User login
Analysis of Predictors and Outcomes of Allogeneic Blood Transfusion After Shoulder Arthroplasty
In shoulder arthroplasty, it is not uncommon for patients to receive postoperative blood transfusions; rates range from 7% to 43%.1-6 Allogeneic blood transfusions (ABTs) are costly and not entirely free of risks.7 The risk for infection has decreased because of improved screening and risk reduction strategies, but there are still significant risks associated with ABTs, such as clerical errors, acute and delayed hemolytic reactions, graft-versus-host reactions, transfusion-related acute lung injury, and anaphylaxis.8-10 As use of shoulder arthroplasty continues to increase, the importance of minimizing unnecessary transfusions is growing as well.7
Predictive factors for ABT have been explored in other orthopedic settings, yet little has been done in shoulder arthroplasty.1-6,11-15 Previous shoulder arthroplasty studies have shown that low preoperative hemoglobin (Hb) levels are independent risk factors for postoperative blood transfusion. However, there is debate over the significance of other variables, such as procedure type, age, sex, and medical comorbidities. Further, prior studies were limited by relatively small samples from single institutions; the largest series included fewer than 600 patients.1-6
We conducted a study to determine predictors of ABT in a large cohort of patients admitted to US hospitals for shoulder arthroplasty. We also wanted to evaluate the effect of ABT on postoperative outcomes, including inpatient mortality, adverse events, prolonged hospital stay, and nonroutine discharge. According to the null hypothesis, in shoulder arthroplasty there will be no difference in risk factors between patients who require ABT and those who did not, after accounting for confounding variables.
Materials and Methods
This study was exempt from institutional review board approval, as all data were appropriately deidentified before use in this project. We used the Nationwide Inpatient Sample (NIS) to retrospectively study the period 2002–2011, from which all demographic, clinical, and resource use data were derived.16 NIS, an annual survey conducted by the Agency for Healthcare Research and Quality (AHRQ) since 1988, has generated a huge amount of data, forming the largest all-payer inpatient care database in the United States. Yearly samples contain discharge data from about 8 million hospital stays at more than 1000 hospitals across 46 states, approximating a 20% random sample of all hospital discharges at participating institutions.17 These data are then weighted to generate statistically valid national estimates.
The NIS database uses International Classification of Diseases, Ninth Edition, Clinical Modification (ICD-9-CM) codes to identify 15 medical diagnoses up to the year 2008 and a maximum of 25 medical diagnoses and 15 procedures thereafter. In addition, the database includes information on patient and hospital characteristics as well as inpatient outcomes such as length of stay, total hospitalization charges, and discharge disposition.18,19 Given its large sample size and data volume, NIS is a powerful tool in the analysis of data associated with a multitude of medical diagnoses and procedures.20
We used the NIS database to study a population of 422,371 patients (age, >18 years) who underwent total shoulder arthroplasty (TSA) or hemiarthroplasty (HSA) between 2002 and 2011. ICD-9-CM procedure codes for TSA (81.80, 81.88) and HSA (81.81) were used to identify this population. We also analyzed data for reverse TSA for the year 2011. Then we divided our target population into 2 different cohorts: patients who did not receive any blood transfusion products and patients who received a transfusion of allogeneic packed cells (ICD-9-CM code 99.04 was used to identify the latter cohort).
In this study, normal distribution of the dataset was assumed, given the large sample size. The 2 cohorts were evaluated through bivariate analysis using the Pearson χ2 test for categorical data and the independent-samples t test for continuous data. The extent to which diagnosis, age, race, sex, and medical comorbidities were predictive of blood transfusion after TSA or HSA was evaluated through multivariate binary logistic regression analysis. Statistical significance was set at P < .05. All statistical analyses and data modeling were performed with SPSS Version 22.0.
Results
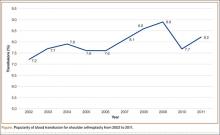
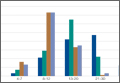
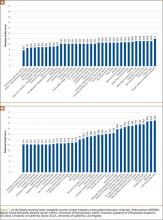
Using the NIS database, we stratified an estimated 422,371 patients who presented for shoulder arthroplasty between January 1, 2002, and December 31, 2011, into a TSA cohort (59.3%) and an HSA cohort (40.7%). Eight percent (33,889) of all patients received an ABT; the proportion of patients who received ABT was higher (P < .001) for the HSA cohort (55.6%) than the TSA cohort (39.4%). Further, the rate of ABT after shoulder arthroplasty showed an upward inclination (Figure).
Demographically, patients who received ABT tended (P < .001) to be older (74±11 years vs 68±11 years) and of a minority race (black or Hispanic) and to fall in either the lowest range of median household income (21.5% vs 20.7%; ≤$38,999) or the highest (27.3% vs 25.4%; ≥$63,000). Shoulder arthroplasty with ABT occurred more often (P < .001) at hospitals that were urban (13.3% vs 11.3%), medium in size (27.3% vs 23.4%), and nonteaching (56.2% vs 54.3%). In addition, ABT was used more often (P < .001) in patients with a primary diagnosis of fracture (43.1% vs 14.3%) or fracture nonunion (4.4% vs 2.1%). These groups also had a longer (P < .001) hospital stay (5.0±4.3 days vs 2.5±2.2 days). Table 1 summarizes these findings.
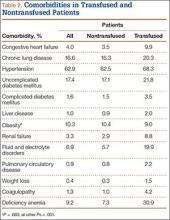
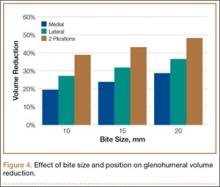
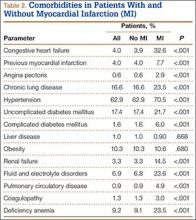
The 2 cohorts were then analyzed for presence of medical comorbidities (Table 2). Patients who required ABT during shoulder arthroplasty had a significantly (P < .001) higher prevalence of congestive heart failure, chronic lung disease, hypertension, uncomplicated and complicated diabetes mellitus, liver disease, renal failure, fluid and electrolyte disorders, pulmonary circulatory disease, weight loss, coagulopathy, and deficiency anemia.
In multivariate regression modeling (Table 3), demographic predictors of ABT (P < .001) included increasing age (odds ratio [OR], 1.03 per year; 95% confidence interval [95% CI], 1.03-1.03), female sex (OR, 1.55; 95% CI, 1.51-1.60), and minority race (black or Hispanic). Odds of requiring ABT were higher for patients with Medicare (OR, 1.25; 95% CI, 1.20-1.30) and patients with Medicaid (OR, 1.63; 95% CI, 1.51-1.77) than for patients with private insurance.
ABT was more likely to be required (P < .001) in patients with a primary diagnosis of fracture (OR, 4.49; 95% CI, 4.34-4.65), avascular necrosis (OR, 2.06; 95% CI, 1.91-2.22), rheumatoid arthritis (OR, 1.91; 95% CI, 1.72-2.12), fracture nonunion (OR, 3.55; 95% CI, 3.33-3.79), or rotator cuff arthropathy (OR, 1.47; 95% CI, 1.41-1.54) than for patients with osteoarthritis. Moreover, compared with patients having HSA, patients having TSA were more likely to require ABT (OR, 1.20; 95% CI, 1.17-1.24). According to the analysis restricted to the year 2011, compared with patients having anatomical TSAs, patients having reverse TSAs were 1.6 times more likely (P < .001) to require ABT (OR, 1.63; 95% CI, 1.50-1.79).
With the exception of obesity, all comorbidities were significant (P < .001) independent predictors of ABT after shoulder arthroplasty: deficiency anemia (OR, 3.42; 95% CI, 3.32-3.52), coagulopathy (OR, 2.54; 95% CI, 2.36-2.73), fluid and electrolyte disorders (OR, 1.91; 95% CI, 1.84-1.97), and weight loss (OR, 1.78; 95% CI, 1.58-2.00).
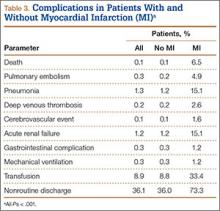
Patients who received ABT were more likely to experience adverse events (OR, 1.74; 95% CI, 1.68-1.81), prolonged hospital stay (OR, 3.21; 95% CI, 3.12-3.30), and nonroutine discharge (OR, 1.77; 95% CI, 1.72-1.82) (Table 4). There was no difference in mortality between the 2 cohorts.
Discussion
There is an abundance of literature on blood transfusions in hip and knee arthroplasty, but there are few articles on ABT in shoulder arthroplasty, and they all report data from single institutions with relatively low caseloads.1,2,11-13,15,21 In the present study, we investigated ABT in shoulder arthroplasty from the perspective of a multi-institutional database with a caseload of more than 400,000. Given the rapidly increasing rates of shoulder arthroplasty, it is important to further examine this issue to minimize unnecessary blood transfusion and its associated risks and costs.7
We found that 8% of patients who had shoulder arthroplasty received ABT, which is consistent with previously reported transfusion rates (range, 7%-43%).1-6 Rates of ABT after shoulder arthroplasty have continued to rise. The exception, a decrease during the year 2010, can be explained by increased efforts to more rigidly follow transfusion indication guidelines to reduce the number of potentially unnecessary ABTs.21-24 Our study also identified numerous significant independent predictors of ABT in shoulder arthroplasty: age, sex, race, insurance status, procedure type, primary diagnoses, and multiple medical comorbidities.
Demographics
According to our analysis, more than 80% of patients who received ABT were over age 65 years, which aligns with what several other studies have demonstrated: Increasing age is a predictor of ABT, despite higher rates of comorbidities and lower preoperative Hb levels in this population.1,2,4,5,25-27 Consistent with previous work, female sex was predictive of ABT.2,5 It has been suggested that females are more likely predisposed to ABT because of lower preoperative Hb and smaller blood mass.2,5,28 Interestingly, our study showed a higher likelihood of ABT in both black and Hispanic populations. Further, patients with Medicare or Medicaid were more likely to receive ABT.
Primary Diagnosis
Although patients with a primary diagnosis of osteoarthritis constitute the majority of patients who undergo shoulder arthroplasty, our analysis showed that patients with a diagnosis of proximal humerus fracture were more likely to receive ABT. This finding is reasonable given studies showing the high prevalence of proximal humerus fractures in elderly women.29,30 Similarly, patients with a humerus fracture nonunion were more likely to receive a blood transfusion, which is unsurprising given the increased complexity associated with arthroplasty in this predominately elderly population.31 Interestingly, compared with patients with osteoarthritis, patients with any one of the other primary diagnoses were more likely to require a transfusion—proximal humerus fracture being the most significant, followed by humerus fracture nonunion, avascular necrosis, rheumatoid arthritis, and rotator cuff arthropathy.
Type of Arthroplasty
Bivariate analysis revealed that 55.6% of the patients who received ABT underwent HSA; the other 44.4% underwent TSA. The effect of primary diagnosis on procedure choice likely played a role in this finding. HSA indications include humerus fracture, which has been associated with increased ABT, whereas patients with osteoarthritis requiring TSA are significantly less likely to require ABT, as reflected in this analysis.7,32-34 Previous studies have failed to show a difference in blood transfusion rates between TSA and HSA.2,4-6,35 Conversely, with confounding factors controlled for, multivariate logistic regression analysis showed that TSA was 1.2 times more likely than HSA to require ABT, which could be explained by the increased operative time, case complexity, and blood loss that may be associated with the glenoid exposure.36,37 With analysis restricted to the year 2011, patients with reverse TSAs were 1.6 times more likely than patients with anatomical TSAs to receive a blood transfusion (OR, 1.63; 95% CI, 1.50-1.79). Although this finding differs from what was previously reported, it fits given that patients having reverse TSAs are often older and may present with a more significant comorbidity profile.3 In addition, there are the increased technical surgical aspects associated with “salvage surgery” for challenging indications such as cuff arthropathy and failed previous arthroplasty.38-41
Medical Comorbidities
Patients who received ABT were more likely to present with numerous medical comorbidities. Previous studies have indicated that the presence of multiple medical comorbidities significantly increased blood transfusion rates, possibly by working synergistically.42 All studies of blood transfusion in shoulder arthroplasty concluded that lower preoperative Hb was an independent predictor.1-6 Schumer and colleagues4 reported a 4-fold increase in likelihood of blood transfusion in patients with a preoperative Hb level less than 12.5 g/dL. In addition, Millett and colleagues6 showed a 20-fold increase in likelihood of transfusion in patients with a preoperative Hb level less than 11.0 g/dL compared with patients with a level higher than 13.0 g/dL. Patients with a Hb level between 11.0 and 13.0 g/dL showed a 5-fold increase in likelihood of transfusion.6 We should note that correction of preoperative anemia through various pharmacologic methods (eg, erythropoietin, intravenous iron supplementation) has been shown to decrease postoperative transfusion rates.43,44 Although we could not include preoperative Hb levels in the present study, given inherent limitations in using NIS, our multivariate analysis showed that preoperative deficiency anemia and coagulopathy were the most significant predictors of ABT.
In addition, the multivariate logistic regression model showed that both cardiac disease and diabetes were independent predictors of ABT, confirming data reported by Ahmadi and colleagues.1 Although not as well characterized in other studies, in the current analysis multiple other medical comorbidities, including fluid and electrolyte abnormalities, weight loss, liver disease, renal failure, and chronic lung disease, had significant predictive value. Contrarily, obesity significantly decreased the odds of ABT, likely because of higher baseline blood volume in obese patients.
Patient Outcomes
Patients who undergo shoulder arthroplasty with ABT are more likely to experience adverse events or a prolonged hospital stay and are more often discharged to a nursing home or an extended-care facility. In this population, however, deaths did not occur at a significantly higher rate—similar to what was found for patients who underwent hip or knee arthroplasty with blood transfusions.45
Little has been done to investigate the effect of pharmacologic agents on the need for perioperative ABT for orthopedic shoulder procedures. Aprotinin, tranexamic acid, epoetin-α, and aminocaproic acid have all been effective in limiting ABT during the perioperative period in various orthopedic hip, knee, and spine procedures.9,46-53 Given the increased morbidity associated with ABT, it may be beneficial to use similar methods to limit blood loss in high-risk patients undergoing shoulder arthroplasty.
Study Limitations
NIS has intrinsic limitations. Given its massive volume, it is subject to errors in both data entry and clinical coding. Moreover, the database lacks data that would have been useful in our study: preoperative Hb levels, intraoperative course, number of units transfused, total blood loss, use of blood conservation techniques, transfusion protocols, and severity of comorbidities. Reverse TSA was given a unique ICD-9-CM code in October 2010, so 2011 was the only year we were able to examine the relationship between reverse TSA and transfusions. Further, our analysis was unable to identify any medications, including chronic anticoagulants or postoperative prophylaxis, that have been shown to significantly affect blood transfusion rates.54 Yet, there are obvious advantages to using the NIS database, as previously outlined across the medical landscape.
Conclusion
Our results confirmed previous findings and identified new predictors of ABT in shoulder arthroplasty in a large cohort. We examined demographics and perioperative complications while identifying predictors of ABT use. Patients who received ABT were older, female, and nonwhite and were covered by Medicare or Medicaid insurance, and many had a primary diagnosis of proximal humerus fracture. The ABT cohort had numerous medical comorbidities, including deficiency anemia and coagulopathy. Identifying this patient population is a prerequisite to educating patients while minimizing unnecessary risks and costs.
Using NIS data on a population of 422,371 patients who underwent shoulder arthroplasty, we identified the 5 likeliest predictors of ABT: fracture, fracture nonunion, deficiency anemia, coagulopathy, and avascular necrosis. Of the identified variables associated with ABT, deficiency anemia may be the most amenable to treatment; therefore, there may be benefit in delaying elective shoulder arthroplasty in this cohort. Given these findings, it is important to identify at-risk patients before surgery, with the intent to provide education and minimize risk.
1. Ahmadi S, Lawrence TM, Sahota S, et al. The incidence and risk factors for blood transfusion in revision shoulder arthroplasty: our institution’s experience and review of the literature. J Shoulder Elbow Surg. 2014;23(1):43-48.
2. Sperling JW, Duncan SF, Cofield RH, Schleck CD, Harmsen WS. Incidence and risk factors for blood transfusion in shoulder arthroplasty. J Shoulder Elbow Surg. 2005;14(6):599-601.
3. Hardy JC, Hung M, Snow BJ, et al. Blood transfusion associated with shoulder arthroplasty. J Shoulder Elbow Surg. 2013;22(2):233-239.
4. Schumer RA, Chae JS, Markert RJ, Sprott D, Crosby LA. Predicting transfusion in shoulder arthroplasty. J Shoulder Elbow Surg. 2010;19(1):91-96.
5. Gruson KI, Accousti KJ, Parsons BO, Pillai G, Flatow EL. Transfusion after shoulder arthroplasty: an analysis of rates and risk factors. J Shoulder Elbow Surg. 2009;18(2):225-230.
6. Millett PJ, Porramatikul M, Chen N, Zurakowski D, Warner JJ. Analysis of transfusion predictors in shoulder arthroplasty. J Bone Joint Surg Am. 2006;88(6):1223-1230.
7. Kim SH, Wise BL, Zhang Y, Szabo RM. Increasing incidence of shoulder arthroplasty in the United States. J Bone Joint Surg Am. 2011;93(24):2249-2254.
8. Ceccherini-Nelli L, Filipponi F, Mosca F, Campa M. The risk of contracting an infectious disease from blood transfusion. Transplantation Proc. 2004;36(3):680-682.
9. Friedman R, Homering M, Holberg G, Berkowitz SD. Allogeneic blood transfusions and postoperative infections after total hip or knee arthroplasty. J Bone Joint Surg Am. 2014;96(4):272-278.
10. Hatzidakis AM, Mendlick RM, McKillip T, Reddy RL, Garvin KL. Preoperative autologous donation for total joint arthroplasty. An analysis of risk factors for allogenic transfusion. J Bone Joint Surg Am. 2000;82(1):89-100.
11. Park JH, Rasouli MR, Mortazavi SM, Tokarski AT, Maltenfort MG, Parvizi J. Predictors of perioperative blood loss in total joint arthroplasty. J Bone Joint Surg Am. 2013;95(19):1777-1783.
12. Aderinto J, Brenkel IJ. Pre-operative predictors of the requirement for blood transfusion following total hip replacement. J Bone Joint Surg Br. 2004;86(7):970-973.
13. Browne JA, Adib F, Brown TE, Novicoff WM. Transfusion rates are increasing following total hip arthroplasty: risk factors and outcomes. J Arthroplasty. 2013;28(8 suppl):34-37.
14. Yoshihara H, Yoneoka D. Predictors of allogeneic blood transfusion in spinal fusion in the United States, 2004–2009. Spine. 2014;39(4):304-310.
15. Noticewala MS, Nyce JD, Wang W, Geller JA, Macaulay W. Predicting need for allogeneic transfusion after total knee arthroplasty. J Arthroplasty. 2012;27(6):961-967.
16. Griffin JW, Novicoff WM, Browne JA, Brockmeier SF. Obstructive sleep apnea as a risk factor after shoulder arthroplasty. J Shoulder Elbow Surg. 2013;22(12):e6-e9.
17. Maynard C, Sales AE. Changes in the use of coronary artery revascularization procedures in the Department of Veterans Affairs, the National Hospital Discharge Survey, and the Nationwide Inpatient Sample, 1991–1999. BMC Health Serv Res. 2003;3(1):12.
18. Pereira BM, Chan PH, Weinstein PR, Fishman RA. Cerebral protection during reperfusion with superoxide dismutase in focal cerebral ischemia. Adv Neurol. 1990;52:97-103.
19. Hambright D, Henderson RA, Cook C, Worrell T, Moorman CT, Bolognesi MP. A comparison of perioperative outcomes in patients with and without rheumatoid arthritis after receiving a total shoulder replacement arthroplasty. J Shoulder Elbow Surg. 2011;20(1):77-85.
20. Ponce BA, Menendez ME, Oladeji LO, Soldado F. Diabetes as a risk factor for poorer early postoperative outcomes after shoulder arthroplasty. J Shoulder Elbow Surg. 2014;23(5):671-678.
21. Pierson JL, Hannon TJ, Earles DR. A blood-conservation algorithm to reduce blood transfusions after total hip and knee arthroplasty. J Bone Joint Surg Am. 2004;86(7):1512-1518.
22. Martinez V, Monsaingeon-Lion A, Cherif K, Judet T, Chauvin M, Fletcher D. Transfusion strategy for primary knee and hip arthroplasty: impact of an algorithm to lower transfusion rates and hospital costs. Br J Anaesth. 2007;99(6):794-800.
23. Helm AT, Karski MT, Parsons SJ, Sampath JS, Bale RS. A strategy for reducing blood-transfusion requirements in elective orthopaedic surgery. Audit of an algorithm for arthroplasty of the lower limb. J Bone Joint Surg Br. 2003;85(4):484-489.
24. Watts CD, Pagnano MW. Minimising blood loss and transfusion in contemporary hip and knee arthroplasty. J Bone Joint Surg Br. 2012;94(11 suppl A):8-10.
25. Guralnik JM, Eisenstaedt RS, Ferrucci L, Klein HG, Woodman RC. Prevalence of anemia in persons 65 years and older in the United States: evidence for a high rate of unexplained anemia. Blood. 2004;104(8):2263-2268.
26. Rogers MA, Blumberg N, Heal JM, Langa KM. Utilization of blood transfusion among older adults in the United States. Transfusion. 2011;51(4):710-718.
27. Cobain TJ, Vamvakas EC, Wells A, Titlestad K. A survey of the demographics of blood use. Transfusion Med. 2007;17(1):1-15.
28. Fosco M, Di Fiore M. Factors predicting blood transfusion in different surgical procedures for degenerative spine disease. Eur Rev Med Pharmacol Sci. 2012;16(13):1853-1858.
29. Handoll HH, Ollivere BJ, Rollins KE. Interventions for treating proximal humeral fractures in adults. Cochrane Database Syst Rev. 2012;12:CD000434.
30. Neuhaus V, Swellengrebel CH, Bossen JK, Ring D. What are the factors influencing outcome among patients admitted to a hospital with a proximal humeral fracture? Clin Orthop Relat Res. 2013;471(5):1698-1706.
31. Volgas DA, Stannard JP, Alonso JE. Nonunions of the humerus. Clin Orthop Relat Res. 2004;(419):46-50.
32. Chambers L, Dines JS, Lorich DG, Dines DM. Hemiarthroplasty for proximal humerus fractures. Curr Rev Musculoskeletal Med. 2013;6(1):57-62.
33. Jain NB, Hocker S, Pietrobon R, Guller U, Bathia N, Higgins LD. Total arthroplasty versus hemiarthroplasty for glenohumeral osteoarthritis: role of provider volume. J Shoulder Elbow Surg. 2005;14(4):361-367.
34. Izquierdo R, Voloshin I, Edwards S, et al. Treatment of glenohumeral osteoarthritis. J Am Acad Orthop Surg. 2010;18(6):375-382.
35. Shields E, Iannuzzi JC, Thorsness R, Noyes K, Voloshin I. Perioperative complications after hemiarthroplasty and total shoulder arthroplasty are equivalent. J Shoulder Elbow Surg. 2014;23(10):1449-1453.
36. Gartsman GM, Roddey TS, Hammerman SM. Shoulder arthroplasty with or without resurfacing of the glenoid in patients who have osteoarthritis. J Bone Joint Surg Am. 2000;82(1):26-34.
37. Singh A, Yian EH, Dillon MT, Takayanagi M, Burke MF, Navarro RA. The effect of surgeon and hospital volume on shoulder arthroplasty perioperative quality metrics. J Shoulder Elbow Surg. 2014;23(8):1187-1194.
38. Groh GI, Groh GM. Complications rates, reoperation rates, and the learning curve in reverse shoulder arthroplasty. J Shoulder Elbow Surg. 2014;23(3):388-394.
39. Boileau P, Gonzalez JF, Chuinard C, Bicknell R, Walch G. Reverse total shoulder arthroplasty after failed rotator cuff surgery. J Shoulder Elbow Surg. 2009;18(4):600-606.
40. Boileau P, Watkinson D, Hatzidakis AM, Hovorka I. Neer Award 2005: the Grammont reverse shoulder prosthesis: results in cuff tear arthritis, fracture sequelae, and revision arthroplasty. J Shoulder Elbow Surg. 2006;15(5):527-540.
41. Boileau P, Watkinson DJ, Hatzidakis AM, Balg F. Grammont reverse prosthesis: design, rationale, and biomechanics. J Shoulder Elbow Surg. 2005;14(1 suppl S):147S-161S.
42. Pola E, Papaleo P, Santoliquido A, Gasparini G, Aulisa L, De Santis E. Clinical factors associated with an increased risk of perioperative blood transfusion in nonanemic patients undergoing total hip arthroplasty. J Bone Joint Surg Am. 2004;86(1):57-61.
43. Lin DM, Lin ES, Tran MH. Efficacy and safety of erythropoietin and intravenous iron in perioperative blood management: a systematic review. Transfusion Med Rev. 2013;27(4):221-234.
44. Muñoz M, Gómez-Ramírez S, Cuenca J, et al. Very-short-term perioperative intravenous iron administration and postoperative outcome in major orthopedic surgery: a pooled analysis of observational data from 2547 patients. Transfusion. 2014;54(2):289-299.
45. Danninger T, Rasul R, Poeran J, et al. Blood transfusions in total hip and knee arthroplasty: an analysis of outcomes. ScientificWorldJournal. 2014;2014:623460.
46. Baldus CR, Bridwell KH, Lenke LG, Okubadejo GO. Can we safely reduce blood loss during lumbar pedicle subtraction osteotomy procedures using tranexamic acid or aprotinin? A comparative study with controls. Spine. 2010;35(2):235-239.
47. Chang CH, Chang Y, Chen DW, Ueng SW, Lee MS. Topical tranexamic acid reduces blood loss and transfusion rates associated with primary total hip arthroplasty. Clin Orthop Relat Res. 2014;472(5):1552-1557.
48. Delasotta LA, Orozco F, Jafari SM, Blair JL, Ong A. Should we use preoperative epoetin-alpha in the mildly anemic patient undergoing simultaneous total knee arthroplasty? Open Orthop J. 2013;7:47-50.
49. Delasotta LA, Rangavajjula A, Frank ML, Blair J, Orozco F, Ong A. The use of preoperative epoetin-alpha in revision hip arthroplasty. Open Orthop J. 2012;6:179-183.
50. Kelley TC, Tucker KK, Adams MJ, Dalury DF. Use of tranexamic acid results in decreased blood loss and decreased transfusions in patients undergoing staged bilateral total knee arthroplasty. Transfusion. 2014;54(1):26-30.
51. Martin JG, Cassatt KB, Kincaid-Cinnamon KA, Westendorf DS, Garton AS, Lemke JH. Topical administration of tranexamic acid in primary total hip and total knee arthroplasty. J Arthroplasty. 2014;29(5):889-894.
52. Tzortzopoulou A, Cepeda MS, Schumann R, Carr DB. Antifibrinolytic agents for reducing blood loss in scoliosis surgery in children. Cochrane Database Syst Rev. 2008(3):CD006883.
53. Zhang H, Chen J, Chen F, Que W. The effect of tranexamic acid on blood loss and use of blood products in total knee arthroplasty: a meta-analysis. Knee Surg Sports Traumatol Arthrosc. 2012;20(9):1742-1752.
54. Bong MR, Patel V, Chang E, Issack PS, Hebert R, Di Cesare PE. Risks associated with blood transfusion after total knee arthroplasty. J Arthroplasty. 2004;19(3):281-287.
In shoulder arthroplasty, it is not uncommon for patients to receive postoperative blood transfusions; rates range from 7% to 43%.1-6 Allogeneic blood transfusions (ABTs) are costly and not entirely free of risks.7 The risk for infection has decreased because of improved screening and risk reduction strategies, but there are still significant risks associated with ABTs, such as clerical errors, acute and delayed hemolytic reactions, graft-versus-host reactions, transfusion-related acute lung injury, and anaphylaxis.8-10 As use of shoulder arthroplasty continues to increase, the importance of minimizing unnecessary transfusions is growing as well.7
Predictive factors for ABT have been explored in other orthopedic settings, yet little has been done in shoulder arthroplasty.1-6,11-15 Previous shoulder arthroplasty studies have shown that low preoperative hemoglobin (Hb) levels are independent risk factors for postoperative blood transfusion. However, there is debate over the significance of other variables, such as procedure type, age, sex, and medical comorbidities. Further, prior studies were limited by relatively small samples from single institutions; the largest series included fewer than 600 patients.1-6
We conducted a study to determine predictors of ABT in a large cohort of patients admitted to US hospitals for shoulder arthroplasty. We also wanted to evaluate the effect of ABT on postoperative outcomes, including inpatient mortality, adverse events, prolonged hospital stay, and nonroutine discharge. According to the null hypothesis, in shoulder arthroplasty there will be no difference in risk factors between patients who require ABT and those who did not, after accounting for confounding variables.
Materials and Methods
This study was exempt from institutional review board approval, as all data were appropriately deidentified before use in this project. We used the Nationwide Inpatient Sample (NIS) to retrospectively study the period 2002–2011, from which all demographic, clinical, and resource use data were derived.16 NIS, an annual survey conducted by the Agency for Healthcare Research and Quality (AHRQ) since 1988, has generated a huge amount of data, forming the largest all-payer inpatient care database in the United States. Yearly samples contain discharge data from about 8 million hospital stays at more than 1000 hospitals across 46 states, approximating a 20% random sample of all hospital discharges at participating institutions.17 These data are then weighted to generate statistically valid national estimates.
The NIS database uses International Classification of Diseases, Ninth Edition, Clinical Modification (ICD-9-CM) codes to identify 15 medical diagnoses up to the year 2008 and a maximum of 25 medical diagnoses and 15 procedures thereafter. In addition, the database includes information on patient and hospital characteristics as well as inpatient outcomes such as length of stay, total hospitalization charges, and discharge disposition.18,19 Given its large sample size and data volume, NIS is a powerful tool in the analysis of data associated with a multitude of medical diagnoses and procedures.20
We used the NIS database to study a population of 422,371 patients (age, >18 years) who underwent total shoulder arthroplasty (TSA) or hemiarthroplasty (HSA) between 2002 and 2011. ICD-9-CM procedure codes for TSA (81.80, 81.88) and HSA (81.81) were used to identify this population. We also analyzed data for reverse TSA for the year 2011. Then we divided our target population into 2 different cohorts: patients who did not receive any blood transfusion products and patients who received a transfusion of allogeneic packed cells (ICD-9-CM code 99.04 was used to identify the latter cohort).
In this study, normal distribution of the dataset was assumed, given the large sample size. The 2 cohorts were evaluated through bivariate analysis using the Pearson χ2 test for categorical data and the independent-samples t test for continuous data. The extent to which diagnosis, age, race, sex, and medical comorbidities were predictive of blood transfusion after TSA or HSA was evaluated through multivariate binary logistic regression analysis. Statistical significance was set at P < .05. All statistical analyses and data modeling were performed with SPSS Version 22.0.
Results
Using the NIS database, we stratified an estimated 422,371 patients who presented for shoulder arthroplasty between January 1, 2002, and December 31, 2011, into a TSA cohort (59.3%) and an HSA cohort (40.7%). Eight percent (33,889) of all patients received an ABT; the proportion of patients who received ABT was higher (P < .001) for the HSA cohort (55.6%) than the TSA cohort (39.4%). Further, the rate of ABT after shoulder arthroplasty showed an upward inclination (Figure).
Demographically, patients who received ABT tended (P < .001) to be older (74±11 years vs 68±11 years) and of a minority race (black or Hispanic) and to fall in either the lowest range of median household income (21.5% vs 20.7%; ≤$38,999) or the highest (27.3% vs 25.4%; ≥$63,000). Shoulder arthroplasty with ABT occurred more often (P < .001) at hospitals that were urban (13.3% vs 11.3%), medium in size (27.3% vs 23.4%), and nonteaching (56.2% vs 54.3%). In addition, ABT was used more often (P < .001) in patients with a primary diagnosis of fracture (43.1% vs 14.3%) or fracture nonunion (4.4% vs 2.1%). These groups also had a longer (P < .001) hospital stay (5.0±4.3 days vs 2.5±2.2 days). Table 1 summarizes these findings.
The 2 cohorts were then analyzed for presence of medical comorbidities (Table 2). Patients who required ABT during shoulder arthroplasty had a significantly (P < .001) higher prevalence of congestive heart failure, chronic lung disease, hypertension, uncomplicated and complicated diabetes mellitus, liver disease, renal failure, fluid and electrolyte disorders, pulmonary circulatory disease, weight loss, coagulopathy, and deficiency anemia.
In multivariate regression modeling (Table 3), demographic predictors of ABT (P < .001) included increasing age (odds ratio [OR], 1.03 per year; 95% confidence interval [95% CI], 1.03-1.03), female sex (OR, 1.55; 95% CI, 1.51-1.60), and minority race (black or Hispanic). Odds of requiring ABT were higher for patients with Medicare (OR, 1.25; 95% CI, 1.20-1.30) and patients with Medicaid (OR, 1.63; 95% CI, 1.51-1.77) than for patients with private insurance.
ABT was more likely to be required (P < .001) in patients with a primary diagnosis of fracture (OR, 4.49; 95% CI, 4.34-4.65), avascular necrosis (OR, 2.06; 95% CI, 1.91-2.22), rheumatoid arthritis (OR, 1.91; 95% CI, 1.72-2.12), fracture nonunion (OR, 3.55; 95% CI, 3.33-3.79), or rotator cuff arthropathy (OR, 1.47; 95% CI, 1.41-1.54) than for patients with osteoarthritis. Moreover, compared with patients having HSA, patients having TSA were more likely to require ABT (OR, 1.20; 95% CI, 1.17-1.24). According to the analysis restricted to the year 2011, compared with patients having anatomical TSAs, patients having reverse TSAs were 1.6 times more likely (P < .001) to require ABT (OR, 1.63; 95% CI, 1.50-1.79).
With the exception of obesity, all comorbidities were significant (P < .001) independent predictors of ABT after shoulder arthroplasty: deficiency anemia (OR, 3.42; 95% CI, 3.32-3.52), coagulopathy (OR, 2.54; 95% CI, 2.36-2.73), fluid and electrolyte disorders (OR, 1.91; 95% CI, 1.84-1.97), and weight loss (OR, 1.78; 95% CI, 1.58-2.00).
Patients who received ABT were more likely to experience adverse events (OR, 1.74; 95% CI, 1.68-1.81), prolonged hospital stay (OR, 3.21; 95% CI, 3.12-3.30), and nonroutine discharge (OR, 1.77; 95% CI, 1.72-1.82) (Table 4). There was no difference in mortality between the 2 cohorts.
Discussion
There is an abundance of literature on blood transfusions in hip and knee arthroplasty, but there are few articles on ABT in shoulder arthroplasty, and they all report data from single institutions with relatively low caseloads.1,2,11-13,15,21 In the present study, we investigated ABT in shoulder arthroplasty from the perspective of a multi-institutional database with a caseload of more than 400,000. Given the rapidly increasing rates of shoulder arthroplasty, it is important to further examine this issue to minimize unnecessary blood transfusion and its associated risks and costs.7
We found that 8% of patients who had shoulder arthroplasty received ABT, which is consistent with previously reported transfusion rates (range, 7%-43%).1-6 Rates of ABT after shoulder arthroplasty have continued to rise. The exception, a decrease during the year 2010, can be explained by increased efforts to more rigidly follow transfusion indication guidelines to reduce the number of potentially unnecessary ABTs.21-24 Our study also identified numerous significant independent predictors of ABT in shoulder arthroplasty: age, sex, race, insurance status, procedure type, primary diagnoses, and multiple medical comorbidities.
Demographics
According to our analysis, more than 80% of patients who received ABT were over age 65 years, which aligns with what several other studies have demonstrated: Increasing age is a predictor of ABT, despite higher rates of comorbidities and lower preoperative Hb levels in this population.1,2,4,5,25-27 Consistent with previous work, female sex was predictive of ABT.2,5 It has been suggested that females are more likely predisposed to ABT because of lower preoperative Hb and smaller blood mass.2,5,28 Interestingly, our study showed a higher likelihood of ABT in both black and Hispanic populations. Further, patients with Medicare or Medicaid were more likely to receive ABT.
Primary Diagnosis
Although patients with a primary diagnosis of osteoarthritis constitute the majority of patients who undergo shoulder arthroplasty, our analysis showed that patients with a diagnosis of proximal humerus fracture were more likely to receive ABT. This finding is reasonable given studies showing the high prevalence of proximal humerus fractures in elderly women.29,30 Similarly, patients with a humerus fracture nonunion were more likely to receive a blood transfusion, which is unsurprising given the increased complexity associated with arthroplasty in this predominately elderly population.31 Interestingly, compared with patients with osteoarthritis, patients with any one of the other primary diagnoses were more likely to require a transfusion—proximal humerus fracture being the most significant, followed by humerus fracture nonunion, avascular necrosis, rheumatoid arthritis, and rotator cuff arthropathy.
Type of Arthroplasty
Bivariate analysis revealed that 55.6% of the patients who received ABT underwent HSA; the other 44.4% underwent TSA. The effect of primary diagnosis on procedure choice likely played a role in this finding. HSA indications include humerus fracture, which has been associated with increased ABT, whereas patients with osteoarthritis requiring TSA are significantly less likely to require ABT, as reflected in this analysis.7,32-34 Previous studies have failed to show a difference in blood transfusion rates between TSA and HSA.2,4-6,35 Conversely, with confounding factors controlled for, multivariate logistic regression analysis showed that TSA was 1.2 times more likely than HSA to require ABT, which could be explained by the increased operative time, case complexity, and blood loss that may be associated with the glenoid exposure.36,37 With analysis restricted to the year 2011, patients with reverse TSAs were 1.6 times more likely than patients with anatomical TSAs to receive a blood transfusion (OR, 1.63; 95% CI, 1.50-1.79). Although this finding differs from what was previously reported, it fits given that patients having reverse TSAs are often older and may present with a more significant comorbidity profile.3 In addition, there are the increased technical surgical aspects associated with “salvage surgery” for challenging indications such as cuff arthropathy and failed previous arthroplasty.38-41
Medical Comorbidities
Patients who received ABT were more likely to present with numerous medical comorbidities. Previous studies have indicated that the presence of multiple medical comorbidities significantly increased blood transfusion rates, possibly by working synergistically.42 All studies of blood transfusion in shoulder arthroplasty concluded that lower preoperative Hb was an independent predictor.1-6 Schumer and colleagues4 reported a 4-fold increase in likelihood of blood transfusion in patients with a preoperative Hb level less than 12.5 g/dL. In addition, Millett and colleagues6 showed a 20-fold increase in likelihood of transfusion in patients with a preoperative Hb level less than 11.0 g/dL compared with patients with a level higher than 13.0 g/dL. Patients with a Hb level between 11.0 and 13.0 g/dL showed a 5-fold increase in likelihood of transfusion.6 We should note that correction of preoperative anemia through various pharmacologic methods (eg, erythropoietin, intravenous iron supplementation) has been shown to decrease postoperative transfusion rates.43,44 Although we could not include preoperative Hb levels in the present study, given inherent limitations in using NIS, our multivariate analysis showed that preoperative deficiency anemia and coagulopathy were the most significant predictors of ABT.
In addition, the multivariate logistic regression model showed that both cardiac disease and diabetes were independent predictors of ABT, confirming data reported by Ahmadi and colleagues.1 Although not as well characterized in other studies, in the current analysis multiple other medical comorbidities, including fluid and electrolyte abnormalities, weight loss, liver disease, renal failure, and chronic lung disease, had significant predictive value. Contrarily, obesity significantly decreased the odds of ABT, likely because of higher baseline blood volume in obese patients.
Patient Outcomes
Patients who undergo shoulder arthroplasty with ABT are more likely to experience adverse events or a prolonged hospital stay and are more often discharged to a nursing home or an extended-care facility. In this population, however, deaths did not occur at a significantly higher rate—similar to what was found for patients who underwent hip or knee arthroplasty with blood transfusions.45
Little has been done to investigate the effect of pharmacologic agents on the need for perioperative ABT for orthopedic shoulder procedures. Aprotinin, tranexamic acid, epoetin-α, and aminocaproic acid have all been effective in limiting ABT during the perioperative period in various orthopedic hip, knee, and spine procedures.9,46-53 Given the increased morbidity associated with ABT, it may be beneficial to use similar methods to limit blood loss in high-risk patients undergoing shoulder arthroplasty.
Study Limitations
NIS has intrinsic limitations. Given its massive volume, it is subject to errors in both data entry and clinical coding. Moreover, the database lacks data that would have been useful in our study: preoperative Hb levels, intraoperative course, number of units transfused, total blood loss, use of blood conservation techniques, transfusion protocols, and severity of comorbidities. Reverse TSA was given a unique ICD-9-CM code in October 2010, so 2011 was the only year we were able to examine the relationship between reverse TSA and transfusions. Further, our analysis was unable to identify any medications, including chronic anticoagulants or postoperative prophylaxis, that have been shown to significantly affect blood transfusion rates.54 Yet, there are obvious advantages to using the NIS database, as previously outlined across the medical landscape.
Conclusion
Our results confirmed previous findings and identified new predictors of ABT in shoulder arthroplasty in a large cohort. We examined demographics and perioperative complications while identifying predictors of ABT use. Patients who received ABT were older, female, and nonwhite and were covered by Medicare or Medicaid insurance, and many had a primary diagnosis of proximal humerus fracture. The ABT cohort had numerous medical comorbidities, including deficiency anemia and coagulopathy. Identifying this patient population is a prerequisite to educating patients while minimizing unnecessary risks and costs.
Using NIS data on a population of 422,371 patients who underwent shoulder arthroplasty, we identified the 5 likeliest predictors of ABT: fracture, fracture nonunion, deficiency anemia, coagulopathy, and avascular necrosis. Of the identified variables associated with ABT, deficiency anemia may be the most amenable to treatment; therefore, there may be benefit in delaying elective shoulder arthroplasty in this cohort. Given these findings, it is important to identify at-risk patients before surgery, with the intent to provide education and minimize risk.
In shoulder arthroplasty, it is not uncommon for patients to receive postoperative blood transfusions; rates range from 7% to 43%.1-6 Allogeneic blood transfusions (ABTs) are costly and not entirely free of risks.7 The risk for infection has decreased because of improved screening and risk reduction strategies, but there are still significant risks associated with ABTs, such as clerical errors, acute and delayed hemolytic reactions, graft-versus-host reactions, transfusion-related acute lung injury, and anaphylaxis.8-10 As use of shoulder arthroplasty continues to increase, the importance of minimizing unnecessary transfusions is growing as well.7
Predictive factors for ABT have been explored in other orthopedic settings, yet little has been done in shoulder arthroplasty.1-6,11-15 Previous shoulder arthroplasty studies have shown that low preoperative hemoglobin (Hb) levels are independent risk factors for postoperative blood transfusion. However, there is debate over the significance of other variables, such as procedure type, age, sex, and medical comorbidities. Further, prior studies were limited by relatively small samples from single institutions; the largest series included fewer than 600 patients.1-6
We conducted a study to determine predictors of ABT in a large cohort of patients admitted to US hospitals for shoulder arthroplasty. We also wanted to evaluate the effect of ABT on postoperative outcomes, including inpatient mortality, adverse events, prolonged hospital stay, and nonroutine discharge. According to the null hypothesis, in shoulder arthroplasty there will be no difference in risk factors between patients who require ABT and those who did not, after accounting for confounding variables.
Materials and Methods
This study was exempt from institutional review board approval, as all data were appropriately deidentified before use in this project. We used the Nationwide Inpatient Sample (NIS) to retrospectively study the period 2002–2011, from which all demographic, clinical, and resource use data were derived.16 NIS, an annual survey conducted by the Agency for Healthcare Research and Quality (AHRQ) since 1988, has generated a huge amount of data, forming the largest all-payer inpatient care database in the United States. Yearly samples contain discharge data from about 8 million hospital stays at more than 1000 hospitals across 46 states, approximating a 20% random sample of all hospital discharges at participating institutions.17 These data are then weighted to generate statistically valid national estimates.
The NIS database uses International Classification of Diseases, Ninth Edition, Clinical Modification (ICD-9-CM) codes to identify 15 medical diagnoses up to the year 2008 and a maximum of 25 medical diagnoses and 15 procedures thereafter. In addition, the database includes information on patient and hospital characteristics as well as inpatient outcomes such as length of stay, total hospitalization charges, and discharge disposition.18,19 Given its large sample size and data volume, NIS is a powerful tool in the analysis of data associated with a multitude of medical diagnoses and procedures.20
We used the NIS database to study a population of 422,371 patients (age, >18 years) who underwent total shoulder arthroplasty (TSA) or hemiarthroplasty (HSA) between 2002 and 2011. ICD-9-CM procedure codes for TSA (81.80, 81.88) and HSA (81.81) were used to identify this population. We also analyzed data for reverse TSA for the year 2011. Then we divided our target population into 2 different cohorts: patients who did not receive any blood transfusion products and patients who received a transfusion of allogeneic packed cells (ICD-9-CM code 99.04 was used to identify the latter cohort).
In this study, normal distribution of the dataset was assumed, given the large sample size. The 2 cohorts were evaluated through bivariate analysis using the Pearson χ2 test for categorical data and the independent-samples t test for continuous data. The extent to which diagnosis, age, race, sex, and medical comorbidities were predictive of blood transfusion after TSA or HSA was evaluated through multivariate binary logistic regression analysis. Statistical significance was set at P < .05. All statistical analyses and data modeling were performed with SPSS Version 22.0.
Results
Using the NIS database, we stratified an estimated 422,371 patients who presented for shoulder arthroplasty between January 1, 2002, and December 31, 2011, into a TSA cohort (59.3%) and an HSA cohort (40.7%). Eight percent (33,889) of all patients received an ABT; the proportion of patients who received ABT was higher (P < .001) for the HSA cohort (55.6%) than the TSA cohort (39.4%). Further, the rate of ABT after shoulder arthroplasty showed an upward inclination (Figure).
Demographically, patients who received ABT tended (P < .001) to be older (74±11 years vs 68±11 years) and of a minority race (black or Hispanic) and to fall in either the lowest range of median household income (21.5% vs 20.7%; ≤$38,999) or the highest (27.3% vs 25.4%; ≥$63,000). Shoulder arthroplasty with ABT occurred more often (P < .001) at hospitals that were urban (13.3% vs 11.3%), medium in size (27.3% vs 23.4%), and nonteaching (56.2% vs 54.3%). In addition, ABT was used more often (P < .001) in patients with a primary diagnosis of fracture (43.1% vs 14.3%) or fracture nonunion (4.4% vs 2.1%). These groups also had a longer (P < .001) hospital stay (5.0±4.3 days vs 2.5±2.2 days). Table 1 summarizes these findings.
The 2 cohorts were then analyzed for presence of medical comorbidities (Table 2). Patients who required ABT during shoulder arthroplasty had a significantly (P < .001) higher prevalence of congestive heart failure, chronic lung disease, hypertension, uncomplicated and complicated diabetes mellitus, liver disease, renal failure, fluid and electrolyte disorders, pulmonary circulatory disease, weight loss, coagulopathy, and deficiency anemia.
In multivariate regression modeling (Table 3), demographic predictors of ABT (P < .001) included increasing age (odds ratio [OR], 1.03 per year; 95% confidence interval [95% CI], 1.03-1.03), female sex (OR, 1.55; 95% CI, 1.51-1.60), and minority race (black or Hispanic). Odds of requiring ABT were higher for patients with Medicare (OR, 1.25; 95% CI, 1.20-1.30) and patients with Medicaid (OR, 1.63; 95% CI, 1.51-1.77) than for patients with private insurance.
ABT was more likely to be required (P < .001) in patients with a primary diagnosis of fracture (OR, 4.49; 95% CI, 4.34-4.65), avascular necrosis (OR, 2.06; 95% CI, 1.91-2.22), rheumatoid arthritis (OR, 1.91; 95% CI, 1.72-2.12), fracture nonunion (OR, 3.55; 95% CI, 3.33-3.79), or rotator cuff arthropathy (OR, 1.47; 95% CI, 1.41-1.54) than for patients with osteoarthritis. Moreover, compared with patients having HSA, patients having TSA were more likely to require ABT (OR, 1.20; 95% CI, 1.17-1.24). According to the analysis restricted to the year 2011, compared with patients having anatomical TSAs, patients having reverse TSAs were 1.6 times more likely (P < .001) to require ABT (OR, 1.63; 95% CI, 1.50-1.79).
With the exception of obesity, all comorbidities were significant (P < .001) independent predictors of ABT after shoulder arthroplasty: deficiency anemia (OR, 3.42; 95% CI, 3.32-3.52), coagulopathy (OR, 2.54; 95% CI, 2.36-2.73), fluid and electrolyte disorders (OR, 1.91; 95% CI, 1.84-1.97), and weight loss (OR, 1.78; 95% CI, 1.58-2.00).
Patients who received ABT were more likely to experience adverse events (OR, 1.74; 95% CI, 1.68-1.81), prolonged hospital stay (OR, 3.21; 95% CI, 3.12-3.30), and nonroutine discharge (OR, 1.77; 95% CI, 1.72-1.82) (Table 4). There was no difference in mortality between the 2 cohorts.
Discussion
There is an abundance of literature on blood transfusions in hip and knee arthroplasty, but there are few articles on ABT in shoulder arthroplasty, and they all report data from single institutions with relatively low caseloads.1,2,11-13,15,21 In the present study, we investigated ABT in shoulder arthroplasty from the perspective of a multi-institutional database with a caseload of more than 400,000. Given the rapidly increasing rates of shoulder arthroplasty, it is important to further examine this issue to minimize unnecessary blood transfusion and its associated risks and costs.7
We found that 8% of patients who had shoulder arthroplasty received ABT, which is consistent with previously reported transfusion rates (range, 7%-43%).1-6 Rates of ABT after shoulder arthroplasty have continued to rise. The exception, a decrease during the year 2010, can be explained by increased efforts to more rigidly follow transfusion indication guidelines to reduce the number of potentially unnecessary ABTs.21-24 Our study also identified numerous significant independent predictors of ABT in shoulder arthroplasty: age, sex, race, insurance status, procedure type, primary diagnoses, and multiple medical comorbidities.
Demographics
According to our analysis, more than 80% of patients who received ABT were over age 65 years, which aligns with what several other studies have demonstrated: Increasing age is a predictor of ABT, despite higher rates of comorbidities and lower preoperative Hb levels in this population.1,2,4,5,25-27 Consistent with previous work, female sex was predictive of ABT.2,5 It has been suggested that females are more likely predisposed to ABT because of lower preoperative Hb and smaller blood mass.2,5,28 Interestingly, our study showed a higher likelihood of ABT in both black and Hispanic populations. Further, patients with Medicare or Medicaid were more likely to receive ABT.
Primary Diagnosis
Although patients with a primary diagnosis of osteoarthritis constitute the majority of patients who undergo shoulder arthroplasty, our analysis showed that patients with a diagnosis of proximal humerus fracture were more likely to receive ABT. This finding is reasonable given studies showing the high prevalence of proximal humerus fractures in elderly women.29,30 Similarly, patients with a humerus fracture nonunion were more likely to receive a blood transfusion, which is unsurprising given the increased complexity associated with arthroplasty in this predominately elderly population.31 Interestingly, compared with patients with osteoarthritis, patients with any one of the other primary diagnoses were more likely to require a transfusion—proximal humerus fracture being the most significant, followed by humerus fracture nonunion, avascular necrosis, rheumatoid arthritis, and rotator cuff arthropathy.
Type of Arthroplasty
Bivariate analysis revealed that 55.6% of the patients who received ABT underwent HSA; the other 44.4% underwent TSA. The effect of primary diagnosis on procedure choice likely played a role in this finding. HSA indications include humerus fracture, which has been associated with increased ABT, whereas patients with osteoarthritis requiring TSA are significantly less likely to require ABT, as reflected in this analysis.7,32-34 Previous studies have failed to show a difference in blood transfusion rates between TSA and HSA.2,4-6,35 Conversely, with confounding factors controlled for, multivariate logistic regression analysis showed that TSA was 1.2 times more likely than HSA to require ABT, which could be explained by the increased operative time, case complexity, and blood loss that may be associated with the glenoid exposure.36,37 With analysis restricted to the year 2011, patients with reverse TSAs were 1.6 times more likely than patients with anatomical TSAs to receive a blood transfusion (OR, 1.63; 95% CI, 1.50-1.79). Although this finding differs from what was previously reported, it fits given that patients having reverse TSAs are often older and may present with a more significant comorbidity profile.3 In addition, there are the increased technical surgical aspects associated with “salvage surgery” for challenging indications such as cuff arthropathy and failed previous arthroplasty.38-41
Medical Comorbidities
Patients who received ABT were more likely to present with numerous medical comorbidities. Previous studies have indicated that the presence of multiple medical comorbidities significantly increased blood transfusion rates, possibly by working synergistically.42 All studies of blood transfusion in shoulder arthroplasty concluded that lower preoperative Hb was an independent predictor.1-6 Schumer and colleagues4 reported a 4-fold increase in likelihood of blood transfusion in patients with a preoperative Hb level less than 12.5 g/dL. In addition, Millett and colleagues6 showed a 20-fold increase in likelihood of transfusion in patients with a preoperative Hb level less than 11.0 g/dL compared with patients with a level higher than 13.0 g/dL. Patients with a Hb level between 11.0 and 13.0 g/dL showed a 5-fold increase in likelihood of transfusion.6 We should note that correction of preoperative anemia through various pharmacologic methods (eg, erythropoietin, intravenous iron supplementation) has been shown to decrease postoperative transfusion rates.43,44 Although we could not include preoperative Hb levels in the present study, given inherent limitations in using NIS, our multivariate analysis showed that preoperative deficiency anemia and coagulopathy were the most significant predictors of ABT.
In addition, the multivariate logistic regression model showed that both cardiac disease and diabetes were independent predictors of ABT, confirming data reported by Ahmadi and colleagues.1 Although not as well characterized in other studies, in the current analysis multiple other medical comorbidities, including fluid and electrolyte abnormalities, weight loss, liver disease, renal failure, and chronic lung disease, had significant predictive value. Contrarily, obesity significantly decreased the odds of ABT, likely because of higher baseline blood volume in obese patients.
Patient Outcomes
Patients who undergo shoulder arthroplasty with ABT are more likely to experience adverse events or a prolonged hospital stay and are more often discharged to a nursing home or an extended-care facility. In this population, however, deaths did not occur at a significantly higher rate—similar to what was found for patients who underwent hip or knee arthroplasty with blood transfusions.45
Little has been done to investigate the effect of pharmacologic agents on the need for perioperative ABT for orthopedic shoulder procedures. Aprotinin, tranexamic acid, epoetin-α, and aminocaproic acid have all been effective in limiting ABT during the perioperative period in various orthopedic hip, knee, and spine procedures.9,46-53 Given the increased morbidity associated with ABT, it may be beneficial to use similar methods to limit blood loss in high-risk patients undergoing shoulder arthroplasty.
Study Limitations
NIS has intrinsic limitations. Given its massive volume, it is subject to errors in both data entry and clinical coding. Moreover, the database lacks data that would have been useful in our study: preoperative Hb levels, intraoperative course, number of units transfused, total blood loss, use of blood conservation techniques, transfusion protocols, and severity of comorbidities. Reverse TSA was given a unique ICD-9-CM code in October 2010, so 2011 was the only year we were able to examine the relationship between reverse TSA and transfusions. Further, our analysis was unable to identify any medications, including chronic anticoagulants or postoperative prophylaxis, that have been shown to significantly affect blood transfusion rates.54 Yet, there are obvious advantages to using the NIS database, as previously outlined across the medical landscape.
Conclusion
Our results confirmed previous findings and identified new predictors of ABT in shoulder arthroplasty in a large cohort. We examined demographics and perioperative complications while identifying predictors of ABT use. Patients who received ABT were older, female, and nonwhite and were covered by Medicare or Medicaid insurance, and many had a primary diagnosis of proximal humerus fracture. The ABT cohort had numerous medical comorbidities, including deficiency anemia and coagulopathy. Identifying this patient population is a prerequisite to educating patients while minimizing unnecessary risks and costs.
Using NIS data on a population of 422,371 patients who underwent shoulder arthroplasty, we identified the 5 likeliest predictors of ABT: fracture, fracture nonunion, deficiency anemia, coagulopathy, and avascular necrosis. Of the identified variables associated with ABT, deficiency anemia may be the most amenable to treatment; therefore, there may be benefit in delaying elective shoulder arthroplasty in this cohort. Given these findings, it is important to identify at-risk patients before surgery, with the intent to provide education and minimize risk.
1. Ahmadi S, Lawrence TM, Sahota S, et al. The incidence and risk factors for blood transfusion in revision shoulder arthroplasty: our institution’s experience and review of the literature. J Shoulder Elbow Surg. 2014;23(1):43-48.
2. Sperling JW, Duncan SF, Cofield RH, Schleck CD, Harmsen WS. Incidence and risk factors for blood transfusion in shoulder arthroplasty. J Shoulder Elbow Surg. 2005;14(6):599-601.
3. Hardy JC, Hung M, Snow BJ, et al. Blood transfusion associated with shoulder arthroplasty. J Shoulder Elbow Surg. 2013;22(2):233-239.
4. Schumer RA, Chae JS, Markert RJ, Sprott D, Crosby LA. Predicting transfusion in shoulder arthroplasty. J Shoulder Elbow Surg. 2010;19(1):91-96.
5. Gruson KI, Accousti KJ, Parsons BO, Pillai G, Flatow EL. Transfusion after shoulder arthroplasty: an analysis of rates and risk factors. J Shoulder Elbow Surg. 2009;18(2):225-230.
6. Millett PJ, Porramatikul M, Chen N, Zurakowski D, Warner JJ. Analysis of transfusion predictors in shoulder arthroplasty. J Bone Joint Surg Am. 2006;88(6):1223-1230.
7. Kim SH, Wise BL, Zhang Y, Szabo RM. Increasing incidence of shoulder arthroplasty in the United States. J Bone Joint Surg Am. 2011;93(24):2249-2254.
8. Ceccherini-Nelli L, Filipponi F, Mosca F, Campa M. The risk of contracting an infectious disease from blood transfusion. Transplantation Proc. 2004;36(3):680-682.
9. Friedman R, Homering M, Holberg G, Berkowitz SD. Allogeneic blood transfusions and postoperative infections after total hip or knee arthroplasty. J Bone Joint Surg Am. 2014;96(4):272-278.
10. Hatzidakis AM, Mendlick RM, McKillip T, Reddy RL, Garvin KL. Preoperative autologous donation for total joint arthroplasty. An analysis of risk factors for allogenic transfusion. J Bone Joint Surg Am. 2000;82(1):89-100.
11. Park JH, Rasouli MR, Mortazavi SM, Tokarski AT, Maltenfort MG, Parvizi J. Predictors of perioperative blood loss in total joint arthroplasty. J Bone Joint Surg Am. 2013;95(19):1777-1783.
12. Aderinto J, Brenkel IJ. Pre-operative predictors of the requirement for blood transfusion following total hip replacement. J Bone Joint Surg Br. 2004;86(7):970-973.
13. Browne JA, Adib F, Brown TE, Novicoff WM. Transfusion rates are increasing following total hip arthroplasty: risk factors and outcomes. J Arthroplasty. 2013;28(8 suppl):34-37.
14. Yoshihara H, Yoneoka D. Predictors of allogeneic blood transfusion in spinal fusion in the United States, 2004–2009. Spine. 2014;39(4):304-310.
15. Noticewala MS, Nyce JD, Wang W, Geller JA, Macaulay W. Predicting need for allogeneic transfusion after total knee arthroplasty. J Arthroplasty. 2012;27(6):961-967.
16. Griffin JW, Novicoff WM, Browne JA, Brockmeier SF. Obstructive sleep apnea as a risk factor after shoulder arthroplasty. J Shoulder Elbow Surg. 2013;22(12):e6-e9.
17. Maynard C, Sales AE. Changes in the use of coronary artery revascularization procedures in the Department of Veterans Affairs, the National Hospital Discharge Survey, and the Nationwide Inpatient Sample, 1991–1999. BMC Health Serv Res. 2003;3(1):12.
18. Pereira BM, Chan PH, Weinstein PR, Fishman RA. Cerebral protection during reperfusion with superoxide dismutase in focal cerebral ischemia. Adv Neurol. 1990;52:97-103.
19. Hambright D, Henderson RA, Cook C, Worrell T, Moorman CT, Bolognesi MP. A comparison of perioperative outcomes in patients with and without rheumatoid arthritis after receiving a total shoulder replacement arthroplasty. J Shoulder Elbow Surg. 2011;20(1):77-85.
20. Ponce BA, Menendez ME, Oladeji LO, Soldado F. Diabetes as a risk factor for poorer early postoperative outcomes after shoulder arthroplasty. J Shoulder Elbow Surg. 2014;23(5):671-678.
21. Pierson JL, Hannon TJ, Earles DR. A blood-conservation algorithm to reduce blood transfusions after total hip and knee arthroplasty. J Bone Joint Surg Am. 2004;86(7):1512-1518.
22. Martinez V, Monsaingeon-Lion A, Cherif K, Judet T, Chauvin M, Fletcher D. Transfusion strategy for primary knee and hip arthroplasty: impact of an algorithm to lower transfusion rates and hospital costs. Br J Anaesth. 2007;99(6):794-800.
23. Helm AT, Karski MT, Parsons SJ, Sampath JS, Bale RS. A strategy for reducing blood-transfusion requirements in elective orthopaedic surgery. Audit of an algorithm for arthroplasty of the lower limb. J Bone Joint Surg Br. 2003;85(4):484-489.
24. Watts CD, Pagnano MW. Minimising blood loss and transfusion in contemporary hip and knee arthroplasty. J Bone Joint Surg Br. 2012;94(11 suppl A):8-10.
25. Guralnik JM, Eisenstaedt RS, Ferrucci L, Klein HG, Woodman RC. Prevalence of anemia in persons 65 years and older in the United States: evidence for a high rate of unexplained anemia. Blood. 2004;104(8):2263-2268.
26. Rogers MA, Blumberg N, Heal JM, Langa KM. Utilization of blood transfusion among older adults in the United States. Transfusion. 2011;51(4):710-718.
27. Cobain TJ, Vamvakas EC, Wells A, Titlestad K. A survey of the demographics of blood use. Transfusion Med. 2007;17(1):1-15.
28. Fosco M, Di Fiore M. Factors predicting blood transfusion in different surgical procedures for degenerative spine disease. Eur Rev Med Pharmacol Sci. 2012;16(13):1853-1858.
29. Handoll HH, Ollivere BJ, Rollins KE. Interventions for treating proximal humeral fractures in adults. Cochrane Database Syst Rev. 2012;12:CD000434.
30. Neuhaus V, Swellengrebel CH, Bossen JK, Ring D. What are the factors influencing outcome among patients admitted to a hospital with a proximal humeral fracture? Clin Orthop Relat Res. 2013;471(5):1698-1706.
31. Volgas DA, Stannard JP, Alonso JE. Nonunions of the humerus. Clin Orthop Relat Res. 2004;(419):46-50.
32. Chambers L, Dines JS, Lorich DG, Dines DM. Hemiarthroplasty for proximal humerus fractures. Curr Rev Musculoskeletal Med. 2013;6(1):57-62.
33. Jain NB, Hocker S, Pietrobon R, Guller U, Bathia N, Higgins LD. Total arthroplasty versus hemiarthroplasty for glenohumeral osteoarthritis: role of provider volume. J Shoulder Elbow Surg. 2005;14(4):361-367.
34. Izquierdo R, Voloshin I, Edwards S, et al. Treatment of glenohumeral osteoarthritis. J Am Acad Orthop Surg. 2010;18(6):375-382.
35. Shields E, Iannuzzi JC, Thorsness R, Noyes K, Voloshin I. Perioperative complications after hemiarthroplasty and total shoulder arthroplasty are equivalent. J Shoulder Elbow Surg. 2014;23(10):1449-1453.
36. Gartsman GM, Roddey TS, Hammerman SM. Shoulder arthroplasty with or without resurfacing of the glenoid in patients who have osteoarthritis. J Bone Joint Surg Am. 2000;82(1):26-34.
37. Singh A, Yian EH, Dillon MT, Takayanagi M, Burke MF, Navarro RA. The effect of surgeon and hospital volume on shoulder arthroplasty perioperative quality metrics. J Shoulder Elbow Surg. 2014;23(8):1187-1194.
38. Groh GI, Groh GM. Complications rates, reoperation rates, and the learning curve in reverse shoulder arthroplasty. J Shoulder Elbow Surg. 2014;23(3):388-394.
39. Boileau P, Gonzalez JF, Chuinard C, Bicknell R, Walch G. Reverse total shoulder arthroplasty after failed rotator cuff surgery. J Shoulder Elbow Surg. 2009;18(4):600-606.
40. Boileau P, Watkinson D, Hatzidakis AM, Hovorka I. Neer Award 2005: the Grammont reverse shoulder prosthesis: results in cuff tear arthritis, fracture sequelae, and revision arthroplasty. J Shoulder Elbow Surg. 2006;15(5):527-540.
41. Boileau P, Watkinson DJ, Hatzidakis AM, Balg F. Grammont reverse prosthesis: design, rationale, and biomechanics. J Shoulder Elbow Surg. 2005;14(1 suppl S):147S-161S.
42. Pola E, Papaleo P, Santoliquido A, Gasparini G, Aulisa L, De Santis E. Clinical factors associated with an increased risk of perioperative blood transfusion in nonanemic patients undergoing total hip arthroplasty. J Bone Joint Surg Am. 2004;86(1):57-61.
43. Lin DM, Lin ES, Tran MH. Efficacy and safety of erythropoietin and intravenous iron in perioperative blood management: a systematic review. Transfusion Med Rev. 2013;27(4):221-234.
44. Muñoz M, Gómez-Ramírez S, Cuenca J, et al. Very-short-term perioperative intravenous iron administration and postoperative outcome in major orthopedic surgery: a pooled analysis of observational data from 2547 patients. Transfusion. 2014;54(2):289-299.
45. Danninger T, Rasul R, Poeran J, et al. Blood transfusions in total hip and knee arthroplasty: an analysis of outcomes. ScientificWorldJournal. 2014;2014:623460.
46. Baldus CR, Bridwell KH, Lenke LG, Okubadejo GO. Can we safely reduce blood loss during lumbar pedicle subtraction osteotomy procedures using tranexamic acid or aprotinin? A comparative study with controls. Spine. 2010;35(2):235-239.
47. Chang CH, Chang Y, Chen DW, Ueng SW, Lee MS. Topical tranexamic acid reduces blood loss and transfusion rates associated with primary total hip arthroplasty. Clin Orthop Relat Res. 2014;472(5):1552-1557.
48. Delasotta LA, Orozco F, Jafari SM, Blair JL, Ong A. Should we use preoperative epoetin-alpha in the mildly anemic patient undergoing simultaneous total knee arthroplasty? Open Orthop J. 2013;7:47-50.
49. Delasotta LA, Rangavajjula A, Frank ML, Blair J, Orozco F, Ong A. The use of preoperative epoetin-alpha in revision hip arthroplasty. Open Orthop J. 2012;6:179-183.
50. Kelley TC, Tucker KK, Adams MJ, Dalury DF. Use of tranexamic acid results in decreased blood loss and decreased transfusions in patients undergoing staged bilateral total knee arthroplasty. Transfusion. 2014;54(1):26-30.
51. Martin JG, Cassatt KB, Kincaid-Cinnamon KA, Westendorf DS, Garton AS, Lemke JH. Topical administration of tranexamic acid in primary total hip and total knee arthroplasty. J Arthroplasty. 2014;29(5):889-894.
52. Tzortzopoulou A, Cepeda MS, Schumann R, Carr DB. Antifibrinolytic agents for reducing blood loss in scoliosis surgery in children. Cochrane Database Syst Rev. 2008(3):CD006883.
53. Zhang H, Chen J, Chen F, Que W. The effect of tranexamic acid on blood loss and use of blood products in total knee arthroplasty: a meta-analysis. Knee Surg Sports Traumatol Arthrosc. 2012;20(9):1742-1752.
54. Bong MR, Patel V, Chang E, Issack PS, Hebert R, Di Cesare PE. Risks associated with blood transfusion after total knee arthroplasty. J Arthroplasty. 2004;19(3):281-287.
1. Ahmadi S, Lawrence TM, Sahota S, et al. The incidence and risk factors for blood transfusion in revision shoulder arthroplasty: our institution’s experience and review of the literature. J Shoulder Elbow Surg. 2014;23(1):43-48.
2. Sperling JW, Duncan SF, Cofield RH, Schleck CD, Harmsen WS. Incidence and risk factors for blood transfusion in shoulder arthroplasty. J Shoulder Elbow Surg. 2005;14(6):599-601.
3. Hardy JC, Hung M, Snow BJ, et al. Blood transfusion associated with shoulder arthroplasty. J Shoulder Elbow Surg. 2013;22(2):233-239.
4. Schumer RA, Chae JS, Markert RJ, Sprott D, Crosby LA. Predicting transfusion in shoulder arthroplasty. J Shoulder Elbow Surg. 2010;19(1):91-96.
5. Gruson KI, Accousti KJ, Parsons BO, Pillai G, Flatow EL. Transfusion after shoulder arthroplasty: an analysis of rates and risk factors. J Shoulder Elbow Surg. 2009;18(2):225-230.
6. Millett PJ, Porramatikul M, Chen N, Zurakowski D, Warner JJ. Analysis of transfusion predictors in shoulder arthroplasty. J Bone Joint Surg Am. 2006;88(6):1223-1230.
7. Kim SH, Wise BL, Zhang Y, Szabo RM. Increasing incidence of shoulder arthroplasty in the United States. J Bone Joint Surg Am. 2011;93(24):2249-2254.
8. Ceccherini-Nelli L, Filipponi F, Mosca F, Campa M. The risk of contracting an infectious disease from blood transfusion. Transplantation Proc. 2004;36(3):680-682.
9. Friedman R, Homering M, Holberg G, Berkowitz SD. Allogeneic blood transfusions and postoperative infections after total hip or knee arthroplasty. J Bone Joint Surg Am. 2014;96(4):272-278.
10. Hatzidakis AM, Mendlick RM, McKillip T, Reddy RL, Garvin KL. Preoperative autologous donation for total joint arthroplasty. An analysis of risk factors for allogenic transfusion. J Bone Joint Surg Am. 2000;82(1):89-100.
11. Park JH, Rasouli MR, Mortazavi SM, Tokarski AT, Maltenfort MG, Parvizi J. Predictors of perioperative blood loss in total joint arthroplasty. J Bone Joint Surg Am. 2013;95(19):1777-1783.
12. Aderinto J, Brenkel IJ. Pre-operative predictors of the requirement for blood transfusion following total hip replacement. J Bone Joint Surg Br. 2004;86(7):970-973.
13. Browne JA, Adib F, Brown TE, Novicoff WM. Transfusion rates are increasing following total hip arthroplasty: risk factors and outcomes. J Arthroplasty. 2013;28(8 suppl):34-37.
14. Yoshihara H, Yoneoka D. Predictors of allogeneic blood transfusion in spinal fusion in the United States, 2004–2009. Spine. 2014;39(4):304-310.
15. Noticewala MS, Nyce JD, Wang W, Geller JA, Macaulay W. Predicting need for allogeneic transfusion after total knee arthroplasty. J Arthroplasty. 2012;27(6):961-967.
16. Griffin JW, Novicoff WM, Browne JA, Brockmeier SF. Obstructive sleep apnea as a risk factor after shoulder arthroplasty. J Shoulder Elbow Surg. 2013;22(12):e6-e9.
17. Maynard C, Sales AE. Changes in the use of coronary artery revascularization procedures in the Department of Veterans Affairs, the National Hospital Discharge Survey, and the Nationwide Inpatient Sample, 1991–1999. BMC Health Serv Res. 2003;3(1):12.
18. Pereira BM, Chan PH, Weinstein PR, Fishman RA. Cerebral protection during reperfusion with superoxide dismutase in focal cerebral ischemia. Adv Neurol. 1990;52:97-103.
19. Hambright D, Henderson RA, Cook C, Worrell T, Moorman CT, Bolognesi MP. A comparison of perioperative outcomes in patients with and without rheumatoid arthritis after receiving a total shoulder replacement arthroplasty. J Shoulder Elbow Surg. 2011;20(1):77-85.
20. Ponce BA, Menendez ME, Oladeji LO, Soldado F. Diabetes as a risk factor for poorer early postoperative outcomes after shoulder arthroplasty. J Shoulder Elbow Surg. 2014;23(5):671-678.
21. Pierson JL, Hannon TJ, Earles DR. A blood-conservation algorithm to reduce blood transfusions after total hip and knee arthroplasty. J Bone Joint Surg Am. 2004;86(7):1512-1518.
22. Martinez V, Monsaingeon-Lion A, Cherif K, Judet T, Chauvin M, Fletcher D. Transfusion strategy for primary knee and hip arthroplasty: impact of an algorithm to lower transfusion rates and hospital costs. Br J Anaesth. 2007;99(6):794-800.
23. Helm AT, Karski MT, Parsons SJ, Sampath JS, Bale RS. A strategy for reducing blood-transfusion requirements in elective orthopaedic surgery. Audit of an algorithm for arthroplasty of the lower limb. J Bone Joint Surg Br. 2003;85(4):484-489.
24. Watts CD, Pagnano MW. Minimising blood loss and transfusion in contemporary hip and knee arthroplasty. J Bone Joint Surg Br. 2012;94(11 suppl A):8-10.
25. Guralnik JM, Eisenstaedt RS, Ferrucci L, Klein HG, Woodman RC. Prevalence of anemia in persons 65 years and older in the United States: evidence for a high rate of unexplained anemia. Blood. 2004;104(8):2263-2268.
26. Rogers MA, Blumberg N, Heal JM, Langa KM. Utilization of blood transfusion among older adults in the United States. Transfusion. 2011;51(4):710-718.
27. Cobain TJ, Vamvakas EC, Wells A, Titlestad K. A survey of the demographics of blood use. Transfusion Med. 2007;17(1):1-15.
28. Fosco M, Di Fiore M. Factors predicting blood transfusion in different surgical procedures for degenerative spine disease. Eur Rev Med Pharmacol Sci. 2012;16(13):1853-1858.
29. Handoll HH, Ollivere BJ, Rollins KE. Interventions for treating proximal humeral fractures in adults. Cochrane Database Syst Rev. 2012;12:CD000434.
30. Neuhaus V, Swellengrebel CH, Bossen JK, Ring D. What are the factors influencing outcome among patients admitted to a hospital with a proximal humeral fracture? Clin Orthop Relat Res. 2013;471(5):1698-1706.
31. Volgas DA, Stannard JP, Alonso JE. Nonunions of the humerus. Clin Orthop Relat Res. 2004;(419):46-50.
32. Chambers L, Dines JS, Lorich DG, Dines DM. Hemiarthroplasty for proximal humerus fractures. Curr Rev Musculoskeletal Med. 2013;6(1):57-62.
33. Jain NB, Hocker S, Pietrobon R, Guller U, Bathia N, Higgins LD. Total arthroplasty versus hemiarthroplasty for glenohumeral osteoarthritis: role of provider volume. J Shoulder Elbow Surg. 2005;14(4):361-367.
34. Izquierdo R, Voloshin I, Edwards S, et al. Treatment of glenohumeral osteoarthritis. J Am Acad Orthop Surg. 2010;18(6):375-382.
35. Shields E, Iannuzzi JC, Thorsness R, Noyes K, Voloshin I. Perioperative complications after hemiarthroplasty and total shoulder arthroplasty are equivalent. J Shoulder Elbow Surg. 2014;23(10):1449-1453.
36. Gartsman GM, Roddey TS, Hammerman SM. Shoulder arthroplasty with or without resurfacing of the glenoid in patients who have osteoarthritis. J Bone Joint Surg Am. 2000;82(1):26-34.
37. Singh A, Yian EH, Dillon MT, Takayanagi M, Burke MF, Navarro RA. The effect of surgeon and hospital volume on shoulder arthroplasty perioperative quality metrics. J Shoulder Elbow Surg. 2014;23(8):1187-1194.
38. Groh GI, Groh GM. Complications rates, reoperation rates, and the learning curve in reverse shoulder arthroplasty. J Shoulder Elbow Surg. 2014;23(3):388-394.
39. Boileau P, Gonzalez JF, Chuinard C, Bicknell R, Walch G. Reverse total shoulder arthroplasty after failed rotator cuff surgery. J Shoulder Elbow Surg. 2009;18(4):600-606.
40. Boileau P, Watkinson D, Hatzidakis AM, Hovorka I. Neer Award 2005: the Grammont reverse shoulder prosthesis: results in cuff tear arthritis, fracture sequelae, and revision arthroplasty. J Shoulder Elbow Surg. 2006;15(5):527-540.
41. Boileau P, Watkinson DJ, Hatzidakis AM, Balg F. Grammont reverse prosthesis: design, rationale, and biomechanics. J Shoulder Elbow Surg. 2005;14(1 suppl S):147S-161S.
42. Pola E, Papaleo P, Santoliquido A, Gasparini G, Aulisa L, De Santis E. Clinical factors associated with an increased risk of perioperative blood transfusion in nonanemic patients undergoing total hip arthroplasty. J Bone Joint Surg Am. 2004;86(1):57-61.
43. Lin DM, Lin ES, Tran MH. Efficacy and safety of erythropoietin and intravenous iron in perioperative blood management: a systematic review. Transfusion Med Rev. 2013;27(4):221-234.
44. Muñoz M, Gómez-Ramírez S, Cuenca J, et al. Very-short-term perioperative intravenous iron administration and postoperative outcome in major orthopedic surgery: a pooled analysis of observational data from 2547 patients. Transfusion. 2014;54(2):289-299.
45. Danninger T, Rasul R, Poeran J, et al. Blood transfusions in total hip and knee arthroplasty: an analysis of outcomes. ScientificWorldJournal. 2014;2014:623460.
46. Baldus CR, Bridwell KH, Lenke LG, Okubadejo GO. Can we safely reduce blood loss during lumbar pedicle subtraction osteotomy procedures using tranexamic acid or aprotinin? A comparative study with controls. Spine. 2010;35(2):235-239.
47. Chang CH, Chang Y, Chen DW, Ueng SW, Lee MS. Topical tranexamic acid reduces blood loss and transfusion rates associated with primary total hip arthroplasty. Clin Orthop Relat Res. 2014;472(5):1552-1557.
48. Delasotta LA, Orozco F, Jafari SM, Blair JL, Ong A. Should we use preoperative epoetin-alpha in the mildly anemic patient undergoing simultaneous total knee arthroplasty? Open Orthop J. 2013;7:47-50.
49. Delasotta LA, Rangavajjula A, Frank ML, Blair J, Orozco F, Ong A. The use of preoperative epoetin-alpha in revision hip arthroplasty. Open Orthop J. 2012;6:179-183.
50. Kelley TC, Tucker KK, Adams MJ, Dalury DF. Use of tranexamic acid results in decreased blood loss and decreased transfusions in patients undergoing staged bilateral total knee arthroplasty. Transfusion. 2014;54(1):26-30.
51. Martin JG, Cassatt KB, Kincaid-Cinnamon KA, Westendorf DS, Garton AS, Lemke JH. Topical administration of tranexamic acid in primary total hip and total knee arthroplasty. J Arthroplasty. 2014;29(5):889-894.
52. Tzortzopoulou A, Cepeda MS, Schumann R, Carr DB. Antifibrinolytic agents for reducing blood loss in scoliosis surgery in children. Cochrane Database Syst Rev. 2008(3):CD006883.
53. Zhang H, Chen J, Chen F, Que W. The effect of tranexamic acid on blood loss and use of blood products in total knee arthroplasty: a meta-analysis. Knee Surg Sports Traumatol Arthrosc. 2012;20(9):1742-1752.
54. Bong MR, Patel V, Chang E, Issack PS, Hebert R, Di Cesare PE. Risks associated with blood transfusion after total knee arthroplasty. J Arthroplasty. 2004;19(3):281-287.
Is the Orthopedic Fellowship Interview Process Broken? A Survey of Program Directors and Residents
Over the past several decades, an increasing number of orthopedic surgery residents have pursued fellowship training.1 This inclination parallels market trends toward subspecialization.2-5 In 1984, 83% of orthopedics job announcements were for general orthopedists. Twenty-five years later, almost 70% of orthopedic opportunities were for fellowship-trained surgeons.6 Further, between 1990 and 2006, the proportion of practicing orthopedic generalists decreased from 44% to 29%.3 In 2007, the American Academy of Orthopaedic Surgery (AAOS) reported 90% of graduating residents were planning to pursue fellowship training.7 Reasons for the explosion in subspecialty training are plentiful and well documented.2-5 Subspecialty positions now dominate the job market, further reinforcing incentives for residents to pursue fellowship training.
The past several decades have seen numerous changes in the orthopedic fellowship interview process. Early on, it was largely unregulated, dependent on personal and professional connections, and flush with the classic “exploding offer” (residents were given a fellowship offer that expired within hours or days). In the 1980s, as the number of fellowship applications surged, the Accreditation Council for Graduate Medical Education (ACGME) pushed for a more regulated process.8 To further standardize the system, the American Orthopaedic Association (AOA), the AAOS, and several other specialty organizations created the Orthopaedic Fellowship Match Program Initiative in 2008.9 Currently, all orthopedic specialties are represented in either the San Francisco Match Program or National Residency Match Program.
As the system currently stands, postgraduate year 4 (PGY-4) residents are required to interview across the country to secure postgraduate training. This process necessitates residents’ absence from their program, reducing educational opportunities and placing potential continuity-of-care constraints on the residency program. Despite the growing competitiveness for fellowship positions, the increasing number of fellowships available, the rising educational debt of residents, and the limitations of the 80-hour work week, the impact of the interview process on both residents and residency programs has received minimal attention.
We conducted a study to elucidate the impact of the fellowship interview process on residents and residency programs. We hypothesized the time and financial costs for fellowship interviews would be substantial.
Materials and Methods
We obtained institutional review board (IRB) approval for this study. Then, in April 2014, we sent 2 mixed-response questionnaires to orthopedic surgery residency directors and residents. There were 8 items on the director questionnaire and 11 on the resident questionnaire. The surveys were designed to determine the impact of the fellowship interview process on residents and residency programs with respect to finances, time, education, and continuity of care. Each survey had at least 1 free-response question, providing the opportunity to recommend changes to the interview process. The surveys were reviewed and approved by our IRB.
An email was sent to 155 orthopedic surgery program directors or their secretaries. The email asked that the director complete the director questionnaire and that the resident questionnaire be forwarded to senior-level residents, PGY-4s and PGY-5s, who had completed the fellowship interview process. Forty-five (29%) of the 155 directors responded, as did 129 (estimated 9.5%) of an estimated 1354 potential PGY-4s and PGY-5s.10
The Survey Monkey surveys could be completed over a 3-week period. All responses were anonymous. Using Survey Monkey, we aggregated individual responses into predefined clusters before performing statistical analysis. Descriptive statistics were generated with Microsoft Excel.
Results
Survey respondents represented all the orthopedic subspecialties (Table). Seventy-eight percent of residents applied to at least 13 programs (average, 19) (Figure 1). Ninety-two percent received at least 8 interview offers (average, 14). Eighty-three percent attended 8 or more interviews (average, 11). Seventy-one percent of all interviews were granted when requested, and 79% of all interviews were attended when offered.
Residents spent an average of $5875 (range, $500-$12,000+) on the fellowship interview process (Figure 2). The highest percentage of respondents, 39.5%, selected an average expense between $4000 and $6000. Forty-nine percent of residents borrowed money (from credit cards, additional loans, family members) to pay their expenses.
Average number of days away from residency programs was 11, with 86% of residents missing more than 8 days (Figure 1). About one-third of residents reported being away from their home program for almost 2 weeks during the interview season. Further, 74% of residents wanted changes made to the fellowship application process.
Thirty-seven (82%) of the 45 program directors were from academic programs, the other 8 from community-based programs. Average number of residents in programs per year was 4 (73% of the programs had 4-6 residents per year). Respondents rated the disruption caused by residents’ interview absences from 1 (least disruptive) to 10 (most disruptive) (Figure 3); the average rating was over 7 (high level of disruption). Although 9% of directors thought the process caused little or no disruption (rating, 1-3), 62% thought it extremely disruptive (rating, 8-10).
Thirty-one (69%) of the 45 directors agreed that the fellowship interview process should undergo fundamental change. Asked about possible solutions to current complaints, 60% of the directors agreed that interviews should be conducted in a central location. Of the directors who thought fundamental change was needed, 59% indicated AAOS and other specialty societies together should lead the change in the fellowship interview process.
Both residents and program directors were given the opportunity to write in suggestions regarding how to improve the fellowship interview process. Suggestions were made by 85 (66%) of the 129 residents and 24 (53%) of the 45 directors (Appendix).
Discussion
Graduating residents are entering a health care environment in which they must be financially conscious because of increasing education debt and decreasing reimbursement prospects.3 Nevertheless, an overwhelming majority of residents delay entering practice to pursue fellowship training—an estimated opportunity cost of $350,000.3 Minimal attention has been given to the potential costs of the fellowship interview process.
Our study results highlight that time away from residency training, financial costs associated with the fellowship interview process, and disruption of the residency program are substantial. On average, residents applied to 19 programs, received 14 interview offers, attended 11 interviews, were away from residency training 11 days, and spent $5875 on travel. The great majority of both residents and program directors wanted changes in the current paradigm governing the orthopedic fellowship interview process.
It is reasonable to think that the number of days residents spend away on interviews would reduce the time available for education and patient care. Although unknown, it is plausible that residents of programs outside major metropolitan centers and residents who apply to more competitive fellowships may be forced to spend even more time away from training. Outside the focus of this study are the impact that residents’ absence might have on their education and the impact of this absence on the people who do the residents’ work while they are away.
Mean fellowship expense was similar to that reported by residents pursuing a pediatric general surgery fellowship ($6974) or a plastic surgery fellowship ($6100).11,12 Unfortunately, we were unable to determine if average cost is influenced by choice of fellowship specialty or location of residency program. Regardless, fellowship cost may impose an additional financial burden on residents. According to the Association of American Medical Colleges (AAMC), the median salary for PGY-4 residents was $56,380 in 2013. Therefore, on average, the fellowship process consumes more than 10% of a resident’s pretax salary. For perspective, this equates to more than $40,000 for a practicing orthopedic surgeon with a median salary of $413,000.13 With an average medical student graduate debt of $175,000 and continuing decreases in reimbursement, further financial hardships to newly graduating residents cannot be understated.5,11,12
Almost 70% of program directors thought the fellowship process significantly disrupted their program. Reasons given for this disruption mainly involved residents’ time away from the program and the resulting strains placed on maintaining adequate coverage for patient care. The overall disruption score of 7.4 out of 10 was consistent with the great majority thinking that the fellowship process negatively affects their residency program. Altering the fellowship interview process may provide unintended benefits to programs and program directors.
Both program directors and residents communicated that change is needed, but there was little consensus regarding how to effect change and who should lead. This lack of consensus highlights how important it is for the various orthopedic leadership committees to actively and collectively participate in discussions about redefining the system. It has been proposed that it would be ideal for the AOA to lead the change, as the AOA consists of a representative cohort of academic orthopedists and leaders across the spectrum of all fellowship specialties.14 Given the abundant concern of both residents and program directors, we find it prudent to issue a call to arms of sorts to the AAOS and the individual orthopedic subspecialty societies to work together on a common goal that would benefit residents, programs, and subspecialties within orthopedics.
In trying to understand the challenges that residents, program directors, and programs face, as well as the inherent complexity of the current system, we incorporated respondents’ write-in comments into suggested ways of improving the fellowship interview process. These comments had broad perspectives but overall were consistent with the survey results (Appendix).
Technology
Health care is continually finding new ways to take advantage of technological advances. This is occurring with the fellowship interview schema. Numerous disciplines are using videoconferencing platforms (eg, Skype) to conduct interviews. This practice is becoming more commonplace in the business sector. In a recent survey, more than 60% of human resource managers reported conducting video interviews.15 Two independent residency programs have used video interviews with mixed success.16,17
Another technological change requested by residents is the creation and updating of fellowship web pages with standardized information. Such a service may prove useful to residents researching a program and may even lead to limiting the number of programs residents apply to, as they may be able to dial in on exactly what distinguishes one program from another before traveling for an interview. A recent study of orthopedic sports medicine fellowship programs found that most of these programs lacked pertinent information on their websites.18 Important information regarding case logs from current and former fellows; number of faculty, residents, and fellows; and schedules and facilities of interview sites are a few of the online data points that may help residents differentiate particular programs.19,20 Questions like these are often asked at interviews and site visits. Having accurate information easily available online may reduce or eliminate the need to travel to a site for such information. Standardizing information would also increase transparency among available fellowships. Although not specifically mentioned, organizational software that improves the productivity of the process may help limit the large number of programs applied to, the interviews offered and attended, the days away, and the financial costs without reducing the match rate.
Timing and Location
The issue of timing—with respect to geographical or meteorological concerns—was another recurring theme among respondents. Numerous respondents indicated that certain programs located in geographic proximity tried to minimize travel by offering interviews around the same time. This coordination potentially minimizes travel expenses and time away from the residency program by allowing residents to interview at multiple locations during a single trip per region. The sports medicine fellowship process was identified as a good example of aligning interviews based on geography. Several respondents suggested an option that also reflects the practice of nonsurgical fellowships—delaying the interview season to bypass potential weather concerns. Winter 2013–2014 saw the most flight delays or cancellations in more than a decade; about 50% of all flights scheduled between December and February were delayed or canceled.21 Beyond the additional factor of more time away or missing an interview because of the weather are safety concerns related to the weather. One resident reported having a motor vehicle accident while traveling to an interview in poor weather conditions (Appendix).
National Meetings
Each orthopedic subspecialty has numerous national meetings. Many programs offer applicants the opportunity to interview at these meetings. One respondent mentioned that the annual meeting of the Orthopaedic Trauma Association offers trauma applicants the opportunity to interview with multiple programs. It might be beneficial to endorse this practice on a larger scale to help reduce travel and time away. We recognize that visiting individual programs is an important aspect of the match process, but doing so on a targeted level may make more sense, increasing financial efficiency and reducing time away from programs.
Proposed Solution
A combined proposed solution that can be implemented without a radical overhaul or significant investments might involve moving the interview season to early spring, switching to a 2-tiered system with a centralized first round of interview screening coinciding with subspecialty national meetings or the AAOS annual meeting, and standardizing online information for all orthopedic fellowship programs. A 2-tiered interview process would allow programs and candidates to obtain exposure to a significant number of programs in the first round without incurring significant costs and then would impose a cap on the number of programs to visit. This would level the playing field between candidates with more time and money and candidates who are more constrained in their training environment and finances. A stopgap or adjunct to residents or fellowship programs unable to attend a centralized meeting would be to combine technological tools, such as Internet-based videoconferencing (Skype), before site visits by residents. After this first round of introductions and interviews, residents could then decide on a limited number of programs to formally visit, attend, and ultimately rank. This proposed system would still be able to function within the confines of the match, and it would benefit from the protections offered to residents and programs. Although capping the number of interviews attended by residents clearly can lower costs across the board, we recognize the difficulty of enforcing such a requirement. These potential changes to the system are not exhaustive, and we hope this work will serve as a springboard to further discussion.
Our study had several inherent weaknesses. Our data came from survey responses, which reflect the perspectives only of the responding residents and program directors. Unfortunately, a small number of orthopedic residents responded to this survey, so there was a potential for bias. However, we think the central themes discovered in this survey are only echoes of the concerns of the larger population of residents and program directors. Our hope in designing such a study was to bring to light some of the discrepancies in the fellowship interview process, the goal being to stimulate interest among the orthopedic leadership representing future orthopedic surgeons. More study is needed to clarify if these issues are reflective of a larger segment of residents and program directors. In addition, action may be needed to fully elucidate the intricate interworking of the fellowship process in order to maximize the interest of the orthopedic surgeons who are seeking fellowship training. Another study limitation was the potential for recall bias in the more senior PGY-5 residents, who were further from the interview process than PGY-4 respondents were. Because of the need for anonymity with the surveys, we could not link some findings (eg, program impact, cost, time away) to individual programs or different specialty fellowships. Although it appears there is a desire for a more cost-effective system, given the financial pressures on medical students and residents, the desire to match increases costs because students are likely to attend more interviews than actually needed. Our proposed solution does not take into account residents’ behavior with respect to the current match system. For example, the prevailing thought is that interviewing at more programs increases the likelihood of matching into a desired subspecialty. Despite these study limitations, we think our results identified important points for discussion, investigation, and potential action by orthopedic leadership.
Conclusion
The challenge of critiquing and improving the orthopedic fellowship process requires the same courageous leadership that was recommended almost a decade ago.14 In this study, we tried to elucidate the impact of the PGY-4 fellowship interview process with respect to residents and residency programs. Our results highlight that time away from residency training, financial costs associated with the fellowship interview process, and disruption of the residency program are substantial and that both residents and program directors want changes made. Leadership needs to further investigate alternatives to the current process to lessen the impact on all parties in this important process.
1. Simon MA. Evolution of the present status of orthopaedic surgery fellowships. J Bone Joint Surg Am. 1998;80(12):1826-1829.
2. Brunworth LS, Chintalapani SR, Gray RR, Cardoso R, Owens PW. Resident selection of hand surgery fellowships: a survey of the 2011, 2012, and 2013 hand fellowship graduates. Hand. 2013;8(2):164-171.
3. Gaskill T, Cook C, Nunley J, Mather RC. The financial impact of orthopaedic fellowship training. J Bone Joint Surg Am. 2009;91(7):1814-1821.
4. Sarmiento A. Additional thoughts on orthopedic residency and fellowships. Orthopedics. 2010;33(10):712-713.
5. Griffin SM, Stoneback JW. Navigating the Orthopaedic Trauma Fellowship Match from a candidate’s perspective. J Orthop Trauma. 2011;25(suppl 3):S101-S103.
6. Morrell NT, Mercer DM, Moneim MS. Trends in the orthopedic job market and the importance of fellowship subspecialty training. Orthopedics. 2012;35(4):e555-e560.
7. Iorio R, Robb WJ, Healy WL, et al. Orthopaedic surgeon workforce and volume assessment for total hip and knee replacement in the United States: preparing for an epidemic. J Bone Joint Surg Am. 2008;90(7):1598-1605.
8. Emery SE, Guss D, Kuremsky MA, Hamlin BR, Herndon JH, Rubash HE. Resident education versus fellowship training—conflict or synergy? AOA critical issues. J Bone Joint Surg Am. 2012;94(21):e159.
9. Harner CD, Ranawat AS, Niederle M, et al. AOA symposium. Current state of fellowship hiring: is a universal match necessary? Is it possible? J Bone Joint Surg Am. 2008;90(6):1375-1384.
10. Ranawat A, Nunley RM, Genuario JW, Sharan AD, Mehta S; Washington Health Policy Fellows. Current state of the fellowship hiring process: Are we in 1957 or 2007? AAOS Now. 2007;1(8).
11. Little DC, Yoder SM, Grikscheit TC, et al. Cost considerations and applicant characteristics for the Pediatric Surgery Match. J Pediatr Surg. 2005;40(1):69-73.
12. Claiborne JR, Crantford JC, Swett KR, David LR. The Plastic Surgery Match: predicting success and improving the process. Ann Plast Surg. 2013;70(6):698-703.
13. Kane L, Peckham C. Medscape Physician Compensation Report 2014. http://www.medscape.com/features/slideshow/compensation/2014/public/overview. Published April 15, 2014. Accessed September 26, 2015.
14. Swiontkowski MF. A simple formula for continued improvement in orthopaedic surgery postgraduate training: courageous leadership. J Bone Joint Surg Am. 2008;90(6):1175.
15. Survey: six in 10 companies conduct video job interviews [news release]. http://www.prnewswire.com/news-releases/survey-six-in-10-companies-conduct-video-job-interviews-167973406.html. Published August 30, 2012. Accessed September 26, 2015.
16. Kerfoot BP, Asher KP, McCullough DL. Financial and educational costs of the residency interview process for urology applicants. Urology. 2008;71(6):990-994.
17. Edje L, Miller C, Kiefer J, Oram D. Using Skype as an alternative for residency selection interviews. J Grad Med Educ. 2013;5(3):503-505.
18. Mulcahey MK, Gosselin MM, Fadale PD. Evaluation of the content and accessibility of web sites for accredited orthopaedic sports medicine fellowships. J Bone Joint Surg Am. 2013;95(12):e85.
19. Gaeta TJ, Birkhahn RH, Lamont D, Banga N, Bove JJ. Aspects of residency programs’ web sites important to student applicants. Acad Emerg Med. 2005;12(1):89-92.
20. Mahler SA, Wagner MJ, Church A, Sokolosky M, Cline DM. Importance of residency program web sites to emergency medicine applicants. J Emerg Med. 2009;36(1):83-88.
21. Davies A. Winter’s toll: 1 million flights cancelled or delayed, costing travelers $5.3 billion. Business Insider. http://www.businessinsider.com/winter-flights-cancelled-delayed-cost-2014-3. Published March 3, 2014. Accessed September 26, 2015.
Over the past several decades, an increasing number of orthopedic surgery residents have pursued fellowship training.1 This inclination parallels market trends toward subspecialization.2-5 In 1984, 83% of orthopedics job announcements were for general orthopedists. Twenty-five years later, almost 70% of orthopedic opportunities were for fellowship-trained surgeons.6 Further, between 1990 and 2006, the proportion of practicing orthopedic generalists decreased from 44% to 29%.3 In 2007, the American Academy of Orthopaedic Surgery (AAOS) reported 90% of graduating residents were planning to pursue fellowship training.7 Reasons for the explosion in subspecialty training are plentiful and well documented.2-5 Subspecialty positions now dominate the job market, further reinforcing incentives for residents to pursue fellowship training.
The past several decades have seen numerous changes in the orthopedic fellowship interview process. Early on, it was largely unregulated, dependent on personal and professional connections, and flush with the classic “exploding offer” (residents were given a fellowship offer that expired within hours or days). In the 1980s, as the number of fellowship applications surged, the Accreditation Council for Graduate Medical Education (ACGME) pushed for a more regulated process.8 To further standardize the system, the American Orthopaedic Association (AOA), the AAOS, and several other specialty organizations created the Orthopaedic Fellowship Match Program Initiative in 2008.9 Currently, all orthopedic specialties are represented in either the San Francisco Match Program or National Residency Match Program.
As the system currently stands, postgraduate year 4 (PGY-4) residents are required to interview across the country to secure postgraduate training. This process necessitates residents’ absence from their program, reducing educational opportunities and placing potential continuity-of-care constraints on the residency program. Despite the growing competitiveness for fellowship positions, the increasing number of fellowships available, the rising educational debt of residents, and the limitations of the 80-hour work week, the impact of the interview process on both residents and residency programs has received minimal attention.
We conducted a study to elucidate the impact of the fellowship interview process on residents and residency programs. We hypothesized the time and financial costs for fellowship interviews would be substantial.
Materials and Methods
We obtained institutional review board (IRB) approval for this study. Then, in April 2014, we sent 2 mixed-response questionnaires to orthopedic surgery residency directors and residents. There were 8 items on the director questionnaire and 11 on the resident questionnaire. The surveys were designed to determine the impact of the fellowship interview process on residents and residency programs with respect to finances, time, education, and continuity of care. Each survey had at least 1 free-response question, providing the opportunity to recommend changes to the interview process. The surveys were reviewed and approved by our IRB.
An email was sent to 155 orthopedic surgery program directors or their secretaries. The email asked that the director complete the director questionnaire and that the resident questionnaire be forwarded to senior-level residents, PGY-4s and PGY-5s, who had completed the fellowship interview process. Forty-five (29%) of the 155 directors responded, as did 129 (estimated 9.5%) of an estimated 1354 potential PGY-4s and PGY-5s.10
The Survey Monkey surveys could be completed over a 3-week period. All responses were anonymous. Using Survey Monkey, we aggregated individual responses into predefined clusters before performing statistical analysis. Descriptive statistics were generated with Microsoft Excel.
Results
Survey respondents represented all the orthopedic subspecialties (Table). Seventy-eight percent of residents applied to at least 13 programs (average, 19) (Figure 1). Ninety-two percent received at least 8 interview offers (average, 14). Eighty-three percent attended 8 or more interviews (average, 11). Seventy-one percent of all interviews were granted when requested, and 79% of all interviews were attended when offered.
Residents spent an average of $5875 (range, $500-$12,000+) on the fellowship interview process (Figure 2). The highest percentage of respondents, 39.5%, selected an average expense between $4000 and $6000. Forty-nine percent of residents borrowed money (from credit cards, additional loans, family members) to pay their expenses.
Average number of days away from residency programs was 11, with 86% of residents missing more than 8 days (Figure 1). About one-third of residents reported being away from their home program for almost 2 weeks during the interview season. Further, 74% of residents wanted changes made to the fellowship application process.
Thirty-seven (82%) of the 45 program directors were from academic programs, the other 8 from community-based programs. Average number of residents in programs per year was 4 (73% of the programs had 4-6 residents per year). Respondents rated the disruption caused by residents’ interview absences from 1 (least disruptive) to 10 (most disruptive) (Figure 3); the average rating was over 7 (high level of disruption). Although 9% of directors thought the process caused little or no disruption (rating, 1-3), 62% thought it extremely disruptive (rating, 8-10).
Thirty-one (69%) of the 45 directors agreed that the fellowship interview process should undergo fundamental change. Asked about possible solutions to current complaints, 60% of the directors agreed that interviews should be conducted in a central location. Of the directors who thought fundamental change was needed, 59% indicated AAOS and other specialty societies together should lead the change in the fellowship interview process.
Both residents and program directors were given the opportunity to write in suggestions regarding how to improve the fellowship interview process. Suggestions were made by 85 (66%) of the 129 residents and 24 (53%) of the 45 directors (Appendix).
Discussion
Graduating residents are entering a health care environment in which they must be financially conscious because of increasing education debt and decreasing reimbursement prospects.3 Nevertheless, an overwhelming majority of residents delay entering practice to pursue fellowship training—an estimated opportunity cost of $350,000.3 Minimal attention has been given to the potential costs of the fellowship interview process.
Our study results highlight that time away from residency training, financial costs associated with the fellowship interview process, and disruption of the residency program are substantial. On average, residents applied to 19 programs, received 14 interview offers, attended 11 interviews, were away from residency training 11 days, and spent $5875 on travel. The great majority of both residents and program directors wanted changes in the current paradigm governing the orthopedic fellowship interview process.
It is reasonable to think that the number of days residents spend away on interviews would reduce the time available for education and patient care. Although unknown, it is plausible that residents of programs outside major metropolitan centers and residents who apply to more competitive fellowships may be forced to spend even more time away from training. Outside the focus of this study are the impact that residents’ absence might have on their education and the impact of this absence on the people who do the residents’ work while they are away.
Mean fellowship expense was similar to that reported by residents pursuing a pediatric general surgery fellowship ($6974) or a plastic surgery fellowship ($6100).11,12 Unfortunately, we were unable to determine if average cost is influenced by choice of fellowship specialty or location of residency program. Regardless, fellowship cost may impose an additional financial burden on residents. According to the Association of American Medical Colleges (AAMC), the median salary for PGY-4 residents was $56,380 in 2013. Therefore, on average, the fellowship process consumes more than 10% of a resident’s pretax salary. For perspective, this equates to more than $40,000 for a practicing orthopedic surgeon with a median salary of $413,000.13 With an average medical student graduate debt of $175,000 and continuing decreases in reimbursement, further financial hardships to newly graduating residents cannot be understated.5,11,12
Almost 70% of program directors thought the fellowship process significantly disrupted their program. Reasons given for this disruption mainly involved residents’ time away from the program and the resulting strains placed on maintaining adequate coverage for patient care. The overall disruption score of 7.4 out of 10 was consistent with the great majority thinking that the fellowship process negatively affects their residency program. Altering the fellowship interview process may provide unintended benefits to programs and program directors.
Both program directors and residents communicated that change is needed, but there was little consensus regarding how to effect change and who should lead. This lack of consensus highlights how important it is for the various orthopedic leadership committees to actively and collectively participate in discussions about redefining the system. It has been proposed that it would be ideal for the AOA to lead the change, as the AOA consists of a representative cohort of academic orthopedists and leaders across the spectrum of all fellowship specialties.14 Given the abundant concern of both residents and program directors, we find it prudent to issue a call to arms of sorts to the AAOS and the individual orthopedic subspecialty societies to work together on a common goal that would benefit residents, programs, and subspecialties within orthopedics.
In trying to understand the challenges that residents, program directors, and programs face, as well as the inherent complexity of the current system, we incorporated respondents’ write-in comments into suggested ways of improving the fellowship interview process. These comments had broad perspectives but overall were consistent with the survey results (Appendix).
Technology
Health care is continually finding new ways to take advantage of technological advances. This is occurring with the fellowship interview schema. Numerous disciplines are using videoconferencing platforms (eg, Skype) to conduct interviews. This practice is becoming more commonplace in the business sector. In a recent survey, more than 60% of human resource managers reported conducting video interviews.15 Two independent residency programs have used video interviews with mixed success.16,17
Another technological change requested by residents is the creation and updating of fellowship web pages with standardized information. Such a service may prove useful to residents researching a program and may even lead to limiting the number of programs residents apply to, as they may be able to dial in on exactly what distinguishes one program from another before traveling for an interview. A recent study of orthopedic sports medicine fellowship programs found that most of these programs lacked pertinent information on their websites.18 Important information regarding case logs from current and former fellows; number of faculty, residents, and fellows; and schedules and facilities of interview sites are a few of the online data points that may help residents differentiate particular programs.19,20 Questions like these are often asked at interviews and site visits. Having accurate information easily available online may reduce or eliminate the need to travel to a site for such information. Standardizing information would also increase transparency among available fellowships. Although not specifically mentioned, organizational software that improves the productivity of the process may help limit the large number of programs applied to, the interviews offered and attended, the days away, and the financial costs without reducing the match rate.
Timing and Location
The issue of timing—with respect to geographical or meteorological concerns—was another recurring theme among respondents. Numerous respondents indicated that certain programs located in geographic proximity tried to minimize travel by offering interviews around the same time. This coordination potentially minimizes travel expenses and time away from the residency program by allowing residents to interview at multiple locations during a single trip per region. The sports medicine fellowship process was identified as a good example of aligning interviews based on geography. Several respondents suggested an option that also reflects the practice of nonsurgical fellowships—delaying the interview season to bypass potential weather concerns. Winter 2013–2014 saw the most flight delays or cancellations in more than a decade; about 50% of all flights scheduled between December and February were delayed or canceled.21 Beyond the additional factor of more time away or missing an interview because of the weather are safety concerns related to the weather. One resident reported having a motor vehicle accident while traveling to an interview in poor weather conditions (Appendix).
National Meetings
Each orthopedic subspecialty has numerous national meetings. Many programs offer applicants the opportunity to interview at these meetings. One respondent mentioned that the annual meeting of the Orthopaedic Trauma Association offers trauma applicants the opportunity to interview with multiple programs. It might be beneficial to endorse this practice on a larger scale to help reduce travel and time away. We recognize that visiting individual programs is an important aspect of the match process, but doing so on a targeted level may make more sense, increasing financial efficiency and reducing time away from programs.
Proposed Solution
A combined proposed solution that can be implemented without a radical overhaul or significant investments might involve moving the interview season to early spring, switching to a 2-tiered system with a centralized first round of interview screening coinciding with subspecialty national meetings or the AAOS annual meeting, and standardizing online information for all orthopedic fellowship programs. A 2-tiered interview process would allow programs and candidates to obtain exposure to a significant number of programs in the first round without incurring significant costs and then would impose a cap on the number of programs to visit. This would level the playing field between candidates with more time and money and candidates who are more constrained in their training environment and finances. A stopgap or adjunct to residents or fellowship programs unable to attend a centralized meeting would be to combine technological tools, such as Internet-based videoconferencing (Skype), before site visits by residents. After this first round of introductions and interviews, residents could then decide on a limited number of programs to formally visit, attend, and ultimately rank. This proposed system would still be able to function within the confines of the match, and it would benefit from the protections offered to residents and programs. Although capping the number of interviews attended by residents clearly can lower costs across the board, we recognize the difficulty of enforcing such a requirement. These potential changes to the system are not exhaustive, and we hope this work will serve as a springboard to further discussion.
Our study had several inherent weaknesses. Our data came from survey responses, which reflect the perspectives only of the responding residents and program directors. Unfortunately, a small number of orthopedic residents responded to this survey, so there was a potential for bias. However, we think the central themes discovered in this survey are only echoes of the concerns of the larger population of residents and program directors. Our hope in designing such a study was to bring to light some of the discrepancies in the fellowship interview process, the goal being to stimulate interest among the orthopedic leadership representing future orthopedic surgeons. More study is needed to clarify if these issues are reflective of a larger segment of residents and program directors. In addition, action may be needed to fully elucidate the intricate interworking of the fellowship process in order to maximize the interest of the orthopedic surgeons who are seeking fellowship training. Another study limitation was the potential for recall bias in the more senior PGY-5 residents, who were further from the interview process than PGY-4 respondents were. Because of the need for anonymity with the surveys, we could not link some findings (eg, program impact, cost, time away) to individual programs or different specialty fellowships. Although it appears there is a desire for a more cost-effective system, given the financial pressures on medical students and residents, the desire to match increases costs because students are likely to attend more interviews than actually needed. Our proposed solution does not take into account residents’ behavior with respect to the current match system. For example, the prevailing thought is that interviewing at more programs increases the likelihood of matching into a desired subspecialty. Despite these study limitations, we think our results identified important points for discussion, investigation, and potential action by orthopedic leadership.
Conclusion
The challenge of critiquing and improving the orthopedic fellowship process requires the same courageous leadership that was recommended almost a decade ago.14 In this study, we tried to elucidate the impact of the PGY-4 fellowship interview process with respect to residents and residency programs. Our results highlight that time away from residency training, financial costs associated with the fellowship interview process, and disruption of the residency program are substantial and that both residents and program directors want changes made. Leadership needs to further investigate alternatives to the current process to lessen the impact on all parties in this important process.
Over the past several decades, an increasing number of orthopedic surgery residents have pursued fellowship training.1 This inclination parallels market trends toward subspecialization.2-5 In 1984, 83% of orthopedics job announcements were for general orthopedists. Twenty-five years later, almost 70% of orthopedic opportunities were for fellowship-trained surgeons.6 Further, between 1990 and 2006, the proportion of practicing orthopedic generalists decreased from 44% to 29%.3 In 2007, the American Academy of Orthopaedic Surgery (AAOS) reported 90% of graduating residents were planning to pursue fellowship training.7 Reasons for the explosion in subspecialty training are plentiful and well documented.2-5 Subspecialty positions now dominate the job market, further reinforcing incentives for residents to pursue fellowship training.
The past several decades have seen numerous changes in the orthopedic fellowship interview process. Early on, it was largely unregulated, dependent on personal and professional connections, and flush with the classic “exploding offer” (residents were given a fellowship offer that expired within hours or days). In the 1980s, as the number of fellowship applications surged, the Accreditation Council for Graduate Medical Education (ACGME) pushed for a more regulated process.8 To further standardize the system, the American Orthopaedic Association (AOA), the AAOS, and several other specialty organizations created the Orthopaedic Fellowship Match Program Initiative in 2008.9 Currently, all orthopedic specialties are represented in either the San Francisco Match Program or National Residency Match Program.
As the system currently stands, postgraduate year 4 (PGY-4) residents are required to interview across the country to secure postgraduate training. This process necessitates residents’ absence from their program, reducing educational opportunities and placing potential continuity-of-care constraints on the residency program. Despite the growing competitiveness for fellowship positions, the increasing number of fellowships available, the rising educational debt of residents, and the limitations of the 80-hour work week, the impact of the interview process on both residents and residency programs has received minimal attention.
We conducted a study to elucidate the impact of the fellowship interview process on residents and residency programs. We hypothesized the time and financial costs for fellowship interviews would be substantial.
Materials and Methods
We obtained institutional review board (IRB) approval for this study. Then, in April 2014, we sent 2 mixed-response questionnaires to orthopedic surgery residency directors and residents. There were 8 items on the director questionnaire and 11 on the resident questionnaire. The surveys were designed to determine the impact of the fellowship interview process on residents and residency programs with respect to finances, time, education, and continuity of care. Each survey had at least 1 free-response question, providing the opportunity to recommend changes to the interview process. The surveys were reviewed and approved by our IRB.
An email was sent to 155 orthopedic surgery program directors or their secretaries. The email asked that the director complete the director questionnaire and that the resident questionnaire be forwarded to senior-level residents, PGY-4s and PGY-5s, who had completed the fellowship interview process. Forty-five (29%) of the 155 directors responded, as did 129 (estimated 9.5%) of an estimated 1354 potential PGY-4s and PGY-5s.10
The Survey Monkey surveys could be completed over a 3-week period. All responses were anonymous. Using Survey Monkey, we aggregated individual responses into predefined clusters before performing statistical analysis. Descriptive statistics were generated with Microsoft Excel.
Results
Survey respondents represented all the orthopedic subspecialties (Table). Seventy-eight percent of residents applied to at least 13 programs (average, 19) (Figure 1). Ninety-two percent received at least 8 interview offers (average, 14). Eighty-three percent attended 8 or more interviews (average, 11). Seventy-one percent of all interviews were granted when requested, and 79% of all interviews were attended when offered.
Residents spent an average of $5875 (range, $500-$12,000+) on the fellowship interview process (Figure 2). The highest percentage of respondents, 39.5%, selected an average expense between $4000 and $6000. Forty-nine percent of residents borrowed money (from credit cards, additional loans, family members) to pay their expenses.
Average number of days away from residency programs was 11, with 86% of residents missing more than 8 days (Figure 1). About one-third of residents reported being away from their home program for almost 2 weeks during the interview season. Further, 74% of residents wanted changes made to the fellowship application process.
Thirty-seven (82%) of the 45 program directors were from academic programs, the other 8 from community-based programs. Average number of residents in programs per year was 4 (73% of the programs had 4-6 residents per year). Respondents rated the disruption caused by residents’ interview absences from 1 (least disruptive) to 10 (most disruptive) (Figure 3); the average rating was over 7 (high level of disruption). Although 9% of directors thought the process caused little or no disruption (rating, 1-3), 62% thought it extremely disruptive (rating, 8-10).
Thirty-one (69%) of the 45 directors agreed that the fellowship interview process should undergo fundamental change. Asked about possible solutions to current complaints, 60% of the directors agreed that interviews should be conducted in a central location. Of the directors who thought fundamental change was needed, 59% indicated AAOS and other specialty societies together should lead the change in the fellowship interview process.
Both residents and program directors were given the opportunity to write in suggestions regarding how to improve the fellowship interview process. Suggestions were made by 85 (66%) of the 129 residents and 24 (53%) of the 45 directors (Appendix).
Discussion
Graduating residents are entering a health care environment in which they must be financially conscious because of increasing education debt and decreasing reimbursement prospects.3 Nevertheless, an overwhelming majority of residents delay entering practice to pursue fellowship training—an estimated opportunity cost of $350,000.3 Minimal attention has been given to the potential costs of the fellowship interview process.
Our study results highlight that time away from residency training, financial costs associated with the fellowship interview process, and disruption of the residency program are substantial. On average, residents applied to 19 programs, received 14 interview offers, attended 11 interviews, were away from residency training 11 days, and spent $5875 on travel. The great majority of both residents and program directors wanted changes in the current paradigm governing the orthopedic fellowship interview process.
It is reasonable to think that the number of days residents spend away on interviews would reduce the time available for education and patient care. Although unknown, it is plausible that residents of programs outside major metropolitan centers and residents who apply to more competitive fellowships may be forced to spend even more time away from training. Outside the focus of this study are the impact that residents’ absence might have on their education and the impact of this absence on the people who do the residents’ work while they are away.
Mean fellowship expense was similar to that reported by residents pursuing a pediatric general surgery fellowship ($6974) or a plastic surgery fellowship ($6100).11,12 Unfortunately, we were unable to determine if average cost is influenced by choice of fellowship specialty or location of residency program. Regardless, fellowship cost may impose an additional financial burden on residents. According to the Association of American Medical Colleges (AAMC), the median salary for PGY-4 residents was $56,380 in 2013. Therefore, on average, the fellowship process consumes more than 10% of a resident’s pretax salary. For perspective, this equates to more than $40,000 for a practicing orthopedic surgeon with a median salary of $413,000.13 With an average medical student graduate debt of $175,000 and continuing decreases in reimbursement, further financial hardships to newly graduating residents cannot be understated.5,11,12
Almost 70% of program directors thought the fellowship process significantly disrupted their program. Reasons given for this disruption mainly involved residents’ time away from the program and the resulting strains placed on maintaining adequate coverage for patient care. The overall disruption score of 7.4 out of 10 was consistent with the great majority thinking that the fellowship process negatively affects their residency program. Altering the fellowship interview process may provide unintended benefits to programs and program directors.
Both program directors and residents communicated that change is needed, but there was little consensus regarding how to effect change and who should lead. This lack of consensus highlights how important it is for the various orthopedic leadership committees to actively and collectively participate in discussions about redefining the system. It has been proposed that it would be ideal for the AOA to lead the change, as the AOA consists of a representative cohort of academic orthopedists and leaders across the spectrum of all fellowship specialties.14 Given the abundant concern of both residents and program directors, we find it prudent to issue a call to arms of sorts to the AAOS and the individual orthopedic subspecialty societies to work together on a common goal that would benefit residents, programs, and subspecialties within orthopedics.
In trying to understand the challenges that residents, program directors, and programs face, as well as the inherent complexity of the current system, we incorporated respondents’ write-in comments into suggested ways of improving the fellowship interview process. These comments had broad perspectives but overall were consistent with the survey results (Appendix).
Technology
Health care is continually finding new ways to take advantage of technological advances. This is occurring with the fellowship interview schema. Numerous disciplines are using videoconferencing platforms (eg, Skype) to conduct interviews. This practice is becoming more commonplace in the business sector. In a recent survey, more than 60% of human resource managers reported conducting video interviews.15 Two independent residency programs have used video interviews with mixed success.16,17
Another technological change requested by residents is the creation and updating of fellowship web pages with standardized information. Such a service may prove useful to residents researching a program and may even lead to limiting the number of programs residents apply to, as they may be able to dial in on exactly what distinguishes one program from another before traveling for an interview. A recent study of orthopedic sports medicine fellowship programs found that most of these programs lacked pertinent information on their websites.18 Important information regarding case logs from current and former fellows; number of faculty, residents, and fellows; and schedules and facilities of interview sites are a few of the online data points that may help residents differentiate particular programs.19,20 Questions like these are often asked at interviews and site visits. Having accurate information easily available online may reduce or eliminate the need to travel to a site for such information. Standardizing information would also increase transparency among available fellowships. Although not specifically mentioned, organizational software that improves the productivity of the process may help limit the large number of programs applied to, the interviews offered and attended, the days away, and the financial costs without reducing the match rate.
Timing and Location
The issue of timing—with respect to geographical or meteorological concerns—was another recurring theme among respondents. Numerous respondents indicated that certain programs located in geographic proximity tried to minimize travel by offering interviews around the same time. This coordination potentially minimizes travel expenses and time away from the residency program by allowing residents to interview at multiple locations during a single trip per region. The sports medicine fellowship process was identified as a good example of aligning interviews based on geography. Several respondents suggested an option that also reflects the practice of nonsurgical fellowships—delaying the interview season to bypass potential weather concerns. Winter 2013–2014 saw the most flight delays or cancellations in more than a decade; about 50% of all flights scheduled between December and February were delayed or canceled.21 Beyond the additional factor of more time away or missing an interview because of the weather are safety concerns related to the weather. One resident reported having a motor vehicle accident while traveling to an interview in poor weather conditions (Appendix).
National Meetings
Each orthopedic subspecialty has numerous national meetings. Many programs offer applicants the opportunity to interview at these meetings. One respondent mentioned that the annual meeting of the Orthopaedic Trauma Association offers trauma applicants the opportunity to interview with multiple programs. It might be beneficial to endorse this practice on a larger scale to help reduce travel and time away. We recognize that visiting individual programs is an important aspect of the match process, but doing so on a targeted level may make more sense, increasing financial efficiency and reducing time away from programs.
Proposed Solution
A combined proposed solution that can be implemented without a radical overhaul or significant investments might involve moving the interview season to early spring, switching to a 2-tiered system with a centralized first round of interview screening coinciding with subspecialty national meetings or the AAOS annual meeting, and standardizing online information for all orthopedic fellowship programs. A 2-tiered interview process would allow programs and candidates to obtain exposure to a significant number of programs in the first round without incurring significant costs and then would impose a cap on the number of programs to visit. This would level the playing field between candidates with more time and money and candidates who are more constrained in their training environment and finances. A stopgap or adjunct to residents or fellowship programs unable to attend a centralized meeting would be to combine technological tools, such as Internet-based videoconferencing (Skype), before site visits by residents. After this first round of introductions and interviews, residents could then decide on a limited number of programs to formally visit, attend, and ultimately rank. This proposed system would still be able to function within the confines of the match, and it would benefit from the protections offered to residents and programs. Although capping the number of interviews attended by residents clearly can lower costs across the board, we recognize the difficulty of enforcing such a requirement. These potential changes to the system are not exhaustive, and we hope this work will serve as a springboard to further discussion.
Our study had several inherent weaknesses. Our data came from survey responses, which reflect the perspectives only of the responding residents and program directors. Unfortunately, a small number of orthopedic residents responded to this survey, so there was a potential for bias. However, we think the central themes discovered in this survey are only echoes of the concerns of the larger population of residents and program directors. Our hope in designing such a study was to bring to light some of the discrepancies in the fellowship interview process, the goal being to stimulate interest among the orthopedic leadership representing future orthopedic surgeons. More study is needed to clarify if these issues are reflective of a larger segment of residents and program directors. In addition, action may be needed to fully elucidate the intricate interworking of the fellowship process in order to maximize the interest of the orthopedic surgeons who are seeking fellowship training. Another study limitation was the potential for recall bias in the more senior PGY-5 residents, who were further from the interview process than PGY-4 respondents were. Because of the need for anonymity with the surveys, we could not link some findings (eg, program impact, cost, time away) to individual programs or different specialty fellowships. Although it appears there is a desire for a more cost-effective system, given the financial pressures on medical students and residents, the desire to match increases costs because students are likely to attend more interviews than actually needed. Our proposed solution does not take into account residents’ behavior with respect to the current match system. For example, the prevailing thought is that interviewing at more programs increases the likelihood of matching into a desired subspecialty. Despite these study limitations, we think our results identified important points for discussion, investigation, and potential action by orthopedic leadership.
Conclusion
The challenge of critiquing and improving the orthopedic fellowship process requires the same courageous leadership that was recommended almost a decade ago.14 In this study, we tried to elucidate the impact of the PGY-4 fellowship interview process with respect to residents and residency programs. Our results highlight that time away from residency training, financial costs associated with the fellowship interview process, and disruption of the residency program are substantial and that both residents and program directors want changes made. Leadership needs to further investigate alternatives to the current process to lessen the impact on all parties in this important process.
1. Simon MA. Evolution of the present status of orthopaedic surgery fellowships. J Bone Joint Surg Am. 1998;80(12):1826-1829.
2. Brunworth LS, Chintalapani SR, Gray RR, Cardoso R, Owens PW. Resident selection of hand surgery fellowships: a survey of the 2011, 2012, and 2013 hand fellowship graduates. Hand. 2013;8(2):164-171.
3. Gaskill T, Cook C, Nunley J, Mather RC. The financial impact of orthopaedic fellowship training. J Bone Joint Surg Am. 2009;91(7):1814-1821.
4. Sarmiento A. Additional thoughts on orthopedic residency and fellowships. Orthopedics. 2010;33(10):712-713.
5. Griffin SM, Stoneback JW. Navigating the Orthopaedic Trauma Fellowship Match from a candidate’s perspective. J Orthop Trauma. 2011;25(suppl 3):S101-S103.
6. Morrell NT, Mercer DM, Moneim MS. Trends in the orthopedic job market and the importance of fellowship subspecialty training. Orthopedics. 2012;35(4):e555-e560.
7. Iorio R, Robb WJ, Healy WL, et al. Orthopaedic surgeon workforce and volume assessment for total hip and knee replacement in the United States: preparing for an epidemic. J Bone Joint Surg Am. 2008;90(7):1598-1605.
8. Emery SE, Guss D, Kuremsky MA, Hamlin BR, Herndon JH, Rubash HE. Resident education versus fellowship training—conflict or synergy? AOA critical issues. J Bone Joint Surg Am. 2012;94(21):e159.
9. Harner CD, Ranawat AS, Niederle M, et al. AOA symposium. Current state of fellowship hiring: is a universal match necessary? Is it possible? J Bone Joint Surg Am. 2008;90(6):1375-1384.
10. Ranawat A, Nunley RM, Genuario JW, Sharan AD, Mehta S; Washington Health Policy Fellows. Current state of the fellowship hiring process: Are we in 1957 or 2007? AAOS Now. 2007;1(8).
11. Little DC, Yoder SM, Grikscheit TC, et al. Cost considerations and applicant characteristics for the Pediatric Surgery Match. J Pediatr Surg. 2005;40(1):69-73.
12. Claiborne JR, Crantford JC, Swett KR, David LR. The Plastic Surgery Match: predicting success and improving the process. Ann Plast Surg. 2013;70(6):698-703.
13. Kane L, Peckham C. Medscape Physician Compensation Report 2014. http://www.medscape.com/features/slideshow/compensation/2014/public/overview. Published April 15, 2014. Accessed September 26, 2015.
14. Swiontkowski MF. A simple formula for continued improvement in orthopaedic surgery postgraduate training: courageous leadership. J Bone Joint Surg Am. 2008;90(6):1175.
15. Survey: six in 10 companies conduct video job interviews [news release]. http://www.prnewswire.com/news-releases/survey-six-in-10-companies-conduct-video-job-interviews-167973406.html. Published August 30, 2012. Accessed September 26, 2015.
16. Kerfoot BP, Asher KP, McCullough DL. Financial and educational costs of the residency interview process for urology applicants. Urology. 2008;71(6):990-994.
17. Edje L, Miller C, Kiefer J, Oram D. Using Skype as an alternative for residency selection interviews. J Grad Med Educ. 2013;5(3):503-505.
18. Mulcahey MK, Gosselin MM, Fadale PD. Evaluation of the content and accessibility of web sites for accredited orthopaedic sports medicine fellowships. J Bone Joint Surg Am. 2013;95(12):e85.
19. Gaeta TJ, Birkhahn RH, Lamont D, Banga N, Bove JJ. Aspects of residency programs’ web sites important to student applicants. Acad Emerg Med. 2005;12(1):89-92.
20. Mahler SA, Wagner MJ, Church A, Sokolosky M, Cline DM. Importance of residency program web sites to emergency medicine applicants. J Emerg Med. 2009;36(1):83-88.
21. Davies A. Winter’s toll: 1 million flights cancelled or delayed, costing travelers $5.3 billion. Business Insider. http://www.businessinsider.com/winter-flights-cancelled-delayed-cost-2014-3. Published March 3, 2014. Accessed September 26, 2015.
1. Simon MA. Evolution of the present status of orthopaedic surgery fellowships. J Bone Joint Surg Am. 1998;80(12):1826-1829.
2. Brunworth LS, Chintalapani SR, Gray RR, Cardoso R, Owens PW. Resident selection of hand surgery fellowships: a survey of the 2011, 2012, and 2013 hand fellowship graduates. Hand. 2013;8(2):164-171.
3. Gaskill T, Cook C, Nunley J, Mather RC. The financial impact of orthopaedic fellowship training. J Bone Joint Surg Am. 2009;91(7):1814-1821.
4. Sarmiento A. Additional thoughts on orthopedic residency and fellowships. Orthopedics. 2010;33(10):712-713.
5. Griffin SM, Stoneback JW. Navigating the Orthopaedic Trauma Fellowship Match from a candidate’s perspective. J Orthop Trauma. 2011;25(suppl 3):S101-S103.
6. Morrell NT, Mercer DM, Moneim MS. Trends in the orthopedic job market and the importance of fellowship subspecialty training. Orthopedics. 2012;35(4):e555-e560.
7. Iorio R, Robb WJ, Healy WL, et al. Orthopaedic surgeon workforce and volume assessment for total hip and knee replacement in the United States: preparing for an epidemic. J Bone Joint Surg Am. 2008;90(7):1598-1605.
8. Emery SE, Guss D, Kuremsky MA, Hamlin BR, Herndon JH, Rubash HE. Resident education versus fellowship training—conflict or synergy? AOA critical issues. J Bone Joint Surg Am. 2012;94(21):e159.
9. Harner CD, Ranawat AS, Niederle M, et al. AOA symposium. Current state of fellowship hiring: is a universal match necessary? Is it possible? J Bone Joint Surg Am. 2008;90(6):1375-1384.
10. Ranawat A, Nunley RM, Genuario JW, Sharan AD, Mehta S; Washington Health Policy Fellows. Current state of the fellowship hiring process: Are we in 1957 or 2007? AAOS Now. 2007;1(8).
11. Little DC, Yoder SM, Grikscheit TC, et al. Cost considerations and applicant characteristics for the Pediatric Surgery Match. J Pediatr Surg. 2005;40(1):69-73.
12. Claiborne JR, Crantford JC, Swett KR, David LR. The Plastic Surgery Match: predicting success and improving the process. Ann Plast Surg. 2013;70(6):698-703.
13. Kane L, Peckham C. Medscape Physician Compensation Report 2014. http://www.medscape.com/features/slideshow/compensation/2014/public/overview. Published April 15, 2014. Accessed September 26, 2015.
14. Swiontkowski MF. A simple formula for continued improvement in orthopaedic surgery postgraduate training: courageous leadership. J Bone Joint Surg Am. 2008;90(6):1175.
15. Survey: six in 10 companies conduct video job interviews [news release]. http://www.prnewswire.com/news-releases/survey-six-in-10-companies-conduct-video-job-interviews-167973406.html. Published August 30, 2012. Accessed September 26, 2015.
16. Kerfoot BP, Asher KP, McCullough DL. Financial and educational costs of the residency interview process for urology applicants. Urology. 2008;71(6):990-994.
17. Edje L, Miller C, Kiefer J, Oram D. Using Skype as an alternative for residency selection interviews. J Grad Med Educ. 2013;5(3):503-505.
18. Mulcahey MK, Gosselin MM, Fadale PD. Evaluation of the content and accessibility of web sites for accredited orthopaedic sports medicine fellowships. J Bone Joint Surg Am. 2013;95(12):e85.
19. Gaeta TJ, Birkhahn RH, Lamont D, Banga N, Bove JJ. Aspects of residency programs’ web sites important to student applicants. Acad Emerg Med. 2005;12(1):89-92.
20. Mahler SA, Wagner MJ, Church A, Sokolosky M, Cline DM. Importance of residency program web sites to emergency medicine applicants. J Emerg Med. 2009;36(1):83-88.
21. Davies A. Winter’s toll: 1 million flights cancelled or delayed, costing travelers $5.3 billion. Business Insider. http://www.businessinsider.com/winter-flights-cancelled-delayed-cost-2014-3. Published March 3, 2014. Accessed September 26, 2015.
The Effect of Arthroscopic Rotator Interval Closure on Glenohumeral Volume
Since Neer described the rotator interval in 1970, its closure, often used in conjunction with capsulorrhaphy, has become an important surgical technique in managing shoulder instability.1-11 Numerous studies have sought to define the function of the rotator interval.1-3,6-20 The etiology of lesions of the rotator interval has been debated, and there is evidence that such lesions may be in part congenital.21 Increased rotator interval depth and width, along with increased size of the distended inferior and posteroinferior joint capsule on magnetic resonance arthrography, have been reported in cases of multidirectional shoulder instability.22 However, confusion remains about the role of the rotator interval in shoulder instability and about the effect its closure has on shoulder function. No one knows the degree of volume reduction that results from closure of the rotator interval and whether medial and lateral sutures differ in the volume reduction achieved.
Cadaveric studies have shown that the rotator interval has an important role in shoulder motion.6,13-16,19,20,23 Harryman and colleagues13 found that sectioning the coracohumeral ligament (CHL) increased shoulder range of motion (ROM), and medial-to-lateral closure of the rotator interval restricted motion in all planes. Most notably, interval closure limited inferior translation in the adducted shoulder, posterior translation in the flexed adducted shoulder, and external rotation in the neutral position. Subsequent studies,17,18 using rotator interval closure combined with thermal capsulorrhaphy, confirmed the results reported by Harryman and colleagues.13
More recent cadaveric studies using superior-to-inferior rotator interval closures have shown a decrease in anterior translation but not posterior translation.14-16,19-21 A superior-to-inferior interval closure technique limited external rotation less than a medial-to-lateral closure did.13-16,19-21 The majority of arthroscopically described rotator interval closures involve a superior-to-inferior technique and use 2 or 3 sutures.1,3,9-11
Plausinis and colleagues15 examined the effects of an isolated medial, an isolated lateral, and a medial combined with a lateral closure of the rotator interval. They noted that all 3 methods limited anterior translation and motion by means of 6° flexion and 10° external rotation; however, there was no statistical difference between methods. They also found that occasionally the medial interval closure resulted in massive loss of external rotation. Earlier, Jost and colleagues14 noted that a medial rotator interval could cause this massive loss by tethering the CHL, resulting in a medial-to-lateral imbrication of the CHL.
Arthroscopic rotator interval closure has clinically demonstrated an additive effect on shoulder stability. The recurrence rate was lower for arthroscopic Bankart repair combined with arthroscopic rotator interval closure (8%) than for arthroscopic Bankart repair alone (13%).24 In addition, time to recurrent dislocation was longer (42 vs 13 months) for the group that underwent the combination of Bankart repair and rotator interval closure. Regarding the concern about loss of motion after arthroscopic rotator interval closure, Chiang and colleagues25 recently noted no significant loss of motion 5 years after arthroscopic Bankart repair with rotator interval closure.
What effect rotator interval closure has on intra-articular glenohumeral volume (GHV) remains unknown. Using a cadaveric model, Yamamoto and colleagues20 showed that decreasing GHV can increase the responsiveness of the glenohumeral joint to the intra-articular pressure. Thus, reducing the volume can improve stability in vitro by increasing the magnitude of negative pressure stabilizing the glenohumeral joint.
We conducted a study to quantify the effects of arthroscopic rotator interval closure on capsular volume and to determine whether medial and lateral interval closures resulted in different degrees of volume reduction. Our hypothesis was that shoulder volume would be significantly reduced by closing the rotator interval.
Materials and Methods
Previous studies have not specifically evaluated GHV after rotator interval closure. Our power analysis was performed with data from a study by Karas and colleagues,26 who evaluated GHV after capsular plication. To detect a capsular volume reduction of 20% per stitch, with a 2-sided 5% significance level and a power of 80%, we needed a sample size of 5 specimens per group.
After receiving institutional review board approval for this study, we obtained 10 cadaveric shoulders (5 matched pairs). Exclusion criteria included arthroscopic evaluation revealing a full-thickness rotator cuff tear or significant osteoarthritis. Two shoulders had full-thickness cuff tears, leaving 8 shoulders to be tested; 6 of these were matched pairs. The shoulders were from 1 man (matched pair) and 4 women (2 matched pairs). Age ranged from 38 to 70 years (mean, 59.6 years). Differences in material properties between the specimens were accounted for by using primarily matched pairs.
The 2 study groups consisted of 4 shoulders each. After specimens were thawed, the skin, subcutaneous tissues, and periscapular muscles were removed from the shoulder. Only the capsule, biceps, and rotator cuff remained. For measurement purposes, the shoulders were mounted in a vice clamp in a beach-chair orientation. We placed a total of 2 portals with fully threaded 8.25-mm cannulas (Arthrex, Naples, Florida). A standard posterior portal was placed in the soft spot. A low anterior portal was then placed just superior to the subscapularis tendon. For arthroscopic examination and instrumentation in a saline environment, the shoulders were rotated into the lateral decubitus position, with suspension in 30° abduction and 20° forward flexion, by a rope attached to a pin in the distal shaft of the humerus.
In both groups, medial and lateral stitches with No. 2 FiberWire (Arthrex) were used to close the interval. The medial interval closure stitch was placed more than 10 mm away from the glenoid to prevent unpredictable CHL tethering; the lateral closure stitch was placed 10 mm lateral to the medial stitch (Figure 1).14 All sutures were placed intra-articularly under direct arthroscopic visualization, similar to the methods described in the literature.1,3,9-11 Sutures were passed through the superior glenohumeral ligament (SGHL) and through the upper subscapularis using a suture shuttle (SutureLasso; Arthrex) and Penetrator II Suture Retriever (Arthrex). The upper subscapularis was incorporated because of the unpredictable nature of the middle glenohumeral ligament (MGHL). Both rotator interval sutures were placed before tying either. In the medial group, the medial stitch was tied first, using alternating half-hitches, followed by the lateral stitch. In the lateral group, the lateral stitch was tied first, followed by the medial stitch. GHV was measured at baseline and after tying each stitch. Dr. Ponce instrumented all shoulders.
Modifying a beach-chair technique described by Miller and colleagues,27 we used a viscous fatty-acid sulfate solution, liquid soap, to measure GHV.27-29 A small slit in line with the fibers was made in the supraspinatus tendon just lateral to the musculotendinous junction. A 3-way stop-cock was placed into the joint though this defect. A 20-mL syringe with a 16-gauge needle was used to inject the soap. The needle was inserted into the rotator cuff interval, and the viscous solution was injected in 5-mL increments until there was active extravasation through the supraspinatus cannula (Figure 2). This technique, the “volcano method,” marked the maximum capacity of the joint. The joint was then copiously irrigated with normal saline and suctioned until all normal saline was evacuated. Dr. Rosenzweig took 2 measurements on each shoulder, and their mean was used for analysis.
The baseline measurement was taken with the 2 working cannulas in the shoulder joint. Measurements were obtained with cannulas to simulate normal clinical conditions. Subsequent measurements were done with the cannulas in place and inserted up to the same thread each time so as not to change the volume. The capsule and the rotator cuff were then dissected from the humerus so the size of the capsulolabral plication could be directly evaluated. Methylene blue was used to mark the capsular suture holes before removing the sutures. With use of a caliper, the size of the plication bite was measured (in millimeters).
Statistical Analysis
The primary outcome was percent reduction in GHV as a function of number of plications and size of plication. When only the first plication was tightened, the effect of position (medial or lateral) was also of interest. Percent volume reduction was calculated as (original – new) / original × 100. SAS 8.02 (SAS Institute, Cary, North Carolina) was used to fit a repeated random-intercept regression model for each outcome. This technique properly accounts for the paired nature of the specimens and the repeated measures (baseline plus 2 plications). Model fit was assessed by the method of difference in log likelihood.
Results
In the medial group, GHV was reduced by a mean of 24.2% with a single medial stitch; in the lateral group, GHV was reduced by a mean of 35.1% (Figure 3). The difference was significant (P < .02). In the medial group, when a second lateral stitch was used, GHV was reduced by another 18.7%; in the lateral group, when a medial stitch was added, GHV was reduced by another 11.4%. Final GHV for the medial and lateral groups was 42.9% and 46.5%, respectively. There was no statistical difference in final GHV, regardless of which stitch was placed first. When the 2 groups were combined, GHV was reduced by 44.9% with use of medial and lateral rotator interval closure stitches.
Mean amount of tissue purchased, or “bite size,” was 18 mm with a lateral suture and 15 mm with a medial suture (P < .05). In addition, an increase in bite size to GHV reduction was essentially linear, where an increase in bite size of 1 mm reduced GHV by about 1% (Figure 4).
Discussion
Although there have been numerous clinical series and biomechanical studies focused on isolated rotator interval closure (or its use as an adjunct) in shoulder stabilization, the precise function of the rotator interval remains poorly understood.1-3,6-11,19 Consequently, the in vivo effects of interval closure are unknown.
Initial studies proposed that rotator interval closure limited inferior and posterior translation.30 More recent studies have demonstrated that rotator interval closure confers little effect on posterior instability but increases anterior stability in cadaveric models.15,16 Clinical series have provided evidence that rotator interval closure can increase anterior stability.1,3,7,9,12 In a series of isolated rotator interval closures for multidirectional instability, Field and colleagues12 found that preoperative anterior and inferior symptoms predominated over posterior symptoms. Isolated closure of the rotator interval resulted in 100% excellent results with no cases of recurrent instability. Moon and colleagues31 reported that arthroscopic rotator interval closure with or without inferior capsular plication in multidirectional instability and predominant symptomatic inferior instability has shown benefit by improving function and stability. Other clinical reports of rotator interval closure in conjunction with arthroscopic Bankart repair have suggested it has an additive effect on anterior shoulder stability without limiting motion.24,25
In our study, arthroscopic closure of the rotator interval with 2 superior-to-inferior stitches reduced intracapsular volume by 45%. Even though open capsular shifts use different surgical techniques, similar technique volume reduction studies have reported reductions between 34% and 54% with open shifts.27,30 It is unknown if the stability resulting from decreased GHV is primarily from increasing intra-articular pressures or from restricting ROM, or from a combination of both. In shoulders with multidirectional instability, the joint volume may be increased, the joint capsule may be enlarged, or the glenohumeral ligaments may be lax and thin.4,6,32,33 Yamamoto and colleagues19 stated that intra-articular pressure is determined by 3 factors: load, joint volume, and material properties of the capsule. Load is a constant; joint volume and material properties can be changed.19 In our study, material properties were controlled by using a majority of matched specimens. Regardless of the stabilizing mechanism, our study results demonstrated that arthroscopic rotator interval closure may be a powerful tool in reducing shoulder volume, a consistent principle of surgical techniques used in reestablishing shoulder stability.19,20
When a single rotator interval closure stitch was used, volume reduction with a lateral stitch was superior to that with a medial stitch. This finding is logical, as anatomically the dimensions of the rotator interval are larger laterally as the CHL fans out to insert on the greater and lesser tuberosities.14 This finding has also been reported in open capsular shifts for multidirectional instability, with a lateral humeral shift having a larger volume reduction than a medial glenoid shift.27 Miller and colleagues27 used the image of a cone, with its larger opening facing the humerus and narrower side facing the glenoid, to illustrate this difference in open capsular shifts.
Our study also showed a larger volume reduction with 2 rotator interval closure stitches than with a single interval stitch. As ROM testing has not shown a difference between results with 1 and 2 sutures, we recommend a minimum of 2 sutures for arthroscopic rotator interval closure.15 If a single plication stitch is preferred, a lateral stitch (vs a medial stitch) can be used for a significantly larger reduction in shoulder volume. We think this is because of a larger amount of capsule being purchased with lateral closure (Figure 5). However, if a medial stitch is used, it is important to not place it too near the glenoid to avoid CHL tethering and subsequent excessive loss of external rotation.15
This study had several weaknesses. First, it was a cadaveric study, and use of specimens not known to have instability or specific rotator interval injury may make generalization to a clinical situation difficult. Second, although our power analysis called for 5 shoulders in each group, full-thickness rotator cuff tears rendered 2 shoulders unusable. This reduced our sample sizes and potentially decreased the power of the study, though the data demonstrated statistically significant differences. Third, we did not compare the effects of an open medial-to-lateral imbrication of the rotator interval on intracapsular volume with the effects of our arthroscopic method. We also did not assess our specimens’ ROM, effects of interval closure stitches on shoulder stability, or glenohumeral contact surface pressures, as these factors have already been studied.13-19 Instead, we focused on the effects of rotator interval closure on intracapsular volume, which had not been quantified until now. The clinical significance of such a volume reduction is unknown, especially with respect to influence on ROM, but the degree of volume reduction was larger than with previously reported arthroscopic instability repairs and smaller than with open capsular shifts, demonstrating that it may be a powerful tool in restoring stability in an unstable shoulder.26-30,34 Fourth, the role of isolated rotator interval closure is poorly defined, as only 1 clinical series of isolated rotator interval closure has been reported thus far.12 It has been far more common for rotator interval closure to be used with Bankart repair or capsulorrhaphy.1-3,7-9
In a cadaveric study by Provencher and colleagues,16 open rotator interval closure with medial-to-lateral imbrication of the interval altered shoulder kinematics differently from what occurred with arthroscopic closure of the MGHL to the SGHL, resulting in superior-to-inferior shift. Comparing the 2 methods may therefore be inappropriate. Currently we reserve rotator interval closure for infrequent cases of revision instability and cases in which glenoid bone loss is marginal (5%-15%) and there is a willingness to potentially sacrifice ROM to restore stability and avoid an open stabilization procedure. Continued investigation into the clinical role of rotator interval closure in shoulder stability is needed. We should identify the pathology in a patient with instability and use this technique as an adjuvant to other stabilization procedures.
Conclusion
Arthroscopic rotator interval closure with 2 plication stitches is a powerful tool in reducing the intracapsular volume of the shoulder. If a single plication stitch is preferred, a lateral rotator interval closure stitch (vs a medial stitch) can be used for a larger reduction in shoulder volume.
1. Creighton RA, Romeo AA, Brown FM, Hayden JK, Verma NN. Revision arthroscopic shoulder instability repair. Arthroscopy. 2007;23(7):703-709.
2. Gartsman GM, Roddey TS, Hammerman SM. Arthroscopic treatment of anterior-inferior glenohumeral instability. Two to five-year follow-up. J Bone Joint Surg Am. 2000;82(7):991-1003.
3. Gartsman GM, Taverna E, Hammerman SM. Arthroscopic rotator interval repair in glenohumeral instability: description of an operative technique. Arthroscopy. 1999;15(3):330-332.
4. Neer CS 2nd, Foster CR. Inferior capsular shift for involuntary inferior and multidirectional instability of the shoulder: a preliminary report. J Bone Joint Surg Am. 1980;62(6):897-908.
5. Neer CS 2nd. Displaced proximal humerus fractures: I. Classification and evaluation. J Bone Joint Surg Am. 1970;52(6):1077-1089.
6. Nobuhara K, Ikeda H. Rotator interval lesion. Clin Orthop. 1987;(223):44-50.
7. Rowe CR, Zarins B, Ciullo JV. Recurrent anterior dislocation of the shoulder after surgical repair. J Bone Joint Surg Am. 1984;66(2):159-168.
8. Rowe CR, Zarins B. Recurrent transient subluxation of the shoulder. J Bone Joint Surg Am. 1981;63(6):863-872.
9. Stokes DA, Savoie FH, Field LD. Arthroscopic repair of anterior glenohumeral instability and rotator interval lesions. Orthop Clin North Am. 2003;34(4):529-539.
10. Taverna E, Sansone V, Battistella F. Arthroscopic rotator interval repair: the three-step all-inside technique. Arthroscopy. 2004;20 Suppl 2:105-109.
11. Treacy SH, Field LD, Savoie FH. Rotator interval capsule closure: an arthroscopic technique. Arthroscopy. 1997;13(1):103-106.
12. Field LD, Warren RF, O’Brien SJ, Altcheck DW, Wickiewicz TL. Isolated closure of rotator interval defects for shoulder instability. Am J Sports Med. 1995;23(5):557-563.
13. Harryman DT 2nd, Sidles JA, Harris SL, Matsen FA 3rd. The role of the rotator interval capsule in passive motion and stability of the shoulder. J Bone Joint Surg Am. 1992;74(1):53-66.
14. Jost B, Koch PP, Gerber C. Anatomy and functional aspects of the rotator interval. J Shoulder Elbow Surg. 2000;9(4):336-341.
15. Plausinis D, Bravman JT, Heywood C, Kummer FJ, Kwon YM, Jazrawi LM. Arthroscopic rotator interval closure: effect of sutures on glenohumeral motion and anterior-posterior translation. Am J Sports Med. 2006;34(10):1656-1661.
16. Provencher MT, Mologne TS, Hongo M, Zhao K, Tasto JP, An KN. Arthroscopic versus open rotator interval closure: biomechanical evaluation of stability and motion. Arthroscopy. 2007;23(6):583-592.
17. Selecky MT, Tibone JE, Yang BY, et al. Glenohumeral joint translation after thermal capsuloplasty of the rotator interval. J Shoulder Elbow Surg. 2003;12(2):139-143.
18. Wolf R, Zheng N, Iero J, Weichel D. The effects of thermal capsulorrhaphy and rotator interval closure on multidirectional laxity in the glenohumeral joint: a cadaveric biomechanical study. Arthroscopy. 2004;20(10):1044-1049.
19. Yamamoto N, Itoi E, Tuoheti Y, et al. Effect of rotator interval closure on glenohumeral stability and motion: a cadaveric study. J Shoulder Elbow Surg. 2006;15(6):750-758.
20. Yamamoto N, Itoi E, Tuoheti Y, et al. The effect of the inferior capsular shift on shoulder intra-articular pressure: a cadaveric study. Am J Sports Med. 2006;34(6):939-944.
21. Cole BJ, Rodeo SA, O’Brien SJ, et al. The anatomy and histology of the rotator interval capsule of the shoulder. Clin Orthop. 2001;(390):129-137.
22. Lee HJ, Kim NR, Moon SG, Ko SM, Park JY. Multidirectional instability of the shoulder: rotator interval dimension and capsular laxity evaluation using MR arthrography. Skeletal Radiol. 2013;42(2):231-238.
23. Warner JP, Deng X, Warren RF, Torzilli PA, O’Brien SJ. Superoinferior translation in intact and vented glenohumeral joint. J Shoulder Elbow Surg. 1993;2(2):99-105.
24. Chechik O, Maman E, Dolkart O, Khashan M, Shabtai L, Mozes G. Arthroscopic rotator interval closure in shoulder instability repair: a retrospective study. J Shoulder Elbow Surg. 2010;19(7):1056-1062.
25. Chiang, E, Wang J, Wang S, et al. Arthroscopic posteroinferior capsular plication and rotator interval closure after Bankart repair in patients with traumatic anterior glenohumeral instability—a minimum follow-up of 5 years. Injury. 2010;41(10):1075-1078.
26. Karas SG, Creighton RA, DeMorat GJ. Glenohumeral volume reduction in arthroscopic shoulder reconstruction: a cadaveric analysis of suture plication and thermal capsulorrhaphy. Arthroscopy. 2004;20(2):179-184.
27. Miller MD, Larsen KM, Luke T, Leis HT, Plancher KD. Anterior capsular shift volume reduction: an in vitro comparison of 3 techniques. J Shoulder Elbow Surg. 2003;12(4):350-354.
28. Luke TA, Rovner AD, Karas SG, Hawkins RJ, Plancher KD. Volumetric change in the shoulder capsule after open inferior capsular shift versus arthroscopic thermal capsular shrinkage: a cadaveric model. J Shoulder Elbow Surg. 2004;13(2):146-149.
29. Ponce BA, Rosenzweig SD, Thompson KJ, Tokish J. Sequential volume reduction with capsular plications: relationship between cumulative size of plications and volumetric reduction for multidirectional instability of the shoulder. Am J Sports Med. 2011;39(3):526-531.
30. Lubowitz J, Bartolozzi A, Rubenstein D, et al. How much does inferior capsular shift reduce shoulder volume? Clin Orthop. 1996;(328):86-90.
31. Moon YL, Singh H, Yang H, Chul LK. Arthroscopic rotator interval closure by purse string suture for symptomatic inferior shoulder instability. Orthopedics. 2011;34(4).
32. Jerosch J, Castro WH. Shoulder instability in Ehlers-Danlos syndrome: an indication for surgical treatment? Acta Orthop Belg. 1990;56(2):451-453.
33. Schenk TJ, Brems JJ. Multidirectional instability of the shoulder: pathophysiology, diagnosis, and management. J Am Acad Orthop Surg. 1998;6(1):65-72.
34. Cohen SB, Wiley W, Goradia VK, Pearson S, Miller MD. Anterior capsulorrhaphy: an in vitro comparison of volume reduction. Arthroscopic plication versus open capsular shift. Arthroscopy. 2005;21(6):659-664.
Since Neer described the rotator interval in 1970, its closure, often used in conjunction with capsulorrhaphy, has become an important surgical technique in managing shoulder instability.1-11 Numerous studies have sought to define the function of the rotator interval.1-3,6-20 The etiology of lesions of the rotator interval has been debated, and there is evidence that such lesions may be in part congenital.21 Increased rotator interval depth and width, along with increased size of the distended inferior and posteroinferior joint capsule on magnetic resonance arthrography, have been reported in cases of multidirectional shoulder instability.22 However, confusion remains about the role of the rotator interval in shoulder instability and about the effect its closure has on shoulder function. No one knows the degree of volume reduction that results from closure of the rotator interval and whether medial and lateral sutures differ in the volume reduction achieved.
Cadaveric studies have shown that the rotator interval has an important role in shoulder motion.6,13-16,19,20,23 Harryman and colleagues13 found that sectioning the coracohumeral ligament (CHL) increased shoulder range of motion (ROM), and medial-to-lateral closure of the rotator interval restricted motion in all planes. Most notably, interval closure limited inferior translation in the adducted shoulder, posterior translation in the flexed adducted shoulder, and external rotation in the neutral position. Subsequent studies,17,18 using rotator interval closure combined with thermal capsulorrhaphy, confirmed the results reported by Harryman and colleagues.13
More recent cadaveric studies using superior-to-inferior rotator interval closures have shown a decrease in anterior translation but not posterior translation.14-16,19-21 A superior-to-inferior interval closure technique limited external rotation less than a medial-to-lateral closure did.13-16,19-21 The majority of arthroscopically described rotator interval closures involve a superior-to-inferior technique and use 2 or 3 sutures.1,3,9-11
Plausinis and colleagues15 examined the effects of an isolated medial, an isolated lateral, and a medial combined with a lateral closure of the rotator interval. They noted that all 3 methods limited anterior translation and motion by means of 6° flexion and 10° external rotation; however, there was no statistical difference between methods. They also found that occasionally the medial interval closure resulted in massive loss of external rotation. Earlier, Jost and colleagues14 noted that a medial rotator interval could cause this massive loss by tethering the CHL, resulting in a medial-to-lateral imbrication of the CHL.
Arthroscopic rotator interval closure has clinically demonstrated an additive effect on shoulder stability. The recurrence rate was lower for arthroscopic Bankart repair combined with arthroscopic rotator interval closure (8%) than for arthroscopic Bankart repair alone (13%).24 In addition, time to recurrent dislocation was longer (42 vs 13 months) for the group that underwent the combination of Bankart repair and rotator interval closure. Regarding the concern about loss of motion after arthroscopic rotator interval closure, Chiang and colleagues25 recently noted no significant loss of motion 5 years after arthroscopic Bankart repair with rotator interval closure.
What effect rotator interval closure has on intra-articular glenohumeral volume (GHV) remains unknown. Using a cadaveric model, Yamamoto and colleagues20 showed that decreasing GHV can increase the responsiveness of the glenohumeral joint to the intra-articular pressure. Thus, reducing the volume can improve stability in vitro by increasing the magnitude of negative pressure stabilizing the glenohumeral joint.
We conducted a study to quantify the effects of arthroscopic rotator interval closure on capsular volume and to determine whether medial and lateral interval closures resulted in different degrees of volume reduction. Our hypothesis was that shoulder volume would be significantly reduced by closing the rotator interval.
Materials and Methods
Previous studies have not specifically evaluated GHV after rotator interval closure. Our power analysis was performed with data from a study by Karas and colleagues,26 who evaluated GHV after capsular plication. To detect a capsular volume reduction of 20% per stitch, with a 2-sided 5% significance level and a power of 80%, we needed a sample size of 5 specimens per group.
After receiving institutional review board approval for this study, we obtained 10 cadaveric shoulders (5 matched pairs). Exclusion criteria included arthroscopic evaluation revealing a full-thickness rotator cuff tear or significant osteoarthritis. Two shoulders had full-thickness cuff tears, leaving 8 shoulders to be tested; 6 of these were matched pairs. The shoulders were from 1 man (matched pair) and 4 women (2 matched pairs). Age ranged from 38 to 70 years (mean, 59.6 years). Differences in material properties between the specimens were accounted for by using primarily matched pairs.
The 2 study groups consisted of 4 shoulders each. After specimens were thawed, the skin, subcutaneous tissues, and periscapular muscles were removed from the shoulder. Only the capsule, biceps, and rotator cuff remained. For measurement purposes, the shoulders were mounted in a vice clamp in a beach-chair orientation. We placed a total of 2 portals with fully threaded 8.25-mm cannulas (Arthrex, Naples, Florida). A standard posterior portal was placed in the soft spot. A low anterior portal was then placed just superior to the subscapularis tendon. For arthroscopic examination and instrumentation in a saline environment, the shoulders were rotated into the lateral decubitus position, with suspension in 30° abduction and 20° forward flexion, by a rope attached to a pin in the distal shaft of the humerus.
In both groups, medial and lateral stitches with No. 2 FiberWire (Arthrex) were used to close the interval. The medial interval closure stitch was placed more than 10 mm away from the glenoid to prevent unpredictable CHL tethering; the lateral closure stitch was placed 10 mm lateral to the medial stitch (Figure 1).14 All sutures were placed intra-articularly under direct arthroscopic visualization, similar to the methods described in the literature.1,3,9-11 Sutures were passed through the superior glenohumeral ligament (SGHL) and through the upper subscapularis using a suture shuttle (SutureLasso; Arthrex) and Penetrator II Suture Retriever (Arthrex). The upper subscapularis was incorporated because of the unpredictable nature of the middle glenohumeral ligament (MGHL). Both rotator interval sutures were placed before tying either. In the medial group, the medial stitch was tied first, using alternating half-hitches, followed by the lateral stitch. In the lateral group, the lateral stitch was tied first, followed by the medial stitch. GHV was measured at baseline and after tying each stitch. Dr. Ponce instrumented all shoulders.
Modifying a beach-chair technique described by Miller and colleagues,27 we used a viscous fatty-acid sulfate solution, liquid soap, to measure GHV.27-29 A small slit in line with the fibers was made in the supraspinatus tendon just lateral to the musculotendinous junction. A 3-way stop-cock was placed into the joint though this defect. A 20-mL syringe with a 16-gauge needle was used to inject the soap. The needle was inserted into the rotator cuff interval, and the viscous solution was injected in 5-mL increments until there was active extravasation through the supraspinatus cannula (Figure 2). This technique, the “volcano method,” marked the maximum capacity of the joint. The joint was then copiously irrigated with normal saline and suctioned until all normal saline was evacuated. Dr. Rosenzweig took 2 measurements on each shoulder, and their mean was used for analysis.
The baseline measurement was taken with the 2 working cannulas in the shoulder joint. Measurements were obtained with cannulas to simulate normal clinical conditions. Subsequent measurements were done with the cannulas in place and inserted up to the same thread each time so as not to change the volume. The capsule and the rotator cuff were then dissected from the humerus so the size of the capsulolabral plication could be directly evaluated. Methylene blue was used to mark the capsular suture holes before removing the sutures. With use of a caliper, the size of the plication bite was measured (in millimeters).
Statistical Analysis
The primary outcome was percent reduction in GHV as a function of number of plications and size of plication. When only the first plication was tightened, the effect of position (medial or lateral) was also of interest. Percent volume reduction was calculated as (original – new) / original × 100. SAS 8.02 (SAS Institute, Cary, North Carolina) was used to fit a repeated random-intercept regression model for each outcome. This technique properly accounts for the paired nature of the specimens and the repeated measures (baseline plus 2 plications). Model fit was assessed by the method of difference in log likelihood.
Results
In the medial group, GHV was reduced by a mean of 24.2% with a single medial stitch; in the lateral group, GHV was reduced by a mean of 35.1% (Figure 3). The difference was significant (P < .02). In the medial group, when a second lateral stitch was used, GHV was reduced by another 18.7%; in the lateral group, when a medial stitch was added, GHV was reduced by another 11.4%. Final GHV for the medial and lateral groups was 42.9% and 46.5%, respectively. There was no statistical difference in final GHV, regardless of which stitch was placed first. When the 2 groups were combined, GHV was reduced by 44.9% with use of medial and lateral rotator interval closure stitches.
Mean amount of tissue purchased, or “bite size,” was 18 mm with a lateral suture and 15 mm with a medial suture (P < .05). In addition, an increase in bite size to GHV reduction was essentially linear, where an increase in bite size of 1 mm reduced GHV by about 1% (Figure 4).
Discussion
Although there have been numerous clinical series and biomechanical studies focused on isolated rotator interval closure (or its use as an adjunct) in shoulder stabilization, the precise function of the rotator interval remains poorly understood.1-3,6-11,19 Consequently, the in vivo effects of interval closure are unknown.
Initial studies proposed that rotator interval closure limited inferior and posterior translation.30 More recent studies have demonstrated that rotator interval closure confers little effect on posterior instability but increases anterior stability in cadaveric models.15,16 Clinical series have provided evidence that rotator interval closure can increase anterior stability.1,3,7,9,12 In a series of isolated rotator interval closures for multidirectional instability, Field and colleagues12 found that preoperative anterior and inferior symptoms predominated over posterior symptoms. Isolated closure of the rotator interval resulted in 100% excellent results with no cases of recurrent instability. Moon and colleagues31 reported that arthroscopic rotator interval closure with or without inferior capsular plication in multidirectional instability and predominant symptomatic inferior instability has shown benefit by improving function and stability. Other clinical reports of rotator interval closure in conjunction with arthroscopic Bankart repair have suggested it has an additive effect on anterior shoulder stability without limiting motion.24,25
In our study, arthroscopic closure of the rotator interval with 2 superior-to-inferior stitches reduced intracapsular volume by 45%. Even though open capsular shifts use different surgical techniques, similar technique volume reduction studies have reported reductions between 34% and 54% with open shifts.27,30 It is unknown if the stability resulting from decreased GHV is primarily from increasing intra-articular pressures or from restricting ROM, or from a combination of both. In shoulders with multidirectional instability, the joint volume may be increased, the joint capsule may be enlarged, or the glenohumeral ligaments may be lax and thin.4,6,32,33 Yamamoto and colleagues19 stated that intra-articular pressure is determined by 3 factors: load, joint volume, and material properties of the capsule. Load is a constant; joint volume and material properties can be changed.19 In our study, material properties were controlled by using a majority of matched specimens. Regardless of the stabilizing mechanism, our study results demonstrated that arthroscopic rotator interval closure may be a powerful tool in reducing shoulder volume, a consistent principle of surgical techniques used in reestablishing shoulder stability.19,20
When a single rotator interval closure stitch was used, volume reduction with a lateral stitch was superior to that with a medial stitch. This finding is logical, as anatomically the dimensions of the rotator interval are larger laterally as the CHL fans out to insert on the greater and lesser tuberosities.14 This finding has also been reported in open capsular shifts for multidirectional instability, with a lateral humeral shift having a larger volume reduction than a medial glenoid shift.27 Miller and colleagues27 used the image of a cone, with its larger opening facing the humerus and narrower side facing the glenoid, to illustrate this difference in open capsular shifts.
Our study also showed a larger volume reduction with 2 rotator interval closure stitches than with a single interval stitch. As ROM testing has not shown a difference between results with 1 and 2 sutures, we recommend a minimum of 2 sutures for arthroscopic rotator interval closure.15 If a single plication stitch is preferred, a lateral stitch (vs a medial stitch) can be used for a significantly larger reduction in shoulder volume. We think this is because of a larger amount of capsule being purchased with lateral closure (Figure 5). However, if a medial stitch is used, it is important to not place it too near the glenoid to avoid CHL tethering and subsequent excessive loss of external rotation.15
This study had several weaknesses. First, it was a cadaveric study, and use of specimens not known to have instability or specific rotator interval injury may make generalization to a clinical situation difficult. Second, although our power analysis called for 5 shoulders in each group, full-thickness rotator cuff tears rendered 2 shoulders unusable. This reduced our sample sizes and potentially decreased the power of the study, though the data demonstrated statistically significant differences. Third, we did not compare the effects of an open medial-to-lateral imbrication of the rotator interval on intracapsular volume with the effects of our arthroscopic method. We also did not assess our specimens’ ROM, effects of interval closure stitches on shoulder stability, or glenohumeral contact surface pressures, as these factors have already been studied.13-19 Instead, we focused on the effects of rotator interval closure on intracapsular volume, which had not been quantified until now. The clinical significance of such a volume reduction is unknown, especially with respect to influence on ROM, but the degree of volume reduction was larger than with previously reported arthroscopic instability repairs and smaller than with open capsular shifts, demonstrating that it may be a powerful tool in restoring stability in an unstable shoulder.26-30,34 Fourth, the role of isolated rotator interval closure is poorly defined, as only 1 clinical series of isolated rotator interval closure has been reported thus far.12 It has been far more common for rotator interval closure to be used with Bankart repair or capsulorrhaphy.1-3,7-9
In a cadaveric study by Provencher and colleagues,16 open rotator interval closure with medial-to-lateral imbrication of the interval altered shoulder kinematics differently from what occurred with arthroscopic closure of the MGHL to the SGHL, resulting in superior-to-inferior shift. Comparing the 2 methods may therefore be inappropriate. Currently we reserve rotator interval closure for infrequent cases of revision instability and cases in which glenoid bone loss is marginal (5%-15%) and there is a willingness to potentially sacrifice ROM to restore stability and avoid an open stabilization procedure. Continued investigation into the clinical role of rotator interval closure in shoulder stability is needed. We should identify the pathology in a patient with instability and use this technique as an adjuvant to other stabilization procedures.
Conclusion
Arthroscopic rotator interval closure with 2 plication stitches is a powerful tool in reducing the intracapsular volume of the shoulder. If a single plication stitch is preferred, a lateral rotator interval closure stitch (vs a medial stitch) can be used for a larger reduction in shoulder volume.
Since Neer described the rotator interval in 1970, its closure, often used in conjunction with capsulorrhaphy, has become an important surgical technique in managing shoulder instability.1-11 Numerous studies have sought to define the function of the rotator interval.1-3,6-20 The etiology of lesions of the rotator interval has been debated, and there is evidence that such lesions may be in part congenital.21 Increased rotator interval depth and width, along with increased size of the distended inferior and posteroinferior joint capsule on magnetic resonance arthrography, have been reported in cases of multidirectional shoulder instability.22 However, confusion remains about the role of the rotator interval in shoulder instability and about the effect its closure has on shoulder function. No one knows the degree of volume reduction that results from closure of the rotator interval and whether medial and lateral sutures differ in the volume reduction achieved.
Cadaveric studies have shown that the rotator interval has an important role in shoulder motion.6,13-16,19,20,23 Harryman and colleagues13 found that sectioning the coracohumeral ligament (CHL) increased shoulder range of motion (ROM), and medial-to-lateral closure of the rotator interval restricted motion in all planes. Most notably, interval closure limited inferior translation in the adducted shoulder, posterior translation in the flexed adducted shoulder, and external rotation in the neutral position. Subsequent studies,17,18 using rotator interval closure combined with thermal capsulorrhaphy, confirmed the results reported by Harryman and colleagues.13
More recent cadaveric studies using superior-to-inferior rotator interval closures have shown a decrease in anterior translation but not posterior translation.14-16,19-21 A superior-to-inferior interval closure technique limited external rotation less than a medial-to-lateral closure did.13-16,19-21 The majority of arthroscopically described rotator interval closures involve a superior-to-inferior technique and use 2 or 3 sutures.1,3,9-11
Plausinis and colleagues15 examined the effects of an isolated medial, an isolated lateral, and a medial combined with a lateral closure of the rotator interval. They noted that all 3 methods limited anterior translation and motion by means of 6° flexion and 10° external rotation; however, there was no statistical difference between methods. They also found that occasionally the medial interval closure resulted in massive loss of external rotation. Earlier, Jost and colleagues14 noted that a medial rotator interval could cause this massive loss by tethering the CHL, resulting in a medial-to-lateral imbrication of the CHL.
Arthroscopic rotator interval closure has clinically demonstrated an additive effect on shoulder stability. The recurrence rate was lower for arthroscopic Bankart repair combined with arthroscopic rotator interval closure (8%) than for arthroscopic Bankart repair alone (13%).24 In addition, time to recurrent dislocation was longer (42 vs 13 months) for the group that underwent the combination of Bankart repair and rotator interval closure. Regarding the concern about loss of motion after arthroscopic rotator interval closure, Chiang and colleagues25 recently noted no significant loss of motion 5 years after arthroscopic Bankart repair with rotator interval closure.
What effect rotator interval closure has on intra-articular glenohumeral volume (GHV) remains unknown. Using a cadaveric model, Yamamoto and colleagues20 showed that decreasing GHV can increase the responsiveness of the glenohumeral joint to the intra-articular pressure. Thus, reducing the volume can improve stability in vitro by increasing the magnitude of negative pressure stabilizing the glenohumeral joint.
We conducted a study to quantify the effects of arthroscopic rotator interval closure on capsular volume and to determine whether medial and lateral interval closures resulted in different degrees of volume reduction. Our hypothesis was that shoulder volume would be significantly reduced by closing the rotator interval.
Materials and Methods
Previous studies have not specifically evaluated GHV after rotator interval closure. Our power analysis was performed with data from a study by Karas and colleagues,26 who evaluated GHV after capsular plication. To detect a capsular volume reduction of 20% per stitch, with a 2-sided 5% significance level and a power of 80%, we needed a sample size of 5 specimens per group.
After receiving institutional review board approval for this study, we obtained 10 cadaveric shoulders (5 matched pairs). Exclusion criteria included arthroscopic evaluation revealing a full-thickness rotator cuff tear or significant osteoarthritis. Two shoulders had full-thickness cuff tears, leaving 8 shoulders to be tested; 6 of these were matched pairs. The shoulders were from 1 man (matched pair) and 4 women (2 matched pairs). Age ranged from 38 to 70 years (mean, 59.6 years). Differences in material properties between the specimens were accounted for by using primarily matched pairs.
The 2 study groups consisted of 4 shoulders each. After specimens were thawed, the skin, subcutaneous tissues, and periscapular muscles were removed from the shoulder. Only the capsule, biceps, and rotator cuff remained. For measurement purposes, the shoulders were mounted in a vice clamp in a beach-chair orientation. We placed a total of 2 portals with fully threaded 8.25-mm cannulas (Arthrex, Naples, Florida). A standard posterior portal was placed in the soft spot. A low anterior portal was then placed just superior to the subscapularis tendon. For arthroscopic examination and instrumentation in a saline environment, the shoulders were rotated into the lateral decubitus position, with suspension in 30° abduction and 20° forward flexion, by a rope attached to a pin in the distal shaft of the humerus.
In both groups, medial and lateral stitches with No. 2 FiberWire (Arthrex) were used to close the interval. The medial interval closure stitch was placed more than 10 mm away from the glenoid to prevent unpredictable CHL tethering; the lateral closure stitch was placed 10 mm lateral to the medial stitch (Figure 1).14 All sutures were placed intra-articularly under direct arthroscopic visualization, similar to the methods described in the literature.1,3,9-11 Sutures were passed through the superior glenohumeral ligament (SGHL) and through the upper subscapularis using a suture shuttle (SutureLasso; Arthrex) and Penetrator II Suture Retriever (Arthrex). The upper subscapularis was incorporated because of the unpredictable nature of the middle glenohumeral ligament (MGHL). Both rotator interval sutures were placed before tying either. In the medial group, the medial stitch was tied first, using alternating half-hitches, followed by the lateral stitch. In the lateral group, the lateral stitch was tied first, followed by the medial stitch. GHV was measured at baseline and after tying each stitch. Dr. Ponce instrumented all shoulders.
Modifying a beach-chair technique described by Miller and colleagues,27 we used a viscous fatty-acid sulfate solution, liquid soap, to measure GHV.27-29 A small slit in line with the fibers was made in the supraspinatus tendon just lateral to the musculotendinous junction. A 3-way stop-cock was placed into the joint though this defect. A 20-mL syringe with a 16-gauge needle was used to inject the soap. The needle was inserted into the rotator cuff interval, and the viscous solution was injected in 5-mL increments until there was active extravasation through the supraspinatus cannula (Figure 2). This technique, the “volcano method,” marked the maximum capacity of the joint. The joint was then copiously irrigated with normal saline and suctioned until all normal saline was evacuated. Dr. Rosenzweig took 2 measurements on each shoulder, and their mean was used for analysis.
The baseline measurement was taken with the 2 working cannulas in the shoulder joint. Measurements were obtained with cannulas to simulate normal clinical conditions. Subsequent measurements were done with the cannulas in place and inserted up to the same thread each time so as not to change the volume. The capsule and the rotator cuff were then dissected from the humerus so the size of the capsulolabral plication could be directly evaluated. Methylene blue was used to mark the capsular suture holes before removing the sutures. With use of a caliper, the size of the plication bite was measured (in millimeters).
Statistical Analysis
The primary outcome was percent reduction in GHV as a function of number of plications and size of plication. When only the first plication was tightened, the effect of position (medial or lateral) was also of interest. Percent volume reduction was calculated as (original – new) / original × 100. SAS 8.02 (SAS Institute, Cary, North Carolina) was used to fit a repeated random-intercept regression model for each outcome. This technique properly accounts for the paired nature of the specimens and the repeated measures (baseline plus 2 plications). Model fit was assessed by the method of difference in log likelihood.
Results
In the medial group, GHV was reduced by a mean of 24.2% with a single medial stitch; in the lateral group, GHV was reduced by a mean of 35.1% (Figure 3). The difference was significant (P < .02). In the medial group, when a second lateral stitch was used, GHV was reduced by another 18.7%; in the lateral group, when a medial stitch was added, GHV was reduced by another 11.4%. Final GHV for the medial and lateral groups was 42.9% and 46.5%, respectively. There was no statistical difference in final GHV, regardless of which stitch was placed first. When the 2 groups were combined, GHV was reduced by 44.9% with use of medial and lateral rotator interval closure stitches.
Mean amount of tissue purchased, or “bite size,” was 18 mm with a lateral suture and 15 mm with a medial suture (P < .05). In addition, an increase in bite size to GHV reduction was essentially linear, where an increase in bite size of 1 mm reduced GHV by about 1% (Figure 4).
Discussion
Although there have been numerous clinical series and biomechanical studies focused on isolated rotator interval closure (or its use as an adjunct) in shoulder stabilization, the precise function of the rotator interval remains poorly understood.1-3,6-11,19 Consequently, the in vivo effects of interval closure are unknown.
Initial studies proposed that rotator interval closure limited inferior and posterior translation.30 More recent studies have demonstrated that rotator interval closure confers little effect on posterior instability but increases anterior stability in cadaveric models.15,16 Clinical series have provided evidence that rotator interval closure can increase anterior stability.1,3,7,9,12 In a series of isolated rotator interval closures for multidirectional instability, Field and colleagues12 found that preoperative anterior and inferior symptoms predominated over posterior symptoms. Isolated closure of the rotator interval resulted in 100% excellent results with no cases of recurrent instability. Moon and colleagues31 reported that arthroscopic rotator interval closure with or without inferior capsular plication in multidirectional instability and predominant symptomatic inferior instability has shown benefit by improving function and stability. Other clinical reports of rotator interval closure in conjunction with arthroscopic Bankart repair have suggested it has an additive effect on anterior shoulder stability without limiting motion.24,25
In our study, arthroscopic closure of the rotator interval with 2 superior-to-inferior stitches reduced intracapsular volume by 45%. Even though open capsular shifts use different surgical techniques, similar technique volume reduction studies have reported reductions between 34% and 54% with open shifts.27,30 It is unknown if the stability resulting from decreased GHV is primarily from increasing intra-articular pressures or from restricting ROM, or from a combination of both. In shoulders with multidirectional instability, the joint volume may be increased, the joint capsule may be enlarged, or the glenohumeral ligaments may be lax and thin.4,6,32,33 Yamamoto and colleagues19 stated that intra-articular pressure is determined by 3 factors: load, joint volume, and material properties of the capsule. Load is a constant; joint volume and material properties can be changed.19 In our study, material properties were controlled by using a majority of matched specimens. Regardless of the stabilizing mechanism, our study results demonstrated that arthroscopic rotator interval closure may be a powerful tool in reducing shoulder volume, a consistent principle of surgical techniques used in reestablishing shoulder stability.19,20
When a single rotator interval closure stitch was used, volume reduction with a lateral stitch was superior to that with a medial stitch. This finding is logical, as anatomically the dimensions of the rotator interval are larger laterally as the CHL fans out to insert on the greater and lesser tuberosities.14 This finding has also been reported in open capsular shifts for multidirectional instability, with a lateral humeral shift having a larger volume reduction than a medial glenoid shift.27 Miller and colleagues27 used the image of a cone, with its larger opening facing the humerus and narrower side facing the glenoid, to illustrate this difference in open capsular shifts.
Our study also showed a larger volume reduction with 2 rotator interval closure stitches than with a single interval stitch. As ROM testing has not shown a difference between results with 1 and 2 sutures, we recommend a minimum of 2 sutures for arthroscopic rotator interval closure.15 If a single plication stitch is preferred, a lateral stitch (vs a medial stitch) can be used for a significantly larger reduction in shoulder volume. We think this is because of a larger amount of capsule being purchased with lateral closure (Figure 5). However, if a medial stitch is used, it is important to not place it too near the glenoid to avoid CHL tethering and subsequent excessive loss of external rotation.15
This study had several weaknesses. First, it was a cadaveric study, and use of specimens not known to have instability or specific rotator interval injury may make generalization to a clinical situation difficult. Second, although our power analysis called for 5 shoulders in each group, full-thickness rotator cuff tears rendered 2 shoulders unusable. This reduced our sample sizes and potentially decreased the power of the study, though the data demonstrated statistically significant differences. Third, we did not compare the effects of an open medial-to-lateral imbrication of the rotator interval on intracapsular volume with the effects of our arthroscopic method. We also did not assess our specimens’ ROM, effects of interval closure stitches on shoulder stability, or glenohumeral contact surface pressures, as these factors have already been studied.13-19 Instead, we focused on the effects of rotator interval closure on intracapsular volume, which had not been quantified until now. The clinical significance of such a volume reduction is unknown, especially with respect to influence on ROM, but the degree of volume reduction was larger than with previously reported arthroscopic instability repairs and smaller than with open capsular shifts, demonstrating that it may be a powerful tool in restoring stability in an unstable shoulder.26-30,34 Fourth, the role of isolated rotator interval closure is poorly defined, as only 1 clinical series of isolated rotator interval closure has been reported thus far.12 It has been far more common for rotator interval closure to be used with Bankart repair or capsulorrhaphy.1-3,7-9
In a cadaveric study by Provencher and colleagues,16 open rotator interval closure with medial-to-lateral imbrication of the interval altered shoulder kinematics differently from what occurred with arthroscopic closure of the MGHL to the SGHL, resulting in superior-to-inferior shift. Comparing the 2 methods may therefore be inappropriate. Currently we reserve rotator interval closure for infrequent cases of revision instability and cases in which glenoid bone loss is marginal (5%-15%) and there is a willingness to potentially sacrifice ROM to restore stability and avoid an open stabilization procedure. Continued investigation into the clinical role of rotator interval closure in shoulder stability is needed. We should identify the pathology in a patient with instability and use this technique as an adjuvant to other stabilization procedures.
Conclusion
Arthroscopic rotator interval closure with 2 plication stitches is a powerful tool in reducing the intracapsular volume of the shoulder. If a single plication stitch is preferred, a lateral rotator interval closure stitch (vs a medial stitch) can be used for a larger reduction in shoulder volume.
1. Creighton RA, Romeo AA, Brown FM, Hayden JK, Verma NN. Revision arthroscopic shoulder instability repair. Arthroscopy. 2007;23(7):703-709.
2. Gartsman GM, Roddey TS, Hammerman SM. Arthroscopic treatment of anterior-inferior glenohumeral instability. Two to five-year follow-up. J Bone Joint Surg Am. 2000;82(7):991-1003.
3. Gartsman GM, Taverna E, Hammerman SM. Arthroscopic rotator interval repair in glenohumeral instability: description of an operative technique. Arthroscopy. 1999;15(3):330-332.
4. Neer CS 2nd, Foster CR. Inferior capsular shift for involuntary inferior and multidirectional instability of the shoulder: a preliminary report. J Bone Joint Surg Am. 1980;62(6):897-908.
5. Neer CS 2nd. Displaced proximal humerus fractures: I. Classification and evaluation. J Bone Joint Surg Am. 1970;52(6):1077-1089.
6. Nobuhara K, Ikeda H. Rotator interval lesion. Clin Orthop. 1987;(223):44-50.
7. Rowe CR, Zarins B, Ciullo JV. Recurrent anterior dislocation of the shoulder after surgical repair. J Bone Joint Surg Am. 1984;66(2):159-168.
8. Rowe CR, Zarins B. Recurrent transient subluxation of the shoulder. J Bone Joint Surg Am. 1981;63(6):863-872.
9. Stokes DA, Savoie FH, Field LD. Arthroscopic repair of anterior glenohumeral instability and rotator interval lesions. Orthop Clin North Am. 2003;34(4):529-539.
10. Taverna E, Sansone V, Battistella F. Arthroscopic rotator interval repair: the three-step all-inside technique. Arthroscopy. 2004;20 Suppl 2:105-109.
11. Treacy SH, Field LD, Savoie FH. Rotator interval capsule closure: an arthroscopic technique. Arthroscopy. 1997;13(1):103-106.
12. Field LD, Warren RF, O’Brien SJ, Altcheck DW, Wickiewicz TL. Isolated closure of rotator interval defects for shoulder instability. Am J Sports Med. 1995;23(5):557-563.
13. Harryman DT 2nd, Sidles JA, Harris SL, Matsen FA 3rd. The role of the rotator interval capsule in passive motion and stability of the shoulder. J Bone Joint Surg Am. 1992;74(1):53-66.
14. Jost B, Koch PP, Gerber C. Anatomy and functional aspects of the rotator interval. J Shoulder Elbow Surg. 2000;9(4):336-341.
15. Plausinis D, Bravman JT, Heywood C, Kummer FJ, Kwon YM, Jazrawi LM. Arthroscopic rotator interval closure: effect of sutures on glenohumeral motion and anterior-posterior translation. Am J Sports Med. 2006;34(10):1656-1661.
16. Provencher MT, Mologne TS, Hongo M, Zhao K, Tasto JP, An KN. Arthroscopic versus open rotator interval closure: biomechanical evaluation of stability and motion. Arthroscopy. 2007;23(6):583-592.
17. Selecky MT, Tibone JE, Yang BY, et al. Glenohumeral joint translation after thermal capsuloplasty of the rotator interval. J Shoulder Elbow Surg. 2003;12(2):139-143.
18. Wolf R, Zheng N, Iero J, Weichel D. The effects of thermal capsulorrhaphy and rotator interval closure on multidirectional laxity in the glenohumeral joint: a cadaveric biomechanical study. Arthroscopy. 2004;20(10):1044-1049.
19. Yamamoto N, Itoi E, Tuoheti Y, et al. Effect of rotator interval closure on glenohumeral stability and motion: a cadaveric study. J Shoulder Elbow Surg. 2006;15(6):750-758.
20. Yamamoto N, Itoi E, Tuoheti Y, et al. The effect of the inferior capsular shift on shoulder intra-articular pressure: a cadaveric study. Am J Sports Med. 2006;34(6):939-944.
21. Cole BJ, Rodeo SA, O’Brien SJ, et al. The anatomy and histology of the rotator interval capsule of the shoulder. Clin Orthop. 2001;(390):129-137.
22. Lee HJ, Kim NR, Moon SG, Ko SM, Park JY. Multidirectional instability of the shoulder: rotator interval dimension and capsular laxity evaluation using MR arthrography. Skeletal Radiol. 2013;42(2):231-238.
23. Warner JP, Deng X, Warren RF, Torzilli PA, O’Brien SJ. Superoinferior translation in intact and vented glenohumeral joint. J Shoulder Elbow Surg. 1993;2(2):99-105.
24. Chechik O, Maman E, Dolkart O, Khashan M, Shabtai L, Mozes G. Arthroscopic rotator interval closure in shoulder instability repair: a retrospective study. J Shoulder Elbow Surg. 2010;19(7):1056-1062.
25. Chiang, E, Wang J, Wang S, et al. Arthroscopic posteroinferior capsular plication and rotator interval closure after Bankart repair in patients with traumatic anterior glenohumeral instability—a minimum follow-up of 5 years. Injury. 2010;41(10):1075-1078.
26. Karas SG, Creighton RA, DeMorat GJ. Glenohumeral volume reduction in arthroscopic shoulder reconstruction: a cadaveric analysis of suture plication and thermal capsulorrhaphy. Arthroscopy. 2004;20(2):179-184.
27. Miller MD, Larsen KM, Luke T, Leis HT, Plancher KD. Anterior capsular shift volume reduction: an in vitro comparison of 3 techniques. J Shoulder Elbow Surg. 2003;12(4):350-354.
28. Luke TA, Rovner AD, Karas SG, Hawkins RJ, Plancher KD. Volumetric change in the shoulder capsule after open inferior capsular shift versus arthroscopic thermal capsular shrinkage: a cadaveric model. J Shoulder Elbow Surg. 2004;13(2):146-149.
29. Ponce BA, Rosenzweig SD, Thompson KJ, Tokish J. Sequential volume reduction with capsular plications: relationship between cumulative size of plications and volumetric reduction for multidirectional instability of the shoulder. Am J Sports Med. 2011;39(3):526-531.
30. Lubowitz J, Bartolozzi A, Rubenstein D, et al. How much does inferior capsular shift reduce shoulder volume? Clin Orthop. 1996;(328):86-90.
31. Moon YL, Singh H, Yang H, Chul LK. Arthroscopic rotator interval closure by purse string suture for symptomatic inferior shoulder instability. Orthopedics. 2011;34(4).
32. Jerosch J, Castro WH. Shoulder instability in Ehlers-Danlos syndrome: an indication for surgical treatment? Acta Orthop Belg. 1990;56(2):451-453.
33. Schenk TJ, Brems JJ. Multidirectional instability of the shoulder: pathophysiology, diagnosis, and management. J Am Acad Orthop Surg. 1998;6(1):65-72.
34. Cohen SB, Wiley W, Goradia VK, Pearson S, Miller MD. Anterior capsulorrhaphy: an in vitro comparison of volume reduction. Arthroscopic plication versus open capsular shift. Arthroscopy. 2005;21(6):659-664.
1. Creighton RA, Romeo AA, Brown FM, Hayden JK, Verma NN. Revision arthroscopic shoulder instability repair. Arthroscopy. 2007;23(7):703-709.
2. Gartsman GM, Roddey TS, Hammerman SM. Arthroscopic treatment of anterior-inferior glenohumeral instability. Two to five-year follow-up. J Bone Joint Surg Am. 2000;82(7):991-1003.
3. Gartsman GM, Taverna E, Hammerman SM. Arthroscopic rotator interval repair in glenohumeral instability: description of an operative technique. Arthroscopy. 1999;15(3):330-332.
4. Neer CS 2nd, Foster CR. Inferior capsular shift for involuntary inferior and multidirectional instability of the shoulder: a preliminary report. J Bone Joint Surg Am. 1980;62(6):897-908.
5. Neer CS 2nd. Displaced proximal humerus fractures: I. Classification and evaluation. J Bone Joint Surg Am. 1970;52(6):1077-1089.
6. Nobuhara K, Ikeda H. Rotator interval lesion. Clin Orthop. 1987;(223):44-50.
7. Rowe CR, Zarins B, Ciullo JV. Recurrent anterior dislocation of the shoulder after surgical repair. J Bone Joint Surg Am. 1984;66(2):159-168.
8. Rowe CR, Zarins B. Recurrent transient subluxation of the shoulder. J Bone Joint Surg Am. 1981;63(6):863-872.
9. Stokes DA, Savoie FH, Field LD. Arthroscopic repair of anterior glenohumeral instability and rotator interval lesions. Orthop Clin North Am. 2003;34(4):529-539.
10. Taverna E, Sansone V, Battistella F. Arthroscopic rotator interval repair: the three-step all-inside technique. Arthroscopy. 2004;20 Suppl 2:105-109.
11. Treacy SH, Field LD, Savoie FH. Rotator interval capsule closure: an arthroscopic technique. Arthroscopy. 1997;13(1):103-106.
12. Field LD, Warren RF, O’Brien SJ, Altcheck DW, Wickiewicz TL. Isolated closure of rotator interval defects for shoulder instability. Am J Sports Med. 1995;23(5):557-563.
13. Harryman DT 2nd, Sidles JA, Harris SL, Matsen FA 3rd. The role of the rotator interval capsule in passive motion and stability of the shoulder. J Bone Joint Surg Am. 1992;74(1):53-66.
14. Jost B, Koch PP, Gerber C. Anatomy and functional aspects of the rotator interval. J Shoulder Elbow Surg. 2000;9(4):336-341.
15. Plausinis D, Bravman JT, Heywood C, Kummer FJ, Kwon YM, Jazrawi LM. Arthroscopic rotator interval closure: effect of sutures on glenohumeral motion and anterior-posterior translation. Am J Sports Med. 2006;34(10):1656-1661.
16. Provencher MT, Mologne TS, Hongo M, Zhao K, Tasto JP, An KN. Arthroscopic versus open rotator interval closure: biomechanical evaluation of stability and motion. Arthroscopy. 2007;23(6):583-592.
17. Selecky MT, Tibone JE, Yang BY, et al. Glenohumeral joint translation after thermal capsuloplasty of the rotator interval. J Shoulder Elbow Surg. 2003;12(2):139-143.
18. Wolf R, Zheng N, Iero J, Weichel D. The effects of thermal capsulorrhaphy and rotator interval closure on multidirectional laxity in the glenohumeral joint: a cadaveric biomechanical study. Arthroscopy. 2004;20(10):1044-1049.
19. Yamamoto N, Itoi E, Tuoheti Y, et al. Effect of rotator interval closure on glenohumeral stability and motion: a cadaveric study. J Shoulder Elbow Surg. 2006;15(6):750-758.
20. Yamamoto N, Itoi E, Tuoheti Y, et al. The effect of the inferior capsular shift on shoulder intra-articular pressure: a cadaveric study. Am J Sports Med. 2006;34(6):939-944.
21. Cole BJ, Rodeo SA, O’Brien SJ, et al. The anatomy and histology of the rotator interval capsule of the shoulder. Clin Orthop. 2001;(390):129-137.
22. Lee HJ, Kim NR, Moon SG, Ko SM, Park JY. Multidirectional instability of the shoulder: rotator interval dimension and capsular laxity evaluation using MR arthrography. Skeletal Radiol. 2013;42(2):231-238.
23. Warner JP, Deng X, Warren RF, Torzilli PA, O’Brien SJ. Superoinferior translation in intact and vented glenohumeral joint. J Shoulder Elbow Surg. 1993;2(2):99-105.
24. Chechik O, Maman E, Dolkart O, Khashan M, Shabtai L, Mozes G. Arthroscopic rotator interval closure in shoulder instability repair: a retrospective study. J Shoulder Elbow Surg. 2010;19(7):1056-1062.
25. Chiang, E, Wang J, Wang S, et al. Arthroscopic posteroinferior capsular plication and rotator interval closure after Bankart repair in patients with traumatic anterior glenohumeral instability—a minimum follow-up of 5 years. Injury. 2010;41(10):1075-1078.
26. Karas SG, Creighton RA, DeMorat GJ. Glenohumeral volume reduction in arthroscopic shoulder reconstruction: a cadaveric analysis of suture plication and thermal capsulorrhaphy. Arthroscopy. 2004;20(2):179-184.
27. Miller MD, Larsen KM, Luke T, Leis HT, Plancher KD. Anterior capsular shift volume reduction: an in vitro comparison of 3 techniques. J Shoulder Elbow Surg. 2003;12(4):350-354.
28. Luke TA, Rovner AD, Karas SG, Hawkins RJ, Plancher KD. Volumetric change in the shoulder capsule after open inferior capsular shift versus arthroscopic thermal capsular shrinkage: a cadaveric model. J Shoulder Elbow Surg. 2004;13(2):146-149.
29. Ponce BA, Rosenzweig SD, Thompson KJ, Tokish J. Sequential volume reduction with capsular plications: relationship between cumulative size of plications and volumetric reduction for multidirectional instability of the shoulder. Am J Sports Med. 2011;39(3):526-531.
30. Lubowitz J, Bartolozzi A, Rubenstein D, et al. How much does inferior capsular shift reduce shoulder volume? Clin Orthop. 1996;(328):86-90.
31. Moon YL, Singh H, Yang H, Chul LK. Arthroscopic rotator interval closure by purse string suture for symptomatic inferior shoulder instability. Orthopedics. 2011;34(4).
32. Jerosch J, Castro WH. Shoulder instability in Ehlers-Danlos syndrome: an indication for surgical treatment? Acta Orthop Belg. 1990;56(2):451-453.
33. Schenk TJ, Brems JJ. Multidirectional instability of the shoulder: pathophysiology, diagnosis, and management. J Am Acad Orthop Surg. 1998;6(1):65-72.
34. Cohen SB, Wiley W, Goradia VK, Pearson S, Miller MD. Anterior capsulorrhaphy: an in vitro comparison of volume reduction. Arthroscopic plication versus open capsular shift. Arthroscopy. 2005;21(6):659-664.
Risk Factors for In-Hospital Myocardial Infarction After Shoulder Arthroplasty
The incidence of shoulder arthroplasty in the United States is increasing annually,1-3 and the majority of these operations occur in older patients.4-6 Elderly patients with cardiovascular, pulmonary, cerebral, renal, and hepatic disease are increasingly susceptible to numerous surgical complications.4 Myocardial infarction (MI) is a complication that occurs in 0.7% of noncardiac surgeries. This figure increases to 1.1% in patients with coronary artery disease.7-11 Perioperative MI increases morbidity and mortality,8 and perioperative cardiac morbidity is the leading cause of death after anesthesia and surgery.12 The financial effects of perioperative cardiac morbidity and mortality must also be considered. A 2009 claims analysis study estimated charges associated with a perioperative MI at $15,000 and the cost of cardiac death at $21,909.13
Cardiovascular complications are associated with a significant degree of morbidity and mortality in patients who undergo arthroplasty.14-16 Although studies have elucidated 30- and 90-day morbidity and mortality rates after shoulder arthroplasty, in hip and knee arthroplasty17-19 little has been done to determine predictors of perioperative MI in a representative database of patients. Given the increasing incidence of shoulder arthroplasty in the United States, the elective nature of this procedure, and the percentage of the US population with cardiovascular risk factors,20 it is important to establish predictors of perioperative MI to ensure patients and physicians have the necessary resources to make informed decisions.
We conducted a study to examine the risk factors for perioperative MI in a large cohort of patients admitted for shoulder arthroplasty to US hospitals. We wanted to evaluate the association between perioperative MI and shoulder arthroplasty with respect to demographics, primary diagnosis, medical comorbidities, and perioperative complications. Specifically, we tested the null hypothesis that, among patients undergoing shoulder arthroplasty, and accounting for confounding variables, there would be no difference in risk factors for patients who have a perioperative MI.
Materials and Methods
This study was exempt from approval by our institutional review board. All data used in this project were deidentified before use.
Nationwide Inpatient Sample (NIS)
The Nationwide Inpatient Sample (NIS), an annual survey of hospitals, is conducted by the Healthcare Cost and Utilization Project (HCUP) and sponsored by the Agency for Healthcare Research and Quality (AHRQ). This database is the largest publicly available all-payer inpatient discharge database in the United States.21 Sampling 8 million hospital stays each year, NIS includes information from a representative batch of 20% of US hospitals. In 2011, 46 states and 1045 hospitals contributed information to the database, representing 97% of the US population.22 This large sample allows researchers to analyze a robust set of medical conditions and uncommon treatments. The survey, conducted each year since 1988, includes demographic, clinical, and resource use data.23 Discharge weight files are provided by NIS to arrive at valid national estimates.
This database is particularly useful because it provides information on up to 25 medical diagnoses and 15 procedures, which are recorded with International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) codes. Researchers can use this database to analyze patient and hospital characteristics as well as inpatient outcomes.24,25 Numerous studies have used NIS to address pertinent queries across the medical landscape.22,26
Patient Selection and Analysis
We used NIS to isolate a population of 422,371 adults (≥18 years old) who underwent total shoulder arthroplasty (TSA) or hemi–shoulder arthroplasty (HSA) between January 1, 2002 and December 31, 2011. We then placed the patients in this population into 1 of 2 cohorts. The first cohort had an acute MI during the perioperative period after TSA, and the second, larger cohort did not have an acute MI after TSA. Acute MI was identified using ICD-9-CM code 410.xx. To identify a population of shoulder arthroplasty patients, we included discharges with an ICD-9-CM procedure code of 81.80 or 81.88 (both TSA) or 81.81 (HSA) in the sample. We then considered the degree to which each of 5 variables—primary diagnosis, age, sex, race, and select medical comorbidities—was predictive of in-hospital MI after TSA.
Statistical Analysis
Given the large sample used in this study, normal distribution of data was assumed. Using bivariate analysis, Pearson χ2 test for categorical data, and independent-samples t test for continuous data, we compared the nonacute MI and acute MI groups. Multivariable binary logistic regression analyses allowed us to isolate the extent that primary diagnosis, age, sex, race, and medical comorbidities were predictors of acute MI after shoulder arthroplasty. Statistical significance was set at P < .05. SPSS Version 22.0 (SPSS, Chicago, Illinois) was used for all statistical analyses and data modeling.
Results
Between January 1, 2002 and December 31, 2011, an estimated total of 422,371 patients underwent shoulder arthroplasty (59.3% TSA, 40.7% HSA). Of these patients, 1174 (0.28%) had a perioperative MI, and 421,197 (99.72%) did not (Table 1). Patients with a primary diagnosis of proximal humerus fracture (33.8% vs 16.6%; P < .001) or rotator cuff arthropathy (10.1% vs 9.9%; P < .001) were more likely than patients with other diagnoses to have an in-hospital MI.
Our review of the demographics found that patients who underwent shoulder arthroplasty and had a perioperative MI were likely older (75±8.9 years vs 69±11 years; P < .001), Caucasian (94.2% vs 91.9%; P = .002), male (43.2% vs 39.7%; P = .013), in the highest median household income bracket of $63,000 or more (30.8% vs 25.6%; P < .001), and using Medicare (80.9% vs 66.3%; P < .001). They were more likely to be treated in a medical center of medium size (25.6% vs 23.7%; P = .042) or larger (61.8% vs 61.2%; P = .042). MIs occurred more often in urban environments (91.4% vs 88.5%; P = .002) and in HSA patients (55% vs 40.6%; P < .001), resulting in longer hospital stays (9.4±7.9 days vs 2.7±2.5 days; P < .001) and higher probability of death (6.5% vs 0.1%; P < .001).
We then analyzed the 2 cohorts for medical comorbidities (Table 2). Patients in the MI cohort presented with a significantly higher incidence of congestive heart failure, previous MI, angina pectoris, chronic lung disease, hypertension, diabetes, renal failure, fluid and electrolyte disorders, pulmonary circulatory disease, coagulopathy, and deficiency anemia (P < .001) but not liver disease and obesity. Bivariate analysis of perioperative outcomes (Table 3) indicated that these patients also had a statistically higher rate of numerous other complications: pulmonary embolism (4.9% vs 0.2%; P < .001), pneumonia (15.1% vs 1.2%; P < .001), deep venous thrombosis (2.6% vs 0.2%; P < .001), cerebrovascular event (1.6% vs 0.1%; P < .001), acute renal failure (15.1% vs 1.2%; P < .001), gastrointestinal complication (1.2% vs 0.3%; P < .001), mechanical ventilation (1.2% vs 0.3%; P < .001), transfusion (33.4% vs 8.8%; P < .001), and nonroutine discharge (73.3% vs 36.0%; P < .001).
Multivariable logistic regression analysis was performed to determine independent predictors of perioperative MI after shoulder arthroplasty (Table 4). Patients with a primary diagnosis of proximal humerus fracture (odds ratio [OR], 1.38; 95% confidence interval [CI], 1.15-1.65; P < .001) were more likely than patients with a primary diagnosis of osteoarthritis to have an MI. The odds of postoperative MI increased with age (OR, 1.04 per year; 95% CI, 1.03-1.05; P < .001) and were higher in males (OR, 1.72; 95% CI, 1.52-1.96; P < .001). Compared with Caucasians, African Americans (OR, 0.19; 95% CI, 0.09-0.40; P < .001) were less likely to have an in-hospital MI after shoulder arthroplasty. After shoulder arthroplasty, the odds of MI in the perioperative period increased with each subsequent day of care (OR, 1.10; 95% CI, 1.10-1.11; P < .001).
Regarding independent comorbidities, multivariable logistic regression analysis also determined that history of congestive heart failure (OR, 4.86; 95% CI, 4.20-5.61; P < .001), angina pectoris (OR, 2.90; 95% CI, 2.02-4.17; P < .001), complicated diabetes (OR, 1.96; 95% CI, 1.49-2.57; P < .001), renal failure (OR, 1.42; 95% CI, 1.17-1.72; P < .001), fluid and electrolyte disorders (OR, 1.42; 95% CI, 1.21-1.67; P < .001), and deficiency anemia (OR, 1.62; 95% CI, 1.40-1.88; P < .001) were significant predictors of perioperative MI after shoulder arthroplasty.
Discussion
Results of other studies have elucidated 30- and 90-day mortality rates and postoperative complications after shoulder arthroplasty, but, relative to hip and knee arthroplasty,17-19 little has been done to determine predictors of perioperative MI in a large sample of shoulder arthroplasty patients. Given the increasing rates of shoulder arthroplasty1-3 and the demographics of this population,4-6 it is likely that postoperative cardiovascular events will increase in frequency. We found that, in order of decreasing significance, the top 4 risk predictors for acute MI after shoulder arthroplasty were congestive heart failure, angina pectoralis, complicated diabetes mellitus, and male sex. Other pertinent risk factors included older age, Caucasian ethnicity, and a primary diagnosis of proximal humerus fracture. The rate of acute MI in patients who were older than 75 years when they underwent HSA for proximal humerus fracture was 0.80%.
Demographics
We found that patients who had an acute MI after shoulder arthroplasty were likely older, male, and Caucasian. Age and male sex are well-established risk factors for increased cardiac complications after arthroplasty.27-29 Previous studies have indicated that the rate of cardiac events increases in arthroplasty patients older than 65 years.19,28,29 In our study, more than 50% of the patients who had an acute perioperative MI were older than 85 years. Less explainable is the increased occurrence of acute MI in Caucasian patients and wealthy patients, given that minorities in the United States have higher rates of cardiovascular disease.30 Shoulder arthroplasty is an elective procedure, more likely to be undertaken by Caucasians. Therefore, at-risk minority groups and financially challenged groups may be less likely to have this procedure.
Primary Diagnosis
In this series, patients with a primary diagnosis of proximal humerus fracture were more likely to have an in-hospital MI. This finding is consistent with previous studies indicating a higher rate of complications for proximal humerus fracture patients than for shoulder arthroplasty patients.31,32 Given that more than 75% of patients who present with a proximal humerus fracture are older than 70 years, it would be prudent to examine operative indications after this diagnosis,33 particularly as benefit from surgery for fractures has not been definitively demonstrated.34-37
Comorbidities
Many of the patients in our MI cohort presented with congestive heart failure, angina pectoris, complicated diabetes, renal failure, fluid and electrolyte disorders, or deficiency anemia. This is in keeping with other studies indicating that preexisting cardiovascular morbidity increases the rate of MI after various forms of arthroplasty.7-11 Patients in our MI cohort were also susceptible to a variety of post-MI perioperative complications, including pulmonary embolism, pneumonia, deep venous thrombosis, cerebrovascular event, acute renal failure, gastrointestinal complication, mechanical ventilation, transfusion, and nonroutine discharge, and their incidence of death was higher. These findings are consistent with reports that postoperative cardiovascular complications increase the degree of morbidity and mortality in arthroplasty patients.14-16 It is also worth noting that the odds of MI in the perioperative period increase with each subsequent day of care. This is understandable given that patients presenting with numerous comorbidities are at increased risk for perioperative complications38 resulting in hospital readmission.39
The literature indicates that MI occurs as a complication in 0.7% of patients who undergo noncardiac surgery,7 though some series have shown it is more prevalent after arthroplasty procedures.28,40 MI significantly increases the rate of perioperative morbidity and mortality,8 and perioperative cardiac morbidity is a leading cause of death after anesthesia and surgery.12 Furthermore, the most common cause of death after lower extremity arthroplasty is cardiovascular-related.41,42 In patients who presented for elective hip arthroplasty, cardiorespiratory disease was one of the main risk factors (with older age and male sex) shown to increase perioperative mortality.43
Perioperative cardiovascular complications increase postoperative morbidity and mortality.12 The rate of cardiovascular complications after shoulder arthroplasty ranges from 0.8% to 2.6%, and the incidence of MI hovers between 0.3% and 0.9%.17,19,28,40,44 A recent study in 793 patients found that, over a 30-day period, cardiovascular complications accounted for more than one-fourth of all complications.17 Singh and colleagues19 analyzed cardiopulmonary complications after primary shoulder arthroplasty in a total of 3480 patients (4019 arthroplasties) and found this group had a 90-day cardiac morbidity (MI, congestive heart failure, arrhythmia) rate of 2.6%. In that study, a Deyo-Charlson index of 1 or more was a significant independent risk factor for cardiac complications following surgery. Scores on this weighted index of 17 comorbidities are used to assess the complexities of a patient population. Given the severity of cardiovascular perioperative complications, it is important to preoperatively identify high-risk population groups and sufficiently study and optimize patients before shoulder arthroplasty.
There is much debate about the effectiveness of perioperative β-blockers in reducing perioperative cardiac morbidity and mortality.45-48 Such a discussion is outside of the scope of this article, but it may be prudent to seek a cardiology consultation for patients presenting with risk factors for perioperative MI. β-Blockers may prove useful in reducing cardiac morbidity in high-risk patients after noncardiac surgery.45,49
Many limitations are inherent in studies that use a nationally represented database such as NIS, which we used in this study. It is highly likely that NIS does not capture all potential postoperative complications, as this database is very large and subject to errors in data entry and clinical coding. In addition, detailed clinical information (eg, severity of certain comorbid diseases before shoulder arthroplasty, details about the intraoperative course) was not readily available for analysis. Another limitation, which may have led to an underestimate of complication rates, was our not being able to obtain information about postdischarge complications.
Despite these limitations, NIS and other databases have helped researchers answer questions about low-incidence conditions and generalize findings to a national population. In the present study, we analyzed 2 cohorts, patients with and without acute MI after shoulder arthroplasty, to determine predictors for and complications of postarthroplasty MI. We identified numerous predictors for acute MI: congestive heart failure, angina pectoris, complicated diabetes, renal failure, fluid and electrolyte disorders, and deficiency anemia prior to arthroplasty. As perioperative MI is associated with significant morbidity,14-16 it would be wise to screen patients for such comorbid conditions, assess the severity of these conditions, and offer shoulder arthroplasty with prudence.
Conclusion
The top 4 predictors for acute MI after shoulder arthroplasty were congestive heart failure, angina pectoralis, complicated diabetes mellitus, and male sex. Other pertinent risk factors included older age, Caucasian ethnicity, and primary diagnosis of proximal humerus fracture. Surgeons and patients must be aware of predictors for adverse surgical outcomes such as perioperative MI and understand the extent to which these events increase perioperative morbidity and mortality.
1. Day JS, Lau E, Ong KL, Williams GR, Ramsey ML, Kurtz SM. Prevalence and projections of total shoulder and elbow arthroplasty in the United States to 2015. J Shoulder Elbow Surg. 2010;19(8):1115-1120.
2. Kim SH, Wise BL, Zhang Y, Szabo RM. Increasing incidence of shoulder arthroplasty in the United States. J Bone Joint Surg Am. 2011;93(24):2249-2254.
3. Kurtz SM, Lau E, Ong K, Zhao K, Kelly M, Bozic KJ. Future young patient demand for primary and revision joint replacement: national projections from 2010 to 2030. Clin Orthop. 2009;467(10):2606-2612.
4. Boettcher WG. Total hip arthroplasties in the elderly. Morbidity, mortality, and cost effectiveness. Clin Orthop. 1992;(274):30-34.
5. Greenfield S, Apolone G, McNeil BJ, Cleary PD. The importance of co-existent disease in the occurrence of postoperative complications and one-year recovery in patients undergoing total hip replacement. Comorbidity and outcomes after hip replacement. Med Care. 1993;31(2):141-154.
6. Kreder HJ, Williams JI, Jaglal S, Hu R, Axcell T, Stephen D. Are complication rates for elective primary total hip arthroplasty in Ontario related to surgeon and hospital volumes? A preliminary investigation. Can J Surg. 1998;41(6):431-437.
7. Botto F, Alonso-Coello P, Chan MT, et al. Myocardial injury after noncardiac surgery: a large, international, prospective cohort study establishing diagnostic criteria, characteristics, predictors, and 30-day outcomes. Anesthesiology. 2014;120(3):564-578.
8. Mangano DT, Browner WS, Hollenberg M, London MJ, Tubau JF, Tateo IM. Association of perioperative myocardial ischemia with cardiac morbidity and mortality in men undergoing noncardiac surgery. The Study of Perioperative Ischemia Research Group. N Engl J Med. 1990;323(26):1781-1788.
9. Tarhan S, Moffitt EA, Taylor WF, Giuliani ER. Myocardial infarction after general anesthesia. JAMA. 1972;220(11):1451-1454.
10. Landesberg G, Mosseri M, Zahger D, et al. Myocardial infarction after vascular surgery: the role of prolonged stress-induced, ST depression-type ischemia. J Am Coll Cardiol. 2001;37(7):1839-1845.
11. van Waes JA, Nathoe HM, de Graaff JC, et al. Myocardial injury after noncardiac surgery and its association with short-term mortality. Circulation. 2013;127(23):2264-2271.
12. Mangano DT. Perioperative cardiac morbidity. Anesthesiology. 1990;72(1):153-184.
13. Fleisher LA, Corbett W, Berry C, Poldermans D. Cost-effectiveness of differing perioperative beta-blockade strategies in vascular surgery patients. J Cardiothorac Vasc Anesth. 2004;18(1):7-13.
14. Aynardi M, Pulido L, Parvizi J, Sharkey PF, Rothman RH. Early mortality after modern total hip arthroplasty. Clin Orthop. 2009;467(1):213-218.
15. Gangireddy C, Rectenwald JR, Upchurch GR, et al. Risk factors and clinical impact of postoperative symptomatic venous thromboembolism. J Vasc Surg. 2007;45(2):335-341.
16. Baser O, Supina D, Sengupta N, Wang L, Kwong L. Impact of postoperative venous thromboembolism on Medicare recipients undergoing total hip replacement or total knee replacement surgery. Am J Health Syst Pharm. 2010;67(17):1438-1445.
17. Fehringer EV, Mikuls TR, Michaud KD, Henderson WG, O’Dell JR. Shoulder arthroplasties have fewer complications than hip or knee arthroplasties in US veterans. Clin Orthop. 2010;468(3):717-722.
18. Farmer KW, Hammond JW, Queale WS, Keyurapan E, McFarland EG. Shoulder arthroplasty versus hip and knee arthroplasties: a comparison of outcomes. Clin Orthop. 2007;(455):183-189.
19. Singh JA, Sperling JW, Cofield RH. Cardiopulmonary complications after primary shoulder arthroplasty: a cohort study. Semin Arthritis Rheum. 2012;41(5):689-697.
20. Go AS, Mozaffarian D, Roger VL, et al; American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics—2014 update: a report from the American Heart Association. Circulation. 2014;129(3):e28-e292.
21. Lin CA, Kuo AC, Takemoto S. Comorbidities and perioperative complications in HIV-positive patients undergoing primary total hip and knee arthroplasty. J Bone Joint Surg Am. 2013;95(11):1028-1036.
22. Maynard C, Sales AE. Changes in the use of coronary artery revascularization procedures in the Department of Veterans Affairs, the National Hospital Discharge Survey, and the Nationwide Inpatient Sample, 1991–1999. BMC Health Serv Res. 2003;3(1):12.
23. Griffin JW, Novicoff WM, Browne JA, Brockmeier SF. Obstructive sleep apnea as a risk factor after shoulder arthroplasty. J Shoulder Elbow Surg. 2013;22(12):e6-e9.
24. Hambright D, Henderson RA, Cook C, Worrell T, Moorman CT, Bolognesi MP. A comparison of perioperative outcomes in patients with and without rheumatoid arthritis after receiving a total shoulder replacement arthroplasty. J Shoulder Elbow Surg. 2011;20(1):77-85.
25. Odum SM, Troyer JL, Kelly MP, Dedini RD, Bozic KJ. A cost-utility analysis comparing the cost-effectiveness of simultaneous and staged bilateral total knee arthroplasty. J Bone Joint Surg Am. 2013;95(16):1441-1449.
26. Ponce BA, Menendez ME, Oladeji LO, Soldado F. Diabetes as a risk factor for poorer early postoperative outcomes after shoulder arthroplasty. J Shoulder Elbow Surg. 2014;23(5):671-678.
27. Alfonso DT, Toussaint RJ, Alfonso BD, Strauss EJ, Steiger DT, Di Cesare PE. Nonsurgical complications after total hip and knee arthroplasty. Am J Orthop. 2006;35(11):503-510.
28. Mantilla CB, Horlocker TT, Schroeder DR, Berry DJ, Brown DL. Frequency of myocardial infarction, pulmonary embolism, deep venous thrombosis, and death following primary hip or knee arthroplasty. Anesthesiology. 2002;96(5):1140-1146.
29. Singh JA, Jensen MR, Harmsen WS, Gabriel SE, Lewallen DG. Cardiac and thromboembolic complications and mortality in patients undergoing total hip and total knee arthroplasty. Ann Rheum Dis. 2011;70(12):2082-2088.
30. Kurian AK, Cardarelli KM. Racial and ethnic differences in cardiovascular disease risk factors: a systematic review. Ethn Dis. 2007;17(1):143-152.
31. Zhang AL, Schairer WW, Feeley BT. Hospital readmissions after surgical treatment of proximal humerus fractures: is arthroplasty safer than open reduction internal fixation? Clin Orthop. 2014;472(8):2317-2324.
32. Schairer WW, Zhang AL, Feeley BT. Hospital readmissions after primary shoulder arthroplasty. J Shoulder Elbow Surg. 2014;23(9):1349-1355.
33. de Kruijf M, Vroemen JP, de Leur K, van der Voort EA, Vos DI, Van der Laan L. Proximal fractures of the humerus in patients older than 75 years of age: should we consider operative treatment? J Orthop Traumatol. 2014;15(2):111-115.
34. Hauschild O, Konrad G, Audige L, et al. Operative versus non-operative treatment for two-part surgical neck fractures of the proximal humerus. Arch Orthop Trauma Surg. 2013;133(10):1385-1393.
35. Hanson B, Neidenbach P, de Boer P, Stengel D. Functional outcomes after nonoperative management of fractures of the proximal humerus. J Shoulder Elbow Surg. 2009;18(4):612-621.
36. Handoll HH, Ollivere BJ, Rollins KE. Interventions for treating proximal humeral fractures in adults. Cochrane Database Syst Rev. 2012;12:CD000434.
37. Court-Brown CM, Cattermole H, McQueen MM. Impacted valgus fractures (B1.1) of the proximal humerus. The results of non-operative treatment. J Bone Joint Surg Br. 2002;84(4):504-508.
38. Chalmers PN, Gupta AK, Rahman Z, Bruce B, Romeo AA, Nicholson GP. Predictors of early complications of total shoulder arthroplasty. J Arthroplasty. 2014;29(4):856-860.
39. Mahoney A, Bosco JA 3rd, Zuckerman JD. Readmission after shoulder arthroplasty. J Shoulder Elbow Surg. 2014;23(3):377-381.
40. Khan SK, Malviya A, Muller SD, et al. Reduced short-term complications and mortality following Enhanced Recovery primary hip and knee arthroplasty: results from 6,000 consecutive procedures. Acta Orthop. 2014;85(1):26-31.
41. Paavolainen P, Pukkala E, Pulkkinen P, Visuri T. Causes of death after total hip arthroplasty: a nationwide cohort study with 24,638 patients. J Arthroplasty. 2002;17(3):274-281.
42. Sharrock NE, Cazan MG, Hargett MJ, Williams-Russo P, Wilson PD Jr. Changes in mortality after total hip and knee arthroplasty over a ten-year period. Anesth Analg. 1995;80(2):242-248.
43. Parvizi J, Johnson BG, Rowland C, Ereth MH, Lewallen DG. Thirty-day mortality after elective total hip arthroplasty. J Bone Joint Surg Am. 2001;83(10):1524-1528.
44. Morris MJ, Molli RG, Berend KR, Lombardi AV Jr. Mortality and perioperative complications after unicompartmental knee arthroplasty. Knee. 2013;20(3):218-220.
45. Lindenauer PK, Pekow P, Wang K, Mamidi DK, Gutierrez B, Benjamin EM. Perioperative beta-blocker therapy and mortality after major noncardiac surgery. N Engl J Med. 2005;353(4):349-361.
46. Wijeysundera DN, Beattie WS, Wijeysundera HC, Yun L, Austin PC, Ko DT. Duration of preoperative beta-blockade and outcomes after major elective noncardiac surgery. Can J Cardiol. 2014;30(2):217-223.
47. Andersson C, Merie C, Jorgensen M, et al. Association of beta-blocker therapy with risks of adverse cardiovascular events and deaths in patients with ischemic heart disease undergoing noncardiac surgery: a Danish nationwide cohort study. JAMA Int Med. 2014;174(3):336-344.
48. Bakker EJ, Ravensbergen NJ, Poldermans D. Perioperative cardiac evaluation, monitoring, and risk reduction strategies in noncardiac surgery patients. Curr Opin Crit Care. 2011;17(5):409-415.
49. Auerbach AD, Goldman L. Beta-blockers and reduction of cardiac events in noncardiac surgery: scientific review. JAMA. 2002;287(11):1435-1444.
The incidence of shoulder arthroplasty in the United States is increasing annually,1-3 and the majority of these operations occur in older patients.4-6 Elderly patients with cardiovascular, pulmonary, cerebral, renal, and hepatic disease are increasingly susceptible to numerous surgical complications.4 Myocardial infarction (MI) is a complication that occurs in 0.7% of noncardiac surgeries. This figure increases to 1.1% in patients with coronary artery disease.7-11 Perioperative MI increases morbidity and mortality,8 and perioperative cardiac morbidity is the leading cause of death after anesthesia and surgery.12 The financial effects of perioperative cardiac morbidity and mortality must also be considered. A 2009 claims analysis study estimated charges associated with a perioperative MI at $15,000 and the cost of cardiac death at $21,909.13
Cardiovascular complications are associated with a significant degree of morbidity and mortality in patients who undergo arthroplasty.14-16 Although studies have elucidated 30- and 90-day morbidity and mortality rates after shoulder arthroplasty, in hip and knee arthroplasty17-19 little has been done to determine predictors of perioperative MI in a representative database of patients. Given the increasing incidence of shoulder arthroplasty in the United States, the elective nature of this procedure, and the percentage of the US population with cardiovascular risk factors,20 it is important to establish predictors of perioperative MI to ensure patients and physicians have the necessary resources to make informed decisions.
We conducted a study to examine the risk factors for perioperative MI in a large cohort of patients admitted for shoulder arthroplasty to US hospitals. We wanted to evaluate the association between perioperative MI and shoulder arthroplasty with respect to demographics, primary diagnosis, medical comorbidities, and perioperative complications. Specifically, we tested the null hypothesis that, among patients undergoing shoulder arthroplasty, and accounting for confounding variables, there would be no difference in risk factors for patients who have a perioperative MI.
Materials and Methods
This study was exempt from approval by our institutional review board. All data used in this project were deidentified before use.
Nationwide Inpatient Sample (NIS)
The Nationwide Inpatient Sample (NIS), an annual survey of hospitals, is conducted by the Healthcare Cost and Utilization Project (HCUP) and sponsored by the Agency for Healthcare Research and Quality (AHRQ). This database is the largest publicly available all-payer inpatient discharge database in the United States.21 Sampling 8 million hospital stays each year, NIS includes information from a representative batch of 20% of US hospitals. In 2011, 46 states and 1045 hospitals contributed information to the database, representing 97% of the US population.22 This large sample allows researchers to analyze a robust set of medical conditions and uncommon treatments. The survey, conducted each year since 1988, includes demographic, clinical, and resource use data.23 Discharge weight files are provided by NIS to arrive at valid national estimates.
This database is particularly useful because it provides information on up to 25 medical diagnoses and 15 procedures, which are recorded with International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) codes. Researchers can use this database to analyze patient and hospital characteristics as well as inpatient outcomes.24,25 Numerous studies have used NIS to address pertinent queries across the medical landscape.22,26
Patient Selection and Analysis
We used NIS to isolate a population of 422,371 adults (≥18 years old) who underwent total shoulder arthroplasty (TSA) or hemi–shoulder arthroplasty (HSA) between January 1, 2002 and December 31, 2011. We then placed the patients in this population into 1 of 2 cohorts. The first cohort had an acute MI during the perioperative period after TSA, and the second, larger cohort did not have an acute MI after TSA. Acute MI was identified using ICD-9-CM code 410.xx. To identify a population of shoulder arthroplasty patients, we included discharges with an ICD-9-CM procedure code of 81.80 or 81.88 (both TSA) or 81.81 (HSA) in the sample. We then considered the degree to which each of 5 variables—primary diagnosis, age, sex, race, and select medical comorbidities—was predictive of in-hospital MI after TSA.
Statistical Analysis
Given the large sample used in this study, normal distribution of data was assumed. Using bivariate analysis, Pearson χ2 test for categorical data, and independent-samples t test for continuous data, we compared the nonacute MI and acute MI groups. Multivariable binary logistic regression analyses allowed us to isolate the extent that primary diagnosis, age, sex, race, and medical comorbidities were predictors of acute MI after shoulder arthroplasty. Statistical significance was set at P < .05. SPSS Version 22.0 (SPSS, Chicago, Illinois) was used for all statistical analyses and data modeling.
Results
Between January 1, 2002 and December 31, 2011, an estimated total of 422,371 patients underwent shoulder arthroplasty (59.3% TSA, 40.7% HSA). Of these patients, 1174 (0.28%) had a perioperative MI, and 421,197 (99.72%) did not (Table 1). Patients with a primary diagnosis of proximal humerus fracture (33.8% vs 16.6%; P < .001) or rotator cuff arthropathy (10.1% vs 9.9%; P < .001) were more likely than patients with other diagnoses to have an in-hospital MI.
Our review of the demographics found that patients who underwent shoulder arthroplasty and had a perioperative MI were likely older (75±8.9 years vs 69±11 years; P < .001), Caucasian (94.2% vs 91.9%; P = .002), male (43.2% vs 39.7%; P = .013), in the highest median household income bracket of $63,000 or more (30.8% vs 25.6%; P < .001), and using Medicare (80.9% vs 66.3%; P < .001). They were more likely to be treated in a medical center of medium size (25.6% vs 23.7%; P = .042) or larger (61.8% vs 61.2%; P = .042). MIs occurred more often in urban environments (91.4% vs 88.5%; P = .002) and in HSA patients (55% vs 40.6%; P < .001), resulting in longer hospital stays (9.4±7.9 days vs 2.7±2.5 days; P < .001) and higher probability of death (6.5% vs 0.1%; P < .001).
We then analyzed the 2 cohorts for medical comorbidities (Table 2). Patients in the MI cohort presented with a significantly higher incidence of congestive heart failure, previous MI, angina pectoris, chronic lung disease, hypertension, diabetes, renal failure, fluid and electrolyte disorders, pulmonary circulatory disease, coagulopathy, and deficiency anemia (P < .001) but not liver disease and obesity. Bivariate analysis of perioperative outcomes (Table 3) indicated that these patients also had a statistically higher rate of numerous other complications: pulmonary embolism (4.9% vs 0.2%; P < .001), pneumonia (15.1% vs 1.2%; P < .001), deep venous thrombosis (2.6% vs 0.2%; P < .001), cerebrovascular event (1.6% vs 0.1%; P < .001), acute renal failure (15.1% vs 1.2%; P < .001), gastrointestinal complication (1.2% vs 0.3%; P < .001), mechanical ventilation (1.2% vs 0.3%; P < .001), transfusion (33.4% vs 8.8%; P < .001), and nonroutine discharge (73.3% vs 36.0%; P < .001).
Multivariable logistic regression analysis was performed to determine independent predictors of perioperative MI after shoulder arthroplasty (Table 4). Patients with a primary diagnosis of proximal humerus fracture (odds ratio [OR], 1.38; 95% confidence interval [CI], 1.15-1.65; P < .001) were more likely than patients with a primary diagnosis of osteoarthritis to have an MI. The odds of postoperative MI increased with age (OR, 1.04 per year; 95% CI, 1.03-1.05; P < .001) and were higher in males (OR, 1.72; 95% CI, 1.52-1.96; P < .001). Compared with Caucasians, African Americans (OR, 0.19; 95% CI, 0.09-0.40; P < .001) were less likely to have an in-hospital MI after shoulder arthroplasty. After shoulder arthroplasty, the odds of MI in the perioperative period increased with each subsequent day of care (OR, 1.10; 95% CI, 1.10-1.11; P < .001).
Regarding independent comorbidities, multivariable logistic regression analysis also determined that history of congestive heart failure (OR, 4.86; 95% CI, 4.20-5.61; P < .001), angina pectoris (OR, 2.90; 95% CI, 2.02-4.17; P < .001), complicated diabetes (OR, 1.96; 95% CI, 1.49-2.57; P < .001), renal failure (OR, 1.42; 95% CI, 1.17-1.72; P < .001), fluid and electrolyte disorders (OR, 1.42; 95% CI, 1.21-1.67; P < .001), and deficiency anemia (OR, 1.62; 95% CI, 1.40-1.88; P < .001) were significant predictors of perioperative MI after shoulder arthroplasty.
Discussion
Results of other studies have elucidated 30- and 90-day mortality rates and postoperative complications after shoulder arthroplasty, but, relative to hip and knee arthroplasty,17-19 little has been done to determine predictors of perioperative MI in a large sample of shoulder arthroplasty patients. Given the increasing rates of shoulder arthroplasty1-3 and the demographics of this population,4-6 it is likely that postoperative cardiovascular events will increase in frequency. We found that, in order of decreasing significance, the top 4 risk predictors for acute MI after shoulder arthroplasty were congestive heart failure, angina pectoralis, complicated diabetes mellitus, and male sex. Other pertinent risk factors included older age, Caucasian ethnicity, and a primary diagnosis of proximal humerus fracture. The rate of acute MI in patients who were older than 75 years when they underwent HSA for proximal humerus fracture was 0.80%.
Demographics
We found that patients who had an acute MI after shoulder arthroplasty were likely older, male, and Caucasian. Age and male sex are well-established risk factors for increased cardiac complications after arthroplasty.27-29 Previous studies have indicated that the rate of cardiac events increases in arthroplasty patients older than 65 years.19,28,29 In our study, more than 50% of the patients who had an acute perioperative MI were older than 85 years. Less explainable is the increased occurrence of acute MI in Caucasian patients and wealthy patients, given that minorities in the United States have higher rates of cardiovascular disease.30 Shoulder arthroplasty is an elective procedure, more likely to be undertaken by Caucasians. Therefore, at-risk minority groups and financially challenged groups may be less likely to have this procedure.
Primary Diagnosis
In this series, patients with a primary diagnosis of proximal humerus fracture were more likely to have an in-hospital MI. This finding is consistent with previous studies indicating a higher rate of complications for proximal humerus fracture patients than for shoulder arthroplasty patients.31,32 Given that more than 75% of patients who present with a proximal humerus fracture are older than 70 years, it would be prudent to examine operative indications after this diagnosis,33 particularly as benefit from surgery for fractures has not been definitively demonstrated.34-37
Comorbidities
Many of the patients in our MI cohort presented with congestive heart failure, angina pectoris, complicated diabetes, renal failure, fluid and electrolyte disorders, or deficiency anemia. This is in keeping with other studies indicating that preexisting cardiovascular morbidity increases the rate of MI after various forms of arthroplasty.7-11 Patients in our MI cohort were also susceptible to a variety of post-MI perioperative complications, including pulmonary embolism, pneumonia, deep venous thrombosis, cerebrovascular event, acute renal failure, gastrointestinal complication, mechanical ventilation, transfusion, and nonroutine discharge, and their incidence of death was higher. These findings are consistent with reports that postoperative cardiovascular complications increase the degree of morbidity and mortality in arthroplasty patients.14-16 It is also worth noting that the odds of MI in the perioperative period increase with each subsequent day of care. This is understandable given that patients presenting with numerous comorbidities are at increased risk for perioperative complications38 resulting in hospital readmission.39
The literature indicates that MI occurs as a complication in 0.7% of patients who undergo noncardiac surgery,7 though some series have shown it is more prevalent after arthroplasty procedures.28,40 MI significantly increases the rate of perioperative morbidity and mortality,8 and perioperative cardiac morbidity is a leading cause of death after anesthesia and surgery.12 Furthermore, the most common cause of death after lower extremity arthroplasty is cardiovascular-related.41,42 In patients who presented for elective hip arthroplasty, cardiorespiratory disease was one of the main risk factors (with older age and male sex) shown to increase perioperative mortality.43
Perioperative cardiovascular complications increase postoperative morbidity and mortality.12 The rate of cardiovascular complications after shoulder arthroplasty ranges from 0.8% to 2.6%, and the incidence of MI hovers between 0.3% and 0.9%.17,19,28,40,44 A recent study in 793 patients found that, over a 30-day period, cardiovascular complications accounted for more than one-fourth of all complications.17 Singh and colleagues19 analyzed cardiopulmonary complications after primary shoulder arthroplasty in a total of 3480 patients (4019 arthroplasties) and found this group had a 90-day cardiac morbidity (MI, congestive heart failure, arrhythmia) rate of 2.6%. In that study, a Deyo-Charlson index of 1 or more was a significant independent risk factor for cardiac complications following surgery. Scores on this weighted index of 17 comorbidities are used to assess the complexities of a patient population. Given the severity of cardiovascular perioperative complications, it is important to preoperatively identify high-risk population groups and sufficiently study and optimize patients before shoulder arthroplasty.
There is much debate about the effectiveness of perioperative β-blockers in reducing perioperative cardiac morbidity and mortality.45-48 Such a discussion is outside of the scope of this article, but it may be prudent to seek a cardiology consultation for patients presenting with risk factors for perioperative MI. β-Blockers may prove useful in reducing cardiac morbidity in high-risk patients after noncardiac surgery.45,49
Many limitations are inherent in studies that use a nationally represented database such as NIS, which we used in this study. It is highly likely that NIS does not capture all potential postoperative complications, as this database is very large and subject to errors in data entry and clinical coding. In addition, detailed clinical information (eg, severity of certain comorbid diseases before shoulder arthroplasty, details about the intraoperative course) was not readily available for analysis. Another limitation, which may have led to an underestimate of complication rates, was our not being able to obtain information about postdischarge complications.
Despite these limitations, NIS and other databases have helped researchers answer questions about low-incidence conditions and generalize findings to a national population. In the present study, we analyzed 2 cohorts, patients with and without acute MI after shoulder arthroplasty, to determine predictors for and complications of postarthroplasty MI. We identified numerous predictors for acute MI: congestive heart failure, angina pectoris, complicated diabetes, renal failure, fluid and electrolyte disorders, and deficiency anemia prior to arthroplasty. As perioperative MI is associated with significant morbidity,14-16 it would be wise to screen patients for such comorbid conditions, assess the severity of these conditions, and offer shoulder arthroplasty with prudence.
Conclusion
The top 4 predictors for acute MI after shoulder arthroplasty were congestive heart failure, angina pectoralis, complicated diabetes mellitus, and male sex. Other pertinent risk factors included older age, Caucasian ethnicity, and primary diagnosis of proximal humerus fracture. Surgeons and patients must be aware of predictors for adverse surgical outcomes such as perioperative MI and understand the extent to which these events increase perioperative morbidity and mortality.
The incidence of shoulder arthroplasty in the United States is increasing annually,1-3 and the majority of these operations occur in older patients.4-6 Elderly patients with cardiovascular, pulmonary, cerebral, renal, and hepatic disease are increasingly susceptible to numerous surgical complications.4 Myocardial infarction (MI) is a complication that occurs in 0.7% of noncardiac surgeries. This figure increases to 1.1% in patients with coronary artery disease.7-11 Perioperative MI increases morbidity and mortality,8 and perioperative cardiac morbidity is the leading cause of death after anesthesia and surgery.12 The financial effects of perioperative cardiac morbidity and mortality must also be considered. A 2009 claims analysis study estimated charges associated with a perioperative MI at $15,000 and the cost of cardiac death at $21,909.13
Cardiovascular complications are associated with a significant degree of morbidity and mortality in patients who undergo arthroplasty.14-16 Although studies have elucidated 30- and 90-day morbidity and mortality rates after shoulder arthroplasty, in hip and knee arthroplasty17-19 little has been done to determine predictors of perioperative MI in a representative database of patients. Given the increasing incidence of shoulder arthroplasty in the United States, the elective nature of this procedure, and the percentage of the US population with cardiovascular risk factors,20 it is important to establish predictors of perioperative MI to ensure patients and physicians have the necessary resources to make informed decisions.
We conducted a study to examine the risk factors for perioperative MI in a large cohort of patients admitted for shoulder arthroplasty to US hospitals. We wanted to evaluate the association between perioperative MI and shoulder arthroplasty with respect to demographics, primary diagnosis, medical comorbidities, and perioperative complications. Specifically, we tested the null hypothesis that, among patients undergoing shoulder arthroplasty, and accounting for confounding variables, there would be no difference in risk factors for patients who have a perioperative MI.
Materials and Methods
This study was exempt from approval by our institutional review board. All data used in this project were deidentified before use.
Nationwide Inpatient Sample (NIS)
The Nationwide Inpatient Sample (NIS), an annual survey of hospitals, is conducted by the Healthcare Cost and Utilization Project (HCUP) and sponsored by the Agency for Healthcare Research and Quality (AHRQ). This database is the largest publicly available all-payer inpatient discharge database in the United States.21 Sampling 8 million hospital stays each year, NIS includes information from a representative batch of 20% of US hospitals. In 2011, 46 states and 1045 hospitals contributed information to the database, representing 97% of the US population.22 This large sample allows researchers to analyze a robust set of medical conditions and uncommon treatments. The survey, conducted each year since 1988, includes demographic, clinical, and resource use data.23 Discharge weight files are provided by NIS to arrive at valid national estimates.
This database is particularly useful because it provides information on up to 25 medical diagnoses and 15 procedures, which are recorded with International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) codes. Researchers can use this database to analyze patient and hospital characteristics as well as inpatient outcomes.24,25 Numerous studies have used NIS to address pertinent queries across the medical landscape.22,26
Patient Selection and Analysis
We used NIS to isolate a population of 422,371 adults (≥18 years old) who underwent total shoulder arthroplasty (TSA) or hemi–shoulder arthroplasty (HSA) between January 1, 2002 and December 31, 2011. We then placed the patients in this population into 1 of 2 cohorts. The first cohort had an acute MI during the perioperative period after TSA, and the second, larger cohort did not have an acute MI after TSA. Acute MI was identified using ICD-9-CM code 410.xx. To identify a population of shoulder arthroplasty patients, we included discharges with an ICD-9-CM procedure code of 81.80 or 81.88 (both TSA) or 81.81 (HSA) in the sample. We then considered the degree to which each of 5 variables—primary diagnosis, age, sex, race, and select medical comorbidities—was predictive of in-hospital MI after TSA.
Statistical Analysis
Given the large sample used in this study, normal distribution of data was assumed. Using bivariate analysis, Pearson χ2 test for categorical data, and independent-samples t test for continuous data, we compared the nonacute MI and acute MI groups. Multivariable binary logistic regression analyses allowed us to isolate the extent that primary diagnosis, age, sex, race, and medical comorbidities were predictors of acute MI after shoulder arthroplasty. Statistical significance was set at P < .05. SPSS Version 22.0 (SPSS, Chicago, Illinois) was used for all statistical analyses and data modeling.
Results
Between January 1, 2002 and December 31, 2011, an estimated total of 422,371 patients underwent shoulder arthroplasty (59.3% TSA, 40.7% HSA). Of these patients, 1174 (0.28%) had a perioperative MI, and 421,197 (99.72%) did not (Table 1). Patients with a primary diagnosis of proximal humerus fracture (33.8% vs 16.6%; P < .001) or rotator cuff arthropathy (10.1% vs 9.9%; P < .001) were more likely than patients with other diagnoses to have an in-hospital MI.
Our review of the demographics found that patients who underwent shoulder arthroplasty and had a perioperative MI were likely older (75±8.9 years vs 69±11 years; P < .001), Caucasian (94.2% vs 91.9%; P = .002), male (43.2% vs 39.7%; P = .013), in the highest median household income bracket of $63,000 or more (30.8% vs 25.6%; P < .001), and using Medicare (80.9% vs 66.3%; P < .001). They were more likely to be treated in a medical center of medium size (25.6% vs 23.7%; P = .042) or larger (61.8% vs 61.2%; P = .042). MIs occurred more often in urban environments (91.4% vs 88.5%; P = .002) and in HSA patients (55% vs 40.6%; P < .001), resulting in longer hospital stays (9.4±7.9 days vs 2.7±2.5 days; P < .001) and higher probability of death (6.5% vs 0.1%; P < .001).
We then analyzed the 2 cohorts for medical comorbidities (Table 2). Patients in the MI cohort presented with a significantly higher incidence of congestive heart failure, previous MI, angina pectoris, chronic lung disease, hypertension, diabetes, renal failure, fluid and electrolyte disorders, pulmonary circulatory disease, coagulopathy, and deficiency anemia (P < .001) but not liver disease and obesity. Bivariate analysis of perioperative outcomes (Table 3) indicated that these patients also had a statistically higher rate of numerous other complications: pulmonary embolism (4.9% vs 0.2%; P < .001), pneumonia (15.1% vs 1.2%; P < .001), deep venous thrombosis (2.6% vs 0.2%; P < .001), cerebrovascular event (1.6% vs 0.1%; P < .001), acute renal failure (15.1% vs 1.2%; P < .001), gastrointestinal complication (1.2% vs 0.3%; P < .001), mechanical ventilation (1.2% vs 0.3%; P < .001), transfusion (33.4% vs 8.8%; P < .001), and nonroutine discharge (73.3% vs 36.0%; P < .001).
Multivariable logistic regression analysis was performed to determine independent predictors of perioperative MI after shoulder arthroplasty (Table 4). Patients with a primary diagnosis of proximal humerus fracture (odds ratio [OR], 1.38; 95% confidence interval [CI], 1.15-1.65; P < .001) were more likely than patients with a primary diagnosis of osteoarthritis to have an MI. The odds of postoperative MI increased with age (OR, 1.04 per year; 95% CI, 1.03-1.05; P < .001) and were higher in males (OR, 1.72; 95% CI, 1.52-1.96; P < .001). Compared with Caucasians, African Americans (OR, 0.19; 95% CI, 0.09-0.40; P < .001) were less likely to have an in-hospital MI after shoulder arthroplasty. After shoulder arthroplasty, the odds of MI in the perioperative period increased with each subsequent day of care (OR, 1.10; 95% CI, 1.10-1.11; P < .001).
Regarding independent comorbidities, multivariable logistic regression analysis also determined that history of congestive heart failure (OR, 4.86; 95% CI, 4.20-5.61; P < .001), angina pectoris (OR, 2.90; 95% CI, 2.02-4.17; P < .001), complicated diabetes (OR, 1.96; 95% CI, 1.49-2.57; P < .001), renal failure (OR, 1.42; 95% CI, 1.17-1.72; P < .001), fluid and electrolyte disorders (OR, 1.42; 95% CI, 1.21-1.67; P < .001), and deficiency anemia (OR, 1.62; 95% CI, 1.40-1.88; P < .001) were significant predictors of perioperative MI after shoulder arthroplasty.
Discussion
Results of other studies have elucidated 30- and 90-day mortality rates and postoperative complications after shoulder arthroplasty, but, relative to hip and knee arthroplasty,17-19 little has been done to determine predictors of perioperative MI in a large sample of shoulder arthroplasty patients. Given the increasing rates of shoulder arthroplasty1-3 and the demographics of this population,4-6 it is likely that postoperative cardiovascular events will increase in frequency. We found that, in order of decreasing significance, the top 4 risk predictors for acute MI after shoulder arthroplasty were congestive heart failure, angina pectoralis, complicated diabetes mellitus, and male sex. Other pertinent risk factors included older age, Caucasian ethnicity, and a primary diagnosis of proximal humerus fracture. The rate of acute MI in patients who were older than 75 years when they underwent HSA for proximal humerus fracture was 0.80%.
Demographics
We found that patients who had an acute MI after shoulder arthroplasty were likely older, male, and Caucasian. Age and male sex are well-established risk factors for increased cardiac complications after arthroplasty.27-29 Previous studies have indicated that the rate of cardiac events increases in arthroplasty patients older than 65 years.19,28,29 In our study, more than 50% of the patients who had an acute perioperative MI were older than 85 years. Less explainable is the increased occurrence of acute MI in Caucasian patients and wealthy patients, given that minorities in the United States have higher rates of cardiovascular disease.30 Shoulder arthroplasty is an elective procedure, more likely to be undertaken by Caucasians. Therefore, at-risk minority groups and financially challenged groups may be less likely to have this procedure.
Primary Diagnosis
In this series, patients with a primary diagnosis of proximal humerus fracture were more likely to have an in-hospital MI. This finding is consistent with previous studies indicating a higher rate of complications for proximal humerus fracture patients than for shoulder arthroplasty patients.31,32 Given that more than 75% of patients who present with a proximal humerus fracture are older than 70 years, it would be prudent to examine operative indications after this diagnosis,33 particularly as benefit from surgery for fractures has not been definitively demonstrated.34-37
Comorbidities
Many of the patients in our MI cohort presented with congestive heart failure, angina pectoris, complicated diabetes, renal failure, fluid and electrolyte disorders, or deficiency anemia. This is in keeping with other studies indicating that preexisting cardiovascular morbidity increases the rate of MI after various forms of arthroplasty.7-11 Patients in our MI cohort were also susceptible to a variety of post-MI perioperative complications, including pulmonary embolism, pneumonia, deep venous thrombosis, cerebrovascular event, acute renal failure, gastrointestinal complication, mechanical ventilation, transfusion, and nonroutine discharge, and their incidence of death was higher. These findings are consistent with reports that postoperative cardiovascular complications increase the degree of morbidity and mortality in arthroplasty patients.14-16 It is also worth noting that the odds of MI in the perioperative period increase with each subsequent day of care. This is understandable given that patients presenting with numerous comorbidities are at increased risk for perioperative complications38 resulting in hospital readmission.39
The literature indicates that MI occurs as a complication in 0.7% of patients who undergo noncardiac surgery,7 though some series have shown it is more prevalent after arthroplasty procedures.28,40 MI significantly increases the rate of perioperative morbidity and mortality,8 and perioperative cardiac morbidity is a leading cause of death after anesthesia and surgery.12 Furthermore, the most common cause of death after lower extremity arthroplasty is cardiovascular-related.41,42 In patients who presented for elective hip arthroplasty, cardiorespiratory disease was one of the main risk factors (with older age and male sex) shown to increase perioperative mortality.43
Perioperative cardiovascular complications increase postoperative morbidity and mortality.12 The rate of cardiovascular complications after shoulder arthroplasty ranges from 0.8% to 2.6%, and the incidence of MI hovers between 0.3% and 0.9%.17,19,28,40,44 A recent study in 793 patients found that, over a 30-day period, cardiovascular complications accounted for more than one-fourth of all complications.17 Singh and colleagues19 analyzed cardiopulmonary complications after primary shoulder arthroplasty in a total of 3480 patients (4019 arthroplasties) and found this group had a 90-day cardiac morbidity (MI, congestive heart failure, arrhythmia) rate of 2.6%. In that study, a Deyo-Charlson index of 1 or more was a significant independent risk factor for cardiac complications following surgery. Scores on this weighted index of 17 comorbidities are used to assess the complexities of a patient population. Given the severity of cardiovascular perioperative complications, it is important to preoperatively identify high-risk population groups and sufficiently study and optimize patients before shoulder arthroplasty.
There is much debate about the effectiveness of perioperative β-blockers in reducing perioperative cardiac morbidity and mortality.45-48 Such a discussion is outside of the scope of this article, but it may be prudent to seek a cardiology consultation for patients presenting with risk factors for perioperative MI. β-Blockers may prove useful in reducing cardiac morbidity in high-risk patients after noncardiac surgery.45,49
Many limitations are inherent in studies that use a nationally represented database such as NIS, which we used in this study. It is highly likely that NIS does not capture all potential postoperative complications, as this database is very large and subject to errors in data entry and clinical coding. In addition, detailed clinical information (eg, severity of certain comorbid diseases before shoulder arthroplasty, details about the intraoperative course) was not readily available for analysis. Another limitation, which may have led to an underestimate of complication rates, was our not being able to obtain information about postdischarge complications.
Despite these limitations, NIS and other databases have helped researchers answer questions about low-incidence conditions and generalize findings to a national population. In the present study, we analyzed 2 cohorts, patients with and without acute MI after shoulder arthroplasty, to determine predictors for and complications of postarthroplasty MI. We identified numerous predictors for acute MI: congestive heart failure, angina pectoris, complicated diabetes, renal failure, fluid and electrolyte disorders, and deficiency anemia prior to arthroplasty. As perioperative MI is associated with significant morbidity,14-16 it would be wise to screen patients for such comorbid conditions, assess the severity of these conditions, and offer shoulder arthroplasty with prudence.
Conclusion
The top 4 predictors for acute MI after shoulder arthroplasty were congestive heart failure, angina pectoralis, complicated diabetes mellitus, and male sex. Other pertinent risk factors included older age, Caucasian ethnicity, and primary diagnosis of proximal humerus fracture. Surgeons and patients must be aware of predictors for adverse surgical outcomes such as perioperative MI and understand the extent to which these events increase perioperative morbidity and mortality.
1. Day JS, Lau E, Ong KL, Williams GR, Ramsey ML, Kurtz SM. Prevalence and projections of total shoulder and elbow arthroplasty in the United States to 2015. J Shoulder Elbow Surg. 2010;19(8):1115-1120.
2. Kim SH, Wise BL, Zhang Y, Szabo RM. Increasing incidence of shoulder arthroplasty in the United States. J Bone Joint Surg Am. 2011;93(24):2249-2254.
3. Kurtz SM, Lau E, Ong K, Zhao K, Kelly M, Bozic KJ. Future young patient demand for primary and revision joint replacement: national projections from 2010 to 2030. Clin Orthop. 2009;467(10):2606-2612.
4. Boettcher WG. Total hip arthroplasties in the elderly. Morbidity, mortality, and cost effectiveness. Clin Orthop. 1992;(274):30-34.
5. Greenfield S, Apolone G, McNeil BJ, Cleary PD. The importance of co-existent disease in the occurrence of postoperative complications and one-year recovery in patients undergoing total hip replacement. Comorbidity and outcomes after hip replacement. Med Care. 1993;31(2):141-154.
6. Kreder HJ, Williams JI, Jaglal S, Hu R, Axcell T, Stephen D. Are complication rates for elective primary total hip arthroplasty in Ontario related to surgeon and hospital volumes? A preliminary investigation. Can J Surg. 1998;41(6):431-437.
7. Botto F, Alonso-Coello P, Chan MT, et al. Myocardial injury after noncardiac surgery: a large, international, prospective cohort study establishing diagnostic criteria, characteristics, predictors, and 30-day outcomes. Anesthesiology. 2014;120(3):564-578.
8. Mangano DT, Browner WS, Hollenberg M, London MJ, Tubau JF, Tateo IM. Association of perioperative myocardial ischemia with cardiac morbidity and mortality in men undergoing noncardiac surgery. The Study of Perioperative Ischemia Research Group. N Engl J Med. 1990;323(26):1781-1788.
9. Tarhan S, Moffitt EA, Taylor WF, Giuliani ER. Myocardial infarction after general anesthesia. JAMA. 1972;220(11):1451-1454.
10. Landesberg G, Mosseri M, Zahger D, et al. Myocardial infarction after vascular surgery: the role of prolonged stress-induced, ST depression-type ischemia. J Am Coll Cardiol. 2001;37(7):1839-1845.
11. van Waes JA, Nathoe HM, de Graaff JC, et al. Myocardial injury after noncardiac surgery and its association with short-term mortality. Circulation. 2013;127(23):2264-2271.
12. Mangano DT. Perioperative cardiac morbidity. Anesthesiology. 1990;72(1):153-184.
13. Fleisher LA, Corbett W, Berry C, Poldermans D. Cost-effectiveness of differing perioperative beta-blockade strategies in vascular surgery patients. J Cardiothorac Vasc Anesth. 2004;18(1):7-13.
14. Aynardi M, Pulido L, Parvizi J, Sharkey PF, Rothman RH. Early mortality after modern total hip arthroplasty. Clin Orthop. 2009;467(1):213-218.
15. Gangireddy C, Rectenwald JR, Upchurch GR, et al. Risk factors and clinical impact of postoperative symptomatic venous thromboembolism. J Vasc Surg. 2007;45(2):335-341.
16. Baser O, Supina D, Sengupta N, Wang L, Kwong L. Impact of postoperative venous thromboembolism on Medicare recipients undergoing total hip replacement or total knee replacement surgery. Am J Health Syst Pharm. 2010;67(17):1438-1445.
17. Fehringer EV, Mikuls TR, Michaud KD, Henderson WG, O’Dell JR. Shoulder arthroplasties have fewer complications than hip or knee arthroplasties in US veterans. Clin Orthop. 2010;468(3):717-722.
18. Farmer KW, Hammond JW, Queale WS, Keyurapan E, McFarland EG. Shoulder arthroplasty versus hip and knee arthroplasties: a comparison of outcomes. Clin Orthop. 2007;(455):183-189.
19. Singh JA, Sperling JW, Cofield RH. Cardiopulmonary complications after primary shoulder arthroplasty: a cohort study. Semin Arthritis Rheum. 2012;41(5):689-697.
20. Go AS, Mozaffarian D, Roger VL, et al; American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics—2014 update: a report from the American Heart Association. Circulation. 2014;129(3):e28-e292.
21. Lin CA, Kuo AC, Takemoto S. Comorbidities and perioperative complications in HIV-positive patients undergoing primary total hip and knee arthroplasty. J Bone Joint Surg Am. 2013;95(11):1028-1036.
22. Maynard C, Sales AE. Changes in the use of coronary artery revascularization procedures in the Department of Veterans Affairs, the National Hospital Discharge Survey, and the Nationwide Inpatient Sample, 1991–1999. BMC Health Serv Res. 2003;3(1):12.
23. Griffin JW, Novicoff WM, Browne JA, Brockmeier SF. Obstructive sleep apnea as a risk factor after shoulder arthroplasty. J Shoulder Elbow Surg. 2013;22(12):e6-e9.
24. Hambright D, Henderson RA, Cook C, Worrell T, Moorman CT, Bolognesi MP. A comparison of perioperative outcomes in patients with and without rheumatoid arthritis after receiving a total shoulder replacement arthroplasty. J Shoulder Elbow Surg. 2011;20(1):77-85.
25. Odum SM, Troyer JL, Kelly MP, Dedini RD, Bozic KJ. A cost-utility analysis comparing the cost-effectiveness of simultaneous and staged bilateral total knee arthroplasty. J Bone Joint Surg Am. 2013;95(16):1441-1449.
26. Ponce BA, Menendez ME, Oladeji LO, Soldado F. Diabetes as a risk factor for poorer early postoperative outcomes after shoulder arthroplasty. J Shoulder Elbow Surg. 2014;23(5):671-678.
27. Alfonso DT, Toussaint RJ, Alfonso BD, Strauss EJ, Steiger DT, Di Cesare PE. Nonsurgical complications after total hip and knee arthroplasty. Am J Orthop. 2006;35(11):503-510.
28. Mantilla CB, Horlocker TT, Schroeder DR, Berry DJ, Brown DL. Frequency of myocardial infarction, pulmonary embolism, deep venous thrombosis, and death following primary hip or knee arthroplasty. Anesthesiology. 2002;96(5):1140-1146.
29. Singh JA, Jensen MR, Harmsen WS, Gabriel SE, Lewallen DG. Cardiac and thromboembolic complications and mortality in patients undergoing total hip and total knee arthroplasty. Ann Rheum Dis. 2011;70(12):2082-2088.
30. Kurian AK, Cardarelli KM. Racial and ethnic differences in cardiovascular disease risk factors: a systematic review. Ethn Dis. 2007;17(1):143-152.
31. Zhang AL, Schairer WW, Feeley BT. Hospital readmissions after surgical treatment of proximal humerus fractures: is arthroplasty safer than open reduction internal fixation? Clin Orthop. 2014;472(8):2317-2324.
32. Schairer WW, Zhang AL, Feeley BT. Hospital readmissions after primary shoulder arthroplasty. J Shoulder Elbow Surg. 2014;23(9):1349-1355.
33. de Kruijf M, Vroemen JP, de Leur K, van der Voort EA, Vos DI, Van der Laan L. Proximal fractures of the humerus in patients older than 75 years of age: should we consider operative treatment? J Orthop Traumatol. 2014;15(2):111-115.
34. Hauschild O, Konrad G, Audige L, et al. Operative versus non-operative treatment for two-part surgical neck fractures of the proximal humerus. Arch Orthop Trauma Surg. 2013;133(10):1385-1393.
35. Hanson B, Neidenbach P, de Boer P, Stengel D. Functional outcomes after nonoperative management of fractures of the proximal humerus. J Shoulder Elbow Surg. 2009;18(4):612-621.
36. Handoll HH, Ollivere BJ, Rollins KE. Interventions for treating proximal humeral fractures in adults. Cochrane Database Syst Rev. 2012;12:CD000434.
37. Court-Brown CM, Cattermole H, McQueen MM. Impacted valgus fractures (B1.1) of the proximal humerus. The results of non-operative treatment. J Bone Joint Surg Br. 2002;84(4):504-508.
38. Chalmers PN, Gupta AK, Rahman Z, Bruce B, Romeo AA, Nicholson GP. Predictors of early complications of total shoulder arthroplasty. J Arthroplasty. 2014;29(4):856-860.
39. Mahoney A, Bosco JA 3rd, Zuckerman JD. Readmission after shoulder arthroplasty. J Shoulder Elbow Surg. 2014;23(3):377-381.
40. Khan SK, Malviya A, Muller SD, et al. Reduced short-term complications and mortality following Enhanced Recovery primary hip and knee arthroplasty: results from 6,000 consecutive procedures. Acta Orthop. 2014;85(1):26-31.
41. Paavolainen P, Pukkala E, Pulkkinen P, Visuri T. Causes of death after total hip arthroplasty: a nationwide cohort study with 24,638 patients. J Arthroplasty. 2002;17(3):274-281.
42. Sharrock NE, Cazan MG, Hargett MJ, Williams-Russo P, Wilson PD Jr. Changes in mortality after total hip and knee arthroplasty over a ten-year period. Anesth Analg. 1995;80(2):242-248.
43. Parvizi J, Johnson BG, Rowland C, Ereth MH, Lewallen DG. Thirty-day mortality after elective total hip arthroplasty. J Bone Joint Surg Am. 2001;83(10):1524-1528.
44. Morris MJ, Molli RG, Berend KR, Lombardi AV Jr. Mortality and perioperative complications after unicompartmental knee arthroplasty. Knee. 2013;20(3):218-220.
45. Lindenauer PK, Pekow P, Wang K, Mamidi DK, Gutierrez B, Benjamin EM. Perioperative beta-blocker therapy and mortality after major noncardiac surgery. N Engl J Med. 2005;353(4):349-361.
46. Wijeysundera DN, Beattie WS, Wijeysundera HC, Yun L, Austin PC, Ko DT. Duration of preoperative beta-blockade and outcomes after major elective noncardiac surgery. Can J Cardiol. 2014;30(2):217-223.
47. Andersson C, Merie C, Jorgensen M, et al. Association of beta-blocker therapy with risks of adverse cardiovascular events and deaths in patients with ischemic heart disease undergoing noncardiac surgery: a Danish nationwide cohort study. JAMA Int Med. 2014;174(3):336-344.
48. Bakker EJ, Ravensbergen NJ, Poldermans D. Perioperative cardiac evaluation, monitoring, and risk reduction strategies in noncardiac surgery patients. Curr Opin Crit Care. 2011;17(5):409-415.
49. Auerbach AD, Goldman L. Beta-blockers and reduction of cardiac events in noncardiac surgery: scientific review. JAMA. 2002;287(11):1435-1444.
1. Day JS, Lau E, Ong KL, Williams GR, Ramsey ML, Kurtz SM. Prevalence and projections of total shoulder and elbow arthroplasty in the United States to 2015. J Shoulder Elbow Surg. 2010;19(8):1115-1120.
2. Kim SH, Wise BL, Zhang Y, Szabo RM. Increasing incidence of shoulder arthroplasty in the United States. J Bone Joint Surg Am. 2011;93(24):2249-2254.
3. Kurtz SM, Lau E, Ong K, Zhao K, Kelly M, Bozic KJ. Future young patient demand for primary and revision joint replacement: national projections from 2010 to 2030. Clin Orthop. 2009;467(10):2606-2612.
4. Boettcher WG. Total hip arthroplasties in the elderly. Morbidity, mortality, and cost effectiveness. Clin Orthop. 1992;(274):30-34.
5. Greenfield S, Apolone G, McNeil BJ, Cleary PD. The importance of co-existent disease in the occurrence of postoperative complications and one-year recovery in patients undergoing total hip replacement. Comorbidity and outcomes after hip replacement. Med Care. 1993;31(2):141-154.
6. Kreder HJ, Williams JI, Jaglal S, Hu R, Axcell T, Stephen D. Are complication rates for elective primary total hip arthroplasty in Ontario related to surgeon and hospital volumes? A preliminary investigation. Can J Surg. 1998;41(6):431-437.
7. Botto F, Alonso-Coello P, Chan MT, et al. Myocardial injury after noncardiac surgery: a large, international, prospective cohort study establishing diagnostic criteria, characteristics, predictors, and 30-day outcomes. Anesthesiology. 2014;120(3):564-578.
8. Mangano DT, Browner WS, Hollenberg M, London MJ, Tubau JF, Tateo IM. Association of perioperative myocardial ischemia with cardiac morbidity and mortality in men undergoing noncardiac surgery. The Study of Perioperative Ischemia Research Group. N Engl J Med. 1990;323(26):1781-1788.
9. Tarhan S, Moffitt EA, Taylor WF, Giuliani ER. Myocardial infarction after general anesthesia. JAMA. 1972;220(11):1451-1454.
10. Landesberg G, Mosseri M, Zahger D, et al. Myocardial infarction after vascular surgery: the role of prolonged stress-induced, ST depression-type ischemia. J Am Coll Cardiol. 2001;37(7):1839-1845.
11. van Waes JA, Nathoe HM, de Graaff JC, et al. Myocardial injury after noncardiac surgery and its association with short-term mortality. Circulation. 2013;127(23):2264-2271.
12. Mangano DT. Perioperative cardiac morbidity. Anesthesiology. 1990;72(1):153-184.
13. Fleisher LA, Corbett W, Berry C, Poldermans D. Cost-effectiveness of differing perioperative beta-blockade strategies in vascular surgery patients. J Cardiothorac Vasc Anesth. 2004;18(1):7-13.
14. Aynardi M, Pulido L, Parvizi J, Sharkey PF, Rothman RH. Early mortality after modern total hip arthroplasty. Clin Orthop. 2009;467(1):213-218.
15. Gangireddy C, Rectenwald JR, Upchurch GR, et al. Risk factors and clinical impact of postoperative symptomatic venous thromboembolism. J Vasc Surg. 2007;45(2):335-341.
16. Baser O, Supina D, Sengupta N, Wang L, Kwong L. Impact of postoperative venous thromboembolism on Medicare recipients undergoing total hip replacement or total knee replacement surgery. Am J Health Syst Pharm. 2010;67(17):1438-1445.
17. Fehringer EV, Mikuls TR, Michaud KD, Henderson WG, O’Dell JR. Shoulder arthroplasties have fewer complications than hip or knee arthroplasties in US veterans. Clin Orthop. 2010;468(3):717-722.
18. Farmer KW, Hammond JW, Queale WS, Keyurapan E, McFarland EG. Shoulder arthroplasty versus hip and knee arthroplasties: a comparison of outcomes. Clin Orthop. 2007;(455):183-189.
19. Singh JA, Sperling JW, Cofield RH. Cardiopulmonary complications after primary shoulder arthroplasty: a cohort study. Semin Arthritis Rheum. 2012;41(5):689-697.
20. Go AS, Mozaffarian D, Roger VL, et al; American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics—2014 update: a report from the American Heart Association. Circulation. 2014;129(3):e28-e292.
21. Lin CA, Kuo AC, Takemoto S. Comorbidities and perioperative complications in HIV-positive patients undergoing primary total hip and knee arthroplasty. J Bone Joint Surg Am. 2013;95(11):1028-1036.
22. Maynard C, Sales AE. Changes in the use of coronary artery revascularization procedures in the Department of Veterans Affairs, the National Hospital Discharge Survey, and the Nationwide Inpatient Sample, 1991–1999. BMC Health Serv Res. 2003;3(1):12.
23. Griffin JW, Novicoff WM, Browne JA, Brockmeier SF. Obstructive sleep apnea as a risk factor after shoulder arthroplasty. J Shoulder Elbow Surg. 2013;22(12):e6-e9.
24. Hambright D, Henderson RA, Cook C, Worrell T, Moorman CT, Bolognesi MP. A comparison of perioperative outcomes in patients with and without rheumatoid arthritis after receiving a total shoulder replacement arthroplasty. J Shoulder Elbow Surg. 2011;20(1):77-85.
25. Odum SM, Troyer JL, Kelly MP, Dedini RD, Bozic KJ. A cost-utility analysis comparing the cost-effectiveness of simultaneous and staged bilateral total knee arthroplasty. J Bone Joint Surg Am. 2013;95(16):1441-1449.
26. Ponce BA, Menendez ME, Oladeji LO, Soldado F. Diabetes as a risk factor for poorer early postoperative outcomes after shoulder arthroplasty. J Shoulder Elbow Surg. 2014;23(5):671-678.
27. Alfonso DT, Toussaint RJ, Alfonso BD, Strauss EJ, Steiger DT, Di Cesare PE. Nonsurgical complications after total hip and knee arthroplasty. Am J Orthop. 2006;35(11):503-510.
28. Mantilla CB, Horlocker TT, Schroeder DR, Berry DJ, Brown DL. Frequency of myocardial infarction, pulmonary embolism, deep venous thrombosis, and death following primary hip or knee arthroplasty. Anesthesiology. 2002;96(5):1140-1146.
29. Singh JA, Jensen MR, Harmsen WS, Gabriel SE, Lewallen DG. Cardiac and thromboembolic complications and mortality in patients undergoing total hip and total knee arthroplasty. Ann Rheum Dis. 2011;70(12):2082-2088.
30. Kurian AK, Cardarelli KM. Racial and ethnic differences in cardiovascular disease risk factors: a systematic review. Ethn Dis. 2007;17(1):143-152.
31. Zhang AL, Schairer WW, Feeley BT. Hospital readmissions after surgical treatment of proximal humerus fractures: is arthroplasty safer than open reduction internal fixation? Clin Orthop. 2014;472(8):2317-2324.
32. Schairer WW, Zhang AL, Feeley BT. Hospital readmissions after primary shoulder arthroplasty. J Shoulder Elbow Surg. 2014;23(9):1349-1355.
33. de Kruijf M, Vroemen JP, de Leur K, van der Voort EA, Vos DI, Van der Laan L. Proximal fractures of the humerus in patients older than 75 years of age: should we consider operative treatment? J Orthop Traumatol. 2014;15(2):111-115.
34. Hauschild O, Konrad G, Audige L, et al. Operative versus non-operative treatment for two-part surgical neck fractures of the proximal humerus. Arch Orthop Trauma Surg. 2013;133(10):1385-1393.
35. Hanson B, Neidenbach P, de Boer P, Stengel D. Functional outcomes after nonoperative management of fractures of the proximal humerus. J Shoulder Elbow Surg. 2009;18(4):612-621.
36. Handoll HH, Ollivere BJ, Rollins KE. Interventions for treating proximal humeral fractures in adults. Cochrane Database Syst Rev. 2012;12:CD000434.
37. Court-Brown CM, Cattermole H, McQueen MM. Impacted valgus fractures (B1.1) of the proximal humerus. The results of non-operative treatment. J Bone Joint Surg Br. 2002;84(4):504-508.
38. Chalmers PN, Gupta AK, Rahman Z, Bruce B, Romeo AA, Nicholson GP. Predictors of early complications of total shoulder arthroplasty. J Arthroplasty. 2014;29(4):856-860.
39. Mahoney A, Bosco JA 3rd, Zuckerman JD. Readmission after shoulder arthroplasty. J Shoulder Elbow Surg. 2014;23(3):377-381.
40. Khan SK, Malviya A, Muller SD, et al. Reduced short-term complications and mortality following Enhanced Recovery primary hip and knee arthroplasty: results from 6,000 consecutive procedures. Acta Orthop. 2014;85(1):26-31.
41. Paavolainen P, Pukkala E, Pulkkinen P, Visuri T. Causes of death after total hip arthroplasty: a nationwide cohort study with 24,638 patients. J Arthroplasty. 2002;17(3):274-281.
42. Sharrock NE, Cazan MG, Hargett MJ, Williams-Russo P, Wilson PD Jr. Changes in mortality after total hip and knee arthroplasty over a ten-year period. Anesth Analg. 1995;80(2):242-248.
43. Parvizi J, Johnson BG, Rowland C, Ereth MH, Lewallen DG. Thirty-day mortality after elective total hip arthroplasty. J Bone Joint Surg Am. 2001;83(10):1524-1528.
44. Morris MJ, Molli RG, Berend KR, Lombardi AV Jr. Mortality and perioperative complications after unicompartmental knee arthroplasty. Knee. 2013;20(3):218-220.
45. Lindenauer PK, Pekow P, Wang K, Mamidi DK, Gutierrez B, Benjamin EM. Perioperative beta-blocker therapy and mortality after major noncardiac surgery. N Engl J Med. 2005;353(4):349-361.
46. Wijeysundera DN, Beattie WS, Wijeysundera HC, Yun L, Austin PC, Ko DT. Duration of preoperative beta-blockade and outcomes after major elective noncardiac surgery. Can J Cardiol. 2014;30(2):217-223.
47. Andersson C, Merie C, Jorgensen M, et al. Association of beta-blocker therapy with risks of adverse cardiovascular events and deaths in patients with ischemic heart disease undergoing noncardiac surgery: a Danish nationwide cohort study. JAMA Int Med. 2014;174(3):336-344.
48. Bakker EJ, Ravensbergen NJ, Poldermans D. Perioperative cardiac evaluation, monitoring, and risk reduction strategies in noncardiac surgery patients. Curr Opin Crit Care. 2011;17(5):409-415.
49. Auerbach AD, Goldman L. Beta-blockers and reduction of cardiac events in noncardiac surgery: scientific review. JAMA. 2002;287(11):1435-1444.
Assessing the Reading Level of Online Sarcoma Patient Education Materials
The diagnosis of cancer is a life-changing event for the patient as well as the patient’s family, friends, and relatives. Once diagnosed, most cancer patients want more information about their prognosis, future procedures, and/or treatment options.1 Receiving such information has been shown to reduce patient anxiety, increase patient satisfaction with care, and improve self-care.2-6 With the evolution of the Internet, patients in general7-9 and, specifically, cancer patients10-17 have turned to websites and online patient education materials (PEMs) to gather more health information.
For online PEMs to convey health information, their reading level must match the health literacy of the individuals who access them. Health literacy is the ability of an individual to gather and comprehend information about their condition to make the best decisions for their health.18 According to a report by the Institute of Medicine, 90 million American adults cannot properly use the US health care system because they do not possess adequate health literacy.18 Additionally, 36% of adults in the United States have basic or less-than-basic health literacy.19 This is starkly contrasted with the 12% of US adults who have proficient health literacy. A 2012 survey showed that about 31% of individuals who look for health information on the Internet have a high school education or less.8 In order to address the low health literacy of adults, the National Institutes of Health (NIH) has recommended that online PEMs be written at a sixth- to seventh-grade reading level.20
Unfortunately, many online PEMs related to certain cancer21-25 and orthopedic conditions26-31 do not meet NIH recommendations. Only 1 study has specifically looked at PEMs related to an orthopedic cancer condition.32 Lam and colleagues32 evaluated the readability of osteosarcoma PEMs from 56 websites using only 2 readability instruments and identified 86% of the websites as having a greater than eighth-grade reading level. No study has thoroughly assessed the readability of PEMs about bone and soft-tissue sarcomas and related conditions nor has any used 10 different readability instruments. Since each readability instrument has different variables (eg, sentence length, number of paragraphs, or number of complex words), averaging the scores of 10 of these instruments may result in less bias.
The purpose of this study was to evaluate the readability of online PEMs concerning bone and soft-tissue sarcomas and related conditions. The online PEMs came from websites that sarcoma patients may visit to obtain information about their condition. Our hypothesis was that the majority of these online PEMs will have a higher reading level than the NIH recommendations.
Materials and Methods
In May 2013, we identified online PEMs that included background, diagnosis, tests, or treatments for bone and soft-tissue sarcomas and conditions that mimic bone sarcoma. We included articles from the Tumors section of the American Academy of Orthopaedic Surgeons (AAOS) website.33 A second source of online PEMs came from a list of academic training centers created through the American Medical Association’s Fellowship and Residency Electronic Internet Database (FREIDA) with search criteria narrowed to orthopedic surgery. If we did not find PEMs of bone and soft-tissue cancers in the orthopedic department of a given academic training center’s website, we searched its cancer center website. We chose 4 programs with PEMs relevant to bone and soft-tissue sarcomas from each region in FREIDA for a balanced representation, except for the Territory region because it had only 1 academic training center and no relevant PEMs. Specialized websites, including Bonetumor.org, Sarcoma Alliance (Sarcomaalliance.org), and Sarcoma Foundation of America (Curesarcoma.org), were also evaluated. Within the Sarcoma Specialists section of the Sarcoma Alliance website,34 sarcoma specialists who were not identified from the FREIDA search for academic training centers were selected for review.
Because 8 of 10 individuals looking for health information on the Internet start their investigation at search engines, we also looked for PEMs through a Google search (Google.com) of bone cancer, and evaluated the first 10 hits for PEMs.8 Of these 10 hits, 8 had relevant PEMs, which we searched for additional PEMs about bone and soft-tissue cancers and related conditions. We also conducted a Google search of the most common bone sarcoma and soft-tissue sarcoma, osteosarcoma and malignant fibrous histiocytoma, respectively, and found 2 additional websites with relevant PEMs. LaCoursiere and colleagues35 surveyed cancer patients who used the Internet and found that they preferred WebMD (Webmd.com) and Medscape (Medscape.com) as sources for content about their medical condition.35 WebMD had been identified in the Google search, and we gathered the PEMs from Medscape also. It is worth noting that some of these websites are written for patients as well as clinicians.
Text from these PEMs were copied and pasted into separate Microsoft Word documents (Microsoft, Redmond, Washington). Advertisements, pictures, picture text, hyperlinks, copyright notices, page navigation links, paragraphs with no text, and any text that was not related to the given condition were deleted from the document to format the text for the readability software. Then, each Microsoft Word document was uploaded into the software package Readability Studio Professional (RSP) Edition Version 2012.1 for Windows (Oleander Software, Vandalia, Ohio). The 10 distinct readability instruments that were used to gauge the readability of each document were the Flesch Reading Ease score (FRE), the New Fog Count, the New Automated Readability Index, the Coleman-Liau Index (CLI), the Fry readability graph, the New Dale-Chall formula (NDC), the Gunning Frequency of Gobbledygook (Gunning FOG), the Powers-Sumner-Kearl formula, the Simple Measure of Gobbledygook (SMOG), and the Raygor Estimate Graph.
The FRE’s formula takes the average number of words per sentence and average number of syllables per word to compute a score ranging from 0 to 100 with 0 being the hardest to read.36 The New Fog Count tallies the number of sentences, easy words, and hard words (polysyllables) to calculate the grade level of the document.37 The New Automated Readability Index takes the average characters per word and average words per sentence to calculate a grade level for the document.37 The CLI randomly samples a few hundred words from the document, averages the number of letters and sentences per sample, and calculates an estimated grade level.38 The Fry readability graph selects samples of 100 words from the document, averages the number of syllables and sentences per 100 words, plots these data points on a graph, with the intersection determining the reading level.39 The NDC uses a list of 3000 familiar words that most fourth-grade students know.40 The percentage of difficult words, which are not on the list of familiar words, and the average sentence length in words are used to calculate the reading grade level of the document. The Gunning FOG uses the average sentence length in words and the percentage of hard words from a sample of at least 100 words to determine the reading grade level of the document.41 The Powers-Sumner-Kearl formula uses the average sentence length and percentage of monosyllables from a 100-word sample passage to calculate the reading grade level.42 The SMOG formula counts the number of polysyllabic words from 30 sentences and calculates the reading grade level of the document.43 In contrast to other formulas that test for 50% to 75% comprehension, the SMOG formula tests for 100% comprehension. As a result, the SMOG formula generally assigns a reading level 2 grades higher than the Dale-Chall level. The Raygor Estimate Graph selects a 100-word passage, counts the number of sentences and number of words with 6 or more letters, and plots the 2 variables on a graph to determine the reading grade level.44 The software package calculated the results from each reading instrument and reported the mean grade level score
for each document.
Results
We identified a total of 72 websites with relevant PEMs and included them in this study. Of these 72 websites, 36 websites were academic training centers, 10 were Google search hits, and 21 were from the Sarcoma Alliance list of sarcoma specialists. The remaining 5 websites were AAOS, Bonetumor.org, Sarcoma Alliance, Sarcoma Foundation of America, and Medscape. A list of conditions and treatments that were considered relevant PEMs is found in Appendix 1. A total of 774 articles were obtained from the 72 websites.
None of the websites had a mean readability score of 7 (seventh grade) or lower (Figures 1A, 1B). Mid-America Sarcoma Institute’s PEMs had the lowest mean readability score, 8.9. The lowest readability score was 5.3, which the New Fog Count readability instrument calculated for Vanderbilt University Medical Center’s (VUMC’s) PEMs (Appendix 2). The mean readability score of all websites was 11.4 (range, 8.9-15.5) (Appendix 2).
Seventy of 72 websites (97%) had PEMs that were fairly difficult or difficult, according to the FRE analysis (Figure 2). The American Cancer Society and Mid-America Sarcoma Institute had PEMs that were written in plain English. Sixty-nine of 72 websites (96%) had PEMs with a readability score of 10 or higher, according to the Raygor readability estimate (Figure 3). Using this instrument, the scores of the American Cancer Society and the University of Pennsylvania–Joan Karnell Cancer Center were 9; Mid-America Sarcoma Institute’s score was 8.
Discussion
Many cancer patients have turned to websites and online PEMs to gather health information about their condition.10-17 Basch and colleagues10 reported almost a decade ago that 44% of cancer patients, as well as 60% of their companions, used the Internet to find cancer-related information.10 When LaCoursiere and colleagues35 surveyed cancer patients, they found that patients handled their condition better and had less anxiety and uncertainty after using the Internet to find health information and support.35 In addition, many orthopedic patients, specifically 46% of orthopedic community outpatients,45 consult the Internet for information about their condition and future surgical procedures.46,47
This study comprehensively evaluated the readability of online PEMs of bone and soft-tissue sarcomas and related conditions by using 10 different readability instruments. After identifying 72 websites and 774 articles, we found that all 72 websites’ PEMs had a mean readability score that did not meet the NIH recommendation of writing PEMs at a sixth- to seventh-grade reading level. These results are consistent with studies evaluating the readability of online PEMs related to other cancer conditions21-25 and other orthopedic conditions.26-31
The combination of low health literacy of many US adults and high reading grade levels of the majority of online PEMs is not conducive to patients’ better understanding their condition(s). Even individuals with high reading skills prefer information that is simpler to read.48 In many areas of medicine, there is evidence that patients’ understanding of their condition has a positive impact on health outcomes, well-being, and the patient–physician relationship.49-61 Regarding cancer patients, Davis and colleagues54 and Peterson and colleagues57 showed that lower health literacy contributes to less knowledge and lower rates of breast54 and colorectal cancer57 screening tests. Even low health literacy of family caregivers of cancer patients can result in increased stress and lack of communication of important medical information between caregiver and physician.52 Among cancer patients, poor health literacy has been associated with mental distress60 as well as decreased compliance with treatment and lower involvement in clinical trials.55
The disparity between patients’ health literacy and the readability of online PEMs needs to be addressed by finding methods to improve patients’ understanding of their condition and to lower the readability scores of online PEMs. Better communication between patient and physician may improve patients’ comprehension of their condition and different aspects of their care.59,62-66 Doak and colleagues63 recommend giving cancer patients the most important information first; presenting information to patients in smaller doses; intermittently asking patients questions; and incorporating graphs, tables, and drawings into communication with patients.63 Additionally, allowing patients to repeat information they have just received/heard to the physician is another useful tool to improve patient education.62,64-66
Another way to address the disparity between patients’ health literacy and the readability of online PEMs is to reduce the reading grade level of existing PEMs. According to results from this study and others, the majority of online PEMs are above the reading grade level of a significant number of US adults. Many available and inexpensive readability instruments allow authors to assess their articles’ readability. Many writing guidelines also exist to help authors improve the readability of their PEMs.20,64,67-71 Living Word Vocabulary70 and Plain Language71 help authors replace complex words or medical terms with simpler words.29 Visual aids, audio, and video help patients with low health literacy remember the information.64
Efforts to improve PEM readability are effective. Of all the websites reviewed, VUMC was identified as having PEMs with the lowest readability score (5.3). This score was reported by the New Fog Count readability instrument, which accounts for the number of sentences, easy words, and hard words. In 2011, VUMC formed the Department of Patient Education to review and update its online and printed PEMs to make sure patients could read them.72 Additionally, the mean readability scores of the websites of the National Cancer Institute and MedlinePlus are in the top 50% of the websites included in this study. The NIH sponsors both sites, which follow the NIH guidelines for writing online PEMs at a reading level suitable for individuals with lower health literacy.20 These materials serve as potential models to improve the readability of PEMs, and, thus, help patients to better understand their condition, medical procedures, and/or treatment options.
To illustrate ways to improve the reading grade level of PEMs, we used the article “Ewing’s Sarcoma” from the AAOS website73 and followed the NIH guidelines to improve the reading grade level of the article.20 We identified complex words and defined them at an eighth-grade reading level. If that word was mentioned later in the article, simpler terminology was used instead of the initial complex word. For example, Ewing’s sarcoma was defined early and then referred to as bone tumor later in the article. We also identified every word that was 3 syllables or longer and used Microsoft Word’s thesaurus to replace those words with ones that were less than 3 syllables. Lastly, all sentences longer than 15 words were rewritten to be less than 15 words. After making these 3 changes to the article, the mean reading grade level dropped from 11.2 to 7.3.
This study has limitations. First, some readability instruments evaluate the number of syllables per word or polysyllabic words as part of their formula and, thus, can underestimate or overestimate the reading grade level of a document. Some readability formulas consider medical terms such as ulna, femur, or carpal as “easy” words because they have 2 syllables, but many laypersons may not comprehend these words. On the other hand, some readability formulas consider medical terms such as medications, diagnosis, or radiation as “hard” words because they contain 3 or more syllables, but the majority of laypersons likely comprehend these words. Second, the reading level of the patient population accessing those online sites was not assessed. Third, the readability instruments in this study did not evaluate the accuracy of the content, pictures, or tables of the PEMs. However, using 10 readability instruments allowed evaluation of many different readability aspects of the text. Fourth, because some websites identified in this study, such as Bonetumor.org, were written for patients as well as clinicians, the reading grade level of these sites may be higher than that of those sites written just for patients.
Conclusion
Because many orthopedic cancer patients rely on the Internet as a source of information, the need for online PEMs to match the reading skills of the patient population who accesses them is vital. However, this study shows that many organizations, academic training centers, and other entities need to update their online PEMs because all PEMs in this study had a mean readability grade level higher than the NIH recommendation. Further research needs to evaluate the effectiveness of other media, such as video, illustrations, and audio, to provide health information to patients. With many guidelines available that provide plans and advice to improve the readability of PEMs, research also must assess the most effective plans and advice in order to allow authors to focus their attention on 1 set of guidelines to improve the readability of their PEMs.
1. Piredda M, Rocci L, Gualandi R, Petitti T, Vincenzi B, De Marinis MG. Survey on learning needs and preferred sources of information to meet these needs in Italian oncology patients receiving chemotherapy. Eur J Oncol Nurs. 2008;12(2):120-126.
2. Fernsler JI, Cannon CA. The whys of patient education. Semin Oncol Nurs. 1991;7(2):79-86.
3. Glimelius B, Birgegård G, Hoffman K, Kvale G, Sjödén PO. Information to and communication with cancer patients: improvements and psychosocial correlates in a comprehensive care program for patients and their relatives. Patient Educ Couns. 1995;25(2):171-182.
4. Harris KA. The informational needs of patients with cancer and their families. Cancer Pract. 1998;6(1):39-46.
5. Jensen AB, Madsen B, Andersen P, Rose C. Information for cancer patients entering a clinical trial--an evaluation of an information strategy. Eur J Cancer. 1993;29A(16):2235-2238.
6. Wells ME, McQuellon RP, Hinkle JS, Cruz JM. Reducing anxiety in newly diagnosed cancer patients: a pilot program. Cancer Pract. 1995;3(2):100-104.
7. Diaz JA, Griffith RA, Ng JJ, Reinert SE, Friedmann PD, Moulton AW. Patients’ use of the Internet for medical information. J Gen Intern Med. 2002;17(3):180-185.
8. Fox S, Duggan M. Health Online 2013. Pew Research Center’s Internet and American Life Project. www.pewinternet.org/~/media//Files/Reports/PIP_HealthOnline.pdf. Published January 15, 2013. Accessed November 18. 2014.
9. Schwartz KL, Roe T, Northrup J, Meza J, Seifeldin R, Neale AV. Family medicine patients’ use of the Internet for health information: a MetroNet study. J Am Board Fam Med. 2006;19(1):39-45.
10. Basch EM, Thaler HT, Shi W, Yakren S, Schrag D. Use of information resources by patients with cancer and their companions. Cancer. 2004;100(11):2476-2483.
11. Huang GJ, Penson DF. Internet health resources and the cancer patient. Cancer Invest. 2008;26(2):202-207.
12. Metz JM, Devine P, Denittis A, et al. A multi-institutional study of Internet utilization by radiation oncology patients. Int J Radiat Oncol Biol Phys. 2003;56(4):1201-1205.
13. Peterson MW, Fretz PC. Patient use of the internet for information in a lung cancer clinic. Chest. 2003;123(2):452-457.
14. Satterlund MJ, McCaul KD, Sandgren AK. Information gathering over time by breast cancer patients. J Med Internet Res. 2003;5(3):e15.
15. Tustin N. The role of patient satisfaction in online health information seeking. J Health Commun. 2010;15(1):3-17.
16. Van de Poll-Franse LV, Van Eenbergen MC. Internet use by cancer survivors: current use and future wishes. Support Care Cancer. 2008;16(10):1189-1195.
17. Ziebland S, Chapple A, Dumelow C, Evans J, Prinjha S, Rozmovits L. How the internet affects patients’ experience of cancer: a qualitative study. BMJ. 2004;328(7439):564.
18. Committee on Health Literacy, Board on Neuroscience and Behavioral Health, Institute of Medicine. Nielsen-Bohlman L, Panzer AM, Kindig DA, eds. Health Literacy: A Prescription to End Confusion. Washington, DC: National Academies Press; 2004. Available at: www.nap.edu/openbook.php?record_id=10883. Accessed November 18, 2014.
19. Kutner M, Greenberg E, Ying J, Paulsen C. The Health Literacy of America’s Adults: Results from the 2003 National Assessment of Adult Literacy. NCES 2006-483. US Department of Education. Washington, DC: National Center for Education Statistics; 2006. Available at: www.nces.ed.gov/pubs2006/2006483.pdf. Accessed November 18, 2014.
20. How to write easy-to-read health materials. MedlinePlus website. www.nlm.nih.gov/medlineplus/etr.html. Updated February 13, 2013. Accessed November 18, 2014.
21. Ellimoottil C, Polcari A, Kadlec A, Gupta G. Readability of websites containing information about prostate cancer treatment options. J Urol. 2012;188(6):2171-2175.
22. Friedman DB, Hoffman-Goetz L, Arocha JF. Health literacy and the World Wide Web: comparing the readability of leading incident cancers on the Internet. Med Inform Internet Med. 2006;31(1):67-87.
23. Hoppe IC. Readability of patient information regarding breast cancer prevention from the Web site of the National Cancer Institute. J Cancer Educ. 2010;25(4):490-492.
24. Misra P, Kasabwala K, Agarwal N, Eloy JA, Liu JK. Readability analysis of internet-based patient information regarding skull base tumors. J Neurooncol. 2012;109(3):573-580.
25. Stinson JN, White M, Breakey V, et al. Perspectives on quality and content of information on the internet for adolescents with cancer. Pediatr Blood Cancer. 2011;57(1):97-104.
26. Badarudeen S, Sabharwal S. Readability of patient education materials from the American Academy of Orthopaedic Surgeons and Pediatric Orthopaedic Society of North America web sites. J Bone Joint Surg Am. 2008;90(1):199-204.
27. Bluman EM, Foley RP, Chiodo CP. Readability of the Patient Education Section of the AOFAS Website. Foot Ankle Int. 2009;30(4):287-291.
28. Polishchuk DL, Hashem J, Sabharwal S. Readability of online patient education materials on adult reconstruction Web sites. J Arthroplasty. 2012;27(5):716-719.
29. Sabharwal S, Badarudeen S, Unes Kunju S. Readability of online patient education materials from the AAOS web site. Clin Orthop. 2008;466(5):1245-1250.
30. Vives M, Young L, Sabharwal S. Readability of spine-related patient education materials from subspecialty organization and spine practitioner websites. Spine. 2009;34(25):2826-2831.
31. Wang SW, Capo JT, Orillaza N. Readability and comprehensibility of patient education material in hand-related web sites. J Hand Surg Am. 2009;34(7):1308-1315.
32. Lam CG, Roter DL, Cohen KJ. Survey of quality, readability, and social reach of websites on osteosarcoma in adolescents. Patient Educ Couns. 2013;90(1):82-87.
33. Tumors. Quinn RH, ed. OrthoInfo. American Academy of Orthopaedic Surgeons website. http://orthoinfo.aaos.org/menus/tumors.cfm. Accessed November 18, 2014.
34. Sarcoma specialists. Sarcoma Alliance website. sarcomaalliance.org/sarcoma-centers. Accessed November 18, 2014.
35. LaCoursiere SP, Knobf MT, McCorkle R. Cancer patients’ self-reported attitudes about the Internet. J Med Internet Res. 2005;7(3):e22.
36. Test your document’s readability. Microsoft Office website. office.microsoft.com/en-us/word-help/test-your-document-s-readability-HP010148506.aspx. Accessed November 18, 2014.
37. Kincaid JP, Fishburne RP, Rogers RL, Chissom BS. Derivation of new readability formulas (Automated Readability Index, Fog Count and Flesch Reading Ease Formula) for Navy enlisted personnel. Naval Technical Training Command. Research Branch Report 8-75. www.dtic.mil/dtic/tr/fulltext/u2/a006655.pdf. Published February 1975. Accessed November 18, 2014.
38. Coleman M, Liau TL. A computer readability formula designed for machine scoring. J Appl Psychol. 1975;60(2):283-284.
39. Fry E. Fry’s readability graph: clarifications, validity, and extension to Level 17. J Reading. 1977;21(3):242-252.
40. Chall JS, Dale E. Manual for the New Dale-Chall Readability Formula. Cambridge, MA: Brookline Books; 1995.
41. Gunning R. The Technique of Clear Writing. Rev. ed. New York, NY: McGraw-Hill; 1968.
42. Powers RD, Sumner WA, Kearl BE. A recalculation of four adult readability formulas. J Educ Psychol. 1958;49(2):99-105.
43. McLaughlin GH. SMOG grading—a new readability formula. J Reading. 1969;22,639-646.
44. Raygor L. The Raygor readability estimate: a quick and easy way to determine difficulty. In: Pearson PD, Hansen J, eds. Reading Theory, Research and Practice. Twenty-Sixth Yearbook of the National Reading Conference. Clemson, SC: National Reading Conference Inc; 1977:259-263.
45. Krempec J, Hall J, Biermann JS. Internet use by patients in orthopaedic surgery. Iowa Orthop J. 2003;23:80-82.
46. Beall MS, Golladay GJ, Greenfield ML, Hensinger RN, Biermann JS. Use of the Internet by pediatric orthopaedic outpatients. J Pediatr Orthop. 2002;22(2):261-264.
47. Beall MS, Beall MS, Greenfield ML, Biermann JS. Patient Internet use in a community outpatient orthopaedic practice. Iowa Orthop J. 2002;22:103-107.
48. Davis TC, Bocchini JA, Fredrickson D, et al. Parent comprehension of polio vaccine information pamphlets. Pediatrics. 1996;97(6 Pt 1):804-810.
49. Apter AJ, Wan F, Reisine S, et al. The association of health literacy with adherence and outcomes in moderate-severe asthma. J Allergy Clin Immunol. 2013;132(2):321-327.
50. Baker DW, Parker RM, Williams MV, Clark WS. Health literacy and the risk of hospital admission. J Gen Intern Med. 1998;13(12):791-798.
51. Berkman ND, Sheridan SL, Donahue KE, Halpern DJ, Crotty K. Low health literacy and health outcomes: an updated systematic review. Ann Intern Med. 2011;155(2):97-107.
52. Bevan JL, Pecchioni LL. Understanding the impact of family caregiver cancer literacy on patient health outcomes. Patient Educ Couns. 2008;71(3):356-364.
53. Corey MR, St Julien J, Miller C, et al. Patient education level affects functionality and long term mortality after major lower extremity amputation. Am J Surg. 2012;204(5):626-630.
54. Davis TC, Arnold C, Berkel HJ, Nandy I, Jackson RH, Glass J. Knowledge and attitude on screening mammography among low-literate, low-income women. Cancer. 1996;78(9):1912-1920.
55. Davis TC, Williams MV, Marin E, Parker RM, Glass J. Health literacy and cancer communication. CA Cancer J Clin. 2002;52(3):134-149.
56. Freedman RB, Jones SK, Lin A, Robin AL, Muir KW. Influence of parental health literacy and dosing responsibility on pediatric glaucoma medication adherence. Arch Ophthalmol. 2012;130(3):306-311.
57. Peterson NB, Dwyer KA, Mulvaney SA, Dietrich MS, Rothman RL. The influence of health literacy on colorectal cancer screening knowledge, beliefs and behavior. J Natl Med Assoc. 2007;99(10):1105-1112.
58. Peterson PN, Shetterly SM, Clarke CL, et al. Health literacy and outcomes among patients with heart failure. JAMA. 2011;305(16):1695-1701.
59. Rosas-salazar C, Apter AJ, Canino G, Celedón JC. Health literacy and asthma. J Allergy Clin Immunol. 2012;129(4):935-942.
60. Song L, Mishel M, Bensen JT, et al. How does health literacy affect quality of life among men with newly diagnosed clinically localized prostate cancer? Findings from the North Carolina-Louisiana Prostate Cancer Project (PCaP). Cancer. 2012;118(15):3842-3851.
61. Williams MV, Davis T, Parker RM, Weiss BD. The role of health literacy in patient-physician communication. Fam Med. 2002;34(5):383-389.
62. Badarudeen S, Sabharwal S. Assessing readability of patient education materials: current role in orthopaedics. Clin Orthop. 2010;468(10):2572-2580.
63. Doak CC, Doak LG, Friedell GH, Meade CD. Improving comprehension for cancer patients with low literacy skills: strategies for clinicians. CA Cancer J Clin. 1998;48(3):151-162.
64. Doak CC, Doak LG, Root JH. Teaching Patients With Low Literacy Skills. 2nd ed. Philadelphia, PA: JB Lippincott Company; 1996.
65. Kemp EC, Floyd MR, McCord-Duncan E, Lang F. Patients prefer the method of “tell back-collaborative inquiry” to assess understanding of medical information. J Am Board Fam Med. 2008;21(1):24-30.
66. Kripalani S, Bengtzen R, Henderson LE, Jacobson TA. Clinical research in low-literacy populations: using teach-back to assess comprehension of informed consent and privacy information. IRB. 2008;30(2):13-19.
67. Centers for Disease Control and Prevention. Simply Put: A Guide For Creating Easy-to-Understand Materials. 3rd ed. Atlanta, GA: Strategic and Proactive Communication Branch, Centers for Disease Control and Prevention, US Dept of Health and Human Services; 2009.
68. National Institutes of Health, National Cancer Institute. Clear & Simple: Developing Effective Print Materials for Low-Literate Readers. Devcompage website. http://devcompage.com/wp-content/uploads/2010/12/Clear_n_Simple.pdf Published March 2, 1998. Accessed December 1, 2014.
69. Weiss BD. Health Literacy and Patient Safety: Help Patients Understand. 2nd ed. Chicago, IL: American Medical Association and AMA Foundation; 2007:35-41.
70. Dale E, O’Rourke J. The Living Word Vocabulary. Newington, CT: World Book-Childcraft International, 1981.
71. Word suggestions. Plain Language website. www.plainlanguage.gov/howto/wordsuggestions/index.cfm. Accessed November 18, 2014.
72. Rivers K. Initiative aims to enhance patient communication materials. Reporter: Vanderbilt University Medical Center’s Weekly Newspaper. April 28, 2011. http://www.mc.vanderbilt.edu/reporter/index.html?ID=10649. Accessed November 18, 2014.
73. Ewing’s sarcoma. OrthoInfo. American Academy of Orthopaedic Surgeons website. http://orthoinfo.aaos.org/topic.cfm?topic=A00082. Last reviewed September 2011. Accessed November 18, 2014.
The diagnosis of cancer is a life-changing event for the patient as well as the patient’s family, friends, and relatives. Once diagnosed, most cancer patients want more information about their prognosis, future procedures, and/or treatment options.1 Receiving such information has been shown to reduce patient anxiety, increase patient satisfaction with care, and improve self-care.2-6 With the evolution of the Internet, patients in general7-9 and, specifically, cancer patients10-17 have turned to websites and online patient education materials (PEMs) to gather more health information.
For online PEMs to convey health information, their reading level must match the health literacy of the individuals who access them. Health literacy is the ability of an individual to gather and comprehend information about their condition to make the best decisions for their health.18 According to a report by the Institute of Medicine, 90 million American adults cannot properly use the US health care system because they do not possess adequate health literacy.18 Additionally, 36% of adults in the United States have basic or less-than-basic health literacy.19 This is starkly contrasted with the 12% of US adults who have proficient health literacy. A 2012 survey showed that about 31% of individuals who look for health information on the Internet have a high school education or less.8 In order to address the low health literacy of adults, the National Institutes of Health (NIH) has recommended that online PEMs be written at a sixth- to seventh-grade reading level.20
Unfortunately, many online PEMs related to certain cancer21-25 and orthopedic conditions26-31 do not meet NIH recommendations. Only 1 study has specifically looked at PEMs related to an orthopedic cancer condition.32 Lam and colleagues32 evaluated the readability of osteosarcoma PEMs from 56 websites using only 2 readability instruments and identified 86% of the websites as having a greater than eighth-grade reading level. No study has thoroughly assessed the readability of PEMs about bone and soft-tissue sarcomas and related conditions nor has any used 10 different readability instruments. Since each readability instrument has different variables (eg, sentence length, number of paragraphs, or number of complex words), averaging the scores of 10 of these instruments may result in less bias.
The purpose of this study was to evaluate the readability of online PEMs concerning bone and soft-tissue sarcomas and related conditions. The online PEMs came from websites that sarcoma patients may visit to obtain information about their condition. Our hypothesis was that the majority of these online PEMs will have a higher reading level than the NIH recommendations.
Materials and Methods
In May 2013, we identified online PEMs that included background, diagnosis, tests, or treatments for bone and soft-tissue sarcomas and conditions that mimic bone sarcoma. We included articles from the Tumors section of the American Academy of Orthopaedic Surgeons (AAOS) website.33 A second source of online PEMs came from a list of academic training centers created through the American Medical Association’s Fellowship and Residency Electronic Internet Database (FREIDA) with search criteria narrowed to orthopedic surgery. If we did not find PEMs of bone and soft-tissue cancers in the orthopedic department of a given academic training center’s website, we searched its cancer center website. We chose 4 programs with PEMs relevant to bone and soft-tissue sarcomas from each region in FREIDA for a balanced representation, except for the Territory region because it had only 1 academic training center and no relevant PEMs. Specialized websites, including Bonetumor.org, Sarcoma Alliance (Sarcomaalliance.org), and Sarcoma Foundation of America (Curesarcoma.org), were also evaluated. Within the Sarcoma Specialists section of the Sarcoma Alliance website,34 sarcoma specialists who were not identified from the FREIDA search for academic training centers were selected for review.
Because 8 of 10 individuals looking for health information on the Internet start their investigation at search engines, we also looked for PEMs through a Google search (Google.com) of bone cancer, and evaluated the first 10 hits for PEMs.8 Of these 10 hits, 8 had relevant PEMs, which we searched for additional PEMs about bone and soft-tissue cancers and related conditions. We also conducted a Google search of the most common bone sarcoma and soft-tissue sarcoma, osteosarcoma and malignant fibrous histiocytoma, respectively, and found 2 additional websites with relevant PEMs. LaCoursiere and colleagues35 surveyed cancer patients who used the Internet and found that they preferred WebMD (Webmd.com) and Medscape (Medscape.com) as sources for content about their medical condition.35 WebMD had been identified in the Google search, and we gathered the PEMs from Medscape also. It is worth noting that some of these websites are written for patients as well as clinicians.
Text from these PEMs were copied and pasted into separate Microsoft Word documents (Microsoft, Redmond, Washington). Advertisements, pictures, picture text, hyperlinks, copyright notices, page navigation links, paragraphs with no text, and any text that was not related to the given condition were deleted from the document to format the text for the readability software. Then, each Microsoft Word document was uploaded into the software package Readability Studio Professional (RSP) Edition Version 2012.1 for Windows (Oleander Software, Vandalia, Ohio). The 10 distinct readability instruments that were used to gauge the readability of each document were the Flesch Reading Ease score (FRE), the New Fog Count, the New Automated Readability Index, the Coleman-Liau Index (CLI), the Fry readability graph, the New Dale-Chall formula (NDC), the Gunning Frequency of Gobbledygook (Gunning FOG), the Powers-Sumner-Kearl formula, the Simple Measure of Gobbledygook (SMOG), and the Raygor Estimate Graph.
The FRE’s formula takes the average number of words per sentence and average number of syllables per word to compute a score ranging from 0 to 100 with 0 being the hardest to read.36 The New Fog Count tallies the number of sentences, easy words, and hard words (polysyllables) to calculate the grade level of the document.37 The New Automated Readability Index takes the average characters per word and average words per sentence to calculate a grade level for the document.37 The CLI randomly samples a few hundred words from the document, averages the number of letters and sentences per sample, and calculates an estimated grade level.38 The Fry readability graph selects samples of 100 words from the document, averages the number of syllables and sentences per 100 words, plots these data points on a graph, with the intersection determining the reading level.39 The NDC uses a list of 3000 familiar words that most fourth-grade students know.40 The percentage of difficult words, which are not on the list of familiar words, and the average sentence length in words are used to calculate the reading grade level of the document. The Gunning FOG uses the average sentence length in words and the percentage of hard words from a sample of at least 100 words to determine the reading grade level of the document.41 The Powers-Sumner-Kearl formula uses the average sentence length and percentage of monosyllables from a 100-word sample passage to calculate the reading grade level.42 The SMOG formula counts the number of polysyllabic words from 30 sentences and calculates the reading grade level of the document.43 In contrast to other formulas that test for 50% to 75% comprehension, the SMOG formula tests for 100% comprehension. As a result, the SMOG formula generally assigns a reading level 2 grades higher than the Dale-Chall level. The Raygor Estimate Graph selects a 100-word passage, counts the number of sentences and number of words with 6 or more letters, and plots the 2 variables on a graph to determine the reading grade level.44 The software package calculated the results from each reading instrument and reported the mean grade level score
for each document.
Results
We identified a total of 72 websites with relevant PEMs and included them in this study. Of these 72 websites, 36 websites were academic training centers, 10 were Google search hits, and 21 were from the Sarcoma Alliance list of sarcoma specialists. The remaining 5 websites were AAOS, Bonetumor.org, Sarcoma Alliance, Sarcoma Foundation of America, and Medscape. A list of conditions and treatments that were considered relevant PEMs is found in Appendix 1. A total of 774 articles were obtained from the 72 websites.
None of the websites had a mean readability score of 7 (seventh grade) or lower (Figures 1A, 1B). Mid-America Sarcoma Institute’s PEMs had the lowest mean readability score, 8.9. The lowest readability score was 5.3, which the New Fog Count readability instrument calculated for Vanderbilt University Medical Center’s (VUMC’s) PEMs (Appendix 2). The mean readability score of all websites was 11.4 (range, 8.9-15.5) (Appendix 2).
Seventy of 72 websites (97%) had PEMs that were fairly difficult or difficult, according to the FRE analysis (Figure 2). The American Cancer Society and Mid-America Sarcoma Institute had PEMs that were written in plain English. Sixty-nine of 72 websites (96%) had PEMs with a readability score of 10 or higher, according to the Raygor readability estimate (Figure 3). Using this instrument, the scores of the American Cancer Society and the University of Pennsylvania–Joan Karnell Cancer Center were 9; Mid-America Sarcoma Institute’s score was 8.
Discussion
Many cancer patients have turned to websites and online PEMs to gather health information about their condition.10-17 Basch and colleagues10 reported almost a decade ago that 44% of cancer patients, as well as 60% of their companions, used the Internet to find cancer-related information.10 When LaCoursiere and colleagues35 surveyed cancer patients, they found that patients handled their condition better and had less anxiety and uncertainty after using the Internet to find health information and support.35 In addition, many orthopedic patients, specifically 46% of orthopedic community outpatients,45 consult the Internet for information about their condition and future surgical procedures.46,47
This study comprehensively evaluated the readability of online PEMs of bone and soft-tissue sarcomas and related conditions by using 10 different readability instruments. After identifying 72 websites and 774 articles, we found that all 72 websites’ PEMs had a mean readability score that did not meet the NIH recommendation of writing PEMs at a sixth- to seventh-grade reading level. These results are consistent with studies evaluating the readability of online PEMs related to other cancer conditions21-25 and other orthopedic conditions.26-31
The combination of low health literacy of many US adults and high reading grade levels of the majority of online PEMs is not conducive to patients’ better understanding their condition(s). Even individuals with high reading skills prefer information that is simpler to read.48 In many areas of medicine, there is evidence that patients’ understanding of their condition has a positive impact on health outcomes, well-being, and the patient–physician relationship.49-61 Regarding cancer patients, Davis and colleagues54 and Peterson and colleagues57 showed that lower health literacy contributes to less knowledge and lower rates of breast54 and colorectal cancer57 screening tests. Even low health literacy of family caregivers of cancer patients can result in increased stress and lack of communication of important medical information between caregiver and physician.52 Among cancer patients, poor health literacy has been associated with mental distress60 as well as decreased compliance with treatment and lower involvement in clinical trials.55
The disparity between patients’ health literacy and the readability of online PEMs needs to be addressed by finding methods to improve patients’ understanding of their condition and to lower the readability scores of online PEMs. Better communication between patient and physician may improve patients’ comprehension of their condition and different aspects of their care.59,62-66 Doak and colleagues63 recommend giving cancer patients the most important information first; presenting information to patients in smaller doses; intermittently asking patients questions; and incorporating graphs, tables, and drawings into communication with patients.63 Additionally, allowing patients to repeat information they have just received/heard to the physician is another useful tool to improve patient education.62,64-66
Another way to address the disparity between patients’ health literacy and the readability of online PEMs is to reduce the reading grade level of existing PEMs. According to results from this study and others, the majority of online PEMs are above the reading grade level of a significant number of US adults. Many available and inexpensive readability instruments allow authors to assess their articles’ readability. Many writing guidelines also exist to help authors improve the readability of their PEMs.20,64,67-71 Living Word Vocabulary70 and Plain Language71 help authors replace complex words or medical terms with simpler words.29 Visual aids, audio, and video help patients with low health literacy remember the information.64
Efforts to improve PEM readability are effective. Of all the websites reviewed, VUMC was identified as having PEMs with the lowest readability score (5.3). This score was reported by the New Fog Count readability instrument, which accounts for the number of sentences, easy words, and hard words. In 2011, VUMC formed the Department of Patient Education to review and update its online and printed PEMs to make sure patients could read them.72 Additionally, the mean readability scores of the websites of the National Cancer Institute and MedlinePlus are in the top 50% of the websites included in this study. The NIH sponsors both sites, which follow the NIH guidelines for writing online PEMs at a reading level suitable for individuals with lower health literacy.20 These materials serve as potential models to improve the readability of PEMs, and, thus, help patients to better understand their condition, medical procedures, and/or treatment options.
To illustrate ways to improve the reading grade level of PEMs, we used the article “Ewing’s Sarcoma” from the AAOS website73 and followed the NIH guidelines to improve the reading grade level of the article.20 We identified complex words and defined them at an eighth-grade reading level. If that word was mentioned later in the article, simpler terminology was used instead of the initial complex word. For example, Ewing’s sarcoma was defined early and then referred to as bone tumor later in the article. We also identified every word that was 3 syllables or longer and used Microsoft Word’s thesaurus to replace those words with ones that were less than 3 syllables. Lastly, all sentences longer than 15 words were rewritten to be less than 15 words. After making these 3 changes to the article, the mean reading grade level dropped from 11.2 to 7.3.
This study has limitations. First, some readability instruments evaluate the number of syllables per word or polysyllabic words as part of their formula and, thus, can underestimate or overestimate the reading grade level of a document. Some readability formulas consider medical terms such as ulna, femur, or carpal as “easy” words because they have 2 syllables, but many laypersons may not comprehend these words. On the other hand, some readability formulas consider medical terms such as medications, diagnosis, or radiation as “hard” words because they contain 3 or more syllables, but the majority of laypersons likely comprehend these words. Second, the reading level of the patient population accessing those online sites was not assessed. Third, the readability instruments in this study did not evaluate the accuracy of the content, pictures, or tables of the PEMs. However, using 10 readability instruments allowed evaluation of many different readability aspects of the text. Fourth, because some websites identified in this study, such as Bonetumor.org, were written for patients as well as clinicians, the reading grade level of these sites may be higher than that of those sites written just for patients.
Conclusion
Because many orthopedic cancer patients rely on the Internet as a source of information, the need for online PEMs to match the reading skills of the patient population who accesses them is vital. However, this study shows that many organizations, academic training centers, and other entities need to update their online PEMs because all PEMs in this study had a mean readability grade level higher than the NIH recommendation. Further research needs to evaluate the effectiveness of other media, such as video, illustrations, and audio, to provide health information to patients. With many guidelines available that provide plans and advice to improve the readability of PEMs, research also must assess the most effective plans and advice in order to allow authors to focus their attention on 1 set of guidelines to improve the readability of their PEMs.
The diagnosis of cancer is a life-changing event for the patient as well as the patient’s family, friends, and relatives. Once diagnosed, most cancer patients want more information about their prognosis, future procedures, and/or treatment options.1 Receiving such information has been shown to reduce patient anxiety, increase patient satisfaction with care, and improve self-care.2-6 With the evolution of the Internet, patients in general7-9 and, specifically, cancer patients10-17 have turned to websites and online patient education materials (PEMs) to gather more health information.
For online PEMs to convey health information, their reading level must match the health literacy of the individuals who access them. Health literacy is the ability of an individual to gather and comprehend information about their condition to make the best decisions for their health.18 According to a report by the Institute of Medicine, 90 million American adults cannot properly use the US health care system because they do not possess adequate health literacy.18 Additionally, 36% of adults in the United States have basic or less-than-basic health literacy.19 This is starkly contrasted with the 12% of US adults who have proficient health literacy. A 2012 survey showed that about 31% of individuals who look for health information on the Internet have a high school education or less.8 In order to address the low health literacy of adults, the National Institutes of Health (NIH) has recommended that online PEMs be written at a sixth- to seventh-grade reading level.20
Unfortunately, many online PEMs related to certain cancer21-25 and orthopedic conditions26-31 do not meet NIH recommendations. Only 1 study has specifically looked at PEMs related to an orthopedic cancer condition.32 Lam and colleagues32 evaluated the readability of osteosarcoma PEMs from 56 websites using only 2 readability instruments and identified 86% of the websites as having a greater than eighth-grade reading level. No study has thoroughly assessed the readability of PEMs about bone and soft-tissue sarcomas and related conditions nor has any used 10 different readability instruments. Since each readability instrument has different variables (eg, sentence length, number of paragraphs, or number of complex words), averaging the scores of 10 of these instruments may result in less bias.
The purpose of this study was to evaluate the readability of online PEMs concerning bone and soft-tissue sarcomas and related conditions. The online PEMs came from websites that sarcoma patients may visit to obtain information about their condition. Our hypothesis was that the majority of these online PEMs will have a higher reading level than the NIH recommendations.
Materials and Methods
In May 2013, we identified online PEMs that included background, diagnosis, tests, or treatments for bone and soft-tissue sarcomas and conditions that mimic bone sarcoma. We included articles from the Tumors section of the American Academy of Orthopaedic Surgeons (AAOS) website.33 A second source of online PEMs came from a list of academic training centers created through the American Medical Association’s Fellowship and Residency Electronic Internet Database (FREIDA) with search criteria narrowed to orthopedic surgery. If we did not find PEMs of bone and soft-tissue cancers in the orthopedic department of a given academic training center’s website, we searched its cancer center website. We chose 4 programs with PEMs relevant to bone and soft-tissue sarcomas from each region in FREIDA for a balanced representation, except for the Territory region because it had only 1 academic training center and no relevant PEMs. Specialized websites, including Bonetumor.org, Sarcoma Alliance (Sarcomaalliance.org), and Sarcoma Foundation of America (Curesarcoma.org), were also evaluated. Within the Sarcoma Specialists section of the Sarcoma Alliance website,34 sarcoma specialists who were not identified from the FREIDA search for academic training centers were selected for review.
Because 8 of 10 individuals looking for health information on the Internet start their investigation at search engines, we also looked for PEMs through a Google search (Google.com) of bone cancer, and evaluated the first 10 hits for PEMs.8 Of these 10 hits, 8 had relevant PEMs, which we searched for additional PEMs about bone and soft-tissue cancers and related conditions. We also conducted a Google search of the most common bone sarcoma and soft-tissue sarcoma, osteosarcoma and malignant fibrous histiocytoma, respectively, and found 2 additional websites with relevant PEMs. LaCoursiere and colleagues35 surveyed cancer patients who used the Internet and found that they preferred WebMD (Webmd.com) and Medscape (Medscape.com) as sources for content about their medical condition.35 WebMD had been identified in the Google search, and we gathered the PEMs from Medscape also. It is worth noting that some of these websites are written for patients as well as clinicians.
Text from these PEMs were copied and pasted into separate Microsoft Word documents (Microsoft, Redmond, Washington). Advertisements, pictures, picture text, hyperlinks, copyright notices, page navigation links, paragraphs with no text, and any text that was not related to the given condition were deleted from the document to format the text for the readability software. Then, each Microsoft Word document was uploaded into the software package Readability Studio Professional (RSP) Edition Version 2012.1 for Windows (Oleander Software, Vandalia, Ohio). The 10 distinct readability instruments that were used to gauge the readability of each document were the Flesch Reading Ease score (FRE), the New Fog Count, the New Automated Readability Index, the Coleman-Liau Index (CLI), the Fry readability graph, the New Dale-Chall formula (NDC), the Gunning Frequency of Gobbledygook (Gunning FOG), the Powers-Sumner-Kearl formula, the Simple Measure of Gobbledygook (SMOG), and the Raygor Estimate Graph.
The FRE’s formula takes the average number of words per sentence and average number of syllables per word to compute a score ranging from 0 to 100 with 0 being the hardest to read.36 The New Fog Count tallies the number of sentences, easy words, and hard words (polysyllables) to calculate the grade level of the document.37 The New Automated Readability Index takes the average characters per word and average words per sentence to calculate a grade level for the document.37 The CLI randomly samples a few hundred words from the document, averages the number of letters and sentences per sample, and calculates an estimated grade level.38 The Fry readability graph selects samples of 100 words from the document, averages the number of syllables and sentences per 100 words, plots these data points on a graph, with the intersection determining the reading level.39 The NDC uses a list of 3000 familiar words that most fourth-grade students know.40 The percentage of difficult words, which are not on the list of familiar words, and the average sentence length in words are used to calculate the reading grade level of the document. The Gunning FOG uses the average sentence length in words and the percentage of hard words from a sample of at least 100 words to determine the reading grade level of the document.41 The Powers-Sumner-Kearl formula uses the average sentence length and percentage of monosyllables from a 100-word sample passage to calculate the reading grade level.42 The SMOG formula counts the number of polysyllabic words from 30 sentences and calculates the reading grade level of the document.43 In contrast to other formulas that test for 50% to 75% comprehension, the SMOG formula tests for 100% comprehension. As a result, the SMOG formula generally assigns a reading level 2 grades higher than the Dale-Chall level. The Raygor Estimate Graph selects a 100-word passage, counts the number of sentences and number of words with 6 or more letters, and plots the 2 variables on a graph to determine the reading grade level.44 The software package calculated the results from each reading instrument and reported the mean grade level score
for each document.
Results
We identified a total of 72 websites with relevant PEMs and included them in this study. Of these 72 websites, 36 websites were academic training centers, 10 were Google search hits, and 21 were from the Sarcoma Alliance list of sarcoma specialists. The remaining 5 websites were AAOS, Bonetumor.org, Sarcoma Alliance, Sarcoma Foundation of America, and Medscape. A list of conditions and treatments that were considered relevant PEMs is found in Appendix 1. A total of 774 articles were obtained from the 72 websites.
None of the websites had a mean readability score of 7 (seventh grade) or lower (Figures 1A, 1B). Mid-America Sarcoma Institute’s PEMs had the lowest mean readability score, 8.9. The lowest readability score was 5.3, which the New Fog Count readability instrument calculated for Vanderbilt University Medical Center’s (VUMC’s) PEMs (Appendix 2). The mean readability score of all websites was 11.4 (range, 8.9-15.5) (Appendix 2).
Seventy of 72 websites (97%) had PEMs that were fairly difficult or difficult, according to the FRE analysis (Figure 2). The American Cancer Society and Mid-America Sarcoma Institute had PEMs that were written in plain English. Sixty-nine of 72 websites (96%) had PEMs with a readability score of 10 or higher, according to the Raygor readability estimate (Figure 3). Using this instrument, the scores of the American Cancer Society and the University of Pennsylvania–Joan Karnell Cancer Center were 9; Mid-America Sarcoma Institute’s score was 8.
Discussion
Many cancer patients have turned to websites and online PEMs to gather health information about their condition.10-17 Basch and colleagues10 reported almost a decade ago that 44% of cancer patients, as well as 60% of their companions, used the Internet to find cancer-related information.10 When LaCoursiere and colleagues35 surveyed cancer patients, they found that patients handled their condition better and had less anxiety and uncertainty after using the Internet to find health information and support.35 In addition, many orthopedic patients, specifically 46% of orthopedic community outpatients,45 consult the Internet for information about their condition and future surgical procedures.46,47
This study comprehensively evaluated the readability of online PEMs of bone and soft-tissue sarcomas and related conditions by using 10 different readability instruments. After identifying 72 websites and 774 articles, we found that all 72 websites’ PEMs had a mean readability score that did not meet the NIH recommendation of writing PEMs at a sixth- to seventh-grade reading level. These results are consistent with studies evaluating the readability of online PEMs related to other cancer conditions21-25 and other orthopedic conditions.26-31
The combination of low health literacy of many US adults and high reading grade levels of the majority of online PEMs is not conducive to patients’ better understanding their condition(s). Even individuals with high reading skills prefer information that is simpler to read.48 In many areas of medicine, there is evidence that patients’ understanding of their condition has a positive impact on health outcomes, well-being, and the patient–physician relationship.49-61 Regarding cancer patients, Davis and colleagues54 and Peterson and colleagues57 showed that lower health literacy contributes to less knowledge and lower rates of breast54 and colorectal cancer57 screening tests. Even low health literacy of family caregivers of cancer patients can result in increased stress and lack of communication of important medical information between caregiver and physician.52 Among cancer patients, poor health literacy has been associated with mental distress60 as well as decreased compliance with treatment and lower involvement in clinical trials.55
The disparity between patients’ health literacy and the readability of online PEMs needs to be addressed by finding methods to improve patients’ understanding of their condition and to lower the readability scores of online PEMs. Better communication between patient and physician may improve patients’ comprehension of their condition and different aspects of their care.59,62-66 Doak and colleagues63 recommend giving cancer patients the most important information first; presenting information to patients in smaller doses; intermittently asking patients questions; and incorporating graphs, tables, and drawings into communication with patients.63 Additionally, allowing patients to repeat information they have just received/heard to the physician is another useful tool to improve patient education.62,64-66
Another way to address the disparity between patients’ health literacy and the readability of online PEMs is to reduce the reading grade level of existing PEMs. According to results from this study and others, the majority of online PEMs are above the reading grade level of a significant number of US adults. Many available and inexpensive readability instruments allow authors to assess their articles’ readability. Many writing guidelines also exist to help authors improve the readability of their PEMs.20,64,67-71 Living Word Vocabulary70 and Plain Language71 help authors replace complex words or medical terms with simpler words.29 Visual aids, audio, and video help patients with low health literacy remember the information.64
Efforts to improve PEM readability are effective. Of all the websites reviewed, VUMC was identified as having PEMs with the lowest readability score (5.3). This score was reported by the New Fog Count readability instrument, which accounts for the number of sentences, easy words, and hard words. In 2011, VUMC formed the Department of Patient Education to review and update its online and printed PEMs to make sure patients could read them.72 Additionally, the mean readability scores of the websites of the National Cancer Institute and MedlinePlus are in the top 50% of the websites included in this study. The NIH sponsors both sites, which follow the NIH guidelines for writing online PEMs at a reading level suitable for individuals with lower health literacy.20 These materials serve as potential models to improve the readability of PEMs, and, thus, help patients to better understand their condition, medical procedures, and/or treatment options.
To illustrate ways to improve the reading grade level of PEMs, we used the article “Ewing’s Sarcoma” from the AAOS website73 and followed the NIH guidelines to improve the reading grade level of the article.20 We identified complex words and defined them at an eighth-grade reading level. If that word was mentioned later in the article, simpler terminology was used instead of the initial complex word. For example, Ewing’s sarcoma was defined early and then referred to as bone tumor later in the article. We also identified every word that was 3 syllables or longer and used Microsoft Word’s thesaurus to replace those words with ones that were less than 3 syllables. Lastly, all sentences longer than 15 words were rewritten to be less than 15 words. After making these 3 changes to the article, the mean reading grade level dropped from 11.2 to 7.3.
This study has limitations. First, some readability instruments evaluate the number of syllables per word or polysyllabic words as part of their formula and, thus, can underestimate or overestimate the reading grade level of a document. Some readability formulas consider medical terms such as ulna, femur, or carpal as “easy” words because they have 2 syllables, but many laypersons may not comprehend these words. On the other hand, some readability formulas consider medical terms such as medications, diagnosis, or radiation as “hard” words because they contain 3 or more syllables, but the majority of laypersons likely comprehend these words. Second, the reading level of the patient population accessing those online sites was not assessed. Third, the readability instruments in this study did not evaluate the accuracy of the content, pictures, or tables of the PEMs. However, using 10 readability instruments allowed evaluation of many different readability aspects of the text. Fourth, because some websites identified in this study, such as Bonetumor.org, were written for patients as well as clinicians, the reading grade level of these sites may be higher than that of those sites written just for patients.
Conclusion
Because many orthopedic cancer patients rely on the Internet as a source of information, the need for online PEMs to match the reading skills of the patient population who accesses them is vital. However, this study shows that many organizations, academic training centers, and other entities need to update their online PEMs because all PEMs in this study had a mean readability grade level higher than the NIH recommendation. Further research needs to evaluate the effectiveness of other media, such as video, illustrations, and audio, to provide health information to patients. With many guidelines available that provide plans and advice to improve the readability of PEMs, research also must assess the most effective plans and advice in order to allow authors to focus their attention on 1 set of guidelines to improve the readability of their PEMs.
1. Piredda M, Rocci L, Gualandi R, Petitti T, Vincenzi B, De Marinis MG. Survey on learning needs and preferred sources of information to meet these needs in Italian oncology patients receiving chemotherapy. Eur J Oncol Nurs. 2008;12(2):120-126.
2. Fernsler JI, Cannon CA. The whys of patient education. Semin Oncol Nurs. 1991;7(2):79-86.
3. Glimelius B, Birgegård G, Hoffman K, Kvale G, Sjödén PO. Information to and communication with cancer patients: improvements and psychosocial correlates in a comprehensive care program for patients and their relatives. Patient Educ Couns. 1995;25(2):171-182.
4. Harris KA. The informational needs of patients with cancer and their families. Cancer Pract. 1998;6(1):39-46.
5. Jensen AB, Madsen B, Andersen P, Rose C. Information for cancer patients entering a clinical trial--an evaluation of an information strategy. Eur J Cancer. 1993;29A(16):2235-2238.
6. Wells ME, McQuellon RP, Hinkle JS, Cruz JM. Reducing anxiety in newly diagnosed cancer patients: a pilot program. Cancer Pract. 1995;3(2):100-104.
7. Diaz JA, Griffith RA, Ng JJ, Reinert SE, Friedmann PD, Moulton AW. Patients’ use of the Internet for medical information. J Gen Intern Med. 2002;17(3):180-185.
8. Fox S, Duggan M. Health Online 2013. Pew Research Center’s Internet and American Life Project. www.pewinternet.org/~/media//Files/Reports/PIP_HealthOnline.pdf. Published January 15, 2013. Accessed November 18. 2014.
9. Schwartz KL, Roe T, Northrup J, Meza J, Seifeldin R, Neale AV. Family medicine patients’ use of the Internet for health information: a MetroNet study. J Am Board Fam Med. 2006;19(1):39-45.
10. Basch EM, Thaler HT, Shi W, Yakren S, Schrag D. Use of information resources by patients with cancer and their companions. Cancer. 2004;100(11):2476-2483.
11. Huang GJ, Penson DF. Internet health resources and the cancer patient. Cancer Invest. 2008;26(2):202-207.
12. Metz JM, Devine P, Denittis A, et al. A multi-institutional study of Internet utilization by radiation oncology patients. Int J Radiat Oncol Biol Phys. 2003;56(4):1201-1205.
13. Peterson MW, Fretz PC. Patient use of the internet for information in a lung cancer clinic. Chest. 2003;123(2):452-457.
14. Satterlund MJ, McCaul KD, Sandgren AK. Information gathering over time by breast cancer patients. J Med Internet Res. 2003;5(3):e15.
15. Tustin N. The role of patient satisfaction in online health information seeking. J Health Commun. 2010;15(1):3-17.
16. Van de Poll-Franse LV, Van Eenbergen MC. Internet use by cancer survivors: current use and future wishes. Support Care Cancer. 2008;16(10):1189-1195.
17. Ziebland S, Chapple A, Dumelow C, Evans J, Prinjha S, Rozmovits L. How the internet affects patients’ experience of cancer: a qualitative study. BMJ. 2004;328(7439):564.
18. Committee on Health Literacy, Board on Neuroscience and Behavioral Health, Institute of Medicine. Nielsen-Bohlman L, Panzer AM, Kindig DA, eds. Health Literacy: A Prescription to End Confusion. Washington, DC: National Academies Press; 2004. Available at: www.nap.edu/openbook.php?record_id=10883. Accessed November 18, 2014.
19. Kutner M, Greenberg E, Ying J, Paulsen C. The Health Literacy of America’s Adults: Results from the 2003 National Assessment of Adult Literacy. NCES 2006-483. US Department of Education. Washington, DC: National Center for Education Statistics; 2006. Available at: www.nces.ed.gov/pubs2006/2006483.pdf. Accessed November 18, 2014.
20. How to write easy-to-read health materials. MedlinePlus website. www.nlm.nih.gov/medlineplus/etr.html. Updated February 13, 2013. Accessed November 18, 2014.
21. Ellimoottil C, Polcari A, Kadlec A, Gupta G. Readability of websites containing information about prostate cancer treatment options. J Urol. 2012;188(6):2171-2175.
22. Friedman DB, Hoffman-Goetz L, Arocha JF. Health literacy and the World Wide Web: comparing the readability of leading incident cancers on the Internet. Med Inform Internet Med. 2006;31(1):67-87.
23. Hoppe IC. Readability of patient information regarding breast cancer prevention from the Web site of the National Cancer Institute. J Cancer Educ. 2010;25(4):490-492.
24. Misra P, Kasabwala K, Agarwal N, Eloy JA, Liu JK. Readability analysis of internet-based patient information regarding skull base tumors. J Neurooncol. 2012;109(3):573-580.
25. Stinson JN, White M, Breakey V, et al. Perspectives on quality and content of information on the internet for adolescents with cancer. Pediatr Blood Cancer. 2011;57(1):97-104.
26. Badarudeen S, Sabharwal S. Readability of patient education materials from the American Academy of Orthopaedic Surgeons and Pediatric Orthopaedic Society of North America web sites. J Bone Joint Surg Am. 2008;90(1):199-204.
27. Bluman EM, Foley RP, Chiodo CP. Readability of the Patient Education Section of the AOFAS Website. Foot Ankle Int. 2009;30(4):287-291.
28. Polishchuk DL, Hashem J, Sabharwal S. Readability of online patient education materials on adult reconstruction Web sites. J Arthroplasty. 2012;27(5):716-719.
29. Sabharwal S, Badarudeen S, Unes Kunju S. Readability of online patient education materials from the AAOS web site. Clin Orthop. 2008;466(5):1245-1250.
30. Vives M, Young L, Sabharwal S. Readability of spine-related patient education materials from subspecialty organization and spine practitioner websites. Spine. 2009;34(25):2826-2831.
31. Wang SW, Capo JT, Orillaza N. Readability and comprehensibility of patient education material in hand-related web sites. J Hand Surg Am. 2009;34(7):1308-1315.
32. Lam CG, Roter DL, Cohen KJ. Survey of quality, readability, and social reach of websites on osteosarcoma in adolescents. Patient Educ Couns. 2013;90(1):82-87.
33. Tumors. Quinn RH, ed. OrthoInfo. American Academy of Orthopaedic Surgeons website. http://orthoinfo.aaos.org/menus/tumors.cfm. Accessed November 18, 2014.
34. Sarcoma specialists. Sarcoma Alliance website. sarcomaalliance.org/sarcoma-centers. Accessed November 18, 2014.
35. LaCoursiere SP, Knobf MT, McCorkle R. Cancer patients’ self-reported attitudes about the Internet. J Med Internet Res. 2005;7(3):e22.
36. Test your document’s readability. Microsoft Office website. office.microsoft.com/en-us/word-help/test-your-document-s-readability-HP010148506.aspx. Accessed November 18, 2014.
37. Kincaid JP, Fishburne RP, Rogers RL, Chissom BS. Derivation of new readability formulas (Automated Readability Index, Fog Count and Flesch Reading Ease Formula) for Navy enlisted personnel. Naval Technical Training Command. Research Branch Report 8-75. www.dtic.mil/dtic/tr/fulltext/u2/a006655.pdf. Published February 1975. Accessed November 18, 2014.
38. Coleman M, Liau TL. A computer readability formula designed for machine scoring. J Appl Psychol. 1975;60(2):283-284.
39. Fry E. Fry’s readability graph: clarifications, validity, and extension to Level 17. J Reading. 1977;21(3):242-252.
40. Chall JS, Dale E. Manual for the New Dale-Chall Readability Formula. Cambridge, MA: Brookline Books; 1995.
41. Gunning R. The Technique of Clear Writing. Rev. ed. New York, NY: McGraw-Hill; 1968.
42. Powers RD, Sumner WA, Kearl BE. A recalculation of four adult readability formulas. J Educ Psychol. 1958;49(2):99-105.
43. McLaughlin GH. SMOG grading—a new readability formula. J Reading. 1969;22,639-646.
44. Raygor L. The Raygor readability estimate: a quick and easy way to determine difficulty. In: Pearson PD, Hansen J, eds. Reading Theory, Research and Practice. Twenty-Sixth Yearbook of the National Reading Conference. Clemson, SC: National Reading Conference Inc; 1977:259-263.
45. Krempec J, Hall J, Biermann JS. Internet use by patients in orthopaedic surgery. Iowa Orthop J. 2003;23:80-82.
46. Beall MS, Golladay GJ, Greenfield ML, Hensinger RN, Biermann JS. Use of the Internet by pediatric orthopaedic outpatients. J Pediatr Orthop. 2002;22(2):261-264.
47. Beall MS, Beall MS, Greenfield ML, Biermann JS. Patient Internet use in a community outpatient orthopaedic practice. Iowa Orthop J. 2002;22:103-107.
48. Davis TC, Bocchini JA, Fredrickson D, et al. Parent comprehension of polio vaccine information pamphlets. Pediatrics. 1996;97(6 Pt 1):804-810.
49. Apter AJ, Wan F, Reisine S, et al. The association of health literacy with adherence and outcomes in moderate-severe asthma. J Allergy Clin Immunol. 2013;132(2):321-327.
50. Baker DW, Parker RM, Williams MV, Clark WS. Health literacy and the risk of hospital admission. J Gen Intern Med. 1998;13(12):791-798.
51. Berkman ND, Sheridan SL, Donahue KE, Halpern DJ, Crotty K. Low health literacy and health outcomes: an updated systematic review. Ann Intern Med. 2011;155(2):97-107.
52. Bevan JL, Pecchioni LL. Understanding the impact of family caregiver cancer literacy on patient health outcomes. Patient Educ Couns. 2008;71(3):356-364.
53. Corey MR, St Julien J, Miller C, et al. Patient education level affects functionality and long term mortality after major lower extremity amputation. Am J Surg. 2012;204(5):626-630.
54. Davis TC, Arnold C, Berkel HJ, Nandy I, Jackson RH, Glass J. Knowledge and attitude on screening mammography among low-literate, low-income women. Cancer. 1996;78(9):1912-1920.
55. Davis TC, Williams MV, Marin E, Parker RM, Glass J. Health literacy and cancer communication. CA Cancer J Clin. 2002;52(3):134-149.
56. Freedman RB, Jones SK, Lin A, Robin AL, Muir KW. Influence of parental health literacy and dosing responsibility on pediatric glaucoma medication adherence. Arch Ophthalmol. 2012;130(3):306-311.
57. Peterson NB, Dwyer KA, Mulvaney SA, Dietrich MS, Rothman RL. The influence of health literacy on colorectal cancer screening knowledge, beliefs and behavior. J Natl Med Assoc. 2007;99(10):1105-1112.
58. Peterson PN, Shetterly SM, Clarke CL, et al. Health literacy and outcomes among patients with heart failure. JAMA. 2011;305(16):1695-1701.
59. Rosas-salazar C, Apter AJ, Canino G, Celedón JC. Health literacy and asthma. J Allergy Clin Immunol. 2012;129(4):935-942.
60. Song L, Mishel M, Bensen JT, et al. How does health literacy affect quality of life among men with newly diagnosed clinically localized prostate cancer? Findings from the North Carolina-Louisiana Prostate Cancer Project (PCaP). Cancer. 2012;118(15):3842-3851.
61. Williams MV, Davis T, Parker RM, Weiss BD. The role of health literacy in patient-physician communication. Fam Med. 2002;34(5):383-389.
62. Badarudeen S, Sabharwal S. Assessing readability of patient education materials: current role in orthopaedics. Clin Orthop. 2010;468(10):2572-2580.
63. Doak CC, Doak LG, Friedell GH, Meade CD. Improving comprehension for cancer patients with low literacy skills: strategies for clinicians. CA Cancer J Clin. 1998;48(3):151-162.
64. Doak CC, Doak LG, Root JH. Teaching Patients With Low Literacy Skills. 2nd ed. Philadelphia, PA: JB Lippincott Company; 1996.
65. Kemp EC, Floyd MR, McCord-Duncan E, Lang F. Patients prefer the method of “tell back-collaborative inquiry” to assess understanding of medical information. J Am Board Fam Med. 2008;21(1):24-30.
66. Kripalani S, Bengtzen R, Henderson LE, Jacobson TA. Clinical research in low-literacy populations: using teach-back to assess comprehension of informed consent and privacy information. IRB. 2008;30(2):13-19.
67. Centers for Disease Control and Prevention. Simply Put: A Guide For Creating Easy-to-Understand Materials. 3rd ed. Atlanta, GA: Strategic and Proactive Communication Branch, Centers for Disease Control and Prevention, US Dept of Health and Human Services; 2009.
68. National Institutes of Health, National Cancer Institute. Clear & Simple: Developing Effective Print Materials for Low-Literate Readers. Devcompage website. http://devcompage.com/wp-content/uploads/2010/12/Clear_n_Simple.pdf Published March 2, 1998. Accessed December 1, 2014.
69. Weiss BD. Health Literacy and Patient Safety: Help Patients Understand. 2nd ed. Chicago, IL: American Medical Association and AMA Foundation; 2007:35-41.
70. Dale E, O’Rourke J. The Living Word Vocabulary. Newington, CT: World Book-Childcraft International, 1981.
71. Word suggestions. Plain Language website. www.plainlanguage.gov/howto/wordsuggestions/index.cfm. Accessed November 18, 2014.
72. Rivers K. Initiative aims to enhance patient communication materials. Reporter: Vanderbilt University Medical Center’s Weekly Newspaper. April 28, 2011. http://www.mc.vanderbilt.edu/reporter/index.html?ID=10649. Accessed November 18, 2014.
73. Ewing’s sarcoma. OrthoInfo. American Academy of Orthopaedic Surgeons website. http://orthoinfo.aaos.org/topic.cfm?topic=A00082. Last reviewed September 2011. Accessed November 18, 2014.
1. Piredda M, Rocci L, Gualandi R, Petitti T, Vincenzi B, De Marinis MG. Survey on learning needs and preferred sources of information to meet these needs in Italian oncology patients receiving chemotherapy. Eur J Oncol Nurs. 2008;12(2):120-126.
2. Fernsler JI, Cannon CA. The whys of patient education. Semin Oncol Nurs. 1991;7(2):79-86.
3. Glimelius B, Birgegård G, Hoffman K, Kvale G, Sjödén PO. Information to and communication with cancer patients: improvements and psychosocial correlates in a comprehensive care program for patients and their relatives. Patient Educ Couns. 1995;25(2):171-182.
4. Harris KA. The informational needs of patients with cancer and their families. Cancer Pract. 1998;6(1):39-46.
5. Jensen AB, Madsen B, Andersen P, Rose C. Information for cancer patients entering a clinical trial--an evaluation of an information strategy. Eur J Cancer. 1993;29A(16):2235-2238.
6. Wells ME, McQuellon RP, Hinkle JS, Cruz JM. Reducing anxiety in newly diagnosed cancer patients: a pilot program. Cancer Pract. 1995;3(2):100-104.
7. Diaz JA, Griffith RA, Ng JJ, Reinert SE, Friedmann PD, Moulton AW. Patients’ use of the Internet for medical information. J Gen Intern Med. 2002;17(3):180-185.
8. Fox S, Duggan M. Health Online 2013. Pew Research Center’s Internet and American Life Project. www.pewinternet.org/~/media//Files/Reports/PIP_HealthOnline.pdf. Published January 15, 2013. Accessed November 18. 2014.
9. Schwartz KL, Roe T, Northrup J, Meza J, Seifeldin R, Neale AV. Family medicine patients’ use of the Internet for health information: a MetroNet study. J Am Board Fam Med. 2006;19(1):39-45.
10. Basch EM, Thaler HT, Shi W, Yakren S, Schrag D. Use of information resources by patients with cancer and their companions. Cancer. 2004;100(11):2476-2483.
11. Huang GJ, Penson DF. Internet health resources and the cancer patient. Cancer Invest. 2008;26(2):202-207.
12. Metz JM, Devine P, Denittis A, et al. A multi-institutional study of Internet utilization by radiation oncology patients. Int J Radiat Oncol Biol Phys. 2003;56(4):1201-1205.
13. Peterson MW, Fretz PC. Patient use of the internet for information in a lung cancer clinic. Chest. 2003;123(2):452-457.
14. Satterlund MJ, McCaul KD, Sandgren AK. Information gathering over time by breast cancer patients. J Med Internet Res. 2003;5(3):e15.
15. Tustin N. The role of patient satisfaction in online health information seeking. J Health Commun. 2010;15(1):3-17.
16. Van de Poll-Franse LV, Van Eenbergen MC. Internet use by cancer survivors: current use and future wishes. Support Care Cancer. 2008;16(10):1189-1195.
17. Ziebland S, Chapple A, Dumelow C, Evans J, Prinjha S, Rozmovits L. How the internet affects patients’ experience of cancer: a qualitative study. BMJ. 2004;328(7439):564.
18. Committee on Health Literacy, Board on Neuroscience and Behavioral Health, Institute of Medicine. Nielsen-Bohlman L, Panzer AM, Kindig DA, eds. Health Literacy: A Prescription to End Confusion. Washington, DC: National Academies Press; 2004. Available at: www.nap.edu/openbook.php?record_id=10883. Accessed November 18, 2014.
19. Kutner M, Greenberg E, Ying J, Paulsen C. The Health Literacy of America’s Adults: Results from the 2003 National Assessment of Adult Literacy. NCES 2006-483. US Department of Education. Washington, DC: National Center for Education Statistics; 2006. Available at: www.nces.ed.gov/pubs2006/2006483.pdf. Accessed November 18, 2014.
20. How to write easy-to-read health materials. MedlinePlus website. www.nlm.nih.gov/medlineplus/etr.html. Updated February 13, 2013. Accessed November 18, 2014.
21. Ellimoottil C, Polcari A, Kadlec A, Gupta G. Readability of websites containing information about prostate cancer treatment options. J Urol. 2012;188(6):2171-2175.
22. Friedman DB, Hoffman-Goetz L, Arocha JF. Health literacy and the World Wide Web: comparing the readability of leading incident cancers on the Internet. Med Inform Internet Med. 2006;31(1):67-87.
23. Hoppe IC. Readability of patient information regarding breast cancer prevention from the Web site of the National Cancer Institute. J Cancer Educ. 2010;25(4):490-492.
24. Misra P, Kasabwala K, Agarwal N, Eloy JA, Liu JK. Readability analysis of internet-based patient information regarding skull base tumors. J Neurooncol. 2012;109(3):573-580.
25. Stinson JN, White M, Breakey V, et al. Perspectives on quality and content of information on the internet for adolescents with cancer. Pediatr Blood Cancer. 2011;57(1):97-104.
26. Badarudeen S, Sabharwal S. Readability of patient education materials from the American Academy of Orthopaedic Surgeons and Pediatric Orthopaedic Society of North America web sites. J Bone Joint Surg Am. 2008;90(1):199-204.
27. Bluman EM, Foley RP, Chiodo CP. Readability of the Patient Education Section of the AOFAS Website. Foot Ankle Int. 2009;30(4):287-291.
28. Polishchuk DL, Hashem J, Sabharwal S. Readability of online patient education materials on adult reconstruction Web sites. J Arthroplasty. 2012;27(5):716-719.
29. Sabharwal S, Badarudeen S, Unes Kunju S. Readability of online patient education materials from the AAOS web site. Clin Orthop. 2008;466(5):1245-1250.
30. Vives M, Young L, Sabharwal S. Readability of spine-related patient education materials from subspecialty organization and spine practitioner websites. Spine. 2009;34(25):2826-2831.
31. Wang SW, Capo JT, Orillaza N. Readability and comprehensibility of patient education material in hand-related web sites. J Hand Surg Am. 2009;34(7):1308-1315.
32. Lam CG, Roter DL, Cohen KJ. Survey of quality, readability, and social reach of websites on osteosarcoma in adolescents. Patient Educ Couns. 2013;90(1):82-87.
33. Tumors. Quinn RH, ed. OrthoInfo. American Academy of Orthopaedic Surgeons website. http://orthoinfo.aaos.org/menus/tumors.cfm. Accessed November 18, 2014.
34. Sarcoma specialists. Sarcoma Alliance website. sarcomaalliance.org/sarcoma-centers. Accessed November 18, 2014.
35. LaCoursiere SP, Knobf MT, McCorkle R. Cancer patients’ self-reported attitudes about the Internet. J Med Internet Res. 2005;7(3):e22.
36. Test your document’s readability. Microsoft Office website. office.microsoft.com/en-us/word-help/test-your-document-s-readability-HP010148506.aspx. Accessed November 18, 2014.
37. Kincaid JP, Fishburne RP, Rogers RL, Chissom BS. Derivation of new readability formulas (Automated Readability Index, Fog Count and Flesch Reading Ease Formula) for Navy enlisted personnel. Naval Technical Training Command. Research Branch Report 8-75. www.dtic.mil/dtic/tr/fulltext/u2/a006655.pdf. Published February 1975. Accessed November 18, 2014.
38. Coleman M, Liau TL. A computer readability formula designed for machine scoring. J Appl Psychol. 1975;60(2):283-284.
39. Fry E. Fry’s readability graph: clarifications, validity, and extension to Level 17. J Reading. 1977;21(3):242-252.
40. Chall JS, Dale E. Manual for the New Dale-Chall Readability Formula. Cambridge, MA: Brookline Books; 1995.
41. Gunning R. The Technique of Clear Writing. Rev. ed. New York, NY: McGraw-Hill; 1968.
42. Powers RD, Sumner WA, Kearl BE. A recalculation of four adult readability formulas. J Educ Psychol. 1958;49(2):99-105.
43. McLaughlin GH. SMOG grading—a new readability formula. J Reading. 1969;22,639-646.
44. Raygor L. The Raygor readability estimate: a quick and easy way to determine difficulty. In: Pearson PD, Hansen J, eds. Reading Theory, Research and Practice. Twenty-Sixth Yearbook of the National Reading Conference. Clemson, SC: National Reading Conference Inc; 1977:259-263.
45. Krempec J, Hall J, Biermann JS. Internet use by patients in orthopaedic surgery. Iowa Orthop J. 2003;23:80-82.
46. Beall MS, Golladay GJ, Greenfield ML, Hensinger RN, Biermann JS. Use of the Internet by pediatric orthopaedic outpatients. J Pediatr Orthop. 2002;22(2):261-264.
47. Beall MS, Beall MS, Greenfield ML, Biermann JS. Patient Internet use in a community outpatient orthopaedic practice. Iowa Orthop J. 2002;22:103-107.
48. Davis TC, Bocchini JA, Fredrickson D, et al. Parent comprehension of polio vaccine information pamphlets. Pediatrics. 1996;97(6 Pt 1):804-810.
49. Apter AJ, Wan F, Reisine S, et al. The association of health literacy with adherence and outcomes in moderate-severe asthma. J Allergy Clin Immunol. 2013;132(2):321-327.
50. Baker DW, Parker RM, Williams MV, Clark WS. Health literacy and the risk of hospital admission. J Gen Intern Med. 1998;13(12):791-798.
51. Berkman ND, Sheridan SL, Donahue KE, Halpern DJ, Crotty K. Low health literacy and health outcomes: an updated systematic review. Ann Intern Med. 2011;155(2):97-107.
52. Bevan JL, Pecchioni LL. Understanding the impact of family caregiver cancer literacy on patient health outcomes. Patient Educ Couns. 2008;71(3):356-364.
53. Corey MR, St Julien J, Miller C, et al. Patient education level affects functionality and long term mortality after major lower extremity amputation. Am J Surg. 2012;204(5):626-630.
54. Davis TC, Arnold C, Berkel HJ, Nandy I, Jackson RH, Glass J. Knowledge and attitude on screening mammography among low-literate, low-income women. Cancer. 1996;78(9):1912-1920.
55. Davis TC, Williams MV, Marin E, Parker RM, Glass J. Health literacy and cancer communication. CA Cancer J Clin. 2002;52(3):134-149.
56. Freedman RB, Jones SK, Lin A, Robin AL, Muir KW. Influence of parental health literacy and dosing responsibility on pediatric glaucoma medication adherence. Arch Ophthalmol. 2012;130(3):306-311.
57. Peterson NB, Dwyer KA, Mulvaney SA, Dietrich MS, Rothman RL. The influence of health literacy on colorectal cancer screening knowledge, beliefs and behavior. J Natl Med Assoc. 2007;99(10):1105-1112.
58. Peterson PN, Shetterly SM, Clarke CL, et al. Health literacy and outcomes among patients with heart failure. JAMA. 2011;305(16):1695-1701.
59. Rosas-salazar C, Apter AJ, Canino G, Celedón JC. Health literacy and asthma. J Allergy Clin Immunol. 2012;129(4):935-942.
60. Song L, Mishel M, Bensen JT, et al. How does health literacy affect quality of life among men with newly diagnosed clinically localized prostate cancer? Findings from the North Carolina-Louisiana Prostate Cancer Project (PCaP). Cancer. 2012;118(15):3842-3851.
61. Williams MV, Davis T, Parker RM, Weiss BD. The role of health literacy in patient-physician communication. Fam Med. 2002;34(5):383-389.
62. Badarudeen S, Sabharwal S. Assessing readability of patient education materials: current role in orthopaedics. Clin Orthop. 2010;468(10):2572-2580.
63. Doak CC, Doak LG, Friedell GH, Meade CD. Improving comprehension for cancer patients with low literacy skills: strategies for clinicians. CA Cancer J Clin. 1998;48(3):151-162.
64. Doak CC, Doak LG, Root JH. Teaching Patients With Low Literacy Skills. 2nd ed. Philadelphia, PA: JB Lippincott Company; 1996.
65. Kemp EC, Floyd MR, McCord-Duncan E, Lang F. Patients prefer the method of “tell back-collaborative inquiry” to assess understanding of medical information. J Am Board Fam Med. 2008;21(1):24-30.
66. Kripalani S, Bengtzen R, Henderson LE, Jacobson TA. Clinical research in low-literacy populations: using teach-back to assess comprehension of informed consent and privacy information. IRB. 2008;30(2):13-19.
67. Centers for Disease Control and Prevention. Simply Put: A Guide For Creating Easy-to-Understand Materials. 3rd ed. Atlanta, GA: Strategic and Proactive Communication Branch, Centers for Disease Control and Prevention, US Dept of Health and Human Services; 2009.
68. National Institutes of Health, National Cancer Institute. Clear & Simple: Developing Effective Print Materials for Low-Literate Readers. Devcompage website. http://devcompage.com/wp-content/uploads/2010/12/Clear_n_Simple.pdf Published March 2, 1998. Accessed December 1, 2014.
69. Weiss BD. Health Literacy and Patient Safety: Help Patients Understand. 2nd ed. Chicago, IL: American Medical Association and AMA Foundation; 2007:35-41.
70. Dale E, O’Rourke J. The Living Word Vocabulary. Newington, CT: World Book-Childcraft International, 1981.
71. Word suggestions. Plain Language website. www.plainlanguage.gov/howto/wordsuggestions/index.cfm. Accessed November 18, 2014.
72. Rivers K. Initiative aims to enhance patient communication materials. Reporter: Vanderbilt University Medical Center’s Weekly Newspaper. April 28, 2011. http://www.mc.vanderbilt.edu/reporter/index.html?ID=10649. Accessed November 18, 2014.
73. Ewing’s sarcoma. OrthoInfo. American Academy of Orthopaedic Surgeons website. http://orthoinfo.aaos.org/topic.cfm?topic=A00082. Last reviewed September 2011. Accessed November 18, 2014.