User login
Patient unhappiness with migraine prophylaxis options abounds
VALENCIA, SPAIN – How dissatisfied are migraine patients with their prophylactic medication options?
Here’s a clue: Roughly two-thirds of migraine patients who initiate topiramate, a beta-blocker, or a tricyclic antidepressant for prophylaxis have given up on prophylactic therapy altogether within 9 months, Dr. Robert Lenz reported at the International Headache Congress.
Moreover, treatment gaps longer than 90 days in duration are the rule rather than the exception within the first year after starting prophylactic therapy. Seventy-eight percent of patients who start on topiramate for migraine prophylaxis have such a gap in therapy within the first year, as do 80% who start on a beta-blocker, and 85% on a tricyclic antidepressant. These prolonged treatment gaps occur early: a mean of 95 days after commencing treatment. And only 9%-12% of patients restart prophylaxis after a gap, added Dr. Lenz of Amgen in Thousand Oaks, Calif.
He presented an analysis of patterns of prophylaxis use in 107,122 migraine patients who initiated therapy during 2008-2011. Forty-nine percent went on topiramate for this purpose, 30% on a tricyclic antidepressant, and 21% on a beta-blocker. The researchers used Medicare claims data as well as information from the Truven Health Analytics MarketScan Commercial Claims and Encounters database.
Despite the generally poor persistence with prophylactic therapy documented in this study, few patients were interested in switching to a different preventive medication or adding a second one. Indeed, only 12%-14% of patients did so within the first year after starting prophylaxis, Dr. Lenz noted at the meeting, which was sponsored by the International Headache Society and the American Headache Society.
Eighty-one percent of patients who started on migraine prophylaxis also used acute migraine medications during the first year. Forty-eight percent of the study population filled an average of 4.4 prescriptions for a triptan during that period, whereas 38% used a triptan in the year prior to going on prophylaxis.
More disturbingly, Dr. Lenz said, 53% of patients who initiated prophylactic migraine therapy also received opioids during that first year; indeed, they filled an average of 6.1 prescriptions for opioids during the year. However, upon exclusion of patients who carried a diagnosis of another chronic pain condition, such as low back pain, arthritis, or neuropathic pain, 40% of the remaining patients who started migraine prophylaxis received opioids during the study year, filling an average of 3.8 prescriptions.
The study was funded by Dr. Lenz’ employer, Amgen, which is among the pharmaceutical companies developing a new class of monoclonal antibodies to calcitonin gene-related peptide (CGRP) for the prevention of migraine.
VALENCIA, SPAIN – How dissatisfied are migraine patients with their prophylactic medication options?
Here’s a clue: Roughly two-thirds of migraine patients who initiate topiramate, a beta-blocker, or a tricyclic antidepressant for prophylaxis have given up on prophylactic therapy altogether within 9 months, Dr. Robert Lenz reported at the International Headache Congress.
Moreover, treatment gaps longer than 90 days in duration are the rule rather than the exception within the first year after starting prophylactic therapy. Seventy-eight percent of patients who start on topiramate for migraine prophylaxis have such a gap in therapy within the first year, as do 80% who start on a beta-blocker, and 85% on a tricyclic antidepressant. These prolonged treatment gaps occur early: a mean of 95 days after commencing treatment. And only 9%-12% of patients restart prophylaxis after a gap, added Dr. Lenz of Amgen in Thousand Oaks, Calif.
He presented an analysis of patterns of prophylaxis use in 107,122 migraine patients who initiated therapy during 2008-2011. Forty-nine percent went on topiramate for this purpose, 30% on a tricyclic antidepressant, and 21% on a beta-blocker. The researchers used Medicare claims data as well as information from the Truven Health Analytics MarketScan Commercial Claims and Encounters database.
Despite the generally poor persistence with prophylactic therapy documented in this study, few patients were interested in switching to a different preventive medication or adding a second one. Indeed, only 12%-14% of patients did so within the first year after starting prophylaxis, Dr. Lenz noted at the meeting, which was sponsored by the International Headache Society and the American Headache Society.
Eighty-one percent of patients who started on migraine prophylaxis also used acute migraine medications during the first year. Forty-eight percent of the study population filled an average of 4.4 prescriptions for a triptan during that period, whereas 38% used a triptan in the year prior to going on prophylaxis.
More disturbingly, Dr. Lenz said, 53% of patients who initiated prophylactic migraine therapy also received opioids during that first year; indeed, they filled an average of 6.1 prescriptions for opioids during the year. However, upon exclusion of patients who carried a diagnosis of another chronic pain condition, such as low back pain, arthritis, or neuropathic pain, 40% of the remaining patients who started migraine prophylaxis received opioids during the study year, filling an average of 3.8 prescriptions.
The study was funded by Dr. Lenz’ employer, Amgen, which is among the pharmaceutical companies developing a new class of monoclonal antibodies to calcitonin gene-related peptide (CGRP) for the prevention of migraine.
VALENCIA, SPAIN – How dissatisfied are migraine patients with their prophylactic medication options?
Here’s a clue: Roughly two-thirds of migraine patients who initiate topiramate, a beta-blocker, or a tricyclic antidepressant for prophylaxis have given up on prophylactic therapy altogether within 9 months, Dr. Robert Lenz reported at the International Headache Congress.
Moreover, treatment gaps longer than 90 days in duration are the rule rather than the exception within the first year after starting prophylactic therapy. Seventy-eight percent of patients who start on topiramate for migraine prophylaxis have such a gap in therapy within the first year, as do 80% who start on a beta-blocker, and 85% on a tricyclic antidepressant. These prolonged treatment gaps occur early: a mean of 95 days after commencing treatment. And only 9%-12% of patients restart prophylaxis after a gap, added Dr. Lenz of Amgen in Thousand Oaks, Calif.
He presented an analysis of patterns of prophylaxis use in 107,122 migraine patients who initiated therapy during 2008-2011. Forty-nine percent went on topiramate for this purpose, 30% on a tricyclic antidepressant, and 21% on a beta-blocker. The researchers used Medicare claims data as well as information from the Truven Health Analytics MarketScan Commercial Claims and Encounters database.
Despite the generally poor persistence with prophylactic therapy documented in this study, few patients were interested in switching to a different preventive medication or adding a second one. Indeed, only 12%-14% of patients did so within the first year after starting prophylaxis, Dr. Lenz noted at the meeting, which was sponsored by the International Headache Society and the American Headache Society.
Eighty-one percent of patients who started on migraine prophylaxis also used acute migraine medications during the first year. Forty-eight percent of the study population filled an average of 4.4 prescriptions for a triptan during that period, whereas 38% used a triptan in the year prior to going on prophylaxis.
More disturbingly, Dr. Lenz said, 53% of patients who initiated prophylactic migraine therapy also received opioids during that first year; indeed, they filled an average of 6.1 prescriptions for opioids during the year. However, upon exclusion of patients who carried a diagnosis of another chronic pain condition, such as low back pain, arthritis, or neuropathic pain, 40% of the remaining patients who started migraine prophylaxis received opioids during the study year, filling an average of 3.8 prescriptions.
The study was funded by Dr. Lenz’ employer, Amgen, which is among the pharmaceutical companies developing a new class of monoclonal antibodies to calcitonin gene-related peptide (CGRP) for the prevention of migraine.
AT IHC 2015
Key clinical point: Migraine patients are deeply dissatisfied with their current options for prophylactic therapy.
Major finding: About one-third of patients who initiate prophylactic drug therapy for migraine are still on preventive therapy 9 months later.
Data source: An analysis of claims data for more than 107,000 U.S. patients who initiated prophylactic migraine therapy.
Disclosures: The study was sponsored by Amgen and presented by a company employee.
Botox effective in pediatric chronic migraine
VALENCIA, SPAIN – OnabotulinumtoxinA injections are safe, well tolerated, and effective for chronic migraine in adolescents refractory to medications, Dr. Ilya Bragin reported at the International Headache Congress.
“Overall, botulinum toxin type A proved to be an excellent treatment option for this group,” said Dr. Bragin, a neurologist at the State University of New York Upstate Medical Center at Syracuse.
He reported on 19 adolescents, mean age 15.7 years, seen for chronic migraine at a pediatric headache clinic. All had failed to respond to multiple standard medications, including amitriptyline and topiramate. The teens were treated with onabotulinumtoxinA (Botox) injections using the standard Food and Drug Administration–specified adult protocol.
This was off-label therapy, since Botox is FDA approved for the treatment of headaches only in adults. However, as was noted by numerous speakers at the meeting, which was sponsored by the International Headache Society and the American Headache Society, the great majority of drug prescribing for pediatric headaches is off label.
Indeed, the only FDA-approved medications for pediatric migraine are topiramate (Topamax) and almotriptan (Axert) for use in patients 12 years of age and older, rizatriptan (Maxalt) starting as early as age 6 years, and – as of May 2015 – Treximet, a proprietary sumatriptan/naproxen sodium combination approved for use in patients as young as 12 years of age.
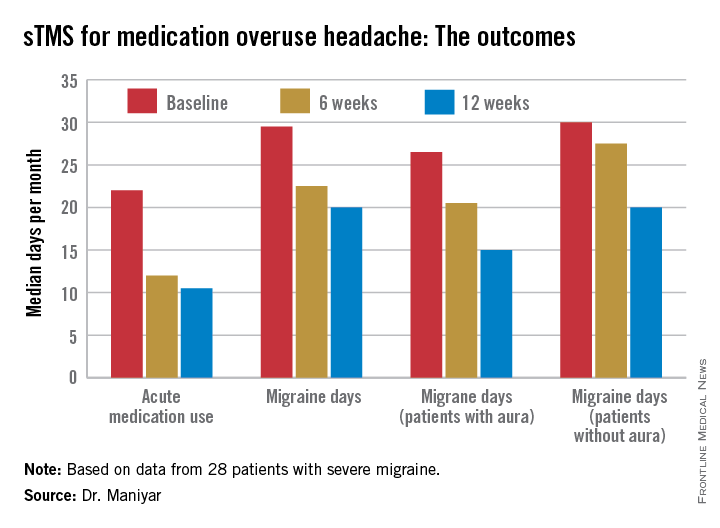
Dr. Bragin reported that after an average of two injection cycles, the adolescents’ migraine severity decreased by a mean of nearly 3 points on a 1-10 visual analog scale, from 7.7 at baseline to 4.8. Mean headache frequency fell from 24.8 days per month to 15.
Drilling down further into the results, 12 patients had severe migraine at baseline as defined by a pain score of 7-10. After treatment, 5 of the 12 were still categorized as severe, 1 moderate, 4 mild – meaning they had a pain score of 1-3 – and 2 patients became headache free.
Among the seven patients with moderate chronic migraine at baseline, five remained in the moderate category post treatment, one became mild, and one patient experienced headache resolution.
Thus, although the study sample size was limited, it appears from these results that adolescents with the most severe chronic migraine at baseline see the most benefit, the neurologist observed.
Dr. Bragin reported having no financial conflicts regarding this study.
VALENCIA, SPAIN – OnabotulinumtoxinA injections are safe, well tolerated, and effective for chronic migraine in adolescents refractory to medications, Dr. Ilya Bragin reported at the International Headache Congress.
“Overall, botulinum toxin type A proved to be an excellent treatment option for this group,” said Dr. Bragin, a neurologist at the State University of New York Upstate Medical Center at Syracuse.
He reported on 19 adolescents, mean age 15.7 years, seen for chronic migraine at a pediatric headache clinic. All had failed to respond to multiple standard medications, including amitriptyline and topiramate. The teens were treated with onabotulinumtoxinA (Botox) injections using the standard Food and Drug Administration–specified adult protocol.
This was off-label therapy, since Botox is FDA approved for the treatment of headaches only in adults. However, as was noted by numerous speakers at the meeting, which was sponsored by the International Headache Society and the American Headache Society, the great majority of drug prescribing for pediatric headaches is off label.
Indeed, the only FDA-approved medications for pediatric migraine are topiramate (Topamax) and almotriptan (Axert) for use in patients 12 years of age and older, rizatriptan (Maxalt) starting as early as age 6 years, and – as of May 2015 – Treximet, a proprietary sumatriptan/naproxen sodium combination approved for use in patients as young as 12 years of age.
Dr. Bragin reported that after an average of two injection cycles, the adolescents’ migraine severity decreased by a mean of nearly 3 points on a 1-10 visual analog scale, from 7.7 at baseline to 4.8. Mean headache frequency fell from 24.8 days per month to 15.
Drilling down further into the results, 12 patients had severe migraine at baseline as defined by a pain score of 7-10. After treatment, 5 of the 12 were still categorized as severe, 1 moderate, 4 mild – meaning they had a pain score of 1-3 – and 2 patients became headache free.
Among the seven patients with moderate chronic migraine at baseline, five remained in the moderate category post treatment, one became mild, and one patient experienced headache resolution.
Thus, although the study sample size was limited, it appears from these results that adolescents with the most severe chronic migraine at baseline see the most benefit, the neurologist observed.
Dr. Bragin reported having no financial conflicts regarding this study.
VALENCIA, SPAIN – OnabotulinumtoxinA injections are safe, well tolerated, and effective for chronic migraine in adolescents refractory to medications, Dr. Ilya Bragin reported at the International Headache Congress.
“Overall, botulinum toxin type A proved to be an excellent treatment option for this group,” said Dr. Bragin, a neurologist at the State University of New York Upstate Medical Center at Syracuse.
He reported on 19 adolescents, mean age 15.7 years, seen for chronic migraine at a pediatric headache clinic. All had failed to respond to multiple standard medications, including amitriptyline and topiramate. The teens were treated with onabotulinumtoxinA (Botox) injections using the standard Food and Drug Administration–specified adult protocol.
This was off-label therapy, since Botox is FDA approved for the treatment of headaches only in adults. However, as was noted by numerous speakers at the meeting, which was sponsored by the International Headache Society and the American Headache Society, the great majority of drug prescribing for pediatric headaches is off label.
Indeed, the only FDA-approved medications for pediatric migraine are topiramate (Topamax) and almotriptan (Axert) for use in patients 12 years of age and older, rizatriptan (Maxalt) starting as early as age 6 years, and – as of May 2015 – Treximet, a proprietary sumatriptan/naproxen sodium combination approved for use in patients as young as 12 years of age.
Dr. Bragin reported that after an average of two injection cycles, the adolescents’ migraine severity decreased by a mean of nearly 3 points on a 1-10 visual analog scale, from 7.7 at baseline to 4.8. Mean headache frequency fell from 24.8 days per month to 15.
Drilling down further into the results, 12 patients had severe migraine at baseline as defined by a pain score of 7-10. After treatment, 5 of the 12 were still categorized as severe, 1 moderate, 4 mild – meaning they had a pain score of 1-3 – and 2 patients became headache free.
Among the seven patients with moderate chronic migraine at baseline, five remained in the moderate category post treatment, one became mild, and one patient experienced headache resolution.
Thus, although the study sample size was limited, it appears from these results that adolescents with the most severe chronic migraine at baseline see the most benefit, the neurologist observed.
Dr. Bragin reported having no financial conflicts regarding this study.
AT IHC 2015
Key clinical point: OnabotulinumtoxinA injections appear to be well tolerated and effective for refractory chronic migraine in adolescents.
Major finding: Mean pain scores on a 1-10 scale improved from 7.7 at baseline to 4.8 after 2 standard injection cycles.
Data source: A retrospective case series of 19 adolescents treated for refractory chronic migraine at a pediatric headache clinic.
Disclosures: The presenter reported having no financial conflicts regarding this unsponsored study.
TAVI embolic protection device shows favorable safety, efficacy
PARIS – Using the TriGuard embolic protection device during transcatheter aortic valve implantation resulted in a fivefold reduction in the incidence of new postprocedural neurologic deficits, compared with unprotected TAVI in the randomized DEFLECT III trial.
Moreover, TriGuard-protected TAVI patients showed a 44% reduction in the volume of new brain lesions on diffusion-weighted MRI at discharge, coupled with a 45% greater likelihood of freedom from any cerebral ischemic lesions, compared with the unprotected controls, Dr. Andreas Baumbach reported at the annual congress of the European Association of Percutaneous Cardiovascular Interventions.
The TriGuard is a filter/deflector device that covers all three cerebral arteries, and thus all brain territories. It consists of a single-wire nitinol frame and mesh filter with a pore size of 130 microns, enabling it to deflect cerebral emboli during TAVI while allowing maximal blood flow. The device is approved and commercially available in Europe but remains investigational in the United States
TriGuard is delivered by a 9 Fr sheath from the femoral artery and maintained during the TAVI procedure by a stabilizer in the innominate artery. Although introducing an additional element into TAVI raises the theoretic possibility of safety concerns, no safety signal was seen in DEFLECT III. In-hospital rates of death, stroke, bleeding, acute kidney injury, and major vascular complications didn’t differ between TriGuard recipients and controls in the 85-patient study.
TriGuard is designed to address an emerging concern as TAVI’s popularity soars and the procedure is extended to younger, lower–surgical risk patients: namely, the high rate of new embolic lesions seen on brain imaging post procedure. Such lesions have been linked to cognitive decline, noted Dr. Baumbach, professor of interventional cardiology at the University of Bristol (England).
Focusing on outcomes in the 89% of TriGuard recipients whose device remained in position and thus provided complete three-vessel coverage throughout TAVI, he said that group had significantly fewer new neurologic deficits on the National Institutes of Health Stroke Scale at discharge than the control group, by a margin of 3.1% versus 15.4%. Twenty-seven percent of the TriGuard group had no new ischemic brain lesions at discharge as determined by diffusion-weighted MRI, compared with 11.5% of controls who underwent TAVI with no cerebral protection. The TriGuard group also had significantly better scores at discharge on the Montreal Cognitive Assessment and on a delayed memory task.
Of particular importance was the finding that 30 days post procedure the TriGuard group was 2.27-fold more likely to have recovered normal cognitive function, compared with unprotected controls, the cardiologist continued. What that means is that 45% of the TriGuard group had an age-normalized Montreal Cognitive Assessment score greater than 26 out of a possible 30, compared with just 20% of controls.
Another intriguing finding was that roughly 10% of patients in both study arms developed new cerebral emboli between their discharge and 30-day follow-up brain imaging studies. By definition, such emboli can’t be prevented using the TriGuard, but this finding identifies the postprocedural period as one of continued elevated embolic risk, Dr. Baumbach said.
DEFLECT III was a 13-center, 30-day, exploratory, prospective, randomized trial designed to establish benchmark rates for the upcoming larger pivotal REFLECT randomized trial. Simultaneous with Dr. Baumbach’s presentation in Paris, the final DEFLECT III results were published online (Eur. Heart J. 2015 May 19. [doi:10.1093/eurheartj/ehv191]). Session cochair Dr. Stephan Windecker of the University of Bern (Switzerland) noted that there’s a discrepancy between imaging results, which identify brain lesions in 70% or more of patients post-TAVI, and the vastly lower stroke rate – roughly 3% – associated with TAVI in contemporary series. He asked Dr. Baumbach how this can be reconciled: Is imaging too sensitive, or are cardiologists missing lots of smaller strokes?
“What we’ve learned is the emboli we see have a large spectrum of clinical sequelae,” Dr . Baumbach replied. “Of course, the volume of lesions you have in the brain matters, but so does the location of the infarct. Volume alone doesn’t give us the whole answer regarding the severity of these lesions. But what is clear after this study is that we cannot ignore these lesions. They may not produce overt stroke that we as cardiologists are able to detect, but they certainly produce neurologic deficits that a neurologist can see, and they do so in about 15% of unprotected procedures. And this has been seen with surgical aortic valve replacement as well.”
He reported serving on the scientific advisory board for and receiving research grants from Keystone Health, which produces TriGuard.
PARIS – Using the TriGuard embolic protection device during transcatheter aortic valve implantation resulted in a fivefold reduction in the incidence of new postprocedural neurologic deficits, compared with unprotected TAVI in the randomized DEFLECT III trial.
Moreover, TriGuard-protected TAVI patients showed a 44% reduction in the volume of new brain lesions on diffusion-weighted MRI at discharge, coupled with a 45% greater likelihood of freedom from any cerebral ischemic lesions, compared with the unprotected controls, Dr. Andreas Baumbach reported at the annual congress of the European Association of Percutaneous Cardiovascular Interventions.
The TriGuard is a filter/deflector device that covers all three cerebral arteries, and thus all brain territories. It consists of a single-wire nitinol frame and mesh filter with a pore size of 130 microns, enabling it to deflect cerebral emboli during TAVI while allowing maximal blood flow. The device is approved and commercially available in Europe but remains investigational in the United States
TriGuard is delivered by a 9 Fr sheath from the femoral artery and maintained during the TAVI procedure by a stabilizer in the innominate artery. Although introducing an additional element into TAVI raises the theoretic possibility of safety concerns, no safety signal was seen in DEFLECT III. In-hospital rates of death, stroke, bleeding, acute kidney injury, and major vascular complications didn’t differ between TriGuard recipients and controls in the 85-patient study.
TriGuard is designed to address an emerging concern as TAVI’s popularity soars and the procedure is extended to younger, lower–surgical risk patients: namely, the high rate of new embolic lesions seen on brain imaging post procedure. Such lesions have been linked to cognitive decline, noted Dr. Baumbach, professor of interventional cardiology at the University of Bristol (England).
Focusing on outcomes in the 89% of TriGuard recipients whose device remained in position and thus provided complete three-vessel coverage throughout TAVI, he said that group had significantly fewer new neurologic deficits on the National Institutes of Health Stroke Scale at discharge than the control group, by a margin of 3.1% versus 15.4%. Twenty-seven percent of the TriGuard group had no new ischemic brain lesions at discharge as determined by diffusion-weighted MRI, compared with 11.5% of controls who underwent TAVI with no cerebral protection. The TriGuard group also had significantly better scores at discharge on the Montreal Cognitive Assessment and on a delayed memory task.
Of particular importance was the finding that 30 days post procedure the TriGuard group was 2.27-fold more likely to have recovered normal cognitive function, compared with unprotected controls, the cardiologist continued. What that means is that 45% of the TriGuard group had an age-normalized Montreal Cognitive Assessment score greater than 26 out of a possible 30, compared with just 20% of controls.
Another intriguing finding was that roughly 10% of patients in both study arms developed new cerebral emboli between their discharge and 30-day follow-up brain imaging studies. By definition, such emboli can’t be prevented using the TriGuard, but this finding identifies the postprocedural period as one of continued elevated embolic risk, Dr. Baumbach said.
DEFLECT III was a 13-center, 30-day, exploratory, prospective, randomized trial designed to establish benchmark rates for the upcoming larger pivotal REFLECT randomized trial. Simultaneous with Dr. Baumbach’s presentation in Paris, the final DEFLECT III results were published online (Eur. Heart J. 2015 May 19. [doi:10.1093/eurheartj/ehv191]). Session cochair Dr. Stephan Windecker of the University of Bern (Switzerland) noted that there’s a discrepancy between imaging results, which identify brain lesions in 70% or more of patients post-TAVI, and the vastly lower stroke rate – roughly 3% – associated with TAVI in contemporary series. He asked Dr. Baumbach how this can be reconciled: Is imaging too sensitive, or are cardiologists missing lots of smaller strokes?
“What we’ve learned is the emboli we see have a large spectrum of clinical sequelae,” Dr . Baumbach replied. “Of course, the volume of lesions you have in the brain matters, but so does the location of the infarct. Volume alone doesn’t give us the whole answer regarding the severity of these lesions. But what is clear after this study is that we cannot ignore these lesions. They may not produce overt stroke that we as cardiologists are able to detect, but they certainly produce neurologic deficits that a neurologist can see, and they do so in about 15% of unprotected procedures. And this has been seen with surgical aortic valve replacement as well.”
He reported serving on the scientific advisory board for and receiving research grants from Keystone Health, which produces TriGuard.
PARIS – Using the TriGuard embolic protection device during transcatheter aortic valve implantation resulted in a fivefold reduction in the incidence of new postprocedural neurologic deficits, compared with unprotected TAVI in the randomized DEFLECT III trial.
Moreover, TriGuard-protected TAVI patients showed a 44% reduction in the volume of new brain lesions on diffusion-weighted MRI at discharge, coupled with a 45% greater likelihood of freedom from any cerebral ischemic lesions, compared with the unprotected controls, Dr. Andreas Baumbach reported at the annual congress of the European Association of Percutaneous Cardiovascular Interventions.
The TriGuard is a filter/deflector device that covers all three cerebral arteries, and thus all brain territories. It consists of a single-wire nitinol frame and mesh filter with a pore size of 130 microns, enabling it to deflect cerebral emboli during TAVI while allowing maximal blood flow. The device is approved and commercially available in Europe but remains investigational in the United States
TriGuard is delivered by a 9 Fr sheath from the femoral artery and maintained during the TAVI procedure by a stabilizer in the innominate artery. Although introducing an additional element into TAVI raises the theoretic possibility of safety concerns, no safety signal was seen in DEFLECT III. In-hospital rates of death, stroke, bleeding, acute kidney injury, and major vascular complications didn’t differ between TriGuard recipients and controls in the 85-patient study.
TriGuard is designed to address an emerging concern as TAVI’s popularity soars and the procedure is extended to younger, lower–surgical risk patients: namely, the high rate of new embolic lesions seen on brain imaging post procedure. Such lesions have been linked to cognitive decline, noted Dr. Baumbach, professor of interventional cardiology at the University of Bristol (England).
Focusing on outcomes in the 89% of TriGuard recipients whose device remained in position and thus provided complete three-vessel coverage throughout TAVI, he said that group had significantly fewer new neurologic deficits on the National Institutes of Health Stroke Scale at discharge than the control group, by a margin of 3.1% versus 15.4%. Twenty-seven percent of the TriGuard group had no new ischemic brain lesions at discharge as determined by diffusion-weighted MRI, compared with 11.5% of controls who underwent TAVI with no cerebral protection. The TriGuard group also had significantly better scores at discharge on the Montreal Cognitive Assessment and on a delayed memory task.
Of particular importance was the finding that 30 days post procedure the TriGuard group was 2.27-fold more likely to have recovered normal cognitive function, compared with unprotected controls, the cardiologist continued. What that means is that 45% of the TriGuard group had an age-normalized Montreal Cognitive Assessment score greater than 26 out of a possible 30, compared with just 20% of controls.
Another intriguing finding was that roughly 10% of patients in both study arms developed new cerebral emboli between their discharge and 30-day follow-up brain imaging studies. By definition, such emboli can’t be prevented using the TriGuard, but this finding identifies the postprocedural period as one of continued elevated embolic risk, Dr. Baumbach said.
DEFLECT III was a 13-center, 30-day, exploratory, prospective, randomized trial designed to establish benchmark rates for the upcoming larger pivotal REFLECT randomized trial. Simultaneous with Dr. Baumbach’s presentation in Paris, the final DEFLECT III results were published online (Eur. Heart J. 2015 May 19. [doi:10.1093/eurheartj/ehv191]). Session cochair Dr. Stephan Windecker of the University of Bern (Switzerland) noted that there’s a discrepancy between imaging results, which identify brain lesions in 70% or more of patients post-TAVI, and the vastly lower stroke rate – roughly 3% – associated with TAVI in contemporary series. He asked Dr. Baumbach how this can be reconciled: Is imaging too sensitive, or are cardiologists missing lots of smaller strokes?
“What we’ve learned is the emboli we see have a large spectrum of clinical sequelae,” Dr . Baumbach replied. “Of course, the volume of lesions you have in the brain matters, but so does the location of the infarct. Volume alone doesn’t give us the whole answer regarding the severity of these lesions. But what is clear after this study is that we cannot ignore these lesions. They may not produce overt stroke that we as cardiologists are able to detect, but they certainly produce neurologic deficits that a neurologist can see, and they do so in about 15% of unprotected procedures. And this has been seen with surgical aortic valve replacement as well.”
He reported serving on the scientific advisory board for and receiving research grants from Keystone Health, which produces TriGuard.
AT EUROPCR 2015
Key clinical point: Transcatheter aortic valve implantation has underappreciated adverse neurologic and cognitive consequences that can largely be avoided via an effective embolic protection device.
Major finding: Fifteen percent of patients showed new neurologic deficits on the NIH Stroke Scale after TAVI without cerebrovascular protection, a rate fivefold higher than in patients who used the TriGuard embolic protection device.
Data source: DEFLECT III, which randomized 85 participants to TAVI with the TriGuard embolic protection device or unprotected TAVI.
Disclosures: The DEFLECT III study was funded by Keystone Health. The presenter is on the company’s scientific advisory board.
Worsening migraine in pregnancy linked to adverse outcomes
VALENCIA, SPAIN – Women who present with acute severe migraine during pregnancy are at increased risk for adverse pregnancy outcomes and should be seen in a high-risk pregnancy clinic, Dr. Tracy B. Grossman asserted at the International Headache Congress.
“We should not be seeing these patients in a regular ob.gyn./generalist’s office because oftentimes we need input from neurology, and we need extra surveillance for both the fetus and the mother,” she said at the meeting sponsored by the International Headache Society and the American Headache Society.
Dr. Grossman presented a retrospective study of 90 consecutive pregnant patients who presented with acute severe migraine and obtained a neurology consult at Montefiore Medical Center, New York, where she is an ob.gyn. resident.
“These patients are different from most migraine patients because most migraine patients actually see improvement of symptoms during pregnancy. So this is a special group of patients with worsening and refractory migraine,” she noted in an interview.
Most were in their third trimester and had migraine without aura. Twenty-four presented with status migrainosus, a migraine for 15 or more days a month for more than 3 months.
Forty-nine of the 90 patients (54%) experienced one or more adverse pregnancy outcomes. Of note, the 28% preterm delivery rate was nearly three times the national average of 11% as reported by the March of Dimes. The preeclampsia rate was 20.5%, compared with a national rate of just 3%-4%. The 19.2% low birth weight rate was more than double the 8% national average. The cesarean section rate was 30.8%.
The study hypothesis was that the migraine-with-aura group would have higher preeclampsia, preterm delivery, and low birth weight rates, as has been reported by some other investigators in what is a sparse literature. Not so, Dr. Grossman said, because most of these patients didn’t have aura.
“So it can’t be purely an aura/vascular phenomenon that’s resulting in these adverse outcomes. These high rates of adverse pregnancy outcomes aren’t easily explainable. There’s something going on here that we haven’t teased out yet as to why these migraine patients are special,” she continued.
Their risk of adverse pregnancy outcomes wasn’t related to the headache medications they took. Sixty-two patients received a combination of oral and intravenous therapy with acetaminophen, metoclopramide, and dihydroergotamine. But 30% of patients were briefly on barbiturates, and 30% were on oxycodone or codeine; these are drugs of concern during pregnancy, yet there was no associated increase in adverse pregnancy outcomes, compared with the women who weren’t on those drugs or who indeed weren’t on any headache medications at all.
Dr. Grossman’s own therapeutic preference in patients with severe migraine in pregnancy is a peripheral nerve block with bupivacaine and lidocaine.
“It works for the majority of people – we don’t quite know why – and it’s a local therapy that avoids fetal exposure to systemic medications,” she observed.
Dr. Grossman reported no financial conflicts with regard to her study, which was carried out without industry support.
VALENCIA, SPAIN – Women who present with acute severe migraine during pregnancy are at increased risk for adverse pregnancy outcomes and should be seen in a high-risk pregnancy clinic, Dr. Tracy B. Grossman asserted at the International Headache Congress.
“We should not be seeing these patients in a regular ob.gyn./generalist’s office because oftentimes we need input from neurology, and we need extra surveillance for both the fetus and the mother,” she said at the meeting sponsored by the International Headache Society and the American Headache Society.
Dr. Grossman presented a retrospective study of 90 consecutive pregnant patients who presented with acute severe migraine and obtained a neurology consult at Montefiore Medical Center, New York, where she is an ob.gyn. resident.
“These patients are different from most migraine patients because most migraine patients actually see improvement of symptoms during pregnancy. So this is a special group of patients with worsening and refractory migraine,” she noted in an interview.
Most were in their third trimester and had migraine without aura. Twenty-four presented with status migrainosus, a migraine for 15 or more days a month for more than 3 months.
Forty-nine of the 90 patients (54%) experienced one or more adverse pregnancy outcomes. Of note, the 28% preterm delivery rate was nearly three times the national average of 11% as reported by the March of Dimes. The preeclampsia rate was 20.5%, compared with a national rate of just 3%-4%. The 19.2% low birth weight rate was more than double the 8% national average. The cesarean section rate was 30.8%.
The study hypothesis was that the migraine-with-aura group would have higher preeclampsia, preterm delivery, and low birth weight rates, as has been reported by some other investigators in what is a sparse literature. Not so, Dr. Grossman said, because most of these patients didn’t have aura.
“So it can’t be purely an aura/vascular phenomenon that’s resulting in these adverse outcomes. These high rates of adverse pregnancy outcomes aren’t easily explainable. There’s something going on here that we haven’t teased out yet as to why these migraine patients are special,” she continued.
Their risk of adverse pregnancy outcomes wasn’t related to the headache medications they took. Sixty-two patients received a combination of oral and intravenous therapy with acetaminophen, metoclopramide, and dihydroergotamine. But 30% of patients were briefly on barbiturates, and 30% were on oxycodone or codeine; these are drugs of concern during pregnancy, yet there was no associated increase in adverse pregnancy outcomes, compared with the women who weren’t on those drugs or who indeed weren’t on any headache medications at all.
Dr. Grossman’s own therapeutic preference in patients with severe migraine in pregnancy is a peripheral nerve block with bupivacaine and lidocaine.
“It works for the majority of people – we don’t quite know why – and it’s a local therapy that avoids fetal exposure to systemic medications,” she observed.
Dr. Grossman reported no financial conflicts with regard to her study, which was carried out without industry support.
VALENCIA, SPAIN – Women who present with acute severe migraine during pregnancy are at increased risk for adverse pregnancy outcomes and should be seen in a high-risk pregnancy clinic, Dr. Tracy B. Grossman asserted at the International Headache Congress.
“We should not be seeing these patients in a regular ob.gyn./generalist’s office because oftentimes we need input from neurology, and we need extra surveillance for both the fetus and the mother,” she said at the meeting sponsored by the International Headache Society and the American Headache Society.
Dr. Grossman presented a retrospective study of 90 consecutive pregnant patients who presented with acute severe migraine and obtained a neurology consult at Montefiore Medical Center, New York, where she is an ob.gyn. resident.
“These patients are different from most migraine patients because most migraine patients actually see improvement of symptoms during pregnancy. So this is a special group of patients with worsening and refractory migraine,” she noted in an interview.
Most were in their third trimester and had migraine without aura. Twenty-four presented with status migrainosus, a migraine for 15 or more days a month for more than 3 months.
Forty-nine of the 90 patients (54%) experienced one or more adverse pregnancy outcomes. Of note, the 28% preterm delivery rate was nearly three times the national average of 11% as reported by the March of Dimes. The preeclampsia rate was 20.5%, compared with a national rate of just 3%-4%. The 19.2% low birth weight rate was more than double the 8% national average. The cesarean section rate was 30.8%.
The study hypothesis was that the migraine-with-aura group would have higher preeclampsia, preterm delivery, and low birth weight rates, as has been reported by some other investigators in what is a sparse literature. Not so, Dr. Grossman said, because most of these patients didn’t have aura.
“So it can’t be purely an aura/vascular phenomenon that’s resulting in these adverse outcomes. These high rates of adverse pregnancy outcomes aren’t easily explainable. There’s something going on here that we haven’t teased out yet as to why these migraine patients are special,” she continued.
Their risk of adverse pregnancy outcomes wasn’t related to the headache medications they took. Sixty-two patients received a combination of oral and intravenous therapy with acetaminophen, metoclopramide, and dihydroergotamine. But 30% of patients were briefly on barbiturates, and 30% were on oxycodone or codeine; these are drugs of concern during pregnancy, yet there was no associated increase in adverse pregnancy outcomes, compared with the women who weren’t on those drugs or who indeed weren’t on any headache medications at all.
Dr. Grossman’s own therapeutic preference in patients with severe migraine in pregnancy is a peripheral nerve block with bupivacaine and lidocaine.
“It works for the majority of people – we don’t quite know why – and it’s a local therapy that avoids fetal exposure to systemic medications,” she observed.
Dr. Grossman reported no financial conflicts with regard to her study, which was carried out without industry support.
AT IHC 2015
Key clinical point: Patients who present with acute severe migraine during pregnancy should be seen in a high-risk pregnancy clinic.
Major finding: Forty-nine of 90 (54%) consecutive pregnant women who presented with acute severe migraine subsequently had one or more adverse pregnancy outcomes.
Data source: This was a retrospective single-center study.
Disclosures: Dr. Grossman reported having no financial conflicts regarding this study, carried out free of commercial support.
IHC: In medication overuse headache, think ‘stress reduction’
VALENCIA, SPAIN – Medication overuse headache is strongly associated with sky-high stress levels and several unhealthy – yet modifiable – lifestyle behaviors, according to a large Danish population-based study.
“High stress plus smoking, low physical activity, or obesity has synergistic effects in medication overuse headache. So stress reduction is highly relevant in medication overuse headache [MOH] management,” Dr. Rigmor H. Jensen reported at the International Headache Congress.
Stopping the offending medications remains central to the successful treatment of MOH. And while bearing in mind that association doesn’t prove causality, the findings of the Danish study suggest that stress reduction and lifestyle modification, in addition to their many recognized mental and physical health benefits, might also have some MOH-specific effects, according to Dr. Jensen, professor of neurology and director of the Danish Headache Center at the University of Copenhagen.
She presented the results of a questionnaire survey sent to a representative sample comprising 129,150 Danish adults. The survey focused on headache symptoms, lifestyle, and stress as measured by the validated 10-question perceived stress scale. The survey response rate was 53%, a figure so high as to likely elicit envy among U.S. researchers.
A total of 3.4% of the 68,518 respondents were classified as having chronic headache based upon self-report of headache on at least 15 days per month for 3 months. This group was then further categorized as having MOH by the standard International Classification of Headache Disorders definition – as did 1.8% of the total study population – or chronic headache without medication overuse.
Of adults with MOH, 58% scored in the top fifth of the total study population in terms of stress level. Those with chronic headache without medication overuse were less well represented at the high end of the stress spectrum: 46% of them were in the top stress quintile, as were 18% of people without chronic headache, she reported at the meeting sponsored by the International Headache Society and the American Headache Society.
Dr. Jensen and coinvestigators examined the relationship between MOH, stress level, and five unhealthy lifestyle behaviors: daily smoking, excessive alcohol intake, physical inactivity, obesity, and illicit drug use. In multivariate logistic regression analysis, smoking, physical inactivity, and obesity were strongly associated with MOH, while excessive drinking and illicit drug use were not.
Women in the top quintile for stress were 3.8-fold more likely to have MOH if they were a smoker than if they smoked but were in any of the four lower-stress quintiles, 3.5-fold more likely to have MOH if sedentary and high rather than lower stress, and 2.9-fold more likely if obese. For men in the top quintile for stress, the respective odds ratios for MOH were all in the 5-5.6 range for the three lifestyles.
After controlling for stress level, the odds of having MOH were greatest among individuals with all three unhealthy behaviors, compared with those with none: a 5.1-fold increase among men and 2.8-fold increase in women.
In terms of a theoretic goal for stress reduction that might be a useful target as part of the long-term management of MOH, it appears from the Danish data that patients wouldn’t need to attain super-relaxed, below-average stress levels. The independent association between stress and MOH was statistically significant only for individuals in the top two stress quintiles. Men in the fifth or top quintile for stress were 10.3-fold more likely to have MOH than those in the first quintile, while those in the fourth quintile were 4.3-fold more likely to have MOH than those in the first. In women the associations were less dramatic: a 3.9-fold increased risk of MOH if they were in the fifth stress quintile and a 2-fold increase in the fourth.
This study was funded by Danish governmental research agencies. Dr. Jensen reported having no financial conflicts.
VALENCIA, SPAIN – Medication overuse headache is strongly associated with sky-high stress levels and several unhealthy – yet modifiable – lifestyle behaviors, according to a large Danish population-based study.
“High stress plus smoking, low physical activity, or obesity has synergistic effects in medication overuse headache. So stress reduction is highly relevant in medication overuse headache [MOH] management,” Dr. Rigmor H. Jensen reported at the International Headache Congress.
Stopping the offending medications remains central to the successful treatment of MOH. And while bearing in mind that association doesn’t prove causality, the findings of the Danish study suggest that stress reduction and lifestyle modification, in addition to their many recognized mental and physical health benefits, might also have some MOH-specific effects, according to Dr. Jensen, professor of neurology and director of the Danish Headache Center at the University of Copenhagen.
She presented the results of a questionnaire survey sent to a representative sample comprising 129,150 Danish adults. The survey focused on headache symptoms, lifestyle, and stress as measured by the validated 10-question perceived stress scale. The survey response rate was 53%, a figure so high as to likely elicit envy among U.S. researchers.
A total of 3.4% of the 68,518 respondents were classified as having chronic headache based upon self-report of headache on at least 15 days per month for 3 months. This group was then further categorized as having MOH by the standard International Classification of Headache Disorders definition – as did 1.8% of the total study population – or chronic headache without medication overuse.
Of adults with MOH, 58% scored in the top fifth of the total study population in terms of stress level. Those with chronic headache without medication overuse were less well represented at the high end of the stress spectrum: 46% of them were in the top stress quintile, as were 18% of people without chronic headache, she reported at the meeting sponsored by the International Headache Society and the American Headache Society.
Dr. Jensen and coinvestigators examined the relationship between MOH, stress level, and five unhealthy lifestyle behaviors: daily smoking, excessive alcohol intake, physical inactivity, obesity, and illicit drug use. In multivariate logistic regression analysis, smoking, physical inactivity, and obesity were strongly associated with MOH, while excessive drinking and illicit drug use were not.
Women in the top quintile for stress were 3.8-fold more likely to have MOH if they were a smoker than if they smoked but were in any of the four lower-stress quintiles, 3.5-fold more likely to have MOH if sedentary and high rather than lower stress, and 2.9-fold more likely if obese. For men in the top quintile for stress, the respective odds ratios for MOH were all in the 5-5.6 range for the three lifestyles.
After controlling for stress level, the odds of having MOH were greatest among individuals with all three unhealthy behaviors, compared with those with none: a 5.1-fold increase among men and 2.8-fold increase in women.
In terms of a theoretic goal for stress reduction that might be a useful target as part of the long-term management of MOH, it appears from the Danish data that patients wouldn’t need to attain super-relaxed, below-average stress levels. The independent association between stress and MOH was statistically significant only for individuals in the top two stress quintiles. Men in the fifth or top quintile for stress were 10.3-fold more likely to have MOH than those in the first quintile, while those in the fourth quintile were 4.3-fold more likely to have MOH than those in the first. In women the associations were less dramatic: a 3.9-fold increased risk of MOH if they were in the fifth stress quintile and a 2-fold increase in the fourth.
This study was funded by Danish governmental research agencies. Dr. Jensen reported having no financial conflicts.
VALENCIA, SPAIN – Medication overuse headache is strongly associated with sky-high stress levels and several unhealthy – yet modifiable – lifestyle behaviors, according to a large Danish population-based study.
“High stress plus smoking, low physical activity, or obesity has synergistic effects in medication overuse headache. So stress reduction is highly relevant in medication overuse headache [MOH] management,” Dr. Rigmor H. Jensen reported at the International Headache Congress.
Stopping the offending medications remains central to the successful treatment of MOH. And while bearing in mind that association doesn’t prove causality, the findings of the Danish study suggest that stress reduction and lifestyle modification, in addition to their many recognized mental and physical health benefits, might also have some MOH-specific effects, according to Dr. Jensen, professor of neurology and director of the Danish Headache Center at the University of Copenhagen.
She presented the results of a questionnaire survey sent to a representative sample comprising 129,150 Danish adults. The survey focused on headache symptoms, lifestyle, and stress as measured by the validated 10-question perceived stress scale. The survey response rate was 53%, a figure so high as to likely elicit envy among U.S. researchers.
A total of 3.4% of the 68,518 respondents were classified as having chronic headache based upon self-report of headache on at least 15 days per month for 3 months. This group was then further categorized as having MOH by the standard International Classification of Headache Disorders definition – as did 1.8% of the total study population – or chronic headache without medication overuse.
Of adults with MOH, 58% scored in the top fifth of the total study population in terms of stress level. Those with chronic headache without medication overuse were less well represented at the high end of the stress spectrum: 46% of them were in the top stress quintile, as were 18% of people without chronic headache, she reported at the meeting sponsored by the International Headache Society and the American Headache Society.
Dr. Jensen and coinvestigators examined the relationship between MOH, stress level, and five unhealthy lifestyle behaviors: daily smoking, excessive alcohol intake, physical inactivity, obesity, and illicit drug use. In multivariate logistic regression analysis, smoking, physical inactivity, and obesity were strongly associated with MOH, while excessive drinking and illicit drug use were not.
Women in the top quintile for stress were 3.8-fold more likely to have MOH if they were a smoker than if they smoked but were in any of the four lower-stress quintiles, 3.5-fold more likely to have MOH if sedentary and high rather than lower stress, and 2.9-fold more likely if obese. For men in the top quintile for stress, the respective odds ratios for MOH were all in the 5-5.6 range for the three lifestyles.
After controlling for stress level, the odds of having MOH were greatest among individuals with all three unhealthy behaviors, compared with those with none: a 5.1-fold increase among men and 2.8-fold increase in women.
In terms of a theoretic goal for stress reduction that might be a useful target as part of the long-term management of MOH, it appears from the Danish data that patients wouldn’t need to attain super-relaxed, below-average stress levels. The independent association between stress and MOH was statistically significant only for individuals in the top two stress quintiles. Men in the fifth or top quintile for stress were 10.3-fold more likely to have MOH than those in the first quintile, while those in the fourth quintile were 4.3-fold more likely to have MOH than those in the first. In women the associations were less dramatic: a 3.9-fold increased risk of MOH if they were in the fifth stress quintile and a 2-fold increase in the fourth.
This study was funded by Danish governmental research agencies. Dr. Jensen reported having no financial conflicts.
AT IHC 2015
Key clinical point: High stress and unhealthy behaviors figure prominently in people with medication overuse headache – and addressing those issues can only help.
Major finding: Fifty-eight percent of Danes with medication overuse headache are in the top 20% of the population in terms of perceived stress level.
Data source: This was a population-based, cross-sectional study of 68,518 Danish adults, 1.8% of whom had medication overuse headache.
Disclosures: This study was funded by Danish governmental research agencies. The presenter reported having no financial conflicts.
Paclitaxel balloon achieves unprecedented success in complex PAD
PARIS – A proprietary drug-coated percutaneous angioplasty balloon has achieved unprecedented clinical success in treating long lesions in the superficial femoral arteries.
“The 360-day primary patency rate of 91.1% and clinically driven target lesion revascularization rate of 6% are unmatched for this complex patient subgroup,” Dr. Dierk Scheinert declared in presenting the results of the IN.PACT Global Study Long Lesion Imaging Cohort at the annual congress of the European Association of Percutaneous Cardiovascular Interventions.
The 157 patients who comprise the long lesion cohort are among 1,538 patients with various forms of complex peripheral arterial disease being treated at 64 sites on four continents under the IN.PACT Global Clinical Study, which focuses on the use of Medtronic’s Admiral paclitaxel-coated balloon for percutaneous transluminal angioplasty. Upon balloon inflation, the paclitaxel binds to the vessel wall, penetrating deep into the adventitia in order to interfere with smooth muscle cell proliferation and neointimal hyperplasia. The drug remains in the vessel wall at therapeutic levels for up to 6 months.
The Food and Drug Administration approved the balloon for lesions up to 18 cm in length in native superficial femoral or popliteal arteries. Dr. Scheinert presented a study that sought to broaden that indication: The average lesion length in the 157 participants was 26.4 cm.
The majority of the target lesions, 83%, were de novo, 60% were total occlusions, and 72% featured calcification. These were patients with substantial comorbidities: 41% had diabetes, 88% hypertension, 52% coronary heart disease, 77% dyslipidemia, and 34% were current smokers, noted Dr. Scheinert, professor and chairman of the department of vascular medicine and angiology at the University of Leipzig (Germany).
The mean diameter of the target stenosis was 90% predilatation and 39% afterwards. Provisional stents were implanted in 33% of the 100 patients with lesion lengths of 15-25 cm and 53% in those with lesions longer than 25 cm.
The 12-month primary patency rate, defined as freedom from clinically driven target lesion revascularization and restenosis as determined by a central core lab, varied by lesion length: 97.7% for 15- to 25-cm-long lesions compared with 79.2% for longer ones.
The major adverse event rate – a composite of all-cause mortality, major target limb amputation, clinically driven target vessel revascularization, or thrombosis – was 11.9%.
Of note, the impressive 91.1% primary patency rate at 12 months fell off sharply to 80.7% at 390 days, prompting comment from session cochair Dr. Elazer Edelman.
“I am struck by what happened to these patients at the end of 1 year. Not to detract in any way from the value and importance of what you’ve shown, but the fact that at 1 year we get this precipitous decline is something we as a group ought to be investigating,” said Dr. Edelman, a cardiologist who is professor of health sciences and technology at the Massachusetts Institute of Technology, professor of medicine at Harvard University, and director of the Harvard-MIT Biomedical Engineering Center.
“We have to be a little bit patient,” Dr. Scheinert replied. “We’re going to see some long-term follow-up data beginning this fall. The positive message here is that at 6 months we see almost no events, which is an achievement because typically we already see the first step down in the results curve by then. And we see patency results at 12 months that we haven’t seen with any other therapy so far.”
Dr. Edelman retorted that while it’s clear this innovative therapy has delayed the period of vulnerability to patency loss, “Your work cannot continue to be impactful if we don’t focus on where the loss of patency occurs.”
“When we talk about adjunctive therapy, we need to think now about something innovative. We need to start thinking about how to give drugs from 6 to 12 to 18 months,” he asserted.
Dr. Scheinert is on the scientific advisory board for Medtronic, which funded the study, as well as on advisory boards for more than a dozen other medical companies.
PARIS – A proprietary drug-coated percutaneous angioplasty balloon has achieved unprecedented clinical success in treating long lesions in the superficial femoral arteries.
“The 360-day primary patency rate of 91.1% and clinically driven target lesion revascularization rate of 6% are unmatched for this complex patient subgroup,” Dr. Dierk Scheinert declared in presenting the results of the IN.PACT Global Study Long Lesion Imaging Cohort at the annual congress of the European Association of Percutaneous Cardiovascular Interventions.
The 157 patients who comprise the long lesion cohort are among 1,538 patients with various forms of complex peripheral arterial disease being treated at 64 sites on four continents under the IN.PACT Global Clinical Study, which focuses on the use of Medtronic’s Admiral paclitaxel-coated balloon for percutaneous transluminal angioplasty. Upon balloon inflation, the paclitaxel binds to the vessel wall, penetrating deep into the adventitia in order to interfere with smooth muscle cell proliferation and neointimal hyperplasia. The drug remains in the vessel wall at therapeutic levels for up to 6 months.
The Food and Drug Administration approved the balloon for lesions up to 18 cm in length in native superficial femoral or popliteal arteries. Dr. Scheinert presented a study that sought to broaden that indication: The average lesion length in the 157 participants was 26.4 cm.
The majority of the target lesions, 83%, were de novo, 60% were total occlusions, and 72% featured calcification. These were patients with substantial comorbidities: 41% had diabetes, 88% hypertension, 52% coronary heart disease, 77% dyslipidemia, and 34% were current smokers, noted Dr. Scheinert, professor and chairman of the department of vascular medicine and angiology at the University of Leipzig (Germany).
The mean diameter of the target stenosis was 90% predilatation and 39% afterwards. Provisional stents were implanted in 33% of the 100 patients with lesion lengths of 15-25 cm and 53% in those with lesions longer than 25 cm.
The 12-month primary patency rate, defined as freedom from clinically driven target lesion revascularization and restenosis as determined by a central core lab, varied by lesion length: 97.7% for 15- to 25-cm-long lesions compared with 79.2% for longer ones.
The major adverse event rate – a composite of all-cause mortality, major target limb amputation, clinically driven target vessel revascularization, or thrombosis – was 11.9%.
Of note, the impressive 91.1% primary patency rate at 12 months fell off sharply to 80.7% at 390 days, prompting comment from session cochair Dr. Elazer Edelman.
“I am struck by what happened to these patients at the end of 1 year. Not to detract in any way from the value and importance of what you’ve shown, but the fact that at 1 year we get this precipitous decline is something we as a group ought to be investigating,” said Dr. Edelman, a cardiologist who is professor of health sciences and technology at the Massachusetts Institute of Technology, professor of medicine at Harvard University, and director of the Harvard-MIT Biomedical Engineering Center.
“We have to be a little bit patient,” Dr. Scheinert replied. “We’re going to see some long-term follow-up data beginning this fall. The positive message here is that at 6 months we see almost no events, which is an achievement because typically we already see the first step down in the results curve by then. And we see patency results at 12 months that we haven’t seen with any other therapy so far.”
Dr. Edelman retorted that while it’s clear this innovative therapy has delayed the period of vulnerability to patency loss, “Your work cannot continue to be impactful if we don’t focus on where the loss of patency occurs.”
“When we talk about adjunctive therapy, we need to think now about something innovative. We need to start thinking about how to give drugs from 6 to 12 to 18 months,” he asserted.
Dr. Scheinert is on the scientific advisory board for Medtronic, which funded the study, as well as on advisory boards for more than a dozen other medical companies.
PARIS – A proprietary drug-coated percutaneous angioplasty balloon has achieved unprecedented clinical success in treating long lesions in the superficial femoral arteries.
“The 360-day primary patency rate of 91.1% and clinically driven target lesion revascularization rate of 6% are unmatched for this complex patient subgroup,” Dr. Dierk Scheinert declared in presenting the results of the IN.PACT Global Study Long Lesion Imaging Cohort at the annual congress of the European Association of Percutaneous Cardiovascular Interventions.
The 157 patients who comprise the long lesion cohort are among 1,538 patients with various forms of complex peripheral arterial disease being treated at 64 sites on four continents under the IN.PACT Global Clinical Study, which focuses on the use of Medtronic’s Admiral paclitaxel-coated balloon for percutaneous transluminal angioplasty. Upon balloon inflation, the paclitaxel binds to the vessel wall, penetrating deep into the adventitia in order to interfere with smooth muscle cell proliferation and neointimal hyperplasia. The drug remains in the vessel wall at therapeutic levels for up to 6 months.
The Food and Drug Administration approved the balloon for lesions up to 18 cm in length in native superficial femoral or popliteal arteries. Dr. Scheinert presented a study that sought to broaden that indication: The average lesion length in the 157 participants was 26.4 cm.
The majority of the target lesions, 83%, were de novo, 60% were total occlusions, and 72% featured calcification. These were patients with substantial comorbidities: 41% had diabetes, 88% hypertension, 52% coronary heart disease, 77% dyslipidemia, and 34% were current smokers, noted Dr. Scheinert, professor and chairman of the department of vascular medicine and angiology at the University of Leipzig (Germany).
The mean diameter of the target stenosis was 90% predilatation and 39% afterwards. Provisional stents were implanted in 33% of the 100 patients with lesion lengths of 15-25 cm and 53% in those with lesions longer than 25 cm.
The 12-month primary patency rate, defined as freedom from clinically driven target lesion revascularization and restenosis as determined by a central core lab, varied by lesion length: 97.7% for 15- to 25-cm-long lesions compared with 79.2% for longer ones.
The major adverse event rate – a composite of all-cause mortality, major target limb amputation, clinically driven target vessel revascularization, or thrombosis – was 11.9%.
Of note, the impressive 91.1% primary patency rate at 12 months fell off sharply to 80.7% at 390 days, prompting comment from session cochair Dr. Elazer Edelman.
“I am struck by what happened to these patients at the end of 1 year. Not to detract in any way from the value and importance of what you’ve shown, but the fact that at 1 year we get this precipitous decline is something we as a group ought to be investigating,” said Dr. Edelman, a cardiologist who is professor of health sciences and technology at the Massachusetts Institute of Technology, professor of medicine at Harvard University, and director of the Harvard-MIT Biomedical Engineering Center.
“We have to be a little bit patient,” Dr. Scheinert replied. “We’re going to see some long-term follow-up data beginning this fall. The positive message here is that at 6 months we see almost no events, which is an achievement because typically we already see the first step down in the results curve by then. And we see patency results at 12 months that we haven’t seen with any other therapy so far.”
Dr. Edelman retorted that while it’s clear this innovative therapy has delayed the period of vulnerability to patency loss, “Your work cannot continue to be impactful if we don’t focus on where the loss of patency occurs.”
“When we talk about adjunctive therapy, we need to think now about something innovative. We need to start thinking about how to give drugs from 6 to 12 to 18 months,” he asserted.
Dr. Scheinert is on the scientific advisory board for Medtronic, which funded the study, as well as on advisory boards for more than a dozen other medical companies.
AT EuroPCR
Key clinical point: A paclitaxel-coated angioplasty balloon results in 12-month patency rates never before seen in patients with peripheral arterial disease involving long lesions in the superficial femoral arteries.
Major finding: The 12-month primary patency rate was 91.1% in patients whose average lesion length was 26.4 cm.
Data source: This prospective, multinational study included 157 subjects with complex superficial femoral artery lesions at least 15 cm in length.
Disclosures: The IN.PACT Global Study is funded by Medtronic. The presenter is on the company’s scientific advisory board.
NOTION: TAVI has edge in patients at low surgical risk
PARIS – The writing is on the wall: 2-year results of the NOTION* trial suggest that transcatheter aortic valve replacement is the future – and already in selected cases, the present – preferred therapy for aortic stenosis in patients at low surgical risk.
NOTION was a multicenter, prospective, nonblinded, randomized trial, the first-ever study to compare less-invasive transcatheter aortic valve implantation (TAVI, also called transcatheter aortic valve replacement, or TAVR) and traditional surgical aortic valve replacement (SAVR) in a truly low-surgical-risk population. The 280 participants had a median Society of Thoracic Surgeons score of 3 and no major comorbid conditions.
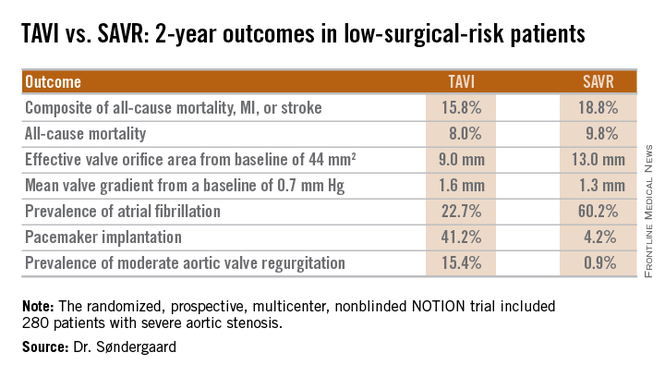
At 2 years’ follow-up, the TAVI group had significantly larger valve orifice areas and lower gradients, along with lower rates of life-threatening bleeding, cardiogenic stroke, and severe kidney injury than did the SAVR group. Moreover, the TAVI group showed a strong favorable trend in terms of the primary composite endpoint comprising all-cause mortality, MI, or stroke, although the advantage didn’t achieve statistical significance because of the relatively small study size, Dr. Lars Søndergaard said at the annual congress of the European Association of Percutaneous Cardiovascular Interventions.
“Longer-term data on durability and more randomized controlled trials are needed before we adopt routine use of TAVI in low-risk patients, but I think it’s reasonable to offer TAVI in selected low-risk patients today,” concluded Dr. Søndergaard of the University of Copenhagen.
Session cochair Dr. William Wijns noted that NOTION, which utilized Medtronics’ self-expanding CoreValve for TAVI, began in 2009 and thus used an early iteration of the device. One might reasonably expect that the study results would be substantially more strongly in favor of TAVI had the contemporary version of the CoreValve been employed, observed Dr. Wijns, codirector of the cardiovascular center in Aalst, Belgium.
Dr. Søndergaard concurred. While the prevalence of moderate aortic regurgitation at 2 years in the TAVI arm of NOTION was 15.4%, in part because the valves were routinely placed under echocardiographic guidance, current-generation TAVI valves placed under CT guidance have a 1%-5% rate of moderate regurgitation. And while 41% of the TAVI group in NOTION had a pacemaker at 2 years, other studies show the rate drops to roughly 10% with the newest version of the CoreValve.
Dr. Søndergaard and coinvestigators plan to follow the NOTION participants for 10 years, issuing periodic updates. That’s a welcome development because patients at low surgical risk constitute the largest portion of those with significant aortic stenosis. Many of them are young enough that they should have a substantial remaining lifespan after aortic valve replacement, so it will be important to establish TAVI’s long-term durability.
The NOTION trial was funded by the Danish Heart Foundation. Dr. Søndergaard reported having no financial conflicts.
*Correction, 6/1/2015: An earlier version of this article misstated the name of the NOTION trial.
PARIS – The writing is on the wall: 2-year results of the NOTION* trial suggest that transcatheter aortic valve replacement is the future – and already in selected cases, the present – preferred therapy for aortic stenosis in patients at low surgical risk.
NOTION was a multicenter, prospective, nonblinded, randomized trial, the first-ever study to compare less-invasive transcatheter aortic valve implantation (TAVI, also called transcatheter aortic valve replacement, or TAVR) and traditional surgical aortic valve replacement (SAVR) in a truly low-surgical-risk population. The 280 participants had a median Society of Thoracic Surgeons score of 3 and no major comorbid conditions.
At 2 years’ follow-up, the TAVI group had significantly larger valve orifice areas and lower gradients, along with lower rates of life-threatening bleeding, cardiogenic stroke, and severe kidney injury than did the SAVR group. Moreover, the TAVI group showed a strong favorable trend in terms of the primary composite endpoint comprising all-cause mortality, MI, or stroke, although the advantage didn’t achieve statistical significance because of the relatively small study size, Dr. Lars Søndergaard said at the annual congress of the European Association of Percutaneous Cardiovascular Interventions.
“Longer-term data on durability and more randomized controlled trials are needed before we adopt routine use of TAVI in low-risk patients, but I think it’s reasonable to offer TAVI in selected low-risk patients today,” concluded Dr. Søndergaard of the University of Copenhagen.

Session cochair Dr. William Wijns noted that NOTION, which utilized Medtronics’ self-expanding CoreValve for TAVI, began in 2009 and thus used an early iteration of the device. One might reasonably expect that the study results would be substantially more strongly in favor of TAVI had the contemporary version of the CoreValve been employed, observed Dr. Wijns, codirector of the cardiovascular center in Aalst, Belgium.
Dr. Søndergaard concurred. While the prevalence of moderate aortic regurgitation at 2 years in the TAVI arm of NOTION was 15.4%, in part because the valves were routinely placed under echocardiographic guidance, current-generation TAVI valves placed under CT guidance have a 1%-5% rate of moderate regurgitation. And while 41% of the TAVI group in NOTION had a pacemaker at 2 years, other studies show the rate drops to roughly 10% with the newest version of the CoreValve.
Dr. Søndergaard and coinvestigators plan to follow the NOTION participants for 10 years, issuing periodic updates. That’s a welcome development because patients at low surgical risk constitute the largest portion of those with significant aortic stenosis. Many of them are young enough that they should have a substantial remaining lifespan after aortic valve replacement, so it will be important to establish TAVI’s long-term durability.
The NOTION trial was funded by the Danish Heart Foundation. Dr. Søndergaard reported having no financial conflicts.
*Correction, 6/1/2015: An earlier version of this article misstated the name of the NOTION trial.
PARIS – The writing is on the wall: 2-year results of the NOTION* trial suggest that transcatheter aortic valve replacement is the future – and already in selected cases, the present – preferred therapy for aortic stenosis in patients at low surgical risk.
NOTION was a multicenter, prospective, nonblinded, randomized trial, the first-ever study to compare less-invasive transcatheter aortic valve implantation (TAVI, also called transcatheter aortic valve replacement, or TAVR) and traditional surgical aortic valve replacement (SAVR) in a truly low-surgical-risk population. The 280 participants had a median Society of Thoracic Surgeons score of 3 and no major comorbid conditions.
At 2 years’ follow-up, the TAVI group had significantly larger valve orifice areas and lower gradients, along with lower rates of life-threatening bleeding, cardiogenic stroke, and severe kidney injury than did the SAVR group. Moreover, the TAVI group showed a strong favorable trend in terms of the primary composite endpoint comprising all-cause mortality, MI, or stroke, although the advantage didn’t achieve statistical significance because of the relatively small study size, Dr. Lars Søndergaard said at the annual congress of the European Association of Percutaneous Cardiovascular Interventions.
“Longer-term data on durability and more randomized controlled trials are needed before we adopt routine use of TAVI in low-risk patients, but I think it’s reasonable to offer TAVI in selected low-risk patients today,” concluded Dr. Søndergaard of the University of Copenhagen.

Session cochair Dr. William Wijns noted that NOTION, which utilized Medtronics’ self-expanding CoreValve for TAVI, began in 2009 and thus used an early iteration of the device. One might reasonably expect that the study results would be substantially more strongly in favor of TAVI had the contemporary version of the CoreValve been employed, observed Dr. Wijns, codirector of the cardiovascular center in Aalst, Belgium.
Dr. Søndergaard concurred. While the prevalence of moderate aortic regurgitation at 2 years in the TAVI arm of NOTION was 15.4%, in part because the valves were routinely placed under echocardiographic guidance, current-generation TAVI valves placed under CT guidance have a 1%-5% rate of moderate regurgitation. And while 41% of the TAVI group in NOTION had a pacemaker at 2 years, other studies show the rate drops to roughly 10% with the newest version of the CoreValve.
Dr. Søndergaard and coinvestigators plan to follow the NOTION participants for 10 years, issuing periodic updates. That’s a welcome development because patients at low surgical risk constitute the largest portion of those with significant aortic stenosis. Many of them are young enough that they should have a substantial remaining lifespan after aortic valve replacement, so it will be important to establish TAVI’s long-term durability.
The NOTION trial was funded by the Danish Heart Foundation. Dr. Søndergaard reported having no financial conflicts.
*Correction, 6/1/2015: An earlier version of this article misstated the name of the NOTION trial.
AT EUROPCR
Key clinical point: At 2 years, outcomes of TAVI look as good as and in some domains better than outcomes of surgical aortic valve replacement in low-surgical-risk patients.
Major finding: The 2-year composite outcome of all-cause mortality, MI, or stroke occurred in 15.8% of the TAVI group compared with 18.8% of surgically treated patients.
Data source: The randomized, prospective, multicenter, nonblinded NOTION trial includes 280 low-surgical-risk patients with severe aortic stenosis.
Disclosures: The NOTION trial was sponsored by the Danish Heart Foundation. The presenter reported having no financial conflicts.
PAS: Start sulfonylureas before genetic testing in neonatal diabetes
SAN DIEGO – Any child diagnosed with neonatal diabetes should undergo genetic testing for monogenic mutations as quickly as possible, and a case can be made for switching from insulin injections to empiric oral sulfonylurea therapy while awaiting the test results, Dr. Siri A. Greeley asserted at the annual meeting of the Pediatric Academic Societies.
“I think the situation is analogous to congenital hypothyroidism, where early treatment is really important while the brain is still developing. I think sulfonylureas’ effects on brain function are age dependent. That’s one of my main rationales for starting sulfonylurea therapy early on, especially if you’re having trouble getting the genetic testing done,” explained Dr. Greeley, a pediatric endocrinologist at the University of Chicago.
Diabetes diagnosed before age 6 months is nearly always genetic in origin. Heterozygous activating mutations of the adenosine triphosphate (ATP)-sensitive potassium channel (KATP) cause up to 60% of diabetes diagnosed before 6 months of age. Sulfonylurea therapy is typically highly effective in these children, and the sooner they start therapy the better their outcomes, both in terms of glycemic control and neurodevelopmental endpoints, he said.
In his recent retrospective observational study of 58 patients enrolled in the University of Chicago Monogenic Diabetes Registry who had neonatal diabetes involving the KCNJ11 subunit of the KATP channel, hemoglobin A1c fell from an average of 8.5% on insulin to 6.2% after the transition to sulfonylurea therapy. Age at initiation of sulfonylureas showed a linear correlation with the dose required at follow-up. Indeed, all 10 patients who needed additional glucose-lowering medications along with their sulfonylurea had started on sulfonylurea therapy at age 13 years or older (Diabetologia 2015 April 17 [Epub ahead of print] PMID:25877689).
A likely possible explanation for the observed decline in sensitivity to sulfonylureas with an older starting age is that insulin-treated patients experience loss of beta cell mass over time, according to Dr. Greeley.
A syndrome known by the acronym DEND, for developmental delay, epilepsy, and neonatal diabetes, affects 20%-30% of patients with neonatal diabetes related to mutations in the KATP channel. These channels are widely expressed in the brain.
The first hint that early initiation of sulfonylureas in children with KATP-related neonatal diabetes may have beneficial neurodevelopmental effects has been provided by a study Dr. Greeley and coinvestigators conducted in 19 patients, aged 2-20 years, with KATP channel mutations. Eight had diabetes only, eight had DEND, all eight of whom had potent V59M/A mutations, and three had other KATP-related mutations associated with milder neurodevelopmental impairment. Upon testing via the age-standardized Beery-Buktenica Developmental Test of Visual-Motor Integration, the investigators found that the group with diabetes only had normal scores while those with V59M/A mutations scored quite low. The key finding: Age at sulfonylurea initiation was inversely associated with scores on the Beery test. Those who started on the medication before 1 year of age had near-normal scores (Diabetes Care 2012;35:2086-8).
That’s not definitive evidence, Dr. Greeley observed. It needs to be confirmed with larger patient numbers. But it does warrant at least considering preemptive sulfonylurea therapy while awaiting genetic test results in patients with neonatal diabetes, he said.
He noted that in his retrospective study of 154 subjects diagnosed with diabetes before age 6 months, barriers to expeditious genetic testing, mainly consisting of obstacles created by insurance companies, resulted in a median time between clinical diagnosis and genetic diagnosis of 10.4 weeks (J. Clin. Endocrinol. Metab. 2014;99:E2709-14).
Neonatal diabetes is a rare condition. Most of the culprit mutations are de novo, not inherited. With an incidence of roughly 1 in 100,000 births, the rate is in line with other disorders that are part of standard newborn screening, such as maple syrup urine disease, Dr. Greeley observed.
More than 70% of all cases of neonatal diabetes are due to mutations at one of four loci: 6q24, KCNJ11, INS, or ABCC8. It’s important to order genetic testing from a laboratory that includes a comprehensive 6q24 analysis in its panel; many don’t, creating the potential for a false-negative test result, the pediatric endocrinologist cautioned.
Dr. Greeley’s research is funded by the National Institutes of Health, the American Diabetes Association, and the Kovler Family Foundation. He reported having no financial conflicts.
SAN DIEGO – Any child diagnosed with neonatal diabetes should undergo genetic testing for monogenic mutations as quickly as possible, and a case can be made for switching from insulin injections to empiric oral sulfonylurea therapy while awaiting the test results, Dr. Siri A. Greeley asserted at the annual meeting of the Pediatric Academic Societies.
“I think the situation is analogous to congenital hypothyroidism, where early treatment is really important while the brain is still developing. I think sulfonylureas’ effects on brain function are age dependent. That’s one of my main rationales for starting sulfonylurea therapy early on, especially if you’re having trouble getting the genetic testing done,” explained Dr. Greeley, a pediatric endocrinologist at the University of Chicago.
Diabetes diagnosed before age 6 months is nearly always genetic in origin. Heterozygous activating mutations of the adenosine triphosphate (ATP)-sensitive potassium channel (KATP) cause up to 60% of diabetes diagnosed before 6 months of age. Sulfonylurea therapy is typically highly effective in these children, and the sooner they start therapy the better their outcomes, both in terms of glycemic control and neurodevelopmental endpoints, he said.
In his recent retrospective observational study of 58 patients enrolled in the University of Chicago Monogenic Diabetes Registry who had neonatal diabetes involving the KCNJ11 subunit of the KATP channel, hemoglobin A1c fell from an average of 8.5% on insulin to 6.2% after the transition to sulfonylurea therapy. Age at initiation of sulfonylureas showed a linear correlation with the dose required at follow-up. Indeed, all 10 patients who needed additional glucose-lowering medications along with their sulfonylurea had started on sulfonylurea therapy at age 13 years or older (Diabetologia 2015 April 17 [Epub ahead of print] PMID:25877689).
A likely possible explanation for the observed decline in sensitivity to sulfonylureas with an older starting age is that insulin-treated patients experience loss of beta cell mass over time, according to Dr. Greeley.
A syndrome known by the acronym DEND, for developmental delay, epilepsy, and neonatal diabetes, affects 20%-30% of patients with neonatal diabetes related to mutations in the KATP channel. These channels are widely expressed in the brain.
The first hint that early initiation of sulfonylureas in children with KATP-related neonatal diabetes may have beneficial neurodevelopmental effects has been provided by a study Dr. Greeley and coinvestigators conducted in 19 patients, aged 2-20 years, with KATP channel mutations. Eight had diabetes only, eight had DEND, all eight of whom had potent V59M/A mutations, and three had other KATP-related mutations associated with milder neurodevelopmental impairment. Upon testing via the age-standardized Beery-Buktenica Developmental Test of Visual-Motor Integration, the investigators found that the group with diabetes only had normal scores while those with V59M/A mutations scored quite low. The key finding: Age at sulfonylurea initiation was inversely associated with scores on the Beery test. Those who started on the medication before 1 year of age had near-normal scores (Diabetes Care 2012;35:2086-8).
That’s not definitive evidence, Dr. Greeley observed. It needs to be confirmed with larger patient numbers. But it does warrant at least considering preemptive sulfonylurea therapy while awaiting genetic test results in patients with neonatal diabetes, he said.
He noted that in his retrospective study of 154 subjects diagnosed with diabetes before age 6 months, barriers to expeditious genetic testing, mainly consisting of obstacles created by insurance companies, resulted in a median time between clinical diagnosis and genetic diagnosis of 10.4 weeks (J. Clin. Endocrinol. Metab. 2014;99:E2709-14).
Neonatal diabetes is a rare condition. Most of the culprit mutations are de novo, not inherited. With an incidence of roughly 1 in 100,000 births, the rate is in line with other disorders that are part of standard newborn screening, such as maple syrup urine disease, Dr. Greeley observed.
More than 70% of all cases of neonatal diabetes are due to mutations at one of four loci: 6q24, KCNJ11, INS, or ABCC8. It’s important to order genetic testing from a laboratory that includes a comprehensive 6q24 analysis in its panel; many don’t, creating the potential for a false-negative test result, the pediatric endocrinologist cautioned.
Dr. Greeley’s research is funded by the National Institutes of Health, the American Diabetes Association, and the Kovler Family Foundation. He reported having no financial conflicts.
SAN DIEGO – Any child diagnosed with neonatal diabetes should undergo genetic testing for monogenic mutations as quickly as possible, and a case can be made for switching from insulin injections to empiric oral sulfonylurea therapy while awaiting the test results, Dr. Siri A. Greeley asserted at the annual meeting of the Pediatric Academic Societies.
“I think the situation is analogous to congenital hypothyroidism, where early treatment is really important while the brain is still developing. I think sulfonylureas’ effects on brain function are age dependent. That’s one of my main rationales for starting sulfonylurea therapy early on, especially if you’re having trouble getting the genetic testing done,” explained Dr. Greeley, a pediatric endocrinologist at the University of Chicago.
Diabetes diagnosed before age 6 months is nearly always genetic in origin. Heterozygous activating mutations of the adenosine triphosphate (ATP)-sensitive potassium channel (KATP) cause up to 60% of diabetes diagnosed before 6 months of age. Sulfonylurea therapy is typically highly effective in these children, and the sooner they start therapy the better their outcomes, both in terms of glycemic control and neurodevelopmental endpoints, he said.
In his recent retrospective observational study of 58 patients enrolled in the University of Chicago Monogenic Diabetes Registry who had neonatal diabetes involving the KCNJ11 subunit of the KATP channel, hemoglobin A1c fell from an average of 8.5% on insulin to 6.2% after the transition to sulfonylurea therapy. Age at initiation of sulfonylureas showed a linear correlation with the dose required at follow-up. Indeed, all 10 patients who needed additional glucose-lowering medications along with their sulfonylurea had started on sulfonylurea therapy at age 13 years or older (Diabetologia 2015 April 17 [Epub ahead of print] PMID:25877689).
A likely possible explanation for the observed decline in sensitivity to sulfonylureas with an older starting age is that insulin-treated patients experience loss of beta cell mass over time, according to Dr. Greeley.
A syndrome known by the acronym DEND, for developmental delay, epilepsy, and neonatal diabetes, affects 20%-30% of patients with neonatal diabetes related to mutations in the KATP channel. These channels are widely expressed in the brain.
The first hint that early initiation of sulfonylureas in children with KATP-related neonatal diabetes may have beneficial neurodevelopmental effects has been provided by a study Dr. Greeley and coinvestigators conducted in 19 patients, aged 2-20 years, with KATP channel mutations. Eight had diabetes only, eight had DEND, all eight of whom had potent V59M/A mutations, and three had other KATP-related mutations associated with milder neurodevelopmental impairment. Upon testing via the age-standardized Beery-Buktenica Developmental Test of Visual-Motor Integration, the investigators found that the group with diabetes only had normal scores while those with V59M/A mutations scored quite low. The key finding: Age at sulfonylurea initiation was inversely associated with scores on the Beery test. Those who started on the medication before 1 year of age had near-normal scores (Diabetes Care 2012;35:2086-8).
That’s not definitive evidence, Dr. Greeley observed. It needs to be confirmed with larger patient numbers. But it does warrant at least considering preemptive sulfonylurea therapy while awaiting genetic test results in patients with neonatal diabetes, he said.
He noted that in his retrospective study of 154 subjects diagnosed with diabetes before age 6 months, barriers to expeditious genetic testing, mainly consisting of obstacles created by insurance companies, resulted in a median time between clinical diagnosis and genetic diagnosis of 10.4 weeks (J. Clin. Endocrinol. Metab. 2014;99:E2709-14).
Neonatal diabetes is a rare condition. Most of the culprit mutations are de novo, not inherited. With an incidence of roughly 1 in 100,000 births, the rate is in line with other disorders that are part of standard newborn screening, such as maple syrup urine disease, Dr. Greeley observed.
More than 70% of all cases of neonatal diabetes are due to mutations at one of four loci: 6q24, KCNJ11, INS, or ABCC8. It’s important to order genetic testing from a laboratory that includes a comprehensive 6q24 analysis in its panel; many don’t, creating the potential for a false-negative test result, the pediatric endocrinologist cautioned.
Dr. Greeley’s research is funded by the National Institutes of Health, the American Diabetes Association, and the Kovler Family Foundation. He reported having no financial conflicts.
EXPERT ANALYSIS FROM THE PAS ANNUAL MEETING
AAS: Acute suicidal affective disturbance proposed as new diagnosis
ATLANTA – Among all deaths by suicide, 15%-20% are attributable to a previously undescribed entity that now has a name: acute suicidal affective disturbance, Thomas Joiner, Ph.D., said at the annual conference of the American Association of Suicidology.
“It’s real, it’s fairly common, and it’s extremely dangerous. We think it ranks up there among the most serious of conditions – more likely to result in death than the manic phase of bipolar disorder or schizophrenia, for example,” said Dr. Joiner, who first identified and characterized the condition, named it, plays a pivotal role in ongoing multicenter collaborative research efforts targeting it, and vows to see acute suicidal affective disturbance, or ASAD, included in the DSM-6.
ASAD is a concept compatible with Dr. Joiner’s “Interpersonal Theory of Suicide,” which has taken the field of suicidology by storm. But ASAD , he stressed, is not a theoretical construct.
“ASAD exists as an entity, as a true object in nature that I worry over because it kills people and we can’t diagnose it currently because there’s a gap in the nomenclature. We’re proposing this condition to fill that gap,” explained Dr. Joiner, professor of psychology at Florida State University, Tallahassee.
Roughly 80% of all deaths by suicide can be attributed to a recognized mental health disorder, such as major depressive disorder, the depressive phase of bipolar disorder, anorexia nervosa, schizophrenia, or borderline personality disorder. Dr. Joiner presented highlights of three separate studies now in press – one conducted in Veterans Affairs outpatients, another in high-risk university undergraduates, and a third involving nearly 7,700 Mayo Clinic psychiatric inpatients and more than 3,400 U.S. Army high-risk patients – all of which support the construct validity of ASAD as a distinct entity that correlates negatively with impulsivity and is unrelated to alcohol use disorders or mood disorders.
In addition, Dr. Joiner continued, three different research teams – his own; the Military Suicide Research Consortium, which he codirects; and the U.S. Army STARRS group – have identified subgroups of patients at very high suicide risk who are characterized by ASAD symptoms that are discernible from those of established clinical entities (see list for proposed ASAD criteria).
“We intend to put ASAD into DSM-6. We know that’s going to be a battle, and we’re ready for it. We’re determined to fight it,” the psychologist said.
If ASAD were to eventually become a diagnosable condition, the clinical implications would be far-reaching, he observed. It would be a billable entity. Also, its existence in the formal psychiatric nomenclature would alert clinicians to be vigilant regarding ASAD; that’s crucial because recurrences can be lethal, and recognition of the prodromal overarousal symptoms may head off a full-blown crisis.
“If we’re right about ASAD, it would imply that mood disorders and ASAD are distinct, that their onset and remission patterns are different, thus supporting the rationale of suicide-specific treatments like CAMS [collaborative assessment and management of suicidality] and DBT [dialectical behavior therapy]. It would suggest the need for intensive and long-lasting therapies, because a recurrence can cost people their lives,” he continued.
Should ASAD become legitimized as a diagnosis, it would shore up the public health argument in favor of suicide-means restriction as a strategy for forestalling suicidal action.
“ASAD is a time-limited arousal state. You can’t sustain it for more than an hour or a few hours. It’ll abate with the passage of time,” according to Dr. Joiner.
In response to an audience question, he said it’s his clinical impression – not yet supported by data – that the most common comorbid conditions in patients with ASAD are anxiety disorders and personality disorders. Also, ASAD doesn’t appear to be age dependent: “We think it can emerge at any point in the lifespan.”
Dr. Joiner emphasized that many questions regarding ASAD remain unanswered.
“We have a long way to go,” he conceded. “And I’m not talking about years here, I’m talking about decades. The effort is going to be primarily research oriented. It’s a scientific effort. We’ve got to clear the hurdle scientifically, and we haven’t done that yet, although we’ve gotten a start.”
He reported having no financial conflicts regarding his presentation.
Proposed criteria for ASAD
1. A sudden surge in suicidal intent occurring over the course of minutes, hours, or days rather than weeks or months.
2. One or both of two alienation criteria: a) severe social withdrawal defined by extreme disgust with others or perceived liability to others; b) marked self-alienation manifested as self-disgust or the view that one’s selfhood is an onerous burden.
3. The perception that numbers 1 and 2 are hopelessly intractable.
4. At least two of the following manifestations of overarousal: insomnia, nightmares, irritability, agitation.
5. Exclusion criteria: The above is not due to exacerbation of a mood disorder or ingestion of alcohol or another substance.
Source: Dr. Joiner
ATLANTA – Among all deaths by suicide, 15%-20% are attributable to a previously undescribed entity that now has a name: acute suicidal affective disturbance, Thomas Joiner, Ph.D., said at the annual conference of the American Association of Suicidology.
“It’s real, it’s fairly common, and it’s extremely dangerous. We think it ranks up there among the most serious of conditions – more likely to result in death than the manic phase of bipolar disorder or schizophrenia, for example,” said Dr. Joiner, who first identified and characterized the condition, named it, plays a pivotal role in ongoing multicenter collaborative research efforts targeting it, and vows to see acute suicidal affective disturbance, or ASAD, included in the DSM-6.
ASAD is a concept compatible with Dr. Joiner’s “Interpersonal Theory of Suicide,” which has taken the field of suicidology by storm. But ASAD , he stressed, is not a theoretical construct.
“ASAD exists as an entity, as a true object in nature that I worry over because it kills people and we can’t diagnose it currently because there’s a gap in the nomenclature. We’re proposing this condition to fill that gap,” explained Dr. Joiner, professor of psychology at Florida State University, Tallahassee.
Roughly 80% of all deaths by suicide can be attributed to a recognized mental health disorder, such as major depressive disorder, the depressive phase of bipolar disorder, anorexia nervosa, schizophrenia, or borderline personality disorder. Dr. Joiner presented highlights of three separate studies now in press – one conducted in Veterans Affairs outpatients, another in high-risk university undergraduates, and a third involving nearly 7,700 Mayo Clinic psychiatric inpatients and more than 3,400 U.S. Army high-risk patients – all of which support the construct validity of ASAD as a distinct entity that correlates negatively with impulsivity and is unrelated to alcohol use disorders or mood disorders.
In addition, Dr. Joiner continued, three different research teams – his own; the Military Suicide Research Consortium, which he codirects; and the U.S. Army STARRS group – have identified subgroups of patients at very high suicide risk who are characterized by ASAD symptoms that are discernible from those of established clinical entities (see list for proposed ASAD criteria).
“We intend to put ASAD into DSM-6. We know that’s going to be a battle, and we’re ready for it. We’re determined to fight it,” the psychologist said.
If ASAD were to eventually become a diagnosable condition, the clinical implications would be far-reaching, he observed. It would be a billable entity. Also, its existence in the formal psychiatric nomenclature would alert clinicians to be vigilant regarding ASAD; that’s crucial because recurrences can be lethal, and recognition of the prodromal overarousal symptoms may head off a full-blown crisis.
“If we’re right about ASAD, it would imply that mood disorders and ASAD are distinct, that their onset and remission patterns are different, thus supporting the rationale of suicide-specific treatments like CAMS [collaborative assessment and management of suicidality] and DBT [dialectical behavior therapy]. It would suggest the need for intensive and long-lasting therapies, because a recurrence can cost people their lives,” he continued.
Should ASAD become legitimized as a diagnosis, it would shore up the public health argument in favor of suicide-means restriction as a strategy for forestalling suicidal action.
“ASAD is a time-limited arousal state. You can’t sustain it for more than an hour or a few hours. It’ll abate with the passage of time,” according to Dr. Joiner.
In response to an audience question, he said it’s his clinical impression – not yet supported by data – that the most common comorbid conditions in patients with ASAD are anxiety disorders and personality disorders. Also, ASAD doesn’t appear to be age dependent: “We think it can emerge at any point in the lifespan.”
Dr. Joiner emphasized that many questions regarding ASAD remain unanswered.
“We have a long way to go,” he conceded. “And I’m not talking about years here, I’m talking about decades. The effort is going to be primarily research oriented. It’s a scientific effort. We’ve got to clear the hurdle scientifically, and we haven’t done that yet, although we’ve gotten a start.”
He reported having no financial conflicts regarding his presentation.
Proposed criteria for ASAD
1. A sudden surge in suicidal intent occurring over the course of minutes, hours, or days rather than weeks or months.
2. One or both of two alienation criteria: a) severe social withdrawal defined by extreme disgust with others or perceived liability to others; b) marked self-alienation manifested as self-disgust or the view that one’s selfhood is an onerous burden.
3. The perception that numbers 1 and 2 are hopelessly intractable.
4. At least two of the following manifestations of overarousal: insomnia, nightmares, irritability, agitation.
5. Exclusion criteria: The above is not due to exacerbation of a mood disorder or ingestion of alcohol or another substance.
Source: Dr. Joiner
ATLANTA – Among all deaths by suicide, 15%-20% are attributable to a previously undescribed entity that now has a name: acute suicidal affective disturbance, Thomas Joiner, Ph.D., said at the annual conference of the American Association of Suicidology.
“It’s real, it’s fairly common, and it’s extremely dangerous. We think it ranks up there among the most serious of conditions – more likely to result in death than the manic phase of bipolar disorder or schizophrenia, for example,” said Dr. Joiner, who first identified and characterized the condition, named it, plays a pivotal role in ongoing multicenter collaborative research efforts targeting it, and vows to see acute suicidal affective disturbance, or ASAD, included in the DSM-6.
ASAD is a concept compatible with Dr. Joiner’s “Interpersonal Theory of Suicide,” which has taken the field of suicidology by storm. But ASAD , he stressed, is not a theoretical construct.
“ASAD exists as an entity, as a true object in nature that I worry over because it kills people and we can’t diagnose it currently because there’s a gap in the nomenclature. We’re proposing this condition to fill that gap,” explained Dr. Joiner, professor of psychology at Florida State University, Tallahassee.
Roughly 80% of all deaths by suicide can be attributed to a recognized mental health disorder, such as major depressive disorder, the depressive phase of bipolar disorder, anorexia nervosa, schizophrenia, or borderline personality disorder. Dr. Joiner presented highlights of three separate studies now in press – one conducted in Veterans Affairs outpatients, another in high-risk university undergraduates, and a third involving nearly 7,700 Mayo Clinic psychiatric inpatients and more than 3,400 U.S. Army high-risk patients – all of which support the construct validity of ASAD as a distinct entity that correlates negatively with impulsivity and is unrelated to alcohol use disorders or mood disorders.
In addition, Dr. Joiner continued, three different research teams – his own; the Military Suicide Research Consortium, which he codirects; and the U.S. Army STARRS group – have identified subgroups of patients at very high suicide risk who are characterized by ASAD symptoms that are discernible from those of established clinical entities (see list for proposed ASAD criteria).
“We intend to put ASAD into DSM-6. We know that’s going to be a battle, and we’re ready for it. We’re determined to fight it,” the psychologist said.
If ASAD were to eventually become a diagnosable condition, the clinical implications would be far-reaching, he observed. It would be a billable entity. Also, its existence in the formal psychiatric nomenclature would alert clinicians to be vigilant regarding ASAD; that’s crucial because recurrences can be lethal, and recognition of the prodromal overarousal symptoms may head off a full-blown crisis.
“If we’re right about ASAD, it would imply that mood disorders and ASAD are distinct, that their onset and remission patterns are different, thus supporting the rationale of suicide-specific treatments like CAMS [collaborative assessment and management of suicidality] and DBT [dialectical behavior therapy]. It would suggest the need for intensive and long-lasting therapies, because a recurrence can cost people their lives,” he continued.
Should ASAD become legitimized as a diagnosis, it would shore up the public health argument in favor of suicide-means restriction as a strategy for forestalling suicidal action.
“ASAD is a time-limited arousal state. You can’t sustain it for more than an hour or a few hours. It’ll abate with the passage of time,” according to Dr. Joiner.
In response to an audience question, he said it’s his clinical impression – not yet supported by data – that the most common comorbid conditions in patients with ASAD are anxiety disorders and personality disorders. Also, ASAD doesn’t appear to be age dependent: “We think it can emerge at any point in the lifespan.”
Dr. Joiner emphasized that many questions regarding ASAD remain unanswered.
“We have a long way to go,” he conceded. “And I’m not talking about years here, I’m talking about decades. The effort is going to be primarily research oriented. It’s a scientific effort. We’ve got to clear the hurdle scientifically, and we haven’t done that yet, although we’ve gotten a start.”
He reported having no financial conflicts regarding his presentation.
Proposed criteria for ASAD
1. A sudden surge in suicidal intent occurring over the course of minutes, hours, or days rather than weeks or months.
2. One or both of two alienation criteria: a) severe social withdrawal defined by extreme disgust with others or perceived liability to others; b) marked self-alienation manifested as self-disgust or the view that one’s selfhood is an onerous burden.
3. The perception that numbers 1 and 2 are hopelessly intractable.
4. At least two of the following manifestations of overarousal: insomnia, nightmares, irritability, agitation.
5. Exclusion criteria: The above is not due to exacerbation of a mood disorder or ingestion of alcohol or another substance.
Source: Dr. Joiner
EXPERT ANALYSIS FROM THE ANNUAL AAS CONFERENCE
IHC: Transcranial magnetic stimulation effective for medication overuse headache
VALENCIA, SPAIN – Single-pulse transcranial magnetic stimulation shows promise for treatment of migraine with medication overuse headache, according to a pilot study.
Twenty-eight severely affected patients participated in this prospective, open-label study of single-pulse transcranial magnetic stimulation (sTMS). They were instructed to use the device for at least 3 months, with an option to continue for another 3 months.
The proprietary SpringTMS system was approved by the Food and Drug Administration and the U.K.’s National Institute for Clinical Excellence for treatment of acute migraine on the basis of a positive randomized, double-blind, sham-controlled clinical trial (Lancet Neurol. 2010;9:373-80), but it hasn’t previously been evaluated in the common and clinically challenging setting of migraine complicated by medication overuse, Dr. Farooq Maniyar noted at the International Headache Congress.
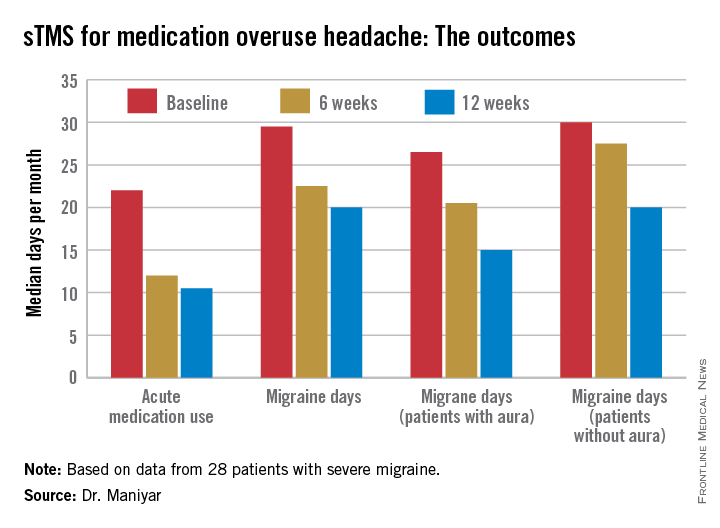
The reasons participants gave for enrolling in the study varied. Most felt their current medications had intolerable side effects and/or weren’t sufficiently beneficial. Some preferred the possibility of a nondrug method of managing their headaches, explained Dr. Maniyar, a consulting neurologist at Basildon and Thurrock University Hospitals NHS Foundation Trust, Basildon, England, and the Royal London Hospital of Barts Health NHS Trust.
The key results include:
• Twenty-four of 28 patients (86%) reported a reduction in their days of acute medication use per month, while 2 patients reported an increase in acute medications.
• Nineteen patients (68%) experienced fewer migraine days per month, and 7 of the 19 had a 50% or greater reduction.
• The number of patients who rated their migraine pain as excruciating or severe plunged from 19 at baseline to 3 at 3 months, an 84% drop.
• Self-reported headache attack duration, which averaged 1 hour at baseline, decreased in 15 patients, remained unchanged in 9, and increased in 4.
• The Headache Impact Test (HIT-6) score, which reflects patient perception of the impact the migraine headaches have on daily life, was rated severe at baseline – meaning a score of at least 60 – by 26 of 28 participants. At 3 months, this number had decreased from 26 to 18.
“I think it’s fair to say that sTMS may be a useful option in treatment of medication overuse headache,” Dr. Maniyar declared at the meeting sponsored by the International Headache Society and the American Headache Society.
The sTMS regimen was beneficial in migraine patients with or without aura. Although the 12 patients who had migraine with aura showed a consistent trend for better outcomes, Dr. Maniyar said the patient numbers in the study were too small to draw definite conclusions on this score.
After 3 months, five patients discontinued sTMS because of inconvenience or lack of benefit. Four others were lost to follow-up. Of the remaining 19, 16 reported reduced days of acute medication usage at 6 months, compared with baseline. HIT-6 scores in the 19 patients with 6-month treatment data were comparable to their scores at 3 months.
No adverse events occurred in connection with the study.
Dr. Maniyar said the study’s major limitations are its relatively small sample size – the results need a confirmatory study – and the open-label, uncontrolled study design.
“We can’t rule out the possibility of a placebo effect for sTMS,” he observed.
The portable, noninvasive SpringTMS device delivers a brief pulse of magnetic energy to the back of the head in order to disrupt the migraine headache. The daily treatment regimen for the first month involved giving two pulses, followed 15 minutes later by another two, then repeating the sequence roughly 12 hours later. In addition, patients were instructed to use the device at the onset of headaches. Patients were allowed to use a maximum of 16 pulses per day in month 1, 24 per day in month 2, and up to 32 daily during month 3.
The study was sponsored by eNeura, which markets the SpringTMS device. Dr. Maniyar reported serving as a consultant to the company.
VALENCIA, SPAIN – Single-pulse transcranial magnetic stimulation shows promise for treatment of migraine with medication overuse headache, according to a pilot study.
Twenty-eight severely affected patients participated in this prospective, open-label study of single-pulse transcranial magnetic stimulation (sTMS). They were instructed to use the device for at least 3 months, with an option to continue for another 3 months.
The proprietary SpringTMS system was approved by the Food and Drug Administration and the U.K.’s National Institute for Clinical Excellence for treatment of acute migraine on the basis of a positive randomized, double-blind, sham-controlled clinical trial (Lancet Neurol. 2010;9:373-80), but it hasn’t previously been evaluated in the common and clinically challenging setting of migraine complicated by medication overuse, Dr. Farooq Maniyar noted at the International Headache Congress.

The reasons participants gave for enrolling in the study varied. Most felt their current medications had intolerable side effects and/or weren’t sufficiently beneficial. Some preferred the possibility of a nondrug method of managing their headaches, explained Dr. Maniyar, a consulting neurologist at Basildon and Thurrock University Hospitals NHS Foundation Trust, Basildon, England, and the Royal London Hospital of Barts Health NHS Trust.
The key results include:
• Twenty-four of 28 patients (86%) reported a reduction in their days of acute medication use per month, while 2 patients reported an increase in acute medications.
• Nineteen patients (68%) experienced fewer migraine days per month, and 7 of the 19 had a 50% or greater reduction.
• The number of patients who rated their migraine pain as excruciating or severe plunged from 19 at baseline to 3 at 3 months, an 84% drop.
• Self-reported headache attack duration, which averaged 1 hour at baseline, decreased in 15 patients, remained unchanged in 9, and increased in 4.
• The Headache Impact Test (HIT-6) score, which reflects patient perception of the impact the migraine headaches have on daily life, was rated severe at baseline – meaning a score of at least 60 – by 26 of 28 participants. At 3 months, this number had decreased from 26 to 18.
“I think it’s fair to say that sTMS may be a useful option in treatment of medication overuse headache,” Dr. Maniyar declared at the meeting sponsored by the International Headache Society and the American Headache Society.
The sTMS regimen was beneficial in migraine patients with or without aura. Although the 12 patients who had migraine with aura showed a consistent trend for better outcomes, Dr. Maniyar said the patient numbers in the study were too small to draw definite conclusions on this score.
After 3 months, five patients discontinued sTMS because of inconvenience or lack of benefit. Four others were lost to follow-up. Of the remaining 19, 16 reported reduced days of acute medication usage at 6 months, compared with baseline. HIT-6 scores in the 19 patients with 6-month treatment data were comparable to their scores at 3 months.
No adverse events occurred in connection with the study.
Dr. Maniyar said the study’s major limitations are its relatively small sample size – the results need a confirmatory study – and the open-label, uncontrolled study design.
“We can’t rule out the possibility of a placebo effect for sTMS,” he observed.
The portable, noninvasive SpringTMS device delivers a brief pulse of magnetic energy to the back of the head in order to disrupt the migraine headache. The daily treatment regimen for the first month involved giving two pulses, followed 15 minutes later by another two, then repeating the sequence roughly 12 hours later. In addition, patients were instructed to use the device at the onset of headaches. Patients were allowed to use a maximum of 16 pulses per day in month 1, 24 per day in month 2, and up to 32 daily during month 3.
The study was sponsored by eNeura, which markets the SpringTMS device. Dr. Maniyar reported serving as a consultant to the company.
VALENCIA, SPAIN – Single-pulse transcranial magnetic stimulation shows promise for treatment of migraine with medication overuse headache, according to a pilot study.
Twenty-eight severely affected patients participated in this prospective, open-label study of single-pulse transcranial magnetic stimulation (sTMS). They were instructed to use the device for at least 3 months, with an option to continue for another 3 months.
The proprietary SpringTMS system was approved by the Food and Drug Administration and the U.K.’s National Institute for Clinical Excellence for treatment of acute migraine on the basis of a positive randomized, double-blind, sham-controlled clinical trial (Lancet Neurol. 2010;9:373-80), but it hasn’t previously been evaluated in the common and clinically challenging setting of migraine complicated by medication overuse, Dr. Farooq Maniyar noted at the International Headache Congress.

The reasons participants gave for enrolling in the study varied. Most felt their current medications had intolerable side effects and/or weren’t sufficiently beneficial. Some preferred the possibility of a nondrug method of managing their headaches, explained Dr. Maniyar, a consulting neurologist at Basildon and Thurrock University Hospitals NHS Foundation Trust, Basildon, England, and the Royal London Hospital of Barts Health NHS Trust.
The key results include:
• Twenty-four of 28 patients (86%) reported a reduction in their days of acute medication use per month, while 2 patients reported an increase in acute medications.
• Nineteen patients (68%) experienced fewer migraine days per month, and 7 of the 19 had a 50% or greater reduction.
• The number of patients who rated their migraine pain as excruciating or severe plunged from 19 at baseline to 3 at 3 months, an 84% drop.
• Self-reported headache attack duration, which averaged 1 hour at baseline, decreased in 15 patients, remained unchanged in 9, and increased in 4.
• The Headache Impact Test (HIT-6) score, which reflects patient perception of the impact the migraine headaches have on daily life, was rated severe at baseline – meaning a score of at least 60 – by 26 of 28 participants. At 3 months, this number had decreased from 26 to 18.
“I think it’s fair to say that sTMS may be a useful option in treatment of medication overuse headache,” Dr. Maniyar declared at the meeting sponsored by the International Headache Society and the American Headache Society.
The sTMS regimen was beneficial in migraine patients with or without aura. Although the 12 patients who had migraine with aura showed a consistent trend for better outcomes, Dr. Maniyar said the patient numbers in the study were too small to draw definite conclusions on this score.
After 3 months, five patients discontinued sTMS because of inconvenience or lack of benefit. Four others were lost to follow-up. Of the remaining 19, 16 reported reduced days of acute medication usage at 6 months, compared with baseline. HIT-6 scores in the 19 patients with 6-month treatment data were comparable to their scores at 3 months.
No adverse events occurred in connection with the study.
Dr. Maniyar said the study’s major limitations are its relatively small sample size – the results need a confirmatory study – and the open-label, uncontrolled study design.
“We can’t rule out the possibility of a placebo effect for sTMS,” he observed.
The portable, noninvasive SpringTMS device delivers a brief pulse of magnetic energy to the back of the head in order to disrupt the migraine headache. The daily treatment regimen for the first month involved giving two pulses, followed 15 minutes later by another two, then repeating the sequence roughly 12 hours later. In addition, patients were instructed to use the device at the onset of headaches. Patients were allowed to use a maximum of 16 pulses per day in month 1, 24 per day in month 2, and up to 32 daily during month 3.
The study was sponsored by eNeura, which markets the SpringTMS device. Dr. Maniyar reported serving as a consultant to the company.
AT IHC 2015
Key clinical point:The SpringTMS device shows promising results as a safe and effective nondrug therapeutic option in migraine with medication overuse headache.
Major finding: The median number of days of acute medication use per month went from 22 at baseline to 12 after 6 weeks of self-treatment with single-pulse transcranial magnetic stimulation to 10.5 days after 3 months.
Data source: This was a prospective, open-label, uncontrolled pilot study involving 28 patients with severe migraine complicated by medication overuse headache.
Disclosures: The study was sponsored by eNeura, which markets the SpringTMS device. The presenter reported serving as a consultant to the company.