User login
Doug Brunk is a San Diego-based award-winning reporter who began covering health care in 1991. Before joining the company, he wrote for the health sciences division of Columbia University and was an associate editor at Contemporary Long Term Care magazine when it won a Jesse H. Neal Award. His work has been syndicated by the Los Angeles Times and he is the author of two books related to the University of Kentucky Wildcats men's basketball program. Doug has a master’s degree in magazine journalism from the S.I. Newhouse School of Public Communications at Syracuse University. Follow him on Twitter @dougbrunk.
Low serum uric acid levels protect against progressions of renal disease
SAN DIEGO – Patients who achieve a serum uric acid level of less than 6 mg/dL based on current gout management guidelines demonstrated a 37% reduction in progression of renal disease, a large retrospective study showed.
"There are numerous studies showing that people with renal disease can develop hyperuricemia," Dr. Gerald D. Levy said during a press briefing at the annual meeting of the American College of Rheumatology. "Some of them will also develop gout. There are a few small studies showing that in humans, you can reverse hyperuricemia with urate lowering therapy and make an impact in renal disease. We wanted to see if this is true."
Dr. Levy of the division of rheumatology in the department of internal medicine at Kaiser Permanente Medical Group, Downey, Calif., was the lead investigators in a study of 111,992 Kaiser Permanente Southern California patients with a serum uric acid (SUA) level of 7 mg/dL or greater from Jan. 1, 2002, to Dec. 31, 2010. Patients with at least 12 months of health plan membership, including drug benefit prior to the index date, were studied. All patients had at least one SUA and glomerular filtration rate (GFR) level measurement in the 6-month period prior to the index date and at least one SUA and one GFR in the follow-up period following the index date. Primary outcome events were at least a 30% worsening of renal function, initiation of dialysis, having a GFR of less than 15 mL/min, and undergoing a kidney transplant.
Patients with a new diagnosis of cancer were excluded from the analysis, as were those with HIV, glomerulonephritis, and/or organ transplant other than a kidney transplant.
Dr. Levy reported results from 16,186 patients who were divided into three groups: those who were never treated with urate-lowering therapy (ULT; 11,192); those who were treated with ULT less than 80% of the time from the index date to the end of follow-up period (3,902); and those who were treated with ULT 80% of the time or more from the index date to the end of the follow-up period (1,092). Of the three treatment groups, those who were treated with ULT 80% of the time or more during the study tended to be older and have more comorbid conditions, compared with the other two groups. They also began their ULT therapy earlier.
Among all patients combined, factors significantly associated with renal disease progression included having diabetes (hazard ratio, 1.96), hypertension (HR, 1.50), heart failure (HR, 1.39), previous hospitalizations (HR, 1.33), and being female (HR, 1.49) and older (HR, 1.03). The researchers found that time on ULT was not significantly associated with a reduction in renal disease progression outcome events (HR, 1.27, among those on ULT less than 80% of the time during the study vs. HR, 1.08, among those on ULT 80% of the time or more during the study). However, patients who achieved an SUA level below 6 mg/dL – a treatment goal in the 2012 ACR guidelines for management of gout – demonstrated a 37% reduction in renal disease progression (HR, 0.63; P less than .0001).
Dr. Levy acknowledged certain limitations of the study, including its retrospective design and the fact that patients with stage 4 and 5 chronic kidney disease were not included. "This is an important area, because if we can delay the worsening of renal disease in these folks, perhaps we’re abetting dialysis, which is growing by leaps and bounds in this country," he said. "Each dialysis patient now costs about $80,000 per year to take care of. If we could push that back even for a few years it would have a tremendous impact."
Dr. Levy had no relevant financial conflicts to disclose.
SAN DIEGO – Patients who achieve a serum uric acid level of less than 6 mg/dL based on current gout management guidelines demonstrated a 37% reduction in progression of renal disease, a large retrospective study showed.
"There are numerous studies showing that people with renal disease can develop hyperuricemia," Dr. Gerald D. Levy said during a press briefing at the annual meeting of the American College of Rheumatology. "Some of them will also develop gout. There are a few small studies showing that in humans, you can reverse hyperuricemia with urate lowering therapy and make an impact in renal disease. We wanted to see if this is true."
Dr. Levy of the division of rheumatology in the department of internal medicine at Kaiser Permanente Medical Group, Downey, Calif., was the lead investigators in a study of 111,992 Kaiser Permanente Southern California patients with a serum uric acid (SUA) level of 7 mg/dL or greater from Jan. 1, 2002, to Dec. 31, 2010. Patients with at least 12 months of health plan membership, including drug benefit prior to the index date, were studied. All patients had at least one SUA and glomerular filtration rate (GFR) level measurement in the 6-month period prior to the index date and at least one SUA and one GFR in the follow-up period following the index date. Primary outcome events were at least a 30% worsening of renal function, initiation of dialysis, having a GFR of less than 15 mL/min, and undergoing a kidney transplant.
Patients with a new diagnosis of cancer were excluded from the analysis, as were those with HIV, glomerulonephritis, and/or organ transplant other than a kidney transplant.
Dr. Levy reported results from 16,186 patients who were divided into three groups: those who were never treated with urate-lowering therapy (ULT; 11,192); those who were treated with ULT less than 80% of the time from the index date to the end of follow-up period (3,902); and those who were treated with ULT 80% of the time or more from the index date to the end of the follow-up period (1,092). Of the three treatment groups, those who were treated with ULT 80% of the time or more during the study tended to be older and have more comorbid conditions, compared with the other two groups. They also began their ULT therapy earlier.
Among all patients combined, factors significantly associated with renal disease progression included having diabetes (hazard ratio, 1.96), hypertension (HR, 1.50), heart failure (HR, 1.39), previous hospitalizations (HR, 1.33), and being female (HR, 1.49) and older (HR, 1.03). The researchers found that time on ULT was not significantly associated with a reduction in renal disease progression outcome events (HR, 1.27, among those on ULT less than 80% of the time during the study vs. HR, 1.08, among those on ULT 80% of the time or more during the study). However, patients who achieved an SUA level below 6 mg/dL – a treatment goal in the 2012 ACR guidelines for management of gout – demonstrated a 37% reduction in renal disease progression (HR, 0.63; P less than .0001).
Dr. Levy acknowledged certain limitations of the study, including its retrospective design and the fact that patients with stage 4 and 5 chronic kidney disease were not included. "This is an important area, because if we can delay the worsening of renal disease in these folks, perhaps we’re abetting dialysis, which is growing by leaps and bounds in this country," he said. "Each dialysis patient now costs about $80,000 per year to take care of. If we could push that back even for a few years it would have a tremendous impact."
Dr. Levy had no relevant financial conflicts to disclose.
SAN DIEGO – Patients who achieve a serum uric acid level of less than 6 mg/dL based on current gout management guidelines demonstrated a 37% reduction in progression of renal disease, a large retrospective study showed.
"There are numerous studies showing that people with renal disease can develop hyperuricemia," Dr. Gerald D. Levy said during a press briefing at the annual meeting of the American College of Rheumatology. "Some of them will also develop gout. There are a few small studies showing that in humans, you can reverse hyperuricemia with urate lowering therapy and make an impact in renal disease. We wanted to see if this is true."
Dr. Levy of the division of rheumatology in the department of internal medicine at Kaiser Permanente Medical Group, Downey, Calif., was the lead investigators in a study of 111,992 Kaiser Permanente Southern California patients with a serum uric acid (SUA) level of 7 mg/dL or greater from Jan. 1, 2002, to Dec. 31, 2010. Patients with at least 12 months of health plan membership, including drug benefit prior to the index date, were studied. All patients had at least one SUA and glomerular filtration rate (GFR) level measurement in the 6-month period prior to the index date and at least one SUA and one GFR in the follow-up period following the index date. Primary outcome events were at least a 30% worsening of renal function, initiation of dialysis, having a GFR of less than 15 mL/min, and undergoing a kidney transplant.
Patients with a new diagnosis of cancer were excluded from the analysis, as were those with HIV, glomerulonephritis, and/or organ transplant other than a kidney transplant.
Dr. Levy reported results from 16,186 patients who were divided into three groups: those who were never treated with urate-lowering therapy (ULT; 11,192); those who were treated with ULT less than 80% of the time from the index date to the end of follow-up period (3,902); and those who were treated with ULT 80% of the time or more from the index date to the end of the follow-up period (1,092). Of the three treatment groups, those who were treated with ULT 80% of the time or more during the study tended to be older and have more comorbid conditions, compared with the other two groups. They also began their ULT therapy earlier.
Among all patients combined, factors significantly associated with renal disease progression included having diabetes (hazard ratio, 1.96), hypertension (HR, 1.50), heart failure (HR, 1.39), previous hospitalizations (HR, 1.33), and being female (HR, 1.49) and older (HR, 1.03). The researchers found that time on ULT was not significantly associated with a reduction in renal disease progression outcome events (HR, 1.27, among those on ULT less than 80% of the time during the study vs. HR, 1.08, among those on ULT 80% of the time or more during the study). However, patients who achieved an SUA level below 6 mg/dL – a treatment goal in the 2012 ACR guidelines for management of gout – demonstrated a 37% reduction in renal disease progression (HR, 0.63; P less than .0001).
Dr. Levy acknowledged certain limitations of the study, including its retrospective design and the fact that patients with stage 4 and 5 chronic kidney disease were not included. "This is an important area, because if we can delay the worsening of renal disease in these folks, perhaps we’re abetting dialysis, which is growing by leaps and bounds in this country," he said. "Each dialysis patient now costs about $80,000 per year to take care of. If we could push that back even for a few years it would have a tremendous impact."
Dr. Levy had no relevant financial conflicts to disclose.
AT THE ACR ANNUAL MEETING
Major finding: Patients who achieved a serum uric acid level below 6 mg/dL – a treatment goal in the 2012 ACR guidelines for management of gout – demonstrated a 37% reduction in renal disease progression (HR, 0.63; P less than .0001).
Data source: A study of 16,186 patients who were divided into three groups: those who were never treated with urate-lowering therapy (ULT; 11,192), those who were treated with ULT less than 80% of the time from the index date to the end of follow-up period (3,902), and those who were treated with ULT 80% of the time or more from the index date to the end of the follow-up period (1,092).
Disclosures: Dr. Levy said that he had no relevant financial conflicts to disclose.
Ceftaroline fosamil cleared over 80% of bacterial skin infections
DENVER – The clinical success of ceftaroline fosamil exceeded 80% in patients with acute bacterial skin and skin structure infections, including those with underlying comorbidities and those with infections caused by methicillin-resistant Staphylococcus aureus, a multicenter, industry-funded trial demonstrated.
"At one time, MRSA infections used to be a tertiary care phenomenon," Dr. Ananthakrishnan Ramani said in an interview during a poster session at the annual Interscience Conference on Antimicrobial Agents and Chemotherapy.
"Nowadays, MRSA has become a problem in the community as well. We have seen that it’s no longer bacteremia and sepsis, but also bacterial skin and skin structure infections. Ceftaroline fosamil is the only [Food and Drug Administration]–approved cephalosporin which has activity against MRSA. That’s why this medicine is fascinating to me as an internal medicine physician and as an infectious diseases doc, because we know cephalosporins very well," he said.
Developed by Oakland, Calif.–based Cerexa Inc., ceftaroline fosamil was approved in 2010 for the treatment of acute bacterial skin and skin structure infections (ABSSSIs) and community-acquired bacterial pneumonia. The purpose of the current trial, known as CAPTURE, was to retrospectively evaluate information on the routine clinical use of intravenous ceftaroline fosamil in the United States.
At the meeting, Dr. Ramani and his associates presented data limited to 1,030 patients with ABSSSI who were treated with the agent at one of 38 centers from September 2011 through February 2013. They focused their analysis on patient demographics, disease characteristics, antibiotics, pathogens, and outcomes.
Of the 1,030 patients with ABSSSI, mean age was 58 years, mean body mass index was 34 kg/m2, and 54% were male. Nearly two-thirds (73%) were overweight or obese and 46% had diabetes mellitus. The three most common types of infection were deep/extensive cellulitis (59%), major abscesses (19%), and infected ulcers (15%); the most common infections sites were the leg/thigh (47%) and foot (24%) and infected surgical wounds (13%).
Prior to administration of ceftaroline fosamil, 77% of the patients had received antibiotics, and concurrent antibiotics, mostly clindamycin, were used in 37% of cases, said Dr. Ramani, who practices in Catskill, N.Y.
The overall clinical success rate was 85%. It was slightly lower for patients with diabetes (83%) and slightly higher for obese patients (88%). When analyzed by infection type, ceftaroline fosamil therapy achieved clinical success in 86% of major abscesses, 85% of deep/excessive cellulitis cases, 79% of infected surgical wounds, and 79% of infected ulcers, he said.
The researchers also found that clinical success was achieved in 80% of patients with diabetes and MRSA and in 83% of obese patients with MRSA. In addition, clinical success in MRSA infections with ceftaroline fosamil monotherapy was 83%, while success with concurrent therapy was 76%.
Cerexa funded the trial. Dr. Ramani has received research funding from the company.
DENVER – The clinical success of ceftaroline fosamil exceeded 80% in patients with acute bacterial skin and skin structure infections, including those with underlying comorbidities and those with infections caused by methicillin-resistant Staphylococcus aureus, a multicenter, industry-funded trial demonstrated.
"At one time, MRSA infections used to be a tertiary care phenomenon," Dr. Ananthakrishnan Ramani said in an interview during a poster session at the annual Interscience Conference on Antimicrobial Agents and Chemotherapy.
"Nowadays, MRSA has become a problem in the community as well. We have seen that it’s no longer bacteremia and sepsis, but also bacterial skin and skin structure infections. Ceftaroline fosamil is the only [Food and Drug Administration]–approved cephalosporin which has activity against MRSA. That’s why this medicine is fascinating to me as an internal medicine physician and as an infectious diseases doc, because we know cephalosporins very well," he said.
Developed by Oakland, Calif.–based Cerexa Inc., ceftaroline fosamil was approved in 2010 for the treatment of acute bacterial skin and skin structure infections (ABSSSIs) and community-acquired bacterial pneumonia. The purpose of the current trial, known as CAPTURE, was to retrospectively evaluate information on the routine clinical use of intravenous ceftaroline fosamil in the United States.
At the meeting, Dr. Ramani and his associates presented data limited to 1,030 patients with ABSSSI who were treated with the agent at one of 38 centers from September 2011 through February 2013. They focused their analysis on patient demographics, disease characteristics, antibiotics, pathogens, and outcomes.
Of the 1,030 patients with ABSSSI, mean age was 58 years, mean body mass index was 34 kg/m2, and 54% were male. Nearly two-thirds (73%) were overweight or obese and 46% had diabetes mellitus. The three most common types of infection were deep/extensive cellulitis (59%), major abscesses (19%), and infected ulcers (15%); the most common infections sites were the leg/thigh (47%) and foot (24%) and infected surgical wounds (13%).
Prior to administration of ceftaroline fosamil, 77% of the patients had received antibiotics, and concurrent antibiotics, mostly clindamycin, were used in 37% of cases, said Dr. Ramani, who practices in Catskill, N.Y.
The overall clinical success rate was 85%. It was slightly lower for patients with diabetes (83%) and slightly higher for obese patients (88%). When analyzed by infection type, ceftaroline fosamil therapy achieved clinical success in 86% of major abscesses, 85% of deep/excessive cellulitis cases, 79% of infected surgical wounds, and 79% of infected ulcers, he said.
The researchers also found that clinical success was achieved in 80% of patients with diabetes and MRSA and in 83% of obese patients with MRSA. In addition, clinical success in MRSA infections with ceftaroline fosamil monotherapy was 83%, while success with concurrent therapy was 76%.
Cerexa funded the trial. Dr. Ramani has received research funding from the company.
DENVER – The clinical success of ceftaroline fosamil exceeded 80% in patients with acute bacterial skin and skin structure infections, including those with underlying comorbidities and those with infections caused by methicillin-resistant Staphylococcus aureus, a multicenter, industry-funded trial demonstrated.
"At one time, MRSA infections used to be a tertiary care phenomenon," Dr. Ananthakrishnan Ramani said in an interview during a poster session at the annual Interscience Conference on Antimicrobial Agents and Chemotherapy.
"Nowadays, MRSA has become a problem in the community as well. We have seen that it’s no longer bacteremia and sepsis, but also bacterial skin and skin structure infections. Ceftaroline fosamil is the only [Food and Drug Administration]–approved cephalosporin which has activity against MRSA. That’s why this medicine is fascinating to me as an internal medicine physician and as an infectious diseases doc, because we know cephalosporins very well," he said.
Developed by Oakland, Calif.–based Cerexa Inc., ceftaroline fosamil was approved in 2010 for the treatment of acute bacterial skin and skin structure infections (ABSSSIs) and community-acquired bacterial pneumonia. The purpose of the current trial, known as CAPTURE, was to retrospectively evaluate information on the routine clinical use of intravenous ceftaroline fosamil in the United States.
At the meeting, Dr. Ramani and his associates presented data limited to 1,030 patients with ABSSSI who were treated with the agent at one of 38 centers from September 2011 through February 2013. They focused their analysis on patient demographics, disease characteristics, antibiotics, pathogens, and outcomes.
Of the 1,030 patients with ABSSSI, mean age was 58 years, mean body mass index was 34 kg/m2, and 54% were male. Nearly two-thirds (73%) were overweight or obese and 46% had diabetes mellitus. The three most common types of infection were deep/extensive cellulitis (59%), major abscesses (19%), and infected ulcers (15%); the most common infections sites were the leg/thigh (47%) and foot (24%) and infected surgical wounds (13%).
Prior to administration of ceftaroline fosamil, 77% of the patients had received antibiotics, and concurrent antibiotics, mostly clindamycin, were used in 37% of cases, said Dr. Ramani, who practices in Catskill, N.Y.
The overall clinical success rate was 85%. It was slightly lower for patients with diabetes (83%) and slightly higher for obese patients (88%). When analyzed by infection type, ceftaroline fosamil therapy achieved clinical success in 86% of major abscesses, 85% of deep/excessive cellulitis cases, 79% of infected surgical wounds, and 79% of infected ulcers, he said.
The researchers also found that clinical success was achieved in 80% of patients with diabetes and MRSA and in 83% of obese patients with MRSA. In addition, clinical success in MRSA infections with ceftaroline fosamil monotherapy was 83%, while success with concurrent therapy was 76%.
Cerexa funded the trial. Dr. Ramani has received research funding from the company.
AT ICAAC 2013
Major finding: In patients with acute bacterial skin and skin structure infections who were treated with ceftaroline fosamil, the overall clinical success rate was 85%. Clinical success in methicillin-resistant Staphylococcus aureus infections was 83%.
Data source: Retrospective analysis of 1,030 patients with ABSSSI who were treated from September 2011 through February of 2013.
Disclosures: Cerexa funded the trial. Dr. Ramani has received research funding from the company.
Many gout patients not reaching treatment goals
SAN DIEGO – A high percentage of gout patients treated by rheumatologists do not meet the treatment goals established by the American College of Rheumatology, even after 6 months of higher-dose urate-lowering therapy, results from a national survey found.
"These findings suggest that even among rheumatologists, gout management may not be optimal and may be inadequately aggressive in the most severe patients," Dr. Max Hamburger said at the annual meeting of the American College of Rheumatology. "It seems that there is further study needed to determine the long-term impact of the new ACR guidelines."
In 2012 the ACR published updated guidelines for the management of gout and hyperuricemia (Arth. Care and Res. 2012;64:1431-46). The recommendations included a call for treat-to-target serum uric acid (sUA) of below 6 mg/dL at a minimum, and below 5 mg/dL in select patients. "The intent of this treat-to-target was to durably improve signs and symptoms of gout and also to address palpable and visible tophi," said Dr. Hamburger, a rheumatologist who practices in Melville, N.Y. "The extent to which current practice among rheumatologists aligns with the guidelines is unknown. The areas in which the guidelines may help improve gout treatment also remains to be determined."
He and his associates set out to assess symptoms, treatment, and outcomes among gout patients treated by rheumatologists in the United States and to identify gaps that might exist in current practice with the new ACR recommendations. They recruited a national sample of rheumatologists to report gout patient encounters prospectively during Jan. 15 to Feb. 22, 2013. Rheumatologists were eligible for the study if they were board certified or board eligible in rheumatology, if they spent at least 70% of their time on patient care, if they were in practice for at least 2 years, and if they saw at least four gout patients per month.
The researchers collected anonymous patient data, including demographics, history with the rheumatology practice, gout symptoms and severity, rheumatologist assessment of disease control, and gout medications and treatment changes at the time of each visit. They applied the ACR working case scenarios and grouped patients by increasing level of disease severity. Patients in the scenarios 1-3 group had intermittent symptoms and no tophi (mild disease); patients in the scenarios 4-6 group had intermittent symptoms and 1 tophus or more (moderate disease), and patients in the scenarios 7-9 group had chronic tophaceous gouty arthropathy (more severe disease). Higher-dose ULT was defined as greater than 300 mg/day of allopurinol or 80 mg or more per day of febuxostat (Uloric).
Dr. Hamburger reported results from 127 rheumatologists who received 2,380 valid patient encounter forms. Most of the patients (79%) were male, their mean age was 61 years, and 72% were seen by a rheumatologist for 6 months or longer. Based on ACR scenario groupings, 68% were in the scenarios 1-3 group, 4% were in the scenarios 4-6 group, and 28% were in the scenarios 7-9 group.
Most patients in the scenarios 1-3 group were judged by the rheumatologists to have controlled disease, compared with 91% of patients in the scenarios 4-6 group and 81% of patients in the scenarios 7-9 group. In addition, 14% of patients in the scenarios 1-3 group were on higher-dose ULT, compared with 28% in the scenarios 4-6 group and 40% in the scenarios 7-9 group. Nearly one-quarter of all patients (24%) were on higher-dose ULT.
Among patients on higher-dose ULT, 45% of those in the scenarios 1-3 group had an sUA greater than 6 mg/dL, compared with 53% in the scenarios 4-6 group and 61% in the scenarios 7-9 group. "Despite elevated sUA, 45% of encounters did not result in an increased ULT dose or treatment change at this visit," Dr. Hamburger said.
Even with 6 months or more at higher-dose ULT, only 55% of patients overall had an sUA at or below guideline recommendations of 6 mg/dL, including only 40% of patients in the scenarios 7-9 group. In addition, 16% of patients overall had an sUA between 6 and 6.8 mg/dL, despite being on higher-dose ULT for 6 months or longer.
Dr. Hamburger acknowledged certain limitations of the study, including the fact that "rheumatologist participation in this market research may be biased based on willingness to participate in online data collection over the reporting period," he said. In addition, "a varying number of encounter forms were provided by each participant and based on estimated patient volume. Not all participants reported on 100% of their patients during the reporting period."
Dr. Hamburger disclosed that he is a speaker for Savient Pharmaceuticals, which markets the gout drug pegloticase (Krystexxa), and Takeda Pharmaceuticals, which markets febuxostat and colchicine (Colcrys). Funding for the market research used in the study was provided by Savient.
SAN DIEGO – A high percentage of gout patients treated by rheumatologists do not meet the treatment goals established by the American College of Rheumatology, even after 6 months of higher-dose urate-lowering therapy, results from a national survey found.
"These findings suggest that even among rheumatologists, gout management may not be optimal and may be inadequately aggressive in the most severe patients," Dr. Max Hamburger said at the annual meeting of the American College of Rheumatology. "It seems that there is further study needed to determine the long-term impact of the new ACR guidelines."
In 2012 the ACR published updated guidelines for the management of gout and hyperuricemia (Arth. Care and Res. 2012;64:1431-46). The recommendations included a call for treat-to-target serum uric acid (sUA) of below 6 mg/dL at a minimum, and below 5 mg/dL in select patients. "The intent of this treat-to-target was to durably improve signs and symptoms of gout and also to address palpable and visible tophi," said Dr. Hamburger, a rheumatologist who practices in Melville, N.Y. "The extent to which current practice among rheumatologists aligns with the guidelines is unknown. The areas in which the guidelines may help improve gout treatment also remains to be determined."
He and his associates set out to assess symptoms, treatment, and outcomes among gout patients treated by rheumatologists in the United States and to identify gaps that might exist in current practice with the new ACR recommendations. They recruited a national sample of rheumatologists to report gout patient encounters prospectively during Jan. 15 to Feb. 22, 2013. Rheumatologists were eligible for the study if they were board certified or board eligible in rheumatology, if they spent at least 70% of their time on patient care, if they were in practice for at least 2 years, and if they saw at least four gout patients per month.
The researchers collected anonymous patient data, including demographics, history with the rheumatology practice, gout symptoms and severity, rheumatologist assessment of disease control, and gout medications and treatment changes at the time of each visit. They applied the ACR working case scenarios and grouped patients by increasing level of disease severity. Patients in the scenarios 1-3 group had intermittent symptoms and no tophi (mild disease); patients in the scenarios 4-6 group had intermittent symptoms and 1 tophus or more (moderate disease), and patients in the scenarios 7-9 group had chronic tophaceous gouty arthropathy (more severe disease). Higher-dose ULT was defined as greater than 300 mg/day of allopurinol or 80 mg or more per day of febuxostat (Uloric).
Dr. Hamburger reported results from 127 rheumatologists who received 2,380 valid patient encounter forms. Most of the patients (79%) were male, their mean age was 61 years, and 72% were seen by a rheumatologist for 6 months or longer. Based on ACR scenario groupings, 68% were in the scenarios 1-3 group, 4% were in the scenarios 4-6 group, and 28% were in the scenarios 7-9 group.
Most patients in the scenarios 1-3 group were judged by the rheumatologists to have controlled disease, compared with 91% of patients in the scenarios 4-6 group and 81% of patients in the scenarios 7-9 group. In addition, 14% of patients in the scenarios 1-3 group were on higher-dose ULT, compared with 28% in the scenarios 4-6 group and 40% in the scenarios 7-9 group. Nearly one-quarter of all patients (24%) were on higher-dose ULT.
Among patients on higher-dose ULT, 45% of those in the scenarios 1-3 group had an sUA greater than 6 mg/dL, compared with 53% in the scenarios 4-6 group and 61% in the scenarios 7-9 group. "Despite elevated sUA, 45% of encounters did not result in an increased ULT dose or treatment change at this visit," Dr. Hamburger said.
Even with 6 months or more at higher-dose ULT, only 55% of patients overall had an sUA at or below guideline recommendations of 6 mg/dL, including only 40% of patients in the scenarios 7-9 group. In addition, 16% of patients overall had an sUA between 6 and 6.8 mg/dL, despite being on higher-dose ULT for 6 months or longer.
Dr. Hamburger acknowledged certain limitations of the study, including the fact that "rheumatologist participation in this market research may be biased based on willingness to participate in online data collection over the reporting period," he said. In addition, "a varying number of encounter forms were provided by each participant and based on estimated patient volume. Not all participants reported on 100% of their patients during the reporting period."
Dr. Hamburger disclosed that he is a speaker for Savient Pharmaceuticals, which markets the gout drug pegloticase (Krystexxa), and Takeda Pharmaceuticals, which markets febuxostat and colchicine (Colcrys). Funding for the market research used in the study was provided by Savient.
SAN DIEGO – A high percentage of gout patients treated by rheumatologists do not meet the treatment goals established by the American College of Rheumatology, even after 6 months of higher-dose urate-lowering therapy, results from a national survey found.
"These findings suggest that even among rheumatologists, gout management may not be optimal and may be inadequately aggressive in the most severe patients," Dr. Max Hamburger said at the annual meeting of the American College of Rheumatology. "It seems that there is further study needed to determine the long-term impact of the new ACR guidelines."
In 2012 the ACR published updated guidelines for the management of gout and hyperuricemia (Arth. Care and Res. 2012;64:1431-46). The recommendations included a call for treat-to-target serum uric acid (sUA) of below 6 mg/dL at a minimum, and below 5 mg/dL in select patients. "The intent of this treat-to-target was to durably improve signs and symptoms of gout and also to address palpable and visible tophi," said Dr. Hamburger, a rheumatologist who practices in Melville, N.Y. "The extent to which current practice among rheumatologists aligns with the guidelines is unknown. The areas in which the guidelines may help improve gout treatment also remains to be determined."
He and his associates set out to assess symptoms, treatment, and outcomes among gout patients treated by rheumatologists in the United States and to identify gaps that might exist in current practice with the new ACR recommendations. They recruited a national sample of rheumatologists to report gout patient encounters prospectively during Jan. 15 to Feb. 22, 2013. Rheumatologists were eligible for the study if they were board certified or board eligible in rheumatology, if they spent at least 70% of their time on patient care, if they were in practice for at least 2 years, and if they saw at least four gout patients per month.
The researchers collected anonymous patient data, including demographics, history with the rheumatology practice, gout symptoms and severity, rheumatologist assessment of disease control, and gout medications and treatment changes at the time of each visit. They applied the ACR working case scenarios and grouped patients by increasing level of disease severity. Patients in the scenarios 1-3 group had intermittent symptoms and no tophi (mild disease); patients in the scenarios 4-6 group had intermittent symptoms and 1 tophus or more (moderate disease), and patients in the scenarios 7-9 group had chronic tophaceous gouty arthropathy (more severe disease). Higher-dose ULT was defined as greater than 300 mg/day of allopurinol or 80 mg or more per day of febuxostat (Uloric).
Dr. Hamburger reported results from 127 rheumatologists who received 2,380 valid patient encounter forms. Most of the patients (79%) were male, their mean age was 61 years, and 72% were seen by a rheumatologist for 6 months or longer. Based on ACR scenario groupings, 68% were in the scenarios 1-3 group, 4% were in the scenarios 4-6 group, and 28% were in the scenarios 7-9 group.
Most patients in the scenarios 1-3 group were judged by the rheumatologists to have controlled disease, compared with 91% of patients in the scenarios 4-6 group and 81% of patients in the scenarios 7-9 group. In addition, 14% of patients in the scenarios 1-3 group were on higher-dose ULT, compared with 28% in the scenarios 4-6 group and 40% in the scenarios 7-9 group. Nearly one-quarter of all patients (24%) were on higher-dose ULT.
Among patients on higher-dose ULT, 45% of those in the scenarios 1-3 group had an sUA greater than 6 mg/dL, compared with 53% in the scenarios 4-6 group and 61% in the scenarios 7-9 group. "Despite elevated sUA, 45% of encounters did not result in an increased ULT dose or treatment change at this visit," Dr. Hamburger said.
Even with 6 months or more at higher-dose ULT, only 55% of patients overall had an sUA at or below guideline recommendations of 6 mg/dL, including only 40% of patients in the scenarios 7-9 group. In addition, 16% of patients overall had an sUA between 6 and 6.8 mg/dL, despite being on higher-dose ULT for 6 months or longer.
Dr. Hamburger acknowledged certain limitations of the study, including the fact that "rheumatologist participation in this market research may be biased based on willingness to participate in online data collection over the reporting period," he said. In addition, "a varying number of encounter forms were provided by each participant and based on estimated patient volume. Not all participants reported on 100% of their patients during the reporting period."
Dr. Hamburger disclosed that he is a speaker for Savient Pharmaceuticals, which markets the gout drug pegloticase (Krystexxa), and Takeda Pharmaceuticals, which markets febuxostat and colchicine (Colcrys). Funding for the market research used in the study was provided by Savient.
AT THE ACR ANNUAL MEETING
Major finding: Even with 6 months or more at higher-dose urate-lowering therapy, only 55% of gout patients overall had a serum uric acid level at or below ACR guideline recommendations of 6.0 mg/dL, including only 40% of patients with the most severe disease.
Data source: A study of 127 rheumatologists who reported on encounters with 2,380 gout patients during Jan. 15 to Feb. 22, 2013.
Disclosures: Dr. Hamburger disclosed that he is a speaker for Savient Pharmaceuticals and Takeda Pharmaceuticals. Funding for the market research used in the study was provided by Savient.
Distribution of female pelvic medicine fellowships varied
LAS VEGAS – The geographic distribution of female pelvic medicine and reconstructive surgery fellowships approved by the Accreditation Council for Graduate Medical Education varies widely in the United States, results from an analysis demonstrated.
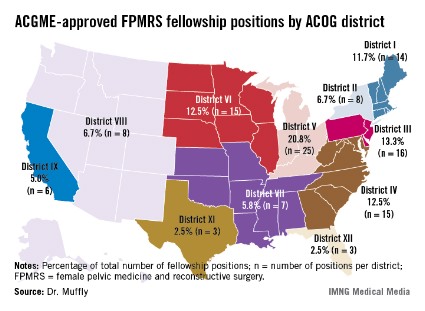
Such variation "may limit the choices for residents wishing to pursue further training in female pelvic medicine and reconstructive surgery," Dr. Tyler Muffly said in an interview during a poster session at the annual meeting of the American Urogynecologic Society. "It also means that patients in some areas are going to have to travel further to get to a referral center."
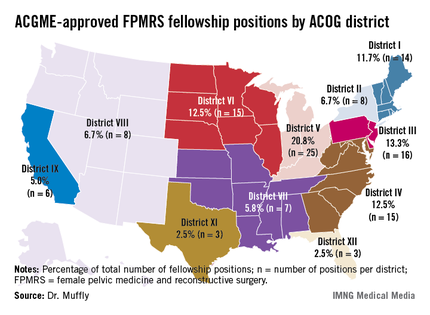
In an effort to determine the distribution of first-year female pelvic medicine and reconstructive surgery (FPMRS) fellowship positions according to population within states and within districts of the American Congress of Obstetrics and Gynecology, Dr. Muffly and his associates used data from the 2010 U.S. Census and from the number of 2012 female pelvic medicine and reconstructive surgery fellowship positions available through the match, which were obtained from the Accreditation Council for Graduate Medical Education (ACGME). They rounded off population data to the nearest 1,000 persons.
Dr. Muffly of the division of female pelvic medicine and reconstructive surgery (FPMRS) at the University of Colorado, Aurora, reported that there was a mean of 9.6 FPMRS fellowship positions per ACOG district and a mean of 2.3 FPMRS programs per ACOG district. More than two-thirds of fellowship programs were located east of the Mississippi River (67.5% vs. 32.5% west of that spot), with the greatest number clustered in ACOG District V, which consists of Michigan, Indiana, Ohio, and Kentucky (20.8%).
The researchers observed a significant difference between ACOG districts based on the number of FPMRS fellowship programs (P = .04) as well as a difference in total population between districts (P less than .0001). Specifically, Florida (ACOG District XII) had the highest proportion of women overall (P = .001) while California (ACOG District IX) had the highest proportion of postmenopausal women (P less than .001).
When Dr. Muffly and his associates analyzed the distribution of FPMRS fellowship positions within ACOG districts, a statistically significant difference was observed in the number of FPMRS fellowship positions per district (P = .03). For example, there were only 8 positions in ACOG District VIII (Arizona, Colorado, Hawaii, Idaho, Montana, Nevada, New Mexico, Oregon, Utah, Washington, and Wyoming), compared with 16 positions in ACOG District III (Delaware, New Jersey, and Pennsylvania).
"The findings suggest that future FPMRS fellowships might be started in the West or in high-density states like Florida or California where there are many postmenopausal women," Dr. Muffly concluded. "There are fellowships in all 11 ACOG districts. Physicians in the West or in the high plains states might have to look a little harder for a fellowship or a referral center for female pelvic medicine and reconstructive surgery."
Dr. Muffly said he had no relevant financial disclosures.
LAS VEGAS – The geographic distribution of female pelvic medicine and reconstructive surgery fellowships approved by the Accreditation Council for Graduate Medical Education varies widely in the United States, results from an analysis demonstrated.
Such variation "may limit the choices for residents wishing to pursue further training in female pelvic medicine and reconstructive surgery," Dr. Tyler Muffly said in an interview during a poster session at the annual meeting of the American Urogynecologic Society. "It also means that patients in some areas are going to have to travel further to get to a referral center."

In an effort to determine the distribution of first-year female pelvic medicine and reconstructive surgery (FPMRS) fellowship positions according to population within states and within districts of the American Congress of Obstetrics and Gynecology, Dr. Muffly and his associates used data from the 2010 U.S. Census and from the number of 2012 female pelvic medicine and reconstructive surgery fellowship positions available through the match, which were obtained from the Accreditation Council for Graduate Medical Education (ACGME). They rounded off population data to the nearest 1,000 persons.
Dr. Muffly of the division of female pelvic medicine and reconstructive surgery (FPMRS) at the University of Colorado, Aurora, reported that there was a mean of 9.6 FPMRS fellowship positions per ACOG district and a mean of 2.3 FPMRS programs per ACOG district. More than two-thirds of fellowship programs were located east of the Mississippi River (67.5% vs. 32.5% west of that spot), with the greatest number clustered in ACOG District V, which consists of Michigan, Indiana, Ohio, and Kentucky (20.8%).
The researchers observed a significant difference between ACOG districts based on the number of FPMRS fellowship programs (P = .04) as well as a difference in total population between districts (P less than .0001). Specifically, Florida (ACOG District XII) had the highest proportion of women overall (P = .001) while California (ACOG District IX) had the highest proportion of postmenopausal women (P less than .001).
When Dr. Muffly and his associates analyzed the distribution of FPMRS fellowship positions within ACOG districts, a statistically significant difference was observed in the number of FPMRS fellowship positions per district (P = .03). For example, there were only 8 positions in ACOG District VIII (Arizona, Colorado, Hawaii, Idaho, Montana, Nevada, New Mexico, Oregon, Utah, Washington, and Wyoming), compared with 16 positions in ACOG District III (Delaware, New Jersey, and Pennsylvania).
"The findings suggest that future FPMRS fellowships might be started in the West or in high-density states like Florida or California where there are many postmenopausal women," Dr. Muffly concluded. "There are fellowships in all 11 ACOG districts. Physicians in the West or in the high plains states might have to look a little harder for a fellowship or a referral center for female pelvic medicine and reconstructive surgery."
Dr. Muffly said he had no relevant financial disclosures.
LAS VEGAS – The geographic distribution of female pelvic medicine and reconstructive surgery fellowships approved by the Accreditation Council for Graduate Medical Education varies widely in the United States, results from an analysis demonstrated.
Such variation "may limit the choices for residents wishing to pursue further training in female pelvic medicine and reconstructive surgery," Dr. Tyler Muffly said in an interview during a poster session at the annual meeting of the American Urogynecologic Society. "It also means that patients in some areas are going to have to travel further to get to a referral center."

In an effort to determine the distribution of first-year female pelvic medicine and reconstructive surgery (FPMRS) fellowship positions according to population within states and within districts of the American Congress of Obstetrics and Gynecology, Dr. Muffly and his associates used data from the 2010 U.S. Census and from the number of 2012 female pelvic medicine and reconstructive surgery fellowship positions available through the match, which were obtained from the Accreditation Council for Graduate Medical Education (ACGME). They rounded off population data to the nearest 1,000 persons.
Dr. Muffly of the division of female pelvic medicine and reconstructive surgery (FPMRS) at the University of Colorado, Aurora, reported that there was a mean of 9.6 FPMRS fellowship positions per ACOG district and a mean of 2.3 FPMRS programs per ACOG district. More than two-thirds of fellowship programs were located east of the Mississippi River (67.5% vs. 32.5% west of that spot), with the greatest number clustered in ACOG District V, which consists of Michigan, Indiana, Ohio, and Kentucky (20.8%).
The researchers observed a significant difference between ACOG districts based on the number of FPMRS fellowship programs (P = .04) as well as a difference in total population between districts (P less than .0001). Specifically, Florida (ACOG District XII) had the highest proportion of women overall (P = .001) while California (ACOG District IX) had the highest proportion of postmenopausal women (P less than .001).
When Dr. Muffly and his associates analyzed the distribution of FPMRS fellowship positions within ACOG districts, a statistically significant difference was observed in the number of FPMRS fellowship positions per district (P = .03). For example, there were only 8 positions in ACOG District VIII (Arizona, Colorado, Hawaii, Idaho, Montana, Nevada, New Mexico, Oregon, Utah, Washington, and Wyoming), compared with 16 positions in ACOG District III (Delaware, New Jersey, and Pennsylvania).
"The findings suggest that future FPMRS fellowships might be started in the West or in high-density states like Florida or California where there are many postmenopausal women," Dr. Muffly concluded. "There are fellowships in all 11 ACOG districts. Physicians in the West or in the high plains states might have to look a little harder for a fellowship or a referral center for female pelvic medicine and reconstructive surgery."
Dr. Muffly said he had no relevant financial disclosures.
AT THE AUGS ANNUAL MEETING
Major finding: More than two-thirds of fellowship programs in female pelvic medicine and reconstructive surgery were located east of the Mississippi River (67.5% vs. 32.5% west of that spot), with the greatest number clustered in American Congress of Obstetrics and Gynecology District V, which consists of Michigan, Indiana, Ohio, and Kentucky (20.8%).
Data source: An analysis of data from the 2010 U.S. Census and from the number of 2012 female pelvic medicine and reconstructive surgery fellowship positions available through the match.
Disclosures: Dr. Muffly said he had no relevant financial disclosures.
Bracing lessened patellofemoral pain in OA
SAN DIEGO – Patients with osteoarthritis of the knee who wore a patellofemoral brace for 6 weeks experienced a significant reduction in pain and in bone marrow lesion volumes in the patellofemoral region, compared with those who did not wear the brace, a multicenter trial showed.
"There’s a pressing need for nonsurgical intervention for knee osteoarthritis," Dr. David T. Felson said in a press briefing at the annual meeting of the American College of Rheumatology.
"There are no currently approved structure-modifying treatments. This has been a focus of studies that have been testing modifying treatments on hyaline cartilage, which changes slowly, necessitating expensive, long-term, large trials. Even so, mechanopathology such as that caused by malalignment or meniscal tears may make it impossible to protect cartilage in existing OA," he noted.
Dr. Felson, director of the Research in Osteoarthritis in Manchester group at the University of Manchester (England) and professor of medicine at Boston University, went on to note that bone marrow lesions (BMLs) "have been well shown to predict later cartilage loss in that location and correlate with pain and its severity. Recently, we showed that BMLs fluctuate in volume in as little as 6 weeks. Further, one small trial has suggested that zoledronic acid may shrink BMLs and reduce knee pain. That leads us to suggest that BMLs may be a viable treatment target in OA."
The patellofemoral joint "is a major source of knee pain in OA, and there has been little study of the efficacy of PF braces," he continued. "In a body mechanics study, PF bracing has been shown to increase the contact area of the PF joint. It may thereby lower the contact stress and shrink BMLs."
He and his associates set out to determine whether bracing would improve pain and lessen the volume of BMLs in patients with knee OA. They enrolled 126 patients with a mean age of 55 years whose knee pain had been present daily for the previous 3 months. Half of the patients wore a soft neoprene PF brace for a mean of 7.3 hours per day, while the other half did not.
All study participants "had to have at least a score of 40 on a 0-100 mm visual analogue scale (VAS) for nominated aggravating activity likely to originate in the PF joint," Dr. Felson said. "They had to have pain with activities such as stair climbing, kneeling, prolonged sitting or squatting, [and] they also had to have a radiographic KL [Kellgren-Lawrence] score of grade 2 or 3 in the PF joint. That score had to be greater than the KL score for the tibiofemoral compartments. They also had to undergo a clinical exam by a trained physiotherapist to confirm PF joint tenderness."
The researchers performed contrast-enhanced knee MRIs at baseline and at 6 weeks. The primary symptom outcome measure was VAS pain during the patients’ nominated aggravating activity, while the primary structural outcome measure was BML volume in the PF joint as assessed on sagittal precontrast view.
At 6 weeks, Dr. Felson reported that patients in the no-brace group had a mean reduction in their VAS pain of 1.3, compared with a reduction of 18.2 in the braced group, a mean between-group difference of 16.9 that reached statistical significance (P less than .001).
As for PF BML volume, patients in the no-brace group showed a slight increase in volume (mean, 102.7 mm3), while the braced group showed a significant decrease in PF BML volume (mean, –554.9 mm3), for a mean between-group difference of 657.6 mm3 that reached statistical significance (P = .02). "That represents about a 25% decrease in volume," Dr. Felson said.
No differences were observed between the two groups in terms of tibiofemoral BML volume or in synovitis volume.
Dr. Felson acknowledged certain limitations of the study, including its 6-week design. "OA is a long-term chronic disease," he said. "We don’t know what relevance our findings have for longer-term structure changes of the knee."
The researchers stated that they had no relevant financial conflicts to disclose.
SAN DIEGO – Patients with osteoarthritis of the knee who wore a patellofemoral brace for 6 weeks experienced a significant reduction in pain and in bone marrow lesion volumes in the patellofemoral region, compared with those who did not wear the brace, a multicenter trial showed.
"There’s a pressing need for nonsurgical intervention for knee osteoarthritis," Dr. David T. Felson said in a press briefing at the annual meeting of the American College of Rheumatology.
"There are no currently approved structure-modifying treatments. This has been a focus of studies that have been testing modifying treatments on hyaline cartilage, which changes slowly, necessitating expensive, long-term, large trials. Even so, mechanopathology such as that caused by malalignment or meniscal tears may make it impossible to protect cartilage in existing OA," he noted.
Dr. Felson, director of the Research in Osteoarthritis in Manchester group at the University of Manchester (England) and professor of medicine at Boston University, went on to note that bone marrow lesions (BMLs) "have been well shown to predict later cartilage loss in that location and correlate with pain and its severity. Recently, we showed that BMLs fluctuate in volume in as little as 6 weeks. Further, one small trial has suggested that zoledronic acid may shrink BMLs and reduce knee pain. That leads us to suggest that BMLs may be a viable treatment target in OA."
The patellofemoral joint "is a major source of knee pain in OA, and there has been little study of the efficacy of PF braces," he continued. "In a body mechanics study, PF bracing has been shown to increase the contact area of the PF joint. It may thereby lower the contact stress and shrink BMLs."
He and his associates set out to determine whether bracing would improve pain and lessen the volume of BMLs in patients with knee OA. They enrolled 126 patients with a mean age of 55 years whose knee pain had been present daily for the previous 3 months. Half of the patients wore a soft neoprene PF brace for a mean of 7.3 hours per day, while the other half did not.
All study participants "had to have at least a score of 40 on a 0-100 mm visual analogue scale (VAS) for nominated aggravating activity likely to originate in the PF joint," Dr. Felson said. "They had to have pain with activities such as stair climbing, kneeling, prolonged sitting or squatting, [and] they also had to have a radiographic KL [Kellgren-Lawrence] score of grade 2 or 3 in the PF joint. That score had to be greater than the KL score for the tibiofemoral compartments. They also had to undergo a clinical exam by a trained physiotherapist to confirm PF joint tenderness."
The researchers performed contrast-enhanced knee MRIs at baseline and at 6 weeks. The primary symptom outcome measure was VAS pain during the patients’ nominated aggravating activity, while the primary structural outcome measure was BML volume in the PF joint as assessed on sagittal precontrast view.
At 6 weeks, Dr. Felson reported that patients in the no-brace group had a mean reduction in their VAS pain of 1.3, compared with a reduction of 18.2 in the braced group, a mean between-group difference of 16.9 that reached statistical significance (P less than .001).
As for PF BML volume, patients in the no-brace group showed a slight increase in volume (mean, 102.7 mm3), while the braced group showed a significant decrease in PF BML volume (mean, –554.9 mm3), for a mean between-group difference of 657.6 mm3 that reached statistical significance (P = .02). "That represents about a 25% decrease in volume," Dr. Felson said.
No differences were observed between the two groups in terms of tibiofemoral BML volume or in synovitis volume.
Dr. Felson acknowledged certain limitations of the study, including its 6-week design. "OA is a long-term chronic disease," he said. "We don’t know what relevance our findings have for longer-term structure changes of the knee."
The researchers stated that they had no relevant financial conflicts to disclose.
SAN DIEGO – Patients with osteoarthritis of the knee who wore a patellofemoral brace for 6 weeks experienced a significant reduction in pain and in bone marrow lesion volumes in the patellofemoral region, compared with those who did not wear the brace, a multicenter trial showed.
"There’s a pressing need for nonsurgical intervention for knee osteoarthritis," Dr. David T. Felson said in a press briefing at the annual meeting of the American College of Rheumatology.
"There are no currently approved structure-modifying treatments. This has been a focus of studies that have been testing modifying treatments on hyaline cartilage, which changes slowly, necessitating expensive, long-term, large trials. Even so, mechanopathology such as that caused by malalignment or meniscal tears may make it impossible to protect cartilage in existing OA," he noted.
Dr. Felson, director of the Research in Osteoarthritis in Manchester group at the University of Manchester (England) and professor of medicine at Boston University, went on to note that bone marrow lesions (BMLs) "have been well shown to predict later cartilage loss in that location and correlate with pain and its severity. Recently, we showed that BMLs fluctuate in volume in as little as 6 weeks. Further, one small trial has suggested that zoledronic acid may shrink BMLs and reduce knee pain. That leads us to suggest that BMLs may be a viable treatment target in OA."
The patellofemoral joint "is a major source of knee pain in OA, and there has been little study of the efficacy of PF braces," he continued. "In a body mechanics study, PF bracing has been shown to increase the contact area of the PF joint. It may thereby lower the contact stress and shrink BMLs."
He and his associates set out to determine whether bracing would improve pain and lessen the volume of BMLs in patients with knee OA. They enrolled 126 patients with a mean age of 55 years whose knee pain had been present daily for the previous 3 months. Half of the patients wore a soft neoprene PF brace for a mean of 7.3 hours per day, while the other half did not.
All study participants "had to have at least a score of 40 on a 0-100 mm visual analogue scale (VAS) for nominated aggravating activity likely to originate in the PF joint," Dr. Felson said. "They had to have pain with activities such as stair climbing, kneeling, prolonged sitting or squatting, [and] they also had to have a radiographic KL [Kellgren-Lawrence] score of grade 2 or 3 in the PF joint. That score had to be greater than the KL score for the tibiofemoral compartments. They also had to undergo a clinical exam by a trained physiotherapist to confirm PF joint tenderness."
The researchers performed contrast-enhanced knee MRIs at baseline and at 6 weeks. The primary symptom outcome measure was VAS pain during the patients’ nominated aggravating activity, while the primary structural outcome measure was BML volume in the PF joint as assessed on sagittal precontrast view.
At 6 weeks, Dr. Felson reported that patients in the no-brace group had a mean reduction in their VAS pain of 1.3, compared with a reduction of 18.2 in the braced group, a mean between-group difference of 16.9 that reached statistical significance (P less than .001).
As for PF BML volume, patients in the no-brace group showed a slight increase in volume (mean, 102.7 mm3), while the braced group showed a significant decrease in PF BML volume (mean, –554.9 mm3), for a mean between-group difference of 657.6 mm3 that reached statistical significance (P = .02). "That represents about a 25% decrease in volume," Dr. Felson said.
No differences were observed between the two groups in terms of tibiofemoral BML volume or in synovitis volume.
Dr. Felson acknowledged certain limitations of the study, including its 6-week design. "OA is a long-term chronic disease," he said. "We don’t know what relevance our findings have for longer-term structure changes of the knee."
The researchers stated that they had no relevant financial conflicts to disclose.
AT THE ACR ANNUAL MEETING
Major finding: Patients who wore a patellofemoral brace over the course of 6 weeks had a significant reduction in patellofemoral pain as measured by a visual analogue scale compared with those who did not wear a brace (reductions of 18.2 and 1.3, respectively; P less than .001).
Data source: 126 patients with a mean age of 55 years who had knee pain present daily for the previous 3 months. Half of the patients wore a soft neoprene PF brace for a mean of 7.3 hours per day, while the other half did not.
Disclosures: The researchers had no relevant financial conflicts to disclose.
Surgical treatments for failed midurethral sling compared
LAS VEGAS – In a study of patients who experienced a failed midurethral sling, urethral bulking injection was associated with a greater than threefold increased risk of failure compared with a repeat midurethral sling procedure, a retrospective analysis showed.
In addition, the diagnosis of intrinsic sphincter deficiency conferred a greater than fourfold risk of failure compared with patients without the diagnosis, regardless of which procedure was performed.
Those are findings from the largest cohort study to date evaluating failure of midurethral sling (MUS), and the only one to include both repeat MUS procedures and urethral bulking injections.
"This study provides important baseline data for surgeons when faced with MUS failure," Dr. Anthony Gaddi said at the annual meeting of the American Urogynecologic Society. "Prospective, randomized data with validated subjective and objective outcomes is warranted."
In an effort to compare the efficacy and safety of a repeat MUS procedure with urethral bulking injection after failed primary MUS, Dr. Gaddi and his associates performed an electronic chart review of patients from the Southern California Permanente Medical Group who underwent MUS for stress urinary incontinence (SUI) between 2008 and 2011.
The primary outcome was a measure of subjective failure, defined as a complaint of SUI, or objective failure, defined as documentation of a positive cough stress test, urodynamic stress incontinence, or reoperation for SUI, said Dr. Gaddi of the department of obstetrics and gynecology at the University of California, Irvine. Secondary outcomes included perioperative complications and adverse events.
For the 7,412 MUS procedures performed between 2008 and 2011, there were 165 repeat procedures for sling failure. Of these, 98 were repeat MUS procedures and 67 were urethral bulking injections. The mean age of patients was 58 years, their mean body mass index was 29.3 kg/m2, 65% were menopausal, and 59% were white.
Dr. Gaddi reported that there were 11 failures in the MUS group (11.2%), compared with 26 failures in the bulking group (38.8%), a difference that reached significance (P less than .01).
In multivariable logistic regression analysis, patients who underwent urethral bulking injections experienced a 3.7-fold increased risk of failure compared with those in the repeat MUS group. In addition, patients with a preoperative diagnosis of intrinsic sphincter deficiency experienced a 4.45-fold higher risk of failure compared with those who had no such deficiency, regardless of which procedure was performed.
Perioperative complications were similar between the two groups, "suggesting that both are safe options in this cohort," Dr. Gaddi said.
He acknowledged certain limitations of the study, including its retrospective design, and "difficulty standardizing our definition of failure. The low number of complications among our repeat procedures limits conclusions that can be made about safety."
Dr. Gaddi said that he had no relevant financial conflicts to disclose.
LAS VEGAS – In a study of patients who experienced a failed midurethral sling, urethral bulking injection was associated with a greater than threefold increased risk of failure compared with a repeat midurethral sling procedure, a retrospective analysis showed.
In addition, the diagnosis of intrinsic sphincter deficiency conferred a greater than fourfold risk of failure compared with patients without the diagnosis, regardless of which procedure was performed.
Those are findings from the largest cohort study to date evaluating failure of midurethral sling (MUS), and the only one to include both repeat MUS procedures and urethral bulking injections.
"This study provides important baseline data for surgeons when faced with MUS failure," Dr. Anthony Gaddi said at the annual meeting of the American Urogynecologic Society. "Prospective, randomized data with validated subjective and objective outcomes is warranted."
In an effort to compare the efficacy and safety of a repeat MUS procedure with urethral bulking injection after failed primary MUS, Dr. Gaddi and his associates performed an electronic chart review of patients from the Southern California Permanente Medical Group who underwent MUS for stress urinary incontinence (SUI) between 2008 and 2011.
The primary outcome was a measure of subjective failure, defined as a complaint of SUI, or objective failure, defined as documentation of a positive cough stress test, urodynamic stress incontinence, or reoperation for SUI, said Dr. Gaddi of the department of obstetrics and gynecology at the University of California, Irvine. Secondary outcomes included perioperative complications and adverse events.
For the 7,412 MUS procedures performed between 2008 and 2011, there were 165 repeat procedures for sling failure. Of these, 98 were repeat MUS procedures and 67 were urethral bulking injections. The mean age of patients was 58 years, their mean body mass index was 29.3 kg/m2, 65% were menopausal, and 59% were white.
Dr. Gaddi reported that there were 11 failures in the MUS group (11.2%), compared with 26 failures in the bulking group (38.8%), a difference that reached significance (P less than .01).
In multivariable logistic regression analysis, patients who underwent urethral bulking injections experienced a 3.7-fold increased risk of failure compared with those in the repeat MUS group. In addition, patients with a preoperative diagnosis of intrinsic sphincter deficiency experienced a 4.45-fold higher risk of failure compared with those who had no such deficiency, regardless of which procedure was performed.
Perioperative complications were similar between the two groups, "suggesting that both are safe options in this cohort," Dr. Gaddi said.
He acknowledged certain limitations of the study, including its retrospective design, and "difficulty standardizing our definition of failure. The low number of complications among our repeat procedures limits conclusions that can be made about safety."
Dr. Gaddi said that he had no relevant financial conflicts to disclose.
LAS VEGAS – In a study of patients who experienced a failed midurethral sling, urethral bulking injection was associated with a greater than threefold increased risk of failure compared with a repeat midurethral sling procedure, a retrospective analysis showed.
In addition, the diagnosis of intrinsic sphincter deficiency conferred a greater than fourfold risk of failure compared with patients without the diagnosis, regardless of which procedure was performed.
Those are findings from the largest cohort study to date evaluating failure of midurethral sling (MUS), and the only one to include both repeat MUS procedures and urethral bulking injections.
"This study provides important baseline data for surgeons when faced with MUS failure," Dr. Anthony Gaddi said at the annual meeting of the American Urogynecologic Society. "Prospective, randomized data with validated subjective and objective outcomes is warranted."
In an effort to compare the efficacy and safety of a repeat MUS procedure with urethral bulking injection after failed primary MUS, Dr. Gaddi and his associates performed an electronic chart review of patients from the Southern California Permanente Medical Group who underwent MUS for stress urinary incontinence (SUI) between 2008 and 2011.
The primary outcome was a measure of subjective failure, defined as a complaint of SUI, or objective failure, defined as documentation of a positive cough stress test, urodynamic stress incontinence, or reoperation for SUI, said Dr. Gaddi of the department of obstetrics and gynecology at the University of California, Irvine. Secondary outcomes included perioperative complications and adverse events.
For the 7,412 MUS procedures performed between 2008 and 2011, there were 165 repeat procedures for sling failure. Of these, 98 were repeat MUS procedures and 67 were urethral bulking injections. The mean age of patients was 58 years, their mean body mass index was 29.3 kg/m2, 65% were menopausal, and 59% were white.
Dr. Gaddi reported that there were 11 failures in the MUS group (11.2%), compared with 26 failures in the bulking group (38.8%), a difference that reached significance (P less than .01).
In multivariable logistic regression analysis, patients who underwent urethral bulking injections experienced a 3.7-fold increased risk of failure compared with those in the repeat MUS group. In addition, patients with a preoperative diagnosis of intrinsic sphincter deficiency experienced a 4.45-fold higher risk of failure compared with those who had no such deficiency, regardless of which procedure was performed.
Perioperative complications were similar between the two groups, "suggesting that both are safe options in this cohort," Dr. Gaddi said.
He acknowledged certain limitations of the study, including its retrospective design, and "difficulty standardizing our definition of failure. The low number of complications among our repeat procedures limits conclusions that can be made about safety."
Dr. Gaddi said that he had no relevant financial conflicts to disclose.
AT THE AUGS ANNUAL MEETING
Major finding: In multivariable logistic regression analysis, patients who underwent urethral bulking injections for a failed midurethral sling experienced a 3.7-fold increased risk of failure, compared with those who underwent a repeat midurethral sling procedure.
Data source: A single-center study of 165 repeat procedures for midurethral sling failure performed between 2008 and 2011.
Disclosures: Dr. Gaddi said that he had no relevant financial conflicts to disclose.
Regular exercise added to days of perfect health in OA patients
SAN DIEGO – Osteoarthritis patients who meet federal guidelines for exercise have more days of perfect health per year than do their sedentary counterparts, results from a novel study suggest.
"Our findings support interventions that help older adults to increase their physical activity level, even if guidelines are not fully met," Dr. Kai Sun said during a press briefing at the annual meeting of the American College of Rheumatology. "Increased physical activity could essentially translate into better quality of life and increased time spent in good health. We expect that this benefit would translate into lower overall health care costs."
Osteoarthritis is "a major cause of disability, and its prevalence is on the rise due to the aging population and the obesity epidemic. Inactivity is also a major problem in the United States. This is a significant economic burden as well because of the disability and the many health issues associated with inactivity as well as lost productivity and diminished quality of life," noted Dr. Sun, a medical resident and research trainee at Northwestern Feinberg School of Medicine, Chicago.
The study involved analysis of data from the National Institutes of Health–funded Osteoarthritis Initiative (OAI) to gather information on the physical activity levels of more than 4,700 adults, most of whom were aged older than 65 years and more than 40% of whom had a body mass index over 30 kg/m2, with or at risk for knee osteoarthritis.
The researchers set out to determine whether meeting the 2008 physical activity guidelines from the Department of Health and Human Services would translate into better overall quality of life and whether interventions to improve physical activity level correlate to better quality-adjusted life years (QALYs).The HHS guidelines recommend 150 minutes of moderate to vigorous activity per week performed in sessions lasting at least 10 minutes each. "An example of moderate activity would be walking briskly as if you were late to an appointment," Dr. Sun said.
The researchers used accelerometers to measure the physical activity level in 1,794 OAI study participants over the course of 1 week and placed them into one of three groups: 235 who met the HHS exercise guidelines of 150 minutes of moderate to vigorous activity, 763 who were "insufficiently active" (engaging in some but fewer than 150 minutes of moderate to vigorous exercise per week), and 796 who were inactive (engaging in no moderate to vigorous exercise per week). Health-related utility scores used to calculate QALYs were measured at the beginning of the study and 2 years later.
Overall, QALYs were significantly better with increasing level of physical activity, Dr. Sun reported. "This was a graded relationship, meaning that [people] in the middle group who had some [level of exercise] but not enough to meet guidelines had significantly better overall quality of life compared with the inactive group," she said. Specifically, after adjustment for socioeconomic and health factors over the 2-year period, people who met the HHS exercise guidelines had QALYs that were 0.11 higher compared with those who were inactive. At the same time, those in the insufficiently active group had QALYs that were 0.058 higher compared with those who were inactive.
"The most active group experienced the most benefit," Dr. Sun said. "The improvement was meaningful and translated into roughly an additional 10-20 days of perfect health in a year. We estimate that if an intervention could move someone out of the inactive group and cost less than $1,450 per person per year, that intervention would be considered cost-effective."
The researchers had no relevant financial conflicts to disclose.
SAN DIEGO – Osteoarthritis patients who meet federal guidelines for exercise have more days of perfect health per year than do their sedentary counterparts, results from a novel study suggest.
"Our findings support interventions that help older adults to increase their physical activity level, even if guidelines are not fully met," Dr. Kai Sun said during a press briefing at the annual meeting of the American College of Rheumatology. "Increased physical activity could essentially translate into better quality of life and increased time spent in good health. We expect that this benefit would translate into lower overall health care costs."
Osteoarthritis is "a major cause of disability, and its prevalence is on the rise due to the aging population and the obesity epidemic. Inactivity is also a major problem in the United States. This is a significant economic burden as well because of the disability and the many health issues associated with inactivity as well as lost productivity and diminished quality of life," noted Dr. Sun, a medical resident and research trainee at Northwestern Feinberg School of Medicine, Chicago.
The study involved analysis of data from the National Institutes of Health–funded Osteoarthritis Initiative (OAI) to gather information on the physical activity levels of more than 4,700 adults, most of whom were aged older than 65 years and more than 40% of whom had a body mass index over 30 kg/m2, with or at risk for knee osteoarthritis.
The researchers set out to determine whether meeting the 2008 physical activity guidelines from the Department of Health and Human Services would translate into better overall quality of life and whether interventions to improve physical activity level correlate to better quality-adjusted life years (QALYs).The HHS guidelines recommend 150 minutes of moderate to vigorous activity per week performed in sessions lasting at least 10 minutes each. "An example of moderate activity would be walking briskly as if you were late to an appointment," Dr. Sun said.
The researchers used accelerometers to measure the physical activity level in 1,794 OAI study participants over the course of 1 week and placed them into one of three groups: 235 who met the HHS exercise guidelines of 150 minutes of moderate to vigorous activity, 763 who were "insufficiently active" (engaging in some but fewer than 150 minutes of moderate to vigorous exercise per week), and 796 who were inactive (engaging in no moderate to vigorous exercise per week). Health-related utility scores used to calculate QALYs were measured at the beginning of the study and 2 years later.
Overall, QALYs were significantly better with increasing level of physical activity, Dr. Sun reported. "This was a graded relationship, meaning that [people] in the middle group who had some [level of exercise] but not enough to meet guidelines had significantly better overall quality of life compared with the inactive group," she said. Specifically, after adjustment for socioeconomic and health factors over the 2-year period, people who met the HHS exercise guidelines had QALYs that were 0.11 higher compared with those who were inactive. At the same time, those in the insufficiently active group had QALYs that were 0.058 higher compared with those who were inactive.
"The most active group experienced the most benefit," Dr. Sun said. "The improvement was meaningful and translated into roughly an additional 10-20 days of perfect health in a year. We estimate that if an intervention could move someone out of the inactive group and cost less than $1,450 per person per year, that intervention would be considered cost-effective."
The researchers had no relevant financial conflicts to disclose.
SAN DIEGO – Osteoarthritis patients who meet federal guidelines for exercise have more days of perfect health per year than do their sedentary counterparts, results from a novel study suggest.
"Our findings support interventions that help older adults to increase their physical activity level, even if guidelines are not fully met," Dr. Kai Sun said during a press briefing at the annual meeting of the American College of Rheumatology. "Increased physical activity could essentially translate into better quality of life and increased time spent in good health. We expect that this benefit would translate into lower overall health care costs."
Osteoarthritis is "a major cause of disability, and its prevalence is on the rise due to the aging population and the obesity epidemic. Inactivity is also a major problem in the United States. This is a significant economic burden as well because of the disability and the many health issues associated with inactivity as well as lost productivity and diminished quality of life," noted Dr. Sun, a medical resident and research trainee at Northwestern Feinberg School of Medicine, Chicago.
The study involved analysis of data from the National Institutes of Health–funded Osteoarthritis Initiative (OAI) to gather information on the physical activity levels of more than 4,700 adults, most of whom were aged older than 65 years and more than 40% of whom had a body mass index over 30 kg/m2, with or at risk for knee osteoarthritis.
The researchers set out to determine whether meeting the 2008 physical activity guidelines from the Department of Health and Human Services would translate into better overall quality of life and whether interventions to improve physical activity level correlate to better quality-adjusted life years (QALYs).The HHS guidelines recommend 150 minutes of moderate to vigorous activity per week performed in sessions lasting at least 10 minutes each. "An example of moderate activity would be walking briskly as if you were late to an appointment," Dr. Sun said.
The researchers used accelerometers to measure the physical activity level in 1,794 OAI study participants over the course of 1 week and placed them into one of three groups: 235 who met the HHS exercise guidelines of 150 minutes of moderate to vigorous activity, 763 who were "insufficiently active" (engaging in some but fewer than 150 minutes of moderate to vigorous exercise per week), and 796 who were inactive (engaging in no moderate to vigorous exercise per week). Health-related utility scores used to calculate QALYs were measured at the beginning of the study and 2 years later.
Overall, QALYs were significantly better with increasing level of physical activity, Dr. Sun reported. "This was a graded relationship, meaning that [people] in the middle group who had some [level of exercise] but not enough to meet guidelines had significantly better overall quality of life compared with the inactive group," she said. Specifically, after adjustment for socioeconomic and health factors over the 2-year period, people who met the HHS exercise guidelines had QALYs that were 0.11 higher compared with those who were inactive. At the same time, those in the insufficiently active group had QALYs that were 0.058 higher compared with those who were inactive.
"The most active group experienced the most benefit," Dr. Sun said. "The improvement was meaningful and translated into roughly an additional 10-20 days of perfect health in a year. We estimate that if an intervention could move someone out of the inactive group and cost less than $1,450 per person per year, that intervention would be considered cost-effective."
The researchers had no relevant financial conflicts to disclose.
AT THE ACR ANNUAL MEETING
Major finding: Over the course of 2 years, people with, or at risk for, osteoarthritis who met the HHS exercise guidelines for adults had quality-adjusted life years that that were 0.11 higher compared with those who were inactive.
Data source: A nationwide study of 1,794 participants in the National Institutes of Health–funded Osteoarthritis Initiative.
Disclosures: The researchers had no relevant financial conflicts to disclose.
Cost of Botox vs. anticholinergics for urge urinary incontinence
LAS VEGAS – Onabotulinum toxin A and anticholinergic medications have similar cost effectiveness in the first 6 months of treatment for urge urinary incontinence, results from a multicenter randomized trial showed.
However, if costs and outcomes are considered through 9 months, Botox has significantly lower costs than anticholinergics but similar urge urinary incontinence (UUI) control, Dr. Anthony G. Visco reported at the annual meeting of the American Urogynecologic Society.
On behalf of the National Institute of Child Health and Human Development–funded Pelvic Floor Disorders Network, Dr. Visco presented findings from an analysis of 231 women who participated in the ABC trial, which directly compared the safety and efficacy of a 6-month regimen of anticholinergic medications (solifenacin or trospium) to a single 100-U injection of Botox (N. Engl. J. Med. 2012;367:1803-13).
The current study sought to compare the cost effectiveness of anticholinergic medications and Botox for the management of urge urinary incontinence (UUI). The researchers adopted a societal cost perspective and included both direct costs such as physician visits/procedures and medication costs, as well as indirect costs such as incontinence pads, laundry use, and time lost from work.
Patients were randomized to one of the two groups and were followed for 6 months. At each month the researchers obtained bladder diaries, including the Overactive Bladder Questionnaire (OABq) and the Patient Global Symptom Control instrument (PGSC). At 6 months, all anticholinergic pills were stopped. Dr. Visco and his associates followed the patients up to an additional 6 months "to look at the effect of Botox using the PGSC scores to determine the duration of adequate symptom control," he said.
The researchers adopted two different measures of efficacy. In the first 6 months, efficacy outcome was the average reduction in UUI episodes, and utilities were obtained from the OABq completed at baseline through 6 months; quality-adjusted life-years were calculated and annualized from 6 months to a full year.
Between 6 and 9 months, the efficacy outcome was adequate symptom control as measured by the PGSC. "We chose 9 months because that was a period of time when half the participants in the Botox group still had adequate symptom control," explained Dr. Visco, chief of urogynecology and female pelvic medicine and reconstructive surgery at Duke University, Durham, N.C.
Cost analyses assumed that Botox patients incurred no additional costs between months 6 and 9 while those on anticholinergics incurred an additional 3 months of medication costs.
At 6 months the direct medical costs were similar between the anticholinergic and Botox groups ($1,339 vs. $1,266), as were the indirect costs ($150 vs. $106). Both treatments decreased the amount of UUI episodes by 3.3 per day. Both groups also had improvements in total quality-adjusted life-years (0.702 vs. 0.707) and in the annualized cost per quality-adjusted life-year ($57,890 vs. $55,869).
Dr. Visco noted that 74% of study participants who received Botox had adequate symptom control at 6 months. That decreased to 55% at the 9-month mark.
The cost-effectiveness analysis through 9 months revealed that anticholinergics cost $1,942 while Botox cost $1,266. Months of symptom control also were similar between the groups (a mean of 6.36 vs. 6.13, respectively), but in the analysis of cost per month of adequate symptom control, anticholinergics were significantly more expensive: $305 vs. $207 for Botox (P value less than .0001).
Dr. Visco acknowledged certain limitations of the study, including the fact that true cost data were not available beyond 6 months and that the researchers evaluated only solifenacin or trospium. In addition, "quality-adjusted life-year data were limited to the first 6 months, and we are unable to comment on the cost effectiveness of multiple injections," he said. "Anticholinergic assumptions were also made between 6 and 9 months."
Dr. Visco said he had no relevant financial disclosures.
LAS VEGAS – Onabotulinum toxin A and anticholinergic medications have similar cost effectiveness in the first 6 months of treatment for urge urinary incontinence, results from a multicenter randomized trial showed.
However, if costs and outcomes are considered through 9 months, Botox has significantly lower costs than anticholinergics but similar urge urinary incontinence (UUI) control, Dr. Anthony G. Visco reported at the annual meeting of the American Urogynecologic Society.
On behalf of the National Institute of Child Health and Human Development–funded Pelvic Floor Disorders Network, Dr. Visco presented findings from an analysis of 231 women who participated in the ABC trial, which directly compared the safety and efficacy of a 6-month regimen of anticholinergic medications (solifenacin or trospium) to a single 100-U injection of Botox (N. Engl. J. Med. 2012;367:1803-13).
The current study sought to compare the cost effectiveness of anticholinergic medications and Botox for the management of urge urinary incontinence (UUI). The researchers adopted a societal cost perspective and included both direct costs such as physician visits/procedures and medication costs, as well as indirect costs such as incontinence pads, laundry use, and time lost from work.
Patients were randomized to one of the two groups and were followed for 6 months. At each month the researchers obtained bladder diaries, including the Overactive Bladder Questionnaire (OABq) and the Patient Global Symptom Control instrument (PGSC). At 6 months, all anticholinergic pills were stopped. Dr. Visco and his associates followed the patients up to an additional 6 months "to look at the effect of Botox using the PGSC scores to determine the duration of adequate symptom control," he said.
The researchers adopted two different measures of efficacy. In the first 6 months, efficacy outcome was the average reduction in UUI episodes, and utilities were obtained from the OABq completed at baseline through 6 months; quality-adjusted life-years were calculated and annualized from 6 months to a full year.
Between 6 and 9 months, the efficacy outcome was adequate symptom control as measured by the PGSC. "We chose 9 months because that was a period of time when half the participants in the Botox group still had adequate symptom control," explained Dr. Visco, chief of urogynecology and female pelvic medicine and reconstructive surgery at Duke University, Durham, N.C.
Cost analyses assumed that Botox patients incurred no additional costs between months 6 and 9 while those on anticholinergics incurred an additional 3 months of medication costs.
At 6 months the direct medical costs were similar between the anticholinergic and Botox groups ($1,339 vs. $1,266), as were the indirect costs ($150 vs. $106). Both treatments decreased the amount of UUI episodes by 3.3 per day. Both groups also had improvements in total quality-adjusted life-years (0.702 vs. 0.707) and in the annualized cost per quality-adjusted life-year ($57,890 vs. $55,869).
Dr. Visco noted that 74% of study participants who received Botox had adequate symptom control at 6 months. That decreased to 55% at the 9-month mark.
The cost-effectiveness analysis through 9 months revealed that anticholinergics cost $1,942 while Botox cost $1,266. Months of symptom control also were similar between the groups (a mean of 6.36 vs. 6.13, respectively), but in the analysis of cost per month of adequate symptom control, anticholinergics were significantly more expensive: $305 vs. $207 for Botox (P value less than .0001).
Dr. Visco acknowledged certain limitations of the study, including the fact that true cost data were not available beyond 6 months and that the researchers evaluated only solifenacin or trospium. In addition, "quality-adjusted life-year data were limited to the first 6 months, and we are unable to comment on the cost effectiveness of multiple injections," he said. "Anticholinergic assumptions were also made between 6 and 9 months."
Dr. Visco said he had no relevant financial disclosures.
LAS VEGAS – Onabotulinum toxin A and anticholinergic medications have similar cost effectiveness in the first 6 months of treatment for urge urinary incontinence, results from a multicenter randomized trial showed.
However, if costs and outcomes are considered through 9 months, Botox has significantly lower costs than anticholinergics but similar urge urinary incontinence (UUI) control, Dr. Anthony G. Visco reported at the annual meeting of the American Urogynecologic Society.
On behalf of the National Institute of Child Health and Human Development–funded Pelvic Floor Disorders Network, Dr. Visco presented findings from an analysis of 231 women who participated in the ABC trial, which directly compared the safety and efficacy of a 6-month regimen of anticholinergic medications (solifenacin or trospium) to a single 100-U injection of Botox (N. Engl. J. Med. 2012;367:1803-13).
The current study sought to compare the cost effectiveness of anticholinergic medications and Botox for the management of urge urinary incontinence (UUI). The researchers adopted a societal cost perspective and included both direct costs such as physician visits/procedures and medication costs, as well as indirect costs such as incontinence pads, laundry use, and time lost from work.
Patients were randomized to one of the two groups and were followed for 6 months. At each month the researchers obtained bladder diaries, including the Overactive Bladder Questionnaire (OABq) and the Patient Global Symptom Control instrument (PGSC). At 6 months, all anticholinergic pills were stopped. Dr. Visco and his associates followed the patients up to an additional 6 months "to look at the effect of Botox using the PGSC scores to determine the duration of adequate symptom control," he said.
The researchers adopted two different measures of efficacy. In the first 6 months, efficacy outcome was the average reduction in UUI episodes, and utilities were obtained from the OABq completed at baseline through 6 months; quality-adjusted life-years were calculated and annualized from 6 months to a full year.
Between 6 and 9 months, the efficacy outcome was adequate symptom control as measured by the PGSC. "We chose 9 months because that was a period of time when half the participants in the Botox group still had adequate symptom control," explained Dr. Visco, chief of urogynecology and female pelvic medicine and reconstructive surgery at Duke University, Durham, N.C.
Cost analyses assumed that Botox patients incurred no additional costs between months 6 and 9 while those on anticholinergics incurred an additional 3 months of medication costs.
At 6 months the direct medical costs were similar between the anticholinergic and Botox groups ($1,339 vs. $1,266), as were the indirect costs ($150 vs. $106). Both treatments decreased the amount of UUI episodes by 3.3 per day. Both groups also had improvements in total quality-adjusted life-years (0.702 vs. 0.707) and in the annualized cost per quality-adjusted life-year ($57,890 vs. $55,869).
Dr. Visco noted that 74% of study participants who received Botox had adequate symptom control at 6 months. That decreased to 55% at the 9-month mark.
The cost-effectiveness analysis through 9 months revealed that anticholinergics cost $1,942 while Botox cost $1,266. Months of symptom control also were similar between the groups (a mean of 6.36 vs. 6.13, respectively), but in the analysis of cost per month of adequate symptom control, anticholinergics were significantly more expensive: $305 vs. $207 for Botox (P value less than .0001).
Dr. Visco acknowledged certain limitations of the study, including the fact that true cost data were not available beyond 6 months and that the researchers evaluated only solifenacin or trospium. In addition, "quality-adjusted life-year data were limited to the first 6 months, and we are unable to comment on the cost effectiveness of multiple injections," he said. "Anticholinergic assumptions were also made between 6 and 9 months."
Dr. Visco said he had no relevant financial disclosures.
AT THE AUGS ANNUAL MEETING
Major finding: In an analysis of cost per month of adequate symptom control for urge urinary incontinence through 9 months of treatment, anticholinergics were significantly more expensive than Botox ($305 vs. $207; P value less than .0001).
Data source: A study of 231 women who participated in the ABC trial, which directly compared the safety and efficacy of a 6-month regimen of anticholinergic medications (solifenacin or trospium) to a single 100-U injection of Botox.
Disclosures: Dr. Visco said he had no relevant financial disclosures.
One in five women undergoes urogynecologic surgery
LAS VEGAS – Among women with private health insurance in this country, the lifetime risk of surgery for either stress urinary incontinence or pelvic organ prolapse stands at 20.2%, results from a large analysis demonstrated.
"This means that one out of every five women in the United States will undergo a urogynecologic procedure by the age of 80," Dr. Jennifer M. Wu said at the annual meeting of the American Urogynecologic Society. "This high rate highlights the public health burden of pelvic floor disorders, exposes the need for improved prevention strategies, and underscores the importance of effective long-term surgical interventions."
A commonly referenced statistic is that the lifetime risk of surgery for either stress urinary incontinence (SUI) or pelvic organ prolapse (POP) is 11%, but this was based on a study of 395 patients who underwent surgery in 1995 in the Northwest (Obstet. Gynecol. 1997;89:501-6).
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Since 1995, several factors may have increased this risk. These include "the integration of midurethral slings and vaginal mesh prolapse procedures, regional differences in surgery rates, and the greater number of elderly women," explained Dr. Wu of the department of obstetrics and gynecology and the Center for Women’s Health Research at the University of North Carolina at Chapel Hill. "Thus, the objective of our study was to estimate the cumulative risk of SUI or POP surgery over a woman’s lifetime until the age of 80."
The researchers used the Market Scan Commercial Claims and Encounters database with the Medicare Supplemental, which contains de-identified inpatient and outpatient claims data from about 100 employer-based insurance plans in the United States. They included women aged 18 years and older and identified any SUI or POP surgery performed between 2007 and 2011 based on CPT codes. This resulted in a study population of 51.8 million women. Of these, 311,070 underwent surgery for either SUI or POP.
To estimate age-specific incidence rates for each age between 18 and 80 years, Dr. Wu and her associates multiplied age-specific prevalence rates by the proportion of cases that were incident. To estimate the proportion of all procedures that were repeat surgeries, they evaluated women who had a SUI or POP surgery in 2011 and "looked back" 5 years to identify prior surgeries. Finally, they used Monte Carlo simulations to determine the cumulative lifetime risk of either incontinence or prolapse surgery with 95% confidence intervals.
Dr. Wu reported that the cumulative lifetime risk of either SUI or POP surgery was 20.2% (95% CI, 19.2%-21.1%). The cumulative lifetime risk of SUI was 14.5% (95% CI, 13.4%-15.5%), and the risk of POP surgery was 13.7% (95% CI, 12.6%-14.8%). The cumulative lifetime risk for SUI or POP surgery increased with age in a stepwise fashion, from 11.4% by age 60 years to 15.9% by age 70 and 20.2% by age 80, she noted.
To place the risk of any primary surgery for SUI or POP in perspective, Dr. Wu drew a comparison with the lifetime risk estimates for colon cancer (4.8%), lung cancer (6.3%), breast cancer (14.8%), and any type of cancer (41.3%). "Granted, incontinence and prolapse are not life-threatening conditions, but this 20% risk highlights how common surgeries are for pelvic floor disorders, despite the fact that they’re widely underrecognized," she noted.
She acknowledged certain limitations of the study, including the fact that she and her associates were unable to review actual medical records to confirm the rate of prior surgeries. "In addition, we assumed a closed cohort and no competing risks such that all women live until the age of 80," she said. "Also, these results reflect women with private insurance and may not be generalizable to those who are uninsured or underinsured."
Dr. Wu disclosed that she is a consultant for Procter & Gamble.
Dr. David M. Jaspan comments: Direct to consumer advertising has enabled more women to feel comfortable discussing incontinence with their health care providers, which is great. Industry had inundated general ob.gyn. offices with the opportunity to attend short courses to learn "mesh procedures," which may not have been great. Therefore, I am not surprised that the numbers of women with private insurance undergoing urogynecologic surgery are so high.
These advanced procedures should be undertaken by those surgeons with sufficient training and experience to properly counsel women about the risk and benefit of a native tissue repair, compared with a graft placement. In addition, surgeons should have a volume of experience and training that qualifies them to provide this detailed counseling and should perform an appropriate preoperative evaluation to determine the absolute need for a procedure. More procedures may have brought more risk to women.
I applaud the American College of Obstetricians and Gynecologists for the 2011 ACOG Committee Opinion #513, which says, "Surgeons performing complex pelvic floor reconstructive surgery should have adequate experience and training in native tissue repairs as well as repairs using mesh augmentation specific to each device, should have a thorough understanding of pelvic anatomy, and should be able to counsel patients regarding the risk/benefit ratio on the use of mesh compared with native tissue repairs" (Obstet. Gynecol. 2011;118:1459-64).
In its 2011 Safety Communication, the Food and Drug Administration said that transvaginal placement of surgical mesh for POP is of "continuing serious concern." In the document, the FDA restated its 2008 recommendation that health care providers "inform patients about the potential for serious complications and the effect on quality of life, including pain during sexual intercourse, scarring, and narrowing of the vaginal wall in POP repair using surgical mesh; and provide a copy of the patient labeling from the surgical mesh manufacturer if available."
The FDA also made several new recommendations for health care providers in its report:
- Surgeons should recognize that, in most cases, POP can be treated successfully without mesh, thus avoiding the risk of mesh-related complications.
- Surgeons should choose mesh surgery only after weighing the risks and benefits of surgery with mesh versus all surgical and nonsurgical alternatives.
- Removal of mesh may involve multiple surgical procedures and significantly impair the patient's quality of life. Complete removal of mesh may not be possible and may not result in complete resolution of complications, including pain.
Patient counseling needs to include discussion of alternative native tissue repair and the fact that synthetic mesh is permanent, according to ACOG. Other issues to discuss include that vaginal bleeding, pain, and dyspareunia may be related to vaginal mesh; Patients reporting such symptoms should undergo a thorough vaginal exam, including an exam under anesthesia if necessary.
Dr. David M. Jaspan is chairman of ob.gyn. for the Einstein Health Care Network in Philadelphia. He was asked to comment on Dr. Wu's study. Dr. Jaspan said that he had no financial disclosures.
Remember to ask about pelvic floor issues
Dr. Meadow Maze Good comments: This is a well done and important study that highlights the magnitude of pelvic floor disorders in women. Previous studies indicate that women delay or do not seek care for pelvic floor disorders and incorrectly think that these problems are a normal result of aging.
These data presented at the American Urogynecologic Society meeting by Dr. Wu and her colleagues reinforce the need for increased public awareness and education regarding pelvic floor disorders as well as available treatment options, including nonsurgical and surgical options. Additionally, this study points out that the physicians treating women of all ages including the primary care provider (family practice physicians and obstetrician/gynecologists) should query women about possible pelvic floor symptoms, including incontinence, prolapse, and sexual function.
It is especially important for the ob.gyn. to ask their patient about these symptoms if there is planning underway for a hysterectomy for other causes. This is an exciting time for the newly board-certifiable field of female pelvic medicine and reconstructive surgery. As the aging population of women with prolapse and incontinence increases, fellowship programs are graduating physicians knowledgeable in this specific field who are dedicated to taking care of women with these treatable conditions.
Meadow Maze Good, D.O., is a fellow in female pelvic medicine and reconstructive surgery at the University of Texas Southwestern Medical Center, Dallas. Dr. Good, who is also the chair of the Junior Fellow Congress Advisory Council of the American Congress of Obstetricians and Gynecologists, was asked to comment on Dr. Wu's study. She said she had no relevant financial disclosures.
Dr. David M. Jaspan comments: Direct to consumer advertising has enabled more women to feel comfortable discussing incontinence with their health care providers, which is great. Industry had inundated general ob.gyn. offices with the opportunity to attend short courses to learn "mesh procedures," which may not have been great. Therefore, I am not surprised that the numbers of women with private insurance undergoing urogynecologic surgery are so high.
These advanced procedures should be undertaken by those surgeons with sufficient training and experience to properly counsel women about the risk and benefit of a native tissue repair, compared with a graft placement. In addition, surgeons should have a volume of experience and training that qualifies them to provide this detailed counseling and should perform an appropriate preoperative evaluation to determine the absolute need for a procedure. More procedures may have brought more risk to women.
I applaud the American College of Obstetricians and Gynecologists for the 2011 ACOG Committee Opinion #513, which says, "Surgeons performing complex pelvic floor reconstructive surgery should have adequate experience and training in native tissue repairs as well as repairs using mesh augmentation specific to each device, should have a thorough understanding of pelvic anatomy, and should be able to counsel patients regarding the risk/benefit ratio on the use of mesh compared with native tissue repairs" (Obstet. Gynecol. 2011;118:1459-64).
In its 2011 Safety Communication, the Food and Drug Administration said that transvaginal placement of surgical mesh for POP is of "continuing serious concern." In the document, the FDA restated its 2008 recommendation that health care providers "inform patients about the potential for serious complications and the effect on quality of life, including pain during sexual intercourse, scarring, and narrowing of the vaginal wall in POP repair using surgical mesh; and provide a copy of the patient labeling from the surgical mesh manufacturer if available."
The FDA also made several new recommendations for health care providers in its report:
- Surgeons should recognize that, in most cases, POP can be treated successfully without mesh, thus avoiding the risk of mesh-related complications.
- Surgeons should choose mesh surgery only after weighing the risks and benefits of surgery with mesh versus all surgical and nonsurgical alternatives.
- Removal of mesh may involve multiple surgical procedures and significantly impair the patient's quality of life. Complete removal of mesh may not be possible and may not result in complete resolution of complications, including pain.
Patient counseling needs to include discussion of alternative native tissue repair and the fact that synthetic mesh is permanent, according to ACOG. Other issues to discuss include that vaginal bleeding, pain, and dyspareunia may be related to vaginal mesh; Patients reporting such symptoms should undergo a thorough vaginal exam, including an exam under anesthesia if necessary.
Dr. David M. Jaspan is chairman of ob.gyn. for the Einstein Health Care Network in Philadelphia. He was asked to comment on Dr. Wu's study. Dr. Jaspan said that he had no financial disclosures.
Remember to ask about pelvic floor issues
Dr. Meadow Maze Good comments: This is a well done and important study that highlights the magnitude of pelvic floor disorders in women. Previous studies indicate that women delay or do not seek care for pelvic floor disorders and incorrectly think that these problems are a normal result of aging.
These data presented at the American Urogynecologic Society meeting by Dr. Wu and her colleagues reinforce the need for increased public awareness and education regarding pelvic floor disorders as well as available treatment options, including nonsurgical and surgical options. Additionally, this study points out that the physicians treating women of all ages including the primary care provider (family practice physicians and obstetrician/gynecologists) should query women about possible pelvic floor symptoms, including incontinence, prolapse, and sexual function.
It is especially important for the ob.gyn. to ask their patient about these symptoms if there is planning underway for a hysterectomy for other causes. This is an exciting time for the newly board-certifiable field of female pelvic medicine and reconstructive surgery. As the aging population of women with prolapse and incontinence increases, fellowship programs are graduating physicians knowledgeable in this specific field who are dedicated to taking care of women with these treatable conditions.
Meadow Maze Good, D.O., is a fellow in female pelvic medicine and reconstructive surgery at the University of Texas Southwestern Medical Center, Dallas. Dr. Good, who is also the chair of the Junior Fellow Congress Advisory Council of the American Congress of Obstetricians and Gynecologists, was asked to comment on Dr. Wu's study. She said she had no relevant financial disclosures.
Dr. David M. Jaspan comments: Direct to consumer advertising has enabled more women to feel comfortable discussing incontinence with their health care providers, which is great. Industry had inundated general ob.gyn. offices with the opportunity to attend short courses to learn "mesh procedures," which may not have been great. Therefore, I am not surprised that the numbers of women with private insurance undergoing urogynecologic surgery are so high.
These advanced procedures should be undertaken by those surgeons with sufficient training and experience to properly counsel women about the risk and benefit of a native tissue repair, compared with a graft placement. In addition, surgeons should have a volume of experience and training that qualifies them to provide this detailed counseling and should perform an appropriate preoperative evaluation to determine the absolute need for a procedure. More procedures may have brought more risk to women.
I applaud the American College of Obstetricians and Gynecologists for the 2011 ACOG Committee Opinion #513, which says, "Surgeons performing complex pelvic floor reconstructive surgery should have adequate experience and training in native tissue repairs as well as repairs using mesh augmentation specific to each device, should have a thorough understanding of pelvic anatomy, and should be able to counsel patients regarding the risk/benefit ratio on the use of mesh compared with native tissue repairs" (Obstet. Gynecol. 2011;118:1459-64).
In its 2011 Safety Communication, the Food and Drug Administration said that transvaginal placement of surgical mesh for POP is of "continuing serious concern." In the document, the FDA restated its 2008 recommendation that health care providers "inform patients about the potential for serious complications and the effect on quality of life, including pain during sexual intercourse, scarring, and narrowing of the vaginal wall in POP repair using surgical mesh; and provide a copy of the patient labeling from the surgical mesh manufacturer if available."
The FDA also made several new recommendations for health care providers in its report:
- Surgeons should recognize that, in most cases, POP can be treated successfully without mesh, thus avoiding the risk of mesh-related complications.
- Surgeons should choose mesh surgery only after weighing the risks and benefits of surgery with mesh versus all surgical and nonsurgical alternatives.
- Removal of mesh may involve multiple surgical procedures and significantly impair the patient's quality of life. Complete removal of mesh may not be possible and may not result in complete resolution of complications, including pain.
Patient counseling needs to include discussion of alternative native tissue repair and the fact that synthetic mesh is permanent, according to ACOG. Other issues to discuss include that vaginal bleeding, pain, and dyspareunia may be related to vaginal mesh; Patients reporting such symptoms should undergo a thorough vaginal exam, including an exam under anesthesia if necessary.
Dr. David M. Jaspan is chairman of ob.gyn. for the Einstein Health Care Network in Philadelphia. He was asked to comment on Dr. Wu's study. Dr. Jaspan said that he had no financial disclosures.
Remember to ask about pelvic floor issues
Dr. Meadow Maze Good comments: This is a well done and important study that highlights the magnitude of pelvic floor disorders in women. Previous studies indicate that women delay or do not seek care for pelvic floor disorders and incorrectly think that these problems are a normal result of aging.
These data presented at the American Urogynecologic Society meeting by Dr. Wu and her colleagues reinforce the need for increased public awareness and education regarding pelvic floor disorders as well as available treatment options, including nonsurgical and surgical options. Additionally, this study points out that the physicians treating women of all ages including the primary care provider (family practice physicians and obstetrician/gynecologists) should query women about possible pelvic floor symptoms, including incontinence, prolapse, and sexual function.
It is especially important for the ob.gyn. to ask their patient about these symptoms if there is planning underway for a hysterectomy for other causes. This is an exciting time for the newly board-certifiable field of female pelvic medicine and reconstructive surgery. As the aging population of women with prolapse and incontinence increases, fellowship programs are graduating physicians knowledgeable in this specific field who are dedicated to taking care of women with these treatable conditions.
Meadow Maze Good, D.O., is a fellow in female pelvic medicine and reconstructive surgery at the University of Texas Southwestern Medical Center, Dallas. Dr. Good, who is also the chair of the Junior Fellow Congress Advisory Council of the American Congress of Obstetricians and Gynecologists, was asked to comment on Dr. Wu's study. She said she had no relevant financial disclosures.
LAS VEGAS – Among women with private health insurance in this country, the lifetime risk of surgery for either stress urinary incontinence or pelvic organ prolapse stands at 20.2%, results from a large analysis demonstrated.
"This means that one out of every five women in the United States will undergo a urogynecologic procedure by the age of 80," Dr. Jennifer M. Wu said at the annual meeting of the American Urogynecologic Society. "This high rate highlights the public health burden of pelvic floor disorders, exposes the need for improved prevention strategies, and underscores the importance of effective long-term surgical interventions."
A commonly referenced statistic is that the lifetime risk of surgery for either stress urinary incontinence (SUI) or pelvic organ prolapse (POP) is 11%, but this was based on a study of 395 patients who underwent surgery in 1995 in the Northwest (Obstet. Gynecol. 1997;89:501-6).
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Since 1995, several factors may have increased this risk. These include "the integration of midurethral slings and vaginal mesh prolapse procedures, regional differences in surgery rates, and the greater number of elderly women," explained Dr. Wu of the department of obstetrics and gynecology and the Center for Women’s Health Research at the University of North Carolina at Chapel Hill. "Thus, the objective of our study was to estimate the cumulative risk of SUI or POP surgery over a woman’s lifetime until the age of 80."
The researchers used the Market Scan Commercial Claims and Encounters database with the Medicare Supplemental, which contains de-identified inpatient and outpatient claims data from about 100 employer-based insurance plans in the United States. They included women aged 18 years and older and identified any SUI or POP surgery performed between 2007 and 2011 based on CPT codes. This resulted in a study population of 51.8 million women. Of these, 311,070 underwent surgery for either SUI or POP.
To estimate age-specific incidence rates for each age between 18 and 80 years, Dr. Wu and her associates multiplied age-specific prevalence rates by the proportion of cases that were incident. To estimate the proportion of all procedures that were repeat surgeries, they evaluated women who had a SUI or POP surgery in 2011 and "looked back" 5 years to identify prior surgeries. Finally, they used Monte Carlo simulations to determine the cumulative lifetime risk of either incontinence or prolapse surgery with 95% confidence intervals.
Dr. Wu reported that the cumulative lifetime risk of either SUI or POP surgery was 20.2% (95% CI, 19.2%-21.1%). The cumulative lifetime risk of SUI was 14.5% (95% CI, 13.4%-15.5%), and the risk of POP surgery was 13.7% (95% CI, 12.6%-14.8%). The cumulative lifetime risk for SUI or POP surgery increased with age in a stepwise fashion, from 11.4% by age 60 years to 15.9% by age 70 and 20.2% by age 80, she noted.
To place the risk of any primary surgery for SUI or POP in perspective, Dr. Wu drew a comparison with the lifetime risk estimates for colon cancer (4.8%), lung cancer (6.3%), breast cancer (14.8%), and any type of cancer (41.3%). "Granted, incontinence and prolapse are not life-threatening conditions, but this 20% risk highlights how common surgeries are for pelvic floor disorders, despite the fact that they’re widely underrecognized," she noted.
She acknowledged certain limitations of the study, including the fact that she and her associates were unable to review actual medical records to confirm the rate of prior surgeries. "In addition, we assumed a closed cohort and no competing risks such that all women live until the age of 80," she said. "Also, these results reflect women with private insurance and may not be generalizable to those who are uninsured or underinsured."
Dr. Wu disclosed that she is a consultant for Procter & Gamble.
LAS VEGAS – Among women with private health insurance in this country, the lifetime risk of surgery for either stress urinary incontinence or pelvic organ prolapse stands at 20.2%, results from a large analysis demonstrated.
"This means that one out of every five women in the United States will undergo a urogynecologic procedure by the age of 80," Dr. Jennifer M. Wu said at the annual meeting of the American Urogynecologic Society. "This high rate highlights the public health burden of pelvic floor disorders, exposes the need for improved prevention strategies, and underscores the importance of effective long-term surgical interventions."
A commonly referenced statistic is that the lifetime risk of surgery for either stress urinary incontinence (SUI) or pelvic organ prolapse (POP) is 11%, but this was based on a study of 395 patients who underwent surgery in 1995 in the Northwest (Obstet. Gynecol. 1997;89:501-6).
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Since 1995, several factors may have increased this risk. These include "the integration of midurethral slings and vaginal mesh prolapse procedures, regional differences in surgery rates, and the greater number of elderly women," explained Dr. Wu of the department of obstetrics and gynecology and the Center for Women’s Health Research at the University of North Carolina at Chapel Hill. "Thus, the objective of our study was to estimate the cumulative risk of SUI or POP surgery over a woman’s lifetime until the age of 80."
The researchers used the Market Scan Commercial Claims and Encounters database with the Medicare Supplemental, which contains de-identified inpatient and outpatient claims data from about 100 employer-based insurance plans in the United States. They included women aged 18 years and older and identified any SUI or POP surgery performed between 2007 and 2011 based on CPT codes. This resulted in a study population of 51.8 million women. Of these, 311,070 underwent surgery for either SUI or POP.
To estimate age-specific incidence rates for each age between 18 and 80 years, Dr. Wu and her associates multiplied age-specific prevalence rates by the proportion of cases that were incident. To estimate the proportion of all procedures that were repeat surgeries, they evaluated women who had a SUI or POP surgery in 2011 and "looked back" 5 years to identify prior surgeries. Finally, they used Monte Carlo simulations to determine the cumulative lifetime risk of either incontinence or prolapse surgery with 95% confidence intervals.
Dr. Wu reported that the cumulative lifetime risk of either SUI or POP surgery was 20.2% (95% CI, 19.2%-21.1%). The cumulative lifetime risk of SUI was 14.5% (95% CI, 13.4%-15.5%), and the risk of POP surgery was 13.7% (95% CI, 12.6%-14.8%). The cumulative lifetime risk for SUI or POP surgery increased with age in a stepwise fashion, from 11.4% by age 60 years to 15.9% by age 70 and 20.2% by age 80, she noted.
To place the risk of any primary surgery for SUI or POP in perspective, Dr. Wu drew a comparison with the lifetime risk estimates for colon cancer (4.8%), lung cancer (6.3%), breast cancer (14.8%), and any type of cancer (41.3%). "Granted, incontinence and prolapse are not life-threatening conditions, but this 20% risk highlights how common surgeries are for pelvic floor disorders, despite the fact that they’re widely underrecognized," she noted.
She acknowledged certain limitations of the study, including the fact that she and her associates were unable to review actual medical records to confirm the rate of prior surgeries. "In addition, we assumed a closed cohort and no competing risks such that all women live until the age of 80," she said. "Also, these results reflect women with private insurance and may not be generalizable to those who are uninsured or underinsured."
Dr. Wu disclosed that she is a consultant for Procter & Gamble.
AT THE AUGS ANNUAL MEETING
Major finding: The cumulative lifetime risk of surgery for either stress urinary incontinence (SUI) or pelvic organ prolapse (POP) was 20.2% (95%CI 19.2%, 21.1%).
Data source: Claims data from 311,070 women in the United States who underwent any SUI or POP surgery between 2007 and 2011.
Disclosures: Dr. Wu disclosed that she is a consultant for Procter & Gamble.
Treat-to-target Approach for Psoriatic Arthritis Beneficial
SAN DIEGO – Compared with standard care, intensive management of psoriatic arthritis using a treat-to-target approach significantly improved joint and skin outcomes for patients newly diagnosed with the disease, results from a multicenter, randomized controlled trial showed.
"Treating to target works in this disease, and it’s going to result in better long-term outcomes," lead investigator Dr. Philip Helliwell said during a press briefing at the annual meeting of the American College of Rheumatology.
Dr. Helliwell and his associates at eight centers in the United Kingdom randomized 206 patients with early psoriatic arthritis to standard care or intensive management, and followed them for 48 weeks. Patients in the standard care group were treated by a rheumatologist with no set protocol and no limitations, while those in the intensive management group followed a strict treatment protocol with escalation of therapy if minimal disease activity criteria were not met.
Patients in the intensive management group were started on methotrexate with rapid escalation to a dose of *25 mg/week after 6 weeks if they tolerated the drug. If they did not meet minimal disease criteria after 12 weeks, they received a more powerful combination of disease-modifying antirheumatic drugs (DMARDs).
After another 12 weeks, patients in the intensive management group were given anti–tumor necrosis factor therapy if they had three or more tender joints. If they had fewer than three tender or swollen joints but did not meet the minimal disease activity criteria, they were given methotrexate and an alternative DMARD. Patients in the standard care group were treated with DMARDs, but with no set time limits for drug therapy escalation or measurements to reach.
The primary outcome measures were the proportion of patients in both groups who achieved ACR20, ACR50, and ACR70 criteria for disease activity, which represent disease improvement of 20%, 50%, and 70%, respectively. Dr. Helliwell reported that compared with the standard care group, a higher proportion of patients in the intensive management group achieved ACR20 (62% vs. 45%, respectively), ACR50 (51% vs. 25%), and ACR70 (38% vs. 17%). A higher proportion of patients in the intensive management group also achieved a Psoriasis Area and Severity Index 75 compared with their counterparts in the standard care group (59% vs. 33%).
Research in psoriatic arthritis has "lagged behind that of rheumatoid arthritis for years in terms of pathogenesis and treatment paradigms," noted Dr. Helliwell, a senior lecturer in rheumatology at the Institute of Rheumatic and Musculoskeletal Medicine at the University of Leeds (England). "I think the study we’ve reported brings psoriatic arthritis right up to date alongside RA."
He said that up to one-third of people with psoriasis will develop psoriatic arthritis, "so it’s not an insignificant arthritis. It’s often not recognized. One survey we did of people with psoriasis in the community in the U.K. found that up to half of the people with psoriatic arthritis didn’t know they had it. They’d seen their doctor for various reasons and had been told they’d had another form of arthritis, or they were fobbed off with other diagnoses."
The researchers have yet to perform a cost analysis comparing the two treatment groups.
The study was funded by Arthritis Research UK and Pfizer. Dr. Helliwell disclosed that he has received consulting fees from Pfizer.
*Correction 11/11/13: A previous version of this story misstated the methotrexate dosage used in the study. This version has been updated to reflect the correct dosage.
SAN DIEGO – Compared with standard care, intensive management of psoriatic arthritis using a treat-to-target approach significantly improved joint and skin outcomes for patients newly diagnosed with the disease, results from a multicenter, randomized controlled trial showed.
"Treating to target works in this disease, and it’s going to result in better long-term outcomes," lead investigator Dr. Philip Helliwell said during a press briefing at the annual meeting of the American College of Rheumatology.
Dr. Helliwell and his associates at eight centers in the United Kingdom randomized 206 patients with early psoriatic arthritis to standard care or intensive management, and followed them for 48 weeks. Patients in the standard care group were treated by a rheumatologist with no set protocol and no limitations, while those in the intensive management group followed a strict treatment protocol with escalation of therapy if minimal disease activity criteria were not met.
Patients in the intensive management group were started on methotrexate with rapid escalation to a dose of *25 mg/week after 6 weeks if they tolerated the drug. If they did not meet minimal disease criteria after 12 weeks, they received a more powerful combination of disease-modifying antirheumatic drugs (DMARDs).
After another 12 weeks, patients in the intensive management group were given anti–tumor necrosis factor therapy if they had three or more tender joints. If they had fewer than three tender or swollen joints but did not meet the minimal disease activity criteria, they were given methotrexate and an alternative DMARD. Patients in the standard care group were treated with DMARDs, but with no set time limits for drug therapy escalation or measurements to reach.
The primary outcome measures were the proportion of patients in both groups who achieved ACR20, ACR50, and ACR70 criteria for disease activity, which represent disease improvement of 20%, 50%, and 70%, respectively. Dr. Helliwell reported that compared with the standard care group, a higher proportion of patients in the intensive management group achieved ACR20 (62% vs. 45%, respectively), ACR50 (51% vs. 25%), and ACR70 (38% vs. 17%). A higher proportion of patients in the intensive management group also achieved a Psoriasis Area and Severity Index 75 compared with their counterparts in the standard care group (59% vs. 33%).
Research in psoriatic arthritis has "lagged behind that of rheumatoid arthritis for years in terms of pathogenesis and treatment paradigms," noted Dr. Helliwell, a senior lecturer in rheumatology at the Institute of Rheumatic and Musculoskeletal Medicine at the University of Leeds (England). "I think the study we’ve reported brings psoriatic arthritis right up to date alongside RA."
He said that up to one-third of people with psoriasis will develop psoriatic arthritis, "so it’s not an insignificant arthritis. It’s often not recognized. One survey we did of people with psoriasis in the community in the U.K. found that up to half of the people with psoriatic arthritis didn’t know they had it. They’d seen their doctor for various reasons and had been told they’d had another form of arthritis, or they were fobbed off with other diagnoses."
The researchers have yet to perform a cost analysis comparing the two treatment groups.
The study was funded by Arthritis Research UK and Pfizer. Dr. Helliwell disclosed that he has received consulting fees from Pfizer.
*Correction 11/11/13: A previous version of this story misstated the methotrexate dosage used in the study. This version has been updated to reflect the correct dosage.
SAN DIEGO – Compared with standard care, intensive management of psoriatic arthritis using a treat-to-target approach significantly improved joint and skin outcomes for patients newly diagnosed with the disease, results from a multicenter, randomized controlled trial showed.
"Treating to target works in this disease, and it’s going to result in better long-term outcomes," lead investigator Dr. Philip Helliwell said during a press briefing at the annual meeting of the American College of Rheumatology.
Dr. Helliwell and his associates at eight centers in the United Kingdom randomized 206 patients with early psoriatic arthritis to standard care or intensive management, and followed them for 48 weeks. Patients in the standard care group were treated by a rheumatologist with no set protocol and no limitations, while those in the intensive management group followed a strict treatment protocol with escalation of therapy if minimal disease activity criteria were not met.
Patients in the intensive management group were started on methotrexate with rapid escalation to a dose of *25 mg/week after 6 weeks if they tolerated the drug. If they did not meet minimal disease criteria after 12 weeks, they received a more powerful combination of disease-modifying antirheumatic drugs (DMARDs).
After another 12 weeks, patients in the intensive management group were given anti–tumor necrosis factor therapy if they had three or more tender joints. If they had fewer than three tender or swollen joints but did not meet the minimal disease activity criteria, they were given methotrexate and an alternative DMARD. Patients in the standard care group were treated with DMARDs, but with no set time limits for drug therapy escalation or measurements to reach.
The primary outcome measures were the proportion of patients in both groups who achieved ACR20, ACR50, and ACR70 criteria for disease activity, which represent disease improvement of 20%, 50%, and 70%, respectively. Dr. Helliwell reported that compared with the standard care group, a higher proportion of patients in the intensive management group achieved ACR20 (62% vs. 45%, respectively), ACR50 (51% vs. 25%), and ACR70 (38% vs. 17%). A higher proportion of patients in the intensive management group also achieved a Psoriasis Area and Severity Index 75 compared with their counterparts in the standard care group (59% vs. 33%).
Research in psoriatic arthritis has "lagged behind that of rheumatoid arthritis for years in terms of pathogenesis and treatment paradigms," noted Dr. Helliwell, a senior lecturer in rheumatology at the Institute of Rheumatic and Musculoskeletal Medicine at the University of Leeds (England). "I think the study we’ve reported brings psoriatic arthritis right up to date alongside RA."
He said that up to one-third of people with psoriasis will develop psoriatic arthritis, "so it’s not an insignificant arthritis. It’s often not recognized. One survey we did of people with psoriasis in the community in the U.K. found that up to half of the people with psoriatic arthritis didn’t know they had it. They’d seen their doctor for various reasons and had been told they’d had another form of arthritis, or they were fobbed off with other diagnoses."
The researchers have yet to perform a cost analysis comparing the two treatment groups.
The study was funded by Arthritis Research UK and Pfizer. Dr. Helliwell disclosed that he has received consulting fees from Pfizer.
*Correction 11/11/13: A previous version of this story misstated the methotrexate dosage used in the study. This version has been updated to reflect the correct dosage.
AT THE ACR ANNUAL MEETING