User login
Consider centralized pain in patients with rheumatic disease
Las Vegas – A fibromyalgia survey may provide important information about the degree to which patients with rheumatic disease experience centralized pain. This information may guide treatment decisions, said Daniel J. Clauw, MD, professor of anesthesiology, rheumatology, and psychiatry and director of the Chronic Pain and Fatigue Research Center at the University of Michigan in Ann Arbor.
The questionnaire that Dr. Clauw uses is a patient self-report survey for the assessment of fibromyalgia based on criteria in the 2011 modification of the American College of Rheumatology preliminary diagnostic criteria for fibromyalgia. In it, he asks patients to report where they experience pain throughout the body and symptoms such as fatigue, sleep problems, and memory problems. The survey predicts outcomes of surgery for osteoarthritis better than x-rays, MRI scans, or psychological factors do, he said.
Physicians should ask every patient with chronic pain, including patients with OA, rheumatoid arthritis, or lupus, to complete the survey, Dr. Clauw said at the annual Perspectives in Rheumatic Diseases held by Global Academy for Medical Education. “This score will tell you the degree to which their central nervous system is augmenting or amplifying what is going on in their body,” he said. “And the higher their score is, the more you should treat them like you would someone with fibromyalgia, even if their underlying disease might be an autoimmune disease.”
Physicians should not use a cutoff of 13 points on the fibromyalgia measure to define whether a patient has the disease, as has been done in the past, he said. The threshold is arbitrary, he said. “We should not think about fibromyalgia as ‘yes’ or ‘no.’ We should think of the degree of fibromyalgia that people have.”
A poor relationship between pain and imaging
Some patients who have severe knee OA on imaging walk without pain. Other patients have normal x-rays, but severe pain. “There is a terrible relationship between what you see on a knee x-ray or an MRI and whether someone has pain,” Dr. Clauw said. Furthermore, the poor relationship between imaging and pain is common across chronic pain conditions, he said.
This phenomenon may occur because pain manifests in different ways, similar to there being multiple ways to adjust the volume of an electric guitar, he said. How hard the strings are strummed affects the volume. But so does the amplifier setting. “In these centralized pain conditions, the problem is an amplifier problem, not a guitar problem,” he said. “The amplifier, i.e., the central nervous system, is set too high.”
Researchers have found that people who have severe OA of the knee on x-ray but do not experience pain “have a very low amplifier setting,” he said. That is, they are nontender and less sensitive to pain. Most of these patients are men. “On average, men have a much lower amplifier setting than women,” he said. “This is also why ... women have 1.5 to 2 times the rate of any type of chronic pain than men, because on average women have a higher amplifier setting. ... In OA, at any given age, men and women have the exact same percentage of radiographic OA. But if you look at the clinical condition of OA, it is always two-thirds women, one-third men.”
Opioid responsiveness
To examine whether fibromyalgia survey results correlate with outcomes after knee and hip arthroplasty, Dr. Clauw and colleagues conducted a prospective, observational cohort study that included approximately 500 people. Patients completed the questionnaire on the day of surgery.
Patients with higher levels of fibromyalgia were less responsive to opioids. “For each 1-point increase in the fibromyalgia score, people needed about one more hydrocodone tablet in the first 24-48 hours to control their pain,” he said (Anesthesiology. 2013 Dec;119[6]:1434-43). In addition, each 1-point increase in the fibromyalgia score made people about 25% less likely to have a 50% improvement in knee pain level after 6 months (Arthritis Rheumatol. 2015 May;67[5]:1386-94). The correlations were independent of psychological factors. In addition, the associations were linear. “There was nothing magical about a fibromyalgia score of 13,” Dr. Clauw said.
Dr. Clauw is a coauthor of a study to be presented at the 2019 American College of Rheumatology/Association of Rheumatology Professionals annual meeting that found pain centralization in patients with RA is associated with poor response to disease-modifying antirheumatic drugs (DMARDs).
Prior studies in patients with RA have found that the degree of fibromyalgia is a better predictor of pain and disability than erythrocyte sedimentation rate or the number of swollen joints.
Diagnosed cases are the “tip of the iceberg”
Researchers at Dr. Clauw’s institution have identified dozens of patients undergoing knee surgery who met criteria for fibromyalgia but had not received the diagnosis. “This is at the University of Michigan, which is the epicenter for fibromyalgia research. If we are not seeing fibromyalgia superimposed on OA in our patients, no one is seeing it,” he said.
Patients with diagnosed fibromyalgia are “the tip of the iceberg,” he said. “There are far greater numbers of individuals whose primary diagnosis is OA, RA, lupus, ankylosing spondylitis, cancer pain, or sickle cell disease that have the same fundamental problem as fibromyalgia patients. But you do not see it because you label them as having an autoimmune disease or osteoarthritis. And that is at your peril and at their peril. Because treating that individual as if all of their pain and other symptoms are due to a problem out on the periphery will not make that person better.”
Patients with high levels of centralized pain may be less responsive to peripherally directed therapies such as surgery or injections, Dr. Clauw said. Pharmacologic options for patients with centralized pain include gabapentinoids (e.g., pregabalin and gabapentin), serotonin-norepinephrine reuptake inhibitors (e.g., duloxetine and milnacipran), and tricyclic compounds (e.g., amitriptyline and cyclobenzaprine), he said. “Opioids are going to be quite unlikely to help these individuals,” he said. “In fact, it is likely that opioids will make this kind of pain worse.”
Dr. Clauw is a consultant for Aptinyx, Daiichi Sankyo, Eli Lilly, Intec Pharma, Pfizer, Samumed, Theravance, Tonix, and Zynerba Pharma. He has received grant or research support from Aptinyx and Pfizer and is an expert witness.
Global Academy for Medical Education and this news organization are owned by the same parent company.
Las Vegas – A fibromyalgia survey may provide important information about the degree to which patients with rheumatic disease experience centralized pain. This information may guide treatment decisions, said Daniel J. Clauw, MD, professor of anesthesiology, rheumatology, and psychiatry and director of the Chronic Pain and Fatigue Research Center at the University of Michigan in Ann Arbor.
The questionnaire that Dr. Clauw uses is a patient self-report survey for the assessment of fibromyalgia based on criteria in the 2011 modification of the American College of Rheumatology preliminary diagnostic criteria for fibromyalgia. In it, he asks patients to report where they experience pain throughout the body and symptoms such as fatigue, sleep problems, and memory problems. The survey predicts outcomes of surgery for osteoarthritis better than x-rays, MRI scans, or psychological factors do, he said.
Physicians should ask every patient with chronic pain, including patients with OA, rheumatoid arthritis, or lupus, to complete the survey, Dr. Clauw said at the annual Perspectives in Rheumatic Diseases held by Global Academy for Medical Education. “This score will tell you the degree to which their central nervous system is augmenting or amplifying what is going on in their body,” he said. “And the higher their score is, the more you should treat them like you would someone with fibromyalgia, even if their underlying disease might be an autoimmune disease.”
Physicians should not use a cutoff of 13 points on the fibromyalgia measure to define whether a patient has the disease, as has been done in the past, he said. The threshold is arbitrary, he said. “We should not think about fibromyalgia as ‘yes’ or ‘no.’ We should think of the degree of fibromyalgia that people have.”
A poor relationship between pain and imaging
Some patients who have severe knee OA on imaging walk without pain. Other patients have normal x-rays, but severe pain. “There is a terrible relationship between what you see on a knee x-ray or an MRI and whether someone has pain,” Dr. Clauw said. Furthermore, the poor relationship between imaging and pain is common across chronic pain conditions, he said.
This phenomenon may occur because pain manifests in different ways, similar to there being multiple ways to adjust the volume of an electric guitar, he said. How hard the strings are strummed affects the volume. But so does the amplifier setting. “In these centralized pain conditions, the problem is an amplifier problem, not a guitar problem,” he said. “The amplifier, i.e., the central nervous system, is set too high.”
Researchers have found that people who have severe OA of the knee on x-ray but do not experience pain “have a very low amplifier setting,” he said. That is, they are nontender and less sensitive to pain. Most of these patients are men. “On average, men have a much lower amplifier setting than women,” he said. “This is also why ... women have 1.5 to 2 times the rate of any type of chronic pain than men, because on average women have a higher amplifier setting. ... In OA, at any given age, men and women have the exact same percentage of radiographic OA. But if you look at the clinical condition of OA, it is always two-thirds women, one-third men.”
Opioid responsiveness
To examine whether fibromyalgia survey results correlate with outcomes after knee and hip arthroplasty, Dr. Clauw and colleagues conducted a prospective, observational cohort study that included approximately 500 people. Patients completed the questionnaire on the day of surgery.
Patients with higher levels of fibromyalgia were less responsive to opioids. “For each 1-point increase in the fibromyalgia score, people needed about one more hydrocodone tablet in the first 24-48 hours to control their pain,” he said (Anesthesiology. 2013 Dec;119[6]:1434-43). In addition, each 1-point increase in the fibromyalgia score made people about 25% less likely to have a 50% improvement in knee pain level after 6 months (Arthritis Rheumatol. 2015 May;67[5]:1386-94). The correlations were independent of psychological factors. In addition, the associations were linear. “There was nothing magical about a fibromyalgia score of 13,” Dr. Clauw said.
Dr. Clauw is a coauthor of a study to be presented at the 2019 American College of Rheumatology/Association of Rheumatology Professionals annual meeting that found pain centralization in patients with RA is associated with poor response to disease-modifying antirheumatic drugs (DMARDs).
Prior studies in patients with RA have found that the degree of fibromyalgia is a better predictor of pain and disability than erythrocyte sedimentation rate or the number of swollen joints.
Diagnosed cases are the “tip of the iceberg”
Researchers at Dr. Clauw’s institution have identified dozens of patients undergoing knee surgery who met criteria for fibromyalgia but had not received the diagnosis. “This is at the University of Michigan, which is the epicenter for fibromyalgia research. If we are not seeing fibromyalgia superimposed on OA in our patients, no one is seeing it,” he said.
Patients with diagnosed fibromyalgia are “the tip of the iceberg,” he said. “There are far greater numbers of individuals whose primary diagnosis is OA, RA, lupus, ankylosing spondylitis, cancer pain, or sickle cell disease that have the same fundamental problem as fibromyalgia patients. But you do not see it because you label them as having an autoimmune disease or osteoarthritis. And that is at your peril and at their peril. Because treating that individual as if all of their pain and other symptoms are due to a problem out on the periphery will not make that person better.”
Patients with high levels of centralized pain may be less responsive to peripherally directed therapies such as surgery or injections, Dr. Clauw said. Pharmacologic options for patients with centralized pain include gabapentinoids (e.g., pregabalin and gabapentin), serotonin-norepinephrine reuptake inhibitors (e.g., duloxetine and milnacipran), and tricyclic compounds (e.g., amitriptyline and cyclobenzaprine), he said. “Opioids are going to be quite unlikely to help these individuals,” he said. “In fact, it is likely that opioids will make this kind of pain worse.”
Dr. Clauw is a consultant for Aptinyx, Daiichi Sankyo, Eli Lilly, Intec Pharma, Pfizer, Samumed, Theravance, Tonix, and Zynerba Pharma. He has received grant or research support from Aptinyx and Pfizer and is an expert witness.
Global Academy for Medical Education and this news organization are owned by the same parent company.
Las Vegas – A fibromyalgia survey may provide important information about the degree to which patients with rheumatic disease experience centralized pain. This information may guide treatment decisions, said Daniel J. Clauw, MD, professor of anesthesiology, rheumatology, and psychiatry and director of the Chronic Pain and Fatigue Research Center at the University of Michigan in Ann Arbor.
The questionnaire that Dr. Clauw uses is a patient self-report survey for the assessment of fibromyalgia based on criteria in the 2011 modification of the American College of Rheumatology preliminary diagnostic criteria for fibromyalgia. In it, he asks patients to report where they experience pain throughout the body and symptoms such as fatigue, sleep problems, and memory problems. The survey predicts outcomes of surgery for osteoarthritis better than x-rays, MRI scans, or psychological factors do, he said.
Physicians should ask every patient with chronic pain, including patients with OA, rheumatoid arthritis, or lupus, to complete the survey, Dr. Clauw said at the annual Perspectives in Rheumatic Diseases held by Global Academy for Medical Education. “This score will tell you the degree to which their central nervous system is augmenting or amplifying what is going on in their body,” he said. “And the higher their score is, the more you should treat them like you would someone with fibromyalgia, even if their underlying disease might be an autoimmune disease.”
Physicians should not use a cutoff of 13 points on the fibromyalgia measure to define whether a patient has the disease, as has been done in the past, he said. The threshold is arbitrary, he said. “We should not think about fibromyalgia as ‘yes’ or ‘no.’ We should think of the degree of fibromyalgia that people have.”
A poor relationship between pain and imaging
Some patients who have severe knee OA on imaging walk without pain. Other patients have normal x-rays, but severe pain. “There is a terrible relationship between what you see on a knee x-ray or an MRI and whether someone has pain,” Dr. Clauw said. Furthermore, the poor relationship between imaging and pain is common across chronic pain conditions, he said.
This phenomenon may occur because pain manifests in different ways, similar to there being multiple ways to adjust the volume of an electric guitar, he said. How hard the strings are strummed affects the volume. But so does the amplifier setting. “In these centralized pain conditions, the problem is an amplifier problem, not a guitar problem,” he said. “The amplifier, i.e., the central nervous system, is set too high.”
Researchers have found that people who have severe OA of the knee on x-ray but do not experience pain “have a very low amplifier setting,” he said. That is, they are nontender and less sensitive to pain. Most of these patients are men. “On average, men have a much lower amplifier setting than women,” he said. “This is also why ... women have 1.5 to 2 times the rate of any type of chronic pain than men, because on average women have a higher amplifier setting. ... In OA, at any given age, men and women have the exact same percentage of radiographic OA. But if you look at the clinical condition of OA, it is always two-thirds women, one-third men.”
Opioid responsiveness
To examine whether fibromyalgia survey results correlate with outcomes after knee and hip arthroplasty, Dr. Clauw and colleagues conducted a prospective, observational cohort study that included approximately 500 people. Patients completed the questionnaire on the day of surgery.
Patients with higher levels of fibromyalgia were less responsive to opioids. “For each 1-point increase in the fibromyalgia score, people needed about one more hydrocodone tablet in the first 24-48 hours to control their pain,” he said (Anesthesiology. 2013 Dec;119[6]:1434-43). In addition, each 1-point increase in the fibromyalgia score made people about 25% less likely to have a 50% improvement in knee pain level after 6 months (Arthritis Rheumatol. 2015 May;67[5]:1386-94). The correlations were independent of psychological factors. In addition, the associations were linear. “There was nothing magical about a fibromyalgia score of 13,” Dr. Clauw said.
Dr. Clauw is a coauthor of a study to be presented at the 2019 American College of Rheumatology/Association of Rheumatology Professionals annual meeting that found pain centralization in patients with RA is associated with poor response to disease-modifying antirheumatic drugs (DMARDs).
Prior studies in patients with RA have found that the degree of fibromyalgia is a better predictor of pain and disability than erythrocyte sedimentation rate or the number of swollen joints.
Diagnosed cases are the “tip of the iceberg”
Researchers at Dr. Clauw’s institution have identified dozens of patients undergoing knee surgery who met criteria for fibromyalgia but had not received the diagnosis. “This is at the University of Michigan, which is the epicenter for fibromyalgia research. If we are not seeing fibromyalgia superimposed on OA in our patients, no one is seeing it,” he said.
Patients with diagnosed fibromyalgia are “the tip of the iceberg,” he said. “There are far greater numbers of individuals whose primary diagnosis is OA, RA, lupus, ankylosing spondylitis, cancer pain, or sickle cell disease that have the same fundamental problem as fibromyalgia patients. But you do not see it because you label them as having an autoimmune disease or osteoarthritis. And that is at your peril and at their peril. Because treating that individual as if all of their pain and other symptoms are due to a problem out on the periphery will not make that person better.”
Patients with high levels of centralized pain may be less responsive to peripherally directed therapies such as surgery or injections, Dr. Clauw said. Pharmacologic options for patients with centralized pain include gabapentinoids (e.g., pregabalin and gabapentin), serotonin-norepinephrine reuptake inhibitors (e.g., duloxetine and milnacipran), and tricyclic compounds (e.g., amitriptyline and cyclobenzaprine), he said. “Opioids are going to be quite unlikely to help these individuals,” he said. “In fact, it is likely that opioids will make this kind of pain worse.”
Dr. Clauw is a consultant for Aptinyx, Daiichi Sankyo, Eli Lilly, Intec Pharma, Pfizer, Samumed, Theravance, Tonix, and Zynerba Pharma. He has received grant or research support from Aptinyx and Pfizer and is an expert witness.
Global Academy for Medical Education and this news organization are owned by the same parent company.
EXPERT ANALYSIS FROM PRD 2019
Corticosteroid use in pregnancy linked to preterm birth
Pregnant women taking oral corticosteroids for rheumatoid arthritis may be at increased risk of preterm birth, according to research published online Sept. 30 in Rheumatology.
A study of 528 pregnant women with rheumatoid arthritis enrolled in the MotherToBaby Pregnancy Studies found that those taking a daily dose of 10 mg or more of prednisone equivalent – representing a mean cumulative dose of 2,208.6 mg over the first 139 days of pregnancy – had 4.77-fold higher odds of preterm birth, compared with those not taking oral corticosteroids. Women on medium doses – with a mean cumulative dose of 883 mg – had 81% higher odds of preterm birth, while those on low cumulative doses of 264.9 mg showed a nonsignificant 38% increase in preterm birth risk.
Women who did not use oral corticosteroids before day 140 of pregnancy had a 2.2% risk of early preterm birth. Among women with low use of oral corticosteroids, the risk was 3.4%, among those with medium use the risk was 3.3%, but among those with high use the risk was 26.7%.
After day 140 of gestation, there was a nonsignificant 64% increase in the risk for preterm birth with any use of oral corticosteroids, compared with no use. But among women taking 10 mg or more of prednisone equivalent per day, the risk was 2.45-fold higher, whereas those taking under 10 mg showed no significant increase in risk.
“Systemic corticosteroid use has been associated with serious infection in pregnant women and serious and nonserious infection in individuals with autoimmune diseases, independent of other immunosuppressive medications, especially for doses of 10 mg of prednisone equivalent per day and greater,” wrote Kristin Palmsten, ScD, a research investigator with HealthPartners Institute in Minneapolis, Minn., and coauthors.
Given that intrauterine infection is believed to contribute to preterm birth, some have suggested that the immunosuppressive effects of oral corticosteroids could be associated with an increased risk of preterm birth because of subclinical intra-amniotic infection, they wrote.
However, they noted that there was a lack of information on the effect of dose and timing of oral corticosteroids during pregnancy on the risk of preterm birth.
The authors acknowledged that dosage of oral corticosteroids during pregnancy was linked to disease activity, which was itself associated with preterm birth risk. They adjusted for self-assessed rheumatoid arthritis severity at enrollment, which was generally during the first trimester, and found that this did attenuate the association with preterm birth.
“Ideally, we would have measures of disease severity at the time of every medication start, stop, or dose change to account for time-varying confounding later in pregnancy,” they wrote.
The study did not find any effect of biologic or nonbiologic disease-modifying antirheumatic drugs, either before or after the first 140 days of gestation.
The authors also looked at pregnancy outcomes among women with inflammatory bowel disease and asthma who were taking corticosteroids for those conditions.
While noting that these estimates were “imprecise,” they did see the suggestion of an increase in preterm birth among women taking oral corticosteroids for asthma, especially when used in the first half of pregnancy. There was also a suggestion of increased preterm birth risk associated with high oral corticosteroid use for inflammatory bowel disease, but these estimates were unadjusted, they noted.
“Overall, IBD and asthma exploratory analyses align with the direction of the associations in the RA analysis despite limitations of precision and inability to adjust for IBD severity,” they wrote.
The conclusions to be drawn from the study are limited by its small size, the investigators noted, as well as a lack of information on the type of rheumatoid arthritis and longitudinal disease severity. They added that while the hypothesized mechanism of action linking oral corticosteroid use to preterm birth was subclinical intrauterine infection, they did not have access to placental pathology to confirm this.
The study was supported by the National Institutes of Health, and the MotherToBaby Pregnancy Studies are supported by research grants from a number of pharmaceutical companies. No other conflicts of interest were declared.
SOURCE: Palmsten K et al. Rheumatology 2019 Sep 30. doi: 10.1093/rheumatology/kez405.
Pregnant women taking oral corticosteroids for rheumatoid arthritis may be at increased risk of preterm birth, according to research published online Sept. 30 in Rheumatology.
A study of 528 pregnant women with rheumatoid arthritis enrolled in the MotherToBaby Pregnancy Studies found that those taking a daily dose of 10 mg or more of prednisone equivalent – representing a mean cumulative dose of 2,208.6 mg over the first 139 days of pregnancy – had 4.77-fold higher odds of preterm birth, compared with those not taking oral corticosteroids. Women on medium doses – with a mean cumulative dose of 883 mg – had 81% higher odds of preterm birth, while those on low cumulative doses of 264.9 mg showed a nonsignificant 38% increase in preterm birth risk.
Women who did not use oral corticosteroids before day 140 of pregnancy had a 2.2% risk of early preterm birth. Among women with low use of oral corticosteroids, the risk was 3.4%, among those with medium use the risk was 3.3%, but among those with high use the risk was 26.7%.
After day 140 of gestation, there was a nonsignificant 64% increase in the risk for preterm birth with any use of oral corticosteroids, compared with no use. But among women taking 10 mg or more of prednisone equivalent per day, the risk was 2.45-fold higher, whereas those taking under 10 mg showed no significant increase in risk.
“Systemic corticosteroid use has been associated with serious infection in pregnant women and serious and nonserious infection in individuals with autoimmune diseases, independent of other immunosuppressive medications, especially for doses of 10 mg of prednisone equivalent per day and greater,” wrote Kristin Palmsten, ScD, a research investigator with HealthPartners Institute in Minneapolis, Minn., and coauthors.
Given that intrauterine infection is believed to contribute to preterm birth, some have suggested that the immunosuppressive effects of oral corticosteroids could be associated with an increased risk of preterm birth because of subclinical intra-amniotic infection, they wrote.
However, they noted that there was a lack of information on the effect of dose and timing of oral corticosteroids during pregnancy on the risk of preterm birth.
The authors acknowledged that dosage of oral corticosteroids during pregnancy was linked to disease activity, which was itself associated with preterm birth risk. They adjusted for self-assessed rheumatoid arthritis severity at enrollment, which was generally during the first trimester, and found that this did attenuate the association with preterm birth.
“Ideally, we would have measures of disease severity at the time of every medication start, stop, or dose change to account for time-varying confounding later in pregnancy,” they wrote.
The study did not find any effect of biologic or nonbiologic disease-modifying antirheumatic drugs, either before or after the first 140 days of gestation.
The authors also looked at pregnancy outcomes among women with inflammatory bowel disease and asthma who were taking corticosteroids for those conditions.
While noting that these estimates were “imprecise,” they did see the suggestion of an increase in preterm birth among women taking oral corticosteroids for asthma, especially when used in the first half of pregnancy. There was also a suggestion of increased preterm birth risk associated with high oral corticosteroid use for inflammatory bowel disease, but these estimates were unadjusted, they noted.
“Overall, IBD and asthma exploratory analyses align with the direction of the associations in the RA analysis despite limitations of precision and inability to adjust for IBD severity,” they wrote.
The conclusions to be drawn from the study are limited by its small size, the investigators noted, as well as a lack of information on the type of rheumatoid arthritis and longitudinal disease severity. They added that while the hypothesized mechanism of action linking oral corticosteroid use to preterm birth was subclinical intrauterine infection, they did not have access to placental pathology to confirm this.
The study was supported by the National Institutes of Health, and the MotherToBaby Pregnancy Studies are supported by research grants from a number of pharmaceutical companies. No other conflicts of interest were declared.
SOURCE: Palmsten K et al. Rheumatology 2019 Sep 30. doi: 10.1093/rheumatology/kez405.
Pregnant women taking oral corticosteroids for rheumatoid arthritis may be at increased risk of preterm birth, according to research published online Sept. 30 in Rheumatology.
A study of 528 pregnant women with rheumatoid arthritis enrolled in the MotherToBaby Pregnancy Studies found that those taking a daily dose of 10 mg or more of prednisone equivalent – representing a mean cumulative dose of 2,208.6 mg over the first 139 days of pregnancy – had 4.77-fold higher odds of preterm birth, compared with those not taking oral corticosteroids. Women on medium doses – with a mean cumulative dose of 883 mg – had 81% higher odds of preterm birth, while those on low cumulative doses of 264.9 mg showed a nonsignificant 38% increase in preterm birth risk.
Women who did not use oral corticosteroids before day 140 of pregnancy had a 2.2% risk of early preterm birth. Among women with low use of oral corticosteroids, the risk was 3.4%, among those with medium use the risk was 3.3%, but among those with high use the risk was 26.7%.
After day 140 of gestation, there was a nonsignificant 64% increase in the risk for preterm birth with any use of oral corticosteroids, compared with no use. But among women taking 10 mg or more of prednisone equivalent per day, the risk was 2.45-fold higher, whereas those taking under 10 mg showed no significant increase in risk.
“Systemic corticosteroid use has been associated with serious infection in pregnant women and serious and nonserious infection in individuals with autoimmune diseases, independent of other immunosuppressive medications, especially for doses of 10 mg of prednisone equivalent per day and greater,” wrote Kristin Palmsten, ScD, a research investigator with HealthPartners Institute in Minneapolis, Minn., and coauthors.
Given that intrauterine infection is believed to contribute to preterm birth, some have suggested that the immunosuppressive effects of oral corticosteroids could be associated with an increased risk of preterm birth because of subclinical intra-amniotic infection, they wrote.
However, they noted that there was a lack of information on the effect of dose and timing of oral corticosteroids during pregnancy on the risk of preterm birth.
The authors acknowledged that dosage of oral corticosteroids during pregnancy was linked to disease activity, which was itself associated with preterm birth risk. They adjusted for self-assessed rheumatoid arthritis severity at enrollment, which was generally during the first trimester, and found that this did attenuate the association with preterm birth.
“Ideally, we would have measures of disease severity at the time of every medication start, stop, or dose change to account for time-varying confounding later in pregnancy,” they wrote.
The study did not find any effect of biologic or nonbiologic disease-modifying antirheumatic drugs, either before or after the first 140 days of gestation.
The authors also looked at pregnancy outcomes among women with inflammatory bowel disease and asthma who were taking corticosteroids for those conditions.
While noting that these estimates were “imprecise,” they did see the suggestion of an increase in preterm birth among women taking oral corticosteroids for asthma, especially when used in the first half of pregnancy. There was also a suggestion of increased preterm birth risk associated with high oral corticosteroid use for inflammatory bowel disease, but these estimates were unadjusted, they noted.
“Overall, IBD and asthma exploratory analyses align with the direction of the associations in the RA analysis despite limitations of precision and inability to adjust for IBD severity,” they wrote.
The conclusions to be drawn from the study are limited by its small size, the investigators noted, as well as a lack of information on the type of rheumatoid arthritis and longitudinal disease severity. They added that while the hypothesized mechanism of action linking oral corticosteroid use to preterm birth was subclinical intrauterine infection, they did not have access to placental pathology to confirm this.
The study was supported by the National Institutes of Health, and the MotherToBaby Pregnancy Studies are supported by research grants from a number of pharmaceutical companies. No other conflicts of interest were declared.
SOURCE: Palmsten K et al. Rheumatology 2019 Sep 30. doi: 10.1093/rheumatology/kez405.
FROM RHEUMATOLOGY
Clinical Presentation of Rheumatoid Arthritis
Inflammatory arthritis induced by ICIs can persist after therapy
according to a new study of long-term outcomes of immune-related adverse events published in Annals of the Rheumatic Diseases.
“This study is one of the largest longitudinal reports to date of patients with ICI-induced IA and the first to evaluate persistence of ICI-induced IA and identify influential factors on outcome,” wrote Tawnie J. Braaten, MD, and coauthors. “Continued clinical and translational investigation on larger longitudinal cohorts will allow for increased understanding of pathophysiology and determination of the best clinical care for patients with ICI-induced IA.”
Dr. Braaten conducted the study at Johns Hopkins University, Baltimore, when she was a postdoctoral fellow there, and she is now in the division of rheumatology at the University of Utah, Salt Lake City.
To determine how long IA can persist after patients cease ICI therapy, along with factors associated with its persistence, the researchers studied 60 patients who were referred to the Johns Hopkins Arthritis Center for IA caused by ICIs. The patients – 32 females and 28 males – had a median follow-up of 9 months after ICI cessation.
Of the 51 patients with 3-month follow-up data, 70.6% had active IA. Of the 41 patients with 6-month follow-up data, 48.8% had active IA. All told, 53.3% of patients had active IA at their last follow-up visit, which occurred anywhere from 1 to 24 months after stopping ICI therapy.
According to univariable analysis, arthritis was less likely to improve in patients with a longer duration of ICI exposure (hazard ratio, 0.93; 95% confidence interval, 0.87-0.99; P = .02), in patients receiving combination ICI therapy (HR, 0.29; 95% CI, 0.12-0.72; P = .008) and in patients with a history of other immune-related adverse events (HR, 0.61; 95% CI, 0.39-0.95; P = .03).
The authors acknowledged their study’s limitations, including a potential selection bias for symptomatic individuals and the possibility that persistent IA sufferers may have pursued follow-up for longer periods of time. In addition, they noted that some patients were omitted from analysis if they were on a blinded clinical trial or had been receiving an investigational immunotherapy agent.
The study was funded via a grant from Bristol-Myers Squibb, an arthritis fellowship award from AbbVie, and additional financial support from the Camille Julia Morgan Arthritis Research and Education Fund, the Jerome L. Greene Foundation, and the National Institutes of Health. The authors reported various conflicts of interest, including receiving honoraria, grants, and research funding from numerous pharmaceutical companies.
SOURCE: Braaten TJ et al. Ann Rheum Dis. 2019 Sep 20. doi: 10.1136/annrheumdis-2019-216109.
according to a new study of long-term outcomes of immune-related adverse events published in Annals of the Rheumatic Diseases.
“This study is one of the largest longitudinal reports to date of patients with ICI-induced IA and the first to evaluate persistence of ICI-induced IA and identify influential factors on outcome,” wrote Tawnie J. Braaten, MD, and coauthors. “Continued clinical and translational investigation on larger longitudinal cohorts will allow for increased understanding of pathophysiology and determination of the best clinical care for patients with ICI-induced IA.”
Dr. Braaten conducted the study at Johns Hopkins University, Baltimore, when she was a postdoctoral fellow there, and she is now in the division of rheumatology at the University of Utah, Salt Lake City.
To determine how long IA can persist after patients cease ICI therapy, along with factors associated with its persistence, the researchers studied 60 patients who were referred to the Johns Hopkins Arthritis Center for IA caused by ICIs. The patients – 32 females and 28 males – had a median follow-up of 9 months after ICI cessation.
Of the 51 patients with 3-month follow-up data, 70.6% had active IA. Of the 41 patients with 6-month follow-up data, 48.8% had active IA. All told, 53.3% of patients had active IA at their last follow-up visit, which occurred anywhere from 1 to 24 months after stopping ICI therapy.
According to univariable analysis, arthritis was less likely to improve in patients with a longer duration of ICI exposure (hazard ratio, 0.93; 95% confidence interval, 0.87-0.99; P = .02), in patients receiving combination ICI therapy (HR, 0.29; 95% CI, 0.12-0.72; P = .008) and in patients with a history of other immune-related adverse events (HR, 0.61; 95% CI, 0.39-0.95; P = .03).
The authors acknowledged their study’s limitations, including a potential selection bias for symptomatic individuals and the possibility that persistent IA sufferers may have pursued follow-up for longer periods of time. In addition, they noted that some patients were omitted from analysis if they were on a blinded clinical trial or had been receiving an investigational immunotherapy agent.
The study was funded via a grant from Bristol-Myers Squibb, an arthritis fellowship award from AbbVie, and additional financial support from the Camille Julia Morgan Arthritis Research and Education Fund, the Jerome L. Greene Foundation, and the National Institutes of Health. The authors reported various conflicts of interest, including receiving honoraria, grants, and research funding from numerous pharmaceutical companies.
SOURCE: Braaten TJ et al. Ann Rheum Dis. 2019 Sep 20. doi: 10.1136/annrheumdis-2019-216109.
according to a new study of long-term outcomes of immune-related adverse events published in Annals of the Rheumatic Diseases.
“This study is one of the largest longitudinal reports to date of patients with ICI-induced IA and the first to evaluate persistence of ICI-induced IA and identify influential factors on outcome,” wrote Tawnie J. Braaten, MD, and coauthors. “Continued clinical and translational investigation on larger longitudinal cohorts will allow for increased understanding of pathophysiology and determination of the best clinical care for patients with ICI-induced IA.”
Dr. Braaten conducted the study at Johns Hopkins University, Baltimore, when she was a postdoctoral fellow there, and she is now in the division of rheumatology at the University of Utah, Salt Lake City.
To determine how long IA can persist after patients cease ICI therapy, along with factors associated with its persistence, the researchers studied 60 patients who were referred to the Johns Hopkins Arthritis Center for IA caused by ICIs. The patients – 32 females and 28 males – had a median follow-up of 9 months after ICI cessation.
Of the 51 patients with 3-month follow-up data, 70.6% had active IA. Of the 41 patients with 6-month follow-up data, 48.8% had active IA. All told, 53.3% of patients had active IA at their last follow-up visit, which occurred anywhere from 1 to 24 months after stopping ICI therapy.
According to univariable analysis, arthritis was less likely to improve in patients with a longer duration of ICI exposure (hazard ratio, 0.93; 95% confidence interval, 0.87-0.99; P = .02), in patients receiving combination ICI therapy (HR, 0.29; 95% CI, 0.12-0.72; P = .008) and in patients with a history of other immune-related adverse events (HR, 0.61; 95% CI, 0.39-0.95; P = .03).
The authors acknowledged their study’s limitations, including a potential selection bias for symptomatic individuals and the possibility that persistent IA sufferers may have pursued follow-up for longer periods of time. In addition, they noted that some patients were omitted from analysis if they were on a blinded clinical trial or had been receiving an investigational immunotherapy agent.
The study was funded via a grant from Bristol-Myers Squibb, an arthritis fellowship award from AbbVie, and additional financial support from the Camille Julia Morgan Arthritis Research and Education Fund, the Jerome L. Greene Foundation, and the National Institutes of Health. The authors reported various conflicts of interest, including receiving honoraria, grants, and research funding from numerous pharmaceutical companies.
SOURCE: Braaten TJ et al. Ann Rheum Dis. 2019 Sep 20. doi: 10.1136/annrheumdis-2019-216109.
FROM ANNALS OF THE RHEUMATIC DISEASES
Overview and Pathophysiology of Rheumatoid Arthritis
Prior authorizations for infusibles cause delays, toxicities
Rheumatologist Zachary S. Wallace, MD, knew just how prior authorization requirements were impacting his staff time and work flow when he embarked on a study several years ago. Managing authorizations for infusible medications alone was about to become a full-time job for one of the administrative assistants in the rheumatology unit at Massachusetts General Hospital in Boston.
His research questions concerned patients. “There’s a lot of talk about how much onus prior authorization requirements put on providers and the practice,” Dr. Wallace said. ”I was interested in understanding what impact [these requirements] have on patients themselves.”
Dr. Wallace led a review of the EHRs of 225 patients for whom an infusible medication such as rituximab and infliximab was ordered by 1 of the 16 physicians in the rheumatology unit between July 2016 and June 2018. The findings – that patients who needed prior authorizations for infusible medications had a significantly longer time to treatment initiation and higher prednisone-equivalent glucocorticoid exposure – were reported online in Arthritis Care & Research.
Among patients whose authorizations were initially denied, these differences were “pretty drastic,” Dr. Wallace said. The median time to receiving a first infusion was 50 days, compared with 27 days when permission was not required, and glucocorticoid exposure during the 3 months following the request was 605 mg versus 160 mg.
Among patients whose authorizations were not denied, the median time to first infusion was 31 days, compared with 27 days when authorization was not required, and the mean glucocorticoid exposure over 3 months was 364 mg versus 160 mg.
“I hope that our findings will help facilitate discussions with insurance providers, pharmacy benefit managers, and state and federal legislators about the need to address the impact that prior authorization requirements have on patients and providers,” said Dr. Wallace, also of the clinical epidemiology program in the division of rheumatology, allergy and immunology at Massachusetts General, and an assistant professor of medicine at Harvard Medical School, Boston.
Of the 225 patients for whom an infusible medication was ordered, 71% required preauthorization. Of these, 79% were approved and 21% were denied after the first request. And in a finding that Dr. Wallace called “somewhat surprising,” 82% of the authorizations originally denied were approved after appeal.
All told, prior authorizations for infusible medications were eventually approved in all but a small number of cases. “We go through all this effort to get these prior authorizations approved, and 96% of the time, they were ultimately approved,” he said in an interview.
Christopher Phillips, MD, a community rheumatologist in Paducah, Ky., who serves as chair of the insurance subcommittee of the American College of Rheumatology’s committee on rheumatologic care, said the findings “give further credence” to rheumatologists’ concerns. “We know [from our own experiences] that prior authorizations delay care, and we know that delays can cause harm to patients. We now have hard data backing up this assertion.”
Regarding the high number of authorization approvals, “there’s an argument to be made that for certain treatments and certain conditions where the success rate of appeals is high enough, you shouldn’t be subjecting these treatments to these [preauthorization] policies,” he said.
Calls for prior authorization reform
Most patients in the study (71%) had private insurance. But the findings also have implications for Medicare, Dr. Wallace said, as recent federal policies have expanded Medicare Advantage plans’ authority to use prior authorization in conjunction with step therapy for medications administered under Part B. Step therapy favors primary use of what insurers deem the most cost-effective therapies.
The ACR is one of almost 370 physician, patient, and health care organizations that are urging Congress to pass a bipartisan bill aimed at streamlining and standardizing prior authorization under the Medicare Advantage program. The legislation – Improving Seniors’ Timely Access to Care Act of 2019 (H.R. 3107) – was introduced by Reps. Suzan DelBene (D-Wash.), Mike Kelly (R-Pa.), Roger Marshall, MD (R-Kan.), and Ami Bera, MD (D-Calif.).
The bill calls for the creation of an electronic prior authorization program and a “real-time process for items and services that are routinely approved,” as well as greater Centers for Medicare & Medicaid Services oversight on how Medicare Advantage plans use prior authorization. Plans would be required to report to the CMS on the extent of their use of prior authorization and the rate of approvals or denials. They would also be held accountable for making timely prior authorization determinations and providing rationales for denials, according to a letter to Congress cosigned by the ACR.
In a press release about the legislation, Paula Marchetta, MD, president of the ACR, said that “the unregulated use of prior authorization has devolved into a time-consuming and obstructive process that often stalls or outright revokes patient access to medically necessary therapies.” She added that “many health care plans now use prior authorization indiscriminately.”
Cathryn Donaldson, director of communications for America’s Health Insurance Plans (AHIP), said in an email that prior authorization is used for less than 15% of covered services, and that, along with step therapy, it “helps ensure that patients receive care that is safe, effective, and necessary.” AHIP “knows that prior authorization can be improved,” she said, and is committed to streamlining the process.
A demonstration project on the automation of various parts of prior authorization is being coordinated with health information technology companies, plans, and providers, she noted.
The federal legislation is based at least partly on a consensus statement drafted by AHIP, the American Medical Association, and four other organizations representing hospitals, medical groups, and health plans on ways to improve the prior authorization process. Among the items mentioned in the statement is that “regular review” of services subject to prior authorization could help identify therapies that “no longer warrant” prior authorization because of low denial rates.
Outside of Medicare Advantage, the AMA is aware of at least 85 bills being introduced in states this year that address utilization management in commercial plans. Nearly all these bills attempt to reform prior authorization programs in some way, according to R. J. Mills, media relations coordinator for the AMA.
Rheumatologic patients hard hit
Off-label medication use was the most common reason (82%) for a prior authorization denial in the Massachusetts General study, even though 78% of the patients for whom infusible medications were prescribed had a condition with no Food and Drug Administration–approved treatment. Having such a condition was associated with 120% or 190% higher odds of having a denial in unadjusted and adjusted (for age and sex) analyses, Dr. Wallace and colleagues reported.
Moreover, nearly half (48%) of the patients with denials had already tried or were currently taking an oral disease-modifying antirheumatic drug, such as methotrexate.
The majority of denials were for the use of rituximab (70%), followed by infliximab (12%) and tocilizumab (12%). Most of the denials (79%) were appealed successfully through a peer-to-peer discussion. In five cases, the insurer’s preferred drug (for example, adalimumab) had to be used rather than the requested infusion (for example, infliximab).
Infused medications, many of which are biologics, are among the most expensive drugs prescribed for patients with rheumatic diseases. They were easiest for Dr. Wallace to study because of the way prior authorizations are handled in his unit, but prior authorization requirements are “widespread” in rheumatology practices across treatment types, he and Dr. Phillips said.
“Some of our relatively inexpensive treatments are subject to prior authorization requirements,” Dr. Phillips said. “We hear stories about prednisone needing a prior authorization sometimes.”
With respect to infusible medications, the insurance subcommittee is hearing from ACR members about seemingly increasing numbers of both clinical coverage reviews – for example, reviews of prior treatments – and site-of-care restrictions, Dr. Phillips noted. “Some carriers are insisting on infusions in non-hospital-based settings, for cost savings, or on home infusions, which are concerning because of [possible] infusion reactions and medical service availability.”
The application of step therapy to rheumatologic patients is troubling because of the “often unique medical circumstances of the patient,” Dr. Phillips said. “There are enough differences among the [tumor necrosis factor] antagonists, for instance, that make one more appropriate for a certain patient than another. Those differences are not brought into consideration with these policies.”
There are other ways in which prior authorization processes “are not well informed medically,” he said, recalling a case brought to the attention of the subcommittee in which a patient prescribed a biologic drug for psoriatic arthritis was denied authorization because “the documentation did not include a [disease activity measure] that is specific to RA and not used for psoriatic arthritis.”
It is not uncommon for authorizations for infusible medications to take 2 weeks or longer to secure – even when initially approved. In the AMA’s 2018 Prior Authorization Physician Survey, 65% reported waiting at least 1 business day for a decision and 26% reported waiting at least 3 business days for responses. “With infusibles, we’re absolutely dealing with a much longer time,” Dr. Phillips said.
In Dr. Wallace’s study, the finding that prior authorizations facilitated greater prednisone-equivalent glucocorticoid exposure is important, he and his colleagues wrote, because these medications may put patients at higher risk of infection, cardiovascular disease, and diabetes – even in low doses and with short-term use. Notably, the median delay to the initiation of treatment was 29 days, regardless of prior authorization requirements. Dr. Wallace said the delays “likely reflect a combination of factors” – including infusion center waiting lists and patient-level factors – and that his team is “thinking about how to facilitate better access [to their practice’s infusion center] for those who are approved for treatment.”
The most common conditions for which infused medication was ordered were inflammatory arthritis (32%), vasculitis (23%), and IgG4-related disease (17%). The 225 patients in the study had an average age of 53 years.
Dr. Wallace reported that he has no relevant financial disclosures.
SOURCE: Wallace ZS et al. Arthritis Care Res. 2019 Sep 10. doi: 10.1002/acr.24062.
Rheumatologist Zachary S. Wallace, MD, knew just how prior authorization requirements were impacting his staff time and work flow when he embarked on a study several years ago. Managing authorizations for infusible medications alone was about to become a full-time job for one of the administrative assistants in the rheumatology unit at Massachusetts General Hospital in Boston.
His research questions concerned patients. “There’s a lot of talk about how much onus prior authorization requirements put on providers and the practice,” Dr. Wallace said. ”I was interested in understanding what impact [these requirements] have on patients themselves.”
Dr. Wallace led a review of the EHRs of 225 patients for whom an infusible medication such as rituximab and infliximab was ordered by 1 of the 16 physicians in the rheumatology unit between July 2016 and June 2018. The findings – that patients who needed prior authorizations for infusible medications had a significantly longer time to treatment initiation and higher prednisone-equivalent glucocorticoid exposure – were reported online in Arthritis Care & Research.
Among patients whose authorizations were initially denied, these differences were “pretty drastic,” Dr. Wallace said. The median time to receiving a first infusion was 50 days, compared with 27 days when permission was not required, and glucocorticoid exposure during the 3 months following the request was 605 mg versus 160 mg.
Among patients whose authorizations were not denied, the median time to first infusion was 31 days, compared with 27 days when authorization was not required, and the mean glucocorticoid exposure over 3 months was 364 mg versus 160 mg.
“I hope that our findings will help facilitate discussions with insurance providers, pharmacy benefit managers, and state and federal legislators about the need to address the impact that prior authorization requirements have on patients and providers,” said Dr. Wallace, also of the clinical epidemiology program in the division of rheumatology, allergy and immunology at Massachusetts General, and an assistant professor of medicine at Harvard Medical School, Boston.
Of the 225 patients for whom an infusible medication was ordered, 71% required preauthorization. Of these, 79% were approved and 21% were denied after the first request. And in a finding that Dr. Wallace called “somewhat surprising,” 82% of the authorizations originally denied were approved after appeal.
All told, prior authorizations for infusible medications were eventually approved in all but a small number of cases. “We go through all this effort to get these prior authorizations approved, and 96% of the time, they were ultimately approved,” he said in an interview.
Christopher Phillips, MD, a community rheumatologist in Paducah, Ky., who serves as chair of the insurance subcommittee of the American College of Rheumatology’s committee on rheumatologic care, said the findings “give further credence” to rheumatologists’ concerns. “We know [from our own experiences] that prior authorizations delay care, and we know that delays can cause harm to patients. We now have hard data backing up this assertion.”
Regarding the high number of authorization approvals, “there’s an argument to be made that for certain treatments and certain conditions where the success rate of appeals is high enough, you shouldn’t be subjecting these treatments to these [preauthorization] policies,” he said.
Calls for prior authorization reform
Most patients in the study (71%) had private insurance. But the findings also have implications for Medicare, Dr. Wallace said, as recent federal policies have expanded Medicare Advantage plans’ authority to use prior authorization in conjunction with step therapy for medications administered under Part B. Step therapy favors primary use of what insurers deem the most cost-effective therapies.
The ACR is one of almost 370 physician, patient, and health care organizations that are urging Congress to pass a bipartisan bill aimed at streamlining and standardizing prior authorization under the Medicare Advantage program. The legislation – Improving Seniors’ Timely Access to Care Act of 2019 (H.R. 3107) – was introduced by Reps. Suzan DelBene (D-Wash.), Mike Kelly (R-Pa.), Roger Marshall, MD (R-Kan.), and Ami Bera, MD (D-Calif.).
The bill calls for the creation of an electronic prior authorization program and a “real-time process for items and services that are routinely approved,” as well as greater Centers for Medicare & Medicaid Services oversight on how Medicare Advantage plans use prior authorization. Plans would be required to report to the CMS on the extent of their use of prior authorization and the rate of approvals or denials. They would also be held accountable for making timely prior authorization determinations and providing rationales for denials, according to a letter to Congress cosigned by the ACR.
In a press release about the legislation, Paula Marchetta, MD, president of the ACR, said that “the unregulated use of prior authorization has devolved into a time-consuming and obstructive process that often stalls or outright revokes patient access to medically necessary therapies.” She added that “many health care plans now use prior authorization indiscriminately.”
Cathryn Donaldson, director of communications for America’s Health Insurance Plans (AHIP), said in an email that prior authorization is used for less than 15% of covered services, and that, along with step therapy, it “helps ensure that patients receive care that is safe, effective, and necessary.” AHIP “knows that prior authorization can be improved,” she said, and is committed to streamlining the process.
A demonstration project on the automation of various parts of prior authorization is being coordinated with health information technology companies, plans, and providers, she noted.
The federal legislation is based at least partly on a consensus statement drafted by AHIP, the American Medical Association, and four other organizations representing hospitals, medical groups, and health plans on ways to improve the prior authorization process. Among the items mentioned in the statement is that “regular review” of services subject to prior authorization could help identify therapies that “no longer warrant” prior authorization because of low denial rates.
Outside of Medicare Advantage, the AMA is aware of at least 85 bills being introduced in states this year that address utilization management in commercial plans. Nearly all these bills attempt to reform prior authorization programs in some way, according to R. J. Mills, media relations coordinator for the AMA.
Rheumatologic patients hard hit
Off-label medication use was the most common reason (82%) for a prior authorization denial in the Massachusetts General study, even though 78% of the patients for whom infusible medications were prescribed had a condition with no Food and Drug Administration–approved treatment. Having such a condition was associated with 120% or 190% higher odds of having a denial in unadjusted and adjusted (for age and sex) analyses, Dr. Wallace and colleagues reported.
Moreover, nearly half (48%) of the patients with denials had already tried or were currently taking an oral disease-modifying antirheumatic drug, such as methotrexate.
The majority of denials were for the use of rituximab (70%), followed by infliximab (12%) and tocilizumab (12%). Most of the denials (79%) were appealed successfully through a peer-to-peer discussion. In five cases, the insurer’s preferred drug (for example, adalimumab) had to be used rather than the requested infusion (for example, infliximab).
Infused medications, many of which are biologics, are among the most expensive drugs prescribed for patients with rheumatic diseases. They were easiest for Dr. Wallace to study because of the way prior authorizations are handled in his unit, but prior authorization requirements are “widespread” in rheumatology practices across treatment types, he and Dr. Phillips said.
“Some of our relatively inexpensive treatments are subject to prior authorization requirements,” Dr. Phillips said. “We hear stories about prednisone needing a prior authorization sometimes.”
With respect to infusible medications, the insurance subcommittee is hearing from ACR members about seemingly increasing numbers of both clinical coverage reviews – for example, reviews of prior treatments – and site-of-care restrictions, Dr. Phillips noted. “Some carriers are insisting on infusions in non-hospital-based settings, for cost savings, or on home infusions, which are concerning because of [possible] infusion reactions and medical service availability.”
The application of step therapy to rheumatologic patients is troubling because of the “often unique medical circumstances of the patient,” Dr. Phillips said. “There are enough differences among the [tumor necrosis factor] antagonists, for instance, that make one more appropriate for a certain patient than another. Those differences are not brought into consideration with these policies.”
There are other ways in which prior authorization processes “are not well informed medically,” he said, recalling a case brought to the attention of the subcommittee in which a patient prescribed a biologic drug for psoriatic arthritis was denied authorization because “the documentation did not include a [disease activity measure] that is specific to RA and not used for psoriatic arthritis.”
It is not uncommon for authorizations for infusible medications to take 2 weeks or longer to secure – even when initially approved. In the AMA’s 2018 Prior Authorization Physician Survey, 65% reported waiting at least 1 business day for a decision and 26% reported waiting at least 3 business days for responses. “With infusibles, we’re absolutely dealing with a much longer time,” Dr. Phillips said.
In Dr. Wallace’s study, the finding that prior authorizations facilitated greater prednisone-equivalent glucocorticoid exposure is important, he and his colleagues wrote, because these medications may put patients at higher risk of infection, cardiovascular disease, and diabetes – even in low doses and with short-term use. Notably, the median delay to the initiation of treatment was 29 days, regardless of prior authorization requirements. Dr. Wallace said the delays “likely reflect a combination of factors” – including infusion center waiting lists and patient-level factors – and that his team is “thinking about how to facilitate better access [to their practice’s infusion center] for those who are approved for treatment.”
The most common conditions for which infused medication was ordered were inflammatory arthritis (32%), vasculitis (23%), and IgG4-related disease (17%). The 225 patients in the study had an average age of 53 years.
Dr. Wallace reported that he has no relevant financial disclosures.
SOURCE: Wallace ZS et al. Arthritis Care Res. 2019 Sep 10. doi: 10.1002/acr.24062.
Rheumatologist Zachary S. Wallace, MD, knew just how prior authorization requirements were impacting his staff time and work flow when he embarked on a study several years ago. Managing authorizations for infusible medications alone was about to become a full-time job for one of the administrative assistants in the rheumatology unit at Massachusetts General Hospital in Boston.
His research questions concerned patients. “There’s a lot of talk about how much onus prior authorization requirements put on providers and the practice,” Dr. Wallace said. ”I was interested in understanding what impact [these requirements] have on patients themselves.”
Dr. Wallace led a review of the EHRs of 225 patients for whom an infusible medication such as rituximab and infliximab was ordered by 1 of the 16 physicians in the rheumatology unit between July 2016 and June 2018. The findings – that patients who needed prior authorizations for infusible medications had a significantly longer time to treatment initiation and higher prednisone-equivalent glucocorticoid exposure – were reported online in Arthritis Care & Research.
Among patients whose authorizations were initially denied, these differences were “pretty drastic,” Dr. Wallace said. The median time to receiving a first infusion was 50 days, compared with 27 days when permission was not required, and glucocorticoid exposure during the 3 months following the request was 605 mg versus 160 mg.
Among patients whose authorizations were not denied, the median time to first infusion was 31 days, compared with 27 days when authorization was not required, and the mean glucocorticoid exposure over 3 months was 364 mg versus 160 mg.
“I hope that our findings will help facilitate discussions with insurance providers, pharmacy benefit managers, and state and federal legislators about the need to address the impact that prior authorization requirements have on patients and providers,” said Dr. Wallace, also of the clinical epidemiology program in the division of rheumatology, allergy and immunology at Massachusetts General, and an assistant professor of medicine at Harvard Medical School, Boston.
Of the 225 patients for whom an infusible medication was ordered, 71% required preauthorization. Of these, 79% were approved and 21% were denied after the first request. And in a finding that Dr. Wallace called “somewhat surprising,” 82% of the authorizations originally denied were approved after appeal.
All told, prior authorizations for infusible medications were eventually approved in all but a small number of cases. “We go through all this effort to get these prior authorizations approved, and 96% of the time, they were ultimately approved,” he said in an interview.
Christopher Phillips, MD, a community rheumatologist in Paducah, Ky., who serves as chair of the insurance subcommittee of the American College of Rheumatology’s committee on rheumatologic care, said the findings “give further credence” to rheumatologists’ concerns. “We know [from our own experiences] that prior authorizations delay care, and we know that delays can cause harm to patients. We now have hard data backing up this assertion.”
Regarding the high number of authorization approvals, “there’s an argument to be made that for certain treatments and certain conditions where the success rate of appeals is high enough, you shouldn’t be subjecting these treatments to these [preauthorization] policies,” he said.
Calls for prior authorization reform
Most patients in the study (71%) had private insurance. But the findings also have implications for Medicare, Dr. Wallace said, as recent federal policies have expanded Medicare Advantage plans’ authority to use prior authorization in conjunction with step therapy for medications administered under Part B. Step therapy favors primary use of what insurers deem the most cost-effective therapies.
The ACR is one of almost 370 physician, patient, and health care organizations that are urging Congress to pass a bipartisan bill aimed at streamlining and standardizing prior authorization under the Medicare Advantage program. The legislation – Improving Seniors’ Timely Access to Care Act of 2019 (H.R. 3107) – was introduced by Reps. Suzan DelBene (D-Wash.), Mike Kelly (R-Pa.), Roger Marshall, MD (R-Kan.), and Ami Bera, MD (D-Calif.).
The bill calls for the creation of an electronic prior authorization program and a “real-time process for items and services that are routinely approved,” as well as greater Centers for Medicare & Medicaid Services oversight on how Medicare Advantage plans use prior authorization. Plans would be required to report to the CMS on the extent of their use of prior authorization and the rate of approvals or denials. They would also be held accountable for making timely prior authorization determinations and providing rationales for denials, according to a letter to Congress cosigned by the ACR.
In a press release about the legislation, Paula Marchetta, MD, president of the ACR, said that “the unregulated use of prior authorization has devolved into a time-consuming and obstructive process that often stalls or outright revokes patient access to medically necessary therapies.” She added that “many health care plans now use prior authorization indiscriminately.”
Cathryn Donaldson, director of communications for America’s Health Insurance Plans (AHIP), said in an email that prior authorization is used for less than 15% of covered services, and that, along with step therapy, it “helps ensure that patients receive care that is safe, effective, and necessary.” AHIP “knows that prior authorization can be improved,” she said, and is committed to streamlining the process.
A demonstration project on the automation of various parts of prior authorization is being coordinated with health information technology companies, plans, and providers, she noted.
The federal legislation is based at least partly on a consensus statement drafted by AHIP, the American Medical Association, and four other organizations representing hospitals, medical groups, and health plans on ways to improve the prior authorization process. Among the items mentioned in the statement is that “regular review” of services subject to prior authorization could help identify therapies that “no longer warrant” prior authorization because of low denial rates.
Outside of Medicare Advantage, the AMA is aware of at least 85 bills being introduced in states this year that address utilization management in commercial plans. Nearly all these bills attempt to reform prior authorization programs in some way, according to R. J. Mills, media relations coordinator for the AMA.
Rheumatologic patients hard hit
Off-label medication use was the most common reason (82%) for a prior authorization denial in the Massachusetts General study, even though 78% of the patients for whom infusible medications were prescribed had a condition with no Food and Drug Administration–approved treatment. Having such a condition was associated with 120% or 190% higher odds of having a denial in unadjusted and adjusted (for age and sex) analyses, Dr. Wallace and colleagues reported.
Moreover, nearly half (48%) of the patients with denials had already tried or were currently taking an oral disease-modifying antirheumatic drug, such as methotrexate.
The majority of denials were for the use of rituximab (70%), followed by infliximab (12%) and tocilizumab (12%). Most of the denials (79%) were appealed successfully through a peer-to-peer discussion. In five cases, the insurer’s preferred drug (for example, adalimumab) had to be used rather than the requested infusion (for example, infliximab).
Infused medications, many of which are biologics, are among the most expensive drugs prescribed for patients with rheumatic diseases. They were easiest for Dr. Wallace to study because of the way prior authorizations are handled in his unit, but prior authorization requirements are “widespread” in rheumatology practices across treatment types, he and Dr. Phillips said.
“Some of our relatively inexpensive treatments are subject to prior authorization requirements,” Dr. Phillips said. “We hear stories about prednisone needing a prior authorization sometimes.”
With respect to infusible medications, the insurance subcommittee is hearing from ACR members about seemingly increasing numbers of both clinical coverage reviews – for example, reviews of prior treatments – and site-of-care restrictions, Dr. Phillips noted. “Some carriers are insisting on infusions in non-hospital-based settings, for cost savings, or on home infusions, which are concerning because of [possible] infusion reactions and medical service availability.”
The application of step therapy to rheumatologic patients is troubling because of the “often unique medical circumstances of the patient,” Dr. Phillips said. “There are enough differences among the [tumor necrosis factor] antagonists, for instance, that make one more appropriate for a certain patient than another. Those differences are not brought into consideration with these policies.”
There are other ways in which prior authorization processes “are not well informed medically,” he said, recalling a case brought to the attention of the subcommittee in which a patient prescribed a biologic drug for psoriatic arthritis was denied authorization because “the documentation did not include a [disease activity measure] that is specific to RA and not used for psoriatic arthritis.”
It is not uncommon for authorizations for infusible medications to take 2 weeks or longer to secure – even when initially approved. In the AMA’s 2018 Prior Authorization Physician Survey, 65% reported waiting at least 1 business day for a decision and 26% reported waiting at least 3 business days for responses. “With infusibles, we’re absolutely dealing with a much longer time,” Dr. Phillips said.
In Dr. Wallace’s study, the finding that prior authorizations facilitated greater prednisone-equivalent glucocorticoid exposure is important, he and his colleagues wrote, because these medications may put patients at higher risk of infection, cardiovascular disease, and diabetes – even in low doses and with short-term use. Notably, the median delay to the initiation of treatment was 29 days, regardless of prior authorization requirements. Dr. Wallace said the delays “likely reflect a combination of factors” – including infusion center waiting lists and patient-level factors – and that his team is “thinking about how to facilitate better access [to their practice’s infusion center] for those who are approved for treatment.”
The most common conditions for which infused medication was ordered were inflammatory arthritis (32%), vasculitis (23%), and IgG4-related disease (17%). The 225 patients in the study had an average age of 53 years.
Dr. Wallace reported that he has no relevant financial disclosures.
SOURCE: Wallace ZS et al. Arthritis Care Res. 2019 Sep 10. doi: 10.1002/acr.24062.
FROM ARTHRITIS CARE & RESEARCH
ACR looks for continued growth in awareness of rheumatic diseases with latest annual campaign
With the help of a key celebrity spokesperson, the American College of Rheumatology is hoping Rheumatic Disease Awareness Month (RDAM) will continue to raise the profile of the related illnesses and help more patients recognize their symptoms and get on the road to treatment.
This year, the celebrity tapped to lead the now-annual campaign that began in September 2016 is tennis champion Venus Williams, who has been competing while battling Sjögren’s syndrome. She was diagnosed in 2011.
Having a high-profile individual like Venus Williams will “bring more awareness to not a specific illness but just rheumatic diseases in general,” Suleman Bhana, MD, chair of the ACR’s marketing and communications committee, said in an interview, adding that it is a way to help get attention from patients and their family members to be more aware of what they are facing when afflicted with a rheumatic disease.
It also helps to drive traffic to a website (simpletasks.org) set up to help build a community around rheumatic diseases, he noted.
“By signing up on the website, patients and others can get connected with wellness articles and can be informed about any advocacy opportunities in their local area or the national level,” said Dr. Bhana, a rheumatologist with Crystal Run Healthcare in Middletown, N.Y. “It helps us a lot from an ACR point of view just by getting the word out about rheumatic diseases.”
Dr. Bhana said that part of the success of the RDAM campaign is being measured by the number of people who sign up for the website. “Last year we had about 1,600 people sign up, which was more than double from the previous year and we are hoping to do even better this year now that we have Venus Williams as our spokesperson.”
But the campaign involves more than just a celebrity spokesperson and an online community to build awareness for rheumatic diseases and their associated symptoms and treatments. The ACR is working with patients to make sure their voices are heard on Capitol Hill to help ensure proper access to medical care and those treatments.
This year, the ACR – along with more than 100 advocates – met with members of Congress to push for policies that will benefit people with rheumatic diseases. One key item on the agenda targets the use of step therapy.
“This year the ACR’s Government Affairs Committee had a list of several pieces of legislation that they are looking at heavily. [One bill is about] having brakes on what is called step therapy, which is a fairly egregious practice by many insurers that forces patients to use different medications than what the physician and patient decide in their mutual doctor-patient relationship before they can use the medicine that was originally decided upon,” he said. “The reasons for this practice mostly are financial in that insurers get kickbacks from certain pharmaceutical companies, and that is what they term as preferred drugs. It’s a lot more than just cost savings. It’s about the insurers financially benefiting from patients rather than doing the right thing and preserving the doctor-patient relationship.”
One bill in particular that has been highlighted by the ACR is the Safe Step Act of 2019 (H.R. 2279), introduced by Rep. Raul Ruiz, MD (D-Calif.), and Rep. Brad Wenstrup, DPM (R-Ohio), would put limits on step therapy and create a clear process for patients and physicians to seek exemptions.
Other legislative actions that patients and the ACR were advocating for during the visits for RDAM included the EMPOWER for Health Act (H.R. 2781), which would help increase the number of pediatric subspecialists, including pediatric rheumatologists, through loan repayment for health professionals who agree to work at least 2 years in pediatric medicine, and the REDI Act (H.R. 1554), which would defer the accumulation on student loan interest while future doctors serve in a medical internship or residency program.
“The reality of pediatrics is that they don’t get well compensated as a pediatric rheumatologist subspecialist, so there is an ultimate financial disincentive for training pediatricians to go into rheumatology,” Dr. Bhana said. “So we are trying to get support for loan repayment for pediatric rheumatologists or rheumatology fellowship programs, which may help to incentivize young pediatricians to go into rheumatology to at least expand access to care.”
The RDAM campaign also provides useful contacts for an annual survey that the ACR conducts. This year’s survey found that access issues for both physicians and treatments persist, informing the agenda for the advocacy on Capitol Hill.
For example, the 2019 survey found that more than 60% of respondents had to wait at least 31 days from a physician referral to an initial rheumatologist appointment. Dr. Bhana noted that, in his practice, the wait can be 3 months or longer. A little more than 36% of respondents were able to get that initial appointment after referral in 30 days or less.
Additionally, nearly 47% of respondents were subjected to step therapy in the past year.
Treatment pricing was also raised as a concern in the survey, with a little more than 57% of respondents reporting difficulty affording treatments in the past year, and 25% reporting annual out-of-pocket spending at greater than $1,000, with more than 6% reporting annual out-of-pocket spending of greater than $5,000.
Despite all the challenges that lay ahead, Dr. Bhana believes that, as the campaign continues in its fourth year, it is having a positive impact.
“I think there is more awareness among patients to look up their symptoms and prompt their providers to look into, if not test for, rheumatic diseases, direct referrals, and I think because of these campaigns, we’ve gotten some indication that from a social media awareness that more patients are talking about it, about trying to get into seeing a physician.”
With the help of a key celebrity spokesperson, the American College of Rheumatology is hoping Rheumatic Disease Awareness Month (RDAM) will continue to raise the profile of the related illnesses and help more patients recognize their symptoms and get on the road to treatment.
This year, the celebrity tapped to lead the now-annual campaign that began in September 2016 is tennis champion Venus Williams, who has been competing while battling Sjögren’s syndrome. She was diagnosed in 2011.
Having a high-profile individual like Venus Williams will “bring more awareness to not a specific illness but just rheumatic diseases in general,” Suleman Bhana, MD, chair of the ACR’s marketing and communications committee, said in an interview, adding that it is a way to help get attention from patients and their family members to be more aware of what they are facing when afflicted with a rheumatic disease.
It also helps to drive traffic to a website (simpletasks.org) set up to help build a community around rheumatic diseases, he noted.
“By signing up on the website, patients and others can get connected with wellness articles and can be informed about any advocacy opportunities in their local area or the national level,” said Dr. Bhana, a rheumatologist with Crystal Run Healthcare in Middletown, N.Y. “It helps us a lot from an ACR point of view just by getting the word out about rheumatic diseases.”
Dr. Bhana said that part of the success of the RDAM campaign is being measured by the number of people who sign up for the website. “Last year we had about 1,600 people sign up, which was more than double from the previous year and we are hoping to do even better this year now that we have Venus Williams as our spokesperson.”
But the campaign involves more than just a celebrity spokesperson and an online community to build awareness for rheumatic diseases and their associated symptoms and treatments. The ACR is working with patients to make sure their voices are heard on Capitol Hill to help ensure proper access to medical care and those treatments.
This year, the ACR – along with more than 100 advocates – met with members of Congress to push for policies that will benefit people with rheumatic diseases. One key item on the agenda targets the use of step therapy.
“This year the ACR’s Government Affairs Committee had a list of several pieces of legislation that they are looking at heavily. [One bill is about] having brakes on what is called step therapy, which is a fairly egregious practice by many insurers that forces patients to use different medications than what the physician and patient decide in their mutual doctor-patient relationship before they can use the medicine that was originally decided upon,” he said. “The reasons for this practice mostly are financial in that insurers get kickbacks from certain pharmaceutical companies, and that is what they term as preferred drugs. It’s a lot more than just cost savings. It’s about the insurers financially benefiting from patients rather than doing the right thing and preserving the doctor-patient relationship.”
One bill in particular that has been highlighted by the ACR is the Safe Step Act of 2019 (H.R. 2279), introduced by Rep. Raul Ruiz, MD (D-Calif.), and Rep. Brad Wenstrup, DPM (R-Ohio), would put limits on step therapy and create a clear process for patients and physicians to seek exemptions.
Other legislative actions that patients and the ACR were advocating for during the visits for RDAM included the EMPOWER for Health Act (H.R. 2781), which would help increase the number of pediatric subspecialists, including pediatric rheumatologists, through loan repayment for health professionals who agree to work at least 2 years in pediatric medicine, and the REDI Act (H.R. 1554), which would defer the accumulation on student loan interest while future doctors serve in a medical internship or residency program.
“The reality of pediatrics is that they don’t get well compensated as a pediatric rheumatologist subspecialist, so there is an ultimate financial disincentive for training pediatricians to go into rheumatology,” Dr. Bhana said. “So we are trying to get support for loan repayment for pediatric rheumatologists or rheumatology fellowship programs, which may help to incentivize young pediatricians to go into rheumatology to at least expand access to care.”
The RDAM campaign also provides useful contacts for an annual survey that the ACR conducts. This year’s survey found that access issues for both physicians and treatments persist, informing the agenda for the advocacy on Capitol Hill.
For example, the 2019 survey found that more than 60% of respondents had to wait at least 31 days from a physician referral to an initial rheumatologist appointment. Dr. Bhana noted that, in his practice, the wait can be 3 months or longer. A little more than 36% of respondents were able to get that initial appointment after referral in 30 days or less.
Additionally, nearly 47% of respondents were subjected to step therapy in the past year.
Treatment pricing was also raised as a concern in the survey, with a little more than 57% of respondents reporting difficulty affording treatments in the past year, and 25% reporting annual out-of-pocket spending at greater than $1,000, with more than 6% reporting annual out-of-pocket spending of greater than $5,000.
Despite all the challenges that lay ahead, Dr. Bhana believes that, as the campaign continues in its fourth year, it is having a positive impact.
“I think there is more awareness among patients to look up their symptoms and prompt their providers to look into, if not test for, rheumatic diseases, direct referrals, and I think because of these campaigns, we’ve gotten some indication that from a social media awareness that more patients are talking about it, about trying to get into seeing a physician.”
With the help of a key celebrity spokesperson, the American College of Rheumatology is hoping Rheumatic Disease Awareness Month (RDAM) will continue to raise the profile of the related illnesses and help more patients recognize their symptoms and get on the road to treatment.
This year, the celebrity tapped to lead the now-annual campaign that began in September 2016 is tennis champion Venus Williams, who has been competing while battling Sjögren’s syndrome. She was diagnosed in 2011.
Having a high-profile individual like Venus Williams will “bring more awareness to not a specific illness but just rheumatic diseases in general,” Suleman Bhana, MD, chair of the ACR’s marketing and communications committee, said in an interview, adding that it is a way to help get attention from patients and their family members to be more aware of what they are facing when afflicted with a rheumatic disease.
It also helps to drive traffic to a website (simpletasks.org) set up to help build a community around rheumatic diseases, he noted.
“By signing up on the website, patients and others can get connected with wellness articles and can be informed about any advocacy opportunities in their local area or the national level,” said Dr. Bhana, a rheumatologist with Crystal Run Healthcare in Middletown, N.Y. “It helps us a lot from an ACR point of view just by getting the word out about rheumatic diseases.”
Dr. Bhana said that part of the success of the RDAM campaign is being measured by the number of people who sign up for the website. “Last year we had about 1,600 people sign up, which was more than double from the previous year and we are hoping to do even better this year now that we have Venus Williams as our spokesperson.”
But the campaign involves more than just a celebrity spokesperson and an online community to build awareness for rheumatic diseases and their associated symptoms and treatments. The ACR is working with patients to make sure their voices are heard on Capitol Hill to help ensure proper access to medical care and those treatments.
This year, the ACR – along with more than 100 advocates – met with members of Congress to push for policies that will benefit people with rheumatic diseases. One key item on the agenda targets the use of step therapy.
“This year the ACR’s Government Affairs Committee had a list of several pieces of legislation that they are looking at heavily. [One bill is about] having brakes on what is called step therapy, which is a fairly egregious practice by many insurers that forces patients to use different medications than what the physician and patient decide in their mutual doctor-patient relationship before they can use the medicine that was originally decided upon,” he said. “The reasons for this practice mostly are financial in that insurers get kickbacks from certain pharmaceutical companies, and that is what they term as preferred drugs. It’s a lot more than just cost savings. It’s about the insurers financially benefiting from patients rather than doing the right thing and preserving the doctor-patient relationship.”
One bill in particular that has been highlighted by the ACR is the Safe Step Act of 2019 (H.R. 2279), introduced by Rep. Raul Ruiz, MD (D-Calif.), and Rep. Brad Wenstrup, DPM (R-Ohio), would put limits on step therapy and create a clear process for patients and physicians to seek exemptions.
Other legislative actions that patients and the ACR were advocating for during the visits for RDAM included the EMPOWER for Health Act (H.R. 2781), which would help increase the number of pediatric subspecialists, including pediatric rheumatologists, through loan repayment for health professionals who agree to work at least 2 years in pediatric medicine, and the REDI Act (H.R. 1554), which would defer the accumulation on student loan interest while future doctors serve in a medical internship or residency program.
“The reality of pediatrics is that they don’t get well compensated as a pediatric rheumatologist subspecialist, so there is an ultimate financial disincentive for training pediatricians to go into rheumatology,” Dr. Bhana said. “So we are trying to get support for loan repayment for pediatric rheumatologists or rheumatology fellowship programs, which may help to incentivize young pediatricians to go into rheumatology to at least expand access to care.”
The RDAM campaign also provides useful contacts for an annual survey that the ACR conducts. This year’s survey found that access issues for both physicians and treatments persist, informing the agenda for the advocacy on Capitol Hill.
For example, the 2019 survey found that more than 60% of respondents had to wait at least 31 days from a physician referral to an initial rheumatologist appointment. Dr. Bhana noted that, in his practice, the wait can be 3 months or longer. A little more than 36% of respondents were able to get that initial appointment after referral in 30 days or less.
Additionally, nearly 47% of respondents were subjected to step therapy in the past year.
Treatment pricing was also raised as a concern in the survey, with a little more than 57% of respondents reporting difficulty affording treatments in the past year, and 25% reporting annual out-of-pocket spending at greater than $1,000, with more than 6% reporting annual out-of-pocket spending of greater than $5,000.
Despite all the challenges that lay ahead, Dr. Bhana believes that, as the campaign continues in its fourth year, it is having a positive impact.
“I think there is more awareness among patients to look up their symptoms and prompt their providers to look into, if not test for, rheumatic diseases, direct referrals, and I think because of these campaigns, we’ve gotten some indication that from a social media awareness that more patients are talking about it, about trying to get into seeing a physician.”
U.S. increases in RA burden outpace global averages
according to a new analysis of RA activity in 195 countries.
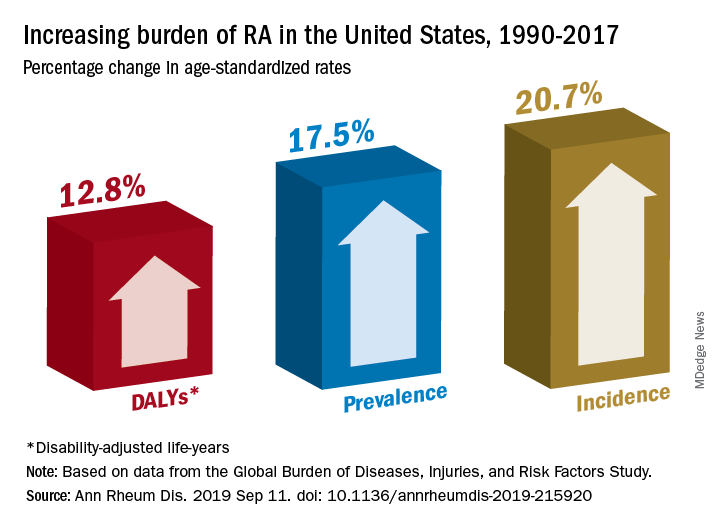
Percentage changes in the incidence, prevalence, and disability-adjusted life-year (DALY) rates for RA all reached double digits in the United States over the study period, but global increases for those measures stayed in the single digits, except for DALY, which did not increase, Saeid Safiri, PhD, of Tabriz (Iran) University of Medical Sciences and associates wrote in Annals of the Rheumatic Diseases.
Data from the Global Burden of Diseases, Injuries, and Risk Factors Study (GBD) 2017 show that RA incidence in the United States had the largest increase (20.7%) among the three measures, rising from 19.3 cases per 100,000 population in 1990 to 23.4 in 2017. Overall incidence for the 195 countries included in the study went from 13.7 to 14.9 cases per 100,000, for an increase of 8.2%, the investigators reported.
That pattern largely repeats for prevalence: an increase of 17.5% in the United States as the number of cases went from 336.5 per 100,000 in 1990 to 395.5 in 2017, and an increase of 7.4% globally, with the number of prevalent cases rising from 229.6 to 246.5 per 100,000, they said.
The DALY numbers – think of each DALY as 1 lost year of “healthy” life, the World Health Organization says – tell a somewhat different story. The United States had DALY rate of 53.2 per 100,000 in 1990, but by 2017 it had climbed to 60 per 100,000, an increase of 12.8%. Over that same time period, the global rate fell by 3.6% as it went from 44.9 to 43.3, Dr. Safiri and associates reported.
That long-term decline does, however, disguise a more recent trend. The global DALY rate “decreased from 1990 to 2012 but then increased and reached higher than expected levels in the following 5 years to 2017,” they wrote.
RA rates in the United States in 2017 were, as noted, above average, but they were not the highest. The United Kingdom achieved the RA trifecta of highest incidence (27.5 per 100,000), highest prevalence (471.8 per 100,000), and highest DALY rate (73 per 100,000) among the 195 countries in the study. At the other end of the three scales, Indonesia had the lowest incidence (5.6 per 100,000) and prevalence (91.1 per 100,000), and Sri Lanka had the lowest DALY rate (14.2 per 100,000), they said.
“Age-standardized prevalence and incidence rates are overall increasing globally. Increasing population awareness regarding RA, its risk factors and the importance of early diagnosis and treatment with disease-modifying agents is warranted to reduce the future burden of this condition,” the research team concluded.
GBD is funded by the Bill and Melinda Gates Foundation. The current analysis also was supported by Social Determinants of Health Research Center, Shahid Beheshti University of Medical Sciences in Tehran, Iran. The investigators did not declare any conflicts of interest.
SOURCE: Safiri S et al. Ann Rheum Dis. 2019 Sep 11. doi: 10.1136/annrheumdis-2019-215920.
according to a new analysis of RA activity in 195 countries.

Percentage changes in the incidence, prevalence, and disability-adjusted life-year (DALY) rates for RA all reached double digits in the United States over the study period, but global increases for those measures stayed in the single digits, except for DALY, which did not increase, Saeid Safiri, PhD, of Tabriz (Iran) University of Medical Sciences and associates wrote in Annals of the Rheumatic Diseases.
Data from the Global Burden of Diseases, Injuries, and Risk Factors Study (GBD) 2017 show that RA incidence in the United States had the largest increase (20.7%) among the three measures, rising from 19.3 cases per 100,000 population in 1990 to 23.4 in 2017. Overall incidence for the 195 countries included in the study went from 13.7 to 14.9 cases per 100,000, for an increase of 8.2%, the investigators reported.
That pattern largely repeats for prevalence: an increase of 17.5% in the United States as the number of cases went from 336.5 per 100,000 in 1990 to 395.5 in 2017, and an increase of 7.4% globally, with the number of prevalent cases rising from 229.6 to 246.5 per 100,000, they said.
The DALY numbers – think of each DALY as 1 lost year of “healthy” life, the World Health Organization says – tell a somewhat different story. The United States had DALY rate of 53.2 per 100,000 in 1990, but by 2017 it had climbed to 60 per 100,000, an increase of 12.8%. Over that same time period, the global rate fell by 3.6% as it went from 44.9 to 43.3, Dr. Safiri and associates reported.
That long-term decline does, however, disguise a more recent trend. The global DALY rate “decreased from 1990 to 2012 but then increased and reached higher than expected levels in the following 5 years to 2017,” they wrote.
RA rates in the United States in 2017 were, as noted, above average, but they were not the highest. The United Kingdom achieved the RA trifecta of highest incidence (27.5 per 100,000), highest prevalence (471.8 per 100,000), and highest DALY rate (73 per 100,000) among the 195 countries in the study. At the other end of the three scales, Indonesia had the lowest incidence (5.6 per 100,000) and prevalence (91.1 per 100,000), and Sri Lanka had the lowest DALY rate (14.2 per 100,000), they said.
“Age-standardized prevalence and incidence rates are overall increasing globally. Increasing population awareness regarding RA, its risk factors and the importance of early diagnosis and treatment with disease-modifying agents is warranted to reduce the future burden of this condition,” the research team concluded.
GBD is funded by the Bill and Melinda Gates Foundation. The current analysis also was supported by Social Determinants of Health Research Center, Shahid Beheshti University of Medical Sciences in Tehran, Iran. The investigators did not declare any conflicts of interest.
SOURCE: Safiri S et al. Ann Rheum Dis. 2019 Sep 11. doi: 10.1136/annrheumdis-2019-215920.
according to a new analysis of RA activity in 195 countries.

Percentage changes in the incidence, prevalence, and disability-adjusted life-year (DALY) rates for RA all reached double digits in the United States over the study period, but global increases for those measures stayed in the single digits, except for DALY, which did not increase, Saeid Safiri, PhD, of Tabriz (Iran) University of Medical Sciences and associates wrote in Annals of the Rheumatic Diseases.
Data from the Global Burden of Diseases, Injuries, and Risk Factors Study (GBD) 2017 show that RA incidence in the United States had the largest increase (20.7%) among the three measures, rising from 19.3 cases per 100,000 population in 1990 to 23.4 in 2017. Overall incidence for the 195 countries included in the study went from 13.7 to 14.9 cases per 100,000, for an increase of 8.2%, the investigators reported.
That pattern largely repeats for prevalence: an increase of 17.5% in the United States as the number of cases went from 336.5 per 100,000 in 1990 to 395.5 in 2017, and an increase of 7.4% globally, with the number of prevalent cases rising from 229.6 to 246.5 per 100,000, they said.
The DALY numbers – think of each DALY as 1 lost year of “healthy” life, the World Health Organization says – tell a somewhat different story. The United States had DALY rate of 53.2 per 100,000 in 1990, but by 2017 it had climbed to 60 per 100,000, an increase of 12.8%. Over that same time period, the global rate fell by 3.6% as it went from 44.9 to 43.3, Dr. Safiri and associates reported.
That long-term decline does, however, disguise a more recent trend. The global DALY rate “decreased from 1990 to 2012 but then increased and reached higher than expected levels in the following 5 years to 2017,” they wrote.
RA rates in the United States in 2017 were, as noted, above average, but they were not the highest. The United Kingdom achieved the RA trifecta of highest incidence (27.5 per 100,000), highest prevalence (471.8 per 100,000), and highest DALY rate (73 per 100,000) among the 195 countries in the study. At the other end of the three scales, Indonesia had the lowest incidence (5.6 per 100,000) and prevalence (91.1 per 100,000), and Sri Lanka had the lowest DALY rate (14.2 per 100,000), they said.
“Age-standardized prevalence and incidence rates are overall increasing globally. Increasing population awareness regarding RA, its risk factors and the importance of early diagnosis and treatment with disease-modifying agents is warranted to reduce the future burden of this condition,” the research team concluded.
GBD is funded by the Bill and Melinda Gates Foundation. The current analysis also was supported by Social Determinants of Health Research Center, Shahid Beheshti University of Medical Sciences in Tehran, Iran. The investigators did not declare any conflicts of interest.
SOURCE: Safiri S et al. Ann Rheum Dis. 2019 Sep 11. doi: 10.1136/annrheumdis-2019-215920.
FROM ANNALS OF THE RHEUMATIC DISEASES
Cannabidiol may interact with rheumatologic drugs
A number of medications commonly prescribed by rheumatologists may interact with cannabidiol oil, investigators at the Imperial College Healthcare NHS Trust, London, reported.
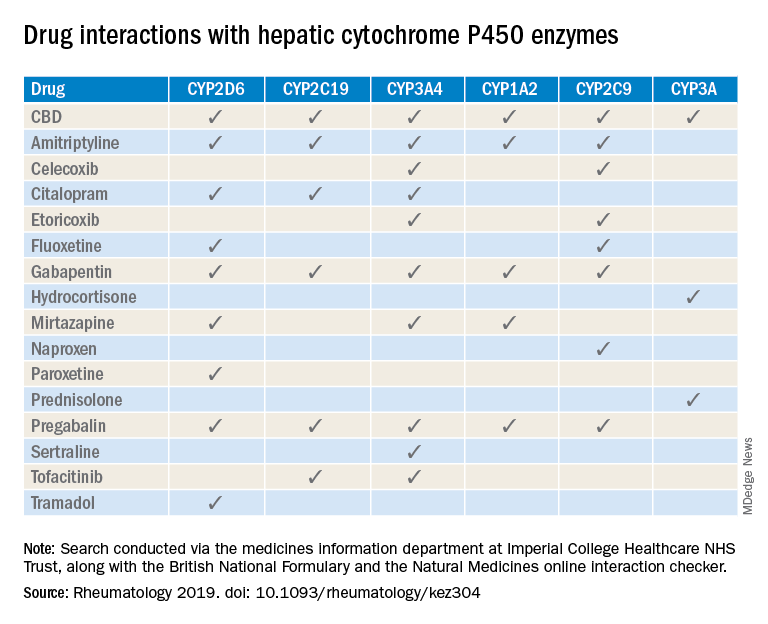
“Patients are increasingly requesting information concerning the safety of CBD oil,” Taryn Youngstein, MD, and associates said in letter to the editor in Rheumatology, but current guidelines on the use of medical cannabis do “not address the potential interactions between CBD oil and medicines frequently used in the rheumatology clinic.”
The most important potential CBD interaction, they suggested, may be with corticosteroids. Hydrocortisone and prednisolone both inhibit the cytochrome P450 enzyme CYP3A, but CBD is a potent inhibitor of CYP3A, so “concomitant use may decrease glucocorticoid clearance and increase risk of systemic [corticosteroid] side effects,” the investigators wrote.
CBD also is known to inhibit the cytochrome P450 isozymes CYP2C9, CYP2D6, CYP2C19, CYP3A4, and CYP1A2, which, alone or in combination, are involved in the metabolization of naproxen, tramadol, amitriptyline, and tofacitinib (Xeljanz), according to a literature search done via the college’s medicine information department that also used the British National Formulary and the Natural Medicines online interaction checker.
The Janus kinase inhibitor tofacitinib is included among the possible interactions, but the other Food and Drug Administration–approved JAK inhibitor, baricitinib (Olumiant), is primarily metabolized by the kidneys and should not have significant interaction with CBD, Dr. Youngstein and associates said. Most of the conventional synthetic and biologic disease-modifying antirheumatic drugs, including methotrexate, hydroxychloroquine, adalimumab (Humira), and abatacept (Orencia), also are expected to be relatively free from CBD interactions.
This first published report on interactions between CBD oil and common rheumatology medications “highlights the importance of taking comprehensive drug histories, by asking directly about drugs considered alternative medicines and food supplements,” they said.
The investigators declared no conflicts of interest, and there was no specific funding for the study.
SOURCE: Wilson-Morkeh H et al. Rheumatology. 2019 July 29. doi: 10.1093/rheumatology/kez304.
A number of medications commonly prescribed by rheumatologists may interact with cannabidiol oil, investigators at the Imperial College Healthcare NHS Trust, London, reported.

“Patients are increasingly requesting information concerning the safety of CBD oil,” Taryn Youngstein, MD, and associates said in letter to the editor in Rheumatology, but current guidelines on the use of medical cannabis do “not address the potential interactions between CBD oil and medicines frequently used in the rheumatology clinic.”
The most important potential CBD interaction, they suggested, may be with corticosteroids. Hydrocortisone and prednisolone both inhibit the cytochrome P450 enzyme CYP3A, but CBD is a potent inhibitor of CYP3A, so “concomitant use may decrease glucocorticoid clearance and increase risk of systemic [corticosteroid] side effects,” the investigators wrote.
CBD also is known to inhibit the cytochrome P450 isozymes CYP2C9, CYP2D6, CYP2C19, CYP3A4, and CYP1A2, which, alone or in combination, are involved in the metabolization of naproxen, tramadol, amitriptyline, and tofacitinib (Xeljanz), according to a literature search done via the college’s medicine information department that also used the British National Formulary and the Natural Medicines online interaction checker.
The Janus kinase inhibitor tofacitinib is included among the possible interactions, but the other Food and Drug Administration–approved JAK inhibitor, baricitinib (Olumiant), is primarily metabolized by the kidneys and should not have significant interaction with CBD, Dr. Youngstein and associates said. Most of the conventional synthetic and biologic disease-modifying antirheumatic drugs, including methotrexate, hydroxychloroquine, adalimumab (Humira), and abatacept (Orencia), also are expected to be relatively free from CBD interactions.
This first published report on interactions between CBD oil and common rheumatology medications “highlights the importance of taking comprehensive drug histories, by asking directly about drugs considered alternative medicines and food supplements,” they said.
The investigators declared no conflicts of interest, and there was no specific funding for the study.
SOURCE: Wilson-Morkeh H et al. Rheumatology. 2019 July 29. doi: 10.1093/rheumatology/kez304.
A number of medications commonly prescribed by rheumatologists may interact with cannabidiol oil, investigators at the Imperial College Healthcare NHS Trust, London, reported.

“Patients are increasingly requesting information concerning the safety of CBD oil,” Taryn Youngstein, MD, and associates said in letter to the editor in Rheumatology, but current guidelines on the use of medical cannabis do “not address the potential interactions between CBD oil and medicines frequently used in the rheumatology clinic.”
The most important potential CBD interaction, they suggested, may be with corticosteroids. Hydrocortisone and prednisolone both inhibit the cytochrome P450 enzyme CYP3A, but CBD is a potent inhibitor of CYP3A, so “concomitant use may decrease glucocorticoid clearance and increase risk of systemic [corticosteroid] side effects,” the investigators wrote.
CBD also is known to inhibit the cytochrome P450 isozymes CYP2C9, CYP2D6, CYP2C19, CYP3A4, and CYP1A2, which, alone or in combination, are involved in the metabolization of naproxen, tramadol, amitriptyline, and tofacitinib (Xeljanz), according to a literature search done via the college’s medicine information department that also used the British National Formulary and the Natural Medicines online interaction checker.
The Janus kinase inhibitor tofacitinib is included among the possible interactions, but the other Food and Drug Administration–approved JAK inhibitor, baricitinib (Olumiant), is primarily metabolized by the kidneys and should not have significant interaction with CBD, Dr. Youngstein and associates said. Most of the conventional synthetic and biologic disease-modifying antirheumatic drugs, including methotrexate, hydroxychloroquine, adalimumab (Humira), and abatacept (Orencia), also are expected to be relatively free from CBD interactions.
This first published report on interactions between CBD oil and common rheumatology medications “highlights the importance of taking comprehensive drug histories, by asking directly about drugs considered alternative medicines and food supplements,” they said.
The investigators declared no conflicts of interest, and there was no specific funding for the study.
SOURCE: Wilson-Morkeh H et al. Rheumatology. 2019 July 29. doi: 10.1093/rheumatology/kez304.
FROM RHEUMATOLOGY
Prior DMARD use in RA may limit adalimumab treatment response
A history of using multiple conventional synthetic disease-modifying antirheumatic drugs (DMARDs) is a key predictor for poorer response to adalimumab therapy in rheumatoid arthritis patients, according to data from a pair of studies with a total of 274 patients.
Although patients with RA who have failed methotrexate or tumor necrosis factor inhibitor therapy respond less than methotrexate-naive patients, “it remains unknown if response to the first biologic DMARD, in particular a [tumor necrosis factor inhibitor], depends on disease duration or prior numbers of failed [conventional synthetic] DMARDs,” wrote Daniel Aletaha, MD, of the Medical University of Vienna and colleagues.
In a study published in Annals of the Rheumatic Diseases, the researchers reviewed data from two randomized, controlled trials of patients with RA. In the larger trial of 207 adults (known as DE019), past use of two or more conventional synthetic DMARDs was associated with less improvement in 28-joint Disease Activity Score using C-reactive protein (DAS28-CRP) after 24 weeks of adalimumab (Humira), compared with use of one or no DMARDs (–1.8 vs. –2.2, respectively). Similarly, disease activity and disability scores improved significantly less in patients who had used two or more DMARDs, compared with those who used one or no DMARDs, according to the Simplified Disease Activity Index (SDAI; –22.1 vs. –26.9) and the Health Assessment Questionnaire Disability Index (HAQ-DI; –0.43 vs. –0.64).
The researchers also examined the role of disease duration on treatment response. Overall, patients with disease duration greater than 10 years showed more improvement at 24 weeks than did those with disease duration less than 1 year, based on HAQ-DI scores (1.1 vs. 0.7), but final scores on the SDAI and DAS28-CRP were not significantly different between those with disease duration greater than 10 years and those with duration of less than 1 year. These results suggest that the impact of DMARDs holds true regardless of disease duration, the researchers noted.
The findings were similar with regard to number of prior conventional synthetic DMARDs and the effects of disease duration in the second trial of 67 patients, known as the ARMADA study.
The study findings were limited by several factors, including the post hoc analysis design, use of only adalimumab data, and the small number of patients in several subgroups, the researchers noted. However, the results support the need for more standardized treatment guidelines and suggest that RA patients who fail to respond to methotrexate soon after RA diagnosis may benefit most from adding adalimumab, they said.
“Furthermore, these findings should be considered in future trials when defining inclusion criteria not only by duration of disease but also by number of prior DMARDs,” they concluded.
The study was sponsored by AbbVie, which markets adalimumab. Dr. Aletaha disclosed grants and consulting fees from AbbVie, as well as other pharmaceutical companies. Four of the authors were current or former employees of AbbVie, and some other authors also reported financial relationships with the company.
SOURCE: Aletaha D et al. Ann Rheum Dis. 2019 Aug 21. doi: 10.1136/annrheumdis-2018-214918.
A history of using multiple conventional synthetic disease-modifying antirheumatic drugs (DMARDs) is a key predictor for poorer response to adalimumab therapy in rheumatoid arthritis patients, according to data from a pair of studies with a total of 274 patients.
Although patients with RA who have failed methotrexate or tumor necrosis factor inhibitor therapy respond less than methotrexate-naive patients, “it remains unknown if response to the first biologic DMARD, in particular a [tumor necrosis factor inhibitor], depends on disease duration or prior numbers of failed [conventional synthetic] DMARDs,” wrote Daniel Aletaha, MD, of the Medical University of Vienna and colleagues.
In a study published in Annals of the Rheumatic Diseases, the researchers reviewed data from two randomized, controlled trials of patients with RA. In the larger trial of 207 adults (known as DE019), past use of two or more conventional synthetic DMARDs was associated with less improvement in 28-joint Disease Activity Score using C-reactive protein (DAS28-CRP) after 24 weeks of adalimumab (Humira), compared with use of one or no DMARDs (–1.8 vs. –2.2, respectively). Similarly, disease activity and disability scores improved significantly less in patients who had used two or more DMARDs, compared with those who used one or no DMARDs, according to the Simplified Disease Activity Index (SDAI; –22.1 vs. –26.9) and the Health Assessment Questionnaire Disability Index (HAQ-DI; –0.43 vs. –0.64).
The researchers also examined the role of disease duration on treatment response. Overall, patients with disease duration greater than 10 years showed more improvement at 24 weeks than did those with disease duration less than 1 year, based on HAQ-DI scores (1.1 vs. 0.7), but final scores on the SDAI and DAS28-CRP were not significantly different between those with disease duration greater than 10 years and those with duration of less than 1 year. These results suggest that the impact of DMARDs holds true regardless of disease duration, the researchers noted.
The findings were similar with regard to number of prior conventional synthetic DMARDs and the effects of disease duration in the second trial of 67 patients, known as the ARMADA study.
The study findings were limited by several factors, including the post hoc analysis design, use of only adalimumab data, and the small number of patients in several subgroups, the researchers noted. However, the results support the need for more standardized treatment guidelines and suggest that RA patients who fail to respond to methotrexate soon after RA diagnosis may benefit most from adding adalimumab, they said.
“Furthermore, these findings should be considered in future trials when defining inclusion criteria not only by duration of disease but also by number of prior DMARDs,” they concluded.
The study was sponsored by AbbVie, which markets adalimumab. Dr. Aletaha disclosed grants and consulting fees from AbbVie, as well as other pharmaceutical companies. Four of the authors were current or former employees of AbbVie, and some other authors also reported financial relationships with the company.
SOURCE: Aletaha D et al. Ann Rheum Dis. 2019 Aug 21. doi: 10.1136/annrheumdis-2018-214918.
A history of using multiple conventional synthetic disease-modifying antirheumatic drugs (DMARDs) is a key predictor for poorer response to adalimumab therapy in rheumatoid arthritis patients, according to data from a pair of studies with a total of 274 patients.
Although patients with RA who have failed methotrexate or tumor necrosis factor inhibitor therapy respond less than methotrexate-naive patients, “it remains unknown if response to the first biologic DMARD, in particular a [tumor necrosis factor inhibitor], depends on disease duration or prior numbers of failed [conventional synthetic] DMARDs,” wrote Daniel Aletaha, MD, of the Medical University of Vienna and colleagues.
In a study published in Annals of the Rheumatic Diseases, the researchers reviewed data from two randomized, controlled trials of patients with RA. In the larger trial of 207 adults (known as DE019), past use of two or more conventional synthetic DMARDs was associated with less improvement in 28-joint Disease Activity Score using C-reactive protein (DAS28-CRP) after 24 weeks of adalimumab (Humira), compared with use of one or no DMARDs (–1.8 vs. –2.2, respectively). Similarly, disease activity and disability scores improved significantly less in patients who had used two or more DMARDs, compared with those who used one or no DMARDs, according to the Simplified Disease Activity Index (SDAI; –22.1 vs. –26.9) and the Health Assessment Questionnaire Disability Index (HAQ-DI; –0.43 vs. –0.64).
The researchers also examined the role of disease duration on treatment response. Overall, patients with disease duration greater than 10 years showed more improvement at 24 weeks than did those with disease duration less than 1 year, based on HAQ-DI scores (1.1 vs. 0.7), but final scores on the SDAI and DAS28-CRP were not significantly different between those with disease duration greater than 10 years and those with duration of less than 1 year. These results suggest that the impact of DMARDs holds true regardless of disease duration, the researchers noted.
The findings were similar with regard to number of prior conventional synthetic DMARDs and the effects of disease duration in the second trial of 67 patients, known as the ARMADA study.
The study findings were limited by several factors, including the post hoc analysis design, use of only adalimumab data, and the small number of patients in several subgroups, the researchers noted. However, the results support the need for more standardized treatment guidelines and suggest that RA patients who fail to respond to methotrexate soon after RA diagnosis may benefit most from adding adalimumab, they said.
“Furthermore, these findings should be considered in future trials when defining inclusion criteria not only by duration of disease but also by number of prior DMARDs,” they concluded.
The study was sponsored by AbbVie, which markets adalimumab. Dr. Aletaha disclosed grants and consulting fees from AbbVie, as well as other pharmaceutical companies. Four of the authors were current or former employees of AbbVie, and some other authors also reported financial relationships with the company.
SOURCE: Aletaha D et al. Ann Rheum Dis. 2019 Aug 21. doi: 10.1136/annrheumdis-2018-214918.
FROM ANNALS OF THE RHEUMATIC DISEASES






