User login
Direct Anterior Versus Posterior Simultaneous Bilateral Total Hip Arthroplasties: No Major Differences at 90 Days
End-stage osteoarthritis of the hip is a debilitating disease that is reliably treated with total hip arthroplasty (THA).1 Up to 35% of patients who undergo THA eventually require contralateral THA.2,3 In patients who present with advanced bilateral disease and undergo unilateral THA, the risk of ultimately requiring a contralateral procedure is as high as 97%.3-6 In patients with bilateral hip disease, function is not fully optimized until both hips have been replaced, particularly in the setting of fixed flexion contractures.7-9 Naturally, there has been some interest in simultaneous bilateral THAs for select patients.
The potential benefits of bilateral THAs over staged procedures include faster overall rehabilitation, exposure to a single anesthetic, reduced hospital length of stay (LOS), and cost savings.10-12 However, opinion on recommending bilateral THAs is mixed. Although bilateral procedures historically have been fraught with perioperative complications,13,14 advances in surgical and anesthetic techniques have led to improved outcomes.15 Whether surgical approach is a factor in these outcomes is unclear.
The popularity of the direct anterior (DA) approach for THA has increased in recent years.16 Although the relative advantages of various approaches remain in debate, one potential benefit of the DA approach is supine positioning, which allows simultaneous bilateral THAs to be performed without the need for repositioning before proceeding with the contralateral side. However, simultaneous bilateral THAs performed through the DA approach and those performed through other surgical approaches are lacking in comparative outcomes data.17In this study, we evaluated operative times, transfusion requirements, hospital discharge data, and 90-day complication rates in patients who had simultaneous bilateral THAs through either the DA approach or the posterior approach.
Methods
Study Design
This single-center study was conducted at the Mayo Clinic in Rochester, Minnesota. After obtaining approval from our Institutional Review Board, we performed a retrospective cohort analysis. We used our institution’s total joint registry to identify all patients who underwent simultaneous bilateral THAs through either the DA approach or the posterior approach. The first bilateral THAs to use the DA approach at our institution were performed in 2012. To ensure that the DA and posterior groups’ perioperative management would be similar, we included only cases performed between 2012 and 2014.
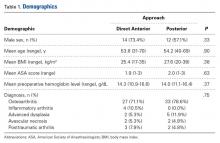
There were 19 patients in the DA group and 21 in the posterior group. The groups were similar in mean age (54 vs 54 years; P = .90), sex (73% vs 57% males; P = .33), body mass index (BMI; 25 vs 28 kg/m2; P = .38), preoperative hemoglobin level (14.3 vs 14.0 g/dL; P = .37), preoperative diagnosis (71.1% vs 78.6% degenerative joint disease; P = .75), and American Society of Anesthesiologists (ASA) score (1.9 vs 2.0; P = .63) (Table 1).
Patient Care
All cases were performed by 1 of 3 dedicated arthroplasty surgeons (Dr. Taunton, Dr. Sierra, Dr. Trousdale). Dr. Taunton exclusively uses the DA approach, and Dr. Sierra and Dr. Trousdale exclusively use the posterior approach. Patients in both groups received preoperative medical clearance and attended the same preoperative education class.
Patients in the DA group were positioned supine on an orthopedic table that allows hyperextension and adduction of the operative leg. Both hips were prepared and draped simultaneously. The most symptomatic hip was operated on first, with a sterile drape covering the contralateral hip. Between hips, fluoroscopy was moved to the other side of the operative suite, but no changes in positioning or preparation were necessary. A deep drain was placed on each side, and then was removed the morning of postoperative day 1. The same set of instruments was used on both sides.
Patients in the posterior group were positioned lateral on a regular operative table with hip rests. The most symptomatic hip was operated on first. After wound closure and dressing application, the patient was flipped to allow access to the contralateral hip and was prepared and draped again. The same instruments were used on each side. Drains were not used.
All patients received the same comprehensive multimodal pain management, which combined general and epidural anesthesia (remaining in place until postoperative day 2) and included an oral pain regimen of scheduled acetaminophen and as-needed tramadol and oxycodone. In all cases, intraoperative blood salvage and intravenous tranexamic acid (1 g at time of incision on first hip, 1 g at wound closure on second hip) were used. Preoperative autologous blood donation was not used. For deep vein thrombosis prophylaxis, patients were treated with bilateral sequential compression devices while hospitalized, but chemoprophylaxis was different between groups. Patients in the DA group received prophylactic low-molecular-weight heparin for 10 days, followed by twice-daily aspirin (325 mg) for 4 weeks. Patients in the posterior group received warfarin (goal international normalized ratio, 1.7-2.2) for 3 weeks, followed by twice-daily aspirin (325 mg) for 3 weeks. The decision to transfuse allogenic red blood cells was made by the treating surgeon, based on standardized hospital protocols, wherein patients are transfused for hemoglobin levels under 7.0 g/dL, or for hemoglobin levels less than 8.0 g/dL in the presence of persistent symptoms. All patients received care on an orthopedic specialty floor and were assisted by the same physical therapists. Discharge disposition was coordinated with the same group of social workers.
Two to 3 months after surgery, patients returned for routine examination and radiographs. All patients were followed up for at least 90 days.
Statistical Analysis
All outcomes were analyzed with appropriate summary statistics. Chi-square tests or logistic regression analyses (for categorical outcomes) were used to compare baseline covariates with perioperative outcomes, and 2-sample tests or Wilcoxon rank-sum tests were used to compare outcomes measured on a continuous scale. Odds ratios (ORs) with 95% confidence intervals (CIs) were calculated as appropriate. Operative time was calculated by adding time from incision to wound closure for both hips (room turnover time between hips was not included). Anesthesia time was defined as total time patients were in the operating room. All statistical tests were 2-sided, and the threshold for statistical significance was set at α = 0.05.
Results
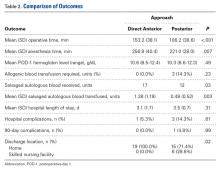
Compared with patients who underwent simultaneous bilateral THAs through the posterior approach, patients who underwent simultaneous bilateral THAs through the DA approach had longer mean operative times (153 vs 106 min; P < .001) and anesthesia times (257 vs 221 min; P = .007). The 2 groups’ hospital stays were similar in length (3.1 vs 3.5 days; P = .31), but patients in the DA group were more likely to be discharged home (100.0% vs 71.4%; P = .02) (Table 2).
Patients in the DA group were more likely to have sufficient intraoperative blood salvage for autologous transfusion (89.5% vs 57.1%; OR, 6.4; 95% CI, 1.16-34.94; P = .03) (Table 3) and received more mean units of salvaged autologous blood (1.4 vs 0.5; P = .003) (Table 2). Allogenic blood was not given to any patients in the DA group, but 3 patients in the posterior group (14.3%) required allogenic blood transfusion (P = .23) (Table 2). Salvaged autologous and allogenic blood transfusion was not associated with sex, age 60 years or older, or hospital LOS of 4 days or more (Table 3). The groups’ mean hemoglobin levels, measured the morning of postoperative day 1, were similar: 10.6 g/dL (range, 8.5-12.4 g/dL) for the DA group and 10.3 g/dL (range 8.6-12.3 g/dL) for the posterior group (Table 2).
In-hospital complications were uncommon in both groups (5% vs 14%; P = .61) (Table 2). One patient in the posterior group sustained a unilateral dislocation the day of surgery, and closed reduction was required; other complications (1 ileus, 2 tachyarrhythmias) did not require intervention. Ninety-day complications were also rare; 1 patient in the posterior group developed a hematoma with wound drainage, and this was successfully managed conservatively. There were no reoperations or readmissions in either group (Table 2).
Discussion
Although bilateral procedures account for less than 1% of THAs in the United States,11 debate about their role in patients with severe bilateral hip disease continues. The potential benefits of a single episode of care must be weighed against the slightly increased risk for systemic complications.7,10-15 Recent innovations in perioperative management have been shown to minimize complications,15 but it is unclear whether surgical approach affects perioperative outcomes. Our goals in this study were to evaluate operative times, transfusion requirements, hospital discharge data, and 90-day complication rates in patients who underwent simultaneous bilateral THAs through either the DA approach or the posterior approach.
Patients in our DA group had longer operative and anesthesia times. Other studies have found longer operative times for the DA approach relative to the posterior approach in unilateral THAs.18 One potential benefit of the DA approach in the setting of simultaneous bilateral THAs is the ability to prepare and drape both sides before surgery and thereby keep the interruption between hips to a minimum. In the present study, however, time saved during turnover between hips was overshadowed by the time added for each THA.
Although it was uncommon for complications to occur within 90 days after surgery in this study, many patients are needed to fully investigate these rare occurrences. Because of inherent selection bias, these risks are difficult to directly compare in patients who undergo unilateral procedures. Although small studies have failed to clarify the issue,7,19,20 a recent review of the almost 20,000 bilateral THA cases in the US Nationwide Inpatient Sample database found that bilateral (vs unilateral) THAs were associated with increased risk of local and systemic complications.11 Therefore, bilateral THAs should be reserved for select cases, with attention given to excluding patients with preexisting cardiopulmonary disease and providing appropriate preoperative counseling.
Most studies have reported a higher transfusion rate in bilateral THAs relative to staged procedures.7,21-23 Allogenic blood transfusion leads to immune suppression, coagulopathy, and other systemic effects in general, and has been specifically associated with infection in patients who undergo total joint arthroplasty.24-29 Parvizi and colleagues17 reported reduced blood loss and fewer blood transfusions in patients who had simultaneous bilateral THAs through the DA approach, compared with the direct lateral approach. Patients in our DA group received more salvaged autologous blood, which we suppose was a function of longer operative times. However, postoperative hemoglobin levels and allogenic blood transfusion rates were statistically similar between the 2 groups. It is important to consider the increased risk of required allogenic blood transfusion associated with simultaneous bilateral THAs, but it is not fully clear if this risk is lower in THAs performed through the DA approach relative to other approaches. In our experience, the required transfusion risk is limited in DA and posterior approaches with use of contemporary perioperative blood management strategies.
Although hospital LOS is longer with simultaneous bilateral THAs than with unilateral THAs, historically it is shorter than the combined LOS of staged bilateral THAs.20 Patients in our study had a relatively short LOS after bilateral THAs, and there was no difference in LOS between groups. However, patients were more likely to be discharged home after bilateral THAs through the DA approach vs the posterior approach. Although discharge location was not affected by age, sex, ASA score, or LOS, unrecognized social factors unrelated to surgical approach likely influenced this finding.
This study should be interpreted in light of important limitations. Foremost, although data were prospectively collected, we examined them retrospectively. Thus, it is possible there may be unaccounted for differences between our DA and posterior THA groups. For example, the DA and posterior approaches were used by different surgeons with differing experience, technique, and preferences, all of which could have affected outcomes. Furthermore, our sample was relatively small (simultaneous bilateral THAs are performed relatively infrequently). Last, although anesthesia, pain management, blood conservation, and physical therapy were similar for the 2 groups, there was no standardized protocol for determining eligibility for simultaneous bilateral THAs.
In conclusion, we found that simultaneous bilateral THAs can be safely performed through either the DA approach or the posterior approach. Although the transition between hips is shorter with the DA approach, this time savings is overshadowed by the increased duration of each procedure. Transfusion rates are low in both groups, and in-hospital and 90-day complications are quite rare. Furthermore, patients can routinely be discharged home without elevating readmission rates. We will continue to perform simultaneous bilateral THAs through the DA approach or the posterior approach, according to surgeon preference.
Am J Orthop. 2016;45(6):E373-E378. Copyright Frontline Medical Communications Inc. 2016. All rights reserved.
1. Learmonth ID, Young C, Rorabeck C. The operation of the century: total hip replacement. Lancet. 2007;370(9597):1508-1519.
2. Sayeed SA, Johnson AJ, Jaffe DE, Mont MA. Incidence of contralateral THA after index THA for osteoarthritis. Clin Orthop Relat Res. 2012;470(2):535-540.
3. Sayeed SA, Trousdale RT, Barnes SA, Kaufman KR, Pagnano MW. Joint arthroplasty within 10 years after primary Charnley total hip arthroplasty. Am J Orthop. 2009;38(8):E141-E143.
4. Goker B, Doughan AM, Schnitzer TJ, Block JA. Quantification of progressive joint space narrowing in osteoarthritis of the hip: longitudinal analysis of the contralateral hip after total hip arthroplasty. Arthritis Rheum. 2000;43(5):988-994.
5. Husted H, Overgaard S, Laursen JO, et al. Need for bilateral arthroplasty for coxarthrosis. 1,477 replacements in 1,199 patients followed for 0-14 years. Acta Orthop Scand. 1996;67(5):421-423.
6. Ritter MA, Carr K, Herbst SA, et al. Outcome of the contralateral hip following total hip arthroplasty for osteoarthritis. J Arthroplasty. 1996;11(3):242-246.
7. Alfaro- Adrián J, Bayona F, Rech JA, Murray DW. One- or two-stage bilateral total hip replacement. J Arthroplasty. 1999;14(4):439-445.
8. Wykman A, Olsson E. Walking ability after total hip replacement. A comparison of gait analysis in unilateral and bilateral cases. J Bone Joint Surg Br. 1992;74(1):53-56.
9. Yoshii T, Jinno T, Morita S, et al. Postoperative hip motion and functional recovery after simultaneous bilateral total hip arthroplasty for bilateral osteoarthritis. J Orthop Sci. 2009;14(2):161-166.
10. Lorenze M, Huo MH, Zatorski LE, Keggi KJ. A comparison of the cost effectiveness of one-stage versus two-stage bilateral total hip replacement. Orthopedics. 1998;21(12):1249-1252.
11. Rasouli MR, Maltenfort MG, Ross D, Hozack WJ, Memtsoudis SG, Parvizi J. Perioperative morbidity and mortality following bilateral total hip arthroplasty. J Arthroplasty. 2014;29(1):142-148.
12. Reuben JD, Meyers SJ, Cox DD, Elliott M, Watson M, Shim SD. Cost comparison between bilateral simultaneous, staged, and unilateral total joint arthroplasty. J Arthroplasty. 1998;13(2):172-179.
13. Bracy D, Wroblewski BM. Bilateral Charnley arthroplasty as a single procedure. A report on 400 patients. J Bone Joint Surg Br. 1981;63(3):354-356.
14. Ritter MA, Randolph JC. Bilateral total hip arthroplasty: a simultaneous procedure. Acta Orthop Scand. 1976;47(2):203-208.
15. Ritter MA, Stringer EA. Bilateral total hip arthroplasty: a single procedure. Clin Orthop Relat Res. 1980;(149):185-190.
16. Matta JM, Shahrdar C, Ferguson T. Single-incision anterior approach for total hip arthroplasty on an orthopaedic table. Clin Orthop Relat Res. 2005;(441):115-124.
17. Parvizi J, Rasouli MR, Jaberi M, et al. Does the surgical approach in one stage bilateral total hip arthroplasty affect blood loss? Int Orthop. 2013;37(12):2357-2362.
18. Poehling-Monaghan KL, Kamath AF, Taunton MJ, Pagnano MW. Direct anterior versus miniposterior THA with the same advanced perioperative protocols: surprising early clinical results. Clin Orthop Relat Res. 2015;473(2):623-631.
19. Macaulay W, Salvati EA, Sculco TP, Pellicci PM. Single-stage bilateral total hip arthroplasty. J Am Acad Orthop Surg. 2002;10(3):217-221.
20. Romagnoli S, Zacchetti S, Perazzo P, Verde F, Banfi G, Viganò M. Simultaneous bilateral total hip arthroplasties do not lead to higher complication or allogeneic transfusion rates compared to unilateral procedures. Int Orthop. 2013;37(11):2125-2130.
21. Salvati EA, Hughes P, Lachiewicz P. Bilateral total hip-replacement arthroplasty in one stage. J Bone Joint Surg Am. 1978;60(5):640-644.
22. Parvizi J, Chaudhry S, Rasouli MR, et al. Who needs autologous blood donation in joint replacement? J Knee Surg. 2011;24(1):25-31.
23. Parvizi J, Mui A, Purtill JJ, Sharkey PF, Hozack WJ, Rothman RH. Total joint arthroplasty: when do fatal or near-fatal complications occur? J Bone Joint Surg Am. 2007;89(1):27-32.
24. Blair SD, Janvrin SB, McCollum CN, Greenhalgh RM. Effect of early blood transfusion on gastrointestinal haemorrhage. Br J Surg. 1986;73(10):783-785.
25. Blumberg N, Heal JM. Immunomodulation by blood transfusion: an evolving scientific and clinical challenge. Am J Med. 1996;101(3):299-308.
26. Hébert PC, Wells G, Blajchman MA, et al. A multicenter, randomized, controlled clinical trial of transfusion requirements in critical care. Transfusion Requirements in Critical Care Investigators, Canadian Critical Care Trials Group. N Engl J Med. 1999;340(6):409-417.
27. Iturbe T, Cornudella R, de Miguel R, et al. Hypercoagulability state in hip and knee surgery: influence of ABO antigenic system and allogenic transfusion. Transfus Sci. 1999;20(1):17-20.
28. Murphy P, Heal JM, Blumberg N. Infection or suspected infection after hip replacement surgery with autologous or homologous blood transfusions. Transfusion. 1991;31(3):212-217.
29. Watts CD, Pagnano MW. Minimising blood loss and transfusion in contemporary hip and knee arthroplasty. J Bone Joint Surg Br. 2012;94(11 suppl A):8-10.
End-stage osteoarthritis of the hip is a debilitating disease that is reliably treated with total hip arthroplasty (THA).1 Up to 35% of patients who undergo THA eventually require contralateral THA.2,3 In patients who present with advanced bilateral disease and undergo unilateral THA, the risk of ultimately requiring a contralateral procedure is as high as 97%.3-6 In patients with bilateral hip disease, function is not fully optimized until both hips have been replaced, particularly in the setting of fixed flexion contractures.7-9 Naturally, there has been some interest in simultaneous bilateral THAs for select patients.
The potential benefits of bilateral THAs over staged procedures include faster overall rehabilitation, exposure to a single anesthetic, reduced hospital length of stay (LOS), and cost savings.10-12 However, opinion on recommending bilateral THAs is mixed. Although bilateral procedures historically have been fraught with perioperative complications,13,14 advances in surgical and anesthetic techniques have led to improved outcomes.15 Whether surgical approach is a factor in these outcomes is unclear.
The popularity of the direct anterior (DA) approach for THA has increased in recent years.16 Although the relative advantages of various approaches remain in debate, one potential benefit of the DA approach is supine positioning, which allows simultaneous bilateral THAs to be performed without the need for repositioning before proceeding with the contralateral side. However, simultaneous bilateral THAs performed through the DA approach and those performed through other surgical approaches are lacking in comparative outcomes data.17In this study, we evaluated operative times, transfusion requirements, hospital discharge data, and 90-day complication rates in patients who had simultaneous bilateral THAs through either the DA approach or the posterior approach.
Methods
Study Design
This single-center study was conducted at the Mayo Clinic in Rochester, Minnesota. After obtaining approval from our Institutional Review Board, we performed a retrospective cohort analysis. We used our institution’s total joint registry to identify all patients who underwent simultaneous bilateral THAs through either the DA approach or the posterior approach. The first bilateral THAs to use the DA approach at our institution were performed in 2012. To ensure that the DA and posterior groups’ perioperative management would be similar, we included only cases performed between 2012 and 2014.
There were 19 patients in the DA group and 21 in the posterior group. The groups were similar in mean age (54 vs 54 years; P = .90), sex (73% vs 57% males; P = .33), body mass index (BMI; 25 vs 28 kg/m2; P = .38), preoperative hemoglobin level (14.3 vs 14.0 g/dL; P = .37), preoperative diagnosis (71.1% vs 78.6% degenerative joint disease; P = .75), and American Society of Anesthesiologists (ASA) score (1.9 vs 2.0; P = .63) (Table 1).
Patient Care
All cases were performed by 1 of 3 dedicated arthroplasty surgeons (Dr. Taunton, Dr. Sierra, Dr. Trousdale). Dr. Taunton exclusively uses the DA approach, and Dr. Sierra and Dr. Trousdale exclusively use the posterior approach. Patients in both groups received preoperative medical clearance and attended the same preoperative education class.
Patients in the DA group were positioned supine on an orthopedic table that allows hyperextension and adduction of the operative leg. Both hips were prepared and draped simultaneously. The most symptomatic hip was operated on first, with a sterile drape covering the contralateral hip. Between hips, fluoroscopy was moved to the other side of the operative suite, but no changes in positioning or preparation were necessary. A deep drain was placed on each side, and then was removed the morning of postoperative day 1. The same set of instruments was used on both sides.
Patients in the posterior group were positioned lateral on a regular operative table with hip rests. The most symptomatic hip was operated on first. After wound closure and dressing application, the patient was flipped to allow access to the contralateral hip and was prepared and draped again. The same instruments were used on each side. Drains were not used.
All patients received the same comprehensive multimodal pain management, which combined general and epidural anesthesia (remaining in place until postoperative day 2) and included an oral pain regimen of scheduled acetaminophen and as-needed tramadol and oxycodone. In all cases, intraoperative blood salvage and intravenous tranexamic acid (1 g at time of incision on first hip, 1 g at wound closure on second hip) were used. Preoperative autologous blood donation was not used. For deep vein thrombosis prophylaxis, patients were treated with bilateral sequential compression devices while hospitalized, but chemoprophylaxis was different between groups. Patients in the DA group received prophylactic low-molecular-weight heparin for 10 days, followed by twice-daily aspirin (325 mg) for 4 weeks. Patients in the posterior group received warfarin (goal international normalized ratio, 1.7-2.2) for 3 weeks, followed by twice-daily aspirin (325 mg) for 3 weeks. The decision to transfuse allogenic red blood cells was made by the treating surgeon, based on standardized hospital protocols, wherein patients are transfused for hemoglobin levels under 7.0 g/dL, or for hemoglobin levels less than 8.0 g/dL in the presence of persistent symptoms. All patients received care on an orthopedic specialty floor and were assisted by the same physical therapists. Discharge disposition was coordinated with the same group of social workers.
Two to 3 months after surgery, patients returned for routine examination and radiographs. All patients were followed up for at least 90 days.
Statistical Analysis
All outcomes were analyzed with appropriate summary statistics. Chi-square tests or logistic regression analyses (for categorical outcomes) were used to compare baseline covariates with perioperative outcomes, and 2-sample tests or Wilcoxon rank-sum tests were used to compare outcomes measured on a continuous scale. Odds ratios (ORs) with 95% confidence intervals (CIs) were calculated as appropriate. Operative time was calculated by adding time from incision to wound closure for both hips (room turnover time between hips was not included). Anesthesia time was defined as total time patients were in the operating room. All statistical tests were 2-sided, and the threshold for statistical significance was set at α = 0.05.
Results
Compared with patients who underwent simultaneous bilateral THAs through the posterior approach, patients who underwent simultaneous bilateral THAs through the DA approach had longer mean operative times (153 vs 106 min; P < .001) and anesthesia times (257 vs 221 min; P = .007). The 2 groups’ hospital stays were similar in length (3.1 vs 3.5 days; P = .31), but patients in the DA group were more likely to be discharged home (100.0% vs 71.4%; P = .02) (Table 2).
Patients in the DA group were more likely to have sufficient intraoperative blood salvage for autologous transfusion (89.5% vs 57.1%; OR, 6.4; 95% CI, 1.16-34.94; P = .03) (Table 3) and received more mean units of salvaged autologous blood (1.4 vs 0.5; P = .003) (Table 2). Allogenic blood was not given to any patients in the DA group, but 3 patients in the posterior group (14.3%) required allogenic blood transfusion (P = .23) (Table 2). Salvaged autologous and allogenic blood transfusion was not associated with sex, age 60 years or older, or hospital LOS of 4 days or more (Table 3). The groups’ mean hemoglobin levels, measured the morning of postoperative day 1, were similar: 10.6 g/dL (range, 8.5-12.4 g/dL) for the DA group and 10.3 g/dL (range 8.6-12.3 g/dL) for the posterior group (Table 2).
In-hospital complications were uncommon in both groups (5% vs 14%; P = .61) (Table 2). One patient in the posterior group sustained a unilateral dislocation the day of surgery, and closed reduction was required; other complications (1 ileus, 2 tachyarrhythmias) did not require intervention. Ninety-day complications were also rare; 1 patient in the posterior group developed a hematoma with wound drainage, and this was successfully managed conservatively. There were no reoperations or readmissions in either group (Table 2).
Discussion
Although bilateral procedures account for less than 1% of THAs in the United States,11 debate about their role in patients with severe bilateral hip disease continues. The potential benefits of a single episode of care must be weighed against the slightly increased risk for systemic complications.7,10-15 Recent innovations in perioperative management have been shown to minimize complications,15 but it is unclear whether surgical approach affects perioperative outcomes. Our goals in this study were to evaluate operative times, transfusion requirements, hospital discharge data, and 90-day complication rates in patients who underwent simultaneous bilateral THAs through either the DA approach or the posterior approach.
Patients in our DA group had longer operative and anesthesia times. Other studies have found longer operative times for the DA approach relative to the posterior approach in unilateral THAs.18 One potential benefit of the DA approach in the setting of simultaneous bilateral THAs is the ability to prepare and drape both sides before surgery and thereby keep the interruption between hips to a minimum. In the present study, however, time saved during turnover between hips was overshadowed by the time added for each THA.
Although it was uncommon for complications to occur within 90 days after surgery in this study, many patients are needed to fully investigate these rare occurrences. Because of inherent selection bias, these risks are difficult to directly compare in patients who undergo unilateral procedures. Although small studies have failed to clarify the issue,7,19,20 a recent review of the almost 20,000 bilateral THA cases in the US Nationwide Inpatient Sample database found that bilateral (vs unilateral) THAs were associated with increased risk of local and systemic complications.11 Therefore, bilateral THAs should be reserved for select cases, with attention given to excluding patients with preexisting cardiopulmonary disease and providing appropriate preoperative counseling.
Most studies have reported a higher transfusion rate in bilateral THAs relative to staged procedures.7,21-23 Allogenic blood transfusion leads to immune suppression, coagulopathy, and other systemic effects in general, and has been specifically associated with infection in patients who undergo total joint arthroplasty.24-29 Parvizi and colleagues17 reported reduced blood loss and fewer blood transfusions in patients who had simultaneous bilateral THAs through the DA approach, compared with the direct lateral approach. Patients in our DA group received more salvaged autologous blood, which we suppose was a function of longer operative times. However, postoperative hemoglobin levels and allogenic blood transfusion rates were statistically similar between the 2 groups. It is important to consider the increased risk of required allogenic blood transfusion associated with simultaneous bilateral THAs, but it is not fully clear if this risk is lower in THAs performed through the DA approach relative to other approaches. In our experience, the required transfusion risk is limited in DA and posterior approaches with use of contemporary perioperative blood management strategies.
Although hospital LOS is longer with simultaneous bilateral THAs than with unilateral THAs, historically it is shorter than the combined LOS of staged bilateral THAs.20 Patients in our study had a relatively short LOS after bilateral THAs, and there was no difference in LOS between groups. However, patients were more likely to be discharged home after bilateral THAs through the DA approach vs the posterior approach. Although discharge location was not affected by age, sex, ASA score, or LOS, unrecognized social factors unrelated to surgical approach likely influenced this finding.
This study should be interpreted in light of important limitations. Foremost, although data were prospectively collected, we examined them retrospectively. Thus, it is possible there may be unaccounted for differences between our DA and posterior THA groups. For example, the DA and posterior approaches were used by different surgeons with differing experience, technique, and preferences, all of which could have affected outcomes. Furthermore, our sample was relatively small (simultaneous bilateral THAs are performed relatively infrequently). Last, although anesthesia, pain management, blood conservation, and physical therapy were similar for the 2 groups, there was no standardized protocol for determining eligibility for simultaneous bilateral THAs.
In conclusion, we found that simultaneous bilateral THAs can be safely performed through either the DA approach or the posterior approach. Although the transition between hips is shorter with the DA approach, this time savings is overshadowed by the increased duration of each procedure. Transfusion rates are low in both groups, and in-hospital and 90-day complications are quite rare. Furthermore, patients can routinely be discharged home without elevating readmission rates. We will continue to perform simultaneous bilateral THAs through the DA approach or the posterior approach, according to surgeon preference.
Am J Orthop. 2016;45(6):E373-E378. Copyright Frontline Medical Communications Inc. 2016. All rights reserved.
End-stage osteoarthritis of the hip is a debilitating disease that is reliably treated with total hip arthroplasty (THA).1 Up to 35% of patients who undergo THA eventually require contralateral THA.2,3 In patients who present with advanced bilateral disease and undergo unilateral THA, the risk of ultimately requiring a contralateral procedure is as high as 97%.3-6 In patients with bilateral hip disease, function is not fully optimized until both hips have been replaced, particularly in the setting of fixed flexion contractures.7-9 Naturally, there has been some interest in simultaneous bilateral THAs for select patients.
The potential benefits of bilateral THAs over staged procedures include faster overall rehabilitation, exposure to a single anesthetic, reduced hospital length of stay (LOS), and cost savings.10-12 However, opinion on recommending bilateral THAs is mixed. Although bilateral procedures historically have been fraught with perioperative complications,13,14 advances in surgical and anesthetic techniques have led to improved outcomes.15 Whether surgical approach is a factor in these outcomes is unclear.
The popularity of the direct anterior (DA) approach for THA has increased in recent years.16 Although the relative advantages of various approaches remain in debate, one potential benefit of the DA approach is supine positioning, which allows simultaneous bilateral THAs to be performed without the need for repositioning before proceeding with the contralateral side. However, simultaneous bilateral THAs performed through the DA approach and those performed through other surgical approaches are lacking in comparative outcomes data.17In this study, we evaluated operative times, transfusion requirements, hospital discharge data, and 90-day complication rates in patients who had simultaneous bilateral THAs through either the DA approach or the posterior approach.
Methods
Study Design
This single-center study was conducted at the Mayo Clinic in Rochester, Minnesota. After obtaining approval from our Institutional Review Board, we performed a retrospective cohort analysis. We used our institution’s total joint registry to identify all patients who underwent simultaneous bilateral THAs through either the DA approach or the posterior approach. The first bilateral THAs to use the DA approach at our institution were performed in 2012. To ensure that the DA and posterior groups’ perioperative management would be similar, we included only cases performed between 2012 and 2014.
There were 19 patients in the DA group and 21 in the posterior group. The groups were similar in mean age (54 vs 54 years; P = .90), sex (73% vs 57% males; P = .33), body mass index (BMI; 25 vs 28 kg/m2; P = .38), preoperative hemoglobin level (14.3 vs 14.0 g/dL; P = .37), preoperative diagnosis (71.1% vs 78.6% degenerative joint disease; P = .75), and American Society of Anesthesiologists (ASA) score (1.9 vs 2.0; P = .63) (Table 1).
Patient Care
All cases were performed by 1 of 3 dedicated arthroplasty surgeons (Dr. Taunton, Dr. Sierra, Dr. Trousdale). Dr. Taunton exclusively uses the DA approach, and Dr. Sierra and Dr. Trousdale exclusively use the posterior approach. Patients in both groups received preoperative medical clearance and attended the same preoperative education class.
Patients in the DA group were positioned supine on an orthopedic table that allows hyperextension and adduction of the operative leg. Both hips were prepared and draped simultaneously. The most symptomatic hip was operated on first, with a sterile drape covering the contralateral hip. Between hips, fluoroscopy was moved to the other side of the operative suite, but no changes in positioning or preparation were necessary. A deep drain was placed on each side, and then was removed the morning of postoperative day 1. The same set of instruments was used on both sides.
Patients in the posterior group were positioned lateral on a regular operative table with hip rests. The most symptomatic hip was operated on first. After wound closure and dressing application, the patient was flipped to allow access to the contralateral hip and was prepared and draped again. The same instruments were used on each side. Drains were not used.
All patients received the same comprehensive multimodal pain management, which combined general and epidural anesthesia (remaining in place until postoperative day 2) and included an oral pain regimen of scheduled acetaminophen and as-needed tramadol and oxycodone. In all cases, intraoperative blood salvage and intravenous tranexamic acid (1 g at time of incision on first hip, 1 g at wound closure on second hip) were used. Preoperative autologous blood donation was not used. For deep vein thrombosis prophylaxis, patients were treated with bilateral sequential compression devices while hospitalized, but chemoprophylaxis was different between groups. Patients in the DA group received prophylactic low-molecular-weight heparin for 10 days, followed by twice-daily aspirin (325 mg) for 4 weeks. Patients in the posterior group received warfarin (goal international normalized ratio, 1.7-2.2) for 3 weeks, followed by twice-daily aspirin (325 mg) for 3 weeks. The decision to transfuse allogenic red blood cells was made by the treating surgeon, based on standardized hospital protocols, wherein patients are transfused for hemoglobin levels under 7.0 g/dL, or for hemoglobin levels less than 8.0 g/dL in the presence of persistent symptoms. All patients received care on an orthopedic specialty floor and were assisted by the same physical therapists. Discharge disposition was coordinated with the same group of social workers.
Two to 3 months after surgery, patients returned for routine examination and radiographs. All patients were followed up for at least 90 days.
Statistical Analysis
All outcomes were analyzed with appropriate summary statistics. Chi-square tests or logistic regression analyses (for categorical outcomes) were used to compare baseline covariates with perioperative outcomes, and 2-sample tests or Wilcoxon rank-sum tests were used to compare outcomes measured on a continuous scale. Odds ratios (ORs) with 95% confidence intervals (CIs) were calculated as appropriate. Operative time was calculated by adding time from incision to wound closure for both hips (room turnover time between hips was not included). Anesthesia time was defined as total time patients were in the operating room. All statistical tests were 2-sided, and the threshold for statistical significance was set at α = 0.05.
Results
Compared with patients who underwent simultaneous bilateral THAs through the posterior approach, patients who underwent simultaneous bilateral THAs through the DA approach had longer mean operative times (153 vs 106 min; P < .001) and anesthesia times (257 vs 221 min; P = .007). The 2 groups’ hospital stays were similar in length (3.1 vs 3.5 days; P = .31), but patients in the DA group were more likely to be discharged home (100.0% vs 71.4%; P = .02) (Table 2).
Patients in the DA group were more likely to have sufficient intraoperative blood salvage for autologous transfusion (89.5% vs 57.1%; OR, 6.4; 95% CI, 1.16-34.94; P = .03) (Table 3) and received more mean units of salvaged autologous blood (1.4 vs 0.5; P = .003) (Table 2). Allogenic blood was not given to any patients in the DA group, but 3 patients in the posterior group (14.3%) required allogenic blood transfusion (P = .23) (Table 2). Salvaged autologous and allogenic blood transfusion was not associated with sex, age 60 years or older, or hospital LOS of 4 days or more (Table 3). The groups’ mean hemoglobin levels, measured the morning of postoperative day 1, were similar: 10.6 g/dL (range, 8.5-12.4 g/dL) for the DA group and 10.3 g/dL (range 8.6-12.3 g/dL) for the posterior group (Table 2).
In-hospital complications were uncommon in both groups (5% vs 14%; P = .61) (Table 2). One patient in the posterior group sustained a unilateral dislocation the day of surgery, and closed reduction was required; other complications (1 ileus, 2 tachyarrhythmias) did not require intervention. Ninety-day complications were also rare; 1 patient in the posterior group developed a hematoma with wound drainage, and this was successfully managed conservatively. There were no reoperations or readmissions in either group (Table 2).
Discussion
Although bilateral procedures account for less than 1% of THAs in the United States,11 debate about their role in patients with severe bilateral hip disease continues. The potential benefits of a single episode of care must be weighed against the slightly increased risk for systemic complications.7,10-15 Recent innovations in perioperative management have been shown to minimize complications,15 but it is unclear whether surgical approach affects perioperative outcomes. Our goals in this study were to evaluate operative times, transfusion requirements, hospital discharge data, and 90-day complication rates in patients who underwent simultaneous bilateral THAs through either the DA approach or the posterior approach.
Patients in our DA group had longer operative and anesthesia times. Other studies have found longer operative times for the DA approach relative to the posterior approach in unilateral THAs.18 One potential benefit of the DA approach in the setting of simultaneous bilateral THAs is the ability to prepare and drape both sides before surgery and thereby keep the interruption between hips to a minimum. In the present study, however, time saved during turnover between hips was overshadowed by the time added for each THA.
Although it was uncommon for complications to occur within 90 days after surgery in this study, many patients are needed to fully investigate these rare occurrences. Because of inherent selection bias, these risks are difficult to directly compare in patients who undergo unilateral procedures. Although small studies have failed to clarify the issue,7,19,20 a recent review of the almost 20,000 bilateral THA cases in the US Nationwide Inpatient Sample database found that bilateral (vs unilateral) THAs were associated with increased risk of local and systemic complications.11 Therefore, bilateral THAs should be reserved for select cases, with attention given to excluding patients with preexisting cardiopulmonary disease and providing appropriate preoperative counseling.
Most studies have reported a higher transfusion rate in bilateral THAs relative to staged procedures.7,21-23 Allogenic blood transfusion leads to immune suppression, coagulopathy, and other systemic effects in general, and has been specifically associated with infection in patients who undergo total joint arthroplasty.24-29 Parvizi and colleagues17 reported reduced blood loss and fewer blood transfusions in patients who had simultaneous bilateral THAs through the DA approach, compared with the direct lateral approach. Patients in our DA group received more salvaged autologous blood, which we suppose was a function of longer operative times. However, postoperative hemoglobin levels and allogenic blood transfusion rates were statistically similar between the 2 groups. It is important to consider the increased risk of required allogenic blood transfusion associated with simultaneous bilateral THAs, but it is not fully clear if this risk is lower in THAs performed through the DA approach relative to other approaches. In our experience, the required transfusion risk is limited in DA and posterior approaches with use of contemporary perioperative blood management strategies.
Although hospital LOS is longer with simultaneous bilateral THAs than with unilateral THAs, historically it is shorter than the combined LOS of staged bilateral THAs.20 Patients in our study had a relatively short LOS after bilateral THAs, and there was no difference in LOS between groups. However, patients were more likely to be discharged home after bilateral THAs through the DA approach vs the posterior approach. Although discharge location was not affected by age, sex, ASA score, or LOS, unrecognized social factors unrelated to surgical approach likely influenced this finding.
This study should be interpreted in light of important limitations. Foremost, although data were prospectively collected, we examined them retrospectively. Thus, it is possible there may be unaccounted for differences between our DA and posterior THA groups. For example, the DA and posterior approaches were used by different surgeons with differing experience, technique, and preferences, all of which could have affected outcomes. Furthermore, our sample was relatively small (simultaneous bilateral THAs are performed relatively infrequently). Last, although anesthesia, pain management, blood conservation, and physical therapy were similar for the 2 groups, there was no standardized protocol for determining eligibility for simultaneous bilateral THAs.
In conclusion, we found that simultaneous bilateral THAs can be safely performed through either the DA approach or the posterior approach. Although the transition between hips is shorter with the DA approach, this time savings is overshadowed by the increased duration of each procedure. Transfusion rates are low in both groups, and in-hospital and 90-day complications are quite rare. Furthermore, patients can routinely be discharged home without elevating readmission rates. We will continue to perform simultaneous bilateral THAs through the DA approach or the posterior approach, according to surgeon preference.
Am J Orthop. 2016;45(6):E373-E378. Copyright Frontline Medical Communications Inc. 2016. All rights reserved.
1. Learmonth ID, Young C, Rorabeck C. The operation of the century: total hip replacement. Lancet. 2007;370(9597):1508-1519.
2. Sayeed SA, Johnson AJ, Jaffe DE, Mont MA. Incidence of contralateral THA after index THA for osteoarthritis. Clin Orthop Relat Res. 2012;470(2):535-540.
3. Sayeed SA, Trousdale RT, Barnes SA, Kaufman KR, Pagnano MW. Joint arthroplasty within 10 years after primary Charnley total hip arthroplasty. Am J Orthop. 2009;38(8):E141-E143.
4. Goker B, Doughan AM, Schnitzer TJ, Block JA. Quantification of progressive joint space narrowing in osteoarthritis of the hip: longitudinal analysis of the contralateral hip after total hip arthroplasty. Arthritis Rheum. 2000;43(5):988-994.
5. Husted H, Overgaard S, Laursen JO, et al. Need for bilateral arthroplasty for coxarthrosis. 1,477 replacements in 1,199 patients followed for 0-14 years. Acta Orthop Scand. 1996;67(5):421-423.
6. Ritter MA, Carr K, Herbst SA, et al. Outcome of the contralateral hip following total hip arthroplasty for osteoarthritis. J Arthroplasty. 1996;11(3):242-246.
7. Alfaro- Adrián J, Bayona F, Rech JA, Murray DW. One- or two-stage bilateral total hip replacement. J Arthroplasty. 1999;14(4):439-445.
8. Wykman A, Olsson E. Walking ability after total hip replacement. A comparison of gait analysis in unilateral and bilateral cases. J Bone Joint Surg Br. 1992;74(1):53-56.
9. Yoshii T, Jinno T, Morita S, et al. Postoperative hip motion and functional recovery after simultaneous bilateral total hip arthroplasty for bilateral osteoarthritis. J Orthop Sci. 2009;14(2):161-166.
10. Lorenze M, Huo MH, Zatorski LE, Keggi KJ. A comparison of the cost effectiveness of one-stage versus two-stage bilateral total hip replacement. Orthopedics. 1998;21(12):1249-1252.
11. Rasouli MR, Maltenfort MG, Ross D, Hozack WJ, Memtsoudis SG, Parvizi J. Perioperative morbidity and mortality following bilateral total hip arthroplasty. J Arthroplasty. 2014;29(1):142-148.
12. Reuben JD, Meyers SJ, Cox DD, Elliott M, Watson M, Shim SD. Cost comparison between bilateral simultaneous, staged, and unilateral total joint arthroplasty. J Arthroplasty. 1998;13(2):172-179.
13. Bracy D, Wroblewski BM. Bilateral Charnley arthroplasty as a single procedure. A report on 400 patients. J Bone Joint Surg Br. 1981;63(3):354-356.
14. Ritter MA, Randolph JC. Bilateral total hip arthroplasty: a simultaneous procedure. Acta Orthop Scand. 1976;47(2):203-208.
15. Ritter MA, Stringer EA. Bilateral total hip arthroplasty: a single procedure. Clin Orthop Relat Res. 1980;(149):185-190.
16. Matta JM, Shahrdar C, Ferguson T. Single-incision anterior approach for total hip arthroplasty on an orthopaedic table. Clin Orthop Relat Res. 2005;(441):115-124.
17. Parvizi J, Rasouli MR, Jaberi M, et al. Does the surgical approach in one stage bilateral total hip arthroplasty affect blood loss? Int Orthop. 2013;37(12):2357-2362.
18. Poehling-Monaghan KL, Kamath AF, Taunton MJ, Pagnano MW. Direct anterior versus miniposterior THA with the same advanced perioperative protocols: surprising early clinical results. Clin Orthop Relat Res. 2015;473(2):623-631.
19. Macaulay W, Salvati EA, Sculco TP, Pellicci PM. Single-stage bilateral total hip arthroplasty. J Am Acad Orthop Surg. 2002;10(3):217-221.
20. Romagnoli S, Zacchetti S, Perazzo P, Verde F, Banfi G, Viganò M. Simultaneous bilateral total hip arthroplasties do not lead to higher complication or allogeneic transfusion rates compared to unilateral procedures. Int Orthop. 2013;37(11):2125-2130.
21. Salvati EA, Hughes P, Lachiewicz P. Bilateral total hip-replacement arthroplasty in one stage. J Bone Joint Surg Am. 1978;60(5):640-644.
22. Parvizi J, Chaudhry S, Rasouli MR, et al. Who needs autologous blood donation in joint replacement? J Knee Surg. 2011;24(1):25-31.
23. Parvizi J, Mui A, Purtill JJ, Sharkey PF, Hozack WJ, Rothman RH. Total joint arthroplasty: when do fatal or near-fatal complications occur? J Bone Joint Surg Am. 2007;89(1):27-32.
24. Blair SD, Janvrin SB, McCollum CN, Greenhalgh RM. Effect of early blood transfusion on gastrointestinal haemorrhage. Br J Surg. 1986;73(10):783-785.
25. Blumberg N, Heal JM. Immunomodulation by blood transfusion: an evolving scientific and clinical challenge. Am J Med. 1996;101(3):299-308.
26. Hébert PC, Wells G, Blajchman MA, et al. A multicenter, randomized, controlled clinical trial of transfusion requirements in critical care. Transfusion Requirements in Critical Care Investigators, Canadian Critical Care Trials Group. N Engl J Med. 1999;340(6):409-417.
27. Iturbe T, Cornudella R, de Miguel R, et al. Hypercoagulability state in hip and knee surgery: influence of ABO antigenic system and allogenic transfusion. Transfus Sci. 1999;20(1):17-20.
28. Murphy P, Heal JM, Blumberg N. Infection or suspected infection after hip replacement surgery with autologous or homologous blood transfusions. Transfusion. 1991;31(3):212-217.
29. Watts CD, Pagnano MW. Minimising blood loss and transfusion in contemporary hip and knee arthroplasty. J Bone Joint Surg Br. 2012;94(11 suppl A):8-10.
1. Learmonth ID, Young C, Rorabeck C. The operation of the century: total hip replacement. Lancet. 2007;370(9597):1508-1519.
2. Sayeed SA, Johnson AJ, Jaffe DE, Mont MA. Incidence of contralateral THA after index THA for osteoarthritis. Clin Orthop Relat Res. 2012;470(2):535-540.
3. Sayeed SA, Trousdale RT, Barnes SA, Kaufman KR, Pagnano MW. Joint arthroplasty within 10 years after primary Charnley total hip arthroplasty. Am J Orthop. 2009;38(8):E141-E143.
4. Goker B, Doughan AM, Schnitzer TJ, Block JA. Quantification of progressive joint space narrowing in osteoarthritis of the hip: longitudinal analysis of the contralateral hip after total hip arthroplasty. Arthritis Rheum. 2000;43(5):988-994.
5. Husted H, Overgaard S, Laursen JO, et al. Need for bilateral arthroplasty for coxarthrosis. 1,477 replacements in 1,199 patients followed for 0-14 years. Acta Orthop Scand. 1996;67(5):421-423.
6. Ritter MA, Carr K, Herbst SA, et al. Outcome of the contralateral hip following total hip arthroplasty for osteoarthritis. J Arthroplasty. 1996;11(3):242-246.
7. Alfaro- Adrián J, Bayona F, Rech JA, Murray DW. One- or two-stage bilateral total hip replacement. J Arthroplasty. 1999;14(4):439-445.
8. Wykman A, Olsson E. Walking ability after total hip replacement. A comparison of gait analysis in unilateral and bilateral cases. J Bone Joint Surg Br. 1992;74(1):53-56.
9. Yoshii T, Jinno T, Morita S, et al. Postoperative hip motion and functional recovery after simultaneous bilateral total hip arthroplasty for bilateral osteoarthritis. J Orthop Sci. 2009;14(2):161-166.
10. Lorenze M, Huo MH, Zatorski LE, Keggi KJ. A comparison of the cost effectiveness of one-stage versus two-stage bilateral total hip replacement. Orthopedics. 1998;21(12):1249-1252.
11. Rasouli MR, Maltenfort MG, Ross D, Hozack WJ, Memtsoudis SG, Parvizi J. Perioperative morbidity and mortality following bilateral total hip arthroplasty. J Arthroplasty. 2014;29(1):142-148.
12. Reuben JD, Meyers SJ, Cox DD, Elliott M, Watson M, Shim SD. Cost comparison between bilateral simultaneous, staged, and unilateral total joint arthroplasty. J Arthroplasty. 1998;13(2):172-179.
13. Bracy D, Wroblewski BM. Bilateral Charnley arthroplasty as a single procedure. A report on 400 patients. J Bone Joint Surg Br. 1981;63(3):354-356.
14. Ritter MA, Randolph JC. Bilateral total hip arthroplasty: a simultaneous procedure. Acta Orthop Scand. 1976;47(2):203-208.
15. Ritter MA, Stringer EA. Bilateral total hip arthroplasty: a single procedure. Clin Orthop Relat Res. 1980;(149):185-190.
16. Matta JM, Shahrdar C, Ferguson T. Single-incision anterior approach for total hip arthroplasty on an orthopaedic table. Clin Orthop Relat Res. 2005;(441):115-124.
17. Parvizi J, Rasouli MR, Jaberi M, et al. Does the surgical approach in one stage bilateral total hip arthroplasty affect blood loss? Int Orthop. 2013;37(12):2357-2362.
18. Poehling-Monaghan KL, Kamath AF, Taunton MJ, Pagnano MW. Direct anterior versus miniposterior THA with the same advanced perioperative protocols: surprising early clinical results. Clin Orthop Relat Res. 2015;473(2):623-631.
19. Macaulay W, Salvati EA, Sculco TP, Pellicci PM. Single-stage bilateral total hip arthroplasty. J Am Acad Orthop Surg. 2002;10(3):217-221.
20. Romagnoli S, Zacchetti S, Perazzo P, Verde F, Banfi G, Viganò M. Simultaneous bilateral total hip arthroplasties do not lead to higher complication or allogeneic transfusion rates compared to unilateral procedures. Int Orthop. 2013;37(11):2125-2130.
21. Salvati EA, Hughes P, Lachiewicz P. Bilateral total hip-replacement arthroplasty in one stage. J Bone Joint Surg Am. 1978;60(5):640-644.
22. Parvizi J, Chaudhry S, Rasouli MR, et al. Who needs autologous blood donation in joint replacement? J Knee Surg. 2011;24(1):25-31.
23. Parvizi J, Mui A, Purtill JJ, Sharkey PF, Hozack WJ, Rothman RH. Total joint arthroplasty: when do fatal or near-fatal complications occur? J Bone Joint Surg Am. 2007;89(1):27-32.
24. Blair SD, Janvrin SB, McCollum CN, Greenhalgh RM. Effect of early blood transfusion on gastrointestinal haemorrhage. Br J Surg. 1986;73(10):783-785.
25. Blumberg N, Heal JM. Immunomodulation by blood transfusion: an evolving scientific and clinical challenge. Am J Med. 1996;101(3):299-308.
26. Hébert PC, Wells G, Blajchman MA, et al. A multicenter, randomized, controlled clinical trial of transfusion requirements in critical care. Transfusion Requirements in Critical Care Investigators, Canadian Critical Care Trials Group. N Engl J Med. 1999;340(6):409-417.
27. Iturbe T, Cornudella R, de Miguel R, et al. Hypercoagulability state in hip and knee surgery: influence of ABO antigenic system and allogenic transfusion. Transfus Sci. 1999;20(1):17-20.
28. Murphy P, Heal JM, Blumberg N. Infection or suspected infection after hip replacement surgery with autologous or homologous blood transfusions. Transfusion. 1991;31(3):212-217.
29. Watts CD, Pagnano MW. Minimising blood loss and transfusion in contemporary hip and knee arthroplasty. J Bone Joint Surg Br. 2012;94(11 suppl A):8-10.