User login
Metformin trims mother’s weight, but not baby’s
In obese pregnant women without diabetes, daily metformin reduces maternal weight gain, but not infant birth weight, results of the MOP trial show.
The incidence of preeclampsia was also lower with metformin than with placebo (3.0% vs. 11.3%; odds ratio, 0.24; P =.001).
There was no significant difference between the two groups in the incidence of other pregnancy complications or of adverse fetal or neonatal outcomes, Argyro Syngelaki, Ph.D., of King’s College Hospital in London, and her colleagues reported (N Engl J Med. 2016 Feb 3;374:434-43.).
Attempts at reducing the incidence of pregnancy complications associated with obesity have focused on dietary and lifestyle interventions but have generally been unsuccessful, she noted.
Additionally, the recent randomized EMPOWaR (Effect of Metformin on Maternal and Fetal Outcomes) trial failed to show that metformin use was associated with less gestational weight gain and a lower incidence of preeclampsia than did placebo.
EMPOWaR, however, used a body mass index (BMI) cutoff of 30 kg/m2 and a 2.5-g dose of metformin, compared with a BMI cutoff of 35 and the 3.0-g dose used in the MOP (Metformin in Obese Non-diabetic Pregnant Women) trial, Dr. Syngelaki observed.
Adherence to metformin was also higher in the MOP trial. Nearly 66% of women took a minimum metformin dose of 2.5 g for at least 50% of the days between randomization and delivery, compared with 2.5 g of metformin being used for only 38% of the days in the same period in EMPOWaR. The proportion of patients who were in this dose subgroup in the EMPOWaR trial was not specified.
MOP investigators randomly assigned 400 women with a singleton pregnancy to metformin or placebo from 12-18 weeks of gestation until delivery. All women underwent a 75-g oral glucose tolerance test at 28 weeks’ gestation, with metformin and placebo stopped for 1 week before the test. Women with abnormal oral glucose tolerance test results continued the assigned study regimen, and began home glucose monitoring. Insulin was added to their regimen, if target blood-glucose values were not achieved.
The metformin and placebo groups were well matched at baseline with respect to median maternal age (32.9 years vs. 30.8 years), median BMI (38.6 kg/m2 vs. 38.4 kg/m2), and spontaneous conception (97.5% vs. 98.0%).
Metformin did not reduce the primary outcome of median neonatal birth-weight z score, compared with placebo (0.05 vs. 0.17; P = .66) or the incidence of large for gestational age neonates (16.8% vs. 15.4%; P = .79), the MOP investigators reported.
There were no significant differences between the metformin and placebo groups in rates of miscarriage (zero vs. three cases), stillbirths (one case vs. two cases), or neonatal death (zero vs. one case).
The median maternal gestational weight gain, however, was lower with metformin than placebo (4.6 kg vs 6.3 kg; P less than .001), as was the incidence of preeclampsia.
The authors acknowledged that a limitation of the study was that it was not adequately powered for the secondary outcomes, but said the preeclampsia finding is compatible with several previous studies reporting that the prevalence of this potentially deadly complication increased with increasing prepregnancy BMI and gestational weight gain.
Side effects such as nausea, vomiting, and headache were as expected during gestation, although the incidence was significantly higher in the metformin group. Still, there was no significant between-group differences with regard to the decision to continue with the full dose, reduce the dose, or stop the study regimen.
“The rate of adherence was considerably higher among women taking the full dose of 3.0 g per day than among those taking less than 2.0 g per day, which suggests that adherence was not driven by the presence or absence of side effects, but by the motivation of the patients to adhere to the demands of the study,” the investigators wrote.
The Fetal Medicine Foundation funded the study. The investigators reported having no financial disclosures.
In obese pregnant women without diabetes, daily metformin reduces maternal weight gain, but not infant birth weight, results of the MOP trial show.
The incidence of preeclampsia was also lower with metformin than with placebo (3.0% vs. 11.3%; odds ratio, 0.24; P =.001).
There was no significant difference between the two groups in the incidence of other pregnancy complications or of adverse fetal or neonatal outcomes, Argyro Syngelaki, Ph.D., of King’s College Hospital in London, and her colleagues reported (N Engl J Med. 2016 Feb 3;374:434-43.).
Attempts at reducing the incidence of pregnancy complications associated with obesity have focused on dietary and lifestyle interventions but have generally been unsuccessful, she noted.
Additionally, the recent randomized EMPOWaR (Effect of Metformin on Maternal and Fetal Outcomes) trial failed to show that metformin use was associated with less gestational weight gain and a lower incidence of preeclampsia than did placebo.
EMPOWaR, however, used a body mass index (BMI) cutoff of 30 kg/m2 and a 2.5-g dose of metformin, compared with a BMI cutoff of 35 and the 3.0-g dose used in the MOP (Metformin in Obese Non-diabetic Pregnant Women) trial, Dr. Syngelaki observed.
Adherence to metformin was also higher in the MOP trial. Nearly 66% of women took a minimum metformin dose of 2.5 g for at least 50% of the days between randomization and delivery, compared with 2.5 g of metformin being used for only 38% of the days in the same period in EMPOWaR. The proportion of patients who were in this dose subgroup in the EMPOWaR trial was not specified.
MOP investigators randomly assigned 400 women with a singleton pregnancy to metformin or placebo from 12-18 weeks of gestation until delivery. All women underwent a 75-g oral glucose tolerance test at 28 weeks’ gestation, with metformin and placebo stopped for 1 week before the test. Women with abnormal oral glucose tolerance test results continued the assigned study regimen, and began home glucose monitoring. Insulin was added to their regimen, if target blood-glucose values were not achieved.
The metformin and placebo groups were well matched at baseline with respect to median maternal age (32.9 years vs. 30.8 years), median BMI (38.6 kg/m2 vs. 38.4 kg/m2), and spontaneous conception (97.5% vs. 98.0%).
Metformin did not reduce the primary outcome of median neonatal birth-weight z score, compared with placebo (0.05 vs. 0.17; P = .66) or the incidence of large for gestational age neonates (16.8% vs. 15.4%; P = .79), the MOP investigators reported.
There were no significant differences between the metformin and placebo groups in rates of miscarriage (zero vs. three cases), stillbirths (one case vs. two cases), or neonatal death (zero vs. one case).
The median maternal gestational weight gain, however, was lower with metformin than placebo (4.6 kg vs 6.3 kg; P less than .001), as was the incidence of preeclampsia.
The authors acknowledged that a limitation of the study was that it was not adequately powered for the secondary outcomes, but said the preeclampsia finding is compatible with several previous studies reporting that the prevalence of this potentially deadly complication increased with increasing prepregnancy BMI and gestational weight gain.
Side effects such as nausea, vomiting, and headache were as expected during gestation, although the incidence was significantly higher in the metformin group. Still, there was no significant between-group differences with regard to the decision to continue with the full dose, reduce the dose, or stop the study regimen.
“The rate of adherence was considerably higher among women taking the full dose of 3.0 g per day than among those taking less than 2.0 g per day, which suggests that adherence was not driven by the presence or absence of side effects, but by the motivation of the patients to adhere to the demands of the study,” the investigators wrote.
The Fetal Medicine Foundation funded the study. The investigators reported having no financial disclosures.
In obese pregnant women without diabetes, daily metformin reduces maternal weight gain, but not infant birth weight, results of the MOP trial show.
The incidence of preeclampsia was also lower with metformin than with placebo (3.0% vs. 11.3%; odds ratio, 0.24; P =.001).
There was no significant difference between the two groups in the incidence of other pregnancy complications or of adverse fetal or neonatal outcomes, Argyro Syngelaki, Ph.D., of King’s College Hospital in London, and her colleagues reported (N Engl J Med. 2016 Feb 3;374:434-43.).
Attempts at reducing the incidence of pregnancy complications associated with obesity have focused on dietary and lifestyle interventions but have generally been unsuccessful, she noted.
Additionally, the recent randomized EMPOWaR (Effect of Metformin on Maternal and Fetal Outcomes) trial failed to show that metformin use was associated with less gestational weight gain and a lower incidence of preeclampsia than did placebo.
EMPOWaR, however, used a body mass index (BMI) cutoff of 30 kg/m2 and a 2.5-g dose of metformin, compared with a BMI cutoff of 35 and the 3.0-g dose used in the MOP (Metformin in Obese Non-diabetic Pregnant Women) trial, Dr. Syngelaki observed.
Adherence to metformin was also higher in the MOP trial. Nearly 66% of women took a minimum metformin dose of 2.5 g for at least 50% of the days between randomization and delivery, compared with 2.5 g of metformin being used for only 38% of the days in the same period in EMPOWaR. The proportion of patients who were in this dose subgroup in the EMPOWaR trial was not specified.
MOP investigators randomly assigned 400 women with a singleton pregnancy to metformin or placebo from 12-18 weeks of gestation until delivery. All women underwent a 75-g oral glucose tolerance test at 28 weeks’ gestation, with metformin and placebo stopped for 1 week before the test. Women with abnormal oral glucose tolerance test results continued the assigned study regimen, and began home glucose monitoring. Insulin was added to their regimen, if target blood-glucose values were not achieved.
The metformin and placebo groups were well matched at baseline with respect to median maternal age (32.9 years vs. 30.8 years), median BMI (38.6 kg/m2 vs. 38.4 kg/m2), and spontaneous conception (97.5% vs. 98.0%).
Metformin did not reduce the primary outcome of median neonatal birth-weight z score, compared with placebo (0.05 vs. 0.17; P = .66) or the incidence of large for gestational age neonates (16.8% vs. 15.4%; P = .79), the MOP investigators reported.
There were no significant differences between the metformin and placebo groups in rates of miscarriage (zero vs. three cases), stillbirths (one case vs. two cases), or neonatal death (zero vs. one case).
The median maternal gestational weight gain, however, was lower with metformin than placebo (4.6 kg vs 6.3 kg; P less than .001), as was the incidence of preeclampsia.
The authors acknowledged that a limitation of the study was that it was not adequately powered for the secondary outcomes, but said the preeclampsia finding is compatible with several previous studies reporting that the prevalence of this potentially deadly complication increased with increasing prepregnancy BMI and gestational weight gain.
Side effects such as nausea, vomiting, and headache were as expected during gestation, although the incidence was significantly higher in the metformin group. Still, there was no significant between-group differences with regard to the decision to continue with the full dose, reduce the dose, or stop the study regimen.
“The rate of adherence was considerably higher among women taking the full dose of 3.0 g per day than among those taking less than 2.0 g per day, which suggests that adherence was not driven by the presence or absence of side effects, but by the motivation of the patients to adhere to the demands of the study,” the investigators wrote.
The Fetal Medicine Foundation funded the study. The investigators reported having no financial disclosures.
FROM NEW ENGLAND JOURNAL OF MEDICINE
Key clinical point: Antenatal metformin use reduces maternal weight gain, but not neonatal birth weight.
Major finding: The median maternal gestational weight gain was 4.6 kg with metformin and 6.3 kg with placebo (P less than .001).
Data source: Double-blind, randomized trial in 400 obese pregnant women without diabetes.
Disclosures: The Fetal Medicine Foundation funded the study. The investigators reported having no financial disclosures.
Office visits still common before colonoscopy
Nearly 30% of outpatient colonoscopies for colon cancer screening or polyp surveillance were preceded by a gastroenterology office visit, with that number reaching a staggering 50.5% in the South, according to an analysis of 842,849 middle-aged adults.
Patients with a higher Charlson Comorbidity Index (CCI) also were more likely to have an office visit. Still, 66.4% of patients with an office visit had a CCI of 0.
“Although the precolonoscopy office visits added a modest $36 per colonoscopy in this population, there are an estimated 7 million screening colonoscopies performed in the United States annually, so the cumulative costs are significant,” reported Dr. Kevin Riggs of Johns Hopkins University, Baltimore, and associates reported (JAMA. 2016 Feb 2. doi:10.1001/jama2015.15278).
The cost of precolonoscopy office visits has received little attention despite scrutiny of the high cost of colonoscopy in the United States and the availability of open-access colonoscopy since the 1990s, the authors noted.
The primary limitation of the study was the inability to determine the exact circumstances of the office visits. Patients with a diagnosis of colon cancer or inflammatory bowel disease in the prior 12 months were excluded from the analysis.
The study was funded by the National Institutes of Health and the National Cancer Institute. Dr. Riggs’ salary is supported by an NIH grant. Coauthor and colleague Dr. Craig Pollack reported stock ownership in the Advisory Board Company and that his salary is supported by a grant from the NCI.
Nearly 30% of outpatient colonoscopies for colon cancer screening or polyp surveillance were preceded by a gastroenterology office visit, with that number reaching a staggering 50.5% in the South, according to an analysis of 842,849 middle-aged adults.
Patients with a higher Charlson Comorbidity Index (CCI) also were more likely to have an office visit. Still, 66.4% of patients with an office visit had a CCI of 0.
“Although the precolonoscopy office visits added a modest $36 per colonoscopy in this population, there are an estimated 7 million screening colonoscopies performed in the United States annually, so the cumulative costs are significant,” reported Dr. Kevin Riggs of Johns Hopkins University, Baltimore, and associates reported (JAMA. 2016 Feb 2. doi:10.1001/jama2015.15278).
The cost of precolonoscopy office visits has received little attention despite scrutiny of the high cost of colonoscopy in the United States and the availability of open-access colonoscopy since the 1990s, the authors noted.
The primary limitation of the study was the inability to determine the exact circumstances of the office visits. Patients with a diagnosis of colon cancer or inflammatory bowel disease in the prior 12 months were excluded from the analysis.
The study was funded by the National Institutes of Health and the National Cancer Institute. Dr. Riggs’ salary is supported by an NIH grant. Coauthor and colleague Dr. Craig Pollack reported stock ownership in the Advisory Board Company and that his salary is supported by a grant from the NCI.
Nearly 30% of outpatient colonoscopies for colon cancer screening or polyp surveillance were preceded by a gastroenterology office visit, with that number reaching a staggering 50.5% in the South, according to an analysis of 842,849 middle-aged adults.
Patients with a higher Charlson Comorbidity Index (CCI) also were more likely to have an office visit. Still, 66.4% of patients with an office visit had a CCI of 0.
“Although the precolonoscopy office visits added a modest $36 per colonoscopy in this population, there are an estimated 7 million screening colonoscopies performed in the United States annually, so the cumulative costs are significant,” reported Dr. Kevin Riggs of Johns Hopkins University, Baltimore, and associates reported (JAMA. 2016 Feb 2. doi:10.1001/jama2015.15278).
The cost of precolonoscopy office visits has received little attention despite scrutiny of the high cost of colonoscopy in the United States and the availability of open-access colonoscopy since the 1990s, the authors noted.
The primary limitation of the study was the inability to determine the exact circumstances of the office visits. Patients with a diagnosis of colon cancer or inflammatory bowel disease in the prior 12 months were excluded from the analysis.
The study was funded by the National Institutes of Health and the National Cancer Institute. Dr. Riggs’ salary is supported by an NIH grant. Coauthor and colleague Dr. Craig Pollack reported stock ownership in the Advisory Board Company and that his salary is supported by a grant from the NCI.
FROM JAMA
Minimal residual disease a powerful prognostic factor in AML
The presence of minimal residual disease predicts relapse in patients with NMP1-mutated acute myeloid leukemia and is superior to currently used molecular genetic markers in determining whether these patients should be considered for stem cell transplantation, a new study has found.
At 3 years, patients with minimal residual disease (MRD) had a significantly greater risk of relapse than those with no MRD (82% vs. 30%; univariate hazard ratio, 4.80; P less than .001) and a lower rate of survival (24% vs. 75%; univariate HR, 4.38; P less than .001), Adam Ivey of King’s College London reported (N Engl J Med. 2016; doi:10.1056/NEJMoa1507471).
In an editorial that accompanied the study Dr. Michael J. Burke from the Children’s Hospital of Wisconsin in Milwaukee wrote, “Time will tell, but this moment may prove to be a pivotal one in the assessment of minimal residual disease to assign treatment in patients with AML” (N Engl J Med. 2016; doi:10.1056/NEJMe1515525).
In adult AML, assessment of MRD has taken a back seat to analyses of cytogenetic and molecular lesions in determining a patient’s risk and treatment strategy. Typically, allogeneic stem cell transplantation is used for patients with high-risk features such as chromosome 3, 5, or 7 abnormalities or the FLT3-internal tandem duplication (ITD) mutation, while chemotherapy alone is used for low-risk disease.
The role of transplantation is unclear, however, for cytogenetically standard-risk patients, which includes those with a mutation in the gene encoding nucleophosmin (NPM1).
To address this issue, the investigators used a reverse-transcriptase quantitative polymerase chain reaction assay to evaluate 2,569 bone marrow and peripheral-blood samples from 346 patients with NPM1 mutations who had completed two cycles of induction chemotherapy in the U.K. National Cancer Research Institute AML17 trial.
MRD, defined as persistence of NPM1-mutated transcripts in peripheral blood, was present in 15% of patients after the second chemotherapy cycle.
Patients with MRD were significantly more likely than those without MRD to have a high U.K. Medical Research Council clinical risk score and to carry the FLT3-ITD mutation.
On univariate analysis, the risk of relapse was significantly higher with the presence of MRD in peripheral blood, an increased white cell count, and with the DNMT3A and FLT3-ITD mutations.
Only the presence of MRD and an elevated white cell count significantly predicted survival, Mr. Ivey reported.
“We could find no specific molecular subgroup consisting of 10 patients or more that had a rate of survival less than 52%; in contrast, the rate in the group with the presence of minimal residual disease was 24%,” he observed.
In multivariate analysis, the presence of MRD was the only significant prognostic factor for relapse (HR, 5.09; P less than .001) or death (HR, 4.84; P less than .001).
The results were validated in an independent cohort of 91 AML17 study patients. It confirmed that MRD in peripheral blood predicts worse outcome at 2 years than the absence of MRD, with a cumulative incidence of relapse of 70% vs. 31% (P = .001) and overall survival rates of 40% vs. 87% (P = .001), reported the investigators, including senior author Professor David Grimwade, also from King’s College London.
The clinical implications of these results “are substantive” because NPM-1 mutated AML is the most common subtype of AML and because of the uncertainty over the best treatment strategy for patients typically classified as standard risk, editorialist Dr. Burke observed.
“Now with the ability to reclassify standard-risk or low-risk patients as high-risk on the basis of the persistent expression of mutant NPM1 transcripts, it may be possible that stem-cell transplantation is a better approach in patients who otherwise would be treated with chemotherapy alone and that transplantation may be avoidable in high-risk patients who have no evidence of minimal residual disease,” he wrote. “Such predictions will need to be tested prospectively.”
The presence of MRD is also known to be an important independent prognostic factor in acute lymphoblastic leukemia, but since AML has a greater molecular heterogeneity, routine MRD assessment has not been as quickly adopted in AML, Dr. Burke noted.
The Children’s Oncology Group, however, recently adopted MRD assessment by flow cytometry to further stratify children with newly diagnosed AML after first induction therapy into low-risk or high-risk groups.
The study was supported by grants from Bloodwise and the National Institute for Health Research. Mr. Ivey and Dr. Burke reported having no disclosures.
The presence of minimal residual disease predicts relapse in patients with NMP1-mutated acute myeloid leukemia and is superior to currently used molecular genetic markers in determining whether these patients should be considered for stem cell transplantation, a new study has found.
At 3 years, patients with minimal residual disease (MRD) had a significantly greater risk of relapse than those with no MRD (82% vs. 30%; univariate hazard ratio, 4.80; P less than .001) and a lower rate of survival (24% vs. 75%; univariate HR, 4.38; P less than .001), Adam Ivey of King’s College London reported (N Engl J Med. 2016; doi:10.1056/NEJMoa1507471).
In an editorial that accompanied the study Dr. Michael J. Burke from the Children’s Hospital of Wisconsin in Milwaukee wrote, “Time will tell, but this moment may prove to be a pivotal one in the assessment of minimal residual disease to assign treatment in patients with AML” (N Engl J Med. 2016; doi:10.1056/NEJMe1515525).
In adult AML, assessment of MRD has taken a back seat to analyses of cytogenetic and molecular lesions in determining a patient’s risk and treatment strategy. Typically, allogeneic stem cell transplantation is used for patients with high-risk features such as chromosome 3, 5, or 7 abnormalities or the FLT3-internal tandem duplication (ITD) mutation, while chemotherapy alone is used for low-risk disease.
The role of transplantation is unclear, however, for cytogenetically standard-risk patients, which includes those with a mutation in the gene encoding nucleophosmin (NPM1).
To address this issue, the investigators used a reverse-transcriptase quantitative polymerase chain reaction assay to evaluate 2,569 bone marrow and peripheral-blood samples from 346 patients with NPM1 mutations who had completed two cycles of induction chemotherapy in the U.K. National Cancer Research Institute AML17 trial.
MRD, defined as persistence of NPM1-mutated transcripts in peripheral blood, was present in 15% of patients after the second chemotherapy cycle.
Patients with MRD were significantly more likely than those without MRD to have a high U.K. Medical Research Council clinical risk score and to carry the FLT3-ITD mutation.
On univariate analysis, the risk of relapse was significantly higher with the presence of MRD in peripheral blood, an increased white cell count, and with the DNMT3A and FLT3-ITD mutations.
Only the presence of MRD and an elevated white cell count significantly predicted survival, Mr. Ivey reported.
“We could find no specific molecular subgroup consisting of 10 patients or more that had a rate of survival less than 52%; in contrast, the rate in the group with the presence of minimal residual disease was 24%,” he observed.
In multivariate analysis, the presence of MRD was the only significant prognostic factor for relapse (HR, 5.09; P less than .001) or death (HR, 4.84; P less than .001).
The results were validated in an independent cohort of 91 AML17 study patients. It confirmed that MRD in peripheral blood predicts worse outcome at 2 years than the absence of MRD, with a cumulative incidence of relapse of 70% vs. 31% (P = .001) and overall survival rates of 40% vs. 87% (P = .001), reported the investigators, including senior author Professor David Grimwade, also from King’s College London.
The clinical implications of these results “are substantive” because NPM-1 mutated AML is the most common subtype of AML and because of the uncertainty over the best treatment strategy for patients typically classified as standard risk, editorialist Dr. Burke observed.
“Now with the ability to reclassify standard-risk or low-risk patients as high-risk on the basis of the persistent expression of mutant NPM1 transcripts, it may be possible that stem-cell transplantation is a better approach in patients who otherwise would be treated with chemotherapy alone and that transplantation may be avoidable in high-risk patients who have no evidence of minimal residual disease,” he wrote. “Such predictions will need to be tested prospectively.”
The presence of MRD is also known to be an important independent prognostic factor in acute lymphoblastic leukemia, but since AML has a greater molecular heterogeneity, routine MRD assessment has not been as quickly adopted in AML, Dr. Burke noted.
The Children’s Oncology Group, however, recently adopted MRD assessment by flow cytometry to further stratify children with newly diagnosed AML after first induction therapy into low-risk or high-risk groups.
The study was supported by grants from Bloodwise and the National Institute for Health Research. Mr. Ivey and Dr. Burke reported having no disclosures.
The presence of minimal residual disease predicts relapse in patients with NMP1-mutated acute myeloid leukemia and is superior to currently used molecular genetic markers in determining whether these patients should be considered for stem cell transplantation, a new study has found.
At 3 years, patients with minimal residual disease (MRD) had a significantly greater risk of relapse than those with no MRD (82% vs. 30%; univariate hazard ratio, 4.80; P less than .001) and a lower rate of survival (24% vs. 75%; univariate HR, 4.38; P less than .001), Adam Ivey of King’s College London reported (N Engl J Med. 2016; doi:10.1056/NEJMoa1507471).
In an editorial that accompanied the study Dr. Michael J. Burke from the Children’s Hospital of Wisconsin in Milwaukee wrote, “Time will tell, but this moment may prove to be a pivotal one in the assessment of minimal residual disease to assign treatment in patients with AML” (N Engl J Med. 2016; doi:10.1056/NEJMe1515525).
In adult AML, assessment of MRD has taken a back seat to analyses of cytogenetic and molecular lesions in determining a patient’s risk and treatment strategy. Typically, allogeneic stem cell transplantation is used for patients with high-risk features such as chromosome 3, 5, or 7 abnormalities or the FLT3-internal tandem duplication (ITD) mutation, while chemotherapy alone is used for low-risk disease.
The role of transplantation is unclear, however, for cytogenetically standard-risk patients, which includes those with a mutation in the gene encoding nucleophosmin (NPM1).
To address this issue, the investigators used a reverse-transcriptase quantitative polymerase chain reaction assay to evaluate 2,569 bone marrow and peripheral-blood samples from 346 patients with NPM1 mutations who had completed two cycles of induction chemotherapy in the U.K. National Cancer Research Institute AML17 trial.
MRD, defined as persistence of NPM1-mutated transcripts in peripheral blood, was present in 15% of patients after the second chemotherapy cycle.
Patients with MRD were significantly more likely than those without MRD to have a high U.K. Medical Research Council clinical risk score and to carry the FLT3-ITD mutation.
On univariate analysis, the risk of relapse was significantly higher with the presence of MRD in peripheral blood, an increased white cell count, and with the DNMT3A and FLT3-ITD mutations.
Only the presence of MRD and an elevated white cell count significantly predicted survival, Mr. Ivey reported.
“We could find no specific molecular subgroup consisting of 10 patients or more that had a rate of survival less than 52%; in contrast, the rate in the group with the presence of minimal residual disease was 24%,” he observed.
In multivariate analysis, the presence of MRD was the only significant prognostic factor for relapse (HR, 5.09; P less than .001) or death (HR, 4.84; P less than .001).
The results were validated in an independent cohort of 91 AML17 study patients. It confirmed that MRD in peripheral blood predicts worse outcome at 2 years than the absence of MRD, with a cumulative incidence of relapse of 70% vs. 31% (P = .001) and overall survival rates of 40% vs. 87% (P = .001), reported the investigators, including senior author Professor David Grimwade, also from King’s College London.
The clinical implications of these results “are substantive” because NPM-1 mutated AML is the most common subtype of AML and because of the uncertainty over the best treatment strategy for patients typically classified as standard risk, editorialist Dr. Burke observed.
“Now with the ability to reclassify standard-risk or low-risk patients as high-risk on the basis of the persistent expression of mutant NPM1 transcripts, it may be possible that stem-cell transplantation is a better approach in patients who otherwise would be treated with chemotherapy alone and that transplantation may be avoidable in high-risk patients who have no evidence of minimal residual disease,” he wrote. “Such predictions will need to be tested prospectively.”
The presence of MRD is also known to be an important independent prognostic factor in acute lymphoblastic leukemia, but since AML has a greater molecular heterogeneity, routine MRD assessment has not been as quickly adopted in AML, Dr. Burke noted.
The Children’s Oncology Group, however, recently adopted MRD assessment by flow cytometry to further stratify children with newly diagnosed AML after first induction therapy into low-risk or high-risk groups.
The study was supported by grants from Bloodwise and the National Institute for Health Research. Mr. Ivey and Dr. Burke reported having no disclosures.
FROM THE NEW ENGLAND JOURNAL OF MEDICINE
Key clinical point: The presence of minimal residual disease provides powerful prognostic information independent of other risk factors in patients with NPM1-mutated AML.
Major finding: Minimal residual disease was associated with a significantly greater risk of relapse than absence of MRD (82% vs. 30%; hazard ratio, 4.80; P less than .001) and a lower rate of survival (24% vs. 75%; HR, 4.38, P less than .001).
Data source: Analysis of 346 patients with NPM1-mutated AML.
Disclosures: The study was supported by grants from Bloodwise and the National Institute for Health Research. Mr. Ivey and Dr. Burke reported having no disclosures.
Research refining radiomic features for lung cancer screening
SAN DIEGO – A series of radiomics-derived imaging features may improve the diagnostic accuracy of low-dose CT lung cancer screening and help predict which nodules are at risk of becoming cancers.
“We are providing pretty compelling evidence that there is some utility in this science,” Matthew Schabath, Ph.D., said at a conference on lung cancer translational science sponsored by the American Association for Cancer Research and the International Association for the Study of Lung Cancer.
Radiomics is an emerging field that uses high-throughput extraction to identify hundreds of quantitative features from standard computed tomography (CT) images and mines that data to develop diagnostic, predictive, or prognostic models.
Radiologists first identify a region of interest (ROI) on the CT scan containing either the whole tumor or spatially explicit regions of the tumor called “habitats.” These ROIs are then segmented via computer software before being rendered in three dimensions. Quantitative features are extracted from the rendered volumes and entered into the models, along with other clinical and patient data.
“Right now our tool box is about 219, but by the end of the year we are hoping to have close to 1,000 radiomic features we can extract from a 3-D rendered nodule or tumor,” said Dr. Schabath, of the Moffitt Cancer Center in Tampa, Fla.
Although not without its own challenges, radiomics is a far cry from the current practice that relies on a single CT feature, nodule size, and clinical guidelines to evaluate and follow-up pulmonary nodules, none of which provides clinicians tools to accurately predict the risk or probability of lung cancer development.
CT images are typically thought of as pictures, but in radiomics, “the images are data. That’s really the underlying principle,” he said.
Led by Dr. Robert Gillies, often referred to as the father of radiomics, the researchers extracted and analyzed the 219 radiomic features from nodules in 196 lung cancer cases and in 392 controls who had a positive but benign nodule at the baseline scan and were matched for age, sex, smoking status, and race.
The post hoc, nested case-control study used images and data from the pivotal National Lung Screening Trial, which identified a 20% reduction in lung cancer mortality for low-dose CT screening compared with chest x-rays, but with a 96% false-positive rate, which also highlighted the challenges of LDCT as a screening tool.
Two classes of features were extracted from the images: semantic features, which are commonly used in radiology to describe ROIs, and agnostic features, which are mathematically extracted quantitative descriptors that capture lesion heterogeneity.
Univariable analyses were used to identify statistically significant features (threshold P value less than .05) and a backward elimination process (threshold P less than 0.1) performed to generate the final set of features, Dr. Schabath said.
Separate analyses were performed for predictive and diagnostic features.
In the risk prediction model, eight “highly informative features” were identified, Dr. Schabath said. Five were agnostic and three were semantic – circularity of the nodule, volume, and distance from or pleural attachment.
The receiver operating characteristic (ROC) area under the curve for the model was 0.92, with 75% sensitivity and 89% specificity. When the model included only patient demographics, it was no better than flipping a coin for predicting nodules at risk of becoming cancerous (ROC 0.58), he said.
Six highly informative features were identified in the agnostic model, which extracted features from the nodules found at the first and second follow-up interval, Dr. Schabath said. Three were agnostic and three semantic – longest diameter, volume, and distance from or pleural attachment.
The ROC for the diagnostic model was 0.89, with 74% sensitivity and 89% specificity.
When an additional analysis was performed using a nodule threshold of less than 15 mm to account for nodule growth over time and smaller nodule size at baseline in controls, the ROC and specificity held steady, but sensitivity dropped off to 59%, he said.
“I think we’re showing a rigorous [statistical] approach by identifying really unique, highly informative features,” Dr. Schabath concluded.
The overlap of volume and distance from or pleural attachment in both the diagnostic and predictive models suggests “there might be something very important about these two features,” he added.
Dr. Schabath stressed that the findings are preliminary and said additional analyses will be run before the results are ready for prime time. Long-term goals are to implement radiomic-based decision support tools and models into radiology reading rooms.
“In the future, we envision that all medical images will be converted to mineable data with the process of radiomics as part of standard of care,” Dr. Gillies said in an interview. “Such data have already shown promise to increase the precision and accuracy of diagnostic images, and hence, will increasingly be used in therapy decision support.”
Among the many challenges that first need to be resolved are that images are often captured with settings and filters that can be different even within a single institution. The inconsistency adds noise to the data that are extracted by computers.
“Hence, the most robust data we have today are generated by radiologists themselves, although this has its own challenges of being time-consuming with inter-reader variability,” Dr. Gillies noted.
Another major challenge is sharing of the image data. Right now, radiomics is practiced at only a few research hospitals and thus, building large cohort studies requires that the images be moved across site. In the future, the researchers anticipate that software can be deployed across sites to enable radiomic feature extraction, which would mean that only the extracted data will have to be shared, he said.
SAN DIEGO – A series of radiomics-derived imaging features may improve the diagnostic accuracy of low-dose CT lung cancer screening and help predict which nodules are at risk of becoming cancers.
“We are providing pretty compelling evidence that there is some utility in this science,” Matthew Schabath, Ph.D., said at a conference on lung cancer translational science sponsored by the American Association for Cancer Research and the International Association for the Study of Lung Cancer.
Radiomics is an emerging field that uses high-throughput extraction to identify hundreds of quantitative features from standard computed tomography (CT) images and mines that data to develop diagnostic, predictive, or prognostic models.
Radiologists first identify a region of interest (ROI) on the CT scan containing either the whole tumor or spatially explicit regions of the tumor called “habitats.” These ROIs are then segmented via computer software before being rendered in three dimensions. Quantitative features are extracted from the rendered volumes and entered into the models, along with other clinical and patient data.
“Right now our tool box is about 219, but by the end of the year we are hoping to have close to 1,000 radiomic features we can extract from a 3-D rendered nodule or tumor,” said Dr. Schabath, of the Moffitt Cancer Center in Tampa, Fla.
Although not without its own challenges, radiomics is a far cry from the current practice that relies on a single CT feature, nodule size, and clinical guidelines to evaluate and follow-up pulmonary nodules, none of which provides clinicians tools to accurately predict the risk or probability of lung cancer development.
CT images are typically thought of as pictures, but in radiomics, “the images are data. That’s really the underlying principle,” he said.
Led by Dr. Robert Gillies, often referred to as the father of radiomics, the researchers extracted and analyzed the 219 radiomic features from nodules in 196 lung cancer cases and in 392 controls who had a positive but benign nodule at the baseline scan and were matched for age, sex, smoking status, and race.
The post hoc, nested case-control study used images and data from the pivotal National Lung Screening Trial, which identified a 20% reduction in lung cancer mortality for low-dose CT screening compared with chest x-rays, but with a 96% false-positive rate, which also highlighted the challenges of LDCT as a screening tool.
Two classes of features were extracted from the images: semantic features, which are commonly used in radiology to describe ROIs, and agnostic features, which are mathematically extracted quantitative descriptors that capture lesion heterogeneity.
Univariable analyses were used to identify statistically significant features (threshold P value less than .05) and a backward elimination process (threshold P less than 0.1) performed to generate the final set of features, Dr. Schabath said.
Separate analyses were performed for predictive and diagnostic features.
In the risk prediction model, eight “highly informative features” were identified, Dr. Schabath said. Five were agnostic and three were semantic – circularity of the nodule, volume, and distance from or pleural attachment.
The receiver operating characteristic (ROC) area under the curve for the model was 0.92, with 75% sensitivity and 89% specificity. When the model included only patient demographics, it was no better than flipping a coin for predicting nodules at risk of becoming cancerous (ROC 0.58), he said.
Six highly informative features were identified in the agnostic model, which extracted features from the nodules found at the first and second follow-up interval, Dr. Schabath said. Three were agnostic and three semantic – longest diameter, volume, and distance from or pleural attachment.
The ROC for the diagnostic model was 0.89, with 74% sensitivity and 89% specificity.
When an additional analysis was performed using a nodule threshold of less than 15 mm to account for nodule growth over time and smaller nodule size at baseline in controls, the ROC and specificity held steady, but sensitivity dropped off to 59%, he said.
“I think we’re showing a rigorous [statistical] approach by identifying really unique, highly informative features,” Dr. Schabath concluded.
The overlap of volume and distance from or pleural attachment in both the diagnostic and predictive models suggests “there might be something very important about these two features,” he added.
Dr. Schabath stressed that the findings are preliminary and said additional analyses will be run before the results are ready for prime time. Long-term goals are to implement radiomic-based decision support tools and models into radiology reading rooms.
“In the future, we envision that all medical images will be converted to mineable data with the process of radiomics as part of standard of care,” Dr. Gillies said in an interview. “Such data have already shown promise to increase the precision and accuracy of diagnostic images, and hence, will increasingly be used in therapy decision support.”
Among the many challenges that first need to be resolved are that images are often captured with settings and filters that can be different even within a single institution. The inconsistency adds noise to the data that are extracted by computers.
“Hence, the most robust data we have today are generated by radiologists themselves, although this has its own challenges of being time-consuming with inter-reader variability,” Dr. Gillies noted.
Another major challenge is sharing of the image data. Right now, radiomics is practiced at only a few research hospitals and thus, building large cohort studies requires that the images be moved across site. In the future, the researchers anticipate that software can be deployed across sites to enable radiomic feature extraction, which would mean that only the extracted data will have to be shared, he said.
SAN DIEGO – A series of radiomics-derived imaging features may improve the diagnostic accuracy of low-dose CT lung cancer screening and help predict which nodules are at risk of becoming cancers.
“We are providing pretty compelling evidence that there is some utility in this science,” Matthew Schabath, Ph.D., said at a conference on lung cancer translational science sponsored by the American Association for Cancer Research and the International Association for the Study of Lung Cancer.
Radiomics is an emerging field that uses high-throughput extraction to identify hundreds of quantitative features from standard computed tomography (CT) images and mines that data to develop diagnostic, predictive, or prognostic models.
Radiologists first identify a region of interest (ROI) on the CT scan containing either the whole tumor or spatially explicit regions of the tumor called “habitats.” These ROIs are then segmented via computer software before being rendered in three dimensions. Quantitative features are extracted from the rendered volumes and entered into the models, along with other clinical and patient data.
“Right now our tool box is about 219, but by the end of the year we are hoping to have close to 1,000 radiomic features we can extract from a 3-D rendered nodule or tumor,” said Dr. Schabath, of the Moffitt Cancer Center in Tampa, Fla.
Although not without its own challenges, radiomics is a far cry from the current practice that relies on a single CT feature, nodule size, and clinical guidelines to evaluate and follow-up pulmonary nodules, none of which provides clinicians tools to accurately predict the risk or probability of lung cancer development.
CT images are typically thought of as pictures, but in radiomics, “the images are data. That’s really the underlying principle,” he said.
Led by Dr. Robert Gillies, often referred to as the father of radiomics, the researchers extracted and analyzed the 219 radiomic features from nodules in 196 lung cancer cases and in 392 controls who had a positive but benign nodule at the baseline scan and were matched for age, sex, smoking status, and race.
The post hoc, nested case-control study used images and data from the pivotal National Lung Screening Trial, which identified a 20% reduction in lung cancer mortality for low-dose CT screening compared with chest x-rays, but with a 96% false-positive rate, which also highlighted the challenges of LDCT as a screening tool.
Two classes of features were extracted from the images: semantic features, which are commonly used in radiology to describe ROIs, and agnostic features, which are mathematically extracted quantitative descriptors that capture lesion heterogeneity.
Univariable analyses were used to identify statistically significant features (threshold P value less than .05) and a backward elimination process (threshold P less than 0.1) performed to generate the final set of features, Dr. Schabath said.
Separate analyses were performed for predictive and diagnostic features.
In the risk prediction model, eight “highly informative features” were identified, Dr. Schabath said. Five were agnostic and three were semantic – circularity of the nodule, volume, and distance from or pleural attachment.
The receiver operating characteristic (ROC) area under the curve for the model was 0.92, with 75% sensitivity and 89% specificity. When the model included only patient demographics, it was no better than flipping a coin for predicting nodules at risk of becoming cancerous (ROC 0.58), he said.
Six highly informative features were identified in the agnostic model, which extracted features from the nodules found at the first and second follow-up interval, Dr. Schabath said. Three were agnostic and three semantic – longest diameter, volume, and distance from or pleural attachment.
The ROC for the diagnostic model was 0.89, with 74% sensitivity and 89% specificity.
When an additional analysis was performed using a nodule threshold of less than 15 mm to account for nodule growth over time and smaller nodule size at baseline in controls, the ROC and specificity held steady, but sensitivity dropped off to 59%, he said.
“I think we’re showing a rigorous [statistical] approach by identifying really unique, highly informative features,” Dr. Schabath concluded.
The overlap of volume and distance from or pleural attachment in both the diagnostic and predictive models suggests “there might be something very important about these two features,” he added.
Dr. Schabath stressed that the findings are preliminary and said additional analyses will be run before the results are ready for prime time. Long-term goals are to implement radiomic-based decision support tools and models into radiology reading rooms.
“In the future, we envision that all medical images will be converted to mineable data with the process of radiomics as part of standard of care,” Dr. Gillies said in an interview. “Such data have already shown promise to increase the precision and accuracy of diagnostic images, and hence, will increasingly be used in therapy decision support.”
Among the many challenges that first need to be resolved are that images are often captured with settings and filters that can be different even within a single institution. The inconsistency adds noise to the data that are extracted by computers.
“Hence, the most robust data we have today are generated by radiologists themselves, although this has its own challenges of being time-consuming with inter-reader variability,” Dr. Gillies noted.
Another major challenge is sharing of the image data. Right now, radiomics is practiced at only a few research hospitals and thus, building large cohort studies requires that the images be moved across site. In the future, the researchers anticipate that software can be deployed across sites to enable radiomic feature extraction, which would mean that only the extracted data will have to be shared, he said.
AT AN AACR/IASLC JOINT CONFERENCE
Key clinical point: Non-invasive radiomics is showing potential for reducing false positives by differentiating between benign and cancerous lung nodules and quantitatively predicting future risk of lung cancer incidence.
Major finding: The receiver operating characteristic area under the curve for the risk prediction model was 0.92, with 75% sensitivity and 89% specificity.
Data source: Post hoc case-control analysis in 588 persons at high risk for lung cancer in the National Lung Screening Trial.
Disclosures: Dr. Schabath reported having no relevant conflicts of interest. Dr. Gillies reported serving as a speaker for HealthMyne.
Deciphering lung cancer biomarkers shrouded in tobacco smoke
SAN DIEGO – Tobacco researchers are getting one step closer to identifying young smokers particularly susceptible to the carcinogenic effects of smoking, by using large-scale epidemiology studies and DNA adducts data.
“This is not lung cancer screening; this is not early detection of lung cancer; this is detection of susceptible, high-risk individuals, so they can be targeted for cessation, surveillance, and prevention,” said Stephen S. Hecht, Ph.D., professor of cancer prevention at the University of Minnesota Masonic Cancer Center in Minneapolis.
While nicotine itself is not a carcinogen, each puff of tobacco smoke delivers a mixture of more than 70 established carcinogens, including the potent lung-specific carcinogen 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK).
Some of these carcinogens are excreted, but many will undergo metabolic activation and interact with a patient’s DNA.
The product of this interaction, called DNA adducts, causes persistent miscoding during DNA replication, leading to the thousands of mutations we see in lung cancer, he explained at a conference on lung cancer translational science sponsored by the American Association for Cancer Research and the International Association for the Study of Lung Cancer.
The biomarkers of tobacco exposure and metabolism are very well developed, with blood and urinary biomarker panels applied in many studies and validated by mass spectrometry.
Using the Shanghai cohort study, the researchers identified three urinary biomarkers – PheT (r-1,t-2,3,c-4-tetrahydroxy-1,2,3,4-tetrahydrophenanthrene), total NNAL (a metabolite of NNK and its glucuronides), and total cotinine (a surrogate for nicotine that includes cotinine and its glucuronides) – that were significantly associated with lung cancer in 950 smokers, even after adjustment for smoking duration and intensity (Cancer Res. 2011;71:6749-57).
A more recent, deeper dive of 735 of those urinary samples, still stable about 20 years after they were taken, looked specifically at polymorphisms in cytochrome P450 2A6 (CYP2A6), the primary enzyme responsible for the oxidation of nicotine and cotinine.
Data currently in press show that CYP2A6 poor metabolizers had a lower risk of lung cancer than the combined group of normal, intermediate, or slow metabolizers (odds ratio, 0.64; P value for trend = .034).
“This is logical because poor metabolizers of nicotine have more unchanged nicotine on board, so they don’t need to smoke as intensely in order to get more nicotine because that nicotine is remaining to a greater extent in its natural form, rather than being metabolized into cotinine, which is not addictive,” Dr. Hecht said.
Recent genomewide-association studies by Dr. Hecht and his colleagues of smokers in the Multiethnic Cohort Study revealed that Japanese Americans had the highest number of low nicotine CYP2A6 metabolizing genotypes of the five ethnic groups analyzed, consistent with their low lung cancer risk.
Urinary biomarker studies showed that levels of total NNAL, 3-hydroxy phenanthrene, a biomarker of polycyclic aromatic hydrocarbon uptake, and S-phenylmercapturic acid (SPMA), a biomarker of benzene exposure, were also significantly higher among African Americans than whites and significantly lower in Japanese Americans than whites, he said.
The findings are consistent with the initial study results published a decade ago (N Engl J Med. 2006;354:333-342), showing that at low levels of smoking (10 cigarettes per day), Japanese Americans and Hispanics had one-third the risk of lung cancer of African Americans or Native Hawaiians (P less than .001). The differences disappeared at higher levels of smoking (30 cigarettes per day).
Results from the new analyses for Native Hawaiians and Hispanics, however, did not fit this pattern, and “we’re not sure what’s going on there and need to do more work,” Dr. Hecht said.
Nicotine equivalent levels in Native Hawaiians were actually lower than would be expected based on their high lung cancer risk, while Hispanics had higher levels than would be expected based on their low risk.
Interestingly, urinary levels of 3-hydroxypropylmercapturic acid (3-HPMA), a metabolite of acrolein, were found to be unusually high in Native Hawaiians and relatively low in Hispanics, “which may play into their lung cancer risk because acrolein is a very strong toxicant and there is evidence to suggest it is involved in lung cancer,” he said.
While tobacco exposure and metabolism biomarkers have hit their stride, less well developed are the DNA adducts and repair biomarkers that can predict lung cancer susceptibility. This is a critical step because of the role DNA adducts play in the formation of lung cancer mutations, Dr. Hecht said.
A smoker may have a high tobacco exposure as indicated by the urinary biomarkers, but have a low metabolism to form DNA adducts or high DNA repair, which would mean their DNA adduct levels would be low. Conversely, another smoker with low exposure and poor DNA repair may have higher DNA adduct levels, and thus, a greater risk for developing lung cancer.
In addition, the exposure biomarkers are not entirely predictive and thus, will need to be combined with multiple DNA adducts and repair if susceptible smokers are to be identified and targeted for state-of-the art cessation approaches and surveillance, he said.
Using high-level mass spectrometry, the researchers have been able to readily measure formaldehyde DNA adducts and tobacco-specific 4-hydroxy-1-(3-pyridyl)-1-butanone (HPB)–releasing DNA adducts in human oral cells. Levels of the HPB adduct were unusually high at 12-45 pmol/mg, which is similar to what is seen in animals when exposed to NNK at a much higher dose than smokers take in.
“So this is a real lead,” said Dr. Hecht, who reported having no relevant conflicts of interest.
SAN DIEGO – Tobacco researchers are getting one step closer to identifying young smokers particularly susceptible to the carcinogenic effects of smoking, by using large-scale epidemiology studies and DNA adducts data.
“This is not lung cancer screening; this is not early detection of lung cancer; this is detection of susceptible, high-risk individuals, so they can be targeted for cessation, surveillance, and prevention,” said Stephen S. Hecht, Ph.D., professor of cancer prevention at the University of Minnesota Masonic Cancer Center in Minneapolis.
While nicotine itself is not a carcinogen, each puff of tobacco smoke delivers a mixture of more than 70 established carcinogens, including the potent lung-specific carcinogen 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK).
Some of these carcinogens are excreted, but many will undergo metabolic activation and interact with a patient’s DNA.
The product of this interaction, called DNA adducts, causes persistent miscoding during DNA replication, leading to the thousands of mutations we see in lung cancer, he explained at a conference on lung cancer translational science sponsored by the American Association for Cancer Research and the International Association for the Study of Lung Cancer.
The biomarkers of tobacco exposure and metabolism are very well developed, with blood and urinary biomarker panels applied in many studies and validated by mass spectrometry.
Using the Shanghai cohort study, the researchers identified three urinary biomarkers – PheT (r-1,t-2,3,c-4-tetrahydroxy-1,2,3,4-tetrahydrophenanthrene), total NNAL (a metabolite of NNK and its glucuronides), and total cotinine (a surrogate for nicotine that includes cotinine and its glucuronides) – that were significantly associated with lung cancer in 950 smokers, even after adjustment for smoking duration and intensity (Cancer Res. 2011;71:6749-57).
A more recent, deeper dive of 735 of those urinary samples, still stable about 20 years after they were taken, looked specifically at polymorphisms in cytochrome P450 2A6 (CYP2A6), the primary enzyme responsible for the oxidation of nicotine and cotinine.
Data currently in press show that CYP2A6 poor metabolizers had a lower risk of lung cancer than the combined group of normal, intermediate, or slow metabolizers (odds ratio, 0.64; P value for trend = .034).
“This is logical because poor metabolizers of nicotine have more unchanged nicotine on board, so they don’t need to smoke as intensely in order to get more nicotine because that nicotine is remaining to a greater extent in its natural form, rather than being metabolized into cotinine, which is not addictive,” Dr. Hecht said.
Recent genomewide-association studies by Dr. Hecht and his colleagues of smokers in the Multiethnic Cohort Study revealed that Japanese Americans had the highest number of low nicotine CYP2A6 metabolizing genotypes of the five ethnic groups analyzed, consistent with their low lung cancer risk.
Urinary biomarker studies showed that levels of total NNAL, 3-hydroxy phenanthrene, a biomarker of polycyclic aromatic hydrocarbon uptake, and S-phenylmercapturic acid (SPMA), a biomarker of benzene exposure, were also significantly higher among African Americans than whites and significantly lower in Japanese Americans than whites, he said.
The findings are consistent with the initial study results published a decade ago (N Engl J Med. 2006;354:333-342), showing that at low levels of smoking (10 cigarettes per day), Japanese Americans and Hispanics had one-third the risk of lung cancer of African Americans or Native Hawaiians (P less than .001). The differences disappeared at higher levels of smoking (30 cigarettes per day).
Results from the new analyses for Native Hawaiians and Hispanics, however, did not fit this pattern, and “we’re not sure what’s going on there and need to do more work,” Dr. Hecht said.
Nicotine equivalent levels in Native Hawaiians were actually lower than would be expected based on their high lung cancer risk, while Hispanics had higher levels than would be expected based on their low risk.
Interestingly, urinary levels of 3-hydroxypropylmercapturic acid (3-HPMA), a metabolite of acrolein, were found to be unusually high in Native Hawaiians and relatively low in Hispanics, “which may play into their lung cancer risk because acrolein is a very strong toxicant and there is evidence to suggest it is involved in lung cancer,” he said.
While tobacco exposure and metabolism biomarkers have hit their stride, less well developed are the DNA adducts and repair biomarkers that can predict lung cancer susceptibility. This is a critical step because of the role DNA adducts play in the formation of lung cancer mutations, Dr. Hecht said.
A smoker may have a high tobacco exposure as indicated by the urinary biomarkers, but have a low metabolism to form DNA adducts or high DNA repair, which would mean their DNA adduct levels would be low. Conversely, another smoker with low exposure and poor DNA repair may have higher DNA adduct levels, and thus, a greater risk for developing lung cancer.
In addition, the exposure biomarkers are not entirely predictive and thus, will need to be combined with multiple DNA adducts and repair if susceptible smokers are to be identified and targeted for state-of-the art cessation approaches and surveillance, he said.
Using high-level mass spectrometry, the researchers have been able to readily measure formaldehyde DNA adducts and tobacco-specific 4-hydroxy-1-(3-pyridyl)-1-butanone (HPB)–releasing DNA adducts in human oral cells. Levels of the HPB adduct were unusually high at 12-45 pmol/mg, which is similar to what is seen in animals when exposed to NNK at a much higher dose than smokers take in.
“So this is a real lead,” said Dr. Hecht, who reported having no relevant conflicts of interest.
SAN DIEGO – Tobacco researchers are getting one step closer to identifying young smokers particularly susceptible to the carcinogenic effects of smoking, by using large-scale epidemiology studies and DNA adducts data.
“This is not lung cancer screening; this is not early detection of lung cancer; this is detection of susceptible, high-risk individuals, so they can be targeted for cessation, surveillance, and prevention,” said Stephen S. Hecht, Ph.D., professor of cancer prevention at the University of Minnesota Masonic Cancer Center in Minneapolis.
While nicotine itself is not a carcinogen, each puff of tobacco smoke delivers a mixture of more than 70 established carcinogens, including the potent lung-specific carcinogen 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK).
Some of these carcinogens are excreted, but many will undergo metabolic activation and interact with a patient’s DNA.
The product of this interaction, called DNA adducts, causes persistent miscoding during DNA replication, leading to the thousands of mutations we see in lung cancer, he explained at a conference on lung cancer translational science sponsored by the American Association for Cancer Research and the International Association for the Study of Lung Cancer.
The biomarkers of tobacco exposure and metabolism are very well developed, with blood and urinary biomarker panels applied in many studies and validated by mass spectrometry.
Using the Shanghai cohort study, the researchers identified three urinary biomarkers – PheT (r-1,t-2,3,c-4-tetrahydroxy-1,2,3,4-tetrahydrophenanthrene), total NNAL (a metabolite of NNK and its glucuronides), and total cotinine (a surrogate for nicotine that includes cotinine and its glucuronides) – that were significantly associated with lung cancer in 950 smokers, even after adjustment for smoking duration and intensity (Cancer Res. 2011;71:6749-57).
A more recent, deeper dive of 735 of those urinary samples, still stable about 20 years after they were taken, looked specifically at polymorphisms in cytochrome P450 2A6 (CYP2A6), the primary enzyme responsible for the oxidation of nicotine and cotinine.
Data currently in press show that CYP2A6 poor metabolizers had a lower risk of lung cancer than the combined group of normal, intermediate, or slow metabolizers (odds ratio, 0.64; P value for trend = .034).
“This is logical because poor metabolizers of nicotine have more unchanged nicotine on board, so they don’t need to smoke as intensely in order to get more nicotine because that nicotine is remaining to a greater extent in its natural form, rather than being metabolized into cotinine, which is not addictive,” Dr. Hecht said.
Recent genomewide-association studies by Dr. Hecht and his colleagues of smokers in the Multiethnic Cohort Study revealed that Japanese Americans had the highest number of low nicotine CYP2A6 metabolizing genotypes of the five ethnic groups analyzed, consistent with their low lung cancer risk.
Urinary biomarker studies showed that levels of total NNAL, 3-hydroxy phenanthrene, a biomarker of polycyclic aromatic hydrocarbon uptake, and S-phenylmercapturic acid (SPMA), a biomarker of benzene exposure, were also significantly higher among African Americans than whites and significantly lower in Japanese Americans than whites, he said.
The findings are consistent with the initial study results published a decade ago (N Engl J Med. 2006;354:333-342), showing that at low levels of smoking (10 cigarettes per day), Japanese Americans and Hispanics had one-third the risk of lung cancer of African Americans or Native Hawaiians (P less than .001). The differences disappeared at higher levels of smoking (30 cigarettes per day).
Results from the new analyses for Native Hawaiians and Hispanics, however, did not fit this pattern, and “we’re not sure what’s going on there and need to do more work,” Dr. Hecht said.
Nicotine equivalent levels in Native Hawaiians were actually lower than would be expected based on their high lung cancer risk, while Hispanics had higher levels than would be expected based on their low risk.
Interestingly, urinary levels of 3-hydroxypropylmercapturic acid (3-HPMA), a metabolite of acrolein, were found to be unusually high in Native Hawaiians and relatively low in Hispanics, “which may play into their lung cancer risk because acrolein is a very strong toxicant and there is evidence to suggest it is involved in lung cancer,” he said.
While tobacco exposure and metabolism biomarkers have hit their stride, less well developed are the DNA adducts and repair biomarkers that can predict lung cancer susceptibility. This is a critical step because of the role DNA adducts play in the formation of lung cancer mutations, Dr. Hecht said.
A smoker may have a high tobacco exposure as indicated by the urinary biomarkers, but have a low metabolism to form DNA adducts or high DNA repair, which would mean their DNA adduct levels would be low. Conversely, another smoker with low exposure and poor DNA repair may have higher DNA adduct levels, and thus, a greater risk for developing lung cancer.
In addition, the exposure biomarkers are not entirely predictive and thus, will need to be combined with multiple DNA adducts and repair if susceptible smokers are to be identified and targeted for state-of-the art cessation approaches and surveillance, he said.
Using high-level mass spectrometry, the researchers have been able to readily measure formaldehyde DNA adducts and tobacco-specific 4-hydroxy-1-(3-pyridyl)-1-butanone (HPB)–releasing DNA adducts in human oral cells. Levels of the HPB adduct were unusually high at 12-45 pmol/mg, which is similar to what is seen in animals when exposed to NNK at a much higher dose than smokers take in.
“So this is a real lead,” said Dr. Hecht, who reported having no relevant conflicts of interest.
AT AN AACR/IASLC JOINT CONFERENCE
Lymphedema microsurgery gaining momentum
CHICAGO – Microsurgery does not cure lymphedema, in most cases. But, in most cases, it does improve the severity of lymphedema and reduce the complications of this chronic and debilitating disease. And “it certainly improves patients’ quality of life,” lymphedema treatment pioneer Dr. David W. Chang said at the 40th annual Northwestern Vascular Symposium.
Surgical treatment for limb lymphedema has come into its own since a lymphovenous shunt was first used in a dog model in 1962, with Dr. Chang and others now anastomosing subdermal lymphatics to subdermal venules less than 0.8 mm in diameter. The rationale behind “super-microsurgery” is that venous pressure is low in the subdermal venules and has minimal back flow, he said.
One of the big problems early on was knowing exactly where the lymphatic vessels were, but newer technology like indocyanine green (ICG) lymphangiography helps visualize functioning lymphatic channels for potential bypass and determine the severity of the disease. Understanding the disease stage is key to selecting the appropriate surgical procedure.
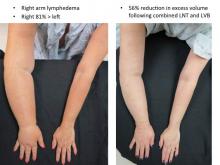
Lymphovenous bypass (LVB) is best in patients with stage 1 or 2 upper extremity lymphedema, while lymph node transfer (LNT) works for patients who are poor candidates for LVB or require combined breast reconstruction, said Dr. Chang, a plastic surgeon with the University of Chicago.
More recently, Dr. Chang has begun combining LVB and LNT, particularly for the more severe cases with stage 3 or 4 upper or lower extremity disease.
In Dr. Chang’s first 100 consecutive LVB cases while at the M.D. Anderson Cancer Center in Houston, quantitative improvement occurred in 74% of patients, symptom improvement in 96%, and the average volume differential reduction was 42% at 12 months (Plast Reconstr Surg. 2013 Nov;132:1305-14). The reduction was significantly larger in patients with earlier stage 1 or 2 vs. later stage 3 or 4 disease (61% vs. 17%).
During lymphovenous bypass, ICG is injected into the dermis of the web space and the superficial lymphatics evaluated with near-infrared fluorescence. It is easy to identify discrete functioning lymphatic channels in early-stage disease, but in late-stage disease significant dermal back flow is present, Dr. Chang said.
Dissection is performed under the microscope in the superficial subcutaneous plane to locate a good venule and lymph channel. Lymphatics are confirmed with isosulfan blue and ICG, and once the bypass site is determined, the lymphatic is anastomosed to the venule using 11-0 or 12-0 nylon, preferably in an end-to-side fashion. It’s thought this creates a more favorable flow pattern for the lymph to empty into the venule than an end-to-end anastomosis, he observed.
After the anastomosis is complete, patency is confirmed with isosulfan blue and ICG and the incision is closed under the microscope to ensure that the delicate anastomosis isn’t damaged. To avoid shear injury to the anastomosis, the limb is wrapped postoperatively for about a month without use of compression garments, he said.
Lymph node transfer (LNT) is increasingly being offered at centers to provide relief from lymphedema, although the mechanism by which it works is yet unclear; either the healthy lymph nodes act as a sponge to absorb lymphatic fluid or they induce lymphangiogenesis. Experience has shown, however, that rather than just grafting the lymph nodes, they need to be harvested with a vascular pedicle before transfer and anastomosed to the recipient artery and vein, although reconnecting the actual lymphatics may not be necessary, Dr. Chang observed.
Despite its popularity as a donor site, Dr. Chang said he is reluctant to use the groin because of the potential for iatrogenic lymphedema and prefers to harvest the supraclavicular nodes based off the transverse cervical artery. The external jugular vein can be harvested with the nodes if adequate venae comitantes are not present with the artery. Dissection of this flap can be difficult and care should be taken not to injure the lymphatic ducts, he noted.
It is also important to excise all scar tissue in the recipient site as this can impair lymphatic flow and inhibits lymphangiogenesis. If it is difficult to access or remove the scar, the vascularized lymph nodes are best placed just distal on the limb to the site of lymphatic obstruction, he added.
A recent meta-analysis (Plast Reconstr Surg. 2014 Apr;133:905-13) in five LNT studies reported that 91% of patients had a quantitative improvement, 78% discontinued compression garments, and complications were infection (8%), lymphorrhea (15%), and need for additional procedures (36%). There was great heterogeneity between studies, so the results should be interpreted with caution, Dr. Chang advised.
LNT is frequently combined with autologous breast reconstruction in patients with breast cancer, who comprise a significant percentage of Dr. Chang’s practice. The overall incidence of arm lymphedema after breast cancer can range from 8% to 56% at 2 years’ post-surgery, with the risk higher among women undergoing axillary lymph node dissection and/or axillary radiation.
Outcomes with combined LNT and breast reconstruction have been favorable, with one series reporting evidence of improved lymphatic flow on lymphoscintigraphy in five of six cases and one-third of patients no longer needing compression therapy (Ann Surg. 2012 Mar;255:468-73).
In cases where the patient requires a large skin paddle or seeks breast reconstruction after a previous mastectomy, lateral superficial groin lymph nodes can be harvested for transfer, leaving the deeper lymph nodes that drain the leg behind, Dr. Chang said. The nodes are usually clustered at the junction of the superior inferior epigastric and superficial circumflex iliac veins.
When combining LNT with breast reconstruction, this tissue is harvested together with the free abdominal flap used to reconstruct the breast. The superficial circumflex iliac vein is anastomosed in the axilla in addition to the arterial and venous anastomosis of the deep inferior epigastric vessels to the internal mammary vessels for the breast reconstruction. Reverse lymphatic mapping with technetium and ICG is used to decrease the risk of donor site lymphedema.
An algorithmic approach to simultaneous LNT with microvascular breast reconstruction proposed by Dr. Chang resulted in a 47% reduction in mean volume differential 12 months after reconstruction in 29 consecutive patients with refractory lymphedema following breast cancer treatment. These early results also showed no flap losses or donor-site lymphedema and donor-site wound complications in six patients (21%) that resolved with conservative measures (Ann Surg Oncol. 2015 Sep;22:2919-24).
The holy grail may be to strike lymphedema before it develops. To that end, Italian surgeons have proposed the Lymphatic Microsurgical Preventing Healing Approach (LYMPHA), which involves anastomosing arm lymphatics to a collateral branch of the axillary vein at the time of nodal dissection.
Over more than 4 years’ follow-up, only 3 of 74 breast cancer patients who underwent axillary nodal dissection with LYMPHA developed lymphedema, translating into a an exceptionally low 4% risk of lymphedema (Microsurgery. 2014 Sep;34:421-4). However, this approach is controversial because of unknown oncological risk and the uncertainty of its effectiveness in patients who may receive radiation after the surgery, Dr. Chang said in an interview.
Although these techniques show promise, currently no optimal solution exists and more research is needed to better understand lymphatic anatomy and physiology and the pathophysiology of lymphedema, concluded Dr. Chang, who reported no relevant conflicts of interest.
CHICAGO – Microsurgery does not cure lymphedema, in most cases. But, in most cases, it does improve the severity of lymphedema and reduce the complications of this chronic and debilitating disease. And “it certainly improves patients’ quality of life,” lymphedema treatment pioneer Dr. David W. Chang said at the 40th annual Northwestern Vascular Symposium.
Surgical treatment for limb lymphedema has come into its own since a lymphovenous shunt was first used in a dog model in 1962, with Dr. Chang and others now anastomosing subdermal lymphatics to subdermal venules less than 0.8 mm in diameter. The rationale behind “super-microsurgery” is that venous pressure is low in the subdermal venules and has minimal back flow, he said.
One of the big problems early on was knowing exactly where the lymphatic vessels were, but newer technology like indocyanine green (ICG) lymphangiography helps visualize functioning lymphatic channels for potential bypass and determine the severity of the disease. Understanding the disease stage is key to selecting the appropriate surgical procedure.
Lymphovenous bypass (LVB) is best in patients with stage 1 or 2 upper extremity lymphedema, while lymph node transfer (LNT) works for patients who are poor candidates for LVB or require combined breast reconstruction, said Dr. Chang, a plastic surgeon with the University of Chicago.
More recently, Dr. Chang has begun combining LVB and LNT, particularly for the more severe cases with stage 3 or 4 upper or lower extremity disease.
In Dr. Chang’s first 100 consecutive LVB cases while at the M.D. Anderson Cancer Center in Houston, quantitative improvement occurred in 74% of patients, symptom improvement in 96%, and the average volume differential reduction was 42% at 12 months (Plast Reconstr Surg. 2013 Nov;132:1305-14). The reduction was significantly larger in patients with earlier stage 1 or 2 vs. later stage 3 or 4 disease (61% vs. 17%).
During lymphovenous bypass, ICG is injected into the dermis of the web space and the superficial lymphatics evaluated with near-infrared fluorescence. It is easy to identify discrete functioning lymphatic channels in early-stage disease, but in late-stage disease significant dermal back flow is present, Dr. Chang said.
Dissection is performed under the microscope in the superficial subcutaneous plane to locate a good venule and lymph channel. Lymphatics are confirmed with isosulfan blue and ICG, and once the bypass site is determined, the lymphatic is anastomosed to the venule using 11-0 or 12-0 nylon, preferably in an end-to-side fashion. It’s thought this creates a more favorable flow pattern for the lymph to empty into the venule than an end-to-end anastomosis, he observed.
After the anastomosis is complete, patency is confirmed with isosulfan blue and ICG and the incision is closed under the microscope to ensure that the delicate anastomosis isn’t damaged. To avoid shear injury to the anastomosis, the limb is wrapped postoperatively for about a month without use of compression garments, he said.
Lymph node transfer (LNT) is increasingly being offered at centers to provide relief from lymphedema, although the mechanism by which it works is yet unclear; either the healthy lymph nodes act as a sponge to absorb lymphatic fluid or they induce lymphangiogenesis. Experience has shown, however, that rather than just grafting the lymph nodes, they need to be harvested with a vascular pedicle before transfer and anastomosed to the recipient artery and vein, although reconnecting the actual lymphatics may not be necessary, Dr. Chang observed.
Despite its popularity as a donor site, Dr. Chang said he is reluctant to use the groin because of the potential for iatrogenic lymphedema and prefers to harvest the supraclavicular nodes based off the transverse cervical artery. The external jugular vein can be harvested with the nodes if adequate venae comitantes are not present with the artery. Dissection of this flap can be difficult and care should be taken not to injure the lymphatic ducts, he noted.
It is also important to excise all scar tissue in the recipient site as this can impair lymphatic flow and inhibits lymphangiogenesis. If it is difficult to access or remove the scar, the vascularized lymph nodes are best placed just distal on the limb to the site of lymphatic obstruction, he added.
A recent meta-analysis (Plast Reconstr Surg. 2014 Apr;133:905-13) in five LNT studies reported that 91% of patients had a quantitative improvement, 78% discontinued compression garments, and complications were infection (8%), lymphorrhea (15%), and need for additional procedures (36%). There was great heterogeneity between studies, so the results should be interpreted with caution, Dr. Chang advised.
LNT is frequently combined with autologous breast reconstruction in patients with breast cancer, who comprise a significant percentage of Dr. Chang’s practice. The overall incidence of arm lymphedema after breast cancer can range from 8% to 56% at 2 years’ post-surgery, with the risk higher among women undergoing axillary lymph node dissection and/or axillary radiation.
Outcomes with combined LNT and breast reconstruction have been favorable, with one series reporting evidence of improved lymphatic flow on lymphoscintigraphy in five of six cases and one-third of patients no longer needing compression therapy (Ann Surg. 2012 Mar;255:468-73).
In cases where the patient requires a large skin paddle or seeks breast reconstruction after a previous mastectomy, lateral superficial groin lymph nodes can be harvested for transfer, leaving the deeper lymph nodes that drain the leg behind, Dr. Chang said. The nodes are usually clustered at the junction of the superior inferior epigastric and superficial circumflex iliac veins.
When combining LNT with breast reconstruction, this tissue is harvested together with the free abdominal flap used to reconstruct the breast. The superficial circumflex iliac vein is anastomosed in the axilla in addition to the arterial and venous anastomosis of the deep inferior epigastric vessels to the internal mammary vessels for the breast reconstruction. Reverse lymphatic mapping with technetium and ICG is used to decrease the risk of donor site lymphedema.
An algorithmic approach to simultaneous LNT with microvascular breast reconstruction proposed by Dr. Chang resulted in a 47% reduction in mean volume differential 12 months after reconstruction in 29 consecutive patients with refractory lymphedema following breast cancer treatment. These early results also showed no flap losses or donor-site lymphedema and donor-site wound complications in six patients (21%) that resolved with conservative measures (Ann Surg Oncol. 2015 Sep;22:2919-24).
The holy grail may be to strike lymphedema before it develops. To that end, Italian surgeons have proposed the Lymphatic Microsurgical Preventing Healing Approach (LYMPHA), which involves anastomosing arm lymphatics to a collateral branch of the axillary vein at the time of nodal dissection.
Over more than 4 years’ follow-up, only 3 of 74 breast cancer patients who underwent axillary nodal dissection with LYMPHA developed lymphedema, translating into a an exceptionally low 4% risk of lymphedema (Microsurgery. 2014 Sep;34:421-4). However, this approach is controversial because of unknown oncological risk and the uncertainty of its effectiveness in patients who may receive radiation after the surgery, Dr. Chang said in an interview.
Although these techniques show promise, currently no optimal solution exists and more research is needed to better understand lymphatic anatomy and physiology and the pathophysiology of lymphedema, concluded Dr. Chang, who reported no relevant conflicts of interest.
CHICAGO – Microsurgery does not cure lymphedema, in most cases. But, in most cases, it does improve the severity of lymphedema and reduce the complications of this chronic and debilitating disease. And “it certainly improves patients’ quality of life,” lymphedema treatment pioneer Dr. David W. Chang said at the 40th annual Northwestern Vascular Symposium.
Surgical treatment for limb lymphedema has come into its own since a lymphovenous shunt was first used in a dog model in 1962, with Dr. Chang and others now anastomosing subdermal lymphatics to subdermal venules less than 0.8 mm in diameter. The rationale behind “super-microsurgery” is that venous pressure is low in the subdermal venules and has minimal back flow, he said.
One of the big problems early on was knowing exactly where the lymphatic vessels were, but newer technology like indocyanine green (ICG) lymphangiography helps visualize functioning lymphatic channels for potential bypass and determine the severity of the disease. Understanding the disease stage is key to selecting the appropriate surgical procedure.
Lymphovenous bypass (LVB) is best in patients with stage 1 or 2 upper extremity lymphedema, while lymph node transfer (LNT) works for patients who are poor candidates for LVB or require combined breast reconstruction, said Dr. Chang, a plastic surgeon with the University of Chicago.
More recently, Dr. Chang has begun combining LVB and LNT, particularly for the more severe cases with stage 3 or 4 upper or lower extremity disease.
In Dr. Chang’s first 100 consecutive LVB cases while at the M.D. Anderson Cancer Center in Houston, quantitative improvement occurred in 74% of patients, symptom improvement in 96%, and the average volume differential reduction was 42% at 12 months (Plast Reconstr Surg. 2013 Nov;132:1305-14). The reduction was significantly larger in patients with earlier stage 1 or 2 vs. later stage 3 or 4 disease (61% vs. 17%).
During lymphovenous bypass, ICG is injected into the dermis of the web space and the superficial lymphatics evaluated with near-infrared fluorescence. It is easy to identify discrete functioning lymphatic channels in early-stage disease, but in late-stage disease significant dermal back flow is present, Dr. Chang said.
Dissection is performed under the microscope in the superficial subcutaneous plane to locate a good venule and lymph channel. Lymphatics are confirmed with isosulfan blue and ICG, and once the bypass site is determined, the lymphatic is anastomosed to the venule using 11-0 or 12-0 nylon, preferably in an end-to-side fashion. It’s thought this creates a more favorable flow pattern for the lymph to empty into the venule than an end-to-end anastomosis, he observed.
After the anastomosis is complete, patency is confirmed with isosulfan blue and ICG and the incision is closed under the microscope to ensure that the delicate anastomosis isn’t damaged. To avoid shear injury to the anastomosis, the limb is wrapped postoperatively for about a month without use of compression garments, he said.
Lymph node transfer (LNT) is increasingly being offered at centers to provide relief from lymphedema, although the mechanism by which it works is yet unclear; either the healthy lymph nodes act as a sponge to absorb lymphatic fluid or they induce lymphangiogenesis. Experience has shown, however, that rather than just grafting the lymph nodes, they need to be harvested with a vascular pedicle before transfer and anastomosed to the recipient artery and vein, although reconnecting the actual lymphatics may not be necessary, Dr. Chang observed.
Despite its popularity as a donor site, Dr. Chang said he is reluctant to use the groin because of the potential for iatrogenic lymphedema and prefers to harvest the supraclavicular nodes based off the transverse cervical artery. The external jugular vein can be harvested with the nodes if adequate venae comitantes are not present with the artery. Dissection of this flap can be difficult and care should be taken not to injure the lymphatic ducts, he noted.
It is also important to excise all scar tissue in the recipient site as this can impair lymphatic flow and inhibits lymphangiogenesis. If it is difficult to access or remove the scar, the vascularized lymph nodes are best placed just distal on the limb to the site of lymphatic obstruction, he added.
A recent meta-analysis (Plast Reconstr Surg. 2014 Apr;133:905-13) in five LNT studies reported that 91% of patients had a quantitative improvement, 78% discontinued compression garments, and complications were infection (8%), lymphorrhea (15%), and need for additional procedures (36%). There was great heterogeneity between studies, so the results should be interpreted with caution, Dr. Chang advised.
LNT is frequently combined with autologous breast reconstruction in patients with breast cancer, who comprise a significant percentage of Dr. Chang’s practice. The overall incidence of arm lymphedema after breast cancer can range from 8% to 56% at 2 years’ post-surgery, with the risk higher among women undergoing axillary lymph node dissection and/or axillary radiation.
Outcomes with combined LNT and breast reconstruction have been favorable, with one series reporting evidence of improved lymphatic flow on lymphoscintigraphy in five of six cases and one-third of patients no longer needing compression therapy (Ann Surg. 2012 Mar;255:468-73).
In cases where the patient requires a large skin paddle or seeks breast reconstruction after a previous mastectomy, lateral superficial groin lymph nodes can be harvested for transfer, leaving the deeper lymph nodes that drain the leg behind, Dr. Chang said. The nodes are usually clustered at the junction of the superior inferior epigastric and superficial circumflex iliac veins.
When combining LNT with breast reconstruction, this tissue is harvested together with the free abdominal flap used to reconstruct the breast. The superficial circumflex iliac vein is anastomosed in the axilla in addition to the arterial and venous anastomosis of the deep inferior epigastric vessels to the internal mammary vessels for the breast reconstruction. Reverse lymphatic mapping with technetium and ICG is used to decrease the risk of donor site lymphedema.
An algorithmic approach to simultaneous LNT with microvascular breast reconstruction proposed by Dr. Chang resulted in a 47% reduction in mean volume differential 12 months after reconstruction in 29 consecutive patients with refractory lymphedema following breast cancer treatment. These early results also showed no flap losses or donor-site lymphedema and donor-site wound complications in six patients (21%) that resolved with conservative measures (Ann Surg Oncol. 2015 Sep;22:2919-24).
The holy grail may be to strike lymphedema before it develops. To that end, Italian surgeons have proposed the Lymphatic Microsurgical Preventing Healing Approach (LYMPHA), which involves anastomosing arm lymphatics to a collateral branch of the axillary vein at the time of nodal dissection.
Over more than 4 years’ follow-up, only 3 of 74 breast cancer patients who underwent axillary nodal dissection with LYMPHA developed lymphedema, translating into a an exceptionally low 4% risk of lymphedema (Microsurgery. 2014 Sep;34:421-4). However, this approach is controversial because of unknown oncological risk and the uncertainty of its effectiveness in patients who may receive radiation after the surgery, Dr. Chang said in an interview.
Although these techniques show promise, currently no optimal solution exists and more research is needed to better understand lymphatic anatomy and physiology and the pathophysiology of lymphedema, concluded Dr. Chang, who reported no relevant conflicts of interest.
EXPERT ANALYSIS AT THE NORTHWESTERN VASCULAR SYMPOSIUM
Endocrine Society Issues First-ever Guidelines for Primary Adrenal Insufficiency
New guidelines on the diagnosis and management of primary adrenal insufficiency stress the importance of early recognition and the need to prevent life-threatening adrenal crises in these patients.
These are the first clinical practice guidelines on primary adrenal insufficiency (PAI), also known as Addison’s disease, issued by Endocrine Society (J Clin Endocrinol Metab. 2016 Jan 13:jc20151710 [Epub ahead of print]).
“Because it’s a rare disease and symptoms can mimic common conditions, adrenal insufficiency is often, at least initially, overlooked,” guideline co-author Dr. Deborah Merke, a senior investigator with the National Institutes of Health Clinical Center in Bethesda, Md., said. “So the main goal of these clinical practice guidelines is to improve patient care.”
The guidelines suggest clinicians should have a low diagnostic threshold in acutely ill patients with unexplained symptoms or signs suggestive of PAI such as volume depletion, hypotension, hyponatremia, hyperkalemia, fever, abdominal pain, hyperpigmentation, or, especially in children, hypoglycemia.
This low diagnostic threshold for PAI should also be extended to pregnant women with unexplained persistent nausea, fatigue, and hypotension.
For adult patients with a suspected adrenal crisis, an immediate parenteral injection of hydrocortisone 100 mg should be given, followed by appropriate fluid resuscitation and 200 mg of hydrocortisone for 24 hours, according to the guidelines, which were co-sponsored by the European Society of Endocrinology and American Association for Clinical Chemistry.
Despite a known association between adrenal crisis and mortality, there is a knowledge gap regarding how to prevent, recognize, and reduce the risk of these life-threatening events, Dr. Merke said.
To that end, the task force has taken a page from the diabetes community in recommending all PAI patients carry steroid emergency identification cards and be equipped with a glucocorticoid injection kit for emergency use and be educated on how to use it.
The guidelines also advocate education about stress dosing to counter the increased demand for corticosteroids during periods of stress, which can encompass something as common as the flu.
“Just like diabetics carry around emergency medicines, it’s important for patients with adrenal insufficiency to carry around an emergency kit and to realize that should they start to get sick, they need to increase their doses,” she said. “There often seems to be a lack of awareness among physicians as well that these patients have a potentially life-threatening condition, should they get a common illness.”
One of the key unanswered clinical questions the task force sought to address was whether the widely used high-dose (250 mcg) corticotropin stimulation test, also known as the adrenocorticotropin (ACTH) or short Synacthen test, should be replaced by the low-dose test (1 mcg) to diagnosis PAI.
Despite a review of published data and a systematic review commissioned by the task force, “We didn’t come up with much scientific evidence to say we should be changing the historic standard,” Dr. Merke said.
The systematic review identified only five studies of high-dose corticotropin testing specifically in PAI and none of low-dose testing. The low-dose test has shown higher sensitivity in the detection of adrenal insufficiency in critically ill patients and secondary adrenal insufficiency, but the limited available data suggest it does not provide better diagnostic accuracy for PAI than the high-dose test.
As a result, the guidelines recommend the standard, short corticotropin test (250 mcg for adults and children aged at least 2 years) as the “gold standard” diagnostic test to establish a PAI diagnosis.
The low-dose (1 mcg) test is recommended only when corticotropin is in short supply, which is not typically a problem in the United States, she said.
If corticotropin testing isn’t feasible, a combination of a morning plasma ACTH and cortisol levels (less than 5 mcg/dL) can be used as an initial screening, though confirmatory testing with corticotropin stimulation is strongly recommended.
Glucocorticoid therapy is recommended in all patients with confirmed PAI based on the highest quality of evidence, with a clear preference given for the short-acting steroids, Dr. Merke observed.
Hydrocortisone 15 mg-25 mg or cortisone acetate 20 mg-35 mg given in two to three divided doses per day is suggested for adults, with the highest dose to be given in the morning. Once- or twice-daily prednisolone 3 mg-5 mg is suggested as an alternative.
Hydrocortisone is also suggested over cortisone acetate, prednisolone, or prednisone for pregnant women and recommended for children (about 8 mg/m2 per day), but the evidence supporting these items was of low quality.
The guidelines suggest against using dexamethasone, the longest-acting glucocorticoid, because of the potential long-term side effects of overt-treatment and the frequent appearance of cushingoid side effects. They also recommend against dexamethasone in pregnant women because it is not inactivated in the placenta.
The guidelines are also quite clear in their suggestion against hormonal monitoring of glucocorticoid replacement and instead favor adjusting treatment based only on clinical response.
“This is a very important suggestion that we made because often clinicians use ACTH to adjust doses and this commonly results in overreplacement and there are side effects to overreplacement,” including weight gain, insomnia, and peripheral edema, Dr. Merke said.
A second systematic review commissioned by the task force involving 15 observational studies of glucocorticoid replacement regimens uncovered very sparse data on mortality, bone density, and incidence of adrenal crisis.
It has been suggested that newer extended-release and dual-release glucocorticoid formulations may result in higher health-reality quality of life than once-, twice-, or thrice-daily regimens, but once again, the evidence was insufficient to support a specific recommendation.
Dr. Merke acknowledged that many of the guidelines recommendations were ungraded or best practices, reflecting the lack of randomized clinical trials in PAI.
“I think that’s why it was so important for us to do this,” she said. “We had a group of experts that were very familiar with this disease providing guidance, but I think it’s also one reason why physicians out there in practice get confused about exactly what to do because of the lack of hard evidence. ... It does certainly cry for the need for more studies in these rare diseases.”
The guidelines were funded by the Endocrine Society, and the authors reported receiving no external funding or remuneration.
New guidelines on the diagnosis and management of primary adrenal insufficiency stress the importance of early recognition and the need to prevent life-threatening adrenal crises in these patients.
These are the first clinical practice guidelines on primary adrenal insufficiency (PAI), also known as Addison’s disease, issued by Endocrine Society (J Clin Endocrinol Metab. 2016 Jan 13:jc20151710 [Epub ahead of print]).
“Because it’s a rare disease and symptoms can mimic common conditions, adrenal insufficiency is often, at least initially, overlooked,” guideline co-author Dr. Deborah Merke, a senior investigator with the National Institutes of Health Clinical Center in Bethesda, Md., said. “So the main goal of these clinical practice guidelines is to improve patient care.”
The guidelines suggest clinicians should have a low diagnostic threshold in acutely ill patients with unexplained symptoms or signs suggestive of PAI such as volume depletion, hypotension, hyponatremia, hyperkalemia, fever, abdominal pain, hyperpigmentation, or, especially in children, hypoglycemia.
This low diagnostic threshold for PAI should also be extended to pregnant women with unexplained persistent nausea, fatigue, and hypotension.
For adult patients with a suspected adrenal crisis, an immediate parenteral injection of hydrocortisone 100 mg should be given, followed by appropriate fluid resuscitation and 200 mg of hydrocortisone for 24 hours, according to the guidelines, which were co-sponsored by the European Society of Endocrinology and American Association for Clinical Chemistry.
Despite a known association between adrenal crisis and mortality, there is a knowledge gap regarding how to prevent, recognize, and reduce the risk of these life-threatening events, Dr. Merke said.
To that end, the task force has taken a page from the diabetes community in recommending all PAI patients carry steroid emergency identification cards and be equipped with a glucocorticoid injection kit for emergency use and be educated on how to use it.
The guidelines also advocate education about stress dosing to counter the increased demand for corticosteroids during periods of stress, which can encompass something as common as the flu.
“Just like diabetics carry around emergency medicines, it’s important for patients with adrenal insufficiency to carry around an emergency kit and to realize that should they start to get sick, they need to increase their doses,” she said. “There often seems to be a lack of awareness among physicians as well that these patients have a potentially life-threatening condition, should they get a common illness.”
One of the key unanswered clinical questions the task force sought to address was whether the widely used high-dose (250 mcg) corticotropin stimulation test, also known as the adrenocorticotropin (ACTH) or short Synacthen test, should be replaced by the low-dose test (1 mcg) to diagnosis PAI.
Despite a review of published data and a systematic review commissioned by the task force, “We didn’t come up with much scientific evidence to say we should be changing the historic standard,” Dr. Merke said.
The systematic review identified only five studies of high-dose corticotropin testing specifically in PAI and none of low-dose testing. The low-dose test has shown higher sensitivity in the detection of adrenal insufficiency in critically ill patients and secondary adrenal insufficiency, but the limited available data suggest it does not provide better diagnostic accuracy for PAI than the high-dose test.
As a result, the guidelines recommend the standard, short corticotropin test (250 mcg for adults and children aged at least 2 years) as the “gold standard” diagnostic test to establish a PAI diagnosis.
The low-dose (1 mcg) test is recommended only when corticotropin is in short supply, which is not typically a problem in the United States, she said.
If corticotropin testing isn’t feasible, a combination of a morning plasma ACTH and cortisol levels (less than 5 mcg/dL) can be used as an initial screening, though confirmatory testing with corticotropin stimulation is strongly recommended.
Glucocorticoid therapy is recommended in all patients with confirmed PAI based on the highest quality of evidence, with a clear preference given for the short-acting steroids, Dr. Merke observed.
Hydrocortisone 15 mg-25 mg or cortisone acetate 20 mg-35 mg given in two to three divided doses per day is suggested for adults, with the highest dose to be given in the morning. Once- or twice-daily prednisolone 3 mg-5 mg is suggested as an alternative.
Hydrocortisone is also suggested over cortisone acetate, prednisolone, or prednisone for pregnant women and recommended for children (about 8 mg/m2 per day), but the evidence supporting these items was of low quality.
The guidelines suggest against using dexamethasone, the longest-acting glucocorticoid, because of the potential long-term side effects of overt-treatment and the frequent appearance of cushingoid side effects. They also recommend against dexamethasone in pregnant women because it is not inactivated in the placenta.
The guidelines are also quite clear in their suggestion against hormonal monitoring of glucocorticoid replacement and instead favor adjusting treatment based only on clinical response.
“This is a very important suggestion that we made because often clinicians use ACTH to adjust doses and this commonly results in overreplacement and there are side effects to overreplacement,” including weight gain, insomnia, and peripheral edema, Dr. Merke said.
A second systematic review commissioned by the task force involving 15 observational studies of glucocorticoid replacement regimens uncovered very sparse data on mortality, bone density, and incidence of adrenal crisis.
It has been suggested that newer extended-release and dual-release glucocorticoid formulations may result in higher health-reality quality of life than once-, twice-, or thrice-daily regimens, but once again, the evidence was insufficient to support a specific recommendation.
Dr. Merke acknowledged that many of the guidelines recommendations were ungraded or best practices, reflecting the lack of randomized clinical trials in PAI.
“I think that’s why it was so important for us to do this,” she said. “We had a group of experts that were very familiar with this disease providing guidance, but I think it’s also one reason why physicians out there in practice get confused about exactly what to do because of the lack of hard evidence. ... It does certainly cry for the need for more studies in these rare diseases.”
The guidelines were funded by the Endocrine Society, and the authors reported receiving no external funding or remuneration.
New guidelines on the diagnosis and management of primary adrenal insufficiency stress the importance of early recognition and the need to prevent life-threatening adrenal crises in these patients.
These are the first clinical practice guidelines on primary adrenal insufficiency (PAI), also known as Addison’s disease, issued by Endocrine Society (J Clin Endocrinol Metab. 2016 Jan 13:jc20151710 [Epub ahead of print]).
“Because it’s a rare disease and symptoms can mimic common conditions, adrenal insufficiency is often, at least initially, overlooked,” guideline co-author Dr. Deborah Merke, a senior investigator with the National Institutes of Health Clinical Center in Bethesda, Md., said. “So the main goal of these clinical practice guidelines is to improve patient care.”
The guidelines suggest clinicians should have a low diagnostic threshold in acutely ill patients with unexplained symptoms or signs suggestive of PAI such as volume depletion, hypotension, hyponatremia, hyperkalemia, fever, abdominal pain, hyperpigmentation, or, especially in children, hypoglycemia.
This low diagnostic threshold for PAI should also be extended to pregnant women with unexplained persistent nausea, fatigue, and hypotension.
For adult patients with a suspected adrenal crisis, an immediate parenteral injection of hydrocortisone 100 mg should be given, followed by appropriate fluid resuscitation and 200 mg of hydrocortisone for 24 hours, according to the guidelines, which were co-sponsored by the European Society of Endocrinology and American Association for Clinical Chemistry.
Despite a known association between adrenal crisis and mortality, there is a knowledge gap regarding how to prevent, recognize, and reduce the risk of these life-threatening events, Dr. Merke said.
To that end, the task force has taken a page from the diabetes community in recommending all PAI patients carry steroid emergency identification cards and be equipped with a glucocorticoid injection kit for emergency use and be educated on how to use it.
The guidelines also advocate education about stress dosing to counter the increased demand for corticosteroids during periods of stress, which can encompass something as common as the flu.
“Just like diabetics carry around emergency medicines, it’s important for patients with adrenal insufficiency to carry around an emergency kit and to realize that should they start to get sick, they need to increase their doses,” she said. “There often seems to be a lack of awareness among physicians as well that these patients have a potentially life-threatening condition, should they get a common illness.”
One of the key unanswered clinical questions the task force sought to address was whether the widely used high-dose (250 mcg) corticotropin stimulation test, also known as the adrenocorticotropin (ACTH) or short Synacthen test, should be replaced by the low-dose test (1 mcg) to diagnosis PAI.
Despite a review of published data and a systematic review commissioned by the task force, “We didn’t come up with much scientific evidence to say we should be changing the historic standard,” Dr. Merke said.
The systematic review identified only five studies of high-dose corticotropin testing specifically in PAI and none of low-dose testing. The low-dose test has shown higher sensitivity in the detection of adrenal insufficiency in critically ill patients and secondary adrenal insufficiency, but the limited available data suggest it does not provide better diagnostic accuracy for PAI than the high-dose test.
As a result, the guidelines recommend the standard, short corticotropin test (250 mcg for adults and children aged at least 2 years) as the “gold standard” diagnostic test to establish a PAI diagnosis.
The low-dose (1 mcg) test is recommended only when corticotropin is in short supply, which is not typically a problem in the United States, she said.
If corticotropin testing isn’t feasible, a combination of a morning plasma ACTH and cortisol levels (less than 5 mcg/dL) can be used as an initial screening, though confirmatory testing with corticotropin stimulation is strongly recommended.
Glucocorticoid therapy is recommended in all patients with confirmed PAI based on the highest quality of evidence, with a clear preference given for the short-acting steroids, Dr. Merke observed.
Hydrocortisone 15 mg-25 mg or cortisone acetate 20 mg-35 mg given in two to three divided doses per day is suggested for adults, with the highest dose to be given in the morning. Once- or twice-daily prednisolone 3 mg-5 mg is suggested as an alternative.
Hydrocortisone is also suggested over cortisone acetate, prednisolone, or prednisone for pregnant women and recommended for children (about 8 mg/m2 per day), but the evidence supporting these items was of low quality.
The guidelines suggest against using dexamethasone, the longest-acting glucocorticoid, because of the potential long-term side effects of overt-treatment and the frequent appearance of cushingoid side effects. They also recommend against dexamethasone in pregnant women because it is not inactivated in the placenta.
The guidelines are also quite clear in their suggestion against hormonal monitoring of glucocorticoid replacement and instead favor adjusting treatment based only on clinical response.
“This is a very important suggestion that we made because often clinicians use ACTH to adjust doses and this commonly results in overreplacement and there are side effects to overreplacement,” including weight gain, insomnia, and peripheral edema, Dr. Merke said.
A second systematic review commissioned by the task force involving 15 observational studies of glucocorticoid replacement regimens uncovered very sparse data on mortality, bone density, and incidence of adrenal crisis.
It has been suggested that newer extended-release and dual-release glucocorticoid formulations may result in higher health-reality quality of life than once-, twice-, or thrice-daily regimens, but once again, the evidence was insufficient to support a specific recommendation.
Dr. Merke acknowledged that many of the guidelines recommendations were ungraded or best practices, reflecting the lack of randomized clinical trials in PAI.
“I think that’s why it was so important for us to do this,” she said. “We had a group of experts that were very familiar with this disease providing guidance, but I think it’s also one reason why physicians out there in practice get confused about exactly what to do because of the lack of hard evidence. ... It does certainly cry for the need for more studies in these rare diseases.”
The guidelines were funded by the Endocrine Society, and the authors reported receiving no external funding or remuneration.
FROM THE JOURNAL OF CLINICAL ENDOCRINOLOGY & METABOLISM
Endocrine Society issues first-ever guidelines for primary adrenal insufficiency
New guidelines on the diagnosis and management of primary adrenal insufficiency stress the importance of early recognition and the need to prevent life-threatening adrenal crises in these patients.
These are the first clinical practice guidelines on primary adrenal insufficiency (PAI), also known as Addison’s disease, issued by Endocrine Society (J Clin Endocrinol Metab. 2016 Jan 13:jc20151710 [Epub ahead of print]).
“Because it’s a rare disease and symptoms can mimic common conditions, adrenal insufficiency is often, at least initially, overlooked,” guideline co-author Dr. Deborah Merke, a senior investigator with the National Institutes of Health Clinical Center in Bethesda, Md., said. “So the main goal of these clinical practice guidelines is to improve patient care.”
The guidelines suggest clinicians should have a low diagnostic threshold in acutely ill patients with unexplained symptoms or signs suggestive of PAI such as volume depletion, hypotension, hyponatremia, hyperkalemia, fever, abdominal pain, hyperpigmentation, or, especially in children, hypoglycemia.
This low diagnostic threshold for PAI should also be extended to pregnant women with unexplained persistent nausea, fatigue, and hypotension.
For adult patients with a suspected adrenal crisis, an immediate parenteral injection of hydrocortisone 100 mg should be given, followed by appropriate fluid resuscitation and 200 mg of hydrocortisone for 24 hours, according to the guidelines, which were co-sponsored by the European Society of Endocrinology and American Association for Clinical Chemistry.
Despite a known association between adrenal crisis and mortality, there is a knowledge gap regarding how to prevent, recognize, and reduce the risk of these life-threatening events, Dr. Merke said.
To that end, the task force has taken a page from the diabetes community in recommending all PAI patients carry steroid emergency identification cards and be equipped with a glucocorticoid injection kit for emergency use and be educated on how to use it.
The guidelines also advocate education about stress dosing to counter the increased demand for corticosteroids during periods of stress, which can encompass something as common as the flu.
“Just like diabetics carry around emergency medicines, it’s important for patients with adrenal insufficiency to carry around an emergency kit and to realize that should they start to get sick, they need to increase their doses,” she said. “There often seems to be a lack of awareness among physicians as well that these patients have a potentially life-threatening condition, should they get a common illness.”
One of the key unanswered clinical questions the task force sought to address was whether the widely used high-dose (250 mcg) corticotropin stimulation test, also known as the adrenocorticotropin (ACTH) or short Synacthen test, should be replaced by the low-dose test (1 mcg) to diagnosis PAI.
Despite a review of published data and a systematic review commissioned by the task force, “We didn’t come up with much scientific evidence to say we should be changing the historic standard,” Dr. Merke said.
The systematic review identified only five studies of high-dose corticotropin testing specifically in PAI and none of low-dose testing. The low-dose test has shown higher sensitivity in the detection of adrenal insufficiency in critically ill patients and secondary adrenal insufficiency, but the limited available data suggest it does not provide better diagnostic accuracy for PAI than the high-dose test.
As a result, the guidelines recommend the standard, short corticotropin test (250 mcg for adults and children aged at least 2 years) as the “gold standard” diagnostic test to establish a PAI diagnosis.
The low-dose (1 mcg) test is recommended only when corticotropin is in short supply, which is not typically a problem in the United States, she said.
If corticotropin testing isn’t feasible, a combination of a morning plasma ACTH and cortisol levels (less than 5 mcg/dL) can be used as an initial screening, though confirmatory testing with corticotropin stimulation is strongly recommended.
Glucocorticoid therapy is recommended in all patients with confirmed PAI based on the highest quality of evidence, with a clear preference given for the short-acting steroids, Dr. Merke observed.
Hydrocortisone 15 mg-25 mg or cortisone acetate 20 mg-35 mg given in two to three divided doses per day is suggested for adults, with the highest dose to be given in the morning. Once- or twice-daily prednisolone 3 mg-5 mg is suggested as an alternative.
Hydrocortisone is also suggested over cortisone acetate, prednisolone, or prednisone for pregnant women and recommended for children (about 8 mg/m2 per day), but the evidence supporting these items was of low quality.
The guidelines suggest against using dexamethasone, the longest-acting glucocorticoid, because of the potential long-term side effects of overt-treatment and the frequent appearance of cushingoid side effects. They also recommend against dexamethasone in pregnant women because it is not inactivated in the placenta.
The guidelines are also quite clear in their suggestion against hormonal monitoring of glucocorticoid replacement and instead favor adjusting treatment based only on clinical response.
“This is a very important suggestion that we made because often clinicians use ACTH to adjust doses and this commonly results in overreplacement and there are side effects to overreplacement,” including weight gain, insomnia, and peripheral edema, Dr. Merke said.
A second systematic review commissioned by the task force involving 15 observational studies of glucocorticoid replacement regimens uncovered very sparse data on mortality, bone density, and incidence of adrenal crisis.
It has been suggested that newer extended-release and dual-release glucocorticoid formulations may result in higher health-reality quality of life than once-, twice-, or thrice-daily regimens, but once again, the evidence was insufficient to support a specific recommendation.
Dr. Merke acknowledged that many of the guidelines recommendations were ungraded or best practices, reflecting the lack of randomized clinical trials in PAI.
“I think that’s why it was so important for us to do this,” she said. “We had a group of experts that were very familiar with this disease providing guidance, but I think it’s also one reason why physicians out there in practice get confused about exactly what to do because of the lack of hard evidence. ... It does certainly cry for the need for more studies in these rare diseases.”
The guidelines were funded by the Endocrine Society, and the authors reported receiving no external funding or remuneration.
New guidelines on the diagnosis and management of primary adrenal insufficiency stress the importance of early recognition and the need to prevent life-threatening adrenal crises in these patients.
These are the first clinical practice guidelines on primary adrenal insufficiency (PAI), also known as Addison’s disease, issued by Endocrine Society (J Clin Endocrinol Metab. 2016 Jan 13:jc20151710 [Epub ahead of print]).
“Because it’s a rare disease and symptoms can mimic common conditions, adrenal insufficiency is often, at least initially, overlooked,” guideline co-author Dr. Deborah Merke, a senior investigator with the National Institutes of Health Clinical Center in Bethesda, Md., said. “So the main goal of these clinical practice guidelines is to improve patient care.”
The guidelines suggest clinicians should have a low diagnostic threshold in acutely ill patients with unexplained symptoms or signs suggestive of PAI such as volume depletion, hypotension, hyponatremia, hyperkalemia, fever, abdominal pain, hyperpigmentation, or, especially in children, hypoglycemia.
This low diagnostic threshold for PAI should also be extended to pregnant women with unexplained persistent nausea, fatigue, and hypotension.
For adult patients with a suspected adrenal crisis, an immediate parenteral injection of hydrocortisone 100 mg should be given, followed by appropriate fluid resuscitation and 200 mg of hydrocortisone for 24 hours, according to the guidelines, which were co-sponsored by the European Society of Endocrinology and American Association for Clinical Chemistry.
Despite a known association between adrenal crisis and mortality, there is a knowledge gap regarding how to prevent, recognize, and reduce the risk of these life-threatening events, Dr. Merke said.
To that end, the task force has taken a page from the diabetes community in recommending all PAI patients carry steroid emergency identification cards and be equipped with a glucocorticoid injection kit for emergency use and be educated on how to use it.
The guidelines also advocate education about stress dosing to counter the increased demand for corticosteroids during periods of stress, which can encompass something as common as the flu.
“Just like diabetics carry around emergency medicines, it’s important for patients with adrenal insufficiency to carry around an emergency kit and to realize that should they start to get sick, they need to increase their doses,” she said. “There often seems to be a lack of awareness among physicians as well that these patients have a potentially life-threatening condition, should they get a common illness.”
One of the key unanswered clinical questions the task force sought to address was whether the widely used high-dose (250 mcg) corticotropin stimulation test, also known as the adrenocorticotropin (ACTH) or short Synacthen test, should be replaced by the low-dose test (1 mcg) to diagnosis PAI.
Despite a review of published data and a systematic review commissioned by the task force, “We didn’t come up with much scientific evidence to say we should be changing the historic standard,” Dr. Merke said.
The systematic review identified only five studies of high-dose corticotropin testing specifically in PAI and none of low-dose testing. The low-dose test has shown higher sensitivity in the detection of adrenal insufficiency in critically ill patients and secondary adrenal insufficiency, but the limited available data suggest it does not provide better diagnostic accuracy for PAI than the high-dose test.
As a result, the guidelines recommend the standard, short corticotropin test (250 mcg for adults and children aged at least 2 years) as the “gold standard” diagnostic test to establish a PAI diagnosis.
The low-dose (1 mcg) test is recommended only when corticotropin is in short supply, which is not typically a problem in the United States, she said.
If corticotropin testing isn’t feasible, a combination of a morning plasma ACTH and cortisol levels (less than 5 mcg/dL) can be used as an initial screening, though confirmatory testing with corticotropin stimulation is strongly recommended.
Glucocorticoid therapy is recommended in all patients with confirmed PAI based on the highest quality of evidence, with a clear preference given for the short-acting steroids, Dr. Merke observed.
Hydrocortisone 15 mg-25 mg or cortisone acetate 20 mg-35 mg given in two to three divided doses per day is suggested for adults, with the highest dose to be given in the morning. Once- or twice-daily prednisolone 3 mg-5 mg is suggested as an alternative.
Hydrocortisone is also suggested over cortisone acetate, prednisolone, or prednisone for pregnant women and recommended for children (about 8 mg/m2 per day), but the evidence supporting these items was of low quality.
The guidelines suggest against using dexamethasone, the longest-acting glucocorticoid, because of the potential long-term side effects of overt-treatment and the frequent appearance of cushingoid side effects. They also recommend against dexamethasone in pregnant women because it is not inactivated in the placenta.
The guidelines are also quite clear in their suggestion against hormonal monitoring of glucocorticoid replacement and instead favor adjusting treatment based only on clinical response.
“This is a very important suggestion that we made because often clinicians use ACTH to adjust doses and this commonly results in overreplacement and there are side effects to overreplacement,” including weight gain, insomnia, and peripheral edema, Dr. Merke said.
A second systematic review commissioned by the task force involving 15 observational studies of glucocorticoid replacement regimens uncovered very sparse data on mortality, bone density, and incidence of adrenal crisis.
It has been suggested that newer extended-release and dual-release glucocorticoid formulations may result in higher health-reality quality of life than once-, twice-, or thrice-daily regimens, but once again, the evidence was insufficient to support a specific recommendation.
Dr. Merke acknowledged that many of the guidelines recommendations were ungraded or best practices, reflecting the lack of randomized clinical trials in PAI.
“I think that’s why it was so important for us to do this,” she said. “We had a group of experts that were very familiar with this disease providing guidance, but I think it’s also one reason why physicians out there in practice get confused about exactly what to do because of the lack of hard evidence. ... It does certainly cry for the need for more studies in these rare diseases.”
The guidelines were funded by the Endocrine Society, and the authors reported receiving no external funding or remuneration.
New guidelines on the diagnosis and management of primary adrenal insufficiency stress the importance of early recognition and the need to prevent life-threatening adrenal crises in these patients.
These are the first clinical practice guidelines on primary adrenal insufficiency (PAI), also known as Addison’s disease, issued by Endocrine Society (J Clin Endocrinol Metab. 2016 Jan 13:jc20151710 [Epub ahead of print]).
“Because it’s a rare disease and symptoms can mimic common conditions, adrenal insufficiency is often, at least initially, overlooked,” guideline co-author Dr. Deborah Merke, a senior investigator with the National Institutes of Health Clinical Center in Bethesda, Md., said. “So the main goal of these clinical practice guidelines is to improve patient care.”
The guidelines suggest clinicians should have a low diagnostic threshold in acutely ill patients with unexplained symptoms or signs suggestive of PAI such as volume depletion, hypotension, hyponatremia, hyperkalemia, fever, abdominal pain, hyperpigmentation, or, especially in children, hypoglycemia.
This low diagnostic threshold for PAI should also be extended to pregnant women with unexplained persistent nausea, fatigue, and hypotension.
For adult patients with a suspected adrenal crisis, an immediate parenteral injection of hydrocortisone 100 mg should be given, followed by appropriate fluid resuscitation and 200 mg of hydrocortisone for 24 hours, according to the guidelines, which were co-sponsored by the European Society of Endocrinology and American Association for Clinical Chemistry.
Despite a known association between adrenal crisis and mortality, there is a knowledge gap regarding how to prevent, recognize, and reduce the risk of these life-threatening events, Dr. Merke said.
To that end, the task force has taken a page from the diabetes community in recommending all PAI patients carry steroid emergency identification cards and be equipped with a glucocorticoid injection kit for emergency use and be educated on how to use it.
The guidelines also advocate education about stress dosing to counter the increased demand for corticosteroids during periods of stress, which can encompass something as common as the flu.
“Just like diabetics carry around emergency medicines, it’s important for patients with adrenal insufficiency to carry around an emergency kit and to realize that should they start to get sick, they need to increase their doses,” she said. “There often seems to be a lack of awareness among physicians as well that these patients have a potentially life-threatening condition, should they get a common illness.”
One of the key unanswered clinical questions the task force sought to address was whether the widely used high-dose (250 mcg) corticotropin stimulation test, also known as the adrenocorticotropin (ACTH) or short Synacthen test, should be replaced by the low-dose test (1 mcg) to diagnosis PAI.
Despite a review of published data and a systematic review commissioned by the task force, “We didn’t come up with much scientific evidence to say we should be changing the historic standard,” Dr. Merke said.
The systematic review identified only five studies of high-dose corticotropin testing specifically in PAI and none of low-dose testing. The low-dose test has shown higher sensitivity in the detection of adrenal insufficiency in critically ill patients and secondary adrenal insufficiency, but the limited available data suggest it does not provide better diagnostic accuracy for PAI than the high-dose test.
As a result, the guidelines recommend the standard, short corticotropin test (250 mcg for adults and children aged at least 2 years) as the “gold standard” diagnostic test to establish a PAI diagnosis.
The low-dose (1 mcg) test is recommended only when corticotropin is in short supply, which is not typically a problem in the United States, she said.
If corticotropin testing isn’t feasible, a combination of a morning plasma ACTH and cortisol levels (less than 5 mcg/dL) can be used as an initial screening, though confirmatory testing with corticotropin stimulation is strongly recommended.
Glucocorticoid therapy is recommended in all patients with confirmed PAI based on the highest quality of evidence, with a clear preference given for the short-acting steroids, Dr. Merke observed.
Hydrocortisone 15 mg-25 mg or cortisone acetate 20 mg-35 mg given in two to three divided doses per day is suggested for adults, with the highest dose to be given in the morning. Once- or twice-daily prednisolone 3 mg-5 mg is suggested as an alternative.
Hydrocortisone is also suggested over cortisone acetate, prednisolone, or prednisone for pregnant women and recommended for children (about 8 mg/m2 per day), but the evidence supporting these items was of low quality.
The guidelines suggest against using dexamethasone, the longest-acting glucocorticoid, because of the potential long-term side effects of overt-treatment and the frequent appearance of cushingoid side effects. They also recommend against dexamethasone in pregnant women because it is not inactivated in the placenta.
The guidelines are also quite clear in their suggestion against hormonal monitoring of glucocorticoid replacement and instead favor adjusting treatment based only on clinical response.
“This is a very important suggestion that we made because often clinicians use ACTH to adjust doses and this commonly results in overreplacement and there are side effects to overreplacement,” including weight gain, insomnia, and peripheral edema, Dr. Merke said.
A second systematic review commissioned by the task force involving 15 observational studies of glucocorticoid replacement regimens uncovered very sparse data on mortality, bone density, and incidence of adrenal crisis.
It has been suggested that newer extended-release and dual-release glucocorticoid formulations may result in higher health-reality quality of life than once-, twice-, or thrice-daily regimens, but once again, the evidence was insufficient to support a specific recommendation.
Dr. Merke acknowledged that many of the guidelines recommendations were ungraded or best practices, reflecting the lack of randomized clinical trials in PAI.
“I think that’s why it was so important for us to do this,” she said. “We had a group of experts that were very familiar with this disease providing guidance, but I think it’s also one reason why physicians out there in practice get confused about exactly what to do because of the lack of hard evidence. ... It does certainly cry for the need for more studies in these rare diseases.”
The guidelines were funded by the Endocrine Society, and the authors reported receiving no external funding or remuneration.
FROM THE JOURNAL OF CLINICAL ENDOCRINOLOGY & METABOLISM
Making sense of the expanded myeloma treatment landscape
ORLANDO – The moment the Food and Drug Administration approved daratumumab, ixazomib, and elotuzumab in rapid-fire succession over 15 days in November 2015, Dr. S. Vincent Rajkumar’s phone started ringing.
As with other multiple myeloma experts, three common questions kept cropping up:
• For previously untreated patients, should we add bortezomib to lenalidomide plus dexamethasone (Rd) based on the S0777 results?
• For previously treated patients, should we add ixazomib or elotuzumab to Rd?
• Should we add daratumumab to frontline therapy right out of the box?
Daratumumab (Darzalex), ixazomib (Ninlaro), and elotuzumab (Empliciti) are welcome additions to the armamentarium, but the problem with this plethora of riches is that numerous treatments already exist for frontline multiple myeloma, observed Dr. Rajkumar, professor of medicine at the Mayo Clinic in Rochester, Minn.
In fact, the National Comprehensive Cancer Network guidelines list 22 possible newly diagnosed myeloma regimens that can be potentially recommended for patients.
“This definitely leads to confusion in the community. And this was the result of the fact that we didn’t have a single, good randomized trial with a survival benefit of a modern therapy against another modern therapy,” Dr. Rajkumar said at the annual meeting of the American Society of Hematology during a joint FDA/ASH symposium on the three newly approved agents.
This quandary was solved at ASH with phase III randomized data from the Southwest Oncology Group S0777 study showing a significant overall survival advantage with a triplet of bortezomib (Velcade), lenalidomide (Revlimid), and dexamethasone (VRd) followed by continuous Rd maintenance compared with Rd alone and ongoing maintenance in untreated patients who did not intend to receive stem cell transplant, he said.
Median overall survival was 75 months for the triplet vs. 64 months for the Rd doublet (hazard ratio, 0.709; two-sided log-rank P = .0250), and median PFS 43 months vs. 30 months (HR, 0.712; one-sided P = .0018), study author Dr. Brian Durie, of Cedars-Sinai Comprehensive Cancer Center in Los Angeles, reported (Abstract 25).
The VRd triplet is already in use in the United States, but based on the S0777 results, many groups, including the Mayo Clinic, have changed treatment guidelines and now “prefer bortezomib, len-dex for frontline therapy, not just in transplant candidates, but also in non-transplant candidates,” Dr. Rajkumar said.
In countries where VRd is not possible, bortezomib, thalidomide, and dexamethasone (VTd) is a second option.
Rd is an appropriate therapy for non-transplant candidates who are frail or aged 75 years or older, he said, adding that there is no need to add bortezomib for patients already on Rd and doing well.
“If your patient is doing well on a doublet, leave them alone,” Dr. Rajkumar advised.
Similarly, for patients with relapsed myeloma who are doing well on Rd, there isn’t “an urgent need” to add ixazomib or elotuzumab, but rather, he said, “We can reserve those for when the patient progresses.”
Ixazomib is approved in combination with Rd after at least one prior therapy, but the oral proteasome inhibitor may have a role in the frontline treatment of standard-risk patients. It is a very simple regimen, just three pills a month, and “the side effect profile is outstanding; virtually difficult to tell who’s taking placebo and who’s taking drug,” Dr. Rajkumar observed.
In addition, some patients may not have access to bortezomib because of insurance reasons or can’t drive to the clinic once a week to get the shot, while others may be too frail to get an intravenous or subcutaneous shot or may have neuropathy.
“For whatever reason, I think it is reasonable to keep in mind that we may have a situation where we can use ixazomib/len-dex in clinical practice if the patient’s best interests so dictate,” he said.
For high-risk patients (deletion 17p or translocations t(4;14), t(14;16), t(14;20), VRd or VTd are obvious upfront choices. Based on four phase II trials and the ASPIRE results in the relapsed and refractory setting, however, the Mayo Clinic has already decided that the recently approved second-generation proteasome inhibitor carfilzomib (Kyprolis) plus Rd is also worth considering.
Adding a monoclonal antibody such as elotuzumab or daratumumab to a VRd triplet or ixazomib, lenalidomide, and dexamethasone (IRd) triplet may be another way to improve outcomes in high-risk patients, who still die with a median overall survival of 3 years, Dr. Rajkumar said. This strategy is already being used in the ongoing SWOG S1211 study.
For maintenance therapy after VRd or VTd and autologous stem cell transplant, he recommended lenalidomide for standard-risk patients and bortezomib-based maintenance for high-risk patients, but said ixazomib-based maintenance with the addition of monoclonal antibodies may also have a role in high-risk patients.
What may be more important going forward is how these three drugs will be used in clinical trials, Dr. Rajkumar observed.
“We’d rather put all patients on clinical trials than any of the recommendations I made,” he said. “The problem is that clinical trials have to be appropriately designed.”
Several phase III trials are already ongoing comparing a doublet versus a triplet (IRd vs. Rd, elotuzumab-Rd vs. Rd, and daratumumab-Rd vs. Rd) in the frontline setting, so the key question for future trials is which triplet: VRd, KRd, elotuzumab-Rd, or daratumumab-Rd, and to what endpoint.
Progression-free survival can remain a primary endpoint for comparing two triplets in the frontline, but PFS alone is not enough in the maintenance setting and investigators should look to other primary endpoints such as PFS2, PFS1 vs. PFS2, overall survival with a higher type 1 error than currently used, or PFS plus validated patient-reported or quality of life outcomes, Dr. Rajkumar said.
Relapsed/refractory disease
Speaking on how the three new agents fit into the relapsed or refractory space,Dr. Paul Richardson, of Dana-Farber Cancer Institute, Boston, said three-drug platforms are emerging as a standard of care for relapsed or refractory disease after studies have shown time and time again they are better than doublets.
He highlighted phase III data reported at ASH by Dr. Philippe Moreau from TOURMALINE-MM1 (Abstract 727) showing a 35% improvement in PFS with weekly oral ixazomib plus lenalidomide-dexamethasone vs. Rd alone in relapsed and/or refractory multiple myeloma.
This translated into a median 6-month gain in PFS compared with an almost 9-month PFS benefit seen in ASPIRE with carfilzomib plus Rd, but cross-trial comparisons should be approached with some caution and both hazard ratios were very robust, he said. In addition, as previously observed, ixazomib is remarkably well tolerated.
“I think ixazomib, particularly in older patients and particularly in patients with high-risk disease, will be very useful in the context of the three-drug or even greater combinations. So there’s a strong rationale for its use,” Dr. Richardson said.
He went on to say that elotuzumab has shown remarkable anti-myeloma activity in the relapsed and refractory setting, improving both the overall response rate and PFS when used in combination with Ld vs. Ld alone in the ELOQUENT-2 trial. Updated results from ELOQUENT-2 were presented at the ASH meeting (Abstract 28).
A PFS benefit was also seen when elotuzumab was added to bortezomib and dexamethasone, with a 24% reduction in the risk of disease progression or death reported in a study presented at ASH by myeloma expert Dr. Antonio Palumbo (Abstract 510).
“My point in showing this is that when you think of elotuzumab being used with lenalidomide and dexamethasone in relapse, many of our patients are actually on them as maintenance when it occurs, therefore elotuzumab may have a role in combination, for example, with proteasome inhibitors in this same setting,” Dr. Richardson said.
Several pomalidomide-based triple therapy combinations have been evaluated in advanced relapsed or refractory myeloma, with a phase II study (Abstract 506) reported that morning at ASH showing the third-generation immunomodulatory drug (IMiD) pomalidomide induced responses in 60% of heavily pretreated patients when partnered with pembrolizumab and dexamethasone.
Combination strategies with daratumumab are also very provocative, particularly in the context of IMiDs, he noted. A phase Ib study reported in the same early morning session by Dr. Ajai Chari (Abstract 508) had a “very encouraging” overall response rate of 71% with daratumumab plus pomalidomide and dexamethasone in heavily pretreated patients, including 43% very good partial responses or better, and an overall response rate of 67% among double-refractory patients.
“Daratumumab and elotuzumab, in my view, as first-in-class monoclonal antibodies, are paradigm-changing agents,” Dr. Richardson concluded. “They provide us with this mutation-driven ability to overdrive the impact of those mutations and the important point is that they prescribe an entirely non-crossresistant strategy that can be easily added to existing platforms of drugs.”
Dr. Rajkumar reported discussion of off-label drug use for elotuzumab, daratumumab, ixazomib, and carfilzomib in untreated myeloma, maintenance, and early relapse. Dr. Richardson reported membership on a board of directors or advisory committee for Millennium Takeda, Celgene, Janssen, Bristol-Myers Squibb, and Novartis, and research funding from Millennium Takeda and Celgene.
ORLANDO – The moment the Food and Drug Administration approved daratumumab, ixazomib, and elotuzumab in rapid-fire succession over 15 days in November 2015, Dr. S. Vincent Rajkumar’s phone started ringing.
As with other multiple myeloma experts, three common questions kept cropping up:
• For previously untreated patients, should we add bortezomib to lenalidomide plus dexamethasone (Rd) based on the S0777 results?
• For previously treated patients, should we add ixazomib or elotuzumab to Rd?
• Should we add daratumumab to frontline therapy right out of the box?
Daratumumab (Darzalex), ixazomib (Ninlaro), and elotuzumab (Empliciti) are welcome additions to the armamentarium, but the problem with this plethora of riches is that numerous treatments already exist for frontline multiple myeloma, observed Dr. Rajkumar, professor of medicine at the Mayo Clinic in Rochester, Minn.
In fact, the National Comprehensive Cancer Network guidelines list 22 possible newly diagnosed myeloma regimens that can be potentially recommended for patients.
“This definitely leads to confusion in the community. And this was the result of the fact that we didn’t have a single, good randomized trial with a survival benefit of a modern therapy against another modern therapy,” Dr. Rajkumar said at the annual meeting of the American Society of Hematology during a joint FDA/ASH symposium on the three newly approved agents.
This quandary was solved at ASH with phase III randomized data from the Southwest Oncology Group S0777 study showing a significant overall survival advantage with a triplet of bortezomib (Velcade), lenalidomide (Revlimid), and dexamethasone (VRd) followed by continuous Rd maintenance compared with Rd alone and ongoing maintenance in untreated patients who did not intend to receive stem cell transplant, he said.
Median overall survival was 75 months for the triplet vs. 64 months for the Rd doublet (hazard ratio, 0.709; two-sided log-rank P = .0250), and median PFS 43 months vs. 30 months (HR, 0.712; one-sided P = .0018), study author Dr. Brian Durie, of Cedars-Sinai Comprehensive Cancer Center in Los Angeles, reported (Abstract 25).
The VRd triplet is already in use in the United States, but based on the S0777 results, many groups, including the Mayo Clinic, have changed treatment guidelines and now “prefer bortezomib, len-dex for frontline therapy, not just in transplant candidates, but also in non-transplant candidates,” Dr. Rajkumar said.
In countries where VRd is not possible, bortezomib, thalidomide, and dexamethasone (VTd) is a second option.
Rd is an appropriate therapy for non-transplant candidates who are frail or aged 75 years or older, he said, adding that there is no need to add bortezomib for patients already on Rd and doing well.
“If your patient is doing well on a doublet, leave them alone,” Dr. Rajkumar advised.
Similarly, for patients with relapsed myeloma who are doing well on Rd, there isn’t “an urgent need” to add ixazomib or elotuzumab, but rather, he said, “We can reserve those for when the patient progresses.”
Ixazomib is approved in combination with Rd after at least one prior therapy, but the oral proteasome inhibitor may have a role in the frontline treatment of standard-risk patients. It is a very simple regimen, just three pills a month, and “the side effect profile is outstanding; virtually difficult to tell who’s taking placebo and who’s taking drug,” Dr. Rajkumar observed.
In addition, some patients may not have access to bortezomib because of insurance reasons or can’t drive to the clinic once a week to get the shot, while others may be too frail to get an intravenous or subcutaneous shot or may have neuropathy.
“For whatever reason, I think it is reasonable to keep in mind that we may have a situation where we can use ixazomib/len-dex in clinical practice if the patient’s best interests so dictate,” he said.
For high-risk patients (deletion 17p or translocations t(4;14), t(14;16), t(14;20), VRd or VTd are obvious upfront choices. Based on four phase II trials and the ASPIRE results in the relapsed and refractory setting, however, the Mayo Clinic has already decided that the recently approved second-generation proteasome inhibitor carfilzomib (Kyprolis) plus Rd is also worth considering.
Adding a monoclonal antibody such as elotuzumab or daratumumab to a VRd triplet or ixazomib, lenalidomide, and dexamethasone (IRd) triplet may be another way to improve outcomes in high-risk patients, who still die with a median overall survival of 3 years, Dr. Rajkumar said. This strategy is already being used in the ongoing SWOG S1211 study.
For maintenance therapy after VRd or VTd and autologous stem cell transplant, he recommended lenalidomide for standard-risk patients and bortezomib-based maintenance for high-risk patients, but said ixazomib-based maintenance with the addition of monoclonal antibodies may also have a role in high-risk patients.
What may be more important going forward is how these three drugs will be used in clinical trials, Dr. Rajkumar observed.
“We’d rather put all patients on clinical trials than any of the recommendations I made,” he said. “The problem is that clinical trials have to be appropriately designed.”
Several phase III trials are already ongoing comparing a doublet versus a triplet (IRd vs. Rd, elotuzumab-Rd vs. Rd, and daratumumab-Rd vs. Rd) in the frontline setting, so the key question for future trials is which triplet: VRd, KRd, elotuzumab-Rd, or daratumumab-Rd, and to what endpoint.
Progression-free survival can remain a primary endpoint for comparing two triplets in the frontline, but PFS alone is not enough in the maintenance setting and investigators should look to other primary endpoints such as PFS2, PFS1 vs. PFS2, overall survival with a higher type 1 error than currently used, or PFS plus validated patient-reported or quality of life outcomes, Dr. Rajkumar said.
Relapsed/refractory disease
Speaking on how the three new agents fit into the relapsed or refractory space,Dr. Paul Richardson, of Dana-Farber Cancer Institute, Boston, said three-drug platforms are emerging as a standard of care for relapsed or refractory disease after studies have shown time and time again they are better than doublets.
He highlighted phase III data reported at ASH by Dr. Philippe Moreau from TOURMALINE-MM1 (Abstract 727) showing a 35% improvement in PFS with weekly oral ixazomib plus lenalidomide-dexamethasone vs. Rd alone in relapsed and/or refractory multiple myeloma.
This translated into a median 6-month gain in PFS compared with an almost 9-month PFS benefit seen in ASPIRE with carfilzomib plus Rd, but cross-trial comparisons should be approached with some caution and both hazard ratios were very robust, he said. In addition, as previously observed, ixazomib is remarkably well tolerated.
“I think ixazomib, particularly in older patients and particularly in patients with high-risk disease, will be very useful in the context of the three-drug or even greater combinations. So there’s a strong rationale for its use,” Dr. Richardson said.
He went on to say that elotuzumab has shown remarkable anti-myeloma activity in the relapsed and refractory setting, improving both the overall response rate and PFS when used in combination with Ld vs. Ld alone in the ELOQUENT-2 trial. Updated results from ELOQUENT-2 were presented at the ASH meeting (Abstract 28).
A PFS benefit was also seen when elotuzumab was added to bortezomib and dexamethasone, with a 24% reduction in the risk of disease progression or death reported in a study presented at ASH by myeloma expert Dr. Antonio Palumbo (Abstract 510).
“My point in showing this is that when you think of elotuzumab being used with lenalidomide and dexamethasone in relapse, many of our patients are actually on them as maintenance when it occurs, therefore elotuzumab may have a role in combination, for example, with proteasome inhibitors in this same setting,” Dr. Richardson said.
Several pomalidomide-based triple therapy combinations have been evaluated in advanced relapsed or refractory myeloma, with a phase II study (Abstract 506) reported that morning at ASH showing the third-generation immunomodulatory drug (IMiD) pomalidomide induced responses in 60% of heavily pretreated patients when partnered with pembrolizumab and dexamethasone.
Combination strategies with daratumumab are also very provocative, particularly in the context of IMiDs, he noted. A phase Ib study reported in the same early morning session by Dr. Ajai Chari (Abstract 508) had a “very encouraging” overall response rate of 71% with daratumumab plus pomalidomide and dexamethasone in heavily pretreated patients, including 43% very good partial responses or better, and an overall response rate of 67% among double-refractory patients.
“Daratumumab and elotuzumab, in my view, as first-in-class monoclonal antibodies, are paradigm-changing agents,” Dr. Richardson concluded. “They provide us with this mutation-driven ability to overdrive the impact of those mutations and the important point is that they prescribe an entirely non-crossresistant strategy that can be easily added to existing platforms of drugs.”
Dr. Rajkumar reported discussion of off-label drug use for elotuzumab, daratumumab, ixazomib, and carfilzomib in untreated myeloma, maintenance, and early relapse. Dr. Richardson reported membership on a board of directors or advisory committee for Millennium Takeda, Celgene, Janssen, Bristol-Myers Squibb, and Novartis, and research funding from Millennium Takeda and Celgene.
ORLANDO – The moment the Food and Drug Administration approved daratumumab, ixazomib, and elotuzumab in rapid-fire succession over 15 days in November 2015, Dr. S. Vincent Rajkumar’s phone started ringing.
As with other multiple myeloma experts, three common questions kept cropping up:
• For previously untreated patients, should we add bortezomib to lenalidomide plus dexamethasone (Rd) based on the S0777 results?
• For previously treated patients, should we add ixazomib or elotuzumab to Rd?
• Should we add daratumumab to frontline therapy right out of the box?
Daratumumab (Darzalex), ixazomib (Ninlaro), and elotuzumab (Empliciti) are welcome additions to the armamentarium, but the problem with this plethora of riches is that numerous treatments already exist for frontline multiple myeloma, observed Dr. Rajkumar, professor of medicine at the Mayo Clinic in Rochester, Minn.
In fact, the National Comprehensive Cancer Network guidelines list 22 possible newly diagnosed myeloma regimens that can be potentially recommended for patients.
“This definitely leads to confusion in the community. And this was the result of the fact that we didn’t have a single, good randomized trial with a survival benefit of a modern therapy against another modern therapy,” Dr. Rajkumar said at the annual meeting of the American Society of Hematology during a joint FDA/ASH symposium on the three newly approved agents.
This quandary was solved at ASH with phase III randomized data from the Southwest Oncology Group S0777 study showing a significant overall survival advantage with a triplet of bortezomib (Velcade), lenalidomide (Revlimid), and dexamethasone (VRd) followed by continuous Rd maintenance compared with Rd alone and ongoing maintenance in untreated patients who did not intend to receive stem cell transplant, he said.
Median overall survival was 75 months for the triplet vs. 64 months for the Rd doublet (hazard ratio, 0.709; two-sided log-rank P = .0250), and median PFS 43 months vs. 30 months (HR, 0.712; one-sided P = .0018), study author Dr. Brian Durie, of Cedars-Sinai Comprehensive Cancer Center in Los Angeles, reported (Abstract 25).
The VRd triplet is already in use in the United States, but based on the S0777 results, many groups, including the Mayo Clinic, have changed treatment guidelines and now “prefer bortezomib, len-dex for frontline therapy, not just in transplant candidates, but also in non-transplant candidates,” Dr. Rajkumar said.
In countries where VRd is not possible, bortezomib, thalidomide, and dexamethasone (VTd) is a second option.
Rd is an appropriate therapy for non-transplant candidates who are frail or aged 75 years or older, he said, adding that there is no need to add bortezomib for patients already on Rd and doing well.
“If your patient is doing well on a doublet, leave them alone,” Dr. Rajkumar advised.
Similarly, for patients with relapsed myeloma who are doing well on Rd, there isn’t “an urgent need” to add ixazomib or elotuzumab, but rather, he said, “We can reserve those for when the patient progresses.”
Ixazomib is approved in combination with Rd after at least one prior therapy, but the oral proteasome inhibitor may have a role in the frontline treatment of standard-risk patients. It is a very simple regimen, just three pills a month, and “the side effect profile is outstanding; virtually difficult to tell who’s taking placebo and who’s taking drug,” Dr. Rajkumar observed.
In addition, some patients may not have access to bortezomib because of insurance reasons or can’t drive to the clinic once a week to get the shot, while others may be too frail to get an intravenous or subcutaneous shot or may have neuropathy.
“For whatever reason, I think it is reasonable to keep in mind that we may have a situation where we can use ixazomib/len-dex in clinical practice if the patient’s best interests so dictate,” he said.
For high-risk patients (deletion 17p or translocations t(4;14), t(14;16), t(14;20), VRd or VTd are obvious upfront choices. Based on four phase II trials and the ASPIRE results in the relapsed and refractory setting, however, the Mayo Clinic has already decided that the recently approved second-generation proteasome inhibitor carfilzomib (Kyprolis) plus Rd is also worth considering.
Adding a monoclonal antibody such as elotuzumab or daratumumab to a VRd triplet or ixazomib, lenalidomide, and dexamethasone (IRd) triplet may be another way to improve outcomes in high-risk patients, who still die with a median overall survival of 3 years, Dr. Rajkumar said. This strategy is already being used in the ongoing SWOG S1211 study.
For maintenance therapy after VRd or VTd and autologous stem cell transplant, he recommended lenalidomide for standard-risk patients and bortezomib-based maintenance for high-risk patients, but said ixazomib-based maintenance with the addition of monoclonal antibodies may also have a role in high-risk patients.
What may be more important going forward is how these three drugs will be used in clinical trials, Dr. Rajkumar observed.
“We’d rather put all patients on clinical trials than any of the recommendations I made,” he said. “The problem is that clinical trials have to be appropriately designed.”
Several phase III trials are already ongoing comparing a doublet versus a triplet (IRd vs. Rd, elotuzumab-Rd vs. Rd, and daratumumab-Rd vs. Rd) in the frontline setting, so the key question for future trials is which triplet: VRd, KRd, elotuzumab-Rd, or daratumumab-Rd, and to what endpoint.
Progression-free survival can remain a primary endpoint for comparing two triplets in the frontline, but PFS alone is not enough in the maintenance setting and investigators should look to other primary endpoints such as PFS2, PFS1 vs. PFS2, overall survival with a higher type 1 error than currently used, or PFS plus validated patient-reported or quality of life outcomes, Dr. Rajkumar said.
Relapsed/refractory disease
Speaking on how the three new agents fit into the relapsed or refractory space,Dr. Paul Richardson, of Dana-Farber Cancer Institute, Boston, said three-drug platforms are emerging as a standard of care for relapsed or refractory disease after studies have shown time and time again they are better than doublets.
He highlighted phase III data reported at ASH by Dr. Philippe Moreau from TOURMALINE-MM1 (Abstract 727) showing a 35% improvement in PFS with weekly oral ixazomib plus lenalidomide-dexamethasone vs. Rd alone in relapsed and/or refractory multiple myeloma.
This translated into a median 6-month gain in PFS compared with an almost 9-month PFS benefit seen in ASPIRE with carfilzomib plus Rd, but cross-trial comparisons should be approached with some caution and both hazard ratios were very robust, he said. In addition, as previously observed, ixazomib is remarkably well tolerated.
“I think ixazomib, particularly in older patients and particularly in patients with high-risk disease, will be very useful in the context of the three-drug or even greater combinations. So there’s a strong rationale for its use,” Dr. Richardson said.
He went on to say that elotuzumab has shown remarkable anti-myeloma activity in the relapsed and refractory setting, improving both the overall response rate and PFS when used in combination with Ld vs. Ld alone in the ELOQUENT-2 trial. Updated results from ELOQUENT-2 were presented at the ASH meeting (Abstract 28).
A PFS benefit was also seen when elotuzumab was added to bortezomib and dexamethasone, with a 24% reduction in the risk of disease progression or death reported in a study presented at ASH by myeloma expert Dr. Antonio Palumbo (Abstract 510).
“My point in showing this is that when you think of elotuzumab being used with lenalidomide and dexamethasone in relapse, many of our patients are actually on them as maintenance when it occurs, therefore elotuzumab may have a role in combination, for example, with proteasome inhibitors in this same setting,” Dr. Richardson said.
Several pomalidomide-based triple therapy combinations have been evaluated in advanced relapsed or refractory myeloma, with a phase II study (Abstract 506) reported that morning at ASH showing the third-generation immunomodulatory drug (IMiD) pomalidomide induced responses in 60% of heavily pretreated patients when partnered with pembrolizumab and dexamethasone.
Combination strategies with daratumumab are also very provocative, particularly in the context of IMiDs, he noted. A phase Ib study reported in the same early morning session by Dr. Ajai Chari (Abstract 508) had a “very encouraging” overall response rate of 71% with daratumumab plus pomalidomide and dexamethasone in heavily pretreated patients, including 43% very good partial responses or better, and an overall response rate of 67% among double-refractory patients.
“Daratumumab and elotuzumab, in my view, as first-in-class monoclonal antibodies, are paradigm-changing agents,” Dr. Richardson concluded. “They provide us with this mutation-driven ability to overdrive the impact of those mutations and the important point is that they prescribe an entirely non-crossresistant strategy that can be easily added to existing platforms of drugs.”
Dr. Rajkumar reported discussion of off-label drug use for elotuzumab, daratumumab, ixazomib, and carfilzomib in untreated myeloma, maintenance, and early relapse. Dr. Richardson reported membership on a board of directors or advisory committee for Millennium Takeda, Celgene, Janssen, Bristol-Myers Squibb, and Novartis, and research funding from Millennium Takeda and Celgene.
EXPERT ANALYSIS FROM ASH 2015
Cancer prevention field riding high into the new year
The new year has us all looking forward and the cancer prevention community is no exception.
In a special report entitled “Transforming Cancer Prevention through Precision Medicine and Immune-Oncology,” a team of experts offer a brief look at what we can expect in the near future for cancer prevention research, including a Pre-Cancer Genome Atlas (PCGA), and highlight some of the recent advances shaping their optimism.
“Just as precision therapy and immunotherapy are transforming cancer treatment, precision medicine and immunoprevention approaches are being translated to the clinic and showing great promise. We stand at the edge of a new frontier that will include comprehensively characterizing the molecular and cellular events that drive premalignant progression (e.g. PCGA),” Dr. Scott M. Lippman, director of the University of California San Diego Moores Cancer Center, and his coauthors wrote (Cancer Prev Res. 2016;9:2-10).
The report details some of the clinical firsts in 2015 including genomic studies suggesting that clonal hematopoiesis is a premalignant state for blood cancer, the first precision medicine trial in cancer prevention (EPOC) reporting that loss of heterozygosity can predict which patients with premalignant mouth lesions are most likely to develop oral cancer, and the U.S. Preventive Services Task Force recommending low-dose aspirin for colorectal cancer prevention based on age and risk.
Randomized trials have also suggested that a single dose of human papillomavirus vaccine can provide durable protection against HPV infection. Tumor biology studies established new chemoprevention for familial adenomatous polyposis syndrome and universal tumor screening guidelines based on DNA mismatch repair mutations and microsatellite instability for colorectal cancer in patients with Lynch syndrome.
Further, remarkable advances have been made in liquid biopsy technology, high-throughput functional screening, and computational biology methods and algorithms that “provide unprecedented opportunities to interrogate the biology of premalignancy...” they noted.
In an American Association for Cancer Research blog post, Dr. Lippman acknowledges that not everyone is the same page when it comes to the underlying principles of cancer prevention.
A “contentious” paper published at the start of 2015 suggested that variations in cancer risk are due to random mutations or what might otherwise be called bad luck. The new year was heralded in by a second paper, however, that came to roughly the opposite conclusion or that most cancers are preventable.
In February, an AACR Cancer Prevention Summit will bring together various stakeholders to discuss the current state of cancer prevention and to identify top priorities and research directions for the field, he noted.
The authors acknowledged grant support from the National Institutes of Health/National Cancer Institute.
The new year has us all looking forward and the cancer prevention community is no exception.
In a special report entitled “Transforming Cancer Prevention through Precision Medicine and Immune-Oncology,” a team of experts offer a brief look at what we can expect in the near future for cancer prevention research, including a Pre-Cancer Genome Atlas (PCGA), and highlight some of the recent advances shaping their optimism.
“Just as precision therapy and immunotherapy are transforming cancer treatment, precision medicine and immunoprevention approaches are being translated to the clinic and showing great promise. We stand at the edge of a new frontier that will include comprehensively characterizing the molecular and cellular events that drive premalignant progression (e.g. PCGA),” Dr. Scott M. Lippman, director of the University of California San Diego Moores Cancer Center, and his coauthors wrote (Cancer Prev Res. 2016;9:2-10).
The report details some of the clinical firsts in 2015 including genomic studies suggesting that clonal hematopoiesis is a premalignant state for blood cancer, the first precision medicine trial in cancer prevention (EPOC) reporting that loss of heterozygosity can predict which patients with premalignant mouth lesions are most likely to develop oral cancer, and the U.S. Preventive Services Task Force recommending low-dose aspirin for colorectal cancer prevention based on age and risk.
Randomized trials have also suggested that a single dose of human papillomavirus vaccine can provide durable protection against HPV infection. Tumor biology studies established new chemoprevention for familial adenomatous polyposis syndrome and universal tumor screening guidelines based on DNA mismatch repair mutations and microsatellite instability for colorectal cancer in patients with Lynch syndrome.
Further, remarkable advances have been made in liquid biopsy technology, high-throughput functional screening, and computational biology methods and algorithms that “provide unprecedented opportunities to interrogate the biology of premalignancy...” they noted.
In an American Association for Cancer Research blog post, Dr. Lippman acknowledges that not everyone is the same page when it comes to the underlying principles of cancer prevention.
A “contentious” paper published at the start of 2015 suggested that variations in cancer risk are due to random mutations or what might otherwise be called bad luck. The new year was heralded in by a second paper, however, that came to roughly the opposite conclusion or that most cancers are preventable.
In February, an AACR Cancer Prevention Summit will bring together various stakeholders to discuss the current state of cancer prevention and to identify top priorities and research directions for the field, he noted.
The authors acknowledged grant support from the National Institutes of Health/National Cancer Institute.
The new year has us all looking forward and the cancer prevention community is no exception.
In a special report entitled “Transforming Cancer Prevention through Precision Medicine and Immune-Oncology,” a team of experts offer a brief look at what we can expect in the near future for cancer prevention research, including a Pre-Cancer Genome Atlas (PCGA), and highlight some of the recent advances shaping their optimism.
“Just as precision therapy and immunotherapy are transforming cancer treatment, precision medicine and immunoprevention approaches are being translated to the clinic and showing great promise. We stand at the edge of a new frontier that will include comprehensively characterizing the molecular and cellular events that drive premalignant progression (e.g. PCGA),” Dr. Scott M. Lippman, director of the University of California San Diego Moores Cancer Center, and his coauthors wrote (Cancer Prev Res. 2016;9:2-10).
The report details some of the clinical firsts in 2015 including genomic studies suggesting that clonal hematopoiesis is a premalignant state for blood cancer, the first precision medicine trial in cancer prevention (EPOC) reporting that loss of heterozygosity can predict which patients with premalignant mouth lesions are most likely to develop oral cancer, and the U.S. Preventive Services Task Force recommending low-dose aspirin for colorectal cancer prevention based on age and risk.
Randomized trials have also suggested that a single dose of human papillomavirus vaccine can provide durable protection against HPV infection. Tumor biology studies established new chemoprevention for familial adenomatous polyposis syndrome and universal tumor screening guidelines based on DNA mismatch repair mutations and microsatellite instability for colorectal cancer in patients with Lynch syndrome.
Further, remarkable advances have been made in liquid biopsy technology, high-throughput functional screening, and computational biology methods and algorithms that “provide unprecedented opportunities to interrogate the biology of premalignancy...” they noted.
In an American Association for Cancer Research blog post, Dr. Lippman acknowledges that not everyone is the same page when it comes to the underlying principles of cancer prevention.
A “contentious” paper published at the start of 2015 suggested that variations in cancer risk are due to random mutations or what might otherwise be called bad luck. The new year was heralded in by a second paper, however, that came to roughly the opposite conclusion or that most cancers are preventable.
In February, an AACR Cancer Prevention Summit will bring together various stakeholders to discuss the current state of cancer prevention and to identify top priorities and research directions for the field, he noted.
The authors acknowledged grant support from the National Institutes of Health/National Cancer Institute.
FROM CANCER PREVENTION RESEARCH