User login
Neurotransmitter-based diagnosis and treatment: A hypothesis (Part 3)
Optimal diagnosis and treatment of psychiatric illness requires clinicians to be able to connect mental and physical symptoms. Direct brain neurotransmitter testing is presently in its infancy and the science of defining the underlying mechanisms of psychiatric disorders lags behind the obvious clinical needs. We are not yet equipped to clearly recognize which neurotransmitters cause which symptoms. In this article series, we suggest an indirect way of judging neurotransmitter activity by recognizing specific mental and physical symptoms connected by common biology. Here we present hypothetical clinical cases to emphasize a possible way of analyzing symptoms in order to identify underlying pathology and guide more effective treatment. The descriptions we present in this series do not reflect the entire set of symptoms caused by the neurotransmitters we discuss; we created them based on what is presently known (or suspected). Additional research is needed to confirm or disprove the hypothesis we present. We argue that in cases of multiple psychiatric disorders and chronic pain, the development and approval of medications currently is based on an umbrella descriptive diagnoses, and disregards the various underlying causes of such conditions. Similar to how the many types of pneumonias are treated differently depending on the infective agent, we suggested the same possible causative approach to various types of depression and pain.
Part 1 of this series (
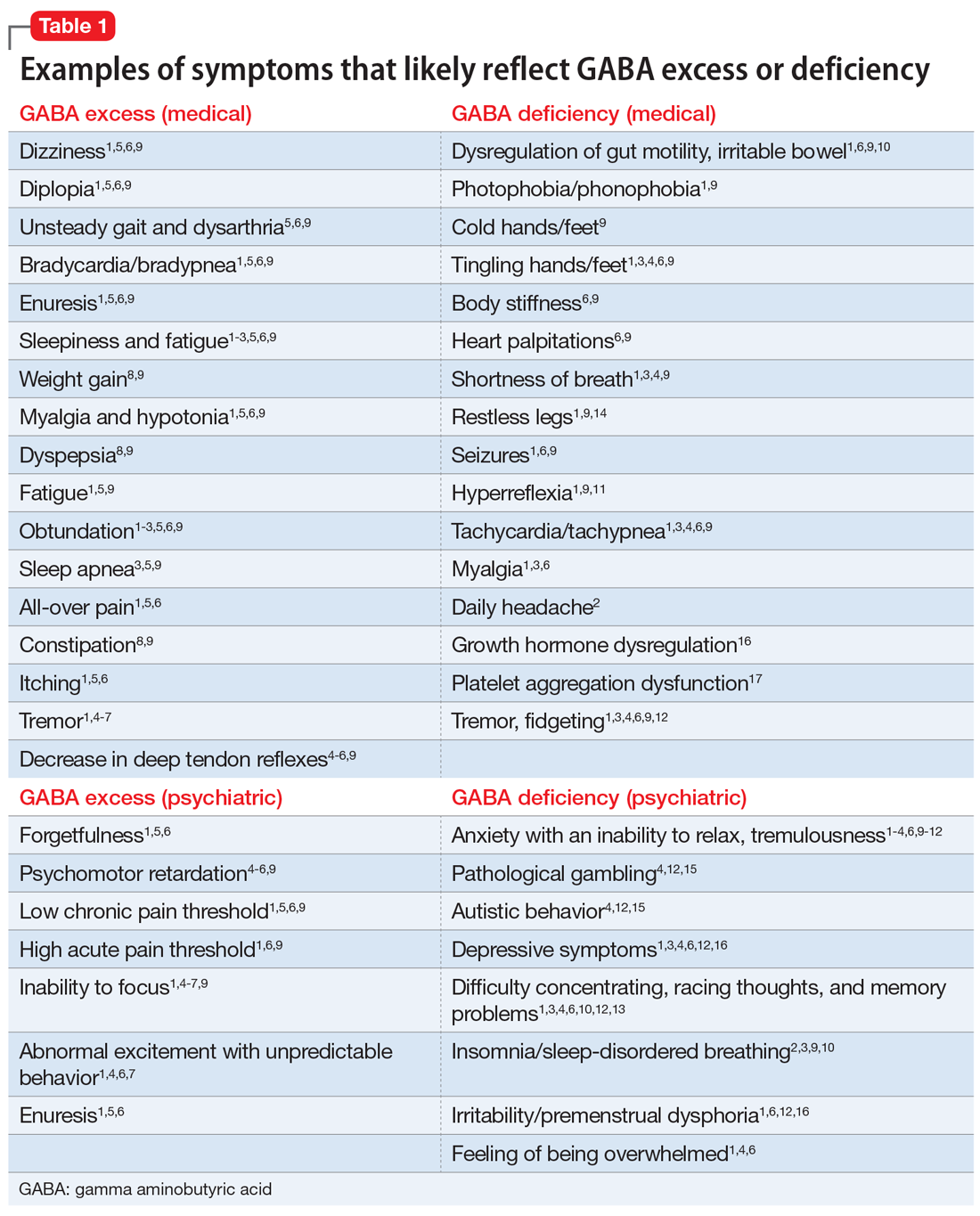
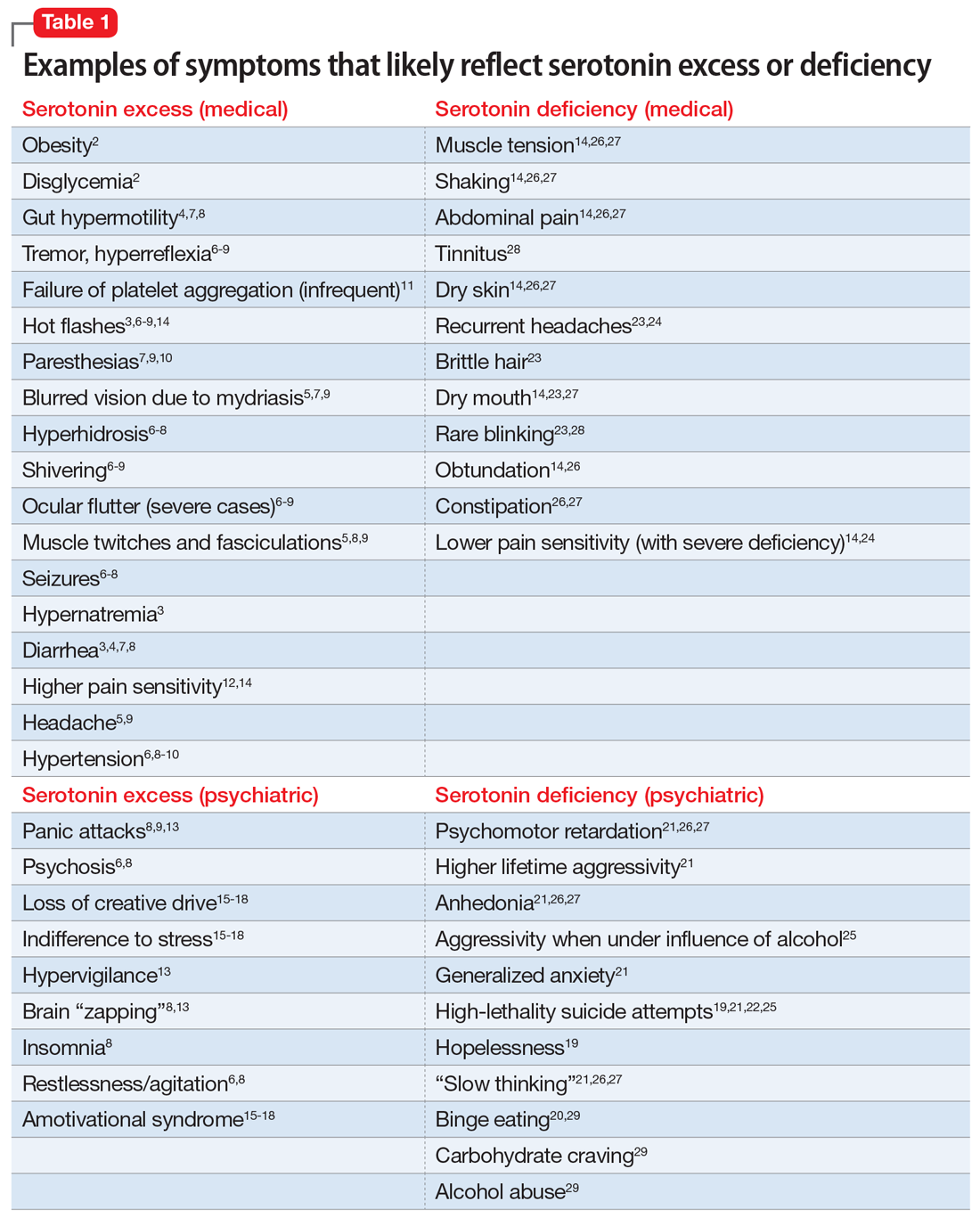
GABA excess (Table 11-9)
Ms. V is brought to your office by a friend. She complains of pain all over her body, itchiness, inability to focus, and dizziness.1,5,6,9 She is puzzled by how little pain she feels when she cuts her finger but by how much pain she is in every day, though her clinicians have not discovered a reason for her pain.1,6,9 She states that her fatigue is so severe that she can sleep 15 hours a day.1-6,9 Her obstructive and central sleep apnea have been treated, but this did not improve her fatigue.3,5,9 She is forgetful and has been diagnosed with depression, though she says she does not feel depressed.1,5,6 Nothing is pleasant to her, but she is prone to abnormal excitement and unpredictable behavior.1,4,6,7
A physical exam shows slow breathing, bradycardia, decreased deep tendon reflexes, and decreased muscle tone.1,5,6,9 Ms. V complains of double vision1,5,6,9 and problems with gait and balance,5,6,9 as well as tremors.1,4-7 She experienced enuresis well into adulthood1,5,6,9 and is prone to weight gain, dyspepsia, and constipation.8,9 She cannot understand people who have anxiety, and is prone to melancholy.4-6,9 Ms. V had been treated with electroconvulsive therapy in the past but states that she “had to have so much electricity, they gave up on me.”
Impression. Ms. V exhibits multiple symptoms associated with GABA excess. Dopaminergic medications such as methylphenidate or amphetamines may be helpful, as they suppress GABA. GABAergic medications and supplements should be avoided in such a patient. Noradrenergic medications including antidepressants with corresponding activity or vasopressors may be beneficial. Suppression of glutamate increases GABA, which is why ketamine in any formulation should be avoided in a patient such as Ms. V.
GABA deficiency (Table 11-4,6,9-17)
Mr. N complains of depression,1,3,4,6,12,16 pain all over his body, tingling in his hands and feet,1,6,9 a constant dull headache,2 and severe insomnia.2,3,9,10 He cannot control his anxiety and, in general, has problems relaxing. In the office, he is jumpy, tremulous, and fidgety during the interview and examination.1,3,4,6,9,12 His muscle tone is high1,9,11 and he feels stiff.6,9 Mr. N’s pupils are narrow1,9; he is hyper-reflexive1,9,11 and reports “Klonopin withdrawal seizures.”1,6,9 He loves alcohol because “it makes me feel good” and helps with his mind, which otherwise “never stops.”1,6,13 Mr. N is frequently anxious and very sensitive to pain, especially when he is upset. He was diagnosed with fibromyalgia by his primary care doctor, who says that irritable bowel is common in patients like him.1,6 His anxiety disables him.1-4,6,9-12 His sister reports that in addition to having difficulty relaxing, Mr. N is easily frustrated and sleeps poorly because he says he has racing thoughts.10 She mentions that her brother’s gambling addiction endangered his finances on several occasions4,12,15 and he was suspected of having autism spectrum disorder.4,12 Mr. N is frequently overwhelmed, including during your interview.1,3,4,6 He is sensitive to light and noise1,9 and complains of palpitations1,3,4,6,9 and frequent shortness of breath.1,3,4,9 He mentions his hands and feet often are cold, especially when he is anxious.1,3,4,6,9 Not eating at regular times makes his symptoms even worse. Mr. N commonly feels depressed, but his anxiety is more bothersome.1,3,4,6,12,16 His ongoing complaints include difficulty concentrating and memory problems,3,4,12,13 as well as a constant feeling of being overwhelmed.1,3,4,6 His restless leg syndrome requires ongoing treatment.1,9,14 Though uncommon, Mr. N has episodes of slowing and weakness, which are associated with growth hormone problems.16 In the past, he experienced gut motility dysregulation9,10 and prolonged bleeding that worried his doctors.17
Impression. Mr. N shows multiple symptoms associated with GABA deficiency. The deficiency of GABA activity ultimately causes an increase in norepinephrine and dopamine firing; therefore, symptoms of GABA deficiency are partially aligned with symptoms of dopamine and norepinephrine excess. GABAergic medications would be most beneficial for such patients. Anticonvulsants (eg, gabapentin and pregabalin) are preferable. Acamprostate may be considered. For long-term use, benzodiapines are as problematic as opioids and should be avoided, if possible. The use of opioids in such patients is especially counterproductive. Some supplements and vitamins may enhance GABA activity. Avoiding bupropion and stimulants would be wise. Ketamine in any formulation would be a good choice in this scenario. Sedating antipsychotic medications have a promise for patients such as Mr. N. The muscle relaxant baclofen frequently helps with these patients’ pain, anxiety, and sleep.
Continue to: Glutamate excess
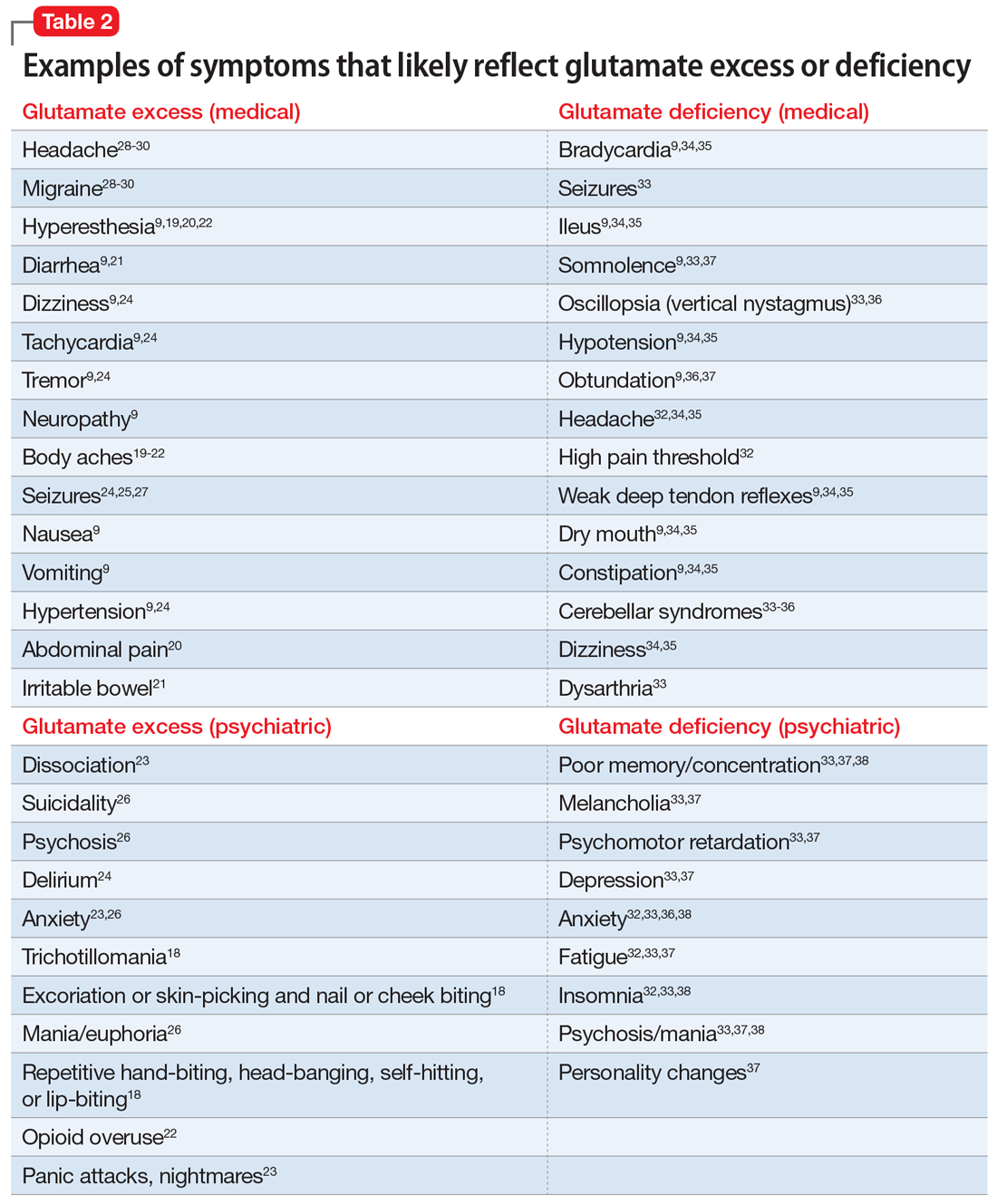
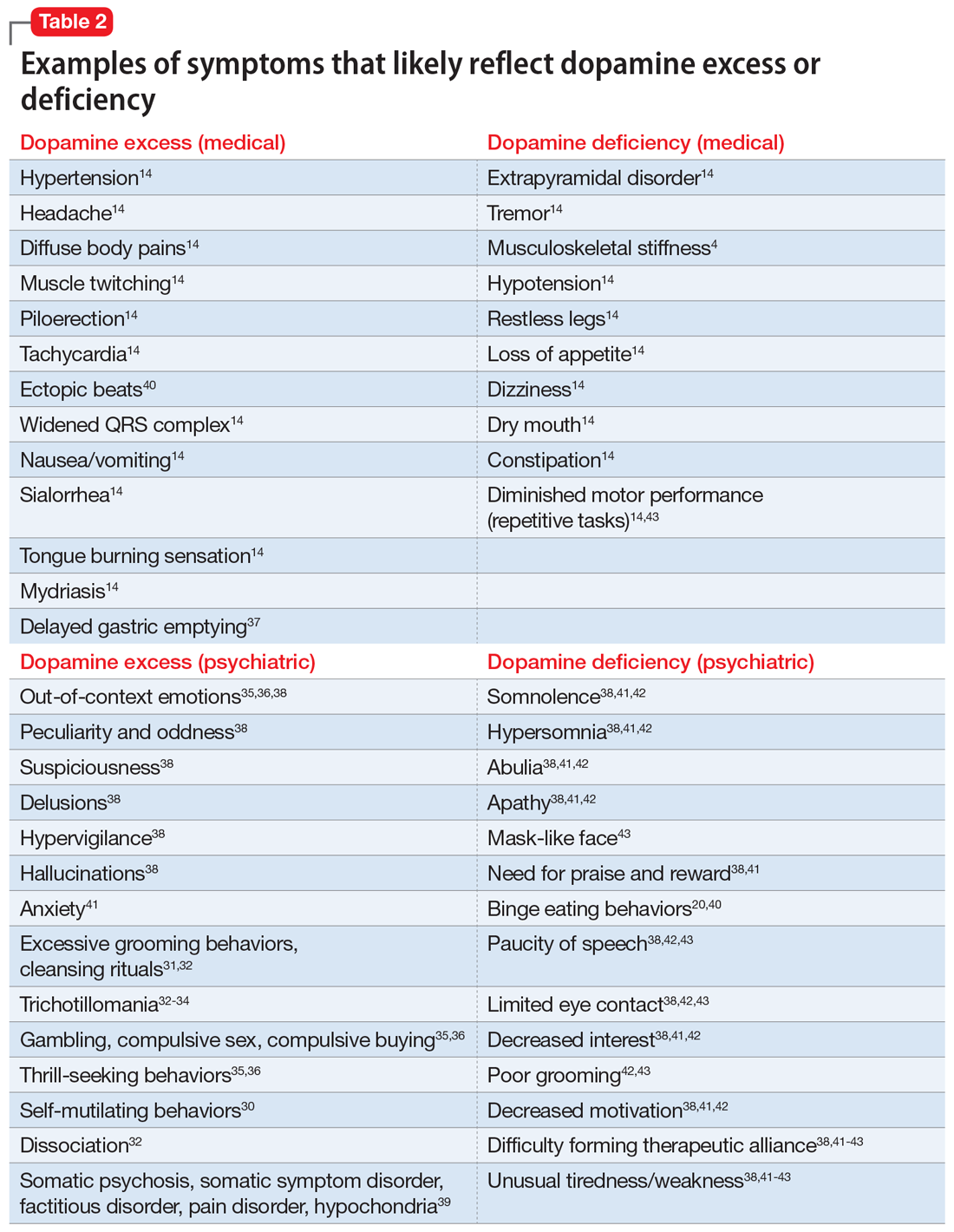
Glutamate excess (Table 29,18-30)
Mr. B is anxious and bites his fingernails and cheek while you interview him.18 He has scars on his lower arms that were caused by years of picking his skin.18 He complains of headache28-30 and deep muscle, whole body,19-23 and abdominal pain.20 Both hyperesthesia (he calls it “fibromyalgia”)9,19,20,22 and irritable bowel syndrome flare up if he eats Chinese food that contains monosodium glutamate.21 This also increases nausea, vomiting, and hypertensive episodes.9,19,20,22,24,26 Mr. B developed and received treatment for opioid use disorder after being prescribed morphine for the treatment of fibromyalgia.22 He is being treated for posttraumatic stress disorder at the VA hospital and is bitter that his flashbacks are not controlled.23 Once, he experienced a frank psychosis.26 He commonly experiences dissociative symptoms and suicidality.23,26 The sensations of crawling skin,18 panic attacks, and nightmares complicate his life.23 Mr. B is angry that his “incompetent” psychiatrist stopped his diazepam and that it “almost killed him” by causing delirium.24 He suffers from severe neuropathic pain in his feet and says that his pain, depression, and anxiety respond especially well to ketamine treatment.9,23,26 He is prone to euphoria and has had several manic episodes.26 In childhood, his parents brought him to a psychiatrist to address episodes of head-banging and self-hitting.18 Mr. B developed seizures; presently, they are controlled, but he remains chronically dizzy.9,24,25,27 He claims that his headaches and migraines respond only to methadone and that sumatriptan makes them worse, especially in prolonged treatment.28-30 He is tachycardic, tremulous, and makes you feel deeply uneasy.9,24
Impression. Mr. B has many symptoms of glutamate hyperactivity. The use of N-methyl-
Glutamate deficiency (Table 29,32-38)
Mr. Z feels dull, fatigued, and unhappy.32,33,37 He is overweight and moves slowly. Sometimes he is so slow and clumsy that he seems obtunded.9,36,37 He states that his peripheral neuropathy does not cause him pain, though his neurodiagnostic results are unfavorable.32 Mr. Z’s overall pain threshold is high, and he is unhappy with people who complain about pain because “who cares?”32 His memory and concentration were never good.33,37,38 He suffers from insomnia and is frequently miserable and disheartened.32,33,38 People view him as melancholic.33,37 Mr. Z is mildly depressed, but he experiences aggressive outbursts37,38 and bouts of anxiety,32,33,36,38 psychosis, and mania.33,37,38 He is visibly confused37 and says it is easy for him to get disoriented and lost.37,38 His medical history includes long-term constipation and several episodes of ileus.9,34,35 His childhood-onset seizures are controlled presently.33 He complains of frequent bouts of dizziness and headache.32,34,35 On physical exam, Mr. Z has dry mouth, hypotension, diminished deep tendon reflexes, and bradycardia.9,34,35 He sought a consultation from an ophthalmologist to evaluate an eye movement problem.33,36 No cause was found, but the ophthalmologist thought this problem might have the same underlying mechanism as his dysarthria.33 Mr. Z’s balance is bothersome, but his podiatrist was unable to help him to correct his abnormal gait.33-36 A friend who came with Mr. Z mentioned she had noticed personality changes in him over the last several months.37
Impression. Mr. Z exhibits multiple signs of low glutamatergic function. Amino acid taurine has been shown in rodents to increase brain levels of both GABA and glutamate. Glutamate is metabolized into GABA, so low glutamate and low GABA symptoms overlap. Glutamine, which is present in meat, fish, eggs, dairy, wheat, and some vegetables, is converted in the body into glutamate and may be considered for a patient with low glutamate function. The medication approach to such a patient would be similar to the treatment of a low GABA patient and includes glutamate-enhancing magnesium and dextromethorphan.
Rarely is just 1 neurotransmitter involved
Most real-world patients have mixed presentations with more than 1 neurotransmitter implicated in the pathology of their symptoms. A clinician’s ability to dissect the clinical picture and select an appropriate treatment must be based on history and observed behavior because no lab results or reliable tests are presently available.
Continue to: The most studied...
The most studied neurotransmitter in depression and anxiety is serotonin, and for many years psychiatrists have paid too much attention to it. Similarly, pain physicians have been overly focused on the opioid system. Excessive attention to these neurochemicals has overshadowed multiple other (no less impactful) neurotransmitters. Dopamine is frequently not attended to by many physicians who treat chronic pain. Psychiatrists also may overlook underlying endorphin or glutamate dysfunction in patients with psychiatric illness.
Nonpharmacologic approaches can affect neurotransmitters
With all the emphasis on pharmacologic treatments, it is important to remember that nonpharmacologic modalities such as exercise, diet, hydrotherapy, acupuncture, and psychotherapy can help normalize neurotransmitter function in the brain and ultimately help patients with chronic conditions. Careful use of nutritional supplements and vitamins may also be beneficial.
A hypothesis for future research
Multiple peripheral and central mechanisms define various chronic pain and psychiatric symptoms and disorders, including depression, anxiety, and fibromyalgia. The variety of mechanisms of pathologic mood and pain perception may be expressed to a different extent and in countless combinations in individual patients. This, in part, explains the variable responses to the same treatment observed in similar patients, or even in the same patient.
Clinicians should always remember that depression and anxiety as well as chronic pain (including fibromyalgia and chronic headache) are not a representation of a single condition but are the result of an assembly of different syndromes; therefore, 1 treatment does not fit all patients. Pain is ultimately recognized and comprehended centrally, making it very much a neuropsychiatric field. The optimal treatment for 2 patients with similar pain or psychiatric symptoms may be drastically different due to different underlying mechanisms that can be distinguished by looking at the symptoms other than “pain” or “depression.”
Remembering that every neurotransmitter deficiency or excess has an identifiable clinical correlation is important. Basing a treatment approach on a specific clinical presentation in a particular depressed or chronic pain patient would assure a more successful and reliable outcome.
Continue to: This 3-part series...
This 3-part series was designed to bring attention to a notion that diagnosis and treatment of diverse conditions such as “depression,” “anxiety,” or “chronic pain” should be based on clinically identifiable symptoms that may suggest specific neurotransmitter(s) involved in a specific type of each of these conditions. However, there are no well-recognized, well-established, reliable, or validated syndromes described in this series. The collection of symptoms associated with the various neurotransmitters described in this series is not complete. We have assembled what is described in the literature as a suggestion for future research.
Bottom Line
Both high and low levels of gamma aminobutyric acid (GABA) and glutamate may be associated with certain psychiatric and medical symptoms and disorders. An astute clinician may judge which neurotransmitter is dysfunctional based on the patient’s presentation, and tailor treatment accordingly.
Related Resources
- Arbuck DM, Salmerón JM, Mueller R. Neurotransmitter-based diagnosis and treatment: a hypothesis (part 1). Current Psychiatry. 2022;21(5):30-36. doi:10.12788/cp.0242
- Arbuck DM, Salmerón JM, Mueller R. Neurotransmitter-based diagnosis and treatment: a hypothesis (part 2). Current Psychiatry. 2022;21(6):28-33. doi:10.12788/cp.0253
Drug Brand Names
Acamprostate • Campral
Amantadine • Gocovri
Bupropion • Wellbutrin
Clonazepam • Klonopin
Clonidine • Catapres
Diazepam • Valium
Gabapentin • Neurontin
Ketamine • Ketalar
Memantine • Namenda
Methylphenidate • Concerta
Morphine • Kadian
Pregabalin • Lyrica
Sumatriptan • Imitrex
Tizanidine • Zanaflex
1. Petroff OA. GABA and glutamate in the human brain. Neuroscientist. 2002;8(6):562-573.
2. Winkelman JW, Buxton OM, Jensen JE, et al. Reduced brain GABA in primary insomnia: preliminary data from 4T proton magnetic resonance spectroscopy (1H-MRS). Sleep. 2008;31(11):1499-1506.
3. Pereira AC, Mao X, Jiang CS, et al. Dorsolateral prefrontal cortex GABA deficit in older adults with sleep-disordered breathing. Proc Natl Acad Sci U S A. 2017;114(38):10250-10255.
4. Schür RR, Draisma LW, Wijnen JP, et al. Brain GABA levels across psychiatric disorders: a systematic literature review and meta-analysis of (1) H-MRS studies. Hum Brain Mapp. 2016;37(9):3337-3352.
5. Evoy KE, Morrison MD, Saklad SR. Abuse and misuse of pregabalin and gabapentin. Drugs. 2017;77(4):403-426.
6. Mersfelder TL, Nichols WH. Gabapentin: abuse, dependence, and withdrawal. Ann Pharmacother. 2016;50(3):229-233.
7. Bremner JD. Traumatic stress: effects on the brain. Dialogues Clin Neurosci. 2006;8(4):445-461.
8. Kelly JR, Kennedy PJ, Cryan JF, et al. Breaking down the barriers: the gut microbiome, intestinal permeability, and stress-related psychiatric disorders. Front Cell Neurosci. 2015;9:392.
9. Guyton AC, Hall JE. Guyton and Hall Textbook of Medical Physiology. 12th ed. Elsevier; 2011:550-551,692-693.
10. Evrensel A, Ceylan ME. The gut-brain axis: the missing link in depression. Clin Psychopharmacol Neurosci. 2015;13(3):239-244.
11. Vianello M, Tavolato B, Giometto B. Glutamic acid decarboxylase autoantibodies and neurological disorders. Neurol Sci. 2002;23(4):145-151.
12. Marin O. Interneuron dysfunction in psychiatric disorders. Nat Rev Neurosci. 2012;13(2):107-120.
13. Huang D, Liu D, Yin J, et al. Glutamate-glutamine and GABA in the brain of normal aged and patients with cognitive impairment. Eur Radiol. 2017;27(7):2698-2705.
14. Jiménez-Jiménez FJ, Alonso-Navarro H, García-Martín E, et al. Neurochemical features of idiopathic restless legs syndrome. Sleep Med Rev. 2019;45:70-87.
15. Mick I, Ramos AC, Myers J, et al. Evidence for GABA-A receptor dysregulation in gambling disorder: correlation with impulsivity. Addict Biol. 2017;22(6):1601-1609.
16. Brambilla P, Perez J, Barale F, et al. Gabaergic dysfunction in mood disorders. Molecular Psychiatry. 2003;8:721-737.
17. Kaneez FS, Saeed SA. Investigating GABA and its function in platelets as compared to neurons. Platelets. 2009;20(5):328-333.
18. Paholpak P, Mendez MF. Trichotillomania as a manifestation of dementia. Case Rep Psychiatry. 2016;2016:9782702.
19. Miranda A, Peles S, Rudolph C, et al. Altered visceral sensation in response to somatic pain in the rat. Gastroenterology. 2004;126(4):1082-1089.
20. Skyba DA, King EW, Sluka KA. Effects of NMDA and non-NMDA ionotropic glutamate receptor antagonists on the development and maintenance of hyperalgesia induced by repeated intramuscular injection of acidic saline. Pain. 2002;98(1-2):69-78.
21. Holton KF, Taren DL, Thomson CA, et al. The effect of dietary glutamate on fibromyalgia and irritable bowel symptoms. Clin Exp Rheumatol. 2012;30(6 Suppl 74):10-70.
22. Sekiya Y, Nakagawa T, Ozawa T, et al. Facilitation of morphine withdrawal symptoms and morphine-induced conditioned place preference by a glutamate transporter inhibitor DL-threo-beta-benzyloxy aspartate in rats. Eur J Pharmacol. 2004;485(1-3):201-210.
23. Bestha D, Soliman L, Blankenship K. et al. The walking wounded: emerging treatments for PTSD. Curr Psychiatry Rep. 2018;20(10):94.
24. Tsuda M, Shimizu N, Suzuki T. Contribution of glutamate receptors to benzodiazepine withdrawal signs. Jpn J Pharmacol. 1999;81(1):1-6.
25. Spravato [package insert]. Janssen Pharmaceuticals, Inc; 2019.
26. Mattingly GW, Anderson RH. Intranasal ketamine. Current Psychiatry. 2019;18(5):31-38.
27. Buckingham SC, Campbell SL, Haas BR, et al. Glutamate release by primary brain tumors induces epileptic activity. Nat Med. 2011;17(10):1269-1275.
28. Ferrari A, Spaccapelo L, Pinetti D, et al. Effective prophylactic treatment of migraines lower plasma glutamate levels. Cephalalgia. 2009;29(4):423-429.
29. Vieira DS, Naffah-Mazzacoratti Mda G, Zukerman E, et al. Glutamate levels in cerebrospinal fluid and triptans overuse in chronic migraine. Headache. 2007;47(6):842-847.
30. Chan K, MaassenVanDenBrink A. Glutamate receptor antagonists in the management of migraine. Drugs. 2014;74:1165-1176.
31. Pappa S, Tsouli S, Apostolou G, et al. Effects of amantadine on tardive dyskinesia: a randomized, double-blind, placebo-controlled study. Clin Neuropharmacol. 2010;33(6):271-275.
32. Kraal AZ, Arvanitis NR, Jaeger AP, et al. Could dietary glutamate play a role in psychiatric distress? Neuro Psych. 2020;79:13-19.
33. Levite M. Glutamate receptor antibodies in neurological diseases: anti-AMPA-GluR3 antibodies, Anti-NMDA-NR1 antibodies, Anti-NMDA-NR2A/B antibodies, Anti-mGluR1 antibodies or Anti-mGluR5 antibodies are present in subpopulations of patients with either: epilepsy, encephalitis, cerebellar ataxia, systemic lupus erythematosus (SLE) and neuropsychiatric SLE, Sjogren’s syndrome, schizophrenia, mania or stroke. These autoimmune anti-glutamate receptor antibodies can bind neurons in few brain regions, activate glutamate receptors, decrease glutamate receptor’s expression, impair glutamate-induced signaling and function, activate blood brain barrier endothelial cells, kill neurons, damage the brain, induce behavioral/psychiatric/cognitive abnormalities and ataxia in animal models, and can be removed or silenced in some patients by immunotherapy. J Neural Transm (Vienna). 2014;121(8):1029-1075.
34. Lancaster E. CNS syndromes associated with antibodies against metabotropic receptors. Curr Opin Neurol. 2017;30:354-360.
35. Sillevis Smitt P, Kinoshita A, De Leeuw B, et al. Paraneoplastic cerebellar ataxia due to autoantibodies against a glutamate receptor. N Engl J Med. 2000;342(1):21-27.
36. Marignier R, Chenevier F, Rogemond V, et al. Metabotropic glutamate receptor type 1 autoantibody-associated cerebellitis: a primary autoimmune disease? Arch Neurol. 2010;67(5):627-630.
37. Lancaster E, Martinez-Hernandez E, Titulaer MJ, et al. Antibodies to metabotropic glutamate receptor 5 in the Ophelia syndrome. Neurology. 2011;77:1698-1701.
38. Mat A, Adler H, Merwick A, et al. Ophelia syndrome with metabotropic glutamate receptor 5 antibodies in CSF. Neurology. 2013;80(14):1349-1350.
Optimal diagnosis and treatment of psychiatric illness requires clinicians to be able to connect mental and physical symptoms. Direct brain neurotransmitter testing is presently in its infancy and the science of defining the underlying mechanisms of psychiatric disorders lags behind the obvious clinical needs. We are not yet equipped to clearly recognize which neurotransmitters cause which symptoms. In this article series, we suggest an indirect way of judging neurotransmitter activity by recognizing specific mental and physical symptoms connected by common biology. Here we present hypothetical clinical cases to emphasize a possible way of analyzing symptoms in order to identify underlying pathology and guide more effective treatment. The descriptions we present in this series do not reflect the entire set of symptoms caused by the neurotransmitters we discuss; we created them based on what is presently known (or suspected). Additional research is needed to confirm or disprove the hypothesis we present. We argue that in cases of multiple psychiatric disorders and chronic pain, the development and approval of medications currently is based on an umbrella descriptive diagnoses, and disregards the various underlying causes of such conditions. Similar to how the many types of pneumonias are treated differently depending on the infective agent, we suggested the same possible causative approach to various types of depression and pain.
Part 1 of this series (
GABA excess (Table 11-9)
Ms. V is brought to your office by a friend. She complains of pain all over her body, itchiness, inability to focus, and dizziness.1,5,6,9 She is puzzled by how little pain she feels when she cuts her finger but by how much pain she is in every day, though her clinicians have not discovered a reason for her pain.1,6,9 She states that her fatigue is so severe that she can sleep 15 hours a day.1-6,9 Her obstructive and central sleep apnea have been treated, but this did not improve her fatigue.3,5,9 She is forgetful and has been diagnosed with depression, though she says she does not feel depressed.1,5,6 Nothing is pleasant to her, but she is prone to abnormal excitement and unpredictable behavior.1,4,6,7
A physical exam shows slow breathing, bradycardia, decreased deep tendon reflexes, and decreased muscle tone.1,5,6,9 Ms. V complains of double vision1,5,6,9 and problems with gait and balance,5,6,9 as well as tremors.1,4-7 She experienced enuresis well into adulthood1,5,6,9 and is prone to weight gain, dyspepsia, and constipation.8,9 She cannot understand people who have anxiety, and is prone to melancholy.4-6,9 Ms. V had been treated with electroconvulsive therapy in the past but states that she “had to have so much electricity, they gave up on me.”
Impression. Ms. V exhibits multiple symptoms associated with GABA excess. Dopaminergic medications such as methylphenidate or amphetamines may be helpful, as they suppress GABA. GABAergic medications and supplements should be avoided in such a patient. Noradrenergic medications including antidepressants with corresponding activity or vasopressors may be beneficial. Suppression of glutamate increases GABA, which is why ketamine in any formulation should be avoided in a patient such as Ms. V.
GABA deficiency (Table 11-4,6,9-17)
Mr. N complains of depression,1,3,4,6,12,16 pain all over his body, tingling in his hands and feet,1,6,9 a constant dull headache,2 and severe insomnia.2,3,9,10 He cannot control his anxiety and, in general, has problems relaxing. In the office, he is jumpy, tremulous, and fidgety during the interview and examination.1,3,4,6,9,12 His muscle tone is high1,9,11 and he feels stiff.6,9 Mr. N’s pupils are narrow1,9; he is hyper-reflexive1,9,11 and reports “Klonopin withdrawal seizures.”1,6,9 He loves alcohol because “it makes me feel good” and helps with his mind, which otherwise “never stops.”1,6,13 Mr. N is frequently anxious and very sensitive to pain, especially when he is upset. He was diagnosed with fibromyalgia by his primary care doctor, who says that irritable bowel is common in patients like him.1,6 His anxiety disables him.1-4,6,9-12 His sister reports that in addition to having difficulty relaxing, Mr. N is easily frustrated and sleeps poorly because he says he has racing thoughts.10 She mentions that her brother’s gambling addiction endangered his finances on several occasions4,12,15 and he was suspected of having autism spectrum disorder.4,12 Mr. N is frequently overwhelmed, including during your interview.1,3,4,6 He is sensitive to light and noise1,9 and complains of palpitations1,3,4,6,9 and frequent shortness of breath.1,3,4,9 He mentions his hands and feet often are cold, especially when he is anxious.1,3,4,6,9 Not eating at regular times makes his symptoms even worse. Mr. N commonly feels depressed, but his anxiety is more bothersome.1,3,4,6,12,16 His ongoing complaints include difficulty concentrating and memory problems,3,4,12,13 as well as a constant feeling of being overwhelmed.1,3,4,6 His restless leg syndrome requires ongoing treatment.1,9,14 Though uncommon, Mr. N has episodes of slowing and weakness, which are associated with growth hormone problems.16 In the past, he experienced gut motility dysregulation9,10 and prolonged bleeding that worried his doctors.17
Impression. Mr. N shows multiple symptoms associated with GABA deficiency. The deficiency of GABA activity ultimately causes an increase in norepinephrine and dopamine firing; therefore, symptoms of GABA deficiency are partially aligned with symptoms of dopamine and norepinephrine excess. GABAergic medications would be most beneficial for such patients. Anticonvulsants (eg, gabapentin and pregabalin) are preferable. Acamprostate may be considered. For long-term use, benzodiapines are as problematic as opioids and should be avoided, if possible. The use of opioids in such patients is especially counterproductive. Some supplements and vitamins may enhance GABA activity. Avoiding bupropion and stimulants would be wise. Ketamine in any formulation would be a good choice in this scenario. Sedating antipsychotic medications have a promise for patients such as Mr. N. The muscle relaxant baclofen frequently helps with these patients’ pain, anxiety, and sleep.
Continue to: Glutamate excess
Glutamate excess (Table 29,18-30)
Mr. B is anxious and bites his fingernails and cheek while you interview him.18 He has scars on his lower arms that were caused by years of picking his skin.18 He complains of headache28-30 and deep muscle, whole body,19-23 and abdominal pain.20 Both hyperesthesia (he calls it “fibromyalgia”)9,19,20,22 and irritable bowel syndrome flare up if he eats Chinese food that contains monosodium glutamate.21 This also increases nausea, vomiting, and hypertensive episodes.9,19,20,22,24,26 Mr. B developed and received treatment for opioid use disorder after being prescribed morphine for the treatment of fibromyalgia.22 He is being treated for posttraumatic stress disorder at the VA hospital and is bitter that his flashbacks are not controlled.23 Once, he experienced a frank psychosis.26 He commonly experiences dissociative symptoms and suicidality.23,26 The sensations of crawling skin,18 panic attacks, and nightmares complicate his life.23 Mr. B is angry that his “incompetent” psychiatrist stopped his diazepam and that it “almost killed him” by causing delirium.24 He suffers from severe neuropathic pain in his feet and says that his pain, depression, and anxiety respond especially well to ketamine treatment.9,23,26 He is prone to euphoria and has had several manic episodes.26 In childhood, his parents brought him to a psychiatrist to address episodes of head-banging and self-hitting.18 Mr. B developed seizures; presently, they are controlled, but he remains chronically dizzy.9,24,25,27 He claims that his headaches and migraines respond only to methadone and that sumatriptan makes them worse, especially in prolonged treatment.28-30 He is tachycardic, tremulous, and makes you feel deeply uneasy.9,24
Impression. Mr. B has many symptoms of glutamate hyperactivity. The use of N-methyl-
Glutamate deficiency (Table 29,32-38)
Mr. Z feels dull, fatigued, and unhappy.32,33,37 He is overweight and moves slowly. Sometimes he is so slow and clumsy that he seems obtunded.9,36,37 He states that his peripheral neuropathy does not cause him pain, though his neurodiagnostic results are unfavorable.32 Mr. Z’s overall pain threshold is high, and he is unhappy with people who complain about pain because “who cares?”32 His memory and concentration were never good.33,37,38 He suffers from insomnia and is frequently miserable and disheartened.32,33,38 People view him as melancholic.33,37 Mr. Z is mildly depressed, but he experiences aggressive outbursts37,38 and bouts of anxiety,32,33,36,38 psychosis, and mania.33,37,38 He is visibly confused37 and says it is easy for him to get disoriented and lost.37,38 His medical history includes long-term constipation and several episodes of ileus.9,34,35 His childhood-onset seizures are controlled presently.33 He complains of frequent bouts of dizziness and headache.32,34,35 On physical exam, Mr. Z has dry mouth, hypotension, diminished deep tendon reflexes, and bradycardia.9,34,35 He sought a consultation from an ophthalmologist to evaluate an eye movement problem.33,36 No cause was found, but the ophthalmologist thought this problem might have the same underlying mechanism as his dysarthria.33 Mr. Z’s balance is bothersome, but his podiatrist was unable to help him to correct his abnormal gait.33-36 A friend who came with Mr. Z mentioned she had noticed personality changes in him over the last several months.37
Impression. Mr. Z exhibits multiple signs of low glutamatergic function. Amino acid taurine has been shown in rodents to increase brain levels of both GABA and glutamate. Glutamate is metabolized into GABA, so low glutamate and low GABA symptoms overlap. Glutamine, which is present in meat, fish, eggs, dairy, wheat, and some vegetables, is converted in the body into glutamate and may be considered for a patient with low glutamate function. The medication approach to such a patient would be similar to the treatment of a low GABA patient and includes glutamate-enhancing magnesium and dextromethorphan.
Rarely is just 1 neurotransmitter involved
Most real-world patients have mixed presentations with more than 1 neurotransmitter implicated in the pathology of their symptoms. A clinician’s ability to dissect the clinical picture and select an appropriate treatment must be based on history and observed behavior because no lab results or reliable tests are presently available.
Continue to: The most studied...
The most studied neurotransmitter in depression and anxiety is serotonin, and for many years psychiatrists have paid too much attention to it. Similarly, pain physicians have been overly focused on the opioid system. Excessive attention to these neurochemicals has overshadowed multiple other (no less impactful) neurotransmitters. Dopamine is frequently not attended to by many physicians who treat chronic pain. Psychiatrists also may overlook underlying endorphin or glutamate dysfunction in patients with psychiatric illness.
Nonpharmacologic approaches can affect neurotransmitters
With all the emphasis on pharmacologic treatments, it is important to remember that nonpharmacologic modalities such as exercise, diet, hydrotherapy, acupuncture, and psychotherapy can help normalize neurotransmitter function in the brain and ultimately help patients with chronic conditions. Careful use of nutritional supplements and vitamins may also be beneficial.
A hypothesis for future research
Multiple peripheral and central mechanisms define various chronic pain and psychiatric symptoms and disorders, including depression, anxiety, and fibromyalgia. The variety of mechanisms of pathologic mood and pain perception may be expressed to a different extent and in countless combinations in individual patients. This, in part, explains the variable responses to the same treatment observed in similar patients, or even in the same patient.
Clinicians should always remember that depression and anxiety as well as chronic pain (including fibromyalgia and chronic headache) are not a representation of a single condition but are the result of an assembly of different syndromes; therefore, 1 treatment does not fit all patients. Pain is ultimately recognized and comprehended centrally, making it very much a neuropsychiatric field. The optimal treatment for 2 patients with similar pain or psychiatric symptoms may be drastically different due to different underlying mechanisms that can be distinguished by looking at the symptoms other than “pain” or “depression.”
Remembering that every neurotransmitter deficiency or excess has an identifiable clinical correlation is important. Basing a treatment approach on a specific clinical presentation in a particular depressed or chronic pain patient would assure a more successful and reliable outcome.
Continue to: This 3-part series...
This 3-part series was designed to bring attention to a notion that diagnosis and treatment of diverse conditions such as “depression,” “anxiety,” or “chronic pain” should be based on clinically identifiable symptoms that may suggest specific neurotransmitter(s) involved in a specific type of each of these conditions. However, there are no well-recognized, well-established, reliable, or validated syndromes described in this series. The collection of symptoms associated with the various neurotransmitters described in this series is not complete. We have assembled what is described in the literature as a suggestion for future research.
Bottom Line
Both high and low levels of gamma aminobutyric acid (GABA) and glutamate may be associated with certain psychiatric and medical symptoms and disorders. An astute clinician may judge which neurotransmitter is dysfunctional based on the patient’s presentation, and tailor treatment accordingly.
Related Resources
- Arbuck DM, Salmerón JM, Mueller R. Neurotransmitter-based diagnosis and treatment: a hypothesis (part 1). Current Psychiatry. 2022;21(5):30-36. doi:10.12788/cp.0242
- Arbuck DM, Salmerón JM, Mueller R. Neurotransmitter-based diagnosis and treatment: a hypothesis (part 2). Current Psychiatry. 2022;21(6):28-33. doi:10.12788/cp.0253
Drug Brand Names
Acamprostate • Campral
Amantadine • Gocovri
Bupropion • Wellbutrin
Clonazepam • Klonopin
Clonidine • Catapres
Diazepam • Valium
Gabapentin • Neurontin
Ketamine • Ketalar
Memantine • Namenda
Methylphenidate • Concerta
Morphine • Kadian
Pregabalin • Lyrica
Sumatriptan • Imitrex
Tizanidine • Zanaflex
Optimal diagnosis and treatment of psychiatric illness requires clinicians to be able to connect mental and physical symptoms. Direct brain neurotransmitter testing is presently in its infancy and the science of defining the underlying mechanisms of psychiatric disorders lags behind the obvious clinical needs. We are not yet equipped to clearly recognize which neurotransmitters cause which symptoms. In this article series, we suggest an indirect way of judging neurotransmitter activity by recognizing specific mental and physical symptoms connected by common biology. Here we present hypothetical clinical cases to emphasize a possible way of analyzing symptoms in order to identify underlying pathology and guide more effective treatment. The descriptions we present in this series do not reflect the entire set of symptoms caused by the neurotransmitters we discuss; we created them based on what is presently known (or suspected). Additional research is needed to confirm or disprove the hypothesis we present. We argue that in cases of multiple psychiatric disorders and chronic pain, the development and approval of medications currently is based on an umbrella descriptive diagnoses, and disregards the various underlying causes of such conditions. Similar to how the many types of pneumonias are treated differently depending on the infective agent, we suggested the same possible causative approach to various types of depression and pain.
Part 1 of this series (
GABA excess (Table 11-9)
Ms. V is brought to your office by a friend. She complains of pain all over her body, itchiness, inability to focus, and dizziness.1,5,6,9 She is puzzled by how little pain she feels when she cuts her finger but by how much pain she is in every day, though her clinicians have not discovered a reason for her pain.1,6,9 She states that her fatigue is so severe that she can sleep 15 hours a day.1-6,9 Her obstructive and central sleep apnea have been treated, but this did not improve her fatigue.3,5,9 She is forgetful and has been diagnosed with depression, though she says she does not feel depressed.1,5,6 Nothing is pleasant to her, but she is prone to abnormal excitement and unpredictable behavior.1,4,6,7
A physical exam shows slow breathing, bradycardia, decreased deep tendon reflexes, and decreased muscle tone.1,5,6,9 Ms. V complains of double vision1,5,6,9 and problems with gait and balance,5,6,9 as well as tremors.1,4-7 She experienced enuresis well into adulthood1,5,6,9 and is prone to weight gain, dyspepsia, and constipation.8,9 She cannot understand people who have anxiety, and is prone to melancholy.4-6,9 Ms. V had been treated with electroconvulsive therapy in the past but states that she “had to have so much electricity, they gave up on me.”
Impression. Ms. V exhibits multiple symptoms associated with GABA excess. Dopaminergic medications such as methylphenidate or amphetamines may be helpful, as they suppress GABA. GABAergic medications and supplements should be avoided in such a patient. Noradrenergic medications including antidepressants with corresponding activity or vasopressors may be beneficial. Suppression of glutamate increases GABA, which is why ketamine in any formulation should be avoided in a patient such as Ms. V.
GABA deficiency (Table 11-4,6,9-17)
Mr. N complains of depression,1,3,4,6,12,16 pain all over his body, tingling in his hands and feet,1,6,9 a constant dull headache,2 and severe insomnia.2,3,9,10 He cannot control his anxiety and, in general, has problems relaxing. In the office, he is jumpy, tremulous, and fidgety during the interview and examination.1,3,4,6,9,12 His muscle tone is high1,9,11 and he feels stiff.6,9 Mr. N’s pupils are narrow1,9; he is hyper-reflexive1,9,11 and reports “Klonopin withdrawal seizures.”1,6,9 He loves alcohol because “it makes me feel good” and helps with his mind, which otherwise “never stops.”1,6,13 Mr. N is frequently anxious and very sensitive to pain, especially when he is upset. He was diagnosed with fibromyalgia by his primary care doctor, who says that irritable bowel is common in patients like him.1,6 His anxiety disables him.1-4,6,9-12 His sister reports that in addition to having difficulty relaxing, Mr. N is easily frustrated and sleeps poorly because he says he has racing thoughts.10 She mentions that her brother’s gambling addiction endangered his finances on several occasions4,12,15 and he was suspected of having autism spectrum disorder.4,12 Mr. N is frequently overwhelmed, including during your interview.1,3,4,6 He is sensitive to light and noise1,9 and complains of palpitations1,3,4,6,9 and frequent shortness of breath.1,3,4,9 He mentions his hands and feet often are cold, especially when he is anxious.1,3,4,6,9 Not eating at regular times makes his symptoms even worse. Mr. N commonly feels depressed, but his anxiety is more bothersome.1,3,4,6,12,16 His ongoing complaints include difficulty concentrating and memory problems,3,4,12,13 as well as a constant feeling of being overwhelmed.1,3,4,6 His restless leg syndrome requires ongoing treatment.1,9,14 Though uncommon, Mr. N has episodes of slowing and weakness, which are associated with growth hormone problems.16 In the past, he experienced gut motility dysregulation9,10 and prolonged bleeding that worried his doctors.17
Impression. Mr. N shows multiple symptoms associated with GABA deficiency. The deficiency of GABA activity ultimately causes an increase in norepinephrine and dopamine firing; therefore, symptoms of GABA deficiency are partially aligned with symptoms of dopamine and norepinephrine excess. GABAergic medications would be most beneficial for such patients. Anticonvulsants (eg, gabapentin and pregabalin) are preferable. Acamprostate may be considered. For long-term use, benzodiapines are as problematic as opioids and should be avoided, if possible. The use of opioids in such patients is especially counterproductive. Some supplements and vitamins may enhance GABA activity. Avoiding bupropion and stimulants would be wise. Ketamine in any formulation would be a good choice in this scenario. Sedating antipsychotic medications have a promise for patients such as Mr. N. The muscle relaxant baclofen frequently helps with these patients’ pain, anxiety, and sleep.
Continue to: Glutamate excess
Glutamate excess (Table 29,18-30)
Mr. B is anxious and bites his fingernails and cheek while you interview him.18 He has scars on his lower arms that were caused by years of picking his skin.18 He complains of headache28-30 and deep muscle, whole body,19-23 and abdominal pain.20 Both hyperesthesia (he calls it “fibromyalgia”)9,19,20,22 and irritable bowel syndrome flare up if he eats Chinese food that contains monosodium glutamate.21 This also increases nausea, vomiting, and hypertensive episodes.9,19,20,22,24,26 Mr. B developed and received treatment for opioid use disorder after being prescribed morphine for the treatment of fibromyalgia.22 He is being treated for posttraumatic stress disorder at the VA hospital and is bitter that his flashbacks are not controlled.23 Once, he experienced a frank psychosis.26 He commonly experiences dissociative symptoms and suicidality.23,26 The sensations of crawling skin,18 panic attacks, and nightmares complicate his life.23 Mr. B is angry that his “incompetent” psychiatrist stopped his diazepam and that it “almost killed him” by causing delirium.24 He suffers from severe neuropathic pain in his feet and says that his pain, depression, and anxiety respond especially well to ketamine treatment.9,23,26 He is prone to euphoria and has had several manic episodes.26 In childhood, his parents brought him to a psychiatrist to address episodes of head-banging and self-hitting.18 Mr. B developed seizures; presently, they are controlled, but he remains chronically dizzy.9,24,25,27 He claims that his headaches and migraines respond only to methadone and that sumatriptan makes them worse, especially in prolonged treatment.28-30 He is tachycardic, tremulous, and makes you feel deeply uneasy.9,24
Impression. Mr. B has many symptoms of glutamate hyperactivity. The use of N-methyl-
Glutamate deficiency (Table 29,32-38)
Mr. Z feels dull, fatigued, and unhappy.32,33,37 He is overweight and moves slowly. Sometimes he is so slow and clumsy that he seems obtunded.9,36,37 He states that his peripheral neuropathy does not cause him pain, though his neurodiagnostic results are unfavorable.32 Mr. Z’s overall pain threshold is high, and he is unhappy with people who complain about pain because “who cares?”32 His memory and concentration were never good.33,37,38 He suffers from insomnia and is frequently miserable and disheartened.32,33,38 People view him as melancholic.33,37 Mr. Z is mildly depressed, but he experiences aggressive outbursts37,38 and bouts of anxiety,32,33,36,38 psychosis, and mania.33,37,38 He is visibly confused37 and says it is easy for him to get disoriented and lost.37,38 His medical history includes long-term constipation and several episodes of ileus.9,34,35 His childhood-onset seizures are controlled presently.33 He complains of frequent bouts of dizziness and headache.32,34,35 On physical exam, Mr. Z has dry mouth, hypotension, diminished deep tendon reflexes, and bradycardia.9,34,35 He sought a consultation from an ophthalmologist to evaluate an eye movement problem.33,36 No cause was found, but the ophthalmologist thought this problem might have the same underlying mechanism as his dysarthria.33 Mr. Z’s balance is bothersome, but his podiatrist was unable to help him to correct his abnormal gait.33-36 A friend who came with Mr. Z mentioned she had noticed personality changes in him over the last several months.37
Impression. Mr. Z exhibits multiple signs of low glutamatergic function. Amino acid taurine has been shown in rodents to increase brain levels of both GABA and glutamate. Glutamate is metabolized into GABA, so low glutamate and low GABA symptoms overlap. Glutamine, which is present in meat, fish, eggs, dairy, wheat, and some vegetables, is converted in the body into glutamate and may be considered for a patient with low glutamate function. The medication approach to such a patient would be similar to the treatment of a low GABA patient and includes glutamate-enhancing magnesium and dextromethorphan.
Rarely is just 1 neurotransmitter involved
Most real-world patients have mixed presentations with more than 1 neurotransmitter implicated in the pathology of their symptoms. A clinician’s ability to dissect the clinical picture and select an appropriate treatment must be based on history and observed behavior because no lab results or reliable tests are presently available.
Continue to: The most studied...
The most studied neurotransmitter in depression and anxiety is serotonin, and for many years psychiatrists have paid too much attention to it. Similarly, pain physicians have been overly focused on the opioid system. Excessive attention to these neurochemicals has overshadowed multiple other (no less impactful) neurotransmitters. Dopamine is frequently not attended to by many physicians who treat chronic pain. Psychiatrists also may overlook underlying endorphin or glutamate dysfunction in patients with psychiatric illness.
Nonpharmacologic approaches can affect neurotransmitters
With all the emphasis on pharmacologic treatments, it is important to remember that nonpharmacologic modalities such as exercise, diet, hydrotherapy, acupuncture, and psychotherapy can help normalize neurotransmitter function in the brain and ultimately help patients with chronic conditions. Careful use of nutritional supplements and vitamins may also be beneficial.
A hypothesis for future research
Multiple peripheral and central mechanisms define various chronic pain and psychiatric symptoms and disorders, including depression, anxiety, and fibromyalgia. The variety of mechanisms of pathologic mood and pain perception may be expressed to a different extent and in countless combinations in individual patients. This, in part, explains the variable responses to the same treatment observed in similar patients, or even in the same patient.
Clinicians should always remember that depression and anxiety as well as chronic pain (including fibromyalgia and chronic headache) are not a representation of a single condition but are the result of an assembly of different syndromes; therefore, 1 treatment does not fit all patients. Pain is ultimately recognized and comprehended centrally, making it very much a neuropsychiatric field. The optimal treatment for 2 patients with similar pain or psychiatric symptoms may be drastically different due to different underlying mechanisms that can be distinguished by looking at the symptoms other than “pain” or “depression.”
Remembering that every neurotransmitter deficiency or excess has an identifiable clinical correlation is important. Basing a treatment approach on a specific clinical presentation in a particular depressed or chronic pain patient would assure a more successful and reliable outcome.
Continue to: This 3-part series...
This 3-part series was designed to bring attention to a notion that diagnosis and treatment of diverse conditions such as “depression,” “anxiety,” or “chronic pain” should be based on clinically identifiable symptoms that may suggest specific neurotransmitter(s) involved in a specific type of each of these conditions. However, there are no well-recognized, well-established, reliable, or validated syndromes described in this series. The collection of symptoms associated with the various neurotransmitters described in this series is not complete. We have assembled what is described in the literature as a suggestion for future research.
Bottom Line
Both high and low levels of gamma aminobutyric acid (GABA) and glutamate may be associated with certain psychiatric and medical symptoms and disorders. An astute clinician may judge which neurotransmitter is dysfunctional based on the patient’s presentation, and tailor treatment accordingly.
Related Resources
- Arbuck DM, Salmerón JM, Mueller R. Neurotransmitter-based diagnosis and treatment: a hypothesis (part 1). Current Psychiatry. 2022;21(5):30-36. doi:10.12788/cp.0242
- Arbuck DM, Salmerón JM, Mueller R. Neurotransmitter-based diagnosis and treatment: a hypothesis (part 2). Current Psychiatry. 2022;21(6):28-33. doi:10.12788/cp.0253
Drug Brand Names
Acamprostate • Campral
Amantadine • Gocovri
Bupropion • Wellbutrin
Clonazepam • Klonopin
Clonidine • Catapres
Diazepam • Valium
Gabapentin • Neurontin
Ketamine • Ketalar
Memantine • Namenda
Methylphenidate • Concerta
Morphine • Kadian
Pregabalin • Lyrica
Sumatriptan • Imitrex
Tizanidine • Zanaflex
1. Petroff OA. GABA and glutamate in the human brain. Neuroscientist. 2002;8(6):562-573.
2. Winkelman JW, Buxton OM, Jensen JE, et al. Reduced brain GABA in primary insomnia: preliminary data from 4T proton magnetic resonance spectroscopy (1H-MRS). Sleep. 2008;31(11):1499-1506.
3. Pereira AC, Mao X, Jiang CS, et al. Dorsolateral prefrontal cortex GABA deficit in older adults with sleep-disordered breathing. Proc Natl Acad Sci U S A. 2017;114(38):10250-10255.
4. Schür RR, Draisma LW, Wijnen JP, et al. Brain GABA levels across psychiatric disorders: a systematic literature review and meta-analysis of (1) H-MRS studies. Hum Brain Mapp. 2016;37(9):3337-3352.
5. Evoy KE, Morrison MD, Saklad SR. Abuse and misuse of pregabalin and gabapentin. Drugs. 2017;77(4):403-426.
6. Mersfelder TL, Nichols WH. Gabapentin: abuse, dependence, and withdrawal. Ann Pharmacother. 2016;50(3):229-233.
7. Bremner JD. Traumatic stress: effects on the brain. Dialogues Clin Neurosci. 2006;8(4):445-461.
8. Kelly JR, Kennedy PJ, Cryan JF, et al. Breaking down the barriers: the gut microbiome, intestinal permeability, and stress-related psychiatric disorders. Front Cell Neurosci. 2015;9:392.
9. Guyton AC, Hall JE. Guyton and Hall Textbook of Medical Physiology. 12th ed. Elsevier; 2011:550-551,692-693.
10. Evrensel A, Ceylan ME. The gut-brain axis: the missing link in depression. Clin Psychopharmacol Neurosci. 2015;13(3):239-244.
11. Vianello M, Tavolato B, Giometto B. Glutamic acid decarboxylase autoantibodies and neurological disorders. Neurol Sci. 2002;23(4):145-151.
12. Marin O. Interneuron dysfunction in psychiatric disorders. Nat Rev Neurosci. 2012;13(2):107-120.
13. Huang D, Liu D, Yin J, et al. Glutamate-glutamine and GABA in the brain of normal aged and patients with cognitive impairment. Eur Radiol. 2017;27(7):2698-2705.
14. Jiménez-Jiménez FJ, Alonso-Navarro H, García-Martín E, et al. Neurochemical features of idiopathic restless legs syndrome. Sleep Med Rev. 2019;45:70-87.
15. Mick I, Ramos AC, Myers J, et al. Evidence for GABA-A receptor dysregulation in gambling disorder: correlation with impulsivity. Addict Biol. 2017;22(6):1601-1609.
16. Brambilla P, Perez J, Barale F, et al. Gabaergic dysfunction in mood disorders. Molecular Psychiatry. 2003;8:721-737.
17. Kaneez FS, Saeed SA. Investigating GABA and its function in platelets as compared to neurons. Platelets. 2009;20(5):328-333.
18. Paholpak P, Mendez MF. Trichotillomania as a manifestation of dementia. Case Rep Psychiatry. 2016;2016:9782702.
19. Miranda A, Peles S, Rudolph C, et al. Altered visceral sensation in response to somatic pain in the rat. Gastroenterology. 2004;126(4):1082-1089.
20. Skyba DA, King EW, Sluka KA. Effects of NMDA and non-NMDA ionotropic glutamate receptor antagonists on the development and maintenance of hyperalgesia induced by repeated intramuscular injection of acidic saline. Pain. 2002;98(1-2):69-78.
21. Holton KF, Taren DL, Thomson CA, et al. The effect of dietary glutamate on fibromyalgia and irritable bowel symptoms. Clin Exp Rheumatol. 2012;30(6 Suppl 74):10-70.
22. Sekiya Y, Nakagawa T, Ozawa T, et al. Facilitation of morphine withdrawal symptoms and morphine-induced conditioned place preference by a glutamate transporter inhibitor DL-threo-beta-benzyloxy aspartate in rats. Eur J Pharmacol. 2004;485(1-3):201-210.
23. Bestha D, Soliman L, Blankenship K. et al. The walking wounded: emerging treatments for PTSD. Curr Psychiatry Rep. 2018;20(10):94.
24. Tsuda M, Shimizu N, Suzuki T. Contribution of glutamate receptors to benzodiazepine withdrawal signs. Jpn J Pharmacol. 1999;81(1):1-6.
25. Spravato [package insert]. Janssen Pharmaceuticals, Inc; 2019.
26. Mattingly GW, Anderson RH. Intranasal ketamine. Current Psychiatry. 2019;18(5):31-38.
27. Buckingham SC, Campbell SL, Haas BR, et al. Glutamate release by primary brain tumors induces epileptic activity. Nat Med. 2011;17(10):1269-1275.
28. Ferrari A, Spaccapelo L, Pinetti D, et al. Effective prophylactic treatment of migraines lower plasma glutamate levels. Cephalalgia. 2009;29(4):423-429.
29. Vieira DS, Naffah-Mazzacoratti Mda G, Zukerman E, et al. Glutamate levels in cerebrospinal fluid and triptans overuse in chronic migraine. Headache. 2007;47(6):842-847.
30. Chan K, MaassenVanDenBrink A. Glutamate receptor antagonists in the management of migraine. Drugs. 2014;74:1165-1176.
31. Pappa S, Tsouli S, Apostolou G, et al. Effects of amantadine on tardive dyskinesia: a randomized, double-blind, placebo-controlled study. Clin Neuropharmacol. 2010;33(6):271-275.
32. Kraal AZ, Arvanitis NR, Jaeger AP, et al. Could dietary glutamate play a role in psychiatric distress? Neuro Psych. 2020;79:13-19.
33. Levite M. Glutamate receptor antibodies in neurological diseases: anti-AMPA-GluR3 antibodies, Anti-NMDA-NR1 antibodies, Anti-NMDA-NR2A/B antibodies, Anti-mGluR1 antibodies or Anti-mGluR5 antibodies are present in subpopulations of patients with either: epilepsy, encephalitis, cerebellar ataxia, systemic lupus erythematosus (SLE) and neuropsychiatric SLE, Sjogren’s syndrome, schizophrenia, mania or stroke. These autoimmune anti-glutamate receptor antibodies can bind neurons in few brain regions, activate glutamate receptors, decrease glutamate receptor’s expression, impair glutamate-induced signaling and function, activate blood brain barrier endothelial cells, kill neurons, damage the brain, induce behavioral/psychiatric/cognitive abnormalities and ataxia in animal models, and can be removed or silenced in some patients by immunotherapy. J Neural Transm (Vienna). 2014;121(8):1029-1075.
34. Lancaster E. CNS syndromes associated with antibodies against metabotropic receptors. Curr Opin Neurol. 2017;30:354-360.
35. Sillevis Smitt P, Kinoshita A, De Leeuw B, et al. Paraneoplastic cerebellar ataxia due to autoantibodies against a glutamate receptor. N Engl J Med. 2000;342(1):21-27.
36. Marignier R, Chenevier F, Rogemond V, et al. Metabotropic glutamate receptor type 1 autoantibody-associated cerebellitis: a primary autoimmune disease? Arch Neurol. 2010;67(5):627-630.
37. Lancaster E, Martinez-Hernandez E, Titulaer MJ, et al. Antibodies to metabotropic glutamate receptor 5 in the Ophelia syndrome. Neurology. 2011;77:1698-1701.
38. Mat A, Adler H, Merwick A, et al. Ophelia syndrome with metabotropic glutamate receptor 5 antibodies in CSF. Neurology. 2013;80(14):1349-1350.
1. Petroff OA. GABA and glutamate in the human brain. Neuroscientist. 2002;8(6):562-573.
2. Winkelman JW, Buxton OM, Jensen JE, et al. Reduced brain GABA in primary insomnia: preliminary data from 4T proton magnetic resonance spectroscopy (1H-MRS). Sleep. 2008;31(11):1499-1506.
3. Pereira AC, Mao X, Jiang CS, et al. Dorsolateral prefrontal cortex GABA deficit in older adults with sleep-disordered breathing. Proc Natl Acad Sci U S A. 2017;114(38):10250-10255.
4. Schür RR, Draisma LW, Wijnen JP, et al. Brain GABA levels across psychiatric disorders: a systematic literature review and meta-analysis of (1) H-MRS studies. Hum Brain Mapp. 2016;37(9):3337-3352.
5. Evoy KE, Morrison MD, Saklad SR. Abuse and misuse of pregabalin and gabapentin. Drugs. 2017;77(4):403-426.
6. Mersfelder TL, Nichols WH. Gabapentin: abuse, dependence, and withdrawal. Ann Pharmacother. 2016;50(3):229-233.
7. Bremner JD. Traumatic stress: effects on the brain. Dialogues Clin Neurosci. 2006;8(4):445-461.
8. Kelly JR, Kennedy PJ, Cryan JF, et al. Breaking down the barriers: the gut microbiome, intestinal permeability, and stress-related psychiatric disorders. Front Cell Neurosci. 2015;9:392.
9. Guyton AC, Hall JE. Guyton and Hall Textbook of Medical Physiology. 12th ed. Elsevier; 2011:550-551,692-693.
10. Evrensel A, Ceylan ME. The gut-brain axis: the missing link in depression. Clin Psychopharmacol Neurosci. 2015;13(3):239-244.
11. Vianello M, Tavolato B, Giometto B. Glutamic acid decarboxylase autoantibodies and neurological disorders. Neurol Sci. 2002;23(4):145-151.
12. Marin O. Interneuron dysfunction in psychiatric disorders. Nat Rev Neurosci. 2012;13(2):107-120.
13. Huang D, Liu D, Yin J, et al. Glutamate-glutamine and GABA in the brain of normal aged and patients with cognitive impairment. Eur Radiol. 2017;27(7):2698-2705.
14. Jiménez-Jiménez FJ, Alonso-Navarro H, García-Martín E, et al. Neurochemical features of idiopathic restless legs syndrome. Sleep Med Rev. 2019;45:70-87.
15. Mick I, Ramos AC, Myers J, et al. Evidence for GABA-A receptor dysregulation in gambling disorder: correlation with impulsivity. Addict Biol. 2017;22(6):1601-1609.
16. Brambilla P, Perez J, Barale F, et al. Gabaergic dysfunction in mood disorders. Molecular Psychiatry. 2003;8:721-737.
17. Kaneez FS, Saeed SA. Investigating GABA and its function in platelets as compared to neurons. Platelets. 2009;20(5):328-333.
18. Paholpak P, Mendez MF. Trichotillomania as a manifestation of dementia. Case Rep Psychiatry. 2016;2016:9782702.
19. Miranda A, Peles S, Rudolph C, et al. Altered visceral sensation in response to somatic pain in the rat. Gastroenterology. 2004;126(4):1082-1089.
20. Skyba DA, King EW, Sluka KA. Effects of NMDA and non-NMDA ionotropic glutamate receptor antagonists on the development and maintenance of hyperalgesia induced by repeated intramuscular injection of acidic saline. Pain. 2002;98(1-2):69-78.
21. Holton KF, Taren DL, Thomson CA, et al. The effect of dietary glutamate on fibromyalgia and irritable bowel symptoms. Clin Exp Rheumatol. 2012;30(6 Suppl 74):10-70.
22. Sekiya Y, Nakagawa T, Ozawa T, et al. Facilitation of morphine withdrawal symptoms and morphine-induced conditioned place preference by a glutamate transporter inhibitor DL-threo-beta-benzyloxy aspartate in rats. Eur J Pharmacol. 2004;485(1-3):201-210.
23. Bestha D, Soliman L, Blankenship K. et al. The walking wounded: emerging treatments for PTSD. Curr Psychiatry Rep. 2018;20(10):94.
24. Tsuda M, Shimizu N, Suzuki T. Contribution of glutamate receptors to benzodiazepine withdrawal signs. Jpn J Pharmacol. 1999;81(1):1-6.
25. Spravato [package insert]. Janssen Pharmaceuticals, Inc; 2019.
26. Mattingly GW, Anderson RH. Intranasal ketamine. Current Psychiatry. 2019;18(5):31-38.
27. Buckingham SC, Campbell SL, Haas BR, et al. Glutamate release by primary brain tumors induces epileptic activity. Nat Med. 2011;17(10):1269-1275.
28. Ferrari A, Spaccapelo L, Pinetti D, et al. Effective prophylactic treatment of migraines lower plasma glutamate levels. Cephalalgia. 2009;29(4):423-429.
29. Vieira DS, Naffah-Mazzacoratti Mda G, Zukerman E, et al. Glutamate levels in cerebrospinal fluid and triptans overuse in chronic migraine. Headache. 2007;47(6):842-847.
30. Chan K, MaassenVanDenBrink A. Glutamate receptor antagonists in the management of migraine. Drugs. 2014;74:1165-1176.
31. Pappa S, Tsouli S, Apostolou G, et al. Effects of amantadine on tardive dyskinesia: a randomized, double-blind, placebo-controlled study. Clin Neuropharmacol. 2010;33(6):271-275.
32. Kraal AZ, Arvanitis NR, Jaeger AP, et al. Could dietary glutamate play a role in psychiatric distress? Neuro Psych. 2020;79:13-19.
33. Levite M. Glutamate receptor antibodies in neurological diseases: anti-AMPA-GluR3 antibodies, Anti-NMDA-NR1 antibodies, Anti-NMDA-NR2A/B antibodies, Anti-mGluR1 antibodies or Anti-mGluR5 antibodies are present in subpopulations of patients with either: epilepsy, encephalitis, cerebellar ataxia, systemic lupus erythematosus (SLE) and neuropsychiatric SLE, Sjogren’s syndrome, schizophrenia, mania or stroke. These autoimmune anti-glutamate receptor antibodies can bind neurons in few brain regions, activate glutamate receptors, decrease glutamate receptor’s expression, impair glutamate-induced signaling and function, activate blood brain barrier endothelial cells, kill neurons, damage the brain, induce behavioral/psychiatric/cognitive abnormalities and ataxia in animal models, and can be removed or silenced in some patients by immunotherapy. J Neural Transm (Vienna). 2014;121(8):1029-1075.
34. Lancaster E. CNS syndromes associated with antibodies against metabotropic receptors. Curr Opin Neurol. 2017;30:354-360.
35. Sillevis Smitt P, Kinoshita A, De Leeuw B, et al. Paraneoplastic cerebellar ataxia due to autoantibodies against a glutamate receptor. N Engl J Med. 2000;342(1):21-27.
36. Marignier R, Chenevier F, Rogemond V, et al. Metabotropic glutamate receptor type 1 autoantibody-associated cerebellitis: a primary autoimmune disease? Arch Neurol. 2010;67(5):627-630.
37. Lancaster E, Martinez-Hernandez E, Titulaer MJ, et al. Antibodies to metabotropic glutamate receptor 5 in the Ophelia syndrome. Neurology. 2011;77:1698-1701.
38. Mat A, Adler H, Merwick A, et al. Ophelia syndrome with metabotropic glutamate receptor 5 antibodies in CSF. Neurology. 2013;80(14):1349-1350.
Neurotransmitter-based diagnosis and treatment: A hypothesis (Part 2)
There is a need to connect mental and physical symptoms in the diagnosis and treatment of psychiatric disorders. Obviously, we are not yet equipped to clearly recognize which neurotransmitters cause which symptoms. The science of defining the underlying mechanisms is lagging behind the clinical needs. However, in this article, we present a few hypothetical clinical cases to emphasize a possible way of analyzing symptoms in order to identify underlying pathology and guide more effective treatment. Our descriptions do not reflect the entire set of symptoms caused by these neurotransmitters; we created them based on what is presently known (or suspected). Additional research is needed to confirm or disprove the hypotheses we present.
In Part 1 (
Endorphin excess (Table 11-16)
Ms. R is a frustrated chronic pain patient who bitterly complains that despite having seen more than 20 physicians, she does not have an answer to what causes her “all over” pain and headache.4,5,11 She does not believe that all her laboratory test are normal, and insists that “something is missing.” She aches all over but says she can actually tolerate more pain than others and experiences only a little discomfort during an electromyogram or dental interventions. Though Ms. R is not very susceptible to acute pain,4,5,9,16 pain all over without an identifiable cause is part of her life.4,5,11 She says that listening to music and social interactions help decrease her pain.4,5,10 Ms. R states that opioid medications do not help her pain, though she has a history of opioid overuse and opioid-induced hyperalgesia.6,11,16
Ms. R tends to overdo pleasureful activities to achieve satisfaction.2 She says exercise is particularly satisfying, to the point that she experiences euphoria and a loss of time.9 She is angry that her neurologist suggested she see a psychiatrist. Her depression bothers her more than her anxiety.2,5,7
Ms. R clearly has a self-image problem, alternating between high and low self-esteem. She has a low appetite1,12,14-16 and sleeps excessively.2,4,7,9,10 Her mother privately tells you that Ms. R has a history of childhood sexual abuse and lagged in life due to a lack of motivation. Ms. R used to self-mutilate “to feel normal.”12 Her primary care physician chronically addresses Ms. R’s poorly explained cholestasis and pruritus8 as well as dysregulation of blood pressure and heart rate, both of which tend to be low.12,13,16
Impression. Ms. R shows multiple symptoms associated with endorphin excess. A trial of an opioid antagonist may be reasonable. Dopamine blockade helps with endorphin suppression and also may be used for this patient. Using a low starting dose and a slow titration of such medications would be beneficial due to frequent intolerance issues, especially nausea. Gamma aminobutyric acid-ergic medications modulate the opioid system and may be considered. A serotonin-norepinephrine reuptake inhibitor (SNRI) or mirtazapine may help patients such as Ms. R to control mood and pain through norepinephrine’s influence on endorphins.
Endorphin deficiency (Table 11,16-24)
Mr. J complains of low back pain, diffuse body pain, depression, and moodiness.19,20,24 He is sluggish and plagued by psychomotor retardation.24 All his life, a heightened perception of pain has caused him problems,19,20 but has not stopped him from engaging in self-mutilation
Continue to: Mr. J responds to treatment...
Mr. J responds to treatment with opioids16,20 but comments that his mood, and not necessarily his pain, improves when he takes these medications.20 He tends to overuse his pain medications, and had run into trouble with his previous pain management physician. Nitrous oxide is remarkably effective during dental procedures.19 Acupuncture helps to control his pain and mood.17 Exercise is also rewarding.18
Mr. J has difficulty achieving orgasm, a decreased sexual drive, and emotional sensitivity.24 He is impulsive.19,20,24 His baseline mood is low-grade; anxiety bothers him more than depression.23,24 Mr. J is thin, has a poor appetite,1,16 and sleeps poorly.24 His primary care physician struggles to help Mr. J to control dysregulation of his heart rate, blood pressure,21 and urinary retention,16,22 as well as episodes of hypoglycemia.1,16 He reluctantly admits to abusing alcohol, but explains that it helps with his mood and pain better than his prescribed medications.18,23
Impression. Mr. J exhibits multiple symptoms associated with endorphin deficiency. Short-term use of opioids is warranted, but he should avoid long-term opioid use, and he and his physician should work together to establish strict control of their intake. Buprenorphine would be the opioid of choice for such a patient. Psychiatric treatment, including for alcohol use disorder, should be a mandatory part of his treatment regimen. Behavioral therapy with a focus on finding healthy ways to achieve gratification would be effective. Alternative treatments such as acupuncture may be of value.
Norepinephrine excess (Table 216,25-30)
Mr. G comes to the office irritable and angry28,30 because no one can help him with his intractable headaches.
Comment. Norepinephrine and dopamine functions are connected through common neuronal and glial uptake mechanisms. This is a foundation of norepinephrine excess symptoms crossing over with symptoms of dopamine deficiency.
Continue to: Impression
Impression. Mr. G shows multiple symptoms associated with norepinephrine excess. It is important to avoid caffeine intake in patients with clinical signs of excessive norepinephrine. Beta-blockers and alpha-2 agonists work well in patients such as Mr. G. Benzodiazepines indirectly decrease norepinephrine activity, but need to be used carefully due to the potential for misuse and addiction. In particular, short-acting benzodiazepines such as alprazolam and lorazepam must be avoided due to the induction of CNS instability with rapidly changing medication blood levels. Chlordiazepoxide may be a good choice for a patient such as Mr. G because it has the fewest adverse effects and the lowest abuse potential compared with other benzodiazepines. Avoid SNRIs in such a patient. Using mood-stabilizing antipsychotic medications may be especially warranted in treating Mr. G’s depression and pain.
Norepinephrine deficiency (Table 216,26,31-39)
Two years ago, Ms. A was diagnosed with chronic fatigue31 and fibromyalgia. She also had been diagnosed with depression and attention-deficit/hyperactivity disorder (ADHD). She presents with concerns of “brain fog,” no energy, low sex drive, and daytime sleepiness.33,35 Allodynia is widespread.16,36,37 Ms. A suffers from bulimia; she eats once a day but is still overweight.26 She has orthostatic hypotension in addition to baseline low blood pressure and bradycardia.16,38,39 Her pupils are almost pinpoint, even when she does not take opioid medications.
Comment. As mentioned earlier, because of the norepinephrine/dopamine relationship, symptoms of excess dopamine overlap with symptoms of norepinephrine deficiency.
Impression. Ms. A shows multiple symptoms associated with norepinephrine deficiency. The use of noradrenergic antidepressants (such as SNRIs and mirtazapine)26 and stimulants may be warranted. Physical exercise, participating in social activities, massage, acupuncture, and family support may help with Ms. A’s pain as well as her depression, as might vasopressors.
In Part 3, we will address gamma aminobutyric acid and glutamate.
Bottom Line
Both high and low levels of endorphins and norepinephrine may be associated with certain psychiatric and medical symptoms and disorders. An astute clinician may judge which neurotransmitter is dysfunctional based on the patient’s presentation, and tailor treatment accordingly.
Related Resources
- Arbuck DM, Salmerón JM, Mueller R. Neurotransmitter-based diagnosis and treatment: a hypothesis (Part 1). Current Psychiatry. 2022;21(5):30-36. doi:10.12788/cp.0242
Drug Brand Names
Alprazolam • Xanax
Chlordiazepoxide • Librium
Lorazepam • Ativan
Mirtazapine • Remeron
1. Applyard SM, Hayward M, Young JI, et al. A role for the endogenous opioid beta-endorphin in energy homeostasis. Endocrinology. 2003;144(5):1753-1760.
2. Craft LL, Perna FM. The benefits of exercise for the clinically depressed. Prim Care Companion J Clin Psychiatry. 2004;6(3):104-111.
3. Dabo F, Nyberg F, Qin Zhou, et al. Plasma levels of beta-endorphin during pregnancy and use of labor analgesia. Reprod Sci. 2010;17(8):742-747.
4. Dunbar RI, Kaskatis K, MacDonald I, et al. Performance of music elevates pain threshold and positive affect: implications for the evolutionary function of music. Evol Psychol. 2012;10(4):688-702.
5. Dunbar RIM, Baron R, Frangou A, et al. Social laughter is correlated with an elevated pain threshold. Proc Biol Sci. 2012;279(1731):1161-1167.
6. Grisel JE, Bartels JL, Allen SA, et al. Influence of beta-Endorphin on anxious behavior in mice: interaction with EtOH. Psychopharmacology (Berl). 2008;200(1):105-115.
7. Zorrilla EP, DeRubeis RJ, Redei E. High self-esteem, hardiness, and affective stability are associated with higher basal pituitary-adrenal hormone levels. Psychoneuroendocrinology. 1995;20(6):591-601.
8. Li X, Zhu J, Tao Y, et al. Elevated endogenous opioids in obstructive jaundice: the possible skin mechanisms. Med Hypotheses. 2018;116:119-121.
9. Hicks SD, Jacob P, Perez O, et al. The transcriptional signature of a runner’s high. Med Sci Sports Exerc. 2019;51(5):970-978.
10. Dunbar RIM. The anatomy of friendship. Trends Cogn Sci. 2018;22(1):32-51.
11. Stephan BC, Parsa FD. Avoiding opioids and their harmful side effects in the postoperative patient: exogenous opioids, endogenous endorphins, wellness, mood, and their relation to postoperative pain. Hawaii J Med Public Health. 2016;75(3):63-70.
12. Cuthbert BN, Holaday JW, Meyerhoff J, et al. Intravenous beta-endorphin: behavioral and physiological effects in conscious monkeys. Peptides. 1989;10(4):729-734.
13. Levin ER, Mills S, Weber MA. Endogenous opioids and opiate antagonists modulate the blood pressure of the spontaneously hypertensive rat. Peptides. 1986;(6):977-981.
14. Davis JM, Lowy MT, Yim GK, et al. Relationship between plasma concentrations of immunoreactive beta-endorphin and food intake in rats. Peptides. 1983;4(1):79-83.
15. Leibowitz SF, Hor L. Endorphinergic and alpha-noradrenergic systems in the paraventricular nucleus: effects on eating behavior. Peptides. 1982;3(3): 421-428.
16. Hall JE, Guyton AC. Textbook of Medical Physiology. 12th ed. Spanish version. Elsevier; 2011:587-588.
17. Han JS. Acupuncture and endorphins. Neurosci Lett. 2004;361(1-3):258-261.
18. Harte JL, Eifert GH, Smith R. The effects of running and meditation on beta-endorphin, corticotropin-releasing hormone and cortisol in plasma, and on mood. Biol Psychol. 1995;40(3):251-265.
19. Petrizzo R, Mohr J, Mantione K, et al. The role of endogenous morphine and nitric oxide in pain management. Pract Pain Manag. 2014;14(9).
20. Sprouse-Blum AS, Smith G, Sugai D, et al. Understanding endorphins and their importance in pain management. Hawaii Med J. 2010;69(3):70-100.
21. Dontsov AV. The influence of deficit of endogenous neuropeptides on the clinical course of coronary artery disease. Klin Med (Mosk). 2017;95(2):127-131. In Russian.
22. Dray A, Metsch R, Davis TP. Endorphins and the central inhibition of urinary bladder motility. Peptides. 1984;5(3):645-647.
23. Zalewska-Kaszubska J, Czarnecka E. Deficit in beta-endorphin peptide and tendency to alcohol abuse. Peptides. 2005;26(4):701-705.
24. McLay RN, Pan W, Kastin AJ. Effects of peptides on animal and human behavior: a review of studies published in the first twenty years of the journal Peptides. Peptides. 2001;22(12):2181-2255.
25. Wong-Riley MT. Neuroscience Secrets. 1st ed. Spanish version. Hanley & Belfus; 1999:424-428.
26. Brewerton TD. Clinical Handbook of Eating Disorders: An Integrated Approach. CRC Press; 2004:257-281.
27. Winklewski PJ, Radkowski M, Wszedybyl-Winklewska M, et al. Stress response, brain noradrenergic system and cognition. Adv Exp Med Biol. 2017;980:67-74.
28. McCall JG, Al-Hasani R, Siuda ER, et al. Engagement of the locus coeruleus noradrenergic system mediates stress-induced anxiety. Neuron. 2015;87(3):605-620.
29. Wszedybyl-Winklewska M, Wolf J, Szarmach A, et al. Central sympathetic nervous system reinforcement in obstructive sleep apnoea. Sleep Med Rev. 2018;39:143-154.
30. Yamamoto K, Shinba T, Yoshii M. Psychiatric symptoms of noradrenergic dysfunction: a pathophysiological view. Psychiatry Clin Neurosci. 2014;201(68):1-20.
31. Stone EA, Lin Y, Sarfraz Y, et al. The role of the central noradrenergic system in behavioral inhibition. Brain Res Rev. 2011;67(1-2):193-208.
32. Haddjeri N, Blier P, de Montigny C. Effect of the alpha-2 adrenoceptor antagonist mirtazapine on the 5-hydroxytryptamine system in the rat brain. J Pharmacol Exp Ther. 1996;277:861-871.
33. De Carvalho D, Patrone LG, Taxini CL, et al. Neurochemical and electrical modulation of the locus coeruleus: contribution to CO2 drive to breathe. Front Physiol. 2014;5(288):1-13.
34. Markianos M, Evangelopoulos ME, Koutsis G, et al. Evidence for involvement of central noradrenergic activity in crying proneness. J Neuropsychiatry Clin Neurosci. 2011;23:403-408.
35. Cao S, Fisher DW, Yu T, et al. The link between chronic pain and Alzheimer’s disease. J Neuroinflammation. 2019;(16):204-215.
36. Caraci F, Merlo S, Drago F, et al. Rescue of noradrenergic system as a novel pharmacological strategy in the treatment of chronic pain: focus on microglia activation. Front Pharmacol. 2019;(10):1024.
37. Hayashida KI, Obata H. Strategies to treat chronic pain and strengthen impaired descending noradrenergic inhibitory system. Int J Mol Sci. 2019;20(4):822.
38. Kur’yanova EV, Tryasuchev AV, Stupin VO, et al. Effect of atropine on adrenergic responsiveness of erythrocyte and heart rhythm variability in outbred rats with stimulation of the central neurotransmitter systems. Bull Exp Biol Med. 2018;165(5):165(5):597-601.
39. Peterson AC, Li CR. Noradrenergic dysfunction in Alzheimer’s and Parkinson’s disease: an overview of imaging studies. Front Aging Neurosci. 2018;(10):127.
There is a need to connect mental and physical symptoms in the diagnosis and treatment of psychiatric disorders. Obviously, we are not yet equipped to clearly recognize which neurotransmitters cause which symptoms. The science of defining the underlying mechanisms is lagging behind the clinical needs. However, in this article, we present a few hypothetical clinical cases to emphasize a possible way of analyzing symptoms in order to identify underlying pathology and guide more effective treatment. Our descriptions do not reflect the entire set of symptoms caused by these neurotransmitters; we created them based on what is presently known (or suspected). Additional research is needed to confirm or disprove the hypotheses we present.
In Part 1 (
Endorphin excess (Table 11-16)
Ms. R is a frustrated chronic pain patient who bitterly complains that despite having seen more than 20 physicians, she does not have an answer to what causes her “all over” pain and headache.4,5,11 She does not believe that all her laboratory test are normal, and insists that “something is missing.” She aches all over but says she can actually tolerate more pain than others and experiences only a little discomfort during an electromyogram or dental interventions. Though Ms. R is not very susceptible to acute pain,4,5,9,16 pain all over without an identifiable cause is part of her life.4,5,11 She says that listening to music and social interactions help decrease her pain.4,5,10 Ms. R states that opioid medications do not help her pain, though she has a history of opioid overuse and opioid-induced hyperalgesia.6,11,16
Ms. R tends to overdo pleasureful activities to achieve satisfaction.2 She says exercise is particularly satisfying, to the point that she experiences euphoria and a loss of time.9 She is angry that her neurologist suggested she see a psychiatrist. Her depression bothers her more than her anxiety.2,5,7
Ms. R clearly has a self-image problem, alternating between high and low self-esteem. She has a low appetite1,12,14-16 and sleeps excessively.2,4,7,9,10 Her mother privately tells you that Ms. R has a history of childhood sexual abuse and lagged in life due to a lack of motivation. Ms. R used to self-mutilate “to feel normal.”12 Her primary care physician chronically addresses Ms. R’s poorly explained cholestasis and pruritus8 as well as dysregulation of blood pressure and heart rate, both of which tend to be low.12,13,16
Impression. Ms. R shows multiple symptoms associated with endorphin excess. A trial of an opioid antagonist may be reasonable. Dopamine blockade helps with endorphin suppression and also may be used for this patient. Using a low starting dose and a slow titration of such medications would be beneficial due to frequent intolerance issues, especially nausea. Gamma aminobutyric acid-ergic medications modulate the opioid system and may be considered. A serotonin-norepinephrine reuptake inhibitor (SNRI) or mirtazapine may help patients such as Ms. R to control mood and pain through norepinephrine’s influence on endorphins.
Endorphin deficiency (Table 11,16-24)
Mr. J complains of low back pain, diffuse body pain, depression, and moodiness.19,20,24 He is sluggish and plagued by psychomotor retardation.24 All his life, a heightened perception of pain has caused him problems,19,20 but has not stopped him from engaging in self-mutilation
Continue to: Mr. J responds to treatment...
Mr. J responds to treatment with opioids16,20 but comments that his mood, and not necessarily his pain, improves when he takes these medications.20 He tends to overuse his pain medications, and had run into trouble with his previous pain management physician. Nitrous oxide is remarkably effective during dental procedures.19 Acupuncture helps to control his pain and mood.17 Exercise is also rewarding.18
Mr. J has difficulty achieving orgasm, a decreased sexual drive, and emotional sensitivity.24 He is impulsive.19,20,24 His baseline mood is low-grade; anxiety bothers him more than depression.23,24 Mr. J is thin, has a poor appetite,1,16 and sleeps poorly.24 His primary care physician struggles to help Mr. J to control dysregulation of his heart rate, blood pressure,21 and urinary retention,16,22 as well as episodes of hypoglycemia.1,16 He reluctantly admits to abusing alcohol, but explains that it helps with his mood and pain better than his prescribed medications.18,23
Impression. Mr. J exhibits multiple symptoms associated with endorphin deficiency. Short-term use of opioids is warranted, but he should avoid long-term opioid use, and he and his physician should work together to establish strict control of their intake. Buprenorphine would be the opioid of choice for such a patient. Psychiatric treatment, including for alcohol use disorder, should be a mandatory part of his treatment regimen. Behavioral therapy with a focus on finding healthy ways to achieve gratification would be effective. Alternative treatments such as acupuncture may be of value.
Norepinephrine excess (Table 216,25-30)
Mr. G comes to the office irritable and angry28,30 because no one can help him with his intractable headaches.
Comment. Norepinephrine and dopamine functions are connected through common neuronal and glial uptake mechanisms. This is a foundation of norepinephrine excess symptoms crossing over with symptoms of dopamine deficiency.
Continue to: Impression
Impression. Mr. G shows multiple symptoms associated with norepinephrine excess. It is important to avoid caffeine intake in patients with clinical signs of excessive norepinephrine. Beta-blockers and alpha-2 agonists work well in patients such as Mr. G. Benzodiazepines indirectly decrease norepinephrine activity, but need to be used carefully due to the potential for misuse and addiction. In particular, short-acting benzodiazepines such as alprazolam and lorazepam must be avoided due to the induction of CNS instability with rapidly changing medication blood levels. Chlordiazepoxide may be a good choice for a patient such as Mr. G because it has the fewest adverse effects and the lowest abuse potential compared with other benzodiazepines. Avoid SNRIs in such a patient. Using mood-stabilizing antipsychotic medications may be especially warranted in treating Mr. G’s depression and pain.
Norepinephrine deficiency (Table 216,26,31-39)
Two years ago, Ms. A was diagnosed with chronic fatigue31 and fibromyalgia. She also had been diagnosed with depression and attention-deficit/hyperactivity disorder (ADHD). She presents with concerns of “brain fog,” no energy, low sex drive, and daytime sleepiness.33,35 Allodynia is widespread.16,36,37 Ms. A suffers from bulimia; she eats once a day but is still overweight.26 She has orthostatic hypotension in addition to baseline low blood pressure and bradycardia.16,38,39 Her pupils are almost pinpoint, even when she does not take opioid medications.
Comment. As mentioned earlier, because of the norepinephrine/dopamine relationship, symptoms of excess dopamine overlap with symptoms of norepinephrine deficiency.
Impression. Ms. A shows multiple symptoms associated with norepinephrine deficiency. The use of noradrenergic antidepressants (such as SNRIs and mirtazapine)26 and stimulants may be warranted. Physical exercise, participating in social activities, massage, acupuncture, and family support may help with Ms. A’s pain as well as her depression, as might vasopressors.
In Part 3, we will address gamma aminobutyric acid and glutamate.
Bottom Line
Both high and low levels of endorphins and norepinephrine may be associated with certain psychiatric and medical symptoms and disorders. An astute clinician may judge which neurotransmitter is dysfunctional based on the patient’s presentation, and tailor treatment accordingly.
Related Resources
- Arbuck DM, Salmerón JM, Mueller R. Neurotransmitter-based diagnosis and treatment: a hypothesis (Part 1). Current Psychiatry. 2022;21(5):30-36. doi:10.12788/cp.0242
Drug Brand Names
Alprazolam • Xanax
Chlordiazepoxide • Librium
Lorazepam • Ativan
Mirtazapine • Remeron
There is a need to connect mental and physical symptoms in the diagnosis and treatment of psychiatric disorders. Obviously, we are not yet equipped to clearly recognize which neurotransmitters cause which symptoms. The science of defining the underlying mechanisms is lagging behind the clinical needs. However, in this article, we present a few hypothetical clinical cases to emphasize a possible way of analyzing symptoms in order to identify underlying pathology and guide more effective treatment. Our descriptions do not reflect the entire set of symptoms caused by these neurotransmitters; we created them based on what is presently known (or suspected). Additional research is needed to confirm or disprove the hypotheses we present.
In Part 1 (
Endorphin excess (Table 11-16)
Ms. R is a frustrated chronic pain patient who bitterly complains that despite having seen more than 20 physicians, she does not have an answer to what causes her “all over” pain and headache.4,5,11 She does not believe that all her laboratory test are normal, and insists that “something is missing.” She aches all over but says she can actually tolerate more pain than others and experiences only a little discomfort during an electromyogram or dental interventions. Though Ms. R is not very susceptible to acute pain,4,5,9,16 pain all over without an identifiable cause is part of her life.4,5,11 She says that listening to music and social interactions help decrease her pain.4,5,10 Ms. R states that opioid medications do not help her pain, though she has a history of opioid overuse and opioid-induced hyperalgesia.6,11,16
Ms. R tends to overdo pleasureful activities to achieve satisfaction.2 She says exercise is particularly satisfying, to the point that she experiences euphoria and a loss of time.9 She is angry that her neurologist suggested she see a psychiatrist. Her depression bothers her more than her anxiety.2,5,7
Ms. R clearly has a self-image problem, alternating between high and low self-esteem. She has a low appetite1,12,14-16 and sleeps excessively.2,4,7,9,10 Her mother privately tells you that Ms. R has a history of childhood sexual abuse and lagged in life due to a lack of motivation. Ms. R used to self-mutilate “to feel normal.”12 Her primary care physician chronically addresses Ms. R’s poorly explained cholestasis and pruritus8 as well as dysregulation of blood pressure and heart rate, both of which tend to be low.12,13,16
Impression. Ms. R shows multiple symptoms associated with endorphin excess. A trial of an opioid antagonist may be reasonable. Dopamine blockade helps with endorphin suppression and also may be used for this patient. Using a low starting dose and a slow titration of such medications would be beneficial due to frequent intolerance issues, especially nausea. Gamma aminobutyric acid-ergic medications modulate the opioid system and may be considered. A serotonin-norepinephrine reuptake inhibitor (SNRI) or mirtazapine may help patients such as Ms. R to control mood and pain through norepinephrine’s influence on endorphins.
Endorphin deficiency (Table 11,16-24)
Mr. J complains of low back pain, diffuse body pain, depression, and moodiness.19,20,24 He is sluggish and plagued by psychomotor retardation.24 All his life, a heightened perception of pain has caused him problems,19,20 but has not stopped him from engaging in self-mutilation
Continue to: Mr. J responds to treatment...
Mr. J responds to treatment with opioids16,20 but comments that his mood, and not necessarily his pain, improves when he takes these medications.20 He tends to overuse his pain medications, and had run into trouble with his previous pain management physician. Nitrous oxide is remarkably effective during dental procedures.19 Acupuncture helps to control his pain and mood.17 Exercise is also rewarding.18
Mr. J has difficulty achieving orgasm, a decreased sexual drive, and emotional sensitivity.24 He is impulsive.19,20,24 His baseline mood is low-grade; anxiety bothers him more than depression.23,24 Mr. J is thin, has a poor appetite,1,16 and sleeps poorly.24 His primary care physician struggles to help Mr. J to control dysregulation of his heart rate, blood pressure,21 and urinary retention,16,22 as well as episodes of hypoglycemia.1,16 He reluctantly admits to abusing alcohol, but explains that it helps with his mood and pain better than his prescribed medications.18,23
Impression. Mr. J exhibits multiple symptoms associated with endorphin deficiency. Short-term use of opioids is warranted, but he should avoid long-term opioid use, and he and his physician should work together to establish strict control of their intake. Buprenorphine would be the opioid of choice for such a patient. Psychiatric treatment, including for alcohol use disorder, should be a mandatory part of his treatment regimen. Behavioral therapy with a focus on finding healthy ways to achieve gratification would be effective. Alternative treatments such as acupuncture may be of value.
Norepinephrine excess (Table 216,25-30)
Mr. G comes to the office irritable and angry28,30 because no one can help him with his intractable headaches.
Comment. Norepinephrine and dopamine functions are connected through common neuronal and glial uptake mechanisms. This is a foundation of norepinephrine excess symptoms crossing over with symptoms of dopamine deficiency.
Continue to: Impression
Impression. Mr. G shows multiple symptoms associated with norepinephrine excess. It is important to avoid caffeine intake in patients with clinical signs of excessive norepinephrine. Beta-blockers and alpha-2 agonists work well in patients such as Mr. G. Benzodiazepines indirectly decrease norepinephrine activity, but need to be used carefully due to the potential for misuse and addiction. In particular, short-acting benzodiazepines such as alprazolam and lorazepam must be avoided due to the induction of CNS instability with rapidly changing medication blood levels. Chlordiazepoxide may be a good choice for a patient such as Mr. G because it has the fewest adverse effects and the lowest abuse potential compared with other benzodiazepines. Avoid SNRIs in such a patient. Using mood-stabilizing antipsychotic medications may be especially warranted in treating Mr. G’s depression and pain.
Norepinephrine deficiency (Table 216,26,31-39)
Two years ago, Ms. A was diagnosed with chronic fatigue31 and fibromyalgia. She also had been diagnosed with depression and attention-deficit/hyperactivity disorder (ADHD). She presents with concerns of “brain fog,” no energy, low sex drive, and daytime sleepiness.33,35 Allodynia is widespread.16,36,37 Ms. A suffers from bulimia; she eats once a day but is still overweight.26 She has orthostatic hypotension in addition to baseline low blood pressure and bradycardia.16,38,39 Her pupils are almost pinpoint, even when she does not take opioid medications.
Comment. As mentioned earlier, because of the norepinephrine/dopamine relationship, symptoms of excess dopamine overlap with symptoms of norepinephrine deficiency.
Impression. Ms. A shows multiple symptoms associated with norepinephrine deficiency. The use of noradrenergic antidepressants (such as SNRIs and mirtazapine)26 and stimulants may be warranted. Physical exercise, participating in social activities, massage, acupuncture, and family support may help with Ms. A’s pain as well as her depression, as might vasopressors.
In Part 3, we will address gamma aminobutyric acid and glutamate.
Bottom Line
Both high and low levels of endorphins and norepinephrine may be associated with certain psychiatric and medical symptoms and disorders. An astute clinician may judge which neurotransmitter is dysfunctional based on the patient’s presentation, and tailor treatment accordingly.
Related Resources
- Arbuck DM, Salmerón JM, Mueller R. Neurotransmitter-based diagnosis and treatment: a hypothesis (Part 1). Current Psychiatry. 2022;21(5):30-36. doi:10.12788/cp.0242
Drug Brand Names
Alprazolam • Xanax
Chlordiazepoxide • Librium
Lorazepam • Ativan
Mirtazapine • Remeron
1. Applyard SM, Hayward M, Young JI, et al. A role for the endogenous opioid beta-endorphin in energy homeostasis. Endocrinology. 2003;144(5):1753-1760.
2. Craft LL, Perna FM. The benefits of exercise for the clinically depressed. Prim Care Companion J Clin Psychiatry. 2004;6(3):104-111.
3. Dabo F, Nyberg F, Qin Zhou, et al. Plasma levels of beta-endorphin during pregnancy and use of labor analgesia. Reprod Sci. 2010;17(8):742-747.
4. Dunbar RI, Kaskatis K, MacDonald I, et al. Performance of music elevates pain threshold and positive affect: implications for the evolutionary function of music. Evol Psychol. 2012;10(4):688-702.
5. Dunbar RIM, Baron R, Frangou A, et al. Social laughter is correlated with an elevated pain threshold. Proc Biol Sci. 2012;279(1731):1161-1167.
6. Grisel JE, Bartels JL, Allen SA, et al. Influence of beta-Endorphin on anxious behavior in mice: interaction with EtOH. Psychopharmacology (Berl). 2008;200(1):105-115.
7. Zorrilla EP, DeRubeis RJ, Redei E. High self-esteem, hardiness, and affective stability are associated with higher basal pituitary-adrenal hormone levels. Psychoneuroendocrinology. 1995;20(6):591-601.
8. Li X, Zhu J, Tao Y, et al. Elevated endogenous opioids in obstructive jaundice: the possible skin mechanisms. Med Hypotheses. 2018;116:119-121.
9. Hicks SD, Jacob P, Perez O, et al. The transcriptional signature of a runner’s high. Med Sci Sports Exerc. 2019;51(5):970-978.
10. Dunbar RIM. The anatomy of friendship. Trends Cogn Sci. 2018;22(1):32-51.
11. Stephan BC, Parsa FD. Avoiding opioids and their harmful side effects in the postoperative patient: exogenous opioids, endogenous endorphins, wellness, mood, and their relation to postoperative pain. Hawaii J Med Public Health. 2016;75(3):63-70.
12. Cuthbert BN, Holaday JW, Meyerhoff J, et al. Intravenous beta-endorphin: behavioral and physiological effects in conscious monkeys. Peptides. 1989;10(4):729-734.
13. Levin ER, Mills S, Weber MA. Endogenous opioids and opiate antagonists modulate the blood pressure of the spontaneously hypertensive rat. Peptides. 1986;(6):977-981.
14. Davis JM, Lowy MT, Yim GK, et al. Relationship between plasma concentrations of immunoreactive beta-endorphin and food intake in rats. Peptides. 1983;4(1):79-83.
15. Leibowitz SF, Hor L. Endorphinergic and alpha-noradrenergic systems in the paraventricular nucleus: effects on eating behavior. Peptides. 1982;3(3): 421-428.
16. Hall JE, Guyton AC. Textbook of Medical Physiology. 12th ed. Spanish version. Elsevier; 2011:587-588.
17. Han JS. Acupuncture and endorphins. Neurosci Lett. 2004;361(1-3):258-261.
18. Harte JL, Eifert GH, Smith R. The effects of running and meditation on beta-endorphin, corticotropin-releasing hormone and cortisol in plasma, and on mood. Biol Psychol. 1995;40(3):251-265.
19. Petrizzo R, Mohr J, Mantione K, et al. The role of endogenous morphine and nitric oxide in pain management. Pract Pain Manag. 2014;14(9).
20. Sprouse-Blum AS, Smith G, Sugai D, et al. Understanding endorphins and their importance in pain management. Hawaii Med J. 2010;69(3):70-100.
21. Dontsov AV. The influence of deficit of endogenous neuropeptides on the clinical course of coronary artery disease. Klin Med (Mosk). 2017;95(2):127-131. In Russian.
22. Dray A, Metsch R, Davis TP. Endorphins and the central inhibition of urinary bladder motility. Peptides. 1984;5(3):645-647.
23. Zalewska-Kaszubska J, Czarnecka E. Deficit in beta-endorphin peptide and tendency to alcohol abuse. Peptides. 2005;26(4):701-705.
24. McLay RN, Pan W, Kastin AJ. Effects of peptides on animal and human behavior: a review of studies published in the first twenty years of the journal Peptides. Peptides. 2001;22(12):2181-2255.
25. Wong-Riley MT. Neuroscience Secrets. 1st ed. Spanish version. Hanley & Belfus; 1999:424-428.
26. Brewerton TD. Clinical Handbook of Eating Disorders: An Integrated Approach. CRC Press; 2004:257-281.
27. Winklewski PJ, Radkowski M, Wszedybyl-Winklewska M, et al. Stress response, brain noradrenergic system and cognition. Adv Exp Med Biol. 2017;980:67-74.
28. McCall JG, Al-Hasani R, Siuda ER, et al. Engagement of the locus coeruleus noradrenergic system mediates stress-induced anxiety. Neuron. 2015;87(3):605-620.
29. Wszedybyl-Winklewska M, Wolf J, Szarmach A, et al. Central sympathetic nervous system reinforcement in obstructive sleep apnoea. Sleep Med Rev. 2018;39:143-154.
30. Yamamoto K, Shinba T, Yoshii M. Psychiatric symptoms of noradrenergic dysfunction: a pathophysiological view. Psychiatry Clin Neurosci. 2014;201(68):1-20.
31. Stone EA, Lin Y, Sarfraz Y, et al. The role of the central noradrenergic system in behavioral inhibition. Brain Res Rev. 2011;67(1-2):193-208.
32. Haddjeri N, Blier P, de Montigny C. Effect of the alpha-2 adrenoceptor antagonist mirtazapine on the 5-hydroxytryptamine system in the rat brain. J Pharmacol Exp Ther. 1996;277:861-871.
33. De Carvalho D, Patrone LG, Taxini CL, et al. Neurochemical and electrical modulation of the locus coeruleus: contribution to CO2 drive to breathe. Front Physiol. 2014;5(288):1-13.
34. Markianos M, Evangelopoulos ME, Koutsis G, et al. Evidence for involvement of central noradrenergic activity in crying proneness. J Neuropsychiatry Clin Neurosci. 2011;23:403-408.
35. Cao S, Fisher DW, Yu T, et al. The link between chronic pain and Alzheimer’s disease. J Neuroinflammation. 2019;(16):204-215.
36. Caraci F, Merlo S, Drago F, et al. Rescue of noradrenergic system as a novel pharmacological strategy in the treatment of chronic pain: focus on microglia activation. Front Pharmacol. 2019;(10):1024.
37. Hayashida KI, Obata H. Strategies to treat chronic pain and strengthen impaired descending noradrenergic inhibitory system. Int J Mol Sci. 2019;20(4):822.
38. Kur’yanova EV, Tryasuchev AV, Stupin VO, et al. Effect of atropine on adrenergic responsiveness of erythrocyte and heart rhythm variability in outbred rats with stimulation of the central neurotransmitter systems. Bull Exp Biol Med. 2018;165(5):165(5):597-601.
39. Peterson AC, Li CR. Noradrenergic dysfunction in Alzheimer’s and Parkinson’s disease: an overview of imaging studies. Front Aging Neurosci. 2018;(10):127.
1. Applyard SM, Hayward M, Young JI, et al. A role for the endogenous opioid beta-endorphin in energy homeostasis. Endocrinology. 2003;144(5):1753-1760.
2. Craft LL, Perna FM. The benefits of exercise for the clinically depressed. Prim Care Companion J Clin Psychiatry. 2004;6(3):104-111.
3. Dabo F, Nyberg F, Qin Zhou, et al. Plasma levels of beta-endorphin during pregnancy and use of labor analgesia. Reprod Sci. 2010;17(8):742-747.
4. Dunbar RI, Kaskatis K, MacDonald I, et al. Performance of music elevates pain threshold and positive affect: implications for the evolutionary function of music. Evol Psychol. 2012;10(4):688-702.
5. Dunbar RIM, Baron R, Frangou A, et al. Social laughter is correlated with an elevated pain threshold. Proc Biol Sci. 2012;279(1731):1161-1167.
6. Grisel JE, Bartels JL, Allen SA, et al. Influence of beta-Endorphin on anxious behavior in mice: interaction with EtOH. Psychopharmacology (Berl). 2008;200(1):105-115.
7. Zorrilla EP, DeRubeis RJ, Redei E. High self-esteem, hardiness, and affective stability are associated with higher basal pituitary-adrenal hormone levels. Psychoneuroendocrinology. 1995;20(6):591-601.
8. Li X, Zhu J, Tao Y, et al. Elevated endogenous opioids in obstructive jaundice: the possible skin mechanisms. Med Hypotheses. 2018;116:119-121.
9. Hicks SD, Jacob P, Perez O, et al. The transcriptional signature of a runner’s high. Med Sci Sports Exerc. 2019;51(5):970-978.
10. Dunbar RIM. The anatomy of friendship. Trends Cogn Sci. 2018;22(1):32-51.
11. Stephan BC, Parsa FD. Avoiding opioids and their harmful side effects in the postoperative patient: exogenous opioids, endogenous endorphins, wellness, mood, and their relation to postoperative pain. Hawaii J Med Public Health. 2016;75(3):63-70.
12. Cuthbert BN, Holaday JW, Meyerhoff J, et al. Intravenous beta-endorphin: behavioral and physiological effects in conscious monkeys. Peptides. 1989;10(4):729-734.
13. Levin ER, Mills S, Weber MA. Endogenous opioids and opiate antagonists modulate the blood pressure of the spontaneously hypertensive rat. Peptides. 1986;(6):977-981.
14. Davis JM, Lowy MT, Yim GK, et al. Relationship between plasma concentrations of immunoreactive beta-endorphin and food intake in rats. Peptides. 1983;4(1):79-83.
15. Leibowitz SF, Hor L. Endorphinergic and alpha-noradrenergic systems in the paraventricular nucleus: effects on eating behavior. Peptides. 1982;3(3): 421-428.
16. Hall JE, Guyton AC. Textbook of Medical Physiology. 12th ed. Spanish version. Elsevier; 2011:587-588.
17. Han JS. Acupuncture and endorphins. Neurosci Lett. 2004;361(1-3):258-261.
18. Harte JL, Eifert GH, Smith R. The effects of running and meditation on beta-endorphin, corticotropin-releasing hormone and cortisol in plasma, and on mood. Biol Psychol. 1995;40(3):251-265.
19. Petrizzo R, Mohr J, Mantione K, et al. The role of endogenous morphine and nitric oxide in pain management. Pract Pain Manag. 2014;14(9).
20. Sprouse-Blum AS, Smith G, Sugai D, et al. Understanding endorphins and their importance in pain management. Hawaii Med J. 2010;69(3):70-100.
21. Dontsov AV. The influence of deficit of endogenous neuropeptides on the clinical course of coronary artery disease. Klin Med (Mosk). 2017;95(2):127-131. In Russian.
22. Dray A, Metsch R, Davis TP. Endorphins and the central inhibition of urinary bladder motility. Peptides. 1984;5(3):645-647.
23. Zalewska-Kaszubska J, Czarnecka E. Deficit in beta-endorphin peptide and tendency to alcohol abuse. Peptides. 2005;26(4):701-705.
24. McLay RN, Pan W, Kastin AJ. Effects of peptides on animal and human behavior: a review of studies published in the first twenty years of the journal Peptides. Peptides. 2001;22(12):2181-2255.
25. Wong-Riley MT. Neuroscience Secrets. 1st ed. Spanish version. Hanley & Belfus; 1999:424-428.
26. Brewerton TD. Clinical Handbook of Eating Disorders: An Integrated Approach. CRC Press; 2004:257-281.
27. Winklewski PJ, Radkowski M, Wszedybyl-Winklewska M, et al. Stress response, brain noradrenergic system and cognition. Adv Exp Med Biol. 2017;980:67-74.
28. McCall JG, Al-Hasani R, Siuda ER, et al. Engagement of the locus coeruleus noradrenergic system mediates stress-induced anxiety. Neuron. 2015;87(3):605-620.
29. Wszedybyl-Winklewska M, Wolf J, Szarmach A, et al. Central sympathetic nervous system reinforcement in obstructive sleep apnoea. Sleep Med Rev. 2018;39:143-154.
30. Yamamoto K, Shinba T, Yoshii M. Psychiatric symptoms of noradrenergic dysfunction: a pathophysiological view. Psychiatry Clin Neurosci. 2014;201(68):1-20.
31. Stone EA, Lin Y, Sarfraz Y, et al. The role of the central noradrenergic system in behavioral inhibition. Brain Res Rev. 2011;67(1-2):193-208.
32. Haddjeri N, Blier P, de Montigny C. Effect of the alpha-2 adrenoceptor antagonist mirtazapine on the 5-hydroxytryptamine system in the rat brain. J Pharmacol Exp Ther. 1996;277:861-871.
33. De Carvalho D, Patrone LG, Taxini CL, et al. Neurochemical and electrical modulation of the locus coeruleus: contribution to CO2 drive to breathe. Front Physiol. 2014;5(288):1-13.
34. Markianos M, Evangelopoulos ME, Koutsis G, et al. Evidence for involvement of central noradrenergic activity in crying proneness. J Neuropsychiatry Clin Neurosci. 2011;23:403-408.
35. Cao S, Fisher DW, Yu T, et al. The link between chronic pain and Alzheimer’s disease. J Neuroinflammation. 2019;(16):204-215.
36. Caraci F, Merlo S, Drago F, et al. Rescue of noradrenergic system as a novel pharmacological strategy in the treatment of chronic pain: focus on microglia activation. Front Pharmacol. 2019;(10):1024.
37. Hayashida KI, Obata H. Strategies to treat chronic pain and strengthen impaired descending noradrenergic inhibitory system. Int J Mol Sci. 2019;20(4):822.
38. Kur’yanova EV, Tryasuchev AV, Stupin VO, et al. Effect of atropine on adrenergic responsiveness of erythrocyte and heart rhythm variability in outbred rats with stimulation of the central neurotransmitter systems. Bull Exp Biol Med. 2018;165(5):165(5):597-601.
39. Peterson AC, Li CR. Noradrenergic dysfunction in Alzheimer’s and Parkinson’s disease: an overview of imaging studies. Front Aging Neurosci. 2018;(10):127.
Neurotransmitter-based diagnosis and treatment: A hypothesis (Part 1)
It is unfortunate that, in some clinical areas, medical conditions are still treated by name and not based on the underlying pathological process. It would be odd in 2022 to treat “dropsy” instead of heart or kidney disease (2 very different causes of edema). Similarly, if the FDA had been approving drugs 150 years ago, we would have medications on label for “dementia praecox,” not schizophrenia or Alzheimer disease. With the help of DSM-5, psychiatry still resides in the descriptive symptomatic world of disorders.
In the United States, thanks to Freud, psychiatric symptoms became separated from medical symptoms, which made it more difficult to associate psychiatric manifestations with the underlying pathophysiology. Though the physical manifestations that parallel emotional symptoms—such as the dry mouth of anxiety, the tremor and leg weakness of fear, the constipation and blurry vision of depression, the breathing difficulty of anger, the abdominal pain of stress, the blushing of shyness, the palpitations of flashbacks, and endless others—are well known, the present classification of psychiatric disorders is blind to it. Neurochemical causes of gastrointestinal spasm or muscle tension are better researched than underlying central neurochemistry, though the same neurotransmitters drive them.
Can the biochemistry of psychiatric symptoms be judged on the basis of peripheral symptoms? Can the mental manifestations be connected to biological causation, and vice versa? Would psychiatrists be better off selecting treatments by recognizing involved neurotransmitters instead of addressing descriptive “depression, anxiety, and psychosis”? Each of these clinical syndromes may be caused by entirely different underlying neuronal mechanisms. Such mechanisms could be suggested if medical symptoms (which are measurable and objective) would become part of the psychiatric diagnosis. Is treating the “cough” sufficient, or would recognition that tuberculosis caused the cough guide better treatment? Is it time to abandon descriptive conditions and replace them with a specific “mechanism-based” viewpoint?
Ample research has shown that serotonin, dopamine, norepinephrine, endorphins, glutamate, and gamma aminobutyric acid (GABA) are the neurotransmitters most responsible in the process of both psychiatric disorders and chronic pain. These neurotransmitters are involved in much more than emotions (including the feeling of pain). An abundance of medical symptom clusters point toward which neurotransmitter dysfunction may be leading in specific cases of distinct types of depression, psychosis, anxiety, or “chronic pain.” Even presently, there are medications available (both for FDA-approved indications and off-label) that can be used to regulate these neurotransmitters, allowing practitioners to target the possible biological underlining of psychiatric or pain pathology. Hopefully, in the not-so-distant future, there will be specific medications for serotonin, dopamine, and noradrenergic depression as well as for GABAergic anxiety, endorphin psychosis, noradrenergic insomnia, and similar conditions.
Numerous neurotransmitters may be connected to both depression and pain in all their forms. These include (but are not limited to) prostaglandins, bradykinins, substance P, potassium, magnesium, calcium, histamine, adenosine triphosphate, calcitonin gene-related peptide (CGRP), nitric oxide (NO), cholecystokinin 7 (CCK7), neurotrophic growth factor (NGF), neurotensin, acetylcholine (Ach), oxytocin, cannabinoids, and others. These have not been researched sufficiently to identify their clinical presentation of excessive or insufficient availability at the sites of neurotransmission. It is difficult to draw conclusions about what kind of clinical symptoms they may cause (outside of pain), and therefore, they are not addressed in this article.
Both high and low levels of certain neurotransmitters may be associated with psychiatric conditions and chronic pain. Too much is as bad as too little.1 This applies to both quantity of neurotransmitters as well as quality of the corresponding receptor activity. An astute clinician may judge which neurotransmitter is dysfunctional based on the patient’s presentation. Reading indirect signs of bodily functions is a basic clinical skill that should not be forgotten, even in the time of advanced technology.
A different way of viewing psychiatric disorders
In this article, we present 4 hypothetical clinical cases to emphasize a possible way of analyzing symptoms to identify underlying pathology and guide more effective treatment. In no way do these descriptions reflect the entire set of symptoms caused by neurotransmitters; we created them based on what is presently known or suspected, and extensive research is required to confirm or disprove what we describe here.
Continue to: There are no well-recognized...
There are no well-recognized, well-established, reliable, or validated syndromes described in our work. Our goal is to suggest an alternative way of looking at psychiatric disorders by viewing syndromal presentation through the lens of specific neurotransmitters. The collection of symptoms associated with various neurotransmitters as presented in this hypothesis is not complete. We have assembled what is described in the literature as a suggestion for specific future research. We simplified these clinical presentations by omitting scenarios in which a specific neurotransmitter increases in one area but not another. For example, all the symptoms of dopamine excess we describe would not have to occur concurrently in the same patient, but they may develop in certain patients depending on which dopaminergic pathway is exhibiting excess activity. Such distinctions may be established only by exhaustive research not yet conducted.
Our proposal may seem radical, but it truly is not. For example, if we know that dopamine excess may cause seizures, psychosis, and blood pressure elevation, why not consider dopamine excess as an underlying cause in a patient with depression who exhibits these symptoms simultaneously? And why not call it “dopamine excess syndrome”? We already have “serotonin syndrome” for a patient experiencing a serotonin storm. However, using the same logic, it should be called “serotonin excess syndrome.” And if we know of “serotonin excess syndrome,” why not consider “serotonin deficiency syndrome”?
In Part 1 of this article, we discuss serotonin and dopamine. Table 1 outlines medical and psychiatric symptoms that likely reflect serotonin excess2-18 and deficiency,14,19-29 and Table 2 lists symptoms that likely reflect dopamine excess14,30-41 and deficiency.4,14,20,38,40-43 In Part 2 we will touch on endorphins and norepinephrine, and in Part 3 we will conclude by looking at GABA and glutamate.
Serotonin excess (Table 12-18)
On a recent office visit, Ms. H reports that most of the time she does not feel much of anything, but she still experiences panic attacks8,9,13,15 and is easily agitated.6,8 Her mother died recently, and Ms. H is concerned that she did not grieve.15-18 She failed her last semester in college and was indifferent to her failure.18 She sleeps poorly,8 is failing her creative classes, and wonders why she has lost her artistic inclination.16-18 Ms. H has difficulty with amotivation, planning, social interactions, and speech.16,17 All of those symptoms worsened after she was prescribed fluoxetine approximately 1 year ago for her “blues.” Ms. H is obese and continues to gain weight,2 though she frequently has diarrhea,3,4,7,8 loud peristalsis, and abdominal cramps.4,7,8 She sweats easily6-8 and her heart frequently races.8,9 Additionally, Ms. H’s primary care physician told her that she has “borderline diabetes.”2 She is prone to frequent bruising11 and is easy to shake, even when she is experiencing minimal anxiety.6-9 Ms. H had consulted with a neurologist because of unusual electrical “zapping” in her brain and muscle twitches.5,8,9,13 She had experienced a seizure as a child, but this was possibly related to hypernatremia,2 and she has not taken any anticonvulsant medication for several years.8 She exhibits hyperactive deep tendon reflexes and tremors5,7,9 and blinks frequently.6,9 She experiences hot flashes,3,6-8,14 does not tolerate heat, and prefers cooler weather.8,9 Her pains and aches,12,14 to which she has been prone all of her life, have recently become much worse, and she was diagnosed with fibromyalgia in part because she frequently feels stiff all over.10 She complains of strange tingling and prickling sensations in her hands and feet, especially when anxious.7,9,10 Her headaches also worsened and may be precipitated by bright light, as her pupils are usually dilated.5,7,9 Her hypertension is fairly controlled with medication.6,8-10 Ms. H says she experienced a psychotic episode when she was in her mid-teens,6,8 but reassures you that “she is not that bad now,” although she remains hypervigilant.13 Also while in her teens, Ms. H was treated with paroxetine and experienced restlessness, agitation, delirium, tachycardia, fluctuating blood pressure, diaphoresis, diarrhea, and neuromuscular excitation, which prompted discontinuation of the antidepressant.5-7,9,10
Impression. Ms. H exhibits symptoms associated with serotonin hyperactivity. Discontinuing and avoiding selective serotonin reuptake inhibitors (SSRIs) would be prudent; prescribing an anticonvulsant would be reasonable. Using a GABAergic medication to suppress serotonin (eg, baclofen) is likely to help. Avoiding dopaminergic medications is a must. Antidepressive antipsychotics would be logical to use. The use of serotonin-suppressing medications may be considered. One may argue for the use of beta-blockers in such a patient.
Continue to: Serotonin deficiency
Serotonin deficiency (Table 114,19-29)
Mr. A is chronically depressed, hopeless,19 and easily angered.21 He does not believe anyone can help him.19 You are concerned for his safety because he had attempted to end his life by shooting himself in the chest.19,21,22,25 Even when he’s not particularly depressed, Mr. A does not enjoy much of anything.21,26,27 He becomes particularly agitated when he drinks alcohol,25 which unfortunately is common for him.29 He engages in binge eating to feel better; he knows this is not healthy but he cannot control his behavior.20,29 Mr. A is poorly compliant with his medications, even with a blood thinner, which he was prescribed due to an episode of deep vein thrombosis. He complains of chronic daily headaches and episodic migraines.23,24 He rarely blinks,23,28 his skin is dry and cool, his hair is brittle,23 his mouth is dry,14,23,27 and he constantly licks his chapped lips.14,26,27 Mr. A frequently has general body pain26,31 but is dismissive of his body aches and completely stops reporting pain when his depression gets particularly severe. When depressed, he is slow in movement and thinking.14,21,26,27 He is more concerned with anxiety than depression.21 Mr. A is plagued by constipation, abdominal pain, muscle tension, and episodes of shaking.14,26,27 He also frequently complains about chronic tinnitus.28
Impression. Mr. A shows symptoms associated with serotonin hypoactivity. SSRIs and any other antidepressants with serotonin activity would be an obvious choice for treatment. A mood-stabilizing antipsychotic with serotonin activity would be welcome in treatment. Thyroid hormone supplementation may be of value, especially if thyroid stimulating hormone level is high. Light therapy, a diet with food that contains tryptophan, psychotherapy, and exercise are desirable. Avoiding benzodiazepines would be a good idea.
Dopamine excess (Table 214,30-41)
Ms. L presents with complaints of “fibromyalgia” and “daily headaches,”14 and also dissociation (finding herself in places when she does not know how she got there) and “out-of-body experiences.”32 She is odd, and states that people do not understand her and that she is “different.”38 Her friend, who is present at the appointment, elaborates on Ms. L’s bizarreness and oddness in behavior, out-of-context emotions, suspiciousness, paranoia, and possible hallucinations.35,36,38 Ms. L discloses frequent diffuse body pains, headaches, nausea, excessive salivation, and tongue burning, as well as muscle twitching.14 Sex worsens her headaches and body pain. She reports seizures that are not registered on EEG. In the office, she is suspicious, exhibits odd posturing, tends to misinterpret your words, and makes you feel uncomfortable. Anxiety38 and multiple obsessive-compulsive symptoms, especially excessive cleaning and grooming, complicate Ms. L’s life.31,32,34 On examination, she is hypertensive, and she has scars caused by self-cutting and skin picking on her arms.30-32 An electrocardiogram shows an elevated heart rate, widened QRS complex, and ectopic heartbeats.14 Ms. L has experienced trichotillomania since adolescence32-34 and her fingernails are bitten to the skin.34 She has difficulty with impulse control, and thrill-seeking is a prominent part of her life, mainly via gambling, compulsive sex, and compulsive buying.35,36 She also says she experiences indigestion and delayed gastric emptying.37
Impression. Ms. L exhibits multiple symptoms associated with dopamine excess. Dopamine antagonists should be considered and may help not only with her psychiatric symptoms but also with her pain symptoms. Bupropion (as a dopamine agonist), caffeine, and stimulants should be avoided.
Excessive dopamine is, in extreme cases, associated with somatic psychosis, somatic symptom disorder, factitious disorder, pain disorder, and hypochondria.39 It may come with odd and bizarre/peculiar symptoms out of proportion with objectively identified pathology. These symptoms are common in chronic pain and headache patients, and need to be addressed by appropriate use of dopamine antagonizing medications.39
Continue to: Dopamine deficiency
Dopamine deficiency (Table 24,14,20,38,40-43)
Mr. W experiences widespread pain, including chronic back pain, headaches, and abdominal pain. He also has substantial anhedonia, lack of interest, procrastination, and hypersomnia.41,42 He is apathetic and has difficulty getting up in the morning.41,42 Unusual tiredness and weakness drive him to overuse caffeine; he states that 5 Mountain Dews and 4 cups of regular coffee a day make his headaches bearable.38,41-43 Sex also improves his headaches. Since childhood, he has taken stimulants for attention-deficit/hyperactivity disorder. He reports that occasional use of cocaine helps ease his pain and depression. Mr. W’s wife is concerned with her husband’s low sexual drive and alcohol consumption, and discloses that he has periodic trouble with gambling. Mr. W was forced into psychotherapy but never was able to work productively with his therapist.38,41-43 He loves eating and cannot control his weight.40 This contrasts with episodic anorexia he experienced when he was younger.20 His face is usually emotionless.43 Mr. W is prone to constipation.14 His restless leg syndrome and periodic limb movement disorder are so bad that his wife refuses to share a bed with him.14 He is clumsy and has a problem with repetitive motor tasks.43 A paucity of speech, limited eye contact, poor grooming, and difficulty forming therapeutic alliances have long been part of Mr. W’s history.38,42,43 On physical examination, he has a dry mouth; he is stiff, tremulous, and hypotensive.14
Impression. Mr. W shows multiple symptoms associated with dopamine deficiency. Bupropion may be reasonable to consider. Dopamine augmentation via the use of stimulants is warranted in such patients, especially if stimulants had not been tried before (lisdexamfetamine would be a good choice to minimize addictive potential). For a patient with dopamine deficiency, levodopa may improve more than just restless legs. Amantadine may improve dopaminergic signaling through the accelerated dopamine release and decrease in presynaptic uptake, so this medication may be carefully tried.44 Pain treatment would not be successful for Mr. W without simultaneous treatment for his substance use.
Bottom Line
Both high and low levels of serotonin and dopamine may be associated with certain psychiatric and medical symptoms and disorders. An astute clinician may judge which neurotransmitter is dysfunctional based on the patient’s presentation, and tailor treatment accordingly.
Related Resources
- Abell SR, El-Mallakh RS. Serotonin-mediated anxiety: How to recognize and treat it. Current Psychiatry. 2021;20(11):37-40. doi:10.12788/cp.0168
Drug Brand Names
Amantadine • Gocovri
Baclofen • Ozobax
Bupropion • Wellbutrin
Fluoxetine • Prozac
Lisdexamfetamine • Vyvanse
Paroxetine • Paxil
1. Stahl SM. Dazzled by the dominions of dopamine: clinical roles of D3, D2, and D1 receptors. CNS Spectr. 2017;22(4):305-311.
2. Young RL, Lumsden AL, Martin AM, et al. Augmented capacity for peripheral serotonin release in human obesity. Int J Obes (Lond). 2018;42(11):1880-1889.
3. Ahlman H. Serotonin and carcinoid tumors. J Cardiovasc Pharmacol. 1985;7(Suppl 7):S79-S85.
4. Terry N, Margolis KG. Serotonergic mechanisms regulating the GI tract: experimental evidence and therapeutic relevance. Handb Exp Pharmacol. 2017;239:319-342.
5. Prakash S, Belani P, Trivedi A. Headache as a presenting feature in patients with serotonin syndrome: a case series. Cephalalgia. 2014;34(2):148-153.
6. van Ewijk CE, Jacobs GE, Girbes ARJ. Unsuspected serotonin toxicity in the ICU. Ann Intensive Care. 2016;6(1):85.
7. Pedavally S, Fugate JE, Rabinstein AA. Serotonin syndrome in the intensive care unit: clinical presentations and precipitating medications. Neurocrit Care. 2014;21(1):108-113.
8. Nguyen H, Pan A, Smollin C, et al. An 11-year retrospective review of cyproheptadine use in serotonin syndrome cases reported to the California Poison Control System. J Clin Pharm Ther. 2019;44(2):327-334.
9. Ansari H, Kouti L. Drug interaction and serotonin toxicity with opioid use: another reason to avoid opioids in headache and migraine treatment. Curr Pain Headache Rep. 2016;20(8):50.
10. Ott M, Mannchen JK, Jamshidi F, et al. Management of severe arterial hypertension associated with serotonin syndrome: a case report analysis based on systematic review techniques. Ther Adv Psychopharmacol. 2019;9:2045125318818814. doi:10.1177/2045125318818814
11. Cerrito F, Lazzaro MP, Gaudio E, et al. 5HT2-receptors and serotonin release: their role in human platelet aggregation. Life Sci. 1993;53(3):209-215.
12. Ohayon MM. Pain sensitivity, depression, and sleep deprivation: links with serotoninergic dysfunction. J Psychiatr Res. 2009;43(16):1243-1245.
13. Maron E, Shlik J. Serotonin function in panic disorder: important, but why? Neuropsychopharmacology. 2006;31(1):1-11.
14. Hall JE, Guyton AC. Textbook of Medical Physiology. 12th ed. Spanish version. Elsevier; 2011:120,199,201-204,730-740.
15. Garland EJ, Baerg EA. Amotivational syndrome associated with selective serotonin reuptake inhibitors in children and adolescents. J Child Adolesc Psychopharmacol. 2001;11(2):181-186.
16. George MS, Trimble MR. A fluvoxamine-induced frontal lobe syndrome in a patient with comorbid Gilles de la Tourette’s syndrome and obsessive compulsive disorder. J Clin Psychiatry. 1992;53(10):379-380.
17. Hoehn-Saric R, Harris GJ, Pearlson GD, et al. A fluoxetine-induced frontal lobe syndrome in an obsessive compulsive patient. J Clin Psychiatry. 1991;52(3):131-133.
18. Hoehn-Saric R, Lipsey JR, McLeod DR. Apathy and indifference in patients on fluvoxamine and fluoxetine. J Clin Psychopharmacol. 1990;10(5):343-345.
19. Samuelsson M, Jokinen J, Nordström AL, et al. CSF 5-HIAA, suicide intent and hopelessness in the prediction of early suicide in male high-risk suicide attempters. Acta Psychiatr Scand. 2006;113(1):44-47.
20. Brewerton TD. Clinical Handbook of Eating Disorders: An Integrated Approach. CRC Press; 2004:257-281.
21. Mann JJ, Oquendo M, Underwood MD, et al. The neurobiology of suicide risk: a review for the clinician. J Clin Psychiatry. 1999;60 Suppl 2:7-116.
22. Mann JJ, Malone KM. Cerebrospinal fluid amines and higher-lethality suicide attempts in depressed inpatients. Biol Psychiatry. 1997;41(2):162-171.
23. Joseph R, Welch KM, D’Andrea G. Serotonergic hypofunction in migraine: a synthesis of evidence based on platelet dense body dysfunction. Cephalalgia. 1989;9(4):293-299.
24. Pakalnis A, Splaingard M, Splaingard D, et al. Serotonin effects on sleep and emotional disorders in adolescent migraine. Headache. 2009;49(10):1486-1492.
25. Virkkunen M, Goldman D, Nielsen DA, et al. Low brain serotonin turnover rate (low CSF 5-HIAA) and impulsive violence. J Psychiatry Neurosci. 1995;20(4):271-275.
26. Liu Y, Zhao J, Fan X, et al. Dysfunction in serotonergic and noradrenergic systems and somatic symptoms in psychiatric disorders. Front Psychiatry. 2019;10:286.
27. Ginsburg GS, Riddle MA, Davies M. Somatic symptoms in children and adolescents with anxiety disorders. J Am Acad Child Adolesc Psychiatry. 2006;45(10):1179-1187.
28. O’Malley PG, Jackson JL, Santoro J, et al. Antidepressant therapy for unexplained symptoms and symptom syndromes. J Fam Pract. 1999;48(12):980-990.
29. Fortuna JL. Sweet preference, sugar addiction and the familial history of alcohol dependence: shared neural pathways and genes. J Psychoactive Drugs. 2010;42(2):147-151.
30. Stanley B, Sher L, Wilson S, et al. Non-suicidal self-injurious behavior, endogenous opioids and monoamine neurotransmitters. J Affect Disord. 2010;124(1-2):134-140.
31. Graybiel AM, Saka E. A genetic basis for obsessive grooming. Neuron. 2002;33(1):1-2.
32. Tse W, Hälbig TD. Skin picking in Parkinson’s disease: a behavioral side-effect of dopaminergic treatment? Psychiatry Clin Neurosci. 2010;64(2):214.
33. Ayaydın H. Probable emergence of symptoms of trichotillomania by atomoxetine: a case report. Psychiatry and Clinical Psychopharmacology. 2019;29(2)220-222.
34. Paholpak P, Mendez MF. Trichotillomania as a manifestation of dementia. Case Rep Psychiatry. 2016;2016:9782702. doi:10.1155/2016/9782702
35. Clark CA, Dagher A. The role of dopamine in risk taking: a specific look at Parkinson’s disease and gambling. Front Behav Neurosci. 2014;8:196.
36. Norbury A, Husain M. Sensation-seeking: dopaminergic modulation and risk for psychopathology. Behav Brain Res. 2015;288:79-93.
37. Chen TS, Chang FY. Elevated serum dopamine increases while coffee consumption decreases the occurrence of reddish streaks in the intact stomach. J Gastroenterol Hepatol. 2013;28(12):1810-1814.
38. Wong-Riley MT. Neuroscience Secrets. 1st edition. Spanish version. Hanley & Belfus; 1999:420-429.
39. Arbuck DM. Antipsychotics, dopamine, and pain. Current Psychiatry. 2020;19(1):25-29,31.
40. Bello NT, Hajnal A. Dopamine and binge eating behaviors. Pharmacol Biochem Behav. 2010;97(1):25-33.
41. Velligan DI, Weiden PJ, Sajatovic M, et al; Expert Consensus Panel on Adherence Problems in Serious and Persistent Mental Illness. The expert consensus guideline series: adherence problems in patients with serious and persistent mental illness. J Clin Psychiatry. 2009;70 Suppl 4:1-46.
42. Milev P, Ho BC, Arndt S, et al. Predictive values of neurocognition and negative symptoms on functional outcome in schizophrenia: a longitudinal first-episode study with 7-year follow-up. Am J Psychiatry. 2005;162(3):495-506.
43. Gepshtein S, Li X, Snider J, et al. Dopamine function and the efficiency of human movement. J Cogn Neurosci. 2014;26(3):645-657.
44. Scarff JR. The ABCDs of treating tardive dyskinesia. Current Psychiatry. 2020;19(4):21,55.
It is unfortunate that, in some clinical areas, medical conditions are still treated by name and not based on the underlying pathological process. It would be odd in 2022 to treat “dropsy” instead of heart or kidney disease (2 very different causes of edema). Similarly, if the FDA had been approving drugs 150 years ago, we would have medications on label for “dementia praecox,” not schizophrenia or Alzheimer disease. With the help of DSM-5, psychiatry still resides in the descriptive symptomatic world of disorders.
In the United States, thanks to Freud, psychiatric symptoms became separated from medical symptoms, which made it more difficult to associate psychiatric manifestations with the underlying pathophysiology. Though the physical manifestations that parallel emotional symptoms—such as the dry mouth of anxiety, the tremor and leg weakness of fear, the constipation and blurry vision of depression, the breathing difficulty of anger, the abdominal pain of stress, the blushing of shyness, the palpitations of flashbacks, and endless others—are well known, the present classification of psychiatric disorders is blind to it. Neurochemical causes of gastrointestinal spasm or muscle tension are better researched than underlying central neurochemistry, though the same neurotransmitters drive them.
Can the biochemistry of psychiatric symptoms be judged on the basis of peripheral symptoms? Can the mental manifestations be connected to biological causation, and vice versa? Would psychiatrists be better off selecting treatments by recognizing involved neurotransmitters instead of addressing descriptive “depression, anxiety, and psychosis”? Each of these clinical syndromes may be caused by entirely different underlying neuronal mechanisms. Such mechanisms could be suggested if medical symptoms (which are measurable and objective) would become part of the psychiatric diagnosis. Is treating the “cough” sufficient, or would recognition that tuberculosis caused the cough guide better treatment? Is it time to abandon descriptive conditions and replace them with a specific “mechanism-based” viewpoint?
Ample research has shown that serotonin, dopamine, norepinephrine, endorphins, glutamate, and gamma aminobutyric acid (GABA) are the neurotransmitters most responsible in the process of both psychiatric disorders and chronic pain. These neurotransmitters are involved in much more than emotions (including the feeling of pain). An abundance of medical symptom clusters point toward which neurotransmitter dysfunction may be leading in specific cases of distinct types of depression, psychosis, anxiety, or “chronic pain.” Even presently, there are medications available (both for FDA-approved indications and off-label) that can be used to regulate these neurotransmitters, allowing practitioners to target the possible biological underlining of psychiatric or pain pathology. Hopefully, in the not-so-distant future, there will be specific medications for serotonin, dopamine, and noradrenergic depression as well as for GABAergic anxiety, endorphin psychosis, noradrenergic insomnia, and similar conditions.
Numerous neurotransmitters may be connected to both depression and pain in all their forms. These include (but are not limited to) prostaglandins, bradykinins, substance P, potassium, magnesium, calcium, histamine, adenosine triphosphate, calcitonin gene-related peptide (CGRP), nitric oxide (NO), cholecystokinin 7 (CCK7), neurotrophic growth factor (NGF), neurotensin, acetylcholine (Ach), oxytocin, cannabinoids, and others. These have not been researched sufficiently to identify their clinical presentation of excessive or insufficient availability at the sites of neurotransmission. It is difficult to draw conclusions about what kind of clinical symptoms they may cause (outside of pain), and therefore, they are not addressed in this article.
Both high and low levels of certain neurotransmitters may be associated with psychiatric conditions and chronic pain. Too much is as bad as too little.1 This applies to both quantity of neurotransmitters as well as quality of the corresponding receptor activity. An astute clinician may judge which neurotransmitter is dysfunctional based on the patient’s presentation. Reading indirect signs of bodily functions is a basic clinical skill that should not be forgotten, even in the time of advanced technology.
A different way of viewing psychiatric disorders
In this article, we present 4 hypothetical clinical cases to emphasize a possible way of analyzing symptoms to identify underlying pathology and guide more effective treatment. In no way do these descriptions reflect the entire set of symptoms caused by neurotransmitters; we created them based on what is presently known or suspected, and extensive research is required to confirm or disprove what we describe here.
Continue to: There are no well-recognized...
There are no well-recognized, well-established, reliable, or validated syndromes described in our work. Our goal is to suggest an alternative way of looking at psychiatric disorders by viewing syndromal presentation through the lens of specific neurotransmitters. The collection of symptoms associated with various neurotransmitters as presented in this hypothesis is not complete. We have assembled what is described in the literature as a suggestion for specific future research. We simplified these clinical presentations by omitting scenarios in which a specific neurotransmitter increases in one area but not another. For example, all the symptoms of dopamine excess we describe would not have to occur concurrently in the same patient, but they may develop in certain patients depending on which dopaminergic pathway is exhibiting excess activity. Such distinctions may be established only by exhaustive research not yet conducted.
Our proposal may seem radical, but it truly is not. For example, if we know that dopamine excess may cause seizures, psychosis, and blood pressure elevation, why not consider dopamine excess as an underlying cause in a patient with depression who exhibits these symptoms simultaneously? And why not call it “dopamine excess syndrome”? We already have “serotonin syndrome” for a patient experiencing a serotonin storm. However, using the same logic, it should be called “serotonin excess syndrome.” And if we know of “serotonin excess syndrome,” why not consider “serotonin deficiency syndrome”?
In Part 1 of this article, we discuss serotonin and dopamine. Table 1 outlines medical and psychiatric symptoms that likely reflect serotonin excess2-18 and deficiency,14,19-29 and Table 2 lists symptoms that likely reflect dopamine excess14,30-41 and deficiency.4,14,20,38,40-43 In Part 2 we will touch on endorphins and norepinephrine, and in Part 3 we will conclude by looking at GABA and glutamate.
Serotonin excess (Table 12-18)
On a recent office visit, Ms. H reports that most of the time she does not feel much of anything, but she still experiences panic attacks8,9,13,15 and is easily agitated.6,8 Her mother died recently, and Ms. H is concerned that she did not grieve.15-18 She failed her last semester in college and was indifferent to her failure.18 She sleeps poorly,8 is failing her creative classes, and wonders why she has lost her artistic inclination.16-18 Ms. H has difficulty with amotivation, planning, social interactions, and speech.16,17 All of those symptoms worsened after she was prescribed fluoxetine approximately 1 year ago for her “blues.” Ms. H is obese and continues to gain weight,2 though she frequently has diarrhea,3,4,7,8 loud peristalsis, and abdominal cramps.4,7,8 She sweats easily6-8 and her heart frequently races.8,9 Additionally, Ms. H’s primary care physician told her that she has “borderline diabetes.”2 She is prone to frequent bruising11 and is easy to shake, even when she is experiencing minimal anxiety.6-9 Ms. H had consulted with a neurologist because of unusual electrical “zapping” in her brain and muscle twitches.5,8,9,13 She had experienced a seizure as a child, but this was possibly related to hypernatremia,2 and she has not taken any anticonvulsant medication for several years.8 She exhibits hyperactive deep tendon reflexes and tremors5,7,9 and blinks frequently.6,9 She experiences hot flashes,3,6-8,14 does not tolerate heat, and prefers cooler weather.8,9 Her pains and aches,12,14 to which she has been prone all of her life, have recently become much worse, and she was diagnosed with fibromyalgia in part because she frequently feels stiff all over.10 She complains of strange tingling and prickling sensations in her hands and feet, especially when anxious.7,9,10 Her headaches also worsened and may be precipitated by bright light, as her pupils are usually dilated.5,7,9 Her hypertension is fairly controlled with medication.6,8-10 Ms. H says she experienced a psychotic episode when she was in her mid-teens,6,8 but reassures you that “she is not that bad now,” although she remains hypervigilant.13 Also while in her teens, Ms. H was treated with paroxetine and experienced restlessness, agitation, delirium, tachycardia, fluctuating blood pressure, diaphoresis, diarrhea, and neuromuscular excitation, which prompted discontinuation of the antidepressant.5-7,9,10
Impression. Ms. H exhibits symptoms associated with serotonin hyperactivity. Discontinuing and avoiding selective serotonin reuptake inhibitors (SSRIs) would be prudent; prescribing an anticonvulsant would be reasonable. Using a GABAergic medication to suppress serotonin (eg, baclofen) is likely to help. Avoiding dopaminergic medications is a must. Antidepressive antipsychotics would be logical to use. The use of serotonin-suppressing medications may be considered. One may argue for the use of beta-blockers in such a patient.
Continue to: Serotonin deficiency
Serotonin deficiency (Table 114,19-29)
Mr. A is chronically depressed, hopeless,19 and easily angered.21 He does not believe anyone can help him.19 You are concerned for his safety because he had attempted to end his life by shooting himself in the chest.19,21,22,25 Even when he’s not particularly depressed, Mr. A does not enjoy much of anything.21,26,27 He becomes particularly agitated when he drinks alcohol,25 which unfortunately is common for him.29 He engages in binge eating to feel better; he knows this is not healthy but he cannot control his behavior.20,29 Mr. A is poorly compliant with his medications, even with a blood thinner, which he was prescribed due to an episode of deep vein thrombosis. He complains of chronic daily headaches and episodic migraines.23,24 He rarely blinks,23,28 his skin is dry and cool, his hair is brittle,23 his mouth is dry,14,23,27 and he constantly licks his chapped lips.14,26,27 Mr. A frequently has general body pain26,31 but is dismissive of his body aches and completely stops reporting pain when his depression gets particularly severe. When depressed, he is slow in movement and thinking.14,21,26,27 He is more concerned with anxiety than depression.21 Mr. A is plagued by constipation, abdominal pain, muscle tension, and episodes of shaking.14,26,27 He also frequently complains about chronic tinnitus.28
Impression. Mr. A shows symptoms associated with serotonin hypoactivity. SSRIs and any other antidepressants with serotonin activity would be an obvious choice for treatment. A mood-stabilizing antipsychotic with serotonin activity would be welcome in treatment. Thyroid hormone supplementation may be of value, especially if thyroid stimulating hormone level is high. Light therapy, a diet with food that contains tryptophan, psychotherapy, and exercise are desirable. Avoiding benzodiazepines would be a good idea.
Dopamine excess (Table 214,30-41)
Ms. L presents with complaints of “fibromyalgia” and “daily headaches,”14 and also dissociation (finding herself in places when she does not know how she got there) and “out-of-body experiences.”32 She is odd, and states that people do not understand her and that she is “different.”38 Her friend, who is present at the appointment, elaborates on Ms. L’s bizarreness and oddness in behavior, out-of-context emotions, suspiciousness, paranoia, and possible hallucinations.35,36,38 Ms. L discloses frequent diffuse body pains, headaches, nausea, excessive salivation, and tongue burning, as well as muscle twitching.14 Sex worsens her headaches and body pain. She reports seizures that are not registered on EEG. In the office, she is suspicious, exhibits odd posturing, tends to misinterpret your words, and makes you feel uncomfortable. Anxiety38 and multiple obsessive-compulsive symptoms, especially excessive cleaning and grooming, complicate Ms. L’s life.31,32,34 On examination, she is hypertensive, and she has scars caused by self-cutting and skin picking on her arms.30-32 An electrocardiogram shows an elevated heart rate, widened QRS complex, and ectopic heartbeats.14 Ms. L has experienced trichotillomania since adolescence32-34 and her fingernails are bitten to the skin.34 She has difficulty with impulse control, and thrill-seeking is a prominent part of her life, mainly via gambling, compulsive sex, and compulsive buying.35,36 She also says she experiences indigestion and delayed gastric emptying.37
Impression. Ms. L exhibits multiple symptoms associated with dopamine excess. Dopamine antagonists should be considered and may help not only with her psychiatric symptoms but also with her pain symptoms. Bupropion (as a dopamine agonist), caffeine, and stimulants should be avoided.
Excessive dopamine is, in extreme cases, associated with somatic psychosis, somatic symptom disorder, factitious disorder, pain disorder, and hypochondria.39 It may come with odd and bizarre/peculiar symptoms out of proportion with objectively identified pathology. These symptoms are common in chronic pain and headache patients, and need to be addressed by appropriate use of dopamine antagonizing medications.39
Continue to: Dopamine deficiency
Dopamine deficiency (Table 24,14,20,38,40-43)
Mr. W experiences widespread pain, including chronic back pain, headaches, and abdominal pain. He also has substantial anhedonia, lack of interest, procrastination, and hypersomnia.41,42 He is apathetic and has difficulty getting up in the morning.41,42 Unusual tiredness and weakness drive him to overuse caffeine; he states that 5 Mountain Dews and 4 cups of regular coffee a day make his headaches bearable.38,41-43 Sex also improves his headaches. Since childhood, he has taken stimulants for attention-deficit/hyperactivity disorder. He reports that occasional use of cocaine helps ease his pain and depression. Mr. W’s wife is concerned with her husband’s low sexual drive and alcohol consumption, and discloses that he has periodic trouble with gambling. Mr. W was forced into psychotherapy but never was able to work productively with his therapist.38,41-43 He loves eating and cannot control his weight.40 This contrasts with episodic anorexia he experienced when he was younger.20 His face is usually emotionless.43 Mr. W is prone to constipation.14 His restless leg syndrome and periodic limb movement disorder are so bad that his wife refuses to share a bed with him.14 He is clumsy and has a problem with repetitive motor tasks.43 A paucity of speech, limited eye contact, poor grooming, and difficulty forming therapeutic alliances have long been part of Mr. W’s history.38,42,43 On physical examination, he has a dry mouth; he is stiff, tremulous, and hypotensive.14
Impression. Mr. W shows multiple symptoms associated with dopamine deficiency. Bupropion may be reasonable to consider. Dopamine augmentation via the use of stimulants is warranted in such patients, especially if stimulants had not been tried before (lisdexamfetamine would be a good choice to minimize addictive potential). For a patient with dopamine deficiency, levodopa may improve more than just restless legs. Amantadine may improve dopaminergic signaling through the accelerated dopamine release and decrease in presynaptic uptake, so this medication may be carefully tried.44 Pain treatment would not be successful for Mr. W without simultaneous treatment for his substance use.
Bottom Line
Both high and low levels of serotonin and dopamine may be associated with certain psychiatric and medical symptoms and disorders. An astute clinician may judge which neurotransmitter is dysfunctional based on the patient’s presentation, and tailor treatment accordingly.
Related Resources
- Abell SR, El-Mallakh RS. Serotonin-mediated anxiety: How to recognize and treat it. Current Psychiatry. 2021;20(11):37-40. doi:10.12788/cp.0168
Drug Brand Names
Amantadine • Gocovri
Baclofen • Ozobax
Bupropion • Wellbutrin
Fluoxetine • Prozac
Lisdexamfetamine • Vyvanse
Paroxetine • Paxil
It is unfortunate that, in some clinical areas, medical conditions are still treated by name and not based on the underlying pathological process. It would be odd in 2022 to treat “dropsy” instead of heart or kidney disease (2 very different causes of edema). Similarly, if the FDA had been approving drugs 150 years ago, we would have medications on label for “dementia praecox,” not schizophrenia or Alzheimer disease. With the help of DSM-5, psychiatry still resides in the descriptive symptomatic world of disorders.
In the United States, thanks to Freud, psychiatric symptoms became separated from medical symptoms, which made it more difficult to associate psychiatric manifestations with the underlying pathophysiology. Though the physical manifestations that parallel emotional symptoms—such as the dry mouth of anxiety, the tremor and leg weakness of fear, the constipation and blurry vision of depression, the breathing difficulty of anger, the abdominal pain of stress, the blushing of shyness, the palpitations of flashbacks, and endless others—are well known, the present classification of psychiatric disorders is blind to it. Neurochemical causes of gastrointestinal spasm or muscle tension are better researched than underlying central neurochemistry, though the same neurotransmitters drive them.
Can the biochemistry of psychiatric symptoms be judged on the basis of peripheral symptoms? Can the mental manifestations be connected to biological causation, and vice versa? Would psychiatrists be better off selecting treatments by recognizing involved neurotransmitters instead of addressing descriptive “depression, anxiety, and psychosis”? Each of these clinical syndromes may be caused by entirely different underlying neuronal mechanisms. Such mechanisms could be suggested if medical symptoms (which are measurable and objective) would become part of the psychiatric diagnosis. Is treating the “cough” sufficient, or would recognition that tuberculosis caused the cough guide better treatment? Is it time to abandon descriptive conditions and replace them with a specific “mechanism-based” viewpoint?
Ample research has shown that serotonin, dopamine, norepinephrine, endorphins, glutamate, and gamma aminobutyric acid (GABA) are the neurotransmitters most responsible in the process of both psychiatric disorders and chronic pain. These neurotransmitters are involved in much more than emotions (including the feeling of pain). An abundance of medical symptom clusters point toward which neurotransmitter dysfunction may be leading in specific cases of distinct types of depression, psychosis, anxiety, or “chronic pain.” Even presently, there are medications available (both for FDA-approved indications and off-label) that can be used to regulate these neurotransmitters, allowing practitioners to target the possible biological underlining of psychiatric or pain pathology. Hopefully, in the not-so-distant future, there will be specific medications for serotonin, dopamine, and noradrenergic depression as well as for GABAergic anxiety, endorphin psychosis, noradrenergic insomnia, and similar conditions.
Numerous neurotransmitters may be connected to both depression and pain in all their forms. These include (but are not limited to) prostaglandins, bradykinins, substance P, potassium, magnesium, calcium, histamine, adenosine triphosphate, calcitonin gene-related peptide (CGRP), nitric oxide (NO), cholecystokinin 7 (CCK7), neurotrophic growth factor (NGF), neurotensin, acetylcholine (Ach), oxytocin, cannabinoids, and others. These have not been researched sufficiently to identify their clinical presentation of excessive or insufficient availability at the sites of neurotransmission. It is difficult to draw conclusions about what kind of clinical symptoms they may cause (outside of pain), and therefore, they are not addressed in this article.
Both high and low levels of certain neurotransmitters may be associated with psychiatric conditions and chronic pain. Too much is as bad as too little.1 This applies to both quantity of neurotransmitters as well as quality of the corresponding receptor activity. An astute clinician may judge which neurotransmitter is dysfunctional based on the patient’s presentation. Reading indirect signs of bodily functions is a basic clinical skill that should not be forgotten, even in the time of advanced technology.
A different way of viewing psychiatric disorders
In this article, we present 4 hypothetical clinical cases to emphasize a possible way of analyzing symptoms to identify underlying pathology and guide more effective treatment. In no way do these descriptions reflect the entire set of symptoms caused by neurotransmitters; we created them based on what is presently known or suspected, and extensive research is required to confirm or disprove what we describe here.
Continue to: There are no well-recognized...
There are no well-recognized, well-established, reliable, or validated syndromes described in our work. Our goal is to suggest an alternative way of looking at psychiatric disorders by viewing syndromal presentation through the lens of specific neurotransmitters. The collection of symptoms associated with various neurotransmitters as presented in this hypothesis is not complete. We have assembled what is described in the literature as a suggestion for specific future research. We simplified these clinical presentations by omitting scenarios in which a specific neurotransmitter increases in one area but not another. For example, all the symptoms of dopamine excess we describe would not have to occur concurrently in the same patient, but they may develop in certain patients depending on which dopaminergic pathway is exhibiting excess activity. Such distinctions may be established only by exhaustive research not yet conducted.
Our proposal may seem radical, but it truly is not. For example, if we know that dopamine excess may cause seizures, psychosis, and blood pressure elevation, why not consider dopamine excess as an underlying cause in a patient with depression who exhibits these symptoms simultaneously? And why not call it “dopamine excess syndrome”? We already have “serotonin syndrome” for a patient experiencing a serotonin storm. However, using the same logic, it should be called “serotonin excess syndrome.” And if we know of “serotonin excess syndrome,” why not consider “serotonin deficiency syndrome”?
In Part 1 of this article, we discuss serotonin and dopamine. Table 1 outlines medical and psychiatric symptoms that likely reflect serotonin excess2-18 and deficiency,14,19-29 and Table 2 lists symptoms that likely reflect dopamine excess14,30-41 and deficiency.4,14,20,38,40-43 In Part 2 we will touch on endorphins and norepinephrine, and in Part 3 we will conclude by looking at GABA and glutamate.
Serotonin excess (Table 12-18)
On a recent office visit, Ms. H reports that most of the time she does not feel much of anything, but she still experiences panic attacks8,9,13,15 and is easily agitated.6,8 Her mother died recently, and Ms. H is concerned that she did not grieve.15-18 She failed her last semester in college and was indifferent to her failure.18 She sleeps poorly,8 is failing her creative classes, and wonders why she has lost her artistic inclination.16-18 Ms. H has difficulty with amotivation, planning, social interactions, and speech.16,17 All of those symptoms worsened after she was prescribed fluoxetine approximately 1 year ago for her “blues.” Ms. H is obese and continues to gain weight,2 though she frequently has diarrhea,3,4,7,8 loud peristalsis, and abdominal cramps.4,7,8 She sweats easily6-8 and her heart frequently races.8,9 Additionally, Ms. H’s primary care physician told her that she has “borderline diabetes.”2 She is prone to frequent bruising11 and is easy to shake, even when she is experiencing minimal anxiety.6-9 Ms. H had consulted with a neurologist because of unusual electrical “zapping” in her brain and muscle twitches.5,8,9,13 She had experienced a seizure as a child, but this was possibly related to hypernatremia,2 and she has not taken any anticonvulsant medication for several years.8 She exhibits hyperactive deep tendon reflexes and tremors5,7,9 and blinks frequently.6,9 She experiences hot flashes,3,6-8,14 does not tolerate heat, and prefers cooler weather.8,9 Her pains and aches,12,14 to which she has been prone all of her life, have recently become much worse, and she was diagnosed with fibromyalgia in part because she frequently feels stiff all over.10 She complains of strange tingling and prickling sensations in her hands and feet, especially when anxious.7,9,10 Her headaches also worsened and may be precipitated by bright light, as her pupils are usually dilated.5,7,9 Her hypertension is fairly controlled with medication.6,8-10 Ms. H says she experienced a psychotic episode when she was in her mid-teens,6,8 but reassures you that “she is not that bad now,” although she remains hypervigilant.13 Also while in her teens, Ms. H was treated with paroxetine and experienced restlessness, agitation, delirium, tachycardia, fluctuating blood pressure, diaphoresis, diarrhea, and neuromuscular excitation, which prompted discontinuation of the antidepressant.5-7,9,10
Impression. Ms. H exhibits symptoms associated with serotonin hyperactivity. Discontinuing and avoiding selective serotonin reuptake inhibitors (SSRIs) would be prudent; prescribing an anticonvulsant would be reasonable. Using a GABAergic medication to suppress serotonin (eg, baclofen) is likely to help. Avoiding dopaminergic medications is a must. Antidepressive antipsychotics would be logical to use. The use of serotonin-suppressing medications may be considered. One may argue for the use of beta-blockers in such a patient.
Continue to: Serotonin deficiency
Serotonin deficiency (Table 114,19-29)
Mr. A is chronically depressed, hopeless,19 and easily angered.21 He does not believe anyone can help him.19 You are concerned for his safety because he had attempted to end his life by shooting himself in the chest.19,21,22,25 Even when he’s not particularly depressed, Mr. A does not enjoy much of anything.21,26,27 He becomes particularly agitated when he drinks alcohol,25 which unfortunately is common for him.29 He engages in binge eating to feel better; he knows this is not healthy but he cannot control his behavior.20,29 Mr. A is poorly compliant with his medications, even with a blood thinner, which he was prescribed due to an episode of deep vein thrombosis. He complains of chronic daily headaches and episodic migraines.23,24 He rarely blinks,23,28 his skin is dry and cool, his hair is brittle,23 his mouth is dry,14,23,27 and he constantly licks his chapped lips.14,26,27 Mr. A frequently has general body pain26,31 but is dismissive of his body aches and completely stops reporting pain when his depression gets particularly severe. When depressed, he is slow in movement and thinking.14,21,26,27 He is more concerned with anxiety than depression.21 Mr. A is plagued by constipation, abdominal pain, muscle tension, and episodes of shaking.14,26,27 He also frequently complains about chronic tinnitus.28
Impression. Mr. A shows symptoms associated with serotonin hypoactivity. SSRIs and any other antidepressants with serotonin activity would be an obvious choice for treatment. A mood-stabilizing antipsychotic with serotonin activity would be welcome in treatment. Thyroid hormone supplementation may be of value, especially if thyroid stimulating hormone level is high. Light therapy, a diet with food that contains tryptophan, psychotherapy, and exercise are desirable. Avoiding benzodiazepines would be a good idea.
Dopamine excess (Table 214,30-41)
Ms. L presents with complaints of “fibromyalgia” and “daily headaches,”14 and also dissociation (finding herself in places when she does not know how she got there) and “out-of-body experiences.”32 She is odd, and states that people do not understand her and that she is “different.”38 Her friend, who is present at the appointment, elaborates on Ms. L’s bizarreness and oddness in behavior, out-of-context emotions, suspiciousness, paranoia, and possible hallucinations.35,36,38 Ms. L discloses frequent diffuse body pains, headaches, nausea, excessive salivation, and tongue burning, as well as muscle twitching.14 Sex worsens her headaches and body pain. She reports seizures that are not registered on EEG. In the office, she is suspicious, exhibits odd posturing, tends to misinterpret your words, and makes you feel uncomfortable. Anxiety38 and multiple obsessive-compulsive symptoms, especially excessive cleaning and grooming, complicate Ms. L’s life.31,32,34 On examination, she is hypertensive, and she has scars caused by self-cutting and skin picking on her arms.30-32 An electrocardiogram shows an elevated heart rate, widened QRS complex, and ectopic heartbeats.14 Ms. L has experienced trichotillomania since adolescence32-34 and her fingernails are bitten to the skin.34 She has difficulty with impulse control, and thrill-seeking is a prominent part of her life, mainly via gambling, compulsive sex, and compulsive buying.35,36 She also says she experiences indigestion and delayed gastric emptying.37
Impression. Ms. L exhibits multiple symptoms associated with dopamine excess. Dopamine antagonists should be considered and may help not only with her psychiatric symptoms but also with her pain symptoms. Bupropion (as a dopamine agonist), caffeine, and stimulants should be avoided.
Excessive dopamine is, in extreme cases, associated with somatic psychosis, somatic symptom disorder, factitious disorder, pain disorder, and hypochondria.39 It may come with odd and bizarre/peculiar symptoms out of proportion with objectively identified pathology. These symptoms are common in chronic pain and headache patients, and need to be addressed by appropriate use of dopamine antagonizing medications.39
Continue to: Dopamine deficiency
Dopamine deficiency (Table 24,14,20,38,40-43)
Mr. W experiences widespread pain, including chronic back pain, headaches, and abdominal pain. He also has substantial anhedonia, lack of interest, procrastination, and hypersomnia.41,42 He is apathetic and has difficulty getting up in the morning.41,42 Unusual tiredness and weakness drive him to overuse caffeine; he states that 5 Mountain Dews and 4 cups of regular coffee a day make his headaches bearable.38,41-43 Sex also improves his headaches. Since childhood, he has taken stimulants for attention-deficit/hyperactivity disorder. He reports that occasional use of cocaine helps ease his pain and depression. Mr. W’s wife is concerned with her husband’s low sexual drive and alcohol consumption, and discloses that he has periodic trouble with gambling. Mr. W was forced into psychotherapy but never was able to work productively with his therapist.38,41-43 He loves eating and cannot control his weight.40 This contrasts with episodic anorexia he experienced when he was younger.20 His face is usually emotionless.43 Mr. W is prone to constipation.14 His restless leg syndrome and periodic limb movement disorder are so bad that his wife refuses to share a bed with him.14 He is clumsy and has a problem with repetitive motor tasks.43 A paucity of speech, limited eye contact, poor grooming, and difficulty forming therapeutic alliances have long been part of Mr. W’s history.38,42,43 On physical examination, he has a dry mouth; he is stiff, tremulous, and hypotensive.14
Impression. Mr. W shows multiple symptoms associated with dopamine deficiency. Bupropion may be reasonable to consider. Dopamine augmentation via the use of stimulants is warranted in such patients, especially if stimulants had not been tried before (lisdexamfetamine would be a good choice to minimize addictive potential). For a patient with dopamine deficiency, levodopa may improve more than just restless legs. Amantadine may improve dopaminergic signaling through the accelerated dopamine release and decrease in presynaptic uptake, so this medication may be carefully tried.44 Pain treatment would not be successful for Mr. W without simultaneous treatment for his substance use.
Bottom Line
Both high and low levels of serotonin and dopamine may be associated with certain psychiatric and medical symptoms and disorders. An astute clinician may judge which neurotransmitter is dysfunctional based on the patient’s presentation, and tailor treatment accordingly.
Related Resources
- Abell SR, El-Mallakh RS. Serotonin-mediated anxiety: How to recognize and treat it. Current Psychiatry. 2021;20(11):37-40. doi:10.12788/cp.0168
Drug Brand Names
Amantadine • Gocovri
Baclofen • Ozobax
Bupropion • Wellbutrin
Fluoxetine • Prozac
Lisdexamfetamine • Vyvanse
Paroxetine • Paxil
1. Stahl SM. Dazzled by the dominions of dopamine: clinical roles of D3, D2, and D1 receptors. CNS Spectr. 2017;22(4):305-311.
2. Young RL, Lumsden AL, Martin AM, et al. Augmented capacity for peripheral serotonin release in human obesity. Int J Obes (Lond). 2018;42(11):1880-1889.
3. Ahlman H. Serotonin and carcinoid tumors. J Cardiovasc Pharmacol. 1985;7(Suppl 7):S79-S85.
4. Terry N, Margolis KG. Serotonergic mechanisms regulating the GI tract: experimental evidence and therapeutic relevance. Handb Exp Pharmacol. 2017;239:319-342.
5. Prakash S, Belani P, Trivedi A. Headache as a presenting feature in patients with serotonin syndrome: a case series. Cephalalgia. 2014;34(2):148-153.
6. van Ewijk CE, Jacobs GE, Girbes ARJ. Unsuspected serotonin toxicity in the ICU. Ann Intensive Care. 2016;6(1):85.
7. Pedavally S, Fugate JE, Rabinstein AA. Serotonin syndrome in the intensive care unit: clinical presentations and precipitating medications. Neurocrit Care. 2014;21(1):108-113.
8. Nguyen H, Pan A, Smollin C, et al. An 11-year retrospective review of cyproheptadine use in serotonin syndrome cases reported to the California Poison Control System. J Clin Pharm Ther. 2019;44(2):327-334.
9. Ansari H, Kouti L. Drug interaction and serotonin toxicity with opioid use: another reason to avoid opioids in headache and migraine treatment. Curr Pain Headache Rep. 2016;20(8):50.
10. Ott M, Mannchen JK, Jamshidi F, et al. Management of severe arterial hypertension associated with serotonin syndrome: a case report analysis based on systematic review techniques. Ther Adv Psychopharmacol. 2019;9:2045125318818814. doi:10.1177/2045125318818814
11. Cerrito F, Lazzaro MP, Gaudio E, et al. 5HT2-receptors and serotonin release: their role in human platelet aggregation. Life Sci. 1993;53(3):209-215.
12. Ohayon MM. Pain sensitivity, depression, and sleep deprivation: links with serotoninergic dysfunction. J Psychiatr Res. 2009;43(16):1243-1245.
13. Maron E, Shlik J. Serotonin function in panic disorder: important, but why? Neuropsychopharmacology. 2006;31(1):1-11.
14. Hall JE, Guyton AC. Textbook of Medical Physiology. 12th ed. Spanish version. Elsevier; 2011:120,199,201-204,730-740.
15. Garland EJ, Baerg EA. Amotivational syndrome associated with selective serotonin reuptake inhibitors in children and adolescents. J Child Adolesc Psychopharmacol. 2001;11(2):181-186.
16. George MS, Trimble MR. A fluvoxamine-induced frontal lobe syndrome in a patient with comorbid Gilles de la Tourette’s syndrome and obsessive compulsive disorder. J Clin Psychiatry. 1992;53(10):379-380.
17. Hoehn-Saric R, Harris GJ, Pearlson GD, et al. A fluoxetine-induced frontal lobe syndrome in an obsessive compulsive patient. J Clin Psychiatry. 1991;52(3):131-133.
18. Hoehn-Saric R, Lipsey JR, McLeod DR. Apathy and indifference in patients on fluvoxamine and fluoxetine. J Clin Psychopharmacol. 1990;10(5):343-345.
19. Samuelsson M, Jokinen J, Nordström AL, et al. CSF 5-HIAA, suicide intent and hopelessness in the prediction of early suicide in male high-risk suicide attempters. Acta Psychiatr Scand. 2006;113(1):44-47.
20. Brewerton TD. Clinical Handbook of Eating Disorders: An Integrated Approach. CRC Press; 2004:257-281.
21. Mann JJ, Oquendo M, Underwood MD, et al. The neurobiology of suicide risk: a review for the clinician. J Clin Psychiatry. 1999;60 Suppl 2:7-116.
22. Mann JJ, Malone KM. Cerebrospinal fluid amines and higher-lethality suicide attempts in depressed inpatients. Biol Psychiatry. 1997;41(2):162-171.
23. Joseph R, Welch KM, D’Andrea G. Serotonergic hypofunction in migraine: a synthesis of evidence based on platelet dense body dysfunction. Cephalalgia. 1989;9(4):293-299.
24. Pakalnis A, Splaingard M, Splaingard D, et al. Serotonin effects on sleep and emotional disorders in adolescent migraine. Headache. 2009;49(10):1486-1492.
25. Virkkunen M, Goldman D, Nielsen DA, et al. Low brain serotonin turnover rate (low CSF 5-HIAA) and impulsive violence. J Psychiatry Neurosci. 1995;20(4):271-275.
26. Liu Y, Zhao J, Fan X, et al. Dysfunction in serotonergic and noradrenergic systems and somatic symptoms in psychiatric disorders. Front Psychiatry. 2019;10:286.
27. Ginsburg GS, Riddle MA, Davies M. Somatic symptoms in children and adolescents with anxiety disorders. J Am Acad Child Adolesc Psychiatry. 2006;45(10):1179-1187.
28. O’Malley PG, Jackson JL, Santoro J, et al. Antidepressant therapy for unexplained symptoms and symptom syndromes. J Fam Pract. 1999;48(12):980-990.
29. Fortuna JL. Sweet preference, sugar addiction and the familial history of alcohol dependence: shared neural pathways and genes. J Psychoactive Drugs. 2010;42(2):147-151.
30. Stanley B, Sher L, Wilson S, et al. Non-suicidal self-injurious behavior, endogenous opioids and monoamine neurotransmitters. J Affect Disord. 2010;124(1-2):134-140.
31. Graybiel AM, Saka E. A genetic basis for obsessive grooming. Neuron. 2002;33(1):1-2.
32. Tse W, Hälbig TD. Skin picking in Parkinson’s disease: a behavioral side-effect of dopaminergic treatment? Psychiatry Clin Neurosci. 2010;64(2):214.
33. Ayaydın H. Probable emergence of symptoms of trichotillomania by atomoxetine: a case report. Psychiatry and Clinical Psychopharmacology. 2019;29(2)220-222.
34. Paholpak P, Mendez MF. Trichotillomania as a manifestation of dementia. Case Rep Psychiatry. 2016;2016:9782702. doi:10.1155/2016/9782702
35. Clark CA, Dagher A. The role of dopamine in risk taking: a specific look at Parkinson’s disease and gambling. Front Behav Neurosci. 2014;8:196.
36. Norbury A, Husain M. Sensation-seeking: dopaminergic modulation and risk for psychopathology. Behav Brain Res. 2015;288:79-93.
37. Chen TS, Chang FY. Elevated serum dopamine increases while coffee consumption decreases the occurrence of reddish streaks in the intact stomach. J Gastroenterol Hepatol. 2013;28(12):1810-1814.
38. Wong-Riley MT. Neuroscience Secrets. 1st edition. Spanish version. Hanley & Belfus; 1999:420-429.
39. Arbuck DM. Antipsychotics, dopamine, and pain. Current Psychiatry. 2020;19(1):25-29,31.
40. Bello NT, Hajnal A. Dopamine and binge eating behaviors. Pharmacol Biochem Behav. 2010;97(1):25-33.
41. Velligan DI, Weiden PJ, Sajatovic M, et al; Expert Consensus Panel on Adherence Problems in Serious and Persistent Mental Illness. The expert consensus guideline series: adherence problems in patients with serious and persistent mental illness. J Clin Psychiatry. 2009;70 Suppl 4:1-46.
42. Milev P, Ho BC, Arndt S, et al. Predictive values of neurocognition and negative symptoms on functional outcome in schizophrenia: a longitudinal first-episode study with 7-year follow-up. Am J Psychiatry. 2005;162(3):495-506.
43. Gepshtein S, Li X, Snider J, et al. Dopamine function and the efficiency of human movement. J Cogn Neurosci. 2014;26(3):645-657.
44. Scarff JR. The ABCDs of treating tardive dyskinesia. Current Psychiatry. 2020;19(4):21,55.
1. Stahl SM. Dazzled by the dominions of dopamine: clinical roles of D3, D2, and D1 receptors. CNS Spectr. 2017;22(4):305-311.
2. Young RL, Lumsden AL, Martin AM, et al. Augmented capacity for peripheral serotonin release in human obesity. Int J Obes (Lond). 2018;42(11):1880-1889.
3. Ahlman H. Serotonin and carcinoid tumors. J Cardiovasc Pharmacol. 1985;7(Suppl 7):S79-S85.
4. Terry N, Margolis KG. Serotonergic mechanisms regulating the GI tract: experimental evidence and therapeutic relevance. Handb Exp Pharmacol. 2017;239:319-342.
5. Prakash S, Belani P, Trivedi A. Headache as a presenting feature in patients with serotonin syndrome: a case series. Cephalalgia. 2014;34(2):148-153.
6. van Ewijk CE, Jacobs GE, Girbes ARJ. Unsuspected serotonin toxicity in the ICU. Ann Intensive Care. 2016;6(1):85.
7. Pedavally S, Fugate JE, Rabinstein AA. Serotonin syndrome in the intensive care unit: clinical presentations and precipitating medications. Neurocrit Care. 2014;21(1):108-113.
8. Nguyen H, Pan A, Smollin C, et al. An 11-year retrospective review of cyproheptadine use in serotonin syndrome cases reported to the California Poison Control System. J Clin Pharm Ther. 2019;44(2):327-334.
9. Ansari H, Kouti L. Drug interaction and serotonin toxicity with opioid use: another reason to avoid opioids in headache and migraine treatment. Curr Pain Headache Rep. 2016;20(8):50.
10. Ott M, Mannchen JK, Jamshidi F, et al. Management of severe arterial hypertension associated with serotonin syndrome: a case report analysis based on systematic review techniques. Ther Adv Psychopharmacol. 2019;9:2045125318818814. doi:10.1177/2045125318818814
11. Cerrito F, Lazzaro MP, Gaudio E, et al. 5HT2-receptors and serotonin release: their role in human platelet aggregation. Life Sci. 1993;53(3):209-215.
12. Ohayon MM. Pain sensitivity, depression, and sleep deprivation: links with serotoninergic dysfunction. J Psychiatr Res. 2009;43(16):1243-1245.
13. Maron E, Shlik J. Serotonin function in panic disorder: important, but why? Neuropsychopharmacology. 2006;31(1):1-11.
14. Hall JE, Guyton AC. Textbook of Medical Physiology. 12th ed. Spanish version. Elsevier; 2011:120,199,201-204,730-740.
15. Garland EJ, Baerg EA. Amotivational syndrome associated with selective serotonin reuptake inhibitors in children and adolescents. J Child Adolesc Psychopharmacol. 2001;11(2):181-186.
16. George MS, Trimble MR. A fluvoxamine-induced frontal lobe syndrome in a patient with comorbid Gilles de la Tourette’s syndrome and obsessive compulsive disorder. J Clin Psychiatry. 1992;53(10):379-380.
17. Hoehn-Saric R, Harris GJ, Pearlson GD, et al. A fluoxetine-induced frontal lobe syndrome in an obsessive compulsive patient. J Clin Psychiatry. 1991;52(3):131-133.
18. Hoehn-Saric R, Lipsey JR, McLeod DR. Apathy and indifference in patients on fluvoxamine and fluoxetine. J Clin Psychopharmacol. 1990;10(5):343-345.
19. Samuelsson M, Jokinen J, Nordström AL, et al. CSF 5-HIAA, suicide intent and hopelessness in the prediction of early suicide in male high-risk suicide attempters. Acta Psychiatr Scand. 2006;113(1):44-47.
20. Brewerton TD. Clinical Handbook of Eating Disorders: An Integrated Approach. CRC Press; 2004:257-281.
21. Mann JJ, Oquendo M, Underwood MD, et al. The neurobiology of suicide risk: a review for the clinician. J Clin Psychiatry. 1999;60 Suppl 2:7-116.
22. Mann JJ, Malone KM. Cerebrospinal fluid amines and higher-lethality suicide attempts in depressed inpatients. Biol Psychiatry. 1997;41(2):162-171.
23. Joseph R, Welch KM, D’Andrea G. Serotonergic hypofunction in migraine: a synthesis of evidence based on platelet dense body dysfunction. Cephalalgia. 1989;9(4):293-299.
24. Pakalnis A, Splaingard M, Splaingard D, et al. Serotonin effects on sleep and emotional disorders in adolescent migraine. Headache. 2009;49(10):1486-1492.
25. Virkkunen M, Goldman D, Nielsen DA, et al. Low brain serotonin turnover rate (low CSF 5-HIAA) and impulsive violence. J Psychiatry Neurosci. 1995;20(4):271-275.
26. Liu Y, Zhao J, Fan X, et al. Dysfunction in serotonergic and noradrenergic systems and somatic symptoms in psychiatric disorders. Front Psychiatry. 2019;10:286.
27. Ginsburg GS, Riddle MA, Davies M. Somatic symptoms in children and adolescents with anxiety disorders. J Am Acad Child Adolesc Psychiatry. 2006;45(10):1179-1187.
28. O’Malley PG, Jackson JL, Santoro J, et al. Antidepressant therapy for unexplained symptoms and symptom syndromes. J Fam Pract. 1999;48(12):980-990.
29. Fortuna JL. Sweet preference, sugar addiction and the familial history of alcohol dependence: shared neural pathways and genes. J Psychoactive Drugs. 2010;42(2):147-151.
30. Stanley B, Sher L, Wilson S, et al. Non-suicidal self-injurious behavior, endogenous opioids and monoamine neurotransmitters. J Affect Disord. 2010;124(1-2):134-140.
31. Graybiel AM, Saka E. A genetic basis for obsessive grooming. Neuron. 2002;33(1):1-2.
32. Tse W, Hälbig TD. Skin picking in Parkinson’s disease: a behavioral side-effect of dopaminergic treatment? Psychiatry Clin Neurosci. 2010;64(2):214.
33. Ayaydın H. Probable emergence of symptoms of trichotillomania by atomoxetine: a case report. Psychiatry and Clinical Psychopharmacology. 2019;29(2)220-222.
34. Paholpak P, Mendez MF. Trichotillomania as a manifestation of dementia. Case Rep Psychiatry. 2016;2016:9782702. doi:10.1155/2016/9782702
35. Clark CA, Dagher A. The role of dopamine in risk taking: a specific look at Parkinson’s disease and gambling. Front Behav Neurosci. 2014;8:196.
36. Norbury A, Husain M. Sensation-seeking: dopaminergic modulation and risk for psychopathology. Behav Brain Res. 2015;288:79-93.
37. Chen TS, Chang FY. Elevated serum dopamine increases while coffee consumption decreases the occurrence of reddish streaks in the intact stomach. J Gastroenterol Hepatol. 2013;28(12):1810-1814.
38. Wong-Riley MT. Neuroscience Secrets. 1st edition. Spanish version. Hanley & Belfus; 1999:420-429.
39. Arbuck DM. Antipsychotics, dopamine, and pain. Current Psychiatry. 2020;19(1):25-29,31.
40. Bello NT, Hajnal A. Dopamine and binge eating behaviors. Pharmacol Biochem Behav. 2010;97(1):25-33.
41. Velligan DI, Weiden PJ, Sajatovic M, et al; Expert Consensus Panel on Adherence Problems in Serious and Persistent Mental Illness. The expert consensus guideline series: adherence problems in patients with serious and persistent mental illness. J Clin Psychiatry. 2009;70 Suppl 4:1-46.
42. Milev P, Ho BC, Arndt S, et al. Predictive values of neurocognition and negative symptoms on functional outcome in schizophrenia: a longitudinal first-episode study with 7-year follow-up. Am J Psychiatry. 2005;162(3):495-506.
43. Gepshtein S, Li X, Snider J, et al. Dopamine function and the efficiency of human movement. J Cogn Neurosci. 2014;26(3):645-657.
44. Scarff JR. The ABCDs of treating tardive dyskinesia. Current Psychiatry. 2020;19(4):21,55.