User login
Calculate an STS score before all aortic valve replacements
SNOWMASS, COLO. – The Society of Thoracic Surgeons Predicted Risk of Mortality score draws criticism when it’s used to help decide whether a patient should undergo surgical or transcatheter aortic valve replacement, because the algorithm excludes plenty of information pertinent to that task. Yet for now it’s an indispensible tool for cardiologists and surgeons alike, Dr. Vinod H. Thourani asserted at the Annual Cardiovascular Conference at Snowmass.
"Calculate an STS score for all patients, as annoying as it is. The goal should be to fit the patient with the best operation, not to force the patient into one technique or the other. The STS score is the only thing we have right now. Work is underway on a new TAVR risk scoring system, but it’s not ready yet," said Dr. Thourani, associate director of cardiothoracic surgery at Emory University, Atlanta.
The STS score was originally developed to predict the risk of mortality in coronary artery bypass graft (CABG) patients. But despite the score’s limitations when applied to patients requiring aortic valve replacement, a new analysis of the massive STS national database demonstrates that the tool is surprisingly accurate for this purpose.
"In reality, the STS score does a pretty good job of predicting what you’re going to do in open surgery. Everybody poo-poos the score, but here we’ve got data on 142,000 patients undergoing isolated surgical aortic valve replacement, and it’s actually pretty close. So I think you can use the STS score for prediction of surgical valve mortality," according to Dr. Thourani.
Among the 141,905 patients in the STS database who underwent isolated surgical aortic valve replacement (SAVR) during roughly the past 6 years, 6% were high risk as defined by an STS predicted risk of mortality score greater than 8%. Another 14% were intermediate risk, with an STS score of 4%-8%. The remaining 80% were low risk, with an STS score of less than 4%.
In a soon-to-be-published report by Dr. Thourani and his coinvestigators, the median postoperative length of stay was 6.0 days in the group with a low-risk STS score, 8.0 days in the intermediate-risk patients, and 9.0 days in those with an STS score greater than 8%. Actual national in-hospital mortality in SAVR patients with an STS score of less than 4% was 1.4%, whereas the mean and median STS for predicted in-hospital mortality in this cohort was 1.7% and 1.5%, respectively. In-hospital mortality in the intermediate-risk group was 5.1%; the mean and median STS scores in this group were 5.5% and 5.2%. And in the high-risk group, in-hospital mortality was 11.8%, compared with 13.7% and 11.2% mean and median predicted rates based on STS scores.
At Emory and other multidisciplinary heart centers around the country, patients needing aortic valve replacement who have an STS score below 4% typically get SAVR with either a stented or sutureless valve. Intermediate-risk patients – those with an STS score of 4%-8% – get SAVR or are enrolled in a mid-risk TAVR clinical trial, provided it’s an option at that site. High-risk patients with an STS score greater than 8% have the option of an open or mini-SAVR, although Dr. Thourani said most of his high-risk patients now undergo TAVR. Extremely high-risk patients having an STS score greater than 15% aren’t candidates for surgery; their options are commercially available TAVR or palliation via balloon valvuloplasty and/or medical therapy.
Patients in need of aortic valve replacement who present to a multidisciplinary heart center undergo an extensive battery of tests aimed at helping the team decide whether SAVR or TAVR is best for that individual. These tests assess relevant factors not included in the STS score algorithm. For example, frailty assessment is not incorporated in the STS score, yet most patients in need of a new aortic valve are elderly. At Emory, patients being evaluated for aortic valve replacement undergo five different measures of frailty: gait speed, grip strength, activities of daily living assessment, nutrition, and the mini–mental status examination. If someone fails three of these five measures, Dr. Thourani rules out the option of SAVR.
Other routine tests include a computed tomography (CT) scan to evaluate the ascending aorta and femoral and iliac arteries for calcification, echocardiography to determine annular sizing, carotid duplex ultrasound, pulmonary function tests, and cardiac catheterization.
TAVR is the best option for patients with porcelain aorta, a hostile chest due to prior radiation therapy, end-stage renal disease, severe lung disease, advanced liver disease, prior CABG surgery with an internal mammary artery graft crossing the midline, more than two prior sternotomies, or moderate dementia.
Dementia is a major issue for patients in their 80s with aortic stenosis warranting valve replacement. Mildly demented patients are suitable for either SAVR or TAVR. Those with severe dementia aren’t candidates for either procedure and are best managed with medication or balloon valvuloplasty.
"We no longer operate on patients with moderate dementia. They sundown really badly. We’ve gone to TAVR in these patients," Dr. Thourani said.
He reported serving as a consultant to Edwards Lifesciences, Sorin, and St. Jude Medical.
SNOWMASS, COLO. – The Society of Thoracic Surgeons Predicted Risk of Mortality score draws criticism when it’s used to help decide whether a patient should undergo surgical or transcatheter aortic valve replacement, because the algorithm excludes plenty of information pertinent to that task. Yet for now it’s an indispensible tool for cardiologists and surgeons alike, Dr. Vinod H. Thourani asserted at the Annual Cardiovascular Conference at Snowmass.
"Calculate an STS score for all patients, as annoying as it is. The goal should be to fit the patient with the best operation, not to force the patient into one technique or the other. The STS score is the only thing we have right now. Work is underway on a new TAVR risk scoring system, but it’s not ready yet," said Dr. Thourani, associate director of cardiothoracic surgery at Emory University, Atlanta.
The STS score was originally developed to predict the risk of mortality in coronary artery bypass graft (CABG) patients. But despite the score’s limitations when applied to patients requiring aortic valve replacement, a new analysis of the massive STS national database demonstrates that the tool is surprisingly accurate for this purpose.
"In reality, the STS score does a pretty good job of predicting what you’re going to do in open surgery. Everybody poo-poos the score, but here we’ve got data on 142,000 patients undergoing isolated surgical aortic valve replacement, and it’s actually pretty close. So I think you can use the STS score for prediction of surgical valve mortality," according to Dr. Thourani.
Among the 141,905 patients in the STS database who underwent isolated surgical aortic valve replacement (SAVR) during roughly the past 6 years, 6% were high risk as defined by an STS predicted risk of mortality score greater than 8%. Another 14% were intermediate risk, with an STS score of 4%-8%. The remaining 80% were low risk, with an STS score of less than 4%.
In a soon-to-be-published report by Dr. Thourani and his coinvestigators, the median postoperative length of stay was 6.0 days in the group with a low-risk STS score, 8.0 days in the intermediate-risk patients, and 9.0 days in those with an STS score greater than 8%. Actual national in-hospital mortality in SAVR patients with an STS score of less than 4% was 1.4%, whereas the mean and median STS for predicted in-hospital mortality in this cohort was 1.7% and 1.5%, respectively. In-hospital mortality in the intermediate-risk group was 5.1%; the mean and median STS scores in this group were 5.5% and 5.2%. And in the high-risk group, in-hospital mortality was 11.8%, compared with 13.7% and 11.2% mean and median predicted rates based on STS scores.
At Emory and other multidisciplinary heart centers around the country, patients needing aortic valve replacement who have an STS score below 4% typically get SAVR with either a stented or sutureless valve. Intermediate-risk patients – those with an STS score of 4%-8% – get SAVR or are enrolled in a mid-risk TAVR clinical trial, provided it’s an option at that site. High-risk patients with an STS score greater than 8% have the option of an open or mini-SAVR, although Dr. Thourani said most of his high-risk patients now undergo TAVR. Extremely high-risk patients having an STS score greater than 15% aren’t candidates for surgery; their options are commercially available TAVR or palliation via balloon valvuloplasty and/or medical therapy.
Patients in need of aortic valve replacement who present to a multidisciplinary heart center undergo an extensive battery of tests aimed at helping the team decide whether SAVR or TAVR is best for that individual. These tests assess relevant factors not included in the STS score algorithm. For example, frailty assessment is not incorporated in the STS score, yet most patients in need of a new aortic valve are elderly. At Emory, patients being evaluated for aortic valve replacement undergo five different measures of frailty: gait speed, grip strength, activities of daily living assessment, nutrition, and the mini–mental status examination. If someone fails three of these five measures, Dr. Thourani rules out the option of SAVR.
Other routine tests include a computed tomography (CT) scan to evaluate the ascending aorta and femoral and iliac arteries for calcification, echocardiography to determine annular sizing, carotid duplex ultrasound, pulmonary function tests, and cardiac catheterization.
TAVR is the best option for patients with porcelain aorta, a hostile chest due to prior radiation therapy, end-stage renal disease, severe lung disease, advanced liver disease, prior CABG surgery with an internal mammary artery graft crossing the midline, more than two prior sternotomies, or moderate dementia.
Dementia is a major issue for patients in their 80s with aortic stenosis warranting valve replacement. Mildly demented patients are suitable for either SAVR or TAVR. Those with severe dementia aren’t candidates for either procedure and are best managed with medication or balloon valvuloplasty.
"We no longer operate on patients with moderate dementia. They sundown really badly. We’ve gone to TAVR in these patients," Dr. Thourani said.
He reported serving as a consultant to Edwards Lifesciences, Sorin, and St. Jude Medical.
SNOWMASS, COLO. – The Society of Thoracic Surgeons Predicted Risk of Mortality score draws criticism when it’s used to help decide whether a patient should undergo surgical or transcatheter aortic valve replacement, because the algorithm excludes plenty of information pertinent to that task. Yet for now it’s an indispensible tool for cardiologists and surgeons alike, Dr. Vinod H. Thourani asserted at the Annual Cardiovascular Conference at Snowmass.
"Calculate an STS score for all patients, as annoying as it is. The goal should be to fit the patient with the best operation, not to force the patient into one technique or the other. The STS score is the only thing we have right now. Work is underway on a new TAVR risk scoring system, but it’s not ready yet," said Dr. Thourani, associate director of cardiothoracic surgery at Emory University, Atlanta.
The STS score was originally developed to predict the risk of mortality in coronary artery bypass graft (CABG) patients. But despite the score’s limitations when applied to patients requiring aortic valve replacement, a new analysis of the massive STS national database demonstrates that the tool is surprisingly accurate for this purpose.
"In reality, the STS score does a pretty good job of predicting what you’re going to do in open surgery. Everybody poo-poos the score, but here we’ve got data on 142,000 patients undergoing isolated surgical aortic valve replacement, and it’s actually pretty close. So I think you can use the STS score for prediction of surgical valve mortality," according to Dr. Thourani.
Among the 141,905 patients in the STS database who underwent isolated surgical aortic valve replacement (SAVR) during roughly the past 6 years, 6% were high risk as defined by an STS predicted risk of mortality score greater than 8%. Another 14% were intermediate risk, with an STS score of 4%-8%. The remaining 80% were low risk, with an STS score of less than 4%.
In a soon-to-be-published report by Dr. Thourani and his coinvestigators, the median postoperative length of stay was 6.0 days in the group with a low-risk STS score, 8.0 days in the intermediate-risk patients, and 9.0 days in those with an STS score greater than 8%. Actual national in-hospital mortality in SAVR patients with an STS score of less than 4% was 1.4%, whereas the mean and median STS for predicted in-hospital mortality in this cohort was 1.7% and 1.5%, respectively. In-hospital mortality in the intermediate-risk group was 5.1%; the mean and median STS scores in this group were 5.5% and 5.2%. And in the high-risk group, in-hospital mortality was 11.8%, compared with 13.7% and 11.2% mean and median predicted rates based on STS scores.
At Emory and other multidisciplinary heart centers around the country, patients needing aortic valve replacement who have an STS score below 4% typically get SAVR with either a stented or sutureless valve. Intermediate-risk patients – those with an STS score of 4%-8% – get SAVR or are enrolled in a mid-risk TAVR clinical trial, provided it’s an option at that site. High-risk patients with an STS score greater than 8% have the option of an open or mini-SAVR, although Dr. Thourani said most of his high-risk patients now undergo TAVR. Extremely high-risk patients having an STS score greater than 15% aren’t candidates for surgery; their options are commercially available TAVR or palliation via balloon valvuloplasty and/or medical therapy.
Patients in need of aortic valve replacement who present to a multidisciplinary heart center undergo an extensive battery of tests aimed at helping the team decide whether SAVR or TAVR is best for that individual. These tests assess relevant factors not included in the STS score algorithm. For example, frailty assessment is not incorporated in the STS score, yet most patients in need of a new aortic valve are elderly. At Emory, patients being evaluated for aortic valve replacement undergo five different measures of frailty: gait speed, grip strength, activities of daily living assessment, nutrition, and the mini–mental status examination. If someone fails three of these five measures, Dr. Thourani rules out the option of SAVR.
Other routine tests include a computed tomography (CT) scan to evaluate the ascending aorta and femoral and iliac arteries for calcification, echocardiography to determine annular sizing, carotid duplex ultrasound, pulmonary function tests, and cardiac catheterization.
TAVR is the best option for patients with porcelain aorta, a hostile chest due to prior radiation therapy, end-stage renal disease, severe lung disease, advanced liver disease, prior CABG surgery with an internal mammary artery graft crossing the midline, more than two prior sternotomies, or moderate dementia.
Dementia is a major issue for patients in their 80s with aortic stenosis warranting valve replacement. Mildly demented patients are suitable for either SAVR or TAVR. Those with severe dementia aren’t candidates for either procedure and are best managed with medication or balloon valvuloplasty.
"We no longer operate on patients with moderate dementia. They sundown really badly. We’ve gone to TAVR in these patients," Dr. Thourani said.
He reported serving as a consultant to Edwards Lifesciences, Sorin, and St. Jude Medical.
EXPERT ANALYSIS FROM THE CARDIOVASCULAR CONFERENCE AT SNOWMASS
Preoperative organ dysfunction worsens SAVR outcomes
SNOWMASS, COLO. – The presence of preoperative dysfunction in more than any one of four key organ systems profoundly reduces survival in patients undergoing surgical aortic valve replacement, a study showed.
"If you have two or more dysfunctional organ systems, you really need to think about what you’re doing for this patient. At 5 years, only about 40% of these patients are alive. It makes a lot of sense to me to say that if you have a patient with severe COPD [chronic obstructive pulmonary disease] and renal dysfunction, that patient should probably never get a surgical valve," Dr. Vinod H. Thourani said at the Annual Cardiovascular Conference at Snowmass.
In a retrospective analysis of a registry with prospectively entered data, 29% of 1,759 patients who underwent surgical aortic valve replacement (SAVR) with or without coronary artery bypass grafting at Emory University during 2002-2010 had preoperative dysfunction of one or more of four organ systems under scrutiny. Eighty-five patients had severe COPD, as defined by a forced expiratory volume in 1 second (FEV1) that was less than 50% of predicted, 140 had chronic renal failure, 149 had a prior stroke, and 241 had heart failure with a left ventricular ejection less than 35%.
Patients with chronic renal failure had far and away the worst 30-day and long-term outcomes. Half were dead within 3 years. The 7-year survival rate was just 11.7%.
The second-worst outcomes were seen in patients with severe COPD preoperatively. Their 7-year survival rate was 30.8%.
"Anyone with an FEV1 below about 40% becomes a higher-risk surgical candidate; think instead of TAVR [transcatheter aortic valve replacement],"advised Dr. Thourani of the division of cardiothoracic surgery at Emory University, Atlanta.
In contrast, outcomes in patients with either heart failure or prior stroke "were not that bad," he said, pointing to 7-year survival rates of 55.9% and 48.6%, respectively.
Ninety-five patients (5.4%) in this recently published study (Ann. Thorac. Surg. 2013;95:838-45) had more than one dysfunctional organ system prior to SAVR. Median survival in patients without dysfunction in any of the four organ systems was 8.2 years and counting. With one dysfunctional organ, it was still good at 7.2 years. However, with two dysfunctional organ systems, the median survival dropped precipitously to 4.1 years. With three dysfunctional organ systems, it was 5.9 years.
Dr. Thourin serves as a consultant to Edwards Lifesciences, Sorin, and St. Jude Medical.
SNOWMASS, COLO. – The presence of preoperative dysfunction in more than any one of four key organ systems profoundly reduces survival in patients undergoing surgical aortic valve replacement, a study showed.
"If you have two or more dysfunctional organ systems, you really need to think about what you’re doing for this patient. At 5 years, only about 40% of these patients are alive. It makes a lot of sense to me to say that if you have a patient with severe COPD [chronic obstructive pulmonary disease] and renal dysfunction, that patient should probably never get a surgical valve," Dr. Vinod H. Thourani said at the Annual Cardiovascular Conference at Snowmass.
In a retrospective analysis of a registry with prospectively entered data, 29% of 1,759 patients who underwent surgical aortic valve replacement (SAVR) with or without coronary artery bypass grafting at Emory University during 2002-2010 had preoperative dysfunction of one or more of four organ systems under scrutiny. Eighty-five patients had severe COPD, as defined by a forced expiratory volume in 1 second (FEV1) that was less than 50% of predicted, 140 had chronic renal failure, 149 had a prior stroke, and 241 had heart failure with a left ventricular ejection less than 35%.
Patients with chronic renal failure had far and away the worst 30-day and long-term outcomes. Half were dead within 3 years. The 7-year survival rate was just 11.7%.
The second-worst outcomes were seen in patients with severe COPD preoperatively. Their 7-year survival rate was 30.8%.
"Anyone with an FEV1 below about 40% becomes a higher-risk surgical candidate; think instead of TAVR [transcatheter aortic valve replacement],"advised Dr. Thourani of the division of cardiothoracic surgery at Emory University, Atlanta.
In contrast, outcomes in patients with either heart failure or prior stroke "were not that bad," he said, pointing to 7-year survival rates of 55.9% and 48.6%, respectively.
Ninety-five patients (5.4%) in this recently published study (Ann. Thorac. Surg. 2013;95:838-45) had more than one dysfunctional organ system prior to SAVR. Median survival in patients without dysfunction in any of the four organ systems was 8.2 years and counting. With one dysfunctional organ, it was still good at 7.2 years. However, with two dysfunctional organ systems, the median survival dropped precipitously to 4.1 years. With three dysfunctional organ systems, it was 5.9 years.
Dr. Thourin serves as a consultant to Edwards Lifesciences, Sorin, and St. Jude Medical.
SNOWMASS, COLO. – The presence of preoperative dysfunction in more than any one of four key organ systems profoundly reduces survival in patients undergoing surgical aortic valve replacement, a study showed.
"If you have two or more dysfunctional organ systems, you really need to think about what you’re doing for this patient. At 5 years, only about 40% of these patients are alive. It makes a lot of sense to me to say that if you have a patient with severe COPD [chronic obstructive pulmonary disease] and renal dysfunction, that patient should probably never get a surgical valve," Dr. Vinod H. Thourani said at the Annual Cardiovascular Conference at Snowmass.
In a retrospective analysis of a registry with prospectively entered data, 29% of 1,759 patients who underwent surgical aortic valve replacement (SAVR) with or without coronary artery bypass grafting at Emory University during 2002-2010 had preoperative dysfunction of one or more of four organ systems under scrutiny. Eighty-five patients had severe COPD, as defined by a forced expiratory volume in 1 second (FEV1) that was less than 50% of predicted, 140 had chronic renal failure, 149 had a prior stroke, and 241 had heart failure with a left ventricular ejection less than 35%.
Patients with chronic renal failure had far and away the worst 30-day and long-term outcomes. Half were dead within 3 years. The 7-year survival rate was just 11.7%.
The second-worst outcomes were seen in patients with severe COPD preoperatively. Their 7-year survival rate was 30.8%.
"Anyone with an FEV1 below about 40% becomes a higher-risk surgical candidate; think instead of TAVR [transcatheter aortic valve replacement],"advised Dr. Thourani of the division of cardiothoracic surgery at Emory University, Atlanta.
In contrast, outcomes in patients with either heart failure or prior stroke "were not that bad," he said, pointing to 7-year survival rates of 55.9% and 48.6%, respectively.
Ninety-five patients (5.4%) in this recently published study (Ann. Thorac. Surg. 2013;95:838-45) had more than one dysfunctional organ system prior to SAVR. Median survival in patients without dysfunction in any of the four organ systems was 8.2 years and counting. With one dysfunctional organ, it was still good at 7.2 years. However, with two dysfunctional organ systems, the median survival dropped precipitously to 4.1 years. With three dysfunctional organ systems, it was 5.9 years.
Dr. Thourin serves as a consultant to Edwards Lifesciences, Sorin, and St. Jude Medical.
EXPERT ANALYSIS FROM THE CARDIOVASCULAR CONFERENCE AT SNOWMASS
New tools for stroke prediction in atrial fibrillation
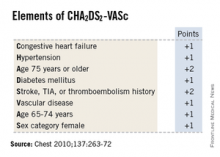
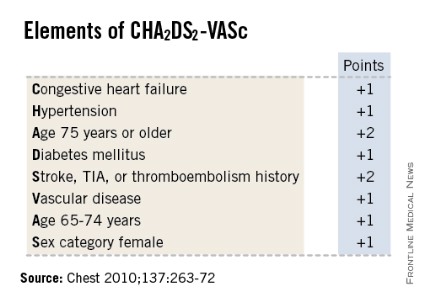
SNOWMASS, COLO. – High-sensitivity troponin T and brain natriuretic peptide levels are better predictors of stroke risk in patients with atrial fibrillation than the CHA2DS2-VASc score that will replace the CHADS2 score in the forthcoming revised American College of Cardiology/American Heart Association guidelines.
Recent evidence indicates the biomarkers may be novel tools for improved stroke prediction in atrial fibrillation (AF), with prognostic value above and beyond that provided by the CHA2DS2-VASc scores.
These findings raise important unanswered questions about the relationship between AF and stroke. Conventional wisdom has held that left atrial thrombus is the cause of most strokes in patients with AF. But it’s not that simple, Dr. Bernard J. Gersh asserted at the Annual Cardiovascular Conference at Snowmass.
"What are we measuring with these biomarkers? This is what we really don’t understand. What has high-sensitivity troponin T got to do with left atrial thrombus?" asked Dr. Gersh, professor of medicine at the Mayo Clinic in Rochester, Minn.
It seems increasingly clear that it’s not just the atrial arrhythmia that’s important in stroke risk, it’s also the company AF keeps. In a substantial but still uncertain proportion of patients, AF is a marker of vascular disease burden expressed through atrial and vascular endothelial dysfunction, vascular inflammation, left atrial dilatation and fibrosis, and a hypercoagulable state, the cardiologist continued.
He was a coinvestigator on a couple of recent groundbreaking studies that show the prognostic power of biomarkers in predicting both stroke risk and cardiac death in AF patients.
In one report, the investigators looked at baseline high-sensitivity troponin T (hsTnT) levels, clinical risk factors for stroke, and CHA2DS2-VASc scores in 12,892 patients with AF who were randomized to apixaban or warfarin in the prospective, double-blind ARISTOTLE (Apixaban for the Prevention of Stroke in Subjects With Atrial Fibrillation) trial (N. Engl. J. Med. 2011;365:981-92). During a median 1.9 years of follow-up, patients in the highest quartile for baseline hsTnT had roughly a twofold greater risk of stroke or systemic embolism than did those in the lowest quartile.
Moreover, patients with a low-risk CHA2DS2-VASc score of 0 or 1 but in the top quartile for hsTnT, with a level in excess of 13 ng/L, had a very substantial stroke rate of 2.7% per year despite anticoagulation with apixaban (Eliquis) or warfarin. The relationship was even stronger for cardiac death, where subjects with a low-risk CHA2DS2-VASc score who were in the top quartile for hsTnT had a 6% annual risk. A higher baseline hsTnT was also independently associated with sharply increased risk of major bleeding in a multivariate regression analysis (J. Am. Coll. Cardiol. 2014;63:52-61).
In another recently published study, he and his international coworkers showed in ARISTOTLE participants with baseline N-terminal pro-B-type natriuretic peptide (NT-proBNP) levels that this biomarker also improved stroke prediction in AF, providing added value to CHA2DS2-VASc scores. Subjects in the top quartile for baseline NT-proBNP had an adjusted 2.35-fold greater risk of stroke or systemic embolism than those in the lowest quartile, irrespective of CHA2DS2-VASc score. They also had a 2.5-fold greater risk of cardiac death (J. Am. Coll. Cardiol. 2013;61:2274-84).
A study Dr. Gersh highlighted as "extremely interesting" involved the use of brain natriuretic peptide (BNP) as a marker to rule out delayed AF in stroke patients. The study, led by investigators at the University Hospital Center of Nice (France) and known as TARGET-AF, included 300 consecutive acute stroke patients with no history of AF and no AF on their baseline ECG. During a median 6.8 days of in-hospital Holter monitoring, 17% of the stroke patients developed newly diagnosed AF.
The strongest predictor of delayed AF was baseline plasma BNP. It outperformed the CHA2DS2-VASc score and all the other parameters examined, including anterior circulation location of the stroke, P-wave initial force, gender, National Institutes of Health Stroke Scale score, age, left atrial dilatation, and Score for the Targeting of AF (STAF) score. A BNP level greater than 131 pg/mL had a 98.1% sensitivity, 71.4% specificity, and 99.4% negative predictive value for delayed AF.
"Our data indicate that a BNP level of 131 pg/mL or less might rule out delayed AF in stroke survivors and could be included in algorithms for AF detection," the French investigators concluded (J. Stroke Cerebrovasc. Dis. 2013;22:e103-10).
There is plenty of direct evidence from transesophageal echocardiography studies and other sources that a substantial proportion of thromboemboli are directly the result of AF. However, indirect evidence points to additional causal factors. For example, there is a high incidence of thromboembolic events in AF patients without left atrial appendage thrombus. Plus, in natural history studies patients with AF without additional risk factors have a low incidence of stroke. And CHADS2 and CHA2DS2-VASc scores predict vascular events but don’t correlate with left atrial appendage thrombus, Dr. Gersh noted.
He said the CHA2DS2-VASc score is clearly an improvement over CHADS2, and its adoption in the forthcoming ACC/AHA guidelines is to be welcomed. The CHA2DS2-VASc score increases the number of patients considered at significant risk of stroke and therefore warranting anticoagulation. For example, in a large Danish registry of nearly 48,000 AF patients with a CHADS2 score of 0-1 not on anticoagulation, patients with a CHADS2 score of 1 but a CHA2DS2-VASc score of 2 had twice the stroke risk of patients with a CHA2DS2-VASc of 1 (Thromb. Haemost. 2012;107:1172-9).
That being said, neither risk score is all that impressive. The C-statistic, a measure of a test’s predictive power, is 0.56 for CHADS2 and it was 0.62 for CHA2DS2-VASc in the ARISTOTLE analysis. To put those figures in perspective, a coin toss has a C-statistic of 0.50.
"The individual predictive values are not good. We use CHADS2 and CHA2DS2-VASc in practice and in the guidelines, but we should not pretend they are highly predictive. We need new risk stratification schemes," according to Dr. Gersh.
He reported serving as an adviser to Boston Scientific and St. Jude Medical.
SNOWMASS, COLO. – High-sensitivity troponin T and brain natriuretic peptide levels are better predictors of stroke risk in patients with atrial fibrillation than the CHA2DS2-VASc score that will replace the CHADS2 score in the forthcoming revised American College of Cardiology/American Heart Association guidelines.
Recent evidence indicates the biomarkers may be novel tools for improved stroke prediction in atrial fibrillation (AF), with prognostic value above and beyond that provided by the CHA2DS2-VASc scores.
These findings raise important unanswered questions about the relationship between AF and stroke. Conventional wisdom has held that left atrial thrombus is the cause of most strokes in patients with AF. But it’s not that simple, Dr. Bernard J. Gersh asserted at the Annual Cardiovascular Conference at Snowmass.
"What are we measuring with these biomarkers? This is what we really don’t understand. What has high-sensitivity troponin T got to do with left atrial thrombus?" asked Dr. Gersh, professor of medicine at the Mayo Clinic in Rochester, Minn.
It seems increasingly clear that it’s not just the atrial arrhythmia that’s important in stroke risk, it’s also the company AF keeps. In a substantial but still uncertain proportion of patients, AF is a marker of vascular disease burden expressed through atrial and vascular endothelial dysfunction, vascular inflammation, left atrial dilatation and fibrosis, and a hypercoagulable state, the cardiologist continued.
He was a coinvestigator on a couple of recent groundbreaking studies that show the prognostic power of biomarkers in predicting both stroke risk and cardiac death in AF patients.
In one report, the investigators looked at baseline high-sensitivity troponin T (hsTnT) levels, clinical risk factors for stroke, and CHA2DS2-VASc scores in 12,892 patients with AF who were randomized to apixaban or warfarin in the prospective, double-blind ARISTOTLE (Apixaban for the Prevention of Stroke in Subjects With Atrial Fibrillation) trial (N. Engl. J. Med. 2011;365:981-92). During a median 1.9 years of follow-up, patients in the highest quartile for baseline hsTnT had roughly a twofold greater risk of stroke or systemic embolism than did those in the lowest quartile.
Moreover, patients with a low-risk CHA2DS2-VASc score of 0 or 1 but in the top quartile for hsTnT, with a level in excess of 13 ng/L, had a very substantial stroke rate of 2.7% per year despite anticoagulation with apixaban (Eliquis) or warfarin. The relationship was even stronger for cardiac death, where subjects with a low-risk CHA2DS2-VASc score who were in the top quartile for hsTnT had a 6% annual risk. A higher baseline hsTnT was also independently associated with sharply increased risk of major bleeding in a multivariate regression analysis (J. Am. Coll. Cardiol. 2014;63:52-61).
In another recently published study, he and his international coworkers showed in ARISTOTLE participants with baseline N-terminal pro-B-type natriuretic peptide (NT-proBNP) levels that this biomarker also improved stroke prediction in AF, providing added value to CHA2DS2-VASc scores. Subjects in the top quartile for baseline NT-proBNP had an adjusted 2.35-fold greater risk of stroke or systemic embolism than those in the lowest quartile, irrespective of CHA2DS2-VASc score. They also had a 2.5-fold greater risk of cardiac death (J. Am. Coll. Cardiol. 2013;61:2274-84).
A study Dr. Gersh highlighted as "extremely interesting" involved the use of brain natriuretic peptide (BNP) as a marker to rule out delayed AF in stroke patients. The study, led by investigators at the University Hospital Center of Nice (France) and known as TARGET-AF, included 300 consecutive acute stroke patients with no history of AF and no AF on their baseline ECG. During a median 6.8 days of in-hospital Holter monitoring, 17% of the stroke patients developed newly diagnosed AF.
The strongest predictor of delayed AF was baseline plasma BNP. It outperformed the CHA2DS2-VASc score and all the other parameters examined, including anterior circulation location of the stroke, P-wave initial force, gender, National Institutes of Health Stroke Scale score, age, left atrial dilatation, and Score for the Targeting of AF (STAF) score. A BNP level greater than 131 pg/mL had a 98.1% sensitivity, 71.4% specificity, and 99.4% negative predictive value for delayed AF.
"Our data indicate that a BNP level of 131 pg/mL or less might rule out delayed AF in stroke survivors and could be included in algorithms for AF detection," the French investigators concluded (J. Stroke Cerebrovasc. Dis. 2013;22:e103-10).
There is plenty of direct evidence from transesophageal echocardiography studies and other sources that a substantial proportion of thromboemboli are directly the result of AF. However, indirect evidence points to additional causal factors. For example, there is a high incidence of thromboembolic events in AF patients without left atrial appendage thrombus. Plus, in natural history studies patients with AF without additional risk factors have a low incidence of stroke. And CHADS2 and CHA2DS2-VASc scores predict vascular events but don’t correlate with left atrial appendage thrombus, Dr. Gersh noted.
He said the CHA2DS2-VASc score is clearly an improvement over CHADS2, and its adoption in the forthcoming ACC/AHA guidelines is to be welcomed. The CHA2DS2-VASc score increases the number of patients considered at significant risk of stroke and therefore warranting anticoagulation. For example, in a large Danish registry of nearly 48,000 AF patients with a CHADS2 score of 0-1 not on anticoagulation, patients with a CHADS2 score of 1 but a CHA2DS2-VASc score of 2 had twice the stroke risk of patients with a CHA2DS2-VASc of 1 (Thromb. Haemost. 2012;107:1172-9).
That being said, neither risk score is all that impressive. The C-statistic, a measure of a test’s predictive power, is 0.56 for CHADS2 and it was 0.62 for CHA2DS2-VASc in the ARISTOTLE analysis. To put those figures in perspective, a coin toss has a C-statistic of 0.50.
"The individual predictive values are not good. We use CHADS2 and CHA2DS2-VASc in practice and in the guidelines, but we should not pretend they are highly predictive. We need new risk stratification schemes," according to Dr. Gersh.
He reported serving as an adviser to Boston Scientific and St. Jude Medical.
SNOWMASS, COLO. – High-sensitivity troponin T and brain natriuretic peptide levels are better predictors of stroke risk in patients with atrial fibrillation than the CHA2DS2-VASc score that will replace the CHADS2 score in the forthcoming revised American College of Cardiology/American Heart Association guidelines.
Recent evidence indicates the biomarkers may be novel tools for improved stroke prediction in atrial fibrillation (AF), with prognostic value above and beyond that provided by the CHA2DS2-VASc scores.
These findings raise important unanswered questions about the relationship between AF and stroke. Conventional wisdom has held that left atrial thrombus is the cause of most strokes in patients with AF. But it’s not that simple, Dr. Bernard J. Gersh asserted at the Annual Cardiovascular Conference at Snowmass.
"What are we measuring with these biomarkers? This is what we really don’t understand. What has high-sensitivity troponin T got to do with left atrial thrombus?" asked Dr. Gersh, professor of medicine at the Mayo Clinic in Rochester, Minn.
It seems increasingly clear that it’s not just the atrial arrhythmia that’s important in stroke risk, it’s also the company AF keeps. In a substantial but still uncertain proportion of patients, AF is a marker of vascular disease burden expressed through atrial and vascular endothelial dysfunction, vascular inflammation, left atrial dilatation and fibrosis, and a hypercoagulable state, the cardiologist continued.
He was a coinvestigator on a couple of recent groundbreaking studies that show the prognostic power of biomarkers in predicting both stroke risk and cardiac death in AF patients.
In one report, the investigators looked at baseline high-sensitivity troponin T (hsTnT) levels, clinical risk factors for stroke, and CHA2DS2-VASc scores in 12,892 patients with AF who were randomized to apixaban or warfarin in the prospective, double-blind ARISTOTLE (Apixaban for the Prevention of Stroke in Subjects With Atrial Fibrillation) trial (N. Engl. J. Med. 2011;365:981-92). During a median 1.9 years of follow-up, patients in the highest quartile for baseline hsTnT had roughly a twofold greater risk of stroke or systemic embolism than did those in the lowest quartile.
Moreover, patients with a low-risk CHA2DS2-VASc score of 0 or 1 but in the top quartile for hsTnT, with a level in excess of 13 ng/L, had a very substantial stroke rate of 2.7% per year despite anticoagulation with apixaban (Eliquis) or warfarin. The relationship was even stronger for cardiac death, where subjects with a low-risk CHA2DS2-VASc score who were in the top quartile for hsTnT had a 6% annual risk. A higher baseline hsTnT was also independently associated with sharply increased risk of major bleeding in a multivariate regression analysis (J. Am. Coll. Cardiol. 2014;63:52-61).
In another recently published study, he and his international coworkers showed in ARISTOTLE participants with baseline N-terminal pro-B-type natriuretic peptide (NT-proBNP) levels that this biomarker also improved stroke prediction in AF, providing added value to CHA2DS2-VASc scores. Subjects in the top quartile for baseline NT-proBNP had an adjusted 2.35-fold greater risk of stroke or systemic embolism than those in the lowest quartile, irrespective of CHA2DS2-VASc score. They also had a 2.5-fold greater risk of cardiac death (J. Am. Coll. Cardiol. 2013;61:2274-84).
A study Dr. Gersh highlighted as "extremely interesting" involved the use of brain natriuretic peptide (BNP) as a marker to rule out delayed AF in stroke patients. The study, led by investigators at the University Hospital Center of Nice (France) and known as TARGET-AF, included 300 consecutive acute stroke patients with no history of AF and no AF on their baseline ECG. During a median 6.8 days of in-hospital Holter monitoring, 17% of the stroke patients developed newly diagnosed AF.
The strongest predictor of delayed AF was baseline plasma BNP. It outperformed the CHA2DS2-VASc score and all the other parameters examined, including anterior circulation location of the stroke, P-wave initial force, gender, National Institutes of Health Stroke Scale score, age, left atrial dilatation, and Score for the Targeting of AF (STAF) score. A BNP level greater than 131 pg/mL had a 98.1% sensitivity, 71.4% specificity, and 99.4% negative predictive value for delayed AF.
"Our data indicate that a BNP level of 131 pg/mL or less might rule out delayed AF in stroke survivors and could be included in algorithms for AF detection," the French investigators concluded (J. Stroke Cerebrovasc. Dis. 2013;22:e103-10).
There is plenty of direct evidence from transesophageal echocardiography studies and other sources that a substantial proportion of thromboemboli are directly the result of AF. However, indirect evidence points to additional causal factors. For example, there is a high incidence of thromboembolic events in AF patients without left atrial appendage thrombus. Plus, in natural history studies patients with AF without additional risk factors have a low incidence of stroke. And CHADS2 and CHA2DS2-VASc scores predict vascular events but don’t correlate with left atrial appendage thrombus, Dr. Gersh noted.
He said the CHA2DS2-VASc score is clearly an improvement over CHADS2, and its adoption in the forthcoming ACC/AHA guidelines is to be welcomed. The CHA2DS2-VASc score increases the number of patients considered at significant risk of stroke and therefore warranting anticoagulation. For example, in a large Danish registry of nearly 48,000 AF patients with a CHADS2 score of 0-1 not on anticoagulation, patients with a CHADS2 score of 1 but a CHA2DS2-VASc score of 2 had twice the stroke risk of patients with a CHA2DS2-VASc of 1 (Thromb. Haemost. 2012;107:1172-9).
That being said, neither risk score is all that impressive. The C-statistic, a measure of a test’s predictive power, is 0.56 for CHADS2 and it was 0.62 for CHA2DS2-VASc in the ARISTOTLE analysis. To put those figures in perspective, a coin toss has a C-statistic of 0.50.
"The individual predictive values are not good. We use CHADS2 and CHA2DS2-VASc in practice and in the guidelines, but we should not pretend they are highly predictive. We need new risk stratification schemes," according to Dr. Gersh.
He reported serving as an adviser to Boston Scientific and St. Jude Medical.
EXPERT ANALYSIS FROM THE CARDIOVASCULAR CONFERENCE AT SNOWMASS
Aldosterone agonists earn new respect in HF guidelines
SNOWMASS, COLO. – The latest heart failure guidelines from the American College of Cardiology/American Heart Association place a new emphasis on aldosterone antagonists as a central aspect of the management of symptomatic or previously symptomatic heart failure with reduced ejection fraction – while underscoring important caveats to their use.
Aldosterone antagonist therapy earns the strongest possible designation in the guidelines: a Class I/Level of Evidence A recommendation. This is based on data from multiple randomized trials showing that, used appropriately, these agents result in a 30% relative risk reduction in mortality and a 35% reduction in the relative risk of heart failure hospitalization, with a number needed to treat for 36 months of just six patients to prevent one additional death. Those figures place the aldosterone antagonists on a par with the other Class I/A heart failure medications – beta-blockers, ACE inhibitors or angiotensin receptor blockers, and hydralazine/isosorbide dinitrate in African Americans – in terms of benefits (see chart).

"These data are quite striking," Dr. Clyde W. Yancy observed in presenting highlights of the 2013 ACC/AHA guidelines at the Annual Cardiovascular Conference at Snowmass.
"For many years, we’ve functioned in a space where we thought there’s not that much we can do for heart failure, and I would now argue stridently against that. You can see the incredibly low numbers needed to treat here. Only a handful of patients need to be exposed to these therapies to derive a significant benefit on mortality. These are data we should incorporate in our clinical practice without exclusion," declared Dr. Yancy, who chaired the heart failure guideline-writing committee.
The important caveat regarding the aldosterone antagonists is that they should be used only in patients with an estimated glomerular filtration rate greater than 30 mL/min per 1.73 m2 and a serum potassium level below 5.0 mEq/dL. Otherwise that Class I/A recommendation plummets to III/B, meaning the treatment is inappropriate and potentially harmful, continued Dr. Yancy, professor of medicine and of medical social sciences and chief of cardiology at Northwestern University, Chicago.
The guidelines emphasize the imperative to implement what has come to be termed guideline-directed medical therapy, known by the abbreviation GDMT. The panel found persuasive an analysis showing that heart failure patients with reduced ejection fraction who were on two of seven evidence-based, guideline-directed management interventions had an adjusted 38% reduction in 2-year mortality risk compared with those on none or one, while those on three interventions had a 62% decrease in the odds of mortality and patients on four or more had mortality reductions of about 70% (J. Am. Heart Assoc. 2012;1:16-26).
The seven interventions are beta-blockers, ACE inhibitors or ARBs, aldosterone antagonists, anticoagulation for atrial fibrillation, cardiac resynchronization therapy, implantable cardioverter-defibrillators, and heart failure education for eligible patients.
The guidelines advise strongly against the combined use of an ACE inhibitor and ARB. It’s an either/or treatment strategy. Studies indicate there is no additive benefit with the combination, only an increased risk of side effects.
An important innovation in the guidelines is the new prominence afforded to heart failure with preserved ejection fraction, known as HFpEF (pronounced heff-peff).
"What’s most different in the new heart failure guidelines is that we have uploaded HFpEF to the front page," said Dr. Yancy. "We want you to appreciate how important it is. We recognize that there’s no evidence-based intervention that changes its natural history; rather, the focus is on identification and treatment of the comorbidities. It’s important to emphasize that this is a novel way of thinking about heart failure for a very important iteration of that disease."
Among the other highlights of the guidelines is a clarification of the current role for biomarker-guided heart failure therapy. B-type natriuretic peptide (BNP) or N-terminal pro-BNP measurements are deemed useful in making the diagnosis of heart failure as well as in establishing prognosis.
Serial measurements can be used to titrate GDMT to optimal doses. But there are as yet no data to show that using the biomarkers to titrate GDMT to higher doses improves mortality.
The 2013 ACC/AHA Guideline for the Management of Heart Failure was developed in collaboration with the American Academy of Family Physicians, the American College of Chest Physicians, the Heart Rhythm Society, and the International Society for Heart and Lung Transplantation (J. Am. Coll. Cardiol. 2013;62:e147-239).
Dr. Yancy reported having no financial conflicts.
Dr. Jun Chiong, FCCP, comments: The new 2013 ACCF/AHA Guideline for the Management of Heart Failure provides a fresh and comprehensive guide to evaluation and management of heart failure patients. The guideline has new areas that are going to be quite helpful for the providers. The indications for aldosterone antagonists are broadened for symptomatic HFpEF patients NYHA class II, III, and IV patients. Creatinine values needed to be = 2.5 mg/dL in men or= 2.0 mg/dL in women, and potassium =5.0 mEq/L are highlighted along with the necessity for careful monitoring of potassium, renal function, and diuretic dosing at initiation follow-up. Routine combined use of an ACE inhibitor, ARB, and aldosterone antagonist is considered potentially harmful and is not recommended.
SNOWMASS, COLO. – The latest heart failure guidelines from the American College of Cardiology/American Heart Association place a new emphasis on aldosterone antagonists as a central aspect of the management of symptomatic or previously symptomatic heart failure with reduced ejection fraction – while underscoring important caveats to their use.
Aldosterone antagonist therapy earns the strongest possible designation in the guidelines: a Class I/Level of Evidence A recommendation. This is based on data from multiple randomized trials showing that, used appropriately, these agents result in a 30% relative risk reduction in mortality and a 35% reduction in the relative risk of heart failure hospitalization, with a number needed to treat for 36 months of just six patients to prevent one additional death. Those figures place the aldosterone antagonists on a par with the other Class I/A heart failure medications – beta-blockers, ACE inhibitors or angiotensin receptor blockers, and hydralazine/isosorbide dinitrate in African Americans – in terms of benefits (see chart).

"These data are quite striking," Dr. Clyde W. Yancy observed in presenting highlights of the 2013 ACC/AHA guidelines at the Annual Cardiovascular Conference at Snowmass.
"For many years, we’ve functioned in a space where we thought there’s not that much we can do for heart failure, and I would now argue stridently against that. You can see the incredibly low numbers needed to treat here. Only a handful of patients need to be exposed to these therapies to derive a significant benefit on mortality. These are data we should incorporate in our clinical practice without exclusion," declared Dr. Yancy, who chaired the heart failure guideline-writing committee.
The important caveat regarding the aldosterone antagonists is that they should be used only in patients with an estimated glomerular filtration rate greater than 30 mL/min per 1.73 m2 and a serum potassium level below 5.0 mEq/dL. Otherwise that Class I/A recommendation plummets to III/B, meaning the treatment is inappropriate and potentially harmful, continued Dr. Yancy, professor of medicine and of medical social sciences and chief of cardiology at Northwestern University, Chicago.
The guidelines emphasize the imperative to implement what has come to be termed guideline-directed medical therapy, known by the abbreviation GDMT. The panel found persuasive an analysis showing that heart failure patients with reduced ejection fraction who were on two of seven evidence-based, guideline-directed management interventions had an adjusted 38% reduction in 2-year mortality risk compared with those on none or one, while those on three interventions had a 62% decrease in the odds of mortality and patients on four or more had mortality reductions of about 70% (J. Am. Heart Assoc. 2012;1:16-26).
The seven interventions are beta-blockers, ACE inhibitors or ARBs, aldosterone antagonists, anticoagulation for atrial fibrillation, cardiac resynchronization therapy, implantable cardioverter-defibrillators, and heart failure education for eligible patients.
The guidelines advise strongly against the combined use of an ACE inhibitor and ARB. It’s an either/or treatment strategy. Studies indicate there is no additive benefit with the combination, only an increased risk of side effects.
An important innovation in the guidelines is the new prominence afforded to heart failure with preserved ejection fraction, known as HFpEF (pronounced heff-peff).
"What’s most different in the new heart failure guidelines is that we have uploaded HFpEF to the front page," said Dr. Yancy. "We want you to appreciate how important it is. We recognize that there’s no evidence-based intervention that changes its natural history; rather, the focus is on identification and treatment of the comorbidities. It’s important to emphasize that this is a novel way of thinking about heart failure for a very important iteration of that disease."
Among the other highlights of the guidelines is a clarification of the current role for biomarker-guided heart failure therapy. B-type natriuretic peptide (BNP) or N-terminal pro-BNP measurements are deemed useful in making the diagnosis of heart failure as well as in establishing prognosis.
Serial measurements can be used to titrate GDMT to optimal doses. But there are as yet no data to show that using the biomarkers to titrate GDMT to higher doses improves mortality.
The 2013 ACC/AHA Guideline for the Management of Heart Failure was developed in collaboration with the American Academy of Family Physicians, the American College of Chest Physicians, the Heart Rhythm Society, and the International Society for Heart and Lung Transplantation (J. Am. Coll. Cardiol. 2013;62:e147-239).
Dr. Yancy reported having no financial conflicts.
Dr. Jun Chiong, FCCP, comments: The new 2013 ACCF/AHA Guideline for the Management of Heart Failure provides a fresh and comprehensive guide to evaluation and management of heart failure patients. The guideline has new areas that are going to be quite helpful for the providers. The indications for aldosterone antagonists are broadened for symptomatic HFpEF patients NYHA class II, III, and IV patients. Creatinine values needed to be = 2.5 mg/dL in men or= 2.0 mg/dL in women, and potassium =5.0 mEq/L are highlighted along with the necessity for careful monitoring of potassium, renal function, and diuretic dosing at initiation follow-up. Routine combined use of an ACE inhibitor, ARB, and aldosterone antagonist is considered potentially harmful and is not recommended.
SNOWMASS, COLO. – The latest heart failure guidelines from the American College of Cardiology/American Heart Association place a new emphasis on aldosterone antagonists as a central aspect of the management of symptomatic or previously symptomatic heart failure with reduced ejection fraction – while underscoring important caveats to their use.
Aldosterone antagonist therapy earns the strongest possible designation in the guidelines: a Class I/Level of Evidence A recommendation. This is based on data from multiple randomized trials showing that, used appropriately, these agents result in a 30% relative risk reduction in mortality and a 35% reduction in the relative risk of heart failure hospitalization, with a number needed to treat for 36 months of just six patients to prevent one additional death. Those figures place the aldosterone antagonists on a par with the other Class I/A heart failure medications – beta-blockers, ACE inhibitors or angiotensin receptor blockers, and hydralazine/isosorbide dinitrate in African Americans – in terms of benefits (see chart).

"These data are quite striking," Dr. Clyde W. Yancy observed in presenting highlights of the 2013 ACC/AHA guidelines at the Annual Cardiovascular Conference at Snowmass.
"For many years, we’ve functioned in a space where we thought there’s not that much we can do for heart failure, and I would now argue stridently against that. You can see the incredibly low numbers needed to treat here. Only a handful of patients need to be exposed to these therapies to derive a significant benefit on mortality. These are data we should incorporate in our clinical practice without exclusion," declared Dr. Yancy, who chaired the heart failure guideline-writing committee.
The important caveat regarding the aldosterone antagonists is that they should be used only in patients with an estimated glomerular filtration rate greater than 30 mL/min per 1.73 m2 and a serum potassium level below 5.0 mEq/dL. Otherwise that Class I/A recommendation plummets to III/B, meaning the treatment is inappropriate and potentially harmful, continued Dr. Yancy, professor of medicine and of medical social sciences and chief of cardiology at Northwestern University, Chicago.
The guidelines emphasize the imperative to implement what has come to be termed guideline-directed medical therapy, known by the abbreviation GDMT. The panel found persuasive an analysis showing that heart failure patients with reduced ejection fraction who were on two of seven evidence-based, guideline-directed management interventions had an adjusted 38% reduction in 2-year mortality risk compared with those on none or one, while those on three interventions had a 62% decrease in the odds of mortality and patients on four or more had mortality reductions of about 70% (J. Am. Heart Assoc. 2012;1:16-26).
The seven interventions are beta-blockers, ACE inhibitors or ARBs, aldosterone antagonists, anticoagulation for atrial fibrillation, cardiac resynchronization therapy, implantable cardioverter-defibrillators, and heart failure education for eligible patients.
The guidelines advise strongly against the combined use of an ACE inhibitor and ARB. It’s an either/or treatment strategy. Studies indicate there is no additive benefit with the combination, only an increased risk of side effects.
An important innovation in the guidelines is the new prominence afforded to heart failure with preserved ejection fraction, known as HFpEF (pronounced heff-peff).
"What’s most different in the new heart failure guidelines is that we have uploaded HFpEF to the front page," said Dr. Yancy. "We want you to appreciate how important it is. We recognize that there’s no evidence-based intervention that changes its natural history; rather, the focus is on identification and treatment of the comorbidities. It’s important to emphasize that this is a novel way of thinking about heart failure for a very important iteration of that disease."
Among the other highlights of the guidelines is a clarification of the current role for biomarker-guided heart failure therapy. B-type natriuretic peptide (BNP) or N-terminal pro-BNP measurements are deemed useful in making the diagnosis of heart failure as well as in establishing prognosis.
Serial measurements can be used to titrate GDMT to optimal doses. But there are as yet no data to show that using the biomarkers to titrate GDMT to higher doses improves mortality.
The 2013 ACC/AHA Guideline for the Management of Heart Failure was developed in collaboration with the American Academy of Family Physicians, the American College of Chest Physicians, the Heart Rhythm Society, and the International Society for Heart and Lung Transplantation (J. Am. Coll. Cardiol. 2013;62:e147-239).
Dr. Yancy reported having no financial conflicts.
Dr. Jun Chiong, FCCP, comments: The new 2013 ACCF/AHA Guideline for the Management of Heart Failure provides a fresh and comprehensive guide to evaluation and management of heart failure patients. The guideline has new areas that are going to be quite helpful for the providers. The indications for aldosterone antagonists are broadened for symptomatic HFpEF patients NYHA class II, III, and IV patients. Creatinine values needed to be = 2.5 mg/dL in men or= 2.0 mg/dL in women, and potassium =5.0 mEq/L are highlighted along with the necessity for careful monitoring of potassium, renal function, and diuretic dosing at initiation follow-up. Routine combined use of an ACE inhibitor, ARB, and aldosterone antagonist is considered potentially harmful and is not recommended.
Colchicine may provide potent cardiac protection
SNOWMASS, COLO. – Evidence from three observational studies suggests colchicine has a strong protective effect against cardiovascular events in gout patients.
These data add to mounting evidence that the venerable 2,400-year-old medication also reduces the incidence of cardiac events in patients at elevated risk who don’t have gout, Dr. Michael H. Pillinger said at the Winter Rheumatology Symposium sponsored by the American College of Rheumatology.
He was a coinvestigator in the three observational studies, two of which are ongoing with only interim results available.
The first of these observational studies was a retrospective, cross-sectional pilot study of 1,288 gout patients in the New York Harbor Healthcare System Veterans Affairs database. The demographics, baseline comorbidities, and cardiovascular risk factors in the 576 colchicine users and 712 nonusers were closely similar. The key finding in this snapshot study: the prevalence of a history of acute MI was 1.2% in the colchicine users, compared with 2.6% in the non-users with gout, for a significant 54% relative risk reduction (J. Rheumatol. 2012;39:1458-64).
"That degree of risk reduction seems too good to be believed, and it probably is," according to Dr. Pillinger, a rheumatologist and director of the crystal diseases study group at New York University.
But the next observational study showed a similar-size benefit. This was a retrospective cohort study of New York VA gout patients. It included only gout patients who met American College of Rheumatology diagnostic criteria as confirmed by manual chart review. There were 410 colchicine users with a collective 1,184 years of active use and another 682 years of lapse time, along with 234 colchicine nonusers with 1,041 years of follow-up time. Again, baseline demographics and comorbidities were remarkably similar for the two groups.
In an interim analysis, the incidence of acute MI was 0.7% among active users of colchicine, 2.0% in lapsed former users, and 3.1% in the nonuser controls. This translated to an incidence rate of 0.003 MIs per person-year in the colchicine users, 0.007 per person-year in the controls, and 0.009 MIs per person-year during a combined 1,723 person-years in the combined control group plus lapsed former users, for relative risk reductions of 57% and 67%, respectively. Still, the final results aren’t in yet, and this study is limited by a small number of events to date, its retrospective design, and the potential for confounding by indication, Dr. Pillinger noted.
Gout patients on colchicine in these two VA studies were on 0.6-1.2 mg/day rather than the now-standard 0.5 mg.
The latest observational study is a retrospective cohort study being conducted in collaboration with Dr. Peter Berger, chair of cardiology at the Geisinger Health System in Danville, Pa. To date, it includes 3,064 gout patients. The MI incidence thus far is 6.3/100 person-years in the colchicine users and 11.2/100 person-years among lapsed users. After controlling for potential confounders such as age, hypertension, and diabetes in a logistic regression analysis, however, the trend for reduced MI risk in the colchicine users hasn’t yet reached significance. Stay tuned, Dr. Pillinger said.
The mechanistic rationale by which colchicine might reduce cardiovascular events in gout patients lies in the fact that it is an anti-inflammatory drug and atherosclerosis is a powerfully inflammatory process. Colchicine is known to suppress production of TNF-alpha, interleukin-1beta, and other inflammatory cytokines by neutrophils, macrophages, and endothelial cells. These cell types are present in atherosclerotic plaque, the rheumatologist explained.
By the same rationale, colchicine might well be cardioprotective in individuals without gout. One strong piece of supporting evidence comes from a 3-year, randomized, observer-blinded clinical trial in which 532 Australian patients with stable coronary artery disease on background statin and antiplatelet therapy received 0.5 mg/day of colchicine or not. The composite primary endpoint comprised acute coronary syndrome, out-of-hospital cardiac arrest, or noncardioembolic ischemic stroke occurred in 5.3% of the colchicine group, compared with 16.0% of controls. That’s a 67% relative risk reduction, with a highly favorable number-needed-to-treat of 11 (J. Am. Coll. Cardiol. 2013;61:404-10).
Dr. Pillinger reported being the recipient of research grants from Takeda, which markets colchicine (Colcrys), and Savient, which markets the gout drug pegloticase (Krystexxa).
SNOWMASS, COLO. – Evidence from three observational studies suggests colchicine has a strong protective effect against cardiovascular events in gout patients.
These data add to mounting evidence that the venerable 2,400-year-old medication also reduces the incidence of cardiac events in patients at elevated risk who don’t have gout, Dr. Michael H. Pillinger said at the Winter Rheumatology Symposium sponsored by the American College of Rheumatology.
He was a coinvestigator in the three observational studies, two of which are ongoing with only interim results available.
The first of these observational studies was a retrospective, cross-sectional pilot study of 1,288 gout patients in the New York Harbor Healthcare System Veterans Affairs database. The demographics, baseline comorbidities, and cardiovascular risk factors in the 576 colchicine users and 712 nonusers were closely similar. The key finding in this snapshot study: the prevalence of a history of acute MI was 1.2% in the colchicine users, compared with 2.6% in the non-users with gout, for a significant 54% relative risk reduction (J. Rheumatol. 2012;39:1458-64).
"That degree of risk reduction seems too good to be believed, and it probably is," according to Dr. Pillinger, a rheumatologist and director of the crystal diseases study group at New York University.
But the next observational study showed a similar-size benefit. This was a retrospective cohort study of New York VA gout patients. It included only gout patients who met American College of Rheumatology diagnostic criteria as confirmed by manual chart review. There were 410 colchicine users with a collective 1,184 years of active use and another 682 years of lapse time, along with 234 colchicine nonusers with 1,041 years of follow-up time. Again, baseline demographics and comorbidities were remarkably similar for the two groups.
In an interim analysis, the incidence of acute MI was 0.7% among active users of colchicine, 2.0% in lapsed former users, and 3.1% in the nonuser controls. This translated to an incidence rate of 0.003 MIs per person-year in the colchicine users, 0.007 per person-year in the controls, and 0.009 MIs per person-year during a combined 1,723 person-years in the combined control group plus lapsed former users, for relative risk reductions of 57% and 67%, respectively. Still, the final results aren’t in yet, and this study is limited by a small number of events to date, its retrospective design, and the potential for confounding by indication, Dr. Pillinger noted.
Gout patients on colchicine in these two VA studies were on 0.6-1.2 mg/day rather than the now-standard 0.5 mg.
The latest observational study is a retrospective cohort study being conducted in collaboration with Dr. Peter Berger, chair of cardiology at the Geisinger Health System in Danville, Pa. To date, it includes 3,064 gout patients. The MI incidence thus far is 6.3/100 person-years in the colchicine users and 11.2/100 person-years among lapsed users. After controlling for potential confounders such as age, hypertension, and diabetes in a logistic regression analysis, however, the trend for reduced MI risk in the colchicine users hasn’t yet reached significance. Stay tuned, Dr. Pillinger said.
The mechanistic rationale by which colchicine might reduce cardiovascular events in gout patients lies in the fact that it is an anti-inflammatory drug and atherosclerosis is a powerfully inflammatory process. Colchicine is known to suppress production of TNF-alpha, interleukin-1beta, and other inflammatory cytokines by neutrophils, macrophages, and endothelial cells. These cell types are present in atherosclerotic plaque, the rheumatologist explained.
By the same rationale, colchicine might well be cardioprotective in individuals without gout. One strong piece of supporting evidence comes from a 3-year, randomized, observer-blinded clinical trial in which 532 Australian patients with stable coronary artery disease on background statin and antiplatelet therapy received 0.5 mg/day of colchicine or not. The composite primary endpoint comprised acute coronary syndrome, out-of-hospital cardiac arrest, or noncardioembolic ischemic stroke occurred in 5.3% of the colchicine group, compared with 16.0% of controls. That’s a 67% relative risk reduction, with a highly favorable number-needed-to-treat of 11 (J. Am. Coll. Cardiol. 2013;61:404-10).
Dr. Pillinger reported being the recipient of research grants from Takeda, which markets colchicine (Colcrys), and Savient, which markets the gout drug pegloticase (Krystexxa).
SNOWMASS, COLO. – Evidence from three observational studies suggests colchicine has a strong protective effect against cardiovascular events in gout patients.
These data add to mounting evidence that the venerable 2,400-year-old medication also reduces the incidence of cardiac events in patients at elevated risk who don’t have gout, Dr. Michael H. Pillinger said at the Winter Rheumatology Symposium sponsored by the American College of Rheumatology.
He was a coinvestigator in the three observational studies, two of which are ongoing with only interim results available.
The first of these observational studies was a retrospective, cross-sectional pilot study of 1,288 gout patients in the New York Harbor Healthcare System Veterans Affairs database. The demographics, baseline comorbidities, and cardiovascular risk factors in the 576 colchicine users and 712 nonusers were closely similar. The key finding in this snapshot study: the prevalence of a history of acute MI was 1.2% in the colchicine users, compared with 2.6% in the non-users with gout, for a significant 54% relative risk reduction (J. Rheumatol. 2012;39:1458-64).
"That degree of risk reduction seems too good to be believed, and it probably is," according to Dr. Pillinger, a rheumatologist and director of the crystal diseases study group at New York University.
But the next observational study showed a similar-size benefit. This was a retrospective cohort study of New York VA gout patients. It included only gout patients who met American College of Rheumatology diagnostic criteria as confirmed by manual chart review. There were 410 colchicine users with a collective 1,184 years of active use and another 682 years of lapse time, along with 234 colchicine nonusers with 1,041 years of follow-up time. Again, baseline demographics and comorbidities were remarkably similar for the two groups.
In an interim analysis, the incidence of acute MI was 0.7% among active users of colchicine, 2.0% in lapsed former users, and 3.1% in the nonuser controls. This translated to an incidence rate of 0.003 MIs per person-year in the colchicine users, 0.007 per person-year in the controls, and 0.009 MIs per person-year during a combined 1,723 person-years in the combined control group plus lapsed former users, for relative risk reductions of 57% and 67%, respectively. Still, the final results aren’t in yet, and this study is limited by a small number of events to date, its retrospective design, and the potential for confounding by indication, Dr. Pillinger noted.
Gout patients on colchicine in these two VA studies were on 0.6-1.2 mg/day rather than the now-standard 0.5 mg.
The latest observational study is a retrospective cohort study being conducted in collaboration with Dr. Peter Berger, chair of cardiology at the Geisinger Health System in Danville, Pa. To date, it includes 3,064 gout patients. The MI incidence thus far is 6.3/100 person-years in the colchicine users and 11.2/100 person-years among lapsed users. After controlling for potential confounders such as age, hypertension, and diabetes in a logistic regression analysis, however, the trend for reduced MI risk in the colchicine users hasn’t yet reached significance. Stay tuned, Dr. Pillinger said.
The mechanistic rationale by which colchicine might reduce cardiovascular events in gout patients lies in the fact that it is an anti-inflammatory drug and atherosclerosis is a powerfully inflammatory process. Colchicine is known to suppress production of TNF-alpha, interleukin-1beta, and other inflammatory cytokines by neutrophils, macrophages, and endothelial cells. These cell types are present in atherosclerotic plaque, the rheumatologist explained.
By the same rationale, colchicine might well be cardioprotective in individuals without gout. One strong piece of supporting evidence comes from a 3-year, randomized, observer-blinded clinical trial in which 532 Australian patients with stable coronary artery disease on background statin and antiplatelet therapy received 0.5 mg/day of colchicine or not. The composite primary endpoint comprised acute coronary syndrome, out-of-hospital cardiac arrest, or noncardioembolic ischemic stroke occurred in 5.3% of the colchicine group, compared with 16.0% of controls. That’s a 67% relative risk reduction, with a highly favorable number-needed-to-treat of 11 (J. Am. Coll. Cardiol. 2013;61:404-10).
Dr. Pillinger reported being the recipient of research grants from Takeda, which markets colchicine (Colcrys), and Savient, which markets the gout drug pegloticase (Krystexxa).
EXPERT ANALYSIS FROM THE WINTER RHEUMATOLOGY SYMPOSIUM
Sublingual immunotherapy is coming soon
KEYSTONE, COLO. – Sublingual immunotherapy is finally coming.
Allergy therapy using rapidly dissolving oral tablets instead of subcutaneous injections has been approved in Europe for years. With Food and Drug Administration approval of sublingual immunotherapy tablets for the treatment of grass and ragweed allergies considered highly likely later this spring, the expectation is that patients, their referring physicians, and allergists will have many questions about this game-changing therapeutic innovation.
Dr. Harold S. Nelson, who closely follows developments in the field, provided answers at a meeting on allergy and respiratory diseases sponsored by National Jewish Health.
Among his key points:
• The effectiveness of sublingual immunotherapy (SLIT) for allergic rhinitis and allergic asthma is now thoroughly established. So are the optimal dosing regimens: SLIT tablets are dosed once daily at 30 times the optimal subcutaneous immunotherapy (SCIT) once-monthly maintenance dose. In other words, over the course of a month, a patient on SLIT will take a roughly 30 times greater dose of grass or ragweed allergen than will a patient on SCIT.
• SLIT for grass allergy will be approved for patients aged 5-65, while SLIT for ragweed will receive an indication for 18- to 65-year-olds.
• SLIT, like conventional subcutaneous immunotherapy, is disease-modifying therapy, which prevents new sensitization and progression to asthma.
• The optimal duration of SLIT is 3-4 years, which typically produces 7-8 years of persisting benefit before retreatment is needed.
• SCIT results in faster clinical improvement than does SLIT. And at least through the first 12-15 months, SCIT also appears to be significantly more effective.
• The use of SLIT in combination with mixes of other readily available pollen extracts is not supported by any evidence of efficacy.
• The big advantages SLIT offers over SCIT are convenience and safety. Although in U.S. clinical trials 1 in every 200-300 SLIT-treated patients experienced mild systemic reactions – typically with the first dose no fatal or near-fatal anaphylactic reactions have occurred. That’s why SLIT will be approved for at-home use after a first in-office observed dose. However, the FDA will mandate that SLIT prescriptions be accompanied by coprescription of an epinephrine autoinjector, according to Dr. Nelson of National Jewish Health in Denver and professor of medicine at the University of Colorado at Denver.
Once SLIT products win FDA approval, the therapy will get a CPT code and become, for the first time, a billable treatment – a most welcome development. But Dr. Nelson emphasized that SLIT’s approval will also create a new dilemma for physicians and their many patients with multiple allergies, say, to trees, dogs, and molds in addition to grasses or ragweed.
"Something everybody’s going to have to decide is where to position this treatment," Dr. Nelson said. "Most of the companies have no plans to take SLIT beyond the standardized extracts, which means grass, ragweed, house dust mite, and cat. You’re probably never going to have SLIT for cottonwood or juniper. And it seems unlikely that anyone is going to put a patient on tablets and injections at the same time. So it’s a decision that will have to be made for every patient: whether the ability to treat grass and ragweed, and later, house dust mite and cat, is sufficient for that patient. Because if it’s not, then probably the patient is still a candidate for SCIT."
The strategy of the companies developing SLIT is not that oral therapy is supposed to be a replacement for SCIT, but rather that it provides an immunotherapy option for patients who currently don’t receive it because they balk at the inconvenience of monthly in-office injections, he continued.
"The idea is that if these people are told, ‘You can just take a tablet at home,’ they’ll opt to get at least their allergies to grass and ragweed treated," Dr. Nelson explained.
Compliance and treatment persistence are going to be issues with SLIT, as documented in a Dutch retrospective study of 3,690 patients placed on SLIT and 2,796 who received SCIT. Only 23% of patients on SCIT stayed on treatment for the recommended 3 years. While that’s hardly a stellar adherence rate, it was actually more than three times better than with SLIT, where the rate was just 7%. The median duration of adherence with SCIT was 1.7 years, compared with 0.6 years for SLIT. The main reason patients stopped SCIT was the inconvenience, while the No. 1 reason people gave up on SLIT was ineffectiveness (J. Allergy Clin. Immunol. 2013;132:353-60).
To be fair, the Dutch study is a worst-case scenario for SLIT, according to Dr. Nelson. There are data showing adherence to SLIT is best when patients are routinely seen in the office every 3 months, apparently not the case in the Dutch study.
In a soon-to-be-published report, Dr. Nelson has reviewed 11 randomized head-to-head-comparison studies of SLIT versus SCIT and found them consistently uninformative. Most often, the deck was stacked against SLIT because it was given only three times per week and/or in too-low doses. In his view, there is only one enlightening comparative study, a recent randomized trial in which 40 Danish patients allergic to grass pollen received optimally dosed SLIT, SCIT, or neither for 15 months, with the same company’s standardized injectable and tablet Timothy grass preparations being used.
After 15 months, both treatments were effective, clinically as well as immunologically, compared with the no-treatment controls, with the benefits becoming significant in the first 1-3 months. However, the improvements in IgG4, IgE-blocking factor, facilitated antigen presentation, and the basal activation test were generally twice as great in the SCIT group. Moreover, the symptomatic response to nasal challenge – the only measure of clinical response utilized in the study was significantly better than in controls only with SCIT (Clin. Exp. Allergy 2014;44:417-28).
"This is the best comparative study we have, and it may be the best we’ll get. Here both treatments are being given optimally, and it’s very clear that at least in the first year, SCIT beats SLIT. It looks as though SLIT is trying to catch up late but doesn’t quite get there through 15 months. The investigators have stored frozen cells, so we can look forward to data on changes in regulatory T cells and suppression of Th2 cells in further publications," Dr. Nelson said.
Of note, an analysis of seven phase III clinical trials totaling nearly 2,700 adults and children showed that roughly half of them experienced transient local adverse reactions to grass SLIT. The reactions usually began on day 1, lasted 30-60 minutes, and recurred with the first seven or so daily doses. The reactions predominantly involved itching of the mouth or throat. About 10% of patients reported a sensation of swelling in the mouth that wasn’t visible to observers and tended to last longer than a week.
"Some people are surprised at the high incidence of these local, transient adverse reactions," he commented.
A common practice among American allergists is off-label sublingual administration of mixtures of eight or more pollen extracts. But a randomized, double-blind, placebo-controlled clinical trial in which Dr. Nelson was senior coinvestigator suggested this may be counterproductive when such mixtures are given in conjunction with Timothy pollen extract. A SLIT combination of Timothy pollen and nine additional pollen allergen extracts performed significantly worse than Timothy alone at the same dose; in fact, it failed to outdistance placebo in most endpoints (J. Allergy Clin. Immunol. 2009;124:150-6).
"This was a small, 56-patient study that clearly needs to be replicated, but there’s no financial backing for it. This is rather critical, since many people doing off-label sublingual immunotherapy using multiple allergen extracts think that they know what they’re doing. I hope I’ve made the point that they don’t know what dose to use, and there’s no evidence that multiple allergen mixes are really effective," Dr. Nelson said.
Grass allergies are the most common seasonal allergies in the United States. The three standardized SLIT products under FDA review, all of which have been approved in Europe for years, are Grastek, a Timothy grass extract, and Ragwitek, both developed by Merck in partnership with ALK of Denmark, and Oralair, a five-grass product developed by the French company Stallergenes. Oralair, to be marketed in the United States by Greer, contains Timothy grass allergen as well as extracts of four other temperate pasture grasses. Of note, Bermuda and Bahia grasses, common causes of seasonal allergy, aren’t included in Oralair or Grastek.
The companies have pursued different dosing strategies. ALK recommends taking Grastek continuously year-round. Stallergenes recommends starting Oralair a few months before the start of grass allergy season and stopping when the pollen season is over. In one 3-year study, 633 grass-allergic patients were randomized to Oralair or placebo starting 2 or 4 months prior to the pollen season. The reduction in adjusted symptom scores was similar with 2 vs. 4 months of therapy in advance of the allergy season (J. Allergy Clin. Immunol. 2011;128:559-66). That’s an important finding because it means preseasonally treated patients can purchase 8 weeks fewer tablets, the allergist noted.
Dr. Nelson’s prediction that these three SLIT products are headed for FDA approval this spring stems from enthusiastic endorsements by the agency’s Allergenic Products Advisory Committee. The SLIT grass allergy products were recommended unanimously, and the ragweed SLIT also received a strongly favorable vote.
He reported serving as a consultant to Merck, Pearl Therapeutic, and Circassia.
KEYSTONE, COLO. – Sublingual immunotherapy is finally coming.
Allergy therapy using rapidly dissolving oral tablets instead of subcutaneous injections has been approved in Europe for years. With Food and Drug Administration approval of sublingual immunotherapy tablets for the treatment of grass and ragweed allergies considered highly likely later this spring, the expectation is that patients, their referring physicians, and allergists will have many questions about this game-changing therapeutic innovation.
Dr. Harold S. Nelson, who closely follows developments in the field, provided answers at a meeting on allergy and respiratory diseases sponsored by National Jewish Health.
Among his key points:
• The effectiveness of sublingual immunotherapy (SLIT) for allergic rhinitis and allergic asthma is now thoroughly established. So are the optimal dosing regimens: SLIT tablets are dosed once daily at 30 times the optimal subcutaneous immunotherapy (SCIT) once-monthly maintenance dose. In other words, over the course of a month, a patient on SLIT will take a roughly 30 times greater dose of grass or ragweed allergen than will a patient on SCIT.
• SLIT for grass allergy will be approved for patients aged 5-65, while SLIT for ragweed will receive an indication for 18- to 65-year-olds.
• SLIT, like conventional subcutaneous immunotherapy, is disease-modifying therapy, which prevents new sensitization and progression to asthma.
• The optimal duration of SLIT is 3-4 years, which typically produces 7-8 years of persisting benefit before retreatment is needed.
• SCIT results in faster clinical improvement than does SLIT. And at least through the first 12-15 months, SCIT also appears to be significantly more effective.
• The use of SLIT in combination with mixes of other readily available pollen extracts is not supported by any evidence of efficacy.
• The big advantages SLIT offers over SCIT are convenience and safety. Although in U.S. clinical trials 1 in every 200-300 SLIT-treated patients experienced mild systemic reactions – typically with the first dose no fatal or near-fatal anaphylactic reactions have occurred. That’s why SLIT will be approved for at-home use after a first in-office observed dose. However, the FDA will mandate that SLIT prescriptions be accompanied by coprescription of an epinephrine autoinjector, according to Dr. Nelson of National Jewish Health in Denver and professor of medicine at the University of Colorado at Denver.
Once SLIT products win FDA approval, the therapy will get a CPT code and become, for the first time, a billable treatment – a most welcome development. But Dr. Nelson emphasized that SLIT’s approval will also create a new dilemma for physicians and their many patients with multiple allergies, say, to trees, dogs, and molds in addition to grasses or ragweed.
"Something everybody’s going to have to decide is where to position this treatment," Dr. Nelson said. "Most of the companies have no plans to take SLIT beyond the standardized extracts, which means grass, ragweed, house dust mite, and cat. You’re probably never going to have SLIT for cottonwood or juniper. And it seems unlikely that anyone is going to put a patient on tablets and injections at the same time. So it’s a decision that will have to be made for every patient: whether the ability to treat grass and ragweed, and later, house dust mite and cat, is sufficient for that patient. Because if it’s not, then probably the patient is still a candidate for SCIT."
The strategy of the companies developing SLIT is not that oral therapy is supposed to be a replacement for SCIT, but rather that it provides an immunotherapy option for patients who currently don’t receive it because they balk at the inconvenience of monthly in-office injections, he continued.
"The idea is that if these people are told, ‘You can just take a tablet at home,’ they’ll opt to get at least their allergies to grass and ragweed treated," Dr. Nelson explained.
Compliance and treatment persistence are going to be issues with SLIT, as documented in a Dutch retrospective study of 3,690 patients placed on SLIT and 2,796 who received SCIT. Only 23% of patients on SCIT stayed on treatment for the recommended 3 years. While that’s hardly a stellar adherence rate, it was actually more than three times better than with SLIT, where the rate was just 7%. The median duration of adherence with SCIT was 1.7 years, compared with 0.6 years for SLIT. The main reason patients stopped SCIT was the inconvenience, while the No. 1 reason people gave up on SLIT was ineffectiveness (J. Allergy Clin. Immunol. 2013;132:353-60).
To be fair, the Dutch study is a worst-case scenario for SLIT, according to Dr. Nelson. There are data showing adherence to SLIT is best when patients are routinely seen in the office every 3 months, apparently not the case in the Dutch study.
In a soon-to-be-published report, Dr. Nelson has reviewed 11 randomized head-to-head-comparison studies of SLIT versus SCIT and found them consistently uninformative. Most often, the deck was stacked against SLIT because it was given only three times per week and/or in too-low doses. In his view, there is only one enlightening comparative study, a recent randomized trial in which 40 Danish patients allergic to grass pollen received optimally dosed SLIT, SCIT, or neither for 15 months, with the same company’s standardized injectable and tablet Timothy grass preparations being used.
After 15 months, both treatments were effective, clinically as well as immunologically, compared with the no-treatment controls, with the benefits becoming significant in the first 1-3 months. However, the improvements in IgG4, IgE-blocking factor, facilitated antigen presentation, and the basal activation test were generally twice as great in the SCIT group. Moreover, the symptomatic response to nasal challenge – the only measure of clinical response utilized in the study was significantly better than in controls only with SCIT (Clin. Exp. Allergy 2014;44:417-28).
"This is the best comparative study we have, and it may be the best we’ll get. Here both treatments are being given optimally, and it’s very clear that at least in the first year, SCIT beats SLIT. It looks as though SLIT is trying to catch up late but doesn’t quite get there through 15 months. The investigators have stored frozen cells, so we can look forward to data on changes in regulatory T cells and suppression of Th2 cells in further publications," Dr. Nelson said.
Of note, an analysis of seven phase III clinical trials totaling nearly 2,700 adults and children showed that roughly half of them experienced transient local adverse reactions to grass SLIT. The reactions usually began on day 1, lasted 30-60 minutes, and recurred with the first seven or so daily doses. The reactions predominantly involved itching of the mouth or throat. About 10% of patients reported a sensation of swelling in the mouth that wasn’t visible to observers and tended to last longer than a week.
"Some people are surprised at the high incidence of these local, transient adverse reactions," he commented.
A common practice among American allergists is off-label sublingual administration of mixtures of eight or more pollen extracts. But a randomized, double-blind, placebo-controlled clinical trial in which Dr. Nelson was senior coinvestigator suggested this may be counterproductive when such mixtures are given in conjunction with Timothy pollen extract. A SLIT combination of Timothy pollen and nine additional pollen allergen extracts performed significantly worse than Timothy alone at the same dose; in fact, it failed to outdistance placebo in most endpoints (J. Allergy Clin. Immunol. 2009;124:150-6).
"This was a small, 56-patient study that clearly needs to be replicated, but there’s no financial backing for it. This is rather critical, since many people doing off-label sublingual immunotherapy using multiple allergen extracts think that they know what they’re doing. I hope I’ve made the point that they don’t know what dose to use, and there’s no evidence that multiple allergen mixes are really effective," Dr. Nelson said.
Grass allergies are the most common seasonal allergies in the United States. The three standardized SLIT products under FDA review, all of which have been approved in Europe for years, are Grastek, a Timothy grass extract, and Ragwitek, both developed by Merck in partnership with ALK of Denmark, and Oralair, a five-grass product developed by the French company Stallergenes. Oralair, to be marketed in the United States by Greer, contains Timothy grass allergen as well as extracts of four other temperate pasture grasses. Of note, Bermuda and Bahia grasses, common causes of seasonal allergy, aren’t included in Oralair or Grastek.
The companies have pursued different dosing strategies. ALK recommends taking Grastek continuously year-round. Stallergenes recommends starting Oralair a few months before the start of grass allergy season and stopping when the pollen season is over. In one 3-year study, 633 grass-allergic patients were randomized to Oralair or placebo starting 2 or 4 months prior to the pollen season. The reduction in adjusted symptom scores was similar with 2 vs. 4 months of therapy in advance of the allergy season (J. Allergy Clin. Immunol. 2011;128:559-66). That’s an important finding because it means preseasonally treated patients can purchase 8 weeks fewer tablets, the allergist noted.
Dr. Nelson’s prediction that these three SLIT products are headed for FDA approval this spring stems from enthusiastic endorsements by the agency’s Allergenic Products Advisory Committee. The SLIT grass allergy products were recommended unanimously, and the ragweed SLIT also received a strongly favorable vote.
He reported serving as a consultant to Merck, Pearl Therapeutic, and Circassia.
KEYSTONE, COLO. – Sublingual immunotherapy is finally coming.
Allergy therapy using rapidly dissolving oral tablets instead of subcutaneous injections has been approved in Europe for years. With Food and Drug Administration approval of sublingual immunotherapy tablets for the treatment of grass and ragweed allergies considered highly likely later this spring, the expectation is that patients, their referring physicians, and allergists will have many questions about this game-changing therapeutic innovation.
Dr. Harold S. Nelson, who closely follows developments in the field, provided answers at a meeting on allergy and respiratory diseases sponsored by National Jewish Health.
Among his key points:
• The effectiveness of sublingual immunotherapy (SLIT) for allergic rhinitis and allergic asthma is now thoroughly established. So are the optimal dosing regimens: SLIT tablets are dosed once daily at 30 times the optimal subcutaneous immunotherapy (SCIT) once-monthly maintenance dose. In other words, over the course of a month, a patient on SLIT will take a roughly 30 times greater dose of grass or ragweed allergen than will a patient on SCIT.
• SLIT for grass allergy will be approved for patients aged 5-65, while SLIT for ragweed will receive an indication for 18- to 65-year-olds.
• SLIT, like conventional subcutaneous immunotherapy, is disease-modifying therapy, which prevents new sensitization and progression to asthma.
• The optimal duration of SLIT is 3-4 years, which typically produces 7-8 years of persisting benefit before retreatment is needed.
• SCIT results in faster clinical improvement than does SLIT. And at least through the first 12-15 months, SCIT also appears to be significantly more effective.
• The use of SLIT in combination with mixes of other readily available pollen extracts is not supported by any evidence of efficacy.
• The big advantages SLIT offers over SCIT are convenience and safety. Although in U.S. clinical trials 1 in every 200-300 SLIT-treated patients experienced mild systemic reactions – typically with the first dose no fatal or near-fatal anaphylactic reactions have occurred. That’s why SLIT will be approved for at-home use after a first in-office observed dose. However, the FDA will mandate that SLIT prescriptions be accompanied by coprescription of an epinephrine autoinjector, according to Dr. Nelson of National Jewish Health in Denver and professor of medicine at the University of Colorado at Denver.
Once SLIT products win FDA approval, the therapy will get a CPT code and become, for the first time, a billable treatment – a most welcome development. But Dr. Nelson emphasized that SLIT’s approval will also create a new dilemma for physicians and their many patients with multiple allergies, say, to trees, dogs, and molds in addition to grasses or ragweed.
"Something everybody’s going to have to decide is where to position this treatment," Dr. Nelson said. "Most of the companies have no plans to take SLIT beyond the standardized extracts, which means grass, ragweed, house dust mite, and cat. You’re probably never going to have SLIT for cottonwood or juniper. And it seems unlikely that anyone is going to put a patient on tablets and injections at the same time. So it’s a decision that will have to be made for every patient: whether the ability to treat grass and ragweed, and later, house dust mite and cat, is sufficient for that patient. Because if it’s not, then probably the patient is still a candidate for SCIT."
The strategy of the companies developing SLIT is not that oral therapy is supposed to be a replacement for SCIT, but rather that it provides an immunotherapy option for patients who currently don’t receive it because they balk at the inconvenience of monthly in-office injections, he continued.
"The idea is that if these people are told, ‘You can just take a tablet at home,’ they’ll opt to get at least their allergies to grass and ragweed treated," Dr. Nelson explained.
Compliance and treatment persistence are going to be issues with SLIT, as documented in a Dutch retrospective study of 3,690 patients placed on SLIT and 2,796 who received SCIT. Only 23% of patients on SCIT stayed on treatment for the recommended 3 years. While that’s hardly a stellar adherence rate, it was actually more than three times better than with SLIT, where the rate was just 7%. The median duration of adherence with SCIT was 1.7 years, compared with 0.6 years for SLIT. The main reason patients stopped SCIT was the inconvenience, while the No. 1 reason people gave up on SLIT was ineffectiveness (J. Allergy Clin. Immunol. 2013;132:353-60).
To be fair, the Dutch study is a worst-case scenario for SLIT, according to Dr. Nelson. There are data showing adherence to SLIT is best when patients are routinely seen in the office every 3 months, apparently not the case in the Dutch study.
In a soon-to-be-published report, Dr. Nelson has reviewed 11 randomized head-to-head-comparison studies of SLIT versus SCIT and found them consistently uninformative. Most often, the deck was stacked against SLIT because it was given only three times per week and/or in too-low doses. In his view, there is only one enlightening comparative study, a recent randomized trial in which 40 Danish patients allergic to grass pollen received optimally dosed SLIT, SCIT, or neither for 15 months, with the same company’s standardized injectable and tablet Timothy grass preparations being used.
After 15 months, both treatments were effective, clinically as well as immunologically, compared with the no-treatment controls, with the benefits becoming significant in the first 1-3 months. However, the improvements in IgG4, IgE-blocking factor, facilitated antigen presentation, and the basal activation test were generally twice as great in the SCIT group. Moreover, the symptomatic response to nasal challenge – the only measure of clinical response utilized in the study was significantly better than in controls only with SCIT (Clin. Exp. Allergy 2014;44:417-28).
"This is the best comparative study we have, and it may be the best we’ll get. Here both treatments are being given optimally, and it’s very clear that at least in the first year, SCIT beats SLIT. It looks as though SLIT is trying to catch up late but doesn’t quite get there through 15 months. The investigators have stored frozen cells, so we can look forward to data on changes in regulatory T cells and suppression of Th2 cells in further publications," Dr. Nelson said.
Of note, an analysis of seven phase III clinical trials totaling nearly 2,700 adults and children showed that roughly half of them experienced transient local adverse reactions to grass SLIT. The reactions usually began on day 1, lasted 30-60 minutes, and recurred with the first seven or so daily doses. The reactions predominantly involved itching of the mouth or throat. About 10% of patients reported a sensation of swelling in the mouth that wasn’t visible to observers and tended to last longer than a week.
"Some people are surprised at the high incidence of these local, transient adverse reactions," he commented.
A common practice among American allergists is off-label sublingual administration of mixtures of eight or more pollen extracts. But a randomized, double-blind, placebo-controlled clinical trial in which Dr. Nelson was senior coinvestigator suggested this may be counterproductive when such mixtures are given in conjunction with Timothy pollen extract. A SLIT combination of Timothy pollen and nine additional pollen allergen extracts performed significantly worse than Timothy alone at the same dose; in fact, it failed to outdistance placebo in most endpoints (J. Allergy Clin. Immunol. 2009;124:150-6).
"This was a small, 56-patient study that clearly needs to be replicated, but there’s no financial backing for it. This is rather critical, since many people doing off-label sublingual immunotherapy using multiple allergen extracts think that they know what they’re doing. I hope I’ve made the point that they don’t know what dose to use, and there’s no evidence that multiple allergen mixes are really effective," Dr. Nelson said.
Grass allergies are the most common seasonal allergies in the United States. The three standardized SLIT products under FDA review, all of which have been approved in Europe for years, are Grastek, a Timothy grass extract, and Ragwitek, both developed by Merck in partnership with ALK of Denmark, and Oralair, a five-grass product developed by the French company Stallergenes. Oralair, to be marketed in the United States by Greer, contains Timothy grass allergen as well as extracts of four other temperate pasture grasses. Of note, Bermuda and Bahia grasses, common causes of seasonal allergy, aren’t included in Oralair or Grastek.
The companies have pursued different dosing strategies. ALK recommends taking Grastek continuously year-round. Stallergenes recommends starting Oralair a few months before the start of grass allergy season and stopping when the pollen season is over. In one 3-year study, 633 grass-allergic patients were randomized to Oralair or placebo starting 2 or 4 months prior to the pollen season. The reduction in adjusted symptom scores was similar with 2 vs. 4 months of therapy in advance of the allergy season (J. Allergy Clin. Immunol. 2011;128:559-66). That’s an important finding because it means preseasonally treated patients can purchase 8 weeks fewer tablets, the allergist noted.
Dr. Nelson’s prediction that these three SLIT products are headed for FDA approval this spring stems from enthusiastic endorsements by the agency’s Allergenic Products Advisory Committee. The SLIT grass allergy products were recommended unanimously, and the ragweed SLIT also received a strongly favorable vote.
He reported serving as a consultant to Merck, Pearl Therapeutic, and Circassia.
EXPERT ANALYSIS FROM THE PULMONARY AND ALLERGY UPDATE
Minimalist transfemoral TAVR winning surgical converts
SNOWMASS, COLO. – Familiarity with the option of minimalist transfemoral transcatheter aortic valve replacement is causing some top surgeons to broaden their use of nonsurgical valve replacement.
"This minimalist transfemoral TAVR is something we’ve done quite a lot of. As a surgeon, I sort of hate to say it, but this to me has made a huge difference in whether I make a patient surgical or transcatheter," Dr. Vinod H. Thourani said at the Annual Cardiovascular Conference at Snowmass.
"It’s me and a cardiology resident doing a minimalist transfemoral TAVR in the cath lab in an awake patient with fentanyl and Versed [midazolam] and a 14- to 24-French sheath. There’s no incision and the patients don’t go to the ICU. Thirty to forty percent of them go straight to their private room after their valve is implanted, if their groin is okay in the postop cath lab area," explained Dr. Thourani, associate director of cardiothoracic surgery at Emory University, Atlanta.
In 200 patients who’ve undergone the procedure at the hands of Dr. Thourani and his Emory colleagues, there has been zero in-hospital mortality. Most patients are home in 2-3 days.
On the other hand, the surgical approach is seeing advances as well, he continued. One technologic development he’s particularly enthusiastic about is the surgical sutureless valve.
"This is a whole new class of valves. My cardiologists, when I call them and tell them I put a sutureless valve in their patient, have no clue what we’re talking about," according to the surgeon.
Two major U.S. clinical trials are ongoing for the investigational sutureless Sorin Perceval and Edwards Intuity valves. These truly sutureless valves are crimped. The surgeon performs an open sternotomy, cross clamps the aorta, makes an aortotomy, then telescopes the valve down through the aortotomy. The Intuity valve is deployed via a brief balloon inflation to size it properly. The Perceval valve contains nitinol, so all that the surgeon has to do is put warm saline on it and the valve starts working.
"On my last Edwards Intuity valve, just last week, the cross clamp time was 35 minutes. Just to give you some perspective, the cross clamp time for a stented valve is 60-65 minutes and about 90 minutes for a stentless valve," Dr. Thourani explained.
He serves as a consultant to Edwards Lifesciences, Sorin, and St. Jude Medical.
SNOWMASS, COLO. – Familiarity with the option of minimalist transfemoral transcatheter aortic valve replacement is causing some top surgeons to broaden their use of nonsurgical valve replacement.
"This minimalist transfemoral TAVR is something we’ve done quite a lot of. As a surgeon, I sort of hate to say it, but this to me has made a huge difference in whether I make a patient surgical or transcatheter," Dr. Vinod H. Thourani said at the Annual Cardiovascular Conference at Snowmass.
"It’s me and a cardiology resident doing a minimalist transfemoral TAVR in the cath lab in an awake patient with fentanyl and Versed [midazolam] and a 14- to 24-French sheath. There’s no incision and the patients don’t go to the ICU. Thirty to forty percent of them go straight to their private room after their valve is implanted, if their groin is okay in the postop cath lab area," explained Dr. Thourani, associate director of cardiothoracic surgery at Emory University, Atlanta.
In 200 patients who’ve undergone the procedure at the hands of Dr. Thourani and his Emory colleagues, there has been zero in-hospital mortality. Most patients are home in 2-3 days.
On the other hand, the surgical approach is seeing advances as well, he continued. One technologic development he’s particularly enthusiastic about is the surgical sutureless valve.
"This is a whole new class of valves. My cardiologists, when I call them and tell them I put a sutureless valve in their patient, have no clue what we’re talking about," according to the surgeon.
Two major U.S. clinical trials are ongoing for the investigational sutureless Sorin Perceval and Edwards Intuity valves. These truly sutureless valves are crimped. The surgeon performs an open sternotomy, cross clamps the aorta, makes an aortotomy, then telescopes the valve down through the aortotomy. The Intuity valve is deployed via a brief balloon inflation to size it properly. The Perceval valve contains nitinol, so all that the surgeon has to do is put warm saline on it and the valve starts working.
"On my last Edwards Intuity valve, just last week, the cross clamp time was 35 minutes. Just to give you some perspective, the cross clamp time for a stented valve is 60-65 minutes and about 90 minutes for a stentless valve," Dr. Thourani explained.
He serves as a consultant to Edwards Lifesciences, Sorin, and St. Jude Medical.
SNOWMASS, COLO. – Familiarity with the option of minimalist transfemoral transcatheter aortic valve replacement is causing some top surgeons to broaden their use of nonsurgical valve replacement.
"This minimalist transfemoral TAVR is something we’ve done quite a lot of. As a surgeon, I sort of hate to say it, but this to me has made a huge difference in whether I make a patient surgical or transcatheter," Dr. Vinod H. Thourani said at the Annual Cardiovascular Conference at Snowmass.
"It’s me and a cardiology resident doing a minimalist transfemoral TAVR in the cath lab in an awake patient with fentanyl and Versed [midazolam] and a 14- to 24-French sheath. There’s no incision and the patients don’t go to the ICU. Thirty to forty percent of them go straight to their private room after their valve is implanted, if their groin is okay in the postop cath lab area," explained Dr. Thourani, associate director of cardiothoracic surgery at Emory University, Atlanta.
In 200 patients who’ve undergone the procedure at the hands of Dr. Thourani and his Emory colleagues, there has been zero in-hospital mortality. Most patients are home in 2-3 days.
On the other hand, the surgical approach is seeing advances as well, he continued. One technologic development he’s particularly enthusiastic about is the surgical sutureless valve.
"This is a whole new class of valves. My cardiologists, when I call them and tell them I put a sutureless valve in their patient, have no clue what we’re talking about," according to the surgeon.
Two major U.S. clinical trials are ongoing for the investigational sutureless Sorin Perceval and Edwards Intuity valves. These truly sutureless valves are crimped. The surgeon performs an open sternotomy, cross clamps the aorta, makes an aortotomy, then telescopes the valve down through the aortotomy. The Intuity valve is deployed via a brief balloon inflation to size it properly. The Perceval valve contains nitinol, so all that the surgeon has to do is put warm saline on it and the valve starts working.
"On my last Edwards Intuity valve, just last week, the cross clamp time was 35 minutes. Just to give you some perspective, the cross clamp time for a stented valve is 60-65 minutes and about 90 minutes for a stentless valve," Dr. Thourani explained.
He serves as a consultant to Edwards Lifesciences, Sorin, and St. Jude Medical.
EXPERT ANALYSIS FROM THE CARDIOVASCULAR CONFERENCE AT SNOWMASS
Physician/patient communication on ICDs deemed problematic
SNOWMASS, COLO. – Cardiologist-patient discussions at the time of decision making about implantable cardioverter-defibrillator therapy are fraught with a disturbing degree of misrepresentation and omission, according to a recent study involving patient focus groups and formal scrutiny of observed physician-patient interactions.
"Our study demonstrates that patient-physician communication about ICDs is characterized by patient misinformation and a lack of attention to psychosocial and long-term risks by the prescribing physicians. ... Training of cardiologists on information exchange with patients may promote informed decision making and preempt threats to patient quality of life," concluded Dr. Paul J. Hauptman and his coinvestigators at St. Louis University.
At the Annual Cardiovascular Conference at Snowmass, Dr. Clyde W. Yancy highlighted the Hauptman study for shining a spotlight on an area in which the quality of communication with patients apparently leaves much to be desired. The study has drawn interest at the federally funded Patient-Centered Outcomes Research Institute (PCORI), where Dr. Yancy, a former president of the American Heart Association, is part of the leadership. Dr. Joe V. Selby, PCORI executive director, recently announced plans to spend roughly $1 billion on patient-centered comparative effectiveness research projects over the next 2 years.
The Hauptman study involved eight focus groups with a total of 41 ICD recipients in three different metropolitan areas as well as observation of 11 cardiologists as they each conducted two discussions about ICDs with individual device candidates.
Trained observers faulted 18 of the 22 cardiologist-patient interviews because the cardiologists didn’t address, minimized, or outright denied quality of life issues and the long-term consequences of ICD therapy, including increased risks for anxiety and depression as well as for inappropriate shocks.
Among the actual statements by cardiologists extracted from the physician-patient discussions were these: "The ICD will only go off if you are in VT/VF," "Inadvertent shock is not detrimental," "Inappropriate shock ... almost never happens," and "These devices are well tested and rarely have problems, so don’t worry about it." Cardiologists frequently compared the ICD to a seat belt, a safety net, or "an ambulance that follows you around." The first mention of a quality of life issue occurred on average halfway through the preimplantation discussions, which averaged 24 minutes in length. Although an increased prevalence of depression secondary to inappropriate ICD discharge or change in body image has been well documented in multiple studies, this possibility was discussed in only 1 of the 22 physician-patient interactions.
"No cardiologist discussed the epidemiological or clinical data on the prevalence of actual lifesaving ICD shocks, the prevalence of patients who ever require a shock, or the risk for death, despite ICD shock," the investigators noted (JAMA Intern. Med. 2013;173:571-7).
In the 2-hour-long patient focus groups, which were conducted with a different population than in the observed cardiologist-patient discussions, only 2 of 41 patients with ICDs could recall preimplantation mention of the possibility of depression or other emotional consequences of having an ICD. Only 5 of 41 patients could recall having a preimplantation discussion about inappropriate shocks. Two remembered mention of the possibility of device recalls.
The small sample size is a limitation of this study. Nevertheless, these rather disheartening data constitute some of the little evidence available on the information exchange between patients and cardiologists surrounding decision-making about ICDs, noted Dr. Yancy, professor of medicine and of medical social sciences as well as chief of cardiology at Northwestern University, Chicago.
"It just continues to be very startling that when you sit down with patients and understand what prism they’re using to see the data, you see information like this," he commented.
Another speaker, Dr. Bernard J. Gersh, was less circumspect. He was left shaking his head following Dr. Yancy’s overview of the Hauptman study findings.
"I was absolutely flabbergasted by the ICD patient information data. I was amazed and disappointed," said Dr. Gersh, professor of medicine at the Mayo Clinic in Rochester, Minn.
Dr. Yancy reported having no financial conflicts. Dr. Gersh is on the advisory boards of St. Jude Medical and Boston Scientific.
SNOWMASS, COLO. – Cardiologist-patient discussions at the time of decision making about implantable cardioverter-defibrillator therapy are fraught with a disturbing degree of misrepresentation and omission, according to a recent study involving patient focus groups and formal scrutiny of observed physician-patient interactions.
"Our study demonstrates that patient-physician communication about ICDs is characterized by patient misinformation and a lack of attention to psychosocial and long-term risks by the prescribing physicians. ... Training of cardiologists on information exchange with patients may promote informed decision making and preempt threats to patient quality of life," concluded Dr. Paul J. Hauptman and his coinvestigators at St. Louis University.
At the Annual Cardiovascular Conference at Snowmass, Dr. Clyde W. Yancy highlighted the Hauptman study for shining a spotlight on an area in which the quality of communication with patients apparently leaves much to be desired. The study has drawn interest at the federally funded Patient-Centered Outcomes Research Institute (PCORI), where Dr. Yancy, a former president of the American Heart Association, is part of the leadership. Dr. Joe V. Selby, PCORI executive director, recently announced plans to spend roughly $1 billion on patient-centered comparative effectiveness research projects over the next 2 years.
The Hauptman study involved eight focus groups with a total of 41 ICD recipients in three different metropolitan areas as well as observation of 11 cardiologists as they each conducted two discussions about ICDs with individual device candidates.
Trained observers faulted 18 of the 22 cardiologist-patient interviews because the cardiologists didn’t address, minimized, or outright denied quality of life issues and the long-term consequences of ICD therapy, including increased risks for anxiety and depression as well as for inappropriate shocks.
Among the actual statements by cardiologists extracted from the physician-patient discussions were these: "The ICD will only go off if you are in VT/VF," "Inadvertent shock is not detrimental," "Inappropriate shock ... almost never happens," and "These devices are well tested and rarely have problems, so don’t worry about it." Cardiologists frequently compared the ICD to a seat belt, a safety net, or "an ambulance that follows you around." The first mention of a quality of life issue occurred on average halfway through the preimplantation discussions, which averaged 24 minutes in length. Although an increased prevalence of depression secondary to inappropriate ICD discharge or change in body image has been well documented in multiple studies, this possibility was discussed in only 1 of the 22 physician-patient interactions.
"No cardiologist discussed the epidemiological or clinical data on the prevalence of actual lifesaving ICD shocks, the prevalence of patients who ever require a shock, or the risk for death, despite ICD shock," the investigators noted (JAMA Intern. Med. 2013;173:571-7).
In the 2-hour-long patient focus groups, which were conducted with a different population than in the observed cardiologist-patient discussions, only 2 of 41 patients with ICDs could recall preimplantation mention of the possibility of depression or other emotional consequences of having an ICD. Only 5 of 41 patients could recall having a preimplantation discussion about inappropriate shocks. Two remembered mention of the possibility of device recalls.
The small sample size is a limitation of this study. Nevertheless, these rather disheartening data constitute some of the little evidence available on the information exchange between patients and cardiologists surrounding decision-making about ICDs, noted Dr. Yancy, professor of medicine and of medical social sciences as well as chief of cardiology at Northwestern University, Chicago.
"It just continues to be very startling that when you sit down with patients and understand what prism they’re using to see the data, you see information like this," he commented.
Another speaker, Dr. Bernard J. Gersh, was less circumspect. He was left shaking his head following Dr. Yancy’s overview of the Hauptman study findings.
"I was absolutely flabbergasted by the ICD patient information data. I was amazed and disappointed," said Dr. Gersh, professor of medicine at the Mayo Clinic in Rochester, Minn.
Dr. Yancy reported having no financial conflicts. Dr. Gersh is on the advisory boards of St. Jude Medical and Boston Scientific.
SNOWMASS, COLO. – Cardiologist-patient discussions at the time of decision making about implantable cardioverter-defibrillator therapy are fraught with a disturbing degree of misrepresentation and omission, according to a recent study involving patient focus groups and formal scrutiny of observed physician-patient interactions.
"Our study demonstrates that patient-physician communication about ICDs is characterized by patient misinformation and a lack of attention to psychosocial and long-term risks by the prescribing physicians. ... Training of cardiologists on information exchange with patients may promote informed decision making and preempt threats to patient quality of life," concluded Dr. Paul J. Hauptman and his coinvestigators at St. Louis University.
At the Annual Cardiovascular Conference at Snowmass, Dr. Clyde W. Yancy highlighted the Hauptman study for shining a spotlight on an area in which the quality of communication with patients apparently leaves much to be desired. The study has drawn interest at the federally funded Patient-Centered Outcomes Research Institute (PCORI), where Dr. Yancy, a former president of the American Heart Association, is part of the leadership. Dr. Joe V. Selby, PCORI executive director, recently announced plans to spend roughly $1 billion on patient-centered comparative effectiveness research projects over the next 2 years.
The Hauptman study involved eight focus groups with a total of 41 ICD recipients in three different metropolitan areas as well as observation of 11 cardiologists as they each conducted two discussions about ICDs with individual device candidates.
Trained observers faulted 18 of the 22 cardiologist-patient interviews because the cardiologists didn’t address, minimized, or outright denied quality of life issues and the long-term consequences of ICD therapy, including increased risks for anxiety and depression as well as for inappropriate shocks.
Among the actual statements by cardiologists extracted from the physician-patient discussions were these: "The ICD will only go off if you are in VT/VF," "Inadvertent shock is not detrimental," "Inappropriate shock ... almost never happens," and "These devices are well tested and rarely have problems, so don’t worry about it." Cardiologists frequently compared the ICD to a seat belt, a safety net, or "an ambulance that follows you around." The first mention of a quality of life issue occurred on average halfway through the preimplantation discussions, which averaged 24 minutes in length. Although an increased prevalence of depression secondary to inappropriate ICD discharge or change in body image has been well documented in multiple studies, this possibility was discussed in only 1 of the 22 physician-patient interactions.
"No cardiologist discussed the epidemiological or clinical data on the prevalence of actual lifesaving ICD shocks, the prevalence of patients who ever require a shock, or the risk for death, despite ICD shock," the investigators noted (JAMA Intern. Med. 2013;173:571-7).
In the 2-hour-long patient focus groups, which were conducted with a different population than in the observed cardiologist-patient discussions, only 2 of 41 patients with ICDs could recall preimplantation mention of the possibility of depression or other emotional consequences of having an ICD. Only 5 of 41 patients could recall having a preimplantation discussion about inappropriate shocks. Two remembered mention of the possibility of device recalls.
The small sample size is a limitation of this study. Nevertheless, these rather disheartening data constitute some of the little evidence available on the information exchange between patients and cardiologists surrounding decision-making about ICDs, noted Dr. Yancy, professor of medicine and of medical social sciences as well as chief of cardiology at Northwestern University, Chicago.
"It just continues to be very startling that when you sit down with patients and understand what prism they’re using to see the data, you see information like this," he commented.
Another speaker, Dr. Bernard J. Gersh, was less circumspect. He was left shaking his head following Dr. Yancy’s overview of the Hauptman study findings.
"I was absolutely flabbergasted by the ICD patient information data. I was amazed and disappointed," said Dr. Gersh, professor of medicine at the Mayo Clinic in Rochester, Minn.
Dr. Yancy reported having no financial conflicts. Dr. Gersh is on the advisory boards of St. Jude Medical and Boston Scientific.
EXPERT ANALYSIS FROM THE CARDIOVASCULAR CONFERENCE AT SNOWMASS
Arthroplasty for rheumatoid arthritis doesn’t boost cardiovascular risk
SNOWMASS, COLO. – During a recent 15-year period in which the annual arthroplasty rate for osteoarthritis and other noninflammatory arthritides doubled, the arthroplasty rate for rheumatoid arthritis actually declined. Moreover, the mean age at the time of arthroplasty for RA rose.
"In a time frame when utilization of total knee and total hip replacement for osteoarthritis is really skyrocketing, with younger and younger patients, I think this speaks to something pretty good going on with our RA patients," Dr. Susan M. Goodman observed at the Winter Rheumatology Symposium sponsored by the American College of Rheumatology.
She presented data from a soon to be published study of nearly 2.8 million arthroplasties included in 10 state databases. The arthroplasty rate for noninflammatory arthritis – the great majority of which is osteoarthritis (OA) – zoomed from 124.5/100,000 population in 1991 to 247.5/100,000 in 2005.
Meanwhile the rate of arthroplasty for RA fell slightly, albeit statistically significantly, from 4.6 to 4.5 per 100,000. The mean age at the time of arthroplasty for RA rose from 63.4 years in 1991 to 64.9 years in 2005, reported Dr. Goodman, a rheumatologist at the Hospital for Special Surgery in New York.
She turned to data from other sources to address issues related to the morbidity of arthroplasty for RA.
For example, it’s well documented that rheumatoid arthritis is associated with elevated cardiovascular risk, such that the typical RA patient has a cardiovascular morbidity burden comparable to that of someone without RA who’s 5 years older. So what does this mean for the many RA patients who come into the hospital for total hip or knee replacement?
Surprisingly, nothing. That is, data from multiple sources indicate RA patients are at no greater perioperative risk of cardiovascular events than are patients with OA undergoing the same procedures.
The take-away message? "Clearly we’re doing something right in managing our patients with RA," Dr. Goodman commented.
Similarly, an analysis of 7.75 million patients in the Nationwide Inpatient Sample database found that among RA patients undergoing intermediate-risk noncardiac surgery, such as total joint arthroplasty, the perioperative cardiovascular event rate was 0.34%, significantly less than the 1.07% rate in diabetic patients undergoing intermediate-risk surgeries. Moreover, perioperative mortality was 0.30% in the RA patients, less than half the 0.65% figure in diabetics (Arthritis Rheum. 2012;64:2429-37). These findings disproved the study hypothesis, which was that the two groups would have similar cardiovascular event rates, since both diseases are – unlike osteoarthritis – systemic inflammatory conditions associated with increased cardiovascular mortality.
"I think this means that we as rheumatologists are taking better care of our patients than the endocrinologists next door whose patients have a similar atherosclerotic burden," she continued.
Dr. Goodman was a coinvestigator in a population-based study of 351,103 total knee replacements and 157,775 total hip replacements done at 400 hospitals during 2006-2010. This retrospective analysis of an administrative database included 11,755 total knee and 5,400 total hip replacements for RA.
The prevalence of a prior history of MI, peripheral vascular disease, or cerebrovascular disease was closely similar in the RA and OA patients undergoing surgery. However, the prevalence of baseline COPD was significantly greater in the RA patients, at roughly 17.5%, or an absolute 3%-4% more than in the osteoarthritis patients.
The 30-day rates of cardiac events, venous thromboembolism, and cerebrovascular events were closely similar in the RA and OA arthroplasty patients. The RA patients undergoing total hip replacement had significantly higher rates of pulmonary compromise, infections, blood product transfusions, mechanical ventilation, and length of stay than did OA patients (Clin. Exp. Rheumatol. 2013;31:889-95). The RA patients with total knee replacement differed from their OA counterparts only in terms of greater need for transfusions and lengthier hospital stays (J. Arthroplasty 2014;29:308-13).
However, an analysis of the Hospital for Special Surgery experience failed to confirm the increased complication risks found in this study of a large administrative database. This retrospective review of adverse events within 6 months of total knee replacement in 156 RA patients and 318 OA controls showed no differences between the two groups in pneumonia, other infections, or venous thromboembolism. Moreover, the reoperation rate was 2.5% in the RA patients, compared with 8.8% in the OA group.
"The advantage of a smaller study like this is you really know who has RA and you have a lot of very granular information about the drugs they’re taking. The disadvantage, of course, is that you’re really not powered to look at major adverse events. But boy, there wasn’t even a hint of an increase in the complication rate amongst these RA patients," according to Dr. Goodman.
In a study she presented at the 2013 European Congress of Rheumatology, she compared 2-year outcomes post arthroplasty in RA and OA patients in the contemporary era of high use of biologic agents and traditional DMARDs for RA. The 178 RA patients who had total knee replacement had significantly greater comorbidities preoperatively than the 5,206 OA patients. Yet by 2 years postoperatively, they had fully caught up in terms of improved Western Ontario and McMaster Osteoarthritis Index (WOMAC) function and pain scores.
Total hip replacement was a very different story. The 202 RA patients were four times more likely to have poor WOMAC functional outcome and three times more likely to have poor pain outcome scores at 2 years, compared with 5,810 OA patients. In a multivariate analysis, higher expectations for surgery, better preoperative mental health, and more advanced education were associated with better 2-year outcomes.
"I’m not sure why our hip replacement patients with RA aren’t doing as well as the knee replacement patients, but they’re clearly not," according to the rheumatologist.
She reported having no financial disclosures.
SNOWMASS, COLO. – During a recent 15-year period in which the annual arthroplasty rate for osteoarthritis and other noninflammatory arthritides doubled, the arthroplasty rate for rheumatoid arthritis actually declined. Moreover, the mean age at the time of arthroplasty for RA rose.
"In a time frame when utilization of total knee and total hip replacement for osteoarthritis is really skyrocketing, with younger and younger patients, I think this speaks to something pretty good going on with our RA patients," Dr. Susan M. Goodman observed at the Winter Rheumatology Symposium sponsored by the American College of Rheumatology.
She presented data from a soon to be published study of nearly 2.8 million arthroplasties included in 10 state databases. The arthroplasty rate for noninflammatory arthritis – the great majority of which is osteoarthritis (OA) – zoomed from 124.5/100,000 population in 1991 to 247.5/100,000 in 2005.
Meanwhile the rate of arthroplasty for RA fell slightly, albeit statistically significantly, from 4.6 to 4.5 per 100,000. The mean age at the time of arthroplasty for RA rose from 63.4 years in 1991 to 64.9 years in 2005, reported Dr. Goodman, a rheumatologist at the Hospital for Special Surgery in New York.
She turned to data from other sources to address issues related to the morbidity of arthroplasty for RA.
For example, it’s well documented that rheumatoid arthritis is associated with elevated cardiovascular risk, such that the typical RA patient has a cardiovascular morbidity burden comparable to that of someone without RA who’s 5 years older. So what does this mean for the many RA patients who come into the hospital for total hip or knee replacement?
Surprisingly, nothing. That is, data from multiple sources indicate RA patients are at no greater perioperative risk of cardiovascular events than are patients with OA undergoing the same procedures.
The take-away message? "Clearly we’re doing something right in managing our patients with RA," Dr. Goodman commented.
Similarly, an analysis of 7.75 million patients in the Nationwide Inpatient Sample database found that among RA patients undergoing intermediate-risk noncardiac surgery, such as total joint arthroplasty, the perioperative cardiovascular event rate was 0.34%, significantly less than the 1.07% rate in diabetic patients undergoing intermediate-risk surgeries. Moreover, perioperative mortality was 0.30% in the RA patients, less than half the 0.65% figure in diabetics (Arthritis Rheum. 2012;64:2429-37). These findings disproved the study hypothesis, which was that the two groups would have similar cardiovascular event rates, since both diseases are – unlike osteoarthritis – systemic inflammatory conditions associated with increased cardiovascular mortality.
"I think this means that we as rheumatologists are taking better care of our patients than the endocrinologists next door whose patients have a similar atherosclerotic burden," she continued.
Dr. Goodman was a coinvestigator in a population-based study of 351,103 total knee replacements and 157,775 total hip replacements done at 400 hospitals during 2006-2010. This retrospective analysis of an administrative database included 11,755 total knee and 5,400 total hip replacements for RA.
The prevalence of a prior history of MI, peripheral vascular disease, or cerebrovascular disease was closely similar in the RA and OA patients undergoing surgery. However, the prevalence of baseline COPD was significantly greater in the RA patients, at roughly 17.5%, or an absolute 3%-4% more than in the osteoarthritis patients.
The 30-day rates of cardiac events, venous thromboembolism, and cerebrovascular events were closely similar in the RA and OA arthroplasty patients. The RA patients undergoing total hip replacement had significantly higher rates of pulmonary compromise, infections, blood product transfusions, mechanical ventilation, and length of stay than did OA patients (Clin. Exp. Rheumatol. 2013;31:889-95). The RA patients with total knee replacement differed from their OA counterparts only in terms of greater need for transfusions and lengthier hospital stays (J. Arthroplasty 2014;29:308-13).
However, an analysis of the Hospital for Special Surgery experience failed to confirm the increased complication risks found in this study of a large administrative database. This retrospective review of adverse events within 6 months of total knee replacement in 156 RA patients and 318 OA controls showed no differences between the two groups in pneumonia, other infections, or venous thromboembolism. Moreover, the reoperation rate was 2.5% in the RA patients, compared with 8.8% in the OA group.
"The advantage of a smaller study like this is you really know who has RA and you have a lot of very granular information about the drugs they’re taking. The disadvantage, of course, is that you’re really not powered to look at major adverse events. But boy, there wasn’t even a hint of an increase in the complication rate amongst these RA patients," according to Dr. Goodman.
In a study she presented at the 2013 European Congress of Rheumatology, she compared 2-year outcomes post arthroplasty in RA and OA patients in the contemporary era of high use of biologic agents and traditional DMARDs for RA. The 178 RA patients who had total knee replacement had significantly greater comorbidities preoperatively than the 5,206 OA patients. Yet by 2 years postoperatively, they had fully caught up in terms of improved Western Ontario and McMaster Osteoarthritis Index (WOMAC) function and pain scores.
Total hip replacement was a very different story. The 202 RA patients were four times more likely to have poor WOMAC functional outcome and three times more likely to have poor pain outcome scores at 2 years, compared with 5,810 OA patients. In a multivariate analysis, higher expectations for surgery, better preoperative mental health, and more advanced education were associated with better 2-year outcomes.
"I’m not sure why our hip replacement patients with RA aren’t doing as well as the knee replacement patients, but they’re clearly not," according to the rheumatologist.
She reported having no financial disclosures.
SNOWMASS, COLO. – During a recent 15-year period in which the annual arthroplasty rate for osteoarthritis and other noninflammatory arthritides doubled, the arthroplasty rate for rheumatoid arthritis actually declined. Moreover, the mean age at the time of arthroplasty for RA rose.
"In a time frame when utilization of total knee and total hip replacement for osteoarthritis is really skyrocketing, with younger and younger patients, I think this speaks to something pretty good going on with our RA patients," Dr. Susan M. Goodman observed at the Winter Rheumatology Symposium sponsored by the American College of Rheumatology.
She presented data from a soon to be published study of nearly 2.8 million arthroplasties included in 10 state databases. The arthroplasty rate for noninflammatory arthritis – the great majority of which is osteoarthritis (OA) – zoomed from 124.5/100,000 population in 1991 to 247.5/100,000 in 2005.
Meanwhile the rate of arthroplasty for RA fell slightly, albeit statistically significantly, from 4.6 to 4.5 per 100,000. The mean age at the time of arthroplasty for RA rose from 63.4 years in 1991 to 64.9 years in 2005, reported Dr. Goodman, a rheumatologist at the Hospital for Special Surgery in New York.
She turned to data from other sources to address issues related to the morbidity of arthroplasty for RA.
For example, it’s well documented that rheumatoid arthritis is associated with elevated cardiovascular risk, such that the typical RA patient has a cardiovascular morbidity burden comparable to that of someone without RA who’s 5 years older. So what does this mean for the many RA patients who come into the hospital for total hip or knee replacement?
Surprisingly, nothing. That is, data from multiple sources indicate RA patients are at no greater perioperative risk of cardiovascular events than are patients with OA undergoing the same procedures.
The take-away message? "Clearly we’re doing something right in managing our patients with RA," Dr. Goodman commented.
Similarly, an analysis of 7.75 million patients in the Nationwide Inpatient Sample database found that among RA patients undergoing intermediate-risk noncardiac surgery, such as total joint arthroplasty, the perioperative cardiovascular event rate was 0.34%, significantly less than the 1.07% rate in diabetic patients undergoing intermediate-risk surgeries. Moreover, perioperative mortality was 0.30% in the RA patients, less than half the 0.65% figure in diabetics (Arthritis Rheum. 2012;64:2429-37). These findings disproved the study hypothesis, which was that the two groups would have similar cardiovascular event rates, since both diseases are – unlike osteoarthritis – systemic inflammatory conditions associated with increased cardiovascular mortality.
"I think this means that we as rheumatologists are taking better care of our patients than the endocrinologists next door whose patients have a similar atherosclerotic burden," she continued.
Dr. Goodman was a coinvestigator in a population-based study of 351,103 total knee replacements and 157,775 total hip replacements done at 400 hospitals during 2006-2010. This retrospective analysis of an administrative database included 11,755 total knee and 5,400 total hip replacements for RA.
The prevalence of a prior history of MI, peripheral vascular disease, or cerebrovascular disease was closely similar in the RA and OA patients undergoing surgery. However, the prevalence of baseline COPD was significantly greater in the RA patients, at roughly 17.5%, or an absolute 3%-4% more than in the osteoarthritis patients.
The 30-day rates of cardiac events, venous thromboembolism, and cerebrovascular events were closely similar in the RA and OA arthroplasty patients. The RA patients undergoing total hip replacement had significantly higher rates of pulmonary compromise, infections, blood product transfusions, mechanical ventilation, and length of stay than did OA patients (Clin. Exp. Rheumatol. 2013;31:889-95). The RA patients with total knee replacement differed from their OA counterparts only in terms of greater need for transfusions and lengthier hospital stays (J. Arthroplasty 2014;29:308-13).
However, an analysis of the Hospital for Special Surgery experience failed to confirm the increased complication risks found in this study of a large administrative database. This retrospective review of adverse events within 6 months of total knee replacement in 156 RA patients and 318 OA controls showed no differences between the two groups in pneumonia, other infections, or venous thromboembolism. Moreover, the reoperation rate was 2.5% in the RA patients, compared with 8.8% in the OA group.
"The advantage of a smaller study like this is you really know who has RA and you have a lot of very granular information about the drugs they’re taking. The disadvantage, of course, is that you’re really not powered to look at major adverse events. But boy, there wasn’t even a hint of an increase in the complication rate amongst these RA patients," according to Dr. Goodman.
In a study she presented at the 2013 European Congress of Rheumatology, she compared 2-year outcomes post arthroplasty in RA and OA patients in the contemporary era of high use of biologic agents and traditional DMARDs for RA. The 178 RA patients who had total knee replacement had significantly greater comorbidities preoperatively than the 5,206 OA patients. Yet by 2 years postoperatively, they had fully caught up in terms of improved Western Ontario and McMaster Osteoarthritis Index (WOMAC) function and pain scores.
Total hip replacement was a very different story. The 202 RA patients were four times more likely to have poor WOMAC functional outcome and three times more likely to have poor pain outcome scores at 2 years, compared with 5,810 OA patients. In a multivariate analysis, higher expectations for surgery, better preoperative mental health, and more advanced education were associated with better 2-year outcomes.
"I’m not sure why our hip replacement patients with RA aren’t doing as well as the knee replacement patients, but they’re clearly not," according to the rheumatologist.
She reported having no financial disclosures.
EXPERT ANALYSIS FROM THE WINTER RHEUMATOLOGY SYMPOSIUM
Statin in Childhood Reduces CHD Risk in Familial Hypercholesterolemia
DALLAS – Roughly 10 years after going on statin therapy as children or adolescents, a group of Dutch patients with familial hypercholesterolemia already has a significantly lower cardiovascular event rate than their affected parents did at the same age.
"Statin therapy in childhood seems to be effective in reducing CHD [coronary heart disease] risk in individuals with FH [familial hypercholesterolemia], although longer follow-up is needed to confirm these results," Dr. Marjet J.A.M. Braamskamp declared at the American Heart Association scientific sessions.
The 214 Dutch youths with FH started on statin therapy at age 8-18 years as part of a randomized clinical trial. None has experienced a cardiovascular event by age 30. In contrast, their affected parents, for whom statins weren’t available until they were well into adulthood, already had a 7% CHD event rate by age 30, a statistically significant difference, reported Dr. Braamskamp of the Academic Medical Center, Amsterdam.
In an interview, she said the plan is to continue to follow this cohort of young adults who started statin therapy in childhood at least until age 50. By that age, 55% of their fathers with FH and 24% of their mothers with FH had known CHD. While none of the FH mothers died of CHD before age 60, 16% of the FH fathers did, at an average age of 35.9 years.
Current European and American guidelines recommend statin therapy starting at age 8 or 10 years in children with the molecular or clinical diagnosis of FH and elevated cholesterol levels. Those guidelines were issued based upon recognition that children with FH have elevated cholesterol levels from birth onward, coupled with evidence from short-term randomized trials demonstrating that statins are safe and effective for lipid-lowering in such patients. The Dutch follow-up study provides evidence to suggest this early-treatment strategy also pays off in terms of reduced CHD risk.
The study was supported by the Dutch Heart Foundation. Dr. Braamskamp reported having no financial conflicts.
DALLAS – Roughly 10 years after going on statin therapy as children or adolescents, a group of Dutch patients with familial hypercholesterolemia already has a significantly lower cardiovascular event rate than their affected parents did at the same age.
"Statin therapy in childhood seems to be effective in reducing CHD [coronary heart disease] risk in individuals with FH [familial hypercholesterolemia], although longer follow-up is needed to confirm these results," Dr. Marjet J.A.M. Braamskamp declared at the American Heart Association scientific sessions.
The 214 Dutch youths with FH started on statin therapy at age 8-18 years as part of a randomized clinical trial. None has experienced a cardiovascular event by age 30. In contrast, their affected parents, for whom statins weren’t available until they were well into adulthood, already had a 7% CHD event rate by age 30, a statistically significant difference, reported Dr. Braamskamp of the Academic Medical Center, Amsterdam.
In an interview, she said the plan is to continue to follow this cohort of young adults who started statin therapy in childhood at least until age 50. By that age, 55% of their fathers with FH and 24% of their mothers with FH had known CHD. While none of the FH mothers died of CHD before age 60, 16% of the FH fathers did, at an average age of 35.9 years.
Current European and American guidelines recommend statin therapy starting at age 8 or 10 years in children with the molecular or clinical diagnosis of FH and elevated cholesterol levels. Those guidelines were issued based upon recognition that children with FH have elevated cholesterol levels from birth onward, coupled with evidence from short-term randomized trials demonstrating that statins are safe and effective for lipid-lowering in such patients. The Dutch follow-up study provides evidence to suggest this early-treatment strategy also pays off in terms of reduced CHD risk.
The study was supported by the Dutch Heart Foundation. Dr. Braamskamp reported having no financial conflicts.
DALLAS – Roughly 10 years after going on statin therapy as children or adolescents, a group of Dutch patients with familial hypercholesterolemia already has a significantly lower cardiovascular event rate than their affected parents did at the same age.
"Statin therapy in childhood seems to be effective in reducing CHD [coronary heart disease] risk in individuals with FH [familial hypercholesterolemia], although longer follow-up is needed to confirm these results," Dr. Marjet J.A.M. Braamskamp declared at the American Heart Association scientific sessions.
The 214 Dutch youths with FH started on statin therapy at age 8-18 years as part of a randomized clinical trial. None has experienced a cardiovascular event by age 30. In contrast, their affected parents, for whom statins weren’t available until they were well into adulthood, already had a 7% CHD event rate by age 30, a statistically significant difference, reported Dr. Braamskamp of the Academic Medical Center, Amsterdam.
In an interview, she said the plan is to continue to follow this cohort of young adults who started statin therapy in childhood at least until age 50. By that age, 55% of their fathers with FH and 24% of their mothers with FH had known CHD. While none of the FH mothers died of CHD before age 60, 16% of the FH fathers did, at an average age of 35.9 years.
Current European and American guidelines recommend statin therapy starting at age 8 or 10 years in children with the molecular or clinical diagnosis of FH and elevated cholesterol levels. Those guidelines were issued based upon recognition that children with FH have elevated cholesterol levels from birth onward, coupled with evidence from short-term randomized trials demonstrating that statins are safe and effective for lipid-lowering in such patients. The Dutch follow-up study provides evidence to suggest this early-treatment strategy also pays off in terms of reduced CHD risk.
The study was supported by the Dutch Heart Foundation. Dr. Braamskamp reported having no financial conflicts.
AT THE AHA SCIENTIFIC SESSIONS