User login
Referral Patterns for Chronic Groin Pain and Athletic Pubalgia/Sports Hernia: Magnetic Resonance Imaging Findings, Treatment, and Outcomes
The past 3 decades have seen an evolution in the understanding, diagnosis, and treatment of groin pain, both chronic and acute, in athletes and non-athletes alike. Groin pain and groin injury are common. Most cases are transient, with patients returning to their activities within weeks or months. There has also been increasing awareness of a definitive population of patients who do not get better, or who improve and plateau before reaching preinjury level of performance.1-3 Several authors have brought more attention to the injury, introducing vocabulary, theories, diagnostic testing, and diagnoses, which now constitute a knowledge base.1,3-5
As stated in almost every article on groin pain and diagnosis, lack of cohesive agreement and vocabulary, and consistent protocols and procedures, has abounded, making general understanding and agreement in this area inconsistent.1,6-8In this article, members of a tertiary-care group specializing in chronic groin pain, athletic pubalgia (sports hernia), and inguinal herniorrhaphy outline their clinical examination, diagnostic algorithm, imaging protocol, treatment strategy, and outcomes for a population of patients referred by physicians and allied health professionals for a suspected diagnosis of athletic pubalgia.
Background
The pubic symphysis acts as a stabilizing central anchor with elaborate involvement of the anterior structures, including the rectus abdominis, adductor longus, and inguinal ligaments.3,7,9 Literature from Europe, Australia, and the United States has described groin pain, mostly in professional athletes, involving these pubic structures and attachments. Several publications have been addressing chronic groin pain, and each has its own diagnostic algorithm, imaging protocol, and treatment strategy.3,6,9-18
Terminology specific to groin pain in athletes is not new, and has a varied history dating to the early 20th century. Terms such as sportsman hernia19 and subsequently sports hernia20, have recently been embraced by the lay population. In 1999, Gibbon21 described shearing of the common adductor–rectus abdominis anatomical and functional unit and referenced a 1902 anatomical text that describes vertical ligamentous fibers contiguous with rectus sheath and adductor muscles, both attaching to the pubis. Injury to this region is the basis of pubalgia, a term originally used in 1984 by Brunet to describe a pain syndrome at the pubis.22
Many authors have proposed replacing sports hernia with athletic pubalgia.1,3,6,7,10,14,18,23 These terms refer to a group of musculoskeletal processes that occur in and around the pubic symphysis and that share similar mechanisms of injury and common clinical manifestations. The condition was originally described in high-performance athletes, and at one point the term sports hernia was reserved for this patient population.5 According to many authors, presence of an inguinal hernia excludes the diagnosis.1,2,5Magnetic resonance imaging (MRI) has helped to advance and define our understanding of the injury.10 As the history of the literature suggests, earlier concepts of chronic pain focused either on the medial aspect of the inguinal canal and its structures or on the pubic attachments. Many specialists in the area have concluded that the chronic groin pain injury can and often does embody both elements.3,9 Correlation with MRI findings, injury seen during surgical procedures, and cadaveric studies have directed our understanding to a structure, the pre-pubic aponeurotic complex (P-PAC), or rectus aponeurotic plate.12,24,25 Anatomically, the P-PAC, which has several fascial components, attaches posteriorly to the pubic bone and, to a degree, the pubic symphyseal cartilaginous disc. Major contributions to the P-PAC are fibers from the rectus abdominis tendon, the medial aspect of the transversalis and internal oblique muscles (the conjoint tendon, according to some), the inguinal ligament, and the adductor longus tendon.26When communicating with referring physicians, we use the term athletic pubalgia to indicate a specific injury. The athletic pubalgia injury can be defined as serial microtearing,1 or complete tearing, of the posterior attachment of the P-PAC off the anterior pubis.3,10 Complete tearing or displacement can occur unilaterally or across the midline to the other side. As athletic pubalgia is a specific anatomical injury rather than a broad category of findings, an additional pathologic diagnosis, such as inguinal hernia, does not exclude the diagnosis of athletic pubalgia. Unfortunately, the terms sports hernia and sportsman hernia, commonly used in the media and in professional communities, have largely confused the broader understanding of nuances and of the differences between the specific injuries and MRI findings.18
Our Experience
In our practice, we see groin pain patients referred by internists, physiatrists, physical therapists, trainers, general surgeons, urologists, gynecologists, and orthopedic surgeons. In many cases, patients have been through several consultations and work-ups, as their pain syndrome does not fall under a specific category. Patients without inguinal hernia, hip injury, urologic, or gynecologic issues typically are referred to a physiatrist or a physical therapist. Often, there are marginal improvements with physical therapy, but in some cases the injury never completely resolves, and the patient continues to have pain with activity or return to sports.
Most of our patients are nonprofessional athletes, men and women who range widely in age and participate casually or regularly in sporting events. Most lack the rigorous training, conditioning, and close supervision that professional athletes receive. Many other patients are nonprofessional but elite athletes who train 7 days a week for marathons, ultramarathons, triathlons, obstacle course races (“mudders”), and similar events.
Work-Up
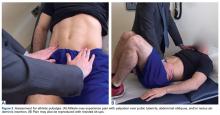
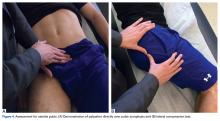
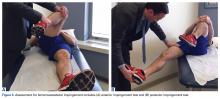
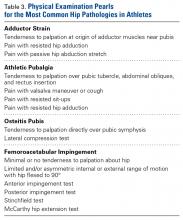
A single algorithm is used for all patients initially referred to the surgeon’s office for pelvic or groin pain. The initial interview directs attention to injury onset and mechanism, duration of rest or physical therapy after surgery, pain quality and pain levels, and antagonistic movements and positions. Examination starts with assessment for inguinal, femoral, and umbilical hernias. Resisted sit-up, leg-raise, adduction, and hip assessment tests are performed. The P-PAC is examined with a maneuver similar to the one used for inguinal hernia, as it allows for better assessment of the transversalis fascia (over the direct space) to determine if the inguinal canal floor is attenuated and bulges forward with the Valsalva maneuver. Then, the lateral aspect of the rectus muscle is assessed for pain, usually with the head raised to contract the muscle, to determine tenderness along the lateral border. The rectus edge is traced down to the pubis at its attachment, the superolateral border of the P-PAC. Examination proceeds medially, over the rectus attachment, toward the pubic symphysis, continuing the assessment for tenderness. Laterally, the conjoint tendon and inguinal ligament medial attachments are assessed at the level of the pubic tubercle, which represents the lateral border of the P-PAC. Finally, the examination continues to the inferior border with assessment of the adductor longus attachment, which is best performed with the leg in an adducted position. In the acute or semiacute setting (pain within 1 year of injury onset), tenderness is often elicited. With long-standing injuries, pain is often not elicited, but the patient experiences pain along this axis during activity or afterward.
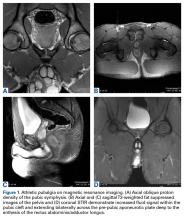
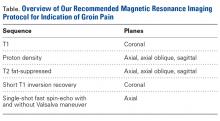
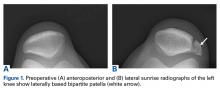
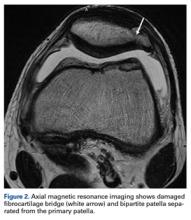
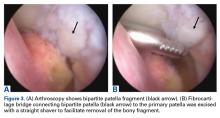
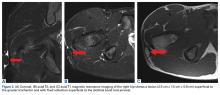
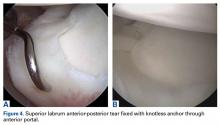
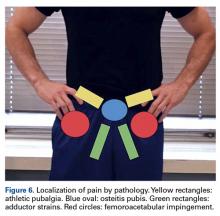
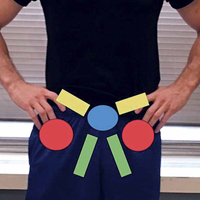
Patients with positive history and physical examination findings proceed through an MRI protocol designed to detect pathology of the pubic symphysis, hips, and inguinal canals (Figures 1A-1D).
Treatment
Patients who report sustaining an acute groin injury within the previous 6 months are treated nonoperatively. A combination of rest, nonsteroidal anti-inflammatory drugs, and physical therapy is generally recommended.2,10 In cases of failed nonoperative management, patients are evaluated for surgery. No single operation is recommended for all patients.1,6,14,27,28 (Larson26 recently reviewed results from several trials involving a variety of surgical repairs and found return-to-sports rates ranging from 80% to 100%.) Findings from the physical examination and from the properly protocolled MRI examination are used in planning surgery to correct any pathology that could be contributing to symptoms or destabilization of the structures attaching to the pubis. Disruption of the P-PAC from the pubis would be repaired, for example. Additional injuries, such as partial or complete detachment of the conjoint tendon or inguinal ligament, may be repaired as well. If the transversalis fascia is attenuated and bulging forward, the inguinal floor is closed. Adductor longus tendon pathology is addressed, most commonly with partial tendinolysis. Often, concomitant inguinal hernias are found, and these may be repaired in open fashion while other maneuvers are being performed, or laparoscopically.
Materials and Methods
After receiving study approval from our Institutional Review Board, we retrospectively searched for all MRIs performed by our radiology department between March 1, 2011 and March 31, 2013 on patients referred for an indication of groin pain, sports hernia, or athletic pubalgia. Patients were excluded if they were younger than 18 years any time during their care. Some patients previously or subsequently underwent computed tomography or ultrasonography. MRIs were reviewed and positive findings were compiled in a database. Charts were reviewed to identify which patients in the dataset underwent surgery, after MRI, to address their presenting chief complaint. Surgery date and procedure(s) performed were recorded. Patients were interviewed by telephone as part of the in-office postoperative follow-up.
Results
One hundred nineteen MRIs were performed on 117 patients (97 men, 83%). Mean age was 39.8 years. Seventy-nine patients (68%) had an MRI finding of athletic pubalgia, 67 (57%) had an acetabular labral tear in one or both hip joints, and 41 (35%) had a true inguinal hernia. Concomitant findings were common: 47 cases of athletic pubalgia and labral tear(s), 28 cases of athletic pubalgia and inguinal hernia, and 15 cases of all 3 (athletic pubalgia, labral tear, inguinal hernia).
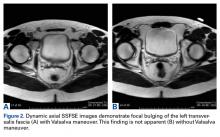
Use of breath-hold axial single-shot fast spin-echo sequences with and without the Valsalva maneuver increased sensitivity in detecting pathologies—inguinal hernia and Gilmore groin in particular. On 24 of the 119 MRIs, the Valsalva maneuver either revealed the finding or made it significantly more apparent.
Of all patients referred for MRI for chronic groin pain, 48 (41%) subsequently underwent surgery. In 29 surgeries, the rectus abdominis, adductor longus, and/or pre-pubic aponeurotic plate were repaired; in 13 cases, herniorrhaphy was performed as well; in 2 cases, masses involving the spermatic cord were removed.
The most common surgery (30 cases) was herniorrhaphy, which was performed as a single procedure, multiple procedures, or in combination with procedures not related to a true hernia. Eighteen patients underwent surgery only for hernia repair.
Of the 79 patients with MRI-positive athletic pubalgia, 39 subsequently underwent surgery, and 31 (79%) of these were followed up by telephone. Mean duration of rest after surgery was 6.2 weeks. Twelve patients (39%) had physical therapy after surgery, some as early as 4 weeks, and some have continued their therapy since surgery. Of the 31 patients who were followed up after surgery, 23 (74%) resumed previous activity levels. Return to previous activity level took these patients a mean of 17.9 weeks. When asked if outcomes satisfied their expectations, 28 patients (90%) said yes, and 3 said no.
Forty patients with MRI-positive athletic pubalgia were nonoperatively treated, and 28 (70%) of these patients were followed up. In this group, mean duration of rest after surgery was 6.9 weeks. Thirteen patients (46%) participated in physical therapy, for a mean duration of 10.8 weeks. Of the patients followed up, 19 (68%) returned to previous activity level. Twenty-one patients (75%) were satisfied with their outcome.
Discussion
Diagnosis and treatment of chronic groin pain have had a long, confusing, and frustrating history for both patients and the medical professionals who provide them with care.3,6,7,10 Historically, the problem has been, in part, the lack of diagnostic capabilities. Currently, however, pubalgia MRI protocol allows the exact pathology to be demonstrated.3 As already noted, concomitant hip pathology or inguinal hernia is not unusual8; any structural abnormality in the area is a potential destabilizer of the structures attached to the pubis.18 Solving only one of these issues may offer only partial resolution of symptoms and thereby reduce the rate of successful treatment of groin pain.
Diagnostic algorithms are being developed. In addition, nonoperative treatments are being tried for some of the issues. Physicians are giving diagnostic and therapeutic steroid injections in the pubic cleft, along the rectus abdominis/adductor longus complex, or posterior to the P-PAC. Platelet-rich plasma injection therapy has had limited success.29This article provides a snapshot of what a tertiary-care group of physicians specializing in chronic groin pain sees in an unfiltered setting. We think this is instructive for several reasons.
First, many patients in our population have visited a multitude of specialists without receiving a diagnosis or being referred appropriately. Simply, many specialists do not know the next step in treating groin pain and thus do not make the appropriate referral. Until recently, the literature has not been helpful. It has poorly described the constellation of injuries comprising chronic groin pain. More significantly, groin injuries have been presented as ambiguous injuries lacking effective treatment. Over the past decade, however, abundant literature on the correlation of these injuries with specific MRI findings has made the case otherwise.
Second, a specific MRI pubalgia protocol is needed. Inability to make a correct diagnosis, because of improper MRI, continues to add to the confusion surrounding the injury and undoubtedly prolongs the general medical community’s thinking that diagnosis and treatment of chronic groin pain are elusive. Our data support this point in many ways. Although all patients in this study were seen by a medical professional before coming to our office, none had received a diagnosis of occult hernia or attenuated transversalis fascia; nevertheless, we identified inguinal hernia, Gilmore groin, or both in 44% of these patients. These findings are not surprising, as MRI was the crucial link in diagnosis. In addition, the point made by other groin pain specialists—that a hernia precludes a pubalgia diagnosis1,2,5—is not supported by our data. Inguinal hernia can and does exist in conjunction with pubalgia. More than half the patients in our study had a combined diagnosis. We contend that, much as hip labral pathology occurs concomitantly with pubalgia,23 inguinal hernia may be a predisposing factor as well. A defect in the direct or indirect space can destabilize the area and place additional strain on the pubic attachments.
In our experience, the dynamic Valsalva sequence improves detection of true hernias and anterior abdominal wall deficiencies and should be included in each protocol for the evaluation of acute or chronic groin pain.
Shear forces and injury at the pubis can occur outside professional athletics. Our patient population is nonprofessional athletes, teenagers to retirees, and all can develop athletic pubalgia. Ninety percent of surveyed patients who received a diagnosis and were treated surgically were satisfied with their outcomes.
Am J Orthop. 2017;46(4):E251-E256. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
1. Meyers WC, Lanfranco A, Castellanos A. Surgical management of chronic lower abdominal and groin pain in high-performance athletes. Curr Sports Med Rep. 2002;1(5):301-305.
2. Ahumada LA, Ashruf S, Espinosa-de-los-Monteros A, et al. Athletic pubalgia: definition and surgical treatment. Ann Plast Surg. 2005;55(4):393-396.
3. Omar IM, Zoga AC, Kavanagh EC, et al. Athletic pubalgia and “sports hernia”: optimal MR imaging technique and findings. Radiographics. 2008;28(5):1415-1438.
4. Gilmore OJA. Gilmore’s groin: ten years experience of groin disruption—a previously unsolved problem in sportsmen. Sports Med Soft Tissue Trauma. 1991;3:12-14.
5. Meyers WC, Foley DP, Garrett WE, Lohnes JH, Mandlebaum BR. Management of severe lower abdominal or inguinal pain in high-performance athletes. PAIN (Performing Athletes with Abdominal or Inguinal Neuromuscular Pain Study Group). Am J Sports Med. 2000;28(1):2-8.
6. Kavanagh EC, Koulouris G, Ford S, McMahon P, Johnson C, Eustace SJ. MR imaging of groin pain in the athlete. Semin Musculoskelet Radiol. 2006;10(3):197-207.
7. Cunningham PM, Brennan D, O’Connell M, MacMahon P, O’Neill P, Eustace S. Patterns of bone and soft-tissue injury at the symphysis pubis in soccer players: observations at MRI. AJR Am J Roentgenol. 2007;188(3):W291-W296.
8. Zoga AC, Kavanagh EC, Omar IM, et al. Athletic pubalgia and the “sports hernia”: MR imaging findings. Radiology. 2008;247(3):797-807.
9. Koulouris G. Imaging review of groin pain in elite athletes: an anatomic approach to imaging findings. AJR Am J Roentgenol. 2008;191(4):962-972.
10. Albers SL, Spritzer CE, Garrett WE Jr, Meyers WC. MR findings in athletes with pubalgia. Skeletal Radiol. 2001;30(5):270-277.
11. Brennan D, O’Connell MJ, Ryan M, et al. Secondary cleft sign as a marker of injury in athletes with groin pain: MR image appearance and interpretation. Radiology. 2005;235(1):162-167.
12. Robinson P, Salehi F, Grainger A, et al. Cadaveric and MRI study of the musculotendinous contributions to the capsule of the symphysis pubis. AJR Am J Roentgenol. 2007;188(5):W440-W445.
13. Schilders E, Talbot JC, Robinson P, Dimitrakopoulou A, Gibbon WW, Bismil Q. Adductor-related groin pain in recreational athletes. J Bone Joint Surg Am. 2009;91(10):2455-2460.
14. Davies AG, Clarke AW, Gilmore J, Wotherspoon M, Connell DA. Review: imaging of groin pain in the athlete. Skeletal Radiol. 2010;39(7):629-644.
15. Mullens FE, Zoga AC, Morrison WB, Meyers WC. Review of MRI technique and imaging findings in athletic pubalgia and the “sports hernia.” Eur J Radiol. 2012;81(12):3780-3792.
16. Zoga AC, Meyers WC. Magnetic resonance imaging for pain after surgical treatment for athletic pubalgia and the “sports hernia.” Semin Musculoskelet Radiol. 2011;15(4):372-382.
17. Beer E. Periostitis of symphysis and descending rami of pubes following suprapubic operations. Int J Med Surg. 1924;37(5):224-225.
18. MacMahon PJ, Hogan BA, Shelly MJ, Eustace SJ, Kavanagh EC. Imaging of groin pain. Magn Reson Imaging Clin N Am. 2009;17(4):655-666.
19. Malycha P, Lovell G. Inguinal surgery in athletes with chronic groin pain: the ‘sportsman’s’ hernia. Aust N Z J Surg. 1992;62(2):123-125.
20. Hackney RG. The sports hernia: a cause of chronic groin pain. Br J Sports Med. 1993;27(1):58-62.
21. Gibbon WW. Groin pain in athletes. Lancet. 1999;353(9162):1444-1445.
22. Brunet B, Brunet-Geudj E, Genety J. La pubalgie: syndrome “fourre-tout” pur une plus grande riguer diagnostique et therapeutique. Intantanes Medicaux. 1984;55:25-30.
23. Lischuk AW, Dorantes TM, Wong W, Haims AH. Imaging of sports-related hip and groin injuries. Sports Health. 2010;2(3):252-261.
24. Gibbon WW, Hession PR. Diseases of the pubis and pubic symphysis: MR imaging appearances. AJR Am J Roentgenol. 1997;169(3):849-853.
25. Gamble JG, Simmons SC, Freedman M. The symphysis pubis. Anatomic and pathologic considerations. Clin Orthop Relat Res. 1986;(203):261-272.
26. Larson CM. Sports hernia/athletic pubalgia: evaluation and management. Sports Health. 2014;6(2):139-144.
27. Maffulli N, Loppini M, Longo UG, Denaro V. Bilateral mini-invasive adductor tenotomy for the management of chronic unilateral adductor longus tendinopathy in athletes. Am J Sports Med. 2012;40(8):1880-1886.
28. Schilders E, Dimitrakopoulou A, Cooke M, Bismil Q, Cooke C. Effectiveness of a selective partial adductor release for chronic adductor-related groin pain in professional athletes. Am J Sports Med. 2013;41(3):603-607.
29. Scholten PM, Massimi S, Dahmen N, Diamond J, Wyss J. Successful treatment of athletic pubalgia in a lacrosse player with ultrasound-guided needle tenotomy and platelet-rich plasma injection: a case report. PM R. 2015;7(1):79-83.
The past 3 decades have seen an evolution in the understanding, diagnosis, and treatment of groin pain, both chronic and acute, in athletes and non-athletes alike. Groin pain and groin injury are common. Most cases are transient, with patients returning to their activities within weeks or months. There has also been increasing awareness of a definitive population of patients who do not get better, or who improve and plateau before reaching preinjury level of performance.1-3 Several authors have brought more attention to the injury, introducing vocabulary, theories, diagnostic testing, and diagnoses, which now constitute a knowledge base.1,3-5
As stated in almost every article on groin pain and diagnosis, lack of cohesive agreement and vocabulary, and consistent protocols and procedures, has abounded, making general understanding and agreement in this area inconsistent.1,6-8In this article, members of a tertiary-care group specializing in chronic groin pain, athletic pubalgia (sports hernia), and inguinal herniorrhaphy outline their clinical examination, diagnostic algorithm, imaging protocol, treatment strategy, and outcomes for a population of patients referred by physicians and allied health professionals for a suspected diagnosis of athletic pubalgia.
Background
The pubic symphysis acts as a stabilizing central anchor with elaborate involvement of the anterior structures, including the rectus abdominis, adductor longus, and inguinal ligaments.3,7,9 Literature from Europe, Australia, and the United States has described groin pain, mostly in professional athletes, involving these pubic structures and attachments. Several publications have been addressing chronic groin pain, and each has its own diagnostic algorithm, imaging protocol, and treatment strategy.3,6,9-18
Terminology specific to groin pain in athletes is not new, and has a varied history dating to the early 20th century. Terms such as sportsman hernia19 and subsequently sports hernia20, have recently been embraced by the lay population. In 1999, Gibbon21 described shearing of the common adductor–rectus abdominis anatomical and functional unit and referenced a 1902 anatomical text that describes vertical ligamentous fibers contiguous with rectus sheath and adductor muscles, both attaching to the pubis. Injury to this region is the basis of pubalgia, a term originally used in 1984 by Brunet to describe a pain syndrome at the pubis.22
Many authors have proposed replacing sports hernia with athletic pubalgia.1,3,6,7,10,14,18,23 These terms refer to a group of musculoskeletal processes that occur in and around the pubic symphysis and that share similar mechanisms of injury and common clinical manifestations. The condition was originally described in high-performance athletes, and at one point the term sports hernia was reserved for this patient population.5 According to many authors, presence of an inguinal hernia excludes the diagnosis.1,2,5Magnetic resonance imaging (MRI) has helped to advance and define our understanding of the injury.10 As the history of the literature suggests, earlier concepts of chronic pain focused either on the medial aspect of the inguinal canal and its structures or on the pubic attachments. Many specialists in the area have concluded that the chronic groin pain injury can and often does embody both elements.3,9 Correlation with MRI findings, injury seen during surgical procedures, and cadaveric studies have directed our understanding to a structure, the pre-pubic aponeurotic complex (P-PAC), or rectus aponeurotic plate.12,24,25 Anatomically, the P-PAC, which has several fascial components, attaches posteriorly to the pubic bone and, to a degree, the pubic symphyseal cartilaginous disc. Major contributions to the P-PAC are fibers from the rectus abdominis tendon, the medial aspect of the transversalis and internal oblique muscles (the conjoint tendon, according to some), the inguinal ligament, and the adductor longus tendon.26When communicating with referring physicians, we use the term athletic pubalgia to indicate a specific injury. The athletic pubalgia injury can be defined as serial microtearing,1 or complete tearing, of the posterior attachment of the P-PAC off the anterior pubis.3,10 Complete tearing or displacement can occur unilaterally or across the midline to the other side. As athletic pubalgia is a specific anatomical injury rather than a broad category of findings, an additional pathologic diagnosis, such as inguinal hernia, does not exclude the diagnosis of athletic pubalgia. Unfortunately, the terms sports hernia and sportsman hernia, commonly used in the media and in professional communities, have largely confused the broader understanding of nuances and of the differences between the specific injuries and MRI findings.18
Our Experience
In our practice, we see groin pain patients referred by internists, physiatrists, physical therapists, trainers, general surgeons, urologists, gynecologists, and orthopedic surgeons. In many cases, patients have been through several consultations and work-ups, as their pain syndrome does not fall under a specific category. Patients without inguinal hernia, hip injury, urologic, or gynecologic issues typically are referred to a physiatrist or a physical therapist. Often, there are marginal improvements with physical therapy, but in some cases the injury never completely resolves, and the patient continues to have pain with activity or return to sports.
Most of our patients are nonprofessional athletes, men and women who range widely in age and participate casually or regularly in sporting events. Most lack the rigorous training, conditioning, and close supervision that professional athletes receive. Many other patients are nonprofessional but elite athletes who train 7 days a week for marathons, ultramarathons, triathlons, obstacle course races (“mudders”), and similar events.
Work-Up
A single algorithm is used for all patients initially referred to the surgeon’s office for pelvic or groin pain. The initial interview directs attention to injury onset and mechanism, duration of rest or physical therapy after surgery, pain quality and pain levels, and antagonistic movements and positions. Examination starts with assessment for inguinal, femoral, and umbilical hernias. Resisted sit-up, leg-raise, adduction, and hip assessment tests are performed. The P-PAC is examined with a maneuver similar to the one used for inguinal hernia, as it allows for better assessment of the transversalis fascia (over the direct space) to determine if the inguinal canal floor is attenuated and bulges forward with the Valsalva maneuver. Then, the lateral aspect of the rectus muscle is assessed for pain, usually with the head raised to contract the muscle, to determine tenderness along the lateral border. The rectus edge is traced down to the pubis at its attachment, the superolateral border of the P-PAC. Examination proceeds medially, over the rectus attachment, toward the pubic symphysis, continuing the assessment for tenderness. Laterally, the conjoint tendon and inguinal ligament medial attachments are assessed at the level of the pubic tubercle, which represents the lateral border of the P-PAC. Finally, the examination continues to the inferior border with assessment of the adductor longus attachment, which is best performed with the leg in an adducted position. In the acute or semiacute setting (pain within 1 year of injury onset), tenderness is often elicited. With long-standing injuries, pain is often not elicited, but the patient experiences pain along this axis during activity or afterward.
Patients with positive history and physical examination findings proceed through an MRI protocol designed to detect pathology of the pubic symphysis, hips, and inguinal canals (Figures 1A-1D).
Treatment
Patients who report sustaining an acute groin injury within the previous 6 months are treated nonoperatively. A combination of rest, nonsteroidal anti-inflammatory drugs, and physical therapy is generally recommended.2,10 In cases of failed nonoperative management, patients are evaluated for surgery. No single operation is recommended for all patients.1,6,14,27,28 (Larson26 recently reviewed results from several trials involving a variety of surgical repairs and found return-to-sports rates ranging from 80% to 100%.) Findings from the physical examination and from the properly protocolled MRI examination are used in planning surgery to correct any pathology that could be contributing to symptoms or destabilization of the structures attaching to the pubis. Disruption of the P-PAC from the pubis would be repaired, for example. Additional injuries, such as partial or complete detachment of the conjoint tendon or inguinal ligament, may be repaired as well. If the transversalis fascia is attenuated and bulging forward, the inguinal floor is closed. Adductor longus tendon pathology is addressed, most commonly with partial tendinolysis. Often, concomitant inguinal hernias are found, and these may be repaired in open fashion while other maneuvers are being performed, or laparoscopically.
Materials and Methods
After receiving study approval from our Institutional Review Board, we retrospectively searched for all MRIs performed by our radiology department between March 1, 2011 and March 31, 2013 on patients referred for an indication of groin pain, sports hernia, or athletic pubalgia. Patients were excluded if they were younger than 18 years any time during their care. Some patients previously or subsequently underwent computed tomography or ultrasonography. MRIs were reviewed and positive findings were compiled in a database. Charts were reviewed to identify which patients in the dataset underwent surgery, after MRI, to address their presenting chief complaint. Surgery date and procedure(s) performed were recorded. Patients were interviewed by telephone as part of the in-office postoperative follow-up.
Results
One hundred nineteen MRIs were performed on 117 patients (97 men, 83%). Mean age was 39.8 years. Seventy-nine patients (68%) had an MRI finding of athletic pubalgia, 67 (57%) had an acetabular labral tear in one or both hip joints, and 41 (35%) had a true inguinal hernia. Concomitant findings were common: 47 cases of athletic pubalgia and labral tear(s), 28 cases of athletic pubalgia and inguinal hernia, and 15 cases of all 3 (athletic pubalgia, labral tear, inguinal hernia).
Use of breath-hold axial single-shot fast spin-echo sequences with and without the Valsalva maneuver increased sensitivity in detecting pathologies—inguinal hernia and Gilmore groin in particular. On 24 of the 119 MRIs, the Valsalva maneuver either revealed the finding or made it significantly more apparent.
Of all patients referred for MRI for chronic groin pain, 48 (41%) subsequently underwent surgery. In 29 surgeries, the rectus abdominis, adductor longus, and/or pre-pubic aponeurotic plate were repaired; in 13 cases, herniorrhaphy was performed as well; in 2 cases, masses involving the spermatic cord were removed.
The most common surgery (30 cases) was herniorrhaphy, which was performed as a single procedure, multiple procedures, or in combination with procedures not related to a true hernia. Eighteen patients underwent surgery only for hernia repair.
Of the 79 patients with MRI-positive athletic pubalgia, 39 subsequently underwent surgery, and 31 (79%) of these were followed up by telephone. Mean duration of rest after surgery was 6.2 weeks. Twelve patients (39%) had physical therapy after surgery, some as early as 4 weeks, and some have continued their therapy since surgery. Of the 31 patients who were followed up after surgery, 23 (74%) resumed previous activity levels. Return to previous activity level took these patients a mean of 17.9 weeks. When asked if outcomes satisfied their expectations, 28 patients (90%) said yes, and 3 said no.
Forty patients with MRI-positive athletic pubalgia were nonoperatively treated, and 28 (70%) of these patients were followed up. In this group, mean duration of rest after surgery was 6.9 weeks. Thirteen patients (46%) participated in physical therapy, for a mean duration of 10.8 weeks. Of the patients followed up, 19 (68%) returned to previous activity level. Twenty-one patients (75%) were satisfied with their outcome.
Discussion
Diagnosis and treatment of chronic groin pain have had a long, confusing, and frustrating history for both patients and the medical professionals who provide them with care.3,6,7,10 Historically, the problem has been, in part, the lack of diagnostic capabilities. Currently, however, pubalgia MRI protocol allows the exact pathology to be demonstrated.3 As already noted, concomitant hip pathology or inguinal hernia is not unusual8; any structural abnormality in the area is a potential destabilizer of the structures attached to the pubis.18 Solving only one of these issues may offer only partial resolution of symptoms and thereby reduce the rate of successful treatment of groin pain.
Diagnostic algorithms are being developed. In addition, nonoperative treatments are being tried for some of the issues. Physicians are giving diagnostic and therapeutic steroid injections in the pubic cleft, along the rectus abdominis/adductor longus complex, or posterior to the P-PAC. Platelet-rich plasma injection therapy has had limited success.29This article provides a snapshot of what a tertiary-care group of physicians specializing in chronic groin pain sees in an unfiltered setting. We think this is instructive for several reasons.
First, many patients in our population have visited a multitude of specialists without receiving a diagnosis or being referred appropriately. Simply, many specialists do not know the next step in treating groin pain and thus do not make the appropriate referral. Until recently, the literature has not been helpful. It has poorly described the constellation of injuries comprising chronic groin pain. More significantly, groin injuries have been presented as ambiguous injuries lacking effective treatment. Over the past decade, however, abundant literature on the correlation of these injuries with specific MRI findings has made the case otherwise.
Second, a specific MRI pubalgia protocol is needed. Inability to make a correct diagnosis, because of improper MRI, continues to add to the confusion surrounding the injury and undoubtedly prolongs the general medical community’s thinking that diagnosis and treatment of chronic groin pain are elusive. Our data support this point in many ways. Although all patients in this study were seen by a medical professional before coming to our office, none had received a diagnosis of occult hernia or attenuated transversalis fascia; nevertheless, we identified inguinal hernia, Gilmore groin, or both in 44% of these patients. These findings are not surprising, as MRI was the crucial link in diagnosis. In addition, the point made by other groin pain specialists—that a hernia precludes a pubalgia diagnosis1,2,5—is not supported by our data. Inguinal hernia can and does exist in conjunction with pubalgia. More than half the patients in our study had a combined diagnosis. We contend that, much as hip labral pathology occurs concomitantly with pubalgia,23 inguinal hernia may be a predisposing factor as well. A defect in the direct or indirect space can destabilize the area and place additional strain on the pubic attachments.
In our experience, the dynamic Valsalva sequence improves detection of true hernias and anterior abdominal wall deficiencies and should be included in each protocol for the evaluation of acute or chronic groin pain.
Shear forces and injury at the pubis can occur outside professional athletics. Our patient population is nonprofessional athletes, teenagers to retirees, and all can develop athletic pubalgia. Ninety percent of surveyed patients who received a diagnosis and were treated surgically were satisfied with their outcomes.
Am J Orthop. 2017;46(4):E251-E256. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
The past 3 decades have seen an evolution in the understanding, diagnosis, and treatment of groin pain, both chronic and acute, in athletes and non-athletes alike. Groin pain and groin injury are common. Most cases are transient, with patients returning to their activities within weeks or months. There has also been increasing awareness of a definitive population of patients who do not get better, or who improve and plateau before reaching preinjury level of performance.1-3 Several authors have brought more attention to the injury, introducing vocabulary, theories, diagnostic testing, and diagnoses, which now constitute a knowledge base.1,3-5
As stated in almost every article on groin pain and diagnosis, lack of cohesive agreement and vocabulary, and consistent protocols and procedures, has abounded, making general understanding and agreement in this area inconsistent.1,6-8In this article, members of a tertiary-care group specializing in chronic groin pain, athletic pubalgia (sports hernia), and inguinal herniorrhaphy outline their clinical examination, diagnostic algorithm, imaging protocol, treatment strategy, and outcomes for a population of patients referred by physicians and allied health professionals for a suspected diagnosis of athletic pubalgia.
Background
The pubic symphysis acts as a stabilizing central anchor with elaborate involvement of the anterior structures, including the rectus abdominis, adductor longus, and inguinal ligaments.3,7,9 Literature from Europe, Australia, and the United States has described groin pain, mostly in professional athletes, involving these pubic structures and attachments. Several publications have been addressing chronic groin pain, and each has its own diagnostic algorithm, imaging protocol, and treatment strategy.3,6,9-18
Terminology specific to groin pain in athletes is not new, and has a varied history dating to the early 20th century. Terms such as sportsman hernia19 and subsequently sports hernia20, have recently been embraced by the lay population. In 1999, Gibbon21 described shearing of the common adductor–rectus abdominis anatomical and functional unit and referenced a 1902 anatomical text that describes vertical ligamentous fibers contiguous with rectus sheath and adductor muscles, both attaching to the pubis. Injury to this region is the basis of pubalgia, a term originally used in 1984 by Brunet to describe a pain syndrome at the pubis.22
Many authors have proposed replacing sports hernia with athletic pubalgia.1,3,6,7,10,14,18,23 These terms refer to a group of musculoskeletal processes that occur in and around the pubic symphysis and that share similar mechanisms of injury and common clinical manifestations. The condition was originally described in high-performance athletes, and at one point the term sports hernia was reserved for this patient population.5 According to many authors, presence of an inguinal hernia excludes the diagnosis.1,2,5Magnetic resonance imaging (MRI) has helped to advance and define our understanding of the injury.10 As the history of the literature suggests, earlier concepts of chronic pain focused either on the medial aspect of the inguinal canal and its structures or on the pubic attachments. Many specialists in the area have concluded that the chronic groin pain injury can and often does embody both elements.3,9 Correlation with MRI findings, injury seen during surgical procedures, and cadaveric studies have directed our understanding to a structure, the pre-pubic aponeurotic complex (P-PAC), or rectus aponeurotic plate.12,24,25 Anatomically, the P-PAC, which has several fascial components, attaches posteriorly to the pubic bone and, to a degree, the pubic symphyseal cartilaginous disc. Major contributions to the P-PAC are fibers from the rectus abdominis tendon, the medial aspect of the transversalis and internal oblique muscles (the conjoint tendon, according to some), the inguinal ligament, and the adductor longus tendon.26When communicating with referring physicians, we use the term athletic pubalgia to indicate a specific injury. The athletic pubalgia injury can be defined as serial microtearing,1 or complete tearing, of the posterior attachment of the P-PAC off the anterior pubis.3,10 Complete tearing or displacement can occur unilaterally or across the midline to the other side. As athletic pubalgia is a specific anatomical injury rather than a broad category of findings, an additional pathologic diagnosis, such as inguinal hernia, does not exclude the diagnosis of athletic pubalgia. Unfortunately, the terms sports hernia and sportsman hernia, commonly used in the media and in professional communities, have largely confused the broader understanding of nuances and of the differences between the specific injuries and MRI findings.18
Our Experience
In our practice, we see groin pain patients referred by internists, physiatrists, physical therapists, trainers, general surgeons, urologists, gynecologists, and orthopedic surgeons. In many cases, patients have been through several consultations and work-ups, as their pain syndrome does not fall under a specific category. Patients without inguinal hernia, hip injury, urologic, or gynecologic issues typically are referred to a physiatrist or a physical therapist. Often, there are marginal improvements with physical therapy, but in some cases the injury never completely resolves, and the patient continues to have pain with activity or return to sports.
Most of our patients are nonprofessional athletes, men and women who range widely in age and participate casually or regularly in sporting events. Most lack the rigorous training, conditioning, and close supervision that professional athletes receive. Many other patients are nonprofessional but elite athletes who train 7 days a week for marathons, ultramarathons, triathlons, obstacle course races (“mudders”), and similar events.
Work-Up
A single algorithm is used for all patients initially referred to the surgeon’s office for pelvic or groin pain. The initial interview directs attention to injury onset and mechanism, duration of rest or physical therapy after surgery, pain quality and pain levels, and antagonistic movements and positions. Examination starts with assessment for inguinal, femoral, and umbilical hernias. Resisted sit-up, leg-raise, adduction, and hip assessment tests are performed. The P-PAC is examined with a maneuver similar to the one used for inguinal hernia, as it allows for better assessment of the transversalis fascia (over the direct space) to determine if the inguinal canal floor is attenuated and bulges forward with the Valsalva maneuver. Then, the lateral aspect of the rectus muscle is assessed for pain, usually with the head raised to contract the muscle, to determine tenderness along the lateral border. The rectus edge is traced down to the pubis at its attachment, the superolateral border of the P-PAC. Examination proceeds medially, over the rectus attachment, toward the pubic symphysis, continuing the assessment for tenderness. Laterally, the conjoint tendon and inguinal ligament medial attachments are assessed at the level of the pubic tubercle, which represents the lateral border of the P-PAC. Finally, the examination continues to the inferior border with assessment of the adductor longus attachment, which is best performed with the leg in an adducted position. In the acute or semiacute setting (pain within 1 year of injury onset), tenderness is often elicited. With long-standing injuries, pain is often not elicited, but the patient experiences pain along this axis during activity or afterward.
Patients with positive history and physical examination findings proceed through an MRI protocol designed to detect pathology of the pubic symphysis, hips, and inguinal canals (Figures 1A-1D).
Treatment
Patients who report sustaining an acute groin injury within the previous 6 months are treated nonoperatively. A combination of rest, nonsteroidal anti-inflammatory drugs, and physical therapy is generally recommended.2,10 In cases of failed nonoperative management, patients are evaluated for surgery. No single operation is recommended for all patients.1,6,14,27,28 (Larson26 recently reviewed results from several trials involving a variety of surgical repairs and found return-to-sports rates ranging from 80% to 100%.) Findings from the physical examination and from the properly protocolled MRI examination are used in planning surgery to correct any pathology that could be contributing to symptoms or destabilization of the structures attaching to the pubis. Disruption of the P-PAC from the pubis would be repaired, for example. Additional injuries, such as partial or complete detachment of the conjoint tendon or inguinal ligament, may be repaired as well. If the transversalis fascia is attenuated and bulging forward, the inguinal floor is closed. Adductor longus tendon pathology is addressed, most commonly with partial tendinolysis. Often, concomitant inguinal hernias are found, and these may be repaired in open fashion while other maneuvers are being performed, or laparoscopically.
Materials and Methods
After receiving study approval from our Institutional Review Board, we retrospectively searched for all MRIs performed by our radiology department between March 1, 2011 and March 31, 2013 on patients referred for an indication of groin pain, sports hernia, or athletic pubalgia. Patients were excluded if they were younger than 18 years any time during their care. Some patients previously or subsequently underwent computed tomography or ultrasonography. MRIs were reviewed and positive findings were compiled in a database. Charts were reviewed to identify which patients in the dataset underwent surgery, after MRI, to address their presenting chief complaint. Surgery date and procedure(s) performed were recorded. Patients were interviewed by telephone as part of the in-office postoperative follow-up.
Results
One hundred nineteen MRIs were performed on 117 patients (97 men, 83%). Mean age was 39.8 years. Seventy-nine patients (68%) had an MRI finding of athletic pubalgia, 67 (57%) had an acetabular labral tear in one or both hip joints, and 41 (35%) had a true inguinal hernia. Concomitant findings were common: 47 cases of athletic pubalgia and labral tear(s), 28 cases of athletic pubalgia and inguinal hernia, and 15 cases of all 3 (athletic pubalgia, labral tear, inguinal hernia).
Use of breath-hold axial single-shot fast spin-echo sequences with and without the Valsalva maneuver increased sensitivity in detecting pathologies—inguinal hernia and Gilmore groin in particular. On 24 of the 119 MRIs, the Valsalva maneuver either revealed the finding or made it significantly more apparent.
Of all patients referred for MRI for chronic groin pain, 48 (41%) subsequently underwent surgery. In 29 surgeries, the rectus abdominis, adductor longus, and/or pre-pubic aponeurotic plate were repaired; in 13 cases, herniorrhaphy was performed as well; in 2 cases, masses involving the spermatic cord were removed.
The most common surgery (30 cases) was herniorrhaphy, which was performed as a single procedure, multiple procedures, or in combination with procedures not related to a true hernia. Eighteen patients underwent surgery only for hernia repair.
Of the 79 patients with MRI-positive athletic pubalgia, 39 subsequently underwent surgery, and 31 (79%) of these were followed up by telephone. Mean duration of rest after surgery was 6.2 weeks. Twelve patients (39%) had physical therapy after surgery, some as early as 4 weeks, and some have continued their therapy since surgery. Of the 31 patients who were followed up after surgery, 23 (74%) resumed previous activity levels. Return to previous activity level took these patients a mean of 17.9 weeks. When asked if outcomes satisfied their expectations, 28 patients (90%) said yes, and 3 said no.
Forty patients with MRI-positive athletic pubalgia were nonoperatively treated, and 28 (70%) of these patients were followed up. In this group, mean duration of rest after surgery was 6.9 weeks. Thirteen patients (46%) participated in physical therapy, for a mean duration of 10.8 weeks. Of the patients followed up, 19 (68%) returned to previous activity level. Twenty-one patients (75%) were satisfied with their outcome.
Discussion
Diagnosis and treatment of chronic groin pain have had a long, confusing, and frustrating history for both patients and the medical professionals who provide them with care.3,6,7,10 Historically, the problem has been, in part, the lack of diagnostic capabilities. Currently, however, pubalgia MRI protocol allows the exact pathology to be demonstrated.3 As already noted, concomitant hip pathology or inguinal hernia is not unusual8; any structural abnormality in the area is a potential destabilizer of the structures attached to the pubis.18 Solving only one of these issues may offer only partial resolution of symptoms and thereby reduce the rate of successful treatment of groin pain.
Diagnostic algorithms are being developed. In addition, nonoperative treatments are being tried for some of the issues. Physicians are giving diagnostic and therapeutic steroid injections in the pubic cleft, along the rectus abdominis/adductor longus complex, or posterior to the P-PAC. Platelet-rich plasma injection therapy has had limited success.29This article provides a snapshot of what a tertiary-care group of physicians specializing in chronic groin pain sees in an unfiltered setting. We think this is instructive for several reasons.
First, many patients in our population have visited a multitude of specialists without receiving a diagnosis or being referred appropriately. Simply, many specialists do not know the next step in treating groin pain and thus do not make the appropriate referral. Until recently, the literature has not been helpful. It has poorly described the constellation of injuries comprising chronic groin pain. More significantly, groin injuries have been presented as ambiguous injuries lacking effective treatment. Over the past decade, however, abundant literature on the correlation of these injuries with specific MRI findings has made the case otherwise.
Second, a specific MRI pubalgia protocol is needed. Inability to make a correct diagnosis, because of improper MRI, continues to add to the confusion surrounding the injury and undoubtedly prolongs the general medical community’s thinking that diagnosis and treatment of chronic groin pain are elusive. Our data support this point in many ways. Although all patients in this study were seen by a medical professional before coming to our office, none had received a diagnosis of occult hernia or attenuated transversalis fascia; nevertheless, we identified inguinal hernia, Gilmore groin, or both in 44% of these patients. These findings are not surprising, as MRI was the crucial link in diagnosis. In addition, the point made by other groin pain specialists—that a hernia precludes a pubalgia diagnosis1,2,5—is not supported by our data. Inguinal hernia can and does exist in conjunction with pubalgia. More than half the patients in our study had a combined diagnosis. We contend that, much as hip labral pathology occurs concomitantly with pubalgia,23 inguinal hernia may be a predisposing factor as well. A defect in the direct or indirect space can destabilize the area and place additional strain on the pubic attachments.
In our experience, the dynamic Valsalva sequence improves detection of true hernias and anterior abdominal wall deficiencies and should be included in each protocol for the evaluation of acute or chronic groin pain.
Shear forces and injury at the pubis can occur outside professional athletics. Our patient population is nonprofessional athletes, teenagers to retirees, and all can develop athletic pubalgia. Ninety percent of surveyed patients who received a diagnosis and were treated surgically were satisfied with their outcomes.
Am J Orthop. 2017;46(4):E251-E256. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
1. Meyers WC, Lanfranco A, Castellanos A. Surgical management of chronic lower abdominal and groin pain in high-performance athletes. Curr Sports Med Rep. 2002;1(5):301-305.
2. Ahumada LA, Ashruf S, Espinosa-de-los-Monteros A, et al. Athletic pubalgia: definition and surgical treatment. Ann Plast Surg. 2005;55(4):393-396.
3. Omar IM, Zoga AC, Kavanagh EC, et al. Athletic pubalgia and “sports hernia”: optimal MR imaging technique and findings. Radiographics. 2008;28(5):1415-1438.
4. Gilmore OJA. Gilmore’s groin: ten years experience of groin disruption—a previously unsolved problem in sportsmen. Sports Med Soft Tissue Trauma. 1991;3:12-14.
5. Meyers WC, Foley DP, Garrett WE, Lohnes JH, Mandlebaum BR. Management of severe lower abdominal or inguinal pain in high-performance athletes. PAIN (Performing Athletes with Abdominal or Inguinal Neuromuscular Pain Study Group). Am J Sports Med. 2000;28(1):2-8.
6. Kavanagh EC, Koulouris G, Ford S, McMahon P, Johnson C, Eustace SJ. MR imaging of groin pain in the athlete. Semin Musculoskelet Radiol. 2006;10(3):197-207.
7. Cunningham PM, Brennan D, O’Connell M, MacMahon P, O’Neill P, Eustace S. Patterns of bone and soft-tissue injury at the symphysis pubis in soccer players: observations at MRI. AJR Am J Roentgenol. 2007;188(3):W291-W296.
8. Zoga AC, Kavanagh EC, Omar IM, et al. Athletic pubalgia and the “sports hernia”: MR imaging findings. Radiology. 2008;247(3):797-807.
9. Koulouris G. Imaging review of groin pain in elite athletes: an anatomic approach to imaging findings. AJR Am J Roentgenol. 2008;191(4):962-972.
10. Albers SL, Spritzer CE, Garrett WE Jr, Meyers WC. MR findings in athletes with pubalgia. Skeletal Radiol. 2001;30(5):270-277.
11. Brennan D, O’Connell MJ, Ryan M, et al. Secondary cleft sign as a marker of injury in athletes with groin pain: MR image appearance and interpretation. Radiology. 2005;235(1):162-167.
12. Robinson P, Salehi F, Grainger A, et al. Cadaveric and MRI study of the musculotendinous contributions to the capsule of the symphysis pubis. AJR Am J Roentgenol. 2007;188(5):W440-W445.
13. Schilders E, Talbot JC, Robinson P, Dimitrakopoulou A, Gibbon WW, Bismil Q. Adductor-related groin pain in recreational athletes. J Bone Joint Surg Am. 2009;91(10):2455-2460.
14. Davies AG, Clarke AW, Gilmore J, Wotherspoon M, Connell DA. Review: imaging of groin pain in the athlete. Skeletal Radiol. 2010;39(7):629-644.
15. Mullens FE, Zoga AC, Morrison WB, Meyers WC. Review of MRI technique and imaging findings in athletic pubalgia and the “sports hernia.” Eur J Radiol. 2012;81(12):3780-3792.
16. Zoga AC, Meyers WC. Magnetic resonance imaging for pain after surgical treatment for athletic pubalgia and the “sports hernia.” Semin Musculoskelet Radiol. 2011;15(4):372-382.
17. Beer E. Periostitis of symphysis and descending rami of pubes following suprapubic operations. Int J Med Surg. 1924;37(5):224-225.
18. MacMahon PJ, Hogan BA, Shelly MJ, Eustace SJ, Kavanagh EC. Imaging of groin pain. Magn Reson Imaging Clin N Am. 2009;17(4):655-666.
19. Malycha P, Lovell G. Inguinal surgery in athletes with chronic groin pain: the ‘sportsman’s’ hernia. Aust N Z J Surg. 1992;62(2):123-125.
20. Hackney RG. The sports hernia: a cause of chronic groin pain. Br J Sports Med. 1993;27(1):58-62.
21. Gibbon WW. Groin pain in athletes. Lancet. 1999;353(9162):1444-1445.
22. Brunet B, Brunet-Geudj E, Genety J. La pubalgie: syndrome “fourre-tout” pur une plus grande riguer diagnostique et therapeutique. Intantanes Medicaux. 1984;55:25-30.
23. Lischuk AW, Dorantes TM, Wong W, Haims AH. Imaging of sports-related hip and groin injuries. Sports Health. 2010;2(3):252-261.
24. Gibbon WW, Hession PR. Diseases of the pubis and pubic symphysis: MR imaging appearances. AJR Am J Roentgenol. 1997;169(3):849-853.
25. Gamble JG, Simmons SC, Freedman M. The symphysis pubis. Anatomic and pathologic considerations. Clin Orthop Relat Res. 1986;(203):261-272.
26. Larson CM. Sports hernia/athletic pubalgia: evaluation and management. Sports Health. 2014;6(2):139-144.
27. Maffulli N, Loppini M, Longo UG, Denaro V. Bilateral mini-invasive adductor tenotomy for the management of chronic unilateral adductor longus tendinopathy in athletes. Am J Sports Med. 2012;40(8):1880-1886.
28. Schilders E, Dimitrakopoulou A, Cooke M, Bismil Q, Cooke C. Effectiveness of a selective partial adductor release for chronic adductor-related groin pain in professional athletes. Am J Sports Med. 2013;41(3):603-607.
29. Scholten PM, Massimi S, Dahmen N, Diamond J, Wyss J. Successful treatment of athletic pubalgia in a lacrosse player with ultrasound-guided needle tenotomy and platelet-rich plasma injection: a case report. PM R. 2015;7(1):79-83.
1. Meyers WC, Lanfranco A, Castellanos A. Surgical management of chronic lower abdominal and groin pain in high-performance athletes. Curr Sports Med Rep. 2002;1(5):301-305.
2. Ahumada LA, Ashruf S, Espinosa-de-los-Monteros A, et al. Athletic pubalgia: definition and surgical treatment. Ann Plast Surg. 2005;55(4):393-396.
3. Omar IM, Zoga AC, Kavanagh EC, et al. Athletic pubalgia and “sports hernia”: optimal MR imaging technique and findings. Radiographics. 2008;28(5):1415-1438.
4. Gilmore OJA. Gilmore’s groin: ten years experience of groin disruption—a previously unsolved problem in sportsmen. Sports Med Soft Tissue Trauma. 1991;3:12-14.
5. Meyers WC, Foley DP, Garrett WE, Lohnes JH, Mandlebaum BR. Management of severe lower abdominal or inguinal pain in high-performance athletes. PAIN (Performing Athletes with Abdominal or Inguinal Neuromuscular Pain Study Group). Am J Sports Med. 2000;28(1):2-8.
6. Kavanagh EC, Koulouris G, Ford S, McMahon P, Johnson C, Eustace SJ. MR imaging of groin pain in the athlete. Semin Musculoskelet Radiol. 2006;10(3):197-207.
7. Cunningham PM, Brennan D, O’Connell M, MacMahon P, O’Neill P, Eustace S. Patterns of bone and soft-tissue injury at the symphysis pubis in soccer players: observations at MRI. AJR Am J Roentgenol. 2007;188(3):W291-W296.
8. Zoga AC, Kavanagh EC, Omar IM, et al. Athletic pubalgia and the “sports hernia”: MR imaging findings. Radiology. 2008;247(3):797-807.
9. Koulouris G. Imaging review of groin pain in elite athletes: an anatomic approach to imaging findings. AJR Am J Roentgenol. 2008;191(4):962-972.
10. Albers SL, Spritzer CE, Garrett WE Jr, Meyers WC. MR findings in athletes with pubalgia. Skeletal Radiol. 2001;30(5):270-277.
11. Brennan D, O’Connell MJ, Ryan M, et al. Secondary cleft sign as a marker of injury in athletes with groin pain: MR image appearance and interpretation. Radiology. 2005;235(1):162-167.
12. Robinson P, Salehi F, Grainger A, et al. Cadaveric and MRI study of the musculotendinous contributions to the capsule of the symphysis pubis. AJR Am J Roentgenol. 2007;188(5):W440-W445.
13. Schilders E, Talbot JC, Robinson P, Dimitrakopoulou A, Gibbon WW, Bismil Q. Adductor-related groin pain in recreational athletes. J Bone Joint Surg Am. 2009;91(10):2455-2460.
14. Davies AG, Clarke AW, Gilmore J, Wotherspoon M, Connell DA. Review: imaging of groin pain in the athlete. Skeletal Radiol. 2010;39(7):629-644.
15. Mullens FE, Zoga AC, Morrison WB, Meyers WC. Review of MRI technique and imaging findings in athletic pubalgia and the “sports hernia.” Eur J Radiol. 2012;81(12):3780-3792.
16. Zoga AC, Meyers WC. Magnetic resonance imaging for pain after surgical treatment for athletic pubalgia and the “sports hernia.” Semin Musculoskelet Radiol. 2011;15(4):372-382.
17. Beer E. Periostitis of symphysis and descending rami of pubes following suprapubic operations. Int J Med Surg. 1924;37(5):224-225.
18. MacMahon PJ, Hogan BA, Shelly MJ, Eustace SJ, Kavanagh EC. Imaging of groin pain. Magn Reson Imaging Clin N Am. 2009;17(4):655-666.
19. Malycha P, Lovell G. Inguinal surgery in athletes with chronic groin pain: the ‘sportsman’s’ hernia. Aust N Z J Surg. 1992;62(2):123-125.
20. Hackney RG. The sports hernia: a cause of chronic groin pain. Br J Sports Med. 1993;27(1):58-62.
21. Gibbon WW. Groin pain in athletes. Lancet. 1999;353(9162):1444-1445.
22. Brunet B, Brunet-Geudj E, Genety J. La pubalgie: syndrome “fourre-tout” pur une plus grande riguer diagnostique et therapeutique. Intantanes Medicaux. 1984;55:25-30.
23. Lischuk AW, Dorantes TM, Wong W, Haims AH. Imaging of sports-related hip and groin injuries. Sports Health. 2010;2(3):252-261.
24. Gibbon WW, Hession PR. Diseases of the pubis and pubic symphysis: MR imaging appearances. AJR Am J Roentgenol. 1997;169(3):849-853.
25. Gamble JG, Simmons SC, Freedman M. The symphysis pubis. Anatomic and pathologic considerations. Clin Orthop Relat Res. 1986;(203):261-272.
26. Larson CM. Sports hernia/athletic pubalgia: evaluation and management. Sports Health. 2014;6(2):139-144.
27. Maffulli N, Loppini M, Longo UG, Denaro V. Bilateral mini-invasive adductor tenotomy for the management of chronic unilateral adductor longus tendinopathy in athletes. Am J Sports Med. 2012;40(8):1880-1886.
28. Schilders E, Dimitrakopoulou A, Cooke M, Bismil Q, Cooke C. Effectiveness of a selective partial adductor release for chronic adductor-related groin pain in professional athletes. Am J Sports Med. 2013;41(3):603-607.
29. Scholten PM, Massimi S, Dahmen N, Diamond J, Wyss J. Successful treatment of athletic pubalgia in a lacrosse player with ultrasound-guided needle tenotomy and platelet-rich plasma injection: a case report. PM R. 2015;7(1):79-83.
The End of a Season
Spring, it symbolizes a new beginning. The smell of fresh cut grass hangs in the air, and it’s my favorite time of the sports year. A new season has begun in baseball, and the NHL and NBA playoffs are underway. As a new season begins, two more draw to a close. In this fan’s opinion, there is nothing quite as exciting as playoff hockey, and this month AJO hopes to “Capital-ize” on that excitement by presenting the hockey issue.
In “The Ice Hockey Issue”, Popkin and colleagues present a review of upper extremity injuries in hockey, which will serve as a guide for sports medicine physicians covering hockey games. There’s even a segment covering dental and ocular injuries, in case you don’t have a dentist or ophthalmologist handy. While we typically no longer publish case reports, Degen and colleagues present a unique report detailing an unusual injury to a prominent NHL goaltender. AJO presents it to expand your diagnostic differential for neck injuries.
I had another reason in mind when I mentioned the end of a season in this month’s editorial. The new AJO has seen a lot of changes, and it is our Editorial Team’s goal to continuously improve the journal and to provide timely features that are directly relevant to your practice. We’ve updated our website, and we’ve added some features, such as QR codes and take-home points, to improve your reading experience. But our ability to further enhance the journal is limited in print, and our web statistics show that a large percentage of our readers view the articles on their smartphones.
As I’ve written before, these are challenging times for printed media. The digital age has arrived and technology has made traditional publications less appealing. Our younger readers now demand a portable, electronic, media-rich publication that provides information that directly benefits their practices. To provide this, we envision a digital journal that is immersed in a learning environment, with videos, technique guides, and supplementary materials just a click away.
A few months back, AJO tested the digital waters. Our trial met with a positive response, and so, it is with great excitement that we announce that beginning in 2018, AJO will be the first orthopedic journal to go “All Digital.”
To further our goal of creating material that directly impacts your practice, we will present each feature review article as a learning module. The articles will feature extensive photos and videos, PowerPoint presentations for download, test questions, and patient information sheets. We will publish authors’ preference cards and postoperative protocols.
We’re currently developing applications and tools to improve your interactive experience. In the coming months, look for announcements regarding new strategic partnerships and features that will become mainstays of our electronic environment.
I hope you share the excitement of a new beginning in the digital era. I know the transition will provide a greatly enhanced, valuable resource that will change the way we utilize journals in our practice.
Am J Orthop. 2017;46(3):122. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
Spring, it symbolizes a new beginning. The smell of fresh cut grass hangs in the air, and it’s my favorite time of the sports year. A new season has begun in baseball, and the NHL and NBA playoffs are underway. As a new season begins, two more draw to a close. In this fan’s opinion, there is nothing quite as exciting as playoff hockey, and this month AJO hopes to “Capital-ize” on that excitement by presenting the hockey issue.
In “The Ice Hockey Issue”, Popkin and colleagues present a review of upper extremity injuries in hockey, which will serve as a guide for sports medicine physicians covering hockey games. There’s even a segment covering dental and ocular injuries, in case you don’t have a dentist or ophthalmologist handy. While we typically no longer publish case reports, Degen and colleagues present a unique report detailing an unusual injury to a prominent NHL goaltender. AJO presents it to expand your diagnostic differential for neck injuries.
I had another reason in mind when I mentioned the end of a season in this month’s editorial. The new AJO has seen a lot of changes, and it is our Editorial Team’s goal to continuously improve the journal and to provide timely features that are directly relevant to your practice. We’ve updated our website, and we’ve added some features, such as QR codes and take-home points, to improve your reading experience. But our ability to further enhance the journal is limited in print, and our web statistics show that a large percentage of our readers view the articles on their smartphones.
As I’ve written before, these are challenging times for printed media. The digital age has arrived and technology has made traditional publications less appealing. Our younger readers now demand a portable, electronic, media-rich publication that provides information that directly benefits their practices. To provide this, we envision a digital journal that is immersed in a learning environment, with videos, technique guides, and supplementary materials just a click away.
A few months back, AJO tested the digital waters. Our trial met with a positive response, and so, it is with great excitement that we announce that beginning in 2018, AJO will be the first orthopedic journal to go “All Digital.”
To further our goal of creating material that directly impacts your practice, we will present each feature review article as a learning module. The articles will feature extensive photos and videos, PowerPoint presentations for download, test questions, and patient information sheets. We will publish authors’ preference cards and postoperative protocols.
We’re currently developing applications and tools to improve your interactive experience. In the coming months, look for announcements regarding new strategic partnerships and features that will become mainstays of our electronic environment.
I hope you share the excitement of a new beginning in the digital era. I know the transition will provide a greatly enhanced, valuable resource that will change the way we utilize journals in our practice.
Am J Orthop. 2017;46(3):122. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
Spring, it symbolizes a new beginning. The smell of fresh cut grass hangs in the air, and it’s my favorite time of the sports year. A new season has begun in baseball, and the NHL and NBA playoffs are underway. As a new season begins, two more draw to a close. In this fan’s opinion, there is nothing quite as exciting as playoff hockey, and this month AJO hopes to “Capital-ize” on that excitement by presenting the hockey issue.
In “The Ice Hockey Issue”, Popkin and colleagues present a review of upper extremity injuries in hockey, which will serve as a guide for sports medicine physicians covering hockey games. There’s even a segment covering dental and ocular injuries, in case you don’t have a dentist or ophthalmologist handy. While we typically no longer publish case reports, Degen and colleagues present a unique report detailing an unusual injury to a prominent NHL goaltender. AJO presents it to expand your diagnostic differential for neck injuries.
I had another reason in mind when I mentioned the end of a season in this month’s editorial. The new AJO has seen a lot of changes, and it is our Editorial Team’s goal to continuously improve the journal and to provide timely features that are directly relevant to your practice. We’ve updated our website, and we’ve added some features, such as QR codes and take-home points, to improve your reading experience. But our ability to further enhance the journal is limited in print, and our web statistics show that a large percentage of our readers view the articles on their smartphones.
As I’ve written before, these are challenging times for printed media. The digital age has arrived and technology has made traditional publications less appealing. Our younger readers now demand a portable, electronic, media-rich publication that provides information that directly benefits their practices. To provide this, we envision a digital journal that is immersed in a learning environment, with videos, technique guides, and supplementary materials just a click away.
A few months back, AJO tested the digital waters. Our trial met with a positive response, and so, it is with great excitement that we announce that beginning in 2018, AJO will be the first orthopedic journal to go “All Digital.”
To further our goal of creating material that directly impacts your practice, we will present each feature review article as a learning module. The articles will feature extensive photos and videos, PowerPoint presentations for download, test questions, and patient information sheets. We will publish authors’ preference cards and postoperative protocols.
We’re currently developing applications and tools to improve your interactive experience. In the coming months, look for announcements regarding new strategic partnerships and features that will become mainstays of our electronic environment.
I hope you share the excitement of a new beginning in the digital era. I know the transition will provide a greatly enhanced, valuable resource that will change the way we utilize journals in our practice.
Am J Orthop. 2017;46(3):122. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
Head, Neck, and Shoulder Injuries in Ice Hockey: Current Concepts
Take-Home Points
- Hockey is a high-speed collision sport with one of the highest injury rates among all sports.
- Use of a helmet with visors or full-face shields significantly reduces the risk for eye injury.
- Broken portions of teeth should be found and placed in a protective medium such as saline, saliva, or milk for transport.
- A player with unresolved concussion symptoms should not be allowed to return to the ice.
- Shoulder dominance, which determines stick grip, is an important consideration in the treatment of shoulder instability in an ice hockey player.
On a surface of ice in Windsor, Nova Scotia in the middle of the 19th century, the modern game of ice hockey evolved.1 A blend of hurley, a Gaelic sport, and lacrosse, from the native Mi’kmaq culture, the sport of ice hockey gained rapidly in popularity throughout Canada and is now the country’s national sport. Hockey quickly spread to the United States and then Europe. It is presently played in 77 countries across the world.2
Hockey players can reach speeds of up to 48 km (~30 miles) per hour on razor-sharp skates on an ice surface surrounded by rigid plastic composite boards topped with plexiglass.3 They use sticks made of wood, aluminum, or a composite material to advance a 6-ounce vulcanized rubber puck on the opposing goal, and this puck sometimes reaches speeds over 160 km (~100 miles) per hour. Older, male players are allowed to make physical contact with their opposing counterparts to separate them from the puck (body-checking). Not surprisingly, the potential risk for injury in hockey is high. At the 2010 Winter Olympics, men’s ice hockey players had the highest rate of injury of any other competitors there—more than 30% were affected.4
Hockey is played and enjoyed by athletes ranging widely in age. Youth hockey leagues accept players as young as 5 years. Hockey can become a lifelong recreational activity. In North America, old timers’ leagues have many players up to age 70 years.6 According to International Ice Hockey Federation data for 2016, more than 543,000 and 639,500 people play hockey in the United States and Canada, respectively.2 Most of the rules, protective equipment, skates, ice surfaces, and goal sizes are the same in men’s and women’s hockey.7 The major difference is in body-checking—this practice is not allowed at any age in women’s ice hockey.
In this article, we review the evaluation and management of common head, neck, and shoulder hockey injuries for physicians who provide medical support and coverage for youth, amateur, and senior hockey teams.
Evaluation and Management of Common Hockey Injuries
Eye Injuries
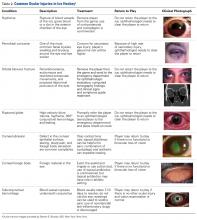
Although eye injuries are less common than musculoskeletal injuries and concussions in hockey, they are a serious risk for recreational and competitive players alike. Furthermore, recovery may be difficult, and eye injuries can have serious lifelong consequences.8 In hockey, the most commonly reported eye injuries are periorbital contusions and lacerations, hyphema, corneal and conjunctival abrasions, orbital fractures, and ruptured globes (Table 2).9,10
As a contact sport, hockey often involves high-impact, blunt-force trauma. The trauma in hockey results from collisions with other players, the boards, hockey sticks, and pucks. It is therefore not surprising that the most common ocular injuries in this sport are periorbital contusions. Although most contusions cause only mild swelling and ecchymosis of the soft tissues around the eye, there is potential for serious consequences. In a Scandinavia study, Leivo and colleagues10 found that 9% of patients who sustained a periocular contusion also had a clinically significant secondary diagnosis, such as retinal tear or hemorrhage, eyelid laceration, vitreous hemorrhage, or retinal detachment. Although the study was hospital-based, and therefore biased toward more severe cases, its findings highlight the potential severity of eye injuries in hockey. Furthermore, the study found that the majority of players who sustained blunt trauma to the eye itself required lifelong follow-up because of increased risk for glaucoma. This is particularly true for hyphema, as this finding indicates significant damage to intraocular tissues.10Players can also sustain fractures of the orbital bones, including orbital blowout fractures. Typical signs and symptoms of blowout fractures include diplopia, proptosis or enophthalmos, infraorbital hypoesthesia, painful and decreased extraocular movement (particularly upgaze), and palpable crepitance caused by sinus air entering the lower eyelid.11 If orbital fracture is suspected, as it should be in any case in which the injured player experiences pain with eye movement or diplopia, the player should be referred to the ED for computed tomography (CT) and ophthalmologic evaluation.12 Continued participation seriously risks making the injury much worse, particularly should another impact occur. In addition, given the impact needed to cause orbital fractures, consideration must be given to the potential for a coexisting concussion injury.
Severe direct trauma to the eye—from a puck, a stick, or a fist—can result in a ruptured globe, a particularly serious injury that requires immediate surgical attention. Signs and symptoms of a ruptured globe are rarely subtle, but associated eyelid swelling or laceration may obscure the injury, delaying proper diagnosis and treatment. More obvious signs include severely reduced vision, hemorrhagic chemosis (swelling) of the conjunctiva, and an irregular or peaked pupil. If a rupture or any significant intraocular injury is suspected, it is crucial to avoid applying any pressure to the globe, as this can significantly worsen the damage to the intraocular tissues. Use of a helmet with protective shields and cages attached markedly reduces the risk for such injuries.13All eye injuries require prompt assessment, which allows for appropriate management and prevention of secondary damage.14 Initial evaluation of a patient with ocular trauma should begin with external examination for lacerations, swelling, or orbital rim step-off deformity. The physician should also check visual acuity in order to assess for significant vision impairment (counting fingers or reading a sign in the arena; confrontation visual fields). This should be done before attending to any periocular injuries, with the uninjured side serving as a control. Next, the physician should assess the extraocular eye movements as well as the size, shape, and reactivity of the pupils. Particular attention should be paid to detecting any deficit in extraocular movement or irregularity in pupil size, shape, or reactivity, as such findings are highly suggestive of serious injury to the globe.13 Hyphema (blood in anterior chamber of eye anterior to pupil) should be suspected if vision is reduced and the pupil cannot be clearly visualized. However, a bright red clot is not always apparent at time of injury or if the amount of blood is small. An irregular pupil, or a pupil that does not constrict well to light, is also a red flag for serious contusion injury to the eye, and requires ophthalmologic evaluation. It is important to keep in mind that blunt trauma severe enough to produce hyphema or an irregular and poorly reactive pupil is often associated with retinal damage as well, including retinal edema or detachment.
Minor injuries (eg, small foreign bodies, minor periocular contusions and lacerations) can often be managed rink-side. Foreign bodies not embedded in the cornea, but lodged under the upper eyelid, can sometimes be removed by everting the eyelid and sweeping with a moistened cotton swab or using diffuse, sterile saline irrigation.11 Corneal abrasions generally cause severe pain, photophobia, and tearing and are easily diagnosed with use of topical fluorescein and a blue light. A topical anesthetic can be extremely helpful in this setting, as it allows for proper pain-free evaluation, but should never be used in an ongoing manner for pain relief. Small lacerations of the brow can be sutured with 5-0 or 6-0 nylon or closed with 2-Octyl cyanoacrylate tissue adhesive (Dermabond). Eyelid lacerations, unless very small, are best managed by an ophthalmologist; care must be taken to rule out injury to the deeper orbital tissues and eye. If serious injury is suspected, or the eye cannot be appropriately evaluated, it should be stabilized and protected with a protective shield or plastic cup, and the player should be transferred to an ED for appropriate ophthalmologic evaluation.13Most eye injuries are accidental, caused by sticks or deflected pucks, but 18% are acquired in fights.8 Use of visors or full-face cages effectively minimizes the rate of eye injuries.8,13,15,16 In a cohort study of 282 elite amateur ice hockey players, the risk of eye injury was 4.7 times higher in players without face protection than in players who used half-face shields; there were no eye injuries in players who used full-face protection.13 For visors to prevent eye injury, they must be positioned to cover the eyes and the lower edge of the nose in all projections.10
Dental Injuries
The incidence and type of facial and dental injuries depend directly on the type of face protection used.11,17,18 In a study of face, head, and neck injuries in elite amateur ice hockey players, Stuart and colleagues13 found game-related injury rates of 158.9 per 1000 player-hours in players without face protection, 73.5 in players who used half-face shields, and 23.2 in players who used full-face shields. Players who wore full-face shields had facial, head, and neck injury rates of only 23.2 per 1000 player-game hours.13 Other studies clearly support the important role face shields play in lowering injury risk in hockey. Face and head injuries account for 20% to 40% of all hockey-related injuries,3,16,19 and dental injuries up to 11.5%.20 In a study from Finland, Lahti and colleagues19 found that over a 2-year period, 479 hockey players sustained injuries, including 650 separate dental injuries. The most commonly diagnosed dental injury was an uncomplicated crown fracture, and the most common cause was a hit with a hockey stick, which accounted for 52.7% and 40.3% of dental injuries in games and practices, respectively.19
In the management of dental fractures, the broken portions of teeth should be found and placed in a transportation-protective medium, such as saline, saliva, or milk,16 which can improve functional and esthetic replacement outcomes.21,22 Loose pieces of teeth should not be left in the player’s mouth. The residual tooth should be stabilized and exposure to air and occlusion limited. Dental fractures can affect the enamel, the enamel and dentin structures (uncomplicated fracture), or enamel, dentin, and pulp (complicated).23 Fractures involving only the enamel do not require urgent dental evaluation. Dentin or pulp involvement may cause temperature and air sensitivity.23 If a tooth is air-sensitive, the player should be referred to a specialist immediately.11
Direct trauma can cause instability without displacement (subluxation) or complete displacement of the tooth from its alveolar socket (avulsion).23 An avulsed tooth should be handled by the crown to avoid further damage to the root and periodontal ligament.16,24 The tooth should be rinsed gently with saline and reimplanted in its socket, ideally within 5 to 10 minutes,23with the athlete biting down gently on gauze to hold the tooth in place. A 1-mL supraperiosteal infiltration of 1% or 2% lidocaine hydrochloride (1:100,000 epinephrine) can be given into the apex of the tooth being anesthetized (Figure 1).
Concussions
A concussion is a “complex pathophysiological process affecting the brain, induced by traumatic biomechanical forces.”25 Concussion is largely a functional disturbance instead of a structural injury, owing to the rotational and/or shearing forces involved. Many studies have identified concussion as the most common type of injury in all of youth hockey.26 Concussions account for up to 19% of all injuries in men’s collegiate hockey.3
Concussion can be challenging to diagnose on the ice. The most important factor in concussion management is symptom reporting by the athlete.27 Despite significant efforts in education and awareness, student athletes, especially hockey players, withhold reporting a possible concussion.28 Reasons for underreporting include fear of letting down other players and coaches, thinking the injury is not severe enough to warrant evaluation, and fear of losing standing with the current team or future teams.28
As postinjury concussion assessments are ideal when comparisons can be made with preseason (baseline) scores, preseason testing is becoming standard in professional, college, junior, and high school hockey. This testing involves the Sport Concussion Assessment Tool, 3rd edition (SCAT3), and the King-Devick (K-D) test.30,31 Some youth leagues have baseline testing as well, though the frequency of baseline testing in their players is controversial,32 as the adolescent mind’s processing speed and memory increase exponentially.33 For these younger athletes, it may be necessary to perform baseline testing more frequently than annually.32 A physician can use baseline test results to help diagnose a concussion at the rink and then track the athlete’s recovery and help with return-to-play decisions.29 Vision involves almost half of the brain’s circuits,34 including areas vulnerable to head impact. A neuro-ophthalmologic test can assess for irregularities in accommodation, convergence, ocular muscle balance, pursuit, and saccades.29 The K-D test is a visual performance examination that allows easy and objective assessment of eye movements. Use of both the K-D test and the SCAT3 at the rink may increase the number of concussions detected.29,35 We recommend that physicians use both tests to assess for concussion at the hockey rink.
Initial treatment involves a period of physical rest and relative cognitive rest. Acute worsening of symptoms warrants urgent imaging to rule out a subdural or subarachnoid bleed. Once a player is symptom-free, a graded return-to-play protocol should be followed (Table 4).
On the prevention side, great efforts have been made to improve hockey helmets. (Some manufacturers claim to have made concussion-proof helmets, but there is no evidence supporting this claim.6) Numerous investigators have reported a lower overall injury rate in players who wear a helmet and a full-face shield.6,13 In addition, rule changes aimed at decreasing head contact have been implemented to decrease the incidence of sport-related concussions.36 Moreover, education on proper helmet use and wear should be emphasized. A study of the effects of hockey helmet fit on cervical motion found that 7 (39%) of 18 players wore a game or competition helmet so loosely that it could be removed without unbuttoning its chinstrap.37 Improperly worn helmets cannot prevent injury as well as properly worn helmets can.
Cervical Spine Injuries
Whereas American football is associated with a higher annual number of nonfatal catastrophic neck injuries, hockey has a 3 to 6 times higher incidence of cervical spine injuries and spinal cord damage.38,39 A Canadian Ice Hockey Spinal Injuries Registry review of the period 2006 to 2011 identified 44 cervical spine injuries, 7.3 per year on average.40 Severe injury, defined as complete motor and sensory loss, complete motor loss and incomplete sensory, or complete motor loss, occurred in 4 (9.1%) of the 44 injured players. In hockey, a major mechanism of cervical spine injury is an axial load to the slightly flexed spine.39 Of 355 hockey-related cervical spine injuries in a Canada study, 95 (35.5%) were caused by a check from behind.40,41 The Canadian neurosurgeons’ work led to rule changes prohibiting checks from behind, and this prohibition has reduced the incidence of cervical spine injuries in ice hockey.38,40
Team physicians should be comfortable managing serious neck and spine injuries on the ice. Initial evaluation should follow the standard ABCs (airway, breathing, circulation). The physician places a hand on each side of the head to stabilize the neck until the initial examination is complete. The goal is to minimize cervical spine motion until transportation to the hospital for advanced imaging and definitive treatment.37 The decision to remove or leave on the helmet is now controversial. Hockey helmets differ from football helmets in that their chinstraps do not afford significant cervical stabilization, and the helmets have less padding and cover less of the head; in addition, a shockingly high percentage of hockey players do not wear properly fitting helmets.37 In one study, 3-dimensional motion analysis of a hockey player during the logroll technique showed less transverse and sagittal cervical plane motion with the helmet removed than with the helmet (properly fitting or not) in place; the authors recommended removing the helmet to limit extraneous cervical spine motion during the technique.37 However, 2 other studies found that helmet removal can result in significantly increased cervical spine motion of the immobilized hockey player.42,43Recommendation 4 of the recently released interassociation consensus statement of the National Athletic Trainers’ Association reads, “Protective athletic equipment should be removed before transport to an emergency facility for an athlete-patient with suspected cervical spine instability.”44 This represents a shift from leaving the helmet and shoulder pads in place. For ice hockey players with suspected cervical spine injury, more research is needed on cervical motion during the entire sequence—partial logrolls, spine-boarding, placement of cervical collar before or after logroll, and different immobilization techniques for transport.37
The athlete must be carefully transferred to a spine board with either logroll or lift-and-slide. Although an extrication cervical collar can be placed before the spine board is placed, the effectiveness of this collar in executing the spine-board transfer is not proven.45 When the player is on the spine board, the head can be secured with pads and straps en route to the hospital.
Return-to-Play Criteria for Cervical Spine Injuries There is no clear consensus on return-to-play guidelines for cervical spine injuries in athletes.46
Shoulder Injuries
For hockey players, the upper extremity traditionally has been considered a well-protected area.48 However, shoulder pads are considerably more flexible in hockey than in football and other collision sports. In addition, hockey gloves allow a fair amount of motion for stick handling, and the wrist may be in maximal flexion or extension when a hit against the boards or the ice occurs. Open-ice checking, board collisions, and hockey stick use have been postulated as reasons for the high incidence of upper extremity injuries in hockey. Researchers in Finland found that upper extremity injuries accounted for up to 31% of all hockey injuries.49 More than 50% of these injuries resulted from checking or board collisions. Furthermore, study findings highlighted a low rate of injury in younger players and indicated the rate increases with age.49,50
In hockey players, the acromioclavicular (AC) joint is the most commonly injured shoulder structure.51 The mechanism of injury can be a board collision or an open-ice hit, but most often is a direct blow to the shoulder. The collision disrupts the AC joint and can sprain or tear the coracoclavicular ligaments. The Rockwood classification is used to categorize AC joint injuries (Figure 2).
Initial management involves icing the AC joint and placing a sling for comfort. Type I and type II injuries can be managed with progressive range-of-motion (ROM) exercises, strengthening, cryotherapy, and a period of rest. Treatment of type III injuries remains controversial,52 but in hockey players these injuries are almost always treated nonoperatively. Return to play requires full motion, normal strength, and minimal discomfort. Players return a few days to 2 weeks after a grade I injury; recovery from grade II injuries may take 2 to 3 weeks, and recovery from grade III injuries, 6 to 12 weeks. Surgical treatment is usually required in type IV and type V injuries, but we have had experience treating these injuries nonoperatively in high-level players. AC joint reinjury in hockey players is common, and surgical treatment should be approached cautiously, as delayed fracture after return to sport has been reported.53 Special precautions should be taken in collision athletes who undergo AC joint reconstruction. In the anatomical reconstruction described by Carofino and Mazzocca,54 2 holes are drilled in the clavicle; these holes are a potential source of fracture when the collision athlete returns to sport (Figure 3).
Clavicle fracture is another common hockey injury.55 Studies have shown clavicle fractures proportionally occur most often in people 15 to 19 years old.49 The injury presents with pain and deformity over the clavicle; in more severe fractures, skin tenting is identified. Initial management of suspected clavicle fracture includes cryotherapy, sling, and radiographs. Radiographs should include an AP view and then a 45° cephalad view, which eliminates overshadowing from the ribs. Most clavicle fractures are successfully managed nonoperatively, though there is evidence that significantly displaced or comminuted fractures have better union rates and shoulder function when treated with open reduction and internal fixation.56 After a clavicle fracture, return to skating and noncontact practice usually takes 8 weeks, with return to full contact occurring around 12 weeks.
Sternoclavicular injuries are relatively uncommon, but potentially serious. Special attention should also be given to adolescent athletes with sternoclavicular pain. Although sternoclavicular dislocations have been reported in hockey players, instead these likely are fractures involving the medial clavicle physis.57
The shoulder is the most commonly dislocated major joint, and the incidence of shoulder dislocation in elite hockey players is 8% to 21%.50,58 Anterior shoulder instability occurs from a fall with the shoulder in an abducted, externally rotated and extended position or from a direct anteriorly placed impact to the posterior shoulder. We recommend taking players off the ice for evaluation. Depending on physician comfort, the shoulder can be reduced in the training room, and the athlete sent for radiographs after reduction. If resources or support for closed reduction is not available at the rink, the athlete should be sent to the ED. Initial radiographic evaluation of a player with shoulder injury begins with plain radiographs, including a true AP (Grashey) view with the humerus in neutral, internal, and external rotation and an axillary view. The axillary radiograph is crucial in determining anterior or posterior dislocation. If the patient cannot tolerate the pain associated with having an axillary radiograph taken, a Velpeau radiograph can be used. This radiograph is taken with the patient’s arm in a sling and with the patient leaning back 30° while the x-ray beam is directed superior to inferior.
CT is performed for a suspected osseous injury. CT is more accurate than plain radiographs in showing glenoid and humeral fractures in the acute setting as well as the amount of bone loss in the case of chronic instability. Magnetic resonance arthrography is the imaging modality of choice for the diagnoses of capsulolabral injury.
After shoulder reduction, treatment with a sling, cryotherapy, and a nonsteroidal anti-inflammatory drug is initiated. In a Minnesota study of nonoperative management of shoulder instability, 9 of 10 hockey players were able to return to play the same season, and 6 of the 10 required surgery at the end of the season.59
Compared with noncontact athletes, hockey players and other collision athletes are at increased risk for recurrence.60-62 For collision athletes who want to continue playing their sport after recurrent instability, surgery is recommended. A shoulder instability study in Toronto found that more than 54% of 24 professional hockey players had associated Hill-Sachs lesions, but only 3 shoulders (12.5%) had glenoid defects.50 Arthroscopic and open techniques both demonstrate good results, and identification of bone loss can help determine which surgery to recommend.63 Hockey players can usually return to sport 6 months after shoulder stabilization.
Another important consideration in managing shoulder instability in hockey players is shoulder dominance, which determines stick grip. A left-handed player places the right hand on top of the stick for support, but most of the motion associated with shooting the puck—including abduction and external rotation—occurs with the left shoulder. Thus, a left-handed player with a history of previous left-side shoulder dislocation may dislocate with each shot, but a right-handed player with left shoulder instability may have considerably less trouble on the ice.58Shoulder and rotator cuff contusions (RCCs) occur in hockey and other collision sports.49,64 RCCs almost always result from a direct blow to the shoulder, and present with shoulder function loss, weakness, and pain.
Summary
Hockey is a high-speed collision sport with one of the highest injury rates among all sports. Physicians caring for youth, amateur, and senior hockey teams see a range of acute head, neck, and shoulder injuries. Although treatment of eye injuries, dental injuries, and concussions is not always considered orthopedic care, an orthopedic surgeon who is covering hockey needs to be comfortable managing these injuries acutely. Quality rink-side care minimizes the impact of the injury, maximizes the functional result, and expedites the safe return of the injured player back to the ice.
Am J Orthop. 2017;46(3):123-134. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
1. Vaughan G. The Puck Starts Here: The Origin of Canada’s Great Winter Game, Ice Hockey. Fredericton, Canada: Goose Lane Editions; 1996.
2. IIHF member national associations. International Ice Hockey Federation website. http://www.iihf.com/iihf-home/the-iihf/members. Accessed April 6, 2017.
3. Flik K, Lyman S, Marx RG. American collegiate men’s ice hockey: an analysis of injuries. Am J Sports Med. 2005;33(2):183-187.
4. Engebretsen L, Steffen K, Alonso JM, et al. Sports injuries and illnesses during the Winter Olympic Games 2010. Br J Sports Med. 2010;44(11):772-780.
5. Deits J, Yard EE, Collins CL, Fields SK, Comstock RD. Patients with ice hockey injuries presenting to US emergency departments, 1990-2006. J Athl Train. 2010;45(5):467-474.
6. Brooks A, Loud KJ, Brenner JS, et al. Reducing injury risk from body checking in boys’ youth ice hockey. Pediatrics. 2014;133(6):1151-1157.
7. Agel J, Harvey EJ. A 7-year review of men’s and women’s ice hockey injuries in the NCAA. Can J Surg. 2010;53(5):319-323.
8. Micieli JA, Zurakowski D, Ahmed, II. Impact of visors on eye and orbital injuries in the National Hockey League. Can J Ophthalmol. 2014;49(3):243-248.
9. Pashby TJ. Ocular injuries in hockey. Int Ophthalmol Clin. 1988;28(3):228-231.
10. Leivo T, Haavisto AK, Sahraravand A. Sports-related eye injuries: the current picture. Acta Ophthalmol. 2015;93(3):224-231.
11. Cohn RM, Alaia MJ, Strauss EJ, Feldman AF. Rink-side management of ice hockey related injuries to the face, neck, and chest. Bull Hosp Jt Dis. 2013;71(4):253-256.
12. Reehal P. Facial injury in sport. Curr Sports Med Rep. 2010;9(1):27-34.
13. Stuart MJ, Smith AM, Malo-Ortiguera SA, Fischer TL, Larson DR. A comparison of facial protection and the incidence of head, neck, and facial injuries in Junior A hockey players. A function of individual playing time. Am J Sports Med. 2002;30(1):39-44.
14. MacEwen CJ, McLatchie GR. Eye injuries in sport. Scott Med J. 2010;55(2):22-24.
15. Stevens ST, Lassonde M, de Beaumont L, Keenan JP. The effect of visors on head and facial injury in National Hockey League players. J Sci Med Sport. 2006;9(3):238-242.
16. Moslener MD, Wadsworth LT. Ice hockey: a team physician’s perspective. Curr Sports Med Rep. 2010;9(3):134-138.
17. LaPrade RF, Burnett QM, Zarzour R, Moss R. The effect of the mandatory use of face masks on facial lacerations and head and neck injuries in ice hockey. A prospective study. Am J Sports Med. 1995;23(6):773-775.
18. Benson BW, Mohtadi NG, Rose MS, Meeuwisse WH. Head and neck injuries among ice hockey players wearing full face shields vs half face shields. JAMA. 1999;282(24):2328-2332.
19. Lahti H, Sane J, Ylipaavalniemi P. Dental injuries in ice hockey games and training. Med Sci Sports Exerc. 2002;34(3):400-402.
20. Sane J, Ylipaavalniemi P, Leppanen H. Maxillofacial and dental ice hockey injuries. Med Sci Sports Exerc. 1988;20(2):202-207.
21. Emerich K, Kaczmarek J. First aid for dental trauma caused by sports activities: state of knowledge, treatment and prevention. Sports Med. 2010;40(5):361-366.
22. Rosenberg H, Rosenberg H, Hickey M. Emergency management of a traumatic tooth avulsion. Ann Emerg Med. 2011;57(4):375-377.
23. Young EJ, Macias CR, Stephens L. Common dental injury management in athletes. Sports Health. 2015;7(3):250-255.
24. Andersson L, Andreasen JO, Day P, et al. International Association of Dental Traumatology guidelines for the management of traumatic dental injuries: 2. Avulsion of permanent teeth. Dent Traumatol. 2012;28(2):88-96.
25. McCrory P, Meeuwisse W, Johnston K, et al. Consensus statement on concussion in sport 3rd International Conference on Concussion in Sport held in Zurich, November 2008. Clin J Sport Med. 2009;19(3):185-200.
26. Schneider KJ, Meeuwisse WH, Kang J, Schneider GM, Emery CA. Preseason reports of neck pain, dizziness, and headache as risk factors for concussion in male youth ice hockey players. Clin J Sport Med. 2013;23(4):267-272.
27. McCrory P, Meeuwisse WH, Aubry M, et al. Consensus statement on concussion in sport: the 4th International Conference on Concussion in Sport held in Zurich, November 2012. Br J Sports Med. 2013;47(5):250-258.
28. Delaney JS, Lamfookon C, Bloom GA, Al-Kashmiri A, Correa JA. Why university athletes choose not to reveal their concussion symptoms during a practice or game. Clin J Sport Med. 2015;25(2):113-125.
29. Ventura RE, Balcer LJ, Galetta SL. The concussion toolbox: the role of vision in the assessment of concussion. Semin Neurol. 2015;35(5):599-606.
30. Vartiainen MV, Holm A, Peltonen K, Luoto TM, Iverson GL, Hokkanen L. King-Devick test normative reference values for professional male ice hockey players. Scand J Med Sci Sports. 2015;25(3):e327-e330.
31. Galetta MS, Galetta KM, McCrossin J, et al. Saccades and memory: baseline associations of the King-Devick and SCAT2 SAC tests in professional ice hockey players. J Neurol Sci. 2013;328(1-2):28-31.
32. Vernau BT, Grady MF, Goodman A, et al. Oculomotor and neurocognitive assessment of youth ice hockey players: baseline associations and observations after concussion. Dev Neuropsychol. 2015;40(1):7-11.
33. Fry AF, Hale S. Relationships among processing speed, working memory, and fluid intelligence in children. Biol Psychol. 2000;54(1-3):1-34.
34. Felleman DJ, Van Essen DC. Distributed hierarchical processing in the primate cerebral cortex. Cereb Cortex. 1991;1(1):1-47.
35. Guskiewicz KM, Register-Mihalik J, McCrory P, et al. Evidence-based approach to revising the SCAT2: introducing the SCAT3. Br J Sports Med. 2013;47(5):289-293.
36. Smith AM, Stuart MJ, Dodick DW, et al. Ice Hockey Summit II: zero tolerance for head hits and fighting. Curr Sports Med Rep. 2015;14(2):135-144.
37. Mihalik JP, Beard JR, Petschauer MA, Prentice WE, Guskiewicz KM. Effect of ice hockey helmet fit on cervical spine motion during an emergency log roll procedure. Clin J Sport Med. 2008;18(5):394-398.
38. Banerjee R, Palumbo MA, Fadale PD. Catastrophic cervical spine injuries in the collision sport athlete, part 1: epidemiology, functional anatomy, and diagnosis. Am J Sports Med. 2004;32(4):1077-1087.
39. Reynen PD, Clancy WG Jr. Cervical spine injury, hockey helmets, and face masks. Am J Sports Med. 1994;22(2):167-170.
40. Tator CH, Provvidenza C, Cassidy JD. Update and overview of spinal injuries in Canadian ice hockey, 1943 to 2011: the continuing need for injury prevention and education. Clin J Sport Med. 2016;26(3):232-238.
41. Tator CH, Edmonds VE, Lapczak L, Tator IB. Spinal injuries in ice hockey players, 1966-1987. Can J Surg. 1991;34(1):63-69.
42. Laprade RF, Schnetzler KA, Broxterman RJ, Wentorf F, Gilbert TJ. Cervical spine alignment in the immobilized ice hockey player. A computed tomographic analysis of the effects of helmet removal. Am J Sports Med. 2000;28(6):800-803.
43. Metz CM, Kuhn JE, Greenfield ML. Cervical spine alignment in immobilized hockey players: radiographic analysis with and without helmets and shoulder pads. Clin J Sport Med. 1998;8(2):92-95.
44. National Athletic Trainers’ Association. Appropriate prehospital management of the spine-injured athlete: updated from 1998 document. http://www.nata.org/sites/default/files/Executive-Summary-Spine-Injury-updated.pdf. Updated August 5, 2015. Accessed April 6, 2017.
45. Del Rossi G, Heffernan TP, Horodyski M, Rechtine GR. The effectiveness of extrication collars tested during the execution of spine-board transfer techniques. Spine J. 2004;4(6):619-623.
46. Morganti C, Sweeney CA, Albanese SA, Burak C, Hosea T, Connolly PJ. Return to play after cervical spine injury. Spine. 2001;26(10):1131-1136.
47. Huang P, Anissipour A, McGee W, Lemak L. Return-to-play recommendations after cervical, thoracic, and lumbar spine injuries: a comprehensive review. Sports Health. 2016;8(1):19-25.
48. Shindle MK, Marx RG, Kelly BT, Bisson L, Burke CJ 3rd. Hockey injuries: a pediatric sport update. Curr Opin Pediatr. 2010;22(1):54-60.
49. Molsa J, Kujala U, Myllynen P, Torstila I, Airaksinen O. Injuries to the upper extremity in ice hockey: analysis of a series of 760 injuries. Am J Sports Med. 2003;31(5):751-757.
50. Dwyer T, Petrera M, Bleakney R, Theodoropoulos JS. Shoulder instability in ice hockey players: incidence, mechanism, and MRI findings. Clin Sports Med. 2013;32(4):803-813.
51. LaPrade RF, Wijdicks CA, Griffith CJ. Division I intercollegiate ice hockey team coverage. Br J Sports Med. 2009;43(13):1000-1005.
52. Willimon SC, Gaskill TR, Millett PJ. Acromioclavicular joint injuries: anatomy, diagnosis, and treatment. Phys Sportsmed. 2011;39(1):116-122.
53. Martetschlager F, Horan MP, Warth RJ, Millett PJ. Complications after anatomic fixation and reconstruction of the coracoclavicular ligaments. Am J Sports Med. 2013;41(12):2896-2903.
54. Carofino BC, Mazzocca AD. The anatomic coracoclavicular ligament reconstruction: surgical technique and indications. J Shoulder Elbow Surg. 2010;19(2 suppl):37-46.
55. Laprade RF, Surowiec RK, Sochanska AN, et al. Epidemiology, identification, treatment and return to play of musculoskeletal-based ice hockey injuries. Br J Sports Med. 2014;48(1):4-10.
56. Canadian Orthopaedic Trauma Society. Nonoperative treatment compared with plate fixation of displaced midshaft clavicular fractures. A multicenter, randomized clinical trial. J Bone Joint Surg Am. 2007;89(1):1-10.
57. Lee JT, Nasreddine AY, Black EM, Bae DS, Kocher MS. Posterior sternoclavicular joint injuries in skeletally immature patients. J Pediatr Orthop. 2014;34(4):369-375.
58. Hovelius L. Shoulder dislocation in Swedish ice hockey players. Am J Sports Med. 1978;6(6):373-377.
59. Buss DD, Lynch GP, Meyer CP, Huber SM, Freehill MQ. Nonoperative management for in-season athletes with anterior shoulder instability. Am J Sports Med. 2004;32(6):1430-1433.
60. Mazzocca AD, Brown FM Jr, Carreira DS, Hayden J, Romeo AA. Arthroscopic anterior shoulder stabilization of collision and contact athletes. Am J Sports Med. 2005;33(1):52-60.
61. Harris JD, Romeo AA. Arthroscopic management of the contact athlete with instability. Clin Sports Med. 2013;32(4):709-730.
62. Cho NS, Hwang JC, Rhee YG. Arthroscopic stabilization in anterior shoulder instability: collision athletes versus noncollision athletes. Arthroscopy. 2006;22(9):947-953.
63. Griffin JW, Brockmeier SF. Shoulder instability with concomitant bone loss in the athlete. Orthop Clin North Am. 2015;46(1):89-103.
64. Cohen SB, Towers JD, Bradley JP. Rotator cuff contusions of the shoulder in professional football players: epidemiology and magnetic resonance imaging findings. Am J Sports Med. 2007;35(3):442-447.
65. Lorentzon R, Wedrèn H, Pietilä T. Incidence, nature, and causes of ice hockey injuries. A three-year prospective study of a Swedish elite ice hockey team. Am J Sports Med. 1988;16(4):392-396.
66. Stuart MJ, Smith A. Injuries in Junior A ice hockey. A three-year prospective study. Am J Sports Med. 1995;23(4):458-461.
67. Voaklander DC, Saunders LD, Quinney HA, Macnab RB. Epidemiology of recreational and old-timer ice hockey injuries. Clin J Sport Med. 1996;6(1):15-21.
68. Mölsä J, Airaksinen O, Näsman O, Torstila I. Ice hockey injuries in Finland. A prospective epidemiologic study. Am J Sports Med. 1997;25(4):495-499.
69. Ferrara MS, Schurr KT. Intercollegiate ice hockey injuries: a casual analysis. Clin J Sport Med. 1999;9(1):30-33.
70. Pinto M, Kuhn JE, Greenfield ML, Hawkins RJ. Prospective analysis of ice hockey injuries at the Junior A level over the course of one season. Clin J Sport Med. 1999;9(2):70-74.
71. Emery CA, Meeuwisse WH. Injury rates, risk factors, and mechanisms of injury in minor hockey. Am J Sports Med. 2006;34(12):1960-1969.
72. Kuzuhara K, Shimamoto H, Mase Y. Ice hockey injuries in a Japanese elite team: a 3-year prospective study. J Athl Train. 2009;44(2):208-214.
73. Rishiraj N, Lloyd-Smith R, Lorenz T, Niven B, Michel M. University men’s ice hockey: rates and risk of injuries over 6-years. J Sports Med Phys Fitness. 2009;49(2):159-166.
74. Tuominen M, Stuart MJ, Aubry M, Kannus P, Parkkari J. Injuries in men’s international ice hockey: a 7-year study of the International Ice Hockey Federation Adult World Championship Tournaments and Olympic Winter Games. Br J Sports Med. 2015;49(1):30-36.
75. Heckman JD, Bucholz RW. In: Rockwood CA, Green DP, Heckman JD, Bucholz RW, eds. Rockwood and Green’s Fractures in Adults, Volume 1. Philadelphia, PA: Lippincott Williams & Wilkins; 2001.
Take-Home Points
- Hockey is a high-speed collision sport with one of the highest injury rates among all sports.
- Use of a helmet with visors or full-face shields significantly reduces the risk for eye injury.
- Broken portions of teeth should be found and placed in a protective medium such as saline, saliva, or milk for transport.
- A player with unresolved concussion symptoms should not be allowed to return to the ice.
- Shoulder dominance, which determines stick grip, is an important consideration in the treatment of shoulder instability in an ice hockey player.
On a surface of ice in Windsor, Nova Scotia in the middle of the 19th century, the modern game of ice hockey evolved.1 A blend of hurley, a Gaelic sport, and lacrosse, from the native Mi’kmaq culture, the sport of ice hockey gained rapidly in popularity throughout Canada and is now the country’s national sport. Hockey quickly spread to the United States and then Europe. It is presently played in 77 countries across the world.2
Hockey players can reach speeds of up to 48 km (~30 miles) per hour on razor-sharp skates on an ice surface surrounded by rigid plastic composite boards topped with plexiglass.3 They use sticks made of wood, aluminum, or a composite material to advance a 6-ounce vulcanized rubber puck on the opposing goal, and this puck sometimes reaches speeds over 160 km (~100 miles) per hour. Older, male players are allowed to make physical contact with their opposing counterparts to separate them from the puck (body-checking). Not surprisingly, the potential risk for injury in hockey is high. At the 2010 Winter Olympics, men’s ice hockey players had the highest rate of injury of any other competitors there—more than 30% were affected.4
Hockey is played and enjoyed by athletes ranging widely in age. Youth hockey leagues accept players as young as 5 years. Hockey can become a lifelong recreational activity. In North America, old timers’ leagues have many players up to age 70 years.6 According to International Ice Hockey Federation data for 2016, more than 543,000 and 639,500 people play hockey in the United States and Canada, respectively.2 Most of the rules, protective equipment, skates, ice surfaces, and goal sizes are the same in men’s and women’s hockey.7 The major difference is in body-checking—this practice is not allowed at any age in women’s ice hockey.
In this article, we review the evaluation and management of common head, neck, and shoulder hockey injuries for physicians who provide medical support and coverage for youth, amateur, and senior hockey teams.
Evaluation and Management of Common Hockey Injuries
Eye Injuries
Although eye injuries are less common than musculoskeletal injuries and concussions in hockey, they are a serious risk for recreational and competitive players alike. Furthermore, recovery may be difficult, and eye injuries can have serious lifelong consequences.8 In hockey, the most commonly reported eye injuries are periorbital contusions and lacerations, hyphema, corneal and conjunctival abrasions, orbital fractures, and ruptured globes (Table 2).9,10
As a contact sport, hockey often involves high-impact, blunt-force trauma. The trauma in hockey results from collisions with other players, the boards, hockey sticks, and pucks. It is therefore not surprising that the most common ocular injuries in this sport are periorbital contusions. Although most contusions cause only mild swelling and ecchymosis of the soft tissues around the eye, there is potential for serious consequences. In a Scandinavia study, Leivo and colleagues10 found that 9% of patients who sustained a periocular contusion also had a clinically significant secondary diagnosis, such as retinal tear or hemorrhage, eyelid laceration, vitreous hemorrhage, or retinal detachment. Although the study was hospital-based, and therefore biased toward more severe cases, its findings highlight the potential severity of eye injuries in hockey. Furthermore, the study found that the majority of players who sustained blunt trauma to the eye itself required lifelong follow-up because of increased risk for glaucoma. This is particularly true for hyphema, as this finding indicates significant damage to intraocular tissues.10Players can also sustain fractures of the orbital bones, including orbital blowout fractures. Typical signs and symptoms of blowout fractures include diplopia, proptosis or enophthalmos, infraorbital hypoesthesia, painful and decreased extraocular movement (particularly upgaze), and palpable crepitance caused by sinus air entering the lower eyelid.11 If orbital fracture is suspected, as it should be in any case in which the injured player experiences pain with eye movement or diplopia, the player should be referred to the ED for computed tomography (CT) and ophthalmologic evaluation.12 Continued participation seriously risks making the injury much worse, particularly should another impact occur. In addition, given the impact needed to cause orbital fractures, consideration must be given to the potential for a coexisting concussion injury.
Severe direct trauma to the eye—from a puck, a stick, or a fist—can result in a ruptured globe, a particularly serious injury that requires immediate surgical attention. Signs and symptoms of a ruptured globe are rarely subtle, but associated eyelid swelling or laceration may obscure the injury, delaying proper diagnosis and treatment. More obvious signs include severely reduced vision, hemorrhagic chemosis (swelling) of the conjunctiva, and an irregular or peaked pupil. If a rupture or any significant intraocular injury is suspected, it is crucial to avoid applying any pressure to the globe, as this can significantly worsen the damage to the intraocular tissues. Use of a helmet with protective shields and cages attached markedly reduces the risk for such injuries.13All eye injuries require prompt assessment, which allows for appropriate management and prevention of secondary damage.14 Initial evaluation of a patient with ocular trauma should begin with external examination for lacerations, swelling, or orbital rim step-off deformity. The physician should also check visual acuity in order to assess for significant vision impairment (counting fingers or reading a sign in the arena; confrontation visual fields). This should be done before attending to any periocular injuries, with the uninjured side serving as a control. Next, the physician should assess the extraocular eye movements as well as the size, shape, and reactivity of the pupils. Particular attention should be paid to detecting any deficit in extraocular movement or irregularity in pupil size, shape, or reactivity, as such findings are highly suggestive of serious injury to the globe.13 Hyphema (blood in anterior chamber of eye anterior to pupil) should be suspected if vision is reduced and the pupil cannot be clearly visualized. However, a bright red clot is not always apparent at time of injury or if the amount of blood is small. An irregular pupil, or a pupil that does not constrict well to light, is also a red flag for serious contusion injury to the eye, and requires ophthalmologic evaluation. It is important to keep in mind that blunt trauma severe enough to produce hyphema or an irregular and poorly reactive pupil is often associated with retinal damage as well, including retinal edema or detachment.
Minor injuries (eg, small foreign bodies, minor periocular contusions and lacerations) can often be managed rink-side. Foreign bodies not embedded in the cornea, but lodged under the upper eyelid, can sometimes be removed by everting the eyelid and sweeping with a moistened cotton swab or using diffuse, sterile saline irrigation.11 Corneal abrasions generally cause severe pain, photophobia, and tearing and are easily diagnosed with use of topical fluorescein and a blue light. A topical anesthetic can be extremely helpful in this setting, as it allows for proper pain-free evaluation, but should never be used in an ongoing manner for pain relief. Small lacerations of the brow can be sutured with 5-0 or 6-0 nylon or closed with 2-Octyl cyanoacrylate tissue adhesive (Dermabond). Eyelid lacerations, unless very small, are best managed by an ophthalmologist; care must be taken to rule out injury to the deeper orbital tissues and eye. If serious injury is suspected, or the eye cannot be appropriately evaluated, it should be stabilized and protected with a protective shield or plastic cup, and the player should be transferred to an ED for appropriate ophthalmologic evaluation.13Most eye injuries are accidental, caused by sticks or deflected pucks, but 18% are acquired in fights.8 Use of visors or full-face cages effectively minimizes the rate of eye injuries.8,13,15,16 In a cohort study of 282 elite amateur ice hockey players, the risk of eye injury was 4.7 times higher in players without face protection than in players who used half-face shields; there were no eye injuries in players who used full-face protection.13 For visors to prevent eye injury, they must be positioned to cover the eyes and the lower edge of the nose in all projections.10
Dental Injuries
The incidence and type of facial and dental injuries depend directly on the type of face protection used.11,17,18 In a study of face, head, and neck injuries in elite amateur ice hockey players, Stuart and colleagues13 found game-related injury rates of 158.9 per 1000 player-hours in players without face protection, 73.5 in players who used half-face shields, and 23.2 in players who used full-face shields. Players who wore full-face shields had facial, head, and neck injury rates of only 23.2 per 1000 player-game hours.13 Other studies clearly support the important role face shields play in lowering injury risk in hockey. Face and head injuries account for 20% to 40% of all hockey-related injuries,3,16,19 and dental injuries up to 11.5%.20 In a study from Finland, Lahti and colleagues19 found that over a 2-year period, 479 hockey players sustained injuries, including 650 separate dental injuries. The most commonly diagnosed dental injury was an uncomplicated crown fracture, and the most common cause was a hit with a hockey stick, which accounted for 52.7% and 40.3% of dental injuries in games and practices, respectively.19
In the management of dental fractures, the broken portions of teeth should be found and placed in a transportation-protective medium, such as saline, saliva, or milk,16 which can improve functional and esthetic replacement outcomes.21,22 Loose pieces of teeth should not be left in the player’s mouth. The residual tooth should be stabilized and exposure to air and occlusion limited. Dental fractures can affect the enamel, the enamel and dentin structures (uncomplicated fracture), or enamel, dentin, and pulp (complicated).23 Fractures involving only the enamel do not require urgent dental evaluation. Dentin or pulp involvement may cause temperature and air sensitivity.23 If a tooth is air-sensitive, the player should be referred to a specialist immediately.11
Direct trauma can cause instability without displacement (subluxation) or complete displacement of the tooth from its alveolar socket (avulsion).23 An avulsed tooth should be handled by the crown to avoid further damage to the root and periodontal ligament.16,24 The tooth should be rinsed gently with saline and reimplanted in its socket, ideally within 5 to 10 minutes,23with the athlete biting down gently on gauze to hold the tooth in place. A 1-mL supraperiosteal infiltration of 1% or 2% lidocaine hydrochloride (1:100,000 epinephrine) can be given into the apex of the tooth being anesthetized (Figure 1).
Concussions
A concussion is a “complex pathophysiological process affecting the brain, induced by traumatic biomechanical forces.”25 Concussion is largely a functional disturbance instead of a structural injury, owing to the rotational and/or shearing forces involved. Many studies have identified concussion as the most common type of injury in all of youth hockey.26 Concussions account for up to 19% of all injuries in men’s collegiate hockey.3
Concussion can be challenging to diagnose on the ice. The most important factor in concussion management is symptom reporting by the athlete.27 Despite significant efforts in education and awareness, student athletes, especially hockey players, withhold reporting a possible concussion.28 Reasons for underreporting include fear of letting down other players and coaches, thinking the injury is not severe enough to warrant evaluation, and fear of losing standing with the current team or future teams.28
As postinjury concussion assessments are ideal when comparisons can be made with preseason (baseline) scores, preseason testing is becoming standard in professional, college, junior, and high school hockey. This testing involves the Sport Concussion Assessment Tool, 3rd edition (SCAT3), and the King-Devick (K-D) test.30,31 Some youth leagues have baseline testing as well, though the frequency of baseline testing in their players is controversial,32 as the adolescent mind’s processing speed and memory increase exponentially.33 For these younger athletes, it may be necessary to perform baseline testing more frequently than annually.32 A physician can use baseline test results to help diagnose a concussion at the rink and then track the athlete’s recovery and help with return-to-play decisions.29 Vision involves almost half of the brain’s circuits,34 including areas vulnerable to head impact. A neuro-ophthalmologic test can assess for irregularities in accommodation, convergence, ocular muscle balance, pursuit, and saccades.29 The K-D test is a visual performance examination that allows easy and objective assessment of eye movements. Use of both the K-D test and the SCAT3 at the rink may increase the number of concussions detected.29,35 We recommend that physicians use both tests to assess for concussion at the hockey rink.
Initial treatment involves a period of physical rest and relative cognitive rest. Acute worsening of symptoms warrants urgent imaging to rule out a subdural or subarachnoid bleed. Once a player is symptom-free, a graded return-to-play protocol should be followed (Table 4).
On the prevention side, great efforts have been made to improve hockey helmets. (Some manufacturers claim to have made concussion-proof helmets, but there is no evidence supporting this claim.6) Numerous investigators have reported a lower overall injury rate in players who wear a helmet and a full-face shield.6,13 In addition, rule changes aimed at decreasing head contact have been implemented to decrease the incidence of sport-related concussions.36 Moreover, education on proper helmet use and wear should be emphasized. A study of the effects of hockey helmet fit on cervical motion found that 7 (39%) of 18 players wore a game or competition helmet so loosely that it could be removed without unbuttoning its chinstrap.37 Improperly worn helmets cannot prevent injury as well as properly worn helmets can.
Cervical Spine Injuries
Whereas American football is associated with a higher annual number of nonfatal catastrophic neck injuries, hockey has a 3 to 6 times higher incidence of cervical spine injuries and spinal cord damage.38,39 A Canadian Ice Hockey Spinal Injuries Registry review of the period 2006 to 2011 identified 44 cervical spine injuries, 7.3 per year on average.40 Severe injury, defined as complete motor and sensory loss, complete motor loss and incomplete sensory, or complete motor loss, occurred in 4 (9.1%) of the 44 injured players. In hockey, a major mechanism of cervical spine injury is an axial load to the slightly flexed spine.39 Of 355 hockey-related cervical spine injuries in a Canada study, 95 (35.5%) were caused by a check from behind.40,41 The Canadian neurosurgeons’ work led to rule changes prohibiting checks from behind, and this prohibition has reduced the incidence of cervical spine injuries in ice hockey.38,40
Team physicians should be comfortable managing serious neck and spine injuries on the ice. Initial evaluation should follow the standard ABCs (airway, breathing, circulation). The physician places a hand on each side of the head to stabilize the neck until the initial examination is complete. The goal is to minimize cervical spine motion until transportation to the hospital for advanced imaging and definitive treatment.37 The decision to remove or leave on the helmet is now controversial. Hockey helmets differ from football helmets in that their chinstraps do not afford significant cervical stabilization, and the helmets have less padding and cover less of the head; in addition, a shockingly high percentage of hockey players do not wear properly fitting helmets.37 In one study, 3-dimensional motion analysis of a hockey player during the logroll technique showed less transverse and sagittal cervical plane motion with the helmet removed than with the helmet (properly fitting or not) in place; the authors recommended removing the helmet to limit extraneous cervical spine motion during the technique.37 However, 2 other studies found that helmet removal can result in significantly increased cervical spine motion of the immobilized hockey player.42,43Recommendation 4 of the recently released interassociation consensus statement of the National Athletic Trainers’ Association reads, “Protective athletic equipment should be removed before transport to an emergency facility for an athlete-patient with suspected cervical spine instability.”44 This represents a shift from leaving the helmet and shoulder pads in place. For ice hockey players with suspected cervical spine injury, more research is needed on cervical motion during the entire sequence—partial logrolls, spine-boarding, placement of cervical collar before or after logroll, and different immobilization techniques for transport.37
The athlete must be carefully transferred to a spine board with either logroll or lift-and-slide. Although an extrication cervical collar can be placed before the spine board is placed, the effectiveness of this collar in executing the spine-board transfer is not proven.45 When the player is on the spine board, the head can be secured with pads and straps en route to the hospital.
Return-to-Play Criteria for Cervical Spine Injuries There is no clear consensus on return-to-play guidelines for cervical spine injuries in athletes.46
Shoulder Injuries
For hockey players, the upper extremity traditionally has been considered a well-protected area.48 However, shoulder pads are considerably more flexible in hockey than in football and other collision sports. In addition, hockey gloves allow a fair amount of motion for stick handling, and the wrist may be in maximal flexion or extension when a hit against the boards or the ice occurs. Open-ice checking, board collisions, and hockey stick use have been postulated as reasons for the high incidence of upper extremity injuries in hockey. Researchers in Finland found that upper extremity injuries accounted for up to 31% of all hockey injuries.49 More than 50% of these injuries resulted from checking or board collisions. Furthermore, study findings highlighted a low rate of injury in younger players and indicated the rate increases with age.49,50
In hockey players, the acromioclavicular (AC) joint is the most commonly injured shoulder structure.51 The mechanism of injury can be a board collision or an open-ice hit, but most often is a direct blow to the shoulder. The collision disrupts the AC joint and can sprain or tear the coracoclavicular ligaments. The Rockwood classification is used to categorize AC joint injuries (Figure 2).
Initial management involves icing the AC joint and placing a sling for comfort. Type I and type II injuries can be managed with progressive range-of-motion (ROM) exercises, strengthening, cryotherapy, and a period of rest. Treatment of type III injuries remains controversial,52 but in hockey players these injuries are almost always treated nonoperatively. Return to play requires full motion, normal strength, and minimal discomfort. Players return a few days to 2 weeks after a grade I injury; recovery from grade II injuries may take 2 to 3 weeks, and recovery from grade III injuries, 6 to 12 weeks. Surgical treatment is usually required in type IV and type V injuries, but we have had experience treating these injuries nonoperatively in high-level players. AC joint reinjury in hockey players is common, and surgical treatment should be approached cautiously, as delayed fracture after return to sport has been reported.53 Special precautions should be taken in collision athletes who undergo AC joint reconstruction. In the anatomical reconstruction described by Carofino and Mazzocca,54 2 holes are drilled in the clavicle; these holes are a potential source of fracture when the collision athlete returns to sport (Figure 3).
Clavicle fracture is another common hockey injury.55 Studies have shown clavicle fractures proportionally occur most often in people 15 to 19 years old.49 The injury presents with pain and deformity over the clavicle; in more severe fractures, skin tenting is identified. Initial management of suspected clavicle fracture includes cryotherapy, sling, and radiographs. Radiographs should include an AP view and then a 45° cephalad view, which eliminates overshadowing from the ribs. Most clavicle fractures are successfully managed nonoperatively, though there is evidence that significantly displaced or comminuted fractures have better union rates and shoulder function when treated with open reduction and internal fixation.56 After a clavicle fracture, return to skating and noncontact practice usually takes 8 weeks, with return to full contact occurring around 12 weeks.
Sternoclavicular injuries are relatively uncommon, but potentially serious. Special attention should also be given to adolescent athletes with sternoclavicular pain. Although sternoclavicular dislocations have been reported in hockey players, instead these likely are fractures involving the medial clavicle physis.57
The shoulder is the most commonly dislocated major joint, and the incidence of shoulder dislocation in elite hockey players is 8% to 21%.50,58 Anterior shoulder instability occurs from a fall with the shoulder in an abducted, externally rotated and extended position or from a direct anteriorly placed impact to the posterior shoulder. We recommend taking players off the ice for evaluation. Depending on physician comfort, the shoulder can be reduced in the training room, and the athlete sent for radiographs after reduction. If resources or support for closed reduction is not available at the rink, the athlete should be sent to the ED. Initial radiographic evaluation of a player with shoulder injury begins with plain radiographs, including a true AP (Grashey) view with the humerus in neutral, internal, and external rotation and an axillary view. The axillary radiograph is crucial in determining anterior or posterior dislocation. If the patient cannot tolerate the pain associated with having an axillary radiograph taken, a Velpeau radiograph can be used. This radiograph is taken with the patient’s arm in a sling and with the patient leaning back 30° while the x-ray beam is directed superior to inferior.
CT is performed for a suspected osseous injury. CT is more accurate than plain radiographs in showing glenoid and humeral fractures in the acute setting as well as the amount of bone loss in the case of chronic instability. Magnetic resonance arthrography is the imaging modality of choice for the diagnoses of capsulolabral injury.
After shoulder reduction, treatment with a sling, cryotherapy, and a nonsteroidal anti-inflammatory drug is initiated. In a Minnesota study of nonoperative management of shoulder instability, 9 of 10 hockey players were able to return to play the same season, and 6 of the 10 required surgery at the end of the season.59
Compared with noncontact athletes, hockey players and other collision athletes are at increased risk for recurrence.60-62 For collision athletes who want to continue playing their sport after recurrent instability, surgery is recommended. A shoulder instability study in Toronto found that more than 54% of 24 professional hockey players had associated Hill-Sachs lesions, but only 3 shoulders (12.5%) had glenoid defects.50 Arthroscopic and open techniques both demonstrate good results, and identification of bone loss can help determine which surgery to recommend.63 Hockey players can usually return to sport 6 months after shoulder stabilization.
Another important consideration in managing shoulder instability in hockey players is shoulder dominance, which determines stick grip. A left-handed player places the right hand on top of the stick for support, but most of the motion associated with shooting the puck—including abduction and external rotation—occurs with the left shoulder. Thus, a left-handed player with a history of previous left-side shoulder dislocation may dislocate with each shot, but a right-handed player with left shoulder instability may have considerably less trouble on the ice.58Shoulder and rotator cuff contusions (RCCs) occur in hockey and other collision sports.49,64 RCCs almost always result from a direct blow to the shoulder, and present with shoulder function loss, weakness, and pain.
Summary
Hockey is a high-speed collision sport with one of the highest injury rates among all sports. Physicians caring for youth, amateur, and senior hockey teams see a range of acute head, neck, and shoulder injuries. Although treatment of eye injuries, dental injuries, and concussions is not always considered orthopedic care, an orthopedic surgeon who is covering hockey needs to be comfortable managing these injuries acutely. Quality rink-side care minimizes the impact of the injury, maximizes the functional result, and expedites the safe return of the injured player back to the ice.
Am J Orthop. 2017;46(3):123-134. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
Take-Home Points
- Hockey is a high-speed collision sport with one of the highest injury rates among all sports.
- Use of a helmet with visors or full-face shields significantly reduces the risk for eye injury.
- Broken portions of teeth should be found and placed in a protective medium such as saline, saliva, or milk for transport.
- A player with unresolved concussion symptoms should not be allowed to return to the ice.
- Shoulder dominance, which determines stick grip, is an important consideration in the treatment of shoulder instability in an ice hockey player.
On a surface of ice in Windsor, Nova Scotia in the middle of the 19th century, the modern game of ice hockey evolved.1 A blend of hurley, a Gaelic sport, and lacrosse, from the native Mi’kmaq culture, the sport of ice hockey gained rapidly in popularity throughout Canada and is now the country’s national sport. Hockey quickly spread to the United States and then Europe. It is presently played in 77 countries across the world.2
Hockey players can reach speeds of up to 48 km (~30 miles) per hour on razor-sharp skates on an ice surface surrounded by rigid plastic composite boards topped with plexiglass.3 They use sticks made of wood, aluminum, or a composite material to advance a 6-ounce vulcanized rubber puck on the opposing goal, and this puck sometimes reaches speeds over 160 km (~100 miles) per hour. Older, male players are allowed to make physical contact with their opposing counterparts to separate them from the puck (body-checking). Not surprisingly, the potential risk for injury in hockey is high. At the 2010 Winter Olympics, men’s ice hockey players had the highest rate of injury of any other competitors there—more than 30% were affected.4
Hockey is played and enjoyed by athletes ranging widely in age. Youth hockey leagues accept players as young as 5 years. Hockey can become a lifelong recreational activity. In North America, old timers’ leagues have many players up to age 70 years.6 According to International Ice Hockey Federation data for 2016, more than 543,000 and 639,500 people play hockey in the United States and Canada, respectively.2 Most of the rules, protective equipment, skates, ice surfaces, and goal sizes are the same in men’s and women’s hockey.7 The major difference is in body-checking—this practice is not allowed at any age in women’s ice hockey.
In this article, we review the evaluation and management of common head, neck, and shoulder hockey injuries for physicians who provide medical support and coverage for youth, amateur, and senior hockey teams.
Evaluation and Management of Common Hockey Injuries
Eye Injuries
Although eye injuries are less common than musculoskeletal injuries and concussions in hockey, they are a serious risk for recreational and competitive players alike. Furthermore, recovery may be difficult, and eye injuries can have serious lifelong consequences.8 In hockey, the most commonly reported eye injuries are periorbital contusions and lacerations, hyphema, corneal and conjunctival abrasions, orbital fractures, and ruptured globes (Table 2).9,10
As a contact sport, hockey often involves high-impact, blunt-force trauma. The trauma in hockey results from collisions with other players, the boards, hockey sticks, and pucks. It is therefore not surprising that the most common ocular injuries in this sport are periorbital contusions. Although most contusions cause only mild swelling and ecchymosis of the soft tissues around the eye, there is potential for serious consequences. In a Scandinavia study, Leivo and colleagues10 found that 9% of patients who sustained a periocular contusion also had a clinically significant secondary diagnosis, such as retinal tear or hemorrhage, eyelid laceration, vitreous hemorrhage, or retinal detachment. Although the study was hospital-based, and therefore biased toward more severe cases, its findings highlight the potential severity of eye injuries in hockey. Furthermore, the study found that the majority of players who sustained blunt trauma to the eye itself required lifelong follow-up because of increased risk for glaucoma. This is particularly true for hyphema, as this finding indicates significant damage to intraocular tissues.10Players can also sustain fractures of the orbital bones, including orbital blowout fractures. Typical signs and symptoms of blowout fractures include diplopia, proptosis or enophthalmos, infraorbital hypoesthesia, painful and decreased extraocular movement (particularly upgaze), and palpable crepitance caused by sinus air entering the lower eyelid.11 If orbital fracture is suspected, as it should be in any case in which the injured player experiences pain with eye movement or diplopia, the player should be referred to the ED for computed tomography (CT) and ophthalmologic evaluation.12 Continued participation seriously risks making the injury much worse, particularly should another impact occur. In addition, given the impact needed to cause orbital fractures, consideration must be given to the potential for a coexisting concussion injury.
Severe direct trauma to the eye—from a puck, a stick, or a fist—can result in a ruptured globe, a particularly serious injury that requires immediate surgical attention. Signs and symptoms of a ruptured globe are rarely subtle, but associated eyelid swelling or laceration may obscure the injury, delaying proper diagnosis and treatment. More obvious signs include severely reduced vision, hemorrhagic chemosis (swelling) of the conjunctiva, and an irregular or peaked pupil. If a rupture or any significant intraocular injury is suspected, it is crucial to avoid applying any pressure to the globe, as this can significantly worsen the damage to the intraocular tissues. Use of a helmet with protective shields and cages attached markedly reduces the risk for such injuries.13All eye injuries require prompt assessment, which allows for appropriate management and prevention of secondary damage.14 Initial evaluation of a patient with ocular trauma should begin with external examination for lacerations, swelling, or orbital rim step-off deformity. The physician should also check visual acuity in order to assess for significant vision impairment (counting fingers or reading a sign in the arena; confrontation visual fields). This should be done before attending to any periocular injuries, with the uninjured side serving as a control. Next, the physician should assess the extraocular eye movements as well as the size, shape, and reactivity of the pupils. Particular attention should be paid to detecting any deficit in extraocular movement or irregularity in pupil size, shape, or reactivity, as such findings are highly suggestive of serious injury to the globe.13 Hyphema (blood in anterior chamber of eye anterior to pupil) should be suspected if vision is reduced and the pupil cannot be clearly visualized. However, a bright red clot is not always apparent at time of injury or if the amount of blood is small. An irregular pupil, or a pupil that does not constrict well to light, is also a red flag for serious contusion injury to the eye, and requires ophthalmologic evaluation. It is important to keep in mind that blunt trauma severe enough to produce hyphema or an irregular and poorly reactive pupil is often associated with retinal damage as well, including retinal edema or detachment.
Minor injuries (eg, small foreign bodies, minor periocular contusions and lacerations) can often be managed rink-side. Foreign bodies not embedded in the cornea, but lodged under the upper eyelid, can sometimes be removed by everting the eyelid and sweeping with a moistened cotton swab or using diffuse, sterile saline irrigation.11 Corneal abrasions generally cause severe pain, photophobia, and tearing and are easily diagnosed with use of topical fluorescein and a blue light. A topical anesthetic can be extremely helpful in this setting, as it allows for proper pain-free evaluation, but should never be used in an ongoing manner for pain relief. Small lacerations of the brow can be sutured with 5-0 or 6-0 nylon or closed with 2-Octyl cyanoacrylate tissue adhesive (Dermabond). Eyelid lacerations, unless very small, are best managed by an ophthalmologist; care must be taken to rule out injury to the deeper orbital tissues and eye. If serious injury is suspected, or the eye cannot be appropriately evaluated, it should be stabilized and protected with a protective shield or plastic cup, and the player should be transferred to an ED for appropriate ophthalmologic evaluation.13Most eye injuries are accidental, caused by sticks or deflected pucks, but 18% are acquired in fights.8 Use of visors or full-face cages effectively minimizes the rate of eye injuries.8,13,15,16 In a cohort study of 282 elite amateur ice hockey players, the risk of eye injury was 4.7 times higher in players without face protection than in players who used half-face shields; there were no eye injuries in players who used full-face protection.13 For visors to prevent eye injury, they must be positioned to cover the eyes and the lower edge of the nose in all projections.10
Dental Injuries
The incidence and type of facial and dental injuries depend directly on the type of face protection used.11,17,18 In a study of face, head, and neck injuries in elite amateur ice hockey players, Stuart and colleagues13 found game-related injury rates of 158.9 per 1000 player-hours in players without face protection, 73.5 in players who used half-face shields, and 23.2 in players who used full-face shields. Players who wore full-face shields had facial, head, and neck injury rates of only 23.2 per 1000 player-game hours.13 Other studies clearly support the important role face shields play in lowering injury risk in hockey. Face and head injuries account for 20% to 40% of all hockey-related injuries,3,16,19 and dental injuries up to 11.5%.20 In a study from Finland, Lahti and colleagues19 found that over a 2-year period, 479 hockey players sustained injuries, including 650 separate dental injuries. The most commonly diagnosed dental injury was an uncomplicated crown fracture, and the most common cause was a hit with a hockey stick, which accounted for 52.7% and 40.3% of dental injuries in games and practices, respectively.19
In the management of dental fractures, the broken portions of teeth should be found and placed in a transportation-protective medium, such as saline, saliva, or milk,16 which can improve functional and esthetic replacement outcomes.21,22 Loose pieces of teeth should not be left in the player’s mouth. The residual tooth should be stabilized and exposure to air and occlusion limited. Dental fractures can affect the enamel, the enamel and dentin structures (uncomplicated fracture), or enamel, dentin, and pulp (complicated).23 Fractures involving only the enamel do not require urgent dental evaluation. Dentin or pulp involvement may cause temperature and air sensitivity.23 If a tooth is air-sensitive, the player should be referred to a specialist immediately.11
Direct trauma can cause instability without displacement (subluxation) or complete displacement of the tooth from its alveolar socket (avulsion).23 An avulsed tooth should be handled by the crown to avoid further damage to the root and periodontal ligament.16,24 The tooth should be rinsed gently with saline and reimplanted in its socket, ideally within 5 to 10 minutes,23with the athlete biting down gently on gauze to hold the tooth in place. A 1-mL supraperiosteal infiltration of 1% or 2% lidocaine hydrochloride (1:100,000 epinephrine) can be given into the apex of the tooth being anesthetized (Figure 1).
Concussions
A concussion is a “complex pathophysiological process affecting the brain, induced by traumatic biomechanical forces.”25 Concussion is largely a functional disturbance instead of a structural injury, owing to the rotational and/or shearing forces involved. Many studies have identified concussion as the most common type of injury in all of youth hockey.26 Concussions account for up to 19% of all injuries in men’s collegiate hockey.3
Concussion can be challenging to diagnose on the ice. The most important factor in concussion management is symptom reporting by the athlete.27 Despite significant efforts in education and awareness, student athletes, especially hockey players, withhold reporting a possible concussion.28 Reasons for underreporting include fear of letting down other players and coaches, thinking the injury is not severe enough to warrant evaluation, and fear of losing standing with the current team or future teams.28
As postinjury concussion assessments are ideal when comparisons can be made with preseason (baseline) scores, preseason testing is becoming standard in professional, college, junior, and high school hockey. This testing involves the Sport Concussion Assessment Tool, 3rd edition (SCAT3), and the King-Devick (K-D) test.30,31 Some youth leagues have baseline testing as well, though the frequency of baseline testing in their players is controversial,32 as the adolescent mind’s processing speed and memory increase exponentially.33 For these younger athletes, it may be necessary to perform baseline testing more frequently than annually.32 A physician can use baseline test results to help diagnose a concussion at the rink and then track the athlete’s recovery and help with return-to-play decisions.29 Vision involves almost half of the brain’s circuits,34 including areas vulnerable to head impact. A neuro-ophthalmologic test can assess for irregularities in accommodation, convergence, ocular muscle balance, pursuit, and saccades.29 The K-D test is a visual performance examination that allows easy and objective assessment of eye movements. Use of both the K-D test and the SCAT3 at the rink may increase the number of concussions detected.29,35 We recommend that physicians use both tests to assess for concussion at the hockey rink.
Initial treatment involves a period of physical rest and relative cognitive rest. Acute worsening of symptoms warrants urgent imaging to rule out a subdural or subarachnoid bleed. Once a player is symptom-free, a graded return-to-play protocol should be followed (Table 4).
On the prevention side, great efforts have been made to improve hockey helmets. (Some manufacturers claim to have made concussion-proof helmets, but there is no evidence supporting this claim.6) Numerous investigators have reported a lower overall injury rate in players who wear a helmet and a full-face shield.6,13 In addition, rule changes aimed at decreasing head contact have been implemented to decrease the incidence of sport-related concussions.36 Moreover, education on proper helmet use and wear should be emphasized. A study of the effects of hockey helmet fit on cervical motion found that 7 (39%) of 18 players wore a game or competition helmet so loosely that it could be removed without unbuttoning its chinstrap.37 Improperly worn helmets cannot prevent injury as well as properly worn helmets can.
Cervical Spine Injuries
Whereas American football is associated with a higher annual number of nonfatal catastrophic neck injuries, hockey has a 3 to 6 times higher incidence of cervical spine injuries and spinal cord damage.38,39 A Canadian Ice Hockey Spinal Injuries Registry review of the period 2006 to 2011 identified 44 cervical spine injuries, 7.3 per year on average.40 Severe injury, defined as complete motor and sensory loss, complete motor loss and incomplete sensory, or complete motor loss, occurred in 4 (9.1%) of the 44 injured players. In hockey, a major mechanism of cervical spine injury is an axial load to the slightly flexed spine.39 Of 355 hockey-related cervical spine injuries in a Canada study, 95 (35.5%) were caused by a check from behind.40,41 The Canadian neurosurgeons’ work led to rule changes prohibiting checks from behind, and this prohibition has reduced the incidence of cervical spine injuries in ice hockey.38,40
Team physicians should be comfortable managing serious neck and spine injuries on the ice. Initial evaluation should follow the standard ABCs (airway, breathing, circulation). The physician places a hand on each side of the head to stabilize the neck until the initial examination is complete. The goal is to minimize cervical spine motion until transportation to the hospital for advanced imaging and definitive treatment.37 The decision to remove or leave on the helmet is now controversial. Hockey helmets differ from football helmets in that their chinstraps do not afford significant cervical stabilization, and the helmets have less padding and cover less of the head; in addition, a shockingly high percentage of hockey players do not wear properly fitting helmets.37 In one study, 3-dimensional motion analysis of a hockey player during the logroll technique showed less transverse and sagittal cervical plane motion with the helmet removed than with the helmet (properly fitting or not) in place; the authors recommended removing the helmet to limit extraneous cervical spine motion during the technique.37 However, 2 other studies found that helmet removal can result in significantly increased cervical spine motion of the immobilized hockey player.42,43Recommendation 4 of the recently released interassociation consensus statement of the National Athletic Trainers’ Association reads, “Protective athletic equipment should be removed before transport to an emergency facility for an athlete-patient with suspected cervical spine instability.”44 This represents a shift from leaving the helmet and shoulder pads in place. For ice hockey players with suspected cervical spine injury, more research is needed on cervical motion during the entire sequence—partial logrolls, spine-boarding, placement of cervical collar before or after logroll, and different immobilization techniques for transport.37
The athlete must be carefully transferred to a spine board with either logroll or lift-and-slide. Although an extrication cervical collar can be placed before the spine board is placed, the effectiveness of this collar in executing the spine-board transfer is not proven.45 When the player is on the spine board, the head can be secured with pads and straps en route to the hospital.
Return-to-Play Criteria for Cervical Spine Injuries There is no clear consensus on return-to-play guidelines for cervical spine injuries in athletes.46
Shoulder Injuries
For hockey players, the upper extremity traditionally has been considered a well-protected area.48 However, shoulder pads are considerably more flexible in hockey than in football and other collision sports. In addition, hockey gloves allow a fair amount of motion for stick handling, and the wrist may be in maximal flexion or extension when a hit against the boards or the ice occurs. Open-ice checking, board collisions, and hockey stick use have been postulated as reasons for the high incidence of upper extremity injuries in hockey. Researchers in Finland found that upper extremity injuries accounted for up to 31% of all hockey injuries.49 More than 50% of these injuries resulted from checking or board collisions. Furthermore, study findings highlighted a low rate of injury in younger players and indicated the rate increases with age.49,50
In hockey players, the acromioclavicular (AC) joint is the most commonly injured shoulder structure.51 The mechanism of injury can be a board collision or an open-ice hit, but most often is a direct blow to the shoulder. The collision disrupts the AC joint and can sprain or tear the coracoclavicular ligaments. The Rockwood classification is used to categorize AC joint injuries (Figure 2).
Initial management involves icing the AC joint and placing a sling for comfort. Type I and type II injuries can be managed with progressive range-of-motion (ROM) exercises, strengthening, cryotherapy, and a period of rest. Treatment of type III injuries remains controversial,52 but in hockey players these injuries are almost always treated nonoperatively. Return to play requires full motion, normal strength, and minimal discomfort. Players return a few days to 2 weeks after a grade I injury; recovery from grade II injuries may take 2 to 3 weeks, and recovery from grade III injuries, 6 to 12 weeks. Surgical treatment is usually required in type IV and type V injuries, but we have had experience treating these injuries nonoperatively in high-level players. AC joint reinjury in hockey players is common, and surgical treatment should be approached cautiously, as delayed fracture after return to sport has been reported.53 Special precautions should be taken in collision athletes who undergo AC joint reconstruction. In the anatomical reconstruction described by Carofino and Mazzocca,54 2 holes are drilled in the clavicle; these holes are a potential source of fracture when the collision athlete returns to sport (Figure 3).
Clavicle fracture is another common hockey injury.55 Studies have shown clavicle fractures proportionally occur most often in people 15 to 19 years old.49 The injury presents with pain and deformity over the clavicle; in more severe fractures, skin tenting is identified. Initial management of suspected clavicle fracture includes cryotherapy, sling, and radiographs. Radiographs should include an AP view and then a 45° cephalad view, which eliminates overshadowing from the ribs. Most clavicle fractures are successfully managed nonoperatively, though there is evidence that significantly displaced or comminuted fractures have better union rates and shoulder function when treated with open reduction and internal fixation.56 After a clavicle fracture, return to skating and noncontact practice usually takes 8 weeks, with return to full contact occurring around 12 weeks.
Sternoclavicular injuries are relatively uncommon, but potentially serious. Special attention should also be given to adolescent athletes with sternoclavicular pain. Although sternoclavicular dislocations have been reported in hockey players, instead these likely are fractures involving the medial clavicle physis.57
The shoulder is the most commonly dislocated major joint, and the incidence of shoulder dislocation in elite hockey players is 8% to 21%.50,58 Anterior shoulder instability occurs from a fall with the shoulder in an abducted, externally rotated and extended position or from a direct anteriorly placed impact to the posterior shoulder. We recommend taking players off the ice for evaluation. Depending on physician comfort, the shoulder can be reduced in the training room, and the athlete sent for radiographs after reduction. If resources or support for closed reduction is not available at the rink, the athlete should be sent to the ED. Initial radiographic evaluation of a player with shoulder injury begins with plain radiographs, including a true AP (Grashey) view with the humerus in neutral, internal, and external rotation and an axillary view. The axillary radiograph is crucial in determining anterior or posterior dislocation. If the patient cannot tolerate the pain associated with having an axillary radiograph taken, a Velpeau radiograph can be used. This radiograph is taken with the patient’s arm in a sling and with the patient leaning back 30° while the x-ray beam is directed superior to inferior.
CT is performed for a suspected osseous injury. CT is more accurate than plain radiographs in showing glenoid and humeral fractures in the acute setting as well as the amount of bone loss in the case of chronic instability. Magnetic resonance arthrography is the imaging modality of choice for the diagnoses of capsulolabral injury.
After shoulder reduction, treatment with a sling, cryotherapy, and a nonsteroidal anti-inflammatory drug is initiated. In a Minnesota study of nonoperative management of shoulder instability, 9 of 10 hockey players were able to return to play the same season, and 6 of the 10 required surgery at the end of the season.59
Compared with noncontact athletes, hockey players and other collision athletes are at increased risk for recurrence.60-62 For collision athletes who want to continue playing their sport after recurrent instability, surgery is recommended. A shoulder instability study in Toronto found that more than 54% of 24 professional hockey players had associated Hill-Sachs lesions, but only 3 shoulders (12.5%) had glenoid defects.50 Arthroscopic and open techniques both demonstrate good results, and identification of bone loss can help determine which surgery to recommend.63 Hockey players can usually return to sport 6 months after shoulder stabilization.
Another important consideration in managing shoulder instability in hockey players is shoulder dominance, which determines stick grip. A left-handed player places the right hand on top of the stick for support, but most of the motion associated with shooting the puck—including abduction and external rotation—occurs with the left shoulder. Thus, a left-handed player with a history of previous left-side shoulder dislocation may dislocate with each shot, but a right-handed player with left shoulder instability may have considerably less trouble on the ice.58Shoulder and rotator cuff contusions (RCCs) occur in hockey and other collision sports.49,64 RCCs almost always result from a direct blow to the shoulder, and present with shoulder function loss, weakness, and pain.
Summary
Hockey is a high-speed collision sport with one of the highest injury rates among all sports. Physicians caring for youth, amateur, and senior hockey teams see a range of acute head, neck, and shoulder injuries. Although treatment of eye injuries, dental injuries, and concussions is not always considered orthopedic care, an orthopedic surgeon who is covering hockey needs to be comfortable managing these injuries acutely. Quality rink-side care minimizes the impact of the injury, maximizes the functional result, and expedites the safe return of the injured player back to the ice.
Am J Orthop. 2017;46(3):123-134. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
1. Vaughan G. The Puck Starts Here: The Origin of Canada’s Great Winter Game, Ice Hockey. Fredericton, Canada: Goose Lane Editions; 1996.
2. IIHF member national associations. International Ice Hockey Federation website. http://www.iihf.com/iihf-home/the-iihf/members. Accessed April 6, 2017.
3. Flik K, Lyman S, Marx RG. American collegiate men’s ice hockey: an analysis of injuries. Am J Sports Med. 2005;33(2):183-187.
4. Engebretsen L, Steffen K, Alonso JM, et al. Sports injuries and illnesses during the Winter Olympic Games 2010. Br J Sports Med. 2010;44(11):772-780.
5. Deits J, Yard EE, Collins CL, Fields SK, Comstock RD. Patients with ice hockey injuries presenting to US emergency departments, 1990-2006. J Athl Train. 2010;45(5):467-474.
6. Brooks A, Loud KJ, Brenner JS, et al. Reducing injury risk from body checking in boys’ youth ice hockey. Pediatrics. 2014;133(6):1151-1157.
7. Agel J, Harvey EJ. A 7-year review of men’s and women’s ice hockey injuries in the NCAA. Can J Surg. 2010;53(5):319-323.
8. Micieli JA, Zurakowski D, Ahmed, II. Impact of visors on eye and orbital injuries in the National Hockey League. Can J Ophthalmol. 2014;49(3):243-248.
9. Pashby TJ. Ocular injuries in hockey. Int Ophthalmol Clin. 1988;28(3):228-231.
10. Leivo T, Haavisto AK, Sahraravand A. Sports-related eye injuries: the current picture. Acta Ophthalmol. 2015;93(3):224-231.
11. Cohn RM, Alaia MJ, Strauss EJ, Feldman AF. Rink-side management of ice hockey related injuries to the face, neck, and chest. Bull Hosp Jt Dis. 2013;71(4):253-256.
12. Reehal P. Facial injury in sport. Curr Sports Med Rep. 2010;9(1):27-34.
13. Stuart MJ, Smith AM, Malo-Ortiguera SA, Fischer TL, Larson DR. A comparison of facial protection and the incidence of head, neck, and facial injuries in Junior A hockey players. A function of individual playing time. Am J Sports Med. 2002;30(1):39-44.
14. MacEwen CJ, McLatchie GR. Eye injuries in sport. Scott Med J. 2010;55(2):22-24.
15. Stevens ST, Lassonde M, de Beaumont L, Keenan JP. The effect of visors on head and facial injury in National Hockey League players. J Sci Med Sport. 2006;9(3):238-242.
16. Moslener MD, Wadsworth LT. Ice hockey: a team physician’s perspective. Curr Sports Med Rep. 2010;9(3):134-138.
17. LaPrade RF, Burnett QM, Zarzour R, Moss R. The effect of the mandatory use of face masks on facial lacerations and head and neck injuries in ice hockey. A prospective study. Am J Sports Med. 1995;23(6):773-775.
18. Benson BW, Mohtadi NG, Rose MS, Meeuwisse WH. Head and neck injuries among ice hockey players wearing full face shields vs half face shields. JAMA. 1999;282(24):2328-2332.
19. Lahti H, Sane J, Ylipaavalniemi P. Dental injuries in ice hockey games and training. Med Sci Sports Exerc. 2002;34(3):400-402.
20. Sane J, Ylipaavalniemi P, Leppanen H. Maxillofacial and dental ice hockey injuries. Med Sci Sports Exerc. 1988;20(2):202-207.
21. Emerich K, Kaczmarek J. First aid for dental trauma caused by sports activities: state of knowledge, treatment and prevention. Sports Med. 2010;40(5):361-366.
22. Rosenberg H, Rosenberg H, Hickey M. Emergency management of a traumatic tooth avulsion. Ann Emerg Med. 2011;57(4):375-377.
23. Young EJ, Macias CR, Stephens L. Common dental injury management in athletes. Sports Health. 2015;7(3):250-255.
24. Andersson L, Andreasen JO, Day P, et al. International Association of Dental Traumatology guidelines for the management of traumatic dental injuries: 2. Avulsion of permanent teeth. Dent Traumatol. 2012;28(2):88-96.
25. McCrory P, Meeuwisse W, Johnston K, et al. Consensus statement on concussion in sport 3rd International Conference on Concussion in Sport held in Zurich, November 2008. Clin J Sport Med. 2009;19(3):185-200.
26. Schneider KJ, Meeuwisse WH, Kang J, Schneider GM, Emery CA. Preseason reports of neck pain, dizziness, and headache as risk factors for concussion in male youth ice hockey players. Clin J Sport Med. 2013;23(4):267-272.
27. McCrory P, Meeuwisse WH, Aubry M, et al. Consensus statement on concussion in sport: the 4th International Conference on Concussion in Sport held in Zurich, November 2012. Br J Sports Med. 2013;47(5):250-258.
28. Delaney JS, Lamfookon C, Bloom GA, Al-Kashmiri A, Correa JA. Why university athletes choose not to reveal their concussion symptoms during a practice or game. Clin J Sport Med. 2015;25(2):113-125.
29. Ventura RE, Balcer LJ, Galetta SL. The concussion toolbox: the role of vision in the assessment of concussion. Semin Neurol. 2015;35(5):599-606.
30. Vartiainen MV, Holm A, Peltonen K, Luoto TM, Iverson GL, Hokkanen L. King-Devick test normative reference values for professional male ice hockey players. Scand J Med Sci Sports. 2015;25(3):e327-e330.
31. Galetta MS, Galetta KM, McCrossin J, et al. Saccades and memory: baseline associations of the King-Devick and SCAT2 SAC tests in professional ice hockey players. J Neurol Sci. 2013;328(1-2):28-31.
32. Vernau BT, Grady MF, Goodman A, et al. Oculomotor and neurocognitive assessment of youth ice hockey players: baseline associations and observations after concussion. Dev Neuropsychol. 2015;40(1):7-11.
33. Fry AF, Hale S. Relationships among processing speed, working memory, and fluid intelligence in children. Biol Psychol. 2000;54(1-3):1-34.
34. Felleman DJ, Van Essen DC. Distributed hierarchical processing in the primate cerebral cortex. Cereb Cortex. 1991;1(1):1-47.
35. Guskiewicz KM, Register-Mihalik J, McCrory P, et al. Evidence-based approach to revising the SCAT2: introducing the SCAT3. Br J Sports Med. 2013;47(5):289-293.
36. Smith AM, Stuart MJ, Dodick DW, et al. Ice Hockey Summit II: zero tolerance for head hits and fighting. Curr Sports Med Rep. 2015;14(2):135-144.
37. Mihalik JP, Beard JR, Petschauer MA, Prentice WE, Guskiewicz KM. Effect of ice hockey helmet fit on cervical spine motion during an emergency log roll procedure. Clin J Sport Med. 2008;18(5):394-398.
38. Banerjee R, Palumbo MA, Fadale PD. Catastrophic cervical spine injuries in the collision sport athlete, part 1: epidemiology, functional anatomy, and diagnosis. Am J Sports Med. 2004;32(4):1077-1087.
39. Reynen PD, Clancy WG Jr. Cervical spine injury, hockey helmets, and face masks. Am J Sports Med. 1994;22(2):167-170.
40. Tator CH, Provvidenza C, Cassidy JD. Update and overview of spinal injuries in Canadian ice hockey, 1943 to 2011: the continuing need for injury prevention and education. Clin J Sport Med. 2016;26(3):232-238.
41. Tator CH, Edmonds VE, Lapczak L, Tator IB. Spinal injuries in ice hockey players, 1966-1987. Can J Surg. 1991;34(1):63-69.
42. Laprade RF, Schnetzler KA, Broxterman RJ, Wentorf F, Gilbert TJ. Cervical spine alignment in the immobilized ice hockey player. A computed tomographic analysis of the effects of helmet removal. Am J Sports Med. 2000;28(6):800-803.
43. Metz CM, Kuhn JE, Greenfield ML. Cervical spine alignment in immobilized hockey players: radiographic analysis with and without helmets and shoulder pads. Clin J Sport Med. 1998;8(2):92-95.
44. National Athletic Trainers’ Association. Appropriate prehospital management of the spine-injured athlete: updated from 1998 document. http://www.nata.org/sites/default/files/Executive-Summary-Spine-Injury-updated.pdf. Updated August 5, 2015. Accessed April 6, 2017.
45. Del Rossi G, Heffernan TP, Horodyski M, Rechtine GR. The effectiveness of extrication collars tested during the execution of spine-board transfer techniques. Spine J. 2004;4(6):619-623.
46. Morganti C, Sweeney CA, Albanese SA, Burak C, Hosea T, Connolly PJ. Return to play after cervical spine injury. Spine. 2001;26(10):1131-1136.
47. Huang P, Anissipour A, McGee W, Lemak L. Return-to-play recommendations after cervical, thoracic, and lumbar spine injuries: a comprehensive review. Sports Health. 2016;8(1):19-25.
48. Shindle MK, Marx RG, Kelly BT, Bisson L, Burke CJ 3rd. Hockey injuries: a pediatric sport update. Curr Opin Pediatr. 2010;22(1):54-60.
49. Molsa J, Kujala U, Myllynen P, Torstila I, Airaksinen O. Injuries to the upper extremity in ice hockey: analysis of a series of 760 injuries. Am J Sports Med. 2003;31(5):751-757.
50. Dwyer T, Petrera M, Bleakney R, Theodoropoulos JS. Shoulder instability in ice hockey players: incidence, mechanism, and MRI findings. Clin Sports Med. 2013;32(4):803-813.
51. LaPrade RF, Wijdicks CA, Griffith CJ. Division I intercollegiate ice hockey team coverage. Br J Sports Med. 2009;43(13):1000-1005.
52. Willimon SC, Gaskill TR, Millett PJ. Acromioclavicular joint injuries: anatomy, diagnosis, and treatment. Phys Sportsmed. 2011;39(1):116-122.
53. Martetschlager F, Horan MP, Warth RJ, Millett PJ. Complications after anatomic fixation and reconstruction of the coracoclavicular ligaments. Am J Sports Med. 2013;41(12):2896-2903.
54. Carofino BC, Mazzocca AD. The anatomic coracoclavicular ligament reconstruction: surgical technique and indications. J Shoulder Elbow Surg. 2010;19(2 suppl):37-46.
55. Laprade RF, Surowiec RK, Sochanska AN, et al. Epidemiology, identification, treatment and return to play of musculoskeletal-based ice hockey injuries. Br J Sports Med. 2014;48(1):4-10.
56. Canadian Orthopaedic Trauma Society. Nonoperative treatment compared with plate fixation of displaced midshaft clavicular fractures. A multicenter, randomized clinical trial. J Bone Joint Surg Am. 2007;89(1):1-10.
57. Lee JT, Nasreddine AY, Black EM, Bae DS, Kocher MS. Posterior sternoclavicular joint injuries in skeletally immature patients. J Pediatr Orthop. 2014;34(4):369-375.
58. Hovelius L. Shoulder dislocation in Swedish ice hockey players. Am J Sports Med. 1978;6(6):373-377.
59. Buss DD, Lynch GP, Meyer CP, Huber SM, Freehill MQ. Nonoperative management for in-season athletes with anterior shoulder instability. Am J Sports Med. 2004;32(6):1430-1433.
60. Mazzocca AD, Brown FM Jr, Carreira DS, Hayden J, Romeo AA. Arthroscopic anterior shoulder stabilization of collision and contact athletes. Am J Sports Med. 2005;33(1):52-60.
61. Harris JD, Romeo AA. Arthroscopic management of the contact athlete with instability. Clin Sports Med. 2013;32(4):709-730.
62. Cho NS, Hwang JC, Rhee YG. Arthroscopic stabilization in anterior shoulder instability: collision athletes versus noncollision athletes. Arthroscopy. 2006;22(9):947-953.
63. Griffin JW, Brockmeier SF. Shoulder instability with concomitant bone loss in the athlete. Orthop Clin North Am. 2015;46(1):89-103.
64. Cohen SB, Towers JD, Bradley JP. Rotator cuff contusions of the shoulder in professional football players: epidemiology and magnetic resonance imaging findings. Am J Sports Med. 2007;35(3):442-447.
65. Lorentzon R, Wedrèn H, Pietilä T. Incidence, nature, and causes of ice hockey injuries. A three-year prospective study of a Swedish elite ice hockey team. Am J Sports Med. 1988;16(4):392-396.
66. Stuart MJ, Smith A. Injuries in Junior A ice hockey. A three-year prospective study. Am J Sports Med. 1995;23(4):458-461.
67. Voaklander DC, Saunders LD, Quinney HA, Macnab RB. Epidemiology of recreational and old-timer ice hockey injuries. Clin J Sport Med. 1996;6(1):15-21.
68. Mölsä J, Airaksinen O, Näsman O, Torstila I. Ice hockey injuries in Finland. A prospective epidemiologic study. Am J Sports Med. 1997;25(4):495-499.
69. Ferrara MS, Schurr KT. Intercollegiate ice hockey injuries: a casual analysis. Clin J Sport Med. 1999;9(1):30-33.
70. Pinto M, Kuhn JE, Greenfield ML, Hawkins RJ. Prospective analysis of ice hockey injuries at the Junior A level over the course of one season. Clin J Sport Med. 1999;9(2):70-74.
71. Emery CA, Meeuwisse WH. Injury rates, risk factors, and mechanisms of injury in minor hockey. Am J Sports Med. 2006;34(12):1960-1969.
72. Kuzuhara K, Shimamoto H, Mase Y. Ice hockey injuries in a Japanese elite team: a 3-year prospective study. J Athl Train. 2009;44(2):208-214.
73. Rishiraj N, Lloyd-Smith R, Lorenz T, Niven B, Michel M. University men’s ice hockey: rates and risk of injuries over 6-years. J Sports Med Phys Fitness. 2009;49(2):159-166.
74. Tuominen M, Stuart MJ, Aubry M, Kannus P, Parkkari J. Injuries in men’s international ice hockey: a 7-year study of the International Ice Hockey Federation Adult World Championship Tournaments and Olympic Winter Games. Br J Sports Med. 2015;49(1):30-36.
75. Heckman JD, Bucholz RW. In: Rockwood CA, Green DP, Heckman JD, Bucholz RW, eds. Rockwood and Green’s Fractures in Adults, Volume 1. Philadelphia, PA: Lippincott Williams & Wilkins; 2001.
1. Vaughan G. The Puck Starts Here: The Origin of Canada’s Great Winter Game, Ice Hockey. Fredericton, Canada: Goose Lane Editions; 1996.
2. IIHF member national associations. International Ice Hockey Federation website. http://www.iihf.com/iihf-home/the-iihf/members. Accessed April 6, 2017.
3. Flik K, Lyman S, Marx RG. American collegiate men’s ice hockey: an analysis of injuries. Am J Sports Med. 2005;33(2):183-187.
4. Engebretsen L, Steffen K, Alonso JM, et al. Sports injuries and illnesses during the Winter Olympic Games 2010. Br J Sports Med. 2010;44(11):772-780.
5. Deits J, Yard EE, Collins CL, Fields SK, Comstock RD. Patients with ice hockey injuries presenting to US emergency departments, 1990-2006. J Athl Train. 2010;45(5):467-474.
6. Brooks A, Loud KJ, Brenner JS, et al. Reducing injury risk from body checking in boys’ youth ice hockey. Pediatrics. 2014;133(6):1151-1157.
7. Agel J, Harvey EJ. A 7-year review of men’s and women’s ice hockey injuries in the NCAA. Can J Surg. 2010;53(5):319-323.
8. Micieli JA, Zurakowski D, Ahmed, II. Impact of visors on eye and orbital injuries in the National Hockey League. Can J Ophthalmol. 2014;49(3):243-248.
9. Pashby TJ. Ocular injuries in hockey. Int Ophthalmol Clin. 1988;28(3):228-231.
10. Leivo T, Haavisto AK, Sahraravand A. Sports-related eye injuries: the current picture. Acta Ophthalmol. 2015;93(3):224-231.
11. Cohn RM, Alaia MJ, Strauss EJ, Feldman AF. Rink-side management of ice hockey related injuries to the face, neck, and chest. Bull Hosp Jt Dis. 2013;71(4):253-256.
12. Reehal P. Facial injury in sport. Curr Sports Med Rep. 2010;9(1):27-34.
13. Stuart MJ, Smith AM, Malo-Ortiguera SA, Fischer TL, Larson DR. A comparison of facial protection and the incidence of head, neck, and facial injuries in Junior A hockey players. A function of individual playing time. Am J Sports Med. 2002;30(1):39-44.
14. MacEwen CJ, McLatchie GR. Eye injuries in sport. Scott Med J. 2010;55(2):22-24.
15. Stevens ST, Lassonde M, de Beaumont L, Keenan JP. The effect of visors on head and facial injury in National Hockey League players. J Sci Med Sport. 2006;9(3):238-242.
16. Moslener MD, Wadsworth LT. Ice hockey: a team physician’s perspective. Curr Sports Med Rep. 2010;9(3):134-138.
17. LaPrade RF, Burnett QM, Zarzour R, Moss R. The effect of the mandatory use of face masks on facial lacerations and head and neck injuries in ice hockey. A prospective study. Am J Sports Med. 1995;23(6):773-775.
18. Benson BW, Mohtadi NG, Rose MS, Meeuwisse WH. Head and neck injuries among ice hockey players wearing full face shields vs half face shields. JAMA. 1999;282(24):2328-2332.
19. Lahti H, Sane J, Ylipaavalniemi P. Dental injuries in ice hockey games and training. Med Sci Sports Exerc. 2002;34(3):400-402.
20. Sane J, Ylipaavalniemi P, Leppanen H. Maxillofacial and dental ice hockey injuries. Med Sci Sports Exerc. 1988;20(2):202-207.
21. Emerich K, Kaczmarek J. First aid for dental trauma caused by sports activities: state of knowledge, treatment and prevention. Sports Med. 2010;40(5):361-366.
22. Rosenberg H, Rosenberg H, Hickey M. Emergency management of a traumatic tooth avulsion. Ann Emerg Med. 2011;57(4):375-377.
23. Young EJ, Macias CR, Stephens L. Common dental injury management in athletes. Sports Health. 2015;7(3):250-255.
24. Andersson L, Andreasen JO, Day P, et al. International Association of Dental Traumatology guidelines for the management of traumatic dental injuries: 2. Avulsion of permanent teeth. Dent Traumatol. 2012;28(2):88-96.
25. McCrory P, Meeuwisse W, Johnston K, et al. Consensus statement on concussion in sport 3rd International Conference on Concussion in Sport held in Zurich, November 2008. Clin J Sport Med. 2009;19(3):185-200.
26. Schneider KJ, Meeuwisse WH, Kang J, Schneider GM, Emery CA. Preseason reports of neck pain, dizziness, and headache as risk factors for concussion in male youth ice hockey players. Clin J Sport Med. 2013;23(4):267-272.
27. McCrory P, Meeuwisse WH, Aubry M, et al. Consensus statement on concussion in sport: the 4th International Conference on Concussion in Sport held in Zurich, November 2012. Br J Sports Med. 2013;47(5):250-258.
28. Delaney JS, Lamfookon C, Bloom GA, Al-Kashmiri A, Correa JA. Why university athletes choose not to reveal their concussion symptoms during a practice or game. Clin J Sport Med. 2015;25(2):113-125.
29. Ventura RE, Balcer LJ, Galetta SL. The concussion toolbox: the role of vision in the assessment of concussion. Semin Neurol. 2015;35(5):599-606.
30. Vartiainen MV, Holm A, Peltonen K, Luoto TM, Iverson GL, Hokkanen L. King-Devick test normative reference values for professional male ice hockey players. Scand J Med Sci Sports. 2015;25(3):e327-e330.
31. Galetta MS, Galetta KM, McCrossin J, et al. Saccades and memory: baseline associations of the King-Devick and SCAT2 SAC tests in professional ice hockey players. J Neurol Sci. 2013;328(1-2):28-31.
32. Vernau BT, Grady MF, Goodman A, et al. Oculomotor and neurocognitive assessment of youth ice hockey players: baseline associations and observations after concussion. Dev Neuropsychol. 2015;40(1):7-11.
33. Fry AF, Hale S. Relationships among processing speed, working memory, and fluid intelligence in children. Biol Psychol. 2000;54(1-3):1-34.
34. Felleman DJ, Van Essen DC. Distributed hierarchical processing in the primate cerebral cortex. Cereb Cortex. 1991;1(1):1-47.
35. Guskiewicz KM, Register-Mihalik J, McCrory P, et al. Evidence-based approach to revising the SCAT2: introducing the SCAT3. Br J Sports Med. 2013;47(5):289-293.
36. Smith AM, Stuart MJ, Dodick DW, et al. Ice Hockey Summit II: zero tolerance for head hits and fighting. Curr Sports Med Rep. 2015;14(2):135-144.
37. Mihalik JP, Beard JR, Petschauer MA, Prentice WE, Guskiewicz KM. Effect of ice hockey helmet fit on cervical spine motion during an emergency log roll procedure. Clin J Sport Med. 2008;18(5):394-398.
38. Banerjee R, Palumbo MA, Fadale PD. Catastrophic cervical spine injuries in the collision sport athlete, part 1: epidemiology, functional anatomy, and diagnosis. Am J Sports Med. 2004;32(4):1077-1087.
39. Reynen PD, Clancy WG Jr. Cervical spine injury, hockey helmets, and face masks. Am J Sports Med. 1994;22(2):167-170.
40. Tator CH, Provvidenza C, Cassidy JD. Update and overview of spinal injuries in Canadian ice hockey, 1943 to 2011: the continuing need for injury prevention and education. Clin J Sport Med. 2016;26(3):232-238.
41. Tator CH, Edmonds VE, Lapczak L, Tator IB. Spinal injuries in ice hockey players, 1966-1987. Can J Surg. 1991;34(1):63-69.
42. Laprade RF, Schnetzler KA, Broxterman RJ, Wentorf F, Gilbert TJ. Cervical spine alignment in the immobilized ice hockey player. A computed tomographic analysis of the effects of helmet removal. Am J Sports Med. 2000;28(6):800-803.
43. Metz CM, Kuhn JE, Greenfield ML. Cervical spine alignment in immobilized hockey players: radiographic analysis with and without helmets and shoulder pads. Clin J Sport Med. 1998;8(2):92-95.
44. National Athletic Trainers’ Association. Appropriate prehospital management of the spine-injured athlete: updated from 1998 document. http://www.nata.org/sites/default/files/Executive-Summary-Spine-Injury-updated.pdf. Updated August 5, 2015. Accessed April 6, 2017.
45. Del Rossi G, Heffernan TP, Horodyski M, Rechtine GR. The effectiveness of extrication collars tested during the execution of spine-board transfer techniques. Spine J. 2004;4(6):619-623.
46. Morganti C, Sweeney CA, Albanese SA, Burak C, Hosea T, Connolly PJ. Return to play after cervical spine injury. Spine. 2001;26(10):1131-1136.
47. Huang P, Anissipour A, McGee W, Lemak L. Return-to-play recommendations after cervical, thoracic, and lumbar spine injuries: a comprehensive review. Sports Health. 2016;8(1):19-25.
48. Shindle MK, Marx RG, Kelly BT, Bisson L, Burke CJ 3rd. Hockey injuries: a pediatric sport update. Curr Opin Pediatr. 2010;22(1):54-60.
49. Molsa J, Kujala U, Myllynen P, Torstila I, Airaksinen O. Injuries to the upper extremity in ice hockey: analysis of a series of 760 injuries. Am J Sports Med. 2003;31(5):751-757.
50. Dwyer T, Petrera M, Bleakney R, Theodoropoulos JS. Shoulder instability in ice hockey players: incidence, mechanism, and MRI findings. Clin Sports Med. 2013;32(4):803-813.
51. LaPrade RF, Wijdicks CA, Griffith CJ. Division I intercollegiate ice hockey team coverage. Br J Sports Med. 2009;43(13):1000-1005.
52. Willimon SC, Gaskill TR, Millett PJ. Acromioclavicular joint injuries: anatomy, diagnosis, and treatment. Phys Sportsmed. 2011;39(1):116-122.
53. Martetschlager F, Horan MP, Warth RJ, Millett PJ. Complications after anatomic fixation and reconstruction of the coracoclavicular ligaments. Am J Sports Med. 2013;41(12):2896-2903.
54. Carofino BC, Mazzocca AD. The anatomic coracoclavicular ligament reconstruction: surgical technique and indications. J Shoulder Elbow Surg. 2010;19(2 suppl):37-46.
55. Laprade RF, Surowiec RK, Sochanska AN, et al. Epidemiology, identification, treatment and return to play of musculoskeletal-based ice hockey injuries. Br J Sports Med. 2014;48(1):4-10.
56. Canadian Orthopaedic Trauma Society. Nonoperative treatment compared with plate fixation of displaced midshaft clavicular fractures. A multicenter, randomized clinical trial. J Bone Joint Surg Am. 2007;89(1):1-10.
57. Lee JT, Nasreddine AY, Black EM, Bae DS, Kocher MS. Posterior sternoclavicular joint injuries in skeletally immature patients. J Pediatr Orthop. 2014;34(4):369-375.
58. Hovelius L. Shoulder dislocation in Swedish ice hockey players. Am J Sports Med. 1978;6(6):373-377.
59. Buss DD, Lynch GP, Meyer CP, Huber SM, Freehill MQ. Nonoperative management for in-season athletes with anterior shoulder instability. Am J Sports Med. 2004;32(6):1430-1433.
60. Mazzocca AD, Brown FM Jr, Carreira DS, Hayden J, Romeo AA. Arthroscopic anterior shoulder stabilization of collision and contact athletes. Am J Sports Med. 2005;33(1):52-60.
61. Harris JD, Romeo AA. Arthroscopic management of the contact athlete with instability. Clin Sports Med. 2013;32(4):709-730.
62. Cho NS, Hwang JC, Rhee YG. Arthroscopic stabilization in anterior shoulder instability: collision athletes versus noncollision athletes. Arthroscopy. 2006;22(9):947-953.
63. Griffin JW, Brockmeier SF. Shoulder instability with concomitant bone loss in the athlete. Orthop Clin North Am. 2015;46(1):89-103.
64. Cohen SB, Towers JD, Bradley JP. Rotator cuff contusions of the shoulder in professional football players: epidemiology and magnetic resonance imaging findings. Am J Sports Med. 2007;35(3):442-447.
65. Lorentzon R, Wedrèn H, Pietilä T. Incidence, nature, and causes of ice hockey injuries. A three-year prospective study of a Swedish elite ice hockey team. Am J Sports Med. 1988;16(4):392-396.
66. Stuart MJ, Smith A. Injuries in Junior A ice hockey. A three-year prospective study. Am J Sports Med. 1995;23(4):458-461.
67. Voaklander DC, Saunders LD, Quinney HA, Macnab RB. Epidemiology of recreational and old-timer ice hockey injuries. Clin J Sport Med. 1996;6(1):15-21.
68. Mölsä J, Airaksinen O, Näsman O, Torstila I. Ice hockey injuries in Finland. A prospective epidemiologic study. Am J Sports Med. 1997;25(4):495-499.
69. Ferrara MS, Schurr KT. Intercollegiate ice hockey injuries: a casual analysis. Clin J Sport Med. 1999;9(1):30-33.
70. Pinto M, Kuhn JE, Greenfield ML, Hawkins RJ. Prospective analysis of ice hockey injuries at the Junior A level over the course of one season. Clin J Sport Med. 1999;9(2):70-74.
71. Emery CA, Meeuwisse WH. Injury rates, risk factors, and mechanisms of injury in minor hockey. Am J Sports Med. 2006;34(12):1960-1969.
72. Kuzuhara K, Shimamoto H, Mase Y. Ice hockey injuries in a Japanese elite team: a 3-year prospective study. J Athl Train. 2009;44(2):208-214.
73. Rishiraj N, Lloyd-Smith R, Lorenz T, Niven B, Michel M. University men’s ice hockey: rates and risk of injuries over 6-years. J Sports Med Phys Fitness. 2009;49(2):159-166.
74. Tuominen M, Stuart MJ, Aubry M, Kannus P, Parkkari J. Injuries in men’s international ice hockey: a 7-year study of the International Ice Hockey Federation Adult World Championship Tournaments and Olympic Winter Games. Br J Sports Med. 2015;49(1):30-36.
75. Heckman JD, Bucholz RW. In: Rockwood CA, Green DP, Heckman JD, Bucholz RW, eds. Rockwood and Green’s Fractures in Adults, Volume 1. Philadelphia, PA: Lippincott Williams & Wilkins; 2001.
Arthroscopic Excision of Bipartite Patella With Preservation of Lateral Retinaculum in an Adolescent Ice Hockey Player
Take-Home Points
- Bipartite patella is an asymptomatic anatomical variant.
- Occasionally, some adolescent athletes can present with AKP, resulting in decreased participation and performance.
- Bipartite patella is classified in type I, inferior pole; type II, lateral margin; and type III, superior lateral pole, depending on where the accessory patellar fragment is.
- Nonoperative treatment is advocated first. If symptoms persist surgical treatment should be attempted.
In 2% to 3% of the general population, the finding of bipartite patella on knee radiographs is often incidental.1,2 During development, the patella normally originates in a primary ossification center. Occasionally, secondary ossification centers emerge around the margins of the primary center and typically join that center. In some cases, the secondary2 center remains separated, leading to patella partita and an accessory patellar fragment.3,4
The bipartite patella is connected to the primary patella by fibrocartilage. The fibrous attachment may become irritated or separated as a result of trauma, overuse, or strenuous activity.1,5-7 Saupe classification of bipartite patella is based on accessory patellar fragment location: type I, inferior pole; type II, lateral margin; and type III, superior lateral pole.8 When an individual with a bipartite patella becomes symptomatic, anterior knee pain (AKP) is the most common complaint—it has been described in adolescent athletes in numerous sports.7,9-11For most patients, first-line treatment is nonoperative management. A typical regimen includes reduced activity, use of nonsteroidal anti-inflammatory drugs, physical therapy, and isometric quadriceps-strengthening exercises.1,12 Other nonoperative approaches described in the literature are immobilization,5,10 steroid and anesthetic injection, and ultrasound therapy.13 If symptoms do not improve, surgical treatment should be considered. Surgical treatment options include open excision of fragment,3,9,12 arthroscopic excision of fragment,7,14,15 tension band wiring,5,16 open reduction and internal fixation,17 open or arthroscopic vastus lateralis release,18-20 and lateral retinacular release.21 However, the optimal surgical option remains controversial.
In this case report, we present a modification of an arthroscopic surgical technique for excising a symptomatic bipartite patella and report midterm clinical outcomes. The patient provided written informed consent for print and electronic publication of this report.
Case Report
A 16-year-old elite male ice hockey player presented to clinic with a 2-week history of left AKP. He could not recall a specific injury that triggered the symptoms. Radiographs were obtained at an outside institution, and knee patellar fracture was diagnosed. The patient, placed in a straight-leg immobilizer, later presented to a referral clinic for a second opinion and further evaluation. Physical examination revealed significant tenderness to palpation of the lateral aspect of the patella. Range of motion was symmetric and fully intact. Patellar mobility was excellent. However, the patient could not perform a straight-leg raise because of the pain.
We obtained anteroposterior and lateral radiographs (Figures 1A, 1B), which showed evidence of a Saupe type III bipartite patella with separation at the superolateral pole.
Two years later, the patient returned with left AKP, again localized to the lateral aspect of the patella, over the bipartite fragment. The pain was significant with compression. Given the patient’s history, arthroscopic excision of the bipartite patella was recommended. After discussing all treatment options, the patient elected to proceed with the surgery.
Surgical Technique
The patient was positioned supine on the operating table. Medial and lateral parapatellar arthroscopic portals were created. Menisci, cruciate ligaments, and tibiofemoral articular cartilage were arthroscopically visualized and determined to be normal. The bipartite patella was easily visualized, and notably loose when probed. Grade 2 chondromalacia was present diffusely throughout the bipartite patella and on the far lateral aspect of the patella, at the fragment interface.
Attention was then turned to arthroscopic removal of the accessory patellar fragment (Figures 3A, 3B).
Postoperative Rehabilitation
Rehabilitation focused on protection of the healing patella and accelerated rehabilitation for early return to play. Range-of-motion exercises and stationary bicycling were initiated on postoperative day 1. Weight-bearing was allowed as tolerated. Quadriceps sets, straight-leg raises, and ankle pumps were performed 5 times daily for 6 weeks. Six weeks after surgery, the patient was cleared, and he returned to full on-ice activities.
Outcomes
This study was approved by an Institutional Review Board. Preoperative and postoperative outcomes were obtained and stored in a data registry. The patient’s Lysholm score22 improved from 71 before surgery to 100 at 31-month follow-up. In addition, his subjective International Knee Documentation Committee score23 improved from 65.5 before surgery to 72.4 after surgery. At follow-up, patient satisfaction with outcome was 10/10. In addition, the patient had returned to playing hockey at a higher national level without functional limitation.
Discussion
The most important finding in this case is that arthroscopic excision of a bipartite patella with preservation of the lateral retinaculum in an elite adolescent hockey player resulted in improved subjective clinical outcomes scores and early return to competition. Arthroscopic excision was favored over open excision in this patient because of potential quicker recovery,14 less pain, and expedited return to competition. In addition, previous arthroscopic techniques were modified to shorten postoperative rehabilitation. The modified technique included preservation of the lateral retinaculum and total arthroscopic excision of the accessory bipartite patella fragment.
Although results of open techniques have been favorable,3,8,9 these procedures are far more invasive than arthroscopic techniques and may result in loss of quadriceps strength and prolonged rehabilitation.18 Weckström and colleagues12 followed 25 male military recruits for a minimum of 10 years after open excision of symptomatic bipartite patella. Mean Kujala score was 95 (range, 75-100), and median visual analog scale score for knee pain was 1.0 (range, 0.0-6.0). In a study by Bourne and Bianco,3 13 of 16 patients who were followed for an average of 7 years experienced complete pain relief with an average recovery time of 2 months.
Other studies have described the arthroscopic excision technique for symptomatic bipartite patella,7,14,15 but outcomes are underreported, especially for follow-ups longer than 2 years. Felli and colleagues7 described a case of arthroscopic excision and lateral release in a 23-year-old female professional volleyball player; at 1-year follow-up, the patient was symptom-free and back to full athletic participation. Azarbod and colleagues14 also reported on a patient who was symptom-free, 6 weeks after arthroscopic excision of bipartite patella. Carney and colleagues15 indicated that successful excision of bipartite patella was evident on 6-month radiographic follow-up. Our 31-month follow-up is the longest of any study on arthroscopic excision of bipartite patella. Clinical outcomes were excellent both in our patient’s case and in the earlier studies.
Our patient was a high-level hockey player who wanted to return to competition as quickly as possible. Conservative management, including physical therapy, initially resolved his symptoms and allowed him to resume on-ice activities after 6 weeks. In time, however, his symptoms returned and began limiting his on-ice performance. Arthroscopic removal of the bipartite patella accessory fragment allowed him to return to full on-ice activities after 6 weeks. His case provides evidence that arthroscopic management of bipartite patella with preservation of the vastus lateralis and lateral retinaculum may be an excellent treatment option for patients who want to return to athletics as quickly as possible.
Our technique of arthroscopic excision with preservation of lateral retinaculum is an excellent treatment option for symptomatic bipartite patella. This option, combined with an aggressive rehabilitation protocol, allows for pain relief and expedited return to competition.
Am J Orthop. 2017;46(3):135-138. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
1. Atesok K, Doral MN, Lowe J, Finsterbush A. Symptomatic bipartite patella: treatment alternatives. J Am Acad Orthop Surg. 2008;16(8):455-461.
2. Insall J. Current concepts review: patellar pain. J Bone Joint Surg Am. 1982;64(1):147-152.
3. Bourne MH, Bianco AJ Jr. Bipartite patella in the adolescent: results of surgical excision. J Pediatr Orthop. 1990;10(1):69-73.
4. Oohashi Y, Koshino T, Oohashi Y. Clinical features and classification of bipartite or tripartite patella. Knee Surg Sports Traumatol Arthrosc. 2010;18(11):1465-1469.
5. Okuno H, Sugita T, Kawamata T, Ohnuma M, Yamada N, Yoshizumi Y. Traumatic separation of a type I bipartite patella: a report of four knees. Clin Orthop Relat Res. 2004;(420):257-260.
6. Yoo JH, Kim EH, Ryu HK. Arthroscopic removal of separated bipartite patella causing snapping knee syndrome. Orthopedics. 2008;31(7):717.
7. Felli L, Fiore M, Biglieni L. Arthroscopic treatment of symptomatic bipartite patella. Knee Surg Sports Traumatol Arthrosc. 2011;19(3):398-399.
8. Green WT Jr. Painful bipartite patellae. A report of three cases. Clin Orthop Relat Res. 1975;(110):197-200.
9. Ishikawa H, Sakurai A, Hirata S, et al. Painful bipartite patella in young athletes. The diagnostic value of skyline views taken in squatting position and the results of surgical excision. Clin Orthop Relat Res. 1994;(305):223-228.
10. Stocker RL, van Laer L. Injury of a bipartite patella in a young upcoming sportsman. Arch Orthop Trauma Surg. 2011;131(1):75-78.
11. Wong CK. Bipartite patella in a young athlete. J Orthop Sports Phys Ther. 2009;39(7):560.
12. Weckström M, Parviainen M, Pihlajamäki HK. Excision of painful bipartite patella: good long-term outcome in young adults. Clin Orthop Relat Res. 2008;466(11):2848-2855.
13. Kumahashi N, Uchio Y, Iwasa J, Kawasaki K, Adachi N, Ochi M. Bone union of painful bipartite patella after treatment with low-intensity pulsed ultrasound: report of two cases. Knee. 2008;15(1):50-53.
14. Azarbod P, Agar G, Patel V. Arthroscopic excision of a painful bipartite patella fragment. Arthroscopy. 2005;21(8):1006.
15. Carney J, Thompson D, O’Daniel J, Cassidy J. Arthroscopic excision of a painful bipartite patella fragment. Am J Orthop. 2010;39(1):40-43.
16. Tauber M, Matis N, Resch H. Traumatic separation of an uncommon bipartite patella type: a case report. Knee Surg Sports Traumatol Arthrosc. 2007;15(1):83-87.
17. Werner S, Durkan M, Jones J, Quilici S, Crawford D. Symptomatic bipartite patella: three subtypes, three representative cases. J Knee Surg. 2013;26(suppl 1):S72-S76.
18. Adachi N, Ochi M, Yamaguchi H, Uchio Y, Kuriwaka M. Vastus lateralis release for painful bipartite patella. Arthroscopy. 2002;18(4):404-411.
19. Maeno S, Hashimoto D, Otani T, Masumoto K, Hui C. The “coiling-up procedure”: a novel technique for extra-articular arthroscopy. Arthroscopy. 2010;26(11):1551-1555.
20. Ogata K. Painful bipartite patella. A new approach to operative treatment. J Bone Joint Surg Am. 1994;76(4):573-578.
21. Mori Y, Okumo H, Iketani H, Kuroki Y. Efficacy of lateral retinacular release for painful bipartite patella. Am J Sports Med. 1995;23(1):13-18.
22. Lysholm J, Gillquist J. Evaluation of knee ligament surgery results with special emphasis on use of a scoring scale. Am J Sports Med. 1982;10(3):150-154
23. Grevnerts HT, Terwee CB, Kvist J. The measurement properties of the IKDC-subjective knee form. Knee Surg Sports Traumatol Arthrosc. 2015;23(12):3698-3706.
Take-Home Points
- Bipartite patella is an asymptomatic anatomical variant.
- Occasionally, some adolescent athletes can present with AKP, resulting in decreased participation and performance.
- Bipartite patella is classified in type I, inferior pole; type II, lateral margin; and type III, superior lateral pole, depending on where the accessory patellar fragment is.
- Nonoperative treatment is advocated first. If symptoms persist surgical treatment should be attempted.
In 2% to 3% of the general population, the finding of bipartite patella on knee radiographs is often incidental.1,2 During development, the patella normally originates in a primary ossification center. Occasionally, secondary ossification centers emerge around the margins of the primary center and typically join that center. In some cases, the secondary2 center remains separated, leading to patella partita and an accessory patellar fragment.3,4
The bipartite patella is connected to the primary patella by fibrocartilage. The fibrous attachment may become irritated or separated as a result of trauma, overuse, or strenuous activity.1,5-7 Saupe classification of bipartite patella is based on accessory patellar fragment location: type I, inferior pole; type II, lateral margin; and type III, superior lateral pole.8 When an individual with a bipartite patella becomes symptomatic, anterior knee pain (AKP) is the most common complaint—it has been described in adolescent athletes in numerous sports.7,9-11For most patients, first-line treatment is nonoperative management. A typical regimen includes reduced activity, use of nonsteroidal anti-inflammatory drugs, physical therapy, and isometric quadriceps-strengthening exercises.1,12 Other nonoperative approaches described in the literature are immobilization,5,10 steroid and anesthetic injection, and ultrasound therapy.13 If symptoms do not improve, surgical treatment should be considered. Surgical treatment options include open excision of fragment,3,9,12 arthroscopic excision of fragment,7,14,15 tension band wiring,5,16 open reduction and internal fixation,17 open or arthroscopic vastus lateralis release,18-20 and lateral retinacular release.21 However, the optimal surgical option remains controversial.
In this case report, we present a modification of an arthroscopic surgical technique for excising a symptomatic bipartite patella and report midterm clinical outcomes. The patient provided written informed consent for print and electronic publication of this report.
Case Report
A 16-year-old elite male ice hockey player presented to clinic with a 2-week history of left AKP. He could not recall a specific injury that triggered the symptoms. Radiographs were obtained at an outside institution, and knee patellar fracture was diagnosed. The patient, placed in a straight-leg immobilizer, later presented to a referral clinic for a second opinion and further evaluation. Physical examination revealed significant tenderness to palpation of the lateral aspect of the patella. Range of motion was symmetric and fully intact. Patellar mobility was excellent. However, the patient could not perform a straight-leg raise because of the pain.
We obtained anteroposterior and lateral radiographs (Figures 1A, 1B), which showed evidence of a Saupe type III bipartite patella with separation at the superolateral pole.
Two years later, the patient returned with left AKP, again localized to the lateral aspect of the patella, over the bipartite fragment. The pain was significant with compression. Given the patient’s history, arthroscopic excision of the bipartite patella was recommended. After discussing all treatment options, the patient elected to proceed with the surgery.
Surgical Technique
The patient was positioned supine on the operating table. Medial and lateral parapatellar arthroscopic portals were created. Menisci, cruciate ligaments, and tibiofemoral articular cartilage were arthroscopically visualized and determined to be normal. The bipartite patella was easily visualized, and notably loose when probed. Grade 2 chondromalacia was present diffusely throughout the bipartite patella and on the far lateral aspect of the patella, at the fragment interface.
Attention was then turned to arthroscopic removal of the accessory patellar fragment (Figures 3A, 3B).
Postoperative Rehabilitation
Rehabilitation focused on protection of the healing patella and accelerated rehabilitation for early return to play. Range-of-motion exercises and stationary bicycling were initiated on postoperative day 1. Weight-bearing was allowed as tolerated. Quadriceps sets, straight-leg raises, and ankle pumps were performed 5 times daily for 6 weeks. Six weeks after surgery, the patient was cleared, and he returned to full on-ice activities.
Outcomes
This study was approved by an Institutional Review Board. Preoperative and postoperative outcomes were obtained and stored in a data registry. The patient’s Lysholm score22 improved from 71 before surgery to 100 at 31-month follow-up. In addition, his subjective International Knee Documentation Committee score23 improved from 65.5 before surgery to 72.4 after surgery. At follow-up, patient satisfaction with outcome was 10/10. In addition, the patient had returned to playing hockey at a higher national level without functional limitation.
Discussion
The most important finding in this case is that arthroscopic excision of a bipartite patella with preservation of the lateral retinaculum in an elite adolescent hockey player resulted in improved subjective clinical outcomes scores and early return to competition. Arthroscopic excision was favored over open excision in this patient because of potential quicker recovery,14 less pain, and expedited return to competition. In addition, previous arthroscopic techniques were modified to shorten postoperative rehabilitation. The modified technique included preservation of the lateral retinaculum and total arthroscopic excision of the accessory bipartite patella fragment.
Although results of open techniques have been favorable,3,8,9 these procedures are far more invasive than arthroscopic techniques and may result in loss of quadriceps strength and prolonged rehabilitation.18 Weckström and colleagues12 followed 25 male military recruits for a minimum of 10 years after open excision of symptomatic bipartite patella. Mean Kujala score was 95 (range, 75-100), and median visual analog scale score for knee pain was 1.0 (range, 0.0-6.0). In a study by Bourne and Bianco,3 13 of 16 patients who were followed for an average of 7 years experienced complete pain relief with an average recovery time of 2 months.
Other studies have described the arthroscopic excision technique for symptomatic bipartite patella,7,14,15 but outcomes are underreported, especially for follow-ups longer than 2 years. Felli and colleagues7 described a case of arthroscopic excision and lateral release in a 23-year-old female professional volleyball player; at 1-year follow-up, the patient was symptom-free and back to full athletic participation. Azarbod and colleagues14 also reported on a patient who was symptom-free, 6 weeks after arthroscopic excision of bipartite patella. Carney and colleagues15 indicated that successful excision of bipartite patella was evident on 6-month radiographic follow-up. Our 31-month follow-up is the longest of any study on arthroscopic excision of bipartite patella. Clinical outcomes were excellent both in our patient’s case and in the earlier studies.
Our patient was a high-level hockey player who wanted to return to competition as quickly as possible. Conservative management, including physical therapy, initially resolved his symptoms and allowed him to resume on-ice activities after 6 weeks. In time, however, his symptoms returned and began limiting his on-ice performance. Arthroscopic removal of the bipartite patella accessory fragment allowed him to return to full on-ice activities after 6 weeks. His case provides evidence that arthroscopic management of bipartite patella with preservation of the vastus lateralis and lateral retinaculum may be an excellent treatment option for patients who want to return to athletics as quickly as possible.
Our technique of arthroscopic excision with preservation of lateral retinaculum is an excellent treatment option for symptomatic bipartite patella. This option, combined with an aggressive rehabilitation protocol, allows for pain relief and expedited return to competition.
Am J Orthop. 2017;46(3):135-138. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
Take-Home Points
- Bipartite patella is an asymptomatic anatomical variant.
- Occasionally, some adolescent athletes can present with AKP, resulting in decreased participation and performance.
- Bipartite patella is classified in type I, inferior pole; type II, lateral margin; and type III, superior lateral pole, depending on where the accessory patellar fragment is.
- Nonoperative treatment is advocated first. If symptoms persist surgical treatment should be attempted.
In 2% to 3% of the general population, the finding of bipartite patella on knee radiographs is often incidental.1,2 During development, the patella normally originates in a primary ossification center. Occasionally, secondary ossification centers emerge around the margins of the primary center and typically join that center. In some cases, the secondary2 center remains separated, leading to patella partita and an accessory patellar fragment.3,4
The bipartite patella is connected to the primary patella by fibrocartilage. The fibrous attachment may become irritated or separated as a result of trauma, overuse, or strenuous activity.1,5-7 Saupe classification of bipartite patella is based on accessory patellar fragment location: type I, inferior pole; type II, lateral margin; and type III, superior lateral pole.8 When an individual with a bipartite patella becomes symptomatic, anterior knee pain (AKP) is the most common complaint—it has been described in adolescent athletes in numerous sports.7,9-11For most patients, first-line treatment is nonoperative management. A typical regimen includes reduced activity, use of nonsteroidal anti-inflammatory drugs, physical therapy, and isometric quadriceps-strengthening exercises.1,12 Other nonoperative approaches described in the literature are immobilization,5,10 steroid and anesthetic injection, and ultrasound therapy.13 If symptoms do not improve, surgical treatment should be considered. Surgical treatment options include open excision of fragment,3,9,12 arthroscopic excision of fragment,7,14,15 tension band wiring,5,16 open reduction and internal fixation,17 open or arthroscopic vastus lateralis release,18-20 and lateral retinacular release.21 However, the optimal surgical option remains controversial.
In this case report, we present a modification of an arthroscopic surgical technique for excising a symptomatic bipartite patella and report midterm clinical outcomes. The patient provided written informed consent for print and electronic publication of this report.
Case Report
A 16-year-old elite male ice hockey player presented to clinic with a 2-week history of left AKP. He could not recall a specific injury that triggered the symptoms. Radiographs were obtained at an outside institution, and knee patellar fracture was diagnosed. The patient, placed in a straight-leg immobilizer, later presented to a referral clinic for a second opinion and further evaluation. Physical examination revealed significant tenderness to palpation of the lateral aspect of the patella. Range of motion was symmetric and fully intact. Patellar mobility was excellent. However, the patient could not perform a straight-leg raise because of the pain.
We obtained anteroposterior and lateral radiographs (Figures 1A, 1B), which showed evidence of a Saupe type III bipartite patella with separation at the superolateral pole.
Two years later, the patient returned with left AKP, again localized to the lateral aspect of the patella, over the bipartite fragment. The pain was significant with compression. Given the patient’s history, arthroscopic excision of the bipartite patella was recommended. After discussing all treatment options, the patient elected to proceed with the surgery.
Surgical Technique
The patient was positioned supine on the operating table. Medial and lateral parapatellar arthroscopic portals were created. Menisci, cruciate ligaments, and tibiofemoral articular cartilage were arthroscopically visualized and determined to be normal. The bipartite patella was easily visualized, and notably loose when probed. Grade 2 chondromalacia was present diffusely throughout the bipartite patella and on the far lateral aspect of the patella, at the fragment interface.
Attention was then turned to arthroscopic removal of the accessory patellar fragment (Figures 3A, 3B).
Postoperative Rehabilitation
Rehabilitation focused on protection of the healing patella and accelerated rehabilitation for early return to play. Range-of-motion exercises and stationary bicycling were initiated on postoperative day 1. Weight-bearing was allowed as tolerated. Quadriceps sets, straight-leg raises, and ankle pumps were performed 5 times daily for 6 weeks. Six weeks after surgery, the patient was cleared, and he returned to full on-ice activities.
Outcomes
This study was approved by an Institutional Review Board. Preoperative and postoperative outcomes were obtained and stored in a data registry. The patient’s Lysholm score22 improved from 71 before surgery to 100 at 31-month follow-up. In addition, his subjective International Knee Documentation Committee score23 improved from 65.5 before surgery to 72.4 after surgery. At follow-up, patient satisfaction with outcome was 10/10. In addition, the patient had returned to playing hockey at a higher national level without functional limitation.
Discussion
The most important finding in this case is that arthroscopic excision of a bipartite patella with preservation of the lateral retinaculum in an elite adolescent hockey player resulted in improved subjective clinical outcomes scores and early return to competition. Arthroscopic excision was favored over open excision in this patient because of potential quicker recovery,14 less pain, and expedited return to competition. In addition, previous arthroscopic techniques were modified to shorten postoperative rehabilitation. The modified technique included preservation of the lateral retinaculum and total arthroscopic excision of the accessory bipartite patella fragment.
Although results of open techniques have been favorable,3,8,9 these procedures are far more invasive than arthroscopic techniques and may result in loss of quadriceps strength and prolonged rehabilitation.18 Weckström and colleagues12 followed 25 male military recruits for a minimum of 10 years after open excision of symptomatic bipartite patella. Mean Kujala score was 95 (range, 75-100), and median visual analog scale score for knee pain was 1.0 (range, 0.0-6.0). In a study by Bourne and Bianco,3 13 of 16 patients who were followed for an average of 7 years experienced complete pain relief with an average recovery time of 2 months.
Other studies have described the arthroscopic excision technique for symptomatic bipartite patella,7,14,15 but outcomes are underreported, especially for follow-ups longer than 2 years. Felli and colleagues7 described a case of arthroscopic excision and lateral release in a 23-year-old female professional volleyball player; at 1-year follow-up, the patient was symptom-free and back to full athletic participation. Azarbod and colleagues14 also reported on a patient who was symptom-free, 6 weeks after arthroscopic excision of bipartite patella. Carney and colleagues15 indicated that successful excision of bipartite patella was evident on 6-month radiographic follow-up. Our 31-month follow-up is the longest of any study on arthroscopic excision of bipartite patella. Clinical outcomes were excellent both in our patient’s case and in the earlier studies.
Our patient was a high-level hockey player who wanted to return to competition as quickly as possible. Conservative management, including physical therapy, initially resolved his symptoms and allowed him to resume on-ice activities after 6 weeks. In time, however, his symptoms returned and began limiting his on-ice performance. Arthroscopic removal of the bipartite patella accessory fragment allowed him to return to full on-ice activities after 6 weeks. His case provides evidence that arthroscopic management of bipartite patella with preservation of the vastus lateralis and lateral retinaculum may be an excellent treatment option for patients who want to return to athletics as quickly as possible.
Our technique of arthroscopic excision with preservation of lateral retinaculum is an excellent treatment option for symptomatic bipartite patella. This option, combined with an aggressive rehabilitation protocol, allows for pain relief and expedited return to competition.
Am J Orthop. 2017;46(3):135-138. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
1. Atesok K, Doral MN, Lowe J, Finsterbush A. Symptomatic bipartite patella: treatment alternatives. J Am Acad Orthop Surg. 2008;16(8):455-461.
2. Insall J. Current concepts review: patellar pain. J Bone Joint Surg Am. 1982;64(1):147-152.
3. Bourne MH, Bianco AJ Jr. Bipartite patella in the adolescent: results of surgical excision. J Pediatr Orthop. 1990;10(1):69-73.
4. Oohashi Y, Koshino T, Oohashi Y. Clinical features and classification of bipartite or tripartite patella. Knee Surg Sports Traumatol Arthrosc. 2010;18(11):1465-1469.
5. Okuno H, Sugita T, Kawamata T, Ohnuma M, Yamada N, Yoshizumi Y. Traumatic separation of a type I bipartite patella: a report of four knees. Clin Orthop Relat Res. 2004;(420):257-260.
6. Yoo JH, Kim EH, Ryu HK. Arthroscopic removal of separated bipartite patella causing snapping knee syndrome. Orthopedics. 2008;31(7):717.
7. Felli L, Fiore M, Biglieni L. Arthroscopic treatment of symptomatic bipartite patella. Knee Surg Sports Traumatol Arthrosc. 2011;19(3):398-399.
8. Green WT Jr. Painful bipartite patellae. A report of three cases. Clin Orthop Relat Res. 1975;(110):197-200.
9. Ishikawa H, Sakurai A, Hirata S, et al. Painful bipartite patella in young athletes. The diagnostic value of skyline views taken in squatting position and the results of surgical excision. Clin Orthop Relat Res. 1994;(305):223-228.
10. Stocker RL, van Laer L. Injury of a bipartite patella in a young upcoming sportsman. Arch Orthop Trauma Surg. 2011;131(1):75-78.
11. Wong CK. Bipartite patella in a young athlete. J Orthop Sports Phys Ther. 2009;39(7):560.
12. Weckström M, Parviainen M, Pihlajamäki HK. Excision of painful bipartite patella: good long-term outcome in young adults. Clin Orthop Relat Res. 2008;466(11):2848-2855.
13. Kumahashi N, Uchio Y, Iwasa J, Kawasaki K, Adachi N, Ochi M. Bone union of painful bipartite patella after treatment with low-intensity pulsed ultrasound: report of two cases. Knee. 2008;15(1):50-53.
14. Azarbod P, Agar G, Patel V. Arthroscopic excision of a painful bipartite patella fragment. Arthroscopy. 2005;21(8):1006.
15. Carney J, Thompson D, O’Daniel J, Cassidy J. Arthroscopic excision of a painful bipartite patella fragment. Am J Orthop. 2010;39(1):40-43.
16. Tauber M, Matis N, Resch H. Traumatic separation of an uncommon bipartite patella type: a case report. Knee Surg Sports Traumatol Arthrosc. 2007;15(1):83-87.
17. Werner S, Durkan M, Jones J, Quilici S, Crawford D. Symptomatic bipartite patella: three subtypes, three representative cases. J Knee Surg. 2013;26(suppl 1):S72-S76.
18. Adachi N, Ochi M, Yamaguchi H, Uchio Y, Kuriwaka M. Vastus lateralis release for painful bipartite patella. Arthroscopy. 2002;18(4):404-411.
19. Maeno S, Hashimoto D, Otani T, Masumoto K, Hui C. The “coiling-up procedure”: a novel technique for extra-articular arthroscopy. Arthroscopy. 2010;26(11):1551-1555.
20. Ogata K. Painful bipartite patella. A new approach to operative treatment. J Bone Joint Surg Am. 1994;76(4):573-578.
21. Mori Y, Okumo H, Iketani H, Kuroki Y. Efficacy of lateral retinacular release for painful bipartite patella. Am J Sports Med. 1995;23(1):13-18.
22. Lysholm J, Gillquist J. Evaluation of knee ligament surgery results with special emphasis on use of a scoring scale. Am J Sports Med. 1982;10(3):150-154
23. Grevnerts HT, Terwee CB, Kvist J. The measurement properties of the IKDC-subjective knee form. Knee Surg Sports Traumatol Arthrosc. 2015;23(12):3698-3706.
1. Atesok K, Doral MN, Lowe J, Finsterbush A. Symptomatic bipartite patella: treatment alternatives. J Am Acad Orthop Surg. 2008;16(8):455-461.
2. Insall J. Current concepts review: patellar pain. J Bone Joint Surg Am. 1982;64(1):147-152.
3. Bourne MH, Bianco AJ Jr. Bipartite patella in the adolescent: results of surgical excision. J Pediatr Orthop. 1990;10(1):69-73.
4. Oohashi Y, Koshino T, Oohashi Y. Clinical features and classification of bipartite or tripartite patella. Knee Surg Sports Traumatol Arthrosc. 2010;18(11):1465-1469.
5. Okuno H, Sugita T, Kawamata T, Ohnuma M, Yamada N, Yoshizumi Y. Traumatic separation of a type I bipartite patella: a report of four knees. Clin Orthop Relat Res. 2004;(420):257-260.
6. Yoo JH, Kim EH, Ryu HK. Arthroscopic removal of separated bipartite patella causing snapping knee syndrome. Orthopedics. 2008;31(7):717.
7. Felli L, Fiore M, Biglieni L. Arthroscopic treatment of symptomatic bipartite patella. Knee Surg Sports Traumatol Arthrosc. 2011;19(3):398-399.
8. Green WT Jr. Painful bipartite patellae. A report of three cases. Clin Orthop Relat Res. 1975;(110):197-200.
9. Ishikawa H, Sakurai A, Hirata S, et al. Painful bipartite patella in young athletes. The diagnostic value of skyline views taken in squatting position and the results of surgical excision. Clin Orthop Relat Res. 1994;(305):223-228.
10. Stocker RL, van Laer L. Injury of a bipartite patella in a young upcoming sportsman. Arch Orthop Trauma Surg. 2011;131(1):75-78.
11. Wong CK. Bipartite patella in a young athlete. J Orthop Sports Phys Ther. 2009;39(7):560.
12. Weckström M, Parviainen M, Pihlajamäki HK. Excision of painful bipartite patella: good long-term outcome in young adults. Clin Orthop Relat Res. 2008;466(11):2848-2855.
13. Kumahashi N, Uchio Y, Iwasa J, Kawasaki K, Adachi N, Ochi M. Bone union of painful bipartite patella after treatment with low-intensity pulsed ultrasound: report of two cases. Knee. 2008;15(1):50-53.
14. Azarbod P, Agar G, Patel V. Arthroscopic excision of a painful bipartite patella fragment. Arthroscopy. 2005;21(8):1006.
15. Carney J, Thompson D, O’Daniel J, Cassidy J. Arthroscopic excision of a painful bipartite patella fragment. Am J Orthop. 2010;39(1):40-43.
16. Tauber M, Matis N, Resch H. Traumatic separation of an uncommon bipartite patella type: a case report. Knee Surg Sports Traumatol Arthrosc. 2007;15(1):83-87.
17. Werner S, Durkan M, Jones J, Quilici S, Crawford D. Symptomatic bipartite patella: three subtypes, three representative cases. J Knee Surg. 2013;26(suppl 1):S72-S76.
18. Adachi N, Ochi M, Yamaguchi H, Uchio Y, Kuriwaka M. Vastus lateralis release for painful bipartite patella. Arthroscopy. 2002;18(4):404-411.
19. Maeno S, Hashimoto D, Otani T, Masumoto K, Hui C. The “coiling-up procedure”: a novel technique for extra-articular arthroscopy. Arthroscopy. 2010;26(11):1551-1555.
20. Ogata K. Painful bipartite patella. A new approach to operative treatment. J Bone Joint Surg Am. 1994;76(4):573-578.
21. Mori Y, Okumo H, Iketani H, Kuroki Y. Efficacy of lateral retinacular release for painful bipartite patella. Am J Sports Med. 1995;23(1):13-18.
22. Lysholm J, Gillquist J. Evaluation of knee ligament surgery results with special emphasis on use of a scoring scale. Am J Sports Med. 1982;10(3):150-154
23. Grevnerts HT, Terwee CB, Kvist J. The measurement properties of the IKDC-subjective knee form. Knee Surg Sports Traumatol Arthrosc. 2015;23(12):3698-3706.
Internal Carotid Artery Dissection After Indirect Blunt Cervical Trauma in an Ice Hockey Goaltender
Take-Home Points
- ICA dissections may occur from direct or indirect trauma.
- Symptoms can be mild, including a persistent headache.
- High clinical suspicion is required for diagnosis when symptoms are mild.
- Neuroimaging is required for definitive diagnosis.
- Conservative management with serial imaging can yield successful outcomes.
Cervical artery dissection (CAD) is an uncommon but potentially life-threatening condition that accounts for a high proportion of ischemic strokes in patients under the age of 45 years.1-4 The extracranial internal carotid arteries (ICAs) and vertebral arteries are most commonly involved; dissections can occur after either direct trauma to the neck, or indirect trauma resulting in acute hyperextension or hyperflexion.4-7 ICA dissection can be difficult to diagnose because of the varying symptomatology. Clinical presentation depends on stenosis location, degree of luminal narrowing, and presence or absence of ischemic stroke. Neurologic symptoms may be delayed, and misdiagnosis of an isolated soft-tissue contusion, whiplash, can be made in the setting of indirect cervical trauma.
Although this entity is well described in the literature,2,3,5,8 there are few reported cases of injuries sustained during high-intensity athletic competition. In this case report, we describe the symptoms, physical examination findings, diagnostic imaging results, and treatment of a young male athlete who presented with delayed-onset symptoms of ICA dissection resulting from indirect cervical trauma sustained during an ice hockey game. We discuss the importance of a high level of clinical suspicion in the diagnosis of neck injuries sustained during athletic competition, as well as the need for early vascular imaging for diagnosis. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
The patient was a right-handed 32-year-old professional hockey goaltender. Four days before diagnosis, his goaltending mask and attached neck-protector were inadvertently lifted by another player’s stick just as a puck traveling at high speed struck him in the neck, to the right of the larynx, causing acute neck hyperextension. He immediately experienced discomfort and fell to the ice, saying he was “dizzy and light-headed.” Play was stopped, and medical personnel attended to him. His symptoms resolved, and he resumed play without any notable deficits. The next day, he noted discomfort at the impact site, but no additional symptoms, and received a presumptive diagnosis of cervical soft-tissue contusion. Continuing to participate in hockey that day, he did not develop any symptoms other than superficial cervical discomfort. However, the next morning, he presented complaining of severe right frontotemporal headache, which had persisted overnight. Orthopedic examination revealed palpable tenderness over the anterior cervical musculature, including the sternocleidomastoid and strap muscles. There was no appreciable hematoma in the contused area. Cervical range of motion was otherwise preserved. Cervical spine examination, including dermatomal and myotomal examination, was normal, as was cranial nerve examination. However, given the headache intensity and the recency of the injury, the potential for vascular or neurologic injury was considered. A neurology consultation was obtained, and arrangements were made for advanced cross-sectional imaging.
On further evaluation, the patient denied loss of consciousness, seizure, vomiting, amnesia, visual disturbance, language or cognitive impairment, balance or coordination difficulties, or any appreciable face or limb weakness. Review of systems was otherwise negative. Detailed neurologic examination did not reveal any cranial nerve deficits, and pupils were 3 mm, equal, and normally responsive to light and accommodation. Muscular tone and strength were symmetric and full in the upper and lower extremities. Gait, coordination, and response to vibration and temperature sensation were all preserved.
Magnetic resonance imaging of the head and neck was normal, but magnetic resonance angiography (MRA) of the neck showed a 1-cm-long region of the ICA, before piercing the petrous bone, with evidence of dissection.
Given the normal neurologic examination, and no evidence of brain infarction or other neurovascular complications, the acute ICA dissection was managed with antiplatelet therapy using aspirin (325 mg/d). In addition, the patient was advised to refrain from strenuous physical activity and to present to the hospital immediately if symptoms worsened or any neurologic impairment developed. Follow-up and repeat MRA were planned to monitor healing progression.
Two weeks after injury, the patient returned for follow-up. His headache and neck pain had resolved. Physical examination findings were unchanged, and there were no notable neurologic deficits. Repeat MRA findings were essentially unchanged, except for slightly increased luminal stenosis, exceeding 50% (Figure 2), attributable to intramural hematoma formation.
At 6-week follow-up, the patient had no clinical symptoms and no recurrence of headaches.
Discussion
In cases of direct (blunt) or indirect cervical trauma, CAD should be considered, as it carries a risk of potentially debilitating ischemic stroke in otherwise healthy young patients. Fortunately, CAD is rare; its annual incidence is 1 in 100,000, occurring in 0.08% to 1.2% of blunt trauma cases.9
As symptoms of ICA dissection can vary depending on stenosis severity, diagnosis can be challenging. The classically associated triad of symptoms includes unilateral head, facial, or neck pain accompanied by partial Horner syndrome with progression to cerebral or retinal ischemia. However, these symptoms occur in less than a third of patients with ICA dissection.2 Neck pain may occur secondary to blunt cervical trauma, consistent with a cervical soft-tissue contusion; however, it may have more severe implications and should be carefully monitored, particularly if accompanied by additional symptoms, such as headache. Headaches, which are present in 44% to 69% of patients, are often unilateral and constant. Either headache or neck pain in isolation is relatively uncommon, occurring in <10% of cases,2 though retrospective reviews of delayed-onset ICA dissection found atypical headache or neck pain in 100% of patients,11 indicating that persistent symptoms should be further evaluated.
More commonly, patients present with neurologic symptoms, particularly Horner syndrome, which is caused by the disruption of the sympathetic nerve fibers adjacent to the ICA, resulting in ipsilateral ptosis and miosis. In addition, patients may present with cranial nerve palsies, most commonly involving cranial nerve XII (the hypoglossal nerve), resulting in tongue weakness and abnormal taste. These and other neurologic findings associated with retinal or cerebral ischemia should raise clinical suspicion for the injury and prompt computed tomography or MRA evaluation.
MRA has largely replaced conventional angiography for the diagnosis of CAD. As MRA is noninvasive, it allows for improved visualization of luminal narrowing and for evaluation of the arterial wall and intramural hematoma.2 Because of the potential for devastating sequelae with missed or delayed diagnosis, several authors have become proponents of early aggressive screening for detection of these injuries.9 Postdiagnostic treatment depends on the presence of neurologic symptoms. Management is directed toward limiting neurologic deficits; anticoagulant or antiplatelet agents are used to prevent thromboembolic events. A randomized controlled trial and other studies have failed to find any appreciable difference in subsequent rates of stroke or associated complications with use of either class of medication.8,12 Conventionally, treatment is continued for 3 to 6 months, depending on clinical resolution. Endovascular or surgical intervention typically is reserved for extreme luminal narrowing, conditions that are preventing anticoagulation, an expanding area of dissection with a persistent pseudoaneurysm, and cases of failed medical management with subsequent ischemic stroke.2The literature includes several case reports involving indirect trauma in recreational athletes. First, a 31-year-old woman sustained an ICA dissection secondary to a head injury that occurred during a soccer match; she presented with headache, altered sense of taste, and objective findings of ptosis and miosis consistent with Horner syndrome.13 Second, a 39-year-old man had an ICA dissection after a snowboarding fall that caused neck hyperextension; he presented with periocular headache, ptosis, and miosis.6 Third, 3 people who participated in CrossFit training sustained ICA dissection.7 They presented with varying degrees of neurologic symptoms: ptosis and miosis; right-side upper extremity ataxia; and visual distortion and receptive aphasia. Our patient’s ICA dissection resulted from indirect trauma that caused sudden hyperextension and lateral flexion in response to contact from a hockey puck. However, his case is unique in that symptoms onset was delayed, and there were no associated neurologic findings on clinical presentation. His case should raise awareness of this potential diagnosis, even in the absence of overt neurologic findings. In addition, the patient’s return to sport at 8 weeks was facilitated by full clinical resolution of symptoms and thorough radiographic documentation of improved intramural narrowing. Finally, to our knowledge this is the first report of this injury in a professional athlete.
Conclusion
We have reported the case of a 32-year-old professional hockey goaltender who presented with isolated, persistent, worsening headache of delayed onset after ICA dissection. The ICA dissection resulted from indirect trauma, with reaction to a puck causing acute hyperextension and rotational injury. To our knowledge, this is the first report of a case of ICA dissection in an athlete, lacking neurologic examination findings that could aid in the diagnosis. The index of suspicion for CAD should be high after direct or indirect cervical trauma when patients present with unilateral neck pain or headache, even in the absence of neurologic findings, as stroke is a catastrophic but preventable complication.
Am J Orthop. 2017;46(3):E139-E143. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
1. Mohan IV. Current optimal assessment and management of carotid and vertebral spontaneous and traumatic dissection. Angiology. 2014;65(4):274-283.
2. Patel RR, Adam R, Maldjian C, Lincoln CM, Yuen A, Arneja A. Cervical carotid artery dissection: current review of diagnosis and treatment. Cardiol Rev. 2012;20(3):145-152.
3. Biller J, Sacco RL, Albuquerque FC, et al; American Heart Association Stroke Council. Cervical arterial dissections and association with cervical manipulative therapy: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2014;45(10):3155-3174.
4. Fukunaga N, Hanaoka M, Sato K. Asymptomatic common carotid artery dissection caused by blunt injury. Emerg Med J. 2011;28(1):50.
5. Chen J, Zhou X, Li C, Cheung BM. Risk of stroke due to spontaneous cervical artery dissection. Intern Med. 2013;52(19):2237-2240.
6. Kalantzis G, Georgalas I, Chang BY, Ong C, El-Hindy N. An unusual case of traumatic internal carotid artery dissection during snowboarding. J Sports Sci Med. 2014;13(2):451-453.
7. Lu A, Shen P, Lee P, et al. CrossFit-related cervical internal carotid artery dissection. Emerg Radiol. 2015;22(4):449-452.
8. CADISS Trial Investigators, Markus HS, Hayter E, Levi C, Feldman A, Venables G, Norris J. Antiplatelet treatment compared with anticoagulation treatment for cervical artery dissection (CADISS): a randomised trial. Lancet Neurol. 2015;14(4):361-367.
9. van Wessem KJ, Meijer JM, Leenen LP, van der Worp HB, Moll FL, de Borst GJ. Blunt traumatic carotid artery dissection still a pitfall? The rationale for aggressive screening. Eur J Trauma Emerg Surg. 2011;37(2):147-154.
10. Haneline M, Triano J. Cervical artery dissection. A comparison of highly dynamic mechanisms: manipulation versus motor vehicle collision. J Manipulative Physiol Ther. 2005;28(1):57-63.
11. Thomas LC, Rivett DA, Attia JR, Levi C. Risk factors and clinical presentation of cervical arterial dissection: preliminary results of a prospective case-control study. J Orthop Sports Phys Ther. 2015;45(7):503-511.
12. Lyrer P, Engelter S. Antithrombotic drugs for carotid artery dissection. Cochrane Database Syst Rev. 2010;(10):CD000255.
13. Creavin ST, Rice CM, Pollentine A, Cowburn P. Carotid artery dissection presenting with isolated headache and Horner syndrome after minor head injury. Am J Emerg Med. 2012;30(9):2103.e5-e7.
Take-Home Points
- ICA dissections may occur from direct or indirect trauma.
- Symptoms can be mild, including a persistent headache.
- High clinical suspicion is required for diagnosis when symptoms are mild.
- Neuroimaging is required for definitive diagnosis.
- Conservative management with serial imaging can yield successful outcomes.
Cervical artery dissection (CAD) is an uncommon but potentially life-threatening condition that accounts for a high proportion of ischemic strokes in patients under the age of 45 years.1-4 The extracranial internal carotid arteries (ICAs) and vertebral arteries are most commonly involved; dissections can occur after either direct trauma to the neck, or indirect trauma resulting in acute hyperextension or hyperflexion.4-7 ICA dissection can be difficult to diagnose because of the varying symptomatology. Clinical presentation depends on stenosis location, degree of luminal narrowing, and presence or absence of ischemic stroke. Neurologic symptoms may be delayed, and misdiagnosis of an isolated soft-tissue contusion, whiplash, can be made in the setting of indirect cervical trauma.
Although this entity is well described in the literature,2,3,5,8 there are few reported cases of injuries sustained during high-intensity athletic competition. In this case report, we describe the symptoms, physical examination findings, diagnostic imaging results, and treatment of a young male athlete who presented with delayed-onset symptoms of ICA dissection resulting from indirect cervical trauma sustained during an ice hockey game. We discuss the importance of a high level of clinical suspicion in the diagnosis of neck injuries sustained during athletic competition, as well as the need for early vascular imaging for diagnosis. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
The patient was a right-handed 32-year-old professional hockey goaltender. Four days before diagnosis, his goaltending mask and attached neck-protector were inadvertently lifted by another player’s stick just as a puck traveling at high speed struck him in the neck, to the right of the larynx, causing acute neck hyperextension. He immediately experienced discomfort and fell to the ice, saying he was “dizzy and light-headed.” Play was stopped, and medical personnel attended to him. His symptoms resolved, and he resumed play without any notable deficits. The next day, he noted discomfort at the impact site, but no additional symptoms, and received a presumptive diagnosis of cervical soft-tissue contusion. Continuing to participate in hockey that day, he did not develop any symptoms other than superficial cervical discomfort. However, the next morning, he presented complaining of severe right frontotemporal headache, which had persisted overnight. Orthopedic examination revealed palpable tenderness over the anterior cervical musculature, including the sternocleidomastoid and strap muscles. There was no appreciable hematoma in the contused area. Cervical range of motion was otherwise preserved. Cervical spine examination, including dermatomal and myotomal examination, was normal, as was cranial nerve examination. However, given the headache intensity and the recency of the injury, the potential for vascular or neurologic injury was considered. A neurology consultation was obtained, and arrangements were made for advanced cross-sectional imaging.
On further evaluation, the patient denied loss of consciousness, seizure, vomiting, amnesia, visual disturbance, language or cognitive impairment, balance or coordination difficulties, or any appreciable face or limb weakness. Review of systems was otherwise negative. Detailed neurologic examination did not reveal any cranial nerve deficits, and pupils were 3 mm, equal, and normally responsive to light and accommodation. Muscular tone and strength were symmetric and full in the upper and lower extremities. Gait, coordination, and response to vibration and temperature sensation were all preserved.
Magnetic resonance imaging of the head and neck was normal, but magnetic resonance angiography (MRA) of the neck showed a 1-cm-long region of the ICA, before piercing the petrous bone, with evidence of dissection.
Given the normal neurologic examination, and no evidence of brain infarction or other neurovascular complications, the acute ICA dissection was managed with antiplatelet therapy using aspirin (325 mg/d). In addition, the patient was advised to refrain from strenuous physical activity and to present to the hospital immediately if symptoms worsened or any neurologic impairment developed. Follow-up and repeat MRA were planned to monitor healing progression.
Two weeks after injury, the patient returned for follow-up. His headache and neck pain had resolved. Physical examination findings were unchanged, and there were no notable neurologic deficits. Repeat MRA findings were essentially unchanged, except for slightly increased luminal stenosis, exceeding 50% (Figure 2), attributable to intramural hematoma formation.
At 6-week follow-up, the patient had no clinical symptoms and no recurrence of headaches.
Discussion
In cases of direct (blunt) or indirect cervical trauma, CAD should be considered, as it carries a risk of potentially debilitating ischemic stroke in otherwise healthy young patients. Fortunately, CAD is rare; its annual incidence is 1 in 100,000, occurring in 0.08% to 1.2% of blunt trauma cases.9
As symptoms of ICA dissection can vary depending on stenosis severity, diagnosis can be challenging. The classically associated triad of symptoms includes unilateral head, facial, or neck pain accompanied by partial Horner syndrome with progression to cerebral or retinal ischemia. However, these symptoms occur in less than a third of patients with ICA dissection.2 Neck pain may occur secondary to blunt cervical trauma, consistent with a cervical soft-tissue contusion; however, it may have more severe implications and should be carefully monitored, particularly if accompanied by additional symptoms, such as headache. Headaches, which are present in 44% to 69% of patients, are often unilateral and constant. Either headache or neck pain in isolation is relatively uncommon, occurring in <10% of cases,2 though retrospective reviews of delayed-onset ICA dissection found atypical headache or neck pain in 100% of patients,11 indicating that persistent symptoms should be further evaluated.
More commonly, patients present with neurologic symptoms, particularly Horner syndrome, which is caused by the disruption of the sympathetic nerve fibers adjacent to the ICA, resulting in ipsilateral ptosis and miosis. In addition, patients may present with cranial nerve palsies, most commonly involving cranial nerve XII (the hypoglossal nerve), resulting in tongue weakness and abnormal taste. These and other neurologic findings associated with retinal or cerebral ischemia should raise clinical suspicion for the injury and prompt computed tomography or MRA evaluation.
MRA has largely replaced conventional angiography for the diagnosis of CAD. As MRA is noninvasive, it allows for improved visualization of luminal narrowing and for evaluation of the arterial wall and intramural hematoma.2 Because of the potential for devastating sequelae with missed or delayed diagnosis, several authors have become proponents of early aggressive screening for detection of these injuries.9 Postdiagnostic treatment depends on the presence of neurologic symptoms. Management is directed toward limiting neurologic deficits; anticoagulant or antiplatelet agents are used to prevent thromboembolic events. A randomized controlled trial and other studies have failed to find any appreciable difference in subsequent rates of stroke or associated complications with use of either class of medication.8,12 Conventionally, treatment is continued for 3 to 6 months, depending on clinical resolution. Endovascular or surgical intervention typically is reserved for extreme luminal narrowing, conditions that are preventing anticoagulation, an expanding area of dissection with a persistent pseudoaneurysm, and cases of failed medical management with subsequent ischemic stroke.2The literature includes several case reports involving indirect trauma in recreational athletes. First, a 31-year-old woman sustained an ICA dissection secondary to a head injury that occurred during a soccer match; she presented with headache, altered sense of taste, and objective findings of ptosis and miosis consistent with Horner syndrome.13 Second, a 39-year-old man had an ICA dissection after a snowboarding fall that caused neck hyperextension; he presented with periocular headache, ptosis, and miosis.6 Third, 3 people who participated in CrossFit training sustained ICA dissection.7 They presented with varying degrees of neurologic symptoms: ptosis and miosis; right-side upper extremity ataxia; and visual distortion and receptive aphasia. Our patient’s ICA dissection resulted from indirect trauma that caused sudden hyperextension and lateral flexion in response to contact from a hockey puck. However, his case is unique in that symptoms onset was delayed, and there were no associated neurologic findings on clinical presentation. His case should raise awareness of this potential diagnosis, even in the absence of overt neurologic findings. In addition, the patient’s return to sport at 8 weeks was facilitated by full clinical resolution of symptoms and thorough radiographic documentation of improved intramural narrowing. Finally, to our knowledge this is the first report of this injury in a professional athlete.
Conclusion
We have reported the case of a 32-year-old professional hockey goaltender who presented with isolated, persistent, worsening headache of delayed onset after ICA dissection. The ICA dissection resulted from indirect trauma, with reaction to a puck causing acute hyperextension and rotational injury. To our knowledge, this is the first report of a case of ICA dissection in an athlete, lacking neurologic examination findings that could aid in the diagnosis. The index of suspicion for CAD should be high after direct or indirect cervical trauma when patients present with unilateral neck pain or headache, even in the absence of neurologic findings, as stroke is a catastrophic but preventable complication.
Am J Orthop. 2017;46(3):E139-E143. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
Take-Home Points
- ICA dissections may occur from direct or indirect trauma.
- Symptoms can be mild, including a persistent headache.
- High clinical suspicion is required for diagnosis when symptoms are mild.
- Neuroimaging is required for definitive diagnosis.
- Conservative management with serial imaging can yield successful outcomes.
Cervical artery dissection (CAD) is an uncommon but potentially life-threatening condition that accounts for a high proportion of ischemic strokes in patients under the age of 45 years.1-4 The extracranial internal carotid arteries (ICAs) and vertebral arteries are most commonly involved; dissections can occur after either direct trauma to the neck, or indirect trauma resulting in acute hyperextension or hyperflexion.4-7 ICA dissection can be difficult to diagnose because of the varying symptomatology. Clinical presentation depends on stenosis location, degree of luminal narrowing, and presence or absence of ischemic stroke. Neurologic symptoms may be delayed, and misdiagnosis of an isolated soft-tissue contusion, whiplash, can be made in the setting of indirect cervical trauma.
Although this entity is well described in the literature,2,3,5,8 there are few reported cases of injuries sustained during high-intensity athletic competition. In this case report, we describe the symptoms, physical examination findings, diagnostic imaging results, and treatment of a young male athlete who presented with delayed-onset symptoms of ICA dissection resulting from indirect cervical trauma sustained during an ice hockey game. We discuss the importance of a high level of clinical suspicion in the diagnosis of neck injuries sustained during athletic competition, as well as the need for early vascular imaging for diagnosis. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
The patient was a right-handed 32-year-old professional hockey goaltender. Four days before diagnosis, his goaltending mask and attached neck-protector were inadvertently lifted by another player’s stick just as a puck traveling at high speed struck him in the neck, to the right of the larynx, causing acute neck hyperextension. He immediately experienced discomfort and fell to the ice, saying he was “dizzy and light-headed.” Play was stopped, and medical personnel attended to him. His symptoms resolved, and he resumed play without any notable deficits. The next day, he noted discomfort at the impact site, but no additional symptoms, and received a presumptive diagnosis of cervical soft-tissue contusion. Continuing to participate in hockey that day, he did not develop any symptoms other than superficial cervical discomfort. However, the next morning, he presented complaining of severe right frontotemporal headache, which had persisted overnight. Orthopedic examination revealed palpable tenderness over the anterior cervical musculature, including the sternocleidomastoid and strap muscles. There was no appreciable hematoma in the contused area. Cervical range of motion was otherwise preserved. Cervical spine examination, including dermatomal and myotomal examination, was normal, as was cranial nerve examination. However, given the headache intensity and the recency of the injury, the potential for vascular or neurologic injury was considered. A neurology consultation was obtained, and arrangements were made for advanced cross-sectional imaging.
On further evaluation, the patient denied loss of consciousness, seizure, vomiting, amnesia, visual disturbance, language or cognitive impairment, balance or coordination difficulties, or any appreciable face or limb weakness. Review of systems was otherwise negative. Detailed neurologic examination did not reveal any cranial nerve deficits, and pupils were 3 mm, equal, and normally responsive to light and accommodation. Muscular tone and strength were symmetric and full in the upper and lower extremities. Gait, coordination, and response to vibration and temperature sensation were all preserved.
Magnetic resonance imaging of the head and neck was normal, but magnetic resonance angiography (MRA) of the neck showed a 1-cm-long region of the ICA, before piercing the petrous bone, with evidence of dissection.
Given the normal neurologic examination, and no evidence of brain infarction or other neurovascular complications, the acute ICA dissection was managed with antiplatelet therapy using aspirin (325 mg/d). In addition, the patient was advised to refrain from strenuous physical activity and to present to the hospital immediately if symptoms worsened or any neurologic impairment developed. Follow-up and repeat MRA were planned to monitor healing progression.
Two weeks after injury, the patient returned for follow-up. His headache and neck pain had resolved. Physical examination findings were unchanged, and there were no notable neurologic deficits. Repeat MRA findings were essentially unchanged, except for slightly increased luminal stenosis, exceeding 50% (Figure 2), attributable to intramural hematoma formation.
At 6-week follow-up, the patient had no clinical symptoms and no recurrence of headaches.
Discussion
In cases of direct (blunt) or indirect cervical trauma, CAD should be considered, as it carries a risk of potentially debilitating ischemic stroke in otherwise healthy young patients. Fortunately, CAD is rare; its annual incidence is 1 in 100,000, occurring in 0.08% to 1.2% of blunt trauma cases.9
As symptoms of ICA dissection can vary depending on stenosis severity, diagnosis can be challenging. The classically associated triad of symptoms includes unilateral head, facial, or neck pain accompanied by partial Horner syndrome with progression to cerebral or retinal ischemia. However, these symptoms occur in less than a third of patients with ICA dissection.2 Neck pain may occur secondary to blunt cervical trauma, consistent with a cervical soft-tissue contusion; however, it may have more severe implications and should be carefully monitored, particularly if accompanied by additional symptoms, such as headache. Headaches, which are present in 44% to 69% of patients, are often unilateral and constant. Either headache or neck pain in isolation is relatively uncommon, occurring in <10% of cases,2 though retrospective reviews of delayed-onset ICA dissection found atypical headache or neck pain in 100% of patients,11 indicating that persistent symptoms should be further evaluated.
More commonly, patients present with neurologic symptoms, particularly Horner syndrome, which is caused by the disruption of the sympathetic nerve fibers adjacent to the ICA, resulting in ipsilateral ptosis and miosis. In addition, patients may present with cranial nerve palsies, most commonly involving cranial nerve XII (the hypoglossal nerve), resulting in tongue weakness and abnormal taste. These and other neurologic findings associated with retinal or cerebral ischemia should raise clinical suspicion for the injury and prompt computed tomography or MRA evaluation.
MRA has largely replaced conventional angiography for the diagnosis of CAD. As MRA is noninvasive, it allows for improved visualization of luminal narrowing and for evaluation of the arterial wall and intramural hematoma.2 Because of the potential for devastating sequelae with missed or delayed diagnosis, several authors have become proponents of early aggressive screening for detection of these injuries.9 Postdiagnostic treatment depends on the presence of neurologic symptoms. Management is directed toward limiting neurologic deficits; anticoagulant or antiplatelet agents are used to prevent thromboembolic events. A randomized controlled trial and other studies have failed to find any appreciable difference in subsequent rates of stroke or associated complications with use of either class of medication.8,12 Conventionally, treatment is continued for 3 to 6 months, depending on clinical resolution. Endovascular or surgical intervention typically is reserved for extreme luminal narrowing, conditions that are preventing anticoagulation, an expanding area of dissection with a persistent pseudoaneurysm, and cases of failed medical management with subsequent ischemic stroke.2The literature includes several case reports involving indirect trauma in recreational athletes. First, a 31-year-old woman sustained an ICA dissection secondary to a head injury that occurred during a soccer match; she presented with headache, altered sense of taste, and objective findings of ptosis and miosis consistent with Horner syndrome.13 Second, a 39-year-old man had an ICA dissection after a snowboarding fall that caused neck hyperextension; he presented with periocular headache, ptosis, and miosis.6 Third, 3 people who participated in CrossFit training sustained ICA dissection.7 They presented with varying degrees of neurologic symptoms: ptosis and miosis; right-side upper extremity ataxia; and visual distortion and receptive aphasia. Our patient’s ICA dissection resulted from indirect trauma that caused sudden hyperextension and lateral flexion in response to contact from a hockey puck. However, his case is unique in that symptoms onset was delayed, and there were no associated neurologic findings on clinical presentation. His case should raise awareness of this potential diagnosis, even in the absence of overt neurologic findings. In addition, the patient’s return to sport at 8 weeks was facilitated by full clinical resolution of symptoms and thorough radiographic documentation of improved intramural narrowing. Finally, to our knowledge this is the first report of this injury in a professional athlete.
Conclusion
We have reported the case of a 32-year-old professional hockey goaltender who presented with isolated, persistent, worsening headache of delayed onset after ICA dissection. The ICA dissection resulted from indirect trauma, with reaction to a puck causing acute hyperextension and rotational injury. To our knowledge, this is the first report of a case of ICA dissection in an athlete, lacking neurologic examination findings that could aid in the diagnosis. The index of suspicion for CAD should be high after direct or indirect cervical trauma when patients present with unilateral neck pain or headache, even in the absence of neurologic findings, as stroke is a catastrophic but preventable complication.
Am J Orthop. 2017;46(3):E139-E143. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
1. Mohan IV. Current optimal assessment and management of carotid and vertebral spontaneous and traumatic dissection. Angiology. 2014;65(4):274-283.
2. Patel RR, Adam R, Maldjian C, Lincoln CM, Yuen A, Arneja A. Cervical carotid artery dissection: current review of diagnosis and treatment. Cardiol Rev. 2012;20(3):145-152.
3. Biller J, Sacco RL, Albuquerque FC, et al; American Heart Association Stroke Council. Cervical arterial dissections and association with cervical manipulative therapy: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2014;45(10):3155-3174.
4. Fukunaga N, Hanaoka M, Sato K. Asymptomatic common carotid artery dissection caused by blunt injury. Emerg Med J. 2011;28(1):50.
5. Chen J, Zhou X, Li C, Cheung BM. Risk of stroke due to spontaneous cervical artery dissection. Intern Med. 2013;52(19):2237-2240.
6. Kalantzis G, Georgalas I, Chang BY, Ong C, El-Hindy N. An unusual case of traumatic internal carotid artery dissection during snowboarding. J Sports Sci Med. 2014;13(2):451-453.
7. Lu A, Shen P, Lee P, et al. CrossFit-related cervical internal carotid artery dissection. Emerg Radiol. 2015;22(4):449-452.
8. CADISS Trial Investigators, Markus HS, Hayter E, Levi C, Feldman A, Venables G, Norris J. Antiplatelet treatment compared with anticoagulation treatment for cervical artery dissection (CADISS): a randomised trial. Lancet Neurol. 2015;14(4):361-367.
9. van Wessem KJ, Meijer JM, Leenen LP, van der Worp HB, Moll FL, de Borst GJ. Blunt traumatic carotid artery dissection still a pitfall? The rationale for aggressive screening. Eur J Trauma Emerg Surg. 2011;37(2):147-154.
10. Haneline M, Triano J. Cervical artery dissection. A comparison of highly dynamic mechanisms: manipulation versus motor vehicle collision. J Manipulative Physiol Ther. 2005;28(1):57-63.
11. Thomas LC, Rivett DA, Attia JR, Levi C. Risk factors and clinical presentation of cervical arterial dissection: preliminary results of a prospective case-control study. J Orthop Sports Phys Ther. 2015;45(7):503-511.
12. Lyrer P, Engelter S. Antithrombotic drugs for carotid artery dissection. Cochrane Database Syst Rev. 2010;(10):CD000255.
13. Creavin ST, Rice CM, Pollentine A, Cowburn P. Carotid artery dissection presenting with isolated headache and Horner syndrome after minor head injury. Am J Emerg Med. 2012;30(9):2103.e5-e7.
1. Mohan IV. Current optimal assessment and management of carotid and vertebral spontaneous and traumatic dissection. Angiology. 2014;65(4):274-283.
2. Patel RR, Adam R, Maldjian C, Lincoln CM, Yuen A, Arneja A. Cervical carotid artery dissection: current review of diagnosis and treatment. Cardiol Rev. 2012;20(3):145-152.
3. Biller J, Sacco RL, Albuquerque FC, et al; American Heart Association Stroke Council. Cervical arterial dissections and association with cervical manipulative therapy: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2014;45(10):3155-3174.
4. Fukunaga N, Hanaoka M, Sato K. Asymptomatic common carotid artery dissection caused by blunt injury. Emerg Med J. 2011;28(1):50.
5. Chen J, Zhou X, Li C, Cheung BM. Risk of stroke due to spontaneous cervical artery dissection. Intern Med. 2013;52(19):2237-2240.
6. Kalantzis G, Georgalas I, Chang BY, Ong C, El-Hindy N. An unusual case of traumatic internal carotid artery dissection during snowboarding. J Sports Sci Med. 2014;13(2):451-453.
7. Lu A, Shen P, Lee P, et al. CrossFit-related cervical internal carotid artery dissection. Emerg Radiol. 2015;22(4):449-452.
8. CADISS Trial Investigators, Markus HS, Hayter E, Levi C, Feldman A, Venables G, Norris J. Antiplatelet treatment compared with anticoagulation treatment for cervical artery dissection (CADISS): a randomised trial. Lancet Neurol. 2015;14(4):361-367.
9. van Wessem KJ, Meijer JM, Leenen LP, van der Worp HB, Moll FL, de Borst GJ. Blunt traumatic carotid artery dissection still a pitfall? The rationale for aggressive screening. Eur J Trauma Emerg Surg. 2011;37(2):147-154.
10. Haneline M, Triano J. Cervical artery dissection. A comparison of highly dynamic mechanisms: manipulation versus motor vehicle collision. J Manipulative Physiol Ther. 2005;28(1):57-63.
11. Thomas LC, Rivett DA, Attia JR, Levi C. Risk factors and clinical presentation of cervical arterial dissection: preliminary results of a prospective case-control study. J Orthop Sports Phys Ther. 2015;45(7):503-511.
12. Lyrer P, Engelter S. Antithrombotic drugs for carotid artery dissection. Cochrane Database Syst Rev. 2010;(10):CD000255.
13. Creavin ST, Rice CM, Pollentine A, Cowburn P. Carotid artery dissection presenting with isolated headache and Horner syndrome after minor head injury. Am J Emerg Med. 2012;30(9):2103.e5-e7.
Encapsulated Fat Necrosis Lesion Caused by Morel-Lavallée Lesion in a Professional Ice Hockey Player
Take-Home Points
- ML lesions usually occur with high-energy injuries and have been reported in wrestlers, football players, and other athlete populations.
- Encapsulated fat necrosis lesions are usually attributable to trauma and disruption of the blood supply in the subcutaneous area, which occurs with ML lesions.
- Encapsulated fat necrosis lesions are rare; only 65 have been reported.
- Encapsulated fat necrosis lesions are characterized by massive fat necrosis encapsulated by fibrous tissue.
- Most are small and asymptomatic; however, in some cases, athletes can develop symptoms from frequent impacts to the region where the lesions are located.
What would become known as the Morel-Lavallée (ML) lesion was first reported in 1853 by French physician Maurice Morel-Lavallée. He described a proximal thigh soft-tissue injury that resulted in a hemolymphatic collection between superficial fascial planes. Deforming forces of pressure and shear result in an internal degloving injury in which subcutaneous tissue is stripped from the fascia and replaced with a hematoma or, less commonly, necrotic fat.1-4 The injury can take several weeks to heal. Up to one-third of such injuries are initially missed because of the initial ecchymosis covering the injured area.5
ML lesions usually occur with high-energy injuries and have been reported in wrestlers,6 football players,7-9 and other athlete populations. ML lesions usually occur about the knee, the site of the sheer mechanism in these athletes’ sports. Tejwani and colleagues9 reported on 24 National Football League (NFL) players (27 knees). These elite athletes typically were able to return to practice and game play long before complete resolution of their lesions.
Nodular cystic fat necrosis was first described by Przyjemski and Schuster10 in 1977. The terms encapsulated fat necrosis lesions and mobile encapsulated lipomas11 were introduced later. Clinically, these entities usually present as lesions on the lower limbs of young men and middle-aged women and can range in size from 1 mm to 35 mm. Most of these lesions are mobile.11 They are usually attributable to trauma and disruption of the blood supply in the subcutaneous area, which occurs with ML lesions. Trauma accounts for the usual occurrence in the lower extremities, though only 40% of patients recall a precipitating event.12 Histologically, these lesions are characterized by massive fat necrosis encapsulated by fibrous tissue.13In this article, we report the case of a professional ice hockey player who presented with an ML lesion of the hip and then developed a symptomatic encapsulated fat necrosis lesion that required surgical removal. To our knowledge, this is the first reported case of an encapsulated fat necrosis lesion caused by an ML lesion in an athlete. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 21-year-old professional hockey player presented with a history of pain from a mass on his right hip. He first noticed the lesion, just lateral to the greater trochanter, about 3 years earlier. The mass appeared after he sustained a shearing-type injury to the lateral aspect of the hip. At the time, there was significant swelling along the lateral aspect, with ecchymosis that resolved over 2 months. The mass, diagnosed as an ML lesion, resolved with nonoperative treatment. However, in the area where the swelling had occurred, a hard mobile mass remained. At times, this mass became painful when direct pressure was applied, as when he hit the boards while playing hockey, or when he lay on his right side or used a roller in the training room. He rated the pain as a 4 on a 1-to-10 scale and said the mass was mobile and had not changed in size or consistency.
Physical examination revealed a palpable mass over the lateral aspect of the hip, over the greater trochanter. The mass, about 3 cm in diameter (Figure 1), was mobile in a subcutaneous pocket, consistent with an old ML lesion.
Options discussed with the patient included use of ice, activity modification, and use of protective padded equipment. As the patient had tried these treatments before and was still intermittently having pain with direct pressure, he asked for surgical removal of the mass.
For the surgery, the patient was positioned in the lateral decubitus position with his right hip facing up. The right hip and thigh were prepared and draped in sterile fashion. An incision 4 cm in length was made directly over the mass, along the lateral aspect of the hip, over the greater trochanter. The incision was taken through skin and subcutaneous tissue down to the deep fascia. The fascia was incised longitudinally in line with the overlying skin incision. As soon as the incision was made through the fascia, the mass was easily seen. The 3-cm × 2-cm × 1-cm mass was free, not attached to any underlying soft tissue (Figure 3).
Discussion
We have described a case of symptomatic encapsulated fat necrosis lesion caused by an ML lesion in a professional hockey player. The ML lesion had resolved with nonoperative treatment (compression), but a subcutaneous pocket remained at the lesion site. Given the patient’s lesion site and occupation as a hockey player, pain with direct pressure on this lesion was a concern.
Long-standing ML lesions have 3 common patterns on MRI.14 A central region, encapsulated partially or completely by a peripheral ring of fibrous tissue or hemosiderin, shows signal properties consistent with a seroma, a homogeneous hemorrhagic collection, or a heterogeneous hemorrhagic collection. In our patient’s case, MRI was used to characterize the mobile mass for operative planning. Although thin strands or lobules of fat have been found within ML lesions, this case was the first to demonstrate a sequestered mass of necrotic fat.
Most football players who develop ML lesions on their knees do not wear kneepads.7-9 Of the 24 NFL players in the study by Tejwani and colleagues,9 52% were successfully treated with compression wrap, cryotherapy, and motion exercises. The rest, however, were treated with aspiration, and 11% underwent doxycycline sclerodesis for recurrent fluid collection. After treatment, all of their players were able to return to football. Their outcomes are consistent with that of our patient, who was treated with compression wrap and returned to hockey without any other intervention.
After our patient’s ML lesion resolved, he developed an encapsulated fat necrosis lesion from the disruption of the blood supply in the subcutaneous pocket. Encapsulated fat necrosis lesions are rare; only 65 have been reported.13,15 Clinically, these lesions are single or multiple pale-yellow encapsulated nodes.13 Most are small and asymptomatic; however, in some cases, athletes can develop symptoms from frequent impacts to the region where the lesions are located.
The literature includes 1 report of an adolescent football player who developed multiple encapsulated fat necrosis lesions 4 months after landing on another player’s cleats.15 The patient, who was having pain with direct pressure during squatting and kneeling, elected to have the lesions surgically removed. These lesions are rare and usually asymptomatic,11 but our patient had his lesion surgically removed to address the pain induced by the direct impacts that came with playing professional hockey. Surgical removal is the treatment for symptomatic encapsulated fat necrosis lesions. Other than 1 case of recurrence after excision,16 these lesions have an excellent prognosis.
Conclusion
Our patient, a professional hockey player, underwent successful surgical removal of a symptomatic encapsulated fat necrosis lesion that had developed from an ML lesion.
Am J Orthop. 2017;46(3):E144-E147. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
1. Aguiar RO, Viegas FC, Fernandez RY, Trudell D, Haghighi P, Resnick D. The prepatellar bursa: cadaveric investigation of regional anatomy with MRI after sonographically guided bursography. AJR Am J Roentgenol. 2007;188(4):W355-W358.
2. Hak DJ, Olson SA, Matta JM. Diagnosis and management of closed internal degloving injuries associated with pelvic and acetabular fractures: the Morel-Lavallée lesion. J Trauma. 1997;42(6):1046-1051.
3. Hudson DA, Knottenbelt JD, Krige JE. Closed degloving injuries: results following conservative surgery. Plast Reconstr Surg. 1992;89(5):853-855.
4. Mellado JM, Bencardino JT. Morel-Lavallée lesion: review with emphasis on MR imaging. Magn Reson Imaging Clin North Am. 2005;13(4):775-782.
5. Dye SF, Campagna-Pinto D, Dye CC, Shifflett S, Eiman T. Soft-tissue anatomy anterior to the human patella. J Bone Joint Surg Am. 2003;85(6):1012-1017.
6. Northam MC, Gaskin CM. Presumed prepatellar fibrosis in collegiate wrestlers: imaging findings and clinical correlation. Skeletal Radiol. 2015;44(2):271-277.
7. Anakwenze OA, Trivedi V, Goodman AM, Ganley TJ. Concealed degloving injury (the Morel-Lavallée lesion) in childhood sports: a case report. J Bone Joint Surg Am. 2011;93(24):e148.
8. Matava MJ, Ellis E, Shah NR, Pogue D, Williams T. Morel-Lavallée lesion in a professional American football player. Am J Orthop. 2010;39(3):144-147.
9. Tejwani SG, Cohen SB, Bradley JP. Management of Morel-Lavallee lesion of the knee: twenty-seven cases in the National Football League. Am J Sports Med. 2007;35(7):1162-1167.
10. Przyjemski CJ, Schuster SR. Nodular-cystic fat necrosis. J Pediatr. 1977;91(4):605-607.
11. Kiryu H, Rikihisa W, Furue M. Encapsulated fat necrosis—a clinicopathological study of 8 cases and a literature review. J Cutan Pathol. 2000;27(1):19-23.
12. Santos-Juanes J, Coto P, Galache C, Sánchez del Rio J, Soto de Delás J. Encapsulated fat necrosis: a form of traumatic panniculitis. J Eur Acad Dermatol Venereol. 2007;21(3):405-406.
13. Sempau L, Sambucetty PS, Garcia JL, Sixto BG, Morán AG, Prieto MA. Mobile encapsulated lipoma. Int J Dermatol. 2012;51(4):448-450.
14. Mellado JM, Pérez del Palomar L, Díaz L, Ramos A, Saurí A. Long-standing Morel-Lavallée lesions of the trochanteric region and proximal thigh: MRI features in five patients. AJR Am J Roentgenol. 2004;182(5):1289-1294.
15. Sole JS, Wisniewski SJ, Dahm DL, Bond J, Smith J. Posttraumatic fat necrosis presenting as prepatellar loose bodies in an adolescent football player. PM R. 2014;6(8):749-752.
16. Felipo F, Vaquero M, del Agua C. Pseudotumoral encapsulated fat necrosis with diffuse pseudomembranous degeneration. J Cutan Pathol. 2004;31(8):565-567.
Take-Home Points
- ML lesions usually occur with high-energy injuries and have been reported in wrestlers, football players, and other athlete populations.
- Encapsulated fat necrosis lesions are usually attributable to trauma and disruption of the blood supply in the subcutaneous area, which occurs with ML lesions.
- Encapsulated fat necrosis lesions are rare; only 65 have been reported.
- Encapsulated fat necrosis lesions are characterized by massive fat necrosis encapsulated by fibrous tissue.
- Most are small and asymptomatic; however, in some cases, athletes can develop symptoms from frequent impacts to the region where the lesions are located.
What would become known as the Morel-Lavallée (ML) lesion was first reported in 1853 by French physician Maurice Morel-Lavallée. He described a proximal thigh soft-tissue injury that resulted in a hemolymphatic collection between superficial fascial planes. Deforming forces of pressure and shear result in an internal degloving injury in which subcutaneous tissue is stripped from the fascia and replaced with a hematoma or, less commonly, necrotic fat.1-4 The injury can take several weeks to heal. Up to one-third of such injuries are initially missed because of the initial ecchymosis covering the injured area.5
ML lesions usually occur with high-energy injuries and have been reported in wrestlers,6 football players,7-9 and other athlete populations. ML lesions usually occur about the knee, the site of the sheer mechanism in these athletes’ sports. Tejwani and colleagues9 reported on 24 National Football League (NFL) players (27 knees). These elite athletes typically were able to return to practice and game play long before complete resolution of their lesions.
Nodular cystic fat necrosis was first described by Przyjemski and Schuster10 in 1977. The terms encapsulated fat necrosis lesions and mobile encapsulated lipomas11 were introduced later. Clinically, these entities usually present as lesions on the lower limbs of young men and middle-aged women and can range in size from 1 mm to 35 mm. Most of these lesions are mobile.11 They are usually attributable to trauma and disruption of the blood supply in the subcutaneous area, which occurs with ML lesions. Trauma accounts for the usual occurrence in the lower extremities, though only 40% of patients recall a precipitating event.12 Histologically, these lesions are characterized by massive fat necrosis encapsulated by fibrous tissue.13In this article, we report the case of a professional ice hockey player who presented with an ML lesion of the hip and then developed a symptomatic encapsulated fat necrosis lesion that required surgical removal. To our knowledge, this is the first reported case of an encapsulated fat necrosis lesion caused by an ML lesion in an athlete. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 21-year-old professional hockey player presented with a history of pain from a mass on his right hip. He first noticed the lesion, just lateral to the greater trochanter, about 3 years earlier. The mass appeared after he sustained a shearing-type injury to the lateral aspect of the hip. At the time, there was significant swelling along the lateral aspect, with ecchymosis that resolved over 2 months. The mass, diagnosed as an ML lesion, resolved with nonoperative treatment. However, in the area where the swelling had occurred, a hard mobile mass remained. At times, this mass became painful when direct pressure was applied, as when he hit the boards while playing hockey, or when he lay on his right side or used a roller in the training room. He rated the pain as a 4 on a 1-to-10 scale and said the mass was mobile and had not changed in size or consistency.
Physical examination revealed a palpable mass over the lateral aspect of the hip, over the greater trochanter. The mass, about 3 cm in diameter (Figure 1), was mobile in a subcutaneous pocket, consistent with an old ML lesion.
Options discussed with the patient included use of ice, activity modification, and use of protective padded equipment. As the patient had tried these treatments before and was still intermittently having pain with direct pressure, he asked for surgical removal of the mass.
For the surgery, the patient was positioned in the lateral decubitus position with his right hip facing up. The right hip and thigh were prepared and draped in sterile fashion. An incision 4 cm in length was made directly over the mass, along the lateral aspect of the hip, over the greater trochanter. The incision was taken through skin and subcutaneous tissue down to the deep fascia. The fascia was incised longitudinally in line with the overlying skin incision. As soon as the incision was made through the fascia, the mass was easily seen. The 3-cm × 2-cm × 1-cm mass was free, not attached to any underlying soft tissue (Figure 3).
Discussion
We have described a case of symptomatic encapsulated fat necrosis lesion caused by an ML lesion in a professional hockey player. The ML lesion had resolved with nonoperative treatment (compression), but a subcutaneous pocket remained at the lesion site. Given the patient’s lesion site and occupation as a hockey player, pain with direct pressure on this lesion was a concern.
Long-standing ML lesions have 3 common patterns on MRI.14 A central region, encapsulated partially or completely by a peripheral ring of fibrous tissue or hemosiderin, shows signal properties consistent with a seroma, a homogeneous hemorrhagic collection, or a heterogeneous hemorrhagic collection. In our patient’s case, MRI was used to characterize the mobile mass for operative planning. Although thin strands or lobules of fat have been found within ML lesions, this case was the first to demonstrate a sequestered mass of necrotic fat.
Most football players who develop ML lesions on their knees do not wear kneepads.7-9 Of the 24 NFL players in the study by Tejwani and colleagues,9 52% were successfully treated with compression wrap, cryotherapy, and motion exercises. The rest, however, were treated with aspiration, and 11% underwent doxycycline sclerodesis for recurrent fluid collection. After treatment, all of their players were able to return to football. Their outcomes are consistent with that of our patient, who was treated with compression wrap and returned to hockey without any other intervention.
After our patient’s ML lesion resolved, he developed an encapsulated fat necrosis lesion from the disruption of the blood supply in the subcutaneous pocket. Encapsulated fat necrosis lesions are rare; only 65 have been reported.13,15 Clinically, these lesions are single or multiple pale-yellow encapsulated nodes.13 Most are small and asymptomatic; however, in some cases, athletes can develop symptoms from frequent impacts to the region where the lesions are located.
The literature includes 1 report of an adolescent football player who developed multiple encapsulated fat necrosis lesions 4 months after landing on another player’s cleats.15 The patient, who was having pain with direct pressure during squatting and kneeling, elected to have the lesions surgically removed. These lesions are rare and usually asymptomatic,11 but our patient had his lesion surgically removed to address the pain induced by the direct impacts that came with playing professional hockey. Surgical removal is the treatment for symptomatic encapsulated fat necrosis lesions. Other than 1 case of recurrence after excision,16 these lesions have an excellent prognosis.
Conclusion
Our patient, a professional hockey player, underwent successful surgical removal of a symptomatic encapsulated fat necrosis lesion that had developed from an ML lesion.
Am J Orthop. 2017;46(3):E144-E147. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
Take-Home Points
- ML lesions usually occur with high-energy injuries and have been reported in wrestlers, football players, and other athlete populations.
- Encapsulated fat necrosis lesions are usually attributable to trauma and disruption of the blood supply in the subcutaneous area, which occurs with ML lesions.
- Encapsulated fat necrosis lesions are rare; only 65 have been reported.
- Encapsulated fat necrosis lesions are characterized by massive fat necrosis encapsulated by fibrous tissue.
- Most are small and asymptomatic; however, in some cases, athletes can develop symptoms from frequent impacts to the region where the lesions are located.
What would become known as the Morel-Lavallée (ML) lesion was first reported in 1853 by French physician Maurice Morel-Lavallée. He described a proximal thigh soft-tissue injury that resulted in a hemolymphatic collection between superficial fascial planes. Deforming forces of pressure and shear result in an internal degloving injury in which subcutaneous tissue is stripped from the fascia and replaced with a hematoma or, less commonly, necrotic fat.1-4 The injury can take several weeks to heal. Up to one-third of such injuries are initially missed because of the initial ecchymosis covering the injured area.5
ML lesions usually occur with high-energy injuries and have been reported in wrestlers,6 football players,7-9 and other athlete populations. ML lesions usually occur about the knee, the site of the sheer mechanism in these athletes’ sports. Tejwani and colleagues9 reported on 24 National Football League (NFL) players (27 knees). These elite athletes typically were able to return to practice and game play long before complete resolution of their lesions.
Nodular cystic fat necrosis was first described by Przyjemski and Schuster10 in 1977. The terms encapsulated fat necrosis lesions and mobile encapsulated lipomas11 were introduced later. Clinically, these entities usually present as lesions on the lower limbs of young men and middle-aged women and can range in size from 1 mm to 35 mm. Most of these lesions are mobile.11 They are usually attributable to trauma and disruption of the blood supply in the subcutaneous area, which occurs with ML lesions. Trauma accounts for the usual occurrence in the lower extremities, though only 40% of patients recall a precipitating event.12 Histologically, these lesions are characterized by massive fat necrosis encapsulated by fibrous tissue.13In this article, we report the case of a professional ice hockey player who presented with an ML lesion of the hip and then developed a symptomatic encapsulated fat necrosis lesion that required surgical removal. To our knowledge, this is the first reported case of an encapsulated fat necrosis lesion caused by an ML lesion in an athlete. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 21-year-old professional hockey player presented with a history of pain from a mass on his right hip. He first noticed the lesion, just lateral to the greater trochanter, about 3 years earlier. The mass appeared after he sustained a shearing-type injury to the lateral aspect of the hip. At the time, there was significant swelling along the lateral aspect, with ecchymosis that resolved over 2 months. The mass, diagnosed as an ML lesion, resolved with nonoperative treatment. However, in the area where the swelling had occurred, a hard mobile mass remained. At times, this mass became painful when direct pressure was applied, as when he hit the boards while playing hockey, or when he lay on his right side or used a roller in the training room. He rated the pain as a 4 on a 1-to-10 scale and said the mass was mobile and had not changed in size or consistency.
Physical examination revealed a palpable mass over the lateral aspect of the hip, over the greater trochanter. The mass, about 3 cm in diameter (Figure 1), was mobile in a subcutaneous pocket, consistent with an old ML lesion.
Options discussed with the patient included use of ice, activity modification, and use of protective padded equipment. As the patient had tried these treatments before and was still intermittently having pain with direct pressure, he asked for surgical removal of the mass.
For the surgery, the patient was positioned in the lateral decubitus position with his right hip facing up. The right hip and thigh were prepared and draped in sterile fashion. An incision 4 cm in length was made directly over the mass, along the lateral aspect of the hip, over the greater trochanter. The incision was taken through skin and subcutaneous tissue down to the deep fascia. The fascia was incised longitudinally in line with the overlying skin incision. As soon as the incision was made through the fascia, the mass was easily seen. The 3-cm × 2-cm × 1-cm mass was free, not attached to any underlying soft tissue (Figure 3).
Discussion
We have described a case of symptomatic encapsulated fat necrosis lesion caused by an ML lesion in a professional hockey player. The ML lesion had resolved with nonoperative treatment (compression), but a subcutaneous pocket remained at the lesion site. Given the patient’s lesion site and occupation as a hockey player, pain with direct pressure on this lesion was a concern.
Long-standing ML lesions have 3 common patterns on MRI.14 A central region, encapsulated partially or completely by a peripheral ring of fibrous tissue or hemosiderin, shows signal properties consistent with a seroma, a homogeneous hemorrhagic collection, or a heterogeneous hemorrhagic collection. In our patient’s case, MRI was used to characterize the mobile mass for operative planning. Although thin strands or lobules of fat have been found within ML lesions, this case was the first to demonstrate a sequestered mass of necrotic fat.
Most football players who develop ML lesions on their knees do not wear kneepads.7-9 Of the 24 NFL players in the study by Tejwani and colleagues,9 52% were successfully treated with compression wrap, cryotherapy, and motion exercises. The rest, however, were treated with aspiration, and 11% underwent doxycycline sclerodesis for recurrent fluid collection. After treatment, all of their players were able to return to football. Their outcomes are consistent with that of our patient, who was treated with compression wrap and returned to hockey without any other intervention.
After our patient’s ML lesion resolved, he developed an encapsulated fat necrosis lesion from the disruption of the blood supply in the subcutaneous pocket. Encapsulated fat necrosis lesions are rare; only 65 have been reported.13,15 Clinically, these lesions are single or multiple pale-yellow encapsulated nodes.13 Most are small and asymptomatic; however, in some cases, athletes can develop symptoms from frequent impacts to the region where the lesions are located.
The literature includes 1 report of an adolescent football player who developed multiple encapsulated fat necrosis lesions 4 months after landing on another player’s cleats.15 The patient, who was having pain with direct pressure during squatting and kneeling, elected to have the lesions surgically removed. These lesions are rare and usually asymptomatic,11 but our patient had his lesion surgically removed to address the pain induced by the direct impacts that came with playing professional hockey. Surgical removal is the treatment for symptomatic encapsulated fat necrosis lesions. Other than 1 case of recurrence after excision,16 these lesions have an excellent prognosis.
Conclusion
Our patient, a professional hockey player, underwent successful surgical removal of a symptomatic encapsulated fat necrosis lesion that had developed from an ML lesion.
Am J Orthop. 2017;46(3):E144-E147. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
1. Aguiar RO, Viegas FC, Fernandez RY, Trudell D, Haghighi P, Resnick D. The prepatellar bursa: cadaveric investigation of regional anatomy with MRI after sonographically guided bursography. AJR Am J Roentgenol. 2007;188(4):W355-W358.
2. Hak DJ, Olson SA, Matta JM. Diagnosis and management of closed internal degloving injuries associated with pelvic and acetabular fractures: the Morel-Lavallée lesion. J Trauma. 1997;42(6):1046-1051.
3. Hudson DA, Knottenbelt JD, Krige JE. Closed degloving injuries: results following conservative surgery. Plast Reconstr Surg. 1992;89(5):853-855.
4. Mellado JM, Bencardino JT. Morel-Lavallée lesion: review with emphasis on MR imaging. Magn Reson Imaging Clin North Am. 2005;13(4):775-782.
5. Dye SF, Campagna-Pinto D, Dye CC, Shifflett S, Eiman T. Soft-tissue anatomy anterior to the human patella. J Bone Joint Surg Am. 2003;85(6):1012-1017.
6. Northam MC, Gaskin CM. Presumed prepatellar fibrosis in collegiate wrestlers: imaging findings and clinical correlation. Skeletal Radiol. 2015;44(2):271-277.
7. Anakwenze OA, Trivedi V, Goodman AM, Ganley TJ. Concealed degloving injury (the Morel-Lavallée lesion) in childhood sports: a case report. J Bone Joint Surg Am. 2011;93(24):e148.
8. Matava MJ, Ellis E, Shah NR, Pogue D, Williams T. Morel-Lavallée lesion in a professional American football player. Am J Orthop. 2010;39(3):144-147.
9. Tejwani SG, Cohen SB, Bradley JP. Management of Morel-Lavallee lesion of the knee: twenty-seven cases in the National Football League. Am J Sports Med. 2007;35(7):1162-1167.
10. Przyjemski CJ, Schuster SR. Nodular-cystic fat necrosis. J Pediatr. 1977;91(4):605-607.
11. Kiryu H, Rikihisa W, Furue M. Encapsulated fat necrosis—a clinicopathological study of 8 cases and a literature review. J Cutan Pathol. 2000;27(1):19-23.
12. Santos-Juanes J, Coto P, Galache C, Sánchez del Rio J, Soto de Delás J. Encapsulated fat necrosis: a form of traumatic panniculitis. J Eur Acad Dermatol Venereol. 2007;21(3):405-406.
13. Sempau L, Sambucetty PS, Garcia JL, Sixto BG, Morán AG, Prieto MA. Mobile encapsulated lipoma. Int J Dermatol. 2012;51(4):448-450.
14. Mellado JM, Pérez del Palomar L, Díaz L, Ramos A, Saurí A. Long-standing Morel-Lavallée lesions of the trochanteric region and proximal thigh: MRI features in five patients. AJR Am J Roentgenol. 2004;182(5):1289-1294.
15. Sole JS, Wisniewski SJ, Dahm DL, Bond J, Smith J. Posttraumatic fat necrosis presenting as prepatellar loose bodies in an adolescent football player. PM R. 2014;6(8):749-752.
16. Felipo F, Vaquero M, del Agua C. Pseudotumoral encapsulated fat necrosis with diffuse pseudomembranous degeneration. J Cutan Pathol. 2004;31(8):565-567.
1. Aguiar RO, Viegas FC, Fernandez RY, Trudell D, Haghighi P, Resnick D. The prepatellar bursa: cadaveric investigation of regional anatomy with MRI after sonographically guided bursography. AJR Am J Roentgenol. 2007;188(4):W355-W358.
2. Hak DJ, Olson SA, Matta JM. Diagnosis and management of closed internal degloving injuries associated with pelvic and acetabular fractures: the Morel-Lavallée lesion. J Trauma. 1997;42(6):1046-1051.
3. Hudson DA, Knottenbelt JD, Krige JE. Closed degloving injuries: results following conservative surgery. Plast Reconstr Surg. 1992;89(5):853-855.
4. Mellado JM, Bencardino JT. Morel-Lavallée lesion: review with emphasis on MR imaging. Magn Reson Imaging Clin North Am. 2005;13(4):775-782.
5. Dye SF, Campagna-Pinto D, Dye CC, Shifflett S, Eiman T. Soft-tissue anatomy anterior to the human patella. J Bone Joint Surg Am. 2003;85(6):1012-1017.
6. Northam MC, Gaskin CM. Presumed prepatellar fibrosis in collegiate wrestlers: imaging findings and clinical correlation. Skeletal Radiol. 2015;44(2):271-277.
7. Anakwenze OA, Trivedi V, Goodman AM, Ganley TJ. Concealed degloving injury (the Morel-Lavallée lesion) in childhood sports: a case report. J Bone Joint Surg Am. 2011;93(24):e148.
8. Matava MJ, Ellis E, Shah NR, Pogue D, Williams T. Morel-Lavallée lesion in a professional American football player. Am J Orthop. 2010;39(3):144-147.
9. Tejwani SG, Cohen SB, Bradley JP. Management of Morel-Lavallee lesion of the knee: twenty-seven cases in the National Football League. Am J Sports Med. 2007;35(7):1162-1167.
10. Przyjemski CJ, Schuster SR. Nodular-cystic fat necrosis. J Pediatr. 1977;91(4):605-607.
11. Kiryu H, Rikihisa W, Furue M. Encapsulated fat necrosis—a clinicopathological study of 8 cases and a literature review. J Cutan Pathol. 2000;27(1):19-23.
12. Santos-Juanes J, Coto P, Galache C, Sánchez del Rio J, Soto de Delás J. Encapsulated fat necrosis: a form of traumatic panniculitis. J Eur Acad Dermatol Venereol. 2007;21(3):405-406.
13. Sempau L, Sambucetty PS, Garcia JL, Sixto BG, Morán AG, Prieto MA. Mobile encapsulated lipoma. Int J Dermatol. 2012;51(4):448-450.
14. Mellado JM, Pérez del Palomar L, Díaz L, Ramos A, Saurí A. Long-standing Morel-Lavallée lesions of the trochanteric region and proximal thigh: MRI features in five patients. AJR Am J Roentgenol. 2004;182(5):1289-1294.
15. Sole JS, Wisniewski SJ, Dahm DL, Bond J, Smith J. Posttraumatic fat necrosis presenting as prepatellar loose bodies in an adolescent football player. PM R. 2014;6(8):749-752.
16. Felipo F, Vaquero M, del Agua C. Pseudotumoral encapsulated fat necrosis with diffuse pseudomembranous degeneration. J Cutan Pathol. 2004;31(8):565-567.
Systemic Hypothermia as Treatment for an Acute Cervical Spinal Cord Injury in a Professional Football Player: 9-Year Follow-Up
Take-Home Points
- Importance of on-field management.
- Preseason drilling of spinal injury management.
- Early and rapid intervention.
- Possible benefit of moderate systemic hypothermia as treatment for acute cervical injury.
In 2010, we reported the case of a professional American football player who sustained a complete cervical spinal cord injury (SCI) while tackling an opposing player.1 He received prompt medical and surgical care based on then-current recommendations, but was also treated with systemic hypothermia soon after his injury. Although systemic hypothermia had been used in the management of other neurologic injuries at that time, it had not been used in humans with acute SCI, except as described in 2 case reports.2,3 However, Dietrich4 described early emerging animal data on the efficacy of systemic hypothermia for acute SCI. We now provide a clinical update on our patient, who provided written informed consent for print and electronic publication of this case report.
Case Report
During a National Football League game, the player sustained a C3–C4 fracture-dislocation after a helmet-to-helmet hit on an opposing player. He fell face down on the ground and did not move. The team’s physician and trainer rushed to the player’s side, immediately assessed him, and initiated the emergency spinal resuscitation protocol.
As per protocol, the assigned team leader took charge of managing the player’s head to maintain in-line traction with the helmet in place until the head was secured in place on a backboard designed to accommodate the helmet.
Complete motor paralysis and sensory loss (American Spinal Injury Association [ASIA] level A) were noted below the clavicles during physical examination by the head athletic trainer and 2 independent physicians, and by self-report.
On arrival at the hospital, the patient had a core temperature of 98°F, which is substantially lower than the average core temperature (≤101.7°F) of an active football player.6He had a normal level of consciousness and normal cranial nerve function but remained without any voluntary motor function in the extremities and still had no sensation below the clavicles, except crude pressure sensation in one hand while in the emergency department. After the helmet and shoulder pads were removed, per National Athletic Trainers’ Association (NATA) protocol7 (Figure 2), he was stabilized, and a hard cervical collar was placed. A lateral radiograph (Figure 4) showed a C3–C4 facet dislocation with about 46% anterior translation of C3 on C4 and obvious disruption of the facets.
About 3 hours after injury, the patient was taken to the operating room. Although closed reduction improved alignment dramatically, it failed to completely reduce the dislocated left C3–C4 facet. An hour later, anterior C3–C4 discectomy was performed from the front with instrumented anterior interbody fusion. This was immediately followed by posterior decompressive laminectomy, bilateral facet reduction, and fusion with instrumentation. Surgery was completed within about 4 hours, almost exactly 7 hours after injury. Anesthesia records indicated a core temperature range of 94.1°F to 95.3°F with passive cooling during surgery. CT and MRI performed within 4 hours after surgery showed excellent cord decompression.
The next morning, about 14.5 hours after injury, the patient demonstrated a flicker of the adductor muscles of the lower extremities. An examination an hour later revealed 1/5 quadriceps, 2/5 adductors, and 1/5 gastrocnemius/soleus. A nurse’s hourly examinations and the surgeon’s repeat examinations revealed no other motor function. Sensory function was more difficult to evaluate because of sedation, but rudimentary sensation was noted throughout the lower extremities, and proprioception and vibratory sensation were noted as well. With passive cooling, it was difficult to consistently maintain moderate hypothermia; the patient’s core temperature ranged from 94.8°F to 98.8°F by 6:00 a.m. Therefore, the decision was made to place a Cordis sheath in the left femoral vein and introduce an intra-vena cava cooling catheter through it. This catheter was highly effective in maintaining the patient’s temperature at about 92.5°F.
Over the next 36 hours, the patient demonstrated increased motor activity in the upper and lower extremities: 1/5 biceps, 2-3/5 triceps, 3/5 quadriceps. He was slowly rewarmed and, on postoperative day 3, extubated.
At 2 years, the patient underwent another anterior-only cervical procedure: The inferior adjacent segment (C4–C5) was fused because of neck pain and deformity.
With respect to the original injury and the evolution in cord appearance, the patient had solid arthrodesis from C3–C5 with instrumentation in good position. There was evidence of loss of lordosis at C5–C6 with disk dessication and broad-based bulging. The spinal cord had evidence of myelomalacia; this was noted when the patient was in rehabilitation, 1 month after injury. The 2-cm × 11-mm area of myelomalacia was directly posterior to the fused C3–C4 interval (original MRI, Figure 5; 2-week MRI, Figure 6).
Conclusion
At the time this player was injured, use of systemic hypothermia with standard therapy for acute SCI was unique and controversial. Since then, smaller randomized human studies have described the tolerable safety profile, efficacy, and potential benefits of this intervention in acute SCI in humans.8-10 Now, modest systemic hypothermia can be one of many tools considered in the treatment of acute SCI. Before it can become the standard of care, however, additional larger prospective randomized studies need to be completed.
Am J Orthop. 2017;46(2):E79-E82. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
1. Cappuccino A, Bisson LJ, Carpenter B, Marzo J, Dietrich WD 3rd, Cappuccino H. The use of systemic hypothermia for the treatment of an acute cervical spinal cord injury in a professional football player. Spine. 2010;35(2):E57-E62.
2. Goldstein J. Lowering body temp shows promise for trauma treatment. Spinal Cord Injury Information Pages news blog. http://www.sci-info-pages.com/2006/05/lowering-body-temp-shows-promise-for.html. Published May 3, 2006. Accessed March 19, 2009.
3. Hartemink KJ, Wisselink W, Rauwerda JA, Girbes AR, Polderman KH. Novel applications of therapeutic hypothermia: report of three cases. Crit Care. 2004;8(5):R343-R346.
4. Dietrich WD. Presidential address presented at: 34th Annual Meeting of the Cervical Spine Research Society; November 30, 2006; Palm Beach, FL.
5. Bracken MB, Shepard MJ, Collins WF, et al. A randomized, controlled trial of methylprednisolone or naloxone in the treatment of acute spinal-cord injury. Results of the second National Acute Spinal Cord Injury Study. N Engl J Med. 1990;322(20):1405-1411.
6. Horodyski MB, LuCante K, Escobar E, et al. Intermittent Cool, Dry Air Underneath Football Shoulder Pads Assists in Temperature Homeostasis. In: The American Orthopaedic Society for Sports Medicine Proceedings 2008; 87-88.
7. Kleiner DM, Almquist JL, Bailes J, et al; Inter-Association Task Force for Appropriate Care of the Spine-Injured Athlete. Prehospital Care of the Spine-Injured Athlete. Dallas, TX: National Athletic Trainers’ Association; 2001. http://www.msata.org/Resources/Documents/PreHospitalCare4SpineInjuredAthlete.pdf. Published March 2001. Accessed January 10, 2017.
8. Dididze M, Green BA, Dietrich WD, Vanni S, Wang MY, Levi AD. Systemic hypothermia in acute cervical spinal cord injury: a case-controlled study. Spinal Cord. 2013;51(5):395-400.
9. Levi AD, Casella G, Green BA, et al. Clinical outcomes using modest intravascular hypothermia after acute cervical spinal cord injury. Neurosurgery. 2010;66(4):670-677.
10. Levi AD, Green BA, Wang MY, et al. Clinical application of modest hypothermia after spinal cord injury. J Neurotrauma. 2009;26(3):407-415.
Take-Home Points
- Importance of on-field management.
- Preseason drilling of spinal injury management.
- Early and rapid intervention.
- Possible benefit of moderate systemic hypothermia as treatment for acute cervical injury.
In 2010, we reported the case of a professional American football player who sustained a complete cervical spinal cord injury (SCI) while tackling an opposing player.1 He received prompt medical and surgical care based on then-current recommendations, but was also treated with systemic hypothermia soon after his injury. Although systemic hypothermia had been used in the management of other neurologic injuries at that time, it had not been used in humans with acute SCI, except as described in 2 case reports.2,3 However, Dietrich4 described early emerging animal data on the efficacy of systemic hypothermia for acute SCI. We now provide a clinical update on our patient, who provided written informed consent for print and electronic publication of this case report.
Case Report
During a National Football League game, the player sustained a C3–C4 fracture-dislocation after a helmet-to-helmet hit on an opposing player. He fell face down on the ground and did not move. The team’s physician and trainer rushed to the player’s side, immediately assessed him, and initiated the emergency spinal resuscitation protocol.
As per protocol, the assigned team leader took charge of managing the player’s head to maintain in-line traction with the helmet in place until the head was secured in place on a backboard designed to accommodate the helmet.
Complete motor paralysis and sensory loss (American Spinal Injury Association [ASIA] level A) were noted below the clavicles during physical examination by the head athletic trainer and 2 independent physicians, and by self-report.
On arrival at the hospital, the patient had a core temperature of 98°F, which is substantially lower than the average core temperature (≤101.7°F) of an active football player.6He had a normal level of consciousness and normal cranial nerve function but remained without any voluntary motor function in the extremities and still had no sensation below the clavicles, except crude pressure sensation in one hand while in the emergency department. After the helmet and shoulder pads were removed, per National Athletic Trainers’ Association (NATA) protocol7 (Figure 2), he was stabilized, and a hard cervical collar was placed. A lateral radiograph (Figure 4) showed a C3–C4 facet dislocation with about 46% anterior translation of C3 on C4 and obvious disruption of the facets.
About 3 hours after injury, the patient was taken to the operating room. Although closed reduction improved alignment dramatically, it failed to completely reduce the dislocated left C3–C4 facet. An hour later, anterior C3–C4 discectomy was performed from the front with instrumented anterior interbody fusion. This was immediately followed by posterior decompressive laminectomy, bilateral facet reduction, and fusion with instrumentation. Surgery was completed within about 4 hours, almost exactly 7 hours after injury. Anesthesia records indicated a core temperature range of 94.1°F to 95.3°F with passive cooling during surgery. CT and MRI performed within 4 hours after surgery showed excellent cord decompression.
The next morning, about 14.5 hours after injury, the patient demonstrated a flicker of the adductor muscles of the lower extremities. An examination an hour later revealed 1/5 quadriceps, 2/5 adductors, and 1/5 gastrocnemius/soleus. A nurse’s hourly examinations and the surgeon’s repeat examinations revealed no other motor function. Sensory function was more difficult to evaluate because of sedation, but rudimentary sensation was noted throughout the lower extremities, and proprioception and vibratory sensation were noted as well. With passive cooling, it was difficult to consistently maintain moderate hypothermia; the patient’s core temperature ranged from 94.8°F to 98.8°F by 6:00 a.m. Therefore, the decision was made to place a Cordis sheath in the left femoral vein and introduce an intra-vena cava cooling catheter through it. This catheter was highly effective in maintaining the patient’s temperature at about 92.5°F.
Over the next 36 hours, the patient demonstrated increased motor activity in the upper and lower extremities: 1/5 biceps, 2-3/5 triceps, 3/5 quadriceps. He was slowly rewarmed and, on postoperative day 3, extubated.
At 2 years, the patient underwent another anterior-only cervical procedure: The inferior adjacent segment (C4–C5) was fused because of neck pain and deformity.
With respect to the original injury and the evolution in cord appearance, the patient had solid arthrodesis from C3–C5 with instrumentation in good position. There was evidence of loss of lordosis at C5–C6 with disk dessication and broad-based bulging. The spinal cord had evidence of myelomalacia; this was noted when the patient was in rehabilitation, 1 month after injury. The 2-cm × 11-mm area of myelomalacia was directly posterior to the fused C3–C4 interval (original MRI, Figure 5; 2-week MRI, Figure 6).
Conclusion
At the time this player was injured, use of systemic hypothermia with standard therapy for acute SCI was unique and controversial. Since then, smaller randomized human studies have described the tolerable safety profile, efficacy, and potential benefits of this intervention in acute SCI in humans.8-10 Now, modest systemic hypothermia can be one of many tools considered in the treatment of acute SCI. Before it can become the standard of care, however, additional larger prospective randomized studies need to be completed.
Am J Orthop. 2017;46(2):E79-E82. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
Take-Home Points
- Importance of on-field management.
- Preseason drilling of spinal injury management.
- Early and rapid intervention.
- Possible benefit of moderate systemic hypothermia as treatment for acute cervical injury.
In 2010, we reported the case of a professional American football player who sustained a complete cervical spinal cord injury (SCI) while tackling an opposing player.1 He received prompt medical and surgical care based on then-current recommendations, but was also treated with systemic hypothermia soon after his injury. Although systemic hypothermia had been used in the management of other neurologic injuries at that time, it had not been used in humans with acute SCI, except as described in 2 case reports.2,3 However, Dietrich4 described early emerging animal data on the efficacy of systemic hypothermia for acute SCI. We now provide a clinical update on our patient, who provided written informed consent for print and electronic publication of this case report.
Case Report
During a National Football League game, the player sustained a C3–C4 fracture-dislocation after a helmet-to-helmet hit on an opposing player. He fell face down on the ground and did not move. The team’s physician and trainer rushed to the player’s side, immediately assessed him, and initiated the emergency spinal resuscitation protocol.
As per protocol, the assigned team leader took charge of managing the player’s head to maintain in-line traction with the helmet in place until the head was secured in place on a backboard designed to accommodate the helmet.
Complete motor paralysis and sensory loss (American Spinal Injury Association [ASIA] level A) were noted below the clavicles during physical examination by the head athletic trainer and 2 independent physicians, and by self-report.
On arrival at the hospital, the patient had a core temperature of 98°F, which is substantially lower than the average core temperature (≤101.7°F) of an active football player.6He had a normal level of consciousness and normal cranial nerve function but remained without any voluntary motor function in the extremities and still had no sensation below the clavicles, except crude pressure sensation in one hand while in the emergency department. After the helmet and shoulder pads were removed, per National Athletic Trainers’ Association (NATA) protocol7 (Figure 2), he was stabilized, and a hard cervical collar was placed. A lateral radiograph (Figure 4) showed a C3–C4 facet dislocation with about 46% anterior translation of C3 on C4 and obvious disruption of the facets.
About 3 hours after injury, the patient was taken to the operating room. Although closed reduction improved alignment dramatically, it failed to completely reduce the dislocated left C3–C4 facet. An hour later, anterior C3–C4 discectomy was performed from the front with instrumented anterior interbody fusion. This was immediately followed by posterior decompressive laminectomy, bilateral facet reduction, and fusion with instrumentation. Surgery was completed within about 4 hours, almost exactly 7 hours after injury. Anesthesia records indicated a core temperature range of 94.1°F to 95.3°F with passive cooling during surgery. CT and MRI performed within 4 hours after surgery showed excellent cord decompression.
The next morning, about 14.5 hours after injury, the patient demonstrated a flicker of the adductor muscles of the lower extremities. An examination an hour later revealed 1/5 quadriceps, 2/5 adductors, and 1/5 gastrocnemius/soleus. A nurse’s hourly examinations and the surgeon’s repeat examinations revealed no other motor function. Sensory function was more difficult to evaluate because of sedation, but rudimentary sensation was noted throughout the lower extremities, and proprioception and vibratory sensation were noted as well. With passive cooling, it was difficult to consistently maintain moderate hypothermia; the patient’s core temperature ranged from 94.8°F to 98.8°F by 6:00 a.m. Therefore, the decision was made to place a Cordis sheath in the left femoral vein and introduce an intra-vena cava cooling catheter through it. This catheter was highly effective in maintaining the patient’s temperature at about 92.5°F.
Over the next 36 hours, the patient demonstrated increased motor activity in the upper and lower extremities: 1/5 biceps, 2-3/5 triceps, 3/5 quadriceps. He was slowly rewarmed and, on postoperative day 3, extubated.
At 2 years, the patient underwent another anterior-only cervical procedure: The inferior adjacent segment (C4–C5) was fused because of neck pain and deformity.
With respect to the original injury and the evolution in cord appearance, the patient had solid arthrodesis from C3–C5 with instrumentation in good position. There was evidence of loss of lordosis at C5–C6 with disk dessication and broad-based bulging. The spinal cord had evidence of myelomalacia; this was noted when the patient was in rehabilitation, 1 month after injury. The 2-cm × 11-mm area of myelomalacia was directly posterior to the fused C3–C4 interval (original MRI, Figure 5; 2-week MRI, Figure 6).
Conclusion
At the time this player was injured, use of systemic hypothermia with standard therapy for acute SCI was unique and controversial. Since then, smaller randomized human studies have described the tolerable safety profile, efficacy, and potential benefits of this intervention in acute SCI in humans.8-10 Now, modest systemic hypothermia can be one of many tools considered in the treatment of acute SCI. Before it can become the standard of care, however, additional larger prospective randomized studies need to be completed.
Am J Orthop. 2017;46(2):E79-E82. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
1. Cappuccino A, Bisson LJ, Carpenter B, Marzo J, Dietrich WD 3rd, Cappuccino H. The use of systemic hypothermia for the treatment of an acute cervical spinal cord injury in a professional football player. Spine. 2010;35(2):E57-E62.
2. Goldstein J. Lowering body temp shows promise for trauma treatment. Spinal Cord Injury Information Pages news blog. http://www.sci-info-pages.com/2006/05/lowering-body-temp-shows-promise-for.html. Published May 3, 2006. Accessed March 19, 2009.
3. Hartemink KJ, Wisselink W, Rauwerda JA, Girbes AR, Polderman KH. Novel applications of therapeutic hypothermia: report of three cases. Crit Care. 2004;8(5):R343-R346.
4. Dietrich WD. Presidential address presented at: 34th Annual Meeting of the Cervical Spine Research Society; November 30, 2006; Palm Beach, FL.
5. Bracken MB, Shepard MJ, Collins WF, et al. A randomized, controlled trial of methylprednisolone or naloxone in the treatment of acute spinal-cord injury. Results of the second National Acute Spinal Cord Injury Study. N Engl J Med. 1990;322(20):1405-1411.
6. Horodyski MB, LuCante K, Escobar E, et al. Intermittent Cool, Dry Air Underneath Football Shoulder Pads Assists in Temperature Homeostasis. In: The American Orthopaedic Society for Sports Medicine Proceedings 2008; 87-88.
7. Kleiner DM, Almquist JL, Bailes J, et al; Inter-Association Task Force for Appropriate Care of the Spine-Injured Athlete. Prehospital Care of the Spine-Injured Athlete. Dallas, TX: National Athletic Trainers’ Association; 2001. http://www.msata.org/Resources/Documents/PreHospitalCare4SpineInjuredAthlete.pdf. Published March 2001. Accessed January 10, 2017.
8. Dididze M, Green BA, Dietrich WD, Vanni S, Wang MY, Levi AD. Systemic hypothermia in acute cervical spinal cord injury: a case-controlled study. Spinal Cord. 2013;51(5):395-400.
9. Levi AD, Casella G, Green BA, et al. Clinical outcomes using modest intravascular hypothermia after acute cervical spinal cord injury. Neurosurgery. 2010;66(4):670-677.
10. Levi AD, Green BA, Wang MY, et al. Clinical application of modest hypothermia after spinal cord injury. J Neurotrauma. 2009;26(3):407-415.
1. Cappuccino A, Bisson LJ, Carpenter B, Marzo J, Dietrich WD 3rd, Cappuccino H. The use of systemic hypothermia for the treatment of an acute cervical spinal cord injury in a professional football player. Spine. 2010;35(2):E57-E62.
2. Goldstein J. Lowering body temp shows promise for trauma treatment. Spinal Cord Injury Information Pages news blog. http://www.sci-info-pages.com/2006/05/lowering-body-temp-shows-promise-for.html. Published May 3, 2006. Accessed March 19, 2009.
3. Hartemink KJ, Wisselink W, Rauwerda JA, Girbes AR, Polderman KH. Novel applications of therapeutic hypothermia: report of three cases. Crit Care. 2004;8(5):R343-R346.
4. Dietrich WD. Presidential address presented at: 34th Annual Meeting of the Cervical Spine Research Society; November 30, 2006; Palm Beach, FL.
5. Bracken MB, Shepard MJ, Collins WF, et al. A randomized, controlled trial of methylprednisolone or naloxone in the treatment of acute spinal-cord injury. Results of the second National Acute Spinal Cord Injury Study. N Engl J Med. 1990;322(20):1405-1411.
6. Horodyski MB, LuCante K, Escobar E, et al. Intermittent Cool, Dry Air Underneath Football Shoulder Pads Assists in Temperature Homeostasis. In: The American Orthopaedic Society for Sports Medicine Proceedings 2008; 87-88.
7. Kleiner DM, Almquist JL, Bailes J, et al; Inter-Association Task Force for Appropriate Care of the Spine-Injured Athlete. Prehospital Care of the Spine-Injured Athlete. Dallas, TX: National Athletic Trainers’ Association; 2001. http://www.msata.org/Resources/Documents/PreHospitalCare4SpineInjuredAthlete.pdf. Published March 2001. Accessed January 10, 2017.
8. Dididze M, Green BA, Dietrich WD, Vanni S, Wang MY, Levi AD. Systemic hypothermia in acute cervical spinal cord injury: a case-controlled study. Spinal Cord. 2013;51(5):395-400.
9. Levi AD, Casella G, Green BA, et al. Clinical outcomes using modest intravascular hypothermia after acute cervical spinal cord injury. Neurosurgery. 2010;66(4):670-677.
10. Levi AD, Green BA, Wang MY, et al. Clinical application of modest hypothermia after spinal cord injury. J Neurotrauma. 2009;26(3):407-415.
Pronator Teres Myotendinous Tear
Take-Home Points
- Pronator teres muscle injuries are rare.
- Injury can be mistaken for MUCL injury in athletes.
- Tenderness and weak/painful forearm pronation are common findings.
- MRI confirms the diagnosis and helps grade the muscle strain injury.
- Conservative treatment is recommended and prognosis is excellent even for high-grade strains.
Pronator teres muscle strain is a rare sporting injury reported only in cricket players, and now in a golfer whose forearm experienced an eccentric force during resisted elbow flexion and pronation.1,2 The injury occurs when the sporting club or racket strikes the ground during a swing, impeding forward progress and subjecting the pronator teres muscle to eccentric forces in excess of what it can withstand. The pronator teres, one of several muscles that comprise the flexor wad of the forearm, consists of 2 heads, originating proximally from the medical epicondyle and attaching distally to the shaft of the radius on its lateral surface and just distal to the supinator. The oblique orientation of the muscle belly allows it to serve in its primary rotatory role as the main pronator of the forearm. Injuries to the soft tissue of the medial forearm are common in both elite and recreational athletes, especially in racket and club sports.3 Often, these injuries are related to overuse and chronic fatigue of the surrounding soft tissue—caused by repetitive flexing, gripping, or swinging. Even when identified early, these injuries can result in a significant loss of training time.4 In this article, we report a case of pronator teres muscle tear at the myotendinous junction. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A right-hand–dominant 36-year-old man presented to the clinic with pain on the medial side of his right elbow after sustaining an injury to the elbow while playing golf several days earlier. The patient, an advertising executive, was playing recreational golf several times a month and had no significant medical history or previous symptoms related to the elbow. Initial pain symptoms began during a second round of play, immediately after the patient miss-hit an iron shot, making contact mostly with the ground and causing the club to forcefully stop. The pain was on the medial side of the elbow and forearm. The patient noted progressive swelling and bruising at the pain site and development of forearm weakness. Physical examination during the clinic presentation revealed ecchymosis on the anterior medial forearm, medial elbow, and medial triceps (Figure 1).
Noncontrast magnetic resonance imaging (MRI) showed a high-grade partial tear of the pronator teres myotendinous junction (Figures 2A-2C).
The patient was instructed to rest the elbow from strenuous activity, golf in particular, for 4 weeks. Physical therapy for ROM and forearm strengthening of the surrounding flexor wad was initiated at 2 weeks and continued for 4 weeks. The patient was advised to take over-the-counter nonsteroidal anti-inflammatory drugs as needed for comfort. On repeat examination at 4 weeks, with tenderness or weakness with pronation absent and full ROM regained, the patient was released back to full activity. He was able to return to golf and reported being symptom-free and having no sense of weakness or loss of control.
Discussion
A tear of the pronator teres is an exceedingly rare injury. Our results with conservative treatment and a full return to previous activity level are consistent with the only other case reported in the literature.5 In contrast to our patient, the previous patient sustained a tear of the pronator teres after a prolonged period of batting during a recreational cricket match.
Our patient’s pronator teres injury occurred at the myotendinous junction, a muscle-tendon transition zone often susceptible to injury. What is unusual for this athletic medial elbow injury is that the patient reported no previous symptoms, and it appears that, though the surrounding muscle may have been fatigued by overuse from the round of golf earlier that day, the pathology was caused by an acute eccentric force. During a golf swing, tremendous forces are put on the entire body, from the lower extremities to the forearm and the fingers. Successful completion of the transfer of energy from the golf club to the ball requires both proper technique and proper functioning of key muscles. Specifically, parameters such as ball positioning, club angle, and wrist control play a major role.6 Altered forearm positioning or swing arc can significantly affect club head velocity and energy transfer without putting more stress on the golfer.7 Therefore, it is easy to understand how prolonged or extended play may fatigue the surrounding elbow muscles, leading to altered technique and increased susceptibility to acute injury. Biomechanical analysis of shoulder motion can provide a helpful baseline for assessing injury-related changes in golf swing and developing specific exercise and rehabilitation programs.8,9Although injury to the pronator teres is rare, sport physicians should be aware that, after a valgus stress or force, bruising and swelling along the medial elbow do not always indicate a medial ulnar collateral ligament (MUCL) tear or medial epicondylitis. The key examination findings that differentiate this injury from a MUCL injury are the exact location of pain, the milking maneuver for MUCL incompetence, and the extensive bruising over the muscle course of the pronator teres. MRI plays a pivotal role in proper diagnosis.4 In addition, MRI allows for evaluation of any concomitant injuries that may be obscuring the clinical presentation.
Successful treatment of such injuries is important for both elite and recreational athletes. With rest and physical therapy, our patient recovered from this rare isolated injury to the pronator teres with complete resolution of symptoms and full ROM. In the literature, we found no other reports of isolated full-thickness myotendinous rupture of the pronator teres or avulsion from the medial epicondyle. Therefore, it is unclear whether the same outcome can be expected with conservative therapy. However, because of the good outcomes for partial-thickness injuries treated conservatively and the lack of robust tendinous tissue to repair at the myotendinous junction, we recommend an initial course of conservative treatment. Sports physicians should be aware of this exceedingly rare injury to the elbow and understand the large forces experienced by the soft tissues of the forearm during the golf swing.9,10
Conclusion
Pronator teres muscle strain is a rare sporting injury reported in cricket and golf players. The elbow experiences a large eccentric force during resisted elbow flexion and pronation. The injury appears to occur when the sporting club or racket strikes the ground during a forceful swing impeding forward progress of the arm. The injury can be confused with a MUCL injury, or exacerbation of medial epicondylitis. Physical examination reveals bruising and tenderness over the course of the pronator teres, often distal to the elbow. Advanced imaging confirms the diagnosis and helps grade the severity of muscle strain. Treatment is often conservative, with return to function and sport after 4 to 6 weeks of rest and restricted activities. The patient in this case report had complete return to sporting function, with no residual weakness or pain.
Am J Orthop. 2017;46(2):E105-E107. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
1. Field LD, Savoie FH. Common elbow injuries in sport. Sports Med. 1998;26(3):193-205.
2. Loomer RL. Elbow injuries in athletes. Can J Appl Sport Sci. 1982;7(3):164-166.
3. Dines JS, Bedi A, Williams PN, et al. Tennis injuries: epidemiology, pathophysiology, and treatment. J Am Acad Orthop Surg. 2015;23(3):181-189.
4. Banks KP, Ly JQ, Beall DP, Grayson DE, Bancroft LW, Tall MA. Overuse injuries of the upper extremity in the competitive athlete: magnetic resonance imaging findings associated with repetitive trauma. Curr Probl Diagn Radiol. 2005;34(4):127-142.
5. Niebulski HZ, Richardson ML. High-grade pronator teres tear in a cricket batsman. Radiol Case Rep. 2015;6(3):540.
6. Zhang X, Shan G. Where do golf drive swings go wrong? Factors influencing driver swing consistency. Scand J Med Sci Sports. 2014;24(5):749-757.
7. Nesbit SM, McGinnis RS. Kinetic constrained optimization of the golf swing hub path. J Sports Sci Med. 2014;13(4):859-873.
8. Helton MS. Conservative treatment of a proximal full-thickness biceps brachii muscle tear in a special operations soldier. Phys Ther. 2014;94(4):571-577.
9. Mitchell K, Banks S, Morgan D, Sugaya H. Shoulder motions during the golf swing in male amateur golfers. J Orthop Sports Phys Ther. 2003;33(4):196-203.
10. Grimshaw P, Giles A, Tong R, Grimmer K. Lower back and elbow injuries in golf. Sports Med. 2002;32(10):655-666.
Take-Home Points
- Pronator teres muscle injuries are rare.
- Injury can be mistaken for MUCL injury in athletes.
- Tenderness and weak/painful forearm pronation are common findings.
- MRI confirms the diagnosis and helps grade the muscle strain injury.
- Conservative treatment is recommended and prognosis is excellent even for high-grade strains.
Pronator teres muscle strain is a rare sporting injury reported only in cricket players, and now in a golfer whose forearm experienced an eccentric force during resisted elbow flexion and pronation.1,2 The injury occurs when the sporting club or racket strikes the ground during a swing, impeding forward progress and subjecting the pronator teres muscle to eccentric forces in excess of what it can withstand. The pronator teres, one of several muscles that comprise the flexor wad of the forearm, consists of 2 heads, originating proximally from the medical epicondyle and attaching distally to the shaft of the radius on its lateral surface and just distal to the supinator. The oblique orientation of the muscle belly allows it to serve in its primary rotatory role as the main pronator of the forearm. Injuries to the soft tissue of the medial forearm are common in both elite and recreational athletes, especially in racket and club sports.3 Often, these injuries are related to overuse and chronic fatigue of the surrounding soft tissue—caused by repetitive flexing, gripping, or swinging. Even when identified early, these injuries can result in a significant loss of training time.4 In this article, we report a case of pronator teres muscle tear at the myotendinous junction. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A right-hand–dominant 36-year-old man presented to the clinic with pain on the medial side of his right elbow after sustaining an injury to the elbow while playing golf several days earlier. The patient, an advertising executive, was playing recreational golf several times a month and had no significant medical history or previous symptoms related to the elbow. Initial pain symptoms began during a second round of play, immediately after the patient miss-hit an iron shot, making contact mostly with the ground and causing the club to forcefully stop. The pain was on the medial side of the elbow and forearm. The patient noted progressive swelling and bruising at the pain site and development of forearm weakness. Physical examination during the clinic presentation revealed ecchymosis on the anterior medial forearm, medial elbow, and medial triceps (Figure 1).
Noncontrast magnetic resonance imaging (MRI) showed a high-grade partial tear of the pronator teres myotendinous junction (Figures 2A-2C).
The patient was instructed to rest the elbow from strenuous activity, golf in particular, for 4 weeks. Physical therapy for ROM and forearm strengthening of the surrounding flexor wad was initiated at 2 weeks and continued for 4 weeks. The patient was advised to take over-the-counter nonsteroidal anti-inflammatory drugs as needed for comfort. On repeat examination at 4 weeks, with tenderness or weakness with pronation absent and full ROM regained, the patient was released back to full activity. He was able to return to golf and reported being symptom-free and having no sense of weakness or loss of control.
Discussion
A tear of the pronator teres is an exceedingly rare injury. Our results with conservative treatment and a full return to previous activity level are consistent with the only other case reported in the literature.5 In contrast to our patient, the previous patient sustained a tear of the pronator teres after a prolonged period of batting during a recreational cricket match.
Our patient’s pronator teres injury occurred at the myotendinous junction, a muscle-tendon transition zone often susceptible to injury. What is unusual for this athletic medial elbow injury is that the patient reported no previous symptoms, and it appears that, though the surrounding muscle may have been fatigued by overuse from the round of golf earlier that day, the pathology was caused by an acute eccentric force. During a golf swing, tremendous forces are put on the entire body, from the lower extremities to the forearm and the fingers. Successful completion of the transfer of energy from the golf club to the ball requires both proper technique and proper functioning of key muscles. Specifically, parameters such as ball positioning, club angle, and wrist control play a major role.6 Altered forearm positioning or swing arc can significantly affect club head velocity and energy transfer without putting more stress on the golfer.7 Therefore, it is easy to understand how prolonged or extended play may fatigue the surrounding elbow muscles, leading to altered technique and increased susceptibility to acute injury. Biomechanical analysis of shoulder motion can provide a helpful baseline for assessing injury-related changes in golf swing and developing specific exercise and rehabilitation programs.8,9Although injury to the pronator teres is rare, sport physicians should be aware that, after a valgus stress or force, bruising and swelling along the medial elbow do not always indicate a medial ulnar collateral ligament (MUCL) tear or medial epicondylitis. The key examination findings that differentiate this injury from a MUCL injury are the exact location of pain, the milking maneuver for MUCL incompetence, and the extensive bruising over the muscle course of the pronator teres. MRI plays a pivotal role in proper diagnosis.4 In addition, MRI allows for evaluation of any concomitant injuries that may be obscuring the clinical presentation.
Successful treatment of such injuries is important for both elite and recreational athletes. With rest and physical therapy, our patient recovered from this rare isolated injury to the pronator teres with complete resolution of symptoms and full ROM. In the literature, we found no other reports of isolated full-thickness myotendinous rupture of the pronator teres or avulsion from the medial epicondyle. Therefore, it is unclear whether the same outcome can be expected with conservative therapy. However, because of the good outcomes for partial-thickness injuries treated conservatively and the lack of robust tendinous tissue to repair at the myotendinous junction, we recommend an initial course of conservative treatment. Sports physicians should be aware of this exceedingly rare injury to the elbow and understand the large forces experienced by the soft tissues of the forearm during the golf swing.9,10
Conclusion
Pronator teres muscle strain is a rare sporting injury reported in cricket and golf players. The elbow experiences a large eccentric force during resisted elbow flexion and pronation. The injury appears to occur when the sporting club or racket strikes the ground during a forceful swing impeding forward progress of the arm. The injury can be confused with a MUCL injury, or exacerbation of medial epicondylitis. Physical examination reveals bruising and tenderness over the course of the pronator teres, often distal to the elbow. Advanced imaging confirms the diagnosis and helps grade the severity of muscle strain. Treatment is often conservative, with return to function and sport after 4 to 6 weeks of rest and restricted activities. The patient in this case report had complete return to sporting function, with no residual weakness or pain.
Am J Orthop. 2017;46(2):E105-E107. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
Take-Home Points
- Pronator teres muscle injuries are rare.
- Injury can be mistaken for MUCL injury in athletes.
- Tenderness and weak/painful forearm pronation are common findings.
- MRI confirms the diagnosis and helps grade the muscle strain injury.
- Conservative treatment is recommended and prognosis is excellent even for high-grade strains.
Pronator teres muscle strain is a rare sporting injury reported only in cricket players, and now in a golfer whose forearm experienced an eccentric force during resisted elbow flexion and pronation.1,2 The injury occurs when the sporting club or racket strikes the ground during a swing, impeding forward progress and subjecting the pronator teres muscle to eccentric forces in excess of what it can withstand. The pronator teres, one of several muscles that comprise the flexor wad of the forearm, consists of 2 heads, originating proximally from the medical epicondyle and attaching distally to the shaft of the radius on its lateral surface and just distal to the supinator. The oblique orientation of the muscle belly allows it to serve in its primary rotatory role as the main pronator of the forearm. Injuries to the soft tissue of the medial forearm are common in both elite and recreational athletes, especially in racket and club sports.3 Often, these injuries are related to overuse and chronic fatigue of the surrounding soft tissue—caused by repetitive flexing, gripping, or swinging. Even when identified early, these injuries can result in a significant loss of training time.4 In this article, we report a case of pronator teres muscle tear at the myotendinous junction. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A right-hand–dominant 36-year-old man presented to the clinic with pain on the medial side of his right elbow after sustaining an injury to the elbow while playing golf several days earlier. The patient, an advertising executive, was playing recreational golf several times a month and had no significant medical history or previous symptoms related to the elbow. Initial pain symptoms began during a second round of play, immediately after the patient miss-hit an iron shot, making contact mostly with the ground and causing the club to forcefully stop. The pain was on the medial side of the elbow and forearm. The patient noted progressive swelling and bruising at the pain site and development of forearm weakness. Physical examination during the clinic presentation revealed ecchymosis on the anterior medial forearm, medial elbow, and medial triceps (Figure 1).
Noncontrast magnetic resonance imaging (MRI) showed a high-grade partial tear of the pronator teres myotendinous junction (Figures 2A-2C).
The patient was instructed to rest the elbow from strenuous activity, golf in particular, for 4 weeks. Physical therapy for ROM and forearm strengthening of the surrounding flexor wad was initiated at 2 weeks and continued for 4 weeks. The patient was advised to take over-the-counter nonsteroidal anti-inflammatory drugs as needed for comfort. On repeat examination at 4 weeks, with tenderness or weakness with pronation absent and full ROM regained, the patient was released back to full activity. He was able to return to golf and reported being symptom-free and having no sense of weakness or loss of control.
Discussion
A tear of the pronator teres is an exceedingly rare injury. Our results with conservative treatment and a full return to previous activity level are consistent with the only other case reported in the literature.5 In contrast to our patient, the previous patient sustained a tear of the pronator teres after a prolonged period of batting during a recreational cricket match.
Our patient’s pronator teres injury occurred at the myotendinous junction, a muscle-tendon transition zone often susceptible to injury. What is unusual for this athletic medial elbow injury is that the patient reported no previous symptoms, and it appears that, though the surrounding muscle may have been fatigued by overuse from the round of golf earlier that day, the pathology was caused by an acute eccentric force. During a golf swing, tremendous forces are put on the entire body, from the lower extremities to the forearm and the fingers. Successful completion of the transfer of energy from the golf club to the ball requires both proper technique and proper functioning of key muscles. Specifically, parameters such as ball positioning, club angle, and wrist control play a major role.6 Altered forearm positioning or swing arc can significantly affect club head velocity and energy transfer without putting more stress on the golfer.7 Therefore, it is easy to understand how prolonged or extended play may fatigue the surrounding elbow muscles, leading to altered technique and increased susceptibility to acute injury. Biomechanical analysis of shoulder motion can provide a helpful baseline for assessing injury-related changes in golf swing and developing specific exercise and rehabilitation programs.8,9Although injury to the pronator teres is rare, sport physicians should be aware that, after a valgus stress or force, bruising and swelling along the medial elbow do not always indicate a medial ulnar collateral ligament (MUCL) tear or medial epicondylitis. The key examination findings that differentiate this injury from a MUCL injury are the exact location of pain, the milking maneuver for MUCL incompetence, and the extensive bruising over the muscle course of the pronator teres. MRI plays a pivotal role in proper diagnosis.4 In addition, MRI allows for evaluation of any concomitant injuries that may be obscuring the clinical presentation.
Successful treatment of such injuries is important for both elite and recreational athletes. With rest and physical therapy, our patient recovered from this rare isolated injury to the pronator teres with complete resolution of symptoms and full ROM. In the literature, we found no other reports of isolated full-thickness myotendinous rupture of the pronator teres or avulsion from the medial epicondyle. Therefore, it is unclear whether the same outcome can be expected with conservative therapy. However, because of the good outcomes for partial-thickness injuries treated conservatively and the lack of robust tendinous tissue to repair at the myotendinous junction, we recommend an initial course of conservative treatment. Sports physicians should be aware of this exceedingly rare injury to the elbow and understand the large forces experienced by the soft tissues of the forearm during the golf swing.9,10
Conclusion
Pronator teres muscle strain is a rare sporting injury reported in cricket and golf players. The elbow experiences a large eccentric force during resisted elbow flexion and pronation. The injury appears to occur when the sporting club or racket strikes the ground during a forceful swing impeding forward progress of the arm. The injury can be confused with a MUCL injury, or exacerbation of medial epicondylitis. Physical examination reveals bruising and tenderness over the course of the pronator teres, often distal to the elbow. Advanced imaging confirms the diagnosis and helps grade the severity of muscle strain. Treatment is often conservative, with return to function and sport after 4 to 6 weeks of rest and restricted activities. The patient in this case report had complete return to sporting function, with no residual weakness or pain.
Am J Orthop. 2017;46(2):E105-E107. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
1. Field LD, Savoie FH. Common elbow injuries in sport. Sports Med. 1998;26(3):193-205.
2. Loomer RL. Elbow injuries in athletes. Can J Appl Sport Sci. 1982;7(3):164-166.
3. Dines JS, Bedi A, Williams PN, et al. Tennis injuries: epidemiology, pathophysiology, and treatment. J Am Acad Orthop Surg. 2015;23(3):181-189.
4. Banks KP, Ly JQ, Beall DP, Grayson DE, Bancroft LW, Tall MA. Overuse injuries of the upper extremity in the competitive athlete: magnetic resonance imaging findings associated with repetitive trauma. Curr Probl Diagn Radiol. 2005;34(4):127-142.
5. Niebulski HZ, Richardson ML. High-grade pronator teres tear in a cricket batsman. Radiol Case Rep. 2015;6(3):540.
6. Zhang X, Shan G. Where do golf drive swings go wrong? Factors influencing driver swing consistency. Scand J Med Sci Sports. 2014;24(5):749-757.
7. Nesbit SM, McGinnis RS. Kinetic constrained optimization of the golf swing hub path. J Sports Sci Med. 2014;13(4):859-873.
8. Helton MS. Conservative treatment of a proximal full-thickness biceps brachii muscle tear in a special operations soldier. Phys Ther. 2014;94(4):571-577.
9. Mitchell K, Banks S, Morgan D, Sugaya H. Shoulder motions during the golf swing in male amateur golfers. J Orthop Sports Phys Ther. 2003;33(4):196-203.
10. Grimshaw P, Giles A, Tong R, Grimmer K. Lower back and elbow injuries in golf. Sports Med. 2002;32(10):655-666.
1. Field LD, Savoie FH. Common elbow injuries in sport. Sports Med. 1998;26(3):193-205.
2. Loomer RL. Elbow injuries in athletes. Can J Appl Sport Sci. 1982;7(3):164-166.
3. Dines JS, Bedi A, Williams PN, et al. Tennis injuries: epidemiology, pathophysiology, and treatment. J Am Acad Orthop Surg. 2015;23(3):181-189.
4. Banks KP, Ly JQ, Beall DP, Grayson DE, Bancroft LW, Tall MA. Overuse injuries of the upper extremity in the competitive athlete: magnetic resonance imaging findings associated with repetitive trauma. Curr Probl Diagn Radiol. 2005;34(4):127-142.
5. Niebulski HZ, Richardson ML. High-grade pronator teres tear in a cricket batsman. Radiol Case Rep. 2015;6(3):540.
6. Zhang X, Shan G. Where do golf drive swings go wrong? Factors influencing driver swing consistency. Scand J Med Sci Sports. 2014;24(5):749-757.
7. Nesbit SM, McGinnis RS. Kinetic constrained optimization of the golf swing hub path. J Sports Sci Med. 2014;13(4):859-873.
8. Helton MS. Conservative treatment of a proximal full-thickness biceps brachii muscle tear in a special operations soldier. Phys Ther. 2014;94(4):571-577.
9. Mitchell K, Banks S, Morgan D, Sugaya H. Shoulder motions during the golf swing in male amateur golfers. J Orthop Sports Phys Ther. 2003;33(4):196-203.
10. Grimshaw P, Giles A, Tong R, Grimmer K. Lower back and elbow injuries in golf. Sports Med. 2002;32(10):655-666.
Management of Proximal Biceps Pathology in Overhead Athletes: What Is the Role of Biceps Tenodesis?
Take Home Points
- Outcomes after SLAP repair remain guarded.
- Physical examination is key in determining proper management of biceps pathology.
- When performing SLAP repair, knotless technology may prevent future cartilage or rotator cuff injury.
- Revision of SLAP repair is best handled with biceps tenodesis.
- Subpectoral biceps tenodesis avoids residual groove pain.
In recent decades, the long head of the biceps (LHB) tendon has been recognized as a pain generator in the shoulder of throwing athletes. The LHB muscle and its role in glenohumeral kinematics remains largely in question. The LHB tendon varies in size but most commonly is 5 mm to 6mm in diameter and about 9 cm in length, inserting on the superior labrum and supraglenoid tubercle after traveling through the bicipital groove.1 The many conditions that can develop along the course of the biceps tendon include overall biceps tendonitis, biceps tendon subluxation or instability, and injuries to the superior anterior to posterior area of the labrum.
These injuries can occur in young overhead athletes as well as manual laborers and older overhead recreational athletes. Pitching is the most common activity that leads to proximal biceps tendon disorders. The 6 phases of the pitch are linked in a kinetic chain that generates energy that is then translated to high velocity. The amount of force that is exerted on the shoulder during pitching and especially after ball release is impressive, and the athlete’s shoulder changes in many ways as it adapts to the motion.2-5 The late-cocking and deceleration phases are most commonly associated with proximal biceps pathology and the “peel-back” phenomenon. Other common activities that lead to biceps tendon issues in a young population are volleyball, baseball, tennis, softball, swimming, and cricket. Shoulder arthroscopies performed in older patients show degenerative biceps and labrum tears, which should be treated appropriately but perhaps different from how they are treated in overhead athletes.6-8 Further, many professional athletes have asymptomatic superior labrum anterior-posterior (SLAP) tears.9
Mechanism of Injury
Overhead throwing is commonly thought to be the mechanism by which lesions are created in the biceps–labrum complex (BLC). Pitching in particular generates incredible force and torque within the shoulder. In professional pitchers, the resulting throwing speed creates forces regularly in excess of 1000 N.3 These forces effect internal compensatory changes and internal derangement of the BLC. These changes often involve internal rotation deficits and alterations in the rotator cuff, which may contribute to glenohumeral instability and altered joint kinematics.10
Repetitive overhead activity is largely considered the mechanism of injury in this population, though more specific mechanisms have been described, including the peel-back mechanism11 and the posterior superior glenoid impingement. There is little evidence that preventive programs have any effect on decreasing the incidence of SLAP tears in overhead athletes.
Preoperative Evaluation
Preoperative evaluation is arguably the most important step in treating a patient with persistent or recurrent symptoms consistent with a SLAP tear. Evaluation includes thorough history, physical examination, and review of any prior injuries or surgical procedures. The physical examination should focus on maneuvers that define where the problem is occurring. Although SLAP tears are most common in this population, disorders of the biceps tendon within the groove, including inflammation and instability, should be ruled out with physical examination and advanced imaging. Palpation for groove tenderness, impingement-type complaints, internal rotation loss, and SLAP provocative testing are crucial in the diagnosis.12,13 The cause of symptoms may be multifactorial and include the often encountered concomitant pathology of rotator cuff tears, internal impingement, and instability.
Standard radiographs (Grashey anteroposterior, scapular/lateral, axillary lateral) and magnetic resonance imaging (MRI) with or without arthrography can be helpful in identifying and characterizing most SLAP tears as well as failed SLAP tear repairs. However, MRI is often positive for SLAP tears in asymptomatic patients, and diagnosing SLAP tears with MRI is often a challenge.14 MRI can help in determining concomitant pathology, including rotator cuff injury and cysts causing nerve compression. Correlation with clinical examination and patient history is most crucial. Conservative treatment (rest, activity modification, use of oral anti-inflammatory medications) typically is attempted and coordinated with respect to the athlete’s season of play.15,16
Classification
In overhead throwing athletes, SLAP tears typically are associated with anterior shoulder pain. Associated shoulder instability and significant glenohumeral dysfunction are not uncommon in athletes with lesions of the BLC. In 1985, Andrews and colleagues17 were the first to describe SLAP tears in overhead athletes (73 patients). Later, Snyder and colleagues18,19 further classified these lesions into 4 types based on tear stability and location, and they coined the acronym SLAP (Figure 1).
Type I lesions typically are described as fraying at the inner margin of the labrum and are common in throwers, even asymptomatic throwers. Type II lesions, separations of the biceps and labrum from the superior glenoid (≥5 mm of excursion), are the most commonly occurring and treated variant in throwing athletes.20-22 Intraoperative evaluation for a peel-back lesion (placing the arm in abduction with external rotation), rather than for a sulcus of 1 mm to 2 mm, may confirm a type II SLAP tear.20,23,24 It is often important to consider the direction of tear propagation as well. Type III lesions include those with an intact BLC (but with a bucket-handle tear of the superior labral complex and an intact biceps tendon), whereas type IV lesions involve additional extension of the tear into the biceps tendon.18,19The classification systems are well defined. Nevertheless, management of SLAP lesions remains controversial.
Options for Surgical Treatment
SLAP Tear Repair—Outcomes
The incidence of SLAP tear repairs has increased dramatically in recent years.6,25 There are various SLAP tear repair methods, but the most common consists of repairing the labrum and biceps anchor. Management of type II SLAP lesions remains controversial. Several prospective studies have found overall improvement after SLAP tear repair.26-31 Other series have reported less encouraging outcomes, including dissatisfaction with persistent pain and inability to return to throwing.28,32 A 2010 systematic review found that the percentage of patients who returned to their preinjury level of play was only 64%, and outcomes for overhead throwing athletes were even worse—only 22% to 60% of these patients returned to their previous level.33 The right surgery for SLAP tears in this population continues to be an area of uncertainty for many surgeons.
Failed SLAP tear repairs (poor outcomes) have become common in overhead throwing athletes. The reasons for these failed repairs are unclear, but several possible explanations have been offered. One is that labral repair may result in permanent alterations in pitching biomechanics, which may lead to an inability to regain velocity and command.3 Another is that the athlete’s shoulder may remain unstable even after repair.10Hardware complications are a significant concern in this high-level population. Suture anchor pullout or iatrogenic cartilage damage may occur during instrumentation or as a result of suture anchor reactive changes. In addition, there are several reports of glenoid osteochondrolysis (Figure 2) caused by prominent hardware or prominent knots.34-39
Stiffness after SLAP tear repair is a significant problem, with most patients taking up to 6 months to regain full motion.26,48 Overtensioning of the labrum and the glenohumeral ligaments may be the cause, and the solution may be to place anchors posterior (vs anterior) to the biceps insertion. In a large prospective military study, mean forward flexion and external rotation were reduced at final follow-up.31 These outcomes are less acceptable to overhead throwing athletes, who rely on motion for high-end throwing activities.
Primary Biceps Tenodesis—Outcomes
A 2015 database study found a 1.7-fold increase in biceps tenodesis over the preceding 5 years.49 However, relatively few procedures included in the study were performed in patients age younger than 30 years. For many older non-overhead throwers with type II tears, SLAP tear repair has become less popular as a treatment option.32 There is a dearth of knowledge about the outcomes of subpectoral biceps tenodesis as a primary treatment for biceps tendonitis and an associated SLAP tear. Although type I tears historically have been treated with débridement, débridement is seldom used for concomitant biceps tendonitis. It should be coupled with careful clinical examination.
In recent years, biceps tenodesis has been proposed as an alternative to repair for SLAP tears, particularly in older patients.24,44 For obvious reasons, however, there has been some trepidation about performing biceps tenodesis in throwing athletes. Some authors have proposed biceps tenodesis as primary treatment for isolated SLAP tears. Boileau and colleagues44 compared the outcomes of treatment of isolated type II SLAP lesions in 25 consecutive patients. For 10 patients, repair involved suture anchors; for the other 15, arthroscopic biceps tenodesis was performed with an absorbable interference screw. Six of the 10 suture anchor patients were disappointed with their outcome (persistent pain or inability to return to sport), whereas 14 of the 15 biceps tenodesis patients were satisfied. The authors concluded that arthroscopic biceps tenodesis is an effective alternative to repair for type II SLAP lesions, though their study was not isolated to overhead athletes (tenodesis group mean age, 52 years).
In a 2014 series of cases, Ek and colleagues50 reported good outcomes of SLAP tear repair and biceps tenodesis. Again, though, tenodesis was used in older patients, and repair in younger, more active patients, with no high-level athletes in either group. There was no difference in return to sport between groups. In a study of patients who underwent primary biceps tenodesis, Gupta and colleagues51 found 80% excellent outcomes (improved shoulder outcome scores) in select SLAP tear patients, including 8 athletes, 88% of whom were overhead athletes. Gottschalk and colleagues52 reported on differences in prospectively collected outcome data (age, sex, SLAP lesion type II or IV) for primary biceps tenodesis in a series of 33 patients. Twenty-six of the 29 patients who completed follow-up returned to their previous level of activity. These studies suggest that primary biceps tenodesis may be an alternative with lower failure rates in the treatment of SLAP tears in middle-aged patients, and in overhead athletes, though additional specific studies are needed to focus on overhead athletes on a larger scale.
Revision SLAP Tear Repair Versus Biceps Tenodesis
Failed arthroscopic SLAP tear repairs, which are increasingly common, present a unique treatment challenge. In a 2013 prospective cohort series, Gupta and colleagues46 found excellent clinical outcomes of subpectoral biceps tenodesis for failed type II SLAP tears. The authors reported a postoperative SANE (Single Assessment Numeric Evaluation) score of 70.4%, an SST (Simple Shoulder Test) score of 9.33, and an ASES (American Shoulder and Elbow Surgeons) score of 77.96, along with reasonable health-related quality-of-life scores. Werner and colleagues53 evaluated 2-year outcomes of biceps tenodesis performed after SLAP tear repair in 24 patients and found a return to almost normal range of motion as well as good clinical outcome scores. Significantly worse outcomes were found for patients with open worker’s compensation claims.
McCormick and colleagues26 prospectively evaluated the efficacy of biceps tenodesis for failed type II SLAP tear repair in 46 patients. Improvement was noted across all outcome assessments during follow-up (mean, 3.6 years). From these findings, we might conclude that biceps tenodesis is a more predictable option for failed SLAP tear repair, and that it has a relatively low complication rate. However, most investigators have used a heterogeneous patient population, as opposed to overhead athletes specifically. To our knowledge, no one has evaluated the specific population of overhead throwers with failed SLAP tear repairs. In addition, no one has conducted randomized controlled trials comparing débridement, biceps tenodesis, and repair for failed SLAP tear repairs.
Postoperative Considerations
When overhead athletes and their surgeons are considering surgical options, they must take rehabilitation and return to play into account. Many surgeons think the possible marginal clinical benefit of SLAP tear repair may not be worth the protracted rehabilitation. In most practices, rehabilitation after biceps tenodesis is less involved. Discussing the advantages and disadvantages of these 2 procedures can be helpful in decision making.
Dein and colleagues54 reported the case of a middle-aged pitcher who sustained a fracture after biceps tenodesis with an interference screw. Cases like this are concerning. Surgeons should consider altering the rehabilitation regimen when planning postoperative care in cases of biceps tenodesis in throwers. Other reported complications of open tenodesis are deep infection, thrombosis, postoperative stiffness, and nerve injury.55-58
Consequences for Overhead Throwers
The unknown role of the BLC leaves surgeons wary when considering biceps tenodesis for elite athletes. Some have postulated that removing the intra-articular portion of the LHB may cause microinstability and alter joint kinematics.10,59-61 Others have suggested the biceps is desynchronized from the other musculature and is not functionally important.62 Disruption of one portion of the superior labrum may result in instability on the opposite side of the glenoid.10,61 Biomechanical studies, both cadaveric and in vivo, have tried to create proper loads to the LHB and evaluate the kinematics of the shoulder before and after biceps tenodesis and SLAP tear repair.59,60 Using a cadaveric model, Strauss and colleagues63 found that type II SLAP lesions resulted in increased glenohumeral translation compared with baseline. Biceps tenodesis did not restore normal translation, but this did not negatively affect stability in the presence of a SLAP lesion. The consensus is that the role of the biceps is controversial at best.
Several studies have used electromyography (EMG) to evaluate LHB functioning. In 2014, Chalmers and colleagues59 used surface EMG and motion analysis to evaluate 18 pitchers: 6 underwent SLAP tear repair, 5 underwent biceps tenodesis, and 7 were uninjured controls. There were no significant differences in the activity of the LHB muscle, the short head of the biceps muscle, the deltoid, the infraspinatus, or the latissimus among the 3 groups. Motion analysis showed that the normal pattern of muscular activation within the LHB muscle was more closely restored by biceps tenodesis than by SLAP tear repair. In addition, thoracic rotation patterns were significantly more altered in the SLAP tear repair patients than in the uninjured controls. As the authors noted, given the low frequency with which biceps tenodesis is performed in overhead athletes, it is unlikely that larger scale studies will be conducted without a multicenter effort.
Recommendations and Our Preferred Technique
Which surgical option is best for treating symptomatic SLAP lesions in overhead athletes remains unclear. Many athletes struggle to return to high-level play after SLAP tear repair. Whether the same is true after biceps tenodesis is yet to be determined because of the low frequency with which biceps tenodesis is performed in high-level overhead athletes. The options for fixation, technique, and fixation location are equally broad. In this section, we outline our general line of thinking for cases of proximal biceps pathology.
In each case, we perform glenohumeral arthroscopy to evaluate the BLC and identify any other pathology. For overhead athletes who are younger than 30 years and lack bicipital groove pain or signs of gross tendinopathy, we favor arthroscopic SLAP tear repair. Repair is usually performed through an anterior working portal for suture passage and a Wilmington portal for anchor placement. We use knotless technology to achieve stable fixation and stay posterior to the biceps anchor insertion.
For the prevention of any potential pain from the bicipital groove in carefully selected patients—recreational overhead athletes and patients who want a less involved surgical recovery—we favor open subpectoral biceps tenodesis rather than arthroscopic tenodesis. The outcomes of biceps tenodesis are consistent, according to the literature.47,57,64 Moreover, the open approach is favored for the incidence of postoperative stiffness in the arthroscopic population.65 Tendons can be fixed with multiple procedures, including soft-tissue tenodesis, interference screw fixation, and surface anchors. We favor using a tenodesis screw in the subpectoral location, as outlined by Mazzocca and colleagues.64Our algorithm for SLAP lesions is evolving with our understanding of this complex disease process. For young overhead throwers with type II SLAP lesions, we favor arthroscopic SLAP tear repair with knotless technology. For older recreational overhead athletes, we favor biceps tenodesis in the subpectoral region after diagnostic arthroscopy plus biceps tenotomy with or without additional SLAP tear fixation, depending on the stability of the biceps anchor (Figures 4A, 4B).
Conclusion
Overhead athletes who present with symptomatic SLAP lesions often provide a treatment dilemma. Although SLAP tear repair historically has been standard treatment, biceps tenodesis represents a consistent surgical option with low complication rates and low revision rates. It is likely that, as additional data on glenohumeral kinematics and outcomes in young athletes become available, improved decision-making algorithms will follow.
Am J Orthop. 2017;46(1):E71-E78. Copyright Frontline Medical Communications Inc. 2016. All rights reserved.
1. Elser F, Braun S, Dewing CB, Giphart JE, Millett PJ. Anatomy, function, injuries, and treatment of the long head of the biceps brachii tendon. Arthroscopy. 2011;27(4):581-592.
2. Fedoriw WW, Ramkumar P, McCulloch PC, Lintner DM. Return to play after treatment of superior labral tears in professional baseball players. Am J Sports Med. 2014;42(5):1155-1160.
3. Fleisig GS, Andrews JR, Dillman CJ, Escamilla RF. Kinetics of baseball pitching with implications about injury mechanisms. Am J Sports Med. 1995;23(2):233-239.
4. Aydin N, Sirin E, Arya A. Superior labrum anterior to posterior lesions of the shoulder: diagnosis and arthroscopic management. World J Orthop. 2014;5(3):344-350.
5. Barber A, Field LD, Ryu R. Biceps tendon and superior labrum injuries: decision-marking. J Bone Joint Surg Am. 2007;89(8):1844-1855.
6. Onyekwelu I, Khatib O, Zuckerman JD, Rokito AS, Kwon YW. The rising incidence of arthroscopic superior labrum anterior and posterior (SLAP) repairs. J Shoulder Elbow Surg. 2012;21(6):728-731.
7. Patterson BM, Creighton RA, Spang JT, Roberson JR, Kamath GV. Surgical trends in the treatment of superior labrum anterior and posterior lesions of the shoulder: analysis of data from the American Board of Orthopaedic Surgery Certification Examination Database. Am J Sports Med. 2014;42(8):1904-1910.
8. Walton DM, Sadi J. Identifying SLAP lesions: a meta-analysis of clinical tests and exercise in clinical reasoning. Phys Ther Sport. 2008;9(4):167-176.
9. Lesniak BP, Baraga MG, Jose J, Smith MK, Cunningham S, Kaplan LD. Glenohumeral findings on magnetic resonance imaging correlate with innings pitched in asymptomatic pitchers. Am J Sports Med. 2013;41(9):2022-2027.
10. Mihata T, McGarry MH, Tibone JE, Fitzpatrick MJ, Kinoshita M, Lee TQ. Biomechanical assessment of type II superior labral anterior-posterior (SLAP) lesions associated with anterior shoulder capsular laxity as seen in throwers: a cadaveric study. Am J Sports Med. 2008;36(8):1604-1610.
11. Burkhart SS, Morgan CD, Kibler WB. The disabled throwing shoulder: spectrum of pathology. Part II: evaluation and treatment of SLAP lesions in throwers. Arthroscopy. 2003;19(5):531-539.
12. Meserve BB, Cleland JA, Boucher TR. A meta-analysis examining clinical test utility for assessing superior labral anterior posterior lesions. Am J Sports Med. 2009;37(11):2252-2258.
13. Pandya NK, Colton A, Webner D, Sennett B, Huffman GR. Physical examination and magnetic resonance imaging in the diagnosis of superior labrum anterior-posterior lesions of the shoulder: a sensitivity analysis. Arthroscopy. 2008;24(3):311-317.
14. Amin MF, Youssef AO. The diagnostic value of magnetic resonance arthrography of the shoulder in detection and grading of SLAP lesions: comparison with arthroscopic findings. Eur J Radiol. 2012;81(9):2343-2347.
15. Cook C, Beaty S, Kissenberth MJ, Siffri P, Pill SG, Hawkins RJ. Diagnostic accuracy of five orthopedic clinical tests for diagnosis of superior labrum anterior posterior (SLAP) lesions. J Shoulder Elbow Surg. 2012;21(1):13-22.
16. Edwards SL, Lee JA, Bell JE, et al. Nonoperative treatment of superior labrum anterior posterior tears: improvements in pain, function, and quality of life. Am J Sports Med. 2010;38(7):1456-1461.
17. Andrews JR, Carson WG Jr, McLeod WD. Glenoid labrum tears related to the long head of the biceps. Am J Sports Med. 1985;13(5):337-341.
18. Snyder SJ, Karzel RP, Del Pizzo W, Ferkel RD, Friedman MJ. SLAP lesions of the shoulder. Arthroscopy. 1990;6(4):274-279.
19. Snyder SJ, Banas MP, Karzel RP. An analysis of 140 injuries to the superior glenoid labrum. J Shoulder Elbow Surg. 1995;4(4):243-248.
20. Morgan CD, Burkhart SS, Palmeri M, Gillespie M. Type II SLAP lesions: three subtypes and their relationships to superior instability and rotator cuff tears. Arthroscopy. 1998;14(6):553-565.
21. Weber SC, Martin DF, Seiler JG 3rd, Harrast JJ. Superior labrum anterior and posterior lesions of the shoulder: incidence rates, complications, and outcomes as reported by American Board of Orthopedic Surgery. Part II candidates. Am J Sports Med. 2012;40(7):1538-1543.
22. Keener JD, Brophy RH. Superior labral tears of the shoulder: pathogenesis, evaluation, and treatment. J Am Acad Orthop Surg. 2009;17(10):627-637.
23. Chen CH, Hsu KY, Chen WJ, Shih CH. Incidence and severity of biceps long head tendon lesion in patients with complete rotator cuff tears. J Trauma. 2005;58(6):1189-1193.
24. Nho SJ, Strauss EJ, Lenart BA, et al. Long head of the biceps tendinopathy: diagnosis and management. J Am Acad Orthop Surg. 2010;18(11):645-656.
25. Zhang AL, Kreulen C, Ngo SS, Hame SL, Wang JC, Gamradt SC. Demographic trends in arthroscopic SLAP repair in the United States. Am J Sports Med. 2012;40(5):1144-1147.
26. McCormick F, Bhatia S, Chalmers P, Gupta A, Verma N, Romeo AA. The management of type II superior labral anterior to posterior injuries. Orthop Clin North Am. 2014;45(1):121-128.
27. Brockmeier SF, Voos JE, Williams RJ 3rd, Altchek DW, Cordasco FA, Allen AA; Hospital for Special Surgery Sports Medicine and Shoulder Service. Outcomes after arthroscopic repair of type-II SLAP lesions. J Bone Joint Surg Am. 2009;91(7):1595-1603.
28. Boileau P, Parratte S, Chuinard C, Roussanne Y, Shia D, Bicknell R. Arthroscopic treatment of isolated type II SLAP lesions: biceps tenodesis as an alternative to reinsertion. Am J Sports Med. 2009;37(5):929-936.
29. Denard PJ, Lädermann A, Burkhart SS. Long-term outcome after arthroscopic repair of type II SLAP lesions: results according to age and workers’ compensation status. Arthroscopy. 2012;28(4):451-457.
30. Friel NA, Karas V, Slabaugh MA, Cole BJ. Outcomes of type II superior labrum, anterior to posterior (SLAP) repair: prospective evaluation at a minimum two-year follow-up. J Shoulder Elbow Surg. 2010;19(6):859-867.
31. Provencher MT, McCormick F, Dewing C, McIntire S, Solomon D. A prospective analysis of 179 type 2 superior labrum anterior and posterior repairs: outcomes and factors associated with success and failure. Am J Sports Med. 2013;41(4):880-886.
32. Gupta AK, Chalmers PN, Klosterman EL, et al. Subpectoral biceps tenodesis for bicipital tendonitis with SLAP tear. Orthopedics. 2015;38(1):e48-e53.
33. Gorantla K, Gill C, Wright RW. The outcome of type II SLAP repair: a systematic review. Arthroscopy. 2010;26(4):537-545.
34. Katz LM, Hsu S, Miller SL, et al. Poor outcomes after SLAP repair: descriptive analysis and prognosis. Arthroscopy. 2009;25(8):849-855.
35. Park MJ, Hsu JE, Harper C, Sennett BJ, Huffman GR. Poly-L/D-lactic acid anchors are associated with reoperation and failure of SLAP repairs. Arthroscopy. 2011;27(10):1335-1340.
36. Sassmannshausen G, Sukay M, Mair SD. Broken or dislodged poly-L-lactic acid bioabsorbable tacks in patients after SLAP lesion surgery. Arthroscopy. 2006;22(6):615-619.
37. Uggen C, Wei A, Glousman RE, et al. Biomechanical comparison of knotless anchor repair versus simple suture repair for type II SLAP lesions. Arthroscopy. 2009;25(10):1085-1092.
38. Weber SC. Surgical management of the failed SLAP repair. Sports Med Arthrosc. 2010;18(3):162-166.
39. Wilkerson JP, Zvijac JE, Uribe JW, Schürhoff MR, Green JB. Failure of polymerized lactic acid tacks in shoulder surgery. J Shoulder Elbow Surg. 2003;12(2):117-121.
40. Weber S. Surgical management of the failed SLAP lesion. Arthroscopy. 2008;24(suppl):e8-e9.
41. Schrøder CP, Skare O, Gjengedal E, Uppheim G, Reikerås O, Brox JI. Long-term results after SLAP repair: a 5-year follow-up study of 107 patients with comparison of patients aged over and under 40 years. Arthroscopy. 2012;28(11):1601-1607.
42. Mazzocca AD, Cote MP, Arciero CL, Romeo AA, Arciero RA. Clinical outcomes after subpectoral biceps tenodesis with an interference screw. Am J Sports Med. 2008;36(10):1922-1929.
43. Mazzocca AD, McCarthy MB, Ledgard FA, et al. Histomorphologic changes of the long head of the biceps tendon in common shoulder pathologies. Arthroscopy. 2013;29(6):972-981.
44. Boileau P, Parratte S, Chuinard C, Roussanne Y, Shia D, Bicknell R. Arthroscopic treatment of isolated type II SLAP lesions: biceps tenodesis as an alternative to reinsertion. Am J Sports Med. 2009;37(5):929-936.
45. Boileau P, Krishnan SG, Coste JS, Walch G. Arthroscopic biceps tenodesis: a new technique using bioabsorbable interference screw fixation. Arthroscopy. 2002;18(9):1002-1012.
46. Gupta AK, Bruce B, Klosterman EL, McCormick F, Harris J, Romeo AA. Subpectoral biceps tenodesis for failed type II SLAP repair. Orthopedics. 2013;36(6):e723-e728.
47. Provencher MT, LeClere LE, Romeo AA. Subpectoral biceps tenodesis. Sports Med Arthrosc. 2008;16(3):170-176.
48. McCarty LP 3rd, Buss DD, Datta MW, Freehill MQ, Giveans MR. Complications observed following labral or rotator cuff repair with use of poly-L-lactic acid implants. J Bone Joint Surg Am. 2013;95(6):507-511.
49. Werner BC, Brockmeier SF, Gwathmey FW. Trends in long head biceps tenodesis. Am J Sports Med. 2015;43(3):570-578.
50. Ek ET, Shi LL, Tompson JD, Freehill MT, Warner JJ. Surgical treatment of isolated type II superior labrum anterior-posterior (SLAP) lesions: repair versus biceps tenodesis. J Shoulder Elbow Surg. 2014;23(7):1059-1065.
51. Gupta AK, Chalmers PN, Klosterman EL, et al. Subpectoral biceps tenodesis for bicipital tendonitis with SLAP tear. Orthopedics. 2015;38(1):e48-e53.
52. Gottschalk MB, Karas SG, Ghattas TN, Burdette R. Subpectoral biceps tenodesis for the treatment of type II and IV superior labral anterior and posterior lesions. Am J Sports Med. 2014;42(9):2128-2135.
53. Werner BC, Pehlivan HC, Hart JM, et al. Biceps tenodesis is a viable option for salvage of failed SLAP repair. J Shoulder Elbow Surg. 2014;23(8):e179-e184.
54. Dein EJ, Huri G, Gordon JC, McFarland EG. A humerus fracture in a baseball pitcher after biceps tenodesis [published correction appears in Am J Sports Med. 2014;42(6):NP39]. Am J Sports Med. 2014;42(4):877-879.
55. Nho SJ, Reiff SN, Verma NN, Slabaugh MA, Mazzocca AD, Romeo AA. Complications associated with subpectoral biceps tenodesis: low rates of incidence following surgery. J Shoulder Elbow Surg. 2010;19(5):764-768.
56. Osbahr DC, Diamond AB, Speer KP. The cosmetic appearance of the biceps muscle after long-head tenotomy versus tenodesis. Arthroscopy. 2002;18(5):483-487.
57. Romeo AA, Mazzocca AD, Tauro JC. Arthroscopic biceps tenodesis. Arthroscopy. 2004;20(2):206-213.
58. Ma H, Van Heest A, Glisson C, Patel S. Musculocutaneous nerve entrapment: an unusual complication after biceps tenodesis. Am J Sports Med. 2009;37(12):2467-2469.
59. Chalmers PN, Trombley R, Cip J, et al. Postoperative restoration of upper extremity motion and neuromuscular control during the overhand pitch: evaluation of tenodesis and repair for superior labral anterior-posterior tears. Am J Sports Med. 2014;42(12):2825-2836.
60. Giphart JE, Elser F, Dewing CB, Torry MR, Millett PJ. The long head of the biceps tendon has minimal effect on in vivo glenohumeral kinematics: a biplane fluoroscopy study. Am J Sports Med. 2012;40(1):202-212.
61. Grossman MG, Tibone JE, McGarry MH, Schneider DJ, Veneziani S, Lee TQ. A cadaveric model of the throwing shoulder: a possible etiology of superior labrum anterior-to-posterior lesions. J Bone Joint Surg Am. 2005;87(4):824-831.
62. Hawkes DH, Alizadehkhaiyat O, Fisher AC, Kemp GJ, Roebuck MM, Frostick SP. Normal shoulder muscular activation and co-ordination during a shoulder elevation task based on activities of daily living: an electromyographic study. J Orthop Res. 2012;30(1):53-60.
63. Strauss EJ, Salata MJ, Sershon RA, et al. Role of the superior labrum after biceps tenodesis in glenohumeral stability. J Shoulder Elbow Surg. 2014;23(4):485-491.
64. Mazzocca AD, Bicos J, Santangelo S, Romeo AA, Arciero RA. The biomechanical evaluation of four fixation techniques for proximal biceps tenodesis. Arthroscopy. 2005;21(11):1296-1306.
65. Werner BC, Pehlivan HC, Hart JM, et al. Increased incidence of postoperative stiffness after arthroscopic compared with open biceps tenodesis. Arthroscopy. 2014;30(9):1075-1084.
66. Denard PJ, Dai X, Hanypsiak BT, Burkhart SS. Anatomy of the biceps tendon: implications for restoring physiological length–tension relation during biceps tenodesis with interference screw fixation. Arthroscopy. 2012;28(10):1352-1358.
67. Mazzocca AD, Rios CG, Romeo AA, Arciero RA. Subpectoral biceps tenodesis with interference screw fixation. Arthroscopy. 2005;21(7):896.
Take Home Points
- Outcomes after SLAP repair remain guarded.
- Physical examination is key in determining proper management of biceps pathology.
- When performing SLAP repair, knotless technology may prevent future cartilage or rotator cuff injury.
- Revision of SLAP repair is best handled with biceps tenodesis.
- Subpectoral biceps tenodesis avoids residual groove pain.
In recent decades, the long head of the biceps (LHB) tendon has been recognized as a pain generator in the shoulder of throwing athletes. The LHB muscle and its role in glenohumeral kinematics remains largely in question. The LHB tendon varies in size but most commonly is 5 mm to 6mm in diameter and about 9 cm in length, inserting on the superior labrum and supraglenoid tubercle after traveling through the bicipital groove.1 The many conditions that can develop along the course of the biceps tendon include overall biceps tendonitis, biceps tendon subluxation or instability, and injuries to the superior anterior to posterior area of the labrum.
These injuries can occur in young overhead athletes as well as manual laborers and older overhead recreational athletes. Pitching is the most common activity that leads to proximal biceps tendon disorders. The 6 phases of the pitch are linked in a kinetic chain that generates energy that is then translated to high velocity. The amount of force that is exerted on the shoulder during pitching and especially after ball release is impressive, and the athlete’s shoulder changes in many ways as it adapts to the motion.2-5 The late-cocking and deceleration phases are most commonly associated with proximal biceps pathology and the “peel-back” phenomenon. Other common activities that lead to biceps tendon issues in a young population are volleyball, baseball, tennis, softball, swimming, and cricket. Shoulder arthroscopies performed in older patients show degenerative biceps and labrum tears, which should be treated appropriately but perhaps different from how they are treated in overhead athletes.6-8 Further, many professional athletes have asymptomatic superior labrum anterior-posterior (SLAP) tears.9
Mechanism of Injury
Overhead throwing is commonly thought to be the mechanism by which lesions are created in the biceps–labrum complex (BLC). Pitching in particular generates incredible force and torque within the shoulder. In professional pitchers, the resulting throwing speed creates forces regularly in excess of 1000 N.3 These forces effect internal compensatory changes and internal derangement of the BLC. These changes often involve internal rotation deficits and alterations in the rotator cuff, which may contribute to glenohumeral instability and altered joint kinematics.10
Repetitive overhead activity is largely considered the mechanism of injury in this population, though more specific mechanisms have been described, including the peel-back mechanism11 and the posterior superior glenoid impingement. There is little evidence that preventive programs have any effect on decreasing the incidence of SLAP tears in overhead athletes.
Preoperative Evaluation
Preoperative evaluation is arguably the most important step in treating a patient with persistent or recurrent symptoms consistent with a SLAP tear. Evaluation includes thorough history, physical examination, and review of any prior injuries or surgical procedures. The physical examination should focus on maneuvers that define where the problem is occurring. Although SLAP tears are most common in this population, disorders of the biceps tendon within the groove, including inflammation and instability, should be ruled out with physical examination and advanced imaging. Palpation for groove tenderness, impingement-type complaints, internal rotation loss, and SLAP provocative testing are crucial in the diagnosis.12,13 The cause of symptoms may be multifactorial and include the often encountered concomitant pathology of rotator cuff tears, internal impingement, and instability.
Standard radiographs (Grashey anteroposterior, scapular/lateral, axillary lateral) and magnetic resonance imaging (MRI) with or without arthrography can be helpful in identifying and characterizing most SLAP tears as well as failed SLAP tear repairs. However, MRI is often positive for SLAP tears in asymptomatic patients, and diagnosing SLAP tears with MRI is often a challenge.14 MRI can help in determining concomitant pathology, including rotator cuff injury and cysts causing nerve compression. Correlation with clinical examination and patient history is most crucial. Conservative treatment (rest, activity modification, use of oral anti-inflammatory medications) typically is attempted and coordinated with respect to the athlete’s season of play.15,16
Classification
In overhead throwing athletes, SLAP tears typically are associated with anterior shoulder pain. Associated shoulder instability and significant glenohumeral dysfunction are not uncommon in athletes with lesions of the BLC. In 1985, Andrews and colleagues17 were the first to describe SLAP tears in overhead athletes (73 patients). Later, Snyder and colleagues18,19 further classified these lesions into 4 types based on tear stability and location, and they coined the acronym SLAP (Figure 1).
Type I lesions typically are described as fraying at the inner margin of the labrum and are common in throwers, even asymptomatic throwers. Type II lesions, separations of the biceps and labrum from the superior glenoid (≥5 mm of excursion), are the most commonly occurring and treated variant in throwing athletes.20-22 Intraoperative evaluation for a peel-back lesion (placing the arm in abduction with external rotation), rather than for a sulcus of 1 mm to 2 mm, may confirm a type II SLAP tear.20,23,24 It is often important to consider the direction of tear propagation as well. Type III lesions include those with an intact BLC (but with a bucket-handle tear of the superior labral complex and an intact biceps tendon), whereas type IV lesions involve additional extension of the tear into the biceps tendon.18,19The classification systems are well defined. Nevertheless, management of SLAP lesions remains controversial.
Options for Surgical Treatment
SLAP Tear Repair—Outcomes
The incidence of SLAP tear repairs has increased dramatically in recent years.6,25 There are various SLAP tear repair methods, but the most common consists of repairing the labrum and biceps anchor. Management of type II SLAP lesions remains controversial. Several prospective studies have found overall improvement after SLAP tear repair.26-31 Other series have reported less encouraging outcomes, including dissatisfaction with persistent pain and inability to return to throwing.28,32 A 2010 systematic review found that the percentage of patients who returned to their preinjury level of play was only 64%, and outcomes for overhead throwing athletes were even worse—only 22% to 60% of these patients returned to their previous level.33 The right surgery for SLAP tears in this population continues to be an area of uncertainty for many surgeons.
Failed SLAP tear repairs (poor outcomes) have become common in overhead throwing athletes. The reasons for these failed repairs are unclear, but several possible explanations have been offered. One is that labral repair may result in permanent alterations in pitching biomechanics, which may lead to an inability to regain velocity and command.3 Another is that the athlete’s shoulder may remain unstable even after repair.10Hardware complications are a significant concern in this high-level population. Suture anchor pullout or iatrogenic cartilage damage may occur during instrumentation or as a result of suture anchor reactive changes. In addition, there are several reports of glenoid osteochondrolysis (Figure 2) caused by prominent hardware or prominent knots.34-39
Stiffness after SLAP tear repair is a significant problem, with most patients taking up to 6 months to regain full motion.26,48 Overtensioning of the labrum and the glenohumeral ligaments may be the cause, and the solution may be to place anchors posterior (vs anterior) to the biceps insertion. In a large prospective military study, mean forward flexion and external rotation were reduced at final follow-up.31 These outcomes are less acceptable to overhead throwing athletes, who rely on motion for high-end throwing activities.
Primary Biceps Tenodesis—Outcomes
A 2015 database study found a 1.7-fold increase in biceps tenodesis over the preceding 5 years.49 However, relatively few procedures included in the study were performed in patients age younger than 30 years. For many older non-overhead throwers with type II tears, SLAP tear repair has become less popular as a treatment option.32 There is a dearth of knowledge about the outcomes of subpectoral biceps tenodesis as a primary treatment for biceps tendonitis and an associated SLAP tear. Although type I tears historically have been treated with débridement, débridement is seldom used for concomitant biceps tendonitis. It should be coupled with careful clinical examination.
In recent years, biceps tenodesis has been proposed as an alternative to repair for SLAP tears, particularly in older patients.24,44 For obvious reasons, however, there has been some trepidation about performing biceps tenodesis in throwing athletes. Some authors have proposed biceps tenodesis as primary treatment for isolated SLAP tears. Boileau and colleagues44 compared the outcomes of treatment of isolated type II SLAP lesions in 25 consecutive patients. For 10 patients, repair involved suture anchors; for the other 15, arthroscopic biceps tenodesis was performed with an absorbable interference screw. Six of the 10 suture anchor patients were disappointed with their outcome (persistent pain or inability to return to sport), whereas 14 of the 15 biceps tenodesis patients were satisfied. The authors concluded that arthroscopic biceps tenodesis is an effective alternative to repair for type II SLAP lesions, though their study was not isolated to overhead athletes (tenodesis group mean age, 52 years).
In a 2014 series of cases, Ek and colleagues50 reported good outcomes of SLAP tear repair and biceps tenodesis. Again, though, tenodesis was used in older patients, and repair in younger, more active patients, with no high-level athletes in either group. There was no difference in return to sport between groups. In a study of patients who underwent primary biceps tenodesis, Gupta and colleagues51 found 80% excellent outcomes (improved shoulder outcome scores) in select SLAP tear patients, including 8 athletes, 88% of whom were overhead athletes. Gottschalk and colleagues52 reported on differences in prospectively collected outcome data (age, sex, SLAP lesion type II or IV) for primary biceps tenodesis in a series of 33 patients. Twenty-six of the 29 patients who completed follow-up returned to their previous level of activity. These studies suggest that primary biceps tenodesis may be an alternative with lower failure rates in the treatment of SLAP tears in middle-aged patients, and in overhead athletes, though additional specific studies are needed to focus on overhead athletes on a larger scale.
Revision SLAP Tear Repair Versus Biceps Tenodesis
Failed arthroscopic SLAP tear repairs, which are increasingly common, present a unique treatment challenge. In a 2013 prospective cohort series, Gupta and colleagues46 found excellent clinical outcomes of subpectoral biceps tenodesis for failed type II SLAP tears. The authors reported a postoperative SANE (Single Assessment Numeric Evaluation) score of 70.4%, an SST (Simple Shoulder Test) score of 9.33, and an ASES (American Shoulder and Elbow Surgeons) score of 77.96, along with reasonable health-related quality-of-life scores. Werner and colleagues53 evaluated 2-year outcomes of biceps tenodesis performed after SLAP tear repair in 24 patients and found a return to almost normal range of motion as well as good clinical outcome scores. Significantly worse outcomes were found for patients with open worker’s compensation claims.
McCormick and colleagues26 prospectively evaluated the efficacy of biceps tenodesis for failed type II SLAP tear repair in 46 patients. Improvement was noted across all outcome assessments during follow-up (mean, 3.6 years). From these findings, we might conclude that biceps tenodesis is a more predictable option for failed SLAP tear repair, and that it has a relatively low complication rate. However, most investigators have used a heterogeneous patient population, as opposed to overhead athletes specifically. To our knowledge, no one has evaluated the specific population of overhead throwers with failed SLAP tear repairs. In addition, no one has conducted randomized controlled trials comparing débridement, biceps tenodesis, and repair for failed SLAP tear repairs.
Postoperative Considerations
When overhead athletes and their surgeons are considering surgical options, they must take rehabilitation and return to play into account. Many surgeons think the possible marginal clinical benefit of SLAP tear repair may not be worth the protracted rehabilitation. In most practices, rehabilitation after biceps tenodesis is less involved. Discussing the advantages and disadvantages of these 2 procedures can be helpful in decision making.
Dein and colleagues54 reported the case of a middle-aged pitcher who sustained a fracture after biceps tenodesis with an interference screw. Cases like this are concerning. Surgeons should consider altering the rehabilitation regimen when planning postoperative care in cases of biceps tenodesis in throwers. Other reported complications of open tenodesis are deep infection, thrombosis, postoperative stiffness, and nerve injury.55-58
Consequences for Overhead Throwers
The unknown role of the BLC leaves surgeons wary when considering biceps tenodesis for elite athletes. Some have postulated that removing the intra-articular portion of the LHB may cause microinstability and alter joint kinematics.10,59-61 Others have suggested the biceps is desynchronized from the other musculature and is not functionally important.62 Disruption of one portion of the superior labrum may result in instability on the opposite side of the glenoid.10,61 Biomechanical studies, both cadaveric and in vivo, have tried to create proper loads to the LHB and evaluate the kinematics of the shoulder before and after biceps tenodesis and SLAP tear repair.59,60 Using a cadaveric model, Strauss and colleagues63 found that type II SLAP lesions resulted in increased glenohumeral translation compared with baseline. Biceps tenodesis did not restore normal translation, but this did not negatively affect stability in the presence of a SLAP lesion. The consensus is that the role of the biceps is controversial at best.
Several studies have used electromyography (EMG) to evaluate LHB functioning. In 2014, Chalmers and colleagues59 used surface EMG and motion analysis to evaluate 18 pitchers: 6 underwent SLAP tear repair, 5 underwent biceps tenodesis, and 7 were uninjured controls. There were no significant differences in the activity of the LHB muscle, the short head of the biceps muscle, the deltoid, the infraspinatus, or the latissimus among the 3 groups. Motion analysis showed that the normal pattern of muscular activation within the LHB muscle was more closely restored by biceps tenodesis than by SLAP tear repair. In addition, thoracic rotation patterns were significantly more altered in the SLAP tear repair patients than in the uninjured controls. As the authors noted, given the low frequency with which biceps tenodesis is performed in overhead athletes, it is unlikely that larger scale studies will be conducted without a multicenter effort.
Recommendations and Our Preferred Technique
Which surgical option is best for treating symptomatic SLAP lesions in overhead athletes remains unclear. Many athletes struggle to return to high-level play after SLAP tear repair. Whether the same is true after biceps tenodesis is yet to be determined because of the low frequency with which biceps tenodesis is performed in high-level overhead athletes. The options for fixation, technique, and fixation location are equally broad. In this section, we outline our general line of thinking for cases of proximal biceps pathology.
In each case, we perform glenohumeral arthroscopy to evaluate the BLC and identify any other pathology. For overhead athletes who are younger than 30 years and lack bicipital groove pain or signs of gross tendinopathy, we favor arthroscopic SLAP tear repair. Repair is usually performed through an anterior working portal for suture passage and a Wilmington portal for anchor placement. We use knotless technology to achieve stable fixation and stay posterior to the biceps anchor insertion.
For the prevention of any potential pain from the bicipital groove in carefully selected patients—recreational overhead athletes and patients who want a less involved surgical recovery—we favor open subpectoral biceps tenodesis rather than arthroscopic tenodesis. The outcomes of biceps tenodesis are consistent, according to the literature.47,57,64 Moreover, the open approach is favored for the incidence of postoperative stiffness in the arthroscopic population.65 Tendons can be fixed with multiple procedures, including soft-tissue tenodesis, interference screw fixation, and surface anchors. We favor using a tenodesis screw in the subpectoral location, as outlined by Mazzocca and colleagues.64Our algorithm for SLAP lesions is evolving with our understanding of this complex disease process. For young overhead throwers with type II SLAP lesions, we favor arthroscopic SLAP tear repair with knotless technology. For older recreational overhead athletes, we favor biceps tenodesis in the subpectoral region after diagnostic arthroscopy plus biceps tenotomy with or without additional SLAP tear fixation, depending on the stability of the biceps anchor (Figures 4A, 4B).
Conclusion
Overhead athletes who present with symptomatic SLAP lesions often provide a treatment dilemma. Although SLAP tear repair historically has been standard treatment, biceps tenodesis represents a consistent surgical option with low complication rates and low revision rates. It is likely that, as additional data on glenohumeral kinematics and outcomes in young athletes become available, improved decision-making algorithms will follow.
Am J Orthop. 2017;46(1):E71-E78. Copyright Frontline Medical Communications Inc. 2016. All rights reserved.
Take Home Points
- Outcomes after SLAP repair remain guarded.
- Physical examination is key in determining proper management of biceps pathology.
- When performing SLAP repair, knotless technology may prevent future cartilage or rotator cuff injury.
- Revision of SLAP repair is best handled with biceps tenodesis.
- Subpectoral biceps tenodesis avoids residual groove pain.
In recent decades, the long head of the biceps (LHB) tendon has been recognized as a pain generator in the shoulder of throwing athletes. The LHB muscle and its role in glenohumeral kinematics remains largely in question. The LHB tendon varies in size but most commonly is 5 mm to 6mm in diameter and about 9 cm in length, inserting on the superior labrum and supraglenoid tubercle after traveling through the bicipital groove.1 The many conditions that can develop along the course of the biceps tendon include overall biceps tendonitis, biceps tendon subluxation or instability, and injuries to the superior anterior to posterior area of the labrum.
These injuries can occur in young overhead athletes as well as manual laborers and older overhead recreational athletes. Pitching is the most common activity that leads to proximal biceps tendon disorders. The 6 phases of the pitch are linked in a kinetic chain that generates energy that is then translated to high velocity. The amount of force that is exerted on the shoulder during pitching and especially after ball release is impressive, and the athlete’s shoulder changes in many ways as it adapts to the motion.2-5 The late-cocking and deceleration phases are most commonly associated with proximal biceps pathology and the “peel-back” phenomenon. Other common activities that lead to biceps tendon issues in a young population are volleyball, baseball, tennis, softball, swimming, and cricket. Shoulder arthroscopies performed in older patients show degenerative biceps and labrum tears, which should be treated appropriately but perhaps different from how they are treated in overhead athletes.6-8 Further, many professional athletes have asymptomatic superior labrum anterior-posterior (SLAP) tears.9
Mechanism of Injury
Overhead throwing is commonly thought to be the mechanism by which lesions are created in the biceps–labrum complex (BLC). Pitching in particular generates incredible force and torque within the shoulder. In professional pitchers, the resulting throwing speed creates forces regularly in excess of 1000 N.3 These forces effect internal compensatory changes and internal derangement of the BLC. These changes often involve internal rotation deficits and alterations in the rotator cuff, which may contribute to glenohumeral instability and altered joint kinematics.10
Repetitive overhead activity is largely considered the mechanism of injury in this population, though more specific mechanisms have been described, including the peel-back mechanism11 and the posterior superior glenoid impingement. There is little evidence that preventive programs have any effect on decreasing the incidence of SLAP tears in overhead athletes.
Preoperative Evaluation
Preoperative evaluation is arguably the most important step in treating a patient with persistent or recurrent symptoms consistent with a SLAP tear. Evaluation includes thorough history, physical examination, and review of any prior injuries or surgical procedures. The physical examination should focus on maneuvers that define where the problem is occurring. Although SLAP tears are most common in this population, disorders of the biceps tendon within the groove, including inflammation and instability, should be ruled out with physical examination and advanced imaging. Palpation for groove tenderness, impingement-type complaints, internal rotation loss, and SLAP provocative testing are crucial in the diagnosis.12,13 The cause of symptoms may be multifactorial and include the often encountered concomitant pathology of rotator cuff tears, internal impingement, and instability.
Standard radiographs (Grashey anteroposterior, scapular/lateral, axillary lateral) and magnetic resonance imaging (MRI) with or without arthrography can be helpful in identifying and characterizing most SLAP tears as well as failed SLAP tear repairs. However, MRI is often positive for SLAP tears in asymptomatic patients, and diagnosing SLAP tears with MRI is often a challenge.14 MRI can help in determining concomitant pathology, including rotator cuff injury and cysts causing nerve compression. Correlation with clinical examination and patient history is most crucial. Conservative treatment (rest, activity modification, use of oral anti-inflammatory medications) typically is attempted and coordinated with respect to the athlete’s season of play.15,16
Classification
In overhead throwing athletes, SLAP tears typically are associated with anterior shoulder pain. Associated shoulder instability and significant glenohumeral dysfunction are not uncommon in athletes with lesions of the BLC. In 1985, Andrews and colleagues17 were the first to describe SLAP tears in overhead athletes (73 patients). Later, Snyder and colleagues18,19 further classified these lesions into 4 types based on tear stability and location, and they coined the acronym SLAP (Figure 1).
Type I lesions typically are described as fraying at the inner margin of the labrum and are common in throwers, even asymptomatic throwers. Type II lesions, separations of the biceps and labrum from the superior glenoid (≥5 mm of excursion), are the most commonly occurring and treated variant in throwing athletes.20-22 Intraoperative evaluation for a peel-back lesion (placing the arm in abduction with external rotation), rather than for a sulcus of 1 mm to 2 mm, may confirm a type II SLAP tear.20,23,24 It is often important to consider the direction of tear propagation as well. Type III lesions include those with an intact BLC (but with a bucket-handle tear of the superior labral complex and an intact biceps tendon), whereas type IV lesions involve additional extension of the tear into the biceps tendon.18,19The classification systems are well defined. Nevertheless, management of SLAP lesions remains controversial.
Options for Surgical Treatment
SLAP Tear Repair—Outcomes
The incidence of SLAP tear repairs has increased dramatically in recent years.6,25 There are various SLAP tear repair methods, but the most common consists of repairing the labrum and biceps anchor. Management of type II SLAP lesions remains controversial. Several prospective studies have found overall improvement after SLAP tear repair.26-31 Other series have reported less encouraging outcomes, including dissatisfaction with persistent pain and inability to return to throwing.28,32 A 2010 systematic review found that the percentage of patients who returned to their preinjury level of play was only 64%, and outcomes for overhead throwing athletes were even worse—only 22% to 60% of these patients returned to their previous level.33 The right surgery for SLAP tears in this population continues to be an area of uncertainty for many surgeons.
Failed SLAP tear repairs (poor outcomes) have become common in overhead throwing athletes. The reasons for these failed repairs are unclear, but several possible explanations have been offered. One is that labral repair may result in permanent alterations in pitching biomechanics, which may lead to an inability to regain velocity and command.3 Another is that the athlete’s shoulder may remain unstable even after repair.10Hardware complications are a significant concern in this high-level population. Suture anchor pullout or iatrogenic cartilage damage may occur during instrumentation or as a result of suture anchor reactive changes. In addition, there are several reports of glenoid osteochondrolysis (Figure 2) caused by prominent hardware or prominent knots.34-39
Stiffness after SLAP tear repair is a significant problem, with most patients taking up to 6 months to regain full motion.26,48 Overtensioning of the labrum and the glenohumeral ligaments may be the cause, and the solution may be to place anchors posterior (vs anterior) to the biceps insertion. In a large prospective military study, mean forward flexion and external rotation were reduced at final follow-up.31 These outcomes are less acceptable to overhead throwing athletes, who rely on motion for high-end throwing activities.
Primary Biceps Tenodesis—Outcomes
A 2015 database study found a 1.7-fold increase in biceps tenodesis over the preceding 5 years.49 However, relatively few procedures included in the study were performed in patients age younger than 30 years. For many older non-overhead throwers with type II tears, SLAP tear repair has become less popular as a treatment option.32 There is a dearth of knowledge about the outcomes of subpectoral biceps tenodesis as a primary treatment for biceps tendonitis and an associated SLAP tear. Although type I tears historically have been treated with débridement, débridement is seldom used for concomitant biceps tendonitis. It should be coupled with careful clinical examination.
In recent years, biceps tenodesis has been proposed as an alternative to repair for SLAP tears, particularly in older patients.24,44 For obvious reasons, however, there has been some trepidation about performing biceps tenodesis in throwing athletes. Some authors have proposed biceps tenodesis as primary treatment for isolated SLAP tears. Boileau and colleagues44 compared the outcomes of treatment of isolated type II SLAP lesions in 25 consecutive patients. For 10 patients, repair involved suture anchors; for the other 15, arthroscopic biceps tenodesis was performed with an absorbable interference screw. Six of the 10 suture anchor patients were disappointed with their outcome (persistent pain or inability to return to sport), whereas 14 of the 15 biceps tenodesis patients were satisfied. The authors concluded that arthroscopic biceps tenodesis is an effective alternative to repair for type II SLAP lesions, though their study was not isolated to overhead athletes (tenodesis group mean age, 52 years).
In a 2014 series of cases, Ek and colleagues50 reported good outcomes of SLAP tear repair and biceps tenodesis. Again, though, tenodesis was used in older patients, and repair in younger, more active patients, with no high-level athletes in either group. There was no difference in return to sport between groups. In a study of patients who underwent primary biceps tenodesis, Gupta and colleagues51 found 80% excellent outcomes (improved shoulder outcome scores) in select SLAP tear patients, including 8 athletes, 88% of whom were overhead athletes. Gottschalk and colleagues52 reported on differences in prospectively collected outcome data (age, sex, SLAP lesion type II or IV) for primary biceps tenodesis in a series of 33 patients. Twenty-six of the 29 patients who completed follow-up returned to their previous level of activity. These studies suggest that primary biceps tenodesis may be an alternative with lower failure rates in the treatment of SLAP tears in middle-aged patients, and in overhead athletes, though additional specific studies are needed to focus on overhead athletes on a larger scale.
Revision SLAP Tear Repair Versus Biceps Tenodesis
Failed arthroscopic SLAP tear repairs, which are increasingly common, present a unique treatment challenge. In a 2013 prospective cohort series, Gupta and colleagues46 found excellent clinical outcomes of subpectoral biceps tenodesis for failed type II SLAP tears. The authors reported a postoperative SANE (Single Assessment Numeric Evaluation) score of 70.4%, an SST (Simple Shoulder Test) score of 9.33, and an ASES (American Shoulder and Elbow Surgeons) score of 77.96, along with reasonable health-related quality-of-life scores. Werner and colleagues53 evaluated 2-year outcomes of biceps tenodesis performed after SLAP tear repair in 24 patients and found a return to almost normal range of motion as well as good clinical outcome scores. Significantly worse outcomes were found for patients with open worker’s compensation claims.
McCormick and colleagues26 prospectively evaluated the efficacy of biceps tenodesis for failed type II SLAP tear repair in 46 patients. Improvement was noted across all outcome assessments during follow-up (mean, 3.6 years). From these findings, we might conclude that biceps tenodesis is a more predictable option for failed SLAP tear repair, and that it has a relatively low complication rate. However, most investigators have used a heterogeneous patient population, as opposed to overhead athletes specifically. To our knowledge, no one has evaluated the specific population of overhead throwers with failed SLAP tear repairs. In addition, no one has conducted randomized controlled trials comparing débridement, biceps tenodesis, and repair for failed SLAP tear repairs.
Postoperative Considerations
When overhead athletes and their surgeons are considering surgical options, they must take rehabilitation and return to play into account. Many surgeons think the possible marginal clinical benefit of SLAP tear repair may not be worth the protracted rehabilitation. In most practices, rehabilitation after biceps tenodesis is less involved. Discussing the advantages and disadvantages of these 2 procedures can be helpful in decision making.
Dein and colleagues54 reported the case of a middle-aged pitcher who sustained a fracture after biceps tenodesis with an interference screw. Cases like this are concerning. Surgeons should consider altering the rehabilitation regimen when planning postoperative care in cases of biceps tenodesis in throwers. Other reported complications of open tenodesis are deep infection, thrombosis, postoperative stiffness, and nerve injury.55-58
Consequences for Overhead Throwers
The unknown role of the BLC leaves surgeons wary when considering biceps tenodesis for elite athletes. Some have postulated that removing the intra-articular portion of the LHB may cause microinstability and alter joint kinematics.10,59-61 Others have suggested the biceps is desynchronized from the other musculature and is not functionally important.62 Disruption of one portion of the superior labrum may result in instability on the opposite side of the glenoid.10,61 Biomechanical studies, both cadaveric and in vivo, have tried to create proper loads to the LHB and evaluate the kinematics of the shoulder before and after biceps tenodesis and SLAP tear repair.59,60 Using a cadaveric model, Strauss and colleagues63 found that type II SLAP lesions resulted in increased glenohumeral translation compared with baseline. Biceps tenodesis did not restore normal translation, but this did not negatively affect stability in the presence of a SLAP lesion. The consensus is that the role of the biceps is controversial at best.
Several studies have used electromyography (EMG) to evaluate LHB functioning. In 2014, Chalmers and colleagues59 used surface EMG and motion analysis to evaluate 18 pitchers: 6 underwent SLAP tear repair, 5 underwent biceps tenodesis, and 7 were uninjured controls. There were no significant differences in the activity of the LHB muscle, the short head of the biceps muscle, the deltoid, the infraspinatus, or the latissimus among the 3 groups. Motion analysis showed that the normal pattern of muscular activation within the LHB muscle was more closely restored by biceps tenodesis than by SLAP tear repair. In addition, thoracic rotation patterns were significantly more altered in the SLAP tear repair patients than in the uninjured controls. As the authors noted, given the low frequency with which biceps tenodesis is performed in overhead athletes, it is unlikely that larger scale studies will be conducted without a multicenter effort.
Recommendations and Our Preferred Technique
Which surgical option is best for treating symptomatic SLAP lesions in overhead athletes remains unclear. Many athletes struggle to return to high-level play after SLAP tear repair. Whether the same is true after biceps tenodesis is yet to be determined because of the low frequency with which biceps tenodesis is performed in high-level overhead athletes. The options for fixation, technique, and fixation location are equally broad. In this section, we outline our general line of thinking for cases of proximal biceps pathology.
In each case, we perform glenohumeral arthroscopy to evaluate the BLC and identify any other pathology. For overhead athletes who are younger than 30 years and lack bicipital groove pain or signs of gross tendinopathy, we favor arthroscopic SLAP tear repair. Repair is usually performed through an anterior working portal for suture passage and a Wilmington portal for anchor placement. We use knotless technology to achieve stable fixation and stay posterior to the biceps anchor insertion.
For the prevention of any potential pain from the bicipital groove in carefully selected patients—recreational overhead athletes and patients who want a less involved surgical recovery—we favor open subpectoral biceps tenodesis rather than arthroscopic tenodesis. The outcomes of biceps tenodesis are consistent, according to the literature.47,57,64 Moreover, the open approach is favored for the incidence of postoperative stiffness in the arthroscopic population.65 Tendons can be fixed with multiple procedures, including soft-tissue tenodesis, interference screw fixation, and surface anchors. We favor using a tenodesis screw in the subpectoral location, as outlined by Mazzocca and colleagues.64Our algorithm for SLAP lesions is evolving with our understanding of this complex disease process. For young overhead throwers with type II SLAP lesions, we favor arthroscopic SLAP tear repair with knotless technology. For older recreational overhead athletes, we favor biceps tenodesis in the subpectoral region after diagnostic arthroscopy plus biceps tenotomy with or without additional SLAP tear fixation, depending on the stability of the biceps anchor (Figures 4A, 4B).
Conclusion
Overhead athletes who present with symptomatic SLAP lesions often provide a treatment dilemma. Although SLAP tear repair historically has been standard treatment, biceps tenodesis represents a consistent surgical option with low complication rates and low revision rates. It is likely that, as additional data on glenohumeral kinematics and outcomes in young athletes become available, improved decision-making algorithms will follow.
Am J Orthop. 2017;46(1):E71-E78. Copyright Frontline Medical Communications Inc. 2016. All rights reserved.
1. Elser F, Braun S, Dewing CB, Giphart JE, Millett PJ. Anatomy, function, injuries, and treatment of the long head of the biceps brachii tendon. Arthroscopy. 2011;27(4):581-592.
2. Fedoriw WW, Ramkumar P, McCulloch PC, Lintner DM. Return to play after treatment of superior labral tears in professional baseball players. Am J Sports Med. 2014;42(5):1155-1160.
3. Fleisig GS, Andrews JR, Dillman CJ, Escamilla RF. Kinetics of baseball pitching with implications about injury mechanisms. Am J Sports Med. 1995;23(2):233-239.
4. Aydin N, Sirin E, Arya A. Superior labrum anterior to posterior lesions of the shoulder: diagnosis and arthroscopic management. World J Orthop. 2014;5(3):344-350.
5. Barber A, Field LD, Ryu R. Biceps tendon and superior labrum injuries: decision-marking. J Bone Joint Surg Am. 2007;89(8):1844-1855.
6. Onyekwelu I, Khatib O, Zuckerman JD, Rokito AS, Kwon YW. The rising incidence of arthroscopic superior labrum anterior and posterior (SLAP) repairs. J Shoulder Elbow Surg. 2012;21(6):728-731.
7. Patterson BM, Creighton RA, Spang JT, Roberson JR, Kamath GV. Surgical trends in the treatment of superior labrum anterior and posterior lesions of the shoulder: analysis of data from the American Board of Orthopaedic Surgery Certification Examination Database. Am J Sports Med. 2014;42(8):1904-1910.
8. Walton DM, Sadi J. Identifying SLAP lesions: a meta-analysis of clinical tests and exercise in clinical reasoning. Phys Ther Sport. 2008;9(4):167-176.
9. Lesniak BP, Baraga MG, Jose J, Smith MK, Cunningham S, Kaplan LD. Glenohumeral findings on magnetic resonance imaging correlate with innings pitched in asymptomatic pitchers. Am J Sports Med. 2013;41(9):2022-2027.
10. Mihata T, McGarry MH, Tibone JE, Fitzpatrick MJ, Kinoshita M, Lee TQ. Biomechanical assessment of type II superior labral anterior-posterior (SLAP) lesions associated with anterior shoulder capsular laxity as seen in throwers: a cadaveric study. Am J Sports Med. 2008;36(8):1604-1610.
11. Burkhart SS, Morgan CD, Kibler WB. The disabled throwing shoulder: spectrum of pathology. Part II: evaluation and treatment of SLAP lesions in throwers. Arthroscopy. 2003;19(5):531-539.
12. Meserve BB, Cleland JA, Boucher TR. A meta-analysis examining clinical test utility for assessing superior labral anterior posterior lesions. Am J Sports Med. 2009;37(11):2252-2258.
13. Pandya NK, Colton A, Webner D, Sennett B, Huffman GR. Physical examination and magnetic resonance imaging in the diagnosis of superior labrum anterior-posterior lesions of the shoulder: a sensitivity analysis. Arthroscopy. 2008;24(3):311-317.
14. Amin MF, Youssef AO. The diagnostic value of magnetic resonance arthrography of the shoulder in detection and grading of SLAP lesions: comparison with arthroscopic findings. Eur J Radiol. 2012;81(9):2343-2347.
15. Cook C, Beaty S, Kissenberth MJ, Siffri P, Pill SG, Hawkins RJ. Diagnostic accuracy of five orthopedic clinical tests for diagnosis of superior labrum anterior posterior (SLAP) lesions. J Shoulder Elbow Surg. 2012;21(1):13-22.
16. Edwards SL, Lee JA, Bell JE, et al. Nonoperative treatment of superior labrum anterior posterior tears: improvements in pain, function, and quality of life. Am J Sports Med. 2010;38(7):1456-1461.
17. Andrews JR, Carson WG Jr, McLeod WD. Glenoid labrum tears related to the long head of the biceps. Am J Sports Med. 1985;13(5):337-341.
18. Snyder SJ, Karzel RP, Del Pizzo W, Ferkel RD, Friedman MJ. SLAP lesions of the shoulder. Arthroscopy. 1990;6(4):274-279.
19. Snyder SJ, Banas MP, Karzel RP. An analysis of 140 injuries to the superior glenoid labrum. J Shoulder Elbow Surg. 1995;4(4):243-248.
20. Morgan CD, Burkhart SS, Palmeri M, Gillespie M. Type II SLAP lesions: three subtypes and their relationships to superior instability and rotator cuff tears. Arthroscopy. 1998;14(6):553-565.
21. Weber SC, Martin DF, Seiler JG 3rd, Harrast JJ. Superior labrum anterior and posterior lesions of the shoulder: incidence rates, complications, and outcomes as reported by American Board of Orthopedic Surgery. Part II candidates. Am J Sports Med. 2012;40(7):1538-1543.
22. Keener JD, Brophy RH. Superior labral tears of the shoulder: pathogenesis, evaluation, and treatment. J Am Acad Orthop Surg. 2009;17(10):627-637.
23. Chen CH, Hsu KY, Chen WJ, Shih CH. Incidence and severity of biceps long head tendon lesion in patients with complete rotator cuff tears. J Trauma. 2005;58(6):1189-1193.
24. Nho SJ, Strauss EJ, Lenart BA, et al. Long head of the biceps tendinopathy: diagnosis and management. J Am Acad Orthop Surg. 2010;18(11):645-656.
25. Zhang AL, Kreulen C, Ngo SS, Hame SL, Wang JC, Gamradt SC. Demographic trends in arthroscopic SLAP repair in the United States. Am J Sports Med. 2012;40(5):1144-1147.
26. McCormick F, Bhatia S, Chalmers P, Gupta A, Verma N, Romeo AA. The management of type II superior labral anterior to posterior injuries. Orthop Clin North Am. 2014;45(1):121-128.
27. Brockmeier SF, Voos JE, Williams RJ 3rd, Altchek DW, Cordasco FA, Allen AA; Hospital for Special Surgery Sports Medicine and Shoulder Service. Outcomes after arthroscopic repair of type-II SLAP lesions. J Bone Joint Surg Am. 2009;91(7):1595-1603.
28. Boileau P, Parratte S, Chuinard C, Roussanne Y, Shia D, Bicknell R. Arthroscopic treatment of isolated type II SLAP lesions: biceps tenodesis as an alternative to reinsertion. Am J Sports Med. 2009;37(5):929-936.
29. Denard PJ, Lädermann A, Burkhart SS. Long-term outcome after arthroscopic repair of type II SLAP lesions: results according to age and workers’ compensation status. Arthroscopy. 2012;28(4):451-457.
30. Friel NA, Karas V, Slabaugh MA, Cole BJ. Outcomes of type II superior labrum, anterior to posterior (SLAP) repair: prospective evaluation at a minimum two-year follow-up. J Shoulder Elbow Surg. 2010;19(6):859-867.
31. Provencher MT, McCormick F, Dewing C, McIntire S, Solomon D. A prospective analysis of 179 type 2 superior labrum anterior and posterior repairs: outcomes and factors associated with success and failure. Am J Sports Med. 2013;41(4):880-886.
32. Gupta AK, Chalmers PN, Klosterman EL, et al. Subpectoral biceps tenodesis for bicipital tendonitis with SLAP tear. Orthopedics. 2015;38(1):e48-e53.
33. Gorantla K, Gill C, Wright RW. The outcome of type II SLAP repair: a systematic review. Arthroscopy. 2010;26(4):537-545.
34. Katz LM, Hsu S, Miller SL, et al. Poor outcomes after SLAP repair: descriptive analysis and prognosis. Arthroscopy. 2009;25(8):849-855.
35. Park MJ, Hsu JE, Harper C, Sennett BJ, Huffman GR. Poly-L/D-lactic acid anchors are associated with reoperation and failure of SLAP repairs. Arthroscopy. 2011;27(10):1335-1340.
36. Sassmannshausen G, Sukay M, Mair SD. Broken or dislodged poly-L-lactic acid bioabsorbable tacks in patients after SLAP lesion surgery. Arthroscopy. 2006;22(6):615-619.
37. Uggen C, Wei A, Glousman RE, et al. Biomechanical comparison of knotless anchor repair versus simple suture repair for type II SLAP lesions. Arthroscopy. 2009;25(10):1085-1092.
38. Weber SC. Surgical management of the failed SLAP repair. Sports Med Arthrosc. 2010;18(3):162-166.
39. Wilkerson JP, Zvijac JE, Uribe JW, Schürhoff MR, Green JB. Failure of polymerized lactic acid tacks in shoulder surgery. J Shoulder Elbow Surg. 2003;12(2):117-121.
40. Weber S. Surgical management of the failed SLAP lesion. Arthroscopy. 2008;24(suppl):e8-e9.
41. Schrøder CP, Skare O, Gjengedal E, Uppheim G, Reikerås O, Brox JI. Long-term results after SLAP repair: a 5-year follow-up study of 107 patients with comparison of patients aged over and under 40 years. Arthroscopy. 2012;28(11):1601-1607.
42. Mazzocca AD, Cote MP, Arciero CL, Romeo AA, Arciero RA. Clinical outcomes after subpectoral biceps tenodesis with an interference screw. Am J Sports Med. 2008;36(10):1922-1929.
43. Mazzocca AD, McCarthy MB, Ledgard FA, et al. Histomorphologic changes of the long head of the biceps tendon in common shoulder pathologies. Arthroscopy. 2013;29(6):972-981.
44. Boileau P, Parratte S, Chuinard C, Roussanne Y, Shia D, Bicknell R. Arthroscopic treatment of isolated type II SLAP lesions: biceps tenodesis as an alternative to reinsertion. Am J Sports Med. 2009;37(5):929-936.
45. Boileau P, Krishnan SG, Coste JS, Walch G. Arthroscopic biceps tenodesis: a new technique using bioabsorbable interference screw fixation. Arthroscopy. 2002;18(9):1002-1012.
46. Gupta AK, Bruce B, Klosterman EL, McCormick F, Harris J, Romeo AA. Subpectoral biceps tenodesis for failed type II SLAP repair. Orthopedics. 2013;36(6):e723-e728.
47. Provencher MT, LeClere LE, Romeo AA. Subpectoral biceps tenodesis. Sports Med Arthrosc. 2008;16(3):170-176.
48. McCarty LP 3rd, Buss DD, Datta MW, Freehill MQ, Giveans MR. Complications observed following labral or rotator cuff repair with use of poly-L-lactic acid implants. J Bone Joint Surg Am. 2013;95(6):507-511.
49. Werner BC, Brockmeier SF, Gwathmey FW. Trends in long head biceps tenodesis. Am J Sports Med. 2015;43(3):570-578.
50. Ek ET, Shi LL, Tompson JD, Freehill MT, Warner JJ. Surgical treatment of isolated type II superior labrum anterior-posterior (SLAP) lesions: repair versus biceps tenodesis. J Shoulder Elbow Surg. 2014;23(7):1059-1065.
51. Gupta AK, Chalmers PN, Klosterman EL, et al. Subpectoral biceps tenodesis for bicipital tendonitis with SLAP tear. Orthopedics. 2015;38(1):e48-e53.
52. Gottschalk MB, Karas SG, Ghattas TN, Burdette R. Subpectoral biceps tenodesis for the treatment of type II and IV superior labral anterior and posterior lesions. Am J Sports Med. 2014;42(9):2128-2135.
53. Werner BC, Pehlivan HC, Hart JM, et al. Biceps tenodesis is a viable option for salvage of failed SLAP repair. J Shoulder Elbow Surg. 2014;23(8):e179-e184.
54. Dein EJ, Huri G, Gordon JC, McFarland EG. A humerus fracture in a baseball pitcher after biceps tenodesis [published correction appears in Am J Sports Med. 2014;42(6):NP39]. Am J Sports Med. 2014;42(4):877-879.
55. Nho SJ, Reiff SN, Verma NN, Slabaugh MA, Mazzocca AD, Romeo AA. Complications associated with subpectoral biceps tenodesis: low rates of incidence following surgery. J Shoulder Elbow Surg. 2010;19(5):764-768.
56. Osbahr DC, Diamond AB, Speer KP. The cosmetic appearance of the biceps muscle after long-head tenotomy versus tenodesis. Arthroscopy. 2002;18(5):483-487.
57. Romeo AA, Mazzocca AD, Tauro JC. Arthroscopic biceps tenodesis. Arthroscopy. 2004;20(2):206-213.
58. Ma H, Van Heest A, Glisson C, Patel S. Musculocutaneous nerve entrapment: an unusual complication after biceps tenodesis. Am J Sports Med. 2009;37(12):2467-2469.
59. Chalmers PN, Trombley R, Cip J, et al. Postoperative restoration of upper extremity motion and neuromuscular control during the overhand pitch: evaluation of tenodesis and repair for superior labral anterior-posterior tears. Am J Sports Med. 2014;42(12):2825-2836.
60. Giphart JE, Elser F, Dewing CB, Torry MR, Millett PJ. The long head of the biceps tendon has minimal effect on in vivo glenohumeral kinematics: a biplane fluoroscopy study. Am J Sports Med. 2012;40(1):202-212.
61. Grossman MG, Tibone JE, McGarry MH, Schneider DJ, Veneziani S, Lee TQ. A cadaveric model of the throwing shoulder: a possible etiology of superior labrum anterior-to-posterior lesions. J Bone Joint Surg Am. 2005;87(4):824-831.
62. Hawkes DH, Alizadehkhaiyat O, Fisher AC, Kemp GJ, Roebuck MM, Frostick SP. Normal shoulder muscular activation and co-ordination during a shoulder elevation task based on activities of daily living: an electromyographic study. J Orthop Res. 2012;30(1):53-60.
63. Strauss EJ, Salata MJ, Sershon RA, et al. Role of the superior labrum after biceps tenodesis in glenohumeral stability. J Shoulder Elbow Surg. 2014;23(4):485-491.
64. Mazzocca AD, Bicos J, Santangelo S, Romeo AA, Arciero RA. The biomechanical evaluation of four fixation techniques for proximal biceps tenodesis. Arthroscopy. 2005;21(11):1296-1306.
65. Werner BC, Pehlivan HC, Hart JM, et al. Increased incidence of postoperative stiffness after arthroscopic compared with open biceps tenodesis. Arthroscopy. 2014;30(9):1075-1084.
66. Denard PJ, Dai X, Hanypsiak BT, Burkhart SS. Anatomy of the biceps tendon: implications for restoring physiological length–tension relation during biceps tenodesis with interference screw fixation. Arthroscopy. 2012;28(10):1352-1358.
67. Mazzocca AD, Rios CG, Romeo AA, Arciero RA. Subpectoral biceps tenodesis with interference screw fixation. Arthroscopy. 2005;21(7):896.
1. Elser F, Braun S, Dewing CB, Giphart JE, Millett PJ. Anatomy, function, injuries, and treatment of the long head of the biceps brachii tendon. Arthroscopy. 2011;27(4):581-592.
2. Fedoriw WW, Ramkumar P, McCulloch PC, Lintner DM. Return to play after treatment of superior labral tears in professional baseball players. Am J Sports Med. 2014;42(5):1155-1160.
3. Fleisig GS, Andrews JR, Dillman CJ, Escamilla RF. Kinetics of baseball pitching with implications about injury mechanisms. Am J Sports Med. 1995;23(2):233-239.
4. Aydin N, Sirin E, Arya A. Superior labrum anterior to posterior lesions of the shoulder: diagnosis and arthroscopic management. World J Orthop. 2014;5(3):344-350.
5. Barber A, Field LD, Ryu R. Biceps tendon and superior labrum injuries: decision-marking. J Bone Joint Surg Am. 2007;89(8):1844-1855.
6. Onyekwelu I, Khatib O, Zuckerman JD, Rokito AS, Kwon YW. The rising incidence of arthroscopic superior labrum anterior and posterior (SLAP) repairs. J Shoulder Elbow Surg. 2012;21(6):728-731.
7. Patterson BM, Creighton RA, Spang JT, Roberson JR, Kamath GV. Surgical trends in the treatment of superior labrum anterior and posterior lesions of the shoulder: analysis of data from the American Board of Orthopaedic Surgery Certification Examination Database. Am J Sports Med. 2014;42(8):1904-1910.
8. Walton DM, Sadi J. Identifying SLAP lesions: a meta-analysis of clinical tests and exercise in clinical reasoning. Phys Ther Sport. 2008;9(4):167-176.
9. Lesniak BP, Baraga MG, Jose J, Smith MK, Cunningham S, Kaplan LD. Glenohumeral findings on magnetic resonance imaging correlate with innings pitched in asymptomatic pitchers. Am J Sports Med. 2013;41(9):2022-2027.
10. Mihata T, McGarry MH, Tibone JE, Fitzpatrick MJ, Kinoshita M, Lee TQ. Biomechanical assessment of type II superior labral anterior-posterior (SLAP) lesions associated with anterior shoulder capsular laxity as seen in throwers: a cadaveric study. Am J Sports Med. 2008;36(8):1604-1610.
11. Burkhart SS, Morgan CD, Kibler WB. The disabled throwing shoulder: spectrum of pathology. Part II: evaluation and treatment of SLAP lesions in throwers. Arthroscopy. 2003;19(5):531-539.
12. Meserve BB, Cleland JA, Boucher TR. A meta-analysis examining clinical test utility for assessing superior labral anterior posterior lesions. Am J Sports Med. 2009;37(11):2252-2258.
13. Pandya NK, Colton A, Webner D, Sennett B, Huffman GR. Physical examination and magnetic resonance imaging in the diagnosis of superior labrum anterior-posterior lesions of the shoulder: a sensitivity analysis. Arthroscopy. 2008;24(3):311-317.
14. Amin MF, Youssef AO. The diagnostic value of magnetic resonance arthrography of the shoulder in detection and grading of SLAP lesions: comparison with arthroscopic findings. Eur J Radiol. 2012;81(9):2343-2347.
15. Cook C, Beaty S, Kissenberth MJ, Siffri P, Pill SG, Hawkins RJ. Diagnostic accuracy of five orthopedic clinical tests for diagnosis of superior labrum anterior posterior (SLAP) lesions. J Shoulder Elbow Surg. 2012;21(1):13-22.
16. Edwards SL, Lee JA, Bell JE, et al. Nonoperative treatment of superior labrum anterior posterior tears: improvements in pain, function, and quality of life. Am J Sports Med. 2010;38(7):1456-1461.
17. Andrews JR, Carson WG Jr, McLeod WD. Glenoid labrum tears related to the long head of the biceps. Am J Sports Med. 1985;13(5):337-341.
18. Snyder SJ, Karzel RP, Del Pizzo W, Ferkel RD, Friedman MJ. SLAP lesions of the shoulder. Arthroscopy. 1990;6(4):274-279.
19. Snyder SJ, Banas MP, Karzel RP. An analysis of 140 injuries to the superior glenoid labrum. J Shoulder Elbow Surg. 1995;4(4):243-248.
20. Morgan CD, Burkhart SS, Palmeri M, Gillespie M. Type II SLAP lesions: three subtypes and their relationships to superior instability and rotator cuff tears. Arthroscopy. 1998;14(6):553-565.
21. Weber SC, Martin DF, Seiler JG 3rd, Harrast JJ. Superior labrum anterior and posterior lesions of the shoulder: incidence rates, complications, and outcomes as reported by American Board of Orthopedic Surgery. Part II candidates. Am J Sports Med. 2012;40(7):1538-1543.
22. Keener JD, Brophy RH. Superior labral tears of the shoulder: pathogenesis, evaluation, and treatment. J Am Acad Orthop Surg. 2009;17(10):627-637.
23. Chen CH, Hsu KY, Chen WJ, Shih CH. Incidence and severity of biceps long head tendon lesion in patients with complete rotator cuff tears. J Trauma. 2005;58(6):1189-1193.
24. Nho SJ, Strauss EJ, Lenart BA, et al. Long head of the biceps tendinopathy: diagnosis and management. J Am Acad Orthop Surg. 2010;18(11):645-656.
25. Zhang AL, Kreulen C, Ngo SS, Hame SL, Wang JC, Gamradt SC. Demographic trends in arthroscopic SLAP repair in the United States. Am J Sports Med. 2012;40(5):1144-1147.
26. McCormick F, Bhatia S, Chalmers P, Gupta A, Verma N, Romeo AA. The management of type II superior labral anterior to posterior injuries. Orthop Clin North Am. 2014;45(1):121-128.
27. Brockmeier SF, Voos JE, Williams RJ 3rd, Altchek DW, Cordasco FA, Allen AA; Hospital for Special Surgery Sports Medicine and Shoulder Service. Outcomes after arthroscopic repair of type-II SLAP lesions. J Bone Joint Surg Am. 2009;91(7):1595-1603.
28. Boileau P, Parratte S, Chuinard C, Roussanne Y, Shia D, Bicknell R. Arthroscopic treatment of isolated type II SLAP lesions: biceps tenodesis as an alternative to reinsertion. Am J Sports Med. 2009;37(5):929-936.
29. Denard PJ, Lädermann A, Burkhart SS. Long-term outcome after arthroscopic repair of type II SLAP lesions: results according to age and workers’ compensation status. Arthroscopy. 2012;28(4):451-457.
30. Friel NA, Karas V, Slabaugh MA, Cole BJ. Outcomes of type II superior labrum, anterior to posterior (SLAP) repair: prospective evaluation at a minimum two-year follow-up. J Shoulder Elbow Surg. 2010;19(6):859-867.
31. Provencher MT, McCormick F, Dewing C, McIntire S, Solomon D. A prospective analysis of 179 type 2 superior labrum anterior and posterior repairs: outcomes and factors associated with success and failure. Am J Sports Med. 2013;41(4):880-886.
32. Gupta AK, Chalmers PN, Klosterman EL, et al. Subpectoral biceps tenodesis for bicipital tendonitis with SLAP tear. Orthopedics. 2015;38(1):e48-e53.
33. Gorantla K, Gill C, Wright RW. The outcome of type II SLAP repair: a systematic review. Arthroscopy. 2010;26(4):537-545.
34. Katz LM, Hsu S, Miller SL, et al. Poor outcomes after SLAP repair: descriptive analysis and prognosis. Arthroscopy. 2009;25(8):849-855.
35. Park MJ, Hsu JE, Harper C, Sennett BJ, Huffman GR. Poly-L/D-lactic acid anchors are associated with reoperation and failure of SLAP repairs. Arthroscopy. 2011;27(10):1335-1340.
36. Sassmannshausen G, Sukay M, Mair SD. Broken or dislodged poly-L-lactic acid bioabsorbable tacks in patients after SLAP lesion surgery. Arthroscopy. 2006;22(6):615-619.
37. Uggen C, Wei A, Glousman RE, et al. Biomechanical comparison of knotless anchor repair versus simple suture repair for type II SLAP lesions. Arthroscopy. 2009;25(10):1085-1092.
38. Weber SC. Surgical management of the failed SLAP repair. Sports Med Arthrosc. 2010;18(3):162-166.
39. Wilkerson JP, Zvijac JE, Uribe JW, Schürhoff MR, Green JB. Failure of polymerized lactic acid tacks in shoulder surgery. J Shoulder Elbow Surg. 2003;12(2):117-121.
40. Weber S. Surgical management of the failed SLAP lesion. Arthroscopy. 2008;24(suppl):e8-e9.
41. Schrøder CP, Skare O, Gjengedal E, Uppheim G, Reikerås O, Brox JI. Long-term results after SLAP repair: a 5-year follow-up study of 107 patients with comparison of patients aged over and under 40 years. Arthroscopy. 2012;28(11):1601-1607.
42. Mazzocca AD, Cote MP, Arciero CL, Romeo AA, Arciero RA. Clinical outcomes after subpectoral biceps tenodesis with an interference screw. Am J Sports Med. 2008;36(10):1922-1929.
43. Mazzocca AD, McCarthy MB, Ledgard FA, et al. Histomorphologic changes of the long head of the biceps tendon in common shoulder pathologies. Arthroscopy. 2013;29(6):972-981.
44. Boileau P, Parratte S, Chuinard C, Roussanne Y, Shia D, Bicknell R. Arthroscopic treatment of isolated type II SLAP lesions: biceps tenodesis as an alternative to reinsertion. Am J Sports Med. 2009;37(5):929-936.
45. Boileau P, Krishnan SG, Coste JS, Walch G. Arthroscopic biceps tenodesis: a new technique using bioabsorbable interference screw fixation. Arthroscopy. 2002;18(9):1002-1012.
46. Gupta AK, Bruce B, Klosterman EL, McCormick F, Harris J, Romeo AA. Subpectoral biceps tenodesis for failed type II SLAP repair. Orthopedics. 2013;36(6):e723-e728.
47. Provencher MT, LeClere LE, Romeo AA. Subpectoral biceps tenodesis. Sports Med Arthrosc. 2008;16(3):170-176.
48. McCarty LP 3rd, Buss DD, Datta MW, Freehill MQ, Giveans MR. Complications observed following labral or rotator cuff repair with use of poly-L-lactic acid implants. J Bone Joint Surg Am. 2013;95(6):507-511.
49. Werner BC, Brockmeier SF, Gwathmey FW. Trends in long head biceps tenodesis. Am J Sports Med. 2015;43(3):570-578.
50. Ek ET, Shi LL, Tompson JD, Freehill MT, Warner JJ. Surgical treatment of isolated type II superior labrum anterior-posterior (SLAP) lesions: repair versus biceps tenodesis. J Shoulder Elbow Surg. 2014;23(7):1059-1065.
51. Gupta AK, Chalmers PN, Klosterman EL, et al. Subpectoral biceps tenodesis for bicipital tendonitis with SLAP tear. Orthopedics. 2015;38(1):e48-e53.
52. Gottschalk MB, Karas SG, Ghattas TN, Burdette R. Subpectoral biceps tenodesis for the treatment of type II and IV superior labral anterior and posterior lesions. Am J Sports Med. 2014;42(9):2128-2135.
53. Werner BC, Pehlivan HC, Hart JM, et al. Biceps tenodesis is a viable option for salvage of failed SLAP repair. J Shoulder Elbow Surg. 2014;23(8):e179-e184.
54. Dein EJ, Huri G, Gordon JC, McFarland EG. A humerus fracture in a baseball pitcher after biceps tenodesis [published correction appears in Am J Sports Med. 2014;42(6):NP39]. Am J Sports Med. 2014;42(4):877-879.
55. Nho SJ, Reiff SN, Verma NN, Slabaugh MA, Mazzocca AD, Romeo AA. Complications associated with subpectoral biceps tenodesis: low rates of incidence following surgery. J Shoulder Elbow Surg. 2010;19(5):764-768.
56. Osbahr DC, Diamond AB, Speer KP. The cosmetic appearance of the biceps muscle after long-head tenotomy versus tenodesis. Arthroscopy. 2002;18(5):483-487.
57. Romeo AA, Mazzocca AD, Tauro JC. Arthroscopic biceps tenodesis. Arthroscopy. 2004;20(2):206-213.
58. Ma H, Van Heest A, Glisson C, Patel S. Musculocutaneous nerve entrapment: an unusual complication after biceps tenodesis. Am J Sports Med. 2009;37(12):2467-2469.
59. Chalmers PN, Trombley R, Cip J, et al. Postoperative restoration of upper extremity motion and neuromuscular control during the overhand pitch: evaluation of tenodesis and repair for superior labral anterior-posterior tears. Am J Sports Med. 2014;42(12):2825-2836.
60. Giphart JE, Elser F, Dewing CB, Torry MR, Millett PJ. The long head of the biceps tendon has minimal effect on in vivo glenohumeral kinematics: a biplane fluoroscopy study. Am J Sports Med. 2012;40(1):202-212.
61. Grossman MG, Tibone JE, McGarry MH, Schneider DJ, Veneziani S, Lee TQ. A cadaveric model of the throwing shoulder: a possible etiology of superior labrum anterior-to-posterior lesions. J Bone Joint Surg Am. 2005;87(4):824-831.
62. Hawkes DH, Alizadehkhaiyat O, Fisher AC, Kemp GJ, Roebuck MM, Frostick SP. Normal shoulder muscular activation and co-ordination during a shoulder elevation task based on activities of daily living: an electromyographic study. J Orthop Res. 2012;30(1):53-60.
63. Strauss EJ, Salata MJ, Sershon RA, et al. Role of the superior labrum after biceps tenodesis in glenohumeral stability. J Shoulder Elbow Surg. 2014;23(4):485-491.
64. Mazzocca AD, Bicos J, Santangelo S, Romeo AA, Arciero RA. The biomechanical evaluation of four fixation techniques for proximal biceps tenodesis. Arthroscopy. 2005;21(11):1296-1306.
65. Werner BC, Pehlivan HC, Hart JM, et al. Increased incidence of postoperative stiffness after arthroscopic compared with open biceps tenodesis. Arthroscopy. 2014;30(9):1075-1084.
66. Denard PJ, Dai X, Hanypsiak BT, Burkhart SS. Anatomy of the biceps tendon: implications for restoring physiological length–tension relation during biceps tenodesis with interference screw fixation. Arthroscopy. 2012;28(10):1352-1358.
67. Mazzocca AD, Rios CG, Romeo AA, Arciero RA. Subpectoral biceps tenodesis with interference screw fixation. Arthroscopy. 2005;21(7):896.
Mastering the Physical Examination of the Athlete’s Hip
Take-Home Points
- Perform a comprehensive examination to determine intra-articular pathology as well as potential extra-articular sources of hip and pelvic pain.
- Adductor strains can be prevented with adequate rehabilitation focused on correcting predisposing factors (ie, adductor weakness or tightness, limited range of motion, and core imbalance).
- Athletic pubalgia is diagnosed when tenderness can be elicited over the pubic tubercle.
- Osteitis pubis is diagnosed with pain over the pubic symphysis.
- FAI and labral injury classically present with a C-sign but can also present with lateral hip pain, buttock pain, low back pain, anterior thigh pain, and knee pain.
Hip and groin pain is a common finding among athletes of all ages and activity levels. Such pain most often occurs among athletes in sports such as football, hockey, rugby, soccer, and ballet, which demand frequent cutting, pivoting, and acceleration.1-4 Previously, pain about the hip and groin was attributed to muscular strains and soft-tissue contusions, but improvements in physical examination skills, imaging modalities, and disease-specific treatment options have led to increased recognition of hip injuries as a significant source of disability in the athletic population.5,6 These injuries make up 6% or more of all sports injuries, and the rate is increasing.7-9
In this review, we describe precise methods for evaluating the athlete’s hip or groin with an emphasis on recognizing the most common extra-articular and intra-articular pathologies, including adductor strains, athletic pubalgia, osteitis pubis, and femoroacetabular impingement (FAI) with labral tears.
Hip Pathoanatomy
The first step in determining the etiology of pain is to establish if there is true pathology of the hip joint and surrounding structures, or if the pain is referred from another source.
Patient History
The physical examination is guided by the patient’s history. Important patient-specific factors to be ascertained include age, sport(s) played, competition level, seasonal timing, and effect of the injury on performance. Regarding presenting symptoms, attention should be given to pain location, timing (acute vs chronic), onset, nature (clicking, catching, instability), and precipitating factors. Acute-onset pain with muscle contraction or stretching, possibly accompanied by an audible pop, is likely musculotendinous in origin. Insidious-onset dull aching pain that worsens with activity more commonly involves intra-articular processes. Most classically, this pain occurs deep in the groin and is demonstrated by the C sign: The patient cups a hand with its fingers pointing toward the anterior groin at the level of the greater trochanter (Figure 1).11
A comprehensive hip evaluation can be performed with the patient in the standing, seated, supine, lateral, and prone positions, as previously described (Table 2).6,12,13
Extra-Articular Hip Pathologies
Adductor Strains
The adductor muscle group includes the adductor magnus, adductor brevis, gracilis, obturator externus, pectineus, and adductor longus, which is the most commonly strained. Adductor strains are the most common cause of groin pain in athletes, and usually occur in sports that require forceful eccentric contraction of the adductors.14 Among professional soccer players, adductor strains represent almost one fourth of all muscle injuries and result in lost playing time averaging 2 weeks and an 18% reinjury rate.15 These injuries are particularly detrimental to performance because the adductor muscles help stabilize the pelvis during closed-chain activities.3 Diagnosis and adequate rehabilitation focused on correcting predisposing factors (eg, adductor weakness or tightness, loss of hip range of motion, core imbalance) are paramount in reinjury prevention.16,17
On presentation, athletes complain of aching groin or medial thigh pain. The examiner should assess for swelling or ecchymosis. There typically is tenderness to palpation at or near the origin on the pubic bones, with pain exacerbated with resisted adduction and passive stretch into abduction during examination. Palpation of adductors requires proper exposure and is most easily performed with the patient supine and the lower extremity in a figure-of-4 position (Figure 2A).
Athletic Pubalgia
Athletic pubalgia, also known as sports hernia or core muscle injury, is an injury to the soft tissues of the lower abdominal or posterior inguinal wall. Although not fully understood, the condition is considered the result of repetitive trunk hyperextension and thigh hyperabduction resulting in shearing at the pubic symphysis where there is a muscle imbalance between the strong proximal thigh muscles and weaker abdominals. This condition is more common in men and typically is insidious in onset with a prolonged course recalcitrant to nonoperative treatment.18 In studies of chronic groin pain in athletes, the rate of athletic pubalgia as the primary etiology ranges from 39% to 85%.9,19,20
Patients typically complain of increasing pain in the lower abdominal and proximal adductors during activity. Symptoms include unilateral or bilateral lower abdominal pain, which can radiate toward the perineum, rectus muscle, and proximal adductors during sport but usually abates with rest.18 Athletes endorse they are not capable of playing at their full athletic potential. Symptoms are initiated with sudden forceful movements, as in sit-ups, sprints, and valsalva maneuvers like coughs and sneezes. Valsalva maneuvers worsen pain in about 10% of patients.21-23On physical examination with the patient supine, tenderness can be elicited over the pubic tubercle, abdominal obliques, and/or rectus abdominis insertion (Figure 3A). Athletes may also have tenderness at the adductor longus tendon origin at or near the pubic symphysis, which may make the diagnosis difficult to distinguish from an adductor strain.
Osteitis Pubis
Osteitis pubis is a painful overuse injury that results in noninfectious inflammation of the pubic symphysis from increased motion at this normally stable immobile joint.3 As with athletic pubalgia, the exact mechanism is unclear, but likely it is similar to the repetitive stress placed on the pubic symphysis by unequal forces of the abdominal and adductor muscles.24 The disease can result in bony erosions and cartilage breakdown with irregularity of the pubic symphysis.
Athletes may complain of anterior and medial groin pain that can radiate to the lower abdominal muscles, perineum, inguinal region, and medial thigh. Walking, pelvic motion, adductor stretching, abdominal muscle exercises, and standing up can exacerbate pain.24 Some cases involve impaired internal or external rotation of the hip, sacroiliac joint dysfunction, or adductor and abductor muscle weakness.25The distinguishing feature of osteitis pubis is pain over the pubic symphysis with direct palpation (Figure 4A). Examination maneuvers that place stress on the pubic symphysis can aid in diagnosis.26
Intra-Articular Hip Pathology: Femoroacetabular Impingement
In athletes, FAI is a leading cause of intra-articular pathology, which can lead to labral tears.28,29 FAI lesions include cam-type impingement from an aspherical femoral head and pincer impingement from acetabular overcoverage, both of which limit internal rotation and cause acetabular rim abutment, which damages the labrum.
Athletes present with activity-related groin or hip pain that is exacerbated by hip flexion and internal rotation, with possible mechanical symptoms from labral tearing.30 However, the pain distribution varies. In a study by Clohisy and colleagues,31 of patients with symptomatic FAI that required surgical intervention, 88% had groin pain, 67% had lateral hip pain, 35% had anterior thigh pain, 29% had buttock pain, 27% had knee pain, and 23% had low back pain.
Careful attention should be given to range of motion in FAI patients, as they can usually flex their hip to 90° to 110°, and in this position there is limited internal rotation and asymmetric external rotation relative to the contralateral leg.32 The anterior impingement test is one of the most reliable tests for FAI (Figure 5A).32 With the patient supine, the hip is dynamically flexed to 90°, adducted, and internally rotated. A positive test elicits deep anterior groin pain that generally replicates the patient’s symptoms.29
Conclusion
Careful, directed history taking and physical examination are essential in narrowing the diagnostic possibilities before initiating a workup for the common intra-articular and extra-articular causes of hip and groin pain in athletes.
Am J Orthop. 2017;46(1):10-16. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
1. Boyd KT, Peirce NS, Batt ME. Common hip injuries in sport. Sports Med. 1997;24(4):273-288.
2. Duthon VB, Charbonnier C, Kolo FC, et al. Correlation of clinical and magnetic resonance imaging findings in hips of elite female ballet dancers. Arthroscopy. 2013;29(3):411-419.
3. Prather H, Cheng A. Diagnosis and treatment of hip girdle pain in the athlete. PM R. 2016;8(3 suppl):S45-S60.
4. Larson CM. Sports hernia/athletic pubalgia: evaluation and management. Sports Health. 2014;6(2):139-144.
5. Bizzini M, Notzli HP, Maffiuletti NA. Femoroacetabular impingement in professional ice hockey players: a case series of 5 athletes after open surgical decompression of the hip. Am J Sports Med. 2007;35(11):1955-1959.
6. Lynch TS, Terry MA, Bedi A, Kelly BT. Hip arthroscopic surgery: patient evaluation, current indications, and outcomes. Am J Sports Med. 2013;41(5):1174-1189.
7. Anderson K, Strickland SM, Warren R. Hip and groin injuries in athletes. Am J Sports Med. 2001;29(4):521-533.
8. Fon LJ, Spence RA. Sportsman’s hernia. Br J Surg. 2000;87(5):545-552.
9. Kluin J, den Hoed PT, van Linschoten R, IJzerman JC, van Steensel CJ. Endoscopic evaluation and treatment of groin pain in the athlete. Am J Sports Med. 2004;32(4):944-949.
10. Ward D, Parvizi J. Management of hip pain in young adults. Orthop Clin North Am. 2016;47(3):485-496.
11. Byrd JW. Hip arthroscopy. J Am Acad Orthop Surg. 2006;14(7):433-444.
12. Martin HD, Palmer IJ. History and physical examination of the hip: the basics. Curr Rev Musculoskelet Med. 2013;6(3):219-225.
13. Shindle MK, Voos JE, Nho SJ, Heyworth BE, Kelly BT. Arthroscopic management of labral tears in the hip. J Bone Joint Surg Am. 2008;90(suppl 4):2-19.
14. Morelli V, Smith V. Groin injuries in athletes. Am Fam Physician. 2001;64(8):1405-1414.
15. Ekstrand J, Hagglund M, Walden M. Epidemiology of muscle injuries in professional football (soccer). Am J Sports Med. 2011;39(6):1226-1232.
16. Ekstrand J, Gillquist J. The avoidability of soccer injuries. Int J Sports Med. 1983;4(2):124-128.
17. Tyler TF, Nicholas SJ, Campbell RJ, McHugh MP. The association of hip strength and flexibility with the incidence of adductor muscle strains in professional ice hockey players. Am J Sports Med. 2001;29(2):124-128.
18. Farber AJ, Wilckens JH. Sports hernia: diagnosis and therapeutic approach. J Am Acad Orthop Surg. 2007;15(8):507-514.
19. De Paulis F, Cacchio A, Michelini O, Damiani A, Saggini R. Sports injuries in the pelvis and hip: diagnostic imaging. Eur J Radiol. 1998;27(suppl 1):S49-S59.
20. Lovell G. The diagnosis of chronic groin pain in athletes: a review of 189 cases. Aust J Sci Med Sport. 1995;27(suppl 1):76-79.
21. Strosberg DS, Ellis TJ, Renton DB. The role of femoroacetabular impingement in core muscle injury/athletic pubalgia: diagnosis and management. Front Surg. 2016;3:6.
22. Meyers WC, Foley DP, Garrett WE, Lohnes JH, Mandlebaum BR. Management of severe lower abdominal or inguinal pain in high-performance athletes. PAIN (Performing Athletes with Abdominal or Inguinal Neuromuscular Pain Study Group). Am J Sports Med. 2000;28(1):2-8.
23. Ahumada LA, Ashruf S, Espinosa-de-los-Monteros A, et al. Athletic pubalgia: definition and surgical treatment. Ann Plast Surg. 2005;55(4):393-396.
24. Angoules AG. Osteitis pubis in elite athletes: diagnostic and therapeutic approach. World J Orthop. 2015;6(9):672-679.
25. Hiti CJ, Stevens KJ, Jamati MK, Garza D, Matheson GO. Athletic osteitis pubis. Sports Med. 2011;41(5):361-376.
26. Mehin R, Meek R, O’Brien P, Blachut P. Surgery for osteitis pubis. Can J Surg. 2006;49(3):170-176.
27. Grace JN, Sim FH, Shives TC, Coventry MB. Wedge resection of the symphysis pubis for the treatment of osteitis pubis. J Bone Joint Surg Am. 1989;71(3):358-364.
28. Amanatullah DF, Antkowiak T, Pillay K, et al. Femoroacetabular impingement: current concepts in diagnosis and treatment. Orthopedics. 2015;38(3):185-199.
29. Ganz R, Parvizi J, Beck M, Leunig M, Nötzli H, Siebenrock KA. Femoroacetabular impingement: a cause for osteoarthritis of the hip. Clin Orthop Relat Res. 2003;(417):112-120.
30. Redmond JM, Gupta A, Hammarstedt JE, Stake CE, Dunne KF, Domb BG. Labral injury: radiographic predictors at the time of hip arthroscopy. Arthroscopy. 2015;31(1):51-56.
31. Clohisy JC, Knaus ER, Hunt DM, Lesher JM, Harris-Hayes M, Prather H. Clinical presentation of patients with symptomatic anterior hip impingement. Clin Orthop Relat Res. 2009;467(3):638-644.
32. Klaue K, Durnin CW, Ganz R. The acetabular rim syndrome. A clinical presentation of dysplasia of the hip. J Bone Joint Surg Br. 1991;73(3):423-429.
33. Philippon MJ, Schenker ML. Arthroscopy for the treatment of femoroacetabular impingement in the athlete. Clin Sports Med. 2006;25(2):299-308.
34. McCarthy JC, Lee JA. Hip arthroscopy: indications, outcomes, and complications. Instr Course Lect. 2006;55:301-308.
Take-Home Points
- Perform a comprehensive examination to determine intra-articular pathology as well as potential extra-articular sources of hip and pelvic pain.
- Adductor strains can be prevented with adequate rehabilitation focused on correcting predisposing factors (ie, adductor weakness or tightness, limited range of motion, and core imbalance).
- Athletic pubalgia is diagnosed when tenderness can be elicited over the pubic tubercle.
- Osteitis pubis is diagnosed with pain over the pubic symphysis.
- FAI and labral injury classically present with a C-sign but can also present with lateral hip pain, buttock pain, low back pain, anterior thigh pain, and knee pain.
Hip and groin pain is a common finding among athletes of all ages and activity levels. Such pain most often occurs among athletes in sports such as football, hockey, rugby, soccer, and ballet, which demand frequent cutting, pivoting, and acceleration.1-4 Previously, pain about the hip and groin was attributed to muscular strains and soft-tissue contusions, but improvements in physical examination skills, imaging modalities, and disease-specific treatment options have led to increased recognition of hip injuries as a significant source of disability in the athletic population.5,6 These injuries make up 6% or more of all sports injuries, and the rate is increasing.7-9
In this review, we describe precise methods for evaluating the athlete’s hip or groin with an emphasis on recognizing the most common extra-articular and intra-articular pathologies, including adductor strains, athletic pubalgia, osteitis pubis, and femoroacetabular impingement (FAI) with labral tears.
Hip Pathoanatomy
The first step in determining the etiology of pain is to establish if there is true pathology of the hip joint and surrounding structures, or if the pain is referred from another source.
Patient History
The physical examination is guided by the patient’s history. Important patient-specific factors to be ascertained include age, sport(s) played, competition level, seasonal timing, and effect of the injury on performance. Regarding presenting symptoms, attention should be given to pain location, timing (acute vs chronic), onset, nature (clicking, catching, instability), and precipitating factors. Acute-onset pain with muscle contraction or stretching, possibly accompanied by an audible pop, is likely musculotendinous in origin. Insidious-onset dull aching pain that worsens with activity more commonly involves intra-articular processes. Most classically, this pain occurs deep in the groin and is demonstrated by the C sign: The patient cups a hand with its fingers pointing toward the anterior groin at the level of the greater trochanter (Figure 1).11
A comprehensive hip evaluation can be performed with the patient in the standing, seated, supine, lateral, and prone positions, as previously described (Table 2).6,12,13
Extra-Articular Hip Pathologies
Adductor Strains
The adductor muscle group includes the adductor magnus, adductor brevis, gracilis, obturator externus, pectineus, and adductor longus, which is the most commonly strained. Adductor strains are the most common cause of groin pain in athletes, and usually occur in sports that require forceful eccentric contraction of the adductors.14 Among professional soccer players, adductor strains represent almost one fourth of all muscle injuries and result in lost playing time averaging 2 weeks and an 18% reinjury rate.15 These injuries are particularly detrimental to performance because the adductor muscles help stabilize the pelvis during closed-chain activities.3 Diagnosis and adequate rehabilitation focused on correcting predisposing factors (eg, adductor weakness or tightness, loss of hip range of motion, core imbalance) are paramount in reinjury prevention.16,17
On presentation, athletes complain of aching groin or medial thigh pain. The examiner should assess for swelling or ecchymosis. There typically is tenderness to palpation at or near the origin on the pubic bones, with pain exacerbated with resisted adduction and passive stretch into abduction during examination. Palpation of adductors requires proper exposure and is most easily performed with the patient supine and the lower extremity in a figure-of-4 position (Figure 2A).
Athletic Pubalgia
Athletic pubalgia, also known as sports hernia or core muscle injury, is an injury to the soft tissues of the lower abdominal or posterior inguinal wall. Although not fully understood, the condition is considered the result of repetitive trunk hyperextension and thigh hyperabduction resulting in shearing at the pubic symphysis where there is a muscle imbalance between the strong proximal thigh muscles and weaker abdominals. This condition is more common in men and typically is insidious in onset with a prolonged course recalcitrant to nonoperative treatment.18 In studies of chronic groin pain in athletes, the rate of athletic pubalgia as the primary etiology ranges from 39% to 85%.9,19,20
Patients typically complain of increasing pain in the lower abdominal and proximal adductors during activity. Symptoms include unilateral or bilateral lower abdominal pain, which can radiate toward the perineum, rectus muscle, and proximal adductors during sport but usually abates with rest.18 Athletes endorse they are not capable of playing at their full athletic potential. Symptoms are initiated with sudden forceful movements, as in sit-ups, sprints, and valsalva maneuvers like coughs and sneezes. Valsalva maneuvers worsen pain in about 10% of patients.21-23On physical examination with the patient supine, tenderness can be elicited over the pubic tubercle, abdominal obliques, and/or rectus abdominis insertion (Figure 3A). Athletes may also have tenderness at the adductor longus tendon origin at or near the pubic symphysis, which may make the diagnosis difficult to distinguish from an adductor strain.
Osteitis Pubis
Osteitis pubis is a painful overuse injury that results in noninfectious inflammation of the pubic symphysis from increased motion at this normally stable immobile joint.3 As with athletic pubalgia, the exact mechanism is unclear, but likely it is similar to the repetitive stress placed on the pubic symphysis by unequal forces of the abdominal and adductor muscles.24 The disease can result in bony erosions and cartilage breakdown with irregularity of the pubic symphysis.
Athletes may complain of anterior and medial groin pain that can radiate to the lower abdominal muscles, perineum, inguinal region, and medial thigh. Walking, pelvic motion, adductor stretching, abdominal muscle exercises, and standing up can exacerbate pain.24 Some cases involve impaired internal or external rotation of the hip, sacroiliac joint dysfunction, or adductor and abductor muscle weakness.25The distinguishing feature of osteitis pubis is pain over the pubic symphysis with direct palpation (Figure 4A). Examination maneuvers that place stress on the pubic symphysis can aid in diagnosis.26
Intra-Articular Hip Pathology: Femoroacetabular Impingement
In athletes, FAI is a leading cause of intra-articular pathology, which can lead to labral tears.28,29 FAI lesions include cam-type impingement from an aspherical femoral head and pincer impingement from acetabular overcoverage, both of which limit internal rotation and cause acetabular rim abutment, which damages the labrum.
Athletes present with activity-related groin or hip pain that is exacerbated by hip flexion and internal rotation, with possible mechanical symptoms from labral tearing.30 However, the pain distribution varies. In a study by Clohisy and colleagues,31 of patients with symptomatic FAI that required surgical intervention, 88% had groin pain, 67% had lateral hip pain, 35% had anterior thigh pain, 29% had buttock pain, 27% had knee pain, and 23% had low back pain.
Careful attention should be given to range of motion in FAI patients, as they can usually flex their hip to 90° to 110°, and in this position there is limited internal rotation and asymmetric external rotation relative to the contralateral leg.32 The anterior impingement test is one of the most reliable tests for FAI (Figure 5A).32 With the patient supine, the hip is dynamically flexed to 90°, adducted, and internally rotated. A positive test elicits deep anterior groin pain that generally replicates the patient’s symptoms.29
Conclusion
Careful, directed history taking and physical examination are essential in narrowing the diagnostic possibilities before initiating a workup for the common intra-articular and extra-articular causes of hip and groin pain in athletes.
Am J Orthop. 2017;46(1):10-16. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
Take-Home Points
- Perform a comprehensive examination to determine intra-articular pathology as well as potential extra-articular sources of hip and pelvic pain.
- Adductor strains can be prevented with adequate rehabilitation focused on correcting predisposing factors (ie, adductor weakness or tightness, limited range of motion, and core imbalance).
- Athletic pubalgia is diagnosed when tenderness can be elicited over the pubic tubercle.
- Osteitis pubis is diagnosed with pain over the pubic symphysis.
- FAI and labral injury classically present with a C-sign but can also present with lateral hip pain, buttock pain, low back pain, anterior thigh pain, and knee pain.
Hip and groin pain is a common finding among athletes of all ages and activity levels. Such pain most often occurs among athletes in sports such as football, hockey, rugby, soccer, and ballet, which demand frequent cutting, pivoting, and acceleration.1-4 Previously, pain about the hip and groin was attributed to muscular strains and soft-tissue contusions, but improvements in physical examination skills, imaging modalities, and disease-specific treatment options have led to increased recognition of hip injuries as a significant source of disability in the athletic population.5,6 These injuries make up 6% or more of all sports injuries, and the rate is increasing.7-9
In this review, we describe precise methods for evaluating the athlete’s hip or groin with an emphasis on recognizing the most common extra-articular and intra-articular pathologies, including adductor strains, athletic pubalgia, osteitis pubis, and femoroacetabular impingement (FAI) with labral tears.
Hip Pathoanatomy
The first step in determining the etiology of pain is to establish if there is true pathology of the hip joint and surrounding structures, or if the pain is referred from another source.
Patient History
The physical examination is guided by the patient’s history. Important patient-specific factors to be ascertained include age, sport(s) played, competition level, seasonal timing, and effect of the injury on performance. Regarding presenting symptoms, attention should be given to pain location, timing (acute vs chronic), onset, nature (clicking, catching, instability), and precipitating factors. Acute-onset pain with muscle contraction or stretching, possibly accompanied by an audible pop, is likely musculotendinous in origin. Insidious-onset dull aching pain that worsens with activity more commonly involves intra-articular processes. Most classically, this pain occurs deep in the groin and is demonstrated by the C sign: The patient cups a hand with its fingers pointing toward the anterior groin at the level of the greater trochanter (Figure 1).11
A comprehensive hip evaluation can be performed with the patient in the standing, seated, supine, lateral, and prone positions, as previously described (Table 2).6,12,13
Extra-Articular Hip Pathologies
Adductor Strains
The adductor muscle group includes the adductor magnus, adductor brevis, gracilis, obturator externus, pectineus, and adductor longus, which is the most commonly strained. Adductor strains are the most common cause of groin pain in athletes, and usually occur in sports that require forceful eccentric contraction of the adductors.14 Among professional soccer players, adductor strains represent almost one fourth of all muscle injuries and result in lost playing time averaging 2 weeks and an 18% reinjury rate.15 These injuries are particularly detrimental to performance because the adductor muscles help stabilize the pelvis during closed-chain activities.3 Diagnosis and adequate rehabilitation focused on correcting predisposing factors (eg, adductor weakness or tightness, loss of hip range of motion, core imbalance) are paramount in reinjury prevention.16,17
On presentation, athletes complain of aching groin or medial thigh pain. The examiner should assess for swelling or ecchymosis. There typically is tenderness to palpation at or near the origin on the pubic bones, with pain exacerbated with resisted adduction and passive stretch into abduction during examination. Palpation of adductors requires proper exposure and is most easily performed with the patient supine and the lower extremity in a figure-of-4 position (Figure 2A).
Athletic Pubalgia
Athletic pubalgia, also known as sports hernia or core muscle injury, is an injury to the soft tissues of the lower abdominal or posterior inguinal wall. Although not fully understood, the condition is considered the result of repetitive trunk hyperextension and thigh hyperabduction resulting in shearing at the pubic symphysis where there is a muscle imbalance between the strong proximal thigh muscles and weaker abdominals. This condition is more common in men and typically is insidious in onset with a prolonged course recalcitrant to nonoperative treatment.18 In studies of chronic groin pain in athletes, the rate of athletic pubalgia as the primary etiology ranges from 39% to 85%.9,19,20
Patients typically complain of increasing pain in the lower abdominal and proximal adductors during activity. Symptoms include unilateral or bilateral lower abdominal pain, which can radiate toward the perineum, rectus muscle, and proximal adductors during sport but usually abates with rest.18 Athletes endorse they are not capable of playing at their full athletic potential. Symptoms are initiated with sudden forceful movements, as in sit-ups, sprints, and valsalva maneuvers like coughs and sneezes. Valsalva maneuvers worsen pain in about 10% of patients.21-23On physical examination with the patient supine, tenderness can be elicited over the pubic tubercle, abdominal obliques, and/or rectus abdominis insertion (Figure 3A). Athletes may also have tenderness at the adductor longus tendon origin at or near the pubic symphysis, which may make the diagnosis difficult to distinguish from an adductor strain.
Osteitis Pubis
Osteitis pubis is a painful overuse injury that results in noninfectious inflammation of the pubic symphysis from increased motion at this normally stable immobile joint.3 As with athletic pubalgia, the exact mechanism is unclear, but likely it is similar to the repetitive stress placed on the pubic symphysis by unequal forces of the abdominal and adductor muscles.24 The disease can result in bony erosions and cartilage breakdown with irregularity of the pubic symphysis.
Athletes may complain of anterior and medial groin pain that can radiate to the lower abdominal muscles, perineum, inguinal region, and medial thigh. Walking, pelvic motion, adductor stretching, abdominal muscle exercises, and standing up can exacerbate pain.24 Some cases involve impaired internal or external rotation of the hip, sacroiliac joint dysfunction, or adductor and abductor muscle weakness.25The distinguishing feature of osteitis pubis is pain over the pubic symphysis with direct palpation (Figure 4A). Examination maneuvers that place stress on the pubic symphysis can aid in diagnosis.26
Intra-Articular Hip Pathology: Femoroacetabular Impingement
In athletes, FAI is a leading cause of intra-articular pathology, which can lead to labral tears.28,29 FAI lesions include cam-type impingement from an aspherical femoral head and pincer impingement from acetabular overcoverage, both of which limit internal rotation and cause acetabular rim abutment, which damages the labrum.
Athletes present with activity-related groin or hip pain that is exacerbated by hip flexion and internal rotation, with possible mechanical symptoms from labral tearing.30 However, the pain distribution varies. In a study by Clohisy and colleagues,31 of patients with symptomatic FAI that required surgical intervention, 88% had groin pain, 67% had lateral hip pain, 35% had anterior thigh pain, 29% had buttock pain, 27% had knee pain, and 23% had low back pain.
Careful attention should be given to range of motion in FAI patients, as they can usually flex their hip to 90° to 110°, and in this position there is limited internal rotation and asymmetric external rotation relative to the contralateral leg.32 The anterior impingement test is one of the most reliable tests for FAI (Figure 5A).32 With the patient supine, the hip is dynamically flexed to 90°, adducted, and internally rotated. A positive test elicits deep anterior groin pain that generally replicates the patient’s symptoms.29
Conclusion
Careful, directed history taking and physical examination are essential in narrowing the diagnostic possibilities before initiating a workup for the common intra-articular and extra-articular causes of hip and groin pain in athletes.
Am J Orthop. 2017;46(1):10-16. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
1. Boyd KT, Peirce NS, Batt ME. Common hip injuries in sport. Sports Med. 1997;24(4):273-288.
2. Duthon VB, Charbonnier C, Kolo FC, et al. Correlation of clinical and magnetic resonance imaging findings in hips of elite female ballet dancers. Arthroscopy. 2013;29(3):411-419.
3. Prather H, Cheng A. Diagnosis and treatment of hip girdle pain in the athlete. PM R. 2016;8(3 suppl):S45-S60.
4. Larson CM. Sports hernia/athletic pubalgia: evaluation and management. Sports Health. 2014;6(2):139-144.
5. Bizzini M, Notzli HP, Maffiuletti NA. Femoroacetabular impingement in professional ice hockey players: a case series of 5 athletes after open surgical decompression of the hip. Am J Sports Med. 2007;35(11):1955-1959.
6. Lynch TS, Terry MA, Bedi A, Kelly BT. Hip arthroscopic surgery: patient evaluation, current indications, and outcomes. Am J Sports Med. 2013;41(5):1174-1189.
7. Anderson K, Strickland SM, Warren R. Hip and groin injuries in athletes. Am J Sports Med. 2001;29(4):521-533.
8. Fon LJ, Spence RA. Sportsman’s hernia. Br J Surg. 2000;87(5):545-552.
9. Kluin J, den Hoed PT, van Linschoten R, IJzerman JC, van Steensel CJ. Endoscopic evaluation and treatment of groin pain in the athlete. Am J Sports Med. 2004;32(4):944-949.
10. Ward D, Parvizi J. Management of hip pain in young adults. Orthop Clin North Am. 2016;47(3):485-496.
11. Byrd JW. Hip arthroscopy. J Am Acad Orthop Surg. 2006;14(7):433-444.
12. Martin HD, Palmer IJ. History and physical examination of the hip: the basics. Curr Rev Musculoskelet Med. 2013;6(3):219-225.
13. Shindle MK, Voos JE, Nho SJ, Heyworth BE, Kelly BT. Arthroscopic management of labral tears in the hip. J Bone Joint Surg Am. 2008;90(suppl 4):2-19.
14. Morelli V, Smith V. Groin injuries in athletes. Am Fam Physician. 2001;64(8):1405-1414.
15. Ekstrand J, Hagglund M, Walden M. Epidemiology of muscle injuries in professional football (soccer). Am J Sports Med. 2011;39(6):1226-1232.
16. Ekstrand J, Gillquist J. The avoidability of soccer injuries. Int J Sports Med. 1983;4(2):124-128.
17. Tyler TF, Nicholas SJ, Campbell RJ, McHugh MP. The association of hip strength and flexibility with the incidence of adductor muscle strains in professional ice hockey players. Am J Sports Med. 2001;29(2):124-128.
18. Farber AJ, Wilckens JH. Sports hernia: diagnosis and therapeutic approach. J Am Acad Orthop Surg. 2007;15(8):507-514.
19. De Paulis F, Cacchio A, Michelini O, Damiani A, Saggini R. Sports injuries in the pelvis and hip: diagnostic imaging. Eur J Radiol. 1998;27(suppl 1):S49-S59.
20. Lovell G. The diagnosis of chronic groin pain in athletes: a review of 189 cases. Aust J Sci Med Sport. 1995;27(suppl 1):76-79.
21. Strosberg DS, Ellis TJ, Renton DB. The role of femoroacetabular impingement in core muscle injury/athletic pubalgia: diagnosis and management. Front Surg. 2016;3:6.
22. Meyers WC, Foley DP, Garrett WE, Lohnes JH, Mandlebaum BR. Management of severe lower abdominal or inguinal pain in high-performance athletes. PAIN (Performing Athletes with Abdominal or Inguinal Neuromuscular Pain Study Group). Am J Sports Med. 2000;28(1):2-8.
23. Ahumada LA, Ashruf S, Espinosa-de-los-Monteros A, et al. Athletic pubalgia: definition and surgical treatment. Ann Plast Surg. 2005;55(4):393-396.
24. Angoules AG. Osteitis pubis in elite athletes: diagnostic and therapeutic approach. World J Orthop. 2015;6(9):672-679.
25. Hiti CJ, Stevens KJ, Jamati MK, Garza D, Matheson GO. Athletic osteitis pubis. Sports Med. 2011;41(5):361-376.
26. Mehin R, Meek R, O’Brien P, Blachut P. Surgery for osteitis pubis. Can J Surg. 2006;49(3):170-176.
27. Grace JN, Sim FH, Shives TC, Coventry MB. Wedge resection of the symphysis pubis for the treatment of osteitis pubis. J Bone Joint Surg Am. 1989;71(3):358-364.
28. Amanatullah DF, Antkowiak T, Pillay K, et al. Femoroacetabular impingement: current concepts in diagnosis and treatment. Orthopedics. 2015;38(3):185-199.
29. Ganz R, Parvizi J, Beck M, Leunig M, Nötzli H, Siebenrock KA. Femoroacetabular impingement: a cause for osteoarthritis of the hip. Clin Orthop Relat Res. 2003;(417):112-120.
30. Redmond JM, Gupta A, Hammarstedt JE, Stake CE, Dunne KF, Domb BG. Labral injury: radiographic predictors at the time of hip arthroscopy. Arthroscopy. 2015;31(1):51-56.
31. Clohisy JC, Knaus ER, Hunt DM, Lesher JM, Harris-Hayes M, Prather H. Clinical presentation of patients with symptomatic anterior hip impingement. Clin Orthop Relat Res. 2009;467(3):638-644.
32. Klaue K, Durnin CW, Ganz R. The acetabular rim syndrome. A clinical presentation of dysplasia of the hip. J Bone Joint Surg Br. 1991;73(3):423-429.
33. Philippon MJ, Schenker ML. Arthroscopy for the treatment of femoroacetabular impingement in the athlete. Clin Sports Med. 2006;25(2):299-308.
34. McCarthy JC, Lee JA. Hip arthroscopy: indications, outcomes, and complications. Instr Course Lect. 2006;55:301-308.
1. Boyd KT, Peirce NS, Batt ME. Common hip injuries in sport. Sports Med. 1997;24(4):273-288.
2. Duthon VB, Charbonnier C, Kolo FC, et al. Correlation of clinical and magnetic resonance imaging findings in hips of elite female ballet dancers. Arthroscopy. 2013;29(3):411-419.
3. Prather H, Cheng A. Diagnosis and treatment of hip girdle pain in the athlete. PM R. 2016;8(3 suppl):S45-S60.
4. Larson CM. Sports hernia/athletic pubalgia: evaluation and management. Sports Health. 2014;6(2):139-144.
5. Bizzini M, Notzli HP, Maffiuletti NA. Femoroacetabular impingement in professional ice hockey players: a case series of 5 athletes after open surgical decompression of the hip. Am J Sports Med. 2007;35(11):1955-1959.
6. Lynch TS, Terry MA, Bedi A, Kelly BT. Hip arthroscopic surgery: patient evaluation, current indications, and outcomes. Am J Sports Med. 2013;41(5):1174-1189.
7. Anderson K, Strickland SM, Warren R. Hip and groin injuries in athletes. Am J Sports Med. 2001;29(4):521-533.
8. Fon LJ, Spence RA. Sportsman’s hernia. Br J Surg. 2000;87(5):545-552.
9. Kluin J, den Hoed PT, van Linschoten R, IJzerman JC, van Steensel CJ. Endoscopic evaluation and treatment of groin pain in the athlete. Am J Sports Med. 2004;32(4):944-949.
10. Ward D, Parvizi J. Management of hip pain in young adults. Orthop Clin North Am. 2016;47(3):485-496.
11. Byrd JW. Hip arthroscopy. J Am Acad Orthop Surg. 2006;14(7):433-444.
12. Martin HD, Palmer IJ. History and physical examination of the hip: the basics. Curr Rev Musculoskelet Med. 2013;6(3):219-225.
13. Shindle MK, Voos JE, Nho SJ, Heyworth BE, Kelly BT. Arthroscopic management of labral tears in the hip. J Bone Joint Surg Am. 2008;90(suppl 4):2-19.
14. Morelli V, Smith V. Groin injuries in athletes. Am Fam Physician. 2001;64(8):1405-1414.
15. Ekstrand J, Hagglund M, Walden M. Epidemiology of muscle injuries in professional football (soccer). Am J Sports Med. 2011;39(6):1226-1232.
16. Ekstrand J, Gillquist J. The avoidability of soccer injuries. Int J Sports Med. 1983;4(2):124-128.
17. Tyler TF, Nicholas SJ, Campbell RJ, McHugh MP. The association of hip strength and flexibility with the incidence of adductor muscle strains in professional ice hockey players. Am J Sports Med. 2001;29(2):124-128.
18. Farber AJ, Wilckens JH. Sports hernia: diagnosis and therapeutic approach. J Am Acad Orthop Surg. 2007;15(8):507-514.
19. De Paulis F, Cacchio A, Michelini O, Damiani A, Saggini R. Sports injuries in the pelvis and hip: diagnostic imaging. Eur J Radiol. 1998;27(suppl 1):S49-S59.
20. Lovell G. The diagnosis of chronic groin pain in athletes: a review of 189 cases. Aust J Sci Med Sport. 1995;27(suppl 1):76-79.
21. Strosberg DS, Ellis TJ, Renton DB. The role of femoroacetabular impingement in core muscle injury/athletic pubalgia: diagnosis and management. Front Surg. 2016;3:6.
22. Meyers WC, Foley DP, Garrett WE, Lohnes JH, Mandlebaum BR. Management of severe lower abdominal or inguinal pain in high-performance athletes. PAIN (Performing Athletes with Abdominal or Inguinal Neuromuscular Pain Study Group). Am J Sports Med. 2000;28(1):2-8.
23. Ahumada LA, Ashruf S, Espinosa-de-los-Monteros A, et al. Athletic pubalgia: definition and surgical treatment. Ann Plast Surg. 2005;55(4):393-396.
24. Angoules AG. Osteitis pubis in elite athletes: diagnostic and therapeutic approach. World J Orthop. 2015;6(9):672-679.
25. Hiti CJ, Stevens KJ, Jamati MK, Garza D, Matheson GO. Athletic osteitis pubis. Sports Med. 2011;41(5):361-376.
26. Mehin R, Meek R, O’Brien P, Blachut P. Surgery for osteitis pubis. Can J Surg. 2006;49(3):170-176.
27. Grace JN, Sim FH, Shives TC, Coventry MB. Wedge resection of the symphysis pubis for the treatment of osteitis pubis. J Bone Joint Surg Am. 1989;71(3):358-364.
28. Amanatullah DF, Antkowiak T, Pillay K, et al. Femoroacetabular impingement: current concepts in diagnosis and treatment. Orthopedics. 2015;38(3):185-199.
29. Ganz R, Parvizi J, Beck M, Leunig M, Nötzli H, Siebenrock KA. Femoroacetabular impingement: a cause for osteoarthritis of the hip. Clin Orthop Relat Res. 2003;(417):112-120.
30. Redmond JM, Gupta A, Hammarstedt JE, Stake CE, Dunne KF, Domb BG. Labral injury: radiographic predictors at the time of hip arthroscopy. Arthroscopy. 2015;31(1):51-56.
31. Clohisy JC, Knaus ER, Hunt DM, Lesher JM, Harris-Hayes M, Prather H. Clinical presentation of patients with symptomatic anterior hip impingement. Clin Orthop Relat Res. 2009;467(3):638-644.
32. Klaue K, Durnin CW, Ganz R. The acetabular rim syndrome. A clinical presentation of dysplasia of the hip. J Bone Joint Surg Br. 1991;73(3):423-429.
33. Philippon MJ, Schenker ML. Arthroscopy for the treatment of femoroacetabular impingement in the athlete. Clin Sports Med. 2006;25(2):299-308.
34. McCarthy JC, Lee JA. Hip arthroscopy: indications, outcomes, and complications. Instr Course Lect. 2006;55:301-308.