User login
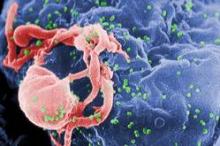
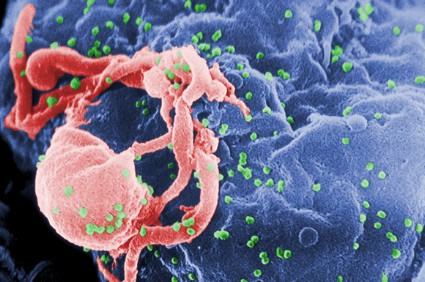
HIV-1 has gained virulence over the course of the epidemic
Even as antiretroviral therapies began making inroads into the AIDS epidemic, the virulence of HIV-1 increased and the time from seroconversion to a significant CD4-cell drop was cut in half, investigators reported at the Conference on Retroviruses and Opportunistic Infections.
"These results have important public health implications, as higher viremial levels are associated with higher risk of transmission. Based on published formulas, our estimated increase of 0.4 log10 copies/mL in viral set point corresponds to a potential 44% increase in transmissibility," reported Dr. Giota Touloumi, of Athens University, in a briefing.
"We all know that the HIV-1 is characterized by huge genetic diversity, and different strains of the virus may differ in virulence," said coinvestigator and lead author Dr. Nikos Pantazis, also from Athens University. Dr. Pantazis presented the data in a plenary session.
In the era before antiretroviral therapy (ART), HIV virulence was measured directly by time to the development of AIDS and death. In the ART era, however, clinicians must rely on marker-based proxies of virulence, such as CD4 seroconversion, CD4 slope (i.e., rate of decline), and viral load set point.
"The picture we have from published research is mixed, with conflicting results, and [some] studies suggest that the HIV virulence has increased, others declare it stable, and others say the virulence is even decreasing," he said.
To see whether the virulence of HIV-1 has changed over the course of the epidemic, the investigators reviewed data from CASCADE, a collaboration between the investigators of 26 cohorts of persons with well-estimated dates of HIV seroconversion. They did not include data on African cohorts, patients with seroconversion from 2009 on, or children.
They censored follow-up either at the time of ART initiation or at the onset of clinical AIDS. A total of 15,875 cohort members met the study criteria.
The authors estimated the CD4 counts at seroconversion declined from about 770/mcL in the early 1980s to about 570/mcL after 2000. The virulence appeared to plateau after the turn of the millennium, they noted.
The rate of CD4-cell loss (CD4 slope) was relatively stable up to 1996, but accelerated from 1996 through 2004. In addition, the estimated HIV set point (viral load) increased from 4.05 log10 copies/mL in 1980 to 4.50 in 2002, suggesting a more virulent virus.
The results are compatible with both a 2012 meta-analysis (AIDS 2012;26:193-205 [doi: 10.1097/QAD.0b013e32834db418]) and a 2007 evolutionary hypothesis (Proc. Natl. Acad. Sci. USA 2007;104:17441-6), Dr. Pantazis said.
The combined findings of a drop in CD4 cell count of about 200 cells/mcL over 30 years and an apparent increase in CD4 slope translated into an estimated 50% decrease in the time from seroconversion to 350 CD4 cells/mcL, from 7 years in a 1980 seroconverter to 3.4 years in a 2004 seroconverter, he said.
Although the study was subject to residual confounding bias, such as changes in methods for viral load and CD4 cell count assays and quantification, and difficulties in pinpointing the time of seroconversion, a wide range of sensitivity analyses furnished qualitatively similar results, Dr. Pantazis said.
The study was supported by a grant from the European Union. Dr. Pantazis, Dr. Touloumi, and their colleagues reported having no financial disclosures.
Even as antiretroviral therapies began making inroads into the AIDS epidemic, the virulence of HIV-1 increased and the time from seroconversion to a significant CD4-cell drop was cut in half, investigators reported at the Conference on Retroviruses and Opportunistic Infections.
"These results have important public health implications, as higher viremial levels are associated with higher risk of transmission. Based on published formulas, our estimated increase of 0.4 log10 copies/mL in viral set point corresponds to a potential 44% increase in transmissibility," reported Dr. Giota Touloumi, of Athens University, in a briefing.
"We all know that the HIV-1 is characterized by huge genetic diversity, and different strains of the virus may differ in virulence," said coinvestigator and lead author Dr. Nikos Pantazis, also from Athens University. Dr. Pantazis presented the data in a plenary session.
In the era before antiretroviral therapy (ART), HIV virulence was measured directly by time to the development of AIDS and death. In the ART era, however, clinicians must rely on marker-based proxies of virulence, such as CD4 seroconversion, CD4 slope (i.e., rate of decline), and viral load set point.
"The picture we have from published research is mixed, with conflicting results, and [some] studies suggest that the HIV virulence has increased, others declare it stable, and others say the virulence is even decreasing," he said.
To see whether the virulence of HIV-1 has changed over the course of the epidemic, the investigators reviewed data from CASCADE, a collaboration between the investigators of 26 cohorts of persons with well-estimated dates of HIV seroconversion. They did not include data on African cohorts, patients with seroconversion from 2009 on, or children.
They censored follow-up either at the time of ART initiation or at the onset of clinical AIDS. A total of 15,875 cohort members met the study criteria.
The authors estimated the CD4 counts at seroconversion declined from about 770/mcL in the early 1980s to about 570/mcL after 2000. The virulence appeared to plateau after the turn of the millennium, they noted.
The rate of CD4-cell loss (CD4 slope) was relatively stable up to 1996, but accelerated from 1996 through 2004. In addition, the estimated HIV set point (viral load) increased from 4.05 log10 copies/mL in 1980 to 4.50 in 2002, suggesting a more virulent virus.
The results are compatible with both a 2012 meta-analysis (AIDS 2012;26:193-205 [doi: 10.1097/QAD.0b013e32834db418]) and a 2007 evolutionary hypothesis (Proc. Natl. Acad. Sci. USA 2007;104:17441-6), Dr. Pantazis said.
The combined findings of a drop in CD4 cell count of about 200 cells/mcL over 30 years and an apparent increase in CD4 slope translated into an estimated 50% decrease in the time from seroconversion to 350 CD4 cells/mcL, from 7 years in a 1980 seroconverter to 3.4 years in a 2004 seroconverter, he said.
Although the study was subject to residual confounding bias, such as changes in methods for viral load and CD4 cell count assays and quantification, and difficulties in pinpointing the time of seroconversion, a wide range of sensitivity analyses furnished qualitatively similar results, Dr. Pantazis said.
The study was supported by a grant from the European Union. Dr. Pantazis, Dr. Touloumi, and their colleagues reported having no financial disclosures.
Even as antiretroviral therapies began making inroads into the AIDS epidemic, the virulence of HIV-1 increased and the time from seroconversion to a significant CD4-cell drop was cut in half, investigators reported at the Conference on Retroviruses and Opportunistic Infections.
"These results have important public health implications, as higher viremial levels are associated with higher risk of transmission. Based on published formulas, our estimated increase of 0.4 log10 copies/mL in viral set point corresponds to a potential 44% increase in transmissibility," reported Dr. Giota Touloumi, of Athens University, in a briefing.
"We all know that the HIV-1 is characterized by huge genetic diversity, and different strains of the virus may differ in virulence," said coinvestigator and lead author Dr. Nikos Pantazis, also from Athens University. Dr. Pantazis presented the data in a plenary session.
In the era before antiretroviral therapy (ART), HIV virulence was measured directly by time to the development of AIDS and death. In the ART era, however, clinicians must rely on marker-based proxies of virulence, such as CD4 seroconversion, CD4 slope (i.e., rate of decline), and viral load set point.
"The picture we have from published research is mixed, with conflicting results, and [some] studies suggest that the HIV virulence has increased, others declare it stable, and others say the virulence is even decreasing," he said.
To see whether the virulence of HIV-1 has changed over the course of the epidemic, the investigators reviewed data from CASCADE, a collaboration between the investigators of 26 cohorts of persons with well-estimated dates of HIV seroconversion. They did not include data on African cohorts, patients with seroconversion from 2009 on, or children.
They censored follow-up either at the time of ART initiation or at the onset of clinical AIDS. A total of 15,875 cohort members met the study criteria.
The authors estimated the CD4 counts at seroconversion declined from about 770/mcL in the early 1980s to about 570/mcL after 2000. The virulence appeared to plateau after the turn of the millennium, they noted.
The rate of CD4-cell loss (CD4 slope) was relatively stable up to 1996, but accelerated from 1996 through 2004. In addition, the estimated HIV set point (viral load) increased from 4.05 log10 copies/mL in 1980 to 4.50 in 2002, suggesting a more virulent virus.
The results are compatible with both a 2012 meta-analysis (AIDS 2012;26:193-205 [doi: 10.1097/QAD.0b013e32834db418]) and a 2007 evolutionary hypothesis (Proc. Natl. Acad. Sci. USA 2007;104:17441-6), Dr. Pantazis said.
The combined findings of a drop in CD4 cell count of about 200 cells/mcL over 30 years and an apparent increase in CD4 slope translated into an estimated 50% decrease in the time from seroconversion to 350 CD4 cells/mcL, from 7 years in a 1980 seroconverter to 3.4 years in a 2004 seroconverter, he said.
Although the study was subject to residual confounding bias, such as changes in methods for viral load and CD4 cell count assays and quantification, and difficulties in pinpointing the time of seroconversion, a wide range of sensitivity analyses furnished qualitatively similar results, Dr. Pantazis said.
The study was supported by a grant from the European Union. Dr. Pantazis, Dr. Touloumi, and their colleagues reported having no financial disclosures.
FROM CROI 2014
Major finding: An increase in the HIV-1 viral set point from 1980 through 2000 translates into an estimated 44% increase in viral transmissibility.
Data source: Retrospective study of 15,875 HIV-positive patients in 1 of 26 cohorts.
Disclosures: The study was supported by a grant from the European Union. Dr. Pantazis, Dr. Touloumi, and their colleagues reported having no financial disclosures.
New Drugs Trump Interferon in HCV Therapy
The era of interferon and ribavirin in the treatment of hepatitis C viral infections appears to be drawing to a close, and few clinicians will mourn the passing of the effective but highly toxic combination, investigators said at the Conference on Retroviruses and Opportunistic Infections.
In patients with hepatitis C virus infection alone or HCV with HIV coinfection, a host of new interferon-free drugs and new combinations are transforming therapy, reported Dr. Jean-Michel Pawlotsky, professor of medicine at the University of Paris-Est.
"Hepatitis C is living a real therapeutic revolution. Everything is changing very fast. We’re now getting infection cure rates higher than 90% with the classes of drugs we have," he said at a briefing.
STARTVerso4
With all of the drugs, the sustained virologic response (SVR) rates "are exactly the same in coinfected patients as they are in monoinfected patients," said Dr. Douglas Dieterich of Mt. Sinai Medical Center, New York.
For example, a combination of the protease inhibitor faldaprevir with pegylated interferon alfa-2a plus ribavirin (PR) produced SVR rates at week 4 of follow-up (SVR4) of 74% in HCV/HIV coinfected patients, said Dr. Dietrich, a principal investigator for the STARTVerso4 trial.
In this phase III open-label trial, 308 patients with HCV/HIV coinfection who were treatment naive or relapsed after prior interferon-based therapy were randomly assigned to receive faldaprevir 120 mg daily for 24 weeks or 240 mg for 12 or 24 weeks according to on-treatment response. In both arms, faldaprevir was given on a PR backbone, with duration guided by response to therapy.
For the primary endpoint of SVR12, the investigators saw a 72% rate, with no significant difference between the two dose groups, compared with approximately 80% in monoinfected patients in other phase III studies. There was also no difference in efficacy between patients with or without cirrhosis, he said.
Adverse events included mild hyperbilirubinemia in some patients and interferon side effects.
Simeprevir in coinfection
Dr. Dietrich was also the lead on study C212, which looked at simeprevir (Olysio) on a PR backbone in coinfected patients. The results were similar to those seen with faldaprevir (73.6% overall SVR12).
Interestingly, the presence of the simeprevir-resistant q80K polymorphism did not make a difference in response rates, he said. Among monoinfected patients in prior studies, those with q80k polymorphism had significantly lower SVR rates. The adverse events were also similar to those seen in patients with monoinfection.
PHOTON-1
The PHOTON-1 trial evaluated the first interferon-free regimen (sofosbuvir plus ribavirin) in patients with HCV genotypes 1-3 and HIV.
In this study, patients with HCV and stable HIV infection received sofosbuvir 400 mg and ribavirin 1,000-1,200 mg daily. Treatment-naive patients with HCV genotype 1 and treatment-experienced patients with genotypes 2 or 3 received treatment for 24 weeks, while treatment-naive genotype 2/3 patients received 12 weeks of treatment. Patients on multiple antiretroviral (ART) regimens and those with compensated cirrhosis were included in the study.
The primary efficacy endpoint, SVR12, was achieved in 88% of treatment-naive genotype 2 patients and 67% of genotype 3 patients. In genotype 1 patients, the SVR was approximately 70%, Dr. Dietrich said.
Adverse events were general and limited to anemias, headache, and other symptoms commonly seen with HCV therapies, he added.
SYNERGY trial
Dr. Anita Kohli presented final results from the SYNERGY trial, which looked at combination oral HCV therapy for 6 or 12 weeks (SVR4 results from this trial were presented at the 2013 Liver Meeting).
In this phase II prospective cohort study, 60 treatment-naive patients with HCV genotype 1 were enrolled into one of three arms to receive either sofosbuvir 400 mg with ledipasvir 90 mg once daily in a fixed-dose combination for 12 weeks (arm A); the same fixed-dose combination plus the non-nucleoside NS5B inhibitor GS-9669 500 mg/day for 6 weeks (arm B); or the fixed-dose combination plus the NS3 protease inhibitor GS-9451 80 mg/day for 6 weeks.
The SVR12 rate among the patients on sofosbuvir/ledipasvir alone (arm A) was 100%, the rate in arm B was 95%, and the rate in arm C was 100%.
"We find these results very promising," said Dr. Kohli of the National Institutes of Health.
She noted that all patients in the trial were treatment naïve, and that all stages of liver disease were included in the 12-week treatment arm, but cirrhotic patients were excluded from the 6-week arms.
"These regimens are very simple. They’re one, two, or three pills a day," she noted. In addition, "our patient population is one that has been historically very difficult to treat, that is, predominantly African American," she noted.
Most of the patients had genotype 1a with a high viral load, and 25%-30% of patients in all treatment arms had advanced-stage liver disease, she added.
PEARL-III
The PEARL III trial looked at 419 treatment-naive, noncirrhotic patients with HCV genotype 1b, who were randomly assigned to receive either a ritonavir-boosted protease inhibitor (ABT-450) with ABT-267, which is an inhibitor of HCV NS5A, coformulated into a single pill; or ABT-333, a non-nucleoside polymerase inhibitor, with or without ribavirin.
In the ribavirin-containing arm, SVR12 was 99.5%, compared with 99% among controls. There was only one virologic failure in the study, and two patients who did not achieve SVR4 were lost to follow-up at week 12, noted Dr. Daniel Cohen of AbbVie Pharmaceuticals.
Adverse events included predominantly mild headache and fatigue in about 25% of patients, with slightly more events seen in the ribavirin combination arm.
STARTVerso4 was sponsored by Boehringer Ingelheim. C212 was sponsored by Janssen. SYNERGY was supported by the National Institutes of Health and Gilead Sciences. Dr. Cohen is employed by AbbVie, which sponsored PEARL III.
The era of interferon and ribavirin in the treatment of hepatitis C viral infections appears to be drawing to a close, and few clinicians will mourn the passing of the effective but highly toxic combination, investigators said at the Conference on Retroviruses and Opportunistic Infections.
In patients with hepatitis C virus infection alone or HCV with HIV coinfection, a host of new interferon-free drugs and new combinations are transforming therapy, reported Dr. Jean-Michel Pawlotsky, professor of medicine at the University of Paris-Est.
"Hepatitis C is living a real therapeutic revolution. Everything is changing very fast. We’re now getting infection cure rates higher than 90% with the classes of drugs we have," he said at a briefing.
STARTVerso4
With all of the drugs, the sustained virologic response (SVR) rates "are exactly the same in coinfected patients as they are in monoinfected patients," said Dr. Douglas Dieterich of Mt. Sinai Medical Center, New York.
For example, a combination of the protease inhibitor faldaprevir with pegylated interferon alfa-2a plus ribavirin (PR) produced SVR rates at week 4 of follow-up (SVR4) of 74% in HCV/HIV coinfected patients, said Dr. Dietrich, a principal investigator for the STARTVerso4 trial.
In this phase III open-label trial, 308 patients with HCV/HIV coinfection who were treatment naive or relapsed after prior interferon-based therapy were randomly assigned to receive faldaprevir 120 mg daily for 24 weeks or 240 mg for 12 or 24 weeks according to on-treatment response. In both arms, faldaprevir was given on a PR backbone, with duration guided by response to therapy.
For the primary endpoint of SVR12, the investigators saw a 72% rate, with no significant difference between the two dose groups, compared with approximately 80% in monoinfected patients in other phase III studies. There was also no difference in efficacy between patients with or without cirrhosis, he said.
Adverse events included mild hyperbilirubinemia in some patients and interferon side effects.
Simeprevir in coinfection
Dr. Dietrich was also the lead on study C212, which looked at simeprevir (Olysio) on a PR backbone in coinfected patients. The results were similar to those seen with faldaprevir (73.6% overall SVR12).
Interestingly, the presence of the simeprevir-resistant q80K polymorphism did not make a difference in response rates, he said. Among monoinfected patients in prior studies, those with q80k polymorphism had significantly lower SVR rates. The adverse events were also similar to those seen in patients with monoinfection.
PHOTON-1
The PHOTON-1 trial evaluated the first interferon-free regimen (sofosbuvir plus ribavirin) in patients with HCV genotypes 1-3 and HIV.
In this study, patients with HCV and stable HIV infection received sofosbuvir 400 mg and ribavirin 1,000-1,200 mg daily. Treatment-naive patients with HCV genotype 1 and treatment-experienced patients with genotypes 2 or 3 received treatment for 24 weeks, while treatment-naive genotype 2/3 patients received 12 weeks of treatment. Patients on multiple antiretroviral (ART) regimens and those with compensated cirrhosis were included in the study.
The primary efficacy endpoint, SVR12, was achieved in 88% of treatment-naive genotype 2 patients and 67% of genotype 3 patients. In genotype 1 patients, the SVR was approximately 70%, Dr. Dietrich said.
Adverse events were general and limited to anemias, headache, and other symptoms commonly seen with HCV therapies, he added.
SYNERGY trial
Dr. Anita Kohli presented final results from the SYNERGY trial, which looked at combination oral HCV therapy for 6 or 12 weeks (SVR4 results from this trial were presented at the 2013 Liver Meeting).
In this phase II prospective cohort study, 60 treatment-naive patients with HCV genotype 1 were enrolled into one of three arms to receive either sofosbuvir 400 mg with ledipasvir 90 mg once daily in a fixed-dose combination for 12 weeks (arm A); the same fixed-dose combination plus the non-nucleoside NS5B inhibitor GS-9669 500 mg/day for 6 weeks (arm B); or the fixed-dose combination plus the NS3 protease inhibitor GS-9451 80 mg/day for 6 weeks.
The SVR12 rate among the patients on sofosbuvir/ledipasvir alone (arm A) was 100%, the rate in arm B was 95%, and the rate in arm C was 100%.
"We find these results very promising," said Dr. Kohli of the National Institutes of Health.
She noted that all patients in the trial were treatment naïve, and that all stages of liver disease were included in the 12-week treatment arm, but cirrhotic patients were excluded from the 6-week arms.
"These regimens are very simple. They’re one, two, or three pills a day," she noted. In addition, "our patient population is one that has been historically very difficult to treat, that is, predominantly African American," she noted.
Most of the patients had genotype 1a with a high viral load, and 25%-30% of patients in all treatment arms had advanced-stage liver disease, she added.
PEARL-III
The PEARL III trial looked at 419 treatment-naive, noncirrhotic patients with HCV genotype 1b, who were randomly assigned to receive either a ritonavir-boosted protease inhibitor (ABT-450) with ABT-267, which is an inhibitor of HCV NS5A, coformulated into a single pill; or ABT-333, a non-nucleoside polymerase inhibitor, with or without ribavirin.
In the ribavirin-containing arm, SVR12 was 99.5%, compared with 99% among controls. There was only one virologic failure in the study, and two patients who did not achieve SVR4 were lost to follow-up at week 12, noted Dr. Daniel Cohen of AbbVie Pharmaceuticals.
Adverse events included predominantly mild headache and fatigue in about 25% of patients, with slightly more events seen in the ribavirin combination arm.
STARTVerso4 was sponsored by Boehringer Ingelheim. C212 was sponsored by Janssen. SYNERGY was supported by the National Institutes of Health and Gilead Sciences. Dr. Cohen is employed by AbbVie, which sponsored PEARL III.
The era of interferon and ribavirin in the treatment of hepatitis C viral infections appears to be drawing to a close, and few clinicians will mourn the passing of the effective but highly toxic combination, investigators said at the Conference on Retroviruses and Opportunistic Infections.
In patients with hepatitis C virus infection alone or HCV with HIV coinfection, a host of new interferon-free drugs and new combinations are transforming therapy, reported Dr. Jean-Michel Pawlotsky, professor of medicine at the University of Paris-Est.
"Hepatitis C is living a real therapeutic revolution. Everything is changing very fast. We’re now getting infection cure rates higher than 90% with the classes of drugs we have," he said at a briefing.
STARTVerso4
With all of the drugs, the sustained virologic response (SVR) rates "are exactly the same in coinfected patients as they are in monoinfected patients," said Dr. Douglas Dieterich of Mt. Sinai Medical Center, New York.
For example, a combination of the protease inhibitor faldaprevir with pegylated interferon alfa-2a plus ribavirin (PR) produced SVR rates at week 4 of follow-up (SVR4) of 74% in HCV/HIV coinfected patients, said Dr. Dietrich, a principal investigator for the STARTVerso4 trial.
In this phase III open-label trial, 308 patients with HCV/HIV coinfection who were treatment naive or relapsed after prior interferon-based therapy were randomly assigned to receive faldaprevir 120 mg daily for 24 weeks or 240 mg for 12 or 24 weeks according to on-treatment response. In both arms, faldaprevir was given on a PR backbone, with duration guided by response to therapy.
For the primary endpoint of SVR12, the investigators saw a 72% rate, with no significant difference between the two dose groups, compared with approximately 80% in monoinfected patients in other phase III studies. There was also no difference in efficacy between patients with or without cirrhosis, he said.
Adverse events included mild hyperbilirubinemia in some patients and interferon side effects.
Simeprevir in coinfection
Dr. Dietrich was also the lead on study C212, which looked at simeprevir (Olysio) on a PR backbone in coinfected patients. The results were similar to those seen with faldaprevir (73.6% overall SVR12).
Interestingly, the presence of the simeprevir-resistant q80K polymorphism did not make a difference in response rates, he said. Among monoinfected patients in prior studies, those with q80k polymorphism had significantly lower SVR rates. The adverse events were also similar to those seen in patients with monoinfection.
PHOTON-1
The PHOTON-1 trial evaluated the first interferon-free regimen (sofosbuvir plus ribavirin) in patients with HCV genotypes 1-3 and HIV.
In this study, patients with HCV and stable HIV infection received sofosbuvir 400 mg and ribavirin 1,000-1,200 mg daily. Treatment-naive patients with HCV genotype 1 and treatment-experienced patients with genotypes 2 or 3 received treatment for 24 weeks, while treatment-naive genotype 2/3 patients received 12 weeks of treatment. Patients on multiple antiretroviral (ART) regimens and those with compensated cirrhosis were included in the study.
The primary efficacy endpoint, SVR12, was achieved in 88% of treatment-naive genotype 2 patients and 67% of genotype 3 patients. In genotype 1 patients, the SVR was approximately 70%, Dr. Dietrich said.
Adverse events were general and limited to anemias, headache, and other symptoms commonly seen with HCV therapies, he added.
SYNERGY trial
Dr. Anita Kohli presented final results from the SYNERGY trial, which looked at combination oral HCV therapy for 6 or 12 weeks (SVR4 results from this trial were presented at the 2013 Liver Meeting).
In this phase II prospective cohort study, 60 treatment-naive patients with HCV genotype 1 were enrolled into one of three arms to receive either sofosbuvir 400 mg with ledipasvir 90 mg once daily in a fixed-dose combination for 12 weeks (arm A); the same fixed-dose combination plus the non-nucleoside NS5B inhibitor GS-9669 500 mg/day for 6 weeks (arm B); or the fixed-dose combination plus the NS3 protease inhibitor GS-9451 80 mg/day for 6 weeks.
The SVR12 rate among the patients on sofosbuvir/ledipasvir alone (arm A) was 100%, the rate in arm B was 95%, and the rate in arm C was 100%.
"We find these results very promising," said Dr. Kohli of the National Institutes of Health.
She noted that all patients in the trial were treatment naïve, and that all stages of liver disease were included in the 12-week treatment arm, but cirrhotic patients were excluded from the 6-week arms.
"These regimens are very simple. They’re one, two, or three pills a day," she noted. In addition, "our patient population is one that has been historically very difficult to treat, that is, predominantly African American," she noted.
Most of the patients had genotype 1a with a high viral load, and 25%-30% of patients in all treatment arms had advanced-stage liver disease, she added.
PEARL-III
The PEARL III trial looked at 419 treatment-naive, noncirrhotic patients with HCV genotype 1b, who were randomly assigned to receive either a ritonavir-boosted protease inhibitor (ABT-450) with ABT-267, which is an inhibitor of HCV NS5A, coformulated into a single pill; or ABT-333, a non-nucleoside polymerase inhibitor, with or without ribavirin.
In the ribavirin-containing arm, SVR12 was 99.5%, compared with 99% among controls. There was only one virologic failure in the study, and two patients who did not achieve SVR4 were lost to follow-up at week 12, noted Dr. Daniel Cohen of AbbVie Pharmaceuticals.
Adverse events included predominantly mild headache and fatigue in about 25% of patients, with slightly more events seen in the ribavirin combination arm.
STARTVerso4 was sponsored by Boehringer Ingelheim. C212 was sponsored by Janssen. SYNERGY was supported by the National Institutes of Health and Gilead Sciences. Dr. Cohen is employed by AbbVie, which sponsored PEARL III.
FROM CROI 2014
New drugs trump interferon in HCV therapy
The era of interferon and ribavirin in the treatment of hepatitis C viral infections appears to be drawing to a close, and few clinicians will mourn the passing of the effective but highly toxic combination, investigators said at the Conference on Retroviruses and Opportunistic Infections.
In patients with hepatitis C virus infection alone or HCV with HIV coinfection, a host of new interferon-free drugs and new combinations are transforming therapy, reported Dr. Jean-Michel Pawlotsky, professor of medicine at the University of Paris-Est.
"Hepatitis C is living a real therapeutic revolution. Everything is changing very fast. We’re now getting infection cure rates higher than 90% with the classes of drugs we have," he said at a briefing.
STARTVerso4
With all of the drugs, the sustained virologic response (SVR) rates "are exactly the same in coinfected patients as they are in monoinfected patients," said Dr. Douglas Dieterich of Mt. Sinai Medical Center, New York.
For example, a combination of the protease inhibitor faldaprevir with pegylated interferon alfa-2a plus ribavirin (PR) produced SVR rates at week 4 of follow-up (SVR4) of 74% in HCV/HIV coinfected patients, said Dr. Dietrich, a principal investigator for the STARTVerso4 trial.
In this phase III open-label trial, 308 patients with HCV/HIV coinfection who were treatment naive or relapsed after prior interferon-based therapy were randomly assigned to receive faldaprevir 120 mg daily for 24 weeks or 240 mg for 12 or 24 weeks according to on-treatment response. In both arms, faldaprevir was given on a PR backbone, with duration guided by response to therapy.
For the primary endpoint of SVR12, the investigators saw a 72% rate, with no significant difference between the two dose groups, compared with approximately 80% in monoinfected patients in other phase III studies. There was also no difference in efficacy between patients with or without cirrhosis, he said.
Adverse events included mild hyperbilirubinemia in some patients and interferon side effects.
Simeprevir in coinfection
Dr. Dietrich was also the lead on study C212, which looked at simeprevir (Olysio) on a PR backbone in coinfected patients. The results were similar to those seen with faldaprevir (73.6% overall SVR12).
Interestingly, the presence of the simeprevir-resistant q80K polymorphism did not make a difference in response rates, he said. Among monoinfected patients in prior studies, those with q80k polymorphism had significantly lower SVR rates. The adverse events were also similar to those seen in patients with monoinfection.
PHOTON-1
The PHOTON-1 trial evaluated the first interferon-free regimen (sofosbuvir plus ribavirin) in patients with HCV genotypes 1-3 and HIV.
In this study, patients with HCV and stable HIV infection received sofosbuvir 400 mg and ribavirin 1,000-1,200 mg daily. Treatment-naive patients with HCV genotype 1 and treatment-experienced patients with genotypes 2 or 3 received treatment for 24 weeks, while treatment-naive genotype 2/3 patients received 12 weeks of treatment. Patients on multiple antiretroviral (ART) regimens and those with compensated cirrhosis were included in the study.
The primary efficacy endpoint, SVR12, was achieved in 88% of treatment-naive genotype 2 patients and 67% of genotype 3 patients. In genotype 1 patients, the SVR was approximately 70%, Dr. Dietrich said.
Adverse events were general and limited to anemias, headache, and other symptoms commonly seen with HCV therapies, he added.
SYNERGY trial
Dr. Anita Kohli presented final results from the SYNERGY trial, which looked at combination oral HCV therapy for 6 or 12 weeks (SVR4 results from this trial were presented at the 2013 Liver Meeting).
In this phase II prospective cohort study, 60 treatment-naive patients with HCV genotype 1 were enrolled into one of three arms to receive either sofosbuvir 400 mg with ledipasvir 90 mg once daily in a fixed-dose combination for 12 weeks (arm A); the same fixed-dose combination plus the non-nucleoside NS5B inhibitor GS-9669 500 mg/day for 6 weeks (arm B); or the fixed-dose combination plus the NS3 protease inhibitor GS-9451 80 mg/day for 6 weeks.
The SVR12 rate among the patients on sofosbuvir/ledipasvir alone (arm A) was 100%, the rate in arm B was 95%, and the rate in arm C was 100%.
"We find these results very promising," said Dr. Kohli of the National Institutes of Health.
She noted that all patients in the trial were treatment naïve, and that all stages of liver disease were included in the 12-week treatment arm, but cirrhotic patients were excluded from the 6-week arms.
"These regimens are very simple. They’re one, two, or three pills a day," she noted. In addition, "our patient population is one that has been historically very difficult to treat, that is, predominantly African American," she noted.
Most of the patients had genotype 1a with a high viral load, and 25%-30% of patients in all treatment arms had advanced-stage liver disease, she added.
PEARL-III
The PEARL III trial looked at 419 treatment-naive, noncirrhotic patients with HCV genotype 1b, who were randomly assigned to receive either a ritonavir-boosted protease inhibitor (ABT-450) with ABT-267, which is an inhibitor of HCV NS5A, coformulated into a single pill; or ABT-333, a non-nucleoside polymerase inhibitor, with or without ribavirin.
In the ribavirin-containing arm, SVR12 was 99.5%, compared with 99% among controls. There was only one virologic failure in the study, and two patients who did not achieve SVR4 were lost to follow-up at week 12, noted Dr. Daniel Cohen of AbbVie Pharmaceuticals.
Adverse events included predominantly mild headache and fatigue in about 25% of patients, with slightly more events seen in the ribavirin combination arm.
STARTVerso4 was sponsored by Boehringer Ingelheim. C212 was sponsored by Janssen. SYNERGY was supported by the National Institutes of Health and Gilead Sciences. Dr. Cohen is employed by AbbVie, which sponsored PEARL III.
The era of interferon and ribavirin in the treatment of hepatitis C viral infections appears to be drawing to a close, and few clinicians will mourn the passing of the effective but highly toxic combination, investigators said at the Conference on Retroviruses and Opportunistic Infections.
In patients with hepatitis C virus infection alone or HCV with HIV coinfection, a host of new interferon-free drugs and new combinations are transforming therapy, reported Dr. Jean-Michel Pawlotsky, professor of medicine at the University of Paris-Est.
"Hepatitis C is living a real therapeutic revolution. Everything is changing very fast. We’re now getting infection cure rates higher than 90% with the classes of drugs we have," he said at a briefing.
STARTVerso4
With all of the drugs, the sustained virologic response (SVR) rates "are exactly the same in coinfected patients as they are in monoinfected patients," said Dr. Douglas Dieterich of Mt. Sinai Medical Center, New York.
For example, a combination of the protease inhibitor faldaprevir with pegylated interferon alfa-2a plus ribavirin (PR) produced SVR rates at week 4 of follow-up (SVR4) of 74% in HCV/HIV coinfected patients, said Dr. Dietrich, a principal investigator for the STARTVerso4 trial.
In this phase III open-label trial, 308 patients with HCV/HIV coinfection who were treatment naive or relapsed after prior interferon-based therapy were randomly assigned to receive faldaprevir 120 mg daily for 24 weeks or 240 mg for 12 or 24 weeks according to on-treatment response. In both arms, faldaprevir was given on a PR backbone, with duration guided by response to therapy.
For the primary endpoint of SVR12, the investigators saw a 72% rate, with no significant difference between the two dose groups, compared with approximately 80% in monoinfected patients in other phase III studies. There was also no difference in efficacy between patients with or without cirrhosis, he said.
Adverse events included mild hyperbilirubinemia in some patients and interferon side effects.
Simeprevir in coinfection
Dr. Dietrich was also the lead on study C212, which looked at simeprevir (Olysio) on a PR backbone in coinfected patients. The results were similar to those seen with faldaprevir (73.6% overall SVR12).
Interestingly, the presence of the simeprevir-resistant q80K polymorphism did not make a difference in response rates, he said. Among monoinfected patients in prior studies, those with q80k polymorphism had significantly lower SVR rates. The adverse events were also similar to those seen in patients with monoinfection.
PHOTON-1
The PHOTON-1 trial evaluated the first interferon-free regimen (sofosbuvir plus ribavirin) in patients with HCV genotypes 1-3 and HIV.
In this study, patients with HCV and stable HIV infection received sofosbuvir 400 mg and ribavirin 1,000-1,200 mg daily. Treatment-naive patients with HCV genotype 1 and treatment-experienced patients with genotypes 2 or 3 received treatment for 24 weeks, while treatment-naive genotype 2/3 patients received 12 weeks of treatment. Patients on multiple antiretroviral (ART) regimens and those with compensated cirrhosis were included in the study.
The primary efficacy endpoint, SVR12, was achieved in 88% of treatment-naive genotype 2 patients and 67% of genotype 3 patients. In genotype 1 patients, the SVR was approximately 70%, Dr. Dietrich said.
Adverse events were general and limited to anemias, headache, and other symptoms commonly seen with HCV therapies, he added.
SYNERGY trial
Dr. Anita Kohli presented final results from the SYNERGY trial, which looked at combination oral HCV therapy for 6 or 12 weeks (SVR4 results from this trial were presented at the 2013 Liver Meeting).
In this phase II prospective cohort study, 60 treatment-naive patients with HCV genotype 1 were enrolled into one of three arms to receive either sofosbuvir 400 mg with ledipasvir 90 mg once daily in a fixed-dose combination for 12 weeks (arm A); the same fixed-dose combination plus the non-nucleoside NS5B inhibitor GS-9669 500 mg/day for 6 weeks (arm B); or the fixed-dose combination plus the NS3 protease inhibitor GS-9451 80 mg/day for 6 weeks.
The SVR12 rate among the patients on sofosbuvir/ledipasvir alone (arm A) was 100%, the rate in arm B was 95%, and the rate in arm C was 100%.
"We find these results very promising," said Dr. Kohli of the National Institutes of Health.
She noted that all patients in the trial were treatment naïve, and that all stages of liver disease were included in the 12-week treatment arm, but cirrhotic patients were excluded from the 6-week arms.
"These regimens are very simple. They’re one, two, or three pills a day," she noted. In addition, "our patient population is one that has been historically very difficult to treat, that is, predominantly African American," she noted.
Most of the patients had genotype 1a with a high viral load, and 25%-30% of patients in all treatment arms had advanced-stage liver disease, she added.
PEARL-III
The PEARL III trial looked at 419 treatment-naive, noncirrhotic patients with HCV genotype 1b, who were randomly assigned to receive either a ritonavir-boosted protease inhibitor (ABT-450) with ABT-267, which is an inhibitor of HCV NS5A, coformulated into a single pill; or ABT-333, a non-nucleoside polymerase inhibitor, with or without ribavirin.
In the ribavirin-containing arm, SVR12 was 99.5%, compared with 99% among controls. There was only one virologic failure in the study, and two patients who did not achieve SVR4 were lost to follow-up at week 12, noted Dr. Daniel Cohen of AbbVie Pharmaceuticals.
Adverse events included predominantly mild headache and fatigue in about 25% of patients, with slightly more events seen in the ribavirin combination arm.
STARTVerso4 was sponsored by Boehringer Ingelheim. C212 was sponsored by Janssen. SYNERGY was supported by the National Institutes of Health and Gilead Sciences. Dr. Cohen is employed by AbbVie, which sponsored PEARL III.
The era of interferon and ribavirin in the treatment of hepatitis C viral infections appears to be drawing to a close, and few clinicians will mourn the passing of the effective but highly toxic combination, investigators said at the Conference on Retroviruses and Opportunistic Infections.
In patients with hepatitis C virus infection alone or HCV with HIV coinfection, a host of new interferon-free drugs and new combinations are transforming therapy, reported Dr. Jean-Michel Pawlotsky, professor of medicine at the University of Paris-Est.
"Hepatitis C is living a real therapeutic revolution. Everything is changing very fast. We’re now getting infection cure rates higher than 90% with the classes of drugs we have," he said at a briefing.
STARTVerso4
With all of the drugs, the sustained virologic response (SVR) rates "are exactly the same in coinfected patients as they are in monoinfected patients," said Dr. Douglas Dieterich of Mt. Sinai Medical Center, New York.
For example, a combination of the protease inhibitor faldaprevir with pegylated interferon alfa-2a plus ribavirin (PR) produced SVR rates at week 4 of follow-up (SVR4) of 74% in HCV/HIV coinfected patients, said Dr. Dietrich, a principal investigator for the STARTVerso4 trial.
In this phase III open-label trial, 308 patients with HCV/HIV coinfection who were treatment naive or relapsed after prior interferon-based therapy were randomly assigned to receive faldaprevir 120 mg daily for 24 weeks or 240 mg for 12 or 24 weeks according to on-treatment response. In both arms, faldaprevir was given on a PR backbone, with duration guided by response to therapy.
For the primary endpoint of SVR12, the investigators saw a 72% rate, with no significant difference between the two dose groups, compared with approximately 80% in monoinfected patients in other phase III studies. There was also no difference in efficacy between patients with or without cirrhosis, he said.
Adverse events included mild hyperbilirubinemia in some patients and interferon side effects.
Simeprevir in coinfection
Dr. Dietrich was also the lead on study C212, which looked at simeprevir (Olysio) on a PR backbone in coinfected patients. The results were similar to those seen with faldaprevir (73.6% overall SVR12).
Interestingly, the presence of the simeprevir-resistant q80K polymorphism did not make a difference in response rates, he said. Among monoinfected patients in prior studies, those with q80k polymorphism had significantly lower SVR rates. The adverse events were also similar to those seen in patients with monoinfection.
PHOTON-1
The PHOTON-1 trial evaluated the first interferon-free regimen (sofosbuvir plus ribavirin) in patients with HCV genotypes 1-3 and HIV.
In this study, patients with HCV and stable HIV infection received sofosbuvir 400 mg and ribavirin 1,000-1,200 mg daily. Treatment-naive patients with HCV genotype 1 and treatment-experienced patients with genotypes 2 or 3 received treatment for 24 weeks, while treatment-naive genotype 2/3 patients received 12 weeks of treatment. Patients on multiple antiretroviral (ART) regimens and those with compensated cirrhosis were included in the study.
The primary efficacy endpoint, SVR12, was achieved in 88% of treatment-naive genotype 2 patients and 67% of genotype 3 patients. In genotype 1 patients, the SVR was approximately 70%, Dr. Dietrich said.
Adverse events were general and limited to anemias, headache, and other symptoms commonly seen with HCV therapies, he added.
SYNERGY trial
Dr. Anita Kohli presented final results from the SYNERGY trial, which looked at combination oral HCV therapy for 6 or 12 weeks (SVR4 results from this trial were presented at the 2013 Liver Meeting).
In this phase II prospective cohort study, 60 treatment-naive patients with HCV genotype 1 were enrolled into one of three arms to receive either sofosbuvir 400 mg with ledipasvir 90 mg once daily in a fixed-dose combination for 12 weeks (arm A); the same fixed-dose combination plus the non-nucleoside NS5B inhibitor GS-9669 500 mg/day for 6 weeks (arm B); or the fixed-dose combination plus the NS3 protease inhibitor GS-9451 80 mg/day for 6 weeks.
The SVR12 rate among the patients on sofosbuvir/ledipasvir alone (arm A) was 100%, the rate in arm B was 95%, and the rate in arm C was 100%.
"We find these results very promising," said Dr. Kohli of the National Institutes of Health.
She noted that all patients in the trial were treatment naïve, and that all stages of liver disease were included in the 12-week treatment arm, but cirrhotic patients were excluded from the 6-week arms.
"These regimens are very simple. They’re one, two, or three pills a day," she noted. In addition, "our patient population is one that has been historically very difficult to treat, that is, predominantly African American," she noted.
Most of the patients had genotype 1a with a high viral load, and 25%-30% of patients in all treatment arms had advanced-stage liver disease, she added.
PEARL-III
The PEARL III trial looked at 419 treatment-naive, noncirrhotic patients with HCV genotype 1b, who were randomly assigned to receive either a ritonavir-boosted protease inhibitor (ABT-450) with ABT-267, which is an inhibitor of HCV NS5A, coformulated into a single pill; or ABT-333, a non-nucleoside polymerase inhibitor, with or without ribavirin.
In the ribavirin-containing arm, SVR12 was 99.5%, compared with 99% among controls. There was only one virologic failure in the study, and two patients who did not achieve SVR4 were lost to follow-up at week 12, noted Dr. Daniel Cohen of AbbVie Pharmaceuticals.
Adverse events included predominantly mild headache and fatigue in about 25% of patients, with slightly more events seen in the ribavirin combination arm.
STARTVerso4 was sponsored by Boehringer Ingelheim. C212 was sponsored by Janssen. SYNERGY was supported by the National Institutes of Health and Gilead Sciences. Dr. Cohen is employed by AbbVie, which sponsored PEARL III.
FROM CROI 2014
Major finding: In a phase II study 100% of treatment naive patients with hepatitis C genotype 1 had an SVR12 with once-daily combination pill.
Data source: Five clinical trials presented in oral abstract sessions.
Disclosures: STARTVerso4 was sponsored by Boehringer Ingelheim. Study C212 was sponsored by Janssen. SYNERGY was supported by the National Institutes of Health and Gilead Sciences. Dr. Cohen is employed by AbbVie, which sponsored PEARL III.
Targeted cancer therapies pose unique perioperative challenges
SCOTTSDALE, ARIZ. – Patients on targeted cancer therapies require special handling in the perioperative period, according to oncologist Sunil K. Sahai.
In addition to the known cardiotoxic side effects of DNA-damaging chemotherapy drugs such as anthracyclines and alkylating agents, newer drugs directed toward specific molecular targets on cancerous tumors have their own side effects that can complicate surgery or recovery, said Dr. Sahai of the University of Texas M.D. Anderson Cancer Center in Houston.
It can be a challenge even for oncologists to stay current with new cancer therapies and their side effect profiles, Dr. Sahai said at a meeting on perioperative medicine sponsored by the University of Miami.
"When M.D. Anderson was founded in the 1940s as the Texas Tumor Institute, there were three chemotherapy drugs on the market. Last year our formulary had 150 different chemotherapy drugs, " he said.
There are four major classes of targeted agents, each with its own associated adverse effects:
• Selective estrogen receptor modulators, such as tamoxifen and toremifene.
• Aromatase inhibitors – letrozole, anastrozole, and exemestane.
• Monoclonal antibodies – cetuximab, bevacizumab, trastuzumab, and others.
• Tyrosine kinase inhibitors (TKIs) – imatinib, dasatinib, sunitinib, and others.
Dr. Sahai presented case examples to illustrate the challenges of perioperative management of patients on targeted therapies. When a 67-year-old morbidly obese woman taking tamoxifen for the prevention of breast cancer presents with acute cholecystitis requiring urgent laparoscopic surgery, for example, Dr. Sahai said he would recommend 30 days of postoperative low-molecular-weight heparin injections to prevent venous thromboembolic events (VTEs).
"Patients who are on tamoxifen have a [baseline] relative risk of a VTE of between 3 and 7," he noted.
The combination of tamoxifen, obesity, and a history of breast cancer in a patient undergoing abdominal surgery suggests a high risk for VTE and a need for prophylaxis, he said.
He said he would not, however, recommend stopping tamoxifen without a documented discussion between the oncologist and the patient.
"Most oncologists are okay with stopping tamoxifen for 5-7 days during a procedure, but most of them are not willing to go for more than 14 days of stopping it," he said.
There are no data to support the practice; rather, it’s a matter of personal preference, he added.
Cardiotoxic agents
The cardiotoxic effects of older chemotherapy agents such as the anthracycline doxorubicin are well known, but newer agents, such as the monoclonal antibody trastuzumab (Herceptin) also have documented cardiotoxicities, although their long-term effects will not be known until the drugs have been on the market longer, Dr. Sahai pointed out.
Thus, a patient with colorectal cancer treated with an anthracycline is at increased risk for cardiomyopathy and heart failure, and if he receives a monoclonal antibody, he is at increased risk for ischemic cardiomyopathy. The combination of an anthracycline and trastuzumab (also used to treat gastric cancers) can increase the risk for heart failure by up to 28%, Dr. Sahai noted.
He also advised his colleagues to monitor patients receiving TKIs (most frequently prescribed for hematologic malignancies) for cardiac rhythm disturbances and drug interactions.
TKIs can prolong the QTc interval, predisposing patients to torsades de pointes, a form of ventricular tachycardia that can cause sudden death.
In addition, TKIs can cause pleural effusions, and are associated with difficult-to-control hypertension, Dr. Sahai said.
Decisions, decisions
If patients treated for cancer present for evaluation before noncardiac surgery with symptoms of cardiovascular disease such as chest pain, shortness of breath, or dyspnea on exertion, the clinician should first determine whether the symptoms appeared before, during, or after cancer therapy.
If the symptoms appeared before treatment, the patients should be evaluated for cardiovascular disease according to 2007 American College of Cardiology/American Heart Association perioperative evaluation guidelines, Dr. Sahai said.
If the cancer therapy included radiation or chemotherapy known to have cardiovascular side effects, patients should be tested for conditions that are most likely associated with their treatment history, whether myocardial ischemia, cardiomyopathies, arrhythmias, valvular disease, or a combination, he advised.
Dr. Sahai reported having no relevant financial disclosures.
SCOTTSDALE, ARIZ. – Patients on targeted cancer therapies require special handling in the perioperative period, according to oncologist Sunil K. Sahai.
In addition to the known cardiotoxic side effects of DNA-damaging chemotherapy drugs such as anthracyclines and alkylating agents, newer drugs directed toward specific molecular targets on cancerous tumors have their own side effects that can complicate surgery or recovery, said Dr. Sahai of the University of Texas M.D. Anderson Cancer Center in Houston.
It can be a challenge even for oncologists to stay current with new cancer therapies and their side effect profiles, Dr. Sahai said at a meeting on perioperative medicine sponsored by the University of Miami.
"When M.D. Anderson was founded in the 1940s as the Texas Tumor Institute, there were three chemotherapy drugs on the market. Last year our formulary had 150 different chemotherapy drugs, " he said.
There are four major classes of targeted agents, each with its own associated adverse effects:
• Selective estrogen receptor modulators, such as tamoxifen and toremifene.
• Aromatase inhibitors – letrozole, anastrozole, and exemestane.
• Monoclonal antibodies – cetuximab, bevacizumab, trastuzumab, and others.
• Tyrosine kinase inhibitors (TKIs) – imatinib, dasatinib, sunitinib, and others.
Dr. Sahai presented case examples to illustrate the challenges of perioperative management of patients on targeted therapies. When a 67-year-old morbidly obese woman taking tamoxifen for the prevention of breast cancer presents with acute cholecystitis requiring urgent laparoscopic surgery, for example, Dr. Sahai said he would recommend 30 days of postoperative low-molecular-weight heparin injections to prevent venous thromboembolic events (VTEs).
"Patients who are on tamoxifen have a [baseline] relative risk of a VTE of between 3 and 7," he noted.
The combination of tamoxifen, obesity, and a history of breast cancer in a patient undergoing abdominal surgery suggests a high risk for VTE and a need for prophylaxis, he said.
He said he would not, however, recommend stopping tamoxifen without a documented discussion between the oncologist and the patient.
"Most oncologists are okay with stopping tamoxifen for 5-7 days during a procedure, but most of them are not willing to go for more than 14 days of stopping it," he said.
There are no data to support the practice; rather, it’s a matter of personal preference, he added.
Cardiotoxic agents
The cardiotoxic effects of older chemotherapy agents such as the anthracycline doxorubicin are well known, but newer agents, such as the monoclonal antibody trastuzumab (Herceptin) also have documented cardiotoxicities, although their long-term effects will not be known until the drugs have been on the market longer, Dr. Sahai pointed out.
Thus, a patient with colorectal cancer treated with an anthracycline is at increased risk for cardiomyopathy and heart failure, and if he receives a monoclonal antibody, he is at increased risk for ischemic cardiomyopathy. The combination of an anthracycline and trastuzumab (also used to treat gastric cancers) can increase the risk for heart failure by up to 28%, Dr. Sahai noted.
He also advised his colleagues to monitor patients receiving TKIs (most frequently prescribed for hematologic malignancies) for cardiac rhythm disturbances and drug interactions.
TKIs can prolong the QTc interval, predisposing patients to torsades de pointes, a form of ventricular tachycardia that can cause sudden death.
In addition, TKIs can cause pleural effusions, and are associated with difficult-to-control hypertension, Dr. Sahai said.
Decisions, decisions
If patients treated for cancer present for evaluation before noncardiac surgery with symptoms of cardiovascular disease such as chest pain, shortness of breath, or dyspnea on exertion, the clinician should first determine whether the symptoms appeared before, during, or after cancer therapy.
If the symptoms appeared before treatment, the patients should be evaluated for cardiovascular disease according to 2007 American College of Cardiology/American Heart Association perioperative evaluation guidelines, Dr. Sahai said.
If the cancer therapy included radiation or chemotherapy known to have cardiovascular side effects, patients should be tested for conditions that are most likely associated with their treatment history, whether myocardial ischemia, cardiomyopathies, arrhythmias, valvular disease, or a combination, he advised.
Dr. Sahai reported having no relevant financial disclosures.
SCOTTSDALE, ARIZ. – Patients on targeted cancer therapies require special handling in the perioperative period, according to oncologist Sunil K. Sahai.
In addition to the known cardiotoxic side effects of DNA-damaging chemotherapy drugs such as anthracyclines and alkylating agents, newer drugs directed toward specific molecular targets on cancerous tumors have their own side effects that can complicate surgery or recovery, said Dr. Sahai of the University of Texas M.D. Anderson Cancer Center in Houston.
It can be a challenge even for oncologists to stay current with new cancer therapies and their side effect profiles, Dr. Sahai said at a meeting on perioperative medicine sponsored by the University of Miami.
"When M.D. Anderson was founded in the 1940s as the Texas Tumor Institute, there were three chemotherapy drugs on the market. Last year our formulary had 150 different chemotherapy drugs, " he said.
There are four major classes of targeted agents, each with its own associated adverse effects:
• Selective estrogen receptor modulators, such as tamoxifen and toremifene.
• Aromatase inhibitors – letrozole, anastrozole, and exemestane.
• Monoclonal antibodies – cetuximab, bevacizumab, trastuzumab, and others.
• Tyrosine kinase inhibitors (TKIs) – imatinib, dasatinib, sunitinib, and others.
Dr. Sahai presented case examples to illustrate the challenges of perioperative management of patients on targeted therapies. When a 67-year-old morbidly obese woman taking tamoxifen for the prevention of breast cancer presents with acute cholecystitis requiring urgent laparoscopic surgery, for example, Dr. Sahai said he would recommend 30 days of postoperative low-molecular-weight heparin injections to prevent venous thromboembolic events (VTEs).
"Patients who are on tamoxifen have a [baseline] relative risk of a VTE of between 3 and 7," he noted.
The combination of tamoxifen, obesity, and a history of breast cancer in a patient undergoing abdominal surgery suggests a high risk for VTE and a need for prophylaxis, he said.
He said he would not, however, recommend stopping tamoxifen without a documented discussion between the oncologist and the patient.
"Most oncologists are okay with stopping tamoxifen for 5-7 days during a procedure, but most of them are not willing to go for more than 14 days of stopping it," he said.
There are no data to support the practice; rather, it’s a matter of personal preference, he added.
Cardiotoxic agents
The cardiotoxic effects of older chemotherapy agents such as the anthracycline doxorubicin are well known, but newer agents, such as the monoclonal antibody trastuzumab (Herceptin) also have documented cardiotoxicities, although their long-term effects will not be known until the drugs have been on the market longer, Dr. Sahai pointed out.
Thus, a patient with colorectal cancer treated with an anthracycline is at increased risk for cardiomyopathy and heart failure, and if he receives a monoclonal antibody, he is at increased risk for ischemic cardiomyopathy. The combination of an anthracycline and trastuzumab (also used to treat gastric cancers) can increase the risk for heart failure by up to 28%, Dr. Sahai noted.
He also advised his colleagues to monitor patients receiving TKIs (most frequently prescribed for hematologic malignancies) for cardiac rhythm disturbances and drug interactions.
TKIs can prolong the QTc interval, predisposing patients to torsades de pointes, a form of ventricular tachycardia that can cause sudden death.
In addition, TKIs can cause pleural effusions, and are associated with difficult-to-control hypertension, Dr. Sahai said.
Decisions, decisions
If patients treated for cancer present for evaluation before noncardiac surgery with symptoms of cardiovascular disease such as chest pain, shortness of breath, or dyspnea on exertion, the clinician should first determine whether the symptoms appeared before, during, or after cancer therapy.
If the symptoms appeared before treatment, the patients should be evaluated for cardiovascular disease according to 2007 American College of Cardiology/American Heart Association perioperative evaluation guidelines, Dr. Sahai said.
If the cancer therapy included radiation or chemotherapy known to have cardiovascular side effects, patients should be tested for conditions that are most likely associated with their treatment history, whether myocardial ischemia, cardiomyopathies, arrhythmias, valvular disease, or a combination, he advised.
Dr. Sahai reported having no relevant financial disclosures.
EXPERT ANALYSIS FROM THE PERIOPERATIVE MEDICINE SUMMIT
Overall survival from recurrent head and neck cancer double among HPV+ patients
SCOTTSDALE, ARIZ. – Patients positive for the human papillomavirus have nearly twice the overall survival rate from recurrent oropharyngeal cancers as HPV-negative patients, Dr. Carole Fakhry reported at the 2014 Multidisciplinary Head and Neck Cancer Symposium.
Two years after a diagnosis of recurrence, 54.6% of HPV-positive patients were alive, compared with 27.6% of HPV-negative patients (P less than .001), according to a retrospective analysis of data from two clinical trials of 181 patients with stage III-IV oropharyngeal squamous cell carcinomas and known HPV status (measured by p16 protein expression).
"Tumor p16 status is independently associated with overall survival among oropharyngeal cancer patients with disease progression," said Dr. Fakhry of Johns Hopkins Medicine in Baltimore.
The analysis shows that "unquestionably, HPV-positive patients have a different molecular disease than their HPV-negative, tobacco-related counterparts. They are different with respect to specific tumor suppressor genes, and they are different in respect to specific activating oncogenes," noted Dr. Ezra Cohen of the University of California San Diego Moores Cancer Center, who was the invited discussant.
Dr. Fakhry and her colleagues looked at data on patients treated in the RTOG 0129 and 0522 trials.
Median time to progression was similar between the groups (8.2 months for HPV+ patients and 7.3 months for HPV–; P = .67), with the majority of disease progressions occurring within the first year (65% and 63%, respectively), reported Dr. Fakhry at the symposium, cosponsored by the American Society for Radiation Oncology and American Society of Clinical Oncology.
Factors associated with better overall survival in multivariate analysis included HPV+ status, salvage surgery, local-regional vs. distant progression, lower T stage at enrollment, and less than 20 smoking pack-years.
The study was supported by the National Cancer Institute and Bristol-Myers Squibb. Dr. Fakhry reported having no financial disclosures. Dr. Cohen disclosed serving as a consultant and adviser to BMS.
SCOTTSDALE, ARIZ. – Patients positive for the human papillomavirus have nearly twice the overall survival rate from recurrent oropharyngeal cancers as HPV-negative patients, Dr. Carole Fakhry reported at the 2014 Multidisciplinary Head and Neck Cancer Symposium.
Two years after a diagnosis of recurrence, 54.6% of HPV-positive patients were alive, compared with 27.6% of HPV-negative patients (P less than .001), according to a retrospective analysis of data from two clinical trials of 181 patients with stage III-IV oropharyngeal squamous cell carcinomas and known HPV status (measured by p16 protein expression).
"Tumor p16 status is independently associated with overall survival among oropharyngeal cancer patients with disease progression," said Dr. Fakhry of Johns Hopkins Medicine in Baltimore.
The analysis shows that "unquestionably, HPV-positive patients have a different molecular disease than their HPV-negative, tobacco-related counterparts. They are different with respect to specific tumor suppressor genes, and they are different in respect to specific activating oncogenes," noted Dr. Ezra Cohen of the University of California San Diego Moores Cancer Center, who was the invited discussant.
Dr. Fakhry and her colleagues looked at data on patients treated in the RTOG 0129 and 0522 trials.
Median time to progression was similar between the groups (8.2 months for HPV+ patients and 7.3 months for HPV–; P = .67), with the majority of disease progressions occurring within the first year (65% and 63%, respectively), reported Dr. Fakhry at the symposium, cosponsored by the American Society for Radiation Oncology and American Society of Clinical Oncology.
Factors associated with better overall survival in multivariate analysis included HPV+ status, salvage surgery, local-regional vs. distant progression, lower T stage at enrollment, and less than 20 smoking pack-years.
The study was supported by the National Cancer Institute and Bristol-Myers Squibb. Dr. Fakhry reported having no financial disclosures. Dr. Cohen disclosed serving as a consultant and adviser to BMS.
SCOTTSDALE, ARIZ. – Patients positive for the human papillomavirus have nearly twice the overall survival rate from recurrent oropharyngeal cancers as HPV-negative patients, Dr. Carole Fakhry reported at the 2014 Multidisciplinary Head and Neck Cancer Symposium.
Two years after a diagnosis of recurrence, 54.6% of HPV-positive patients were alive, compared with 27.6% of HPV-negative patients (P less than .001), according to a retrospective analysis of data from two clinical trials of 181 patients with stage III-IV oropharyngeal squamous cell carcinomas and known HPV status (measured by p16 protein expression).
"Tumor p16 status is independently associated with overall survival among oropharyngeal cancer patients with disease progression," said Dr. Fakhry of Johns Hopkins Medicine in Baltimore.
The analysis shows that "unquestionably, HPV-positive patients have a different molecular disease than their HPV-negative, tobacco-related counterparts. They are different with respect to specific tumor suppressor genes, and they are different in respect to specific activating oncogenes," noted Dr. Ezra Cohen of the University of California San Diego Moores Cancer Center, who was the invited discussant.
Dr. Fakhry and her colleagues looked at data on patients treated in the RTOG 0129 and 0522 trials.
Median time to progression was similar between the groups (8.2 months for HPV+ patients and 7.3 months for HPV–; P = .67), with the majority of disease progressions occurring within the first year (65% and 63%, respectively), reported Dr. Fakhry at the symposium, cosponsored by the American Society for Radiation Oncology and American Society of Clinical Oncology.
Factors associated with better overall survival in multivariate analysis included HPV+ status, salvage surgery, local-regional vs. distant progression, lower T stage at enrollment, and less than 20 smoking pack-years.
The study was supported by the National Cancer Institute and Bristol-Myers Squibb. Dr. Fakhry reported having no financial disclosures. Dr. Cohen disclosed serving as a consultant and adviser to BMS.
AT THE 2014 HEAD AND NECK CANCER SYMPOSIUM
Major finding: Two years after a diagnosis of recurrence of oropharyngeal cancer, 54.6% of HPV-positive patients were alive, compared with 27.6% of HPV-negative patients
Data source: Retrospective analysis of 181 patients with known HPV status in the RTOG 0129 and 0522 trials.
Disclosures: The study was supported by the National Cancer Institute and Bristol-Myers Squibb. Dr. Fakhry reported having no financial disclosures. Dr. Cohen disclosed serving as a consultant and adviser to BMS.
Humidification mitigates radiation-induced mucositis, but compliance is a problem
SCOTTSDALE, ARIZ. – A home humidification device can reduce the symptom burden of mucositis in patients undergoing radiation for head and neck cancers, but the technology only works when patients actually use it, reported investigators at the Multidisciplinary Head and Neck Cancer Symposium.
In a randomized phase III trial, patients assigned to daily humidification of the mouth and throat beginning on the first day of radiation had a 45% reduction in risk for acute hospitalization and had about half the symptom-related hospital days of patients who did not receive daily humidification, reported Dr. Andrew Macann from Auckland (New Zealand) City Hospital.
However, compliance with the humidification protocol was spotty, with only 42% of patients assigned to the therapy using it according to study protocol, Dr. Macann noted.
"The efficacy signals were seen across clinician-reported, independent, and patient-reported outcomes, and although in the main these signals were seen in the per-protocol analysis, the result which is perhaps most influential in considering whether domiciliary humidification could be cost effective, was the reduction in inpatient hospital days, where there was significant reduction in both the intention-to-treat and per protocol analyses," he said at the symposium, cosponsored by the American Society for Radiation Oncology and the American Society of Clinical Oncology .
The device used in the study was a humidifier/flow generator with a plastic face apparatus that delivered 44 mg of water per liter of air at a rate of 30 L/min. The flow was designed to slightly exceed inspiratory flow so that there was no entrainment of nonhumidified air.
In the trial, conducted by the Trans Tasman Radiation Oncology Group, 210 patients with cancers of the nasopharynx, oropharynx, oral cavity, larynx or hypopharynx were randomly assigned to receive humidification plus the institutional standard of care for mucositis management, or standard of care alone. Patients assigned to humidification were supposed to continue on the protocol until resolution of the ulcerative component of clinical mucositis.
A total of 103 patients assigned to humidification and 100 controls were available for the intention-to-treat (ITT) analysis.
Humidification compliance was electronically recorded, with full compliance consisting of more than 4 hours of daily use. The investigators calculated compliance ratios based on the number of full compliance days divided by the total days from the start of therapy to resolution of ulcerative mucositis. They determined high compliance to be a ratio greater than 0.67, and medium compliance to be a ratio of 0.34-0.66. High and medium compliers (23 and 20 patients, respectively) were included in the per-protocol analysis.
Although the humidification protocol did not meet the primary endpoint (area under the curve for a clinical mucositis score of 2 or greater according to Common Terminology Criteria for Adverse Events) in either the ITT or per-protocol analysis, there was a significant reduction in clinician-assessed functional mucositis symptom burden among the compliant patients (P = .009).
Additionally, total days in hospital were significantly lower among patients on the experimental protocol in both the ITT and per-protocol analyses. Control patients spent a geometric mean 4.10 days in hospital compared with 2.32 in ITT (P = .017), and 1.65 in per-protocol (P = .006). The investigators calculated that all patients treated with humidification spent only 57% of the hospital days of controls, and that compliant patients spent only 40% of those days.
Compliant patients were also significantly less likely than controls to require a feeding tube, with an odds ratio for never needing a tube of 2.50 (P = .035).
Patient-reported impression of symptom burden as rated on the McMaster University Head and Neck Questionnaire trended toward favoring the humidification protocol but there were no significant differences between the groups.
Simple strategies such as humidification can add value to head and neck cancer therapy, commented Dr. Paul M. Harari of the University of Wisconsin, Madison.
"I would love to see this humidification developed in a way that would be more compliant for patients, because I have no doubt that it could be valuable," he said.
The study was funded by the New Zealand Ministry of Science and Innovation, Fisher and Paykel Healthcare, Baxter Healthcare, and Auckland Hospital Charitable Trust. Dr. Macann and Dr. Harari reported having no conflicts of interest.
SCOTTSDALE, ARIZ. – A home humidification device can reduce the symptom burden of mucositis in patients undergoing radiation for head and neck cancers, but the technology only works when patients actually use it, reported investigators at the Multidisciplinary Head and Neck Cancer Symposium.
In a randomized phase III trial, patients assigned to daily humidification of the mouth and throat beginning on the first day of radiation had a 45% reduction in risk for acute hospitalization and had about half the symptom-related hospital days of patients who did not receive daily humidification, reported Dr. Andrew Macann from Auckland (New Zealand) City Hospital.
However, compliance with the humidification protocol was spotty, with only 42% of patients assigned to the therapy using it according to study protocol, Dr. Macann noted.
"The efficacy signals were seen across clinician-reported, independent, and patient-reported outcomes, and although in the main these signals were seen in the per-protocol analysis, the result which is perhaps most influential in considering whether domiciliary humidification could be cost effective, was the reduction in inpatient hospital days, where there was significant reduction in both the intention-to-treat and per protocol analyses," he said at the symposium, cosponsored by the American Society for Radiation Oncology and the American Society of Clinical Oncology .
The device used in the study was a humidifier/flow generator with a plastic face apparatus that delivered 44 mg of water per liter of air at a rate of 30 L/min. The flow was designed to slightly exceed inspiratory flow so that there was no entrainment of nonhumidified air.
In the trial, conducted by the Trans Tasman Radiation Oncology Group, 210 patients with cancers of the nasopharynx, oropharynx, oral cavity, larynx or hypopharynx were randomly assigned to receive humidification plus the institutional standard of care for mucositis management, or standard of care alone. Patients assigned to humidification were supposed to continue on the protocol until resolution of the ulcerative component of clinical mucositis.
A total of 103 patients assigned to humidification and 100 controls were available for the intention-to-treat (ITT) analysis.
Humidification compliance was electronically recorded, with full compliance consisting of more than 4 hours of daily use. The investigators calculated compliance ratios based on the number of full compliance days divided by the total days from the start of therapy to resolution of ulcerative mucositis. They determined high compliance to be a ratio greater than 0.67, and medium compliance to be a ratio of 0.34-0.66. High and medium compliers (23 and 20 patients, respectively) were included in the per-protocol analysis.
Although the humidification protocol did not meet the primary endpoint (area under the curve for a clinical mucositis score of 2 or greater according to Common Terminology Criteria for Adverse Events) in either the ITT or per-protocol analysis, there was a significant reduction in clinician-assessed functional mucositis symptom burden among the compliant patients (P = .009).
Additionally, total days in hospital were significantly lower among patients on the experimental protocol in both the ITT and per-protocol analyses. Control patients spent a geometric mean 4.10 days in hospital compared with 2.32 in ITT (P = .017), and 1.65 in per-protocol (P = .006). The investigators calculated that all patients treated with humidification spent only 57% of the hospital days of controls, and that compliant patients spent only 40% of those days.
Compliant patients were also significantly less likely than controls to require a feeding tube, with an odds ratio for never needing a tube of 2.50 (P = .035).
Patient-reported impression of symptom burden as rated on the McMaster University Head and Neck Questionnaire trended toward favoring the humidification protocol but there were no significant differences between the groups.
Simple strategies such as humidification can add value to head and neck cancer therapy, commented Dr. Paul M. Harari of the University of Wisconsin, Madison.
"I would love to see this humidification developed in a way that would be more compliant for patients, because I have no doubt that it could be valuable," he said.
The study was funded by the New Zealand Ministry of Science and Innovation, Fisher and Paykel Healthcare, Baxter Healthcare, and Auckland Hospital Charitable Trust. Dr. Macann and Dr. Harari reported having no conflicts of interest.
SCOTTSDALE, ARIZ. – A home humidification device can reduce the symptom burden of mucositis in patients undergoing radiation for head and neck cancers, but the technology only works when patients actually use it, reported investigators at the Multidisciplinary Head and Neck Cancer Symposium.
In a randomized phase III trial, patients assigned to daily humidification of the mouth and throat beginning on the first day of radiation had a 45% reduction in risk for acute hospitalization and had about half the symptom-related hospital days of patients who did not receive daily humidification, reported Dr. Andrew Macann from Auckland (New Zealand) City Hospital.
However, compliance with the humidification protocol was spotty, with only 42% of patients assigned to the therapy using it according to study protocol, Dr. Macann noted.
"The efficacy signals were seen across clinician-reported, independent, and patient-reported outcomes, and although in the main these signals were seen in the per-protocol analysis, the result which is perhaps most influential in considering whether domiciliary humidification could be cost effective, was the reduction in inpatient hospital days, where there was significant reduction in both the intention-to-treat and per protocol analyses," he said at the symposium, cosponsored by the American Society for Radiation Oncology and the American Society of Clinical Oncology .
The device used in the study was a humidifier/flow generator with a plastic face apparatus that delivered 44 mg of water per liter of air at a rate of 30 L/min. The flow was designed to slightly exceed inspiratory flow so that there was no entrainment of nonhumidified air.
In the trial, conducted by the Trans Tasman Radiation Oncology Group, 210 patients with cancers of the nasopharynx, oropharynx, oral cavity, larynx or hypopharynx were randomly assigned to receive humidification plus the institutional standard of care for mucositis management, or standard of care alone. Patients assigned to humidification were supposed to continue on the protocol until resolution of the ulcerative component of clinical mucositis.
A total of 103 patients assigned to humidification and 100 controls were available for the intention-to-treat (ITT) analysis.
Humidification compliance was electronically recorded, with full compliance consisting of more than 4 hours of daily use. The investigators calculated compliance ratios based on the number of full compliance days divided by the total days from the start of therapy to resolution of ulcerative mucositis. They determined high compliance to be a ratio greater than 0.67, and medium compliance to be a ratio of 0.34-0.66. High and medium compliers (23 and 20 patients, respectively) were included in the per-protocol analysis.
Although the humidification protocol did not meet the primary endpoint (area under the curve for a clinical mucositis score of 2 or greater according to Common Terminology Criteria for Adverse Events) in either the ITT or per-protocol analysis, there was a significant reduction in clinician-assessed functional mucositis symptom burden among the compliant patients (P = .009).
Additionally, total days in hospital were significantly lower among patients on the experimental protocol in both the ITT and per-protocol analyses. Control patients spent a geometric mean 4.10 days in hospital compared with 2.32 in ITT (P = .017), and 1.65 in per-protocol (P = .006). The investigators calculated that all patients treated with humidification spent only 57% of the hospital days of controls, and that compliant patients spent only 40% of those days.
Compliant patients were also significantly less likely than controls to require a feeding tube, with an odds ratio for never needing a tube of 2.50 (P = .035).
Patient-reported impression of symptom burden as rated on the McMaster University Head and Neck Questionnaire trended toward favoring the humidification protocol but there were no significant differences between the groups.
Simple strategies such as humidification can add value to head and neck cancer therapy, commented Dr. Paul M. Harari of the University of Wisconsin, Madison.
"I would love to see this humidification developed in a way that would be more compliant for patients, because I have no doubt that it could be valuable," he said.
The study was funded by the New Zealand Ministry of Science and Innovation, Fisher and Paykel Healthcare, Baxter Healthcare, and Auckland Hospital Charitable Trust. Dr. Macann and Dr. Harari reported having no conflicts of interest.
AT THE HEAD AND NECK CANCER SYMPOSIUM
Major finding: Patients with radiation-treated head and neck cancers who received daily humidification had approximately half of the hospital days for mucositis of controls treated with standard care.
Data source: Randomized phase III trial of 203 patients treated with radiation for head and neck cancers.
Disclosures: The study was funded by the New Zealand Ministry of Science & Innovation, Fisher and Paykel Healthcare, Baxter Healthcare, and Auckland Hospital Charitable Trust. Dr. Macann and Dr. Harari reported having no conflicts of interest.
Gland-sparing technique safe in tonsillar, tongue cancers
SCOTTSDALE, ARIZ. – Although radiation oncologists have typically worried that, in patients with oral cancers, leaving contralateral submandibular glands untreated could lead to tumor involvement of nearby lymph nodes, those worries may soon be put to rest, suggest results of a small retrospective study.
Among 71 patients with locally advanced cancers of the tongue base or tonsils who underwent radiation therapy that avoided targeting the contralateral submandibular glands, there were no cancer recurrences in contralateral level 1B nodes after a median 27.3 months of follow-up, reported Dr. Tyler Robin of the University of Colorado at Denver, Aurora.
"We’re interested in sparing the contralateral submandibular gland because we’re interested in minimizing xerostomia. Xerostomia is a significant morbidity of head and neck cancer radiotherapy, and it has substantial impact on patient quality of life," Dr. Robin said at the Multidisciplinary Head and Neck Cancer Symposium.
Intensity-modulated radiation therapy (IMRT) allows treatment beams to be shaped to avoid the parotid glands with no subsequent increase in regional lymph node failures and preservation of parotid salivary flow. But patient-reported xerostomia and quality-of-life outcomes with parotid-sparing techniques have been mixed, Dr. Robin said.
"Interestingly, an earlier study looking at predictors of xerostomia found that dose to the submandibular gland was a stronger predictor of xerostomia than dose to the parotids, and this may be because of the role of the submandibular gland in unstimulated salivary flow," he said.
The submandibular gland is located near level IB lymph nodes, but the risk of contralateral level IB involvement in oropharyngeal (OP) cancers is low, on the order of 0%-2%. Gland-sparing therapy with IMRT has previously been shown to mitigate xerostomia and to be safe, but primarily in patients with early-stage disease, prompting the investigators to examine whether it would also be safe and effective in patients with locally advanced tumors.
The question is particularly relevant at a time when the epidemiology of OP cancers is shifting toward patients who are positive for the human papillomavirus, who are more likely to have good therapeutic outcomes and who may live for many decades beyond an initial diagnosis, Dr. Robin said at the symposium cosponsored by the American Society for Radiation Oncology and the American Society of Clinical Oncology.
His team reviewed records of 71 patients treated for primary OP cancers at the University of Colorado and at Memorial Sloan-Kettering Cancer Center in New York.
In all, 40 patients had tonsillar cancers, 28 had base-of-tongue lesions, and 3 had cancers involving both sites.
They considered gland-sparing procedures as those in which total doses delivered to the contralateral submandibular gland during bilateral neck radiotherapy were not more than 39 Gy.
Of the 71 patients, 61 (85.9%) had stage IVA disease, 6 (8.5%) had stage III cancers, and 3 (4.2%) had stage IVB disease. The majority of patients had significant nodal involvement, with 46 (64.8%) having stage N2b; 7 (9.9%) N2c; and 3 (4.2%) having staging N3 disease.
The respective mean and median doses to the contralateral glands were 33.04 and 34.21 Gy.
At median follow-up of 27.3 months, there were 12 treatment failures: 1 local, 6 regional, and 5 distant failures. However, there were no cases of disease recurrence in contralateral level IB nodes, the investigators found. There was, however, one documented case of recurrence in contralateral level IIa lymph nodes.
"We believe this is evidence that contralateral submandibular gland sparing can be feasible and safe even in advanced node-positive head and neck cancers, including base- of-tongue lesions," Dr. Robin said,
The data suggest the need for a large prospective trial specifically addressing the safety and efficacy of contralateral submandibular gland-sparing therapy in patients with locally advanced head and neck cancers. Such studies should incorporate existing xerostomia-based quality-of-life assessments and formal sialometry studies, he added.
While the study shows that contralateral submandibular gland sparing is feasible, it raises the question of whether the technique might increase the dose to the muscles of the floor of the mouth, said Dr. Harry Quon of Johns Hopkins University in Baltimore, the invited discussant.
"There is emerging data that our chemoradiation approaches, with long-term follow-up maybe increases [patients’] risk of death, and one hypothesis that has been put forward is chronic aspiration with secondary injury to the lungs," he said.
Radiation injury to the floor-of-mouth muscles appears to be a significant risk factor for aspiration, indicating that future conformal approaches to treating cancers of the tonsils and tongue base should attempt to avoid delivering excessive doses to the midline mucosa, Dr. Quon said.
The study was internally funded. Dr. Robin and Dr. Quon reported having no financial disclosures.
SCOTTSDALE, ARIZ. – Although radiation oncologists have typically worried that, in patients with oral cancers, leaving contralateral submandibular glands untreated could lead to tumor involvement of nearby lymph nodes, those worries may soon be put to rest, suggest results of a small retrospective study.
Among 71 patients with locally advanced cancers of the tongue base or tonsils who underwent radiation therapy that avoided targeting the contralateral submandibular glands, there were no cancer recurrences in contralateral level 1B nodes after a median 27.3 months of follow-up, reported Dr. Tyler Robin of the University of Colorado at Denver, Aurora.
"We’re interested in sparing the contralateral submandibular gland because we’re interested in minimizing xerostomia. Xerostomia is a significant morbidity of head and neck cancer radiotherapy, and it has substantial impact on patient quality of life," Dr. Robin said at the Multidisciplinary Head and Neck Cancer Symposium.
Intensity-modulated radiation therapy (IMRT) allows treatment beams to be shaped to avoid the parotid glands with no subsequent increase in regional lymph node failures and preservation of parotid salivary flow. But patient-reported xerostomia and quality-of-life outcomes with parotid-sparing techniques have been mixed, Dr. Robin said.
"Interestingly, an earlier study looking at predictors of xerostomia found that dose to the submandibular gland was a stronger predictor of xerostomia than dose to the parotids, and this may be because of the role of the submandibular gland in unstimulated salivary flow," he said.
The submandibular gland is located near level IB lymph nodes, but the risk of contralateral level IB involvement in oropharyngeal (OP) cancers is low, on the order of 0%-2%. Gland-sparing therapy with IMRT has previously been shown to mitigate xerostomia and to be safe, but primarily in patients with early-stage disease, prompting the investigators to examine whether it would also be safe and effective in patients with locally advanced tumors.
The question is particularly relevant at a time when the epidemiology of OP cancers is shifting toward patients who are positive for the human papillomavirus, who are more likely to have good therapeutic outcomes and who may live for many decades beyond an initial diagnosis, Dr. Robin said at the symposium cosponsored by the American Society for Radiation Oncology and the American Society of Clinical Oncology.
His team reviewed records of 71 patients treated for primary OP cancers at the University of Colorado and at Memorial Sloan-Kettering Cancer Center in New York.
In all, 40 patients had tonsillar cancers, 28 had base-of-tongue lesions, and 3 had cancers involving both sites.
They considered gland-sparing procedures as those in which total doses delivered to the contralateral submandibular gland during bilateral neck radiotherapy were not more than 39 Gy.
Of the 71 patients, 61 (85.9%) had stage IVA disease, 6 (8.5%) had stage III cancers, and 3 (4.2%) had stage IVB disease. The majority of patients had significant nodal involvement, with 46 (64.8%) having stage N2b; 7 (9.9%) N2c; and 3 (4.2%) having staging N3 disease.
The respective mean and median doses to the contralateral glands were 33.04 and 34.21 Gy.
At median follow-up of 27.3 months, there were 12 treatment failures: 1 local, 6 regional, and 5 distant failures. However, there were no cases of disease recurrence in contralateral level IB nodes, the investigators found. There was, however, one documented case of recurrence in contralateral level IIa lymph nodes.
"We believe this is evidence that contralateral submandibular gland sparing can be feasible and safe even in advanced node-positive head and neck cancers, including base- of-tongue lesions," Dr. Robin said,
The data suggest the need for a large prospective trial specifically addressing the safety and efficacy of contralateral submandibular gland-sparing therapy in patients with locally advanced head and neck cancers. Such studies should incorporate existing xerostomia-based quality-of-life assessments and formal sialometry studies, he added.
While the study shows that contralateral submandibular gland sparing is feasible, it raises the question of whether the technique might increase the dose to the muscles of the floor of the mouth, said Dr. Harry Quon of Johns Hopkins University in Baltimore, the invited discussant.
"There is emerging data that our chemoradiation approaches, with long-term follow-up maybe increases [patients’] risk of death, and one hypothesis that has been put forward is chronic aspiration with secondary injury to the lungs," he said.
Radiation injury to the floor-of-mouth muscles appears to be a significant risk factor for aspiration, indicating that future conformal approaches to treating cancers of the tonsils and tongue base should attempt to avoid delivering excessive doses to the midline mucosa, Dr. Quon said.
The study was internally funded. Dr. Robin and Dr. Quon reported having no financial disclosures.
SCOTTSDALE, ARIZ. – Although radiation oncologists have typically worried that, in patients with oral cancers, leaving contralateral submandibular glands untreated could lead to tumor involvement of nearby lymph nodes, those worries may soon be put to rest, suggest results of a small retrospective study.
Among 71 patients with locally advanced cancers of the tongue base or tonsils who underwent radiation therapy that avoided targeting the contralateral submandibular glands, there were no cancer recurrences in contralateral level 1B nodes after a median 27.3 months of follow-up, reported Dr. Tyler Robin of the University of Colorado at Denver, Aurora.
"We’re interested in sparing the contralateral submandibular gland because we’re interested in minimizing xerostomia. Xerostomia is a significant morbidity of head and neck cancer radiotherapy, and it has substantial impact on patient quality of life," Dr. Robin said at the Multidisciplinary Head and Neck Cancer Symposium.
Intensity-modulated radiation therapy (IMRT) allows treatment beams to be shaped to avoid the parotid glands with no subsequent increase in regional lymph node failures and preservation of parotid salivary flow. But patient-reported xerostomia and quality-of-life outcomes with parotid-sparing techniques have been mixed, Dr. Robin said.
"Interestingly, an earlier study looking at predictors of xerostomia found that dose to the submandibular gland was a stronger predictor of xerostomia than dose to the parotids, and this may be because of the role of the submandibular gland in unstimulated salivary flow," he said.
The submandibular gland is located near level IB lymph nodes, but the risk of contralateral level IB involvement in oropharyngeal (OP) cancers is low, on the order of 0%-2%. Gland-sparing therapy with IMRT has previously been shown to mitigate xerostomia and to be safe, but primarily in patients with early-stage disease, prompting the investigators to examine whether it would also be safe and effective in patients with locally advanced tumors.
The question is particularly relevant at a time when the epidemiology of OP cancers is shifting toward patients who are positive for the human papillomavirus, who are more likely to have good therapeutic outcomes and who may live for many decades beyond an initial diagnosis, Dr. Robin said at the symposium cosponsored by the American Society for Radiation Oncology and the American Society of Clinical Oncology.
His team reviewed records of 71 patients treated for primary OP cancers at the University of Colorado and at Memorial Sloan-Kettering Cancer Center in New York.
In all, 40 patients had tonsillar cancers, 28 had base-of-tongue lesions, and 3 had cancers involving both sites.
They considered gland-sparing procedures as those in which total doses delivered to the contralateral submandibular gland during bilateral neck radiotherapy were not more than 39 Gy.
Of the 71 patients, 61 (85.9%) had stage IVA disease, 6 (8.5%) had stage III cancers, and 3 (4.2%) had stage IVB disease. The majority of patients had significant nodal involvement, with 46 (64.8%) having stage N2b; 7 (9.9%) N2c; and 3 (4.2%) having staging N3 disease.
The respective mean and median doses to the contralateral glands were 33.04 and 34.21 Gy.
At median follow-up of 27.3 months, there were 12 treatment failures: 1 local, 6 regional, and 5 distant failures. However, there were no cases of disease recurrence in contralateral level IB nodes, the investigators found. There was, however, one documented case of recurrence in contralateral level IIa lymph nodes.
"We believe this is evidence that contralateral submandibular gland sparing can be feasible and safe even in advanced node-positive head and neck cancers, including base- of-tongue lesions," Dr. Robin said,
The data suggest the need for a large prospective trial specifically addressing the safety and efficacy of contralateral submandibular gland-sparing therapy in patients with locally advanced head and neck cancers. Such studies should incorporate existing xerostomia-based quality-of-life assessments and formal sialometry studies, he added.
While the study shows that contralateral submandibular gland sparing is feasible, it raises the question of whether the technique might increase the dose to the muscles of the floor of the mouth, said Dr. Harry Quon of Johns Hopkins University in Baltimore, the invited discussant.
"There is emerging data that our chemoradiation approaches, with long-term follow-up maybe increases [patients’] risk of death, and one hypothesis that has been put forward is chronic aspiration with secondary injury to the lungs," he said.
Radiation injury to the floor-of-mouth muscles appears to be a significant risk factor for aspiration, indicating that future conformal approaches to treating cancers of the tonsils and tongue base should attempt to avoid delivering excessive doses to the midline mucosa, Dr. Quon said.
The study was internally funded. Dr. Robin and Dr. Quon reported having no financial disclosures.
AT THE HEAD AND NECK CANCER SYMPOSIUM
Major finding: Among patients with oropharyngeal cancers treated with conformal radiation sparing the contralateral submandibular gland, there were no contralateral lymph node recurrences.
Data source: Retrospective analysis of 71 patients.
Disclosures: The study was internally funded. Dr. Robin and Dr. Quon reported having no financial disclosures.
CAR-T cells drive ALL into remission
NEW ORLEANS – Modified T cells continue to show their mettle against treatment-refractory leukemias, based on study results presented at the annual meeting of the American Society of Hematology.
Among children and young adults with heavily pretreated relapsed or refractory acute lymphoblastic leukemia (ALL), chimeric antigen receptor (CAR) T cells targeted against the CD19 receptor produced complete responses in 10 of 16 patients, including 3 patients with primary, treatment-refractory ALL who had never previously been in remission, reported Dr. Daniel W. Lee III of the National Cancer Institute in Bethesda, Md.
"We were able to clear CNS [central nervous system] leukemia using CAR-T cells alone," Dr. Lee said.
In a second study, CD19-targeted T cells induced molecular remissions in adults with B-lineage ALL refractory to chemotherapy, said Dr. Marco L. Davila from the cellular therapeutics center at Memorial Sloan-Kettering Cancer Center (MSKCC) in New York City.
The immunotherapy produced a complete response (CR) in 12 of 16 patients, and a complete molecular response (CRm) in 12.
Dr. Davila commented that CAR-T cell therapy appears to be a good therapeutic choice for relapsed/refractory B-ALL.
"Especially in light of the data that we see in indolent lymphomas, where the response rates have not been nearly as great, I would speculate that there may be something about this disease that makes it particularly well suited to the second-generation CAR-T cell therapy," he said.
In both studies, therapy with CAR-T cells served as a bridge to stem cell transplantation for several patients.
Different CAR-T flavors
Each research team used its own variation on CAR-T cell therapy. The NCI investigators collected peripheral blood mononuclear cells (PBMCs) from patients via apheresis and used a gamma retrovirus to introduce into effector cells a genetic sequence targeting the CD19 receptor on malignant cells. The NCI version also uses CD28 costimulatory signaling to boost cell-killing effects.
The patients are conditioned with fludarabine and cyclophosphamide, and the treated T-cells are reinfused into the patients 11 days after harvesting.
In the phase I study, 15 patients with relapsed or refractory ALL and 1 with diffuse large B-cell lymphoma were treated. Eight of the patients had undergone at least one hematopoietic stem cell transplant, and all had received total body irradiation. Four had previously received another form of immunotherapy. The patients had to have been at least 100 days post transplant, with no graft-vs.-host disease.
T-cell expansion and transduction was feasible in this heavily pretreated population. All but two patients had an adequate or good expression of CAR-T cells. These patients were treated nonetheless, and one went on to have a minimal-residual disease (MRD) negative response, Dr. Lee noted.
In all, 10 of the patients had complete responses: All of these patients had ALL, and three had never previously achieved a remission. The patient with non-Hodgkin’s lymphoma did not have a significant treatment response.
Of eight patients who were negative for MRD after therapy, six went on to have stem cell transplants, with no unexpected toxicities.
As in other CAR-T cell studies, the chief toxicities seen included grade 4 neutropenia lasting longer than 2 weeks in nine patients, and the cytokine-release syndrome in four patients (grade 3 in two patients and grade 4 in two patients). One patient with the cytokine-release syndrome had cardiac arrest but was successfully resuscitated.
The cytokine-release syndrome was found to be associated with interleukin-6 (IL-6) and could be ameliorated with the IL-6 blocking agent tocilizumab (Actemra). The severity of cytokine-release syndrome did not correlate with tumor burden, the researchers noted.
MSKCC Study
Dr. Davila and his colleagues used a slightly different CAR-T cell construction, also with CD28 costimulation, to treat B-ALL in adults who either had refractory or relapsed disease (MRD-positive) or who were in their first complete remission. Patients who were positive for the Philadelphia chromosome (Ph+) and those who had extramedullary disease, CNS leukemia, or were in relapse after allogeneic stem cell transplant were all eligible.
He presented data on 16 patients with B-ALL with long-term follow-up: 14 patients had a complete response, with an average time to complete response of 24.5 days.
Seven patients in the MSKCC study have gone on to allogeneic stem cell transplants; three patients in complete remission were not eligible for transplant because of medical contraindications, and one additional patient was being evaluated for transplant. There have been no post-transplant relapses to date, with the longest follow-up out to 2 years, Dr. Davila said.
As in the NCI study, the cytokine-release syndrome was a common toxicity. The investigators initially tried to manage it with steroids but found that it came at the cost of lymphotoxicity that caused a marked decline in serum T-cells. They subsequently switched to tocilizumab, which was effective at treating the syndrome without lymphotoxicity.
Dr. Lee’s study was supported by the National Cancer Institute and St. Baldrick’s Foundation. He discussed off-label use of CAR-T cells. Dr. Lee reported having no conflicts of interest. Dr. Davila’s study was supported by MSKCC. He reported having no conflicts of interest.
NEW ORLEANS – Modified T cells continue to show their mettle against treatment-refractory leukemias, based on study results presented at the annual meeting of the American Society of Hematology.
Among children and young adults with heavily pretreated relapsed or refractory acute lymphoblastic leukemia (ALL), chimeric antigen receptor (CAR) T cells targeted against the CD19 receptor produced complete responses in 10 of 16 patients, including 3 patients with primary, treatment-refractory ALL who had never previously been in remission, reported Dr. Daniel W. Lee III of the National Cancer Institute in Bethesda, Md.
"We were able to clear CNS [central nervous system] leukemia using CAR-T cells alone," Dr. Lee said.
In a second study, CD19-targeted T cells induced molecular remissions in adults with B-lineage ALL refractory to chemotherapy, said Dr. Marco L. Davila from the cellular therapeutics center at Memorial Sloan-Kettering Cancer Center (MSKCC) in New York City.
The immunotherapy produced a complete response (CR) in 12 of 16 patients, and a complete molecular response (CRm) in 12.
Dr. Davila commented that CAR-T cell therapy appears to be a good therapeutic choice for relapsed/refractory B-ALL.
"Especially in light of the data that we see in indolent lymphomas, where the response rates have not been nearly as great, I would speculate that there may be something about this disease that makes it particularly well suited to the second-generation CAR-T cell therapy," he said.
In both studies, therapy with CAR-T cells served as a bridge to stem cell transplantation for several patients.
Different CAR-T flavors
Each research team used its own variation on CAR-T cell therapy. The NCI investigators collected peripheral blood mononuclear cells (PBMCs) from patients via apheresis and used a gamma retrovirus to introduce into effector cells a genetic sequence targeting the CD19 receptor on malignant cells. The NCI version also uses CD28 costimulatory signaling to boost cell-killing effects.
The patients are conditioned with fludarabine and cyclophosphamide, and the treated T-cells are reinfused into the patients 11 days after harvesting.
In the phase I study, 15 patients with relapsed or refractory ALL and 1 with diffuse large B-cell lymphoma were treated. Eight of the patients had undergone at least one hematopoietic stem cell transplant, and all had received total body irradiation. Four had previously received another form of immunotherapy. The patients had to have been at least 100 days post transplant, with no graft-vs.-host disease.
T-cell expansion and transduction was feasible in this heavily pretreated population. All but two patients had an adequate or good expression of CAR-T cells. These patients were treated nonetheless, and one went on to have a minimal-residual disease (MRD) negative response, Dr. Lee noted.
In all, 10 of the patients had complete responses: All of these patients had ALL, and three had never previously achieved a remission. The patient with non-Hodgkin’s lymphoma did not have a significant treatment response.
Of eight patients who were negative for MRD after therapy, six went on to have stem cell transplants, with no unexpected toxicities.
As in other CAR-T cell studies, the chief toxicities seen included grade 4 neutropenia lasting longer than 2 weeks in nine patients, and the cytokine-release syndrome in four patients (grade 3 in two patients and grade 4 in two patients). One patient with the cytokine-release syndrome had cardiac arrest but was successfully resuscitated.
The cytokine-release syndrome was found to be associated with interleukin-6 (IL-6) and could be ameliorated with the IL-6 blocking agent tocilizumab (Actemra). The severity of cytokine-release syndrome did not correlate with tumor burden, the researchers noted.
MSKCC Study
Dr. Davila and his colleagues used a slightly different CAR-T cell construction, also with CD28 costimulation, to treat B-ALL in adults who either had refractory or relapsed disease (MRD-positive) or who were in their first complete remission. Patients who were positive for the Philadelphia chromosome (Ph+) and those who had extramedullary disease, CNS leukemia, or were in relapse after allogeneic stem cell transplant were all eligible.
He presented data on 16 patients with B-ALL with long-term follow-up: 14 patients had a complete response, with an average time to complete response of 24.5 days.
Seven patients in the MSKCC study have gone on to allogeneic stem cell transplants; three patients in complete remission were not eligible for transplant because of medical contraindications, and one additional patient was being evaluated for transplant. There have been no post-transplant relapses to date, with the longest follow-up out to 2 years, Dr. Davila said.
As in the NCI study, the cytokine-release syndrome was a common toxicity. The investigators initially tried to manage it with steroids but found that it came at the cost of lymphotoxicity that caused a marked decline in serum T-cells. They subsequently switched to tocilizumab, which was effective at treating the syndrome without lymphotoxicity.
Dr. Lee’s study was supported by the National Cancer Institute and St. Baldrick’s Foundation. He discussed off-label use of CAR-T cells. Dr. Lee reported having no conflicts of interest. Dr. Davila’s study was supported by MSKCC. He reported having no conflicts of interest.
NEW ORLEANS – Modified T cells continue to show their mettle against treatment-refractory leukemias, based on study results presented at the annual meeting of the American Society of Hematology.
Among children and young adults with heavily pretreated relapsed or refractory acute lymphoblastic leukemia (ALL), chimeric antigen receptor (CAR) T cells targeted against the CD19 receptor produced complete responses in 10 of 16 patients, including 3 patients with primary, treatment-refractory ALL who had never previously been in remission, reported Dr. Daniel W. Lee III of the National Cancer Institute in Bethesda, Md.
"We were able to clear CNS [central nervous system] leukemia using CAR-T cells alone," Dr. Lee said.
In a second study, CD19-targeted T cells induced molecular remissions in adults with B-lineage ALL refractory to chemotherapy, said Dr. Marco L. Davila from the cellular therapeutics center at Memorial Sloan-Kettering Cancer Center (MSKCC) in New York City.
The immunotherapy produced a complete response (CR) in 12 of 16 patients, and a complete molecular response (CRm) in 12.
Dr. Davila commented that CAR-T cell therapy appears to be a good therapeutic choice for relapsed/refractory B-ALL.
"Especially in light of the data that we see in indolent lymphomas, where the response rates have not been nearly as great, I would speculate that there may be something about this disease that makes it particularly well suited to the second-generation CAR-T cell therapy," he said.
In both studies, therapy with CAR-T cells served as a bridge to stem cell transplantation for several patients.
Different CAR-T flavors
Each research team used its own variation on CAR-T cell therapy. The NCI investigators collected peripheral blood mononuclear cells (PBMCs) from patients via apheresis and used a gamma retrovirus to introduce into effector cells a genetic sequence targeting the CD19 receptor on malignant cells. The NCI version also uses CD28 costimulatory signaling to boost cell-killing effects.
The patients are conditioned with fludarabine and cyclophosphamide, and the treated T-cells are reinfused into the patients 11 days after harvesting.
In the phase I study, 15 patients with relapsed or refractory ALL and 1 with diffuse large B-cell lymphoma were treated. Eight of the patients had undergone at least one hematopoietic stem cell transplant, and all had received total body irradiation. Four had previously received another form of immunotherapy. The patients had to have been at least 100 days post transplant, with no graft-vs.-host disease.
T-cell expansion and transduction was feasible in this heavily pretreated population. All but two patients had an adequate or good expression of CAR-T cells. These patients were treated nonetheless, and one went on to have a minimal-residual disease (MRD) negative response, Dr. Lee noted.
In all, 10 of the patients had complete responses: All of these patients had ALL, and three had never previously achieved a remission. The patient with non-Hodgkin’s lymphoma did not have a significant treatment response.
Of eight patients who were negative for MRD after therapy, six went on to have stem cell transplants, with no unexpected toxicities.
As in other CAR-T cell studies, the chief toxicities seen included grade 4 neutropenia lasting longer than 2 weeks in nine patients, and the cytokine-release syndrome in four patients (grade 3 in two patients and grade 4 in two patients). One patient with the cytokine-release syndrome had cardiac arrest but was successfully resuscitated.
The cytokine-release syndrome was found to be associated with interleukin-6 (IL-6) and could be ameliorated with the IL-6 blocking agent tocilizumab (Actemra). The severity of cytokine-release syndrome did not correlate with tumor burden, the researchers noted.
MSKCC Study
Dr. Davila and his colleagues used a slightly different CAR-T cell construction, also with CD28 costimulation, to treat B-ALL in adults who either had refractory or relapsed disease (MRD-positive) or who were in their first complete remission. Patients who were positive for the Philadelphia chromosome (Ph+) and those who had extramedullary disease, CNS leukemia, or were in relapse after allogeneic stem cell transplant were all eligible.
He presented data on 16 patients with B-ALL with long-term follow-up: 14 patients had a complete response, with an average time to complete response of 24.5 days.
Seven patients in the MSKCC study have gone on to allogeneic stem cell transplants; three patients in complete remission were not eligible for transplant because of medical contraindications, and one additional patient was being evaluated for transplant. There have been no post-transplant relapses to date, with the longest follow-up out to 2 years, Dr. Davila said.
As in the NCI study, the cytokine-release syndrome was a common toxicity. The investigators initially tried to manage it with steroids but found that it came at the cost of lymphotoxicity that caused a marked decline in serum T-cells. They subsequently switched to tocilizumab, which was effective at treating the syndrome without lymphotoxicity.
Dr. Lee’s study was supported by the National Cancer Institute and St. Baldrick’s Foundation. He discussed off-label use of CAR-T cells. Dr. Lee reported having no conflicts of interest. Dr. Davila’s study was supported by MSKCC. He reported having no conflicts of interest.
AT ASH 2013
Major finding: Anti-CD19 chimeric antigen receptor T cells induced complete responses in 10 of 16 children and young adults with relapsed/refractory acute lymphoblastic leukemia. In a second study, CD19-targeted T cells induced complete molecular responses in 12 of 16 adults with B-lineage ALL refractory to chemotherapy.
Data source: Phase I studies of 2 novel CAR-T cell therapeutic strategies in a total of 32 patients.
Disclosures: Dr. Lee’s study was supported by the National Cancer Institute and St. Baldrick’s Foundation. He discussed off-label use of CAR-T cells. Dr. Lee reported having no conflicts of interest. Dr. Davila’s study was supported by Memorial Sloan-Kettering Cancer Center. He reported having no conflicts of interest.
Sickle cell crises curtailed with experimental cellular adhesion inhibitor
NEW ORLEANS – An experimental cellular adhesion inhibitor was successful at reducing the severity and duration of vaso-occlusive crises in patients with sickle cell disease.
In a phase II trial of 76 patients with sickle cell disease, patients randomized to receive the pan-selectin inhibitor GMI 1070 early in their hospitalization for a vaso-occlusive crisis (VOC) had shorter lengths of stay and needed significantly lower cumulative doses of narcotics for pain control than did patients randomized to placebo, reported Dr. Marilyn J. Telen, chief of the hematology division at Duke University, Durham, N.C.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
"We see somewhere between 75,000 and 90,000 admissions [annually] for acute painful vaso-occlusive crisis among this patient population. Indeed, these crises are the most common and essentially the archetypal presentation of sickle cell disease. Nevertheless, up till this time, treatment for these crises or VOC in sickle cell disease, remain only supportive, focusing largely on using narcotics for symptom relief, and then other measures, some of which are used to counteract the ill effects of narcotics," said Dr. Telen at the annual meeting of the American Society of Hematology.
GMI 1070 (being developed by GlycoMimetics, in partnership with Pfizer) is a synthetic molecule designed to inhibit the glycoprotein cellular-adhesion molecules involved in inflammation. In previous studies, the drug has been shown to be safe, and in a mouse model of VOC, was successful at restoring blood flow, Dr. Telen said. The drug has received both orphan drug and fast-track status from the Food and Drug Administration, according to GlycoMimetics.
Dr. Telen and her colleagues enrolled 76 patients aged 12-51 years with sickle cell disease and randomized them to receive a loading dose of GMI 1070 delivered intravenously (43 patients), followed by up to 14 subsequent doses delivered every 12 hours, or placebo (33 patients), with other treatment left to the discretion of the participating institutions. After an interim pharmacokinetic analysis showed that the drug did not reach target nadir levels, the dose was doubled.
All 76 patients reached the primary endpoint of VOC resolution, defined as a composite of decreased pain, termination of the need for intravenous opioids, patient and physician agreement on the ability to discharge the patient, and actual hospital discharge.
A total of 58 patients continued on the assigned drug until they either reached the primary endpoint criteria or received the maximum number of doses allowed. The remaining 18 patients discontinued the drug either for adverse events, no improvement by day 5 on the assigned drug, or other reasons.
In an analysis pooling all patients assigned to GMI 1070, including those who started out on the lower dose, there was a consistent reduction over placebo in the mean time to resolution of VOC: 103 hours vs. 144 hours for patients treated with placebo. This difference was not statistically significant, however.
A Kaplan-Meier analysis showed a median time to resolution of 69.6 hours for GMI 1070, compared with 139 hours with placebo, a difference that was not significant.
There was an 83% reduction in the secondary endpoint of cumulative opioid analgesics administered during hospitalization, a difference that was statistically significant (P =.010). There was also a reduction by 84 hours in the median time to discharge, and by 55 hours in the mean time to discharge, among patients treated with the active drug, compared with those on placebo. These differences, while large, were not significant, Dr. Telen said.
She noted that although most of the endpoints in this study failed to reach statistical significance, the separation of the curves between the placebo- and GMI 1070–treated patients began early, usually within 2 days of the start of treatment.
Total adverse event rates, including serious events and those deemed to be treatment related, were comparable between the two study arms for all subgroups.
Dr. Telen noted that because the population of patients enrolled was more clinically diverse than the available literature would suggest, the study was underpowered to detect differences, given the size of the sample. She predicted that given the size of the effects seen, statistical significance would emerge in a larger study.
GlycoMimetics is currently working with Pfizer to develop a phase III trial of GM 1070 for this indication.
The study was supported by GlycoMimetics. Dr. Telen is a consultant to the company, and several coauthors are employees of the company.
NEW ORLEANS – An experimental cellular adhesion inhibitor was successful at reducing the severity and duration of vaso-occlusive crises in patients with sickle cell disease.
In a phase II trial of 76 patients with sickle cell disease, patients randomized to receive the pan-selectin inhibitor GMI 1070 early in their hospitalization for a vaso-occlusive crisis (VOC) had shorter lengths of stay and needed significantly lower cumulative doses of narcotics for pain control than did patients randomized to placebo, reported Dr. Marilyn J. Telen, chief of the hematology division at Duke University, Durham, N.C.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
"We see somewhere between 75,000 and 90,000 admissions [annually] for acute painful vaso-occlusive crisis among this patient population. Indeed, these crises are the most common and essentially the archetypal presentation of sickle cell disease. Nevertheless, up till this time, treatment for these crises or VOC in sickle cell disease, remain only supportive, focusing largely on using narcotics for symptom relief, and then other measures, some of which are used to counteract the ill effects of narcotics," said Dr. Telen at the annual meeting of the American Society of Hematology.
GMI 1070 (being developed by GlycoMimetics, in partnership with Pfizer) is a synthetic molecule designed to inhibit the glycoprotein cellular-adhesion molecules involved in inflammation. In previous studies, the drug has been shown to be safe, and in a mouse model of VOC, was successful at restoring blood flow, Dr. Telen said. The drug has received both orphan drug and fast-track status from the Food and Drug Administration, according to GlycoMimetics.
Dr. Telen and her colleagues enrolled 76 patients aged 12-51 years with sickle cell disease and randomized them to receive a loading dose of GMI 1070 delivered intravenously (43 patients), followed by up to 14 subsequent doses delivered every 12 hours, or placebo (33 patients), with other treatment left to the discretion of the participating institutions. After an interim pharmacokinetic analysis showed that the drug did not reach target nadir levels, the dose was doubled.
All 76 patients reached the primary endpoint of VOC resolution, defined as a composite of decreased pain, termination of the need for intravenous opioids, patient and physician agreement on the ability to discharge the patient, and actual hospital discharge.
A total of 58 patients continued on the assigned drug until they either reached the primary endpoint criteria or received the maximum number of doses allowed. The remaining 18 patients discontinued the drug either for adverse events, no improvement by day 5 on the assigned drug, or other reasons.
In an analysis pooling all patients assigned to GMI 1070, including those who started out on the lower dose, there was a consistent reduction over placebo in the mean time to resolution of VOC: 103 hours vs. 144 hours for patients treated with placebo. This difference was not statistically significant, however.
A Kaplan-Meier analysis showed a median time to resolution of 69.6 hours for GMI 1070, compared with 139 hours with placebo, a difference that was not significant.
There was an 83% reduction in the secondary endpoint of cumulative opioid analgesics administered during hospitalization, a difference that was statistically significant (P =.010). There was also a reduction by 84 hours in the median time to discharge, and by 55 hours in the mean time to discharge, among patients treated with the active drug, compared with those on placebo. These differences, while large, were not significant, Dr. Telen said.
She noted that although most of the endpoints in this study failed to reach statistical significance, the separation of the curves between the placebo- and GMI 1070–treated patients began early, usually within 2 days of the start of treatment.
Total adverse event rates, including serious events and those deemed to be treatment related, were comparable between the two study arms for all subgroups.
Dr. Telen noted that because the population of patients enrolled was more clinically diverse than the available literature would suggest, the study was underpowered to detect differences, given the size of the sample. She predicted that given the size of the effects seen, statistical significance would emerge in a larger study.
GlycoMimetics is currently working with Pfizer to develop a phase III trial of GM 1070 for this indication.
The study was supported by GlycoMimetics. Dr. Telen is a consultant to the company, and several coauthors are employees of the company.
NEW ORLEANS – An experimental cellular adhesion inhibitor was successful at reducing the severity and duration of vaso-occlusive crises in patients with sickle cell disease.
In a phase II trial of 76 patients with sickle cell disease, patients randomized to receive the pan-selectin inhibitor GMI 1070 early in their hospitalization for a vaso-occlusive crisis (VOC) had shorter lengths of stay and needed significantly lower cumulative doses of narcotics for pain control than did patients randomized to placebo, reported Dr. Marilyn J. Telen, chief of the hematology division at Duke University, Durham, N.C.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
"We see somewhere between 75,000 and 90,000 admissions [annually] for acute painful vaso-occlusive crisis among this patient population. Indeed, these crises are the most common and essentially the archetypal presentation of sickle cell disease. Nevertheless, up till this time, treatment for these crises or VOC in sickle cell disease, remain only supportive, focusing largely on using narcotics for symptom relief, and then other measures, some of which are used to counteract the ill effects of narcotics," said Dr. Telen at the annual meeting of the American Society of Hematology.
GMI 1070 (being developed by GlycoMimetics, in partnership with Pfizer) is a synthetic molecule designed to inhibit the glycoprotein cellular-adhesion molecules involved in inflammation. In previous studies, the drug has been shown to be safe, and in a mouse model of VOC, was successful at restoring blood flow, Dr. Telen said. The drug has received both orphan drug and fast-track status from the Food and Drug Administration, according to GlycoMimetics.
Dr. Telen and her colleagues enrolled 76 patients aged 12-51 years with sickle cell disease and randomized them to receive a loading dose of GMI 1070 delivered intravenously (43 patients), followed by up to 14 subsequent doses delivered every 12 hours, or placebo (33 patients), with other treatment left to the discretion of the participating institutions. After an interim pharmacokinetic analysis showed that the drug did not reach target nadir levels, the dose was doubled.
All 76 patients reached the primary endpoint of VOC resolution, defined as a composite of decreased pain, termination of the need for intravenous opioids, patient and physician agreement on the ability to discharge the patient, and actual hospital discharge.
A total of 58 patients continued on the assigned drug until they either reached the primary endpoint criteria or received the maximum number of doses allowed. The remaining 18 patients discontinued the drug either for adverse events, no improvement by day 5 on the assigned drug, or other reasons.
In an analysis pooling all patients assigned to GMI 1070, including those who started out on the lower dose, there was a consistent reduction over placebo in the mean time to resolution of VOC: 103 hours vs. 144 hours for patients treated with placebo. This difference was not statistically significant, however.
A Kaplan-Meier analysis showed a median time to resolution of 69.6 hours for GMI 1070, compared with 139 hours with placebo, a difference that was not significant.
There was an 83% reduction in the secondary endpoint of cumulative opioid analgesics administered during hospitalization, a difference that was statistically significant (P =.010). There was also a reduction by 84 hours in the median time to discharge, and by 55 hours in the mean time to discharge, among patients treated with the active drug, compared with those on placebo. These differences, while large, were not significant, Dr. Telen said.
She noted that although most of the endpoints in this study failed to reach statistical significance, the separation of the curves between the placebo- and GMI 1070–treated patients began early, usually within 2 days of the start of treatment.
Total adverse event rates, including serious events and those deemed to be treatment related, were comparable between the two study arms for all subgroups.
Dr. Telen noted that because the population of patients enrolled was more clinically diverse than the available literature would suggest, the study was underpowered to detect differences, given the size of the sample. She predicted that given the size of the effects seen, statistical significance would emerge in a larger study.
GlycoMimetics is currently working with Pfizer to develop a phase III trial of GM 1070 for this indication.
The study was supported by GlycoMimetics. Dr. Telen is a consultant to the company, and several coauthors are employees of the company.
AT ASH 2013
Major finding: The mean time to resolution of vaso-occlusive crisis in patients with sickle cell disease was 103 hours for patients treated with GMI 1070 vs. 144 for those treated with placebo.
Data source: A randomized, double blind multicenter study of 76 patients aged 12-51.
Disclosures: The study was supported by GlycoMimetics. Dr. Telen is a consultant to the company, and several coauthors are employees of the company.
Gene therapy for SCID-X1 may successfully reset immune system
NEW ORLEANS – Tweaking experimental gene therapy for X-linked severe combined immunodeficiency may help to restore patient immune function while reducing the risk for subsequent leukemias.
In a small, multinational phase I/II trial, seven of nine children with SCID-X1 showed evidence of T-cell recovery and function, as well as a lower risk for promoting growth of leukemic cells, when a self-inactivating gamma-retroviral vector (SCID-2) was used to promote reconstitution of the child’s immune system without insertional oncogenesis, reported Dr. Sung-Yun Pai at the annual meeting of the American Society of Hematology.
"Outcomes for boys who do not have well-matched donors are suboptimal, and it’s particularly for these boys that we are targeting gene therapy," said Dr. Pai, of Boston Children’s Hospital and the Dana-Farber Cancer Institute, Boston.
In previous gene therapy trials, investigators used the Moloney leukemia virus (MLV)-based gamma-retroviral vector (SCID-1) with strong promoters and enhancers to express an IL-2 receptor that reconstituted the immune system successfully in 18 of 20 boys.
However, 5 of the 20 boys developed T-cell acute lymphoblastic leukemia; 1 of the children died, and the remaining 4 were successfully treated.
The investigators found that in the patients with leukemia, the SCID-1 vector had inserted into a chromosomal region close to proto-oncogenes such as LMO2, and the enhancers were driving expression of the neighboring oncogene, promoting expression of aberrant T cells. The vector was subsequently modified with the goal of improved safety but similar efficacy to the original, said Dr. Pai. The strong viral enhancers were removed to prevent accident enhancement should the inserted genes manage to find their way into oncogenes.
The phase I/II study is being conducted in London, Paris, Boston, Cincinnati, and Los Angeles, and to date has enrolled 9 male children of a planned 20.
Of the 9, one child died from a preexisting adenoviral infection before immune recovery was complete, and one child did not have engraftment of the gene-marked cells and went on to transplant.
"The other patients have 9 months to 36 months of follow-up, they have evidence of T-cell recovery, of T-cell function, have cleared SCID-related infections, and are all out of hospital, healthy at home, [and] leading essentially normal lives."
When the investigators looked at the comparative efficacy of the SCID-1 and SCID-2 vectors, they saw that 6 months after gene therapy, there was no significant difference in the median number of T cells generated.
"It’s far too early to comment on whether this vector will truly be safer in terms of leukemia," Dr. Pai said, noting that in the SCID-1 trial the leukemias developed 3-5 years after gene therapy, and the longest follow-up in the SCID-2 study is only 3 years.
The investigators are, however, conducting molecular surrogate safety analyses looking at gene insertion sites from the blood of patients treated with SCID-1 and are comparing those sites with the vector-insertion sites in cells from patients in SCID-2.
Looking at a global genomewide map of integrations, they found no significant differences between SCID-1 and SCID-2. However, when they focused on 38 genes that are to be proto-oncogenes in lymphoid cancer, they found that significantly more integration of the modified genes occurred in proximity to the oncogenes in SCID-1 than in SCID-2 (P = .003).
"We hope that these data suggest that the modified SCID vector will show less capacity to drive aberrant cell growth and lead to less leukemogenesis," said Dr. Pai.
SCID-X1 is caused by inherited mutations in the gamma subunit of the interleukin (IL)-2 receptor. As a result, males are born without T lymphocytes or natural killer cells. Without a bone marrow or stem cell transplantation, children with the disease die early from opportunistic or community-acquired infections.
"These are really paradigm-changing results for mortally wounded children," said Dr. Laurence James Neil Cooper of the University of Texas M.D. Anderson Cancer Center in Houston, who moderated the briefing but was not involved in the study.
The trial is being sponsored by Children’s Hospital Boston, Cincinnati Children’s Hospital Medical Center, and the University of California, Los Angeles. Dr. Pai and Dr. Cooper reported having no relevant conflicts of interest.
NEW ORLEANS – Tweaking experimental gene therapy for X-linked severe combined immunodeficiency may help to restore patient immune function while reducing the risk for subsequent leukemias.
In a small, multinational phase I/II trial, seven of nine children with SCID-X1 showed evidence of T-cell recovery and function, as well as a lower risk for promoting growth of leukemic cells, when a self-inactivating gamma-retroviral vector (SCID-2) was used to promote reconstitution of the child’s immune system without insertional oncogenesis, reported Dr. Sung-Yun Pai at the annual meeting of the American Society of Hematology.
"Outcomes for boys who do not have well-matched donors are suboptimal, and it’s particularly for these boys that we are targeting gene therapy," said Dr. Pai, of Boston Children’s Hospital and the Dana-Farber Cancer Institute, Boston.
In previous gene therapy trials, investigators used the Moloney leukemia virus (MLV)-based gamma-retroviral vector (SCID-1) with strong promoters and enhancers to express an IL-2 receptor that reconstituted the immune system successfully in 18 of 20 boys.
However, 5 of the 20 boys developed T-cell acute lymphoblastic leukemia; 1 of the children died, and the remaining 4 were successfully treated.
The investigators found that in the patients with leukemia, the SCID-1 vector had inserted into a chromosomal region close to proto-oncogenes such as LMO2, and the enhancers were driving expression of the neighboring oncogene, promoting expression of aberrant T cells. The vector was subsequently modified with the goal of improved safety but similar efficacy to the original, said Dr. Pai. The strong viral enhancers were removed to prevent accident enhancement should the inserted genes manage to find their way into oncogenes.
The phase I/II study is being conducted in London, Paris, Boston, Cincinnati, and Los Angeles, and to date has enrolled 9 male children of a planned 20.
Of the 9, one child died from a preexisting adenoviral infection before immune recovery was complete, and one child did not have engraftment of the gene-marked cells and went on to transplant.
"The other patients have 9 months to 36 months of follow-up, they have evidence of T-cell recovery, of T-cell function, have cleared SCID-related infections, and are all out of hospital, healthy at home, [and] leading essentially normal lives."
When the investigators looked at the comparative efficacy of the SCID-1 and SCID-2 vectors, they saw that 6 months after gene therapy, there was no significant difference in the median number of T cells generated.
"It’s far too early to comment on whether this vector will truly be safer in terms of leukemia," Dr. Pai said, noting that in the SCID-1 trial the leukemias developed 3-5 years after gene therapy, and the longest follow-up in the SCID-2 study is only 3 years.
The investigators are, however, conducting molecular surrogate safety analyses looking at gene insertion sites from the blood of patients treated with SCID-1 and are comparing those sites with the vector-insertion sites in cells from patients in SCID-2.
Looking at a global genomewide map of integrations, they found no significant differences between SCID-1 and SCID-2. However, when they focused on 38 genes that are to be proto-oncogenes in lymphoid cancer, they found that significantly more integration of the modified genes occurred in proximity to the oncogenes in SCID-1 than in SCID-2 (P = .003).
"We hope that these data suggest that the modified SCID vector will show less capacity to drive aberrant cell growth and lead to less leukemogenesis," said Dr. Pai.
SCID-X1 is caused by inherited mutations in the gamma subunit of the interleukin (IL)-2 receptor. As a result, males are born without T lymphocytes or natural killer cells. Without a bone marrow or stem cell transplantation, children with the disease die early from opportunistic or community-acquired infections.
"These are really paradigm-changing results for mortally wounded children," said Dr. Laurence James Neil Cooper of the University of Texas M.D. Anderson Cancer Center in Houston, who moderated the briefing but was not involved in the study.
The trial is being sponsored by Children’s Hospital Boston, Cincinnati Children’s Hospital Medical Center, and the University of California, Los Angeles. Dr. Pai and Dr. Cooper reported having no relevant conflicts of interest.
NEW ORLEANS – Tweaking experimental gene therapy for X-linked severe combined immunodeficiency may help to restore patient immune function while reducing the risk for subsequent leukemias.
In a small, multinational phase I/II trial, seven of nine children with SCID-X1 showed evidence of T-cell recovery and function, as well as a lower risk for promoting growth of leukemic cells, when a self-inactivating gamma-retroviral vector (SCID-2) was used to promote reconstitution of the child’s immune system without insertional oncogenesis, reported Dr. Sung-Yun Pai at the annual meeting of the American Society of Hematology.
"Outcomes for boys who do not have well-matched donors are suboptimal, and it’s particularly for these boys that we are targeting gene therapy," said Dr. Pai, of Boston Children’s Hospital and the Dana-Farber Cancer Institute, Boston.
In previous gene therapy trials, investigators used the Moloney leukemia virus (MLV)-based gamma-retroviral vector (SCID-1) with strong promoters and enhancers to express an IL-2 receptor that reconstituted the immune system successfully in 18 of 20 boys.
However, 5 of the 20 boys developed T-cell acute lymphoblastic leukemia; 1 of the children died, and the remaining 4 were successfully treated.
The investigators found that in the patients with leukemia, the SCID-1 vector had inserted into a chromosomal region close to proto-oncogenes such as LMO2, and the enhancers were driving expression of the neighboring oncogene, promoting expression of aberrant T cells. The vector was subsequently modified with the goal of improved safety but similar efficacy to the original, said Dr. Pai. The strong viral enhancers were removed to prevent accident enhancement should the inserted genes manage to find their way into oncogenes.
The phase I/II study is being conducted in London, Paris, Boston, Cincinnati, and Los Angeles, and to date has enrolled 9 male children of a planned 20.
Of the 9, one child died from a preexisting adenoviral infection before immune recovery was complete, and one child did not have engraftment of the gene-marked cells and went on to transplant.
"The other patients have 9 months to 36 months of follow-up, they have evidence of T-cell recovery, of T-cell function, have cleared SCID-related infections, and are all out of hospital, healthy at home, [and] leading essentially normal lives."
When the investigators looked at the comparative efficacy of the SCID-1 and SCID-2 vectors, they saw that 6 months after gene therapy, there was no significant difference in the median number of T cells generated.
"It’s far too early to comment on whether this vector will truly be safer in terms of leukemia," Dr. Pai said, noting that in the SCID-1 trial the leukemias developed 3-5 years after gene therapy, and the longest follow-up in the SCID-2 study is only 3 years.
The investigators are, however, conducting molecular surrogate safety analyses looking at gene insertion sites from the blood of patients treated with SCID-1 and are comparing those sites with the vector-insertion sites in cells from patients in SCID-2.
Looking at a global genomewide map of integrations, they found no significant differences between SCID-1 and SCID-2. However, when they focused on 38 genes that are to be proto-oncogenes in lymphoid cancer, they found that significantly more integration of the modified genes occurred in proximity to the oncogenes in SCID-1 than in SCID-2 (P = .003).
"We hope that these data suggest that the modified SCID vector will show less capacity to drive aberrant cell growth and lead to less leukemogenesis," said Dr. Pai.
SCID-X1 is caused by inherited mutations in the gamma subunit of the interleukin (IL)-2 receptor. As a result, males are born without T lymphocytes or natural killer cells. Without a bone marrow or stem cell transplantation, children with the disease die early from opportunistic or community-acquired infections.
"These are really paradigm-changing results for mortally wounded children," said Dr. Laurence James Neil Cooper of the University of Texas M.D. Anderson Cancer Center in Houston, who moderated the briefing but was not involved in the study.
The trial is being sponsored by Children’s Hospital Boston, Cincinnati Children’s Hospital Medical Center, and the University of California, Los Angeles. Dr. Pai and Dr. Cooper reported having no relevant conflicts of interest.
AT ASH 2013
Major finding: Of nine boys with X-linked severe combined immunodeficiency who were treated with gene therapy, seven patients have evidence of T-cell function, have cleared SCID-related infections, and are out of hospital.
Data source: Preliminary results of a prospective phase I/II clinical trial of nine children.
Disclosures: The trial is being sponsored by Children’s Hospital Boston, Cincinnati Children’s Hospital Medical Center, and the University of California, Los Angeles. Dr. Pai and Dr. Cooper reported having no relevant conflicts of interest.