meals and travel. Although the DOJ settlement required that each company disclose on its website, beginning in 2008, the names and locations of its paid consultants and the amounts paid, the settlement did not stipulate how long this must be continued. Of the 13 companies, only 6 (Stryker, DePuy, Smith & Nephew, Medtronic, Wright, Exactech) continued listing and updating surgeon disclosure information.
As the companies differed in how they defined surgeon consulting services, we defined surgeon-consultant payments as the sum of consulting payments, royalty payments, and research support. We searched for each surgeon-consultant’s name in the AAOS orthopedic disclosure program database.7 From the database, we determined whether the surgeon was a member of AAOS. All members were then categorized into those who disclosed all their payments, those who incompletely disclosed their payments, those who did not disclose any payments, and those who did not provide any information. They were then subdivided into those who had and had not participated in CME activities at the AAOS annual meeting in 2011 (participants were listed in the meeting proceedings). This does not take into account AAOS members who presented at other AAOS-sponsored CME courses during 2011 and who therefore were required to disclose. The information was categorized by company, payment amount, and overall. To simplify matters and deal with varying corporate categories, we divided payments into 4 amount groups: less than $10,000, $10,000 to $100,000, $100,001 to $1 million, and more than $1 million. Some orthopedic companies reported surgeon payments as categorical rather than exact amounts. In these cases, we coded the payment as the midpoint of the range.
Results
Overall, 549 AAOS members received payments of more than $1000 from at least 1 of the 6 companies. Of these surgeons, 307 (56%) fully disclosed their payments, and 242 (44%) did not (Table 1). Of the 32 surgeons who were on 2 corporate payment lists, 24 disclosed both companies, 6 disclosed only 1 company, and 2 failed to disclose either company. AAOS members who did not disclose payments received less than $10,000 (average, $3706) in 37% of cases (Table 2), between $10,000 and $100,000 (average, $34,025) in 54% of cases, between $100,001 and $1 million (average, $290,505) in 8% of cases, and more than $1 million (average, $5,126,000) in less than 1% of cases.
Number of consultants, number of surgeons not disclosing payments, and value of these payments varied from company to company (Table 3). The company with the most consultants listed 185 AAOS members, of which 37% had not disclosed payments (average, $39,604). Second was the company that listed 108 members; 39% had not disclosed payments (average, $38,426). The third company listed 102 members, of which 56% had not disclosed payments (average, $217,340). The company with the fourth most consultants listed 84 members; 43% had not disclosed payments (average, $9841). Next to last was the company listing 42 members, of which 52% had not disclosed payments (average, $160,634). The company with the fewest consultants listed 28 members; 61% had not disclosed payments (average $85,388).
Of AAOS members who attended the 2011 annual meeting, 94% fully disclosed industry payments (Table 1). Only 7% of the membership either failed to disclose or incompletely disclosed this relationship. In 36 cases (26%), members disclosed a financial relationship with at least 1 orthopedic company, but this relationship was not listed on the company’s website. One of the companies was responsible for 47% of the underreporting.
Discussion
In this study, we evaluated whether surgeons fully disclosed (on the website for the AAOS disclosure program) payments they received from orthopedic companies. Overall compliance was poor, with 44% of surgeons not disclosing payments. The percentage of surgeons disclosing corporate relationships and payments received varied among orthopedic companies. It is unclear whether this reflects partial reporting, or AAOS disclosure policy being mandatory only for select members rather than the entire membership.
This study had a few limitations, none of which had a substantive impact on the results or conclusions. First, we could not determine how many AAOS members who were required to disclose actually disclosed. There is no mechanism for determining which members are involved in activities that require disclosure. Nonetheless, the intent of the policy is to make collaborations between orthopedic surgeons and industry transparent in order to address concerns about potential conflicts of interest. That 44% of AAOS members did not disclose their relationships cannot be considered a success. Second, information was available on the websites of only 6 of the top 15 orthopedic companies—a result stemming from the DOJ’s failure to specify how long these companies must continue posting disclosures. In this study, the lowest nondisclosure rate was 37%, and there is no reason to suspect that any other group of surgeon-consultants would be any more compliant with AAOS’s policy.
There are few reports on the effects of the DOJ settlement on the behavior of surgeon-consultants who are AAOS members. Hockenberry and colleagues10 found that, since the settlement, surgeon payments have increased, number of consultants has decreased, and the proportion of consultants from academia has increased. They thought their findings confirmed concerns that orthopedic device makers would deliberately select high-volume orthopedic surgeons as consultants in order to increase sales of their implants and gain market share at the expense of their competitors. The authors thought that AAOS had some power to address disclosure through its influence on its members, but that influence may not be enough. Jegede and colleagues11 found that a significant percentage (41%) of orthopedic surgeons who received corporate payments and presented at the AAOS annual meeting were inconsistent in submitting disclosure information. Results of the present study suggest that AAOS policy is weak and does not adequately address the issue and provide full transparency, either within the organization or to the public, of all its members’ industry relationships.
As the preeminent provider of musculoskeletal education to orthopedic surgeons and others, and with a membership totaling almost 39,000, AAOS is one of the most important orthopedic societies in the world. AAOS has clearly stated that one of its goals is to increase transparency by making its surgeon disclosure program available to AAOS members and the public. However, it can be completely transparent only if all its members are required to disclose their corporate relationships. This study demonstrated that AAOS’s policy of mandatory disclosure for select members and voluntary disclosure for all other members is ineffective. We found that 44% of members failed to disclose industry-derived payments. This inadequate level of compliance runs contrary to the AAOS goal of increasing transparency of surgeon–industry consulting by making its surgeon disclosure program available to AAOS members and the public. The AAOS disclosure program and the potential consequences of noncompliance need to be reevaluated by the organization if it wants its program to succeed.
1. Crowninshield RD, Callaghan JJ. The orthopaedic profession and the industry partnership. Clin Orthop Relat Res. 2007;(457):73-77.
2. White AP, Vaccaro AR, Zdeblick T. Counterpoint: physician–industry relationships can be ethically established, and conflicts of interest can be ethically managed. Spine. 2007;32(11 suppl):S53-S57.
3. Steinbrook R. Online disclosure of physician–industry relationships. N Engl J Med. 2009;360(4):325-327.
4. Steinbrook R. Disclosure of industry payments to physicians. N Engl J Med. 2008;359(6):559-561.
5. Weinfurt KP, Friedman JY, Dinan MA, et al. Disclosing conflicts of interest in clinical research: views of institutional review boards, conflict of interest committees, and investigators. J Law Med Ethics. 2006;34(3):581-591, 481.
6. US Attorney’s Office, District of New Jersey. Monitoring and deferred prosecution agreements terminated with companies in hip and knee replacement industry [press release]. Federal Bureau of Investigation, Newark Division website. http://www.fbi.gov/newark/press-releases/2009/nk033009a.htm. March 30, 2009. Accessed May 13, 2015.
7. AAOS mandatory disclosure policy. American Academy of Orthopaedic Surgeons website. http://www.aaos.org/about/policies/DisclosurePolicy.asp. Adopted February 2007. Revised December 2009, February 2012. Accessed May 13, 2015.
8. The AAOS orthopaedic disclosure program. American Academy of Orthopaedic Surgeons website. http://www7.aaos.org/education/disclosure. Accessed May 13, 2015.
9. Top 15 ortho companies by revenue [based on 2011 full-year financials]. OrthoStreams website. http://orthostreams.com/top-15-ortho-companies-by-revenue/http://orthostreams.com/2012/03/the-top-15-orthopedic-companies-ranked-by-2011 revenue/. Accessed May 13, 2015.
10. Hockenberry JM, Weigel P, Auerbach A, Cram P. Financial payments by orthopedic device makers to orthopedic surgeons. Arch Intern Med. 2011;171(19):1759-1765.
11. Jegede KA, Ju B, Miller CP, Whang P, Grauer JN. Quantifying the variability of financial disclosure information reported by authors presenting research at multiple sports medicine conferences. Am J Orthop. 2011;40(11):583-587.
The relationship and collaboration between orthopedic surgeons and the orthopedic industry are considerable. Orthopedic surgeons can provide companies with important clinical input into the design of implants, facilitate commercialization of innovations developed by clinician entrepreneurs, and help provide rapid dissemination of new technologies.1,2 However, these relationships can result in conflicts of interest, thereby influencing the physicians’ judgment and choices and ultimately patient care.3,4 Making these potential conflicts transparent through physician disclosures is an accepted way to limit the negative effects of these relationships.5 The relationship between orthopedic surgeons and industry was brought to the forefront in 2007 with a settlement between the US Department of Justice (DOJ) and the 5 largest orthopedic implant makers.6 Among other things, this settlement required that each company publicly disclose on its website, beginning in 2008, the names and locations of all surgeons and organizations it paid, and how much. The DOJ settlement was one of the impetuses that led many orthopedic societies to adopt either voluntary or mandatory disclosure policies for their members.
In 2007, the American Academy of Orthopaedic Surgeons (AAOS) developed an orthopedic disclosure program to promote transparency and confidence in its educational programs and decisions.7 One of the 2 main purposes of the disclosure program is “streamlining the disclosure process for orthopedic surgeons and others involved in organizational governance, all formats of continuing medical education [CME] and authors of enduring materials, clinical practice guidelines (CPG) and appropriate use criteria (AUC) development and editors-in-chief and editorial boards, from whom disclosure is required.”8 Disclosure is mandatory only for participants in the AAOS CME programs (including any podium or poster presentation) or authors of enduring materials; members of the AAOS Board of Directors, Board of Councilors, Board of Specialty Societies, councils, cabinets, committees, project teams, or other AAOS governance groups; editors-in-chief and editorial boards; and AAOS guideline development workgroups. Members who fail to disclose are informed they cannot participate in AAOS activities. All other members of the organization are not required to disclose any industry-related relationships, and any disclosure is completely voluntary.7 This seems contrary to the second main goal of the disclosure policy: “increase transparency throughout AAOS by making this disclosure program available to the public and to AAOS members.”8
We conducted a study to compare the disclosures posted by the top orthopedic companies with the disclosures made by their surgeon-consultants and to determine how many of these surgeons have disclosed this information on the AAOS website.
Materials and Methods
On November 26, 2012, we reviewed the websites of the top 13 orthopedic device companies by revenue (Stryker, DePuy Orthopaedics, Zimmer Holdings, Smith & Nephew, Synthes, Medtronic Spine, Biomet, DJO Global, Orthofix, NuVasive, Wright Medical Group, ArthroCare, Exactech)9 to identify their surgeon-consultants for 2011. We excluded non-US surgeons (DOJ disclosure not required), revenues under $1000, and reimbursement for meals and travel. Although the DOJ settlement required that each company disclose on its website, beginning in 2008, the names and locations of its paid consultants and the amounts paid, the settlement did not stipulate how long this must be continued. Of the 13 companies, only 6 (Stryker, DePuy, Smith & Nephew, Medtronic, Wright, Exactech) continued listing and updating surgeon disclosure information.
As the companies differed in how they defined surgeon consulting services, we defined surgeon-consultant payments as the sum of consulting payments, royalty payments, and research support. We searched for each surgeon-consultant’s name in the AAOS orthopedic disclosure program database.7 From the database, we determined whether the surgeon was a member of AAOS. All members were then categorized into those who disclosed all their payments, those who incompletely disclosed their payments, those who did not disclose any payments, and those who did not provide any information. They were then subdivided into those who had and had not participated in CME activities at the AAOS annual meeting in 2011 (participants were listed in the meeting proceedings). This does not take into account AAOS members who presented at other AAOS-sponsored CME courses during 2011 and who therefore were required to disclose. The information was categorized by company, payment amount, and overall. To simplify matters and deal with varying corporate categories, we divided payments into 4 amount groups: less than $10,000, $10,000 to $100,000, $100,001 to $1 million, and more than $1 million. Some orthopedic companies reported surgeon payments as categorical rather than exact amounts. In these cases, we coded the payment as the midpoint of the range.
Results
Overall, 549 AAOS members received payments of more than $1000 from at least 1 of the 6 companies. Of these surgeons, 307 (56%) fully disclosed their payments, and 242 (44%) did not (Table 1). Of the 32 surgeons who were on 2 corporate payment lists, 24 disclosed both companies, 6 disclosed only 1 company, and 2 failed to disclose either company. AAOS members who did not disclose payments received less than $10,000 (average, $3706) in 37% of cases (Table 2), between $10,000 and $100,000 (average, $34,025) in 54% of cases, between $100,001 and $1 million (average, $290,505) in 8% of cases, and more than $1 million (average, $5,126,000) in less than 1% of cases.
Number of consultants, number of surgeons not disclosing payments, and value of these payments varied from company to company (Table 3). The company with the most consultants listed 185 AAOS members, of which 37% had not disclosed payments (average, $39,604). Second was the company that listed 108 members; 39% had not disclosed payments (average, $38,426). The third company listed 102 members, of which 56% had not disclosed payments (average, $217,340). The company with the fourth most consultants listed 84 members; 43% had not disclosed payments (average, $9841). Next to last was the company listing 42 members, of which 52% had not disclosed payments (average, $160,634). The company with the fewest consultants listed 28 members; 61% had not disclosed payments (average $85,388).
Of AAOS members who attended the 2011 annual meeting, 94% fully disclosed industry payments (Table 1). Only 7% of the membership either failed to disclose or incompletely disclosed this relationship. In 36 cases (26%), members disclosed a financial relationship with at least 1 orthopedic company, but this relationship was not listed on the company’s website. One of the companies was responsible for 47% of the underreporting.
Discussion
In this study, we evaluated whether surgeons fully disclosed (on the website for the AAOS disclosure program) payments they received from orthopedic companies. Overall compliance was poor, with 44% of surgeons not disclosing payments. The percentage of surgeons disclosing corporate relationships and payments received varied among orthopedic companies. It is unclear whether this reflects partial reporting, or AAOS disclosure policy being mandatory only for select members rather than the entire membership.
This study had a few limitations, none of which had a substantive impact on the results or conclusions. First, we could not determine how many AAOS members who were required to disclose actually disclosed. There is no mechanism for determining which members are involved in activities that require disclosure. Nonetheless, the intent of the policy is to make collaborations between orthopedic surgeons and industry transparent in order to address concerns about potential conflicts of interest. That 44% of AAOS members did not disclose their relationships cannot be considered a success. Second, information was available on the websites of only 6 of the top 15 orthopedic companies—a result stemming from the DOJ’s failure to specify how long these companies must continue posting disclosures. In this study, the lowest nondisclosure rate was 37%, and there is no reason to suspect that any other group of surgeon-consultants would be any more compliant with AAOS’s policy.
There are few reports on the effects of the DOJ settlement on the behavior of surgeon-consultants who are AAOS members. Hockenberry and colleagues10 found that, since the settlement, surgeon payments have increased, number of consultants has decreased, and the proportion of consultants from academia has increased. They thought their findings confirmed concerns that orthopedic device makers would deliberately select high-volume orthopedic surgeons as consultants in order to increase sales of their implants and gain market share at the expense of their competitors. The authors thought that AAOS had some power to address disclosure through its influence on its members, but that influence may not be enough. Jegede and colleagues11 found that a significant percentage (41%) of orthopedic surgeons who received corporate payments and presented at the AAOS annual meeting were inconsistent in submitting disclosure information. Results of the present study suggest that AAOS policy is weak and does not adequately address the issue and provide full transparency, either within the organization or to the public, of all its members’ industry relationships.
As the preeminent provider of musculoskeletal education to orthopedic surgeons and others, and with a membership totaling almost 39,000, AAOS is one of the most important orthopedic societies in the world. AAOS has clearly stated that one of its goals is to increase transparency by making its surgeon disclosure program available to AAOS members and the public. However, it can be completely transparent only if all its members are required to disclose their corporate relationships. This study demonstrated that AAOS’s policy of mandatory disclosure for select members and voluntary disclosure for all other members is ineffective. We found that 44% of members failed to disclose industry-derived payments. This inadequate level of compliance runs contrary to the AAOS goal of increasing transparency of surgeon–industry consulting by making its surgeon disclosure program available to AAOS members and the public. The AAOS disclosure program and the potential consequences of noncompliance need to be reevaluated by the organization if it wants its program to succeed.
The relationship and collaboration between orthopedic surgeons and the orthopedic industry are considerable. Orthopedic surgeons can provide companies with important clinical input into the design of implants, facilitate commercialization of innovations developed by clinician entrepreneurs, and help provide rapid dissemination of new technologies.1,2 However, these relationships can result in conflicts of interest, thereby influencing the physicians’ judgment and choices and ultimately patient care.3,4 Making these potential conflicts transparent through physician disclosures is an accepted way to limit the negative effects of these relationships.5 The relationship between orthopedic surgeons and industry was brought to the forefront in 2007 with a settlement between the US Department of Justice (DOJ) and the 5 largest orthopedic implant makers.6 Among other things, this settlement required that each company publicly disclose on its website, beginning in 2008, the names and locations of all surgeons and organizations it paid, and how much. The DOJ settlement was one of the impetuses that led many orthopedic societies to adopt either voluntary or mandatory disclosure policies for their members.
In 2007, the American Academy of Orthopaedic Surgeons (AAOS) developed an orthopedic disclosure program to promote transparency and confidence in its educational programs and decisions.7 One of the 2 main purposes of the disclosure program is “streamlining the disclosure process for orthopedic surgeons and others involved in organizational governance, all formats of continuing medical education [CME] and authors of enduring materials, clinical practice guidelines (CPG) and appropriate use criteria (AUC) development and editors-in-chief and editorial boards, from whom disclosure is required.”8 Disclosure is mandatory only for participants in the AAOS CME programs (including any podium or poster presentation) or authors of enduring materials; members of the AAOS Board of Directors, Board of Councilors, Board of Specialty Societies, councils, cabinets, committees, project teams, or other AAOS governance groups; editors-in-chief and editorial boards; and AAOS guideline development workgroups. Members who fail to disclose are informed they cannot participate in AAOS activities. All other members of the organization are not required to disclose any industry-related relationships, and any disclosure is completely voluntary.7 This seems contrary to the second main goal of the disclosure policy: “increase transparency throughout AAOS by making this disclosure program available to the public and to AAOS members.”8
We conducted a study to compare the disclosures posted by the top orthopedic companies with the disclosures made by their surgeon-consultants and to determine how many of these surgeons have disclosed this information on the AAOS website.
Materials and Methods
On November 26, 2012, we reviewed the websites of the top 13 orthopedic device companies by revenue (Stryker, DePuy Orthopaedics, Zimmer Holdings, Smith & Nephew, Synthes, Medtronic Spine, Biomet, DJO Global, Orthofix, NuVasive, Wright Medical Group, ArthroCare, Exactech)9 to identify their surgeon-consultants for 2011. We excluded non-US surgeons (DOJ disclosure not required), revenues under $1000, and reimbursement for meals and travel. Although the DOJ settlement required that each company disclose on its website, beginning in 2008, the names and locations of its paid consultants and the amounts paid, the settlement did not stipulate how long this must be continued. Of the 13 companies, only 6 (Stryker, DePuy, Smith & Nephew, Medtronic, Wright, Exactech) continued listing and updating surgeon disclosure information.
As the companies differed in how they defined surgeon consulting services, we defined surgeon-consultant payments as the sum of consulting payments, royalty payments, and research support. We searched for each surgeon-consultant’s name in the AAOS orthopedic disclosure program database.7 From the database, we determined whether the surgeon was a member of AAOS. All members were then categorized into those who disclosed all their payments, those who incompletely disclosed their payments, those who did not disclose any payments, and those who did not provide any information. They were then subdivided into those who had and had not participated in CME activities at the AAOS annual meeting in 2011 (participants were listed in the meeting proceedings). This does not take into account AAOS members who presented at other AAOS-sponsored CME courses during 2011 and who therefore were required to disclose. The information was categorized by company, payment amount, and overall. To simplify matters and deal with varying corporate categories, we divided payments into 4 amount groups: less than $10,000, $10,000 to $100,000, $100,001 to $1 million, and more than $1 million. Some orthopedic companies reported surgeon payments as categorical rather than exact amounts. In these cases, we coded the payment as the midpoint of the range.
Results
Overall, 549 AAOS members received payments of more than $1000 from at least 1 of the 6 companies. Of these surgeons, 307 (56%) fully disclosed their payments, and 242 (44%) did not (Table 1). Of the 32 surgeons who were on 2 corporate payment lists, 24 disclosed both companies, 6 disclosed only 1 company, and 2 failed to disclose either company. AAOS members who did not disclose payments received less than $10,000 (average, $3706) in 37% of cases (Table 2), between $10,000 and $100,000 (average, $34,025) in 54% of cases, between $100,001 and $1 million (average, $290,505) in 8% of cases, and more than $1 million (average, $5,126,000) in less than 1% of cases.
Number of consultants, number of surgeons not disclosing payments, and value of these payments varied from company to company (Table 3). The company with the most consultants listed 185 AAOS members, of which 37% had not disclosed payments (average, $39,604). Second was the company that listed 108 members; 39% had not disclosed payments (average, $38,426). The third company listed 102 members, of which 56% had not disclosed payments (average, $217,340). The company with the fourth most consultants listed 84 members; 43% had not disclosed payments (average, $9841). Next to last was the company listing 42 members, of which 52% had not disclosed payments (average, $160,634). The company with the fewest consultants listed 28 members; 61% had not disclosed payments (average $85,388).
Of AAOS members who attended the 2011 annual meeting, 94% fully disclosed industry payments (Table 1). Only 7% of the membership either failed to disclose or incompletely disclosed this relationship. In 36 cases (26%), members disclosed a financial relationship with at least 1 orthopedic company, but this relationship was not listed on the company’s website. One of the companies was responsible for 47% of the underreporting.
Discussion
In this study, we evaluated whether surgeons fully disclosed (on the website for the AAOS disclosure program) payments they received from orthopedic companies. Overall compliance was poor, with 44% of surgeons not disclosing payments. The percentage of surgeons disclosing corporate relationships and payments received varied among orthopedic companies. It is unclear whether this reflects partial reporting, or AAOS disclosure policy being mandatory only for select members rather than the entire membership.
This study had a few limitations, none of which had a substantive impact on the results or conclusions. First, we could not determine how many AAOS members who were required to disclose actually disclosed. There is no mechanism for determining which members are involved in activities that require disclosure. Nonetheless, the intent of the policy is to make collaborations between orthopedic surgeons and industry transparent in order to address concerns about potential conflicts of interest. That 44% of AAOS members did not disclose their relationships cannot be considered a success. Second, information was available on the websites of only 6 of the top 15 orthopedic companies—a result stemming from the DOJ’s failure to specify how long these companies must continue posting disclosures. In this study, the lowest nondisclosure rate was 37%, and there is no reason to suspect that any other group of surgeon-consultants would be any more compliant with AAOS’s policy.
There are few reports on the effects of the DOJ settlement on the behavior of surgeon-consultants who are AAOS members. Hockenberry and colleagues10 found that, since the settlement, surgeon payments have increased, number of consultants has decreased, and the proportion of consultants from academia has increased. They thought their findings confirmed concerns that orthopedic device makers would deliberately select high-volume orthopedic surgeons as consultants in order to increase sales of their implants and gain market share at the expense of their competitors. The authors thought that AAOS had some power to address disclosure through its influence on its members, but that influence may not be enough. Jegede and colleagues11 found that a significant percentage (41%) of orthopedic surgeons who received corporate payments and presented at the AAOS annual meeting were inconsistent in submitting disclosure information. Results of the present study suggest that AAOS policy is weak and does not adequately address the issue and provide full transparency, either within the organization or to the public, of all its members’ industry relationships.
As the preeminent provider of musculoskeletal education to orthopedic surgeons and others, and with a membership totaling almost 39,000, AAOS is one of the most important orthopedic societies in the world. AAOS has clearly stated that one of its goals is to increase transparency by making its surgeon disclosure program available to AAOS members and the public. However, it can be completely transparent only if all its members are required to disclose their corporate relationships. This study demonstrated that AAOS’s policy of mandatory disclosure for select members and voluntary disclosure for all other members is ineffective. We found that 44% of members failed to disclose industry-derived payments. This inadequate level of compliance runs contrary to the AAOS goal of increasing transparency of surgeon–industry consulting by making its surgeon disclosure program available to AAOS members and the public. The AAOS disclosure program and the potential consequences of noncompliance need to be reevaluated by the organization if it wants its program to succeed.
1. Crowninshield RD, Callaghan JJ. The orthopaedic profession and the industry partnership. Clin Orthop Relat Res. 2007;(457):73-77.
2. White AP, Vaccaro AR, Zdeblick T. Counterpoint: physician–industry relationships can be ethically established, and conflicts of interest can be ethically managed. Spine. 2007;32(11 suppl):S53-S57.
3. Steinbrook R. Online disclosure of physician–industry relationships. N Engl J Med. 2009;360(4):325-327.
4. Steinbrook R. Disclosure of industry payments to physicians. N Engl J Med. 2008;359(6):559-561.
5. Weinfurt KP, Friedman JY, Dinan MA, et al. Disclosing conflicts of interest in clinical research: views of institutional review boards, conflict of interest committees, and investigators. J Law Med Ethics. 2006;34(3):581-591, 481.
6. US Attorney’s Office, District of New Jersey. Monitoring and deferred prosecution agreements terminated with companies in hip and knee replacement industry [press release]. Federal Bureau of Investigation, Newark Division website. http://www.fbi.gov/newark/press-releases/2009/nk033009a.htm. March 30, 2009. Accessed May 13, 2015.
7. AAOS mandatory disclosure policy. American Academy of Orthopaedic Surgeons website. http://www.aaos.org/about/policies/DisclosurePolicy.asp. Adopted February 2007. Revised December 2009, February 2012. Accessed May 13, 2015.
8. The AAOS orthopaedic disclosure program. American Academy of Orthopaedic Surgeons website. http://www7.aaos.org/education/disclosure. Accessed May 13, 2015.
9. Top 15 ortho companies by revenue [based on 2011 full-year financials]. OrthoStreams website. http://orthostreams.com/top-15-ortho-companies-by-revenue/http://orthostreams.com/2012/03/the-top-15-orthopedic-companies-ranked-by-2011 revenue/. Accessed May 13, 2015.
10. Hockenberry JM, Weigel P, Auerbach A, Cram P. Financial payments by orthopedic device makers to orthopedic surgeons. Arch Intern Med. 2011;171(19):1759-1765.
11. Jegede KA, Ju B, Miller CP, Whang P, Grauer JN. Quantifying the variability of financial disclosure information reported by authors presenting research at multiple sports medicine conferences. Am J Orthop. 2011;40(11):583-587.
1. Crowninshield RD, Callaghan JJ. The orthopaedic profession and the industry partnership. Clin Orthop Relat Res. 2007;(457):73-77.
2. White AP, Vaccaro AR, Zdeblick T. Counterpoint: physician–industry relationships can be ethically established, and conflicts of interest can be ethically managed. Spine. 2007;32(11 suppl):S53-S57.
3. Steinbrook R. Online disclosure of physician–industry relationships. N Engl J Med. 2009;360(4):325-327.
4. Steinbrook R. Disclosure of industry payments to physicians. N Engl J Med. 2008;359(6):559-561.
5. Weinfurt KP, Friedman JY, Dinan MA, et al. Disclosing conflicts of interest in clinical research: views of institutional review boards, conflict of interest committees, and investigators. J Law Med Ethics. 2006;34(3):581-591, 481.
6. US Attorney’s Office, District of New Jersey. Monitoring and deferred prosecution agreements terminated with companies in hip and knee replacement industry [press release]. Federal Bureau of Investigation, Newark Division website. http://www.fbi.gov/newark/press-releases/2009/nk033009a.htm. March 30, 2009. Accessed May 13, 2015.
7. AAOS mandatory disclosure policy. American Academy of Orthopaedic Surgeons website. http://www.aaos.org/about/policies/DisclosurePolicy.asp. Adopted February 2007. Revised December 2009, February 2012. Accessed May 13, 2015.
8. The AAOS orthopaedic disclosure program. American Academy of Orthopaedic Surgeons website. http://www7.aaos.org/education/disclosure. Accessed May 13, 2015.
9. Top 15 ortho companies by revenue [based on 2011 full-year financials]. OrthoStreams website. http://orthostreams.com/top-15-ortho-companies-by-revenue/http://orthostreams.com/2012/03/the-top-15-orthopedic-companies-ranked-by-2011 revenue/. Accessed May 13, 2015.
10. Hockenberry JM, Weigel P, Auerbach A, Cram P. Financial payments by orthopedic device makers to orthopedic surgeons. Arch Intern Med. 2011;171(19):1759-1765.
11. Jegede KA, Ju B, Miller CP, Whang P, Grauer JN. Quantifying the variability of financial disclosure information reported by authors presenting research at multiple sports medicine conferences. Am J Orthop. 2011;40(11):583-587.
Image-Based Techniques for Percutaneous Iliosacral Screw Start-Site Localization
Iliosacral (SI) screws remain the standard of care for the vast majority of posterior pelvic ring disruptions.1,2 However, despite their routine use, the procedure remains technically demanding with repeated cases of aberrant screw placement and complications.3,4 Sacral morphology is extremely variable within a patient population and affects accurate placement and trajectory of percutaneous screws.5 Classically, it is taught that the external starting position/landmark is at an intersection point of the greater trochanter and the anterior superior iliac spine (ASIS). While this “one size fits all” approach will certainly help to coordinate a start position, it is our experience that multiple stab incisions are necessary to find the optimal start site. To our knowledge, the most common image-based technique used to guide start-point localization and placement of SI screws begins with drawing a virtual sacrum on the patient’s side, guided by the lateral image.5 This article provides a novel image-based technique to be used with, or as a replacement for, the traditional technique.
Techniques
The patient is brought to the operating room and placed supine on a radiolucent operating table. If the closed reduction of the pelvic ring is successful or can be achieved via anterior manipulation/traction, posterior percutaneous pinning is planned. Either a rolled towel or a bag of saline is used as a bolster and placed midline underneath the sacrum and lumbar spine to help “bump” the pelvis and improve the range of motion for the surgeon’s drill. The patient is brought to the edge of the table when possible (ie, a posterior ring injury requiring fixation from only 1 side) to further enhance drill motion. If bilateral screws are planned, surgeons must be careful not to position 1 side at the expense of screw placement on the contralateral side. Nitrous-based anesthetic agents are avoided, because they may collect in the bowel and obscure good radiographic visualization. Arms are placed perpendicular to the body to facilitate the inlet view. Pre-preparation anteroposterior pelvis, inlet, and outlet views are obtained to assure ability to accurately and safely assess landmarks on all projections, and to mark the C-arm position and angles. This process helps decrease “useless” radiographs obtained during the procedure. Acceptable inlet radiographs show the anterior cortex of the S1 body superimposed on the S2 body. Acceptable outlet radiographs show the superior pubic symphysis at the level of the S2 foramen and visualization of the S1/S2 sacral foramen.6 The patient is then prepared in the standard fashion. Reduction maneuvers are performed and, if acceptable alignment is achieved, posterior percutaneous screw placement begins.
Technique 1
To our knowledge, the most common image-based technique used to guide start-point localization and placement of SI screws begins with drawing a virtual sacrum on the patient’s side using the lateral image. The fluoroscopic machine is set up in a lateral position.5 A free guide wire is superimposed upon the iliac cortical density and anterior sacral slope, which is marked on the skin (Figure 1). The superior portion of S1, as well as the posterior sacral slope, can be marked as well. This process has outlined the sacrum and provides an external landmark for the “safe zone” for screw placement. The operation proceeds in the standard fashion using inlet, outlet, and lateral radiographs. However, the externally drawn sacrum can aid as a reference during guide-pin placement.
Technique 2
This technique takes into account bone anatomy and soft-tissue coverage. It is helpful to think of the abdomen/pelvis as a box. The anterior abdomen represents the top of the box and the lateral buttock represents the side of the box. The corner of the imaginary box is where the abdomen begins to slope down and transitions laterally to become the buttock. This will be referenced as the “down-sloping point” and typically corresponds to the level of the iliac crest (Figure 2).
To begin, a standard cannulated screw guide wire is placed flush on the skin of the abdomen. An inlet fluoroscopy image is taken with the guide pin on the abdomen. Imagine that the resulting image represents the planned screw trajectory (Figure 3A). When the position of the guide wire is deemed adequate, a line is marked on the abdomen, using a pen, directly adjacent to the guide wire. This line represents inlet line 1 (Figure 2). The line must continue laterally until the down-sloping point. The sagittal angle of the imaginary inlet fluoroscopic beam is noted, and a guide wire is placed in the same sagittal orientation flush with the skin on the lateral buttock (Figure 3B). The guide wire must be placed so that it intersects with the first line at the down-sloping point. The skin on the lateral aspect of buttock is marked with a second line, which represents inlet line 2 (Figure 2).
The same process is repeated using an outlet view to create outlet lines 1 and 2 (Figures 4A, 4B). At this point there are 4 lines drawn on the patient (Figure 2). A stab incision is made at the intersection of the 2 lines drawn on the lateral buttock; this represents the skin start point, labeled “start incision” (Figure 2). The procedure continues in standard fashion.
The 4 external reference lines serve multiple purposes. First, the lines mark the true lateral start point for the pin at the level of the skin. This contrasts with the standard technique in which bony landmarks are marked on the skin and the surgeon must estimate a point on the skin that will provide an appropriate trajectory to the bony start point on the ilium. Further, the lines can also be used to reorient the cartesian plane so that adjustments can be isolated to a single plane, ensuring movements only alter the position on a single radiographic view (Figure 5).
Discussion
Despite the widespread use of percutaneous screw placement for posterior pelvic ring injuries, this remains a technically demanding surgery. Recent data suggest patient pelvic anatomy is extremely varied, especially the sacrum.7 Further, screw trajectories vary depending on surgical goals, fracture pattern, and number of screws. Taken together, this implies that there is no perfect universal starting site along the external ilium. Therefore, while classic teaching states to begin screw insertion within the vicinity of the intersection of the greater trochanter and the ASIS, it is our experience that this location is often not ideal.
The inlet, outlet, and lateral radiographs are all vital to assess correct trajectory of the guide pin and drill prior to final screw insertion, but the start site remains a critical step to assure a successful surgical outcome. We present 2 techniques, used together or separately, that allow the surgeon to place the initial guide pin more accurately for percutanous iliosacral screws. Though not specifically examined in this study, we think technique 2 has the potential to save operative time and use less fluoroscopic imaging because a lateral image is not required until later in the case. Technique 2 identifies the start point at the level of the skin. This is in contrast to technique 1, which identifies the desired sacral target and requires a surgeon to select a skin start site that will provide an optimal trajectory towards the desired target. Judging trajectory can be difficult, particularly in obese patients, and technique 2 eliminates this extra variable.
It is also important to consider that criteria-based nonorthogonal imaging is required for percutaneous screw placement. In these cases, it is more difficult to judge trajectory corrections because the fluoroscopic beam cannot guide perpendicular corrections as it can in operations that use orthogonal imaging. Adjustments made perpendicular to the fluoroscopic beam will change trajectory in multiple planes.8 Moreover, because the standard cartesian frame of reference is rotated, understanding the location of the sacrum in space can be especially challenging. When using the first technique, sacral landmarks are delineated, and a virtual sacrum drawn on the patient’s exterior helps with orientation. In the second technique, the ideal pin placement is mapped, and the external reference lines guide uniplanar changes. For example, the line drawn co-planar with the inlet view is essentially marking the sacral slope. Therefore, by following this line, uniplanar changes in the cranial and caudal direction are achieved on the outlet view (Figure 5). Because this line is also in reference to the already known ideal pin placement, ideal pin placement can be maintained in 1 radiographic projection while changing the start site in the appropriate direction. In a similar fashion, the co-planar line identified on the inlet view can be used on the outlet image to affect uniplanar changes in the anteroposterior direction. This technique effectively minimizes disorientation when placing percutaneous SI screws. This can be particularly beneficial when placing screws in the prone position.
Conclusion
We have shown 2 techniques that are routinely used at our institution to help identify an accurate starting position for percutaneous screw placement in posterior pelvic ring injuries. Even experienced traumatologists can more quickly and accurately identify the correct stab incisions leading to more confidently placed screws. Further, we believe understanding the usage of fluoroscopy and the concepts involved in drawing the lines enhance trainees’ comprehension of the complex anatomy of the sacrum.
1. Matta JM, Saucedo T. Internal fixation of pelvic ring fractures. Clin Orthop Relat Res. 1989;242:83-97.
2. Routt ML Jr, Kregor PJ, Simonian PT, Mayo KA. Early results of percutaneous iliosacral screws placed with the patient in the supine position. J Orthop Trauma. 1995;9(3):207-214.
3. Sagi HC, Lindvall EM. Inadvertent intraforaminal iliosacral screw placement despite apparent appropriate positioning on intraoperative fluoroscopy.
J Orthop Trauma. 2005;19(2):130-133.
4. Routt ML Jr, Simonian PT, Mills WJ. Iliosacral screw fixation: early complications of the percutaneous technique. J Orthop Trauma. 1997;11(8):584-589.
5. Routt ML Jr, Simonian PT, Agnew SG, Mann FA. Radiographic recognition of the sacral alar slope for optimal placement of iliosacral screws: a cadaveric and clinical study. J Orthop Trauma. 1996;10(3):171-177.
6. Gardner MJ, Ferrell ED, Nork SE, Segina DN, Routt ML Jr. Percutaneous placement of iliosacral screws without electrodiagnostic monitoring. J Trauma. 2009;66(5):1411-1415.
7. Miller AN, Routt ML Jr. Variations in sacral morphology and implications for iliosacral screw fixation. J Am Acad Orthop Surg. 2012;20(1):8-16.
8. Graves ML, Routt ML. Iliosacral screw placement: are uniplanar changes realistic based on standard fluoroscopic imaging? J Orthop Trauma. 2011;71(1):204-208.
Iliosacral (SI) screws remain the standard of care for the vast majority of posterior pelvic ring disruptions.1,2 However, despite their routine use, the procedure remains technically demanding with repeated cases of aberrant screw placement and complications.3,4 Sacral morphology is extremely variable within a patient population and affects accurate placement and trajectory of percutaneous screws.5 Classically, it is taught that the external starting position/landmark is at an intersection point of the greater trochanter and the anterior superior iliac spine (ASIS). While this “one size fits all” approach will certainly help to coordinate a start position, it is our experience that multiple stab incisions are necessary to find the optimal start site. To our knowledge, the most common image-based technique used to guide start-point localization and placement of SI screws begins with drawing a virtual sacrum on the patient’s side, guided by the lateral image.5 This article provides a novel image-based technique to be used with, or as a replacement for, the traditional technique.
Techniques
The patient is brought to the operating room and placed supine on a radiolucent operating table. If the closed reduction of the pelvic ring is successful or can be achieved via anterior manipulation/traction, posterior percutaneous pinning is planned. Either a rolled towel or a bag of saline is used as a bolster and placed midline underneath the sacrum and lumbar spine to help “bump” the pelvis and improve the range of motion for the surgeon’s drill. The patient is brought to the edge of the table when possible (ie, a posterior ring injury requiring fixation from only 1 side) to further enhance drill motion. If bilateral screws are planned, surgeons must be careful not to position 1 side at the expense of screw placement on the contralateral side. Nitrous-based anesthetic agents are avoided, because they may collect in the bowel and obscure good radiographic visualization. Arms are placed perpendicular to the body to facilitate the inlet view. Pre-preparation anteroposterior pelvis, inlet, and outlet views are obtained to assure ability to accurately and safely assess landmarks on all projections, and to mark the C-arm position and angles. This process helps decrease “useless” radiographs obtained during the procedure. Acceptable inlet radiographs show the anterior cortex of the S1 body superimposed on the S2 body. Acceptable outlet radiographs show the superior pubic symphysis at the level of the S2 foramen and visualization of the S1/S2 sacral foramen.6 The patient is then prepared in the standard fashion. Reduction maneuvers are performed and, if acceptable alignment is achieved, posterior percutaneous screw placement begins.
Technique 1
To our knowledge, the most common image-based technique used to guide start-point localization and placement of SI screws begins with drawing a virtual sacrum on the patient’s side using the lateral image. The fluoroscopic machine is set up in a lateral position.5 A free guide wire is superimposed upon the iliac cortical density and anterior sacral slope, which is marked on the skin (Figure 1). The superior portion of S1, as well as the posterior sacral slope, can be marked as well. This process has outlined the sacrum and provides an external landmark for the “safe zone” for screw placement. The operation proceeds in the standard fashion using inlet, outlet, and lateral radiographs. However, the externally drawn sacrum can aid as a reference during guide-pin placement.
Technique 2
This technique takes into account bone anatomy and soft-tissue coverage. It is helpful to think of the abdomen/pelvis as a box. The anterior abdomen represents the top of the box and the lateral buttock represents the side of the box. The corner of the imaginary box is where the abdomen begins to slope down and transitions laterally to become the buttock. This will be referenced as the “down-sloping point” and typically corresponds to the level of the iliac crest (Figure 2).
To begin, a standard cannulated screw guide wire is placed flush on the skin of the abdomen. An inlet fluoroscopy image is taken with the guide pin on the abdomen. Imagine that the resulting image represents the planned screw trajectory (Figure 3A). When the position of the guide wire is deemed adequate, a line is marked on the abdomen, using a pen, directly adjacent to the guide wire. This line represents inlet line 1 (Figure 2). The line must continue laterally until the down-sloping point. The sagittal angle of the imaginary inlet fluoroscopic beam is noted, and a guide wire is placed in the same sagittal orientation flush with the skin on the lateral buttock (Figure 3B). The guide wire must be placed so that it intersects with the first line at the down-sloping point. The skin on the lateral aspect of buttock is marked with a second line, which represents inlet line 2 (Figure 2).
The same process is repeated using an outlet view to create outlet lines 1 and 2 (Figures 4A, 4B). At this point there are 4 lines drawn on the patient (Figure 2). A stab incision is made at the intersection of the 2 lines drawn on the lateral buttock; this represents the skin start point, labeled “start incision” (Figure 2). The procedure continues in standard fashion.
The 4 external reference lines serve multiple purposes. First, the lines mark the true lateral start point for the pin at the level of the skin. This contrasts with the standard technique in which bony landmarks are marked on the skin and the surgeon must estimate a point on the skin that will provide an appropriate trajectory to the bony start point on the ilium. Further, the lines can also be used to reorient the cartesian plane so that adjustments can be isolated to a single plane, ensuring movements only alter the position on a single radiographic view (Figure 5).
Discussion
Despite the widespread use of percutaneous screw placement for posterior pelvic ring injuries, this remains a technically demanding surgery. Recent data suggest patient pelvic anatomy is extremely varied, especially the sacrum.7 Further, screw trajectories vary depending on surgical goals, fracture pattern, and number of screws. Taken together, this implies that there is no perfect universal starting site along the external ilium. Therefore, while classic teaching states to begin screw insertion within the vicinity of the intersection of the greater trochanter and the ASIS, it is our experience that this location is often not ideal.
The inlet, outlet, and lateral radiographs are all vital to assess correct trajectory of the guide pin and drill prior to final screw insertion, but the start site remains a critical step to assure a successful surgical outcome. We present 2 techniques, used together or separately, that allow the surgeon to place the initial guide pin more accurately for percutanous iliosacral screws. Though not specifically examined in this study, we think technique 2 has the potential to save operative time and use less fluoroscopic imaging because a lateral image is not required until later in the case. Technique 2 identifies the start point at the level of the skin. This is in contrast to technique 1, which identifies the desired sacral target and requires a surgeon to select a skin start site that will provide an optimal trajectory towards the desired target. Judging trajectory can be difficult, particularly in obese patients, and technique 2 eliminates this extra variable.
It is also important to consider that criteria-based nonorthogonal imaging is required for percutaneous screw placement. In these cases, it is more difficult to judge trajectory corrections because the fluoroscopic beam cannot guide perpendicular corrections as it can in operations that use orthogonal imaging. Adjustments made perpendicular to the fluoroscopic beam will change trajectory in multiple planes.8 Moreover, because the standard cartesian frame of reference is rotated, understanding the location of the sacrum in space can be especially challenging. When using the first technique, sacral landmarks are delineated, and a virtual sacrum drawn on the patient’s exterior helps with orientation. In the second technique, the ideal pin placement is mapped, and the external reference lines guide uniplanar changes. For example, the line drawn co-planar with the inlet view is essentially marking the sacral slope. Therefore, by following this line, uniplanar changes in the cranial and caudal direction are achieved on the outlet view (Figure 5). Because this line is also in reference to the already known ideal pin placement, ideal pin placement can be maintained in 1 radiographic projection while changing the start site in the appropriate direction. In a similar fashion, the co-planar line identified on the inlet view can be used on the outlet image to affect uniplanar changes in the anteroposterior direction. This technique effectively minimizes disorientation when placing percutaneous SI screws. This can be particularly beneficial when placing screws in the prone position.
Conclusion
We have shown 2 techniques that are routinely used at our institution to help identify an accurate starting position for percutaneous screw placement in posterior pelvic ring injuries. Even experienced traumatologists can more quickly and accurately identify the correct stab incisions leading to more confidently placed screws. Further, we believe understanding the usage of fluoroscopy and the concepts involved in drawing the lines enhance trainees’ comprehension of the complex anatomy of the sacrum.
Iliosacral (SI) screws remain the standard of care for the vast majority of posterior pelvic ring disruptions.1,2 However, despite their routine use, the procedure remains technically demanding with repeated cases of aberrant screw placement and complications.3,4 Sacral morphology is extremely variable within a patient population and affects accurate placement and trajectory of percutaneous screws.5 Classically, it is taught that the external starting position/landmark is at an intersection point of the greater trochanter and the anterior superior iliac spine (ASIS). While this “one size fits all” approach will certainly help to coordinate a start position, it is our experience that multiple stab incisions are necessary to find the optimal start site. To our knowledge, the most common image-based technique used to guide start-point localization and placement of SI screws begins with drawing a virtual sacrum on the patient’s side, guided by the lateral image.5 This article provides a novel image-based technique to be used with, or as a replacement for, the traditional technique.
Techniques
The patient is brought to the operating room and placed supine on a radiolucent operating table. If the closed reduction of the pelvic ring is successful or can be achieved via anterior manipulation/traction, posterior percutaneous pinning is planned. Either a rolled towel or a bag of saline is used as a bolster and placed midline underneath the sacrum and lumbar spine to help “bump” the pelvis and improve the range of motion for the surgeon’s drill. The patient is brought to the edge of the table when possible (ie, a posterior ring injury requiring fixation from only 1 side) to further enhance drill motion. If bilateral screws are planned, surgeons must be careful not to position 1 side at the expense of screw placement on the contralateral side. Nitrous-based anesthetic agents are avoided, because they may collect in the bowel and obscure good radiographic visualization. Arms are placed perpendicular to the body to facilitate the inlet view. Pre-preparation anteroposterior pelvis, inlet, and outlet views are obtained to assure ability to accurately and safely assess landmarks on all projections, and to mark the C-arm position and angles. This process helps decrease “useless” radiographs obtained during the procedure. Acceptable inlet radiographs show the anterior cortex of the S1 body superimposed on the S2 body. Acceptable outlet radiographs show the superior pubic symphysis at the level of the S2 foramen and visualization of the S1/S2 sacral foramen.6 The patient is then prepared in the standard fashion. Reduction maneuvers are performed and, if acceptable alignment is achieved, posterior percutaneous screw placement begins.
Technique 1
To our knowledge, the most common image-based technique used to guide start-point localization and placement of SI screws begins with drawing a virtual sacrum on the patient’s side using the lateral image. The fluoroscopic machine is set up in a lateral position.5 A free guide wire is superimposed upon the iliac cortical density and anterior sacral slope, which is marked on the skin (Figure 1). The superior portion of S1, as well as the posterior sacral slope, can be marked as well. This process has outlined the sacrum and provides an external landmark for the “safe zone” for screw placement. The operation proceeds in the standard fashion using inlet, outlet, and lateral radiographs. However, the externally drawn sacrum can aid as a reference during guide-pin placement.
Technique 2
This technique takes into account bone anatomy and soft-tissue coverage. It is helpful to think of the abdomen/pelvis as a box. The anterior abdomen represents the top of the box and the lateral buttock represents the side of the box. The corner of the imaginary box is where the abdomen begins to slope down and transitions laterally to become the buttock. This will be referenced as the “down-sloping point” and typically corresponds to the level of the iliac crest (Figure 2).
To begin, a standard cannulated screw guide wire is placed flush on the skin of the abdomen. An inlet fluoroscopy image is taken with the guide pin on the abdomen. Imagine that the resulting image represents the planned screw trajectory (Figure 3A). When the position of the guide wire is deemed adequate, a line is marked on the abdomen, using a pen, directly adjacent to the guide wire. This line represents inlet line 1 (Figure 2). The line must continue laterally until the down-sloping point. The sagittal angle of the imaginary inlet fluoroscopic beam is noted, and a guide wire is placed in the same sagittal orientation flush with the skin on the lateral buttock (Figure 3B). The guide wire must be placed so that it intersects with the first line at the down-sloping point. The skin on the lateral aspect of buttock is marked with a second line, which represents inlet line 2 (Figure 2).
The same process is repeated using an outlet view to create outlet lines 1 and 2 (Figures 4A, 4B). At this point there are 4 lines drawn on the patient (Figure 2). A stab incision is made at the intersection of the 2 lines drawn on the lateral buttock; this represents the skin start point, labeled “start incision” (Figure 2). The procedure continues in standard fashion.
The 4 external reference lines serve multiple purposes. First, the lines mark the true lateral start point for the pin at the level of the skin. This contrasts with the standard technique in which bony landmarks are marked on the skin and the surgeon must estimate a point on the skin that will provide an appropriate trajectory to the bony start point on the ilium. Further, the lines can also be used to reorient the cartesian plane so that adjustments can be isolated to a single plane, ensuring movements only alter the position on a single radiographic view (Figure 5).
Discussion
Despite the widespread use of percutaneous screw placement for posterior pelvic ring injuries, this remains a technically demanding surgery. Recent data suggest patient pelvic anatomy is extremely varied, especially the sacrum.7 Further, screw trajectories vary depending on surgical goals, fracture pattern, and number of screws. Taken together, this implies that there is no perfect universal starting site along the external ilium. Therefore, while classic teaching states to begin screw insertion within the vicinity of the intersection of the greater trochanter and the ASIS, it is our experience that this location is often not ideal.
The inlet, outlet, and lateral radiographs are all vital to assess correct trajectory of the guide pin and drill prior to final screw insertion, but the start site remains a critical step to assure a successful surgical outcome. We present 2 techniques, used together or separately, that allow the surgeon to place the initial guide pin more accurately for percutanous iliosacral screws. Though not specifically examined in this study, we think technique 2 has the potential to save operative time and use less fluoroscopic imaging because a lateral image is not required until later in the case. Technique 2 identifies the start point at the level of the skin. This is in contrast to technique 1, which identifies the desired sacral target and requires a surgeon to select a skin start site that will provide an optimal trajectory towards the desired target. Judging trajectory can be difficult, particularly in obese patients, and technique 2 eliminates this extra variable.
It is also important to consider that criteria-based nonorthogonal imaging is required for percutaneous screw placement. In these cases, it is more difficult to judge trajectory corrections because the fluoroscopic beam cannot guide perpendicular corrections as it can in operations that use orthogonal imaging. Adjustments made perpendicular to the fluoroscopic beam will change trajectory in multiple planes.8 Moreover, because the standard cartesian frame of reference is rotated, understanding the location of the sacrum in space can be especially challenging. When using the first technique, sacral landmarks are delineated, and a virtual sacrum drawn on the patient’s exterior helps with orientation. In the second technique, the ideal pin placement is mapped, and the external reference lines guide uniplanar changes. For example, the line drawn co-planar with the inlet view is essentially marking the sacral slope. Therefore, by following this line, uniplanar changes in the cranial and caudal direction are achieved on the outlet view (Figure 5). Because this line is also in reference to the already known ideal pin placement, ideal pin placement can be maintained in 1 radiographic projection while changing the start site in the appropriate direction. In a similar fashion, the co-planar line identified on the inlet view can be used on the outlet image to affect uniplanar changes in the anteroposterior direction. This technique effectively minimizes disorientation when placing percutaneous SI screws. This can be particularly beneficial when placing screws in the prone position.
Conclusion
We have shown 2 techniques that are routinely used at our institution to help identify an accurate starting position for percutaneous screw placement in posterior pelvic ring injuries. Even experienced traumatologists can more quickly and accurately identify the correct stab incisions leading to more confidently placed screws. Further, we believe understanding the usage of fluoroscopy and the concepts involved in drawing the lines enhance trainees’ comprehension of the complex anatomy of the sacrum.
1. Matta JM, Saucedo T. Internal fixation of pelvic ring fractures. Clin Orthop Relat Res. 1989;242:83-97.
2. Routt ML Jr, Kregor PJ, Simonian PT, Mayo KA. Early results of percutaneous iliosacral screws placed with the patient in the supine position. J Orthop Trauma. 1995;9(3):207-214.
3. Sagi HC, Lindvall EM. Inadvertent intraforaminal iliosacral screw placement despite apparent appropriate positioning on intraoperative fluoroscopy.
J Orthop Trauma. 2005;19(2):130-133.
4. Routt ML Jr, Simonian PT, Mills WJ. Iliosacral screw fixation: early complications of the percutaneous technique. J Orthop Trauma. 1997;11(8):584-589.
5. Routt ML Jr, Simonian PT, Agnew SG, Mann FA. Radiographic recognition of the sacral alar slope for optimal placement of iliosacral screws: a cadaveric and clinical study. J Orthop Trauma. 1996;10(3):171-177.
6. Gardner MJ, Ferrell ED, Nork SE, Segina DN, Routt ML Jr. Percutaneous placement of iliosacral screws without electrodiagnostic monitoring. J Trauma. 2009;66(5):1411-1415.
7. Miller AN, Routt ML Jr. Variations in sacral morphology and implications for iliosacral screw fixation. J Am Acad Orthop Surg. 2012;20(1):8-16.
8. Graves ML, Routt ML. Iliosacral screw placement: are uniplanar changes realistic based on standard fluoroscopic imaging? J Orthop Trauma. 2011;71(1):204-208.
1. Matta JM, Saucedo T. Internal fixation of pelvic ring fractures. Clin Orthop Relat Res. 1989;242:83-97.
2. Routt ML Jr, Kregor PJ, Simonian PT, Mayo KA. Early results of percutaneous iliosacral screws placed with the patient in the supine position. J Orthop Trauma. 1995;9(3):207-214.
3. Sagi HC, Lindvall EM. Inadvertent intraforaminal iliosacral screw placement despite apparent appropriate positioning on intraoperative fluoroscopy.
J Orthop Trauma. 2005;19(2):130-133.
4. Routt ML Jr, Simonian PT, Mills WJ. Iliosacral screw fixation: early complications of the percutaneous technique. J Orthop Trauma. 1997;11(8):584-589.
5. Routt ML Jr, Simonian PT, Agnew SG, Mann FA. Radiographic recognition of the sacral alar slope for optimal placement of iliosacral screws: a cadaveric and clinical study. J Orthop Trauma. 1996;10(3):171-177.
6. Gardner MJ, Ferrell ED, Nork SE, Segina DN, Routt ML Jr. Percutaneous placement of iliosacral screws without electrodiagnostic monitoring. J Trauma. 2009;66(5):1411-1415.
7. Miller AN, Routt ML Jr. Variations in sacral morphology and implications for iliosacral screw fixation. J Am Acad Orthop Surg. 2012;20(1):8-16.
8. Graves ML, Routt ML. Iliosacral screw placement: are uniplanar changes realistic based on standard fluoroscopic imaging? J Orthop Trauma. 2011;71(1):204-208.
Pain: Updates on Diagnostic and Treatment Modalities
IS ACETAMINOPHEN EFFECTIVE IN EASING BACK PAIN AND KNEE PAIN?
Machado GC, Maher CG, Ferreira PH, et al. Efficacy and safety of paracetamol for spinal pain and osteoarthritis: systematic review and meta-analysis of randomised placebo controlled trials. BMJ. 2015;350:h1225. doi: 10.1136/bmj.h1225.
Acetaminophen is ineffective in treating lower back pain and provides minimal short-term benefit for people with osteoarthritis, a systematic review of 13 randomized, placebo-controlled trials reports.
Two independent reviewers extracted data on pain, disability, and quality of life, as well as adverse effects, patient adherence, and use of rescue medication, and found high-quality evidence that:
• Acetaminophen is ineffective for reducing pain intensity and disability, or improving quality of life in patients with low back pain.
• Acetaminophen provides significant, but not clinically important, benefit for pain and disability in patients with hip or knee osteoarthritis.
• Patients taking acetaminophen are nearly four times more likely to have abnormal results on liver function tests.
COMMENTARY
This study adds to the literature a less potent effect of acetaminophen than we have previously assumed,1,2 suggesting a significant but not clinically important effect on pain. This result is at odds with the experience of many clinicians, who use acetaminophen regularly as a first-line agent for pain. When there is a dissonance between clinical experience and emerging evidence, one has to ask why. The explanation here may be that acetaminophen, NSAIDs, and opioid analgesics all have their problems and all seem to work better for some patients than others. In clinical practice, we often start with acetaminophen, which works for some patients, and go on to other agents for those in whom acetaminophen does not provide sufficient pain control. Studies that report a small mean effect may not detect the significant effect that can occur for many patients but gets hidden in the mean (which includes patients in whom there is no effect). I am reminded of the statistician who drowned in a river with a mean depth of 3 feet. A common clinical approach, often starting with well-tolerated acetaminophen and then progressing to other agents when needed, still seems sound. —NS
REFERENCES
1. Bannuru RR, Schmid CH, Kent DM, et al. Comparative effectiveness of pharmacologic interventions for knee osteoarthritis: a systematic review and network meta-analysis. Ann Intern Med. 2015;162(1):46-54. doi: 10.7326/M14-1231.
2. Williams CM, Maher CG, Latimer J, et al. Efficacy of paracetamol for acute low-back pain: a double-blind, randomised controlled trial. Lancet. 2014;384(9954):1586-1596. doi: 10.1016/S0140-6736(14)60805-9.
Continue for Back pain: Does early imaging improve outcomes? >>
BACK PAIN: DOES EARLY IMAGING IMPROVE OUTCOMES?
Jarvik JG, Gold LS, Comstock BA, et al. Association of early imaging for back pain with clinical outcomes in older adults. JAMA. 2015;313(11):1143-1153.
Early imaging for back pain is not associated with better one-year outcomes among patients ages 65 and older, according to a prospective cohort study of 5,239 older patients with a new primary care visit for back pain.
Investigators compared function and pain at the 12-month follow-up visit among 1,174 patients who had early radiographs, 349 patients who had early MRI/CT, and 3,719 controls.
The primary outcome was back or leg pain-related disability, as measured by a back and leg pain disability score. The mean score showed no significant differences between groups.
COMMENTARY
Many guidelines suggest consideration of imaging early on in the diagnosis of low back pain in older adults due to the high prevalence of important underlying causes such as cancer.1 This study looked at an older population and showed, as has been demonstrated in younger populations, that there is no advantage to early imaging with either x-ray or MRI, though costs were about 25% higher. —NS
REFERENCE
1. Davis PC, Wippold FJ II, Brunberg JA, et al. ACR Appropriateness Criteria on low back pain. J Am Coll Radiol. 2009;6(6):401-407.
Continue for anti-inflammatory drugs and antithrombotic therapy >>
ANTI-INFLAMMATORY DRUGS AND ANTITHROMBOTIC THERAPY
Schjerning Olsen AM, Gislason GH, McGettigan P, et al. Association of NSAID use with risk of bleeding and cardiovascular events in patients receiving antithrombotic therapy after myocardial infarction. JAMA. 2015;313(8):805-814.
Combining prescription NSAIDs with antithrombotic therapy following myocardial infarction (MI) increases the risk for bleeding and excess thrombotic events, according to a study of 61,971 MI patients with ongoing antithrombotic therapy.
During an average of 3.5 years’ follow-up, patients who had taken NSAIDs had increased rates of bleeding and cardiovascular events, with incidence rates per 100-person years as follows:
The study authors note that clinicians should use caution when prescribing NSAIDs to patients who have recently experienced MI.
COMMENTARY
The use of NSAIDs in patients who have had coronary disease has been an area of concern for almost a decade. In 2007, the American Heart Association issued a scientific advisory update discouraging use of COX-2 inhibitors in patients with coronary disease and concluding that more data are needed on the cardiovascular safety of conventional NSAIDs.1 Non-COX-2 selective NSAIDs, such as naproxen, appear to have a better cardiovascular safety profile than those with more COX-2 inhibition. They do carry an increased risk for bleeding. The study reviewed above suggests that patients who have been given NSAIDs, even for a short amount of time, have an increased risk for both bleeding and cardiovascular events, reminding us to carefully weigh the risk and benefit of using these commonly prescribed medications. —NS
REFERENCE
1. Antman EM, Bennett JS, Daugherty A, Furberg C, Roberts H, Taubert KA; American Heart Association. Use of nonsteroidal anti-inflammatory drugs: an update for clinicians: a scientific statement from the American Heart Association. Circulation. 2007;115(12):1634-1642.
Continue for chronic fatigue syndrome gets a new name >>
CHRONIC FATIGUE SYNDROME GETS A NEW NAME
Institute of Medicine. Beyond myalgic encephalomyelitis/chronic fatigue syndrome: redefining an illness. www.iom.edu/~/media/Files/Report%20Files/2015/MECFS/MECFS_ReportBrief.pdf. Accessed June 4, 2015.
Myalgic encephalomyelitis/chronic fatigue syndrome has a new name and more clearcut diagnostic criteria following a report by the Institute of Medicine. The new name, systemic exertion intolerance disease (SEID), better reflects how exertion exacerbates symptoms.
According to the report, the proposed diagnostic criteria for SEID is all three of the following:
• A substantial reduction or impairment in the ability to engage in pre-illness levels of occupational, educational, social, or personal activities that persists for more than six months and is accompanied by fatigue, which is often profound, is of new or definite onset (not lifelong), is not the result of ongoing excessive exertion, and is not substantially alleviated by rest
• Post-exertional malaise
• Unrefreshing sleep
Plus, at least one of the following:
• Cognitive impairment
• Orthostatic intolerance
The group also recommended that a new code be assigned in the ICD-10 that is not linked to chronic fatigue or neurasthenia.
COMMENTARY
Systemic exertion intolerance disease (SEID) will likely take some time to be integrated into practice as the new name for this condition. This change will also refocus attention on this difficult illness, which has always been challenging because the symptoms of SEID are nonspecific and overlap with many other illnesses, from hypothyroidism to depression. The guidelines are a welcome addition to the literature, giving us better direction in diagnosing a difficult disease. —NS
Continue for a review of most effective treatments for knee OA >>
REVIEW: MOST EFFECTIVE TREATMENTS FOR KNEE OA
Bannuru RR, Schmid CH, Kent DM, Vet al. Comparative effectiveness of pharmacologic interventions for knee osteoarthritis: a systematic review and network meta-analysis. Ann Intern Med. 2015;162(1):46-54. doi: 10.7326/M14-1231.
Intra-articular hyaluronic acid offers the best relief for pain in patients with knee osteoarthritis (OA), a meta-analysis of 137 studies with 33,243 subjects reports.
Researchers reviewed randomized trials of adults with knee OA that compared two or more treatments, including acetaminophen, diclofenac, ibuprofen, naproxen, celecoxib, intra-articular (IA) corticosteroids, IA hyaluronic acid, oral placebo, and IA placebo. They found for pain, stiffness, and function all treatments fared better than oral placebo.
• For pain, IA hyaluronic acid was most effective (0.63); acetaminophen was least effective (0.18).
• For function, all of the treatments were superior to oral placebo except IA corticosteroids.
• For stiffness, there was no significant difference among the different treatments.
COMMENTARY
The decision about which medicine to use to treat a patient with osteoarthritis is made on an individual basis, based on effectiveness for pain, as well as safety and cost considerations. Acetaminophen, which is the least effective pain agent studied, probably deserves its place as the most commonly used analgesic for OA based on safety and cost. IA treatments were in general more effective than oral treatments, though it is important to recognize that these studies looked at months, not years, of treatment of OA, and most of our patients are treated over a course of years. —NS
IS ACETAMINOPHEN EFFECTIVE IN EASING BACK PAIN AND KNEE PAIN?
Machado GC, Maher CG, Ferreira PH, et al. Efficacy and safety of paracetamol for spinal pain and osteoarthritis: systematic review and meta-analysis of randomised placebo controlled trials. BMJ. 2015;350:h1225. doi: 10.1136/bmj.h1225.
Acetaminophen is ineffective in treating lower back pain and provides minimal short-term benefit for people with osteoarthritis, a systematic review of 13 randomized, placebo-controlled trials reports.
Two independent reviewers extracted data on pain, disability, and quality of life, as well as adverse effects, patient adherence, and use of rescue medication, and found high-quality evidence that:
• Acetaminophen is ineffective for reducing pain intensity and disability, or improving quality of life in patients with low back pain.
• Acetaminophen provides significant, but not clinically important, benefit for pain and disability in patients with hip or knee osteoarthritis.
• Patients taking acetaminophen are nearly four times more likely to have abnormal results on liver function tests.
COMMENTARY
This study adds to the literature a less potent effect of acetaminophen than we have previously assumed,1,2 suggesting a significant but not clinically important effect on pain. This result is at odds with the experience of many clinicians, who use acetaminophen regularly as a first-line agent for pain. When there is a dissonance between clinical experience and emerging evidence, one has to ask why. The explanation here may be that acetaminophen, NSAIDs, and opioid analgesics all have their problems and all seem to work better for some patients than others. In clinical practice, we often start with acetaminophen, which works for some patients, and go on to other agents for those in whom acetaminophen does not provide sufficient pain control. Studies that report a small mean effect may not detect the significant effect that can occur for many patients but gets hidden in the mean (which includes patients in whom there is no effect). I am reminded of the statistician who drowned in a river with a mean depth of 3 feet. A common clinical approach, often starting with well-tolerated acetaminophen and then progressing to other agents when needed, still seems sound. —NS
REFERENCES
1. Bannuru RR, Schmid CH, Kent DM, et al. Comparative effectiveness of pharmacologic interventions for knee osteoarthritis: a systematic review and network meta-analysis. Ann Intern Med. 2015;162(1):46-54. doi: 10.7326/M14-1231.
2. Williams CM, Maher CG, Latimer J, et al. Efficacy of paracetamol for acute low-back pain: a double-blind, randomised controlled trial. Lancet. 2014;384(9954):1586-1596. doi: 10.1016/S0140-6736(14)60805-9.
Continue for Back pain: Does early imaging improve outcomes? >>
BACK PAIN: DOES EARLY IMAGING IMPROVE OUTCOMES?
Jarvik JG, Gold LS, Comstock BA, et al. Association of early imaging for back pain with clinical outcomes in older adults. JAMA. 2015;313(11):1143-1153.
Early imaging for back pain is not associated with better one-year outcomes among patients ages 65 and older, according to a prospective cohort study of 5,239 older patients with a new primary care visit for back pain.
Investigators compared function and pain at the 12-month follow-up visit among 1,174 patients who had early radiographs, 349 patients who had early MRI/CT, and 3,719 controls.
The primary outcome was back or leg pain-related disability, as measured by a back and leg pain disability score. The mean score showed no significant differences between groups.
COMMENTARY
Many guidelines suggest consideration of imaging early on in the diagnosis of low back pain in older adults due to the high prevalence of important underlying causes such as cancer.1 This study looked at an older population and showed, as has been demonstrated in younger populations, that there is no advantage to early imaging with either x-ray or MRI, though costs were about 25% higher. —NS
REFERENCE
1. Davis PC, Wippold FJ II, Brunberg JA, et al. ACR Appropriateness Criteria on low back pain. J Am Coll Radiol. 2009;6(6):401-407.
Continue for anti-inflammatory drugs and antithrombotic therapy >>
ANTI-INFLAMMATORY DRUGS AND ANTITHROMBOTIC THERAPY
Schjerning Olsen AM, Gislason GH, McGettigan P, et al. Association of NSAID use with risk of bleeding and cardiovascular events in patients receiving antithrombotic therapy after myocardial infarction. JAMA. 2015;313(8):805-814.
Combining prescription NSAIDs with antithrombotic therapy following myocardial infarction (MI) increases the risk for bleeding and excess thrombotic events, according to a study of 61,971 MI patients with ongoing antithrombotic therapy.
During an average of 3.5 years’ follow-up, patients who had taken NSAIDs had increased rates of bleeding and cardiovascular events, with incidence rates per 100-person years as follows:
The study authors note that clinicians should use caution when prescribing NSAIDs to patients who have recently experienced MI.
COMMENTARY
The use of NSAIDs in patients who have had coronary disease has been an area of concern for almost a decade. In 2007, the American Heart Association issued a scientific advisory update discouraging use of COX-2 inhibitors in patients with coronary disease and concluding that more data are needed on the cardiovascular safety of conventional NSAIDs.1 Non-COX-2 selective NSAIDs, such as naproxen, appear to have a better cardiovascular safety profile than those with more COX-2 inhibition. They do carry an increased risk for bleeding. The study reviewed above suggests that patients who have been given NSAIDs, even for a short amount of time, have an increased risk for both bleeding and cardiovascular events, reminding us to carefully weigh the risk and benefit of using these commonly prescribed medications. —NS
REFERENCE
1. Antman EM, Bennett JS, Daugherty A, Furberg C, Roberts H, Taubert KA; American Heart Association. Use of nonsteroidal anti-inflammatory drugs: an update for clinicians: a scientific statement from the American Heart Association. Circulation. 2007;115(12):1634-1642.
Continue for chronic fatigue syndrome gets a new name >>
CHRONIC FATIGUE SYNDROME GETS A NEW NAME
Institute of Medicine. Beyond myalgic encephalomyelitis/chronic fatigue syndrome: redefining an illness. www.iom.edu/~/media/Files/Report%20Files/2015/MECFS/MECFS_ReportBrief.pdf. Accessed June 4, 2015.
Myalgic encephalomyelitis/chronic fatigue syndrome has a new name and more clearcut diagnostic criteria following a report by the Institute of Medicine. The new name, systemic exertion intolerance disease (SEID), better reflects how exertion exacerbates symptoms.
According to the report, the proposed diagnostic criteria for SEID is all three of the following:
• A substantial reduction or impairment in the ability to engage in pre-illness levels of occupational, educational, social, or personal activities that persists for more than six months and is accompanied by fatigue, which is often profound, is of new or definite onset (not lifelong), is not the result of ongoing excessive exertion, and is not substantially alleviated by rest
• Post-exertional malaise
• Unrefreshing sleep
Plus, at least one of the following:
• Cognitive impairment
• Orthostatic intolerance
The group also recommended that a new code be assigned in the ICD-10 that is not linked to chronic fatigue or neurasthenia.
COMMENTARY
Systemic exertion intolerance disease (SEID) will likely take some time to be integrated into practice as the new name for this condition. This change will also refocus attention on this difficult illness, which has always been challenging because the symptoms of SEID are nonspecific and overlap with many other illnesses, from hypothyroidism to depression. The guidelines are a welcome addition to the literature, giving us better direction in diagnosing a difficult disease. —NS
Continue for a review of most effective treatments for knee OA >>
REVIEW: MOST EFFECTIVE TREATMENTS FOR KNEE OA
Bannuru RR, Schmid CH, Kent DM, Vet al. Comparative effectiveness of pharmacologic interventions for knee osteoarthritis: a systematic review and network meta-analysis. Ann Intern Med. 2015;162(1):46-54. doi: 10.7326/M14-1231.
Intra-articular hyaluronic acid offers the best relief for pain in patients with knee osteoarthritis (OA), a meta-analysis of 137 studies with 33,243 subjects reports.
Researchers reviewed randomized trials of adults with knee OA that compared two or more treatments, including acetaminophen, diclofenac, ibuprofen, naproxen, celecoxib, intra-articular (IA) corticosteroids, IA hyaluronic acid, oral placebo, and IA placebo. They found for pain, stiffness, and function all treatments fared better than oral placebo.
• For pain, IA hyaluronic acid was most effective (0.63); acetaminophen was least effective (0.18).
• For function, all of the treatments were superior to oral placebo except IA corticosteroids.
• For stiffness, there was no significant difference among the different treatments.
COMMENTARY
The decision about which medicine to use to treat a patient with osteoarthritis is made on an individual basis, based on effectiveness for pain, as well as safety and cost considerations. Acetaminophen, which is the least effective pain agent studied, probably deserves its place as the most commonly used analgesic for OA based on safety and cost. IA treatments were in general more effective than oral treatments, though it is important to recognize that these studies looked at months, not years, of treatment of OA, and most of our patients are treated over a course of years. —NS
IS ACETAMINOPHEN EFFECTIVE IN EASING BACK PAIN AND KNEE PAIN?
Machado GC, Maher CG, Ferreira PH, et al. Efficacy and safety of paracetamol for spinal pain and osteoarthritis: systematic review and meta-analysis of randomised placebo controlled trials. BMJ. 2015;350:h1225. doi: 10.1136/bmj.h1225.
Acetaminophen is ineffective in treating lower back pain and provides minimal short-term benefit for people with osteoarthritis, a systematic review of 13 randomized, placebo-controlled trials reports.
Two independent reviewers extracted data on pain, disability, and quality of life, as well as adverse effects, patient adherence, and use of rescue medication, and found high-quality evidence that:
• Acetaminophen is ineffective for reducing pain intensity and disability, or improving quality of life in patients with low back pain.
• Acetaminophen provides significant, but not clinically important, benefit for pain and disability in patients with hip or knee osteoarthritis.
• Patients taking acetaminophen are nearly four times more likely to have abnormal results on liver function tests.
COMMENTARY
This study adds to the literature a less potent effect of acetaminophen than we have previously assumed,1,2 suggesting a significant but not clinically important effect on pain. This result is at odds with the experience of many clinicians, who use acetaminophen regularly as a first-line agent for pain. When there is a dissonance between clinical experience and emerging evidence, one has to ask why. The explanation here may be that acetaminophen, NSAIDs, and opioid analgesics all have their problems and all seem to work better for some patients than others. In clinical practice, we often start with acetaminophen, which works for some patients, and go on to other agents for those in whom acetaminophen does not provide sufficient pain control. Studies that report a small mean effect may not detect the significant effect that can occur for many patients but gets hidden in the mean (which includes patients in whom there is no effect). I am reminded of the statistician who drowned in a river with a mean depth of 3 feet. A common clinical approach, often starting with well-tolerated acetaminophen and then progressing to other agents when needed, still seems sound. —NS
REFERENCES
1. Bannuru RR, Schmid CH, Kent DM, et al. Comparative effectiveness of pharmacologic interventions for knee osteoarthritis: a systematic review and network meta-analysis. Ann Intern Med. 2015;162(1):46-54. doi: 10.7326/M14-1231.
2. Williams CM, Maher CG, Latimer J, et al. Efficacy of paracetamol for acute low-back pain: a double-blind, randomised controlled trial. Lancet. 2014;384(9954):1586-1596. doi: 10.1016/S0140-6736(14)60805-9.
Continue for Back pain: Does early imaging improve outcomes? >>
BACK PAIN: DOES EARLY IMAGING IMPROVE OUTCOMES?
Jarvik JG, Gold LS, Comstock BA, et al. Association of early imaging for back pain with clinical outcomes in older adults. JAMA. 2015;313(11):1143-1153.
Early imaging for back pain is not associated with better one-year outcomes among patients ages 65 and older, according to a prospective cohort study of 5,239 older patients with a new primary care visit for back pain.
Investigators compared function and pain at the 12-month follow-up visit among 1,174 patients who had early radiographs, 349 patients who had early MRI/CT, and 3,719 controls.
The primary outcome was back or leg pain-related disability, as measured by a back and leg pain disability score. The mean score showed no significant differences between groups.
COMMENTARY
Many guidelines suggest consideration of imaging early on in the diagnosis of low back pain in older adults due to the high prevalence of important underlying causes such as cancer.1 This study looked at an older population and showed, as has been demonstrated in younger populations, that there is no advantage to early imaging with either x-ray or MRI, though costs were about 25% higher. —NS
REFERENCE
1. Davis PC, Wippold FJ II, Brunberg JA, et al. ACR Appropriateness Criteria on low back pain. J Am Coll Radiol. 2009;6(6):401-407.
Continue for anti-inflammatory drugs and antithrombotic therapy >>
ANTI-INFLAMMATORY DRUGS AND ANTITHROMBOTIC THERAPY
Schjerning Olsen AM, Gislason GH, McGettigan P, et al. Association of NSAID use with risk of bleeding and cardiovascular events in patients receiving antithrombotic therapy after myocardial infarction. JAMA. 2015;313(8):805-814.
Combining prescription NSAIDs with antithrombotic therapy following myocardial infarction (MI) increases the risk for bleeding and excess thrombotic events, according to a study of 61,971 MI patients with ongoing antithrombotic therapy.
During an average of 3.5 years’ follow-up, patients who had taken NSAIDs had increased rates of bleeding and cardiovascular events, with incidence rates per 100-person years as follows:
The study authors note that clinicians should use caution when prescribing NSAIDs to patients who have recently experienced MI.
COMMENTARY
The use of NSAIDs in patients who have had coronary disease has been an area of concern for almost a decade. In 2007, the American Heart Association issued a scientific advisory update discouraging use of COX-2 inhibitors in patients with coronary disease and concluding that more data are needed on the cardiovascular safety of conventional NSAIDs.1 Non-COX-2 selective NSAIDs, such as naproxen, appear to have a better cardiovascular safety profile than those with more COX-2 inhibition. They do carry an increased risk for bleeding. The study reviewed above suggests that patients who have been given NSAIDs, even for a short amount of time, have an increased risk for both bleeding and cardiovascular events, reminding us to carefully weigh the risk and benefit of using these commonly prescribed medications. —NS
REFERENCE
1. Antman EM, Bennett JS, Daugherty A, Furberg C, Roberts H, Taubert KA; American Heart Association. Use of nonsteroidal anti-inflammatory drugs: an update for clinicians: a scientific statement from the American Heart Association. Circulation. 2007;115(12):1634-1642.
Continue for chronic fatigue syndrome gets a new name >>
CHRONIC FATIGUE SYNDROME GETS A NEW NAME
Institute of Medicine. Beyond myalgic encephalomyelitis/chronic fatigue syndrome: redefining an illness. www.iom.edu/~/media/Files/Report%20Files/2015/MECFS/MECFS_ReportBrief.pdf. Accessed June 4, 2015.
Myalgic encephalomyelitis/chronic fatigue syndrome has a new name and more clearcut diagnostic criteria following a report by the Institute of Medicine. The new name, systemic exertion intolerance disease (SEID), better reflects how exertion exacerbates symptoms.
According to the report, the proposed diagnostic criteria for SEID is all three of the following:
• A substantial reduction or impairment in the ability to engage in pre-illness levels of occupational, educational, social, or personal activities that persists for more than six months and is accompanied by fatigue, which is often profound, is of new or definite onset (not lifelong), is not the result of ongoing excessive exertion, and is not substantially alleviated by rest
• Post-exertional malaise
• Unrefreshing sleep
Plus, at least one of the following:
• Cognitive impairment
• Orthostatic intolerance
The group also recommended that a new code be assigned in the ICD-10 that is not linked to chronic fatigue or neurasthenia.
COMMENTARY
Systemic exertion intolerance disease (SEID) will likely take some time to be integrated into practice as the new name for this condition. This change will also refocus attention on this difficult illness, which has always been challenging because the symptoms of SEID are nonspecific and overlap with many other illnesses, from hypothyroidism to depression. The guidelines are a welcome addition to the literature, giving us better direction in diagnosing a difficult disease. —NS
Continue for a review of most effective treatments for knee OA >>
REVIEW: MOST EFFECTIVE TREATMENTS FOR KNEE OA
Bannuru RR, Schmid CH, Kent DM, Vet al. Comparative effectiveness of pharmacologic interventions for knee osteoarthritis: a systematic review and network meta-analysis. Ann Intern Med. 2015;162(1):46-54. doi: 10.7326/M14-1231.
Intra-articular hyaluronic acid offers the best relief for pain in patients with knee osteoarthritis (OA), a meta-analysis of 137 studies with 33,243 subjects reports.
Researchers reviewed randomized trials of adults with knee OA that compared two or more treatments, including acetaminophen, diclofenac, ibuprofen, naproxen, celecoxib, intra-articular (IA) corticosteroids, IA hyaluronic acid, oral placebo, and IA placebo. They found for pain, stiffness, and function all treatments fared better than oral placebo.
• For pain, IA hyaluronic acid was most effective (0.63); acetaminophen was least effective (0.18).
• For function, all of the treatments were superior to oral placebo except IA corticosteroids.
• For stiffness, there was no significant difference among the different treatments.
COMMENTARY
The decision about which medicine to use to treat a patient with osteoarthritis is made on an individual basis, based on effectiveness for pain, as well as safety and cost considerations. Acetaminophen, which is the least effective pain agent studied, probably deserves its place as the most commonly used analgesic for OA based on safety and cost. IA treatments were in general more effective than oral treatments, though it is important to recognize that these studies looked at months, not years, of treatment of OA, and most of our patients are treated over a course of years. —NS
Advances in Stem Cell Research Lead to Osteoarthritis Treatment?
Researchers at the University of York in the United Kingdom, along with research colleagues at the Erasmus Medical Centre in Rotterdam, have identified individual stem cells that can regenerate tissue, cartilage, and bone, according to a study published June 9 in Stem Cell Reports.
Lead researcher Paul Genever, PhD, Senior Lecturer in the Department of Biology, and Head of the York site of the Arthritis Research UK Tissue Engineering Centre, said, “While stem cell therapy is an exciting new development for the treatment for osteoarthritis, up to now it has been something of a lottery because we did not know the precise properties of each of the cells.”
The study authors isolated individual marrow stromal cells and analyzed their different properties. This allowed the researchers to identify stem cells that are capable of repairing damaged cartilage or joint tissue. The York team also isolated a rare subset of stem cells in bone marrow that, while having no capability for tissue repair, appeared to have a prominent role in immune function.
“This project has helped us to establish which cells are good at regenerating tissue, cartilage, and bone, respectively. It will help in the search to develop more targeted therapies for arthritis patients, ” stated Dr. Genever.
Coauthor James Fox, PhD, said, “Working with colleagues across the Arthritis Research UK Tissue Engineering Centre will help to bring our discovery closer to patient treatment.”
Suggested Reading
James S, Fox J, Afsari F, et al. Multiparameter analysis of human bone marrow stromal cells identifies distinct immunomodulatory and differentiation-competent subtypes. Stem Cell Reports. 2015;4(6):1004-1015.
Researchers at the University of York in the United Kingdom, along with research colleagues at the Erasmus Medical Centre in Rotterdam, have identified individual stem cells that can regenerate tissue, cartilage, and bone, according to a study published June 9 in Stem Cell Reports.
Lead researcher Paul Genever, PhD, Senior Lecturer in the Department of Biology, and Head of the York site of the Arthritis Research UK Tissue Engineering Centre, said, “While stem cell therapy is an exciting new development for the treatment for osteoarthritis, up to now it has been something of a lottery because we did not know the precise properties of each of the cells.”
The study authors isolated individual marrow stromal cells and analyzed their different properties. This allowed the researchers to identify stem cells that are capable of repairing damaged cartilage or joint tissue. The York team also isolated a rare subset of stem cells in bone marrow that, while having no capability for tissue repair, appeared to have a prominent role in immune function.
“This project has helped us to establish which cells are good at regenerating tissue, cartilage, and bone, respectively. It will help in the search to develop more targeted therapies for arthritis patients, ” stated Dr. Genever.
Coauthor James Fox, PhD, said, “Working with colleagues across the Arthritis Research UK Tissue Engineering Centre will help to bring our discovery closer to patient treatment.”
Researchers at the University of York in the United Kingdom, along with research colleagues at the Erasmus Medical Centre in Rotterdam, have identified individual stem cells that can regenerate tissue, cartilage, and bone, according to a study published June 9 in Stem Cell Reports.
Lead researcher Paul Genever, PhD, Senior Lecturer in the Department of Biology, and Head of the York site of the Arthritis Research UK Tissue Engineering Centre, said, “While stem cell therapy is an exciting new development for the treatment for osteoarthritis, up to now it has been something of a lottery because we did not know the precise properties of each of the cells.”
The study authors isolated individual marrow stromal cells and analyzed their different properties. This allowed the researchers to identify stem cells that are capable of repairing damaged cartilage or joint tissue. The York team also isolated a rare subset of stem cells in bone marrow that, while having no capability for tissue repair, appeared to have a prominent role in immune function.
“This project has helped us to establish which cells are good at regenerating tissue, cartilage, and bone, respectively. It will help in the search to develop more targeted therapies for arthritis patients, ” stated Dr. Genever.
Coauthor James Fox, PhD, said, “Working with colleagues across the Arthritis Research UK Tissue Engineering Centre will help to bring our discovery closer to patient treatment.”
Suggested Reading
James S, Fox J, Afsari F, et al. Multiparameter analysis of human bone marrow stromal cells identifies distinct immunomodulatory and differentiation-competent subtypes. Stem Cell Reports. 2015;4(6):1004-1015.
Suggested Reading
James S, Fox J, Afsari F, et al. Multiparameter analysis of human bone marrow stromal cells identifies distinct immunomodulatory and differentiation-competent subtypes. Stem Cell Reports. 2015;4(6):1004-1015.
Stronger Muscle Mass Equated With Healthier Bone Development
Lean mass gained during childhood is positively associated with bone size and trabecular volumetric bone mineral density at ages 6 and 7, according to a study published online ahead of print in the June issue of Bone.
For this study, detailed measurements of 200 children enrolled in the Southampton Women’s Survey were taken soon after birth and again at ages 6 and 7. Scanning equipment was used to assess bone mineral density, shape and size of the tibia, and body composition.
“Bone strength and size is important because they are significant factors in long-term osteoporosis and fracture risk,” said Rebecca Moon, BSc, lead investigator of the study.
The researchers found no relationship between fat mass and bone development, indicating that it is not an important factor in childhood skeletal strength. The investigators also found that the relationship between changes in lean muscle and bone development was stronger in girls than in boys, despite the ages of the children, ruling out the onset of puberty as a factor.
“A 10% increase in peak bone mass will delay the onset of osteoporosis by 13 years. These findings point to the importance of early childhood physical activity to optimize muscle and bone growth,” said Dr. Moon.
Suggested Reading
Moon RJ, Cole ZA, Crozier SR, et al. Longitudinal changes in lean mass predict pQCT measures of tibial geometry and mineralization at ages 6-7 years. Bone. 2015;75:105-110.
Lean mass gained during childhood is positively associated with bone size and trabecular volumetric bone mineral density at ages 6 and 7, according to a study published online ahead of print in the June issue of Bone.
For this study, detailed measurements of 200 children enrolled in the Southampton Women’s Survey were taken soon after birth and again at ages 6 and 7. Scanning equipment was used to assess bone mineral density, shape and size of the tibia, and body composition.
“Bone strength and size is important because they are significant factors in long-term osteoporosis and fracture risk,” said Rebecca Moon, BSc, lead investigator of the study.
The researchers found no relationship between fat mass and bone development, indicating that it is not an important factor in childhood skeletal strength. The investigators also found that the relationship between changes in lean muscle and bone development was stronger in girls than in boys, despite the ages of the children, ruling out the onset of puberty as a factor.
“A 10% increase in peak bone mass will delay the onset of osteoporosis by 13 years. These findings point to the importance of early childhood physical activity to optimize muscle and bone growth,” said Dr. Moon.
Lean mass gained during childhood is positively associated with bone size and trabecular volumetric bone mineral density at ages 6 and 7, according to a study published online ahead of print in the June issue of Bone.
For this study, detailed measurements of 200 children enrolled in the Southampton Women’s Survey were taken soon after birth and again at ages 6 and 7. Scanning equipment was used to assess bone mineral density, shape and size of the tibia, and body composition.
“Bone strength and size is important because they are significant factors in long-term osteoporosis and fracture risk,” said Rebecca Moon, BSc, lead investigator of the study.
The researchers found no relationship between fat mass and bone development, indicating that it is not an important factor in childhood skeletal strength. The investigators also found that the relationship between changes in lean muscle and bone development was stronger in girls than in boys, despite the ages of the children, ruling out the onset of puberty as a factor.
“A 10% increase in peak bone mass will delay the onset of osteoporosis by 13 years. These findings point to the importance of early childhood physical activity to optimize muscle and bone growth,” said Dr. Moon.
Suggested Reading
Moon RJ, Cole ZA, Crozier SR, et al. Longitudinal changes in lean mass predict pQCT measures of tibial geometry and mineralization at ages 6-7 years. Bone. 2015;75:105-110.
Suggested Reading
Moon RJ, Cole ZA, Crozier SR, et al. Longitudinal changes in lean mass predict pQCT measures of tibial geometry and mineralization at ages 6-7 years. Bone. 2015;75:105-110.
Poor Sleep, Negative Attitude, Amplify Pain in Knee Osteoarthritis
Patients with knee osteoarthritis (OA) who have poor sleep habits display greater central sensitization of pain, according to a study published online ahead of print June 4 in Arthritis Care & Research. Study findings also showed that OA patients who catastrophize had increased central sensitization that was associated with greater pain.
“Our study is the largest and most comprehensive examination of the relationship between sleep disturbance, catastrophizing, and central sensitization in knee OA,” stated lead author Claudia Campbell, PhD, an Associate Professor of Psychiatry and Behavioral Sciences at Johns Hopkins University School of Medicine in Baltimore.
The case-controlled study included 208 participants who were categorized according to 4 groups: patients who have OA and insomnia, patients who have OA and normal sleep habits, healthy controls with insomnia, and healthy controls without a pain syndrome and normal sleep. In all, 72% of the study’s participants were female.
Participants completed multimodal sleep assessments (eg, questionnaire, diary, actigraphy, and polysmnography) and extensive evaluation of pain using clinical measures and quantitative sensory testing to evaluate associations between central sensitization, catastrophizing, and insomnia.
Results showed that the participants with knee OA and insomnia had the greatest amount of central sensitization compared with controls. The team found patients with poor sleep and high catastrophizing scores reported increased levels of central sensitization. In turn, central sensitization was significantly associated with increased clinical pain.
“While no causal processes may be determined from this study, our data suggest that those with low sleep efficiency and higher catastrophizing have the greatest central sensitization. Understanding the intricate relationship between sleep, central sensitization, and catastrophizing has important clinical implications for treating those with chronic pain conditions such as knee OA,” Dr. Campbell stated.
Suggested Reading
Campbell CM, Buenaver LF, Finan P, et al. Sleep, pain catastrophizing and central sensitization in knee osteoarthritis patients with and without insomnia. Arthritis Care Res. 2015 June 4. [Epub ahead of print]
Patients with knee osteoarthritis (OA) who have poor sleep habits display greater central sensitization of pain, according to a study published online ahead of print June 4 in Arthritis Care & Research. Study findings also showed that OA patients who catastrophize had increased central sensitization that was associated with greater pain.
“Our study is the largest and most comprehensive examination of the relationship between sleep disturbance, catastrophizing, and central sensitization in knee OA,” stated lead author Claudia Campbell, PhD, an Associate Professor of Psychiatry and Behavioral Sciences at Johns Hopkins University School of Medicine in Baltimore.
The case-controlled study included 208 participants who were categorized according to 4 groups: patients who have OA and insomnia, patients who have OA and normal sleep habits, healthy controls with insomnia, and healthy controls without a pain syndrome and normal sleep. In all, 72% of the study’s participants were female.
Participants completed multimodal sleep assessments (eg, questionnaire, diary, actigraphy, and polysmnography) and extensive evaluation of pain using clinical measures and quantitative sensory testing to evaluate associations between central sensitization, catastrophizing, and insomnia.
Results showed that the participants with knee OA and insomnia had the greatest amount of central sensitization compared with controls. The team found patients with poor sleep and high catastrophizing scores reported increased levels of central sensitization. In turn, central sensitization was significantly associated with increased clinical pain.
“While no causal processes may be determined from this study, our data suggest that those with low sleep efficiency and higher catastrophizing have the greatest central sensitization. Understanding the intricate relationship between sleep, central sensitization, and catastrophizing has important clinical implications for treating those with chronic pain conditions such as knee OA,” Dr. Campbell stated.
Patients with knee osteoarthritis (OA) who have poor sleep habits display greater central sensitization of pain, according to a study published online ahead of print June 4 in Arthritis Care & Research. Study findings also showed that OA patients who catastrophize had increased central sensitization that was associated with greater pain.
“Our study is the largest and most comprehensive examination of the relationship between sleep disturbance, catastrophizing, and central sensitization in knee OA,” stated lead author Claudia Campbell, PhD, an Associate Professor of Psychiatry and Behavioral Sciences at Johns Hopkins University School of Medicine in Baltimore.
The case-controlled study included 208 participants who were categorized according to 4 groups: patients who have OA and insomnia, patients who have OA and normal sleep habits, healthy controls with insomnia, and healthy controls without a pain syndrome and normal sleep. In all, 72% of the study’s participants were female.
Participants completed multimodal sleep assessments (eg, questionnaire, diary, actigraphy, and polysmnography) and extensive evaluation of pain using clinical measures and quantitative sensory testing to evaluate associations between central sensitization, catastrophizing, and insomnia.
Results showed that the participants with knee OA and insomnia had the greatest amount of central sensitization compared with controls. The team found patients with poor sleep and high catastrophizing scores reported increased levels of central sensitization. In turn, central sensitization was significantly associated with increased clinical pain.
“While no causal processes may be determined from this study, our data suggest that those with low sleep efficiency and higher catastrophizing have the greatest central sensitization. Understanding the intricate relationship between sleep, central sensitization, and catastrophizing has important clinical implications for treating those with chronic pain conditions such as knee OA,” Dr. Campbell stated.
Suggested Reading
Campbell CM, Buenaver LF, Finan P, et al. Sleep, pain catastrophizing and central sensitization in knee osteoarthritis patients with and without insomnia. Arthritis Care Res. 2015 June 4. [Epub ahead of print]
Suggested Reading
Campbell CM, Buenaver LF, Finan P, et al. Sleep, pain catastrophizing and central sensitization in knee osteoarthritis patients with and without insomnia. Arthritis Care Res. 2015 June 4. [Epub ahead of print]