User login
Lifestyle Changes Cut Heart and Cancer Risks
Meeting a greater number of the American Heart Association’s seven ideal cardiovascular health components is a twofer: not only is it associated with a reduced incidence of cardiovascular disease, but with a lower incidence of several major types of cancer as well.
By the same token, a separate study, this one in the cancer literature, has recently documented that individuals who adhere more faithfully to the American Cancer Society’s recommended lifestyle behaviors have sharply reduced mortality from cardiovascular disease as well as a lower risk of death due to cancer during long-term followup.
The two groups’ health promotion recommendations share many similarities. Both emphasize the importance of normal body weight, physical activity, and a healthy diet.
The new data documenting crossover benefits from the health promotion guidelines put forth by two major organizations focused on separate diseases are expected to bring renewed energy to collaborative AHA/ACS public health efforts.
"These findings can be used to simplify public health messages by emphasizing a core set of risk factors for prevention of multiple chronic diseases," said Christina M. Shay, Ph.D., of the University of Oklahoma in Oklahoma City.
Such a stripped-down core message ought to help quell public frustration with often-conflicting health advice and contradictory medical recommendations, she added.
In 2004 the AHA, ACS, and American Diabetes Association formed the Preventive Health Partnership, an ongoing joint effort to reduce the burden of cardiovascular disease, stroke, cancer, and diabetes. The partnership has led to public health campaigns, public service announcements, and advocacy efforts.
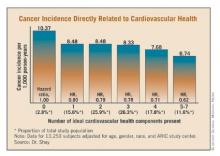
Dr. Shay and coworkers recently examined the anticancer impact of adherence to the AHA’s seven ideal cardiovascular health components using the Atherosclerosis In Communities (ARIC) study cohort. ARIC is a prospective, multicenter, population-based study involving roughly 16,000 middle-aged white and African American men and women who have been reexamined at 3-year intervals. ARIC is rare among cardiovascular studies in that it has prospectively collected reliable data on cancer incidence.
The AHA’s seven ideal cardiovascular health components are a body mass index less than 25 kg/m2, nonsmoking for at least the last 12 months, an untreated total cholesterol below 200 mg/dL, untreated blood pressure below 120/80 mm Hg, untreated fasting blood glucose of less than 100 mg/dL, at least 150 minutes per week of moderate or 75 minutes of vigorous physical activity, and a healthy diet score.
The healthy diet score requires meeting four out of the following five criteria: consumption of at least 4.5 cups of fruits and vegetables daily, eating two or more servings of fish per week, sodium intake of less than 1,500 mg per day, not more than 36 ounces of sugar-sweetened beverages per week, and at least three servings of whole grains daily.
This ARIC analysis involved 13,253 subjects with a mean baseline age of 54.1 years. The incidence of four major cancers – breast, prostate, lung, and colon – was monitored for 1978-2006. The risk of incident cancer dropped in stepwise fashion as a greater number of the ideal cardiovascular health components were met, such that individuals with five to seven of the components had an adjusted risk that was 38% less than that of individuals with none of the ideal health components.
The inverse relationship between the number of ideal cardiovascular health components met and cancer risk was strongest for lung cancer. It was also significant for prostate and colon cancer, but not breast cancer.
Dr. Donald M. Lloyd-Jones calls the new findings "incredibly important."
"What I think is really exciting and striking is the whole concept of trying to get everybody to just take one step forward. But that first step looks to be really key. Going from zero to one cardiovascular health component brings the biggest drop in risk, and that’s true both for cardiovascular disease and cancer. Maybe it’s because in just doing that one thing you actually pull a lot of other components from poor up to intermediate in a way that can’t be detected by this type of analysis," observed Dr. Lloyd-Jones, chair of the department of preventive medicine at Northwestern University, Chicago.
The seven ideal cardiovascular health components were developed in conjunction with an AHA campaign to improve the cardiovascular health of Americans by 20% by the year 2020.
In another recent ARIC analysis, a separate group of investigators showed the 20-year incidence of cardiovascular disease was only one-tenth as high in subjects with six of the ideal cardiovascular health components as it was in those with none. As was the case in the cancer study by Dr. Shay and colleagues, the incidence of cardiovascular disease also decreased in stepwise fashion with an increasing number of cardiovascular health components being met (J. Am. Coll. Cardiol. 2011;57:1690-6).
Meanwhile, investigators at the American Cancer Society recently showed that a high level of adherence to cancer prevention recommendations regarding obesity, diet, physical activity, and alcohol consumption was associated with a 48% reduction in cardiovascular mortality in men and a 58% reduction in women. A high degree of adherence was also associated with a 30% reduction in cancer mortality in men and a 24% decrease in women (Cancer Epidemiol. Biomarkers Prev. 2011;20:1089-97).
The study involved 14 years of follow-up of 111,966 nonsmoking participants in the Cancer Prevention Study-II Nutrition Cohort.
Dr. Shay noted that because the relationship with alcohol intake appears to be different for cancer and cardiovascular disease, what to recommend regarding alcohol consumption will be an important topic of discussion in drawing up any new joint AHA/ACS health promotion message.
Comments for this article by Dr. Shay and Dr. Lloyd-Jones were made at the annual scientific sessions of the American Heart Association in Atlanta. Dr. Shay and Dr. Lloyd-Jones declared having no financial conflicts.
Meeting a greater number of the American Heart Association’s seven ideal cardiovascular health components is a twofer: not only is it associated with a reduced incidence of cardiovascular disease, but with a lower incidence of several major types of cancer as well.
By the same token, a separate study, this one in the cancer literature, has recently documented that individuals who adhere more faithfully to the American Cancer Society’s recommended lifestyle behaviors have sharply reduced mortality from cardiovascular disease as well as a lower risk of death due to cancer during long-term followup.
The two groups’ health promotion recommendations share many similarities. Both emphasize the importance of normal body weight, physical activity, and a healthy diet.
The new data documenting crossover benefits from the health promotion guidelines put forth by two major organizations focused on separate diseases are expected to bring renewed energy to collaborative AHA/ACS public health efforts.
"These findings can be used to simplify public health messages by emphasizing a core set of risk factors for prevention of multiple chronic diseases," said Christina M. Shay, Ph.D., of the University of Oklahoma in Oklahoma City.
Such a stripped-down core message ought to help quell public frustration with often-conflicting health advice and contradictory medical recommendations, she added.
In 2004 the AHA, ACS, and American Diabetes Association formed the Preventive Health Partnership, an ongoing joint effort to reduce the burden of cardiovascular disease, stroke, cancer, and diabetes. The partnership has led to public health campaigns, public service announcements, and advocacy efforts.
Dr. Shay and coworkers recently examined the anticancer impact of adherence to the AHA’s seven ideal cardiovascular health components using the Atherosclerosis In Communities (ARIC) study cohort. ARIC is a prospective, multicenter, population-based study involving roughly 16,000 middle-aged white and African American men and women who have been reexamined at 3-year intervals. ARIC is rare among cardiovascular studies in that it has prospectively collected reliable data on cancer incidence.
The AHA’s seven ideal cardiovascular health components are a body mass index less than 25 kg/m2, nonsmoking for at least the last 12 months, an untreated total cholesterol below 200 mg/dL, untreated blood pressure below 120/80 mm Hg, untreated fasting blood glucose of less than 100 mg/dL, at least 150 minutes per week of moderate or 75 minutes of vigorous physical activity, and a healthy diet score.
The healthy diet score requires meeting four out of the following five criteria: consumption of at least 4.5 cups of fruits and vegetables daily, eating two or more servings of fish per week, sodium intake of less than 1,500 mg per day, not more than 36 ounces of sugar-sweetened beverages per week, and at least three servings of whole grains daily.
This ARIC analysis involved 13,253 subjects with a mean baseline age of 54.1 years. The incidence of four major cancers – breast, prostate, lung, and colon – was monitored for 1978-2006. The risk of incident cancer dropped in stepwise fashion as a greater number of the ideal cardiovascular health components were met, such that individuals with five to seven of the components had an adjusted risk that was 38% less than that of individuals with none of the ideal health components.
The inverse relationship between the number of ideal cardiovascular health components met and cancer risk was strongest for lung cancer. It was also significant for prostate and colon cancer, but not breast cancer.
Dr. Donald M. Lloyd-Jones calls the new findings "incredibly important."
"What I think is really exciting and striking is the whole concept of trying to get everybody to just take one step forward. But that first step looks to be really key. Going from zero to one cardiovascular health component brings the biggest drop in risk, and that’s true both for cardiovascular disease and cancer. Maybe it’s because in just doing that one thing you actually pull a lot of other components from poor up to intermediate in a way that can’t be detected by this type of analysis," observed Dr. Lloyd-Jones, chair of the department of preventive medicine at Northwestern University, Chicago.
The seven ideal cardiovascular health components were developed in conjunction with an AHA campaign to improve the cardiovascular health of Americans by 20% by the year 2020.
In another recent ARIC analysis, a separate group of investigators showed the 20-year incidence of cardiovascular disease was only one-tenth as high in subjects with six of the ideal cardiovascular health components as it was in those with none. As was the case in the cancer study by Dr. Shay and colleagues, the incidence of cardiovascular disease also decreased in stepwise fashion with an increasing number of cardiovascular health components being met (J. Am. Coll. Cardiol. 2011;57:1690-6).
Meanwhile, investigators at the American Cancer Society recently showed that a high level of adherence to cancer prevention recommendations regarding obesity, diet, physical activity, and alcohol consumption was associated with a 48% reduction in cardiovascular mortality in men and a 58% reduction in women. A high degree of adherence was also associated with a 30% reduction in cancer mortality in men and a 24% decrease in women (Cancer Epidemiol. Biomarkers Prev. 2011;20:1089-97).
The study involved 14 years of follow-up of 111,966 nonsmoking participants in the Cancer Prevention Study-II Nutrition Cohort.
Dr. Shay noted that because the relationship with alcohol intake appears to be different for cancer and cardiovascular disease, what to recommend regarding alcohol consumption will be an important topic of discussion in drawing up any new joint AHA/ACS health promotion message.
Comments for this article by Dr. Shay and Dr. Lloyd-Jones were made at the annual scientific sessions of the American Heart Association in Atlanta. Dr. Shay and Dr. Lloyd-Jones declared having no financial conflicts.
Meeting a greater number of the American Heart Association’s seven ideal cardiovascular health components is a twofer: not only is it associated with a reduced incidence of cardiovascular disease, but with a lower incidence of several major types of cancer as well.
By the same token, a separate study, this one in the cancer literature, has recently documented that individuals who adhere more faithfully to the American Cancer Society’s recommended lifestyle behaviors have sharply reduced mortality from cardiovascular disease as well as a lower risk of death due to cancer during long-term followup.
The two groups’ health promotion recommendations share many similarities. Both emphasize the importance of normal body weight, physical activity, and a healthy diet.
The new data documenting crossover benefits from the health promotion guidelines put forth by two major organizations focused on separate diseases are expected to bring renewed energy to collaborative AHA/ACS public health efforts.
"These findings can be used to simplify public health messages by emphasizing a core set of risk factors for prevention of multiple chronic diseases," said Christina M. Shay, Ph.D., of the University of Oklahoma in Oklahoma City.
Such a stripped-down core message ought to help quell public frustration with often-conflicting health advice and contradictory medical recommendations, she added.
In 2004 the AHA, ACS, and American Diabetes Association formed the Preventive Health Partnership, an ongoing joint effort to reduce the burden of cardiovascular disease, stroke, cancer, and diabetes. The partnership has led to public health campaigns, public service announcements, and advocacy efforts.
Dr. Shay and coworkers recently examined the anticancer impact of adherence to the AHA’s seven ideal cardiovascular health components using the Atherosclerosis In Communities (ARIC) study cohort. ARIC is a prospective, multicenter, population-based study involving roughly 16,000 middle-aged white and African American men and women who have been reexamined at 3-year intervals. ARIC is rare among cardiovascular studies in that it has prospectively collected reliable data on cancer incidence.
The AHA’s seven ideal cardiovascular health components are a body mass index less than 25 kg/m2, nonsmoking for at least the last 12 months, an untreated total cholesterol below 200 mg/dL, untreated blood pressure below 120/80 mm Hg, untreated fasting blood glucose of less than 100 mg/dL, at least 150 minutes per week of moderate or 75 minutes of vigorous physical activity, and a healthy diet score.
The healthy diet score requires meeting four out of the following five criteria: consumption of at least 4.5 cups of fruits and vegetables daily, eating two or more servings of fish per week, sodium intake of less than 1,500 mg per day, not more than 36 ounces of sugar-sweetened beverages per week, and at least three servings of whole grains daily.
This ARIC analysis involved 13,253 subjects with a mean baseline age of 54.1 years. The incidence of four major cancers – breast, prostate, lung, and colon – was monitored for 1978-2006. The risk of incident cancer dropped in stepwise fashion as a greater number of the ideal cardiovascular health components were met, such that individuals with five to seven of the components had an adjusted risk that was 38% less than that of individuals with none of the ideal health components.
The inverse relationship between the number of ideal cardiovascular health components met and cancer risk was strongest for lung cancer. It was also significant for prostate and colon cancer, but not breast cancer.
Dr. Donald M. Lloyd-Jones calls the new findings "incredibly important."
"What I think is really exciting and striking is the whole concept of trying to get everybody to just take one step forward. But that first step looks to be really key. Going from zero to one cardiovascular health component brings the biggest drop in risk, and that’s true both for cardiovascular disease and cancer. Maybe it’s because in just doing that one thing you actually pull a lot of other components from poor up to intermediate in a way that can’t be detected by this type of analysis," observed Dr. Lloyd-Jones, chair of the department of preventive medicine at Northwestern University, Chicago.
The seven ideal cardiovascular health components were developed in conjunction with an AHA campaign to improve the cardiovascular health of Americans by 20% by the year 2020.
In another recent ARIC analysis, a separate group of investigators showed the 20-year incidence of cardiovascular disease was only one-tenth as high in subjects with six of the ideal cardiovascular health components as it was in those with none. As was the case in the cancer study by Dr. Shay and colleagues, the incidence of cardiovascular disease also decreased in stepwise fashion with an increasing number of cardiovascular health components being met (J. Am. Coll. Cardiol. 2011;57:1690-6).
Meanwhile, investigators at the American Cancer Society recently showed that a high level of adherence to cancer prevention recommendations regarding obesity, diet, physical activity, and alcohol consumption was associated with a 48% reduction in cardiovascular mortality in men and a 58% reduction in women. A high degree of adherence was also associated with a 30% reduction in cancer mortality in men and a 24% decrease in women (Cancer Epidemiol. Biomarkers Prev. 2011;20:1089-97).
The study involved 14 years of follow-up of 111,966 nonsmoking participants in the Cancer Prevention Study-II Nutrition Cohort.
Dr. Shay noted that because the relationship with alcohol intake appears to be different for cancer and cardiovascular disease, what to recommend regarding alcohol consumption will be an important topic of discussion in drawing up any new joint AHA/ACS health promotion message.
Comments for this article by Dr. Shay and Dr. Lloyd-Jones were made at the annual scientific sessions of the American Heart Association in Atlanta. Dr. Shay and Dr. Lloyd-Jones declared having no financial conflicts.
Study Shows Ramipril Aids Walking With PAD
ORLANDO – The ACE inhibitor ramipril boosted both walking ability and quality of life in patients with peripheral arterial disease in a double-blind, randomized trial.
"The magnitude of these effects is greater than that reported for conventional medical therapies," Dr. Anna A. Ahimastos observed when presenting the study findings at the annual meeting of the American Heart Association.
She reported on 343 patients with peripheral arterial disease (PAD) who were randomized to 24 weeks of ramipril at 10 mg once daily or placebo. They averaged 65 years of age, 80% were men, the baseline ankle-brachial index was 0.56, and 36% had diabetes.
At 24 weeks, the average pain-free walking time (PFWT) in the ramipril group had increased by 87%, compared with a baseline of 131 seconds. The maximum walk time (MWT) improved by 139% from a baseline of 229 seconds. This corresponded to a clinically meaningful 172-meter increase in walking distance on a standardized treadmill test conducted at a speed of 3.2 km/hr and a 12% gradient, according to Dr. Ahimastos of the Baker IDI Heart and Diabetes Institute at Alfred Hospital in Melbourne. In contrast, both PFWT and MWT decreased modestly over the course of 24 weeks in the control group.
A finding of particular importance in this impaired population was the documented improvement in quality of life that accompanied 24 weeks of ramipril, she continued. Daily functional capacity as measured by the WIQ (Walking Impairment Questionnaire) score domains of walking distance, speed, and stair climbing improved by 184%-213%. Scores on the SF-36 (36-Item Short Form) physical function component showed a significant 7% improvement from a baseline average of 38.5.
Results of this study are consistent with those of an earlier randomized, double-blind pilot study that Dr. Ahimastos and coworkers conducted in 40 patients (Ann. Intern. Med. 2006;144:660-4).
The pilot study showed average gains of 164% in PFWT and 243% in MWT with 24 weeks of ramipril, a magnitude of benefit roughly twice that seen in the new, much larger trial. This is probably because the pilot study employed quite restrictive inclusion criteria, whereas the new study included a broader spectrum of PAD patients, including those with diabetes and with aortoiliac or infrainguinal disease.
Outcomes in the two randomized trials of ramipril for PAD – gains of 87%-164% in PFWT and 139%-243% in MWT – compare favorably with the results of placebo-controlled studies of conventional therapies. The phosphodiesterase-3 inhibitor cilostazol has shown 32%-82% improvements in PFWT and MWT. Pentoxifylline, another phosphodiesterase inhibitor, produced a 12% gain in MWT. And exercise training has shown a 150% improvement in both MWT and PFWT. However, the compliance rate with exercise is quite low in the PAD population, she noted.
Audience members who have grown accustomed to negative clinical trials being reported for PAD were enthusiastic at the prospect of finally gaining an additional effective medical therapy for this difficult condition. And they were curious as to Dr. Ahimastos’s thoughts on the mechanism of benefit.
She replied that at this point she can only speculate, since clinical trials don’t provide answers regarding mechanisms and the data from the new study were unblinded only the week prior to her presentation. She noted that ACE inhibitors have antiangiogenic and vasodilatory effects. The enhanced nitric oxide release and the drugs’ effects on bradykinin might also be relevant.
The improvement in walking doesn’t appear to stem from ramipril’s blood pressure–lowering effect, however, as the blood pressure reductions were minimal – less than 3 mm Hg in both systolic and diastolic blood pressure – and the degree of blood pressure reduction in individual patients didn’t correlate with their magnitude of walking improvement.
She and her colleagues don’t know whether angiotensin-receptor blockers would have similar clinical benefits on walking in PAD patients. That will prove difficult to study. For ethical reasons, future clinical trials are unlikely, as there is now evidence-based agreement among experts that patients with PAD should be on an ACE inhibitor or ARB anyway for cardiovascular protection.
The study was funded by Australia’s National Heart Foundation. Dr. Ahimastos declared having no financial conflicts.
ORLANDO – The ACE inhibitor ramipril boosted both walking ability and quality of life in patients with peripheral arterial disease in a double-blind, randomized trial.
"The magnitude of these effects is greater than that reported for conventional medical therapies," Dr. Anna A. Ahimastos observed when presenting the study findings at the annual meeting of the American Heart Association.
She reported on 343 patients with peripheral arterial disease (PAD) who were randomized to 24 weeks of ramipril at 10 mg once daily or placebo. They averaged 65 years of age, 80% were men, the baseline ankle-brachial index was 0.56, and 36% had diabetes.
At 24 weeks, the average pain-free walking time (PFWT) in the ramipril group had increased by 87%, compared with a baseline of 131 seconds. The maximum walk time (MWT) improved by 139% from a baseline of 229 seconds. This corresponded to a clinically meaningful 172-meter increase in walking distance on a standardized treadmill test conducted at a speed of 3.2 km/hr and a 12% gradient, according to Dr. Ahimastos of the Baker IDI Heart and Diabetes Institute at Alfred Hospital in Melbourne. In contrast, both PFWT and MWT decreased modestly over the course of 24 weeks in the control group.
A finding of particular importance in this impaired population was the documented improvement in quality of life that accompanied 24 weeks of ramipril, she continued. Daily functional capacity as measured by the WIQ (Walking Impairment Questionnaire) score domains of walking distance, speed, and stair climbing improved by 184%-213%. Scores on the SF-36 (36-Item Short Form) physical function component showed a significant 7% improvement from a baseline average of 38.5.
Results of this study are consistent with those of an earlier randomized, double-blind pilot study that Dr. Ahimastos and coworkers conducted in 40 patients (Ann. Intern. Med. 2006;144:660-4).
The pilot study showed average gains of 164% in PFWT and 243% in MWT with 24 weeks of ramipril, a magnitude of benefit roughly twice that seen in the new, much larger trial. This is probably because the pilot study employed quite restrictive inclusion criteria, whereas the new study included a broader spectrum of PAD patients, including those with diabetes and with aortoiliac or infrainguinal disease.
Outcomes in the two randomized trials of ramipril for PAD – gains of 87%-164% in PFWT and 139%-243% in MWT – compare favorably with the results of placebo-controlled studies of conventional therapies. The phosphodiesterase-3 inhibitor cilostazol has shown 32%-82% improvements in PFWT and MWT. Pentoxifylline, another phosphodiesterase inhibitor, produced a 12% gain in MWT. And exercise training has shown a 150% improvement in both MWT and PFWT. However, the compliance rate with exercise is quite low in the PAD population, she noted.
Audience members who have grown accustomed to negative clinical trials being reported for PAD were enthusiastic at the prospect of finally gaining an additional effective medical therapy for this difficult condition. And they were curious as to Dr. Ahimastos’s thoughts on the mechanism of benefit.
She replied that at this point she can only speculate, since clinical trials don’t provide answers regarding mechanisms and the data from the new study were unblinded only the week prior to her presentation. She noted that ACE inhibitors have antiangiogenic and vasodilatory effects. The enhanced nitric oxide release and the drugs’ effects on bradykinin might also be relevant.
The improvement in walking doesn’t appear to stem from ramipril’s blood pressure–lowering effect, however, as the blood pressure reductions were minimal – less than 3 mm Hg in both systolic and diastolic blood pressure – and the degree of blood pressure reduction in individual patients didn’t correlate with their magnitude of walking improvement.
She and her colleagues don’t know whether angiotensin-receptor blockers would have similar clinical benefits on walking in PAD patients. That will prove difficult to study. For ethical reasons, future clinical trials are unlikely, as there is now evidence-based agreement among experts that patients with PAD should be on an ACE inhibitor or ARB anyway for cardiovascular protection.
The study was funded by Australia’s National Heart Foundation. Dr. Ahimastos declared having no financial conflicts.
ORLANDO – The ACE inhibitor ramipril boosted both walking ability and quality of life in patients with peripheral arterial disease in a double-blind, randomized trial.
"The magnitude of these effects is greater than that reported for conventional medical therapies," Dr. Anna A. Ahimastos observed when presenting the study findings at the annual meeting of the American Heart Association.
She reported on 343 patients with peripheral arterial disease (PAD) who were randomized to 24 weeks of ramipril at 10 mg once daily or placebo. They averaged 65 years of age, 80% were men, the baseline ankle-brachial index was 0.56, and 36% had diabetes.
At 24 weeks, the average pain-free walking time (PFWT) in the ramipril group had increased by 87%, compared with a baseline of 131 seconds. The maximum walk time (MWT) improved by 139% from a baseline of 229 seconds. This corresponded to a clinically meaningful 172-meter increase in walking distance on a standardized treadmill test conducted at a speed of 3.2 km/hr and a 12% gradient, according to Dr. Ahimastos of the Baker IDI Heart and Diabetes Institute at Alfred Hospital in Melbourne. In contrast, both PFWT and MWT decreased modestly over the course of 24 weeks in the control group.
A finding of particular importance in this impaired population was the documented improvement in quality of life that accompanied 24 weeks of ramipril, she continued. Daily functional capacity as measured by the WIQ (Walking Impairment Questionnaire) score domains of walking distance, speed, and stair climbing improved by 184%-213%. Scores on the SF-36 (36-Item Short Form) physical function component showed a significant 7% improvement from a baseline average of 38.5.
Results of this study are consistent with those of an earlier randomized, double-blind pilot study that Dr. Ahimastos and coworkers conducted in 40 patients (Ann. Intern. Med. 2006;144:660-4).
The pilot study showed average gains of 164% in PFWT and 243% in MWT with 24 weeks of ramipril, a magnitude of benefit roughly twice that seen in the new, much larger trial. This is probably because the pilot study employed quite restrictive inclusion criteria, whereas the new study included a broader spectrum of PAD patients, including those with diabetes and with aortoiliac or infrainguinal disease.
Outcomes in the two randomized trials of ramipril for PAD – gains of 87%-164% in PFWT and 139%-243% in MWT – compare favorably with the results of placebo-controlled studies of conventional therapies. The phosphodiesterase-3 inhibitor cilostazol has shown 32%-82% improvements in PFWT and MWT. Pentoxifylline, another phosphodiesterase inhibitor, produced a 12% gain in MWT. And exercise training has shown a 150% improvement in both MWT and PFWT. However, the compliance rate with exercise is quite low in the PAD population, she noted.
Audience members who have grown accustomed to negative clinical trials being reported for PAD were enthusiastic at the prospect of finally gaining an additional effective medical therapy for this difficult condition. And they were curious as to Dr. Ahimastos’s thoughts on the mechanism of benefit.
She replied that at this point she can only speculate, since clinical trials don’t provide answers regarding mechanisms and the data from the new study were unblinded only the week prior to her presentation. She noted that ACE inhibitors have antiangiogenic and vasodilatory effects. The enhanced nitric oxide release and the drugs’ effects on bradykinin might also be relevant.
The improvement in walking doesn’t appear to stem from ramipril’s blood pressure–lowering effect, however, as the blood pressure reductions were minimal – less than 3 mm Hg in both systolic and diastolic blood pressure – and the degree of blood pressure reduction in individual patients didn’t correlate with their magnitude of walking improvement.
She and her colleagues don’t know whether angiotensin-receptor blockers would have similar clinical benefits on walking in PAD patients. That will prove difficult to study. For ethical reasons, future clinical trials are unlikely, as there is now evidence-based agreement among experts that patients with PAD should be on an ACE inhibitor or ARB anyway for cardiovascular protection.
The study was funded by Australia’s National Heart Foundation. Dr. Ahimastos declared having no financial conflicts.
Major Finding: A 24-week regimen of ramipril resulted in an average 87% improvement in pain-free walking time, a 139% gain in maximum walking time, and significantly enhanced quality of life in patients with peripheral artery disease.
Data Source: A double-blind, placebo-controlled, randomized clinical trial in 343 PAD patients.
Disclosures: No financial conflicts were declared.
U.S. Oncologists Confront Pressure to Curb Cancer Costs.
Cancer care has emerged as a prime target in efforts to contain health care costs.
Health policy makers have painted a bull’s-eye on oncology. Cancer therapy costs are skyrocketing, and the care itself is sometimes seen as fragmented and unsupported by persuasive evidence of effectiveness.
Moreover, cost hasn’t typically been a consideration for American oncologists. The prevailing ethos has been that they have a duty to offer a patient any possible treatment yielding a net benefit, regardless of the cost to society.
That stance is no longer tenable, experts said in interviews and presentations at the recent San Antonio Breast Cancer Symposium.
"The growth in health care spending is unsustainable. Like it or not, efforts to control costs will increase. The question isn’t ‘Should cost be a consideration in the management of cancer?’ but rather ‘How will cost decisions be made and who will be responsible?’ " said Dr. Michael J. Hassett, a medical oncologist at the Dana-Farber Cancer Institute and Harvard Medical School, Boston.
Dr. Thomas J. Smith, the Harry J. Duffey Family Professor of Palliative Care at Johns Hopkins University, Baltimore, concurred.
"I’ve just spent the past couple of days talking to major insurers about the new Medicare/Medicaid innovations program, and everyone says oncology is a major target," said Dr. Smith, director of palliative care at Johns Hopkins Medicine. "It’s 15%-25% of their insurance costs. And there are demonstration projects showing that episode-based payment and salaried physicians work just as well and cost less."
The Association of American Medical Colleges plans to make "cost-conscious use of society’s resources" the next scientific competency required of medical school graduates, added Dr. Smith. "So we’re going to have to figure this out and teach it to the next generation, even if we ourselves didn’t get it right."
A defining experience for Dr. Eric P. Winer came when he and other international experts met in Portugal to draw up consensus guidelines on the treatment of advanced breast cancer.
"It was a surprising moment that seems to keep coming back to me over and over again: Many of our European colleagues were quite open in saying that newer drugs that might lead to a fairly modest survival advantage would probably not be used in their countries if the drugs were costly," recalled Dr. Winer, director of the breast oncology center at Dana-Farber and a professor of medicine at Harvard.
"That’s very different from the approach taken in the [United States] until now, although I think we’re really looking to make changes here," he said. "My own view is, if we don’t take control of this as the people who are providing care, then we will lose all control."
Yet if clinicians are to assume responsibility for considering costs in making cancer treatment decisions, they will have to traverse an ethical mine field, given their multiple potentially conflicting responsibilities as patient advocates, business owners, and citizen-taxpayers, cautioned Dr. Hassett.
Money and Outcomes Don’t Match
In 2009, nearly 18% of the U.S. gross domestic product was spent on health care. Analysts at the National Cancer Institute estimate that direct medical spending on cancer care in the U.S. amounted to $124.5 billion in 2010, with breast cancer care – accounting for $16.5 billion, or 13% of the total – leading the way. By 2020, just 8 years from now, they project that direct medical spending for cancer care will approach $158 billion annually, with breast cancer accounting for up to $25 billion of that figure (Cancer Epidemiol. Biomarkers Prev. 2011;20:2006-14).
How is that money being spent? A separate study concluded that total Medicare fee-for-service spending for breast cancer care during the initial year after diagnosis amounted to $1.06 billion in 2002. Surgery accounted for 25% of that payout, followed by chemotherapy at 15%, radiation therapy at 11%, and other inpatient care at 18% (J. Natl. Cancer Inst. 2008;100:888-97).
Unfortunately, all that spending isn’t buying better outcomes. By a variety of yardsticks, including 5-year overall survival and potential years of life lost due to malignancy, Americans fare no better and in some cases do worse than citizens of countries spending far less per capita on health care, said Dr. Hassett.
In a soon-to-be-published study, he and his coinvestigators analyzed total per-patient expenditures for all Medicare Part A and Part B services for breast cancer during the year following diagnosis of the malignancy in more than 15,000 women aged 65-70 years. Patients in the lowest quintile, with a median 1-year spending of $17,315, had a 5-year overall survival of 88%, identical to that in women in the highest spending quintile, at $26,808. Both quintiles had an identical 81% rate of adherence to 27 National Comprehensive Cancer Network guideline-based quality measures.
How Much Is Too Much per QALY?
Incremental cost-effectiveness analysis is an increasingly popular tool for helping determine whether an intervention provides value for money spent, according to Elena B. Elkin, Ph.D., of the Health Outcomes Research Group at Memorial Sloan-Kettering Cancer Center, New York.
For example, breast cancer cost-effectiveness studies published in recent years have concluded that the use of raloxifene (Evista) to reduce breast cancer risk among white women starting at age 55 comes at a price tag of $22,000 per quality-adjusted life year (QALY) gained. Similarly, the use of adjuvant letrozole (Femara) instead of anastrozole (Arimidex) in patients with hormone receptor–positive breast cancer has a cost of $26,000 per QALY.
At the higher end of the spectrum, bevacizumab (Avastin) plus paclitaxel vs. paclitaxel alone in women with HER2-positive metastatic breast cancer has a cost-effectiveness of $280,000 per QALY; ixabepilone (Ixempra) plus capecitabine (Xeloda) vs. capecitabine alone in the setting of taxane- and anthracycline-resistant metastatic breast cancer has been calculated to carry a price tag of $360,000 per QALY; and digital screening mammography instead of film for all women aged 40 or older costs an estimated $930,000 per QALY gained, according to Dr. Elkin.
There is no "right" answer as to what constitutes good value for money spent on health care. A widespread view held since the early 1980s is that less than $50,000 per QALY is a favorably low incremental cost-effectiveness ratio and thus good value, whereas $50,000-$100,000 per QALY is a grey area and a judgment call. But those rules of thumb are "certainly outdated," said Dr. Elkin.
She noted that Dr. Smith and Dr. Bruce E. Hillner have calculated that by adjusting for health care inflation, an incremental cost-effectiveness ratio of $50,000 per QALY in 1982 equates to $197,000 per QALY in 2007 dollars. Moreover, using the World Health Organization definition of good value for health care money spent (that is, a figure not more than three times a nation’s per capita gross domestic product), then $140,100 per QALY in 2008 U.S. dollars would be a reasonable threshold (J. Clin. Oncol. 2009;27:2111-3).
Impact on Care Worries Oncologists
There are indications that many oncologists are concerned about the exponentially rising cost of cancer care but leery about the possible unintended consequences of efforts to control costs, such as jeopardizing quality or access.
A national survey of medical oncologists conducted by researchers at Tufts University showed that 84% said that patients’ out-of-pocket costs influence their treatment recommendations. Some 56% indicated the cost of new cancer drugs influences their treatment recommendations. Only 29% believe that more cost-sharing by patients for cancer drugs is needed. And 80% of those surveyed want to see more use of cost-effectiveness data in coverage and payment decisions (Health Aff. [Millwood] 2010;29:196-202).
When asked who should determine whether a drug provides good value, 60% of the medical oncologists responded that physicians should make this determination; 57% said nonprofit organizations, 37% said patients, 21% named the government, and 6% said insurance companies.
"I think that’s interesting, because in reality it’s probably the reverse of what actually happens," according to Dr. Hassett.
Earlier Adoption of Trial Results Urged
Dr. Laura J. Esserman suggested that one novel way to curb cancer care costs is through selective early adoption of persuasive clinical trial findings while ongoing definitive studies are still being completed. She cited intraoperative radiation therapy during breast-conserving surgery as a case in point.
Results of the international TARGIT-A (Targeted Intraoperative Radiotherapy) trial, in which 2,232 patients undergoing lumpectomy were randomized to intraoperative radiotherapy (IORT) or standard external-beam radiation, showed closely similar 4-year local recurrence rates in the conserved breast: 1.2% in the IORT group and 0.95% with external-beam radiation (Lancet 2010;376:91-102). Yet IORT costs $6,400 less and provides enormous quality of life advantages, as it replaces the conventional 6 weeks of near-daily radiation therapy with 30 minutes of intraoperative treatment.
"To wake up from your surgery and be done is a wonderful thing," commented Dr. Esserman, professor of surgery and radiology and director of the breast care center at the University of California, San Francisco.
More than 70,000 American women per year who have been diagnosed with breast cancer fit the profile of the TARGIT-A population, she said. Yet some authorities urge holding off on widespread adoption of IORT until results are in from ongoing, large, randomized trials of mastectomy vs. lumpectomy and various forms of radiation, which will take another decade or more.
"The possible harm of early adoption is negligible, and the cost of not intervening is close to $2 billion per year. I strongly feel IORT should be adopted now. I don’t think the results are going to change. We’ve already adopted it at our center, and a registry trial is being organized to help people adopt it nationally," said Dr. Esserman.
A major obstacle here is Medicare’s decision to make IORT part of a bundled-care program, which means that physicians who perform radiation therapy intraoperatively won’t get paid more for it. "This will provide an enormous disincentive to the use of IORT. The big losers here are the patients," she said.
Another opportunity to save money would be to follow the U.S. Preventive Services Task Force guidelines on mammography screening for breast cancer, rather than routinely conducting annual mammography, as many physicians still advocate, Dr. Esserman continued.
"We could save $5 billion per year simply by following those guidelines. And there are now many papers demonstrating that approach certainly is very cost effective. You don’t find any significant increase in advanced cancers with annual screening," she said.
Five More Proposals to Slow Costs
Dr. Smith proposed the following five changes in oncologists’ behavior aimed at slowing the rise in cancer care costs:
• Target surveillance testing with serum tumor markers and imaging in accord with NCCN guidelines. Recommendations to cut back on surveillance testing ought to be incorporated into the American Society of Clinical Oncology’s Quality Oncology Practice Initiative (QOPI), a program of proven effectiveness in changing medical oncologists’ behavior.
• Switch to palliative care in patients with disease progression despite three consecutive chemotherapy regimens. The US Oncology Network’s pathway for metastatic non–small cell lung cancer, which takes this approach, has been shown to reduce treatment costs by 35% with exactly the same survival as in patients treated off pathway – and with better quality of life stemming from a doubled length of stay in hospice care (J. Oncol. Pract. 2010;6:12-18). The same research group reported that a similarly structured pathway for metastatic colon cancer led to a reduction in treatment costs by one-third, along with a significant 6.8-month improvement in survival compared with off-pathway treatment (Am. J. Manag. Care 2011;May [suppl. 5 Developing]: SP45-52).
• Limit chemotherapy for most patients with advanced metastatic solid tumors to patients with good performance status. This would markedly decrease the use of chemotherapy at the end of life.
• Substitute a reduction in chemotherapy dose for the current routine use of colony-stimulating factors in patients with metastatic solid cancers. "We are 3% of the world’s population and we use 75% of the world’s colony-stimulating factors. And 90% of that use isn’t supported by ASCO guidelines," the oncologist asserted. "I think that’s going to be a big target going forward. We simply can’t afford $3,500 per injection for a drug that helps with supportive care but doesn’t improve survival."
• Sequential monotherapies rather than combination chemotherapies as second- and third-line treatment for metastatic cancer. The available data suggest patients will live just as long, but with fewer toxic effects – and at lower cost.
Personalized Medicine and Palliative Care
Dr. Hassett cautioned that although conventional wisdom holds that the emerging field of "personalized medicine" will favorably impact health care costs by providing more selective therapy and improved clinical outcomes, that’s by no means a slam dunk.
"I’m just not sure. I think there’s a chance that personalized medicine could actually increase costs by introducing more expensive tests, new and more expensive drugs, and more complexity into the system," he said.
One thing he is sure of, however, is that any successful effort to reduce the rate of growth in cancer care spending will necessarily have to address the hot-button issue of end-of-life care. One-tenth of all Medicare dollars are spent on care during the final 28 days of life.
Dr. Smith concurred, adding that ASCO has a soon-to-be-published Provisional Clinical Opinion declaring that all oncologists should integrate palliative care into their usual cancer care programs.
"That should be the norm. We have to look at how we spend that money for end-of-life [care] because if we don’t fix that part, we won’t have money for adjuvant therapy and neoadjuvant therapy and funding for major research," he warned.
None of the experts cited in this article declared having any financial conflicts.
Cancer care has emerged as a prime target in efforts to contain health care costs.
Health policy makers have painted a bull’s-eye on oncology. Cancer therapy costs are skyrocketing, and the care itself is sometimes seen as fragmented and unsupported by persuasive evidence of effectiveness.
Moreover, cost hasn’t typically been a consideration for American oncologists. The prevailing ethos has been that they have a duty to offer a patient any possible treatment yielding a net benefit, regardless of the cost to society.
That stance is no longer tenable, experts said in interviews and presentations at the recent San Antonio Breast Cancer Symposium.
"The growth in health care spending is unsustainable. Like it or not, efforts to control costs will increase. The question isn’t ‘Should cost be a consideration in the management of cancer?’ but rather ‘How will cost decisions be made and who will be responsible?’ " said Dr. Michael J. Hassett, a medical oncologist at the Dana-Farber Cancer Institute and Harvard Medical School, Boston.
Dr. Thomas J. Smith, the Harry J. Duffey Family Professor of Palliative Care at Johns Hopkins University, Baltimore, concurred.
"I’ve just spent the past couple of days talking to major insurers about the new Medicare/Medicaid innovations program, and everyone says oncology is a major target," said Dr. Smith, director of palliative care at Johns Hopkins Medicine. "It’s 15%-25% of their insurance costs. And there are demonstration projects showing that episode-based payment and salaried physicians work just as well and cost less."
The Association of American Medical Colleges plans to make "cost-conscious use of society’s resources" the next scientific competency required of medical school graduates, added Dr. Smith. "So we’re going to have to figure this out and teach it to the next generation, even if we ourselves didn’t get it right."
A defining experience for Dr. Eric P. Winer came when he and other international experts met in Portugal to draw up consensus guidelines on the treatment of advanced breast cancer.
"It was a surprising moment that seems to keep coming back to me over and over again: Many of our European colleagues were quite open in saying that newer drugs that might lead to a fairly modest survival advantage would probably not be used in their countries if the drugs were costly," recalled Dr. Winer, director of the breast oncology center at Dana-Farber and a professor of medicine at Harvard.
"That’s very different from the approach taken in the [United States] until now, although I think we’re really looking to make changes here," he said. "My own view is, if we don’t take control of this as the people who are providing care, then we will lose all control."
Yet if clinicians are to assume responsibility for considering costs in making cancer treatment decisions, they will have to traverse an ethical mine field, given their multiple potentially conflicting responsibilities as patient advocates, business owners, and citizen-taxpayers, cautioned Dr. Hassett.
Money and Outcomes Don’t Match
In 2009, nearly 18% of the U.S. gross domestic product was spent on health care. Analysts at the National Cancer Institute estimate that direct medical spending on cancer care in the U.S. amounted to $124.5 billion in 2010, with breast cancer care – accounting for $16.5 billion, or 13% of the total – leading the way. By 2020, just 8 years from now, they project that direct medical spending for cancer care will approach $158 billion annually, with breast cancer accounting for up to $25 billion of that figure (Cancer Epidemiol. Biomarkers Prev. 2011;20:2006-14).
How is that money being spent? A separate study concluded that total Medicare fee-for-service spending for breast cancer care during the initial year after diagnosis amounted to $1.06 billion in 2002. Surgery accounted for 25% of that payout, followed by chemotherapy at 15%, radiation therapy at 11%, and other inpatient care at 18% (J. Natl. Cancer Inst. 2008;100:888-97).
Unfortunately, all that spending isn’t buying better outcomes. By a variety of yardsticks, including 5-year overall survival and potential years of life lost due to malignancy, Americans fare no better and in some cases do worse than citizens of countries spending far less per capita on health care, said Dr. Hassett.
In a soon-to-be-published study, he and his coinvestigators analyzed total per-patient expenditures for all Medicare Part A and Part B services for breast cancer during the year following diagnosis of the malignancy in more than 15,000 women aged 65-70 years. Patients in the lowest quintile, with a median 1-year spending of $17,315, had a 5-year overall survival of 88%, identical to that in women in the highest spending quintile, at $26,808. Both quintiles had an identical 81% rate of adherence to 27 National Comprehensive Cancer Network guideline-based quality measures.
How Much Is Too Much per QALY?
Incremental cost-effectiveness analysis is an increasingly popular tool for helping determine whether an intervention provides value for money spent, according to Elena B. Elkin, Ph.D., of the Health Outcomes Research Group at Memorial Sloan-Kettering Cancer Center, New York.
For example, breast cancer cost-effectiveness studies published in recent years have concluded that the use of raloxifene (Evista) to reduce breast cancer risk among white women starting at age 55 comes at a price tag of $22,000 per quality-adjusted life year (QALY) gained. Similarly, the use of adjuvant letrozole (Femara) instead of anastrozole (Arimidex) in patients with hormone receptor–positive breast cancer has a cost of $26,000 per QALY.
At the higher end of the spectrum, bevacizumab (Avastin) plus paclitaxel vs. paclitaxel alone in women with HER2-positive metastatic breast cancer has a cost-effectiveness of $280,000 per QALY; ixabepilone (Ixempra) plus capecitabine (Xeloda) vs. capecitabine alone in the setting of taxane- and anthracycline-resistant metastatic breast cancer has been calculated to carry a price tag of $360,000 per QALY; and digital screening mammography instead of film for all women aged 40 or older costs an estimated $930,000 per QALY gained, according to Dr. Elkin.
There is no "right" answer as to what constitutes good value for money spent on health care. A widespread view held since the early 1980s is that less than $50,000 per QALY is a favorably low incremental cost-effectiveness ratio and thus good value, whereas $50,000-$100,000 per QALY is a grey area and a judgment call. But those rules of thumb are "certainly outdated," said Dr. Elkin.
She noted that Dr. Smith and Dr. Bruce E. Hillner have calculated that by adjusting for health care inflation, an incremental cost-effectiveness ratio of $50,000 per QALY in 1982 equates to $197,000 per QALY in 2007 dollars. Moreover, using the World Health Organization definition of good value for health care money spent (that is, a figure not more than three times a nation’s per capita gross domestic product), then $140,100 per QALY in 2008 U.S. dollars would be a reasonable threshold (J. Clin. Oncol. 2009;27:2111-3).
Impact on Care Worries Oncologists
There are indications that many oncologists are concerned about the exponentially rising cost of cancer care but leery about the possible unintended consequences of efforts to control costs, such as jeopardizing quality or access.
A national survey of medical oncologists conducted by researchers at Tufts University showed that 84% said that patients’ out-of-pocket costs influence their treatment recommendations. Some 56% indicated the cost of new cancer drugs influences their treatment recommendations. Only 29% believe that more cost-sharing by patients for cancer drugs is needed. And 80% of those surveyed want to see more use of cost-effectiveness data in coverage and payment decisions (Health Aff. [Millwood] 2010;29:196-202).
When asked who should determine whether a drug provides good value, 60% of the medical oncologists responded that physicians should make this determination; 57% said nonprofit organizations, 37% said patients, 21% named the government, and 6% said insurance companies.
"I think that’s interesting, because in reality it’s probably the reverse of what actually happens," according to Dr. Hassett.
Earlier Adoption of Trial Results Urged
Dr. Laura J. Esserman suggested that one novel way to curb cancer care costs is through selective early adoption of persuasive clinical trial findings while ongoing definitive studies are still being completed. She cited intraoperative radiation therapy during breast-conserving surgery as a case in point.
Results of the international TARGIT-A (Targeted Intraoperative Radiotherapy) trial, in which 2,232 patients undergoing lumpectomy were randomized to intraoperative radiotherapy (IORT) or standard external-beam radiation, showed closely similar 4-year local recurrence rates in the conserved breast: 1.2% in the IORT group and 0.95% with external-beam radiation (Lancet 2010;376:91-102). Yet IORT costs $6,400 less and provides enormous quality of life advantages, as it replaces the conventional 6 weeks of near-daily radiation therapy with 30 minutes of intraoperative treatment.
"To wake up from your surgery and be done is a wonderful thing," commented Dr. Esserman, professor of surgery and radiology and director of the breast care center at the University of California, San Francisco.
More than 70,000 American women per year who have been diagnosed with breast cancer fit the profile of the TARGIT-A population, she said. Yet some authorities urge holding off on widespread adoption of IORT until results are in from ongoing, large, randomized trials of mastectomy vs. lumpectomy and various forms of radiation, which will take another decade or more.
"The possible harm of early adoption is negligible, and the cost of not intervening is close to $2 billion per year. I strongly feel IORT should be adopted now. I don’t think the results are going to change. We’ve already adopted it at our center, and a registry trial is being organized to help people adopt it nationally," said Dr. Esserman.
A major obstacle here is Medicare’s decision to make IORT part of a bundled-care program, which means that physicians who perform radiation therapy intraoperatively won’t get paid more for it. "This will provide an enormous disincentive to the use of IORT. The big losers here are the patients," she said.
Another opportunity to save money would be to follow the U.S. Preventive Services Task Force guidelines on mammography screening for breast cancer, rather than routinely conducting annual mammography, as many physicians still advocate, Dr. Esserman continued.
"We could save $5 billion per year simply by following those guidelines. And there are now many papers demonstrating that approach certainly is very cost effective. You don’t find any significant increase in advanced cancers with annual screening," she said.
Five More Proposals to Slow Costs
Dr. Smith proposed the following five changes in oncologists’ behavior aimed at slowing the rise in cancer care costs:
• Target surveillance testing with serum tumor markers and imaging in accord with NCCN guidelines. Recommendations to cut back on surveillance testing ought to be incorporated into the American Society of Clinical Oncology’s Quality Oncology Practice Initiative (QOPI), a program of proven effectiveness in changing medical oncologists’ behavior.
• Switch to palliative care in patients with disease progression despite three consecutive chemotherapy regimens. The US Oncology Network’s pathway for metastatic non–small cell lung cancer, which takes this approach, has been shown to reduce treatment costs by 35% with exactly the same survival as in patients treated off pathway – and with better quality of life stemming from a doubled length of stay in hospice care (J. Oncol. Pract. 2010;6:12-18). The same research group reported that a similarly structured pathway for metastatic colon cancer led to a reduction in treatment costs by one-third, along with a significant 6.8-month improvement in survival compared with off-pathway treatment (Am. J. Manag. Care 2011;May [suppl. 5 Developing]: SP45-52).
• Limit chemotherapy for most patients with advanced metastatic solid tumors to patients with good performance status. This would markedly decrease the use of chemotherapy at the end of life.
• Substitute a reduction in chemotherapy dose for the current routine use of colony-stimulating factors in patients with metastatic solid cancers. "We are 3% of the world’s population and we use 75% of the world’s colony-stimulating factors. And 90% of that use isn’t supported by ASCO guidelines," the oncologist asserted. "I think that’s going to be a big target going forward. We simply can’t afford $3,500 per injection for a drug that helps with supportive care but doesn’t improve survival."
• Sequential monotherapies rather than combination chemotherapies as second- and third-line treatment for metastatic cancer. The available data suggest patients will live just as long, but with fewer toxic effects – and at lower cost.
Personalized Medicine and Palliative Care
Dr. Hassett cautioned that although conventional wisdom holds that the emerging field of "personalized medicine" will favorably impact health care costs by providing more selective therapy and improved clinical outcomes, that’s by no means a slam dunk.
"I’m just not sure. I think there’s a chance that personalized medicine could actually increase costs by introducing more expensive tests, new and more expensive drugs, and more complexity into the system," he said.
One thing he is sure of, however, is that any successful effort to reduce the rate of growth in cancer care spending will necessarily have to address the hot-button issue of end-of-life care. One-tenth of all Medicare dollars are spent on care during the final 28 days of life.
Dr. Smith concurred, adding that ASCO has a soon-to-be-published Provisional Clinical Opinion declaring that all oncologists should integrate palliative care into their usual cancer care programs.
"That should be the norm. We have to look at how we spend that money for end-of-life [care] because if we don’t fix that part, we won’t have money for adjuvant therapy and neoadjuvant therapy and funding for major research," he warned.
None of the experts cited in this article declared having any financial conflicts.
Cancer care has emerged as a prime target in efforts to contain health care costs.
Health policy makers have painted a bull’s-eye on oncology. Cancer therapy costs are skyrocketing, and the care itself is sometimes seen as fragmented and unsupported by persuasive evidence of effectiveness.
Moreover, cost hasn’t typically been a consideration for American oncologists. The prevailing ethos has been that they have a duty to offer a patient any possible treatment yielding a net benefit, regardless of the cost to society.
That stance is no longer tenable, experts said in interviews and presentations at the recent San Antonio Breast Cancer Symposium.
"The growth in health care spending is unsustainable. Like it or not, efforts to control costs will increase. The question isn’t ‘Should cost be a consideration in the management of cancer?’ but rather ‘How will cost decisions be made and who will be responsible?’ " said Dr. Michael J. Hassett, a medical oncologist at the Dana-Farber Cancer Institute and Harvard Medical School, Boston.
Dr. Thomas J. Smith, the Harry J. Duffey Family Professor of Palliative Care at Johns Hopkins University, Baltimore, concurred.
"I’ve just spent the past couple of days talking to major insurers about the new Medicare/Medicaid innovations program, and everyone says oncology is a major target," said Dr. Smith, director of palliative care at Johns Hopkins Medicine. "It’s 15%-25% of their insurance costs. And there are demonstration projects showing that episode-based payment and salaried physicians work just as well and cost less."
The Association of American Medical Colleges plans to make "cost-conscious use of society’s resources" the next scientific competency required of medical school graduates, added Dr. Smith. "So we’re going to have to figure this out and teach it to the next generation, even if we ourselves didn’t get it right."
A defining experience for Dr. Eric P. Winer came when he and other international experts met in Portugal to draw up consensus guidelines on the treatment of advanced breast cancer.
"It was a surprising moment that seems to keep coming back to me over and over again: Many of our European colleagues were quite open in saying that newer drugs that might lead to a fairly modest survival advantage would probably not be used in their countries if the drugs were costly," recalled Dr. Winer, director of the breast oncology center at Dana-Farber and a professor of medicine at Harvard.
"That’s very different from the approach taken in the [United States] until now, although I think we’re really looking to make changes here," he said. "My own view is, if we don’t take control of this as the people who are providing care, then we will lose all control."
Yet if clinicians are to assume responsibility for considering costs in making cancer treatment decisions, they will have to traverse an ethical mine field, given their multiple potentially conflicting responsibilities as patient advocates, business owners, and citizen-taxpayers, cautioned Dr. Hassett.
Money and Outcomes Don’t Match
In 2009, nearly 18% of the U.S. gross domestic product was spent on health care. Analysts at the National Cancer Institute estimate that direct medical spending on cancer care in the U.S. amounted to $124.5 billion in 2010, with breast cancer care – accounting for $16.5 billion, or 13% of the total – leading the way. By 2020, just 8 years from now, they project that direct medical spending for cancer care will approach $158 billion annually, with breast cancer accounting for up to $25 billion of that figure (Cancer Epidemiol. Biomarkers Prev. 2011;20:2006-14).
How is that money being spent? A separate study concluded that total Medicare fee-for-service spending for breast cancer care during the initial year after diagnosis amounted to $1.06 billion in 2002. Surgery accounted for 25% of that payout, followed by chemotherapy at 15%, radiation therapy at 11%, and other inpatient care at 18% (J. Natl. Cancer Inst. 2008;100:888-97).
Unfortunately, all that spending isn’t buying better outcomes. By a variety of yardsticks, including 5-year overall survival and potential years of life lost due to malignancy, Americans fare no better and in some cases do worse than citizens of countries spending far less per capita on health care, said Dr. Hassett.
In a soon-to-be-published study, he and his coinvestigators analyzed total per-patient expenditures for all Medicare Part A and Part B services for breast cancer during the year following diagnosis of the malignancy in more than 15,000 women aged 65-70 years. Patients in the lowest quintile, with a median 1-year spending of $17,315, had a 5-year overall survival of 88%, identical to that in women in the highest spending quintile, at $26,808. Both quintiles had an identical 81% rate of adherence to 27 National Comprehensive Cancer Network guideline-based quality measures.
How Much Is Too Much per QALY?
Incremental cost-effectiveness analysis is an increasingly popular tool for helping determine whether an intervention provides value for money spent, according to Elena B. Elkin, Ph.D., of the Health Outcomes Research Group at Memorial Sloan-Kettering Cancer Center, New York.
For example, breast cancer cost-effectiveness studies published in recent years have concluded that the use of raloxifene (Evista) to reduce breast cancer risk among white women starting at age 55 comes at a price tag of $22,000 per quality-adjusted life year (QALY) gained. Similarly, the use of adjuvant letrozole (Femara) instead of anastrozole (Arimidex) in patients with hormone receptor–positive breast cancer has a cost of $26,000 per QALY.
At the higher end of the spectrum, bevacizumab (Avastin) plus paclitaxel vs. paclitaxel alone in women with HER2-positive metastatic breast cancer has a cost-effectiveness of $280,000 per QALY; ixabepilone (Ixempra) plus capecitabine (Xeloda) vs. capecitabine alone in the setting of taxane- and anthracycline-resistant metastatic breast cancer has been calculated to carry a price tag of $360,000 per QALY; and digital screening mammography instead of film for all women aged 40 or older costs an estimated $930,000 per QALY gained, according to Dr. Elkin.
There is no "right" answer as to what constitutes good value for money spent on health care. A widespread view held since the early 1980s is that less than $50,000 per QALY is a favorably low incremental cost-effectiveness ratio and thus good value, whereas $50,000-$100,000 per QALY is a grey area and a judgment call. But those rules of thumb are "certainly outdated," said Dr. Elkin.
She noted that Dr. Smith and Dr. Bruce E. Hillner have calculated that by adjusting for health care inflation, an incremental cost-effectiveness ratio of $50,000 per QALY in 1982 equates to $197,000 per QALY in 2007 dollars. Moreover, using the World Health Organization definition of good value for health care money spent (that is, a figure not more than three times a nation’s per capita gross domestic product), then $140,100 per QALY in 2008 U.S. dollars would be a reasonable threshold (J. Clin. Oncol. 2009;27:2111-3).
Impact on Care Worries Oncologists
There are indications that many oncologists are concerned about the exponentially rising cost of cancer care but leery about the possible unintended consequences of efforts to control costs, such as jeopardizing quality or access.
A national survey of medical oncologists conducted by researchers at Tufts University showed that 84% said that patients’ out-of-pocket costs influence their treatment recommendations. Some 56% indicated the cost of new cancer drugs influences their treatment recommendations. Only 29% believe that more cost-sharing by patients for cancer drugs is needed. And 80% of those surveyed want to see more use of cost-effectiveness data in coverage and payment decisions (Health Aff. [Millwood] 2010;29:196-202).
When asked who should determine whether a drug provides good value, 60% of the medical oncologists responded that physicians should make this determination; 57% said nonprofit organizations, 37% said patients, 21% named the government, and 6% said insurance companies.
"I think that’s interesting, because in reality it’s probably the reverse of what actually happens," according to Dr. Hassett.
Earlier Adoption of Trial Results Urged
Dr. Laura J. Esserman suggested that one novel way to curb cancer care costs is through selective early adoption of persuasive clinical trial findings while ongoing definitive studies are still being completed. She cited intraoperative radiation therapy during breast-conserving surgery as a case in point.
Results of the international TARGIT-A (Targeted Intraoperative Radiotherapy) trial, in which 2,232 patients undergoing lumpectomy were randomized to intraoperative radiotherapy (IORT) or standard external-beam radiation, showed closely similar 4-year local recurrence rates in the conserved breast: 1.2% in the IORT group and 0.95% with external-beam radiation (Lancet 2010;376:91-102). Yet IORT costs $6,400 less and provides enormous quality of life advantages, as it replaces the conventional 6 weeks of near-daily radiation therapy with 30 minutes of intraoperative treatment.
"To wake up from your surgery and be done is a wonderful thing," commented Dr. Esserman, professor of surgery and radiology and director of the breast care center at the University of California, San Francisco.
More than 70,000 American women per year who have been diagnosed with breast cancer fit the profile of the TARGIT-A population, she said. Yet some authorities urge holding off on widespread adoption of IORT until results are in from ongoing, large, randomized trials of mastectomy vs. lumpectomy and various forms of radiation, which will take another decade or more.
"The possible harm of early adoption is negligible, and the cost of not intervening is close to $2 billion per year. I strongly feel IORT should be adopted now. I don’t think the results are going to change. We’ve already adopted it at our center, and a registry trial is being organized to help people adopt it nationally," said Dr. Esserman.
A major obstacle here is Medicare’s decision to make IORT part of a bundled-care program, which means that physicians who perform radiation therapy intraoperatively won’t get paid more for it. "This will provide an enormous disincentive to the use of IORT. The big losers here are the patients," she said.
Another opportunity to save money would be to follow the U.S. Preventive Services Task Force guidelines on mammography screening for breast cancer, rather than routinely conducting annual mammography, as many physicians still advocate, Dr. Esserman continued.
"We could save $5 billion per year simply by following those guidelines. And there are now many papers demonstrating that approach certainly is very cost effective. You don’t find any significant increase in advanced cancers with annual screening," she said.
Five More Proposals to Slow Costs
Dr. Smith proposed the following five changes in oncologists’ behavior aimed at slowing the rise in cancer care costs:
• Target surveillance testing with serum tumor markers and imaging in accord with NCCN guidelines. Recommendations to cut back on surveillance testing ought to be incorporated into the American Society of Clinical Oncology’s Quality Oncology Practice Initiative (QOPI), a program of proven effectiveness in changing medical oncologists’ behavior.
• Switch to palliative care in patients with disease progression despite three consecutive chemotherapy regimens. The US Oncology Network’s pathway for metastatic non–small cell lung cancer, which takes this approach, has been shown to reduce treatment costs by 35% with exactly the same survival as in patients treated off pathway – and with better quality of life stemming from a doubled length of stay in hospice care (J. Oncol. Pract. 2010;6:12-18). The same research group reported that a similarly structured pathway for metastatic colon cancer led to a reduction in treatment costs by one-third, along with a significant 6.8-month improvement in survival compared with off-pathway treatment (Am. J. Manag. Care 2011;May [suppl. 5 Developing]: SP45-52).
• Limit chemotherapy for most patients with advanced metastatic solid tumors to patients with good performance status. This would markedly decrease the use of chemotherapy at the end of life.
• Substitute a reduction in chemotherapy dose for the current routine use of colony-stimulating factors in patients with metastatic solid cancers. "We are 3% of the world’s population and we use 75% of the world’s colony-stimulating factors. And 90% of that use isn’t supported by ASCO guidelines," the oncologist asserted. "I think that’s going to be a big target going forward. We simply can’t afford $3,500 per injection for a drug that helps with supportive care but doesn’t improve survival."
• Sequential monotherapies rather than combination chemotherapies as second- and third-line treatment for metastatic cancer. The available data suggest patients will live just as long, but with fewer toxic effects – and at lower cost.
Personalized Medicine and Palliative Care
Dr. Hassett cautioned that although conventional wisdom holds that the emerging field of "personalized medicine" will favorably impact health care costs by providing more selective therapy and improved clinical outcomes, that’s by no means a slam dunk.
"I’m just not sure. I think there’s a chance that personalized medicine could actually increase costs by introducing more expensive tests, new and more expensive drugs, and more complexity into the system," he said.
One thing he is sure of, however, is that any successful effort to reduce the rate of growth in cancer care spending will necessarily have to address the hot-button issue of end-of-life care. One-tenth of all Medicare dollars are spent on care during the final 28 days of life.
Dr. Smith concurred, adding that ASCO has a soon-to-be-published Provisional Clinical Opinion declaring that all oncologists should integrate palliative care into their usual cancer care programs.
"That should be the norm. We have to look at how we spend that money for end-of-life [care] because if we don’t fix that part, we won’t have money for adjuvant therapy and neoadjuvant therapy and funding for major research," he warned.
None of the experts cited in this article declared having any financial conflicts.
Diet May Reverse 30 Years of Blood Pressure Aging
A healthful dietary pattern may effectively turn back the clock for aging arteries and blunt the dramatic age-related rise in the prevalence of hypertension seen in the United States and other industrialized countries.
That’s been suspected for decades on the basis of epidemiologic studies. More than 80 years ago, for example, observers reported that German vegetarian Trappist monks didn’t experience the classic blood pressure rise with age, while German meat-eating Benedictine monks did (Med. Klin. 1930;25:929-31).
Only much more recently, however, have randomized controlled dietary trials been performed making the same point. The two most extensively studied and best-established of these healthful dietary patterns are the Dietary Approaches to Stop Hypertension (DASH) low-sodium diet and variations of the Mediterranean diet.
"To be provocative, you could say the DASH diet and low sodium abolishes 30 years of blood pressure aging in 4 weeks," said Dr. Frank M. Sacks, professor of cardiovascular disease prevention at the Harvard School of Public Health, Boston.
This was demonstrated when he and his colleagues randomized 412 subjects, ages 23-76, 41% of whom had stage 1 hypertension, to follow a DASH diet or a typical American diet for 90 days. During that period, subjects in each group were randomly assigned to a high-, intermediate-, or low-sodium diet for 30 days each in crossover fashion. Their weight was held constant by manipulating caloric consumption.
During the 30 days of high sodium intake (3.5 g per day), the slope for mean systolic blood pressure in the control group rose by 0.33 mm Hg per year of life.
In contrast, the slope for the DASH diet group while on a low-sodium diet of 1.2 g/day was 0 mm Hg per year. That is, mean systolic blood pressure during that month was 125 mm Hg in subjects in the oldest quintile, ages 55-76, and exactly the same in participants in the youngest quintile, ages 23-41 (Am. J. Cardiol. 2004;94:222-7).
"That’s because the effects of diet are much stronger in older people than younger people. The good effect of diet is stronger, but the bad effect is stronger, too," said Dr. Sacks, chair of the steering committee for the National Heart, Lung, and Blood Institute–sponsored DASH-Sodium trial.
Indeed, among study participants older than age 45 years with mild hypertension, the switch from the control diet with high salt intake to a month of the DASH diet with low salt intake resulted in a 15 mm Hg reduction in mean systolic blood pressure.
"That’s rather huge for a dietary therapy with no weight loss. The effect was about double the blood pressure reduction seen with the DASH diet and low sodium in the younger hypertensives," he notes.
The DASH diet is similar to what the American Heart Association recommends in guidelines for cardiovascular health and dietary management of hypertension (Hypertension 2006;47:296-308). The DASH diet emphasizes poultry, fish, nuts, and beans instead of red meat. It also makes extensive use of low- and non-fat dairy products, fruits and vegetables, and whole grains. A user-friendly website, www.dashforhealth.com, exists to help consumers get started. Other websites, for example, www.mayoclinic.com/health/dash-diet-recipes/RE00089, are a good source for cooking suggestions.
The traditional Mediterranean diet has much in common with the DASH diet. Dr. Sacks credits Dr. Katherine Esposito of the Second University of Naples and coworkers with having done much of the heavy lifting in terms of randomized trials. Most recently, Dr. Esposito and coinvestigators conducted a meta-analysis of 50 epidemiologic studies and randomized controlled trials of the effects of the Mediterranean diet on metabolic syndrome in more than 534,000 subjects.
The investigators demonstrated that adherence to the Mediterranean diet protected against the development of metabolic syndrome. Moreover, in patients with metabolic syndrome the diet significantly reduced both systolic and diastolic blood pressure, triglycerides, blood glucose, waist circumference, and inflammatory markers, while raising HDL (J. Am. Coll. Cardiol. 2011;57:1299-313).
In other work, Dr. Esposito and coworkers have shown in a randomized trial that adherence to the Mediterranean diet enabled one-third of obese men with erectile dysfunction to regain their sexual activity (Public Health Nutr. 2006;9:1118-20). A similar improvement in sexual function was documented in a separate 2-year randomized trial in women with metabolic syndrome and a diagnosis of female sexual dysfunction (Int. J. Impot. Res. 2007;19:486-91).
These improvements in sexual function presumably stem from the Mediterranean diet’s salutary effects on endothelial function (Am. J. Clin. Nutr. 2009;90:263-8). Similarly, the DASH investigators have shown that the DASH diet improves endothelial function in the microvasculature, notes Dr. Sacks.
The pathophysiology of hypertension is complex. Obesity plays an important role, as does renal aging, which affects sodium retention. The stiffness of large arteries increases with age, while compliance – pulsatile flow – diminishes. These vascular changes, which result from calcification and smooth muscle hyperplasia, are generally thought to be irreversible. Not so.
"If you’ve had a chance to feel those calcified arteries in an elderly person, you say, ‘Wow, these things are like metal pipes, how can that change?’ But functionally they can change," according to Dr. Sacks.
In the NHLBI-funded Exercise and Nutrition Interventions for Cardiovascular Health (ENCORE) study, investigators at Duke University showed that the DASH diet significantly improved central arterial stiffness as measured by pulse wave velocity, as well as left ventricular hypertrophy, baroreflex sensitivity, and both systolic and diastolic blood pressures. And randomization to the DASH diet plus a weight loss program resulted in larger improvements than diet alone (Arch. Intern. Med. 2010;170:126-35).
"Aging arteries can be rejuvenated by diet treatment. Youthful compliance of conduit arteries can be restored, at least partially. Improved endothelial function occurs in the microvasculature. And hypertension can be completely treated or prevented by optimal diets over a short period of time," Dr. Sacks concluded at the annual scientific sessions of the American Heart Association in Orlando.
He and Dr. Esposito reported having no financial conflicts.
A healthful dietary pattern may effectively turn back the clock for aging arteries and blunt the dramatic age-related rise in the prevalence of hypertension seen in the United States and other industrialized countries.
That’s been suspected for decades on the basis of epidemiologic studies. More than 80 years ago, for example, observers reported that German vegetarian Trappist monks didn’t experience the classic blood pressure rise with age, while German meat-eating Benedictine monks did (Med. Klin. 1930;25:929-31).
Only much more recently, however, have randomized controlled dietary trials been performed making the same point. The two most extensively studied and best-established of these healthful dietary patterns are the Dietary Approaches to Stop Hypertension (DASH) low-sodium diet and variations of the Mediterranean diet.
"To be provocative, you could say the DASH diet and low sodium abolishes 30 years of blood pressure aging in 4 weeks," said Dr. Frank M. Sacks, professor of cardiovascular disease prevention at the Harvard School of Public Health, Boston.
This was demonstrated when he and his colleagues randomized 412 subjects, ages 23-76, 41% of whom had stage 1 hypertension, to follow a DASH diet or a typical American diet for 90 days. During that period, subjects in each group were randomly assigned to a high-, intermediate-, or low-sodium diet for 30 days each in crossover fashion. Their weight was held constant by manipulating caloric consumption.
During the 30 days of high sodium intake (3.5 g per day), the slope for mean systolic blood pressure in the control group rose by 0.33 mm Hg per year of life.
In contrast, the slope for the DASH diet group while on a low-sodium diet of 1.2 g/day was 0 mm Hg per year. That is, mean systolic blood pressure during that month was 125 mm Hg in subjects in the oldest quintile, ages 55-76, and exactly the same in participants in the youngest quintile, ages 23-41 (Am. J. Cardiol. 2004;94:222-7).
"That’s because the effects of diet are much stronger in older people than younger people. The good effect of diet is stronger, but the bad effect is stronger, too," said Dr. Sacks, chair of the steering committee for the National Heart, Lung, and Blood Institute–sponsored DASH-Sodium trial.
Indeed, among study participants older than age 45 years with mild hypertension, the switch from the control diet with high salt intake to a month of the DASH diet with low salt intake resulted in a 15 mm Hg reduction in mean systolic blood pressure.
"That’s rather huge for a dietary therapy with no weight loss. The effect was about double the blood pressure reduction seen with the DASH diet and low sodium in the younger hypertensives," he notes.
The DASH diet is similar to what the American Heart Association recommends in guidelines for cardiovascular health and dietary management of hypertension (Hypertension 2006;47:296-308). The DASH diet emphasizes poultry, fish, nuts, and beans instead of red meat. It also makes extensive use of low- and non-fat dairy products, fruits and vegetables, and whole grains. A user-friendly website, www.dashforhealth.com, exists to help consumers get started. Other websites, for example, www.mayoclinic.com/health/dash-diet-recipes/RE00089, are a good source for cooking suggestions.
The traditional Mediterranean diet has much in common with the DASH diet. Dr. Sacks credits Dr. Katherine Esposito of the Second University of Naples and coworkers with having done much of the heavy lifting in terms of randomized trials. Most recently, Dr. Esposito and coinvestigators conducted a meta-analysis of 50 epidemiologic studies and randomized controlled trials of the effects of the Mediterranean diet on metabolic syndrome in more than 534,000 subjects.
The investigators demonstrated that adherence to the Mediterranean diet protected against the development of metabolic syndrome. Moreover, in patients with metabolic syndrome the diet significantly reduced both systolic and diastolic blood pressure, triglycerides, blood glucose, waist circumference, and inflammatory markers, while raising HDL (J. Am. Coll. Cardiol. 2011;57:1299-313).
In other work, Dr. Esposito and coworkers have shown in a randomized trial that adherence to the Mediterranean diet enabled one-third of obese men with erectile dysfunction to regain their sexual activity (Public Health Nutr. 2006;9:1118-20). A similar improvement in sexual function was documented in a separate 2-year randomized trial in women with metabolic syndrome and a diagnosis of female sexual dysfunction (Int. J. Impot. Res. 2007;19:486-91).
These improvements in sexual function presumably stem from the Mediterranean diet’s salutary effects on endothelial function (Am. J. Clin. Nutr. 2009;90:263-8). Similarly, the DASH investigators have shown that the DASH diet improves endothelial function in the microvasculature, notes Dr. Sacks.
The pathophysiology of hypertension is complex. Obesity plays an important role, as does renal aging, which affects sodium retention. The stiffness of large arteries increases with age, while compliance – pulsatile flow – diminishes. These vascular changes, which result from calcification and smooth muscle hyperplasia, are generally thought to be irreversible. Not so.
"If you’ve had a chance to feel those calcified arteries in an elderly person, you say, ‘Wow, these things are like metal pipes, how can that change?’ But functionally they can change," according to Dr. Sacks.
In the NHLBI-funded Exercise and Nutrition Interventions for Cardiovascular Health (ENCORE) study, investigators at Duke University showed that the DASH diet significantly improved central arterial stiffness as measured by pulse wave velocity, as well as left ventricular hypertrophy, baroreflex sensitivity, and both systolic and diastolic blood pressures. And randomization to the DASH diet plus a weight loss program resulted in larger improvements than diet alone (Arch. Intern. Med. 2010;170:126-35).
"Aging arteries can be rejuvenated by diet treatment. Youthful compliance of conduit arteries can be restored, at least partially. Improved endothelial function occurs in the microvasculature. And hypertension can be completely treated or prevented by optimal diets over a short period of time," Dr. Sacks concluded at the annual scientific sessions of the American Heart Association in Orlando.
He and Dr. Esposito reported having no financial conflicts.
A healthful dietary pattern may effectively turn back the clock for aging arteries and blunt the dramatic age-related rise in the prevalence of hypertension seen in the United States and other industrialized countries.
That’s been suspected for decades on the basis of epidemiologic studies. More than 80 years ago, for example, observers reported that German vegetarian Trappist monks didn’t experience the classic blood pressure rise with age, while German meat-eating Benedictine monks did (Med. Klin. 1930;25:929-31).
Only much more recently, however, have randomized controlled dietary trials been performed making the same point. The two most extensively studied and best-established of these healthful dietary patterns are the Dietary Approaches to Stop Hypertension (DASH) low-sodium diet and variations of the Mediterranean diet.
"To be provocative, you could say the DASH diet and low sodium abolishes 30 years of blood pressure aging in 4 weeks," said Dr. Frank M. Sacks, professor of cardiovascular disease prevention at the Harvard School of Public Health, Boston.
This was demonstrated when he and his colleagues randomized 412 subjects, ages 23-76, 41% of whom had stage 1 hypertension, to follow a DASH diet or a typical American diet for 90 days. During that period, subjects in each group were randomly assigned to a high-, intermediate-, or low-sodium diet for 30 days each in crossover fashion. Their weight was held constant by manipulating caloric consumption.
During the 30 days of high sodium intake (3.5 g per day), the slope for mean systolic blood pressure in the control group rose by 0.33 mm Hg per year of life.
In contrast, the slope for the DASH diet group while on a low-sodium diet of 1.2 g/day was 0 mm Hg per year. That is, mean systolic blood pressure during that month was 125 mm Hg in subjects in the oldest quintile, ages 55-76, and exactly the same in participants in the youngest quintile, ages 23-41 (Am. J. Cardiol. 2004;94:222-7).
"That’s because the effects of diet are much stronger in older people than younger people. The good effect of diet is stronger, but the bad effect is stronger, too," said Dr. Sacks, chair of the steering committee for the National Heart, Lung, and Blood Institute–sponsored DASH-Sodium trial.
Indeed, among study participants older than age 45 years with mild hypertension, the switch from the control diet with high salt intake to a month of the DASH diet with low salt intake resulted in a 15 mm Hg reduction in mean systolic blood pressure.
"That’s rather huge for a dietary therapy with no weight loss. The effect was about double the blood pressure reduction seen with the DASH diet and low sodium in the younger hypertensives," he notes.
The DASH diet is similar to what the American Heart Association recommends in guidelines for cardiovascular health and dietary management of hypertension (Hypertension 2006;47:296-308). The DASH diet emphasizes poultry, fish, nuts, and beans instead of red meat. It also makes extensive use of low- and non-fat dairy products, fruits and vegetables, and whole grains. A user-friendly website, www.dashforhealth.com, exists to help consumers get started. Other websites, for example, www.mayoclinic.com/health/dash-diet-recipes/RE00089, are a good source for cooking suggestions.
The traditional Mediterranean diet has much in common with the DASH diet. Dr. Sacks credits Dr. Katherine Esposito of the Second University of Naples and coworkers with having done much of the heavy lifting in terms of randomized trials. Most recently, Dr. Esposito and coinvestigators conducted a meta-analysis of 50 epidemiologic studies and randomized controlled trials of the effects of the Mediterranean diet on metabolic syndrome in more than 534,000 subjects.
The investigators demonstrated that adherence to the Mediterranean diet protected against the development of metabolic syndrome. Moreover, in patients with metabolic syndrome the diet significantly reduced both systolic and diastolic blood pressure, triglycerides, blood glucose, waist circumference, and inflammatory markers, while raising HDL (J. Am. Coll. Cardiol. 2011;57:1299-313).
In other work, Dr. Esposito and coworkers have shown in a randomized trial that adherence to the Mediterranean diet enabled one-third of obese men with erectile dysfunction to regain their sexual activity (Public Health Nutr. 2006;9:1118-20). A similar improvement in sexual function was documented in a separate 2-year randomized trial in women with metabolic syndrome and a diagnosis of female sexual dysfunction (Int. J. Impot. Res. 2007;19:486-91).
These improvements in sexual function presumably stem from the Mediterranean diet’s salutary effects on endothelial function (Am. J. Clin. Nutr. 2009;90:263-8). Similarly, the DASH investigators have shown that the DASH diet improves endothelial function in the microvasculature, notes Dr. Sacks.
The pathophysiology of hypertension is complex. Obesity plays an important role, as does renal aging, which affects sodium retention. The stiffness of large arteries increases with age, while compliance – pulsatile flow – diminishes. These vascular changes, which result from calcification and smooth muscle hyperplasia, are generally thought to be irreversible. Not so.
"If you’ve had a chance to feel those calcified arteries in an elderly person, you say, ‘Wow, these things are like metal pipes, how can that change?’ But functionally they can change," according to Dr. Sacks.
In the NHLBI-funded Exercise and Nutrition Interventions for Cardiovascular Health (ENCORE) study, investigators at Duke University showed that the DASH diet significantly improved central arterial stiffness as measured by pulse wave velocity, as well as left ventricular hypertrophy, baroreflex sensitivity, and both systolic and diastolic blood pressures. And randomization to the DASH diet plus a weight loss program resulted in larger improvements than diet alone (Arch. Intern. Med. 2010;170:126-35).
"Aging arteries can be rejuvenated by diet treatment. Youthful compliance of conduit arteries can be restored, at least partially. Improved endothelial function occurs in the microvasculature. And hypertension can be completely treated or prevented by optimal diets over a short period of time," Dr. Sacks concluded at the annual scientific sessions of the American Heart Association in Orlando.
He and Dr. Esposito reported having no financial conflicts.
ASSERT: Subclinical Atrial Fib Boosts Stroke Risk
Asymptomatic episodes of atrial fibrillation are common in elderly hypertensive patients with pacemakers and no history of clinical AF – and these subclinical atrial tachyarrhythmias are associated with a sharply increased risk of subsequent ischemic stroke.
These were the two major findings of the large, international, randomized ASSERT (Asymptomatic Atrial Fibrillation and Stroke Evaluation in Pacemaker Patients and the Atrial Fibrillation Reduction Atrial Pacing Trial) study.
An important secondary finding was that, disappointingly, continuous atrial overdrive pacing using a programmable algorithm to maintain a paced atrium did not prevent the development of clinical AF, nor did it modify stroke risk, according to Dr. Jeff S. Healey of the Population Health Research Institute at McMaster University in Hamilton, Ont.
ASSERT enrolled 2,580 patients in 23 countries. All were aged 65 years or older, were hypertensive, and had recently received a dual-chamber pacemaker or implantable cardioverter-defibrillator. None of the subjects had a history of AF at enrollment.
In the first 3 months of follow-up, 10.1% of study participants developed device-detected subclinical AF (defined as an atrial rate in excess of 190 beats per minute lasting for more than 6 minutes). As a practical matter, it’s worth noting that the median time to implanted device–detected asymptomatic AF in this subgroup was 36 days. Thus, several days of negative Holter monitoring would have made for false reassurance, the cardiologist noted.
The subgroup with asymptomatic AF during the first 3 months had a 5.6-fold increased risk of developing clinical AF during a mean 2.5 years of prospective follow-up. Moreover, the rate of ischemic stroke or systemic embolism in patients with subclinical AF in the first 3 months was 1.7% per year, compared with 0.69% annually in the rest of the ASSERT participants.
The population attributable risk (PAR) of stroke or systemic embolism associated with asymptomatic AF in the first 3 months was 13%. Of note, that’s similar to the PAR of stroke that is associated with clinical AF in the Framingham Heart Study.
In a multivariate analysis adjusted for the standard predictors of stroke, device-detected subclinical AF during the first 3 months of the study was independently associated with a 2.5-fold increased subsequent risk of stroke. And this may well underestimate the true magnitude of risk, since more than half of ASSERT participants were on aspirin at baseline and 18% of those with early subclinical AF received warfarin during the follow-up period.
Both of these venerable drugs are clearly effective in reducing stroke risk in patients with established clinical AF, although it’s not known whether these agents also have a net benefit in patients with subclinical AF. A randomized trial testing this hypothesis by using these drugs or newer anticoagulants should be a priority, Dr. Healey said (N. Engl. J. Med. 2012;366:120-9).
Subjects with a CHADS2 score greater than 2 plus subclinical AF detected in the first 3 months of the study had a rate of ischemic stroke or systemic embolism of 3.7% per year, compared with 0.97% per year in patients with a similarly elevated CHADS2 score but no early subclinical atrial tachyarrhythmia.
In addition to the 10% of ASSERT participants who developed subclinical AF during the initial 3 months of follow-up, another 24% did so later.
Episodes of asymptomatic AF briefer than 6 minutes were not catalogued in the study, so it remains unknown if they, too, are associated with increased subsequent stroke risk.
At the study’s outset, all subjects with pacemakers were randomized to continuous atrial overdrive pacing or to having this feature switched off for the duration. The atrial pacing intervention did not affect the rate of development of clinical AF, although the trial was underpowered for this outcome.
The ASSERT study was supported by St. Jude Medical. Dr. Healey disclosed that he has served as a paid consultant to that company, Boehringer Ingelheim, and Bayer, as well receiving grant support from AstraZeneca, Boston Scientific, and Boehringer Ingelheim. Dr. Lamas declared having no relevant financial interests.
This is a robust study which convincingly demonstrates that device-detected subclinical AF that was picked up in the initial few months after placement of a dual-chamber pacemaker or defibrillator is independently associated with a more-than-doubled annualized risk of stroke or peripheral embolism.
But the question of cause vs. effect remains open: Are these asymptomatic episodes of AF actually causing cardioembolic stroke, or are they merely a marker of stroke risk, possibly reflecting myocardial fibrosis or structural heart disease?
It’s noteworthy that the lengthier the longest episode of subclinical AF in the first 3 months of the ASSERT study, the greater the subsequent stroke risk. Among those whose longest episode lasted more than 17.7 hours, the risk of stroke or systemic embolism was increased nearly fivefold.
The ASSERT investigators have identified a large and interesting population at increased risk of stroke. But until definitive, randomized clinical trials have been carried out demonstrating that anticoagulant therapy is justified for patients with short episodes of device-detected AF, I’m going to continue relying upon the CHADS2 score for guidance in deciding upon whether to prescribe a prophylactic anticoagulant. I’ll consider applying the CHADS2 metric to patients with subclinical episodes of AF lasting for hours.
Gervasio Lamas, M.D., is chairman of medicine at Mount Sinai Medical Center in Miami Beach. In his editorial on the ASSERT study (N. Engl. J. Med. 2012;366:178-80) from which these comments are adapted, he declared having no relevant financial interests.
This is a robust study which convincingly demonstrates that device-detected subclinical AF that was picked up in the initial few months after placement of a dual-chamber pacemaker or defibrillator is independently associated with a more-than-doubled annualized risk of stroke or peripheral embolism.
But the question of cause vs. effect remains open: Are these asymptomatic episodes of AF actually causing cardioembolic stroke, or are they merely a marker of stroke risk, possibly reflecting myocardial fibrosis or structural heart disease?
It’s noteworthy that the lengthier the longest episode of subclinical AF in the first 3 months of the ASSERT study, the greater the subsequent stroke risk. Among those whose longest episode lasted more than 17.7 hours, the risk of stroke or systemic embolism was increased nearly fivefold.
The ASSERT investigators have identified a large and interesting population at increased risk of stroke. But until definitive, randomized clinical trials have been carried out demonstrating that anticoagulant therapy is justified for patients with short episodes of device-detected AF, I’m going to continue relying upon the CHADS2 score for guidance in deciding upon whether to prescribe a prophylactic anticoagulant. I’ll consider applying the CHADS2 metric to patients with subclinical episodes of AF lasting for hours.
Gervasio Lamas, M.D., is chairman of medicine at Mount Sinai Medical Center in Miami Beach. In his editorial on the ASSERT study (N. Engl. J. Med. 2012;366:178-80) from which these comments are adapted, he declared having no relevant financial interests.
This is a robust study which convincingly demonstrates that device-detected subclinical AF that was picked up in the initial few months after placement of a dual-chamber pacemaker or defibrillator is independently associated with a more-than-doubled annualized risk of stroke or peripheral embolism.
But the question of cause vs. effect remains open: Are these asymptomatic episodes of AF actually causing cardioembolic stroke, or are they merely a marker of stroke risk, possibly reflecting myocardial fibrosis or structural heart disease?
It’s noteworthy that the lengthier the longest episode of subclinical AF in the first 3 months of the ASSERT study, the greater the subsequent stroke risk. Among those whose longest episode lasted more than 17.7 hours, the risk of stroke or systemic embolism was increased nearly fivefold.
The ASSERT investigators have identified a large and interesting population at increased risk of stroke. But until definitive, randomized clinical trials have been carried out demonstrating that anticoagulant therapy is justified for patients with short episodes of device-detected AF, I’m going to continue relying upon the CHADS2 score for guidance in deciding upon whether to prescribe a prophylactic anticoagulant. I’ll consider applying the CHADS2 metric to patients with subclinical episodes of AF lasting for hours.
Gervasio Lamas, M.D., is chairman of medicine at Mount Sinai Medical Center in Miami Beach. In his editorial on the ASSERT study (N. Engl. J. Med. 2012;366:178-80) from which these comments are adapted, he declared having no relevant financial interests.
Asymptomatic episodes of atrial fibrillation are common in elderly hypertensive patients with pacemakers and no history of clinical AF – and these subclinical atrial tachyarrhythmias are associated with a sharply increased risk of subsequent ischemic stroke.
These were the two major findings of the large, international, randomized ASSERT (Asymptomatic Atrial Fibrillation and Stroke Evaluation in Pacemaker Patients and the Atrial Fibrillation Reduction Atrial Pacing Trial) study.
An important secondary finding was that, disappointingly, continuous atrial overdrive pacing using a programmable algorithm to maintain a paced atrium did not prevent the development of clinical AF, nor did it modify stroke risk, according to Dr. Jeff S. Healey of the Population Health Research Institute at McMaster University in Hamilton, Ont.
ASSERT enrolled 2,580 patients in 23 countries. All were aged 65 years or older, were hypertensive, and had recently received a dual-chamber pacemaker or implantable cardioverter-defibrillator. None of the subjects had a history of AF at enrollment.
In the first 3 months of follow-up, 10.1% of study participants developed device-detected subclinical AF (defined as an atrial rate in excess of 190 beats per minute lasting for more than 6 minutes). As a practical matter, it’s worth noting that the median time to implanted device–detected asymptomatic AF in this subgroup was 36 days. Thus, several days of negative Holter monitoring would have made for false reassurance, the cardiologist noted.
The subgroup with asymptomatic AF during the first 3 months had a 5.6-fold increased risk of developing clinical AF during a mean 2.5 years of prospective follow-up. Moreover, the rate of ischemic stroke or systemic embolism in patients with subclinical AF in the first 3 months was 1.7% per year, compared with 0.69% annually in the rest of the ASSERT participants.
The population attributable risk (PAR) of stroke or systemic embolism associated with asymptomatic AF in the first 3 months was 13%. Of note, that’s similar to the PAR of stroke that is associated with clinical AF in the Framingham Heart Study.
In a multivariate analysis adjusted for the standard predictors of stroke, device-detected subclinical AF during the first 3 months of the study was independently associated with a 2.5-fold increased subsequent risk of stroke. And this may well underestimate the true magnitude of risk, since more than half of ASSERT participants were on aspirin at baseline and 18% of those with early subclinical AF received warfarin during the follow-up period.
Both of these venerable drugs are clearly effective in reducing stroke risk in patients with established clinical AF, although it’s not known whether these agents also have a net benefit in patients with subclinical AF. A randomized trial testing this hypothesis by using these drugs or newer anticoagulants should be a priority, Dr. Healey said (N. Engl. J. Med. 2012;366:120-9).
Subjects with a CHADS2 score greater than 2 plus subclinical AF detected in the first 3 months of the study had a rate of ischemic stroke or systemic embolism of 3.7% per year, compared with 0.97% per year in patients with a similarly elevated CHADS2 score but no early subclinical atrial tachyarrhythmia.
In addition to the 10% of ASSERT participants who developed subclinical AF during the initial 3 months of follow-up, another 24% did so later.
Episodes of asymptomatic AF briefer than 6 minutes were not catalogued in the study, so it remains unknown if they, too, are associated with increased subsequent stroke risk.
At the study’s outset, all subjects with pacemakers were randomized to continuous atrial overdrive pacing or to having this feature switched off for the duration. The atrial pacing intervention did not affect the rate of development of clinical AF, although the trial was underpowered for this outcome.
The ASSERT study was supported by St. Jude Medical. Dr. Healey disclosed that he has served as a paid consultant to that company, Boehringer Ingelheim, and Bayer, as well receiving grant support from AstraZeneca, Boston Scientific, and Boehringer Ingelheim. Dr. Lamas declared having no relevant financial interests.
Asymptomatic episodes of atrial fibrillation are common in elderly hypertensive patients with pacemakers and no history of clinical AF – and these subclinical atrial tachyarrhythmias are associated with a sharply increased risk of subsequent ischemic stroke.
These were the two major findings of the large, international, randomized ASSERT (Asymptomatic Atrial Fibrillation and Stroke Evaluation in Pacemaker Patients and the Atrial Fibrillation Reduction Atrial Pacing Trial) study.
An important secondary finding was that, disappointingly, continuous atrial overdrive pacing using a programmable algorithm to maintain a paced atrium did not prevent the development of clinical AF, nor did it modify stroke risk, according to Dr. Jeff S. Healey of the Population Health Research Institute at McMaster University in Hamilton, Ont.
ASSERT enrolled 2,580 patients in 23 countries. All were aged 65 years or older, were hypertensive, and had recently received a dual-chamber pacemaker or implantable cardioverter-defibrillator. None of the subjects had a history of AF at enrollment.
In the first 3 months of follow-up, 10.1% of study participants developed device-detected subclinical AF (defined as an atrial rate in excess of 190 beats per minute lasting for more than 6 minutes). As a practical matter, it’s worth noting that the median time to implanted device–detected asymptomatic AF in this subgroup was 36 days. Thus, several days of negative Holter monitoring would have made for false reassurance, the cardiologist noted.
The subgroup with asymptomatic AF during the first 3 months had a 5.6-fold increased risk of developing clinical AF during a mean 2.5 years of prospective follow-up. Moreover, the rate of ischemic stroke or systemic embolism in patients with subclinical AF in the first 3 months was 1.7% per year, compared with 0.69% annually in the rest of the ASSERT participants.
The population attributable risk (PAR) of stroke or systemic embolism associated with asymptomatic AF in the first 3 months was 13%. Of note, that’s similar to the PAR of stroke that is associated with clinical AF in the Framingham Heart Study.
In a multivariate analysis adjusted for the standard predictors of stroke, device-detected subclinical AF during the first 3 months of the study was independently associated with a 2.5-fold increased subsequent risk of stroke. And this may well underestimate the true magnitude of risk, since more than half of ASSERT participants were on aspirin at baseline and 18% of those with early subclinical AF received warfarin during the follow-up period.
Both of these venerable drugs are clearly effective in reducing stroke risk in patients with established clinical AF, although it’s not known whether these agents also have a net benefit in patients with subclinical AF. A randomized trial testing this hypothesis by using these drugs or newer anticoagulants should be a priority, Dr. Healey said (N. Engl. J. Med. 2012;366:120-9).
Subjects with a CHADS2 score greater than 2 plus subclinical AF detected in the first 3 months of the study had a rate of ischemic stroke or systemic embolism of 3.7% per year, compared with 0.97% per year in patients with a similarly elevated CHADS2 score but no early subclinical atrial tachyarrhythmia.
In addition to the 10% of ASSERT participants who developed subclinical AF during the initial 3 months of follow-up, another 24% did so later.
Episodes of asymptomatic AF briefer than 6 minutes were not catalogued in the study, so it remains unknown if they, too, are associated with increased subsequent stroke risk.
At the study’s outset, all subjects with pacemakers were randomized to continuous atrial overdrive pacing or to having this feature switched off for the duration. The atrial pacing intervention did not affect the rate of development of clinical AF, although the trial was underpowered for this outcome.
The ASSERT study was supported by St. Jude Medical. Dr. Healey disclosed that he has served as a paid consultant to that company, Boehringer Ingelheim, and Bayer, as well receiving grant support from AstraZeneca, Boston Scientific, and Boehringer Ingelheim. Dr. Lamas declared having no relevant financial interests.
FROM THE NEW ENGLAND JOURNAL OF MEDICINE
Major Finding: Elderly hypertensive patients with no history of atrial fibrillation are at a 2.5-fold increased risk of subsequent cardioembolic stroke or systemic embolism if they exhibit device-detected asymptomatic AF in the first few months after receiving a dual-chamber pacemaker or implantable cardioverter-defibrillator.
Data Source: The 2,580-patient randomized, prospective ASSERT study.
Disclosures: ASSERT was supported by St. Jude Medical. Dr. Healey disclosed that he has served as a paid consultant to that company, Boehringer Ingelheim, and Bayer, as well receiving grant support from AstraZeneca, Boston Scientific, and Boehringer Ingelheim.
Ondansetron Quells Methotrexate-Induced Nausea
LISBON – Getting an earful of complaints of nausea from your patients on oral methotrexate therapy?
Consider bracketing the antifolate drug with the antiemetic ondansetron before and afterward, Dr. Richard B. Warren suggested at the congress.
"One successful means of helping patients overcome their nausea that we’ve used a lot in Manchester is ondansetron. For some people it can literally sort out the nausea altogether," said Dr. Warren, senior lecturer and honorary consultant dermatologist at the University of Manchester (England).
The dosing is 8 mg of ondansetron (Zofran) – a serotonin receptor antagonist used to treat nausea caused by chemotherapy, radiation therapy, and/or surgery – administered 2 hours before and once again 12 hours after taking methotrexate.
Nausea is a common nuisance side effect of the drug. It affects roughly one-quarter of patients, typically beginning about 12 hours after they take their weekly dose. The nausea can last for up to 72 hours.
In addition to prophylactic ondansetron, other strategies that may be effective in overcoming methotrexate-induced nausea include the following:
– Divide the dose.
– Have patients take their oral methotrexate with the evening meal.
– Consider switching to subcutaneous methotrexate. "It has been shown to be more efficacious, and with less in the way of adverse events than oral methotrexate," Dr. Warren said.
He declared having no relevant financial interests.
LISBON – Getting an earful of complaints of nausea from your patients on oral methotrexate therapy?
Consider bracketing the antifolate drug with the antiemetic ondansetron before and afterward, Dr. Richard B. Warren suggested at the congress.
"One successful means of helping patients overcome their nausea that we’ve used a lot in Manchester is ondansetron. For some people it can literally sort out the nausea altogether," said Dr. Warren, senior lecturer and honorary consultant dermatologist at the University of Manchester (England).
The dosing is 8 mg of ondansetron (Zofran) – a serotonin receptor antagonist used to treat nausea caused by chemotherapy, radiation therapy, and/or surgery – administered 2 hours before and once again 12 hours after taking methotrexate.
Nausea is a common nuisance side effect of the drug. It affects roughly one-quarter of patients, typically beginning about 12 hours after they take their weekly dose. The nausea can last for up to 72 hours.
In addition to prophylactic ondansetron, other strategies that may be effective in overcoming methotrexate-induced nausea include the following:
– Divide the dose.
– Have patients take their oral methotrexate with the evening meal.
– Consider switching to subcutaneous methotrexate. "It has been shown to be more efficacious, and with less in the way of adverse events than oral methotrexate," Dr. Warren said.
He declared having no relevant financial interests.
LISBON – Getting an earful of complaints of nausea from your patients on oral methotrexate therapy?
Consider bracketing the antifolate drug with the antiemetic ondansetron before and afterward, Dr. Richard B. Warren suggested at the congress.
"One successful means of helping patients overcome their nausea that we’ve used a lot in Manchester is ondansetron. For some people it can literally sort out the nausea altogether," said Dr. Warren, senior lecturer and honorary consultant dermatologist at the University of Manchester (England).
The dosing is 8 mg of ondansetron (Zofran) – a serotonin receptor antagonist used to treat nausea caused by chemotherapy, radiation therapy, and/or surgery – administered 2 hours before and once again 12 hours after taking methotrexate.
Nausea is a common nuisance side effect of the drug. It affects roughly one-quarter of patients, typically beginning about 12 hours after they take their weekly dose. The nausea can last for up to 72 hours.
In addition to prophylactic ondansetron, other strategies that may be effective in overcoming methotrexate-induced nausea include the following:
– Divide the dose.
– Have patients take their oral methotrexate with the evening meal.
– Consider switching to subcutaneous methotrexate. "It has been shown to be more efficacious, and with less in the way of adverse events than oral methotrexate," Dr. Warren said.
He declared having no relevant financial interests.
EXPERT OPINION FROM THE ANNUAL CONGRESS OF THE EUROPEAN ACADEMY OF DERMATOLOGY AND VENEREOLOGY
Program Curbs Shoulder Morbidity Post Breast Cancer
SAN ANTONIO – Long-term upper-extremity pain and functional impairment following breast cancer surgery are reduced by an innovative physical therapy–centered surveillance program, according to a 5-year prospective study.
The program was developed at the breast care center of Walter Reed National Military Medical Center, Bethesda, Md. It begins with a preoperative patient interview and structured evaluation by a physical therapist. The assessment includes what physical therapists call an upper-quarter screen, which evaluates shoulder range of motion, strength, and arm volume. Self-reported pain, fatigue, function, and activity level are recorded.
At that time, the patient is also instructed in a home exercise program she is to start 2 weeks post surgery. These are simple range-of-motion exercises focused on forward flexion overhead; abduction; internal and external rotation of the glenohumeral joint; and scapular retraction, Nicole L. Stout explained at the San Antonio Breast Cancer Symposium.
The patient is subsequently seen by the physical therapist (in conjunction with her regular follow-up visits with an oncologist or surgeon) at 1, 3, 6, 9, 12, and 60 months post surgery. If upper-extremity dysfunction worsens, more intensive interventions are introduced.
The rationale for this prospective surveillance program lies in an understanding that the shoulder and arm impairments caused by breast cancer surgery and radiation therapy are typically subtle initially, but if left unidentified and uncorrected, they often progress to serious rotator cuff damage, "frozen" shoulder, and debilitating lymphedema, according to Ms. Stout, a civilian research physical therapist at the military center.
She had previously presented her 1-year prospective outcomes. This time around, she presented prospective 5-year follow-up data on 86 breast cancer patients who participated in the program. This is the first prospective cohort study in the United States to track breast cancer–related upper-extremity morbidity and functional outcomes for this long a time period.
The 12-month and 5-year visits included formal assessment of upper-extremity outcomes using the Harvard Alumni Activity Survey, the Upper Limb Disability Questionnaire, and the Short Form-36.
At 5 years, 11% of the women had stage I/II lymphedema and 38% had subclinical lymphedema (defined as less than 3% arm volume swelling as measured by a Perometer). These results compare favorably with those of published studies on lymphedema, which have reported long-term rates of stage I-III arm swelling in the 40%-60% range, she noted.
In all, 18% of patients reported arm numbness at 5 years, 17% noted shoulder pain, 3% had neck pain, and 1% had chest wall pain.
One-third of subjects reported fatigue of level 3 or more on a 10-point visual analog scale. Again, other investigators have documented long-term rates of clinically significant fatigue in the 50%-60% range.
At 5 years post surgery, patients demonstrated less impairment of physical activity than at preoperative baseline. At baseline, 33% had significant limitations in terms of carrying heavy objects, performing household chores, and other physical activities. After 5 years of prospective surveillance and tailored physical therapy, only 20% of patients had physical activity limitations.
However, 68% of patients reported experiencing some restrictions on their social activity at 5 years, and 52% experienced limitations on their recreational activities.
The study was funded by the medical center. Ms. Stout declared having no financial conflicts of interest.
SAN ANTONIO – Long-term upper-extremity pain and functional impairment following breast cancer surgery are reduced by an innovative physical therapy–centered surveillance program, according to a 5-year prospective study.
The program was developed at the breast care center of Walter Reed National Military Medical Center, Bethesda, Md. It begins with a preoperative patient interview and structured evaluation by a physical therapist. The assessment includes what physical therapists call an upper-quarter screen, which evaluates shoulder range of motion, strength, and arm volume. Self-reported pain, fatigue, function, and activity level are recorded.
At that time, the patient is also instructed in a home exercise program she is to start 2 weeks post surgery. These are simple range-of-motion exercises focused on forward flexion overhead; abduction; internal and external rotation of the glenohumeral joint; and scapular retraction, Nicole L. Stout explained at the San Antonio Breast Cancer Symposium.
The patient is subsequently seen by the physical therapist (in conjunction with her regular follow-up visits with an oncologist or surgeon) at 1, 3, 6, 9, 12, and 60 months post surgery. If upper-extremity dysfunction worsens, more intensive interventions are introduced.
The rationale for this prospective surveillance program lies in an understanding that the shoulder and arm impairments caused by breast cancer surgery and radiation therapy are typically subtle initially, but if left unidentified and uncorrected, they often progress to serious rotator cuff damage, "frozen" shoulder, and debilitating lymphedema, according to Ms. Stout, a civilian research physical therapist at the military center.
She had previously presented her 1-year prospective outcomes. This time around, she presented prospective 5-year follow-up data on 86 breast cancer patients who participated in the program. This is the first prospective cohort study in the United States to track breast cancer–related upper-extremity morbidity and functional outcomes for this long a time period.
The 12-month and 5-year visits included formal assessment of upper-extremity outcomes using the Harvard Alumni Activity Survey, the Upper Limb Disability Questionnaire, and the Short Form-36.
At 5 years, 11% of the women had stage I/II lymphedema and 38% had subclinical lymphedema (defined as less than 3% arm volume swelling as measured by a Perometer). These results compare favorably with those of published studies on lymphedema, which have reported long-term rates of stage I-III arm swelling in the 40%-60% range, she noted.
In all, 18% of patients reported arm numbness at 5 years, 17% noted shoulder pain, 3% had neck pain, and 1% had chest wall pain.
One-third of subjects reported fatigue of level 3 or more on a 10-point visual analog scale. Again, other investigators have documented long-term rates of clinically significant fatigue in the 50%-60% range.
At 5 years post surgery, patients demonstrated less impairment of physical activity than at preoperative baseline. At baseline, 33% had significant limitations in terms of carrying heavy objects, performing household chores, and other physical activities. After 5 years of prospective surveillance and tailored physical therapy, only 20% of patients had physical activity limitations.
However, 68% of patients reported experiencing some restrictions on their social activity at 5 years, and 52% experienced limitations on their recreational activities.
The study was funded by the medical center. Ms. Stout declared having no financial conflicts of interest.
SAN ANTONIO – Long-term upper-extremity pain and functional impairment following breast cancer surgery are reduced by an innovative physical therapy–centered surveillance program, according to a 5-year prospective study.
The program was developed at the breast care center of Walter Reed National Military Medical Center, Bethesda, Md. It begins with a preoperative patient interview and structured evaluation by a physical therapist. The assessment includes what physical therapists call an upper-quarter screen, which evaluates shoulder range of motion, strength, and arm volume. Self-reported pain, fatigue, function, and activity level are recorded.
At that time, the patient is also instructed in a home exercise program she is to start 2 weeks post surgery. These are simple range-of-motion exercises focused on forward flexion overhead; abduction; internal and external rotation of the glenohumeral joint; and scapular retraction, Nicole L. Stout explained at the San Antonio Breast Cancer Symposium.
The patient is subsequently seen by the physical therapist (in conjunction with her regular follow-up visits with an oncologist or surgeon) at 1, 3, 6, 9, 12, and 60 months post surgery. If upper-extremity dysfunction worsens, more intensive interventions are introduced.
The rationale for this prospective surveillance program lies in an understanding that the shoulder and arm impairments caused by breast cancer surgery and radiation therapy are typically subtle initially, but if left unidentified and uncorrected, they often progress to serious rotator cuff damage, "frozen" shoulder, and debilitating lymphedema, according to Ms. Stout, a civilian research physical therapist at the military center.
She had previously presented her 1-year prospective outcomes. This time around, she presented prospective 5-year follow-up data on 86 breast cancer patients who participated in the program. This is the first prospective cohort study in the United States to track breast cancer–related upper-extremity morbidity and functional outcomes for this long a time period.
The 12-month and 5-year visits included formal assessment of upper-extremity outcomes using the Harvard Alumni Activity Survey, the Upper Limb Disability Questionnaire, and the Short Form-36.
At 5 years, 11% of the women had stage I/II lymphedema and 38% had subclinical lymphedema (defined as less than 3% arm volume swelling as measured by a Perometer). These results compare favorably with those of published studies on lymphedema, which have reported long-term rates of stage I-III arm swelling in the 40%-60% range, she noted.
In all, 18% of patients reported arm numbness at 5 years, 17% noted shoulder pain, 3% had neck pain, and 1% had chest wall pain.
One-third of subjects reported fatigue of level 3 or more on a 10-point visual analog scale. Again, other investigators have documented long-term rates of clinically significant fatigue in the 50%-60% range.
At 5 years post surgery, patients demonstrated less impairment of physical activity than at preoperative baseline. At baseline, 33% had significant limitations in terms of carrying heavy objects, performing household chores, and other physical activities. After 5 years of prospective surveillance and tailored physical therapy, only 20% of patients had physical activity limitations.
However, 68% of patients reported experiencing some restrictions on their social activity at 5 years, and 52% experienced limitations on their recreational activities.
The study was funded by the medical center. Ms. Stout declared having no financial conflicts of interest.
FROM THE SAN ANTONIO BREAST CANCER SYMPOSIUM
Major Finding: After 5 years of participation in a surveillance/early intervention program for upper-extremity morbidity, only 11% of a group of breast cancer patients had stage I/II lymphedema, sharply lower than the 40%-60% long-term rates reported by other investigators.
Data Source: A 5-year prospective, uncontrolled, observational study to date involving 86 breast cancer patients.
Disclosures: The study was funded by the Walter Reed National Military Medical Center. Ms. Stout declared having no financial conflicts.
The Whys and How of Stopping Biologics
The vast majority of medical talks on biologic therapy for psoriasis focus on starting and maintaining the medications. Stopping biologics is a seldom-discussed topic, yet one that physicians often need to address.
"We’ve all got patients who are on biologic therapy who are completely clear of their psoriasis, and you’re constantly wondering, ‘Should I stop the biologic? Do they need to have that treatment anymore? Can I reduce the dosing frequency?’ The simplest answer is that in most cases, it’s probably not a good idea to stop unless there’s a good reason for doing so. It has been shown in most of the big clinical trials that if you stop therapy, the psoriasis will relapse at some point," Dr. Christopher Griffiths said at the annual congress of the European Academy of Dermatology and Venereology.
"With long-term therapy beyond 6-12 months, can biologics be stopped or produce remission? In most cases, no. And there’s no biomarker for disease activity as of yet to guide us," added Dr. Griffiths, professor of dermatology and associate dean for research at the University of Manchester (England).
Compelling reasons to stop biologic therapy include failure to achieve or maintain significant clinical improvement, serious adverse events, impending elective major surgery, certain vaccinations, and pregnancy. Dr. Griffiths highlighted the following points:
• Lack of Effectiveness. Today’s biologics are not curative. In a recent French report, only 31 of 86 psoriasis patients (36%) who started on etanercept (Enbrel), infliximab (Remicade), or efalizumab were still on the same biologic agent 24 months later (J. Dermatol. Treat. 2011;22:151-2). Similarly, the Danish national psoriasis database experience has been that roughly 40% of patients started on etanercept or adalimumab (Humira) were still on the medication 4 years later, as were 70% of those who started on infliximab (Br. J. Dermatol. 2011;164:1091-6).
The good news, Dr. Griffiths continued, is that lack of effectiveness for anti–tumor necrosis factor agents is not a class effect. He and his coinvestigators have reported that 21 of 31 psoriasis patients who switched to adalimumab from another anti-TNF biologic for lack of efficacy met the NICE (U.K. National Institute for Health and Clinical Excellence) criteria for adalimumab treatment continuation at 16 weeks (Br. J. Dermatol. 2010;163:859-62).
"Each anti-TNF drug is different, so if you fail one it doesn’t mean you’re going to fail another. That’s a very important point, and a very strong argument in your favor when dealing with payers. And it’s an observation that can only come from real-life data from a cohort study; you’re not going to get that information from clinical trials," the dermatologist explained.
The NICE criteria for continuation of biologic therapy bring an element of strict objectivity to treatment decision making in a rationed health care system. For British psoriasis patients to continue on a given biologic agent, they have to demonstrate a PASI 75 response (that is, a 75% improvement over the baseline Psoriasis Area and Severity Index score) at 16 weeks, or a PASI 50 response plus a 5-point drop in the Dermatology Life Quality Index (DLQI).
When physicians switch biologics, Dr. Griffiths recommends that they skip a lengthy washout period because of the associated risk of a severe, hard-to-control flare. His practice is to stop one and start another.
• Major Elective Surgery. There is a dearth of data on stopping biologics in psoriasis patients who plan to undergo elective surgery. The best practice for now, in Dr. Griffiths’ view, is to follow the British Society for Rheumatology guidance, which is based on an extensive biologics register the group maintains for rheumatoid arthritis.
The rheumatologists’ advice is to halt effective biologic therapy only for truly major surgery, and to stop anti-TNF drugs more than four half-lives before the operation. That’s 2-3 weeks beforehand for etanercept, 6-8 weeks for adalimumab, and 4-6 weeks for infliximab. There are no firm data for ustekinumab (Stelara) as yet, but experts advise halting it 12 weeks before surgery. Biologic therapy should be resumed as soon as possible postoperatively.
• Pregnancy. Again, a paucity of data exists on biologics for psoriasis in pregnancy. But the data accumulating in the British Society for Rheumatology Biologics Register (BSRBR) is reassuring. Although the rheumatologists recommend that pregnancy be avoided in patients on biologics, the experience to date in pregnant rheumatoid arthritis patients suggests there is little to no risk to the fetus. Breastfeeding should be avoided by women on biologic therapy other than infliximab, which is not excreted in breast milk, Dr. Griffiths continued.
• Vaccinations. British Association of Dermatologists guidelines on the use of biologics for psoriasis (Br. J. Dermatol. 2009;161:987-1019), which Dr. Griffiths coauthored and which will be updated in late 2012, recommend avoiding giving live or attenuated virus vaccines within 2 weeks prior to starting a patient on biologic therapy, or while the patient is on a biologic, or for up to 6 months after the patient stops the biologic. Inactivated virus vaccines are safe for patients on biologic therapy, although the antibody response will be somewhat less robust in a patient on an anti-TNF agent than in other individuals.
"But we advise – as should you – that all patients on biologics should receive influenza and pneumococcal vaccines. It’s just good clinical practice because these patients are by definition in a high-risk category," he said.
• Stopping and Restarting Biologics. It’s well established that etanercept can be used intermittently with good results. That is, a patient might use etanercept to good effect for 6 months, stop it, then restart when relapse occurs, and the agent will still remain effective. The same typically holds true for alefacept (Amevive).
In contrast, infliximab can realistically be used for only a single course of treatment. If a patient goes off the drug and later goes back on it, the chances of regaining a response are very low because of the formation of blocking antibodies.
The picture regarding the intermittent use of adalimumab is less clear. There are documented cases in which this agent hasn’t been effective any longer upon second usage.
There is good new evidence, presented by Dr. Griffiths elsewhere during the congress, that ustekinumab can be restarted after a hiatus with very good results.
Dr. Griffiths disclosed that he serves on the advisory boards for and has received research grants from numerous pharmaceutical companies.
The vast majority of medical talks on biologic therapy for psoriasis focus on starting and maintaining the medications. Stopping biologics is a seldom-discussed topic, yet one that physicians often need to address.
"We’ve all got patients who are on biologic therapy who are completely clear of their psoriasis, and you’re constantly wondering, ‘Should I stop the biologic? Do they need to have that treatment anymore? Can I reduce the dosing frequency?’ The simplest answer is that in most cases, it’s probably not a good idea to stop unless there’s a good reason for doing so. It has been shown in most of the big clinical trials that if you stop therapy, the psoriasis will relapse at some point," Dr. Christopher Griffiths said at the annual congress of the European Academy of Dermatology and Venereology.
"With long-term therapy beyond 6-12 months, can biologics be stopped or produce remission? In most cases, no. And there’s no biomarker for disease activity as of yet to guide us," added Dr. Griffiths, professor of dermatology and associate dean for research at the University of Manchester (England).
Compelling reasons to stop biologic therapy include failure to achieve or maintain significant clinical improvement, serious adverse events, impending elective major surgery, certain vaccinations, and pregnancy. Dr. Griffiths highlighted the following points:
• Lack of Effectiveness. Today’s biologics are not curative. In a recent French report, only 31 of 86 psoriasis patients (36%) who started on etanercept (Enbrel), infliximab (Remicade), or efalizumab were still on the same biologic agent 24 months later (J. Dermatol. Treat. 2011;22:151-2). Similarly, the Danish national psoriasis database experience has been that roughly 40% of patients started on etanercept or adalimumab (Humira) were still on the medication 4 years later, as were 70% of those who started on infliximab (Br. J. Dermatol. 2011;164:1091-6).
The good news, Dr. Griffiths continued, is that lack of effectiveness for anti–tumor necrosis factor agents is not a class effect. He and his coinvestigators have reported that 21 of 31 psoriasis patients who switched to adalimumab from another anti-TNF biologic for lack of efficacy met the NICE (U.K. National Institute for Health and Clinical Excellence) criteria for adalimumab treatment continuation at 16 weeks (Br. J. Dermatol. 2010;163:859-62).
"Each anti-TNF drug is different, so if you fail one it doesn’t mean you’re going to fail another. That’s a very important point, and a very strong argument in your favor when dealing with payers. And it’s an observation that can only come from real-life data from a cohort study; you’re not going to get that information from clinical trials," the dermatologist explained.
The NICE criteria for continuation of biologic therapy bring an element of strict objectivity to treatment decision making in a rationed health care system. For British psoriasis patients to continue on a given biologic agent, they have to demonstrate a PASI 75 response (that is, a 75% improvement over the baseline Psoriasis Area and Severity Index score) at 16 weeks, or a PASI 50 response plus a 5-point drop in the Dermatology Life Quality Index (DLQI).
When physicians switch biologics, Dr. Griffiths recommends that they skip a lengthy washout period because of the associated risk of a severe, hard-to-control flare. His practice is to stop one and start another.
• Major Elective Surgery. There is a dearth of data on stopping biologics in psoriasis patients who plan to undergo elective surgery. The best practice for now, in Dr. Griffiths’ view, is to follow the British Society for Rheumatology guidance, which is based on an extensive biologics register the group maintains for rheumatoid arthritis.
The rheumatologists’ advice is to halt effective biologic therapy only for truly major surgery, and to stop anti-TNF drugs more than four half-lives before the operation. That’s 2-3 weeks beforehand for etanercept, 6-8 weeks for adalimumab, and 4-6 weeks for infliximab. There are no firm data for ustekinumab (Stelara) as yet, but experts advise halting it 12 weeks before surgery. Biologic therapy should be resumed as soon as possible postoperatively.
• Pregnancy. Again, a paucity of data exists on biologics for psoriasis in pregnancy. But the data accumulating in the British Society for Rheumatology Biologics Register (BSRBR) is reassuring. Although the rheumatologists recommend that pregnancy be avoided in patients on biologics, the experience to date in pregnant rheumatoid arthritis patients suggests there is little to no risk to the fetus. Breastfeeding should be avoided by women on biologic therapy other than infliximab, which is not excreted in breast milk, Dr. Griffiths continued.
• Vaccinations. British Association of Dermatologists guidelines on the use of biologics for psoriasis (Br. J. Dermatol. 2009;161:987-1019), which Dr. Griffiths coauthored and which will be updated in late 2012, recommend avoiding giving live or attenuated virus vaccines within 2 weeks prior to starting a patient on biologic therapy, or while the patient is on a biologic, or for up to 6 months after the patient stops the biologic. Inactivated virus vaccines are safe for patients on biologic therapy, although the antibody response will be somewhat less robust in a patient on an anti-TNF agent than in other individuals.
"But we advise – as should you – that all patients on biologics should receive influenza and pneumococcal vaccines. It’s just good clinical practice because these patients are by definition in a high-risk category," he said.
• Stopping and Restarting Biologics. It’s well established that etanercept can be used intermittently with good results. That is, a patient might use etanercept to good effect for 6 months, stop it, then restart when relapse occurs, and the agent will still remain effective. The same typically holds true for alefacept (Amevive).
In contrast, infliximab can realistically be used for only a single course of treatment. If a patient goes off the drug and later goes back on it, the chances of regaining a response are very low because of the formation of blocking antibodies.
The picture regarding the intermittent use of adalimumab is less clear. There are documented cases in which this agent hasn’t been effective any longer upon second usage.
There is good new evidence, presented by Dr. Griffiths elsewhere during the congress, that ustekinumab can be restarted after a hiatus with very good results.
Dr. Griffiths disclosed that he serves on the advisory boards for and has received research grants from numerous pharmaceutical companies.
The vast majority of medical talks on biologic therapy for psoriasis focus on starting and maintaining the medications. Stopping biologics is a seldom-discussed topic, yet one that physicians often need to address.
"We’ve all got patients who are on biologic therapy who are completely clear of their psoriasis, and you’re constantly wondering, ‘Should I stop the biologic? Do they need to have that treatment anymore? Can I reduce the dosing frequency?’ The simplest answer is that in most cases, it’s probably not a good idea to stop unless there’s a good reason for doing so. It has been shown in most of the big clinical trials that if you stop therapy, the psoriasis will relapse at some point," Dr. Christopher Griffiths said at the annual congress of the European Academy of Dermatology and Venereology.
"With long-term therapy beyond 6-12 months, can biologics be stopped or produce remission? In most cases, no. And there’s no biomarker for disease activity as of yet to guide us," added Dr. Griffiths, professor of dermatology and associate dean for research at the University of Manchester (England).
Compelling reasons to stop biologic therapy include failure to achieve or maintain significant clinical improvement, serious adverse events, impending elective major surgery, certain vaccinations, and pregnancy. Dr. Griffiths highlighted the following points:
• Lack of Effectiveness. Today’s biologics are not curative. In a recent French report, only 31 of 86 psoriasis patients (36%) who started on etanercept (Enbrel), infliximab (Remicade), or efalizumab were still on the same biologic agent 24 months later (J. Dermatol. Treat. 2011;22:151-2). Similarly, the Danish national psoriasis database experience has been that roughly 40% of patients started on etanercept or adalimumab (Humira) were still on the medication 4 years later, as were 70% of those who started on infliximab (Br. J. Dermatol. 2011;164:1091-6).
The good news, Dr. Griffiths continued, is that lack of effectiveness for anti–tumor necrosis factor agents is not a class effect. He and his coinvestigators have reported that 21 of 31 psoriasis patients who switched to adalimumab from another anti-TNF biologic for lack of efficacy met the NICE (U.K. National Institute for Health and Clinical Excellence) criteria for adalimumab treatment continuation at 16 weeks (Br. J. Dermatol. 2010;163:859-62).
"Each anti-TNF drug is different, so if you fail one it doesn’t mean you’re going to fail another. That’s a very important point, and a very strong argument in your favor when dealing with payers. And it’s an observation that can only come from real-life data from a cohort study; you’re not going to get that information from clinical trials," the dermatologist explained.
The NICE criteria for continuation of biologic therapy bring an element of strict objectivity to treatment decision making in a rationed health care system. For British psoriasis patients to continue on a given biologic agent, they have to demonstrate a PASI 75 response (that is, a 75% improvement over the baseline Psoriasis Area and Severity Index score) at 16 weeks, or a PASI 50 response plus a 5-point drop in the Dermatology Life Quality Index (DLQI).
When physicians switch biologics, Dr. Griffiths recommends that they skip a lengthy washout period because of the associated risk of a severe, hard-to-control flare. His practice is to stop one and start another.
• Major Elective Surgery. There is a dearth of data on stopping biologics in psoriasis patients who plan to undergo elective surgery. The best practice for now, in Dr. Griffiths’ view, is to follow the British Society for Rheumatology guidance, which is based on an extensive biologics register the group maintains for rheumatoid arthritis.
The rheumatologists’ advice is to halt effective biologic therapy only for truly major surgery, and to stop anti-TNF drugs more than four half-lives before the operation. That’s 2-3 weeks beforehand for etanercept, 6-8 weeks for adalimumab, and 4-6 weeks for infliximab. There are no firm data for ustekinumab (Stelara) as yet, but experts advise halting it 12 weeks before surgery. Biologic therapy should be resumed as soon as possible postoperatively.
• Pregnancy. Again, a paucity of data exists on biologics for psoriasis in pregnancy. But the data accumulating in the British Society for Rheumatology Biologics Register (BSRBR) is reassuring. Although the rheumatologists recommend that pregnancy be avoided in patients on biologics, the experience to date in pregnant rheumatoid arthritis patients suggests there is little to no risk to the fetus. Breastfeeding should be avoided by women on biologic therapy other than infliximab, which is not excreted in breast milk, Dr. Griffiths continued.
• Vaccinations. British Association of Dermatologists guidelines on the use of biologics for psoriasis (Br. J. Dermatol. 2009;161:987-1019), which Dr. Griffiths coauthored and which will be updated in late 2012, recommend avoiding giving live or attenuated virus vaccines within 2 weeks prior to starting a patient on biologic therapy, or while the patient is on a biologic, or for up to 6 months after the patient stops the biologic. Inactivated virus vaccines are safe for patients on biologic therapy, although the antibody response will be somewhat less robust in a patient on an anti-TNF agent than in other individuals.
"But we advise – as should you – that all patients on biologics should receive influenza and pneumococcal vaccines. It’s just good clinical practice because these patients are by definition in a high-risk category," he said.
• Stopping and Restarting Biologics. It’s well established that etanercept can be used intermittently with good results. That is, a patient might use etanercept to good effect for 6 months, stop it, then restart when relapse occurs, and the agent will still remain effective. The same typically holds true for alefacept (Amevive).
In contrast, infliximab can realistically be used for only a single course of treatment. If a patient goes off the drug and later goes back on it, the chances of regaining a response are very low because of the formation of blocking antibodies.
The picture regarding the intermittent use of adalimumab is less clear. There are documented cases in which this agent hasn’t been effective any longer upon second usage.
There is good new evidence, presented by Dr. Griffiths elsewhere during the congress, that ustekinumab can be restarted after a hiatus with very good results.
Dr. Griffiths disclosed that he serves on the advisory boards for and has received research grants from numerous pharmaceutical companies.
Pegfilgrastim Costly, Unnecessary in Some Breast Cancer Patients
SAN ANTONIO – Curbing colony-stimulating factor use at the start of dose-dense chemotherapy in Medicare patients with hormone receptor–positive breast cancer would save nearly $40 million per year in a population unlikely to gain meaningful benefit from such therapy, according to a new study.
The use of first-cycle colony-stimulating factor (CSF) therapy took off in 2002, Dr. Dawn L. Hershman told attendees at the San Antonio Breast Cancer Symposium. That was the year the benefits of dose-dense chemotherapy were reported, and pegfilgrastim (Neulasta), a granulocyte CSF that was easier to use than those previously available, received marketing approval from the Food and Drug Administration.
A new analysis of Medicare data shows that first-cycle use of CSFs in patients with stage I-III breast cancer jumped from 13% during 1998-2002 to 68% in 2002-2005, reported Dr. Hershman of Columbia University, New York.
In the last half-decade, however, multiple major studies have convincingly shown that the benefits of dose-dense chemotherapy with first-cycle CSFs are confined to breast cancer patients with hormone receptor–negative tumors, according to Dr. Hershman.
For example, one meta-analysis of studies comparing dose-dense to standard chemotherapy in 3,337 estrogen receptor–negative patients showed that dose-dense chemotherapy yielded a 29% reduction in recurrence risk and a 16% improvement in overall survival. In 1,344 estrogen receptor–positive patients, in contrast, dose-dense chemotherapy provided a nonsignificant 8% reduction in recurrence risk and no hint of a benefit in terms of overall survival (J. Natl. Cancer Inst. 2010;102:1845-54).
For the new study, Dr. Hershman and her coauthors identified 10,773 patients in a combined Surveillance, Epidemiology, and End Results (SEER)-Medicare database who were over age 65 with stage I-III breast cancer diagnosed during 1998-2005, and who had at least one chemotherapy claim within 6 months of diagnosis. Of these, 5,266 had a CSF claim during therapy. First-cycle CSF use increased from 13% to 68% over the study period, and the use of pegfilgrastim jumped from 4% to 84%.
"We’re very quick in oncology to adopt, and we never pull back – even when there’s evidence against," Dr. Hershman said. "If we have to make decisions in terms of controlling costs, we shouldn’t be giving drugs that are costly and have no benefit."
Dr. Thomas J. Smith, chair of the session at which Dr. Hershman presented the new study, took the argument further, citing Dr. Hershman’s own earlier study suggesting that CSF therapy in elderly women may actually cause significant harm. In an analysis in which she merged Medicare and SEER databases, Dr. Hershman had reported a twofold increased risk of acute myeloid leukemia and myelodysplastic syndrome in elderly women who received a CSF compared with those who did not (J. Natl. Cancer Inst. 2007;99:196-205).
"All that nearly $40 million per year in Medicare expenditures for colony-stimulating factors is buying is toxicity. You’re not buying any disease benefit," argued Dr. Smith, director of palliative care and Harry J. Duffey Family Professor of Palliative Care at Johns Hopkins University, Baltimore.
He proposed that it’s time to consider putting the use of CSFs and dose-dense chemotherapy in elderly patients with hormone receptor–positive tumors on the Quality Oncology Practice Initiative's "do not use" list.
"My thought would be at a minimum we should explain the very small, at-most 8% relative benefit, and the risks. And most people, I think, given the numbers, would say, ‘I’ll take regular chemotherapy, thank you,’ " Dr. Smith predicted.
Dr. Hershman declared having no financial conflicts.
SAN ANTONIO – Curbing colony-stimulating factor use at the start of dose-dense chemotherapy in Medicare patients with hormone receptor–positive breast cancer would save nearly $40 million per year in a population unlikely to gain meaningful benefit from such therapy, according to a new study.
The use of first-cycle colony-stimulating factor (CSF) therapy took off in 2002, Dr. Dawn L. Hershman told attendees at the San Antonio Breast Cancer Symposium. That was the year the benefits of dose-dense chemotherapy were reported, and pegfilgrastim (Neulasta), a granulocyte CSF that was easier to use than those previously available, received marketing approval from the Food and Drug Administration.
A new analysis of Medicare data shows that first-cycle use of CSFs in patients with stage I-III breast cancer jumped from 13% during 1998-2002 to 68% in 2002-2005, reported Dr. Hershman of Columbia University, New York.
In the last half-decade, however, multiple major studies have convincingly shown that the benefits of dose-dense chemotherapy with first-cycle CSFs are confined to breast cancer patients with hormone receptor–negative tumors, according to Dr. Hershman.
For example, one meta-analysis of studies comparing dose-dense to standard chemotherapy in 3,337 estrogen receptor–negative patients showed that dose-dense chemotherapy yielded a 29% reduction in recurrence risk and a 16% improvement in overall survival. In 1,344 estrogen receptor–positive patients, in contrast, dose-dense chemotherapy provided a nonsignificant 8% reduction in recurrence risk and no hint of a benefit in terms of overall survival (J. Natl. Cancer Inst. 2010;102:1845-54).
For the new study, Dr. Hershman and her coauthors identified 10,773 patients in a combined Surveillance, Epidemiology, and End Results (SEER)-Medicare database who were over age 65 with stage I-III breast cancer diagnosed during 1998-2005, and who had at least one chemotherapy claim within 6 months of diagnosis. Of these, 5,266 had a CSF claim during therapy. First-cycle CSF use increased from 13% to 68% over the study period, and the use of pegfilgrastim jumped from 4% to 84%.
"We’re very quick in oncology to adopt, and we never pull back – even when there’s evidence against," Dr. Hershman said. "If we have to make decisions in terms of controlling costs, we shouldn’t be giving drugs that are costly and have no benefit."
Dr. Thomas J. Smith, chair of the session at which Dr. Hershman presented the new study, took the argument further, citing Dr. Hershman’s own earlier study suggesting that CSF therapy in elderly women may actually cause significant harm. In an analysis in which she merged Medicare and SEER databases, Dr. Hershman had reported a twofold increased risk of acute myeloid leukemia and myelodysplastic syndrome in elderly women who received a CSF compared with those who did not (J. Natl. Cancer Inst. 2007;99:196-205).
"All that nearly $40 million per year in Medicare expenditures for colony-stimulating factors is buying is toxicity. You’re not buying any disease benefit," argued Dr. Smith, director of palliative care and Harry J. Duffey Family Professor of Palliative Care at Johns Hopkins University, Baltimore.
He proposed that it’s time to consider putting the use of CSFs and dose-dense chemotherapy in elderly patients with hormone receptor–positive tumors on the Quality Oncology Practice Initiative's "do not use" list.
"My thought would be at a minimum we should explain the very small, at-most 8% relative benefit, and the risks. And most people, I think, given the numbers, would say, ‘I’ll take regular chemotherapy, thank you,’ " Dr. Smith predicted.
Dr. Hershman declared having no financial conflicts.
SAN ANTONIO – Curbing colony-stimulating factor use at the start of dose-dense chemotherapy in Medicare patients with hormone receptor–positive breast cancer would save nearly $40 million per year in a population unlikely to gain meaningful benefit from such therapy, according to a new study.
The use of first-cycle colony-stimulating factor (CSF) therapy took off in 2002, Dr. Dawn L. Hershman told attendees at the San Antonio Breast Cancer Symposium. That was the year the benefits of dose-dense chemotherapy were reported, and pegfilgrastim (Neulasta), a granulocyte CSF that was easier to use than those previously available, received marketing approval from the Food and Drug Administration.
A new analysis of Medicare data shows that first-cycle use of CSFs in patients with stage I-III breast cancer jumped from 13% during 1998-2002 to 68% in 2002-2005, reported Dr. Hershman of Columbia University, New York.
In the last half-decade, however, multiple major studies have convincingly shown that the benefits of dose-dense chemotherapy with first-cycle CSFs are confined to breast cancer patients with hormone receptor–negative tumors, according to Dr. Hershman.
For example, one meta-analysis of studies comparing dose-dense to standard chemotherapy in 3,337 estrogen receptor–negative patients showed that dose-dense chemotherapy yielded a 29% reduction in recurrence risk and a 16% improvement in overall survival. In 1,344 estrogen receptor–positive patients, in contrast, dose-dense chemotherapy provided a nonsignificant 8% reduction in recurrence risk and no hint of a benefit in terms of overall survival (J. Natl. Cancer Inst. 2010;102:1845-54).
For the new study, Dr. Hershman and her coauthors identified 10,773 patients in a combined Surveillance, Epidemiology, and End Results (SEER)-Medicare database who were over age 65 with stage I-III breast cancer diagnosed during 1998-2005, and who had at least one chemotherapy claim within 6 months of diagnosis. Of these, 5,266 had a CSF claim during therapy. First-cycle CSF use increased from 13% to 68% over the study period, and the use of pegfilgrastim jumped from 4% to 84%.
"We’re very quick in oncology to adopt, and we never pull back – even when there’s evidence against," Dr. Hershman said. "If we have to make decisions in terms of controlling costs, we shouldn’t be giving drugs that are costly and have no benefit."
Dr. Thomas J. Smith, chair of the session at which Dr. Hershman presented the new study, took the argument further, citing Dr. Hershman’s own earlier study suggesting that CSF therapy in elderly women may actually cause significant harm. In an analysis in which she merged Medicare and SEER databases, Dr. Hershman had reported a twofold increased risk of acute myeloid leukemia and myelodysplastic syndrome in elderly women who received a CSF compared with those who did not (J. Natl. Cancer Inst. 2007;99:196-205).
"All that nearly $40 million per year in Medicare expenditures for colony-stimulating factors is buying is toxicity. You’re not buying any disease benefit," argued Dr. Smith, director of palliative care and Harry J. Duffey Family Professor of Palliative Care at Johns Hopkins University, Baltimore.
He proposed that it’s time to consider putting the use of CSFs and dose-dense chemotherapy in elderly patients with hormone receptor–positive tumors on the Quality Oncology Practice Initiative's "do not use" list.
"My thought would be at a minimum we should explain the very small, at-most 8% relative benefit, and the risks. And most people, I think, given the numbers, would say, ‘I’ll take regular chemotherapy, thank you,’ " Dr. Smith predicted.
Dr. Hershman declared having no financial conflicts.
FROM THE SAN ANTONIO BREAST CANCER SYMPOSIUM
Major Finding: Cutting out prophylactic colony-stimulating factor use with dose-dense chemotherapy in elderly women with hormone receptor–positive breast cancer would save Medicare nearly $40 million annually in expenditures for colony-stimulating factors.
Data Source: Analysis of a SEER-Medicare database.
Disclosures: Dr. Hershman declared having no financial conflicts.
Trial Underway of Ixmyelocel-T Therapy for Limb Ischemia
ORLANDO – A large trial of a novel cellular expansion therapy will soon get underway in patients with critical limb ischemia patients and no revascularization options.
Based upon favorable results in initial safety and efficacy studies, the REVIVE-CLI trial of ixmyelocel-T will involve 80 U.S. centers and 594 no-option critical limb ischemia patients with tissue loss at baseline. The primary efficacy end point will be amputation-free survival at 12 months, Dr. William Marston said at the annual scientific sessions of the American Heart Association.
Ixmyelocel-T relies on a 50 mL aspirate of a patient’s bone marrow cells obtained from the ischial tuberosity during a 15-minute office procedure. Over the next 12 days, an automated process is used to expand the cell types believed to be beneficial in critical limb ischemia. The cells are then reintroduced into the patient via 20 intramuscular injections, 0.5 mL each, into the foot, calf, and lower thigh. The procedure takes 20 minutes and is performed in an office setting, explained Dr. Marston, professor of surgery and chief of the division of vascular surgery at the University of North Carolina, Chapel Hill.
The benefits of the process were noted in a double-blind, placebo-controlled, multicenter study involving 72 subjects with infrainguinal occlusive arterial disease deemed not amenable to revascularization. Half of the patients had diabetes. Participants were randomized 2:1 to ixmyelocel-T or to placebo injections. The primary end point was death, major amputation on the treated limb, doubling of wound size from baseline, or new gangrene. During 12 months of follow-up, the primary end point occurred in 67% of controls and in 40% of the ixmyelocel-T group, for a 62% relative reduction in risk.
The major driver of the significant difference in the end point was the change in the wound size. Baseline wounds were present in 45 subjects. Total wound surface area doubled in 5 of 29 ixmyelocel-T–treated patients with baseline wounds, as compared to 7 of 16 controls.
The secondary efficacy end point was amputation-free survival. One-third of controls and one-quarter of ixmyelocel-T–treated patients had an amputation during the study period. The study wasn’t powered to draw conclusions regarding amputation-free survival, according to the surgeon.
The treatment and control groups did not differ in terms of adverse events or study withdrawals due to adverse events.
The laboratory process reduces RBCs, T and B lymphocytes, and CD45+ granulocytes while achieving a 200-fold expansion of monocytes into activated macrophages and a 50-fold increase in mesenchymal stem cells. The mechanism of benefit of ixmyelocel-T, based upon preclinical data, is believed to involve remodeling of ischemic tissue, modulation of inflammation, and promotion of angiogenesis.
"There’s quite a bit of upregulation of tumor necrosis factor-alpha, interleukins, and other proinflammatory cytokines in these patients. We think that this combination of cells that are reintroduced may have benefits other than angiogenesis that may be very important in wound patients," Dr. Marston said.
Other approaches to cellular therapy in critical limb ischemia are being pursued by other groups. Some involve harvesting large quantities of bone marrow under general anesthesia, which Dr. Marston sees as less practical than the ixmyelocel-T strategy.
Dr. Marston declared that he serves as a scientific advisor to Aastrom Biosciences, which is developing ixmyelocel-T.
ORLANDO – A large trial of a novel cellular expansion therapy will soon get underway in patients with critical limb ischemia patients and no revascularization options.
Based upon favorable results in initial safety and efficacy studies, the REVIVE-CLI trial of ixmyelocel-T will involve 80 U.S. centers and 594 no-option critical limb ischemia patients with tissue loss at baseline. The primary efficacy end point will be amputation-free survival at 12 months, Dr. William Marston said at the annual scientific sessions of the American Heart Association.
Ixmyelocel-T relies on a 50 mL aspirate of a patient’s bone marrow cells obtained from the ischial tuberosity during a 15-minute office procedure. Over the next 12 days, an automated process is used to expand the cell types believed to be beneficial in critical limb ischemia. The cells are then reintroduced into the patient via 20 intramuscular injections, 0.5 mL each, into the foot, calf, and lower thigh. The procedure takes 20 minutes and is performed in an office setting, explained Dr. Marston, professor of surgery and chief of the division of vascular surgery at the University of North Carolina, Chapel Hill.
The benefits of the process were noted in a double-blind, placebo-controlled, multicenter study involving 72 subjects with infrainguinal occlusive arterial disease deemed not amenable to revascularization. Half of the patients had diabetes. Participants were randomized 2:1 to ixmyelocel-T or to placebo injections. The primary end point was death, major amputation on the treated limb, doubling of wound size from baseline, or new gangrene. During 12 months of follow-up, the primary end point occurred in 67% of controls and in 40% of the ixmyelocel-T group, for a 62% relative reduction in risk.
The major driver of the significant difference in the end point was the change in the wound size. Baseline wounds were present in 45 subjects. Total wound surface area doubled in 5 of 29 ixmyelocel-T–treated patients with baseline wounds, as compared to 7 of 16 controls.
The secondary efficacy end point was amputation-free survival. One-third of controls and one-quarter of ixmyelocel-T–treated patients had an amputation during the study period. The study wasn’t powered to draw conclusions regarding amputation-free survival, according to the surgeon.
The treatment and control groups did not differ in terms of adverse events or study withdrawals due to adverse events.
The laboratory process reduces RBCs, T and B lymphocytes, and CD45+ granulocytes while achieving a 200-fold expansion of monocytes into activated macrophages and a 50-fold increase in mesenchymal stem cells. The mechanism of benefit of ixmyelocel-T, based upon preclinical data, is believed to involve remodeling of ischemic tissue, modulation of inflammation, and promotion of angiogenesis.
"There’s quite a bit of upregulation of tumor necrosis factor-alpha, interleukins, and other proinflammatory cytokines in these patients. We think that this combination of cells that are reintroduced may have benefits other than angiogenesis that may be very important in wound patients," Dr. Marston said.
Other approaches to cellular therapy in critical limb ischemia are being pursued by other groups. Some involve harvesting large quantities of bone marrow under general anesthesia, which Dr. Marston sees as less practical than the ixmyelocel-T strategy.
Dr. Marston declared that he serves as a scientific advisor to Aastrom Biosciences, which is developing ixmyelocel-T.
ORLANDO – A large trial of a novel cellular expansion therapy will soon get underway in patients with critical limb ischemia patients and no revascularization options.
Based upon favorable results in initial safety and efficacy studies, the REVIVE-CLI trial of ixmyelocel-T will involve 80 U.S. centers and 594 no-option critical limb ischemia patients with tissue loss at baseline. The primary efficacy end point will be amputation-free survival at 12 months, Dr. William Marston said at the annual scientific sessions of the American Heart Association.
Ixmyelocel-T relies on a 50 mL aspirate of a patient’s bone marrow cells obtained from the ischial tuberosity during a 15-minute office procedure. Over the next 12 days, an automated process is used to expand the cell types believed to be beneficial in critical limb ischemia. The cells are then reintroduced into the patient via 20 intramuscular injections, 0.5 mL each, into the foot, calf, and lower thigh. The procedure takes 20 minutes and is performed in an office setting, explained Dr. Marston, professor of surgery and chief of the division of vascular surgery at the University of North Carolina, Chapel Hill.
The benefits of the process were noted in a double-blind, placebo-controlled, multicenter study involving 72 subjects with infrainguinal occlusive arterial disease deemed not amenable to revascularization. Half of the patients had diabetes. Participants were randomized 2:1 to ixmyelocel-T or to placebo injections. The primary end point was death, major amputation on the treated limb, doubling of wound size from baseline, or new gangrene. During 12 months of follow-up, the primary end point occurred in 67% of controls and in 40% of the ixmyelocel-T group, for a 62% relative reduction in risk.
The major driver of the significant difference in the end point was the change in the wound size. Baseline wounds were present in 45 subjects. Total wound surface area doubled in 5 of 29 ixmyelocel-T–treated patients with baseline wounds, as compared to 7 of 16 controls.
The secondary efficacy end point was amputation-free survival. One-third of controls and one-quarter of ixmyelocel-T–treated patients had an amputation during the study period. The study wasn’t powered to draw conclusions regarding amputation-free survival, according to the surgeon.
The treatment and control groups did not differ in terms of adverse events or study withdrawals due to adverse events.
The laboratory process reduces RBCs, T and B lymphocytes, and CD45+ granulocytes while achieving a 200-fold expansion of monocytes into activated macrophages and a 50-fold increase in mesenchymal stem cells. The mechanism of benefit of ixmyelocel-T, based upon preclinical data, is believed to involve remodeling of ischemic tissue, modulation of inflammation, and promotion of angiogenesis.
"There’s quite a bit of upregulation of tumor necrosis factor-alpha, interleukins, and other proinflammatory cytokines in these patients. We think that this combination of cells that are reintroduced may have benefits other than angiogenesis that may be very important in wound patients," Dr. Marston said.
Other approaches to cellular therapy in critical limb ischemia are being pursued by other groups. Some involve harvesting large quantities of bone marrow under general anesthesia, which Dr. Marston sees as less practical than the ixmyelocel-T strategy.
Dr. Marston declared that he serves as a scientific advisor to Aastrom Biosciences, which is developing ixmyelocel-T.
FROM THE ANNUAL SCIENTIFIC SESSIONS OF THE AMERICAN HEART ASSOCIATION
Major Finding: During 12 months of follow-up, the primary end point of death, major amputation on the treated limb, doubling of wound size from baseline, or new gangrene occurred in 67% of controls and in 40% of the ixmyelocel-T group.
Data Source: A double-blind, randomized clinical trial of 72 subjects with infrainguinal occlusive arterial disease deemed not amenable to revascularization.
Disclosures: The study was sponsored by Aastrom Biosciences, which is developing ixmyelocel-T. Dr. Marston declared that he serves as a scientific advisor to Aastrom Biosciences.