User login
Man, 56, With Wrist Pain After a Fall
A white man, age 56, presented to his primary care clinician with wrist pain and swelling. Two days earlier, he had fallen from a step stool and landed on his right wrist. He treated the pain by resting, elevating his arm, applying ice, and taking ibuprofen 800 mg tid. He said he had lost strength in his hand and arm and was experiencing numbness and tingling in his right hand and fingers.
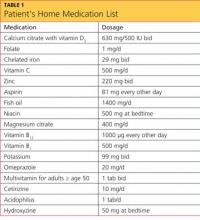
The patient’s medical history included hypertension, type 2 diabetes mellitus, morbid obesity, obstructive sleep apnea, asthma, carpel tunnel syndrome, and peripheral neuropathy. His surgical history was significant for duodenal switch gastric bypass surgery, performed eight years earlier, and his weight at the time of presentation was 200 lb; before his gastric bypass, he weighed 385 lb. Since the surgery, his hypertension, diabetes, asthma, and sleep apnea had all resolved. Table 1 shows a list of medications he was taking at the time of presentation.
The patient, a registered nurse, had been married for 30 years and had one child. He had quit smoking 15 years earlier, with a 43–pack-year smoking history. He reported social drinking but denied any recreational drug use. He was unaware of having any allergies to food or medication.
His vital signs on presentation were blood pressure, 110/75 mm Hg; heart rate, 53 beats/min; respiration, 18 breaths/min; O2 saturation, 97% on room air; and temperature, 97.5°F.
Physical exam revealed that the patient’s right wrist was ecchymotic and swollen with +1 pitting edema. The skin was warm and dry to the touch. Decreased range of motion was noted in the right wrist, compared with the left. Pain with point tenderness was noted at the right lateral wrist. Pulses were +3 with capillary refill of less than 3 seconds. The rest of the exam was unremarkable.
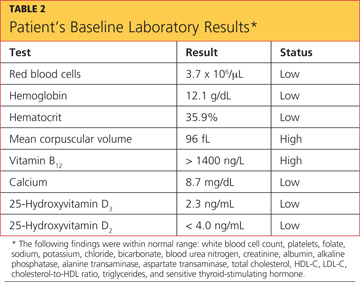
The differential diagnosis included fracture secondary to the fall, osteoporosis, osteopenia, osteomalacia, Paget’s disease, tumor, infection, and sprain or strain of the wrist. A wrist x-ray was ordered, as were the following baseline labs: complete blood count with differential (CBC), vitamin B12 and folate levels, blood chemistry, lipid profile, liver profile, total vitamin D, and sensitive thyroid-stimulating hormone. Test results are shown in Table 2.
X-ray of the wrist showed fracture only, making it possible to rule out Paget’s disease (ie, no patchy white areas noted in the bone) and tumor (no masses seen) as the immediate cause of fracture. Normal body temperature and normal white blood cell count eliminated the possibility of infection.
Because the patient was only 56 and had a history of bariatric surgery, further testing was pursued to investigate a cause for the weakened bone. Bone mineral density (BMD) testing revealed the following results:
• The lumbar spine in frontal projection measured 0.968 g/cm2 with a T-score of –2.2 and a Z-score of –2.2.
• Total BMD of the left hip was 0.863 g/cm2 with a T-score of –1.7 and a Z-score of –1.4.
• Total BMD of the left femoral neck was 0.863 g/cm2 with a T-score of 1.7 and a Z-score of –1.1.
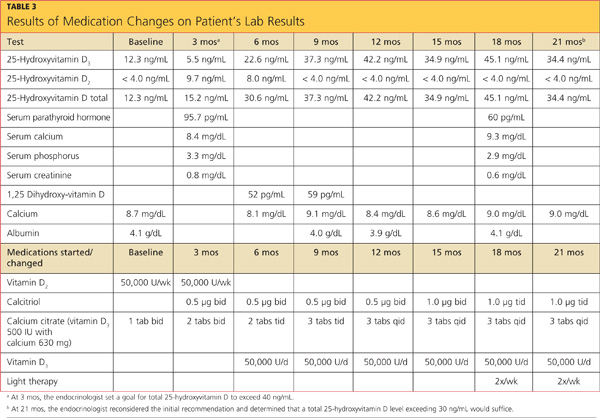
These findings suggested osteopenia1,2 (not osteoporosis) in all sites, with a 12% decrease of BMD in the spine (suggesting increased risk for spinal fracture) and a 16.3% decrease of BMD in the hip since the patient’s most recent bone scan five years earlier (radiologist’s report). Other abnormal findings were elevated parathyroid hormone (PTH) serum, 95.7 pg/mL (reference range, 10 to 65 pg/mL); low total calcium serum, 8.7 mg/dL (reference range, 8.9 to 10.2 mg/dL), and low 25-hydroxyvitamin D total, 12.3 ng/mL (reference range, 25 to 80 ng/mL).
A 2010 clinical practice guideline from the Endocrine Society3 specifies that after malabsorptive surgery, vitamin D and calcium supplementation should be adjusted by a qualified medical professional, based on serum markers and measures of bone density. An endocrinologist who was consulted at the patient’s initial visit prescribed the following medications: vitamin D2, 50,000 U/wk PO; combined calcium citrate (vitamin D3) 500 IU with calcium 630 mg, 1 tab bid; and calcitriol 0.5 μg bid.
The patient’s final diagnosis was osteomalacia secondary to gastric bypass surgery. (See “Making the Diagnosis of Osteomalacia.”4-6)

DISCUSSION
According to 2008 data from the World Health Organization (WHO),7 1.4 billion persons older than 20 worldwide were overweight, and 200 million men and 300 million women were considered obese—meaning that one in every 10 adults worldwide is overweight or obese. In 2010, the WHO reports, 40 million children younger than 5 worldwide were considered overweight.7 Health care providers need to be prepared to care for the increasing number of patients who will undergo bariatric surgeries to treat obesity and its related comorbidities.8
Postoperative follow-up for the malabsorption deficiencies related to bariatric procedures should be performed every six months, including obtaining levels of alkaline phosphatase and others previously discussed. In addition, the Endocrine Society guideline3 recommends measuring levels of vitamin B12, albumin, pre-albumin, iron, and ferritin, and obtaining a CBC, a liver profile, glucose reading, creatinine measurement, and a metabolic profile at one month and two months after surgery, then every six months until two years after surgery, then annually if findings are stable.
Furthermore, the Endocrine Society3 recommends obtaining zinc levels every six months for the first year, then annually. An annual vitamin A level is optional.9 Yearly bone density testing is recommended until the patient’s BMD is deemed stable.3
Additionally, Koch and Finelli10 recommend performing the following labs postoperatively: hemoglobin A1C every three months; copper, magnesium, whole blood thiamine, vitamin B12, and a 24-hour urinary calcium every six months for the first three years, then once a year if findings remain stable.
Use of alcohol should be discouraged among patients who have undergone bariatric surgery, as its use alters micronutrient requirements and metabolism. Alcohol consumption may also contribute to dumping syndrome (ie, rapid gastric emptying).11
Any patient with a history of malabsorptive bypass surgery who complains of neurologic, visual, or skin disorders, anemia, or edema may require a further workup to rule out other absorptive deficiencies. These include vitamins A, E, and B12, zinc, folate, thiamine, niacin, selenium, and ferritin.10
Osteomalacia
Metabolic bone diseases can result from genetics, dietary factors, medication use, surgery, or hormonal irregularities. They alter the normal biochemical reactions in bone structure.
The three most common forms of metabolic bone disease are osteoporosis, osteopenia, and osteomalacia. The WHO diagnostic classifications and associated T-scores for bone mineral density1,2 indicate a T-score above –1.0 as normal. A score between –1.0 and –2.5 is indicative of osteopenia, and a score below –2.5 indicates osteoporosis. A T-score below –2.5 in the patient with a history of fragility fracture indicates severe osteoporosis.1,2
In osteomalacia, bone volume remains unchanged, but mineralization of osteoid in the mature compact and spongy bone is either delayed or inadequate. The remolding cycle continues unchanged in the formation of osteoid, but mineral calcification and deposition do not occur.3-5
Osteomalacia is normally considered a rare disorder, but it may become more common as increasing numbers of patients undergo gastric bypass operations.12,13 Primary care practitioners should monitor for this condition in such patients before serious bone loss or other problems develop.9,13,14
Vitamin D deficiency (see “Vitamin D Metabolism,”4,15-19 below), whether or not the result of gastric bypass surgery, is a major risk factor for osteomalacia. Disorders of the small bowel, the hepatobiliary system, and the pancreas are all common causes of vitamin D deficiency. Liver disease interferes with the metabolism of vitamin D. Diseases of the pancreas may cause a deficiency of bile salts, which are vital for the intestinal absorption of vitamin D.17

Restriction and Malabsorption
The case patient had undergone a gastric bypass (duodenal switch), in which a large portion of the stomach is removed and a large part of the small bowel rerouted—with both parts of the procedure causing malabsorption.11 It is in the small bowel that absorption of vitamin D and calcium takes place.
The duodenal switch gastric bypass surgery causes both restriction and malabsorption. Though similar to a biliopancreatic diversion, the duodenal switch preserves the distal stomach and the pylorus20 by way of a sleeve gastrectomy that is performed to reduce the gastric reservoir; the common channel length after revision is 100 cm, not 50 cm (as in conventional biliopancreatic diversion).13 The sleeve gastrectomy involves removal of parietal cells, reducing production of hydrochloric acid (which is necessary to break down food), and hindering the absorption of certain nutrients, including the fat-soluble vitamins, vitamin B12, and iron.12 Patients who take H2-blockers or proton pump inhibitors experience an additional decrease in the production and availability of HCl and may have an increased risk for fracture.14,20,21
In addition to its biliopancreatic diversion component, the duodenal switch diverts a large portion of the small bowel, with food restricted from moving through it. Vitamin D and protein are normally absorbed at the jejunum and ileum, but only when bile salts are present; after a duodenal switch, bile and pancreatic enzymes are not introduced into the small intestines until 75 to 100 cm before they reach the large intestine. Thus, absorption of vitamin D, protein, calcium, and other nutrients is impaired.20,22
Since phosphorus and magnesium are also absorbed at the sites of the duodenum and jejunum, malabsorption of these nutrients may occur in a patient who has undergone a duodenal switch. Although vitamin B12 is absorbed at the site of the distal ileum, it also requires gastric acid to free it from the food. Zinc absorption, which normally occurs at the site of the jejunum, may be impaired after duodenal switch surgery, and calcium supplementation, though essential, may further reduce zinc absorption.9 Iron absorption requires HCl, facilitated by the presence of vitamin C. Use of H2-blockers and proton pump inhibitors may impair iron metabolism, resulting in anemia.20
In a randomized controlled trial, Aasheim et al23 compared the effects of Roux-en-Y gastric bypass with those of duodenal switch gastric bypass on patients’ vitamin metabolism. The researchers concluded that patients who undergo a duodenal switch are at greater risk for vitamin A and D deficiencies in the first year after surgery; and for thiamine deficiency in the months following surgery as a result of malabsorption, compared with patients who undergo Roux-en-Y gastric bypass.20,23
Patient Management
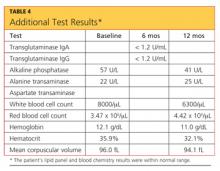
The case patient’s care necessitated consultations with endocrinology, dermatology, and gastroenterology (GI). Table 3 (below) shows the laboratory findings and the medication changes prompted by the patient’s physical exam and lab results. Table 4 lists the findings from other lab studies ordered throughout the patient’s course of treatment.
The endocrinologist was consulted at the first sign of osteopenia, and a workup was soon initiated, followed by treatment. GI was consulted six months after the beginning of treatment, when the patient began to complain of reflux while sleeping and frequent diarrhea throughout the day.
Results of esophagogastroduodenoscopy with biopsy ruled out celiac disease and mucosal ulceration, but a small hiatal hernia that was detected (< 3 cm) was determined to be an aggravating factor for the patient’s reflux. The patient was instructed in lifestyle modifications for hiatal hernia, including the need to remain upright one to two hours after eating before going to sleep to prevent aspiration. The patient was instructed to avoid taking iron and calcium within two hours of each other and to limit his alcohol intake. He was also educated in precautions against falls.
Dermatology was consulted nine months into treatment so that light therapy could be initiated, allowing the patient to take advantage of the body’s natural pathway to manufacture vitamin D3.
CONCLUSION
For post–bariatric surgery patients, primary care practitioners are in a position to coordinate care recommendations from multiple specialists, including those in nutrition, to determine the best course of action.
This case illustrates complications of bariatric surgery (malabsorption of key vitamins and minerals, wrist fracture, osteopenia, osteomalacia) that require diagnosis and treatment. The specialists and the primary care practitioner, along with the patient, had to weigh the risks and benefits of continued proton pump inhibitor use, as such medications can increase the risk for fracture. They also addressed the patient’s anemia and remained attentive to his preventive health care needs.
REFERENCES
1. Brusin JH. Update on bone densitometry. Radiol Technol. 2009;81(2):153BD-170BD.
2. Wilson CR. Essentials of bone densitometry for the medical physicist. Presented at: The American Association of Physicists in Medicine 2003 Annual Meeting; July 22-26, 2003; San Diego, CA.
3. Heber D, Greenway FL, Kaplan LM. et al. Endocrine and nutritional management of the post-bariatric surgery patient: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2010;95(11):4825-4843.
4. Osteomalacia: step-by-step diagnostic approach (2011). http://bestpractice.bmj.com/best-practice/monograph/517/diagnosis/step-by-step.html. Accessed December 18, 2012.
5. Gifre L, Peris P, Monegal A, et al. Osteomalacia revisited : a report on 28 cases. Clin Rheumatol. 2011;30(5):639-645.
6. Bingham CT, Fitzpatrick LA. Noninvasive testing in the diagnosis of osteomalacia. Am J Med. 1993;95(5):519-523.
7. World Health Organization. Obesity and overweight (May 2012). Fact Sheet No 311. www.who.int/mediacentre/factsheets/fs311/en/index.html. Accessed December 18, 2012.
8. Tanner BD, Allen JW. Complications of bariatric surgery: implications for the covering physician. Am Surg. 2009;75(2):103-112.
9. Soleymani T, Tejavanija S, Morgan S. Obesity, bariatric surgery, and bone. Curr Opin Rheumatol. 2011;23(4):396-405.
10. Koch TR, Finelli FC. Postoperative metabolic and nutritional complications of bariatric surgery. Gastroenterol Clin North Am. 2010;39(1):109-124.
11. Manchester S, Roye GD. Bariatric surgery: an overview for dietetics professionals. Nutr Today. 2011;46(6):264-275.
12. Bal BS, Finelli FC, Shope TR, Koch TR. Nutritional deficiencies after bariatric surgery. Nat Rev Endocrinol. 2012;8(9):544-546.
13. Iannelli A, Schneck AS, Dahman M, et al. Two-step laparoscopic duodenal switch for superobesity: a feasibility study. Surg Endosc. 2009;23(10):2385-2389.
14. Lalmohamed A, de Vries F, Bazelier MT, et al. Risk of fracture after bariatric surgery in the United Kingdom: population based, retrospective cohort study. BMJ. 2012;345:e5085.
15. Holrick MF. Vitamin D: important for prevention of osteoporosis, cardiovascular heart disease, type 1 diabetes, autoimmune diseases, and some cancers. South Med J. 2005;98 (10):1024-1027.
16. Kalro BN. Vitamin D and the skeleton. Alt Ther Womens Health. 2009;2(4):25-32.
17. Crowther-Radulewicz CL, McCance KL. Alterations of musculoskeletal function. In: McCance KL, Huether SE, Brashers VL, Rote NS, eds. Pathophysiology: The Biologic Basis for Disease in Adults and Children. 6th ed. Maryland Heights, MO: Mosby Elsevier; 2010:1568-1617.
18. Huether SE. Structure and function of the renal and urologic systems. In: McCance KL, Huether SE, Brashers VL, Rote NS, eds. Pathophysiology: The Biologic Basis for Disease in Adults and Children. 6th ed. Maryland Heights, MO: Mosby Elsevier; 2010:1344-1364.
19. Bhan A, Rao AD, Rao DS. Osteomalacia as a result of vitamin D deficiency. Endocrinol Metab Clin North Am. 2010;39(2):321-331.
20. Decker GA, Swain JM, Crowell MD. Gastrointestinal and nutritional complications after bariatric surgery. Am J Gastroenterol. 2007;102(11):2571-2580.
21. Targownik LE, Lix LM, Metge C, et al. Use of proton pump inhibitors and risk of osteoporosis-related fractures. CMAJ. 2008;179(4):319-326.
22. Ybarra J, Sánchez-Hernández J, Pérez A. Hypovitaminosis D and morbid obesity. Nurs Clin North Am. 2007;42(1):19-27.
23. Aasheim ET, Björkman S, Søvik TT, et al. Vitamin status after bariatric surgery: a randomized study of gastric bypass and duodenal switch. Am J Clin Nutr. 2009;90(1):15-22.
A white man, age 56, presented to his primary care clinician with wrist pain and swelling. Two days earlier, he had fallen from a step stool and landed on his right wrist. He treated the pain by resting, elevating his arm, applying ice, and taking ibuprofen 800 mg tid. He said he had lost strength in his hand and arm and was experiencing numbness and tingling in his right hand and fingers.
The patient’s medical history included hypertension, type 2 diabetes mellitus, morbid obesity, obstructive sleep apnea, asthma, carpel tunnel syndrome, and peripheral neuropathy. His surgical history was significant for duodenal switch gastric bypass surgery, performed eight years earlier, and his weight at the time of presentation was 200 lb; before his gastric bypass, he weighed 385 lb. Since the surgery, his hypertension, diabetes, asthma, and sleep apnea had all resolved. Table 1 shows a list of medications he was taking at the time of presentation.
The patient, a registered nurse, had been married for 30 years and had one child. He had quit smoking 15 years earlier, with a 43–pack-year smoking history. He reported social drinking but denied any recreational drug use. He was unaware of having any allergies to food or medication.
His vital signs on presentation were blood pressure, 110/75 mm Hg; heart rate, 53 beats/min; respiration, 18 breaths/min; O2 saturation, 97% on room air; and temperature, 97.5°F.
Physical exam revealed that the patient’s right wrist was ecchymotic and swollen with +1 pitting edema. The skin was warm and dry to the touch. Decreased range of motion was noted in the right wrist, compared with the left. Pain with point tenderness was noted at the right lateral wrist. Pulses were +3 with capillary refill of less than 3 seconds. The rest of the exam was unremarkable.
The differential diagnosis included fracture secondary to the fall, osteoporosis, osteopenia, osteomalacia, Paget’s disease, tumor, infection, and sprain or strain of the wrist. A wrist x-ray was ordered, as were the following baseline labs: complete blood count with differential (CBC), vitamin B12 and folate levels, blood chemistry, lipid profile, liver profile, total vitamin D, and sensitive thyroid-stimulating hormone. Test results are shown in Table 2.

X-ray of the wrist showed fracture only, making it possible to rule out Paget’s disease (ie, no patchy white areas noted in the bone) and tumor (no masses seen) as the immediate cause of fracture. Normal body temperature and normal white blood cell count eliminated the possibility of infection.
Because the patient was only 56 and had a history of bariatric surgery, further testing was pursued to investigate a cause for the weakened bone. Bone mineral density (BMD) testing revealed the following results:
• The lumbar spine in frontal projection measured 0.968 g/cm2 with a T-score of –2.2 and a Z-score of –2.2.
• Total BMD of the left hip was 0.863 g/cm2 with a T-score of –1.7 and a Z-score of –1.4.
• Total BMD of the left femoral neck was 0.863 g/cm2 with a T-score of 1.7 and a Z-score of –1.1.
These findings suggested osteopenia1,2 (not osteoporosis) in all sites, with a 12% decrease of BMD in the spine (suggesting increased risk for spinal fracture) and a 16.3% decrease of BMD in the hip since the patient’s most recent bone scan five years earlier (radiologist’s report). Other abnormal findings were elevated parathyroid hormone (PTH) serum, 95.7 pg/mL (reference range, 10 to 65 pg/mL); low total calcium serum, 8.7 mg/dL (reference range, 8.9 to 10.2 mg/dL), and low 25-hydroxyvitamin D total, 12.3 ng/mL (reference range, 25 to 80 ng/mL).
A 2010 clinical practice guideline from the Endocrine Society3 specifies that after malabsorptive surgery, vitamin D and calcium supplementation should be adjusted by a qualified medical professional, based on serum markers and measures of bone density. An endocrinologist who was consulted at the patient’s initial visit prescribed the following medications: vitamin D2, 50,000 U/wk PO; combined calcium citrate (vitamin D3) 500 IU with calcium 630 mg, 1 tab bid; and calcitriol 0.5 μg bid.
The patient’s final diagnosis was osteomalacia secondary to gastric bypass surgery. (See “Making the Diagnosis of Osteomalacia.”4-6)

DISCUSSION
According to 2008 data from the World Health Organization (WHO),7 1.4 billion persons older than 20 worldwide were overweight, and 200 million men and 300 million women were considered obese—meaning that one in every 10 adults worldwide is overweight or obese. In 2010, the WHO reports, 40 million children younger than 5 worldwide were considered overweight.7 Health care providers need to be prepared to care for the increasing number of patients who will undergo bariatric surgeries to treat obesity and its related comorbidities.8
Postoperative follow-up for the malabsorption deficiencies related to bariatric procedures should be performed every six months, including obtaining levels of alkaline phosphatase and others previously discussed. In addition, the Endocrine Society guideline3 recommends measuring levels of vitamin B12, albumin, pre-albumin, iron, and ferritin, and obtaining a CBC, a liver profile, glucose reading, creatinine measurement, and a metabolic profile at one month and two months after surgery, then every six months until two years after surgery, then annually if findings are stable.
Furthermore, the Endocrine Society3 recommends obtaining zinc levels every six months for the first year, then annually. An annual vitamin A level is optional.9 Yearly bone density testing is recommended until the patient’s BMD is deemed stable.3
Additionally, Koch and Finelli10 recommend performing the following labs postoperatively: hemoglobin A1C every three months; copper, magnesium, whole blood thiamine, vitamin B12, and a 24-hour urinary calcium every six months for the first three years, then once a year if findings remain stable.
Use of alcohol should be discouraged among patients who have undergone bariatric surgery, as its use alters micronutrient requirements and metabolism. Alcohol consumption may also contribute to dumping syndrome (ie, rapid gastric emptying).11
Any patient with a history of malabsorptive bypass surgery who complains of neurologic, visual, or skin disorders, anemia, or edema may require a further workup to rule out other absorptive deficiencies. These include vitamins A, E, and B12, zinc, folate, thiamine, niacin, selenium, and ferritin.10
Osteomalacia
Metabolic bone diseases can result from genetics, dietary factors, medication use, surgery, or hormonal irregularities. They alter the normal biochemical reactions in bone structure.
The three most common forms of metabolic bone disease are osteoporosis, osteopenia, and osteomalacia. The WHO diagnostic classifications and associated T-scores for bone mineral density1,2 indicate a T-score above –1.0 as normal. A score between –1.0 and –2.5 is indicative of osteopenia, and a score below –2.5 indicates osteoporosis. A T-score below –2.5 in the patient with a history of fragility fracture indicates severe osteoporosis.1,2
In osteomalacia, bone volume remains unchanged, but mineralization of osteoid in the mature compact and spongy bone is either delayed or inadequate. The remolding cycle continues unchanged in the formation of osteoid, but mineral calcification and deposition do not occur.3-5
Osteomalacia is normally considered a rare disorder, but it may become more common as increasing numbers of patients undergo gastric bypass operations.12,13 Primary care practitioners should monitor for this condition in such patients before serious bone loss or other problems develop.9,13,14
Vitamin D deficiency (see “Vitamin D Metabolism,”4,15-19 below), whether or not the result of gastric bypass surgery, is a major risk factor for osteomalacia. Disorders of the small bowel, the hepatobiliary system, and the pancreas are all common causes of vitamin D deficiency. Liver disease interferes with the metabolism of vitamin D. Diseases of the pancreas may cause a deficiency of bile salts, which are vital for the intestinal absorption of vitamin D.17

Restriction and Malabsorption
The case patient had undergone a gastric bypass (duodenal switch), in which a large portion of the stomach is removed and a large part of the small bowel rerouted—with both parts of the procedure causing malabsorption.11 It is in the small bowel that absorption of vitamin D and calcium takes place.
The duodenal switch gastric bypass surgery causes both restriction and malabsorption. Though similar to a biliopancreatic diversion, the duodenal switch preserves the distal stomach and the pylorus20 by way of a sleeve gastrectomy that is performed to reduce the gastric reservoir; the common channel length after revision is 100 cm, not 50 cm (as in conventional biliopancreatic diversion).13 The sleeve gastrectomy involves removal of parietal cells, reducing production of hydrochloric acid (which is necessary to break down food), and hindering the absorption of certain nutrients, including the fat-soluble vitamins, vitamin B12, and iron.12 Patients who take H2-blockers or proton pump inhibitors experience an additional decrease in the production and availability of HCl and may have an increased risk for fracture.14,20,21
In addition to its biliopancreatic diversion component, the duodenal switch diverts a large portion of the small bowel, with food restricted from moving through it. Vitamin D and protein are normally absorbed at the jejunum and ileum, but only when bile salts are present; after a duodenal switch, bile and pancreatic enzymes are not introduced into the small intestines until 75 to 100 cm before they reach the large intestine. Thus, absorption of vitamin D, protein, calcium, and other nutrients is impaired.20,22
Since phosphorus and magnesium are also absorbed at the sites of the duodenum and jejunum, malabsorption of these nutrients may occur in a patient who has undergone a duodenal switch. Although vitamin B12 is absorbed at the site of the distal ileum, it also requires gastric acid to free it from the food. Zinc absorption, which normally occurs at the site of the jejunum, may be impaired after duodenal switch surgery, and calcium supplementation, though essential, may further reduce zinc absorption.9 Iron absorption requires HCl, facilitated by the presence of vitamin C. Use of H2-blockers and proton pump inhibitors may impair iron metabolism, resulting in anemia.20
In a randomized controlled trial, Aasheim et al23 compared the effects of Roux-en-Y gastric bypass with those of duodenal switch gastric bypass on patients’ vitamin metabolism. The researchers concluded that patients who undergo a duodenal switch are at greater risk for vitamin A and D deficiencies in the first year after surgery; and for thiamine deficiency in the months following surgery as a result of malabsorption, compared with patients who undergo Roux-en-Y gastric bypass.20,23
Patient Management
The case patient’s care necessitated consultations with endocrinology, dermatology, and gastroenterology (GI). Table 3 (below) shows the laboratory findings and the medication changes prompted by the patient’s physical exam and lab results. Table 4 lists the findings from other lab studies ordered throughout the patient’s course of treatment.

The endocrinologist was consulted at the first sign of osteopenia, and a workup was soon initiated, followed by treatment. GI was consulted six months after the beginning of treatment, when the patient began to complain of reflux while sleeping and frequent diarrhea throughout the day.
Results of esophagogastroduodenoscopy with biopsy ruled out celiac disease and mucosal ulceration, but a small hiatal hernia that was detected (< 3 cm) was determined to be an aggravating factor for the patient’s reflux. The patient was instructed in lifestyle modifications for hiatal hernia, including the need to remain upright one to two hours after eating before going to sleep to prevent aspiration. The patient was instructed to avoid taking iron and calcium within two hours of each other and to limit his alcohol intake. He was also educated in precautions against falls.
Dermatology was consulted nine months into treatment so that light therapy could be initiated, allowing the patient to take advantage of the body’s natural pathway to manufacture vitamin D3.
CONCLUSION
For post–bariatric surgery patients, primary care practitioners are in a position to coordinate care recommendations from multiple specialists, including those in nutrition, to determine the best course of action.
This case illustrates complications of bariatric surgery (malabsorption of key vitamins and minerals, wrist fracture, osteopenia, osteomalacia) that require diagnosis and treatment. The specialists and the primary care practitioner, along with the patient, had to weigh the risks and benefits of continued proton pump inhibitor use, as such medications can increase the risk for fracture. They also addressed the patient’s anemia and remained attentive to his preventive health care needs.
REFERENCES
1. Brusin JH. Update on bone densitometry. Radiol Technol. 2009;81(2):153BD-170BD.
2. Wilson CR. Essentials of bone densitometry for the medical physicist. Presented at: The American Association of Physicists in Medicine 2003 Annual Meeting; July 22-26, 2003; San Diego, CA.
3. Heber D, Greenway FL, Kaplan LM. et al. Endocrine and nutritional management of the post-bariatric surgery patient: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2010;95(11):4825-4843.
4. Osteomalacia: step-by-step diagnostic approach (2011). http://bestpractice.bmj.com/best-practice/monograph/517/diagnosis/step-by-step.html. Accessed December 18, 2012.
5. Gifre L, Peris P, Monegal A, et al. Osteomalacia revisited : a report on 28 cases. Clin Rheumatol. 2011;30(5):639-645.
6. Bingham CT, Fitzpatrick LA. Noninvasive testing in the diagnosis of osteomalacia. Am J Med. 1993;95(5):519-523.
7. World Health Organization. Obesity and overweight (May 2012). Fact Sheet No 311. www.who.int/mediacentre/factsheets/fs311/en/index.html. Accessed December 18, 2012.
8. Tanner BD, Allen JW. Complications of bariatric surgery: implications for the covering physician. Am Surg. 2009;75(2):103-112.
9. Soleymani T, Tejavanija S, Morgan S. Obesity, bariatric surgery, and bone. Curr Opin Rheumatol. 2011;23(4):396-405.
10. Koch TR, Finelli FC. Postoperative metabolic and nutritional complications of bariatric surgery. Gastroenterol Clin North Am. 2010;39(1):109-124.
11. Manchester S, Roye GD. Bariatric surgery: an overview for dietetics professionals. Nutr Today. 2011;46(6):264-275.
12. Bal BS, Finelli FC, Shope TR, Koch TR. Nutritional deficiencies after bariatric surgery. Nat Rev Endocrinol. 2012;8(9):544-546.
13. Iannelli A, Schneck AS, Dahman M, et al. Two-step laparoscopic duodenal switch for superobesity: a feasibility study. Surg Endosc. 2009;23(10):2385-2389.
14. Lalmohamed A, de Vries F, Bazelier MT, et al. Risk of fracture after bariatric surgery in the United Kingdom: population based, retrospective cohort study. BMJ. 2012;345:e5085.
15. Holrick MF. Vitamin D: important for prevention of osteoporosis, cardiovascular heart disease, type 1 diabetes, autoimmune diseases, and some cancers. South Med J. 2005;98 (10):1024-1027.
16. Kalro BN. Vitamin D and the skeleton. Alt Ther Womens Health. 2009;2(4):25-32.
17. Crowther-Radulewicz CL, McCance KL. Alterations of musculoskeletal function. In: McCance KL, Huether SE, Brashers VL, Rote NS, eds. Pathophysiology: The Biologic Basis for Disease in Adults and Children. 6th ed. Maryland Heights, MO: Mosby Elsevier; 2010:1568-1617.
18. Huether SE. Structure and function of the renal and urologic systems. In: McCance KL, Huether SE, Brashers VL, Rote NS, eds. Pathophysiology: The Biologic Basis for Disease in Adults and Children. 6th ed. Maryland Heights, MO: Mosby Elsevier; 2010:1344-1364.
19. Bhan A, Rao AD, Rao DS. Osteomalacia as a result of vitamin D deficiency. Endocrinol Metab Clin North Am. 2010;39(2):321-331.
20. Decker GA, Swain JM, Crowell MD. Gastrointestinal and nutritional complications after bariatric surgery. Am J Gastroenterol. 2007;102(11):2571-2580.
21. Targownik LE, Lix LM, Metge C, et al. Use of proton pump inhibitors and risk of osteoporosis-related fractures. CMAJ. 2008;179(4):319-326.
22. Ybarra J, Sánchez-Hernández J, Pérez A. Hypovitaminosis D and morbid obesity. Nurs Clin North Am. 2007;42(1):19-27.
23. Aasheim ET, Björkman S, Søvik TT, et al. Vitamin status after bariatric surgery: a randomized study of gastric bypass and duodenal switch. Am J Clin Nutr. 2009;90(1):15-22.
A white man, age 56, presented to his primary care clinician with wrist pain and swelling. Two days earlier, he had fallen from a step stool and landed on his right wrist. He treated the pain by resting, elevating his arm, applying ice, and taking ibuprofen 800 mg tid. He said he had lost strength in his hand and arm and was experiencing numbness and tingling in his right hand and fingers.
The patient’s medical history included hypertension, type 2 diabetes mellitus, morbid obesity, obstructive sleep apnea, asthma, carpel tunnel syndrome, and peripheral neuropathy. His surgical history was significant for duodenal switch gastric bypass surgery, performed eight years earlier, and his weight at the time of presentation was 200 lb; before his gastric bypass, he weighed 385 lb. Since the surgery, his hypertension, diabetes, asthma, and sleep apnea had all resolved. Table 1 shows a list of medications he was taking at the time of presentation.
The patient, a registered nurse, had been married for 30 years and had one child. He had quit smoking 15 years earlier, with a 43–pack-year smoking history. He reported social drinking but denied any recreational drug use. He was unaware of having any allergies to food or medication.
His vital signs on presentation were blood pressure, 110/75 mm Hg; heart rate, 53 beats/min; respiration, 18 breaths/min; O2 saturation, 97% on room air; and temperature, 97.5°F.
Physical exam revealed that the patient’s right wrist was ecchymotic and swollen with +1 pitting edema. The skin was warm and dry to the touch. Decreased range of motion was noted in the right wrist, compared with the left. Pain with point tenderness was noted at the right lateral wrist. Pulses were +3 with capillary refill of less than 3 seconds. The rest of the exam was unremarkable.
The differential diagnosis included fracture secondary to the fall, osteoporosis, osteopenia, osteomalacia, Paget’s disease, tumor, infection, and sprain or strain of the wrist. A wrist x-ray was ordered, as were the following baseline labs: complete blood count with differential (CBC), vitamin B12 and folate levels, blood chemistry, lipid profile, liver profile, total vitamin D, and sensitive thyroid-stimulating hormone. Test results are shown in Table 2.

X-ray of the wrist showed fracture only, making it possible to rule out Paget’s disease (ie, no patchy white areas noted in the bone) and tumor (no masses seen) as the immediate cause of fracture. Normal body temperature and normal white blood cell count eliminated the possibility of infection.
Because the patient was only 56 and had a history of bariatric surgery, further testing was pursued to investigate a cause for the weakened bone. Bone mineral density (BMD) testing revealed the following results:
• The lumbar spine in frontal projection measured 0.968 g/cm2 with a T-score of –2.2 and a Z-score of –2.2.
• Total BMD of the left hip was 0.863 g/cm2 with a T-score of –1.7 and a Z-score of –1.4.
• Total BMD of the left femoral neck was 0.863 g/cm2 with a T-score of 1.7 and a Z-score of –1.1.
These findings suggested osteopenia1,2 (not osteoporosis) in all sites, with a 12% decrease of BMD in the spine (suggesting increased risk for spinal fracture) and a 16.3% decrease of BMD in the hip since the patient’s most recent bone scan five years earlier (radiologist’s report). Other abnormal findings were elevated parathyroid hormone (PTH) serum, 95.7 pg/mL (reference range, 10 to 65 pg/mL); low total calcium serum, 8.7 mg/dL (reference range, 8.9 to 10.2 mg/dL), and low 25-hydroxyvitamin D total, 12.3 ng/mL (reference range, 25 to 80 ng/mL).
A 2010 clinical practice guideline from the Endocrine Society3 specifies that after malabsorptive surgery, vitamin D and calcium supplementation should be adjusted by a qualified medical professional, based on serum markers and measures of bone density. An endocrinologist who was consulted at the patient’s initial visit prescribed the following medications: vitamin D2, 50,000 U/wk PO; combined calcium citrate (vitamin D3) 500 IU with calcium 630 mg, 1 tab bid; and calcitriol 0.5 μg bid.
The patient’s final diagnosis was osteomalacia secondary to gastric bypass surgery. (See “Making the Diagnosis of Osteomalacia.”4-6)

DISCUSSION
According to 2008 data from the World Health Organization (WHO),7 1.4 billion persons older than 20 worldwide were overweight, and 200 million men and 300 million women were considered obese—meaning that one in every 10 adults worldwide is overweight or obese. In 2010, the WHO reports, 40 million children younger than 5 worldwide were considered overweight.7 Health care providers need to be prepared to care for the increasing number of patients who will undergo bariatric surgeries to treat obesity and its related comorbidities.8
Postoperative follow-up for the malabsorption deficiencies related to bariatric procedures should be performed every six months, including obtaining levels of alkaline phosphatase and others previously discussed. In addition, the Endocrine Society guideline3 recommends measuring levels of vitamin B12, albumin, pre-albumin, iron, and ferritin, and obtaining a CBC, a liver profile, glucose reading, creatinine measurement, and a metabolic profile at one month and two months after surgery, then every six months until two years after surgery, then annually if findings are stable.
Furthermore, the Endocrine Society3 recommends obtaining zinc levels every six months for the first year, then annually. An annual vitamin A level is optional.9 Yearly bone density testing is recommended until the patient’s BMD is deemed stable.3
Additionally, Koch and Finelli10 recommend performing the following labs postoperatively: hemoglobin A1C every three months; copper, magnesium, whole blood thiamine, vitamin B12, and a 24-hour urinary calcium every six months for the first three years, then once a year if findings remain stable.
Use of alcohol should be discouraged among patients who have undergone bariatric surgery, as its use alters micronutrient requirements and metabolism. Alcohol consumption may also contribute to dumping syndrome (ie, rapid gastric emptying).11
Any patient with a history of malabsorptive bypass surgery who complains of neurologic, visual, or skin disorders, anemia, or edema may require a further workup to rule out other absorptive deficiencies. These include vitamins A, E, and B12, zinc, folate, thiamine, niacin, selenium, and ferritin.10
Osteomalacia
Metabolic bone diseases can result from genetics, dietary factors, medication use, surgery, or hormonal irregularities. They alter the normal biochemical reactions in bone structure.
The three most common forms of metabolic bone disease are osteoporosis, osteopenia, and osteomalacia. The WHO diagnostic classifications and associated T-scores for bone mineral density1,2 indicate a T-score above –1.0 as normal. A score between –1.0 and –2.5 is indicative of osteopenia, and a score below –2.5 indicates osteoporosis. A T-score below –2.5 in the patient with a history of fragility fracture indicates severe osteoporosis.1,2
In osteomalacia, bone volume remains unchanged, but mineralization of osteoid in the mature compact and spongy bone is either delayed or inadequate. The remolding cycle continues unchanged in the formation of osteoid, but mineral calcification and deposition do not occur.3-5
Osteomalacia is normally considered a rare disorder, but it may become more common as increasing numbers of patients undergo gastric bypass operations.12,13 Primary care practitioners should monitor for this condition in such patients before serious bone loss or other problems develop.9,13,14
Vitamin D deficiency (see “Vitamin D Metabolism,”4,15-19 below), whether or not the result of gastric bypass surgery, is a major risk factor for osteomalacia. Disorders of the small bowel, the hepatobiliary system, and the pancreas are all common causes of vitamin D deficiency. Liver disease interferes with the metabolism of vitamin D. Diseases of the pancreas may cause a deficiency of bile salts, which are vital for the intestinal absorption of vitamin D.17

Restriction and Malabsorption
The case patient had undergone a gastric bypass (duodenal switch), in which a large portion of the stomach is removed and a large part of the small bowel rerouted—with both parts of the procedure causing malabsorption.11 It is in the small bowel that absorption of vitamin D and calcium takes place.
The duodenal switch gastric bypass surgery causes both restriction and malabsorption. Though similar to a biliopancreatic diversion, the duodenal switch preserves the distal stomach and the pylorus20 by way of a sleeve gastrectomy that is performed to reduce the gastric reservoir; the common channel length after revision is 100 cm, not 50 cm (as in conventional biliopancreatic diversion).13 The sleeve gastrectomy involves removal of parietal cells, reducing production of hydrochloric acid (which is necessary to break down food), and hindering the absorption of certain nutrients, including the fat-soluble vitamins, vitamin B12, and iron.12 Patients who take H2-blockers or proton pump inhibitors experience an additional decrease in the production and availability of HCl and may have an increased risk for fracture.14,20,21
In addition to its biliopancreatic diversion component, the duodenal switch diverts a large portion of the small bowel, with food restricted from moving through it. Vitamin D and protein are normally absorbed at the jejunum and ileum, but only when bile salts are present; after a duodenal switch, bile and pancreatic enzymes are not introduced into the small intestines until 75 to 100 cm before they reach the large intestine. Thus, absorption of vitamin D, protein, calcium, and other nutrients is impaired.20,22
Since phosphorus and magnesium are also absorbed at the sites of the duodenum and jejunum, malabsorption of these nutrients may occur in a patient who has undergone a duodenal switch. Although vitamin B12 is absorbed at the site of the distal ileum, it also requires gastric acid to free it from the food. Zinc absorption, which normally occurs at the site of the jejunum, may be impaired after duodenal switch surgery, and calcium supplementation, though essential, may further reduce zinc absorption.9 Iron absorption requires HCl, facilitated by the presence of vitamin C. Use of H2-blockers and proton pump inhibitors may impair iron metabolism, resulting in anemia.20
In a randomized controlled trial, Aasheim et al23 compared the effects of Roux-en-Y gastric bypass with those of duodenal switch gastric bypass on patients’ vitamin metabolism. The researchers concluded that patients who undergo a duodenal switch are at greater risk for vitamin A and D deficiencies in the first year after surgery; and for thiamine deficiency in the months following surgery as a result of malabsorption, compared with patients who undergo Roux-en-Y gastric bypass.20,23
Patient Management
The case patient’s care necessitated consultations with endocrinology, dermatology, and gastroenterology (GI). Table 3 (below) shows the laboratory findings and the medication changes prompted by the patient’s physical exam and lab results. Table 4 lists the findings from other lab studies ordered throughout the patient’s course of treatment.

The endocrinologist was consulted at the first sign of osteopenia, and a workup was soon initiated, followed by treatment. GI was consulted six months after the beginning of treatment, when the patient began to complain of reflux while sleeping and frequent diarrhea throughout the day.
Results of esophagogastroduodenoscopy with biopsy ruled out celiac disease and mucosal ulceration, but a small hiatal hernia that was detected (< 3 cm) was determined to be an aggravating factor for the patient’s reflux. The patient was instructed in lifestyle modifications for hiatal hernia, including the need to remain upright one to two hours after eating before going to sleep to prevent aspiration. The patient was instructed to avoid taking iron and calcium within two hours of each other and to limit his alcohol intake. He was also educated in precautions against falls.
Dermatology was consulted nine months into treatment so that light therapy could be initiated, allowing the patient to take advantage of the body’s natural pathway to manufacture vitamin D3.
CONCLUSION
For post–bariatric surgery patients, primary care practitioners are in a position to coordinate care recommendations from multiple specialists, including those in nutrition, to determine the best course of action.
This case illustrates complications of bariatric surgery (malabsorption of key vitamins and minerals, wrist fracture, osteopenia, osteomalacia) that require diagnosis and treatment. The specialists and the primary care practitioner, along with the patient, had to weigh the risks and benefits of continued proton pump inhibitor use, as such medications can increase the risk for fracture. They also addressed the patient’s anemia and remained attentive to his preventive health care needs.
REFERENCES
1. Brusin JH. Update on bone densitometry. Radiol Technol. 2009;81(2):153BD-170BD.
2. Wilson CR. Essentials of bone densitometry for the medical physicist. Presented at: The American Association of Physicists in Medicine 2003 Annual Meeting; July 22-26, 2003; San Diego, CA.
3. Heber D, Greenway FL, Kaplan LM. et al. Endocrine and nutritional management of the post-bariatric surgery patient: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2010;95(11):4825-4843.
4. Osteomalacia: step-by-step diagnostic approach (2011). http://bestpractice.bmj.com/best-practice/monograph/517/diagnosis/step-by-step.html. Accessed December 18, 2012.
5. Gifre L, Peris P, Monegal A, et al. Osteomalacia revisited : a report on 28 cases. Clin Rheumatol. 2011;30(5):639-645.
6. Bingham CT, Fitzpatrick LA. Noninvasive testing in the diagnosis of osteomalacia. Am J Med. 1993;95(5):519-523.
7. World Health Organization. Obesity and overweight (May 2012). Fact Sheet No 311. www.who.int/mediacentre/factsheets/fs311/en/index.html. Accessed December 18, 2012.
8. Tanner BD, Allen JW. Complications of bariatric surgery: implications for the covering physician. Am Surg. 2009;75(2):103-112.
9. Soleymani T, Tejavanija S, Morgan S. Obesity, bariatric surgery, and bone. Curr Opin Rheumatol. 2011;23(4):396-405.
10. Koch TR, Finelli FC. Postoperative metabolic and nutritional complications of bariatric surgery. Gastroenterol Clin North Am. 2010;39(1):109-124.
11. Manchester S, Roye GD. Bariatric surgery: an overview for dietetics professionals. Nutr Today. 2011;46(6):264-275.
12. Bal BS, Finelli FC, Shope TR, Koch TR. Nutritional deficiencies after bariatric surgery. Nat Rev Endocrinol. 2012;8(9):544-546.
13. Iannelli A, Schneck AS, Dahman M, et al. Two-step laparoscopic duodenal switch for superobesity: a feasibility study. Surg Endosc. 2009;23(10):2385-2389.
14. Lalmohamed A, de Vries F, Bazelier MT, et al. Risk of fracture after bariatric surgery in the United Kingdom: population based, retrospective cohort study. BMJ. 2012;345:e5085.
15. Holrick MF. Vitamin D: important for prevention of osteoporosis, cardiovascular heart disease, type 1 diabetes, autoimmune diseases, and some cancers. South Med J. 2005;98 (10):1024-1027.
16. Kalro BN. Vitamin D and the skeleton. Alt Ther Womens Health. 2009;2(4):25-32.
17. Crowther-Radulewicz CL, McCance KL. Alterations of musculoskeletal function. In: McCance KL, Huether SE, Brashers VL, Rote NS, eds. Pathophysiology: The Biologic Basis for Disease in Adults and Children. 6th ed. Maryland Heights, MO: Mosby Elsevier; 2010:1568-1617.
18. Huether SE. Structure and function of the renal and urologic systems. In: McCance KL, Huether SE, Brashers VL, Rote NS, eds. Pathophysiology: The Biologic Basis for Disease in Adults and Children. 6th ed. Maryland Heights, MO: Mosby Elsevier; 2010:1344-1364.
19. Bhan A, Rao AD, Rao DS. Osteomalacia as a result of vitamin D deficiency. Endocrinol Metab Clin North Am. 2010;39(2):321-331.
20. Decker GA, Swain JM, Crowell MD. Gastrointestinal and nutritional complications after bariatric surgery. Am J Gastroenterol. 2007;102(11):2571-2580.
21. Targownik LE, Lix LM, Metge C, et al. Use of proton pump inhibitors and risk of osteoporosis-related fractures. CMAJ. 2008;179(4):319-326.
22. Ybarra J, Sánchez-Hernández J, Pérez A. Hypovitaminosis D and morbid obesity. Nurs Clin North Am. 2007;42(1):19-27.
23. Aasheim ET, Björkman S, Søvik TT, et al. Vitamin status after bariatric surgery: a randomized study of gastric bypass and duodenal switch. Am J Clin Nutr. 2009;90(1):15-22.
Grand Rounds: Girl, 6, With Facial Weakness
A 6-year-old girl was brought to a pediatric emergency department (ED) in Atlanta by her mother. The mother stated that during the previous hour, she had noticed that her daughter’s face seemed weaker on the right side.
The night before, the child had said, “I can’t blink my eye”; when her mother asked her to demonstrate, the child seemed to be able to blink both eyes appropriately, and she had no further complaints. The next morning, the child complained of the light being too bright and asked to wear her mother’s sunglasses. In the course of the day, she continued to complain of eye discomfort, which she described as “stinging” and “sore.” The mother could see nothing abnormal, but by late afternoon noticed that her daughter’s smile and facial movements were asymmetrical. She immediately took her to the pediatric ED.
The child had no significant medical history and no surgical history. Her vaccination schedule was current, and she denied any recent illnesses. The mother could recall no exposures to infections or tick bites, no rashes, and no trauma to the face or head. The mother and child were visiting Atlanta from northeastern Florida.
The review of systems was negative for headache, fever, chills, rash, earache, sore throat, cough, rhinorrhea, vision changes, weight loss, or change in appetite or disposition. The child was afebrile, and the other vital signs were within normal limits.
Physical examination revealed an alert child who was calm and conversant. Her height was 45” and weight, 43 lb. Otoscopic exam showed normal ears and tympanic membranes with no sign of otitis media or ear pathology. No throat redness, tonsillar enlargement, or lymphadenopathies were noted. Breath sounds were clear, and heart rhythm and rate were regular without murmur.
The patient’s left eye appeared normal, and the right eye was mildly erythematic without drainage or swelling; since corneal abrasion was not suspected, a slit lamp examination was not performed. Upon neurologic examination, right eye ptosis with incomplete lid closure, asymmetrical mouth movement with smile, and a diminished nasal labial fold crease were noted on the right side. When the child was asked to raise her eyebrows and wrinkle her forehead, asymmetrical forehead creases were apparent. All other cranial nerve functions were intact, and motor and sensory responses, including gait and reflexes, were assessed as normal. Unilateral dysfunction of right-sided cranial nerve VII (CN VII), including forehead involvement, was confirmed, consistent with a grade of III to IV on the House-Brackmann (maximum, VI)1,2 facial nerve grading scale.
Based on the rapid onset of unilateral facial nerve paresis (FNP) and an otherwise normal exam, the patient was diagnosed with Bell’s palsy. No further testing was done, and the child was given a dose of oral prednisolone 40 mg in the ED, with a prescription for four more days of oral prednisolone at 15 mg bid. The need for eye protection and lubrication was emphasized to the mother, who was given lubricating eye drops to administer. The mother was also instructed to follow up with the child’s primary care practitioner upon their return to Florida.
The child was seen by her pediatrician three days later. Her facial paresis had not worsened in the interim, and the pediatrician declined to extend the course of corticosteroids or to add an antiviral medication. At the mother’s request, the child was referred to a pediatric otolaryngologist, who saw her the following day and adjusted the treatment plan. The child was prescribed prednisolone elixir 20 mg bid for one week, followed by a tapering dose for the second week. In addition, she was prescribed oral acyclovir 400 mg qid for 10 days. Her mother was instructed to return with the child in one week for audiometry testing.
Discussion
Idiopathic FNP, commonly referred to as Bell’s palsy, is defined as an acute unilateral paresis of the facial nerve without detectable underlying cause.3,4 It most commonly occurs among persons ages 15 to 45, with a prevalence rate of 15 to 30 cases per 100,000 persons. The peak incidence of Bell’s palsy is in the fourth decade of life. Diabetic patients and pregnant women are disproportionately affected by idiopathic FNP.2,5 About 8% to 10% of patients will experience a recurrence of Bell’s palsy within 10 years.2,6
Pediatric FNP can be congenital or acquired. Congenital FNP is most often associated with birth trauma and occurs at a rate of 2.1 cases per 1,000 births. Rare genetic syndromes can also manifest with FNP and will most often present with other syndromic anomalies noted at birth.7
Acquired FNP is two to four times less common in children than adults, with an estimated prevalence of 2.7 per 100,000 patients younger than 10. Children account for only a small proportion of subjects in published studies that address diagnosis and management of FNP.3 While the presentation of FNP is much the same in adults and children, some notable differences in etiology exist.2,3,7-9 Infectious, traumatic, or neoplastic causes of FNP are more common among children than adults and must be distinguished from idiopathic FNP.7,9-11
Decisions regarding diagnostic testing, pharmacologic treatment, and referral must be guided by the history and physical exam, neurologic exam, and clinical judgment. Being able to identify or exclude alarming causes of FNP, such as neoplasm, will aid the primary care practitioner in treatment and referral practices for this condition.
Pathophysiology
CN VII, the facial nerve, has a broad scope of function that incorporates both sensory and motor pathways. The brachial nerve portion of CN VII controls the muscles of voluntary facial expression. CN VII also autonomically innervates the lacrimal gland and submandibular gland and governs sensation from part of the ear as well as taste from the anterior two-thirds of the tongue.4
The precise pathophysiology involved in FNP remains an area of continuing debate, but infectious, vascular, immunologic, and genetic causes have been hypothesized.7,12 Inflammation and subsequent nerve damage along CN VII caused by an infectious process is thought to be the most likely explanation for the pathogenesis of acquired FNP in both adults and children.5,13
Herpes simplex virus 1 (HSV-1) has been suggested as the virus most commonly linked to FNP in both adults and children, but it is unlikely to be the sole cause.5,6,9 Data from a three-year prospective study of FNP cases in children support a relationship between pediatric FNP and HSV-1 infection.14 Other infectious causes implicated in pediatric FNP are Lyme disease, Epstein-Barr, varicella zoster virus, rubella, coxsackie virus, adenovirus, and otitis media.4,7,9
Presentation, History, and Physical Exam
Most children with idiopathic FNP will present with sudden-onset facial asymmetry and may have decreased tearing, loss of the conjunctival reflex (leading to difficulty closing the eye), an inability to hold the lips tightly together, and difficulty keeping food in the mouth. Complaints of otalgia, speech disturbances, hyperacusis, and altered sense of taste are common.2,7 Recent occurrence of an upper respiratory infection is often reported in the history of a pediatric patient with FNP.3,7,15,16
Idiopathic FNP is essentially a diagnosis of exclusion.3,5 A meticulous history must be conducted, including any recent illnesses, trauma to the face or head, vaccines, rashes, and travel. Assessment of the head, eyes, ears, nose, and throat, and a careful neurologic history must be conducted to identify nonidiopathic causes of FNP (see Table 15-7,9). Facial weakness can progress from mild palsy to complete paralysis over one to two weeks5; therefore, a careful history of the progression of facial weakness should be ascertained and documented.5,17
A full neurologic exam is essential. Cranial nerves I through XII should be evaluated; any malfunction of a cranial nerve other than CN VII could be indicative of a tumor or process other than idiopathic FNP. Assessment of facial nerve function is imperative, as this factor is the most important for predicting recovery; it can also aid in formulating a prognosis and directing treatment.5,9,17
The House-Brackmann facial nerve grading system1,2 is considered the gold standard for grading severity of facial paresis9 (see Table 21,2 ). A clear distinction between paresis (partial or incomplete palsy) and paralysis (complete palsy) must be made. Pediatric patients with an incomplete palsy have an improved chance of full recovery.17,18
Any abnormalities in the peripheral neurologic exam should prompt further testing. FNP not involving the forehead musculature, gradual progression of paresis, and weakness in any extremity could be indicative of a central lesion. FNP has been the presenting symptom in various neoplastic processes, including leukemia, cholesteatoma, and astrocytoma.3,7,9
Otitis media is a frequent cause of FNP among children.9-11 Thus, a thorough examination of the ear canal, tympanic membrane, and hearing should be performed. The throat and oropharynx should be inspected, and the parotid gland palpated. Any swelling or abnormalities warrant further investigation.
Lyme disease presenting with FNP is more common in children than adults. This may be related to the increased likelihood for children to be bitten by ticks in the head and neck areas. Frequently, FNP associated with Lyme disease is bilateral—as often as 25% of the time.19 Headache, onset of symptoms during peak Lyme season, or bilateral FNP should raise the clinician’s suspicion for Lyme disease.7,9,19
An accurate assessment of blood pressure is essential, as severe hypertension may be implicated in FNP in children.3,5,7 One literature review reported that hypertension was the origin of FNP in 3% to 17% of affected children.20 Vascular hemorrhage induced by hypertension is thought to cause nerve compression and subsequent FNP.7
A bilateral eye exam is also important. Irritation is likely, and the patient with any suspected corneal abrasion or damage should be referred to an ophthalmologist.6,18
Laboratory Testing and Imaging
Diagnostic testing that facilitates the exclusion of known causes of FNP should be considered, as there is no specific laboratory test to confirm the diagnosis. A complete blood count, Lyme titers, cerebrospinal fluid analysis, CT, and/or MRI may be warranted, based on the clinical presentation.7-9 In children in whom Lyme disease is suspected (ie, those living in tick-endemic areas or with recent tick bites), serologic testing should be performed. Lumbar puncture and an evaluation of cerebrospinal fluid may be necessary in cases in which meningitis cannot be excluded.7,9
Specialized diagnostic tests are not routinely recommended for patients with paresis that is improving. Audiometry and evaluation of the stapedial reflex may help guide treatment decisions for patients whose condition is not improving. In children, the presence or return of the stapedial reflex within three weeks of disease onset is predictive of complete recovery.5 In patients who experience complete paralysis or unimproved paresis, results of electrodiagnostic testing (in particular, evoked facial nerve electroneuronography) can help forecast recovery of facial nerve function.5,17
Treatment and Management
Treatment for FNP in adults is controversial, and even more so for the pediatric patient. Treatment decisions consist of eye care, corticosteroids, antiviral medications, and appropriate referrals.
Eye care. Eye lubrication and protection should be implemented immediately. Protecting the cornea is paramount; thorough lubrication of the eye is the mainstay of treatment.18 Artificial tears should be used frequently during the day, and an ointment should be applied to the eye at night. Use of eye patches is controversial, as they may actually cause corneal injury.7,9 Taping the eye shut at night may prevent trauma during sleep, but this option must be considered carefully.9,18
Corticosteroids. Early initiation of corticosteroids should be considered for all patients with FNP, including children.2,7,9,17 Studies are inconclusive as to whether steroid therapy is beneficial in children with idiopathic FNP. However, two 2010 reviews of pediatric FNP recommend early initiation of steroids for children with acute-onset FNP, particularly when facial paresis is evaluated at a House-Brackmann grade V or VI.7,9 The American Academy of Family Physicians (AAFP) recommends a tapering course of prednisone for all patients, begun as soon as possible.6 The prednisone dosage for pediatric patients is usually 1.0 mg/kg/d, split into two doses, for six days, followed by a tapering dose for four days.5
Antivirals and antibiotic therapy. When an infectious cause of FNP is known, appropriate antibiotic or antiviral therapy should begin. If the patient lives in or has traveled to an area endemic for Lyme disease, empiric treatment may be appropriate. When Ramsay Hunt syndrome is diagnosed or herpetic lesions are visible, antiviral treatment should be initiated.7
Antiviral therapy for idiopathic FNP is the most controversial of the treatment decisions. In 2001, the American Academy of Neurology concluded that no clear benefit from acyclovir could be ascertained, although it might be effective.13 This was affirmed in a recently updated Cochrane review of antiviral therapy for idiopathic FNP.12 Antiviral therapy alone showed no benefit, compared with placebo; however, combined antiviral and corticosteroid therapy was more effective than placebo alone in recovery outcomes. Antivirals may benefit pediatric patients and should be considered early when the cause of FNP is viral or idiopathic.7,9
Referrals. Initial presentation and course of paresis should guide referral patterns for the pediatric patient presenting with FNP. The American Academy of Pediatrics (AAP) recommends referral to an otolaryngologist for any infant or child with FNP.21 The AAFP recommends referral to a specialist for any patient who does not show improvement within two weeks.6
In patients with complete paralysis, early surgical intervention may be considered, and referral should be made promptly for electrodiagnostic testing and surgical consult. In cases in which otitis media causes FNP, myringotomy and tube insertion are indicated, and appropriate referral should be made.7,9
Outcomes
|The prognosis in children with FNP is good, and most will recover completely.2,9-11,22 Idiopathic and infectious etiologies of FNP seem to have the greatest likelihood for complete recovery.10,11,16,17 Recovery appears to be affected by etiology, degree of paresis, and treatment. How these factors coalesce is not fully understood, and up to 20% of children may have mild to moderate residual facial nerve dysfunction.10,11,19,22
The Case Patient
The child’s facial nerve function gradually returned over a three-week period, with no residual deficit (see Figures 1a, 1b, and 1c). Results of the audiometry screening on day 10 were normal, showing a positive stapedial reflex. An MRI, performed four months after the initial paralysis to rule out any tumors, yielded normal results.
This case highlights the differing management of pediatric Bell’s palsy among emergency, pediatric, and specialized providers. This child was managed more aggressively under the care of an otolaryngologist with a two-week course of steroids, antiviral medication for 10 days, and a follow-up MRI to rule out any evidence of a tumor. The need for further research to guide practice in the pediatric patient with Bell’s palsy is apparent.
Conclusion
FNP in the pediatric population is rare and more likely to have an identifiable cause than among adults. Careful examination should reveal differential diagnoses that warrant treatment and referrals. The main causes of FNP that should not be missed are otitis media, hypertension, varicella zoster virus (Ramsay Hunt syndrome), neoplastic processes, and Lyme disease.
Practitioners should have a high index of suspicion for nonidiopathic causes of FNP when a child has a neurologic exam that includes facial paresis of gradual onset, abnormal function of other cranial nerves, lack of forehead muscle weakness, or peripheral abnormalities. In addition to the history and exam, blood work and radiologic imaging can aid the practitioner in ruling in or out nonidiopathic causes of FNP.
Grading of facial palsy severity using the House-Brackmann scale helps guide prognosis and referral choices. Referral to a specialist in otolaryngology is appropriate and recommended by the AAP. Referral should be made to an ophthalmologist if any suspicion of corneal abrasion exists.
Treatment in children should consist of eye care and steroids. Antiviral therapy should be considered on an individualized basis and when evidence of HSV or varicella exists. Parents should be advised about the importance of eye care in a child with FNP (see Table 35-7,9,17,18,22).
The emotional stress associated with FNP can be significant for both children and adults; fear of lifelong facial deformity can be psychologically debilitating. Yet a favorable prognosis for recovery of facial nerve function can be relayed to anxious parents.
1. House JW, Brackmann DE. Facial nerve grading system. Otolaryngol Head Neck Surg. 1985;93(2): 146-147.
2. Finsterer J. Management of peripheral facial nerve palsy. Eur Arch Otorhinolaryngol. 2008;265(7):743-752.
3. Lunan R, Nagarajan L. Bell’s palsy: a guideline proposal following a review of practice. J Paediatr Child Health. 2008;44(4):219-220.
4. Blosser CG, Reider-Demer M. Neurologic disorders. In: Burns CE, Dunn AM, Brady MA, et al, eds. Pediatric Primary Care. 4th ed. St. Louis: Saunders Elsevier; 2008:634-672.
5. Singhi P, Jain V. Bell’s palsy in children. Semin Pediatr Neurol. 2003;10(4):289-297.
6. Tiemstra JD, Khatkhate N. Bell’s palsy: diagnosis and management. Am Fam Physician. 2007;76(7):997-1002.
7. Lorch M, Teach SJ. Facial nerve palsy: Etiology and approach to diagnosis and treatment. Pediatr Emerg Care. 2010;26(10):763-769.
8. El-Hawrani AS, Eng CY, Ahmed SK, et al. General practitioners’ referral pattern for children with acute facial paralysis. J Laryngol Otol. 2005;119(7):540-542.
9. Shargorodsky J, Lin HW, Gopen Q. Facial nerve palsy in the pediatric population. Clin Pediatr (Phila). 2010;49(5):411-417.
10. Wang CH, Chang YC, Shih HM, et al. Facial palsy in children: emergency department management and outcome. Pediatr Emerg Care. 2010;26(2):121-125.
11. Evans AK, Licameli G, Brietzke S, et al. Pediatric facial nerve paralysis: patients, management and outcomes. Int J Pediatr Otorhinolaryngol. 2005;69(11):1521-1528.
12. Lockhart P, Daly F, Pitkethly M, et al. Antiviral treatment for Bell’s palsy (idiopathic facial paralysis). Cochrane Database Syst Rev. 2009;(4):CD001869.
13. Grogan PM, Gronseth GS. Practice parameter: steroids, acyclovir, and surgery for Bell’s palsy (an evidence-based review): report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology. 2001;56(7):830-836.
14. Khine H, Mayers M, Avner JR, et al. Association between herpes simplex virus-1 infection and idiopathic unilateral facial paralysis in children and adolescents. Pediatr Infect Dis J. 2008;27(5):468-469.
15. Tsai HS, Chang LY, Lu CY, et al. Epidemiology and treatment of Bell’s palsy in children in northern Taiwan. J Microbiol Immunol Infect. 2009;42(4):351-356.
16. Cha CI, Hong CK, Park MS, Yeo SG. Comparison of facial nerve paralysis in adults and children. Yonsei Med J. 2008;49(5):725-734.
17. Linder TE, Abdelkafy W, Cavero-Vanek S. The management of peripheral facial nerve palsy: “paresis” versus “paralysis” and sources of ambiguity in study designs. Otol Neurotol. 2010;31(2):319-327.
18. Rahman I, Sadiq SA. Ophthalmic management of facial nerve palsy: a review. Surv Ophthalmol. 2007;52(2):121-144.
19. Skogman BH, Croner S, Odkvist L. Acute facial palsy in children: a 2-year follow-up with focus on Lyme neuroborreliosis. Int J Pediatr Otorhinolaryngol. 2003;67(6):597-602.
20. Siegler RL, Brewer ED, Corneli HM, Thompson JA. Hypertension first seen as facial paralysis: case reports and review of the literature. Pediatrics. 1991;87(3):387-389.
21. Surgical Advisory Panel, American Academy of Pediatrics. Guidelines for referral to pediatric surgical specialists. Pediatrics. 2002;110(1 pt 1):187-191.
22. Chen WX, Wong V. Prognosis of Bell’s palsy in children: analysis of 29 cases. Brain Dev. 2005; 27(7):504-508.
A 6-year-old girl was brought to a pediatric emergency department (ED) in Atlanta by her mother. The mother stated that during the previous hour, she had noticed that her daughter’s face seemed weaker on the right side.
The night before, the child had said, “I can’t blink my eye”; when her mother asked her to demonstrate, the child seemed to be able to blink both eyes appropriately, and she had no further complaints. The next morning, the child complained of the light being too bright and asked to wear her mother’s sunglasses. In the course of the day, she continued to complain of eye discomfort, which she described as “stinging” and “sore.” The mother could see nothing abnormal, but by late afternoon noticed that her daughter’s smile and facial movements were asymmetrical. She immediately took her to the pediatric ED.
The child had no significant medical history and no surgical history. Her vaccination schedule was current, and she denied any recent illnesses. The mother could recall no exposures to infections or tick bites, no rashes, and no trauma to the face or head. The mother and child were visiting Atlanta from northeastern Florida.
The review of systems was negative for headache, fever, chills, rash, earache, sore throat, cough, rhinorrhea, vision changes, weight loss, or change in appetite or disposition. The child was afebrile, and the other vital signs were within normal limits.
Physical examination revealed an alert child who was calm and conversant. Her height was 45” and weight, 43 lb. Otoscopic exam showed normal ears and tympanic membranes with no sign of otitis media or ear pathology. No throat redness, tonsillar enlargement, or lymphadenopathies were noted. Breath sounds were clear, and heart rhythm and rate were regular without murmur.
The patient’s left eye appeared normal, and the right eye was mildly erythematic without drainage or swelling; since corneal abrasion was not suspected, a slit lamp examination was not performed. Upon neurologic examination, right eye ptosis with incomplete lid closure, asymmetrical mouth movement with smile, and a diminished nasal labial fold crease were noted on the right side. When the child was asked to raise her eyebrows and wrinkle her forehead, asymmetrical forehead creases were apparent. All other cranial nerve functions were intact, and motor and sensory responses, including gait and reflexes, were assessed as normal. Unilateral dysfunction of right-sided cranial nerve VII (CN VII), including forehead involvement, was confirmed, consistent with a grade of III to IV on the House-Brackmann (maximum, VI)1,2 facial nerve grading scale.
Based on the rapid onset of unilateral facial nerve paresis (FNP) and an otherwise normal exam, the patient was diagnosed with Bell’s palsy. No further testing was done, and the child was given a dose of oral prednisolone 40 mg in the ED, with a prescription for four more days of oral prednisolone at 15 mg bid. The need for eye protection and lubrication was emphasized to the mother, who was given lubricating eye drops to administer. The mother was also instructed to follow up with the child’s primary care practitioner upon their return to Florida.
The child was seen by her pediatrician three days later. Her facial paresis had not worsened in the interim, and the pediatrician declined to extend the course of corticosteroids or to add an antiviral medication. At the mother’s request, the child was referred to a pediatric otolaryngologist, who saw her the following day and adjusted the treatment plan. The child was prescribed prednisolone elixir 20 mg bid for one week, followed by a tapering dose for the second week. In addition, she was prescribed oral acyclovir 400 mg qid for 10 days. Her mother was instructed to return with the child in one week for audiometry testing.
Discussion
Idiopathic FNP, commonly referred to as Bell’s palsy, is defined as an acute unilateral paresis of the facial nerve without detectable underlying cause.3,4 It most commonly occurs among persons ages 15 to 45, with a prevalence rate of 15 to 30 cases per 100,000 persons. The peak incidence of Bell’s palsy is in the fourth decade of life. Diabetic patients and pregnant women are disproportionately affected by idiopathic FNP.2,5 About 8% to 10% of patients will experience a recurrence of Bell’s palsy within 10 years.2,6
Pediatric FNP can be congenital or acquired. Congenital FNP is most often associated with birth trauma and occurs at a rate of 2.1 cases per 1,000 births. Rare genetic syndromes can also manifest with FNP and will most often present with other syndromic anomalies noted at birth.7
Acquired FNP is two to four times less common in children than adults, with an estimated prevalence of 2.7 per 100,000 patients younger than 10. Children account for only a small proportion of subjects in published studies that address diagnosis and management of FNP.3 While the presentation of FNP is much the same in adults and children, some notable differences in etiology exist.2,3,7-9 Infectious, traumatic, or neoplastic causes of FNP are more common among children than adults and must be distinguished from idiopathic FNP.7,9-11
Decisions regarding diagnostic testing, pharmacologic treatment, and referral must be guided by the history and physical exam, neurologic exam, and clinical judgment. Being able to identify or exclude alarming causes of FNP, such as neoplasm, will aid the primary care practitioner in treatment and referral practices for this condition.
Pathophysiology
CN VII, the facial nerve, has a broad scope of function that incorporates both sensory and motor pathways. The brachial nerve portion of CN VII controls the muscles of voluntary facial expression. CN VII also autonomically innervates the lacrimal gland and submandibular gland and governs sensation from part of the ear as well as taste from the anterior two-thirds of the tongue.4
The precise pathophysiology involved in FNP remains an area of continuing debate, but infectious, vascular, immunologic, and genetic causes have been hypothesized.7,12 Inflammation and subsequent nerve damage along CN VII caused by an infectious process is thought to be the most likely explanation for the pathogenesis of acquired FNP in both adults and children.5,13
Herpes simplex virus 1 (HSV-1) has been suggested as the virus most commonly linked to FNP in both adults and children, but it is unlikely to be the sole cause.5,6,9 Data from a three-year prospective study of FNP cases in children support a relationship between pediatric FNP and HSV-1 infection.14 Other infectious causes implicated in pediatric FNP are Lyme disease, Epstein-Barr, varicella zoster virus, rubella, coxsackie virus, adenovirus, and otitis media.4,7,9
Presentation, History, and Physical Exam
Most children with idiopathic FNP will present with sudden-onset facial asymmetry and may have decreased tearing, loss of the conjunctival reflex (leading to difficulty closing the eye), an inability to hold the lips tightly together, and difficulty keeping food in the mouth. Complaints of otalgia, speech disturbances, hyperacusis, and altered sense of taste are common.2,7 Recent occurrence of an upper respiratory infection is often reported in the history of a pediatric patient with FNP.3,7,15,16
Idiopathic FNP is essentially a diagnosis of exclusion.3,5 A meticulous history must be conducted, including any recent illnesses, trauma to the face or head, vaccines, rashes, and travel. Assessment of the head, eyes, ears, nose, and throat, and a careful neurologic history must be conducted to identify nonidiopathic causes of FNP (see Table 15-7,9). Facial weakness can progress from mild palsy to complete paralysis over one to two weeks5; therefore, a careful history of the progression of facial weakness should be ascertained and documented.5,17
A full neurologic exam is essential. Cranial nerves I through XII should be evaluated; any malfunction of a cranial nerve other than CN VII could be indicative of a tumor or process other than idiopathic FNP. Assessment of facial nerve function is imperative, as this factor is the most important for predicting recovery; it can also aid in formulating a prognosis and directing treatment.5,9,17
The House-Brackmann facial nerve grading system1,2 is considered the gold standard for grading severity of facial paresis9 (see Table 21,2 ). A clear distinction between paresis (partial or incomplete palsy) and paralysis (complete palsy) must be made. Pediatric patients with an incomplete palsy have an improved chance of full recovery.17,18
Any abnormalities in the peripheral neurologic exam should prompt further testing. FNP not involving the forehead musculature, gradual progression of paresis, and weakness in any extremity could be indicative of a central lesion. FNP has been the presenting symptom in various neoplastic processes, including leukemia, cholesteatoma, and astrocytoma.3,7,9
Otitis media is a frequent cause of FNP among children.9-11 Thus, a thorough examination of the ear canal, tympanic membrane, and hearing should be performed. The throat and oropharynx should be inspected, and the parotid gland palpated. Any swelling or abnormalities warrant further investigation.
Lyme disease presenting with FNP is more common in children than adults. This may be related to the increased likelihood for children to be bitten by ticks in the head and neck areas. Frequently, FNP associated with Lyme disease is bilateral—as often as 25% of the time.19 Headache, onset of symptoms during peak Lyme season, or bilateral FNP should raise the clinician’s suspicion for Lyme disease.7,9,19
An accurate assessment of blood pressure is essential, as severe hypertension may be implicated in FNP in children.3,5,7 One literature review reported that hypertension was the origin of FNP in 3% to 17% of affected children.20 Vascular hemorrhage induced by hypertension is thought to cause nerve compression and subsequent FNP.7
A bilateral eye exam is also important. Irritation is likely, and the patient with any suspected corneal abrasion or damage should be referred to an ophthalmologist.6,18
Laboratory Testing and Imaging
Diagnostic testing that facilitates the exclusion of known causes of FNP should be considered, as there is no specific laboratory test to confirm the diagnosis. A complete blood count, Lyme titers, cerebrospinal fluid analysis, CT, and/or MRI may be warranted, based on the clinical presentation.7-9 In children in whom Lyme disease is suspected (ie, those living in tick-endemic areas or with recent tick bites), serologic testing should be performed. Lumbar puncture and an evaluation of cerebrospinal fluid may be necessary in cases in which meningitis cannot be excluded.7,9
Specialized diagnostic tests are not routinely recommended for patients with paresis that is improving. Audiometry and evaluation of the stapedial reflex may help guide treatment decisions for patients whose condition is not improving. In children, the presence or return of the stapedial reflex within three weeks of disease onset is predictive of complete recovery.5 In patients who experience complete paralysis or unimproved paresis, results of electrodiagnostic testing (in particular, evoked facial nerve electroneuronography) can help forecast recovery of facial nerve function.5,17
Treatment and Management
Treatment for FNP in adults is controversial, and even more so for the pediatric patient. Treatment decisions consist of eye care, corticosteroids, antiviral medications, and appropriate referrals.
Eye care. Eye lubrication and protection should be implemented immediately. Protecting the cornea is paramount; thorough lubrication of the eye is the mainstay of treatment.18 Artificial tears should be used frequently during the day, and an ointment should be applied to the eye at night. Use of eye patches is controversial, as they may actually cause corneal injury.7,9 Taping the eye shut at night may prevent trauma during sleep, but this option must be considered carefully.9,18
Corticosteroids. Early initiation of corticosteroids should be considered for all patients with FNP, including children.2,7,9,17 Studies are inconclusive as to whether steroid therapy is beneficial in children with idiopathic FNP. However, two 2010 reviews of pediatric FNP recommend early initiation of steroids for children with acute-onset FNP, particularly when facial paresis is evaluated at a House-Brackmann grade V or VI.7,9 The American Academy of Family Physicians (AAFP) recommends a tapering course of prednisone for all patients, begun as soon as possible.6 The prednisone dosage for pediatric patients is usually 1.0 mg/kg/d, split into two doses, for six days, followed by a tapering dose for four days.5
Antivirals and antibiotic therapy. When an infectious cause of FNP is known, appropriate antibiotic or antiviral therapy should begin. If the patient lives in or has traveled to an area endemic for Lyme disease, empiric treatment may be appropriate. When Ramsay Hunt syndrome is diagnosed or herpetic lesions are visible, antiviral treatment should be initiated.7
Antiviral therapy for idiopathic FNP is the most controversial of the treatment decisions. In 2001, the American Academy of Neurology concluded that no clear benefit from acyclovir could be ascertained, although it might be effective.13 This was affirmed in a recently updated Cochrane review of antiviral therapy for idiopathic FNP.12 Antiviral therapy alone showed no benefit, compared with placebo; however, combined antiviral and corticosteroid therapy was more effective than placebo alone in recovery outcomes. Antivirals may benefit pediatric patients and should be considered early when the cause of FNP is viral or idiopathic.7,9
Referrals. Initial presentation and course of paresis should guide referral patterns for the pediatric patient presenting with FNP. The American Academy of Pediatrics (AAP) recommends referral to an otolaryngologist for any infant or child with FNP.21 The AAFP recommends referral to a specialist for any patient who does not show improvement within two weeks.6
In patients with complete paralysis, early surgical intervention may be considered, and referral should be made promptly for electrodiagnostic testing and surgical consult. In cases in which otitis media causes FNP, myringotomy and tube insertion are indicated, and appropriate referral should be made.7,9
Outcomes
|The prognosis in children with FNP is good, and most will recover completely.2,9-11,22 Idiopathic and infectious etiologies of FNP seem to have the greatest likelihood for complete recovery.10,11,16,17 Recovery appears to be affected by etiology, degree of paresis, and treatment. How these factors coalesce is not fully understood, and up to 20% of children may have mild to moderate residual facial nerve dysfunction.10,11,19,22
The Case Patient
The child’s facial nerve function gradually returned over a three-week period, with no residual deficit (see Figures 1a, 1b, and 1c). Results of the audiometry screening on day 10 were normal, showing a positive stapedial reflex. An MRI, performed four months after the initial paralysis to rule out any tumors, yielded normal results.
This case highlights the differing management of pediatric Bell’s palsy among emergency, pediatric, and specialized providers. This child was managed more aggressively under the care of an otolaryngologist with a two-week course of steroids, antiviral medication for 10 days, and a follow-up MRI to rule out any evidence of a tumor. The need for further research to guide practice in the pediatric patient with Bell’s palsy is apparent.
Conclusion
FNP in the pediatric population is rare and more likely to have an identifiable cause than among adults. Careful examination should reveal differential diagnoses that warrant treatment and referrals. The main causes of FNP that should not be missed are otitis media, hypertension, varicella zoster virus (Ramsay Hunt syndrome), neoplastic processes, and Lyme disease.
Practitioners should have a high index of suspicion for nonidiopathic causes of FNP when a child has a neurologic exam that includes facial paresis of gradual onset, abnormal function of other cranial nerves, lack of forehead muscle weakness, or peripheral abnormalities. In addition to the history and exam, blood work and radiologic imaging can aid the practitioner in ruling in or out nonidiopathic causes of FNP.
Grading of facial palsy severity using the House-Brackmann scale helps guide prognosis and referral choices. Referral to a specialist in otolaryngology is appropriate and recommended by the AAP. Referral should be made to an ophthalmologist if any suspicion of corneal abrasion exists.
Treatment in children should consist of eye care and steroids. Antiviral therapy should be considered on an individualized basis and when evidence of HSV or varicella exists. Parents should be advised about the importance of eye care in a child with FNP (see Table 35-7,9,17,18,22).
The emotional stress associated with FNP can be significant for both children and adults; fear of lifelong facial deformity can be psychologically debilitating. Yet a favorable prognosis for recovery of facial nerve function can be relayed to anxious parents.
A 6-year-old girl was brought to a pediatric emergency department (ED) in Atlanta by her mother. The mother stated that during the previous hour, she had noticed that her daughter’s face seemed weaker on the right side.
The night before, the child had said, “I can’t blink my eye”; when her mother asked her to demonstrate, the child seemed to be able to blink both eyes appropriately, and she had no further complaints. The next morning, the child complained of the light being too bright and asked to wear her mother’s sunglasses. In the course of the day, she continued to complain of eye discomfort, which she described as “stinging” and “sore.” The mother could see nothing abnormal, but by late afternoon noticed that her daughter’s smile and facial movements were asymmetrical. She immediately took her to the pediatric ED.
The child had no significant medical history and no surgical history. Her vaccination schedule was current, and she denied any recent illnesses. The mother could recall no exposures to infections or tick bites, no rashes, and no trauma to the face or head. The mother and child were visiting Atlanta from northeastern Florida.
The review of systems was negative for headache, fever, chills, rash, earache, sore throat, cough, rhinorrhea, vision changes, weight loss, or change in appetite or disposition. The child was afebrile, and the other vital signs were within normal limits.
Physical examination revealed an alert child who was calm and conversant. Her height was 45” and weight, 43 lb. Otoscopic exam showed normal ears and tympanic membranes with no sign of otitis media or ear pathology. No throat redness, tonsillar enlargement, or lymphadenopathies were noted. Breath sounds were clear, and heart rhythm and rate were regular without murmur.
The patient’s left eye appeared normal, and the right eye was mildly erythematic without drainage or swelling; since corneal abrasion was not suspected, a slit lamp examination was not performed. Upon neurologic examination, right eye ptosis with incomplete lid closure, asymmetrical mouth movement with smile, and a diminished nasal labial fold crease were noted on the right side. When the child was asked to raise her eyebrows and wrinkle her forehead, asymmetrical forehead creases were apparent. All other cranial nerve functions were intact, and motor and sensory responses, including gait and reflexes, were assessed as normal. Unilateral dysfunction of right-sided cranial nerve VII (CN VII), including forehead involvement, was confirmed, consistent with a grade of III to IV on the House-Brackmann (maximum, VI)1,2 facial nerve grading scale.
Based on the rapid onset of unilateral facial nerve paresis (FNP) and an otherwise normal exam, the patient was diagnosed with Bell’s palsy. No further testing was done, and the child was given a dose of oral prednisolone 40 mg in the ED, with a prescription for four more days of oral prednisolone at 15 mg bid. The need for eye protection and lubrication was emphasized to the mother, who was given lubricating eye drops to administer. The mother was also instructed to follow up with the child’s primary care practitioner upon their return to Florida.
The child was seen by her pediatrician three days later. Her facial paresis had not worsened in the interim, and the pediatrician declined to extend the course of corticosteroids or to add an antiviral medication. At the mother’s request, the child was referred to a pediatric otolaryngologist, who saw her the following day and adjusted the treatment plan. The child was prescribed prednisolone elixir 20 mg bid for one week, followed by a tapering dose for the second week. In addition, she was prescribed oral acyclovir 400 mg qid for 10 days. Her mother was instructed to return with the child in one week for audiometry testing.
Discussion
Idiopathic FNP, commonly referred to as Bell’s palsy, is defined as an acute unilateral paresis of the facial nerve without detectable underlying cause.3,4 It most commonly occurs among persons ages 15 to 45, with a prevalence rate of 15 to 30 cases per 100,000 persons. The peak incidence of Bell’s palsy is in the fourth decade of life. Diabetic patients and pregnant women are disproportionately affected by idiopathic FNP.2,5 About 8% to 10% of patients will experience a recurrence of Bell’s palsy within 10 years.2,6
Pediatric FNP can be congenital or acquired. Congenital FNP is most often associated with birth trauma and occurs at a rate of 2.1 cases per 1,000 births. Rare genetic syndromes can also manifest with FNP and will most often present with other syndromic anomalies noted at birth.7
Acquired FNP is two to four times less common in children than adults, with an estimated prevalence of 2.7 per 100,000 patients younger than 10. Children account for only a small proportion of subjects in published studies that address diagnosis and management of FNP.3 While the presentation of FNP is much the same in adults and children, some notable differences in etiology exist.2,3,7-9 Infectious, traumatic, or neoplastic causes of FNP are more common among children than adults and must be distinguished from idiopathic FNP.7,9-11
Decisions regarding diagnostic testing, pharmacologic treatment, and referral must be guided by the history and physical exam, neurologic exam, and clinical judgment. Being able to identify or exclude alarming causes of FNP, such as neoplasm, will aid the primary care practitioner in treatment and referral practices for this condition.
Pathophysiology
CN VII, the facial nerve, has a broad scope of function that incorporates both sensory and motor pathways. The brachial nerve portion of CN VII controls the muscles of voluntary facial expression. CN VII also autonomically innervates the lacrimal gland and submandibular gland and governs sensation from part of the ear as well as taste from the anterior two-thirds of the tongue.4
The precise pathophysiology involved in FNP remains an area of continuing debate, but infectious, vascular, immunologic, and genetic causes have been hypothesized.7,12 Inflammation and subsequent nerve damage along CN VII caused by an infectious process is thought to be the most likely explanation for the pathogenesis of acquired FNP in both adults and children.5,13
Herpes simplex virus 1 (HSV-1) has been suggested as the virus most commonly linked to FNP in both adults and children, but it is unlikely to be the sole cause.5,6,9 Data from a three-year prospective study of FNP cases in children support a relationship between pediatric FNP and HSV-1 infection.14 Other infectious causes implicated in pediatric FNP are Lyme disease, Epstein-Barr, varicella zoster virus, rubella, coxsackie virus, adenovirus, and otitis media.4,7,9
Presentation, History, and Physical Exam
Most children with idiopathic FNP will present with sudden-onset facial asymmetry and may have decreased tearing, loss of the conjunctival reflex (leading to difficulty closing the eye), an inability to hold the lips tightly together, and difficulty keeping food in the mouth. Complaints of otalgia, speech disturbances, hyperacusis, and altered sense of taste are common.2,7 Recent occurrence of an upper respiratory infection is often reported in the history of a pediatric patient with FNP.3,7,15,16
Idiopathic FNP is essentially a diagnosis of exclusion.3,5 A meticulous history must be conducted, including any recent illnesses, trauma to the face or head, vaccines, rashes, and travel. Assessment of the head, eyes, ears, nose, and throat, and a careful neurologic history must be conducted to identify nonidiopathic causes of FNP (see Table 15-7,9). Facial weakness can progress from mild palsy to complete paralysis over one to two weeks5; therefore, a careful history of the progression of facial weakness should be ascertained and documented.5,17
A full neurologic exam is essential. Cranial nerves I through XII should be evaluated; any malfunction of a cranial nerve other than CN VII could be indicative of a tumor or process other than idiopathic FNP. Assessment of facial nerve function is imperative, as this factor is the most important for predicting recovery; it can also aid in formulating a prognosis and directing treatment.5,9,17
The House-Brackmann facial nerve grading system1,2 is considered the gold standard for grading severity of facial paresis9 (see Table 21,2 ). A clear distinction between paresis (partial or incomplete palsy) and paralysis (complete palsy) must be made. Pediatric patients with an incomplete palsy have an improved chance of full recovery.17,18
Any abnormalities in the peripheral neurologic exam should prompt further testing. FNP not involving the forehead musculature, gradual progression of paresis, and weakness in any extremity could be indicative of a central lesion. FNP has been the presenting symptom in various neoplastic processes, including leukemia, cholesteatoma, and astrocytoma.3,7,9
Otitis media is a frequent cause of FNP among children.9-11 Thus, a thorough examination of the ear canal, tympanic membrane, and hearing should be performed. The throat and oropharynx should be inspected, and the parotid gland palpated. Any swelling or abnormalities warrant further investigation.
Lyme disease presenting with FNP is more common in children than adults. This may be related to the increased likelihood for children to be bitten by ticks in the head and neck areas. Frequently, FNP associated with Lyme disease is bilateral—as often as 25% of the time.19 Headache, onset of symptoms during peak Lyme season, or bilateral FNP should raise the clinician’s suspicion for Lyme disease.7,9,19
An accurate assessment of blood pressure is essential, as severe hypertension may be implicated in FNP in children.3,5,7 One literature review reported that hypertension was the origin of FNP in 3% to 17% of affected children.20 Vascular hemorrhage induced by hypertension is thought to cause nerve compression and subsequent FNP.7
A bilateral eye exam is also important. Irritation is likely, and the patient with any suspected corneal abrasion or damage should be referred to an ophthalmologist.6,18
Laboratory Testing and Imaging
Diagnostic testing that facilitates the exclusion of known causes of FNP should be considered, as there is no specific laboratory test to confirm the diagnosis. A complete blood count, Lyme titers, cerebrospinal fluid analysis, CT, and/or MRI may be warranted, based on the clinical presentation.7-9 In children in whom Lyme disease is suspected (ie, those living in tick-endemic areas or with recent tick bites), serologic testing should be performed. Lumbar puncture and an evaluation of cerebrospinal fluid may be necessary in cases in which meningitis cannot be excluded.7,9
Specialized diagnostic tests are not routinely recommended for patients with paresis that is improving. Audiometry and evaluation of the stapedial reflex may help guide treatment decisions for patients whose condition is not improving. In children, the presence or return of the stapedial reflex within three weeks of disease onset is predictive of complete recovery.5 In patients who experience complete paralysis or unimproved paresis, results of electrodiagnostic testing (in particular, evoked facial nerve electroneuronography) can help forecast recovery of facial nerve function.5,17
Treatment and Management
Treatment for FNP in adults is controversial, and even more so for the pediatric patient. Treatment decisions consist of eye care, corticosteroids, antiviral medications, and appropriate referrals.
Eye care. Eye lubrication and protection should be implemented immediately. Protecting the cornea is paramount; thorough lubrication of the eye is the mainstay of treatment.18 Artificial tears should be used frequently during the day, and an ointment should be applied to the eye at night. Use of eye patches is controversial, as they may actually cause corneal injury.7,9 Taping the eye shut at night may prevent trauma during sleep, but this option must be considered carefully.9,18
Corticosteroids. Early initiation of corticosteroids should be considered for all patients with FNP, including children.2,7,9,17 Studies are inconclusive as to whether steroid therapy is beneficial in children with idiopathic FNP. However, two 2010 reviews of pediatric FNP recommend early initiation of steroids for children with acute-onset FNP, particularly when facial paresis is evaluated at a House-Brackmann grade V or VI.7,9 The American Academy of Family Physicians (AAFP) recommends a tapering course of prednisone for all patients, begun as soon as possible.6 The prednisone dosage for pediatric patients is usually 1.0 mg/kg/d, split into two doses, for six days, followed by a tapering dose for four days.5
Antivirals and antibiotic therapy. When an infectious cause of FNP is known, appropriate antibiotic or antiviral therapy should begin. If the patient lives in or has traveled to an area endemic for Lyme disease, empiric treatment may be appropriate. When Ramsay Hunt syndrome is diagnosed or herpetic lesions are visible, antiviral treatment should be initiated.7
Antiviral therapy for idiopathic FNP is the most controversial of the treatment decisions. In 2001, the American Academy of Neurology concluded that no clear benefit from acyclovir could be ascertained, although it might be effective.13 This was affirmed in a recently updated Cochrane review of antiviral therapy for idiopathic FNP.12 Antiviral therapy alone showed no benefit, compared with placebo; however, combined antiviral and corticosteroid therapy was more effective than placebo alone in recovery outcomes. Antivirals may benefit pediatric patients and should be considered early when the cause of FNP is viral or idiopathic.7,9
Referrals. Initial presentation and course of paresis should guide referral patterns for the pediatric patient presenting with FNP. The American Academy of Pediatrics (AAP) recommends referral to an otolaryngologist for any infant or child with FNP.21 The AAFP recommends referral to a specialist for any patient who does not show improvement within two weeks.6
In patients with complete paralysis, early surgical intervention may be considered, and referral should be made promptly for electrodiagnostic testing and surgical consult. In cases in which otitis media causes FNP, myringotomy and tube insertion are indicated, and appropriate referral should be made.7,9
Outcomes
|The prognosis in children with FNP is good, and most will recover completely.2,9-11,22 Idiopathic and infectious etiologies of FNP seem to have the greatest likelihood for complete recovery.10,11,16,17 Recovery appears to be affected by etiology, degree of paresis, and treatment. How these factors coalesce is not fully understood, and up to 20% of children may have mild to moderate residual facial nerve dysfunction.10,11,19,22
The Case Patient
The child’s facial nerve function gradually returned over a three-week period, with no residual deficit (see Figures 1a, 1b, and 1c). Results of the audiometry screening on day 10 were normal, showing a positive stapedial reflex. An MRI, performed four months after the initial paralysis to rule out any tumors, yielded normal results.
This case highlights the differing management of pediatric Bell’s palsy among emergency, pediatric, and specialized providers. This child was managed more aggressively under the care of an otolaryngologist with a two-week course of steroids, antiviral medication for 10 days, and a follow-up MRI to rule out any evidence of a tumor. The need for further research to guide practice in the pediatric patient with Bell’s palsy is apparent.
Conclusion
FNP in the pediatric population is rare and more likely to have an identifiable cause than among adults. Careful examination should reveal differential diagnoses that warrant treatment and referrals. The main causes of FNP that should not be missed are otitis media, hypertension, varicella zoster virus (Ramsay Hunt syndrome), neoplastic processes, and Lyme disease.
Practitioners should have a high index of suspicion for nonidiopathic causes of FNP when a child has a neurologic exam that includes facial paresis of gradual onset, abnormal function of other cranial nerves, lack of forehead muscle weakness, or peripheral abnormalities. In addition to the history and exam, blood work and radiologic imaging can aid the practitioner in ruling in or out nonidiopathic causes of FNP.
Grading of facial palsy severity using the House-Brackmann scale helps guide prognosis and referral choices. Referral to a specialist in otolaryngology is appropriate and recommended by the AAP. Referral should be made to an ophthalmologist if any suspicion of corneal abrasion exists.
Treatment in children should consist of eye care and steroids. Antiviral therapy should be considered on an individualized basis and when evidence of HSV or varicella exists. Parents should be advised about the importance of eye care in a child with FNP (see Table 35-7,9,17,18,22).
The emotional stress associated with FNP can be significant for both children and adults; fear of lifelong facial deformity can be psychologically debilitating. Yet a favorable prognosis for recovery of facial nerve function can be relayed to anxious parents.
1. House JW, Brackmann DE. Facial nerve grading system. Otolaryngol Head Neck Surg. 1985;93(2): 146-147.
2. Finsterer J. Management of peripheral facial nerve palsy. Eur Arch Otorhinolaryngol. 2008;265(7):743-752.
3. Lunan R, Nagarajan L. Bell’s palsy: a guideline proposal following a review of practice. J Paediatr Child Health. 2008;44(4):219-220.
4. Blosser CG, Reider-Demer M. Neurologic disorders. In: Burns CE, Dunn AM, Brady MA, et al, eds. Pediatric Primary Care. 4th ed. St. Louis: Saunders Elsevier; 2008:634-672.
5. Singhi P, Jain V. Bell’s palsy in children. Semin Pediatr Neurol. 2003;10(4):289-297.
6. Tiemstra JD, Khatkhate N. Bell’s palsy: diagnosis and management. Am Fam Physician. 2007;76(7):997-1002.
7. Lorch M, Teach SJ. Facial nerve palsy: Etiology and approach to diagnosis and treatment. Pediatr Emerg Care. 2010;26(10):763-769.
8. El-Hawrani AS, Eng CY, Ahmed SK, et al. General practitioners’ referral pattern for children with acute facial paralysis. J Laryngol Otol. 2005;119(7):540-542.
9. Shargorodsky J, Lin HW, Gopen Q. Facial nerve palsy in the pediatric population. Clin Pediatr (Phila). 2010;49(5):411-417.
10. Wang CH, Chang YC, Shih HM, et al. Facial palsy in children: emergency department management and outcome. Pediatr Emerg Care. 2010;26(2):121-125.
11. Evans AK, Licameli G, Brietzke S, et al. Pediatric facial nerve paralysis: patients, management and outcomes. Int J Pediatr Otorhinolaryngol. 2005;69(11):1521-1528.
12. Lockhart P, Daly F, Pitkethly M, et al. Antiviral treatment for Bell’s palsy (idiopathic facial paralysis). Cochrane Database Syst Rev. 2009;(4):CD001869.
13. Grogan PM, Gronseth GS. Practice parameter: steroids, acyclovir, and surgery for Bell’s palsy (an evidence-based review): report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology. 2001;56(7):830-836.
14. Khine H, Mayers M, Avner JR, et al. Association between herpes simplex virus-1 infection and idiopathic unilateral facial paralysis in children and adolescents. Pediatr Infect Dis J. 2008;27(5):468-469.
15. Tsai HS, Chang LY, Lu CY, et al. Epidemiology and treatment of Bell’s palsy in children in northern Taiwan. J Microbiol Immunol Infect. 2009;42(4):351-356.
16. Cha CI, Hong CK, Park MS, Yeo SG. Comparison of facial nerve paralysis in adults and children. Yonsei Med J. 2008;49(5):725-734.
17. Linder TE, Abdelkafy W, Cavero-Vanek S. The management of peripheral facial nerve palsy: “paresis” versus “paralysis” and sources of ambiguity in study designs. Otol Neurotol. 2010;31(2):319-327.
18. Rahman I, Sadiq SA. Ophthalmic management of facial nerve palsy: a review. Surv Ophthalmol. 2007;52(2):121-144.
19. Skogman BH, Croner S, Odkvist L. Acute facial palsy in children: a 2-year follow-up with focus on Lyme neuroborreliosis. Int J Pediatr Otorhinolaryngol. 2003;67(6):597-602.
20. Siegler RL, Brewer ED, Corneli HM, Thompson JA. Hypertension first seen as facial paralysis: case reports and review of the literature. Pediatrics. 1991;87(3):387-389.
21. Surgical Advisory Panel, American Academy of Pediatrics. Guidelines for referral to pediatric surgical specialists. Pediatrics. 2002;110(1 pt 1):187-191.
22. Chen WX, Wong V. Prognosis of Bell’s palsy in children: analysis of 29 cases. Brain Dev. 2005; 27(7):504-508.
1. House JW, Brackmann DE. Facial nerve grading system. Otolaryngol Head Neck Surg. 1985;93(2): 146-147.
2. Finsterer J. Management of peripheral facial nerve palsy. Eur Arch Otorhinolaryngol. 2008;265(7):743-752.
3. Lunan R, Nagarajan L. Bell’s palsy: a guideline proposal following a review of practice. J Paediatr Child Health. 2008;44(4):219-220.
4. Blosser CG, Reider-Demer M. Neurologic disorders. In: Burns CE, Dunn AM, Brady MA, et al, eds. Pediatric Primary Care. 4th ed. St. Louis: Saunders Elsevier; 2008:634-672.
5. Singhi P, Jain V. Bell’s palsy in children. Semin Pediatr Neurol. 2003;10(4):289-297.
6. Tiemstra JD, Khatkhate N. Bell’s palsy: diagnosis and management. Am Fam Physician. 2007;76(7):997-1002.
7. Lorch M, Teach SJ. Facial nerve palsy: Etiology and approach to diagnosis and treatment. Pediatr Emerg Care. 2010;26(10):763-769.
8. El-Hawrani AS, Eng CY, Ahmed SK, et al. General practitioners’ referral pattern for children with acute facial paralysis. J Laryngol Otol. 2005;119(7):540-542.
9. Shargorodsky J, Lin HW, Gopen Q. Facial nerve palsy in the pediatric population. Clin Pediatr (Phila). 2010;49(5):411-417.
10. Wang CH, Chang YC, Shih HM, et al. Facial palsy in children: emergency department management and outcome. Pediatr Emerg Care. 2010;26(2):121-125.
11. Evans AK, Licameli G, Brietzke S, et al. Pediatric facial nerve paralysis: patients, management and outcomes. Int J Pediatr Otorhinolaryngol. 2005;69(11):1521-1528.
12. Lockhart P, Daly F, Pitkethly M, et al. Antiviral treatment for Bell’s palsy (idiopathic facial paralysis). Cochrane Database Syst Rev. 2009;(4):CD001869.
13. Grogan PM, Gronseth GS. Practice parameter: steroids, acyclovir, and surgery for Bell’s palsy (an evidence-based review): report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology. 2001;56(7):830-836.
14. Khine H, Mayers M, Avner JR, et al. Association between herpes simplex virus-1 infection and idiopathic unilateral facial paralysis in children and adolescents. Pediatr Infect Dis J. 2008;27(5):468-469.
15. Tsai HS, Chang LY, Lu CY, et al. Epidemiology and treatment of Bell’s palsy in children in northern Taiwan. J Microbiol Immunol Infect. 2009;42(4):351-356.
16. Cha CI, Hong CK, Park MS, Yeo SG. Comparison of facial nerve paralysis in adults and children. Yonsei Med J. 2008;49(5):725-734.
17. Linder TE, Abdelkafy W, Cavero-Vanek S. The management of peripheral facial nerve palsy: “paresis” versus “paralysis” and sources of ambiguity in study designs. Otol Neurotol. 2010;31(2):319-327.
18. Rahman I, Sadiq SA. Ophthalmic management of facial nerve palsy: a review. Surv Ophthalmol. 2007;52(2):121-144.
19. Skogman BH, Croner S, Odkvist L. Acute facial palsy in children: a 2-year follow-up with focus on Lyme neuroborreliosis. Int J Pediatr Otorhinolaryngol. 2003;67(6):597-602.
20. Siegler RL, Brewer ED, Corneli HM, Thompson JA. Hypertension first seen as facial paralysis: case reports and review of the literature. Pediatrics. 1991;87(3):387-389.
21. Surgical Advisory Panel, American Academy of Pediatrics. Guidelines for referral to pediatric surgical specialists. Pediatrics. 2002;110(1 pt 1):187-191.
22. Chen WX, Wong V. Prognosis of Bell’s palsy in children: analysis of 29 cases. Brain Dev. 2005; 27(7):504-508.
Grand Rounds: Man, 72, With Peeling Penile Skin
A 72-year-old man presented to his primary care provider’s office with complaints of peeling skin on his penis and frequent, burning urination. He said he had first noticed redness on his penis about four days earlier, adding that it was growing worse. He was unsure whether he was truly experiencing frequent urination or just more aware of urinating because of the burning pain. He reported no attempts to treat himself, stating that he was “just keeping an eye on it and hoping it would go away.”
The patient’s medical history was limited to hypertension, for which he was taking valsartan, and allergies, for which he took fexofenadine. His surgical history included a tonsillectomy and appendectomy during his early teens. He had no known allergies to any medications.
The patient was married and retired after an executive career. He and his wife split their residence between New York and Florida during seasonal changes and were living in Florida at the time. He reported social drinking (“on rare occasions, these days”) and smoking an occasional cigar. He reported that he showers only once or twice weekly because of dry skin.
The following vital signs were recorded: blood pressure, 110/72 mm Hg; heart rate, 68 beats/min; respirations, 15/min; temperature, 97.8°F; and O2 saturation, 99% on room air. He was 73” tall and weighed 197 lb, with a BMI of 26.
The patient was alert and oriented. His physical exam was overall unremarkable, with the exception of an uncircumcised penis with redness and inflammation on the glans penis and no discharge noted. The reddened area was bright and shiny with a moist appearance and well-defined borders. The man denied any risk for sexually transmitted disease (STD) and denied any penile discharge. He also denied fever, chills, or arthritis.
Urinalysis performed in the office was negative for a urinary tract infection or for elevated glucose. A laboratory report from six months earlier was reviewed; all findings were within normal range, including the blood glucose level, with special attention paid for possible underlying cause; and the prostate-specific antigen (PSA) level, obtained for possible prostatitis or prostate cancer.
The differential diagnosis included eczema or psoriasis, Zoon’s balanitis, penile cancer, balanitis xerotica obliterans (lichen sclerosus), candidiasis balanitis, and circinate balanitis (as occurs in patients with Reiter’s disease; see table1-5). The absence of circumcision and the patient’s report of infrequent bathing raised concern for a hygiene-related etiology; the final diagnosis, made empirically, was candidiasis balanitis. Regarding an underlying cause, the laboratory order included a urine culture, fasting complete blood count, chemistry panel, and PSA level.
The patient was given instructions to wash the affected area twice daily for one week with a lukewarm weak saline solution (1 tablespoon salt/L water),5,6 gently retracting the foreskin; he was also given a topical antifungal cream7 (ketoconazole 2%, although other choices are discussed below), to be applied two to three times daily until his symptoms resolved.6 He was advised to return in one week if the condition did not improve or grew worse5; referral to dermatology would then be considered. The patient was also advised that in the case of a recurrent episode, dermatology would be consulted. The possibility of circumcision was discussed,8 and the patient was given information about the procedure, with referral to a urologist in the area.
Discussion
Balanitis is an inflammation of the glans penis; balanoposthitis involves the foreskin and prepuce.9-11 Balanitis can occur in men of any age, with etiologies varying with a patient’s age. Typical signs and symptoms include redness and swelling of the glans penis or foreskin, itching and/or pain, urethral discharge, phimosis, swollen lymph nodes, ulceration or plaque appearance, and pain on urination.12
In addition to the differential diagnoses mentioned, several additional conditions can be considered in a man with penile lesions. In older men, it is particularly important to investigate such lesions thoroughly, following the patient until the underlying cause is determined and the best treatment choice is selected. Specialists in dermatology and urology can best identify persistent or chronic lesions and make appropriate treatment recommendations, including possible circumcision.
The condition is commonly associated with absence of circumcision, poor hygiene, and phimosis (the inability to retract the foreskin from the glans penis). Accumulation of glandular secretions (smegma) and sloughed epithelial cells under the foreskin can lead to irritation and subsequent infection.
Uncontrolled or poorly controlled diabetes can be implicated in candidiasis infections.1 Other causes and contributing factors include chemical irritants (eg, soaps, lubricating jelly), edematous conditions (including congestive heart failure, cirrhosis, and nephrosis), drug allergies, morbid obesity, and a number of viruses and other pathogens, including those associated with STDs.12
A more detailed laboratory work-up might include the following:
• Serum glucose test (as part of a diabetes screening; in older men, this inflammatory condition can be a presenting sign of diabetes mellitus6)
• Culture of discharge, if any is present
• Serology test for STDs
• Wet mount with potassium hydroxide (for Candida albicans infection)
• Ultrasound, in severe cases or when urinary obstruction is suspected.
Additionally, in chronic cases, the patient should be referred to dermatology or urology for biopsy.5,9 Testing for anaerobes should also be considered for the patient and his sexual partner; if results are positive, treatment with oral metronidazole (400 mg tid for 10 days) is advised.6
In this patient’s case, the test that would best support an in-office diagnosis of candidiasis balanitis is a wet mount with potassium hydroxide. This was not performed at the time of the case patient’s visit, however; the diagnosis was empirically determined.
Management, Including Patient Education
Treatment of candidiasis balanitis involves routinely cleaning the penis and foreskin, as the case patient was instructed; use of soap, an irritant, should be avoided until the condition is resolved.7,10 Appropriate topical antifungal creams include nystatin, ketoconazole, miconazole, clotrimazole, econazole, and terbinafine, applied two to three times daily for at least 10 days; a cream combining an imidazole with 1% hydrocortisone may be effective for patients with significant inflammation.5,6,8,10,13
The patient should be instructed to:
• Keep the area clean and dry
• Wash twice daily with weak saline solution after removing residual medication and before applying fresh medication
• Wear loose cotton underwear
• Avoid sharing towels or cleaning cloths
• Wash personal items and surfaces, if possible, with disinfectant
• Notify sexual partner(s) that they may need treatment
• Discontinue sexual intercourse until infection is resolved
• Continue treatment for 10 to 14 days, even though relief may occur early
• Follow up with the clinician if no improvement is seen within one week
• Consider circumcision, in case of chronic infection.1,2,8,12
Conclusion
It is important to diagnose balanitis correctly, as this condition can affect sexual and urinary function, and its effects should not be underestimated in older men. Differentiating between infectious, noninfectious, premalignant, and malignant lesions will lead to appropriate care and allow early diagnosis or prevention of curable malignancies.
1. Singh S, Bunker C. Male genital dermatoses in old age. Age Ageing. 2008;37(5):500-504.
2. Thompson IM, Teichman JM, Elston DM, Sea J. Noninfectious penile lesions. Am Fam Physician. 2010;81(2):167-174.
3. Lane JE, Johnson J. Persistent penile patch. Am Fam Physician. 2008;78(9):1081-1082.
4. Gupta S, Malhotra AK, Ajith C. Lichen sclerosus: role of occlusion of the genital skin in the pathogenesis. Indian J Dermatol Venereol Leprol. 2010;76(1):56-58.
5. British Association for Sexual Health and HIV, Clinical Effectiveness Group. 2008 UK National Guideline on the Management of Balanoposthitis. www.bashh.org/documents/2062. Accessed September 22, 2010.
6. Ashton R, Leppard B. Differential Diagnosis in Dermatology. 3rd ed. London: Radcliffe Publishing Ltd; 2004:321.
7. NHS Institute for Innovation and Improvement. Clinical Knowledge Summaries: Balanitis (June 2009). www.cks.nhs.uk/balanitis/management/scenario_balanitis_adults#-378526. Accessed September 22, 2010.
8. Parker J. Management of common fungal infections in primary care. Nurs Stand. 2009;23(43):42-46.
9. Green MB, Bailey PP. Infectious processes: urinary tract infections and sexually transmitted diseases. In: Buttaro TM, Trybulski J, Bailey PP, Sandberg-Cook J, eds. Primary Care: A Collaborative Practice. 3rd ed. St. Louis, MO: Mosby Elsevier; 2008:576-590.
10. Singh-Behl D, Tomecki KJ. Common skins infections 2009. www.clevelandclinicmeded .com/medicalpubs/diseasemanagement/dermatol ogy/common-skin-infections. Accessed September 22, 2010.
11. Ko WT, Adal KA, Tomecki KJ. Infectious diseases. Med Clin North Am. 1998;82:(5):1001-1031.
12. Morgan K, McCance, KL. Alterations of the reproductive systems. In: McCance KL, Huether SE, eds. Pathophysiology: The Biologic Basis for Disease in Adults and Children. 5th ed. St. Louis, MO: Elsevier Mosby; 2006:805-807.
13. Waugh MA, Evans EG, Nayyar KC, Fong R. Clotrimazole (Canestan) in the treatment of candidal balanitis in men: with incidental observations on diabetic candidal balanoposthitis. Br J Vener Dis. 1978;54(3):184-186.
A 72-year-old man presented to his primary care provider’s office with complaints of peeling skin on his penis and frequent, burning urination. He said he had first noticed redness on his penis about four days earlier, adding that it was growing worse. He was unsure whether he was truly experiencing frequent urination or just more aware of urinating because of the burning pain. He reported no attempts to treat himself, stating that he was “just keeping an eye on it and hoping it would go away.”
The patient’s medical history was limited to hypertension, for which he was taking valsartan, and allergies, for which he took fexofenadine. His surgical history included a tonsillectomy and appendectomy during his early teens. He had no known allergies to any medications.
The patient was married and retired after an executive career. He and his wife split their residence between New York and Florida during seasonal changes and were living in Florida at the time. He reported social drinking (“on rare occasions, these days”) and smoking an occasional cigar. He reported that he showers only once or twice weekly because of dry skin.
The following vital signs were recorded: blood pressure, 110/72 mm Hg; heart rate, 68 beats/min; respirations, 15/min; temperature, 97.8°F; and O2 saturation, 99% on room air. He was 73” tall and weighed 197 lb, with a BMI of 26.
The patient was alert and oriented. His physical exam was overall unremarkable, with the exception of an uncircumcised penis with redness and inflammation on the glans penis and no discharge noted. The reddened area was bright and shiny with a moist appearance and well-defined borders. The man denied any risk for sexually transmitted disease (STD) and denied any penile discharge. He also denied fever, chills, or arthritis.
Urinalysis performed in the office was negative for a urinary tract infection or for elevated glucose. A laboratory report from six months earlier was reviewed; all findings were within normal range, including the blood glucose level, with special attention paid for possible underlying cause; and the prostate-specific antigen (PSA) level, obtained for possible prostatitis or prostate cancer.
The differential diagnosis included eczema or psoriasis, Zoon’s balanitis, penile cancer, balanitis xerotica obliterans (lichen sclerosus), candidiasis balanitis, and circinate balanitis (as occurs in patients with Reiter’s disease; see table1-5). The absence of circumcision and the patient’s report of infrequent bathing raised concern for a hygiene-related etiology; the final diagnosis, made empirically, was candidiasis balanitis. Regarding an underlying cause, the laboratory order included a urine culture, fasting complete blood count, chemistry panel, and PSA level.
The patient was given instructions to wash the affected area twice daily for one week with a lukewarm weak saline solution (1 tablespoon salt/L water),5,6 gently retracting the foreskin; he was also given a topical antifungal cream7 (ketoconazole 2%, although other choices are discussed below), to be applied two to three times daily until his symptoms resolved.6 He was advised to return in one week if the condition did not improve or grew worse5; referral to dermatology would then be considered. The patient was also advised that in the case of a recurrent episode, dermatology would be consulted. The possibility of circumcision was discussed,8 and the patient was given information about the procedure, with referral to a urologist in the area.
Discussion
Balanitis is an inflammation of the glans penis; balanoposthitis involves the foreskin and prepuce.9-11 Balanitis can occur in men of any age, with etiologies varying with a patient’s age. Typical signs and symptoms include redness and swelling of the glans penis or foreskin, itching and/or pain, urethral discharge, phimosis, swollen lymph nodes, ulceration or plaque appearance, and pain on urination.12
In addition to the differential diagnoses mentioned, several additional conditions can be considered in a man with penile lesions. In older men, it is particularly important to investigate such lesions thoroughly, following the patient until the underlying cause is determined and the best treatment choice is selected. Specialists in dermatology and urology can best identify persistent or chronic lesions and make appropriate treatment recommendations, including possible circumcision.
The condition is commonly associated with absence of circumcision, poor hygiene, and phimosis (the inability to retract the foreskin from the glans penis). Accumulation of glandular secretions (smegma) and sloughed epithelial cells under the foreskin can lead to irritation and subsequent infection.
Uncontrolled or poorly controlled diabetes can be implicated in candidiasis infections.1 Other causes and contributing factors include chemical irritants (eg, soaps, lubricating jelly), edematous conditions (including congestive heart failure, cirrhosis, and nephrosis), drug allergies, morbid obesity, and a number of viruses and other pathogens, including those associated with STDs.12
A more detailed laboratory work-up might include the following:
• Serum glucose test (as part of a diabetes screening; in older men, this inflammatory condition can be a presenting sign of diabetes mellitus6)
• Culture of discharge, if any is present
• Serology test for STDs
• Wet mount with potassium hydroxide (for Candida albicans infection)
• Ultrasound, in severe cases or when urinary obstruction is suspected.
Additionally, in chronic cases, the patient should be referred to dermatology or urology for biopsy.5,9 Testing for anaerobes should also be considered for the patient and his sexual partner; if results are positive, treatment with oral metronidazole (400 mg tid for 10 days) is advised.6
In this patient’s case, the test that would best support an in-office diagnosis of candidiasis balanitis is a wet mount with potassium hydroxide. This was not performed at the time of the case patient’s visit, however; the diagnosis was empirically determined.
Management, Including Patient Education
Treatment of candidiasis balanitis involves routinely cleaning the penis and foreskin, as the case patient was instructed; use of soap, an irritant, should be avoided until the condition is resolved.7,10 Appropriate topical antifungal creams include nystatin, ketoconazole, miconazole, clotrimazole, econazole, and terbinafine, applied two to three times daily for at least 10 days; a cream combining an imidazole with 1% hydrocortisone may be effective for patients with significant inflammation.5,6,8,10,13
The patient should be instructed to:
• Keep the area clean and dry
• Wash twice daily with weak saline solution after removing residual medication and before applying fresh medication
• Wear loose cotton underwear
• Avoid sharing towels or cleaning cloths
• Wash personal items and surfaces, if possible, with disinfectant
• Notify sexual partner(s) that they may need treatment
• Discontinue sexual intercourse until infection is resolved
• Continue treatment for 10 to 14 days, even though relief may occur early
• Follow up with the clinician if no improvement is seen within one week
• Consider circumcision, in case of chronic infection.1,2,8,12
Conclusion
It is important to diagnose balanitis correctly, as this condition can affect sexual and urinary function, and its effects should not be underestimated in older men. Differentiating between infectious, noninfectious, premalignant, and malignant lesions will lead to appropriate care and allow early diagnosis or prevention of curable malignancies.
A 72-year-old man presented to his primary care provider’s office with complaints of peeling skin on his penis and frequent, burning urination. He said he had first noticed redness on his penis about four days earlier, adding that it was growing worse. He was unsure whether he was truly experiencing frequent urination or just more aware of urinating because of the burning pain. He reported no attempts to treat himself, stating that he was “just keeping an eye on it and hoping it would go away.”
The patient’s medical history was limited to hypertension, for which he was taking valsartan, and allergies, for which he took fexofenadine. His surgical history included a tonsillectomy and appendectomy during his early teens. He had no known allergies to any medications.
The patient was married and retired after an executive career. He and his wife split their residence between New York and Florida during seasonal changes and were living in Florida at the time. He reported social drinking (“on rare occasions, these days”) and smoking an occasional cigar. He reported that he showers only once or twice weekly because of dry skin.
The following vital signs were recorded: blood pressure, 110/72 mm Hg; heart rate, 68 beats/min; respirations, 15/min; temperature, 97.8°F; and O2 saturation, 99% on room air. He was 73” tall and weighed 197 lb, with a BMI of 26.
The patient was alert and oriented. His physical exam was overall unremarkable, with the exception of an uncircumcised penis with redness and inflammation on the glans penis and no discharge noted. The reddened area was bright and shiny with a moist appearance and well-defined borders. The man denied any risk for sexually transmitted disease (STD) and denied any penile discharge. He also denied fever, chills, or arthritis.
Urinalysis performed in the office was negative for a urinary tract infection or for elevated glucose. A laboratory report from six months earlier was reviewed; all findings were within normal range, including the blood glucose level, with special attention paid for possible underlying cause; and the prostate-specific antigen (PSA) level, obtained for possible prostatitis or prostate cancer.
The differential diagnosis included eczema or psoriasis, Zoon’s balanitis, penile cancer, balanitis xerotica obliterans (lichen sclerosus), candidiasis balanitis, and circinate balanitis (as occurs in patients with Reiter’s disease; see table1-5). The absence of circumcision and the patient’s report of infrequent bathing raised concern for a hygiene-related etiology; the final diagnosis, made empirically, was candidiasis balanitis. Regarding an underlying cause, the laboratory order included a urine culture, fasting complete blood count, chemistry panel, and PSA level.
The patient was given instructions to wash the affected area twice daily for one week with a lukewarm weak saline solution (1 tablespoon salt/L water),5,6 gently retracting the foreskin; he was also given a topical antifungal cream7 (ketoconazole 2%, although other choices are discussed below), to be applied two to three times daily until his symptoms resolved.6 He was advised to return in one week if the condition did not improve or grew worse5; referral to dermatology would then be considered. The patient was also advised that in the case of a recurrent episode, dermatology would be consulted. The possibility of circumcision was discussed,8 and the patient was given information about the procedure, with referral to a urologist in the area.
Discussion
Balanitis is an inflammation of the glans penis; balanoposthitis involves the foreskin and prepuce.9-11 Balanitis can occur in men of any age, with etiologies varying with a patient’s age. Typical signs and symptoms include redness and swelling of the glans penis or foreskin, itching and/or pain, urethral discharge, phimosis, swollen lymph nodes, ulceration or plaque appearance, and pain on urination.12
In addition to the differential diagnoses mentioned, several additional conditions can be considered in a man with penile lesions. In older men, it is particularly important to investigate such lesions thoroughly, following the patient until the underlying cause is determined and the best treatment choice is selected. Specialists in dermatology and urology can best identify persistent or chronic lesions and make appropriate treatment recommendations, including possible circumcision.
The condition is commonly associated with absence of circumcision, poor hygiene, and phimosis (the inability to retract the foreskin from the glans penis). Accumulation of glandular secretions (smegma) and sloughed epithelial cells under the foreskin can lead to irritation and subsequent infection.
Uncontrolled or poorly controlled diabetes can be implicated in candidiasis infections.1 Other causes and contributing factors include chemical irritants (eg, soaps, lubricating jelly), edematous conditions (including congestive heart failure, cirrhosis, and nephrosis), drug allergies, morbid obesity, and a number of viruses and other pathogens, including those associated with STDs.12
A more detailed laboratory work-up might include the following:
• Serum glucose test (as part of a diabetes screening; in older men, this inflammatory condition can be a presenting sign of diabetes mellitus6)
• Culture of discharge, if any is present
• Serology test for STDs
• Wet mount with potassium hydroxide (for Candida albicans infection)
• Ultrasound, in severe cases or when urinary obstruction is suspected.
Additionally, in chronic cases, the patient should be referred to dermatology or urology for biopsy.5,9 Testing for anaerobes should also be considered for the patient and his sexual partner; if results are positive, treatment with oral metronidazole (400 mg tid for 10 days) is advised.6
In this patient’s case, the test that would best support an in-office diagnosis of candidiasis balanitis is a wet mount with potassium hydroxide. This was not performed at the time of the case patient’s visit, however; the diagnosis was empirically determined.
Management, Including Patient Education
Treatment of candidiasis balanitis involves routinely cleaning the penis and foreskin, as the case patient was instructed; use of soap, an irritant, should be avoided until the condition is resolved.7,10 Appropriate topical antifungal creams include nystatin, ketoconazole, miconazole, clotrimazole, econazole, and terbinafine, applied two to three times daily for at least 10 days; a cream combining an imidazole with 1% hydrocortisone may be effective for patients with significant inflammation.5,6,8,10,13
The patient should be instructed to:
• Keep the area clean and dry
• Wash twice daily with weak saline solution after removing residual medication and before applying fresh medication
• Wear loose cotton underwear
• Avoid sharing towels or cleaning cloths
• Wash personal items and surfaces, if possible, with disinfectant
• Notify sexual partner(s) that they may need treatment
• Discontinue sexual intercourse until infection is resolved
• Continue treatment for 10 to 14 days, even though relief may occur early
• Follow up with the clinician if no improvement is seen within one week
• Consider circumcision, in case of chronic infection.1,2,8,12
Conclusion
It is important to diagnose balanitis correctly, as this condition can affect sexual and urinary function, and its effects should not be underestimated in older men. Differentiating between infectious, noninfectious, premalignant, and malignant lesions will lead to appropriate care and allow early diagnosis or prevention of curable malignancies.
1. Singh S, Bunker C. Male genital dermatoses in old age. Age Ageing. 2008;37(5):500-504.
2. Thompson IM, Teichman JM, Elston DM, Sea J. Noninfectious penile lesions. Am Fam Physician. 2010;81(2):167-174.
3. Lane JE, Johnson J. Persistent penile patch. Am Fam Physician. 2008;78(9):1081-1082.
4. Gupta S, Malhotra AK, Ajith C. Lichen sclerosus: role of occlusion of the genital skin in the pathogenesis. Indian J Dermatol Venereol Leprol. 2010;76(1):56-58.
5. British Association for Sexual Health and HIV, Clinical Effectiveness Group. 2008 UK National Guideline on the Management of Balanoposthitis. www.bashh.org/documents/2062. Accessed September 22, 2010.
6. Ashton R, Leppard B. Differential Diagnosis in Dermatology. 3rd ed. London: Radcliffe Publishing Ltd; 2004:321.
7. NHS Institute for Innovation and Improvement. Clinical Knowledge Summaries: Balanitis (June 2009). www.cks.nhs.uk/balanitis/management/scenario_balanitis_adults#-378526. Accessed September 22, 2010.
8. Parker J. Management of common fungal infections in primary care. Nurs Stand. 2009;23(43):42-46.
9. Green MB, Bailey PP. Infectious processes: urinary tract infections and sexually transmitted diseases. In: Buttaro TM, Trybulski J, Bailey PP, Sandberg-Cook J, eds. Primary Care: A Collaborative Practice. 3rd ed. St. Louis, MO: Mosby Elsevier; 2008:576-590.
10. Singh-Behl D, Tomecki KJ. Common skins infections 2009. www.clevelandclinicmeded .com/medicalpubs/diseasemanagement/dermatol ogy/common-skin-infections. Accessed September 22, 2010.
11. Ko WT, Adal KA, Tomecki KJ. Infectious diseases. Med Clin North Am. 1998;82:(5):1001-1031.
12. Morgan K, McCance, KL. Alterations of the reproductive systems. In: McCance KL, Huether SE, eds. Pathophysiology: The Biologic Basis for Disease in Adults and Children. 5th ed. St. Louis, MO: Elsevier Mosby; 2006:805-807.
13. Waugh MA, Evans EG, Nayyar KC, Fong R. Clotrimazole (Canestan) in the treatment of candidal balanitis in men: with incidental observations on diabetic candidal balanoposthitis. Br J Vener Dis. 1978;54(3):184-186.
1. Singh S, Bunker C. Male genital dermatoses in old age. Age Ageing. 2008;37(5):500-504.
2. Thompson IM, Teichman JM, Elston DM, Sea J. Noninfectious penile lesions. Am Fam Physician. 2010;81(2):167-174.
3. Lane JE, Johnson J. Persistent penile patch. Am Fam Physician. 2008;78(9):1081-1082.
4. Gupta S, Malhotra AK, Ajith C. Lichen sclerosus: role of occlusion of the genital skin in the pathogenesis. Indian J Dermatol Venereol Leprol. 2010;76(1):56-58.
5. British Association for Sexual Health and HIV, Clinical Effectiveness Group. 2008 UK National Guideline on the Management of Balanoposthitis. www.bashh.org/documents/2062. Accessed September 22, 2010.
6. Ashton R, Leppard B. Differential Diagnosis in Dermatology. 3rd ed. London: Radcliffe Publishing Ltd; 2004:321.
7. NHS Institute for Innovation and Improvement. Clinical Knowledge Summaries: Balanitis (June 2009). www.cks.nhs.uk/balanitis/management/scenario_balanitis_adults#-378526. Accessed September 22, 2010.
8. Parker J. Management of common fungal infections in primary care. Nurs Stand. 2009;23(43):42-46.
9. Green MB, Bailey PP. Infectious processes: urinary tract infections and sexually transmitted diseases. In: Buttaro TM, Trybulski J, Bailey PP, Sandberg-Cook J, eds. Primary Care: A Collaborative Practice. 3rd ed. St. Louis, MO: Mosby Elsevier; 2008:576-590.
10. Singh-Behl D, Tomecki KJ. Common skins infections 2009. www.clevelandclinicmeded .com/medicalpubs/diseasemanagement/dermatol ogy/common-skin-infections. Accessed September 22, 2010.
11. Ko WT, Adal KA, Tomecki KJ. Infectious diseases. Med Clin North Am. 1998;82:(5):1001-1031.
12. Morgan K, McCance, KL. Alterations of the reproductive systems. In: McCance KL, Huether SE, eds. Pathophysiology: The Biologic Basis for Disease in Adults and Children. 5th ed. St. Louis, MO: Elsevier Mosby; 2006:805-807.
13. Waugh MA, Evans EG, Nayyar KC, Fong R. Clotrimazole (Canestan) in the treatment of candidal balanitis in men: with incidental observations on diabetic candidal balanoposthitis. Br J Vener Dis. 1978;54(3):184-186.
Grand Rounds: Man, 29, With Apparent Throat Obstruction
A 29-year-old man presented to the emergency department (ED) with a chief complaint of food stuck in his throat. He reported that he had swallowed a piece of chicken and felt it get stuck. Drinking water to help it go down was unsuccessful.
The patient’s history was positive for childhood asthma and nine years of solid food dysphagia. There was no history of a caustic chemical ingestion or of drug-induced esophagitis. He denied having dyspepsia, heartburn, or chest pain. He was not taking any medications and had no allergies.
When his dysphagia symptoms began nine years ago, he was diagnosed with acid reflux disease, confirmed by an upper gastrointestinal (GI) tract x-ray. Since that time, he reported having to swallow liquid after every bite of food and said he suffered from severe anxiety over fear of choking.
Evaluation in the ED consisted of endoscopic examination by a gastroenterologist. In addition to dislodging a food bolus, the endoscope revealed a narrowed, ringed esophagus with mucosal changes throughout the length of the esophagus (see Figures 1 through 3). Esophageal biopsies were taken, and the esophagus was dilated successfully with a 40-Fr Maloney dilator. The endoscopist detected too much resistance to pass a larger dilator.
Biopsy results revealed eosinophilic esophagitis. The patient was given oral fluticasone propionate. At one-month follow-up, he reported feeling much better. Upper endoscopy revealed some improvement, and the gastroenterologist was able to pass both a 46- and a 48-Fr Maloney dilator with only mild resistance. (The largest Maloney dilator, a 60-Fr dilator, should easily pass through a normal esophagus, according to T. L. Sack, MD, oral communication, June 2009.)
Discussion
Eosinophilic esophagitis (EE) involves the infiltration of the esophageal mucosa with eosinophils, causing edema, inflammation, and eventually, thickening and stenotic changes of the esophageal mucosa.1
The normal esophageal mucosa contains lymphocytes, mast cells, and dendritic cells, which protect the esophagus from invading toxins and microorganisms. Eosinophils are not usually present, but when they are, they can have toxic effects on the esophageal mucosa.2 EE is associated with solid food dysphagia, a direct result of damage to the esophageal mucosa, and other causes that are not clearly understood.
Research findings suggest that symptoms of dysphagia may be caused by degranulating eosinophils and mast cells, which have an antagonistic effect on the muscarinic receptors and cause smooth muscle to contract.3,4 The proposed triggering mechanism of EE is an immunoglobulin E (IgE) immune–mediated response to an allergen.2 Based on results from IgE radioallergosorbent testing (RAST), aeroallergens are more likely than food to act as triggers.5
EE in the Adult Patient
Traditionally, EE has been a condition seen in the pediatric population, with symptoms of nausea, vomiting, and failure to thrive; however, it is becoming increasingly recognized among adults. The typical patient is a man in his 20s or 30s (although cases of EE have been reported among women and older adults) with acute and recurrent solid food dysphagia, with or without food impaction.4
Often the patient reports a history of environmental or food allergies, asthma, rhinitis, or eczema.2,4-6 Researchers have reported the presence of allergic symptoms in at least 50% of patients diagnosed with EE,2 and many patients experience exacerbations associated with seasonal changes.7
GERD may coexist with EE; however, no relationship has been identified between the two.8 EE should be considered in patients with gastrointestinal symptoms that persist despite at least four weeks’ treatment with a proton pump inhibitor (PPI).2
Dysphagia: Differential Diagnosis
Adult patients with esophageal dysphagia usually report the feeling of food getting stuck when they try to swallow.9 Dysphagia may result from a mechanical obstruction or a neuromuscular/motility condition. Patients with mechanical obstructions usually have difficulty swallowing solids, while those with motility disorders tend to have difficulty with both liquids and solids.1,9
Mechanical obstructions may include carcinomas (intrinsic and extrinsic), strictures, or Schatzki rings (small thin mucosal rings of unknown etiology located at the gastroesophageal junction).1,9 Progressive dysphagia to solids over a short period of time is often indicative of esophageal carcinoma. GERD, pill-induced trauma, previous ingestion of a caustic chemical, and radiation are common causes of esophageal stricture formation. For a list of medications that are particularly caustic to the esophageal mucosa, see the table.9,10
Neuromuscular manifestations of dysphagia include achalasia, diffuse esophageal spasm, nutcracker esophagus, and scleroderma.1 These are usually associated with progressive difficulty in swallowing.9
Evaluating the Patient
A thorough patient history can often reveal potential causes of dysphagia and eliminate others. This should include current medications, chronic medical conditions and details regarding their onset and duration, and symptoms associated with dysphagia.9
Physical examination should include palpation of the thyroid because of the potential for a thyroid mass to cause extrinsic compression of the esophagus, palpation of the abdomen for masses or organomegaly, and a complete neurologic evaluation.9
Laboratory tests should be ordered based on the information obtained from the history and physical. Testing may include thyroid studies to eliminate hypothyroid or hyperthyroid causes of dysphagia, and complete blood count (CBC) with differential to rule out inflammatory or infectious processes.9 While eosinophilia may be present in the differential, it is not a universally accepted marker for establishing the diagnosis of EE.2,5 Stools should be checked for occult blood, because a positive finding may suggest esophageal carcinoma.9
Diagnosis
In the primary care setting, a barium esophagram may be used during the initial workup to evaluate the anatomic structures of the esophagus and to differentiate between a mechanical obstruction and a neuromuscular disorder.1,9 This noninvasive test requires the patient to swallow a radiopaque liquid as x-rays are taken.
The gold standard for diagnosing EE, however, is upper endoscopy with biopsy of the esophageal mucosa.6 Endoscopic findings that indicate EE are atypical of GERD; they may include a narrowed, small-caliber esophagus, concentric mucosal rings, proximal stenosis, linear ulcerations, atrophic changes, and white papules associated with eosinophilic microabscesses.6
Although there is no consensus regarding the number of eosinophils that should be present for an accurate diagnosis of EE, microscopic interpretation of the biopsy from both the proximal and the distal esophageal epithelia5 usually shows 15 or more eosinophils per high-power field.2,11 It has been suggested that mucosal biopsies be taken along the entire length of the esophagus, as eosinophilic infiltration may extend from the proximal to the distal esophagus.2
GERD and trauma induced by medication use may also be associated with esophageal eosinophilic infiltration5; however, eosinophils are usually present only in the distal esophageal mucosa3 and are not as abundant as in EE.7 If endoscopy reveals persistent eosinophilia despite four to eight weeks’ treatment with a PPI, the diagnosis of EE is confirmed.2
Treatment
Treatment for EE is still under investigation. Research has examined the association between EE and food allergies or aeroallergens.4 Evaluation by an allergist using skin prick tests or RAST is recommended in the adult patient to help determine the source of the underlying inflammation.5,7 Eliminating any identified allergen should help alleviate symptoms.4
For patients in whom no source of inflammation can be identified, treatment with 1.0 to 2.0 mg/kg/d of oral prednisone for acute exacerbations has been shown to significantly improve symptoms and histology12; however, because of the associated risk for adverse systemic effects, long-term use is not recommended.
In many patients, the inhaled corticosteroid fluticasone has also proved successful in reducing EE—associated inflammation.6 Current evidence supports adult dosing between 880 and 1,760 mcg per day for six to eight weeks, administered with a metered-dose inhaler and no spacer. Fluticasone should be sprayed directly into the mouth and swallowed, after which the patient should take nothing by mouth for 30 minutes.13 Prolonged fluticasone use has been associated with esophageal candidiasis.2 There are currently no recommendations regarding its use as maintenance therapy.
Montelukast, a leukotriene receptor antagonist, has also been shown in some studies to reduce the inflammatory process11; however, one study team recently found it to have no therapeutic effect.13
PPIs may be effective for improving EE symptoms even in the absence of GERD because of the reduced gastric acid production,7 but they do not usually improve EE’s histologic features.3
Use of esophageal dilation in patients with EE is controversial because of an associated risk for perforation.14 If this intervention is to be performed, the patient should be treated in advance with oral corticosteroids to reduce esophageal inflammation.15,16 In addition, the endoscopist should start with small-sized dilators and carefully proceed to larger sizes.11 Critics of esophageal dilation argue that the procedure is only a temporary solution and does nothing for the underlying condition.4,8
Regarding endoscopic surveillance, an interval of at least four weeks between interventions is recommended.13
Role of the Primary Care Clinician
Undiagnosed EE can cause the patient discomfort, frustration, and anxiety, as seen in the case study. Many patients with undiagnosed EE have been exposed to unnecessary medical therapy and antireflux surgery.3 Without proper diagnosis and treatment, EE may worsen, causing complications associated with chronic inflammation (ie, esophageal fibrosis and strictures).2,6
The long-term prognosis of EE is unknown at this time.8 The disease is usually chronic, with periods of remission and exacerbation. With an understanding of EE and appropriate therapies, the primary care practitioner can team with the gastroenterologist to provide effective disease management through endoscopic surveillance and intervention for acute exacerbations. Guidelines recommend that patients be closely followed with regular office visits to reassess symptoms, compliance with therapy, and adverse effects, with the goal of preventing complications associated with EE.13
Conclusion
To effectively evaluate the patient who presents with dysphagia, the primary care provider should have a working knowledge of EE, as well as an understanding of the key elements in the history and physical examination to help ensure an accurate diagnosis. This will facilitate timely referral to a gastroenterologist for endoscopic evaluation, when indicated.
1. McQuaid KR. Gastrointestinal disorders. In: McPhee S, Papadakis M. CURRENT Medical Diagnosis & Treatment 2009. New York: McGraw-Hill: 2009:487-581.
2. Nurko S, Furuta GT. Eosinophilic esophagitis (2006). GI Motility Online. www.nature.com/gimo/contents/pt1/full/gimo49.html. Accessed July 27, 2009.
3. Parfitt JR, Gregor JC, Suskin NG, et al. Eosinophilic esophagitis in adults: distinguishing features from gastroesophageal reflux disease: a study of 41 patients. Mod Pathol. 2006;19(1):90-96.
4. Swoger JM, Weiler CR, Arora AS. Eosinophilic esophagitis: is it all allergies? Mayo Clin Proc. 2007;82(12):1541-1549.
5. Conus S, Simon HU. General laboratory diagnostics of eosinophilic GI diseases. Best Pract Res Clin Gastroenterol. 2008;22(3):441-453.
6. Remedios M, Campbell C, Jones DM, Kerlin P. Eosinophilic esophagitis in adults: clinical, endoscopic, histologic findings, and response to treatment with fluticasone propionate. Gastrointest Endosc. 2006;63(1):3-12.
7. Rothenberg ME. Eosinophilic gastrointestinal disorders (EGID). J Allergy Clin Immunol. 2004; 113(1):11-28.
8. Lucendo AJ, Carrion G, Navarro M, et al. Eosinophilic esophagitis in adults: an emerging disease. Dig Dis Sci. 2004;49(11-12):1884-1888.
9. Spieker MR. Evaluating dysphagia. Am Fam Physician. 2000;61(12):3639-3648.
10. Boyce HW. Drug-induced esophageal damage: diseases of medical progress. Gastrointest Endosc. 1998;47:547-550.
11. Potter JW, Saeian K, Staff D, et al. Eosinophilic esophagitis in adults: an emerging problem with unique esophageal features. Gastrointest Endosc. 2004;59(3):355-361.
12. Schaefer ET, Fitzgerald JF, Molleston JP, et al. Comparison of oral prednisone and topical fluticasone in the treatment of eosinophilic esophagitis: a randomized trial in children. Clin Gastroenterol Hepatol. 2008;6(2):165-173.
13. Furuta GT, Liacouras CA, Collins MH, et al; First International Gastrointestinal Eosinophil Research Symposium (FIGERS) Subcommittees. Eosinophilic esophagitis in children and adults: a systematic review and consensus recommendation for diagnosis and treatment. Gastroenterology. 2007;133(4): 1342-1363.
14. Straumann A, Rossi L, Simon HU, et al. Fragility of the esophageal mucosa: a pathognomonic endoscopic sign of primary eosinophilic esophagitis. Gastrointest Endosc. 2003;57(3):407-412.
15. Hawari R, Pasricha PJ. Images in clinical medicine: eosinophilic esophagitis. N Engl J Med. 2007; 356(20):e20.
16. Leclercq P, Marting A, Gast P. Eosinophilic esophagitis. N Engl J Med. 2007;357(14):1446.
A 29-year-old man presented to the emergency department (ED) with a chief complaint of food stuck in his throat. He reported that he had swallowed a piece of chicken and felt it get stuck. Drinking water to help it go down was unsuccessful.
The patient’s history was positive for childhood asthma and nine years of solid food dysphagia. There was no history of a caustic chemical ingestion or of drug-induced esophagitis. He denied having dyspepsia, heartburn, or chest pain. He was not taking any medications and had no allergies.
When his dysphagia symptoms began nine years ago, he was diagnosed with acid reflux disease, confirmed by an upper gastrointestinal (GI) tract x-ray. Since that time, he reported having to swallow liquid after every bite of food and said he suffered from severe anxiety over fear of choking.
Evaluation in the ED consisted of endoscopic examination by a gastroenterologist. In addition to dislodging a food bolus, the endoscope revealed a narrowed, ringed esophagus with mucosal changes throughout the length of the esophagus (see Figures 1 through 3). Esophageal biopsies were taken, and the esophagus was dilated successfully with a 40-Fr Maloney dilator. The endoscopist detected too much resistance to pass a larger dilator.
Biopsy results revealed eosinophilic esophagitis. The patient was given oral fluticasone propionate. At one-month follow-up, he reported feeling much better. Upper endoscopy revealed some improvement, and the gastroenterologist was able to pass both a 46- and a 48-Fr Maloney dilator with only mild resistance. (The largest Maloney dilator, a 60-Fr dilator, should easily pass through a normal esophagus, according to T. L. Sack, MD, oral communication, June 2009.)
Discussion
Eosinophilic esophagitis (EE) involves the infiltration of the esophageal mucosa with eosinophils, causing edema, inflammation, and eventually, thickening and stenotic changes of the esophageal mucosa.1
The normal esophageal mucosa contains lymphocytes, mast cells, and dendritic cells, which protect the esophagus from invading toxins and microorganisms. Eosinophils are not usually present, but when they are, they can have toxic effects on the esophageal mucosa.2 EE is associated with solid food dysphagia, a direct result of damage to the esophageal mucosa, and other causes that are not clearly understood.
Research findings suggest that symptoms of dysphagia may be caused by degranulating eosinophils and mast cells, which have an antagonistic effect on the muscarinic receptors and cause smooth muscle to contract.3,4 The proposed triggering mechanism of EE is an immunoglobulin E (IgE) immune–mediated response to an allergen.2 Based on results from IgE radioallergosorbent testing (RAST), aeroallergens are more likely than food to act as triggers.5
EE in the Adult Patient
Traditionally, EE has been a condition seen in the pediatric population, with symptoms of nausea, vomiting, and failure to thrive; however, it is becoming increasingly recognized among adults. The typical patient is a man in his 20s or 30s (although cases of EE have been reported among women and older adults) with acute and recurrent solid food dysphagia, with or without food impaction.4
Often the patient reports a history of environmental or food allergies, asthma, rhinitis, or eczema.2,4-6 Researchers have reported the presence of allergic symptoms in at least 50% of patients diagnosed with EE,2 and many patients experience exacerbations associated with seasonal changes.7
GERD may coexist with EE; however, no relationship has been identified between the two.8 EE should be considered in patients with gastrointestinal symptoms that persist despite at least four weeks’ treatment with a proton pump inhibitor (PPI).2
Dysphagia: Differential Diagnosis
Adult patients with esophageal dysphagia usually report the feeling of food getting stuck when they try to swallow.9 Dysphagia may result from a mechanical obstruction or a neuromuscular/motility condition. Patients with mechanical obstructions usually have difficulty swallowing solids, while those with motility disorders tend to have difficulty with both liquids and solids.1,9
Mechanical obstructions may include carcinomas (intrinsic and extrinsic), strictures, or Schatzki rings (small thin mucosal rings of unknown etiology located at the gastroesophageal junction).1,9 Progressive dysphagia to solids over a short period of time is often indicative of esophageal carcinoma. GERD, pill-induced trauma, previous ingestion of a caustic chemical, and radiation are common causes of esophageal stricture formation. For a list of medications that are particularly caustic to the esophageal mucosa, see the table.9,10
Neuromuscular manifestations of dysphagia include achalasia, diffuse esophageal spasm, nutcracker esophagus, and scleroderma.1 These are usually associated with progressive difficulty in swallowing.9
Evaluating the Patient
A thorough patient history can often reveal potential causes of dysphagia and eliminate others. This should include current medications, chronic medical conditions and details regarding their onset and duration, and symptoms associated with dysphagia.9
Physical examination should include palpation of the thyroid because of the potential for a thyroid mass to cause extrinsic compression of the esophagus, palpation of the abdomen for masses or organomegaly, and a complete neurologic evaluation.9
Laboratory tests should be ordered based on the information obtained from the history and physical. Testing may include thyroid studies to eliminate hypothyroid or hyperthyroid causes of dysphagia, and complete blood count (CBC) with differential to rule out inflammatory or infectious processes.9 While eosinophilia may be present in the differential, it is not a universally accepted marker for establishing the diagnosis of EE.2,5 Stools should be checked for occult blood, because a positive finding may suggest esophageal carcinoma.9
Diagnosis
In the primary care setting, a barium esophagram may be used during the initial workup to evaluate the anatomic structures of the esophagus and to differentiate between a mechanical obstruction and a neuromuscular disorder.1,9 This noninvasive test requires the patient to swallow a radiopaque liquid as x-rays are taken.
The gold standard for diagnosing EE, however, is upper endoscopy with biopsy of the esophageal mucosa.6 Endoscopic findings that indicate EE are atypical of GERD; they may include a narrowed, small-caliber esophagus, concentric mucosal rings, proximal stenosis, linear ulcerations, atrophic changes, and white papules associated with eosinophilic microabscesses.6
Although there is no consensus regarding the number of eosinophils that should be present for an accurate diagnosis of EE, microscopic interpretation of the biopsy from both the proximal and the distal esophageal epithelia5 usually shows 15 or more eosinophils per high-power field.2,11 It has been suggested that mucosal biopsies be taken along the entire length of the esophagus, as eosinophilic infiltration may extend from the proximal to the distal esophagus.2
GERD and trauma induced by medication use may also be associated with esophageal eosinophilic infiltration5; however, eosinophils are usually present only in the distal esophageal mucosa3 and are not as abundant as in EE.7 If endoscopy reveals persistent eosinophilia despite four to eight weeks’ treatment with a PPI, the diagnosis of EE is confirmed.2
Treatment
Treatment for EE is still under investigation. Research has examined the association between EE and food allergies or aeroallergens.4 Evaluation by an allergist using skin prick tests or RAST is recommended in the adult patient to help determine the source of the underlying inflammation.5,7 Eliminating any identified allergen should help alleviate symptoms.4
For patients in whom no source of inflammation can be identified, treatment with 1.0 to 2.0 mg/kg/d of oral prednisone for acute exacerbations has been shown to significantly improve symptoms and histology12; however, because of the associated risk for adverse systemic effects, long-term use is not recommended.
In many patients, the inhaled corticosteroid fluticasone has also proved successful in reducing EE—associated inflammation.6 Current evidence supports adult dosing between 880 and 1,760 mcg per day for six to eight weeks, administered with a metered-dose inhaler and no spacer. Fluticasone should be sprayed directly into the mouth and swallowed, after which the patient should take nothing by mouth for 30 minutes.13 Prolonged fluticasone use has been associated with esophageal candidiasis.2 There are currently no recommendations regarding its use as maintenance therapy.
Montelukast, a leukotriene receptor antagonist, has also been shown in some studies to reduce the inflammatory process11; however, one study team recently found it to have no therapeutic effect.13
PPIs may be effective for improving EE symptoms even in the absence of GERD because of the reduced gastric acid production,7 but they do not usually improve EE’s histologic features.3
Use of esophageal dilation in patients with EE is controversial because of an associated risk for perforation.14 If this intervention is to be performed, the patient should be treated in advance with oral corticosteroids to reduce esophageal inflammation.15,16 In addition, the endoscopist should start with small-sized dilators and carefully proceed to larger sizes.11 Critics of esophageal dilation argue that the procedure is only a temporary solution and does nothing for the underlying condition.4,8
Regarding endoscopic surveillance, an interval of at least four weeks between interventions is recommended.13
Role of the Primary Care Clinician
Undiagnosed EE can cause the patient discomfort, frustration, and anxiety, as seen in the case study. Many patients with undiagnosed EE have been exposed to unnecessary medical therapy and antireflux surgery.3 Without proper diagnosis and treatment, EE may worsen, causing complications associated with chronic inflammation (ie, esophageal fibrosis and strictures).2,6
The long-term prognosis of EE is unknown at this time.8 The disease is usually chronic, with periods of remission and exacerbation. With an understanding of EE and appropriate therapies, the primary care practitioner can team with the gastroenterologist to provide effective disease management through endoscopic surveillance and intervention for acute exacerbations. Guidelines recommend that patients be closely followed with regular office visits to reassess symptoms, compliance with therapy, and adverse effects, with the goal of preventing complications associated with EE.13
Conclusion
To effectively evaluate the patient who presents with dysphagia, the primary care provider should have a working knowledge of EE, as well as an understanding of the key elements in the history and physical examination to help ensure an accurate diagnosis. This will facilitate timely referral to a gastroenterologist for endoscopic evaluation, when indicated.
A 29-year-old man presented to the emergency department (ED) with a chief complaint of food stuck in his throat. He reported that he had swallowed a piece of chicken and felt it get stuck. Drinking water to help it go down was unsuccessful.
The patient’s history was positive for childhood asthma and nine years of solid food dysphagia. There was no history of a caustic chemical ingestion or of drug-induced esophagitis. He denied having dyspepsia, heartburn, or chest pain. He was not taking any medications and had no allergies.
When his dysphagia symptoms began nine years ago, he was diagnosed with acid reflux disease, confirmed by an upper gastrointestinal (GI) tract x-ray. Since that time, he reported having to swallow liquid after every bite of food and said he suffered from severe anxiety over fear of choking.
Evaluation in the ED consisted of endoscopic examination by a gastroenterologist. In addition to dislodging a food bolus, the endoscope revealed a narrowed, ringed esophagus with mucosal changes throughout the length of the esophagus (see Figures 1 through 3). Esophageal biopsies were taken, and the esophagus was dilated successfully with a 40-Fr Maloney dilator. The endoscopist detected too much resistance to pass a larger dilator.
Biopsy results revealed eosinophilic esophagitis. The patient was given oral fluticasone propionate. At one-month follow-up, he reported feeling much better. Upper endoscopy revealed some improvement, and the gastroenterologist was able to pass both a 46- and a 48-Fr Maloney dilator with only mild resistance. (The largest Maloney dilator, a 60-Fr dilator, should easily pass through a normal esophagus, according to T. L. Sack, MD, oral communication, June 2009.)
Discussion
Eosinophilic esophagitis (EE) involves the infiltration of the esophageal mucosa with eosinophils, causing edema, inflammation, and eventually, thickening and stenotic changes of the esophageal mucosa.1
The normal esophageal mucosa contains lymphocytes, mast cells, and dendritic cells, which protect the esophagus from invading toxins and microorganisms. Eosinophils are not usually present, but when they are, they can have toxic effects on the esophageal mucosa.2 EE is associated with solid food dysphagia, a direct result of damage to the esophageal mucosa, and other causes that are not clearly understood.
Research findings suggest that symptoms of dysphagia may be caused by degranulating eosinophils and mast cells, which have an antagonistic effect on the muscarinic receptors and cause smooth muscle to contract.3,4 The proposed triggering mechanism of EE is an immunoglobulin E (IgE) immune–mediated response to an allergen.2 Based on results from IgE radioallergosorbent testing (RAST), aeroallergens are more likely than food to act as triggers.5
EE in the Adult Patient
Traditionally, EE has been a condition seen in the pediatric population, with symptoms of nausea, vomiting, and failure to thrive; however, it is becoming increasingly recognized among adults. The typical patient is a man in his 20s or 30s (although cases of EE have been reported among women and older adults) with acute and recurrent solid food dysphagia, with or without food impaction.4
Often the patient reports a history of environmental or food allergies, asthma, rhinitis, or eczema.2,4-6 Researchers have reported the presence of allergic symptoms in at least 50% of patients diagnosed with EE,2 and many patients experience exacerbations associated with seasonal changes.7
GERD may coexist with EE; however, no relationship has been identified between the two.8 EE should be considered in patients with gastrointestinal symptoms that persist despite at least four weeks’ treatment with a proton pump inhibitor (PPI).2
Dysphagia: Differential Diagnosis
Adult patients with esophageal dysphagia usually report the feeling of food getting stuck when they try to swallow.9 Dysphagia may result from a mechanical obstruction or a neuromuscular/motility condition. Patients with mechanical obstructions usually have difficulty swallowing solids, while those with motility disorders tend to have difficulty with both liquids and solids.1,9
Mechanical obstructions may include carcinomas (intrinsic and extrinsic), strictures, or Schatzki rings (small thin mucosal rings of unknown etiology located at the gastroesophageal junction).1,9 Progressive dysphagia to solids over a short period of time is often indicative of esophageal carcinoma. GERD, pill-induced trauma, previous ingestion of a caustic chemical, and radiation are common causes of esophageal stricture formation. For a list of medications that are particularly caustic to the esophageal mucosa, see the table.9,10
Neuromuscular manifestations of dysphagia include achalasia, diffuse esophageal spasm, nutcracker esophagus, and scleroderma.1 These are usually associated with progressive difficulty in swallowing.9
Evaluating the Patient
A thorough patient history can often reveal potential causes of dysphagia and eliminate others. This should include current medications, chronic medical conditions and details regarding their onset and duration, and symptoms associated with dysphagia.9
Physical examination should include palpation of the thyroid because of the potential for a thyroid mass to cause extrinsic compression of the esophagus, palpation of the abdomen for masses or organomegaly, and a complete neurologic evaluation.9
Laboratory tests should be ordered based on the information obtained from the history and physical. Testing may include thyroid studies to eliminate hypothyroid or hyperthyroid causes of dysphagia, and complete blood count (CBC) with differential to rule out inflammatory or infectious processes.9 While eosinophilia may be present in the differential, it is not a universally accepted marker for establishing the diagnosis of EE.2,5 Stools should be checked for occult blood, because a positive finding may suggest esophageal carcinoma.9
Diagnosis
In the primary care setting, a barium esophagram may be used during the initial workup to evaluate the anatomic structures of the esophagus and to differentiate between a mechanical obstruction and a neuromuscular disorder.1,9 This noninvasive test requires the patient to swallow a radiopaque liquid as x-rays are taken.
The gold standard for diagnosing EE, however, is upper endoscopy with biopsy of the esophageal mucosa.6 Endoscopic findings that indicate EE are atypical of GERD; they may include a narrowed, small-caliber esophagus, concentric mucosal rings, proximal stenosis, linear ulcerations, atrophic changes, and white papules associated with eosinophilic microabscesses.6
Although there is no consensus regarding the number of eosinophils that should be present for an accurate diagnosis of EE, microscopic interpretation of the biopsy from both the proximal and the distal esophageal epithelia5 usually shows 15 or more eosinophils per high-power field.2,11 It has been suggested that mucosal biopsies be taken along the entire length of the esophagus, as eosinophilic infiltration may extend from the proximal to the distal esophagus.2
GERD and trauma induced by medication use may also be associated with esophageal eosinophilic infiltration5; however, eosinophils are usually present only in the distal esophageal mucosa3 and are not as abundant as in EE.7 If endoscopy reveals persistent eosinophilia despite four to eight weeks’ treatment with a PPI, the diagnosis of EE is confirmed.2
Treatment
Treatment for EE is still under investigation. Research has examined the association between EE and food allergies or aeroallergens.4 Evaluation by an allergist using skin prick tests or RAST is recommended in the adult patient to help determine the source of the underlying inflammation.5,7 Eliminating any identified allergen should help alleviate symptoms.4
For patients in whom no source of inflammation can be identified, treatment with 1.0 to 2.0 mg/kg/d of oral prednisone for acute exacerbations has been shown to significantly improve symptoms and histology12; however, because of the associated risk for adverse systemic effects, long-term use is not recommended.
In many patients, the inhaled corticosteroid fluticasone has also proved successful in reducing EE—associated inflammation.6 Current evidence supports adult dosing between 880 and 1,760 mcg per day for six to eight weeks, administered with a metered-dose inhaler and no spacer. Fluticasone should be sprayed directly into the mouth and swallowed, after which the patient should take nothing by mouth for 30 minutes.13 Prolonged fluticasone use has been associated with esophageal candidiasis.2 There are currently no recommendations regarding its use as maintenance therapy.
Montelukast, a leukotriene receptor antagonist, has also been shown in some studies to reduce the inflammatory process11; however, one study team recently found it to have no therapeutic effect.13
PPIs may be effective for improving EE symptoms even in the absence of GERD because of the reduced gastric acid production,7 but they do not usually improve EE’s histologic features.3
Use of esophageal dilation in patients with EE is controversial because of an associated risk for perforation.14 If this intervention is to be performed, the patient should be treated in advance with oral corticosteroids to reduce esophageal inflammation.15,16 In addition, the endoscopist should start with small-sized dilators and carefully proceed to larger sizes.11 Critics of esophageal dilation argue that the procedure is only a temporary solution and does nothing for the underlying condition.4,8
Regarding endoscopic surveillance, an interval of at least four weeks between interventions is recommended.13
Role of the Primary Care Clinician
Undiagnosed EE can cause the patient discomfort, frustration, and anxiety, as seen in the case study. Many patients with undiagnosed EE have been exposed to unnecessary medical therapy and antireflux surgery.3 Without proper diagnosis and treatment, EE may worsen, causing complications associated with chronic inflammation (ie, esophageal fibrosis and strictures).2,6
The long-term prognosis of EE is unknown at this time.8 The disease is usually chronic, with periods of remission and exacerbation. With an understanding of EE and appropriate therapies, the primary care practitioner can team with the gastroenterologist to provide effective disease management through endoscopic surveillance and intervention for acute exacerbations. Guidelines recommend that patients be closely followed with regular office visits to reassess symptoms, compliance with therapy, and adverse effects, with the goal of preventing complications associated with EE.13
Conclusion
To effectively evaluate the patient who presents with dysphagia, the primary care provider should have a working knowledge of EE, as well as an understanding of the key elements in the history and physical examination to help ensure an accurate diagnosis. This will facilitate timely referral to a gastroenterologist for endoscopic evaluation, when indicated.
1. McQuaid KR. Gastrointestinal disorders. In: McPhee S, Papadakis M. CURRENT Medical Diagnosis & Treatment 2009. New York: McGraw-Hill: 2009:487-581.
2. Nurko S, Furuta GT. Eosinophilic esophagitis (2006). GI Motility Online. www.nature.com/gimo/contents/pt1/full/gimo49.html. Accessed July 27, 2009.
3. Parfitt JR, Gregor JC, Suskin NG, et al. Eosinophilic esophagitis in adults: distinguishing features from gastroesophageal reflux disease: a study of 41 patients. Mod Pathol. 2006;19(1):90-96.
4. Swoger JM, Weiler CR, Arora AS. Eosinophilic esophagitis: is it all allergies? Mayo Clin Proc. 2007;82(12):1541-1549.
5. Conus S, Simon HU. General laboratory diagnostics of eosinophilic GI diseases. Best Pract Res Clin Gastroenterol. 2008;22(3):441-453.
6. Remedios M, Campbell C, Jones DM, Kerlin P. Eosinophilic esophagitis in adults: clinical, endoscopic, histologic findings, and response to treatment with fluticasone propionate. Gastrointest Endosc. 2006;63(1):3-12.
7. Rothenberg ME. Eosinophilic gastrointestinal disorders (EGID). J Allergy Clin Immunol. 2004; 113(1):11-28.
8. Lucendo AJ, Carrion G, Navarro M, et al. Eosinophilic esophagitis in adults: an emerging disease. Dig Dis Sci. 2004;49(11-12):1884-1888.
9. Spieker MR. Evaluating dysphagia. Am Fam Physician. 2000;61(12):3639-3648.
10. Boyce HW. Drug-induced esophageal damage: diseases of medical progress. Gastrointest Endosc. 1998;47:547-550.
11. Potter JW, Saeian K, Staff D, et al. Eosinophilic esophagitis in adults: an emerging problem with unique esophageal features. Gastrointest Endosc. 2004;59(3):355-361.
12. Schaefer ET, Fitzgerald JF, Molleston JP, et al. Comparison of oral prednisone and topical fluticasone in the treatment of eosinophilic esophagitis: a randomized trial in children. Clin Gastroenterol Hepatol. 2008;6(2):165-173.
13. Furuta GT, Liacouras CA, Collins MH, et al; First International Gastrointestinal Eosinophil Research Symposium (FIGERS) Subcommittees. Eosinophilic esophagitis in children and adults: a systematic review and consensus recommendation for diagnosis and treatment. Gastroenterology. 2007;133(4): 1342-1363.
14. Straumann A, Rossi L, Simon HU, et al. Fragility of the esophageal mucosa: a pathognomonic endoscopic sign of primary eosinophilic esophagitis. Gastrointest Endosc. 2003;57(3):407-412.
15. Hawari R, Pasricha PJ. Images in clinical medicine: eosinophilic esophagitis. N Engl J Med. 2007; 356(20):e20.
16. Leclercq P, Marting A, Gast P. Eosinophilic esophagitis. N Engl J Med. 2007;357(14):1446.
1. McQuaid KR. Gastrointestinal disorders. In: McPhee S, Papadakis M. CURRENT Medical Diagnosis & Treatment 2009. New York: McGraw-Hill: 2009:487-581.
2. Nurko S, Furuta GT. Eosinophilic esophagitis (2006). GI Motility Online. www.nature.com/gimo/contents/pt1/full/gimo49.html. Accessed July 27, 2009.
3. Parfitt JR, Gregor JC, Suskin NG, et al. Eosinophilic esophagitis in adults: distinguishing features from gastroesophageal reflux disease: a study of 41 patients. Mod Pathol. 2006;19(1):90-96.
4. Swoger JM, Weiler CR, Arora AS. Eosinophilic esophagitis: is it all allergies? Mayo Clin Proc. 2007;82(12):1541-1549.
5. Conus S, Simon HU. General laboratory diagnostics of eosinophilic GI diseases. Best Pract Res Clin Gastroenterol. 2008;22(3):441-453.
6. Remedios M, Campbell C, Jones DM, Kerlin P. Eosinophilic esophagitis in adults: clinical, endoscopic, histologic findings, and response to treatment with fluticasone propionate. Gastrointest Endosc. 2006;63(1):3-12.
7. Rothenberg ME. Eosinophilic gastrointestinal disorders (EGID). J Allergy Clin Immunol. 2004; 113(1):11-28.
8. Lucendo AJ, Carrion G, Navarro M, et al. Eosinophilic esophagitis in adults: an emerging disease. Dig Dis Sci. 2004;49(11-12):1884-1888.
9. Spieker MR. Evaluating dysphagia. Am Fam Physician. 2000;61(12):3639-3648.
10. Boyce HW. Drug-induced esophageal damage: diseases of medical progress. Gastrointest Endosc. 1998;47:547-550.
11. Potter JW, Saeian K, Staff D, et al. Eosinophilic esophagitis in adults: an emerging problem with unique esophageal features. Gastrointest Endosc. 2004;59(3):355-361.
12. Schaefer ET, Fitzgerald JF, Molleston JP, et al. Comparison of oral prednisone and topical fluticasone in the treatment of eosinophilic esophagitis: a randomized trial in children. Clin Gastroenterol Hepatol. 2008;6(2):165-173.
13. Furuta GT, Liacouras CA, Collins MH, et al; First International Gastrointestinal Eosinophil Research Symposium (FIGERS) Subcommittees. Eosinophilic esophagitis in children and adults: a systematic review and consensus recommendation for diagnosis and treatment. Gastroenterology. 2007;133(4): 1342-1363.
14. Straumann A, Rossi L, Simon HU, et al. Fragility of the esophageal mucosa: a pathognomonic endoscopic sign of primary eosinophilic esophagitis. Gastrointest Endosc. 2003;57(3):407-412.
15. Hawari R, Pasricha PJ. Images in clinical medicine: eosinophilic esophagitis. N Engl J Med. 2007; 356(20):e20.
16. Leclercq P, Marting A, Gast P. Eosinophilic esophagitis. N Engl J Med. 2007;357(14):1446.