User login
Mitchel is a reporter for MDedge based in the Philadelphia area. He started with the company in 1992, when it was International Medical News Group (IMNG), and has since covered a range of medical specialties. Mitchel trained as a virologist at Roswell Park Memorial Institute in Buffalo, and then worked briefly as a researcher at Boston Children's Hospital before pivoting to journalism as a AAAS Mass Media Fellow in 1980. His first reporting job was with Science Digest magazine, and from the mid-1980s to early-1990s he was a reporter with Medical World News. @mitchelzoler
Meta-Analysis Shows Statins Effective for Primary Prevention
The shifting balance of data for and against broader use of statin treatment tilted again toward more liberal use, with results from a meta-analysis of more than 170,000 participants showing a clear, positive, overall effect from statin treatment in all types of adults, even those with a relatively low baseline risk for major vascular events.
Among people with a 5-year risk of major vascular events lower than 10%, each 1-mmol/L (39-mg/dL) reduction in low-density lipoprotein cholesterol from statin treatment produced an absolute reduction in 11 major vascular events per 1,000 people during 5 years of treatment, reported the Cholesterol Treatment Trialists Collaborators, a team based in Oxford, England, in an article published online May 16 in the Lancet (doi: 10.1016./S0140-6736(12)60367-5).
The analysis showed that "statin therapy significantly reduced the risk of major vascular events in individuals with 5-year risk lower than 10% (in whom the mean risks were 2%-6% for major coronary events plus 3% for other major vascular events), even in those with no previous history of vascular disease, diabetes, or chronic kidney disease." The meta-analysis included data on 174,149 people from 27 controlled trials, and included participants with a broad range of baseline cardiovascular-event risk, ranging from a 5-year risk of less than 5% to a risk of greater than 30%
The degree of relative risk reduction among trial participants with a 5-year risk of less than 5%, or 5% to less than 10%, was roughly similar to the risk reduction seen in participants with higher baseline risk levels, even in those with a baseline 5-year risk of 30% or more. The overall relative risk reduction for all people at all baseline risk levels in the analysis was 21% for each 1-mmol/L reduction in LDL cholesterol achieved with statin treatment. For those with a baseline, 5-year risk of less than 5%, the relative risk reduction was 38% for each 1-mmol/L reduction in LDL cholesterol, and for those with a baseline 5-year risk of 5% to less than 10%, the relative risk reduction from statin use was 31% for this level of LDL reduction.
Based on this new analysis, the authors concluded that the benefits of statin treatment, even in people at low risk, "greatly" outweigh the risks: "Any long-term effects of any small excesses in hemorrhagic strokes and in diagnoses of diabetes [triggered by statin use] are not associated with long-term effects on major vascular events that are sufficiently large to outweigh the persistent benefits of statin therapy," they wrote.
Some members of the study-writing committee said that they received reimbursement of costs to participate in scientific meetings from the pharmaceutical industry. Two of the authors received honoraria from Solvay for lectures related to the meta-analysis.
"These findings confirm the efficacy of statins for primary prevention, resolving concerns about possible serious adverse effects, and potential sources of bias in randomized trials," wrote Dr. Shah Ebrahim and Dr. Juan P. Casas in a comment that accompanied the meta-analysis (Lancet 2012 May 16 [doi: 10.1016/S0140-6736(12)60367-5]).
The new analysis predicted that 6* and 15 major vascular events would be avoided per 1,000 people treated for 5 years in the two lowest-risk categories, respectively, if they achieved a 1-mmol/L cut in their baseline level of LDL cholesterol, which translates into numbers needed to treat of 167 and 67. "These figures look encouraging, and are similar to those for treatment of mild hypertension, which is widely accepted as a primary-care task," they noted in their comment.
But the evidence the meta-analysis provides in favor of statin treatment does not make treatment a slam-dunk, they warned. First, an LDL cholesterol reduction of 1 mmol/L (39 mg/dL) can be hard to achieve in people without evidence of cardiovascular disease, although they note that the evidence presented also gives reassurance about prescription of higher statin doses to "achieve greater benefit and dissipate uncertainty about any potential serious adverse risks of statins."
In addition, expansion of routine statin use for primary prevention to people with a 5-year risk for cardiovascular disease of less than 10% would sharply boost statin prescribing, and might potentially deflect attention away from aggressive statin treatment of higher-risk patients. An even better solution would be more aggressive dietary measures to lower LDL cholesterol, Dr. Ebrahim and Dr. Casas suggested, but they acknowledged that taking such steps on a national basis is hard.
Still, a practical solution would be "to use age as the only indicator for statin prescription, as was originally proposed for the polypill," they suggested, "because most people older than 50 years are likely to be at greater than 10% 10-year risk of cardiovascular disease."
Dr. Ebrahim is an epidemiologist at the London School of Hygiene & Tropical Medicine, and Dr. Casas is an epidemiologist at University College London. They both said they had no relevant disclosures.
*CORRECTION, 5/17/12: An earlier version of this article misstated the number of vascular events avoided per 1,000 patients in this group.
"These findings confirm the efficacy of statins for primary prevention, resolving concerns about possible serious adverse effects, and potential sources of bias in randomized trials," wrote Dr. Shah Ebrahim and Dr. Juan P. Casas in a comment that accompanied the meta-analysis (Lancet 2012 May 16 [doi: 10.1016/S0140-6736(12)60367-5]).
The new analysis predicted that 6* and 15 major vascular events would be avoided per 1,000 people treated for 5 years in the two lowest-risk categories, respectively, if they achieved a 1-mmol/L cut in their baseline level of LDL cholesterol, which translates into numbers needed to treat of 167 and 67. "These figures look encouraging, and are similar to those for treatment of mild hypertension, which is widely accepted as a primary-care task," they noted in their comment.
But the evidence the meta-analysis provides in favor of statin treatment does not make treatment a slam-dunk, they warned. First, an LDL cholesterol reduction of 1 mmol/L (39 mg/dL) can be hard to achieve in people without evidence of cardiovascular disease, although they note that the evidence presented also gives reassurance about prescription of higher statin doses to "achieve greater benefit and dissipate uncertainty about any potential serious adverse risks of statins."
In addition, expansion of routine statin use for primary prevention to people with a 5-year risk for cardiovascular disease of less than 10% would sharply boost statin prescribing, and might potentially deflect attention away from aggressive statin treatment of higher-risk patients. An even better solution would be more aggressive dietary measures to lower LDL cholesterol, Dr. Ebrahim and Dr. Casas suggested, but they acknowledged that taking such steps on a national basis is hard.
Still, a practical solution would be "to use age as the only indicator for statin prescription, as was originally proposed for the polypill," they suggested, "because most people older than 50 years are likely to be at greater than 10% 10-year risk of cardiovascular disease."
Dr. Ebrahim is an epidemiologist at the London School of Hygiene & Tropical Medicine, and Dr. Casas is an epidemiologist at University College London. They both said they had no relevant disclosures.
*CORRECTION, 5/17/12: An earlier version of this article misstated the number of vascular events avoided per 1,000 patients in this group.
"These findings confirm the efficacy of statins for primary prevention, resolving concerns about possible serious adverse effects, and potential sources of bias in randomized trials," wrote Dr. Shah Ebrahim and Dr. Juan P. Casas in a comment that accompanied the meta-analysis (Lancet 2012 May 16 [doi: 10.1016/S0140-6736(12)60367-5]).
The new analysis predicted that 6* and 15 major vascular events would be avoided per 1,000 people treated for 5 years in the two lowest-risk categories, respectively, if they achieved a 1-mmol/L cut in their baseline level of LDL cholesterol, which translates into numbers needed to treat of 167 and 67. "These figures look encouraging, and are similar to those for treatment of mild hypertension, which is widely accepted as a primary-care task," they noted in their comment.
But the evidence the meta-analysis provides in favor of statin treatment does not make treatment a slam-dunk, they warned. First, an LDL cholesterol reduction of 1 mmol/L (39 mg/dL) can be hard to achieve in people without evidence of cardiovascular disease, although they note that the evidence presented also gives reassurance about prescription of higher statin doses to "achieve greater benefit and dissipate uncertainty about any potential serious adverse risks of statins."
In addition, expansion of routine statin use for primary prevention to people with a 5-year risk for cardiovascular disease of less than 10% would sharply boost statin prescribing, and might potentially deflect attention away from aggressive statin treatment of higher-risk patients. An even better solution would be more aggressive dietary measures to lower LDL cholesterol, Dr. Ebrahim and Dr. Casas suggested, but they acknowledged that taking such steps on a national basis is hard.
Still, a practical solution would be "to use age as the only indicator for statin prescription, as was originally proposed for the polypill," they suggested, "because most people older than 50 years are likely to be at greater than 10% 10-year risk of cardiovascular disease."
Dr. Ebrahim is an epidemiologist at the London School of Hygiene & Tropical Medicine, and Dr. Casas is an epidemiologist at University College London. They both said they had no relevant disclosures.
*CORRECTION, 5/17/12: An earlier version of this article misstated the number of vascular events avoided per 1,000 patients in this group.
The shifting balance of data for and against broader use of statin treatment tilted again toward more liberal use, with results from a meta-analysis of more than 170,000 participants showing a clear, positive, overall effect from statin treatment in all types of adults, even those with a relatively low baseline risk for major vascular events.
Among people with a 5-year risk of major vascular events lower than 10%, each 1-mmol/L (39-mg/dL) reduction in low-density lipoprotein cholesterol from statin treatment produced an absolute reduction in 11 major vascular events per 1,000 people during 5 years of treatment, reported the Cholesterol Treatment Trialists Collaborators, a team based in Oxford, England, in an article published online May 16 in the Lancet (doi: 10.1016./S0140-6736(12)60367-5).
The analysis showed that "statin therapy significantly reduced the risk of major vascular events in individuals with 5-year risk lower than 10% (in whom the mean risks were 2%-6% for major coronary events plus 3% for other major vascular events), even in those with no previous history of vascular disease, diabetes, or chronic kidney disease." The meta-analysis included data on 174,149 people from 27 controlled trials, and included participants with a broad range of baseline cardiovascular-event risk, ranging from a 5-year risk of less than 5% to a risk of greater than 30%
The degree of relative risk reduction among trial participants with a 5-year risk of less than 5%, or 5% to less than 10%, was roughly similar to the risk reduction seen in participants with higher baseline risk levels, even in those with a baseline 5-year risk of 30% or more. The overall relative risk reduction for all people at all baseline risk levels in the analysis was 21% for each 1-mmol/L reduction in LDL cholesterol achieved with statin treatment. For those with a baseline, 5-year risk of less than 5%, the relative risk reduction was 38% for each 1-mmol/L reduction in LDL cholesterol, and for those with a baseline 5-year risk of 5% to less than 10%, the relative risk reduction from statin use was 31% for this level of LDL reduction.
Based on this new analysis, the authors concluded that the benefits of statin treatment, even in people at low risk, "greatly" outweigh the risks: "Any long-term effects of any small excesses in hemorrhagic strokes and in diagnoses of diabetes [triggered by statin use] are not associated with long-term effects on major vascular events that are sufficiently large to outweigh the persistent benefits of statin therapy," they wrote.
Some members of the study-writing committee said that they received reimbursement of costs to participate in scientific meetings from the pharmaceutical industry. Two of the authors received honoraria from Solvay for lectures related to the meta-analysis.
The shifting balance of data for and against broader use of statin treatment tilted again toward more liberal use, with results from a meta-analysis of more than 170,000 participants showing a clear, positive, overall effect from statin treatment in all types of adults, even those with a relatively low baseline risk for major vascular events.
Among people with a 5-year risk of major vascular events lower than 10%, each 1-mmol/L (39-mg/dL) reduction in low-density lipoprotein cholesterol from statin treatment produced an absolute reduction in 11 major vascular events per 1,000 people during 5 years of treatment, reported the Cholesterol Treatment Trialists Collaborators, a team based in Oxford, England, in an article published online May 16 in the Lancet (doi: 10.1016./S0140-6736(12)60367-5).
The analysis showed that "statin therapy significantly reduced the risk of major vascular events in individuals with 5-year risk lower than 10% (in whom the mean risks were 2%-6% for major coronary events plus 3% for other major vascular events), even in those with no previous history of vascular disease, diabetes, or chronic kidney disease." The meta-analysis included data on 174,149 people from 27 controlled trials, and included participants with a broad range of baseline cardiovascular-event risk, ranging from a 5-year risk of less than 5% to a risk of greater than 30%
The degree of relative risk reduction among trial participants with a 5-year risk of less than 5%, or 5% to less than 10%, was roughly similar to the risk reduction seen in participants with higher baseline risk levels, even in those with a baseline 5-year risk of 30% or more. The overall relative risk reduction for all people at all baseline risk levels in the analysis was 21% for each 1-mmol/L reduction in LDL cholesterol achieved with statin treatment. For those with a baseline, 5-year risk of less than 5%, the relative risk reduction was 38% for each 1-mmol/L reduction in LDL cholesterol, and for those with a baseline 5-year risk of 5% to less than 10%, the relative risk reduction from statin use was 31% for this level of LDL reduction.
Based on this new analysis, the authors concluded that the benefits of statin treatment, even in people at low risk, "greatly" outweigh the risks: "Any long-term effects of any small excesses in hemorrhagic strokes and in diagnoses of diabetes [triggered by statin use] are not associated with long-term effects on major vascular events that are sufficiently large to outweigh the persistent benefits of statin therapy," they wrote.
Some members of the study-writing committee said that they received reimbursement of costs to participate in scientific meetings from the pharmaceutical industry. Two of the authors received honoraria from Solvay for lectures related to the meta-analysis.
FROM THE LANCET
Gene Markers Linked With Suicidality in Schizophrenia
BALTIMORE – Researchers identified markers in two genes involved in the production of norepinephrine that significantly linked with an increased rate of suicide attempts among patients with schizophrenia in an exploratory study of 241 patients.
If the findings are confirmed in expanded clinical studies, the results could advance physicians’ ability to identify patients with schizophrenia who have an elevated risk for attempting suicide, provide important leads for developing new agents to treat patients at risk for suicide, and help better target existing treatments to suicidal patients who could most benefit from them, Dr. Vincenzo De Luca said at the annual conference of the American Association of Suicidology.
Elevated noradrenergic activity is associated with aggressive behavior, which led Dr. De Luca and his associates to explore the hypothesis that a link exists between genes involved in norepinephrine metabolism and suicidal behavior in patients with schizophrenia, explained Dr. De Luca, a psychiatrist at the University of Toronto. Prior work by his group led to preliminary evidence linking a marker in a gene involved in regulating the hypothalamic-pituitary-adrenal pathway to an increased risk for suicide attempts in patients with schizophrenia (J. Psychopharmacol. 2010;24:677-82).
The current study involved 241 patients who met the DSM-IV criteria for schizophrenia and were recruited from several psychiatric care facilities in the Toronto area. The patients averaged 36 years old, with an average duration of illness of 16 years; 71% were men; and 80% were white. Fifty-three of the patients (22%) had a history of at least one well-documented suicide attempt, a rate that fits with prior reports of suicide attempt rates of 20%-50% among patients with schizophrenia, he said.
A series of analyses showed no demographic or clinical differences between the suicide attempters and nonattempters. However, the genetic analysis showed two very statistically significant differences in the prevalence of specific genetic polymorphisms in two different genes involved in norepinephrine production. One marker was in the gene for tyrosine hydroxylase (the enzyme that converts tyrosine to dopamine), and the second marker was in the gene for dopamine beta-hydroxylase (the enzyme that converts dopamine to norepinephrine).
The tyrosine hydroxylase polymorphism linked with a 3.7-fold increased rate of suicide attempts, compared with patients without the marker. And the dopamine beta-hydroxylase polymorphism linked with a 3.5-fold increased rate of suicide attempts, compared with patients who lacked this marker.
If this finding is confirmed in a larger number of patients, it might mean that these markers could constitute "a predictive test to help clinicians assess a patient’s suicide risk," Dr. De Luca said in an interview. The findings may apply not only to patients with schizophrenia, but also to patients with bipolar disorder, a possibility that also needs assessment in future clinical studies, he said. Key elements in trying to make these genetic links are having "clean," well-characterized, and well-documented data about patients’ clinical status; their diagnosed phenotypes; their suicide-attempt histories; and their ethnicity, as well as a large number of potential genetic markers.
Dr. De Luca said he had no relevant financial disclosures.
BALTIMORE – Researchers identified markers in two genes involved in the production of norepinephrine that significantly linked with an increased rate of suicide attempts among patients with schizophrenia in an exploratory study of 241 patients.
If the findings are confirmed in expanded clinical studies, the results could advance physicians’ ability to identify patients with schizophrenia who have an elevated risk for attempting suicide, provide important leads for developing new agents to treat patients at risk for suicide, and help better target existing treatments to suicidal patients who could most benefit from them, Dr. Vincenzo De Luca said at the annual conference of the American Association of Suicidology.
Elevated noradrenergic activity is associated with aggressive behavior, which led Dr. De Luca and his associates to explore the hypothesis that a link exists between genes involved in norepinephrine metabolism and suicidal behavior in patients with schizophrenia, explained Dr. De Luca, a psychiatrist at the University of Toronto. Prior work by his group led to preliminary evidence linking a marker in a gene involved in regulating the hypothalamic-pituitary-adrenal pathway to an increased risk for suicide attempts in patients with schizophrenia (J. Psychopharmacol. 2010;24:677-82).
The current study involved 241 patients who met the DSM-IV criteria for schizophrenia and were recruited from several psychiatric care facilities in the Toronto area. The patients averaged 36 years old, with an average duration of illness of 16 years; 71% were men; and 80% were white. Fifty-three of the patients (22%) had a history of at least one well-documented suicide attempt, a rate that fits with prior reports of suicide attempt rates of 20%-50% among patients with schizophrenia, he said.
A series of analyses showed no demographic or clinical differences between the suicide attempters and nonattempters. However, the genetic analysis showed two very statistically significant differences in the prevalence of specific genetic polymorphisms in two different genes involved in norepinephrine production. One marker was in the gene for tyrosine hydroxylase (the enzyme that converts tyrosine to dopamine), and the second marker was in the gene for dopamine beta-hydroxylase (the enzyme that converts dopamine to norepinephrine).
The tyrosine hydroxylase polymorphism linked with a 3.7-fold increased rate of suicide attempts, compared with patients without the marker. And the dopamine beta-hydroxylase polymorphism linked with a 3.5-fold increased rate of suicide attempts, compared with patients who lacked this marker.
If this finding is confirmed in a larger number of patients, it might mean that these markers could constitute "a predictive test to help clinicians assess a patient’s suicide risk," Dr. De Luca said in an interview. The findings may apply not only to patients with schizophrenia, but also to patients with bipolar disorder, a possibility that also needs assessment in future clinical studies, he said. Key elements in trying to make these genetic links are having "clean," well-characterized, and well-documented data about patients’ clinical status; their diagnosed phenotypes; their suicide-attempt histories; and their ethnicity, as well as a large number of potential genetic markers.
Dr. De Luca said he had no relevant financial disclosures.
BALTIMORE – Researchers identified markers in two genes involved in the production of norepinephrine that significantly linked with an increased rate of suicide attempts among patients with schizophrenia in an exploratory study of 241 patients.
If the findings are confirmed in expanded clinical studies, the results could advance physicians’ ability to identify patients with schizophrenia who have an elevated risk for attempting suicide, provide important leads for developing new agents to treat patients at risk for suicide, and help better target existing treatments to suicidal patients who could most benefit from them, Dr. Vincenzo De Luca said at the annual conference of the American Association of Suicidology.
Elevated noradrenergic activity is associated with aggressive behavior, which led Dr. De Luca and his associates to explore the hypothesis that a link exists between genes involved in norepinephrine metabolism and suicidal behavior in patients with schizophrenia, explained Dr. De Luca, a psychiatrist at the University of Toronto. Prior work by his group led to preliminary evidence linking a marker in a gene involved in regulating the hypothalamic-pituitary-adrenal pathway to an increased risk for suicide attempts in patients with schizophrenia (J. Psychopharmacol. 2010;24:677-82).
The current study involved 241 patients who met the DSM-IV criteria for schizophrenia and were recruited from several psychiatric care facilities in the Toronto area. The patients averaged 36 years old, with an average duration of illness of 16 years; 71% were men; and 80% were white. Fifty-three of the patients (22%) had a history of at least one well-documented suicide attempt, a rate that fits with prior reports of suicide attempt rates of 20%-50% among patients with schizophrenia, he said.
A series of analyses showed no demographic or clinical differences between the suicide attempters and nonattempters. However, the genetic analysis showed two very statistically significant differences in the prevalence of specific genetic polymorphisms in two different genes involved in norepinephrine production. One marker was in the gene for tyrosine hydroxylase (the enzyme that converts tyrosine to dopamine), and the second marker was in the gene for dopamine beta-hydroxylase (the enzyme that converts dopamine to norepinephrine).
The tyrosine hydroxylase polymorphism linked with a 3.7-fold increased rate of suicide attempts, compared with patients without the marker. And the dopamine beta-hydroxylase polymorphism linked with a 3.5-fold increased rate of suicide attempts, compared with patients who lacked this marker.
If this finding is confirmed in a larger number of patients, it might mean that these markers could constitute "a predictive test to help clinicians assess a patient’s suicide risk," Dr. De Luca said in an interview. The findings may apply not only to patients with schizophrenia, but also to patients with bipolar disorder, a possibility that also needs assessment in future clinical studies, he said. Key elements in trying to make these genetic links are having "clean," well-characterized, and well-documented data about patients’ clinical status; their diagnosed phenotypes; their suicide-attempt histories; and their ethnicity, as well as a large number of potential genetic markers.
Dr. De Luca said he had no relevant financial disclosures.
FROM THE ANNUALCONFERENCE OF THE AMERICAN ASSOCIATION OF SUICIDOLOGY
Major Finding: A tyrosine hydroxylase polymorphism and a dopamine beta-hydroxylase polymorphism were each linked with a roughly fourfold increased rate of suicide attempts.
Data Source: Data came from a study of 241 patients with schizophrenia conducted at one Canadian center.
Disclosures: Dr. De Luca said he had no relevant financial disclosures.
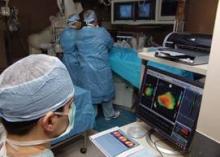
Act 2 Opens for Stem-Cell Heart Treatments
Stem-cell treatment for cardiac disease recently launched into advanced clinical trials, as a flurry of early-phase studies over the last year collectively confirmed the treatment as safe but left its efficacy unresolved.
At least two phase III trials are now underway and others are possibly imminent. But until these pivotal studies begin to yield outcome results in about 4 years, stem-cell treatment remains a question mark – an appealing idea fueled by hints of effectiveness but also dogged by failures that prompt skepticism about its future.
The gush of recent early-phase studies also hint at possible explanations for the variability in the success of stem cell treatment. The studies examined a broad range of cell types and patients. (Click here to see a chart of the studies.) The results imply that select types of bone marrow cells may be more effective as therapy and that the number and potency of stem cells decrease with age.
Clinical testing of stem cells for heart disease has hit its stride more than a decade after the first-in-human report in 2001 of a patient treated following an acute myocardial infarction (Dtsch. Med. Wochenschr. 2001;126:932-8), which was followed by the first randomized clinical trial, also in myocardial infarction patients, reported in 2004 (Lancet 2004;364:141-8).
"So far, stem-cell treatment has been safe in all the areas where it’s been used. I’m very satisfied" with the progress, said Dr. Emerson C. Perin, an interventional cardiologist and medical director of the Stem Cell Center at the Texas Heart Institute and one of the U.S. researchers who has worked longest on stem-cell clinical studies. Those working on clinical investigations of stem cells "haven’t done anything stupid to kill it, and we’ve gone from a crawl to a walk," he said in an interview.
Development of stem cell therapies "has been on a normal track; each step takes time. It’s very similar to what happened with fibrinolytic therapy for treating acute myocardial infarctions" in the 1980s and 1990s, said Dr. Timothy D. Henry, an interventional cardiologist, director of research at the Minneapolis Heart Institute Foundation, and another very active stem cell researcher. "It was a good 10 years before we got lytics up and going. People want [stem cells] to be a magic bullet. I think some people have put unrealistic expectations on stem cells, but it’s like any other treatment. You need to do a trial and find out the relative risks and benefits, and that takes time," he said in an interview.
A different take on the past decade of stem-cell work came from another researcher in the field, Dr. Eduardo Marbán. "The pace of progress has been disappointingly slow, marked by numerous examples of clinical studies prematurely undertaken without benefit of adequate preclinical data. We are lucky that no one has been killed," said Dr. Marbán, professor and director of the Cedars-Sinai Heart Institute in Los Angeles. But despite whatever role luck may have played, Dr. Marbán agreed on the bottom line: "The major accomplishment of the 11-year experience has been the convincing demonstration that most forms of cell therapy are safe, if administered via the intracoronary route." Then he added the elephant in the room: "Efficacy is another matter.
record. Why subject patients to risk in receiving a cell product that has not been extensively validated in vitro and in small and large animals? Yet this has been done over and over again" by investigators running clinical trials of stem cells and other cells for heart disease, Dr. Marbán said in an interview.
Making It to Phase III: REPAIR-AMI/BAMI
The recent surge of study results, and the path to phase III may be best exemplified by the landmark phase II trial done by German investigators, the Reinfusion of Enriched Progenitor Cells and Infarct Remodeling in Acute Myocardial Infarction (REPAIR-AMI) trial (N. Engl. J. Med. 2006;355:1210-21). The multicenter German study randomized 204 patients an average of 4 days following an acute myocardial infarction (MI) to receive either an intracoronary infusion of autologous bone marrow cells processed to enrich for progenitor cells, or placebo.
Last November, at the annual Scientific Sessions of the American Heart Association, the group presented 5-year follow-up results for 200 of the 204 patients. Helped by the lengthy follow-up, the researchers found that the stem-cell treatment cut the cumulative rate of death, recurrent MI, or need for revascularization from 64% in the control arm to 42%, a statistically significant difference in a prespecified end point of the study.
This striking change in a very meaningful clinical outcome contrasted with the modest change in a surrogate end point at 4 months (first reported in the 2006 article), and led to the launch of the largest phase III study of stem cells for heart disease, the Effect of Intracoronary Reinfusion of Bone Marrow Derived Mononuclear Cells (BM-MNC) on All-Cause Mortality in Acute Myocardial Infarction (BAMI), a 3,000-patient, multi-center study funded by the European Union. The study will enroll patients within 3-6 days after reperfusion therapy of an acute MI if they have a left ventricular ejection fraction of 45% or less. The primary end point is a reduction in all-cause mortality, a design that will allow BAMI to use standard care as its control instead of a sham infusion.
"We discussed the end point with the EMA [European Medicines Agency], and they said that if you use only death as the end point we can do it with a state-of-the-art control. I think that is the proper way, because some people believe that intracoronary instrumentation by itself carries a risk that could also affect placebo groups," said Dr. Andreas M. Zeiher, professor and chairman of medicine at Goethe University in Frankfurt, Germany, speaking last November at the American Heart Association meeting.
Dr. Zeiher, who led the team that ran REPAIR-AMI and spearheaded organization of the BAMI trial, also stressed the need to avoid heparin when handling and administering autologous bone marrow cells to achieve optimal results. "Heparin interferes with a process that is absolutely crucial for these cells to extravasate during infusion to enter ischemic tissue," he said during a talk in March at the American College of Cardiology Scientific Session in Chicago. "Heparin in the syringe more or less completely abolishes the migratory capacity of the [bone-marrow] cells." Dr. Zeiher reviewed the methods and results of more than 20 stem-cell studies and found those that used heparin-treated bone-marrow cells had little clinical effect, when studies that avoided heparin or neutralized the drug with serum the cell treatments proved effective. REPAIR-AMI used serum to neutralize heparin, and BAMI will use bivalirudin as an anticoagulant and no heparin, he said.
In contrast to the success in REPAIR-AMI, no efficacy signal occurred in a Norwegian study of bone-marrow mononuclear cells injected into the hearts of 47 acute MI patients in the Autologous Stem-Cell Transplantation in Acute Myocardial Infarction (ASTAMI) study, which coincidently appeared in the same issue of The New England Journal of Medicine as the REPAIR-AMI report (N. Engl. J. Med. 2006;355:1199-209). The lack of success in ASTAMI juxtaposed against the efficacy signal in REPAIR-AMI (a signal later confirmed and strengthened by longer follow-up) provides an example in microcosm of the uneven road that stem cell therapy traversed over the past 11 years. Also worth noting: in ASTAMI the investigators used heparin.
"Heparin interferes with a process that is absolutely crucial for these cells to extravasate during infusion to enter ischemic tissuu," Dr. Andreas M. Zeiher said.
Dr. Zeiher’s heparin explanation for outcome differences among many stem-cell studies is "an interesting hypothesis," said Dr. Robert D. Simari, a cardiologist and professor of medicine at the Mayo Clinic in Rochester, Minn. There are likely several important differences" between the stem-cell trials that have been positive and those that have failed. Trials have differed in their design, patient populations, their stem cell products, and in the end points they have used to measure success, said Dr. Simari, who also chairs the steering committee of the Cardiovascular Cell Therapy Research Network (CCTRN), a research group of seven U.S. centers organized by the National Heart Lung and Blood Institute to run early-phase clinical trials with cell treatments.
Also in the Phase III Act: ACT34-CMI/RENEW
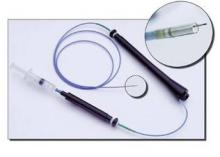
Another cell therapy success story now advancing to a phase III trial is led by Dr. Douglas W. Losordo using autologous CD34+ cells injected into the myocardium of patients with refractory angina. He and his associates published results last August from a phase II study with167 patients, the Double-blind, Prospective, Randomized, Placebo-controlled Study to Determine the Tolerability, Efficacy, Safety, and Dose Range of Intramyocardial Injections of G-CSF Mobilized Auto–CD34+ Cells for Reduction of Angina Episodes in Patients With Refractory Chronic Myocardial Ischemia (ACT34-CMI).
The researchers obtained the autologous cell preparation used in ACT34-CMI by first treating each patient with granulocyte colony stimulating factor daily for 4 or 5 days to mobilize their CD34+ cells. The day following the last dose of drug, each patient underwent leukopheresis to collect mononuclear cells. The cell material then underwent further ex vivo enrichment for CD34+ cells using a commercially-available magnetic cell selection device marketed by Baxter Healthcare. Patients received 100,000 CD34+ cells/kg, 500,000 CD34+ cells/kg, or placebo as intramyocardial injections at 10 sites identified by electromechanical endocardial mapping. Dr. Losordo and his associates said they used CD34+ cells because of evidence these cells, called endothelial progenitor cells, can stimulate neovascularization in ischemic tissue, and improved function in animal models of acute and chronic myocardial ischemia. The patients enrolled in ACT34-CMI all had Canadian Cardiovascular Society class III or IV chronic refractory angina despite optimal medial treatment.
The study, done at 26 U.S. centers, found that at 6 and 12 months after treatment, 54 patients who received the low-dose injections had significantly fewer angina episodes per week than the 53 patients who received placebo infections, the study’s primary end point. Patients who received the higher-dose injections also had fewer angina episodes than the controls, but the difference was not statistically significant at either 6- or 12-month follow-up. The low-dose patients also had statistically significant improvement in their total exercise time in an exercise tolerance test compared with the placebo patients; the high-dose patients also had better exercise times, but not significantly better than the controls.
Speaking at the American College of Cardiology meeting in Chicago in March, Dr. Losordo presented 2-year follow-up data on the patients in ACT34-CMI. At 2 years, the low-dose patients continued to have a significantly lower rate of weekly angina counts than control patients, while the high-dose patients continued to have a numerically lower rate that was not significantly different from the controls. The cumulative, 2-year rate of death, MI, or hospitalization for acute coronary syndrome was 30% in the control patients, 18% in the low-dose patients, and 14% in the high-dose patients. Although these between group differences were not statistically significant, the trends were "in the right direction," said Dr. Losordo, an interventional cardiologist at Northwestern University in Chicago, and vice president for new therapeutic development at Baxter International. Baxter sponsored ACT34-CMI, as well as the phase III study now starting based on the phase II results.
The phase III study, A Prospective, Randomized, Double-blinded, Active-control and Unblinded Standard of Care (SOC) Controlled Study to Determine the Efficacy and Safety of Targeted Intramyocardial Delivery of Autologous CD34+ Cells (Auto-CD34+ Cells) for Increasing Exercise Capacity During Standardized Exercise Testing in Subjects With Refractory Angina Pectoris and Chronic Myocardial Ischemia (RENEW), will enroll about 450 refractory angina patients at 50 U.S. sites, randomizing them to CD34+ treatment, placebo treatment, or no treatment and optimal standard care. The primary efficacy outcome will be change in total exercise time on an exercise tolerance test after 12 months.
Choosing the Right Cells
The success of the ACT34-CMI study, and the decision by Dr. Losordo and Baxter to move on to the phase III RENEW trial, highlights what may be a possible advantage to using selected stem and progenitor cells compared with the strategy of using an unselected cell population, such as the bone-marrow cells used in many of the studies of the past 11 years.
More evidence favoring cell selection came recently in results reported by Dr. Perin from the First Mononuclear Cells Injected in the United States Conducted by the Cardiovascular Cell Therapy Research Network (FOCUS-CCTRN) study, which he presented in March at the American College of Cardiology meeting in Chicago. The phase II study enrolled 92 patients with chronic heart failure and a left ventricular ejection fraction of 45% or less. Patients received a transendocardial injection of 100 million autologous bone-marrow cells or placebo. The primary end points were 6-month changes in left ventricular end systolic volume, in maximal oxygen consumption, and the extent of ischemic myocardial defects measured by single photon emission tomography. The results showed no statistically significant improvement for any of these three end points in the patients treated with bone-marrow cells compared with the controls, and were simultaneously reported in an article published online (JAMA 2012; March 24 [doi:10.1001/jama.2012.418]).
Despite the study’s failure for its prespecified end points, it also showed signals of efficacy and possibly highlighted some important lessons on how to best apply stem cell therapy in the future. On the efficacy side, the 61 patients who received bone-marrow cell injections had an average 1.4% improvement in their left ventricular ejection fraction, compared with an average 1.3% decline in the 31 placebo patients, an overall between-group difference of 2.7% that was statistically significant. Further analysis showed that patients who received bone-marrow cell preparations that had higher levels of either CD34+ cell or CD133+ cells had greater increases in their left ventricular ejection fractions. Also, when the researchers analyzed responses in subgroups divided by their age, patients 62 years old or younger (62 years old was the median age in the study) who received bone-marrow cells had a statistically significant, 4.7% improvement in their left ventricular ejection fraction after 6 months compared with placebo patients, while among patients greater than 62 years old treatment with autologous bone-marrow cells produced no significant improvement in ejection fraction compared with the controls.
"It could very well be that [unfractionated] bone marrow is a weaker product than using a specific cell type," said Dr. Perin. "Now we’ll start using parts of bone marrow, specific cells or cell combinations," he predicted, although he added that additional analysis of the FOCUS-CCTRN data must occur before he and his associates decide which cell types show the most promise. "Even though it’s not answered perfectly, the results definitely point us toward" using more selected types of bone marrow cells, he said.
"The most important lesson FOCUS-CCTRN showed was that as patients get older the number and potency of their stem cells decreases. It showed that if you give cells that aren’t potent they won’t work," said Dr. Henry, a co-investigator of the study and an active member of CCTRN. "That’s a really important insight, because if you treat patients with 100 mg of a beta blocker you get a consistent effect, but if you treat patients with autologous stem cells, every patient gets something different."
Development of stem cell therapies "has been on a normal track; each step takes time. It’s very similar to what happened with fibrinolytic therapy for treating acute myocardial infarctions" in the 1980s and 1990s, said Dr. Timothy D. Henry.
"The FOCUS results showed some evidence of an effect, but there are a number of ways to make it better," Dr. Henry said. One approach is to use selected cells, such as CD34+ cells. Another strategy is to deliver allogenic cells obtained initially from young and healthy donors, a way to avoid the issue of older patients and those with co-morbidities who may have compromised stem cells.
Administering selected or allogenic cells has another potential advantage: it opens the door to commercial involvement with a potentially saleable product, a strategy that can attract the interest of a company willing to shoulder the cost of a phase III trial.
"A challenge with [unprocessed] bone marrow is it’s too easy. Bone marrow is inexpensive, and anyone can do it," Dr. Henry noted. That’s one reason why it took several years for the REPAIR-AMI strategy to advance into a phase III trial, eventually moving forward when it received financial backing from the European Union. In contrast, the promising ACT34-CMI strategy quickly jumped to the phase III level, aided by financial support from Baxter.
But others cautioned against drawing too many inferences from FOCUS-CCTRN because it was a negative study and hence all its results must be considered suspect.
"I do not believe that post-hoc analysis of cell potency should be used to guide future trials. FOCUS was negative; the subgroup analyses and cell potency assays were attempts to glean some positivity from that study after the fact," said Dr. Marbán.
More Phase III Studies Coming, with Commercial Support
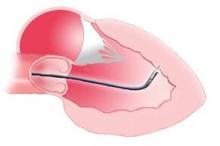
Another example of a selected, allogenic cell preparation with commercial support is the study of mesenchymal precursor cells (MPC), bone-marrow cells obtained from young, healthy donors and delivered by transendocardial injection to 45 patients with cardiomyopathy, New York Heart Association class II or III heart failure, and impaired left ventricular function in a phase II study presented by Dr. Perin last November at the American Heart Association’s Scientific Sessions in Orlando. The MPC preparation he and his associates tested was an "off-the-shelf" stem-cell product made by the Australian-based company Mesoblast.
The injections were safe, and the three different amounts of cells delivered showed various signs of efficacy. The lowest dose of cells tested showed a statistically significant improvement in left ventricular ejection fraction at 3 months compared with placebo; the highest cell dose tested showed a statistically significant improvement in left ventricular end-systolic volume at 12 months compared with the placebo control; and collectively, all three cell doses tested produced a statistically significant 20% reduction in the rate of major adverse coronary events compared with the placebo patients during a follow-up of up to 3 years, Dr. Perin reported last November.
"Given the very positive results we saw in the phase II trial, it would be logical to move on to a bigger trial," said Dr. Perin in an interview, but he added that he was unable to provide details about possible additional trials with this MPC product.
Another commercially-backed stem-cell product poised for a larger trial is cardiopoietic stem cells, made by a proprietary, patented method that uses a patient’s autologous bone-marrow stem cells and treats them in vitro to produce cells that are then injected into a patient’s myocardium where they produce new heart muscle. Researchers at the Mayo Clinic in Rochester, Minn. developed this process, and Mayo licensed it to a Belgian company, Cardio 3 BioSciences.
The company released results in November 2010 from the C-Cure study done in Belgium, Serbia, and Switzerland that enrolled 45-patients with severe ischemic heart failure. Six months after treatment, the 21 patients who received injections of the stem cells had an average 18% relative increase in their left ventricular ejection fraction compared with baseline, versus a 4% relative increase over baseline among 24 control patients. Speaking in March at the American College of Cardiology meeting, Dr. André Terzic, who led the study, provided a few more details from the results. Average ejection fraction in the actively-treated patients rose from 28% at baseline to 33%. The treatment was especially effective for the patients who entered the study with an ejection fraction below 28%, said Dr. Terzic, a professor of medicine and cardiology, and director of the Center for Regenerative Medicine at the Mayo Clinic. The 6-month results also showed other indications of benefit in the patients treated with the cardiopoietic stem cells, including significantly increased left ventricular end systolic volume, and reduced left ventricular mass index.
Cardio 3 Biosciences is now planning to start a "late phase II" or phase III trial with this material, said Dr. Simari, who is not directly involved in these studies.
What Comes Next?
Despite 11 years of work, "it’s still very early in the field. We’re still trying to figure out the cells and out targets," said Dr. Simari. "This is the haze of early days, of learning from groups of 10, 20, or 80 patients. Only in retrospect, and as we get to larger trials will the truth emerge. Right now, there is a lot of work needed and few headlines, and it will probably stay that way for awhile. This is not a game for people with short attention spans.
"The business model for autologous cells remains uncertain," he added. "For allogenic products that are generated from young, healthy donors and made into thousands of doses, the business model is different and the costs might be more reasonable. Hopefully, as our knowledge grows, we will develop treatments that end up costing less."
"I have no doubt that cell treatment is one of the great avenues of future medicine; it’s just a matter of figuring it out," said Dr. Perin. "Having phase III trials now starting is a step forward. Things are starting to come into focus. Unless something totally unforeseen happens, we will eventually have an approved product," he predicted.
Dr. Perin said that he has been a consultant to Amorcyte, Teva, Cytori, and Aldagen. Dr. Henry said that he has served on steering committees for and received research grants from Aastrom and Mesoblast. Dr. Marbán said that he is a founder of and holds equity in Capricor. Dr. Simari said that he had no disclosures. Dr. Zeiher said he is a cofounder of and scientific advisor to t2cure, a company set up to run the BAMI trial, sponsored by the European Union Dr. Losordo is an employee of Baxter International. Dr. Terzic said that he has received research grants from Cardio 3 BioSciences.
Stem-cell treatment for cardiac disease recently launched into advanced clinical trials, as a flurry of early-phase studies over the last year collectively confirmed the treatment as safe but left its efficacy unresolved.
At least two phase III trials are now underway and others are possibly imminent. But until these pivotal studies begin to yield outcome results in about 4 years, stem-cell treatment remains a question mark – an appealing idea fueled by hints of effectiveness but also dogged by failures that prompt skepticism about its future.
The gush of recent early-phase studies also hint at possible explanations for the variability in the success of stem cell treatment. The studies examined a broad range of cell types and patients. (Click here to see a chart of the studies.) The results imply that select types of bone marrow cells may be more effective as therapy and that the number and potency of stem cells decrease with age.
Clinical testing of stem cells for heart disease has hit its stride more than a decade after the first-in-human report in 2001 of a patient treated following an acute myocardial infarction (Dtsch. Med. Wochenschr. 2001;126:932-8), which was followed by the first randomized clinical trial, also in myocardial infarction patients, reported in 2004 (Lancet 2004;364:141-8).
"So far, stem-cell treatment has been safe in all the areas where it’s been used. I’m very satisfied" with the progress, said Dr. Emerson C. Perin, an interventional cardiologist and medical director of the Stem Cell Center at the Texas Heart Institute and one of the U.S. researchers who has worked longest on stem-cell clinical studies. Those working on clinical investigations of stem cells "haven’t done anything stupid to kill it, and we’ve gone from a crawl to a walk," he said in an interview.
Development of stem cell therapies "has been on a normal track; each step takes time. It’s very similar to what happened with fibrinolytic therapy for treating acute myocardial infarctions" in the 1980s and 1990s, said Dr. Timothy D. Henry, an interventional cardiologist, director of research at the Minneapolis Heart Institute Foundation, and another very active stem cell researcher. "It was a good 10 years before we got lytics up and going. People want [stem cells] to be a magic bullet. I think some people have put unrealistic expectations on stem cells, but it’s like any other treatment. You need to do a trial and find out the relative risks and benefits, and that takes time," he said in an interview.
A different take on the past decade of stem-cell work came from another researcher in the field, Dr. Eduardo Marbán. "The pace of progress has been disappointingly slow, marked by numerous examples of clinical studies prematurely undertaken without benefit of adequate preclinical data. We are lucky that no one has been killed," said Dr. Marbán, professor and director of the Cedars-Sinai Heart Institute in Los Angeles. But despite whatever role luck may have played, Dr. Marbán agreed on the bottom line: "The major accomplishment of the 11-year experience has been the convincing demonstration that most forms of cell therapy are safe, if administered via the intracoronary route." Then he added the elephant in the room: "Efficacy is another matter.
record. Why subject patients to risk in receiving a cell product that has not been extensively validated in vitro and in small and large animals? Yet this has been done over and over again" by investigators running clinical trials of stem cells and other cells for heart disease, Dr. Marbán said in an interview.
Making It to Phase III: REPAIR-AMI/BAMI
The recent surge of study results, and the path to phase III may be best exemplified by the landmark phase II trial done by German investigators, the Reinfusion of Enriched Progenitor Cells and Infarct Remodeling in Acute Myocardial Infarction (REPAIR-AMI) trial (N. Engl. J. Med. 2006;355:1210-21). The multicenter German study randomized 204 patients an average of 4 days following an acute myocardial infarction (MI) to receive either an intracoronary infusion of autologous bone marrow cells processed to enrich for progenitor cells, or placebo.
Last November, at the annual Scientific Sessions of the American Heart Association, the group presented 5-year follow-up results for 200 of the 204 patients. Helped by the lengthy follow-up, the researchers found that the stem-cell treatment cut the cumulative rate of death, recurrent MI, or need for revascularization from 64% in the control arm to 42%, a statistically significant difference in a prespecified end point of the study.
This striking change in a very meaningful clinical outcome contrasted with the modest change in a surrogate end point at 4 months (first reported in the 2006 article), and led to the launch of the largest phase III study of stem cells for heart disease, the Effect of Intracoronary Reinfusion of Bone Marrow Derived Mononuclear Cells (BM-MNC) on All-Cause Mortality in Acute Myocardial Infarction (BAMI), a 3,000-patient, multi-center study funded by the European Union. The study will enroll patients within 3-6 days after reperfusion therapy of an acute MI if they have a left ventricular ejection fraction of 45% or less. The primary end point is a reduction in all-cause mortality, a design that will allow BAMI to use standard care as its control instead of a sham infusion.
"We discussed the end point with the EMA [European Medicines Agency], and they said that if you use only death as the end point we can do it with a state-of-the-art control. I think that is the proper way, because some people believe that intracoronary instrumentation by itself carries a risk that could also affect placebo groups," said Dr. Andreas M. Zeiher, professor and chairman of medicine at Goethe University in Frankfurt, Germany, speaking last November at the American Heart Association meeting.
Dr. Zeiher, who led the team that ran REPAIR-AMI and spearheaded organization of the BAMI trial, also stressed the need to avoid heparin when handling and administering autologous bone marrow cells to achieve optimal results. "Heparin interferes with a process that is absolutely crucial for these cells to extravasate during infusion to enter ischemic tissue," he said during a talk in March at the American College of Cardiology Scientific Session in Chicago. "Heparin in the syringe more or less completely abolishes the migratory capacity of the [bone-marrow] cells." Dr. Zeiher reviewed the methods and results of more than 20 stem-cell studies and found those that used heparin-treated bone-marrow cells had little clinical effect, when studies that avoided heparin or neutralized the drug with serum the cell treatments proved effective. REPAIR-AMI used serum to neutralize heparin, and BAMI will use bivalirudin as an anticoagulant and no heparin, he said.
In contrast to the success in REPAIR-AMI, no efficacy signal occurred in a Norwegian study of bone-marrow mononuclear cells injected into the hearts of 47 acute MI patients in the Autologous Stem-Cell Transplantation in Acute Myocardial Infarction (ASTAMI) study, which coincidently appeared in the same issue of The New England Journal of Medicine as the REPAIR-AMI report (N. Engl. J. Med. 2006;355:1199-209). The lack of success in ASTAMI juxtaposed against the efficacy signal in REPAIR-AMI (a signal later confirmed and strengthened by longer follow-up) provides an example in microcosm of the uneven road that stem cell therapy traversed over the past 11 years. Also worth noting: in ASTAMI the investigators used heparin.
"Heparin interferes with a process that is absolutely crucial for these cells to extravasate during infusion to enter ischemic tissuu," Dr. Andreas M. Zeiher said.
Dr. Zeiher’s heparin explanation for outcome differences among many stem-cell studies is "an interesting hypothesis," said Dr. Robert D. Simari, a cardiologist and professor of medicine at the Mayo Clinic in Rochester, Minn. There are likely several important differences" between the stem-cell trials that have been positive and those that have failed. Trials have differed in their design, patient populations, their stem cell products, and in the end points they have used to measure success, said Dr. Simari, who also chairs the steering committee of the Cardiovascular Cell Therapy Research Network (CCTRN), a research group of seven U.S. centers organized by the National Heart Lung and Blood Institute to run early-phase clinical trials with cell treatments.
Also in the Phase III Act: ACT34-CMI/RENEW
Another cell therapy success story now advancing to a phase III trial is led by Dr. Douglas W. Losordo using autologous CD34+ cells injected into the myocardium of patients with refractory angina. He and his associates published results last August from a phase II study with167 patients, the Double-blind, Prospective, Randomized, Placebo-controlled Study to Determine the Tolerability, Efficacy, Safety, and Dose Range of Intramyocardial Injections of G-CSF Mobilized Auto–CD34+ Cells for Reduction of Angina Episodes in Patients With Refractory Chronic Myocardial Ischemia (ACT34-CMI).
The researchers obtained the autologous cell preparation used in ACT34-CMI by first treating each patient with granulocyte colony stimulating factor daily for 4 or 5 days to mobilize their CD34+ cells. The day following the last dose of drug, each patient underwent leukopheresis to collect mononuclear cells. The cell material then underwent further ex vivo enrichment for CD34+ cells using a commercially-available magnetic cell selection device marketed by Baxter Healthcare. Patients received 100,000 CD34+ cells/kg, 500,000 CD34+ cells/kg, or placebo as intramyocardial injections at 10 sites identified by electromechanical endocardial mapping. Dr. Losordo and his associates said they used CD34+ cells because of evidence these cells, called endothelial progenitor cells, can stimulate neovascularization in ischemic tissue, and improved function in animal models of acute and chronic myocardial ischemia. The patients enrolled in ACT34-CMI all had Canadian Cardiovascular Society class III or IV chronic refractory angina despite optimal medial treatment.
The study, done at 26 U.S. centers, found that at 6 and 12 months after treatment, 54 patients who received the low-dose injections had significantly fewer angina episodes per week than the 53 patients who received placebo infections, the study’s primary end point. Patients who received the higher-dose injections also had fewer angina episodes than the controls, but the difference was not statistically significant at either 6- or 12-month follow-up. The low-dose patients also had statistically significant improvement in their total exercise time in an exercise tolerance test compared with the placebo patients; the high-dose patients also had better exercise times, but not significantly better than the controls.
Speaking at the American College of Cardiology meeting in Chicago in March, Dr. Losordo presented 2-year follow-up data on the patients in ACT34-CMI. At 2 years, the low-dose patients continued to have a significantly lower rate of weekly angina counts than control patients, while the high-dose patients continued to have a numerically lower rate that was not significantly different from the controls. The cumulative, 2-year rate of death, MI, or hospitalization for acute coronary syndrome was 30% in the control patients, 18% in the low-dose patients, and 14% in the high-dose patients. Although these between group differences were not statistically significant, the trends were "in the right direction," said Dr. Losordo, an interventional cardiologist at Northwestern University in Chicago, and vice president for new therapeutic development at Baxter International. Baxter sponsored ACT34-CMI, as well as the phase III study now starting based on the phase II results.
The phase III study, A Prospective, Randomized, Double-blinded, Active-control and Unblinded Standard of Care (SOC) Controlled Study to Determine the Efficacy and Safety of Targeted Intramyocardial Delivery of Autologous CD34+ Cells (Auto-CD34+ Cells) for Increasing Exercise Capacity During Standardized Exercise Testing in Subjects With Refractory Angina Pectoris and Chronic Myocardial Ischemia (RENEW), will enroll about 450 refractory angina patients at 50 U.S. sites, randomizing them to CD34+ treatment, placebo treatment, or no treatment and optimal standard care. The primary efficacy outcome will be change in total exercise time on an exercise tolerance test after 12 months.
Choosing the Right Cells
The success of the ACT34-CMI study, and the decision by Dr. Losordo and Baxter to move on to the phase III RENEW trial, highlights what may be a possible advantage to using selected stem and progenitor cells compared with the strategy of using an unselected cell population, such as the bone-marrow cells used in many of the studies of the past 11 years.
More evidence favoring cell selection came recently in results reported by Dr. Perin from the First Mononuclear Cells Injected in the United States Conducted by the Cardiovascular Cell Therapy Research Network (FOCUS-CCTRN) study, which he presented in March at the American College of Cardiology meeting in Chicago. The phase II study enrolled 92 patients with chronic heart failure and a left ventricular ejection fraction of 45% or less. Patients received a transendocardial injection of 100 million autologous bone-marrow cells or placebo. The primary end points were 6-month changes in left ventricular end systolic volume, in maximal oxygen consumption, and the extent of ischemic myocardial defects measured by single photon emission tomography. The results showed no statistically significant improvement for any of these three end points in the patients treated with bone-marrow cells compared with the controls, and were simultaneously reported in an article published online (JAMA 2012; March 24 [doi:10.1001/jama.2012.418]).
Despite the study’s failure for its prespecified end points, it also showed signals of efficacy and possibly highlighted some important lessons on how to best apply stem cell therapy in the future. On the efficacy side, the 61 patients who received bone-marrow cell injections had an average 1.4% improvement in their left ventricular ejection fraction, compared with an average 1.3% decline in the 31 placebo patients, an overall between-group difference of 2.7% that was statistically significant. Further analysis showed that patients who received bone-marrow cell preparations that had higher levels of either CD34+ cell or CD133+ cells had greater increases in their left ventricular ejection fractions. Also, when the researchers analyzed responses in subgroups divided by their age, patients 62 years old or younger (62 years old was the median age in the study) who received bone-marrow cells had a statistically significant, 4.7% improvement in their left ventricular ejection fraction after 6 months compared with placebo patients, while among patients greater than 62 years old treatment with autologous bone-marrow cells produced no significant improvement in ejection fraction compared with the controls.
"It could very well be that [unfractionated] bone marrow is a weaker product than using a specific cell type," said Dr. Perin. "Now we’ll start using parts of bone marrow, specific cells or cell combinations," he predicted, although he added that additional analysis of the FOCUS-CCTRN data must occur before he and his associates decide which cell types show the most promise. "Even though it’s not answered perfectly, the results definitely point us toward" using more selected types of bone marrow cells, he said.
"The most important lesson FOCUS-CCTRN showed was that as patients get older the number and potency of their stem cells decreases. It showed that if you give cells that aren’t potent they won’t work," said Dr. Henry, a co-investigator of the study and an active member of CCTRN. "That’s a really important insight, because if you treat patients with 100 mg of a beta blocker you get a consistent effect, but if you treat patients with autologous stem cells, every patient gets something different."
Development of stem cell therapies "has been on a normal track; each step takes time. It’s very similar to what happened with fibrinolytic therapy for treating acute myocardial infarctions" in the 1980s and 1990s, said Dr. Timothy D. Henry.
"The FOCUS results showed some evidence of an effect, but there are a number of ways to make it better," Dr. Henry said. One approach is to use selected cells, such as CD34+ cells. Another strategy is to deliver allogenic cells obtained initially from young and healthy donors, a way to avoid the issue of older patients and those with co-morbidities who may have compromised stem cells.
Administering selected or allogenic cells has another potential advantage: it opens the door to commercial involvement with a potentially saleable product, a strategy that can attract the interest of a company willing to shoulder the cost of a phase III trial.
"A challenge with [unprocessed] bone marrow is it’s too easy. Bone marrow is inexpensive, and anyone can do it," Dr. Henry noted. That’s one reason why it took several years for the REPAIR-AMI strategy to advance into a phase III trial, eventually moving forward when it received financial backing from the European Union. In contrast, the promising ACT34-CMI strategy quickly jumped to the phase III level, aided by financial support from Baxter.
But others cautioned against drawing too many inferences from FOCUS-CCTRN because it was a negative study and hence all its results must be considered suspect.
"I do not believe that post-hoc analysis of cell potency should be used to guide future trials. FOCUS was negative; the subgroup analyses and cell potency assays were attempts to glean some positivity from that study after the fact," said Dr. Marbán.
More Phase III Studies Coming, with Commercial Support
Another example of a selected, allogenic cell preparation with commercial support is the study of mesenchymal precursor cells (MPC), bone-marrow cells obtained from young, healthy donors and delivered by transendocardial injection to 45 patients with cardiomyopathy, New York Heart Association class II or III heart failure, and impaired left ventricular function in a phase II study presented by Dr. Perin last November at the American Heart Association’s Scientific Sessions in Orlando. The MPC preparation he and his associates tested was an "off-the-shelf" stem-cell product made by the Australian-based company Mesoblast.
The injections were safe, and the three different amounts of cells delivered showed various signs of efficacy. The lowest dose of cells tested showed a statistically significant improvement in left ventricular ejection fraction at 3 months compared with placebo; the highest cell dose tested showed a statistically significant improvement in left ventricular end-systolic volume at 12 months compared with the placebo control; and collectively, all three cell doses tested produced a statistically significant 20% reduction in the rate of major adverse coronary events compared with the placebo patients during a follow-up of up to 3 years, Dr. Perin reported last November.
"Given the very positive results we saw in the phase II trial, it would be logical to move on to a bigger trial," said Dr. Perin in an interview, but he added that he was unable to provide details about possible additional trials with this MPC product.
Another commercially-backed stem-cell product poised for a larger trial is cardiopoietic stem cells, made by a proprietary, patented method that uses a patient’s autologous bone-marrow stem cells and treats them in vitro to produce cells that are then injected into a patient’s myocardium where they produce new heart muscle. Researchers at the Mayo Clinic in Rochester, Minn. developed this process, and Mayo licensed it to a Belgian company, Cardio 3 BioSciences.
The company released results in November 2010 from the C-Cure study done in Belgium, Serbia, and Switzerland that enrolled 45-patients with severe ischemic heart failure. Six months after treatment, the 21 patients who received injections of the stem cells had an average 18% relative increase in their left ventricular ejection fraction compared with baseline, versus a 4% relative increase over baseline among 24 control patients. Speaking in March at the American College of Cardiology meeting, Dr. André Terzic, who led the study, provided a few more details from the results. Average ejection fraction in the actively-treated patients rose from 28% at baseline to 33%. The treatment was especially effective for the patients who entered the study with an ejection fraction below 28%, said Dr. Terzic, a professor of medicine and cardiology, and director of the Center for Regenerative Medicine at the Mayo Clinic. The 6-month results also showed other indications of benefit in the patients treated with the cardiopoietic stem cells, including significantly increased left ventricular end systolic volume, and reduced left ventricular mass index.
Cardio 3 Biosciences is now planning to start a "late phase II" or phase III trial with this material, said Dr. Simari, who is not directly involved in these studies.
What Comes Next?
Despite 11 years of work, "it’s still very early in the field. We’re still trying to figure out the cells and out targets," said Dr. Simari. "This is the haze of early days, of learning from groups of 10, 20, or 80 patients. Only in retrospect, and as we get to larger trials will the truth emerge. Right now, there is a lot of work needed and few headlines, and it will probably stay that way for awhile. This is not a game for people with short attention spans.
"The business model for autologous cells remains uncertain," he added. "For allogenic products that are generated from young, healthy donors and made into thousands of doses, the business model is different and the costs might be more reasonable. Hopefully, as our knowledge grows, we will develop treatments that end up costing less."
"I have no doubt that cell treatment is one of the great avenues of future medicine; it’s just a matter of figuring it out," said Dr. Perin. "Having phase III trials now starting is a step forward. Things are starting to come into focus. Unless something totally unforeseen happens, we will eventually have an approved product," he predicted.
Dr. Perin said that he has been a consultant to Amorcyte, Teva, Cytori, and Aldagen. Dr. Henry said that he has served on steering committees for and received research grants from Aastrom and Mesoblast. Dr. Marbán said that he is a founder of and holds equity in Capricor. Dr. Simari said that he had no disclosures. Dr. Zeiher said he is a cofounder of and scientific advisor to t2cure, a company set up to run the BAMI trial, sponsored by the European Union Dr. Losordo is an employee of Baxter International. Dr. Terzic said that he has received research grants from Cardio 3 BioSciences.
Stem-cell treatment for cardiac disease recently launched into advanced clinical trials, as a flurry of early-phase studies over the last year collectively confirmed the treatment as safe but left its efficacy unresolved.
At least two phase III trials are now underway and others are possibly imminent. But until these pivotal studies begin to yield outcome results in about 4 years, stem-cell treatment remains a question mark – an appealing idea fueled by hints of effectiveness but also dogged by failures that prompt skepticism about its future.
The gush of recent early-phase studies also hint at possible explanations for the variability in the success of stem cell treatment. The studies examined a broad range of cell types and patients. (Click here to see a chart of the studies.) The results imply that select types of bone marrow cells may be more effective as therapy and that the number and potency of stem cells decrease with age.
Clinical testing of stem cells for heart disease has hit its stride more than a decade after the first-in-human report in 2001 of a patient treated following an acute myocardial infarction (Dtsch. Med. Wochenschr. 2001;126:932-8), which was followed by the first randomized clinical trial, also in myocardial infarction patients, reported in 2004 (Lancet 2004;364:141-8).
"So far, stem-cell treatment has been safe in all the areas where it’s been used. I’m very satisfied" with the progress, said Dr. Emerson C. Perin, an interventional cardiologist and medical director of the Stem Cell Center at the Texas Heart Institute and one of the U.S. researchers who has worked longest on stem-cell clinical studies. Those working on clinical investigations of stem cells "haven’t done anything stupid to kill it, and we’ve gone from a crawl to a walk," he said in an interview.
Development of stem cell therapies "has been on a normal track; each step takes time. It’s very similar to what happened with fibrinolytic therapy for treating acute myocardial infarctions" in the 1980s and 1990s, said Dr. Timothy D. Henry, an interventional cardiologist, director of research at the Minneapolis Heart Institute Foundation, and another very active stem cell researcher. "It was a good 10 years before we got lytics up and going. People want [stem cells] to be a magic bullet. I think some people have put unrealistic expectations on stem cells, but it’s like any other treatment. You need to do a trial and find out the relative risks and benefits, and that takes time," he said in an interview.
A different take on the past decade of stem-cell work came from another researcher in the field, Dr. Eduardo Marbán. "The pace of progress has been disappointingly slow, marked by numerous examples of clinical studies prematurely undertaken without benefit of adequate preclinical data. We are lucky that no one has been killed," said Dr. Marbán, professor and director of the Cedars-Sinai Heart Institute in Los Angeles. But despite whatever role luck may have played, Dr. Marbán agreed on the bottom line: "The major accomplishment of the 11-year experience has been the convincing demonstration that most forms of cell therapy are safe, if administered via the intracoronary route." Then he added the elephant in the room: "Efficacy is another matter.
record. Why subject patients to risk in receiving a cell product that has not been extensively validated in vitro and in small and large animals? Yet this has been done over and over again" by investigators running clinical trials of stem cells and other cells for heart disease, Dr. Marbán said in an interview.
Making It to Phase III: REPAIR-AMI/BAMI
The recent surge of study results, and the path to phase III may be best exemplified by the landmark phase II trial done by German investigators, the Reinfusion of Enriched Progenitor Cells and Infarct Remodeling in Acute Myocardial Infarction (REPAIR-AMI) trial (N. Engl. J. Med. 2006;355:1210-21). The multicenter German study randomized 204 patients an average of 4 days following an acute myocardial infarction (MI) to receive either an intracoronary infusion of autologous bone marrow cells processed to enrich for progenitor cells, or placebo.
Last November, at the annual Scientific Sessions of the American Heart Association, the group presented 5-year follow-up results for 200 of the 204 patients. Helped by the lengthy follow-up, the researchers found that the stem-cell treatment cut the cumulative rate of death, recurrent MI, or need for revascularization from 64% in the control arm to 42%, a statistically significant difference in a prespecified end point of the study.
This striking change in a very meaningful clinical outcome contrasted with the modest change in a surrogate end point at 4 months (first reported in the 2006 article), and led to the launch of the largest phase III study of stem cells for heart disease, the Effect of Intracoronary Reinfusion of Bone Marrow Derived Mononuclear Cells (BM-MNC) on All-Cause Mortality in Acute Myocardial Infarction (BAMI), a 3,000-patient, multi-center study funded by the European Union. The study will enroll patients within 3-6 days after reperfusion therapy of an acute MI if they have a left ventricular ejection fraction of 45% or less. The primary end point is a reduction in all-cause mortality, a design that will allow BAMI to use standard care as its control instead of a sham infusion.
"We discussed the end point with the EMA [European Medicines Agency], and they said that if you use only death as the end point we can do it with a state-of-the-art control. I think that is the proper way, because some people believe that intracoronary instrumentation by itself carries a risk that could also affect placebo groups," said Dr. Andreas M. Zeiher, professor and chairman of medicine at Goethe University in Frankfurt, Germany, speaking last November at the American Heart Association meeting.
Dr. Zeiher, who led the team that ran REPAIR-AMI and spearheaded organization of the BAMI trial, also stressed the need to avoid heparin when handling and administering autologous bone marrow cells to achieve optimal results. "Heparin interferes with a process that is absolutely crucial for these cells to extravasate during infusion to enter ischemic tissue," he said during a talk in March at the American College of Cardiology Scientific Session in Chicago. "Heparin in the syringe more or less completely abolishes the migratory capacity of the [bone-marrow] cells." Dr. Zeiher reviewed the methods and results of more than 20 stem-cell studies and found those that used heparin-treated bone-marrow cells had little clinical effect, when studies that avoided heparin or neutralized the drug with serum the cell treatments proved effective. REPAIR-AMI used serum to neutralize heparin, and BAMI will use bivalirudin as an anticoagulant and no heparin, he said.
In contrast to the success in REPAIR-AMI, no efficacy signal occurred in a Norwegian study of bone-marrow mononuclear cells injected into the hearts of 47 acute MI patients in the Autologous Stem-Cell Transplantation in Acute Myocardial Infarction (ASTAMI) study, which coincidently appeared in the same issue of The New England Journal of Medicine as the REPAIR-AMI report (N. Engl. J. Med. 2006;355:1199-209). The lack of success in ASTAMI juxtaposed against the efficacy signal in REPAIR-AMI (a signal later confirmed and strengthened by longer follow-up) provides an example in microcosm of the uneven road that stem cell therapy traversed over the past 11 years. Also worth noting: in ASTAMI the investigators used heparin.
"Heparin interferes with a process that is absolutely crucial for these cells to extravasate during infusion to enter ischemic tissuu," Dr. Andreas M. Zeiher said.
Dr. Zeiher’s heparin explanation for outcome differences among many stem-cell studies is "an interesting hypothesis," said Dr. Robert D. Simari, a cardiologist and professor of medicine at the Mayo Clinic in Rochester, Minn. There are likely several important differences" between the stem-cell trials that have been positive and those that have failed. Trials have differed in their design, patient populations, their stem cell products, and in the end points they have used to measure success, said Dr. Simari, who also chairs the steering committee of the Cardiovascular Cell Therapy Research Network (CCTRN), a research group of seven U.S. centers organized by the National Heart Lung and Blood Institute to run early-phase clinical trials with cell treatments.
Also in the Phase III Act: ACT34-CMI/RENEW
Another cell therapy success story now advancing to a phase III trial is led by Dr. Douglas W. Losordo using autologous CD34+ cells injected into the myocardium of patients with refractory angina. He and his associates published results last August from a phase II study with167 patients, the Double-blind, Prospective, Randomized, Placebo-controlled Study to Determine the Tolerability, Efficacy, Safety, and Dose Range of Intramyocardial Injections of G-CSF Mobilized Auto–CD34+ Cells for Reduction of Angina Episodes in Patients With Refractory Chronic Myocardial Ischemia (ACT34-CMI).
The researchers obtained the autologous cell preparation used in ACT34-CMI by first treating each patient with granulocyte colony stimulating factor daily for 4 or 5 days to mobilize their CD34+ cells. The day following the last dose of drug, each patient underwent leukopheresis to collect mononuclear cells. The cell material then underwent further ex vivo enrichment for CD34+ cells using a commercially-available magnetic cell selection device marketed by Baxter Healthcare. Patients received 100,000 CD34+ cells/kg, 500,000 CD34+ cells/kg, or placebo as intramyocardial injections at 10 sites identified by electromechanical endocardial mapping. Dr. Losordo and his associates said they used CD34+ cells because of evidence these cells, called endothelial progenitor cells, can stimulate neovascularization in ischemic tissue, and improved function in animal models of acute and chronic myocardial ischemia. The patients enrolled in ACT34-CMI all had Canadian Cardiovascular Society class III or IV chronic refractory angina despite optimal medial treatment.
The study, done at 26 U.S. centers, found that at 6 and 12 months after treatment, 54 patients who received the low-dose injections had significantly fewer angina episodes per week than the 53 patients who received placebo infections, the study’s primary end point. Patients who received the higher-dose injections also had fewer angina episodes than the controls, but the difference was not statistically significant at either 6- or 12-month follow-up. The low-dose patients also had statistically significant improvement in their total exercise time in an exercise tolerance test compared with the placebo patients; the high-dose patients also had better exercise times, but not significantly better than the controls.
Speaking at the American College of Cardiology meeting in Chicago in March, Dr. Losordo presented 2-year follow-up data on the patients in ACT34-CMI. At 2 years, the low-dose patients continued to have a significantly lower rate of weekly angina counts than control patients, while the high-dose patients continued to have a numerically lower rate that was not significantly different from the controls. The cumulative, 2-year rate of death, MI, or hospitalization for acute coronary syndrome was 30% in the control patients, 18% in the low-dose patients, and 14% in the high-dose patients. Although these between group differences were not statistically significant, the trends were "in the right direction," said Dr. Losordo, an interventional cardiologist at Northwestern University in Chicago, and vice president for new therapeutic development at Baxter International. Baxter sponsored ACT34-CMI, as well as the phase III study now starting based on the phase II results.
The phase III study, A Prospective, Randomized, Double-blinded, Active-control and Unblinded Standard of Care (SOC) Controlled Study to Determine the Efficacy and Safety of Targeted Intramyocardial Delivery of Autologous CD34+ Cells (Auto-CD34+ Cells) for Increasing Exercise Capacity During Standardized Exercise Testing in Subjects With Refractory Angina Pectoris and Chronic Myocardial Ischemia (RENEW), will enroll about 450 refractory angina patients at 50 U.S. sites, randomizing them to CD34+ treatment, placebo treatment, or no treatment and optimal standard care. The primary efficacy outcome will be change in total exercise time on an exercise tolerance test after 12 months.
Choosing the Right Cells
The success of the ACT34-CMI study, and the decision by Dr. Losordo and Baxter to move on to the phase III RENEW trial, highlights what may be a possible advantage to using selected stem and progenitor cells compared with the strategy of using an unselected cell population, such as the bone-marrow cells used in many of the studies of the past 11 years.
More evidence favoring cell selection came recently in results reported by Dr. Perin from the First Mononuclear Cells Injected in the United States Conducted by the Cardiovascular Cell Therapy Research Network (FOCUS-CCTRN) study, which he presented in March at the American College of Cardiology meeting in Chicago. The phase II study enrolled 92 patients with chronic heart failure and a left ventricular ejection fraction of 45% or less. Patients received a transendocardial injection of 100 million autologous bone-marrow cells or placebo. The primary end points were 6-month changes in left ventricular end systolic volume, in maximal oxygen consumption, and the extent of ischemic myocardial defects measured by single photon emission tomography. The results showed no statistically significant improvement for any of these three end points in the patients treated with bone-marrow cells compared with the controls, and were simultaneously reported in an article published online (JAMA 2012; March 24 [doi:10.1001/jama.2012.418]).
Despite the study’s failure for its prespecified end points, it also showed signals of efficacy and possibly highlighted some important lessons on how to best apply stem cell therapy in the future. On the efficacy side, the 61 patients who received bone-marrow cell injections had an average 1.4% improvement in their left ventricular ejection fraction, compared with an average 1.3% decline in the 31 placebo patients, an overall between-group difference of 2.7% that was statistically significant. Further analysis showed that patients who received bone-marrow cell preparations that had higher levels of either CD34+ cell or CD133+ cells had greater increases in their left ventricular ejection fractions. Also, when the researchers analyzed responses in subgroups divided by their age, patients 62 years old or younger (62 years old was the median age in the study) who received bone-marrow cells had a statistically significant, 4.7% improvement in their left ventricular ejection fraction after 6 months compared with placebo patients, while among patients greater than 62 years old treatment with autologous bone-marrow cells produced no significant improvement in ejection fraction compared with the controls.
"It could very well be that [unfractionated] bone marrow is a weaker product than using a specific cell type," said Dr. Perin. "Now we’ll start using parts of bone marrow, specific cells or cell combinations," he predicted, although he added that additional analysis of the FOCUS-CCTRN data must occur before he and his associates decide which cell types show the most promise. "Even though it’s not answered perfectly, the results definitely point us toward" using more selected types of bone marrow cells, he said.
"The most important lesson FOCUS-CCTRN showed was that as patients get older the number and potency of their stem cells decreases. It showed that if you give cells that aren’t potent they won’t work," said Dr. Henry, a co-investigator of the study and an active member of CCTRN. "That’s a really important insight, because if you treat patients with 100 mg of a beta blocker you get a consistent effect, but if you treat patients with autologous stem cells, every patient gets something different."
Development of stem cell therapies "has been on a normal track; each step takes time. It’s very similar to what happened with fibrinolytic therapy for treating acute myocardial infarctions" in the 1980s and 1990s, said Dr. Timothy D. Henry.
"The FOCUS results showed some evidence of an effect, but there are a number of ways to make it better," Dr. Henry said. One approach is to use selected cells, such as CD34+ cells. Another strategy is to deliver allogenic cells obtained initially from young and healthy donors, a way to avoid the issue of older patients and those with co-morbidities who may have compromised stem cells.
Administering selected or allogenic cells has another potential advantage: it opens the door to commercial involvement with a potentially saleable product, a strategy that can attract the interest of a company willing to shoulder the cost of a phase III trial.
"A challenge with [unprocessed] bone marrow is it’s too easy. Bone marrow is inexpensive, and anyone can do it," Dr. Henry noted. That’s one reason why it took several years for the REPAIR-AMI strategy to advance into a phase III trial, eventually moving forward when it received financial backing from the European Union. In contrast, the promising ACT34-CMI strategy quickly jumped to the phase III level, aided by financial support from Baxter.
But others cautioned against drawing too many inferences from FOCUS-CCTRN because it was a negative study and hence all its results must be considered suspect.
"I do not believe that post-hoc analysis of cell potency should be used to guide future trials. FOCUS was negative; the subgroup analyses and cell potency assays were attempts to glean some positivity from that study after the fact," said Dr. Marbán.
More Phase III Studies Coming, with Commercial Support
Another example of a selected, allogenic cell preparation with commercial support is the study of mesenchymal precursor cells (MPC), bone-marrow cells obtained from young, healthy donors and delivered by transendocardial injection to 45 patients with cardiomyopathy, New York Heart Association class II or III heart failure, and impaired left ventricular function in a phase II study presented by Dr. Perin last November at the American Heart Association’s Scientific Sessions in Orlando. The MPC preparation he and his associates tested was an "off-the-shelf" stem-cell product made by the Australian-based company Mesoblast.
The injections were safe, and the three different amounts of cells delivered showed various signs of efficacy. The lowest dose of cells tested showed a statistically significant improvement in left ventricular ejection fraction at 3 months compared with placebo; the highest cell dose tested showed a statistically significant improvement in left ventricular end-systolic volume at 12 months compared with the placebo control; and collectively, all three cell doses tested produced a statistically significant 20% reduction in the rate of major adverse coronary events compared with the placebo patients during a follow-up of up to 3 years, Dr. Perin reported last November.
"Given the very positive results we saw in the phase II trial, it would be logical to move on to a bigger trial," said Dr. Perin in an interview, but he added that he was unable to provide details about possible additional trials with this MPC product.
Another commercially-backed stem-cell product poised for a larger trial is cardiopoietic stem cells, made by a proprietary, patented method that uses a patient’s autologous bone-marrow stem cells and treats them in vitro to produce cells that are then injected into a patient’s myocardium where they produce new heart muscle. Researchers at the Mayo Clinic in Rochester, Minn. developed this process, and Mayo licensed it to a Belgian company, Cardio 3 BioSciences.
The company released results in November 2010 from the C-Cure study done in Belgium, Serbia, and Switzerland that enrolled 45-patients with severe ischemic heart failure. Six months after treatment, the 21 patients who received injections of the stem cells had an average 18% relative increase in their left ventricular ejection fraction compared with baseline, versus a 4% relative increase over baseline among 24 control patients. Speaking in March at the American College of Cardiology meeting, Dr. André Terzic, who led the study, provided a few more details from the results. Average ejection fraction in the actively-treated patients rose from 28% at baseline to 33%. The treatment was especially effective for the patients who entered the study with an ejection fraction below 28%, said Dr. Terzic, a professor of medicine and cardiology, and director of the Center for Regenerative Medicine at the Mayo Clinic. The 6-month results also showed other indications of benefit in the patients treated with the cardiopoietic stem cells, including significantly increased left ventricular end systolic volume, and reduced left ventricular mass index.
Cardio 3 Biosciences is now planning to start a "late phase II" or phase III trial with this material, said Dr. Simari, who is not directly involved in these studies.
What Comes Next?
Despite 11 years of work, "it’s still very early in the field. We’re still trying to figure out the cells and out targets," said Dr. Simari. "This is the haze of early days, of learning from groups of 10, 20, or 80 patients. Only in retrospect, and as we get to larger trials will the truth emerge. Right now, there is a lot of work needed and few headlines, and it will probably stay that way for awhile. This is not a game for people with short attention spans.
"The business model for autologous cells remains uncertain," he added. "For allogenic products that are generated from young, healthy donors and made into thousands of doses, the business model is different and the costs might be more reasonable. Hopefully, as our knowledge grows, we will develop treatments that end up costing less."
"I have no doubt that cell treatment is one of the great avenues of future medicine; it’s just a matter of figuring it out," said Dr. Perin. "Having phase III trials now starting is a step forward. Things are starting to come into focus. Unless something totally unforeseen happens, we will eventually have an approved product," he predicted.
Dr. Perin said that he has been a consultant to Amorcyte, Teva, Cytori, and Aldagen. Dr. Henry said that he has served on steering committees for and received research grants from Aastrom and Mesoblast. Dr. Marbán said that he is a founder of and holds equity in Capricor. Dr. Simari said that he had no disclosures. Dr. Zeiher said he is a cofounder of and scientific advisor to t2cure, a company set up to run the BAMI trial, sponsored by the European Union Dr. Losordo is an employee of Baxter International. Dr. Terzic said that he has received research grants from Cardio 3 BioSciences.
Carotid Artery Interventions Break Three Ways
Optimal management of carotid stenosis to prevent death and strokes is a work in progress right now, with experts groping for the right balance between carotid endarterectomy, carotid artery stenting, and best medical therapy.
The field is bereft of both conclusive, up-to-date data and – more importantly – wide agreement on data interpretation that clearly tips treatment toward one of these options, so much so that in late January an expert panel organized by the Centers for Medicare and Medicaid Services found itself unable to support with high confidence the application of these treatments to any subgroup of carotid disease patients.
Amid this confusion are the following certainties:
• Use of carotid artery stenting (CAS) on U.S. patients grew substantially since its introduction in the late 1990s and since it began receiving limited Medicare coverage in 2004. Data collected through 2007 showed a greater-than-fourfold increase in CAS use among Medicare beneficiaries, compared with 1998 (Circ. Cardiovasc. Qual. Outcomes 2010;3:15-24).
• Other findings documented a shift in CAS use during the 2000s toward patients with asymptomatic carotid stenosis, with a recent estimate that 70%-90% of CAS patients now fall into that category (Arch. Intern. Med. 2010;170:1225-7).
• The main results of the landmark CREST (Carotid Revascularization Endarterectomy vs. Stenting) trial, first reported 2 years ago (N. Engl. J. Med. 2010;363:11-23), appeared on the surface to show very similar safety and efficacy for CAS and carotid endarterectomy (CEA), but some experts caution that deeper drilling into the results show that this is an oversimplification of how the two treatments compare.
• And many experts agree that best medical therapy has improved in recent years, to the point where it deserves a new appraisal in a large trial that would compare it against carotid revascularization with CAS or CEA in asymptomatic patients. The leaders of CREST themselves have designed a new trial, CREST II, aimed at making this comparison, and have begun to vigorously lobby the National Institutes of Health to support this study.
In the meantime, vascular surgeons, endovascularists, cardiologists, and the other types of physicians who treat patients with carotid disease try as best they can to strike the right balance in how they apply the three management options.
CREST Provides New Guidance
One surgeon who has perhaps given the most thought to sorting out treatment options is Dr. Brajesh K. Lal, a co–principal investigator on CREST, a vascular surgeon at the University of Maryland in Baltimore, and a researcher who spent the past couple of years sorting through CREST’s voluminous data to find new clues to guide patient triage.
A major, recent guide for matching patients with a specific carotid intervention came from his painstaking analysis of carotid angiograms from 1,070 of the CREST patients who underwent CAS. This effort identified four anatomical features that appear to mark patients with an increased rate of periprocedural stroke when they are treated with CAS. (See box.)
Patients with one or more of these carotid anatomical features "should be treated by endarterectomy," Dr. Lal said in an interview. "If I see any one of these in my clinical practice, I will not stent."
Endovascularists were already routinely assessing carotid anatomy before attempting CAS, but prior to Dr. Lal’s report on these new findings in February at the International Stroke Conference in New Orleans, "I’m not so sure that everyone has been using this [anatomical] information," he said. "When we talk about operator experience in stenting, if we don’t have the facts about what increases the stenting risk, then we won’t improve performance. You’d be surprised at what some interventionalists are willing to do," despite a patient’s challenging carotid anatomy. "These data show that you can work around a single 90-degree bend [of the distal carotid artery], but not around two bends. That is the kind of stuff we’re beginning to find out."
Additional, recent CREST analyses that were also reported at the International Stroke Conference revealed other new, important lessons about CAS and CEA.
First, by 2 years after intervention, both CAS and CEA produced roughly comparable low rates of restenosis: a 6.0% rate in the CAS patients who underwent a prespecified, follow-up duplex ultrasound examination of their carotid artery, and 6.3% in the CEA patients (Stroke 2012;43:abstract A3).
The rates were also similar regardless of whether patients were symptomatic before their carotid intervention, Dr. Lal reported.
This finding appears to lay to rest the concern about a major restenosis risk using bare-metal stents in carotid arteries, in contrast to what happens in coronary arteries, Dr. Lal said.
"To my mind, these data are fairly definitive. We followed more than 2,000 patients, and every ultrasound was reviewed and adjudicated. I’m fairly comfortable with these results."
A third CREST analysis that was also reported in February showed that, after adjustment for baseline differences, patients had no significant changes in their rates of periprocedural events during and after CAS throughout the study, from 2000 to 2008 (Stroke 2012;43:abstract A1). "We hoped to show that CAS was getting better [during the 9 years of CREST], but we saw no evidence it got better with time," said George Howard, Dr.P.H., professor and chairman of the department of biostatistics at the University of Alabama at Birmingham. "It would make sense if CAS got better; it’s a new procedure that people learn." But the CREST results showed no evidence of a learning curve, perhaps because the 224 interventionalists who performed CAS in CREST went through a rigorous credentialing process and also performed their first several CAS procedures in CREST during a lead-in phase that was not included in the main outcomes.
Choosing Among the Options
Dr. Lal said that he draws several other lessons from the CREST results to guide his treatment decisions: Patients who are physiologically elderly, those with a large amount of calcified atheroma in their aortic arch, and patients with significant peripheral arterial disease that impedes endovascular access are all poor CAS candidates, he said, adding that he focuses on a patient’s physiological age rather than chronological age.
Patients who are well suited to CAS are those with a younger physiological age, those with a history of radiation treatment, and patients with restenotic carotid lesions.
Although CREST showed worse performance of CAS in women compared with men, "I don’t know why, so it’s hard for me to exclude or offer a particular treatment" based on the patient’s sex, he said. "I treat women just like men, and work through all the other things that I know about to help me decide."
Then there is a third subgroup, patients with carotid disease who he feels "benefit the most" from medical management only. This category primarily includes asymptomatic patients with moderate stenosis (that is, less than 80% carotid occlusion).
His prescription for medical management includes 325 mg of aspirin daily, treatment with a statin, good blood pressure and blood sugar control, smoking cessation, and lifestyle modification. If, despite this, an asymptomatic patient has continued stenotic progression that advances beyond 80%, he will then recommend revascularization.
Dr. Lal said that in his practice today, roughly one-third of carotid-disease patients are on medical management, a third undergo CEA, and a third receive CAS. "We are at a stage of equipoise," for these three options, which is why he helped design and is working to get funding for CREST II.
Dr. Lal personally applies all three treatment options – CAS, CEA, or medical management. Having physicians involved in the decision-making process who are comfortable using each of these options is critical for making a balanced management decision, said Dr. L. Nelson Hopkins, another CREST collaborator and professor and chairman of neurosurgery at the State University of New York at Buffalo.
"The single most important thing we do is have a weekly, multidisciplinary conference to discuss each case, with input from everyone, to decide the best treatment for each [carotid disease] patient," he said when he spoke at ISET 2012, an International Symposium on Endovascular Therapy in Miami Beach in January.
"The most important factors" guiding the decision of whether or not to perform CAS are, does a symptomatic patient have "hot" lesions, what is the arterial tortuosity, and what is the calcification, he said. Other important features include whether the patient has a contralateral carotid occlusion, whether the carotid has a high bifurcation, and whether there are ostial or tandem lesions. "If the anatomy is favorable, CAS is fine even in a 95-year-old," Dr. Hopkins said.
What About Insurance Coverage?
Carotid specialists concede that another, nonmedical issue also plays a big role in deciding how to manage a patient: What will the patient’s health insurance cover?
In its most recent decision on the issue, effective in April 2010, the Centers for Medicare and Medicaid Services (CMS) said that Medicare covers CAS performed on a routine basis for patients with symptomatic carotid disease who are at high risk for CEA and have at least 70% carotid stenosis. CMS also said that Medicare will cover FDA-approved investigations of CAS in symptomatic patients who are at high risk for CEA and have at least 50% stenosis, as well as CAS investigations in asymptomatic patients at high risk for CEA with at least 80% carotid stenosis.
As a result, patients with other forms of carotid disease often do not have insurance coverage for CAS.
"The issue for us is primarily reimbursement. If we can do [a procedure] and be reimbursed, we often stent the patient," said Dr. Carlos H. Timaran, a vascular surgeon and chief of endovascular surgery at the University of Texas Southwestern Medical Center in Dallas.
"For my private practice patients, we assess their risk based on their
[anatomical] and medical criteria. If they are high risk, we usually stent. We have a bias toward stenting because it is faster and easier. Some people say they can do endarterectomy in an hour, but I don’t believe that. I do CEA with residents and fellows, and it takes 2 hours. But with stenting, I can do it in 40 minutes, even with a fellow. If I have three patients with carotid disease to treat [and] if I do CEA on all three, it will take all day. If I do CAS, it will take the morning, or less," he said in an interview.
In addition, "CEA is a nice procedure, but you need anesthesia. CAS is easier, I can do it myself, and I don’t need anesthesia. I am in more control of the case," Dr. Timaran said.
For patients who are eligible for both CAS and CEA, "it also comes down to anatomy," he grants. But if the patient has anatomy favorable to either method, "I offer patients both, and they will probably go for a stent," because patients usually prefer the quicker recovery that stenting offers, compared with CEA.
An interesting footnote to the issue of how to best manage patients who are eligible for CAS under CMS rules is that the CREST results offer no direct guidance on the relative safety and efficacy of CAS and CEA in patients with Medicare coverage. That’s because "the CREST cohort was a conventional-risk cohort; most criteria that would make someone a high surgical risk would have been excluded from CREST," Dr. Thomas G. Brott, professor of neurology at the Mayo Clinic in Jacksonville, Fla., and lead investigator for CREST. "The high-surgical-risk patients who are currently covered by Medicare would not have even been enrolled in CREST," Dr. Brott said in an interview.
Where Does Medical Therapy Fit In?
Although Dr. Timaran sees the potential value of medical therapy only for asymptomatic patients, he also urges caution.
"I don’t think [the efficacy of medical treatment] has been proven, and until it’s proven I don’t think physicians should deny revascularization treatment to these patients," he said. "The standard of care for a patient with severe carotid stenosis is revascularization, even if the patient is asymptomatic, unless there is some other consideration." But, he admitted, "there is little downside to medical treatment in asymptomatic patients, even if they have severe stenosis." That’s because their stroke risk is low regardless of which option a patient chooses.
"I tell asymptomatic patients that ‘based on the data, your risk of stroke is 2% per year, and that risk may be even lower with best medical therapy.’ With a carotid intervention, we can lower their risk to 1% per year." In other words, the patient’s stroke risk is low regardless of which option is chosen. Plus, medical therapy only is the best choice for patients with special risk factors, such as radiation-induced stenosis, Dr. Timaran added.
Other experts see medical treatment alone as an excellent option for many carotid-stenosis patients.
"The majority of patients I see don’t get an intervention; we use medical treatment only," said Dr. Jon S. Matsumura in an interview. "I think that’s true for most vascular surgery practices, and in cardiology and neurology practices. A lot of these patients just need reassurance," said Dr. Matsumura, professor and chairman of vascular surgery at the University of Wisconsin in Madison.
"Most asymptomatic patients [with carotid stenosis] should be treated by best medical therapy, with very few – fewer than 5% – treated by CAS or CEA," said Dr. Frank J. Veith at the International Symposium on Endovascular Therapy (ISET 12) in January. He acknowledged that well-controlled trials done during the 1990s showed that CEA led to superior outcomes compared with medical therapy, but since the early 2000s, "best medical therapy to prevent strokes leapt forward," he said, especially with improved statin therapy. He cited findings from a 2010 prospective study that showed asymptomatic Canadian patients managed starting in 2003 with best medical therapy had an annual stroke rate of 0.7% (Arch. Neurol. 2010;67:180-6).
He singled out four small groups of asymptomatic patients who warrant revascularization: patients with CT or MRI evidence for a history of silent strokes, patients with very-high-grade carotid stenosis that leaves just 1% of the carotid lumen open, those with a contralateral occlusion, and patients who serially experience transient occlusions detected by transcranial Doppler ultrasound examination, said Dr. Veith, professor of surgery at New York
University. Aside from this small fraction of patients with asymptomatic disease, performing interventions on anyone else will "cause more strokes than you’ll prevent," he warned in an interview.
What Will CMS Do?
Dr. Veith’s take on limiting CAS in asymptomatic patients received substantial support early this year in the days leading up to the Jan. 25 meeting of the Medicare Evidence Development and Coverage Advisory Committee (MEDCAC) that heard evidence and opinions for and against expanding Medicare coverage for CAS. In early January, Dr. Veith joined with 40 other vascular surgeons and stroke specialists to urge CMS not to broaden CAS coverage (Eur. J. Vasc. Endovasc. Surg. 2012 Jan. 5 [doi:10.1016/j.ejvs.2011.12.006]). The Society for Vascular Surgeons has also been on the forefront of this issue, urging CMS to maintain appropriate coverage restrictions.
But, as MEDCAC heard during this meeting, the proponents of expanded coverage remain staunch.. "CMS continues to restrict access to CAS to about 10% of patients" with significant carotid stenosis, said Dr. William A. Gray, director of endovascular services at Columbia University in New York, speaking at ISET 2012 in January. "Expanded CAS coverage would allow greater patient access and device development, and therefore create a safer option for patients." Dr. Gray presented his positive views on CAS to MEDCAC in January as well.
At press time, CMS had not announced whether or not the January MEDCAC hearing will result in a change to its CAS coverage policy. But the votes cast by MEDCAC’s membership in January suggested that the issues surrounding management of carotid stenosis remain too unresolved to trigger immediate changes.
Dr. Lal, Dr. Timaran, and Dr. Veith said that they had no disclosures. Dr. Howard said that he has received grant support from Abbott. Dr. Hopkins said that he has financial relationships with Bard, Boston Scientific, Cordis, Abbott Vascular, Toshiba, W.L. Gore, Medtronic, Micrus, St. Jude, Access Closure, and other device companies. Dr. Brott said that he has been a consultant to Edwards Lifesciences. Dr. Matsumura said that he has received research grants from Abbott, Cook, Covidien, Endologix, and W.L. Gore. Dr. Gray said that he has had financial relationships with Boston Scientific, Coherex, Contego, Cordis, and a number of other device companies.
Optimal management of carotid stenosis to prevent death and strokes is a work in progress right now, with experts groping for the right balance between carotid endarterectomy, carotid artery stenting, and best medical therapy.
The field is bereft of both conclusive, up-to-date data and – more importantly – wide agreement on data interpretation that clearly tips treatment toward one of these options, so much so that in late January an expert panel organized by the Centers for Medicare and Medicaid Services found itself unable to support with high confidence the application of these treatments to any subgroup of carotid disease patients.
Amid this confusion are the following certainties:
• Use of carotid artery stenting (CAS) on U.S. patients grew substantially since its introduction in the late 1990s and since it began receiving limited Medicare coverage in 2004. Data collected through 2007 showed a greater-than-fourfold increase in CAS use among Medicare beneficiaries, compared with 1998 (Circ. Cardiovasc. Qual. Outcomes 2010;3:15-24).
• Other findings documented a shift in CAS use during the 2000s toward patients with asymptomatic carotid stenosis, with a recent estimate that 70%-90% of CAS patients now fall into that category (Arch. Intern. Med. 2010;170:1225-7).
• The main results of the landmark CREST (Carotid Revascularization Endarterectomy vs. Stenting) trial, first reported 2 years ago (N. Engl. J. Med. 2010;363:11-23), appeared on the surface to show very similar safety and efficacy for CAS and carotid endarterectomy (CEA), but some experts caution that deeper drilling into the results show that this is an oversimplification of how the two treatments compare.
• And many experts agree that best medical therapy has improved in recent years, to the point where it deserves a new appraisal in a large trial that would compare it against carotid revascularization with CAS or CEA in asymptomatic patients. The leaders of CREST themselves have designed a new trial, CREST II, aimed at making this comparison, and have begun to vigorously lobby the National Institutes of Health to support this study.
In the meantime, vascular surgeons, endovascularists, cardiologists, and the other types of physicians who treat patients with carotid disease try as best they can to strike the right balance in how they apply the three management options.
CREST Provides New Guidance
One surgeon who has perhaps given the most thought to sorting out treatment options is Dr. Brajesh K. Lal, a co–principal investigator on CREST, a vascular surgeon at the University of Maryland in Baltimore, and a researcher who spent the past couple of years sorting through CREST’s voluminous data to find new clues to guide patient triage.
A major, recent guide for matching patients with a specific carotid intervention came from his painstaking analysis of carotid angiograms from 1,070 of the CREST patients who underwent CAS. This effort identified four anatomical features that appear to mark patients with an increased rate of periprocedural stroke when they are treated with CAS. (See box.)
Patients with one or more of these carotid anatomical features "should be treated by endarterectomy," Dr. Lal said in an interview. "If I see any one of these in my clinical practice, I will not stent."
Endovascularists were already routinely assessing carotid anatomy before attempting CAS, but prior to Dr. Lal’s report on these new findings in February at the International Stroke Conference in New Orleans, "I’m not so sure that everyone has been using this [anatomical] information," he said. "When we talk about operator experience in stenting, if we don’t have the facts about what increases the stenting risk, then we won’t improve performance. You’d be surprised at what some interventionalists are willing to do," despite a patient’s challenging carotid anatomy. "These data show that you can work around a single 90-degree bend [of the distal carotid artery], but not around two bends. That is the kind of stuff we’re beginning to find out."
Additional, recent CREST analyses that were also reported at the International Stroke Conference revealed other new, important lessons about CAS and CEA.
First, by 2 years after intervention, both CAS and CEA produced roughly comparable low rates of restenosis: a 6.0% rate in the CAS patients who underwent a prespecified, follow-up duplex ultrasound examination of their carotid artery, and 6.3% in the CEA patients (Stroke 2012;43:abstract A3).
The rates were also similar regardless of whether patients were symptomatic before their carotid intervention, Dr. Lal reported.
This finding appears to lay to rest the concern about a major restenosis risk using bare-metal stents in carotid arteries, in contrast to what happens in coronary arteries, Dr. Lal said.
"To my mind, these data are fairly definitive. We followed more than 2,000 patients, and every ultrasound was reviewed and adjudicated. I’m fairly comfortable with these results."
A third CREST analysis that was also reported in February showed that, after adjustment for baseline differences, patients had no significant changes in their rates of periprocedural events during and after CAS throughout the study, from 2000 to 2008 (Stroke 2012;43:abstract A1). "We hoped to show that CAS was getting better [during the 9 years of CREST], but we saw no evidence it got better with time," said George Howard, Dr.P.H., professor and chairman of the department of biostatistics at the University of Alabama at Birmingham. "It would make sense if CAS got better; it’s a new procedure that people learn." But the CREST results showed no evidence of a learning curve, perhaps because the 224 interventionalists who performed CAS in CREST went through a rigorous credentialing process and also performed their first several CAS procedures in CREST during a lead-in phase that was not included in the main outcomes.
Choosing Among the Options
Dr. Lal said that he draws several other lessons from the CREST results to guide his treatment decisions: Patients who are physiologically elderly, those with a large amount of calcified atheroma in their aortic arch, and patients with significant peripheral arterial disease that impedes endovascular access are all poor CAS candidates, he said, adding that he focuses on a patient’s physiological age rather than chronological age.
Patients who are well suited to CAS are those with a younger physiological age, those with a history of radiation treatment, and patients with restenotic carotid lesions.
Although CREST showed worse performance of CAS in women compared with men, "I don’t know why, so it’s hard for me to exclude or offer a particular treatment" based on the patient’s sex, he said. "I treat women just like men, and work through all the other things that I know about to help me decide."
Then there is a third subgroup, patients with carotid disease who he feels "benefit the most" from medical management only. This category primarily includes asymptomatic patients with moderate stenosis (that is, less than 80% carotid occlusion).
His prescription for medical management includes 325 mg of aspirin daily, treatment with a statin, good blood pressure and blood sugar control, smoking cessation, and lifestyle modification. If, despite this, an asymptomatic patient has continued stenotic progression that advances beyond 80%, he will then recommend revascularization.
Dr. Lal said that in his practice today, roughly one-third of carotid-disease patients are on medical management, a third undergo CEA, and a third receive CAS. "We are at a stage of equipoise," for these three options, which is why he helped design and is working to get funding for CREST II.
Dr. Lal personally applies all three treatment options – CAS, CEA, or medical management. Having physicians involved in the decision-making process who are comfortable using each of these options is critical for making a balanced management decision, said Dr. L. Nelson Hopkins, another CREST collaborator and professor and chairman of neurosurgery at the State University of New York at Buffalo.
"The single most important thing we do is have a weekly, multidisciplinary conference to discuss each case, with input from everyone, to decide the best treatment for each [carotid disease] patient," he said when he spoke at ISET 2012, an International Symposium on Endovascular Therapy in Miami Beach in January.
"The most important factors" guiding the decision of whether or not to perform CAS are, does a symptomatic patient have "hot" lesions, what is the arterial tortuosity, and what is the calcification, he said. Other important features include whether the patient has a contralateral carotid occlusion, whether the carotid has a high bifurcation, and whether there are ostial or tandem lesions. "If the anatomy is favorable, CAS is fine even in a 95-year-old," Dr. Hopkins said.
What About Insurance Coverage?
Carotid specialists concede that another, nonmedical issue also plays a big role in deciding how to manage a patient: What will the patient’s health insurance cover?
In its most recent decision on the issue, effective in April 2010, the Centers for Medicare and Medicaid Services (CMS) said that Medicare covers CAS performed on a routine basis for patients with symptomatic carotid disease who are at high risk for CEA and have at least 70% carotid stenosis. CMS also said that Medicare will cover FDA-approved investigations of CAS in symptomatic patients who are at high risk for CEA and have at least 50% stenosis, as well as CAS investigations in asymptomatic patients at high risk for CEA with at least 80% carotid stenosis.
As a result, patients with other forms of carotid disease often do not have insurance coverage for CAS.
"The issue for us is primarily reimbursement. If we can do [a procedure] and be reimbursed, we often stent the patient," said Dr. Carlos H. Timaran, a vascular surgeon and chief of endovascular surgery at the University of Texas Southwestern Medical Center in Dallas.
"For my private practice patients, we assess their risk based on their
[anatomical] and medical criteria. If they are high risk, we usually stent. We have a bias toward stenting because it is faster and easier. Some people say they can do endarterectomy in an hour, but I don’t believe that. I do CEA with residents and fellows, and it takes 2 hours. But with stenting, I can do it in 40 minutes, even with a fellow. If I have three patients with carotid disease to treat [and] if I do CEA on all three, it will take all day. If I do CAS, it will take the morning, or less," he said in an interview.
In addition, "CEA is a nice procedure, but you need anesthesia. CAS is easier, I can do it myself, and I don’t need anesthesia. I am in more control of the case," Dr. Timaran said.
For patients who are eligible for both CAS and CEA, "it also comes down to anatomy," he grants. But if the patient has anatomy favorable to either method, "I offer patients both, and they will probably go for a stent," because patients usually prefer the quicker recovery that stenting offers, compared with CEA.
An interesting footnote to the issue of how to best manage patients who are eligible for CAS under CMS rules is that the CREST results offer no direct guidance on the relative safety and efficacy of CAS and CEA in patients with Medicare coverage. That’s because "the CREST cohort was a conventional-risk cohort; most criteria that would make someone a high surgical risk would have been excluded from CREST," Dr. Thomas G. Brott, professor of neurology at the Mayo Clinic in Jacksonville, Fla., and lead investigator for CREST. "The high-surgical-risk patients who are currently covered by Medicare would not have even been enrolled in CREST," Dr. Brott said in an interview.
Where Does Medical Therapy Fit In?
Although Dr. Timaran sees the potential value of medical therapy only for asymptomatic patients, he also urges caution.
"I don’t think [the efficacy of medical treatment] has been proven, and until it’s proven I don’t think physicians should deny revascularization treatment to these patients," he said. "The standard of care for a patient with severe carotid stenosis is revascularization, even if the patient is asymptomatic, unless there is some other consideration." But, he admitted, "there is little downside to medical treatment in asymptomatic patients, even if they have severe stenosis." That’s because their stroke risk is low regardless of which option a patient chooses.
"I tell asymptomatic patients that ‘based on the data, your risk of stroke is 2% per year, and that risk may be even lower with best medical therapy.’ With a carotid intervention, we can lower their risk to 1% per year." In other words, the patient’s stroke risk is low regardless of which option is chosen. Plus, medical therapy only is the best choice for patients with special risk factors, such as radiation-induced stenosis, Dr. Timaran added.
Other experts see medical treatment alone as an excellent option for many carotid-stenosis patients.
"The majority of patients I see don’t get an intervention; we use medical treatment only," said Dr. Jon S. Matsumura in an interview. "I think that’s true for most vascular surgery practices, and in cardiology and neurology practices. A lot of these patients just need reassurance," said Dr. Matsumura, professor and chairman of vascular surgery at the University of Wisconsin in Madison.
"Most asymptomatic patients [with carotid stenosis] should be treated by best medical therapy, with very few – fewer than 5% – treated by CAS or CEA," said Dr. Frank J. Veith at the International Symposium on Endovascular Therapy (ISET 12) in January. He acknowledged that well-controlled trials done during the 1990s showed that CEA led to superior outcomes compared with medical therapy, but since the early 2000s, "best medical therapy to prevent strokes leapt forward," he said, especially with improved statin therapy. He cited findings from a 2010 prospective study that showed asymptomatic Canadian patients managed starting in 2003 with best medical therapy had an annual stroke rate of 0.7% (Arch. Neurol. 2010;67:180-6).
He singled out four small groups of asymptomatic patients who warrant revascularization: patients with CT or MRI evidence for a history of silent strokes, patients with very-high-grade carotid stenosis that leaves just 1% of the carotid lumen open, those with a contralateral occlusion, and patients who serially experience transient occlusions detected by transcranial Doppler ultrasound examination, said Dr. Veith, professor of surgery at New York
University. Aside from this small fraction of patients with asymptomatic disease, performing interventions on anyone else will "cause more strokes than you’ll prevent," he warned in an interview.
What Will CMS Do?
Dr. Veith’s take on limiting CAS in asymptomatic patients received substantial support early this year in the days leading up to the Jan. 25 meeting of the Medicare Evidence Development and Coverage Advisory Committee (MEDCAC) that heard evidence and opinions for and against expanding Medicare coverage for CAS. In early January, Dr. Veith joined with 40 other vascular surgeons and stroke specialists to urge CMS not to broaden CAS coverage (Eur. J. Vasc. Endovasc. Surg. 2012 Jan. 5 [doi:10.1016/j.ejvs.2011.12.006]). The Society for Vascular Surgeons has also been on the forefront of this issue, urging CMS to maintain appropriate coverage restrictions.
But, as MEDCAC heard during this meeting, the proponents of expanded coverage remain staunch.. "CMS continues to restrict access to CAS to about 10% of patients" with significant carotid stenosis, said Dr. William A. Gray, director of endovascular services at Columbia University in New York, speaking at ISET 2012 in January. "Expanded CAS coverage would allow greater patient access and device development, and therefore create a safer option for patients." Dr. Gray presented his positive views on CAS to MEDCAC in January as well.
At press time, CMS had not announced whether or not the January MEDCAC hearing will result in a change to its CAS coverage policy. But the votes cast by MEDCAC’s membership in January suggested that the issues surrounding management of carotid stenosis remain too unresolved to trigger immediate changes.
Dr. Lal, Dr. Timaran, and Dr. Veith said that they had no disclosures. Dr. Howard said that he has received grant support from Abbott. Dr. Hopkins said that he has financial relationships with Bard, Boston Scientific, Cordis, Abbott Vascular, Toshiba, W.L. Gore, Medtronic, Micrus, St. Jude, Access Closure, and other device companies. Dr. Brott said that he has been a consultant to Edwards Lifesciences. Dr. Matsumura said that he has received research grants from Abbott, Cook, Covidien, Endologix, and W.L. Gore. Dr. Gray said that he has had financial relationships with Boston Scientific, Coherex, Contego, Cordis, and a number of other device companies.
Optimal management of carotid stenosis to prevent death and strokes is a work in progress right now, with experts groping for the right balance between carotid endarterectomy, carotid artery stenting, and best medical therapy.
The field is bereft of both conclusive, up-to-date data and – more importantly – wide agreement on data interpretation that clearly tips treatment toward one of these options, so much so that in late January an expert panel organized by the Centers for Medicare and Medicaid Services found itself unable to support with high confidence the application of these treatments to any subgroup of carotid disease patients.
Amid this confusion are the following certainties:
• Use of carotid artery stenting (CAS) on U.S. patients grew substantially since its introduction in the late 1990s and since it began receiving limited Medicare coverage in 2004. Data collected through 2007 showed a greater-than-fourfold increase in CAS use among Medicare beneficiaries, compared with 1998 (Circ. Cardiovasc. Qual. Outcomes 2010;3:15-24).
• Other findings documented a shift in CAS use during the 2000s toward patients with asymptomatic carotid stenosis, with a recent estimate that 70%-90% of CAS patients now fall into that category (Arch. Intern. Med. 2010;170:1225-7).
• The main results of the landmark CREST (Carotid Revascularization Endarterectomy vs. Stenting) trial, first reported 2 years ago (N. Engl. J. Med. 2010;363:11-23), appeared on the surface to show very similar safety and efficacy for CAS and carotid endarterectomy (CEA), but some experts caution that deeper drilling into the results show that this is an oversimplification of how the two treatments compare.
• And many experts agree that best medical therapy has improved in recent years, to the point where it deserves a new appraisal in a large trial that would compare it against carotid revascularization with CAS or CEA in asymptomatic patients. The leaders of CREST themselves have designed a new trial, CREST II, aimed at making this comparison, and have begun to vigorously lobby the National Institutes of Health to support this study.
In the meantime, vascular surgeons, endovascularists, cardiologists, and the other types of physicians who treat patients with carotid disease try as best they can to strike the right balance in how they apply the three management options.
CREST Provides New Guidance
One surgeon who has perhaps given the most thought to sorting out treatment options is Dr. Brajesh K. Lal, a co–principal investigator on CREST, a vascular surgeon at the University of Maryland in Baltimore, and a researcher who spent the past couple of years sorting through CREST’s voluminous data to find new clues to guide patient triage.
A major, recent guide for matching patients with a specific carotid intervention came from his painstaking analysis of carotid angiograms from 1,070 of the CREST patients who underwent CAS. This effort identified four anatomical features that appear to mark patients with an increased rate of periprocedural stroke when they are treated with CAS. (See box.)
Patients with one or more of these carotid anatomical features "should be treated by endarterectomy," Dr. Lal said in an interview. "If I see any one of these in my clinical practice, I will not stent."
Endovascularists were already routinely assessing carotid anatomy before attempting CAS, but prior to Dr. Lal’s report on these new findings in February at the International Stroke Conference in New Orleans, "I’m not so sure that everyone has been using this [anatomical] information," he said. "When we talk about operator experience in stenting, if we don’t have the facts about what increases the stenting risk, then we won’t improve performance. You’d be surprised at what some interventionalists are willing to do," despite a patient’s challenging carotid anatomy. "These data show that you can work around a single 90-degree bend [of the distal carotid artery], but not around two bends. That is the kind of stuff we’re beginning to find out."
Additional, recent CREST analyses that were also reported at the International Stroke Conference revealed other new, important lessons about CAS and CEA.
First, by 2 years after intervention, both CAS and CEA produced roughly comparable low rates of restenosis: a 6.0% rate in the CAS patients who underwent a prespecified, follow-up duplex ultrasound examination of their carotid artery, and 6.3% in the CEA patients (Stroke 2012;43:abstract A3).
The rates were also similar regardless of whether patients were symptomatic before their carotid intervention, Dr. Lal reported.
This finding appears to lay to rest the concern about a major restenosis risk using bare-metal stents in carotid arteries, in contrast to what happens in coronary arteries, Dr. Lal said.
"To my mind, these data are fairly definitive. We followed more than 2,000 patients, and every ultrasound was reviewed and adjudicated. I’m fairly comfortable with these results."
A third CREST analysis that was also reported in February showed that, after adjustment for baseline differences, patients had no significant changes in their rates of periprocedural events during and after CAS throughout the study, from 2000 to 2008 (Stroke 2012;43:abstract A1). "We hoped to show that CAS was getting better [during the 9 years of CREST], but we saw no evidence it got better with time," said George Howard, Dr.P.H., professor and chairman of the department of biostatistics at the University of Alabama at Birmingham. "It would make sense if CAS got better; it’s a new procedure that people learn." But the CREST results showed no evidence of a learning curve, perhaps because the 224 interventionalists who performed CAS in CREST went through a rigorous credentialing process and also performed their first several CAS procedures in CREST during a lead-in phase that was not included in the main outcomes.
Choosing Among the Options
Dr. Lal said that he draws several other lessons from the CREST results to guide his treatment decisions: Patients who are physiologically elderly, those with a large amount of calcified atheroma in their aortic arch, and patients with significant peripheral arterial disease that impedes endovascular access are all poor CAS candidates, he said, adding that he focuses on a patient’s physiological age rather than chronological age.
Patients who are well suited to CAS are those with a younger physiological age, those with a history of radiation treatment, and patients with restenotic carotid lesions.
Although CREST showed worse performance of CAS in women compared with men, "I don’t know why, so it’s hard for me to exclude or offer a particular treatment" based on the patient’s sex, he said. "I treat women just like men, and work through all the other things that I know about to help me decide."
Then there is a third subgroup, patients with carotid disease who he feels "benefit the most" from medical management only. This category primarily includes asymptomatic patients with moderate stenosis (that is, less than 80% carotid occlusion).
His prescription for medical management includes 325 mg of aspirin daily, treatment with a statin, good blood pressure and blood sugar control, smoking cessation, and lifestyle modification. If, despite this, an asymptomatic patient has continued stenotic progression that advances beyond 80%, he will then recommend revascularization.
Dr. Lal said that in his practice today, roughly one-third of carotid-disease patients are on medical management, a third undergo CEA, and a third receive CAS. "We are at a stage of equipoise," for these three options, which is why he helped design and is working to get funding for CREST II.
Dr. Lal personally applies all three treatment options – CAS, CEA, or medical management. Having physicians involved in the decision-making process who are comfortable using each of these options is critical for making a balanced management decision, said Dr. L. Nelson Hopkins, another CREST collaborator and professor and chairman of neurosurgery at the State University of New York at Buffalo.
"The single most important thing we do is have a weekly, multidisciplinary conference to discuss each case, with input from everyone, to decide the best treatment for each [carotid disease] patient," he said when he spoke at ISET 2012, an International Symposium on Endovascular Therapy in Miami Beach in January.
"The most important factors" guiding the decision of whether or not to perform CAS are, does a symptomatic patient have "hot" lesions, what is the arterial tortuosity, and what is the calcification, he said. Other important features include whether the patient has a contralateral carotid occlusion, whether the carotid has a high bifurcation, and whether there are ostial or tandem lesions. "If the anatomy is favorable, CAS is fine even in a 95-year-old," Dr. Hopkins said.
What About Insurance Coverage?
Carotid specialists concede that another, nonmedical issue also plays a big role in deciding how to manage a patient: What will the patient’s health insurance cover?
In its most recent decision on the issue, effective in April 2010, the Centers for Medicare and Medicaid Services (CMS) said that Medicare covers CAS performed on a routine basis for patients with symptomatic carotid disease who are at high risk for CEA and have at least 70% carotid stenosis. CMS also said that Medicare will cover FDA-approved investigations of CAS in symptomatic patients who are at high risk for CEA and have at least 50% stenosis, as well as CAS investigations in asymptomatic patients at high risk for CEA with at least 80% carotid stenosis.
As a result, patients with other forms of carotid disease often do not have insurance coverage for CAS.
"The issue for us is primarily reimbursement. If we can do [a procedure] and be reimbursed, we often stent the patient," said Dr. Carlos H. Timaran, a vascular surgeon and chief of endovascular surgery at the University of Texas Southwestern Medical Center in Dallas.
"For my private practice patients, we assess their risk based on their
[anatomical] and medical criteria. If they are high risk, we usually stent. We have a bias toward stenting because it is faster and easier. Some people say they can do endarterectomy in an hour, but I don’t believe that. I do CEA with residents and fellows, and it takes 2 hours. But with stenting, I can do it in 40 minutes, even with a fellow. If I have three patients with carotid disease to treat [and] if I do CEA on all three, it will take all day. If I do CAS, it will take the morning, or less," he said in an interview.
In addition, "CEA is a nice procedure, but you need anesthesia. CAS is easier, I can do it myself, and I don’t need anesthesia. I am in more control of the case," Dr. Timaran said.
For patients who are eligible for both CAS and CEA, "it also comes down to anatomy," he grants. But if the patient has anatomy favorable to either method, "I offer patients both, and they will probably go for a stent," because patients usually prefer the quicker recovery that stenting offers, compared with CEA.
An interesting footnote to the issue of how to best manage patients who are eligible for CAS under CMS rules is that the CREST results offer no direct guidance on the relative safety and efficacy of CAS and CEA in patients with Medicare coverage. That’s because "the CREST cohort was a conventional-risk cohort; most criteria that would make someone a high surgical risk would have been excluded from CREST," Dr. Thomas G. Brott, professor of neurology at the Mayo Clinic in Jacksonville, Fla., and lead investigator for CREST. "The high-surgical-risk patients who are currently covered by Medicare would not have even been enrolled in CREST," Dr. Brott said in an interview.
Where Does Medical Therapy Fit In?
Although Dr. Timaran sees the potential value of medical therapy only for asymptomatic patients, he also urges caution.
"I don’t think [the efficacy of medical treatment] has been proven, and until it’s proven I don’t think physicians should deny revascularization treatment to these patients," he said. "The standard of care for a patient with severe carotid stenosis is revascularization, even if the patient is asymptomatic, unless there is some other consideration." But, he admitted, "there is little downside to medical treatment in asymptomatic patients, even if they have severe stenosis." That’s because their stroke risk is low regardless of which option a patient chooses.
"I tell asymptomatic patients that ‘based on the data, your risk of stroke is 2% per year, and that risk may be even lower with best medical therapy.’ With a carotid intervention, we can lower their risk to 1% per year." In other words, the patient’s stroke risk is low regardless of which option is chosen. Plus, medical therapy only is the best choice for patients with special risk factors, such as radiation-induced stenosis, Dr. Timaran added.
Other experts see medical treatment alone as an excellent option for many carotid-stenosis patients.
"The majority of patients I see don’t get an intervention; we use medical treatment only," said Dr. Jon S. Matsumura in an interview. "I think that’s true for most vascular surgery practices, and in cardiology and neurology practices. A lot of these patients just need reassurance," said Dr. Matsumura, professor and chairman of vascular surgery at the University of Wisconsin in Madison.
"Most asymptomatic patients [with carotid stenosis] should be treated by best medical therapy, with very few – fewer than 5% – treated by CAS or CEA," said Dr. Frank J. Veith at the International Symposium on Endovascular Therapy (ISET 12) in January. He acknowledged that well-controlled trials done during the 1990s showed that CEA led to superior outcomes compared with medical therapy, but since the early 2000s, "best medical therapy to prevent strokes leapt forward," he said, especially with improved statin therapy. He cited findings from a 2010 prospective study that showed asymptomatic Canadian patients managed starting in 2003 with best medical therapy had an annual stroke rate of 0.7% (Arch. Neurol. 2010;67:180-6).
He singled out four small groups of asymptomatic patients who warrant revascularization: patients with CT or MRI evidence for a history of silent strokes, patients with very-high-grade carotid stenosis that leaves just 1% of the carotid lumen open, those with a contralateral occlusion, and patients who serially experience transient occlusions detected by transcranial Doppler ultrasound examination, said Dr. Veith, professor of surgery at New York
University. Aside from this small fraction of patients with asymptomatic disease, performing interventions on anyone else will "cause more strokes than you’ll prevent," he warned in an interview.
What Will CMS Do?
Dr. Veith’s take on limiting CAS in asymptomatic patients received substantial support early this year in the days leading up to the Jan. 25 meeting of the Medicare Evidence Development and Coverage Advisory Committee (MEDCAC) that heard evidence and opinions for and against expanding Medicare coverage for CAS. In early January, Dr. Veith joined with 40 other vascular surgeons and stroke specialists to urge CMS not to broaden CAS coverage (Eur. J. Vasc. Endovasc. Surg. 2012 Jan. 5 [doi:10.1016/j.ejvs.2011.12.006]). The Society for Vascular Surgeons has also been on the forefront of this issue, urging CMS to maintain appropriate coverage restrictions.
But, as MEDCAC heard during this meeting, the proponents of expanded coverage remain staunch.. "CMS continues to restrict access to CAS to about 10% of patients" with significant carotid stenosis, said Dr. William A. Gray, director of endovascular services at Columbia University in New York, speaking at ISET 2012 in January. "Expanded CAS coverage would allow greater patient access and device development, and therefore create a safer option for patients." Dr. Gray presented his positive views on CAS to MEDCAC in January as well.
At press time, CMS had not announced whether or not the January MEDCAC hearing will result in a change to its CAS coverage policy. But the votes cast by MEDCAC’s membership in January suggested that the issues surrounding management of carotid stenosis remain too unresolved to trigger immediate changes.
Dr. Lal, Dr. Timaran, and Dr. Veith said that they had no disclosures. Dr. Howard said that he has received grant support from Abbott. Dr. Hopkins said that he has financial relationships with Bard, Boston Scientific, Cordis, Abbott Vascular, Toshiba, W.L. Gore, Medtronic, Micrus, St. Jude, Access Closure, and other device companies. Dr. Brott said that he has been a consultant to Edwards Lifesciences. Dr. Matsumura said that he has received research grants from Abbott, Cook, Covidien, Endologix, and W.L. Gore. Dr. Gray said that he has had financial relationships with Boston Scientific, Coherex, Contego, Cordis, and a number of other device companies.
U.S. Medicare Leg Amputations Down From 2000 to 2008
CHICAGO – Leg and foot amputations for elderly U.S. patients with peripheral artery disease dropped by more than half during the period 2000-2008 for unknown reasons, based on a review of Medicare data.
During the 9-year period studied, the rate of lower-extremity amputations among U.S. Medicare beneficiaries fell from 9,650/100,000 beneficiaries with peripheral artery disease (PAD) to 4,274/100,000, Dr. W. Schuyler Jones and his associates reported in a poster at the meeting.
Their analysis of the 2000-2008 Medicare data also showed significant regional variations in lower-extremity amputation rates in PAD patients, with the highest rate in New England and the lowest rate in the East North Central region (Illinois, Indiana, Michigan, Ohio, and Wisconsin). Again, no clear explanation exists for this pattern, said Dr. Jones, an interventional cardiologist at Duke University in Durham, N.C., and his associates.
Their analysis of data from the Centers for Medicare & Medicaid Services included 3,354,264 diagnosed with PAD during 2000-2008, of whom 249,310 (7%) underwent amputation of a foot or leg, either below or above the knee. The average age of all PAD patients was 77 years old among both those who underwent amputations and those who did not have this surgery. The analysis identified four factors that were significant, independent predictors of having an amputation: male sex, African American race, and the presence of renal disease or diabetes.
During the 9-year period, the annual incidence of patients newly diagnosed with PAD remained relatively steady, with 398,000 diagnosed in 2000 and 397,000 diagnosed in 2008. The rates showed some year-to-year fluctuation, ranging from a low of 304,000 in 2003 to a high of 517,000 in 2006.
In contrast, the annual incidence of lower-extremity amputations showed a clear downward trajectory, with a sharp drop starting in 2005. During 2000-2004, the annual amputation rate hovered at about 10,000 cases/100,000 patients with PAD, but then fell to 7,455/100,000 in 2005, and fell further to 4,261/100,000 in 2006. During the subsequent 2 years the rate remained at about the same low level first reached in 2006. Roughly similar patterns existed for the subgroups of patients who underwent above-the-knee amputations, below-the-knee amputations, or foot amputations.
The authors of the report performed an analysis of amputation rates by U.S. Census geographic regions for the entire period of 2000-2008 that they adjusted by regional differences in patients’ age, sex, race, comorbidities, and year of amputation. They set the amputation rate in the South Atlantic region as their reference level, and found four regions with significantly higher rates of amputations: New England ran 16% higher, the West South Central region (Arkansas, Louisiana, Oklahoma, and Texas) ran 12% higher, Mid Atlantic (Connecticut, New Jersey, New York, and Pennsylvania) was 8% higher, and the Pacific region (Alaska, California, Hawaii, Oregon, and Washington) was 3% above the reference level. Three other U.S. regions had amputations rates below the reference level, headed by the East North Central which ran 11% below the South Atlantic region, followed by the Mountain region at 10% lower, and the West North Central region (Iowa, Kansas, Minnesota, Missouri, Nebraska, North Dakota, and South Dakota) which was 5% below the reference level.
Dr. Jones said that he had no disclosures.
CHICAGO – Leg and foot amputations for elderly U.S. patients with peripheral artery disease dropped by more than half during the period 2000-2008 for unknown reasons, based on a review of Medicare data.
During the 9-year period studied, the rate of lower-extremity amputations among U.S. Medicare beneficiaries fell from 9,650/100,000 beneficiaries with peripheral artery disease (PAD) to 4,274/100,000, Dr. W. Schuyler Jones and his associates reported in a poster at the meeting.
Their analysis of the 2000-2008 Medicare data also showed significant regional variations in lower-extremity amputation rates in PAD patients, with the highest rate in New England and the lowest rate in the East North Central region (Illinois, Indiana, Michigan, Ohio, and Wisconsin). Again, no clear explanation exists for this pattern, said Dr. Jones, an interventional cardiologist at Duke University in Durham, N.C., and his associates.
Their analysis of data from the Centers for Medicare & Medicaid Services included 3,354,264 diagnosed with PAD during 2000-2008, of whom 249,310 (7%) underwent amputation of a foot or leg, either below or above the knee. The average age of all PAD patients was 77 years old among both those who underwent amputations and those who did not have this surgery. The analysis identified four factors that were significant, independent predictors of having an amputation: male sex, African American race, and the presence of renal disease or diabetes.
During the 9-year period, the annual incidence of patients newly diagnosed with PAD remained relatively steady, with 398,000 diagnosed in 2000 and 397,000 diagnosed in 2008. The rates showed some year-to-year fluctuation, ranging from a low of 304,000 in 2003 to a high of 517,000 in 2006.
In contrast, the annual incidence of lower-extremity amputations showed a clear downward trajectory, with a sharp drop starting in 2005. During 2000-2004, the annual amputation rate hovered at about 10,000 cases/100,000 patients with PAD, but then fell to 7,455/100,000 in 2005, and fell further to 4,261/100,000 in 2006. During the subsequent 2 years the rate remained at about the same low level first reached in 2006. Roughly similar patterns existed for the subgroups of patients who underwent above-the-knee amputations, below-the-knee amputations, or foot amputations.
The authors of the report performed an analysis of amputation rates by U.S. Census geographic regions for the entire period of 2000-2008 that they adjusted by regional differences in patients’ age, sex, race, comorbidities, and year of amputation. They set the amputation rate in the South Atlantic region as their reference level, and found four regions with significantly higher rates of amputations: New England ran 16% higher, the West South Central region (Arkansas, Louisiana, Oklahoma, and Texas) ran 12% higher, Mid Atlantic (Connecticut, New Jersey, New York, and Pennsylvania) was 8% higher, and the Pacific region (Alaska, California, Hawaii, Oregon, and Washington) was 3% above the reference level. Three other U.S. regions had amputations rates below the reference level, headed by the East North Central which ran 11% below the South Atlantic region, followed by the Mountain region at 10% lower, and the West North Central region (Iowa, Kansas, Minnesota, Missouri, Nebraska, North Dakota, and South Dakota) which was 5% below the reference level.
Dr. Jones said that he had no disclosures.
CHICAGO – Leg and foot amputations for elderly U.S. patients with peripheral artery disease dropped by more than half during the period 2000-2008 for unknown reasons, based on a review of Medicare data.
During the 9-year period studied, the rate of lower-extremity amputations among U.S. Medicare beneficiaries fell from 9,650/100,000 beneficiaries with peripheral artery disease (PAD) to 4,274/100,000, Dr. W. Schuyler Jones and his associates reported in a poster at the meeting.
Their analysis of the 2000-2008 Medicare data also showed significant regional variations in lower-extremity amputation rates in PAD patients, with the highest rate in New England and the lowest rate in the East North Central region (Illinois, Indiana, Michigan, Ohio, and Wisconsin). Again, no clear explanation exists for this pattern, said Dr. Jones, an interventional cardiologist at Duke University in Durham, N.C., and his associates.
Their analysis of data from the Centers for Medicare & Medicaid Services included 3,354,264 diagnosed with PAD during 2000-2008, of whom 249,310 (7%) underwent amputation of a foot or leg, either below or above the knee. The average age of all PAD patients was 77 years old among both those who underwent amputations and those who did not have this surgery. The analysis identified four factors that were significant, independent predictors of having an amputation: male sex, African American race, and the presence of renal disease or diabetes.
During the 9-year period, the annual incidence of patients newly diagnosed with PAD remained relatively steady, with 398,000 diagnosed in 2000 and 397,000 diagnosed in 2008. The rates showed some year-to-year fluctuation, ranging from a low of 304,000 in 2003 to a high of 517,000 in 2006.
In contrast, the annual incidence of lower-extremity amputations showed a clear downward trajectory, with a sharp drop starting in 2005. During 2000-2004, the annual amputation rate hovered at about 10,000 cases/100,000 patients with PAD, but then fell to 7,455/100,000 in 2005, and fell further to 4,261/100,000 in 2006. During the subsequent 2 years the rate remained at about the same low level first reached in 2006. Roughly similar patterns existed for the subgroups of patients who underwent above-the-knee amputations, below-the-knee amputations, or foot amputations.
The authors of the report performed an analysis of amputation rates by U.S. Census geographic regions for the entire period of 2000-2008 that they adjusted by regional differences in patients’ age, sex, race, comorbidities, and year of amputation. They set the amputation rate in the South Atlantic region as their reference level, and found four regions with significantly higher rates of amputations: New England ran 16% higher, the West South Central region (Arkansas, Louisiana, Oklahoma, and Texas) ran 12% higher, Mid Atlantic (Connecticut, New Jersey, New York, and Pennsylvania) was 8% higher, and the Pacific region (Alaska, California, Hawaii, Oregon, and Washington) was 3% above the reference level. Three other U.S. regions had amputations rates below the reference level, headed by the East North Central which ran 11% below the South Atlantic region, followed by the Mountain region at 10% lower, and the West North Central region (Iowa, Kansas, Minnesota, Missouri, Nebraska, North Dakota, and South Dakota) which was 5% below the reference level.
Dr. Jones said that he had no disclosures.
FROM THE ANNUAL MEETING OF THE AMERICAN COLLEGE OF CARDIOLOGY
Major Finding: Lower-extremity amputations among Medicare beneficiaries with PAD dropped from 9,650/100,000 in 2000 to 4,274/100,000 in 2008.
Data Source: Data came from an analysis of U.S. Medicare beneficiaries during 2000-2008 using information compiled by the Centers for Medicare & Medicaid Services.
Disclosures: Dr. Jones said that he had no disclosures.
Metformin Resolves Amenorrhea in Antipsychotic-Treated Schizophrenia
PHILADELPHIA – Treatment with metformin led to striking resolution of amenorrhea in women with schizophrenia treated with an antipsychotic drug, in a randomized, controlled study with 84 patients.
Six months of treatment with a standard metformin dosage of 500 mg b.i.d. also led to significant weight loss, lower body mass index, and reduced insulin levels and insulin resistance. It also normalized levels of prolactin and luteinizing hormone, Dr. Ren-Rong Wu said at the annual meeting of the American Psychiatric Association.
Amenorrhea completely resolved in 28 (67%) of 42 women randomized to receive the metformin regimen after 3 months of treatment, compared with resolution in 2 (5%) of 42 women in the placebo arm, a statistically significant difference, reported Dr. Wu, a psychiatrist at the Mental Health Institute of the Second Xiangya Hospital of Central South University in Changsha, China.
"Metformin provides a new option for managing amenorrhea and weight gain" in women with schizophrenia on antipsychotic treatment, she said. The metformin regimen was safe, with an adverse-effect profile similar to that of the placebo arm.
Results from a prior study by Dr. Wu and her associates had documented the efficacy and safety of metformin for producing weight loss in patients with schizophrenia and antipsychotic-induced weight gain (JAMA 2008;299:185-93).
Based on the new results, "it’s reasonable to use metformin to treat women who develop amenorrhea on antipsychotic treatment," commented Dr. Keming Gao, a psychiatrist and clinical director of the mood disorders program at University Hospital Case Medical Center in Cleveland. Although Dr. Wu and her associates maintained their patients on metformin for only 6 months in the reported study, in routine practice it would be reasonable to keep women who safely respond to metformin on the drug for as long as they continue on an antipsychotic regimen, Dr. Gao said in an interview.
Menstrual irregularity is a common side effect of antipsychotic treatment in women, with reports documenting an incidence of 26%-78% (Br. J. Psychiatry 2003;182:199-204). The mechanism of this effect appears to be multifactorial, with antipsychotic drugs causing weight gain and insulin resistance that triggers hyperprolactinemia; the drugs also cause the pituitary to produce elevated levels of luteinizing hormone, Dr. Wu said. Insulin resistance also may have direct effects on ovarian function, she added.
Her study enrolled 84 women with first-episode schizophrenia who had developed amenorrhea while taking clozapine, olanzapine, risperidone, or sulpiride. Patients randomized to the metformin arm started treatment with 500 mg once daily, and if after 3 days they tolerated the drug, their dosage increased to 500 mg b.i.d. Resolution of amenorrhea was the study’s primary end point.
After 6 months on treatment, women in the metformin arm lost an average 2.4 kg of weight, had an average 0.9 kg/m2 reduction in their body mass index, had an average 8.8 microIU/mL drop in their blood insulin level, and had an average 2.0 point reduction in their insulin resistance index. All these changes were statistically significant, compared with baseline levels. In contrast, women in the placebo arm had, on average, significant increases from baseline in weight and body mass index, and had no significant change in their insulin or insulin resistance.
Metformin treatment also produced an average 84.9-mcg/L cut in prolactin levels and a 3.3-IU/L drop in luteinizing hormone levels, both statistically significant changes from baseline, as well as a significant reduction in the ratio of luteinizing hormone to follicle-stimulating hormone. None of these measures changed significantly in women who received placebo.
A series of regression analyses by Dr. Wu and her associates indicated that reduction in body weight was the strongest predictor of restoration of menstrual function in metformin-treated patients, followed by reduction in prolactin level. Normalization of luteinizing hormone and reduction of insulin resistance index also appeared to play significant, independent roles in resolving amenorrhea, she said.
Dr. Wu said that she had no disclosures.
PHILADELPHIA – Treatment with metformin led to striking resolution of amenorrhea in women with schizophrenia treated with an antipsychotic drug, in a randomized, controlled study with 84 patients.
Six months of treatment with a standard metformin dosage of 500 mg b.i.d. also led to significant weight loss, lower body mass index, and reduced insulin levels and insulin resistance. It also normalized levels of prolactin and luteinizing hormone, Dr. Ren-Rong Wu said at the annual meeting of the American Psychiatric Association.
Amenorrhea completely resolved in 28 (67%) of 42 women randomized to receive the metformin regimen after 3 months of treatment, compared with resolution in 2 (5%) of 42 women in the placebo arm, a statistically significant difference, reported Dr. Wu, a psychiatrist at the Mental Health Institute of the Second Xiangya Hospital of Central South University in Changsha, China.
"Metformin provides a new option for managing amenorrhea and weight gain" in women with schizophrenia on antipsychotic treatment, she said. The metformin regimen was safe, with an adverse-effect profile similar to that of the placebo arm.
Results from a prior study by Dr. Wu and her associates had documented the efficacy and safety of metformin for producing weight loss in patients with schizophrenia and antipsychotic-induced weight gain (JAMA 2008;299:185-93).
Based on the new results, "it’s reasonable to use metformin to treat women who develop amenorrhea on antipsychotic treatment," commented Dr. Keming Gao, a psychiatrist and clinical director of the mood disorders program at University Hospital Case Medical Center in Cleveland. Although Dr. Wu and her associates maintained their patients on metformin for only 6 months in the reported study, in routine practice it would be reasonable to keep women who safely respond to metformin on the drug for as long as they continue on an antipsychotic regimen, Dr. Gao said in an interview.
Menstrual irregularity is a common side effect of antipsychotic treatment in women, with reports documenting an incidence of 26%-78% (Br. J. Psychiatry 2003;182:199-204). The mechanism of this effect appears to be multifactorial, with antipsychotic drugs causing weight gain and insulin resistance that triggers hyperprolactinemia; the drugs also cause the pituitary to produce elevated levels of luteinizing hormone, Dr. Wu said. Insulin resistance also may have direct effects on ovarian function, she added.
Her study enrolled 84 women with first-episode schizophrenia who had developed amenorrhea while taking clozapine, olanzapine, risperidone, or sulpiride. Patients randomized to the metformin arm started treatment with 500 mg once daily, and if after 3 days they tolerated the drug, their dosage increased to 500 mg b.i.d. Resolution of amenorrhea was the study’s primary end point.
After 6 months on treatment, women in the metformin arm lost an average 2.4 kg of weight, had an average 0.9 kg/m2 reduction in their body mass index, had an average 8.8 microIU/mL drop in their blood insulin level, and had an average 2.0 point reduction in their insulin resistance index. All these changes were statistically significant, compared with baseline levels. In contrast, women in the placebo arm had, on average, significant increases from baseline in weight and body mass index, and had no significant change in their insulin or insulin resistance.
Metformin treatment also produced an average 84.9-mcg/L cut in prolactin levels and a 3.3-IU/L drop in luteinizing hormone levels, both statistically significant changes from baseline, as well as a significant reduction in the ratio of luteinizing hormone to follicle-stimulating hormone. None of these measures changed significantly in women who received placebo.
A series of regression analyses by Dr. Wu and her associates indicated that reduction in body weight was the strongest predictor of restoration of menstrual function in metformin-treated patients, followed by reduction in prolactin level. Normalization of luteinizing hormone and reduction of insulin resistance index also appeared to play significant, independent roles in resolving amenorrhea, she said.
Dr. Wu said that she had no disclosures.
PHILADELPHIA – Treatment with metformin led to striking resolution of amenorrhea in women with schizophrenia treated with an antipsychotic drug, in a randomized, controlled study with 84 patients.
Six months of treatment with a standard metformin dosage of 500 mg b.i.d. also led to significant weight loss, lower body mass index, and reduced insulin levels and insulin resistance. It also normalized levels of prolactin and luteinizing hormone, Dr. Ren-Rong Wu said at the annual meeting of the American Psychiatric Association.
Amenorrhea completely resolved in 28 (67%) of 42 women randomized to receive the metformin regimen after 3 months of treatment, compared with resolution in 2 (5%) of 42 women in the placebo arm, a statistically significant difference, reported Dr. Wu, a psychiatrist at the Mental Health Institute of the Second Xiangya Hospital of Central South University in Changsha, China.
"Metformin provides a new option for managing amenorrhea and weight gain" in women with schizophrenia on antipsychotic treatment, she said. The metformin regimen was safe, with an adverse-effect profile similar to that of the placebo arm.
Results from a prior study by Dr. Wu and her associates had documented the efficacy and safety of metformin for producing weight loss in patients with schizophrenia and antipsychotic-induced weight gain (JAMA 2008;299:185-93).
Based on the new results, "it’s reasonable to use metformin to treat women who develop amenorrhea on antipsychotic treatment," commented Dr. Keming Gao, a psychiatrist and clinical director of the mood disorders program at University Hospital Case Medical Center in Cleveland. Although Dr. Wu and her associates maintained their patients on metformin for only 6 months in the reported study, in routine practice it would be reasonable to keep women who safely respond to metformin on the drug for as long as they continue on an antipsychotic regimen, Dr. Gao said in an interview.
Menstrual irregularity is a common side effect of antipsychotic treatment in women, with reports documenting an incidence of 26%-78% (Br. J. Psychiatry 2003;182:199-204). The mechanism of this effect appears to be multifactorial, with antipsychotic drugs causing weight gain and insulin resistance that triggers hyperprolactinemia; the drugs also cause the pituitary to produce elevated levels of luteinizing hormone, Dr. Wu said. Insulin resistance also may have direct effects on ovarian function, she added.
Her study enrolled 84 women with first-episode schizophrenia who had developed amenorrhea while taking clozapine, olanzapine, risperidone, or sulpiride. Patients randomized to the metformin arm started treatment with 500 mg once daily, and if after 3 days they tolerated the drug, their dosage increased to 500 mg b.i.d. Resolution of amenorrhea was the study’s primary end point.
After 6 months on treatment, women in the metformin arm lost an average 2.4 kg of weight, had an average 0.9 kg/m2 reduction in their body mass index, had an average 8.8 microIU/mL drop in their blood insulin level, and had an average 2.0 point reduction in their insulin resistance index. All these changes were statistically significant, compared with baseline levels. In contrast, women in the placebo arm had, on average, significant increases from baseline in weight and body mass index, and had no significant change in their insulin or insulin resistance.
Metformin treatment also produced an average 84.9-mcg/L cut in prolactin levels and a 3.3-IU/L drop in luteinizing hormone levels, both statistically significant changes from baseline, as well as a significant reduction in the ratio of luteinizing hormone to follicle-stimulating hormone. None of these measures changed significantly in women who received placebo.
A series of regression analyses by Dr. Wu and her associates indicated that reduction in body weight was the strongest predictor of restoration of menstrual function in metformin-treated patients, followed by reduction in prolactin level. Normalization of luteinizing hormone and reduction of insulin resistance index also appeared to play significant, independent roles in resolving amenorrhea, she said.
Dr. Wu said that she had no disclosures.
FROM THE ANNUAL MEETING OF THE AMERICAN PSYCHIATRIC ASSOCIATION
Major Finding: Metformin treatment resolved amenorrhea in 67% of women with schizophrenia on an antipsychotic, compared with 5% resolution in the placebo arm.
Data Source: Data came from a randomized, controlled study with 84 patients at a single center in China.
Disclosures: Dr. Wu said she had no disclosures.
Short Psychiatric Hospitalizations Linked to Higher Readmissions
PHILADELPHIA – Putting a uniform, brief cap on the number of days that psychiatric patients can remain hospitalized might have the unintended consequence of significantly boosting the rate of short-term hospital readmissions, according to results from a study of more than 12,000 patients at one U.S. hospital.
Psychiatric patients hospitalized for 4 days or fewer had a statistically significant 25% increased rate of readmission in the following 30 days in an analysis that adjusted for many possible demographic and clinical confounders, Dr. John W. Goethe said at the annual meeting of the American Psychiatric Association.
At a time when the Centers for Medicare and Medicaid Services (CMS) is poised to start penalizing hospitals with outlier short-term readmission rates, the finding highlights the complex constellation of factors that affect 30-day readmission rates. The finding also underscores the potent, rock-and-hard-place one-two punch that hospitals and clinicians will soon face when treating psychiatric patients: The pressure from payers to quickly move inpatients out the door and the penalty that CMS will levy if these patients wind up rehospitalized less than a month later.
"Readmission is a complex problem and an extremely crude proxy for the many things that can make a patient’s condition deteriorate," said Dr. Goethe, a psychiatrist and director of the center for research and education at Hartford (Conn.) Hospital.
"Readmission rates are clearly related to length of stay," and trying to assess quality of hospital care with the metric of readmission loses sight of factors contributing to readmissions that can be out of a hospital’s control, he said in an interview.
A length-of-stay cap fixed at something like 4 days "does not take into account a patient’s particular circumstances" and might mandate a hospitalization duration that is "inadequate to address what brought the patient into the hospital," he said. The important role that length of stay plays in readmissions should be "taken into account by policy makers who are planning financial penalties for hospitals with what [they consider] inappropriate readmission rates," Dr. Goethe said.
His study included 12,830 patients with a psychiatric diagnosis who were admitted to Hartford Hospital during January 2002-May 2008. The majority of patients, 71%, were 18-60 years old; 14% were age 17 or younger and 15% were age 61 or older. Patient records showed a length of stay of 4 days or fewer for 13% of pediatric patients, 33% of adults, and 9% of older patients. The rate of readmission within 30 days was 7% for pediatric patients, 10% for adults, and 9% among older patients.
The researchers then performed a series of proportional-hazard regression analyses that calculated the relationship between a 4-day-or-less length of stay and readmission rates. They adjusted for many baseline factors, including age, sex, race and ethnicity, type of psychiatric diagnosis, types of treatment received, and prior hospitalization history. The result showed that patients hospitalized for 5 days or more had a statistically significant 20% reduced rate of 30-day hospital readmission, compared with patients hospitalized for 4 days or fewer. Expressed conversely, patients hospitalized for 4 days or fewer had a 25% higher rate of 30-day readmission than those hospitalized for longer periods.
The analyses identified six other variables that also had a significant, independent effect on 30-day readmission rate: female sex, schizophrenia, and attention-deficit disorder linked with a reduced readmission rate, while benzodiazepine treatment, dementia, and conduct disorder all linked with an increased rate.
Results from a companion study identified other factors that significantly linked with 30-day readmission. Patients who previously had been hospitalized within 30 days, 90 days, or 2 years of the index hospitalization all faced a significantly increased risk for 30-day rehospitalization following the index admission. And patients treated with six or more psychotropic drugs had a significantly increased risk for 30-day readmission, compared with patients who received five or fewer psychotropic drugs.
"To our knowledge, this is the largest patient sample used to address this question" for psychiatric patients, Dr. Goethe said. He cautioned that the analysis did not take into account several other factors that could also affect readmission rates, such as severity of illness, socioeconomic status, social support, quality of life, and adherence to treatment.
Dr. Goethe said he had no disclosures, but his hospital’s research program has received grants from several drug companies.
PHILADELPHIA – Putting a uniform, brief cap on the number of days that psychiatric patients can remain hospitalized might have the unintended consequence of significantly boosting the rate of short-term hospital readmissions, according to results from a study of more than 12,000 patients at one U.S. hospital.
Psychiatric patients hospitalized for 4 days or fewer had a statistically significant 25% increased rate of readmission in the following 30 days in an analysis that adjusted for many possible demographic and clinical confounders, Dr. John W. Goethe said at the annual meeting of the American Psychiatric Association.
At a time when the Centers for Medicare and Medicaid Services (CMS) is poised to start penalizing hospitals with outlier short-term readmission rates, the finding highlights the complex constellation of factors that affect 30-day readmission rates. The finding also underscores the potent, rock-and-hard-place one-two punch that hospitals and clinicians will soon face when treating psychiatric patients: The pressure from payers to quickly move inpatients out the door and the penalty that CMS will levy if these patients wind up rehospitalized less than a month later.
"Readmission is a complex problem and an extremely crude proxy for the many things that can make a patient’s condition deteriorate," said Dr. Goethe, a psychiatrist and director of the center for research and education at Hartford (Conn.) Hospital.
"Readmission rates are clearly related to length of stay," and trying to assess quality of hospital care with the metric of readmission loses sight of factors contributing to readmissions that can be out of a hospital’s control, he said in an interview.
A length-of-stay cap fixed at something like 4 days "does not take into account a patient’s particular circumstances" and might mandate a hospitalization duration that is "inadequate to address what brought the patient into the hospital," he said. The important role that length of stay plays in readmissions should be "taken into account by policy makers who are planning financial penalties for hospitals with what [they consider] inappropriate readmission rates," Dr. Goethe said.
His study included 12,830 patients with a psychiatric diagnosis who were admitted to Hartford Hospital during January 2002-May 2008. The majority of patients, 71%, were 18-60 years old; 14% were age 17 or younger and 15% were age 61 or older. Patient records showed a length of stay of 4 days or fewer for 13% of pediatric patients, 33% of adults, and 9% of older patients. The rate of readmission within 30 days was 7% for pediatric patients, 10% for adults, and 9% among older patients.
The researchers then performed a series of proportional-hazard regression analyses that calculated the relationship between a 4-day-or-less length of stay and readmission rates. They adjusted for many baseline factors, including age, sex, race and ethnicity, type of psychiatric diagnosis, types of treatment received, and prior hospitalization history. The result showed that patients hospitalized for 5 days or more had a statistically significant 20% reduced rate of 30-day hospital readmission, compared with patients hospitalized for 4 days or fewer. Expressed conversely, patients hospitalized for 4 days or fewer had a 25% higher rate of 30-day readmission than those hospitalized for longer periods.
The analyses identified six other variables that also had a significant, independent effect on 30-day readmission rate: female sex, schizophrenia, and attention-deficit disorder linked with a reduced readmission rate, while benzodiazepine treatment, dementia, and conduct disorder all linked with an increased rate.
Results from a companion study identified other factors that significantly linked with 30-day readmission. Patients who previously had been hospitalized within 30 days, 90 days, or 2 years of the index hospitalization all faced a significantly increased risk for 30-day rehospitalization following the index admission. And patients treated with six or more psychotropic drugs had a significantly increased risk for 30-day readmission, compared with patients who received five or fewer psychotropic drugs.
"To our knowledge, this is the largest patient sample used to address this question" for psychiatric patients, Dr. Goethe said. He cautioned that the analysis did not take into account several other factors that could also affect readmission rates, such as severity of illness, socioeconomic status, social support, quality of life, and adherence to treatment.
Dr. Goethe said he had no disclosures, but his hospital’s research program has received grants from several drug companies.
PHILADELPHIA – Putting a uniform, brief cap on the number of days that psychiatric patients can remain hospitalized might have the unintended consequence of significantly boosting the rate of short-term hospital readmissions, according to results from a study of more than 12,000 patients at one U.S. hospital.
Psychiatric patients hospitalized for 4 days or fewer had a statistically significant 25% increased rate of readmission in the following 30 days in an analysis that adjusted for many possible demographic and clinical confounders, Dr. John W. Goethe said at the annual meeting of the American Psychiatric Association.
At a time when the Centers for Medicare and Medicaid Services (CMS) is poised to start penalizing hospitals with outlier short-term readmission rates, the finding highlights the complex constellation of factors that affect 30-day readmission rates. The finding also underscores the potent, rock-and-hard-place one-two punch that hospitals and clinicians will soon face when treating psychiatric patients: The pressure from payers to quickly move inpatients out the door and the penalty that CMS will levy if these patients wind up rehospitalized less than a month later.
"Readmission is a complex problem and an extremely crude proxy for the many things that can make a patient’s condition deteriorate," said Dr. Goethe, a psychiatrist and director of the center for research and education at Hartford (Conn.) Hospital.
"Readmission rates are clearly related to length of stay," and trying to assess quality of hospital care with the metric of readmission loses sight of factors contributing to readmissions that can be out of a hospital’s control, he said in an interview.
A length-of-stay cap fixed at something like 4 days "does not take into account a patient’s particular circumstances" and might mandate a hospitalization duration that is "inadequate to address what brought the patient into the hospital," he said. The important role that length of stay plays in readmissions should be "taken into account by policy makers who are planning financial penalties for hospitals with what [they consider] inappropriate readmission rates," Dr. Goethe said.
His study included 12,830 patients with a psychiatric diagnosis who were admitted to Hartford Hospital during January 2002-May 2008. The majority of patients, 71%, were 18-60 years old; 14% were age 17 or younger and 15% were age 61 or older. Patient records showed a length of stay of 4 days or fewer for 13% of pediatric patients, 33% of adults, and 9% of older patients. The rate of readmission within 30 days was 7% for pediatric patients, 10% for adults, and 9% among older patients.
The researchers then performed a series of proportional-hazard regression analyses that calculated the relationship between a 4-day-or-less length of stay and readmission rates. They adjusted for many baseline factors, including age, sex, race and ethnicity, type of psychiatric diagnosis, types of treatment received, and prior hospitalization history. The result showed that patients hospitalized for 5 days or more had a statistically significant 20% reduced rate of 30-day hospital readmission, compared with patients hospitalized for 4 days or fewer. Expressed conversely, patients hospitalized for 4 days or fewer had a 25% higher rate of 30-day readmission than those hospitalized for longer periods.
The analyses identified six other variables that also had a significant, independent effect on 30-day readmission rate: female sex, schizophrenia, and attention-deficit disorder linked with a reduced readmission rate, while benzodiazepine treatment, dementia, and conduct disorder all linked with an increased rate.
Results from a companion study identified other factors that significantly linked with 30-day readmission. Patients who previously had been hospitalized within 30 days, 90 days, or 2 years of the index hospitalization all faced a significantly increased risk for 30-day rehospitalization following the index admission. And patients treated with six or more psychotropic drugs had a significantly increased risk for 30-day readmission, compared with patients who received five or fewer psychotropic drugs.
"To our knowledge, this is the largest patient sample used to address this question" for psychiatric patients, Dr. Goethe said. He cautioned that the analysis did not take into account several other factors that could also affect readmission rates, such as severity of illness, socioeconomic status, social support, quality of life, and adherence to treatment.
Dr. Goethe said he had no disclosures, but his hospital’s research program has received grants from several drug companies.
FROM THE ANNUAL MEETING OF THE AMERICAN PSYCHIATRIC ASSOCIATION
Major Finding: Psychiatric patients hospitalized for 5 or more days had a 20% reduced 30-day rehospitalization rate, compared with shorter hospitalizations.
Data Source: Data came from an analysis of 12,830 psychiatric patients hospitalized at one U.S. center during 2002-2008.
Disclosures: Dr. Goethe said he had no disclosures, but his hospital’s research program has received grants from several drug companies.
U.S. 2012 Suicides Projected to Cost $48 Billion
BALTIMORE – The 35,000 American suicides that occurred in 2007 as well as attempted suicides that year cost the United States a total of roughly $48 billion in direct and indirect expenses.
This economic impact highlights the potential financial benefit from allocating additional resources toward suicide prevention, Donald S. Shepard, Ph.D., said at the annual conference of the American Association of Suicidology.
"Investment in additional medical and counseling services would be relatively inexpensive and would likely reduce these national costs. It would pay for itself in economic terms, and it would also pay for itself many times over in human terms," said Dr. Shepard, a health economist and professor of health policy at Brandeis University in Waltham, Mass.
Dr. Shepard identified three primary opportunities for suicide prevention that he derived from the analysis:
• Strengthen the capacity of emergency departments to identify patients at risk.
• Strengthen the availability of community-based providers to treat patients at risk.
• Strengthen the linkage between acute care and community-based behavioral-health delivery systems to ensure continuity of care.
"The costs really make the case for an ounce of prevention," said Jerry Reed, Ph.D., director of the Center for the Study and Prevention of Injury, Violence ,and Suicide at the Education Development Center in Waltham, and a collaborator on the study. "If we can connect people with resources in the community and have the resources resolve the burden, we can save what these people contribute to the economic prosperity of the nation," Dr. Reed said. "Further investment in [suicide] prevention would be very productive."
To make their cost estimate, Dr. Shepard, Dr. Reed, and their associates began with the 34,598 U.S. suicides in 2007, the most recent year with complete data available at the time they began their analysis, as well as nonfatal attempted suicides that year. They identified the total direct medical costs for the fatal and nonfatal self-inflicted injuries as $1.3 billion, calculated in 2012 dollars and adjusted to 2012 medical costs. They then added to this their calculated $46.3 billion in indirect costs, primarily from the fatal suicide attempts, a figure that primarily derives from lost future productivity, especially lost wages. The total cost added up to $47.6 billion projected for this year, with 83% accounted for by men and 17% by women.
This estimate is conservative, because the number of completed suicides and nonfatal attempts has risen since 2007, Dr. Shepard said. A manuscript based on this material has been submitted for publication, he added.
Dr. Shepard and Dr. Reed said they had no relevant relevant financial disclosures.
BALTIMORE – The 35,000 American suicides that occurred in 2007 as well as attempted suicides that year cost the United States a total of roughly $48 billion in direct and indirect expenses.
This economic impact highlights the potential financial benefit from allocating additional resources toward suicide prevention, Donald S. Shepard, Ph.D., said at the annual conference of the American Association of Suicidology.
"Investment in additional medical and counseling services would be relatively inexpensive and would likely reduce these national costs. It would pay for itself in economic terms, and it would also pay for itself many times over in human terms," said Dr. Shepard, a health economist and professor of health policy at Brandeis University in Waltham, Mass.
Dr. Shepard identified three primary opportunities for suicide prevention that he derived from the analysis:
• Strengthen the capacity of emergency departments to identify patients at risk.
• Strengthen the availability of community-based providers to treat patients at risk.
• Strengthen the linkage between acute care and community-based behavioral-health delivery systems to ensure continuity of care.
"The costs really make the case for an ounce of prevention," said Jerry Reed, Ph.D., director of the Center for the Study and Prevention of Injury, Violence ,and Suicide at the Education Development Center in Waltham, and a collaborator on the study. "If we can connect people with resources in the community and have the resources resolve the burden, we can save what these people contribute to the economic prosperity of the nation," Dr. Reed said. "Further investment in [suicide] prevention would be very productive."
To make their cost estimate, Dr. Shepard, Dr. Reed, and their associates began with the 34,598 U.S. suicides in 2007, the most recent year with complete data available at the time they began their analysis, as well as nonfatal attempted suicides that year. They identified the total direct medical costs for the fatal and nonfatal self-inflicted injuries as $1.3 billion, calculated in 2012 dollars and adjusted to 2012 medical costs. They then added to this their calculated $46.3 billion in indirect costs, primarily from the fatal suicide attempts, a figure that primarily derives from lost future productivity, especially lost wages. The total cost added up to $47.6 billion projected for this year, with 83% accounted for by men and 17% by women.
This estimate is conservative, because the number of completed suicides and nonfatal attempts has risen since 2007, Dr. Shepard said. A manuscript based on this material has been submitted for publication, he added.
Dr. Shepard and Dr. Reed said they had no relevant relevant financial disclosures.
BALTIMORE – The 35,000 American suicides that occurred in 2007 as well as attempted suicides that year cost the United States a total of roughly $48 billion in direct and indirect expenses.
This economic impact highlights the potential financial benefit from allocating additional resources toward suicide prevention, Donald S. Shepard, Ph.D., said at the annual conference of the American Association of Suicidology.
"Investment in additional medical and counseling services would be relatively inexpensive and would likely reduce these national costs. It would pay for itself in economic terms, and it would also pay for itself many times over in human terms," said Dr. Shepard, a health economist and professor of health policy at Brandeis University in Waltham, Mass.
Dr. Shepard identified three primary opportunities for suicide prevention that he derived from the analysis:
• Strengthen the capacity of emergency departments to identify patients at risk.
• Strengthen the availability of community-based providers to treat patients at risk.
• Strengthen the linkage between acute care and community-based behavioral-health delivery systems to ensure continuity of care.
"The costs really make the case for an ounce of prevention," said Jerry Reed, Ph.D., director of the Center for the Study and Prevention of Injury, Violence ,and Suicide at the Education Development Center in Waltham, and a collaborator on the study. "If we can connect people with resources in the community and have the resources resolve the burden, we can save what these people contribute to the economic prosperity of the nation," Dr. Reed said. "Further investment in [suicide] prevention would be very productive."
To make their cost estimate, Dr. Shepard, Dr. Reed, and their associates began with the 34,598 U.S. suicides in 2007, the most recent year with complete data available at the time they began their analysis, as well as nonfatal attempted suicides that year. They identified the total direct medical costs for the fatal and nonfatal self-inflicted injuries as $1.3 billion, calculated in 2012 dollars and adjusted to 2012 medical costs. They then added to this their calculated $46.3 billion in indirect costs, primarily from the fatal suicide attempts, a figure that primarily derives from lost future productivity, especially lost wages. The total cost added up to $47.6 billion projected for this year, with 83% accounted for by men and 17% by women.
This estimate is conservative, because the number of completed suicides and nonfatal attempts has risen since 2007, Dr. Shepard said. A manuscript based on this material has been submitted for publication, he added.
Dr. Shepard and Dr. Reed said they had no relevant relevant financial disclosures.
FROM THE ANNUAL CONFERENCE OF THE AMERICAN ASSOCIATION OF SUICIDOLOGY
Major Finding: A projection pegged the direct and indirect costs of 2012 U.S. suicides at a total of $47.6 billion.
Data Source: Data came from projections and estimates based on the 2007 U.S. rates of attempted and completed suicides.
Disclosures: Dr. Shepard and Dr. Reed said they had no relevant financial disclosures.
Cognitive-Behavioral Therapy Dropped Suicidal Ideation Rate
BALTIMORE – A cognitive-behavioral intervention showed promise for reducing suicidal ideation among substance users in a pilot, controlled study with 46 patients.
"Preliminary evidence supports the short-term efficacy of CBT [cognitive-behavioral therapy] for suicidal substance users, but more comprehensive data are needed," Mark A. Ilgen, Ph.D., said at the annual conference of the American Association of Suicidology.
The study’s main finding was that among 22 patients assessed 1 month after the end of a 4-week CBT intervention, 3 participants (14%) said they had recent suicidal ideation, compared with 10 of 24 (42%) people from the control arm of the study, a statistically significant difference, said Dr. Ilgen, a psychologist at the University of Michigan in Ann Arbor.
Suicidal ideation and attempts among substance users receive little attention in "standard" addiction-treatment programs. But attention in this area is needed because "substance use disorders are strong risk factors for suicide attempts," he said. Results from prior studies of people receiving substance use treatment showed that 45% reported making at least one suicide attempt, and a third reported suicidal ideation within the prior 2 weeks.
The current study by Dr. Ilgen and his associates began with 56 people enrolled in a residential addiction-treatment program in Waterford, Mich.; many of the residents lived at the center in lieu of going to jail, he noted. The participants in Dr. Ilgen’s study all had a history of at least one suicide attempt and current suicidal ideation. The researchers randomized 29 people into the CBT intervention and 27 into a control education-support program. The intervention consisted of eight sessions, done twice a week for 4 weeks.
The average age of the participants was 32, and they closely split between women and men. About four-fifths were white, 71% had used more than one substance, and 70% had a history of at least two suicide attempts.
Dr. Ilgen modeled the CBT intervention on CBT programs used for depression and substance use that have a focus on avoiding and managing suicidal crises. The program began with a suicide-risk assessment; subsequent sessions included the development of a narrative of past suicide attempts; advice on safety plans, coping skills, and problem solving; identifying reasons for living; and guidance on social support and relapse prevention. Patients in the CBT program attended an average of six of the eight scheduled sessions, while those in the control group attended an average of seven sessions.
Forty-six of the 56 enrolled participants returned for a follow-up assessment 1 month after the end of treatment, and 36 returned for a second assessment 3 months following treatment. At the first follow-up, participants in the CBT program had a significantly reduced prevalence of suicidal ideation, compared with the controls. The CBT participants also had statistically significant reductions in several other measurements of suicidality at 1-month follow-up. A wish to die sentiment was expressed by 8 of the 22 assessed (38%) in the CBT group, vs. 14 of the 24 (58%) controls. The average number of depressive symptoms was 14 in the CBT group and 18.8 in the controls. And the average level of suicide self-efficacy was 133 in the CBT patients, significantly higher than the average of 106 among the controls.
In contrast, the researchers found no statistically significant differences between the two treatment groups at the 3-month follow-up, possibly in part explained by the reduced number of study participants who returned for the second assessment, Dr. Ilgen said.
He said he had no relevant financial disclosures.
BALTIMORE – A cognitive-behavioral intervention showed promise for reducing suicidal ideation among substance users in a pilot, controlled study with 46 patients.
"Preliminary evidence supports the short-term efficacy of CBT [cognitive-behavioral therapy] for suicidal substance users, but more comprehensive data are needed," Mark A. Ilgen, Ph.D., said at the annual conference of the American Association of Suicidology.
The study’s main finding was that among 22 patients assessed 1 month after the end of a 4-week CBT intervention, 3 participants (14%) said they had recent suicidal ideation, compared with 10 of 24 (42%) people from the control arm of the study, a statistically significant difference, said Dr. Ilgen, a psychologist at the University of Michigan in Ann Arbor.
Suicidal ideation and attempts among substance users receive little attention in "standard" addiction-treatment programs. But attention in this area is needed because "substance use disorders are strong risk factors for suicide attempts," he said. Results from prior studies of people receiving substance use treatment showed that 45% reported making at least one suicide attempt, and a third reported suicidal ideation within the prior 2 weeks.
The current study by Dr. Ilgen and his associates began with 56 people enrolled in a residential addiction-treatment program in Waterford, Mich.; many of the residents lived at the center in lieu of going to jail, he noted. The participants in Dr. Ilgen’s study all had a history of at least one suicide attempt and current suicidal ideation. The researchers randomized 29 people into the CBT intervention and 27 into a control education-support program. The intervention consisted of eight sessions, done twice a week for 4 weeks.
The average age of the participants was 32, and they closely split between women and men. About four-fifths were white, 71% had used more than one substance, and 70% had a history of at least two suicide attempts.
Dr. Ilgen modeled the CBT intervention on CBT programs used for depression and substance use that have a focus on avoiding and managing suicidal crises. The program began with a suicide-risk assessment; subsequent sessions included the development of a narrative of past suicide attempts; advice on safety plans, coping skills, and problem solving; identifying reasons for living; and guidance on social support and relapse prevention. Patients in the CBT program attended an average of six of the eight scheduled sessions, while those in the control group attended an average of seven sessions.
Forty-six of the 56 enrolled participants returned for a follow-up assessment 1 month after the end of treatment, and 36 returned for a second assessment 3 months following treatment. At the first follow-up, participants in the CBT program had a significantly reduced prevalence of suicidal ideation, compared with the controls. The CBT participants also had statistically significant reductions in several other measurements of suicidality at 1-month follow-up. A wish to die sentiment was expressed by 8 of the 22 assessed (38%) in the CBT group, vs. 14 of the 24 (58%) controls. The average number of depressive symptoms was 14 in the CBT group and 18.8 in the controls. And the average level of suicide self-efficacy was 133 in the CBT patients, significantly higher than the average of 106 among the controls.
In contrast, the researchers found no statistically significant differences between the two treatment groups at the 3-month follow-up, possibly in part explained by the reduced number of study participants who returned for the second assessment, Dr. Ilgen said.
He said he had no relevant financial disclosures.
BALTIMORE – A cognitive-behavioral intervention showed promise for reducing suicidal ideation among substance users in a pilot, controlled study with 46 patients.
"Preliminary evidence supports the short-term efficacy of CBT [cognitive-behavioral therapy] for suicidal substance users, but more comprehensive data are needed," Mark A. Ilgen, Ph.D., said at the annual conference of the American Association of Suicidology.
The study’s main finding was that among 22 patients assessed 1 month after the end of a 4-week CBT intervention, 3 participants (14%) said they had recent suicidal ideation, compared with 10 of 24 (42%) people from the control arm of the study, a statistically significant difference, said Dr. Ilgen, a psychologist at the University of Michigan in Ann Arbor.
Suicidal ideation and attempts among substance users receive little attention in "standard" addiction-treatment programs. But attention in this area is needed because "substance use disorders are strong risk factors for suicide attempts," he said. Results from prior studies of people receiving substance use treatment showed that 45% reported making at least one suicide attempt, and a third reported suicidal ideation within the prior 2 weeks.
The current study by Dr. Ilgen and his associates began with 56 people enrolled in a residential addiction-treatment program in Waterford, Mich.; many of the residents lived at the center in lieu of going to jail, he noted. The participants in Dr. Ilgen’s study all had a history of at least one suicide attempt and current suicidal ideation. The researchers randomized 29 people into the CBT intervention and 27 into a control education-support program. The intervention consisted of eight sessions, done twice a week for 4 weeks.
The average age of the participants was 32, and they closely split between women and men. About four-fifths were white, 71% had used more than one substance, and 70% had a history of at least two suicide attempts.
Dr. Ilgen modeled the CBT intervention on CBT programs used for depression and substance use that have a focus on avoiding and managing suicidal crises. The program began with a suicide-risk assessment; subsequent sessions included the development of a narrative of past suicide attempts; advice on safety plans, coping skills, and problem solving; identifying reasons for living; and guidance on social support and relapse prevention. Patients in the CBT program attended an average of six of the eight scheduled sessions, while those in the control group attended an average of seven sessions.
Forty-six of the 56 enrolled participants returned for a follow-up assessment 1 month after the end of treatment, and 36 returned for a second assessment 3 months following treatment. At the first follow-up, participants in the CBT program had a significantly reduced prevalence of suicidal ideation, compared with the controls. The CBT participants also had statistically significant reductions in several other measurements of suicidality at 1-month follow-up. A wish to die sentiment was expressed by 8 of the 22 assessed (38%) in the CBT group, vs. 14 of the 24 (58%) controls. The average number of depressive symptoms was 14 in the CBT group and 18.8 in the controls. And the average level of suicide self-efficacy was 133 in the CBT patients, significantly higher than the average of 106 among the controls.
In contrast, the researchers found no statistically significant differences between the two treatment groups at the 3-month follow-up, possibly in part explained by the reduced number of study participants who returned for the second assessment, Dr. Ilgen said.
He said he had no relevant financial disclosures.
FROM THE ANNUAL CONFERENCE OF THE AMERICAN ASSOCIATION OF SUICIDOLOGY
Major Finding: One month after eight sessions of cognitive-behavioral therapy, 14% of patients had suicidal ideation, compared with 42% of controls.
Data Source: Data came from a randomized, controlled study with 46 patients in a U.S. residential addiction-treatment program.
Disclosures: Dr. Ilgen said he had no relevant financial disclosures.
Adolescent Bullies Show Heightened Suicidality
BALTIMORE – Acutely suicidal adolescents who also were bullies had a heightened prevalence of suicidal ideation, substance use, and functional impairment, compared with similar suicidal adolescents who were not bullies, a review of more than 400 American teenagers has shown.
Results from a post hoc analysis of a suicide intervention trial also showed that the bullying behaviors in most study participants resolved over the course of 1 year.
"These findings highlight the importance of specifically assessing for and targeting bullying behavior when treating suicidal adolescents," Cheryl A. King, Ph.D., reported in a poster at the meeting. "The coinciding improvement in mental health suggests that targeting bullying perpetrators for treatment could improve outcomes for both the bullies as well as potential victims," said Dr. King, professor of psychology and director of the institute for human adjustment at the University of Michigan in Ann Arbor.
The post hoc analysis she and her associates ran included 433 of the 448 hospitalized, suicidal adolescents, aged 13-17, who participated in the Youth-Nominated Support Team–Version II intervention study (J. Consulting Clin. Psychology 2009;77:880-93). The investigators randomized suicidal teenagers in a psychiatric hospital to receive either usual care or usual care plus the Youth-Nominated Support Team–Version II intervention, a 3-month treatment based on social support and healthy behavior models. Results from the primary analysis showed very limited positive effects from this intervention, with no effect on suicide attempts and no enduring effect on suicidal ideation scores.
"These findings highlight the importance of specifically assessing for and targeting bullying behavior when treating suicidal adolescents."
For the post hoc analysis, the researchers evaluated 433 of the participating adolescents at baseline on a bullying scale that rated each participant by six criteria: teasing, physical attacks, meanness to others, destroying belongings of others, threats to hurt others, and getting into many fights. Fifty-four (12%) of the adolescents included in this analysis met the assessment’s threshold for being bullies.
The bully subgroup had significantly higher levels of suicidal ideation, substance use, and functional impairment, compared with the other teens in the study who did not meet the bully criteria.
One year after their first assessment, 4 of the original 54 teens who met the bullying criteria continued to have scores that classified them as bullies. In addition, 12 other adolescents who initially had not met the bullying criteria did so when they underwent reassessment at 12 months. Most of those initially identified as bullies showed improvements in their suicidal ideation and substance use during the following 12 months. However, they continued to have greater functional impairment than the nonbullies at 12 months.
The total group of 16 adolescents who met the bullying criteria at 1 year again showed higher levels of suicidal ideation and other suicide risk factors, compared with the nonbullies.
The factors that shape the fluctuating trajectories of bullying in this study population might help identify targets for intervention that could decrease bullying and suicidal behaviors among adolescents, Dr. King and her associates concluded.
Dr. King said she had no relevant financial disclosures.
BALTIMORE – Acutely suicidal adolescents who also were bullies had a heightened prevalence of suicidal ideation, substance use, and functional impairment, compared with similar suicidal adolescents who were not bullies, a review of more than 400 American teenagers has shown.
Results from a post hoc analysis of a suicide intervention trial also showed that the bullying behaviors in most study participants resolved over the course of 1 year.
"These findings highlight the importance of specifically assessing for and targeting bullying behavior when treating suicidal adolescents," Cheryl A. King, Ph.D., reported in a poster at the meeting. "The coinciding improvement in mental health suggests that targeting bullying perpetrators for treatment could improve outcomes for both the bullies as well as potential victims," said Dr. King, professor of psychology and director of the institute for human adjustment at the University of Michigan in Ann Arbor.
The post hoc analysis she and her associates ran included 433 of the 448 hospitalized, suicidal adolescents, aged 13-17, who participated in the Youth-Nominated Support Team–Version II intervention study (J. Consulting Clin. Psychology 2009;77:880-93). The investigators randomized suicidal teenagers in a psychiatric hospital to receive either usual care or usual care plus the Youth-Nominated Support Team–Version II intervention, a 3-month treatment based on social support and healthy behavior models. Results from the primary analysis showed very limited positive effects from this intervention, with no effect on suicide attempts and no enduring effect on suicidal ideation scores.
"These findings highlight the importance of specifically assessing for and targeting bullying behavior when treating suicidal adolescents."
For the post hoc analysis, the researchers evaluated 433 of the participating adolescents at baseline on a bullying scale that rated each participant by six criteria: teasing, physical attacks, meanness to others, destroying belongings of others, threats to hurt others, and getting into many fights. Fifty-four (12%) of the adolescents included in this analysis met the assessment’s threshold for being bullies.
The bully subgroup had significantly higher levels of suicidal ideation, substance use, and functional impairment, compared with the other teens in the study who did not meet the bully criteria.
One year after their first assessment, 4 of the original 54 teens who met the bullying criteria continued to have scores that classified them as bullies. In addition, 12 other adolescents who initially had not met the bullying criteria did so when they underwent reassessment at 12 months. Most of those initially identified as bullies showed improvements in their suicidal ideation and substance use during the following 12 months. However, they continued to have greater functional impairment than the nonbullies at 12 months.
The total group of 16 adolescents who met the bullying criteria at 1 year again showed higher levels of suicidal ideation and other suicide risk factors, compared with the nonbullies.
The factors that shape the fluctuating trajectories of bullying in this study population might help identify targets for intervention that could decrease bullying and suicidal behaviors among adolescents, Dr. King and her associates concluded.
Dr. King said she had no relevant financial disclosures.
BALTIMORE – Acutely suicidal adolescents who also were bullies had a heightened prevalence of suicidal ideation, substance use, and functional impairment, compared with similar suicidal adolescents who were not bullies, a review of more than 400 American teenagers has shown.
Results from a post hoc analysis of a suicide intervention trial also showed that the bullying behaviors in most study participants resolved over the course of 1 year.
"These findings highlight the importance of specifically assessing for and targeting bullying behavior when treating suicidal adolescents," Cheryl A. King, Ph.D., reported in a poster at the meeting. "The coinciding improvement in mental health suggests that targeting bullying perpetrators for treatment could improve outcomes for both the bullies as well as potential victims," said Dr. King, professor of psychology and director of the institute for human adjustment at the University of Michigan in Ann Arbor.
The post hoc analysis she and her associates ran included 433 of the 448 hospitalized, suicidal adolescents, aged 13-17, who participated in the Youth-Nominated Support Team–Version II intervention study (J. Consulting Clin. Psychology 2009;77:880-93). The investigators randomized suicidal teenagers in a psychiatric hospital to receive either usual care or usual care plus the Youth-Nominated Support Team–Version II intervention, a 3-month treatment based on social support and healthy behavior models. Results from the primary analysis showed very limited positive effects from this intervention, with no effect on suicide attempts and no enduring effect on suicidal ideation scores.
"These findings highlight the importance of specifically assessing for and targeting bullying behavior when treating suicidal adolescents."
For the post hoc analysis, the researchers evaluated 433 of the participating adolescents at baseline on a bullying scale that rated each participant by six criteria: teasing, physical attacks, meanness to others, destroying belongings of others, threats to hurt others, and getting into many fights. Fifty-four (12%) of the adolescents included in this analysis met the assessment’s threshold for being bullies.
The bully subgroup had significantly higher levels of suicidal ideation, substance use, and functional impairment, compared with the other teens in the study who did not meet the bully criteria.
One year after their first assessment, 4 of the original 54 teens who met the bullying criteria continued to have scores that classified them as bullies. In addition, 12 other adolescents who initially had not met the bullying criteria did so when they underwent reassessment at 12 months. Most of those initially identified as bullies showed improvements in their suicidal ideation and substance use during the following 12 months. However, they continued to have greater functional impairment than the nonbullies at 12 months.
The total group of 16 adolescents who met the bullying criteria at 1 year again showed higher levels of suicidal ideation and other suicide risk factors, compared with the nonbullies.
The factors that shape the fluctuating trajectories of bullying in this study population might help identify targets for intervention that could decrease bullying and suicidal behaviors among adolescents, Dr. King and her associates concluded.
Dr. King said she had no relevant financial disclosures.
FROM THE ANNUAL CONFERENCE OF THE AMERICAN ASSOCIATION OF SUICIDOLOGY
Major Finding: Sixteen adolescents who met bully criteria at 1 year showed higher levels of suicidal ideation and other suicide risk factors, compared with the nonbullies.
Data Source: The findings came from a post hoc analysis of 433 American adolescents enrolled in a suicide intervention study.
Disclosures: Dr. King said that she had no relevant financial disclosures.