User login
EuroPCR: CT-derived FFR promising in evaluating chest pain
PARIS – Noninvasive measurement of computed tomography–derived fractional flow reserve is a potential game changer in the management of patients with stable chest pain.
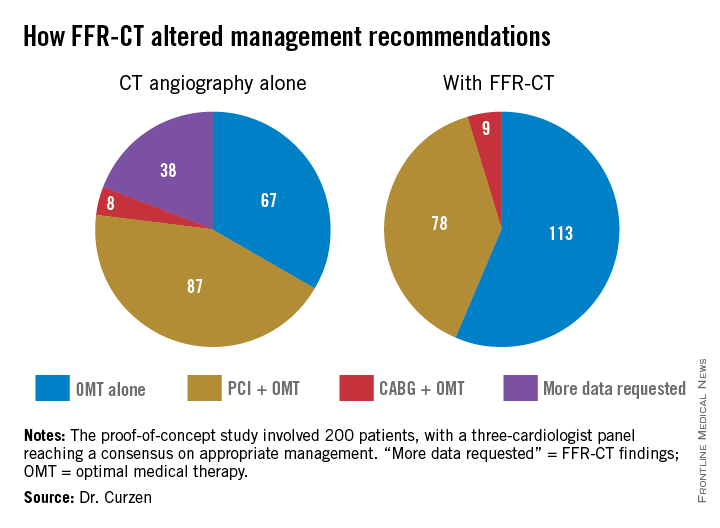
In a 200-patient proof-of-concept study known as FFR-CT RIPCORD, in which three experienced interventional cardiologists initially devised management plans based on coronary anatomy as defined by the results of CT angiography alone, subsequent knowledge of CT-derived fractional flow reserve (FFR-CT) caused them to change their management strategies in fully 36% of cases, Dr. Nick Curzen reported at the annual congress of the European Association of Percutaneous Cardiovascular Interventions.

“If this novel proof-of-concept result can be confirmed in large-scale trials, this suggests that noninvasive FFR-CT can be used as a clinically relevant tool that mimics the well-described ability of invasive FFR to refine management decisions for patients with chest pain that are made by invasive coronary angiography alone. This would indeed have important implications for routine clinical practice. FFR-CT may have potential as a noninvasive default method for simultaneous assessment of coronary anatomy and physiology in angina patients in order to define their management, which would completely change the way we look after them,” observed Dr. Curzen, professor of interventional cardiology at the University of Southampton (England).
EuroPCR codirector Dr. Williams Wijns was favorably impressed by the FFR-CT RIPCORD findings.
“This, I find just stunning. It’s really far reaching. This is a complete change in paradigm. Many patients that today undergo invasive angiography won’t even be sent to the cath lab. The invasive center becomes only for treatment,” commented Dr. Wijns, codirector of the cardiovascular center in Aalst, Belgium.
In FFR-CT RIPCORD, the cardiologists received information about a patient’s history and nonvasive CT angiography findings and were asked to reach consensus in selecting one of four management options: optimal medical therapy (OMT) alone, PCI plus OMT, CABG surgery and OMT, or ‘more information needed’ in the form of FFR findings, which identify those coronary lesions that are actually causing ischemia. Instead of receiving the results of conventional invasive FFR obtained using a pressure wire, however, the cardiologists were provided with the noninvasive FFR-CT findings in all 200 cases.
The resultant changes in management were substantial. Thirty percent of the patients initially slated for PCI were reallocated to OMT alone because no ischemic lesions were present. Twelve percent of patients assigned to OMT-only got reassigned to coronary revascularization. Moreover, in 18% of the PCI group, FFR-CT data led to a change in the vessel or vessels targeted for intervention.
“What particularly impressed me were two of those figures: that one-third of PCI patients are redirected to medical therapy, and – even more impressive to me – is the 18% of PCI patients who had a change in their target vessel. That’s a problem we often have in patients with multivessel disease and intermediate lesions: Sometimes we think, for example, the target is the LAD when in fact it’s another vessel,” commented Dr. Jean Fajadet, codirector of the interventional cardiovascular group at the Clinique Pasteur in Toulouse, France.
Dr. Curzen said the exciting thing about FFR-CT is that it could provide in one fell swoop a standardized way of obtaining both the anatomic and physiologic data necessary for informed clinical decision making, and without exposing patients needlessly to the risks of contrast and radiation exposure entailed in invasive coronary angiography.
“When we assess people with stable angina, if you have a room full of invasive cardiologists, we all do it differently at the moment. It’s crazy. A lot of us will do noninvasive tests like stress echo or MRI or some kind of exercise test, and then refer them for an invasive angiogram where we’ll also do an FFR. Some people will go straight for an angiogram. It’s a real mess. The thing I love about FFR-CT is it would be so slick for patients and their families: You see them in a chest pain clinic or your office and you put them in for this test. They don’t have to waste their time coming back several times for different tests. It’s a really beautiful concept,” Dr. Curzen continued.
Right now the turnaround time on FFR-CT is about 12 hours. The dataset has to be sent off to a supercomputer for a complex modeling analysis before the results come back.
“Of course, if this ever becomes clinically proven, I’m sure the turnaround time would become very quick,” according to the cardiologist.
A cost-effectiveness analysis of FFR-CT versus current standard care is ongoing and the results aren’t yet available. However, Dr. Curzen observed, “The cost to the patient is a very important issue: Who would want to have this done invasively if you have a test that proves you don’t need to have an invasive procedure?”
The FFR-CT RIPCORD study was sponsored by Heartflow. Dr. Curzen reported receiving research support from Heartflow, Boston Scientific, Haemonetics, and Medtronic.
PARIS – Noninvasive measurement of computed tomography–derived fractional flow reserve is a potential game changer in the management of patients with stable chest pain.
In a 200-patient proof-of-concept study known as FFR-CT RIPCORD, in which three experienced interventional cardiologists initially devised management plans based on coronary anatomy as defined by the results of CT angiography alone, subsequent knowledge of CT-derived fractional flow reserve (FFR-CT) caused them to change their management strategies in fully 36% of cases, Dr. Nick Curzen reported at the annual congress of the European Association of Percutaneous Cardiovascular Interventions.

“If this novel proof-of-concept result can be confirmed in large-scale trials, this suggests that noninvasive FFR-CT can be used as a clinically relevant tool that mimics the well-described ability of invasive FFR to refine management decisions for patients with chest pain that are made by invasive coronary angiography alone. This would indeed have important implications for routine clinical practice. FFR-CT may have potential as a noninvasive default method for simultaneous assessment of coronary anatomy and physiology in angina patients in order to define their management, which would completely change the way we look after them,” observed Dr. Curzen, professor of interventional cardiology at the University of Southampton (England).
EuroPCR codirector Dr. Williams Wijns was favorably impressed by the FFR-CT RIPCORD findings.
“This, I find just stunning. It’s really far reaching. This is a complete change in paradigm. Many patients that today undergo invasive angiography won’t even be sent to the cath lab. The invasive center becomes only for treatment,” commented Dr. Wijns, codirector of the cardiovascular center in Aalst, Belgium.
In FFR-CT RIPCORD, the cardiologists received information about a patient’s history and nonvasive CT angiography findings and were asked to reach consensus in selecting one of four management options: optimal medical therapy (OMT) alone, PCI plus OMT, CABG surgery and OMT, or ‘more information needed’ in the form of FFR findings, which identify those coronary lesions that are actually causing ischemia. Instead of receiving the results of conventional invasive FFR obtained using a pressure wire, however, the cardiologists were provided with the noninvasive FFR-CT findings in all 200 cases.
The resultant changes in management were substantial. Thirty percent of the patients initially slated for PCI were reallocated to OMT alone because no ischemic lesions were present. Twelve percent of patients assigned to OMT-only got reassigned to coronary revascularization. Moreover, in 18% of the PCI group, FFR-CT data led to a change in the vessel or vessels targeted for intervention.
“What particularly impressed me were two of those figures: that one-third of PCI patients are redirected to medical therapy, and – even more impressive to me – is the 18% of PCI patients who had a change in their target vessel. That’s a problem we often have in patients with multivessel disease and intermediate lesions: Sometimes we think, for example, the target is the LAD when in fact it’s another vessel,” commented Dr. Jean Fajadet, codirector of the interventional cardiovascular group at the Clinique Pasteur in Toulouse, France.
Dr. Curzen said the exciting thing about FFR-CT is that it could provide in one fell swoop a standardized way of obtaining both the anatomic and physiologic data necessary for informed clinical decision making, and without exposing patients needlessly to the risks of contrast and radiation exposure entailed in invasive coronary angiography.
“When we assess people with stable angina, if you have a room full of invasive cardiologists, we all do it differently at the moment. It’s crazy. A lot of us will do noninvasive tests like stress echo or MRI or some kind of exercise test, and then refer them for an invasive angiogram where we’ll also do an FFR. Some people will go straight for an angiogram. It’s a real mess. The thing I love about FFR-CT is it would be so slick for patients and their families: You see them in a chest pain clinic or your office and you put them in for this test. They don’t have to waste their time coming back several times for different tests. It’s a really beautiful concept,” Dr. Curzen continued.
Right now the turnaround time on FFR-CT is about 12 hours. The dataset has to be sent off to a supercomputer for a complex modeling analysis before the results come back.
“Of course, if this ever becomes clinically proven, I’m sure the turnaround time would become very quick,” according to the cardiologist.
A cost-effectiveness analysis of FFR-CT versus current standard care is ongoing and the results aren’t yet available. However, Dr. Curzen observed, “The cost to the patient is a very important issue: Who would want to have this done invasively if you have a test that proves you don’t need to have an invasive procedure?”
The FFR-CT RIPCORD study was sponsored by Heartflow. Dr. Curzen reported receiving research support from Heartflow, Boston Scientific, Haemonetics, and Medtronic.
PARIS – Noninvasive measurement of computed tomography–derived fractional flow reserve is a potential game changer in the management of patients with stable chest pain.
In a 200-patient proof-of-concept study known as FFR-CT RIPCORD, in which three experienced interventional cardiologists initially devised management plans based on coronary anatomy as defined by the results of CT angiography alone, subsequent knowledge of CT-derived fractional flow reserve (FFR-CT) caused them to change their management strategies in fully 36% of cases, Dr. Nick Curzen reported at the annual congress of the European Association of Percutaneous Cardiovascular Interventions.

“If this novel proof-of-concept result can be confirmed in large-scale trials, this suggests that noninvasive FFR-CT can be used as a clinically relevant tool that mimics the well-described ability of invasive FFR to refine management decisions for patients with chest pain that are made by invasive coronary angiography alone. This would indeed have important implications for routine clinical practice. FFR-CT may have potential as a noninvasive default method for simultaneous assessment of coronary anatomy and physiology in angina patients in order to define their management, which would completely change the way we look after them,” observed Dr. Curzen, professor of interventional cardiology at the University of Southampton (England).
EuroPCR codirector Dr. Williams Wijns was favorably impressed by the FFR-CT RIPCORD findings.
“This, I find just stunning. It’s really far reaching. This is a complete change in paradigm. Many patients that today undergo invasive angiography won’t even be sent to the cath lab. The invasive center becomes only for treatment,” commented Dr. Wijns, codirector of the cardiovascular center in Aalst, Belgium.
In FFR-CT RIPCORD, the cardiologists received information about a patient’s history and nonvasive CT angiography findings and were asked to reach consensus in selecting one of four management options: optimal medical therapy (OMT) alone, PCI plus OMT, CABG surgery and OMT, or ‘more information needed’ in the form of FFR findings, which identify those coronary lesions that are actually causing ischemia. Instead of receiving the results of conventional invasive FFR obtained using a pressure wire, however, the cardiologists were provided with the noninvasive FFR-CT findings in all 200 cases.
The resultant changes in management were substantial. Thirty percent of the patients initially slated for PCI were reallocated to OMT alone because no ischemic lesions were present. Twelve percent of patients assigned to OMT-only got reassigned to coronary revascularization. Moreover, in 18% of the PCI group, FFR-CT data led to a change in the vessel or vessels targeted for intervention.
“What particularly impressed me were two of those figures: that one-third of PCI patients are redirected to medical therapy, and – even more impressive to me – is the 18% of PCI patients who had a change in their target vessel. That’s a problem we often have in patients with multivessel disease and intermediate lesions: Sometimes we think, for example, the target is the LAD when in fact it’s another vessel,” commented Dr. Jean Fajadet, codirector of the interventional cardiovascular group at the Clinique Pasteur in Toulouse, France.
Dr. Curzen said the exciting thing about FFR-CT is that it could provide in one fell swoop a standardized way of obtaining both the anatomic and physiologic data necessary for informed clinical decision making, and without exposing patients needlessly to the risks of contrast and radiation exposure entailed in invasive coronary angiography.
“When we assess people with stable angina, if you have a room full of invasive cardiologists, we all do it differently at the moment. It’s crazy. A lot of us will do noninvasive tests like stress echo or MRI or some kind of exercise test, and then refer them for an invasive angiogram where we’ll also do an FFR. Some people will go straight for an angiogram. It’s a real mess. The thing I love about FFR-CT is it would be so slick for patients and their families: You see them in a chest pain clinic or your office and you put them in for this test. They don’t have to waste their time coming back several times for different tests. It’s a really beautiful concept,” Dr. Curzen continued.
Right now the turnaround time on FFR-CT is about 12 hours. The dataset has to be sent off to a supercomputer for a complex modeling analysis before the results come back.
“Of course, if this ever becomes clinically proven, I’m sure the turnaround time would become very quick,” according to the cardiologist.
A cost-effectiveness analysis of FFR-CT versus current standard care is ongoing and the results aren’t yet available. However, Dr. Curzen observed, “The cost to the patient is a very important issue: Who would want to have this done invasively if you have a test that proves you don’t need to have an invasive procedure?”
The FFR-CT RIPCORD study was sponsored by Heartflow. Dr. Curzen reported receiving research support from Heartflow, Boston Scientific, Haemonetics, and Medtronic.
AT EUROPCR
Key clinical point: Clinically decisive anatomic and physiologic data regarding the coronary arteries of patients with stable angina can be obtained noninvasively with a single test: CT-derived fractional flow reserve.
Major finding: Noninvasive FFR-CT findings resulted in a change in management strategy for 36% of patients with stable angina whose initial treatment plan was based on CT angiography alone.
Data source: A proof-of-concept study involving 200 patients with stable angina and a panel of three experienced interventional cardiologists making consensus decisions regarding their appropriate management.
Disclosures: The FFR-CT RIPCORD study was sponsored by Heartflow. The presenter reported having received research support from the company.
AAS: The ‘sad truth’ about suicide risk assessment scales
ATLANTA – Don’t – repeat, don’t – use risk assessment tools and scales in an effort to predict future suicide in patients who’ve committed intentional self-harm, Dr. Keith Hawton urged at the annual conference of the American Association of Suicidology.
He noted this isn’t simply a matter of his personal opinion; it’s also a strongly worded recommendation in the current U.K. NICE (National Institute for Health and Care Excellence) guidelines on the long-term management of patients who’ve committed self-harm. The various NICE guidelines, which address numerous areas of medical practice and are used to determine what’s reimbursable through the U.K.’s National Health Service, are famously evidence based and concerned with cost-effectiveness.
The NICE guidelines on management of self-harm further advise: “Do not use risk assessment tools and scales to determine who should and should not be offered treatment or who should be discharged from hospital.”
“Those are some fairly contentious statements about risk assessment scales. But those statements are based upon review of the evidence about the effectiveness of risk assessment scales,” according to Dr. Hawton, professor of psychiatry and director of the Centre for Suicide Research at the University of Oxford (England).
“In our country, hospitals have become obsessed with risk assessment. And usually it seems to be about protecting the organization rather than the patient, because so often the results aren’t linked to risk management, which is what we should be talking about,” he observed.
Dr. Hawton and his colleagues provided some of the evidence that led to the NICE guideline committee’s thumbs-down on the use of suicide risk assessment scales in patients who’ve engaged in intentional self-harm. In a study provocatively titled “The sad truth about the SADPERSONS scale,” he and his coinvestigators essentially dismantled SADPERSONS, a widely used screening tool for suicide risk, concluding that it is without value.
The acronym stands for Sex (male), Age (<19 or >45), Depression, Previous attempts, Ethanol abuse, Rational thinking loss, Social supports lacking, Organized plan, No spouse, and Sickness. One point is given for each. Patients who score 7-10 are to be hospitalized, and those with a total of 5 or 6 points should be strongly considered for hospitalization.
Dr. Hawton and coinvestigators tracked 126 consecutive patients who were evaluated for self-harm using the SADPERSONS scale in a general hospital emergency department and then followed them for 6 months. SADPERSONS performed miserably in predicting clinical management outcomes, such as admission to a psychiatric hospital or repetition of self-harm within 6 months. Indeed, the test failed to identify 4 of the 5 patients admitted to a psychiatric hospital, 65 of 70 who were referred from the ED to community psychiatric aftercare, and 28 of 31 who repeated self-harm within 6 months. Thus, its sensitivity as a predictor of repetition of self-harm was a lowly 6.6% (Emerg. Med. J. 2014;31:796-8).
And yet, a 32-hospital U.K. national study conducted by Dr. Hawton and others found that SADPERSONS was the most widely used scale in EDs for risk assessment following self-harm (BMJ Open. 2014 May 2;4:e004732 [doi:10.1136/bmjopen-2013-004732]).
“It’s a very crude tool,” Dr. Hawton said. “How it found its way into common use in clinical practice is beyond me.”
ATLANTA – Don’t – repeat, don’t – use risk assessment tools and scales in an effort to predict future suicide in patients who’ve committed intentional self-harm, Dr. Keith Hawton urged at the annual conference of the American Association of Suicidology.
He noted this isn’t simply a matter of his personal opinion; it’s also a strongly worded recommendation in the current U.K. NICE (National Institute for Health and Care Excellence) guidelines on the long-term management of patients who’ve committed self-harm. The various NICE guidelines, which address numerous areas of medical practice and are used to determine what’s reimbursable through the U.K.’s National Health Service, are famously evidence based and concerned with cost-effectiveness.
The NICE guidelines on management of self-harm further advise: “Do not use risk assessment tools and scales to determine who should and should not be offered treatment or who should be discharged from hospital.”
“Those are some fairly contentious statements about risk assessment scales. But those statements are based upon review of the evidence about the effectiveness of risk assessment scales,” according to Dr. Hawton, professor of psychiatry and director of the Centre for Suicide Research at the University of Oxford (England).
“In our country, hospitals have become obsessed with risk assessment. And usually it seems to be about protecting the organization rather than the patient, because so often the results aren’t linked to risk management, which is what we should be talking about,” he observed.
Dr. Hawton and his colleagues provided some of the evidence that led to the NICE guideline committee’s thumbs-down on the use of suicide risk assessment scales in patients who’ve engaged in intentional self-harm. In a study provocatively titled “The sad truth about the SADPERSONS scale,” he and his coinvestigators essentially dismantled SADPERSONS, a widely used screening tool for suicide risk, concluding that it is without value.
The acronym stands for Sex (male), Age (<19 or >45), Depression, Previous attempts, Ethanol abuse, Rational thinking loss, Social supports lacking, Organized plan, No spouse, and Sickness. One point is given for each. Patients who score 7-10 are to be hospitalized, and those with a total of 5 or 6 points should be strongly considered for hospitalization.
Dr. Hawton and coinvestigators tracked 126 consecutive patients who were evaluated for self-harm using the SADPERSONS scale in a general hospital emergency department and then followed them for 6 months. SADPERSONS performed miserably in predicting clinical management outcomes, such as admission to a psychiatric hospital or repetition of self-harm within 6 months. Indeed, the test failed to identify 4 of the 5 patients admitted to a psychiatric hospital, 65 of 70 who were referred from the ED to community psychiatric aftercare, and 28 of 31 who repeated self-harm within 6 months. Thus, its sensitivity as a predictor of repetition of self-harm was a lowly 6.6% (Emerg. Med. J. 2014;31:796-8).
And yet, a 32-hospital U.K. national study conducted by Dr. Hawton and others found that SADPERSONS was the most widely used scale in EDs for risk assessment following self-harm (BMJ Open. 2014 May 2;4:e004732 [doi:10.1136/bmjopen-2013-004732]).
“It’s a very crude tool,” Dr. Hawton said. “How it found its way into common use in clinical practice is beyond me.”
ATLANTA – Don’t – repeat, don’t – use risk assessment tools and scales in an effort to predict future suicide in patients who’ve committed intentional self-harm, Dr. Keith Hawton urged at the annual conference of the American Association of Suicidology.
He noted this isn’t simply a matter of his personal opinion; it’s also a strongly worded recommendation in the current U.K. NICE (National Institute for Health and Care Excellence) guidelines on the long-term management of patients who’ve committed self-harm. The various NICE guidelines, which address numerous areas of medical practice and are used to determine what’s reimbursable through the U.K.’s National Health Service, are famously evidence based and concerned with cost-effectiveness.
The NICE guidelines on management of self-harm further advise: “Do not use risk assessment tools and scales to determine who should and should not be offered treatment or who should be discharged from hospital.”
“Those are some fairly contentious statements about risk assessment scales. But those statements are based upon review of the evidence about the effectiveness of risk assessment scales,” according to Dr. Hawton, professor of psychiatry and director of the Centre for Suicide Research at the University of Oxford (England).
“In our country, hospitals have become obsessed with risk assessment. And usually it seems to be about protecting the organization rather than the patient, because so often the results aren’t linked to risk management, which is what we should be talking about,” he observed.
Dr. Hawton and his colleagues provided some of the evidence that led to the NICE guideline committee’s thumbs-down on the use of suicide risk assessment scales in patients who’ve engaged in intentional self-harm. In a study provocatively titled “The sad truth about the SADPERSONS scale,” he and his coinvestigators essentially dismantled SADPERSONS, a widely used screening tool for suicide risk, concluding that it is without value.
The acronym stands for Sex (male), Age (<19 or >45), Depression, Previous attempts, Ethanol abuse, Rational thinking loss, Social supports lacking, Organized plan, No spouse, and Sickness. One point is given for each. Patients who score 7-10 are to be hospitalized, and those with a total of 5 or 6 points should be strongly considered for hospitalization.
Dr. Hawton and coinvestigators tracked 126 consecutive patients who were evaluated for self-harm using the SADPERSONS scale in a general hospital emergency department and then followed them for 6 months. SADPERSONS performed miserably in predicting clinical management outcomes, such as admission to a psychiatric hospital or repetition of self-harm within 6 months. Indeed, the test failed to identify 4 of the 5 patients admitted to a psychiatric hospital, 65 of 70 who were referred from the ED to community psychiatric aftercare, and 28 of 31 who repeated self-harm within 6 months. Thus, its sensitivity as a predictor of repetition of self-harm was a lowly 6.6% (Emerg. Med. J. 2014;31:796-8).
And yet, a 32-hospital U.K. national study conducted by Dr. Hawton and others found that SADPERSONS was the most widely used scale in EDs for risk assessment following self-harm (BMJ Open. 2014 May 2;4:e004732 [doi:10.1136/bmjopen-2013-004732]).
“It’s a very crude tool,” Dr. Hawton said. “How it found its way into common use in clinical practice is beyond me.”
EXPERT ANALYSIS FROM THE ANNUAL AAS CONFERENCE
IHC: Real-world data support Botox in chronic migraine
VALENCIA, SPAIN – OnabotulinumtoxinA injections for the treatment of refractory chronic migraine halved the number of days of work per month missed because of migraine while quadrupling the number of crystal-clear, headache-free days in a large, prospective, real-world study.
“OnabotulinumtoxinA [Botox] is a valuable addition to current treatment options in patients with chronic migraine,” Dr. Modar Khalil concluded in summarizing his study findings at the International Headache Congress.
He presented a prospective study of 465 patients with chronic migraine treated with onabotulinumtoxinA in Hull, England.
The results paralleled the findings of the earlier PREEMPT (Phase-3 Research Evaluating Migraine Prophylaxis Therapy) study program which led to marketing approval of onabotulinumtoxinA for chronic migraine in the United Kingdom as well as the United States. However, the Hull patients were more representative of the sort of chronic migraine patients typically seen in tertiary headache clinics. Their chronic migraine was more severe than in the PREEMPT population: 97% of the Hull group had failed three or more preventive therapies at baseline, compared with only one-third of PREEMPT participants, noted Dr. Khalil of the Hull Royal Infirmary.
The Hull patients had a median 4-year history of chronic migraine. Prior to undergoing their first cycle of onabotulinumtoxinA therapy they averaged 27 total headache days and 15 migraine days per month.
The three coprimary study endpoints were at least a 50% reduction in headache days in the first month after onabotulinumtoxinA therapy, compared with the month before, at least a 50% reduction in migraine days, or a doubling of the number of crystal-clear days.
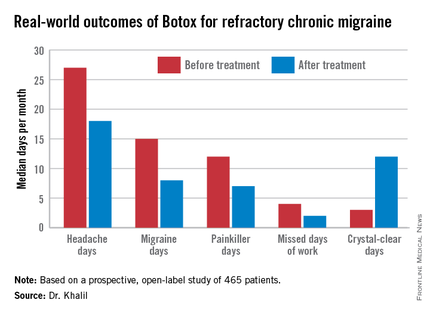
Fifty-nine percent of patients achieved at least one of those targets, 39% reached two, and 21% attained all three. The goal most frequently met was the 50% or greater reduction in monthly migraine days, achieved by 47% of patients. Forty-six percent met the target for an increase in crystal-clear days, while 27% of patients experienced at least a 50% reduction in headache days, Dr. Khalil said at the meeting sponsored by the International Headache Society and the American Headache Society.
The highest response rate was seen in patients with what he termed moderate-frequency chronic migraine as defined by 21-25 days of headache per month. Seventy-four percent of those patients met at least one of the three coprimary endpoints. In contrast, patients with a baseline headache frequency of 26-30 days per month had a 50% response rate, while those with 16-20 headache days had a 59% response rate.
A tougher standard – a 75% or greater reduction in either migraine days or headache days or at least a threefold increase in crystal-clear days – was achieved in one-third of patients. Fourteen percent met any two of the goals, and 6% met all three goals at the higher bar.
The median number of work days missed per month due to headache fell from 4 to 2 in the study population.
The most common adverse event was neck stiffness, reported by 16% of treated patients. In addition, 15% reported injection site pain lasting at least 24 hours, 9% experienced drooping of the upper eyelid, and 1.5% had difficulty swallowing.
Dr. Khalil noted that while a real-world, prospective, observational study such as this offers advantages over a controlled clinical trial, which often enrolls a select patient population, it has the disadvantage of not having an active comparator arm. That’s important because it’s well known that migraine studies tend to have a high placebo response rate.
In other Botox-related news at the IHC Congress, Rami Burstein, Ph.D., received the 2015 International Headache Society Cephalalgia Award given by the editorial board of the journal Cephalalgia for the most important paper in the field of headache medicine published in that journal in the past year. Dr. Burstein, professor of anesthesiology at Harvard Medical School, Boston, earned the honor, which comes with a 10,000 euro award, for his work in elucidating the mechanism of benefit for Botox in migraine (Cephalalgia 2014;34:853-69).
Dr. Khalil’s study was supported by Allergan. He reported having no financial conflicts.
VALENCIA, SPAIN – OnabotulinumtoxinA injections for the treatment of refractory chronic migraine halved the number of days of work per month missed because of migraine while quadrupling the number of crystal-clear, headache-free days in a large, prospective, real-world study.
“OnabotulinumtoxinA [Botox] is a valuable addition to current treatment options in patients with chronic migraine,” Dr. Modar Khalil concluded in summarizing his study findings at the International Headache Congress.
He presented a prospective study of 465 patients with chronic migraine treated with onabotulinumtoxinA in Hull, England.
The results paralleled the findings of the earlier PREEMPT (Phase-3 Research Evaluating Migraine Prophylaxis Therapy) study program which led to marketing approval of onabotulinumtoxinA for chronic migraine in the United Kingdom as well as the United States. However, the Hull patients were more representative of the sort of chronic migraine patients typically seen in tertiary headache clinics. Their chronic migraine was more severe than in the PREEMPT population: 97% of the Hull group had failed three or more preventive therapies at baseline, compared with only one-third of PREEMPT participants, noted Dr. Khalil of the Hull Royal Infirmary.
The Hull patients had a median 4-year history of chronic migraine. Prior to undergoing their first cycle of onabotulinumtoxinA therapy they averaged 27 total headache days and 15 migraine days per month.
The three coprimary study endpoints were at least a 50% reduction in headache days in the first month after onabotulinumtoxinA therapy, compared with the month before, at least a 50% reduction in migraine days, or a doubling of the number of crystal-clear days.

Fifty-nine percent of patients achieved at least one of those targets, 39% reached two, and 21% attained all three. The goal most frequently met was the 50% or greater reduction in monthly migraine days, achieved by 47% of patients. Forty-six percent met the target for an increase in crystal-clear days, while 27% of patients experienced at least a 50% reduction in headache days, Dr. Khalil said at the meeting sponsored by the International Headache Society and the American Headache Society.
The highest response rate was seen in patients with what he termed moderate-frequency chronic migraine as defined by 21-25 days of headache per month. Seventy-four percent of those patients met at least one of the three coprimary endpoints. In contrast, patients with a baseline headache frequency of 26-30 days per month had a 50% response rate, while those with 16-20 headache days had a 59% response rate.
A tougher standard – a 75% or greater reduction in either migraine days or headache days or at least a threefold increase in crystal-clear days – was achieved in one-third of patients. Fourteen percent met any two of the goals, and 6% met all three goals at the higher bar.
The median number of work days missed per month due to headache fell from 4 to 2 in the study population.
The most common adverse event was neck stiffness, reported by 16% of treated patients. In addition, 15% reported injection site pain lasting at least 24 hours, 9% experienced drooping of the upper eyelid, and 1.5% had difficulty swallowing.
Dr. Khalil noted that while a real-world, prospective, observational study such as this offers advantages over a controlled clinical trial, which often enrolls a select patient population, it has the disadvantage of not having an active comparator arm. That’s important because it’s well known that migraine studies tend to have a high placebo response rate.
In other Botox-related news at the IHC Congress, Rami Burstein, Ph.D., received the 2015 International Headache Society Cephalalgia Award given by the editorial board of the journal Cephalalgia for the most important paper in the field of headache medicine published in that journal in the past year. Dr. Burstein, professor of anesthesiology at Harvard Medical School, Boston, earned the honor, which comes with a 10,000 euro award, for his work in elucidating the mechanism of benefit for Botox in migraine (Cephalalgia 2014;34:853-69).
Dr. Khalil’s study was supported by Allergan. He reported having no financial conflicts.
VALENCIA, SPAIN – OnabotulinumtoxinA injections for the treatment of refractory chronic migraine halved the number of days of work per month missed because of migraine while quadrupling the number of crystal-clear, headache-free days in a large, prospective, real-world study.
“OnabotulinumtoxinA [Botox] is a valuable addition to current treatment options in patients with chronic migraine,” Dr. Modar Khalil concluded in summarizing his study findings at the International Headache Congress.
He presented a prospective study of 465 patients with chronic migraine treated with onabotulinumtoxinA in Hull, England.
The results paralleled the findings of the earlier PREEMPT (Phase-3 Research Evaluating Migraine Prophylaxis Therapy) study program which led to marketing approval of onabotulinumtoxinA for chronic migraine in the United Kingdom as well as the United States. However, the Hull patients were more representative of the sort of chronic migraine patients typically seen in tertiary headache clinics. Their chronic migraine was more severe than in the PREEMPT population: 97% of the Hull group had failed three or more preventive therapies at baseline, compared with only one-third of PREEMPT participants, noted Dr. Khalil of the Hull Royal Infirmary.
The Hull patients had a median 4-year history of chronic migraine. Prior to undergoing their first cycle of onabotulinumtoxinA therapy they averaged 27 total headache days and 15 migraine days per month.
The three coprimary study endpoints were at least a 50% reduction in headache days in the first month after onabotulinumtoxinA therapy, compared with the month before, at least a 50% reduction in migraine days, or a doubling of the number of crystal-clear days.

Fifty-nine percent of patients achieved at least one of those targets, 39% reached two, and 21% attained all three. The goal most frequently met was the 50% or greater reduction in monthly migraine days, achieved by 47% of patients. Forty-six percent met the target for an increase in crystal-clear days, while 27% of patients experienced at least a 50% reduction in headache days, Dr. Khalil said at the meeting sponsored by the International Headache Society and the American Headache Society.
The highest response rate was seen in patients with what he termed moderate-frequency chronic migraine as defined by 21-25 days of headache per month. Seventy-four percent of those patients met at least one of the three coprimary endpoints. In contrast, patients with a baseline headache frequency of 26-30 days per month had a 50% response rate, while those with 16-20 headache days had a 59% response rate.
A tougher standard – a 75% or greater reduction in either migraine days or headache days or at least a threefold increase in crystal-clear days – was achieved in one-third of patients. Fourteen percent met any two of the goals, and 6% met all three goals at the higher bar.
The median number of work days missed per month due to headache fell from 4 to 2 in the study population.
The most common adverse event was neck stiffness, reported by 16% of treated patients. In addition, 15% reported injection site pain lasting at least 24 hours, 9% experienced drooping of the upper eyelid, and 1.5% had difficulty swallowing.
Dr. Khalil noted that while a real-world, prospective, observational study such as this offers advantages over a controlled clinical trial, which often enrolls a select patient population, it has the disadvantage of not having an active comparator arm. That’s important because it’s well known that migraine studies tend to have a high placebo response rate.
In other Botox-related news at the IHC Congress, Rami Burstein, Ph.D., received the 2015 International Headache Society Cephalalgia Award given by the editorial board of the journal Cephalalgia for the most important paper in the field of headache medicine published in that journal in the past year. Dr. Burstein, professor of anesthesiology at Harvard Medical School, Boston, earned the honor, which comes with a 10,000 euro award, for his work in elucidating the mechanism of benefit for Botox in migraine (Cephalalgia 2014;34:853-69).
Dr. Khalil’s study was supported by Allergan. He reported having no financial conflicts.
AT IHC 2015
Key clinical point: OnabotulinumtoxinA (Botox) injections have a high treatment success rate in real-world chronic migraine patients who’ve failed to respond to at least three preventive therapies.
Major finding: Fifty-nine percent of refractory chronic migraine patients showed a meaningful response to onabotulinumtoxinA therapy based upon a significant reduction in either monthly total headache or migraine days or an increase in completely headache-free days.
Data source: This was a prospective open-label study involving 465 unselected, real-world, patients with refractory chronic migraine treated with Botox at a tertiary headache center.
Disclosures: The study was supported by Allergan. The presenter reported having no financial conflicts.
PAS: New approaches for cystic fibrosis–related diabetes in development
SAN DIEGO – Promising developments in the treatment and perhaps even prevention of cystic fibrosis–related diabetes are on the horizon – and they’re coming none too soon.
This is a field in need of a kick start, Dr. Antoinette Moran said at the annual meeting of the Pediatric Academic Societies.
She cited her recent review of 664 cystic fibrosis patients treated at the University of Minnesota during 2008-2012. Overall mortality in those with cystic fibrosis–related diabetes (CFRD) was unchanged from the high rates seen in a similar review covering 2003-2008, despite adoption of an institutional policy of aggressive screening for diabetes and early initiation of insulin therapy upon diagnosis of CFRD (Am. J. Respir. Crit. Care Med. 2015;191:194-200).
“Certainly screening and early institution of insulin are critical, but we seem to have come up against a wall. Honestly, at the University of Minnesota there is no way that we can be more aggressive than we already are with screening and with insulin. We have done as much as we can. So we need to think of something different to move this to the next stage,” said Dr. Moran, professor of pediatrics and chief of the division of pediatric endocrinology and diabetes at the University of Minnesota, Minneapolis.
She highlighted what she considers two of the most promising novel areas of CFRD research. One involves high-priority studies laying the groundwork for possible initiation of insulin therapy in cystic fibrosis (CF) patients even before they are diagnosed with CFRD, possibly starting in infancy.
The other major event is the anticipated Food and Drug Administration approval of a fixed-dose combination of ivacaftor and lumacaftor, a combined potentiator and corrector of the CF transmembrane conductance regulator (CFTR). Mutations in this chloride conduction channel are the most common cause of CF. Marketing approval for the new combination agent, which has breakthrough drug designation, is believed to be imminent.
“This is absolutely fascinating and a real game changer in the world of CF: the ability to fix CFTR, the abnormal CF chloride channel,” Dr. Moran said. “It’s expected that virtually every CF patient in the country is going to be on these drugs once the combination is approved.”
A large multicenter postmarketing study known as PROSPECT is in place and ready to start once the ivacaftor/lumacaftor combination receives approval. It will capture patients right before they start the agent and then follow them longitudinally. Dr. Moran is principal investigator for the GIFT (Gastrointestinal/Glucose and Insulin Functional Testing) substudy of PROSPECT. This 75-patient study will entail oral glucose tolerance testing with insulin, glucose, and C-peptide levels obtained at baseline and 1, 6, and 12 months.
The rationale for GIFT comes from the fact that CFTR mutations are present in the pancreatic beta cells of CF patients. An earlier five-patient pilot study conducted by Dr. Moran suggested ivacaftor might improve insulin secretion in patients with CF. This observation raises the question: “If we could start the drugs in very young children, might it prevent the development of diabetes? If the basic CFTR defect impacts beta cell function, it suggests this is going to be the way to get around that impasse – that treatment other than insulin will prevent or at least partially treat CFRD,” she said.
Another approach to preventing CFRD is being pursued by her colleague, Dr. Katie Larson Ode, a pediatric diabetologist at the University of Iowa, Iowa City. She showed that abnormal glucose tolerance was already present in 41% of 6- to 9-year-olds with CF, and among that subgroup, 42% developed early-onset CFRD at an average age of 11 years in girls and 12 years in boys, in contrast to the general Minnesota CF population, where the average age of onset of CFRD is age 23 years.
Moreover, the 59% of 6- to 9-year-olds with normal glucose tolerance had impaired insulin secretion. Their insulin secretion on oral glucose tolerance testing was half that of controls without CF.
This raises a question: Were these CF children born with these defects, or do the abnormalities evolve slowly during childhood? Dr. Ode is attempting to find out by performing annual oral glucose tolerance tests starting in infancy at the time of CF diagnosis. She is looking prospectively at the relationships between glucose and insulin levels and exocrine function, growth, inflammation, and pulmonary function, with the subjects’ unaffected siblings serving as controls.
“If those infants and toddlers with CF have abnormal insulin secretion and if it correlates with worse clinical parameters, we’re really going to have to consider whether we should be starting insulin right away in these babies. After all, we don’t wait to start vitamin D in CF infants until they develop rickets. We treat everything else preventively – we may need to think about doing that with insulin,” said Dr. Moran.
In her 664-patient review of the University of Minnesota experience with CF during 2008-2012, overall mortality in those with CFRD was 1.8 per 100 person-years ,compared with 0.5 per 100 person-years in CF patients without diabetes. In patients with mild CF genotypes, the risk of mortality was 20% in those with CFRD compared with 2% without diabetes. In patients with severe genotypes, overall mortality was 12% in those with CFRD, threefold higher than in those without diabetes.
Dr. Moran reported having financial relationships with Novo Nordisk and Vertex, which is developing ivacaftor/lumacaftor.
SAN DIEGO – Promising developments in the treatment and perhaps even prevention of cystic fibrosis–related diabetes are on the horizon – and they’re coming none too soon.
This is a field in need of a kick start, Dr. Antoinette Moran said at the annual meeting of the Pediatric Academic Societies.
She cited her recent review of 664 cystic fibrosis patients treated at the University of Minnesota during 2008-2012. Overall mortality in those with cystic fibrosis–related diabetes (CFRD) was unchanged from the high rates seen in a similar review covering 2003-2008, despite adoption of an institutional policy of aggressive screening for diabetes and early initiation of insulin therapy upon diagnosis of CFRD (Am. J. Respir. Crit. Care Med. 2015;191:194-200).
“Certainly screening and early institution of insulin are critical, but we seem to have come up against a wall. Honestly, at the University of Minnesota there is no way that we can be more aggressive than we already are with screening and with insulin. We have done as much as we can. So we need to think of something different to move this to the next stage,” said Dr. Moran, professor of pediatrics and chief of the division of pediatric endocrinology and diabetes at the University of Minnesota, Minneapolis.
She highlighted what she considers two of the most promising novel areas of CFRD research. One involves high-priority studies laying the groundwork for possible initiation of insulin therapy in cystic fibrosis (CF) patients even before they are diagnosed with CFRD, possibly starting in infancy.
The other major event is the anticipated Food and Drug Administration approval of a fixed-dose combination of ivacaftor and lumacaftor, a combined potentiator and corrector of the CF transmembrane conductance regulator (CFTR). Mutations in this chloride conduction channel are the most common cause of CF. Marketing approval for the new combination agent, which has breakthrough drug designation, is believed to be imminent.
“This is absolutely fascinating and a real game changer in the world of CF: the ability to fix CFTR, the abnormal CF chloride channel,” Dr. Moran said. “It’s expected that virtually every CF patient in the country is going to be on these drugs once the combination is approved.”
A large multicenter postmarketing study known as PROSPECT is in place and ready to start once the ivacaftor/lumacaftor combination receives approval. It will capture patients right before they start the agent and then follow them longitudinally. Dr. Moran is principal investigator for the GIFT (Gastrointestinal/Glucose and Insulin Functional Testing) substudy of PROSPECT. This 75-patient study will entail oral glucose tolerance testing with insulin, glucose, and C-peptide levels obtained at baseline and 1, 6, and 12 months.
The rationale for GIFT comes from the fact that CFTR mutations are present in the pancreatic beta cells of CF patients. An earlier five-patient pilot study conducted by Dr. Moran suggested ivacaftor might improve insulin secretion in patients with CF. This observation raises the question: “If we could start the drugs in very young children, might it prevent the development of diabetes? If the basic CFTR defect impacts beta cell function, it suggests this is going to be the way to get around that impasse – that treatment other than insulin will prevent or at least partially treat CFRD,” she said.
Another approach to preventing CFRD is being pursued by her colleague, Dr. Katie Larson Ode, a pediatric diabetologist at the University of Iowa, Iowa City. She showed that abnormal glucose tolerance was already present in 41% of 6- to 9-year-olds with CF, and among that subgroup, 42% developed early-onset CFRD at an average age of 11 years in girls and 12 years in boys, in contrast to the general Minnesota CF population, where the average age of onset of CFRD is age 23 years.
Moreover, the 59% of 6- to 9-year-olds with normal glucose tolerance had impaired insulin secretion. Their insulin secretion on oral glucose tolerance testing was half that of controls without CF.
This raises a question: Were these CF children born with these defects, or do the abnormalities evolve slowly during childhood? Dr. Ode is attempting to find out by performing annual oral glucose tolerance tests starting in infancy at the time of CF diagnosis. She is looking prospectively at the relationships between glucose and insulin levels and exocrine function, growth, inflammation, and pulmonary function, with the subjects’ unaffected siblings serving as controls.
“If those infants and toddlers with CF have abnormal insulin secretion and if it correlates with worse clinical parameters, we’re really going to have to consider whether we should be starting insulin right away in these babies. After all, we don’t wait to start vitamin D in CF infants until they develop rickets. We treat everything else preventively – we may need to think about doing that with insulin,” said Dr. Moran.
In her 664-patient review of the University of Minnesota experience with CF during 2008-2012, overall mortality in those with CFRD was 1.8 per 100 person-years ,compared with 0.5 per 100 person-years in CF patients without diabetes. In patients with mild CF genotypes, the risk of mortality was 20% in those with CFRD compared with 2% without diabetes. In patients with severe genotypes, overall mortality was 12% in those with CFRD, threefold higher than in those without diabetes.
Dr. Moran reported having financial relationships with Novo Nordisk and Vertex, which is developing ivacaftor/lumacaftor.
SAN DIEGO – Promising developments in the treatment and perhaps even prevention of cystic fibrosis–related diabetes are on the horizon – and they’re coming none too soon.
This is a field in need of a kick start, Dr. Antoinette Moran said at the annual meeting of the Pediatric Academic Societies.
She cited her recent review of 664 cystic fibrosis patients treated at the University of Minnesota during 2008-2012. Overall mortality in those with cystic fibrosis–related diabetes (CFRD) was unchanged from the high rates seen in a similar review covering 2003-2008, despite adoption of an institutional policy of aggressive screening for diabetes and early initiation of insulin therapy upon diagnosis of CFRD (Am. J. Respir. Crit. Care Med. 2015;191:194-200).
“Certainly screening and early institution of insulin are critical, but we seem to have come up against a wall. Honestly, at the University of Minnesota there is no way that we can be more aggressive than we already are with screening and with insulin. We have done as much as we can. So we need to think of something different to move this to the next stage,” said Dr. Moran, professor of pediatrics and chief of the division of pediatric endocrinology and diabetes at the University of Minnesota, Minneapolis.
She highlighted what she considers two of the most promising novel areas of CFRD research. One involves high-priority studies laying the groundwork for possible initiation of insulin therapy in cystic fibrosis (CF) patients even before they are diagnosed with CFRD, possibly starting in infancy.
The other major event is the anticipated Food and Drug Administration approval of a fixed-dose combination of ivacaftor and lumacaftor, a combined potentiator and corrector of the CF transmembrane conductance regulator (CFTR). Mutations in this chloride conduction channel are the most common cause of CF. Marketing approval for the new combination agent, which has breakthrough drug designation, is believed to be imminent.
“This is absolutely fascinating and a real game changer in the world of CF: the ability to fix CFTR, the abnormal CF chloride channel,” Dr. Moran said. “It’s expected that virtually every CF patient in the country is going to be on these drugs once the combination is approved.”
A large multicenter postmarketing study known as PROSPECT is in place and ready to start once the ivacaftor/lumacaftor combination receives approval. It will capture patients right before they start the agent and then follow them longitudinally. Dr. Moran is principal investigator for the GIFT (Gastrointestinal/Glucose and Insulin Functional Testing) substudy of PROSPECT. This 75-patient study will entail oral glucose tolerance testing with insulin, glucose, and C-peptide levels obtained at baseline and 1, 6, and 12 months.
The rationale for GIFT comes from the fact that CFTR mutations are present in the pancreatic beta cells of CF patients. An earlier five-patient pilot study conducted by Dr. Moran suggested ivacaftor might improve insulin secretion in patients with CF. This observation raises the question: “If we could start the drugs in very young children, might it prevent the development of diabetes? If the basic CFTR defect impacts beta cell function, it suggests this is going to be the way to get around that impasse – that treatment other than insulin will prevent or at least partially treat CFRD,” she said.
Another approach to preventing CFRD is being pursued by her colleague, Dr. Katie Larson Ode, a pediatric diabetologist at the University of Iowa, Iowa City. She showed that abnormal glucose tolerance was already present in 41% of 6- to 9-year-olds with CF, and among that subgroup, 42% developed early-onset CFRD at an average age of 11 years in girls and 12 years in boys, in contrast to the general Minnesota CF population, where the average age of onset of CFRD is age 23 years.
Moreover, the 59% of 6- to 9-year-olds with normal glucose tolerance had impaired insulin secretion. Their insulin secretion on oral glucose tolerance testing was half that of controls without CF.
This raises a question: Were these CF children born with these defects, or do the abnormalities evolve slowly during childhood? Dr. Ode is attempting to find out by performing annual oral glucose tolerance tests starting in infancy at the time of CF diagnosis. She is looking prospectively at the relationships between glucose and insulin levels and exocrine function, growth, inflammation, and pulmonary function, with the subjects’ unaffected siblings serving as controls.
“If those infants and toddlers with CF have abnormal insulin secretion and if it correlates with worse clinical parameters, we’re really going to have to consider whether we should be starting insulin right away in these babies. After all, we don’t wait to start vitamin D in CF infants until they develop rickets. We treat everything else preventively – we may need to think about doing that with insulin,” said Dr. Moran.
In her 664-patient review of the University of Minnesota experience with CF during 2008-2012, overall mortality in those with CFRD was 1.8 per 100 person-years ,compared with 0.5 per 100 person-years in CF patients without diabetes. In patients with mild CF genotypes, the risk of mortality was 20% in those with CFRD compared with 2% without diabetes. In patients with severe genotypes, overall mortality was 12% in those with CFRD, threefold higher than in those without diabetes.
Dr. Moran reported having financial relationships with Novo Nordisk and Vertex, which is developing ivacaftor/lumacaftor.
EXPERT ANALYSIS FROM THE PAS ANNUAL MEETING
Cystic fibrosis–related diabetes requires different approach
SAN DIEGO – Cystic fibrosis–related diabetes is a unique disease, and it requires a different mindset on the part of the treating physician.
“The risk of cardiovascular death drives a lot of the recommendations for management of our patients with type 1 and type 2 diabetes, but this doesn’t apply in cystic fibrosis. Patients with cystic fibrosis–related diabetes do not appear to get macrovascular complications. These patients have other, more important concerns – namely, survival. They die from their CF lung disease. Diabetes is important, but we have to remember that in CF, lung function and nutrition come first. It’s our job to work around that,” Dr. Antoinette Moran asserted at the annual meeting of the Pediatric Academic Societies.
Diabetes is the most common comorbidity associated with CF. And it spells big trouble. It’s associated with pancreatic insufficiency, liver dysfunction, requirement for corticosteroids, and prognostically with undernutrition, worse pulmonary function, and early death, noted Dr. Moran, professor of pediatrics and chief of the division of pediatric endocrinology and diabetes at the University of Minnesota, Minneapolis.
The prevalence of cystic fibrosis–related diabetes (CFRD) is age related. It’s rare in children, but the prevalence climbs to about 15% in adolescents, 40% in 20- to 39-year-olds, and 55% after age 40.
“In fact, more than 80% of CF patients with the most severe mutations have diabetes by the time they’re 40,” according to Dr. Moran, who was lead author of CFRD management guidelines released last year by the International Society for Pediatric and Adolescent Diabetes (Pediatr. Diabetes 2014 Sep;15 Suppl 20:65-76).
CFRD is not an autoimmune disease. Ketones are rare. Glycosylated hemoglobin levels are spuriously low. And the definitive treatment for CFRD is insulin.
“Remember, you’re not just treating hyperglycemia, you’re treating insulin deficiency. Insulin deficiency is really the hallmark of this disease. It is progressive and eventually severe, but not complete – unlike in type 1 diabetes,” she observed. “Treatment of patients in their well state is similar to treating type 1 diabetes in the honeymoon phase. However, during acute illness patients become extremely insulin resistant. It’s a black hole that you can pour insulin into, and sometimes you can’t get them to budge. Then a couple of months later they’re insulin sensitive again.”
Multiple studies have demonstrated that diabetes has a negative impact upon survival in patients with CF. Both hyperglycemia and insulin insufficiency have negative impacts upon the CF lung disease.
Insulin is a potent anabolic hormone that’s necessary for maintenance of body weight and lean body mass, and insulin insufficiency leads to a catabolic state which accelerates pulmonary decline in CF. Studies show that nutritional status and pulmonary function start to decline in CF patients several years before they’re diagnosed with diabetes. Thus, by the time CFRD is diagnosed, patients have already experienced several years of insulin insufficiency, with adverse consequences.
Moreover, when blood glucose levels exceed 144 mg/dL, glucose appears in the airways of CF patients. That’s not good. It probably promotes pulmonary infection. Anecdotal evidence suggests hyperglycemia makes sputum thicker and more difficult to clear as well as boosting bacterial growth. And continuous glucose monitoring studies conducted in patients with CFRD indicate they spend roughly half of each day with a blood glucose in excess of 144 mg/dL.
Aggressive screening and early initiation of insulin therapy help reverse chronic weight loss and reduce mortality in patients with CFRD. The various guidelines recommend annual screening for diabetes in CF patients starting by age 10.
“I personally believe it should begin much earlier than that,” Dr. Moran said, citing a study led by her Minnesota colleague Dr. Katie L. Ode that showed that abnormal glucose tolerance was already present in 41% of children with CF at ages 6-9, and that those children had a high rate of early-onset CFRD (Pediatr. Diabetes 2010 Nov;11:487-92).
The oral glucose tolerance test, performed when the patient is clinically stable, is the screening tool of choice for CFRD.
“It’s not that it’s such a great test – we all know it has problems – but the other tests perform poorly in CF. And a diagnosis based upon an oral glucose tolerance test correlates with prognosis and future outcomes, so you get meaningful data when you do it,” she explained.
Evidence-based guidelines for CFRD put forth jointly by the American Diabetes Association, Cystic Fibrosis Foundation, and Lawson Wilkins Pediatric Endocrinology Society (Diabetes Care 2010;33:2697-2708) emphasize that, unlike in patients without CF, the diagnosis of CFRD can be made while a patient is hospitalized with an acute illness. The criterion is fasting or postprandial hyperglycemia persisting for more than 48 hours after hospitalization.
“Why are we calling this diabetes? These patients have repeated bouts of acute illness. The CF patient you’re seeing today in the hospital may very well be back in 5 months, and again 2 months after that. It’s a frequent event in these patients, and when their diabetes persists for longer than 48 hours it tends to persist for weeks before their need for insulin goes away until the next time they get sick. But most of these patients spend a substantial amount of time each year hyperglycemic. And most importantly, if you use as your date of diagnosis diabetes that’s present at the time of an acute illness, it correlates with microvascular complications and with mortality. So it establishes a meaningful start point for future risk,” Dr. Moran said.
She reported financial relationships with Novo Nordisk and Vertex.
SAN DIEGO – Cystic fibrosis–related diabetes is a unique disease, and it requires a different mindset on the part of the treating physician.
“The risk of cardiovascular death drives a lot of the recommendations for management of our patients with type 1 and type 2 diabetes, but this doesn’t apply in cystic fibrosis. Patients with cystic fibrosis–related diabetes do not appear to get macrovascular complications. These patients have other, more important concerns – namely, survival. They die from their CF lung disease. Diabetes is important, but we have to remember that in CF, lung function and nutrition come first. It’s our job to work around that,” Dr. Antoinette Moran asserted at the annual meeting of the Pediatric Academic Societies.
Diabetes is the most common comorbidity associated with CF. And it spells big trouble. It’s associated with pancreatic insufficiency, liver dysfunction, requirement for corticosteroids, and prognostically with undernutrition, worse pulmonary function, and early death, noted Dr. Moran, professor of pediatrics and chief of the division of pediatric endocrinology and diabetes at the University of Minnesota, Minneapolis.
The prevalence of cystic fibrosis–related diabetes (CFRD) is age related. It’s rare in children, but the prevalence climbs to about 15% in adolescents, 40% in 20- to 39-year-olds, and 55% after age 40.
“In fact, more than 80% of CF patients with the most severe mutations have diabetes by the time they’re 40,” according to Dr. Moran, who was lead author of CFRD management guidelines released last year by the International Society for Pediatric and Adolescent Diabetes (Pediatr. Diabetes 2014 Sep;15 Suppl 20:65-76).
CFRD is not an autoimmune disease. Ketones are rare. Glycosylated hemoglobin levels are spuriously low. And the definitive treatment for CFRD is insulin.
“Remember, you’re not just treating hyperglycemia, you’re treating insulin deficiency. Insulin deficiency is really the hallmark of this disease. It is progressive and eventually severe, but not complete – unlike in type 1 diabetes,” she observed. “Treatment of patients in their well state is similar to treating type 1 diabetes in the honeymoon phase. However, during acute illness patients become extremely insulin resistant. It’s a black hole that you can pour insulin into, and sometimes you can’t get them to budge. Then a couple of months later they’re insulin sensitive again.”
Multiple studies have demonstrated that diabetes has a negative impact upon survival in patients with CF. Both hyperglycemia and insulin insufficiency have negative impacts upon the CF lung disease.
Insulin is a potent anabolic hormone that’s necessary for maintenance of body weight and lean body mass, and insulin insufficiency leads to a catabolic state which accelerates pulmonary decline in CF. Studies show that nutritional status and pulmonary function start to decline in CF patients several years before they’re diagnosed with diabetes. Thus, by the time CFRD is diagnosed, patients have already experienced several years of insulin insufficiency, with adverse consequences.
Moreover, when blood glucose levels exceed 144 mg/dL, glucose appears in the airways of CF patients. That’s not good. It probably promotes pulmonary infection. Anecdotal evidence suggests hyperglycemia makes sputum thicker and more difficult to clear as well as boosting bacterial growth. And continuous glucose monitoring studies conducted in patients with CFRD indicate they spend roughly half of each day with a blood glucose in excess of 144 mg/dL.
Aggressive screening and early initiation of insulin therapy help reverse chronic weight loss and reduce mortality in patients with CFRD. The various guidelines recommend annual screening for diabetes in CF patients starting by age 10.
“I personally believe it should begin much earlier than that,” Dr. Moran said, citing a study led by her Minnesota colleague Dr. Katie L. Ode that showed that abnormal glucose tolerance was already present in 41% of children with CF at ages 6-9, and that those children had a high rate of early-onset CFRD (Pediatr. Diabetes 2010 Nov;11:487-92).
The oral glucose tolerance test, performed when the patient is clinically stable, is the screening tool of choice for CFRD.
“It’s not that it’s such a great test – we all know it has problems – but the other tests perform poorly in CF. And a diagnosis based upon an oral glucose tolerance test correlates with prognosis and future outcomes, so you get meaningful data when you do it,” she explained.
Evidence-based guidelines for CFRD put forth jointly by the American Diabetes Association, Cystic Fibrosis Foundation, and Lawson Wilkins Pediatric Endocrinology Society (Diabetes Care 2010;33:2697-2708) emphasize that, unlike in patients without CF, the diagnosis of CFRD can be made while a patient is hospitalized with an acute illness. The criterion is fasting or postprandial hyperglycemia persisting for more than 48 hours after hospitalization.
“Why are we calling this diabetes? These patients have repeated bouts of acute illness. The CF patient you’re seeing today in the hospital may very well be back in 5 months, and again 2 months after that. It’s a frequent event in these patients, and when their diabetes persists for longer than 48 hours it tends to persist for weeks before their need for insulin goes away until the next time they get sick. But most of these patients spend a substantial amount of time each year hyperglycemic. And most importantly, if you use as your date of diagnosis diabetes that’s present at the time of an acute illness, it correlates with microvascular complications and with mortality. So it establishes a meaningful start point for future risk,” Dr. Moran said.
She reported financial relationships with Novo Nordisk and Vertex.
SAN DIEGO – Cystic fibrosis–related diabetes is a unique disease, and it requires a different mindset on the part of the treating physician.
“The risk of cardiovascular death drives a lot of the recommendations for management of our patients with type 1 and type 2 diabetes, but this doesn’t apply in cystic fibrosis. Patients with cystic fibrosis–related diabetes do not appear to get macrovascular complications. These patients have other, more important concerns – namely, survival. They die from their CF lung disease. Diabetes is important, but we have to remember that in CF, lung function and nutrition come first. It’s our job to work around that,” Dr. Antoinette Moran asserted at the annual meeting of the Pediatric Academic Societies.
Diabetes is the most common comorbidity associated with CF. And it spells big trouble. It’s associated with pancreatic insufficiency, liver dysfunction, requirement for corticosteroids, and prognostically with undernutrition, worse pulmonary function, and early death, noted Dr. Moran, professor of pediatrics and chief of the division of pediatric endocrinology and diabetes at the University of Minnesota, Minneapolis.
The prevalence of cystic fibrosis–related diabetes (CFRD) is age related. It’s rare in children, but the prevalence climbs to about 15% in adolescents, 40% in 20- to 39-year-olds, and 55% after age 40.
“In fact, more than 80% of CF patients with the most severe mutations have diabetes by the time they’re 40,” according to Dr. Moran, who was lead author of CFRD management guidelines released last year by the International Society for Pediatric and Adolescent Diabetes (Pediatr. Diabetes 2014 Sep;15 Suppl 20:65-76).
CFRD is not an autoimmune disease. Ketones are rare. Glycosylated hemoglobin levels are spuriously low. And the definitive treatment for CFRD is insulin.
“Remember, you’re not just treating hyperglycemia, you’re treating insulin deficiency. Insulin deficiency is really the hallmark of this disease. It is progressive and eventually severe, but not complete – unlike in type 1 diabetes,” she observed. “Treatment of patients in their well state is similar to treating type 1 diabetes in the honeymoon phase. However, during acute illness patients become extremely insulin resistant. It’s a black hole that you can pour insulin into, and sometimes you can’t get them to budge. Then a couple of months later they’re insulin sensitive again.”
Multiple studies have demonstrated that diabetes has a negative impact upon survival in patients with CF. Both hyperglycemia and insulin insufficiency have negative impacts upon the CF lung disease.
Insulin is a potent anabolic hormone that’s necessary for maintenance of body weight and lean body mass, and insulin insufficiency leads to a catabolic state which accelerates pulmonary decline in CF. Studies show that nutritional status and pulmonary function start to decline in CF patients several years before they’re diagnosed with diabetes. Thus, by the time CFRD is diagnosed, patients have already experienced several years of insulin insufficiency, with adverse consequences.
Moreover, when blood glucose levels exceed 144 mg/dL, glucose appears in the airways of CF patients. That’s not good. It probably promotes pulmonary infection. Anecdotal evidence suggests hyperglycemia makes sputum thicker and more difficult to clear as well as boosting bacterial growth. And continuous glucose monitoring studies conducted in patients with CFRD indicate they spend roughly half of each day with a blood glucose in excess of 144 mg/dL.
Aggressive screening and early initiation of insulin therapy help reverse chronic weight loss and reduce mortality in patients with CFRD. The various guidelines recommend annual screening for diabetes in CF patients starting by age 10.
“I personally believe it should begin much earlier than that,” Dr. Moran said, citing a study led by her Minnesota colleague Dr. Katie L. Ode that showed that abnormal glucose tolerance was already present in 41% of children with CF at ages 6-9, and that those children had a high rate of early-onset CFRD (Pediatr. Diabetes 2010 Nov;11:487-92).
The oral glucose tolerance test, performed when the patient is clinically stable, is the screening tool of choice for CFRD.
“It’s not that it’s such a great test – we all know it has problems – but the other tests perform poorly in CF. And a diagnosis based upon an oral glucose tolerance test correlates with prognosis and future outcomes, so you get meaningful data when you do it,” she explained.
Evidence-based guidelines for CFRD put forth jointly by the American Diabetes Association, Cystic Fibrosis Foundation, and Lawson Wilkins Pediatric Endocrinology Society (Diabetes Care 2010;33:2697-2708) emphasize that, unlike in patients without CF, the diagnosis of CFRD can be made while a patient is hospitalized with an acute illness. The criterion is fasting or postprandial hyperglycemia persisting for more than 48 hours after hospitalization.
“Why are we calling this diabetes? These patients have repeated bouts of acute illness. The CF patient you’re seeing today in the hospital may very well be back in 5 months, and again 2 months after that. It’s a frequent event in these patients, and when their diabetes persists for longer than 48 hours it tends to persist for weeks before their need for insulin goes away until the next time they get sick. But most of these patients spend a substantial amount of time each year hyperglycemic. And most importantly, if you use as your date of diagnosis diabetes that’s present at the time of an acute illness, it correlates with microvascular complications and with mortality. So it establishes a meaningful start point for future risk,” Dr. Moran said.
She reported financial relationships with Novo Nordisk and Vertex.
EXPERT ANALYSIS FROM THE PAS ANNUAL MEETING
How to code for cystic fibrosis–related diabetes
SAN DIEGO – A question that arises all the time for physicians who find themselves providing care for a patient with cystic fibrosis–related diabetes is, How do I code for it?
No specific code exists for cystic fibrosis–related diabetes (CFRD), even though it is a unique illness and the most common comorbid condition among patients with CF.
“People use a lot of different codes. I use the type 1 diabetes code, and my personal opinion is that there are good reasons for doing so,” Dr. Antoinette Moran said at the annual meeting of the Pediatric Academic Societies.
“For one thing, the patients perform similar tasks as those with type 1 diabetes. They’re taking the same amount of your time and your diabetes educator’s time. But here’s the most important reason: It seems like all around the country, insurance companies are getting more and more restrictive for people who don’t carry a diagnosis of type 1 diabetes. These CFRD patients need to test their blood sugars at least 4 times a day, sometimes 10 times a day. These are patients who do really, really well on insulin pump therapy. We don’t want to be the ones limiting their options just based on what is admittedly an arbitrary code,” explained Dr. Moran, professor of pediatrics and chief of the division of pediatric endocrinology and diabetes at the University of Minnesota – Minneapolis.
She reported financial relationships with Novo Nordisk and Vertex.
SAN DIEGO – A question that arises all the time for physicians who find themselves providing care for a patient with cystic fibrosis–related diabetes is, How do I code for it?
No specific code exists for cystic fibrosis–related diabetes (CFRD), even though it is a unique illness and the most common comorbid condition among patients with CF.
“People use a lot of different codes. I use the type 1 diabetes code, and my personal opinion is that there are good reasons for doing so,” Dr. Antoinette Moran said at the annual meeting of the Pediatric Academic Societies.
“For one thing, the patients perform similar tasks as those with type 1 diabetes. They’re taking the same amount of your time and your diabetes educator’s time. But here’s the most important reason: It seems like all around the country, insurance companies are getting more and more restrictive for people who don’t carry a diagnosis of type 1 diabetes. These CFRD patients need to test their blood sugars at least 4 times a day, sometimes 10 times a day. These are patients who do really, really well on insulin pump therapy. We don’t want to be the ones limiting their options just based on what is admittedly an arbitrary code,” explained Dr. Moran, professor of pediatrics and chief of the division of pediatric endocrinology and diabetes at the University of Minnesota – Minneapolis.
She reported financial relationships with Novo Nordisk and Vertex.
SAN DIEGO – A question that arises all the time for physicians who find themselves providing care for a patient with cystic fibrosis–related diabetes is, How do I code for it?
No specific code exists for cystic fibrosis–related diabetes (CFRD), even though it is a unique illness and the most common comorbid condition among patients with CF.
“People use a lot of different codes. I use the type 1 diabetes code, and my personal opinion is that there are good reasons for doing so,” Dr. Antoinette Moran said at the annual meeting of the Pediatric Academic Societies.
“For one thing, the patients perform similar tasks as those with type 1 diabetes. They’re taking the same amount of your time and your diabetes educator’s time. But here’s the most important reason: It seems like all around the country, insurance companies are getting more and more restrictive for people who don’t carry a diagnosis of type 1 diabetes. These CFRD patients need to test their blood sugars at least 4 times a day, sometimes 10 times a day. These are patients who do really, really well on insulin pump therapy. We don’t want to be the ones limiting their options just based on what is admittedly an arbitrary code,” explained Dr. Moran, professor of pediatrics and chief of the division of pediatric endocrinology and diabetes at the University of Minnesota – Minneapolis.
She reported financial relationships with Novo Nordisk and Vertex.
EXPERT OPINION FROM THE PAS ANNUAL MEETING
PAS: Getting a grip on glucokinase MODY
SAN DIEGO – The two main things to understand about maturity-onset diabetes of the young due to mutations in the glucokinase gene are that this form of diabetes is far more common than you probably realize, and – unique in the world of diabetes – no treatment is warranted, according to Dr. Siri Atma W. Greeley, a pediatric endocrinologist at the University of Chicago.
He cited what he called “a great study” by investigators at the University of Exeter (England), who showed that very, very few patients with glucokinase maturity-onset diabetes of the young (GCK MODY) develop micro- or macrovascular complications. Despite a median 48.6 years of follow-up of 99 patients with GCK MODY, also known as MODY 2, the prevalence and severity of retinopathy, nephropathy, peripheral neuropathy, cardiovascular disease, and peripheral vascular disease weren’t significantly different from controls (JAMA 2014;311:279-86).
“Just remember – these patients do not get diabetes-related complications. And we think that if they do, it might be because they later go on to develop type 2 diabetes; for instance, if they become obese and have a genetic predisposition in addition to having their GCK mutation,” Dr. Greeley said at the annual meeting of the Pediatric Academic Societies.
There’s another reason to spare patients with GCK MODY from lifelong diabetes therapy, besides the facts that treatment is costly, has side effects, and does nothing to minimize their risk of vascular complications because their risk isn’t increased: Namely, pharmacotherapy doesn’t alter glycemic control in these patients. The Exeter group has shown that HbA1c in patients with GCK MODY isn’t affected by treatment with insulin or oral hypoglycemic agents (Diabetologia 2014;57:54-6). That’s because the GCK gene acts as a blood glucose sensor for the pancreas, and a mutation causes the gene to reset blood glucose at a slightly higher than normal level. Treatment is ineffective because the body will relentlessly strive to maintain blood glucose at the reset level, he explained.
Typically, however, patients with GCK MODY are treated – inappropriately – with insulin or oral hypoglycemic agents because they have been misdiagnosed as having type 2 or less commonly type 1 diabetes. Indeed, roughly half of patients with GCK MODY have been on drug therapy for more than 3 months at the time they enroll in the U.S. Monogenic Diabetes Registry maintained by Dr. Greeley and coworkers at the University of Chicago. Some are actually on an insulin pump.
“There’s a lot of unnecessary treatment going on with these patients,” he said.
The only time treatment of GCK MODY is appropriate, the endocrinologist added, is in some affected women during pregnancy in order to control fetal growth.
How common is GCK MODY in pregnancy? The Exeter group has estimated the population prevalence at 1.2 cases per 1,000 pregnancies, or 0.1%, based on genetic testing of participants in the Atlantic Diabetes in Pregnancy Study (Diabetes Care 2014;37:1230-6). However, among more than 61,000 unrelated individuals who have participated in various large-scale gene sequencing projects under the umbrella of the Exome Aggregation Consortium, GCK mutation rates were higher, ranging from 2.71 cases per 1,000 non-Finnish Europeans to 4.93 per 1,000 among Latinos and 5.74 per 1,000 Africans.
More than 90% of all cases of MODY with a known cause are due to mutations in one of four genes: GCK, hepatocyte nuclear factor homeobox 1-alpha (HNF1A), HNF1 homeobox 1B, or HNF4 alpha. Investigators in the U.S. multicenter SEARCH for Diabetes in Youth Study performed gene-sequencing studies in 586 study participants and found the prevalence of mutations in GCK, HNF1A, or HNF4 alpha was 1.2% among the pediatric diabetes population (J. Clin. Endocrinol. Metab. 2013;98:4055-62). That finding has clear implications for clinical practice: “All of you, if you’re seeing any number of diabetes patients, should have at least a handful of cases of MODY,” Dr. Greeley observed.
The hallmark of GCK MODY is mild fasting hyperglycemia present from birth, with a stable HbA1c below 7.8% throughout life. But Dr. Greeley emphasized that there is no set of clinical features that make the diagnosis of GCK MODY or other forms of MODY with 100% certainty. The only way to firmly establish the diagnosis is through genetic testing, which in Dr. Greeley’s view is vastly underutilized. He was coinvestigator in a study that demonstrated that genetic testing for causative mutations in the big-four genes is cost effective in selected circumstances. And at least one major insurer, he added, is starting to come around to that point of view.
Dr. Greeley’s research is funded by the National Institutes of Health, the American Diabetes Association, and the Kovler Family Foundation. He reported having no financial conflicts.
SAN DIEGO – The two main things to understand about maturity-onset diabetes of the young due to mutations in the glucokinase gene are that this form of diabetes is far more common than you probably realize, and – unique in the world of diabetes – no treatment is warranted, according to Dr. Siri Atma W. Greeley, a pediatric endocrinologist at the University of Chicago.
He cited what he called “a great study” by investigators at the University of Exeter (England), who showed that very, very few patients with glucokinase maturity-onset diabetes of the young (GCK MODY) develop micro- or macrovascular complications. Despite a median 48.6 years of follow-up of 99 patients with GCK MODY, also known as MODY 2, the prevalence and severity of retinopathy, nephropathy, peripheral neuropathy, cardiovascular disease, and peripheral vascular disease weren’t significantly different from controls (JAMA 2014;311:279-86).
“Just remember – these patients do not get diabetes-related complications. And we think that if they do, it might be because they later go on to develop type 2 diabetes; for instance, if they become obese and have a genetic predisposition in addition to having their GCK mutation,” Dr. Greeley said at the annual meeting of the Pediatric Academic Societies.
There’s another reason to spare patients with GCK MODY from lifelong diabetes therapy, besides the facts that treatment is costly, has side effects, and does nothing to minimize their risk of vascular complications because their risk isn’t increased: Namely, pharmacotherapy doesn’t alter glycemic control in these patients. The Exeter group has shown that HbA1c in patients with GCK MODY isn’t affected by treatment with insulin or oral hypoglycemic agents (Diabetologia 2014;57:54-6). That’s because the GCK gene acts as a blood glucose sensor for the pancreas, and a mutation causes the gene to reset blood glucose at a slightly higher than normal level. Treatment is ineffective because the body will relentlessly strive to maintain blood glucose at the reset level, he explained.
Typically, however, patients with GCK MODY are treated – inappropriately – with insulin or oral hypoglycemic agents because they have been misdiagnosed as having type 2 or less commonly type 1 diabetes. Indeed, roughly half of patients with GCK MODY have been on drug therapy for more than 3 months at the time they enroll in the U.S. Monogenic Diabetes Registry maintained by Dr. Greeley and coworkers at the University of Chicago. Some are actually on an insulin pump.
“There’s a lot of unnecessary treatment going on with these patients,” he said.
The only time treatment of GCK MODY is appropriate, the endocrinologist added, is in some affected women during pregnancy in order to control fetal growth.
How common is GCK MODY in pregnancy? The Exeter group has estimated the population prevalence at 1.2 cases per 1,000 pregnancies, or 0.1%, based on genetic testing of participants in the Atlantic Diabetes in Pregnancy Study (Diabetes Care 2014;37:1230-6). However, among more than 61,000 unrelated individuals who have participated in various large-scale gene sequencing projects under the umbrella of the Exome Aggregation Consortium, GCK mutation rates were higher, ranging from 2.71 cases per 1,000 non-Finnish Europeans to 4.93 per 1,000 among Latinos and 5.74 per 1,000 Africans.
More than 90% of all cases of MODY with a known cause are due to mutations in one of four genes: GCK, hepatocyte nuclear factor homeobox 1-alpha (HNF1A), HNF1 homeobox 1B, or HNF4 alpha. Investigators in the U.S. multicenter SEARCH for Diabetes in Youth Study performed gene-sequencing studies in 586 study participants and found the prevalence of mutations in GCK, HNF1A, or HNF4 alpha was 1.2% among the pediatric diabetes population (J. Clin. Endocrinol. Metab. 2013;98:4055-62). That finding has clear implications for clinical practice: “All of you, if you’re seeing any number of diabetes patients, should have at least a handful of cases of MODY,” Dr. Greeley observed.
The hallmark of GCK MODY is mild fasting hyperglycemia present from birth, with a stable HbA1c below 7.8% throughout life. But Dr. Greeley emphasized that there is no set of clinical features that make the diagnosis of GCK MODY or other forms of MODY with 100% certainty. The only way to firmly establish the diagnosis is through genetic testing, which in Dr. Greeley’s view is vastly underutilized. He was coinvestigator in a study that demonstrated that genetic testing for causative mutations in the big-four genes is cost effective in selected circumstances. And at least one major insurer, he added, is starting to come around to that point of view.
Dr. Greeley’s research is funded by the National Institutes of Health, the American Diabetes Association, and the Kovler Family Foundation. He reported having no financial conflicts.
SAN DIEGO – The two main things to understand about maturity-onset diabetes of the young due to mutations in the glucokinase gene are that this form of diabetes is far more common than you probably realize, and – unique in the world of diabetes – no treatment is warranted, according to Dr. Siri Atma W. Greeley, a pediatric endocrinologist at the University of Chicago.
He cited what he called “a great study” by investigators at the University of Exeter (England), who showed that very, very few patients with glucokinase maturity-onset diabetes of the young (GCK MODY) develop micro- or macrovascular complications. Despite a median 48.6 years of follow-up of 99 patients with GCK MODY, also known as MODY 2, the prevalence and severity of retinopathy, nephropathy, peripheral neuropathy, cardiovascular disease, and peripheral vascular disease weren’t significantly different from controls (JAMA 2014;311:279-86).
“Just remember – these patients do not get diabetes-related complications. And we think that if they do, it might be because they later go on to develop type 2 diabetes; for instance, if they become obese and have a genetic predisposition in addition to having their GCK mutation,” Dr. Greeley said at the annual meeting of the Pediatric Academic Societies.
There’s another reason to spare patients with GCK MODY from lifelong diabetes therapy, besides the facts that treatment is costly, has side effects, and does nothing to minimize their risk of vascular complications because their risk isn’t increased: Namely, pharmacotherapy doesn’t alter glycemic control in these patients. The Exeter group has shown that HbA1c in patients with GCK MODY isn’t affected by treatment with insulin or oral hypoglycemic agents (Diabetologia 2014;57:54-6). That’s because the GCK gene acts as a blood glucose sensor for the pancreas, and a mutation causes the gene to reset blood glucose at a slightly higher than normal level. Treatment is ineffective because the body will relentlessly strive to maintain blood glucose at the reset level, he explained.
Typically, however, patients with GCK MODY are treated – inappropriately – with insulin or oral hypoglycemic agents because they have been misdiagnosed as having type 2 or less commonly type 1 diabetes. Indeed, roughly half of patients with GCK MODY have been on drug therapy for more than 3 months at the time they enroll in the U.S. Monogenic Diabetes Registry maintained by Dr. Greeley and coworkers at the University of Chicago. Some are actually on an insulin pump.
“There’s a lot of unnecessary treatment going on with these patients,” he said.
The only time treatment of GCK MODY is appropriate, the endocrinologist added, is in some affected women during pregnancy in order to control fetal growth.
How common is GCK MODY in pregnancy? The Exeter group has estimated the population prevalence at 1.2 cases per 1,000 pregnancies, or 0.1%, based on genetic testing of participants in the Atlantic Diabetes in Pregnancy Study (Diabetes Care 2014;37:1230-6). However, among more than 61,000 unrelated individuals who have participated in various large-scale gene sequencing projects under the umbrella of the Exome Aggregation Consortium, GCK mutation rates were higher, ranging from 2.71 cases per 1,000 non-Finnish Europeans to 4.93 per 1,000 among Latinos and 5.74 per 1,000 Africans.
More than 90% of all cases of MODY with a known cause are due to mutations in one of four genes: GCK, hepatocyte nuclear factor homeobox 1-alpha (HNF1A), HNF1 homeobox 1B, or HNF4 alpha. Investigators in the U.S. multicenter SEARCH for Diabetes in Youth Study performed gene-sequencing studies in 586 study participants and found the prevalence of mutations in GCK, HNF1A, or HNF4 alpha was 1.2% among the pediatric diabetes population (J. Clin. Endocrinol. Metab. 2013;98:4055-62). That finding has clear implications for clinical practice: “All of you, if you’re seeing any number of diabetes patients, should have at least a handful of cases of MODY,” Dr. Greeley observed.
The hallmark of GCK MODY is mild fasting hyperglycemia present from birth, with a stable HbA1c below 7.8% throughout life. But Dr. Greeley emphasized that there is no set of clinical features that make the diagnosis of GCK MODY or other forms of MODY with 100% certainty. The only way to firmly establish the diagnosis is through genetic testing, which in Dr. Greeley’s view is vastly underutilized. He was coinvestigator in a study that demonstrated that genetic testing for causative mutations in the big-four genes is cost effective in selected circumstances. And at least one major insurer, he added, is starting to come around to that point of view.
Dr. Greeley’s research is funded by the National Institutes of Health, the American Diabetes Association, and the Kovler Family Foundation. He reported having no financial conflicts.
EXPERT ANALYSIS FROM THE PAS ANNUAL MEETING
PAS: Genetic testing for MODY deemed increasingly cost effective
SAN DIEGO – Routine genetic testing for maturity-onset diabetes of the young has become compellingly cost effective in selected populations of diabetic patients, Dr. Siri Atma W. Greeley asserted at the annual meeting of the Pediatric Academic Societies.
“We’re not quite at the point of saying test everyone with diabetes for monogenic causes, but as the cost of genetic testing goes down that could actually happen,” said Dr. Greeley, a pediatric endocrinologist at the University of Chicago.
Today, however, the genetic testing is greatly underutilized and the insurance industry remains balky about providing coverage. That’s starting to change, though, as Dr. Greeley and other MODY experts meet with insurers and explain the dollars-and-cents benefits. Encouragingly, Aetna has led the way forward by publishing its rules for coverage of MODY genetic testing in 2015.
Dr. Greeley was coauthor of a study that evaluated the cost-effectiveness of testing for mutations in three genes responsible for more than 90% of cases of MODY with a known cause. The study, led by his University of Chicago colleague Dr. Rochelle N. Naylor, involved a hypothetical cohort of 25- to 40-year-olds presumed to have type 2 diabetes. Various studies indicate that roughly 2% of such patients actually turn out to have MODY. The question asked by the researchers was, is it cost effective to find them?
With a 2% MODY prevalence, the answer turned out to be no. However, if the population undergoing genetic testing could be enriched through application of clinical criteria associated with increased risk of MODY, then testing does become cost effective even when it identifies a case only 6% of the time. Under that scenario, at a genetic testing cost of $2,580, the incremental cost-effectiveness ratio is $50,000 per additional quality-adjusted life year, which meets the definition of cost-effectiveness widely accepted by health policy makers.
The improvement in quality-adjusted life years is achieved in the case of MODY on the basis of reduced lifetime costs of treatment and complications because one relatively common type of MODY known as glucokinase MODY, or MODY 2, requires no treatment and has essentially no vascular complications, while other common forms of MODY generally respond well to low-dose sulfonylureas rather than more elaborate insulin regimens.
The analysis also concluded that routine testing in a population with a 2% prevalence of MODY would become cost effective if the price tag for testing decreased to $700, as seems highly plausible with further advances in sequencing. And if the MODY prevalence is 31%, a policy of routine genetic testing for MODY actually becomes cost saving rather than just cost effective (Diabetes Care 2014;37:202-9).
This cost-effectiveness analysis doesn’t take into account the added value of genetic testing in terms of family counseling. MODY is transmitted in autosomal dominant fashion, so first-degree relatives of an affected individual have a 50% chance of inheriting a MODY gene mutation, Dr. Greeley noted.
He assisted University of Chicago laboratory scientists in creating a commercially available panel that tests for more than 40 genes known to cause diabetes, including all of the genes known to cause the various types of MODY.
The American Diabetes Association, in its standards of care for 2015, lists a series of criteria that boost the likelihood of monogenic diabetes above the background 2% rate, including diabetes with negative autoantibodies and no signs of insulin resistance or obesity. Moreover, investigators at the University of Exeter (England) have developed an eight-question MODY probability calculator to guide genetic testing decisions, although this tool requires validation. The Exeter group also has shown that among women with gestational diabetes who meet the dual criteria of a body mass index below 25 kg/m2 plus a fasting blood glucose of 5.5 mmol/L or more, genetic testing will identify one case of glucokinase MODY for every 2.7 such women tested (Diabetes Care 2014;37:1230-6).
Dr. Greeley’s research is funded by the National Institutes of Health, the American Diabetes Association, and the Kovler Family Foundation. He reported having no financial conflicts and emphasized that he has no financial ties to the University of Chicago’s genetic testing lab.
SAN DIEGO – Routine genetic testing for maturity-onset diabetes of the young has become compellingly cost effective in selected populations of diabetic patients, Dr. Siri Atma W. Greeley asserted at the annual meeting of the Pediatric Academic Societies.
“We’re not quite at the point of saying test everyone with diabetes for monogenic causes, but as the cost of genetic testing goes down that could actually happen,” said Dr. Greeley, a pediatric endocrinologist at the University of Chicago.
Today, however, the genetic testing is greatly underutilized and the insurance industry remains balky about providing coverage. That’s starting to change, though, as Dr. Greeley and other MODY experts meet with insurers and explain the dollars-and-cents benefits. Encouragingly, Aetna has led the way forward by publishing its rules for coverage of MODY genetic testing in 2015.
Dr. Greeley was coauthor of a study that evaluated the cost-effectiveness of testing for mutations in three genes responsible for more than 90% of cases of MODY with a known cause. The study, led by his University of Chicago colleague Dr. Rochelle N. Naylor, involved a hypothetical cohort of 25- to 40-year-olds presumed to have type 2 diabetes. Various studies indicate that roughly 2% of such patients actually turn out to have MODY. The question asked by the researchers was, is it cost effective to find them?
With a 2% MODY prevalence, the answer turned out to be no. However, if the population undergoing genetic testing could be enriched through application of clinical criteria associated with increased risk of MODY, then testing does become cost effective even when it identifies a case only 6% of the time. Under that scenario, at a genetic testing cost of $2,580, the incremental cost-effectiveness ratio is $50,000 per additional quality-adjusted life year, which meets the definition of cost-effectiveness widely accepted by health policy makers.
The improvement in quality-adjusted life years is achieved in the case of MODY on the basis of reduced lifetime costs of treatment and complications because one relatively common type of MODY known as glucokinase MODY, or MODY 2, requires no treatment and has essentially no vascular complications, while other common forms of MODY generally respond well to low-dose sulfonylureas rather than more elaborate insulin regimens.
The analysis also concluded that routine testing in a population with a 2% prevalence of MODY would become cost effective if the price tag for testing decreased to $700, as seems highly plausible with further advances in sequencing. And if the MODY prevalence is 31%, a policy of routine genetic testing for MODY actually becomes cost saving rather than just cost effective (Diabetes Care 2014;37:202-9).
This cost-effectiveness analysis doesn’t take into account the added value of genetic testing in terms of family counseling. MODY is transmitted in autosomal dominant fashion, so first-degree relatives of an affected individual have a 50% chance of inheriting a MODY gene mutation, Dr. Greeley noted.
He assisted University of Chicago laboratory scientists in creating a commercially available panel that tests for more than 40 genes known to cause diabetes, including all of the genes known to cause the various types of MODY.
The American Diabetes Association, in its standards of care for 2015, lists a series of criteria that boost the likelihood of monogenic diabetes above the background 2% rate, including diabetes with negative autoantibodies and no signs of insulin resistance or obesity. Moreover, investigators at the University of Exeter (England) have developed an eight-question MODY probability calculator to guide genetic testing decisions, although this tool requires validation. The Exeter group also has shown that among women with gestational diabetes who meet the dual criteria of a body mass index below 25 kg/m2 plus a fasting blood glucose of 5.5 mmol/L or more, genetic testing will identify one case of glucokinase MODY for every 2.7 such women tested (Diabetes Care 2014;37:1230-6).
Dr. Greeley’s research is funded by the National Institutes of Health, the American Diabetes Association, and the Kovler Family Foundation. He reported having no financial conflicts and emphasized that he has no financial ties to the University of Chicago’s genetic testing lab.
SAN DIEGO – Routine genetic testing for maturity-onset diabetes of the young has become compellingly cost effective in selected populations of diabetic patients, Dr. Siri Atma W. Greeley asserted at the annual meeting of the Pediatric Academic Societies.
“We’re not quite at the point of saying test everyone with diabetes for monogenic causes, but as the cost of genetic testing goes down that could actually happen,” said Dr. Greeley, a pediatric endocrinologist at the University of Chicago.
Today, however, the genetic testing is greatly underutilized and the insurance industry remains balky about providing coverage. That’s starting to change, though, as Dr. Greeley and other MODY experts meet with insurers and explain the dollars-and-cents benefits. Encouragingly, Aetna has led the way forward by publishing its rules for coverage of MODY genetic testing in 2015.
Dr. Greeley was coauthor of a study that evaluated the cost-effectiveness of testing for mutations in three genes responsible for more than 90% of cases of MODY with a known cause. The study, led by his University of Chicago colleague Dr. Rochelle N. Naylor, involved a hypothetical cohort of 25- to 40-year-olds presumed to have type 2 diabetes. Various studies indicate that roughly 2% of such patients actually turn out to have MODY. The question asked by the researchers was, is it cost effective to find them?
With a 2% MODY prevalence, the answer turned out to be no. However, if the population undergoing genetic testing could be enriched through application of clinical criteria associated with increased risk of MODY, then testing does become cost effective even when it identifies a case only 6% of the time. Under that scenario, at a genetic testing cost of $2,580, the incremental cost-effectiveness ratio is $50,000 per additional quality-adjusted life year, which meets the definition of cost-effectiveness widely accepted by health policy makers.
The improvement in quality-adjusted life years is achieved in the case of MODY on the basis of reduced lifetime costs of treatment and complications because one relatively common type of MODY known as glucokinase MODY, or MODY 2, requires no treatment and has essentially no vascular complications, while other common forms of MODY generally respond well to low-dose sulfonylureas rather than more elaborate insulin regimens.
The analysis also concluded that routine testing in a population with a 2% prevalence of MODY would become cost effective if the price tag for testing decreased to $700, as seems highly plausible with further advances in sequencing. And if the MODY prevalence is 31%, a policy of routine genetic testing for MODY actually becomes cost saving rather than just cost effective (Diabetes Care 2014;37:202-9).
This cost-effectiveness analysis doesn’t take into account the added value of genetic testing in terms of family counseling. MODY is transmitted in autosomal dominant fashion, so first-degree relatives of an affected individual have a 50% chance of inheriting a MODY gene mutation, Dr. Greeley noted.
He assisted University of Chicago laboratory scientists in creating a commercially available panel that tests for more than 40 genes known to cause diabetes, including all of the genes known to cause the various types of MODY.
The American Diabetes Association, in its standards of care for 2015, lists a series of criteria that boost the likelihood of monogenic diabetes above the background 2% rate, including diabetes with negative autoantibodies and no signs of insulin resistance or obesity. Moreover, investigators at the University of Exeter (England) have developed an eight-question MODY probability calculator to guide genetic testing decisions, although this tool requires validation. The Exeter group also has shown that among women with gestational diabetes who meet the dual criteria of a body mass index below 25 kg/m2 plus a fasting blood glucose of 5.5 mmol/L or more, genetic testing will identify one case of glucokinase MODY for every 2.7 such women tested (Diabetes Care 2014;37:1230-6).
Dr. Greeley’s research is funded by the National Institutes of Health, the American Diabetes Association, and the Kovler Family Foundation. He reported having no financial conflicts and emphasized that he has no financial ties to the University of Chicago’s genetic testing lab.
EXPERT ANALYSIS FROM THE PAS ANNUAL MEETING
PAS: Children’s hospitals take disproportionate financial losses from Medicaid
SAN DIEGO – Freestanding children’s hospitals take a far larger financial hit in serving Medicaid pediatric inpatients than do hospitals of other types, Dr. Jeffrey D. Colvin reported at the annual meeting of the Pediatric Academic Societies.
“Increasing Medicaid reimbursement for hospitals serving large volumes of pediatric inpatients covered by Medicaid should be considered,” asserted Dr. Colvin of Children’s Mercy Hospital in Kansas City, Mo.
He presented a cross-sectional study of the Kids’ Inpatient Database (KID) sponsored by the Agency for Healthcare Research and Quality. The analysis encompassed 843,725 discharges from 1,485 U.S. hospitals. The KID database contains total charges for every discharge. The purpose of the study was to determine the median inpatient net revenue from Medicaid by hospital type.
Hospitals of all types experienced a net financial loss for inpatient care of Medicaid-insured pediatric patients, but the size of the losses varied enormously. Freestanding children’s hospitals had a median annual loss of $9.7 million, compared with $1.8 million per year for children’s hospitals located within a general hospital, $204,100 for non-children’s teaching hospitals, and a median loss of just $28,300 per year for non-children’s non-teaching hospitals.
The rate of uninsured pediatric discharges at children’s hospitals was very low: 1.5% at the freestanding ones and 3.5% at those located within a general hospital.
“For all children’s hospitals, the reduction of uninsured patients under the Affordable Care Act is unlikely to offset the continuing losses from Medicaid,” Dr. Colvin observed.
The children’s hospitals provided a disproportionate amount of Medicaid-insured pediatric inpatient care. Freestanding children’s hospitals accounted for 0.8% of hospitals in the KID database, yet they were responsible for 6% of the discharges. And children’s hospitals within a general hospital composed 3.1% of all hospitals while handling 14% of all Medicaid discharges. In contrast, non-children’s non-teaching hospitals made up 75% of the hospital universe, but were responsible for 41.7% of discharges.
Forty-three percent of Medicaid discharges from freestanding children’s hospitals involved children with what is classified as a complex chronic condition. This was the case for 31% of discharges from children’s hospitals within a general hospital, 16.6% of Medicaid pediatric discharges from non-children’s teaching hospitals, and 8.8% of discharges from non-children’s non-teaching hospitals.
Dr. Colvin predicted that the financial burden on children’s hospitals is likely to worsen, given that under the Affordable Care Act, the Disproportionate Share Hospital Payments program that is part of Medicaid is scheduled to be greatly reduced if not eliminated.
The relationship between hospital charges and costs is convoluted. No single source exists for determining hospital-specific Medicaid revenue-to-cost ratios. Dr. Colvin and coinvestigators determined each hospital’s net Medicaid revenue by first figuring the hospital’s total cost, derived by multiplying total Medicaid inpatient charges by the Agency for Healthcare Research and Quality’s hospital-specific ratio of costs-to-charges. Total costs were then multiplied by the hospital’s ratio of revenue-to-costs as determined from the American Hospital Association and Centers for Medicare & Medicaid Services data. This figure, representing the hospital’s gross revenue from Medicaid, was subtracted from the costs to arrive at net revenue.
“This is really important information to give to policy makers, who seem to think that things will sort of level out under the Affordable Care Act,” said Dr. Megan M. Tschudy of Johns Hopkins University, Baltimore. Dr. Tschudy was session cochair.
Dr. Colvin concurred. “I would very much hope that this information has some kind of policy impact,” he said.
Dr. Colvin reported having no financial conflicts regarding this study, which was conducted without commercial support.
SAN DIEGO – Freestanding children’s hospitals take a far larger financial hit in serving Medicaid pediatric inpatients than do hospitals of other types, Dr. Jeffrey D. Colvin reported at the annual meeting of the Pediatric Academic Societies.
“Increasing Medicaid reimbursement for hospitals serving large volumes of pediatric inpatients covered by Medicaid should be considered,” asserted Dr. Colvin of Children’s Mercy Hospital in Kansas City, Mo.
He presented a cross-sectional study of the Kids’ Inpatient Database (KID) sponsored by the Agency for Healthcare Research and Quality. The analysis encompassed 843,725 discharges from 1,485 U.S. hospitals. The KID database contains total charges for every discharge. The purpose of the study was to determine the median inpatient net revenue from Medicaid by hospital type.
Hospitals of all types experienced a net financial loss for inpatient care of Medicaid-insured pediatric patients, but the size of the losses varied enormously. Freestanding children’s hospitals had a median annual loss of $9.7 million, compared with $1.8 million per year for children’s hospitals located within a general hospital, $204,100 for non-children’s teaching hospitals, and a median loss of just $28,300 per year for non-children’s non-teaching hospitals.
The rate of uninsured pediatric discharges at children’s hospitals was very low: 1.5% at the freestanding ones and 3.5% at those located within a general hospital.
“For all children’s hospitals, the reduction of uninsured patients under the Affordable Care Act is unlikely to offset the continuing losses from Medicaid,” Dr. Colvin observed.
The children’s hospitals provided a disproportionate amount of Medicaid-insured pediatric inpatient care. Freestanding children’s hospitals accounted for 0.8% of hospitals in the KID database, yet they were responsible for 6% of the discharges. And children’s hospitals within a general hospital composed 3.1% of all hospitals while handling 14% of all Medicaid discharges. In contrast, non-children’s non-teaching hospitals made up 75% of the hospital universe, but were responsible for 41.7% of discharges.
Forty-three percent of Medicaid discharges from freestanding children’s hospitals involved children with what is classified as a complex chronic condition. This was the case for 31% of discharges from children’s hospitals within a general hospital, 16.6% of Medicaid pediatric discharges from non-children’s teaching hospitals, and 8.8% of discharges from non-children’s non-teaching hospitals.
Dr. Colvin predicted that the financial burden on children’s hospitals is likely to worsen, given that under the Affordable Care Act, the Disproportionate Share Hospital Payments program that is part of Medicaid is scheduled to be greatly reduced if not eliminated.
The relationship between hospital charges and costs is convoluted. No single source exists for determining hospital-specific Medicaid revenue-to-cost ratios. Dr. Colvin and coinvestigators determined each hospital’s net Medicaid revenue by first figuring the hospital’s total cost, derived by multiplying total Medicaid inpatient charges by the Agency for Healthcare Research and Quality’s hospital-specific ratio of costs-to-charges. Total costs were then multiplied by the hospital’s ratio of revenue-to-costs as determined from the American Hospital Association and Centers for Medicare & Medicaid Services data. This figure, representing the hospital’s gross revenue from Medicaid, was subtracted from the costs to arrive at net revenue.
“This is really important information to give to policy makers, who seem to think that things will sort of level out under the Affordable Care Act,” said Dr. Megan M. Tschudy of Johns Hopkins University, Baltimore. Dr. Tschudy was session cochair.
Dr. Colvin concurred. “I would very much hope that this information has some kind of policy impact,” he said.
Dr. Colvin reported having no financial conflicts regarding this study, which was conducted without commercial support.
SAN DIEGO – Freestanding children’s hospitals take a far larger financial hit in serving Medicaid pediatric inpatients than do hospitals of other types, Dr. Jeffrey D. Colvin reported at the annual meeting of the Pediatric Academic Societies.
“Increasing Medicaid reimbursement for hospitals serving large volumes of pediatric inpatients covered by Medicaid should be considered,” asserted Dr. Colvin of Children’s Mercy Hospital in Kansas City, Mo.
He presented a cross-sectional study of the Kids’ Inpatient Database (KID) sponsored by the Agency for Healthcare Research and Quality. The analysis encompassed 843,725 discharges from 1,485 U.S. hospitals. The KID database contains total charges for every discharge. The purpose of the study was to determine the median inpatient net revenue from Medicaid by hospital type.
Hospitals of all types experienced a net financial loss for inpatient care of Medicaid-insured pediatric patients, but the size of the losses varied enormously. Freestanding children’s hospitals had a median annual loss of $9.7 million, compared with $1.8 million per year for children’s hospitals located within a general hospital, $204,100 for non-children’s teaching hospitals, and a median loss of just $28,300 per year for non-children’s non-teaching hospitals.
The rate of uninsured pediatric discharges at children’s hospitals was very low: 1.5% at the freestanding ones and 3.5% at those located within a general hospital.
“For all children’s hospitals, the reduction of uninsured patients under the Affordable Care Act is unlikely to offset the continuing losses from Medicaid,” Dr. Colvin observed.
The children’s hospitals provided a disproportionate amount of Medicaid-insured pediatric inpatient care. Freestanding children’s hospitals accounted for 0.8% of hospitals in the KID database, yet they were responsible for 6% of the discharges. And children’s hospitals within a general hospital composed 3.1% of all hospitals while handling 14% of all Medicaid discharges. In contrast, non-children’s non-teaching hospitals made up 75% of the hospital universe, but were responsible for 41.7% of discharges.
Forty-three percent of Medicaid discharges from freestanding children’s hospitals involved children with what is classified as a complex chronic condition. This was the case for 31% of discharges from children’s hospitals within a general hospital, 16.6% of Medicaid pediatric discharges from non-children’s teaching hospitals, and 8.8% of discharges from non-children’s non-teaching hospitals.
Dr. Colvin predicted that the financial burden on children’s hospitals is likely to worsen, given that under the Affordable Care Act, the Disproportionate Share Hospital Payments program that is part of Medicaid is scheduled to be greatly reduced if not eliminated.
The relationship between hospital charges and costs is convoluted. No single source exists for determining hospital-specific Medicaid revenue-to-cost ratios. Dr. Colvin and coinvestigators determined each hospital’s net Medicaid revenue by first figuring the hospital’s total cost, derived by multiplying total Medicaid inpatient charges by the Agency for Healthcare Research and Quality’s hospital-specific ratio of costs-to-charges. Total costs were then multiplied by the hospital’s ratio of revenue-to-costs as determined from the American Hospital Association and Centers for Medicare & Medicaid Services data. This figure, representing the hospital’s gross revenue from Medicaid, was subtracted from the costs to arrive at net revenue.
“This is really important information to give to policy makers, who seem to think that things will sort of level out under the Affordable Care Act,” said Dr. Megan M. Tschudy of Johns Hopkins University, Baltimore. Dr. Tschudy was session cochair.
Dr. Colvin concurred. “I would very much hope that this information has some kind of policy impact,” he said.
Dr. Colvin reported having no financial conflicts regarding this study, which was conducted without commercial support.
AT THE PAS ANNUAL MEETING
Key clinical point: Hospitals of all types experienced a net financial loss for inpatient care of Medicaid-insured pediatric patients, but the size of the losses varied enormously, with freestanding children’s hospitals taking the biggest hit.
Major finding: Freestanding children’s hospitals experience a median annual net financial loss for inpatient care of Medicaid-insured patients of $9.7 million, compared with a $28,300 loss for Medicaid pediatric inpatient care at non-teaching non-children’s hospitals.
Data source: A cross-sectional study of 843,725 discharges from 1,485 U.S. hospitals.
Disclosures: The presenter reported having no conflicts of interest. The study was done without commercial support.
PAS: Program curbs excess antibiotic prescribing in NICU
SAN DIEGO – Neonatal intensive care units are famously spendthrift when it comes to antibiotic utilization, but a well-executed antibiotic stewardship strategy can safely reduce unnecessary prescribing – as demonstrated at the 90-bed, level-IIIC neonatal intensive care unit (NICU) at Parkland Memorial Hospital, Dallas.
Results of the Surveillance and Correction of Unnecessary Antibiotic Therapy (SCOUT) study showed that total antibiotic days of therapy (DOT) in the Parkland NICU dropped by 27% following implementation of an antibiotic stewardship program featuring hard stops built into the electronic medical record, Dr. Joseph B. Cantey reported at the annual meeting of the Pediatric Academic Societies.
This overall reduction in antibiotic utilization was accomplished through improvements in the three specific categories of NICU antibiotic use targeted for intervention in the SCOUT study: treatment courses of more than 48 hours for “rule-out sepsis” and treatment lasting longer than 5 days for pneumonia or “culture-negative sepsis.”
The proportion of antibiotic treatment courses for rule-out sepsis that were discontinued by 48 hours when cultures were sterile tripled from 32% to 95% between a 9-month baseline period and a second 9-month period after implementation of the antibiotic stewardship program. The proportion of courses of antibiotics for pneumonia that were limited to 5 days doubled from 36% to 72%. Similarly, there was a doubling in the proportion of treatment courses for “culture-negative sepsis” limited to 5 days, with the rate going from 31% to 62%, said Dr. Cantey, a pediatrics fellow in training at the University of Texas Southwestern Medical Center, Dallas.
During the 9-month baseline surveillance period, in which there were 1,607 patients in the NICU, the total antibiotic use was 343 DOT per 1,000 patient-days. During period two with the intervention in place, there were 895 NICU patients, and 251 DOT per 1,000 patient-days. The DOT, a commonly used measure in the field of infectious diseases, is determined by multiplying the number of doses by the dosing interval. DOTs in patients on multiple antibiotics are additive, he explained.
During the baseline observation period, 94% of all antibiotic use in the NICU was empiric therapy for suspected infection. More specifically, 63% of all antibiotic use was for rule-out sepsis. And while many of the courses of antibiotics given for this reason that exceeded the 48-hour limit during the baseline period did so by only one or two doses, those extra doses added up to 41 DOT per 1,000 patient-years, making this a worthy target for intervention. Together with treatment of pneumonia or “culture-negative sepsis” for longer than 5 days, these three high-yield targets accounted for 87% of all antibiotic use in the NICU during the baseline period, Dr. Cantey said.
The infants occupying the NICU during the two 9-month study periods were virtually identical in terms of their characteristics and reasons for admission.
Antibiotics are the most commonly prescribed medications in NICUs. Their use has been associated with adverse NICU outcomes, including increased risks of necrotizing enterocolitis, late-onset sepsis, and death in infants with birth weights below 1,500 g, as well as an increase in multidrug-resistant organisms, he noted.
In SCOUT, the composite outcome of necrotizing enterocolitis, late-onset sepsis, or death didn’t differ between the two study periods: 17.1% at baseline and 15.8% during the intervention period. Similarly, the incidence of colonization with multidrug-resistant organisms was 1.4% during the baseline period and not significantly different at 1% after implementation of the antibiotic stewardship program. Larger multicenter studies with pooled data will be required to determine whether antibiotic stewardship in NICUs affects neonatal outcomes, Dr. Cantey said.
He added that he and his coinvestigators are now trying to identify additional NICU scenarios in which antibiotics can safely be withheld. One likely candidate: asymptomatic preterm infants exposed to premature rupture of membranes. The investigators also hope to utilize the ongoing prospective surveillance element of Parkland’s NICU antibiotic stewardship program to identify the safe minimum treatment duration for common conditions such as urinary tract infections, sepsis, and necrotizing enterocolitis.
Audience members were effusive in their praise of the SCOUT study and the Parkland program. They wanted to hear more details about how the physician behavior change was accomplished. Dr. Cantey said that the three intervention targets were approved by the NICU medical director and the plan was disseminated to all the neonatologists, nurse practitioners, fellows, and residents. It was important to be able to assure everyone that outcomes would be prospectively monitored closely to ensure safety. The toughest task, he added, was to create the hard stops in the electronic medical record so that, for example, treatment for rule-out sepsis would automatically stop at 48 hours: skilled information technologists were essential.
The SCOUT study was supported by a Gerber Novice Researcher Award from the Gerber Foundation. Dr. Cantey reported having no financial conflicts.
SAN DIEGO – Neonatal intensive care units are famously spendthrift when it comes to antibiotic utilization, but a well-executed antibiotic stewardship strategy can safely reduce unnecessary prescribing – as demonstrated at the 90-bed, level-IIIC neonatal intensive care unit (NICU) at Parkland Memorial Hospital, Dallas.
Results of the Surveillance and Correction of Unnecessary Antibiotic Therapy (SCOUT) study showed that total antibiotic days of therapy (DOT) in the Parkland NICU dropped by 27% following implementation of an antibiotic stewardship program featuring hard stops built into the electronic medical record, Dr. Joseph B. Cantey reported at the annual meeting of the Pediatric Academic Societies.
This overall reduction in antibiotic utilization was accomplished through improvements in the three specific categories of NICU antibiotic use targeted for intervention in the SCOUT study: treatment courses of more than 48 hours for “rule-out sepsis” and treatment lasting longer than 5 days for pneumonia or “culture-negative sepsis.”
The proportion of antibiotic treatment courses for rule-out sepsis that were discontinued by 48 hours when cultures were sterile tripled from 32% to 95% between a 9-month baseline period and a second 9-month period after implementation of the antibiotic stewardship program. The proportion of courses of antibiotics for pneumonia that were limited to 5 days doubled from 36% to 72%. Similarly, there was a doubling in the proportion of treatment courses for “culture-negative sepsis” limited to 5 days, with the rate going from 31% to 62%, said Dr. Cantey, a pediatrics fellow in training at the University of Texas Southwestern Medical Center, Dallas.
During the 9-month baseline surveillance period, in which there were 1,607 patients in the NICU, the total antibiotic use was 343 DOT per 1,000 patient-days. During period two with the intervention in place, there were 895 NICU patients, and 251 DOT per 1,000 patient-days. The DOT, a commonly used measure in the field of infectious diseases, is determined by multiplying the number of doses by the dosing interval. DOTs in patients on multiple antibiotics are additive, he explained.
During the baseline observation period, 94% of all antibiotic use in the NICU was empiric therapy for suspected infection. More specifically, 63% of all antibiotic use was for rule-out sepsis. And while many of the courses of antibiotics given for this reason that exceeded the 48-hour limit during the baseline period did so by only one or two doses, those extra doses added up to 41 DOT per 1,000 patient-years, making this a worthy target for intervention. Together with treatment of pneumonia or “culture-negative sepsis” for longer than 5 days, these three high-yield targets accounted for 87% of all antibiotic use in the NICU during the baseline period, Dr. Cantey said.
The infants occupying the NICU during the two 9-month study periods were virtually identical in terms of their characteristics and reasons for admission.
Antibiotics are the most commonly prescribed medications in NICUs. Their use has been associated with adverse NICU outcomes, including increased risks of necrotizing enterocolitis, late-onset sepsis, and death in infants with birth weights below 1,500 g, as well as an increase in multidrug-resistant organisms, he noted.
In SCOUT, the composite outcome of necrotizing enterocolitis, late-onset sepsis, or death didn’t differ between the two study periods: 17.1% at baseline and 15.8% during the intervention period. Similarly, the incidence of colonization with multidrug-resistant organisms was 1.4% during the baseline period and not significantly different at 1% after implementation of the antibiotic stewardship program. Larger multicenter studies with pooled data will be required to determine whether antibiotic stewardship in NICUs affects neonatal outcomes, Dr. Cantey said.
He added that he and his coinvestigators are now trying to identify additional NICU scenarios in which antibiotics can safely be withheld. One likely candidate: asymptomatic preterm infants exposed to premature rupture of membranes. The investigators also hope to utilize the ongoing prospective surveillance element of Parkland’s NICU antibiotic stewardship program to identify the safe minimum treatment duration for common conditions such as urinary tract infections, sepsis, and necrotizing enterocolitis.
Audience members were effusive in their praise of the SCOUT study and the Parkland program. They wanted to hear more details about how the physician behavior change was accomplished. Dr. Cantey said that the three intervention targets were approved by the NICU medical director and the plan was disseminated to all the neonatologists, nurse practitioners, fellows, and residents. It was important to be able to assure everyone that outcomes would be prospectively monitored closely to ensure safety. The toughest task, he added, was to create the hard stops in the electronic medical record so that, for example, treatment for rule-out sepsis would automatically stop at 48 hours: skilled information technologists were essential.
The SCOUT study was supported by a Gerber Novice Researcher Award from the Gerber Foundation. Dr. Cantey reported having no financial conflicts.
SAN DIEGO – Neonatal intensive care units are famously spendthrift when it comes to antibiotic utilization, but a well-executed antibiotic stewardship strategy can safely reduce unnecessary prescribing – as demonstrated at the 90-bed, level-IIIC neonatal intensive care unit (NICU) at Parkland Memorial Hospital, Dallas.
Results of the Surveillance and Correction of Unnecessary Antibiotic Therapy (SCOUT) study showed that total antibiotic days of therapy (DOT) in the Parkland NICU dropped by 27% following implementation of an antibiotic stewardship program featuring hard stops built into the electronic medical record, Dr. Joseph B. Cantey reported at the annual meeting of the Pediatric Academic Societies.
This overall reduction in antibiotic utilization was accomplished through improvements in the three specific categories of NICU antibiotic use targeted for intervention in the SCOUT study: treatment courses of more than 48 hours for “rule-out sepsis” and treatment lasting longer than 5 days for pneumonia or “culture-negative sepsis.”
The proportion of antibiotic treatment courses for rule-out sepsis that were discontinued by 48 hours when cultures were sterile tripled from 32% to 95% between a 9-month baseline period and a second 9-month period after implementation of the antibiotic stewardship program. The proportion of courses of antibiotics for pneumonia that were limited to 5 days doubled from 36% to 72%. Similarly, there was a doubling in the proportion of treatment courses for “culture-negative sepsis” limited to 5 days, with the rate going from 31% to 62%, said Dr. Cantey, a pediatrics fellow in training at the University of Texas Southwestern Medical Center, Dallas.
During the 9-month baseline surveillance period, in which there were 1,607 patients in the NICU, the total antibiotic use was 343 DOT per 1,000 patient-days. During period two with the intervention in place, there were 895 NICU patients, and 251 DOT per 1,000 patient-days. The DOT, a commonly used measure in the field of infectious diseases, is determined by multiplying the number of doses by the dosing interval. DOTs in patients on multiple antibiotics are additive, he explained.
During the baseline observation period, 94% of all antibiotic use in the NICU was empiric therapy for suspected infection. More specifically, 63% of all antibiotic use was for rule-out sepsis. And while many of the courses of antibiotics given for this reason that exceeded the 48-hour limit during the baseline period did so by only one or two doses, those extra doses added up to 41 DOT per 1,000 patient-years, making this a worthy target for intervention. Together with treatment of pneumonia or “culture-negative sepsis” for longer than 5 days, these three high-yield targets accounted for 87% of all antibiotic use in the NICU during the baseline period, Dr. Cantey said.
The infants occupying the NICU during the two 9-month study periods were virtually identical in terms of their characteristics and reasons for admission.
Antibiotics are the most commonly prescribed medications in NICUs. Their use has been associated with adverse NICU outcomes, including increased risks of necrotizing enterocolitis, late-onset sepsis, and death in infants with birth weights below 1,500 g, as well as an increase in multidrug-resistant organisms, he noted.
In SCOUT, the composite outcome of necrotizing enterocolitis, late-onset sepsis, or death didn’t differ between the two study periods: 17.1% at baseline and 15.8% during the intervention period. Similarly, the incidence of colonization with multidrug-resistant organisms was 1.4% during the baseline period and not significantly different at 1% after implementation of the antibiotic stewardship program. Larger multicenter studies with pooled data will be required to determine whether antibiotic stewardship in NICUs affects neonatal outcomes, Dr. Cantey said.
He added that he and his coinvestigators are now trying to identify additional NICU scenarios in which antibiotics can safely be withheld. One likely candidate: asymptomatic preterm infants exposed to premature rupture of membranes. The investigators also hope to utilize the ongoing prospective surveillance element of Parkland’s NICU antibiotic stewardship program to identify the safe minimum treatment duration for common conditions such as urinary tract infections, sepsis, and necrotizing enterocolitis.
Audience members were effusive in their praise of the SCOUT study and the Parkland program. They wanted to hear more details about how the physician behavior change was accomplished. Dr. Cantey said that the three intervention targets were approved by the NICU medical director and the plan was disseminated to all the neonatologists, nurse practitioners, fellows, and residents. It was important to be able to assure everyone that outcomes would be prospectively monitored closely to ensure safety. The toughest task, he added, was to create the hard stops in the electronic medical record so that, for example, treatment for rule-out sepsis would automatically stop at 48 hours: skilled information technologists were essential.
The SCOUT study was supported by a Gerber Novice Researcher Award from the Gerber Foundation. Dr. Cantey reported having no financial conflicts.
AT THE PAS ANNUAL MEETING
Key clinical point: Total antibiotic utilization in a level-IIIC neonatal intensive care unit was safely reduced by 27% through a formal antibiotic stewardship program.
Major finding: The rate of discontinuation of courses of antibiotics given for “rule-out sepsis” by 48 hours tripled from 32% at baseline to 95% post intervention.
Data source: This prospective study carried out in a 90-bed NICU involved an initial 9-month baseline observation period and a second 9-month period starting after implementation of the stewardship program.
Disclosures: The study was supported by a Gerber Novice Researcher Award from the Gerber Foundation. The presenter reported having no relevant financial disclosures.