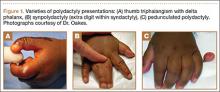
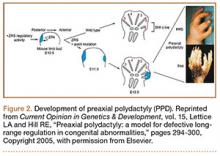
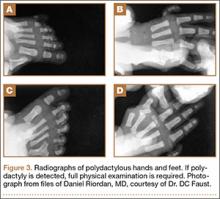
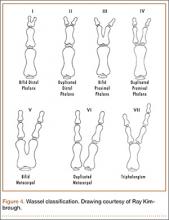
User login
Oral steroids modestly improve acute radiculopathy
A 15-day course of oral steroids modestly improved function but not pain in patients with acute radiculopathy due to a herniated lumbar disk, according to a report published online May 19 in JAMA.
Compared with epidural steroid injections, oral steroids provide similar anti-inflammatory activity but avoid MRI, can be delivered quickly by primary care physicians, avert the risks of spinal injection, and are much cheaper. “Oral steroids are used by many community physicians, have been included in some clinical guidelines, and are noted as a treatment option by some authors,” yet no adequately powered clinical trials of the therapy have been performed until now, said Dr. Harley Goldberg of the department of physical medicine at the Kaiser Permanente Northern California Spine Care Program, San Jose, and his associates.
Their study involved 269 adults at three primary care practices who had leg pain extending below the knee in a nerve root distribution, confirmation of a herniated disk on MRI, and scores of 30 or higher on the 100-point Oswestry Disability Index (ODI). These participants were randomly assigned to receive either daily prednisone capsules (cumulative dose, 600 mg) or matching placebo in addition to usual care and were followed for 1 year.
The primary outcome measure was self-reported score on the ODI at 3 weeks. After adjustment for potential confounders, the mean score was 6.4 points higher with prednisone than with placebo, a significant but modest difference. This benefit persisted at 1-year follow-up, with a mean difference of 7.4 points between the two study groups. In addition, the active-treatment group was significantly more likely to achieve at least a 30-point or 50% improvement in ODI score at 3 weeks (RR, 1.7) and at 1 year (RR, 1.3), and showed significantly greater improvement in the physical component summary score on the Short Form 36 at 3 weeks and on the mental component summary score at 1 year, the investigators said (JAMA 2015;313:1915-23).
There were no differences between the active treatment and the placebo groups in measures of below-the-waist pain at either 3 weeks or 1 year, however, and no differences in the proportion of patients achieving 2- to 5-point improvements in pain scores on a 10-point numerical rating scale at either follow-up assessment. Most importantly, there was no significant between-group difference in the likelihood of undergoing spine surgery during the year following treatment; rates of spine surgery were 9.9% among patients who received prednisone and 9.1% among those who received placebo.
Patients who received active treatment were significantly more likely to report adverse effects (49.2%) than those who received placebo (23.9%), but these were minor and transient. No serious treatment-related adverse events occurred.
“Whether the observed improvement in function (without concomitant improvement in pain) merits use of oral steroids for patients with an acute radiculopathy is a difficult decision and, ultimately, becomes a personal one that must be weighed by individual patients and their physicians,” Dr. Goldberg and his associates said.
This study was supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases. Dr. Goldberg reported having no financial disclosures; one of his associates reported ties to the Orthopaedic Research and Education Foundation, AOSpine, Simpirica, and Intrinsic Orthopedics.
A 15-day course of oral steroids modestly improved function but not pain in patients with acute radiculopathy due to a herniated lumbar disk, according to a report published online May 19 in JAMA.
Compared with epidural steroid injections, oral steroids provide similar anti-inflammatory activity but avoid MRI, can be delivered quickly by primary care physicians, avert the risks of spinal injection, and are much cheaper. “Oral steroids are used by many community physicians, have been included in some clinical guidelines, and are noted as a treatment option by some authors,” yet no adequately powered clinical trials of the therapy have been performed until now, said Dr. Harley Goldberg of the department of physical medicine at the Kaiser Permanente Northern California Spine Care Program, San Jose, and his associates.
Their study involved 269 adults at three primary care practices who had leg pain extending below the knee in a nerve root distribution, confirmation of a herniated disk on MRI, and scores of 30 or higher on the 100-point Oswestry Disability Index (ODI). These participants were randomly assigned to receive either daily prednisone capsules (cumulative dose, 600 mg) or matching placebo in addition to usual care and were followed for 1 year.
The primary outcome measure was self-reported score on the ODI at 3 weeks. After adjustment for potential confounders, the mean score was 6.4 points higher with prednisone than with placebo, a significant but modest difference. This benefit persisted at 1-year follow-up, with a mean difference of 7.4 points between the two study groups. In addition, the active-treatment group was significantly more likely to achieve at least a 30-point or 50% improvement in ODI score at 3 weeks (RR, 1.7) and at 1 year (RR, 1.3), and showed significantly greater improvement in the physical component summary score on the Short Form 36 at 3 weeks and on the mental component summary score at 1 year, the investigators said (JAMA 2015;313:1915-23).
There were no differences between the active treatment and the placebo groups in measures of below-the-waist pain at either 3 weeks or 1 year, however, and no differences in the proportion of patients achieving 2- to 5-point improvements in pain scores on a 10-point numerical rating scale at either follow-up assessment. Most importantly, there was no significant between-group difference in the likelihood of undergoing spine surgery during the year following treatment; rates of spine surgery were 9.9% among patients who received prednisone and 9.1% among those who received placebo.
Patients who received active treatment were significantly more likely to report adverse effects (49.2%) than those who received placebo (23.9%), but these were minor and transient. No serious treatment-related adverse events occurred.
“Whether the observed improvement in function (without concomitant improvement in pain) merits use of oral steroids for patients with an acute radiculopathy is a difficult decision and, ultimately, becomes a personal one that must be weighed by individual patients and their physicians,” Dr. Goldberg and his associates said.
This study was supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases. Dr. Goldberg reported having no financial disclosures; one of his associates reported ties to the Orthopaedic Research and Education Foundation, AOSpine, Simpirica, and Intrinsic Orthopedics.
A 15-day course of oral steroids modestly improved function but not pain in patients with acute radiculopathy due to a herniated lumbar disk, according to a report published online May 19 in JAMA.
Compared with epidural steroid injections, oral steroids provide similar anti-inflammatory activity but avoid MRI, can be delivered quickly by primary care physicians, avert the risks of spinal injection, and are much cheaper. “Oral steroids are used by many community physicians, have been included in some clinical guidelines, and are noted as a treatment option by some authors,” yet no adequately powered clinical trials of the therapy have been performed until now, said Dr. Harley Goldberg of the department of physical medicine at the Kaiser Permanente Northern California Spine Care Program, San Jose, and his associates.
Their study involved 269 adults at three primary care practices who had leg pain extending below the knee in a nerve root distribution, confirmation of a herniated disk on MRI, and scores of 30 or higher on the 100-point Oswestry Disability Index (ODI). These participants were randomly assigned to receive either daily prednisone capsules (cumulative dose, 600 mg) or matching placebo in addition to usual care and were followed for 1 year.
The primary outcome measure was self-reported score on the ODI at 3 weeks. After adjustment for potential confounders, the mean score was 6.4 points higher with prednisone than with placebo, a significant but modest difference. This benefit persisted at 1-year follow-up, with a mean difference of 7.4 points between the two study groups. In addition, the active-treatment group was significantly more likely to achieve at least a 30-point or 50% improvement in ODI score at 3 weeks (RR, 1.7) and at 1 year (RR, 1.3), and showed significantly greater improvement in the physical component summary score on the Short Form 36 at 3 weeks and on the mental component summary score at 1 year, the investigators said (JAMA 2015;313:1915-23).
There were no differences between the active treatment and the placebo groups in measures of below-the-waist pain at either 3 weeks or 1 year, however, and no differences in the proportion of patients achieving 2- to 5-point improvements in pain scores on a 10-point numerical rating scale at either follow-up assessment. Most importantly, there was no significant between-group difference in the likelihood of undergoing spine surgery during the year following treatment; rates of spine surgery were 9.9% among patients who received prednisone and 9.1% among those who received placebo.
Patients who received active treatment were significantly more likely to report adverse effects (49.2%) than those who received placebo (23.9%), but these were minor and transient. No serious treatment-related adverse events occurred.
“Whether the observed improvement in function (without concomitant improvement in pain) merits use of oral steroids for patients with an acute radiculopathy is a difficult decision and, ultimately, becomes a personal one that must be weighed by individual patients and their physicians,” Dr. Goldberg and his associates said.
This study was supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases. Dr. Goldberg reported having no financial disclosures; one of his associates reported ties to the Orthopaedic Research and Education Foundation, AOSpine, Simpirica, and Intrinsic Orthopedics.
FROM JAMA
Key clinical point: A 15-day course of oral steroids modestly improved function but not pain in acute radiculopathy due to a herniated lumbar disk.
Major finding: The primary outcome measure, mean self-reported score on the ODI at 3 weeks, was 6.4 points higher with prednisone than with placebo, a significant but modest difference.
Data source: A randomized, double-blind, placebo-controlled trial involving 269 adults followed for 1 year.
Disclosures: This study was supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases. Dr. Goldberg reported having no financial disclosures; one of his associates reported ties to the Orthopaedic Research and Education Foundation, AOSpine, Simpirica, and Intrinsic Orthopedics.
Orthopedic Residents: What Are We Worth?
The modern age of health care has driven a national interest in quality, health care economics, and proving value. A commonly used definition for quality is value/cost. Defining the value of orthopedic residents is difficult. With changes in the delivery of health care, the implementation of the Affordable Care Act, and an increasing federal deficit, defining the value of orthopedic residents has never been more important.1
Funding for graduate medical education (GME) has been a source of recent intense debate.2-8 From the inception of Medicare and Medicaid services, the value of residents has been recognized, and funding has been provided for resident and fellowship education. In 2012, public tax dollars provided more than $15 billion towards GME, with more than 90% coming from the Center of Medicare and Medicaid Services (CMS).4 This funding was initially established to:
- support the education of physicians
- provide well-trained physicians for future generations
- account for a disproportion of care provided to underfunded patients at teaching hospitals
- account for specialty services (eg, burn centers, trauma centers, emergency psychiatric services) that can be net revenue negative.
The significant cost of these programs, which are almost exclusively government-funded, has been the subject of cost-cutting discussions in Congress since the Balanced Budget Act of 1997 that froze GME funding.9 More recently, the National Commission on Fiscal Responsibility and Reform report authored by the Bowles-Simpson Commission proposed decreases in both direct medical education (DME) and indirect medical education (IME) payments that could total $6 billion by 2015 and $60 billion by 2020.4,7,8 The proposed cuts come on the heels of the Affordable Care Act and the projected significant increase in health care demand.1 It is important to note that private payers do not support GME despite receiving health care provided by residents and fellows.
Despite a track record of producing well-trained and skilled physicians at the end of GME training, several reports from both the public and private sectors have identified weaknesses in the GME system. These include a mismatch between the specialty composition of physician trainees and the population needs, geographic maldistribution of the physician workforce, and a lack of fiscal transparency of GME fund use by hospitals.2 A recent comprehensive report from the Institute of Medicine (IOM) entitled Graduate Medical Education that Meets the Nation’s Health Needs highlights the current issues surrounding GME funding.2 The report made note of several important problems with the current GME system, including:
- The revenue impact and cost savings associated with sponsoring residents are neither tracked nor reported, and they are rarely acknowledged in analyses of GME costs.
- In 1997, Congress capped the number of Medicare-supported physician training slots. Hospitals may add residents beyond the cap but cannot receive additional Medicare payments for those trainees. The cap is equal to each hospital’s number of residents in 1996—essentially freezing the geographic distribution of Medicare-supported residencies without regard for future changes in local or regional health workforce priorities or the geography and demography of the US population.9
- By distributing funds directly to teaching hospitals, the Medicare payment system discourages physician training outside the hospital, in clinical settings where most health care is delivered.
- Because Medicare GME funding is formula-driven, the payments are essentially guaranteed regardless of whether the funded trainees reflect local, national, or regional health needs.
- The system’s only mechanism for ensuring accountability is the requirement that residency programs be accredited. The system does not yield useful data on program outcomes and performance.
- Despite receiving government support for their residency or fellowship training, the graduate physician has no obligation to return this government investment through service.
Some of the IOM’s proposed changes to the system include:
- Updating the GME funding to account for inflation and make GME payments based on accountable performance.
- Phasing out the current GME payment system.
- Specifying funds for “transformational” programs that promote innovation and planning for the future.
- Analyzing and redistributing GME funds based on current population needs and performance metrics.
- Increasing fiscal transparency of the CMS payments and their use by hospitals.
- Establishing a GME center within CMS for ongoing oversight.
The American Orthopaedic Association recently held a forum on GME funding for resident education.10 At that forum, departmental leaders noted the difficulty in securing additional funded resident spots from hospitals and the difficulty in proving residents’ value to the hospital administration. Some in the forum suggested that, in the future, residents may need to pay for their residency like they pay for medical school.
There is very limited published data on the financial value added by orthopedic residents. A recent study examined the service provided by a single junior resident during 2 years of primary orthopedic call.11 They found that the potentially billable services provided by the resident totaled more than $79,000 per year. This only accounted for services delivered while on call every sixth night. This did not account for any surgical assisting or outpatient clinic support.11 This amount is nearly twice the amount provided in DME funds to the hospital for resident support.
Although the care and service that residents deliver is obvious to orthopedic attending physicians, we must “prove” our value through continued research and reporting of services provided by residents. If we do not demonstrate our value to the funders of GME, the government, and Congress, I worry that residents who follow behind us may have to fund their own training. An additional concern is that the current shortage of orthopedic surgeons may be worsened if GME funding is decreased.12 This shortage will be exacerbated by the aging population’s increased need for orthopedic care.13
As health care goes through dramatic changes, orthopedic residents and attending surgeons need to be engaged in the discussion so that we can help shape our future in a way that meets the needs of our patients and continues to allow orthopedic care to be delivered at a high level nationally.
1. Sommers BD, Swartz K, Epstein A. Policy makers should prepare for major uncertainties in Medicaid enrollment, costs, and needs for physicians under health reform. Health Aff (Millwood). 2011;30(11):2186-2193.
2. Eden J, Berwick D, Wilensky G, eds. Graduate Medical Education That Meets the Nation’s Health Needs. Washington, DC: National Academies Press; 2014.
3. Chandra A, Khullar D, Wilensky GR. The economics of graduate medical education. N Engl J Med. 2014;370(25):2357-2360.
4. Iglehart JK. The uncertain future of Medicare and graduate medical education. N Engl J Med. 2011;365(14):1340-1345.
5. Nuckols TK, Bhattacharya J, Wolman DM, Ulmer C, Escarce JJ. Cost implications of reduced work hours and workloads for resident physicians. N Engl J Med. 2009;360(21):2202-2215.
6. Robbins RA, Singarajah CU. IOM releases report on graduate medical education. Southwest J Pulm Crit Care. 2014;9(2):123-125. http://www.swjpcc.com/editorial/2014/8/12/iom-releases-report-on-graduate-medical-education.html. Published August 12, 2014. Accessed March 5, 2015.
7. Iglehart JK. Financing graduate medical education—mounting pressure for reform. N Engl J Med. 2012;366(17):1562-1563.
8. The National Commission on Fiscal Responsibility and Reform. The Moment of Truth: Report of the National Commission on Fiscal Responsibility and Reform. The National Commission on Fiscal Responsibility and Reform website. http://www.fiscalcommission.gov/sites/fiscalcommission.gov/files/documents/TheMomentofTruth12_1_2010.pdf. Published December 2010. Accessed March 5, 2015.
9. Balanced Budget Act of 1997, Pub L No. 105-33, 111 Stat 251.
10. Weinstein S. Departmental Leadship Forum III—Orthopaedic workforce needs: challenges in a changing enviroment. Final Program: 2014 Combined Meeting of The American Orthopaedic Association and the Canadian Orthopaedic Association. American Orthopaedic Association website. https://www.aoassn.org/media/410447/final-program-with-covers.pdf. Published June 2014. Accessed March 5, 2015.
11. Jackson JB, Huntington WP, Frick SL. Assessing the value of work done by an orthopedic resident during call. J Grad Med Educ. 2014;6(3):567-570.
12. Frick SL, Richards BS, Weinstein SL, Beaty JH, Wattenbarger JM. Workforce, work, and advocacy issues in pediatric orthopaedics. J Bone Joint Surg Am. 2010;92(17):e31.
13. Physician supply and demand through 2025: key findings. Association of American Medical Colleges website. https://www.aamc.org/download/153160/data/physician_shortages_to_worsen_without_increases_in_residency_tr.pdf. Published March 3, 2015. Accessed March 5, 2015.
The modern age of health care has driven a national interest in quality, health care economics, and proving value. A commonly used definition for quality is value/cost. Defining the value of orthopedic residents is difficult. With changes in the delivery of health care, the implementation of the Affordable Care Act, and an increasing federal deficit, defining the value of orthopedic residents has never been more important.1
Funding for graduate medical education (GME) has been a source of recent intense debate.2-8 From the inception of Medicare and Medicaid services, the value of residents has been recognized, and funding has been provided for resident and fellowship education. In 2012, public tax dollars provided more than $15 billion towards GME, with more than 90% coming from the Center of Medicare and Medicaid Services (CMS).4 This funding was initially established to:
- support the education of physicians
- provide well-trained physicians for future generations
- account for a disproportion of care provided to underfunded patients at teaching hospitals
- account for specialty services (eg, burn centers, trauma centers, emergency psychiatric services) that can be net revenue negative.
The significant cost of these programs, which are almost exclusively government-funded, has been the subject of cost-cutting discussions in Congress since the Balanced Budget Act of 1997 that froze GME funding.9 More recently, the National Commission on Fiscal Responsibility and Reform report authored by the Bowles-Simpson Commission proposed decreases in both direct medical education (DME) and indirect medical education (IME) payments that could total $6 billion by 2015 and $60 billion by 2020.4,7,8 The proposed cuts come on the heels of the Affordable Care Act and the projected significant increase in health care demand.1 It is important to note that private payers do not support GME despite receiving health care provided by residents and fellows.
Despite a track record of producing well-trained and skilled physicians at the end of GME training, several reports from both the public and private sectors have identified weaknesses in the GME system. These include a mismatch between the specialty composition of physician trainees and the population needs, geographic maldistribution of the physician workforce, and a lack of fiscal transparency of GME fund use by hospitals.2 A recent comprehensive report from the Institute of Medicine (IOM) entitled Graduate Medical Education that Meets the Nation’s Health Needs highlights the current issues surrounding GME funding.2 The report made note of several important problems with the current GME system, including:
- The revenue impact and cost savings associated with sponsoring residents are neither tracked nor reported, and they are rarely acknowledged in analyses of GME costs.
- In 1997, Congress capped the number of Medicare-supported physician training slots. Hospitals may add residents beyond the cap but cannot receive additional Medicare payments for those trainees. The cap is equal to each hospital’s number of residents in 1996—essentially freezing the geographic distribution of Medicare-supported residencies without regard for future changes in local or regional health workforce priorities or the geography and demography of the US population.9
- By distributing funds directly to teaching hospitals, the Medicare payment system discourages physician training outside the hospital, in clinical settings where most health care is delivered.
- Because Medicare GME funding is formula-driven, the payments are essentially guaranteed regardless of whether the funded trainees reflect local, national, or regional health needs.
- The system’s only mechanism for ensuring accountability is the requirement that residency programs be accredited. The system does not yield useful data on program outcomes and performance.
- Despite receiving government support for their residency or fellowship training, the graduate physician has no obligation to return this government investment through service.
Some of the IOM’s proposed changes to the system include:
- Updating the GME funding to account for inflation and make GME payments based on accountable performance.
- Phasing out the current GME payment system.
- Specifying funds for “transformational” programs that promote innovation and planning for the future.
- Analyzing and redistributing GME funds based on current population needs and performance metrics.
- Increasing fiscal transparency of the CMS payments and their use by hospitals.
- Establishing a GME center within CMS for ongoing oversight.
The American Orthopaedic Association recently held a forum on GME funding for resident education.10 At that forum, departmental leaders noted the difficulty in securing additional funded resident spots from hospitals and the difficulty in proving residents’ value to the hospital administration. Some in the forum suggested that, in the future, residents may need to pay for their residency like they pay for medical school.
There is very limited published data on the financial value added by orthopedic residents. A recent study examined the service provided by a single junior resident during 2 years of primary orthopedic call.11 They found that the potentially billable services provided by the resident totaled more than $79,000 per year. This only accounted for services delivered while on call every sixth night. This did not account for any surgical assisting or outpatient clinic support.11 This amount is nearly twice the amount provided in DME funds to the hospital for resident support.
Although the care and service that residents deliver is obvious to orthopedic attending physicians, we must “prove” our value through continued research and reporting of services provided by residents. If we do not demonstrate our value to the funders of GME, the government, and Congress, I worry that residents who follow behind us may have to fund their own training. An additional concern is that the current shortage of orthopedic surgeons may be worsened if GME funding is decreased.12 This shortage will be exacerbated by the aging population’s increased need for orthopedic care.13
As health care goes through dramatic changes, orthopedic residents and attending surgeons need to be engaged in the discussion so that we can help shape our future in a way that meets the needs of our patients and continues to allow orthopedic care to be delivered at a high level nationally.
The modern age of health care has driven a national interest in quality, health care economics, and proving value. A commonly used definition for quality is value/cost. Defining the value of orthopedic residents is difficult. With changes in the delivery of health care, the implementation of the Affordable Care Act, and an increasing federal deficit, defining the value of orthopedic residents has never been more important.1
Funding for graduate medical education (GME) has been a source of recent intense debate.2-8 From the inception of Medicare and Medicaid services, the value of residents has been recognized, and funding has been provided for resident and fellowship education. In 2012, public tax dollars provided more than $15 billion towards GME, with more than 90% coming from the Center of Medicare and Medicaid Services (CMS).4 This funding was initially established to:
- support the education of physicians
- provide well-trained physicians for future generations
- account for a disproportion of care provided to underfunded patients at teaching hospitals
- account for specialty services (eg, burn centers, trauma centers, emergency psychiatric services) that can be net revenue negative.
The significant cost of these programs, which are almost exclusively government-funded, has been the subject of cost-cutting discussions in Congress since the Balanced Budget Act of 1997 that froze GME funding.9 More recently, the National Commission on Fiscal Responsibility and Reform report authored by the Bowles-Simpson Commission proposed decreases in both direct medical education (DME) and indirect medical education (IME) payments that could total $6 billion by 2015 and $60 billion by 2020.4,7,8 The proposed cuts come on the heels of the Affordable Care Act and the projected significant increase in health care demand.1 It is important to note that private payers do not support GME despite receiving health care provided by residents and fellows.
Despite a track record of producing well-trained and skilled physicians at the end of GME training, several reports from both the public and private sectors have identified weaknesses in the GME system. These include a mismatch between the specialty composition of physician trainees and the population needs, geographic maldistribution of the physician workforce, and a lack of fiscal transparency of GME fund use by hospitals.2 A recent comprehensive report from the Institute of Medicine (IOM) entitled Graduate Medical Education that Meets the Nation’s Health Needs highlights the current issues surrounding GME funding.2 The report made note of several important problems with the current GME system, including:
- The revenue impact and cost savings associated with sponsoring residents are neither tracked nor reported, and they are rarely acknowledged in analyses of GME costs.
- In 1997, Congress capped the number of Medicare-supported physician training slots. Hospitals may add residents beyond the cap but cannot receive additional Medicare payments for those trainees. The cap is equal to each hospital’s number of residents in 1996—essentially freezing the geographic distribution of Medicare-supported residencies without regard for future changes in local or regional health workforce priorities or the geography and demography of the US population.9
- By distributing funds directly to teaching hospitals, the Medicare payment system discourages physician training outside the hospital, in clinical settings where most health care is delivered.
- Because Medicare GME funding is formula-driven, the payments are essentially guaranteed regardless of whether the funded trainees reflect local, national, or regional health needs.
- The system’s only mechanism for ensuring accountability is the requirement that residency programs be accredited. The system does not yield useful data on program outcomes and performance.
- Despite receiving government support for their residency or fellowship training, the graduate physician has no obligation to return this government investment through service.
Some of the IOM’s proposed changes to the system include:
- Updating the GME funding to account for inflation and make GME payments based on accountable performance.
- Phasing out the current GME payment system.
- Specifying funds for “transformational” programs that promote innovation and planning for the future.
- Analyzing and redistributing GME funds based on current population needs and performance metrics.
- Increasing fiscal transparency of the CMS payments and their use by hospitals.
- Establishing a GME center within CMS for ongoing oversight.
The American Orthopaedic Association recently held a forum on GME funding for resident education.10 At that forum, departmental leaders noted the difficulty in securing additional funded resident spots from hospitals and the difficulty in proving residents’ value to the hospital administration. Some in the forum suggested that, in the future, residents may need to pay for their residency like they pay for medical school.
There is very limited published data on the financial value added by orthopedic residents. A recent study examined the service provided by a single junior resident during 2 years of primary orthopedic call.11 They found that the potentially billable services provided by the resident totaled more than $79,000 per year. This only accounted for services delivered while on call every sixth night. This did not account for any surgical assisting or outpatient clinic support.11 This amount is nearly twice the amount provided in DME funds to the hospital for resident support.
Although the care and service that residents deliver is obvious to orthopedic attending physicians, we must “prove” our value through continued research and reporting of services provided by residents. If we do not demonstrate our value to the funders of GME, the government, and Congress, I worry that residents who follow behind us may have to fund their own training. An additional concern is that the current shortage of orthopedic surgeons may be worsened if GME funding is decreased.12 This shortage will be exacerbated by the aging population’s increased need for orthopedic care.13
As health care goes through dramatic changes, orthopedic residents and attending surgeons need to be engaged in the discussion so that we can help shape our future in a way that meets the needs of our patients and continues to allow orthopedic care to be delivered at a high level nationally.
1. Sommers BD, Swartz K, Epstein A. Policy makers should prepare for major uncertainties in Medicaid enrollment, costs, and needs for physicians under health reform. Health Aff (Millwood). 2011;30(11):2186-2193.
2. Eden J, Berwick D, Wilensky G, eds. Graduate Medical Education That Meets the Nation’s Health Needs. Washington, DC: National Academies Press; 2014.
3. Chandra A, Khullar D, Wilensky GR. The economics of graduate medical education. N Engl J Med. 2014;370(25):2357-2360.
4. Iglehart JK. The uncertain future of Medicare and graduate medical education. N Engl J Med. 2011;365(14):1340-1345.
5. Nuckols TK, Bhattacharya J, Wolman DM, Ulmer C, Escarce JJ. Cost implications of reduced work hours and workloads for resident physicians. N Engl J Med. 2009;360(21):2202-2215.
6. Robbins RA, Singarajah CU. IOM releases report on graduate medical education. Southwest J Pulm Crit Care. 2014;9(2):123-125. http://www.swjpcc.com/editorial/2014/8/12/iom-releases-report-on-graduate-medical-education.html. Published August 12, 2014. Accessed March 5, 2015.
7. Iglehart JK. Financing graduate medical education—mounting pressure for reform. N Engl J Med. 2012;366(17):1562-1563.
8. The National Commission on Fiscal Responsibility and Reform. The Moment of Truth: Report of the National Commission on Fiscal Responsibility and Reform. The National Commission on Fiscal Responsibility and Reform website. http://www.fiscalcommission.gov/sites/fiscalcommission.gov/files/documents/TheMomentofTruth12_1_2010.pdf. Published December 2010. Accessed March 5, 2015.
9. Balanced Budget Act of 1997, Pub L No. 105-33, 111 Stat 251.
10. Weinstein S. Departmental Leadship Forum III—Orthopaedic workforce needs: challenges in a changing enviroment. Final Program: 2014 Combined Meeting of The American Orthopaedic Association and the Canadian Orthopaedic Association. American Orthopaedic Association website. https://www.aoassn.org/media/410447/final-program-with-covers.pdf. Published June 2014. Accessed March 5, 2015.
11. Jackson JB, Huntington WP, Frick SL. Assessing the value of work done by an orthopedic resident during call. J Grad Med Educ. 2014;6(3):567-570.
12. Frick SL, Richards BS, Weinstein SL, Beaty JH, Wattenbarger JM. Workforce, work, and advocacy issues in pediatric orthopaedics. J Bone Joint Surg Am. 2010;92(17):e31.
13. Physician supply and demand through 2025: key findings. Association of American Medical Colleges website. https://www.aamc.org/download/153160/data/physician_shortages_to_worsen_without_increases_in_residency_tr.pdf. Published March 3, 2015. Accessed March 5, 2015.
1. Sommers BD, Swartz K, Epstein A. Policy makers should prepare for major uncertainties in Medicaid enrollment, costs, and needs for physicians under health reform. Health Aff (Millwood). 2011;30(11):2186-2193.
2. Eden J, Berwick D, Wilensky G, eds. Graduate Medical Education That Meets the Nation’s Health Needs. Washington, DC: National Academies Press; 2014.
3. Chandra A, Khullar D, Wilensky GR. The economics of graduate medical education. N Engl J Med. 2014;370(25):2357-2360.
4. Iglehart JK. The uncertain future of Medicare and graduate medical education. N Engl J Med. 2011;365(14):1340-1345.
5. Nuckols TK, Bhattacharya J, Wolman DM, Ulmer C, Escarce JJ. Cost implications of reduced work hours and workloads for resident physicians. N Engl J Med. 2009;360(21):2202-2215.
6. Robbins RA, Singarajah CU. IOM releases report on graduate medical education. Southwest J Pulm Crit Care. 2014;9(2):123-125. http://www.swjpcc.com/editorial/2014/8/12/iom-releases-report-on-graduate-medical-education.html. Published August 12, 2014. Accessed March 5, 2015.
7. Iglehart JK. Financing graduate medical education—mounting pressure for reform. N Engl J Med. 2012;366(17):1562-1563.
8. The National Commission on Fiscal Responsibility and Reform. The Moment of Truth: Report of the National Commission on Fiscal Responsibility and Reform. The National Commission on Fiscal Responsibility and Reform website. http://www.fiscalcommission.gov/sites/fiscalcommission.gov/files/documents/TheMomentofTruth12_1_2010.pdf. Published December 2010. Accessed March 5, 2015.
9. Balanced Budget Act of 1997, Pub L No. 105-33, 111 Stat 251.
10. Weinstein S. Departmental Leadship Forum III—Orthopaedic workforce needs: challenges in a changing enviroment. Final Program: 2014 Combined Meeting of The American Orthopaedic Association and the Canadian Orthopaedic Association. American Orthopaedic Association website. https://www.aoassn.org/media/410447/final-program-with-covers.pdf. Published June 2014. Accessed March 5, 2015.
11. Jackson JB, Huntington WP, Frick SL. Assessing the value of work done by an orthopedic resident during call. J Grad Med Educ. 2014;6(3):567-570.
12. Frick SL, Richards BS, Weinstein SL, Beaty JH, Wattenbarger JM. Workforce, work, and advocacy issues in pediatric orthopaedics. J Bone Joint Surg Am. 2010;92(17):e31.
13. Physician supply and demand through 2025: key findings. Association of American Medical Colleges website. https://www.aamc.org/download/153160/data/physician_shortages_to_worsen_without_increases_in_residency_tr.pdf. Published March 3, 2015. Accessed March 5, 2015.
Arthroscopic Treatment of Tibial Spine Malunion With Resorbable Screws
Anterior tibial spine fractures are rare, occurring with an incidence of 3 per 100,000 per year.1,2 Historically, this fracture has occurred more frequently in children,3-5 and was considered a condition of skeletal immaturity and the pediatric equivalent of an anterior cruciate ligament (ACL) rupture.6 However, recent literature indicates that this fracture is more common in the adult population than previously thought.7 The tibial spine is an attachment point for the ACL and an avulsion may produce ACL laxity,8 predisposing to further symptomatic laxity and premature osteoarthritis. Nearly 40% of these fractures are associated with concomitant injuries to surrounding structures.9
Meyers and McKeever10,11 originally classified these fractures into 3 groups on the basis of displacement. Type I fractures present with no significant displacement of the anterior margin, type II involve displacement and are hinged, while type III have complete displacement.10,11 More recently, a type IV fracture has been added, involving comminution of the displaced fragment. Nondisplaced fractures are commonly treated with immobilization in varying degrees of extension; this allows the femoral condyles to compress and to reduce the fracture while arthroscopic or open reduction is the preferred method for displaced fractures of the tibial spine.2,4,8,10
We report the case of an 11-year-old boy with a tibial spine fracture that failed conservative management. He developed a subsequent malunion with impingement anteriorly of the tibial spine on the notch, and residual instability of the ACL. The patient’s parents provided written informed consent for print and electronic publication of this case report.
Case Report
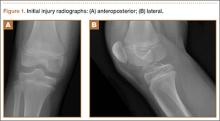
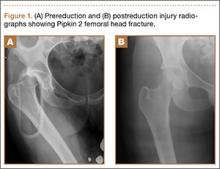
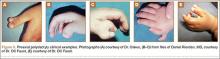
An 11-year-old Caucasian boy was referred to our office for evaluation of right knee injury. He sustained the injury approximately 3 months earlier, and it was determined that he had a tibial spine fracture. Conservative management with immobilization in extension and activity modification was undertaken; however, he was referred for further evaluation because of healing in a malreduced position and residual ACL laxity. Physical examination showed a grade 2A Lachman test (contralateral limb with negative Lachman examination), negative McMurray test, and pain with forced hyperextension; range-of-motion examination showed lack of the terminal 5º of extension. Magnetic resonance and computed tomography imaging from an outside facility showed a skeletally immature individual with a large tibial spine fracture that had healed in a malunited position with the fragment extended on a posterior hinge, creating a large prominence anteriorly (Figures 1A, 1B). Magnetic resonance imaging showed that the ACL fibers were likely to remain intact but would lack appropriate tension secondary to the displacement of the tibial insertion.
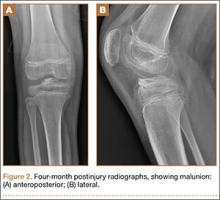
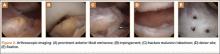
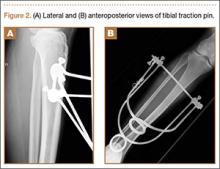
Because of healing in a displaced position, lack of terminal extension, ACL laxity, and subjective complaints of pain, we discussed surgery with the patient and his parents (Figures 2A, 2B). Four months after the initial injury, the patient underwent surgery for a right tibial spine malunion arthroscopic takedown and repair, as well as an intraoperative evaluation of the ACL. Standard arthroscopy was performed, using anterolateral and anteromedial arthroscopic portals, and an accessory medial peripatellar portal. During surgery, a large prominence was noted in the region of the anterior tibial spine (Figure 3A). The ACL fibers maintained a slack position secondary to the elevation of the tibial insertion point, and intraoperative Lachman examination showed anterior translation of the tibia on the femur as the slack was removed from the ACL. During surgery, impingement of the anterior tibial spine along the femoral notch was shown to be significant by taking the knee into near-full extension (Figure 3B). A cam-like effect was noted at the time of impingement with the posterior soft tissues relaxing to accommodate slight further extension.
Based on these findings, we chose to take down the malunited fracture and repair it (Figure 3C). PDS suture (Ethicon, Somerville, New Jersey) was temporarily placed along the intermeniscal ligament and anterior horns of the medial and lateral menisci, using a system of spinal needles to facilitate suture passage. Surgical clamps were hung from the suture to provide traction on the sutures throughout the case, allowing the intermeniscal ligament and menisci to recede anteriorly to improve working space and aid in preventing iatrogenic injury. These sutures were removed at the conclusion of the case. Using a combination of curettes, elevator, and small shaver, we were able to meticulously remove interposed malunited callus to allow for mobilization of the displaced fragment. After removal of the excess bone formation, a typical donor site was created, allowing the displaced spine fragment to be hinged into appropriate alignment (Figure 3D). We were able to maintain a posterior cortical hinge to facilitate this process.
Then, we placed Kirschner wires (K-wires) across the fracture in an antegrade fashion, anterior to the trochlea and notch, using an accessory medial peripatellar starting point percutaneously, under direct visualization to avoid iatrogenic chondral injury. The tibial spine fragment was temporarily maintained in a reduced position with an arthroscopic probe and pinned in place with two 0.062-in K-wires. The fracture was stabilized with 8 resorbable 1.6-mm poly-L-lactic/polyglycolic acid (PLLA/PGA) nails, in varying lengths from 18 mm to 22 mm. Excellent fixation was obtained, and range of motion was tested from 0º to 80º, without movement of the fracture site (Figure 3E). Fluoroscopy with multi-axial views verified adequate fixation and reduction. Further, we examined and noted a taut ACL after fixation. The patient was placed in a long leg cast for 3 weeks at 30º, based upon intraoperative determination of the position of least tension on the fracture fragment.
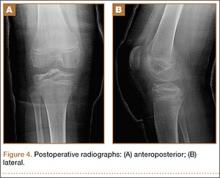
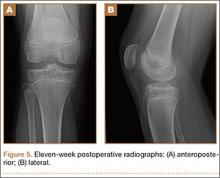
At 3-week follow-up, the patient was progressing well and transitioned from a long leg cast to a hinged knee brace, to allow for early range of motion. Radiographs showed appropriate alignment of the tibial spine fracture with no significant loss of fixation (Figures 4A, 4B). Physical therapy was initiated between 0º and 30º, and flexion was progressively increased over the course of the first 3 weeks. Active and active-assist, closed-chain activities were maintained. Seven weeks postoperatively, the patient displayed continued clinical progression. Radiographs showed interval healing with slight lucency over the anterolateral aspect of the fracture fragment, likely related to the early resorptive process of healing. Physical examination showed movement between 0º and 120º, stable Lachman test, and stable anterior drawer. Crutches were discontinued and hinged knee brace was converted to an ACL brace. By the 11th week, motion had increased to 140º, and radiographs continued to show acceptable alignment and healing (Figures 5A, 5B). The patient was released to return to play as tolerated; however, an ACL brace was recommended during his initial return to provide additional support.
Discussion
In this report, we present an approach for arthroscopic reduction of a malunited tibial spine fracture using resorbable PLLA/PGA nails. The number of polyglycolic nails employed is individualized per case, dependent on the surface area and the quality of the bone within the fractured fragment. Preoperative templating allows for measurements from the fractured fragment to the level of the proximal tibial physis. Based on these measurements, nails are chosen to maximize fixation length and avoid the physis. Despite studies that have examined the effect of transphyseal K-wire pinning or drilling on subsequent growth, there is no consensus about optimal technique. Experiments in animal models indicate that drill injuries destroying less than 8% to 9% of the physis do not impact total bone growth.12,13 Further, temporary crossing of the physeal plate for internal fixation of dislocated joint injuries has not been shown to result in bone bridging or growth disturbance.14,15
Each nail is 1.6 mm in diameter, leaving a small footprint. The nails are used judiciously to provide effective stabilization of the fragment and to maintain a cost-conscious approach. An accessory superomedial peripatellar portal allows an appropriate angle for nail placement. This portal allows access to all regions of the fractured fragment, while an anteromedial and anterolateral portal are used as working and camera portals, respectively. Nails are placed to provide an axis perpendicular to the fracture line to allow appropriate compression. By virtue of the shape of the typical fragment in a tibial spine fracture, the nails vary in insertion angle.
The occurrence of anterior tibial spine fractures is rare, and while several techniques have been described to repair this fracture, there remains a great deal of uncertainty regarding the best course of treatment. A review of the literature finds arthroscopic and open approaches, as well as techniques employing K-wire fixation, metal screw fixation, staple fixation, absorbable fixation, and fixation with sutures passed through the tibial tunnel.16-18
Avulsion fractures of the tibial eminence were treated with open fixation until McLennan8 first reported the benefits of reduction with an arthroscope. Open reduction and internal fixation provide the benefit of direct visualization,9 while arthroscopic reduction offers decreased morbidity and an accelerated recovery of knee functions,8 despite the fact that a higher rate of range-of-motion deficits were seen in patients treated arthroscopically.19 We feel that with proper early rehabilitation to achieve range of motion, the risk of this can be minimal.
Various arthroscopic approaches that improve the accuracy of the reduction and decrease surgical invasiveness have been described. Suture and screw fixation are among the most common methods, and both have resulted in positive outcomes.20-24 Suture fixation of the tibial eminence is technically demanding but offers secure fixation without the need for follow-up hardware removal. Screw fixation results in secure fixation; however, numerous hardware-related issues may necessitate removal. Furthermore, in skeletally immature patients, screw fixation may disturb the growth plate if it crosses an open physis.9
Hunter and Willis25 retrospectively reviewed patients with tibial eminence fractures treated with either screw or suture fixation and found a 44% reoperation rate in the screw-fixation group. Removal was often recommended as a result of hardware-related issues. There was a 13% reoperation rate in the suture-fixation group, which resulted largely from stiffness.25 In a recent review, Gans and colleagues19 reviewed 6 publications comparing screw and suture fixation of tibial eminence fractures and found 82.4% of screw patients had laxity on both the anterior drawer and Lachman tests, compared with 18.8% in the suture-fixation group. This study also noted a slightly higher rate of arthrofibrosis in patients treated with suture fixation.19 Biomechanical studies indicate that suture fixation imparts greater strength under cyclic-loading conditions;26 however, there does not appear to be a difference in ultimate force required for fixation failure.27
Ultimately, both suture and screw fixation result in secure methods of fixation; however, there are often greater issues with screw fixation because of the persistent hardware. Metal has been the most popular method for fracture fixation, and while biodegradable materials have been alluring, adverse tissue reactions have slowed implementation. However, these implants have become increasingly sophisticated, thereby reducing disadvantages.28 Previous biodegradable devices were often composed of a single polymer, and many caused adverse reactions by degrading too quickly or provided no real advantages because they degraded too slowly.29 As the number of polymers approved for internal use and surgical applications continues to rise, so too will the benefits of employing this technology. Furthermore, by including multiple polymers in these implants, one is better able to control the degradation rate, limiting the tissue response.
In this study, we employed PLLA/PGA nails. Studies of PGA implants indicate this molecule degrades at a fast rate resulting in adverse tissue reactions. Adverse reactions in studies of PLLA implants are less frequent because of their slower rate of degradation.29,30 Combining these monomers results in appropriate strength and a controlled degradation rate, reducing the likelihood of adverse reactions. Furthermore, numerous studies have reported that inflammatory responses in children are rare and mild in nature.31,32 Absorbable implants have displayed efficacy in numerous orthopedic settings33-36 and are beneficial in procedures that are not suitable for repeated surgeries, such as reconstruction of the ACL.37 There is some concern about the use of absorbable implants in synovial joints. Polyglycolic acid use in synovial joints may cause foreign-body reactions and may increase the risk of intra-articular dissemination of polymeric debris;38 however, use of a multipolymer construct decreases the likelihood of this occurrence.
Polyglycolic nails confer the advantage over nonresorbable screw fixation because further procedure for hardware removal is not required. Although suture fixation has proved to be beneficial over nonresorbable screw fixation, implantation of resorbable nails appears to have several advantages. In Dr. Estes’ experience, placement of resorbable screws through an accessory superomedial portal is far less technically demanding than placement of suture through the fracture fragment. Further, as sutures are passed from the extra-articular to the intra-articular region of the joint, capsular layers of the knee may inadvertently be bound up in the fixation, predisposing to arthrofibrosis.
At the same time, biodegradable devices are often more costly than alternative forms of treatment; however, a true cost-to-benefit analysis requires consideration of other factors. One of the benefits of biodegradable hardware is that there is no need for follow-up hardware removal. Reports have indicated that up to 91% of patients thought that hardware removal was the most negative aspect of metal implants.39 It is estimated that if the removal rate for metallic implants is higher than 19% to 54%, resorbable implants would be more cost-effective.40 The cost of sutures and screws is variable, however; they are invariably less expensive than biodegradable nails. A study of fracture patients determined that biodegradable implants were cheaper on average after considering the cost of implant removal.40 Ultimately, the hardware choice depends on numerous factors, including surgeon’s discretion; however, biodegradable hardware should not be discounted for financial reasons because the difference in cost is likely negligible.
Conclusion
The approach described in this report offers efficient and secure fixation with resorbable hardware without a reduction in range of motion. Resorbable implants may prove beneficial in the treatment of tibial eminence fractures by offering robust fixation without the concerns associated with permanent hardware.
1. Hargrove R, Parsons S, Payne R. Anterior tibial spine fracture – an easy fracture to miss. Accid Emerg Nurs. 2004;12(3):173-175.
2. Aderinto J, Walmsley P, Keating JF. Fractures of the tibial spine: epidemiology and outcome. Knee. 2008;15(3):164-167.
3. Driessen MJ, Winkelman PA. Fractures of the intercondylar eminence of the tibia in childhood. Neth J Surg. 1984;36(3):69-72.
4. Zaricznyj B. Avulsion fracture of the tibial eminence: treatment by open reduction and pinning. J Bone Joint Surg Am. 1977;59(8):1111-1114.
5. Molander ML, Wallin G, Wikstad I. Fracture of the intercondylar eminence of the tibia: a review of 35 patients. J Bone Joint Surg Br. 1981;63(1):89-91.
6. Kieser DC, Gwynne-Jones D, Dreyer S. Displaced tibial intercondylar eminence fractures. J Orthop Surg. 2011;19(3):292-296.
7. Ishibashi Y, Tsuda E, Sasaki T, Toh S. Magnetic resonance imaging AIDS in detecting concomitant injuries in patients with tibial spine fractures. Clin Orthop. 2005;(434):207-212.
8. McLennan JG. The role of arthroscopic surgery in the treatment of fractures of the intercondylar eminence of the tibia. J Bone Joint Surg Br. 1982;64(4):477-480.
9. Lafrance RM, Giordano B, Goldblatt J, Voloshin I, Maloney M. Pediatric tibial eminence fractures: evaluation and management. J Am Acad Orthop Surg. 2010;18(7):395-405.
10. Meyers MH, McKeever FM. Fracture of the intercondylar eminence of the tibia. J Bone Joint Surg Am. 1959;41(2):209-220.
11. Meyers MH, McKeever FM. Fracture of the intercondylar eminence of the tibia. J Bone Joint Surg Am. 1970;52(8):1677-1684.
12. Garcés GL, Mugica-Garay I, López-González Coviella N, Guerado E. Growth-plate modifications after drilling. J Pediatr Orthop. 1994;14(2):225-228.
13. Janarv PM, Wikström B, Hirsch G. The influence of transphyseal drilling and tendon grafting on bone growth: an experimental study in the rabbit. J Pediatr Orthop. 1998;18(2):149-154.
14. Boelitz R, Dallek M, Meenen NM, Jungbluth KH. Reaction of the epiphyseal groove to groove-crossing bore-wire osteosynthesis. Results of a histomorphologic small animal study. Unfallchirurgie. 1994;20(3):131-137.
15. Yung PS, Lam CY, Ng BK, Lam TP, Cheng JC. Percutaneous transphyseal intramedullary Kirschner wire pinning: a safe and effective procedure for treatment of displaced diaphyseal forearm fracture in children. J Pediatr Orthop. 2004;24(1):7-12.
16. Bong MR, Romero A, Kubiak E, et al. Suture versus screw fixation of displaced tibial eminence fractures: a biomechanical comparison. Arthroscopy. 2005;21(10):1172-1176.
17. Vega JR, Irribarra LA, Baar AK, Iñiguez M, Salgado M, Gana N. Arthroscopic fixation of displaced tibial eminence fractures: a new growth plate-sparing method. Arthroscopy. 2008;24(11):1239-1243.
18. Shepley RW. Arthroscopic treatment of type III tibial spine fractures using absorbable fixation. Orthopedics. 2004;27(7):767-769.
19. Gans I, Baldwin KD, Ganley TJ. Treatment and management outcomes of tibial eminence fractures in pediatric patients: a systematic review. Am J Sports Med. 2013;42(7):1743-1750.
20. Delcogliano A, Chiossi S, Caporaso A, Menghi A, Rinonapoli G. Tibial intercondylar eminence fractures in adults: arthroscopic treatment. Knee Surg Sports Traumatol Arthrosc. 2003;11(4):255-259.
21. Mulhall KJ, Dowdall J, Grannell M, McCabe JP. Tibial spine fractures: an analysis of outcome in surgically treated type III injuries. Injury. 1999;30(4):289-292.
22. Geissler WB, Matthews DE. Arthroscopic suture fixation of displaced tibial eminence fractures. Orthopedics. 1993;16(3):331-333.
23. Mah JY, Otsuka NY, McLean J. An arthroscopic technique for the reduction and fixation of tibial-eminence fractures. J Pediatr Orthop. 1996;16(1):119-121.
24. Reynders P, Reynders K, Broos P. Pediatric and adolescent tibial eminence fractures: arthroscopic cannulated screw fixation. J Trauma. 2002;53(1):49-54.
25. Hunter RE, Willis JA. Arthroscopic fixation of avulsion fractures of the tibial eminence: technique and outcome. Arthroscopy. 2004;20(2):113-121.
26. Eggers AK, Becker C, Weimann A, et al. Biomechanical evaluation of different fixation methods for tibial eminence fractures. Am J Sports Med. 2007;35(3):404-410.
27. Mahar AT, Duncan D, Oka R, Lowry A, Gillingham B, Chambers H. Biomechanical comparison of four different fixation techniques for pediatric tibial eminence avulsion fractures. J Pediatr Orthop. 2008;28(2):159-162.
28. Toro C, Robiony M, Zerman N, Politi M. Resorbable plates in maxillary fixation. A 5-year experience. Minerva Stomatol. 2005;54(4):199-206.
29. Andriano KP, Pohjonen T, Törmälä P. Processing and characterization of absorbable polylactide polymers for use in surgical implants. J Appl Biomater.1994;5(2):133-140.
30. Böstman O, Pihlajamäki H. Clinical biocompatibility of biodegradable orthopaedic implants for internal fixation: a review. Biomaterials. 2000;21(24):2615-2621.
31. Rokkanen PU, Böstman O, Hirvensalo E, et al. Bioabsorbable fixation in orthopaedic surgery and traumatology. Biomaterials. 2000;21(24):2607-2613.
32. Athanasiou KA, Niederauer GG, Agrawal CM. Sterilization, toxicity, biocompatibility and clinical applications of polylactic acid/polyglycolic acid copolymers. Biomaterials. 1996;17(2):93-102.
33. Li ZH, Yu AX, Guo XP, Qi BW, Zhou M, Wang WY. Absorbable implants versus metal implants for the treatment of ankle fractures: A meta-analysis. Exp Ther Med. 2013;5(5):1531-1537.
34. Singh G, Mohammad S, Chak RK, Lepcha N, Singh N, Malkunje LR. Bio-resorbable plates as effective implant in paediatric mandibular fracture. J Maxillofac Oral Surg. 2012;11(4):400-406.
35. Sakamoto Y, Shimizu Y, Nagasao T, Kishi K. Combined use of resorbable poly-L-lactic acid-polyglycolic acid implant and bone cement for treating large orbital floor fractures. J Plast Reconstr Aesthet Surg. 2014;67(3):e88-e90.
36. Benz G, Kallieris D, Seeböck T, McIntosh A, Daum R. Bioresorbable pins and screws in paediatric traumatology. Eur J Pediatr Surg. 1994;4(2):103-107.
37. Gaweda K, Walawski J, Weglowski R, Krzyzanowski W. Comparison of bioabsorbable interference screws and posts for distal fixation in anterior cruciate ligament reconstruction. Int Orthop. 2009;33(1):123-127.
38. Böstman OM. Osteoarthritis of the ankle after foreign-body reaction to absorbable pins and screws: a three- to nine-year follow-up study. J Bone Joint Surg Br. 1998;80(2):333-338.
39. Mittal R, Morley J, Dinopoulos H, Drakoulakis EG, Vermani E, Giannoudis PV. Use of bio-resorbable implants for stabilisation of distal radius fractures: the United Kingdom patients’ perspective. Injury. 2005;36(2):333-338.
40. Böstman OM. Metallic or absorbable fracture fixation devices. A cost minimization analysis. Clin Orthop. 1996;(329):233-239.
Anterior tibial spine fractures are rare, occurring with an incidence of 3 per 100,000 per year.1,2 Historically, this fracture has occurred more frequently in children,3-5 and was considered a condition of skeletal immaturity and the pediatric equivalent of an anterior cruciate ligament (ACL) rupture.6 However, recent literature indicates that this fracture is more common in the adult population than previously thought.7 The tibial spine is an attachment point for the ACL and an avulsion may produce ACL laxity,8 predisposing to further symptomatic laxity and premature osteoarthritis. Nearly 40% of these fractures are associated with concomitant injuries to surrounding structures.9
Meyers and McKeever10,11 originally classified these fractures into 3 groups on the basis of displacement. Type I fractures present with no significant displacement of the anterior margin, type II involve displacement and are hinged, while type III have complete displacement.10,11 More recently, a type IV fracture has been added, involving comminution of the displaced fragment. Nondisplaced fractures are commonly treated with immobilization in varying degrees of extension; this allows the femoral condyles to compress and to reduce the fracture while arthroscopic or open reduction is the preferred method for displaced fractures of the tibial spine.2,4,8,10
We report the case of an 11-year-old boy with a tibial spine fracture that failed conservative management. He developed a subsequent malunion with impingement anteriorly of the tibial spine on the notch, and residual instability of the ACL. The patient’s parents provided written informed consent for print and electronic publication of this case report.
Case Report
An 11-year-old Caucasian boy was referred to our office for evaluation of right knee injury. He sustained the injury approximately 3 months earlier, and it was determined that he had a tibial spine fracture. Conservative management with immobilization in extension and activity modification was undertaken; however, he was referred for further evaluation because of healing in a malreduced position and residual ACL laxity. Physical examination showed a grade 2A Lachman test (contralateral limb with negative Lachman examination), negative McMurray test, and pain with forced hyperextension; range-of-motion examination showed lack of the terminal 5º of extension. Magnetic resonance and computed tomography imaging from an outside facility showed a skeletally immature individual with a large tibial spine fracture that had healed in a malunited position with the fragment extended on a posterior hinge, creating a large prominence anteriorly (Figures 1A, 1B). Magnetic resonance imaging showed that the ACL fibers were likely to remain intact but would lack appropriate tension secondary to the displacement of the tibial insertion.
Because of healing in a displaced position, lack of terminal extension, ACL laxity, and subjective complaints of pain, we discussed surgery with the patient and his parents (Figures 2A, 2B). Four months after the initial injury, the patient underwent surgery for a right tibial spine malunion arthroscopic takedown and repair, as well as an intraoperative evaluation of the ACL. Standard arthroscopy was performed, using anterolateral and anteromedial arthroscopic portals, and an accessory medial peripatellar portal. During surgery, a large prominence was noted in the region of the anterior tibial spine (Figure 3A). The ACL fibers maintained a slack position secondary to the elevation of the tibial insertion point, and intraoperative Lachman examination showed anterior translation of the tibia on the femur as the slack was removed from the ACL. During surgery, impingement of the anterior tibial spine along the femoral notch was shown to be significant by taking the knee into near-full extension (Figure 3B). A cam-like effect was noted at the time of impingement with the posterior soft tissues relaxing to accommodate slight further extension.
Based on these findings, we chose to take down the malunited fracture and repair it (Figure 3C). PDS suture (Ethicon, Somerville, New Jersey) was temporarily placed along the intermeniscal ligament and anterior horns of the medial and lateral menisci, using a system of spinal needles to facilitate suture passage. Surgical clamps were hung from the suture to provide traction on the sutures throughout the case, allowing the intermeniscal ligament and menisci to recede anteriorly to improve working space and aid in preventing iatrogenic injury. These sutures were removed at the conclusion of the case. Using a combination of curettes, elevator, and small shaver, we were able to meticulously remove interposed malunited callus to allow for mobilization of the displaced fragment. After removal of the excess bone formation, a typical donor site was created, allowing the displaced spine fragment to be hinged into appropriate alignment (Figure 3D). We were able to maintain a posterior cortical hinge to facilitate this process.
Then, we placed Kirschner wires (K-wires) across the fracture in an antegrade fashion, anterior to the trochlea and notch, using an accessory medial peripatellar starting point percutaneously, under direct visualization to avoid iatrogenic chondral injury. The tibial spine fragment was temporarily maintained in a reduced position with an arthroscopic probe and pinned in place with two 0.062-in K-wires. The fracture was stabilized with 8 resorbable 1.6-mm poly-L-lactic/polyglycolic acid (PLLA/PGA) nails, in varying lengths from 18 mm to 22 mm. Excellent fixation was obtained, and range of motion was tested from 0º to 80º, without movement of the fracture site (Figure 3E). Fluoroscopy with multi-axial views verified adequate fixation and reduction. Further, we examined and noted a taut ACL after fixation. The patient was placed in a long leg cast for 3 weeks at 30º, based upon intraoperative determination of the position of least tension on the fracture fragment.
At 3-week follow-up, the patient was progressing well and transitioned from a long leg cast to a hinged knee brace, to allow for early range of motion. Radiographs showed appropriate alignment of the tibial spine fracture with no significant loss of fixation (Figures 4A, 4B). Physical therapy was initiated between 0º and 30º, and flexion was progressively increased over the course of the first 3 weeks. Active and active-assist, closed-chain activities were maintained. Seven weeks postoperatively, the patient displayed continued clinical progression. Radiographs showed interval healing with slight lucency over the anterolateral aspect of the fracture fragment, likely related to the early resorptive process of healing. Physical examination showed movement between 0º and 120º, stable Lachman test, and stable anterior drawer. Crutches were discontinued and hinged knee brace was converted to an ACL brace. By the 11th week, motion had increased to 140º, and radiographs continued to show acceptable alignment and healing (Figures 5A, 5B). The patient was released to return to play as tolerated; however, an ACL brace was recommended during his initial return to provide additional support.
Discussion
In this report, we present an approach for arthroscopic reduction of a malunited tibial spine fracture using resorbable PLLA/PGA nails. The number of polyglycolic nails employed is individualized per case, dependent on the surface area and the quality of the bone within the fractured fragment. Preoperative templating allows for measurements from the fractured fragment to the level of the proximal tibial physis. Based on these measurements, nails are chosen to maximize fixation length and avoid the physis. Despite studies that have examined the effect of transphyseal K-wire pinning or drilling on subsequent growth, there is no consensus about optimal technique. Experiments in animal models indicate that drill injuries destroying less than 8% to 9% of the physis do not impact total bone growth.12,13 Further, temporary crossing of the physeal plate for internal fixation of dislocated joint injuries has not been shown to result in bone bridging or growth disturbance.14,15
Each nail is 1.6 mm in diameter, leaving a small footprint. The nails are used judiciously to provide effective stabilization of the fragment and to maintain a cost-conscious approach. An accessory superomedial peripatellar portal allows an appropriate angle for nail placement. This portal allows access to all regions of the fractured fragment, while an anteromedial and anterolateral portal are used as working and camera portals, respectively. Nails are placed to provide an axis perpendicular to the fracture line to allow appropriate compression. By virtue of the shape of the typical fragment in a tibial spine fracture, the nails vary in insertion angle.
The occurrence of anterior tibial spine fractures is rare, and while several techniques have been described to repair this fracture, there remains a great deal of uncertainty regarding the best course of treatment. A review of the literature finds arthroscopic and open approaches, as well as techniques employing K-wire fixation, metal screw fixation, staple fixation, absorbable fixation, and fixation with sutures passed through the tibial tunnel.16-18
Avulsion fractures of the tibial eminence were treated with open fixation until McLennan8 first reported the benefits of reduction with an arthroscope. Open reduction and internal fixation provide the benefit of direct visualization,9 while arthroscopic reduction offers decreased morbidity and an accelerated recovery of knee functions,8 despite the fact that a higher rate of range-of-motion deficits were seen in patients treated arthroscopically.19 We feel that with proper early rehabilitation to achieve range of motion, the risk of this can be minimal.
Various arthroscopic approaches that improve the accuracy of the reduction and decrease surgical invasiveness have been described. Suture and screw fixation are among the most common methods, and both have resulted in positive outcomes.20-24 Suture fixation of the tibial eminence is technically demanding but offers secure fixation without the need for follow-up hardware removal. Screw fixation results in secure fixation; however, numerous hardware-related issues may necessitate removal. Furthermore, in skeletally immature patients, screw fixation may disturb the growth plate if it crosses an open physis.9
Hunter and Willis25 retrospectively reviewed patients with tibial eminence fractures treated with either screw or suture fixation and found a 44% reoperation rate in the screw-fixation group. Removal was often recommended as a result of hardware-related issues. There was a 13% reoperation rate in the suture-fixation group, which resulted largely from stiffness.25 In a recent review, Gans and colleagues19 reviewed 6 publications comparing screw and suture fixation of tibial eminence fractures and found 82.4% of screw patients had laxity on both the anterior drawer and Lachman tests, compared with 18.8% in the suture-fixation group. This study also noted a slightly higher rate of arthrofibrosis in patients treated with suture fixation.19 Biomechanical studies indicate that suture fixation imparts greater strength under cyclic-loading conditions;26 however, there does not appear to be a difference in ultimate force required for fixation failure.27
Ultimately, both suture and screw fixation result in secure methods of fixation; however, there are often greater issues with screw fixation because of the persistent hardware. Metal has been the most popular method for fracture fixation, and while biodegradable materials have been alluring, adverse tissue reactions have slowed implementation. However, these implants have become increasingly sophisticated, thereby reducing disadvantages.28 Previous biodegradable devices were often composed of a single polymer, and many caused adverse reactions by degrading too quickly or provided no real advantages because they degraded too slowly.29 As the number of polymers approved for internal use and surgical applications continues to rise, so too will the benefits of employing this technology. Furthermore, by including multiple polymers in these implants, one is better able to control the degradation rate, limiting the tissue response.
In this study, we employed PLLA/PGA nails. Studies of PGA implants indicate this molecule degrades at a fast rate resulting in adverse tissue reactions. Adverse reactions in studies of PLLA implants are less frequent because of their slower rate of degradation.29,30 Combining these monomers results in appropriate strength and a controlled degradation rate, reducing the likelihood of adverse reactions. Furthermore, numerous studies have reported that inflammatory responses in children are rare and mild in nature.31,32 Absorbable implants have displayed efficacy in numerous orthopedic settings33-36 and are beneficial in procedures that are not suitable for repeated surgeries, such as reconstruction of the ACL.37 There is some concern about the use of absorbable implants in synovial joints. Polyglycolic acid use in synovial joints may cause foreign-body reactions and may increase the risk of intra-articular dissemination of polymeric debris;38 however, use of a multipolymer construct decreases the likelihood of this occurrence.
Polyglycolic nails confer the advantage over nonresorbable screw fixation because further procedure for hardware removal is not required. Although suture fixation has proved to be beneficial over nonresorbable screw fixation, implantation of resorbable nails appears to have several advantages. In Dr. Estes’ experience, placement of resorbable screws through an accessory superomedial portal is far less technically demanding than placement of suture through the fracture fragment. Further, as sutures are passed from the extra-articular to the intra-articular region of the joint, capsular layers of the knee may inadvertently be bound up in the fixation, predisposing to arthrofibrosis.
At the same time, biodegradable devices are often more costly than alternative forms of treatment; however, a true cost-to-benefit analysis requires consideration of other factors. One of the benefits of biodegradable hardware is that there is no need for follow-up hardware removal. Reports have indicated that up to 91% of patients thought that hardware removal was the most negative aspect of metal implants.39 It is estimated that if the removal rate for metallic implants is higher than 19% to 54%, resorbable implants would be more cost-effective.40 The cost of sutures and screws is variable, however; they are invariably less expensive than biodegradable nails. A study of fracture patients determined that biodegradable implants were cheaper on average after considering the cost of implant removal.40 Ultimately, the hardware choice depends on numerous factors, including surgeon’s discretion; however, biodegradable hardware should not be discounted for financial reasons because the difference in cost is likely negligible.
Conclusion
The approach described in this report offers efficient and secure fixation with resorbable hardware without a reduction in range of motion. Resorbable implants may prove beneficial in the treatment of tibial eminence fractures by offering robust fixation without the concerns associated with permanent hardware.
Anterior tibial spine fractures are rare, occurring with an incidence of 3 per 100,000 per year.1,2 Historically, this fracture has occurred more frequently in children,3-5 and was considered a condition of skeletal immaturity and the pediatric equivalent of an anterior cruciate ligament (ACL) rupture.6 However, recent literature indicates that this fracture is more common in the adult population than previously thought.7 The tibial spine is an attachment point for the ACL and an avulsion may produce ACL laxity,8 predisposing to further symptomatic laxity and premature osteoarthritis. Nearly 40% of these fractures are associated with concomitant injuries to surrounding structures.9
Meyers and McKeever10,11 originally classified these fractures into 3 groups on the basis of displacement. Type I fractures present with no significant displacement of the anterior margin, type II involve displacement and are hinged, while type III have complete displacement.10,11 More recently, a type IV fracture has been added, involving comminution of the displaced fragment. Nondisplaced fractures are commonly treated with immobilization in varying degrees of extension; this allows the femoral condyles to compress and to reduce the fracture while arthroscopic or open reduction is the preferred method for displaced fractures of the tibial spine.2,4,8,10
We report the case of an 11-year-old boy with a tibial spine fracture that failed conservative management. He developed a subsequent malunion with impingement anteriorly of the tibial spine on the notch, and residual instability of the ACL. The patient’s parents provided written informed consent for print and electronic publication of this case report.
Case Report
An 11-year-old Caucasian boy was referred to our office for evaluation of right knee injury. He sustained the injury approximately 3 months earlier, and it was determined that he had a tibial spine fracture. Conservative management with immobilization in extension and activity modification was undertaken; however, he was referred for further evaluation because of healing in a malreduced position and residual ACL laxity. Physical examination showed a grade 2A Lachman test (contralateral limb with negative Lachman examination), negative McMurray test, and pain with forced hyperextension; range-of-motion examination showed lack of the terminal 5º of extension. Magnetic resonance and computed tomography imaging from an outside facility showed a skeletally immature individual with a large tibial spine fracture that had healed in a malunited position with the fragment extended on a posterior hinge, creating a large prominence anteriorly (Figures 1A, 1B). Magnetic resonance imaging showed that the ACL fibers were likely to remain intact but would lack appropriate tension secondary to the displacement of the tibial insertion.
Because of healing in a displaced position, lack of terminal extension, ACL laxity, and subjective complaints of pain, we discussed surgery with the patient and his parents (Figures 2A, 2B). Four months after the initial injury, the patient underwent surgery for a right tibial spine malunion arthroscopic takedown and repair, as well as an intraoperative evaluation of the ACL. Standard arthroscopy was performed, using anterolateral and anteromedial arthroscopic portals, and an accessory medial peripatellar portal. During surgery, a large prominence was noted in the region of the anterior tibial spine (Figure 3A). The ACL fibers maintained a slack position secondary to the elevation of the tibial insertion point, and intraoperative Lachman examination showed anterior translation of the tibia on the femur as the slack was removed from the ACL. During surgery, impingement of the anterior tibial spine along the femoral notch was shown to be significant by taking the knee into near-full extension (Figure 3B). A cam-like effect was noted at the time of impingement with the posterior soft tissues relaxing to accommodate slight further extension.
Based on these findings, we chose to take down the malunited fracture and repair it (Figure 3C). PDS suture (Ethicon, Somerville, New Jersey) was temporarily placed along the intermeniscal ligament and anterior horns of the medial and lateral menisci, using a system of spinal needles to facilitate suture passage. Surgical clamps were hung from the suture to provide traction on the sutures throughout the case, allowing the intermeniscal ligament and menisci to recede anteriorly to improve working space and aid in preventing iatrogenic injury. These sutures were removed at the conclusion of the case. Using a combination of curettes, elevator, and small shaver, we were able to meticulously remove interposed malunited callus to allow for mobilization of the displaced fragment. After removal of the excess bone formation, a typical donor site was created, allowing the displaced spine fragment to be hinged into appropriate alignment (Figure 3D). We were able to maintain a posterior cortical hinge to facilitate this process.
Then, we placed Kirschner wires (K-wires) across the fracture in an antegrade fashion, anterior to the trochlea and notch, using an accessory medial peripatellar starting point percutaneously, under direct visualization to avoid iatrogenic chondral injury. The tibial spine fragment was temporarily maintained in a reduced position with an arthroscopic probe and pinned in place with two 0.062-in K-wires. The fracture was stabilized with 8 resorbable 1.6-mm poly-L-lactic/polyglycolic acid (PLLA/PGA) nails, in varying lengths from 18 mm to 22 mm. Excellent fixation was obtained, and range of motion was tested from 0º to 80º, without movement of the fracture site (Figure 3E). Fluoroscopy with multi-axial views verified adequate fixation and reduction. Further, we examined and noted a taut ACL after fixation. The patient was placed in a long leg cast for 3 weeks at 30º, based upon intraoperative determination of the position of least tension on the fracture fragment.
At 3-week follow-up, the patient was progressing well and transitioned from a long leg cast to a hinged knee brace, to allow for early range of motion. Radiographs showed appropriate alignment of the tibial spine fracture with no significant loss of fixation (Figures 4A, 4B). Physical therapy was initiated between 0º and 30º, and flexion was progressively increased over the course of the first 3 weeks. Active and active-assist, closed-chain activities were maintained. Seven weeks postoperatively, the patient displayed continued clinical progression. Radiographs showed interval healing with slight lucency over the anterolateral aspect of the fracture fragment, likely related to the early resorptive process of healing. Physical examination showed movement between 0º and 120º, stable Lachman test, and stable anterior drawer. Crutches were discontinued and hinged knee brace was converted to an ACL brace. By the 11th week, motion had increased to 140º, and radiographs continued to show acceptable alignment and healing (Figures 5A, 5B). The patient was released to return to play as tolerated; however, an ACL brace was recommended during his initial return to provide additional support.
Discussion
In this report, we present an approach for arthroscopic reduction of a malunited tibial spine fracture using resorbable PLLA/PGA nails. The number of polyglycolic nails employed is individualized per case, dependent on the surface area and the quality of the bone within the fractured fragment. Preoperative templating allows for measurements from the fractured fragment to the level of the proximal tibial physis. Based on these measurements, nails are chosen to maximize fixation length and avoid the physis. Despite studies that have examined the effect of transphyseal K-wire pinning or drilling on subsequent growth, there is no consensus about optimal technique. Experiments in animal models indicate that drill injuries destroying less than 8% to 9% of the physis do not impact total bone growth.12,13 Further, temporary crossing of the physeal plate for internal fixation of dislocated joint injuries has not been shown to result in bone bridging or growth disturbance.14,15
Each nail is 1.6 mm in diameter, leaving a small footprint. The nails are used judiciously to provide effective stabilization of the fragment and to maintain a cost-conscious approach. An accessory superomedial peripatellar portal allows an appropriate angle for nail placement. This portal allows access to all regions of the fractured fragment, while an anteromedial and anterolateral portal are used as working and camera portals, respectively. Nails are placed to provide an axis perpendicular to the fracture line to allow appropriate compression. By virtue of the shape of the typical fragment in a tibial spine fracture, the nails vary in insertion angle.
The occurrence of anterior tibial spine fractures is rare, and while several techniques have been described to repair this fracture, there remains a great deal of uncertainty regarding the best course of treatment. A review of the literature finds arthroscopic and open approaches, as well as techniques employing K-wire fixation, metal screw fixation, staple fixation, absorbable fixation, and fixation with sutures passed through the tibial tunnel.16-18
Avulsion fractures of the tibial eminence were treated with open fixation until McLennan8 first reported the benefits of reduction with an arthroscope. Open reduction and internal fixation provide the benefit of direct visualization,9 while arthroscopic reduction offers decreased morbidity and an accelerated recovery of knee functions,8 despite the fact that a higher rate of range-of-motion deficits were seen in patients treated arthroscopically.19 We feel that with proper early rehabilitation to achieve range of motion, the risk of this can be minimal.
Various arthroscopic approaches that improve the accuracy of the reduction and decrease surgical invasiveness have been described. Suture and screw fixation are among the most common methods, and both have resulted in positive outcomes.20-24 Suture fixation of the tibial eminence is technically demanding but offers secure fixation without the need for follow-up hardware removal. Screw fixation results in secure fixation; however, numerous hardware-related issues may necessitate removal. Furthermore, in skeletally immature patients, screw fixation may disturb the growth plate if it crosses an open physis.9
Hunter and Willis25 retrospectively reviewed patients with tibial eminence fractures treated with either screw or suture fixation and found a 44% reoperation rate in the screw-fixation group. Removal was often recommended as a result of hardware-related issues. There was a 13% reoperation rate in the suture-fixation group, which resulted largely from stiffness.25 In a recent review, Gans and colleagues19 reviewed 6 publications comparing screw and suture fixation of tibial eminence fractures and found 82.4% of screw patients had laxity on both the anterior drawer and Lachman tests, compared with 18.8% in the suture-fixation group. This study also noted a slightly higher rate of arthrofibrosis in patients treated with suture fixation.19 Biomechanical studies indicate that suture fixation imparts greater strength under cyclic-loading conditions;26 however, there does not appear to be a difference in ultimate force required for fixation failure.27
Ultimately, both suture and screw fixation result in secure methods of fixation; however, there are often greater issues with screw fixation because of the persistent hardware. Metal has been the most popular method for fracture fixation, and while biodegradable materials have been alluring, adverse tissue reactions have slowed implementation. However, these implants have become increasingly sophisticated, thereby reducing disadvantages.28 Previous biodegradable devices were often composed of a single polymer, and many caused adverse reactions by degrading too quickly or provided no real advantages because they degraded too slowly.29 As the number of polymers approved for internal use and surgical applications continues to rise, so too will the benefits of employing this technology. Furthermore, by including multiple polymers in these implants, one is better able to control the degradation rate, limiting the tissue response.
In this study, we employed PLLA/PGA nails. Studies of PGA implants indicate this molecule degrades at a fast rate resulting in adverse tissue reactions. Adverse reactions in studies of PLLA implants are less frequent because of their slower rate of degradation.29,30 Combining these monomers results in appropriate strength and a controlled degradation rate, reducing the likelihood of adverse reactions. Furthermore, numerous studies have reported that inflammatory responses in children are rare and mild in nature.31,32 Absorbable implants have displayed efficacy in numerous orthopedic settings33-36 and are beneficial in procedures that are not suitable for repeated surgeries, such as reconstruction of the ACL.37 There is some concern about the use of absorbable implants in synovial joints. Polyglycolic acid use in synovial joints may cause foreign-body reactions and may increase the risk of intra-articular dissemination of polymeric debris;38 however, use of a multipolymer construct decreases the likelihood of this occurrence.
Polyglycolic nails confer the advantage over nonresorbable screw fixation because further procedure for hardware removal is not required. Although suture fixation has proved to be beneficial over nonresorbable screw fixation, implantation of resorbable nails appears to have several advantages. In Dr. Estes’ experience, placement of resorbable screws through an accessory superomedial portal is far less technically demanding than placement of suture through the fracture fragment. Further, as sutures are passed from the extra-articular to the intra-articular region of the joint, capsular layers of the knee may inadvertently be bound up in the fixation, predisposing to arthrofibrosis.
At the same time, biodegradable devices are often more costly than alternative forms of treatment; however, a true cost-to-benefit analysis requires consideration of other factors. One of the benefits of biodegradable hardware is that there is no need for follow-up hardware removal. Reports have indicated that up to 91% of patients thought that hardware removal was the most negative aspect of metal implants.39 It is estimated that if the removal rate for metallic implants is higher than 19% to 54%, resorbable implants would be more cost-effective.40 The cost of sutures and screws is variable, however; they are invariably less expensive than biodegradable nails. A study of fracture patients determined that biodegradable implants were cheaper on average after considering the cost of implant removal.40 Ultimately, the hardware choice depends on numerous factors, including surgeon’s discretion; however, biodegradable hardware should not be discounted for financial reasons because the difference in cost is likely negligible.
Conclusion
The approach described in this report offers efficient and secure fixation with resorbable hardware without a reduction in range of motion. Resorbable implants may prove beneficial in the treatment of tibial eminence fractures by offering robust fixation without the concerns associated with permanent hardware.
1. Hargrove R, Parsons S, Payne R. Anterior tibial spine fracture – an easy fracture to miss. Accid Emerg Nurs. 2004;12(3):173-175.
2. Aderinto J, Walmsley P, Keating JF. Fractures of the tibial spine: epidemiology and outcome. Knee. 2008;15(3):164-167.
3. Driessen MJ, Winkelman PA. Fractures of the intercondylar eminence of the tibia in childhood. Neth J Surg. 1984;36(3):69-72.
4. Zaricznyj B. Avulsion fracture of the tibial eminence: treatment by open reduction and pinning. J Bone Joint Surg Am. 1977;59(8):1111-1114.
5. Molander ML, Wallin G, Wikstad I. Fracture of the intercondylar eminence of the tibia: a review of 35 patients. J Bone Joint Surg Br. 1981;63(1):89-91.
6. Kieser DC, Gwynne-Jones D, Dreyer S. Displaced tibial intercondylar eminence fractures. J Orthop Surg. 2011;19(3):292-296.
7. Ishibashi Y, Tsuda E, Sasaki T, Toh S. Magnetic resonance imaging AIDS in detecting concomitant injuries in patients with tibial spine fractures. Clin Orthop. 2005;(434):207-212.
8. McLennan JG. The role of arthroscopic surgery in the treatment of fractures of the intercondylar eminence of the tibia. J Bone Joint Surg Br. 1982;64(4):477-480.
9. Lafrance RM, Giordano B, Goldblatt J, Voloshin I, Maloney M. Pediatric tibial eminence fractures: evaluation and management. J Am Acad Orthop Surg. 2010;18(7):395-405.
10. Meyers MH, McKeever FM. Fracture of the intercondylar eminence of the tibia. J Bone Joint Surg Am. 1959;41(2):209-220.
11. Meyers MH, McKeever FM. Fracture of the intercondylar eminence of the tibia. J Bone Joint Surg Am. 1970;52(8):1677-1684.
12. Garcés GL, Mugica-Garay I, López-González Coviella N, Guerado E. Growth-plate modifications after drilling. J Pediatr Orthop. 1994;14(2):225-228.
13. Janarv PM, Wikström B, Hirsch G. The influence of transphyseal drilling and tendon grafting on bone growth: an experimental study in the rabbit. J Pediatr Orthop. 1998;18(2):149-154.
14. Boelitz R, Dallek M, Meenen NM, Jungbluth KH. Reaction of the epiphyseal groove to groove-crossing bore-wire osteosynthesis. Results of a histomorphologic small animal study. Unfallchirurgie. 1994;20(3):131-137.
15. Yung PS, Lam CY, Ng BK, Lam TP, Cheng JC. Percutaneous transphyseal intramedullary Kirschner wire pinning: a safe and effective procedure for treatment of displaced diaphyseal forearm fracture in children. J Pediatr Orthop. 2004;24(1):7-12.
16. Bong MR, Romero A, Kubiak E, et al. Suture versus screw fixation of displaced tibial eminence fractures: a biomechanical comparison. Arthroscopy. 2005;21(10):1172-1176.
17. Vega JR, Irribarra LA, Baar AK, Iñiguez M, Salgado M, Gana N. Arthroscopic fixation of displaced tibial eminence fractures: a new growth plate-sparing method. Arthroscopy. 2008;24(11):1239-1243.
18. Shepley RW. Arthroscopic treatment of type III tibial spine fractures using absorbable fixation. Orthopedics. 2004;27(7):767-769.
19. Gans I, Baldwin KD, Ganley TJ. Treatment and management outcomes of tibial eminence fractures in pediatric patients: a systematic review. Am J Sports Med. 2013;42(7):1743-1750.
20. Delcogliano A, Chiossi S, Caporaso A, Menghi A, Rinonapoli G. Tibial intercondylar eminence fractures in adults: arthroscopic treatment. Knee Surg Sports Traumatol Arthrosc. 2003;11(4):255-259.
21. Mulhall KJ, Dowdall J, Grannell M, McCabe JP. Tibial spine fractures: an analysis of outcome in surgically treated type III injuries. Injury. 1999;30(4):289-292.
22. Geissler WB, Matthews DE. Arthroscopic suture fixation of displaced tibial eminence fractures. Orthopedics. 1993;16(3):331-333.
23. Mah JY, Otsuka NY, McLean J. An arthroscopic technique for the reduction and fixation of tibial-eminence fractures. J Pediatr Orthop. 1996;16(1):119-121.
24. Reynders P, Reynders K, Broos P. Pediatric and adolescent tibial eminence fractures: arthroscopic cannulated screw fixation. J Trauma. 2002;53(1):49-54.
25. Hunter RE, Willis JA. Arthroscopic fixation of avulsion fractures of the tibial eminence: technique and outcome. Arthroscopy. 2004;20(2):113-121.
26. Eggers AK, Becker C, Weimann A, et al. Biomechanical evaluation of different fixation methods for tibial eminence fractures. Am J Sports Med. 2007;35(3):404-410.
27. Mahar AT, Duncan D, Oka R, Lowry A, Gillingham B, Chambers H. Biomechanical comparison of four different fixation techniques for pediatric tibial eminence avulsion fractures. J Pediatr Orthop. 2008;28(2):159-162.
28. Toro C, Robiony M, Zerman N, Politi M. Resorbable plates in maxillary fixation. A 5-year experience. Minerva Stomatol. 2005;54(4):199-206.
29. Andriano KP, Pohjonen T, Törmälä P. Processing and characterization of absorbable polylactide polymers for use in surgical implants. J Appl Biomater.1994;5(2):133-140.
30. Böstman O, Pihlajamäki H. Clinical biocompatibility of biodegradable orthopaedic implants for internal fixation: a review. Biomaterials. 2000;21(24):2615-2621.
31. Rokkanen PU, Böstman O, Hirvensalo E, et al. Bioabsorbable fixation in orthopaedic surgery and traumatology. Biomaterials. 2000;21(24):2607-2613.
32. Athanasiou KA, Niederauer GG, Agrawal CM. Sterilization, toxicity, biocompatibility and clinical applications of polylactic acid/polyglycolic acid copolymers. Biomaterials. 1996;17(2):93-102.
33. Li ZH, Yu AX, Guo XP, Qi BW, Zhou M, Wang WY. Absorbable implants versus metal implants for the treatment of ankle fractures: A meta-analysis. Exp Ther Med. 2013;5(5):1531-1537.
34. Singh G, Mohammad S, Chak RK, Lepcha N, Singh N, Malkunje LR. Bio-resorbable plates as effective implant in paediatric mandibular fracture. J Maxillofac Oral Surg. 2012;11(4):400-406.
35. Sakamoto Y, Shimizu Y, Nagasao T, Kishi K. Combined use of resorbable poly-L-lactic acid-polyglycolic acid implant and bone cement for treating large orbital floor fractures. J Plast Reconstr Aesthet Surg. 2014;67(3):e88-e90.
36. Benz G, Kallieris D, Seeböck T, McIntosh A, Daum R. Bioresorbable pins and screws in paediatric traumatology. Eur J Pediatr Surg. 1994;4(2):103-107.
37. Gaweda K, Walawski J, Weglowski R, Krzyzanowski W. Comparison of bioabsorbable interference screws and posts for distal fixation in anterior cruciate ligament reconstruction. Int Orthop. 2009;33(1):123-127.
38. Böstman OM. Osteoarthritis of the ankle after foreign-body reaction to absorbable pins and screws: a three- to nine-year follow-up study. J Bone Joint Surg Br. 1998;80(2):333-338.
39. Mittal R, Morley J, Dinopoulos H, Drakoulakis EG, Vermani E, Giannoudis PV. Use of bio-resorbable implants for stabilisation of distal radius fractures: the United Kingdom patients’ perspective. Injury. 2005;36(2):333-338.
40. Böstman OM. Metallic or absorbable fracture fixation devices. A cost minimization analysis. Clin Orthop. 1996;(329):233-239.
1. Hargrove R, Parsons S, Payne R. Anterior tibial spine fracture – an easy fracture to miss. Accid Emerg Nurs. 2004;12(3):173-175.
2. Aderinto J, Walmsley P, Keating JF. Fractures of the tibial spine: epidemiology and outcome. Knee. 2008;15(3):164-167.
3. Driessen MJ, Winkelman PA. Fractures of the intercondylar eminence of the tibia in childhood. Neth J Surg. 1984;36(3):69-72.
4. Zaricznyj B. Avulsion fracture of the tibial eminence: treatment by open reduction and pinning. J Bone Joint Surg Am. 1977;59(8):1111-1114.
5. Molander ML, Wallin G, Wikstad I. Fracture of the intercondylar eminence of the tibia: a review of 35 patients. J Bone Joint Surg Br. 1981;63(1):89-91.
6. Kieser DC, Gwynne-Jones D, Dreyer S. Displaced tibial intercondylar eminence fractures. J Orthop Surg. 2011;19(3):292-296.
7. Ishibashi Y, Tsuda E, Sasaki T, Toh S. Magnetic resonance imaging AIDS in detecting concomitant injuries in patients with tibial spine fractures. Clin Orthop. 2005;(434):207-212.
8. McLennan JG. The role of arthroscopic surgery in the treatment of fractures of the intercondylar eminence of the tibia. J Bone Joint Surg Br. 1982;64(4):477-480.
9. Lafrance RM, Giordano B, Goldblatt J, Voloshin I, Maloney M. Pediatric tibial eminence fractures: evaluation and management. J Am Acad Orthop Surg. 2010;18(7):395-405.
10. Meyers MH, McKeever FM. Fracture of the intercondylar eminence of the tibia. J Bone Joint Surg Am. 1959;41(2):209-220.
11. Meyers MH, McKeever FM. Fracture of the intercondylar eminence of the tibia. J Bone Joint Surg Am. 1970;52(8):1677-1684.
12. Garcés GL, Mugica-Garay I, López-González Coviella N, Guerado E. Growth-plate modifications after drilling. J Pediatr Orthop. 1994;14(2):225-228.
13. Janarv PM, Wikström B, Hirsch G. The influence of transphyseal drilling and tendon grafting on bone growth: an experimental study in the rabbit. J Pediatr Orthop. 1998;18(2):149-154.
14. Boelitz R, Dallek M, Meenen NM, Jungbluth KH. Reaction of the epiphyseal groove to groove-crossing bore-wire osteosynthesis. Results of a histomorphologic small animal study. Unfallchirurgie. 1994;20(3):131-137.
15. Yung PS, Lam CY, Ng BK, Lam TP, Cheng JC. Percutaneous transphyseal intramedullary Kirschner wire pinning: a safe and effective procedure for treatment of displaced diaphyseal forearm fracture in children. J Pediatr Orthop. 2004;24(1):7-12.
16. Bong MR, Romero A, Kubiak E, et al. Suture versus screw fixation of displaced tibial eminence fractures: a biomechanical comparison. Arthroscopy. 2005;21(10):1172-1176.
17. Vega JR, Irribarra LA, Baar AK, Iñiguez M, Salgado M, Gana N. Arthroscopic fixation of displaced tibial eminence fractures: a new growth plate-sparing method. Arthroscopy. 2008;24(11):1239-1243.
18. Shepley RW. Arthroscopic treatment of type III tibial spine fractures using absorbable fixation. Orthopedics. 2004;27(7):767-769.
19. Gans I, Baldwin KD, Ganley TJ. Treatment and management outcomes of tibial eminence fractures in pediatric patients: a systematic review. Am J Sports Med. 2013;42(7):1743-1750.
20. Delcogliano A, Chiossi S, Caporaso A, Menghi A, Rinonapoli G. Tibial intercondylar eminence fractures in adults: arthroscopic treatment. Knee Surg Sports Traumatol Arthrosc. 2003;11(4):255-259.
21. Mulhall KJ, Dowdall J, Grannell M, McCabe JP. Tibial spine fractures: an analysis of outcome in surgically treated type III injuries. Injury. 1999;30(4):289-292.
22. Geissler WB, Matthews DE. Arthroscopic suture fixation of displaced tibial eminence fractures. Orthopedics. 1993;16(3):331-333.
23. Mah JY, Otsuka NY, McLean J. An arthroscopic technique for the reduction and fixation of tibial-eminence fractures. J Pediatr Orthop. 1996;16(1):119-121.
24. Reynders P, Reynders K, Broos P. Pediatric and adolescent tibial eminence fractures: arthroscopic cannulated screw fixation. J Trauma. 2002;53(1):49-54.
25. Hunter RE, Willis JA. Arthroscopic fixation of avulsion fractures of the tibial eminence: technique and outcome. Arthroscopy. 2004;20(2):113-121.
26. Eggers AK, Becker C, Weimann A, et al. Biomechanical evaluation of different fixation methods for tibial eminence fractures. Am J Sports Med. 2007;35(3):404-410.
27. Mahar AT, Duncan D, Oka R, Lowry A, Gillingham B, Chambers H. Biomechanical comparison of four different fixation techniques for pediatric tibial eminence avulsion fractures. J Pediatr Orthop. 2008;28(2):159-162.
28. Toro C, Robiony M, Zerman N, Politi M. Resorbable plates in maxillary fixation. A 5-year experience. Minerva Stomatol. 2005;54(4):199-206.
29. Andriano KP, Pohjonen T, Törmälä P. Processing and characterization of absorbable polylactide polymers for use in surgical implants. J Appl Biomater.1994;5(2):133-140.
30. Böstman O, Pihlajamäki H. Clinical biocompatibility of biodegradable orthopaedic implants for internal fixation: a review. Biomaterials. 2000;21(24):2615-2621.
31. Rokkanen PU, Böstman O, Hirvensalo E, et al. Bioabsorbable fixation in orthopaedic surgery and traumatology. Biomaterials. 2000;21(24):2607-2613.
32. Athanasiou KA, Niederauer GG, Agrawal CM. Sterilization, toxicity, biocompatibility and clinical applications of polylactic acid/polyglycolic acid copolymers. Biomaterials. 1996;17(2):93-102.
33. Li ZH, Yu AX, Guo XP, Qi BW, Zhou M, Wang WY. Absorbable implants versus metal implants for the treatment of ankle fractures: A meta-analysis. Exp Ther Med. 2013;5(5):1531-1537.
34. Singh G, Mohammad S, Chak RK, Lepcha N, Singh N, Malkunje LR. Bio-resorbable plates as effective implant in paediatric mandibular fracture. J Maxillofac Oral Surg. 2012;11(4):400-406.
35. Sakamoto Y, Shimizu Y, Nagasao T, Kishi K. Combined use of resorbable poly-L-lactic acid-polyglycolic acid implant and bone cement for treating large orbital floor fractures. J Plast Reconstr Aesthet Surg. 2014;67(3):e88-e90.
36. Benz G, Kallieris D, Seeböck T, McIntosh A, Daum R. Bioresorbable pins and screws in paediatric traumatology. Eur J Pediatr Surg. 1994;4(2):103-107.
37. Gaweda K, Walawski J, Weglowski R, Krzyzanowski W. Comparison of bioabsorbable interference screws and posts for distal fixation in anterior cruciate ligament reconstruction. Int Orthop. 2009;33(1):123-127.
38. Böstman OM. Osteoarthritis of the ankle after foreign-body reaction to absorbable pins and screws: a three- to nine-year follow-up study. J Bone Joint Surg Br. 1998;80(2):333-338.
39. Mittal R, Morley J, Dinopoulos H, Drakoulakis EG, Vermani E, Giannoudis PV. Use of bio-resorbable implants for stabilisation of distal radius fractures: the United Kingdom patients’ perspective. Injury. 2005;36(2):333-338.
40. Böstman OM. Metallic or absorbable fracture fixation devices. A cost minimization analysis. Clin Orthop. 1996;(329):233-239.
Popliteal Artery Pseudoaneurysm: An Unusual Complication of Tibial Traction
Traction-pin placement is a basic orthopedic skill learned in the early years of residency training. Skeletal traction historically was used as definitive treatment for long-bone fractures, and it is still in use in countries without access to modern medical care.1,2 In current orthopedic practice, proximal tibial and distal femoral traction pins are most commonly used to temporize femoral shaft and acetabular fractures, respectively, before definitive surgical intervention. Although traction-pin placement is common, there are complications that can cause morbidity ranging from skin irritation to death.
In this article, we report on a popliteal artery pseudoaneurysm, a unique complication related to pin placement. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 22-year-old woman with no past medical history was driving a car at 35 miles per hour when she hit a telephone pole. She was wearing a seatbelt. She was taken by ambulance for a trauma evaluation. She sustained a right posterior hip dislocation with an associated Pipkin 2 femoral head fracture (Figures 1A, 1B), a right elbow skin avulsion, and a scalp abrasion.
The patient underwent closed hip reduction under sedation, and a proximal tibial traction pin was placed (Figures 2A, 2B). At our institution, proximal tibial traction pins are placed 2 cm distal and 2 cm posterior to the tibial tubercle without the use of fluoroscopy. The skin leg is prepared and draped in sterile fashion, and a local anesthetic is used to anesthetize the skin and periosteum on the medial and lateral aspects of the leg. A 1.5-cm incision is made laterally, and soft tissue is spread laterally down to the periosteum. Then the traction pin (always the largest nonthreaded pin available) is used to sound the anterior and posterior aspects of the tibia—the goal being to end at the anterior two-thirds/posterior one-third mark. After the pin is advanced through both cortices, the place where the pin will exit the skin is noted, and another incision is made. For this patient, no complications were noted at time of pin placement.
On postinjury day 1, she was taken to the operating room for open reduction and internal fixation of the femoral head fracture through a Smith-Petersen approach. At the same time, a traction pin was removed. Routine postoperative protocols were followed. The patient was started on enoxaparin, was seen by physical therapy on postoperative day 1, and was kept non-weight-bearing on the right lower extremity. Pain was well controlled, and the patient was discharged on postoperative day 3 (postinjury day 4).
Three weeks after surgery, she returned for a scheduled follow-up with complaints of paresthesia and decreased sensation in the right lateral cutaneous femoral nerve distribution and burning pain in the toes. A stiff right ankle was noted. A night splint, amitriptyline, and additional pain medications were ordered.
The patient returned for her scheduled 6-week follow-up reporting she had not taken the enoxaparin because her pharmacy “did not have any.” Her primary care physician had given her “another blood thinner,” which she had taken orally for 1 to 2 weeks. She had complaints of right calf pain, but her hip was pain-free. There was a large amount of calf swelling and tenderness along with mild equinus contracture of the ankle. She was sent to the emergency department (ED) for Doppler ultrasound evaluation for deep venous thrombosis (DVT).
Duplex Doppler imaging of the deep venous system of the lower extremities was performed with visualization from the common femoral veins to the popliteal veins bilaterally. There was normal compressibility, respiratory phasicity, and flow with no intraluminal thrombus. A radiograph of tibia and fibula showed soft-tissue swelling and lucencies from the traction pin but no other abnormalities.
Eight weeks after surgery, the patient returned to the ED because of continuing calf pain. Given that the ultrasound findings had been negative, magnetic resonance imaging (MRI) was ordered, and orthopedic follow-up scheduled. About 10 weeks after surgery, she returned to the ED, still with calf pain, and reported having been unable to go for her MRI because of financial reasons. Physical examination revealed increased calf swelling and a new ecchymosis tracking along the tibia. Repeat ultrasound, performed from the common femoral veins through the popliteal veins, showed a right posterior calf pseudoaneurysm (8.5×4.3×5.3 cm) arising from the popliteal artery about 5 cm below the popliteal fossa. In addition, a large hematoma was seen originating in the upper posterior calf and extending inferiorly (Figure 3).
Computed tomography angiogram confirmed the ultrasound diagnosis of a large (5.6×4.8-cm) right calf pseudoaneurysm arising possibly from a small muscular branch of the proximal tibioperoneal trunk, with a large surrounding hematoma in the posterior compartment of the proximal right calf (Figure 4). Vascular surgery was consulted, and coil embolization of the pseudoaneurysm was performed later that night in the interventional radiology suite.
After the coiling of the pseudoaneurysm, the calf swelling and pain slowly improved. At 12-week orthopedic follow-up, the patient was no longer using any pain medications, and she noted improvement in the right foot’s neuropathic symptoms. Serial casting was prescribed for the equinus contracture of the ankle. She was allowed to start weight-bearing on the right lower extremity. Radiographs at 12 weeks showed collapse of the superomedial aspect of the femoral head with surrounding sclerosis consistent with posttraumatic avascular necrosis.
At final orthopedic follow-up, about 16 weeks after surgery, the patient reported 0/10 pain. Sensation was noted as being intact throughout the right lower extremity but decreased in the tibial nerve distribution. Ankle range of motion was still limited, with 5° of dorsiflexion and 25° of plantar flexion. The hip was pain-free with flexion of 0° to 100°, 10° of internal rotation, and 20° of external rotation. Additional appointments were scheduled, but the patient did not follow up. Two years after initial injury, she returned to the ED for evaluation of rhinorrhea, and no orthopedic complaints were noted.
Discussion
Skeletal traction begins with the insertion of a wire or pin through a bone. It is extremely important to use proper technique in order to minimize the risks associated with pin insertion.3 Potential pitfalls involve the energy transferred into the bone during insertion, the incisions used to place the pin, and injury to surrounding neurovascular structures. For proximal tibial pins, standard technique dictates placing the pin in a lateral-to-medial direction 2 cm posterior to the tibial tubercle and avoiding the dense anterior cortical bone. At our institution, traction pins are placed with a power drill after the patient is given a local anesthetic or is placed under conscious sedation. Which type of anesthesia to use is based on the patient’s overall condition and on the ED attending physician’s willingness to administer conscious sedation.
The 2 most common types of tibial traction involve use of either a large Steinmann pin attached to a metal bow or a Kirschner wire (K-wire) placed under tension before traction. Which to use is the surgeon’s choice. Surgeons at our institution historically have used Steinmann pins. No studies have directly compared fine-wire and Steinmann-pin traction, but with this complication our institution is evaluating a change to tensioned wires. Compared with large Steinmann pins, fine-wire pins create less of a defect in the bone but also bend or break more easily if tension is not applied or if it fails. A fine wire with its smaller surface area may also cut more easily into osteopenic bone than a large-diameter pin would.
Proximal tibial traction typically is indicated for femoral shaft and acetabular fractures. Although the subcutaneous nature of the tibia makes for easier pin placement, the anatomy of the tibia can predispose this bone to complications. Its triangular shape can lead to intracortical rather than the preferred bicortical pin placement. Increased heat caused by intracortical placement can lead to osteonecrosis and even to damage of surrounding soft tissues. Green and Ripley4 found that chronic osteomyelitis typically resulted from intracortical placement of traction pins.
Injury to surrounding soft tissues, either from heat necrosis or from infection introduced through pin sites, can also have consequences. Pin-site infections increase with duration of treatment, though care seldom requires more than pin removal and antibiotics.5,6 More-invasive infections range from cellulitis surrounding the pin site to subcutaneous abscesses. There is 1 report of a Clostridium perfringens infection leading to death only 5 days after pin placement.7
Neurovascular structures are at risk with any orthopedic procedure. With proximal tibial pins in pediatric patients, the peroneal nerve, the anterior tibial artery, and the proximal physis are most at risk. The deep peroneal nerve and the anterior tibial artery run together deep to the anterior compartment, which places them at highest risk with pin insertion. The peroneal and tibial arteries run deep to the deep posterior compartment along with the tibial nerve behind the posterior cortex of the tibia, which makes injury less likely.8
Historically, long-bone fractures were often treated with traction. Kirby and Fitts9 reported on 342 transfixion pins and wires used in the treatment of 233 long-bone fractures between 1943 and 1945. Of the 305 pins/wires observed over the entire treatment period (average, 6 weeks), only 12 (3.93%) developed a complication. There were 4 loose K-wires, 1 broken wire, and 1 bow failure; Steinmann pins were involved in 1 infection and 2 transient peroneal nerve palsies; and 3 Roger Anderson pins loosened. Pin-tract drainage was not included as a complication if it did not also involve localized or general signs of inflammation. The 2 peroneal nerve palsies were associated with medial-to-lateral pin insertion creating a more posterior pin path.
Pins inserted for external fixators of the tibia have injured the anterior tibial vessels and branches of the peroneal and saphenous nerves. A proximal tibial traction pin, in essence a transfixion pin, can cause similar injuries, particularly with imperfect placement (Figure 5).3,10
A pseudoaneurysm is a pulsating, encapsulated hematoma that remains in communication with the lumen of a ruptured or injured vessel. The arterial wall itself is torn or ruptured, and the external wall of the aneurysmal sac consists of outer arterial layers, perivascular tissue, blood clot, or a layer of reactive fibrosis. This contrasts with a true aneurysm, in which all 3 arterial layers (intima, media, adventitia) remain confluent but are dilated beyond their normal diameter. Of all pseudoaneurysms, those caused iatrogenically are the most common and are typically produced by femoral artery catheterization, accounting for 70% to 80% of the incidence.11
Our patient’s injury was most likely caused by an initial error in pin placement before the pin was driven across the tibia. The typical teaching for traction-pin placement involves finding the correct starting point and then using the pin to feel the anterior and posterior surfaces of the bone (described earlier). If the pin slid posteriorly, it may have contacted the artery and caused a small tear that eventually led to the formation of the pseudoaneurysm.
The pseudoaneurysm was not the only complication in the present case. There was also the delay in diagnosis. A standard technique is used to evaluate the lower extremity venous system for DVT. The ultrasonographer starts with the probe as proximal as possible (above the inguinal ligament), ideally proximal to the saphenofemoral junction, and moves distally in 1-cm increments, checking the veins for compressibility, color, and Doppler signal. Unless advised otherwise, the ultrasonographer typically does not examine distal to the knee.12,13 As this patient’s pseudoaneurysm was distal to the knee, it was not found on initial ultrasound, and her inability to obtain her MRI compounded the delay. The second ultrasound identified the pseudoaneurysm. The ultrasonographer examined more distally, given the contrast between the clinical diagnosis of vascular pathology and the negative Doppler study. Computed tomography angiogram confirmed the diagnosis and guided the vascular surgeons in identifying the lesion as a pseudoaneurysm, allowing it to be coiled rather than bypassed.
Duplex ultrasound is the preferred diagnostic modality for imaging pseudoaneurysms. Although our patient’s scan was performed in timely fashion, it did not image the area of pathology. Instead, this patient with multiple orthopedic injuries was scanned for DVT, the most likely cause of her lower extremity swelling. Had a pseudoaneurysm been suspected, the ultrasonographer would have been instructed to image the entire extremity and not just the area where DVT might be found.
Fortunately, despite the treatment delay, the patient recovered well from both the traumatic injuries sustained in the car crash and the likely iatrogenic pseudoaneurysm. Although traction pins are easily and frequently used, they can have complications, which are often preventable. Starting with pin placement itself, there were several opportunities for improving this patient’s care or, at a minimum, reducing the time spent in diagnosis. If the pin had been noticed sliding posteriorly during insertion, extra attention during follow-up visits could have helped identify the injury sooner. Another difficulty in diagnosis was that of obtaining the appropriate outpatient radiology studies which necessitated repeat ED visits. An additional juncture was between the patient’s multiple ED visits for similar complaints. Obtaining advanced imaging sooner could have helped in diagnosing the pseudoaneurysm earlier.
1. Gosselin RA, Heitto M, Zirkle L. Cost-effectiveness of replacing skeletal traction by interlocked intramedullary nailing for femoral shaft fractures in a provincial trauma hospital in Cambodia. Int Orthop. 2009;33(5):1445-1448.
2. Gosselin R, Lavaly D. Perkins traction for adult femoral shaft fractures: a report on 53 patients in Sierra Leone. Int Orthop. 2007;31(5):697-702.
3. Althausen PL, Hak DJ. Lower extremity traction pins: indications, technique, and complications. Am J Orthop. 2002;31(1):43-47.
4. Green SA, Ripley MJ. Chronic osteomyelitis in pin tracks. J Bone Joint Surg Am. 1984;66(7):1092-1098.
5. Nigam V, Jaiswal A, Dhaon BK. Local antibiotics: panacea for long term skeletal traction. Injury. 2005;36(1):199-202.
6. Lethaby A, Temple J, Santy J. Pin site care for preventing infections associated with external bone fixators and pins. Cochrane Database Syst Rev. 2008;(4):CD004551.
7. Taylor BC, Bramwell TJ, Formaini N. Gas gangrene as a result of femoral traction pin placement. Case Rep Orthop. 2011;(2011):459812.
8. Moskovich R. Proximal tibial transfixion for skeletal traction. An anatomic study of neurovascular structures. Clin Orthop. 1987;(214):264-268.
9. Kirby CK, Fitts WT. The incidence of complications in the use of transfixion pins and wires for skeletal traction. Ann Surg. 1946;123(1):27-31.
10. Behrens F, Searls K. External fixation of the tibia. Basic concepts and prospective evaluation. J Bone Joint Surg Br. 1986;68(2):246-254.
11. Sueyoshi E, Sakamoto I, Nakashima K, Minami K, Hayashi K. Visceral and peripheral arterial pseudoaneurysms. AJR Am J Roentgenol. 2005;185(3):741-749.
12. Scoutt LM, Zawin ML, Taylor KJ. Doppler US. Part II. Clinical applications. Radiology. 1990;174(2):309-319.
13. Mitchell DG, Needleman L, Bezzi M, et al. Femoral artery pseudoaneurysm: diagnosis with conventional duplex and color Doppler US. Radiology. 1987;165(3):687-690.
Traction-pin placement is a basic orthopedic skill learned in the early years of residency training. Skeletal traction historically was used as definitive treatment for long-bone fractures, and it is still in use in countries without access to modern medical care.1,2 In current orthopedic practice, proximal tibial and distal femoral traction pins are most commonly used to temporize femoral shaft and acetabular fractures, respectively, before definitive surgical intervention. Although traction-pin placement is common, there are complications that can cause morbidity ranging from skin irritation to death.
In this article, we report on a popliteal artery pseudoaneurysm, a unique complication related to pin placement. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 22-year-old woman with no past medical history was driving a car at 35 miles per hour when she hit a telephone pole. She was wearing a seatbelt. She was taken by ambulance for a trauma evaluation. She sustained a right posterior hip dislocation with an associated Pipkin 2 femoral head fracture (Figures 1A, 1B), a right elbow skin avulsion, and a scalp abrasion.
The patient underwent closed hip reduction under sedation, and a proximal tibial traction pin was placed (Figures 2A, 2B). At our institution, proximal tibial traction pins are placed 2 cm distal and 2 cm posterior to the tibial tubercle without the use of fluoroscopy. The skin leg is prepared and draped in sterile fashion, and a local anesthetic is used to anesthetize the skin and periosteum on the medial and lateral aspects of the leg. A 1.5-cm incision is made laterally, and soft tissue is spread laterally down to the periosteum. Then the traction pin (always the largest nonthreaded pin available) is used to sound the anterior and posterior aspects of the tibia—the goal being to end at the anterior two-thirds/posterior one-third mark. After the pin is advanced through both cortices, the place where the pin will exit the skin is noted, and another incision is made. For this patient, no complications were noted at time of pin placement.
On postinjury day 1, she was taken to the operating room for open reduction and internal fixation of the femoral head fracture through a Smith-Petersen approach. At the same time, a traction pin was removed. Routine postoperative protocols were followed. The patient was started on enoxaparin, was seen by physical therapy on postoperative day 1, and was kept non-weight-bearing on the right lower extremity. Pain was well controlled, and the patient was discharged on postoperative day 3 (postinjury day 4).
Three weeks after surgery, she returned for a scheduled follow-up with complaints of paresthesia and decreased sensation in the right lateral cutaneous femoral nerve distribution and burning pain in the toes. A stiff right ankle was noted. A night splint, amitriptyline, and additional pain medications were ordered.
The patient returned for her scheduled 6-week follow-up reporting she had not taken the enoxaparin because her pharmacy “did not have any.” Her primary care physician had given her “another blood thinner,” which she had taken orally for 1 to 2 weeks. She had complaints of right calf pain, but her hip was pain-free. There was a large amount of calf swelling and tenderness along with mild equinus contracture of the ankle. She was sent to the emergency department (ED) for Doppler ultrasound evaluation for deep venous thrombosis (DVT).
Duplex Doppler imaging of the deep venous system of the lower extremities was performed with visualization from the common femoral veins to the popliteal veins bilaterally. There was normal compressibility, respiratory phasicity, and flow with no intraluminal thrombus. A radiograph of tibia and fibula showed soft-tissue swelling and lucencies from the traction pin but no other abnormalities.
Eight weeks after surgery, the patient returned to the ED because of continuing calf pain. Given that the ultrasound findings had been negative, magnetic resonance imaging (MRI) was ordered, and orthopedic follow-up scheduled. About 10 weeks after surgery, she returned to the ED, still with calf pain, and reported having been unable to go for her MRI because of financial reasons. Physical examination revealed increased calf swelling and a new ecchymosis tracking along the tibia. Repeat ultrasound, performed from the common femoral veins through the popliteal veins, showed a right posterior calf pseudoaneurysm (8.5×4.3×5.3 cm) arising from the popliteal artery about 5 cm below the popliteal fossa. In addition, a large hematoma was seen originating in the upper posterior calf and extending inferiorly (Figure 3).
Computed tomography angiogram confirmed the ultrasound diagnosis of a large (5.6×4.8-cm) right calf pseudoaneurysm arising possibly from a small muscular branch of the proximal tibioperoneal trunk, with a large surrounding hematoma in the posterior compartment of the proximal right calf (Figure 4). Vascular surgery was consulted, and coil embolization of the pseudoaneurysm was performed later that night in the interventional radiology suite.
After the coiling of the pseudoaneurysm, the calf swelling and pain slowly improved. At 12-week orthopedic follow-up, the patient was no longer using any pain medications, and she noted improvement in the right foot’s neuropathic symptoms. Serial casting was prescribed for the equinus contracture of the ankle. She was allowed to start weight-bearing on the right lower extremity. Radiographs at 12 weeks showed collapse of the superomedial aspect of the femoral head with surrounding sclerosis consistent with posttraumatic avascular necrosis.
At final orthopedic follow-up, about 16 weeks after surgery, the patient reported 0/10 pain. Sensation was noted as being intact throughout the right lower extremity but decreased in the tibial nerve distribution. Ankle range of motion was still limited, with 5° of dorsiflexion and 25° of plantar flexion. The hip was pain-free with flexion of 0° to 100°, 10° of internal rotation, and 20° of external rotation. Additional appointments were scheduled, but the patient did not follow up. Two years after initial injury, she returned to the ED for evaluation of rhinorrhea, and no orthopedic complaints were noted.
Discussion
Skeletal traction begins with the insertion of a wire or pin through a bone. It is extremely important to use proper technique in order to minimize the risks associated with pin insertion.3 Potential pitfalls involve the energy transferred into the bone during insertion, the incisions used to place the pin, and injury to surrounding neurovascular structures. For proximal tibial pins, standard technique dictates placing the pin in a lateral-to-medial direction 2 cm posterior to the tibial tubercle and avoiding the dense anterior cortical bone. At our institution, traction pins are placed with a power drill after the patient is given a local anesthetic or is placed under conscious sedation. Which type of anesthesia to use is based on the patient’s overall condition and on the ED attending physician’s willingness to administer conscious sedation.
The 2 most common types of tibial traction involve use of either a large Steinmann pin attached to a metal bow or a Kirschner wire (K-wire) placed under tension before traction. Which to use is the surgeon’s choice. Surgeons at our institution historically have used Steinmann pins. No studies have directly compared fine-wire and Steinmann-pin traction, but with this complication our institution is evaluating a change to tensioned wires. Compared with large Steinmann pins, fine-wire pins create less of a defect in the bone but also bend or break more easily if tension is not applied or if it fails. A fine wire with its smaller surface area may also cut more easily into osteopenic bone than a large-diameter pin would.
Proximal tibial traction typically is indicated for femoral shaft and acetabular fractures. Although the subcutaneous nature of the tibia makes for easier pin placement, the anatomy of the tibia can predispose this bone to complications. Its triangular shape can lead to intracortical rather than the preferred bicortical pin placement. Increased heat caused by intracortical placement can lead to osteonecrosis and even to damage of surrounding soft tissues. Green and Ripley4 found that chronic osteomyelitis typically resulted from intracortical placement of traction pins.
Injury to surrounding soft tissues, either from heat necrosis or from infection introduced through pin sites, can also have consequences. Pin-site infections increase with duration of treatment, though care seldom requires more than pin removal and antibiotics.5,6 More-invasive infections range from cellulitis surrounding the pin site to subcutaneous abscesses. There is 1 report of a Clostridium perfringens infection leading to death only 5 days after pin placement.7
Neurovascular structures are at risk with any orthopedic procedure. With proximal tibial pins in pediatric patients, the peroneal nerve, the anterior tibial artery, and the proximal physis are most at risk. The deep peroneal nerve and the anterior tibial artery run together deep to the anterior compartment, which places them at highest risk with pin insertion. The peroneal and tibial arteries run deep to the deep posterior compartment along with the tibial nerve behind the posterior cortex of the tibia, which makes injury less likely.8
Historically, long-bone fractures were often treated with traction. Kirby and Fitts9 reported on 342 transfixion pins and wires used in the treatment of 233 long-bone fractures between 1943 and 1945. Of the 305 pins/wires observed over the entire treatment period (average, 6 weeks), only 12 (3.93%) developed a complication. There were 4 loose K-wires, 1 broken wire, and 1 bow failure; Steinmann pins were involved in 1 infection and 2 transient peroneal nerve palsies; and 3 Roger Anderson pins loosened. Pin-tract drainage was not included as a complication if it did not also involve localized or general signs of inflammation. The 2 peroneal nerve palsies were associated with medial-to-lateral pin insertion creating a more posterior pin path.
Pins inserted for external fixators of the tibia have injured the anterior tibial vessels and branches of the peroneal and saphenous nerves. A proximal tibial traction pin, in essence a transfixion pin, can cause similar injuries, particularly with imperfect placement (Figure 5).3,10
A pseudoaneurysm is a pulsating, encapsulated hematoma that remains in communication with the lumen of a ruptured or injured vessel. The arterial wall itself is torn or ruptured, and the external wall of the aneurysmal sac consists of outer arterial layers, perivascular tissue, blood clot, or a layer of reactive fibrosis. This contrasts with a true aneurysm, in which all 3 arterial layers (intima, media, adventitia) remain confluent but are dilated beyond their normal diameter. Of all pseudoaneurysms, those caused iatrogenically are the most common and are typically produced by femoral artery catheterization, accounting for 70% to 80% of the incidence.11
Our patient’s injury was most likely caused by an initial error in pin placement before the pin was driven across the tibia. The typical teaching for traction-pin placement involves finding the correct starting point and then using the pin to feel the anterior and posterior surfaces of the bone (described earlier). If the pin slid posteriorly, it may have contacted the artery and caused a small tear that eventually led to the formation of the pseudoaneurysm.
The pseudoaneurysm was not the only complication in the present case. There was also the delay in diagnosis. A standard technique is used to evaluate the lower extremity venous system for DVT. The ultrasonographer starts with the probe as proximal as possible (above the inguinal ligament), ideally proximal to the saphenofemoral junction, and moves distally in 1-cm increments, checking the veins for compressibility, color, and Doppler signal. Unless advised otherwise, the ultrasonographer typically does not examine distal to the knee.12,13 As this patient’s pseudoaneurysm was distal to the knee, it was not found on initial ultrasound, and her inability to obtain her MRI compounded the delay. The second ultrasound identified the pseudoaneurysm. The ultrasonographer examined more distally, given the contrast between the clinical diagnosis of vascular pathology and the negative Doppler study. Computed tomography angiogram confirmed the diagnosis and guided the vascular surgeons in identifying the lesion as a pseudoaneurysm, allowing it to be coiled rather than bypassed.
Duplex ultrasound is the preferred diagnostic modality for imaging pseudoaneurysms. Although our patient’s scan was performed in timely fashion, it did not image the area of pathology. Instead, this patient with multiple orthopedic injuries was scanned for DVT, the most likely cause of her lower extremity swelling. Had a pseudoaneurysm been suspected, the ultrasonographer would have been instructed to image the entire extremity and not just the area where DVT might be found.
Fortunately, despite the treatment delay, the patient recovered well from both the traumatic injuries sustained in the car crash and the likely iatrogenic pseudoaneurysm. Although traction pins are easily and frequently used, they can have complications, which are often preventable. Starting with pin placement itself, there were several opportunities for improving this patient’s care or, at a minimum, reducing the time spent in diagnosis. If the pin had been noticed sliding posteriorly during insertion, extra attention during follow-up visits could have helped identify the injury sooner. Another difficulty in diagnosis was that of obtaining the appropriate outpatient radiology studies which necessitated repeat ED visits. An additional juncture was between the patient’s multiple ED visits for similar complaints. Obtaining advanced imaging sooner could have helped in diagnosing the pseudoaneurysm earlier.
Traction-pin placement is a basic orthopedic skill learned in the early years of residency training. Skeletal traction historically was used as definitive treatment for long-bone fractures, and it is still in use in countries without access to modern medical care.1,2 In current orthopedic practice, proximal tibial and distal femoral traction pins are most commonly used to temporize femoral shaft and acetabular fractures, respectively, before definitive surgical intervention. Although traction-pin placement is common, there are complications that can cause morbidity ranging from skin irritation to death.
In this article, we report on a popliteal artery pseudoaneurysm, a unique complication related to pin placement. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 22-year-old woman with no past medical history was driving a car at 35 miles per hour when she hit a telephone pole. She was wearing a seatbelt. She was taken by ambulance for a trauma evaluation. She sustained a right posterior hip dislocation with an associated Pipkin 2 femoral head fracture (Figures 1A, 1B), a right elbow skin avulsion, and a scalp abrasion.
The patient underwent closed hip reduction under sedation, and a proximal tibial traction pin was placed (Figures 2A, 2B). At our institution, proximal tibial traction pins are placed 2 cm distal and 2 cm posterior to the tibial tubercle without the use of fluoroscopy. The skin leg is prepared and draped in sterile fashion, and a local anesthetic is used to anesthetize the skin and periosteum on the medial and lateral aspects of the leg. A 1.5-cm incision is made laterally, and soft tissue is spread laterally down to the periosteum. Then the traction pin (always the largest nonthreaded pin available) is used to sound the anterior and posterior aspects of the tibia—the goal being to end at the anterior two-thirds/posterior one-third mark. After the pin is advanced through both cortices, the place where the pin will exit the skin is noted, and another incision is made. For this patient, no complications were noted at time of pin placement.
On postinjury day 1, she was taken to the operating room for open reduction and internal fixation of the femoral head fracture through a Smith-Petersen approach. At the same time, a traction pin was removed. Routine postoperative protocols were followed. The patient was started on enoxaparin, was seen by physical therapy on postoperative day 1, and was kept non-weight-bearing on the right lower extremity. Pain was well controlled, and the patient was discharged on postoperative day 3 (postinjury day 4).
Three weeks after surgery, she returned for a scheduled follow-up with complaints of paresthesia and decreased sensation in the right lateral cutaneous femoral nerve distribution and burning pain in the toes. A stiff right ankle was noted. A night splint, amitriptyline, and additional pain medications were ordered.
The patient returned for her scheduled 6-week follow-up reporting she had not taken the enoxaparin because her pharmacy “did not have any.” Her primary care physician had given her “another blood thinner,” which she had taken orally for 1 to 2 weeks. She had complaints of right calf pain, but her hip was pain-free. There was a large amount of calf swelling and tenderness along with mild equinus contracture of the ankle. She was sent to the emergency department (ED) for Doppler ultrasound evaluation for deep venous thrombosis (DVT).
Duplex Doppler imaging of the deep venous system of the lower extremities was performed with visualization from the common femoral veins to the popliteal veins bilaterally. There was normal compressibility, respiratory phasicity, and flow with no intraluminal thrombus. A radiograph of tibia and fibula showed soft-tissue swelling and lucencies from the traction pin but no other abnormalities.
Eight weeks after surgery, the patient returned to the ED because of continuing calf pain. Given that the ultrasound findings had been negative, magnetic resonance imaging (MRI) was ordered, and orthopedic follow-up scheduled. About 10 weeks after surgery, she returned to the ED, still with calf pain, and reported having been unable to go for her MRI because of financial reasons. Physical examination revealed increased calf swelling and a new ecchymosis tracking along the tibia. Repeat ultrasound, performed from the common femoral veins through the popliteal veins, showed a right posterior calf pseudoaneurysm (8.5×4.3×5.3 cm) arising from the popliteal artery about 5 cm below the popliteal fossa. In addition, a large hematoma was seen originating in the upper posterior calf and extending inferiorly (Figure 3).
Computed tomography angiogram confirmed the ultrasound diagnosis of a large (5.6×4.8-cm) right calf pseudoaneurysm arising possibly from a small muscular branch of the proximal tibioperoneal trunk, with a large surrounding hematoma in the posterior compartment of the proximal right calf (Figure 4). Vascular surgery was consulted, and coil embolization of the pseudoaneurysm was performed later that night in the interventional radiology suite.
After the coiling of the pseudoaneurysm, the calf swelling and pain slowly improved. At 12-week orthopedic follow-up, the patient was no longer using any pain medications, and she noted improvement in the right foot’s neuropathic symptoms. Serial casting was prescribed for the equinus contracture of the ankle. She was allowed to start weight-bearing on the right lower extremity. Radiographs at 12 weeks showed collapse of the superomedial aspect of the femoral head with surrounding sclerosis consistent with posttraumatic avascular necrosis.
At final orthopedic follow-up, about 16 weeks after surgery, the patient reported 0/10 pain. Sensation was noted as being intact throughout the right lower extremity but decreased in the tibial nerve distribution. Ankle range of motion was still limited, with 5° of dorsiflexion and 25° of plantar flexion. The hip was pain-free with flexion of 0° to 100°, 10° of internal rotation, and 20° of external rotation. Additional appointments were scheduled, but the patient did not follow up. Two years after initial injury, she returned to the ED for evaluation of rhinorrhea, and no orthopedic complaints were noted.
Discussion
Skeletal traction begins with the insertion of a wire or pin through a bone. It is extremely important to use proper technique in order to minimize the risks associated with pin insertion.3 Potential pitfalls involve the energy transferred into the bone during insertion, the incisions used to place the pin, and injury to surrounding neurovascular structures. For proximal tibial pins, standard technique dictates placing the pin in a lateral-to-medial direction 2 cm posterior to the tibial tubercle and avoiding the dense anterior cortical bone. At our institution, traction pins are placed with a power drill after the patient is given a local anesthetic or is placed under conscious sedation. Which type of anesthesia to use is based on the patient’s overall condition and on the ED attending physician’s willingness to administer conscious sedation.
The 2 most common types of tibial traction involve use of either a large Steinmann pin attached to a metal bow or a Kirschner wire (K-wire) placed under tension before traction. Which to use is the surgeon’s choice. Surgeons at our institution historically have used Steinmann pins. No studies have directly compared fine-wire and Steinmann-pin traction, but with this complication our institution is evaluating a change to tensioned wires. Compared with large Steinmann pins, fine-wire pins create less of a defect in the bone but also bend or break more easily if tension is not applied or if it fails. A fine wire with its smaller surface area may also cut more easily into osteopenic bone than a large-diameter pin would.
Proximal tibial traction typically is indicated for femoral shaft and acetabular fractures. Although the subcutaneous nature of the tibia makes for easier pin placement, the anatomy of the tibia can predispose this bone to complications. Its triangular shape can lead to intracortical rather than the preferred bicortical pin placement. Increased heat caused by intracortical placement can lead to osteonecrosis and even to damage of surrounding soft tissues. Green and Ripley4 found that chronic osteomyelitis typically resulted from intracortical placement of traction pins.
Injury to surrounding soft tissues, either from heat necrosis or from infection introduced through pin sites, can also have consequences. Pin-site infections increase with duration of treatment, though care seldom requires more than pin removal and antibiotics.5,6 More-invasive infections range from cellulitis surrounding the pin site to subcutaneous abscesses. There is 1 report of a Clostridium perfringens infection leading to death only 5 days after pin placement.7
Neurovascular structures are at risk with any orthopedic procedure. With proximal tibial pins in pediatric patients, the peroneal nerve, the anterior tibial artery, and the proximal physis are most at risk. The deep peroneal nerve and the anterior tibial artery run together deep to the anterior compartment, which places them at highest risk with pin insertion. The peroneal and tibial arteries run deep to the deep posterior compartment along with the tibial nerve behind the posterior cortex of the tibia, which makes injury less likely.8
Historically, long-bone fractures were often treated with traction. Kirby and Fitts9 reported on 342 transfixion pins and wires used in the treatment of 233 long-bone fractures between 1943 and 1945. Of the 305 pins/wires observed over the entire treatment period (average, 6 weeks), only 12 (3.93%) developed a complication. There were 4 loose K-wires, 1 broken wire, and 1 bow failure; Steinmann pins were involved in 1 infection and 2 transient peroneal nerve palsies; and 3 Roger Anderson pins loosened. Pin-tract drainage was not included as a complication if it did not also involve localized or general signs of inflammation. The 2 peroneal nerve palsies were associated with medial-to-lateral pin insertion creating a more posterior pin path.
Pins inserted for external fixators of the tibia have injured the anterior tibial vessels and branches of the peroneal and saphenous nerves. A proximal tibial traction pin, in essence a transfixion pin, can cause similar injuries, particularly with imperfect placement (Figure 5).3,10
A pseudoaneurysm is a pulsating, encapsulated hematoma that remains in communication with the lumen of a ruptured or injured vessel. The arterial wall itself is torn or ruptured, and the external wall of the aneurysmal sac consists of outer arterial layers, perivascular tissue, blood clot, or a layer of reactive fibrosis. This contrasts with a true aneurysm, in which all 3 arterial layers (intima, media, adventitia) remain confluent but are dilated beyond their normal diameter. Of all pseudoaneurysms, those caused iatrogenically are the most common and are typically produced by femoral artery catheterization, accounting for 70% to 80% of the incidence.11
Our patient’s injury was most likely caused by an initial error in pin placement before the pin was driven across the tibia. The typical teaching for traction-pin placement involves finding the correct starting point and then using the pin to feel the anterior and posterior surfaces of the bone (described earlier). If the pin slid posteriorly, it may have contacted the artery and caused a small tear that eventually led to the formation of the pseudoaneurysm.
The pseudoaneurysm was not the only complication in the present case. There was also the delay in diagnosis. A standard technique is used to evaluate the lower extremity venous system for DVT. The ultrasonographer starts with the probe as proximal as possible (above the inguinal ligament), ideally proximal to the saphenofemoral junction, and moves distally in 1-cm increments, checking the veins for compressibility, color, and Doppler signal. Unless advised otherwise, the ultrasonographer typically does not examine distal to the knee.12,13 As this patient’s pseudoaneurysm was distal to the knee, it was not found on initial ultrasound, and her inability to obtain her MRI compounded the delay. The second ultrasound identified the pseudoaneurysm. The ultrasonographer examined more distally, given the contrast between the clinical diagnosis of vascular pathology and the negative Doppler study. Computed tomography angiogram confirmed the diagnosis and guided the vascular surgeons in identifying the lesion as a pseudoaneurysm, allowing it to be coiled rather than bypassed.
Duplex ultrasound is the preferred diagnostic modality for imaging pseudoaneurysms. Although our patient’s scan was performed in timely fashion, it did not image the area of pathology. Instead, this patient with multiple orthopedic injuries was scanned for DVT, the most likely cause of her lower extremity swelling. Had a pseudoaneurysm been suspected, the ultrasonographer would have been instructed to image the entire extremity and not just the area where DVT might be found.
Fortunately, despite the treatment delay, the patient recovered well from both the traumatic injuries sustained in the car crash and the likely iatrogenic pseudoaneurysm. Although traction pins are easily and frequently used, they can have complications, which are often preventable. Starting with pin placement itself, there were several opportunities for improving this patient’s care or, at a minimum, reducing the time spent in diagnosis. If the pin had been noticed sliding posteriorly during insertion, extra attention during follow-up visits could have helped identify the injury sooner. Another difficulty in diagnosis was that of obtaining the appropriate outpatient radiology studies which necessitated repeat ED visits. An additional juncture was between the patient’s multiple ED visits for similar complaints. Obtaining advanced imaging sooner could have helped in diagnosing the pseudoaneurysm earlier.
1. Gosselin RA, Heitto M, Zirkle L. Cost-effectiveness of replacing skeletal traction by interlocked intramedullary nailing for femoral shaft fractures in a provincial trauma hospital in Cambodia. Int Orthop. 2009;33(5):1445-1448.
2. Gosselin R, Lavaly D. Perkins traction for adult femoral shaft fractures: a report on 53 patients in Sierra Leone. Int Orthop. 2007;31(5):697-702.
3. Althausen PL, Hak DJ. Lower extremity traction pins: indications, technique, and complications. Am J Orthop. 2002;31(1):43-47.
4. Green SA, Ripley MJ. Chronic osteomyelitis in pin tracks. J Bone Joint Surg Am. 1984;66(7):1092-1098.
5. Nigam V, Jaiswal A, Dhaon BK. Local antibiotics: panacea for long term skeletal traction. Injury. 2005;36(1):199-202.
6. Lethaby A, Temple J, Santy J. Pin site care for preventing infections associated with external bone fixators and pins. Cochrane Database Syst Rev. 2008;(4):CD004551.
7. Taylor BC, Bramwell TJ, Formaini N. Gas gangrene as a result of femoral traction pin placement. Case Rep Orthop. 2011;(2011):459812.
8. Moskovich R. Proximal tibial transfixion for skeletal traction. An anatomic study of neurovascular structures. Clin Orthop. 1987;(214):264-268.
9. Kirby CK, Fitts WT. The incidence of complications in the use of transfixion pins and wires for skeletal traction. Ann Surg. 1946;123(1):27-31.
10. Behrens F, Searls K. External fixation of the tibia. Basic concepts and prospective evaluation. J Bone Joint Surg Br. 1986;68(2):246-254.
11. Sueyoshi E, Sakamoto I, Nakashima K, Minami K, Hayashi K. Visceral and peripheral arterial pseudoaneurysms. AJR Am J Roentgenol. 2005;185(3):741-749.
12. Scoutt LM, Zawin ML, Taylor KJ. Doppler US. Part II. Clinical applications. Radiology. 1990;174(2):309-319.
13. Mitchell DG, Needleman L, Bezzi M, et al. Femoral artery pseudoaneurysm: diagnosis with conventional duplex and color Doppler US. Radiology. 1987;165(3):687-690.
1. Gosselin RA, Heitto M, Zirkle L. Cost-effectiveness of replacing skeletal traction by interlocked intramedullary nailing for femoral shaft fractures in a provincial trauma hospital in Cambodia. Int Orthop. 2009;33(5):1445-1448.
2. Gosselin R, Lavaly D. Perkins traction for adult femoral shaft fractures: a report on 53 patients in Sierra Leone. Int Orthop. 2007;31(5):697-702.
3. Althausen PL, Hak DJ. Lower extremity traction pins: indications, technique, and complications. Am J Orthop. 2002;31(1):43-47.
4. Green SA, Ripley MJ. Chronic osteomyelitis in pin tracks. J Bone Joint Surg Am. 1984;66(7):1092-1098.
5. Nigam V, Jaiswal A, Dhaon BK. Local antibiotics: panacea for long term skeletal traction. Injury. 2005;36(1):199-202.
6. Lethaby A, Temple J, Santy J. Pin site care for preventing infections associated with external bone fixators and pins. Cochrane Database Syst Rev. 2008;(4):CD004551.
7. Taylor BC, Bramwell TJ, Formaini N. Gas gangrene as a result of femoral traction pin placement. Case Rep Orthop. 2011;(2011):459812.
8. Moskovich R. Proximal tibial transfixion for skeletal traction. An anatomic study of neurovascular structures. Clin Orthop. 1987;(214):264-268.
9. Kirby CK, Fitts WT. The incidence of complications in the use of transfixion pins and wires for skeletal traction. Ann Surg. 1946;123(1):27-31.
10. Behrens F, Searls K. External fixation of the tibia. Basic concepts and prospective evaluation. J Bone Joint Surg Br. 1986;68(2):246-254.
11. Sueyoshi E, Sakamoto I, Nakashima K, Minami K, Hayashi K. Visceral and peripheral arterial pseudoaneurysms. AJR Am J Roentgenol. 2005;185(3):741-749.
12. Scoutt LM, Zawin ML, Taylor KJ. Doppler US. Part II. Clinical applications. Radiology. 1990;174(2):309-319.
13. Mitchell DG, Needleman L, Bezzi M, et al. Femoral artery pseudoaneurysm: diagnosis with conventional duplex and color Doppler US. Radiology. 1987;165(3):687-690.
Recurrent Patellar Tendon Rupture in a Patient After Intramedullary Nailing of the Tibia: Reconstruction Using an Achilles Tendon Allograft
Ruptures of the patellar tendon usually occur in patients under age 40 years, with men having a higher incidence than women.1 History of local steroid injection,2,3 total knee arthroplasty,4-8 anterior cruciate ligament reconstruction with central third patellar tendon autograft,9-11 and a variety of systemic diseases are associated with an increased tendency to rupture.12-15 Primary acute ruptures of the patellar tendon can be difficult to repair because of the quality of remaining tissues. In cases of chronic tendon ruptures subject to delayed treatment, additional complications such as tissue contracture and scar-tissue formation are likely to exist.15-17
Complications after intramedullary (IM) nailing of the tibia include infection, compartment syndrome, deep vein thrombosis, thermal necrosis of the bone with alteration of its endosteal architecture, failure of the hardware, malunion, and nonunion.18 The most common complaint after IM nailing of the tibia is chronic anterior knee pain and symptoms similar to tendonitis; incidences as high as 86% have been reported.18-20 Extensive review of the literature found only 2 reports of patellar tendon rupture after IM nailing of the tibia; both cases used a patellar tendon–splitting approach. The first report described patellar tendon rupture 8 years after IM nailing of the tibia during a forced deep-flexion movement.21 Radiographic examination showed the IM nail positioned proud relative to the tibial plateau, impinging upon the patellar tendon. An intraoperative examination confirmed the radiographic findings and found rupture of the patellar tendon to be consistent with the exposed tip of the IM nail. The second report described patellar tendon rupture 2 months postoperatively in a patient with Ehlers-Danlos syndrome, a hereditary disorder characterized by alterations to muscle/tendon tissue and hyperextensible skin.22
Patellar tendon rupture after IM nailing of the tibia is a rare complication. Patellar tendon re-rupture after primary repair in a patient with history of IM tibial nailing has not been reported. This case outlines the progression of such a patient with a recurrent patellar tendon rupture that was successfully reconstructed using an Achilles tendon allograft. The patient’s surgical history of IM tibial nailing through a mid-patellar tendon–splitting approach 4 years prior to initial tendon rupture is noteworthy and potentially predisposed the patient to injury. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 44-year-old woman, 5 ft, 3 in tall, and weighing 129 lb (body mass index, 22.8), with a history of osteoporosis and transverse myelitis, presented with pain and persistent swelling about the left knee. Her baseline ambulatory status required crutches because of decreased sensation and strength in her lower extremity in conjunction with a foot drop; she had mild quadriceps and hamstring muscle weakness but otherwise normal knee function. The patient had been seen 4 years earlier at our facility for IM fixation of a distal tibia fracture through a patellar tendon–splitting approach. The fracture was well healed and showed no signs of complication or nail migration; the nail was not proud.
Initially, the patient was admitted to another hospital through the emergency department for swelling and pain about the left knee. She was believed to have an infection and was placed on antibiotics by the primary care team. An orthopedic evaluation showed induration, edema, and warmth in the patellar tendon region of the left knee. Magnetic resonance imaging (MRI) showed a full-thickness patellar tendon rupture. Aspiration of the knee was performed and cultures were negative; white blood cell, erythrocyte sedimentation rate, and C-reactive protein values were normal. The risks and benefits of various treatments were discussed, and surgical intervention was elected to repair the patellar tendon.
Intraoperative findings showed a massive midsubstance rupture of the patellar tendon, accompanied by medial and lateral retinacular tears and a quadriceps tendon partial rupture; the central aspect of the quadriceps tendon attaching to the patella remained intact. The patella was retracted proximally; no evidence of active infection was present. Good-quality tissue remained attached to both the tibial tuberosity and the inferior pole of the patella. A No. 2 FiberWire suture (Arthrex, Inc, Naples, Florida) was used to run whip stitches in the distal end of the patellar tendon and a second No. 2 FiberWire suture was used to run whip stitches in the proximal aspect of the patellar tendon rupture. The 4 ends of the sutures were tied together, thus re-approximating the distal and proximal ends of the ruptured patellar tendon. No bone drilling was used because the midsubstance tear was amenable to good repair with reasonable expectation of healing based on tissue quality. The quadriceps tendon, which was partially torn, was repaired with a No. 1 Vicryl suture (Ethicon, Somerville, New Jersey). The medial and lateral retinacula were also repaired with a No. 1 Vicryl suture. The suturing scheme effectively re-approximated the knee extensor mechanism, and the patient was placed in a knee immobilizer that permitted no flexion for 6 weeks postoperatively.
After 3 months of gradual improvement with physical therapy, the patient returned for a follow-up visit, concerned that her knee function was beginning to decline. Physical examination showed patella alta with a thinned and diminutive palpable tendon in the patellar tendon region. She was capable of active flexion to 90º and extension to 50º, but beyond 50º, she was unable to actively extend; she was capable of full passive extension. MRI showed a repeat full-thickness patellar tendon tear with retraction from the inferior pole of the patella; previous tears to the quadriceps tendon were healed. Because of the recurrent nature of the injury, the patient’s physical examination, MRI findings, and anticipated poor quality of remaining tendon tissue, patellar tendon reconstruction using a cadaveric Achilles tendon allograft was recommended. The patient chose surgery for potential improvement in knee range of motion, active extension, and ambulation.
The previous anterior midline incision was used and carried down through the subcutaneous tissues where a complete rupture of the patellar tendon was identified. A limited amount of good-quality tendon tissue remained at the medial aspect of the tibial tuberosity. The remaining tissue located at the patella’s inferior pole was nonviable for use in surgical repair. Retinacular contractures were released to bring the patella distally; the trochlear groove was used as the anatomic landmark for the patella resting position. During reconstruction, the knee was placed into 30° of flexion, with the patella located in the trochlear groove, and the cadaveric Achilles tendon was placed on the midline of the patella, where measurements were done to assess proper length and tension (Figure 1).
The patient’s remaining native tissue on the medial aspect of the tibial tuberosity was used to augment the Achilles tendon graft medially. The cadaveric Achilles tendon graft was primarily used to replace the central and lateral aspects of the patellar tendon. Additionally, the calcaneal bone segment at the end of the Achilles tendon graft was removed prior to use. Cadaveric and host tissues at the medial aspect of the tibial tuberosity were sutured together with a No. 1 Vicryl suture (Figure 2). The distal aspect of the cadaveric Achilles tendon was used to re-approximate the patient’s native patellar tendon insertion at the tibial tuberosity. To supplement the graft anchor, a Richards metallic ligament staple (Smith & Nephew, Memphis, Tennessee) was used to fix the distal aspect of the Achilles tendon graft into the tibial tuberosity.
Proper tensioning of the graft was performed by visualizing patella tracking during the arc-of-knee motion and properly suturing the graft to allow for functional range. The proximal aspect of the cadaveric Achilles tendon was sutured into host tissues surrounding the superior pole of the patellar and quadriceps tendon. The edges of the graft were sutured with supplemental No. 1 Vicryl sutures (Figure 3).
Before surgical closure, knee range of motion was checked and noted to be 0º to 100º. The repaired construct was stable and uncompromised throughout the entire range of motion. Patella tracking was central and significantly improved; knee stability was normal to varus and valgus stress.
The patient was placed in a knee immobilizer for 6 weeks before range of motion was allowed. Seven months postoperatively, the patient returned for a follow-up visit, ambulating with 2 forearm crutches, which was her baseline ambulatory status. Physical examination revealed passive range of motion from 0º to 130º, an extension lag of 10º, and 4/5 quadriceps strength. It was recommended the patient continue physical therapy to improve strength and range of motion.
Conclusion
This is the first report in the literature documenting a recurrent patellar tendon rupture after primary repair in a patient with a history of IM tibial nailing. It is also the first report of a cadaveric Achilles tendon allograft used as a solution to this problem. Complete reconstruction of the patellar tendon using an Achilles tendon allograft is a method commonly used for ruptures after total knee arthroplasty.4-7,23,24 This case report highlights the utility of a cadaveric Achilles tendon in the setting of a recurrent patellar tendon rupture with poor remaining tissue quality.
1. Scott WN, Insall JN. Injuries of the knee. In: Rockwood CA Jr, Green DP, Bucholz RW, eds. Fractures in Adults. 3rd ed. Philadelphia, PA: JB Lippincott; 1991: 1799-1914.
2. Clark SC, Jones MW, Choudhury RR, Smith E. Bilateral patellar tendon rupture secondary to repeated local steroid injections. J Accid Emerg Med. 1995;12(4):300-301.
3. Unverferth LJ, Olix ML. The effect of local steroid injections on tendon. J Sports Med. 1973;1(4):31-37.
4. Cadambi A, Engh GA. Use of a semitendinosus tendon autogenous graft for rupture of the patellar ligament after total knee arthroplasty. A report of seven cases. J Bone Joint Surg Am. 1992;74(7):974-979.
5. Emerson RH Jr, Head WC, Malinin TI. Reconstruction of patellar tendon rupture after total knee arthroplasty with an extensor mechanism allograft. Clin Orthop.1990;(260):154-161.
6. Gustillo RB, Thompson R. Quadriceps and patellar tendon ruptures following total knee arthroplasty. In: Rand JA, Dorr LD, eds. Total Arthroplasty of the Knee: Proceedings of the Knee Society, 1985-1986. Rockville, MD: Aspen; 1987: 41-70.
7. Rand JA, Morrey BF, Bryan RS. Patellar tendon rupture after total knee arthroplasty. Clin Orthop. 1989;(244):233-238.
8. Schoderbek RJ, Brown TE, Mulhall KJ, et al. Extensor mechanism disruption after total knee arthroplasty. Clin Orthop. 2006;446:176-185.
9. Bonamo JJ, Krinik RM, Sporn AA. Rupture of the patellar ligament after use of the central third for anterior cruciate reconstruction. A report of two cases. J Bone Joint Surg Am. 1984;66(8):1294-1297.
10. Marumoto JM, Mitsunaga MM, Richardson AB, Medoff RJ, Mayfield GW. Late patellar tendon ruptures after removal of the central third for anterior cruciate ligament reconstruction. A report of two cases. Am J Sports Med. 1996;24(5):698-701.
11. Mickelsen PL, Morgan SJ, Johnson WA, Ferrari JD. Patellar tendon rupture 3 years after anterior cruciate ligament reconstruction with a central one third bone-patellar tendon-bone graft. Arthroscopy. 2001;17(6):648-652.
12. Morgan J, McCarty DJ. Tendon ruptures in patients with systemic lupus erythematosus treated with corticosteroids. Arthritis Rheum. 1974;17(6):1033-1036.
13. Webb LX, Toby EB. Bilateral rupture of the patellar tendon in an otherwise healthy male patient following minor trauma. J Trauma. 1986;26(11):1045-1048.
14. Greis PE, Holmstrom MC, Lahav A. Surgical treatment options for patella tendon rupture, Part I: Acute. Orthopedics. 2005;28(7):672-679.
15. Greis PE, Lahav A, Holstrom MC. Surgical treatment options for patella tendon rupture, part II: chronic. Orthopedics. 2005;28(8):765-769.
16. Lewis PB, Rue JP, Bach BR Jr. Chronic patellar tendon rupture: surgical reconstruction technique using 2 Achilles tendon allografts. J Knee Surg. 2008;21(12):130-135.
17. McNally PD, Marcelli EA. Achilles tendon allograft of a chronic patellar tendon rupture. Arthroscopy. 1998;14(3):340-344.
18. Katsoulis E, Court-Brown C, Giannoudis PV. Incidence and atieology of anterior knee pain after intramedullary nailing of the femur and tibia. J Bone Joint Surg Br. 2006;88(5):576-580.
19. Brumback RJ, Uwagie-Ero S, Lakatos RP, et al. Intramedullary nailing of femoral shaft fractures. Part II: Fracture-healing with static interlocking fixation. J Bone Joint Surg Am. 1988;70(1):1453-1462.
20. Koval KJ, Clapper MF, Brumback RJ, et al. Complications of reamed intramedullary nailing of the tibia. J Orthop Trauma. 1991;5(2):184-189.
21. Kretzler JE, Curtin SL, Wegner DA, Baumgaertner MR, Galloway MT. Patella tendon rupture: a late complication of a tibial nail. Orthopedics. 1995;18(11):1109-1111.
22. Moroney P, McCarthy T, Borton D. Patellar tendon rupture post reamed intra-medullary tibial nail in a patient with Ehlers-Danlos syndrome. A case report. Eur J Orthop Surg Traumatol. 2004;14(1):50-51.
23. Crossett LS, Sinha RK, Sechriest VF, Rubash HE. Reconstruction of a ruptured patellar tendon with achilles tendon allograft following total knee arthroplasty. J Bone Joint Surg Am. 2002;84(8):1354-1361.
24. Falconiero RP, Pallis MP. Chronic rupture of a patellar tendon: a technique for reconstruction with Achilles allograft. Arthroscopy. 1996;12(5):623-626.
Ruptures of the patellar tendon usually occur in patients under age 40 years, with men having a higher incidence than women.1 History of local steroid injection,2,3 total knee arthroplasty,4-8 anterior cruciate ligament reconstruction with central third patellar tendon autograft,9-11 and a variety of systemic diseases are associated with an increased tendency to rupture.12-15 Primary acute ruptures of the patellar tendon can be difficult to repair because of the quality of remaining tissues. In cases of chronic tendon ruptures subject to delayed treatment, additional complications such as tissue contracture and scar-tissue formation are likely to exist.15-17
Complications after intramedullary (IM) nailing of the tibia include infection, compartment syndrome, deep vein thrombosis, thermal necrosis of the bone with alteration of its endosteal architecture, failure of the hardware, malunion, and nonunion.18 The most common complaint after IM nailing of the tibia is chronic anterior knee pain and symptoms similar to tendonitis; incidences as high as 86% have been reported.18-20 Extensive review of the literature found only 2 reports of patellar tendon rupture after IM nailing of the tibia; both cases used a patellar tendon–splitting approach. The first report described patellar tendon rupture 8 years after IM nailing of the tibia during a forced deep-flexion movement.21 Radiographic examination showed the IM nail positioned proud relative to the tibial plateau, impinging upon the patellar tendon. An intraoperative examination confirmed the radiographic findings and found rupture of the patellar tendon to be consistent with the exposed tip of the IM nail. The second report described patellar tendon rupture 2 months postoperatively in a patient with Ehlers-Danlos syndrome, a hereditary disorder characterized by alterations to muscle/tendon tissue and hyperextensible skin.22
Patellar tendon rupture after IM nailing of the tibia is a rare complication. Patellar tendon re-rupture after primary repair in a patient with history of IM tibial nailing has not been reported. This case outlines the progression of such a patient with a recurrent patellar tendon rupture that was successfully reconstructed using an Achilles tendon allograft. The patient’s surgical history of IM tibial nailing through a mid-patellar tendon–splitting approach 4 years prior to initial tendon rupture is noteworthy and potentially predisposed the patient to injury. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 44-year-old woman, 5 ft, 3 in tall, and weighing 129 lb (body mass index, 22.8), with a history of osteoporosis and transverse myelitis, presented with pain and persistent swelling about the left knee. Her baseline ambulatory status required crutches because of decreased sensation and strength in her lower extremity in conjunction with a foot drop; she had mild quadriceps and hamstring muscle weakness but otherwise normal knee function. The patient had been seen 4 years earlier at our facility for IM fixation of a distal tibia fracture through a patellar tendon–splitting approach. The fracture was well healed and showed no signs of complication or nail migration; the nail was not proud.
Initially, the patient was admitted to another hospital through the emergency department for swelling and pain about the left knee. She was believed to have an infection and was placed on antibiotics by the primary care team. An orthopedic evaluation showed induration, edema, and warmth in the patellar tendon region of the left knee. Magnetic resonance imaging (MRI) showed a full-thickness patellar tendon rupture. Aspiration of the knee was performed and cultures were negative; white blood cell, erythrocyte sedimentation rate, and C-reactive protein values were normal. The risks and benefits of various treatments were discussed, and surgical intervention was elected to repair the patellar tendon.
Intraoperative findings showed a massive midsubstance rupture of the patellar tendon, accompanied by medial and lateral retinacular tears and a quadriceps tendon partial rupture; the central aspect of the quadriceps tendon attaching to the patella remained intact. The patella was retracted proximally; no evidence of active infection was present. Good-quality tissue remained attached to both the tibial tuberosity and the inferior pole of the patella. A No. 2 FiberWire suture (Arthrex, Inc, Naples, Florida) was used to run whip stitches in the distal end of the patellar tendon and a second No. 2 FiberWire suture was used to run whip stitches in the proximal aspect of the patellar tendon rupture. The 4 ends of the sutures were tied together, thus re-approximating the distal and proximal ends of the ruptured patellar tendon. No bone drilling was used because the midsubstance tear was amenable to good repair with reasonable expectation of healing based on tissue quality. The quadriceps tendon, which was partially torn, was repaired with a No. 1 Vicryl suture (Ethicon, Somerville, New Jersey). The medial and lateral retinacula were also repaired with a No. 1 Vicryl suture. The suturing scheme effectively re-approximated the knee extensor mechanism, and the patient was placed in a knee immobilizer that permitted no flexion for 6 weeks postoperatively.
After 3 months of gradual improvement with physical therapy, the patient returned for a follow-up visit, concerned that her knee function was beginning to decline. Physical examination showed patella alta with a thinned and diminutive palpable tendon in the patellar tendon region. She was capable of active flexion to 90º and extension to 50º, but beyond 50º, she was unable to actively extend; she was capable of full passive extension. MRI showed a repeat full-thickness patellar tendon tear with retraction from the inferior pole of the patella; previous tears to the quadriceps tendon were healed. Because of the recurrent nature of the injury, the patient’s physical examination, MRI findings, and anticipated poor quality of remaining tendon tissue, patellar tendon reconstruction using a cadaveric Achilles tendon allograft was recommended. The patient chose surgery for potential improvement in knee range of motion, active extension, and ambulation.
The previous anterior midline incision was used and carried down through the subcutaneous tissues where a complete rupture of the patellar tendon was identified. A limited amount of good-quality tendon tissue remained at the medial aspect of the tibial tuberosity. The remaining tissue located at the patella’s inferior pole was nonviable for use in surgical repair. Retinacular contractures were released to bring the patella distally; the trochlear groove was used as the anatomic landmark for the patella resting position. During reconstruction, the knee was placed into 30° of flexion, with the patella located in the trochlear groove, and the cadaveric Achilles tendon was placed on the midline of the patella, where measurements were done to assess proper length and tension (Figure 1).
The patient’s remaining native tissue on the medial aspect of the tibial tuberosity was used to augment the Achilles tendon graft medially. The cadaveric Achilles tendon graft was primarily used to replace the central and lateral aspects of the patellar tendon. Additionally, the calcaneal bone segment at the end of the Achilles tendon graft was removed prior to use. Cadaveric and host tissues at the medial aspect of the tibial tuberosity were sutured together with a No. 1 Vicryl suture (Figure 2). The distal aspect of the cadaveric Achilles tendon was used to re-approximate the patient’s native patellar tendon insertion at the tibial tuberosity. To supplement the graft anchor, a Richards metallic ligament staple (Smith & Nephew, Memphis, Tennessee) was used to fix the distal aspect of the Achilles tendon graft into the tibial tuberosity.
Proper tensioning of the graft was performed by visualizing patella tracking during the arc-of-knee motion and properly suturing the graft to allow for functional range. The proximal aspect of the cadaveric Achilles tendon was sutured into host tissues surrounding the superior pole of the patellar and quadriceps tendon. The edges of the graft were sutured with supplemental No. 1 Vicryl sutures (Figure 3).
Before surgical closure, knee range of motion was checked and noted to be 0º to 100º. The repaired construct was stable and uncompromised throughout the entire range of motion. Patella tracking was central and significantly improved; knee stability was normal to varus and valgus stress.
The patient was placed in a knee immobilizer for 6 weeks before range of motion was allowed. Seven months postoperatively, the patient returned for a follow-up visit, ambulating with 2 forearm crutches, which was her baseline ambulatory status. Physical examination revealed passive range of motion from 0º to 130º, an extension lag of 10º, and 4/5 quadriceps strength. It was recommended the patient continue physical therapy to improve strength and range of motion.
Conclusion
This is the first report in the literature documenting a recurrent patellar tendon rupture after primary repair in a patient with a history of IM tibial nailing. It is also the first report of a cadaveric Achilles tendon allograft used as a solution to this problem. Complete reconstruction of the patellar tendon using an Achilles tendon allograft is a method commonly used for ruptures after total knee arthroplasty.4-7,23,24 This case report highlights the utility of a cadaveric Achilles tendon in the setting of a recurrent patellar tendon rupture with poor remaining tissue quality.
Ruptures of the patellar tendon usually occur in patients under age 40 years, with men having a higher incidence than women.1 History of local steroid injection,2,3 total knee arthroplasty,4-8 anterior cruciate ligament reconstruction with central third patellar tendon autograft,9-11 and a variety of systemic diseases are associated with an increased tendency to rupture.12-15 Primary acute ruptures of the patellar tendon can be difficult to repair because of the quality of remaining tissues. In cases of chronic tendon ruptures subject to delayed treatment, additional complications such as tissue contracture and scar-tissue formation are likely to exist.15-17
Complications after intramedullary (IM) nailing of the tibia include infection, compartment syndrome, deep vein thrombosis, thermal necrosis of the bone with alteration of its endosteal architecture, failure of the hardware, malunion, and nonunion.18 The most common complaint after IM nailing of the tibia is chronic anterior knee pain and symptoms similar to tendonitis; incidences as high as 86% have been reported.18-20 Extensive review of the literature found only 2 reports of patellar tendon rupture after IM nailing of the tibia; both cases used a patellar tendon–splitting approach. The first report described patellar tendon rupture 8 years after IM nailing of the tibia during a forced deep-flexion movement.21 Radiographic examination showed the IM nail positioned proud relative to the tibial plateau, impinging upon the patellar tendon. An intraoperative examination confirmed the radiographic findings and found rupture of the patellar tendon to be consistent with the exposed tip of the IM nail. The second report described patellar tendon rupture 2 months postoperatively in a patient with Ehlers-Danlos syndrome, a hereditary disorder characterized by alterations to muscle/tendon tissue and hyperextensible skin.22
Patellar tendon rupture after IM nailing of the tibia is a rare complication. Patellar tendon re-rupture after primary repair in a patient with history of IM tibial nailing has not been reported. This case outlines the progression of such a patient with a recurrent patellar tendon rupture that was successfully reconstructed using an Achilles tendon allograft. The patient’s surgical history of IM tibial nailing through a mid-patellar tendon–splitting approach 4 years prior to initial tendon rupture is noteworthy and potentially predisposed the patient to injury. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 44-year-old woman, 5 ft, 3 in tall, and weighing 129 lb (body mass index, 22.8), with a history of osteoporosis and transverse myelitis, presented with pain and persistent swelling about the left knee. Her baseline ambulatory status required crutches because of decreased sensation and strength in her lower extremity in conjunction with a foot drop; she had mild quadriceps and hamstring muscle weakness but otherwise normal knee function. The patient had been seen 4 years earlier at our facility for IM fixation of a distal tibia fracture through a patellar tendon–splitting approach. The fracture was well healed and showed no signs of complication or nail migration; the nail was not proud.
Initially, the patient was admitted to another hospital through the emergency department for swelling and pain about the left knee. She was believed to have an infection and was placed on antibiotics by the primary care team. An orthopedic evaluation showed induration, edema, and warmth in the patellar tendon region of the left knee. Magnetic resonance imaging (MRI) showed a full-thickness patellar tendon rupture. Aspiration of the knee was performed and cultures were negative; white blood cell, erythrocyte sedimentation rate, and C-reactive protein values were normal. The risks and benefits of various treatments were discussed, and surgical intervention was elected to repair the patellar tendon.
Intraoperative findings showed a massive midsubstance rupture of the patellar tendon, accompanied by medial and lateral retinacular tears and a quadriceps tendon partial rupture; the central aspect of the quadriceps tendon attaching to the patella remained intact. The patella was retracted proximally; no evidence of active infection was present. Good-quality tissue remained attached to both the tibial tuberosity and the inferior pole of the patella. A No. 2 FiberWire suture (Arthrex, Inc, Naples, Florida) was used to run whip stitches in the distal end of the patellar tendon and a second No. 2 FiberWire suture was used to run whip stitches in the proximal aspect of the patellar tendon rupture. The 4 ends of the sutures were tied together, thus re-approximating the distal and proximal ends of the ruptured patellar tendon. No bone drilling was used because the midsubstance tear was amenable to good repair with reasonable expectation of healing based on tissue quality. The quadriceps tendon, which was partially torn, was repaired with a No. 1 Vicryl suture (Ethicon, Somerville, New Jersey). The medial and lateral retinacula were also repaired with a No. 1 Vicryl suture. The suturing scheme effectively re-approximated the knee extensor mechanism, and the patient was placed in a knee immobilizer that permitted no flexion for 6 weeks postoperatively.
After 3 months of gradual improvement with physical therapy, the patient returned for a follow-up visit, concerned that her knee function was beginning to decline. Physical examination showed patella alta with a thinned and diminutive palpable tendon in the patellar tendon region. She was capable of active flexion to 90º and extension to 50º, but beyond 50º, she was unable to actively extend; she was capable of full passive extension. MRI showed a repeat full-thickness patellar tendon tear with retraction from the inferior pole of the patella; previous tears to the quadriceps tendon were healed. Because of the recurrent nature of the injury, the patient’s physical examination, MRI findings, and anticipated poor quality of remaining tendon tissue, patellar tendon reconstruction using a cadaveric Achilles tendon allograft was recommended. The patient chose surgery for potential improvement in knee range of motion, active extension, and ambulation.
The previous anterior midline incision was used and carried down through the subcutaneous tissues where a complete rupture of the patellar tendon was identified. A limited amount of good-quality tendon tissue remained at the medial aspect of the tibial tuberosity. The remaining tissue located at the patella’s inferior pole was nonviable for use in surgical repair. Retinacular contractures were released to bring the patella distally; the trochlear groove was used as the anatomic landmark for the patella resting position. During reconstruction, the knee was placed into 30° of flexion, with the patella located in the trochlear groove, and the cadaveric Achilles tendon was placed on the midline of the patella, where measurements were done to assess proper length and tension (Figure 1).
The patient’s remaining native tissue on the medial aspect of the tibial tuberosity was used to augment the Achilles tendon graft medially. The cadaveric Achilles tendon graft was primarily used to replace the central and lateral aspects of the patellar tendon. Additionally, the calcaneal bone segment at the end of the Achilles tendon graft was removed prior to use. Cadaveric and host tissues at the medial aspect of the tibial tuberosity were sutured together with a No. 1 Vicryl suture (Figure 2). The distal aspect of the cadaveric Achilles tendon was used to re-approximate the patient’s native patellar tendon insertion at the tibial tuberosity. To supplement the graft anchor, a Richards metallic ligament staple (Smith & Nephew, Memphis, Tennessee) was used to fix the distal aspect of the Achilles tendon graft into the tibial tuberosity.
Proper tensioning of the graft was performed by visualizing patella tracking during the arc-of-knee motion and properly suturing the graft to allow for functional range. The proximal aspect of the cadaveric Achilles tendon was sutured into host tissues surrounding the superior pole of the patellar and quadriceps tendon. The edges of the graft were sutured with supplemental No. 1 Vicryl sutures (Figure 3).
Before surgical closure, knee range of motion was checked and noted to be 0º to 100º. The repaired construct was stable and uncompromised throughout the entire range of motion. Patella tracking was central and significantly improved; knee stability was normal to varus and valgus stress.
The patient was placed in a knee immobilizer for 6 weeks before range of motion was allowed. Seven months postoperatively, the patient returned for a follow-up visit, ambulating with 2 forearm crutches, which was her baseline ambulatory status. Physical examination revealed passive range of motion from 0º to 130º, an extension lag of 10º, and 4/5 quadriceps strength. It was recommended the patient continue physical therapy to improve strength and range of motion.
Conclusion
This is the first report in the literature documenting a recurrent patellar tendon rupture after primary repair in a patient with a history of IM tibial nailing. It is also the first report of a cadaveric Achilles tendon allograft used as a solution to this problem. Complete reconstruction of the patellar tendon using an Achilles tendon allograft is a method commonly used for ruptures after total knee arthroplasty.4-7,23,24 This case report highlights the utility of a cadaveric Achilles tendon in the setting of a recurrent patellar tendon rupture with poor remaining tissue quality.
1. Scott WN, Insall JN. Injuries of the knee. In: Rockwood CA Jr, Green DP, Bucholz RW, eds. Fractures in Adults. 3rd ed. Philadelphia, PA: JB Lippincott; 1991: 1799-1914.
2. Clark SC, Jones MW, Choudhury RR, Smith E. Bilateral patellar tendon rupture secondary to repeated local steroid injections. J Accid Emerg Med. 1995;12(4):300-301.
3. Unverferth LJ, Olix ML. The effect of local steroid injections on tendon. J Sports Med. 1973;1(4):31-37.
4. Cadambi A, Engh GA. Use of a semitendinosus tendon autogenous graft for rupture of the patellar ligament after total knee arthroplasty. A report of seven cases. J Bone Joint Surg Am. 1992;74(7):974-979.
5. Emerson RH Jr, Head WC, Malinin TI. Reconstruction of patellar tendon rupture after total knee arthroplasty with an extensor mechanism allograft. Clin Orthop.1990;(260):154-161.
6. Gustillo RB, Thompson R. Quadriceps and patellar tendon ruptures following total knee arthroplasty. In: Rand JA, Dorr LD, eds. Total Arthroplasty of the Knee: Proceedings of the Knee Society, 1985-1986. Rockville, MD: Aspen; 1987: 41-70.
7. Rand JA, Morrey BF, Bryan RS. Patellar tendon rupture after total knee arthroplasty. Clin Orthop. 1989;(244):233-238.
8. Schoderbek RJ, Brown TE, Mulhall KJ, et al. Extensor mechanism disruption after total knee arthroplasty. Clin Orthop. 2006;446:176-185.
9. Bonamo JJ, Krinik RM, Sporn AA. Rupture of the patellar ligament after use of the central third for anterior cruciate reconstruction. A report of two cases. J Bone Joint Surg Am. 1984;66(8):1294-1297.
10. Marumoto JM, Mitsunaga MM, Richardson AB, Medoff RJ, Mayfield GW. Late patellar tendon ruptures after removal of the central third for anterior cruciate ligament reconstruction. A report of two cases. Am J Sports Med. 1996;24(5):698-701.
11. Mickelsen PL, Morgan SJ, Johnson WA, Ferrari JD. Patellar tendon rupture 3 years after anterior cruciate ligament reconstruction with a central one third bone-patellar tendon-bone graft. Arthroscopy. 2001;17(6):648-652.
12. Morgan J, McCarty DJ. Tendon ruptures in patients with systemic lupus erythematosus treated with corticosteroids. Arthritis Rheum. 1974;17(6):1033-1036.
13. Webb LX, Toby EB. Bilateral rupture of the patellar tendon in an otherwise healthy male patient following minor trauma. J Trauma. 1986;26(11):1045-1048.
14. Greis PE, Holmstrom MC, Lahav A. Surgical treatment options for patella tendon rupture, Part I: Acute. Orthopedics. 2005;28(7):672-679.
15. Greis PE, Lahav A, Holstrom MC. Surgical treatment options for patella tendon rupture, part II: chronic. Orthopedics. 2005;28(8):765-769.
16. Lewis PB, Rue JP, Bach BR Jr. Chronic patellar tendon rupture: surgical reconstruction technique using 2 Achilles tendon allografts. J Knee Surg. 2008;21(12):130-135.
17. McNally PD, Marcelli EA. Achilles tendon allograft of a chronic patellar tendon rupture. Arthroscopy. 1998;14(3):340-344.
18. Katsoulis E, Court-Brown C, Giannoudis PV. Incidence and atieology of anterior knee pain after intramedullary nailing of the femur and tibia. J Bone Joint Surg Br. 2006;88(5):576-580.
19. Brumback RJ, Uwagie-Ero S, Lakatos RP, et al. Intramedullary nailing of femoral shaft fractures. Part II: Fracture-healing with static interlocking fixation. J Bone Joint Surg Am. 1988;70(1):1453-1462.
20. Koval KJ, Clapper MF, Brumback RJ, et al. Complications of reamed intramedullary nailing of the tibia. J Orthop Trauma. 1991;5(2):184-189.
21. Kretzler JE, Curtin SL, Wegner DA, Baumgaertner MR, Galloway MT. Patella tendon rupture: a late complication of a tibial nail. Orthopedics. 1995;18(11):1109-1111.
22. Moroney P, McCarthy T, Borton D. Patellar tendon rupture post reamed intra-medullary tibial nail in a patient with Ehlers-Danlos syndrome. A case report. Eur J Orthop Surg Traumatol. 2004;14(1):50-51.
23. Crossett LS, Sinha RK, Sechriest VF, Rubash HE. Reconstruction of a ruptured patellar tendon with achilles tendon allograft following total knee arthroplasty. J Bone Joint Surg Am. 2002;84(8):1354-1361.
24. Falconiero RP, Pallis MP. Chronic rupture of a patellar tendon: a technique for reconstruction with Achilles allograft. Arthroscopy. 1996;12(5):623-626.
1. Scott WN, Insall JN. Injuries of the knee. In: Rockwood CA Jr, Green DP, Bucholz RW, eds. Fractures in Adults. 3rd ed. Philadelphia, PA: JB Lippincott; 1991: 1799-1914.
2. Clark SC, Jones MW, Choudhury RR, Smith E. Bilateral patellar tendon rupture secondary to repeated local steroid injections. J Accid Emerg Med. 1995;12(4):300-301.
3. Unverferth LJ, Olix ML. The effect of local steroid injections on tendon. J Sports Med. 1973;1(4):31-37.
4. Cadambi A, Engh GA. Use of a semitendinosus tendon autogenous graft for rupture of the patellar ligament after total knee arthroplasty. A report of seven cases. J Bone Joint Surg Am. 1992;74(7):974-979.
5. Emerson RH Jr, Head WC, Malinin TI. Reconstruction of patellar tendon rupture after total knee arthroplasty with an extensor mechanism allograft. Clin Orthop.1990;(260):154-161.
6. Gustillo RB, Thompson R. Quadriceps and patellar tendon ruptures following total knee arthroplasty. In: Rand JA, Dorr LD, eds. Total Arthroplasty of the Knee: Proceedings of the Knee Society, 1985-1986. Rockville, MD: Aspen; 1987: 41-70.
7. Rand JA, Morrey BF, Bryan RS. Patellar tendon rupture after total knee arthroplasty. Clin Orthop. 1989;(244):233-238.
8. Schoderbek RJ, Brown TE, Mulhall KJ, et al. Extensor mechanism disruption after total knee arthroplasty. Clin Orthop. 2006;446:176-185.
9. Bonamo JJ, Krinik RM, Sporn AA. Rupture of the patellar ligament after use of the central third for anterior cruciate reconstruction. A report of two cases. J Bone Joint Surg Am. 1984;66(8):1294-1297.
10. Marumoto JM, Mitsunaga MM, Richardson AB, Medoff RJ, Mayfield GW. Late patellar tendon ruptures after removal of the central third for anterior cruciate ligament reconstruction. A report of two cases. Am J Sports Med. 1996;24(5):698-701.
11. Mickelsen PL, Morgan SJ, Johnson WA, Ferrari JD. Patellar tendon rupture 3 years after anterior cruciate ligament reconstruction with a central one third bone-patellar tendon-bone graft. Arthroscopy. 2001;17(6):648-652.
12. Morgan J, McCarty DJ. Tendon ruptures in patients with systemic lupus erythematosus treated with corticosteroids. Arthritis Rheum. 1974;17(6):1033-1036.
13. Webb LX, Toby EB. Bilateral rupture of the patellar tendon in an otherwise healthy male patient following minor trauma. J Trauma. 1986;26(11):1045-1048.
14. Greis PE, Holmstrom MC, Lahav A. Surgical treatment options for patella tendon rupture, Part I: Acute. Orthopedics. 2005;28(7):672-679.
15. Greis PE, Lahav A, Holstrom MC. Surgical treatment options for patella tendon rupture, part II: chronic. Orthopedics. 2005;28(8):765-769.
16. Lewis PB, Rue JP, Bach BR Jr. Chronic patellar tendon rupture: surgical reconstruction technique using 2 Achilles tendon allografts. J Knee Surg. 2008;21(12):130-135.
17. McNally PD, Marcelli EA. Achilles tendon allograft of a chronic patellar tendon rupture. Arthroscopy. 1998;14(3):340-344.
18. Katsoulis E, Court-Brown C, Giannoudis PV. Incidence and atieology of anterior knee pain after intramedullary nailing of the femur and tibia. J Bone Joint Surg Br. 2006;88(5):576-580.
19. Brumback RJ, Uwagie-Ero S, Lakatos RP, et al. Intramedullary nailing of femoral shaft fractures. Part II: Fracture-healing with static interlocking fixation. J Bone Joint Surg Am. 1988;70(1):1453-1462.
20. Koval KJ, Clapper MF, Brumback RJ, et al. Complications of reamed intramedullary nailing of the tibia. J Orthop Trauma. 1991;5(2):184-189.
21. Kretzler JE, Curtin SL, Wegner DA, Baumgaertner MR, Galloway MT. Patella tendon rupture: a late complication of a tibial nail. Orthopedics. 1995;18(11):1109-1111.
22. Moroney P, McCarthy T, Borton D. Patellar tendon rupture post reamed intra-medullary tibial nail in a patient with Ehlers-Danlos syndrome. A case report. Eur J Orthop Surg Traumatol. 2004;14(1):50-51.
23. Crossett LS, Sinha RK, Sechriest VF, Rubash HE. Reconstruction of a ruptured patellar tendon with achilles tendon allograft following total knee arthroplasty. J Bone Joint Surg Am. 2002;84(8):1354-1361.
24. Falconiero RP, Pallis MP. Chronic rupture of a patellar tendon: a technique for reconstruction with Achilles allograft. Arthroscopy. 1996;12(5):623-626.
Assessment of Scapular Morphology and Surgical Technique as Predictors of Notching in Reverse Shoulder Arthroplasty
Reverse shoulder arthroplasty (RSA) is a treatment option for a spectrum of diseases in shoulders with rotator cuff deficiency. There are distinct morphologic changes in the scapular and glenoid anatomy in patients with chronic rotator cuff tears.1 A muscular imbalance that occurs in the joint as a result of rotator cuff deficiency leads to morphologic changes that eliminate the compressive forces that hold the humeral head against the glenoid.2 RSA effectively stabilizes the glenohumeral joint in shoulders with deficient rotator cuffs.3,4 In early work, Grammont proposed that the glenosphere center of rotation should be medialized (concentric to the central axis of the metaglene or baseplate) and lowered.5 Although the medialized center of rotation in Grammont prostheses decreases shear forces and improves the deltoid lever arm, it also tends to result in mechanical impingement between the superomedial aspect of the humeral polyethylene insert and the scapular neck—so-called inferior scapular notching.6-9
Notching, which has been reported in 50% to 96% of patients who receive a Delta III prosthesis, typically appears within the first few months after surgery but may be seen as late as 14 months after surgery.5,10-12 Postmortem studies have shown that notching corresponds with erosion of the inferior pole of the glenoid and scapular neck, thought to be caused by the polyethylene cup of the implant.13 Although some studies have found that notching stabilizes after 1 year, others have shown notching progressing for up to 4 years after surgery.11,12,14 The clinical relevance of notching continues to be controversial, but notching has been associated with poorer clinical outcomes, polyethylene wear, and local osteolysis. Component loosening has also been reported with notching of grade 3 or more.8,10 Ultimately, there is concern that scapular notching could progress, ultimately leading to late glenoid loosening and potentially catastrophic failure.
Scapular anatomy has become an area of increased focus in rotator cuff disorders and in effects on RSA biomechanics.9 Recent reports have described important scapular morphology variations that suggest more individualized adjustments are needed during RSA.9,15 In addition, some investigators have reported that development of notching appears to depend on the height and inclination of the implanted glenoid component, where an inferior position of the glenosphere leads to less impingement and better range of motion.8,16 Simovitch and colleagues8 found the angle between the glenosphere and scapular neck and the craniocaudal position of the glenosphere to be highly correlated with inferior notching. They combined these 2 parameters into a predictive algorithm that provides a guideline (notching index, <35) for prevention of notching.
We conducted a study to evaluate the scapular notching index as a predictive tool and to consider other factors that may be associated with scapular notching occurring with use of Grammont reverse replacement systems. We hypothesized that patients with a notching index of less than 35 would not develop notching and that patients with an index of more than 35 would have increased incidence and severity of notching.
Materials and Methods
Patients treated with RSA for painful cuff tear arthropathy or irreparable rotator cuff tear with pseudoparesis (inability to actively elevate shoulder >90° in presence of free passive anterior elevation) were included in this retrospective review. All patients were treated between 2006 and 2010 by 1 of 2 established senior shoulder subspecialty surgeons. Patients treated with a Delta (DePuy Orthopaedics, Warsaw, Indiana) or an Aequalis (Tornier, Edina, Minnesota) reverse shoulder implant were included in the study. A standard polyethylene liner was used for all patients. These prostheses have the same neck shaft angle, 155º, as they have similar geometric designs, both based on the Grammont design—semiconstrained inverted with a fixed, lowered, medialized center of rotation. Standard instrumentation was used for all procedures. Patients were excluded if any nonstandard techniques or components were used (constrained or high-mobility liner, glenoid bone grafting). Patients who underwent revision for a previous reverse total arthroplasty, a total shoulder arthroplasty, or a hemiarthroplasty, or for treatment of acute fracture, posttraumatic deformity, or posttraumatic arthritis, were also excluded from our analyses. Minimum follow-up for study inclusion was 24 months.
All procedures were performed with the patient in the semi-beach-chair position and with use of a deltopectoral approach. The glenoid was prepared such that minimal reaming was needed to preserve the subchondral plate. The glenoid baseplate was positioned in the recommended inferior position to minimize notching and optimize functional outcomes.13 After surgery, all patients were managed with a simple soft immobilizer with or without a pillow with the arm at the patient’s side in internal rotation. Immediate passive mobilization was begun under the direction of physical therapists. Passive and active-assisted exercises were continued with gradual progression to independent activities of daily living at 6 weeks. Clinical evaluations were performed before and after surgery by the operating surgeon or independent research nurse. Active forward flexion, passive external rotation, strength, and visual analog scale (VAS) pain scores were reviewed and recorded. Case-specific complications were also reviewed.
Preoperative and postoperative anteroposterior radiographs were evaluated by 2 independent observers (attending surgeon, junior resident). Per standard technique, each radiograph was positioned horizontal to the scapular plane. Of the 91 patients, 66 had preoperative shoulder radiographs of acceptable quality, with complete visualization of scapular morphology. Radiographs were reviewed to measure the scapular neck angle (SNA), inferior scapular notching, prosthesis–scapular neck angle (PSNA), and peg glenoid rim distance (PGRD) (Figure 1). For the 66 patients with acceptable preoperative radiographs, SNA was determined by subtracting preoperative SNA from postoperative PSNA. Postoperative anteroposterior radiographs were used to classify degree of inferior scapular notching based on the Nerot grading scale (0-4). In addition, glenosphere overhang and glenosphere inclination were measured on postoperative radiographs.
The 91 shoulders were sorted into 2 groups based on degree of scapular notching: group 1, Nerot grade 0 (no inferior notching) and grade 1, and group 2, Nerot grades 2, 3, and 4. Group 1 had 37 patients with a size 36 glenosphere, 3 patients with size 38, and 8 patients with size 42; group 2 had 34 patients with a size 36 glenosphere, 1 patient with size 38, and 8 patients with size 42. All measurements were normalized to account for differences in glenosphere size. Groups 1 and 2 were compared on each radiographic parameter (inferior scapular notching, PSNA, PGRD, SNA).
Notching indexes were calculated ([PSNA × 0.13] + PGRD) and compared with the suggested index of 35.8 Simovitch and colleagues8 demonstrated that a notching index of more than 35 had 91% sensitivity and 88% specificity in predicting inferior notching, whereas a notching index of 35 or less avoided inferior notching 91% of the time. In this study, notching index was calculated for each patient, and then the mean values of groups 1 and 2 were compared (Table 1).
The effect of scapular notching and other individual radiographic parameters on outcomes was also evaluated with respect to forward flexion, external rotation, VAS pain score, complications, and external rotation lag sign. Mann-Whitney U test was used to test these variables; Spearman rank test was performed to determine correlation between each variable and scapular notching; logistic regression was used to explore the relationship of variables (PGRD, PSNA) as predictors of Nerot degree of inferior scapular notching, and postoperative complications; and independent-samples t test was used to determine group differences for each variable. For each investigation, the level of significance was set at P < .05. A biostatistician performed all statistical analyses using SPSS Version 19 (IBM, Armonk, New York).
Results
Our study cohort consisted of 91 shoulders. Mean follow-up was 41.8 months (range, 24.0-80.8 months). Seventy-five (82%) of the 91 shoulders developed scapular notching. Mean (SD) SNA on preoperative radiographs, used to assess preoperative scapular morphology, was 103.9° (14.5°). For all 91 shoulders, mean (SD) PSNA was 125.6° (16°), and mean (SD) PGRD was 16 (5.4) mm (Table 1). Inclination measurements were available for 86 patients. Mean (SD) inclination from 90° was 2.5° (10.3°) (range, 21°-30°). Mean (SD) SNA (postoperative PSNA minus preoperative SNA) for the 66 patients with acceptable preoperative radiographs was 24.3° (21.3°) (Table 1). Forty-eight of the 91 shoulders were placed in scapular notching group 1 (16 grade-0 shoulders, 32 grade-1 shoulders); the other 43 shoulders were placed in group 2 (33 grade-2 shoulders, 9 grade-3 shoulders, 1 grade-4 shoulder). Mean follow-up was 40 months for group 1 and 43 months for group 2.
There were no significant differences between groups 1 and 2 in SNA (102.8° vs 105.4°; P = .3), PGRD (15.4 vs 16.8 mm; P = . 47), or PSNA (125.8° vs 125.4°; P = .82) (Table 1). In addition, groups 1 and 2 had no significant differences (P > .05) in glenoid overhang and glenosphere inclination (other possible factors influencing notching).
Mean (SD) notching index was 31.8 (4.4) for group 1 and 33.1 (7.2) for group 2. These values were not significantly different (P = .29) (Table 1, Figure 2).8 Each was below the recommended threshold of 35 for prevention of notching (Table 1, Figure 2).
To try to understand why mean scapular notching index was low for both groups, we examined the contributing factors individually. Our cohort’s mean PGRD of 16.1 mm (15.4 and 16.8 mm for groups 1 and 2, respectively) was significantly lower than the cohort mean reported by Simovitch and colleagues8 (Table 2). Given that PGRD is more strongly weighted in the originally described notching index ([PSNA × 0.13] + PGRD),8 it was the primary driver for our notching index results, even though on average our results demonstrated a PSNA higher than that found by Simovitch and colleagues8 (Table 2; Figures 3, 4). Analyzing PGRD and PSNA together, we found no relationship between these variables and increased severity of inferior notching (Figure 5).
Regarding the effects of notching severity on outcomes in our study cohort, there were no significant differences between groups 1 and 2 in postoperative function, including forward flexion (123° vs 112.4°; P = .11), external rotation (18.8° vs 16.7°; P = .76), positive lag sign (P = .2), and VAS pain scores (1.2 vs 2.1; P = .15). There were also no significant differences between groups in the rate of complications (P = .92). Regression analysis determined that PSNA, PGRD, glenosphere inclination, glenosphere overhang, and implant manufacturer were not significant predictors of complications.
Discussion
RSA has provided good pain relief and restored function in patients with irreparable rotator cuff disease associated with arthritis.5,12,17,18 Scapular notching is a complex, multifactorial process. Nevertheless, surgeons remain cautious about the implications of inferior scapular notching, which is being reported by a significant number of patients. Our cohort’s high incidence of scapular notching (82%) in the early postoperative period clearly highlights the importance of predictive models, such as the notching index.8 Although concerns about consequences of notching have been expressed, notching severity did not affect outcomes or increase complications in this cohort.5,8,11,12,17-19
We conducted this study to examine use of a predictive tool for scapular notching, the notching index, in a large cohort of patients who underwent primary RSA. This index combines 2 well-established factors that contribute to notching—craniocaudal position and PSNA—into a predictive formula based on statistical analyses performed in a prospective cohort study.4,5,8,12,18 In their clinical study, Simovitch and colleagues8 found that both craniocaudal position and PSNA were tightly coupled with inferior scapular notching, and they developed a notching index that accounts for this relationship. We hypothesized that patients with a notching index of less than the recommended 35 would not develop notching and that patients with a notching index of more than 35 would have increased incidence and severity of notching. With our cohort, the recommended index of 35 was not an appropriate threshold predictive of notching. Furthermore, the 35 threshold applied to our cohort had 89% sensitivity and 21% specificity in predicting notching. Although the sensitivity is high, and correctly predicted true instances of notching, the low specificity compromises the precision of the notching formula ([PSNA × 0.13] + PGRD).
From the formula, it can be inferred that higher PSNA values can be compensated for by decreasing PGRD and inferiorizing the glenosphere. However, this recommendation appears limited based on increasing PSNA values, as in our cohort. The previously described notching formula cannot be universally applied to all patients treated with RSA because of the complexity of this relationship and patient-specific anatomy.
We assessed other possible anatomical and surgical factors, specific to scapular morphology, that could contribute to scapular notching. In other studies, reaming that produced an inferior tilt of the glenoid increased the likelihood of inferior notching.8,20,21 Furthermore, we expected less inferior glenoid overhang and smaller glenosphere would predispose patients to more notching.8,12,19 In our cohort, notching grade was not correlated with inferior tilt, glenoid overhang, or glenosphere size, which may be attributed to minimal variability in glenosphere size and a small range of glenosphere overhang.
There were limitations to this study. We examined only 2 types of RSA systems, and they had very similar Grammont designs. Other RSA designs might not have similar shortcomings with respect to inferior notching. In addition, we examined patient cases at a single time point and did not evaluate the effect of notching over time.
Overall, our results suggest that PGRD and PSNA have little effect on development of higher grade notching, particularly with use of Grammont prostheses. With newer surgical techniques, the recommendation is for inferior craniocaudal placement of the glenosphere, but this may not prevent notching with some types of patient-specific scapular morphology. Clearer surgical guidelines and techniques may help delineate the contribution of each parameter causing inferior scapular notching. Surgeons must weigh the evidence to determine how to correct patient-specific glenoid pathology and orient the glenosphere. Recent studies on bony increased-offset reverse shoulder arthroplasty (bio-RSA) techniques or newer prosthetic designs that considerably alter PSNA and the center of rotation may prevent inferior notching and provide a promising alternative to Grammont designs. Ultimately, longer follow-up is also needed to understand the clinical relevance of increased scapular notching.
1. Woodruff MJ, Cohen AP, Bradley JG. Arthroplasty of the shoulder in rheumatoid arthritis with rotator cuff dysfunction. Int Orthop. 2003;27(1):7-10.
2. Inman VT, Saunders JB, Abbott LC. Observations of the function of the shoulder joint. 1944. Clin Orthop. 1996;(330):3-12.
3. Boileau P, Watkinson D, Hatzidakis AM, Hovorka I. Neer Award 2005: the Grammont reverse shoulder prosthesis: results in cuff tear arthritis, fracture sequelae, and revision arthroplasty. J Shoulder Elbow Surg. 2006;15(5):527-540.
4. Boileau P, Watkinson DJ, Hatzidakis AM, Balg F. Grammont reverse prosthesis: design, rationale, and biomechanics. J Shoulder Elbow Surg. 2005;14(1 suppl S):147S-161S.
5. Sirveaux F, Favard L, Oudet D, Huquet D, Walch G, Molé D. Grammont inverted total shoulder arthroplasty in the treatment of glenohumeral osteoarthritis with massive rupture of the cuff. Results of a multicentre study of 80 shoulders. J Bone Joint Surg Br. 2004;86(3):388-395.
6. Kowalsky MS, Galatz LM, Shia DS, Steger-May K, Keener JD. The relationship between scapular notching and reverse shoulder arthroplasty prosthesis design. J Shoulder Elbow Surg. 2012;21(10):1430-1441.
7. Lévigne C, Boileau P, Favard L, et al. Scapular notching in reverse shoulder arthroplasty. J Shoulder Elbow Surg. 2008;17(6):925-935.
8. Simovitch RW, Zumstein MA, Lohri E, Helmy N, Gerber C. Predictors of scapular notching in patients managed with the Delta III reverse total shoulder replacement. J Bone Joint Surg Am. 2007;89(3):588-600.
9. Torrens C, Corrales M, Gonzalez G, Solano A, Caceres E. Morphology of the scapula relative to the reverse shoulder prosthesis. J Orthop Surg (Hong Kong). 2009;17(2):146-150.
10. McFarland EG, Sanguanjit P, Tasaki A, Keyurapan E, Fishman EK, Fayad LM. The reverse shoulder prosthesis: a review of imaging features and complications. Skeletal Radiol. 2006;35(7):488-496.
11. Valenti PH, Boutens D, Nerot C. Delta 3 reversed prosthesis for osteoarthritis with massive rotator cuff tear: long-term results (>5 years). In: Walch G, Boileau P, Molé D, eds. Shoulder Prosthesis: Two to Ten Years Follow-Up. Montpellier, France: Sauramps Medical; 2001:253-259.
12. Werner CM, Steinmann PA, Gilbart M, Gerber C. Treatment of painful pseudoparesis due to irreparable rotator cuff dysfunction with the Delta III reverse-ball-and-socket total shoulder prosthesis. J Bone Joint Surg Am. 2005;87(7):1476-1486.
13. Nyffeler RW, Werner CM, Simmen BR, Gerber C. Analysis of a retrieved Delta III total shoulder prosthesis. J Bone Joint Surg Br. 2004;86(8):1187-1191.
14. Grassi FA, Murena L, Valli F, Alberio R. Six-year experience with the Delta III reverse shoulder prosthesis. J Orthop Surg (Hong Kong). 2009;17(2):151-156.
15. Lévigne C, Garret J, Boileau P, Alami G, Favard L, Walch G. Scapular notching in reverse shoulder arthroplasty: is it important to avoid it and how? Clin Orthop. 2011;469(9):2512-2520.
16. Nyffeler RW, Werner CM, Gerber C. Biomechanical relevance of glenoid component positioning in the reverse Delta III total shoulder prosthesis. J Shoulder Elbow Surg. 2005;14(5):524-528.
17. Rittmeister M, Kerschbaumer F. Grammont reverse total shoulder arthroplasty in patients with rheumatoid arthritis and nonreconstructible rotator cuff lesions. J Shoulder Elbow Surg. 2001;10(1):17-22.
18. Vanhove B, Beugnies A. Grammont’s reverse shoulder prosthesis for rotator cuff arthropathy. A retrospective study of 32 cases. Acta Orthop Belg. 2004;70(3):219-225.
19. Kempton LB, Balasubramaniam M, Ankerson E, Wiater JM. A radiographic analysis of the effects of glenosphere position on scapular notching following reverse total shoulder arthroplasty. J Shoulder Elbow Surg. 2011;20(6):968-974.
20. Gutiérrez S, Greiwe RM, Frankle MA, Siegal S, Lee WE. Biomechanical comparison of component position and hardware failure in the reverse shoulder prosthesis. J Shoulder Elbow Surg. 2007;16(3 suppl):S9-S12.
21. Roche CP, Diep P, Hamilton M, et al. Impact of inferior glenoid tilt, humeral retroversion, bone grafting, and design parameters on muscle length and deltoid wrapping in reverse shoulder arthroplasty. Bull Hosp Jt Dis. 2013;71(4):284-293.
Reverse shoulder arthroplasty (RSA) is a treatment option for a spectrum of diseases in shoulders with rotator cuff deficiency. There are distinct morphologic changes in the scapular and glenoid anatomy in patients with chronic rotator cuff tears.1 A muscular imbalance that occurs in the joint as a result of rotator cuff deficiency leads to morphologic changes that eliminate the compressive forces that hold the humeral head against the glenoid.2 RSA effectively stabilizes the glenohumeral joint in shoulders with deficient rotator cuffs.3,4 In early work, Grammont proposed that the glenosphere center of rotation should be medialized (concentric to the central axis of the metaglene or baseplate) and lowered.5 Although the medialized center of rotation in Grammont prostheses decreases shear forces and improves the deltoid lever arm, it also tends to result in mechanical impingement between the superomedial aspect of the humeral polyethylene insert and the scapular neck—so-called inferior scapular notching.6-9
Notching, which has been reported in 50% to 96% of patients who receive a Delta III prosthesis, typically appears within the first few months after surgery but may be seen as late as 14 months after surgery.5,10-12 Postmortem studies have shown that notching corresponds with erosion of the inferior pole of the glenoid and scapular neck, thought to be caused by the polyethylene cup of the implant.13 Although some studies have found that notching stabilizes after 1 year, others have shown notching progressing for up to 4 years after surgery.11,12,14 The clinical relevance of notching continues to be controversial, but notching has been associated with poorer clinical outcomes, polyethylene wear, and local osteolysis. Component loosening has also been reported with notching of grade 3 or more.8,10 Ultimately, there is concern that scapular notching could progress, ultimately leading to late glenoid loosening and potentially catastrophic failure.
Scapular anatomy has become an area of increased focus in rotator cuff disorders and in effects on RSA biomechanics.9 Recent reports have described important scapular morphology variations that suggest more individualized adjustments are needed during RSA.9,15 In addition, some investigators have reported that development of notching appears to depend on the height and inclination of the implanted glenoid component, where an inferior position of the glenosphere leads to less impingement and better range of motion.8,16 Simovitch and colleagues8 found the angle between the glenosphere and scapular neck and the craniocaudal position of the glenosphere to be highly correlated with inferior notching. They combined these 2 parameters into a predictive algorithm that provides a guideline (notching index, <35) for prevention of notching.
We conducted a study to evaluate the scapular notching index as a predictive tool and to consider other factors that may be associated with scapular notching occurring with use of Grammont reverse replacement systems. We hypothesized that patients with a notching index of less than 35 would not develop notching and that patients with an index of more than 35 would have increased incidence and severity of notching.
Materials and Methods
Patients treated with RSA for painful cuff tear arthropathy or irreparable rotator cuff tear with pseudoparesis (inability to actively elevate shoulder >90° in presence of free passive anterior elevation) were included in this retrospective review. All patients were treated between 2006 and 2010 by 1 of 2 established senior shoulder subspecialty surgeons. Patients treated with a Delta (DePuy Orthopaedics, Warsaw, Indiana) or an Aequalis (Tornier, Edina, Minnesota) reverse shoulder implant were included in the study. A standard polyethylene liner was used for all patients. These prostheses have the same neck shaft angle, 155º, as they have similar geometric designs, both based on the Grammont design—semiconstrained inverted with a fixed, lowered, medialized center of rotation. Standard instrumentation was used for all procedures. Patients were excluded if any nonstandard techniques or components were used (constrained or high-mobility liner, glenoid bone grafting). Patients who underwent revision for a previous reverse total arthroplasty, a total shoulder arthroplasty, or a hemiarthroplasty, or for treatment of acute fracture, posttraumatic deformity, or posttraumatic arthritis, were also excluded from our analyses. Minimum follow-up for study inclusion was 24 months.
All procedures were performed with the patient in the semi-beach-chair position and with use of a deltopectoral approach. The glenoid was prepared such that minimal reaming was needed to preserve the subchondral plate. The glenoid baseplate was positioned in the recommended inferior position to minimize notching and optimize functional outcomes.13 After surgery, all patients were managed with a simple soft immobilizer with or without a pillow with the arm at the patient’s side in internal rotation. Immediate passive mobilization was begun under the direction of physical therapists. Passive and active-assisted exercises were continued with gradual progression to independent activities of daily living at 6 weeks. Clinical evaluations were performed before and after surgery by the operating surgeon or independent research nurse. Active forward flexion, passive external rotation, strength, and visual analog scale (VAS) pain scores were reviewed and recorded. Case-specific complications were also reviewed.
Preoperative and postoperative anteroposterior radiographs were evaluated by 2 independent observers (attending surgeon, junior resident). Per standard technique, each radiograph was positioned horizontal to the scapular plane. Of the 91 patients, 66 had preoperative shoulder radiographs of acceptable quality, with complete visualization of scapular morphology. Radiographs were reviewed to measure the scapular neck angle (SNA), inferior scapular notching, prosthesis–scapular neck angle (PSNA), and peg glenoid rim distance (PGRD) (Figure 1). For the 66 patients with acceptable preoperative radiographs, SNA was determined by subtracting preoperative SNA from postoperative PSNA. Postoperative anteroposterior radiographs were used to classify degree of inferior scapular notching based on the Nerot grading scale (0-4). In addition, glenosphere overhang and glenosphere inclination were measured on postoperative radiographs.
The 91 shoulders were sorted into 2 groups based on degree of scapular notching: group 1, Nerot grade 0 (no inferior notching) and grade 1, and group 2, Nerot grades 2, 3, and 4. Group 1 had 37 patients with a size 36 glenosphere, 3 patients with size 38, and 8 patients with size 42; group 2 had 34 patients with a size 36 glenosphere, 1 patient with size 38, and 8 patients with size 42. All measurements were normalized to account for differences in glenosphere size. Groups 1 and 2 were compared on each radiographic parameter (inferior scapular notching, PSNA, PGRD, SNA).
Notching indexes were calculated ([PSNA × 0.13] + PGRD) and compared with the suggested index of 35.8 Simovitch and colleagues8 demonstrated that a notching index of more than 35 had 91% sensitivity and 88% specificity in predicting inferior notching, whereas a notching index of 35 or less avoided inferior notching 91% of the time. In this study, notching index was calculated for each patient, and then the mean values of groups 1 and 2 were compared (Table 1).
The effect of scapular notching and other individual radiographic parameters on outcomes was also evaluated with respect to forward flexion, external rotation, VAS pain score, complications, and external rotation lag sign. Mann-Whitney U test was used to test these variables; Spearman rank test was performed to determine correlation between each variable and scapular notching; logistic regression was used to explore the relationship of variables (PGRD, PSNA) as predictors of Nerot degree of inferior scapular notching, and postoperative complications; and independent-samples t test was used to determine group differences for each variable. For each investigation, the level of significance was set at P < .05. A biostatistician performed all statistical analyses using SPSS Version 19 (IBM, Armonk, New York).
Results
Our study cohort consisted of 91 shoulders. Mean follow-up was 41.8 months (range, 24.0-80.8 months). Seventy-five (82%) of the 91 shoulders developed scapular notching. Mean (SD) SNA on preoperative radiographs, used to assess preoperative scapular morphology, was 103.9° (14.5°). For all 91 shoulders, mean (SD) PSNA was 125.6° (16°), and mean (SD) PGRD was 16 (5.4) mm (Table 1). Inclination measurements were available for 86 patients. Mean (SD) inclination from 90° was 2.5° (10.3°) (range, 21°-30°). Mean (SD) SNA (postoperative PSNA minus preoperative SNA) for the 66 patients with acceptable preoperative radiographs was 24.3° (21.3°) (Table 1). Forty-eight of the 91 shoulders were placed in scapular notching group 1 (16 grade-0 shoulders, 32 grade-1 shoulders); the other 43 shoulders were placed in group 2 (33 grade-2 shoulders, 9 grade-3 shoulders, 1 grade-4 shoulder). Mean follow-up was 40 months for group 1 and 43 months for group 2.
There were no significant differences between groups 1 and 2 in SNA (102.8° vs 105.4°; P = .3), PGRD (15.4 vs 16.8 mm; P = . 47), or PSNA (125.8° vs 125.4°; P = .82) (Table 1). In addition, groups 1 and 2 had no significant differences (P > .05) in glenoid overhang and glenosphere inclination (other possible factors influencing notching).
Mean (SD) notching index was 31.8 (4.4) for group 1 and 33.1 (7.2) for group 2. These values were not significantly different (P = .29) (Table 1, Figure 2).8 Each was below the recommended threshold of 35 for prevention of notching (Table 1, Figure 2).
To try to understand why mean scapular notching index was low for both groups, we examined the contributing factors individually. Our cohort’s mean PGRD of 16.1 mm (15.4 and 16.8 mm for groups 1 and 2, respectively) was significantly lower than the cohort mean reported by Simovitch and colleagues8 (Table 2). Given that PGRD is more strongly weighted in the originally described notching index ([PSNA × 0.13] + PGRD),8 it was the primary driver for our notching index results, even though on average our results demonstrated a PSNA higher than that found by Simovitch and colleagues8 (Table 2; Figures 3, 4). Analyzing PGRD and PSNA together, we found no relationship between these variables and increased severity of inferior notching (Figure 5).
Regarding the effects of notching severity on outcomes in our study cohort, there were no significant differences between groups 1 and 2 in postoperative function, including forward flexion (123° vs 112.4°; P = .11), external rotation (18.8° vs 16.7°; P = .76), positive lag sign (P = .2), and VAS pain scores (1.2 vs 2.1; P = .15). There were also no significant differences between groups in the rate of complications (P = .92). Regression analysis determined that PSNA, PGRD, glenosphere inclination, glenosphere overhang, and implant manufacturer were not significant predictors of complications.
Discussion
RSA has provided good pain relief and restored function in patients with irreparable rotator cuff disease associated with arthritis.5,12,17,18 Scapular notching is a complex, multifactorial process. Nevertheless, surgeons remain cautious about the implications of inferior scapular notching, which is being reported by a significant number of patients. Our cohort’s high incidence of scapular notching (82%) in the early postoperative period clearly highlights the importance of predictive models, such as the notching index.8 Although concerns about consequences of notching have been expressed, notching severity did not affect outcomes or increase complications in this cohort.5,8,11,12,17-19
We conducted this study to examine use of a predictive tool for scapular notching, the notching index, in a large cohort of patients who underwent primary RSA. This index combines 2 well-established factors that contribute to notching—craniocaudal position and PSNA—into a predictive formula based on statistical analyses performed in a prospective cohort study.4,5,8,12,18 In their clinical study, Simovitch and colleagues8 found that both craniocaudal position and PSNA were tightly coupled with inferior scapular notching, and they developed a notching index that accounts for this relationship. We hypothesized that patients with a notching index of less than the recommended 35 would not develop notching and that patients with a notching index of more than 35 would have increased incidence and severity of notching. With our cohort, the recommended index of 35 was not an appropriate threshold predictive of notching. Furthermore, the 35 threshold applied to our cohort had 89% sensitivity and 21% specificity in predicting notching. Although the sensitivity is high, and correctly predicted true instances of notching, the low specificity compromises the precision of the notching formula ([PSNA × 0.13] + PGRD).
From the formula, it can be inferred that higher PSNA values can be compensated for by decreasing PGRD and inferiorizing the glenosphere. However, this recommendation appears limited based on increasing PSNA values, as in our cohort. The previously described notching formula cannot be universally applied to all patients treated with RSA because of the complexity of this relationship and patient-specific anatomy.
We assessed other possible anatomical and surgical factors, specific to scapular morphology, that could contribute to scapular notching. In other studies, reaming that produced an inferior tilt of the glenoid increased the likelihood of inferior notching.8,20,21 Furthermore, we expected less inferior glenoid overhang and smaller glenosphere would predispose patients to more notching.8,12,19 In our cohort, notching grade was not correlated with inferior tilt, glenoid overhang, or glenosphere size, which may be attributed to minimal variability in glenosphere size and a small range of glenosphere overhang.
There were limitations to this study. We examined only 2 types of RSA systems, and they had very similar Grammont designs. Other RSA designs might not have similar shortcomings with respect to inferior notching. In addition, we examined patient cases at a single time point and did not evaluate the effect of notching over time.
Overall, our results suggest that PGRD and PSNA have little effect on development of higher grade notching, particularly with use of Grammont prostheses. With newer surgical techniques, the recommendation is for inferior craniocaudal placement of the glenosphere, but this may not prevent notching with some types of patient-specific scapular morphology. Clearer surgical guidelines and techniques may help delineate the contribution of each parameter causing inferior scapular notching. Surgeons must weigh the evidence to determine how to correct patient-specific glenoid pathology and orient the glenosphere. Recent studies on bony increased-offset reverse shoulder arthroplasty (bio-RSA) techniques or newer prosthetic designs that considerably alter PSNA and the center of rotation may prevent inferior notching and provide a promising alternative to Grammont designs. Ultimately, longer follow-up is also needed to understand the clinical relevance of increased scapular notching.
Reverse shoulder arthroplasty (RSA) is a treatment option for a spectrum of diseases in shoulders with rotator cuff deficiency. There are distinct morphologic changes in the scapular and glenoid anatomy in patients with chronic rotator cuff tears.1 A muscular imbalance that occurs in the joint as a result of rotator cuff deficiency leads to morphologic changes that eliminate the compressive forces that hold the humeral head against the glenoid.2 RSA effectively stabilizes the glenohumeral joint in shoulders with deficient rotator cuffs.3,4 In early work, Grammont proposed that the glenosphere center of rotation should be medialized (concentric to the central axis of the metaglene or baseplate) and lowered.5 Although the medialized center of rotation in Grammont prostheses decreases shear forces and improves the deltoid lever arm, it also tends to result in mechanical impingement between the superomedial aspect of the humeral polyethylene insert and the scapular neck—so-called inferior scapular notching.6-9
Notching, which has been reported in 50% to 96% of patients who receive a Delta III prosthesis, typically appears within the first few months after surgery but may be seen as late as 14 months after surgery.5,10-12 Postmortem studies have shown that notching corresponds with erosion of the inferior pole of the glenoid and scapular neck, thought to be caused by the polyethylene cup of the implant.13 Although some studies have found that notching stabilizes after 1 year, others have shown notching progressing for up to 4 years after surgery.11,12,14 The clinical relevance of notching continues to be controversial, but notching has been associated with poorer clinical outcomes, polyethylene wear, and local osteolysis. Component loosening has also been reported with notching of grade 3 or more.8,10 Ultimately, there is concern that scapular notching could progress, ultimately leading to late glenoid loosening and potentially catastrophic failure.
Scapular anatomy has become an area of increased focus in rotator cuff disorders and in effects on RSA biomechanics.9 Recent reports have described important scapular morphology variations that suggest more individualized adjustments are needed during RSA.9,15 In addition, some investigators have reported that development of notching appears to depend on the height and inclination of the implanted glenoid component, where an inferior position of the glenosphere leads to less impingement and better range of motion.8,16 Simovitch and colleagues8 found the angle between the glenosphere and scapular neck and the craniocaudal position of the glenosphere to be highly correlated with inferior notching. They combined these 2 parameters into a predictive algorithm that provides a guideline (notching index, <35) for prevention of notching.
We conducted a study to evaluate the scapular notching index as a predictive tool and to consider other factors that may be associated with scapular notching occurring with use of Grammont reverse replacement systems. We hypothesized that patients with a notching index of less than 35 would not develop notching and that patients with an index of more than 35 would have increased incidence and severity of notching.
Materials and Methods
Patients treated with RSA for painful cuff tear arthropathy or irreparable rotator cuff tear with pseudoparesis (inability to actively elevate shoulder >90° in presence of free passive anterior elevation) were included in this retrospective review. All patients were treated between 2006 and 2010 by 1 of 2 established senior shoulder subspecialty surgeons. Patients treated with a Delta (DePuy Orthopaedics, Warsaw, Indiana) or an Aequalis (Tornier, Edina, Minnesota) reverse shoulder implant were included in the study. A standard polyethylene liner was used for all patients. These prostheses have the same neck shaft angle, 155º, as they have similar geometric designs, both based on the Grammont design—semiconstrained inverted with a fixed, lowered, medialized center of rotation. Standard instrumentation was used for all procedures. Patients were excluded if any nonstandard techniques or components were used (constrained or high-mobility liner, glenoid bone grafting). Patients who underwent revision for a previous reverse total arthroplasty, a total shoulder arthroplasty, or a hemiarthroplasty, or for treatment of acute fracture, posttraumatic deformity, or posttraumatic arthritis, were also excluded from our analyses. Minimum follow-up for study inclusion was 24 months.
All procedures were performed with the patient in the semi-beach-chair position and with use of a deltopectoral approach. The glenoid was prepared such that minimal reaming was needed to preserve the subchondral plate. The glenoid baseplate was positioned in the recommended inferior position to minimize notching and optimize functional outcomes.13 After surgery, all patients were managed with a simple soft immobilizer with or without a pillow with the arm at the patient’s side in internal rotation. Immediate passive mobilization was begun under the direction of physical therapists. Passive and active-assisted exercises were continued with gradual progression to independent activities of daily living at 6 weeks. Clinical evaluations were performed before and after surgery by the operating surgeon or independent research nurse. Active forward flexion, passive external rotation, strength, and visual analog scale (VAS) pain scores were reviewed and recorded. Case-specific complications were also reviewed.
Preoperative and postoperative anteroposterior radiographs were evaluated by 2 independent observers (attending surgeon, junior resident). Per standard technique, each radiograph was positioned horizontal to the scapular plane. Of the 91 patients, 66 had preoperative shoulder radiographs of acceptable quality, with complete visualization of scapular morphology. Radiographs were reviewed to measure the scapular neck angle (SNA), inferior scapular notching, prosthesis–scapular neck angle (PSNA), and peg glenoid rim distance (PGRD) (Figure 1). For the 66 patients with acceptable preoperative radiographs, SNA was determined by subtracting preoperative SNA from postoperative PSNA. Postoperative anteroposterior radiographs were used to classify degree of inferior scapular notching based on the Nerot grading scale (0-4). In addition, glenosphere overhang and glenosphere inclination were measured on postoperative radiographs.
The 91 shoulders were sorted into 2 groups based on degree of scapular notching: group 1, Nerot grade 0 (no inferior notching) and grade 1, and group 2, Nerot grades 2, 3, and 4. Group 1 had 37 patients with a size 36 glenosphere, 3 patients with size 38, and 8 patients with size 42; group 2 had 34 patients with a size 36 glenosphere, 1 patient with size 38, and 8 patients with size 42. All measurements were normalized to account for differences in glenosphere size. Groups 1 and 2 were compared on each radiographic parameter (inferior scapular notching, PSNA, PGRD, SNA).
Notching indexes were calculated ([PSNA × 0.13] + PGRD) and compared with the suggested index of 35.8 Simovitch and colleagues8 demonstrated that a notching index of more than 35 had 91% sensitivity and 88% specificity in predicting inferior notching, whereas a notching index of 35 or less avoided inferior notching 91% of the time. In this study, notching index was calculated for each patient, and then the mean values of groups 1 and 2 were compared (Table 1).
The effect of scapular notching and other individual radiographic parameters on outcomes was also evaluated with respect to forward flexion, external rotation, VAS pain score, complications, and external rotation lag sign. Mann-Whitney U test was used to test these variables; Spearman rank test was performed to determine correlation between each variable and scapular notching; logistic regression was used to explore the relationship of variables (PGRD, PSNA) as predictors of Nerot degree of inferior scapular notching, and postoperative complications; and independent-samples t test was used to determine group differences for each variable. For each investigation, the level of significance was set at P < .05. A biostatistician performed all statistical analyses using SPSS Version 19 (IBM, Armonk, New York).
Results
Our study cohort consisted of 91 shoulders. Mean follow-up was 41.8 months (range, 24.0-80.8 months). Seventy-five (82%) of the 91 shoulders developed scapular notching. Mean (SD) SNA on preoperative radiographs, used to assess preoperative scapular morphology, was 103.9° (14.5°). For all 91 shoulders, mean (SD) PSNA was 125.6° (16°), and mean (SD) PGRD was 16 (5.4) mm (Table 1). Inclination measurements were available for 86 patients. Mean (SD) inclination from 90° was 2.5° (10.3°) (range, 21°-30°). Mean (SD) SNA (postoperative PSNA minus preoperative SNA) for the 66 patients with acceptable preoperative radiographs was 24.3° (21.3°) (Table 1). Forty-eight of the 91 shoulders were placed in scapular notching group 1 (16 grade-0 shoulders, 32 grade-1 shoulders); the other 43 shoulders were placed in group 2 (33 grade-2 shoulders, 9 grade-3 shoulders, 1 grade-4 shoulder). Mean follow-up was 40 months for group 1 and 43 months for group 2.
There were no significant differences between groups 1 and 2 in SNA (102.8° vs 105.4°; P = .3), PGRD (15.4 vs 16.8 mm; P = . 47), or PSNA (125.8° vs 125.4°; P = .82) (Table 1). In addition, groups 1 and 2 had no significant differences (P > .05) in glenoid overhang and glenosphere inclination (other possible factors influencing notching).
Mean (SD) notching index was 31.8 (4.4) for group 1 and 33.1 (7.2) for group 2. These values were not significantly different (P = .29) (Table 1, Figure 2).8 Each was below the recommended threshold of 35 for prevention of notching (Table 1, Figure 2).
To try to understand why mean scapular notching index was low for both groups, we examined the contributing factors individually. Our cohort’s mean PGRD of 16.1 mm (15.4 and 16.8 mm for groups 1 and 2, respectively) was significantly lower than the cohort mean reported by Simovitch and colleagues8 (Table 2). Given that PGRD is more strongly weighted in the originally described notching index ([PSNA × 0.13] + PGRD),8 it was the primary driver for our notching index results, even though on average our results demonstrated a PSNA higher than that found by Simovitch and colleagues8 (Table 2; Figures 3, 4). Analyzing PGRD and PSNA together, we found no relationship between these variables and increased severity of inferior notching (Figure 5).
Regarding the effects of notching severity on outcomes in our study cohort, there were no significant differences between groups 1 and 2 in postoperative function, including forward flexion (123° vs 112.4°; P = .11), external rotation (18.8° vs 16.7°; P = .76), positive lag sign (P = .2), and VAS pain scores (1.2 vs 2.1; P = .15). There were also no significant differences between groups in the rate of complications (P = .92). Regression analysis determined that PSNA, PGRD, glenosphere inclination, glenosphere overhang, and implant manufacturer were not significant predictors of complications.
Discussion
RSA has provided good pain relief and restored function in patients with irreparable rotator cuff disease associated with arthritis.5,12,17,18 Scapular notching is a complex, multifactorial process. Nevertheless, surgeons remain cautious about the implications of inferior scapular notching, which is being reported by a significant number of patients. Our cohort’s high incidence of scapular notching (82%) in the early postoperative period clearly highlights the importance of predictive models, such as the notching index.8 Although concerns about consequences of notching have been expressed, notching severity did not affect outcomes or increase complications in this cohort.5,8,11,12,17-19
We conducted this study to examine use of a predictive tool for scapular notching, the notching index, in a large cohort of patients who underwent primary RSA. This index combines 2 well-established factors that contribute to notching—craniocaudal position and PSNA—into a predictive formula based on statistical analyses performed in a prospective cohort study.4,5,8,12,18 In their clinical study, Simovitch and colleagues8 found that both craniocaudal position and PSNA were tightly coupled with inferior scapular notching, and they developed a notching index that accounts for this relationship. We hypothesized that patients with a notching index of less than the recommended 35 would not develop notching and that patients with a notching index of more than 35 would have increased incidence and severity of notching. With our cohort, the recommended index of 35 was not an appropriate threshold predictive of notching. Furthermore, the 35 threshold applied to our cohort had 89% sensitivity and 21% specificity in predicting notching. Although the sensitivity is high, and correctly predicted true instances of notching, the low specificity compromises the precision of the notching formula ([PSNA × 0.13] + PGRD).
From the formula, it can be inferred that higher PSNA values can be compensated for by decreasing PGRD and inferiorizing the glenosphere. However, this recommendation appears limited based on increasing PSNA values, as in our cohort. The previously described notching formula cannot be universally applied to all patients treated with RSA because of the complexity of this relationship and patient-specific anatomy.
We assessed other possible anatomical and surgical factors, specific to scapular morphology, that could contribute to scapular notching. In other studies, reaming that produced an inferior tilt of the glenoid increased the likelihood of inferior notching.8,20,21 Furthermore, we expected less inferior glenoid overhang and smaller glenosphere would predispose patients to more notching.8,12,19 In our cohort, notching grade was not correlated with inferior tilt, glenoid overhang, or glenosphere size, which may be attributed to minimal variability in glenosphere size and a small range of glenosphere overhang.
There were limitations to this study. We examined only 2 types of RSA systems, and they had very similar Grammont designs. Other RSA designs might not have similar shortcomings with respect to inferior notching. In addition, we examined patient cases at a single time point and did not evaluate the effect of notching over time.
Overall, our results suggest that PGRD and PSNA have little effect on development of higher grade notching, particularly with use of Grammont prostheses. With newer surgical techniques, the recommendation is for inferior craniocaudal placement of the glenosphere, but this may not prevent notching with some types of patient-specific scapular morphology. Clearer surgical guidelines and techniques may help delineate the contribution of each parameter causing inferior scapular notching. Surgeons must weigh the evidence to determine how to correct patient-specific glenoid pathology and orient the glenosphere. Recent studies on bony increased-offset reverse shoulder arthroplasty (bio-RSA) techniques or newer prosthetic designs that considerably alter PSNA and the center of rotation may prevent inferior notching and provide a promising alternative to Grammont designs. Ultimately, longer follow-up is also needed to understand the clinical relevance of increased scapular notching.
1. Woodruff MJ, Cohen AP, Bradley JG. Arthroplasty of the shoulder in rheumatoid arthritis with rotator cuff dysfunction. Int Orthop. 2003;27(1):7-10.
2. Inman VT, Saunders JB, Abbott LC. Observations of the function of the shoulder joint. 1944. Clin Orthop. 1996;(330):3-12.
3. Boileau P, Watkinson D, Hatzidakis AM, Hovorka I. Neer Award 2005: the Grammont reverse shoulder prosthesis: results in cuff tear arthritis, fracture sequelae, and revision arthroplasty. J Shoulder Elbow Surg. 2006;15(5):527-540.
4. Boileau P, Watkinson DJ, Hatzidakis AM, Balg F. Grammont reverse prosthesis: design, rationale, and biomechanics. J Shoulder Elbow Surg. 2005;14(1 suppl S):147S-161S.
5. Sirveaux F, Favard L, Oudet D, Huquet D, Walch G, Molé D. Grammont inverted total shoulder arthroplasty in the treatment of glenohumeral osteoarthritis with massive rupture of the cuff. Results of a multicentre study of 80 shoulders. J Bone Joint Surg Br. 2004;86(3):388-395.
6. Kowalsky MS, Galatz LM, Shia DS, Steger-May K, Keener JD. The relationship between scapular notching and reverse shoulder arthroplasty prosthesis design. J Shoulder Elbow Surg. 2012;21(10):1430-1441.
7. Lévigne C, Boileau P, Favard L, et al. Scapular notching in reverse shoulder arthroplasty. J Shoulder Elbow Surg. 2008;17(6):925-935.
8. Simovitch RW, Zumstein MA, Lohri E, Helmy N, Gerber C. Predictors of scapular notching in patients managed with the Delta III reverse total shoulder replacement. J Bone Joint Surg Am. 2007;89(3):588-600.
9. Torrens C, Corrales M, Gonzalez G, Solano A, Caceres E. Morphology of the scapula relative to the reverse shoulder prosthesis. J Orthop Surg (Hong Kong). 2009;17(2):146-150.
10. McFarland EG, Sanguanjit P, Tasaki A, Keyurapan E, Fishman EK, Fayad LM. The reverse shoulder prosthesis: a review of imaging features and complications. Skeletal Radiol. 2006;35(7):488-496.
11. Valenti PH, Boutens D, Nerot C. Delta 3 reversed prosthesis for osteoarthritis with massive rotator cuff tear: long-term results (>5 years). In: Walch G, Boileau P, Molé D, eds. Shoulder Prosthesis: Two to Ten Years Follow-Up. Montpellier, France: Sauramps Medical; 2001:253-259.
12. Werner CM, Steinmann PA, Gilbart M, Gerber C. Treatment of painful pseudoparesis due to irreparable rotator cuff dysfunction with the Delta III reverse-ball-and-socket total shoulder prosthesis. J Bone Joint Surg Am. 2005;87(7):1476-1486.
13. Nyffeler RW, Werner CM, Simmen BR, Gerber C. Analysis of a retrieved Delta III total shoulder prosthesis. J Bone Joint Surg Br. 2004;86(8):1187-1191.
14. Grassi FA, Murena L, Valli F, Alberio R. Six-year experience with the Delta III reverse shoulder prosthesis. J Orthop Surg (Hong Kong). 2009;17(2):151-156.
15. Lévigne C, Garret J, Boileau P, Alami G, Favard L, Walch G. Scapular notching in reverse shoulder arthroplasty: is it important to avoid it and how? Clin Orthop. 2011;469(9):2512-2520.
16. Nyffeler RW, Werner CM, Gerber C. Biomechanical relevance of glenoid component positioning in the reverse Delta III total shoulder prosthesis. J Shoulder Elbow Surg. 2005;14(5):524-528.
17. Rittmeister M, Kerschbaumer F. Grammont reverse total shoulder arthroplasty in patients with rheumatoid arthritis and nonreconstructible rotator cuff lesions. J Shoulder Elbow Surg. 2001;10(1):17-22.
18. Vanhove B, Beugnies A. Grammont’s reverse shoulder prosthesis for rotator cuff arthropathy. A retrospective study of 32 cases. Acta Orthop Belg. 2004;70(3):219-225.
19. Kempton LB, Balasubramaniam M, Ankerson E, Wiater JM. A radiographic analysis of the effects of glenosphere position on scapular notching following reverse total shoulder arthroplasty. J Shoulder Elbow Surg. 2011;20(6):968-974.
20. Gutiérrez S, Greiwe RM, Frankle MA, Siegal S, Lee WE. Biomechanical comparison of component position and hardware failure in the reverse shoulder prosthesis. J Shoulder Elbow Surg. 2007;16(3 suppl):S9-S12.
21. Roche CP, Diep P, Hamilton M, et al. Impact of inferior glenoid tilt, humeral retroversion, bone grafting, and design parameters on muscle length and deltoid wrapping in reverse shoulder arthroplasty. Bull Hosp Jt Dis. 2013;71(4):284-293.
1. Woodruff MJ, Cohen AP, Bradley JG. Arthroplasty of the shoulder in rheumatoid arthritis with rotator cuff dysfunction. Int Orthop. 2003;27(1):7-10.
2. Inman VT, Saunders JB, Abbott LC. Observations of the function of the shoulder joint. 1944. Clin Orthop. 1996;(330):3-12.
3. Boileau P, Watkinson D, Hatzidakis AM, Hovorka I. Neer Award 2005: the Grammont reverse shoulder prosthesis: results in cuff tear arthritis, fracture sequelae, and revision arthroplasty. J Shoulder Elbow Surg. 2006;15(5):527-540.
4. Boileau P, Watkinson DJ, Hatzidakis AM, Balg F. Grammont reverse prosthesis: design, rationale, and biomechanics. J Shoulder Elbow Surg. 2005;14(1 suppl S):147S-161S.
5. Sirveaux F, Favard L, Oudet D, Huquet D, Walch G, Molé D. Grammont inverted total shoulder arthroplasty in the treatment of glenohumeral osteoarthritis with massive rupture of the cuff. Results of a multicentre study of 80 shoulders. J Bone Joint Surg Br. 2004;86(3):388-395.
6. Kowalsky MS, Galatz LM, Shia DS, Steger-May K, Keener JD. The relationship between scapular notching and reverse shoulder arthroplasty prosthesis design. J Shoulder Elbow Surg. 2012;21(10):1430-1441.
7. Lévigne C, Boileau P, Favard L, et al. Scapular notching in reverse shoulder arthroplasty. J Shoulder Elbow Surg. 2008;17(6):925-935.
8. Simovitch RW, Zumstein MA, Lohri E, Helmy N, Gerber C. Predictors of scapular notching in patients managed with the Delta III reverse total shoulder replacement. J Bone Joint Surg Am. 2007;89(3):588-600.
9. Torrens C, Corrales M, Gonzalez G, Solano A, Caceres E. Morphology of the scapula relative to the reverse shoulder prosthesis. J Orthop Surg (Hong Kong). 2009;17(2):146-150.
10. McFarland EG, Sanguanjit P, Tasaki A, Keyurapan E, Fishman EK, Fayad LM. The reverse shoulder prosthesis: a review of imaging features and complications. Skeletal Radiol. 2006;35(7):488-496.
11. Valenti PH, Boutens D, Nerot C. Delta 3 reversed prosthesis for osteoarthritis with massive rotator cuff tear: long-term results (>5 years). In: Walch G, Boileau P, Molé D, eds. Shoulder Prosthesis: Two to Ten Years Follow-Up. Montpellier, France: Sauramps Medical; 2001:253-259.
12. Werner CM, Steinmann PA, Gilbart M, Gerber C. Treatment of painful pseudoparesis due to irreparable rotator cuff dysfunction with the Delta III reverse-ball-and-socket total shoulder prosthesis. J Bone Joint Surg Am. 2005;87(7):1476-1486.
13. Nyffeler RW, Werner CM, Simmen BR, Gerber C. Analysis of a retrieved Delta III total shoulder prosthesis. J Bone Joint Surg Br. 2004;86(8):1187-1191.
14. Grassi FA, Murena L, Valli F, Alberio R. Six-year experience with the Delta III reverse shoulder prosthesis. J Orthop Surg (Hong Kong). 2009;17(2):151-156.
15. Lévigne C, Garret J, Boileau P, Alami G, Favard L, Walch G. Scapular notching in reverse shoulder arthroplasty: is it important to avoid it and how? Clin Orthop. 2011;469(9):2512-2520.
16. Nyffeler RW, Werner CM, Gerber C. Biomechanical relevance of glenoid component positioning in the reverse Delta III total shoulder prosthesis. J Shoulder Elbow Surg. 2005;14(5):524-528.
17. Rittmeister M, Kerschbaumer F. Grammont reverse total shoulder arthroplasty in patients with rheumatoid arthritis and nonreconstructible rotator cuff lesions. J Shoulder Elbow Surg. 2001;10(1):17-22.
18. Vanhove B, Beugnies A. Grammont’s reverse shoulder prosthesis for rotator cuff arthropathy. A retrospective study of 32 cases. Acta Orthop Belg. 2004;70(3):219-225.
19. Kempton LB, Balasubramaniam M, Ankerson E, Wiater JM. A radiographic analysis of the effects of glenosphere position on scapular notching following reverse total shoulder arthroplasty. J Shoulder Elbow Surg. 2011;20(6):968-974.
20. Gutiérrez S, Greiwe RM, Frankle MA, Siegal S, Lee WE. Biomechanical comparison of component position and hardware failure in the reverse shoulder prosthesis. J Shoulder Elbow Surg. 2007;16(3 suppl):S9-S12.
21. Roche CP, Diep P, Hamilton M, et al. Impact of inferior glenoid tilt, humeral retroversion, bone grafting, and design parameters on muscle length and deltoid wrapping in reverse shoulder arthroplasty. Bull Hosp Jt Dis. 2013;71(4):284-293.
Risk Factors for In-Hospital Myocardial Infarction After Shoulder Arthroplasty
The incidence of shoulder arthroplasty in the United States is increasing annually,1-3 and the majority of these operations occur in older patients.4-6 Elderly patients with cardiovascular, pulmonary, cerebral, renal, and hepatic disease are increasingly susceptible to numerous surgical complications.4 Myocardial infarction (MI) is a complication that occurs in 0.7% of noncardiac surgeries. This figure increases to 1.1% in patients with coronary artery disease.7-11 Perioperative MI increases morbidity and mortality,8 and perioperative cardiac morbidity is the leading cause of death after anesthesia and surgery.12 The financial effects of perioperative cardiac morbidity and mortality must also be considered. A 2009 claims analysis study estimated charges associated with a perioperative MI at $15,000 and the cost of cardiac death at $21,909.13
Cardiovascular complications are associated with a significant degree of morbidity and mortality in patients who undergo arthroplasty.14-16 Although studies have elucidated 30- and 90-day morbidity and mortality rates after shoulder arthroplasty, in hip and knee arthroplasty17-19 little has been done to determine predictors of perioperative MI in a representative database of patients. Given the increasing incidence of shoulder arthroplasty in the United States, the elective nature of this procedure, and the percentage of the US population with cardiovascular risk factors,20 it is important to establish predictors of perioperative MI to ensure patients and physicians have the necessary resources to make informed decisions.
We conducted a study to examine the risk factors for perioperative MI in a large cohort of patients admitted for shoulder arthroplasty to US hospitals. We wanted to evaluate the association between perioperative MI and shoulder arthroplasty with respect to demographics, primary diagnosis, medical comorbidities, and perioperative complications. Specifically, we tested the null hypothesis that, among patients undergoing shoulder arthroplasty, and accounting for confounding variables, there would be no difference in risk factors for patients who have a perioperative MI.
Materials and Methods
This study was exempt from approval by our institutional review board. All data used in this project were deidentified before use.
Nationwide Inpatient Sample (NIS)
The Nationwide Inpatient Sample (NIS), an annual survey of hospitals, is conducted by the Healthcare Cost and Utilization Project (HCUP) and sponsored by the Agency for Healthcare Research and Quality (AHRQ). This database is the largest publicly available all-payer inpatient discharge database in the United States.21 Sampling 8 million hospital stays each year, NIS includes information from a representative batch of 20% of US hospitals. In 2011, 46 states and 1045 hospitals contributed information to the database, representing 97% of the US population.22 This large sample allows researchers to analyze a robust set of medical conditions and uncommon treatments. The survey, conducted each year since 1988, includes demographic, clinical, and resource use data.23 Discharge weight files are provided by NIS to arrive at valid national estimates.
This database is particularly useful because it provides information on up to 25 medical diagnoses and 15 procedures, which are recorded with International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) codes. Researchers can use this database to analyze patient and hospital characteristics as well as inpatient outcomes.24,25 Numerous studies have used NIS to address pertinent queries across the medical landscape.22,26
Patient Selection and Analysis
We used NIS to isolate a population of 422,371 adults (≥18 years old) who underwent total shoulder arthroplasty (TSA) or hemi–shoulder arthroplasty (HSA) between January 1, 2002 and December 31, 2011. We then placed the patients in this population into 1 of 2 cohorts. The first cohort had an acute MI during the perioperative period after TSA, and the second, larger cohort did not have an acute MI after TSA. Acute MI was identified using ICD-9-CM code 410.xx. To identify a population of shoulder arthroplasty patients, we included discharges with an ICD-9-CM procedure code of 81.80 or 81.88 (both TSA) or 81.81 (HSA) in the sample. We then considered the degree to which each of 5 variables—primary diagnosis, age, sex, race, and select medical comorbidities—was predictive of in-hospital MI after TSA.
Statistical Analysis
Given the large sample used in this study, normal distribution of data was assumed. Using bivariate analysis, Pearson χ2 test for categorical data, and independent-samples t test for continuous data, we compared the nonacute MI and acute MI groups. Multivariable binary logistic regression analyses allowed us to isolate the extent that primary diagnosis, age, sex, race, and medical comorbidities were predictors of acute MI after shoulder arthroplasty. Statistical significance was set at P < .05. SPSS Version 22.0 (SPSS, Chicago, Illinois) was used for all statistical analyses and data modeling.
Results
Between January 1, 2002 and December 31, 2011, an estimated total of 422,371 patients underwent shoulder arthroplasty (59.3% TSA, 40.7% HSA). Of these patients, 1174 (0.28%) had a perioperative MI, and 421,197 (99.72%) did not (Table 1). Patients with a primary diagnosis of proximal humerus fracture (33.8% vs 16.6%; P < .001) or rotator cuff arthropathy (10.1% vs 9.9%; P < .001) were more likely than patients with other diagnoses to have an in-hospital MI.
Our review of the demographics found that patients who underwent shoulder arthroplasty and had a perioperative MI were likely older (75±8.9 years vs 69±11 years; P < .001), Caucasian (94.2% vs 91.9%; P = .002), male (43.2% vs 39.7%; P = .013), in the highest median household income bracket of $63,000 or more (30.8% vs 25.6%; P < .001), and using Medicare (80.9% vs 66.3%; P < .001). They were more likely to be treated in a medical center of medium size (25.6% vs 23.7%; P = .042) or larger (61.8% vs 61.2%; P = .042). MIs occurred more often in urban environments (91.4% vs 88.5%; P = .002) and in HSA patients (55% vs 40.6%; P < .001), resulting in longer hospital stays (9.4±7.9 days vs 2.7±2.5 days; P < .001) and higher probability of death (6.5% vs 0.1%; P < .001).
We then analyzed the 2 cohorts for medical comorbidities (Table 2). Patients in the MI cohort presented with a significantly higher incidence of congestive heart failure, previous MI, angina pectoris, chronic lung disease, hypertension, diabetes, renal failure, fluid and electrolyte disorders, pulmonary circulatory disease, coagulopathy, and deficiency anemia (P < .001) but not liver disease and obesity. Bivariate analysis of perioperative outcomes (Table 3) indicated that these patients also had a statistically higher rate of numerous other complications: pulmonary embolism (4.9% vs 0.2%; P < .001), pneumonia (15.1% vs 1.2%; P < .001), deep venous thrombosis (2.6% vs 0.2%; P < .001), cerebrovascular event (1.6% vs 0.1%; P < .001), acute renal failure (15.1% vs 1.2%; P < .001), gastrointestinal complication (1.2% vs 0.3%; P < .001), mechanical ventilation (1.2% vs 0.3%; P < .001), transfusion (33.4% vs 8.8%; P < .001), and nonroutine discharge (73.3% vs 36.0%; P < .001).
Multivariable logistic regression analysis was performed to determine independent predictors of perioperative MI after shoulder arthroplasty (Table 4). Patients with a primary diagnosis of proximal humerus fracture (odds ratio [OR], 1.38; 95% confidence interval [CI], 1.15-1.65; P < .001) were more likely than patients with a primary diagnosis of osteoarthritis to have an MI. The odds of postoperative MI increased with age (OR, 1.04 per year; 95% CI, 1.03-1.05; P < .001) and were higher in males (OR, 1.72; 95% CI, 1.52-1.96; P < .001). Compared with Caucasians, African Americans (OR, 0.19; 95% CI, 0.09-0.40; P < .001) were less likely to have an in-hospital MI after shoulder arthroplasty. After shoulder arthroplasty, the odds of MI in the perioperative period increased with each subsequent day of care (OR, 1.10; 95% CI, 1.10-1.11; P < .001).
Regarding independent comorbidities, multivariable logistic regression analysis also determined that history of congestive heart failure (OR, 4.86; 95% CI, 4.20-5.61; P < .001), angina pectoris (OR, 2.90; 95% CI, 2.02-4.17; P < .001), complicated diabetes (OR, 1.96; 95% CI, 1.49-2.57; P < .001), renal failure (OR, 1.42; 95% CI, 1.17-1.72; P < .001), fluid and electrolyte disorders (OR, 1.42; 95% CI, 1.21-1.67; P < .001), and deficiency anemia (OR, 1.62; 95% CI, 1.40-1.88; P < .001) were significant predictors of perioperative MI after shoulder arthroplasty.
Discussion
Results of other studies have elucidated 30- and 90-day mortality rates and postoperative complications after shoulder arthroplasty, but, relative to hip and knee arthroplasty,17-19 little has been done to determine predictors of perioperative MI in a large sample of shoulder arthroplasty patients. Given the increasing rates of shoulder arthroplasty1-3 and the demographics of this population,4-6 it is likely that postoperative cardiovascular events will increase in frequency. We found that, in order of decreasing significance, the top 4 risk predictors for acute MI after shoulder arthroplasty were congestive heart failure, angina pectoralis, complicated diabetes mellitus, and male sex. Other pertinent risk factors included older age, Caucasian ethnicity, and a primary diagnosis of proximal humerus fracture. The rate of acute MI in patients who were older than 75 years when they underwent HSA for proximal humerus fracture was 0.80%.
Demographics
We found that patients who had an acute MI after shoulder arthroplasty were likely older, male, and Caucasian. Age and male sex are well-established risk factors for increased cardiac complications after arthroplasty.27-29 Previous studies have indicated that the rate of cardiac events increases in arthroplasty patients older than 65 years.19,28,29 In our study, more than 50% of the patients who had an acute perioperative MI were older than 85 years. Less explainable is the increased occurrence of acute MI in Caucasian patients and wealthy patients, given that minorities in the United States have higher rates of cardiovascular disease.30 Shoulder arthroplasty is an elective procedure, more likely to be undertaken by Caucasians. Therefore, at-risk minority groups and financially challenged groups may be less likely to have this procedure.
Primary Diagnosis
In this series, patients with a primary diagnosis of proximal humerus fracture were more likely to have an in-hospital MI. This finding is consistent with previous studies indicating a higher rate of complications for proximal humerus fracture patients than for shoulder arthroplasty patients.31,32 Given that more than 75% of patients who present with a proximal humerus fracture are older than 70 years, it would be prudent to examine operative indications after this diagnosis,33 particularly as benefit from surgery for fractures has not been definitively demonstrated.34-37
Comorbidities
Many of the patients in our MI cohort presented with congestive heart failure, angina pectoris, complicated diabetes, renal failure, fluid and electrolyte disorders, or deficiency anemia. This is in keeping with other studies indicating that preexisting cardiovascular morbidity increases the rate of MI after various forms of arthroplasty.7-11 Patients in our MI cohort were also susceptible to a variety of post-MI perioperative complications, including pulmonary embolism, pneumonia, deep venous thrombosis, cerebrovascular event, acute renal failure, gastrointestinal complication, mechanical ventilation, transfusion, and nonroutine discharge, and their incidence of death was higher. These findings are consistent with reports that postoperative cardiovascular complications increase the degree of morbidity and mortality in arthroplasty patients.14-16 It is also worth noting that the odds of MI in the perioperative period increase with each subsequent day of care. This is understandable given that patients presenting with numerous comorbidities are at increased risk for perioperative complications38 resulting in hospital readmission.39
The literature indicates that MI occurs as a complication in 0.7% of patients who undergo noncardiac surgery,7 though some series have shown it is more prevalent after arthroplasty procedures.28,40 MI significantly increases the rate of perioperative morbidity and mortality,8 and perioperative cardiac morbidity is a leading cause of death after anesthesia and surgery.12 Furthermore, the most common cause of death after lower extremity arthroplasty is cardiovascular-related.41,42 In patients who presented for elective hip arthroplasty, cardiorespiratory disease was one of the main risk factors (with older age and male sex) shown to increase perioperative mortality.43
Perioperative cardiovascular complications increase postoperative morbidity and mortality.12 The rate of cardiovascular complications after shoulder arthroplasty ranges from 0.8% to 2.6%, and the incidence of MI hovers between 0.3% and 0.9%.17,19,28,40,44 A recent study in 793 patients found that, over a 30-day period, cardiovascular complications accounted for more than one-fourth of all complications.17 Singh and colleagues19 analyzed cardiopulmonary complications after primary shoulder arthroplasty in a total of 3480 patients (4019 arthroplasties) and found this group had a 90-day cardiac morbidity (MI, congestive heart failure, arrhythmia) rate of 2.6%. In that study, a Deyo-Charlson index of 1 or more was a significant independent risk factor for cardiac complications following surgery. Scores on this weighted index of 17 comorbidities are used to assess the complexities of a patient population. Given the severity of cardiovascular perioperative complications, it is important to preoperatively identify high-risk population groups and sufficiently study and optimize patients before shoulder arthroplasty.
There is much debate about the effectiveness of perioperative β-blockers in reducing perioperative cardiac morbidity and mortality.45-48 Such a discussion is outside of the scope of this article, but it may be prudent to seek a cardiology consultation for patients presenting with risk factors for perioperative MI. β-Blockers may prove useful in reducing cardiac morbidity in high-risk patients after noncardiac surgery.45,49
Many limitations are inherent in studies that use a nationally represented database such as NIS, which we used in this study. It is highly likely that NIS does not capture all potential postoperative complications, as this database is very large and subject to errors in data entry and clinical coding. In addition, detailed clinical information (eg, severity of certain comorbid diseases before shoulder arthroplasty, details about the intraoperative course) was not readily available for analysis. Another limitation, which may have led to an underestimate of complication rates, was our not being able to obtain information about postdischarge complications.
Despite these limitations, NIS and other databases have helped researchers answer questions about low-incidence conditions and generalize findings to a national population. In the present study, we analyzed 2 cohorts, patients with and without acute MI after shoulder arthroplasty, to determine predictors for and complications of postarthroplasty MI. We identified numerous predictors for acute MI: congestive heart failure, angina pectoris, complicated diabetes, renal failure, fluid and electrolyte disorders, and deficiency anemia prior to arthroplasty. As perioperative MI is associated with significant morbidity,14-16 it would be wise to screen patients for such comorbid conditions, assess the severity of these conditions, and offer shoulder arthroplasty with prudence.
Conclusion
The top 4 predictors for acute MI after shoulder arthroplasty were congestive heart failure, angina pectoralis, complicated diabetes mellitus, and male sex. Other pertinent risk factors included older age, Caucasian ethnicity, and primary diagnosis of proximal humerus fracture. Surgeons and patients must be aware of predictors for adverse surgical outcomes such as perioperative MI and understand the extent to which these events increase perioperative morbidity and mortality.
1. Day JS, Lau E, Ong KL, Williams GR, Ramsey ML, Kurtz SM. Prevalence and projections of total shoulder and elbow arthroplasty in the United States to 2015. J Shoulder Elbow Surg. 2010;19(8):1115-1120.
2. Kim SH, Wise BL, Zhang Y, Szabo RM. Increasing incidence of shoulder arthroplasty in the United States. J Bone Joint Surg Am. 2011;93(24):2249-2254.
3. Kurtz SM, Lau E, Ong K, Zhao K, Kelly M, Bozic KJ. Future young patient demand for primary and revision joint replacement: national projections from 2010 to 2030. Clin Orthop. 2009;467(10):2606-2612.
4. Boettcher WG. Total hip arthroplasties in the elderly. Morbidity, mortality, and cost effectiveness. Clin Orthop. 1992;(274):30-34.
5. Greenfield S, Apolone G, McNeil BJ, Cleary PD. The importance of co-existent disease in the occurrence of postoperative complications and one-year recovery in patients undergoing total hip replacement. Comorbidity and outcomes after hip replacement. Med Care. 1993;31(2):141-154.
6. Kreder HJ, Williams JI, Jaglal S, Hu R, Axcell T, Stephen D. Are complication rates for elective primary total hip arthroplasty in Ontario related to surgeon and hospital volumes? A preliminary investigation. Can J Surg. 1998;41(6):431-437.
7. Botto F, Alonso-Coello P, Chan MT, et al. Myocardial injury after noncardiac surgery: a large, international, prospective cohort study establishing diagnostic criteria, characteristics, predictors, and 30-day outcomes. Anesthesiology. 2014;120(3):564-578.
8. Mangano DT, Browner WS, Hollenberg M, London MJ, Tubau JF, Tateo IM. Association of perioperative myocardial ischemia with cardiac morbidity and mortality in men undergoing noncardiac surgery. The Study of Perioperative Ischemia Research Group. N Engl J Med. 1990;323(26):1781-1788.
9. Tarhan S, Moffitt EA, Taylor WF, Giuliani ER. Myocardial infarction after general anesthesia. JAMA. 1972;220(11):1451-1454.
10. Landesberg G, Mosseri M, Zahger D, et al. Myocardial infarction after vascular surgery: the role of prolonged stress-induced, ST depression-type ischemia. J Am Coll Cardiol. 2001;37(7):1839-1845.
11. van Waes JA, Nathoe HM, de Graaff JC, et al. Myocardial injury after noncardiac surgery and its association with short-term mortality. Circulation. 2013;127(23):2264-2271.
12. Mangano DT. Perioperative cardiac morbidity. Anesthesiology. 1990;72(1):153-184.
13. Fleisher LA, Corbett W, Berry C, Poldermans D. Cost-effectiveness of differing perioperative beta-blockade strategies in vascular surgery patients. J Cardiothorac Vasc Anesth. 2004;18(1):7-13.
14. Aynardi M, Pulido L, Parvizi J, Sharkey PF, Rothman RH. Early mortality after modern total hip arthroplasty. Clin Orthop. 2009;467(1):213-218.
15. Gangireddy C, Rectenwald JR, Upchurch GR, et al. Risk factors and clinical impact of postoperative symptomatic venous thromboembolism. J Vasc Surg. 2007;45(2):335-341.
16. Baser O, Supina D, Sengupta N, Wang L, Kwong L. Impact of postoperative venous thromboembolism on Medicare recipients undergoing total hip replacement or total knee replacement surgery. Am J Health Syst Pharm. 2010;67(17):1438-1445.
17. Fehringer EV, Mikuls TR, Michaud KD, Henderson WG, O’Dell JR. Shoulder arthroplasties have fewer complications than hip or knee arthroplasties in US veterans. Clin Orthop. 2010;468(3):717-722.
18. Farmer KW, Hammond JW, Queale WS, Keyurapan E, McFarland EG. Shoulder arthroplasty versus hip and knee arthroplasties: a comparison of outcomes. Clin Orthop. 2007;(455):183-189.
19. Singh JA, Sperling JW, Cofield RH. Cardiopulmonary complications after primary shoulder arthroplasty: a cohort study. Semin Arthritis Rheum. 2012;41(5):689-697.
20. Go AS, Mozaffarian D, Roger VL, et al; American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics—2014 update: a report from the American Heart Association. Circulation. 2014;129(3):e28-e292.
21. Lin CA, Kuo AC, Takemoto S. Comorbidities and perioperative complications in HIV-positive patients undergoing primary total hip and knee arthroplasty. J Bone Joint Surg Am. 2013;95(11):1028-1036.
22. Maynard C, Sales AE. Changes in the use of coronary artery revascularization procedures in the Department of Veterans Affairs, the National Hospital Discharge Survey, and the Nationwide Inpatient Sample, 1991–1999. BMC Health Serv Res. 2003;3(1):12.
23. Griffin JW, Novicoff WM, Browne JA, Brockmeier SF. Obstructive sleep apnea as a risk factor after shoulder arthroplasty. J Shoulder Elbow Surg. 2013;22(12):e6-e9.
24. Hambright D, Henderson RA, Cook C, Worrell T, Moorman CT, Bolognesi MP. A comparison of perioperative outcomes in patients with and without rheumatoid arthritis after receiving a total shoulder replacement arthroplasty. J Shoulder Elbow Surg. 2011;20(1):77-85.
25. Odum SM, Troyer JL, Kelly MP, Dedini RD, Bozic KJ. A cost-utility analysis comparing the cost-effectiveness of simultaneous and staged bilateral total knee arthroplasty. J Bone Joint Surg Am. 2013;95(16):1441-1449.
26. Ponce BA, Menendez ME, Oladeji LO, Soldado F. Diabetes as a risk factor for poorer early postoperative outcomes after shoulder arthroplasty. J Shoulder Elbow Surg. 2014;23(5):671-678.
27. Alfonso DT, Toussaint RJ, Alfonso BD, Strauss EJ, Steiger DT, Di Cesare PE. Nonsurgical complications after total hip and knee arthroplasty. Am J Orthop. 2006;35(11):503-510.
28. Mantilla CB, Horlocker TT, Schroeder DR, Berry DJ, Brown DL. Frequency of myocardial infarction, pulmonary embolism, deep venous thrombosis, and death following primary hip or knee arthroplasty. Anesthesiology. 2002;96(5):1140-1146.
29. Singh JA, Jensen MR, Harmsen WS, Gabriel SE, Lewallen DG. Cardiac and thromboembolic complications and mortality in patients undergoing total hip and total knee arthroplasty. Ann Rheum Dis. 2011;70(12):2082-2088.
30. Kurian AK, Cardarelli KM. Racial and ethnic differences in cardiovascular disease risk factors: a systematic review. Ethn Dis. 2007;17(1):143-152.
31. Zhang AL, Schairer WW, Feeley BT. Hospital readmissions after surgical treatment of proximal humerus fractures: is arthroplasty safer than open reduction internal fixation? Clin Orthop. 2014;472(8):2317-2324.
32. Schairer WW, Zhang AL, Feeley BT. Hospital readmissions after primary shoulder arthroplasty. J Shoulder Elbow Surg. 2014;23(9):1349-1355.
33. de Kruijf M, Vroemen JP, de Leur K, van der Voort EA, Vos DI, Van der Laan L. Proximal fractures of the humerus in patients older than 75 years of age: should we consider operative treatment? J Orthop Traumatol. 2014;15(2):111-115.
34. Hauschild O, Konrad G, Audige L, et al. Operative versus non-operative treatment for two-part surgical neck fractures of the proximal humerus. Arch Orthop Trauma Surg. 2013;133(10):1385-1393.
35. Hanson B, Neidenbach P, de Boer P, Stengel D. Functional outcomes after nonoperative management of fractures of the proximal humerus. J Shoulder Elbow Surg. 2009;18(4):612-621.
36. Handoll HH, Ollivere BJ, Rollins KE. Interventions for treating proximal humeral fractures in adults. Cochrane Database Syst Rev. 2012;12:CD000434.
37. Court-Brown CM, Cattermole H, McQueen MM. Impacted valgus fractures (B1.1) of the proximal humerus. The results of non-operative treatment. J Bone Joint Surg Br. 2002;84(4):504-508.
38. Chalmers PN, Gupta AK, Rahman Z, Bruce B, Romeo AA, Nicholson GP. Predictors of early complications of total shoulder arthroplasty. J Arthroplasty. 2014;29(4):856-860.
39. Mahoney A, Bosco JA 3rd, Zuckerman JD. Readmission after shoulder arthroplasty. J Shoulder Elbow Surg. 2014;23(3):377-381.
40. Khan SK, Malviya A, Muller SD, et al. Reduced short-term complications and mortality following Enhanced Recovery primary hip and knee arthroplasty: results from 6,000 consecutive procedures. Acta Orthop. 2014;85(1):26-31.
41. Paavolainen P, Pukkala E, Pulkkinen P, Visuri T. Causes of death after total hip arthroplasty: a nationwide cohort study with 24,638 patients. J Arthroplasty. 2002;17(3):274-281.
42. Sharrock NE, Cazan MG, Hargett MJ, Williams-Russo P, Wilson PD Jr. Changes in mortality after total hip and knee arthroplasty over a ten-year period. Anesth Analg. 1995;80(2):242-248.
43. Parvizi J, Johnson BG, Rowland C, Ereth MH, Lewallen DG. Thirty-day mortality after elective total hip arthroplasty. J Bone Joint Surg Am. 2001;83(10):1524-1528.
44. Morris MJ, Molli RG, Berend KR, Lombardi AV Jr. Mortality and perioperative complications after unicompartmental knee arthroplasty. Knee. 2013;20(3):218-220.
45. Lindenauer PK, Pekow P, Wang K, Mamidi DK, Gutierrez B, Benjamin EM. Perioperative beta-blocker therapy and mortality after major noncardiac surgery. N Engl J Med. 2005;353(4):349-361.
46. Wijeysundera DN, Beattie WS, Wijeysundera HC, Yun L, Austin PC, Ko DT. Duration of preoperative beta-blockade and outcomes after major elective noncardiac surgery. Can J Cardiol. 2014;30(2):217-223.
47. Andersson C, Merie C, Jorgensen M, et al. Association of beta-blocker therapy with risks of adverse cardiovascular events and deaths in patients with ischemic heart disease undergoing noncardiac surgery: a Danish nationwide cohort study. JAMA Int Med. 2014;174(3):336-344.
48. Bakker EJ, Ravensbergen NJ, Poldermans D. Perioperative cardiac evaluation, monitoring, and risk reduction strategies in noncardiac surgery patients. Curr Opin Crit Care. 2011;17(5):409-415.
49. Auerbach AD, Goldman L. Beta-blockers and reduction of cardiac events in noncardiac surgery: scientific review. JAMA. 2002;287(11):1435-1444.
The incidence of shoulder arthroplasty in the United States is increasing annually,1-3 and the majority of these operations occur in older patients.4-6 Elderly patients with cardiovascular, pulmonary, cerebral, renal, and hepatic disease are increasingly susceptible to numerous surgical complications.4 Myocardial infarction (MI) is a complication that occurs in 0.7% of noncardiac surgeries. This figure increases to 1.1% in patients with coronary artery disease.7-11 Perioperative MI increases morbidity and mortality,8 and perioperative cardiac morbidity is the leading cause of death after anesthesia and surgery.12 The financial effects of perioperative cardiac morbidity and mortality must also be considered. A 2009 claims analysis study estimated charges associated with a perioperative MI at $15,000 and the cost of cardiac death at $21,909.13
Cardiovascular complications are associated with a significant degree of morbidity and mortality in patients who undergo arthroplasty.14-16 Although studies have elucidated 30- and 90-day morbidity and mortality rates after shoulder arthroplasty, in hip and knee arthroplasty17-19 little has been done to determine predictors of perioperative MI in a representative database of patients. Given the increasing incidence of shoulder arthroplasty in the United States, the elective nature of this procedure, and the percentage of the US population with cardiovascular risk factors,20 it is important to establish predictors of perioperative MI to ensure patients and physicians have the necessary resources to make informed decisions.
We conducted a study to examine the risk factors for perioperative MI in a large cohort of patients admitted for shoulder arthroplasty to US hospitals. We wanted to evaluate the association between perioperative MI and shoulder arthroplasty with respect to demographics, primary diagnosis, medical comorbidities, and perioperative complications. Specifically, we tested the null hypothesis that, among patients undergoing shoulder arthroplasty, and accounting for confounding variables, there would be no difference in risk factors for patients who have a perioperative MI.
Materials and Methods
This study was exempt from approval by our institutional review board. All data used in this project were deidentified before use.
Nationwide Inpatient Sample (NIS)
The Nationwide Inpatient Sample (NIS), an annual survey of hospitals, is conducted by the Healthcare Cost and Utilization Project (HCUP) and sponsored by the Agency for Healthcare Research and Quality (AHRQ). This database is the largest publicly available all-payer inpatient discharge database in the United States.21 Sampling 8 million hospital stays each year, NIS includes information from a representative batch of 20% of US hospitals. In 2011, 46 states and 1045 hospitals contributed information to the database, representing 97% of the US population.22 This large sample allows researchers to analyze a robust set of medical conditions and uncommon treatments. The survey, conducted each year since 1988, includes demographic, clinical, and resource use data.23 Discharge weight files are provided by NIS to arrive at valid national estimates.
This database is particularly useful because it provides information on up to 25 medical diagnoses and 15 procedures, which are recorded with International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) codes. Researchers can use this database to analyze patient and hospital characteristics as well as inpatient outcomes.24,25 Numerous studies have used NIS to address pertinent queries across the medical landscape.22,26
Patient Selection and Analysis
We used NIS to isolate a population of 422,371 adults (≥18 years old) who underwent total shoulder arthroplasty (TSA) or hemi–shoulder arthroplasty (HSA) between January 1, 2002 and December 31, 2011. We then placed the patients in this population into 1 of 2 cohorts. The first cohort had an acute MI during the perioperative period after TSA, and the second, larger cohort did not have an acute MI after TSA. Acute MI was identified using ICD-9-CM code 410.xx. To identify a population of shoulder arthroplasty patients, we included discharges with an ICD-9-CM procedure code of 81.80 or 81.88 (both TSA) or 81.81 (HSA) in the sample. We then considered the degree to which each of 5 variables—primary diagnosis, age, sex, race, and select medical comorbidities—was predictive of in-hospital MI after TSA.
Statistical Analysis
Given the large sample used in this study, normal distribution of data was assumed. Using bivariate analysis, Pearson χ2 test for categorical data, and independent-samples t test for continuous data, we compared the nonacute MI and acute MI groups. Multivariable binary logistic regression analyses allowed us to isolate the extent that primary diagnosis, age, sex, race, and medical comorbidities were predictors of acute MI after shoulder arthroplasty. Statistical significance was set at P < .05. SPSS Version 22.0 (SPSS, Chicago, Illinois) was used for all statistical analyses and data modeling.
Results
Between January 1, 2002 and December 31, 2011, an estimated total of 422,371 patients underwent shoulder arthroplasty (59.3% TSA, 40.7% HSA). Of these patients, 1174 (0.28%) had a perioperative MI, and 421,197 (99.72%) did not (Table 1). Patients with a primary diagnosis of proximal humerus fracture (33.8% vs 16.6%; P < .001) or rotator cuff arthropathy (10.1% vs 9.9%; P < .001) were more likely than patients with other diagnoses to have an in-hospital MI.
Our review of the demographics found that patients who underwent shoulder arthroplasty and had a perioperative MI were likely older (75±8.9 years vs 69±11 years; P < .001), Caucasian (94.2% vs 91.9%; P = .002), male (43.2% vs 39.7%; P = .013), in the highest median household income bracket of $63,000 or more (30.8% vs 25.6%; P < .001), and using Medicare (80.9% vs 66.3%; P < .001). They were more likely to be treated in a medical center of medium size (25.6% vs 23.7%; P = .042) or larger (61.8% vs 61.2%; P = .042). MIs occurred more often in urban environments (91.4% vs 88.5%; P = .002) and in HSA patients (55% vs 40.6%; P < .001), resulting in longer hospital stays (9.4±7.9 days vs 2.7±2.5 days; P < .001) and higher probability of death (6.5% vs 0.1%; P < .001).
We then analyzed the 2 cohorts for medical comorbidities (Table 2). Patients in the MI cohort presented with a significantly higher incidence of congestive heart failure, previous MI, angina pectoris, chronic lung disease, hypertension, diabetes, renal failure, fluid and electrolyte disorders, pulmonary circulatory disease, coagulopathy, and deficiency anemia (P < .001) but not liver disease and obesity. Bivariate analysis of perioperative outcomes (Table 3) indicated that these patients also had a statistically higher rate of numerous other complications: pulmonary embolism (4.9% vs 0.2%; P < .001), pneumonia (15.1% vs 1.2%; P < .001), deep venous thrombosis (2.6% vs 0.2%; P < .001), cerebrovascular event (1.6% vs 0.1%; P < .001), acute renal failure (15.1% vs 1.2%; P < .001), gastrointestinal complication (1.2% vs 0.3%; P < .001), mechanical ventilation (1.2% vs 0.3%; P < .001), transfusion (33.4% vs 8.8%; P < .001), and nonroutine discharge (73.3% vs 36.0%; P < .001).
Multivariable logistic regression analysis was performed to determine independent predictors of perioperative MI after shoulder arthroplasty (Table 4). Patients with a primary diagnosis of proximal humerus fracture (odds ratio [OR], 1.38; 95% confidence interval [CI], 1.15-1.65; P < .001) were more likely than patients with a primary diagnosis of osteoarthritis to have an MI. The odds of postoperative MI increased with age (OR, 1.04 per year; 95% CI, 1.03-1.05; P < .001) and were higher in males (OR, 1.72; 95% CI, 1.52-1.96; P < .001). Compared with Caucasians, African Americans (OR, 0.19; 95% CI, 0.09-0.40; P < .001) were less likely to have an in-hospital MI after shoulder arthroplasty. After shoulder arthroplasty, the odds of MI in the perioperative period increased with each subsequent day of care (OR, 1.10; 95% CI, 1.10-1.11; P < .001).
Regarding independent comorbidities, multivariable logistic regression analysis also determined that history of congestive heart failure (OR, 4.86; 95% CI, 4.20-5.61; P < .001), angina pectoris (OR, 2.90; 95% CI, 2.02-4.17; P < .001), complicated diabetes (OR, 1.96; 95% CI, 1.49-2.57; P < .001), renal failure (OR, 1.42; 95% CI, 1.17-1.72; P < .001), fluid and electrolyte disorders (OR, 1.42; 95% CI, 1.21-1.67; P < .001), and deficiency anemia (OR, 1.62; 95% CI, 1.40-1.88; P < .001) were significant predictors of perioperative MI after shoulder arthroplasty.
Discussion
Results of other studies have elucidated 30- and 90-day mortality rates and postoperative complications after shoulder arthroplasty, but, relative to hip and knee arthroplasty,17-19 little has been done to determine predictors of perioperative MI in a large sample of shoulder arthroplasty patients. Given the increasing rates of shoulder arthroplasty1-3 and the demographics of this population,4-6 it is likely that postoperative cardiovascular events will increase in frequency. We found that, in order of decreasing significance, the top 4 risk predictors for acute MI after shoulder arthroplasty were congestive heart failure, angina pectoralis, complicated diabetes mellitus, and male sex. Other pertinent risk factors included older age, Caucasian ethnicity, and a primary diagnosis of proximal humerus fracture. The rate of acute MI in patients who were older than 75 years when they underwent HSA for proximal humerus fracture was 0.80%.
Demographics
We found that patients who had an acute MI after shoulder arthroplasty were likely older, male, and Caucasian. Age and male sex are well-established risk factors for increased cardiac complications after arthroplasty.27-29 Previous studies have indicated that the rate of cardiac events increases in arthroplasty patients older than 65 years.19,28,29 In our study, more than 50% of the patients who had an acute perioperative MI were older than 85 years. Less explainable is the increased occurrence of acute MI in Caucasian patients and wealthy patients, given that minorities in the United States have higher rates of cardiovascular disease.30 Shoulder arthroplasty is an elective procedure, more likely to be undertaken by Caucasians. Therefore, at-risk minority groups and financially challenged groups may be less likely to have this procedure.
Primary Diagnosis
In this series, patients with a primary diagnosis of proximal humerus fracture were more likely to have an in-hospital MI. This finding is consistent with previous studies indicating a higher rate of complications for proximal humerus fracture patients than for shoulder arthroplasty patients.31,32 Given that more than 75% of patients who present with a proximal humerus fracture are older than 70 years, it would be prudent to examine operative indications after this diagnosis,33 particularly as benefit from surgery for fractures has not been definitively demonstrated.34-37
Comorbidities
Many of the patients in our MI cohort presented with congestive heart failure, angina pectoris, complicated diabetes, renal failure, fluid and electrolyte disorders, or deficiency anemia. This is in keeping with other studies indicating that preexisting cardiovascular morbidity increases the rate of MI after various forms of arthroplasty.7-11 Patients in our MI cohort were also susceptible to a variety of post-MI perioperative complications, including pulmonary embolism, pneumonia, deep venous thrombosis, cerebrovascular event, acute renal failure, gastrointestinal complication, mechanical ventilation, transfusion, and nonroutine discharge, and their incidence of death was higher. These findings are consistent with reports that postoperative cardiovascular complications increase the degree of morbidity and mortality in arthroplasty patients.14-16 It is also worth noting that the odds of MI in the perioperative period increase with each subsequent day of care. This is understandable given that patients presenting with numerous comorbidities are at increased risk for perioperative complications38 resulting in hospital readmission.39
The literature indicates that MI occurs as a complication in 0.7% of patients who undergo noncardiac surgery,7 though some series have shown it is more prevalent after arthroplasty procedures.28,40 MI significantly increases the rate of perioperative morbidity and mortality,8 and perioperative cardiac morbidity is a leading cause of death after anesthesia and surgery.12 Furthermore, the most common cause of death after lower extremity arthroplasty is cardiovascular-related.41,42 In patients who presented for elective hip arthroplasty, cardiorespiratory disease was one of the main risk factors (with older age and male sex) shown to increase perioperative mortality.43
Perioperative cardiovascular complications increase postoperative morbidity and mortality.12 The rate of cardiovascular complications after shoulder arthroplasty ranges from 0.8% to 2.6%, and the incidence of MI hovers between 0.3% and 0.9%.17,19,28,40,44 A recent study in 793 patients found that, over a 30-day period, cardiovascular complications accounted for more than one-fourth of all complications.17 Singh and colleagues19 analyzed cardiopulmonary complications after primary shoulder arthroplasty in a total of 3480 patients (4019 arthroplasties) and found this group had a 90-day cardiac morbidity (MI, congestive heart failure, arrhythmia) rate of 2.6%. In that study, a Deyo-Charlson index of 1 or more was a significant independent risk factor for cardiac complications following surgery. Scores on this weighted index of 17 comorbidities are used to assess the complexities of a patient population. Given the severity of cardiovascular perioperative complications, it is important to preoperatively identify high-risk population groups and sufficiently study and optimize patients before shoulder arthroplasty.
There is much debate about the effectiveness of perioperative β-blockers in reducing perioperative cardiac morbidity and mortality.45-48 Such a discussion is outside of the scope of this article, but it may be prudent to seek a cardiology consultation for patients presenting with risk factors for perioperative MI. β-Blockers may prove useful in reducing cardiac morbidity in high-risk patients after noncardiac surgery.45,49
Many limitations are inherent in studies that use a nationally represented database such as NIS, which we used in this study. It is highly likely that NIS does not capture all potential postoperative complications, as this database is very large and subject to errors in data entry and clinical coding. In addition, detailed clinical information (eg, severity of certain comorbid diseases before shoulder arthroplasty, details about the intraoperative course) was not readily available for analysis. Another limitation, which may have led to an underestimate of complication rates, was our not being able to obtain information about postdischarge complications.
Despite these limitations, NIS and other databases have helped researchers answer questions about low-incidence conditions and generalize findings to a national population. In the present study, we analyzed 2 cohorts, patients with and without acute MI after shoulder arthroplasty, to determine predictors for and complications of postarthroplasty MI. We identified numerous predictors for acute MI: congestive heart failure, angina pectoris, complicated diabetes, renal failure, fluid and electrolyte disorders, and deficiency anemia prior to arthroplasty. As perioperative MI is associated with significant morbidity,14-16 it would be wise to screen patients for such comorbid conditions, assess the severity of these conditions, and offer shoulder arthroplasty with prudence.
Conclusion
The top 4 predictors for acute MI after shoulder arthroplasty were congestive heart failure, angina pectoralis, complicated diabetes mellitus, and male sex. Other pertinent risk factors included older age, Caucasian ethnicity, and primary diagnosis of proximal humerus fracture. Surgeons and patients must be aware of predictors for adverse surgical outcomes such as perioperative MI and understand the extent to which these events increase perioperative morbidity and mortality.
The incidence of shoulder arthroplasty in the United States is increasing annually,1-3 and the majority of these operations occur in older patients.4-6 Elderly patients with cardiovascular, pulmonary, cerebral, renal, and hepatic disease are increasingly susceptible to numerous surgical complications.4 Myocardial infarction (MI) is a complication that occurs in 0.7% of noncardiac surgeries. This figure increases to 1.1% in patients with coronary artery disease.7-11 Perioperative MI increases morbidity and mortality,8 and perioperative cardiac morbidity is the leading cause of death after anesthesia and surgery.12 The financial effects of perioperative cardiac morbidity and mortality must also be considered. A 2009 claims analysis study estimated charges associated with a perioperative MI at $15,000 and the cost of cardiac death at $21,909.13
Cardiovascular complications are associated with a significant degree of morbidity and mortality in patients who undergo arthroplasty.14-16 Although studies have elucidated 30- and 90-day morbidity and mortality rates after shoulder arthroplasty, in hip and knee arthroplasty17-19 little has been done to determine predictors of perioperative MI in a representative database of patients. Given the increasing incidence of shoulder arthroplasty in the United States, the elective nature of this procedure, and the percentage of the US population with cardiovascular risk factors,20 it is important to establish predictors of perioperative MI to ensure patients and physicians have the necessary resources to make informed decisions.
We conducted a study to examine the risk factors for perioperative MI in a large cohort of patients admitted for shoulder arthroplasty to US hospitals. We wanted to evaluate the association between perioperative MI and shoulder arthroplasty with respect to demographics, primary diagnosis, medical comorbidities, and perioperative complications. Specifically, we tested the null hypothesis that, among patients undergoing shoulder arthroplasty, and accounting for confounding variables, there would be no difference in risk factors for patients who have a perioperative MI.
Materials and Methods
This study was exempt from approval by our institutional review board. All data used in this project were deidentified before use.
Nationwide Inpatient Sample (NIS)
The Nationwide Inpatient Sample (NIS), an annual survey of hospitals, is conducted by the Healthcare Cost and Utilization Project (HCUP) and sponsored by the Agency for Healthcare Research and Quality (AHRQ). This database is the largest publicly available all-payer inpatient discharge database in the United States.21 Sampling 8 million hospital stays each year, NIS includes information from a representative batch of 20% of US hospitals. In 2011, 46 states and 1045 hospitals contributed information to the database, representing 97% of the US population.22 This large sample allows researchers to analyze a robust set of medical conditions and uncommon treatments. The survey, conducted each year since 1988, includes demographic, clinical, and resource use data.23 Discharge weight files are provided by NIS to arrive at valid national estimates.
This database is particularly useful because it provides information on up to 25 medical diagnoses and 15 procedures, which are recorded with International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) codes. Researchers can use this database to analyze patient and hospital characteristics as well as inpatient outcomes.24,25 Numerous studies have used NIS to address pertinent queries across the medical landscape.22,26
Patient Selection and Analysis
We used NIS to isolate a population of 422,371 adults (≥18 years old) who underwent total shoulder arthroplasty (TSA) or hemi–shoulder arthroplasty (HSA) between January 1, 2002 and December 31, 2011. We then placed the patients in this population into 1 of 2 cohorts. The first cohort had an acute MI during the perioperative period after TSA, and the second, larger cohort did not have an acute MI after TSA. Acute MI was identified using ICD-9-CM code 410.xx. To identify a population of shoulder arthroplasty patients, we included discharges with an ICD-9-CM procedure code of 81.80 or 81.88 (both TSA) or 81.81 (HSA) in the sample. We then considered the degree to which each of 5 variables—primary diagnosis, age, sex, race, and select medical comorbidities—was predictive of in-hospital MI after TSA.
Statistical Analysis
Given the large sample used in this study, normal distribution of data was assumed. Using bivariate analysis, Pearson χ2 test for categorical data, and independent-samples t test for continuous data, we compared the nonacute MI and acute MI groups. Multivariable binary logistic regression analyses allowed us to isolate the extent that primary diagnosis, age, sex, race, and medical comorbidities were predictors of acute MI after shoulder arthroplasty. Statistical significance was set at P < .05. SPSS Version 22.0 (SPSS, Chicago, Illinois) was used for all statistical analyses and data modeling.
Results
Between January 1, 2002 and December 31, 2011, an estimated total of 422,371 patients underwent shoulder arthroplasty (59.3% TSA, 40.7% HSA). Of these patients, 1174 (0.28%) had a perioperative MI, and 421,197 (99.72%) did not (Table 1). Patients with a primary diagnosis of proximal humerus fracture (33.8% vs 16.6%; P < .001) or rotator cuff arthropathy (10.1% vs 9.9%; P < .001) were more likely than patients with other diagnoses to have an in-hospital MI.
Our review of the demographics found that patients who underwent shoulder arthroplasty and had a perioperative MI were likely older (75±8.9 years vs 69±11 years; P < .001), Caucasian (94.2% vs 91.9%; P = .002), male (43.2% vs 39.7%; P = .013), in the highest median household income bracket of $63,000 or more (30.8% vs 25.6%; P < .001), and using Medicare (80.9% vs 66.3%; P < .001). They were more likely to be treated in a medical center of medium size (25.6% vs 23.7%; P = .042) or larger (61.8% vs 61.2%; P = .042). MIs occurred more often in urban environments (91.4% vs 88.5%; P = .002) and in HSA patients (55% vs 40.6%; P < .001), resulting in longer hospital stays (9.4±7.9 days vs 2.7±2.5 days; P < .001) and higher probability of death (6.5% vs 0.1%; P < .001).
We then analyzed the 2 cohorts for medical comorbidities (Table 2). Patients in the MI cohort presented with a significantly higher incidence of congestive heart failure, previous MI, angina pectoris, chronic lung disease, hypertension, diabetes, renal failure, fluid and electrolyte disorders, pulmonary circulatory disease, coagulopathy, and deficiency anemia (P < .001) but not liver disease and obesity. Bivariate analysis of perioperative outcomes (Table 3) indicated that these patients also had a statistically higher rate of numerous other complications: pulmonary embolism (4.9% vs 0.2%; P < .001), pneumonia (15.1% vs 1.2%; P < .001), deep venous thrombosis (2.6% vs 0.2%; P < .001), cerebrovascular event (1.6% vs 0.1%; P < .001), acute renal failure (15.1% vs 1.2%; P < .001), gastrointestinal complication (1.2% vs 0.3%; P < .001), mechanical ventilation (1.2% vs 0.3%; P < .001), transfusion (33.4% vs 8.8%; P < .001), and nonroutine discharge (73.3% vs 36.0%; P < .001).
Multivariable logistic regression analysis was performed to determine independent predictors of perioperative MI after shoulder arthroplasty (Table 4). Patients with a primary diagnosis of proximal humerus fracture (odds ratio [OR], 1.38; 95% confidence interval [CI], 1.15-1.65; P < .001) were more likely than patients with a primary diagnosis of osteoarthritis to have an MI. The odds of postoperative MI increased with age (OR, 1.04 per year; 95% CI, 1.03-1.05; P < .001) and were higher in males (OR, 1.72; 95% CI, 1.52-1.96; P < .001). Compared with Caucasians, African Americans (OR, 0.19; 95% CI, 0.09-0.40; P < .001) were less likely to have an in-hospital MI after shoulder arthroplasty. After shoulder arthroplasty, the odds of MI in the perioperative period increased with each subsequent day of care (OR, 1.10; 95% CI, 1.10-1.11; P < .001).
Regarding independent comorbidities, multivariable logistic regression analysis also determined that history of congestive heart failure (OR, 4.86; 95% CI, 4.20-5.61; P < .001), angina pectoris (OR, 2.90; 95% CI, 2.02-4.17; P < .001), complicated diabetes (OR, 1.96; 95% CI, 1.49-2.57; P < .001), renal failure (OR, 1.42; 95% CI, 1.17-1.72; P < .001), fluid and electrolyte disorders (OR, 1.42; 95% CI, 1.21-1.67; P < .001), and deficiency anemia (OR, 1.62; 95% CI, 1.40-1.88; P < .001) were significant predictors of perioperative MI after shoulder arthroplasty.
Discussion
Results of other studies have elucidated 30- and 90-day mortality rates and postoperative complications after shoulder arthroplasty, but, relative to hip and knee arthroplasty,17-19 little has been done to determine predictors of perioperative MI in a large sample of shoulder arthroplasty patients. Given the increasing rates of shoulder arthroplasty1-3 and the demographics of this population,4-6 it is likely that postoperative cardiovascular events will increase in frequency. We found that, in order of decreasing significance, the top 4 risk predictors for acute MI after shoulder arthroplasty were congestive heart failure, angina pectoralis, complicated diabetes mellitus, and male sex. Other pertinent risk factors included older age, Caucasian ethnicity, and a primary diagnosis of proximal humerus fracture. The rate of acute MI in patients who were older than 75 years when they underwent HSA for proximal humerus fracture was 0.80%.
Demographics
We found that patients who had an acute MI after shoulder arthroplasty were likely older, male, and Caucasian. Age and male sex are well-established risk factors for increased cardiac complications after arthroplasty.27-29 Previous studies have indicated that the rate of cardiac events increases in arthroplasty patients older than 65 years.19,28,29 In our study, more than 50% of the patients who had an acute perioperative MI were older than 85 years. Less explainable is the increased occurrence of acute MI in Caucasian patients and wealthy patients, given that minorities in the United States have higher rates of cardiovascular disease.30 Shoulder arthroplasty is an elective procedure, more likely to be undertaken by Caucasians. Therefore, at-risk minority groups and financially challenged groups may be less likely to have this procedure.
Primary Diagnosis
In this series, patients with a primary diagnosis of proximal humerus fracture were more likely to have an in-hospital MI. This finding is consistent with previous studies indicating a higher rate of complications for proximal humerus fracture patients than for shoulder arthroplasty patients.31,32 Given that more than 75% of patients who present with a proximal humerus fracture are older than 70 years, it would be prudent to examine operative indications after this diagnosis,33 particularly as benefit from surgery for fractures has not been definitively demonstrated.34-37
Comorbidities
Many of the patients in our MI cohort presented with congestive heart failure, angina pectoris, complicated diabetes, renal failure, fluid and electrolyte disorders, or deficiency anemia. This is in keeping with other studies indicating that preexisting cardiovascular morbidity increases the rate of MI after various forms of arthroplasty.7-11 Patients in our MI cohort were also susceptible to a variety of post-MI perioperative complications, including pulmonary embolism, pneumonia, deep venous thrombosis, cerebrovascular event, acute renal failure, gastrointestinal complication, mechanical ventilation, transfusion, and nonroutine discharge, and their incidence of death was higher. These findings are consistent with reports that postoperative cardiovascular complications increase the degree of morbidity and mortality in arthroplasty patients.14-16 It is also worth noting that the odds of MI in the perioperative period increase with each subsequent day of care. This is understandable given that patients presenting with numerous comorbidities are at increased risk for perioperative complications38 resulting in hospital readmission.39
The literature indicates that MI occurs as a complication in 0.7% of patients who undergo noncardiac surgery,7 though some series have shown it is more prevalent after arthroplasty procedures.28,40 MI significantly increases the rate of perioperative morbidity and mortality,8 and perioperative cardiac morbidity is a leading cause of death after anesthesia and surgery.12 Furthermore, the most common cause of death after lower extremity arthroplasty is cardiovascular-related.41,42 In patients who presented for elective hip arthroplasty, cardiorespiratory disease was one of the main risk factors (with older age and male sex) shown to increase perioperative mortality.43
Perioperative cardiovascular complications increase postoperative morbidity and mortality.12 The rate of cardiovascular complications after shoulder arthroplasty ranges from 0.8% to 2.6%, and the incidence of MI hovers between 0.3% and 0.9%.17,19,28,40,44 A recent study in 793 patients found that, over a 30-day period, cardiovascular complications accounted for more than one-fourth of all complications.17 Singh and colleagues19 analyzed cardiopulmonary complications after primary shoulder arthroplasty in a total of 3480 patients (4019 arthroplasties) and found this group had a 90-day cardiac morbidity (MI, congestive heart failure, arrhythmia) rate of 2.6%. In that study, a Deyo-Charlson index of 1 or more was a significant independent risk factor for cardiac complications following surgery. Scores on this weighted index of 17 comorbidities are used to assess the complexities of a patient population. Given the severity of cardiovascular perioperative complications, it is important to preoperatively identify high-risk population groups and sufficiently study and optimize patients before shoulder arthroplasty.
There is much debate about the effectiveness of perioperative β-blockers in reducing perioperative cardiac morbidity and mortality.45-48 Such a discussion is outside of the scope of this article, but it may be prudent to seek a cardiology consultation for patients presenting with risk factors for perioperative MI. β-Blockers may prove useful in reducing cardiac morbidity in high-risk patients after noncardiac surgery.45,49
Many limitations are inherent in studies that use a nationally represented database such as NIS, which we used in this study. It is highly likely that NIS does not capture all potential postoperative complications, as this database is very large and subject to errors in data entry and clinical coding. In addition, detailed clinical information (eg, severity of certain comorbid diseases before shoulder arthroplasty, details about the intraoperative course) was not readily available for analysis. Another limitation, which may have led to an underestimate of complication rates, was our not being able to obtain information about postdischarge complications.
Despite these limitations, NIS and other databases have helped researchers answer questions about low-incidence conditions and generalize findings to a national population. In the present study, we analyzed 2 cohorts, patients with and without acute MI after shoulder arthroplasty, to determine predictors for and complications of postarthroplasty MI. We identified numerous predictors for acute MI: congestive heart failure, angina pectoris, complicated diabetes, renal failure, fluid and electrolyte disorders, and deficiency anemia prior to arthroplasty. As perioperative MI is associated with significant morbidity,14-16 it would be wise to screen patients for such comorbid conditions, assess the severity of these conditions, and offer shoulder arthroplasty with prudence.
Conclusion
The top 4 predictors for acute MI after shoulder arthroplasty were congestive heart failure, angina pectoralis, complicated diabetes mellitus, and male sex. Other pertinent risk factors included older age, Caucasian ethnicity, and primary diagnosis of proximal humerus fracture. Surgeons and patients must be aware of predictors for adverse surgical outcomes such as perioperative MI and understand the extent to which these events increase perioperative morbidity and mortality.
1. Day JS, Lau E, Ong KL, Williams GR, Ramsey ML, Kurtz SM. Prevalence and projections of total shoulder and elbow arthroplasty in the United States to 2015. J Shoulder Elbow Surg. 2010;19(8):1115-1120.
2. Kim SH, Wise BL, Zhang Y, Szabo RM. Increasing incidence of shoulder arthroplasty in the United States. J Bone Joint Surg Am. 2011;93(24):2249-2254.
3. Kurtz SM, Lau E, Ong K, Zhao K, Kelly M, Bozic KJ. Future young patient demand for primary and revision joint replacement: national projections from 2010 to 2030. Clin Orthop. 2009;467(10):2606-2612.
4. Boettcher WG. Total hip arthroplasties in the elderly. Morbidity, mortality, and cost effectiveness. Clin Orthop. 1992;(274):30-34.
5. Greenfield S, Apolone G, McNeil BJ, Cleary PD. The importance of co-existent disease in the occurrence of postoperative complications and one-year recovery in patients undergoing total hip replacement. Comorbidity and outcomes after hip replacement. Med Care. 1993;31(2):141-154.
6. Kreder HJ, Williams JI, Jaglal S, Hu R, Axcell T, Stephen D. Are complication rates for elective primary total hip arthroplasty in Ontario related to surgeon and hospital volumes? A preliminary investigation. Can J Surg. 1998;41(6):431-437.
7. Botto F, Alonso-Coello P, Chan MT, et al. Myocardial injury after noncardiac surgery: a large, international, prospective cohort study establishing diagnostic criteria, characteristics, predictors, and 30-day outcomes. Anesthesiology. 2014;120(3):564-578.
8. Mangano DT, Browner WS, Hollenberg M, London MJ, Tubau JF, Tateo IM. Association of perioperative myocardial ischemia with cardiac morbidity and mortality in men undergoing noncardiac surgery. The Study of Perioperative Ischemia Research Group. N Engl J Med. 1990;323(26):1781-1788.
9. Tarhan S, Moffitt EA, Taylor WF, Giuliani ER. Myocardial infarction after general anesthesia. JAMA. 1972;220(11):1451-1454.
10. Landesberg G, Mosseri M, Zahger D, et al. Myocardial infarction after vascular surgery: the role of prolonged stress-induced, ST depression-type ischemia. J Am Coll Cardiol. 2001;37(7):1839-1845.
11. van Waes JA, Nathoe HM, de Graaff JC, et al. Myocardial injury after noncardiac surgery and its association with short-term mortality. Circulation. 2013;127(23):2264-2271.
12. Mangano DT. Perioperative cardiac morbidity. Anesthesiology. 1990;72(1):153-184.
13. Fleisher LA, Corbett W, Berry C, Poldermans D. Cost-effectiveness of differing perioperative beta-blockade strategies in vascular surgery patients. J Cardiothorac Vasc Anesth. 2004;18(1):7-13.
14. Aynardi M, Pulido L, Parvizi J, Sharkey PF, Rothman RH. Early mortality after modern total hip arthroplasty. Clin Orthop. 2009;467(1):213-218.
15. Gangireddy C, Rectenwald JR, Upchurch GR, et al. Risk factors and clinical impact of postoperative symptomatic venous thromboembolism. J Vasc Surg. 2007;45(2):335-341.
16. Baser O, Supina D, Sengupta N, Wang L, Kwong L. Impact of postoperative venous thromboembolism on Medicare recipients undergoing total hip replacement or total knee replacement surgery. Am J Health Syst Pharm. 2010;67(17):1438-1445.
17. Fehringer EV, Mikuls TR, Michaud KD, Henderson WG, O’Dell JR. Shoulder arthroplasties have fewer complications than hip or knee arthroplasties in US veterans. Clin Orthop. 2010;468(3):717-722.
18. Farmer KW, Hammond JW, Queale WS, Keyurapan E, McFarland EG. Shoulder arthroplasty versus hip and knee arthroplasties: a comparison of outcomes. Clin Orthop. 2007;(455):183-189.
19. Singh JA, Sperling JW, Cofield RH. Cardiopulmonary complications after primary shoulder arthroplasty: a cohort study. Semin Arthritis Rheum. 2012;41(5):689-697.
20. Go AS, Mozaffarian D, Roger VL, et al; American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics—2014 update: a report from the American Heart Association. Circulation. 2014;129(3):e28-e292.
21. Lin CA, Kuo AC, Takemoto S. Comorbidities and perioperative complications in HIV-positive patients undergoing primary total hip and knee arthroplasty. J Bone Joint Surg Am. 2013;95(11):1028-1036.
22. Maynard C, Sales AE. Changes in the use of coronary artery revascularization procedures in the Department of Veterans Affairs, the National Hospital Discharge Survey, and the Nationwide Inpatient Sample, 1991–1999. BMC Health Serv Res. 2003;3(1):12.
23. Griffin JW, Novicoff WM, Browne JA, Brockmeier SF. Obstructive sleep apnea as a risk factor after shoulder arthroplasty. J Shoulder Elbow Surg. 2013;22(12):e6-e9.
24. Hambright D, Henderson RA, Cook C, Worrell T, Moorman CT, Bolognesi MP. A comparison of perioperative outcomes in patients with and without rheumatoid arthritis after receiving a total shoulder replacement arthroplasty. J Shoulder Elbow Surg. 2011;20(1):77-85.
25. Odum SM, Troyer JL, Kelly MP, Dedini RD, Bozic KJ. A cost-utility analysis comparing the cost-effectiveness of simultaneous and staged bilateral total knee arthroplasty. J Bone Joint Surg Am. 2013;95(16):1441-1449.
26. Ponce BA, Menendez ME, Oladeji LO, Soldado F. Diabetes as a risk factor for poorer early postoperative outcomes after shoulder arthroplasty. J Shoulder Elbow Surg. 2014;23(5):671-678.
27. Alfonso DT, Toussaint RJ, Alfonso BD, Strauss EJ, Steiger DT, Di Cesare PE. Nonsurgical complications after total hip and knee arthroplasty. Am J Orthop. 2006;35(11):503-510.
28. Mantilla CB, Horlocker TT, Schroeder DR, Berry DJ, Brown DL. Frequency of myocardial infarction, pulmonary embolism, deep venous thrombosis, and death following primary hip or knee arthroplasty. Anesthesiology. 2002;96(5):1140-1146.
29. Singh JA, Jensen MR, Harmsen WS, Gabriel SE, Lewallen DG. Cardiac and thromboembolic complications and mortality in patients undergoing total hip and total knee arthroplasty. Ann Rheum Dis. 2011;70(12):2082-2088.
30. Kurian AK, Cardarelli KM. Racial and ethnic differences in cardiovascular disease risk factors: a systematic review. Ethn Dis. 2007;17(1):143-152.
31. Zhang AL, Schairer WW, Feeley BT. Hospital readmissions after surgical treatment of proximal humerus fractures: is arthroplasty safer than open reduction internal fixation? Clin Orthop. 2014;472(8):2317-2324.
32. Schairer WW, Zhang AL, Feeley BT. Hospital readmissions after primary shoulder arthroplasty. J Shoulder Elbow Surg. 2014;23(9):1349-1355.
33. de Kruijf M, Vroemen JP, de Leur K, van der Voort EA, Vos DI, Van der Laan L. Proximal fractures of the humerus in patients older than 75 years of age: should we consider operative treatment? J Orthop Traumatol. 2014;15(2):111-115.
34. Hauschild O, Konrad G, Audige L, et al. Operative versus non-operative treatment for two-part surgical neck fractures of the proximal humerus. Arch Orthop Trauma Surg. 2013;133(10):1385-1393.
35. Hanson B, Neidenbach P, de Boer P, Stengel D. Functional outcomes after nonoperative management of fractures of the proximal humerus. J Shoulder Elbow Surg. 2009;18(4):612-621.
36. Handoll HH, Ollivere BJ, Rollins KE. Interventions for treating proximal humeral fractures in adults. Cochrane Database Syst Rev. 2012;12:CD000434.
37. Court-Brown CM, Cattermole H, McQueen MM. Impacted valgus fractures (B1.1) of the proximal humerus. The results of non-operative treatment. J Bone Joint Surg Br. 2002;84(4):504-508.
38. Chalmers PN, Gupta AK, Rahman Z, Bruce B, Romeo AA, Nicholson GP. Predictors of early complications of total shoulder arthroplasty. J Arthroplasty. 2014;29(4):856-860.
39. Mahoney A, Bosco JA 3rd, Zuckerman JD. Readmission after shoulder arthroplasty. J Shoulder Elbow Surg. 2014;23(3):377-381.
40. Khan SK, Malviya A, Muller SD, et al. Reduced short-term complications and mortality following Enhanced Recovery primary hip and knee arthroplasty: results from 6,000 consecutive procedures. Acta Orthop. 2014;85(1):26-31.
41. Paavolainen P, Pukkala E, Pulkkinen P, Visuri T. Causes of death after total hip arthroplasty: a nationwide cohort study with 24,638 patients. J Arthroplasty. 2002;17(3):274-281.
42. Sharrock NE, Cazan MG, Hargett MJ, Williams-Russo P, Wilson PD Jr. Changes in mortality after total hip and knee arthroplasty over a ten-year period. Anesth Analg. 1995;80(2):242-248.
43. Parvizi J, Johnson BG, Rowland C, Ereth MH, Lewallen DG. Thirty-day mortality after elective total hip arthroplasty. J Bone Joint Surg Am. 2001;83(10):1524-1528.
44. Morris MJ, Molli RG, Berend KR, Lombardi AV Jr. Mortality and perioperative complications after unicompartmental knee arthroplasty. Knee. 2013;20(3):218-220.
45. Lindenauer PK, Pekow P, Wang K, Mamidi DK, Gutierrez B, Benjamin EM. Perioperative beta-blocker therapy and mortality after major noncardiac surgery. N Engl J Med. 2005;353(4):349-361.
46. Wijeysundera DN, Beattie WS, Wijeysundera HC, Yun L, Austin PC, Ko DT. Duration of preoperative beta-blockade and outcomes after major elective noncardiac surgery. Can J Cardiol. 2014;30(2):217-223.
47. Andersson C, Merie C, Jorgensen M, et al. Association of beta-blocker therapy with risks of adverse cardiovascular events and deaths in patients with ischemic heart disease undergoing noncardiac surgery: a Danish nationwide cohort study. JAMA Int Med. 2014;174(3):336-344.
48. Bakker EJ, Ravensbergen NJ, Poldermans D. Perioperative cardiac evaluation, monitoring, and risk reduction strategies in noncardiac surgery patients. Curr Opin Crit Care. 2011;17(5):409-415.
49. Auerbach AD, Goldman L. Beta-blockers and reduction of cardiac events in noncardiac surgery: scientific review. JAMA. 2002;287(11):1435-1444.
1. Day JS, Lau E, Ong KL, Williams GR, Ramsey ML, Kurtz SM. Prevalence and projections of total shoulder and elbow arthroplasty in the United States to 2015. J Shoulder Elbow Surg. 2010;19(8):1115-1120.
2. Kim SH, Wise BL, Zhang Y, Szabo RM. Increasing incidence of shoulder arthroplasty in the United States. J Bone Joint Surg Am. 2011;93(24):2249-2254.
3. Kurtz SM, Lau E, Ong K, Zhao K, Kelly M, Bozic KJ. Future young patient demand for primary and revision joint replacement: national projections from 2010 to 2030. Clin Orthop. 2009;467(10):2606-2612.
4. Boettcher WG. Total hip arthroplasties in the elderly. Morbidity, mortality, and cost effectiveness. Clin Orthop. 1992;(274):30-34.
5. Greenfield S, Apolone G, McNeil BJ, Cleary PD. The importance of co-existent disease in the occurrence of postoperative complications and one-year recovery in patients undergoing total hip replacement. Comorbidity and outcomes after hip replacement. Med Care. 1993;31(2):141-154.
6. Kreder HJ, Williams JI, Jaglal S, Hu R, Axcell T, Stephen D. Are complication rates for elective primary total hip arthroplasty in Ontario related to surgeon and hospital volumes? A preliminary investigation. Can J Surg. 1998;41(6):431-437.
7. Botto F, Alonso-Coello P, Chan MT, et al. Myocardial injury after noncardiac surgery: a large, international, prospective cohort study establishing diagnostic criteria, characteristics, predictors, and 30-day outcomes. Anesthesiology. 2014;120(3):564-578.
8. Mangano DT, Browner WS, Hollenberg M, London MJ, Tubau JF, Tateo IM. Association of perioperative myocardial ischemia with cardiac morbidity and mortality in men undergoing noncardiac surgery. The Study of Perioperative Ischemia Research Group. N Engl J Med. 1990;323(26):1781-1788.
9. Tarhan S, Moffitt EA, Taylor WF, Giuliani ER. Myocardial infarction after general anesthesia. JAMA. 1972;220(11):1451-1454.
10. Landesberg G, Mosseri M, Zahger D, et al. Myocardial infarction after vascular surgery: the role of prolonged stress-induced, ST depression-type ischemia. J Am Coll Cardiol. 2001;37(7):1839-1845.
11. van Waes JA, Nathoe HM, de Graaff JC, et al. Myocardial injury after noncardiac surgery and its association with short-term mortality. Circulation. 2013;127(23):2264-2271.
12. Mangano DT. Perioperative cardiac morbidity. Anesthesiology. 1990;72(1):153-184.
13. Fleisher LA, Corbett W, Berry C, Poldermans D. Cost-effectiveness of differing perioperative beta-blockade strategies in vascular surgery patients. J Cardiothorac Vasc Anesth. 2004;18(1):7-13.
14. Aynardi M, Pulido L, Parvizi J, Sharkey PF, Rothman RH. Early mortality after modern total hip arthroplasty. Clin Orthop. 2009;467(1):213-218.
15. Gangireddy C, Rectenwald JR, Upchurch GR, et al. Risk factors and clinical impact of postoperative symptomatic venous thromboembolism. J Vasc Surg. 2007;45(2):335-341.
16. Baser O, Supina D, Sengupta N, Wang L, Kwong L. Impact of postoperative venous thromboembolism on Medicare recipients undergoing total hip replacement or total knee replacement surgery. Am J Health Syst Pharm. 2010;67(17):1438-1445.
17. Fehringer EV, Mikuls TR, Michaud KD, Henderson WG, O’Dell JR. Shoulder arthroplasties have fewer complications than hip or knee arthroplasties in US veterans. Clin Orthop. 2010;468(3):717-722.
18. Farmer KW, Hammond JW, Queale WS, Keyurapan E, McFarland EG. Shoulder arthroplasty versus hip and knee arthroplasties: a comparison of outcomes. Clin Orthop. 2007;(455):183-189.
19. Singh JA, Sperling JW, Cofield RH. Cardiopulmonary complications after primary shoulder arthroplasty: a cohort study. Semin Arthritis Rheum. 2012;41(5):689-697.
20. Go AS, Mozaffarian D, Roger VL, et al; American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics—2014 update: a report from the American Heart Association. Circulation. 2014;129(3):e28-e292.
21. Lin CA, Kuo AC, Takemoto S. Comorbidities and perioperative complications in HIV-positive patients undergoing primary total hip and knee arthroplasty. J Bone Joint Surg Am. 2013;95(11):1028-1036.
22. Maynard C, Sales AE. Changes in the use of coronary artery revascularization procedures in the Department of Veterans Affairs, the National Hospital Discharge Survey, and the Nationwide Inpatient Sample, 1991–1999. BMC Health Serv Res. 2003;3(1):12.
23. Griffin JW, Novicoff WM, Browne JA, Brockmeier SF. Obstructive sleep apnea as a risk factor after shoulder arthroplasty. J Shoulder Elbow Surg. 2013;22(12):e6-e9.
24. Hambright D, Henderson RA, Cook C, Worrell T, Moorman CT, Bolognesi MP. A comparison of perioperative outcomes in patients with and without rheumatoid arthritis after receiving a total shoulder replacement arthroplasty. J Shoulder Elbow Surg. 2011;20(1):77-85.
25. Odum SM, Troyer JL, Kelly MP, Dedini RD, Bozic KJ. A cost-utility analysis comparing the cost-effectiveness of simultaneous and staged bilateral total knee arthroplasty. J Bone Joint Surg Am. 2013;95(16):1441-1449.
26. Ponce BA, Menendez ME, Oladeji LO, Soldado F. Diabetes as a risk factor for poorer early postoperative outcomes after shoulder arthroplasty. J Shoulder Elbow Surg. 2014;23(5):671-678.
27. Alfonso DT, Toussaint RJ, Alfonso BD, Strauss EJ, Steiger DT, Di Cesare PE. Nonsurgical complications after total hip and knee arthroplasty. Am J Orthop. 2006;35(11):503-510.
28. Mantilla CB, Horlocker TT, Schroeder DR, Berry DJ, Brown DL. Frequency of myocardial infarction, pulmonary embolism, deep venous thrombosis, and death following primary hip or knee arthroplasty. Anesthesiology. 2002;96(5):1140-1146.
29. Singh JA, Jensen MR, Harmsen WS, Gabriel SE, Lewallen DG. Cardiac and thromboembolic complications and mortality in patients undergoing total hip and total knee arthroplasty. Ann Rheum Dis. 2011;70(12):2082-2088.
30. Kurian AK, Cardarelli KM. Racial and ethnic differences in cardiovascular disease risk factors: a systematic review. Ethn Dis. 2007;17(1):143-152.
31. Zhang AL, Schairer WW, Feeley BT. Hospital readmissions after surgical treatment of proximal humerus fractures: is arthroplasty safer than open reduction internal fixation? Clin Orthop. 2014;472(8):2317-2324.
32. Schairer WW, Zhang AL, Feeley BT. Hospital readmissions after primary shoulder arthroplasty. J Shoulder Elbow Surg. 2014;23(9):1349-1355.
33. de Kruijf M, Vroemen JP, de Leur K, van der Voort EA, Vos DI, Van der Laan L. Proximal fractures of the humerus in patients older than 75 years of age: should we consider operative treatment? J Orthop Traumatol. 2014;15(2):111-115.
34. Hauschild O, Konrad G, Audige L, et al. Operative versus non-operative treatment for two-part surgical neck fractures of the proximal humerus. Arch Orthop Trauma Surg. 2013;133(10):1385-1393.
35. Hanson B, Neidenbach P, de Boer P, Stengel D. Functional outcomes after nonoperative management of fractures of the proximal humerus. J Shoulder Elbow Surg. 2009;18(4):612-621.
36. Handoll HH, Ollivere BJ, Rollins KE. Interventions for treating proximal humeral fractures in adults. Cochrane Database Syst Rev. 2012;12:CD000434.
37. Court-Brown CM, Cattermole H, McQueen MM. Impacted valgus fractures (B1.1) of the proximal humerus. The results of non-operative treatment. J Bone Joint Surg Br. 2002;84(4):504-508.
38. Chalmers PN, Gupta AK, Rahman Z, Bruce B, Romeo AA, Nicholson GP. Predictors of early complications of total shoulder arthroplasty. J Arthroplasty. 2014;29(4):856-860.
39. Mahoney A, Bosco JA 3rd, Zuckerman JD. Readmission after shoulder arthroplasty. J Shoulder Elbow Surg. 2014;23(3):377-381.
40. Khan SK, Malviya A, Muller SD, et al. Reduced short-term complications and mortality following Enhanced Recovery primary hip and knee arthroplasty: results from 6,000 consecutive procedures. Acta Orthop. 2014;85(1):26-31.
41. Paavolainen P, Pukkala E, Pulkkinen P, Visuri T. Causes of death after total hip arthroplasty: a nationwide cohort study with 24,638 patients. J Arthroplasty. 2002;17(3):274-281.
42. Sharrock NE, Cazan MG, Hargett MJ, Williams-Russo P, Wilson PD Jr. Changes in mortality after total hip and knee arthroplasty over a ten-year period. Anesth Analg. 1995;80(2):242-248.
43. Parvizi J, Johnson BG, Rowland C, Ereth MH, Lewallen DG. Thirty-day mortality after elective total hip arthroplasty. J Bone Joint Surg Am. 2001;83(10):1524-1528.
44. Morris MJ, Molli RG, Berend KR, Lombardi AV Jr. Mortality and perioperative complications after unicompartmental knee arthroplasty. Knee. 2013;20(3):218-220.
45. Lindenauer PK, Pekow P, Wang K, Mamidi DK, Gutierrez B, Benjamin EM. Perioperative beta-blocker therapy and mortality after major noncardiac surgery. N Engl J Med. 2005;353(4):349-361.
46. Wijeysundera DN, Beattie WS, Wijeysundera HC, Yun L, Austin PC, Ko DT. Duration of preoperative beta-blockade and outcomes after major elective noncardiac surgery. Can J Cardiol. 2014;30(2):217-223.
47. Andersson C, Merie C, Jorgensen M, et al. Association of beta-blocker therapy with risks of adverse cardiovascular events and deaths in patients with ischemic heart disease undergoing noncardiac surgery: a Danish nationwide cohort study. JAMA Int Med. 2014;174(3):336-344.
48. Bakker EJ, Ravensbergen NJ, Poldermans D. Perioperative cardiac evaluation, monitoring, and risk reduction strategies in noncardiac surgery patients. Curr Opin Crit Care. 2011;17(5):409-415.
49. Auerbach AD, Goldman L. Beta-blockers and reduction of cardiac events in noncardiac surgery: scientific review. JAMA. 2002;287(11):1435-1444.
Patients’ Perceptions of the Costs of Total Hip and Knee Arthroplasty
Medical economics has been a major sociopolitical issue in the United States for the past 20 years, with concerns focused on increasing medical spending. These costs are projected to continue to rise, from 15.3% of gross domestic product in 2002 to 19.6% in 2017.1
Multiple steps have been taken to help reduce the cost of health care, many of which center on physician reimbursement. The Balanced Budget Act of 1997 worked to control Medicare spending by increasing reimbursement for clinic visits by setting reductions for procedural reimbursements. This specifically affects orthopedic surgeons, who between 1991 and 2002 experienced a 28% reduction in reimbursement, after inflation, for commonly performed orthopedic procedures, including hip and knee arthroplasty.2 Unfortunately, this system does not take into account the value of services as perceived by patients.
Total hip and knee arthroplasty (THA, TKA) are well-established surgical treatments for advanced osteoarthritis of the hip and knee, respectively. Much research has been done on patient satisfaction with these procedures and on their long-term results and cost-effectiveness. These procedures rank among the highest in patient satisfaction, and improvements in technique and technology have steadily improved long-term results. THA and TKA have proved to be cost-effective in appropriately indicated patients.
The demand for THA and TKA is projected to increase by 174% and 673%, respectively, from 2005 to 2030.3 Legislators, payers, health care providers, and patients are understandably concerned about the rising cost of health care and the implications for access to elective surgical procedures. In a recent study by Foran and colleagues,4 surveyed postoperative patients indicated that Medicare reimbursement was “much lower” for arthroplasty than it should be. In addition, they overestimated (compared with national averages) what Medicare reimburses for hip and knee arthroplasty. Many raised concerns that orthopedic surgeons might drop Medicare entirely.4
These misconceptions about reimbursement may stem partly from the inaccessibility of health care cost information. Rosenthal and colleagues5 recently queried a random selection of US hospitals and demonstrated the difficulty in obtaining THA pricing information.
In a system in which consumers and payers are often not one and the same, it is unclear if consumers understand the cost of their health care. We conducted a study to assess patients’ perceptions of the cost of total joint arthroplasty (TJA) and gain insight into their understanding of health care costs and their sense of the value of this elective surgical procedure.
Materials and Methods
After obtaining institutional review board approval and informed consent for this study, we surveyed 284 consecutive patients who underwent THA or TKA at an academic medical center. Patients had either primary or revision surgery performed (by Dr. Hallstrom or Dr. Urquhart) and were surveyed during their first (2-week) postoperative visit, between March 1, 2012 and December 20, 2012.
Surveys were labeled with patient identifiers to facilitate abstraction of data from electronic medical records. Operative reports and discharge summaries were reviewed for data that included sex, age, diagnosis, procedure, surgeon, implant, admission date, and length of stay.
The survey asked for demographic information, including level of education, insurance coverage, and annual household income, and included a question to verify the surgical procedure and a question to determine if the patient had reviewed a hospital billing statement pertaining to the patient’s admission. The survey also included these questions about reimbursement and cost:
- How much do you feel your orthopedic surgeon was reimbursed for your surgery? (EXCLUDING payments to the hospital)
- How much do you think your surgeon gets reimbursed to see you IN THE HOSPITAL after surgery?
- How much do you think your surgeon gets reimbursed per visit to see you IN CLINIC for follow-up during the first 3 months after surgery?
- How much do you think the implant used in your surgery cost?
- How much do you think the hospital was reimbursed for your surgery and admission to the hospital after surgery? (EXCLUDING payments to the surgeon)
- How much do you think it cost the hospital to provide your surgery and admission to the hospital after surgery?
Responses were limited to numeric currency format using a response area as shown in Figure 1. Overall patient satisfaction was elicited with use of a 5-point scale ranging from 1 (very unsatisfied) to 5 (very satisfied). Regarding type of implant used, patients could select from 6 prominent vendors or indicate “other” or “don’t know.” They were also asked which of several factors should primarily determine surgeon reimbursement: overall patient satisfaction, technical difficulty, amount of risk/possible harm, duration/amount of time, and rate of complications. A free-response comments section was provided at the end of the survey.
Data from the survey and the electronic medical records were collected using Research Electronic Data Capture (REDCap; Vanderbilt University, Nashville, Tennessee). Statistical analysis was performed with SAS Version 9.3 (SAS Institute, Cary, North Carolina). Data were screened before further analysis. Patients who provided nonnumeric responses in numeric response fields were excluded from further analysis. Numeric ranges were applied in subsequent analysis using the mean of the range. Implausible responses resulted in the removal of the entire encounter from subsequent analysis.
Demographic data used to define categories for further subgroup analysis are presented as percentages of the group. Medians, means, and interquartile ranges were calculated for all responses regarding reimbursement and cost. Differences in perceptions of reimbursement and cost based on subgroups, including procedure type, diagnosis, education level, and satisfaction, were calculated. Independent-samples Student t tests were used to determine the statistical significance of the differences detected.
Results
Of the 400 eligible patients seen at the first postoperative follow-up, 284 (71%) were enrolled in the study. Mean (SD) age was 62.6 (12.6) years. Of the 284 patients enrolled, 154 (54%) were female. Of the participants who reported their education and income, 125 (44%) had a bachelor’s degree or higher degree, and 68 (23.9%) reported income of more than $100,000 per year. The largest payers reported by patients were private insurance (80%) and Medicare (46%). Additional demographic details are listed in Table 1.
Of the 284 patients enrolled in the study, 159 (56%) had THA, and 88 (31%) had TKA (Table 2). Thirty-seven patients (13%) underwent revision procedures. Only 5 patients (2%) indicated they had reviewed their hospital billing statement from their most recent admission. Two hundred forty-two patients (85%) were satisfied or very satisfied with their procedure.
Regarding the implant used in their surgery, 216 patients (76%) indicated they did not know which company manufactured it. Of the 68 patients (24%) who named a manufacturer, 53 (78%) were correct in their selection (intraoperative records were checked). Patients indicated they thought the implant used in their surgery cost $6447 on average (95% CI, $5581-$7312).
On average, patients thought their surgeon was reimbursed $12,014 (95% CI, $10,845-$13,183) for their procedure, and they estimated that the hospital was reimbursed $28,392 (95% CI, $25,271-$31,512) for their perioperative care and that it cost the hospital $24,389 (95% CI, $21,612-$27,165) to provide it. Means, confidence intervals, medians, and interquartile ranges for parameters of reimbursement and cost are listed in Table 3. Seventy-one patients (25%) thought on average that the hospital took a net loss for each TJA performed, and 146 patients (51%) thought on average that the hospital generated a net profit for each TJA.
On average, patients thought surgeons were reimbursed $11,872 for a THA and $12,263 for a TKA. Patients also estimated a higher hospital cost (THA, $22,981; TKA, $26,998) and reimbursement (THA, $27,366; TKA, $30,230) after TKA than THA. These differences in perceptions of cost and reimbursement for THA and TKA appear in Table 4 and Figure 2.
Statistically significant differences were also found in perceptions of cost and reimbursement based on level of education and overall patient satisfaction. Patients with a bachelor’s degree or higher estimated physician reimbursement at $11,006, whereas patients with a lower level of education estimated reimbursement at $12,890. In addition, patients with a lower level of education gave estimates of hospital cost and reimbursement that were $7698 and $10,799 higher, respectively, than the estimates given by patients with a higher level of education (Table 5, Figure 3). Patients who were satisfied or very satisfied with their overall TJA experience estimated surgeon reimbursement at $11,673. Patients who indicated they were unsatisfied, very unsatisfied, or neutral regarding their overall experience gave a higher estimate of surgeon reimbursement: $14,317 (Table 6, Figure 4).
Because of the small number of enrolled patients who had revision surgery and the high variability in patient responses, there were no meaningful or statistically significant differences in perceptions of cost and reimbursement based on revision or primary surgery.
Patients also estimated substantial additional reimbursements to physicians for services included at no additional charge with the global surgical package. Median estimates were $300 for reimbursement to a physician making rounds in the hospital and $250 for reimbursement for an outpatient follow-up. Only 47 patients (17%) and 35 patients (12%) correctly indicated there is no additional payment for making rounds and outpatient follow-up, respectively. Estimates of these reimbursements varied by education level, procedure, and overall satisfaction (Tables 4–6).
Discussion
The sustainable growth rate (SGR) formula, part of the Balanced Budget Act of 1997, was constructed to manage health care costs in the context of overall economic growth. By 2001, Medicare health care expenditures had begun to outpace economic growth, and the SGR formula dictated a reduction in reimbursement to physicians. Each year over the past decade, Congress has passed legislation providing a temporary reprieve, staving off a drastic reduction of as much as 25% in 2010.6 Despite these adjustments, reimbursement continues to decrease because of overall inflation.
More worrisome is that “more than half of the nearly trillion dollar price tag for expanding coverage under the Affordable Care Act (ACA) will be paid by decreasing spending for the more than 46.3 million individuals covered by Medicare.”7 ACA provisions will also create an Independent Payment Advisory Board (IPAB) to oversee health care costs and reduce Medicare spending when it is expected to exceed target levels.8 As IPAB cannot recommend increasing revenues or changing benefits, and because it is initially prohibited from recommending decreasing payments to hospitals, the decreases will likely have the greatest impact on physician reimbursement.7-9
Health care policy has been a major campaign issue during recent US elections. The public and popular media remain engaged in this important discussion. Although patients, policymakers, and physicians are understandably concerned about cost and access to health care, it is unclear if patients understand the distribution of health care cost and reimbursement.
Other authors have studied patients’ perceptions of physician reimbursement for TJA. Hayden and colleagues10 surveyed 1000 residents of a Texas city. The 121 who responded to the survey thought that fair compensation for performing a TKA was $5080, on average.10 Although this was significantly higher than the actual Medicare reimbursement at the time, a later study, by Foran and colleagues,4 found patients’ estimates of both fair reimbursement and Medicare reimbursement for TJA to be even higher. Foran and colleagues4 surveyed 1120 patients who thought surgeons deserved to be paid $14,358 for THA and $13,322 for TKA, on average. These reimbursement values are nearly an order of magnitude higher than actual reimbursements. For Medicare payments, patients lowered their estimates to $8212 for THA and $7196 for TKA.4
To our knowledge, the present study is the first to use a “postconsumer” survey to assess patients’ perceptions of THA and TKA costs. Our results confirmed that patients substantially overestimated reimbursement for THA and TKA at $11,872 and $12,263, respectively, relative to the average Medicare reimbursements of $1467 and $1530, respectively.11 We also found that patients overestimated both hospital cost and reimbursement for THA at $22,981 and $27,366, respectively, relative to recently published hospital economic analyses showing THA cost and reimbursement to be $11,688 and $15,789, respectively.12 Few patients enrolled in our study demonstrated an understanding of the services included in the global surgical package. Only about 12% of patients correctly indicated there was no additional payment to the physician for initial follow-up appointments. However, patients were fairly accurate in their estimates of implant cost. On average, patients who underwent THA priced their implant at $6823, which is only about 9% higher than the reported median cost of $6072 to $6400.13,14
We also found significant differences in perceptions of cost based on level of education, joint replaced, and overall level of satisfaction. On average, patients with a bachelor’s degree or higher gave estimates of cost and reimbursement that were lower than those given by patients with a lower level of education. Estimates of physician reimbursement and hospital reimbursement and cost were higher from patients who had TKA than from patients who had THA.
Comparing perceptions of reimbursement for appendectomy and coronary artery bypass with perceptions for TJA, Foran and colleagues4 found that patients understood the relative complexity of each procedure, as evidenced by their estimates of fair reimbursement for each. However, in comparing patient estimates for the different components of cost and reimbursement for TJA, we found great variability in understanding. Patients in our study overestimated payments to the hospital by 73% but overestimated the cost of the THA implant by only 9%. However, the same patients overestimated physician reimbursement for THA by about 800%. If these patients’ estimates of reimbursement are considered surrogates for relative value, then physicians, based on actual payments, are grossly undervalued relative to implant manufacturers.
Our study had several limitations. First, the enrolled patients were all seen at one medical center, in Ann Arbor, Michigan, and our results may not be generalizable outside the region. Second, the survey respondents were postoperative patients who had an established relationship with the study’s principal investigators—a relationship that may have been a source of bias in the consideration of reimbursement as a function of value. Third, despite our efforts to carefully design a survey with open-ended responses, the order in which the survey questions were presented may have influenced patient responses. Fourth, the open-ended question design may have had an impact on responses where the correct answer would have required entering 0.00.
Despite these limitations, our study results demonstrated general public misconceptions about cost and reimbursement for common orthopedic procedures. Although more transparency in health care cost information may not immediately result in a more well-informed population,15 our patients, given the opportunity to develop an understanding of the economics of their own medical treatment, may become better prepared to make informed choices regarding changes in health care policy.
1. Kumar S, Ghildayal NS, Shah RN. Examining quality and efficiency of the U.S. healthcare system. Int J Health Care Qual Assur. 2011;24(5):366-388.
2. Hariri S, Bozic KJ, Lavernia C, Prestipino A, Rubash HE. Medicare physician reimbursement: past, present, and future. J Bone Joint Surg Am. 2007;89(11):2536-2546.
3. Kurtz S, Ong K, Lau E, Mowat F, Halpern M. Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. J Bone Joint Surg Am. 2007;89(4):780-785.
4. Foran JR, Sheth NP, Ward SR, et al. Patient perception of physician reimbursement in elective total hip and knee arthroplasty. J Arthroplasty. 2012;27(5):703-709.
5. Rosenthal JA, Lu X, Cram P. Availability of consumer prices from US hospitals for a common surgical procedure. JAMA Intern Med. 2013;173(6):427-432.
6. US Senate Committee on Finance. H.R. 4994: the Medicare and Medicaid Extenders Act of 2010. http://www.finance.senate.gov/legislation/details/?id=9f97aa2e-5056-a032-52d4-8db158b12b11. Accessed March 25, 2015.
7. Zinberg JM. When patients call, will physicians respond? JAMA. 2011;305(19):2011-2012.
8. Jost TS. The Independent Payment Advisory Board. N Engl J Med. 2010;363(2):103-105.
9. US Department of Health and Human Services, Centers for Medicare & Medicaid Services. Estimated financial effects of the “Patient Protection and Affordable Care Act,” as amended. 2010. http://www.cms.gov/Research-Statistics-Data-and-Systems/Research/ActuarialStudies/downloads/PPACA_2010-04-22.pdf. Accessed March 25, 2015.
10. Hayden SA, Hayden D, White LW. The U.S. public’s perceived value of the surgeon’s fee for total knee replacement. Abstract presented at: 75th Annual Meeting of the American Academy of Orthopaedic Surgeons; March 5-9, 2008; San Francisco, CA. Abstract 214.
11. Centers for Medicare & Medicaid Services. Physician Fee Schedule Search Tool. http://www.cms.gov/apps/physician-fee-schedule/search/search-criteria.aspx. Accessed March 25, 2015.
12. Rana AJ, Iorio R, Healy WL. Hospital economics of primary THA decreasing reimbursement and increasing cost, 1990 to 2008. Clin Orthop. 2011;469(2):355-361.
13. Lavernia CJ, Hernandez VH, Rossi MD. Payment analysis of total hip replacement. Curr Opin Orthop. 2007;18(1):23-27.
14. Robinson JC, Pozen A, Tseng S, Bozic KJ. Variability in costs associated with total hip and knee replacement implants. J Bone Joint Surg Am. 2012;94(18):1693-1698.
15. Smolders JM, Van Loon CJ, Rijnberg WJ, Van Susante JL. Patients poorly estimate the overall costs of a total knee arthroplasty and strongly overestimate the surgeon’s fee. Acta Orthop Belg. 2007;73(3):339-344.
Medical economics has been a major sociopolitical issue in the United States for the past 20 years, with concerns focused on increasing medical spending. These costs are projected to continue to rise, from 15.3% of gross domestic product in 2002 to 19.6% in 2017.1
Multiple steps have been taken to help reduce the cost of health care, many of which center on physician reimbursement. The Balanced Budget Act of 1997 worked to control Medicare spending by increasing reimbursement for clinic visits by setting reductions for procedural reimbursements. This specifically affects orthopedic surgeons, who between 1991 and 2002 experienced a 28% reduction in reimbursement, after inflation, for commonly performed orthopedic procedures, including hip and knee arthroplasty.2 Unfortunately, this system does not take into account the value of services as perceived by patients.
Total hip and knee arthroplasty (THA, TKA) are well-established surgical treatments for advanced osteoarthritis of the hip and knee, respectively. Much research has been done on patient satisfaction with these procedures and on their long-term results and cost-effectiveness. These procedures rank among the highest in patient satisfaction, and improvements in technique and technology have steadily improved long-term results. THA and TKA have proved to be cost-effective in appropriately indicated patients.
The demand for THA and TKA is projected to increase by 174% and 673%, respectively, from 2005 to 2030.3 Legislators, payers, health care providers, and patients are understandably concerned about the rising cost of health care and the implications for access to elective surgical procedures. In a recent study by Foran and colleagues,4 surveyed postoperative patients indicated that Medicare reimbursement was “much lower” for arthroplasty than it should be. In addition, they overestimated (compared with national averages) what Medicare reimburses for hip and knee arthroplasty. Many raised concerns that orthopedic surgeons might drop Medicare entirely.4
These misconceptions about reimbursement may stem partly from the inaccessibility of health care cost information. Rosenthal and colleagues5 recently queried a random selection of US hospitals and demonstrated the difficulty in obtaining THA pricing information.
In a system in which consumers and payers are often not one and the same, it is unclear if consumers understand the cost of their health care. We conducted a study to assess patients’ perceptions of the cost of total joint arthroplasty (TJA) and gain insight into their understanding of health care costs and their sense of the value of this elective surgical procedure.
Materials and Methods
After obtaining institutional review board approval and informed consent for this study, we surveyed 284 consecutive patients who underwent THA or TKA at an academic medical center. Patients had either primary or revision surgery performed (by Dr. Hallstrom or Dr. Urquhart) and were surveyed during their first (2-week) postoperative visit, between March 1, 2012 and December 20, 2012.
Surveys were labeled with patient identifiers to facilitate abstraction of data from electronic medical records. Operative reports and discharge summaries were reviewed for data that included sex, age, diagnosis, procedure, surgeon, implant, admission date, and length of stay.
The survey asked for demographic information, including level of education, insurance coverage, and annual household income, and included a question to verify the surgical procedure and a question to determine if the patient had reviewed a hospital billing statement pertaining to the patient’s admission. The survey also included these questions about reimbursement and cost:
- How much do you feel your orthopedic surgeon was reimbursed for your surgery? (EXCLUDING payments to the hospital)
- How much do you think your surgeon gets reimbursed to see you IN THE HOSPITAL after surgery?
- How much do you think your surgeon gets reimbursed per visit to see you IN CLINIC for follow-up during the first 3 months after surgery?
- How much do you think the implant used in your surgery cost?
- How much do you think the hospital was reimbursed for your surgery and admission to the hospital after surgery? (EXCLUDING payments to the surgeon)
- How much do you think it cost the hospital to provide your surgery and admission to the hospital after surgery?
Responses were limited to numeric currency format using a response area as shown in Figure 1. Overall patient satisfaction was elicited with use of a 5-point scale ranging from 1 (very unsatisfied) to 5 (very satisfied). Regarding type of implant used, patients could select from 6 prominent vendors or indicate “other” or “don’t know.” They were also asked which of several factors should primarily determine surgeon reimbursement: overall patient satisfaction, technical difficulty, amount of risk/possible harm, duration/amount of time, and rate of complications. A free-response comments section was provided at the end of the survey.
Data from the survey and the electronic medical records were collected using Research Electronic Data Capture (REDCap; Vanderbilt University, Nashville, Tennessee). Statistical analysis was performed with SAS Version 9.3 (SAS Institute, Cary, North Carolina). Data were screened before further analysis. Patients who provided nonnumeric responses in numeric response fields were excluded from further analysis. Numeric ranges were applied in subsequent analysis using the mean of the range. Implausible responses resulted in the removal of the entire encounter from subsequent analysis.
Demographic data used to define categories for further subgroup analysis are presented as percentages of the group. Medians, means, and interquartile ranges were calculated for all responses regarding reimbursement and cost. Differences in perceptions of reimbursement and cost based on subgroups, including procedure type, diagnosis, education level, and satisfaction, were calculated. Independent-samples Student t tests were used to determine the statistical significance of the differences detected.
Results
Of the 400 eligible patients seen at the first postoperative follow-up, 284 (71%) were enrolled in the study. Mean (SD) age was 62.6 (12.6) years. Of the 284 patients enrolled, 154 (54%) were female. Of the participants who reported their education and income, 125 (44%) had a bachelor’s degree or higher degree, and 68 (23.9%) reported income of more than $100,000 per year. The largest payers reported by patients were private insurance (80%) and Medicare (46%). Additional demographic details are listed in Table 1.
Of the 284 patients enrolled in the study, 159 (56%) had THA, and 88 (31%) had TKA (Table 2). Thirty-seven patients (13%) underwent revision procedures. Only 5 patients (2%) indicated they had reviewed their hospital billing statement from their most recent admission. Two hundred forty-two patients (85%) were satisfied or very satisfied with their procedure.
Regarding the implant used in their surgery, 216 patients (76%) indicated they did not know which company manufactured it. Of the 68 patients (24%) who named a manufacturer, 53 (78%) were correct in their selection (intraoperative records were checked). Patients indicated they thought the implant used in their surgery cost $6447 on average (95% CI, $5581-$7312).
On average, patients thought their surgeon was reimbursed $12,014 (95% CI, $10,845-$13,183) for their procedure, and they estimated that the hospital was reimbursed $28,392 (95% CI, $25,271-$31,512) for their perioperative care and that it cost the hospital $24,389 (95% CI, $21,612-$27,165) to provide it. Means, confidence intervals, medians, and interquartile ranges for parameters of reimbursement and cost are listed in Table 3. Seventy-one patients (25%) thought on average that the hospital took a net loss for each TJA performed, and 146 patients (51%) thought on average that the hospital generated a net profit for each TJA.
On average, patients thought surgeons were reimbursed $11,872 for a THA and $12,263 for a TKA. Patients also estimated a higher hospital cost (THA, $22,981; TKA, $26,998) and reimbursement (THA, $27,366; TKA, $30,230) after TKA than THA. These differences in perceptions of cost and reimbursement for THA and TKA appear in Table 4 and Figure 2.
Statistically significant differences were also found in perceptions of cost and reimbursement based on level of education and overall patient satisfaction. Patients with a bachelor’s degree or higher estimated physician reimbursement at $11,006, whereas patients with a lower level of education estimated reimbursement at $12,890. In addition, patients with a lower level of education gave estimates of hospital cost and reimbursement that were $7698 and $10,799 higher, respectively, than the estimates given by patients with a higher level of education (Table 5, Figure 3). Patients who were satisfied or very satisfied with their overall TJA experience estimated surgeon reimbursement at $11,673. Patients who indicated they were unsatisfied, very unsatisfied, or neutral regarding their overall experience gave a higher estimate of surgeon reimbursement: $14,317 (Table 6, Figure 4).
Because of the small number of enrolled patients who had revision surgery and the high variability in patient responses, there were no meaningful or statistically significant differences in perceptions of cost and reimbursement based on revision or primary surgery.
Patients also estimated substantial additional reimbursements to physicians for services included at no additional charge with the global surgical package. Median estimates were $300 for reimbursement to a physician making rounds in the hospital and $250 for reimbursement for an outpatient follow-up. Only 47 patients (17%) and 35 patients (12%) correctly indicated there is no additional payment for making rounds and outpatient follow-up, respectively. Estimates of these reimbursements varied by education level, procedure, and overall satisfaction (Tables 4–6).
Discussion
The sustainable growth rate (SGR) formula, part of the Balanced Budget Act of 1997, was constructed to manage health care costs in the context of overall economic growth. By 2001, Medicare health care expenditures had begun to outpace economic growth, and the SGR formula dictated a reduction in reimbursement to physicians. Each year over the past decade, Congress has passed legislation providing a temporary reprieve, staving off a drastic reduction of as much as 25% in 2010.6 Despite these adjustments, reimbursement continues to decrease because of overall inflation.
More worrisome is that “more than half of the nearly trillion dollar price tag for expanding coverage under the Affordable Care Act (ACA) will be paid by decreasing spending for the more than 46.3 million individuals covered by Medicare.”7 ACA provisions will also create an Independent Payment Advisory Board (IPAB) to oversee health care costs and reduce Medicare spending when it is expected to exceed target levels.8 As IPAB cannot recommend increasing revenues or changing benefits, and because it is initially prohibited from recommending decreasing payments to hospitals, the decreases will likely have the greatest impact on physician reimbursement.7-9
Health care policy has been a major campaign issue during recent US elections. The public and popular media remain engaged in this important discussion. Although patients, policymakers, and physicians are understandably concerned about cost and access to health care, it is unclear if patients understand the distribution of health care cost and reimbursement.
Other authors have studied patients’ perceptions of physician reimbursement for TJA. Hayden and colleagues10 surveyed 1000 residents of a Texas city. The 121 who responded to the survey thought that fair compensation for performing a TKA was $5080, on average.10 Although this was significantly higher than the actual Medicare reimbursement at the time, a later study, by Foran and colleagues,4 found patients’ estimates of both fair reimbursement and Medicare reimbursement for TJA to be even higher. Foran and colleagues4 surveyed 1120 patients who thought surgeons deserved to be paid $14,358 for THA and $13,322 for TKA, on average. These reimbursement values are nearly an order of magnitude higher than actual reimbursements. For Medicare payments, patients lowered their estimates to $8212 for THA and $7196 for TKA.4
To our knowledge, the present study is the first to use a “postconsumer” survey to assess patients’ perceptions of THA and TKA costs. Our results confirmed that patients substantially overestimated reimbursement for THA and TKA at $11,872 and $12,263, respectively, relative to the average Medicare reimbursements of $1467 and $1530, respectively.11 We also found that patients overestimated both hospital cost and reimbursement for THA at $22,981 and $27,366, respectively, relative to recently published hospital economic analyses showing THA cost and reimbursement to be $11,688 and $15,789, respectively.12 Few patients enrolled in our study demonstrated an understanding of the services included in the global surgical package. Only about 12% of patients correctly indicated there was no additional payment to the physician for initial follow-up appointments. However, patients were fairly accurate in their estimates of implant cost. On average, patients who underwent THA priced their implant at $6823, which is only about 9% higher than the reported median cost of $6072 to $6400.13,14
We also found significant differences in perceptions of cost based on level of education, joint replaced, and overall level of satisfaction. On average, patients with a bachelor’s degree or higher gave estimates of cost and reimbursement that were lower than those given by patients with a lower level of education. Estimates of physician reimbursement and hospital reimbursement and cost were higher from patients who had TKA than from patients who had THA.
Comparing perceptions of reimbursement for appendectomy and coronary artery bypass with perceptions for TJA, Foran and colleagues4 found that patients understood the relative complexity of each procedure, as evidenced by their estimates of fair reimbursement for each. However, in comparing patient estimates for the different components of cost and reimbursement for TJA, we found great variability in understanding. Patients in our study overestimated payments to the hospital by 73% but overestimated the cost of the THA implant by only 9%. However, the same patients overestimated physician reimbursement for THA by about 800%. If these patients’ estimates of reimbursement are considered surrogates for relative value, then physicians, based on actual payments, are grossly undervalued relative to implant manufacturers.
Our study had several limitations. First, the enrolled patients were all seen at one medical center, in Ann Arbor, Michigan, and our results may not be generalizable outside the region. Second, the survey respondents were postoperative patients who had an established relationship with the study’s principal investigators—a relationship that may have been a source of bias in the consideration of reimbursement as a function of value. Third, despite our efforts to carefully design a survey with open-ended responses, the order in which the survey questions were presented may have influenced patient responses. Fourth, the open-ended question design may have had an impact on responses where the correct answer would have required entering 0.00.
Despite these limitations, our study results demonstrated general public misconceptions about cost and reimbursement for common orthopedic procedures. Although more transparency in health care cost information may not immediately result in a more well-informed population,15 our patients, given the opportunity to develop an understanding of the economics of their own medical treatment, may become better prepared to make informed choices regarding changes in health care policy.
Medical economics has been a major sociopolitical issue in the United States for the past 20 years, with concerns focused on increasing medical spending. These costs are projected to continue to rise, from 15.3% of gross domestic product in 2002 to 19.6% in 2017.1
Multiple steps have been taken to help reduce the cost of health care, many of which center on physician reimbursement. The Balanced Budget Act of 1997 worked to control Medicare spending by increasing reimbursement for clinic visits by setting reductions for procedural reimbursements. This specifically affects orthopedic surgeons, who between 1991 and 2002 experienced a 28% reduction in reimbursement, after inflation, for commonly performed orthopedic procedures, including hip and knee arthroplasty.2 Unfortunately, this system does not take into account the value of services as perceived by patients.
Total hip and knee arthroplasty (THA, TKA) are well-established surgical treatments for advanced osteoarthritis of the hip and knee, respectively. Much research has been done on patient satisfaction with these procedures and on their long-term results and cost-effectiveness. These procedures rank among the highest in patient satisfaction, and improvements in technique and technology have steadily improved long-term results. THA and TKA have proved to be cost-effective in appropriately indicated patients.
The demand for THA and TKA is projected to increase by 174% and 673%, respectively, from 2005 to 2030.3 Legislators, payers, health care providers, and patients are understandably concerned about the rising cost of health care and the implications for access to elective surgical procedures. In a recent study by Foran and colleagues,4 surveyed postoperative patients indicated that Medicare reimbursement was “much lower” for arthroplasty than it should be. In addition, they overestimated (compared with national averages) what Medicare reimburses for hip and knee arthroplasty. Many raised concerns that orthopedic surgeons might drop Medicare entirely.4
These misconceptions about reimbursement may stem partly from the inaccessibility of health care cost information. Rosenthal and colleagues5 recently queried a random selection of US hospitals and demonstrated the difficulty in obtaining THA pricing information.
In a system in which consumers and payers are often not one and the same, it is unclear if consumers understand the cost of their health care. We conducted a study to assess patients’ perceptions of the cost of total joint arthroplasty (TJA) and gain insight into their understanding of health care costs and their sense of the value of this elective surgical procedure.
Materials and Methods
After obtaining institutional review board approval and informed consent for this study, we surveyed 284 consecutive patients who underwent THA or TKA at an academic medical center. Patients had either primary or revision surgery performed (by Dr. Hallstrom or Dr. Urquhart) and were surveyed during their first (2-week) postoperative visit, between March 1, 2012 and December 20, 2012.
Surveys were labeled with patient identifiers to facilitate abstraction of data from electronic medical records. Operative reports and discharge summaries were reviewed for data that included sex, age, diagnosis, procedure, surgeon, implant, admission date, and length of stay.
The survey asked for demographic information, including level of education, insurance coverage, and annual household income, and included a question to verify the surgical procedure and a question to determine if the patient had reviewed a hospital billing statement pertaining to the patient’s admission. The survey also included these questions about reimbursement and cost:
- How much do you feel your orthopedic surgeon was reimbursed for your surgery? (EXCLUDING payments to the hospital)
- How much do you think your surgeon gets reimbursed to see you IN THE HOSPITAL after surgery?
- How much do you think your surgeon gets reimbursed per visit to see you IN CLINIC for follow-up during the first 3 months after surgery?
- How much do you think the implant used in your surgery cost?
- How much do you think the hospital was reimbursed for your surgery and admission to the hospital after surgery? (EXCLUDING payments to the surgeon)
- How much do you think it cost the hospital to provide your surgery and admission to the hospital after surgery?
Responses were limited to numeric currency format using a response area as shown in Figure 1. Overall patient satisfaction was elicited with use of a 5-point scale ranging from 1 (very unsatisfied) to 5 (very satisfied). Regarding type of implant used, patients could select from 6 prominent vendors or indicate “other” or “don’t know.” They were also asked which of several factors should primarily determine surgeon reimbursement: overall patient satisfaction, technical difficulty, amount of risk/possible harm, duration/amount of time, and rate of complications. A free-response comments section was provided at the end of the survey.
Data from the survey and the electronic medical records were collected using Research Electronic Data Capture (REDCap; Vanderbilt University, Nashville, Tennessee). Statistical analysis was performed with SAS Version 9.3 (SAS Institute, Cary, North Carolina). Data were screened before further analysis. Patients who provided nonnumeric responses in numeric response fields were excluded from further analysis. Numeric ranges were applied in subsequent analysis using the mean of the range. Implausible responses resulted in the removal of the entire encounter from subsequent analysis.
Demographic data used to define categories for further subgroup analysis are presented as percentages of the group. Medians, means, and interquartile ranges were calculated for all responses regarding reimbursement and cost. Differences in perceptions of reimbursement and cost based on subgroups, including procedure type, diagnosis, education level, and satisfaction, were calculated. Independent-samples Student t tests were used to determine the statistical significance of the differences detected.
Results
Of the 400 eligible patients seen at the first postoperative follow-up, 284 (71%) were enrolled in the study. Mean (SD) age was 62.6 (12.6) years. Of the 284 patients enrolled, 154 (54%) were female. Of the participants who reported their education and income, 125 (44%) had a bachelor’s degree or higher degree, and 68 (23.9%) reported income of more than $100,000 per year. The largest payers reported by patients were private insurance (80%) and Medicare (46%). Additional demographic details are listed in Table 1.
Of the 284 patients enrolled in the study, 159 (56%) had THA, and 88 (31%) had TKA (Table 2). Thirty-seven patients (13%) underwent revision procedures. Only 5 patients (2%) indicated they had reviewed their hospital billing statement from their most recent admission. Two hundred forty-two patients (85%) were satisfied or very satisfied with their procedure.
Regarding the implant used in their surgery, 216 patients (76%) indicated they did not know which company manufactured it. Of the 68 patients (24%) who named a manufacturer, 53 (78%) were correct in their selection (intraoperative records were checked). Patients indicated they thought the implant used in their surgery cost $6447 on average (95% CI, $5581-$7312).
On average, patients thought their surgeon was reimbursed $12,014 (95% CI, $10,845-$13,183) for their procedure, and they estimated that the hospital was reimbursed $28,392 (95% CI, $25,271-$31,512) for their perioperative care and that it cost the hospital $24,389 (95% CI, $21,612-$27,165) to provide it. Means, confidence intervals, medians, and interquartile ranges for parameters of reimbursement and cost are listed in Table 3. Seventy-one patients (25%) thought on average that the hospital took a net loss for each TJA performed, and 146 patients (51%) thought on average that the hospital generated a net profit for each TJA.
On average, patients thought surgeons were reimbursed $11,872 for a THA and $12,263 for a TKA. Patients also estimated a higher hospital cost (THA, $22,981; TKA, $26,998) and reimbursement (THA, $27,366; TKA, $30,230) after TKA than THA. These differences in perceptions of cost and reimbursement for THA and TKA appear in Table 4 and Figure 2.
Statistically significant differences were also found in perceptions of cost and reimbursement based on level of education and overall patient satisfaction. Patients with a bachelor’s degree or higher estimated physician reimbursement at $11,006, whereas patients with a lower level of education estimated reimbursement at $12,890. In addition, patients with a lower level of education gave estimates of hospital cost and reimbursement that were $7698 and $10,799 higher, respectively, than the estimates given by patients with a higher level of education (Table 5, Figure 3). Patients who were satisfied or very satisfied with their overall TJA experience estimated surgeon reimbursement at $11,673. Patients who indicated they were unsatisfied, very unsatisfied, or neutral regarding their overall experience gave a higher estimate of surgeon reimbursement: $14,317 (Table 6, Figure 4).
Because of the small number of enrolled patients who had revision surgery and the high variability in patient responses, there were no meaningful or statistically significant differences in perceptions of cost and reimbursement based on revision or primary surgery.
Patients also estimated substantial additional reimbursements to physicians for services included at no additional charge with the global surgical package. Median estimates were $300 for reimbursement to a physician making rounds in the hospital and $250 for reimbursement for an outpatient follow-up. Only 47 patients (17%) and 35 patients (12%) correctly indicated there is no additional payment for making rounds and outpatient follow-up, respectively. Estimates of these reimbursements varied by education level, procedure, and overall satisfaction (Tables 4–6).
Discussion
The sustainable growth rate (SGR) formula, part of the Balanced Budget Act of 1997, was constructed to manage health care costs in the context of overall economic growth. By 2001, Medicare health care expenditures had begun to outpace economic growth, and the SGR formula dictated a reduction in reimbursement to physicians. Each year over the past decade, Congress has passed legislation providing a temporary reprieve, staving off a drastic reduction of as much as 25% in 2010.6 Despite these adjustments, reimbursement continues to decrease because of overall inflation.
More worrisome is that “more than half of the nearly trillion dollar price tag for expanding coverage under the Affordable Care Act (ACA) will be paid by decreasing spending for the more than 46.3 million individuals covered by Medicare.”7 ACA provisions will also create an Independent Payment Advisory Board (IPAB) to oversee health care costs and reduce Medicare spending when it is expected to exceed target levels.8 As IPAB cannot recommend increasing revenues or changing benefits, and because it is initially prohibited from recommending decreasing payments to hospitals, the decreases will likely have the greatest impact on physician reimbursement.7-9
Health care policy has been a major campaign issue during recent US elections. The public and popular media remain engaged in this important discussion. Although patients, policymakers, and physicians are understandably concerned about cost and access to health care, it is unclear if patients understand the distribution of health care cost and reimbursement.
Other authors have studied patients’ perceptions of physician reimbursement for TJA. Hayden and colleagues10 surveyed 1000 residents of a Texas city. The 121 who responded to the survey thought that fair compensation for performing a TKA was $5080, on average.10 Although this was significantly higher than the actual Medicare reimbursement at the time, a later study, by Foran and colleagues,4 found patients’ estimates of both fair reimbursement and Medicare reimbursement for TJA to be even higher. Foran and colleagues4 surveyed 1120 patients who thought surgeons deserved to be paid $14,358 for THA and $13,322 for TKA, on average. These reimbursement values are nearly an order of magnitude higher than actual reimbursements. For Medicare payments, patients lowered their estimates to $8212 for THA and $7196 for TKA.4
To our knowledge, the present study is the first to use a “postconsumer” survey to assess patients’ perceptions of THA and TKA costs. Our results confirmed that patients substantially overestimated reimbursement for THA and TKA at $11,872 and $12,263, respectively, relative to the average Medicare reimbursements of $1467 and $1530, respectively.11 We also found that patients overestimated both hospital cost and reimbursement for THA at $22,981 and $27,366, respectively, relative to recently published hospital economic analyses showing THA cost and reimbursement to be $11,688 and $15,789, respectively.12 Few patients enrolled in our study demonstrated an understanding of the services included in the global surgical package. Only about 12% of patients correctly indicated there was no additional payment to the physician for initial follow-up appointments. However, patients were fairly accurate in their estimates of implant cost. On average, patients who underwent THA priced their implant at $6823, which is only about 9% higher than the reported median cost of $6072 to $6400.13,14
We also found significant differences in perceptions of cost based on level of education, joint replaced, and overall level of satisfaction. On average, patients with a bachelor’s degree or higher gave estimates of cost and reimbursement that were lower than those given by patients with a lower level of education. Estimates of physician reimbursement and hospital reimbursement and cost were higher from patients who had TKA than from patients who had THA.
Comparing perceptions of reimbursement for appendectomy and coronary artery bypass with perceptions for TJA, Foran and colleagues4 found that patients understood the relative complexity of each procedure, as evidenced by their estimates of fair reimbursement for each. However, in comparing patient estimates for the different components of cost and reimbursement for TJA, we found great variability in understanding. Patients in our study overestimated payments to the hospital by 73% but overestimated the cost of the THA implant by only 9%. However, the same patients overestimated physician reimbursement for THA by about 800%. If these patients’ estimates of reimbursement are considered surrogates for relative value, then physicians, based on actual payments, are grossly undervalued relative to implant manufacturers.
Our study had several limitations. First, the enrolled patients were all seen at one medical center, in Ann Arbor, Michigan, and our results may not be generalizable outside the region. Second, the survey respondents were postoperative patients who had an established relationship with the study’s principal investigators—a relationship that may have been a source of bias in the consideration of reimbursement as a function of value. Third, despite our efforts to carefully design a survey with open-ended responses, the order in which the survey questions were presented may have influenced patient responses. Fourth, the open-ended question design may have had an impact on responses where the correct answer would have required entering 0.00.
Despite these limitations, our study results demonstrated general public misconceptions about cost and reimbursement for common orthopedic procedures. Although more transparency in health care cost information may not immediately result in a more well-informed population,15 our patients, given the opportunity to develop an understanding of the economics of their own medical treatment, may become better prepared to make informed choices regarding changes in health care policy.
1. Kumar S, Ghildayal NS, Shah RN. Examining quality and efficiency of the U.S. healthcare system. Int J Health Care Qual Assur. 2011;24(5):366-388.
2. Hariri S, Bozic KJ, Lavernia C, Prestipino A, Rubash HE. Medicare physician reimbursement: past, present, and future. J Bone Joint Surg Am. 2007;89(11):2536-2546.
3. Kurtz S, Ong K, Lau E, Mowat F, Halpern M. Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. J Bone Joint Surg Am. 2007;89(4):780-785.
4. Foran JR, Sheth NP, Ward SR, et al. Patient perception of physician reimbursement in elective total hip and knee arthroplasty. J Arthroplasty. 2012;27(5):703-709.
5. Rosenthal JA, Lu X, Cram P. Availability of consumer prices from US hospitals for a common surgical procedure. JAMA Intern Med. 2013;173(6):427-432.
6. US Senate Committee on Finance. H.R. 4994: the Medicare and Medicaid Extenders Act of 2010. http://www.finance.senate.gov/legislation/details/?id=9f97aa2e-5056-a032-52d4-8db158b12b11. Accessed March 25, 2015.
7. Zinberg JM. When patients call, will physicians respond? JAMA. 2011;305(19):2011-2012.
8. Jost TS. The Independent Payment Advisory Board. N Engl J Med. 2010;363(2):103-105.
9. US Department of Health and Human Services, Centers for Medicare & Medicaid Services. Estimated financial effects of the “Patient Protection and Affordable Care Act,” as amended. 2010. http://www.cms.gov/Research-Statistics-Data-and-Systems/Research/ActuarialStudies/downloads/PPACA_2010-04-22.pdf. Accessed March 25, 2015.
10. Hayden SA, Hayden D, White LW. The U.S. public’s perceived value of the surgeon’s fee for total knee replacement. Abstract presented at: 75th Annual Meeting of the American Academy of Orthopaedic Surgeons; March 5-9, 2008; San Francisco, CA. Abstract 214.
11. Centers for Medicare & Medicaid Services. Physician Fee Schedule Search Tool. http://www.cms.gov/apps/physician-fee-schedule/search/search-criteria.aspx. Accessed March 25, 2015.
12. Rana AJ, Iorio R, Healy WL. Hospital economics of primary THA decreasing reimbursement and increasing cost, 1990 to 2008. Clin Orthop. 2011;469(2):355-361.
13. Lavernia CJ, Hernandez VH, Rossi MD. Payment analysis of total hip replacement. Curr Opin Orthop. 2007;18(1):23-27.
14. Robinson JC, Pozen A, Tseng S, Bozic KJ. Variability in costs associated with total hip and knee replacement implants. J Bone Joint Surg Am. 2012;94(18):1693-1698.
15. Smolders JM, Van Loon CJ, Rijnberg WJ, Van Susante JL. Patients poorly estimate the overall costs of a total knee arthroplasty and strongly overestimate the surgeon’s fee. Acta Orthop Belg. 2007;73(3):339-344.
1. Kumar S, Ghildayal NS, Shah RN. Examining quality and efficiency of the U.S. healthcare system. Int J Health Care Qual Assur. 2011;24(5):366-388.
2. Hariri S, Bozic KJ, Lavernia C, Prestipino A, Rubash HE. Medicare physician reimbursement: past, present, and future. J Bone Joint Surg Am. 2007;89(11):2536-2546.
3. Kurtz S, Ong K, Lau E, Mowat F, Halpern M. Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. J Bone Joint Surg Am. 2007;89(4):780-785.
4. Foran JR, Sheth NP, Ward SR, et al. Patient perception of physician reimbursement in elective total hip and knee arthroplasty. J Arthroplasty. 2012;27(5):703-709.
5. Rosenthal JA, Lu X, Cram P. Availability of consumer prices from US hospitals for a common surgical procedure. JAMA Intern Med. 2013;173(6):427-432.
6. US Senate Committee on Finance. H.R. 4994: the Medicare and Medicaid Extenders Act of 2010. http://www.finance.senate.gov/legislation/details/?id=9f97aa2e-5056-a032-52d4-8db158b12b11. Accessed March 25, 2015.
7. Zinberg JM. When patients call, will physicians respond? JAMA. 2011;305(19):2011-2012.
8. Jost TS. The Independent Payment Advisory Board. N Engl J Med. 2010;363(2):103-105.
9. US Department of Health and Human Services, Centers for Medicare & Medicaid Services. Estimated financial effects of the “Patient Protection and Affordable Care Act,” as amended. 2010. http://www.cms.gov/Research-Statistics-Data-and-Systems/Research/ActuarialStudies/downloads/PPACA_2010-04-22.pdf. Accessed March 25, 2015.
10. Hayden SA, Hayden D, White LW. The U.S. public’s perceived value of the surgeon’s fee for total knee replacement. Abstract presented at: 75th Annual Meeting of the American Academy of Orthopaedic Surgeons; March 5-9, 2008; San Francisco, CA. Abstract 214.
11. Centers for Medicare & Medicaid Services. Physician Fee Schedule Search Tool. http://www.cms.gov/apps/physician-fee-schedule/search/search-criteria.aspx. Accessed March 25, 2015.
12. Rana AJ, Iorio R, Healy WL. Hospital economics of primary THA decreasing reimbursement and increasing cost, 1990 to 2008. Clin Orthop. 2011;469(2):355-361.
13. Lavernia CJ, Hernandez VH, Rossi MD. Payment analysis of total hip replacement. Curr Opin Orthop. 2007;18(1):23-27.
14. Robinson JC, Pozen A, Tseng S, Bozic KJ. Variability in costs associated with total hip and knee replacement implants. J Bone Joint Surg Am. 2012;94(18):1693-1698.
15. Smolders JM, Van Loon CJ, Rijnberg WJ, Van Susante JL. Patients poorly estimate the overall costs of a total knee arthroplasty and strongly overestimate the surgeon’s fee. Acta Orthop Belg. 2007;73(3):339-344.
Polydactyly of the Hand
Polydactyly is the presence of extra digits. Its incidence is likely underestimated because many practitioners treat simple “nubbins” without referring them to orthopedic specialists.1-3 Polydactyly can be detected by ultrasound as early as 14 weeks’ gestational age, with partial autoamputation seen in most isolated polydactylies.4 The thumb, responsible for 40% of hand function, must be able to oppose the other digits with a stable pinch.5 Polydactyly encumbers this motion when the duplicated digits deviate from normal alignment. Ezaki6 noted that the anatomy is better described as “split” than “duplicated.” There are many dichotomous ways to classify polydactyly: preaxial (radial) versus postaxial (ulnar), thumb versus triphalangeal, simple versus complex (Figure 1). Mixed polydactyly is defined as the presence of preaxial and postaxial polydactyly.7 Surgical management seeks to allow normal hand function and to restore cosmesis.
Epidemiology
Sun and colleagues8 reported the overall polydactyly incidence as 2 per 1000 live births in China from 1998 to 2009, with a slight male predominance; polydactyly was also 3 times more common than syndactyly in this population. Ivy,9 in a 5-year audit of Pennsylvania Department of Health records, found polydactyly to be the fourth most common congenital anomaly after clubfoot, cleft lip/palate, and spina bifida. Thumb duplication occurs in 0.08 to 1.4 per 1000 live births and is more common in American Indians and Asians than in other races.5,10 It occurs in a male-to-female ratio of 2.5 to 1 and is most often unilateral.5 Postaxial polydactyly is predominant in black infants; it is most often inherited in an autosomal dominant fashion, if isolated, or in an autosomal recessive pattern, if syndromic.1 A prospective San Diego study of 11,161 newborns found postaxial type B polydactyly in 1 per 531 live births (1 per 143 black infants, 1 per 1339 white infants); 76% of cases were bilateral, and 86% had a positive family history.3 In patients of non-African descent, it is associated with anomalies in other organs. Central duplication is rare and often autosomal dominant.5,10
Genetics and Development
As early as 1896, the heritability of polydactyly was noted.11 As of 2010, polydactyly has been associated with 310 diseases.12 Ninety-nine genes, most involved in regulation of anterior-posterior formation of the limb bud, have been implicated.12,13
The upper limb begins to form at day 26 in utero.14 Apoptosis in the interdigital necrotic zones results in the formation of individual digits. It is presumed that, in polydactyly, the involved tissue is hypoplastic because of an abnormal interaction between mesoderm and ectoderm.5 Presence of an apical ectodermal ridge determines the formation of a limb bud, and on it the zone of polarizing activity (ZPA) dictates preaxial and postaxial alignment.14,15 The ZPA is located on the posterior zone of the developing limb bud. The levels of GLI3, a zinc finger-containing DNA-binding protein, are highest in the anterior area, and HAND2, a basic helix-loop-helix DNA-binding protein, is found in the ZPA. This polarity promotes sonic hedgehog (Shh) gene expression in the posterior region, which in turn prevents GLI3 cleavage into its repressed form. GLI3R (repressed) and GLI3A (active) concentrations are highest, therefore, in the anterior and posterior portions of the bud, respectively. The GLI3A:GLI3R ratio is responsible for the identity and number of digits in the hand (ie, the thumb develops in regions of high GLI3R). GLI and Shh mutations lead to polydactylous hands with absent thumbs (Figure 2).16
Ciliopathies have also been shown to cause postaxial polydactyly, possibly because of the role that nonmotile cilia play in hedgehog signaling.17 Mutations in Shh genomic regulators cause preaxial polydactyly.18 HoxD activates Shh in the ZPA; HoxD13 mutations are associated with synpolydactyly.16,19 In each of these mutations, Shh production is altered, and some form of polydactyly results.
Associations
Many syndromes have been associated with polydactyly. Not all polydactyly is associated with other disorders, but the more complex the polydactyly, the more likely that other anomalies are present. Every patient who presents with polydactyly should have a full history taken and a physical examination performed (Figure 3). Any patient with syndromic findings or atypical presentations (eg, triphalangism, postaxial polydactyly in a patient of non-African descent, central and index polydactyly) should be referred to a geneticist.
Classifications
The Wassel20 classification describes the anatomical presentation of thumb duplication on the basis of 70 cases in Iowa (Figures 4, 5; Table 1). Because some duplications fall outside the Wassel classification, many researchers have proposed modifications (Figure 6).21-25
The Temtamy and McKusick10 classification, which is the product of geneticists, classifies duplications by grouping genetically related presentations (Table 2). It provides the most commonly used postaxial classification, with type A being a fully developed digit and type B a rudimentary and pedunculated digit, informally referred to as a nubbin. Type B is more common than type A. Given inheritance patterns, it is assumed that type A is likely multifactorial and type B autosomal dominant.10 Thumb polydactyly inheritance is still unclear. The other types of preaxial polydactyly and high degrees of polydactyly are rare but seem to be passed on in an autosomal dominant fashion on pedigree analysis.10
The Stelling and Turek classification presents the duplication from a tissue perspective: Type I duplication is a rudimentary mass devoid of other tissue elements; type II is a subtotal duplication with some normal structures; and type III is a duplication of the entire “osteoarticular column,” including the metacarpal.1 It is interesting to note that histology of type I duplications shows neuroma-like tissue.26-28 Again, normal is a relative term because, in polydactyly, the duplications are hypoplastic and deviated, with anomalous anatomy.
The Rayan classification describes ulnar polydactyly and was derived from a case study series of 148 patients in Oklahoma (Table 3).29
There are also some complex polydactylies that are not easily classified: ulnar dimelia, cleft hand, pentadactyly, and hyperphalangism. Ulnar dimelia, also known as “mirror hand,” is typically 7 digits with no thumb, but other variations are seen. The radius is often absent, and the elbow is abnormal. There is some debate about whether it is a fusion of 2 hands. Pentadactyly, or the 5-fingered hand, appears as 5 triphalangeal digits with no thumb (Figure 7).
Isolated thumb triphalangism might appear similar to pentadactyly. Miura30,31 pointed out that the radial digit in the pentadactylous hand may be opposable (thumb-like) or nonopposable; in his studies, the patients with the opposable thumb had a metacarpal with a proximal epiphysis (Figure 8). Consequently, the triphalangeal thumb metacarpal with a distal epiphysis is true pentadactyly, whereas that with a proximal epiphysis is hyperphalangism (Figure 9). Treatment of these complex polydactylies involves the same underlying principles as for preaxial and postaxial polydactyly, albeit with additional proximal upper extremity considerations.
When to Operate (Timing)
Ezaki6 recommended surgical intervention at age 6 to 9 months, before fine motor skills have developed with the abnormal anatomy. Cortical learning occurs as the child begins prehensile activities before 6 months, but the risks of anesthesia outweigh functional benefits until the child is older. Waiting until 1 year of age is not uncommon, though surgery at an earlier age may be beneficial if the polydactyly affects hand function.32 It is not uncommon to wait with the more balanced thumb polydactylies to assess thumb function. Hypoplasia might also delay surgical intervention until there is enough tissue inventory for reconstruction. Wassel20 noted that surgical intervention ideally occurs before the supernumerary elements displace the normal elements, as tends to happen with growth. Suture ligation is an option in the neonatal unit for some pedunculated digits.33 Studies have shown satisfactory results in adults treated for polydactyly, if the patient presents later than expected.34
Surgical Considerations
Knavel recommended simple excision, stating that “ablation requires no ingenuity and creates no problems.”5 This belief, though true for some duplications, will not lead to the best outcome for more complex polydactylies. The goal of surgery is a stable and well-aligned thumb for pinch and prehensile activity, as well as a cosmetically pleasing hand. Incisions should not be made linearly along the axis of the digit, as the scar will cause deviation with growth.24
Wassel type I polydactyly might appear incidentally as a broad thumb, in which case it requires no intervention (Figure 10). However, in Wassel types I and II polydactyly with deformity, the Bilhaut-Cloquet procedure is useful for both bifid and duplicated phalanges (Figure 11).5,6,30,32,35 Collateral ligaments may need to be released in type II because of difficulty in opposing the tissue. Cosmetic results with Bilhaut-Cloquet are unpredictable. The original technique required symmetrically sized digits; results today have been improved with microtechniques and preservation of an entire nail.36 Another option is ablation of the more hypoplastic osseous element and soft-tissue augmentation of the residual digit. The theme of ablation and augmentation is seen throughout the literature for the surgical treatment of polydactyly (Figure 12).1
For type III polydactyly, the bifid proximal phalanx is narrowed by resection and realigned with osteotomy of the remaining diaphysis. Type IV polydactyly, the most common thumb duplication, often requires advancement of the abductor pollicis brevis to the base of the proximal phalanx to aid in metacarpophalangeal (MCP) stabilization, abduction, and opposition. The metacarpal head, if broad and with 2 facets, can be shaped to form a single articulating surface. The metacarpal, occasionally with the proximal phalanx, often requires realignment by closing wedge osteotomy. Last, tendons on the resected bony elements should be rebalanced on the remaining digit, and anomalous slips must be released. For instance, given a radial insertion of the long flexor tendon on the distal phalanx, the tendon should be moved centrally. A strong flexor or extensor tendon on the amputated digit should be transferred to the remaining digit.24
Types V and VI are treated similarly to type IV, with the addition of a first web space Z-plasty or web widening if there is thenar eminence contracture. Acral transposition has also been described, with transposition of the tip of the ablated digit in place of the tip of the kept digit; this technique is ideal if one digit has a more normal proximal part while the other has a more normal distal part (Figure 13).35
Type VII thumb polydactyly, the type most likely inherited and associated with other disorders, should be treated like type VI. The nail should be preserved; amputation of the distal phalanx is not advised. Resection of the delta phalanx or 1 interphalangeal (IP) joint is an option. Articular surfaces will remodel if done before the age of 1 year. If the thenar eminence is hypoplastic, then Huber transfer of the abductor digiti minimi should be considered.37 Resection of the triphalangeal thumb is also advised, even if the biphalangeal thumb is more hypoplastic, with transfer of the ligaments and tendons, as described earlier.5,6,24,30,32,35
Thumb triphalangism, if isolated, and hyperphalangism in the other digits, can be treated with resection of the delta phalanx or one of the IP joints if it is affecting function or cosmesis.1,6 Wood and Flatt23 recommended early resection of a thumb delta phalanx because of the likelihood of deviation that impedes thumb function. For children, they recommended delta phalanx resection and Kirschner wire fixation for 6 weeks; for adults, they recommended resection or fusion of the joint, with osteotomy as needed for deviation.23,24 For thumb triphalangism, multiple surgeries are the norm, as Wood24 reported in his study of 21 patients who underwent 78 operations in total.
Index polydactyly may present as a simple pedunculated skin tag, which can be simply excised, or as a more complex musculoskeletal duplication. More complex presentations can be treated with procedures similar to those used for the thumb. Typically, the additional digit is radially deviated and angulated, eventually leading to impingement of thumb pinch and the first web space. Ray amputation is also an option if no reconstructive surgery will produce the stable, sensate radial pinch that is essential to hand function.32
Ring-finger polydactyly and long-finger polydactyly are often complicated by some element of syndactyly, resulting in a relative paucity of skin (Figure 14). There is failure of both formation (hypoplasia) and differentiation (syndactyly). The hypoplasia particularly affects the function of these digits by tethering them; multiple surgeries to restore proper hand function are the norm.1 Reconstructive surgery for these digits requires preoperative tissue inventory followed by resection and augmentation; as in syndactyly, skin for coverage is at a premium. Creation of a 3-fingered hand is an option.23
Temtamy and McKusick10 type A little-finger polydactyly is treated similarly to the thumb, with the caveat that hypothenar and intrinsic muscles that insert on the resected little finger are transferred to the remaining digit. In contrast to thumb polydactyly, the extrinsic musculature tends to be in good position. Suture ligation of type B polydactyly, as described by Flatt, is likely more common than orthopedists appreciate, as pediatricians and neonatal unit practitioners commonly perform this procedure in the nursery.1-3 It has been described with 2-0 Vicryl3 (Ethicon, Somerville, New Jersey) and 4-0 silk sutures,32 with the goal of necrosis and autoamputation. Parents should be told the finger generally falls off about 10 days (range, 4-21 days) after ligation.3 Multiple authors have cited a report of exsanguination from suture ligation, but we could not locate the primary source. It is advisable to wait until a patient is 6 months of age if planning to resect the nubbin in the operating room, given the anesthesia risk and the lack of functional impairment. Katz and Linder33 indicated they remove type B polydactyly in the nursery suite used for circumcisions; they use anesthetizing cream on the skin, and sharp excision with a scalpel, followed by direct pressure and Steri-Strip (3M, St. Paul, Minnesota) application. Suture ligation is recommended only if there is a narrow, thin (<2 mm) soft-tissue stalk; any broad or bony stalk necessitates surgical removal to avoid neuroma formation and failure of autonecrosis (Figure 15).27 Other options are a single swipe of a scalpel and elliptical excision; sharp transaction of the digital nerve with subsequent retraction is advised to avoid neuroma formation.2
Barton described ulnar dimelia operations as “spare parts surgery.”1 Extra digits are ablated and a thumb created (Figure 16). The hand might have a digit in relatively good rotational position for thumbplasty, or the principles of pollicization may need to be used. If the patient is already using the hand, the surgeon should note which finger the patient uses as a thumb.24 Any accompanying wrist flexion contracture must be corrected with careful attention to musculotendinous balancing. Because the forearm and elbow, and occasionally even the more proximal limb, will be abnormal in this disorder, multiple surgeries are again the norm.1
Pentadactyly is treated like thumb hypoplasia, with first web space creation.1
Complications
In polydactyly, a reoperation rate of up to 25% has been reported, with most reoperations performed because of residual or subsequent deformity.5,30,31,38 Risk factors for reoperation are type IV thumb duplication, preoperative “zigzag” deformity, and radially deviated thumb elements at presentation.5 The delta phalanx may not show on radiographs until the patient is 18 months old, but functional deformity will worsen as long as it is present. Zigzag deformity may be due to the delta phalanx or to musculotendinous imbalance, such as a radially inserted flexor pollicis longus (FPL) or lack of stable MCP abduction. Miura31 found that careful reconstruction of the joint capsule and thenar muscles from the ablated digit to the remnant digit is the key to a successful initial surgery. Lee and colleagues39 defined zigzag deformity as more than 20° MCP and IP angulation; for cases present before surgery, they recommended FPL relocation by the pullout technique in addition to osteotomies to prevent further interphalangeal deviation (Figures 17, 18).
Abnormal physeal growth, joint instability, and stiffness can all occur. Stiffness is particularly difficult to treat but seldom presents a functional problem. Joint enlargement, which is not uncommon, results from either broad articular surfaces or retained cartilage from the perichondral ring after resection that later ossifies.5,38 Nubbin-type duplications may not fall off after suture ligation, necessitating further excision, and a cosmetic bump is seen after 40% of suture ligations.3 Patillo and Rayan28 and Rayan and Frey29 warned against suture ligation unless the nubbin has a small stalk because of the possibility of infection and gangrene. The excised nubbin tissue is histologically nervous, and there have been reports of painful neuromas in the remaining scar of a ligated nubbin that respond well to excision.26,27,40 It is thought that these painful lesions form because the ligature prevents the digital nerves to the vestigial digit from retracting.27 Nail deformity and IP joint stiffness are seen with the Bilhaut-Cloquet procedure, though often finger function remains satisfactory.
Conclusion
Polydactyly is a common congenital hand abnormality. Its true incidence is unknown because of inconsistent documentation. Surgeons must strive for a functional, cosmetic hand, given a diverse set of possible anomalies. Hypoplasia is the rule; tissue should be ablated and augmented as necessary. Musculotendinous insertions may need to be centralized. Patients’ family members should always be counseled that more surgery may be needed in the future, as further deformity can occur with growth. Surgically corrected thumb duplications will be stiffer, shorter, and thinner than their normal counterparts. Nail ridges are common. However, it should be noted that 88% of these patients are satisfied with their results.41 Some amount of contracture and abnormal function should be expected with index-, long-, and ring-finger duplications. The only remnant of type B postaxial duplications may be a slight discoloration or bump, though stiffness and deformity can happen with a type A deformity. A “duplicated” digit that requires surgical correction will never be completely normal, but acceptable function is routinely achievable.
1. Graham TJ, Ress AM. Finger polydactyly. Hand Clin. 1998;14(1):49-64.
2. Abzug JM, Kozin SH. Treatment of postaxial polydactyly type B. J Hand Surg Am. 2013;38(6):1223-1225.
3. Watson BT, Hennrikus WL. Postaxial type-B polydactyly—prevalence and treatment. J Bone Joint Surg Am. 1997;79(1):65-68.
4. Zimmer EZ, Bronshtein M. Fetal polydactyly diagnosis during early pregnancy: clinical applications. Am J Obstet Gynecol. 2000;183(3):755-758.
5. Cohen MS. Thumb duplication. Hand Clin. 1998;14(1):17-27.
6. Ezaki M. Radial polydactyly. Hand Clin. 1990;6(4):577-588.
7. Nathan PA, Keniston RC. Crossed polydactyly: case report and review of the literature. J Bone Joint Surg Am. 1975;57(6):847-849.
8. Sun G, Xu ZM, Liang JF, Li L, Tang DX. Twelve-year prevalence of common neonatal congenital malformations in Zhejiang Province, China. World J Pediatr. 2011;7(4):331-336.
9. Ivy RH. Congenital anomalies as recorded on birth certificates in the Division of Vital Statistics of the Pennsylvania Department of Health, for the period of 1951–1955, inclusive. Plast Reconstr Surg. 1957;20(5):400-411.
10. Temtamy SA, McKusick VA. Polydactyly as a part of syndromes. In: Bergsma D, ed. Mudge JR, Paul NW, Conde Greene S, associate eds. The Genetics of Hand Malformations. New York, NY: Liss. Birth Defects Original Article Series. 1978;14(3):364-439.
11. Gould W, Pyle L. Anomalies and Curiosities of Medicine. New York, NY: Bell; 1896.
12. Biesecker LG. Polydactyly: how many disorders and how many genes: 2010 update. Dev Dyn. 2011;250(5):931-942.
13. Grzeschik K. Human limb malformations; an approach to the molecular basis of development. Int J Dev Biol. 2001;46(7):983-991.
14. Zaleske DJ. Development of the upper limb. Hand Clin. 1985;1(3):383-390.
15. Beatty E. Upper limb tissue differentiation in the human embryo. Hand Clin. 1985;1(3):391-404.
16. Anderson E, Peluso S, Lettice LA, Hill RE. Human limb abnormalities caused by disruption of hedgehog signaling. Trends Genet. 2012;28(8):364-373.
17. Ware SM, Aygun MG, Heldebrandt F. Spectrum of clinical diseases caused by disorders of primary cilia. Proc Am Thorac Soc. 2011;8(5):444-450.
18. Lettice LA, Hill RE. Preaxial polydactyly: a model for defective long-range regulation in congenital abnormalities. Curr Opin Genet Dev. 2005;15(3):294-300.
19. Al-Qattan MA. Type II familial synpolydactyly: report on two families with an emphasis on variations of expression. Eur J Hum Genet. 2011;19(1):112-114.
20. Wassel HD. The results of surgery for polydactyly of the thumb. Clin Orthop. 1969;(64):175-193.
21. Blauth W, Olason AT. Classification of polydactyly of the hands and feet. Arch Orthop Trauma Surg. 1988;107(6):334-344.
22. Wood VE. Super digit. Hand Clin. 1990;6(4):673-684.
23. Wood VE, Flatt AE. Congenital triangular bones in the hand. J Hand Surg Am. 1977;2(3):179-193.
24. Wood VE. Polydactyly and the triphalangeal thumb. J Hand Surg Am. 1978;3(5):436-444.
25. Zuidam JM, Selles RW, Ananta M, Runia J, Hovius SER. A classification system of radial polydactyly: inclusion of triphalangeal thumb and triplication. J Hand Surg Am. 2008;33(3):373-377.
26. Leber GE, Gosain AK. Surgical excision of pedunculated supernumerary digits prevents traumatic amputation neuromas. Pediatr Dermatol. 2003;20(2):108-112.
27. Mullick S, Borschel GH. A selective approach to treatment of ulnar polydactyly: preventing painful neuroma and incomplete excision. Pediatr Dermatol. 2001;27(1):39-42.
28. Patillo D, Rayan GM. Complications of suture ligation ablation for ulnar polydactyly: a report of two cases. Hand (N Y). 2011;6(1):102-105.
29. Rayan GM, Frey B. Ulnar polydactyly. Plastic Reconstr Surg. 2001;107(6):1449-1454.
30. Miura T. Triphalangeal thumb. Plastic Reconstr Surg. 1976;58(5):587-594.
31. Miura T. Duplicated thumb. Plastic Reconstr Surg. 1982;69(3):470-481.
32. Simmons BP. Polydactyly. Hand Clin. 1985;1(3):545-566.
33. Katz K, Linder N. Postaxial type B polydactyly treated by excision in the neonatal nursery. J Pediatr Orthop. 2011;31(4):448-449.
34. Manohar A, Beard AJ. Outcome of reconstruction for duplication of the thumb in adults aged over 40. Hand Surg. 2011;16(2):207-210.
35. Watt AJ, Chung KC. Duplication. Hand Clin. 2009;25(2):215-228.
36. Tonkin MA. Thumb duplication: concepts and techniques. Clin Orthop Surg. 2012;4(1):1-17.
37. Huber E. Relief operation in the case of paralysis of the median nerve. J Hand Surg Eur. 2004;29(1):35-37.
38. Mih AD. Complications of duplicate thumb reconstruction. Hand Clin. 1998;14(1):143-149.
39. Lee CC, Park HY, Yoon JO, Lee KW. Correction of Wassel type IV thumb duplication with zigzag deformity: results of a new method of flexor pollicis longus tendon relocation. J Hand Surg Eur. 2013;38(3):272-280.
40. Hare PJ. Rudimentary polydactyly. Br J Dermatol. 1954;66(11):402-408.
41. Yen CH, Chan WL, Leung HB, Mak KH. Thumb polydactyly: clinical outcome after reconstruction. J Orthop Surg (Hong Kong). 2006;14(3):295-302.
42. Edmunds JO. A tribute to Daniel C. Riordan, MD (1917–2012). Tulane University School of Medicine, Department of Orthopaedics website. http://tulane.edu/som/departments/orthopaedics/news-and-events/danriordantribute.cfm. Accessed March 31, 2015.
43. Faust DC, Herms R. Daniel C. Riordan, MD, 1917–2012. J Hand Surg Am. 2013;38(1):202-205.
Polydactyly is the presence of extra digits. Its incidence is likely underestimated because many practitioners treat simple “nubbins” without referring them to orthopedic specialists.1-3 Polydactyly can be detected by ultrasound as early as 14 weeks’ gestational age, with partial autoamputation seen in most isolated polydactylies.4 The thumb, responsible for 40% of hand function, must be able to oppose the other digits with a stable pinch.5 Polydactyly encumbers this motion when the duplicated digits deviate from normal alignment. Ezaki6 noted that the anatomy is better described as “split” than “duplicated.” There are many dichotomous ways to classify polydactyly: preaxial (radial) versus postaxial (ulnar), thumb versus triphalangeal, simple versus complex (Figure 1). Mixed polydactyly is defined as the presence of preaxial and postaxial polydactyly.7 Surgical management seeks to allow normal hand function and to restore cosmesis.
Epidemiology
Sun and colleagues8 reported the overall polydactyly incidence as 2 per 1000 live births in China from 1998 to 2009, with a slight male predominance; polydactyly was also 3 times more common than syndactyly in this population. Ivy,9 in a 5-year audit of Pennsylvania Department of Health records, found polydactyly to be the fourth most common congenital anomaly after clubfoot, cleft lip/palate, and spina bifida. Thumb duplication occurs in 0.08 to 1.4 per 1000 live births and is more common in American Indians and Asians than in other races.5,10 It occurs in a male-to-female ratio of 2.5 to 1 and is most often unilateral.5 Postaxial polydactyly is predominant in black infants; it is most often inherited in an autosomal dominant fashion, if isolated, or in an autosomal recessive pattern, if syndromic.1 A prospective San Diego study of 11,161 newborns found postaxial type B polydactyly in 1 per 531 live births (1 per 143 black infants, 1 per 1339 white infants); 76% of cases were bilateral, and 86% had a positive family history.3 In patients of non-African descent, it is associated with anomalies in other organs. Central duplication is rare and often autosomal dominant.5,10
Genetics and Development
As early as 1896, the heritability of polydactyly was noted.11 As of 2010, polydactyly has been associated with 310 diseases.12 Ninety-nine genes, most involved in regulation of anterior-posterior formation of the limb bud, have been implicated.12,13
The upper limb begins to form at day 26 in utero.14 Apoptosis in the interdigital necrotic zones results in the formation of individual digits. It is presumed that, in polydactyly, the involved tissue is hypoplastic because of an abnormal interaction between mesoderm and ectoderm.5 Presence of an apical ectodermal ridge determines the formation of a limb bud, and on it the zone of polarizing activity (ZPA) dictates preaxial and postaxial alignment.14,15 The ZPA is located on the posterior zone of the developing limb bud. The levels of GLI3, a zinc finger-containing DNA-binding protein, are highest in the anterior area, and HAND2, a basic helix-loop-helix DNA-binding protein, is found in the ZPA. This polarity promotes sonic hedgehog (Shh) gene expression in the posterior region, which in turn prevents GLI3 cleavage into its repressed form. GLI3R (repressed) and GLI3A (active) concentrations are highest, therefore, in the anterior and posterior portions of the bud, respectively. The GLI3A:GLI3R ratio is responsible for the identity and number of digits in the hand (ie, the thumb develops in regions of high GLI3R). GLI and Shh mutations lead to polydactylous hands with absent thumbs (Figure 2).16
Ciliopathies have also been shown to cause postaxial polydactyly, possibly because of the role that nonmotile cilia play in hedgehog signaling.17 Mutations in Shh genomic regulators cause preaxial polydactyly.18 HoxD activates Shh in the ZPA; HoxD13 mutations are associated with synpolydactyly.16,19 In each of these mutations, Shh production is altered, and some form of polydactyly results.
Associations
Many syndromes have been associated with polydactyly. Not all polydactyly is associated with other disorders, but the more complex the polydactyly, the more likely that other anomalies are present. Every patient who presents with polydactyly should have a full history taken and a physical examination performed (Figure 3). Any patient with syndromic findings or atypical presentations (eg, triphalangism, postaxial polydactyly in a patient of non-African descent, central and index polydactyly) should be referred to a geneticist.
Classifications
The Wassel20 classification describes the anatomical presentation of thumb duplication on the basis of 70 cases in Iowa (Figures 4, 5; Table 1). Because some duplications fall outside the Wassel classification, many researchers have proposed modifications (Figure 6).21-25
The Temtamy and McKusick10 classification, which is the product of geneticists, classifies duplications by grouping genetically related presentations (Table 2). It provides the most commonly used postaxial classification, with type A being a fully developed digit and type B a rudimentary and pedunculated digit, informally referred to as a nubbin. Type B is more common than type A. Given inheritance patterns, it is assumed that type A is likely multifactorial and type B autosomal dominant.10 Thumb polydactyly inheritance is still unclear. The other types of preaxial polydactyly and high degrees of polydactyly are rare but seem to be passed on in an autosomal dominant fashion on pedigree analysis.10
The Stelling and Turek classification presents the duplication from a tissue perspective: Type I duplication is a rudimentary mass devoid of other tissue elements; type II is a subtotal duplication with some normal structures; and type III is a duplication of the entire “osteoarticular column,” including the metacarpal.1 It is interesting to note that histology of type I duplications shows neuroma-like tissue.26-28 Again, normal is a relative term because, in polydactyly, the duplications are hypoplastic and deviated, with anomalous anatomy.
The Rayan classification describes ulnar polydactyly and was derived from a case study series of 148 patients in Oklahoma (Table 3).29
There are also some complex polydactylies that are not easily classified: ulnar dimelia, cleft hand, pentadactyly, and hyperphalangism. Ulnar dimelia, also known as “mirror hand,” is typically 7 digits with no thumb, but other variations are seen. The radius is often absent, and the elbow is abnormal. There is some debate about whether it is a fusion of 2 hands. Pentadactyly, or the 5-fingered hand, appears as 5 triphalangeal digits with no thumb (Figure 7).
Isolated thumb triphalangism might appear similar to pentadactyly. Miura30,31 pointed out that the radial digit in the pentadactylous hand may be opposable (thumb-like) or nonopposable; in his studies, the patients with the opposable thumb had a metacarpal with a proximal epiphysis (Figure 8). Consequently, the triphalangeal thumb metacarpal with a distal epiphysis is true pentadactyly, whereas that with a proximal epiphysis is hyperphalangism (Figure 9). Treatment of these complex polydactylies involves the same underlying principles as for preaxial and postaxial polydactyly, albeit with additional proximal upper extremity considerations.
When to Operate (Timing)
Ezaki6 recommended surgical intervention at age 6 to 9 months, before fine motor skills have developed with the abnormal anatomy. Cortical learning occurs as the child begins prehensile activities before 6 months, but the risks of anesthesia outweigh functional benefits until the child is older. Waiting until 1 year of age is not uncommon, though surgery at an earlier age may be beneficial if the polydactyly affects hand function.32 It is not uncommon to wait with the more balanced thumb polydactylies to assess thumb function. Hypoplasia might also delay surgical intervention until there is enough tissue inventory for reconstruction. Wassel20 noted that surgical intervention ideally occurs before the supernumerary elements displace the normal elements, as tends to happen with growth. Suture ligation is an option in the neonatal unit for some pedunculated digits.33 Studies have shown satisfactory results in adults treated for polydactyly, if the patient presents later than expected.34
Surgical Considerations
Knavel recommended simple excision, stating that “ablation requires no ingenuity and creates no problems.”5 This belief, though true for some duplications, will not lead to the best outcome for more complex polydactylies. The goal of surgery is a stable and well-aligned thumb for pinch and prehensile activity, as well as a cosmetically pleasing hand. Incisions should not be made linearly along the axis of the digit, as the scar will cause deviation with growth.24
Wassel type I polydactyly might appear incidentally as a broad thumb, in which case it requires no intervention (Figure 10). However, in Wassel types I and II polydactyly with deformity, the Bilhaut-Cloquet procedure is useful for both bifid and duplicated phalanges (Figure 11).5,6,30,32,35 Collateral ligaments may need to be released in type II because of difficulty in opposing the tissue. Cosmetic results with Bilhaut-Cloquet are unpredictable. The original technique required symmetrically sized digits; results today have been improved with microtechniques and preservation of an entire nail.36 Another option is ablation of the more hypoplastic osseous element and soft-tissue augmentation of the residual digit. The theme of ablation and augmentation is seen throughout the literature for the surgical treatment of polydactyly (Figure 12).1
For type III polydactyly, the bifid proximal phalanx is narrowed by resection and realigned with osteotomy of the remaining diaphysis. Type IV polydactyly, the most common thumb duplication, often requires advancement of the abductor pollicis brevis to the base of the proximal phalanx to aid in metacarpophalangeal (MCP) stabilization, abduction, and opposition. The metacarpal head, if broad and with 2 facets, can be shaped to form a single articulating surface. The metacarpal, occasionally with the proximal phalanx, often requires realignment by closing wedge osteotomy. Last, tendons on the resected bony elements should be rebalanced on the remaining digit, and anomalous slips must be released. For instance, given a radial insertion of the long flexor tendon on the distal phalanx, the tendon should be moved centrally. A strong flexor or extensor tendon on the amputated digit should be transferred to the remaining digit.24
Types V and VI are treated similarly to type IV, with the addition of a first web space Z-plasty or web widening if there is thenar eminence contracture. Acral transposition has also been described, with transposition of the tip of the ablated digit in place of the tip of the kept digit; this technique is ideal if one digit has a more normal proximal part while the other has a more normal distal part (Figure 13).35
Type VII thumb polydactyly, the type most likely inherited and associated with other disorders, should be treated like type VI. The nail should be preserved; amputation of the distal phalanx is not advised. Resection of the delta phalanx or 1 interphalangeal (IP) joint is an option. Articular surfaces will remodel if done before the age of 1 year. If the thenar eminence is hypoplastic, then Huber transfer of the abductor digiti minimi should be considered.37 Resection of the triphalangeal thumb is also advised, even if the biphalangeal thumb is more hypoplastic, with transfer of the ligaments and tendons, as described earlier.5,6,24,30,32,35
Thumb triphalangism, if isolated, and hyperphalangism in the other digits, can be treated with resection of the delta phalanx or one of the IP joints if it is affecting function or cosmesis.1,6 Wood and Flatt23 recommended early resection of a thumb delta phalanx because of the likelihood of deviation that impedes thumb function. For children, they recommended delta phalanx resection and Kirschner wire fixation for 6 weeks; for adults, they recommended resection or fusion of the joint, with osteotomy as needed for deviation.23,24 For thumb triphalangism, multiple surgeries are the norm, as Wood24 reported in his study of 21 patients who underwent 78 operations in total.
Index polydactyly may present as a simple pedunculated skin tag, which can be simply excised, or as a more complex musculoskeletal duplication. More complex presentations can be treated with procedures similar to those used for the thumb. Typically, the additional digit is radially deviated and angulated, eventually leading to impingement of thumb pinch and the first web space. Ray amputation is also an option if no reconstructive surgery will produce the stable, sensate radial pinch that is essential to hand function.32
Ring-finger polydactyly and long-finger polydactyly are often complicated by some element of syndactyly, resulting in a relative paucity of skin (Figure 14). There is failure of both formation (hypoplasia) and differentiation (syndactyly). The hypoplasia particularly affects the function of these digits by tethering them; multiple surgeries to restore proper hand function are the norm.1 Reconstructive surgery for these digits requires preoperative tissue inventory followed by resection and augmentation; as in syndactyly, skin for coverage is at a premium. Creation of a 3-fingered hand is an option.23
Temtamy and McKusick10 type A little-finger polydactyly is treated similarly to the thumb, with the caveat that hypothenar and intrinsic muscles that insert on the resected little finger are transferred to the remaining digit. In contrast to thumb polydactyly, the extrinsic musculature tends to be in good position. Suture ligation of type B polydactyly, as described by Flatt, is likely more common than orthopedists appreciate, as pediatricians and neonatal unit practitioners commonly perform this procedure in the nursery.1-3 It has been described with 2-0 Vicryl3 (Ethicon, Somerville, New Jersey) and 4-0 silk sutures,32 with the goal of necrosis and autoamputation. Parents should be told the finger generally falls off about 10 days (range, 4-21 days) after ligation.3 Multiple authors have cited a report of exsanguination from suture ligation, but we could not locate the primary source. It is advisable to wait until a patient is 6 months of age if planning to resect the nubbin in the operating room, given the anesthesia risk and the lack of functional impairment. Katz and Linder33 indicated they remove type B polydactyly in the nursery suite used for circumcisions; they use anesthetizing cream on the skin, and sharp excision with a scalpel, followed by direct pressure and Steri-Strip (3M, St. Paul, Minnesota) application. Suture ligation is recommended only if there is a narrow, thin (<2 mm) soft-tissue stalk; any broad or bony stalk necessitates surgical removal to avoid neuroma formation and failure of autonecrosis (Figure 15).27 Other options are a single swipe of a scalpel and elliptical excision; sharp transaction of the digital nerve with subsequent retraction is advised to avoid neuroma formation.2
Barton described ulnar dimelia operations as “spare parts surgery.”1 Extra digits are ablated and a thumb created (Figure 16). The hand might have a digit in relatively good rotational position for thumbplasty, or the principles of pollicization may need to be used. If the patient is already using the hand, the surgeon should note which finger the patient uses as a thumb.24 Any accompanying wrist flexion contracture must be corrected with careful attention to musculotendinous balancing. Because the forearm and elbow, and occasionally even the more proximal limb, will be abnormal in this disorder, multiple surgeries are again the norm.1
Pentadactyly is treated like thumb hypoplasia, with first web space creation.1
Complications
In polydactyly, a reoperation rate of up to 25% has been reported, with most reoperations performed because of residual or subsequent deformity.5,30,31,38 Risk factors for reoperation are type IV thumb duplication, preoperative “zigzag” deformity, and radially deviated thumb elements at presentation.5 The delta phalanx may not show on radiographs until the patient is 18 months old, but functional deformity will worsen as long as it is present. Zigzag deformity may be due to the delta phalanx or to musculotendinous imbalance, such as a radially inserted flexor pollicis longus (FPL) or lack of stable MCP abduction. Miura31 found that careful reconstruction of the joint capsule and thenar muscles from the ablated digit to the remnant digit is the key to a successful initial surgery. Lee and colleagues39 defined zigzag deformity as more than 20° MCP and IP angulation; for cases present before surgery, they recommended FPL relocation by the pullout technique in addition to osteotomies to prevent further interphalangeal deviation (Figures 17, 18).
Abnormal physeal growth, joint instability, and stiffness can all occur. Stiffness is particularly difficult to treat but seldom presents a functional problem. Joint enlargement, which is not uncommon, results from either broad articular surfaces or retained cartilage from the perichondral ring after resection that later ossifies.5,38 Nubbin-type duplications may not fall off after suture ligation, necessitating further excision, and a cosmetic bump is seen after 40% of suture ligations.3 Patillo and Rayan28 and Rayan and Frey29 warned against suture ligation unless the nubbin has a small stalk because of the possibility of infection and gangrene. The excised nubbin tissue is histologically nervous, and there have been reports of painful neuromas in the remaining scar of a ligated nubbin that respond well to excision.26,27,40 It is thought that these painful lesions form because the ligature prevents the digital nerves to the vestigial digit from retracting.27 Nail deformity and IP joint stiffness are seen with the Bilhaut-Cloquet procedure, though often finger function remains satisfactory.
Conclusion
Polydactyly is a common congenital hand abnormality. Its true incidence is unknown because of inconsistent documentation. Surgeons must strive for a functional, cosmetic hand, given a diverse set of possible anomalies. Hypoplasia is the rule; tissue should be ablated and augmented as necessary. Musculotendinous insertions may need to be centralized. Patients’ family members should always be counseled that more surgery may be needed in the future, as further deformity can occur with growth. Surgically corrected thumb duplications will be stiffer, shorter, and thinner than their normal counterparts. Nail ridges are common. However, it should be noted that 88% of these patients are satisfied with their results.41 Some amount of contracture and abnormal function should be expected with index-, long-, and ring-finger duplications. The only remnant of type B postaxial duplications may be a slight discoloration or bump, though stiffness and deformity can happen with a type A deformity. A “duplicated” digit that requires surgical correction will never be completely normal, but acceptable function is routinely achievable.
Polydactyly is the presence of extra digits. Its incidence is likely underestimated because many practitioners treat simple “nubbins” without referring them to orthopedic specialists.1-3 Polydactyly can be detected by ultrasound as early as 14 weeks’ gestational age, with partial autoamputation seen in most isolated polydactylies.4 The thumb, responsible for 40% of hand function, must be able to oppose the other digits with a stable pinch.5 Polydactyly encumbers this motion when the duplicated digits deviate from normal alignment. Ezaki6 noted that the anatomy is better described as “split” than “duplicated.” There are many dichotomous ways to classify polydactyly: preaxial (radial) versus postaxial (ulnar), thumb versus triphalangeal, simple versus complex (Figure 1). Mixed polydactyly is defined as the presence of preaxial and postaxial polydactyly.7 Surgical management seeks to allow normal hand function and to restore cosmesis.
Epidemiology
Sun and colleagues8 reported the overall polydactyly incidence as 2 per 1000 live births in China from 1998 to 2009, with a slight male predominance; polydactyly was also 3 times more common than syndactyly in this population. Ivy,9 in a 5-year audit of Pennsylvania Department of Health records, found polydactyly to be the fourth most common congenital anomaly after clubfoot, cleft lip/palate, and spina bifida. Thumb duplication occurs in 0.08 to 1.4 per 1000 live births and is more common in American Indians and Asians than in other races.5,10 It occurs in a male-to-female ratio of 2.5 to 1 and is most often unilateral.5 Postaxial polydactyly is predominant in black infants; it is most often inherited in an autosomal dominant fashion, if isolated, or in an autosomal recessive pattern, if syndromic.1 A prospective San Diego study of 11,161 newborns found postaxial type B polydactyly in 1 per 531 live births (1 per 143 black infants, 1 per 1339 white infants); 76% of cases were bilateral, and 86% had a positive family history.3 In patients of non-African descent, it is associated with anomalies in other organs. Central duplication is rare and often autosomal dominant.5,10
Genetics and Development
As early as 1896, the heritability of polydactyly was noted.11 As of 2010, polydactyly has been associated with 310 diseases.12 Ninety-nine genes, most involved in regulation of anterior-posterior formation of the limb bud, have been implicated.12,13
The upper limb begins to form at day 26 in utero.14 Apoptosis in the interdigital necrotic zones results in the formation of individual digits. It is presumed that, in polydactyly, the involved tissue is hypoplastic because of an abnormal interaction between mesoderm and ectoderm.5 Presence of an apical ectodermal ridge determines the formation of a limb bud, and on it the zone of polarizing activity (ZPA) dictates preaxial and postaxial alignment.14,15 The ZPA is located on the posterior zone of the developing limb bud. The levels of GLI3, a zinc finger-containing DNA-binding protein, are highest in the anterior area, and HAND2, a basic helix-loop-helix DNA-binding protein, is found in the ZPA. This polarity promotes sonic hedgehog (Shh) gene expression in the posterior region, which in turn prevents GLI3 cleavage into its repressed form. GLI3R (repressed) and GLI3A (active) concentrations are highest, therefore, in the anterior and posterior portions of the bud, respectively. The GLI3A:GLI3R ratio is responsible for the identity and number of digits in the hand (ie, the thumb develops in regions of high GLI3R). GLI and Shh mutations lead to polydactylous hands with absent thumbs (Figure 2).16
Ciliopathies have also been shown to cause postaxial polydactyly, possibly because of the role that nonmotile cilia play in hedgehog signaling.17 Mutations in Shh genomic regulators cause preaxial polydactyly.18 HoxD activates Shh in the ZPA; HoxD13 mutations are associated with synpolydactyly.16,19 In each of these mutations, Shh production is altered, and some form of polydactyly results.
Associations
Many syndromes have been associated with polydactyly. Not all polydactyly is associated with other disorders, but the more complex the polydactyly, the more likely that other anomalies are present. Every patient who presents with polydactyly should have a full history taken and a physical examination performed (Figure 3). Any patient with syndromic findings or atypical presentations (eg, triphalangism, postaxial polydactyly in a patient of non-African descent, central and index polydactyly) should be referred to a geneticist.
Classifications
The Wassel20 classification describes the anatomical presentation of thumb duplication on the basis of 70 cases in Iowa (Figures 4, 5; Table 1). Because some duplications fall outside the Wassel classification, many researchers have proposed modifications (Figure 6).21-25
The Temtamy and McKusick10 classification, which is the product of geneticists, classifies duplications by grouping genetically related presentations (Table 2). It provides the most commonly used postaxial classification, with type A being a fully developed digit and type B a rudimentary and pedunculated digit, informally referred to as a nubbin. Type B is more common than type A. Given inheritance patterns, it is assumed that type A is likely multifactorial and type B autosomal dominant.10 Thumb polydactyly inheritance is still unclear. The other types of preaxial polydactyly and high degrees of polydactyly are rare but seem to be passed on in an autosomal dominant fashion on pedigree analysis.10
The Stelling and Turek classification presents the duplication from a tissue perspective: Type I duplication is a rudimentary mass devoid of other tissue elements; type II is a subtotal duplication with some normal structures; and type III is a duplication of the entire “osteoarticular column,” including the metacarpal.1 It is interesting to note that histology of type I duplications shows neuroma-like tissue.26-28 Again, normal is a relative term because, in polydactyly, the duplications are hypoplastic and deviated, with anomalous anatomy.
The Rayan classification describes ulnar polydactyly and was derived from a case study series of 148 patients in Oklahoma (Table 3).29
There are also some complex polydactylies that are not easily classified: ulnar dimelia, cleft hand, pentadactyly, and hyperphalangism. Ulnar dimelia, also known as “mirror hand,” is typically 7 digits with no thumb, but other variations are seen. The radius is often absent, and the elbow is abnormal. There is some debate about whether it is a fusion of 2 hands. Pentadactyly, or the 5-fingered hand, appears as 5 triphalangeal digits with no thumb (Figure 7).
Isolated thumb triphalangism might appear similar to pentadactyly. Miura30,31 pointed out that the radial digit in the pentadactylous hand may be opposable (thumb-like) or nonopposable; in his studies, the patients with the opposable thumb had a metacarpal with a proximal epiphysis (Figure 8). Consequently, the triphalangeal thumb metacarpal with a distal epiphysis is true pentadactyly, whereas that with a proximal epiphysis is hyperphalangism (Figure 9). Treatment of these complex polydactylies involves the same underlying principles as for preaxial and postaxial polydactyly, albeit with additional proximal upper extremity considerations.
When to Operate (Timing)
Ezaki6 recommended surgical intervention at age 6 to 9 months, before fine motor skills have developed with the abnormal anatomy. Cortical learning occurs as the child begins prehensile activities before 6 months, but the risks of anesthesia outweigh functional benefits until the child is older. Waiting until 1 year of age is not uncommon, though surgery at an earlier age may be beneficial if the polydactyly affects hand function.32 It is not uncommon to wait with the more balanced thumb polydactylies to assess thumb function. Hypoplasia might also delay surgical intervention until there is enough tissue inventory for reconstruction. Wassel20 noted that surgical intervention ideally occurs before the supernumerary elements displace the normal elements, as tends to happen with growth. Suture ligation is an option in the neonatal unit for some pedunculated digits.33 Studies have shown satisfactory results in adults treated for polydactyly, if the patient presents later than expected.34
Surgical Considerations
Knavel recommended simple excision, stating that “ablation requires no ingenuity and creates no problems.”5 This belief, though true for some duplications, will not lead to the best outcome for more complex polydactylies. The goal of surgery is a stable and well-aligned thumb for pinch and prehensile activity, as well as a cosmetically pleasing hand. Incisions should not be made linearly along the axis of the digit, as the scar will cause deviation with growth.24
Wassel type I polydactyly might appear incidentally as a broad thumb, in which case it requires no intervention (Figure 10). However, in Wassel types I and II polydactyly with deformity, the Bilhaut-Cloquet procedure is useful for both bifid and duplicated phalanges (Figure 11).5,6,30,32,35 Collateral ligaments may need to be released in type II because of difficulty in opposing the tissue. Cosmetic results with Bilhaut-Cloquet are unpredictable. The original technique required symmetrically sized digits; results today have been improved with microtechniques and preservation of an entire nail.36 Another option is ablation of the more hypoplastic osseous element and soft-tissue augmentation of the residual digit. The theme of ablation and augmentation is seen throughout the literature for the surgical treatment of polydactyly (Figure 12).1
For type III polydactyly, the bifid proximal phalanx is narrowed by resection and realigned with osteotomy of the remaining diaphysis. Type IV polydactyly, the most common thumb duplication, often requires advancement of the abductor pollicis brevis to the base of the proximal phalanx to aid in metacarpophalangeal (MCP) stabilization, abduction, and opposition. The metacarpal head, if broad and with 2 facets, can be shaped to form a single articulating surface. The metacarpal, occasionally with the proximal phalanx, often requires realignment by closing wedge osteotomy. Last, tendons on the resected bony elements should be rebalanced on the remaining digit, and anomalous slips must be released. For instance, given a radial insertion of the long flexor tendon on the distal phalanx, the tendon should be moved centrally. A strong flexor or extensor tendon on the amputated digit should be transferred to the remaining digit.24
Types V and VI are treated similarly to type IV, with the addition of a first web space Z-plasty or web widening if there is thenar eminence contracture. Acral transposition has also been described, with transposition of the tip of the ablated digit in place of the tip of the kept digit; this technique is ideal if one digit has a more normal proximal part while the other has a more normal distal part (Figure 13).35
Type VII thumb polydactyly, the type most likely inherited and associated with other disorders, should be treated like type VI. The nail should be preserved; amputation of the distal phalanx is not advised. Resection of the delta phalanx or 1 interphalangeal (IP) joint is an option. Articular surfaces will remodel if done before the age of 1 year. If the thenar eminence is hypoplastic, then Huber transfer of the abductor digiti minimi should be considered.37 Resection of the triphalangeal thumb is also advised, even if the biphalangeal thumb is more hypoplastic, with transfer of the ligaments and tendons, as described earlier.5,6,24,30,32,35
Thumb triphalangism, if isolated, and hyperphalangism in the other digits, can be treated with resection of the delta phalanx or one of the IP joints if it is affecting function or cosmesis.1,6 Wood and Flatt23 recommended early resection of a thumb delta phalanx because of the likelihood of deviation that impedes thumb function. For children, they recommended delta phalanx resection and Kirschner wire fixation for 6 weeks; for adults, they recommended resection or fusion of the joint, with osteotomy as needed for deviation.23,24 For thumb triphalangism, multiple surgeries are the norm, as Wood24 reported in his study of 21 patients who underwent 78 operations in total.
Index polydactyly may present as a simple pedunculated skin tag, which can be simply excised, or as a more complex musculoskeletal duplication. More complex presentations can be treated with procedures similar to those used for the thumb. Typically, the additional digit is radially deviated and angulated, eventually leading to impingement of thumb pinch and the first web space. Ray amputation is also an option if no reconstructive surgery will produce the stable, sensate radial pinch that is essential to hand function.32
Ring-finger polydactyly and long-finger polydactyly are often complicated by some element of syndactyly, resulting in a relative paucity of skin (Figure 14). There is failure of both formation (hypoplasia) and differentiation (syndactyly). The hypoplasia particularly affects the function of these digits by tethering them; multiple surgeries to restore proper hand function are the norm.1 Reconstructive surgery for these digits requires preoperative tissue inventory followed by resection and augmentation; as in syndactyly, skin for coverage is at a premium. Creation of a 3-fingered hand is an option.23
Temtamy and McKusick10 type A little-finger polydactyly is treated similarly to the thumb, with the caveat that hypothenar and intrinsic muscles that insert on the resected little finger are transferred to the remaining digit. In contrast to thumb polydactyly, the extrinsic musculature tends to be in good position. Suture ligation of type B polydactyly, as described by Flatt, is likely more common than orthopedists appreciate, as pediatricians and neonatal unit practitioners commonly perform this procedure in the nursery.1-3 It has been described with 2-0 Vicryl3 (Ethicon, Somerville, New Jersey) and 4-0 silk sutures,32 with the goal of necrosis and autoamputation. Parents should be told the finger generally falls off about 10 days (range, 4-21 days) after ligation.3 Multiple authors have cited a report of exsanguination from suture ligation, but we could not locate the primary source. It is advisable to wait until a patient is 6 months of age if planning to resect the nubbin in the operating room, given the anesthesia risk and the lack of functional impairment. Katz and Linder33 indicated they remove type B polydactyly in the nursery suite used for circumcisions; they use anesthetizing cream on the skin, and sharp excision with a scalpel, followed by direct pressure and Steri-Strip (3M, St. Paul, Minnesota) application. Suture ligation is recommended only if there is a narrow, thin (<2 mm) soft-tissue stalk; any broad or bony stalk necessitates surgical removal to avoid neuroma formation and failure of autonecrosis (Figure 15).27 Other options are a single swipe of a scalpel and elliptical excision; sharp transaction of the digital nerve with subsequent retraction is advised to avoid neuroma formation.2
Barton described ulnar dimelia operations as “spare parts surgery.”1 Extra digits are ablated and a thumb created (Figure 16). The hand might have a digit in relatively good rotational position for thumbplasty, or the principles of pollicization may need to be used. If the patient is already using the hand, the surgeon should note which finger the patient uses as a thumb.24 Any accompanying wrist flexion contracture must be corrected with careful attention to musculotendinous balancing. Because the forearm and elbow, and occasionally even the more proximal limb, will be abnormal in this disorder, multiple surgeries are again the norm.1
Pentadactyly is treated like thumb hypoplasia, with first web space creation.1
Complications
In polydactyly, a reoperation rate of up to 25% has been reported, with most reoperations performed because of residual or subsequent deformity.5,30,31,38 Risk factors for reoperation are type IV thumb duplication, preoperative “zigzag” deformity, and radially deviated thumb elements at presentation.5 The delta phalanx may not show on radiographs until the patient is 18 months old, but functional deformity will worsen as long as it is present. Zigzag deformity may be due to the delta phalanx or to musculotendinous imbalance, such as a radially inserted flexor pollicis longus (FPL) or lack of stable MCP abduction. Miura31 found that careful reconstruction of the joint capsule and thenar muscles from the ablated digit to the remnant digit is the key to a successful initial surgery. Lee and colleagues39 defined zigzag deformity as more than 20° MCP and IP angulation; for cases present before surgery, they recommended FPL relocation by the pullout technique in addition to osteotomies to prevent further interphalangeal deviation (Figures 17, 18).
Abnormal physeal growth, joint instability, and stiffness can all occur. Stiffness is particularly difficult to treat but seldom presents a functional problem. Joint enlargement, which is not uncommon, results from either broad articular surfaces or retained cartilage from the perichondral ring after resection that later ossifies.5,38 Nubbin-type duplications may not fall off after suture ligation, necessitating further excision, and a cosmetic bump is seen after 40% of suture ligations.3 Patillo and Rayan28 and Rayan and Frey29 warned against suture ligation unless the nubbin has a small stalk because of the possibility of infection and gangrene. The excised nubbin tissue is histologically nervous, and there have been reports of painful neuromas in the remaining scar of a ligated nubbin that respond well to excision.26,27,40 It is thought that these painful lesions form because the ligature prevents the digital nerves to the vestigial digit from retracting.27 Nail deformity and IP joint stiffness are seen with the Bilhaut-Cloquet procedure, though often finger function remains satisfactory.
Conclusion
Polydactyly is a common congenital hand abnormality. Its true incidence is unknown because of inconsistent documentation. Surgeons must strive for a functional, cosmetic hand, given a diverse set of possible anomalies. Hypoplasia is the rule; tissue should be ablated and augmented as necessary. Musculotendinous insertions may need to be centralized. Patients’ family members should always be counseled that more surgery may be needed in the future, as further deformity can occur with growth. Surgically corrected thumb duplications will be stiffer, shorter, and thinner than their normal counterparts. Nail ridges are common. However, it should be noted that 88% of these patients are satisfied with their results.41 Some amount of contracture and abnormal function should be expected with index-, long-, and ring-finger duplications. The only remnant of type B postaxial duplications may be a slight discoloration or bump, though stiffness and deformity can happen with a type A deformity. A “duplicated” digit that requires surgical correction will never be completely normal, but acceptable function is routinely achievable.
1. Graham TJ, Ress AM. Finger polydactyly. Hand Clin. 1998;14(1):49-64.
2. Abzug JM, Kozin SH. Treatment of postaxial polydactyly type B. J Hand Surg Am. 2013;38(6):1223-1225.
3. Watson BT, Hennrikus WL. Postaxial type-B polydactyly—prevalence and treatment. J Bone Joint Surg Am. 1997;79(1):65-68.
4. Zimmer EZ, Bronshtein M. Fetal polydactyly diagnosis during early pregnancy: clinical applications. Am J Obstet Gynecol. 2000;183(3):755-758.
5. Cohen MS. Thumb duplication. Hand Clin. 1998;14(1):17-27.
6. Ezaki M. Radial polydactyly. Hand Clin. 1990;6(4):577-588.
7. Nathan PA, Keniston RC. Crossed polydactyly: case report and review of the literature. J Bone Joint Surg Am. 1975;57(6):847-849.
8. Sun G, Xu ZM, Liang JF, Li L, Tang DX. Twelve-year prevalence of common neonatal congenital malformations in Zhejiang Province, China. World J Pediatr. 2011;7(4):331-336.
9. Ivy RH. Congenital anomalies as recorded on birth certificates in the Division of Vital Statistics of the Pennsylvania Department of Health, for the period of 1951–1955, inclusive. Plast Reconstr Surg. 1957;20(5):400-411.
10. Temtamy SA, McKusick VA. Polydactyly as a part of syndromes. In: Bergsma D, ed. Mudge JR, Paul NW, Conde Greene S, associate eds. The Genetics of Hand Malformations. New York, NY: Liss. Birth Defects Original Article Series. 1978;14(3):364-439.
11. Gould W, Pyle L. Anomalies and Curiosities of Medicine. New York, NY: Bell; 1896.
12. Biesecker LG. Polydactyly: how many disorders and how many genes: 2010 update. Dev Dyn. 2011;250(5):931-942.
13. Grzeschik K. Human limb malformations; an approach to the molecular basis of development. Int J Dev Biol. 2001;46(7):983-991.
14. Zaleske DJ. Development of the upper limb. Hand Clin. 1985;1(3):383-390.
15. Beatty E. Upper limb tissue differentiation in the human embryo. Hand Clin. 1985;1(3):391-404.
16. Anderson E, Peluso S, Lettice LA, Hill RE. Human limb abnormalities caused by disruption of hedgehog signaling. Trends Genet. 2012;28(8):364-373.
17. Ware SM, Aygun MG, Heldebrandt F. Spectrum of clinical diseases caused by disorders of primary cilia. Proc Am Thorac Soc. 2011;8(5):444-450.
18. Lettice LA, Hill RE. Preaxial polydactyly: a model for defective long-range regulation in congenital abnormalities. Curr Opin Genet Dev. 2005;15(3):294-300.
19. Al-Qattan MA. Type II familial synpolydactyly: report on two families with an emphasis on variations of expression. Eur J Hum Genet. 2011;19(1):112-114.
20. Wassel HD. The results of surgery for polydactyly of the thumb. Clin Orthop. 1969;(64):175-193.
21. Blauth W, Olason AT. Classification of polydactyly of the hands and feet. Arch Orthop Trauma Surg. 1988;107(6):334-344.
22. Wood VE. Super digit. Hand Clin. 1990;6(4):673-684.
23. Wood VE, Flatt AE. Congenital triangular bones in the hand. J Hand Surg Am. 1977;2(3):179-193.
24. Wood VE. Polydactyly and the triphalangeal thumb. J Hand Surg Am. 1978;3(5):436-444.
25. Zuidam JM, Selles RW, Ananta M, Runia J, Hovius SER. A classification system of radial polydactyly: inclusion of triphalangeal thumb and triplication. J Hand Surg Am. 2008;33(3):373-377.
26. Leber GE, Gosain AK. Surgical excision of pedunculated supernumerary digits prevents traumatic amputation neuromas. Pediatr Dermatol. 2003;20(2):108-112.
27. Mullick S, Borschel GH. A selective approach to treatment of ulnar polydactyly: preventing painful neuroma and incomplete excision. Pediatr Dermatol. 2001;27(1):39-42.
28. Patillo D, Rayan GM. Complications of suture ligation ablation for ulnar polydactyly: a report of two cases. Hand (N Y). 2011;6(1):102-105.
29. Rayan GM, Frey B. Ulnar polydactyly. Plastic Reconstr Surg. 2001;107(6):1449-1454.
30. Miura T. Triphalangeal thumb. Plastic Reconstr Surg. 1976;58(5):587-594.
31. Miura T. Duplicated thumb. Plastic Reconstr Surg. 1982;69(3):470-481.
32. Simmons BP. Polydactyly. Hand Clin. 1985;1(3):545-566.
33. Katz K, Linder N. Postaxial type B polydactyly treated by excision in the neonatal nursery. J Pediatr Orthop. 2011;31(4):448-449.
34. Manohar A, Beard AJ. Outcome of reconstruction for duplication of the thumb in adults aged over 40. Hand Surg. 2011;16(2):207-210.
35. Watt AJ, Chung KC. Duplication. Hand Clin. 2009;25(2):215-228.
36. Tonkin MA. Thumb duplication: concepts and techniques. Clin Orthop Surg. 2012;4(1):1-17.
37. Huber E. Relief operation in the case of paralysis of the median nerve. J Hand Surg Eur. 2004;29(1):35-37.
38. Mih AD. Complications of duplicate thumb reconstruction. Hand Clin. 1998;14(1):143-149.
39. Lee CC, Park HY, Yoon JO, Lee KW. Correction of Wassel type IV thumb duplication with zigzag deformity: results of a new method of flexor pollicis longus tendon relocation. J Hand Surg Eur. 2013;38(3):272-280.
40. Hare PJ. Rudimentary polydactyly. Br J Dermatol. 1954;66(11):402-408.
41. Yen CH, Chan WL, Leung HB, Mak KH. Thumb polydactyly: clinical outcome after reconstruction. J Orthop Surg (Hong Kong). 2006;14(3):295-302.
42. Edmunds JO. A tribute to Daniel C. Riordan, MD (1917–2012). Tulane University School of Medicine, Department of Orthopaedics website. http://tulane.edu/som/departments/orthopaedics/news-and-events/danriordantribute.cfm. Accessed March 31, 2015.
43. Faust DC, Herms R. Daniel C. Riordan, MD, 1917–2012. J Hand Surg Am. 2013;38(1):202-205.
1. Graham TJ, Ress AM. Finger polydactyly. Hand Clin. 1998;14(1):49-64.
2. Abzug JM, Kozin SH. Treatment of postaxial polydactyly type B. J Hand Surg Am. 2013;38(6):1223-1225.
3. Watson BT, Hennrikus WL. Postaxial type-B polydactyly—prevalence and treatment. J Bone Joint Surg Am. 1997;79(1):65-68.
4. Zimmer EZ, Bronshtein M. Fetal polydactyly diagnosis during early pregnancy: clinical applications. Am J Obstet Gynecol. 2000;183(3):755-758.
5. Cohen MS. Thumb duplication. Hand Clin. 1998;14(1):17-27.
6. Ezaki M. Radial polydactyly. Hand Clin. 1990;6(4):577-588.
7. Nathan PA, Keniston RC. Crossed polydactyly: case report and review of the literature. J Bone Joint Surg Am. 1975;57(6):847-849.
8. Sun G, Xu ZM, Liang JF, Li L, Tang DX. Twelve-year prevalence of common neonatal congenital malformations in Zhejiang Province, China. World J Pediatr. 2011;7(4):331-336.
9. Ivy RH. Congenital anomalies as recorded on birth certificates in the Division of Vital Statistics of the Pennsylvania Department of Health, for the period of 1951–1955, inclusive. Plast Reconstr Surg. 1957;20(5):400-411.
10. Temtamy SA, McKusick VA. Polydactyly as a part of syndromes. In: Bergsma D, ed. Mudge JR, Paul NW, Conde Greene S, associate eds. The Genetics of Hand Malformations. New York, NY: Liss. Birth Defects Original Article Series. 1978;14(3):364-439.
11. Gould W, Pyle L. Anomalies and Curiosities of Medicine. New York, NY: Bell; 1896.
12. Biesecker LG. Polydactyly: how many disorders and how many genes: 2010 update. Dev Dyn. 2011;250(5):931-942.
13. Grzeschik K. Human limb malformations; an approach to the molecular basis of development. Int J Dev Biol. 2001;46(7):983-991.
14. Zaleske DJ. Development of the upper limb. Hand Clin. 1985;1(3):383-390.
15. Beatty E. Upper limb tissue differentiation in the human embryo. Hand Clin. 1985;1(3):391-404.
16. Anderson E, Peluso S, Lettice LA, Hill RE. Human limb abnormalities caused by disruption of hedgehog signaling. Trends Genet. 2012;28(8):364-373.
17. Ware SM, Aygun MG, Heldebrandt F. Spectrum of clinical diseases caused by disorders of primary cilia. Proc Am Thorac Soc. 2011;8(5):444-450.
18. Lettice LA, Hill RE. Preaxial polydactyly: a model for defective long-range regulation in congenital abnormalities. Curr Opin Genet Dev. 2005;15(3):294-300.
19. Al-Qattan MA. Type II familial synpolydactyly: report on two families with an emphasis on variations of expression. Eur J Hum Genet. 2011;19(1):112-114.
20. Wassel HD. The results of surgery for polydactyly of the thumb. Clin Orthop. 1969;(64):175-193.
21. Blauth W, Olason AT. Classification of polydactyly of the hands and feet. Arch Orthop Trauma Surg. 1988;107(6):334-344.
22. Wood VE. Super digit. Hand Clin. 1990;6(4):673-684.
23. Wood VE, Flatt AE. Congenital triangular bones in the hand. J Hand Surg Am. 1977;2(3):179-193.
24. Wood VE. Polydactyly and the triphalangeal thumb. J Hand Surg Am. 1978;3(5):436-444.
25. Zuidam JM, Selles RW, Ananta M, Runia J, Hovius SER. A classification system of radial polydactyly: inclusion of triphalangeal thumb and triplication. J Hand Surg Am. 2008;33(3):373-377.
26. Leber GE, Gosain AK. Surgical excision of pedunculated supernumerary digits prevents traumatic amputation neuromas. Pediatr Dermatol. 2003;20(2):108-112.
27. Mullick S, Borschel GH. A selective approach to treatment of ulnar polydactyly: preventing painful neuroma and incomplete excision. Pediatr Dermatol. 2001;27(1):39-42.
28. Patillo D, Rayan GM. Complications of suture ligation ablation for ulnar polydactyly: a report of two cases. Hand (N Y). 2011;6(1):102-105.
29. Rayan GM, Frey B. Ulnar polydactyly. Plastic Reconstr Surg. 2001;107(6):1449-1454.
30. Miura T. Triphalangeal thumb. Plastic Reconstr Surg. 1976;58(5):587-594.
31. Miura T. Duplicated thumb. Plastic Reconstr Surg. 1982;69(3):470-481.
32. Simmons BP. Polydactyly. Hand Clin. 1985;1(3):545-566.
33. Katz K, Linder N. Postaxial type B polydactyly treated by excision in the neonatal nursery. J Pediatr Orthop. 2011;31(4):448-449.
34. Manohar A, Beard AJ. Outcome of reconstruction for duplication of the thumb in adults aged over 40. Hand Surg. 2011;16(2):207-210.
35. Watt AJ, Chung KC. Duplication. Hand Clin. 2009;25(2):215-228.
36. Tonkin MA. Thumb duplication: concepts and techniques. Clin Orthop Surg. 2012;4(1):1-17.
37. Huber E. Relief operation in the case of paralysis of the median nerve. J Hand Surg Eur. 2004;29(1):35-37.
38. Mih AD. Complications of duplicate thumb reconstruction. Hand Clin. 1998;14(1):143-149.
39. Lee CC, Park HY, Yoon JO, Lee KW. Correction of Wassel type IV thumb duplication with zigzag deformity: results of a new method of flexor pollicis longus tendon relocation. J Hand Surg Eur. 2013;38(3):272-280.
40. Hare PJ. Rudimentary polydactyly. Br J Dermatol. 1954;66(11):402-408.
41. Yen CH, Chan WL, Leung HB, Mak KH. Thumb polydactyly: clinical outcome after reconstruction. J Orthop Surg (Hong Kong). 2006;14(3):295-302.
42. Edmunds JO. A tribute to Daniel C. Riordan, MD (1917–2012). Tulane University School of Medicine, Department of Orthopaedics website. http://tulane.edu/som/departments/orthopaedics/news-and-events/danriordantribute.cfm. Accessed March 31, 2015.
43. Faust DC, Herms R. Daniel C. Riordan, MD, 1917–2012. J Hand Surg Am. 2013;38(1):202-205.
Nontraumatic Knee Pain: A Diagnostic & Treatment Guide
Jane, age 42, presents with right knee pain that she’s had for about six months. She denies any trauma. Jane describes the pain as “vague and poorly localized” but worse with activity. She says she started a walking/running program nine months ago, when she was told she was overweight (BMI, 29). She has lost 10 pounds since then and hopes to lose more by continuing to exercise. Further review reveals that Jane has experienced increasing pain while ascending and descending stairs and that the pain is also exacerbated when she stands after prolonged sitting.
If Jane were your patient, what would you include in a physical examination, and how would you diagnose and treat her?
Knee pain is a common presentation in primary care. While traumatic knee pain is frequently addressed in the medical literature, little has been written about chronic nontraumatic nonarthritic knee pain such as Jane’s. Thus, while physical exam tests often lead to the correct diagnosis for traumatic knee pain, there is limited information on the use of such tests to determine the etiology of chronic knee pain.
This review was developed to fill that gap. The pages that follow contain general guidance on the diagnosis and treatment of chronic nontraumatic knee pain. The conditions are presented anatomically—anterior, lateral, medial, or posterior—with common etiologies, history and physical exam findings, and diagnosis and treatment options for each (see Table, page 28).1-31
ANTERIOR KNEE PAIN
Patellofemoral pain syndrome (PFPS)
The most common cause of anterior knee pain, PFPS is a complex entity with an etiology that has not been well described.2 The quadriceps tendon, medial and lateral retinacula, iliotibial band (ITB), vastus medialis and lateralis, and the insertion of the patellar tendon on the anterior tibial tubercle all play a role in proper tracking of the patellofemoral joint; an imbalance in any of these forces leads to abnormal patellar tracking over the femoral condyles, and pain ensues. PFPS can also be secondary to joint overload, in which excessive physical activity (eg, running, lunges, or squats) overloads the patellofemoral joint and causes pain.
Risk factors for PFPS include strength imbalances in the quadriceps, hamstring, and hip muscle groups, and increased training, such as running longer distances.4,32 A recent review showed no relationship between an increased quadriceps (Q)-angle and PFPS, so that is no longer considered a major risk factor.5
Diagnosis. PFPS is a diagnosis of exclusion and is primarily based on history and physical exam. Anterior knee pain that is exacerbated when seated for long periods of time (the “theater sign”) or by descending stairs is a classic indication of PFPS.1 Patients may complain of knee stiffness or “giving out” secondary to sharp knee pain and a sensation of popping or crepitus in the joint. Swelling is not a common finding.2
A recent meta-analysis revealed limited evidence for the use of any specific physical exam tests to diagnose PFPS. But pain during squatting and pain with a patellar tilt test were most consistent with a diagnosis of PFPS. (The patellar tilt test involves lifting the lateral edge of the patella superiorly while the patient lies supine with knee extended; pain with < 20° of lift suggests a tight lateral retinaculum). Conversely, the absence of pain during squatting or the absence of lateral retinacular pain helps rule it out.2 A physical exam of the cruciate and collateral ligaments should be performed in a patient with a history of instability. Radiography is not needed for a diagnosis but may be considered if examination reveals an effusion, the patient is 50 or older, or no improvement occurs after eight to 12 weeks of treatment.33
Treatment. The most effective and strongly supported treatment for PFPS is a six-week physiotherapy program focusing on strengthening the quadriceps and hip muscles and stretching the quadriceps, ITB, hamstrings, and hip flexors.4,5 There is limited information about the use of NSAIDs, but they can be considered for short-term management.2
Patellar taping and bracing have shown some promise as adjunct therapies for PFPS, although the data for both are nonconclusive. There is a paucity of prospective randomized trials of patellar bracing, and a 2012 Cochrane review found limited evidence of its efficacy.34 But a 2014 meta-analysis revealed moderate evidence in support of patellar taping early on to help decrease pain,6 and a recent review suggests that it can be helpful in both the short and long term.7
Taping or bracing may be useful when combined with a tailored physical therapy program. Evidence for treatments such as biofeedback, chiropractic manipulation, and orthotics is limited, and they should be used only as adjunctive therapy.4
When you examine Jane, you find no swelling of the affected knee. You perform the tilt test, which elicits pain. Squatting causes some pain, as well. You diagnose PFPS and provide a referral for six weeks of physiotherapy.
Patellofemoral instability (PFI)
PFI occurs when the patella disengages completely from the trochlear groove.11 PFI’s etiology also relates to the complexity of the patellofemoral joint. Here, too, stability of the joint is achieved with a combination of soft-tissue and bony restraints. At full extension and early flexion of the knee, however, the mechanisms of stability are limited, resulting in increased instability. Other associated factors include Q-angle, lateral pull from a tight ITB, and opposing forces from the vastus lateralis and vastus medialis obliquus (VMO).8-10
Risk factors for PFI. The most common predisposing factors for PFI are trochlear dysplasia, patella alta, and lateralization of the tibial tuberosity or patella.10,11 Older patients, predominately women, have an increased risk for PFI.9 Patients usually have a history of patellar subluxation or dislocation in their youth, with approximately 17% of those who had a first dislocation experiencing a recurrence.9 A family history of PFI is common, as well.10
Diagnosis. Patients with PFI often present with nonspecific anterior knee pain secondary to recurrent dislocation.13 Notable exam findings include
• A positive J sign (noted if the patella suddenly shifts medially during early knee flexion or laterally during full extension)
• Decreased quadriceps (specifically VMO) and hamstring strength and flexibility
• Patellar hypermobility, which should be no more than a quarter to a half of the patellar diameter bilaterally
• Pain during a patellar tilt test
• A positive patellar apprehension test.10 (With the patient lying with the knee flexed to 20°, place thumbs on the medial patella and push laterally; the patient will straighten leg with pain or “apprehension” prior to patellar dislocation.)
Plain radiography should be ordered in all cases to assess for osseous trauma/deformity and to help guide surgical consideration. MRI can provide additional information when significant soft-tissue damage is suspected or the patient does not improve with conservative therapy.8,11
Treatment. A recent Cochrane review showed that conservative treatment (VMO strengthening, bracing, and proprioceptive therapy) prevented future dislocations more effectively than surgical intervention.11 However, surgery is indicated when obvious predisposing anatomic conditions (osteochondral fracture, intra-articular deformity, or a major tear of a medial soft-tissue stabilizer) are clearly shown on imaging.8,11
Patellar tendinopathy
An overuse injury often called “jumper’s knee” because it is associated with high-intensity jumping sports (eg, volleyball and basketball), patellar tendinopathy is an insertional tendinopathy with pain most commonly at the proximal patellar tendon.10 The pathology of the injury, though poorly understood, is believed to result from an impaired healing response to microtears.12,14
Diagnosis. Patients with patellar tendinopathy typically present with anterior suprapatellar pain aggravated by activity. Classically, the pain can occur in any of four phases12
1. Pain isolated after activity
2. Pain that occurs during activity but does not impede activity
3. Pain that occurs both during and after the activity and interferes with competition
4. A complete tendon disruption.
Examination should include an assessment of the patellar tendon for localized thickening, nodularity, crepitus, and focal suprapatellar tenderness. The muscle tendon function should be evaluated by assessing knee mobility and strength of the quads via straight-leg raise, decline squat, or single-leg squats.12 The Victorian Institute of Sport Assessment (VISA) questionnaire can be used to quantify the symptoms and to help track the patient’s progress throughout therapy.31 There are no proven special tests or radiologic studies to aid in the diagnosis of patellar tendinopathy,14 but MRI can be used for further evaluation when findings are equivocal.35
Treatment. A wide range of options, from eccentric training (eg, three sets of 15 repetitions performed twice a day for 12 weeks) and physical therapy to platelet-rich plasma (PRP) injections, sclerosing injections, and surgery, are available for the treatment of patellar tendinopathy.13-15 While no specific data have proven the superiority of any one therapy, expert consensus recommends eccentric exercise as initial therapy, performed for 12 weeks.14,15
It’s also interesting to note that a recently published study showed that three weekly PRP injections helped 75% of patients—all of whom failed to respond to four months of eccentric therapy—return to their presymptom activity level within 90 days.16 Corticosteroid injections should not be used to treat patellar tendinopathy due to the risk for tendon rupture.15 Orthopedic referral for surgical intervention should be considered for patients who fail to respond after three to six months of conservative therapy.14
Next: Lateral knee pain >>
LATERAL KNEE PAIN
Iliotibial band syndrome (ITBS)
A common source of lateral knee pain, ITBS is found particularly in runners, cyclists, and endurance athletes.17-19,36,37 The exact pathophysiology behind this diagnosis is debatable, but the most accepted etiology is inflammation generated from microtrauma to the soft tissues with inadequate healing time, resulting in persistent inflammation. ITBS is often associated with excessive overall running mileage, a sudden increase in mileage, or an abrupt change in training.18,37
Diagnosis. Patients often complain of persistent nontraumatic lateral knee pain that worsens with repetitive knee flexion (eg, running or cycling).17-19,37 A physical exam will often reveal pain over the lateral femoral condyle and a positive Noble’s test (see Figure 1, page 30). A positive Ober’s test (see Figure 2, page 32) is suggestive of ITBS, as well. The sensitivity and specificity of these tests are not well established, but in patients performing repetitive knee flexion activities with subjective lateral knee pain, pain over the lateral femoral condyle and a positive Ober’s and/or Noble’s test suggest an ITBS diagnosis.18 Imaging is not indicated initially, but MRI should be used in refractory cases to rule out other etiologies.17,19
Treatment. First-line therapy for ITBS is conservative,17-19,36,37 often involving a combination of techniques such as refraining from the activity that triggers the pain, NSAIDs, activity modification to reduce the strain over the ITB, myofascial release via foam rollers, and physical therapy focused on stretching the ITB, tensor fasciae latae, and gluteus medius while strengthening the gluteus medius and core muscles.17 No single program has been shown to be better than another.
Corticosteroid injections are second-line therapy and have been shown to improve pain compared with placebo up to two weeks postinjection.17,19 When symptoms persist for more than six months despite conservative treatment, surgical intervention may be indicated.18,19 Patients who experience temporary pain relief with corticosteroid injections often respond best to surgery.36
MEDIAL KNEE PAIN
Medial plica syndrome
Because of its anatomic location, the medial plica—which can be palpated in up to 84% of the population20—is susceptible to impingement by the medial femoral condyle or the patellofemoral joint. Trauma with repetitive knee movement leads to inflammation and thickening of the plica, resulting in medial plica syndrome.20,38 Initial inflammation may be triggered by blunt trauma, a sudden increase in activity, or transient synovitis.22
Diagnosis. Medial plica syndrome is a challenging diagnosis. Patients generally have nonspecific complaints of aching medial knee pain, locking, and catching similar to complaints of a medial meniscal injury.20
Evaluation should include the mediopatellar plica test, which is performed with the patient lying supine with the knee fully extended. Pressure is placed over the inferomedial patellofemoral joint, creating an impingement of the medial plica between the finger and the medial femoral condyle. Elimination or marked diminishing of pain with knee flexion to 90° is considered a positive test.21
A recent systematic review found this test to be more diagnostically accurate than an MRI (sensitivity of the test is 90% and specificity is 89%, vs 77% and 58%, respectively, for MRI) for detection of medial plica syndrome. Ultrasound is almost as accurate, with a sensitivity of 90% and specificity of 83%.39
Treatment of medial plica syndrome centers on physiotherapy and quadriceps strengthening,20 augmented with NSAIDs. Intra-articular corticosteroid injections are considered second-line treatment.20,22 An orthopedics referral is indicated to consider arthroscopic plica removal for refractory cases.20,22
Pes anserine bursitis
The anserine bursal complex, located approximately 5 cm distal to the medial joint line, is formed by the combined insertion of the sartorius, gracilis, and semitendinosus tendons,39 but the exact mechanism of pain is not well understood. Whether the pathophysiology is from an insertional tendonitis or overt bursitis is unknown, and no studies have focused on prevalence or risk factors. What is known is that overweight individuals and women with a wide pelvis seem to have a greater predilection and those with pes planus, diabetes, or knee osteoarthritis are at increased risk.23
Diagnosis. Medial knee pain reproduced on palpation of the anatomic site of insertion of the pes anserine tendon complex supports a diagnosis of pes anserine bursitis, with or without edema. Radiologic studies are not needed but may be helpful if significant bony pathology is suspected. Ultrasound, CT, and MRI are not recommended.23
Treatment. Resting the affected knee, cryotherapy, NSAIDs, and use of a pillow at night to relieve direct bursal pressure are recommended.33 Weight loss in obese patients, treatment of pes planus, and control of diabetes may be helpful, as well. Although the literature is limited and dated, corticosteroid injection has been found to reduce the pain and may be considered as second-line treatment.24-26
POSTERIOR KNEE PAIN
Popliteal (Baker’s) cyst
The popliteal fossa contains six of the numerous bursa of the knee; the bursa beneath the medial head of the gastrocnemius muscle and the semimembranosus tendon is most commonly involved in the formation of a popliteal cyst.40 It is postulated that increased intra-articular pressure forces fluid into the bursa, leading to expansion and pain. This can be idiopathic or secondary to internal derangement or trauma to the knee.41 Older age, a remote history of knee trauma, or a coexisting joint disease such as osteoarthritis, meniscal pathology, or rheumatoid arthritis are significant risk factors for popliteal cysts.27
Diagnosis. Most popliteal cysts are asymptomatic in adults and discovered incidentally after routine imaging to evaluate other knee pathology. However, symptomatic popliteal cysts present as a palpable mass in the popliteal fossa, resulting in pain and limited range of motion.
During the physical exam with the patient lying supine, a medial popliteal mass that is most prominent with the knee fully extended is common. A positive Foucher’s sign (the painful mass is palpated posteriorly in the popliteal fossa with the knee fully extended; pain is relieved and/or the mass reduced in size with knee flexion to 45°) suggests a diagnosis of popliteal cyst.27,28
Radiologic studies are generally not needed to diagnose a popliteal cyst. However, if diagnostic uncertainty remains after the history and physical exam, plain knee radiographs and ultrasound should be obtained. This combination provides complementary information and helps rule out a fracture, arthritis, and thrombosis as the cause of the pain.27 MRI is helpful if the diagnosis is still in doubt or if patients are suspected of having significant internal derangement leading to cyst formation. Arthrography or CT is generally not needed.27,41
Treatment. As popliteal cysts are often associated with other knee pathology, management of the underlying condition often leads to cyst regression. Keeping the knee in flexion can decrease the available space and assist in pain control in the acute phase.27 Cold packs and NSAIDs can also be used initially. Cyst aspiration and intra-articular corticosteroid injection have been shown to be effective for cysts that do not respond to this conservative approach.27 However, addressing and managing the underlying knee pathology (eg, osteoarthritis, meniscal pathology, or rheumatoid arthritis) will prevent popliteal cysts from recurring.
Continue for when the problem is painful knee effusion >>
WHEN THE PROBLEM IS PAINFUL KNEE EFFUSION
Nontraumatic knee effusion can be the primary source of knee pain or the result of underlying pathology. It is mentioned here because clinical suspicion is paramount to diagnosis of a septic joint—a serious cause of painful knee effusion that warrants prompt treatment.
As in other causes of knee pain, a detailed history of the character of the pain is essential. Septic arthritis and crystalline disease (gout, pseudogout) should be suspected in patients without a history of trauma who cannot bear weight. Systemic complaints point to an infection and, with the exception of a possible low-grade fever, are not typically seen in crystalline disease. Notable findings include an erythematous, hot, swollen knee and pain with both active and passive movement.
Plain radiographs of the knee should be ordered to rule out significant trauma or arthritis as the etiology. It is important to perform joint aspiration with synovial fluid analysis. Fluid analysis should include a white blood cell (WBC) count with differential, Gram stain and cultures, and polarized light microscopy (not readily available in an outpatient setting).29
Synovial fluid analysis characteristics suggestive of a septic joint include turbid quality, WBC > 50,000/mL, an elevated protein content, and a low glucose concentration.30 Gram stain and culture will help identify the infectious agent. Orthopedic referral should not be delayed in patients with a suspected infectious joint. Corticosteroids should not be injected during aspiration if infection is being ruled out.
When Jane returns for a follow-up visit eight weeks later, she states that the knee pain has resolved and that she has returned to running. She has lost an additional eight pounds and continues to diet. And, at the advice of her physical therapist, she is continuing her physiotherapy regimen at home to prevent a recurrence of PFPS.
REFERENCES
1. Earl JE, Vetter CS. Patellofemoral pain. Phys Med Rehabil Clin N Am. 2007;18:439-458,viii.
2. McGowan HJ, Beutler A. Patellofemoral syndrome. Essential Evidence Plus. www.essentialevidenceplus.com. Accessed April 14, 2015.
3. Nunes GS, Stapait EL, Kirsten MH, et al. Clinical test for diagnosis of patellofemoral pain syndrome: systematic review with meta-analysis. Phys Ther Sport. 2013;14:54-59.
4. Rixe JA, Glick JE, Brady J, et al. A review of the management of patellofemoral pain syndrome. Phys Sportsmed. 2013;41:19-28.
5. Bolgla LA, Boling MC. An update for the conservative management of patellofemoral pain syndrome: a systematic review of the literature from 2000 to 2010. Int J Sports Phys Ther. 2011;6:112-125.
6. Barton C, Balachandar V, Lack S, et al. Patellar taping for patellofemoral pain: a systematic review and meta-analysis to evaluate clinical outcomes and biomechanical mechanisms. Br J Sports Med. 2014;48:417-424.
7. Dutton RA, Khadavi MJ, Fredericson M. Update on rehabilitation of patellofemoral pain. Curr Sports Med Rep. 2014;13:172-178.
8. Kapur S, Wissman RD, Robertson M, et al. Acute knee dislocation: review of an elusive entity. Curr Probl Diagn Radiol. 2009;38:237-250.
9. Colvin AC, West RV. Patellar instability. J Bone Joint Surg Am. 2008;90:2751-2762.
10. Tscholl PM, Koch PP, Fucentese SF. Treatment options for patellofemoral instability in sports traumatology. Orthop Rev (Pavia). 2013;5:e23.
11. Earhart C, Patel DB, White EA, et al. Transient lateral patellar dislocation: review of imaging findings, patellofemoral anatomy, and treatment options. Emerg Radiol. 2013;20:11-23.
12. Tan SC, Chan O. Achilles and patellar tendinopathy: current understanding of pathophysiology and management. Disabil Rehabil. 2008;30:1608-1615.
13. Gaida JE, Cook J. Treatment options for patellar tendinopathy: critical review. Curr Sports Med Rep. 2011;10:255-270.
14. Rodriguez-Merchan EC. The treatment of patellar tendinopathy. J Orthop Traumatol. 2013;14:77-81.
15. Childress MA, Beutler A. Management of chronic tendon injuries. Am Fam Physician. 2013;87:486-490.
16. Charousset C, Zaoui A, Bellaiche L, et al. Are multiple platelet-rich plasma injections useful for treatment of chronic patellar tendinopathy in athletes? A prospective study. Am J Sports Med. 2014;42:906-911.
17. Strauss EJ, Kim S, Calcei JG, et al. Iliotibial band syndrome: evaluation and management. J Am Acad Orthop Surg. 2011;19:728-736.
18. Bellary SS, Lynch G, Housman B, et al. Medial plica syndrome: a review of the literature. Clin Anat. 2012;25:423-428.
19. Hong JH, Kim JS. Diagnosis of iliotibial band friction syndrome and ultrasound guided steroid injection. Korean J Pain. 2013;26:387-391.
20. Bellary SS, Lynch G, Housman B, et al. Medial plica syndrome: a review of the literature. Clin Anat. 2012;25:423-428.
21. Kim SJ, Jeong JH, Cheon YM, et al. MPP test in the diagnosis of medial patellar plica syndrome. Arthroscopy. 2004;20:1101-1103.
22. Schindler OS. ‘The Sneaky Plica’ revisited: morphology, pathophysiology and treatment of synovial plicae of the knee. Knee Surg Sports Traumatol Arthrosc. 2014;22:247-262.
23. Helfenstein M Jr, Kuromoto J. Anserine syndrome. Rev Bras Rheumatol. 2010;50:313-327.
24. Abeles M. Osteoarthritis of the knee: anserine bursitis as an extra-articular cause of pain. Clin Res. 1983;31:4471-4476.
25. Kang I, Han SW. Anserine bursitis in patients with osteoarthritis of the knee. South Med J. 2000;93:207-209.
26. Yoon HS, Kim SE, Suh YR, et al. Correlation between ultrasonographic findings and the response to corticosteroid injection in pes anserinus tendinobursitis syndrome in knee osteoarthritis patients. J Korean Med Sci. 2005;20:109-112.
27. Stein D, Cantlon M, MacKay B, et al. Cysts about the knee: evaluation and management. J Am Acad Orthop Surg. 2013;21:469-479.
28. Canoso JJ, Goldsmith MR, Gerzof SG, et al. Foucher’s sign of the Baker’s cyst. Ann Rheum Dis. 1987;46:228-232.
29. Palmer T. Knee pain. Essential Evidence Plus. www.essentialevidenceplus.com. Accessed April 14, 2015.
30. Franks AG Jr. Rheumatologic aspects of knee disorders. In: Scott WN, ed. The Knee. St. Louis: Mosby; 1994:315-329.
31. Visentini PJ, Khan KM, Cook JL, et al. The VISA score: an index of severity of symptoms in patients with jumper’s knee (patellar tendinosis). Victorian Institute of Sport Tendon Study Group. J Sci Med Sport. 1998;1:22-28.
32. Halabchi F, Mazaheri R, Seif-Barghi T. Patellofemoral pain syndrome and modifiable intrinsic risk factors; how to assess and address? Asian J Sports Med. 2013;4:85-100.
33. Dixit S, DiFiori JP, Burton M, et al. Management of patellofemoral pain syndrome. Am Fam Physician. 2007;75:194-202.
34. Callaghan MJ, Selfe J. Patellar taping for patellofemoral pain syndrome in adults. Cochrane Database Syst Rev. 2012;4:CD006717.
35. Atanda AJ Jr, Ruiz D, Dodson CC, et al. Approach to the active patient with chronic anterior knee pain. Phys Sportsmed. 2012;40:41-50.
36. Ellis R, Hing W, Reid D. Iliotibial band friction syndrome—a systematic review. Man Ther. 2007;12:200-208.
37. Kirk KL, Kuklo T, Klemme W. Iliotibial band friction syndrome. Orthopedics. 2000;23:1209-1217.
38. Stubbings N, Smith T. Diagnostic test accuracy of clinical and radiological assessments for medial patella plica syndrome: a systematic review and meta-analysis. Knee. 2014;21:486-490.
39. Alvarez-Nemegyei J, Canoso JJ. Evidence-based soft tissue rheumatology IV: anserine bursitis. J Clin Rheumatol. 2004;10:205-206.
40. Fritschy D, Fasel J, Imbert JC, et al. The popliteal cyst. Knee Surg Sports Traumatol Arthrosc. 2006;14:623-628.
41. Handy JR. Popliteal cysts in adults: a review. Semin Arthritis Rheum. 2001;31:108-118.
Jane, age 42, presents with right knee pain that she’s had for about six months. She denies any trauma. Jane describes the pain as “vague and poorly localized” but worse with activity. She says she started a walking/running program nine months ago, when she was told she was overweight (BMI, 29). She has lost 10 pounds since then and hopes to lose more by continuing to exercise. Further review reveals that Jane has experienced increasing pain while ascending and descending stairs and that the pain is also exacerbated when she stands after prolonged sitting.
If Jane were your patient, what would you include in a physical examination, and how would you diagnose and treat her?
Knee pain is a common presentation in primary care. While traumatic knee pain is frequently addressed in the medical literature, little has been written about chronic nontraumatic nonarthritic knee pain such as Jane’s. Thus, while physical exam tests often lead to the correct diagnosis for traumatic knee pain, there is limited information on the use of such tests to determine the etiology of chronic knee pain.
This review was developed to fill that gap. The pages that follow contain general guidance on the diagnosis and treatment of chronic nontraumatic knee pain. The conditions are presented anatomically—anterior, lateral, medial, or posterior—with common etiologies, history and physical exam findings, and diagnosis and treatment options for each (see Table, page 28).1-31
ANTERIOR KNEE PAIN
Patellofemoral pain syndrome (PFPS)
The most common cause of anterior knee pain, PFPS is a complex entity with an etiology that has not been well described.2 The quadriceps tendon, medial and lateral retinacula, iliotibial band (ITB), vastus medialis and lateralis, and the insertion of the patellar tendon on the anterior tibial tubercle all play a role in proper tracking of the patellofemoral joint; an imbalance in any of these forces leads to abnormal patellar tracking over the femoral condyles, and pain ensues. PFPS can also be secondary to joint overload, in which excessive physical activity (eg, running, lunges, or squats) overloads the patellofemoral joint and causes pain.
Risk factors for PFPS include strength imbalances in the quadriceps, hamstring, and hip muscle groups, and increased training, such as running longer distances.4,32 A recent review showed no relationship between an increased quadriceps (Q)-angle and PFPS, so that is no longer considered a major risk factor.5
Diagnosis. PFPS is a diagnosis of exclusion and is primarily based on history and physical exam. Anterior knee pain that is exacerbated when seated for long periods of time (the “theater sign”) or by descending stairs is a classic indication of PFPS.1 Patients may complain of knee stiffness or “giving out” secondary to sharp knee pain and a sensation of popping or crepitus in the joint. Swelling is not a common finding.2
A recent meta-analysis revealed limited evidence for the use of any specific physical exam tests to diagnose PFPS. But pain during squatting and pain with a patellar tilt test were most consistent with a diagnosis of PFPS. (The patellar tilt test involves lifting the lateral edge of the patella superiorly while the patient lies supine with knee extended; pain with < 20° of lift suggests a tight lateral retinaculum). Conversely, the absence of pain during squatting or the absence of lateral retinacular pain helps rule it out.2 A physical exam of the cruciate and collateral ligaments should be performed in a patient with a history of instability. Radiography is not needed for a diagnosis but may be considered if examination reveals an effusion, the patient is 50 or older, or no improvement occurs after eight to 12 weeks of treatment.33
Treatment. The most effective and strongly supported treatment for PFPS is a six-week physiotherapy program focusing on strengthening the quadriceps and hip muscles and stretching the quadriceps, ITB, hamstrings, and hip flexors.4,5 There is limited information about the use of NSAIDs, but they can be considered for short-term management.2
Patellar taping and bracing have shown some promise as adjunct therapies for PFPS, although the data for both are nonconclusive. There is a paucity of prospective randomized trials of patellar bracing, and a 2012 Cochrane review found limited evidence of its efficacy.34 But a 2014 meta-analysis revealed moderate evidence in support of patellar taping early on to help decrease pain,6 and a recent review suggests that it can be helpful in both the short and long term.7
Taping or bracing may be useful when combined with a tailored physical therapy program. Evidence for treatments such as biofeedback, chiropractic manipulation, and orthotics is limited, and they should be used only as adjunctive therapy.4
When you examine Jane, you find no swelling of the affected knee. You perform the tilt test, which elicits pain. Squatting causes some pain, as well. You diagnose PFPS and provide a referral for six weeks of physiotherapy.
Patellofemoral instability (PFI)
PFI occurs when the patella disengages completely from the trochlear groove.11 PFI’s etiology also relates to the complexity of the patellofemoral joint. Here, too, stability of the joint is achieved with a combination of soft-tissue and bony restraints. At full extension and early flexion of the knee, however, the mechanisms of stability are limited, resulting in increased instability. Other associated factors include Q-angle, lateral pull from a tight ITB, and opposing forces from the vastus lateralis and vastus medialis obliquus (VMO).8-10
Risk factors for PFI. The most common predisposing factors for PFI are trochlear dysplasia, patella alta, and lateralization of the tibial tuberosity or patella.10,11 Older patients, predominately women, have an increased risk for PFI.9 Patients usually have a history of patellar subluxation or dislocation in their youth, with approximately 17% of those who had a first dislocation experiencing a recurrence.9 A family history of PFI is common, as well.10
Diagnosis. Patients with PFI often present with nonspecific anterior knee pain secondary to recurrent dislocation.13 Notable exam findings include
• A positive J sign (noted if the patella suddenly shifts medially during early knee flexion or laterally during full extension)
• Decreased quadriceps (specifically VMO) and hamstring strength and flexibility
• Patellar hypermobility, which should be no more than a quarter to a half of the patellar diameter bilaterally
• Pain during a patellar tilt test
• A positive patellar apprehension test.10 (With the patient lying with the knee flexed to 20°, place thumbs on the medial patella and push laterally; the patient will straighten leg with pain or “apprehension” prior to patellar dislocation.)
Plain radiography should be ordered in all cases to assess for osseous trauma/deformity and to help guide surgical consideration. MRI can provide additional information when significant soft-tissue damage is suspected or the patient does not improve with conservative therapy.8,11
Treatment. A recent Cochrane review showed that conservative treatment (VMO strengthening, bracing, and proprioceptive therapy) prevented future dislocations more effectively than surgical intervention.11 However, surgery is indicated when obvious predisposing anatomic conditions (osteochondral fracture, intra-articular deformity, or a major tear of a medial soft-tissue stabilizer) are clearly shown on imaging.8,11
Patellar tendinopathy
An overuse injury often called “jumper’s knee” because it is associated with high-intensity jumping sports (eg, volleyball and basketball), patellar tendinopathy is an insertional tendinopathy with pain most commonly at the proximal patellar tendon.10 The pathology of the injury, though poorly understood, is believed to result from an impaired healing response to microtears.12,14
Diagnosis. Patients with patellar tendinopathy typically present with anterior suprapatellar pain aggravated by activity. Classically, the pain can occur in any of four phases12
1. Pain isolated after activity
2. Pain that occurs during activity but does not impede activity
3. Pain that occurs both during and after the activity and interferes with competition
4. A complete tendon disruption.
Examination should include an assessment of the patellar tendon for localized thickening, nodularity, crepitus, and focal suprapatellar tenderness. The muscle tendon function should be evaluated by assessing knee mobility and strength of the quads via straight-leg raise, decline squat, or single-leg squats.12 The Victorian Institute of Sport Assessment (VISA) questionnaire can be used to quantify the symptoms and to help track the patient’s progress throughout therapy.31 There are no proven special tests or radiologic studies to aid in the diagnosis of patellar tendinopathy,14 but MRI can be used for further evaluation when findings are equivocal.35
Treatment. A wide range of options, from eccentric training (eg, three sets of 15 repetitions performed twice a day for 12 weeks) and physical therapy to platelet-rich plasma (PRP) injections, sclerosing injections, and surgery, are available for the treatment of patellar tendinopathy.13-15 While no specific data have proven the superiority of any one therapy, expert consensus recommends eccentric exercise as initial therapy, performed for 12 weeks.14,15
It’s also interesting to note that a recently published study showed that three weekly PRP injections helped 75% of patients—all of whom failed to respond to four months of eccentric therapy—return to their presymptom activity level within 90 days.16 Corticosteroid injections should not be used to treat patellar tendinopathy due to the risk for tendon rupture.15 Orthopedic referral for surgical intervention should be considered for patients who fail to respond after three to six months of conservative therapy.14
Next: Lateral knee pain >>
LATERAL KNEE PAIN
Iliotibial band syndrome (ITBS)
A common source of lateral knee pain, ITBS is found particularly in runners, cyclists, and endurance athletes.17-19,36,37 The exact pathophysiology behind this diagnosis is debatable, but the most accepted etiology is inflammation generated from microtrauma to the soft tissues with inadequate healing time, resulting in persistent inflammation. ITBS is often associated with excessive overall running mileage, a sudden increase in mileage, or an abrupt change in training.18,37
Diagnosis. Patients often complain of persistent nontraumatic lateral knee pain that worsens with repetitive knee flexion (eg, running or cycling).17-19,37 A physical exam will often reveal pain over the lateral femoral condyle and a positive Noble’s test (see Figure 1, page 30). A positive Ober’s test (see Figure 2, page 32) is suggestive of ITBS, as well. The sensitivity and specificity of these tests are not well established, but in patients performing repetitive knee flexion activities with subjective lateral knee pain, pain over the lateral femoral condyle and a positive Ober’s and/or Noble’s test suggest an ITBS diagnosis.18 Imaging is not indicated initially, but MRI should be used in refractory cases to rule out other etiologies.17,19
Treatment. First-line therapy for ITBS is conservative,17-19,36,37 often involving a combination of techniques such as refraining from the activity that triggers the pain, NSAIDs, activity modification to reduce the strain over the ITB, myofascial release via foam rollers, and physical therapy focused on stretching the ITB, tensor fasciae latae, and gluteus medius while strengthening the gluteus medius and core muscles.17 No single program has been shown to be better than another.
Corticosteroid injections are second-line therapy and have been shown to improve pain compared with placebo up to two weeks postinjection.17,19 When symptoms persist for more than six months despite conservative treatment, surgical intervention may be indicated.18,19 Patients who experience temporary pain relief with corticosteroid injections often respond best to surgery.36
MEDIAL KNEE PAIN
Medial plica syndrome
Because of its anatomic location, the medial plica—which can be palpated in up to 84% of the population20—is susceptible to impingement by the medial femoral condyle or the patellofemoral joint. Trauma with repetitive knee movement leads to inflammation and thickening of the plica, resulting in medial plica syndrome.20,38 Initial inflammation may be triggered by blunt trauma, a sudden increase in activity, or transient synovitis.22
Diagnosis. Medial plica syndrome is a challenging diagnosis. Patients generally have nonspecific complaints of aching medial knee pain, locking, and catching similar to complaints of a medial meniscal injury.20
Evaluation should include the mediopatellar plica test, which is performed with the patient lying supine with the knee fully extended. Pressure is placed over the inferomedial patellofemoral joint, creating an impingement of the medial plica between the finger and the medial femoral condyle. Elimination or marked diminishing of pain with knee flexion to 90° is considered a positive test.21
A recent systematic review found this test to be more diagnostically accurate than an MRI (sensitivity of the test is 90% and specificity is 89%, vs 77% and 58%, respectively, for MRI) for detection of medial plica syndrome. Ultrasound is almost as accurate, with a sensitivity of 90% and specificity of 83%.39
Treatment of medial plica syndrome centers on physiotherapy and quadriceps strengthening,20 augmented with NSAIDs. Intra-articular corticosteroid injections are considered second-line treatment.20,22 An orthopedics referral is indicated to consider arthroscopic plica removal for refractory cases.20,22
Pes anserine bursitis
The anserine bursal complex, located approximately 5 cm distal to the medial joint line, is formed by the combined insertion of the sartorius, gracilis, and semitendinosus tendons,39 but the exact mechanism of pain is not well understood. Whether the pathophysiology is from an insertional tendonitis or overt bursitis is unknown, and no studies have focused on prevalence or risk factors. What is known is that overweight individuals and women with a wide pelvis seem to have a greater predilection and those with pes planus, diabetes, or knee osteoarthritis are at increased risk.23
Diagnosis. Medial knee pain reproduced on palpation of the anatomic site of insertion of the pes anserine tendon complex supports a diagnosis of pes anserine bursitis, with or without edema. Radiologic studies are not needed but may be helpful if significant bony pathology is suspected. Ultrasound, CT, and MRI are not recommended.23
Treatment. Resting the affected knee, cryotherapy, NSAIDs, and use of a pillow at night to relieve direct bursal pressure are recommended.33 Weight loss in obese patients, treatment of pes planus, and control of diabetes may be helpful, as well. Although the literature is limited and dated, corticosteroid injection has been found to reduce the pain and may be considered as second-line treatment.24-26
POSTERIOR KNEE PAIN
Popliteal (Baker’s) cyst
The popliteal fossa contains six of the numerous bursa of the knee; the bursa beneath the medial head of the gastrocnemius muscle and the semimembranosus tendon is most commonly involved in the formation of a popliteal cyst.40 It is postulated that increased intra-articular pressure forces fluid into the bursa, leading to expansion and pain. This can be idiopathic or secondary to internal derangement or trauma to the knee.41 Older age, a remote history of knee trauma, or a coexisting joint disease such as osteoarthritis, meniscal pathology, or rheumatoid arthritis are significant risk factors for popliteal cysts.27
Diagnosis. Most popliteal cysts are asymptomatic in adults and discovered incidentally after routine imaging to evaluate other knee pathology. However, symptomatic popliteal cysts present as a palpable mass in the popliteal fossa, resulting in pain and limited range of motion.
During the physical exam with the patient lying supine, a medial popliteal mass that is most prominent with the knee fully extended is common. A positive Foucher’s sign (the painful mass is palpated posteriorly in the popliteal fossa with the knee fully extended; pain is relieved and/or the mass reduced in size with knee flexion to 45°) suggests a diagnosis of popliteal cyst.27,28
Radiologic studies are generally not needed to diagnose a popliteal cyst. However, if diagnostic uncertainty remains after the history and physical exam, plain knee radiographs and ultrasound should be obtained. This combination provides complementary information and helps rule out a fracture, arthritis, and thrombosis as the cause of the pain.27 MRI is helpful if the diagnosis is still in doubt or if patients are suspected of having significant internal derangement leading to cyst formation. Arthrography or CT is generally not needed.27,41
Treatment. As popliteal cysts are often associated with other knee pathology, management of the underlying condition often leads to cyst regression. Keeping the knee in flexion can decrease the available space and assist in pain control in the acute phase.27 Cold packs and NSAIDs can also be used initially. Cyst aspiration and intra-articular corticosteroid injection have been shown to be effective for cysts that do not respond to this conservative approach.27 However, addressing and managing the underlying knee pathology (eg, osteoarthritis, meniscal pathology, or rheumatoid arthritis) will prevent popliteal cysts from recurring.
Continue for when the problem is painful knee effusion >>
WHEN THE PROBLEM IS PAINFUL KNEE EFFUSION
Nontraumatic knee effusion can be the primary source of knee pain or the result of underlying pathology. It is mentioned here because clinical suspicion is paramount to diagnosis of a septic joint—a serious cause of painful knee effusion that warrants prompt treatment.
As in other causes of knee pain, a detailed history of the character of the pain is essential. Septic arthritis and crystalline disease (gout, pseudogout) should be suspected in patients without a history of trauma who cannot bear weight. Systemic complaints point to an infection and, with the exception of a possible low-grade fever, are not typically seen in crystalline disease. Notable findings include an erythematous, hot, swollen knee and pain with both active and passive movement.
Plain radiographs of the knee should be ordered to rule out significant trauma or arthritis as the etiology. It is important to perform joint aspiration with synovial fluid analysis. Fluid analysis should include a white blood cell (WBC) count with differential, Gram stain and cultures, and polarized light microscopy (not readily available in an outpatient setting).29
Synovial fluid analysis characteristics suggestive of a septic joint include turbid quality, WBC > 50,000/mL, an elevated protein content, and a low glucose concentration.30 Gram stain and culture will help identify the infectious agent. Orthopedic referral should not be delayed in patients with a suspected infectious joint. Corticosteroids should not be injected during aspiration if infection is being ruled out.
When Jane returns for a follow-up visit eight weeks later, she states that the knee pain has resolved and that she has returned to running. She has lost an additional eight pounds and continues to diet. And, at the advice of her physical therapist, she is continuing her physiotherapy regimen at home to prevent a recurrence of PFPS.
REFERENCES
1. Earl JE, Vetter CS. Patellofemoral pain. Phys Med Rehabil Clin N Am. 2007;18:439-458,viii.
2. McGowan HJ, Beutler A. Patellofemoral syndrome. Essential Evidence Plus. www.essentialevidenceplus.com. Accessed April 14, 2015.
3. Nunes GS, Stapait EL, Kirsten MH, et al. Clinical test for diagnosis of patellofemoral pain syndrome: systematic review with meta-analysis. Phys Ther Sport. 2013;14:54-59.
4. Rixe JA, Glick JE, Brady J, et al. A review of the management of patellofemoral pain syndrome. Phys Sportsmed. 2013;41:19-28.
5. Bolgla LA, Boling MC. An update for the conservative management of patellofemoral pain syndrome: a systematic review of the literature from 2000 to 2010. Int J Sports Phys Ther. 2011;6:112-125.
6. Barton C, Balachandar V, Lack S, et al. Patellar taping for patellofemoral pain: a systematic review and meta-analysis to evaluate clinical outcomes and biomechanical mechanisms. Br J Sports Med. 2014;48:417-424.
7. Dutton RA, Khadavi MJ, Fredericson M. Update on rehabilitation of patellofemoral pain. Curr Sports Med Rep. 2014;13:172-178.
8. Kapur S, Wissman RD, Robertson M, et al. Acute knee dislocation: review of an elusive entity. Curr Probl Diagn Radiol. 2009;38:237-250.
9. Colvin AC, West RV. Patellar instability. J Bone Joint Surg Am. 2008;90:2751-2762.
10. Tscholl PM, Koch PP, Fucentese SF. Treatment options for patellofemoral instability in sports traumatology. Orthop Rev (Pavia). 2013;5:e23.
11. Earhart C, Patel DB, White EA, et al. Transient lateral patellar dislocation: review of imaging findings, patellofemoral anatomy, and treatment options. Emerg Radiol. 2013;20:11-23.
12. Tan SC, Chan O. Achilles and patellar tendinopathy: current understanding of pathophysiology and management. Disabil Rehabil. 2008;30:1608-1615.
13. Gaida JE, Cook J. Treatment options for patellar tendinopathy: critical review. Curr Sports Med Rep. 2011;10:255-270.
14. Rodriguez-Merchan EC. The treatment of patellar tendinopathy. J Orthop Traumatol. 2013;14:77-81.
15. Childress MA, Beutler A. Management of chronic tendon injuries. Am Fam Physician. 2013;87:486-490.
16. Charousset C, Zaoui A, Bellaiche L, et al. Are multiple platelet-rich plasma injections useful for treatment of chronic patellar tendinopathy in athletes? A prospective study. Am J Sports Med. 2014;42:906-911.
17. Strauss EJ, Kim S, Calcei JG, et al. Iliotibial band syndrome: evaluation and management. J Am Acad Orthop Surg. 2011;19:728-736.
18. Bellary SS, Lynch G, Housman B, et al. Medial plica syndrome: a review of the literature. Clin Anat. 2012;25:423-428.
19. Hong JH, Kim JS. Diagnosis of iliotibial band friction syndrome and ultrasound guided steroid injection. Korean J Pain. 2013;26:387-391.
20. Bellary SS, Lynch G, Housman B, et al. Medial plica syndrome: a review of the literature. Clin Anat. 2012;25:423-428.
21. Kim SJ, Jeong JH, Cheon YM, et al. MPP test in the diagnosis of medial patellar plica syndrome. Arthroscopy. 2004;20:1101-1103.
22. Schindler OS. ‘The Sneaky Plica’ revisited: morphology, pathophysiology and treatment of synovial plicae of the knee. Knee Surg Sports Traumatol Arthrosc. 2014;22:247-262.
23. Helfenstein M Jr, Kuromoto J. Anserine syndrome. Rev Bras Rheumatol. 2010;50:313-327.
24. Abeles M. Osteoarthritis of the knee: anserine bursitis as an extra-articular cause of pain. Clin Res. 1983;31:4471-4476.
25. Kang I, Han SW. Anserine bursitis in patients with osteoarthritis of the knee. South Med J. 2000;93:207-209.
26. Yoon HS, Kim SE, Suh YR, et al. Correlation between ultrasonographic findings and the response to corticosteroid injection in pes anserinus tendinobursitis syndrome in knee osteoarthritis patients. J Korean Med Sci. 2005;20:109-112.
27. Stein D, Cantlon M, MacKay B, et al. Cysts about the knee: evaluation and management. J Am Acad Orthop Surg. 2013;21:469-479.
28. Canoso JJ, Goldsmith MR, Gerzof SG, et al. Foucher’s sign of the Baker’s cyst. Ann Rheum Dis. 1987;46:228-232.
29. Palmer T. Knee pain. Essential Evidence Plus. www.essentialevidenceplus.com. Accessed April 14, 2015.
30. Franks AG Jr. Rheumatologic aspects of knee disorders. In: Scott WN, ed. The Knee. St. Louis: Mosby; 1994:315-329.
31. Visentini PJ, Khan KM, Cook JL, et al. The VISA score: an index of severity of symptoms in patients with jumper’s knee (patellar tendinosis). Victorian Institute of Sport Tendon Study Group. J Sci Med Sport. 1998;1:22-28.
32. Halabchi F, Mazaheri R, Seif-Barghi T. Patellofemoral pain syndrome and modifiable intrinsic risk factors; how to assess and address? Asian J Sports Med. 2013;4:85-100.
33. Dixit S, DiFiori JP, Burton M, et al. Management of patellofemoral pain syndrome. Am Fam Physician. 2007;75:194-202.
34. Callaghan MJ, Selfe J. Patellar taping for patellofemoral pain syndrome in adults. Cochrane Database Syst Rev. 2012;4:CD006717.
35. Atanda AJ Jr, Ruiz D, Dodson CC, et al. Approach to the active patient with chronic anterior knee pain. Phys Sportsmed. 2012;40:41-50.
36. Ellis R, Hing W, Reid D. Iliotibial band friction syndrome—a systematic review. Man Ther. 2007;12:200-208.
37. Kirk KL, Kuklo T, Klemme W. Iliotibial band friction syndrome. Orthopedics. 2000;23:1209-1217.
38. Stubbings N, Smith T. Diagnostic test accuracy of clinical and radiological assessments for medial patella plica syndrome: a systematic review and meta-analysis. Knee. 2014;21:486-490.
39. Alvarez-Nemegyei J, Canoso JJ. Evidence-based soft tissue rheumatology IV: anserine bursitis. J Clin Rheumatol. 2004;10:205-206.
40. Fritschy D, Fasel J, Imbert JC, et al. The popliteal cyst. Knee Surg Sports Traumatol Arthrosc. 2006;14:623-628.
41. Handy JR. Popliteal cysts in adults: a review. Semin Arthritis Rheum. 2001;31:108-118.
Jane, age 42, presents with right knee pain that she’s had for about six months. She denies any trauma. Jane describes the pain as “vague and poorly localized” but worse with activity. She says she started a walking/running program nine months ago, when she was told she was overweight (BMI, 29). She has lost 10 pounds since then and hopes to lose more by continuing to exercise. Further review reveals that Jane has experienced increasing pain while ascending and descending stairs and that the pain is also exacerbated when she stands after prolonged sitting.
If Jane were your patient, what would you include in a physical examination, and how would you diagnose and treat her?
Knee pain is a common presentation in primary care. While traumatic knee pain is frequently addressed in the medical literature, little has been written about chronic nontraumatic nonarthritic knee pain such as Jane’s. Thus, while physical exam tests often lead to the correct diagnosis for traumatic knee pain, there is limited information on the use of such tests to determine the etiology of chronic knee pain.
This review was developed to fill that gap. The pages that follow contain general guidance on the diagnosis and treatment of chronic nontraumatic knee pain. The conditions are presented anatomically—anterior, lateral, medial, or posterior—with common etiologies, history and physical exam findings, and diagnosis and treatment options for each (see Table, page 28).1-31
ANTERIOR KNEE PAIN
Patellofemoral pain syndrome (PFPS)
The most common cause of anterior knee pain, PFPS is a complex entity with an etiology that has not been well described.2 The quadriceps tendon, medial and lateral retinacula, iliotibial band (ITB), vastus medialis and lateralis, and the insertion of the patellar tendon on the anterior tibial tubercle all play a role in proper tracking of the patellofemoral joint; an imbalance in any of these forces leads to abnormal patellar tracking over the femoral condyles, and pain ensues. PFPS can also be secondary to joint overload, in which excessive physical activity (eg, running, lunges, or squats) overloads the patellofemoral joint and causes pain.
Risk factors for PFPS include strength imbalances in the quadriceps, hamstring, and hip muscle groups, and increased training, such as running longer distances.4,32 A recent review showed no relationship between an increased quadriceps (Q)-angle and PFPS, so that is no longer considered a major risk factor.5
Diagnosis. PFPS is a diagnosis of exclusion and is primarily based on history and physical exam. Anterior knee pain that is exacerbated when seated for long periods of time (the “theater sign”) or by descending stairs is a classic indication of PFPS.1 Patients may complain of knee stiffness or “giving out” secondary to sharp knee pain and a sensation of popping or crepitus in the joint. Swelling is not a common finding.2
A recent meta-analysis revealed limited evidence for the use of any specific physical exam tests to diagnose PFPS. But pain during squatting and pain with a patellar tilt test were most consistent with a diagnosis of PFPS. (The patellar tilt test involves lifting the lateral edge of the patella superiorly while the patient lies supine with knee extended; pain with < 20° of lift suggests a tight lateral retinaculum). Conversely, the absence of pain during squatting or the absence of lateral retinacular pain helps rule it out.2 A physical exam of the cruciate and collateral ligaments should be performed in a patient with a history of instability. Radiography is not needed for a diagnosis but may be considered if examination reveals an effusion, the patient is 50 or older, or no improvement occurs after eight to 12 weeks of treatment.33
Treatment. The most effective and strongly supported treatment for PFPS is a six-week physiotherapy program focusing on strengthening the quadriceps and hip muscles and stretching the quadriceps, ITB, hamstrings, and hip flexors.4,5 There is limited information about the use of NSAIDs, but they can be considered for short-term management.2
Patellar taping and bracing have shown some promise as adjunct therapies for PFPS, although the data for both are nonconclusive. There is a paucity of prospective randomized trials of patellar bracing, and a 2012 Cochrane review found limited evidence of its efficacy.34 But a 2014 meta-analysis revealed moderate evidence in support of patellar taping early on to help decrease pain,6 and a recent review suggests that it can be helpful in both the short and long term.7
Taping or bracing may be useful when combined with a tailored physical therapy program. Evidence for treatments such as biofeedback, chiropractic manipulation, and orthotics is limited, and they should be used only as adjunctive therapy.4
When you examine Jane, you find no swelling of the affected knee. You perform the tilt test, which elicits pain. Squatting causes some pain, as well. You diagnose PFPS and provide a referral for six weeks of physiotherapy.
Patellofemoral instability (PFI)
PFI occurs when the patella disengages completely from the trochlear groove.11 PFI’s etiology also relates to the complexity of the patellofemoral joint. Here, too, stability of the joint is achieved with a combination of soft-tissue and bony restraints. At full extension and early flexion of the knee, however, the mechanisms of stability are limited, resulting in increased instability. Other associated factors include Q-angle, lateral pull from a tight ITB, and opposing forces from the vastus lateralis and vastus medialis obliquus (VMO).8-10
Risk factors for PFI. The most common predisposing factors for PFI are trochlear dysplasia, patella alta, and lateralization of the tibial tuberosity or patella.10,11 Older patients, predominately women, have an increased risk for PFI.9 Patients usually have a history of patellar subluxation or dislocation in their youth, with approximately 17% of those who had a first dislocation experiencing a recurrence.9 A family history of PFI is common, as well.10
Diagnosis. Patients with PFI often present with nonspecific anterior knee pain secondary to recurrent dislocation.13 Notable exam findings include
• A positive J sign (noted if the patella suddenly shifts medially during early knee flexion or laterally during full extension)
• Decreased quadriceps (specifically VMO) and hamstring strength and flexibility
• Patellar hypermobility, which should be no more than a quarter to a half of the patellar diameter bilaterally
• Pain during a patellar tilt test
• A positive patellar apprehension test.10 (With the patient lying with the knee flexed to 20°, place thumbs on the medial patella and push laterally; the patient will straighten leg with pain or “apprehension” prior to patellar dislocation.)
Plain radiography should be ordered in all cases to assess for osseous trauma/deformity and to help guide surgical consideration. MRI can provide additional information when significant soft-tissue damage is suspected or the patient does not improve with conservative therapy.8,11
Treatment. A recent Cochrane review showed that conservative treatment (VMO strengthening, bracing, and proprioceptive therapy) prevented future dislocations more effectively than surgical intervention.11 However, surgery is indicated when obvious predisposing anatomic conditions (osteochondral fracture, intra-articular deformity, or a major tear of a medial soft-tissue stabilizer) are clearly shown on imaging.8,11
Patellar tendinopathy
An overuse injury often called “jumper’s knee” because it is associated with high-intensity jumping sports (eg, volleyball and basketball), patellar tendinopathy is an insertional tendinopathy with pain most commonly at the proximal patellar tendon.10 The pathology of the injury, though poorly understood, is believed to result from an impaired healing response to microtears.12,14
Diagnosis. Patients with patellar tendinopathy typically present with anterior suprapatellar pain aggravated by activity. Classically, the pain can occur in any of four phases12
1. Pain isolated after activity
2. Pain that occurs during activity but does not impede activity
3. Pain that occurs both during and after the activity and interferes with competition
4. A complete tendon disruption.
Examination should include an assessment of the patellar tendon for localized thickening, nodularity, crepitus, and focal suprapatellar tenderness. The muscle tendon function should be evaluated by assessing knee mobility and strength of the quads via straight-leg raise, decline squat, or single-leg squats.12 The Victorian Institute of Sport Assessment (VISA) questionnaire can be used to quantify the symptoms and to help track the patient’s progress throughout therapy.31 There are no proven special tests or radiologic studies to aid in the diagnosis of patellar tendinopathy,14 but MRI can be used for further evaluation when findings are equivocal.35
Treatment. A wide range of options, from eccentric training (eg, three sets of 15 repetitions performed twice a day for 12 weeks) and physical therapy to platelet-rich plasma (PRP) injections, sclerosing injections, and surgery, are available for the treatment of patellar tendinopathy.13-15 While no specific data have proven the superiority of any one therapy, expert consensus recommends eccentric exercise as initial therapy, performed for 12 weeks.14,15
It’s also interesting to note that a recently published study showed that three weekly PRP injections helped 75% of patients—all of whom failed to respond to four months of eccentric therapy—return to their presymptom activity level within 90 days.16 Corticosteroid injections should not be used to treat patellar tendinopathy due to the risk for tendon rupture.15 Orthopedic referral for surgical intervention should be considered for patients who fail to respond after three to six months of conservative therapy.14
Next: Lateral knee pain >>
LATERAL KNEE PAIN
Iliotibial band syndrome (ITBS)
A common source of lateral knee pain, ITBS is found particularly in runners, cyclists, and endurance athletes.17-19,36,37 The exact pathophysiology behind this diagnosis is debatable, but the most accepted etiology is inflammation generated from microtrauma to the soft tissues with inadequate healing time, resulting in persistent inflammation. ITBS is often associated with excessive overall running mileage, a sudden increase in mileage, or an abrupt change in training.18,37
Diagnosis. Patients often complain of persistent nontraumatic lateral knee pain that worsens with repetitive knee flexion (eg, running or cycling).17-19,37 A physical exam will often reveal pain over the lateral femoral condyle and a positive Noble’s test (see Figure 1, page 30). A positive Ober’s test (see Figure 2, page 32) is suggestive of ITBS, as well. The sensitivity and specificity of these tests are not well established, but in patients performing repetitive knee flexion activities with subjective lateral knee pain, pain over the lateral femoral condyle and a positive Ober’s and/or Noble’s test suggest an ITBS diagnosis.18 Imaging is not indicated initially, but MRI should be used in refractory cases to rule out other etiologies.17,19
Treatment. First-line therapy for ITBS is conservative,17-19,36,37 often involving a combination of techniques such as refraining from the activity that triggers the pain, NSAIDs, activity modification to reduce the strain over the ITB, myofascial release via foam rollers, and physical therapy focused on stretching the ITB, tensor fasciae latae, and gluteus medius while strengthening the gluteus medius and core muscles.17 No single program has been shown to be better than another.
Corticosteroid injections are second-line therapy and have been shown to improve pain compared with placebo up to two weeks postinjection.17,19 When symptoms persist for more than six months despite conservative treatment, surgical intervention may be indicated.18,19 Patients who experience temporary pain relief with corticosteroid injections often respond best to surgery.36
MEDIAL KNEE PAIN
Medial plica syndrome
Because of its anatomic location, the medial plica—which can be palpated in up to 84% of the population20—is susceptible to impingement by the medial femoral condyle or the patellofemoral joint. Trauma with repetitive knee movement leads to inflammation and thickening of the plica, resulting in medial plica syndrome.20,38 Initial inflammation may be triggered by blunt trauma, a sudden increase in activity, or transient synovitis.22
Diagnosis. Medial plica syndrome is a challenging diagnosis. Patients generally have nonspecific complaints of aching medial knee pain, locking, and catching similar to complaints of a medial meniscal injury.20
Evaluation should include the mediopatellar plica test, which is performed with the patient lying supine with the knee fully extended. Pressure is placed over the inferomedial patellofemoral joint, creating an impingement of the medial plica between the finger and the medial femoral condyle. Elimination or marked diminishing of pain with knee flexion to 90° is considered a positive test.21
A recent systematic review found this test to be more diagnostically accurate than an MRI (sensitivity of the test is 90% and specificity is 89%, vs 77% and 58%, respectively, for MRI) for detection of medial plica syndrome. Ultrasound is almost as accurate, with a sensitivity of 90% and specificity of 83%.39
Treatment of medial plica syndrome centers on physiotherapy and quadriceps strengthening,20 augmented with NSAIDs. Intra-articular corticosteroid injections are considered second-line treatment.20,22 An orthopedics referral is indicated to consider arthroscopic plica removal for refractory cases.20,22
Pes anserine bursitis
The anserine bursal complex, located approximately 5 cm distal to the medial joint line, is formed by the combined insertion of the sartorius, gracilis, and semitendinosus tendons,39 but the exact mechanism of pain is not well understood. Whether the pathophysiology is from an insertional tendonitis or overt bursitis is unknown, and no studies have focused on prevalence or risk factors. What is known is that overweight individuals and women with a wide pelvis seem to have a greater predilection and those with pes planus, diabetes, or knee osteoarthritis are at increased risk.23
Diagnosis. Medial knee pain reproduced on palpation of the anatomic site of insertion of the pes anserine tendon complex supports a diagnosis of pes anserine bursitis, with or without edema. Radiologic studies are not needed but may be helpful if significant bony pathology is suspected. Ultrasound, CT, and MRI are not recommended.23
Treatment. Resting the affected knee, cryotherapy, NSAIDs, and use of a pillow at night to relieve direct bursal pressure are recommended.33 Weight loss in obese patients, treatment of pes planus, and control of diabetes may be helpful, as well. Although the literature is limited and dated, corticosteroid injection has been found to reduce the pain and may be considered as second-line treatment.24-26
POSTERIOR KNEE PAIN
Popliteal (Baker’s) cyst
The popliteal fossa contains six of the numerous bursa of the knee; the bursa beneath the medial head of the gastrocnemius muscle and the semimembranosus tendon is most commonly involved in the formation of a popliteal cyst.40 It is postulated that increased intra-articular pressure forces fluid into the bursa, leading to expansion and pain. This can be idiopathic or secondary to internal derangement or trauma to the knee.41 Older age, a remote history of knee trauma, or a coexisting joint disease such as osteoarthritis, meniscal pathology, or rheumatoid arthritis are significant risk factors for popliteal cysts.27
Diagnosis. Most popliteal cysts are asymptomatic in adults and discovered incidentally after routine imaging to evaluate other knee pathology. However, symptomatic popliteal cysts present as a palpable mass in the popliteal fossa, resulting in pain and limited range of motion.
During the physical exam with the patient lying supine, a medial popliteal mass that is most prominent with the knee fully extended is common. A positive Foucher’s sign (the painful mass is palpated posteriorly in the popliteal fossa with the knee fully extended; pain is relieved and/or the mass reduced in size with knee flexion to 45°) suggests a diagnosis of popliteal cyst.27,28
Radiologic studies are generally not needed to diagnose a popliteal cyst. However, if diagnostic uncertainty remains after the history and physical exam, plain knee radiographs and ultrasound should be obtained. This combination provides complementary information and helps rule out a fracture, arthritis, and thrombosis as the cause of the pain.27 MRI is helpful if the diagnosis is still in doubt or if patients are suspected of having significant internal derangement leading to cyst formation. Arthrography or CT is generally not needed.27,41
Treatment. As popliteal cysts are often associated with other knee pathology, management of the underlying condition often leads to cyst regression. Keeping the knee in flexion can decrease the available space and assist in pain control in the acute phase.27 Cold packs and NSAIDs can also be used initially. Cyst aspiration and intra-articular corticosteroid injection have been shown to be effective for cysts that do not respond to this conservative approach.27 However, addressing and managing the underlying knee pathology (eg, osteoarthritis, meniscal pathology, or rheumatoid arthritis) will prevent popliteal cysts from recurring.
Continue for when the problem is painful knee effusion >>
WHEN THE PROBLEM IS PAINFUL KNEE EFFUSION
Nontraumatic knee effusion can be the primary source of knee pain or the result of underlying pathology. It is mentioned here because clinical suspicion is paramount to diagnosis of a septic joint—a serious cause of painful knee effusion that warrants prompt treatment.
As in other causes of knee pain, a detailed history of the character of the pain is essential. Septic arthritis and crystalline disease (gout, pseudogout) should be suspected in patients without a history of trauma who cannot bear weight. Systemic complaints point to an infection and, with the exception of a possible low-grade fever, are not typically seen in crystalline disease. Notable findings include an erythematous, hot, swollen knee and pain with both active and passive movement.
Plain radiographs of the knee should be ordered to rule out significant trauma or arthritis as the etiology. It is important to perform joint aspiration with synovial fluid analysis. Fluid analysis should include a white blood cell (WBC) count with differential, Gram stain and cultures, and polarized light microscopy (not readily available in an outpatient setting).29
Synovial fluid analysis characteristics suggestive of a septic joint include turbid quality, WBC > 50,000/mL, an elevated protein content, and a low glucose concentration.30 Gram stain and culture will help identify the infectious agent. Orthopedic referral should not be delayed in patients with a suspected infectious joint. Corticosteroids should not be injected during aspiration if infection is being ruled out.
When Jane returns for a follow-up visit eight weeks later, she states that the knee pain has resolved and that she has returned to running. She has lost an additional eight pounds and continues to diet. And, at the advice of her physical therapist, she is continuing her physiotherapy regimen at home to prevent a recurrence of PFPS.
REFERENCES
1. Earl JE, Vetter CS. Patellofemoral pain. Phys Med Rehabil Clin N Am. 2007;18:439-458,viii.
2. McGowan HJ, Beutler A. Patellofemoral syndrome. Essential Evidence Plus. www.essentialevidenceplus.com. Accessed April 14, 2015.
3. Nunes GS, Stapait EL, Kirsten MH, et al. Clinical test for diagnosis of patellofemoral pain syndrome: systematic review with meta-analysis. Phys Ther Sport. 2013;14:54-59.
4. Rixe JA, Glick JE, Brady J, et al. A review of the management of patellofemoral pain syndrome. Phys Sportsmed. 2013;41:19-28.
5. Bolgla LA, Boling MC. An update for the conservative management of patellofemoral pain syndrome: a systematic review of the literature from 2000 to 2010. Int J Sports Phys Ther. 2011;6:112-125.
6. Barton C, Balachandar V, Lack S, et al. Patellar taping for patellofemoral pain: a systematic review and meta-analysis to evaluate clinical outcomes and biomechanical mechanisms. Br J Sports Med. 2014;48:417-424.
7. Dutton RA, Khadavi MJ, Fredericson M. Update on rehabilitation of patellofemoral pain. Curr Sports Med Rep. 2014;13:172-178.
8. Kapur S, Wissman RD, Robertson M, et al. Acute knee dislocation: review of an elusive entity. Curr Probl Diagn Radiol. 2009;38:237-250.
9. Colvin AC, West RV. Patellar instability. J Bone Joint Surg Am. 2008;90:2751-2762.
10. Tscholl PM, Koch PP, Fucentese SF. Treatment options for patellofemoral instability in sports traumatology. Orthop Rev (Pavia). 2013;5:e23.
11. Earhart C, Patel DB, White EA, et al. Transient lateral patellar dislocation: review of imaging findings, patellofemoral anatomy, and treatment options. Emerg Radiol. 2013;20:11-23.
12. Tan SC, Chan O. Achilles and patellar tendinopathy: current understanding of pathophysiology and management. Disabil Rehabil. 2008;30:1608-1615.
13. Gaida JE, Cook J. Treatment options for patellar tendinopathy: critical review. Curr Sports Med Rep. 2011;10:255-270.
14. Rodriguez-Merchan EC. The treatment of patellar tendinopathy. J Orthop Traumatol. 2013;14:77-81.
15. Childress MA, Beutler A. Management of chronic tendon injuries. Am Fam Physician. 2013;87:486-490.
16. Charousset C, Zaoui A, Bellaiche L, et al. Are multiple platelet-rich plasma injections useful for treatment of chronic patellar tendinopathy in athletes? A prospective study. Am J Sports Med. 2014;42:906-911.
17. Strauss EJ, Kim S, Calcei JG, et al. Iliotibial band syndrome: evaluation and management. J Am Acad Orthop Surg. 2011;19:728-736.
18. Bellary SS, Lynch G, Housman B, et al. Medial plica syndrome: a review of the literature. Clin Anat. 2012;25:423-428.
19. Hong JH, Kim JS. Diagnosis of iliotibial band friction syndrome and ultrasound guided steroid injection. Korean J Pain. 2013;26:387-391.
20. Bellary SS, Lynch G, Housman B, et al. Medial plica syndrome: a review of the literature. Clin Anat. 2012;25:423-428.
21. Kim SJ, Jeong JH, Cheon YM, et al. MPP test in the diagnosis of medial patellar plica syndrome. Arthroscopy. 2004;20:1101-1103.
22. Schindler OS. ‘The Sneaky Plica’ revisited: morphology, pathophysiology and treatment of synovial plicae of the knee. Knee Surg Sports Traumatol Arthrosc. 2014;22:247-262.
23. Helfenstein M Jr, Kuromoto J. Anserine syndrome. Rev Bras Rheumatol. 2010;50:313-327.
24. Abeles M. Osteoarthritis of the knee: anserine bursitis as an extra-articular cause of pain. Clin Res. 1983;31:4471-4476.
25. Kang I, Han SW. Anserine bursitis in patients with osteoarthritis of the knee. South Med J. 2000;93:207-209.
26. Yoon HS, Kim SE, Suh YR, et al. Correlation between ultrasonographic findings and the response to corticosteroid injection in pes anserinus tendinobursitis syndrome in knee osteoarthritis patients. J Korean Med Sci. 2005;20:109-112.
27. Stein D, Cantlon M, MacKay B, et al. Cysts about the knee: evaluation and management. J Am Acad Orthop Surg. 2013;21:469-479.
28. Canoso JJ, Goldsmith MR, Gerzof SG, et al. Foucher’s sign of the Baker’s cyst. Ann Rheum Dis. 1987;46:228-232.
29. Palmer T. Knee pain. Essential Evidence Plus. www.essentialevidenceplus.com. Accessed April 14, 2015.
30. Franks AG Jr. Rheumatologic aspects of knee disorders. In: Scott WN, ed. The Knee. St. Louis: Mosby; 1994:315-329.
31. Visentini PJ, Khan KM, Cook JL, et al. The VISA score: an index of severity of symptoms in patients with jumper’s knee (patellar tendinosis). Victorian Institute of Sport Tendon Study Group. J Sci Med Sport. 1998;1:22-28.
32. Halabchi F, Mazaheri R, Seif-Barghi T. Patellofemoral pain syndrome and modifiable intrinsic risk factors; how to assess and address? Asian J Sports Med. 2013;4:85-100.
33. Dixit S, DiFiori JP, Burton M, et al. Management of patellofemoral pain syndrome. Am Fam Physician. 2007;75:194-202.
34. Callaghan MJ, Selfe J. Patellar taping for patellofemoral pain syndrome in adults. Cochrane Database Syst Rev. 2012;4:CD006717.
35. Atanda AJ Jr, Ruiz D, Dodson CC, et al. Approach to the active patient with chronic anterior knee pain. Phys Sportsmed. 2012;40:41-50.
36. Ellis R, Hing W, Reid D. Iliotibial band friction syndrome—a systematic review. Man Ther. 2007;12:200-208.
37. Kirk KL, Kuklo T, Klemme W. Iliotibial band friction syndrome. Orthopedics. 2000;23:1209-1217.
38. Stubbings N, Smith T. Diagnostic test accuracy of clinical and radiological assessments for medial patella plica syndrome: a systematic review and meta-analysis. Knee. 2014;21:486-490.
39. Alvarez-Nemegyei J, Canoso JJ. Evidence-based soft tissue rheumatology IV: anserine bursitis. J Clin Rheumatol. 2004;10:205-206.
40. Fritschy D, Fasel J, Imbert JC, et al. The popliteal cyst. Knee Surg Sports Traumatol Arthrosc. 2006;14:623-628.
41. Handy JR. Popliteal cysts in adults: a review. Semin Arthritis Rheum. 2001;31:108-118.