User login
Less Trastuzumab Cardiotoxicity in Beta-Blocker Users
ORLANDO – Beta-blockers appear to be cardioprotective in breast cancer patients on trastuzumab, according to a prospective case-control study.
The risk of developing new-onset heart failure or left ventricular dysfunction during 1 year on trastuzumab (Herceptin) was 65% lower in 30 breast cancer patients who were incidentally on a beta-blocker at the start of the monoclonal antibody therapy than in 167 other breast cancer patients who were not, Thomas B. Cook, Ph.D., reported at the annual scientific sessions of the American Heart Association.
The women on a beta-blocker averaged 59 years of age, a full 8 years older than the breast cancer patients not on a beta-blocker. The patients on a beta-blocker also had significantly higher rates of hypertension, diabetes, obesity, and smoking. After adjustment for these traditional cardiovascular risk factors as well as other factors predisposing to heart failure, including prior treatment with anthracyclines or radiation therapy, the beta-blocker group had an adjusted 83% lower risk of developing cardiotoxicity during 1 year of follow-up on trastuzumab (P = .006).
This is a small hypothesis-generating study. The findings warrant a proper randomized controlled trial assessing the impact of prophylactic beta-blocker therapy in breast cancer patients going on trastuzumab, Dr. Cook asserted.
In all, 22 of the 197 breast cancer patients were on an ACE inhibitor at the time they started on trastuzumab. Logistic regression analysis indicated no cardioprotective effect for this class of medication, according to Dr. Cook of the Cleveland Clinic Foundation.
New-onset heart failure was diagnosed in 11% of patients not on a beta-blocker and in none who were. Left ventricular dysfunction – defined as an echocardiographically documented ejection fraction decline of at least 10% – occurred in 40% of trastuzumab users not on a beta-blocker, compared to 20% who were.
A trajectory analysis of serial echocardiographic exams revealed overall significant declines in left ventricular ejection fraction in patients during their year of follow-up on trastuzumab; however, the decline was markedly steeper in those who were not on a beta-blocker.
Interestingly, neither prior treatment with anthracyclines, even in excess of four cycles, nor other prior chemotherapy were predictive of cardiotoxicity in this series of trastuzumab-treated women. Current smoking, however, was associated with a 4.2-fold increased risk.
Dr. Cook reported no financial conflicts.
ORLANDO – Beta-blockers appear to be cardioprotective in breast cancer patients on trastuzumab, according to a prospective case-control study.
The risk of developing new-onset heart failure or left ventricular dysfunction during 1 year on trastuzumab (Herceptin) was 65% lower in 30 breast cancer patients who were incidentally on a beta-blocker at the start of the monoclonal antibody therapy than in 167 other breast cancer patients who were not, Thomas B. Cook, Ph.D., reported at the annual scientific sessions of the American Heart Association.
The women on a beta-blocker averaged 59 years of age, a full 8 years older than the breast cancer patients not on a beta-blocker. The patients on a beta-blocker also had significantly higher rates of hypertension, diabetes, obesity, and smoking. After adjustment for these traditional cardiovascular risk factors as well as other factors predisposing to heart failure, including prior treatment with anthracyclines or radiation therapy, the beta-blocker group had an adjusted 83% lower risk of developing cardiotoxicity during 1 year of follow-up on trastuzumab (P = .006).
This is a small hypothesis-generating study. The findings warrant a proper randomized controlled trial assessing the impact of prophylactic beta-blocker therapy in breast cancer patients going on trastuzumab, Dr. Cook asserted.
In all, 22 of the 197 breast cancer patients were on an ACE inhibitor at the time they started on trastuzumab. Logistic regression analysis indicated no cardioprotective effect for this class of medication, according to Dr. Cook of the Cleveland Clinic Foundation.
New-onset heart failure was diagnosed in 11% of patients not on a beta-blocker and in none who were. Left ventricular dysfunction – defined as an echocardiographically documented ejection fraction decline of at least 10% – occurred in 40% of trastuzumab users not on a beta-blocker, compared to 20% who were.
A trajectory analysis of serial echocardiographic exams revealed overall significant declines in left ventricular ejection fraction in patients during their year of follow-up on trastuzumab; however, the decline was markedly steeper in those who were not on a beta-blocker.
Interestingly, neither prior treatment with anthracyclines, even in excess of four cycles, nor other prior chemotherapy were predictive of cardiotoxicity in this series of trastuzumab-treated women. Current smoking, however, was associated with a 4.2-fold increased risk.
Dr. Cook reported no financial conflicts.
ORLANDO – Beta-blockers appear to be cardioprotective in breast cancer patients on trastuzumab, according to a prospective case-control study.
The risk of developing new-onset heart failure or left ventricular dysfunction during 1 year on trastuzumab (Herceptin) was 65% lower in 30 breast cancer patients who were incidentally on a beta-blocker at the start of the monoclonal antibody therapy than in 167 other breast cancer patients who were not, Thomas B. Cook, Ph.D., reported at the annual scientific sessions of the American Heart Association.
The women on a beta-blocker averaged 59 years of age, a full 8 years older than the breast cancer patients not on a beta-blocker. The patients on a beta-blocker also had significantly higher rates of hypertension, diabetes, obesity, and smoking. After adjustment for these traditional cardiovascular risk factors as well as other factors predisposing to heart failure, including prior treatment with anthracyclines or radiation therapy, the beta-blocker group had an adjusted 83% lower risk of developing cardiotoxicity during 1 year of follow-up on trastuzumab (P = .006).
This is a small hypothesis-generating study. The findings warrant a proper randomized controlled trial assessing the impact of prophylactic beta-blocker therapy in breast cancer patients going on trastuzumab, Dr. Cook asserted.
In all, 22 of the 197 breast cancer patients were on an ACE inhibitor at the time they started on trastuzumab. Logistic regression analysis indicated no cardioprotective effect for this class of medication, according to Dr. Cook of the Cleveland Clinic Foundation.
New-onset heart failure was diagnosed in 11% of patients not on a beta-blocker and in none who were. Left ventricular dysfunction – defined as an echocardiographically documented ejection fraction decline of at least 10% – occurred in 40% of trastuzumab users not on a beta-blocker, compared to 20% who were.
A trajectory analysis of serial echocardiographic exams revealed overall significant declines in left ventricular ejection fraction in patients during their year of follow-up on trastuzumab; however, the decline was markedly steeper in those who were not on a beta-blocker.
Interestingly, neither prior treatment with anthracyclines, even in excess of four cycles, nor other prior chemotherapy were predictive of cardiotoxicity in this series of trastuzumab-treated women. Current smoking, however, was associated with a 4.2-fold increased risk.
Dr. Cook reported no financial conflicts.
FROM THE ANNUAL SCIENTIFIC SESSIONS OF THE AMERICAN HEART ASSOCIATION
Major Finding: Breast cancer patients who were incidentally on a beta-blocker had an adjusted 83% lower risk of developing new-onset heart failure or left ventricular dysfunction during their first year on trastuzumab.
Data Source: A prospective observational study of 197 breast cancer patients followed during 1 year on trastuzumab.
Disclosures: Dr. Cook reported no financial conflicts.
'Transcutaneous Systemic' Clobetasol Reverses Bullous Pemphigoid
LISBON – An intensive regimen of whole-body topical clobetasol may be an effective and better tolerated alternative to standard, high-dose oral prednisone in patients with milder cases of bullous pemphigoid, according to Dr. Marcel F. Jonkman.
"In your daily practice you all use topical clobetasol, but not like this. I call it transcutaneous systemic clobetasol therapy. That means you put it on the entire geography, the whole body surface from your cheeks to your toes, not just on lesional skin," Dr. Jonkman explained at the annual congress of the European Academy of Dermatology and Venereology.
This is a laborious 4-month-long course of therapy. For mild disease – which Dr. Jonkman defined as fewer than 10 new bullae arising during the 3 days before first consultation – patients apply 20 g of clobetasol propionate cream once daily for the first month, then every other day for the second month, twice weekly in the third, and once per week for the fourth month.
Patients with severe bullous pemphigoid follow the same schedule, using 30 g per application rather than 20 g.
"That's really a lot of clobetasol. If you put on 25 g, I expect it's effectively equivalent to 35-40 mg of oral prednisone. Sometimes I get referred patients who are on 80 mg/day of prednisone and they respond to clobetasol therapy," said Dr. Jonkman, professor and chair of the department of dermatology at the University of Groningen (the Netherlands).
This is a decidedly off-label method of applying the superpotent fluorinated topical corticosteroid, he said. And make no mistake, the medication is absorbed systemically to a substantial degree. That's why it is so effective. Within about 5 days after starting daily therapy, the peripheral hypereosinophilia that marks bullous pemphigoid is reversed, with titers falling into the normal range.
Moreover, whole-body topical clobetasol is capable of inducing adrenal gland suppression and other familiar systemic side effects of high-dose corticosteroid therapy, said Dr. Jonkman. The main advantage of topical therapy is it avoids the severe gastritis that often results from long-term, high-dose oral prednisone.
Clinical Experience
He presented his personal experience using transcutaneous systemic clobetasol in 40 patients with mild, and 34 patients with severe, bullous pemphigoid. Patients in the mild disease group had a mean of 3.6 new blisters during the 3 days prior to first consultation; those in the severe bullous pemphigoid group averaged 36.7.
As a clinical pearl, Dr. Jonkman noted that all patients with severe disease and 39 of the 40 with mild disease had prominent itching. "That means if your patient does not have itch, he or she probably doesn’t have bullous pemphigoid. It's such a central part of the disease," he said.
Disease control (absence of new bullae for 3 consecutive days) was achieved in a mean of 26 days in 36 of 40 patients (90%) with mild disease and in 25 of 34 (73.5%) with severe disease. Thus, transcutaneous systemic clobetasol was less effective in patients with severe bullous pemphigoid, even though most of them were on additional medications, most often azathioprine.
"In severe bullous pemphigoid, trans-cutaneous systemic clobetasol is not sufficient, although it has added value as an adjunct," Dr. Jonkman said.
Thirty-six percent of patients in the mild disease group maintained a complete remission off therapy. Another 29% had a complete remission while on therapy only, 15% had a partial remission, and 20% had no remission.
In the severe bullous pemphigoid group, 6% maintained a complete remission off therapy, 35% had a complete remission while on therapy only, 29% had a partial remission, and 30% had no remission.
Relapse occurred in 56% of responders in both groups a mean of 2 months after they completed the 4-month treatment course. The relapses were mild and responded to another round of whole-body clobetasol or, if the patient preferred, oral therapy, according to Dr. Jonkman.
The most common side effect was skin atrophy, seen in 13 of the 74 patients. Striae and purpura were the other main adverse events.
Audience Questions
An audience member at the congress asked if the treatment could trigger steroid-induced diabetes. Dr. Jonkman replied that the complication hasn't arisen in his patients on transcutaneous systemic clobetasol therapy. He said he suspects it's because the 4-month treatment course is too brief to trigger the problem. He has found, however, that some patients with preexisting type 2 diabetes require an additional oral antidiabetic agent.
Another audience member asked if patients should apply the topical steroid on top of intact blisters and the wounds created by broken blisters. This should be done, Dr. Jonkman advised. "The medication won't work on top of an intact blister because it's too far away from the dermis. But because it's systemic therapy it will come from the blood into the skin," he explained.
A third audience member asked if Dr. Jonkman had ever used whole-body clobetasol in patients with pemphigus foliaceous. He replied that he hasn't because the opportunity hasn't arisen, although he said he suspects it would be effective in milder cases.
Background Studies
Dr. Jonkman credited French dermatologists with the idea of using topical corticosteroids to treat patients with bullous pemphigoid. In a landmark multicenter trial nearly a decade ago, researchers randomized 341 bullous pemphigoid patients to 40 g/day of topical clobetasol propionate cream or to oral prednisone for 1 year. Patients with moderate disease received 0.5 mg/kg per day of prednisone, while those with severe disease got 1 mg/kg.
The topical regimen proved superior to oral prednisone in the 188 patients with extensive disease. Disease was controlled at 3 weeks in 99% of patients on clobetasol, significantly higher than the 91% rate with prednisone. Moreover, the 1-year survival rate was 76% in the topical therapy arm, compared with 58% with oral therapy. Severe complications were half as common in the clobetasol arm.
In patients with moderate bullous pemphigoid, the two forms of therapy were similar in terms of disease control at 3 weeks, overall survival, and severe complications (N. Engl. J. Med. 2002;346:321-7).
Dr. Jonkman noted that more recently the French group tweaked their topical regimen, adopting a milder approach similar to his own. In a 312-patient multicenter trial, they randomized participants to 10-30 g/day of clobetasol tapered over a 4-month period or to their earlier regimen of 40 g/day for a full year. The investigators showed that the rate of death or life-threatening adverse events in the 134 patients with moderate disease was reduced by nearly half with the milder regimen (J. Invest. Dermatol. 2009;129:1681-7).
He reported having no relevant financial disclosures.
LISBON – An intensive regimen of whole-body topical clobetasol may be an effective and better tolerated alternative to standard, high-dose oral prednisone in patients with milder cases of bullous pemphigoid, according to Dr. Marcel F. Jonkman.
"In your daily practice you all use topical clobetasol, but not like this. I call it transcutaneous systemic clobetasol therapy. That means you put it on the entire geography, the whole body surface from your cheeks to your toes, not just on lesional skin," Dr. Jonkman explained at the annual congress of the European Academy of Dermatology and Venereology.
This is a laborious 4-month-long course of therapy. For mild disease – which Dr. Jonkman defined as fewer than 10 new bullae arising during the 3 days before first consultation – patients apply 20 g of clobetasol propionate cream once daily for the first month, then every other day for the second month, twice weekly in the third, and once per week for the fourth month.
Patients with severe bullous pemphigoid follow the same schedule, using 30 g per application rather than 20 g.
"That's really a lot of clobetasol. If you put on 25 g, I expect it's effectively equivalent to 35-40 mg of oral prednisone. Sometimes I get referred patients who are on 80 mg/day of prednisone and they respond to clobetasol therapy," said Dr. Jonkman, professor and chair of the department of dermatology at the University of Groningen (the Netherlands).
This is a decidedly off-label method of applying the superpotent fluorinated topical corticosteroid, he said. And make no mistake, the medication is absorbed systemically to a substantial degree. That's why it is so effective. Within about 5 days after starting daily therapy, the peripheral hypereosinophilia that marks bullous pemphigoid is reversed, with titers falling into the normal range.
Moreover, whole-body topical clobetasol is capable of inducing adrenal gland suppression and other familiar systemic side effects of high-dose corticosteroid therapy, said Dr. Jonkman. The main advantage of topical therapy is it avoids the severe gastritis that often results from long-term, high-dose oral prednisone.
Clinical Experience
He presented his personal experience using transcutaneous systemic clobetasol in 40 patients with mild, and 34 patients with severe, bullous pemphigoid. Patients in the mild disease group had a mean of 3.6 new blisters during the 3 days prior to first consultation; those in the severe bullous pemphigoid group averaged 36.7.
As a clinical pearl, Dr. Jonkman noted that all patients with severe disease and 39 of the 40 with mild disease had prominent itching. "That means if your patient does not have itch, he or she probably doesn’t have bullous pemphigoid. It's such a central part of the disease," he said.
Disease control (absence of new bullae for 3 consecutive days) was achieved in a mean of 26 days in 36 of 40 patients (90%) with mild disease and in 25 of 34 (73.5%) with severe disease. Thus, transcutaneous systemic clobetasol was less effective in patients with severe bullous pemphigoid, even though most of them were on additional medications, most often azathioprine.
"In severe bullous pemphigoid, trans-cutaneous systemic clobetasol is not sufficient, although it has added value as an adjunct," Dr. Jonkman said.
Thirty-six percent of patients in the mild disease group maintained a complete remission off therapy. Another 29% had a complete remission while on therapy only, 15% had a partial remission, and 20% had no remission.
In the severe bullous pemphigoid group, 6% maintained a complete remission off therapy, 35% had a complete remission while on therapy only, 29% had a partial remission, and 30% had no remission.
Relapse occurred in 56% of responders in both groups a mean of 2 months after they completed the 4-month treatment course. The relapses were mild and responded to another round of whole-body clobetasol or, if the patient preferred, oral therapy, according to Dr. Jonkman.
The most common side effect was skin atrophy, seen in 13 of the 74 patients. Striae and purpura were the other main adverse events.
Audience Questions
An audience member at the congress asked if the treatment could trigger steroid-induced diabetes. Dr. Jonkman replied that the complication hasn't arisen in his patients on transcutaneous systemic clobetasol therapy. He said he suspects it's because the 4-month treatment course is too brief to trigger the problem. He has found, however, that some patients with preexisting type 2 diabetes require an additional oral antidiabetic agent.
Another audience member asked if patients should apply the topical steroid on top of intact blisters and the wounds created by broken blisters. This should be done, Dr. Jonkman advised. "The medication won't work on top of an intact blister because it's too far away from the dermis. But because it's systemic therapy it will come from the blood into the skin," he explained.
A third audience member asked if Dr. Jonkman had ever used whole-body clobetasol in patients with pemphigus foliaceous. He replied that he hasn't because the opportunity hasn't arisen, although he said he suspects it would be effective in milder cases.
Background Studies
Dr. Jonkman credited French dermatologists with the idea of using topical corticosteroids to treat patients with bullous pemphigoid. In a landmark multicenter trial nearly a decade ago, researchers randomized 341 bullous pemphigoid patients to 40 g/day of topical clobetasol propionate cream or to oral prednisone for 1 year. Patients with moderate disease received 0.5 mg/kg per day of prednisone, while those with severe disease got 1 mg/kg.
The topical regimen proved superior to oral prednisone in the 188 patients with extensive disease. Disease was controlled at 3 weeks in 99% of patients on clobetasol, significantly higher than the 91% rate with prednisone. Moreover, the 1-year survival rate was 76% in the topical therapy arm, compared with 58% with oral therapy. Severe complications were half as common in the clobetasol arm.
In patients with moderate bullous pemphigoid, the two forms of therapy were similar in terms of disease control at 3 weeks, overall survival, and severe complications (N. Engl. J. Med. 2002;346:321-7).
Dr. Jonkman noted that more recently the French group tweaked their topical regimen, adopting a milder approach similar to his own. In a 312-patient multicenter trial, they randomized participants to 10-30 g/day of clobetasol tapered over a 4-month period or to their earlier regimen of 40 g/day for a full year. The investigators showed that the rate of death or life-threatening adverse events in the 134 patients with moderate disease was reduced by nearly half with the milder regimen (J. Invest. Dermatol. 2009;129:1681-7).
He reported having no relevant financial disclosures.
LISBON – An intensive regimen of whole-body topical clobetasol may be an effective and better tolerated alternative to standard, high-dose oral prednisone in patients with milder cases of bullous pemphigoid, according to Dr. Marcel F. Jonkman.
"In your daily practice you all use topical clobetasol, but not like this. I call it transcutaneous systemic clobetasol therapy. That means you put it on the entire geography, the whole body surface from your cheeks to your toes, not just on lesional skin," Dr. Jonkman explained at the annual congress of the European Academy of Dermatology and Venereology.
This is a laborious 4-month-long course of therapy. For mild disease – which Dr. Jonkman defined as fewer than 10 new bullae arising during the 3 days before first consultation – patients apply 20 g of clobetasol propionate cream once daily for the first month, then every other day for the second month, twice weekly in the third, and once per week for the fourth month.
Patients with severe bullous pemphigoid follow the same schedule, using 30 g per application rather than 20 g.
"That's really a lot of clobetasol. If you put on 25 g, I expect it's effectively equivalent to 35-40 mg of oral prednisone. Sometimes I get referred patients who are on 80 mg/day of prednisone and they respond to clobetasol therapy," said Dr. Jonkman, professor and chair of the department of dermatology at the University of Groningen (the Netherlands).
This is a decidedly off-label method of applying the superpotent fluorinated topical corticosteroid, he said. And make no mistake, the medication is absorbed systemically to a substantial degree. That's why it is so effective. Within about 5 days after starting daily therapy, the peripheral hypereosinophilia that marks bullous pemphigoid is reversed, with titers falling into the normal range.
Moreover, whole-body topical clobetasol is capable of inducing adrenal gland suppression and other familiar systemic side effects of high-dose corticosteroid therapy, said Dr. Jonkman. The main advantage of topical therapy is it avoids the severe gastritis that often results from long-term, high-dose oral prednisone.
Clinical Experience
He presented his personal experience using transcutaneous systemic clobetasol in 40 patients with mild, and 34 patients with severe, bullous pemphigoid. Patients in the mild disease group had a mean of 3.6 new blisters during the 3 days prior to first consultation; those in the severe bullous pemphigoid group averaged 36.7.
As a clinical pearl, Dr. Jonkman noted that all patients with severe disease and 39 of the 40 with mild disease had prominent itching. "That means if your patient does not have itch, he or she probably doesn’t have bullous pemphigoid. It's such a central part of the disease," he said.
Disease control (absence of new bullae for 3 consecutive days) was achieved in a mean of 26 days in 36 of 40 patients (90%) with mild disease and in 25 of 34 (73.5%) with severe disease. Thus, transcutaneous systemic clobetasol was less effective in patients with severe bullous pemphigoid, even though most of them were on additional medications, most often azathioprine.
"In severe bullous pemphigoid, trans-cutaneous systemic clobetasol is not sufficient, although it has added value as an adjunct," Dr. Jonkman said.
Thirty-six percent of patients in the mild disease group maintained a complete remission off therapy. Another 29% had a complete remission while on therapy only, 15% had a partial remission, and 20% had no remission.
In the severe bullous pemphigoid group, 6% maintained a complete remission off therapy, 35% had a complete remission while on therapy only, 29% had a partial remission, and 30% had no remission.
Relapse occurred in 56% of responders in both groups a mean of 2 months after they completed the 4-month treatment course. The relapses were mild and responded to another round of whole-body clobetasol or, if the patient preferred, oral therapy, according to Dr. Jonkman.
The most common side effect was skin atrophy, seen in 13 of the 74 patients. Striae and purpura were the other main adverse events.
Audience Questions
An audience member at the congress asked if the treatment could trigger steroid-induced diabetes. Dr. Jonkman replied that the complication hasn't arisen in his patients on transcutaneous systemic clobetasol therapy. He said he suspects it's because the 4-month treatment course is too brief to trigger the problem. He has found, however, that some patients with preexisting type 2 diabetes require an additional oral antidiabetic agent.
Another audience member asked if patients should apply the topical steroid on top of intact blisters and the wounds created by broken blisters. This should be done, Dr. Jonkman advised. "The medication won't work on top of an intact blister because it's too far away from the dermis. But because it's systemic therapy it will come from the blood into the skin," he explained.
A third audience member asked if Dr. Jonkman had ever used whole-body clobetasol in patients with pemphigus foliaceous. He replied that he hasn't because the opportunity hasn't arisen, although he said he suspects it would be effective in milder cases.
Background Studies
Dr. Jonkman credited French dermatologists with the idea of using topical corticosteroids to treat patients with bullous pemphigoid. In a landmark multicenter trial nearly a decade ago, researchers randomized 341 bullous pemphigoid patients to 40 g/day of topical clobetasol propionate cream or to oral prednisone for 1 year. Patients with moderate disease received 0.5 mg/kg per day of prednisone, while those with severe disease got 1 mg/kg.
The topical regimen proved superior to oral prednisone in the 188 patients with extensive disease. Disease was controlled at 3 weeks in 99% of patients on clobetasol, significantly higher than the 91% rate with prednisone. Moreover, the 1-year survival rate was 76% in the topical therapy arm, compared with 58% with oral therapy. Severe complications were half as common in the clobetasol arm.
In patients with moderate bullous pemphigoid, the two forms of therapy were similar in terms of disease control at 3 weeks, overall survival, and severe complications (N. Engl. J. Med. 2002;346:321-7).
Dr. Jonkman noted that more recently the French group tweaked their topical regimen, adopting a milder approach similar to his own. In a 312-patient multicenter trial, they randomized participants to 10-30 g/day of clobetasol tapered over a 4-month period or to their earlier regimen of 40 g/day for a full year. The investigators showed that the rate of death or life-threatening adverse events in the 134 patients with moderate disease was reduced by nearly half with the milder regimen (J. Invest. Dermatol. 2009;129:1681-7).
He reported having no relevant financial disclosures.
FROM THE ANNUAL CONGRESS OF THE EUROPEAN ACADEMY OF DERMATOLOGY AND VENEREOLOGY
Major Finding: Disease control was achieved in a mean of 26 days in 90% of patients with mild disease and in 73.5% with severe disease.
Data Source: A series of 74 bullous pemphigoid patients.
Disclosures: Dr. Jonkman reported no relevant financial disclosures.
New Anticonvulsants Did Not Impair Bone Density in VA Study
SAN DIEGO – Newer anticonvulsants do not appear to be associated with the reduced bone mineral density seen with traditional anticonvulsants, based on results from a retrospective Veterans Affairs study.
Observational studies have linked traditional anticonvulsants, such as phenytoin, carbamazepine, and valproic acid, to reduced bone mineral density (Neurology 2004;62:2051-7). There are few data on this score regarding the newer anticonvulsants, including gabapentin, levetiracetam, lamotrigine, and topiramate, drugs increasingly prescribed not only for seizure disorders but for mood disorders and neuropathic pain as well.
Dr. Richard Lee and coworkers at Duke University Medical Center, Durham, N.C., performed a retrospective study of 1,799 patients who underwent bone mineral density measurement at the Durham VA Medical Center from 2005 through June 2010. The study population comprised 679 patients on newer anticonvulsants and 382 on traditional antiepileptic drugs.
Bone mineral density in patients on traditional anticonvulsants was progressively lower at the lumbar spine, total hip, and femoral neck with each additional 90-day period on medication during the previous 10 years, based on a multivariate logistic regression analysis.
In contrast, bone density increased at the lumbar spine and total hip with exposure to the newer nonenzyme-inducing anticonvulsants. There was a nonsignificant trend for increasing bone density at the femoral neck with greater exposure to these agents. For every 90 days on one of the newer nonenzyme-inducing anticonvulsants (lamotrigine, gabapentin, levetiracetam, pregabalin), dual-energy x-ray absorptiometry T scores increased significantly by 0.13 at the lumbar spine and by 0.087 at the total hip.
Topiramate, unlike the other newer anticonvulsants, is enzyme inducing, yet it also showed favorable bone-boosting trends that did not reach statistical significance.
The analysis was adjusted for age, race, body mass index, alcohol and tobacco use, prior fractures, and medical comorbidities. Dr. Lee said he had no disclosures.
SAN DIEGO – Newer anticonvulsants do not appear to be associated with the reduced bone mineral density seen with traditional anticonvulsants, based on results from a retrospective Veterans Affairs study.
Observational studies have linked traditional anticonvulsants, such as phenytoin, carbamazepine, and valproic acid, to reduced bone mineral density (Neurology 2004;62:2051-7). There are few data on this score regarding the newer anticonvulsants, including gabapentin, levetiracetam, lamotrigine, and topiramate, drugs increasingly prescribed not only for seizure disorders but for mood disorders and neuropathic pain as well.
Dr. Richard Lee and coworkers at Duke University Medical Center, Durham, N.C., performed a retrospective study of 1,799 patients who underwent bone mineral density measurement at the Durham VA Medical Center from 2005 through June 2010. The study population comprised 679 patients on newer anticonvulsants and 382 on traditional antiepileptic drugs.
Bone mineral density in patients on traditional anticonvulsants was progressively lower at the lumbar spine, total hip, and femoral neck with each additional 90-day period on medication during the previous 10 years, based on a multivariate logistic regression analysis.
In contrast, bone density increased at the lumbar spine and total hip with exposure to the newer nonenzyme-inducing anticonvulsants. There was a nonsignificant trend for increasing bone density at the femoral neck with greater exposure to these agents. For every 90 days on one of the newer nonenzyme-inducing anticonvulsants (lamotrigine, gabapentin, levetiracetam, pregabalin), dual-energy x-ray absorptiometry T scores increased significantly by 0.13 at the lumbar spine and by 0.087 at the total hip.
Topiramate, unlike the other newer anticonvulsants, is enzyme inducing, yet it also showed favorable bone-boosting trends that did not reach statistical significance.
The analysis was adjusted for age, race, body mass index, alcohol and tobacco use, prior fractures, and medical comorbidities. Dr. Lee said he had no disclosures.
SAN DIEGO – Newer anticonvulsants do not appear to be associated with the reduced bone mineral density seen with traditional anticonvulsants, based on results from a retrospective Veterans Affairs study.
Observational studies have linked traditional anticonvulsants, such as phenytoin, carbamazepine, and valproic acid, to reduced bone mineral density (Neurology 2004;62:2051-7). There are few data on this score regarding the newer anticonvulsants, including gabapentin, levetiracetam, lamotrigine, and topiramate, drugs increasingly prescribed not only for seizure disorders but for mood disorders and neuropathic pain as well.
Dr. Richard Lee and coworkers at Duke University Medical Center, Durham, N.C., performed a retrospective study of 1,799 patients who underwent bone mineral density measurement at the Durham VA Medical Center from 2005 through June 2010. The study population comprised 679 patients on newer anticonvulsants and 382 on traditional antiepileptic drugs.
Bone mineral density in patients on traditional anticonvulsants was progressively lower at the lumbar spine, total hip, and femoral neck with each additional 90-day period on medication during the previous 10 years, based on a multivariate logistic regression analysis.
In contrast, bone density increased at the lumbar spine and total hip with exposure to the newer nonenzyme-inducing anticonvulsants. There was a nonsignificant trend for increasing bone density at the femoral neck with greater exposure to these agents. For every 90 days on one of the newer nonenzyme-inducing anticonvulsants (lamotrigine, gabapentin, levetiracetam, pregabalin), dual-energy x-ray absorptiometry T scores increased significantly by 0.13 at the lumbar spine and by 0.087 at the total hip.
Topiramate, unlike the other newer anticonvulsants, is enzyme inducing, yet it also showed favorable bone-boosting trends that did not reach statistical significance.
The analysis was adjusted for age, race, body mass index, alcohol and tobacco use, prior fractures, and medical comorbidities. Dr. Lee said he had no disclosures.
From the Annual Meeting of the American Society for Bone and Mineral Research
Analysis Challenges Link Between Vitamin D Deficiency and Cancer
SAN ANTONIO – A new meta-analysis of 16 studies challenges the notion that breast cancer risk is inversely related to serum vitamin D level.
In 10 studies, vitamin D was measured before diagnosis of breast cancer; in the other 6 studies, blood samples were gathered for vitamin D measurement only after the diagnosis.
When data from all 16 studies were pooled, lower vitamin D levels were linked with a significant 1.5-fold increased rate of breast cancer (P less than .001). Thus, low vitamin D might be causally related to breast cancer, and, by extension, vitamin D supplementation might be an effective option for breast cancer prevention. But a major difficulty with this line of thinking arose when the two groups of studies were analyzed separately, Dr. Eitan Amir said at the meeting.
Only 1 of the 10 studies in which vitamin D was measured before diagnosis of breast cancer showed a significant relationship between low levels of vitamin D and subsequent increased likelihood of the malignancy. But all six studies in which serum vitamin D was measured after the diagnosis showed a significant inverse relationship. In the pooled analysis of these six studies, lower serum vitamin D was associated with a highly significant, 2.63-fold increased likelihood of breast cancer (P less than .001), said Dr. Amir of the University of Toronto.
Breast cancer cells have been shown to express vitamin D catalytic enzymes that may interfere with accurate measurement of serum levels of the vitamin, added Dr. Amir, who said he had no disclosures.
SAN ANTONIO – A new meta-analysis of 16 studies challenges the notion that breast cancer risk is inversely related to serum vitamin D level.
In 10 studies, vitamin D was measured before diagnosis of breast cancer; in the other 6 studies, blood samples were gathered for vitamin D measurement only after the diagnosis.
When data from all 16 studies were pooled, lower vitamin D levels were linked with a significant 1.5-fold increased rate of breast cancer (P less than .001). Thus, low vitamin D might be causally related to breast cancer, and, by extension, vitamin D supplementation might be an effective option for breast cancer prevention. But a major difficulty with this line of thinking arose when the two groups of studies were analyzed separately, Dr. Eitan Amir said at the meeting.
Only 1 of the 10 studies in which vitamin D was measured before diagnosis of breast cancer showed a significant relationship between low levels of vitamin D and subsequent increased likelihood of the malignancy. But all six studies in which serum vitamin D was measured after the diagnosis showed a significant inverse relationship. In the pooled analysis of these six studies, lower serum vitamin D was associated with a highly significant, 2.63-fold increased likelihood of breast cancer (P less than .001), said Dr. Amir of the University of Toronto.
Breast cancer cells have been shown to express vitamin D catalytic enzymes that may interfere with accurate measurement of serum levels of the vitamin, added Dr. Amir, who said he had no disclosures.
SAN ANTONIO – A new meta-analysis of 16 studies challenges the notion that breast cancer risk is inversely related to serum vitamin D level.
In 10 studies, vitamin D was measured before diagnosis of breast cancer; in the other 6 studies, blood samples were gathered for vitamin D measurement only after the diagnosis.
When data from all 16 studies were pooled, lower vitamin D levels were linked with a significant 1.5-fold increased rate of breast cancer (P less than .001). Thus, low vitamin D might be causally related to breast cancer, and, by extension, vitamin D supplementation might be an effective option for breast cancer prevention. But a major difficulty with this line of thinking arose when the two groups of studies were analyzed separately, Dr. Eitan Amir said at the meeting.
Only 1 of the 10 studies in which vitamin D was measured before diagnosis of breast cancer showed a significant relationship between low levels of vitamin D and subsequent increased likelihood of the malignancy. But all six studies in which serum vitamin D was measured after the diagnosis showed a significant inverse relationship. In the pooled analysis of these six studies, lower serum vitamin D was associated with a highly significant, 2.63-fold increased likelihood of breast cancer (P less than .001), said Dr. Amir of the University of Toronto.
Breast cancer cells have been shown to express vitamin D catalytic enzymes that may interfere with accurate measurement of serum levels of the vitamin, added Dr. Amir, who said he had no disclosures.
From the San Antonio Breast Cancer Symposium
Early Oophorectomy Linked to Osteoporosis and Arthritis
SAN ANTONIO – Bilateral oophorectomy in women younger than age 45 is associated with a subsequent doubled prevalence of osteoporosis and a similarly elevated rate of arthritis, compared with women with intact ovaries.
The findings from a new analysis of the Third National Health and Nutrition Examination Survey (NHANES III) had a further twist: The likelihood of having low bone mineral density and/or arthritis was even greater in the subgroup of women not on hormone replacement therapy following their surgically-induced abrupt menopause, Anne Marie McCarthy said at the symposium.
“The implication of our findings is that women who've had their ovaries removed at a young age can now be informed about their risk for bone loss over the long term. However, additional studies are needed to determine the frequency of monitoring for osteoporosis and the appropriateness of various preventive strategies in women who've had their ovaries removed,” said Ms. McCarthy, a doctoral candidate in epidemiology at Johns Hopkins University, Baltimore.
The bone mineral density analysis included 3,660 women who underwent femoral neck bone density measurement by dual energy x-ray as part of their participation in NHANES III, which was conducted in a U.S. nationally representative sample in 1988-1994.
The age-standardized mean femoral neck bone density was significantly lower in women with oophorectomy before age 45 than in those with intact ovaries: 0.711 compared with 0.743 g/m
The Johns Hopkins researchers are doing a study in which they're measuring bone mineral density before and after prophylactic oophorectomy in women who carry high-risk BRCA mutations.
The arthritis analysis included 4,039 women. Those who had undergone oophorectomy were significantly more likely to report having been informed by a physician that they have arthritis, by a margin of 45.4% to 32.1% (P less than .001). In the subset of women with oophorectomy before age 45, the prevalence of arthritis was higher at 47.7%. In a multivariate analysis, women with oophorectomy before age 45 had a 1.78-fold increased odds of arthritis compared with those with intact ovaries. If they didn't use hormone therapy, however, those odds rose to 1.99-fold.
The researchers did not study the NHANES III subjects' medical records, so they were unable to say which forms of arthritis were more prevalent in the early oophorectomy group. Ms. McCarthy said the oophorectomy-arthritis association needs confirmation by other studies. There are animal data supporting such a link, she noted.
“We think estrogen is important for the health of cartilage, so losing estrogen can lead to inflammation and damage of cartilage, perhaps,” Ms. McCarthy said.
Prophylactic bilateral oophorectomy is a widely accepted procedure to reduce the risks of breast and ovarian cancer in BRCA mutation carriers. But this indication accounts for only a small fraction of oophorectomies performed in this country. About 600,000 women per year undergo hysterectomy for indications such as fibroids, abnormal bleeding, endometriosis, and uterine prolapse, according to the Centers for Disease Control and Prevention, and about half of them have both ovaries removed at that time to prevent ovarian cancer.
NHANES III was conducted by the CDC. Ms. McCarthy said she had no relevant financial disclosures.
'Women who've had their ovaries removed at a young age can now be informed about their risk for bone loss.'
Source DR. McCARTHY
SAN ANTONIO – Bilateral oophorectomy in women younger than age 45 is associated with a subsequent doubled prevalence of osteoporosis and a similarly elevated rate of arthritis, compared with women with intact ovaries.
The findings from a new analysis of the Third National Health and Nutrition Examination Survey (NHANES III) had a further twist: The likelihood of having low bone mineral density and/or arthritis was even greater in the subgroup of women not on hormone replacement therapy following their surgically-induced abrupt menopause, Anne Marie McCarthy said at the symposium.
“The implication of our findings is that women who've had their ovaries removed at a young age can now be informed about their risk for bone loss over the long term. However, additional studies are needed to determine the frequency of monitoring for osteoporosis and the appropriateness of various preventive strategies in women who've had their ovaries removed,” said Ms. McCarthy, a doctoral candidate in epidemiology at Johns Hopkins University, Baltimore.
The bone mineral density analysis included 3,660 women who underwent femoral neck bone density measurement by dual energy x-ray as part of their participation in NHANES III, which was conducted in a U.S. nationally representative sample in 1988-1994.
The age-standardized mean femoral neck bone density was significantly lower in women with oophorectomy before age 45 than in those with intact ovaries: 0.711 compared with 0.743 g/m
The Johns Hopkins researchers are doing a study in which they're measuring bone mineral density before and after prophylactic oophorectomy in women who carry high-risk BRCA mutations.
The arthritis analysis included 4,039 women. Those who had undergone oophorectomy were significantly more likely to report having been informed by a physician that they have arthritis, by a margin of 45.4% to 32.1% (P less than .001). In the subset of women with oophorectomy before age 45, the prevalence of arthritis was higher at 47.7%. In a multivariate analysis, women with oophorectomy before age 45 had a 1.78-fold increased odds of arthritis compared with those with intact ovaries. If they didn't use hormone therapy, however, those odds rose to 1.99-fold.
The researchers did not study the NHANES III subjects' medical records, so they were unable to say which forms of arthritis were more prevalent in the early oophorectomy group. Ms. McCarthy said the oophorectomy-arthritis association needs confirmation by other studies. There are animal data supporting such a link, she noted.
“We think estrogen is important for the health of cartilage, so losing estrogen can lead to inflammation and damage of cartilage, perhaps,” Ms. McCarthy said.
Prophylactic bilateral oophorectomy is a widely accepted procedure to reduce the risks of breast and ovarian cancer in BRCA mutation carriers. But this indication accounts for only a small fraction of oophorectomies performed in this country. About 600,000 women per year undergo hysterectomy for indications such as fibroids, abnormal bleeding, endometriosis, and uterine prolapse, according to the Centers for Disease Control and Prevention, and about half of them have both ovaries removed at that time to prevent ovarian cancer.
NHANES III was conducted by the CDC. Ms. McCarthy said she had no relevant financial disclosures.
'Women who've had their ovaries removed at a young age can now be informed about their risk for bone loss.'
Source DR. McCARTHY
SAN ANTONIO – Bilateral oophorectomy in women younger than age 45 is associated with a subsequent doubled prevalence of osteoporosis and a similarly elevated rate of arthritis, compared with women with intact ovaries.
The findings from a new analysis of the Third National Health and Nutrition Examination Survey (NHANES III) had a further twist: The likelihood of having low bone mineral density and/or arthritis was even greater in the subgroup of women not on hormone replacement therapy following their surgically-induced abrupt menopause, Anne Marie McCarthy said at the symposium.
“The implication of our findings is that women who've had their ovaries removed at a young age can now be informed about their risk for bone loss over the long term. However, additional studies are needed to determine the frequency of monitoring for osteoporosis and the appropriateness of various preventive strategies in women who've had their ovaries removed,” said Ms. McCarthy, a doctoral candidate in epidemiology at Johns Hopkins University, Baltimore.
The bone mineral density analysis included 3,660 women who underwent femoral neck bone density measurement by dual energy x-ray as part of their participation in NHANES III, which was conducted in a U.S. nationally representative sample in 1988-1994.
The age-standardized mean femoral neck bone density was significantly lower in women with oophorectomy before age 45 than in those with intact ovaries: 0.711 compared with 0.743 g/m
The Johns Hopkins researchers are doing a study in which they're measuring bone mineral density before and after prophylactic oophorectomy in women who carry high-risk BRCA mutations.
The arthritis analysis included 4,039 women. Those who had undergone oophorectomy were significantly more likely to report having been informed by a physician that they have arthritis, by a margin of 45.4% to 32.1% (P less than .001). In the subset of women with oophorectomy before age 45, the prevalence of arthritis was higher at 47.7%. In a multivariate analysis, women with oophorectomy before age 45 had a 1.78-fold increased odds of arthritis compared with those with intact ovaries. If they didn't use hormone therapy, however, those odds rose to 1.99-fold.
The researchers did not study the NHANES III subjects' medical records, so they were unable to say which forms of arthritis were more prevalent in the early oophorectomy group. Ms. McCarthy said the oophorectomy-arthritis association needs confirmation by other studies. There are animal data supporting such a link, she noted.
“We think estrogen is important for the health of cartilage, so losing estrogen can lead to inflammation and damage of cartilage, perhaps,” Ms. McCarthy said.
Prophylactic bilateral oophorectomy is a widely accepted procedure to reduce the risks of breast and ovarian cancer in BRCA mutation carriers. But this indication accounts for only a small fraction of oophorectomies performed in this country. About 600,000 women per year undergo hysterectomy for indications such as fibroids, abnormal bleeding, endometriosis, and uterine prolapse, according to the Centers for Disease Control and Prevention, and about half of them have both ovaries removed at that time to prevent ovarian cancer.
NHANES III was conducted by the CDC. Ms. McCarthy said she had no relevant financial disclosures.
'Women who've had their ovaries removed at a young age can now be informed about their risk for bone loss.'
Source DR. McCARTHY
From the San Antonio Breast Cancer Symposium
Comorbid Psoriasis Adds Hefty Economic Burden
LISBON – Psoriasis and diabetes are expensive diseases, and patients with both conditions experience a synergistic increase in health care utilization and costs that is significantly greater than the incremental economic burden imposed by each disease individually.
In other words, patients with comorbid psoriasis and diabetes have more hospitalizations and outpatient visits over the course of a year than would be expected simply from adding together the increased use typical of patients with psoriasis to that of patients with diabetes, compared with health care use by individuals with neither condition, Dr. Frank Zhang reported at the annual congress of the European Academy of Dermatology and Venereology.
This is an observation with important implications for health economics. These are two common diseases. Psoriasis affects 2%-3% of the world's population, with 260,000 new cases arising per year in the United States alone. Psoriasis predisposes to insulin resistance, and psoriasis patients have a one-third greater risk of diabetes than the general population, noted Dr. Zhang of Celgene Corporation, Summit, N.J.
Because the economic impact of having both diseases had not been addressed, he and his colleagues conducted a retrospective study of 106,128 patient pairs matched for age and gender, one member of each pair having psoriasis and the other free of the disease. The patients were drawn from the Thomson Reuters MarketScan Research Databases for 2004 through June 2009.
Sixteen percent of the psoriasis patients had diabetes, significantly greater than the 13% with diabetes in the control group.
Psoriasis patients with diabetes averaged 17 more hospitalizations per 100 patient-years and 5.8 more outpatient visits per year than did psoriasis patients without diabetes. Furthermore, patients with diabetes and psoriasis averaged five more hospitalizations per 100 patient-years and 6.3 additional outpatient visits per year, compared with nonpsoriatic patients with diabetes.
The most likely explanation for this synergistic health care burden in the dual-diagnosis patient lies in the complexity entailed in managing the two diseases simultaneously, said Dr. Zhang.
Total annual health care costs in patients with both psoriasis and diabetes averaged $19,536, compared with $13,589 for psoriasis-free patients with diabetes and $5,539 in those who had psoriasis but not diabetes.
The study was funded by Celgene, where Dr. Zhang is employed.
LISBON – Psoriasis and diabetes are expensive diseases, and patients with both conditions experience a synergistic increase in health care utilization and costs that is significantly greater than the incremental economic burden imposed by each disease individually.
In other words, patients with comorbid psoriasis and diabetes have more hospitalizations and outpatient visits over the course of a year than would be expected simply from adding together the increased use typical of patients with psoriasis to that of patients with diabetes, compared with health care use by individuals with neither condition, Dr. Frank Zhang reported at the annual congress of the European Academy of Dermatology and Venereology.
This is an observation with important implications for health economics. These are two common diseases. Psoriasis affects 2%-3% of the world's population, with 260,000 new cases arising per year in the United States alone. Psoriasis predisposes to insulin resistance, and psoriasis patients have a one-third greater risk of diabetes than the general population, noted Dr. Zhang of Celgene Corporation, Summit, N.J.
Because the economic impact of having both diseases had not been addressed, he and his colleagues conducted a retrospective study of 106,128 patient pairs matched for age and gender, one member of each pair having psoriasis and the other free of the disease. The patients were drawn from the Thomson Reuters MarketScan Research Databases for 2004 through June 2009.
Sixteen percent of the psoriasis patients had diabetes, significantly greater than the 13% with diabetes in the control group.
Psoriasis patients with diabetes averaged 17 more hospitalizations per 100 patient-years and 5.8 more outpatient visits per year than did psoriasis patients without diabetes. Furthermore, patients with diabetes and psoriasis averaged five more hospitalizations per 100 patient-years and 6.3 additional outpatient visits per year, compared with nonpsoriatic patients with diabetes.
The most likely explanation for this synergistic health care burden in the dual-diagnosis patient lies in the complexity entailed in managing the two diseases simultaneously, said Dr. Zhang.
Total annual health care costs in patients with both psoriasis and diabetes averaged $19,536, compared with $13,589 for psoriasis-free patients with diabetes and $5,539 in those who had psoriasis but not diabetes.
The study was funded by Celgene, where Dr. Zhang is employed.
LISBON – Psoriasis and diabetes are expensive diseases, and patients with both conditions experience a synergistic increase in health care utilization and costs that is significantly greater than the incremental economic burden imposed by each disease individually.
In other words, patients with comorbid psoriasis and diabetes have more hospitalizations and outpatient visits over the course of a year than would be expected simply from adding together the increased use typical of patients with psoriasis to that of patients with diabetes, compared with health care use by individuals with neither condition, Dr. Frank Zhang reported at the annual congress of the European Academy of Dermatology and Venereology.
This is an observation with important implications for health economics. These are two common diseases. Psoriasis affects 2%-3% of the world's population, with 260,000 new cases arising per year in the United States alone. Psoriasis predisposes to insulin resistance, and psoriasis patients have a one-third greater risk of diabetes than the general population, noted Dr. Zhang of Celgene Corporation, Summit, N.J.
Because the economic impact of having both diseases had not been addressed, he and his colleagues conducted a retrospective study of 106,128 patient pairs matched for age and gender, one member of each pair having psoriasis and the other free of the disease. The patients were drawn from the Thomson Reuters MarketScan Research Databases for 2004 through June 2009.
Sixteen percent of the psoriasis patients had diabetes, significantly greater than the 13% with diabetes in the control group.
Psoriasis patients with diabetes averaged 17 more hospitalizations per 100 patient-years and 5.8 more outpatient visits per year than did psoriasis patients without diabetes. Furthermore, patients with diabetes and psoriasis averaged five more hospitalizations per 100 patient-years and 6.3 additional outpatient visits per year, compared with nonpsoriatic patients with diabetes.
The most likely explanation for this synergistic health care burden in the dual-diagnosis patient lies in the complexity entailed in managing the two diseases simultaneously, said Dr. Zhang.
Total annual health care costs in patients with both psoriasis and diabetes averaged $19,536, compared with $13,589 for psoriasis-free patients with diabetes and $5,539 in those who had psoriasis but not diabetes.
The study was funded by Celgene, where Dr. Zhang is employed.
From the Annual Congress of the European Academy of Dermatology and Venereology
Adjuvant Breast Cancer Vaccine in Phase III Trial
SAN ANTONIO – A phase III trial of an adjuvant breast cancer vaccine began enrollment before the end of 2011 as a result of favorable 5-year efficacy and safety data in a phase II study.
In updated phase II results, disease-free survival at a median follow-up of 60 months was 95.9% in 53 patients who received the E75 vaccine with multiple booster inoculations, significantly better than the 79.7% figure in 79 controls, Dr. Timothy J. Vreeland reported at the meeting.
The vaccine, known as NeuVax, is composed of the E75 peptide, which is derived from human epidermal growth factor receptor 2 (HER2), mixed with granulocyte macrophage colony-stimulating factor (GM-CSF). The vaccine has previously been shown to stimulate cytotoxic T cells to specifically target cells expressing HER2.
As a result of lessons learned in the randomized phase II study, the phase III trial will be restricted to patients with lymph node–positive tumors who are clinically disease-free after completing standard therapy. Only patients with low levels of HER2 expression, meaning immunohistochemistry 1+ or 2+, will be eligible.
The E75 vaccine was initially given as an intradermal injection once a month for 6 months. Because of waning immunity noted during phase I and II testing, however, a booster immunization program was initiated. It consists of a booster injection once every 6 months. The booster program will be routine in the phase III trial.
The overall phase II study population consisted of 187 patients with node-positive or high-risk node-negative tumors expressing any level of HER2. The median 5-year disease-free survival in the 108 patients in the vaccine arm was 89.4%, compared with 79.7% in controls, a nonsignificant difference. But the vaccine arm included 55 women who didn't receive booster immunizations. When they were excluded, the 5-year disease-free survival rate climbed to 95.9%, according to Dr. Vreeland, a U.S. Army captain at San Antonio Military Medical Center.
The phase III trial is called PRESENT (Prevention of Recurrence in Early-Stage, Node-Positive Breast Cancer with Low to Intermediate HER2 Expression with NeuVax Treatment).
The NeuVax vaccine has been licensed by the U.S. military to Galena Biopharma. Dr. Vreeland delared having no relevant financial disclosures.
SAN ANTONIO – A phase III trial of an adjuvant breast cancer vaccine began enrollment before the end of 2011 as a result of favorable 5-year efficacy and safety data in a phase II study.
In updated phase II results, disease-free survival at a median follow-up of 60 months was 95.9% in 53 patients who received the E75 vaccine with multiple booster inoculations, significantly better than the 79.7% figure in 79 controls, Dr. Timothy J. Vreeland reported at the meeting.
The vaccine, known as NeuVax, is composed of the E75 peptide, which is derived from human epidermal growth factor receptor 2 (HER2), mixed with granulocyte macrophage colony-stimulating factor (GM-CSF). The vaccine has previously been shown to stimulate cytotoxic T cells to specifically target cells expressing HER2.
As a result of lessons learned in the randomized phase II study, the phase III trial will be restricted to patients with lymph node–positive tumors who are clinically disease-free after completing standard therapy. Only patients with low levels of HER2 expression, meaning immunohistochemistry 1+ or 2+, will be eligible.
The E75 vaccine was initially given as an intradermal injection once a month for 6 months. Because of waning immunity noted during phase I and II testing, however, a booster immunization program was initiated. It consists of a booster injection once every 6 months. The booster program will be routine in the phase III trial.
The overall phase II study population consisted of 187 patients with node-positive or high-risk node-negative tumors expressing any level of HER2. The median 5-year disease-free survival in the 108 patients in the vaccine arm was 89.4%, compared with 79.7% in controls, a nonsignificant difference. But the vaccine arm included 55 women who didn't receive booster immunizations. When they were excluded, the 5-year disease-free survival rate climbed to 95.9%, according to Dr. Vreeland, a U.S. Army captain at San Antonio Military Medical Center.
The phase III trial is called PRESENT (Prevention of Recurrence in Early-Stage, Node-Positive Breast Cancer with Low to Intermediate HER2 Expression with NeuVax Treatment).
The NeuVax vaccine has been licensed by the U.S. military to Galena Biopharma. Dr. Vreeland delared having no relevant financial disclosures.
SAN ANTONIO – A phase III trial of an adjuvant breast cancer vaccine began enrollment before the end of 2011 as a result of favorable 5-year efficacy and safety data in a phase II study.
In updated phase II results, disease-free survival at a median follow-up of 60 months was 95.9% in 53 patients who received the E75 vaccine with multiple booster inoculations, significantly better than the 79.7% figure in 79 controls, Dr. Timothy J. Vreeland reported at the meeting.
The vaccine, known as NeuVax, is composed of the E75 peptide, which is derived from human epidermal growth factor receptor 2 (HER2), mixed with granulocyte macrophage colony-stimulating factor (GM-CSF). The vaccine has previously been shown to stimulate cytotoxic T cells to specifically target cells expressing HER2.
As a result of lessons learned in the randomized phase II study, the phase III trial will be restricted to patients with lymph node–positive tumors who are clinically disease-free after completing standard therapy. Only patients with low levels of HER2 expression, meaning immunohistochemistry 1+ or 2+, will be eligible.
The E75 vaccine was initially given as an intradermal injection once a month for 6 months. Because of waning immunity noted during phase I and II testing, however, a booster immunization program was initiated. It consists of a booster injection once every 6 months. The booster program will be routine in the phase III trial.
The overall phase II study population consisted of 187 patients with node-positive or high-risk node-negative tumors expressing any level of HER2. The median 5-year disease-free survival in the 108 patients in the vaccine arm was 89.4%, compared with 79.7% in controls, a nonsignificant difference. But the vaccine arm included 55 women who didn't receive booster immunizations. When they were excluded, the 5-year disease-free survival rate climbed to 95.9%, according to Dr. Vreeland, a U.S. Army captain at San Antonio Military Medical Center.
The phase III trial is called PRESENT (Prevention of Recurrence in Early-Stage, Node-Positive Breast Cancer with Low to Intermediate HER2 Expression with NeuVax Treatment).
The NeuVax vaccine has been licensed by the U.S. military to Galena Biopharma. Dr. Vreeland delared having no relevant financial disclosures.
From the San Antonio Breast Cancer Symposium
Early Bilateral Oophorectomy Is Linked to Osteoporosis, Arthritis
SAN ANTONIO – Bilateral oophorectomy in women younger than age 45 is associated with a subsequent doubled prevalence of osteoporosis and a similarly elevated rate of arthritis, compared with women with intact ovaries.
These findings from a new analysis of the Third National Health and Nutrition Examination Survey (NHANES III) had a further twist: The likelihood of having low bone mineral density and/or arthritis was even greater in the subgroup of women not on hormone replacement therapy following their surgically-induced abrupt menopause, Anne Marie McCarthy said at the annual meeting.
“The implication of our findings is that women who've had their ovaries removed at a young age can now be informed about their risk for bone loss over the long term. However, additional studies are needed to determine the frequency of monitoring for osteoporosis and the appropriateness of various preventive strategies in women who've had their ovaries removed,” according to Ms. McCarthy, a doctoral candidate in epidemiology at Johns Hopkins University, Baltimore.
The bone mineral density analysis included 3,660 women who underwent femoral neck bone density measurement by dual energy x-ray as part of their participation in NHANES III, which was conducted in a U.S. nationally representative sample in 1988-1994.
The age-standardized mean femoral neck bone density was significantly lower in women with oophorectomy before age 45 than in those with intact ovaries: 0.711 compared with 0.743 g/m
The Johns Hopkins investigators are now in the midst of a study in which they're measuring bone mineral density before and after prophylactic oophorectomy in women who carry high-risk BRCA mutations.
The arthritis analysis included 4,039 women. Those who had undergone oophorectomy were significantly more likely to report having been informed by a physician that they have arthritis, by a margin of 45.4% to 32.1% (P less than .001). Among the subset of women with oophorectomy before age 45, the prevalence of arthritis was even higher at 47.7%. In a multivariate analysis, women with oophorectomy when they were younger than 45 had a 1.78-fold increased odds of arthritis compared with those with intact ovaries. If they didn't use hormone replacement therapy, however, those odds rose to 1.99-fold.
Because the investigators didn't study the NHANES III participants' actual medical records, they were unable to say what specific forms of arthritis were more prevalent in the early oophorectomy group. Also, since to Ms. McCarthy's knowledge this is the first-ever study linking oophorectomy to arthritis, this association needs confirmation by others. There are animal data supporting such a link, she noted.
“One possible mechanism is that we think estrogen is important for the health of cartilage, so losing estrogen can lead to inflammation and damage of cartilage, perhaps,” Ms. McCarthy speculated.
Prophylactic bilateral oophorectomy is a widely accepted procedure to reduce the risks of breast and ovarian cancer in BRCA mutation carriers. But this indication actually accounts for only a small fraction of oophorectomies performed in this country.
About 600,000 women per year undergo hysterectomy for indications including fibroids, abnormal bleeding, endometriosis, and uterine prolapse, according to the Centers for Disease Control and Prevention, and about half of them have both ovaries removed at that time to prevent ovarian cancer.
SAN ANTONIO – Bilateral oophorectomy in women younger than age 45 is associated with a subsequent doubled prevalence of osteoporosis and a similarly elevated rate of arthritis, compared with women with intact ovaries.
These findings from a new analysis of the Third National Health and Nutrition Examination Survey (NHANES III) had a further twist: The likelihood of having low bone mineral density and/or arthritis was even greater in the subgroup of women not on hormone replacement therapy following their surgically-induced abrupt menopause, Anne Marie McCarthy said at the annual meeting.
“The implication of our findings is that women who've had their ovaries removed at a young age can now be informed about their risk for bone loss over the long term. However, additional studies are needed to determine the frequency of monitoring for osteoporosis and the appropriateness of various preventive strategies in women who've had their ovaries removed,” according to Ms. McCarthy, a doctoral candidate in epidemiology at Johns Hopkins University, Baltimore.
The bone mineral density analysis included 3,660 women who underwent femoral neck bone density measurement by dual energy x-ray as part of their participation in NHANES III, which was conducted in a U.S. nationally representative sample in 1988-1994.
The age-standardized mean femoral neck bone density was significantly lower in women with oophorectomy before age 45 than in those with intact ovaries: 0.711 compared with 0.743 g/m
The Johns Hopkins investigators are now in the midst of a study in which they're measuring bone mineral density before and after prophylactic oophorectomy in women who carry high-risk BRCA mutations.
The arthritis analysis included 4,039 women. Those who had undergone oophorectomy were significantly more likely to report having been informed by a physician that they have arthritis, by a margin of 45.4% to 32.1% (P less than .001). Among the subset of women with oophorectomy before age 45, the prevalence of arthritis was even higher at 47.7%. In a multivariate analysis, women with oophorectomy when they were younger than 45 had a 1.78-fold increased odds of arthritis compared with those with intact ovaries. If they didn't use hormone replacement therapy, however, those odds rose to 1.99-fold.
Because the investigators didn't study the NHANES III participants' actual medical records, they were unable to say what specific forms of arthritis were more prevalent in the early oophorectomy group. Also, since to Ms. McCarthy's knowledge this is the first-ever study linking oophorectomy to arthritis, this association needs confirmation by others. There are animal data supporting such a link, she noted.
“One possible mechanism is that we think estrogen is important for the health of cartilage, so losing estrogen can lead to inflammation and damage of cartilage, perhaps,” Ms. McCarthy speculated.
Prophylactic bilateral oophorectomy is a widely accepted procedure to reduce the risks of breast and ovarian cancer in BRCA mutation carriers. But this indication actually accounts for only a small fraction of oophorectomies performed in this country.
About 600,000 women per year undergo hysterectomy for indications including fibroids, abnormal bleeding, endometriosis, and uterine prolapse, according to the Centers for Disease Control and Prevention, and about half of them have both ovaries removed at that time to prevent ovarian cancer.
SAN ANTONIO – Bilateral oophorectomy in women younger than age 45 is associated with a subsequent doubled prevalence of osteoporosis and a similarly elevated rate of arthritis, compared with women with intact ovaries.
These findings from a new analysis of the Third National Health and Nutrition Examination Survey (NHANES III) had a further twist: The likelihood of having low bone mineral density and/or arthritis was even greater in the subgroup of women not on hormone replacement therapy following their surgically-induced abrupt menopause, Anne Marie McCarthy said at the annual meeting.
“The implication of our findings is that women who've had their ovaries removed at a young age can now be informed about their risk for bone loss over the long term. However, additional studies are needed to determine the frequency of monitoring for osteoporosis and the appropriateness of various preventive strategies in women who've had their ovaries removed,” according to Ms. McCarthy, a doctoral candidate in epidemiology at Johns Hopkins University, Baltimore.
The bone mineral density analysis included 3,660 women who underwent femoral neck bone density measurement by dual energy x-ray as part of their participation in NHANES III, which was conducted in a U.S. nationally representative sample in 1988-1994.
The age-standardized mean femoral neck bone density was significantly lower in women with oophorectomy before age 45 than in those with intact ovaries: 0.711 compared with 0.743 g/m
The Johns Hopkins investigators are now in the midst of a study in which they're measuring bone mineral density before and after prophylactic oophorectomy in women who carry high-risk BRCA mutations.
The arthritis analysis included 4,039 women. Those who had undergone oophorectomy were significantly more likely to report having been informed by a physician that they have arthritis, by a margin of 45.4% to 32.1% (P less than .001). Among the subset of women with oophorectomy before age 45, the prevalence of arthritis was even higher at 47.7%. In a multivariate analysis, women with oophorectomy when they were younger than 45 had a 1.78-fold increased odds of arthritis compared with those with intact ovaries. If they didn't use hormone replacement therapy, however, those odds rose to 1.99-fold.
Because the investigators didn't study the NHANES III participants' actual medical records, they were unable to say what specific forms of arthritis were more prevalent in the early oophorectomy group. Also, since to Ms. McCarthy's knowledge this is the first-ever study linking oophorectomy to arthritis, this association needs confirmation by others. There are animal data supporting such a link, she noted.
“One possible mechanism is that we think estrogen is important for the health of cartilage, so losing estrogen can lead to inflammation and damage of cartilage, perhaps,” Ms. McCarthy speculated.
Prophylactic bilateral oophorectomy is a widely accepted procedure to reduce the risks of breast and ovarian cancer in BRCA mutation carriers. But this indication actually accounts for only a small fraction of oophorectomies performed in this country.
About 600,000 women per year undergo hysterectomy for indications including fibroids, abnormal bleeding, endometriosis, and uterine prolapse, according to the Centers for Disease Control and Prevention, and about half of them have both ovaries removed at that time to prevent ovarian cancer.
From the San Antonio Breast Cancer Symposium
Major Finding: The age-standardized mean femoral neck bone
density was significantly lower in women with oophorectomy before age 45
than in those with intact ovaries: 0.711 compared with 0.743 g/m
Data Source:
The Third National Health and Nutrition Examination Survey (NHANES
III), conducted in a nationally representative sample of the U.S.
population. The bone mineral density analysis included 3,660 women, and
the arthritis analysis included 4,039 women.
Disclosures:
NHANES III was conducted by the Centers for Disease Control and
Prevention. Ms. McCarthy said she had no relevant financial disclosures.
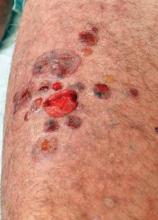
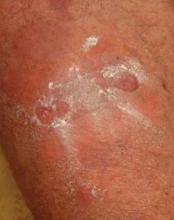
Imiquimod/Abraxane Combo Effective for Skin Mets
SAN ANTONIO – The combination of topical 5% imiquimod plus systemic nanoparticle albumin-bound paclitaxel showed excellent clinical efficacy and was well tolerated for the treatment of cutaneous metastases of breast cancer in a phase II study.
Among the 11 patients who were able to complete the novel combined chemoimmunotherapy regimen, 5 had a complete response, meaning 100% clearance of treated skin lesions at week 24.
In addition, one patient had a partial response, defined as a greater than 50% reduction in the size of the largest treated lesion. Four patients had stable disease, with less than 50% reductions in lesion size. One patient experienced progressive disease, with a 25% increase in target lesion size, Dr. Lupe G. Salazar reported at the San Antonio Breast Cancer Symposium.
Fifteen heavily pretreated breast cancer patients were enrolled in the single-arm, nonrandomized study. All had skin metastases no longer amenable to standard therapies.
The chemoimmunotherapy regimen consisted of three treatment cycles. Each 4-week cycle consisted of application of topical 5% imiquimod to target cutaneous lesions on 4 days per week plus systemic albumin-bound paclitaxel (Abraxane) at 100 mg/m2 on days 1, 8, 15, and 28, explained Dr. Salazar of the University of Washington, Seattle.
Treatment-related toxicities included neutropenia, lymphopenia, anemia, nausea, and fatigue; 34% of toxicities were grade 1, 56% were grade 2, and the remaining 10% were grade 3.
Four of 15 subjects were unable to complete the treatment regimen, having withdrawn due to progression of visceral disease.
The rationale for the imiquimod plus nanoparticle albumin-bound (nab) paclitaxel (Abraxane) therapy derives from previous evidence that imiquimod, a toll-like receptor-7 agonist, has shown clinical efficacy against cutaneous metastases. Imiquimod stimulates secretion of Th1 cytokines and upregulates immune costimulatory molecules at the tumor site. Tumor-specific T cell immunity and tumor growth inhibition are enhanced. Moreover, paclitaxel has been shown to increase serum interferon-gamma levels and boost natural killer cell activity. Thus, the working hypothesis was that nab-paclitaxel would augment imiquimod’s antitumor effects, according to Dr. Salazar.
She and her coinvestigators examined the combination therapy’s impact upon endogenous tumor-specific immunity. They obtained pre- and posttreatment 2-mm skin biopsies from target lesions and were able to demonstrate that the treatment marginally enhanced endogenous immunity to the well-known breast cancer antigens HER2, p53, melanoma-associated antigen 3 (MAGE-3), insulin growth factor binding protein-2 (IGFBP-2), and topoisomerase IIa (TOPO-IIa).
The study was funded by a grant from the National Cancer Institute. Dr. Salazar declared having no relevant financial interests.
SAN ANTONIO – The combination of topical 5% imiquimod plus systemic nanoparticle albumin-bound paclitaxel showed excellent clinical efficacy and was well tolerated for the treatment of cutaneous metastases of breast cancer in a phase II study.
Among the 11 patients who were able to complete the novel combined chemoimmunotherapy regimen, 5 had a complete response, meaning 100% clearance of treated skin lesions at week 24.
In addition, one patient had a partial response, defined as a greater than 50% reduction in the size of the largest treated lesion. Four patients had stable disease, with less than 50% reductions in lesion size. One patient experienced progressive disease, with a 25% increase in target lesion size, Dr. Lupe G. Salazar reported at the San Antonio Breast Cancer Symposium.
Fifteen heavily pretreated breast cancer patients were enrolled in the single-arm, nonrandomized study. All had skin metastases no longer amenable to standard therapies.
The chemoimmunotherapy regimen consisted of three treatment cycles. Each 4-week cycle consisted of application of topical 5% imiquimod to target cutaneous lesions on 4 days per week plus systemic albumin-bound paclitaxel (Abraxane) at 100 mg/m2 on days 1, 8, 15, and 28, explained Dr. Salazar of the University of Washington, Seattle.
Treatment-related toxicities included neutropenia, lymphopenia, anemia, nausea, and fatigue; 34% of toxicities were grade 1, 56% were grade 2, and the remaining 10% were grade 3.
Four of 15 subjects were unable to complete the treatment regimen, having withdrawn due to progression of visceral disease.
The rationale for the imiquimod plus nanoparticle albumin-bound (nab) paclitaxel (Abraxane) therapy derives from previous evidence that imiquimod, a toll-like receptor-7 agonist, has shown clinical efficacy against cutaneous metastases. Imiquimod stimulates secretion of Th1 cytokines and upregulates immune costimulatory molecules at the tumor site. Tumor-specific T cell immunity and tumor growth inhibition are enhanced. Moreover, paclitaxel has been shown to increase serum interferon-gamma levels and boost natural killer cell activity. Thus, the working hypothesis was that nab-paclitaxel would augment imiquimod’s antitumor effects, according to Dr. Salazar.
She and her coinvestigators examined the combination therapy’s impact upon endogenous tumor-specific immunity. They obtained pre- and posttreatment 2-mm skin biopsies from target lesions and were able to demonstrate that the treatment marginally enhanced endogenous immunity to the well-known breast cancer antigens HER2, p53, melanoma-associated antigen 3 (MAGE-3), insulin growth factor binding protein-2 (IGFBP-2), and topoisomerase IIa (TOPO-IIa).
The study was funded by a grant from the National Cancer Institute. Dr. Salazar declared having no relevant financial interests.
SAN ANTONIO – The combination of topical 5% imiquimod plus systemic nanoparticle albumin-bound paclitaxel showed excellent clinical efficacy and was well tolerated for the treatment of cutaneous metastases of breast cancer in a phase II study.
Among the 11 patients who were able to complete the novel combined chemoimmunotherapy regimen, 5 had a complete response, meaning 100% clearance of treated skin lesions at week 24.
In addition, one patient had a partial response, defined as a greater than 50% reduction in the size of the largest treated lesion. Four patients had stable disease, with less than 50% reductions in lesion size. One patient experienced progressive disease, with a 25% increase in target lesion size, Dr. Lupe G. Salazar reported at the San Antonio Breast Cancer Symposium.
Fifteen heavily pretreated breast cancer patients were enrolled in the single-arm, nonrandomized study. All had skin metastases no longer amenable to standard therapies.
The chemoimmunotherapy regimen consisted of three treatment cycles. Each 4-week cycle consisted of application of topical 5% imiquimod to target cutaneous lesions on 4 days per week plus systemic albumin-bound paclitaxel (Abraxane) at 100 mg/m2 on days 1, 8, 15, and 28, explained Dr. Salazar of the University of Washington, Seattle.
Treatment-related toxicities included neutropenia, lymphopenia, anemia, nausea, and fatigue; 34% of toxicities were grade 1, 56% were grade 2, and the remaining 10% were grade 3.
Four of 15 subjects were unable to complete the treatment regimen, having withdrawn due to progression of visceral disease.
The rationale for the imiquimod plus nanoparticle albumin-bound (nab) paclitaxel (Abraxane) therapy derives from previous evidence that imiquimod, a toll-like receptor-7 agonist, has shown clinical efficacy against cutaneous metastases. Imiquimod stimulates secretion of Th1 cytokines and upregulates immune costimulatory molecules at the tumor site. Tumor-specific T cell immunity and tumor growth inhibition are enhanced. Moreover, paclitaxel has been shown to increase serum interferon-gamma levels and boost natural killer cell activity. Thus, the working hypothesis was that nab-paclitaxel would augment imiquimod’s antitumor effects, according to Dr. Salazar.
She and her coinvestigators examined the combination therapy’s impact upon endogenous tumor-specific immunity. They obtained pre- and posttreatment 2-mm skin biopsies from target lesions and were able to demonstrate that the treatment marginally enhanced endogenous immunity to the well-known breast cancer antigens HER2, p53, melanoma-associated antigen 3 (MAGE-3), insulin growth factor binding protein-2 (IGFBP-2), and topoisomerase IIa (TOPO-IIa).
The study was funded by a grant from the National Cancer Institute. Dr. Salazar declared having no relevant financial interests.
FROM THE SAN ANTONIO BREAST CANCER SYMPOSIUM
Major Finding: Among the 11 patients who were able to complete the regimen of topical 5% imiquimod plus systemic albumin-bound paclitaxel, 5 had a complete response, meaning 100% clearance of treated skin lesions at week 24.
Data Source: Fifteen heavily pretreated breast cancer patients enrolled in a single-arm, nonrandomized study.
Disclosures: The study was funded by a grant from the National Cancer Institute. Dr. Salazar declared having no relevant financial interests.
Doctors, Patients Disconnected on Aromatase Inhibitor Compliance
SAN ANTONIO – Physicians tend to broadly overestimate their own breast cancer patients’ compliance with adjuvant aromatase inhibitor therapy, according to a large observational German study.
Among 2,313 postmenopausal breast cancer patients who were enrolled in the ongoing COMPACT (Compliance and Arthralgias in Clinical Therapy) study, 80.5% stated that they took all or nearly all of their prescribed anastrozole tablets during their first year on adjuvant endocrine therapy.
In contrast, 93.2% of their oncologists indicated they believed those same patients had taken all or nearly all of their medication, Dr. Peyman Hadji reported at the San Antonio Breast Cancer Symposium.
Among COMPACT participants, 11.9% indicated that they experienced pre-existing arthralgias within 4 weeks prior to going on the aromatase inhibitor. Another 17% reported new-onset arthralgias after starting on anastrozole, according to Dr. Hadji of the University Hospital of Giessen and Marburg (Germany) GmbH.
Session chair Dr. Thomas J. Smith said that these initial data from the COMPACT study contain a key take-home message: "I think it certainly behooves me as a breast cancer doctor to ask every time, ‘Are you having trouble with these drugs?’ because people do have trouble with these drugs, and they don’t want to bother us."
Another take-home point is that although roughly 12% of breast cancer patients have pre-existing arthralgias, the prescription of an aromatase inhibitor increases the population of affected individuals by close to an absolute 20%, added Dr. Smith, director of palliative care and professor of oncology at Johns Hopkins University, Baltimore.
Dr. Hadji said that going forward, COMPACT will assess the frequency and severity of aromatase inhibitor–associated arthralgias in real-world clinical practice, will chart rates of arthralgia-related treatment noncompliance, and will document the effectiveness and costs of various treatments.
Dr. Smith observed that to date there is a noteworthy lack of good-quality, randomized trial data to guide treatment of aromatase inhibitor–associated arthralgias. Dr. Hadji concurred. He said he and his colleagues have had favorable experiences using NSAIDs, but that’s anecdotal experience. Regarding high-quality data, he cited what he termed a "wonderful" double-blind study showing benefit for acupuncture (J. Clin. Oncol. 2010;28:1154-60).
"The data were really fascinating, but we couldn’t find anybody in our clinic to start doing it. For whatever reasons, oncologists don’t like putting needles into patients," he quipped.
The COMPACT study is sponsored by AstraZeneca. Dr. Hadji serves as a consultant to the company.
SAN ANTONIO – Physicians tend to broadly overestimate their own breast cancer patients’ compliance with adjuvant aromatase inhibitor therapy, according to a large observational German study.
Among 2,313 postmenopausal breast cancer patients who were enrolled in the ongoing COMPACT (Compliance and Arthralgias in Clinical Therapy) study, 80.5% stated that they took all or nearly all of their prescribed anastrozole tablets during their first year on adjuvant endocrine therapy.
In contrast, 93.2% of their oncologists indicated they believed those same patients had taken all or nearly all of their medication, Dr. Peyman Hadji reported at the San Antonio Breast Cancer Symposium.
Among COMPACT participants, 11.9% indicated that they experienced pre-existing arthralgias within 4 weeks prior to going on the aromatase inhibitor. Another 17% reported new-onset arthralgias after starting on anastrozole, according to Dr. Hadji of the University Hospital of Giessen and Marburg (Germany) GmbH.
Session chair Dr. Thomas J. Smith said that these initial data from the COMPACT study contain a key take-home message: "I think it certainly behooves me as a breast cancer doctor to ask every time, ‘Are you having trouble with these drugs?’ because people do have trouble with these drugs, and they don’t want to bother us."
Another take-home point is that although roughly 12% of breast cancer patients have pre-existing arthralgias, the prescription of an aromatase inhibitor increases the population of affected individuals by close to an absolute 20%, added Dr. Smith, director of palliative care and professor of oncology at Johns Hopkins University, Baltimore.
Dr. Hadji said that going forward, COMPACT will assess the frequency and severity of aromatase inhibitor–associated arthralgias in real-world clinical practice, will chart rates of arthralgia-related treatment noncompliance, and will document the effectiveness and costs of various treatments.
Dr. Smith observed that to date there is a noteworthy lack of good-quality, randomized trial data to guide treatment of aromatase inhibitor–associated arthralgias. Dr. Hadji concurred. He said he and his colleagues have had favorable experiences using NSAIDs, but that’s anecdotal experience. Regarding high-quality data, he cited what he termed a "wonderful" double-blind study showing benefit for acupuncture (J. Clin. Oncol. 2010;28:1154-60).
"The data were really fascinating, but we couldn’t find anybody in our clinic to start doing it. For whatever reasons, oncologists don’t like putting needles into patients," he quipped.
The COMPACT study is sponsored by AstraZeneca. Dr. Hadji serves as a consultant to the company.
SAN ANTONIO – Physicians tend to broadly overestimate their own breast cancer patients’ compliance with adjuvant aromatase inhibitor therapy, according to a large observational German study.
Among 2,313 postmenopausal breast cancer patients who were enrolled in the ongoing COMPACT (Compliance and Arthralgias in Clinical Therapy) study, 80.5% stated that they took all or nearly all of their prescribed anastrozole tablets during their first year on adjuvant endocrine therapy.
In contrast, 93.2% of their oncologists indicated they believed those same patients had taken all or nearly all of their medication, Dr. Peyman Hadji reported at the San Antonio Breast Cancer Symposium.
Among COMPACT participants, 11.9% indicated that they experienced pre-existing arthralgias within 4 weeks prior to going on the aromatase inhibitor. Another 17% reported new-onset arthralgias after starting on anastrozole, according to Dr. Hadji of the University Hospital of Giessen and Marburg (Germany) GmbH.
Session chair Dr. Thomas J. Smith said that these initial data from the COMPACT study contain a key take-home message: "I think it certainly behooves me as a breast cancer doctor to ask every time, ‘Are you having trouble with these drugs?’ because people do have trouble with these drugs, and they don’t want to bother us."
Another take-home point is that although roughly 12% of breast cancer patients have pre-existing arthralgias, the prescription of an aromatase inhibitor increases the population of affected individuals by close to an absolute 20%, added Dr. Smith, director of palliative care and professor of oncology at Johns Hopkins University, Baltimore.
Dr. Hadji said that going forward, COMPACT will assess the frequency and severity of aromatase inhibitor–associated arthralgias in real-world clinical practice, will chart rates of arthralgia-related treatment noncompliance, and will document the effectiveness and costs of various treatments.
Dr. Smith observed that to date there is a noteworthy lack of good-quality, randomized trial data to guide treatment of aromatase inhibitor–associated arthralgias. Dr. Hadji concurred. He said he and his colleagues have had favorable experiences using NSAIDs, but that’s anecdotal experience. Regarding high-quality data, he cited what he termed a "wonderful" double-blind study showing benefit for acupuncture (J. Clin. Oncol. 2010;28:1154-60).
"The data were really fascinating, but we couldn’t find anybody in our clinic to start doing it. For whatever reasons, oncologists don’t like putting needles into patients," he quipped.
The COMPACT study is sponsored by AstraZeneca. Dr. Hadji serves as a consultant to the company.
FROM THE SAN ANTONIO BREAST CANCER SYMPOSIUM
Major Finding: During their first year on adjuvant endocrine therapy, 80.5% of early-stage breast cancer patients stated that they took all or nearly all of their prescribed anastrozole tablets, whereas fully 93.2% of their physicians believed the patients had been completely or almost completely compliant.
Data Source: The ongoing large observational German COMPACT study.
Disclosures: COMPACT is sponsored by AstraZeneca. Dr. Hadji serves as a consultant to the company.