User login
Mitchel is a reporter for MDedge based in the Philadelphia area. He started with the company in 1992, when it was International Medical News Group (IMNG), and has since covered a range of medical specialties. Mitchel trained as a virologist at Roswell Park Memorial Institute in Buffalo, and then worked briefly as a researcher at Boston Children's Hospital before pivoting to journalism as a AAAS Mass Media Fellow in 1980. His first reporting job was with Science Digest magazine, and from the mid-1980s to early-1990s he was a reporter with Medical World News. @mitchelzoler
Carotid Artery Interventions Break Three Ways
Optimal management of carotid stenosis to prevent death and strokes is a work in progress right now, with experts groping for the right balance between carotid endarterectomy, carotid artery stenting, and best medical therapy.
The field is bereft of both conclusive, up-to-date data and – more importantly – wide agreement on data interpretation that clearly tips treatment toward one of these options, so much so that in late January an expert panel organized by the Centers for Medicare and Medicaid Services found itself unable to support with high confidence the application of these treatments to any subgroup of carotid disease patients.
Amid this confusion are the following certainties:
- The use of carotid artery stenting (CAS) on U.S. patients grew substantially since its introduction in the late 1990s and since it began receiving limited Medicare coverage in 2004. Data collected through 2007 showed a greater-than-fourfold increase in CAS use among Medicare beneficiaries, compared with 1998 (Circ. Cardiovasc. Qual. Outcomes 2010;3:15-24).
- Other findings documented a shift in CAS use during the 2000s toward patients with asymptomatic carotid stenosis, with a recent estimate that 70%-90% of CAS patients now fall into that category (Arch. Int. Med. 2010;170:1225-7).
- The main results of the landmark CREST (Carotid Revascularization Endarterectomy vs. Stenting) trial, first reported 2 years ago (N. Engl. J. Med. 2010;363:11-23), appeared on the surface to show very similar safety and efficacy for CAS and carotid endarterectomy (CEA), but some experts caution that deeper drilling into the results show that this is an oversimplification of how the two treatments compare.
- And many experts agree that best medical therapy has improved in recent years, to the point where it deserves a new appraisal in a large trial that would compare it against carotid revascularization with CAS or CEA in asymptomatic patients. The leaders of CREST themselves have designed a new trial, CREST II, aimed at making this comparison, and have begun to vigorously lobby the National Institutes of Health to support this study.
In the meantime, vascular surgeons, endovascularists, cardiologists, and the other types of physicians who treat patients with carotid disease try as best they can to strike the right balance in how they apply the three management options.
CREST Provides New Guidance
One surgeon who has perhaps given the most thought to sorting out treatment options is Dr. Brajesh K. Lal, a co–principal investigator on CREST, a vascular surgeon at the University of Maryland in Baltimore, and a researcher who spent the past couple of years sorting through CREST’s voluminous data to find new clues to guide patient triage.
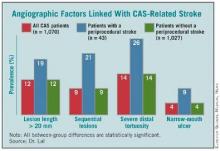
A major, recent guide for matching patients with a specific carotid intervention came from his painstaking analysis of carotid angiograms from 1,070 of the CREST patients who underwent CAS, of whom 43 patients (4%) then went on to have a stroke.
This effort identified the following four anatomical features that appear to mark patients with an increased rate of periprocedural stroke when they are treated with CAS:
- Stenotic lesion more than 20 mm long. The researchers measured lesion length as the distance from the proximal to distal shoulder of the lesion.
- Sequential lesions. This refers to two lesions separated by at least 10 mm of normal vessel.
- Severe distal tortuosity. This was defined as at least two arterial bends of at least 90 degrees that are distal to the lesion.
- Narrow-mouth ulcers. This refers to a small crater or lumen flap that produces a discrete area of contrast extension beyond the normal arterial lumen with a narrow inlet into the ulceration.
Patients with one or more of these carotid anatomical features "should be treated by endarterectomy," Dr. Lal said in an interview. "If I see any one of these in my clinical practice, I will not stent."
The variables assessed in this analysis did not include arterial calcification, as the angiographic imaging routinely collected in the study did not allow calcium quantification, said Dr. Lal. Among the stented patients in the study, the cumulative prevalence of the four significant risk markers was 39%. Patients with none of the four markers had a significantly reduced rate of periprocedural stroke during carotid stenting.
Endovascularists were already routinely assessing carotid anatomy before attempting CAS, but prior to Dr. Lal’s report on these new findings at the International Stroke Conference in New Orleans in February, "I’m not so sure that everyone has been using this [anatomical] information," he said. "When we talk about operator experience in stenting, if we don’t have the facts about what increases the stenting risk, then we won’t improve performance. You’d be surprised at what some interventionalists are willing to do," despite a patient’s challenging carotid anatomy. "These data show that you can work around a single 90-degree bend [of the distal carotid artery], but not around two bends. That is the kind of stuff we’re beginning to find out."
Dr. Lal and his associates plan to further analyze their data and derive a formula that physicians can apply to patients to estimate their periprocedural stroke risk.
Additional, recent CREST analyses that were also reported at the International Stroke Conference revealed other new, important lessons about CAS and CEA.
First, by 2 years after intervention, both CAS and CEA produced roughly comparable low rates of restenosis: a 6.0% rate in the CAS patients who underwent a prespecified, follow-up duplex ultrasound examination of their carotid artery, and 6.3% in the CEA patients (Stroke 2012;43: abstract A3). The rates were also similar regardless of whether patients were symptomatic before their carotid intervention, Dr. Lal reported.
This finding appears to lay to rest the concern about a major restenosis risk using bare-metal stents in carotid arteries, in contrast to what happens in coronary arteries, Dr. Lal said. "To my mind, these data are fairly definitive. We followed more than 2,000 patients, and every ultrasound was reviewed and adjudicated. I’m fairly comfortable with these results."
A third CREST analysis that was also reported in February showed that, after adjustment for baseline differences, patients had no significant changes in their rates of periprocedural events during and after CAS throughout the study, from 2000 to 2008 (Stroke 2012;43: abstract A1). "We hoped to show that CAS was getting better [during the 9 years of CREST], but we saw no evidence it got better with time," said George Howard, Dr.P.H., professor and chairman of the department of biostatistics at the University of Alabama at Birmingham. "It would make sense if CAS got better; it’s a new procedure that people learn." But the CREST results showed no evidence of a learning curve, perhaps because the 224 interventionalists who performed CAS in CREST went through a rigorous credentialing process and also performed their first several CAS procedures in CREST during a lead-in phase that was not included in the main outcomes.
Choosing Among the Options
Dr. Lal said that he draws several other lessons from the CREST results to guide his treatment decisions: Patients who are physiologically elderly, those with a large amount of calcified atheroma in their aortic arch, and patients with significant peripheral arterial disease that impedes endovascular access are all poor CAS candidates, he said, adding that he focuses on a patient’s physiological age rather than chronological age.
Patients who are well suited to CAS are those with a younger physiological age, those with a history of radiation treatment, and patients with restenotic carotid lesions.
Although CREST showed worse performance of CAS in women compared with men, "I don’t know why, so it’s hard for me to exclude or offer a particular treatment" based on the patient’s sex, he said. "I treat women just like men, and work through all the other things that I know about to help me decide."
Then there is a third subgroup, patients with carotid disease who he feels "benefit the most" from medical management only. This category primarily includes asymptomatic patients with moderate stenosis (that is, less than 80% carotid occlusion). His prescription for medical management includes 325 mg of aspirin daily, treatment with a statin, good blood pressure and blood sugar control, smoking cessation, and lifestyle modification. If, despite this, an asymptomatic patient has continued stenotic progression that advances beyond 80%, he will then recommend revascularization.
Dr. Lal said that in his practice today, roughly one-third of carotid-disease patients are on medical management, a third undergo CEA, and a third receive CAS. "We are at a stage of equipoise," for these three options, which is why he helped design and is working to get funding for CREST II.
Dr. Lal personally applies all three treatment options – CAS, CEA, or medical management. Having physicians involved in the decision-making process who are comfortable using each of these options is critical for making a balanced management decision, said Dr. L. Nelson Hopkins, another CREST collaborator and professor and chairman of neurosurgery at the State University of New York at Buffalo.
"The single most important thing we do is have a weekly, multidisciplinary conference to discuss each case, with input from everyone, to decide the best treatment for each [carotid disease] patient," he said when he spoke at ISET 2012, an International Symposium on Endovascular Therapy in Miami Beach in January.
"The most important factors" guiding the decision of whether or not to perform CAS are, does a symptomatic patient has "hot" lesions, what is the arterial tortuosity, and what is the calcification, he said. Other important features include whether the patient has a contralateral carotid occlusion, whether the carotid has a high bifurcation, and whether there are ostial or tandem lesions. "If the anatomy is favorable, CAS is fine even in a 95-year-old," Dr. Hopkins said.
What About Insurance Coverage?
Carotid specialists concede that another, nonmedical issue also plays a big role in deciding how to manage a patient: What will the patient’s health insurance cover?
In its most recent decision on the issue, effective in April 2010, the Centers for Medicare and Medicaid Services (CMS) said that Medicare covers CAS performed on a routine basis for patients with symptomatic carotid disease who are at high risk for CEA and have at least 70% carotid stenosis. CMS also said that Medicare will cover FDA-approved investigations of CAS in symptomatic patients who are at high risk for CEA and have at least 50% stenosis, as well as CAS investigations in asymptomatic patients at high risk for CEA with at least 80% carotid stenosis.
As a result, patients with other forms of carotid disease often do not have insurance coverage for CAS.
"The issue for us is primarily reimbursement. If we can do [a procedure] and be reimbursed, we often stent the patient," said Dr. Carlos H. Timaran, a vascular surgeon and chief of endovascular surgery at the University of Texas Southwestern Medical Center in Dallas.
"For my private practice patients, we assess their risk based on their [anatomical] and medical criteria. If they are high risk, we usually stent. We have a bias toward stenting because it is faster and easier. Some people say they can do endarterectomy in an hour, but I don’t believe that. I do CEA with residents and fellows, and it takes 2 hours. But with stenting, I can do it in 40 minutes, even with a fellow. If I have three patients with carotid disease to treat [and] if I do CEA on all three, it will take all day. If I do CAS, it will take the morning, or less," he said in an interview.
In addition, "CEA is a nice procedure, but you need anesthesia. CAS is easier, I can do it myself, and I don’t need anesthesia. I am in more control of the case," Dr. Timaran said.
For patients who are eligible for both CAS and CEA, "it also comes down to anatomy," he grants. But if the patient has anatomy favorable to either method, "I offer patients both, and they will probably go for a stent," because patients usually prefer the quicker recovery that stenting offers, compared with CEA.
An interesting footnote to the issue of how to best manage patients who are eligible for CAS under CMS rules is that the CREST results offer no direct guidance on the relative safety and efficacy of CAS and CEA in patients with Medicare coverage. That’s because "the CREST cohort was a conventional-risk cohort; most criteria that would make someone a high surgical risk would have been excluded from CREST," Dr. Thomas G. Brott, professor of neurology at the Mayo Clinic in Jacksonville, Fla., and lead investigator for CREST. "The high-surgical-risk patients who are currently covered by Medicare would not have even been enrolled in CREST," Dr. Brott said in an interview.
Where Does Medical Therapy Fit In?
Although Dr. Timaran sees the potential value of medical therapy only for asymptomatic patients, he also urges caution.
"I don’t think [the efficacy of medical treatment] has been proven, and until it’s proven I don’t think physicians should deny revascularization treatment to these patients," he said. "The standard of care for a patient with severe carotid stenosis is revascularization, even if the patient is asymptomatic, unless there is some other consideration." But, he admitted, "there is little downside to medical treatment in asymptomatic patients, even if they have severe stenosis." That’s because their stroke risk is low regardless of which option a patient chooses.
"I tell asymptomatic patients that ‘based on the data, your risk of stroke is 2% per year, and that risk may be even lower with best medical therapy.’ With a carotid intervention, we can lower their risk to 1% per year." In other words, the patient’s stroke risk is low regardless of which option is chosen. Plus, medical therapy only is the best choice for patients with special risk factors, such as radiation-induced stenosis, Dr. Timaran added.
Other experts see medical treatment alone as an excellent option for many carotid-stenosis patients.
"The majority of patients I see don’t get an intervention; we use medical treatment only," said Dr. Jon S. Matsumura in an interview. "I think that’s true for most vascular surgery practices, and in cardiology and neurology practices. A lot of these patients just need reassurance," said Dr. Matsumura, professor and chairman of vascular surgery at the University of Wisconsin in Madison.
"Most asymptomatic patients [with carotid stenosis] should be treated by best medical therapy, with very few – fewer than 5% – treated by CAS or CEA," said Dr. Frank J. Veith at the International Symposium on Endovascular Therapy (ISET 12) in January. He acknowledged that well-controlled trials done during the 1990s showed that CEA led to superior outcomes compared with medical therapy, but since the early 2000s, "best medical therapy to prevent strokes leapt forward," he said, especially with improved statin therapy. He cited findings from a 2010 prospective study that showed asymptomatic Canadian patients managed starting in 2003 with best medical therapy had an annual stroke rate of 0.7% (Arch. Neurol. 2010;67:180-6).
He singled out four small groups of asymptomatic patients who warrant revascularization: patients with CT or MRI evidence for a history of silent strokes, patients with very-high-grade carotid stenosis that leaves just 1% of the carotid lumen open, those with a contralateral occlusion, and patients who serially experience transient occlusions detected by transcranial Doppler ultrasound examination, said Dr. Veith, professor of surgery at New York University. Aside from this small fraction of patients with asymptomatic disease, performing interventions on anyone else will "cause more strokes than you’ll prevent," he warned in an interview.
What Will CMS Do?
Dr. Veith’s take on limiting CAS in asymptomatic patients received substantial support early this year in the days leading up to the Jan. 25 meeting of the Medicare Evidence Development and Coverage Advisory Committee (MEDCAC) that heard evidence and opinions for and against widening of Medicare coverage for CAS. In early January, Dr. Veith joined with 40 other vascular surgeons and stroke specialists to urge CMS not to broaden CAS coverage (Eur. J. Vasc. Endovasc. Surg. 2012 Jan. 5 [doi:10.1016/j.ejvs.2011.12.006]). But, as MEDCAC heard during this meeting, experts differ on this issue.
"CMS continues to restrict access to CAS to about 10% of patients" with significant carotid stenosis, said Dr. William A. Gray, director of endovascular services at Columbia University in New York, speaking at ISET 2012 in January. "Expanded CAS coverage would allow greater patient access and device development, and therefore create a safer option for patients." Dr. Gray presented his positive views on CAS to MEDCAC in January as well.
At press time, CMS had not announced whether or not the January MEDCAC hearing will result in a change to its CAS coverage policy. But the votes cast by MEDCAC’s membership in January suggested that the issues surrounding management of carotid stenosis remain too unresolved to trigger immediate changes.
Dr. Lal, Dr. Timaran, and Dr. Veith said that they had no disclosures. Dr. Howard said that he has received grant support from Abbott. Dr. Hopkins said that he has financial relationships with Bard, Boston Scientific, Cordis, Abbott Vascular, Toshiba, W.L. Gore, Medtronic, Micrus, St. Jude, Access Closure, Osteal, Vascular Dynamics, Square One, Valor Medical, Claret Medical, and Augmenix. Dr. Brott said that he has been a consultant to Edwards Lifesciences. Dr. Matsumura said that he has received research grants from Abbott, Cook, Covidien, Endologix, and W.L. Gore. Dr. Gray said that he has had financial relationships with Boston Scientific, Coherex, Contego, Cordis, Fiatlux 3D, Nexeon, Quantumcor, Abbott Vascular, and Biocardia.
Optimal management of carotid stenosis to prevent death and strokes is a work in progress right now, with experts groping for the right balance between carotid endarterectomy, carotid artery stenting, and best medical therapy.
The field is bereft of both conclusive, up-to-date data and – more importantly – wide agreement on data interpretation that clearly tips treatment toward one of these options, so much so that in late January an expert panel organized by the Centers for Medicare and Medicaid Services found itself unable to support with high confidence the application of these treatments to any subgroup of carotid disease patients.
Amid this confusion are the following certainties:
- The use of carotid artery stenting (CAS) on U.S. patients grew substantially since its introduction in the late 1990s and since it began receiving limited Medicare coverage in 2004. Data collected through 2007 showed a greater-than-fourfold increase in CAS use among Medicare beneficiaries, compared with 1998 (Circ. Cardiovasc. Qual. Outcomes 2010;3:15-24).
- Other findings documented a shift in CAS use during the 2000s toward patients with asymptomatic carotid stenosis, with a recent estimate that 70%-90% of CAS patients now fall into that category (Arch. Int. Med. 2010;170:1225-7).
- The main results of the landmark CREST (Carotid Revascularization Endarterectomy vs. Stenting) trial, first reported 2 years ago (N. Engl. J. Med. 2010;363:11-23), appeared on the surface to show very similar safety and efficacy for CAS and carotid endarterectomy (CEA), but some experts caution that deeper drilling into the results show that this is an oversimplification of how the two treatments compare.
- And many experts agree that best medical therapy has improved in recent years, to the point where it deserves a new appraisal in a large trial that would compare it against carotid revascularization with CAS or CEA in asymptomatic patients. The leaders of CREST themselves have designed a new trial, CREST II, aimed at making this comparison, and have begun to vigorously lobby the National Institutes of Health to support this study.
In the meantime, vascular surgeons, endovascularists, cardiologists, and the other types of physicians who treat patients with carotid disease try as best they can to strike the right balance in how they apply the three management options.
CREST Provides New Guidance
One surgeon who has perhaps given the most thought to sorting out treatment options is Dr. Brajesh K. Lal, a co–principal investigator on CREST, a vascular surgeon at the University of Maryland in Baltimore, and a researcher who spent the past couple of years sorting through CREST’s voluminous data to find new clues to guide patient triage.
A major, recent guide for matching patients with a specific carotid intervention came from his painstaking analysis of carotid angiograms from 1,070 of the CREST patients who underwent CAS, of whom 43 patients (4%) then went on to have a stroke.
This effort identified the following four anatomical features that appear to mark patients with an increased rate of periprocedural stroke when they are treated with CAS:
- Stenotic lesion more than 20 mm long. The researchers measured lesion length as the distance from the proximal to distal shoulder of the lesion.
- Sequential lesions. This refers to two lesions separated by at least 10 mm of normal vessel.
- Severe distal tortuosity. This was defined as at least two arterial bends of at least 90 degrees that are distal to the lesion.
- Narrow-mouth ulcers. This refers to a small crater or lumen flap that produces a discrete area of contrast extension beyond the normal arterial lumen with a narrow inlet into the ulceration.
Patients with one or more of these carotid anatomical features "should be treated by endarterectomy," Dr. Lal said in an interview. "If I see any one of these in my clinical practice, I will not stent."
The variables assessed in this analysis did not include arterial calcification, as the angiographic imaging routinely collected in the study did not allow calcium quantification, said Dr. Lal. Among the stented patients in the study, the cumulative prevalence of the four significant risk markers was 39%. Patients with none of the four markers had a significantly reduced rate of periprocedural stroke during carotid stenting.
Endovascularists were already routinely assessing carotid anatomy before attempting CAS, but prior to Dr. Lal’s report on these new findings at the International Stroke Conference in New Orleans in February, "I’m not so sure that everyone has been using this [anatomical] information," he said. "When we talk about operator experience in stenting, if we don’t have the facts about what increases the stenting risk, then we won’t improve performance. You’d be surprised at what some interventionalists are willing to do," despite a patient’s challenging carotid anatomy. "These data show that you can work around a single 90-degree bend [of the distal carotid artery], but not around two bends. That is the kind of stuff we’re beginning to find out."
Dr. Lal and his associates plan to further analyze their data and derive a formula that physicians can apply to patients to estimate their periprocedural stroke risk.
Additional, recent CREST analyses that were also reported at the International Stroke Conference revealed other new, important lessons about CAS and CEA.
First, by 2 years after intervention, both CAS and CEA produced roughly comparable low rates of restenosis: a 6.0% rate in the CAS patients who underwent a prespecified, follow-up duplex ultrasound examination of their carotid artery, and 6.3% in the CEA patients (Stroke 2012;43: abstract A3). The rates were also similar regardless of whether patients were symptomatic before their carotid intervention, Dr. Lal reported.
This finding appears to lay to rest the concern about a major restenosis risk using bare-metal stents in carotid arteries, in contrast to what happens in coronary arteries, Dr. Lal said. "To my mind, these data are fairly definitive. We followed more than 2,000 patients, and every ultrasound was reviewed and adjudicated. I’m fairly comfortable with these results."
A third CREST analysis that was also reported in February showed that, after adjustment for baseline differences, patients had no significant changes in their rates of periprocedural events during and after CAS throughout the study, from 2000 to 2008 (Stroke 2012;43: abstract A1). "We hoped to show that CAS was getting better [during the 9 years of CREST], but we saw no evidence it got better with time," said George Howard, Dr.P.H., professor and chairman of the department of biostatistics at the University of Alabama at Birmingham. "It would make sense if CAS got better; it’s a new procedure that people learn." But the CREST results showed no evidence of a learning curve, perhaps because the 224 interventionalists who performed CAS in CREST went through a rigorous credentialing process and also performed their first several CAS procedures in CREST during a lead-in phase that was not included in the main outcomes.
Choosing Among the Options
Dr. Lal said that he draws several other lessons from the CREST results to guide his treatment decisions: Patients who are physiologically elderly, those with a large amount of calcified atheroma in their aortic arch, and patients with significant peripheral arterial disease that impedes endovascular access are all poor CAS candidates, he said, adding that he focuses on a patient’s physiological age rather than chronological age.
Patients who are well suited to CAS are those with a younger physiological age, those with a history of radiation treatment, and patients with restenotic carotid lesions.
Although CREST showed worse performance of CAS in women compared with men, "I don’t know why, so it’s hard for me to exclude or offer a particular treatment" based on the patient’s sex, he said. "I treat women just like men, and work through all the other things that I know about to help me decide."
Then there is a third subgroup, patients with carotid disease who he feels "benefit the most" from medical management only. This category primarily includes asymptomatic patients with moderate stenosis (that is, less than 80% carotid occlusion). His prescription for medical management includes 325 mg of aspirin daily, treatment with a statin, good blood pressure and blood sugar control, smoking cessation, and lifestyle modification. If, despite this, an asymptomatic patient has continued stenotic progression that advances beyond 80%, he will then recommend revascularization.
Dr. Lal said that in his practice today, roughly one-third of carotid-disease patients are on medical management, a third undergo CEA, and a third receive CAS. "We are at a stage of equipoise," for these three options, which is why he helped design and is working to get funding for CREST II.
Dr. Lal personally applies all three treatment options – CAS, CEA, or medical management. Having physicians involved in the decision-making process who are comfortable using each of these options is critical for making a balanced management decision, said Dr. L. Nelson Hopkins, another CREST collaborator and professor and chairman of neurosurgery at the State University of New York at Buffalo.
"The single most important thing we do is have a weekly, multidisciplinary conference to discuss each case, with input from everyone, to decide the best treatment for each [carotid disease] patient," he said when he spoke at ISET 2012, an International Symposium on Endovascular Therapy in Miami Beach in January.
"The most important factors" guiding the decision of whether or not to perform CAS are, does a symptomatic patient has "hot" lesions, what is the arterial tortuosity, and what is the calcification, he said. Other important features include whether the patient has a contralateral carotid occlusion, whether the carotid has a high bifurcation, and whether there are ostial or tandem lesions. "If the anatomy is favorable, CAS is fine even in a 95-year-old," Dr. Hopkins said.
What About Insurance Coverage?
Carotid specialists concede that another, nonmedical issue also plays a big role in deciding how to manage a patient: What will the patient’s health insurance cover?
In its most recent decision on the issue, effective in April 2010, the Centers for Medicare and Medicaid Services (CMS) said that Medicare covers CAS performed on a routine basis for patients with symptomatic carotid disease who are at high risk for CEA and have at least 70% carotid stenosis. CMS also said that Medicare will cover FDA-approved investigations of CAS in symptomatic patients who are at high risk for CEA and have at least 50% stenosis, as well as CAS investigations in asymptomatic patients at high risk for CEA with at least 80% carotid stenosis.
As a result, patients with other forms of carotid disease often do not have insurance coverage for CAS.
"The issue for us is primarily reimbursement. If we can do [a procedure] and be reimbursed, we often stent the patient," said Dr. Carlos H. Timaran, a vascular surgeon and chief of endovascular surgery at the University of Texas Southwestern Medical Center in Dallas.
"For my private practice patients, we assess their risk based on their [anatomical] and medical criteria. If they are high risk, we usually stent. We have a bias toward stenting because it is faster and easier. Some people say they can do endarterectomy in an hour, but I don’t believe that. I do CEA with residents and fellows, and it takes 2 hours. But with stenting, I can do it in 40 minutes, even with a fellow. If I have three patients with carotid disease to treat [and] if I do CEA on all three, it will take all day. If I do CAS, it will take the morning, or less," he said in an interview.
In addition, "CEA is a nice procedure, but you need anesthesia. CAS is easier, I can do it myself, and I don’t need anesthesia. I am in more control of the case," Dr. Timaran said.
For patients who are eligible for both CAS and CEA, "it also comes down to anatomy," he grants. But if the patient has anatomy favorable to either method, "I offer patients both, and they will probably go for a stent," because patients usually prefer the quicker recovery that stenting offers, compared with CEA.
An interesting footnote to the issue of how to best manage patients who are eligible for CAS under CMS rules is that the CREST results offer no direct guidance on the relative safety and efficacy of CAS and CEA in patients with Medicare coverage. That’s because "the CREST cohort was a conventional-risk cohort; most criteria that would make someone a high surgical risk would have been excluded from CREST," Dr. Thomas G. Brott, professor of neurology at the Mayo Clinic in Jacksonville, Fla., and lead investigator for CREST. "The high-surgical-risk patients who are currently covered by Medicare would not have even been enrolled in CREST," Dr. Brott said in an interview.
Where Does Medical Therapy Fit In?
Although Dr. Timaran sees the potential value of medical therapy only for asymptomatic patients, he also urges caution.
"I don’t think [the efficacy of medical treatment] has been proven, and until it’s proven I don’t think physicians should deny revascularization treatment to these patients," he said. "The standard of care for a patient with severe carotid stenosis is revascularization, even if the patient is asymptomatic, unless there is some other consideration." But, he admitted, "there is little downside to medical treatment in asymptomatic patients, even if they have severe stenosis." That’s because their stroke risk is low regardless of which option a patient chooses.
"I tell asymptomatic patients that ‘based on the data, your risk of stroke is 2% per year, and that risk may be even lower with best medical therapy.’ With a carotid intervention, we can lower their risk to 1% per year." In other words, the patient’s stroke risk is low regardless of which option is chosen. Plus, medical therapy only is the best choice for patients with special risk factors, such as radiation-induced stenosis, Dr. Timaran added.
Other experts see medical treatment alone as an excellent option for many carotid-stenosis patients.
"The majority of patients I see don’t get an intervention; we use medical treatment only," said Dr. Jon S. Matsumura in an interview. "I think that’s true for most vascular surgery practices, and in cardiology and neurology practices. A lot of these patients just need reassurance," said Dr. Matsumura, professor and chairman of vascular surgery at the University of Wisconsin in Madison.
"Most asymptomatic patients [with carotid stenosis] should be treated by best medical therapy, with very few – fewer than 5% – treated by CAS or CEA," said Dr. Frank J. Veith at the International Symposium on Endovascular Therapy (ISET 12) in January. He acknowledged that well-controlled trials done during the 1990s showed that CEA led to superior outcomes compared with medical therapy, but since the early 2000s, "best medical therapy to prevent strokes leapt forward," he said, especially with improved statin therapy. He cited findings from a 2010 prospective study that showed asymptomatic Canadian patients managed starting in 2003 with best medical therapy had an annual stroke rate of 0.7% (Arch. Neurol. 2010;67:180-6).
He singled out four small groups of asymptomatic patients who warrant revascularization: patients with CT or MRI evidence for a history of silent strokes, patients with very-high-grade carotid stenosis that leaves just 1% of the carotid lumen open, those with a contralateral occlusion, and patients who serially experience transient occlusions detected by transcranial Doppler ultrasound examination, said Dr. Veith, professor of surgery at New York University. Aside from this small fraction of patients with asymptomatic disease, performing interventions on anyone else will "cause more strokes than you’ll prevent," he warned in an interview.
What Will CMS Do?
Dr. Veith’s take on limiting CAS in asymptomatic patients received substantial support early this year in the days leading up to the Jan. 25 meeting of the Medicare Evidence Development and Coverage Advisory Committee (MEDCAC) that heard evidence and opinions for and against widening of Medicare coverage for CAS. In early January, Dr. Veith joined with 40 other vascular surgeons and stroke specialists to urge CMS not to broaden CAS coverage (Eur. J. Vasc. Endovasc. Surg. 2012 Jan. 5 [doi:10.1016/j.ejvs.2011.12.006]). But, as MEDCAC heard during this meeting, experts differ on this issue.
"CMS continues to restrict access to CAS to about 10% of patients" with significant carotid stenosis, said Dr. William A. Gray, director of endovascular services at Columbia University in New York, speaking at ISET 2012 in January. "Expanded CAS coverage would allow greater patient access and device development, and therefore create a safer option for patients." Dr. Gray presented his positive views on CAS to MEDCAC in January as well.
At press time, CMS had not announced whether or not the January MEDCAC hearing will result in a change to its CAS coverage policy. But the votes cast by MEDCAC’s membership in January suggested that the issues surrounding management of carotid stenosis remain too unresolved to trigger immediate changes.
Dr. Lal, Dr. Timaran, and Dr. Veith said that they had no disclosures. Dr. Howard said that he has received grant support from Abbott. Dr. Hopkins said that he has financial relationships with Bard, Boston Scientific, Cordis, Abbott Vascular, Toshiba, W.L. Gore, Medtronic, Micrus, St. Jude, Access Closure, Osteal, Vascular Dynamics, Square One, Valor Medical, Claret Medical, and Augmenix. Dr. Brott said that he has been a consultant to Edwards Lifesciences. Dr. Matsumura said that he has received research grants from Abbott, Cook, Covidien, Endologix, and W.L. Gore. Dr. Gray said that he has had financial relationships with Boston Scientific, Coherex, Contego, Cordis, Fiatlux 3D, Nexeon, Quantumcor, Abbott Vascular, and Biocardia.
Optimal management of carotid stenosis to prevent death and strokes is a work in progress right now, with experts groping for the right balance between carotid endarterectomy, carotid artery stenting, and best medical therapy.
The field is bereft of both conclusive, up-to-date data and – more importantly – wide agreement on data interpretation that clearly tips treatment toward one of these options, so much so that in late January an expert panel organized by the Centers for Medicare and Medicaid Services found itself unable to support with high confidence the application of these treatments to any subgroup of carotid disease patients.
Amid this confusion are the following certainties:
- The use of carotid artery stenting (CAS) on U.S. patients grew substantially since its introduction in the late 1990s and since it began receiving limited Medicare coverage in 2004. Data collected through 2007 showed a greater-than-fourfold increase in CAS use among Medicare beneficiaries, compared with 1998 (Circ. Cardiovasc. Qual. Outcomes 2010;3:15-24).
- Other findings documented a shift in CAS use during the 2000s toward patients with asymptomatic carotid stenosis, with a recent estimate that 70%-90% of CAS patients now fall into that category (Arch. Int. Med. 2010;170:1225-7).
- The main results of the landmark CREST (Carotid Revascularization Endarterectomy vs. Stenting) trial, first reported 2 years ago (N. Engl. J. Med. 2010;363:11-23), appeared on the surface to show very similar safety and efficacy for CAS and carotid endarterectomy (CEA), but some experts caution that deeper drilling into the results show that this is an oversimplification of how the two treatments compare.
- And many experts agree that best medical therapy has improved in recent years, to the point where it deserves a new appraisal in a large trial that would compare it against carotid revascularization with CAS or CEA in asymptomatic patients. The leaders of CREST themselves have designed a new trial, CREST II, aimed at making this comparison, and have begun to vigorously lobby the National Institutes of Health to support this study.
In the meantime, vascular surgeons, endovascularists, cardiologists, and the other types of physicians who treat patients with carotid disease try as best they can to strike the right balance in how they apply the three management options.
CREST Provides New Guidance
One surgeon who has perhaps given the most thought to sorting out treatment options is Dr. Brajesh K. Lal, a co–principal investigator on CREST, a vascular surgeon at the University of Maryland in Baltimore, and a researcher who spent the past couple of years sorting through CREST’s voluminous data to find new clues to guide patient triage.
A major, recent guide for matching patients with a specific carotid intervention came from his painstaking analysis of carotid angiograms from 1,070 of the CREST patients who underwent CAS, of whom 43 patients (4%) then went on to have a stroke.
This effort identified the following four anatomical features that appear to mark patients with an increased rate of periprocedural stroke when they are treated with CAS:
- Stenotic lesion more than 20 mm long. The researchers measured lesion length as the distance from the proximal to distal shoulder of the lesion.
- Sequential lesions. This refers to two lesions separated by at least 10 mm of normal vessel.
- Severe distal tortuosity. This was defined as at least two arterial bends of at least 90 degrees that are distal to the lesion.
- Narrow-mouth ulcers. This refers to a small crater or lumen flap that produces a discrete area of contrast extension beyond the normal arterial lumen with a narrow inlet into the ulceration.
Patients with one or more of these carotid anatomical features "should be treated by endarterectomy," Dr. Lal said in an interview. "If I see any one of these in my clinical practice, I will not stent."
The variables assessed in this analysis did not include arterial calcification, as the angiographic imaging routinely collected in the study did not allow calcium quantification, said Dr. Lal. Among the stented patients in the study, the cumulative prevalence of the four significant risk markers was 39%. Patients with none of the four markers had a significantly reduced rate of periprocedural stroke during carotid stenting.
Endovascularists were already routinely assessing carotid anatomy before attempting CAS, but prior to Dr. Lal’s report on these new findings at the International Stroke Conference in New Orleans in February, "I’m not so sure that everyone has been using this [anatomical] information," he said. "When we talk about operator experience in stenting, if we don’t have the facts about what increases the stenting risk, then we won’t improve performance. You’d be surprised at what some interventionalists are willing to do," despite a patient’s challenging carotid anatomy. "These data show that you can work around a single 90-degree bend [of the distal carotid artery], but not around two bends. That is the kind of stuff we’re beginning to find out."
Dr. Lal and his associates plan to further analyze their data and derive a formula that physicians can apply to patients to estimate their periprocedural stroke risk.
Additional, recent CREST analyses that were also reported at the International Stroke Conference revealed other new, important lessons about CAS and CEA.
First, by 2 years after intervention, both CAS and CEA produced roughly comparable low rates of restenosis: a 6.0% rate in the CAS patients who underwent a prespecified, follow-up duplex ultrasound examination of their carotid artery, and 6.3% in the CEA patients (Stroke 2012;43: abstract A3). The rates were also similar regardless of whether patients were symptomatic before their carotid intervention, Dr. Lal reported.
This finding appears to lay to rest the concern about a major restenosis risk using bare-metal stents in carotid arteries, in contrast to what happens in coronary arteries, Dr. Lal said. "To my mind, these data are fairly definitive. We followed more than 2,000 patients, and every ultrasound was reviewed and adjudicated. I’m fairly comfortable with these results."
A third CREST analysis that was also reported in February showed that, after adjustment for baseline differences, patients had no significant changes in their rates of periprocedural events during and after CAS throughout the study, from 2000 to 2008 (Stroke 2012;43: abstract A1). "We hoped to show that CAS was getting better [during the 9 years of CREST], but we saw no evidence it got better with time," said George Howard, Dr.P.H., professor and chairman of the department of biostatistics at the University of Alabama at Birmingham. "It would make sense if CAS got better; it’s a new procedure that people learn." But the CREST results showed no evidence of a learning curve, perhaps because the 224 interventionalists who performed CAS in CREST went through a rigorous credentialing process and also performed their first several CAS procedures in CREST during a lead-in phase that was not included in the main outcomes.
Choosing Among the Options
Dr. Lal said that he draws several other lessons from the CREST results to guide his treatment decisions: Patients who are physiologically elderly, those with a large amount of calcified atheroma in their aortic arch, and patients with significant peripheral arterial disease that impedes endovascular access are all poor CAS candidates, he said, adding that he focuses on a patient’s physiological age rather than chronological age.
Patients who are well suited to CAS are those with a younger physiological age, those with a history of radiation treatment, and patients with restenotic carotid lesions.
Although CREST showed worse performance of CAS in women compared with men, "I don’t know why, so it’s hard for me to exclude or offer a particular treatment" based on the patient’s sex, he said. "I treat women just like men, and work through all the other things that I know about to help me decide."
Then there is a third subgroup, patients with carotid disease who he feels "benefit the most" from medical management only. This category primarily includes asymptomatic patients with moderate stenosis (that is, less than 80% carotid occlusion). His prescription for medical management includes 325 mg of aspirin daily, treatment with a statin, good blood pressure and blood sugar control, smoking cessation, and lifestyle modification. If, despite this, an asymptomatic patient has continued stenotic progression that advances beyond 80%, he will then recommend revascularization.
Dr. Lal said that in his practice today, roughly one-third of carotid-disease patients are on medical management, a third undergo CEA, and a third receive CAS. "We are at a stage of equipoise," for these three options, which is why he helped design and is working to get funding for CREST II.
Dr. Lal personally applies all three treatment options – CAS, CEA, or medical management. Having physicians involved in the decision-making process who are comfortable using each of these options is critical for making a balanced management decision, said Dr. L. Nelson Hopkins, another CREST collaborator and professor and chairman of neurosurgery at the State University of New York at Buffalo.
"The single most important thing we do is have a weekly, multidisciplinary conference to discuss each case, with input from everyone, to decide the best treatment for each [carotid disease] patient," he said when he spoke at ISET 2012, an International Symposium on Endovascular Therapy in Miami Beach in January.
"The most important factors" guiding the decision of whether or not to perform CAS are, does a symptomatic patient has "hot" lesions, what is the arterial tortuosity, and what is the calcification, he said. Other important features include whether the patient has a contralateral carotid occlusion, whether the carotid has a high bifurcation, and whether there are ostial or tandem lesions. "If the anatomy is favorable, CAS is fine even in a 95-year-old," Dr. Hopkins said.
What About Insurance Coverage?
Carotid specialists concede that another, nonmedical issue also plays a big role in deciding how to manage a patient: What will the patient’s health insurance cover?
In its most recent decision on the issue, effective in April 2010, the Centers for Medicare and Medicaid Services (CMS) said that Medicare covers CAS performed on a routine basis for patients with symptomatic carotid disease who are at high risk for CEA and have at least 70% carotid stenosis. CMS also said that Medicare will cover FDA-approved investigations of CAS in symptomatic patients who are at high risk for CEA and have at least 50% stenosis, as well as CAS investigations in asymptomatic patients at high risk for CEA with at least 80% carotid stenosis.
As a result, patients with other forms of carotid disease often do not have insurance coverage for CAS.
"The issue for us is primarily reimbursement. If we can do [a procedure] and be reimbursed, we often stent the patient," said Dr. Carlos H. Timaran, a vascular surgeon and chief of endovascular surgery at the University of Texas Southwestern Medical Center in Dallas.
"For my private practice patients, we assess their risk based on their [anatomical] and medical criteria. If they are high risk, we usually stent. We have a bias toward stenting because it is faster and easier. Some people say they can do endarterectomy in an hour, but I don’t believe that. I do CEA with residents and fellows, and it takes 2 hours. But with stenting, I can do it in 40 minutes, even with a fellow. If I have three patients with carotid disease to treat [and] if I do CEA on all three, it will take all day. If I do CAS, it will take the morning, or less," he said in an interview.
In addition, "CEA is a nice procedure, but you need anesthesia. CAS is easier, I can do it myself, and I don’t need anesthesia. I am in more control of the case," Dr. Timaran said.
For patients who are eligible for both CAS and CEA, "it also comes down to anatomy," he grants. But if the patient has anatomy favorable to either method, "I offer patients both, and they will probably go for a stent," because patients usually prefer the quicker recovery that stenting offers, compared with CEA.
An interesting footnote to the issue of how to best manage patients who are eligible for CAS under CMS rules is that the CREST results offer no direct guidance on the relative safety and efficacy of CAS and CEA in patients with Medicare coverage. That’s because "the CREST cohort was a conventional-risk cohort; most criteria that would make someone a high surgical risk would have been excluded from CREST," Dr. Thomas G. Brott, professor of neurology at the Mayo Clinic in Jacksonville, Fla., and lead investigator for CREST. "The high-surgical-risk patients who are currently covered by Medicare would not have even been enrolled in CREST," Dr. Brott said in an interview.
Where Does Medical Therapy Fit In?
Although Dr. Timaran sees the potential value of medical therapy only for asymptomatic patients, he also urges caution.
"I don’t think [the efficacy of medical treatment] has been proven, and until it’s proven I don’t think physicians should deny revascularization treatment to these patients," he said. "The standard of care for a patient with severe carotid stenosis is revascularization, even if the patient is asymptomatic, unless there is some other consideration." But, he admitted, "there is little downside to medical treatment in asymptomatic patients, even if they have severe stenosis." That’s because their stroke risk is low regardless of which option a patient chooses.
"I tell asymptomatic patients that ‘based on the data, your risk of stroke is 2% per year, and that risk may be even lower with best medical therapy.’ With a carotid intervention, we can lower their risk to 1% per year." In other words, the patient’s stroke risk is low regardless of which option is chosen. Plus, medical therapy only is the best choice for patients with special risk factors, such as radiation-induced stenosis, Dr. Timaran added.
Other experts see medical treatment alone as an excellent option for many carotid-stenosis patients.
"The majority of patients I see don’t get an intervention; we use medical treatment only," said Dr. Jon S. Matsumura in an interview. "I think that’s true for most vascular surgery practices, and in cardiology and neurology practices. A lot of these patients just need reassurance," said Dr. Matsumura, professor and chairman of vascular surgery at the University of Wisconsin in Madison.
"Most asymptomatic patients [with carotid stenosis] should be treated by best medical therapy, with very few – fewer than 5% – treated by CAS or CEA," said Dr. Frank J. Veith at the International Symposium on Endovascular Therapy (ISET 12) in January. He acknowledged that well-controlled trials done during the 1990s showed that CEA led to superior outcomes compared with medical therapy, but since the early 2000s, "best medical therapy to prevent strokes leapt forward," he said, especially with improved statin therapy. He cited findings from a 2010 prospective study that showed asymptomatic Canadian patients managed starting in 2003 with best medical therapy had an annual stroke rate of 0.7% (Arch. Neurol. 2010;67:180-6).
He singled out four small groups of asymptomatic patients who warrant revascularization: patients with CT or MRI evidence for a history of silent strokes, patients with very-high-grade carotid stenosis that leaves just 1% of the carotid lumen open, those with a contralateral occlusion, and patients who serially experience transient occlusions detected by transcranial Doppler ultrasound examination, said Dr. Veith, professor of surgery at New York University. Aside from this small fraction of patients with asymptomatic disease, performing interventions on anyone else will "cause more strokes than you’ll prevent," he warned in an interview.
What Will CMS Do?
Dr. Veith’s take on limiting CAS in asymptomatic patients received substantial support early this year in the days leading up to the Jan. 25 meeting of the Medicare Evidence Development and Coverage Advisory Committee (MEDCAC) that heard evidence and opinions for and against widening of Medicare coverage for CAS. In early January, Dr. Veith joined with 40 other vascular surgeons and stroke specialists to urge CMS not to broaden CAS coverage (Eur. J. Vasc. Endovasc. Surg. 2012 Jan. 5 [doi:10.1016/j.ejvs.2011.12.006]). But, as MEDCAC heard during this meeting, experts differ on this issue.
"CMS continues to restrict access to CAS to about 10% of patients" with significant carotid stenosis, said Dr. William A. Gray, director of endovascular services at Columbia University in New York, speaking at ISET 2012 in January. "Expanded CAS coverage would allow greater patient access and device development, and therefore create a safer option for patients." Dr. Gray presented his positive views on CAS to MEDCAC in January as well.
At press time, CMS had not announced whether or not the January MEDCAC hearing will result in a change to its CAS coverage policy. But the votes cast by MEDCAC’s membership in January suggested that the issues surrounding management of carotid stenosis remain too unresolved to trigger immediate changes.
Dr. Lal, Dr. Timaran, and Dr. Veith said that they had no disclosures. Dr. Howard said that he has received grant support from Abbott. Dr. Hopkins said that he has financial relationships with Bard, Boston Scientific, Cordis, Abbott Vascular, Toshiba, W.L. Gore, Medtronic, Micrus, St. Jude, Access Closure, Osteal, Vascular Dynamics, Square One, Valor Medical, Claret Medical, and Augmenix. Dr. Brott said that he has been a consultant to Edwards Lifesciences. Dr. Matsumura said that he has received research grants from Abbott, Cook, Covidien, Endologix, and W.L. Gore. Dr. Gray said that he has had financial relationships with Boston Scientific, Coherex, Contego, Cordis, Fiatlux 3D, Nexeon, Quantumcor, Abbott Vascular, and Biocardia.
Epilepsy Incidence High Following Pediatric Stroke
NEW ORLEANS – Pediatric stroke survivors had an absolute risk of 39% for developing epilepsy in the 10 years following their stroke in a review of 371 patients in Northern California.
Two factors significantly boosted a child’s risk of developing epilepsy following a stroke: A seizure at the time of stroke, and a neurologic deficit at the time of hospital discharge following the index stroke, Dr. Christine Fox said at the International Stroke Conference (Stroke 2012;43:A41).
Although these findings have no immediate management implications, the information could help parents know what to expect when their child has a stroke.
Epilepsy "is not always one of the first outcomes we think about when we speak with parents," said Dr. Fox, a neurologist at the University of California, San Francisco. "If there is such a high incidence of epilepsy after stroke, maybe it’s something to talk about with parents," she said in an interview.
The study reviewed used data collected in the Kaiser Pediatric Stroke Study, run by Kaiser Permanente of Northern California, and included children who ranged from neonates to 19 years old and had a clinical presentation and brain imaging results consistent with a stroke. The group included hemorrhagic and ischemic strokes occurring during 1993-2003, and arterial ischemic strokes during 2003-2007. This cohort consisted of 371 children who survived a stroke to hospital discharge, including 110 neonates and 261 older children who had 226 ischemic strokes and 145 hemorrhagic strokes.
During a median follow-up of 4.5 years, 89 of these children developed epilepsy, defined as a confirmed, unprovoked seizure occurring more than 30 days following the index stroke. The researchers excluded 7 of the 89 incident epilepsy cases because they had a history of seizures prior to their stroke. Survivor analysis of the 82 new-onset cases showed an average annual incidence of 6%, with a cumulative 5-year incidence of 23% and a cumulative 10-year rate of 39%, Dr. Fox said.
A multivariate adjusted analysis that controlled for factors such as age, sex, race, and type of stroke identified two parameters linked with a significantly higher risk for developing epilepsy following stroke: a seizure concurrent with the stroke ictus, which boosted the risk of subsequent epilepsy 3.6-fold, and a neurologic deficit at discharge, which increased the risk for later epilepsy twofold.
In contrast to these findings, previous reports documented a much lower incidence of epilepsy in adults following a stroke, with a 3% rate after 1 year, and an 8% rate after 5 years, Dr. Fox said.
Dr. Fox said that she had no disclosures. The conference was sponsored by the American Heart Association.
NEW ORLEANS – Pediatric stroke survivors had an absolute risk of 39% for developing epilepsy in the 10 years following their stroke in a review of 371 patients in Northern California.
Two factors significantly boosted a child’s risk of developing epilepsy following a stroke: A seizure at the time of stroke, and a neurologic deficit at the time of hospital discharge following the index stroke, Dr. Christine Fox said at the International Stroke Conference (Stroke 2012;43:A41).
Although these findings have no immediate management implications, the information could help parents know what to expect when their child has a stroke.
Epilepsy "is not always one of the first outcomes we think about when we speak with parents," said Dr. Fox, a neurologist at the University of California, San Francisco. "If there is such a high incidence of epilepsy after stroke, maybe it’s something to talk about with parents," she said in an interview.
The study reviewed used data collected in the Kaiser Pediatric Stroke Study, run by Kaiser Permanente of Northern California, and included children who ranged from neonates to 19 years old and had a clinical presentation and brain imaging results consistent with a stroke. The group included hemorrhagic and ischemic strokes occurring during 1993-2003, and arterial ischemic strokes during 2003-2007. This cohort consisted of 371 children who survived a stroke to hospital discharge, including 110 neonates and 261 older children who had 226 ischemic strokes and 145 hemorrhagic strokes.
During a median follow-up of 4.5 years, 89 of these children developed epilepsy, defined as a confirmed, unprovoked seizure occurring more than 30 days following the index stroke. The researchers excluded 7 of the 89 incident epilepsy cases because they had a history of seizures prior to their stroke. Survivor analysis of the 82 new-onset cases showed an average annual incidence of 6%, with a cumulative 5-year incidence of 23% and a cumulative 10-year rate of 39%, Dr. Fox said.
A multivariate adjusted analysis that controlled for factors such as age, sex, race, and type of stroke identified two parameters linked with a significantly higher risk for developing epilepsy following stroke: a seizure concurrent with the stroke ictus, which boosted the risk of subsequent epilepsy 3.6-fold, and a neurologic deficit at discharge, which increased the risk for later epilepsy twofold.
In contrast to these findings, previous reports documented a much lower incidence of epilepsy in adults following a stroke, with a 3% rate after 1 year, and an 8% rate after 5 years, Dr. Fox said.
Dr. Fox said that she had no disclosures. The conference was sponsored by the American Heart Association.
NEW ORLEANS – Pediatric stroke survivors had an absolute risk of 39% for developing epilepsy in the 10 years following their stroke in a review of 371 patients in Northern California.
Two factors significantly boosted a child’s risk of developing epilepsy following a stroke: A seizure at the time of stroke, and a neurologic deficit at the time of hospital discharge following the index stroke, Dr. Christine Fox said at the International Stroke Conference (Stroke 2012;43:A41).
Although these findings have no immediate management implications, the information could help parents know what to expect when their child has a stroke.
Epilepsy "is not always one of the first outcomes we think about when we speak with parents," said Dr. Fox, a neurologist at the University of California, San Francisco. "If there is such a high incidence of epilepsy after stroke, maybe it’s something to talk about with parents," she said in an interview.
The study reviewed used data collected in the Kaiser Pediatric Stroke Study, run by Kaiser Permanente of Northern California, and included children who ranged from neonates to 19 years old and had a clinical presentation and brain imaging results consistent with a stroke. The group included hemorrhagic and ischemic strokes occurring during 1993-2003, and arterial ischemic strokes during 2003-2007. This cohort consisted of 371 children who survived a stroke to hospital discharge, including 110 neonates and 261 older children who had 226 ischemic strokes and 145 hemorrhagic strokes.
During a median follow-up of 4.5 years, 89 of these children developed epilepsy, defined as a confirmed, unprovoked seizure occurring more than 30 days following the index stroke. The researchers excluded 7 of the 89 incident epilepsy cases because they had a history of seizures prior to their stroke. Survivor analysis of the 82 new-onset cases showed an average annual incidence of 6%, with a cumulative 5-year incidence of 23% and a cumulative 10-year rate of 39%, Dr. Fox said.
A multivariate adjusted analysis that controlled for factors such as age, sex, race, and type of stroke identified two parameters linked with a significantly higher risk for developing epilepsy following stroke: a seizure concurrent with the stroke ictus, which boosted the risk of subsequent epilepsy 3.6-fold, and a neurologic deficit at discharge, which increased the risk for later epilepsy twofold.
In contrast to these findings, previous reports documented a much lower incidence of epilepsy in adults following a stroke, with a 3% rate after 1 year, and an 8% rate after 5 years, Dr. Fox said.
Dr. Fox said that she had no disclosures. The conference was sponsored by the American Heart Association.
FROM THE INTERNATIONAL STROKE CONFERENCE
Major Finding: During the 10 years following a stroke, the cumulative incidence of epilepsy in children aged 19 years and younger was 39%.
Data Source: The review included 371 pediatric strokes included in the Kaiser Pediatric Stroke Study.
Disclosures: Dr. Fox said that she had no disclosures.
Tenecteplase Edges TPA in Acute Stroke
NEW ORLEANS – The potential advantages of tenecteplase, a modified form of tissue plasminogen activator engineered to outperform the native molecule, finally shone through for treating patients with acute ischemic stroke.
A head-to-head comparison of tenecteplase against recombinant tissue plasminogen activator (TPA; alteplase) showed that the modified molecule produced better reperfusion and better clinical recovery 24 hours after treatment in a randomized trial of 75 Australian patients whose treatment was guided by CT brain imaging, Dr. Bill O’Brien reported at the International Stroke Conference.
Although Dr. O’Brien cautioned that this pilot study did not produce definitive results, the superiority of tenecteplase eclipsed its failure to surpass TPA in a randomized comparison of 112 patients reported in 2010.
The results "support using advanced imaging to identify the patients who are most likely to benefit" from tenecteplase, he said. The use of systematic CT brain imaging to select patients appeared to be the main factor that distinguished this study from the previous trial where tenecteplase failed to show an advantage, said Dr. O’Brien, a stroke physician at John Hunter Hospital in Newcastle, Australia.
Targeted patient selection may also explain why the tenecteplase treatment was so safe, resulting in a trend toward a reduced rate of type 2 parenchymal hemorrhages, compared with TPA, despite the superior thrombolytic activity shown by tenecteplase, he said.
Although the current trial had "compelling results," another study will follow to compare the two drugs by several 3-month outcomes, Dr. O’Brien said in an interview. The successful dosages of tenecteplase tested also have the advantage of being cheaper than the standard TPA dosage, he added.
Developed in the 1990s, tenecteplase resulted from three targeted amino acid substitutions in conventional TPA. This produced a molecule with increased fibrin specificity, and longer plasma half-life due to increased resistance to enzymatic degradation, which meant that it could be administered by an intravenous bolus instead of requiring continuous infusion like TPA. When used to treat myocardial infarctions, tenecteplase has also resulted in fewer bleeding complications than TPA.
The study enrolled 75 patients within 6 hours of onset of an ischemic stroke at any of three Australian hospitals, following selection by CT imaging to identify patients with a small infarct core and a large penumbra of salvageable brain. Researchers randomized patients to receive 0.1 mg/kg tenecteplase or 0.25 mg/kg tenecteplase by an intravenous bolus injection, or standard infusion with 0.9 mg/kg TPA. The patients averaged 70 years old, their average NIHSS (National Institutes of Health Stroke Scale) score at entry was about 14, and patients received treatment an average of about 3 hours after symptom onset.
The reperfusion rate after 24 hours was 78% among all 50 patients treated with tenecteplase, and 55% among the TPA treated patients, a statistically significant difference for one of the study’s two primary end points, Dr. O’Brien said. The second primary end point, change in NIHSS score after 24 hours, improved by an average of 8 points in all 50 tenecteplase patients, and by an average of 3 points in the TPA patients, also a significant difference.
The results appeared to show a dose-response relationship for tenecteplase, with the 25 patients who received the 0.25-mg/kg dosage having better reperfusion rates and reductions in their NIHSS scores than the 25 patients who received a 0.1-mg/kg dosage as well as the patients treated with TPA, but Dr. O’Brien did not report specific numbers from this analysis.
Of the 50 tenecteplase patients, 2 (4%) developed a type 2 parenchymal hemorrhage within 24 hours, compared with 4 (16%) of the 25 TPA-treated patients, a difference that trended toward significance. The rates of symptomatic intracerebral hemorrhages and death at 24 hours were similar with both drugs. But at 90 days after treatment, the combined rate of death or severe disability was 10% in the 50 tenecteplase patients and 25% in the TPA patients, a difference that also trended toward significance.
These findings contrast with a 2010 report from a randomized trial that compared three different tenecteplase dosages with TPA in a multicenter U.S. study of 112 patients (Stroke 2010;41:707-11). The results showed no significant differences between any of the tenecteplase dosages and TPA for any of the outcomes measured out to 90 days after treatment.
The major difference between the U.S. study and the new Australian study was in patient selection, Dr. O’Brien said. "We used imaging to balance the groups," and to select patients who stood to benefit most from effective reperfusion, he said.
Dr. O’Brien said that he and his associates had no disclosures.
NEW ORLEANS – The potential advantages of tenecteplase, a modified form of tissue plasminogen activator engineered to outperform the native molecule, finally shone through for treating patients with acute ischemic stroke.
A head-to-head comparison of tenecteplase against recombinant tissue plasminogen activator (TPA; alteplase) showed that the modified molecule produced better reperfusion and better clinical recovery 24 hours after treatment in a randomized trial of 75 Australian patients whose treatment was guided by CT brain imaging, Dr. Bill O’Brien reported at the International Stroke Conference.
Although Dr. O’Brien cautioned that this pilot study did not produce definitive results, the superiority of tenecteplase eclipsed its failure to surpass TPA in a randomized comparison of 112 patients reported in 2010.
The results "support using advanced imaging to identify the patients who are most likely to benefit" from tenecteplase, he said. The use of systematic CT brain imaging to select patients appeared to be the main factor that distinguished this study from the previous trial where tenecteplase failed to show an advantage, said Dr. O’Brien, a stroke physician at John Hunter Hospital in Newcastle, Australia.
Targeted patient selection may also explain why the tenecteplase treatment was so safe, resulting in a trend toward a reduced rate of type 2 parenchymal hemorrhages, compared with TPA, despite the superior thrombolytic activity shown by tenecteplase, he said.
Although the current trial had "compelling results," another study will follow to compare the two drugs by several 3-month outcomes, Dr. O’Brien said in an interview. The successful dosages of tenecteplase tested also have the advantage of being cheaper than the standard TPA dosage, he added.
Developed in the 1990s, tenecteplase resulted from three targeted amino acid substitutions in conventional TPA. This produced a molecule with increased fibrin specificity, and longer plasma half-life due to increased resistance to enzymatic degradation, which meant that it could be administered by an intravenous bolus instead of requiring continuous infusion like TPA. When used to treat myocardial infarctions, tenecteplase has also resulted in fewer bleeding complications than TPA.
The study enrolled 75 patients within 6 hours of onset of an ischemic stroke at any of three Australian hospitals, following selection by CT imaging to identify patients with a small infarct core and a large penumbra of salvageable brain. Researchers randomized patients to receive 0.1 mg/kg tenecteplase or 0.25 mg/kg tenecteplase by an intravenous bolus injection, or standard infusion with 0.9 mg/kg TPA. The patients averaged 70 years old, their average NIHSS (National Institutes of Health Stroke Scale) score at entry was about 14, and patients received treatment an average of about 3 hours after symptom onset.
The reperfusion rate after 24 hours was 78% among all 50 patients treated with tenecteplase, and 55% among the TPA treated patients, a statistically significant difference for one of the study’s two primary end points, Dr. O’Brien said. The second primary end point, change in NIHSS score after 24 hours, improved by an average of 8 points in all 50 tenecteplase patients, and by an average of 3 points in the TPA patients, also a significant difference.
The results appeared to show a dose-response relationship for tenecteplase, with the 25 patients who received the 0.25-mg/kg dosage having better reperfusion rates and reductions in their NIHSS scores than the 25 patients who received a 0.1-mg/kg dosage as well as the patients treated with TPA, but Dr. O’Brien did not report specific numbers from this analysis.
Of the 50 tenecteplase patients, 2 (4%) developed a type 2 parenchymal hemorrhage within 24 hours, compared with 4 (16%) of the 25 TPA-treated patients, a difference that trended toward significance. The rates of symptomatic intracerebral hemorrhages and death at 24 hours were similar with both drugs. But at 90 days after treatment, the combined rate of death or severe disability was 10% in the 50 tenecteplase patients and 25% in the TPA patients, a difference that also trended toward significance.
These findings contrast with a 2010 report from a randomized trial that compared three different tenecteplase dosages with TPA in a multicenter U.S. study of 112 patients (Stroke 2010;41:707-11). The results showed no significant differences between any of the tenecteplase dosages and TPA for any of the outcomes measured out to 90 days after treatment.
The major difference between the U.S. study and the new Australian study was in patient selection, Dr. O’Brien said. "We used imaging to balance the groups," and to select patients who stood to benefit most from effective reperfusion, he said.
Dr. O’Brien said that he and his associates had no disclosures.
NEW ORLEANS – The potential advantages of tenecteplase, a modified form of tissue plasminogen activator engineered to outperform the native molecule, finally shone through for treating patients with acute ischemic stroke.
A head-to-head comparison of tenecteplase against recombinant tissue plasminogen activator (TPA; alteplase) showed that the modified molecule produced better reperfusion and better clinical recovery 24 hours after treatment in a randomized trial of 75 Australian patients whose treatment was guided by CT brain imaging, Dr. Bill O’Brien reported at the International Stroke Conference.
Although Dr. O’Brien cautioned that this pilot study did not produce definitive results, the superiority of tenecteplase eclipsed its failure to surpass TPA in a randomized comparison of 112 patients reported in 2010.
The results "support using advanced imaging to identify the patients who are most likely to benefit" from tenecteplase, he said. The use of systematic CT brain imaging to select patients appeared to be the main factor that distinguished this study from the previous trial where tenecteplase failed to show an advantage, said Dr. O’Brien, a stroke physician at John Hunter Hospital in Newcastle, Australia.
Targeted patient selection may also explain why the tenecteplase treatment was so safe, resulting in a trend toward a reduced rate of type 2 parenchymal hemorrhages, compared with TPA, despite the superior thrombolytic activity shown by tenecteplase, he said.
Although the current trial had "compelling results," another study will follow to compare the two drugs by several 3-month outcomes, Dr. O’Brien said in an interview. The successful dosages of tenecteplase tested also have the advantage of being cheaper than the standard TPA dosage, he added.
Developed in the 1990s, tenecteplase resulted from three targeted amino acid substitutions in conventional TPA. This produced a molecule with increased fibrin specificity, and longer plasma half-life due to increased resistance to enzymatic degradation, which meant that it could be administered by an intravenous bolus instead of requiring continuous infusion like TPA. When used to treat myocardial infarctions, tenecteplase has also resulted in fewer bleeding complications than TPA.
The study enrolled 75 patients within 6 hours of onset of an ischemic stroke at any of three Australian hospitals, following selection by CT imaging to identify patients with a small infarct core and a large penumbra of salvageable brain. Researchers randomized patients to receive 0.1 mg/kg tenecteplase or 0.25 mg/kg tenecteplase by an intravenous bolus injection, or standard infusion with 0.9 mg/kg TPA. The patients averaged 70 years old, their average NIHSS (National Institutes of Health Stroke Scale) score at entry was about 14, and patients received treatment an average of about 3 hours after symptom onset.
The reperfusion rate after 24 hours was 78% among all 50 patients treated with tenecteplase, and 55% among the TPA treated patients, a statistically significant difference for one of the study’s two primary end points, Dr. O’Brien said. The second primary end point, change in NIHSS score after 24 hours, improved by an average of 8 points in all 50 tenecteplase patients, and by an average of 3 points in the TPA patients, also a significant difference.
The results appeared to show a dose-response relationship for tenecteplase, with the 25 patients who received the 0.25-mg/kg dosage having better reperfusion rates and reductions in their NIHSS scores than the 25 patients who received a 0.1-mg/kg dosage as well as the patients treated with TPA, but Dr. O’Brien did not report specific numbers from this analysis.
Of the 50 tenecteplase patients, 2 (4%) developed a type 2 parenchymal hemorrhage within 24 hours, compared with 4 (16%) of the 25 TPA-treated patients, a difference that trended toward significance. The rates of symptomatic intracerebral hemorrhages and death at 24 hours were similar with both drugs. But at 90 days after treatment, the combined rate of death or severe disability was 10% in the 50 tenecteplase patients and 25% in the TPA patients, a difference that also trended toward significance.
These findings contrast with a 2010 report from a randomized trial that compared three different tenecteplase dosages with TPA in a multicenter U.S. study of 112 patients (Stroke 2010;41:707-11). The results showed no significant differences between any of the tenecteplase dosages and TPA for any of the outcomes measured out to 90 days after treatment.
The major difference between the U.S. study and the new Australian study was in patient selection, Dr. O’Brien said. "We used imaging to balance the groups," and to select patients who stood to benefit most from effective reperfusion, he said.
Dr. O’Brien said that he and his associates had no disclosures.
FROM THE INTERNATIONAL STROKE CONFERENCE
Desensitization Succeeds for Aspirin-Allergic Heart Patients
ORLANDO – Coronary disease patients with a history of aspirin sensitivity who need to start a daily aspirin regimen can safely and quickly undergo desensitization, often as outpatients, that gets them on the drug, according to two case series reported at the annual meeting of the American Academy of Allergy, Asthma, and Immunology.
The trickiest cases are patients with aspirin-exacerbated respiratory disease (AERD), but both of these programs have steered a handful of AERD patients through desensitization safely and effectively.
"Our cardiologists love that we do this for them," Dr. John A. Saryan said while presenting a poster at the meeting. "They started to ask us 6 years ago; they said they really needed aspirin for patients receiving drug-eluting coronary stents. We tried to talk the cardiologists out of it, but then decided to try it and we had no trouble. By now we’ve done 20, 25 patients," said Dr. Saryan, chairman of allergy and immunology at the Lahey Clinic in Burlington, Mass. He presented details from the first 14 patients he and his associates desensitized. "Ten years ago I would have said it’s nuts, I wouldn’t do it," but his recent experience changed his mind, he said in an interview.
"The patients do very well, with no problems; we have no worries about these patients, but the AERD patients are very different. We do them in a spirometry laboratory, and we get a FEV1 [forced expiratory volume in one second] before each desensitization dose," said Dr. H. James Wedner, professor of medicine and chief of the division of allergy and immunology at Washington University, St. Louis. He presented a poster on 23 coronary disease patients he treated for aspirin desensitization since 1996, but added that he has treated additional patients who required aspirin for treatment of arthritis.
Dr. Wedner’s usual protocol involves no prophylactic anti-allergy treatment. Patients identified as having true aspirin sensitivity by their history start on a supervised protocol of aspirin ingestion using a 10-mg/mL solution made by dissolving an Alka-Seltzer tablet in water. Patients start by drinking a 1-mg dose, waiting 15 minutes, and then taking a 10-mg dose, followed by doses of 20 mg, 40 mg, 80 mg, 160 mg, and 325 mg, each delivered 15 minutes apart. Patients who develop a rash as they progress through the doses receive an antihistamine, such as diphenhydramine (Benedryl) or cetirizine (Zyrtec). Three of the 23 patients in the series developed angioedema, two of them safely continued with the protocol. The third patient decided to withdraw from further desensitization, the only patient in the series who did not complete the process, Dr. Wedner reported.
Once patients do this, they can then safely take an aspirin a day. If they miss a dose for more than 48 hours they have to be desensitized again. In Dr. Wedner's series, 3 of the 23 patients needed a second desensitization course. Ten of the courses occurred on an inpatient basis, the other 16 on an outpatient basis.
Patients with a history of AERD go through the same desensitization steps, but with FEV1 monitoring. If their FEV1 drops by 20% or more at any point the protocol stops and the patient goes home but then comes back and continues the protocol the next day, and "they do better," Dr. Wedner said. Although AERD patients have reactions, they can safely proceed through full desensitization, he said. "Some patients drop their FEV1 twice, and it takes 3 days, but ultimately they all get through."
A key preliminary step is taking a careful history to identify patients with true aspirin sensitivity, he added. "There is no skin test or blood test for aspirin sensitivity. It’s all based on history." About 50% of patients Dr. Wedner sees with suspected aspirin sensitivity had been misidentified and don’t need desensitization.
The protocol Dr. Saryan and his associates at Lahey use involves systematic pretreatment with antihistamines, leukotriene-modifying drugs such as montelukast (Singulair) or zileuton (Zyflo), and in one case, prednisone. Many patients received prophylactic drugs from more than one class. The Lahey physicians gear the aggressiveness of the prophylaxis to each patient’s history: the severity of their past aspirin reactions, and their current medical state. "If a coronary patient is unstable, you don’t want them to have a reaction, as respiratory effects can lead to changes in heart rate" or have other cardiac consequences, noted Dr. David E. Riester, an allergist and immunologist at Lahey who also managed these cases.
They modeled their outpatient desensitization protocol on the method reported in 2000 by physicians at Massachusetts General Hospital in Boston (J. Allergy Clin. Immunol. 2000;105:997-1001). The Lahey group has its pharmacy prepare an aspirin solution that gets dosed to a patient starting with 0.1 mg, and then progressing through doses of 0.3 mg, 1 mg, 3 mg, 10 mg, 20 mg, 40 mg, 81 mg, 162 mg, and finishing with 243 or 325 mg. They separate each dose by 20-30 minutes.
For patients with a history of AERD, they do the desensitization in their CCU or ICU, and space out each incremental dose in the sequence by 2-3 hours, doing the protocol over 2 days. They modeled this approach on a 2003 report by Dr. Donald D. Stevenson of the Scripps Clinic, La Jolla, Calif. (Clin. Rev. Allergy Immunol. 2003;24:159-67).
None of the 14 coronary disease patients in the series from Lahey included patients with AERD. All 14 patients underwent successful desensitization, with 3 having a reaction during the process, in one case a respiratory reaction.
Dr. Saryan, Dr. Wedner, and Dr. Riester said that they had no disclosures.
ORLANDO – Coronary disease patients with a history of aspirin sensitivity who need to start a daily aspirin regimen can safely and quickly undergo desensitization, often as outpatients, that gets them on the drug, according to two case series reported at the annual meeting of the American Academy of Allergy, Asthma, and Immunology.
The trickiest cases are patients with aspirin-exacerbated respiratory disease (AERD), but both of these programs have steered a handful of AERD patients through desensitization safely and effectively.
"Our cardiologists love that we do this for them," Dr. John A. Saryan said while presenting a poster at the meeting. "They started to ask us 6 years ago; they said they really needed aspirin for patients receiving drug-eluting coronary stents. We tried to talk the cardiologists out of it, but then decided to try it and we had no trouble. By now we’ve done 20, 25 patients," said Dr. Saryan, chairman of allergy and immunology at the Lahey Clinic in Burlington, Mass. He presented details from the first 14 patients he and his associates desensitized. "Ten years ago I would have said it’s nuts, I wouldn’t do it," but his recent experience changed his mind, he said in an interview.
"The patients do very well, with no problems; we have no worries about these patients, but the AERD patients are very different. We do them in a spirometry laboratory, and we get a FEV1 [forced expiratory volume in one second] before each desensitization dose," said Dr. H. James Wedner, professor of medicine and chief of the division of allergy and immunology at Washington University, St. Louis. He presented a poster on 23 coronary disease patients he treated for aspirin desensitization since 1996, but added that he has treated additional patients who required aspirin for treatment of arthritis.
Dr. Wedner’s usual protocol involves no prophylactic anti-allergy treatment. Patients identified as having true aspirin sensitivity by their history start on a supervised protocol of aspirin ingestion using a 10-mg/mL solution made by dissolving an Alka-Seltzer tablet in water. Patients start by drinking a 1-mg dose, waiting 15 minutes, and then taking a 10-mg dose, followed by doses of 20 mg, 40 mg, 80 mg, 160 mg, and 325 mg, each delivered 15 minutes apart. Patients who develop a rash as they progress through the doses receive an antihistamine, such as diphenhydramine (Benedryl) or cetirizine (Zyrtec). Three of the 23 patients in the series developed angioedema, two of them safely continued with the protocol. The third patient decided to withdraw from further desensitization, the only patient in the series who did not complete the process, Dr. Wedner reported.
Once patients do this, they can then safely take an aspirin a day. If they miss a dose for more than 48 hours they have to be desensitized again. In Dr. Wedner's series, 3 of the 23 patients needed a second desensitization course. Ten of the courses occurred on an inpatient basis, the other 16 on an outpatient basis.
Patients with a history of AERD go through the same desensitization steps, but with FEV1 monitoring. If their FEV1 drops by 20% or more at any point the protocol stops and the patient goes home but then comes back and continues the protocol the next day, and "they do better," Dr. Wedner said. Although AERD patients have reactions, they can safely proceed through full desensitization, he said. "Some patients drop their FEV1 twice, and it takes 3 days, but ultimately they all get through."
A key preliminary step is taking a careful history to identify patients with true aspirin sensitivity, he added. "There is no skin test or blood test for aspirin sensitivity. It’s all based on history." About 50% of patients Dr. Wedner sees with suspected aspirin sensitivity had been misidentified and don’t need desensitization.
The protocol Dr. Saryan and his associates at Lahey use involves systematic pretreatment with antihistamines, leukotriene-modifying drugs such as montelukast (Singulair) or zileuton (Zyflo), and in one case, prednisone. Many patients received prophylactic drugs from more than one class. The Lahey physicians gear the aggressiveness of the prophylaxis to each patient’s history: the severity of their past aspirin reactions, and their current medical state. "If a coronary patient is unstable, you don’t want them to have a reaction, as respiratory effects can lead to changes in heart rate" or have other cardiac consequences, noted Dr. David E. Riester, an allergist and immunologist at Lahey who also managed these cases.
They modeled their outpatient desensitization protocol on the method reported in 2000 by physicians at Massachusetts General Hospital in Boston (J. Allergy Clin. Immunol. 2000;105:997-1001). The Lahey group has its pharmacy prepare an aspirin solution that gets dosed to a patient starting with 0.1 mg, and then progressing through doses of 0.3 mg, 1 mg, 3 mg, 10 mg, 20 mg, 40 mg, 81 mg, 162 mg, and finishing with 243 or 325 mg. They separate each dose by 20-30 minutes.
For patients with a history of AERD, they do the desensitization in their CCU or ICU, and space out each incremental dose in the sequence by 2-3 hours, doing the protocol over 2 days. They modeled this approach on a 2003 report by Dr. Donald D. Stevenson of the Scripps Clinic, La Jolla, Calif. (Clin. Rev. Allergy Immunol. 2003;24:159-67).
None of the 14 coronary disease patients in the series from Lahey included patients with AERD. All 14 patients underwent successful desensitization, with 3 having a reaction during the process, in one case a respiratory reaction.
Dr. Saryan, Dr. Wedner, and Dr. Riester said that they had no disclosures.
ORLANDO – Coronary disease patients with a history of aspirin sensitivity who need to start a daily aspirin regimen can safely and quickly undergo desensitization, often as outpatients, that gets them on the drug, according to two case series reported at the annual meeting of the American Academy of Allergy, Asthma, and Immunology.
The trickiest cases are patients with aspirin-exacerbated respiratory disease (AERD), but both of these programs have steered a handful of AERD patients through desensitization safely and effectively.
"Our cardiologists love that we do this for them," Dr. John A. Saryan said while presenting a poster at the meeting. "They started to ask us 6 years ago; they said they really needed aspirin for patients receiving drug-eluting coronary stents. We tried to talk the cardiologists out of it, but then decided to try it and we had no trouble. By now we’ve done 20, 25 patients," said Dr. Saryan, chairman of allergy and immunology at the Lahey Clinic in Burlington, Mass. He presented details from the first 14 patients he and his associates desensitized. "Ten years ago I would have said it’s nuts, I wouldn’t do it," but his recent experience changed his mind, he said in an interview.
"The patients do very well, with no problems; we have no worries about these patients, but the AERD patients are very different. We do them in a spirometry laboratory, and we get a FEV1 [forced expiratory volume in one second] before each desensitization dose," said Dr. H. James Wedner, professor of medicine and chief of the division of allergy and immunology at Washington University, St. Louis. He presented a poster on 23 coronary disease patients he treated for aspirin desensitization since 1996, but added that he has treated additional patients who required aspirin for treatment of arthritis.
Dr. Wedner’s usual protocol involves no prophylactic anti-allergy treatment. Patients identified as having true aspirin sensitivity by their history start on a supervised protocol of aspirin ingestion using a 10-mg/mL solution made by dissolving an Alka-Seltzer tablet in water. Patients start by drinking a 1-mg dose, waiting 15 minutes, and then taking a 10-mg dose, followed by doses of 20 mg, 40 mg, 80 mg, 160 mg, and 325 mg, each delivered 15 minutes apart. Patients who develop a rash as they progress through the doses receive an antihistamine, such as diphenhydramine (Benedryl) or cetirizine (Zyrtec). Three of the 23 patients in the series developed angioedema, two of them safely continued with the protocol. The third patient decided to withdraw from further desensitization, the only patient in the series who did not complete the process, Dr. Wedner reported.
Once patients do this, they can then safely take an aspirin a day. If they miss a dose for more than 48 hours they have to be desensitized again. In Dr. Wedner's series, 3 of the 23 patients needed a second desensitization course. Ten of the courses occurred on an inpatient basis, the other 16 on an outpatient basis.
Patients with a history of AERD go through the same desensitization steps, but with FEV1 monitoring. If their FEV1 drops by 20% or more at any point the protocol stops and the patient goes home but then comes back and continues the protocol the next day, and "they do better," Dr. Wedner said. Although AERD patients have reactions, they can safely proceed through full desensitization, he said. "Some patients drop their FEV1 twice, and it takes 3 days, but ultimately they all get through."
A key preliminary step is taking a careful history to identify patients with true aspirin sensitivity, he added. "There is no skin test or blood test for aspirin sensitivity. It’s all based on history." About 50% of patients Dr. Wedner sees with suspected aspirin sensitivity had been misidentified and don’t need desensitization.
The protocol Dr. Saryan and his associates at Lahey use involves systematic pretreatment with antihistamines, leukotriene-modifying drugs such as montelukast (Singulair) or zileuton (Zyflo), and in one case, prednisone. Many patients received prophylactic drugs from more than one class. The Lahey physicians gear the aggressiveness of the prophylaxis to each patient’s history: the severity of their past aspirin reactions, and their current medical state. "If a coronary patient is unstable, you don’t want them to have a reaction, as respiratory effects can lead to changes in heart rate" or have other cardiac consequences, noted Dr. David E. Riester, an allergist and immunologist at Lahey who also managed these cases.
They modeled their outpatient desensitization protocol on the method reported in 2000 by physicians at Massachusetts General Hospital in Boston (J. Allergy Clin. Immunol. 2000;105:997-1001). The Lahey group has its pharmacy prepare an aspirin solution that gets dosed to a patient starting with 0.1 mg, and then progressing through doses of 0.3 mg, 1 mg, 3 mg, 10 mg, 20 mg, 40 mg, 81 mg, 162 mg, and finishing with 243 or 325 mg. They separate each dose by 20-30 minutes.
For patients with a history of AERD, they do the desensitization in their CCU or ICU, and space out each incremental dose in the sequence by 2-3 hours, doing the protocol over 2 days. They modeled this approach on a 2003 report by Dr. Donald D. Stevenson of the Scripps Clinic, La Jolla, Calif. (Clin. Rev. Allergy Immunol. 2003;24:159-67).
None of the 14 coronary disease patients in the series from Lahey included patients with AERD. All 14 patients underwent successful desensitization, with 3 having a reaction during the process, in one case a respiratory reaction.
Dr. Saryan, Dr. Wedner, and Dr. Riester said that they had no disclosures.
FROM THE ANNUAL MEETING OF THE AMERICAN ACADEMY OF ALLERGY, ASTHMA, AND IMMUNOLOGY
Major Finding: Coronary disease patients who required aspirin treatment but had a history of aspirin sensitivity safely and successfully underwent desensitization.
Data Source: Data are from two single-center, U.S. cases series, one with 23 coronary disease patients, the second with 14 coronary patients.
Disclosures: Dr. Saryan, Dr. Wedner, and Dr. Riester said that they had no disclosures.
Sublingual Pollen Pill Delivered Durable Allergy Relief
ORLANDO – Patients who underwent 3 consecutive years of desensitization to grass pollen with seasonal use of a daily, sublingual pill maintained their reduced level of grass pollen allergy during the following fourth season despite stopping pill treatment, according to results from a study of 432 patients who stayed in the study for 4 years.
The findings provide the first evidence that sublingual desensitization produces allergy disease modification similar to subcutaneous desensitization, Dr. Hans-Jørgen Malling said during a poster presentation March 4 at the meeting.
"The data show sustained clinical efficacy when you stop treatment," said Dr. Malling, professor of medicine at the University of Copenhagen and chief of the allergy clinic at Gentofte Hospital in nearby Hellerup. "That is disease modifying. If there is no efficacy after you stop treatment, then this treatment will not be viable."
Although long-term efficacy is crucial, "up to now, it seems like sublingual is equivalent to subcutaneous," he said in an interview.
But Dr. Malling also cautioned that a major test will be the ability of sublingual desensitization to maintain allergy desensitization during a second year off treatment – results that will come during the fifth and final year of the study and will be available a year from now.
Prior reports from the current study documented the ability of the sublingual grass pollen pill to produce desensitization in adult patients during each of the 3 years of active treatment (J. Allergy Clin. Immunol. 2011;128:559-66).
During the first 3 years, the oral desensitization regimen led to progressively higher levels of symptom reduction each year, compared with placebo, a pattern that mimicked what typically occurs with subcutaneous desensitization.
The new results showed that during patients’ first year off active treatment, symptom relief showed a trend toward a small dip in efficacy (see box), compared with placebo, although patients also self reported a maintained, positive effect on their total quality of life scores.
During the first year off treatment, the average adjusted symptom score (the study’s primary end point) was 29% below the placebo level among 136 patients who both took the pill starting 2 months before the start of their allergy season and remained in the study. The symptom score was 23% below placebo among the 142 patients who took the pill starting 4 months before the start of their allergy season and remained in the study.
The study initially randomized 633 adult patients with grass pollen allergy at several European centers. Patients began receiving either the active desensitization tablet or placebo at 2 months or 4 months before the onset of their local grass pollen season, and continued daily treatment through the end of the local pollen season. They then resumed the same treatment schedule during the subsequent 2 years. By the end of the fourth year of the study, roughly two-thirds of the initially enrolled patients remained in the study.
The most common adverse effect during treatment was mouth pruritus, followed by throat irritation. All of the adverse effects were mild or moderate, and their incidence and severity steadily declined during the 3 years of active treatment.
No patients in the study had an anaphylactic reaction, unlike what occurs with subcutaneous injections, which are know to cause anaphylaxis occasionally. The entire worldwide experience with the sublingual grass pollen tablet has so far resulted in six episodes of "doubtful" anaphylaxis, an experience that led Dr. Malling to declare that the sublingual tablet "is safer" than subcutaneous injections.
"For some patients, [sublingual] is probably optimal, while for others subcutaneous will be better," Dr. Malling said. Having both options available gives patients a choice of disease-modifying therapy. One advantage of the sublingual tablet is that it precludes the need for patients to travel to a physician’s office for regular injections, he noted, although being on a schedule of regular injections helps ensure compliance.
Stallergenes developed the sublingual tablet, and has marketed it as Oralair in Europe since 2009. The tablet contains pollen extracts from five grass types that are common throughout Europe.
A pivotal trial of the formulation is in progress in the United States, but has not yet progressed to the stage where patients have stopped active, seasonal treatment. Other companies are also conducting clinical trials to test the efficacy and safety of sublingual desensitization tablets for grass pollen and for other allergens. The new results reported by Dr. Malling and his associates are the first to report what happens once patients stop active, annual treatment.
The study was sponsored by Stallergenes, the company that markets the sublingual grass pollen tablet as Oralair. Dr. Malling said that he is a consultant to and has received research support from Stallergenes and from several other drug companies.
ORLANDO – Patients who underwent 3 consecutive years of desensitization to grass pollen with seasonal use of a daily, sublingual pill maintained their reduced level of grass pollen allergy during the following fourth season despite stopping pill treatment, according to results from a study of 432 patients who stayed in the study for 4 years.
The findings provide the first evidence that sublingual desensitization produces allergy disease modification similar to subcutaneous desensitization, Dr. Hans-Jørgen Malling said during a poster presentation March 4 at the meeting.
"The data show sustained clinical efficacy when you stop treatment," said Dr. Malling, professor of medicine at the University of Copenhagen and chief of the allergy clinic at Gentofte Hospital in nearby Hellerup. "That is disease modifying. If there is no efficacy after you stop treatment, then this treatment will not be viable."
Although long-term efficacy is crucial, "up to now, it seems like sublingual is equivalent to subcutaneous," he said in an interview.
But Dr. Malling also cautioned that a major test will be the ability of sublingual desensitization to maintain allergy desensitization during a second year off treatment – results that will come during the fifth and final year of the study and will be available a year from now.
Prior reports from the current study documented the ability of the sublingual grass pollen pill to produce desensitization in adult patients during each of the 3 years of active treatment (J. Allergy Clin. Immunol. 2011;128:559-66).
During the first 3 years, the oral desensitization regimen led to progressively higher levels of symptom reduction each year, compared with placebo, a pattern that mimicked what typically occurs with subcutaneous desensitization.
The new results showed that during patients’ first year off active treatment, symptom relief showed a trend toward a small dip in efficacy (see box), compared with placebo, although patients also self reported a maintained, positive effect on their total quality of life scores.
During the first year off treatment, the average adjusted symptom score (the study’s primary end point) was 29% below the placebo level among 136 patients who both took the pill starting 2 months before the start of their allergy season and remained in the study. The symptom score was 23% below placebo among the 142 patients who took the pill starting 4 months before the start of their allergy season and remained in the study.
The study initially randomized 633 adult patients with grass pollen allergy at several European centers. Patients began receiving either the active desensitization tablet or placebo at 2 months or 4 months before the onset of their local grass pollen season, and continued daily treatment through the end of the local pollen season. They then resumed the same treatment schedule during the subsequent 2 years. By the end of the fourth year of the study, roughly two-thirds of the initially enrolled patients remained in the study.
The most common adverse effect during treatment was mouth pruritus, followed by throat irritation. All of the adverse effects were mild or moderate, and their incidence and severity steadily declined during the 3 years of active treatment.
No patients in the study had an anaphylactic reaction, unlike what occurs with subcutaneous injections, which are know to cause anaphylaxis occasionally. The entire worldwide experience with the sublingual grass pollen tablet has so far resulted in six episodes of "doubtful" anaphylaxis, an experience that led Dr. Malling to declare that the sublingual tablet "is safer" than subcutaneous injections.
"For some patients, [sublingual] is probably optimal, while for others subcutaneous will be better," Dr. Malling said. Having both options available gives patients a choice of disease-modifying therapy. One advantage of the sublingual tablet is that it precludes the need for patients to travel to a physician’s office for regular injections, he noted, although being on a schedule of regular injections helps ensure compliance.
Stallergenes developed the sublingual tablet, and has marketed it as Oralair in Europe since 2009. The tablet contains pollen extracts from five grass types that are common throughout Europe.
A pivotal trial of the formulation is in progress in the United States, but has not yet progressed to the stage where patients have stopped active, seasonal treatment. Other companies are also conducting clinical trials to test the efficacy and safety of sublingual desensitization tablets for grass pollen and for other allergens. The new results reported by Dr. Malling and his associates are the first to report what happens once patients stop active, annual treatment.
The study was sponsored by Stallergenes, the company that markets the sublingual grass pollen tablet as Oralair. Dr. Malling said that he is a consultant to and has received research support from Stallergenes and from several other drug companies.
ORLANDO – Patients who underwent 3 consecutive years of desensitization to grass pollen with seasonal use of a daily, sublingual pill maintained their reduced level of grass pollen allergy during the following fourth season despite stopping pill treatment, according to results from a study of 432 patients who stayed in the study for 4 years.
The findings provide the first evidence that sublingual desensitization produces allergy disease modification similar to subcutaneous desensitization, Dr. Hans-Jørgen Malling said during a poster presentation March 4 at the meeting.
"The data show sustained clinical efficacy when you stop treatment," said Dr. Malling, professor of medicine at the University of Copenhagen and chief of the allergy clinic at Gentofte Hospital in nearby Hellerup. "That is disease modifying. If there is no efficacy after you stop treatment, then this treatment will not be viable."
Although long-term efficacy is crucial, "up to now, it seems like sublingual is equivalent to subcutaneous," he said in an interview.
But Dr. Malling also cautioned that a major test will be the ability of sublingual desensitization to maintain allergy desensitization during a second year off treatment – results that will come during the fifth and final year of the study and will be available a year from now.
Prior reports from the current study documented the ability of the sublingual grass pollen pill to produce desensitization in adult patients during each of the 3 years of active treatment (J. Allergy Clin. Immunol. 2011;128:559-66).
During the first 3 years, the oral desensitization regimen led to progressively higher levels of symptom reduction each year, compared with placebo, a pattern that mimicked what typically occurs with subcutaneous desensitization.
The new results showed that during patients’ first year off active treatment, symptom relief showed a trend toward a small dip in efficacy (see box), compared with placebo, although patients also self reported a maintained, positive effect on their total quality of life scores.
During the first year off treatment, the average adjusted symptom score (the study’s primary end point) was 29% below the placebo level among 136 patients who both took the pill starting 2 months before the start of their allergy season and remained in the study. The symptom score was 23% below placebo among the 142 patients who took the pill starting 4 months before the start of their allergy season and remained in the study.
The study initially randomized 633 adult patients with grass pollen allergy at several European centers. Patients began receiving either the active desensitization tablet or placebo at 2 months or 4 months before the onset of their local grass pollen season, and continued daily treatment through the end of the local pollen season. They then resumed the same treatment schedule during the subsequent 2 years. By the end of the fourth year of the study, roughly two-thirds of the initially enrolled patients remained in the study.
The most common adverse effect during treatment was mouth pruritus, followed by throat irritation. All of the adverse effects were mild or moderate, and their incidence and severity steadily declined during the 3 years of active treatment.
No patients in the study had an anaphylactic reaction, unlike what occurs with subcutaneous injections, which are know to cause anaphylaxis occasionally. The entire worldwide experience with the sublingual grass pollen tablet has so far resulted in six episodes of "doubtful" anaphylaxis, an experience that led Dr. Malling to declare that the sublingual tablet "is safer" than subcutaneous injections.
"For some patients, [sublingual] is probably optimal, while for others subcutaneous will be better," Dr. Malling said. Having both options available gives patients a choice of disease-modifying therapy. One advantage of the sublingual tablet is that it precludes the need for patients to travel to a physician’s office for regular injections, he noted, although being on a schedule of regular injections helps ensure compliance.
Stallergenes developed the sublingual tablet, and has marketed it as Oralair in Europe since 2009. The tablet contains pollen extracts from five grass types that are common throughout Europe.
A pivotal trial of the formulation is in progress in the United States, but has not yet progressed to the stage where patients have stopped active, seasonal treatment. Other companies are also conducting clinical trials to test the efficacy and safety of sublingual desensitization tablets for grass pollen and for other allergens. The new results reported by Dr. Malling and his associates are the first to report what happens once patients stop active, annual treatment.
The study was sponsored by Stallergenes, the company that markets the sublingual grass pollen tablet as Oralair. Dr. Malling said that he is a consultant to and has received research support from Stallergenes and from several other drug companies.
FROM THE ANNUAL MEETING OF THE AMERICAN ACADEMY OF ALLERGY, ASTHMA, AND IMMUNOLOGY
Major Finding: Grass-allergy patients with 3 years of desensitization with a sublingual tablet maintained desensitization in their first season without treatment.
Data Source: A multicenter, randomized, placebo-controlled study that initially enrolled 633 adult patients with grass pollen allergy.
Disclosures: The study was sponsored by Stallergenes, the company that markets the sublingual grass pollen tablet as Oralair. Dr. Malling said that he is a consultant to and has received research support from Stallergenes and from several other drug companies.
'Drip and Ship' Proves Safe for Stroke
NEW ORLEANS – "Drip and ship" is a safe way to manage patients with acute ischemic stroke, according to results from two separate studies.
A review of 145 ischemic stroke patients who began receiving an intravenous infusion of tissue plasminogen activator (TPA) at a community hospital, followed by transport to a regional primary stroke center, showed a 63% rate of good stroke recoveries and a 3% rate of spontaneous intracranial hemorrhage, Dr. Marilyn M. Rymer reported at the International Stroke Conference.
And a review of 129 acute ischemic stroke patients treated by "drip and ship" throughout Minnesota during October 2008–December 2009 showed that initiating a TPA drip at a community hospital and then shifting the patient to a regional center led to outcomes that were generally identical to those of 473 similar patients who received TPA at a primary center and remained there, Dr. Saqib A. Chaudhry and his associates reported in a poster at the meeting, which was sponsored by the American Heart Association.
The review by Dr. Rymer and her associates included 145 consecutive ischemic stroke patients who arrived at the Mid-American Brain and Stroke Institute at Saint Luke’s Hospital in Kansas City, Mo., in 2008-2010. They came from 63 referring hospitals, including 40 hospitals located more than 50 miles away from Saint Luke’s. In all, 29 of the referring hospitals had 25 or fewer beds, indicating that despite their small size, these community facilities had "pretty sophisticated emergency [department] staffing," said Dr. Rymer, the institute’s medical director.
The shipped patients were an average of 68 years old, and their mean National Institutes of Health Stroke Scale score at admission to Mid-America was 10.4. On arrival, 14 (10%) of the shipped patients had blood pressure higher than 180/105 mm Hg, the maximum level recommended for treatment with IV TPA.
Among the 114 patients in the series who were assessed for their modified Rankin Scale (mRS) score at 90 days after their stroke, 72 patients (63%) had an mRS score of 0-2, a "good" outcome. (The 31 patients who were treated during 2008 did not have an mRS score available at 90 days.) Of all 145 patients in the series, 14% (20 patients) died.
Four of the 145 patients (3%) had a spontaneous intracerebral hemorrhage on arrival to the referral center, which in two cases resulted in death. One of these patients arrived with a blood pressure of 183/77 mm Hg; the other three intracranial hemorrhages occurred in patients with blood pressures below the limit for receiving TPA. Six patients (4%) received an inaccurate diagnosis when they were initially evaluated at a community hospital, but all six of these patients had "excellent" clinical outcomes.
These results show that immediate transfer of patients treated with IV TPA is "safe, with a low rate of spontaneous intracranial hemorrhage en route," said Dr. Rymer, who is also a professor of medicine at the University of Missouri–Kansas City. "The 63% rate of good outcomes may, in part, relate to early treatment with IV TPA at the referring hospitals. Hospitals of every size and location can safely treat stroke patients with IV TPA if they have access to consultation and transfer agreements with experienced stroke centers," she concluded.
The Minnesota study used data collected by the Minnesota Hospital Association; researchers identified patients with ischemic stroke and those treated with IV TPA who were then transported to a referral hospital according to the ICD-9-CM codes in their medical records, said Dr. Chaudhry, a researcher at the stroke research center of the University of Minnesota in Minneapolis.
Among more than 21,000 patients with acute ischemic strokes who were seen at Minnesota hospitals during the 14-month study period, 602 received thrombolytic therapy at 30 different hospitals, with 129 (21%) undergoing "drip and ship" management at 13 of these hospitals, while the other 473 (79%) remained at the centers where they began treatment with IV TPA. The patients averaged about 70 years old, and nearly half were women.
Patient profiles of the shipped and unshipped patients were similar, as were most outcomes including in-hospital mortality and length of stay. The only significant difference in clinical outcomes between these two subgroups during hospitalization was in the rate of intracerebral or subarachnoid hemorrhage, which occurred in 9% of the patients who remained at the site where they first received TPA and in 3% of the shipped patients, a statistically significant difference.
"Drip and ship" was also substantially cheaper. Average hospital charges for the untransported patients ran more than $65,000, whereas patients managed by "drip and ship" had average hospitalization charges of just under $48,000, a statistically significant difference.
Dr. Rymer said that she has been a speaker for Genentech and Concentric Medical. Dr. Chaudhry and his associates had no disclosures.
NEW ORLEANS – "Drip and ship" is a safe way to manage patients with acute ischemic stroke, according to results from two separate studies.
A review of 145 ischemic stroke patients who began receiving an intravenous infusion of tissue plasminogen activator (TPA) at a community hospital, followed by transport to a regional primary stroke center, showed a 63% rate of good stroke recoveries and a 3% rate of spontaneous intracranial hemorrhage, Dr. Marilyn M. Rymer reported at the International Stroke Conference.
And a review of 129 acute ischemic stroke patients treated by "drip and ship" throughout Minnesota during October 2008–December 2009 showed that initiating a TPA drip at a community hospital and then shifting the patient to a regional center led to outcomes that were generally identical to those of 473 similar patients who received TPA at a primary center and remained there, Dr. Saqib A. Chaudhry and his associates reported in a poster at the meeting, which was sponsored by the American Heart Association.
The review by Dr. Rymer and her associates included 145 consecutive ischemic stroke patients who arrived at the Mid-American Brain and Stroke Institute at Saint Luke’s Hospital in Kansas City, Mo., in 2008-2010. They came from 63 referring hospitals, including 40 hospitals located more than 50 miles away from Saint Luke’s. In all, 29 of the referring hospitals had 25 or fewer beds, indicating that despite their small size, these community facilities had "pretty sophisticated emergency [department] staffing," said Dr. Rymer, the institute’s medical director.
The shipped patients were an average of 68 years old, and their mean National Institutes of Health Stroke Scale score at admission to Mid-America was 10.4. On arrival, 14 (10%) of the shipped patients had blood pressure higher than 180/105 mm Hg, the maximum level recommended for treatment with IV TPA.
Among the 114 patients in the series who were assessed for their modified Rankin Scale (mRS) score at 90 days after their stroke, 72 patients (63%) had an mRS score of 0-2, a "good" outcome. (The 31 patients who were treated during 2008 did not have an mRS score available at 90 days.) Of all 145 patients in the series, 14% (20 patients) died.
Four of the 145 patients (3%) had a spontaneous intracerebral hemorrhage on arrival to the referral center, which in two cases resulted in death. One of these patients arrived with a blood pressure of 183/77 mm Hg; the other three intracranial hemorrhages occurred in patients with blood pressures below the limit for receiving TPA. Six patients (4%) received an inaccurate diagnosis when they were initially evaluated at a community hospital, but all six of these patients had "excellent" clinical outcomes.
These results show that immediate transfer of patients treated with IV TPA is "safe, with a low rate of spontaneous intracranial hemorrhage en route," said Dr. Rymer, who is also a professor of medicine at the University of Missouri–Kansas City. "The 63% rate of good outcomes may, in part, relate to early treatment with IV TPA at the referring hospitals. Hospitals of every size and location can safely treat stroke patients with IV TPA if they have access to consultation and transfer agreements with experienced stroke centers," she concluded.
The Minnesota study used data collected by the Minnesota Hospital Association; researchers identified patients with ischemic stroke and those treated with IV TPA who were then transported to a referral hospital according to the ICD-9-CM codes in their medical records, said Dr. Chaudhry, a researcher at the stroke research center of the University of Minnesota in Minneapolis.
Among more than 21,000 patients with acute ischemic strokes who were seen at Minnesota hospitals during the 14-month study period, 602 received thrombolytic therapy at 30 different hospitals, with 129 (21%) undergoing "drip and ship" management at 13 of these hospitals, while the other 473 (79%) remained at the centers where they began treatment with IV TPA. The patients averaged about 70 years old, and nearly half were women.
Patient profiles of the shipped and unshipped patients were similar, as were most outcomes including in-hospital mortality and length of stay. The only significant difference in clinical outcomes between these two subgroups during hospitalization was in the rate of intracerebral or subarachnoid hemorrhage, which occurred in 9% of the patients who remained at the site where they first received TPA and in 3% of the shipped patients, a statistically significant difference.
"Drip and ship" was also substantially cheaper. Average hospital charges for the untransported patients ran more than $65,000, whereas patients managed by "drip and ship" had average hospitalization charges of just under $48,000, a statistically significant difference.
Dr. Rymer said that she has been a speaker for Genentech and Concentric Medical. Dr. Chaudhry and his associates had no disclosures.
NEW ORLEANS – "Drip and ship" is a safe way to manage patients with acute ischemic stroke, according to results from two separate studies.
A review of 145 ischemic stroke patients who began receiving an intravenous infusion of tissue plasminogen activator (TPA) at a community hospital, followed by transport to a regional primary stroke center, showed a 63% rate of good stroke recoveries and a 3% rate of spontaneous intracranial hemorrhage, Dr. Marilyn M. Rymer reported at the International Stroke Conference.
And a review of 129 acute ischemic stroke patients treated by "drip and ship" throughout Minnesota during October 2008–December 2009 showed that initiating a TPA drip at a community hospital and then shifting the patient to a regional center led to outcomes that were generally identical to those of 473 similar patients who received TPA at a primary center and remained there, Dr. Saqib A. Chaudhry and his associates reported in a poster at the meeting, which was sponsored by the American Heart Association.
The review by Dr. Rymer and her associates included 145 consecutive ischemic stroke patients who arrived at the Mid-American Brain and Stroke Institute at Saint Luke’s Hospital in Kansas City, Mo., in 2008-2010. They came from 63 referring hospitals, including 40 hospitals located more than 50 miles away from Saint Luke’s. In all, 29 of the referring hospitals had 25 or fewer beds, indicating that despite their small size, these community facilities had "pretty sophisticated emergency [department] staffing," said Dr. Rymer, the institute’s medical director.
The shipped patients were an average of 68 years old, and their mean National Institutes of Health Stroke Scale score at admission to Mid-America was 10.4. On arrival, 14 (10%) of the shipped patients had blood pressure higher than 180/105 mm Hg, the maximum level recommended for treatment with IV TPA.
Among the 114 patients in the series who were assessed for their modified Rankin Scale (mRS) score at 90 days after their stroke, 72 patients (63%) had an mRS score of 0-2, a "good" outcome. (The 31 patients who were treated during 2008 did not have an mRS score available at 90 days.) Of all 145 patients in the series, 14% (20 patients) died.
Four of the 145 patients (3%) had a spontaneous intracerebral hemorrhage on arrival to the referral center, which in two cases resulted in death. One of these patients arrived with a blood pressure of 183/77 mm Hg; the other three intracranial hemorrhages occurred in patients with blood pressures below the limit for receiving TPA. Six patients (4%) received an inaccurate diagnosis when they were initially evaluated at a community hospital, but all six of these patients had "excellent" clinical outcomes.
These results show that immediate transfer of patients treated with IV TPA is "safe, with a low rate of spontaneous intracranial hemorrhage en route," said Dr. Rymer, who is also a professor of medicine at the University of Missouri–Kansas City. "The 63% rate of good outcomes may, in part, relate to early treatment with IV TPA at the referring hospitals. Hospitals of every size and location can safely treat stroke patients with IV TPA if they have access to consultation and transfer agreements with experienced stroke centers," she concluded.
The Minnesota study used data collected by the Minnesota Hospital Association; researchers identified patients with ischemic stroke and those treated with IV TPA who were then transported to a referral hospital according to the ICD-9-CM codes in their medical records, said Dr. Chaudhry, a researcher at the stroke research center of the University of Minnesota in Minneapolis.
Among more than 21,000 patients with acute ischemic strokes who were seen at Minnesota hospitals during the 14-month study period, 602 received thrombolytic therapy at 30 different hospitals, with 129 (21%) undergoing "drip and ship" management at 13 of these hospitals, while the other 473 (79%) remained at the centers where they began treatment with IV TPA. The patients averaged about 70 years old, and nearly half were women.
Patient profiles of the shipped and unshipped patients were similar, as were most outcomes including in-hospital mortality and length of stay. The only significant difference in clinical outcomes between these two subgroups during hospitalization was in the rate of intracerebral or subarachnoid hemorrhage, which occurred in 9% of the patients who remained at the site where they first received TPA and in 3% of the shipped patients, a statistically significant difference.
"Drip and ship" was also substantially cheaper. Average hospital charges for the untransported patients ran more than $65,000, whereas patients managed by "drip and ship" had average hospitalization charges of just under $48,000, a statistically significant difference.
Dr. Rymer said that she has been a speaker for Genentech and Concentric Medical. Dr. Chaudhry and his associates had no disclosures.
FROM THE INTERNATIONAL STROKE CONFERENCE
Major Finding: In
acute ischemic stroke patients, 63% in one review had an mRS score of 0-2 at 90
days after TPA and transfer to a stroke center; in another review, 129 “ship
and drip” patients had outcomes similar to those of 473 patients who had TPA
but remained in place.
Data Source: Data
are from two reviews, one of 145 patients transferred to a single stroke center
in Missouri, and another of more than 21,000
patients in Minnesota.
Disclosures: Dr.
Rymer said that she has been a speaker for Genentech and Concentric Medical.
Dr. Chaudhry and his associates had no disclosures.
New Details Further Blunt the H5N1 Flu Danger
Dr. Ron Fouchier, one of the two researchers who developed and studied mutant forms of avian H5N1 influenza that’s transmissible through the air, provided new details of his findings at a conference this morning in Washington. He explained that the mutant virus is not nearly as deadly or transmissible as many people have supposed.
This new information seems to be, at least in part, at the root of the different conclusions recently reached by the U.S. National Science Advisory Board for Biosecurity (NSABB) and by a group organized by the World Health Organization (WHO) on whether detailed methods of the H5N1 mutant research should be released to the public. During the past few days, the National Institutes of Health called on the NSABB to meet again to hear the new data and see if it would change the Board’s decision to keep the methods sections of the papers under wraps, Dr. Anthony Fauci said at today’s meeting.
"This virus does not kill ferrets that are sneezed on [by ferrets already infected with mutant H5N1], and if it was released it is unlikely that it would spread like wildfire, and to extrapolate that it would spread like wildfire in humans is really farfetched at this stage," said Dr. Fouchier, a researcher at Erasmus University in Rotterdam. "This virus does not spread like a pandemic or seasonal influenza virus," he said in a session that dealt with H5N1 issues during a meeting on Biodefense and Emerging Diseases sponsored by the American Society for Microbiology. He called any notion that the mutant avian H5N1 flu he created could transmit readily in aerosolized form from ferret-to-ferret a "misperception."
In addition, many people have had a second important misperception of the virus he’s studied: The H5N1 mutant strains he created are not highly lethal.
"It’s very clear that H5N1 is highly lethal in chickens, but in mammals that’s not the case." The mutant form of the virus will kill a ferret if you place a large dose of the virus—a million virions—directly into the animals lower respiratory tract. That kills the animal in about 3 days, he said. But if a more modest and typical inoculum gets introduced intranasally to a ferret, the animal simply gets a flu-like illness but recovers. "We saw no severe disease in any of the seven animals that received virus by aerosol," he said.
A third, heartening observation he’s made about how mammalian-transmissible H5N1 behaves is that ferrets exposed to seasonal flu before exposure to the H5N1 mutant "are fully protected against severe disease." His conclusion from this: "It’s unlikely that humans have no cross protection to H5N1, so very few would develop severe disease. Most [people] would be protected by cross-protective immunity."
According to Dr. Fauci, director of the National Institute of Allergy and Infectious Disease, these clarifications from Dr. Fouchier first came to light earlier this month during a meeting on H5N1 convened by the WHO in Geneva. These new data, as well as the recommendations made by the WHO group, led Dr. Fauci to ask the NSABB to reconvene.
"The NIH continues to support the NSABB recommendations regarding the original manuscripts [to publish redacted versions of the papers], and supports revision of the manuscripts to include new data and explicit clarifications of old data," Dr. Fauci said. "There was obviously a disagreement in the recommendations between Geneva and the NSABB. There was a strong feeling to reconvene the NSABB to give them the benefit of the same information and discussion as in Geneva."
Dr. Ron Fouchier, one of the two researchers who developed and studied mutant forms of avian H5N1 influenza that’s transmissible through the air, provided new details of his findings at a conference this morning in Washington. He explained that the mutant virus is not nearly as deadly or transmissible as many people have supposed.
This new information seems to be, at least in part, at the root of the different conclusions recently reached by the U.S. National Science Advisory Board for Biosecurity (NSABB) and by a group organized by the World Health Organization (WHO) on whether detailed methods of the H5N1 mutant research should be released to the public. During the past few days, the National Institutes of Health called on the NSABB to meet again to hear the new data and see if it would change the Board’s decision to keep the methods sections of the papers under wraps, Dr. Anthony Fauci said at today’s meeting.
"This virus does not kill ferrets that are sneezed on [by ferrets already infected with mutant H5N1], and if it was released it is unlikely that it would spread like wildfire, and to extrapolate that it would spread like wildfire in humans is really farfetched at this stage," said Dr. Fouchier, a researcher at Erasmus University in Rotterdam. "This virus does not spread like a pandemic or seasonal influenza virus," he said in a session that dealt with H5N1 issues during a meeting on Biodefense and Emerging Diseases sponsored by the American Society for Microbiology. He called any notion that the mutant avian H5N1 flu he created could transmit readily in aerosolized form from ferret-to-ferret a "misperception."
In addition, many people have had a second important misperception of the virus he’s studied: The H5N1 mutant strains he created are not highly lethal.
"It’s very clear that H5N1 is highly lethal in chickens, but in mammals that’s not the case." The mutant form of the virus will kill a ferret if you place a large dose of the virus—a million virions—directly into the animals lower respiratory tract. That kills the animal in about 3 days, he said. But if a more modest and typical inoculum gets introduced intranasally to a ferret, the animal simply gets a flu-like illness but recovers. "We saw no severe disease in any of the seven animals that received virus by aerosol," he said.
A third, heartening observation he’s made about how mammalian-transmissible H5N1 behaves is that ferrets exposed to seasonal flu before exposure to the H5N1 mutant "are fully protected against severe disease." His conclusion from this: "It’s unlikely that humans have no cross protection to H5N1, so very few would develop severe disease. Most [people] would be protected by cross-protective immunity."
According to Dr. Fauci, director of the National Institute of Allergy and Infectious Disease, these clarifications from Dr. Fouchier first came to light earlier this month during a meeting on H5N1 convened by the WHO in Geneva. These new data, as well as the recommendations made by the WHO group, led Dr. Fauci to ask the NSABB to reconvene.
"The NIH continues to support the NSABB recommendations regarding the original manuscripts [to publish redacted versions of the papers], and supports revision of the manuscripts to include new data and explicit clarifications of old data," Dr. Fauci said. "There was obviously a disagreement in the recommendations between Geneva and the NSABB. There was a strong feeling to reconvene the NSABB to give them the benefit of the same information and discussion as in Geneva."
Dr. Ron Fouchier, one of the two researchers who developed and studied mutant forms of avian H5N1 influenza that’s transmissible through the air, provided new details of his findings at a conference this morning in Washington. He explained that the mutant virus is not nearly as deadly or transmissible as many people have supposed.
This new information seems to be, at least in part, at the root of the different conclusions recently reached by the U.S. National Science Advisory Board for Biosecurity (NSABB) and by a group organized by the World Health Organization (WHO) on whether detailed methods of the H5N1 mutant research should be released to the public. During the past few days, the National Institutes of Health called on the NSABB to meet again to hear the new data and see if it would change the Board’s decision to keep the methods sections of the papers under wraps, Dr. Anthony Fauci said at today’s meeting.
"This virus does not kill ferrets that are sneezed on [by ferrets already infected with mutant H5N1], and if it was released it is unlikely that it would spread like wildfire, and to extrapolate that it would spread like wildfire in humans is really farfetched at this stage," said Dr. Fouchier, a researcher at Erasmus University in Rotterdam. "This virus does not spread like a pandemic or seasonal influenza virus," he said in a session that dealt with H5N1 issues during a meeting on Biodefense and Emerging Diseases sponsored by the American Society for Microbiology. He called any notion that the mutant avian H5N1 flu he created could transmit readily in aerosolized form from ferret-to-ferret a "misperception."
In addition, many people have had a second important misperception of the virus he’s studied: The H5N1 mutant strains he created are not highly lethal.
"It’s very clear that H5N1 is highly lethal in chickens, but in mammals that’s not the case." The mutant form of the virus will kill a ferret if you place a large dose of the virus—a million virions—directly into the animals lower respiratory tract. That kills the animal in about 3 days, he said. But if a more modest and typical inoculum gets introduced intranasally to a ferret, the animal simply gets a flu-like illness but recovers. "We saw no severe disease in any of the seven animals that received virus by aerosol," he said.
A third, heartening observation he’s made about how mammalian-transmissible H5N1 behaves is that ferrets exposed to seasonal flu before exposure to the H5N1 mutant "are fully protected against severe disease." His conclusion from this: "It’s unlikely that humans have no cross protection to H5N1, so very few would develop severe disease. Most [people] would be protected by cross-protective immunity."
According to Dr. Fauci, director of the National Institute of Allergy and Infectious Disease, these clarifications from Dr. Fouchier first came to light earlier this month during a meeting on H5N1 convened by the WHO in Geneva. These new data, as well as the recommendations made by the WHO group, led Dr. Fauci to ask the NSABB to reconvene.
"The NIH continues to support the NSABB recommendations regarding the original manuscripts [to publish redacted versions of the papers], and supports revision of the manuscripts to include new data and explicit clarifications of old data," Dr. Fauci said. "There was obviously a disagreement in the recommendations between Geneva and the NSABB. There was a strong feeling to reconvene the NSABB to give them the benefit of the same information and discussion as in Geneva."
Meta-Analyses Support Influenza Antivirals, Diagnostic Tests
The two most commonly used antiviral drugs for treating influenza infections, oseltamivir and zanamivir, each provide a net benefit to patients compared with no treatment, concluded the authors of a meta-analysis of 74 influenza antiviral observational studies published online Feb. 27 in Annals of Internal Medicine.
And in a separate report published in the same journal, another group of researchers found in a meta-analysis of 159 studies that evaluated rapid influenza diagnostic tests (RIDTs) that these tests have high specificity for positively identifying a patient infected with influenza but low sensitivity, which means that a negative test result cannot reliably rule out influenza infection (Ann. Intern. Med. 2012;156 [Epub ahead of print Feb. 28).
Both findings support existing U.S. recommendations from the Centers for Disease Control and Prevention (CDC) for the use of neuraminidase-inhibitor anti-influenza drugs and the use of RIDTs.
The antivirals meta-analysis, which was commissioned by the World Health Organization, included 51 observational studies that compared treatment with oral oseltamivir to no antiviral therapy. However, not every study looked at the same types of patients or the same outcomes, or had similar methods. For example, three studies assessed mortality rates in hospitalized patients and adjusted for age and comorbidities. This pooled analysis showed a statistically significant, 77% reduction in mortality compared with no antiviral therapy, suggesting that oral oseltamivir may reduce deaths, although the overall grade for the quality of the evidence was deemed low (Ann. Intern. Med. 2012;156 [Epub ahead of print Feb. 28, 2012]).
A second analysis included nine studies that also looked at the effect of oseltamivir on mortality in hospitalized patients, but these studies did not make any adjustments for possible confounders. The meta-analysis showed a more modest, 49% reduction in mortality.
Other parts of the oseltamivir analysis showed that its use reduced the rate of hospitalization among outpatients by 25%, and the duration of fever by approximately 33 hours. Oseltamivir use also reduced the incidence of neuropsychiatric events, as well as other signs and symptoms of influenza infection.
"Our findings indicate that the use of oral oseltamivir for treatment of influenza may provide net benefit by reducing mortality, the duration of symptoms, and complications of influenza. We observed a large, precise effect of oseltamivir on hospitalization" that was "compatible with the imprecise estimate from randomized controlled trials," wrote Dr. Holger J. Schünemann, chairman of the department of clinical epidemiology and biostatistics at McMaster University, Hamilton, Ont., and his associates.
The meta-analysis also included five observational studies and two surveys that compared the second marketed neuraminidase inhibitor, inhaled zanamivir, with no antiviral therapy in patients treated as outpatients. Two studies addressed hospitalization, and showed that patients with confirmed influenza or influenza-like illness who received zanamivir may be less likely to be hospitalized than patients who received no antiviral, a 34% reduction that was not statistically significant. Pooled results from three studies that reported on symptom duration showed an approximately 23-hour reduced duration of symptoms with zanamivir.
"Inhaled zanamivir reduces signs and symptoms, but we judged the overall confidence in the estimates of effect as very low, owing to imprecise and possibly biased data on mortality and hospitalization," the authors said. "A direct comparison between oral oseltamivir and inhaled zanamivir in eight studies showed that zanamivir may have a slight advantage in shortening the duration of signs and symptoms."
The meta-analysis also found that the evidence on the use of oral amantadine is "sparse, but may suggest a benefit from using this agent for treatment of drug-sensitive influenza A infection."
The analysis also suggested that earlier treatment with antivirals, within 48 hours, "may be of greater benefit" than later treatment.
"The potential positive effect of earlier rather than later administration of oseltamivir on death in hospitalized patients, and suggestions that pregnant women, children, and patients who are immunocompromised may also benefit from treatment, are among the key contributions of our study," the authors concluded. In addition, "we found moderate-quality evidence for the reduction in signs and symptoms from treatment with inhaled zanamivir compared with no treatment. The data suggest that oral oseltamivir could provide a net benefit in the treatment of patients with influenza, including a sizable reduction in hospitalized patients, although our confidence in these effects is low."
"Evidence that treatment with a neuraminidase inhibitor offers significant benefit in people with influenza is growing," commented Dr. Timothy R. Peters, a pediatric infectious diseases physician at Wake Forest University, Winston-Salem, N.C. As expected, those benefits are likely greatest in patients at highest risk for severe influenza disease, including pregnant women, young children, and immunocompromised patients. The findings of the meta-analysis generally support the recommendations of the CDC that encourage clinicians to aggressively protect influenza-infected patients at high risk for severe disease by using a neuraminidase inhibitor (MMWR 2011;60:1-24). Once the 2009 H1N1 pandemic ended, "it may be that clinicians do not prescribe neuraminidase inhibitors as aggressively as the CDC recommendations would support," he said.
The meta-analysis of RIDTs included 159 studies that assessed the accuracy of 26 commercially marketed RIDTs; 119 of the studies compared one or more RIDTs against either of the two reference standards for influenza infection, viral culture, or reverse transcriptase-polymerase chain reaction. The analysis showed that specificity rates ranged from 51% to 100%, and that sensitivity rates ranged from 4% to 100%. The pooled sensitivity was 62%, and the pooled specificity was 98%, reported Dr. Caroline Chartrand, a pediatrician at CHU Sainte-Justine in Montreal, and her associates.
"Overall, RIDTs have high specificity, with modest and highly variable sensitivity," the authors concluded. "This means that a positive test is unlikely to be a false positive result." In the presence of a positive RIDT result, "a clinician can confidently make the diagnosis of influenza and begin appropriate infection-control measures and antiviral therapy, if indicated, while forgoing unnecessary additional diagnostic testing and antibiotic prescription. However, a negative RIDT result has a reasonable likelihood of being a false negative and should be confirmed by other laboratory diagnostic tests if the result is likely to affect patient management."
The analysis also showed that RIDTs performed better in children than in adults, with approximately 13% higher sensitivity in children. "This is plausible because young children have higher viral loads and longer viral shedding than adults," the authors said. RIDTs also showed higher sensitivity for detecting influenza A compared with influenza B.
"Overall, no commercial brand of RIDT seemed to perform markedly better or worse than the others, but this finding should be interpreted cautiously because head-to-head comparisons were not done in most studies. Administration of the RIDT by personnel other than a trained laboratory technician does not seem to adversely influence the performance of these tests," they added.
"The most important advantage of RIDTs is their rapid turnaround time. RIDTs fill a void at the point of care that no other test is likely to fill in the near future. As long as clinicians understand the limitations of RIDTs, namely that a negative result is unreliable and should be confirmed by using culture or RT-PCR, RIDTs could enable clinicians to institute prompt infection-control measures, begin antiviral treatment in high-risk populations, and make informed decisions about further diagnostic interventions," the authors concluded.
The meta-analysis results "support the notion that a positive rapid test result is much more helpful to the clinician than a negative result," commented Dr. Peters. "Positive test results can prompt early, appropriate initiation of neuraminidase inhibitor therapy. A negative result may occur in an influenza-infected patient at high risk for severe disease, especially in adults who shed less virus [than children] when infected. Clinicians should be especially suspicious of negative test results at times when they are seeing a lot of influenza disease, and they should be prepared to initiate early neuraminidase inhibitor therapy when their clinical suspicion for influenza is high despite a negative test result," Dr. Peters said.
Dr. Schünemann and his associates, Dr. Chartrand and her associates, and Dr. Peters said that they had no disclosures.
The meta-analysis on the efficacy of oseltamivir and zanamivir for treating influenza, especially in severely infected or high-risk patients, is very important. The controlled clinical trials for these drugs excluded hospitalized patients, pregnant women, and patients at very high risk for complications. But these are precisely the patients who face the greatest risk from influenza and need effective therapy. Because it is unlikely that placebo-controlled, randomized trials of these drugs will ever be done in these populations, our only recourse is to use observational studies for guidance on whether these drugs work in these critical settings.
The observational studies reviewed in this report show the consistent finding that antiviral treatment of influenza infections in patients who need hospitalization or are pregnant prevented death and ICU admissions. The results of these studies, individually, had already led to recommendations for using antivirals in these patients from the Centers for Disease Control and Prevention and from the Infectious Diseases Society of America (Clin. Infect. Dis. 2009;48:1003-32). The new meta-analysis highlights these benefits in a more detailed way. More importantly, the new analysis provides evidence that treating high-risk patients infected with influenza with one of these drugs prevents the outcomes that we care about the most: death, hospitalization, and complications.
Observational studies are always subject to more limitations than properly controlled randomized trials, but the consistency of the evidence and the attempts to control for bias in the meta-analysis improve the quality of the evidence.
I believe that most clinicians are already aware of the CDC recommendations on antiviral use for influenza. In recent years we have seen improved rates of treatment of high-risk patients with an antiviral, but too many patients remain untreated. I worry that some clinicians have only heard the message that the results from randomized trials showed only modest benefits in otherwise healthy influenza patients. These new meta-analysis results should reassure them that there is solid evidence for benefit in high-risk patients.
The meta-analysis of RIDTs involved data that, I think, were generally better known to flu experts. The meta-analysis deals with a wide range of test types and flu seasons, and that is both a strength and weakness of the analysis.
The results show that the relatively low sensitivity of the RIDTs is a consistent finding from year to year and in study to study, and that this produces a low negative predictive value. An important message from this new analysis is that RIDT sensitivity is consistently lower among adult patients. During the 2009 H1N1 pandemic, we saw that many clinicians did not appreciate that a negative test did not rule out influenza. Since then, the CDC has issued statements on how to best use RIDTs in practice, but I’m not sure how much of this misuse has changed.
It is reassuring to see that the positive predictive value of RIDTs is consistently high according to the meta-analysis. RIDTs can be very useful when positive for limiting additional testing and avoiding the use of antibiotics. Results from several studies showed that clinicians change their behavior when they get a positive RIDT result. The low negative predictive value of these tests means that a negative RIDT result should not be the reason to not use antiviral treatment. That is a major shortcoming of RIDTs. The role of RIDT on management of patients with suspected influenza would be greater if and when a more sensitive, point-of-care test becomes available.
ANDREW T. PAVIA, M.D., is a professor and chief of the division of pediatric infectious diseases at the University of Utah in Salt Lake City. He said that he has no disclosures. He made these comments in an interview.
The meta-analysis on the efficacy of oseltamivir and zanamivir for treating influenza, especially in severely infected or high-risk patients, is very important. The controlled clinical trials for these drugs excluded hospitalized patients, pregnant women, and patients at very high risk for complications. But these are precisely the patients who face the greatest risk from influenza and need effective therapy. Because it is unlikely that placebo-controlled, randomized trials of these drugs will ever be done in these populations, our only recourse is to use observational studies for guidance on whether these drugs work in these critical settings.
The observational studies reviewed in this report show the consistent finding that antiviral treatment of influenza infections in patients who need hospitalization or are pregnant prevented death and ICU admissions. The results of these studies, individually, had already led to recommendations for using antivirals in these patients from the Centers for Disease Control and Prevention and from the Infectious Diseases Society of America (Clin. Infect. Dis. 2009;48:1003-32). The new meta-analysis highlights these benefits in a more detailed way. More importantly, the new analysis provides evidence that treating high-risk patients infected with influenza with one of these drugs prevents the outcomes that we care about the most: death, hospitalization, and complications.
Observational studies are always subject to more limitations than properly controlled randomized trials, but the consistency of the evidence and the attempts to control for bias in the meta-analysis improve the quality of the evidence.
I believe that most clinicians are already aware of the CDC recommendations on antiviral use for influenza. In recent years we have seen improved rates of treatment of high-risk patients with an antiviral, but too many patients remain untreated. I worry that some clinicians have only heard the message that the results from randomized trials showed only modest benefits in otherwise healthy influenza patients. These new meta-analysis results should reassure them that there is solid evidence for benefit in high-risk patients.
The meta-analysis of RIDTs involved data that, I think, were generally better known to flu experts. The meta-analysis deals with a wide range of test types and flu seasons, and that is both a strength and weakness of the analysis.
The results show that the relatively low sensitivity of the RIDTs is a consistent finding from year to year and in study to study, and that this produces a low negative predictive value. An important message from this new analysis is that RIDT sensitivity is consistently lower among adult patients. During the 2009 H1N1 pandemic, we saw that many clinicians did not appreciate that a negative test did not rule out influenza. Since then, the CDC has issued statements on how to best use RIDTs in practice, but I’m not sure how much of this misuse has changed.
It is reassuring to see that the positive predictive value of RIDTs is consistently high according to the meta-analysis. RIDTs can be very useful when positive for limiting additional testing and avoiding the use of antibiotics. Results from several studies showed that clinicians change their behavior when they get a positive RIDT result. The low negative predictive value of these tests means that a negative RIDT result should not be the reason to not use antiviral treatment. That is a major shortcoming of RIDTs. The role of RIDT on management of patients with suspected influenza would be greater if and when a more sensitive, point-of-care test becomes available.
ANDREW T. PAVIA, M.D., is a professor and chief of the division of pediatric infectious diseases at the University of Utah in Salt Lake City. He said that he has no disclosures. He made these comments in an interview.
The meta-analysis on the efficacy of oseltamivir and zanamivir for treating influenza, especially in severely infected or high-risk patients, is very important. The controlled clinical trials for these drugs excluded hospitalized patients, pregnant women, and patients at very high risk for complications. But these are precisely the patients who face the greatest risk from influenza and need effective therapy. Because it is unlikely that placebo-controlled, randomized trials of these drugs will ever be done in these populations, our only recourse is to use observational studies for guidance on whether these drugs work in these critical settings.
The observational studies reviewed in this report show the consistent finding that antiviral treatment of influenza infections in patients who need hospitalization or are pregnant prevented death and ICU admissions. The results of these studies, individually, had already led to recommendations for using antivirals in these patients from the Centers for Disease Control and Prevention and from the Infectious Diseases Society of America (Clin. Infect. Dis. 2009;48:1003-32). The new meta-analysis highlights these benefits in a more detailed way. More importantly, the new analysis provides evidence that treating high-risk patients infected with influenza with one of these drugs prevents the outcomes that we care about the most: death, hospitalization, and complications.
Observational studies are always subject to more limitations than properly controlled randomized trials, but the consistency of the evidence and the attempts to control for bias in the meta-analysis improve the quality of the evidence.
I believe that most clinicians are already aware of the CDC recommendations on antiviral use for influenza. In recent years we have seen improved rates of treatment of high-risk patients with an antiviral, but too many patients remain untreated. I worry that some clinicians have only heard the message that the results from randomized trials showed only modest benefits in otherwise healthy influenza patients. These new meta-analysis results should reassure them that there is solid evidence for benefit in high-risk patients.
The meta-analysis of RIDTs involved data that, I think, were generally better known to flu experts. The meta-analysis deals with a wide range of test types and flu seasons, and that is both a strength and weakness of the analysis.
The results show that the relatively low sensitivity of the RIDTs is a consistent finding from year to year and in study to study, and that this produces a low negative predictive value. An important message from this new analysis is that RIDT sensitivity is consistently lower among adult patients. During the 2009 H1N1 pandemic, we saw that many clinicians did not appreciate that a negative test did not rule out influenza. Since then, the CDC has issued statements on how to best use RIDTs in practice, but I’m not sure how much of this misuse has changed.
It is reassuring to see that the positive predictive value of RIDTs is consistently high according to the meta-analysis. RIDTs can be very useful when positive for limiting additional testing and avoiding the use of antibiotics. Results from several studies showed that clinicians change their behavior when they get a positive RIDT result. The low negative predictive value of these tests means that a negative RIDT result should not be the reason to not use antiviral treatment. That is a major shortcoming of RIDTs. The role of RIDT on management of patients with suspected influenza would be greater if and when a more sensitive, point-of-care test becomes available.
ANDREW T. PAVIA, M.D., is a professor and chief of the division of pediatric infectious diseases at the University of Utah in Salt Lake City. He said that he has no disclosures. He made these comments in an interview.
The two most commonly used antiviral drugs for treating influenza infections, oseltamivir and zanamivir, each provide a net benefit to patients compared with no treatment, concluded the authors of a meta-analysis of 74 influenza antiviral observational studies published online Feb. 27 in Annals of Internal Medicine.
And in a separate report published in the same journal, another group of researchers found in a meta-analysis of 159 studies that evaluated rapid influenza diagnostic tests (RIDTs) that these tests have high specificity for positively identifying a patient infected with influenza but low sensitivity, which means that a negative test result cannot reliably rule out influenza infection (Ann. Intern. Med. 2012;156 [Epub ahead of print Feb. 28).
Both findings support existing U.S. recommendations from the Centers for Disease Control and Prevention (CDC) for the use of neuraminidase-inhibitor anti-influenza drugs and the use of RIDTs.
The antivirals meta-analysis, which was commissioned by the World Health Organization, included 51 observational studies that compared treatment with oral oseltamivir to no antiviral therapy. However, not every study looked at the same types of patients or the same outcomes, or had similar methods. For example, three studies assessed mortality rates in hospitalized patients and adjusted for age and comorbidities. This pooled analysis showed a statistically significant, 77% reduction in mortality compared with no antiviral therapy, suggesting that oral oseltamivir may reduce deaths, although the overall grade for the quality of the evidence was deemed low (Ann. Intern. Med. 2012;156 [Epub ahead of print Feb. 28, 2012]).
A second analysis included nine studies that also looked at the effect of oseltamivir on mortality in hospitalized patients, but these studies did not make any adjustments for possible confounders. The meta-analysis showed a more modest, 49% reduction in mortality.
Other parts of the oseltamivir analysis showed that its use reduced the rate of hospitalization among outpatients by 25%, and the duration of fever by approximately 33 hours. Oseltamivir use also reduced the incidence of neuropsychiatric events, as well as other signs and symptoms of influenza infection.
"Our findings indicate that the use of oral oseltamivir for treatment of influenza may provide net benefit by reducing mortality, the duration of symptoms, and complications of influenza. We observed a large, precise effect of oseltamivir on hospitalization" that was "compatible with the imprecise estimate from randomized controlled trials," wrote Dr. Holger J. Schünemann, chairman of the department of clinical epidemiology and biostatistics at McMaster University, Hamilton, Ont., and his associates.
The meta-analysis also included five observational studies and two surveys that compared the second marketed neuraminidase inhibitor, inhaled zanamivir, with no antiviral therapy in patients treated as outpatients. Two studies addressed hospitalization, and showed that patients with confirmed influenza or influenza-like illness who received zanamivir may be less likely to be hospitalized than patients who received no antiviral, a 34% reduction that was not statistically significant. Pooled results from three studies that reported on symptom duration showed an approximately 23-hour reduced duration of symptoms with zanamivir.
"Inhaled zanamivir reduces signs and symptoms, but we judged the overall confidence in the estimates of effect as very low, owing to imprecise and possibly biased data on mortality and hospitalization," the authors said. "A direct comparison between oral oseltamivir and inhaled zanamivir in eight studies showed that zanamivir may have a slight advantage in shortening the duration of signs and symptoms."
The meta-analysis also found that the evidence on the use of oral amantadine is "sparse, but may suggest a benefit from using this agent for treatment of drug-sensitive influenza A infection."
The analysis also suggested that earlier treatment with antivirals, within 48 hours, "may be of greater benefit" than later treatment.
"The potential positive effect of earlier rather than later administration of oseltamivir on death in hospitalized patients, and suggestions that pregnant women, children, and patients who are immunocompromised may also benefit from treatment, are among the key contributions of our study," the authors concluded. In addition, "we found moderate-quality evidence for the reduction in signs and symptoms from treatment with inhaled zanamivir compared with no treatment. The data suggest that oral oseltamivir could provide a net benefit in the treatment of patients with influenza, including a sizable reduction in hospitalized patients, although our confidence in these effects is low."
"Evidence that treatment with a neuraminidase inhibitor offers significant benefit in people with influenza is growing," commented Dr. Timothy R. Peters, a pediatric infectious diseases physician at Wake Forest University, Winston-Salem, N.C. As expected, those benefits are likely greatest in patients at highest risk for severe influenza disease, including pregnant women, young children, and immunocompromised patients. The findings of the meta-analysis generally support the recommendations of the CDC that encourage clinicians to aggressively protect influenza-infected patients at high risk for severe disease by using a neuraminidase inhibitor (MMWR 2011;60:1-24). Once the 2009 H1N1 pandemic ended, "it may be that clinicians do not prescribe neuraminidase inhibitors as aggressively as the CDC recommendations would support," he said.
The meta-analysis of RIDTs included 159 studies that assessed the accuracy of 26 commercially marketed RIDTs; 119 of the studies compared one or more RIDTs against either of the two reference standards for influenza infection, viral culture, or reverse transcriptase-polymerase chain reaction. The analysis showed that specificity rates ranged from 51% to 100%, and that sensitivity rates ranged from 4% to 100%. The pooled sensitivity was 62%, and the pooled specificity was 98%, reported Dr. Caroline Chartrand, a pediatrician at CHU Sainte-Justine in Montreal, and her associates.
"Overall, RIDTs have high specificity, with modest and highly variable sensitivity," the authors concluded. "This means that a positive test is unlikely to be a false positive result." In the presence of a positive RIDT result, "a clinician can confidently make the diagnosis of influenza and begin appropriate infection-control measures and antiviral therapy, if indicated, while forgoing unnecessary additional diagnostic testing and antibiotic prescription. However, a negative RIDT result has a reasonable likelihood of being a false negative and should be confirmed by other laboratory diagnostic tests if the result is likely to affect patient management."
The analysis also showed that RIDTs performed better in children than in adults, with approximately 13% higher sensitivity in children. "This is plausible because young children have higher viral loads and longer viral shedding than adults," the authors said. RIDTs also showed higher sensitivity for detecting influenza A compared with influenza B.
"Overall, no commercial brand of RIDT seemed to perform markedly better or worse than the others, but this finding should be interpreted cautiously because head-to-head comparisons were not done in most studies. Administration of the RIDT by personnel other than a trained laboratory technician does not seem to adversely influence the performance of these tests," they added.
"The most important advantage of RIDTs is their rapid turnaround time. RIDTs fill a void at the point of care that no other test is likely to fill in the near future. As long as clinicians understand the limitations of RIDTs, namely that a negative result is unreliable and should be confirmed by using culture or RT-PCR, RIDTs could enable clinicians to institute prompt infection-control measures, begin antiviral treatment in high-risk populations, and make informed decisions about further diagnostic interventions," the authors concluded.
The meta-analysis results "support the notion that a positive rapid test result is much more helpful to the clinician than a negative result," commented Dr. Peters. "Positive test results can prompt early, appropriate initiation of neuraminidase inhibitor therapy. A negative result may occur in an influenza-infected patient at high risk for severe disease, especially in adults who shed less virus [than children] when infected. Clinicians should be especially suspicious of negative test results at times when they are seeing a lot of influenza disease, and they should be prepared to initiate early neuraminidase inhibitor therapy when their clinical suspicion for influenza is high despite a negative test result," Dr. Peters said.
Dr. Schünemann and his associates, Dr. Chartrand and her associates, and Dr. Peters said that they had no disclosures.
The two most commonly used antiviral drugs for treating influenza infections, oseltamivir and zanamivir, each provide a net benefit to patients compared with no treatment, concluded the authors of a meta-analysis of 74 influenza antiviral observational studies published online Feb. 27 in Annals of Internal Medicine.
And in a separate report published in the same journal, another group of researchers found in a meta-analysis of 159 studies that evaluated rapid influenza diagnostic tests (RIDTs) that these tests have high specificity for positively identifying a patient infected with influenza but low sensitivity, which means that a negative test result cannot reliably rule out influenza infection (Ann. Intern. Med. 2012;156 [Epub ahead of print Feb. 28).
Both findings support existing U.S. recommendations from the Centers for Disease Control and Prevention (CDC) for the use of neuraminidase-inhibitor anti-influenza drugs and the use of RIDTs.
The antivirals meta-analysis, which was commissioned by the World Health Organization, included 51 observational studies that compared treatment with oral oseltamivir to no antiviral therapy. However, not every study looked at the same types of patients or the same outcomes, or had similar methods. For example, three studies assessed mortality rates in hospitalized patients and adjusted for age and comorbidities. This pooled analysis showed a statistically significant, 77% reduction in mortality compared with no antiviral therapy, suggesting that oral oseltamivir may reduce deaths, although the overall grade for the quality of the evidence was deemed low (Ann. Intern. Med. 2012;156 [Epub ahead of print Feb. 28, 2012]).
A second analysis included nine studies that also looked at the effect of oseltamivir on mortality in hospitalized patients, but these studies did not make any adjustments for possible confounders. The meta-analysis showed a more modest, 49% reduction in mortality.
Other parts of the oseltamivir analysis showed that its use reduced the rate of hospitalization among outpatients by 25%, and the duration of fever by approximately 33 hours. Oseltamivir use also reduced the incidence of neuropsychiatric events, as well as other signs and symptoms of influenza infection.
"Our findings indicate that the use of oral oseltamivir for treatment of influenza may provide net benefit by reducing mortality, the duration of symptoms, and complications of influenza. We observed a large, precise effect of oseltamivir on hospitalization" that was "compatible with the imprecise estimate from randomized controlled trials," wrote Dr. Holger J. Schünemann, chairman of the department of clinical epidemiology and biostatistics at McMaster University, Hamilton, Ont., and his associates.
The meta-analysis also included five observational studies and two surveys that compared the second marketed neuraminidase inhibitor, inhaled zanamivir, with no antiviral therapy in patients treated as outpatients. Two studies addressed hospitalization, and showed that patients with confirmed influenza or influenza-like illness who received zanamivir may be less likely to be hospitalized than patients who received no antiviral, a 34% reduction that was not statistically significant. Pooled results from three studies that reported on symptom duration showed an approximately 23-hour reduced duration of symptoms with zanamivir.
"Inhaled zanamivir reduces signs and symptoms, but we judged the overall confidence in the estimates of effect as very low, owing to imprecise and possibly biased data on mortality and hospitalization," the authors said. "A direct comparison between oral oseltamivir and inhaled zanamivir in eight studies showed that zanamivir may have a slight advantage in shortening the duration of signs and symptoms."
The meta-analysis also found that the evidence on the use of oral amantadine is "sparse, but may suggest a benefit from using this agent for treatment of drug-sensitive influenza A infection."
The analysis also suggested that earlier treatment with antivirals, within 48 hours, "may be of greater benefit" than later treatment.
"The potential positive effect of earlier rather than later administration of oseltamivir on death in hospitalized patients, and suggestions that pregnant women, children, and patients who are immunocompromised may also benefit from treatment, are among the key contributions of our study," the authors concluded. In addition, "we found moderate-quality evidence for the reduction in signs and symptoms from treatment with inhaled zanamivir compared with no treatment. The data suggest that oral oseltamivir could provide a net benefit in the treatment of patients with influenza, including a sizable reduction in hospitalized patients, although our confidence in these effects is low."
"Evidence that treatment with a neuraminidase inhibitor offers significant benefit in people with influenza is growing," commented Dr. Timothy R. Peters, a pediatric infectious diseases physician at Wake Forest University, Winston-Salem, N.C. As expected, those benefits are likely greatest in patients at highest risk for severe influenza disease, including pregnant women, young children, and immunocompromised patients. The findings of the meta-analysis generally support the recommendations of the CDC that encourage clinicians to aggressively protect influenza-infected patients at high risk for severe disease by using a neuraminidase inhibitor (MMWR 2011;60:1-24). Once the 2009 H1N1 pandemic ended, "it may be that clinicians do not prescribe neuraminidase inhibitors as aggressively as the CDC recommendations would support," he said.
The meta-analysis of RIDTs included 159 studies that assessed the accuracy of 26 commercially marketed RIDTs; 119 of the studies compared one or more RIDTs against either of the two reference standards for influenza infection, viral culture, or reverse transcriptase-polymerase chain reaction. The analysis showed that specificity rates ranged from 51% to 100%, and that sensitivity rates ranged from 4% to 100%. The pooled sensitivity was 62%, and the pooled specificity was 98%, reported Dr. Caroline Chartrand, a pediatrician at CHU Sainte-Justine in Montreal, and her associates.
"Overall, RIDTs have high specificity, with modest and highly variable sensitivity," the authors concluded. "This means that a positive test is unlikely to be a false positive result." In the presence of a positive RIDT result, "a clinician can confidently make the diagnosis of influenza and begin appropriate infection-control measures and antiviral therapy, if indicated, while forgoing unnecessary additional diagnostic testing and antibiotic prescription. However, a negative RIDT result has a reasonable likelihood of being a false negative and should be confirmed by other laboratory diagnostic tests if the result is likely to affect patient management."
The analysis also showed that RIDTs performed better in children than in adults, with approximately 13% higher sensitivity in children. "This is plausible because young children have higher viral loads and longer viral shedding than adults," the authors said. RIDTs also showed higher sensitivity for detecting influenza A compared with influenza B.
"Overall, no commercial brand of RIDT seemed to perform markedly better or worse than the others, but this finding should be interpreted cautiously because head-to-head comparisons were not done in most studies. Administration of the RIDT by personnel other than a trained laboratory technician does not seem to adversely influence the performance of these tests," they added.
"The most important advantage of RIDTs is their rapid turnaround time. RIDTs fill a void at the point of care that no other test is likely to fill in the near future. As long as clinicians understand the limitations of RIDTs, namely that a negative result is unreliable and should be confirmed by using culture or RT-PCR, RIDTs could enable clinicians to institute prompt infection-control measures, begin antiviral treatment in high-risk populations, and make informed decisions about further diagnostic interventions," the authors concluded.
The meta-analysis results "support the notion that a positive rapid test result is much more helpful to the clinician than a negative result," commented Dr. Peters. "Positive test results can prompt early, appropriate initiation of neuraminidase inhibitor therapy. A negative result may occur in an influenza-infected patient at high risk for severe disease, especially in adults who shed less virus [than children] when infected. Clinicians should be especially suspicious of negative test results at times when they are seeing a lot of influenza disease, and they should be prepared to initiate early neuraminidase inhibitor therapy when their clinical suspicion for influenza is high despite a negative test result," Dr. Peters said.
Dr. Schünemann and his associates, Dr. Chartrand and her associates, and Dr. Peters said that they had no disclosures.
FROM ANNALS OF INTERNAL MEDICINE
U.S. 2011-2012 Flu Season Gets Late Start
The Centers for Disease Control and Prevention officially declared that the U.S. 2011-2012 influenza season had begun Feb. 24, based on data through Feb. 18, the latest opening to the influenza season in 29 years.
For the time being, the season remains in relatively low gear, although it’s gaining some momentum: Only California and Colorado are showing "widespread" flu activity, while 14 states continue to show "sporadic" activity, according to the latest CDC data.
"While seasonal flu activity is currently low, we expect it to increase in coming weeks," said Dr. Joseph Bresee, chief of the CDC’s Epidemiology and Prevention Branch in the Influenza Division. "The U.S. is experiencing a late start to the flu season this year, but activity has increased in the past few weeks."
That pattern "is unusual, but not unprecedented," he said. Over the past 35 years, peak U.S. flu activity occurred in March four times, and in April twice, Dr. Bresee noted.
"We could speculate as to the reasons" for the late and mild season so far, he added, "but flu is inherently unpredictable." Among Dr. Bresee’s possible explanations:
• This year’s flu strains mimic last year’s. The U.S. flu strains circulating so far have been predominantly A/H3N2, constituting more than 70% of infections, followed by the 2009 A/H1N1 pandemic strain and a B strain. That pattern closely mimics the strains that circulated last year. "So, it is possible there is higher immunity to these viruses than last year, and that leads to less transmission," he said.
• More people are vaccinated this year. The pattern of circulating flu so far closely matches the seasonal 2011-2012 vaccine, and vaccine coverage rates continue to trend upward this year. That should translate into less transmission and disease over time, especially as people at high risk for infection and complications continue to get vaccinated.
"I think that increased vaccine coverage is certainly playing a role," he said. As of mid-February, U.S. distribution of the 2011-2012 seasonal flu vaccine reached 132 million doses. While that number is slightly below the rate for all of last season, there is still time before the current season’s number wraps.
As of last November, U.S. flu vaccination rates were running ahead of November 2010. And while no high-risk populations have yet come close to the 80% vaccination rate that is the CDC’s goal, coverage rates in pregnant women and children have trended up "slowly but steadily over the past couple of years," Dr. Bresee said.
• Residual immunity may play a part. Many Americans continue to have high levels of antibody against the 2009 H1N1 pandemic strain, a factor that "probably plays a role in the [low] amount of H1N1 we’re seeing this year," he said.
• Mother Nature may be helping. Finally, the relatively mild winter that has occurred in much of the country is a factor that "might play a role," although Dr. Bresee cautioned that the influence that the weather has on flu transmission is poorly understood.
Other elements that together paint an upbeat picture of the current flu season are easier to document with data.
As of Feb. 18, three children had died as a result of influenza infection. To put that number in perspective, since the CDC first made flu-related pediatric death reportable in 2004, the full seasonal number ranged from 46 in 2005-2006 to 282 in 2009-2010, with 340 pediatric flu-related deaths during the entire 2009 H1N1 pandemic.
Another positive is that so far this season, not a single flu isolate tested has shown resistance to oseltamivir or zanamivir, data that "reinforce the value of these medications, especially for people with serious illness due to underlying health conditions," Dr. Bresee said.
And so far, hospitalization rates for patients with laboratory-confirmed influenza have been low. Since Oct. 1, the CDC tallied a national total of 347 laboratory-confirmed infections that required hospitalization, a rate of 1.3 cases/100,000 people.
The CDC declares the start of the annual flu season when its national surveillance statistics show 3 consecutive weeks when at least 10% of collected respiratory specimens are positive for flu. During the week ending Feb. 18, that percentage was 14.4%, following levels of 15.5% for the week ending Feb. 11, and 10.5% for the week ending Feb. 4.
Dr. Bresee had no disclosures.
The Centers for Disease Control and Prevention officially declared that the U.S. 2011-2012 influenza season had begun Feb. 24, based on data through Feb. 18, the latest opening to the influenza season in 29 years.
For the time being, the season remains in relatively low gear, although it’s gaining some momentum: Only California and Colorado are showing "widespread" flu activity, while 14 states continue to show "sporadic" activity, according to the latest CDC data.
"While seasonal flu activity is currently low, we expect it to increase in coming weeks," said Dr. Joseph Bresee, chief of the CDC’s Epidemiology and Prevention Branch in the Influenza Division. "The U.S. is experiencing a late start to the flu season this year, but activity has increased in the past few weeks."
That pattern "is unusual, but not unprecedented," he said. Over the past 35 years, peak U.S. flu activity occurred in March four times, and in April twice, Dr. Bresee noted.
"We could speculate as to the reasons" for the late and mild season so far, he added, "but flu is inherently unpredictable." Among Dr. Bresee’s possible explanations:
• This year’s flu strains mimic last year’s. The U.S. flu strains circulating so far have been predominantly A/H3N2, constituting more than 70% of infections, followed by the 2009 A/H1N1 pandemic strain and a B strain. That pattern closely mimics the strains that circulated last year. "So, it is possible there is higher immunity to these viruses than last year, and that leads to less transmission," he said.
• More people are vaccinated this year. The pattern of circulating flu so far closely matches the seasonal 2011-2012 vaccine, and vaccine coverage rates continue to trend upward this year. That should translate into less transmission and disease over time, especially as people at high risk for infection and complications continue to get vaccinated.
"I think that increased vaccine coverage is certainly playing a role," he said. As of mid-February, U.S. distribution of the 2011-2012 seasonal flu vaccine reached 132 million doses. While that number is slightly below the rate for all of last season, there is still time before the current season’s number wraps.
As of last November, U.S. flu vaccination rates were running ahead of November 2010. And while no high-risk populations have yet come close to the 80% vaccination rate that is the CDC’s goal, coverage rates in pregnant women and children have trended up "slowly but steadily over the past couple of years," Dr. Bresee said.
• Residual immunity may play a part. Many Americans continue to have high levels of antibody against the 2009 H1N1 pandemic strain, a factor that "probably plays a role in the [low] amount of H1N1 we’re seeing this year," he said.
• Mother Nature may be helping. Finally, the relatively mild winter that has occurred in much of the country is a factor that "might play a role," although Dr. Bresee cautioned that the influence that the weather has on flu transmission is poorly understood.
Other elements that together paint an upbeat picture of the current flu season are easier to document with data.
As of Feb. 18, three children had died as a result of influenza infection. To put that number in perspective, since the CDC first made flu-related pediatric death reportable in 2004, the full seasonal number ranged from 46 in 2005-2006 to 282 in 2009-2010, with 340 pediatric flu-related deaths during the entire 2009 H1N1 pandemic.
Another positive is that so far this season, not a single flu isolate tested has shown resistance to oseltamivir or zanamivir, data that "reinforce the value of these medications, especially for people with serious illness due to underlying health conditions," Dr. Bresee said.
And so far, hospitalization rates for patients with laboratory-confirmed influenza have been low. Since Oct. 1, the CDC tallied a national total of 347 laboratory-confirmed infections that required hospitalization, a rate of 1.3 cases/100,000 people.
The CDC declares the start of the annual flu season when its national surveillance statistics show 3 consecutive weeks when at least 10% of collected respiratory specimens are positive for flu. During the week ending Feb. 18, that percentage was 14.4%, following levels of 15.5% for the week ending Feb. 11, and 10.5% for the week ending Feb. 4.
Dr. Bresee had no disclosures.
The Centers for Disease Control and Prevention officially declared that the U.S. 2011-2012 influenza season had begun Feb. 24, based on data through Feb. 18, the latest opening to the influenza season in 29 years.
For the time being, the season remains in relatively low gear, although it’s gaining some momentum: Only California and Colorado are showing "widespread" flu activity, while 14 states continue to show "sporadic" activity, according to the latest CDC data.
"While seasonal flu activity is currently low, we expect it to increase in coming weeks," said Dr. Joseph Bresee, chief of the CDC’s Epidemiology and Prevention Branch in the Influenza Division. "The U.S. is experiencing a late start to the flu season this year, but activity has increased in the past few weeks."
That pattern "is unusual, but not unprecedented," he said. Over the past 35 years, peak U.S. flu activity occurred in March four times, and in April twice, Dr. Bresee noted.
"We could speculate as to the reasons" for the late and mild season so far, he added, "but flu is inherently unpredictable." Among Dr. Bresee’s possible explanations:
• This year’s flu strains mimic last year’s. The U.S. flu strains circulating so far have been predominantly A/H3N2, constituting more than 70% of infections, followed by the 2009 A/H1N1 pandemic strain and a B strain. That pattern closely mimics the strains that circulated last year. "So, it is possible there is higher immunity to these viruses than last year, and that leads to less transmission," he said.
• More people are vaccinated this year. The pattern of circulating flu so far closely matches the seasonal 2011-2012 vaccine, and vaccine coverage rates continue to trend upward this year. That should translate into less transmission and disease over time, especially as people at high risk for infection and complications continue to get vaccinated.
"I think that increased vaccine coverage is certainly playing a role," he said. As of mid-February, U.S. distribution of the 2011-2012 seasonal flu vaccine reached 132 million doses. While that number is slightly below the rate for all of last season, there is still time before the current season’s number wraps.
As of last November, U.S. flu vaccination rates were running ahead of November 2010. And while no high-risk populations have yet come close to the 80% vaccination rate that is the CDC’s goal, coverage rates in pregnant women and children have trended up "slowly but steadily over the past couple of years," Dr. Bresee said.
• Residual immunity may play a part. Many Americans continue to have high levels of antibody against the 2009 H1N1 pandemic strain, a factor that "probably plays a role in the [low] amount of H1N1 we’re seeing this year," he said.
• Mother Nature may be helping. Finally, the relatively mild winter that has occurred in much of the country is a factor that "might play a role," although Dr. Bresee cautioned that the influence that the weather has on flu transmission is poorly understood.
Other elements that together paint an upbeat picture of the current flu season are easier to document with data.
As of Feb. 18, three children had died as a result of influenza infection. To put that number in perspective, since the CDC first made flu-related pediatric death reportable in 2004, the full seasonal number ranged from 46 in 2005-2006 to 282 in 2009-2010, with 340 pediatric flu-related deaths during the entire 2009 H1N1 pandemic.
Another positive is that so far this season, not a single flu isolate tested has shown resistance to oseltamivir or zanamivir, data that "reinforce the value of these medications, especially for people with serious illness due to underlying health conditions," Dr. Bresee said.
And so far, hospitalization rates for patients with laboratory-confirmed influenza have been low. Since Oct. 1, the CDC tallied a national total of 347 laboratory-confirmed infections that required hospitalization, a rate of 1.3 cases/100,000 people.
The CDC declares the start of the annual flu season when its national surveillance statistics show 3 consecutive weeks when at least 10% of collected respiratory specimens are positive for flu. During the week ending Feb. 18, that percentage was 14.4%, following levels of 15.5% for the week ending Feb. 11, and 10.5% for the week ending Feb. 4.
Dr. Bresee had no disclosures.
FROM A TELECONFERENCE SPONSORED BY THE CENTERS FOR DISEASE CONTROL AND PREVENTION
Major Finding: The Centers for Disease Control and Prevention declared the U.S. influenza season open as of Feb. 18, the latest start in 29 years.
Data Source: U.S. influenza surveillance data compiled by the CDC.
Disclosures: Dr. Bresee had no disclosures.
CT Perfusion in Acute Stroke Questioned
NEW ORLEANS – The extra time needed for CT perfusion imaging in patients with an acute ischemic stroke may not be warranted, based on a retrospective analysis of 418 patients treated at nine U.S. tertiary stroke centers.
The analysis showed that the outcomes in patients assessed using CT perfusion (CTP) were very similar to those in patients worked up with noncontrast CT (NCCT), and that CTP added an average of 48 minutes to the time elapsed between the start of imaging and the completion of the reperfusion procedure, Dr. Rishi Gupta said at the annual International Stroke Conference.
"Additional imaging did not translate into better clinical outcomes or reduced hemorrhage rates, raising the question of whether NCCT is good enough," said Dr. Gupta, a neurologist at Emory University and director of the acute stroke network at Grady Health System, both in Atlanta.
Because the analysis was retrospective, the next step is a prospective, randomized study to compare the impact of NCCT and CT perfusion, Dr. Gupta noted. Despite this limitation, he said he and his associates at Grady Health are convinced by the findings and have already scaled back their use of CTP to rely more on NCCT.
"CTP better demarcates the area of irreversible brain damage, but we stopped doing a lot of CTP because we were cognizant of the time delay. We didn’t feel we got additional, helpful information. We cut back quite a lot on our CTP based on these findings," although a majority of centers that perform endovascular perfusion on acute stroke patients "tolerate the delay and get CTP," he said in an interview. "We need to do a randomized study" to settle the question, he added.
The data set compiled from the nine participating U.S. centers included 418 eligible patients who underwent imaging prior to endovascular reperfusion therapy between September 2009 and December 2011. Of these, 227 (54%) had CTP and 191 (46%) had NCCT.
The study included consecutive patients with an occlusion of the middle cerebral or internal carotid artery treated within 8 hours of symptom onset. The analysis excluded patients with a posterior circulation stroke, those who underwent MRI, and those with a thrombus in their anterior cerebral or distal middle cerebral artery. The patients’ average age was 67 years, and their average National Institutes of Health Stroke Scale score was 18.
The analysis showed successful reperfusion in 65% of the NCCT patients and in 71% of those imaged with CTP. A good clinical outcome – a modified Rankin Scale score of 0-2 at 90 days after hospitalization – was achieved in 37% of the NCCT and 38% of the CTP patients. Mortality was 23% in the NCCT and 21% in the CTP patients. The rates of symptomatic and asymptomatic hemorrhage were also similar in the two subgroups. None of these between-group differences were statistically significant. In a multivariate analysis, the use of CTP was not a significant determinant of a good clinical outcome.
Average time from the start of CT imaging to reperfusion was 175 minutes in the NCCT patients and 223 minutes in the CTP group – an average of 48 minutes additional time with CTP.
Dr. Gupta presented two additional analyses designed to compare outcomes using the two imaging methods in closely matched subgroups. In one analysis, he focused exclusively on the 291 patients in the database who had occlusions at the M1 site of the middle cerebral artery. In these patients, NCCT saved an average of 40 minutes, compared with CTP, and outcomes were not significantly better with CTP. In the second analysis, he categorized patients by their Alberta Stroke Program Early CT Score (ASPECTS). In the subgroup of 198 patients with an ASPECTS of more than 7 on a scale of 10, with 10 being normal, CTP did not lead to significant improvement in outcomes and took an average of 45 minutes longer to reperfusion, compared with NCCT.
Dr. Gupta said that he has been a consultant to Concentric Medical, CoAxia, Rapid Medical, and Codman.
NEW ORLEANS – The extra time needed for CT perfusion imaging in patients with an acute ischemic stroke may not be warranted, based on a retrospective analysis of 418 patients treated at nine U.S. tertiary stroke centers.
The analysis showed that the outcomes in patients assessed using CT perfusion (CTP) were very similar to those in patients worked up with noncontrast CT (NCCT), and that CTP added an average of 48 minutes to the time elapsed between the start of imaging and the completion of the reperfusion procedure, Dr. Rishi Gupta said at the annual International Stroke Conference.
"Additional imaging did not translate into better clinical outcomes or reduced hemorrhage rates, raising the question of whether NCCT is good enough," said Dr. Gupta, a neurologist at Emory University and director of the acute stroke network at Grady Health System, both in Atlanta.
Because the analysis was retrospective, the next step is a prospective, randomized study to compare the impact of NCCT and CT perfusion, Dr. Gupta noted. Despite this limitation, he said he and his associates at Grady Health are convinced by the findings and have already scaled back their use of CTP to rely more on NCCT.
"CTP better demarcates the area of irreversible brain damage, but we stopped doing a lot of CTP because we were cognizant of the time delay. We didn’t feel we got additional, helpful information. We cut back quite a lot on our CTP based on these findings," although a majority of centers that perform endovascular perfusion on acute stroke patients "tolerate the delay and get CTP," he said in an interview. "We need to do a randomized study" to settle the question, he added.
The data set compiled from the nine participating U.S. centers included 418 eligible patients who underwent imaging prior to endovascular reperfusion therapy between September 2009 and December 2011. Of these, 227 (54%) had CTP and 191 (46%) had NCCT.
The study included consecutive patients with an occlusion of the middle cerebral or internal carotid artery treated within 8 hours of symptom onset. The analysis excluded patients with a posterior circulation stroke, those who underwent MRI, and those with a thrombus in their anterior cerebral or distal middle cerebral artery. The patients’ average age was 67 years, and their average National Institutes of Health Stroke Scale score was 18.
The analysis showed successful reperfusion in 65% of the NCCT patients and in 71% of those imaged with CTP. A good clinical outcome – a modified Rankin Scale score of 0-2 at 90 days after hospitalization – was achieved in 37% of the NCCT and 38% of the CTP patients. Mortality was 23% in the NCCT and 21% in the CTP patients. The rates of symptomatic and asymptomatic hemorrhage were also similar in the two subgroups. None of these between-group differences were statistically significant. In a multivariate analysis, the use of CTP was not a significant determinant of a good clinical outcome.
Average time from the start of CT imaging to reperfusion was 175 minutes in the NCCT patients and 223 minutes in the CTP group – an average of 48 minutes additional time with CTP.
Dr. Gupta presented two additional analyses designed to compare outcomes using the two imaging methods in closely matched subgroups. In one analysis, he focused exclusively on the 291 patients in the database who had occlusions at the M1 site of the middle cerebral artery. In these patients, NCCT saved an average of 40 minutes, compared with CTP, and outcomes were not significantly better with CTP. In the second analysis, he categorized patients by their Alberta Stroke Program Early CT Score (ASPECTS). In the subgroup of 198 patients with an ASPECTS of more than 7 on a scale of 10, with 10 being normal, CTP did not lead to significant improvement in outcomes and took an average of 45 minutes longer to reperfusion, compared with NCCT.
Dr. Gupta said that he has been a consultant to Concentric Medical, CoAxia, Rapid Medical, and Codman.
NEW ORLEANS – The extra time needed for CT perfusion imaging in patients with an acute ischemic stroke may not be warranted, based on a retrospective analysis of 418 patients treated at nine U.S. tertiary stroke centers.
The analysis showed that the outcomes in patients assessed using CT perfusion (CTP) were very similar to those in patients worked up with noncontrast CT (NCCT), and that CTP added an average of 48 minutes to the time elapsed between the start of imaging and the completion of the reperfusion procedure, Dr. Rishi Gupta said at the annual International Stroke Conference.
"Additional imaging did not translate into better clinical outcomes or reduced hemorrhage rates, raising the question of whether NCCT is good enough," said Dr. Gupta, a neurologist at Emory University and director of the acute stroke network at Grady Health System, both in Atlanta.
Because the analysis was retrospective, the next step is a prospective, randomized study to compare the impact of NCCT and CT perfusion, Dr. Gupta noted. Despite this limitation, he said he and his associates at Grady Health are convinced by the findings and have already scaled back their use of CTP to rely more on NCCT.
"CTP better demarcates the area of irreversible brain damage, but we stopped doing a lot of CTP because we were cognizant of the time delay. We didn’t feel we got additional, helpful information. We cut back quite a lot on our CTP based on these findings," although a majority of centers that perform endovascular perfusion on acute stroke patients "tolerate the delay and get CTP," he said in an interview. "We need to do a randomized study" to settle the question, he added.
The data set compiled from the nine participating U.S. centers included 418 eligible patients who underwent imaging prior to endovascular reperfusion therapy between September 2009 and December 2011. Of these, 227 (54%) had CTP and 191 (46%) had NCCT.
The study included consecutive patients with an occlusion of the middle cerebral or internal carotid artery treated within 8 hours of symptom onset. The analysis excluded patients with a posterior circulation stroke, those who underwent MRI, and those with a thrombus in their anterior cerebral or distal middle cerebral artery. The patients’ average age was 67 years, and their average National Institutes of Health Stroke Scale score was 18.
The analysis showed successful reperfusion in 65% of the NCCT patients and in 71% of those imaged with CTP. A good clinical outcome – a modified Rankin Scale score of 0-2 at 90 days after hospitalization – was achieved in 37% of the NCCT and 38% of the CTP patients. Mortality was 23% in the NCCT and 21% in the CTP patients. The rates of symptomatic and asymptomatic hemorrhage were also similar in the two subgroups. None of these between-group differences were statistically significant. In a multivariate analysis, the use of CTP was not a significant determinant of a good clinical outcome.
Average time from the start of CT imaging to reperfusion was 175 minutes in the NCCT patients and 223 minutes in the CTP group – an average of 48 minutes additional time with CTP.
Dr. Gupta presented two additional analyses designed to compare outcomes using the two imaging methods in closely matched subgroups. In one analysis, he focused exclusively on the 291 patients in the database who had occlusions at the M1 site of the middle cerebral artery. In these patients, NCCT saved an average of 40 minutes, compared with CTP, and outcomes were not significantly better with CTP. In the second analysis, he categorized patients by their Alberta Stroke Program Early CT Score (ASPECTS). In the subgroup of 198 patients with an ASPECTS of more than 7 on a scale of 10, with 10 being normal, CTP did not lead to significant improvement in outcomes and took an average of 45 minutes longer to reperfusion, compared with NCCT.
Dr. Gupta said that he has been a consultant to Concentric Medical, CoAxia, Rapid Medical, and Codman.
FROM THE ANNUAL INTERNATIONAL STROKE CONFERENCE
Major Finding: CT perfusion imaging of ischemic stroke patients boosted average time to reperfusion by 48 minutes, compared with noncontrast CT, without improving outcomes.
Data Source: A retrospective analysis of 418 consecutive patients with an acute ischemic stroke treated with endovascular reperfusion therapy was performed at nine U.S. centers during 2009-2011.
Disclosures: Dr. Gupta said that he has been a consultant to Concentric Medical, CoAxia, Rapid Medical, and Codman.