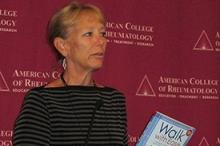User login
Doug Brunk is a San Diego-based award-winning reporter who began covering health care in 1991. Before joining the company, he wrote for the health sciences division of Columbia University and was an associate editor at Contemporary Long Term Care magazine when it won a Jesse H. Neal Award. His work has been syndicated by the Los Angeles Times and he is the author of two books related to the University of Kentucky Wildcats men's basketball program. Doug has a master’s degree in magazine journalism from the S.I. Newhouse School of Public Communications at Syracuse University. Follow him on Twitter @dougbrunk.
Subjective memory complaints predict clinical impairment
SAN DIEGO – Subjective memory complaints at study enrollment predicted clinical memory impairment 8 years later in healthy men aged 60 years and older, findings from a large ongoing study showed.
"This suggests the utility of subjective memory complaints for future prevention trials," Erin L. Abner, Ph.D., said at the Clinical Trials Conference on Alzheimer’s Disease.
She and her associates also found that hypertension, diabetes, black race, and education level emerged as significant risk factors for clinical impairment, while the use of antihypertensive agents and statins emerged as protective factors.
The findings come from centralized follow-up of men enrolled in the PREADVISE (Prevention of Alzheimer’s Disease by Vitamin E and Selenium) trial, which was launched in 2002 as an ancillary to the SELECT (Selenium and Vitamin E in Preventing Prostate Cancer) trial. PREADVISE was designed to assess the effect of vitamin E and selenium on reducing the incidence of Alzheimer’s disease and other neurodegenerative disorders.
The PREADVISE study had four arms: Participants received 400 IU vitamin E per day, 200 mcg of selenium per day, both, or matching placebos. Recruitment began in 2002, and a total of 7,553 nondemented men aged 60 and older participated. In 2008, SELECT was suspended because of an interim futility analysis, and study sites began closing. Two years later, 4,246 PREADVISE participants consented to centralized follow-up by telephone. Of these, 3,701 have been screened to date, said Dr. Abner of the Sanders-Brown Center on Aging and the department of epidemiology at the University of Kentucky, Lexington.
Men were eligible for PREADVISE if they scored at least a 5 on the Memory Impairment Screen (MIS) at baseline. "They received alternating versions of the MIS in subsequent years to minimize learning effects," she said. "If they failed the MIS, they were given the Consortium to Establish a Registry for Alzheimer’s Disease [CERAD] neuropsychological test battery. If their age-adjusted CERAD score was 35 or less, they were referred to a physician for a medical work-up and had their medical records forwarded to us." If they failed the MIS over the phone, they were administered the Modified Telephone Interview for Cognitive Status [TICS-M] test. "If they scored 31 or less on TICS-M, they were advised to seek a medical work-up." Dr. Abner explained. "Although the records were forwarded to us, we were still blinded to treatment status."
The main outcome of interest was clinical impairment, which was defined as impaired cognition indicated by failing scores on the CERAD or the TICS-M. Confirmed impairment was verified by a medical work-up, while suspected impairment was defined as that not yet confirmed. Subjective memory complaints were defined as self-perceived changes in memory that may not be reflected by cognitive testing. "Subjective memory complaints are common in older adults and have been previously reported to predict future cognitive decline," Dr. Abner noted. "All PREADVISE participants were asked at baseline if they had noticed any changes in their memory. Positive responders were also asked if they thought they had more problems with their memory than most people." Thus, participants were classified as having no memory complaint, memory changes, or memory problems at baseline.
The researchers used a stepwise Cox proportional hazards model to identify risk factors associated with the time from baseline to clinical impairment. The predictive variables in the model were both fixed and time dependent. Fixed variables (self-reported) were baseline age, education, black race, Hispanic ethnicity, mother’s age at childbirth, a family history of dementia, and comorbidities. Time-dependent variables were depression, anxiety, alcohol abuse, a high cholesterol level, antihypertensive use, hyperglycemia, thyroid disorder, and sleep apnea.
The mean age of the 3,701 participants was 68 years old, and most (77%) had no memory complaints at baseline. Participants underwent an average of eight annual assessments. Hypertension emerged as the most common comorbidity (34%), with 22% reporting the use of a statin. High cholesterol was the most common time-dependent comorbidity (68%), followed by antihypertensive use (64%), sleep apnea (18%), and depression (11%).
Dr. Abner reported that of the 3,701 men screened to date, 436 (12%) have clinical impairments with 332 having suspected mild cognitive impairment (MCI), 85 confirmed MCI, 4 suspected dementia, and 15 confirmed dementia. The risk of a clinical impairment was significantly increased by an older age at baseline (HR, 1.12 for every 1-year increment); baseline hypertension (HR, 1.96); African American race (HR, 3.24); a high school education or less (HR, 1.66); a history of diabetes (HR, 1.37); and, in the absence of apolipoprotein E4 (apo E4), baseline memory complaint (HR, 1.66 for changes vs. no complaint and HR, 4.48 for problems vs. no complaint). "It turns out that in the absence of apo E4, subjective memory complaints are predictive of clinical impairment," she said. "When apo E4 is present, the hazard ratios are not significant."
The risk of an observed impairment was significantly reduced by the use of antihypertensives (HR, 0.34) and a report of high cholesterol (HR, 0.75).
Dr. Abner acknowledged certain limitations of the study, including the fact that most clinical impairments are not yet confirmed by medical review. In addition, "relevant early to midlife socioeconomic status exposures were not measured, which may mitigate the effect observed for black race," she said. "Also, many of our black participants were recruited from Veterans Affairs sites, so their exposures may be qualitatively different in terms of their risk of head trauma and PTSD [posttraumatic stress disorder]. Overall, the sample was highly educated and healthy."
Screening of the full PREADVISE cohort is expected to be completed in early 2014.
The study is funded by the National Institute on Aging. Dr. Abner said she had no relevant financial conflicts to disclose.
SAN DIEGO – Subjective memory complaints at study enrollment predicted clinical memory impairment 8 years later in healthy men aged 60 years and older, findings from a large ongoing study showed.
"This suggests the utility of subjective memory complaints for future prevention trials," Erin L. Abner, Ph.D., said at the Clinical Trials Conference on Alzheimer’s Disease.
She and her associates also found that hypertension, diabetes, black race, and education level emerged as significant risk factors for clinical impairment, while the use of antihypertensive agents and statins emerged as protective factors.
The findings come from centralized follow-up of men enrolled in the PREADVISE (Prevention of Alzheimer’s Disease by Vitamin E and Selenium) trial, which was launched in 2002 as an ancillary to the SELECT (Selenium and Vitamin E in Preventing Prostate Cancer) trial. PREADVISE was designed to assess the effect of vitamin E and selenium on reducing the incidence of Alzheimer’s disease and other neurodegenerative disorders.
The PREADVISE study had four arms: Participants received 400 IU vitamin E per day, 200 mcg of selenium per day, both, or matching placebos. Recruitment began in 2002, and a total of 7,553 nondemented men aged 60 and older participated. In 2008, SELECT was suspended because of an interim futility analysis, and study sites began closing. Two years later, 4,246 PREADVISE participants consented to centralized follow-up by telephone. Of these, 3,701 have been screened to date, said Dr. Abner of the Sanders-Brown Center on Aging and the department of epidemiology at the University of Kentucky, Lexington.
Men were eligible for PREADVISE if they scored at least a 5 on the Memory Impairment Screen (MIS) at baseline. "They received alternating versions of the MIS in subsequent years to minimize learning effects," she said. "If they failed the MIS, they were given the Consortium to Establish a Registry for Alzheimer’s Disease [CERAD] neuropsychological test battery. If their age-adjusted CERAD score was 35 or less, they were referred to a physician for a medical work-up and had their medical records forwarded to us." If they failed the MIS over the phone, they were administered the Modified Telephone Interview for Cognitive Status [TICS-M] test. "If they scored 31 or less on TICS-M, they were advised to seek a medical work-up." Dr. Abner explained. "Although the records were forwarded to us, we were still blinded to treatment status."
The main outcome of interest was clinical impairment, which was defined as impaired cognition indicated by failing scores on the CERAD or the TICS-M. Confirmed impairment was verified by a medical work-up, while suspected impairment was defined as that not yet confirmed. Subjective memory complaints were defined as self-perceived changes in memory that may not be reflected by cognitive testing. "Subjective memory complaints are common in older adults and have been previously reported to predict future cognitive decline," Dr. Abner noted. "All PREADVISE participants were asked at baseline if they had noticed any changes in their memory. Positive responders were also asked if they thought they had more problems with their memory than most people." Thus, participants were classified as having no memory complaint, memory changes, or memory problems at baseline.
The researchers used a stepwise Cox proportional hazards model to identify risk factors associated with the time from baseline to clinical impairment. The predictive variables in the model were both fixed and time dependent. Fixed variables (self-reported) were baseline age, education, black race, Hispanic ethnicity, mother’s age at childbirth, a family history of dementia, and comorbidities. Time-dependent variables were depression, anxiety, alcohol abuse, a high cholesterol level, antihypertensive use, hyperglycemia, thyroid disorder, and sleep apnea.
The mean age of the 3,701 participants was 68 years old, and most (77%) had no memory complaints at baseline. Participants underwent an average of eight annual assessments. Hypertension emerged as the most common comorbidity (34%), with 22% reporting the use of a statin. High cholesterol was the most common time-dependent comorbidity (68%), followed by antihypertensive use (64%), sleep apnea (18%), and depression (11%).
Dr. Abner reported that of the 3,701 men screened to date, 436 (12%) have clinical impairments with 332 having suspected mild cognitive impairment (MCI), 85 confirmed MCI, 4 suspected dementia, and 15 confirmed dementia. The risk of a clinical impairment was significantly increased by an older age at baseline (HR, 1.12 for every 1-year increment); baseline hypertension (HR, 1.96); African American race (HR, 3.24); a high school education or less (HR, 1.66); a history of diabetes (HR, 1.37); and, in the absence of apolipoprotein E4 (apo E4), baseline memory complaint (HR, 1.66 for changes vs. no complaint and HR, 4.48 for problems vs. no complaint). "It turns out that in the absence of apo E4, subjective memory complaints are predictive of clinical impairment," she said. "When apo E4 is present, the hazard ratios are not significant."
The risk of an observed impairment was significantly reduced by the use of antihypertensives (HR, 0.34) and a report of high cholesterol (HR, 0.75).
Dr. Abner acknowledged certain limitations of the study, including the fact that most clinical impairments are not yet confirmed by medical review. In addition, "relevant early to midlife socioeconomic status exposures were not measured, which may mitigate the effect observed for black race," she said. "Also, many of our black participants were recruited from Veterans Affairs sites, so their exposures may be qualitatively different in terms of their risk of head trauma and PTSD [posttraumatic stress disorder]. Overall, the sample was highly educated and healthy."
Screening of the full PREADVISE cohort is expected to be completed in early 2014.
The study is funded by the National Institute on Aging. Dr. Abner said she had no relevant financial conflicts to disclose.
SAN DIEGO – Subjective memory complaints at study enrollment predicted clinical memory impairment 8 years later in healthy men aged 60 years and older, findings from a large ongoing study showed.
"This suggests the utility of subjective memory complaints for future prevention trials," Erin L. Abner, Ph.D., said at the Clinical Trials Conference on Alzheimer’s Disease.
She and her associates also found that hypertension, diabetes, black race, and education level emerged as significant risk factors for clinical impairment, while the use of antihypertensive agents and statins emerged as protective factors.
The findings come from centralized follow-up of men enrolled in the PREADVISE (Prevention of Alzheimer’s Disease by Vitamin E and Selenium) trial, which was launched in 2002 as an ancillary to the SELECT (Selenium and Vitamin E in Preventing Prostate Cancer) trial. PREADVISE was designed to assess the effect of vitamin E and selenium on reducing the incidence of Alzheimer’s disease and other neurodegenerative disorders.
The PREADVISE study had four arms: Participants received 400 IU vitamin E per day, 200 mcg of selenium per day, both, or matching placebos. Recruitment began in 2002, and a total of 7,553 nondemented men aged 60 and older participated. In 2008, SELECT was suspended because of an interim futility analysis, and study sites began closing. Two years later, 4,246 PREADVISE participants consented to centralized follow-up by telephone. Of these, 3,701 have been screened to date, said Dr. Abner of the Sanders-Brown Center on Aging and the department of epidemiology at the University of Kentucky, Lexington.
Men were eligible for PREADVISE if they scored at least a 5 on the Memory Impairment Screen (MIS) at baseline. "They received alternating versions of the MIS in subsequent years to minimize learning effects," she said. "If they failed the MIS, they were given the Consortium to Establish a Registry for Alzheimer’s Disease [CERAD] neuropsychological test battery. If their age-adjusted CERAD score was 35 or less, they were referred to a physician for a medical work-up and had their medical records forwarded to us." If they failed the MIS over the phone, they were administered the Modified Telephone Interview for Cognitive Status [TICS-M] test. "If they scored 31 or less on TICS-M, they were advised to seek a medical work-up." Dr. Abner explained. "Although the records were forwarded to us, we were still blinded to treatment status."
The main outcome of interest was clinical impairment, which was defined as impaired cognition indicated by failing scores on the CERAD or the TICS-M. Confirmed impairment was verified by a medical work-up, while suspected impairment was defined as that not yet confirmed. Subjective memory complaints were defined as self-perceived changes in memory that may not be reflected by cognitive testing. "Subjective memory complaints are common in older adults and have been previously reported to predict future cognitive decline," Dr. Abner noted. "All PREADVISE participants were asked at baseline if they had noticed any changes in their memory. Positive responders were also asked if they thought they had more problems with their memory than most people." Thus, participants were classified as having no memory complaint, memory changes, or memory problems at baseline.
The researchers used a stepwise Cox proportional hazards model to identify risk factors associated with the time from baseline to clinical impairment. The predictive variables in the model were both fixed and time dependent. Fixed variables (self-reported) were baseline age, education, black race, Hispanic ethnicity, mother’s age at childbirth, a family history of dementia, and comorbidities. Time-dependent variables were depression, anxiety, alcohol abuse, a high cholesterol level, antihypertensive use, hyperglycemia, thyroid disorder, and sleep apnea.
The mean age of the 3,701 participants was 68 years old, and most (77%) had no memory complaints at baseline. Participants underwent an average of eight annual assessments. Hypertension emerged as the most common comorbidity (34%), with 22% reporting the use of a statin. High cholesterol was the most common time-dependent comorbidity (68%), followed by antihypertensive use (64%), sleep apnea (18%), and depression (11%).
Dr. Abner reported that of the 3,701 men screened to date, 436 (12%) have clinical impairments with 332 having suspected mild cognitive impairment (MCI), 85 confirmed MCI, 4 suspected dementia, and 15 confirmed dementia. The risk of a clinical impairment was significantly increased by an older age at baseline (HR, 1.12 for every 1-year increment); baseline hypertension (HR, 1.96); African American race (HR, 3.24); a high school education or less (HR, 1.66); a history of diabetes (HR, 1.37); and, in the absence of apolipoprotein E4 (apo E4), baseline memory complaint (HR, 1.66 for changes vs. no complaint and HR, 4.48 for problems vs. no complaint). "It turns out that in the absence of apo E4, subjective memory complaints are predictive of clinical impairment," she said. "When apo E4 is present, the hazard ratios are not significant."
The risk of an observed impairment was significantly reduced by the use of antihypertensives (HR, 0.34) and a report of high cholesterol (HR, 0.75).
Dr. Abner acknowledged certain limitations of the study, including the fact that most clinical impairments are not yet confirmed by medical review. In addition, "relevant early to midlife socioeconomic status exposures were not measured, which may mitigate the effect observed for black race," she said. "Also, many of our black participants were recruited from Veterans Affairs sites, so their exposures may be qualitatively different in terms of their risk of head trauma and PTSD [posttraumatic stress disorder]. Overall, the sample was highly educated and healthy."
Screening of the full PREADVISE cohort is expected to be completed in early 2014.
The study is funded by the National Institute on Aging. Dr. Abner said she had no relevant financial conflicts to disclose.
AT CTAD
Major finding: The risk of a clinical impairment was significantly increased by an older age at baseline (HR, 1.12 for every 1-year increment); baseline hypertension (HR, 1.96); African American race (HR, 3.24); a high school education or less (HR, 1.66); a history of diabetes (HR, 1.37); and an interaction between baseline memory complaint and apo E4 (when no apo E4 allele was present: HR, 1.66 for changes vs. none and HR 4.48, for problems vs. none).
Data source: A centralized follow-up of 3,701 men enrolled in the PREADVISE trial.
Disclosures: The study is funded by the National Institute on Aging. Dr. Abner said she had no relevant financial conflicts to disclose.
Walking program eased chemo-related joint pain
SAN DIEGO – A 6-week, low-impact walking program relieved joint pain and stiffness and increased the number of minutes per week walking among elderly breast cancer survivors on aromatase inhibitors.
"A breast cancer diagnosis can be an ‘a-ha’ moment for women," Kirsten A. Nyrop, Ph.D, said during a press briefing at the annual meeting of the American College of Rheumatology. "Some of the women with whom we spoke said: ‘My cardiologist told me to walk and my general practitioner told me to walk, but when my oncologist asked me to walk, I started walking.’ "
Dr. Nyrop, of the Thurston Arthritis Research Center at the University of North Carolina at Chapel Hill, and her associates studied 20 patients who were taking an aromatase inhibitor for stage I-III breast cancer and had self-reported joint pain. The walking program they used was the Arthritis Foundation’s Walk With Ease. The program recommends that participants walk 30 minutes per day at least 5 days per week.
"We wanted to be certain we were reaching these women with a message that would resonate with them and a program that they felt was safe, something that they could comfortably do," Dr. Nyrop said.
The researchers administered visual analog scales (VAS) for joint pain, stiffness, and fatigue, as well as Arthritic Self-Efficacy (ASE) scales for joint and pain symptoms. The mean age of study participants was 71 years, 85% were white, and their mean body mass index was 29 kg/m2.
After 6 weeks in the walking program, researchers noticed improvements from baseline in the VAS pain score (effect size = 0.15) and VAS fatigue score (effect size = 0.22); changes in the VAS stiffness score approached statistical significance (effect size = 0.56). "That’s promising," Dr. Nyrop said. Scores on the ASE scales also improved from baseline for pain and fatigue, but the changes did not reach significance.
Significant increases in walking were observed from baseline in terms of the number of times per week walked (mean increase of 1.9 times; effect size = 0.68), number of minutes per walk (mean increase of 8.3 minutes; effect size = 0.48), and total number of minutes walked per week (mean increase of 62.6 minutes; effect size = 0.53).
One study participant wrote: "I absolutely feel the connection between aromatase inhibitors and exercise. On days I don’t walk, I am stiff, but if I walk, I am not stiff. Keep moving; it really helps."
A larger, follow-up study with a randomized controlled design is underway with funding by the National Cancer Institute.
The study was funded by the University of North Carolina Institute on Aging. Dr. Nyrop said that she had no relevant financial conflicts to disclose.
SAN DIEGO – A 6-week, low-impact walking program relieved joint pain and stiffness and increased the number of minutes per week walking among elderly breast cancer survivors on aromatase inhibitors.
"A breast cancer diagnosis can be an ‘a-ha’ moment for women," Kirsten A. Nyrop, Ph.D, said during a press briefing at the annual meeting of the American College of Rheumatology. "Some of the women with whom we spoke said: ‘My cardiologist told me to walk and my general practitioner told me to walk, but when my oncologist asked me to walk, I started walking.’ "
Dr. Nyrop, of the Thurston Arthritis Research Center at the University of North Carolina at Chapel Hill, and her associates studied 20 patients who were taking an aromatase inhibitor for stage I-III breast cancer and had self-reported joint pain. The walking program they used was the Arthritis Foundation’s Walk With Ease. The program recommends that participants walk 30 minutes per day at least 5 days per week.
"We wanted to be certain we were reaching these women with a message that would resonate with them and a program that they felt was safe, something that they could comfortably do," Dr. Nyrop said.
The researchers administered visual analog scales (VAS) for joint pain, stiffness, and fatigue, as well as Arthritic Self-Efficacy (ASE) scales for joint and pain symptoms. The mean age of study participants was 71 years, 85% were white, and their mean body mass index was 29 kg/m2.
After 6 weeks in the walking program, researchers noticed improvements from baseline in the VAS pain score (effect size = 0.15) and VAS fatigue score (effect size = 0.22); changes in the VAS stiffness score approached statistical significance (effect size = 0.56). "That’s promising," Dr. Nyrop said. Scores on the ASE scales also improved from baseline for pain and fatigue, but the changes did not reach significance.
Significant increases in walking were observed from baseline in terms of the number of times per week walked (mean increase of 1.9 times; effect size = 0.68), number of minutes per walk (mean increase of 8.3 minutes; effect size = 0.48), and total number of minutes walked per week (mean increase of 62.6 minutes; effect size = 0.53).
One study participant wrote: "I absolutely feel the connection between aromatase inhibitors and exercise. On days I don’t walk, I am stiff, but if I walk, I am not stiff. Keep moving; it really helps."
A larger, follow-up study with a randomized controlled design is underway with funding by the National Cancer Institute.
The study was funded by the University of North Carolina Institute on Aging. Dr. Nyrop said that she had no relevant financial conflicts to disclose.
SAN DIEGO – A 6-week, low-impact walking program relieved joint pain and stiffness and increased the number of minutes per week walking among elderly breast cancer survivors on aromatase inhibitors.
"A breast cancer diagnosis can be an ‘a-ha’ moment for women," Kirsten A. Nyrop, Ph.D, said during a press briefing at the annual meeting of the American College of Rheumatology. "Some of the women with whom we spoke said: ‘My cardiologist told me to walk and my general practitioner told me to walk, but when my oncologist asked me to walk, I started walking.’ "
Dr. Nyrop, of the Thurston Arthritis Research Center at the University of North Carolina at Chapel Hill, and her associates studied 20 patients who were taking an aromatase inhibitor for stage I-III breast cancer and had self-reported joint pain. The walking program they used was the Arthritis Foundation’s Walk With Ease. The program recommends that participants walk 30 minutes per day at least 5 days per week.
"We wanted to be certain we were reaching these women with a message that would resonate with them and a program that they felt was safe, something that they could comfortably do," Dr. Nyrop said.
The researchers administered visual analog scales (VAS) for joint pain, stiffness, and fatigue, as well as Arthritic Self-Efficacy (ASE) scales for joint and pain symptoms. The mean age of study participants was 71 years, 85% were white, and their mean body mass index was 29 kg/m2.
After 6 weeks in the walking program, researchers noticed improvements from baseline in the VAS pain score (effect size = 0.15) and VAS fatigue score (effect size = 0.22); changes in the VAS stiffness score approached statistical significance (effect size = 0.56). "That’s promising," Dr. Nyrop said. Scores on the ASE scales also improved from baseline for pain and fatigue, but the changes did not reach significance.
Significant increases in walking were observed from baseline in terms of the number of times per week walked (mean increase of 1.9 times; effect size = 0.68), number of minutes per walk (mean increase of 8.3 minutes; effect size = 0.48), and total number of minutes walked per week (mean increase of 62.6 minutes; effect size = 0.53).
One study participant wrote: "I absolutely feel the connection between aromatase inhibitors and exercise. On days I don’t walk, I am stiff, but if I walk, I am not stiff. Keep moving; it really helps."
A larger, follow-up study with a randomized controlled design is underway with funding by the National Cancer Institute.
The study was funded by the University of North Carolina Institute on Aging. Dr. Nyrop said that she had no relevant financial conflicts to disclose.
AT THE ACR ANNUAL MEETING
Major finding: After 6 weeks in a low-impact walking program, patients experienced improvements from baseline on the visual analog scale (VAS) for pain (effect size = 0.15) and the VAS for fatigue (effect size = 0.22); changes in the VAS stiffness score approached statistical significance (effect size = 0.56).
Data source: A pilot study of 20 patients at the North Carolina Cancer Hospital in Chapel Hill who were taking an aromatase inhibitor for stage I-III breast cancer and had self-reported joint pain.
Disclosures: The study was funded by the University of North Carolina Institute on Aging. Dr. Nyrop said that she had no relevant financial conflicts to disclose.
Cholinesterase inhibitors most effective in mild Alzheimer’s
SAN DIEGO – In a 3-year Swedish study of patients with mild or moderate forms of Alzheimer’s disease who were taking cholinesterase inhibitors, varying outcomes were demonstrated in the different domains of the disease and in the scales used.
The findings show the clinical importance of functional evaluations, even in the mild stages of Alzheimer’s disease (AD), Carina Wattmo, Ph.D., said at the Clinical Trials Conference on Alzheimer’s Disease.
Some randomized trials, such as that of the anti-beta-amyloid antibody solanezumab and the medical food souvenaid, "have shown small but significant positive cognitive effects in mild AD exclusively," said Dr. Wattmo of the clinical memory research unit in the department of clinical sciences at Lund University, Malmö, Sweden.
"Placebo-controlled trials longer than 6 months in untreated AD patients are not allowed for ethical reasons. New, longer studies are most often performed on patients who are on active treatment with cholinesterase inhibitors and/or memantine. Therapies expected to modify disease progression need to be thoroughly evaluated over many years. Knowledge on the expected longitudinal outcome in different stages of AD is scarce."
Dr. Wattmo and her associates analyzed data from the Swedish Alzheimer Treatment Study (SATS), a 3-year, prospective, open-label, nonrandomized multicenter study launched to evaluate the long-term effectiveness of cholinesterase inhibitor treatment in a routine clinical setting. Patients were diagnosed with possible or probable AD; the population included 734 patients with mild AD and 287 with moderate disease.
The patients were assessed after 2 months of therapy and every 6 months over the 3-year period, with the Mini-Mental State Examination (MMSE), the Alzheimer’s Disease Assessment Scale–cognitive subscale (ADAS-cog), the Clinician’s Interview-Based Impression of Change (CIBIC), and functional capacity based on the Instrumental Activities of Daily Living scale (IADL).
The mean age of patients was 75 years, and 64% were female. At baseline, patients in the mild AD group showed an illness duration of 2.9 years vs. 3.4 years in the moderate AD group, a difference that was statistically significant (P = .005). They also had more years of education (9.6 vs. 9.0 years; P less than .001), and a better functional capacity based on the IADL score (14.7 points vs. 19.1 points; P less than .001).
Dr. Wattmo reported 3-year results from 306 patients in the mild AD group and 79 patients in the moderate AD group. The mean change in MMSE was 3.1 points in the mild AD group vs. 4 points in the moderate AD group, a difference that did not reach statistical significance (P = .148). The mean decline in the ADAS-cog was 6.1 points in the mild AD group vs. 13.2 points in the moderate AD group, a difference that reached statistical significance (P less than .001).
In addition, 33% of patients in the mild AD group showed global CIBIC improvement or no change after 3 years of therapy, compared with 15% in the moderate AD group, a difference that was statistically significant (P = .002).
On the IADL, the mean decline was 6.3 points in the mild AD group vs. 7.5 points in the moderate AD group, a difference that was not statistically significant (P = .063).
"Varying outcomes were demonstrated in the different domains and stages of AD in this study from routine clinical practice," Dr. Wattmo concluded. "The outcome could be dependent on the scales used."
Dr. Wattmo said that she had no relevant financial conflicts to disclose.
SAN DIEGO – In a 3-year Swedish study of patients with mild or moderate forms of Alzheimer’s disease who were taking cholinesterase inhibitors, varying outcomes were demonstrated in the different domains of the disease and in the scales used.
The findings show the clinical importance of functional evaluations, even in the mild stages of Alzheimer’s disease (AD), Carina Wattmo, Ph.D., said at the Clinical Trials Conference on Alzheimer’s Disease.
Some randomized trials, such as that of the anti-beta-amyloid antibody solanezumab and the medical food souvenaid, "have shown small but significant positive cognitive effects in mild AD exclusively," said Dr. Wattmo of the clinical memory research unit in the department of clinical sciences at Lund University, Malmö, Sweden.
"Placebo-controlled trials longer than 6 months in untreated AD patients are not allowed for ethical reasons. New, longer studies are most often performed on patients who are on active treatment with cholinesterase inhibitors and/or memantine. Therapies expected to modify disease progression need to be thoroughly evaluated over many years. Knowledge on the expected longitudinal outcome in different stages of AD is scarce."
Dr. Wattmo and her associates analyzed data from the Swedish Alzheimer Treatment Study (SATS), a 3-year, prospective, open-label, nonrandomized multicenter study launched to evaluate the long-term effectiveness of cholinesterase inhibitor treatment in a routine clinical setting. Patients were diagnosed with possible or probable AD; the population included 734 patients with mild AD and 287 with moderate disease.
The patients were assessed after 2 months of therapy and every 6 months over the 3-year period, with the Mini-Mental State Examination (MMSE), the Alzheimer’s Disease Assessment Scale–cognitive subscale (ADAS-cog), the Clinician’s Interview-Based Impression of Change (CIBIC), and functional capacity based on the Instrumental Activities of Daily Living scale (IADL).
The mean age of patients was 75 years, and 64% were female. At baseline, patients in the mild AD group showed an illness duration of 2.9 years vs. 3.4 years in the moderate AD group, a difference that was statistically significant (P = .005). They also had more years of education (9.6 vs. 9.0 years; P less than .001), and a better functional capacity based on the IADL score (14.7 points vs. 19.1 points; P less than .001).
Dr. Wattmo reported 3-year results from 306 patients in the mild AD group and 79 patients in the moderate AD group. The mean change in MMSE was 3.1 points in the mild AD group vs. 4 points in the moderate AD group, a difference that did not reach statistical significance (P = .148). The mean decline in the ADAS-cog was 6.1 points in the mild AD group vs. 13.2 points in the moderate AD group, a difference that reached statistical significance (P less than .001).
In addition, 33% of patients in the mild AD group showed global CIBIC improvement or no change after 3 years of therapy, compared with 15% in the moderate AD group, a difference that was statistically significant (P = .002).
On the IADL, the mean decline was 6.3 points in the mild AD group vs. 7.5 points in the moderate AD group, a difference that was not statistically significant (P = .063).
"Varying outcomes were demonstrated in the different domains and stages of AD in this study from routine clinical practice," Dr. Wattmo concluded. "The outcome could be dependent on the scales used."
Dr. Wattmo said that she had no relevant financial conflicts to disclose.
SAN DIEGO – In a 3-year Swedish study of patients with mild or moderate forms of Alzheimer’s disease who were taking cholinesterase inhibitors, varying outcomes were demonstrated in the different domains of the disease and in the scales used.
The findings show the clinical importance of functional evaluations, even in the mild stages of Alzheimer’s disease (AD), Carina Wattmo, Ph.D., said at the Clinical Trials Conference on Alzheimer’s Disease.
Some randomized trials, such as that of the anti-beta-amyloid antibody solanezumab and the medical food souvenaid, "have shown small but significant positive cognitive effects in mild AD exclusively," said Dr. Wattmo of the clinical memory research unit in the department of clinical sciences at Lund University, Malmö, Sweden.
"Placebo-controlled trials longer than 6 months in untreated AD patients are not allowed for ethical reasons. New, longer studies are most often performed on patients who are on active treatment with cholinesterase inhibitors and/or memantine. Therapies expected to modify disease progression need to be thoroughly evaluated over many years. Knowledge on the expected longitudinal outcome in different stages of AD is scarce."
Dr. Wattmo and her associates analyzed data from the Swedish Alzheimer Treatment Study (SATS), a 3-year, prospective, open-label, nonrandomized multicenter study launched to evaluate the long-term effectiveness of cholinesterase inhibitor treatment in a routine clinical setting. Patients were diagnosed with possible or probable AD; the population included 734 patients with mild AD and 287 with moderate disease.
The patients were assessed after 2 months of therapy and every 6 months over the 3-year period, with the Mini-Mental State Examination (MMSE), the Alzheimer’s Disease Assessment Scale–cognitive subscale (ADAS-cog), the Clinician’s Interview-Based Impression of Change (CIBIC), and functional capacity based on the Instrumental Activities of Daily Living scale (IADL).
The mean age of patients was 75 years, and 64% were female. At baseline, patients in the mild AD group showed an illness duration of 2.9 years vs. 3.4 years in the moderate AD group, a difference that was statistically significant (P = .005). They also had more years of education (9.6 vs. 9.0 years; P less than .001), and a better functional capacity based on the IADL score (14.7 points vs. 19.1 points; P less than .001).
Dr. Wattmo reported 3-year results from 306 patients in the mild AD group and 79 patients in the moderate AD group. The mean change in MMSE was 3.1 points in the mild AD group vs. 4 points in the moderate AD group, a difference that did not reach statistical significance (P = .148). The mean decline in the ADAS-cog was 6.1 points in the mild AD group vs. 13.2 points in the moderate AD group, a difference that reached statistical significance (P less than .001).
In addition, 33% of patients in the mild AD group showed global CIBIC improvement or no change after 3 years of therapy, compared with 15% in the moderate AD group, a difference that was statistically significant (P = .002).
On the IADL, the mean decline was 6.3 points in the mild AD group vs. 7.5 points in the moderate AD group, a difference that was not statistically significant (P = .063).
"Varying outcomes were demonstrated in the different domains and stages of AD in this study from routine clinical practice," Dr. Wattmo concluded. "The outcome could be dependent on the scales used."
Dr. Wattmo said that she had no relevant financial conflicts to disclose.
AT CTAD
Major finding: After 3 years on cholinesterase inhibitors, patients with mild Alzheimer’s disease had a mean decline of 6.1 points on the Alzheimer’s Disease Assessment Scale–cognitive subscale, while those with moderate Alzheimer’s had a mean decline of 13.2 points, a difference that reached statistical significance (P less than .001).
Data source: Data from 385 patients in the nonrandomized, multicenter Swedish Alzheimer Treatment Study (SATS), launched to evaluate the long-term effectiveness of cholinesterase inhibitor treatment in a routine clinical setting.
Disclosures: Dr. Wattmo said that she had no relevant financial conflicts to disclose.
Sorting out exposure to toxic substances can be tricky
SAN DIEGO – The first step in determining whether a patient has been exposed to a toxic substance is to conduct a thorough history, according to Dr. Peter J. Ziemkowski.
"First, get a good employment history," he advised at the annual meeting of the American Academy of Family Physicians. "You want to look at their current job, their longest-held jobs in the past, and any other significant jobs you can identify. Try to identify any exposure types they’ve had. You also have to figure out the time. Some things can present acutely. Other can have a long latency before their presentation."
A question to pose to patients is, Do you have any concerns about any exposure to hazards at work or home? "You want to identify if the chief complaint they’re coming in for had some relation to the exposure they’re concerned about, and any other specific triggers, such as symptoms of unknown etiology," said Dr. Ziemkowski of the department of family and community medicine at Western Michigan University, Kalamazoo.
It’s also important to ask patients about their potential exposure to toxic substances from a nonoccupational standpoint. "We’re talking about exposures from hobbies, such as photographers who did their own darkroom work and were exposed to solvents," Dr. Ziemkowski explained. "Digital photography has actually removed some of those hazards. Other risks come with hobbies such as ceramics. The kilns themselves can produce carbon monoxide. Also, painters can be exposed to arsenic, cadmium, lead, chromium, and mercury," he added.
According to the Bureau of Labor Statistics, approximately 3.5 million nonfatal occupational illnesses and injuries occurred in the United States in 2011. "That required millions of dollars in required time off and work restrictions," said Dr. Ziemkowski.
He also noted several case reports of exposure to methylene chloride, which is used in many industrial processes and in products such as paint strippers and degreasers. "Methylene chloride in these solvents is rapidly metabolized to products including carbon monoxide, which can cause headaches and can precipitate cardiac arrest in susceptible patients," he said. "When they say to use these products in a well-ventilated area, they mean it!"
In addition, patients who work around certain metal products, fiberglass, epoxy, and resin "can get terrible skin reactions. Sometimes the time relation can be fairly distant," he noted.
Patients with a history of exposure to asbestos "really need a full physical exam," Dr. Ziemkowski said. For them, and other patients who present with symptoms potentially linked to a toxic substance, order lab and imaging tests that are specific to the condition. "You certainly have to consider the latency in terms of what you order," he said.
Dr. Ziemkowski recommended several resources for clinicians, including the Centers for Disease Control and Prevention’s Agency for Toxic Substances and Disease Registry, the National Center for Environmental Health, the National Institute for Occupational Safety and Health, and the Occupational Safety and Health Administration. Other organizations dedicated to the topic include the American College of Occupational and Environmental Medicine and the Association of Occupational and Environmental Clinics.
Dr. Ziemkowski said he had no relevant financial conflicts to disclose.
SAN DIEGO – The first step in determining whether a patient has been exposed to a toxic substance is to conduct a thorough history, according to Dr. Peter J. Ziemkowski.
"First, get a good employment history," he advised at the annual meeting of the American Academy of Family Physicians. "You want to look at their current job, their longest-held jobs in the past, and any other significant jobs you can identify. Try to identify any exposure types they’ve had. You also have to figure out the time. Some things can present acutely. Other can have a long latency before their presentation."
A question to pose to patients is, Do you have any concerns about any exposure to hazards at work or home? "You want to identify if the chief complaint they’re coming in for had some relation to the exposure they’re concerned about, and any other specific triggers, such as symptoms of unknown etiology," said Dr. Ziemkowski of the department of family and community medicine at Western Michigan University, Kalamazoo.
It’s also important to ask patients about their potential exposure to toxic substances from a nonoccupational standpoint. "We’re talking about exposures from hobbies, such as photographers who did their own darkroom work and were exposed to solvents," Dr. Ziemkowski explained. "Digital photography has actually removed some of those hazards. Other risks come with hobbies such as ceramics. The kilns themselves can produce carbon monoxide. Also, painters can be exposed to arsenic, cadmium, lead, chromium, and mercury," he added.
According to the Bureau of Labor Statistics, approximately 3.5 million nonfatal occupational illnesses and injuries occurred in the United States in 2011. "That required millions of dollars in required time off and work restrictions," said Dr. Ziemkowski.
He also noted several case reports of exposure to methylene chloride, which is used in many industrial processes and in products such as paint strippers and degreasers. "Methylene chloride in these solvents is rapidly metabolized to products including carbon monoxide, which can cause headaches and can precipitate cardiac arrest in susceptible patients," he said. "When they say to use these products in a well-ventilated area, they mean it!"
In addition, patients who work around certain metal products, fiberglass, epoxy, and resin "can get terrible skin reactions. Sometimes the time relation can be fairly distant," he noted.
Patients with a history of exposure to asbestos "really need a full physical exam," Dr. Ziemkowski said. For them, and other patients who present with symptoms potentially linked to a toxic substance, order lab and imaging tests that are specific to the condition. "You certainly have to consider the latency in terms of what you order," he said.
Dr. Ziemkowski recommended several resources for clinicians, including the Centers for Disease Control and Prevention’s Agency for Toxic Substances and Disease Registry, the National Center for Environmental Health, the National Institute for Occupational Safety and Health, and the Occupational Safety and Health Administration. Other organizations dedicated to the topic include the American College of Occupational and Environmental Medicine and the Association of Occupational and Environmental Clinics.
Dr. Ziemkowski said he had no relevant financial conflicts to disclose.
SAN DIEGO – The first step in determining whether a patient has been exposed to a toxic substance is to conduct a thorough history, according to Dr. Peter J. Ziemkowski.
"First, get a good employment history," he advised at the annual meeting of the American Academy of Family Physicians. "You want to look at their current job, their longest-held jobs in the past, and any other significant jobs you can identify. Try to identify any exposure types they’ve had. You also have to figure out the time. Some things can present acutely. Other can have a long latency before their presentation."
A question to pose to patients is, Do you have any concerns about any exposure to hazards at work or home? "You want to identify if the chief complaint they’re coming in for had some relation to the exposure they’re concerned about, and any other specific triggers, such as symptoms of unknown etiology," said Dr. Ziemkowski of the department of family and community medicine at Western Michigan University, Kalamazoo.
It’s also important to ask patients about their potential exposure to toxic substances from a nonoccupational standpoint. "We’re talking about exposures from hobbies, such as photographers who did their own darkroom work and were exposed to solvents," Dr. Ziemkowski explained. "Digital photography has actually removed some of those hazards. Other risks come with hobbies such as ceramics. The kilns themselves can produce carbon monoxide. Also, painters can be exposed to arsenic, cadmium, lead, chromium, and mercury," he added.
According to the Bureau of Labor Statistics, approximately 3.5 million nonfatal occupational illnesses and injuries occurred in the United States in 2011. "That required millions of dollars in required time off and work restrictions," said Dr. Ziemkowski.
He also noted several case reports of exposure to methylene chloride, which is used in many industrial processes and in products such as paint strippers and degreasers. "Methylene chloride in these solvents is rapidly metabolized to products including carbon monoxide, which can cause headaches and can precipitate cardiac arrest in susceptible patients," he said. "When they say to use these products in a well-ventilated area, they mean it!"
In addition, patients who work around certain metal products, fiberglass, epoxy, and resin "can get terrible skin reactions. Sometimes the time relation can be fairly distant," he noted.
Patients with a history of exposure to asbestos "really need a full physical exam," Dr. Ziemkowski said. For them, and other patients who present with symptoms potentially linked to a toxic substance, order lab and imaging tests that are specific to the condition. "You certainly have to consider the latency in terms of what you order," he said.
Dr. Ziemkowski recommended several resources for clinicians, including the Centers for Disease Control and Prevention’s Agency for Toxic Substances and Disease Registry, the National Center for Environmental Health, the National Institute for Occupational Safety and Health, and the Occupational Safety and Health Administration. Other organizations dedicated to the topic include the American College of Occupational and Environmental Medicine and the Association of Occupational and Environmental Clinics.
Dr. Ziemkowski said he had no relevant financial conflicts to disclose.
EXPERT ANALYSIS AT THE AAFP SCIENTIFIC ASSEMBLY
No sex differences in carotid revascularization outcomes?
SAN FRANCISCO – There were no significant differences in endpoints between men and women after carotid endarterectomy or carotid artery stenting, results from a large registry study showed.
"These data suggest that, contrary to previous reports, women do not have a higher risk of adverse events after carotid revascularization," Dr. Jeffrey Jim said at the Society for Vascular Surgery Annual Meeting. "As such, women may derive similar benefits as men from carotid revascularization."
Carotid endarterectomy is considered by many as the gold standard treatment option for patients with severe internal carotid artery stenosis, said Dr. Jim, a vascular surgeon at Washington University School of Medicine, St. Louis. "Its benefit over best medical therapy has been proven by several landmark randomized controlled trials," he said. "While the efficacy of carotid artery stenting compared with carotid endarterectomy remains highly debated, there is clear utility in patients with select high risk criteria. However, it’s important to remember that gender plays an important role in cardiovascular disease. Epidemiologic studies clearly show that males have a higher stroke incidence as well as prevalence rate compared with women. However, when strokes do happen in women they tend to be more severe."
In terms of revascularization, he continued, available data suggested that women have a higher risk of perioperative adverse events compared with men, "suggesting that they may not benefit as much from revascularization compared with men."
He and other members of the SVS Outcomes Committee set out to evaluate the impact of gender on the outcomes after carotid revascularization (CAE and CAS), with the primary endpoint being composite risk of death, stroke, or MI at 30 days. They used data from 9,865 patients in the SVS Vascular Registry, which was developed in 2005 as a response to CMS approval of CAS. The registry was available to all clinical facilities and individual providers. "There are no specific inclusion or exclusion criteria because it aims to capture real-world results," Dr. Jim said. The registry is closed "but it remains one of the largest databases on carotid revascularization in the country."
Of the 9,865 patients 59% were men. There was no difference in age between sexes (both had a mean age of 71 years), but men were more likely to be symptomatic compared with women (42% vs. 39%, respectively).
For disease etiology in CAS, restenosis was higher in women compared with men (29% vs. 20%), while a greater proportion of men were being treated with radiation compared with women (6.2% vs. 2.6%). For CEA, more men were symptomatic compared with women (39% vs. 36%). "This was primarily driven by the fact that slightly more men than women had a stroke in the past (22% vs. 19%)," he said.
"Among patients overall, men had a slightly higher prevalence of coronary artery disease as well as MI, while women had a higher prevalence of hypertension as well as COPD," Dr. Jim said.
The researchers found no statistically significant differences in the composite endpoint of death, stroke, and MI at 30 days between men and women for either CEA (4.06% vs. 4.07%, respectively) or CAS (6.80% vs. 6.69%). The findings remained similar even after stratification by symptomatology and multivariate risk adjustment.
"In all the different ways we looked at it men and women had similar outcomes," Dr. Jim said. "This data is important. It’s representative of real-world outcomes, but there are limitations. It was an observational study done in a retrospective manner, and there is the potential for reporting bias. The most important limitation is that there is no comparison group of patients treated with best medical therapy. That’s an investigation that needs to be done going forward."
Dr. Jim said that he had no relevant financial conflicts to disclose.
SAN FRANCISCO – There were no significant differences in endpoints between men and women after carotid endarterectomy or carotid artery stenting, results from a large registry study showed.
"These data suggest that, contrary to previous reports, women do not have a higher risk of adverse events after carotid revascularization," Dr. Jeffrey Jim said at the Society for Vascular Surgery Annual Meeting. "As such, women may derive similar benefits as men from carotid revascularization."
Carotid endarterectomy is considered by many as the gold standard treatment option for patients with severe internal carotid artery stenosis, said Dr. Jim, a vascular surgeon at Washington University School of Medicine, St. Louis. "Its benefit over best medical therapy has been proven by several landmark randomized controlled trials," he said. "While the efficacy of carotid artery stenting compared with carotid endarterectomy remains highly debated, there is clear utility in patients with select high risk criteria. However, it’s important to remember that gender plays an important role in cardiovascular disease. Epidemiologic studies clearly show that males have a higher stroke incidence as well as prevalence rate compared with women. However, when strokes do happen in women they tend to be more severe."
In terms of revascularization, he continued, available data suggested that women have a higher risk of perioperative adverse events compared with men, "suggesting that they may not benefit as much from revascularization compared with men."
He and other members of the SVS Outcomes Committee set out to evaluate the impact of gender on the outcomes after carotid revascularization (CAE and CAS), with the primary endpoint being composite risk of death, stroke, or MI at 30 days. They used data from 9,865 patients in the SVS Vascular Registry, which was developed in 2005 as a response to CMS approval of CAS. The registry was available to all clinical facilities and individual providers. "There are no specific inclusion or exclusion criteria because it aims to capture real-world results," Dr. Jim said. The registry is closed "but it remains one of the largest databases on carotid revascularization in the country."
Of the 9,865 patients 59% were men. There was no difference in age between sexes (both had a mean age of 71 years), but men were more likely to be symptomatic compared with women (42% vs. 39%, respectively).
For disease etiology in CAS, restenosis was higher in women compared with men (29% vs. 20%), while a greater proportion of men were being treated with radiation compared with women (6.2% vs. 2.6%). For CEA, more men were symptomatic compared with women (39% vs. 36%). "This was primarily driven by the fact that slightly more men than women had a stroke in the past (22% vs. 19%)," he said.
"Among patients overall, men had a slightly higher prevalence of coronary artery disease as well as MI, while women had a higher prevalence of hypertension as well as COPD," Dr. Jim said.
The researchers found no statistically significant differences in the composite endpoint of death, stroke, and MI at 30 days between men and women for either CEA (4.06% vs. 4.07%, respectively) or CAS (6.80% vs. 6.69%). The findings remained similar even after stratification by symptomatology and multivariate risk adjustment.
"In all the different ways we looked at it men and women had similar outcomes," Dr. Jim said. "This data is important. It’s representative of real-world outcomes, but there are limitations. It was an observational study done in a retrospective manner, and there is the potential for reporting bias. The most important limitation is that there is no comparison group of patients treated with best medical therapy. That’s an investigation that needs to be done going forward."
Dr. Jim said that he had no relevant financial conflicts to disclose.
SAN FRANCISCO – There were no significant differences in endpoints between men and women after carotid endarterectomy or carotid artery stenting, results from a large registry study showed.
"These data suggest that, contrary to previous reports, women do not have a higher risk of adverse events after carotid revascularization," Dr. Jeffrey Jim said at the Society for Vascular Surgery Annual Meeting. "As such, women may derive similar benefits as men from carotid revascularization."
Carotid endarterectomy is considered by many as the gold standard treatment option for patients with severe internal carotid artery stenosis, said Dr. Jim, a vascular surgeon at Washington University School of Medicine, St. Louis. "Its benefit over best medical therapy has been proven by several landmark randomized controlled trials," he said. "While the efficacy of carotid artery stenting compared with carotid endarterectomy remains highly debated, there is clear utility in patients with select high risk criteria. However, it’s important to remember that gender plays an important role in cardiovascular disease. Epidemiologic studies clearly show that males have a higher stroke incidence as well as prevalence rate compared with women. However, when strokes do happen in women they tend to be more severe."
In terms of revascularization, he continued, available data suggested that women have a higher risk of perioperative adverse events compared with men, "suggesting that they may not benefit as much from revascularization compared with men."
He and other members of the SVS Outcomes Committee set out to evaluate the impact of gender on the outcomes after carotid revascularization (CAE and CAS), with the primary endpoint being composite risk of death, stroke, or MI at 30 days. They used data from 9,865 patients in the SVS Vascular Registry, which was developed in 2005 as a response to CMS approval of CAS. The registry was available to all clinical facilities and individual providers. "There are no specific inclusion or exclusion criteria because it aims to capture real-world results," Dr. Jim said. The registry is closed "but it remains one of the largest databases on carotid revascularization in the country."
Of the 9,865 patients 59% were men. There was no difference in age between sexes (both had a mean age of 71 years), but men were more likely to be symptomatic compared with women (42% vs. 39%, respectively).
For disease etiology in CAS, restenosis was higher in women compared with men (29% vs. 20%), while a greater proportion of men were being treated with radiation compared with women (6.2% vs. 2.6%). For CEA, more men were symptomatic compared with women (39% vs. 36%). "This was primarily driven by the fact that slightly more men than women had a stroke in the past (22% vs. 19%)," he said.
"Among patients overall, men had a slightly higher prevalence of coronary artery disease as well as MI, while women had a higher prevalence of hypertension as well as COPD," Dr. Jim said.
The researchers found no statistically significant differences in the composite endpoint of death, stroke, and MI at 30 days between men and women for either CEA (4.06% vs. 4.07%, respectively) or CAS (6.80% vs. 6.69%). The findings remained similar even after stratification by symptomatology and multivariate risk adjustment.
"In all the different ways we looked at it men and women had similar outcomes," Dr. Jim said. "This data is important. It’s representative of real-world outcomes, but there are limitations. It was an observational study done in a retrospective manner, and there is the potential for reporting bias. The most important limitation is that there is no comparison group of patients treated with best medical therapy. That’s an investigation that needs to be done going forward."
Dr. Jim said that he had no relevant financial conflicts to disclose.
Memantine helped to delay driving impairment in early Alzheimer’s
SAN DIEGO – Patients with mild Alzheimer’s disease who took 20 mg/day of memantine for 1 year remained the same or improved their driving ability, compared with placebo-treated patients in a small pilot trial.
Memantine is currently approved for moderate and severe Alzheimer’s disease (AD), but the findings suggest that the agent may have some neuroprotective qualities for people in the early stages of the disease, Anna Lisa Curtis said in an interview at the Clinical Trials Conference on Alzheimer’s Disease.
"In Florida, we have a large population of elderly that are still on the road, including those with early AD," said Ms. Curtis, a research coordinator at Florida Atlantic University (FAU), Boca Raton. "Being able to help maintain an imperative skill like driving for one’s sense of self and [the] ability to have a broader life is important, as is the safety of pedestrians and other motorists, and caregiver burden."
In a study led by Dr. Peter J. Holland, a psychiatrist at FAU, the researchers set out to evaluate the effects of 20 mg/day of memantine on delaying the progression of driving impairment in 60 men and women with mild AD as measured by actual driving and by a battery of neuropsychological tests focused on the cognitive skills necessary for driving, including executive functioning, visuospatial abilities, attention, and orientation. At baseline, 6 months, and 12 months, driving ability was measured with the DriveABLE-On-Road Test. They tested cognition with ADAS-Cog mazes, Fuld Object-Memory Evaluation, Clinical Dementia Rating scale, Trail-Making Tests A and B, the Rey-Osterrieth Complex Figure Test, the Useful Field of View test, and the Motor Free Visual Perception TestVisual Closure Subtest. The primary outcome was the number of subjects in each group who were able to pass the DriveABLE test at month 12. The secondary outcome measures were the changes from baseline to month 12 on the driving-related neuropsychological battery.
The researchers reported findings from 22 patients in the memantine group and 21 patients in the placebo group. Their mean age was 79 years and 62% were male. In terms of driving ability, 100% of patients in the memantine group remained the same or improved, compared with 75% of those in the placebo group, a difference that reached statistical significance (P = .04). In addition, after controlling for baseline ability, patients in the memantine group performed significantly better on the Rey-Osterrieth Complex Figure Test (P = .05) and on the Trails A and B tests (P = .05 and .02, respectively). Outcomes on the other tests were similar between the two groups.
"We did not anticipate that the treatment group would perform as well as it did," Ms. Curtis said. "In a 12-month period with AD, we can see quite a bit of decline, and we were surprised to see people in the memantine group maintaining skills or actually improving in their capacity."
She concluded that the findings warrant further study on the role of memantine in delaying driving impairment in patients with mild AD.
Forest Laboratories funded the study. Ms. Curtis said that she had no relevant financial conflicts to disclose.
SAN DIEGO – Patients with mild Alzheimer’s disease who took 20 mg/day of memantine for 1 year remained the same or improved their driving ability, compared with placebo-treated patients in a small pilot trial.
Memantine is currently approved for moderate and severe Alzheimer’s disease (AD), but the findings suggest that the agent may have some neuroprotective qualities for people in the early stages of the disease, Anna Lisa Curtis said in an interview at the Clinical Trials Conference on Alzheimer’s Disease.
"In Florida, we have a large population of elderly that are still on the road, including those with early AD," said Ms. Curtis, a research coordinator at Florida Atlantic University (FAU), Boca Raton. "Being able to help maintain an imperative skill like driving for one’s sense of self and [the] ability to have a broader life is important, as is the safety of pedestrians and other motorists, and caregiver burden."
In a study led by Dr. Peter J. Holland, a psychiatrist at FAU, the researchers set out to evaluate the effects of 20 mg/day of memantine on delaying the progression of driving impairment in 60 men and women with mild AD as measured by actual driving and by a battery of neuropsychological tests focused on the cognitive skills necessary for driving, including executive functioning, visuospatial abilities, attention, and orientation. At baseline, 6 months, and 12 months, driving ability was measured with the DriveABLE-On-Road Test. They tested cognition with ADAS-Cog mazes, Fuld Object-Memory Evaluation, Clinical Dementia Rating scale, Trail-Making Tests A and B, the Rey-Osterrieth Complex Figure Test, the Useful Field of View test, and the Motor Free Visual Perception TestVisual Closure Subtest. The primary outcome was the number of subjects in each group who were able to pass the DriveABLE test at month 12. The secondary outcome measures were the changes from baseline to month 12 on the driving-related neuropsychological battery.
The researchers reported findings from 22 patients in the memantine group and 21 patients in the placebo group. Their mean age was 79 years and 62% were male. In terms of driving ability, 100% of patients in the memantine group remained the same or improved, compared with 75% of those in the placebo group, a difference that reached statistical significance (P = .04). In addition, after controlling for baseline ability, patients in the memantine group performed significantly better on the Rey-Osterrieth Complex Figure Test (P = .05) and on the Trails A and B tests (P = .05 and .02, respectively). Outcomes on the other tests were similar between the two groups.
"We did not anticipate that the treatment group would perform as well as it did," Ms. Curtis said. "In a 12-month period with AD, we can see quite a bit of decline, and we were surprised to see people in the memantine group maintaining skills or actually improving in their capacity."
She concluded that the findings warrant further study on the role of memantine in delaying driving impairment in patients with mild AD.
Forest Laboratories funded the study. Ms. Curtis said that she had no relevant financial conflicts to disclose.
SAN DIEGO – Patients with mild Alzheimer’s disease who took 20 mg/day of memantine for 1 year remained the same or improved their driving ability, compared with placebo-treated patients in a small pilot trial.
Memantine is currently approved for moderate and severe Alzheimer’s disease (AD), but the findings suggest that the agent may have some neuroprotective qualities for people in the early stages of the disease, Anna Lisa Curtis said in an interview at the Clinical Trials Conference on Alzheimer’s Disease.
"In Florida, we have a large population of elderly that are still on the road, including those with early AD," said Ms. Curtis, a research coordinator at Florida Atlantic University (FAU), Boca Raton. "Being able to help maintain an imperative skill like driving for one’s sense of self and [the] ability to have a broader life is important, as is the safety of pedestrians and other motorists, and caregiver burden."
In a study led by Dr. Peter J. Holland, a psychiatrist at FAU, the researchers set out to evaluate the effects of 20 mg/day of memantine on delaying the progression of driving impairment in 60 men and women with mild AD as measured by actual driving and by a battery of neuropsychological tests focused on the cognitive skills necessary for driving, including executive functioning, visuospatial abilities, attention, and orientation. At baseline, 6 months, and 12 months, driving ability was measured with the DriveABLE-On-Road Test. They tested cognition with ADAS-Cog mazes, Fuld Object-Memory Evaluation, Clinical Dementia Rating scale, Trail-Making Tests A and B, the Rey-Osterrieth Complex Figure Test, the Useful Field of View test, and the Motor Free Visual Perception TestVisual Closure Subtest. The primary outcome was the number of subjects in each group who were able to pass the DriveABLE test at month 12. The secondary outcome measures were the changes from baseline to month 12 on the driving-related neuropsychological battery.
The researchers reported findings from 22 patients in the memantine group and 21 patients in the placebo group. Their mean age was 79 years and 62% were male. In terms of driving ability, 100% of patients in the memantine group remained the same or improved, compared with 75% of those in the placebo group, a difference that reached statistical significance (P = .04). In addition, after controlling for baseline ability, patients in the memantine group performed significantly better on the Rey-Osterrieth Complex Figure Test (P = .05) and on the Trails A and B tests (P = .05 and .02, respectively). Outcomes on the other tests were similar between the two groups.
"We did not anticipate that the treatment group would perform as well as it did," Ms. Curtis said. "In a 12-month period with AD, we can see quite a bit of decline, and we were surprised to see people in the memantine group maintaining skills or actually improving in their capacity."
She concluded that the findings warrant further study on the role of memantine in delaying driving impairment in patients with mild AD.
Forest Laboratories funded the study. Ms. Curtis said that she had no relevant financial conflicts to disclose.
AT CTAD
Major finding: In terms of driving ability, 100% of patients in the memantine group remained the same or improved, compared with 75% of those in the placebo group, a difference that reached statistical significance (P = .04).
Data source: A study of 43 patients with mild Alzheimer’s disease who took 20 mg/day of memantine or placebo for 1 year.
Disclosures: Forest Laboratories funded the study. Ms. Curtis said that she had no relevant financial conflicts to disclose.
Nerve growth factor gene therapy delivered safely in Alzheimer’s patients
SAN DIEGO – Delivery of a gene therapy viral vector designed to express only the gene for human growth factor to the nucleus basalis of Meynert in patients with mild to moderate Alzheimer’s disease appears to be safe and feasible, results from a first-in-human trial demonstrated.
"There are significant deficiencies with the therapies that exist for treating the memory and cognitive impairments of Alzheimer’s disease," Raymond T. Bartus, Ph.D., said at the Clinical Trials Conference on Alzheimer’s Disease.
Currently available cholinesterase inhibitors "are nonselective and suffer from serious dose-limiting side effects," said Dr. Bartus, president of San Diego–based RTBioconsultants. "Their mechanism of action for cholinergic enhancement is suboptimal, and they do not repair neurons, protect against death, or truly restore lost neuronal function. While they work to some extent, they are modest in their level of efficacy and do not improve symptoms in all patients. Moreover, they do nothing to affect the disease progression."
Nerve growth factor neurotrophic therapy, he continued, "should overcome all of these deficiencies and therefore provide a more effective therapy, applying a variation of the cholinergic approach that has shown proof of concept." Decades of research in animal models suggest that nerve growth factor "has remarkable antiapoptotic (i.e., antideath) and reparative properties for certain neurons in the degenerative brain," Dr. Bartus said. "There are at least two major obstacles to apply this technology to Alzheimer’s disease. The first is, Alzheimer’s is an extremely complicated disease, so determining where to target the treatment is especially important. Targeting the cholinergic neurons is compelling because their degeneration is known to contribute significantly to the memory loss."
The second key challenge, he said, is the need for constant exposure to cholinergic neurons that comprise the nucleus basalis of Meynert (NBM) while avoiding exposure to untargeted neuronal populations. This has proven very difficult in past efforts extending back 20 years. In the past decade, gene therapy combined with stereotactic surgery has emerged as a practical enabling technology to accomplish this. On this basis, Dr. Bartus and his associates at Ceregene (where he served as chief scientific officer for more than 10 years) developed a viral gene therapy vector bioengineered to express only the gene for human nerve growth factor (AAV2-NGF, or CERE-110).
"You can think of it as an off-the-shelf biopharmaceutical that when injected into the human brain will induce the target neurons to produce or express and secrete only nerve growth factor and therefore provide support to NBM cholinergic neurons," he explained. "Preclinical studies have demonstrated an orderly dose-response relationship with NGF restricted to targeted basal forebrain cholinergic neurons, and no side effects or toxicity, even after testing very high doses in animals. That was quite surprising and certainly welcome."
Dr. Bartus presented phase I clinical data from 10 patients aged 50-79 years with mild to moderate Alzheimer’s disease who received one of three ascending doses of AAV2-NGF via bilateral stereotactic injection into the nucleus basalis of Meynert: dose A (1.2 x 1010 viral genomes), dose B (5.8 x 1010 viral genomes; five times more than dose A), and dose C (1.2 x 1011 viral genomes; two times more than dose B). Patients were followed at 24 months for safety and feasibility and preliminary efficacy as measured by 18-fluorodeoxyglucose PET scans at baseline, 6, 12, and 24 months; autopsy tissue; and validated neuropsychological tests that included the Alzheimer’s Disease Assessment Scale–Cognitive (ADAS-Cog). Computer graphic software was used to provide a 3-D model of the nucleus basalis of Meynert in an effort to "determine where to distribute CERE-110 in the NBM and how much should be given to achieve optimal NGF exposure."
Dr. Bartus reported that AAV2-NGF was safe and well tolerated through 24 months. Adverse events and serious adverse events "were uneventful," he said. "What you see is a profile that reflects either incidental or unrelated events, or those related to a surgical procedure (e.g., temporary headache)."
PET imaging showed no evidence of accelerated decline, and brain autopsy tissue from three patients who died of unrelated causes confirmed long-term, therapeutically active, gene-mediated NGF expression accurately targeted to the nucleus basalis of Meynert.
The researchers observed some deterioration over time based on the ADAS-Cog and other neuropsychological measures. "There is no clinical evidence that cognitive decline is accelerated, but we can’t speak to whether or not we actually improved rate of decline," Dr. Bartus said.
These findings formed the basis of a phase II trial currently underway that is funded by the National Institutes of Health under the auspices of the Alzheimer’s Disease Collaborative Study. Outcomes from that 24-month trial are expected in 2015.
The phase 1 study was funded by Ceregene, which was acquired in October 2013 by Sangamo BioSciences.
Dr. Bartus disclosed that he is a consultant for Sangamo BioSciences.
SAN DIEGO – Delivery of a gene therapy viral vector designed to express only the gene for human growth factor to the nucleus basalis of Meynert in patients with mild to moderate Alzheimer’s disease appears to be safe and feasible, results from a first-in-human trial demonstrated.
"There are significant deficiencies with the therapies that exist for treating the memory and cognitive impairments of Alzheimer’s disease," Raymond T. Bartus, Ph.D., said at the Clinical Trials Conference on Alzheimer’s Disease.
Currently available cholinesterase inhibitors "are nonselective and suffer from serious dose-limiting side effects," said Dr. Bartus, president of San Diego–based RTBioconsultants. "Their mechanism of action for cholinergic enhancement is suboptimal, and they do not repair neurons, protect against death, or truly restore lost neuronal function. While they work to some extent, they are modest in their level of efficacy and do not improve symptoms in all patients. Moreover, they do nothing to affect the disease progression."
Nerve growth factor neurotrophic therapy, he continued, "should overcome all of these deficiencies and therefore provide a more effective therapy, applying a variation of the cholinergic approach that has shown proof of concept." Decades of research in animal models suggest that nerve growth factor "has remarkable antiapoptotic (i.e., antideath) and reparative properties for certain neurons in the degenerative brain," Dr. Bartus said. "There are at least two major obstacles to apply this technology to Alzheimer’s disease. The first is, Alzheimer’s is an extremely complicated disease, so determining where to target the treatment is especially important. Targeting the cholinergic neurons is compelling because their degeneration is known to contribute significantly to the memory loss."
The second key challenge, he said, is the need for constant exposure to cholinergic neurons that comprise the nucleus basalis of Meynert (NBM) while avoiding exposure to untargeted neuronal populations. This has proven very difficult in past efforts extending back 20 years. In the past decade, gene therapy combined with stereotactic surgery has emerged as a practical enabling technology to accomplish this. On this basis, Dr. Bartus and his associates at Ceregene (where he served as chief scientific officer for more than 10 years) developed a viral gene therapy vector bioengineered to express only the gene for human nerve growth factor (AAV2-NGF, or CERE-110).
"You can think of it as an off-the-shelf biopharmaceutical that when injected into the human brain will induce the target neurons to produce or express and secrete only nerve growth factor and therefore provide support to NBM cholinergic neurons," he explained. "Preclinical studies have demonstrated an orderly dose-response relationship with NGF restricted to targeted basal forebrain cholinergic neurons, and no side effects or toxicity, even after testing very high doses in animals. That was quite surprising and certainly welcome."
Dr. Bartus presented phase I clinical data from 10 patients aged 50-79 years with mild to moderate Alzheimer’s disease who received one of three ascending doses of AAV2-NGF via bilateral stereotactic injection into the nucleus basalis of Meynert: dose A (1.2 x 1010 viral genomes), dose B (5.8 x 1010 viral genomes; five times more than dose A), and dose C (1.2 x 1011 viral genomes; two times more than dose B). Patients were followed at 24 months for safety and feasibility and preliminary efficacy as measured by 18-fluorodeoxyglucose PET scans at baseline, 6, 12, and 24 months; autopsy tissue; and validated neuropsychological tests that included the Alzheimer’s Disease Assessment Scale–Cognitive (ADAS-Cog). Computer graphic software was used to provide a 3-D model of the nucleus basalis of Meynert in an effort to "determine where to distribute CERE-110 in the NBM and how much should be given to achieve optimal NGF exposure."
Dr. Bartus reported that AAV2-NGF was safe and well tolerated through 24 months. Adverse events and serious adverse events "were uneventful," he said. "What you see is a profile that reflects either incidental or unrelated events, or those related to a surgical procedure (e.g., temporary headache)."
PET imaging showed no evidence of accelerated decline, and brain autopsy tissue from three patients who died of unrelated causes confirmed long-term, therapeutically active, gene-mediated NGF expression accurately targeted to the nucleus basalis of Meynert.
The researchers observed some deterioration over time based on the ADAS-Cog and other neuropsychological measures. "There is no clinical evidence that cognitive decline is accelerated, but we can’t speak to whether or not we actually improved rate of decline," Dr. Bartus said.
These findings formed the basis of a phase II trial currently underway that is funded by the National Institutes of Health under the auspices of the Alzheimer’s Disease Collaborative Study. Outcomes from that 24-month trial are expected in 2015.
The phase 1 study was funded by Ceregene, which was acquired in October 2013 by Sangamo BioSciences.
Dr. Bartus disclosed that he is a consultant for Sangamo BioSciences.
SAN DIEGO – Delivery of a gene therapy viral vector designed to express only the gene for human growth factor to the nucleus basalis of Meynert in patients with mild to moderate Alzheimer’s disease appears to be safe and feasible, results from a first-in-human trial demonstrated.
"There are significant deficiencies with the therapies that exist for treating the memory and cognitive impairments of Alzheimer’s disease," Raymond T. Bartus, Ph.D., said at the Clinical Trials Conference on Alzheimer’s Disease.
Currently available cholinesterase inhibitors "are nonselective and suffer from serious dose-limiting side effects," said Dr. Bartus, president of San Diego–based RTBioconsultants. "Their mechanism of action for cholinergic enhancement is suboptimal, and they do not repair neurons, protect against death, or truly restore lost neuronal function. While they work to some extent, they are modest in their level of efficacy and do not improve symptoms in all patients. Moreover, they do nothing to affect the disease progression."
Nerve growth factor neurotrophic therapy, he continued, "should overcome all of these deficiencies and therefore provide a more effective therapy, applying a variation of the cholinergic approach that has shown proof of concept." Decades of research in animal models suggest that nerve growth factor "has remarkable antiapoptotic (i.e., antideath) and reparative properties for certain neurons in the degenerative brain," Dr. Bartus said. "There are at least two major obstacles to apply this technology to Alzheimer’s disease. The first is, Alzheimer’s is an extremely complicated disease, so determining where to target the treatment is especially important. Targeting the cholinergic neurons is compelling because their degeneration is known to contribute significantly to the memory loss."
The second key challenge, he said, is the need for constant exposure to cholinergic neurons that comprise the nucleus basalis of Meynert (NBM) while avoiding exposure to untargeted neuronal populations. This has proven very difficult in past efforts extending back 20 years. In the past decade, gene therapy combined with stereotactic surgery has emerged as a practical enabling technology to accomplish this. On this basis, Dr. Bartus and his associates at Ceregene (where he served as chief scientific officer for more than 10 years) developed a viral gene therapy vector bioengineered to express only the gene for human nerve growth factor (AAV2-NGF, or CERE-110).
"You can think of it as an off-the-shelf biopharmaceutical that when injected into the human brain will induce the target neurons to produce or express and secrete only nerve growth factor and therefore provide support to NBM cholinergic neurons," he explained. "Preclinical studies have demonstrated an orderly dose-response relationship with NGF restricted to targeted basal forebrain cholinergic neurons, and no side effects or toxicity, even after testing very high doses in animals. That was quite surprising and certainly welcome."
Dr. Bartus presented phase I clinical data from 10 patients aged 50-79 years with mild to moderate Alzheimer’s disease who received one of three ascending doses of AAV2-NGF via bilateral stereotactic injection into the nucleus basalis of Meynert: dose A (1.2 x 1010 viral genomes), dose B (5.8 x 1010 viral genomes; five times more than dose A), and dose C (1.2 x 1011 viral genomes; two times more than dose B). Patients were followed at 24 months for safety and feasibility and preliminary efficacy as measured by 18-fluorodeoxyglucose PET scans at baseline, 6, 12, and 24 months; autopsy tissue; and validated neuropsychological tests that included the Alzheimer’s Disease Assessment Scale–Cognitive (ADAS-Cog). Computer graphic software was used to provide a 3-D model of the nucleus basalis of Meynert in an effort to "determine where to distribute CERE-110 in the NBM and how much should be given to achieve optimal NGF exposure."
Dr. Bartus reported that AAV2-NGF was safe and well tolerated through 24 months. Adverse events and serious adverse events "were uneventful," he said. "What you see is a profile that reflects either incidental or unrelated events, or those related to a surgical procedure (e.g., temporary headache)."
PET imaging showed no evidence of accelerated decline, and brain autopsy tissue from three patients who died of unrelated causes confirmed long-term, therapeutically active, gene-mediated NGF expression accurately targeted to the nucleus basalis of Meynert.
The researchers observed some deterioration over time based on the ADAS-Cog and other neuropsychological measures. "There is no clinical evidence that cognitive decline is accelerated, but we can’t speak to whether or not we actually improved rate of decline," Dr. Bartus said.
These findings formed the basis of a phase II trial currently underway that is funded by the National Institutes of Health under the auspices of the Alzheimer’s Disease Collaborative Study. Outcomes from that 24-month trial are expected in 2015.
The phase 1 study was funded by Ceregene, which was acquired in October 2013 by Sangamo BioSciences.
Dr. Bartus disclosed that he is a consultant for Sangamo BioSciences.
AT CTAD
Major finding: At 24 months, human nerve growth factor gene therapy via bilateral stereotactic injection into the nucleus basalis of Meynert was safe and well tolerated, PET imaging showed no evidence of accelerated decline, and brain autopsy tissue from three patients who died of unrelated causes confirmed long-term, therapeutically active, gene-mediated NGF expression.
Data source: A study of 10 patients aged 50-79 years with mild to moderate Alzheimer’s disease who received one of three ascending doses of AAV2-NGF.
Disclosures: Dr. Bartus disclosed that he is a consultant for Sangamo BioSciences.
Combined factors predicted risk of de novo stress urinary incontinence
LAS VEGAS – A prediction model of seven combined risk factors provides a validated, individualized way to determine a patient’s risk for developing de novo stress urinary incontinence following pelvic prolapse surgery, a novel study showed.
"In women without stress urinary incontinence symptoms, prolapse surgery may cause de novo SUI in 16%-51% of patients," Dr. J. Eric Jelovsek said at the annual meeting of the American Urogynecologic Society.
"Recent studies have demonstrated effective prevention strategies, including prophylactic incontinence surgery such as concomitant midurethral sling or Burch urethropexy, and provided refined estimates of the average patient’s risk. While these studies have advanced our knowledge of the overall prevalence of de novo SUI, the risk prediction for a specific patient varies based on individual characteristics. A prediction model that more accurately predicts an individual’s risk may further help customize the shared decision-making process that occurs between a patient and her physician when planning for a concomitant continence operation," Dr. Jelovsek said.
In order to develop and validate a model to predict an individual’s risk of de novo SUI within 12 months of prolapse surgery, Dr. Jelovsek and his associates with the Eunice Kennedy Shriver National Institute of Child Health and Human Development’s Pelvic Floor Disorders Network used data from the OPUS (Outcomes Following Vaginal Prolapse Repair and Midurethral Sling) trial.
To externally validate the model, they also used data from the CARE (Colpopexy and Urinary Reduction Efforts) trial. The investigators identified 12 original preoperative patient and test characteristics commonly used to predict the risk of de novo SUI following surgery from the available data sets. They were increased age, white race, higher vaginal parity, higher body mass index (BMI), current smoker, current diagnosis of diabetes, strenuous physical activity, baseline urgency urinary incontinence symptoms, higher preoperative Pelvic Organ Prolapse Quantification (POP-Q) stage, higher POP-Q point Aa measure, positive preoperative prolapse reduction stress test, and performance of a concomitant retropubic midurethral sling.
"We hypothesized that all risk factors except for the performance of a concomitant [retropubic midurethral sling] would increase the risk of de novo SUI," said Dr. Jelovsek, who is director of the Cleveland Clinic Multidisciplinary Simulation Center. The outcome of the prediction model was defined as development of de novo SUI as determined by the response of "somewhat," "moderately," or "quite a bit" on the Pelvic Floor Distress Inventory (PFDI) questions 20-22, as these responses have been shown to be highly relevant outcomes to patients.
Of the 465 women in the OPUS trial, 457 had SUI data available 12 months after surgery. Of the 12 original risk factors hypothesized to predict de novo SUI, 7 final risk factors were identified that when combined together accurately predicted de novo SUI. They were decreased age, higher vaginal parity, higher BMI, current diagnosis of diabetes, baseline urgency urinary incontinence symptoms, positive preoperative prolapse reduction stress test, and performance of a concomitant retropubic midurethral sling.
"Contrary to our original hypothesis, as age increased, a participant’s risk of experiencing de novo SUI decreased when combined with other factors," Dr. Jelovsek said. The researchers created a calculator using the variables from the model for making predictions in a clinical setting.
He went on to report that the prediction model had useful discrimination between women who ultimately did or did not experience de novo SUI, with a concordance index of 0.73. The accuracy of the model on the entire CARE data set was also significantly better than that of random chance (a concordance index of 0.62) as well as that of using the preoperative prolapse reduction stress test alone (a concordance index of 0.54).
"Our model demonstrates good predictive accuracy, with a concordance index of 0.72 in women undergoing transvaginal prolapse surgery," Dr. Jelovsek said. "This compares favorably to the National Cancer Institute Gail Model for Breast Cancer (a concordance index of 0.59), and the Framingham Cardiovascular Risk Model (a concordance index of 0.72)."
He acknowledged certain limitations of the study, including the fact that although the model was better than random chance in predicting the probability of SUI after abdominal sacral colpopexy and Burch urethropexy in the CARE population, the accuracy was lower. "This may have been because women in the OPUS data set underwent sling with vaginal surgery for prolapse, while those in CARE underwent a Burch procedure and abdominal surgery," he explained.
"It is also possible that abdominal sacral colpopexy places a different amount of risk of de novo stress urinary incontinence on individuals than does vaginal surgery for pelvic organ prolapse. Despite this, a concordance index of 0.62 suggests that the model is also a valuable adjunct for shared decision-making between the clinician and patients prior to abdominal sacral colpopexy with Burch urethropexy surgery."
He said that future studies should include populations of women undergoing minimally invasive colpopexy.
Dr. Jelovsek said he had no relevant financial disclosures.
LAS VEGAS – A prediction model of seven combined risk factors provides a validated, individualized way to determine a patient’s risk for developing de novo stress urinary incontinence following pelvic prolapse surgery, a novel study showed.
"In women without stress urinary incontinence symptoms, prolapse surgery may cause de novo SUI in 16%-51% of patients," Dr. J. Eric Jelovsek said at the annual meeting of the American Urogynecologic Society.
"Recent studies have demonstrated effective prevention strategies, including prophylactic incontinence surgery such as concomitant midurethral sling or Burch urethropexy, and provided refined estimates of the average patient’s risk. While these studies have advanced our knowledge of the overall prevalence of de novo SUI, the risk prediction for a specific patient varies based on individual characteristics. A prediction model that more accurately predicts an individual’s risk may further help customize the shared decision-making process that occurs between a patient and her physician when planning for a concomitant continence operation," Dr. Jelovsek said.
In order to develop and validate a model to predict an individual’s risk of de novo SUI within 12 months of prolapse surgery, Dr. Jelovsek and his associates with the Eunice Kennedy Shriver National Institute of Child Health and Human Development’s Pelvic Floor Disorders Network used data from the OPUS (Outcomes Following Vaginal Prolapse Repair and Midurethral Sling) trial.
To externally validate the model, they also used data from the CARE (Colpopexy and Urinary Reduction Efforts) trial. The investigators identified 12 original preoperative patient and test characteristics commonly used to predict the risk of de novo SUI following surgery from the available data sets. They were increased age, white race, higher vaginal parity, higher body mass index (BMI), current smoker, current diagnosis of diabetes, strenuous physical activity, baseline urgency urinary incontinence symptoms, higher preoperative Pelvic Organ Prolapse Quantification (POP-Q) stage, higher POP-Q point Aa measure, positive preoperative prolapse reduction stress test, and performance of a concomitant retropubic midurethral sling.
"We hypothesized that all risk factors except for the performance of a concomitant [retropubic midurethral sling] would increase the risk of de novo SUI," said Dr. Jelovsek, who is director of the Cleveland Clinic Multidisciplinary Simulation Center. The outcome of the prediction model was defined as development of de novo SUI as determined by the response of "somewhat," "moderately," or "quite a bit" on the Pelvic Floor Distress Inventory (PFDI) questions 20-22, as these responses have been shown to be highly relevant outcomes to patients.
Of the 465 women in the OPUS trial, 457 had SUI data available 12 months after surgery. Of the 12 original risk factors hypothesized to predict de novo SUI, 7 final risk factors were identified that when combined together accurately predicted de novo SUI. They were decreased age, higher vaginal parity, higher BMI, current diagnosis of diabetes, baseline urgency urinary incontinence symptoms, positive preoperative prolapse reduction stress test, and performance of a concomitant retropubic midurethral sling.
"Contrary to our original hypothesis, as age increased, a participant’s risk of experiencing de novo SUI decreased when combined with other factors," Dr. Jelovsek said. The researchers created a calculator using the variables from the model for making predictions in a clinical setting.
He went on to report that the prediction model had useful discrimination between women who ultimately did or did not experience de novo SUI, with a concordance index of 0.73. The accuracy of the model on the entire CARE data set was also significantly better than that of random chance (a concordance index of 0.62) as well as that of using the preoperative prolapse reduction stress test alone (a concordance index of 0.54).
"Our model demonstrates good predictive accuracy, with a concordance index of 0.72 in women undergoing transvaginal prolapse surgery," Dr. Jelovsek said. "This compares favorably to the National Cancer Institute Gail Model for Breast Cancer (a concordance index of 0.59), and the Framingham Cardiovascular Risk Model (a concordance index of 0.72)."
He acknowledged certain limitations of the study, including the fact that although the model was better than random chance in predicting the probability of SUI after abdominal sacral colpopexy and Burch urethropexy in the CARE population, the accuracy was lower. "This may have been because women in the OPUS data set underwent sling with vaginal surgery for prolapse, while those in CARE underwent a Burch procedure and abdominal surgery," he explained.
"It is also possible that abdominal sacral colpopexy places a different amount of risk of de novo stress urinary incontinence on individuals than does vaginal surgery for pelvic organ prolapse. Despite this, a concordance index of 0.62 suggests that the model is also a valuable adjunct for shared decision-making between the clinician and patients prior to abdominal sacral colpopexy with Burch urethropexy surgery."
He said that future studies should include populations of women undergoing minimally invasive colpopexy.
Dr. Jelovsek said he had no relevant financial disclosures.
LAS VEGAS – A prediction model of seven combined risk factors provides a validated, individualized way to determine a patient’s risk for developing de novo stress urinary incontinence following pelvic prolapse surgery, a novel study showed.
"In women without stress urinary incontinence symptoms, prolapse surgery may cause de novo SUI in 16%-51% of patients," Dr. J. Eric Jelovsek said at the annual meeting of the American Urogynecologic Society.
"Recent studies have demonstrated effective prevention strategies, including prophylactic incontinence surgery such as concomitant midurethral sling or Burch urethropexy, and provided refined estimates of the average patient’s risk. While these studies have advanced our knowledge of the overall prevalence of de novo SUI, the risk prediction for a specific patient varies based on individual characteristics. A prediction model that more accurately predicts an individual’s risk may further help customize the shared decision-making process that occurs between a patient and her physician when planning for a concomitant continence operation," Dr. Jelovsek said.
In order to develop and validate a model to predict an individual’s risk of de novo SUI within 12 months of prolapse surgery, Dr. Jelovsek and his associates with the Eunice Kennedy Shriver National Institute of Child Health and Human Development’s Pelvic Floor Disorders Network used data from the OPUS (Outcomes Following Vaginal Prolapse Repair and Midurethral Sling) trial.
To externally validate the model, they also used data from the CARE (Colpopexy and Urinary Reduction Efforts) trial. The investigators identified 12 original preoperative patient and test characteristics commonly used to predict the risk of de novo SUI following surgery from the available data sets. They were increased age, white race, higher vaginal parity, higher body mass index (BMI), current smoker, current diagnosis of diabetes, strenuous physical activity, baseline urgency urinary incontinence symptoms, higher preoperative Pelvic Organ Prolapse Quantification (POP-Q) stage, higher POP-Q point Aa measure, positive preoperative prolapse reduction stress test, and performance of a concomitant retropubic midurethral sling.
"We hypothesized that all risk factors except for the performance of a concomitant [retropubic midurethral sling] would increase the risk of de novo SUI," said Dr. Jelovsek, who is director of the Cleveland Clinic Multidisciplinary Simulation Center. The outcome of the prediction model was defined as development of de novo SUI as determined by the response of "somewhat," "moderately," or "quite a bit" on the Pelvic Floor Distress Inventory (PFDI) questions 20-22, as these responses have been shown to be highly relevant outcomes to patients.
Of the 465 women in the OPUS trial, 457 had SUI data available 12 months after surgery. Of the 12 original risk factors hypothesized to predict de novo SUI, 7 final risk factors were identified that when combined together accurately predicted de novo SUI. They were decreased age, higher vaginal parity, higher BMI, current diagnosis of diabetes, baseline urgency urinary incontinence symptoms, positive preoperative prolapse reduction stress test, and performance of a concomitant retropubic midurethral sling.
"Contrary to our original hypothesis, as age increased, a participant’s risk of experiencing de novo SUI decreased when combined with other factors," Dr. Jelovsek said. The researchers created a calculator using the variables from the model for making predictions in a clinical setting.
He went on to report that the prediction model had useful discrimination between women who ultimately did or did not experience de novo SUI, with a concordance index of 0.73. The accuracy of the model on the entire CARE data set was also significantly better than that of random chance (a concordance index of 0.62) as well as that of using the preoperative prolapse reduction stress test alone (a concordance index of 0.54).
"Our model demonstrates good predictive accuracy, with a concordance index of 0.72 in women undergoing transvaginal prolapse surgery," Dr. Jelovsek said. "This compares favorably to the National Cancer Institute Gail Model for Breast Cancer (a concordance index of 0.59), and the Framingham Cardiovascular Risk Model (a concordance index of 0.72)."
He acknowledged certain limitations of the study, including the fact that although the model was better than random chance in predicting the probability of SUI after abdominal sacral colpopexy and Burch urethropexy in the CARE population, the accuracy was lower. "This may have been because women in the OPUS data set underwent sling with vaginal surgery for prolapse, while those in CARE underwent a Burch procedure and abdominal surgery," he explained.
"It is also possible that abdominal sacral colpopexy places a different amount of risk of de novo stress urinary incontinence on individuals than does vaginal surgery for pelvic organ prolapse. Despite this, a concordance index of 0.62 suggests that the model is also a valuable adjunct for shared decision-making between the clinician and patients prior to abdominal sacral colpopexy with Burch urethropexy surgery."
He said that future studies should include populations of women undergoing minimally invasive colpopexy.
Dr. Jelovsek said he had no relevant financial disclosures.
AT THE AUGS ANNUAL MEETING
Major finding: Of the 12 original risk factors hypothesized to predict de novo SUI, 7 final risk factors were identified that when combined together accurately predicted de novo SUI in patients following pelvic prolapse surgery.
Data source: A study of 457 patients in the OPUS (Outcomes Following Vaginal Prolapse Repair and Midurethral Sling) trial.
Disclosures: Dr. Jelovsek said he had no relevant financial disclosures.
Remission reinduction with rituximab a possibility for ANCA-associated vasculitis
SAN DIEGO – Retreatment of granulomatosis with polyangiitis or microscopic polyangiitis with rituximab may be safe and effective in reinducing remission, a prospective trial has shown.
"The vast majority of patients with ANCA [antineutrophil cytoplasmic antibody]–associated vasculitis are able to achieve disease remission initially," Dr. Eli Miloslavsky said at the annual meeting of the American College of Rheumatology. "However, there’s a high rate of flare, as frequent as 55% over the first 3 years. Therefore, it’s critical to determine the best remission agent in relapsing disease."
Dr. Miloslavsky presented data from 17 patients in the RAVE (Rituximab in ANCA–Associated Vasculitis) trial who received two courses of rituximab (RTX) and were followed for an average of 301 days. Patients with a severe flare were eligible to receive an open-label course of RTX between 6 and 18 months (375 mg/m2 once a week for 4 weeks). Severe flare was defined as having a Birmingham Vasculitis Activity Score for Wegener’s Granulomatosis (BVAS/WG) of greater than 3 or one major BVAS/WG item. Outcomes were complete remission (no disease activity and being off of steroids), complete response (no disease activity and taking 10 g of prednisone or less), remission (no disease activity regardless of the prednisone dose), limited flare (BVAS/WG of 3 or less), and severe flare (BVAS/WG of greater than 3 or one major disease activity item). At baseline, 82% of patients who received two courses of rituximab were proteinase 3 positive and 88% had granulomatosis with polyangiitis as the clinical diagnosis.
Of the 17 patients, 11 (65%) had relapsing disease at study entry. After receiving a second course of RTX, 15 patients (88%) achieved remission in an average of 2 months, 12 (71%) had at least a complete response in an average of 5 months, and 8 (47%) reached complete remission in an average of 6 months, reported Dr. Miloslavsky of the department of rheumatology at Massachusetts General Hospital, Boston.
At the 12-month time point, 13 patients (76%) had achieved complete responses and 8 (47%) had reached complete remission.
Four flares occurred during the study. "They were all limited and the time to flare was approximately 8 months after receiving RTX," he said.
Three severe adverse events occurred, including one death (a patient with diffuse alveolar hemorrhage who did not improve and died 7 weeks after the initial flare), one case of metastatic colon cancer, and one case of severe sinusitis.
Dr. Miloslavsky acknowledged certain limitations of the study, including the small sample size, the lack of a comparison group, the lack of long-term follow-up, and the limited generalizability to myeloperoxidase-ANCA–positive patients.
The trial was funded by the Immune Tolerance Network, which is supported by the National Institute of Allergy and Infectious Diseases. Partial funding was also derived from Genentech and Biogen Idec.
SAN DIEGO – Retreatment of granulomatosis with polyangiitis or microscopic polyangiitis with rituximab may be safe and effective in reinducing remission, a prospective trial has shown.
"The vast majority of patients with ANCA [antineutrophil cytoplasmic antibody]–associated vasculitis are able to achieve disease remission initially," Dr. Eli Miloslavsky said at the annual meeting of the American College of Rheumatology. "However, there’s a high rate of flare, as frequent as 55% over the first 3 years. Therefore, it’s critical to determine the best remission agent in relapsing disease."
Dr. Miloslavsky presented data from 17 patients in the RAVE (Rituximab in ANCA–Associated Vasculitis) trial who received two courses of rituximab (RTX) and were followed for an average of 301 days. Patients with a severe flare were eligible to receive an open-label course of RTX between 6 and 18 months (375 mg/m2 once a week for 4 weeks). Severe flare was defined as having a Birmingham Vasculitis Activity Score for Wegener’s Granulomatosis (BVAS/WG) of greater than 3 or one major BVAS/WG item. Outcomes were complete remission (no disease activity and being off of steroids), complete response (no disease activity and taking 10 g of prednisone or less), remission (no disease activity regardless of the prednisone dose), limited flare (BVAS/WG of 3 or less), and severe flare (BVAS/WG of greater than 3 or one major disease activity item). At baseline, 82% of patients who received two courses of rituximab were proteinase 3 positive and 88% had granulomatosis with polyangiitis as the clinical diagnosis.
Of the 17 patients, 11 (65%) had relapsing disease at study entry. After receiving a second course of RTX, 15 patients (88%) achieved remission in an average of 2 months, 12 (71%) had at least a complete response in an average of 5 months, and 8 (47%) reached complete remission in an average of 6 months, reported Dr. Miloslavsky of the department of rheumatology at Massachusetts General Hospital, Boston.
At the 12-month time point, 13 patients (76%) had achieved complete responses and 8 (47%) had reached complete remission.
Four flares occurred during the study. "They were all limited and the time to flare was approximately 8 months after receiving RTX," he said.
Three severe adverse events occurred, including one death (a patient with diffuse alveolar hemorrhage who did not improve and died 7 weeks after the initial flare), one case of metastatic colon cancer, and one case of severe sinusitis.
Dr. Miloslavsky acknowledged certain limitations of the study, including the small sample size, the lack of a comparison group, the lack of long-term follow-up, and the limited generalizability to myeloperoxidase-ANCA–positive patients.
The trial was funded by the Immune Tolerance Network, which is supported by the National Institute of Allergy and Infectious Diseases. Partial funding was also derived from Genentech and Biogen Idec.
SAN DIEGO – Retreatment of granulomatosis with polyangiitis or microscopic polyangiitis with rituximab may be safe and effective in reinducing remission, a prospective trial has shown.
"The vast majority of patients with ANCA [antineutrophil cytoplasmic antibody]–associated vasculitis are able to achieve disease remission initially," Dr. Eli Miloslavsky said at the annual meeting of the American College of Rheumatology. "However, there’s a high rate of flare, as frequent as 55% over the first 3 years. Therefore, it’s critical to determine the best remission agent in relapsing disease."
Dr. Miloslavsky presented data from 17 patients in the RAVE (Rituximab in ANCA–Associated Vasculitis) trial who received two courses of rituximab (RTX) and were followed for an average of 301 days. Patients with a severe flare were eligible to receive an open-label course of RTX between 6 and 18 months (375 mg/m2 once a week for 4 weeks). Severe flare was defined as having a Birmingham Vasculitis Activity Score for Wegener’s Granulomatosis (BVAS/WG) of greater than 3 or one major BVAS/WG item. Outcomes were complete remission (no disease activity and being off of steroids), complete response (no disease activity and taking 10 g of prednisone or less), remission (no disease activity regardless of the prednisone dose), limited flare (BVAS/WG of 3 or less), and severe flare (BVAS/WG of greater than 3 or one major disease activity item). At baseline, 82% of patients who received two courses of rituximab were proteinase 3 positive and 88% had granulomatosis with polyangiitis as the clinical diagnosis.
Of the 17 patients, 11 (65%) had relapsing disease at study entry. After receiving a second course of RTX, 15 patients (88%) achieved remission in an average of 2 months, 12 (71%) had at least a complete response in an average of 5 months, and 8 (47%) reached complete remission in an average of 6 months, reported Dr. Miloslavsky of the department of rheumatology at Massachusetts General Hospital, Boston.
At the 12-month time point, 13 patients (76%) had achieved complete responses and 8 (47%) had reached complete remission.
Four flares occurred during the study. "They were all limited and the time to flare was approximately 8 months after receiving RTX," he said.
Three severe adverse events occurred, including one death (a patient with diffuse alveolar hemorrhage who did not improve and died 7 weeks after the initial flare), one case of metastatic colon cancer, and one case of severe sinusitis.
Dr. Miloslavsky acknowledged certain limitations of the study, including the small sample size, the lack of a comparison group, the lack of long-term follow-up, and the limited generalizability to myeloperoxidase-ANCA–positive patients.
The trial was funded by the Immune Tolerance Network, which is supported by the National Institute of Allergy and Infectious Diseases. Partial funding was also derived from Genentech and Biogen Idec.
AT THE ACR ANNUAL MEETING
Major finding: Six months after receiving a second course of rituximab, 47% of patients achieved complete remission.
Data source: A study of17 patients in the rituximab in ANCA-associated vasculitis trial who received two courses of rituximab and were followed for an average of 301 days.
Disclosures: The trial was funded by the Immune Tolerance Network, which is supported by the National Institute of Allergy and Infectious Diseases. Partial funding was also derived from Genentech and Biogen Idec.
RA triple therapy wins cost-effectiveness comparison vs. etanercept plus methotrexate
SAN DIEGO – In patients with early rheumatoid arthritis, treatment with a combination of disease-modifying antirheumatic drugs is more cost effective than an immediate or step-up approach using etanercept and methotrexate, a new analysis demonstrated.
"The benefits from all strategies were comparable, but biologics strategies were almost twice as expensive [as] triple strategies, producing incremental cost-effectiveness ratios higher than what most health care settings would find acceptable," Kaleb Michaud, Ph.D., said at the annual meeting of the American College of Rheumatology.
Dr. Michaud of the division of rheumatology and immunology at the University of Nebraska Medical Center, Omaha, and his associates analyzed data from 755 patients enrolled in the randomized, 2-year TEAR (Treatment of Early Aggressive RA) trial (Arthritis Rheum. 2012;64:2824-35), which compared the long-term effectiveness of using a triple therapy approach for treating RA with a strategy of using a etanercept (Enbrel) plus methotrexate. They studied four approaches: immediate triple therapy of methotrexate, sulfasalazine, and hydroxychloroquine; immediate treatment with etanercept and methotrexate; a step-up triple therapy; and a step-up etanercept therapy. The researchers incorporated estimates of annual discontinuation rates of triple therapy and etanercept of 22% and 10%, respectively, based on data from the National Data Bank for Rheumatic Diseases.
The analysis was limited to patients with RA duration of less than 3 years and those with active disease, defined as having four or more swollen joints with seropositivity and/or erosions. The mean disease duration was 3.6 months and the mean Disease Activity Score was 5.8. The researchers used a societal perspective and a Markov cohort model with 3% discounting to estimate quality-adjusted life year (QALY) measurements and the costs associated with therapy approaches in the TEAR trial.
Dr. Michaud, who is also codirector of the National Data Bank for Rheumatic Diseases, reported that the lifetime benefit of all four treatment approaches were comparable, within 0.06 QALYs of each other. However, the therapies that included etanercept were nearly twice as expensive because of the higher cost of the TNF inhibitor. Specifically, the costs were $152,400 in the immediate triple therapy group and $154,900 in the step-up triple therapy group, compared with $269,500 in the step-up etanercept group and $338,100 in the immediate etanercept group. The researchers determined that the lifetime incremental cost-effectiveness ratio of immediate etanercept to immediate triple therapy was $837,100/QALY.
"The immediate triple therapy dominated other arms at 1 and 2 years in this trial, and its results were most sensitive to the costs of etanercept and the reduction in triple therapy discontinuation rates," Dr. Michaud commented. "Our sensitivity analysis did not change our conclusions."
Dr. Michaud disclosed that the study was supported in part by a 2012 investigator award from the Rheumatology Research Foundation.
SAN DIEGO – In patients with early rheumatoid arthritis, treatment with a combination of disease-modifying antirheumatic drugs is more cost effective than an immediate or step-up approach using etanercept and methotrexate, a new analysis demonstrated.
"The benefits from all strategies were comparable, but biologics strategies were almost twice as expensive [as] triple strategies, producing incremental cost-effectiveness ratios higher than what most health care settings would find acceptable," Kaleb Michaud, Ph.D., said at the annual meeting of the American College of Rheumatology.
Dr. Michaud of the division of rheumatology and immunology at the University of Nebraska Medical Center, Omaha, and his associates analyzed data from 755 patients enrolled in the randomized, 2-year TEAR (Treatment of Early Aggressive RA) trial (Arthritis Rheum. 2012;64:2824-35), which compared the long-term effectiveness of using a triple therapy approach for treating RA with a strategy of using a etanercept (Enbrel) plus methotrexate. They studied four approaches: immediate triple therapy of methotrexate, sulfasalazine, and hydroxychloroquine; immediate treatment with etanercept and methotrexate; a step-up triple therapy; and a step-up etanercept therapy. The researchers incorporated estimates of annual discontinuation rates of triple therapy and etanercept of 22% and 10%, respectively, based on data from the National Data Bank for Rheumatic Diseases.
The analysis was limited to patients with RA duration of less than 3 years and those with active disease, defined as having four or more swollen joints with seropositivity and/or erosions. The mean disease duration was 3.6 months and the mean Disease Activity Score was 5.8. The researchers used a societal perspective and a Markov cohort model with 3% discounting to estimate quality-adjusted life year (QALY) measurements and the costs associated with therapy approaches in the TEAR trial.
Dr. Michaud, who is also codirector of the National Data Bank for Rheumatic Diseases, reported that the lifetime benefit of all four treatment approaches were comparable, within 0.06 QALYs of each other. However, the therapies that included etanercept were nearly twice as expensive because of the higher cost of the TNF inhibitor. Specifically, the costs were $152,400 in the immediate triple therapy group and $154,900 in the step-up triple therapy group, compared with $269,500 in the step-up etanercept group and $338,100 in the immediate etanercept group. The researchers determined that the lifetime incremental cost-effectiveness ratio of immediate etanercept to immediate triple therapy was $837,100/QALY.
"The immediate triple therapy dominated other arms at 1 and 2 years in this trial, and its results were most sensitive to the costs of etanercept and the reduction in triple therapy discontinuation rates," Dr. Michaud commented. "Our sensitivity analysis did not change our conclusions."
Dr. Michaud disclosed that the study was supported in part by a 2012 investigator award from the Rheumatology Research Foundation.
SAN DIEGO – In patients with early rheumatoid arthritis, treatment with a combination of disease-modifying antirheumatic drugs is more cost effective than an immediate or step-up approach using etanercept and methotrexate, a new analysis demonstrated.
"The benefits from all strategies were comparable, but biologics strategies were almost twice as expensive [as] triple strategies, producing incremental cost-effectiveness ratios higher than what most health care settings would find acceptable," Kaleb Michaud, Ph.D., said at the annual meeting of the American College of Rheumatology.
Dr. Michaud of the division of rheumatology and immunology at the University of Nebraska Medical Center, Omaha, and his associates analyzed data from 755 patients enrolled in the randomized, 2-year TEAR (Treatment of Early Aggressive RA) trial (Arthritis Rheum. 2012;64:2824-35), which compared the long-term effectiveness of using a triple therapy approach for treating RA with a strategy of using a etanercept (Enbrel) plus methotrexate. They studied four approaches: immediate triple therapy of methotrexate, sulfasalazine, and hydroxychloroquine; immediate treatment with etanercept and methotrexate; a step-up triple therapy; and a step-up etanercept therapy. The researchers incorporated estimates of annual discontinuation rates of triple therapy and etanercept of 22% and 10%, respectively, based on data from the National Data Bank for Rheumatic Diseases.
The analysis was limited to patients with RA duration of less than 3 years and those with active disease, defined as having four or more swollen joints with seropositivity and/or erosions. The mean disease duration was 3.6 months and the mean Disease Activity Score was 5.8. The researchers used a societal perspective and a Markov cohort model with 3% discounting to estimate quality-adjusted life year (QALY) measurements and the costs associated with therapy approaches in the TEAR trial.
Dr. Michaud, who is also codirector of the National Data Bank for Rheumatic Diseases, reported that the lifetime benefit of all four treatment approaches were comparable, within 0.06 QALYs of each other. However, the therapies that included etanercept were nearly twice as expensive because of the higher cost of the TNF inhibitor. Specifically, the costs were $152,400 in the immediate triple therapy group and $154,900 in the step-up triple therapy group, compared with $269,500 in the step-up etanercept group and $338,100 in the immediate etanercept group. The researchers determined that the lifetime incremental cost-effectiveness ratio of immediate etanercept to immediate triple therapy was $837,100/QALY.
"The immediate triple therapy dominated other arms at 1 and 2 years in this trial, and its results were most sensitive to the costs of etanercept and the reduction in triple therapy discontinuation rates," Dr. Michaud commented. "Our sensitivity analysis did not change our conclusions."
Dr. Michaud disclosed that the study was supported in part by a 2012 investigator award from the Rheumatology Research Foundation.
AT THE ACR ANNUAL MEETING
Major finding: The incremental cost-effectiveness ratio of immediate etanercept to immediate triple therapy was $837,100/quality-adjusted life year.
Data source: An analysis of 755 patients enrolled in the randomized, 2-year Treatment of Early Aggressive RA (TEAR) trial and patient-level data from the National Data Bank for Rheumatic Diseases.
Disclosures: Dr. Michaud disclosed that the study was supported in part by a 2012 investigator award from the Rheumatology Research Foundation.