User login
Adverse pregnancy outcomes and later cardiovascular disease
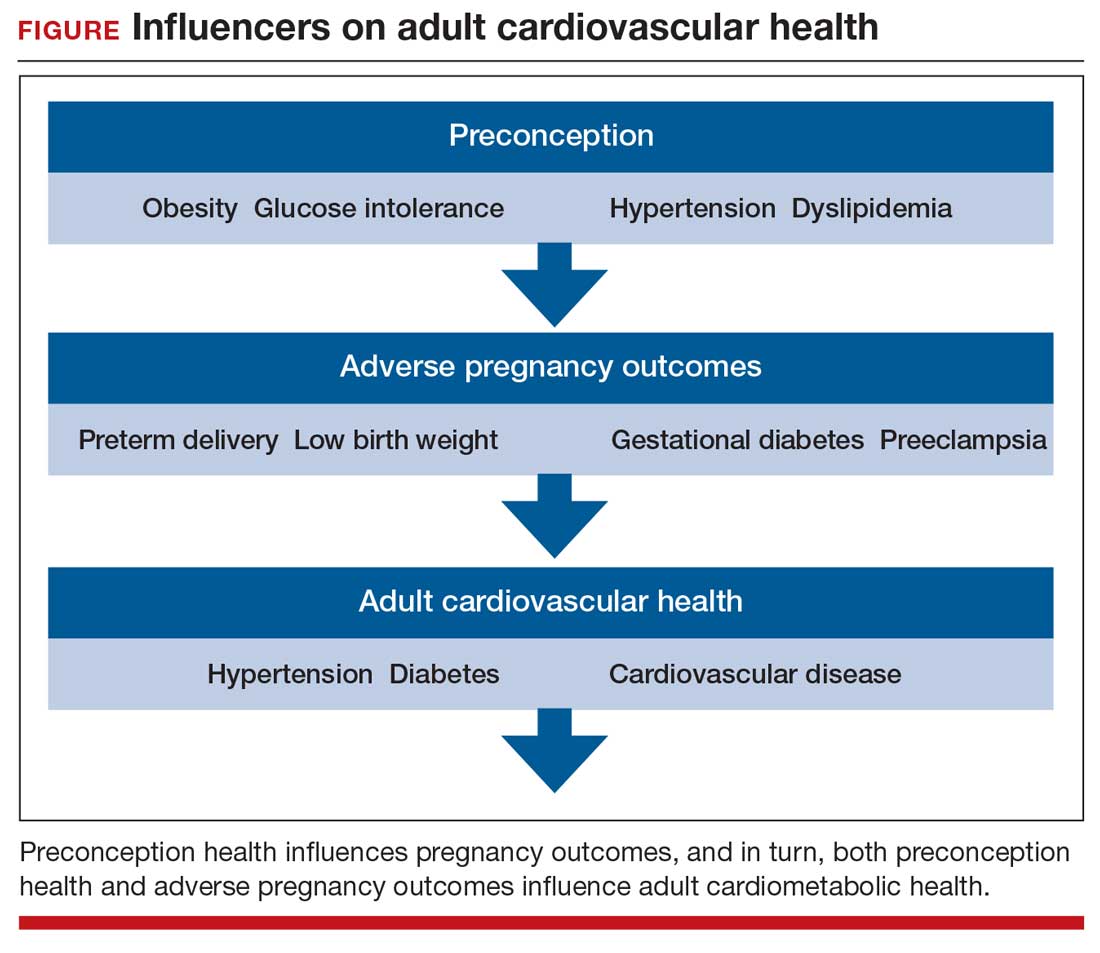
Preconception health influences pregnancy outcomes, and in turn, both preconception health and an APO influence adult cardiometabolic health (FIGURE). This editorial is focused on the link between APOs and later cardiometabolic morbidity and mortality, recognizing that preconception health greatly influences the risk of an APO and lifetime cardiometabolic disease.
Adverse pregnancy outcomes
Major APOs include miscarriage, preterm birth (birth <37 weeks’ gestation), low birth weight (birth weight ≤2,500 g; 5.5 lb), gestational diabetes (GDM), preeclampsia, and placental abruption. In the United States, among all births, reported rates of the following APOs are:1-3
- preterm birth, 10.2%
- low birth weight, 8.3%
- GDM, 6%
- preeclampsia, 5%
- placental abruption, 1%.
Miscarriage occurs in approximately 10% to 15% of pregnancies, influenced by both the age of the woman and the method used to diagnose pregnancy.4 Miscarriage, preterm birth, low birth weight, GDM, preeclampsia, and placental abruption have been reported to be associated with an increased risk of later cardiovascular morbidity and mortality.
APOs and cardiovascular disease
Cardiovascular disease (CVD) affects the majority of people past the age of 60 years and includes 4 major subcategories:
- coronary heart disease, including myocardial infarction, angina, and heart failure
- CVD, stroke, and transient ischemic attack
- peripheral artery disease
- atherosclerosis of the aorta leading to aortic aneurysm.
Multiple meta-analyses report that APOs are associated with CVD in later life. A comprehensive review reported that the risk of CVD was increased following a pregnancy with one of these APOs: severe preeclampsia (odds ratio [OR], 2.74), GDM (OR, 1.68), preterm birth (OR, 1.93), low birth weight (OR, 1.29), and placental abruption (OR, 1.82).5
The link between APOs and CVD may be explained in part by the association of APOs with multiple risk factors for CVD, including chronic hypertension, type 2 diabetes mellitus (T2DM), and dyslipidemia. A meta-analysis of 43 studies reported that, compared with controls, women with a history of preeclampsia have a 3.13 times greater risk of developing chronic hypertension.6 Among women with preeclampsia, approximately 20% will develop hypertension within 15 years.7 A meta-analysis of 20 studies reported that women with a history of GDM had a 9.51-times greater risk of developing T2DM than women without GDM.8 Among women with a history of GDM, over 16 years of follow-up, T2DM was diagnosed in 16.2%, compared with 1.9% of control women.8
CVD prevention—Breastfeeding: An antidote for APOs
Pregnancy stresses both the cardiovascular and metabolic systems. Breastfeeding is an antidote to the stresses imposed by pregnancy. Breastfeeding women have lower blood glucose9 and blood pressure.10
Breastfeeding reduces the risk of CVD. In a study of 100,864 parous Australian women, with a mean age of 60 years, ever breastfeeding was associated a lower risk of CVD hospitalization (adjusted hazard ratio [aHR], 0.86; 95% confidence interval [CI], 0.78–0.96; P = .005) and CVD mortality (aHR, 0.66; 95% CI, 0.49–0.89; P = .006).11
Continue to: CVD prevention—American Heart Association recommendations...
CVD prevention—American Heart Association recommendations
The American Heart Association12 recommends lifestyle interventions to reduce the risk of CVD, including:
- Eat a high-quality diet that includes vegetables, fruit, whole grains, beans, legumes, nuts, plant-based protein, lean animal protein, and fish.
- Limit intake of sugary drinks and foods, fatty or processed meats, full-fat dairy products, eggs, highly processed foods, and tropical oils.
- Exercise at least 150 minutes weekly at a moderate activity level, including muscle-strengthening activity.
- Reduce prolonged intervals of sitting.
- Live a tobacco- and nicotine-free life.
- Strive to maintain a normal body mass index.
- Consider using an activity tracker to monitor activity level.
- After 40 years of age calculate CVD risk using a validated calculator such as the American Cardiology Association risk calculator.13 This calculator uses age, gender, and lipid and blood pressure measurements to calculate the 10-year risk of atherosclerotic CVD, including coronary death, myocardial infarction, and stroke.
Medications to reduce CVD risk
Historically, ObGyns have not routinely prescribed medications to treat hypertension, dyslipidemia, or to prevent diabetes. The recent increase in the valuation of return ambulatory visits and a reduction in the valuation assigned to procedural care may provide ObGyn practices the additional resources needed to manage some chronic diseases. Physician assistants and nurse practitioners may help ObGyn practices to manage hypertension, dyslipidemia, and prediabetes.
Prior to initiating a medicine, counseling about healthy living, including smoking cessation, exercise, heart-healthy diet, and achieving an optimal body mass index is warranted.
For treatment of stage II hypertension, defined as blood pressure (BP) measurements with systolic BP ≥140 mm Hg and diastolic BP ≥90 mm Hg, therapeutic lifestyle interventions include: optimizing weight, following the DASH diet, restricting dietary sodium, physical activity, and reducing alcohol consumption. Medication treatment for essential hypertension is guided by the magnitude of BP reduction needed to achieve normotension. For women with hypertension needing antihypertensive medication and planning another pregnancy in the near future, labetalol or extended-release nifedipine may be first-line medications. For women who have completed their families or who have no immediate plans for pregnancy, an angiotensin-converting enzyme inhibitor, angiotensin receptor blocker, calcium channel blocker, or thiazide diuretic are commonly prescribed.14
For the treatment of elevated low-density lipoprotein (LDL) cholesterol in women who have not had a cardiovascular event, statin therapy is often warranted when both the LDL cholesterol is >100 mg/dL and the woman has a calculated 10-year risk of >10% for a cardiovascular event using the American Heart Association or American College of Cardiology calculator. Most women who meet these criteria will be older than age 40 years and many will be under the care of an internal medicine or family medicine specialist, limiting the role of the ObGyn.15-17
For prevention of diabetes in women with a history of GDM, both weight loss and metformin (1,750 mg daily) have been shown in clinical trials to reduce the risk of developing T2DM.18 Among 350 women with a history of GDM who were followed for 10 years, metformin 850 mg twice daily reduced the risk of developing T2DM by 40% compared with placebo.19 In the same study, lifestyle changes without metformin, including loss of 7% of body weight plus 150 minutes of exercise weekly was associated with a 35% reduction in the risk of developing T2DM.19 Metformin is one of the least expensive prescription medications and is cost-effective for the prevention of T2DM.18
Low-dose aspirin treatment for the prevention of CVD in women who have not had a cardiovascular event must balance a modest reduction in cardiovascular events with a small increased risk of bleeding events. The US Preventive Services Task Force (USPSTF) recommends low-dose aspirin for a limited group of women, those aged 50 to 59 years of age with a 10-year risk of a cardiovascular event >10% who are willing to take aspirin for 10 years. The USPSTF concluded that there is insufficient evidence to recommend low-dose aspirin prevention of CVD in women aged <50 years.20
Continue to: Beyond the fourth trimester...
Beyond the fourth trimester
The fourth trimester is the 12-week period following birth. At the comprehensive postpartum visit, the American College of Obstetricians and Gynecologists (ACOG) recommends that women with APOs be counseled about their increased lifetime risk of maternal cardiometabolic disease.21 In addition, ACOG recommends that at this visit the clinician who will assume primary responsibility for the woman’s ongoing medical care in her primary medical home be clarified. One option is to ensure a high-quality hand-off to an internal medicine or family medicine clinician. Another option is for a clinician in the ObGyn’s office practice, including a physician assistant, nurse practitioner, or office-based ObGyn, to assume some role in the primary care of the woman.
An APO is not only a pregnancy problem
An APO reverberates across a woman’s lifetime, increasing the risk of CVD and diabetes. In the United States the mean age at first birth is 27 years.1 The mean life expectancy of US women is 81 years.22 Following a birth complicated by an APO there are 5 decades of opportunity to improve health through lifestyle changes and medication treatment of obesity, hypertension, dyslipidemia, and hyperglycemia, thereby reducing the risk of CVD.
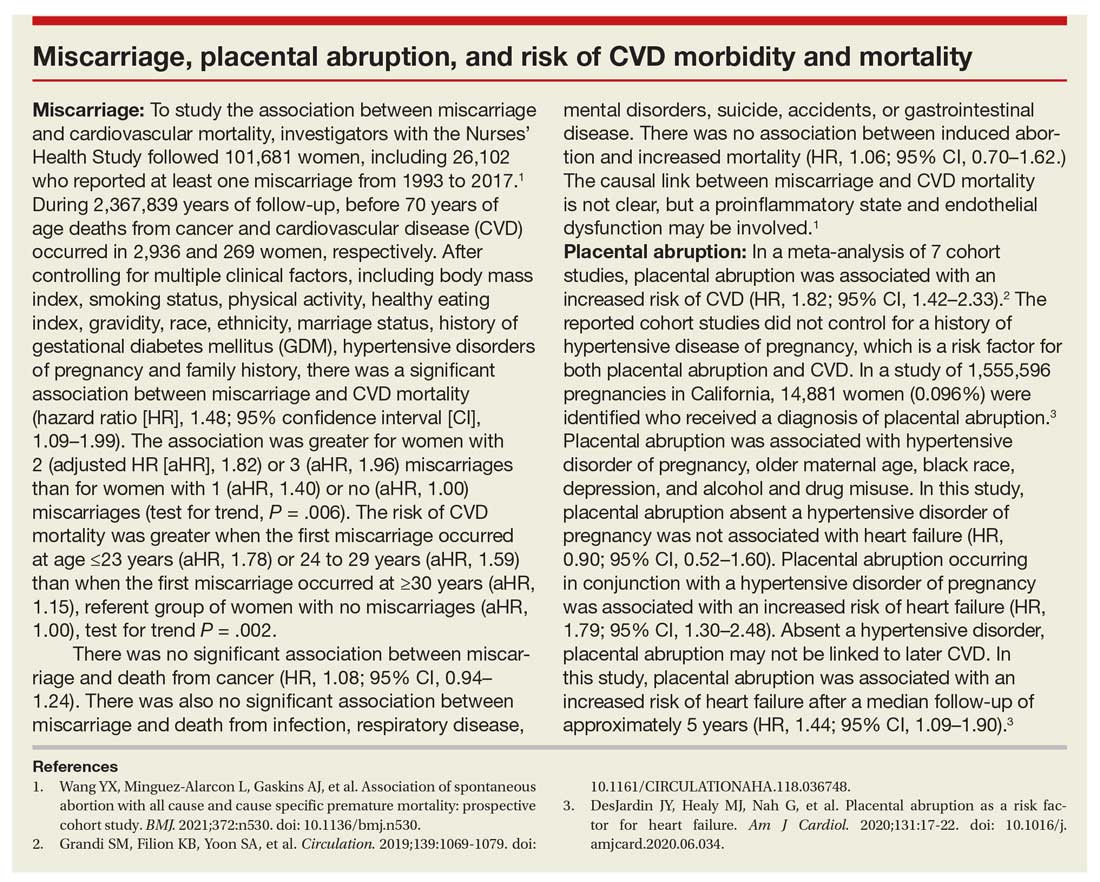
- Martin JA, Hamilton BE, Osterman MJ, et al. Births: final data for 2019. Natl Vital Stat Rep. 2021;70:1-51.
- Deputy NP, Kim SY, Conrey EJ, et al. Prevalence and changes in preexisting diabetes and gestational diabetes among women who had a live birth—United States, 2012-2016. MMWR Morb Mortal Wkly Rep. 2018;67:1201-1207. doi: 10.15585/mmwr.mm6743a2.
- Fingar KR, Mabry-Hernandez I, Ngo-Metzger Q, et al. Delivery hospitalizations involving preeclampsia and eclampsia, 2005–2014. Statistical brief #222. In: Healthcare Cost and Utilization Project (HCUP) Statistical Briefs [Internet]. Agency for Healthcare Research and Quality: Rockville, MD; April 2017.
- Magnus MC, Wilcox AJ, Morken NH, et al. Role of maternal age and pregnancy history in risk of miscarriage: prospective register-based study. BMJ. 2019;364:869.
- Parikh NI, Gonzalez JM, Anderson CAM, et al. Adverse pregnancy outcomes and cardiovascular disease risk: unique opportunities for cardiovascular disease prevention in women. Circulation. 2021;143:e902-e916. doi: 10.1161 /CIR.0000000000000961.
- Brown MC, Best KE, Pearce MS, et al. Cardiovascular disease risk in women with pre-eclampsia: systematic review and meta-analysis. Eur J Epidemiol. 2013;28:1-19. doi: 10.1007/s10654-013- 9762-6.
- Groenfol TK, Zoet GA, Franx A, et al; on behalf of the PREVENT Group. Trajectory of cardiovascular risk factors after hypertensive disorders of pregnancy. Hypertension. 2019;73:171-178. doi: 10.1161/HYPERTENSIONAHA.118.11726.
- Vounzoulaki E, Khunti K, Abner SC, et al. Progression to type 2 diabetes in women with a known history of gestational diabetes: systematic review and meta-analysis. BMJ. 2020;369:m1361. doi: 10.1136/bmj.m1361.
- Tarrant M, Chooniedass R, Fan HSL, et al. Breastfeeding and postpartum glucose regulation among women with prior gestational diabetes: a systematic review. J Hum Lact. 2020;36:723-738. doi: 10.1177/0890334420950259.
- Park S, Choi NK. Breastfeeding and maternal hypertension. Am J Hypertens. 2018;31:615-621. doi: 10.1093/ajh/hpx219.
- Nguyen B, Gale J, Nassar N, et al. Breastfeeding and cardiovascular disease hospitalization and mortality in parous women: evidence from a large Australian cohort study. J Am Heart Assoc. 2019;8:e011056. doi: 10.1161/JAHA.118.011056.
- Eight things you can do to prevent heart disease and stroke. American Heart Association website. https://www.heart.org/en/healthy-living /healthy-lifestyle/prevent-heart-disease-andstroke. Last Reviewed March 14, 2019. Accessed May 19, 2021.
- ASCVD risk estimator plus. American College of Cardiology website. https://tools.acc.org /ascvd-risk-estimator-plus/#!/calculate /estimate/. Accessed May 19, 2021.
- Ferdinand KC, Nasser SA. Management of essential hypertension. Cardiol Clin. 2017;35:231-246. doi: 10.1016/j.ccl.2016.12.005.
- Packard CJ. LDL cholesterol: how low to go? Trends Cardiovasc Med. 2018;28:348-354. doi: 10.1016/j.tcm.2017.12.011.
- Simons L. An updated review of lipid-modifying therapy. Med J Aust. 2019;211:87-92. doi: 10.5694 /mja2.50142.
- Chou R, Dana T, Blazina I, et al. Statins for the prevention of cardiovascular disease in adults: evidence report and systematic review for the US Preventive Services Task Force. JAMA. 2016;316:2008. doi: 10.1001/jama.2015.15629.
- Moin T, Schmittdiel JA, Flory JH, et al. Review of metformin use for type 2 diabetes mellitus prevention. Am J Prev Med. 2018;55:565-574. doi: 10.1016/j.amepre.2018.04.038.
- Aorda VR, Christophi CA, Edelstein SL, et al, for the Diabetes Prevention Program Research Group. The effect of lifestyle intervention and metformin on preventing or delaying diabetes among women with and without gestational diabetes: the Diabetes Prevention Program outcomes study 10-year follow-up. J Clin Endocrinol Metab. 2015;100:1646- 1653. doi: 10.1210/jc.2014-3761.
- Bibbins-Domingo K, U.S. Preventive Services Task Force. Aspirin use of the primary prevention of cardiovascular disease and colorectal cancer: U.S. Preventive Services Task Force Recommendation Statement. Ann Int Med. 2016; 164: 836-845. doi: 10.7326/M16-0577.
- American College of Obstetricians and Gynecologists. ACOG Committee Opinion No. 736: optimizing postpartum care. Obstet Gynecol. 2018;131:e140-e150. doi: 10.1097 /AOG.0000000000002633.
- National Center for Health Statistics. Health, United States, 2017: Table 015. Hyattsville, MD; 2021. https://www.cdc.gov/nchs/data /hus/2017/015.pdf. Accessed May 18, 2021.
Preconception health influences pregnancy outcomes, and in turn, both preconception health and an APO influence adult cardiometabolic health (FIGURE). This editorial is focused on the link between APOs and later cardiometabolic morbidity and mortality, recognizing that preconception health greatly influences the risk of an APO and lifetime cardiometabolic disease.

Adverse pregnancy outcomes
Major APOs include miscarriage, preterm birth (birth <37 weeks’ gestation), low birth weight (birth weight ≤2,500 g; 5.5 lb), gestational diabetes (GDM), preeclampsia, and placental abruption. In the United States, among all births, reported rates of the following APOs are:1-3
- preterm birth, 10.2%
- low birth weight, 8.3%
- GDM, 6%
- preeclampsia, 5%
- placental abruption, 1%.
Miscarriage occurs in approximately 10% to 15% of pregnancies, influenced by both the age of the woman and the method used to diagnose pregnancy.4 Miscarriage, preterm birth, low birth weight, GDM, preeclampsia, and placental abruption have been reported to be associated with an increased risk of later cardiovascular morbidity and mortality.
APOs and cardiovascular disease
Cardiovascular disease (CVD) affects the majority of people past the age of 60 years and includes 4 major subcategories:
- coronary heart disease, including myocardial infarction, angina, and heart failure
- CVD, stroke, and transient ischemic attack
- peripheral artery disease
- atherosclerosis of the aorta leading to aortic aneurysm.
Multiple meta-analyses report that APOs are associated with CVD in later life. A comprehensive review reported that the risk of CVD was increased following a pregnancy with one of these APOs: severe preeclampsia (odds ratio [OR], 2.74), GDM (OR, 1.68), preterm birth (OR, 1.93), low birth weight (OR, 1.29), and placental abruption (OR, 1.82).5
The link between APOs and CVD may be explained in part by the association of APOs with multiple risk factors for CVD, including chronic hypertension, type 2 diabetes mellitus (T2DM), and dyslipidemia. A meta-analysis of 43 studies reported that, compared with controls, women with a history of preeclampsia have a 3.13 times greater risk of developing chronic hypertension.6 Among women with preeclampsia, approximately 20% will develop hypertension within 15 years.7 A meta-analysis of 20 studies reported that women with a history of GDM had a 9.51-times greater risk of developing T2DM than women without GDM.8 Among women with a history of GDM, over 16 years of follow-up, T2DM was diagnosed in 16.2%, compared with 1.9% of control women.8
CVD prevention—Breastfeeding: An antidote for APOs
Pregnancy stresses both the cardiovascular and metabolic systems. Breastfeeding is an antidote to the stresses imposed by pregnancy. Breastfeeding women have lower blood glucose9 and blood pressure.10
Breastfeeding reduces the risk of CVD. In a study of 100,864 parous Australian women, with a mean age of 60 years, ever breastfeeding was associated a lower risk of CVD hospitalization (adjusted hazard ratio [aHR], 0.86; 95% confidence interval [CI], 0.78–0.96; P = .005) and CVD mortality (aHR, 0.66; 95% CI, 0.49–0.89; P = .006).11
Continue to: CVD prevention—American Heart Association recommendations...
CVD prevention—American Heart Association recommendations
The American Heart Association12 recommends lifestyle interventions to reduce the risk of CVD, including:
- Eat a high-quality diet that includes vegetables, fruit, whole grains, beans, legumes, nuts, plant-based protein, lean animal protein, and fish.
- Limit intake of sugary drinks and foods, fatty or processed meats, full-fat dairy products, eggs, highly processed foods, and tropical oils.
- Exercise at least 150 minutes weekly at a moderate activity level, including muscle-strengthening activity.
- Reduce prolonged intervals of sitting.
- Live a tobacco- and nicotine-free life.
- Strive to maintain a normal body mass index.
- Consider using an activity tracker to monitor activity level.
- After 40 years of age calculate CVD risk using a validated calculator such as the American Cardiology Association risk calculator.13 This calculator uses age, gender, and lipid and blood pressure measurements to calculate the 10-year risk of atherosclerotic CVD, including coronary death, myocardial infarction, and stroke.
Medications to reduce CVD risk
Historically, ObGyns have not routinely prescribed medications to treat hypertension, dyslipidemia, or to prevent diabetes. The recent increase in the valuation of return ambulatory visits and a reduction in the valuation assigned to procedural care may provide ObGyn practices the additional resources needed to manage some chronic diseases. Physician assistants and nurse practitioners may help ObGyn practices to manage hypertension, dyslipidemia, and prediabetes.
Prior to initiating a medicine, counseling about healthy living, including smoking cessation, exercise, heart-healthy diet, and achieving an optimal body mass index is warranted.
For treatment of stage II hypertension, defined as blood pressure (BP) measurements with systolic BP ≥140 mm Hg and diastolic BP ≥90 mm Hg, therapeutic lifestyle interventions include: optimizing weight, following the DASH diet, restricting dietary sodium, physical activity, and reducing alcohol consumption. Medication treatment for essential hypertension is guided by the magnitude of BP reduction needed to achieve normotension. For women with hypertension needing antihypertensive medication and planning another pregnancy in the near future, labetalol or extended-release nifedipine may be first-line medications. For women who have completed their families or who have no immediate plans for pregnancy, an angiotensin-converting enzyme inhibitor, angiotensin receptor blocker, calcium channel blocker, or thiazide diuretic are commonly prescribed.14
For the treatment of elevated low-density lipoprotein (LDL) cholesterol in women who have not had a cardiovascular event, statin therapy is often warranted when both the LDL cholesterol is >100 mg/dL and the woman has a calculated 10-year risk of >10% for a cardiovascular event using the American Heart Association or American College of Cardiology calculator. Most women who meet these criteria will be older than age 40 years and many will be under the care of an internal medicine or family medicine specialist, limiting the role of the ObGyn.15-17
For prevention of diabetes in women with a history of GDM, both weight loss and metformin (1,750 mg daily) have been shown in clinical trials to reduce the risk of developing T2DM.18 Among 350 women with a history of GDM who were followed for 10 years, metformin 850 mg twice daily reduced the risk of developing T2DM by 40% compared with placebo.19 In the same study, lifestyle changes without metformin, including loss of 7% of body weight plus 150 minutes of exercise weekly was associated with a 35% reduction in the risk of developing T2DM.19 Metformin is one of the least expensive prescription medications and is cost-effective for the prevention of T2DM.18
Low-dose aspirin treatment for the prevention of CVD in women who have not had a cardiovascular event must balance a modest reduction in cardiovascular events with a small increased risk of bleeding events. The US Preventive Services Task Force (USPSTF) recommends low-dose aspirin for a limited group of women, those aged 50 to 59 years of age with a 10-year risk of a cardiovascular event >10% who are willing to take aspirin for 10 years. The USPSTF concluded that there is insufficient evidence to recommend low-dose aspirin prevention of CVD in women aged <50 years.20
Continue to: Beyond the fourth trimester...
Beyond the fourth trimester
The fourth trimester is the 12-week period following birth. At the comprehensive postpartum visit, the American College of Obstetricians and Gynecologists (ACOG) recommends that women with APOs be counseled about their increased lifetime risk of maternal cardiometabolic disease.21 In addition, ACOG recommends that at this visit the clinician who will assume primary responsibility for the woman’s ongoing medical care in her primary medical home be clarified. One option is to ensure a high-quality hand-off to an internal medicine or family medicine clinician. Another option is for a clinician in the ObGyn’s office practice, including a physician assistant, nurse practitioner, or office-based ObGyn, to assume some role in the primary care of the woman.
An APO is not only a pregnancy problem
An APO reverberates across a woman’s lifetime, increasing the risk of CVD and diabetes. In the United States the mean age at first birth is 27 years.1 The mean life expectancy of US women is 81 years.22 Following a birth complicated by an APO there are 5 decades of opportunity to improve health through lifestyle changes and medication treatment of obesity, hypertension, dyslipidemia, and hyperglycemia, thereby reducing the risk of CVD.

Preconception health influences pregnancy outcomes, and in turn, both preconception health and an APO influence adult cardiometabolic health (FIGURE). This editorial is focused on the link between APOs and later cardiometabolic morbidity and mortality, recognizing that preconception health greatly influences the risk of an APO and lifetime cardiometabolic disease.

Adverse pregnancy outcomes
Major APOs include miscarriage, preterm birth (birth <37 weeks’ gestation), low birth weight (birth weight ≤2,500 g; 5.5 lb), gestational diabetes (GDM), preeclampsia, and placental abruption. In the United States, among all births, reported rates of the following APOs are:1-3
- preterm birth, 10.2%
- low birth weight, 8.3%
- GDM, 6%
- preeclampsia, 5%
- placental abruption, 1%.
Miscarriage occurs in approximately 10% to 15% of pregnancies, influenced by both the age of the woman and the method used to diagnose pregnancy.4 Miscarriage, preterm birth, low birth weight, GDM, preeclampsia, and placental abruption have been reported to be associated with an increased risk of later cardiovascular morbidity and mortality.
APOs and cardiovascular disease
Cardiovascular disease (CVD) affects the majority of people past the age of 60 years and includes 4 major subcategories:
- coronary heart disease, including myocardial infarction, angina, and heart failure
- CVD, stroke, and transient ischemic attack
- peripheral artery disease
- atherosclerosis of the aorta leading to aortic aneurysm.
Multiple meta-analyses report that APOs are associated with CVD in later life. A comprehensive review reported that the risk of CVD was increased following a pregnancy with one of these APOs: severe preeclampsia (odds ratio [OR], 2.74), GDM (OR, 1.68), preterm birth (OR, 1.93), low birth weight (OR, 1.29), and placental abruption (OR, 1.82).5
The link between APOs and CVD may be explained in part by the association of APOs with multiple risk factors for CVD, including chronic hypertension, type 2 diabetes mellitus (T2DM), and dyslipidemia. A meta-analysis of 43 studies reported that, compared with controls, women with a history of preeclampsia have a 3.13 times greater risk of developing chronic hypertension.6 Among women with preeclampsia, approximately 20% will develop hypertension within 15 years.7 A meta-analysis of 20 studies reported that women with a history of GDM had a 9.51-times greater risk of developing T2DM than women without GDM.8 Among women with a history of GDM, over 16 years of follow-up, T2DM was diagnosed in 16.2%, compared with 1.9% of control women.8
CVD prevention—Breastfeeding: An antidote for APOs
Pregnancy stresses both the cardiovascular and metabolic systems. Breastfeeding is an antidote to the stresses imposed by pregnancy. Breastfeeding women have lower blood glucose9 and blood pressure.10
Breastfeeding reduces the risk of CVD. In a study of 100,864 parous Australian women, with a mean age of 60 years, ever breastfeeding was associated a lower risk of CVD hospitalization (adjusted hazard ratio [aHR], 0.86; 95% confidence interval [CI], 0.78–0.96; P = .005) and CVD mortality (aHR, 0.66; 95% CI, 0.49–0.89; P = .006).11
Continue to: CVD prevention—American Heart Association recommendations...
CVD prevention—American Heart Association recommendations
The American Heart Association12 recommends lifestyle interventions to reduce the risk of CVD, including:
- Eat a high-quality diet that includes vegetables, fruit, whole grains, beans, legumes, nuts, plant-based protein, lean animal protein, and fish.
- Limit intake of sugary drinks and foods, fatty or processed meats, full-fat dairy products, eggs, highly processed foods, and tropical oils.
- Exercise at least 150 minutes weekly at a moderate activity level, including muscle-strengthening activity.
- Reduce prolonged intervals of sitting.
- Live a tobacco- and nicotine-free life.
- Strive to maintain a normal body mass index.
- Consider using an activity tracker to monitor activity level.
- After 40 years of age calculate CVD risk using a validated calculator such as the American Cardiology Association risk calculator.13 This calculator uses age, gender, and lipid and blood pressure measurements to calculate the 10-year risk of atherosclerotic CVD, including coronary death, myocardial infarction, and stroke.
Medications to reduce CVD risk
Historically, ObGyns have not routinely prescribed medications to treat hypertension, dyslipidemia, or to prevent diabetes. The recent increase in the valuation of return ambulatory visits and a reduction in the valuation assigned to procedural care may provide ObGyn practices the additional resources needed to manage some chronic diseases. Physician assistants and nurse practitioners may help ObGyn practices to manage hypertension, dyslipidemia, and prediabetes.
Prior to initiating a medicine, counseling about healthy living, including smoking cessation, exercise, heart-healthy diet, and achieving an optimal body mass index is warranted.
For treatment of stage II hypertension, defined as blood pressure (BP) measurements with systolic BP ≥140 mm Hg and diastolic BP ≥90 mm Hg, therapeutic lifestyle interventions include: optimizing weight, following the DASH diet, restricting dietary sodium, physical activity, and reducing alcohol consumption. Medication treatment for essential hypertension is guided by the magnitude of BP reduction needed to achieve normotension. For women with hypertension needing antihypertensive medication and planning another pregnancy in the near future, labetalol or extended-release nifedipine may be first-line medications. For women who have completed their families or who have no immediate plans for pregnancy, an angiotensin-converting enzyme inhibitor, angiotensin receptor blocker, calcium channel blocker, or thiazide diuretic are commonly prescribed.14
For the treatment of elevated low-density lipoprotein (LDL) cholesterol in women who have not had a cardiovascular event, statin therapy is often warranted when both the LDL cholesterol is >100 mg/dL and the woman has a calculated 10-year risk of >10% for a cardiovascular event using the American Heart Association or American College of Cardiology calculator. Most women who meet these criteria will be older than age 40 years and many will be under the care of an internal medicine or family medicine specialist, limiting the role of the ObGyn.15-17
For prevention of diabetes in women with a history of GDM, both weight loss and metformin (1,750 mg daily) have been shown in clinical trials to reduce the risk of developing T2DM.18 Among 350 women with a history of GDM who were followed for 10 years, metformin 850 mg twice daily reduced the risk of developing T2DM by 40% compared with placebo.19 In the same study, lifestyle changes without metformin, including loss of 7% of body weight plus 150 minutes of exercise weekly was associated with a 35% reduction in the risk of developing T2DM.19 Metformin is one of the least expensive prescription medications and is cost-effective for the prevention of T2DM.18
Low-dose aspirin treatment for the prevention of CVD in women who have not had a cardiovascular event must balance a modest reduction in cardiovascular events with a small increased risk of bleeding events. The US Preventive Services Task Force (USPSTF) recommends low-dose aspirin for a limited group of women, those aged 50 to 59 years of age with a 10-year risk of a cardiovascular event >10% who are willing to take aspirin for 10 years. The USPSTF concluded that there is insufficient evidence to recommend low-dose aspirin prevention of CVD in women aged <50 years.20
Continue to: Beyond the fourth trimester...
Beyond the fourth trimester
The fourth trimester is the 12-week period following birth. At the comprehensive postpartum visit, the American College of Obstetricians and Gynecologists (ACOG) recommends that women with APOs be counseled about their increased lifetime risk of maternal cardiometabolic disease.21 In addition, ACOG recommends that at this visit the clinician who will assume primary responsibility for the woman’s ongoing medical care in her primary medical home be clarified. One option is to ensure a high-quality hand-off to an internal medicine or family medicine clinician. Another option is for a clinician in the ObGyn’s office practice, including a physician assistant, nurse practitioner, or office-based ObGyn, to assume some role in the primary care of the woman.
An APO is not only a pregnancy problem
An APO reverberates across a woman’s lifetime, increasing the risk of CVD and diabetes. In the United States the mean age at first birth is 27 years.1 The mean life expectancy of US women is 81 years.22 Following a birth complicated by an APO there are 5 decades of opportunity to improve health through lifestyle changes and medication treatment of obesity, hypertension, dyslipidemia, and hyperglycemia, thereby reducing the risk of CVD.

- Martin JA, Hamilton BE, Osterman MJ, et al. Births: final data for 2019. Natl Vital Stat Rep. 2021;70:1-51.
- Deputy NP, Kim SY, Conrey EJ, et al. Prevalence and changes in preexisting diabetes and gestational diabetes among women who had a live birth—United States, 2012-2016. MMWR Morb Mortal Wkly Rep. 2018;67:1201-1207. doi: 10.15585/mmwr.mm6743a2.
- Fingar KR, Mabry-Hernandez I, Ngo-Metzger Q, et al. Delivery hospitalizations involving preeclampsia and eclampsia, 2005–2014. Statistical brief #222. In: Healthcare Cost and Utilization Project (HCUP) Statistical Briefs [Internet]. Agency for Healthcare Research and Quality: Rockville, MD; April 2017.
- Magnus MC, Wilcox AJ, Morken NH, et al. Role of maternal age and pregnancy history in risk of miscarriage: prospective register-based study. BMJ. 2019;364:869.
- Parikh NI, Gonzalez JM, Anderson CAM, et al. Adverse pregnancy outcomes and cardiovascular disease risk: unique opportunities for cardiovascular disease prevention in women. Circulation. 2021;143:e902-e916. doi: 10.1161 /CIR.0000000000000961.
- Brown MC, Best KE, Pearce MS, et al. Cardiovascular disease risk in women with pre-eclampsia: systematic review and meta-analysis. Eur J Epidemiol. 2013;28:1-19. doi: 10.1007/s10654-013- 9762-6.
- Groenfol TK, Zoet GA, Franx A, et al; on behalf of the PREVENT Group. Trajectory of cardiovascular risk factors after hypertensive disorders of pregnancy. Hypertension. 2019;73:171-178. doi: 10.1161/HYPERTENSIONAHA.118.11726.
- Vounzoulaki E, Khunti K, Abner SC, et al. Progression to type 2 diabetes in women with a known history of gestational diabetes: systematic review and meta-analysis. BMJ. 2020;369:m1361. doi: 10.1136/bmj.m1361.
- Tarrant M, Chooniedass R, Fan HSL, et al. Breastfeeding and postpartum glucose regulation among women with prior gestational diabetes: a systematic review. J Hum Lact. 2020;36:723-738. doi: 10.1177/0890334420950259.
- Park S, Choi NK. Breastfeeding and maternal hypertension. Am J Hypertens. 2018;31:615-621. doi: 10.1093/ajh/hpx219.
- Nguyen B, Gale J, Nassar N, et al. Breastfeeding and cardiovascular disease hospitalization and mortality in parous women: evidence from a large Australian cohort study. J Am Heart Assoc. 2019;8:e011056. doi: 10.1161/JAHA.118.011056.
- Eight things you can do to prevent heart disease and stroke. American Heart Association website. https://www.heart.org/en/healthy-living /healthy-lifestyle/prevent-heart-disease-andstroke. Last Reviewed March 14, 2019. Accessed May 19, 2021.
- ASCVD risk estimator plus. American College of Cardiology website. https://tools.acc.org /ascvd-risk-estimator-plus/#!/calculate /estimate/. Accessed May 19, 2021.
- Ferdinand KC, Nasser SA. Management of essential hypertension. Cardiol Clin. 2017;35:231-246. doi: 10.1016/j.ccl.2016.12.005.
- Packard CJ. LDL cholesterol: how low to go? Trends Cardiovasc Med. 2018;28:348-354. doi: 10.1016/j.tcm.2017.12.011.
- Simons L. An updated review of lipid-modifying therapy. Med J Aust. 2019;211:87-92. doi: 10.5694 /mja2.50142.
- Chou R, Dana T, Blazina I, et al. Statins for the prevention of cardiovascular disease in adults: evidence report and systematic review for the US Preventive Services Task Force. JAMA. 2016;316:2008. doi: 10.1001/jama.2015.15629.
- Moin T, Schmittdiel JA, Flory JH, et al. Review of metformin use for type 2 diabetes mellitus prevention. Am J Prev Med. 2018;55:565-574. doi: 10.1016/j.amepre.2018.04.038.
- Aorda VR, Christophi CA, Edelstein SL, et al, for the Diabetes Prevention Program Research Group. The effect of lifestyle intervention and metformin on preventing or delaying diabetes among women with and without gestational diabetes: the Diabetes Prevention Program outcomes study 10-year follow-up. J Clin Endocrinol Metab. 2015;100:1646- 1653. doi: 10.1210/jc.2014-3761.
- Bibbins-Domingo K, U.S. Preventive Services Task Force. Aspirin use of the primary prevention of cardiovascular disease and colorectal cancer: U.S. Preventive Services Task Force Recommendation Statement. Ann Int Med. 2016; 164: 836-845. doi: 10.7326/M16-0577.
- American College of Obstetricians and Gynecologists. ACOG Committee Opinion No. 736: optimizing postpartum care. Obstet Gynecol. 2018;131:e140-e150. doi: 10.1097 /AOG.0000000000002633.
- National Center for Health Statistics. Health, United States, 2017: Table 015. Hyattsville, MD; 2021. https://www.cdc.gov/nchs/data /hus/2017/015.pdf. Accessed May 18, 2021.
- Martin JA, Hamilton BE, Osterman MJ, et al. Births: final data for 2019. Natl Vital Stat Rep. 2021;70:1-51.
- Deputy NP, Kim SY, Conrey EJ, et al. Prevalence and changes in preexisting diabetes and gestational diabetes among women who had a live birth—United States, 2012-2016. MMWR Morb Mortal Wkly Rep. 2018;67:1201-1207. doi: 10.15585/mmwr.mm6743a2.
- Fingar KR, Mabry-Hernandez I, Ngo-Metzger Q, et al. Delivery hospitalizations involving preeclampsia and eclampsia, 2005–2014. Statistical brief #222. In: Healthcare Cost and Utilization Project (HCUP) Statistical Briefs [Internet]. Agency for Healthcare Research and Quality: Rockville, MD; April 2017.
- Magnus MC, Wilcox AJ, Morken NH, et al. Role of maternal age and pregnancy history in risk of miscarriage: prospective register-based study. BMJ. 2019;364:869.
- Parikh NI, Gonzalez JM, Anderson CAM, et al. Adverse pregnancy outcomes and cardiovascular disease risk: unique opportunities for cardiovascular disease prevention in women. Circulation. 2021;143:e902-e916. doi: 10.1161 /CIR.0000000000000961.
- Brown MC, Best KE, Pearce MS, et al. Cardiovascular disease risk in women with pre-eclampsia: systematic review and meta-analysis. Eur J Epidemiol. 2013;28:1-19. doi: 10.1007/s10654-013- 9762-6.
- Groenfol TK, Zoet GA, Franx A, et al; on behalf of the PREVENT Group. Trajectory of cardiovascular risk factors after hypertensive disorders of pregnancy. Hypertension. 2019;73:171-178. doi: 10.1161/HYPERTENSIONAHA.118.11726.
- Vounzoulaki E, Khunti K, Abner SC, et al. Progression to type 2 diabetes in women with a known history of gestational diabetes: systematic review and meta-analysis. BMJ. 2020;369:m1361. doi: 10.1136/bmj.m1361.
- Tarrant M, Chooniedass R, Fan HSL, et al. Breastfeeding and postpartum glucose regulation among women with prior gestational diabetes: a systematic review. J Hum Lact. 2020;36:723-738. doi: 10.1177/0890334420950259.
- Park S, Choi NK. Breastfeeding and maternal hypertension. Am J Hypertens. 2018;31:615-621. doi: 10.1093/ajh/hpx219.
- Nguyen B, Gale J, Nassar N, et al. Breastfeeding and cardiovascular disease hospitalization and mortality in parous women: evidence from a large Australian cohort study. J Am Heart Assoc. 2019;8:e011056. doi: 10.1161/JAHA.118.011056.
- Eight things you can do to prevent heart disease and stroke. American Heart Association website. https://www.heart.org/en/healthy-living /healthy-lifestyle/prevent-heart-disease-andstroke. Last Reviewed March 14, 2019. Accessed May 19, 2021.
- ASCVD risk estimator plus. American College of Cardiology website. https://tools.acc.org /ascvd-risk-estimator-plus/#!/calculate /estimate/. Accessed May 19, 2021.
- Ferdinand KC, Nasser SA. Management of essential hypertension. Cardiol Clin. 2017;35:231-246. doi: 10.1016/j.ccl.2016.12.005.
- Packard CJ. LDL cholesterol: how low to go? Trends Cardiovasc Med. 2018;28:348-354. doi: 10.1016/j.tcm.2017.12.011.
- Simons L. An updated review of lipid-modifying therapy. Med J Aust. 2019;211:87-92. doi: 10.5694 /mja2.50142.
- Chou R, Dana T, Blazina I, et al. Statins for the prevention of cardiovascular disease in adults: evidence report and systematic review for the US Preventive Services Task Force. JAMA. 2016;316:2008. doi: 10.1001/jama.2015.15629.
- Moin T, Schmittdiel JA, Flory JH, et al. Review of metformin use for type 2 diabetes mellitus prevention. Am J Prev Med. 2018;55:565-574. doi: 10.1016/j.amepre.2018.04.038.
- Aorda VR, Christophi CA, Edelstein SL, et al, for the Diabetes Prevention Program Research Group. The effect of lifestyle intervention and metformin on preventing or delaying diabetes among women with and without gestational diabetes: the Diabetes Prevention Program outcomes study 10-year follow-up. J Clin Endocrinol Metab. 2015;100:1646- 1653. doi: 10.1210/jc.2014-3761.
- Bibbins-Domingo K, U.S. Preventive Services Task Force. Aspirin use of the primary prevention of cardiovascular disease and colorectal cancer: U.S. Preventive Services Task Force Recommendation Statement. Ann Int Med. 2016; 164: 836-845. doi: 10.7326/M16-0577.
- American College of Obstetricians and Gynecologists. ACOG Committee Opinion No. 736: optimizing postpartum care. Obstet Gynecol. 2018;131:e140-e150. doi: 10.1097 /AOG.0000000000002633.
- National Center for Health Statistics. Health, United States, 2017: Table 015. Hyattsville, MD; 2021. https://www.cdc.gov/nchs/data /hus/2017/015.pdf. Accessed May 18, 2021.
Obstetric anal sphincter injury: Prevention and repair
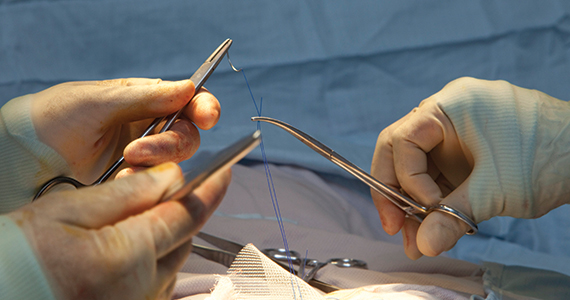
The rate of obstetric anal sphincter injury (OASIS) is approximately 4.4% of vaginal deliveries, with 3.3% 3rd-degree tears and 1.1% 4th-degree tears.1 In the United States in 2019 there were 3,745,540 births—a 31.7% rate of cesarean delivery (CD) and a 68.3% rate of vaginal delivery—resulting in approximately 112,600 births with OASIS.2 A meta-analysis reported that, among 716,031 vaginal births, the risk factors for OASIS included: forceps delivery (relative risk [RR], 3.15), midline episiotomy (RR, 2.88), occiput posterior fetal position (RR, 2.73), vacuum delivery (RR, 2.60), Asian race (RR, 1.87), primiparity (RR, 1.59), mediolateral episiotomy (RR, 1.55), augmentation of labor (RR, 1.46), and epidural anesthesia (RR, 1.21).3 OASIS is associated with an increased risk for developing postpartum perineal pain, anal incontinence, dyspareunia, and wound breakdown.4 Complications following OASIS repair can trigger many follow-up appointments to assess wound healing and provide physical therapy.
This editorial review focuses on evolving recommendations for preventing and repairing OASIS.
The optimal cutting angle for a mediolateral episiotomy is 60 degrees from the midline
For spontaneous vaginal delivery, a policy of restricted episiotomy reduces the risk of OASIS by approximately 30%.5 With an operative vaginal delivery, especially forceps delivery of a large fetus in the occiput posterior position, a mediolateral episiotomy may help to reduce the risk of OASIS, although there are minimal data from clinical trials to support this practice. In one clinical trial, 407 women were randomly assigned to either a mediolateral or midline episiotomy.6 Approximately 25% of the births in both groups were operative deliveries. The mediolateral episiotomy began in the posterior midline of the vaginal introitus and was carried to the right side of the anal sphincter for 3 cm to 4 cm. The midline episiotomy began in the posterior midline of the vagina and was carried 2 cm to 3 cm into the midline perineal tissue. In the women having a midline or mediolateral episiotomy, a 4th-degree tear occurred in 5.5% and 0.4% of births, respectively. For the midline or mediolateral episiotomy, a third-degree tear occurred in 18.4% and 8.6%, respectively. In a prospective cohort study of 1,302 women with an episiotomy and vaginal birth, the rate of OASIS associated with midline or mediolateral episiotomy was 14.8% and 7%, respectively (P<.05).7 In this study, the operative vaginal delivery rate was 11.6% and 15.2% for the women in the midline and mediolateral groups, respectively.
The angle of the mediolateral episiotomy may influence the rate of OASIS and persistent postpartum perineal pain. In one study, 330 nulliparous women who were assessed to need a mediolateral episiotomy at delivery were randomized to an incision with a 40- or 60-degree angle from the midline.8 Prior to incision, a line was drawn on the skin to mark the course of the incision and then infiltrated with 10 mL of lignocaine. The fetal head was delivered with a Ritgen maneuver. The length of the episiotomy averaged 4 cm in both groups. After delivery, the angle of the episiotomy incision was reassessed. The episiotomy incision cut 60 degrees from the midline was measured on average to be 44 degrees from the midline after delivery of the newborn. Similarly, the incision cut at a 40-degree angle was measured to be 24 degrees from the midline after delivery. The rates of OASIS in the women who had a 40- and 60-degree angle incision were 5.5% and 2.4%, respectively (P = .16).
Continue to: Use a prophylactic antibiotic with extended coverage for anaerobes prior to or during your anal sphincter repair...
Use a prophylactic antibiotic with extended coverage for anaerobes prior to or during your anal sphincter repair
Many experts recommend one dose of a prophylactic antibiotic prior to, or during, OASIS repair in order to reduce the risk of wound complications. In a trial 147 women with OASIS were randomly assigned to receive one dose of a second-generation cephalosporin (cefotetan or cefoxitin) with extended anaerobic coverage or a placebo just before repair of the laceration.9 At 2 weeks postpartum, perineal wound complications were significantly lower in women receiving one dose of prophylactic antibiotic with extended anaerobe coverage compared with placebo—8.2% and 24.1%, respectively (P = .037). Additionally, at 2 weeks postpartum, purulent wound discharge was significantly lower in women receiving antibiotic versus placebo, 4% and 17%, respectively (P = .036). Experts writing for the Society of Obstetricians and Gynaecologists of Canada also recommend one dose of cefotetan or cefoxitin.10 Extended anaerobic coverage also can be achieved by administering a single dose of BOTH cefazolin 2 g by intravenous (IV) infusion PLUS metronidazole 500 mg by IV infusion or oral medication.11 For women with severe penicillin allergy, a recommended regimen is gentamicin 5 mg/kg plus clindamycin 900 mg by IV infusion.11 There is evidence that for colorectal or hysterectomy surgery, expanding prophylactic antibiotic coverage of anaerobes with cefazolin PLUS metronidazole significantly reduces postoperative surgical site infection.12,13 Following an OASIS repair, wound breakdown is a catastrophic problem that may take many months to resolve. Administration of a prophylactic antibiotic with extended coverage of anaerobes may help to prevent wound breakdown.
Prioritize identifying and separately repairing the internal anal sphincter
The internal anal sphincter is a smooth muscle that runs along the outside of the rectal wall and thickens into a sphincter toward the anal canal. The internal anal sphincter is thin and grey-white in appearance, like a veil. By contrast, the external anal sphincter is a thick band of red striated muscle tissue. In one study of 3,333 primiparous women with OASIS, an internal anal sphincter injury was detected in 33% of cases.14 In this large cohort, the rate of internal anal sphincter injury with a 3A tear, a 3B tear, a complete tear of the external sphincter and a 4th-degree perineal tear was 22%, 23%, 42%, and 71%, respectively. The internal anal sphincter is important for maintaining rectal continence and is estimated to contribute 50% to 85% of resting anal tone.15 If injury to the internal anal sphincter is detected at a birth with an OASIS, it is important to separately repair the internal anal sphincter to reduce the risk of postpartum rectal incontinence.16
Polyglactin 910 vs Polydioxanone (PDS) Suture—Is PDS the winner?
Polyglactin 910 (Vicryl) is a braided suture that is absorbed within 56 to 70 days. Polydioxanone suture is a long-lasting monofilament suture that is absorbed within 200 days. Many colorectal surgeons and urogynecologists prefer PDS suture for the repair of both the internal and external anal sphincters.16 Authors of one randomized trial of OASIS repair with Vicryl or PDS suture did not report significant differences in most clinical outcomes.17 However, in this study, anal endosonographic imaging of the internal and external anal sphincter demonstrated more internal sphincter defects but not external sphincter defects when the repair was performed with Vicryl rather than PDS. The investigators concluded that comprehensive training of the surgeon, not choice of suture, is probably the most important factor in achieving a good OASIS repair. However, because many subspecialists favor PDS suture for sphincter repair, specialists in obstetrics and gynecology should consider this option.
Continue to: Can your patient access early secondary repair if they develop a perineal laceration wound breakdown?
Can your patient access early secondary repair if they develop a perineal laceration wound breakdown?
The breakdown of an OASIS repair is an obstetric catastrophe with complications that can last many months and sometimes stretch into years. The best approach to a perineal laceration wound breakdown remains controversial. It is optimal if all patients with a wound breakdown can be offered an early secondary repair or healing by secondary intention, permitting the patient to select the best approach for their specific situation.
As noted by the pioneers of early repair of episiotomy dehiscence, Drs. Hankins, Haugh, Gilstrap, Ramin, and others,18-20 conventional doctrine is that an episiotomy repair dehiscence should be managed expectantly, allowing healing by secondary intention and delaying repair of the sphincters for a minimum of 3 to 4 months.21 However, many small case-series report that early secondary repair of a perineal laceration wound breakdown is possible following multiple days of wound preparation prior to the repair, good surgical technique and diligent postoperative follow-up care. One large case series reported on 72 women with complete perineal wound dehiscence who had early secondary repair.22 The median time to complete wound healing following early repair was 28 days. About 36% of the patients had one or more complications, including skin dehiscence, granuloma formation, perineal pain, and sinus formation. A pilot randomized trial reported that, compared with expectant management of a wound breakdown, early repair resulted in a shorter time to wound healing.23
Early repair of perineal wound dehiscence often involves a course of care that extends over multiple weeks. As an example, following a vaginal birth with OASIS and immediate repair, the patient is often discharged from the hospital to home on postpartum day 3. The wound breakdown often is detected between postpartum days 6 to 10. If early secondary repair is selected as the best treatment, 1 to 6 days of daily debridement of the wound is needed to prepare the wound for early secondary repair. The daily debridement required to prepare the wound for early repair is often performed in the hospital, potentially disrupting early mother-newborn bonding. Following the repair, the patient is observed in the hospital for 1 to 3 days and then discharged home with daily wound care and multiple follow-up visits to monitor wound healing. Pelvic floor physical therapy may be initiated when the wound is healed. The prolonged process required for early secondary repair may be best undertaken by a subspecialty practice.24
The surgical repair and postpartum care of OASIS continues to evolve. In your practice you should consider:
- performing a mediolateral episiotomy at a 60-degree angle to reduce the risk of OASIS in situations where there is a high risk of anal sphincter injury, such as in forceps delivery
- using one dose of a prophylactic antibiotic with extended anaerobic coverage, such as cefotetan or cefoxitin
- focus on identifying and separately repairing an internal anal sphincter injury
- using a long-lasting absorbable suture, such as PDS, to repair the internal and external anal sphincters
- ensuring that the patient with a dehiscence following an episiotomy or anal sphincter injury has access to early secondary repair. Standardizing your approach to the prevention and repair of anal sphincter injury will benefit the approximately 112,600 US women who experience OASIS each year. ●
A Cochrane Database Systematic Review reported that moderate-quality evidence showed a decrease in OASIS with the use of intrapartum warm compresses to the perineum and perineal massage.1 Compared with control, intrapartum warm compresses to the perineum did not result in a reduction in first- or second-degree tears, suturing of perineal tears, or use of episiotomy. However, compared with control, intrapartum warm compresses to the perineum was associated with a reduction in OASIS (relative risk [RR], 0.46; 95% confidence interval [CI], 0.27–0.79; 1,799 women; 4 studies; moderate quality evidence; substantial heterogeneity among studies). In addition to a possible reduction in OASIS, warm compresses also may provide the laboring woman, especially those having a natural childbirth, a positive sensory experience and reinforce her perception of the thoughtfulness and caring of her clinicians.
Compared with control, perineal massage was associated with an increase in the rate of an intact perineum (RR, 1.74; 95% CI, 1.11–2.73; 6 studies; 2,618 women; low-quality evidence; substantial heterogeneity among studies) and a decrease in OASIS (RR, 0.49; 95% CI, 0.25–0.94; 5 studies; 2,477 women; moderate quality evidence). Compared with control, perineal massage did not significantly reduce first- or second-degree tears, perineal tears requiring suturing, or the use of episiotomy (very low-quality evidence). Although perineal massage may have benefit, excessive perineal massage likely can contribute to tissue edema and epithelial trauma.
Reference
1. Aasheim V, Nilsen ABC, Reinar LM, et al. Perineal techniques during the second stage of labour for reducing perineal trauma. Cochrane Database Syst Rev. 2017;CD006672.
- Friedman AM, Ananth CV, Prendergast E, et al. Evaluation of third-degree and fourth-degree laceration rates as quality indicators. Obstet Gynecol. 2015;125:927-937.
- Hamilton BE, Martin JA, Osterman MK. Births: Provisional data for 2019. Vital Statistics Rapid Release; No. 8. Hyattsville MD: National Center for Health Statistics; May 2020. https://www.cdc.gov/nchs/data/vsrr/vsrr-8-508.pdf
- Pergialitotis V, Bellos I, Fanaki M, et al. Risk factors for severe perineal trauma during childbirth: an updated meta-analysis. European J Obstet Gynecol Repro Biol. 2020;247:94-100.
- Sultan AH, Kettle C. Diagnosis of perineal trauma. In: Sultan AH, Thakar R, Fenner DE, eds. Perineal and anal sphincter trauma. 1st ed. London, England: Springer-Verlag; 2009:33-51.
- Jiang H, Qian X, Carroli G, et al. Selective versus routine use of episiotomy for vaginal birth. Cochrane Database Syst Rev. 2017;CD000081.
- Coats PM, Chan KK, Wilkins M, et al. A comparison between midline and mediolateral episiotomies. Br J Obstet Gynaecol. 1980;87:408-412.
- Sooklim R, Thinkhamrop J, Lumbiganon P, et al. The outcomes of midline versus medio-lateral episiotomy. Reprod Health. 2007;4:10.
- El-Din AS, Kamal MM, Amin MA. Comparison between two incision angles of mediolateral episiotomy in primiparous women: a randomized controlled trial. J Obstet Gynaecol Res. 2014;40:1877-1882.
- Duggal N, Mercado C, Daniels K, et al. Antibiotic prophylaxis for prevention of postpartum perineal wound complications: a randomized controlled trial. Obstet Gynecol. 2008;111:1268-1273.
- Harvey MA, Pierce M. Obstetrical anal sphincter injuries (OASIS): prevention, recognition and repair. J Obstet Gynecol Can. 2015;37:1131-1148.
- Cox CK, Bugosh MD, Fenner DE, et al. Antibiotic use during repair of obstetrical anal sphincter injury: a qualitative improvement initiative. Int J Gynaecol Obstet. 2021; Epub January 28.
- Deierhoi RJ, Dawes LG, Vick C, et al. Choice of intravenous antibiotic prophylaxis for colorectal surgery does matter. J Am Coll Surg. 2013;217:763-769.
- Till Sr, Morgan DM, Bazzi AA, et al. Reducing surgical site infections after hysterectomy: metronidazole plus cefazolin compared with cephalosporin alone. Am J Obstet Gynecol. 2017;217:187.e1-e11.
- Pihl S, Blomberg M, Uustal E. Internal anal sphincter injury in the immediate postpartum period: prevalence, risk factors and diagnostic methods in the Swedish perineal laceration registry. European J Obst Gynecol Repro Biol. 2020;245:1-6.
- Fornell EU, Matthiesen L, Sjodahl R, et al. Obstetric anal sphincter injury ten years after: subjective and objective long-term effects. BJOG. 2005;112:312-316.
- Sultan AH, Monga AK, Kumar D, et al. Primary repair of obstetric anal sphincter rupture using the overlap technique. Br J Obstet Gynaecol. 1999;106:318-323.
- Williams A, Adams EJ, Tincello DG, et al. How to repair an anal sphincter injury after vaginal delivery: results of a randomised controlled trial. BJOG. 2006;113:201-207.
- Hauth JC, Gilstrap LC, Ward SC, et al. Early repair of an external sphincter ani muscle and rectal mucosal dehiscence. Obstet Gynecol. 1986;67:806-809.
- Hankins GD, Hauth JC, Gilstrap LC, et al. Early repair of episiotomy dehiscence. Obstet Gynecol. 1990;75:48-51.
- Ramin SR, Ramus RM, Little BB, et al. Early repair of episiotomy dehiscence associated with infection. Am J Obstet Gynecol. 1992;167:1104-1107.
- Pritchard JA, MacDonald PC, Gant NF. Williams Obstetrics, 17th ed. Norwalk Connecticut: Appleton-Century-Crofts; 1985:349-350.
- Okeahialam NA, Thakar R, Kleprlikova H, et al. Early re-suturing of dehisced obstetric perineal woulds: a 13-year experience. Eur J Obstet Gynecol Repro Biol. 2020;254:69-73.
- Dudley L, Kettle C, Thomas PW, et al. Perineal resuturing versus expectant management following vaginal delivery complicated by a dehisced wound (PREVIEW): a pilot and feasibility randomised controlled trial. BMJ Open. 2017;7:e012766.
- Lewicky-Gaupp C, Leader-Cramer A, Johnson LL, et al. Wound complications after obstetrical anal sphincter injuries. Obstet Gynecol. 2015;125:1088-1093.
The rate of obstetric anal sphincter injury (OASIS) is approximately 4.4% of vaginal deliveries, with 3.3% 3rd-degree tears and 1.1% 4th-degree tears.1 In the United States in 2019 there were 3,745,540 births—a 31.7% rate of cesarean delivery (CD) and a 68.3% rate of vaginal delivery—resulting in approximately 112,600 births with OASIS.2 A meta-analysis reported that, among 716,031 vaginal births, the risk factors for OASIS included: forceps delivery (relative risk [RR], 3.15), midline episiotomy (RR, 2.88), occiput posterior fetal position (RR, 2.73), vacuum delivery (RR, 2.60), Asian race (RR, 1.87), primiparity (RR, 1.59), mediolateral episiotomy (RR, 1.55), augmentation of labor (RR, 1.46), and epidural anesthesia (RR, 1.21).3 OASIS is associated with an increased risk for developing postpartum perineal pain, anal incontinence, dyspareunia, and wound breakdown.4 Complications following OASIS repair can trigger many follow-up appointments to assess wound healing and provide physical therapy.
This editorial review focuses on evolving recommendations for preventing and repairing OASIS.
The optimal cutting angle for a mediolateral episiotomy is 60 degrees from the midline
For spontaneous vaginal delivery, a policy of restricted episiotomy reduces the risk of OASIS by approximately 30%.5 With an operative vaginal delivery, especially forceps delivery of a large fetus in the occiput posterior position, a mediolateral episiotomy may help to reduce the risk of OASIS, although there are minimal data from clinical trials to support this practice. In one clinical trial, 407 women were randomly assigned to either a mediolateral or midline episiotomy.6 Approximately 25% of the births in both groups were operative deliveries. The mediolateral episiotomy began in the posterior midline of the vaginal introitus and was carried to the right side of the anal sphincter for 3 cm to 4 cm. The midline episiotomy began in the posterior midline of the vagina and was carried 2 cm to 3 cm into the midline perineal tissue. In the women having a midline or mediolateral episiotomy, a 4th-degree tear occurred in 5.5% and 0.4% of births, respectively. For the midline or mediolateral episiotomy, a third-degree tear occurred in 18.4% and 8.6%, respectively. In a prospective cohort study of 1,302 women with an episiotomy and vaginal birth, the rate of OASIS associated with midline or mediolateral episiotomy was 14.8% and 7%, respectively (P<.05).7 In this study, the operative vaginal delivery rate was 11.6% and 15.2% for the women in the midline and mediolateral groups, respectively.
The angle of the mediolateral episiotomy may influence the rate of OASIS and persistent postpartum perineal pain. In one study, 330 nulliparous women who were assessed to need a mediolateral episiotomy at delivery were randomized to an incision with a 40- or 60-degree angle from the midline.8 Prior to incision, a line was drawn on the skin to mark the course of the incision and then infiltrated with 10 mL of lignocaine. The fetal head was delivered with a Ritgen maneuver. The length of the episiotomy averaged 4 cm in both groups. After delivery, the angle of the episiotomy incision was reassessed. The episiotomy incision cut 60 degrees from the midline was measured on average to be 44 degrees from the midline after delivery of the newborn. Similarly, the incision cut at a 40-degree angle was measured to be 24 degrees from the midline after delivery. The rates of OASIS in the women who had a 40- and 60-degree angle incision were 5.5% and 2.4%, respectively (P = .16).
Continue to: Use a prophylactic antibiotic with extended coverage for anaerobes prior to or during your anal sphincter repair...
Use a prophylactic antibiotic with extended coverage for anaerobes prior to or during your anal sphincter repair
Many experts recommend one dose of a prophylactic antibiotic prior to, or during, OASIS repair in order to reduce the risk of wound complications. In a trial 147 women with OASIS were randomly assigned to receive one dose of a second-generation cephalosporin (cefotetan or cefoxitin) with extended anaerobic coverage or a placebo just before repair of the laceration.9 At 2 weeks postpartum, perineal wound complications were significantly lower in women receiving one dose of prophylactic antibiotic with extended anaerobe coverage compared with placebo—8.2% and 24.1%, respectively (P = .037). Additionally, at 2 weeks postpartum, purulent wound discharge was significantly lower in women receiving antibiotic versus placebo, 4% and 17%, respectively (P = .036). Experts writing for the Society of Obstetricians and Gynaecologists of Canada also recommend one dose of cefotetan or cefoxitin.10 Extended anaerobic coverage also can be achieved by administering a single dose of BOTH cefazolin 2 g by intravenous (IV) infusion PLUS metronidazole 500 mg by IV infusion or oral medication.11 For women with severe penicillin allergy, a recommended regimen is gentamicin 5 mg/kg plus clindamycin 900 mg by IV infusion.11 There is evidence that for colorectal or hysterectomy surgery, expanding prophylactic antibiotic coverage of anaerobes with cefazolin PLUS metronidazole significantly reduces postoperative surgical site infection.12,13 Following an OASIS repair, wound breakdown is a catastrophic problem that may take many months to resolve. Administration of a prophylactic antibiotic with extended coverage of anaerobes may help to prevent wound breakdown.
Prioritize identifying and separately repairing the internal anal sphincter
The internal anal sphincter is a smooth muscle that runs along the outside of the rectal wall and thickens into a sphincter toward the anal canal. The internal anal sphincter is thin and grey-white in appearance, like a veil. By contrast, the external anal sphincter is a thick band of red striated muscle tissue. In one study of 3,333 primiparous women with OASIS, an internal anal sphincter injury was detected in 33% of cases.14 In this large cohort, the rate of internal anal sphincter injury with a 3A tear, a 3B tear, a complete tear of the external sphincter and a 4th-degree perineal tear was 22%, 23%, 42%, and 71%, respectively. The internal anal sphincter is important for maintaining rectal continence and is estimated to contribute 50% to 85% of resting anal tone.15 If injury to the internal anal sphincter is detected at a birth with an OASIS, it is important to separately repair the internal anal sphincter to reduce the risk of postpartum rectal incontinence.16
Polyglactin 910 vs Polydioxanone (PDS) Suture—Is PDS the winner?
Polyglactin 910 (Vicryl) is a braided suture that is absorbed within 56 to 70 days. Polydioxanone suture is a long-lasting monofilament suture that is absorbed within 200 days. Many colorectal surgeons and urogynecologists prefer PDS suture for the repair of both the internal and external anal sphincters.16 Authors of one randomized trial of OASIS repair with Vicryl or PDS suture did not report significant differences in most clinical outcomes.17 However, in this study, anal endosonographic imaging of the internal and external anal sphincter demonstrated more internal sphincter defects but not external sphincter defects when the repair was performed with Vicryl rather than PDS. The investigators concluded that comprehensive training of the surgeon, not choice of suture, is probably the most important factor in achieving a good OASIS repair. However, because many subspecialists favor PDS suture for sphincter repair, specialists in obstetrics and gynecology should consider this option.
Continue to: Can your patient access early secondary repair if they develop a perineal laceration wound breakdown?
Can your patient access early secondary repair if they develop a perineal laceration wound breakdown?
The breakdown of an OASIS repair is an obstetric catastrophe with complications that can last many months and sometimes stretch into years. The best approach to a perineal laceration wound breakdown remains controversial. It is optimal if all patients with a wound breakdown can be offered an early secondary repair or healing by secondary intention, permitting the patient to select the best approach for their specific situation.
As noted by the pioneers of early repair of episiotomy dehiscence, Drs. Hankins, Haugh, Gilstrap, Ramin, and others,18-20 conventional doctrine is that an episiotomy repair dehiscence should be managed expectantly, allowing healing by secondary intention and delaying repair of the sphincters for a minimum of 3 to 4 months.21 However, many small case-series report that early secondary repair of a perineal laceration wound breakdown is possible following multiple days of wound preparation prior to the repair, good surgical technique and diligent postoperative follow-up care. One large case series reported on 72 women with complete perineal wound dehiscence who had early secondary repair.22 The median time to complete wound healing following early repair was 28 days. About 36% of the patients had one or more complications, including skin dehiscence, granuloma formation, perineal pain, and sinus formation. A pilot randomized trial reported that, compared with expectant management of a wound breakdown, early repair resulted in a shorter time to wound healing.23
Early repair of perineal wound dehiscence often involves a course of care that extends over multiple weeks. As an example, following a vaginal birth with OASIS and immediate repair, the patient is often discharged from the hospital to home on postpartum day 3. The wound breakdown often is detected between postpartum days 6 to 10. If early secondary repair is selected as the best treatment, 1 to 6 days of daily debridement of the wound is needed to prepare the wound for early secondary repair. The daily debridement required to prepare the wound for early repair is often performed in the hospital, potentially disrupting early mother-newborn bonding. Following the repair, the patient is observed in the hospital for 1 to 3 days and then discharged home with daily wound care and multiple follow-up visits to monitor wound healing. Pelvic floor physical therapy may be initiated when the wound is healed. The prolonged process required for early secondary repair may be best undertaken by a subspecialty practice.24
The surgical repair and postpartum care of OASIS continues to evolve. In your practice you should consider:
- performing a mediolateral episiotomy at a 60-degree angle to reduce the risk of OASIS in situations where there is a high risk of anal sphincter injury, such as in forceps delivery
- using one dose of a prophylactic antibiotic with extended anaerobic coverage, such as cefotetan or cefoxitin
- focus on identifying and separately repairing an internal anal sphincter injury
- using a long-lasting absorbable suture, such as PDS, to repair the internal and external anal sphincters
- ensuring that the patient with a dehiscence following an episiotomy or anal sphincter injury has access to early secondary repair. Standardizing your approach to the prevention and repair of anal sphincter injury will benefit the approximately 112,600 US women who experience OASIS each year. ●
A Cochrane Database Systematic Review reported that moderate-quality evidence showed a decrease in OASIS with the use of intrapartum warm compresses to the perineum and perineal massage.1 Compared with control, intrapartum warm compresses to the perineum did not result in a reduction in first- or second-degree tears, suturing of perineal tears, or use of episiotomy. However, compared with control, intrapartum warm compresses to the perineum was associated with a reduction in OASIS (relative risk [RR], 0.46; 95% confidence interval [CI], 0.27–0.79; 1,799 women; 4 studies; moderate quality evidence; substantial heterogeneity among studies). In addition to a possible reduction in OASIS, warm compresses also may provide the laboring woman, especially those having a natural childbirth, a positive sensory experience and reinforce her perception of the thoughtfulness and caring of her clinicians.
Compared with control, perineal massage was associated with an increase in the rate of an intact perineum (RR, 1.74; 95% CI, 1.11–2.73; 6 studies; 2,618 women; low-quality evidence; substantial heterogeneity among studies) and a decrease in OASIS (RR, 0.49; 95% CI, 0.25–0.94; 5 studies; 2,477 women; moderate quality evidence). Compared with control, perineal massage did not significantly reduce first- or second-degree tears, perineal tears requiring suturing, or the use of episiotomy (very low-quality evidence). Although perineal massage may have benefit, excessive perineal massage likely can contribute to tissue edema and epithelial trauma.
Reference
1. Aasheim V, Nilsen ABC, Reinar LM, et al. Perineal techniques during the second stage of labour for reducing perineal trauma. Cochrane Database Syst Rev. 2017;CD006672.
The rate of obstetric anal sphincter injury (OASIS) is approximately 4.4% of vaginal deliveries, with 3.3% 3rd-degree tears and 1.1% 4th-degree tears.1 In the United States in 2019 there were 3,745,540 births—a 31.7% rate of cesarean delivery (CD) and a 68.3% rate of vaginal delivery—resulting in approximately 112,600 births with OASIS.2 A meta-analysis reported that, among 716,031 vaginal births, the risk factors for OASIS included: forceps delivery (relative risk [RR], 3.15), midline episiotomy (RR, 2.88), occiput posterior fetal position (RR, 2.73), vacuum delivery (RR, 2.60), Asian race (RR, 1.87), primiparity (RR, 1.59), mediolateral episiotomy (RR, 1.55), augmentation of labor (RR, 1.46), and epidural anesthesia (RR, 1.21).3 OASIS is associated with an increased risk for developing postpartum perineal pain, anal incontinence, dyspareunia, and wound breakdown.4 Complications following OASIS repair can trigger many follow-up appointments to assess wound healing and provide physical therapy.
This editorial review focuses on evolving recommendations for preventing and repairing OASIS.
The optimal cutting angle for a mediolateral episiotomy is 60 degrees from the midline
For spontaneous vaginal delivery, a policy of restricted episiotomy reduces the risk of OASIS by approximately 30%.5 With an operative vaginal delivery, especially forceps delivery of a large fetus in the occiput posterior position, a mediolateral episiotomy may help to reduce the risk of OASIS, although there are minimal data from clinical trials to support this practice. In one clinical trial, 407 women were randomly assigned to either a mediolateral or midline episiotomy.6 Approximately 25% of the births in both groups were operative deliveries. The mediolateral episiotomy began in the posterior midline of the vaginal introitus and was carried to the right side of the anal sphincter for 3 cm to 4 cm. The midline episiotomy began in the posterior midline of the vagina and was carried 2 cm to 3 cm into the midline perineal tissue. In the women having a midline or mediolateral episiotomy, a 4th-degree tear occurred in 5.5% and 0.4% of births, respectively. For the midline or mediolateral episiotomy, a third-degree tear occurred in 18.4% and 8.6%, respectively. In a prospective cohort study of 1,302 women with an episiotomy and vaginal birth, the rate of OASIS associated with midline or mediolateral episiotomy was 14.8% and 7%, respectively (P<.05).7 In this study, the operative vaginal delivery rate was 11.6% and 15.2% for the women in the midline and mediolateral groups, respectively.
The angle of the mediolateral episiotomy may influence the rate of OASIS and persistent postpartum perineal pain. In one study, 330 nulliparous women who were assessed to need a mediolateral episiotomy at delivery were randomized to an incision with a 40- or 60-degree angle from the midline.8 Prior to incision, a line was drawn on the skin to mark the course of the incision and then infiltrated with 10 mL of lignocaine. The fetal head was delivered with a Ritgen maneuver. The length of the episiotomy averaged 4 cm in both groups. After delivery, the angle of the episiotomy incision was reassessed. The episiotomy incision cut 60 degrees from the midline was measured on average to be 44 degrees from the midline after delivery of the newborn. Similarly, the incision cut at a 40-degree angle was measured to be 24 degrees from the midline after delivery. The rates of OASIS in the women who had a 40- and 60-degree angle incision were 5.5% and 2.4%, respectively (P = .16).
Continue to: Use a prophylactic antibiotic with extended coverage for anaerobes prior to or during your anal sphincter repair...
Use a prophylactic antibiotic with extended coverage for anaerobes prior to or during your anal sphincter repair
Many experts recommend one dose of a prophylactic antibiotic prior to, or during, OASIS repair in order to reduce the risk of wound complications. In a trial 147 women with OASIS were randomly assigned to receive one dose of a second-generation cephalosporin (cefotetan or cefoxitin) with extended anaerobic coverage or a placebo just before repair of the laceration.9 At 2 weeks postpartum, perineal wound complications were significantly lower in women receiving one dose of prophylactic antibiotic with extended anaerobe coverage compared with placebo—8.2% and 24.1%, respectively (P = .037). Additionally, at 2 weeks postpartum, purulent wound discharge was significantly lower in women receiving antibiotic versus placebo, 4% and 17%, respectively (P = .036). Experts writing for the Society of Obstetricians and Gynaecologists of Canada also recommend one dose of cefotetan or cefoxitin.10 Extended anaerobic coverage also can be achieved by administering a single dose of BOTH cefazolin 2 g by intravenous (IV) infusion PLUS metronidazole 500 mg by IV infusion or oral medication.11 For women with severe penicillin allergy, a recommended regimen is gentamicin 5 mg/kg plus clindamycin 900 mg by IV infusion.11 There is evidence that for colorectal or hysterectomy surgery, expanding prophylactic antibiotic coverage of anaerobes with cefazolin PLUS metronidazole significantly reduces postoperative surgical site infection.12,13 Following an OASIS repair, wound breakdown is a catastrophic problem that may take many months to resolve. Administration of a prophylactic antibiotic with extended coverage of anaerobes may help to prevent wound breakdown.
Prioritize identifying and separately repairing the internal anal sphincter
The internal anal sphincter is a smooth muscle that runs along the outside of the rectal wall and thickens into a sphincter toward the anal canal. The internal anal sphincter is thin and grey-white in appearance, like a veil. By contrast, the external anal sphincter is a thick band of red striated muscle tissue. In one study of 3,333 primiparous women with OASIS, an internal anal sphincter injury was detected in 33% of cases.14 In this large cohort, the rate of internal anal sphincter injury with a 3A tear, a 3B tear, a complete tear of the external sphincter and a 4th-degree perineal tear was 22%, 23%, 42%, and 71%, respectively. The internal anal sphincter is important for maintaining rectal continence and is estimated to contribute 50% to 85% of resting anal tone.15 If injury to the internal anal sphincter is detected at a birth with an OASIS, it is important to separately repair the internal anal sphincter to reduce the risk of postpartum rectal incontinence.16
Polyglactin 910 vs Polydioxanone (PDS) Suture—Is PDS the winner?
Polyglactin 910 (Vicryl) is a braided suture that is absorbed within 56 to 70 days. Polydioxanone suture is a long-lasting monofilament suture that is absorbed within 200 days. Many colorectal surgeons and urogynecologists prefer PDS suture for the repair of both the internal and external anal sphincters.16 Authors of one randomized trial of OASIS repair with Vicryl or PDS suture did not report significant differences in most clinical outcomes.17 However, in this study, anal endosonographic imaging of the internal and external anal sphincter demonstrated more internal sphincter defects but not external sphincter defects when the repair was performed with Vicryl rather than PDS. The investigators concluded that comprehensive training of the surgeon, not choice of suture, is probably the most important factor in achieving a good OASIS repair. However, because many subspecialists favor PDS suture for sphincter repair, specialists in obstetrics and gynecology should consider this option.
Continue to: Can your patient access early secondary repair if they develop a perineal laceration wound breakdown?
Can your patient access early secondary repair if they develop a perineal laceration wound breakdown?
The breakdown of an OASIS repair is an obstetric catastrophe with complications that can last many months and sometimes stretch into years. The best approach to a perineal laceration wound breakdown remains controversial. It is optimal if all patients with a wound breakdown can be offered an early secondary repair or healing by secondary intention, permitting the patient to select the best approach for their specific situation.
As noted by the pioneers of early repair of episiotomy dehiscence, Drs. Hankins, Haugh, Gilstrap, Ramin, and others,18-20 conventional doctrine is that an episiotomy repair dehiscence should be managed expectantly, allowing healing by secondary intention and delaying repair of the sphincters for a minimum of 3 to 4 months.21 However, many small case-series report that early secondary repair of a perineal laceration wound breakdown is possible following multiple days of wound preparation prior to the repair, good surgical technique and diligent postoperative follow-up care. One large case series reported on 72 women with complete perineal wound dehiscence who had early secondary repair.22 The median time to complete wound healing following early repair was 28 days. About 36% of the patients had one or more complications, including skin dehiscence, granuloma formation, perineal pain, and sinus formation. A pilot randomized trial reported that, compared with expectant management of a wound breakdown, early repair resulted in a shorter time to wound healing.23
Early repair of perineal wound dehiscence often involves a course of care that extends over multiple weeks. As an example, following a vaginal birth with OASIS and immediate repair, the patient is often discharged from the hospital to home on postpartum day 3. The wound breakdown often is detected between postpartum days 6 to 10. If early secondary repair is selected as the best treatment, 1 to 6 days of daily debridement of the wound is needed to prepare the wound for early secondary repair. The daily debridement required to prepare the wound for early repair is often performed in the hospital, potentially disrupting early mother-newborn bonding. Following the repair, the patient is observed in the hospital for 1 to 3 days and then discharged home with daily wound care and multiple follow-up visits to monitor wound healing. Pelvic floor physical therapy may be initiated when the wound is healed. The prolonged process required for early secondary repair may be best undertaken by a subspecialty practice.24
The surgical repair and postpartum care of OASIS continues to evolve. In your practice you should consider:
- performing a mediolateral episiotomy at a 60-degree angle to reduce the risk of OASIS in situations where there is a high risk of anal sphincter injury, such as in forceps delivery
- using one dose of a prophylactic antibiotic with extended anaerobic coverage, such as cefotetan or cefoxitin
- focus on identifying and separately repairing an internal anal sphincter injury
- using a long-lasting absorbable suture, such as PDS, to repair the internal and external anal sphincters
- ensuring that the patient with a dehiscence following an episiotomy or anal sphincter injury has access to early secondary repair. Standardizing your approach to the prevention and repair of anal sphincter injury will benefit the approximately 112,600 US women who experience OASIS each year. ●
A Cochrane Database Systematic Review reported that moderate-quality evidence showed a decrease in OASIS with the use of intrapartum warm compresses to the perineum and perineal massage.1 Compared with control, intrapartum warm compresses to the perineum did not result in a reduction in first- or second-degree tears, suturing of perineal tears, or use of episiotomy. However, compared with control, intrapartum warm compresses to the perineum was associated with a reduction in OASIS (relative risk [RR], 0.46; 95% confidence interval [CI], 0.27–0.79; 1,799 women; 4 studies; moderate quality evidence; substantial heterogeneity among studies). In addition to a possible reduction in OASIS, warm compresses also may provide the laboring woman, especially those having a natural childbirth, a positive sensory experience and reinforce her perception of the thoughtfulness and caring of her clinicians.
Compared with control, perineal massage was associated with an increase in the rate of an intact perineum (RR, 1.74; 95% CI, 1.11–2.73; 6 studies; 2,618 women; low-quality evidence; substantial heterogeneity among studies) and a decrease in OASIS (RR, 0.49; 95% CI, 0.25–0.94; 5 studies; 2,477 women; moderate quality evidence). Compared with control, perineal massage did not significantly reduce first- or second-degree tears, perineal tears requiring suturing, or the use of episiotomy (very low-quality evidence). Although perineal massage may have benefit, excessive perineal massage likely can contribute to tissue edema and epithelial trauma.
Reference
1. Aasheim V, Nilsen ABC, Reinar LM, et al. Perineal techniques during the second stage of labour for reducing perineal trauma. Cochrane Database Syst Rev. 2017;CD006672.
- Friedman AM, Ananth CV, Prendergast E, et al. Evaluation of third-degree and fourth-degree laceration rates as quality indicators. Obstet Gynecol. 2015;125:927-937.
- Hamilton BE, Martin JA, Osterman MK. Births: Provisional data for 2019. Vital Statistics Rapid Release; No. 8. Hyattsville MD: National Center for Health Statistics; May 2020. https://www.cdc.gov/nchs/data/vsrr/vsrr-8-508.pdf
- Pergialitotis V, Bellos I, Fanaki M, et al. Risk factors for severe perineal trauma during childbirth: an updated meta-analysis. European J Obstet Gynecol Repro Biol. 2020;247:94-100.
- Sultan AH, Kettle C. Diagnosis of perineal trauma. In: Sultan AH, Thakar R, Fenner DE, eds. Perineal and anal sphincter trauma. 1st ed. London, England: Springer-Verlag; 2009:33-51.
- Jiang H, Qian X, Carroli G, et al. Selective versus routine use of episiotomy for vaginal birth. Cochrane Database Syst Rev. 2017;CD000081.
- Coats PM, Chan KK, Wilkins M, et al. A comparison between midline and mediolateral episiotomies. Br J Obstet Gynaecol. 1980;87:408-412.
- Sooklim R, Thinkhamrop J, Lumbiganon P, et al. The outcomes of midline versus medio-lateral episiotomy. Reprod Health. 2007;4:10.
- El-Din AS, Kamal MM, Amin MA. Comparison between two incision angles of mediolateral episiotomy in primiparous women: a randomized controlled trial. J Obstet Gynaecol Res. 2014;40:1877-1882.
- Duggal N, Mercado C, Daniels K, et al. Antibiotic prophylaxis for prevention of postpartum perineal wound complications: a randomized controlled trial. Obstet Gynecol. 2008;111:1268-1273.
- Harvey MA, Pierce M. Obstetrical anal sphincter injuries (OASIS): prevention, recognition and repair. J Obstet Gynecol Can. 2015;37:1131-1148.
- Cox CK, Bugosh MD, Fenner DE, et al. Antibiotic use during repair of obstetrical anal sphincter injury: a qualitative improvement initiative. Int J Gynaecol Obstet. 2021; Epub January 28.
- Deierhoi RJ, Dawes LG, Vick C, et al. Choice of intravenous antibiotic prophylaxis for colorectal surgery does matter. J Am Coll Surg. 2013;217:763-769.
- Till Sr, Morgan DM, Bazzi AA, et al. Reducing surgical site infections after hysterectomy: metronidazole plus cefazolin compared with cephalosporin alone. Am J Obstet Gynecol. 2017;217:187.e1-e11.
- Pihl S, Blomberg M, Uustal E. Internal anal sphincter injury in the immediate postpartum period: prevalence, risk factors and diagnostic methods in the Swedish perineal laceration registry. European J Obst Gynecol Repro Biol. 2020;245:1-6.
- Fornell EU, Matthiesen L, Sjodahl R, et al. Obstetric anal sphincter injury ten years after: subjective and objective long-term effects. BJOG. 2005;112:312-316.
- Sultan AH, Monga AK, Kumar D, et al. Primary repair of obstetric anal sphincter rupture using the overlap technique. Br J Obstet Gynaecol. 1999;106:318-323.
- Williams A, Adams EJ, Tincello DG, et al. How to repair an anal sphincter injury after vaginal delivery: results of a randomised controlled trial. BJOG. 2006;113:201-207.
- Hauth JC, Gilstrap LC, Ward SC, et al. Early repair of an external sphincter ani muscle and rectal mucosal dehiscence. Obstet Gynecol. 1986;67:806-809.
- Hankins GD, Hauth JC, Gilstrap LC, et al. Early repair of episiotomy dehiscence. Obstet Gynecol. 1990;75:48-51.
- Ramin SR, Ramus RM, Little BB, et al. Early repair of episiotomy dehiscence associated with infection. Am J Obstet Gynecol. 1992;167:1104-1107.
- Pritchard JA, MacDonald PC, Gant NF. Williams Obstetrics, 17th ed. Norwalk Connecticut: Appleton-Century-Crofts; 1985:349-350.
- Okeahialam NA, Thakar R, Kleprlikova H, et al. Early re-suturing of dehisced obstetric perineal woulds: a 13-year experience. Eur J Obstet Gynecol Repro Biol. 2020;254:69-73.
- Dudley L, Kettle C, Thomas PW, et al. Perineal resuturing versus expectant management following vaginal delivery complicated by a dehisced wound (PREVIEW): a pilot and feasibility randomised controlled trial. BMJ Open. 2017;7:e012766.
- Lewicky-Gaupp C, Leader-Cramer A, Johnson LL, et al. Wound complications after obstetrical anal sphincter injuries. Obstet Gynecol. 2015;125:1088-1093.
- Friedman AM, Ananth CV, Prendergast E, et al. Evaluation of third-degree and fourth-degree laceration rates as quality indicators. Obstet Gynecol. 2015;125:927-937.
- Hamilton BE, Martin JA, Osterman MK. Births: Provisional data for 2019. Vital Statistics Rapid Release; No. 8. Hyattsville MD: National Center for Health Statistics; May 2020. https://www.cdc.gov/nchs/data/vsrr/vsrr-8-508.pdf
- Pergialitotis V, Bellos I, Fanaki M, et al. Risk factors for severe perineal trauma during childbirth: an updated meta-analysis. European J Obstet Gynecol Repro Biol. 2020;247:94-100.
- Sultan AH, Kettle C. Diagnosis of perineal trauma. In: Sultan AH, Thakar R, Fenner DE, eds. Perineal and anal sphincter trauma. 1st ed. London, England: Springer-Verlag; 2009:33-51.
- Jiang H, Qian X, Carroli G, et al. Selective versus routine use of episiotomy for vaginal birth. Cochrane Database Syst Rev. 2017;CD000081.
- Coats PM, Chan KK, Wilkins M, et al. A comparison between midline and mediolateral episiotomies. Br J Obstet Gynaecol. 1980;87:408-412.
- Sooklim R, Thinkhamrop J, Lumbiganon P, et al. The outcomes of midline versus medio-lateral episiotomy. Reprod Health. 2007;4:10.
- El-Din AS, Kamal MM, Amin MA. Comparison between two incision angles of mediolateral episiotomy in primiparous women: a randomized controlled trial. J Obstet Gynaecol Res. 2014;40:1877-1882.
- Duggal N, Mercado C, Daniels K, et al. Antibiotic prophylaxis for prevention of postpartum perineal wound complications: a randomized controlled trial. Obstet Gynecol. 2008;111:1268-1273.
- Harvey MA, Pierce M. Obstetrical anal sphincter injuries (OASIS): prevention, recognition and repair. J Obstet Gynecol Can. 2015;37:1131-1148.
- Cox CK, Bugosh MD, Fenner DE, et al. Antibiotic use during repair of obstetrical anal sphincter injury: a qualitative improvement initiative. Int J Gynaecol Obstet. 2021; Epub January 28.
- Deierhoi RJ, Dawes LG, Vick C, et al. Choice of intravenous antibiotic prophylaxis for colorectal surgery does matter. J Am Coll Surg. 2013;217:763-769.
- Till Sr, Morgan DM, Bazzi AA, et al. Reducing surgical site infections after hysterectomy: metronidazole plus cefazolin compared with cephalosporin alone. Am J Obstet Gynecol. 2017;217:187.e1-e11.
- Pihl S, Blomberg M, Uustal E. Internal anal sphincter injury in the immediate postpartum period: prevalence, risk factors and diagnostic methods in the Swedish perineal laceration registry. European J Obst Gynecol Repro Biol. 2020;245:1-6.
- Fornell EU, Matthiesen L, Sjodahl R, et al. Obstetric anal sphincter injury ten years after: subjective and objective long-term effects. BJOG. 2005;112:312-316.
- Sultan AH, Monga AK, Kumar D, et al. Primary repair of obstetric anal sphincter rupture using the overlap technique. Br J Obstet Gynaecol. 1999;106:318-323.
- Williams A, Adams EJ, Tincello DG, et al. How to repair an anal sphincter injury after vaginal delivery: results of a randomised controlled trial. BJOG. 2006;113:201-207.
- Hauth JC, Gilstrap LC, Ward SC, et al. Early repair of an external sphincter ani muscle and rectal mucosal dehiscence. Obstet Gynecol. 1986;67:806-809.
- Hankins GD, Hauth JC, Gilstrap LC, et al. Early repair of episiotomy dehiscence. Obstet Gynecol. 1990;75:48-51.
- Ramin SR, Ramus RM, Little BB, et al. Early repair of episiotomy dehiscence associated with infection. Am J Obstet Gynecol. 1992;167:1104-1107.
- Pritchard JA, MacDonald PC, Gant NF. Williams Obstetrics, 17th ed. Norwalk Connecticut: Appleton-Century-Crofts; 1985:349-350.
- Okeahialam NA, Thakar R, Kleprlikova H, et al. Early re-suturing of dehisced obstetric perineal woulds: a 13-year experience. Eur J Obstet Gynecol Repro Biol. 2020;254:69-73.
- Dudley L, Kettle C, Thomas PW, et al. Perineal resuturing versus expectant management following vaginal delivery complicated by a dehisced wound (PREVIEW): a pilot and feasibility randomised controlled trial. BMJ Open. 2017;7:e012766.
- Lewicky-Gaupp C, Leader-Cramer A, Johnson LL, et al. Wound complications after obstetrical anal sphincter injuries. Obstet Gynecol. 2015;125:1088-1093.
Optimize your treatment of endometriosis by using an FDA-approved hormonal medication
Women with endometriosis often present for medical care for one or more of the following health issues: pelvic pain, infertility, and/or an adnexal cyst (endometrioma). For women with moderate or severe pelvic pain and laparoscopically diagnosed endometriosis, hormone therapy is often necessary to achieve maximal long-term reduction in pain and optimize health. I focus on opportunities to optimize hormonal treatment of endometriosis in this editorial.
When plan A is not working, move expeditiously to plan B
Cyclic or continuous combination estrogen-progestin contraceptives are commonly prescribed to treat pelvic pain caused by endometriosis. Although endometriosis pain may initially improve with estrogen-progestin contraceptives, many women on this medication will eventually report that they have worsening pelvic pain that adversely impacts their daily activities. Surprisingly, clinicians often continue to prescribe estrogen-progestin contraceptives even after the patient reports that the treatment is not effective, and their pain continues to be bothersome.
Patients benefit when they have access to the full range of hormone treatments that have been approved by the FDA for the treatment of moderate to severe pelvic pain caused by endometriosis (TABLE). In the situation where an estrogen-progestin contraceptive is no longer effective at reducing the pelvic pain, I will often offer the patient the option of norethindrone acetate (NEA) or elagolix treatment. My experience is that stopping the estrogen-progestin contraceptive and starting NEA or elagolix will result in a significant decrease in pain symptoms and improvement in the patient’s quality of life.

Other FDA-approved options to treat pelvic pain caused by endometriosis include depot medroxyprogesterone acetate injectable suspension, depot leuprolide acetate, goserelin implant, and danazol. I do not routinely prescribe depot medroxyprogesterone acetate because some patients report new onset or worsening symptoms of depression on the medication. I prescribe depot-leuprolide acetate less often than in the past, because many patients report moderate to severe hypoestrogenic symptoms on this medication. In women taking depot-leuprolide acetate, moderate to severe vasomotor symptoms can be improved by prescribing NEA pills, but the alternative of norethindrone monotherapy is less expensive. I seldom use goserelin or danazol in my practice. The needle required to place the goserelin implant has a diameter of approximately 1.7 mm (16 gauge) or 2.1 mm (14 gauge), for the 3.6 mg and 10 mg doses, respectively. The large diameter of the needle can cause pain and bruising at the implant site. As a comparison, the progestin subdermal implant needle is approximately 2.1 mm in diameter. Danazol is associated with weight gain, and most women prefer to avoid this side effect.
Continue to: Norethindrone acetate...
Norethindrone acetate
NEA 5 mg daily is approved by the FDA to treat endometriosis.1 NEA was approved at a time when large controlled clinical trials were not routinely required for a medicine to be approved. The data to support NEA treatment of pelvic pain caused by endometriosis is based on cohort studies. In a study of 194 women, median age 21 years with moderate to severe pelvic pain and surgically proven endometriosis, the effect of NEA on pelvic pain was explored.2 The initial dose of NEA was 5 mg daily. If the patient did not achieve a reduction in pelvic pain and amenorrhea on the NEA dose of 5 mg daily, the dose was increased by 2.5 mg every 2 weeks, up to a maximum of 15 mg, until amenorrhea and/or a decrease in pelvic pain was achieved. Ninety-five percent of the women in this cohort had previously been treated with an estrogen-progestin contraceptive or a GnRH antagonist and had discontinued those medications because of inadequate control of pelvic pain or because of side effects of the medication.
In this large cohort, 65% of women reported significant improvement in pelvic pain, with a median pain score of 5 before treatment and 0 following NEA treatment. About 55% of the women reported no side effects. The most commonly reported side effects were weight gain (16%; mean weight gain, 3.1 kg), acne (10%), mood lability (9%), hot flashes (8%), depression (6%), scalp hair loss (4%), headache (4%), nausea (3%), and deepening of the voice (1%). (In this study women could report more than one side effect.)
In another cohort study of 52 women with pelvic pain and surgically confirmed endometriosis, NEA treatment resulted in pain relief in 94% of the women.3 Breakthrough bleeding was a common side effect, reported by 58% of participants. The investigators concluded that NEA treatment was a “cost-effective alternative with relatively mild side effects in the treatment of symptomatic endometriosis.” A conclusion which I endorse.
NEA has been reported to effectively treat ovarian endometriomas and rectovaginal endometriosis.4,5 In a cohort of 18 women who had previously had the surgical resection of an ovarian endometriosis cyst and had postoperative recurrence of pelvic pain and ovarian endometriosis, treatment was initiated with an escalating NEA regimen.4 Treatment was initiated with NEA 5 mg daily, with the dosage increased every 2 weeks by 2.5 mg until amenorrhea was established. Most women achieved amenorrhea with NEA 5 mg daily, and 89% had reduced pelvic pain. The investigators reported complete regression of the endometriosis cyst(s) in 74% of the women. In my experience, NEA does not result in complete regression of endometriosis cysts, but it does cause a reduction in cyst diameter and total volume.
In a retrospective cohort study, 61 women with pelvic pain and rectovaginal endometriosis had 5 years of treatment with NEA 2.5 mg or 5.0 mg daily.5 NEA treatment resulted in a decrease in dysmenorrhea, deep dyspareunia, and dyschezia. The most common side effects attributed to NEA treatment were weight gain (30%), vaginal bleeding (23%), decreased libido (11%), headache (9%), bloating or swelling (8%), depression (7%), and acne (5%). In women who had sequential imaging studies, NEA treatment resulted in a decrease in rectovaginal lesion volume, stable disease volume, or an increase in lesion volume in 56%, 32%, and 12% of the women, respectively. The investigators concluded that for women with rectovaginal endometriosis, NEA treatment is a low-cost option for long-term treatment.
In my practice, I do not prescribe NEA at doses greater than 5 mg daily. There are case reports that NEA at a dose of ≥10 mg daily is associated with the development of a hepatic adenoma,6 elevated liver transaminase concentration,7 and jaundice.8 If NEA 5 mg daily is not effective in controlling pelvic pain caused by endometriosis, I stop the NEA and start a GnRH analogue, most often elagolix.
NEA 5 mg is not FDA approved as a contraceptive. However, norethindrone 0.35 mg daily, also known as the “mini-pill”, is approved as a progestin-only contraceptive.9 NEA is rapidly and completely deacetylated to norethindrone, and the disposition of oral NEA is indistinguishable from that of norethindrone.1 Since norethindrone 0.35 mg daily is approved as a contraceptive, it is highly likely that NEA 5 mg has contraceptive properties if taken daily.
Continue to: Elagolix...
Elagolix
Elagolix is FDA approved for the treatment of pelvic pain caused by endometriosis. I reviewed the key studies resulting in FDA approval in the November 2018 issue of
In the Elaris Endometriosis-I study, 872 women with endometriosis and pelvic pain were randomly assigned to treatment with 1 of 2 doses of elagolix (high-dose [200 mg twice daily] and low-dose [150 mg once daily]) or placebo.11 After 3 months of therapy, a clinically meaningful reduction in dysmenorrhea pain was reported by 76%, 46%, and 20% of the women in the high-dose elagolix, low-dose elagolix, and placebo groups, respectively (P<.001 for comparisons of elagolix to placebo). After 3 months of therapy, a clinically meaningful reduction in nonmenstrual pain or decreased or stable use of rescue analgesics was reported by 55%, 50%, and 37% of the women in the high-dose elagolix, low-dose elagolix, and placebo groups, respectively (P<.01 low-dose elagolix vs placebo and P<.001 high-dose elagolix vs placebo).
Hot flashes that were severe enough to be reported as an adverse event by the study participants were reported by 42%, 24%, and 7% of the women in the high-dose elagolix, low-dose elagolix, and placebo groups. Bone density was measured at baseline and after 6 months of treatment. Lumbar bone density changes were -2.61%, -0.32%, and +0.47% and hip femoral neck bone density changes were -1.89%, -0.39%, and +0.02% in the high-dose elagolix, low-dose elagolix, and placebo groups, respectively.
Another large clinical trial of elagolix for the treatment of pelvic pain caused by endometriosis, Elaris EM-II, involving 817 women, produced results very similar to those reported in Elaris EM-I. The elagolix continuation studies, Elaris EM-III and -IV, demonstrated efficacy and safety of elagolix through 12 months of treatment.12
In my 2018 review,10 I noted that elagolix dose adjustment can be utilized to attempt to achieve maximal pain relief with minimal vasomotor symptoms. Elagolix at 200 mg twice daily produces a mean estradiol concentration of 12 pg/mL, whereas elagolix at 150 mg daily resulted in a mean estradiol concentration of 41 pg/mL.13 The estrogen threshold hypothesis posits that in women with endometriosis a stable estradiol concentration of 20 to 30 pg/mL is often associated with decreased pain and fewer vasomotor events.14 To achieve the target estradiol range of 20 to 30 pg/mL, I often initiate elagolix treatment with 200 mg twice daily. This enables a rapid onset of amenorrhea and a reduction in pelvic pain. Once amenorrhea has been achieved and a decrease in pelvic pain has occurred, I adjust the dose downward to 200 mg twice daily on even calendar days of each month and 200 mg once daily on odd calendar days each month. Some women will have continued pain relief and amenorrhea when the dose is further decreased to 200 mg once daily. If bothersome bleeding recurs and/or pain symptoms increase in severity, the dose can be increased to 200 mg twice daily or an alternating regimen of 200 mg twice daily and 200 mg once daily, every 2 days. An alternative to dose adjustment is to combine elagolix with NEA, which can reduce the severity of hot flashes and reduce bone loss caused by hypoestrogenism.15,16
Health insurers and pharmacy benefits managers may require a prior authorization before approving and dispensing elagolix. The prior authorization process can be burdensome for clinicians, consuming limited healthcare resources, contributing to burnout and frustrating patients.17 Elagolix is less expensive than depot-leuprolide acetate and nafarelin nasal spray and somewhat more expensive than a goserelin implant.18,19
Elagolix is not approved as a contraceptive. In the Elaris EM-I and -II trials women were advised to use 2 forms of contraception, although pregnancies did occur. There were 6 pregnancies among 475 women taking elagolix 150 mg daily and 2 pregnancies among 477 women taking elagolix 200 mg twice daily.20 Women taking elagolix should be advised to use a contraceptive, but not an estrogen-progestin contraceptive.
Continue to: Do not use opioids to treat chronic pelvic pain caused by endometriosis...
Do not use opioids to treat chronic pelvic pain caused by endometriosis
One of the greatest public health tragedies of our era is the opioid misuse epidemic. Hundreds of thousands of deaths have been caused by opioid misuse. The Centers for Disease Control and Prevention reported that for the 12-month period ending in May 2020, there were 81,000 opioid-related deaths, the greatest number ever reported in a 12-month period.21 Many authorities believe that in the United States opioid medications have been over-prescribed, contributing to the opioid misuse epidemic. There is little evidence that chronic pelvic pain is optimally managed by chronic treatment with an opioid.22,23 Prescribing opioids to vulnerable individuals to treat chronic pelvic pain may result in opioid dependency and adversely affect the patient’s health. It is best to pledge not to prescribe an opioid medication for a woman with chronic pelvic pain caused by endometriosis. In situations when pelvic pain is difficult to control with hormonal therapy and nonopioid pain medications, referral to a specialty pain practice may be warranted.
Post–conservative surgery hormone treatment reduces pelvic pain recurrence
In a meta-analysis of 14 studies that reported on endometriosis recurrence rates following conservative surgery, recurrence (defined as recurrent pelvic pain or an imaging study showing recurrent endometriosis) was significantly reduced with the use of hormone treatment compared with expectant management or placebo treatment.24 The postoperative relative risk of endometriosis recurrence was reduced by 83% with progestin treatment, 64% with estrogen-progestin contraceptive treatment, and 38% with GnRH analogue treatment. Overall, the number of patients that needed to be treated to prevent one endometriosis recurrence was 10, assuming a recurrence rate of 25% in the placebo treatment or expectant management groups.
For women with pelvic pain caused by endometriosis who develop a recurrence of pelvic pain while on postoperative hormone treatment, it is important for the prescribing clinician to be flexible and consider changing the hormone regimen. For example, if a postoperative patient is treated with a continuous estrogen-progestin contraceptive and develops recurrent pain, I will stop the contraceptive and initiate treatment with either NEA or elagolix.
Capitalize on opportunities to improve the medical care of women with endometriosis
Early diagnosis of endometriosis can be facilitated by recognizing that the condition is a common cause of moderate to severe dysmenorrhea. In 5 studies involving 1,187 women, the mean length of time from onset of pelvic pain symptoms to diagnosis of endometriosis was 8.6 years.25 If a woman with pelvic pain caused by endometriosis has not had sufficient pain relief with one brand of continuous estrogen-progestin contraceptive, it is best not to prescribe an alternative brand but rather to switch to a progestin-only treatment or a GnRH antagonist. If plan A is not working, move expeditiously to plan B. ●
- Aygestin [package insert]. Barr Laboratories: Pomona, NY; 2007.
- Kaser DJ, Missmer SA, Berry KF, et al. Use of norethindrone acetate alone for postoperative suppression of endometriosis symptoms. J Pediatr Adolesc Gynecol. 2012;25:105-108.
- Muneyyirci-Delale O, Karacan M. Effect of norethindrone acetate in the treatment of symptomatic endometriosis. Int J Fertil Womens Med. 1998;43:24-27.
- Muneyyirci-Delale O, Anopa J, Charles C, et al. Medical management of recurrent endometrioma with long-term norethindrone acetate. Int J Women Health. 2012;4:149-154.
- Morotti M, Venturini PL, Biscaldi E, et al. Efficacy and acceptability of long-term norethindrone acetate for the treatment of rectovaginal endometriosis. Eur J Obstet Gynecol Repro Biol. 2017;213:4-10.
- Brady PC, Missmer SA, Laufer MR. Hepatic adenomas in adolescents and young women with endometriosis treated with norethindrone acetate. J Pediatr Adolesc Gynecol. 2017;30:422-424.
- Choudhary NS, Bodh V, Chaudhari S, et al. Norethisterone related drug induced liver injury: a series of 3 cases. J Clin Exp Hepatol. 2017;7:266- 268.
- Perez-Mera RA, Shields CE. Jaundice associated with norethindrone acetate therapy. N Engl J Med. 1962;267:1137-1138.
- Camila [package insert]. Mayne Pharma Inc: Greenville, NC; 2018.
- Barbieri RL. Elagolix: a new treatment for pelvic pain caused by endometriosis. OBG Manag. 2018;30:10,12-14, 20.
- Taylor HS, Giudice LC, Lessey BA, et al. Treatment of endometriosis-associated pain with elagolix, an oral GnRH antagonist. N Engl J Med. 2017;377:28-40.
- Surrey E, Taylor HS, Giudice L, et al. Long-term outcomes of elagolix in women with endometriosis: results from two extension studies. Obstet Gynecol. 2018;132:147-160.
- Orilissa [package insert]. AbbVie Inc; North Chicago, IL; 2018.
- Barbieri RL. Hormonal treatment of endometriosis: the estrogen threshold hypothesis. Am J Obstet Gynecol. 1992;166:740-745.
- Hornstein MD, Surrey ES, Weisberg GW, et al. Leuprolide acetate depot and hormonal add-back in endometriosis: a 12-month study. Lupron Add-Back Study Group. Obstet Gynecol. 1998;91:16-24.
- Gallagher JS, Missmer SA, Hornstein MD, et al. Long-term effects of gonadotropin-releasing hormone agonists and add-back in adolescent endometriosis. J Pediatr Adolesc Gynecol. 2018;31:376- 381.
- Miller A, Shor R, Waites T, et al. Prior authorization reform for better patient care. J Am Coll Cardiol. 2018;71:1937-1939.
- Depot-leuprolide acetate. Good Rx website. https://www.goodrx.com/. Accessed January 22, 2021.
- Goserelin. Good Rx website. https://www .goodrx.com/. Accessed January 22, 2021
- Taylor HS, Giudice LC, Lessey BA, et al. Treatment of endometriosis-associated pain with elagolix, an oral GnRH antagonist. N Engl J Med. 2017;377:28-40.
- Centers for Disease Control and Prevention. Overdose deaths accelerating during COVID19. https://www.cdc.gov/media/releases/2020 /p1218-overdose-deaths-covid-19.html. Reviewed December 18, 2020. Accessed March 24, 2021.
- Till SR, As-Sanie S. 3 cases of chronic pelvic pain with nonsurgical, nonopioid therapies. OBG Manag. 2018;30:41-48.
- Steele A. Opioid use and depression in chronic pelvic pain. Obstet Gynecol Clin North Am. 2014;41:491-501.
- Zakhari A, Delpero E, McKeown S, et al. Endometriosis recurrence following post-operative hormonal suppression: a systematic review and meta-analysis. Hum Reprod Update. 2021;27:96- 107.
- Barbieri RL. Why are there delays in the diagnosis of endometriosis? OBG Manag. 2017;29:8, 10-11, 16.
Women with endometriosis often present for medical care for one or more of the following health issues: pelvic pain, infertility, and/or an adnexal cyst (endometrioma). For women with moderate or severe pelvic pain and laparoscopically diagnosed endometriosis, hormone therapy is often necessary to achieve maximal long-term reduction in pain and optimize health. I focus on opportunities to optimize hormonal treatment of endometriosis in this editorial.
When plan A is not working, move expeditiously to plan B
Cyclic or continuous combination estrogen-progestin contraceptives are commonly prescribed to treat pelvic pain caused by endometriosis. Although endometriosis pain may initially improve with estrogen-progestin contraceptives, many women on this medication will eventually report that they have worsening pelvic pain that adversely impacts their daily activities. Surprisingly, clinicians often continue to prescribe estrogen-progestin contraceptives even after the patient reports that the treatment is not effective, and their pain continues to be bothersome.
Patients benefit when they have access to the full range of hormone treatments that have been approved by the FDA for the treatment of moderate to severe pelvic pain caused by endometriosis (TABLE). In the situation where an estrogen-progestin contraceptive is no longer effective at reducing the pelvic pain, I will often offer the patient the option of norethindrone acetate (NEA) or elagolix treatment. My experience is that stopping the estrogen-progestin contraceptive and starting NEA or elagolix will result in a significant decrease in pain symptoms and improvement in the patient’s quality of life.

Other FDA-approved options to treat pelvic pain caused by endometriosis include depot medroxyprogesterone acetate injectable suspension, depot leuprolide acetate, goserelin implant, and danazol. I do not routinely prescribe depot medroxyprogesterone acetate because some patients report new onset or worsening symptoms of depression on the medication. I prescribe depot-leuprolide acetate less often than in the past, because many patients report moderate to severe hypoestrogenic symptoms on this medication. In women taking depot-leuprolide acetate, moderate to severe vasomotor symptoms can be improved by prescribing NEA pills, but the alternative of norethindrone monotherapy is less expensive. I seldom use goserelin or danazol in my practice. The needle required to place the goserelin implant has a diameter of approximately 1.7 mm (16 gauge) or 2.1 mm (14 gauge), for the 3.6 mg and 10 mg doses, respectively. The large diameter of the needle can cause pain and bruising at the implant site. As a comparison, the progestin subdermal implant needle is approximately 2.1 mm in diameter. Danazol is associated with weight gain, and most women prefer to avoid this side effect.
Continue to: Norethindrone acetate...
Norethindrone acetate
NEA 5 mg daily is approved by the FDA to treat endometriosis.1 NEA was approved at a time when large controlled clinical trials were not routinely required for a medicine to be approved. The data to support NEA treatment of pelvic pain caused by endometriosis is based on cohort studies. In a study of 194 women, median age 21 years with moderate to severe pelvic pain and surgically proven endometriosis, the effect of NEA on pelvic pain was explored.2 The initial dose of NEA was 5 mg daily. If the patient did not achieve a reduction in pelvic pain and amenorrhea on the NEA dose of 5 mg daily, the dose was increased by 2.5 mg every 2 weeks, up to a maximum of 15 mg, until amenorrhea and/or a decrease in pelvic pain was achieved. Ninety-five percent of the women in this cohort had previously been treated with an estrogen-progestin contraceptive or a GnRH antagonist and had discontinued those medications because of inadequate control of pelvic pain or because of side effects of the medication.
In this large cohort, 65% of women reported significant improvement in pelvic pain, with a median pain score of 5 before treatment and 0 following NEA treatment. About 55% of the women reported no side effects. The most commonly reported side effects were weight gain (16%; mean weight gain, 3.1 kg), acne (10%), mood lability (9%), hot flashes (8%), depression (6%), scalp hair loss (4%), headache (4%), nausea (3%), and deepening of the voice (1%). (In this study women could report more than one side effect.)
In another cohort study of 52 women with pelvic pain and surgically confirmed endometriosis, NEA treatment resulted in pain relief in 94% of the women.3 Breakthrough bleeding was a common side effect, reported by 58% of participants. The investigators concluded that NEA treatment was a “cost-effective alternative with relatively mild side effects in the treatment of symptomatic endometriosis.” A conclusion which I endorse.
NEA has been reported to effectively treat ovarian endometriomas and rectovaginal endometriosis.4,5 In a cohort of 18 women who had previously had the surgical resection of an ovarian endometriosis cyst and had postoperative recurrence of pelvic pain and ovarian endometriosis, treatment was initiated with an escalating NEA regimen.4 Treatment was initiated with NEA 5 mg daily, with the dosage increased every 2 weeks by 2.5 mg until amenorrhea was established. Most women achieved amenorrhea with NEA 5 mg daily, and 89% had reduced pelvic pain. The investigators reported complete regression of the endometriosis cyst(s) in 74% of the women. In my experience, NEA does not result in complete regression of endometriosis cysts, but it does cause a reduction in cyst diameter and total volume.
In a retrospective cohort study, 61 women with pelvic pain and rectovaginal endometriosis had 5 years of treatment with NEA 2.5 mg or 5.0 mg daily.5 NEA treatment resulted in a decrease in dysmenorrhea, deep dyspareunia, and dyschezia. The most common side effects attributed to NEA treatment were weight gain (30%), vaginal bleeding (23%), decreased libido (11%), headache (9%), bloating or swelling (8%), depression (7%), and acne (5%). In women who had sequential imaging studies, NEA treatment resulted in a decrease in rectovaginal lesion volume, stable disease volume, or an increase in lesion volume in 56%, 32%, and 12% of the women, respectively. The investigators concluded that for women with rectovaginal endometriosis, NEA treatment is a low-cost option for long-term treatment.
In my practice, I do not prescribe NEA at doses greater than 5 mg daily. There are case reports that NEA at a dose of ≥10 mg daily is associated with the development of a hepatic adenoma,6 elevated liver transaminase concentration,7 and jaundice.8 If NEA 5 mg daily is not effective in controlling pelvic pain caused by endometriosis, I stop the NEA and start a GnRH analogue, most often elagolix.
NEA 5 mg is not FDA approved as a contraceptive. However, norethindrone 0.35 mg daily, also known as the “mini-pill”, is approved as a progestin-only contraceptive.9 NEA is rapidly and completely deacetylated to norethindrone, and the disposition of oral NEA is indistinguishable from that of norethindrone.1 Since norethindrone 0.35 mg daily is approved as a contraceptive, it is highly likely that NEA 5 mg has contraceptive properties if taken daily.
Continue to: Elagolix...
Elagolix
Elagolix is FDA approved for the treatment of pelvic pain caused by endometriosis. I reviewed the key studies resulting in FDA approval in the November 2018 issue of
In the Elaris Endometriosis-I study, 872 women with endometriosis and pelvic pain were randomly assigned to treatment with 1 of 2 doses of elagolix (high-dose [200 mg twice daily] and low-dose [150 mg once daily]) or placebo.11 After 3 months of therapy, a clinically meaningful reduction in dysmenorrhea pain was reported by 76%, 46%, and 20% of the women in the high-dose elagolix, low-dose elagolix, and placebo groups, respectively (P<.001 for comparisons of elagolix to placebo). After 3 months of therapy, a clinically meaningful reduction in nonmenstrual pain or decreased or stable use of rescue analgesics was reported by 55%, 50%, and 37% of the women in the high-dose elagolix, low-dose elagolix, and placebo groups, respectively (P<.01 low-dose elagolix vs placebo and P<.001 high-dose elagolix vs placebo).
Hot flashes that were severe enough to be reported as an adverse event by the study participants were reported by 42%, 24%, and 7% of the women in the high-dose elagolix, low-dose elagolix, and placebo groups. Bone density was measured at baseline and after 6 months of treatment. Lumbar bone density changes were -2.61%, -0.32%, and +0.47% and hip femoral neck bone density changes were -1.89%, -0.39%, and +0.02% in the high-dose elagolix, low-dose elagolix, and placebo groups, respectively.
Another large clinical trial of elagolix for the treatment of pelvic pain caused by endometriosis, Elaris EM-II, involving 817 women, produced results very similar to those reported in Elaris EM-I. The elagolix continuation studies, Elaris EM-III and -IV, demonstrated efficacy and safety of elagolix through 12 months of treatment.12
In my 2018 review,10 I noted that elagolix dose adjustment can be utilized to attempt to achieve maximal pain relief with minimal vasomotor symptoms. Elagolix at 200 mg twice daily produces a mean estradiol concentration of 12 pg/mL, whereas elagolix at 150 mg daily resulted in a mean estradiol concentration of 41 pg/mL.13 The estrogen threshold hypothesis posits that in women with endometriosis a stable estradiol concentration of 20 to 30 pg/mL is often associated with decreased pain and fewer vasomotor events.14 To achieve the target estradiol range of 20 to 30 pg/mL, I often initiate elagolix treatment with 200 mg twice daily. This enables a rapid onset of amenorrhea and a reduction in pelvic pain. Once amenorrhea has been achieved and a decrease in pelvic pain has occurred, I adjust the dose downward to 200 mg twice daily on even calendar days of each month and 200 mg once daily on odd calendar days each month. Some women will have continued pain relief and amenorrhea when the dose is further decreased to 200 mg once daily. If bothersome bleeding recurs and/or pain symptoms increase in severity, the dose can be increased to 200 mg twice daily or an alternating regimen of 200 mg twice daily and 200 mg once daily, every 2 days. An alternative to dose adjustment is to combine elagolix with NEA, which can reduce the severity of hot flashes and reduce bone loss caused by hypoestrogenism.15,16
Health insurers and pharmacy benefits managers may require a prior authorization before approving and dispensing elagolix. The prior authorization process can be burdensome for clinicians, consuming limited healthcare resources, contributing to burnout and frustrating patients.17 Elagolix is less expensive than depot-leuprolide acetate and nafarelin nasal spray and somewhat more expensive than a goserelin implant.18,19
Elagolix is not approved as a contraceptive. In the Elaris EM-I and -II trials women were advised to use 2 forms of contraception, although pregnancies did occur. There were 6 pregnancies among 475 women taking elagolix 150 mg daily and 2 pregnancies among 477 women taking elagolix 200 mg twice daily.20 Women taking elagolix should be advised to use a contraceptive, but not an estrogen-progestin contraceptive.
Continue to: Do not use opioids to treat chronic pelvic pain caused by endometriosis...
Do not use opioids to treat chronic pelvic pain caused by endometriosis
One of the greatest public health tragedies of our era is the opioid misuse epidemic. Hundreds of thousands of deaths have been caused by opioid misuse. The Centers for Disease Control and Prevention reported that for the 12-month period ending in May 2020, there were 81,000 opioid-related deaths, the greatest number ever reported in a 12-month period.21 Many authorities believe that in the United States opioid medications have been over-prescribed, contributing to the opioid misuse epidemic. There is little evidence that chronic pelvic pain is optimally managed by chronic treatment with an opioid.22,23 Prescribing opioids to vulnerable individuals to treat chronic pelvic pain may result in opioid dependency and adversely affect the patient’s health. It is best to pledge not to prescribe an opioid medication for a woman with chronic pelvic pain caused by endometriosis. In situations when pelvic pain is difficult to control with hormonal therapy and nonopioid pain medications, referral to a specialty pain practice may be warranted.
Post–conservative surgery hormone treatment reduces pelvic pain recurrence
In a meta-analysis of 14 studies that reported on endometriosis recurrence rates following conservative surgery, recurrence (defined as recurrent pelvic pain or an imaging study showing recurrent endometriosis) was significantly reduced with the use of hormone treatment compared with expectant management or placebo treatment.24 The postoperative relative risk of endometriosis recurrence was reduced by 83% with progestin treatment, 64% with estrogen-progestin contraceptive treatment, and 38% with GnRH analogue treatment. Overall, the number of patients that needed to be treated to prevent one endometriosis recurrence was 10, assuming a recurrence rate of 25% in the placebo treatment or expectant management groups.
For women with pelvic pain caused by endometriosis who develop a recurrence of pelvic pain while on postoperative hormone treatment, it is important for the prescribing clinician to be flexible and consider changing the hormone regimen. For example, if a postoperative patient is treated with a continuous estrogen-progestin contraceptive and develops recurrent pain, I will stop the contraceptive and initiate treatment with either NEA or elagolix.
Capitalize on opportunities to improve the medical care of women with endometriosis
Early diagnosis of endometriosis can be facilitated by recognizing that the condition is a common cause of moderate to severe dysmenorrhea. In 5 studies involving 1,187 women, the mean length of time from onset of pelvic pain symptoms to diagnosis of endometriosis was 8.6 years.25 If a woman with pelvic pain caused by endometriosis has not had sufficient pain relief with one brand of continuous estrogen-progestin contraceptive, it is best not to prescribe an alternative brand but rather to switch to a progestin-only treatment or a GnRH antagonist. If plan A is not working, move expeditiously to plan B. ●
Women with endometriosis often present for medical care for one or more of the following health issues: pelvic pain, infertility, and/or an adnexal cyst (endometrioma). For women with moderate or severe pelvic pain and laparoscopically diagnosed endometriosis, hormone therapy is often necessary to achieve maximal long-term reduction in pain and optimize health. I focus on opportunities to optimize hormonal treatment of endometriosis in this editorial.
When plan A is not working, move expeditiously to plan B
Cyclic or continuous combination estrogen-progestin contraceptives are commonly prescribed to treat pelvic pain caused by endometriosis. Although endometriosis pain may initially improve with estrogen-progestin contraceptives, many women on this medication will eventually report that they have worsening pelvic pain that adversely impacts their daily activities. Surprisingly, clinicians often continue to prescribe estrogen-progestin contraceptives even after the patient reports that the treatment is not effective, and their pain continues to be bothersome.
Patients benefit when they have access to the full range of hormone treatments that have been approved by the FDA for the treatment of moderate to severe pelvic pain caused by endometriosis (TABLE). In the situation where an estrogen-progestin contraceptive is no longer effective at reducing the pelvic pain, I will often offer the patient the option of norethindrone acetate (NEA) or elagolix treatment. My experience is that stopping the estrogen-progestin contraceptive and starting NEA or elagolix will result in a significant decrease in pain symptoms and improvement in the patient’s quality of life.

Other FDA-approved options to treat pelvic pain caused by endometriosis include depot medroxyprogesterone acetate injectable suspension, depot leuprolide acetate, goserelin implant, and danazol. I do not routinely prescribe depot medroxyprogesterone acetate because some patients report new onset or worsening symptoms of depression on the medication. I prescribe depot-leuprolide acetate less often than in the past, because many patients report moderate to severe hypoestrogenic symptoms on this medication. In women taking depot-leuprolide acetate, moderate to severe vasomotor symptoms can be improved by prescribing NEA pills, but the alternative of norethindrone monotherapy is less expensive. I seldom use goserelin or danazol in my practice. The needle required to place the goserelin implant has a diameter of approximately 1.7 mm (16 gauge) or 2.1 mm (14 gauge), for the 3.6 mg and 10 mg doses, respectively. The large diameter of the needle can cause pain and bruising at the implant site. As a comparison, the progestin subdermal implant needle is approximately 2.1 mm in diameter. Danazol is associated with weight gain, and most women prefer to avoid this side effect.
Continue to: Norethindrone acetate...
Norethindrone acetate
NEA 5 mg daily is approved by the FDA to treat endometriosis.1 NEA was approved at a time when large controlled clinical trials were not routinely required for a medicine to be approved. The data to support NEA treatment of pelvic pain caused by endometriosis is based on cohort studies. In a study of 194 women, median age 21 years with moderate to severe pelvic pain and surgically proven endometriosis, the effect of NEA on pelvic pain was explored.2 The initial dose of NEA was 5 mg daily. If the patient did not achieve a reduction in pelvic pain and amenorrhea on the NEA dose of 5 mg daily, the dose was increased by 2.5 mg every 2 weeks, up to a maximum of 15 mg, until amenorrhea and/or a decrease in pelvic pain was achieved. Ninety-five percent of the women in this cohort had previously been treated with an estrogen-progestin contraceptive or a GnRH antagonist and had discontinued those medications because of inadequate control of pelvic pain or because of side effects of the medication.
In this large cohort, 65% of women reported significant improvement in pelvic pain, with a median pain score of 5 before treatment and 0 following NEA treatment. About 55% of the women reported no side effects. The most commonly reported side effects were weight gain (16%; mean weight gain, 3.1 kg), acne (10%), mood lability (9%), hot flashes (8%), depression (6%), scalp hair loss (4%), headache (4%), nausea (3%), and deepening of the voice (1%). (In this study women could report more than one side effect.)
In another cohort study of 52 women with pelvic pain and surgically confirmed endometriosis, NEA treatment resulted in pain relief in 94% of the women.3 Breakthrough bleeding was a common side effect, reported by 58% of participants. The investigators concluded that NEA treatment was a “cost-effective alternative with relatively mild side effects in the treatment of symptomatic endometriosis.” A conclusion which I endorse.
NEA has been reported to effectively treat ovarian endometriomas and rectovaginal endometriosis.4,5 In a cohort of 18 women who had previously had the surgical resection of an ovarian endometriosis cyst and had postoperative recurrence of pelvic pain and ovarian endometriosis, treatment was initiated with an escalating NEA regimen.4 Treatment was initiated with NEA 5 mg daily, with the dosage increased every 2 weeks by 2.5 mg until amenorrhea was established. Most women achieved amenorrhea with NEA 5 mg daily, and 89% had reduced pelvic pain. The investigators reported complete regression of the endometriosis cyst(s) in 74% of the women. In my experience, NEA does not result in complete regression of endometriosis cysts, but it does cause a reduction in cyst diameter and total volume.
In a retrospective cohort study, 61 women with pelvic pain and rectovaginal endometriosis had 5 years of treatment with NEA 2.5 mg or 5.0 mg daily.5 NEA treatment resulted in a decrease in dysmenorrhea, deep dyspareunia, and dyschezia. The most common side effects attributed to NEA treatment were weight gain (30%), vaginal bleeding (23%), decreased libido (11%), headache (9%), bloating or swelling (8%), depression (7%), and acne (5%). In women who had sequential imaging studies, NEA treatment resulted in a decrease in rectovaginal lesion volume, stable disease volume, or an increase in lesion volume in 56%, 32%, and 12% of the women, respectively. The investigators concluded that for women with rectovaginal endometriosis, NEA treatment is a low-cost option for long-term treatment.
In my practice, I do not prescribe NEA at doses greater than 5 mg daily. There are case reports that NEA at a dose of ≥10 mg daily is associated with the development of a hepatic adenoma,6 elevated liver transaminase concentration,7 and jaundice.8 If NEA 5 mg daily is not effective in controlling pelvic pain caused by endometriosis, I stop the NEA and start a GnRH analogue, most often elagolix.
NEA 5 mg is not FDA approved as a contraceptive. However, norethindrone 0.35 mg daily, also known as the “mini-pill”, is approved as a progestin-only contraceptive.9 NEA is rapidly and completely deacetylated to norethindrone, and the disposition of oral NEA is indistinguishable from that of norethindrone.1 Since norethindrone 0.35 mg daily is approved as a contraceptive, it is highly likely that NEA 5 mg has contraceptive properties if taken daily.
Continue to: Elagolix...
Elagolix
Elagolix is FDA approved for the treatment of pelvic pain caused by endometriosis. I reviewed the key studies resulting in FDA approval in the November 2018 issue of
In the Elaris Endometriosis-I study, 872 women with endometriosis and pelvic pain were randomly assigned to treatment with 1 of 2 doses of elagolix (high-dose [200 mg twice daily] and low-dose [150 mg once daily]) or placebo.11 After 3 months of therapy, a clinically meaningful reduction in dysmenorrhea pain was reported by 76%, 46%, and 20% of the women in the high-dose elagolix, low-dose elagolix, and placebo groups, respectively (P<.001 for comparisons of elagolix to placebo). After 3 months of therapy, a clinically meaningful reduction in nonmenstrual pain or decreased or stable use of rescue analgesics was reported by 55%, 50%, and 37% of the women in the high-dose elagolix, low-dose elagolix, and placebo groups, respectively (P<.01 low-dose elagolix vs placebo and P<.001 high-dose elagolix vs placebo).
Hot flashes that were severe enough to be reported as an adverse event by the study participants were reported by 42%, 24%, and 7% of the women in the high-dose elagolix, low-dose elagolix, and placebo groups. Bone density was measured at baseline and after 6 months of treatment. Lumbar bone density changes were -2.61%, -0.32%, and +0.47% and hip femoral neck bone density changes were -1.89%, -0.39%, and +0.02% in the high-dose elagolix, low-dose elagolix, and placebo groups, respectively.
Another large clinical trial of elagolix for the treatment of pelvic pain caused by endometriosis, Elaris EM-II, involving 817 women, produced results very similar to those reported in Elaris EM-I. The elagolix continuation studies, Elaris EM-III and -IV, demonstrated efficacy and safety of elagolix through 12 months of treatment.12
In my 2018 review,10 I noted that elagolix dose adjustment can be utilized to attempt to achieve maximal pain relief with minimal vasomotor symptoms. Elagolix at 200 mg twice daily produces a mean estradiol concentration of 12 pg/mL, whereas elagolix at 150 mg daily resulted in a mean estradiol concentration of 41 pg/mL.13 The estrogen threshold hypothesis posits that in women with endometriosis a stable estradiol concentration of 20 to 30 pg/mL is often associated with decreased pain and fewer vasomotor events.14 To achieve the target estradiol range of 20 to 30 pg/mL, I often initiate elagolix treatment with 200 mg twice daily. This enables a rapid onset of amenorrhea and a reduction in pelvic pain. Once amenorrhea has been achieved and a decrease in pelvic pain has occurred, I adjust the dose downward to 200 mg twice daily on even calendar days of each month and 200 mg once daily on odd calendar days each month. Some women will have continued pain relief and amenorrhea when the dose is further decreased to 200 mg once daily. If bothersome bleeding recurs and/or pain symptoms increase in severity, the dose can be increased to 200 mg twice daily or an alternating regimen of 200 mg twice daily and 200 mg once daily, every 2 days. An alternative to dose adjustment is to combine elagolix with NEA, which can reduce the severity of hot flashes and reduce bone loss caused by hypoestrogenism.15,16
Health insurers and pharmacy benefits managers may require a prior authorization before approving and dispensing elagolix. The prior authorization process can be burdensome for clinicians, consuming limited healthcare resources, contributing to burnout and frustrating patients.17 Elagolix is less expensive than depot-leuprolide acetate and nafarelin nasal spray and somewhat more expensive than a goserelin implant.18,19
Elagolix is not approved as a contraceptive. In the Elaris EM-I and -II trials women were advised to use 2 forms of contraception, although pregnancies did occur. There were 6 pregnancies among 475 women taking elagolix 150 mg daily and 2 pregnancies among 477 women taking elagolix 200 mg twice daily.20 Women taking elagolix should be advised to use a contraceptive, but not an estrogen-progestin contraceptive.
Continue to: Do not use opioids to treat chronic pelvic pain caused by endometriosis...
Do not use opioids to treat chronic pelvic pain caused by endometriosis
One of the greatest public health tragedies of our era is the opioid misuse epidemic. Hundreds of thousands of deaths have been caused by opioid misuse. The Centers for Disease Control and Prevention reported that for the 12-month period ending in May 2020, there were 81,000 opioid-related deaths, the greatest number ever reported in a 12-month period.21 Many authorities believe that in the United States opioid medications have been over-prescribed, contributing to the opioid misuse epidemic. There is little evidence that chronic pelvic pain is optimally managed by chronic treatment with an opioid.22,23 Prescribing opioids to vulnerable individuals to treat chronic pelvic pain may result in opioid dependency and adversely affect the patient’s health. It is best to pledge not to prescribe an opioid medication for a woman with chronic pelvic pain caused by endometriosis. In situations when pelvic pain is difficult to control with hormonal therapy and nonopioid pain medications, referral to a specialty pain practice may be warranted.
Post–conservative surgery hormone treatment reduces pelvic pain recurrence
In a meta-analysis of 14 studies that reported on endometriosis recurrence rates following conservative surgery, recurrence (defined as recurrent pelvic pain or an imaging study showing recurrent endometriosis) was significantly reduced with the use of hormone treatment compared with expectant management or placebo treatment.24 The postoperative relative risk of endometriosis recurrence was reduced by 83% with progestin treatment, 64% with estrogen-progestin contraceptive treatment, and 38% with GnRH analogue treatment. Overall, the number of patients that needed to be treated to prevent one endometriosis recurrence was 10, assuming a recurrence rate of 25% in the placebo treatment or expectant management groups.
For women with pelvic pain caused by endometriosis who develop a recurrence of pelvic pain while on postoperative hormone treatment, it is important for the prescribing clinician to be flexible and consider changing the hormone regimen. For example, if a postoperative patient is treated with a continuous estrogen-progestin contraceptive and develops recurrent pain, I will stop the contraceptive and initiate treatment with either NEA or elagolix.
Capitalize on opportunities to improve the medical care of women with endometriosis
Early diagnosis of endometriosis can be facilitated by recognizing that the condition is a common cause of moderate to severe dysmenorrhea. In 5 studies involving 1,187 women, the mean length of time from onset of pelvic pain symptoms to diagnosis of endometriosis was 8.6 years.25 If a woman with pelvic pain caused by endometriosis has not had sufficient pain relief with one brand of continuous estrogen-progestin contraceptive, it is best not to prescribe an alternative brand but rather to switch to a progestin-only treatment or a GnRH antagonist. If plan A is not working, move expeditiously to plan B. ●
- Aygestin [package insert]. Barr Laboratories: Pomona, NY; 2007.
- Kaser DJ, Missmer SA, Berry KF, et al. Use of norethindrone acetate alone for postoperative suppression of endometriosis symptoms. J Pediatr Adolesc Gynecol. 2012;25:105-108.
- Muneyyirci-Delale O, Karacan M. Effect of norethindrone acetate in the treatment of symptomatic endometriosis. Int J Fertil Womens Med. 1998;43:24-27.
- Muneyyirci-Delale O, Anopa J, Charles C, et al. Medical management of recurrent endometrioma with long-term norethindrone acetate. Int J Women Health. 2012;4:149-154.
- Morotti M, Venturini PL, Biscaldi E, et al. Efficacy and acceptability of long-term norethindrone acetate for the treatment of rectovaginal endometriosis. Eur J Obstet Gynecol Repro Biol. 2017;213:4-10.
- Brady PC, Missmer SA, Laufer MR. Hepatic adenomas in adolescents and young women with endometriosis treated with norethindrone acetate. J Pediatr Adolesc Gynecol. 2017;30:422-424.
- Choudhary NS, Bodh V, Chaudhari S, et al. Norethisterone related drug induced liver injury: a series of 3 cases. J Clin Exp Hepatol. 2017;7:266- 268.
- Perez-Mera RA, Shields CE. Jaundice associated with norethindrone acetate therapy. N Engl J Med. 1962;267:1137-1138.
- Camila [package insert]. Mayne Pharma Inc: Greenville, NC; 2018.
- Barbieri RL. Elagolix: a new treatment for pelvic pain caused by endometriosis. OBG Manag. 2018;30:10,12-14, 20.
- Taylor HS, Giudice LC, Lessey BA, et al. Treatment of endometriosis-associated pain with elagolix, an oral GnRH antagonist. N Engl J Med. 2017;377:28-40.
- Surrey E, Taylor HS, Giudice L, et al. Long-term outcomes of elagolix in women with endometriosis: results from two extension studies. Obstet Gynecol. 2018;132:147-160.
- Orilissa [package insert]. AbbVie Inc; North Chicago, IL; 2018.
- Barbieri RL. Hormonal treatment of endometriosis: the estrogen threshold hypothesis. Am J Obstet Gynecol. 1992;166:740-745.
- Hornstein MD, Surrey ES, Weisberg GW, et al. Leuprolide acetate depot and hormonal add-back in endometriosis: a 12-month study. Lupron Add-Back Study Group. Obstet Gynecol. 1998;91:16-24.
- Gallagher JS, Missmer SA, Hornstein MD, et al. Long-term effects of gonadotropin-releasing hormone agonists and add-back in adolescent endometriosis. J Pediatr Adolesc Gynecol. 2018;31:376- 381.
- Miller A, Shor R, Waites T, et al. Prior authorization reform for better patient care. J Am Coll Cardiol. 2018;71:1937-1939.
- Depot-leuprolide acetate. Good Rx website. https://www.goodrx.com/. Accessed January 22, 2021.
- Goserelin. Good Rx website. https://www .goodrx.com/. Accessed January 22, 2021
- Taylor HS, Giudice LC, Lessey BA, et al. Treatment of endometriosis-associated pain with elagolix, an oral GnRH antagonist. N Engl J Med. 2017;377:28-40.
- Centers for Disease Control and Prevention. Overdose deaths accelerating during COVID19. https://www.cdc.gov/media/releases/2020 /p1218-overdose-deaths-covid-19.html. Reviewed December 18, 2020. Accessed March 24, 2021.
- Till SR, As-Sanie S. 3 cases of chronic pelvic pain with nonsurgical, nonopioid therapies. OBG Manag. 2018;30:41-48.
- Steele A. Opioid use and depression in chronic pelvic pain. Obstet Gynecol Clin North Am. 2014;41:491-501.
- Zakhari A, Delpero E, McKeown S, et al. Endometriosis recurrence following post-operative hormonal suppression: a systematic review and meta-analysis. Hum Reprod Update. 2021;27:96- 107.
- Barbieri RL. Why are there delays in the diagnosis of endometriosis? OBG Manag. 2017;29:8, 10-11, 16.
- Aygestin [package insert]. Barr Laboratories: Pomona, NY; 2007.
- Kaser DJ, Missmer SA, Berry KF, et al. Use of norethindrone acetate alone for postoperative suppression of endometriosis symptoms. J Pediatr Adolesc Gynecol. 2012;25:105-108.
- Muneyyirci-Delale O, Karacan M. Effect of norethindrone acetate in the treatment of symptomatic endometriosis. Int J Fertil Womens Med. 1998;43:24-27.
- Muneyyirci-Delale O, Anopa J, Charles C, et al. Medical management of recurrent endometrioma with long-term norethindrone acetate. Int J Women Health. 2012;4:149-154.
- Morotti M, Venturini PL, Biscaldi E, et al. Efficacy and acceptability of long-term norethindrone acetate for the treatment of rectovaginal endometriosis. Eur J Obstet Gynecol Repro Biol. 2017;213:4-10.
- Brady PC, Missmer SA, Laufer MR. Hepatic adenomas in adolescents and young women with endometriosis treated with norethindrone acetate. J Pediatr Adolesc Gynecol. 2017;30:422-424.
- Choudhary NS, Bodh V, Chaudhari S, et al. Norethisterone related drug induced liver injury: a series of 3 cases. J Clin Exp Hepatol. 2017;7:266- 268.
- Perez-Mera RA, Shields CE. Jaundice associated with norethindrone acetate therapy. N Engl J Med. 1962;267:1137-1138.
- Camila [package insert]. Mayne Pharma Inc: Greenville, NC; 2018.
- Barbieri RL. Elagolix: a new treatment for pelvic pain caused by endometriosis. OBG Manag. 2018;30:10,12-14, 20.
- Taylor HS, Giudice LC, Lessey BA, et al. Treatment of endometriosis-associated pain with elagolix, an oral GnRH antagonist. N Engl J Med. 2017;377:28-40.
- Surrey E, Taylor HS, Giudice L, et al. Long-term outcomes of elagolix in women with endometriosis: results from two extension studies. Obstet Gynecol. 2018;132:147-160.
- Orilissa [package insert]. AbbVie Inc; North Chicago, IL; 2018.
- Barbieri RL. Hormonal treatment of endometriosis: the estrogen threshold hypothesis. Am J Obstet Gynecol. 1992;166:740-745.
- Hornstein MD, Surrey ES, Weisberg GW, et al. Leuprolide acetate depot and hormonal add-back in endometriosis: a 12-month study. Lupron Add-Back Study Group. Obstet Gynecol. 1998;91:16-24.
- Gallagher JS, Missmer SA, Hornstein MD, et al. Long-term effects of gonadotropin-releasing hormone agonists and add-back in adolescent endometriosis. J Pediatr Adolesc Gynecol. 2018;31:376- 381.
- Miller A, Shor R, Waites T, et al. Prior authorization reform for better patient care. J Am Coll Cardiol. 2018;71:1937-1939.
- Depot-leuprolide acetate. Good Rx website. https://www.goodrx.com/. Accessed January 22, 2021.
- Goserelin. Good Rx website. https://www .goodrx.com/. Accessed January 22, 2021
- Taylor HS, Giudice LC, Lessey BA, et al. Treatment of endometriosis-associated pain with elagolix, an oral GnRH antagonist. N Engl J Med. 2017;377:28-40.
- Centers for Disease Control and Prevention. Overdose deaths accelerating during COVID19. https://www.cdc.gov/media/releases/2020 /p1218-overdose-deaths-covid-19.html. Reviewed December 18, 2020. Accessed March 24, 2021.
- Till SR, As-Sanie S. 3 cases of chronic pelvic pain with nonsurgical, nonopioid therapies. OBG Manag. 2018;30:41-48.
- Steele A. Opioid use and depression in chronic pelvic pain. Obstet Gynecol Clin North Am. 2014;41:491-501.
- Zakhari A, Delpero E, McKeown S, et al. Endometriosis recurrence following post-operative hormonal suppression: a systematic review and meta-analysis. Hum Reprod Update. 2021;27:96- 107.
- Barbieri RL. Why are there delays in the diagnosis of endometriosis? OBG Manag. 2017;29:8, 10-11, 16.
Office-based ambulatory cervical ripening prior to inpatient induction of labor
For women with a Bishop score ≤6, CR is an important first step in planned induction of labor (IOL). CR is believed to reduce the length of labor induction and increase the probability of a vaginal delivery. Historically, CR has been undertaken on a labor unit. However, with an increased rate of labor induction, the resources of the modern labor unit are incredibly stressed. Compounding the problem is the nursing shortage caused by the COVID-19 pandemic, which has resulted in staff being unavailable as they recover from a respiratory infection or are quarantined after an exposure. The COVID-19 pandemic also has motivated many patients to avoid the hospital as much as possible.
Office-based ambulatory CR is an alternative to inpatient CR and has the potential to reduce the use of labor unit resources. When CR is initiated in the office, the patient either is sent home overnight to return to the labor unit for IOL in the morning or is sent home in the morning to return for IOL in the evening or at night. A secondary benefit of office- and home-based CR is that it may increase patient satisfaction with the process of CR. This editorial summarizes the literature supporting office-based ambulatory CR.
Mechanical methods of CR
Contemporary mechanical methods of CR include the transcervical insertion of a Foley catheter, Cook double-balloon CR catheter, Dilapan-S, or laminaria. There are many publications reporting the feasibility of office-based ambulatory CR with transcervical balloon catheters and very few publications reporting on the use of Dilapan-S or laminaria for ambulatory CR.
Foley catheter
Many studies have investigated the effectiveness of transcervical Foley catheter for ambulatory CR. Policiano and colleagues compared the effectiveness of ambulatory versus inpatient Foley catheter CR.1 A total of 130 women with a Bishop score <6 at ≥41 weeks’ gestation were randomly assigned to outpatient or inpatient CR with a transcervical Foley catheter (Covidian Dover Silicon coated latex Foley catheter 16 Fr/5.3 mm diameter). The Foley catheter bulb was distended with 40 mL of a sterile saline solution. The end of the Foley was taped to the patient’s inner thigh. Manual traction was gently applied to the catheter every 6 hours. If the catheter was extruded, the Bishop score was assessed. For a Bishop score <6, the patient was given additional inpatient misoprostol (25 µg vaginally every 4 hours for up to 5 doses). For a Bishop score ≥6, intravenous oxytocin IOL was initiated. At 24 hours if the Foley catheter was still in situ, it was removed. Women were excluded from the study for the following factors: noncephalic presentation, spontaneous labor, hydramnios, nonreassuring cardiotocography (CTG), multiple pregnancy, ruptured membranes, active vaginal bleeding, Streptococcus group B infection, and HIV infection. Prostaglandin CR was not used if the woman had a previous cesarean delivery. No prophylactic antibiotics were administered. After placement of the Foley catheter, reassuring CTG was documented prior to sending the patient home.
Outpatient, compared with inpatient, CR resulted in a mean reduction of 10 hours in the time from admission to delivery. The time from insertion of the Foley catheter to delivery in the outpatient group was 38.2 hours, and 44.9 hours for the inpatient group (P<.01). The cesarean delivery rates were similar in both groups—28% and 38%, respectively. Three cases of chorioamnionitis occurred in each group. These study results support the feasibility of office-based ambulatory CR with a transcervical Foley.
Ausbeck and colleagues randomly assigned 126 nulliparous women with a Bishop score <5, at a gestational age ranging from 39 weeks and 0 days through 41 weeks and 6 days, to outpatient overnight CR or inpatient CR with a transcervical Foley catheter.2 Breech presentation and multiple gestation pregnancies were excluded from the study. The investigators utilized a 16 French Foley catheter and filled the balloon with 30 mL of sterile water. The Foley was taped to the woman’s inner thigh on slight tension. After placement of the Foley catheter at least 20 minutes of CTG monitoring was performed. The women in the outpatient group were given the contact number for the labor unit and advised that they could take acetaminophen for pain. They were advised that they could stay at home if the Foley catheter was expelled. They were admitted to the labor unit at the time scheduled for their IOL.
The mean time from admission to delivery was reduced by 4.3 hours in the outpatient compared with the inpatient CR group (17.4 vs 21.7 hours; P<.01). In the outpatient CR group, 22% of the women were admitted to labor before the time of the scheduled IOL. The cesarean delivery rates were similar in the outpatient and inpatient CR groups (24% vs 33%, P = .32). In the outpatient and inpatient groups, chorioamnionitis was diagnosed in 22% and 13% (P = .16) of the women. The authors concluded that outpatient CR with a transcervical Foley catheter reduced the time from admission to delivery.
Other research groups also have confirmed the feasibility of outpatient CR with a transcervical Foley catheter.3-5
Placement of the Foley catheter can be performed digitally without direct visualization of the cervix or by direct visualization using a vaginal speculum. After placement of the speculum, the cervix is cleansed with a povidone-iodine solution and a sterile ring forceps is used to grasp the catheter and guide it through the cervical os. In one small study, self-reported pain was similar for both digital and direct visualization methods for placement of the balloon catheter.6 When using Foley catheter CR, filling the standard Foley catheter balloon with 60 mL of fluid, rather than 30 to 40 mL of fluid, is rarely associated with balloon rupture and may result in more effective CR.6,7
Continue to: Double-balloon catheter...
Double-balloon catheter
The Cook double-balloon catheter for CR is meant to create pressure on both sides of the cervix, facilitating CR. Studies have reported that the Cook double-balloon catheter can be used for outpatient CR. In one study, 48 women with a low-risk pregnancy, at 37 to 42 weeks’ gestation and a Bishop score <7 were randomly assigned to outpatient or inpatient double-balloon CR.8 Both balloons were filled with 70 to 80 mL of sterile water. CTG monitoring was performed for 20 minutes before and after balloon placement. The women in the outpatient CR group were instructed to return to the labor unit the next day at 8 AM for IOL or earlier if they had regular uterine contractions, rupture of membranes, or vaginal bleeding. Seven percent of the women in the outpatient group returned to the labor unit before 8 AM. After removal of the balloon catheter, women in the outpatient and inpatient groups needed additional misoprostol CR in 12% and 13% of cases, respectively. Outcomes were similar in the two groups, but the study was not powered to identify small differences between the groups.
In another study of outpatient CR with the Cook double-balloon catheter, 695 women with a Bishop score <7, at ≥37 weeks’ gestation, were randomly assigned to outpatient CR with a double-balloon catheter or inpatient CR with dinoprostone (PGE2) (2 mg dinoprostone vaginal gel [Prostin] or dinoprostone 10 mg controlled-release tape (Cervidil).9 Women assigned to dinoprostone CR had CTG monitoring prior to commencing PGE2 CR and at least 30 min of CTG monitoring after insertion of the vaginal PGE2. Women assigned to balloon CR were not admitted to the hospital. CTG was performed prior to insertion of the balloon. After insertion, the two balloons on the catheter were each filled with 80 mL of saline. After catheter insertion CTG monitoring was not routinely performed. The women in the double-balloon catheter group returned to the labor unit 12 hours after insertion to initiate IOL. The primary outcome was composite neonatal morbidity and mortality, including admission to a neonatal intensive care unit (NICU), intubation, cardiac compressions, acidemia, hypoxic ischemic encephalopathy, seizure, infection, pulmonary hypertension, stillbirth, or death.
There was no significant difference in the rate of the primary outcome in the catheter versus the PGE2 group (18.6% and 25.8%; P = .07). Admission to the NICU occurred at rates of 12.6% and 15.5% in the catheter and PGE2 groups. Umbilical cord arterial pH <7.00 at birth occurred at a rate of 3.5% in the catheter group and 9.2% in the PGE2 group. The cesarean delivery rates in the catheter and PGE groups were 32.6% and 25.8%, respectively (P = .24). The investigators concluded that outpatient CR using a double-balloon catheter is safe and feasible for nulliparous women.
Two systematic reviews and meta-analyses reported that outcomes were similar when using the Foley or double-balloon catheter for CR.10,11 The Cook double-balloon CR kit includes a stylet, which can facilitate passing the catheter through the cervix.
Continue to: Dilapan-S and laminaria...
Dilapan-S and laminaria
There are many published studies using Dilapan-S and laminaria for cervical preparation prior to uterine evacuation.12 There are few published studies using Dilapan-S or laminaria for CR prior to IOL. In a pilot study, 21 patients were randomly assigned to outpatient versus inpatient Dilapan-S for CR the night prior to scheduled oxytocin IOL.13 The length of time from initiation of oxytocin to delivery in the outpatient and inpatient groups was similar (11 vs 14 hours, respectively). The outpatient compared with the inpatient group had a shorter length of hospitalization until delivery (51 vs 70 hours).
In other studies of Dilapan-S for CR, the patients remained in the hospital once the dilators were inserted. In one small trial, 41 women were randomized to CR with Dilapan-S or laminaria. As many dilators as could be comfortably tolerated by the patient were inserted.14 The mean numbers of Dilapan-S and laminaria dilators inserted were 4.3 and 9.7, respectively. The morning after the insertion of the dilators, oxytocin IOL was initiated. The times from initiation of oxytocin to delivery for the women in the Dilapan-S and laminaria groups were 11.6 and 15.5 hours, respectively.
An observational study reported on outcomes with Dilapan-S for CR on inpatients.15 In the study 444 women scheduled for IOL at 37 to 40 weeks’ gestation, with a mean baseline Bishop score of 2.9, had Dilapan-S placed for approximately 15 hours prior to oxytocin IOL. The mean number of Dilapan-S dilators that were inserted was 3.8. The study protocol prohibited placing more than 5 cervical dilator devices. The mean Bishop score after removal of the dilators was 6.5. The most common adverse effects of Dilapan-S CR were bleeding (2.7%) and pain (0.2%). The cesarean delivery rate in the cohort was 30.1%. An Apgar score <7 at 5 minutes was recorded for 3 newborns. An umbilical artery pH of <7.10 was observed in 8 newborns.
In a randomized trial performed on inpatients, 419 women undergoing CR were assigned to a Foley balloon or Dilapan-S.16 The vaginal delivery rates were similar in the groups—76% for Foley and 81% for Dilapan-S. Maternal and neonatal adverse effects were similar between the two groups. Compared with Foley catheter, women assigned to Dilapan-S reported greater satisfaction with their CR experience, more sleep, and more ability to perform daily activities.
Misoprostol and dinoprostone
Both misoprostol and dinoprostone are effective for outpatient CR. However, a Cochrane systematic review and meta-analysis concluded that balloon CR, compared with prostaglandin CR, is probably associated with a lower risk of uterine hyperstimulation with concerning fetal heart rate changes.17 Because misoprostol and dinoprostone occasionally can cause uterine hyperstimulation with fetal heart changes, many experts recommend CTG monitoring both before and after administration of misoprostol or dinoprostone for CR.
In a trial of outpatient versus inpatient vaginal PGE2 CR, 425 women at 37 to 42 weeks’ gestation were assigned randomly to outpatient or inpatient CR.18 All women had CTG monitoring for 20 minutes before and after vaginal placement of the PGE2 gel. The PGE2 dose was 2 mg for nulliparous and 1 mg for parous women. The cesarean delivery rates were similar in the outpatient and inpatient groups—22.3% and 22.9%, respectively. Among the women randomized to outpatient CR, 27 women (13%) could not be discharged home after administration of the vaginal PGE2 because of frequent uterine contractions or an abnormal fetal heart rate pattern. In addition, 64 women (30%) in the outpatient group returned to the hospital before scheduled induction because of frequent contractions. Maternal and neonatal complications were similar in the two groups. The investigators concluded that, at the dose and route of prostaglandin utilized in this study, the resultant rates of abnormal fetal heart rate pattern and frequent contractions might reduce the clinical utility of outpatient vaginal prostaglandin CR.
Another study also reported a greater rate of uterine tachysystole with vaginal PGE2 compared with a Foley catheter for CR (9% vs 0%).19 In a Cochrane systematic review of vaginal prostaglandin for CR, compared with placebo, vaginal prostaglandins were associated with a significantly greater rate of uterine hyperstimulation with fetal heart rate changes (4.8% vs 1.0%).20 Other studies also reported the feasibility of outpatient CR with vaginal prostaglandin.21,22
Both oral and vaginal misoprostol have been utilized for outpatient CR. In one study, 87 women with singleton pregnancy at 40 to 42 weeks’ gestation with a Bishop score <6 were randomized to outpatient CR with oral misoprostol (100 µg) or placebo.23 Following administration of the oral misoprostol, the women had 2 hours of CTG monitoring. The treatment was repeated daily for up to 3 days if there was no change in the cervix. If labor occurred, the patient was admitted to the labor unit for oxytocin IOL. The times from first dose of misoprostol or placebo to delivery were 46 and 84 hours (P<.001), respectively.
In another study, 49 women ≥40 weeks’ gestation with a Bishop score <5 were randomly assigned to receive outpatient oral misoprostol 25 µg or 50 µg.24 The dose could be repeated every 3 days over 9 days if ripening or labor had not been achieved. The women had CTG before administration of oral misoprostol. After the misoprostol dose, they had 2 hours of CTG monitoring. The number of doses received by the women assigned to the 50 µg group were 83%, 13%, and 4% for 1, 2, and 3 doses, respectively. The number of doses received by the women assigned to the 25 µg group were 58%, 26%, and 16% for 1, 2, and 3 doses, respectively. The mean intervals from initiation of CR to delivery in the 25 µg and the 50 µg groups were 3.9 and 2.5 days, respectively. The investigators reported no maternal or newborn adverse events, although the study was not powered to detect infrequent events.
Many studies have reported on the feasibility of outpatient CR with vaginal misoprostol.25-30 In one study, 77 women at 40 weeks’ gestation and a Bishop score ≤8 were randomized to a single dose of vaginal misoprostol 25 µg or gentle cervical examination (control).25 The women had 1 hour of CTG monitoring after the intervention. If they had regular contractions they were admitted to the birthing unit. If they had no regular contractions they were discharged home. For nulliparous women, the time from intervention to delivery in the misoprostol group was 4.9 days, and 8.1 days in the control group. For parous women, the times from intervention to delivery in the two groups were 3.8 and 6.9 days, respectively.
Continue to: Inclusion and exclusion criteria for outpatient CR...
Inclusion and exclusion criteria for outpatient CR
Outpatient CR should be limited to low-risk women with a singleton gestation, who have reliable access to transportation from home to the labor unit and have a clear understanding of the instructions for outpatient CR. Patient characteristics that may be utilized to offer office-based CR include:
- singleton pregnancy at 39 weeks’ and 0 days’ gestation through 40 weeks’ and 6 days’ gestation
- cephalic presentation
- Bishop score ≤6.
Women who should be excluded from outpatient CR include those with:
- contraindications to vaginal delivery
- fetal growth restriction
- abnormal umbilical artery Doppler results
- oligo- or polyhydramnios
- multiple gestation
- major fetal anomaly
- recent nonreactive fetal heart rate tracing
- maternal report of decreased fetal movement
- abnormal biophysical profile
- prior cesarean delivery
- recent vaginal bleeding
- gestational diabetes requiring medication treatment
- significant hypertension.
Practices should establish their own inclusion and exclusion criteria for ambulatory CR.
Safety of office-based ambulatory CR among low-risk women
Safety is a complex concept with experts often disagreeing on what level of safety is required to accept a new medical procedure. Establishing the safety of office-based ambulatory CR among low-risk women would require a very large cohort or randomized studies with at least a thousand participants. Only a few large studies focused on the safety of CR have been reported. Sciscione and colleagues reported a large observational study of inpatient transcervical Foley catheter for CR involving 1,905 women.31 They reported no adverse outcomes among term, singleton, uncomplicated pregnancies. They calculated that the 95% confidence interval (CI) for an adverse event was between 0.0% and 0.2%. In a meta-analysis of 26 studies including 5,563 women, the risk of chorioamnionitis during IOL was equivalent with pre-IOL Foley catheter CR (7.2%) or prostaglandin CR (7.2%) (relative risk, 0.96; 95% CI, 0.66–1.38).32
Two systematic reviews have reported that, compared with balloon CR, misoprostol CR is associated with an increased risk of uterine tachysystole.33-34 In a large retrospective study, compared with inpatient CR, outpatient CR with dinoprostone vaginal insert was not associated with an increased risk of newborn admission to the neonatal intensive care unit or a low Apgar score at 5 minutes after birth.35
Will you consider office-based CR in your obstetric practice?
As reviewed in this editorial, evolving data suggest that it is feasible to initiate CR in the office ambulatory setting prior to admission to the labor unit for additional CR or IOL. Many women prefer to complete CR at home after initiation in the office, rather than have CR in a labor unit or hospital setting.36 The transcervical balloon catheter has the most published data supporting the feasibility of ambulatory CR. Compared with misoprostol, the transcervical balloon catheter is associated with a low rate of uterine tachysystole. It may be a preferred method for outpatient CR. If placement of a transcervical balloon catheter is challenging, for example when the patient has a tightly closed cervix, oral misoprostol ambulatory CR may be an option if CTG monitoring is available in the office.
During the COVID pandemic, many in-person office visits have transitioned to virtual visits with the patient in their home. Historically, most cases of CR have been performed on labor and delivery units. It may be time for your practice to consider office-based ambulatory CR for low-risk women planning an IOL. Office-based ambulatory CR is a win for labor nurses who generally prefer to manage laboring patients rather than patients undergoing prolonged in-hospital CR. Outpatient CR is also a win for low-risk patients who prefer to be at home rather than in a labor unit. ●
- Policiano C, Pimenta M, Martins D, et al. Outpatient versus inpatient cervix priming with Foley catheter: a randomized trial. Eur J Obstet Gynecol Repro Biol. 2017;210:1-6.
- Ausbeck EB, Jauk VC, Xue Y, et al. Outpatient Foley catheter for induction of labor in nulliparous women. Obstet Gynecol. 2020;136:597-606.
- Wilkinson C, Adelson P, Turnbull D. A comparison of inpatient with outpatient balloon catheter cervical ripening: a pilot randomized controlled trial. BMC Pregnancy Childbirth. 2015;15:126.
- Sciscione AC, Muench M, Pollock M, et al. Transcervical Foley catheter for preinduction cervical ripening in an outpatient versus inpatient setting. Obstet Gynecol. 2001;98:751-756.
- Henry A, Madan A, Reid R, et al. Outpatient Foley catheter versus inpatient prostaglandin E2 gel for induction of labour: a randomised trial. BMC Pregnancy Childbirth. 2013;13:25.
- Kuhlmann MJ, Spencer N, Garcia-Jasso C, et al. Foley bulb insertion by blind placement compared with direct visualization. Obstet Gynecol. 2021;137:139-145.
- Delaney S, Shaffer BL, Chen YW, et al. Labor induction with a Foley balloon inflated to 30 mL compared with 60 mL. Obstet Gynecol. 2015;115:1239-1245.
- Wilkinson C, Adelson P, Turnbull D. A comparison of inpatient with outpatient balloon catheter cervical ripening: a pilot randomized controlled trial. BMC Pregnancy Childbirth. 2015;15:126.
- Beckmann M, Gibbons K, Flenady V, et al. Induction of labor using prostaglandin E2 as an inpatient versus balloon catheter as an outpatient: a multicenter randomised controlled trial. BJOG. 2020;127:571-579.
- Liu X, Wang Y, Zhange F, et al. Double- versus single-balloon catheters for labour induction and cervical ripening: a meta-analysis. BMC Pregnancy Childbirth. 2019;19:358.
- Yang F, Huan S, Long Y, et al. Double-balloon versus single-balloon catheter for cervical ripening and labor induction: a systematic review and meta-analysis. J Obstet Gynaecol Res. 2018;44: 27-34.
- Goldberg AB, Fortin JA, Drey EA, et al. Cervical preparation before dilation and evacuation using adjunctive misoprostol and mifepristone compared with overnight osmotic dilators alone: a randomized controlled trial. Obstet Gynecol. 2015;126:599-609.
- Upadhyaya NB, Childs KD, Neiger R, et al. Ambulatory cervical ripening in term pregnancy. J Reprod Med. 1999;44:363-366.
- Blumenthal PD, Rmanauskas R. Randomized trial of Dilapan and Laminaria as cervical ripening agents before induction of labor. Obstet Gynecol. 1990;75:365-368.
- Gupta J, Chodankar R, Baev O, et al. Synthetic osmotic dilators in the induction of labour—an international multicenter observational study. Eur J Obstet Gynecol Repro Biol. 2018;229:70-75.
- Saad AF, Villarreal J, Eid J, et al. A randomized controlled trial of Dilapan-S vs Foley balloon for preinduction cervical ripening (DILAFOL trial). Am J Obstet Gynecol. 2019;220:275.e1-e9.
- de Vaan MD, Eikleder MLT, Jozwiak M, et al. Mechanical methods for induction of labour. Cochrane Database Syst Rev. 2019;CD001233.
- Wilkinson C, Bryce R, Adelson P, et al. A randomized controlled trial of outpatient compared with inpatient cervical ripening with prostaglandin E2 (OPRA study). BJOG. 2015;122:94-104.
- Blair R, Harvey MA, Pudwell J, et al. Retrospective comparison of PGE2 vaginal insert and Foley catheter for outpatient cervical ripening. J Obstet Gynaecol Can. 2020;42:1103-1110.
- Thomas J, Fairclough A, Kavanagh J, et al. Vaginal prostaglandin (PGE2 or PGF2alpha) for induction of labour at term. Cochrane Database Syst Rev. 2014;CD003101.
- O’Brien JM, Mercer BM, Cleary NT, et al. Efficacy of outpatient induction with low-dose intravaginal prostaglandin E2: a randomized, doubleblind, placebo controlled trial. Am J Obstet Gynecol. 1995;173:1855-1859.
- Biem SR, Turnell RW, Olatunbosun O, et al. A randomized controlled trial of outpatient versus inpatient labour induction with vaginal controlled-release prostaglandin-E2: effectiveness and satisfaction. J Obstet Gynaecol Can. 2003;25:23-31.
- Gaffaney CA, Saul LL, Rumney PJ, et al. Outpatient oral misoprostol for prolonged pregnancies: a pilot investigation. Am J Perinatol. 2009;26: 673-677.
- Kipikasa JH, Adair CD, Williamson J, et al. Use of misoprostol on an outpatient basis for postdate pregnancy. Int J Gynaecol Obstet. 2005;88:108-111.
- Oboro VO, Tabowei TO. Outpatient misoprostol cervical ripening without subsequent induction of labor to prevent post-term pregnancy. Acta Obstet Gynecol Scand. 2005;84:628-631.
- Stitely ML, Browning J, Fowler M, et al. Outpatient cervical ripening with intravaginal misoprostol. Obstet Gynecol. 2000;96:684-688.
- McKenna DS, Ester JB, Proffitt M, et al. Misoprostol outpatient cervical ripening without subsequent induction of labor: a randomized trial. Obstet Gynecol. 2004;104:579-584.
- PonMalar J, Benjamin SJ, Abraham A, et al. Randomized double-blind placebo controlled study of preinduction cervical priming with 25 µg of misoprostol in the outpatient setting to prevent formal induction of labor. Arch Gynecol Obstet. 2017;295:33-38.
- Chang DW, Velazquez MD, Colyer M, et al. Vaginal misoprostol for cervical ripening at term: comparison of outpatient vs inpatient administration. Obstet Gynecol Surv. 2006;61:167-168.
- Meyer M, Pflum J, Howard D. Outpatient misoprostol compared with dinoprostone gel for preinduction cervical ripening: a randomized controlled trial. Obstet Gynecol. 2005;105:466-472.
- Sciscione AC, Bedder CL, Hoffman MK, et al. The timing of adverse events with Foley catheter preinduction cervical ripening; implications for outpatient use. Am J Perinatol. 2014;31:781-786.
- McMaster K, Sanchez-Ramos L, Kaunitz AM. Evaluation of a transcervical Foley catheter as a source of infection. Obstet Gynecol. 2015;126:539-551.
- Fox NS, Saltzman DH, Roman AS, et al. Intravaginal misoprostol versus Foley catheter for labour induction: a meta-analysis. BJOG. 2011;118: 647-654.
- Hofmeyr GJ, Gulmezoglu AM, Pileggi C. Vaginal misoprostol for cervical ripening and induction of labour. Cochrane Database Syst Rev. 2010:CD000941.
- Salvador SC, Simpson ML, Cundiff GW. Dinoprostone vaginal insert for labour induction: a comparison of outpatient and inpatient settings. J Obstet Gynaecol Can. 2009;31:1028-1034.
- Sutton C, Harding J, Griffin C. Patient attitudes towards outpatient cervical ripening prior to induction of labour at an Australian tertiary hospital. J Obstet Gynaecol. 2016;36:921-928.
For women with a Bishop score ≤6, CR is an important first step in planned induction of labor (IOL). CR is believed to reduce the length of labor induction and increase the probability of a vaginal delivery. Historically, CR has been undertaken on a labor unit. However, with an increased rate of labor induction, the resources of the modern labor unit are incredibly stressed. Compounding the problem is the nursing shortage caused by the COVID-19 pandemic, which has resulted in staff being unavailable as they recover from a respiratory infection or are quarantined after an exposure. The COVID-19 pandemic also has motivated many patients to avoid the hospital as much as possible.
Office-based ambulatory CR is an alternative to inpatient CR and has the potential to reduce the use of labor unit resources. When CR is initiated in the office, the patient either is sent home overnight to return to the labor unit for IOL in the morning or is sent home in the morning to return for IOL in the evening or at night. A secondary benefit of office- and home-based CR is that it may increase patient satisfaction with the process of CR. This editorial summarizes the literature supporting office-based ambulatory CR.
Mechanical methods of CR
Contemporary mechanical methods of CR include the transcervical insertion of a Foley catheter, Cook double-balloon CR catheter, Dilapan-S, or laminaria. There are many publications reporting the feasibility of office-based ambulatory CR with transcervical balloon catheters and very few publications reporting on the use of Dilapan-S or laminaria for ambulatory CR.
Foley catheter
Many studies have investigated the effectiveness of transcervical Foley catheter for ambulatory CR. Policiano and colleagues compared the effectiveness of ambulatory versus inpatient Foley catheter CR.1 A total of 130 women with a Bishop score <6 at ≥41 weeks’ gestation were randomly assigned to outpatient or inpatient CR with a transcervical Foley catheter (Covidian Dover Silicon coated latex Foley catheter 16 Fr/5.3 mm diameter). The Foley catheter bulb was distended with 40 mL of a sterile saline solution. The end of the Foley was taped to the patient’s inner thigh. Manual traction was gently applied to the catheter every 6 hours. If the catheter was extruded, the Bishop score was assessed. For a Bishop score <6, the patient was given additional inpatient misoprostol (25 µg vaginally every 4 hours for up to 5 doses). For a Bishop score ≥6, intravenous oxytocin IOL was initiated. At 24 hours if the Foley catheter was still in situ, it was removed. Women were excluded from the study for the following factors: noncephalic presentation, spontaneous labor, hydramnios, nonreassuring cardiotocography (CTG), multiple pregnancy, ruptured membranes, active vaginal bleeding, Streptococcus group B infection, and HIV infection. Prostaglandin CR was not used if the woman had a previous cesarean delivery. No prophylactic antibiotics were administered. After placement of the Foley catheter, reassuring CTG was documented prior to sending the patient home.
Outpatient, compared with inpatient, CR resulted in a mean reduction of 10 hours in the time from admission to delivery. The time from insertion of the Foley catheter to delivery in the outpatient group was 38.2 hours, and 44.9 hours for the inpatient group (P<.01). The cesarean delivery rates were similar in both groups—28% and 38%, respectively. Three cases of chorioamnionitis occurred in each group. These study results support the feasibility of office-based ambulatory CR with a transcervical Foley.
Ausbeck and colleagues randomly assigned 126 nulliparous women with a Bishop score <5, at a gestational age ranging from 39 weeks and 0 days through 41 weeks and 6 days, to outpatient overnight CR or inpatient CR with a transcervical Foley catheter.2 Breech presentation and multiple gestation pregnancies were excluded from the study. The investigators utilized a 16 French Foley catheter and filled the balloon with 30 mL of sterile water. The Foley was taped to the woman’s inner thigh on slight tension. After placement of the Foley catheter at least 20 minutes of CTG monitoring was performed. The women in the outpatient group were given the contact number for the labor unit and advised that they could take acetaminophen for pain. They were advised that they could stay at home if the Foley catheter was expelled. They were admitted to the labor unit at the time scheduled for their IOL.
The mean time from admission to delivery was reduced by 4.3 hours in the outpatient compared with the inpatient CR group (17.4 vs 21.7 hours; P<.01). In the outpatient CR group, 22% of the women were admitted to labor before the time of the scheduled IOL. The cesarean delivery rates were similar in the outpatient and inpatient CR groups (24% vs 33%, P = .32). In the outpatient and inpatient groups, chorioamnionitis was diagnosed in 22% and 13% (P = .16) of the women. The authors concluded that outpatient CR with a transcervical Foley catheter reduced the time from admission to delivery.
Other research groups also have confirmed the feasibility of outpatient CR with a transcervical Foley catheter.3-5
Placement of the Foley catheter can be performed digitally without direct visualization of the cervix or by direct visualization using a vaginal speculum. After placement of the speculum, the cervix is cleansed with a povidone-iodine solution and a sterile ring forceps is used to grasp the catheter and guide it through the cervical os. In one small study, self-reported pain was similar for both digital and direct visualization methods for placement of the balloon catheter.6 When using Foley catheter CR, filling the standard Foley catheter balloon with 60 mL of fluid, rather than 30 to 40 mL of fluid, is rarely associated with balloon rupture and may result in more effective CR.6,7
Continue to: Double-balloon catheter...
Double-balloon catheter
The Cook double-balloon catheter for CR is meant to create pressure on both sides of the cervix, facilitating CR. Studies have reported that the Cook double-balloon catheter can be used for outpatient CR. In one study, 48 women with a low-risk pregnancy, at 37 to 42 weeks’ gestation and a Bishop score <7 were randomly assigned to outpatient or inpatient double-balloon CR.8 Both balloons were filled with 70 to 80 mL of sterile water. CTG monitoring was performed for 20 minutes before and after balloon placement. The women in the outpatient CR group were instructed to return to the labor unit the next day at 8 AM for IOL or earlier if they had regular uterine contractions, rupture of membranes, or vaginal bleeding. Seven percent of the women in the outpatient group returned to the labor unit before 8 AM. After removal of the balloon catheter, women in the outpatient and inpatient groups needed additional misoprostol CR in 12% and 13% of cases, respectively. Outcomes were similar in the two groups, but the study was not powered to identify small differences between the groups.
In another study of outpatient CR with the Cook double-balloon catheter, 695 women with a Bishop score <7, at ≥37 weeks’ gestation, were randomly assigned to outpatient CR with a double-balloon catheter or inpatient CR with dinoprostone (PGE2) (2 mg dinoprostone vaginal gel [Prostin] or dinoprostone 10 mg controlled-release tape (Cervidil).9 Women assigned to dinoprostone CR had CTG monitoring prior to commencing PGE2 CR and at least 30 min of CTG monitoring after insertion of the vaginal PGE2. Women assigned to balloon CR were not admitted to the hospital. CTG was performed prior to insertion of the balloon. After insertion, the two balloons on the catheter were each filled with 80 mL of saline. After catheter insertion CTG monitoring was not routinely performed. The women in the double-balloon catheter group returned to the labor unit 12 hours after insertion to initiate IOL. The primary outcome was composite neonatal morbidity and mortality, including admission to a neonatal intensive care unit (NICU), intubation, cardiac compressions, acidemia, hypoxic ischemic encephalopathy, seizure, infection, pulmonary hypertension, stillbirth, or death.
There was no significant difference in the rate of the primary outcome in the catheter versus the PGE2 group (18.6% and 25.8%; P = .07). Admission to the NICU occurred at rates of 12.6% and 15.5% in the catheter and PGE2 groups. Umbilical cord arterial pH <7.00 at birth occurred at a rate of 3.5% in the catheter group and 9.2% in the PGE2 group. The cesarean delivery rates in the catheter and PGE groups were 32.6% and 25.8%, respectively (P = .24). The investigators concluded that outpatient CR using a double-balloon catheter is safe and feasible for nulliparous women.
Two systematic reviews and meta-analyses reported that outcomes were similar when using the Foley or double-balloon catheter for CR.10,11 The Cook double-balloon CR kit includes a stylet, which can facilitate passing the catheter through the cervix.
Continue to: Dilapan-S and laminaria...
Dilapan-S and laminaria
There are many published studies using Dilapan-S and laminaria for cervical preparation prior to uterine evacuation.12 There are few published studies using Dilapan-S or laminaria for CR prior to IOL. In a pilot study, 21 patients were randomly assigned to outpatient versus inpatient Dilapan-S for CR the night prior to scheduled oxytocin IOL.13 The length of time from initiation of oxytocin to delivery in the outpatient and inpatient groups was similar (11 vs 14 hours, respectively). The outpatient compared with the inpatient group had a shorter length of hospitalization until delivery (51 vs 70 hours).
In other studies of Dilapan-S for CR, the patients remained in the hospital once the dilators were inserted. In one small trial, 41 women were randomized to CR with Dilapan-S or laminaria. As many dilators as could be comfortably tolerated by the patient were inserted.14 The mean numbers of Dilapan-S and laminaria dilators inserted were 4.3 and 9.7, respectively. The morning after the insertion of the dilators, oxytocin IOL was initiated. The times from initiation of oxytocin to delivery for the women in the Dilapan-S and laminaria groups were 11.6 and 15.5 hours, respectively.
An observational study reported on outcomes with Dilapan-S for CR on inpatients.15 In the study 444 women scheduled for IOL at 37 to 40 weeks’ gestation, with a mean baseline Bishop score of 2.9, had Dilapan-S placed for approximately 15 hours prior to oxytocin IOL. The mean number of Dilapan-S dilators that were inserted was 3.8. The study protocol prohibited placing more than 5 cervical dilator devices. The mean Bishop score after removal of the dilators was 6.5. The most common adverse effects of Dilapan-S CR were bleeding (2.7%) and pain (0.2%). The cesarean delivery rate in the cohort was 30.1%. An Apgar score <7 at 5 minutes was recorded for 3 newborns. An umbilical artery pH of <7.10 was observed in 8 newborns.
In a randomized trial performed on inpatients, 419 women undergoing CR were assigned to a Foley balloon or Dilapan-S.16 The vaginal delivery rates were similar in the groups—76% for Foley and 81% for Dilapan-S. Maternal and neonatal adverse effects were similar between the two groups. Compared with Foley catheter, women assigned to Dilapan-S reported greater satisfaction with their CR experience, more sleep, and more ability to perform daily activities.
Misoprostol and dinoprostone
Both misoprostol and dinoprostone are effective for outpatient CR. However, a Cochrane systematic review and meta-analysis concluded that balloon CR, compared with prostaglandin CR, is probably associated with a lower risk of uterine hyperstimulation with concerning fetal heart rate changes.17 Because misoprostol and dinoprostone occasionally can cause uterine hyperstimulation with fetal heart changes, many experts recommend CTG monitoring both before and after administration of misoprostol or dinoprostone for CR.
In a trial of outpatient versus inpatient vaginal PGE2 CR, 425 women at 37 to 42 weeks’ gestation were assigned randomly to outpatient or inpatient CR.18 All women had CTG monitoring for 20 minutes before and after vaginal placement of the PGE2 gel. The PGE2 dose was 2 mg for nulliparous and 1 mg for parous women. The cesarean delivery rates were similar in the outpatient and inpatient groups—22.3% and 22.9%, respectively. Among the women randomized to outpatient CR, 27 women (13%) could not be discharged home after administration of the vaginal PGE2 because of frequent uterine contractions or an abnormal fetal heart rate pattern. In addition, 64 women (30%) in the outpatient group returned to the hospital before scheduled induction because of frequent contractions. Maternal and neonatal complications were similar in the two groups. The investigators concluded that, at the dose and route of prostaglandin utilized in this study, the resultant rates of abnormal fetal heart rate pattern and frequent contractions might reduce the clinical utility of outpatient vaginal prostaglandin CR.
Another study also reported a greater rate of uterine tachysystole with vaginal PGE2 compared with a Foley catheter for CR (9% vs 0%).19 In a Cochrane systematic review of vaginal prostaglandin for CR, compared with placebo, vaginal prostaglandins were associated with a significantly greater rate of uterine hyperstimulation with fetal heart rate changes (4.8% vs 1.0%).20 Other studies also reported the feasibility of outpatient CR with vaginal prostaglandin.21,22
Both oral and vaginal misoprostol have been utilized for outpatient CR. In one study, 87 women with singleton pregnancy at 40 to 42 weeks’ gestation with a Bishop score <6 were randomized to outpatient CR with oral misoprostol (100 µg) or placebo.23 Following administration of the oral misoprostol, the women had 2 hours of CTG monitoring. The treatment was repeated daily for up to 3 days if there was no change in the cervix. If labor occurred, the patient was admitted to the labor unit for oxytocin IOL. The times from first dose of misoprostol or placebo to delivery were 46 and 84 hours (P<.001), respectively.
In another study, 49 women ≥40 weeks’ gestation with a Bishop score <5 were randomly assigned to receive outpatient oral misoprostol 25 µg or 50 µg.24 The dose could be repeated every 3 days over 9 days if ripening or labor had not been achieved. The women had CTG before administration of oral misoprostol. After the misoprostol dose, they had 2 hours of CTG monitoring. The number of doses received by the women assigned to the 50 µg group were 83%, 13%, and 4% for 1, 2, and 3 doses, respectively. The number of doses received by the women assigned to the 25 µg group were 58%, 26%, and 16% for 1, 2, and 3 doses, respectively. The mean intervals from initiation of CR to delivery in the 25 µg and the 50 µg groups were 3.9 and 2.5 days, respectively. The investigators reported no maternal or newborn adverse events, although the study was not powered to detect infrequent events.
Many studies have reported on the feasibility of outpatient CR with vaginal misoprostol.25-30 In one study, 77 women at 40 weeks’ gestation and a Bishop score ≤8 were randomized to a single dose of vaginal misoprostol 25 µg or gentle cervical examination (control).25 The women had 1 hour of CTG monitoring after the intervention. If they had regular contractions they were admitted to the birthing unit. If they had no regular contractions they were discharged home. For nulliparous women, the time from intervention to delivery in the misoprostol group was 4.9 days, and 8.1 days in the control group. For parous women, the times from intervention to delivery in the two groups were 3.8 and 6.9 days, respectively.
Continue to: Inclusion and exclusion criteria for outpatient CR...
Inclusion and exclusion criteria for outpatient CR
Outpatient CR should be limited to low-risk women with a singleton gestation, who have reliable access to transportation from home to the labor unit and have a clear understanding of the instructions for outpatient CR. Patient characteristics that may be utilized to offer office-based CR include:
- singleton pregnancy at 39 weeks’ and 0 days’ gestation through 40 weeks’ and 6 days’ gestation
- cephalic presentation
- Bishop score ≤6.
Women who should be excluded from outpatient CR include those with:
- contraindications to vaginal delivery
- fetal growth restriction
- abnormal umbilical artery Doppler results
- oligo- or polyhydramnios
- multiple gestation
- major fetal anomaly
- recent nonreactive fetal heart rate tracing
- maternal report of decreased fetal movement
- abnormal biophysical profile
- prior cesarean delivery
- recent vaginal bleeding
- gestational diabetes requiring medication treatment
- significant hypertension.
Practices should establish their own inclusion and exclusion criteria for ambulatory CR.
Safety of office-based ambulatory CR among low-risk women
Safety is a complex concept with experts often disagreeing on what level of safety is required to accept a new medical procedure. Establishing the safety of office-based ambulatory CR among low-risk women would require a very large cohort or randomized studies with at least a thousand participants. Only a few large studies focused on the safety of CR have been reported. Sciscione and colleagues reported a large observational study of inpatient transcervical Foley catheter for CR involving 1,905 women.31 They reported no adverse outcomes among term, singleton, uncomplicated pregnancies. They calculated that the 95% confidence interval (CI) for an adverse event was between 0.0% and 0.2%. In a meta-analysis of 26 studies including 5,563 women, the risk of chorioamnionitis during IOL was equivalent with pre-IOL Foley catheter CR (7.2%) or prostaglandin CR (7.2%) (relative risk, 0.96; 95% CI, 0.66–1.38).32
Two systematic reviews have reported that, compared with balloon CR, misoprostol CR is associated with an increased risk of uterine tachysystole.33-34 In a large retrospective study, compared with inpatient CR, outpatient CR with dinoprostone vaginal insert was not associated with an increased risk of newborn admission to the neonatal intensive care unit or a low Apgar score at 5 minutes after birth.35
Will you consider office-based CR in your obstetric practice?
As reviewed in this editorial, evolving data suggest that it is feasible to initiate CR in the office ambulatory setting prior to admission to the labor unit for additional CR or IOL. Many women prefer to complete CR at home after initiation in the office, rather than have CR in a labor unit or hospital setting.36 The transcervical balloon catheter has the most published data supporting the feasibility of ambulatory CR. Compared with misoprostol, the transcervical balloon catheter is associated with a low rate of uterine tachysystole. It may be a preferred method for outpatient CR. If placement of a transcervical balloon catheter is challenging, for example when the patient has a tightly closed cervix, oral misoprostol ambulatory CR may be an option if CTG monitoring is available in the office.
During the COVID pandemic, many in-person office visits have transitioned to virtual visits with the patient in their home. Historically, most cases of CR have been performed on labor and delivery units. It may be time for your practice to consider office-based ambulatory CR for low-risk women planning an IOL. Office-based ambulatory CR is a win for labor nurses who generally prefer to manage laboring patients rather than patients undergoing prolonged in-hospital CR. Outpatient CR is also a win for low-risk patients who prefer to be at home rather than in a labor unit. ●
For women with a Bishop score ≤6, CR is an important first step in planned induction of labor (IOL). CR is believed to reduce the length of labor induction and increase the probability of a vaginal delivery. Historically, CR has been undertaken on a labor unit. However, with an increased rate of labor induction, the resources of the modern labor unit are incredibly stressed. Compounding the problem is the nursing shortage caused by the COVID-19 pandemic, which has resulted in staff being unavailable as they recover from a respiratory infection or are quarantined after an exposure. The COVID-19 pandemic also has motivated many patients to avoid the hospital as much as possible.
Office-based ambulatory CR is an alternative to inpatient CR and has the potential to reduce the use of labor unit resources. When CR is initiated in the office, the patient either is sent home overnight to return to the labor unit for IOL in the morning or is sent home in the morning to return for IOL in the evening or at night. A secondary benefit of office- and home-based CR is that it may increase patient satisfaction with the process of CR. This editorial summarizes the literature supporting office-based ambulatory CR.
Mechanical methods of CR
Contemporary mechanical methods of CR include the transcervical insertion of a Foley catheter, Cook double-balloon CR catheter, Dilapan-S, or laminaria. There are many publications reporting the feasibility of office-based ambulatory CR with transcervical balloon catheters and very few publications reporting on the use of Dilapan-S or laminaria for ambulatory CR.
Foley catheter
Many studies have investigated the effectiveness of transcervical Foley catheter for ambulatory CR. Policiano and colleagues compared the effectiveness of ambulatory versus inpatient Foley catheter CR.1 A total of 130 women with a Bishop score <6 at ≥41 weeks’ gestation were randomly assigned to outpatient or inpatient CR with a transcervical Foley catheter (Covidian Dover Silicon coated latex Foley catheter 16 Fr/5.3 mm diameter). The Foley catheter bulb was distended with 40 mL of a sterile saline solution. The end of the Foley was taped to the patient’s inner thigh. Manual traction was gently applied to the catheter every 6 hours. If the catheter was extruded, the Bishop score was assessed. For a Bishop score <6, the patient was given additional inpatient misoprostol (25 µg vaginally every 4 hours for up to 5 doses). For a Bishop score ≥6, intravenous oxytocin IOL was initiated. At 24 hours if the Foley catheter was still in situ, it was removed. Women were excluded from the study for the following factors: noncephalic presentation, spontaneous labor, hydramnios, nonreassuring cardiotocography (CTG), multiple pregnancy, ruptured membranes, active vaginal bleeding, Streptococcus group B infection, and HIV infection. Prostaglandin CR was not used if the woman had a previous cesarean delivery. No prophylactic antibiotics were administered. After placement of the Foley catheter, reassuring CTG was documented prior to sending the patient home.
Outpatient, compared with inpatient, CR resulted in a mean reduction of 10 hours in the time from admission to delivery. The time from insertion of the Foley catheter to delivery in the outpatient group was 38.2 hours, and 44.9 hours for the inpatient group (P<.01). The cesarean delivery rates were similar in both groups—28% and 38%, respectively. Three cases of chorioamnionitis occurred in each group. These study results support the feasibility of office-based ambulatory CR with a transcervical Foley.
Ausbeck and colleagues randomly assigned 126 nulliparous women with a Bishop score <5, at a gestational age ranging from 39 weeks and 0 days through 41 weeks and 6 days, to outpatient overnight CR or inpatient CR with a transcervical Foley catheter.2 Breech presentation and multiple gestation pregnancies were excluded from the study. The investigators utilized a 16 French Foley catheter and filled the balloon with 30 mL of sterile water. The Foley was taped to the woman’s inner thigh on slight tension. After placement of the Foley catheter at least 20 minutes of CTG monitoring was performed. The women in the outpatient group were given the contact number for the labor unit and advised that they could take acetaminophen for pain. They were advised that they could stay at home if the Foley catheter was expelled. They were admitted to the labor unit at the time scheduled for their IOL.
The mean time from admission to delivery was reduced by 4.3 hours in the outpatient compared with the inpatient CR group (17.4 vs 21.7 hours; P<.01). In the outpatient CR group, 22% of the women were admitted to labor before the time of the scheduled IOL. The cesarean delivery rates were similar in the outpatient and inpatient CR groups (24% vs 33%, P = .32). In the outpatient and inpatient groups, chorioamnionitis was diagnosed in 22% and 13% (P = .16) of the women. The authors concluded that outpatient CR with a transcervical Foley catheter reduced the time from admission to delivery.
Other research groups also have confirmed the feasibility of outpatient CR with a transcervical Foley catheter.3-5
Placement of the Foley catheter can be performed digitally without direct visualization of the cervix or by direct visualization using a vaginal speculum. After placement of the speculum, the cervix is cleansed with a povidone-iodine solution and a sterile ring forceps is used to grasp the catheter and guide it through the cervical os. In one small study, self-reported pain was similar for both digital and direct visualization methods for placement of the balloon catheter.6 When using Foley catheter CR, filling the standard Foley catheter balloon with 60 mL of fluid, rather than 30 to 40 mL of fluid, is rarely associated with balloon rupture and may result in more effective CR.6,7
Continue to: Double-balloon catheter...
Double-balloon catheter
The Cook double-balloon catheter for CR is meant to create pressure on both sides of the cervix, facilitating CR. Studies have reported that the Cook double-balloon catheter can be used for outpatient CR. In one study, 48 women with a low-risk pregnancy, at 37 to 42 weeks’ gestation and a Bishop score <7 were randomly assigned to outpatient or inpatient double-balloon CR.8 Both balloons were filled with 70 to 80 mL of sterile water. CTG monitoring was performed for 20 minutes before and after balloon placement. The women in the outpatient CR group were instructed to return to the labor unit the next day at 8 AM for IOL or earlier if they had regular uterine contractions, rupture of membranes, or vaginal bleeding. Seven percent of the women in the outpatient group returned to the labor unit before 8 AM. After removal of the balloon catheter, women in the outpatient and inpatient groups needed additional misoprostol CR in 12% and 13% of cases, respectively. Outcomes were similar in the two groups, but the study was not powered to identify small differences between the groups.
In another study of outpatient CR with the Cook double-balloon catheter, 695 women with a Bishop score <7, at ≥37 weeks’ gestation, were randomly assigned to outpatient CR with a double-balloon catheter or inpatient CR with dinoprostone (PGE2) (2 mg dinoprostone vaginal gel [Prostin] or dinoprostone 10 mg controlled-release tape (Cervidil).9 Women assigned to dinoprostone CR had CTG monitoring prior to commencing PGE2 CR and at least 30 min of CTG monitoring after insertion of the vaginal PGE2. Women assigned to balloon CR were not admitted to the hospital. CTG was performed prior to insertion of the balloon. After insertion, the two balloons on the catheter were each filled with 80 mL of saline. After catheter insertion CTG monitoring was not routinely performed. The women in the double-balloon catheter group returned to the labor unit 12 hours after insertion to initiate IOL. The primary outcome was composite neonatal morbidity and mortality, including admission to a neonatal intensive care unit (NICU), intubation, cardiac compressions, acidemia, hypoxic ischemic encephalopathy, seizure, infection, pulmonary hypertension, stillbirth, or death.
There was no significant difference in the rate of the primary outcome in the catheter versus the PGE2 group (18.6% and 25.8%; P = .07). Admission to the NICU occurred at rates of 12.6% and 15.5% in the catheter and PGE2 groups. Umbilical cord arterial pH <7.00 at birth occurred at a rate of 3.5% in the catheter group and 9.2% in the PGE2 group. The cesarean delivery rates in the catheter and PGE groups were 32.6% and 25.8%, respectively (P = .24). The investigators concluded that outpatient CR using a double-balloon catheter is safe and feasible for nulliparous women.
Two systematic reviews and meta-analyses reported that outcomes were similar when using the Foley or double-balloon catheter for CR.10,11 The Cook double-balloon CR kit includes a stylet, which can facilitate passing the catheter through the cervix.
Continue to: Dilapan-S and laminaria...
Dilapan-S and laminaria
There are many published studies using Dilapan-S and laminaria for cervical preparation prior to uterine evacuation.12 There are few published studies using Dilapan-S or laminaria for CR prior to IOL. In a pilot study, 21 patients were randomly assigned to outpatient versus inpatient Dilapan-S for CR the night prior to scheduled oxytocin IOL.13 The length of time from initiation of oxytocin to delivery in the outpatient and inpatient groups was similar (11 vs 14 hours, respectively). The outpatient compared with the inpatient group had a shorter length of hospitalization until delivery (51 vs 70 hours).
In other studies of Dilapan-S for CR, the patients remained in the hospital once the dilators were inserted. In one small trial, 41 women were randomized to CR with Dilapan-S or laminaria. As many dilators as could be comfortably tolerated by the patient were inserted.14 The mean numbers of Dilapan-S and laminaria dilators inserted were 4.3 and 9.7, respectively. The morning after the insertion of the dilators, oxytocin IOL was initiated. The times from initiation of oxytocin to delivery for the women in the Dilapan-S and laminaria groups were 11.6 and 15.5 hours, respectively.
An observational study reported on outcomes with Dilapan-S for CR on inpatients.15 In the study 444 women scheduled for IOL at 37 to 40 weeks’ gestation, with a mean baseline Bishop score of 2.9, had Dilapan-S placed for approximately 15 hours prior to oxytocin IOL. The mean number of Dilapan-S dilators that were inserted was 3.8. The study protocol prohibited placing more than 5 cervical dilator devices. The mean Bishop score after removal of the dilators was 6.5. The most common adverse effects of Dilapan-S CR were bleeding (2.7%) and pain (0.2%). The cesarean delivery rate in the cohort was 30.1%. An Apgar score <7 at 5 minutes was recorded for 3 newborns. An umbilical artery pH of <7.10 was observed in 8 newborns.
In a randomized trial performed on inpatients, 419 women undergoing CR were assigned to a Foley balloon or Dilapan-S.16 The vaginal delivery rates were similar in the groups—76% for Foley and 81% for Dilapan-S. Maternal and neonatal adverse effects were similar between the two groups. Compared with Foley catheter, women assigned to Dilapan-S reported greater satisfaction with their CR experience, more sleep, and more ability to perform daily activities.
Misoprostol and dinoprostone
Both misoprostol and dinoprostone are effective for outpatient CR. However, a Cochrane systematic review and meta-analysis concluded that balloon CR, compared with prostaglandin CR, is probably associated with a lower risk of uterine hyperstimulation with concerning fetal heart rate changes.17 Because misoprostol and dinoprostone occasionally can cause uterine hyperstimulation with fetal heart changes, many experts recommend CTG monitoring both before and after administration of misoprostol or dinoprostone for CR.
In a trial of outpatient versus inpatient vaginal PGE2 CR, 425 women at 37 to 42 weeks’ gestation were assigned randomly to outpatient or inpatient CR.18 All women had CTG monitoring for 20 minutes before and after vaginal placement of the PGE2 gel. The PGE2 dose was 2 mg for nulliparous and 1 mg for parous women. The cesarean delivery rates were similar in the outpatient and inpatient groups—22.3% and 22.9%, respectively. Among the women randomized to outpatient CR, 27 women (13%) could not be discharged home after administration of the vaginal PGE2 because of frequent uterine contractions or an abnormal fetal heart rate pattern. In addition, 64 women (30%) in the outpatient group returned to the hospital before scheduled induction because of frequent contractions. Maternal and neonatal complications were similar in the two groups. The investigators concluded that, at the dose and route of prostaglandin utilized in this study, the resultant rates of abnormal fetal heart rate pattern and frequent contractions might reduce the clinical utility of outpatient vaginal prostaglandin CR.
Another study also reported a greater rate of uterine tachysystole with vaginal PGE2 compared with a Foley catheter for CR (9% vs 0%).19 In a Cochrane systematic review of vaginal prostaglandin for CR, compared with placebo, vaginal prostaglandins were associated with a significantly greater rate of uterine hyperstimulation with fetal heart rate changes (4.8% vs 1.0%).20 Other studies also reported the feasibility of outpatient CR with vaginal prostaglandin.21,22
Both oral and vaginal misoprostol have been utilized for outpatient CR. In one study, 87 women with singleton pregnancy at 40 to 42 weeks’ gestation with a Bishop score <6 were randomized to outpatient CR with oral misoprostol (100 µg) or placebo.23 Following administration of the oral misoprostol, the women had 2 hours of CTG monitoring. The treatment was repeated daily for up to 3 days if there was no change in the cervix. If labor occurred, the patient was admitted to the labor unit for oxytocin IOL. The times from first dose of misoprostol or placebo to delivery were 46 and 84 hours (P<.001), respectively.
In another study, 49 women ≥40 weeks’ gestation with a Bishop score <5 were randomly assigned to receive outpatient oral misoprostol 25 µg or 50 µg.24 The dose could be repeated every 3 days over 9 days if ripening or labor had not been achieved. The women had CTG before administration of oral misoprostol. After the misoprostol dose, they had 2 hours of CTG monitoring. The number of doses received by the women assigned to the 50 µg group were 83%, 13%, and 4% for 1, 2, and 3 doses, respectively. The number of doses received by the women assigned to the 25 µg group were 58%, 26%, and 16% for 1, 2, and 3 doses, respectively. The mean intervals from initiation of CR to delivery in the 25 µg and the 50 µg groups were 3.9 and 2.5 days, respectively. The investigators reported no maternal or newborn adverse events, although the study was not powered to detect infrequent events.
Many studies have reported on the feasibility of outpatient CR with vaginal misoprostol.25-30 In one study, 77 women at 40 weeks’ gestation and a Bishop score ≤8 were randomized to a single dose of vaginal misoprostol 25 µg or gentle cervical examination (control).25 The women had 1 hour of CTG monitoring after the intervention. If they had regular contractions they were admitted to the birthing unit. If they had no regular contractions they were discharged home. For nulliparous women, the time from intervention to delivery in the misoprostol group was 4.9 days, and 8.1 days in the control group. For parous women, the times from intervention to delivery in the two groups were 3.8 and 6.9 days, respectively.
Continue to: Inclusion and exclusion criteria for outpatient CR...
Inclusion and exclusion criteria for outpatient CR
Outpatient CR should be limited to low-risk women with a singleton gestation, who have reliable access to transportation from home to the labor unit and have a clear understanding of the instructions for outpatient CR. Patient characteristics that may be utilized to offer office-based CR include:
- singleton pregnancy at 39 weeks’ and 0 days’ gestation through 40 weeks’ and 6 days’ gestation
- cephalic presentation
- Bishop score ≤6.
Women who should be excluded from outpatient CR include those with:
- contraindications to vaginal delivery
- fetal growth restriction
- abnormal umbilical artery Doppler results
- oligo- or polyhydramnios
- multiple gestation
- major fetal anomaly
- recent nonreactive fetal heart rate tracing
- maternal report of decreased fetal movement
- abnormal biophysical profile
- prior cesarean delivery
- recent vaginal bleeding
- gestational diabetes requiring medication treatment
- significant hypertension.
Practices should establish their own inclusion and exclusion criteria for ambulatory CR.
Safety of office-based ambulatory CR among low-risk women
Safety is a complex concept with experts often disagreeing on what level of safety is required to accept a new medical procedure. Establishing the safety of office-based ambulatory CR among low-risk women would require a very large cohort or randomized studies with at least a thousand participants. Only a few large studies focused on the safety of CR have been reported. Sciscione and colleagues reported a large observational study of inpatient transcervical Foley catheter for CR involving 1,905 women.31 They reported no adverse outcomes among term, singleton, uncomplicated pregnancies. They calculated that the 95% confidence interval (CI) for an adverse event was between 0.0% and 0.2%. In a meta-analysis of 26 studies including 5,563 women, the risk of chorioamnionitis during IOL was equivalent with pre-IOL Foley catheter CR (7.2%) or prostaglandin CR (7.2%) (relative risk, 0.96; 95% CI, 0.66–1.38).32
Two systematic reviews have reported that, compared with balloon CR, misoprostol CR is associated with an increased risk of uterine tachysystole.33-34 In a large retrospective study, compared with inpatient CR, outpatient CR with dinoprostone vaginal insert was not associated with an increased risk of newborn admission to the neonatal intensive care unit or a low Apgar score at 5 minutes after birth.35
Will you consider office-based CR in your obstetric practice?
As reviewed in this editorial, evolving data suggest that it is feasible to initiate CR in the office ambulatory setting prior to admission to the labor unit for additional CR or IOL. Many women prefer to complete CR at home after initiation in the office, rather than have CR in a labor unit or hospital setting.36 The transcervical balloon catheter has the most published data supporting the feasibility of ambulatory CR. Compared with misoprostol, the transcervical balloon catheter is associated with a low rate of uterine tachysystole. It may be a preferred method for outpatient CR. If placement of a transcervical balloon catheter is challenging, for example when the patient has a tightly closed cervix, oral misoprostol ambulatory CR may be an option if CTG monitoring is available in the office.
During the COVID pandemic, many in-person office visits have transitioned to virtual visits with the patient in their home. Historically, most cases of CR have been performed on labor and delivery units. It may be time for your practice to consider office-based ambulatory CR for low-risk women planning an IOL. Office-based ambulatory CR is a win for labor nurses who generally prefer to manage laboring patients rather than patients undergoing prolonged in-hospital CR. Outpatient CR is also a win for low-risk patients who prefer to be at home rather than in a labor unit. ●
- Policiano C, Pimenta M, Martins D, et al. Outpatient versus inpatient cervix priming with Foley catheter: a randomized trial. Eur J Obstet Gynecol Repro Biol. 2017;210:1-6.
- Ausbeck EB, Jauk VC, Xue Y, et al. Outpatient Foley catheter for induction of labor in nulliparous women. Obstet Gynecol. 2020;136:597-606.
- Wilkinson C, Adelson P, Turnbull D. A comparison of inpatient with outpatient balloon catheter cervical ripening: a pilot randomized controlled trial. BMC Pregnancy Childbirth. 2015;15:126.
- Sciscione AC, Muench M, Pollock M, et al. Transcervical Foley catheter for preinduction cervical ripening in an outpatient versus inpatient setting. Obstet Gynecol. 2001;98:751-756.
- Henry A, Madan A, Reid R, et al. Outpatient Foley catheter versus inpatient prostaglandin E2 gel for induction of labour: a randomised trial. BMC Pregnancy Childbirth. 2013;13:25.
- Kuhlmann MJ, Spencer N, Garcia-Jasso C, et al. Foley bulb insertion by blind placement compared with direct visualization. Obstet Gynecol. 2021;137:139-145.
- Delaney S, Shaffer BL, Chen YW, et al. Labor induction with a Foley balloon inflated to 30 mL compared with 60 mL. Obstet Gynecol. 2015;115:1239-1245.
- Wilkinson C, Adelson P, Turnbull D. A comparison of inpatient with outpatient balloon catheter cervical ripening: a pilot randomized controlled trial. BMC Pregnancy Childbirth. 2015;15:126.
- Beckmann M, Gibbons K, Flenady V, et al. Induction of labor using prostaglandin E2 as an inpatient versus balloon catheter as an outpatient: a multicenter randomised controlled trial. BJOG. 2020;127:571-579.
- Liu X, Wang Y, Zhange F, et al. Double- versus single-balloon catheters for labour induction and cervical ripening: a meta-analysis. BMC Pregnancy Childbirth. 2019;19:358.
- Yang F, Huan S, Long Y, et al. Double-balloon versus single-balloon catheter for cervical ripening and labor induction: a systematic review and meta-analysis. J Obstet Gynaecol Res. 2018;44: 27-34.
- Goldberg AB, Fortin JA, Drey EA, et al. Cervical preparation before dilation and evacuation using adjunctive misoprostol and mifepristone compared with overnight osmotic dilators alone: a randomized controlled trial. Obstet Gynecol. 2015;126:599-609.
- Upadhyaya NB, Childs KD, Neiger R, et al. Ambulatory cervical ripening in term pregnancy. J Reprod Med. 1999;44:363-366.
- Blumenthal PD, Rmanauskas R. Randomized trial of Dilapan and Laminaria as cervical ripening agents before induction of labor. Obstet Gynecol. 1990;75:365-368.
- Gupta J, Chodankar R, Baev O, et al. Synthetic osmotic dilators in the induction of labour—an international multicenter observational study. Eur J Obstet Gynecol Repro Biol. 2018;229:70-75.
- Saad AF, Villarreal J, Eid J, et al. A randomized controlled trial of Dilapan-S vs Foley balloon for preinduction cervical ripening (DILAFOL trial). Am J Obstet Gynecol. 2019;220:275.e1-e9.
- de Vaan MD, Eikleder MLT, Jozwiak M, et al. Mechanical methods for induction of labour. Cochrane Database Syst Rev. 2019;CD001233.
- Wilkinson C, Bryce R, Adelson P, et al. A randomized controlled trial of outpatient compared with inpatient cervical ripening with prostaglandin E2 (OPRA study). BJOG. 2015;122:94-104.
- Blair R, Harvey MA, Pudwell J, et al. Retrospective comparison of PGE2 vaginal insert and Foley catheter for outpatient cervical ripening. J Obstet Gynaecol Can. 2020;42:1103-1110.
- Thomas J, Fairclough A, Kavanagh J, et al. Vaginal prostaglandin (PGE2 or PGF2alpha) for induction of labour at term. Cochrane Database Syst Rev. 2014;CD003101.
- O’Brien JM, Mercer BM, Cleary NT, et al. Efficacy of outpatient induction with low-dose intravaginal prostaglandin E2: a randomized, doubleblind, placebo controlled trial. Am J Obstet Gynecol. 1995;173:1855-1859.
- Biem SR, Turnell RW, Olatunbosun O, et al. A randomized controlled trial of outpatient versus inpatient labour induction with vaginal controlled-release prostaglandin-E2: effectiveness and satisfaction. J Obstet Gynaecol Can. 2003;25:23-31.
- Gaffaney CA, Saul LL, Rumney PJ, et al. Outpatient oral misoprostol for prolonged pregnancies: a pilot investigation. Am J Perinatol. 2009;26: 673-677.
- Kipikasa JH, Adair CD, Williamson J, et al. Use of misoprostol on an outpatient basis for postdate pregnancy. Int J Gynaecol Obstet. 2005;88:108-111.
- Oboro VO, Tabowei TO. Outpatient misoprostol cervical ripening without subsequent induction of labor to prevent post-term pregnancy. Acta Obstet Gynecol Scand. 2005;84:628-631.
- Stitely ML, Browning J, Fowler M, et al. Outpatient cervical ripening with intravaginal misoprostol. Obstet Gynecol. 2000;96:684-688.
- McKenna DS, Ester JB, Proffitt M, et al. Misoprostol outpatient cervical ripening without subsequent induction of labor: a randomized trial. Obstet Gynecol. 2004;104:579-584.
- PonMalar J, Benjamin SJ, Abraham A, et al. Randomized double-blind placebo controlled study of preinduction cervical priming with 25 µg of misoprostol in the outpatient setting to prevent formal induction of labor. Arch Gynecol Obstet. 2017;295:33-38.
- Chang DW, Velazquez MD, Colyer M, et al. Vaginal misoprostol for cervical ripening at term: comparison of outpatient vs inpatient administration. Obstet Gynecol Surv. 2006;61:167-168.
- Meyer M, Pflum J, Howard D. Outpatient misoprostol compared with dinoprostone gel for preinduction cervical ripening: a randomized controlled trial. Obstet Gynecol. 2005;105:466-472.
- Sciscione AC, Bedder CL, Hoffman MK, et al. The timing of adverse events with Foley catheter preinduction cervical ripening; implications for outpatient use. Am J Perinatol. 2014;31:781-786.
- McMaster K, Sanchez-Ramos L, Kaunitz AM. Evaluation of a transcervical Foley catheter as a source of infection. Obstet Gynecol. 2015;126:539-551.
- Fox NS, Saltzman DH, Roman AS, et al. Intravaginal misoprostol versus Foley catheter for labour induction: a meta-analysis. BJOG. 2011;118: 647-654.
- Hofmeyr GJ, Gulmezoglu AM, Pileggi C. Vaginal misoprostol for cervical ripening and induction of labour. Cochrane Database Syst Rev. 2010:CD000941.
- Salvador SC, Simpson ML, Cundiff GW. Dinoprostone vaginal insert for labour induction: a comparison of outpatient and inpatient settings. J Obstet Gynaecol Can. 2009;31:1028-1034.
- Sutton C, Harding J, Griffin C. Patient attitudes towards outpatient cervical ripening prior to induction of labour at an Australian tertiary hospital. J Obstet Gynaecol. 2016;36:921-928.
- Policiano C, Pimenta M, Martins D, et al. Outpatient versus inpatient cervix priming with Foley catheter: a randomized trial. Eur J Obstet Gynecol Repro Biol. 2017;210:1-6.
- Ausbeck EB, Jauk VC, Xue Y, et al. Outpatient Foley catheter for induction of labor in nulliparous women. Obstet Gynecol. 2020;136:597-606.
- Wilkinson C, Adelson P, Turnbull D. A comparison of inpatient with outpatient balloon catheter cervical ripening: a pilot randomized controlled trial. BMC Pregnancy Childbirth. 2015;15:126.
- Sciscione AC, Muench M, Pollock M, et al. Transcervical Foley catheter for preinduction cervical ripening in an outpatient versus inpatient setting. Obstet Gynecol. 2001;98:751-756.
- Henry A, Madan A, Reid R, et al. Outpatient Foley catheter versus inpatient prostaglandin E2 gel for induction of labour: a randomised trial. BMC Pregnancy Childbirth. 2013;13:25.
- Kuhlmann MJ, Spencer N, Garcia-Jasso C, et al. Foley bulb insertion by blind placement compared with direct visualization. Obstet Gynecol. 2021;137:139-145.
- Delaney S, Shaffer BL, Chen YW, et al. Labor induction with a Foley balloon inflated to 30 mL compared with 60 mL. Obstet Gynecol. 2015;115:1239-1245.
- Wilkinson C, Adelson P, Turnbull D. A comparison of inpatient with outpatient balloon catheter cervical ripening: a pilot randomized controlled trial. BMC Pregnancy Childbirth. 2015;15:126.
- Beckmann M, Gibbons K, Flenady V, et al. Induction of labor using prostaglandin E2 as an inpatient versus balloon catheter as an outpatient: a multicenter randomised controlled trial. BJOG. 2020;127:571-579.
- Liu X, Wang Y, Zhange F, et al. Double- versus single-balloon catheters for labour induction and cervical ripening: a meta-analysis. BMC Pregnancy Childbirth. 2019;19:358.
- Yang F, Huan S, Long Y, et al. Double-balloon versus single-balloon catheter for cervical ripening and labor induction: a systematic review and meta-analysis. J Obstet Gynaecol Res. 2018;44: 27-34.
- Goldberg AB, Fortin JA, Drey EA, et al. Cervical preparation before dilation and evacuation using adjunctive misoprostol and mifepristone compared with overnight osmotic dilators alone: a randomized controlled trial. Obstet Gynecol. 2015;126:599-609.
- Upadhyaya NB, Childs KD, Neiger R, et al. Ambulatory cervical ripening in term pregnancy. J Reprod Med. 1999;44:363-366.
- Blumenthal PD, Rmanauskas R. Randomized trial of Dilapan and Laminaria as cervical ripening agents before induction of labor. Obstet Gynecol. 1990;75:365-368.
- Gupta J, Chodankar R, Baev O, et al. Synthetic osmotic dilators in the induction of labour—an international multicenter observational study. Eur J Obstet Gynecol Repro Biol. 2018;229:70-75.
- Saad AF, Villarreal J, Eid J, et al. A randomized controlled trial of Dilapan-S vs Foley balloon for preinduction cervical ripening (DILAFOL trial). Am J Obstet Gynecol. 2019;220:275.e1-e9.
- de Vaan MD, Eikleder MLT, Jozwiak M, et al. Mechanical methods for induction of labour. Cochrane Database Syst Rev. 2019;CD001233.
- Wilkinson C, Bryce R, Adelson P, et al. A randomized controlled trial of outpatient compared with inpatient cervical ripening with prostaglandin E2 (OPRA study). BJOG. 2015;122:94-104.
- Blair R, Harvey MA, Pudwell J, et al. Retrospective comparison of PGE2 vaginal insert and Foley catheter for outpatient cervical ripening. J Obstet Gynaecol Can. 2020;42:1103-1110.
- Thomas J, Fairclough A, Kavanagh J, et al. Vaginal prostaglandin (PGE2 or PGF2alpha) for induction of labour at term. Cochrane Database Syst Rev. 2014;CD003101.
- O’Brien JM, Mercer BM, Cleary NT, et al. Efficacy of outpatient induction with low-dose intravaginal prostaglandin E2: a randomized, doubleblind, placebo controlled trial. Am J Obstet Gynecol. 1995;173:1855-1859.
- Biem SR, Turnell RW, Olatunbosun O, et al. A randomized controlled trial of outpatient versus inpatient labour induction with vaginal controlled-release prostaglandin-E2: effectiveness and satisfaction. J Obstet Gynaecol Can. 2003;25:23-31.
- Gaffaney CA, Saul LL, Rumney PJ, et al. Outpatient oral misoprostol for prolonged pregnancies: a pilot investigation. Am J Perinatol. 2009;26: 673-677.
- Kipikasa JH, Adair CD, Williamson J, et al. Use of misoprostol on an outpatient basis for postdate pregnancy. Int J Gynaecol Obstet. 2005;88:108-111.
- Oboro VO, Tabowei TO. Outpatient misoprostol cervical ripening without subsequent induction of labor to prevent post-term pregnancy. Acta Obstet Gynecol Scand. 2005;84:628-631.
- Stitely ML, Browning J, Fowler M, et al. Outpatient cervical ripening with intravaginal misoprostol. Obstet Gynecol. 2000;96:684-688.
- McKenna DS, Ester JB, Proffitt M, et al. Misoprostol outpatient cervical ripening without subsequent induction of labor: a randomized trial. Obstet Gynecol. 2004;104:579-584.
- PonMalar J, Benjamin SJ, Abraham A, et al. Randomized double-blind placebo controlled study of preinduction cervical priming with 25 µg of misoprostol in the outpatient setting to prevent formal induction of labor. Arch Gynecol Obstet. 2017;295:33-38.
- Chang DW, Velazquez MD, Colyer M, et al. Vaginal misoprostol for cervical ripening at term: comparison of outpatient vs inpatient administration. Obstet Gynecol Surv. 2006;61:167-168.
- Meyer M, Pflum J, Howard D. Outpatient misoprostol compared with dinoprostone gel for preinduction cervical ripening: a randomized controlled trial. Obstet Gynecol. 2005;105:466-472.
- Sciscione AC, Bedder CL, Hoffman MK, et al. The timing of adverse events with Foley catheter preinduction cervical ripening; implications for outpatient use. Am J Perinatol. 2014;31:781-786.
- McMaster K, Sanchez-Ramos L, Kaunitz AM. Evaluation of a transcervical Foley catheter as a source of infection. Obstet Gynecol. 2015;126:539-551.
- Fox NS, Saltzman DH, Roman AS, et al. Intravaginal misoprostol versus Foley catheter for labour induction: a meta-analysis. BJOG. 2011;118: 647-654.
- Hofmeyr GJ, Gulmezoglu AM, Pileggi C. Vaginal misoprostol for cervical ripening and induction of labour. Cochrane Database Syst Rev. 2010:CD000941.
- Salvador SC, Simpson ML, Cundiff GW. Dinoprostone vaginal insert for labour induction: a comparison of outpatient and inpatient settings. J Obstet Gynaecol Can. 2009;31:1028-1034.
- Sutton C, Harding J, Griffin C. Patient attitudes towards outpatient cervical ripening prior to induction of labour at an Australian tertiary hospital. J Obstet Gynaecol. 2016;36:921-928.
Cesarean myomectomy: Safe operation or surgical folly?
Uterine leiomyomata (fibroids) are the most common pelvic tumor of women. When women are planning to conceive, and their fibroid(s) are clinically significant, causing abnormal uterine bleeding or bulk symptoms, it is often optimal to remove the uterine tumor(s) before conception. Advances in minimally invasive surgery offer women the option of laparoscopic or robot-assisted myomectomy with a low rate of operative complications, including excessive blood loss and hysterectomy, and a low rate of postoperative complications, including major pelvic adhesions and uterine rupture during subsequent pregnancy.1-3 However, many women become pregnant when they have clinically significant fibroids, and at least one-third of these women will have a cesarean birth.
Important clinical issues are the relative benefits and risks of performing a myomectomy at the time of the cesarean birth, so called cesarean myomectomy. Cesarean myomectomy offers carefully selected women the opportunity to have a cesarean birth and myomectomy in one operation, thereby avoiding a second major operation. Over the past 6 decades, most experts in the United States and the United Kingdom have strongly recommended against myomectomy at the time of cesarean delivery because of the risk of excessive blood loss and hysterectomy. Recently, expert opinion has shifted, especially in continental Europe and Asia, and cesarean myomectomy is now viewed as an acceptable surgical option in a limited number of clinical situations, including removal of pedunculated fibroids, excision of large solitary subserosal fibroids, and to achieve optimal management of the hysterotomy incision.
Decades of expert guidance: Avoid cesarean myomectomy at all costs
Dr. K.S.J. Olah succinctly captured the standard teaching that cesarean myomectomy should be avoided in this personal vignette:
Many years ago as a trainee I removed a subserosal fibroid during a cesarean section that was hanging by a thin stalk on the back of the uterus. The berating I received was severe and disproportionate to the crime. The rule was that myomectomy performed at cesarean section was not just frowned upon but expressly forbidden. It has always been considered foolish to consider removing fibroids at cesarean section, mostly because of the associated morbidity and the risk of haemorrhage requiring hysterectomy.4
Dr. Olah quoted guidance from Shaw’s Textbook of Operative Gynaecology,5 “It should be stressed that myomectomy in pregnancy should be avoided at all costs, including at caesarean section.” However, large case series published over the past 10 years report that, in limited clinical situations, cesarean myomectomy is a viable surgical option, where benefit may outweigh risk.6-14 The current literature has many weaknesses, including failure to specifically identify the indication for the cesarean myomectomy and lack of controlled prospective clinical trials. In almost all cases, cesarean myomectomy is performed after delivery of the fetus and placenta.
Continue to: The pedunculated, FIGO type 7 fibroid...
The pedunculated, FIGO type 7 fibroid
The International Federation of Gynecology and Obstetrics (FIGO) leiomyoma classification system identifies subserosal pedunculated fibroids as type 7 (FIGURE).15 Pedunculated fibroids are attached to the uterus by a stalk that is ≤10% of the mean of the 3 diameters of the fibroid. When a clinically significant pedunculated fibroid, causing bulk symptoms, is encountered at cesarean birth, I recommend that it be removed. This will save many patients a second major operation to perform a myomectomy. The surgical risk of removing a pedunculated is low.
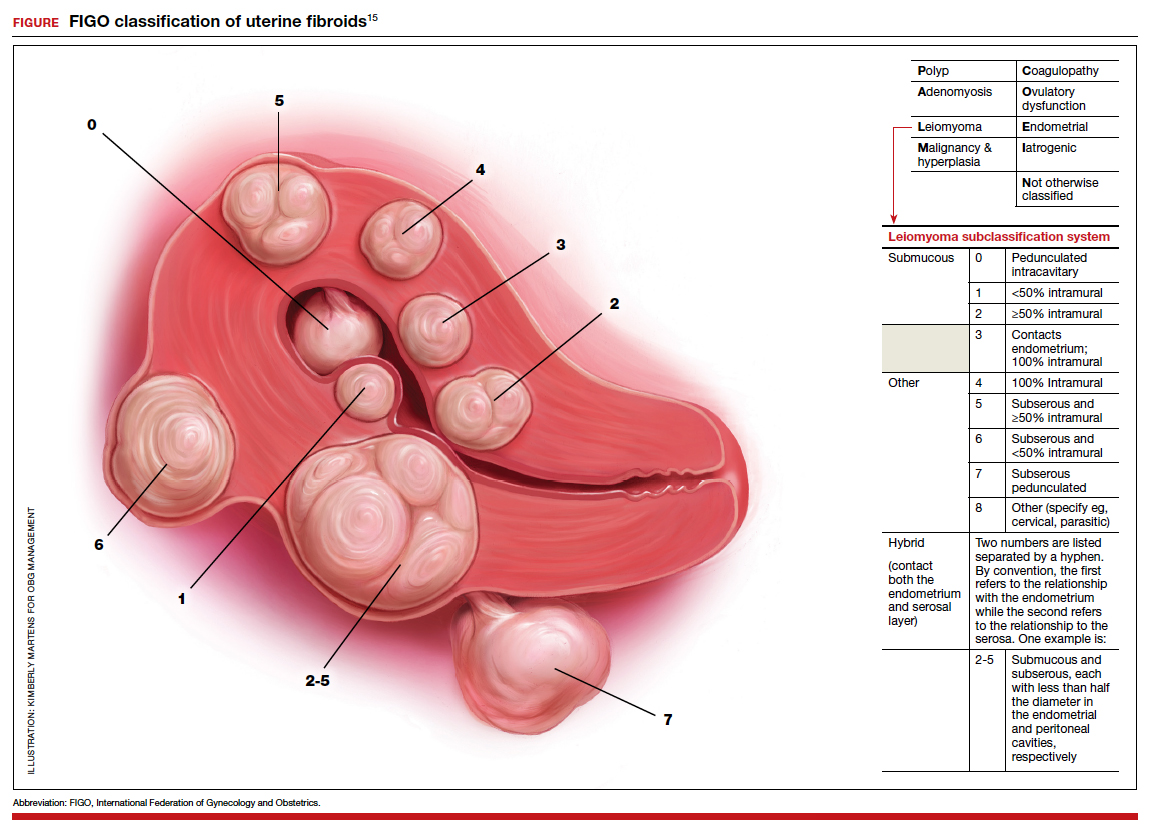
The solitary FIGO type 6 fibroid
Type 6 fibroids are subserosal fibroids with less than 50% of their mass being subserosal. The type 6 fibroid is relatively easy to enucleate from the uterus. Following removal of a type 6 fibroid, closure of the serosal defect is relatively straightforward. In carefully selected cases, if the type 6 fibroid is causing bulk symptoms, cesarean myomectomy may be indicated with a low risk of operative complications.
The FIGO type 2-5 fibroid
The type 2-5 fibroid is a transmural fibroid with significant mass abutting both the endometrial cavity and serosal surface. Excision of a type 2-5 fibroid is likely to result in a large transmyometrial defect that will be more difficult to close and could be associated with greater blood loss. Although data are limited, I would recommend against cesarean myomectomy for type 2-5 fibroids in most clinical situations.
Myomectomy to achieve optimal management of the cesarean hysterotomy incision
Many surgeons performing a cesarean birth for a woman with clinically significant fibroids will plan the hysterotomy incision to avoid the fibroids. However, following delivery and contraction of the uterus, proper closure of the hysterotomy incision may be very difficult without removing a fibroid that is abutting the hysterotomy incision. Surgeons have reported performing myomectomy on lower uterine segment fibroids before making the hysterotomy incision in order to facilitate the hysterotomy incision and closure.16 Myomectomy prior to delivery of the newborn must be associated with additional risks to the fetus. I would prefer to identify an optimal site to perform a hysterotomy, deliver the newborn and placenta, and then consider myomectomy.
Complications associated with cesarean myomectomy
The evidence concerning the complications of cesarean birth plus myomectomy compared with cesarean birth alone in women with fibroids is limited to case series. There are no reported controlled clinical trials to guide practice. The largest single case series reported on 1,242 women with fibroids who had a cesarean birth plus myomectomy compared with 3 control groups, including 200 women without fibroids who had a cesarean birth, 145 women with fibroids who had a cesarean birth and no myomectomy, and 51 women with fibroids who had a cesarean hysterectomy. The investigators reported no significant differences in preoperative to postoperative hemoglobin change, incidence of postoperative fever, or length of hospital stay among the 4 groups.8 The authors concluded that myomectomy during cesarean birth was a safe and effective procedure.
Continue to: A systematic review and meta-analysis reported...
A systematic review and meta-analysis reported on the results of 17 studies which included 4,702 women who had a cesarean myomectomy and 1,843 women with cesarean birth without myomectomy.17 The authors of the meta-analysis noted that most reported case series had excluded women with a high risk of bleeding, including women with placenta previa, placenta accreta, coagulation disorders, and a history of multiple myomectomy operations. The investigators reported that, compared with the control women, the women undergoing cesarean myomectomy had a statistically significant but clinically insignificant decrease in mean hemoglobin concentration (-0.27 g/dL), a significant increase in mean operative time (+15 minutes) and a significant increase in the length of hospital stay (+0.36 days). There was an increase in the need for blood transfusion (risk ratio, 1.45; 95% confidence interval, 1.05–1.99), but only 3% of women undergoing cesarean myomectomy received a blood transfusion. There was no significant difference between the two groups in the incidence of postoperative fever. The authors concluded that cesarean myomectomy is a safe procedure when performed by experienced surgeons with appropriate hemostatic techniques.
Techniques to reduce blood loss at the time of cesarean myomectomy
A detailed review of all the available techniques to reduce blood loss at the time of cesarean myomectomy is beyond the scope of this editorial. All gynecologists know that control of uterine blood flow through the uterine artery, infundibulopelvic vessels and internal iliac artery can help to reduce bleeding at the time of myomectomy. Tourniquets, vascular clamps, and artery ligation all have been reported to be useful at the time of cesarean myomectomy. In addition, intravenous infusion of oxytocin and tranexamic acid is often used at the time of cesarean myomectomy. Direct injection of uterotonics, including carbetocin, oxytocin, and vasopressin, into the uterus also has been reported. Cell saver blood salvage technology has been utilized in a limited number of cases of cesarean myomectomy.8,18,19
Medicine is not a static field
Discoveries and new data help guide advances in medical practice. After 6 decades of strict adherence to the advice that myomectomy in pregnancy should be avoided at all costs, including at caesarean delivery, new data indicate that in carefully selected cases cesarean myomectomy is an acceptable operation. ●
- Pitter MC, Gargiulo AR, Bonaventura LM, et al. Pregnancy outcomes following robot-assisted myomectomy. Hum Reprod. 2013;28:99-108.
- Pitter MC, Srouji SS, Gargiulo AR, et al. Fertility and symptom relief following robot-assisted laparoscopic myomectomy. Obstet Gynecol Int. 2015;2015:967568.
- Huberlant S, Lenot J, Neron M, et al. Fertility and obstetric outcomes after robot-assisted laparoscopic myomectomy. Int J Med Robot. 2020;16:e2059.
- Olah KSJ. Caesarean myomectomy: TE or not TE? BJOG. 2018;125:501.
- Shaw, et al. Textbook of Operative Gynaecology. Edinburgh: Churchill Livingston; 1977.
- Burton CA, Grimes DA, March CM. Surgical management of leiomyomata during pregnancy. Obstet Gynecol. 1989;74:707-709.
- Ortac F, Gungor M, Sonmezer M. Myomectomy during cesarean section. Int J Gynaecol Obstet. 1999;67:189-193.
- Li H, Du J, Jin L, et al. Myomectomy during cesarean section. Acta Obstetricia et Gynecologica. 2009;88:183-186.
- Kwon DH, Song JE, Yoon KR, et al. Obstet Gynecol Sci. 2014;57:367-372.
- Senturk MB, Polat M, Dogan O, et al. Outcome of cesarean myomectomy: is it a safe procedure? Geburtshilfe Frauenheilkd. 2017;77:1200-1206.
- Chauhan AR. Cesarean myomectomy: necessity or opportunity? J Obstet Gynecol India. 2018;68:432-436.
- Sparic R, Kadija S, Stefanovic A, et al. Cesarean myomectomy in modern obstetrics: more light and fewer shadows. J Obstet Gynaecol Res. 2017;43:798-804.
- Ramya T, Sabnis SS, Chitra TV, et al. Cesarean myomectomy: an experience from a tertiary care teaching hospital. J Obstet Gynaecol India. 2019;69:426-430.
- Zhao R, Wang X, Zou L, et al. Outcomes of myomectomy at the time of cesarean section among pregnant women with uterine fibroids: a retrospective cohort study. Biomed Res Int. 2019;7576934.
- Munro MG, Critchley HOD, Fraser IS; FIGO Menstrual Disorders Committee. The two FIGO systems for normal and abnormal uterine bleeding symptoms and classification of causes of abnormal uterine bleeding in the reproductive years: 2018 revisions. In J Gynaecol Obstet. 2018;143:393.
- Omar SZ, Sivanesaratnam V, Damodaran P. Large lower segment myoma—myomectomy at lower segment caesarean section—a report of two cases. Singapore Med J. 1999;40:109-110.
- Goyal M, Dawood AS, Elbohoty SB, et al. Cesarean myomectomy in the last ten years; A true shift from contraindication to indication: a systematic review and meta-analysis. Eur J Obstet Gynecol Reprod Biol. 2021;256:145-157.
- Lin JY, Lee WL, Wang PH, et al. Uterine artery occlusion and myomectomy for treatment of pregnant women with uterine leiomyomas who are undergoing caesarean section. J Obstet Gynecol Res. 2010;36:284-290.
- Alfred E, Joy G, Uduak O, et al. Cesarean myomectomy outcome in a Nigerian hospital district hospital. J Basic Clin Reprod Sci. 2013;2:115-118.

Uterine leiomyomata (fibroids) are the most common pelvic tumor of women. When women are planning to conceive, and their fibroid(s) are clinically significant, causing abnormal uterine bleeding or bulk symptoms, it is often optimal to remove the uterine tumor(s) before conception. Advances in minimally invasive surgery offer women the option of laparoscopic or robot-assisted myomectomy with a low rate of operative complications, including excessive blood loss and hysterectomy, and a low rate of postoperative complications, including major pelvic adhesions and uterine rupture during subsequent pregnancy.1-3 However, many women become pregnant when they have clinically significant fibroids, and at least one-third of these women will have a cesarean birth.
Important clinical issues are the relative benefits and risks of performing a myomectomy at the time of the cesarean birth, so called cesarean myomectomy. Cesarean myomectomy offers carefully selected women the opportunity to have a cesarean birth and myomectomy in one operation, thereby avoiding a second major operation. Over the past 6 decades, most experts in the United States and the United Kingdom have strongly recommended against myomectomy at the time of cesarean delivery because of the risk of excessive blood loss and hysterectomy. Recently, expert opinion has shifted, especially in continental Europe and Asia, and cesarean myomectomy is now viewed as an acceptable surgical option in a limited number of clinical situations, including removal of pedunculated fibroids, excision of large solitary subserosal fibroids, and to achieve optimal management of the hysterotomy incision.
Decades of expert guidance: Avoid cesarean myomectomy at all costs
Dr. K.S.J. Olah succinctly captured the standard teaching that cesarean myomectomy should be avoided in this personal vignette:
Many years ago as a trainee I removed a subserosal fibroid during a cesarean section that was hanging by a thin stalk on the back of the uterus. The berating I received was severe and disproportionate to the crime. The rule was that myomectomy performed at cesarean section was not just frowned upon but expressly forbidden. It has always been considered foolish to consider removing fibroids at cesarean section, mostly because of the associated morbidity and the risk of haemorrhage requiring hysterectomy.4
Dr. Olah quoted guidance from Shaw’s Textbook of Operative Gynaecology,5 “It should be stressed that myomectomy in pregnancy should be avoided at all costs, including at caesarean section.” However, large case series published over the past 10 years report that, in limited clinical situations, cesarean myomectomy is a viable surgical option, where benefit may outweigh risk.6-14 The current literature has many weaknesses, including failure to specifically identify the indication for the cesarean myomectomy and lack of controlled prospective clinical trials. In almost all cases, cesarean myomectomy is performed after delivery of the fetus and placenta.
Continue to: The pedunculated, FIGO type 7 fibroid...
The pedunculated, FIGO type 7 fibroid
The International Federation of Gynecology and Obstetrics (FIGO) leiomyoma classification system identifies subserosal pedunculated fibroids as type 7 (FIGURE).15 Pedunculated fibroids are attached to the uterus by a stalk that is ≤10% of the mean of the 3 diameters of the fibroid. When a clinically significant pedunculated fibroid, causing bulk symptoms, is encountered at cesarean birth, I recommend that it be removed. This will save many patients a second major operation to perform a myomectomy. The surgical risk of removing a pedunculated is low.

The solitary FIGO type 6 fibroid
Type 6 fibroids are subserosal fibroids with less than 50% of their mass being subserosal. The type 6 fibroid is relatively easy to enucleate from the uterus. Following removal of a type 6 fibroid, closure of the serosal defect is relatively straightforward. In carefully selected cases, if the type 6 fibroid is causing bulk symptoms, cesarean myomectomy may be indicated with a low risk of operative complications.
The FIGO type 2-5 fibroid
The type 2-5 fibroid is a transmural fibroid with significant mass abutting both the endometrial cavity and serosal surface. Excision of a type 2-5 fibroid is likely to result in a large transmyometrial defect that will be more difficult to close and could be associated with greater blood loss. Although data are limited, I would recommend against cesarean myomectomy for type 2-5 fibroids in most clinical situations.
Myomectomy to achieve optimal management of the cesarean hysterotomy incision
Many surgeons performing a cesarean birth for a woman with clinically significant fibroids will plan the hysterotomy incision to avoid the fibroids. However, following delivery and contraction of the uterus, proper closure of the hysterotomy incision may be very difficult without removing a fibroid that is abutting the hysterotomy incision. Surgeons have reported performing myomectomy on lower uterine segment fibroids before making the hysterotomy incision in order to facilitate the hysterotomy incision and closure.16 Myomectomy prior to delivery of the newborn must be associated with additional risks to the fetus. I would prefer to identify an optimal site to perform a hysterotomy, deliver the newborn and placenta, and then consider myomectomy.
Complications associated with cesarean myomectomy
The evidence concerning the complications of cesarean birth plus myomectomy compared with cesarean birth alone in women with fibroids is limited to case series. There are no reported controlled clinical trials to guide practice. The largest single case series reported on 1,242 women with fibroids who had a cesarean birth plus myomectomy compared with 3 control groups, including 200 women without fibroids who had a cesarean birth, 145 women with fibroids who had a cesarean birth and no myomectomy, and 51 women with fibroids who had a cesarean hysterectomy. The investigators reported no significant differences in preoperative to postoperative hemoglobin change, incidence of postoperative fever, or length of hospital stay among the 4 groups.8 The authors concluded that myomectomy during cesarean birth was a safe and effective procedure.
Continue to: A systematic review and meta-analysis reported...
A systematic review and meta-analysis reported on the results of 17 studies which included 4,702 women who had a cesarean myomectomy and 1,843 women with cesarean birth without myomectomy.17 The authors of the meta-analysis noted that most reported case series had excluded women with a high risk of bleeding, including women with placenta previa, placenta accreta, coagulation disorders, and a history of multiple myomectomy operations. The investigators reported that, compared with the control women, the women undergoing cesarean myomectomy had a statistically significant but clinically insignificant decrease in mean hemoglobin concentration (-0.27 g/dL), a significant increase in mean operative time (+15 minutes) and a significant increase in the length of hospital stay (+0.36 days). There was an increase in the need for blood transfusion (risk ratio, 1.45; 95% confidence interval, 1.05–1.99), but only 3% of women undergoing cesarean myomectomy received a blood transfusion. There was no significant difference between the two groups in the incidence of postoperative fever. The authors concluded that cesarean myomectomy is a safe procedure when performed by experienced surgeons with appropriate hemostatic techniques.
Techniques to reduce blood loss at the time of cesarean myomectomy
A detailed review of all the available techniques to reduce blood loss at the time of cesarean myomectomy is beyond the scope of this editorial. All gynecologists know that control of uterine blood flow through the uterine artery, infundibulopelvic vessels and internal iliac artery can help to reduce bleeding at the time of myomectomy. Tourniquets, vascular clamps, and artery ligation all have been reported to be useful at the time of cesarean myomectomy. In addition, intravenous infusion of oxytocin and tranexamic acid is often used at the time of cesarean myomectomy. Direct injection of uterotonics, including carbetocin, oxytocin, and vasopressin, into the uterus also has been reported. Cell saver blood salvage technology has been utilized in a limited number of cases of cesarean myomectomy.8,18,19
Medicine is not a static field
Discoveries and new data help guide advances in medical practice. After 6 decades of strict adherence to the advice that myomectomy in pregnancy should be avoided at all costs, including at caesarean delivery, new data indicate that in carefully selected cases cesarean myomectomy is an acceptable operation. ●

Uterine leiomyomata (fibroids) are the most common pelvic tumor of women. When women are planning to conceive, and their fibroid(s) are clinically significant, causing abnormal uterine bleeding or bulk symptoms, it is often optimal to remove the uterine tumor(s) before conception. Advances in minimally invasive surgery offer women the option of laparoscopic or robot-assisted myomectomy with a low rate of operative complications, including excessive blood loss and hysterectomy, and a low rate of postoperative complications, including major pelvic adhesions and uterine rupture during subsequent pregnancy.1-3 However, many women become pregnant when they have clinically significant fibroids, and at least one-third of these women will have a cesarean birth.
Important clinical issues are the relative benefits and risks of performing a myomectomy at the time of the cesarean birth, so called cesarean myomectomy. Cesarean myomectomy offers carefully selected women the opportunity to have a cesarean birth and myomectomy in one operation, thereby avoiding a second major operation. Over the past 6 decades, most experts in the United States and the United Kingdom have strongly recommended against myomectomy at the time of cesarean delivery because of the risk of excessive blood loss and hysterectomy. Recently, expert opinion has shifted, especially in continental Europe and Asia, and cesarean myomectomy is now viewed as an acceptable surgical option in a limited number of clinical situations, including removal of pedunculated fibroids, excision of large solitary subserosal fibroids, and to achieve optimal management of the hysterotomy incision.
Decades of expert guidance: Avoid cesarean myomectomy at all costs
Dr. K.S.J. Olah succinctly captured the standard teaching that cesarean myomectomy should be avoided in this personal vignette:
Many years ago as a trainee I removed a subserosal fibroid during a cesarean section that was hanging by a thin stalk on the back of the uterus. The berating I received was severe and disproportionate to the crime. The rule was that myomectomy performed at cesarean section was not just frowned upon but expressly forbidden. It has always been considered foolish to consider removing fibroids at cesarean section, mostly because of the associated morbidity and the risk of haemorrhage requiring hysterectomy.4
Dr. Olah quoted guidance from Shaw’s Textbook of Operative Gynaecology,5 “It should be stressed that myomectomy in pregnancy should be avoided at all costs, including at caesarean section.” However, large case series published over the past 10 years report that, in limited clinical situations, cesarean myomectomy is a viable surgical option, where benefit may outweigh risk.6-14 The current literature has many weaknesses, including failure to specifically identify the indication for the cesarean myomectomy and lack of controlled prospective clinical trials. In almost all cases, cesarean myomectomy is performed after delivery of the fetus and placenta.
Continue to: The pedunculated, FIGO type 7 fibroid...
The pedunculated, FIGO type 7 fibroid
The International Federation of Gynecology and Obstetrics (FIGO) leiomyoma classification system identifies subserosal pedunculated fibroids as type 7 (FIGURE).15 Pedunculated fibroids are attached to the uterus by a stalk that is ≤10% of the mean of the 3 diameters of the fibroid. When a clinically significant pedunculated fibroid, causing bulk symptoms, is encountered at cesarean birth, I recommend that it be removed. This will save many patients a second major operation to perform a myomectomy. The surgical risk of removing a pedunculated is low.

The solitary FIGO type 6 fibroid
Type 6 fibroids are subserosal fibroids with less than 50% of their mass being subserosal. The type 6 fibroid is relatively easy to enucleate from the uterus. Following removal of a type 6 fibroid, closure of the serosal defect is relatively straightforward. In carefully selected cases, if the type 6 fibroid is causing bulk symptoms, cesarean myomectomy may be indicated with a low risk of operative complications.
The FIGO type 2-5 fibroid
The type 2-5 fibroid is a transmural fibroid with significant mass abutting both the endometrial cavity and serosal surface. Excision of a type 2-5 fibroid is likely to result in a large transmyometrial defect that will be more difficult to close and could be associated with greater blood loss. Although data are limited, I would recommend against cesarean myomectomy for type 2-5 fibroids in most clinical situations.
Myomectomy to achieve optimal management of the cesarean hysterotomy incision
Many surgeons performing a cesarean birth for a woman with clinically significant fibroids will plan the hysterotomy incision to avoid the fibroids. However, following delivery and contraction of the uterus, proper closure of the hysterotomy incision may be very difficult without removing a fibroid that is abutting the hysterotomy incision. Surgeons have reported performing myomectomy on lower uterine segment fibroids before making the hysterotomy incision in order to facilitate the hysterotomy incision and closure.16 Myomectomy prior to delivery of the newborn must be associated with additional risks to the fetus. I would prefer to identify an optimal site to perform a hysterotomy, deliver the newborn and placenta, and then consider myomectomy.
Complications associated with cesarean myomectomy
The evidence concerning the complications of cesarean birth plus myomectomy compared with cesarean birth alone in women with fibroids is limited to case series. There are no reported controlled clinical trials to guide practice. The largest single case series reported on 1,242 women with fibroids who had a cesarean birth plus myomectomy compared with 3 control groups, including 200 women without fibroids who had a cesarean birth, 145 women with fibroids who had a cesarean birth and no myomectomy, and 51 women with fibroids who had a cesarean hysterectomy. The investigators reported no significant differences in preoperative to postoperative hemoglobin change, incidence of postoperative fever, or length of hospital stay among the 4 groups.8 The authors concluded that myomectomy during cesarean birth was a safe and effective procedure.
Continue to: A systematic review and meta-analysis reported...
A systematic review and meta-analysis reported on the results of 17 studies which included 4,702 women who had a cesarean myomectomy and 1,843 women with cesarean birth without myomectomy.17 The authors of the meta-analysis noted that most reported case series had excluded women with a high risk of bleeding, including women with placenta previa, placenta accreta, coagulation disorders, and a history of multiple myomectomy operations. The investigators reported that, compared with the control women, the women undergoing cesarean myomectomy had a statistically significant but clinically insignificant decrease in mean hemoglobin concentration (-0.27 g/dL), a significant increase in mean operative time (+15 minutes) and a significant increase in the length of hospital stay (+0.36 days). There was an increase in the need for blood transfusion (risk ratio, 1.45; 95% confidence interval, 1.05–1.99), but only 3% of women undergoing cesarean myomectomy received a blood transfusion. There was no significant difference between the two groups in the incidence of postoperative fever. The authors concluded that cesarean myomectomy is a safe procedure when performed by experienced surgeons with appropriate hemostatic techniques.
Techniques to reduce blood loss at the time of cesarean myomectomy
A detailed review of all the available techniques to reduce blood loss at the time of cesarean myomectomy is beyond the scope of this editorial. All gynecologists know that control of uterine blood flow through the uterine artery, infundibulopelvic vessels and internal iliac artery can help to reduce bleeding at the time of myomectomy. Tourniquets, vascular clamps, and artery ligation all have been reported to be useful at the time of cesarean myomectomy. In addition, intravenous infusion of oxytocin and tranexamic acid is often used at the time of cesarean myomectomy. Direct injection of uterotonics, including carbetocin, oxytocin, and vasopressin, into the uterus also has been reported. Cell saver blood salvage technology has been utilized in a limited number of cases of cesarean myomectomy.8,18,19
Medicine is not a static field
Discoveries and new data help guide advances in medical practice. After 6 decades of strict adherence to the advice that myomectomy in pregnancy should be avoided at all costs, including at caesarean delivery, new data indicate that in carefully selected cases cesarean myomectomy is an acceptable operation. ●
- Pitter MC, Gargiulo AR, Bonaventura LM, et al. Pregnancy outcomes following robot-assisted myomectomy. Hum Reprod. 2013;28:99-108.
- Pitter MC, Srouji SS, Gargiulo AR, et al. Fertility and symptom relief following robot-assisted laparoscopic myomectomy. Obstet Gynecol Int. 2015;2015:967568.
- Huberlant S, Lenot J, Neron M, et al. Fertility and obstetric outcomes after robot-assisted laparoscopic myomectomy. Int J Med Robot. 2020;16:e2059.
- Olah KSJ. Caesarean myomectomy: TE or not TE? BJOG. 2018;125:501.
- Shaw, et al. Textbook of Operative Gynaecology. Edinburgh: Churchill Livingston; 1977.
- Burton CA, Grimes DA, March CM. Surgical management of leiomyomata during pregnancy. Obstet Gynecol. 1989;74:707-709.
- Ortac F, Gungor M, Sonmezer M. Myomectomy during cesarean section. Int J Gynaecol Obstet. 1999;67:189-193.
- Li H, Du J, Jin L, et al. Myomectomy during cesarean section. Acta Obstetricia et Gynecologica. 2009;88:183-186.
- Kwon DH, Song JE, Yoon KR, et al. Obstet Gynecol Sci. 2014;57:367-372.
- Senturk MB, Polat M, Dogan O, et al. Outcome of cesarean myomectomy: is it a safe procedure? Geburtshilfe Frauenheilkd. 2017;77:1200-1206.
- Chauhan AR. Cesarean myomectomy: necessity or opportunity? J Obstet Gynecol India. 2018;68:432-436.
- Sparic R, Kadija S, Stefanovic A, et al. Cesarean myomectomy in modern obstetrics: more light and fewer shadows. J Obstet Gynaecol Res. 2017;43:798-804.
- Ramya T, Sabnis SS, Chitra TV, et al. Cesarean myomectomy: an experience from a tertiary care teaching hospital. J Obstet Gynaecol India. 2019;69:426-430.
- Zhao R, Wang X, Zou L, et al. Outcomes of myomectomy at the time of cesarean section among pregnant women with uterine fibroids: a retrospective cohort study. Biomed Res Int. 2019;7576934.
- Munro MG, Critchley HOD, Fraser IS; FIGO Menstrual Disorders Committee. The two FIGO systems for normal and abnormal uterine bleeding symptoms and classification of causes of abnormal uterine bleeding in the reproductive years: 2018 revisions. In J Gynaecol Obstet. 2018;143:393.
- Omar SZ, Sivanesaratnam V, Damodaran P. Large lower segment myoma—myomectomy at lower segment caesarean section—a report of two cases. Singapore Med J. 1999;40:109-110.
- Goyal M, Dawood AS, Elbohoty SB, et al. Cesarean myomectomy in the last ten years; A true shift from contraindication to indication: a systematic review and meta-analysis. Eur J Obstet Gynecol Reprod Biol. 2021;256:145-157.
- Lin JY, Lee WL, Wang PH, et al. Uterine artery occlusion and myomectomy for treatment of pregnant women with uterine leiomyomas who are undergoing caesarean section. J Obstet Gynecol Res. 2010;36:284-290.
- Alfred E, Joy G, Uduak O, et al. Cesarean myomectomy outcome in a Nigerian hospital district hospital. J Basic Clin Reprod Sci. 2013;2:115-118.
- Pitter MC, Gargiulo AR, Bonaventura LM, et al. Pregnancy outcomes following robot-assisted myomectomy. Hum Reprod. 2013;28:99-108.
- Pitter MC, Srouji SS, Gargiulo AR, et al. Fertility and symptom relief following robot-assisted laparoscopic myomectomy. Obstet Gynecol Int. 2015;2015:967568.
- Huberlant S, Lenot J, Neron M, et al. Fertility and obstetric outcomes after robot-assisted laparoscopic myomectomy. Int J Med Robot. 2020;16:e2059.
- Olah KSJ. Caesarean myomectomy: TE or not TE? BJOG. 2018;125:501.
- Shaw, et al. Textbook of Operative Gynaecology. Edinburgh: Churchill Livingston; 1977.
- Burton CA, Grimes DA, March CM. Surgical management of leiomyomata during pregnancy. Obstet Gynecol. 1989;74:707-709.
- Ortac F, Gungor M, Sonmezer M. Myomectomy during cesarean section. Int J Gynaecol Obstet. 1999;67:189-193.
- Li H, Du J, Jin L, et al. Myomectomy during cesarean section. Acta Obstetricia et Gynecologica. 2009;88:183-186.
- Kwon DH, Song JE, Yoon KR, et al. Obstet Gynecol Sci. 2014;57:367-372.
- Senturk MB, Polat M, Dogan O, et al. Outcome of cesarean myomectomy: is it a safe procedure? Geburtshilfe Frauenheilkd. 2017;77:1200-1206.
- Chauhan AR. Cesarean myomectomy: necessity or opportunity? J Obstet Gynecol India. 2018;68:432-436.
- Sparic R, Kadija S, Stefanovic A, et al. Cesarean myomectomy in modern obstetrics: more light and fewer shadows. J Obstet Gynaecol Res. 2017;43:798-804.
- Ramya T, Sabnis SS, Chitra TV, et al. Cesarean myomectomy: an experience from a tertiary care teaching hospital. J Obstet Gynaecol India. 2019;69:426-430.
- Zhao R, Wang X, Zou L, et al. Outcomes of myomectomy at the time of cesarean section among pregnant women with uterine fibroids: a retrospective cohort study. Biomed Res Int. 2019;7576934.
- Munro MG, Critchley HOD, Fraser IS; FIGO Menstrual Disorders Committee. The two FIGO systems for normal and abnormal uterine bleeding symptoms and classification of causes of abnormal uterine bleeding in the reproductive years: 2018 revisions. In J Gynaecol Obstet. 2018;143:393.
- Omar SZ, Sivanesaratnam V, Damodaran P. Large lower segment myoma—myomectomy at lower segment caesarean section—a report of two cases. Singapore Med J. 1999;40:109-110.
- Goyal M, Dawood AS, Elbohoty SB, et al. Cesarean myomectomy in the last ten years; A true shift from contraindication to indication: a systematic review and meta-analysis. Eur J Obstet Gynecol Reprod Biol. 2021;256:145-157.
- Lin JY, Lee WL, Wang PH, et al. Uterine artery occlusion and myomectomy for treatment of pregnant women with uterine leiomyomas who are undergoing caesarean section. J Obstet Gynecol Res. 2010;36:284-290.
- Alfred E, Joy G, Uduak O, et al. Cesarean myomectomy outcome in a Nigerian hospital district hospital. J Basic Clin Reprod Sci. 2013;2:115-118.
For obese postmenopausal women, what options may decrease endometrial cancer risk?
Endometrial cancer is the most common gynecologic malignancy, with approximately 59,000 cases diagnosed annually,1 and a lifetime risk of approximately 3.1% in the United States.2 Type I endometrial cancer includes tumors with endometrioid histology that are grade 1 or 2. Type II endometrial cancer includes tumors that have grade 3 endometrioid or nonendometrioid histology, including serous, clear cell, mucinous, squamous transitional cell, mesonephric, and undifferentiated tumors.3 Type I endometrial cancer is hormone sensitive, generally stimulated by estrogen and suppressed by progestins.
Endometrial cancer is diagnosed at a mean age of 63 years,4 and only 15% of cases occur before age 50.5 Women with an elevated body mass index (BMI) have a markedly increased risk of both Types I and II endometrial cancer (TABLE).6 Hence, endometrial cancer is highly prevalent in obese postmenopausal women. For these women health interventions that may reduce the risk of developing endometrial cancer include dieting, physical activity, bariatric surgery, and progestin therapy.

Educating patients is a priority
Many women do not know that postmenopausal bleeding is a sign of endometrial cancer. All postmenopausal women should be advised that if they develop vaginal bleeding they need to be evaluated by a clinician.7 Women who are knowledgeable about the link between postmenopausal vaginal bleeding and endometrial cancer can be encouraged to share this information with their postmenopausal friends in order to reach more people with this important information. All obese postmenopausal women should be advised that weight loss and increased physical activity can reduce the risk of developing endometrial cancer.
How weight loss and physical activity affect risk
Intentional weight loss has been reported to reduce the risk of endometrial cancer in postmenopausal women. As part of the Women’s Health Initiative observational study, 36,794 postmenopausal women aged 50 to 79 years with a uterus had their body weight and height measured at entry into the study and after 3 years of follow-up.8 During the 11 years following study entry, there were 566 incident cases of endometrial cancer. Compared with women who had a stable weight, intentional weight loss of ≥5% was associated with a 40% reduction in the risk of endometrial cancer (hazard ratio [HR], 0.60; 95% confidence interval [CI], 0.42–0.86). Compared with women who had a stable weight, women who had weight gain ≥10% had an increased risk of endometrial cancer (HR, 1.26; 95% CI, 1.00–1.57).
High levels of physical activity may be associated with a decreased risk of endometrial cancer. In one study, compared with a sedentary lifestyle, higher levels of physical activity were reported to be associated with a decreased risk of endometrial cancer.9
Continue to: How bariatric surgery affects risk...
How bariatric surgery affects risk
Many cancers are associated with obesity, including endometrial, breast, colon, pancreas, gallbladder, and renal. Obesity is associated with increased conversion of androgens to estrogens in fat tissue, stimulating excessive endometrial proliferation and increasing the risk of endometrial hyperplasia and cancer. Bariatric surgery reliably causes sustained weight reduction. Multiple studies have reported that bariatric surgery reduces the risk of endometrial cancer.
Schauer and colleagues used data from the Kaiser Permanente health system to identify 22,198 obese people who had undergone bariatric surgery and 66,427 matched controls who were obese but did not have surgery.10 The study population was 81% female, with a mean age of 45 years and a mean BMI of 45 kg/m2. After an average 3.5 years of follow-up there were 2,542 incident cases of cancer, including 322 cases of endometrial cancer. Compared with conventional weight loss treatment, bariatric surgery reduced the risk of endometrial cancer by 50% (HR, 0.50; 95% CI, 0.37–0.67; P<.001).10 In addition, bariatric surgery reduced the risk of colon and pancreatic cancer by 41% and 54%, respectively.10
In the Swedish Obese Subjects (SOS) study, 1,420 women who underwent bariatric surgery and 1,447 matched controls who received conventional obesity treatment were followed for 18 years.11 At study entry, the mean age of the women was approximately 48 years, and the mean BMI was approximately 42 kg/m2. In follow-up there were 76 incident cases of endometrial cancer. Compared with women receiving conventional obesity treatment, women who had bariatric surgery had a non–statistically significant 49% decrease in the risk of developing endometrial cancer (HR, 0.51; 95% CI, 0.24–1.10)
In a systematic review of 5 additional studies (not including publications 10 or 11) of the impact of bariatric surgery on the risk of developing endometrial cancer, the surgery was associated with a 68% risk reduction (odds ratio [OR], 0.32; 95% CI, 0.16–0.63) compared with matched obese women that did not have surgery.12
Although there are no randomized prospective studies showing that bariatric surgery reduces the risk of endometrial cancer, the weight of the observation evidence is strong. In addition, bariatric surgery was reported to reduce all-cause mortality in the SOS study.13 Hence, for obese postmenopausal women, if lifestyle changes do not result in sustained weight loss, bariatric surgery may be an optimal approach to improving health outcomes.
Continue to: Progestin treatment and endometrial cancer risk...
Progestin treatment and endometrial cancer risk
Estrogen stimulates endometrial cell proliferation. Hence, unopposed chronic exposure to estrogen is a major risk factor for developing endometrial hyperplasia and cancer. Progestins block the proliferative effect of estrogen and cause cell differentiation, resulting in stromal decidualization. Progestins also reduce the concentration of estrogen and progesterone receptors and increase the activity of enzymes that convert estradiol to estrone, blocking estrogen-induced endometrial proliferation.14
In women with endometrial hyperplasia, progestins have been shown to be effective in resolving the hyperplasia in approximately 80% of cases. Both oral progestins and the 52-mg levonorgestrel-containing intrauterine device (LNG-IUD) have been reported to be effective in the treatment of endometrial hyperplasia. In a Cochrane systematic review and meta-analysis, the 52-mg LNG-IUD was reported to be somewhat more effective in resolving endometrial hyperplasia than cyclic oral progestins (89% vs 72%, respectively).15
Other studies have also reported that the 52 mg LNG-IUD was more effective than oral progestin therapy for women with complex atypical endometrial hyperplasia.16 There are no large randomized clinical trials of progestin therapy on prevention for future development of endometrial cancer in obese postmenopausal women who have a normal endometrial histology. However, for an obese perimenopausal woman, insertion of a 52-mg LNG-IUD may help to minimize excessive uterine bleeding during the menopause transition and reduce the risk of developing endometrial hyperplasia during the early postmenopause.
We can help our patients reduce their risk of endometrial cancer
Obese postmenopausal women are at increased risk for developing endometrial cancer. Gynecologists play an important role in the prevention and early detection of endometrial cancer. We can make a difference and improve the health of our obese peri- and postmenopausal women by recommending interventions that reduce the risk of endometrial cancer, thereby improving the health of our patients. ●
- American Society of Clinical Oncology. Uterine cancer statistics. https://www.cancer.net/cancer-types/uterine-cancer/statistics#:~:text=This%20year%2C%20an%20
estimated%2065%2C620,cancers%20occur%20in%20the%20endometrium. Accessed November 23, 2020. - Howlader N, Noone AM, Krapcho M, et al (eds). SEER Cancer Statistics Review, 1975-2017. National Cancer Institute: Bethesda, MD. April 15, 2020. https://seer.cancer.gov/csr/1975_2017/. Accessed November 23, 2020.
- Noer MC, Antonsen SL, Ottesen B, et al. Type I versus Type II endometrial cancer: differential impact of comorbidity. Int J Gynecol Cancer. 2018;28:586-593.
- Sorosky JI. Endometrial cancer. Obstet Gynecol. 2008;111:436-437.
- Gallup DG, Stock RJ. Adenocarcinoma of the endometrium in women 40 years of age or younger. Obstet Gynecol. 1984;64:417-420.
- Setiawan VW, Yang HP, Pike MC, et al. Type I and II endometrial cancers: have they different risk factors. J Clin Oncol. 2013;31:2607-2618.
- Saccardi C, Vitagliano A, Marchetti M, et al. Endometrial cancer risk prediction according to indication of diagnostic hysteroscopy in postmenopausal women. Diagnostics (Basel). 2020;10:257.e1-e11.
- Luo J, Chlebowski RT, Hendryx M, et al. Intentional weight loss and endometrial cancer risk. J Clin Oncology. 2017;35:1189-1193.
- Friedenreich CM, Ryder-Burbidge C, McNeil J. Physical activity, obesity and sedentary behavior in cancer etiology: epidemiologic evidence and biological mechanisms. Mol Oncol. August 2, 2020. doi: 10.1001/1878-0261.12772.
- Schauer DP, Feigelson HS, Koebnick C, et al. Bariatric surgery and the risk of cancer in a large multisite cohort. Ann Surg. 2019;269:95-101.
- Anvenden A, Taube M, Peltonen M, et al. Long-term incidence of female-specific cancer after bariatric surgery or usual care in the Swedish Obese Subjects Study. Gynecol Oncol. 2017;145:224-229.
- Winder AA, Kularatna M, MacCormick AD. Does bariatric surgery affect the incidence of endometrial cancer development? A systematic review. Obes Surg. 2018;28:1433-1440.
- Carlsson LM, Sjoholm K, Jacobson P, et al. Life expectancy after bariatric surgery in the Swedish Obese Subjects Study. N Engl J Med. 2020;383:1535-1543.
- Lessey BA, Young SL. In: Strauss JF, Barbieri RL (eds.) Yen and Jaffe’s Reproductive Endocrinology: Physiology, Pathophysiology and Clinical Management. 8th ed. Elsevier Saunders: Philadelphia, PA; 2018:208-212.
- Mittermeier T, Farrant C, Wise MR. Levonorgestrel-releasing intrauterine system for endometrial hyperplasia. Cochrane Database Syst Rev. 2020;CD012658.
- Mandelbaum RS, Ciccone MA, Nusbaum DJ, et al. Progestin therapy for obese women with complex atypical hyperplasia: levonorgestrel-releasing intrauterine device vs systemic therapy. Am J Obstet Gynecol. 2020;223:103.e1-e13.
Endometrial cancer is the most common gynecologic malignancy, with approximately 59,000 cases diagnosed annually,1 and a lifetime risk of approximately 3.1% in the United States.2 Type I endometrial cancer includes tumors with endometrioid histology that are grade 1 or 2. Type II endometrial cancer includes tumors that have grade 3 endometrioid or nonendometrioid histology, including serous, clear cell, mucinous, squamous transitional cell, mesonephric, and undifferentiated tumors.3 Type I endometrial cancer is hormone sensitive, generally stimulated by estrogen and suppressed by progestins.
Endometrial cancer is diagnosed at a mean age of 63 years,4 and only 15% of cases occur before age 50.5 Women with an elevated body mass index (BMI) have a markedly increased risk of both Types I and II endometrial cancer (TABLE).6 Hence, endometrial cancer is highly prevalent in obese postmenopausal women. For these women health interventions that may reduce the risk of developing endometrial cancer include dieting, physical activity, bariatric surgery, and progestin therapy.

Educating patients is a priority
Many women do not know that postmenopausal bleeding is a sign of endometrial cancer. All postmenopausal women should be advised that if they develop vaginal bleeding they need to be evaluated by a clinician.7 Women who are knowledgeable about the link between postmenopausal vaginal bleeding and endometrial cancer can be encouraged to share this information with their postmenopausal friends in order to reach more people with this important information. All obese postmenopausal women should be advised that weight loss and increased physical activity can reduce the risk of developing endometrial cancer.
How weight loss and physical activity affect risk
Intentional weight loss has been reported to reduce the risk of endometrial cancer in postmenopausal women. As part of the Women’s Health Initiative observational study, 36,794 postmenopausal women aged 50 to 79 years with a uterus had their body weight and height measured at entry into the study and after 3 years of follow-up.8 During the 11 years following study entry, there were 566 incident cases of endometrial cancer. Compared with women who had a stable weight, intentional weight loss of ≥5% was associated with a 40% reduction in the risk of endometrial cancer (hazard ratio [HR], 0.60; 95% confidence interval [CI], 0.42–0.86). Compared with women who had a stable weight, women who had weight gain ≥10% had an increased risk of endometrial cancer (HR, 1.26; 95% CI, 1.00–1.57).
High levels of physical activity may be associated with a decreased risk of endometrial cancer. In one study, compared with a sedentary lifestyle, higher levels of physical activity were reported to be associated with a decreased risk of endometrial cancer.9
Continue to: How bariatric surgery affects risk...
How bariatric surgery affects risk
Many cancers are associated with obesity, including endometrial, breast, colon, pancreas, gallbladder, and renal. Obesity is associated with increased conversion of androgens to estrogens in fat tissue, stimulating excessive endometrial proliferation and increasing the risk of endometrial hyperplasia and cancer. Bariatric surgery reliably causes sustained weight reduction. Multiple studies have reported that bariatric surgery reduces the risk of endometrial cancer.
Schauer and colleagues used data from the Kaiser Permanente health system to identify 22,198 obese people who had undergone bariatric surgery and 66,427 matched controls who were obese but did not have surgery.10 The study population was 81% female, with a mean age of 45 years and a mean BMI of 45 kg/m2. After an average 3.5 years of follow-up there were 2,542 incident cases of cancer, including 322 cases of endometrial cancer. Compared with conventional weight loss treatment, bariatric surgery reduced the risk of endometrial cancer by 50% (HR, 0.50; 95% CI, 0.37–0.67; P<.001).10 In addition, bariatric surgery reduced the risk of colon and pancreatic cancer by 41% and 54%, respectively.10
In the Swedish Obese Subjects (SOS) study, 1,420 women who underwent bariatric surgery and 1,447 matched controls who received conventional obesity treatment were followed for 18 years.11 At study entry, the mean age of the women was approximately 48 years, and the mean BMI was approximately 42 kg/m2. In follow-up there were 76 incident cases of endometrial cancer. Compared with women receiving conventional obesity treatment, women who had bariatric surgery had a non–statistically significant 49% decrease in the risk of developing endometrial cancer (HR, 0.51; 95% CI, 0.24–1.10)
In a systematic review of 5 additional studies (not including publications 10 or 11) of the impact of bariatric surgery on the risk of developing endometrial cancer, the surgery was associated with a 68% risk reduction (odds ratio [OR], 0.32; 95% CI, 0.16–0.63) compared with matched obese women that did not have surgery.12
Although there are no randomized prospective studies showing that bariatric surgery reduces the risk of endometrial cancer, the weight of the observation evidence is strong. In addition, bariatric surgery was reported to reduce all-cause mortality in the SOS study.13 Hence, for obese postmenopausal women, if lifestyle changes do not result in sustained weight loss, bariatric surgery may be an optimal approach to improving health outcomes.
Continue to: Progestin treatment and endometrial cancer risk...
Progestin treatment and endometrial cancer risk
Estrogen stimulates endometrial cell proliferation. Hence, unopposed chronic exposure to estrogen is a major risk factor for developing endometrial hyperplasia and cancer. Progestins block the proliferative effect of estrogen and cause cell differentiation, resulting in stromal decidualization. Progestins also reduce the concentration of estrogen and progesterone receptors and increase the activity of enzymes that convert estradiol to estrone, blocking estrogen-induced endometrial proliferation.14
In women with endometrial hyperplasia, progestins have been shown to be effective in resolving the hyperplasia in approximately 80% of cases. Both oral progestins and the 52-mg levonorgestrel-containing intrauterine device (LNG-IUD) have been reported to be effective in the treatment of endometrial hyperplasia. In a Cochrane systematic review and meta-analysis, the 52-mg LNG-IUD was reported to be somewhat more effective in resolving endometrial hyperplasia than cyclic oral progestins (89% vs 72%, respectively).15
Other studies have also reported that the 52 mg LNG-IUD was more effective than oral progestin therapy for women with complex atypical endometrial hyperplasia.16 There are no large randomized clinical trials of progestin therapy on prevention for future development of endometrial cancer in obese postmenopausal women who have a normal endometrial histology. However, for an obese perimenopausal woman, insertion of a 52-mg LNG-IUD may help to minimize excessive uterine bleeding during the menopause transition and reduce the risk of developing endometrial hyperplasia during the early postmenopause.
We can help our patients reduce their risk of endometrial cancer
Obese postmenopausal women are at increased risk for developing endometrial cancer. Gynecologists play an important role in the prevention and early detection of endometrial cancer. We can make a difference and improve the health of our obese peri- and postmenopausal women by recommending interventions that reduce the risk of endometrial cancer, thereby improving the health of our patients. ●
Endometrial cancer is the most common gynecologic malignancy, with approximately 59,000 cases diagnosed annually,1 and a lifetime risk of approximately 3.1% in the United States.2 Type I endometrial cancer includes tumors with endometrioid histology that are grade 1 or 2. Type II endometrial cancer includes tumors that have grade 3 endometrioid or nonendometrioid histology, including serous, clear cell, mucinous, squamous transitional cell, mesonephric, and undifferentiated tumors.3 Type I endometrial cancer is hormone sensitive, generally stimulated by estrogen and suppressed by progestins.
Endometrial cancer is diagnosed at a mean age of 63 years,4 and only 15% of cases occur before age 50.5 Women with an elevated body mass index (BMI) have a markedly increased risk of both Types I and II endometrial cancer (TABLE).6 Hence, endometrial cancer is highly prevalent in obese postmenopausal women. For these women health interventions that may reduce the risk of developing endometrial cancer include dieting, physical activity, bariatric surgery, and progestin therapy.

Educating patients is a priority
Many women do not know that postmenopausal bleeding is a sign of endometrial cancer. All postmenopausal women should be advised that if they develop vaginal bleeding they need to be evaluated by a clinician.7 Women who are knowledgeable about the link between postmenopausal vaginal bleeding and endometrial cancer can be encouraged to share this information with their postmenopausal friends in order to reach more people with this important information. All obese postmenopausal women should be advised that weight loss and increased physical activity can reduce the risk of developing endometrial cancer.
How weight loss and physical activity affect risk
Intentional weight loss has been reported to reduce the risk of endometrial cancer in postmenopausal women. As part of the Women’s Health Initiative observational study, 36,794 postmenopausal women aged 50 to 79 years with a uterus had their body weight and height measured at entry into the study and after 3 years of follow-up.8 During the 11 years following study entry, there were 566 incident cases of endometrial cancer. Compared with women who had a stable weight, intentional weight loss of ≥5% was associated with a 40% reduction in the risk of endometrial cancer (hazard ratio [HR], 0.60; 95% confidence interval [CI], 0.42–0.86). Compared with women who had a stable weight, women who had weight gain ≥10% had an increased risk of endometrial cancer (HR, 1.26; 95% CI, 1.00–1.57).
High levels of physical activity may be associated with a decreased risk of endometrial cancer. In one study, compared with a sedentary lifestyle, higher levels of physical activity were reported to be associated with a decreased risk of endometrial cancer.9
Continue to: How bariatric surgery affects risk...
How bariatric surgery affects risk
Many cancers are associated with obesity, including endometrial, breast, colon, pancreas, gallbladder, and renal. Obesity is associated with increased conversion of androgens to estrogens in fat tissue, stimulating excessive endometrial proliferation and increasing the risk of endometrial hyperplasia and cancer. Bariatric surgery reliably causes sustained weight reduction. Multiple studies have reported that bariatric surgery reduces the risk of endometrial cancer.
Schauer and colleagues used data from the Kaiser Permanente health system to identify 22,198 obese people who had undergone bariatric surgery and 66,427 matched controls who were obese but did not have surgery.10 The study population was 81% female, with a mean age of 45 years and a mean BMI of 45 kg/m2. After an average 3.5 years of follow-up there were 2,542 incident cases of cancer, including 322 cases of endometrial cancer. Compared with conventional weight loss treatment, bariatric surgery reduced the risk of endometrial cancer by 50% (HR, 0.50; 95% CI, 0.37–0.67; P<.001).10 In addition, bariatric surgery reduced the risk of colon and pancreatic cancer by 41% and 54%, respectively.10
In the Swedish Obese Subjects (SOS) study, 1,420 women who underwent bariatric surgery and 1,447 matched controls who received conventional obesity treatment were followed for 18 years.11 At study entry, the mean age of the women was approximately 48 years, and the mean BMI was approximately 42 kg/m2. In follow-up there were 76 incident cases of endometrial cancer. Compared with women receiving conventional obesity treatment, women who had bariatric surgery had a non–statistically significant 49% decrease in the risk of developing endometrial cancer (HR, 0.51; 95% CI, 0.24–1.10)
In a systematic review of 5 additional studies (not including publications 10 or 11) of the impact of bariatric surgery on the risk of developing endometrial cancer, the surgery was associated with a 68% risk reduction (odds ratio [OR], 0.32; 95% CI, 0.16–0.63) compared with matched obese women that did not have surgery.12
Although there are no randomized prospective studies showing that bariatric surgery reduces the risk of endometrial cancer, the weight of the observation evidence is strong. In addition, bariatric surgery was reported to reduce all-cause mortality in the SOS study.13 Hence, for obese postmenopausal women, if lifestyle changes do not result in sustained weight loss, bariatric surgery may be an optimal approach to improving health outcomes.
Continue to: Progestin treatment and endometrial cancer risk...
Progestin treatment and endometrial cancer risk
Estrogen stimulates endometrial cell proliferation. Hence, unopposed chronic exposure to estrogen is a major risk factor for developing endometrial hyperplasia and cancer. Progestins block the proliferative effect of estrogen and cause cell differentiation, resulting in stromal decidualization. Progestins also reduce the concentration of estrogen and progesterone receptors and increase the activity of enzymes that convert estradiol to estrone, blocking estrogen-induced endometrial proliferation.14
In women with endometrial hyperplasia, progestins have been shown to be effective in resolving the hyperplasia in approximately 80% of cases. Both oral progestins and the 52-mg levonorgestrel-containing intrauterine device (LNG-IUD) have been reported to be effective in the treatment of endometrial hyperplasia. In a Cochrane systematic review and meta-analysis, the 52-mg LNG-IUD was reported to be somewhat more effective in resolving endometrial hyperplasia than cyclic oral progestins (89% vs 72%, respectively).15
Other studies have also reported that the 52 mg LNG-IUD was more effective than oral progestin therapy for women with complex atypical endometrial hyperplasia.16 There are no large randomized clinical trials of progestin therapy on prevention for future development of endometrial cancer in obese postmenopausal women who have a normal endometrial histology. However, for an obese perimenopausal woman, insertion of a 52-mg LNG-IUD may help to minimize excessive uterine bleeding during the menopause transition and reduce the risk of developing endometrial hyperplasia during the early postmenopause.
We can help our patients reduce their risk of endometrial cancer
Obese postmenopausal women are at increased risk for developing endometrial cancer. Gynecologists play an important role in the prevention and early detection of endometrial cancer. We can make a difference and improve the health of our obese peri- and postmenopausal women by recommending interventions that reduce the risk of endometrial cancer, thereby improving the health of our patients. ●
- American Society of Clinical Oncology. Uterine cancer statistics. https://www.cancer.net/cancer-types/uterine-cancer/statistics#:~:text=This%20year%2C%20an%20
estimated%2065%2C620,cancers%20occur%20in%20the%20endometrium. Accessed November 23, 2020. - Howlader N, Noone AM, Krapcho M, et al (eds). SEER Cancer Statistics Review, 1975-2017. National Cancer Institute: Bethesda, MD. April 15, 2020. https://seer.cancer.gov/csr/1975_2017/. Accessed November 23, 2020.
- Noer MC, Antonsen SL, Ottesen B, et al. Type I versus Type II endometrial cancer: differential impact of comorbidity. Int J Gynecol Cancer. 2018;28:586-593.
- Sorosky JI. Endometrial cancer. Obstet Gynecol. 2008;111:436-437.
- Gallup DG, Stock RJ. Adenocarcinoma of the endometrium in women 40 years of age or younger. Obstet Gynecol. 1984;64:417-420.
- Setiawan VW, Yang HP, Pike MC, et al. Type I and II endometrial cancers: have they different risk factors. J Clin Oncol. 2013;31:2607-2618.
- Saccardi C, Vitagliano A, Marchetti M, et al. Endometrial cancer risk prediction according to indication of diagnostic hysteroscopy in postmenopausal women. Diagnostics (Basel). 2020;10:257.e1-e11.
- Luo J, Chlebowski RT, Hendryx M, et al. Intentional weight loss and endometrial cancer risk. J Clin Oncology. 2017;35:1189-1193.
- Friedenreich CM, Ryder-Burbidge C, McNeil J. Physical activity, obesity and sedentary behavior in cancer etiology: epidemiologic evidence and biological mechanisms. Mol Oncol. August 2, 2020. doi: 10.1001/1878-0261.12772.
- Schauer DP, Feigelson HS, Koebnick C, et al. Bariatric surgery and the risk of cancer in a large multisite cohort. Ann Surg. 2019;269:95-101.
- Anvenden A, Taube M, Peltonen M, et al. Long-term incidence of female-specific cancer after bariatric surgery or usual care in the Swedish Obese Subjects Study. Gynecol Oncol. 2017;145:224-229.
- Winder AA, Kularatna M, MacCormick AD. Does bariatric surgery affect the incidence of endometrial cancer development? A systematic review. Obes Surg. 2018;28:1433-1440.
- Carlsson LM, Sjoholm K, Jacobson P, et al. Life expectancy after bariatric surgery in the Swedish Obese Subjects Study. N Engl J Med. 2020;383:1535-1543.
- Lessey BA, Young SL. In: Strauss JF, Barbieri RL (eds.) Yen and Jaffe’s Reproductive Endocrinology: Physiology, Pathophysiology and Clinical Management. 8th ed. Elsevier Saunders: Philadelphia, PA; 2018:208-212.
- Mittermeier T, Farrant C, Wise MR. Levonorgestrel-releasing intrauterine system for endometrial hyperplasia. Cochrane Database Syst Rev. 2020;CD012658.
- Mandelbaum RS, Ciccone MA, Nusbaum DJ, et al. Progestin therapy for obese women with complex atypical hyperplasia: levonorgestrel-releasing intrauterine device vs systemic therapy. Am J Obstet Gynecol. 2020;223:103.e1-e13.
- American Society of Clinical Oncology. Uterine cancer statistics. https://www.cancer.net/cancer-types/uterine-cancer/statistics#:~:text=This%20year%2C%20an%20
estimated%2065%2C620,cancers%20occur%20in%20the%20endometrium. Accessed November 23, 2020. - Howlader N, Noone AM, Krapcho M, et al (eds). SEER Cancer Statistics Review, 1975-2017. National Cancer Institute: Bethesda, MD. April 15, 2020. https://seer.cancer.gov/csr/1975_2017/. Accessed November 23, 2020.
- Noer MC, Antonsen SL, Ottesen B, et al. Type I versus Type II endometrial cancer: differential impact of comorbidity. Int J Gynecol Cancer. 2018;28:586-593.
- Sorosky JI. Endometrial cancer. Obstet Gynecol. 2008;111:436-437.
- Gallup DG, Stock RJ. Adenocarcinoma of the endometrium in women 40 years of age or younger. Obstet Gynecol. 1984;64:417-420.
- Setiawan VW, Yang HP, Pike MC, et al. Type I and II endometrial cancers: have they different risk factors. J Clin Oncol. 2013;31:2607-2618.
- Saccardi C, Vitagliano A, Marchetti M, et al. Endometrial cancer risk prediction according to indication of diagnostic hysteroscopy in postmenopausal women. Diagnostics (Basel). 2020;10:257.e1-e11.
- Luo J, Chlebowski RT, Hendryx M, et al. Intentional weight loss and endometrial cancer risk. J Clin Oncology. 2017;35:1189-1193.
- Friedenreich CM, Ryder-Burbidge C, McNeil J. Physical activity, obesity and sedentary behavior in cancer etiology: epidemiologic evidence and biological mechanisms. Mol Oncol. August 2, 2020. doi: 10.1001/1878-0261.12772.
- Schauer DP, Feigelson HS, Koebnick C, et al. Bariatric surgery and the risk of cancer in a large multisite cohort. Ann Surg. 2019;269:95-101.
- Anvenden A, Taube M, Peltonen M, et al. Long-term incidence of female-specific cancer after bariatric surgery or usual care in the Swedish Obese Subjects Study. Gynecol Oncol. 2017;145:224-229.
- Winder AA, Kularatna M, MacCormick AD. Does bariatric surgery affect the incidence of endometrial cancer development? A systematic review. Obes Surg. 2018;28:1433-1440.
- Carlsson LM, Sjoholm K, Jacobson P, et al. Life expectancy after bariatric surgery in the Swedish Obese Subjects Study. N Engl J Med. 2020;383:1535-1543.
- Lessey BA, Young SL. In: Strauss JF, Barbieri RL (eds.) Yen and Jaffe’s Reproductive Endocrinology: Physiology, Pathophysiology and Clinical Management. 8th ed. Elsevier Saunders: Philadelphia, PA; 2018:208-212.
- Mittermeier T, Farrant C, Wise MR. Levonorgestrel-releasing intrauterine system for endometrial hyperplasia. Cochrane Database Syst Rev. 2020;CD012658.
- Mandelbaum RS, Ciccone MA, Nusbaum DJ, et al. Progestin therapy for obese women with complex atypical hyperplasia: levonorgestrel-releasing intrauterine device vs systemic therapy. Am J Obstet Gynecol. 2020;223:103.e1-e13.
9vHPV vaccine: Prevention of oropharyngeal cancer
Surprisingly, in the United States, the most common cancer associated with human papillomavirus (HPV) is oropharyngeal squamous cell cancer (SCC), with one study reporting 15,479 cases among men and 3,428 cases among women in 2015.1 In the same year, the investigators reported 11,788 cases of cervical cancer.1 A public health concern is that cases of oropharyngeal SCC are increasing, while cases of cervical cancer are decreasing. From 1999 to 2015, the rate of oropharyngeal SCC increased annually among both men and women, at rates of 2.7% and 0.8% per year, respectively. By contrast, the rate of cervical cancer decreased by 1.6% per year.1
Although the incidence of HPV-negative oropharyngeal SCC (cases associated with cigarette smoking) has declined by 50% from 1988 to 2004, the incidence of HPV-positive oropharyngeal SCC has increased by 225%, with much of the increase occurring among young, white men.2 HPV infection is a major cause of oropharyngeal SCC at the base of the tongue and tonsils, but not in the soft palate or oropharyngeal walls.3
Most physicians and parents recognize that the 9-valent (9v)HPV vaccine prevents the majority of cervical cancers and precancers in women. Far fewer people realize that there is an important opportunity to prevent a large number of oropharyngeal cancers by improving 9vHPV vaccination in men and women.
Which HPV types are associated with oropharyngeal cancer?
HPV16 is the most common HPV type associated with oropharyngeal SCC. Among these cancer types, greater than 80% harbor HPV16, with greater than 90% harboring HPV16 or 18 and less than 10% of tumors associated with HPV types 31, 33, 45, 52, or 58.4-7
The high prevalence of HPV16 in patients with oropharyngeal cancer raises the question of the HPV status of the intimate partner of the index patient. In one study of 164 people with HPV detected in their oropharyngeal, the partner of the index patient had a low prevalence of high-risk HPV types (1.2%) in oral rinse and gargle samples, similar to the rate in the general population (1.3%).7 This finding is reassuring and suggests that intimate partners of patients with HPV-positive oropharyngeal cancer effectively clear high-risk HPV virus from the oropharynx. The HPV status of the genital tissue of the intimate partner of an index patient with oropharyngeal SCC has not been adequately studied.
Men are more likely than women to harbor oral HPV
Among a sample of 5,501 men and women aged 14 to 69 years from the National Health and Nutrition Examination Survey, oral rinses were obtained and analyzed for the presence of HPV.8 The prevalence of any oral HPV and any oral high-risk HPV was 6.9% and 3.7%, respectively. Oral HPV-16 was detected in 1.6% of men and 0.3% of women. The prevalence of HPV was higher among current smokers, heavy alcohol drinkers, and people with a history of a greater number of sexual partners. In men and women reporting more than 20 lifetime sexual partners, the prevalence of oral HPV was 20%.
In a study of 2,627 men and women aged 18 to 33 years, the prevalence of oral HPV 16/18/6/11 was lower among those vaccinated versus those unvaccinated (0.11% and 1.6%, respectively; P = .008).9 Among men, oral HPV 16/18/6/11 was lower among those vaccinated versus unvaccinated (0.0% and 2.13%, respectively; P = .007).9 The results of this observational study support the important role of vaccination in reducing oral HPV infection.
In 2020, the US Food and Drug Administration (FDA) approved the 9-valent human papillomavirus (9vHPV) vaccine for the prevention of oropharyngeal cancer. The 9vHPV vaccine contains inactive L1 capsid proteins for 9 HPV types, including types 6, 11, 16, 18, 31, 33, 45, 52, and 58. The vaccine stimulates the production of neutralizing antibodies to the capsid protein.
9vHPV is approved for females aged 9 to 45 years to prevent cancers and precancers of the cervix, vulva, vagina, and anus caused by HPV types 16, 18, 31, 33, 45, 52, and 58.1 It is also approved for males aged 9 to 45 years to prevent cancer and precancers of the anus caused by those viral types. In 2020 the 9vHPV vaccine was approved by the FDA to prevent oropharyngeal cancer in males and females. Of note, the FDA reported that, “the oropharyngeal and head and neck cancer indication is approved under accelerated approval based on effectiveness in preventing HPV-related anogenital disease. Continued approval for this indication may be contingent upon verification and description of clinical benefit in a confirmatory trial.”2
The Advisory Committee on Immunization Practices (ACIP) recommends routine vaccination of girls and boys, 11 to 12 years of age.1 Children with a history of sexual abuse or assault can start the vaccine at 9 years of age. Catch-up vaccination is recommended for all females and males through age 26 years. The ACIP recommends shared clinical decision-making regarding vaccination for some adults 27 to 45 years of age. Gynecologists with routine exposure to HPV may have occupational risk that warrants HPV vaccination3 (see “As a gynecologist, should you receive the 9vHPV vaccine?”).
For most individuals who start the vaccine series before age 15, two doses of 9vHPV vaccine are recommended, with the second dose 6 to 12 months following the first dose. For teens and adults aged 15 to 26 years, 3 doses of 9vHPV vaccine are recommended, with the second dose 1 to 2 months later and the third dose 6 months following the first dose. Immunocompromised individuals 9 to 26 years of age, including those with HIV infection, should receive 3 doses of the vaccine.
References
1. Meites E, Szilagyi PG, Chesson HW, et al. Human papillomavirus vaccination for adults: updated recommendations of the Advisory Committee on Immunization Practices. MMWR Morb Mortal Wkly Rep. 2019;68:698-702.
2. Gardasil 9 [package insert]. Whitehouse Station, NJ: Merck & Co. Inc; 2020.
3. Stockdale CK, Einstein MH, Huh WK. ASCCP recommends HPV vaccination for providers. February 19, 2020. https://www.asccp.org/Assets/d3abdb05-25c5-4e58-9cec-05c11fb2b920/637177876310030000/hpv-vaccinemember-announcment-02-19-20-pdf. Accessed October 23, 2020.
Continue to: Vaccinate boys and girls to prevent cancer...
Vaccinate boys and girls to prevent cancer
Most population studies report that males are less likely to receive an HPV vaccine than females. For example, based on the National Health Interview Survey of people aged 18 to 26, the percentage of women who self-reported receiving at least one dose of HPV vaccine was 37% in 2013 and 54% in 2018.10 By contrast, among men, the rates of self-reported vaccination were much lower—8% in 2013 and 27% in 2018.10
The percentage of women who received the recommended number of doses of HPV vaccine (see “9vHPV vaccine: Indications and immunization schedule”) was 26% in 2013 and 35% in 2018.10 For men, these percentages were 2% in 2013 and 9% in 2018.10 These data indicate that, compared with women, men are less likely to receive an HPV vaccination and far less likely to have received the recommended number of doses.
It is heartening that there has been a slow and steady increase in the prevalence of HPV vaccination. In fact, increasing the HPV vaccination rate among both boys and girls has the potential to markedly reduce the incidence of oropharyngeal cancer.
The reasons for the female-male gap in vaccination rates are not fully characterized. For one, parental awareness of the importance of HPV vaccination to prevent cancer among men is limited, and represents an important opportunity for additional public health education. In a qualitative interview study of mothers with children aged 11 to 19, the investigators reported that most mothers were aware that HPV vaccination could prevent cervical cancer in women, but most mothers did not know that HPV causes cancer of the mouth and that vaccination could prevent oropharyngeal cancer in boys and girls.11 Because of this lack of knowledge, the mothers did not think their sons needed to have an HPV vaccine. The research report is aptly titled, “I don’t think he needs the HPV vaccine cause boys can’t have cervical cancer.”11
Clinicians are highly influential in guiding parents to accept HPV vaccination of their children. Offering consistent messaging to parents that HPV vaccination prevents cancer in both women and men, and reducing the out-of-pocket cost of vaccination surely will result in an increase in the vaccination rate of boys and girls. ●
Surgical treatment of tissues infected with human papillomavirus (HPV) often involves the use of laser or electrosurgical devices that generate smoke, which is known to contain HPV nucleic acid sequences and may contain infective virions.1 It is known that HPV nucleic acid sequences are present in surgical smoke. In one study plantar warts were treated with a carbon dioxide laser or electrocoagulation. The vapor produced from the surgery was collected with a dry filter apparatus. Five of 8 laser-derived vapors and 4 of 7 electrocoagulation-derived vapors were positive for HPV DNA. The concentration of HPV DNA was greater with laser than with electrocoagulation treatment.2
It is not known if surgical smoke derived from treatment of HPV-infected tissues contains infective HPV virions. In an experimental bovine model, smoke generated by laser ablation of fibropapillomas was collected. Injection of the contents of the smoke caused cutaneous papillomavirus lesions when inoculated into calves, suggesting that the smoke contained infective HPV virions.3 Although this animal experiment is a proof of principle that surgical smoke generated from treatment of HPVinfected tissue contain virions, it is unclear if surgical smoke generated in gynecologic practice contains HPV virions.
To investigate the prevalence of nasal HPV DNA among gynecologists, 700 physicians in Zhejiang Province, China, completed a questionnaire and provided a nasal swab for HPV DNA analysis.4 Among gynecologists who performed or did not perform LEEP, the prevalence of HPV DNA in the nose was 10% and 3%, respectively. The most common HPV types detected were HPV16 (76%), HPV31 (10%), HPV58 (5%), HPV55 (5%), HPV56 (2%), and HPV59 (2%).4 Among gynecologists who performed LEEP procedures, the prevalence of HPV DNA was 19% for those who did not use a surgical mask, 8% for clinicians who used a standard surgical mask, and 0% for those who used an N95 filtering facepiece respirator, suggesting that an N95 respirator provides the greatest protection from surgical smoke.4 Over 24 months of follow-up, all the gynecologists who had initially tested positive for HPV DNA no longer had detectable nasal HPV DNA. In this study, no gynecologist was diagnosed with an HPV-associated oropharyngeal disease. The investigators concluded that surgical masks, especially an N95 respirator, should be used by gynecologists performing LEEP procedures.
Investigators also have evaluated for the presence of HPV DNA in matched samples from the cervix of 134 patients undergoing loop electrosurgical excision procedure (LEEP) for cervical dysplasia, as well as the smoke generated during the procedure and nasal swabs from the surgeon performing the LEEP.5 HPV DNA was detected in 95% of the cervical samples, 30% of the surgical smoke samples, and 1.5% of the surgeons’ nasal swabs.5 At 6 months of follow-up, the two surgeons who initially had HPV-positive nasal swabs no longer had detected HPV DNA.
Of concern is that otolaryngologists have reported sporadic cases of oropharyngeal squamous cell cancer6 and laryngeal papillomatosis7 in health care workers with frequent and repetitive exposure to HPVs. For example, in one case report, a 53-year-old male gynecologist, nonsmoker, presented to his physician with a lump on the neck.6 The gynecologist had performed more than 3,000 laser ablation or LEEP procedures of dysplastic cervical, vaginal, and vulvar lesions over a span of 20 years.6 Most of the procedures were performed without wearing a mask and in a poorly ventilated procedure room. A computed tomography scan demonstrated a 2.2-cm soft tissue lesion in the right tonsil extending to the right soft palate and a level-2 lymph node. A biopsy of the tonsil confirmed invasive squamous cell carcinoma containing HPV16. He was treated with 35 fractions of radiotherapy and adjuvant cisplatin. Treatment adverse effects included dysphagia and xerostomia, and the patient lost 40 pounds.
Available interventions to reduce exposure of clinicians to HPV virions that may be present in surgical smoke include:
- wearing a fit-tested N95 respirator
- routinely using a smoke evacuation device, and
- ensuring sufficient ventilation in the procedure room.
A new recommendation is to consider 9vHPV vaccination for clinicians who are routinely exposed to HPV virions.8,9 In February 2020, the American Society for Colposcopy and Cervical Pathology recommended that clinicians who are routinely exposed to HPVs consider 9vHPV vaccination.8 This recommendation pertains to all members of the clinical team in the procedure room, including physicians, nurses, and staff. Based on the available data, gynecologists who have not been vaccinated will need to weigh the benefits and costs of receiving a 9vHPV vaccine to protect themselves against an occupational exposure that may adversely impact their health.
References
- Liu Y, Song Y, Hu X, et al. Awareness of surgical smoke hazards and enhancement of surgical smoke prevention among gynecologists. J Cancer. 2019;10:2788-2799.
- Sawchuk WS, Weber PJ, Lowy DR, et al. Infectious papillomavirus in the vapor of warts treated with carbon dioxide laser or electrocoagulation: detection and protection. J Am Acad Dermatol. 1989;21:41-49.
- Garden JM, O’Banion MK, Bakus AD, et al. Viral transmitted by laser-generated plume (aerosol). Arch Dermatol. 2002;138:1303-1307.
- Hu X, Zhou Q, Yu J, et al. Prevalence of HPV infections in surgical smoke exposed gynecologists. Int Arch Occup Environ Health. 2020; Epub September 1. doi: 10.1007 /s00420-020-01568-9.
- Zhou Q, Hu X, Zhou J, et al. Human papillomavirus DNA in surgical smoke during cervical loop electrosurgical excision procedures and its impact on the surgeon. Cancer Manag Res. 2019;11:3643-3654.
- Rioux M, Garland A, Webster D, et al. HPV-positive tonsillar cancer in two laser surgeons: case reports. J Otolaryngol Head Neck Surg. 2013;42:54-57.
- Hallmo P, Naess O. Laryngeal papillomatosis with human papillomavirus DNA contracted by a laser surgeon. Eur Arch Otorhinolaryngol. 1991;248:425-427.
Stockdale CK, Einstein MH, Huh WK. ASCCP recommends HPV vaccination for providers. February 19, 2020. www.asccp.org/Assets/d3abdb05-25c5-4e58-%209cec-05c11fb2b920/637177876310030000/hpv-vaccinemember-announcment-02-19-20-pdf. Accessed October 23, 2020.
- Harrison R, Huh W. Occupational exposure to human papillomavirus and vaccination for health care workers. Obstet Gynecol. 2020;136:663-665
- Van Dyne EA, Henley SJ, Saraiya M, et al. Trends in human papillomavirus-associated cancers--United States, 1999-2015. MMWR. 2018;67:918-924.
- Chaturvedi AK, Engels EA, Pfeiffer RM, et al. Human papillomavirus and rising oropharyngeal cancer incidence in the United States. J Clin Oncol. 2011;29:4294-4301.
- Haeggblom L, Ramqvist T, Tommasino M, et al. Time to change perspective on HPV in oropharyngeal cancer. A systematic review of HPV prevalence per oropharyngeal sub-site the last 3 years. Papillomavirus Research. 2017;4:1-11.
- Kreimer AR, Clifford GM, Boyle P, et al. Human papillomavirus types in head and neck squamous cell carcinomas worldwide: a systematic review. Cancer Epidemiol Biomarkers Prev. 2005;14:467-475.
- D'Souza G, Kreimer AR, Viscidi R, et al. Case-control study of human papillomavirus and oropharyngeal cancer. N Engl J Med. 2007;356:1944-1956.
- de Martel C, Plummer M, Vignat J, et al. Worldwide burden of cancer attributable to HPV by site, country and HPV type. Int J Cancer. 2017;141:664-670.
- D'Souza G, Gross ND, Pai SI, et al. Oral human papillomavirus infection in HPV-positive patients with oropharyngeal cancer and their partners. J Clin Oncol. 2014;32:2408-2415.
- Gillison ML, Broutian T, Pickard RK, et al. Prevalence of oral HPV infection in the United States, 2009-2010. JAMA. 2012;307:693.
- Chaturvedi AK, Graubard BI, Broutian T, et al. Effect of prophylactic human papillomavirus vaccination on oral HPV infections among young adults in the United States. J Clin Oncol. 2018;36:262-267.
- Boersma P, Black LI. Human papillomavirus vaccination among adults aged 18 to 26, 2013-2018. NCHS Data Brief. 2020:1-8.
- Lindsay AC, Delgado D, Valdez MJ, et al. "I don't think he needs the HPV vaccine cause boys can't have cervical cancer": a qualitative study of Latina mothers' (Mis) understandings about human papillomavirus transmission, associated cancers and the vaccine. J Cancer Educ. July 11, 2020. doi: 10.1007/s13187-020-01824-z.
Surprisingly, in the United States, the most common cancer associated with human papillomavirus (HPV) is oropharyngeal squamous cell cancer (SCC), with one study reporting 15,479 cases among men and 3,428 cases among women in 2015.1 In the same year, the investigators reported 11,788 cases of cervical cancer.1 A public health concern is that cases of oropharyngeal SCC are increasing, while cases of cervical cancer are decreasing. From 1999 to 2015, the rate of oropharyngeal SCC increased annually among both men and women, at rates of 2.7% and 0.8% per year, respectively. By contrast, the rate of cervical cancer decreased by 1.6% per year.1
Although the incidence of HPV-negative oropharyngeal SCC (cases associated with cigarette smoking) has declined by 50% from 1988 to 2004, the incidence of HPV-positive oropharyngeal SCC has increased by 225%, with much of the increase occurring among young, white men.2 HPV infection is a major cause of oropharyngeal SCC at the base of the tongue and tonsils, but not in the soft palate or oropharyngeal walls.3
Most physicians and parents recognize that the 9-valent (9v)HPV vaccine prevents the majority of cervical cancers and precancers in women. Far fewer people realize that there is an important opportunity to prevent a large number of oropharyngeal cancers by improving 9vHPV vaccination in men and women.
Which HPV types are associated with oropharyngeal cancer?
HPV16 is the most common HPV type associated with oropharyngeal SCC. Among these cancer types, greater than 80% harbor HPV16, with greater than 90% harboring HPV16 or 18 and less than 10% of tumors associated with HPV types 31, 33, 45, 52, or 58.4-7
The high prevalence of HPV16 in patients with oropharyngeal cancer raises the question of the HPV status of the intimate partner of the index patient. In one study of 164 people with HPV detected in their oropharyngeal, the partner of the index patient had a low prevalence of high-risk HPV types (1.2%) in oral rinse and gargle samples, similar to the rate in the general population (1.3%).7 This finding is reassuring and suggests that intimate partners of patients with HPV-positive oropharyngeal cancer effectively clear high-risk HPV virus from the oropharynx. The HPV status of the genital tissue of the intimate partner of an index patient with oropharyngeal SCC has not been adequately studied.
Men are more likely than women to harbor oral HPV
Among a sample of 5,501 men and women aged 14 to 69 years from the National Health and Nutrition Examination Survey, oral rinses were obtained and analyzed for the presence of HPV.8 The prevalence of any oral HPV and any oral high-risk HPV was 6.9% and 3.7%, respectively. Oral HPV-16 was detected in 1.6% of men and 0.3% of women. The prevalence of HPV was higher among current smokers, heavy alcohol drinkers, and people with a history of a greater number of sexual partners. In men and women reporting more than 20 lifetime sexual partners, the prevalence of oral HPV was 20%.
In a study of 2,627 men and women aged 18 to 33 years, the prevalence of oral HPV 16/18/6/11 was lower among those vaccinated versus those unvaccinated (0.11% and 1.6%, respectively; P = .008).9 Among men, oral HPV 16/18/6/11 was lower among those vaccinated versus unvaccinated (0.0% and 2.13%, respectively; P = .007).9 The results of this observational study support the important role of vaccination in reducing oral HPV infection.
In 2020, the US Food and Drug Administration (FDA) approved the 9-valent human papillomavirus (9vHPV) vaccine for the prevention of oropharyngeal cancer. The 9vHPV vaccine contains inactive L1 capsid proteins for 9 HPV types, including types 6, 11, 16, 18, 31, 33, 45, 52, and 58. The vaccine stimulates the production of neutralizing antibodies to the capsid protein.
9vHPV is approved for females aged 9 to 45 years to prevent cancers and precancers of the cervix, vulva, vagina, and anus caused by HPV types 16, 18, 31, 33, 45, 52, and 58.1 It is also approved for males aged 9 to 45 years to prevent cancer and precancers of the anus caused by those viral types. In 2020 the 9vHPV vaccine was approved by the FDA to prevent oropharyngeal cancer in males and females. Of note, the FDA reported that, “the oropharyngeal and head and neck cancer indication is approved under accelerated approval based on effectiveness in preventing HPV-related anogenital disease. Continued approval for this indication may be contingent upon verification and description of clinical benefit in a confirmatory trial.”2
The Advisory Committee on Immunization Practices (ACIP) recommends routine vaccination of girls and boys, 11 to 12 years of age.1 Children with a history of sexual abuse or assault can start the vaccine at 9 years of age. Catch-up vaccination is recommended for all females and males through age 26 years. The ACIP recommends shared clinical decision-making regarding vaccination for some adults 27 to 45 years of age. Gynecologists with routine exposure to HPV may have occupational risk that warrants HPV vaccination3 (see “As a gynecologist, should you receive the 9vHPV vaccine?”).
For most individuals who start the vaccine series before age 15, two doses of 9vHPV vaccine are recommended, with the second dose 6 to 12 months following the first dose. For teens and adults aged 15 to 26 years, 3 doses of 9vHPV vaccine are recommended, with the second dose 1 to 2 months later and the third dose 6 months following the first dose. Immunocompromised individuals 9 to 26 years of age, including those with HIV infection, should receive 3 doses of the vaccine.
References
1. Meites E, Szilagyi PG, Chesson HW, et al. Human papillomavirus vaccination for adults: updated recommendations of the Advisory Committee on Immunization Practices. MMWR Morb Mortal Wkly Rep. 2019;68:698-702.
2. Gardasil 9 [package insert]. Whitehouse Station, NJ: Merck & Co. Inc; 2020.
3. Stockdale CK, Einstein MH, Huh WK. ASCCP recommends HPV vaccination for providers. February 19, 2020. https://www.asccp.org/Assets/d3abdb05-25c5-4e58-9cec-05c11fb2b920/637177876310030000/hpv-vaccinemember-announcment-02-19-20-pdf. Accessed October 23, 2020.
Continue to: Vaccinate boys and girls to prevent cancer...
Vaccinate boys and girls to prevent cancer
Most population studies report that males are less likely to receive an HPV vaccine than females. For example, based on the National Health Interview Survey of people aged 18 to 26, the percentage of women who self-reported receiving at least one dose of HPV vaccine was 37% in 2013 and 54% in 2018.10 By contrast, among men, the rates of self-reported vaccination were much lower—8% in 2013 and 27% in 2018.10
The percentage of women who received the recommended number of doses of HPV vaccine (see “9vHPV vaccine: Indications and immunization schedule”) was 26% in 2013 and 35% in 2018.10 For men, these percentages were 2% in 2013 and 9% in 2018.10 These data indicate that, compared with women, men are less likely to receive an HPV vaccination and far less likely to have received the recommended number of doses.
It is heartening that there has been a slow and steady increase in the prevalence of HPV vaccination. In fact, increasing the HPV vaccination rate among both boys and girls has the potential to markedly reduce the incidence of oropharyngeal cancer.
The reasons for the female-male gap in vaccination rates are not fully characterized. For one, parental awareness of the importance of HPV vaccination to prevent cancer among men is limited, and represents an important opportunity for additional public health education. In a qualitative interview study of mothers with children aged 11 to 19, the investigators reported that most mothers were aware that HPV vaccination could prevent cervical cancer in women, but most mothers did not know that HPV causes cancer of the mouth and that vaccination could prevent oropharyngeal cancer in boys and girls.11 Because of this lack of knowledge, the mothers did not think their sons needed to have an HPV vaccine. The research report is aptly titled, “I don’t think he needs the HPV vaccine cause boys can’t have cervical cancer.”11
Clinicians are highly influential in guiding parents to accept HPV vaccination of their children. Offering consistent messaging to parents that HPV vaccination prevents cancer in both women and men, and reducing the out-of-pocket cost of vaccination surely will result in an increase in the vaccination rate of boys and girls. ●
Surgical treatment of tissues infected with human papillomavirus (HPV) often involves the use of laser or electrosurgical devices that generate smoke, which is known to contain HPV nucleic acid sequences and may contain infective virions.1 It is known that HPV nucleic acid sequences are present in surgical smoke. In one study plantar warts were treated with a carbon dioxide laser or electrocoagulation. The vapor produced from the surgery was collected with a dry filter apparatus. Five of 8 laser-derived vapors and 4 of 7 electrocoagulation-derived vapors were positive for HPV DNA. The concentration of HPV DNA was greater with laser than with electrocoagulation treatment.2
It is not known if surgical smoke derived from treatment of HPV-infected tissues contains infective HPV virions. In an experimental bovine model, smoke generated by laser ablation of fibropapillomas was collected. Injection of the contents of the smoke caused cutaneous papillomavirus lesions when inoculated into calves, suggesting that the smoke contained infective HPV virions.3 Although this animal experiment is a proof of principle that surgical smoke generated from treatment of HPVinfected tissue contain virions, it is unclear if surgical smoke generated in gynecologic practice contains HPV virions.
To investigate the prevalence of nasal HPV DNA among gynecologists, 700 physicians in Zhejiang Province, China, completed a questionnaire and provided a nasal swab for HPV DNA analysis.4 Among gynecologists who performed or did not perform LEEP, the prevalence of HPV DNA in the nose was 10% and 3%, respectively. The most common HPV types detected were HPV16 (76%), HPV31 (10%), HPV58 (5%), HPV55 (5%), HPV56 (2%), and HPV59 (2%).4 Among gynecologists who performed LEEP procedures, the prevalence of HPV DNA was 19% for those who did not use a surgical mask, 8% for clinicians who used a standard surgical mask, and 0% for those who used an N95 filtering facepiece respirator, suggesting that an N95 respirator provides the greatest protection from surgical smoke.4 Over 24 months of follow-up, all the gynecologists who had initially tested positive for HPV DNA no longer had detectable nasal HPV DNA. In this study, no gynecologist was diagnosed with an HPV-associated oropharyngeal disease. The investigators concluded that surgical masks, especially an N95 respirator, should be used by gynecologists performing LEEP procedures.
Investigators also have evaluated for the presence of HPV DNA in matched samples from the cervix of 134 patients undergoing loop electrosurgical excision procedure (LEEP) for cervical dysplasia, as well as the smoke generated during the procedure and nasal swabs from the surgeon performing the LEEP.5 HPV DNA was detected in 95% of the cervical samples, 30% of the surgical smoke samples, and 1.5% of the surgeons’ nasal swabs.5 At 6 months of follow-up, the two surgeons who initially had HPV-positive nasal swabs no longer had detected HPV DNA.
Of concern is that otolaryngologists have reported sporadic cases of oropharyngeal squamous cell cancer6 and laryngeal papillomatosis7 in health care workers with frequent and repetitive exposure to HPVs. For example, in one case report, a 53-year-old male gynecologist, nonsmoker, presented to his physician with a lump on the neck.6 The gynecologist had performed more than 3,000 laser ablation or LEEP procedures of dysplastic cervical, vaginal, and vulvar lesions over a span of 20 years.6 Most of the procedures were performed without wearing a mask and in a poorly ventilated procedure room. A computed tomography scan demonstrated a 2.2-cm soft tissue lesion in the right tonsil extending to the right soft palate and a level-2 lymph node. A biopsy of the tonsil confirmed invasive squamous cell carcinoma containing HPV16. He was treated with 35 fractions of radiotherapy and adjuvant cisplatin. Treatment adverse effects included dysphagia and xerostomia, and the patient lost 40 pounds.
Available interventions to reduce exposure of clinicians to HPV virions that may be present in surgical smoke include:
- wearing a fit-tested N95 respirator
- routinely using a smoke evacuation device, and
- ensuring sufficient ventilation in the procedure room.
A new recommendation is to consider 9vHPV vaccination for clinicians who are routinely exposed to HPV virions.8,9 In February 2020, the American Society for Colposcopy and Cervical Pathology recommended that clinicians who are routinely exposed to HPVs consider 9vHPV vaccination.8 This recommendation pertains to all members of the clinical team in the procedure room, including physicians, nurses, and staff. Based on the available data, gynecologists who have not been vaccinated will need to weigh the benefits and costs of receiving a 9vHPV vaccine to protect themselves against an occupational exposure that may adversely impact their health.
References
- Liu Y, Song Y, Hu X, et al. Awareness of surgical smoke hazards and enhancement of surgical smoke prevention among gynecologists. J Cancer. 2019;10:2788-2799.
- Sawchuk WS, Weber PJ, Lowy DR, et al. Infectious papillomavirus in the vapor of warts treated with carbon dioxide laser or electrocoagulation: detection and protection. J Am Acad Dermatol. 1989;21:41-49.
- Garden JM, O’Banion MK, Bakus AD, et al. Viral transmitted by laser-generated plume (aerosol). Arch Dermatol. 2002;138:1303-1307.
- Hu X, Zhou Q, Yu J, et al. Prevalence of HPV infections in surgical smoke exposed gynecologists. Int Arch Occup Environ Health. 2020; Epub September 1. doi: 10.1007 /s00420-020-01568-9.
- Zhou Q, Hu X, Zhou J, et al. Human papillomavirus DNA in surgical smoke during cervical loop electrosurgical excision procedures and its impact on the surgeon. Cancer Manag Res. 2019;11:3643-3654.
- Rioux M, Garland A, Webster D, et al. HPV-positive tonsillar cancer in two laser surgeons: case reports. J Otolaryngol Head Neck Surg. 2013;42:54-57.
- Hallmo P, Naess O. Laryngeal papillomatosis with human papillomavirus DNA contracted by a laser surgeon. Eur Arch Otorhinolaryngol. 1991;248:425-427.
Stockdale CK, Einstein MH, Huh WK. ASCCP recommends HPV vaccination for providers. February 19, 2020. www.asccp.org/Assets/d3abdb05-25c5-4e58-%209cec-05c11fb2b920/637177876310030000/hpv-vaccinemember-announcment-02-19-20-pdf. Accessed October 23, 2020.
- Harrison R, Huh W. Occupational exposure to human papillomavirus and vaccination for health care workers. Obstet Gynecol. 2020;136:663-665
Surprisingly, in the United States, the most common cancer associated with human papillomavirus (HPV) is oropharyngeal squamous cell cancer (SCC), with one study reporting 15,479 cases among men and 3,428 cases among women in 2015.1 In the same year, the investigators reported 11,788 cases of cervical cancer.1 A public health concern is that cases of oropharyngeal SCC are increasing, while cases of cervical cancer are decreasing. From 1999 to 2015, the rate of oropharyngeal SCC increased annually among both men and women, at rates of 2.7% and 0.8% per year, respectively. By contrast, the rate of cervical cancer decreased by 1.6% per year.1
Although the incidence of HPV-negative oropharyngeal SCC (cases associated with cigarette smoking) has declined by 50% from 1988 to 2004, the incidence of HPV-positive oropharyngeal SCC has increased by 225%, with much of the increase occurring among young, white men.2 HPV infection is a major cause of oropharyngeal SCC at the base of the tongue and tonsils, but not in the soft palate or oropharyngeal walls.3
Most physicians and parents recognize that the 9-valent (9v)HPV vaccine prevents the majority of cervical cancers and precancers in women. Far fewer people realize that there is an important opportunity to prevent a large number of oropharyngeal cancers by improving 9vHPV vaccination in men and women.
Which HPV types are associated with oropharyngeal cancer?
HPV16 is the most common HPV type associated with oropharyngeal SCC. Among these cancer types, greater than 80% harbor HPV16, with greater than 90% harboring HPV16 or 18 and less than 10% of tumors associated with HPV types 31, 33, 45, 52, or 58.4-7
The high prevalence of HPV16 in patients with oropharyngeal cancer raises the question of the HPV status of the intimate partner of the index patient. In one study of 164 people with HPV detected in their oropharyngeal, the partner of the index patient had a low prevalence of high-risk HPV types (1.2%) in oral rinse and gargle samples, similar to the rate in the general population (1.3%).7 This finding is reassuring and suggests that intimate partners of patients with HPV-positive oropharyngeal cancer effectively clear high-risk HPV virus from the oropharynx. The HPV status of the genital tissue of the intimate partner of an index patient with oropharyngeal SCC has not been adequately studied.
Men are more likely than women to harbor oral HPV
Among a sample of 5,501 men and women aged 14 to 69 years from the National Health and Nutrition Examination Survey, oral rinses were obtained and analyzed for the presence of HPV.8 The prevalence of any oral HPV and any oral high-risk HPV was 6.9% and 3.7%, respectively. Oral HPV-16 was detected in 1.6% of men and 0.3% of women. The prevalence of HPV was higher among current smokers, heavy alcohol drinkers, and people with a history of a greater number of sexual partners. In men and women reporting more than 20 lifetime sexual partners, the prevalence of oral HPV was 20%.
In a study of 2,627 men and women aged 18 to 33 years, the prevalence of oral HPV 16/18/6/11 was lower among those vaccinated versus those unvaccinated (0.11% and 1.6%, respectively; P = .008).9 Among men, oral HPV 16/18/6/11 was lower among those vaccinated versus unvaccinated (0.0% and 2.13%, respectively; P = .007).9 The results of this observational study support the important role of vaccination in reducing oral HPV infection.
In 2020, the US Food and Drug Administration (FDA) approved the 9-valent human papillomavirus (9vHPV) vaccine for the prevention of oropharyngeal cancer. The 9vHPV vaccine contains inactive L1 capsid proteins for 9 HPV types, including types 6, 11, 16, 18, 31, 33, 45, 52, and 58. The vaccine stimulates the production of neutralizing antibodies to the capsid protein.
9vHPV is approved for females aged 9 to 45 years to prevent cancers and precancers of the cervix, vulva, vagina, and anus caused by HPV types 16, 18, 31, 33, 45, 52, and 58.1 It is also approved for males aged 9 to 45 years to prevent cancer and precancers of the anus caused by those viral types. In 2020 the 9vHPV vaccine was approved by the FDA to prevent oropharyngeal cancer in males and females. Of note, the FDA reported that, “the oropharyngeal and head and neck cancer indication is approved under accelerated approval based on effectiveness in preventing HPV-related anogenital disease. Continued approval for this indication may be contingent upon verification and description of clinical benefit in a confirmatory trial.”2
The Advisory Committee on Immunization Practices (ACIP) recommends routine vaccination of girls and boys, 11 to 12 years of age.1 Children with a history of sexual abuse or assault can start the vaccine at 9 years of age. Catch-up vaccination is recommended for all females and males through age 26 years. The ACIP recommends shared clinical decision-making regarding vaccination for some adults 27 to 45 years of age. Gynecologists with routine exposure to HPV may have occupational risk that warrants HPV vaccination3 (see “As a gynecologist, should you receive the 9vHPV vaccine?”).
For most individuals who start the vaccine series before age 15, two doses of 9vHPV vaccine are recommended, with the second dose 6 to 12 months following the first dose. For teens and adults aged 15 to 26 years, 3 doses of 9vHPV vaccine are recommended, with the second dose 1 to 2 months later and the third dose 6 months following the first dose. Immunocompromised individuals 9 to 26 years of age, including those with HIV infection, should receive 3 doses of the vaccine.
References
1. Meites E, Szilagyi PG, Chesson HW, et al. Human papillomavirus vaccination for adults: updated recommendations of the Advisory Committee on Immunization Practices. MMWR Morb Mortal Wkly Rep. 2019;68:698-702.
2. Gardasil 9 [package insert]. Whitehouse Station, NJ: Merck & Co. Inc; 2020.
3. Stockdale CK, Einstein MH, Huh WK. ASCCP recommends HPV vaccination for providers. February 19, 2020. https://www.asccp.org/Assets/d3abdb05-25c5-4e58-9cec-05c11fb2b920/637177876310030000/hpv-vaccinemember-announcment-02-19-20-pdf. Accessed October 23, 2020.
Continue to: Vaccinate boys and girls to prevent cancer...
Vaccinate boys and girls to prevent cancer
Most population studies report that males are less likely to receive an HPV vaccine than females. For example, based on the National Health Interview Survey of people aged 18 to 26, the percentage of women who self-reported receiving at least one dose of HPV vaccine was 37% in 2013 and 54% in 2018.10 By contrast, among men, the rates of self-reported vaccination were much lower—8% in 2013 and 27% in 2018.10
The percentage of women who received the recommended number of doses of HPV vaccine (see “9vHPV vaccine: Indications and immunization schedule”) was 26% in 2013 and 35% in 2018.10 For men, these percentages were 2% in 2013 and 9% in 2018.10 These data indicate that, compared with women, men are less likely to receive an HPV vaccination and far less likely to have received the recommended number of doses.
It is heartening that there has been a slow and steady increase in the prevalence of HPV vaccination. In fact, increasing the HPV vaccination rate among both boys and girls has the potential to markedly reduce the incidence of oropharyngeal cancer.
The reasons for the female-male gap in vaccination rates are not fully characterized. For one, parental awareness of the importance of HPV vaccination to prevent cancer among men is limited, and represents an important opportunity for additional public health education. In a qualitative interview study of mothers with children aged 11 to 19, the investigators reported that most mothers were aware that HPV vaccination could prevent cervical cancer in women, but most mothers did not know that HPV causes cancer of the mouth and that vaccination could prevent oropharyngeal cancer in boys and girls.11 Because of this lack of knowledge, the mothers did not think their sons needed to have an HPV vaccine. The research report is aptly titled, “I don’t think he needs the HPV vaccine cause boys can’t have cervical cancer.”11
Clinicians are highly influential in guiding parents to accept HPV vaccination of their children. Offering consistent messaging to parents that HPV vaccination prevents cancer in both women and men, and reducing the out-of-pocket cost of vaccination surely will result in an increase in the vaccination rate of boys and girls. ●
Surgical treatment of tissues infected with human papillomavirus (HPV) often involves the use of laser or electrosurgical devices that generate smoke, which is known to contain HPV nucleic acid sequences and may contain infective virions.1 It is known that HPV nucleic acid sequences are present in surgical smoke. In one study plantar warts were treated with a carbon dioxide laser or electrocoagulation. The vapor produced from the surgery was collected with a dry filter apparatus. Five of 8 laser-derived vapors and 4 of 7 electrocoagulation-derived vapors were positive for HPV DNA. The concentration of HPV DNA was greater with laser than with electrocoagulation treatment.2
It is not known if surgical smoke derived from treatment of HPV-infected tissues contains infective HPV virions. In an experimental bovine model, smoke generated by laser ablation of fibropapillomas was collected. Injection of the contents of the smoke caused cutaneous papillomavirus lesions when inoculated into calves, suggesting that the smoke contained infective HPV virions.3 Although this animal experiment is a proof of principle that surgical smoke generated from treatment of HPVinfected tissue contain virions, it is unclear if surgical smoke generated in gynecologic practice contains HPV virions.
To investigate the prevalence of nasal HPV DNA among gynecologists, 700 physicians in Zhejiang Province, China, completed a questionnaire and provided a nasal swab for HPV DNA analysis.4 Among gynecologists who performed or did not perform LEEP, the prevalence of HPV DNA in the nose was 10% and 3%, respectively. The most common HPV types detected were HPV16 (76%), HPV31 (10%), HPV58 (5%), HPV55 (5%), HPV56 (2%), and HPV59 (2%).4 Among gynecologists who performed LEEP procedures, the prevalence of HPV DNA was 19% for those who did not use a surgical mask, 8% for clinicians who used a standard surgical mask, and 0% for those who used an N95 filtering facepiece respirator, suggesting that an N95 respirator provides the greatest protection from surgical smoke.4 Over 24 months of follow-up, all the gynecologists who had initially tested positive for HPV DNA no longer had detectable nasal HPV DNA. In this study, no gynecologist was diagnosed with an HPV-associated oropharyngeal disease. The investigators concluded that surgical masks, especially an N95 respirator, should be used by gynecologists performing LEEP procedures.
Investigators also have evaluated for the presence of HPV DNA in matched samples from the cervix of 134 patients undergoing loop electrosurgical excision procedure (LEEP) for cervical dysplasia, as well as the smoke generated during the procedure and nasal swabs from the surgeon performing the LEEP.5 HPV DNA was detected in 95% of the cervical samples, 30% of the surgical smoke samples, and 1.5% of the surgeons’ nasal swabs.5 At 6 months of follow-up, the two surgeons who initially had HPV-positive nasal swabs no longer had detected HPV DNA.
Of concern is that otolaryngologists have reported sporadic cases of oropharyngeal squamous cell cancer6 and laryngeal papillomatosis7 in health care workers with frequent and repetitive exposure to HPVs. For example, in one case report, a 53-year-old male gynecologist, nonsmoker, presented to his physician with a lump on the neck.6 The gynecologist had performed more than 3,000 laser ablation or LEEP procedures of dysplastic cervical, vaginal, and vulvar lesions over a span of 20 years.6 Most of the procedures were performed without wearing a mask and in a poorly ventilated procedure room. A computed tomography scan demonstrated a 2.2-cm soft tissue lesion in the right tonsil extending to the right soft palate and a level-2 lymph node. A biopsy of the tonsil confirmed invasive squamous cell carcinoma containing HPV16. He was treated with 35 fractions of radiotherapy and adjuvant cisplatin. Treatment adverse effects included dysphagia and xerostomia, and the patient lost 40 pounds.
Available interventions to reduce exposure of clinicians to HPV virions that may be present in surgical smoke include:
- wearing a fit-tested N95 respirator
- routinely using a smoke evacuation device, and
- ensuring sufficient ventilation in the procedure room.
A new recommendation is to consider 9vHPV vaccination for clinicians who are routinely exposed to HPV virions.8,9 In February 2020, the American Society for Colposcopy and Cervical Pathology recommended that clinicians who are routinely exposed to HPVs consider 9vHPV vaccination.8 This recommendation pertains to all members of the clinical team in the procedure room, including physicians, nurses, and staff. Based on the available data, gynecologists who have not been vaccinated will need to weigh the benefits and costs of receiving a 9vHPV vaccine to protect themselves against an occupational exposure that may adversely impact their health.
References
- Liu Y, Song Y, Hu X, et al. Awareness of surgical smoke hazards and enhancement of surgical smoke prevention among gynecologists. J Cancer. 2019;10:2788-2799.
- Sawchuk WS, Weber PJ, Lowy DR, et al. Infectious papillomavirus in the vapor of warts treated with carbon dioxide laser or electrocoagulation: detection and protection. J Am Acad Dermatol. 1989;21:41-49.
- Garden JM, O’Banion MK, Bakus AD, et al. Viral transmitted by laser-generated plume (aerosol). Arch Dermatol. 2002;138:1303-1307.
- Hu X, Zhou Q, Yu J, et al. Prevalence of HPV infections in surgical smoke exposed gynecologists. Int Arch Occup Environ Health. 2020; Epub September 1. doi: 10.1007 /s00420-020-01568-9.
- Zhou Q, Hu X, Zhou J, et al. Human papillomavirus DNA in surgical smoke during cervical loop electrosurgical excision procedures and its impact on the surgeon. Cancer Manag Res. 2019;11:3643-3654.
- Rioux M, Garland A, Webster D, et al. HPV-positive tonsillar cancer in two laser surgeons: case reports. J Otolaryngol Head Neck Surg. 2013;42:54-57.
- Hallmo P, Naess O. Laryngeal papillomatosis with human papillomavirus DNA contracted by a laser surgeon. Eur Arch Otorhinolaryngol. 1991;248:425-427.
Stockdale CK, Einstein MH, Huh WK. ASCCP recommends HPV vaccination for providers. February 19, 2020. www.asccp.org/Assets/d3abdb05-25c5-4e58-%209cec-05c11fb2b920/637177876310030000/hpv-vaccinemember-announcment-02-19-20-pdf. Accessed October 23, 2020.
- Harrison R, Huh W. Occupational exposure to human papillomavirus and vaccination for health care workers. Obstet Gynecol. 2020;136:663-665
- Van Dyne EA, Henley SJ, Saraiya M, et al. Trends in human papillomavirus-associated cancers--United States, 1999-2015. MMWR. 2018;67:918-924.
- Chaturvedi AK, Engels EA, Pfeiffer RM, et al. Human papillomavirus and rising oropharyngeal cancer incidence in the United States. J Clin Oncol. 2011;29:4294-4301.
- Haeggblom L, Ramqvist T, Tommasino M, et al. Time to change perspective on HPV in oropharyngeal cancer. A systematic review of HPV prevalence per oropharyngeal sub-site the last 3 years. Papillomavirus Research. 2017;4:1-11.
- Kreimer AR, Clifford GM, Boyle P, et al. Human papillomavirus types in head and neck squamous cell carcinomas worldwide: a systematic review. Cancer Epidemiol Biomarkers Prev. 2005;14:467-475.
- D'Souza G, Kreimer AR, Viscidi R, et al. Case-control study of human papillomavirus and oropharyngeal cancer. N Engl J Med. 2007;356:1944-1956.
- de Martel C, Plummer M, Vignat J, et al. Worldwide burden of cancer attributable to HPV by site, country and HPV type. Int J Cancer. 2017;141:664-670.
- D'Souza G, Gross ND, Pai SI, et al. Oral human papillomavirus infection in HPV-positive patients with oropharyngeal cancer and their partners. J Clin Oncol. 2014;32:2408-2415.
- Gillison ML, Broutian T, Pickard RK, et al. Prevalence of oral HPV infection in the United States, 2009-2010. JAMA. 2012;307:693.
- Chaturvedi AK, Graubard BI, Broutian T, et al. Effect of prophylactic human papillomavirus vaccination on oral HPV infections among young adults in the United States. J Clin Oncol. 2018;36:262-267.
- Boersma P, Black LI. Human papillomavirus vaccination among adults aged 18 to 26, 2013-2018. NCHS Data Brief. 2020:1-8.
- Lindsay AC, Delgado D, Valdez MJ, et al. "I don't think he needs the HPV vaccine cause boys can't have cervical cancer": a qualitative study of Latina mothers' (Mis) understandings about human papillomavirus transmission, associated cancers and the vaccine. J Cancer Educ. July 11, 2020. doi: 10.1007/s13187-020-01824-z.
- Van Dyne EA, Henley SJ, Saraiya M, et al. Trends in human papillomavirus-associated cancers--United States, 1999-2015. MMWR. 2018;67:918-924.
- Chaturvedi AK, Engels EA, Pfeiffer RM, et al. Human papillomavirus and rising oropharyngeal cancer incidence in the United States. J Clin Oncol. 2011;29:4294-4301.
- Haeggblom L, Ramqvist T, Tommasino M, et al. Time to change perspective on HPV in oropharyngeal cancer. A systematic review of HPV prevalence per oropharyngeal sub-site the last 3 years. Papillomavirus Research. 2017;4:1-11.
- Kreimer AR, Clifford GM, Boyle P, et al. Human papillomavirus types in head and neck squamous cell carcinomas worldwide: a systematic review. Cancer Epidemiol Biomarkers Prev. 2005;14:467-475.
- D'Souza G, Kreimer AR, Viscidi R, et al. Case-control study of human papillomavirus and oropharyngeal cancer. N Engl J Med. 2007;356:1944-1956.
- de Martel C, Plummer M, Vignat J, et al. Worldwide burden of cancer attributable to HPV by site, country and HPV type. Int J Cancer. 2017;141:664-670.
- D'Souza G, Gross ND, Pai SI, et al. Oral human papillomavirus infection in HPV-positive patients with oropharyngeal cancer and their partners. J Clin Oncol. 2014;32:2408-2415.
- Gillison ML, Broutian T, Pickard RK, et al. Prevalence of oral HPV infection in the United States, 2009-2010. JAMA. 2012;307:693.
- Chaturvedi AK, Graubard BI, Broutian T, et al. Effect of prophylactic human papillomavirus vaccination on oral HPV infections among young adults in the United States. J Clin Oncol. 2018;36:262-267.
- Boersma P, Black LI. Human papillomavirus vaccination among adults aged 18 to 26, 2013-2018. NCHS Data Brief. 2020:1-8.
- Lindsay AC, Delgado D, Valdez MJ, et al. "I don't think he needs the HPV vaccine cause boys can't have cervical cancer": a qualitative study of Latina mothers' (Mis) understandings about human papillomavirus transmission, associated cancers and the vaccine. J Cancer Educ. July 11, 2020. doi: 10.1007/s13187-020-01824-z.
Please stop using the adjective “elective” to describe the important health services ObGyns provide
During the April 2020 peak of patient admissions to our hospital caused by coronavirus disease 2019 (COVID-19), we severely limited the number of surgical procedures performed to conserve health system resources. During this stressful time, some administrators and physicians began categorizing operations for cancer as "elective" procedures that could be postponed for months. Personally, I think the use of elective to describe cancer surgery is not optimal, even during a pandemic. In reality, the surgeries for patients with cancer were being postponed to ensure that services were available for patients with severe and critical COVID-19 disease, not because the surgeries were "elective." The health system leaders were making the rational decision to prioritize the needs of patients with COVID-19 infections over the needs of patients with cancer. However, they were using an inappropriate description of the rationale for postponing the surgery for patients with cancer—an intellectual short-cut.
This experience prompted me to explore all the medical interventions commonly described as elective. Surprisingly, among medical specialists, obstetricians excel in using the adjective elective to describe our important work. For example, in the medical record we commonly use terms such as “elective induction of labor,” “elective cesarean delivery” (CD) and “elective termination of pregnancy.” I believe it would advance our field if obstetricians stopped using the term elective to describe the important health services we provide.
Stop using the term “elective induction of labor”
Ghartey and Macones recently advocated for all obstetricians to stop using the term elective when describing induction of labor.1 The ARRIVE trial (A Randomized Trial of Induction vs Expectant Management)2 demonstrated that, among nulliparous women at 39 weeks’ gestation, induction of labor resulted in a lower CD rate than expectant management (18.6% vs 22.2%, respectively; relative risk, 0.84; 95% confidence interval [CI], 0.76-0.93). These findings indicate that induction of labor is not elective because it provides a clear health benefit over the alternative of expectant management. Given current expert guidance, induction of labor prior to 39 weeks’ gestation must be based on an accepted medical indication and provide a health benefit; hence, these inductions are medically indicated. Similarly, since induction of labor at 39 weeks’ gestation also provides a clear health benefit it is also medically indicated and not “elective.” Ghartey and Macones conclude1:
"The words we choose to
describe medical interventions
matter. They send a message
to patients, physicians, nurses,
and hospital administrators.
When the term 'elective' is applied to a medical intervention,
it implies that it is not really
necessary. That is certainly not
the case when it comes to 39-
week nulliparous induction. The
ARRIVE trial provides grade A
(good and consistent) evidence
that labor induction provided
benefit with no harm to women
and their infants. These inductions are not 'elective'."
An alternative descriptor is “medically indicated” induction.
Continue to: Stop using the term “elective cesarean delivery”...
Stop using the term “elective cesarean delivery”
I recently searched PubMed for publications using the key words, “elective cesarean delivery,” and more than 7,000 publications were identified by the National Library of Medicine. “Elective cesarean delivery” is clearly an important term used by obstetrical authorities. What do we mean by elective CD?
At 39 weeks’ gestation, a low-risk nulliparous pregnant woman has a limited number of options:
- induction of labor
- expectant management awaiting the onset of labor
- scheduled CD before the onset of labor.
For a low-risk pregnant woman at 39 weeks’ gestation, the American College of Obstetricians and Gynecologists recommends vaginal delivery because it best balances the risks and benefits for the woman and newborn.3 When a low-risk nulliparous pregnant woman asks a clinician about a scheduled CD, we are trained to thoroughly explore the reasons for the woman’s request, including her intellectual, fact-based, concerns about labor and vaginal birth and her emotional reaction to the thought of a vaginal or cesarean birth. In this situation the clinician will provide information about the risks and benefits of vaginal versus CD. In the vast majority of situations, the pregnant woman will agree to attempting vaginal delivery. In one study of 458,767 births, only 0.2% of women choose a “maternal request cesarean delivery.”4
After thorough counseling, if a woman and her clinician jointly agree to schedule a primary CD it will be the result of hours of intensive discussion, not an imprudent and hasty decision. In this case, the delivery is best characterized as a “maternal request cesarean delivery,” not an “elective” CD.
Stop using the terms “elective termination of pregnancy” and “elective abortion”
Janiak and Goldberg have advocated for the elimination of the phrase elective abortion.5 They write5:
"Support for abortion varies
depending on the reason for
the abortion—whether it is
'elective' or 'indicated.' In the
case of abortion, these terms
generally differentiate between
women seeking abortion for
reasons of maternal or fetal
health (an 'indicated abortion')
defined in contrast to women
seeking abortion for other
reasons (an 'elective abortion').
We argue that such a distinction is impossible to operationalize in a just manner. The use
of the phrase 'elective abortion'
promotes the institutionalization of a false hierarchy of need
among abortion patients."
My experience is that pregnant women never seek an abortion based on whimsy. Most pregnant women who consider an abortion struggle greatly with the choice, using reason and judgment to arrive at their final decision. The choice to seek an abortion is always a difficult one, influenced by a constellation of hard facts that impact the woman’s life. Using the term elective to describe an abortion implies a moral judgment and stigmatizes the choice to have an abortion. Janiak and Goldberg conclude by recommending the elimination of the phrase 'elective abortion' in favor of the phrase “induced abortion.”5
Continue to: Time for change...
Time for change
Shockingly, in searching the International Statistical Classification of Diseases and Related Health Problems, 10th revision (ICD10), the word elective is most commonly used in the context of health services provided to pregnant women, including: elective induction of labor (Z34.90), elective cesarean delivery (O82), elective termination of pregnancy (Z33.2), and elective fetal reduction (Z031.30X0). In ICD10, other specialties do not describe the scope of their health services with the adjective elective.
There are many definitions and interpretations of elective. The most benign use of the word in the context of surgery is to contrast procedures that can be scheduled in the future with those that need to be performed urgently. In this context elective only refers to the timing, not the medical necessity, of the procedure. By contrast, describing a procedure as elective may signal that it is not medically necessary and is being performed based on the capricious preference of the patient or physician. Given the confusion and misunderstanding that may be caused by describing our important health services as “elective,” I hope that we can permanently sunset use of the term. ●
- Ghartey J, Macones GA. 39-week nulliparous inductions are not elective. Am J Obstet Gynecol. 2020;222:519-520.
- Grobman WA, Rice MM, Reddy UM, et al. Labor induction versus expectant management in low-risk nulliparous women. N Engl J Med. 2018;379:513-523.
- ACOG Committee Opinion No 761: cesarean delivery on maternal request. Obstet Gynecol. 2019;133.e73-e77.
- Gossman GL, Joesch JM, Tanfer K. Trends in maternal request cesarean delivery from 1991 to 2004. Obstet Gynecol. 2006;108:1506-1516.
- Janiak E, Goldberg AB. Eliminating the phrase “elective abortion”: why language matters. Contraception. 2016;93:89-92.
During the April 2020 peak of patient admissions to our hospital caused by coronavirus disease 2019 (COVID-19), we severely limited the number of surgical procedures performed to conserve health system resources. During this stressful time, some administrators and physicians began categorizing operations for cancer as "elective" procedures that could be postponed for months. Personally, I think the use of elective to describe cancer surgery is not optimal, even during a pandemic. In reality, the surgeries for patients with cancer were being postponed to ensure that services were available for patients with severe and critical COVID-19 disease, not because the surgeries were "elective." The health system leaders were making the rational decision to prioritize the needs of patients with COVID-19 infections over the needs of patients with cancer. However, they were using an inappropriate description of the rationale for postponing the surgery for patients with cancer—an intellectual short-cut.
This experience prompted me to explore all the medical interventions commonly described as elective. Surprisingly, among medical specialists, obstetricians excel in using the adjective elective to describe our important work. For example, in the medical record we commonly use terms such as “elective induction of labor,” “elective cesarean delivery” (CD) and “elective termination of pregnancy.” I believe it would advance our field if obstetricians stopped using the term elective to describe the important health services we provide.
Stop using the term “elective induction of labor”
Ghartey and Macones recently advocated for all obstetricians to stop using the term elective when describing induction of labor.1 The ARRIVE trial (A Randomized Trial of Induction vs Expectant Management)2 demonstrated that, among nulliparous women at 39 weeks’ gestation, induction of labor resulted in a lower CD rate than expectant management (18.6% vs 22.2%, respectively; relative risk, 0.84; 95% confidence interval [CI], 0.76-0.93). These findings indicate that induction of labor is not elective because it provides a clear health benefit over the alternative of expectant management. Given current expert guidance, induction of labor prior to 39 weeks’ gestation must be based on an accepted medical indication and provide a health benefit; hence, these inductions are medically indicated. Similarly, since induction of labor at 39 weeks’ gestation also provides a clear health benefit it is also medically indicated and not “elective.” Ghartey and Macones conclude1:
"The words we choose to
describe medical interventions
matter. They send a message
to patients, physicians, nurses,
and hospital administrators.
When the term 'elective' is applied to a medical intervention,
it implies that it is not really
necessary. That is certainly not
the case when it comes to 39-
week nulliparous induction. The
ARRIVE trial provides grade A
(good and consistent) evidence
that labor induction provided
benefit with no harm to women
and their infants. These inductions are not 'elective'."
An alternative descriptor is “medically indicated” induction.
Continue to: Stop using the term “elective cesarean delivery”...
Stop using the term “elective cesarean delivery”
I recently searched PubMed for publications using the key words, “elective cesarean delivery,” and more than 7,000 publications were identified by the National Library of Medicine. “Elective cesarean delivery” is clearly an important term used by obstetrical authorities. What do we mean by elective CD?
At 39 weeks’ gestation, a low-risk nulliparous pregnant woman has a limited number of options:
- induction of labor
- expectant management awaiting the onset of labor
- scheduled CD before the onset of labor.
For a low-risk pregnant woman at 39 weeks’ gestation, the American College of Obstetricians and Gynecologists recommends vaginal delivery because it best balances the risks and benefits for the woman and newborn.3 When a low-risk nulliparous pregnant woman asks a clinician about a scheduled CD, we are trained to thoroughly explore the reasons for the woman’s request, including her intellectual, fact-based, concerns about labor and vaginal birth and her emotional reaction to the thought of a vaginal or cesarean birth. In this situation the clinician will provide information about the risks and benefits of vaginal versus CD. In the vast majority of situations, the pregnant woman will agree to attempting vaginal delivery. In one study of 458,767 births, only 0.2% of women choose a “maternal request cesarean delivery.”4
After thorough counseling, if a woman and her clinician jointly agree to schedule a primary CD it will be the result of hours of intensive discussion, not an imprudent and hasty decision. In this case, the delivery is best characterized as a “maternal request cesarean delivery,” not an “elective” CD.
Stop using the terms “elective termination of pregnancy” and “elective abortion”
Janiak and Goldberg have advocated for the elimination of the phrase elective abortion.5 They write5:
"Support for abortion varies
depending on the reason for
the abortion—whether it is
'elective' or 'indicated.' In the
case of abortion, these terms
generally differentiate between
women seeking abortion for
reasons of maternal or fetal
health (an 'indicated abortion')
defined in contrast to women
seeking abortion for other
reasons (an 'elective abortion').
We argue that such a distinction is impossible to operationalize in a just manner. The use
of the phrase 'elective abortion'
promotes the institutionalization of a false hierarchy of need
among abortion patients."
My experience is that pregnant women never seek an abortion based on whimsy. Most pregnant women who consider an abortion struggle greatly with the choice, using reason and judgment to arrive at their final decision. The choice to seek an abortion is always a difficult one, influenced by a constellation of hard facts that impact the woman’s life. Using the term elective to describe an abortion implies a moral judgment and stigmatizes the choice to have an abortion. Janiak and Goldberg conclude by recommending the elimination of the phrase 'elective abortion' in favor of the phrase “induced abortion.”5
Continue to: Time for change...
Time for change
Shockingly, in searching the International Statistical Classification of Diseases and Related Health Problems, 10th revision (ICD10), the word elective is most commonly used in the context of health services provided to pregnant women, including: elective induction of labor (Z34.90), elective cesarean delivery (O82), elective termination of pregnancy (Z33.2), and elective fetal reduction (Z031.30X0). In ICD10, other specialties do not describe the scope of their health services with the adjective elective.
There are many definitions and interpretations of elective. The most benign use of the word in the context of surgery is to contrast procedures that can be scheduled in the future with those that need to be performed urgently. In this context elective only refers to the timing, not the medical necessity, of the procedure. By contrast, describing a procedure as elective may signal that it is not medically necessary and is being performed based on the capricious preference of the patient or physician. Given the confusion and misunderstanding that may be caused by describing our important health services as “elective,” I hope that we can permanently sunset use of the term. ●
During the April 2020 peak of patient admissions to our hospital caused by coronavirus disease 2019 (COVID-19), we severely limited the number of surgical procedures performed to conserve health system resources. During this stressful time, some administrators and physicians began categorizing operations for cancer as "elective" procedures that could be postponed for months. Personally, I think the use of elective to describe cancer surgery is not optimal, even during a pandemic. In reality, the surgeries for patients with cancer were being postponed to ensure that services were available for patients with severe and critical COVID-19 disease, not because the surgeries were "elective." The health system leaders were making the rational decision to prioritize the needs of patients with COVID-19 infections over the needs of patients with cancer. However, they were using an inappropriate description of the rationale for postponing the surgery for patients with cancer—an intellectual short-cut.
This experience prompted me to explore all the medical interventions commonly described as elective. Surprisingly, among medical specialists, obstetricians excel in using the adjective elective to describe our important work. For example, in the medical record we commonly use terms such as “elective induction of labor,” “elective cesarean delivery” (CD) and “elective termination of pregnancy.” I believe it would advance our field if obstetricians stopped using the term elective to describe the important health services we provide.
Stop using the term “elective induction of labor”
Ghartey and Macones recently advocated for all obstetricians to stop using the term elective when describing induction of labor.1 The ARRIVE trial (A Randomized Trial of Induction vs Expectant Management)2 demonstrated that, among nulliparous women at 39 weeks’ gestation, induction of labor resulted in a lower CD rate than expectant management (18.6% vs 22.2%, respectively; relative risk, 0.84; 95% confidence interval [CI], 0.76-0.93). These findings indicate that induction of labor is not elective because it provides a clear health benefit over the alternative of expectant management. Given current expert guidance, induction of labor prior to 39 weeks’ gestation must be based on an accepted medical indication and provide a health benefit; hence, these inductions are medically indicated. Similarly, since induction of labor at 39 weeks’ gestation also provides a clear health benefit it is also medically indicated and not “elective.” Ghartey and Macones conclude1:
"The words we choose to
describe medical interventions
matter. They send a message
to patients, physicians, nurses,
and hospital administrators.
When the term 'elective' is applied to a medical intervention,
it implies that it is not really
necessary. That is certainly not
the case when it comes to 39-
week nulliparous induction. The
ARRIVE trial provides grade A
(good and consistent) evidence
that labor induction provided
benefit with no harm to women
and their infants. These inductions are not 'elective'."
An alternative descriptor is “medically indicated” induction.
Continue to: Stop using the term “elective cesarean delivery”...
Stop using the term “elective cesarean delivery”
I recently searched PubMed for publications using the key words, “elective cesarean delivery,” and more than 7,000 publications were identified by the National Library of Medicine. “Elective cesarean delivery” is clearly an important term used by obstetrical authorities. What do we mean by elective CD?
At 39 weeks’ gestation, a low-risk nulliparous pregnant woman has a limited number of options:
- induction of labor
- expectant management awaiting the onset of labor
- scheduled CD before the onset of labor.
For a low-risk pregnant woman at 39 weeks’ gestation, the American College of Obstetricians and Gynecologists recommends vaginal delivery because it best balances the risks and benefits for the woman and newborn.3 When a low-risk nulliparous pregnant woman asks a clinician about a scheduled CD, we are trained to thoroughly explore the reasons for the woman’s request, including her intellectual, fact-based, concerns about labor and vaginal birth and her emotional reaction to the thought of a vaginal or cesarean birth. In this situation the clinician will provide information about the risks and benefits of vaginal versus CD. In the vast majority of situations, the pregnant woman will agree to attempting vaginal delivery. In one study of 458,767 births, only 0.2% of women choose a “maternal request cesarean delivery.”4
After thorough counseling, if a woman and her clinician jointly agree to schedule a primary CD it will be the result of hours of intensive discussion, not an imprudent and hasty decision. In this case, the delivery is best characterized as a “maternal request cesarean delivery,” not an “elective” CD.
Stop using the terms “elective termination of pregnancy” and “elective abortion”
Janiak and Goldberg have advocated for the elimination of the phrase elective abortion.5 They write5:
"Support for abortion varies
depending on the reason for
the abortion—whether it is
'elective' or 'indicated.' In the
case of abortion, these terms
generally differentiate between
women seeking abortion for
reasons of maternal or fetal
health (an 'indicated abortion')
defined in contrast to women
seeking abortion for other
reasons (an 'elective abortion').
We argue that such a distinction is impossible to operationalize in a just manner. The use
of the phrase 'elective abortion'
promotes the institutionalization of a false hierarchy of need
among abortion patients."
My experience is that pregnant women never seek an abortion based on whimsy. Most pregnant women who consider an abortion struggle greatly with the choice, using reason and judgment to arrive at their final decision. The choice to seek an abortion is always a difficult one, influenced by a constellation of hard facts that impact the woman’s life. Using the term elective to describe an abortion implies a moral judgment and stigmatizes the choice to have an abortion. Janiak and Goldberg conclude by recommending the elimination of the phrase 'elective abortion' in favor of the phrase “induced abortion.”5
Continue to: Time for change...
Time for change
Shockingly, in searching the International Statistical Classification of Diseases and Related Health Problems, 10th revision (ICD10), the word elective is most commonly used in the context of health services provided to pregnant women, including: elective induction of labor (Z34.90), elective cesarean delivery (O82), elective termination of pregnancy (Z33.2), and elective fetal reduction (Z031.30X0). In ICD10, other specialties do not describe the scope of their health services with the adjective elective.
There are many definitions and interpretations of elective. The most benign use of the word in the context of surgery is to contrast procedures that can be scheduled in the future with those that need to be performed urgently. In this context elective only refers to the timing, not the medical necessity, of the procedure. By contrast, describing a procedure as elective may signal that it is not medically necessary and is being performed based on the capricious preference of the patient or physician. Given the confusion and misunderstanding that may be caused by describing our important health services as “elective,” I hope that we can permanently sunset use of the term. ●
- Ghartey J, Macones GA. 39-week nulliparous inductions are not elective. Am J Obstet Gynecol. 2020;222:519-520.
- Grobman WA, Rice MM, Reddy UM, et al. Labor induction versus expectant management in low-risk nulliparous women. N Engl J Med. 2018;379:513-523.
- ACOG Committee Opinion No 761: cesarean delivery on maternal request. Obstet Gynecol. 2019;133.e73-e77.
- Gossman GL, Joesch JM, Tanfer K. Trends in maternal request cesarean delivery from 1991 to 2004. Obstet Gynecol. 2006;108:1506-1516.
- Janiak E, Goldberg AB. Eliminating the phrase “elective abortion”: why language matters. Contraception. 2016;93:89-92.
- Ghartey J, Macones GA. 39-week nulliparous inductions are not elective. Am J Obstet Gynecol. 2020;222:519-520.
- Grobman WA, Rice MM, Reddy UM, et al. Labor induction versus expectant management in low-risk nulliparous women. N Engl J Med. 2018;379:513-523.
- ACOG Committee Opinion No 761: cesarean delivery on maternal request. Obstet Gynecol. 2019;133.e73-e77.
- Gossman GL, Joesch JM, Tanfer K. Trends in maternal request cesarean delivery from 1991 to 2004. Obstet Gynecol. 2006;108:1506-1516.
- Janiak E, Goldberg AB. Eliminating the phrase “elective abortion”: why language matters. Contraception. 2016;93:89-92.
Major changes in Medicare billing are planned for January 2021: Some specialties fare better than others
The Centers for Medicare and Medicaid Services (CMS) finalized an increase in the relative value of evaluation and management (E/M) service codes effective January 1, 2021, which results in an overall decrease in the payment for procedural services in the Medicare program. (Due to the mandate for budget neutrality, an increase in relative value units [RVUs] for E/M resulted in a large decrease in the conversion factor—the number of dollars per RVU). This has increased payments for endocrinologists, rheumatologists, and family medicine clinicians and decreased payments for radiologists, pathologists, and surgeons.
In a major win for physicians, CMS proposes to simplify documentation requirements for billing and focus on the complexity of the medical decision making (MDM) or the total time needed to care for the patient on the date of the service as the foundation for determining the relative value of the service. Therefore, there is no more counting bullets—ie, we don’t have to perform a comprehensive physical exam or review of systems to achieve a high level code! Prior to this change, time was only available for coding purposes when counseling and coordination of care was the predominant service (>50%), and only face-to-face time with the patient was considered. Effective January 1, for office and other outpatient services, total time on the calendar date of the encounter will be used. This acknowledges the intensity and value of non–face-to-face work.
Acting through CMS, the federal government influences greatly the US health care system. CMS is an agency in the Department of Health and Human Services that administers the Medicare program and partners with state governments to administer the Health Insurance Exchanges, Medicaid, and the Children’s Health Insurance programs (CHIP).1 In addition, CMS is responsible for enforcing quality care standards in long-term care facilities and clinical laboratories and the implementation of the Health Insurance Portability and Accountability Act.1
In January, CMS plans the following major changes to coding and documentation2,3:
- Selection of the level of E/M service will no longer require documentation of bullet points in the history, physical exam, and MDM. The simplified system allows physicians and qualified health care professionals to code either by total time (both face-to-face and non–face-to-face) on the date of the encounter or by level of MDM.
- For established office patients, 5 levels of office-based evaluation and management services will be retained. CMS had initially proposed to reduce the number of office-based E/M codes from 5 to 3, combining code levels 2, 3, and 4 into 1 code.4 However, after receiving feedback from professional societies and the public, CMS abandoned the plan for radical simplification of coding levels.2,3 Implementation of their proposal would have resulted in the same payment for treatment of a hang nail as for a complex gyn patient with multiple medical problems. Both patient advocacy groups and professional societies argued that incentives originally were misaligned.
- For new office patients, since both 99201 and 99202 require straightforward MDM, the level 1 code (99201) has been eliminated, reducing the number of code levels from 5 to 4.
- History and physical exam will no longer be used to determine code level for office E/M codes. These elements will be required only as medically appropriate. This means that documentation review will no longer focus on “bean counting” the elements in the history and physical exam.
- Following a reassessment of the actual time required to provide E/M services in real-life practice, CMS plans to markedly increase the relative value of office visits for established patients and modestly increase the relative value of office visits for new patients. CMS operates under the principle of “neutral budgeting,” meaning that an increase of the relative value of E/M codes will result in a decrease in the payment for procedural codes. The actual RVUs for procedural services do not change; however, budget neutrality requires a decrease in the dollar conversion factor. The proposed changes will increase the payment for E/M services and decrease payments for procedural services.
Continue to: Refocusing practice on MDM complexity...
Refocusing practice on MDM complexity
The practice of medicine is a calling with great rewards. Prominent among those rewards are improving the health of women, children, and the community, developing deep and trusting relationships with patients, families, and clinical colleagues. The practice of medicine is also replete with a host of punishing administrative burdens, including prior authorizations, clunky electronic medical records, poorly designed quality metrics that are applied to clinicians, and billing compliance rules that emphasize the repetitive documentation of clinical information with minimal value.
Some of the most irritating aspects of medical practice are the CMS rules governing medical record documentation required for billing ambulatory office visits. Current coding compliance focuses on counting the number of systems reviewed in the review of systems; the documentation of past history, social history, and family history; the number of organs and organ elements examined during the physical examination; and the complexity of MDM.
In January 2021, CMS plans to adopt new Current Procedural Terminology (CPT) code descriptors for the office and other outpatient E/M services that sunset most of the “bean-counting” metrics and emphasize the importance of the complexity of MDM in guiding selection of a correct code.2 Beginning in January 2021, clinicians will have the option of selecting an E/M code level based on the total amount of time required to provide the office visit service or the complexity of MDM. When selecting a code level based on MDM the new guidance emphasizes the importance of reviewing notes from other clinicians, reviewing test results, ordering of tests, and discussing and coordinating the care of the patient with other treating physicians. These changes reflect a better understanding of what is most important in good medical practice, promoting better patient care. TABLES 1 and 2 provide the initial guidance from CMS concerning selection of E/M code level based on time and MDM, respectively.2 The guidance for using MDM to select an E/M code level is likely to evolve following implementation, so stay tuned. When using MDM to select a code, 2 of the 3 general categories are required to select that level of service.
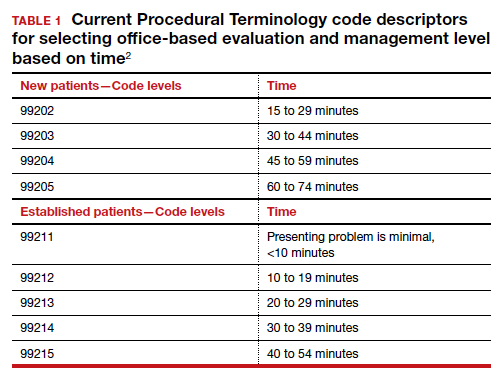
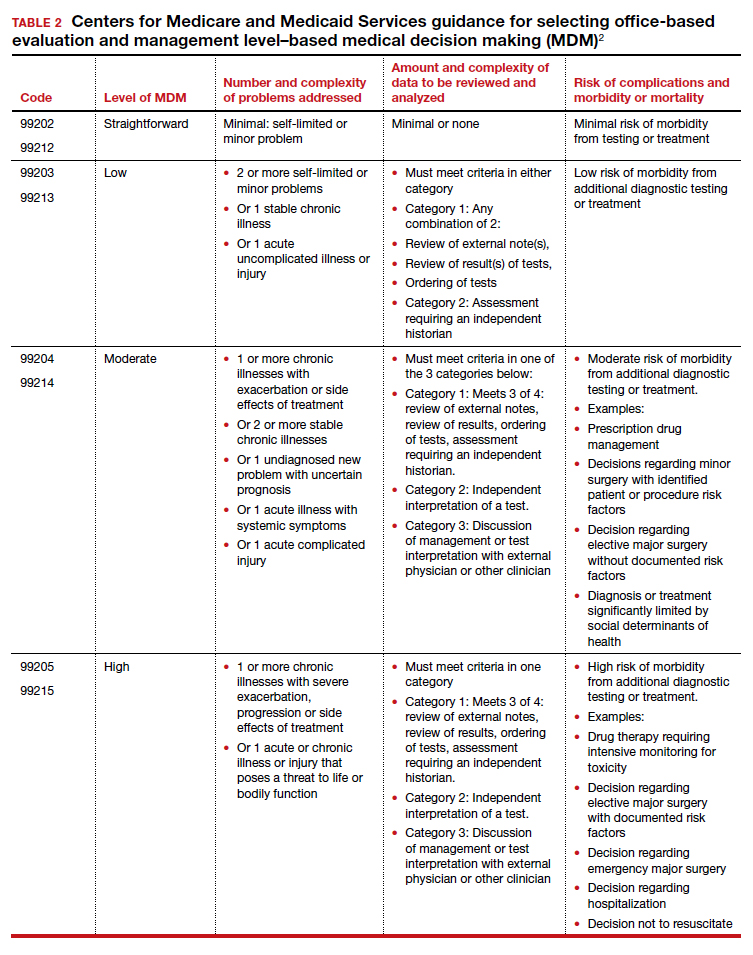
Increase in the valuation of office-based E/M services
The Medicare Physician Fee Schedule uses a resource-based relative value system to determine time and intensity of the work of clinical practice. This system recognizes 3 major factors that influence the resources required to provide a service:
- work of the clinician
- practice expense for technical components
- cost of professional liability insurance.
Many primary care professional associations have long contended that CMS has undervalued office-based E/M services relative to procedures, resulting in the devaluing of primary care practice. After the CPT code descriptors were updated by the CPT editorial panel, 52 specialty societies surveyed their members to provide inputs to CMS on the time and intensity of the office and other outpatient E/M codes as currently practiced. The American Medical Association’s Specialty Society Resource-Based Relative Value Scale Update Committee (RUC) reviewed the surveys and provided new inputs via open comment to CMS. CMS has responded to this feedback with a review of the intensity of clinical work required to provide an ambulatory visit service. In response to the review, CMS proposes to accept the recommendations of the RUC representing the house of medicine and increase the work and practice expense relative value assigned to new and established office visit codes. Overall, the combination of changes in relative values assigned for the work of the clinician and the expense of practice, increases the total value of office-based E/M codes for new patients by 7% to 14% and for established patients from 28% to 46% (see supplemental table in the sidebar at the end of this article).
Continue to: Decreased payments for procedural services...
Decreased payments for procedural services
Medicare is required to offset increased payment in one arena of health care delivery with decreased payment in other arenas of care, thereby achieving “budget-neutrality.” As detailed above, CMS plans to increase Medicare payments for office-based E/M services. Payment for services is calculated by multiplying the total RVUs for a particular service by a “conversion factor” (ie, number of dollars per RVU). To achieve budget-neutrality, CMS has proposed substantially reducing the conversion factor for 2021 (from $36.09 to $32.26), which will effectively decrease Medicare payments for procedural services since their RVUs have not changed. While the AMA RUC and many specialty societies continue to strongly advocate for the E/M work RVU increases to be included in the E/M components of 10- and 90-day global services, CMS has proposed to implement them only for “stand alone” E/M services.
Organizations are lobbying to delay or prevent the planned decrease in conversion factor, which results in substantial declines in payment for procedural services. (See "What do the Medicare billing changes mean for the Obstetrical Bundled services?" at the end of this article.) Due to the economic and clinical practice challenges caused by the coronavirus disease 2019 (COVID-19) pandemic it would be best if CMS did not reduce payments to physicians who are experts in procedural health care, thereby avoiding the risk of reduced access to these vital services.
If the current CMS changes in payment are implemented, endocrinologists, rheumatologists, and family physicians will have an increase in payment, and radiologists, pathologists, and surgeons will have a decrease in payment (TABLE 3).6 Obstetrics and gynecology is projected to have an 8% increase in Medicare payment. However, if an obstetrician-gynecologist derives most of their Medicare payments from surgical procedures, they are likely to have a decrease in payment from Medicare. Other payers will be incorporating the new coding structure for 2021; however, their payment structures and conversion factors are likely to vary. It is important to note that the RVUs for procedures have not changed. The budget neutrality adjustment resulted in a much lower conversion factor and therefore a decrease in payment for those specialties whose RVUs did not increase.
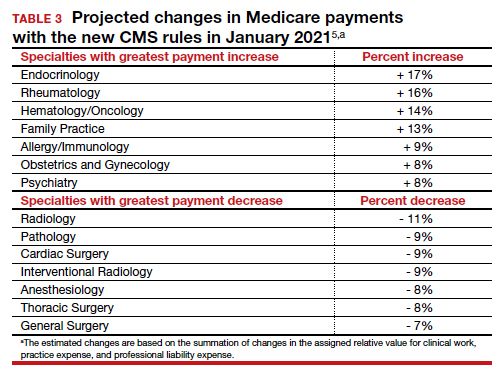
Bottom line
Working through the Medicare, Medicaid, and CHIP programs, CMS can influence greatly the practice of medicine including medical record documentation practices and payment rates for every clinical service. CMS proposes to end the onerous “bean counting” approach to billing compliance and refocus on the complexity of MDM as the foundation for selecting a billing code level. This change is long overdue, valuing the effective management of complex patients in office practice. Hopefully, CMS will reverse the planned reduction in the payment for procedural services, preserving patient access to important health care services. ●
The CY 2020 Medicare Physician Fee Schedule Final Rule was published electronically in the Federal Register on November 1, 2019. This final rule aligns the evaluation and management (E/M) coding and payment with changes recommended by the Current Procedural Terminology (CPT) Editorial Panel and American Medical Association’s (AMA) Specialty Society Resource-Based Relative Value Scale Update Committee (RUC) for office/outpatient E/M visits. Unfortunately, the Centers for Medicare and Medicaid Services (CMS) did not agree with the RUC, AMA, and specialty societies that the E/M payment changes should be applicable across all global services that incorporate E/M visits—despite the fact that the values proposed by the RUC incorporated survey data from 52 specialties, representing most of medicine (including those specialties that predominantly perform procedures). Specifically, CMS expressed the view that the number of E/M visits within the 10- and 90-day global codes, as well as the maternity care bundle, were difficult to validate; therefore, the increased values would not be distributed to those procedural services.
Many professional societies expressed significant concerns about the resulting budget neutrality adjustments that would occur effective January 2021. The great news for ObGyns is that the American College of Obstetricians and Gynecologists (ACOG) was able to respond directly to CMS’s concerns with data to support the number of prenatal visits within the Obstetrical Bundle. Tapping into a de-identified, cloud-based data set of prenatal records—representing more than 1,100 obstetric providers with close to 30,000 recently completed pregnancies—ACOG was able to document both a mean and median number of prenatal visits across a broad geographic, payer, and patient demographic that supported the 13 prenatal visits in the Obstetrical Bundle.
With ACOG’s advocacy and ability to provide data to CMS, the proposed physician fee schedule rule for 2021 has proposed to incorporate the E/M increased reimbursement into the prenatal care codes. Now we urge the CMS to finalize this proposal. Although Medicare pays for a tiny number of pregnancies annually, we hope that all payers, including Medicaid and managed care plans, will agree with this acknowledgement of the increased work of evaluation and management that obstetricians provide during prenatal care. Join ACOG in telling CMS to finalize their proposal to increase the values of the global obstetric codes: https://acog.quorum.us/campaign/28579/.
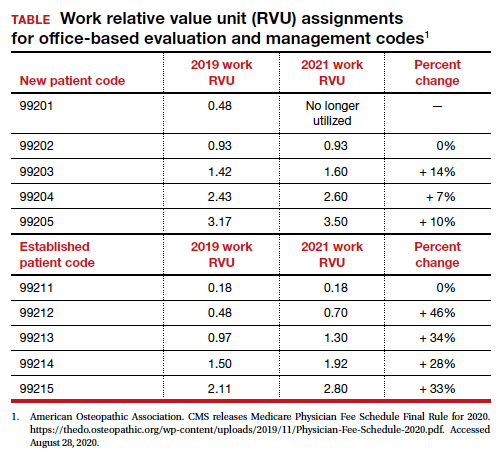
- Centers for Medicare and Medicaid Services. https://www.cms.gov/. Accessed August 28, 2020.
- American Medical Association. CPT Evaluation and Management (E/M) Office or Other Outpatient (99202-99215) and Prolonged Services (99354, 99355, 99356, 99XXX) Code and Guideline Changes. 2019. https://www.ama-assn.org /system/files/2019-06/cpt-office-prolonged-svs -code-changes.pdf. Accessed August 28, 2020.
- The American Academy of Family Physicians. Family medicine updates. Ann Fam Med. 2020;18:84-85. doi: 10.1370/afm.2508.
- Centers for Medicare and Medicaid Services. Final policy, payment and quality provisions changes to the Medicare Physician Fee Schedule for calendar year 2019. November 1, 2018. https://www.cms.gov/newsroom/fact-sheets /final-policy-payment-and-quality-provisionschanges-medicare-physician-fee-schedulecalendar-year. Accessed August 28, 2020.
- Department of Health and Human Services; Centers for Medicare and Medicaid Services. 42 CFR Parts 410, 414, 415, 423, 424, and 425. Federal Register. 2020;85(159). https://www.govinfo.gov /content/pkg/FR-2020-08-17/pdf/2020-17127 .pdf. Accessed August 28, 2020.
The Centers for Medicare and Medicaid Services (CMS) finalized an increase in the relative value of evaluation and management (E/M) service codes effective January 1, 2021, which results in an overall decrease in the payment for procedural services in the Medicare program. (Due to the mandate for budget neutrality, an increase in relative value units [RVUs] for E/M resulted in a large decrease in the conversion factor—the number of dollars per RVU). This has increased payments for endocrinologists, rheumatologists, and family medicine clinicians and decreased payments for radiologists, pathologists, and surgeons.
In a major win for physicians, CMS proposes to simplify documentation requirements for billing and focus on the complexity of the medical decision making (MDM) or the total time needed to care for the patient on the date of the service as the foundation for determining the relative value of the service. Therefore, there is no more counting bullets—ie, we don’t have to perform a comprehensive physical exam or review of systems to achieve a high level code! Prior to this change, time was only available for coding purposes when counseling and coordination of care was the predominant service (>50%), and only face-to-face time with the patient was considered. Effective January 1, for office and other outpatient services, total time on the calendar date of the encounter will be used. This acknowledges the intensity and value of non–face-to-face work.
Acting through CMS, the federal government influences greatly the US health care system. CMS is an agency in the Department of Health and Human Services that administers the Medicare program and partners with state governments to administer the Health Insurance Exchanges, Medicaid, and the Children’s Health Insurance programs (CHIP).1 In addition, CMS is responsible for enforcing quality care standards in long-term care facilities and clinical laboratories and the implementation of the Health Insurance Portability and Accountability Act.1
In January, CMS plans the following major changes to coding and documentation2,3:
- Selection of the level of E/M service will no longer require documentation of bullet points in the history, physical exam, and MDM. The simplified system allows physicians and qualified health care professionals to code either by total time (both face-to-face and non–face-to-face) on the date of the encounter or by level of MDM.
- For established office patients, 5 levels of office-based evaluation and management services will be retained. CMS had initially proposed to reduce the number of office-based E/M codes from 5 to 3, combining code levels 2, 3, and 4 into 1 code.4 However, after receiving feedback from professional societies and the public, CMS abandoned the plan for radical simplification of coding levels.2,3 Implementation of their proposal would have resulted in the same payment for treatment of a hang nail as for a complex gyn patient with multiple medical problems. Both patient advocacy groups and professional societies argued that incentives originally were misaligned.
- For new office patients, since both 99201 and 99202 require straightforward MDM, the level 1 code (99201) has been eliminated, reducing the number of code levels from 5 to 4.
- History and physical exam will no longer be used to determine code level for office E/M codes. These elements will be required only as medically appropriate. This means that documentation review will no longer focus on “bean counting” the elements in the history and physical exam.
- Following a reassessment of the actual time required to provide E/M services in real-life practice, CMS plans to markedly increase the relative value of office visits for established patients and modestly increase the relative value of office visits for new patients. CMS operates under the principle of “neutral budgeting,” meaning that an increase of the relative value of E/M codes will result in a decrease in the payment for procedural codes. The actual RVUs for procedural services do not change; however, budget neutrality requires a decrease in the dollar conversion factor. The proposed changes will increase the payment for E/M services and decrease payments for procedural services.
Continue to: Refocusing practice on MDM complexity...
Refocusing practice on MDM complexity
The practice of medicine is a calling with great rewards. Prominent among those rewards are improving the health of women, children, and the community, developing deep and trusting relationships with patients, families, and clinical colleagues. The practice of medicine is also replete with a host of punishing administrative burdens, including prior authorizations, clunky electronic medical records, poorly designed quality metrics that are applied to clinicians, and billing compliance rules that emphasize the repetitive documentation of clinical information with minimal value.
Some of the most irritating aspects of medical practice are the CMS rules governing medical record documentation required for billing ambulatory office visits. Current coding compliance focuses on counting the number of systems reviewed in the review of systems; the documentation of past history, social history, and family history; the number of organs and organ elements examined during the physical examination; and the complexity of MDM.
In January 2021, CMS plans to adopt new Current Procedural Terminology (CPT) code descriptors for the office and other outpatient E/M services that sunset most of the “bean-counting” metrics and emphasize the importance of the complexity of MDM in guiding selection of a correct code.2 Beginning in January 2021, clinicians will have the option of selecting an E/M code level based on the total amount of time required to provide the office visit service or the complexity of MDM. When selecting a code level based on MDM the new guidance emphasizes the importance of reviewing notes from other clinicians, reviewing test results, ordering of tests, and discussing and coordinating the care of the patient with other treating physicians. These changes reflect a better understanding of what is most important in good medical practice, promoting better patient care. TABLES 1 and 2 provide the initial guidance from CMS concerning selection of E/M code level based on time and MDM, respectively.2 The guidance for using MDM to select an E/M code level is likely to evolve following implementation, so stay tuned. When using MDM to select a code, 2 of the 3 general categories are required to select that level of service.


Increase in the valuation of office-based E/M services
The Medicare Physician Fee Schedule uses a resource-based relative value system to determine time and intensity of the work of clinical practice. This system recognizes 3 major factors that influence the resources required to provide a service:
- work of the clinician
- practice expense for technical components
- cost of professional liability insurance.
Many primary care professional associations have long contended that CMS has undervalued office-based E/M services relative to procedures, resulting in the devaluing of primary care practice. After the CPT code descriptors were updated by the CPT editorial panel, 52 specialty societies surveyed their members to provide inputs to CMS on the time and intensity of the office and other outpatient E/M codes as currently practiced. The American Medical Association’s Specialty Society Resource-Based Relative Value Scale Update Committee (RUC) reviewed the surveys and provided new inputs via open comment to CMS. CMS has responded to this feedback with a review of the intensity of clinical work required to provide an ambulatory visit service. In response to the review, CMS proposes to accept the recommendations of the RUC representing the house of medicine and increase the work and practice expense relative value assigned to new and established office visit codes. Overall, the combination of changes in relative values assigned for the work of the clinician and the expense of practice, increases the total value of office-based E/M codes for new patients by 7% to 14% and for established patients from 28% to 46% (see supplemental table in the sidebar at the end of this article).
Continue to: Decreased payments for procedural services...
Decreased payments for procedural services
Medicare is required to offset increased payment in one arena of health care delivery with decreased payment in other arenas of care, thereby achieving “budget-neutrality.” As detailed above, CMS plans to increase Medicare payments for office-based E/M services. Payment for services is calculated by multiplying the total RVUs for a particular service by a “conversion factor” (ie, number of dollars per RVU). To achieve budget-neutrality, CMS has proposed substantially reducing the conversion factor for 2021 (from $36.09 to $32.26), which will effectively decrease Medicare payments for procedural services since their RVUs have not changed. While the AMA RUC and many specialty societies continue to strongly advocate for the E/M work RVU increases to be included in the E/M components of 10- and 90-day global services, CMS has proposed to implement them only for “stand alone” E/M services.
Organizations are lobbying to delay or prevent the planned decrease in conversion factor, which results in substantial declines in payment for procedural services. (See "What do the Medicare billing changes mean for the Obstetrical Bundled services?" at the end of this article.) Due to the economic and clinical practice challenges caused by the coronavirus disease 2019 (COVID-19) pandemic it would be best if CMS did not reduce payments to physicians who are experts in procedural health care, thereby avoiding the risk of reduced access to these vital services.
If the current CMS changes in payment are implemented, endocrinologists, rheumatologists, and family physicians will have an increase in payment, and radiologists, pathologists, and surgeons will have a decrease in payment (TABLE 3).6 Obstetrics and gynecology is projected to have an 8% increase in Medicare payment. However, if an obstetrician-gynecologist derives most of their Medicare payments from surgical procedures, they are likely to have a decrease in payment from Medicare. Other payers will be incorporating the new coding structure for 2021; however, their payment structures and conversion factors are likely to vary. It is important to note that the RVUs for procedures have not changed. The budget neutrality adjustment resulted in a much lower conversion factor and therefore a decrease in payment for those specialties whose RVUs did not increase.

Bottom line
Working through the Medicare, Medicaid, and CHIP programs, CMS can influence greatly the practice of medicine including medical record documentation practices and payment rates for every clinical service. CMS proposes to end the onerous “bean counting” approach to billing compliance and refocus on the complexity of MDM as the foundation for selecting a billing code level. This change is long overdue, valuing the effective management of complex patients in office practice. Hopefully, CMS will reverse the planned reduction in the payment for procedural services, preserving patient access to important health care services. ●
The CY 2020 Medicare Physician Fee Schedule Final Rule was published electronically in the Federal Register on November 1, 2019. This final rule aligns the evaluation and management (E/M) coding and payment with changes recommended by the Current Procedural Terminology (CPT) Editorial Panel and American Medical Association’s (AMA) Specialty Society Resource-Based Relative Value Scale Update Committee (RUC) for office/outpatient E/M visits. Unfortunately, the Centers for Medicare and Medicaid Services (CMS) did not agree with the RUC, AMA, and specialty societies that the E/M payment changes should be applicable across all global services that incorporate E/M visits—despite the fact that the values proposed by the RUC incorporated survey data from 52 specialties, representing most of medicine (including those specialties that predominantly perform procedures). Specifically, CMS expressed the view that the number of E/M visits within the 10- and 90-day global codes, as well as the maternity care bundle, were difficult to validate; therefore, the increased values would not be distributed to those procedural services.
Many professional societies expressed significant concerns about the resulting budget neutrality adjustments that would occur effective January 2021. The great news for ObGyns is that the American College of Obstetricians and Gynecologists (ACOG) was able to respond directly to CMS’s concerns with data to support the number of prenatal visits within the Obstetrical Bundle. Tapping into a de-identified, cloud-based data set of prenatal records—representing more than 1,100 obstetric providers with close to 30,000 recently completed pregnancies—ACOG was able to document both a mean and median number of prenatal visits across a broad geographic, payer, and patient demographic that supported the 13 prenatal visits in the Obstetrical Bundle.
With ACOG’s advocacy and ability to provide data to CMS, the proposed physician fee schedule rule for 2021 has proposed to incorporate the E/M increased reimbursement into the prenatal care codes. Now we urge the CMS to finalize this proposal. Although Medicare pays for a tiny number of pregnancies annually, we hope that all payers, including Medicaid and managed care plans, will agree with this acknowledgement of the increased work of evaluation and management that obstetricians provide during prenatal care. Join ACOG in telling CMS to finalize their proposal to increase the values of the global obstetric codes: https://acog.quorum.us/campaign/28579/.

The Centers for Medicare and Medicaid Services (CMS) finalized an increase in the relative value of evaluation and management (E/M) service codes effective January 1, 2021, which results in an overall decrease in the payment for procedural services in the Medicare program. (Due to the mandate for budget neutrality, an increase in relative value units [RVUs] for E/M resulted in a large decrease in the conversion factor—the number of dollars per RVU). This has increased payments for endocrinologists, rheumatologists, and family medicine clinicians and decreased payments for radiologists, pathologists, and surgeons.
In a major win for physicians, CMS proposes to simplify documentation requirements for billing and focus on the complexity of the medical decision making (MDM) or the total time needed to care for the patient on the date of the service as the foundation for determining the relative value of the service. Therefore, there is no more counting bullets—ie, we don’t have to perform a comprehensive physical exam or review of systems to achieve a high level code! Prior to this change, time was only available for coding purposes when counseling and coordination of care was the predominant service (>50%), and only face-to-face time with the patient was considered. Effective January 1, for office and other outpatient services, total time on the calendar date of the encounter will be used. This acknowledges the intensity and value of non–face-to-face work.
Acting through CMS, the federal government influences greatly the US health care system. CMS is an agency in the Department of Health and Human Services that administers the Medicare program and partners with state governments to administer the Health Insurance Exchanges, Medicaid, and the Children’s Health Insurance programs (CHIP).1 In addition, CMS is responsible for enforcing quality care standards in long-term care facilities and clinical laboratories and the implementation of the Health Insurance Portability and Accountability Act.1
In January, CMS plans the following major changes to coding and documentation2,3:
- Selection of the level of E/M service will no longer require documentation of bullet points in the history, physical exam, and MDM. The simplified system allows physicians and qualified health care professionals to code either by total time (both face-to-face and non–face-to-face) on the date of the encounter or by level of MDM.
- For established office patients, 5 levels of office-based evaluation and management services will be retained. CMS had initially proposed to reduce the number of office-based E/M codes from 5 to 3, combining code levels 2, 3, and 4 into 1 code.4 However, after receiving feedback from professional societies and the public, CMS abandoned the plan for radical simplification of coding levels.2,3 Implementation of their proposal would have resulted in the same payment for treatment of a hang nail as for a complex gyn patient with multiple medical problems. Both patient advocacy groups and professional societies argued that incentives originally were misaligned.
- For new office patients, since both 99201 and 99202 require straightforward MDM, the level 1 code (99201) has been eliminated, reducing the number of code levels from 5 to 4.
- History and physical exam will no longer be used to determine code level for office E/M codes. These elements will be required only as medically appropriate. This means that documentation review will no longer focus on “bean counting” the elements in the history and physical exam.
- Following a reassessment of the actual time required to provide E/M services in real-life practice, CMS plans to markedly increase the relative value of office visits for established patients and modestly increase the relative value of office visits for new patients. CMS operates under the principle of “neutral budgeting,” meaning that an increase of the relative value of E/M codes will result in a decrease in the payment for procedural codes. The actual RVUs for procedural services do not change; however, budget neutrality requires a decrease in the dollar conversion factor. The proposed changes will increase the payment for E/M services and decrease payments for procedural services.
Continue to: Refocusing practice on MDM complexity...
Refocusing practice on MDM complexity
The practice of medicine is a calling with great rewards. Prominent among those rewards are improving the health of women, children, and the community, developing deep and trusting relationships with patients, families, and clinical colleagues. The practice of medicine is also replete with a host of punishing administrative burdens, including prior authorizations, clunky electronic medical records, poorly designed quality metrics that are applied to clinicians, and billing compliance rules that emphasize the repetitive documentation of clinical information with minimal value.
Some of the most irritating aspects of medical practice are the CMS rules governing medical record documentation required for billing ambulatory office visits. Current coding compliance focuses on counting the number of systems reviewed in the review of systems; the documentation of past history, social history, and family history; the number of organs and organ elements examined during the physical examination; and the complexity of MDM.
In January 2021, CMS plans to adopt new Current Procedural Terminology (CPT) code descriptors for the office and other outpatient E/M services that sunset most of the “bean-counting” metrics and emphasize the importance of the complexity of MDM in guiding selection of a correct code.2 Beginning in January 2021, clinicians will have the option of selecting an E/M code level based on the total amount of time required to provide the office visit service or the complexity of MDM. When selecting a code level based on MDM the new guidance emphasizes the importance of reviewing notes from other clinicians, reviewing test results, ordering of tests, and discussing and coordinating the care of the patient with other treating physicians. These changes reflect a better understanding of what is most important in good medical practice, promoting better patient care. TABLES 1 and 2 provide the initial guidance from CMS concerning selection of E/M code level based on time and MDM, respectively.2 The guidance for using MDM to select an E/M code level is likely to evolve following implementation, so stay tuned. When using MDM to select a code, 2 of the 3 general categories are required to select that level of service.


Increase in the valuation of office-based E/M services
The Medicare Physician Fee Schedule uses a resource-based relative value system to determine time and intensity of the work of clinical practice. This system recognizes 3 major factors that influence the resources required to provide a service:
- work of the clinician
- practice expense for technical components
- cost of professional liability insurance.
Many primary care professional associations have long contended that CMS has undervalued office-based E/M services relative to procedures, resulting in the devaluing of primary care practice. After the CPT code descriptors were updated by the CPT editorial panel, 52 specialty societies surveyed their members to provide inputs to CMS on the time and intensity of the office and other outpatient E/M codes as currently practiced. The American Medical Association’s Specialty Society Resource-Based Relative Value Scale Update Committee (RUC) reviewed the surveys and provided new inputs via open comment to CMS. CMS has responded to this feedback with a review of the intensity of clinical work required to provide an ambulatory visit service. In response to the review, CMS proposes to accept the recommendations of the RUC representing the house of medicine and increase the work and practice expense relative value assigned to new and established office visit codes. Overall, the combination of changes in relative values assigned for the work of the clinician and the expense of practice, increases the total value of office-based E/M codes for new patients by 7% to 14% and for established patients from 28% to 46% (see supplemental table in the sidebar at the end of this article).
Continue to: Decreased payments for procedural services...
Decreased payments for procedural services
Medicare is required to offset increased payment in one arena of health care delivery with decreased payment in other arenas of care, thereby achieving “budget-neutrality.” As detailed above, CMS plans to increase Medicare payments for office-based E/M services. Payment for services is calculated by multiplying the total RVUs for a particular service by a “conversion factor” (ie, number of dollars per RVU). To achieve budget-neutrality, CMS has proposed substantially reducing the conversion factor for 2021 (from $36.09 to $32.26), which will effectively decrease Medicare payments for procedural services since their RVUs have not changed. While the AMA RUC and many specialty societies continue to strongly advocate for the E/M work RVU increases to be included in the E/M components of 10- and 90-day global services, CMS has proposed to implement them only for “stand alone” E/M services.
Organizations are lobbying to delay or prevent the planned decrease in conversion factor, which results in substantial declines in payment for procedural services. (See "What do the Medicare billing changes mean for the Obstetrical Bundled services?" at the end of this article.) Due to the economic and clinical practice challenges caused by the coronavirus disease 2019 (COVID-19) pandemic it would be best if CMS did not reduce payments to physicians who are experts in procedural health care, thereby avoiding the risk of reduced access to these vital services.
If the current CMS changes in payment are implemented, endocrinologists, rheumatologists, and family physicians will have an increase in payment, and radiologists, pathologists, and surgeons will have a decrease in payment (TABLE 3).6 Obstetrics and gynecology is projected to have an 8% increase in Medicare payment. However, if an obstetrician-gynecologist derives most of their Medicare payments from surgical procedures, they are likely to have a decrease in payment from Medicare. Other payers will be incorporating the new coding structure for 2021; however, their payment structures and conversion factors are likely to vary. It is important to note that the RVUs for procedures have not changed. The budget neutrality adjustment resulted in a much lower conversion factor and therefore a decrease in payment for those specialties whose RVUs did not increase.

Bottom line
Working through the Medicare, Medicaid, and CHIP programs, CMS can influence greatly the practice of medicine including medical record documentation practices and payment rates for every clinical service. CMS proposes to end the onerous “bean counting” approach to billing compliance and refocus on the complexity of MDM as the foundation for selecting a billing code level. This change is long overdue, valuing the effective management of complex patients in office practice. Hopefully, CMS will reverse the planned reduction in the payment for procedural services, preserving patient access to important health care services. ●
The CY 2020 Medicare Physician Fee Schedule Final Rule was published electronically in the Federal Register on November 1, 2019. This final rule aligns the evaluation and management (E/M) coding and payment with changes recommended by the Current Procedural Terminology (CPT) Editorial Panel and American Medical Association’s (AMA) Specialty Society Resource-Based Relative Value Scale Update Committee (RUC) for office/outpatient E/M visits. Unfortunately, the Centers for Medicare and Medicaid Services (CMS) did not agree with the RUC, AMA, and specialty societies that the E/M payment changes should be applicable across all global services that incorporate E/M visits—despite the fact that the values proposed by the RUC incorporated survey data from 52 specialties, representing most of medicine (including those specialties that predominantly perform procedures). Specifically, CMS expressed the view that the number of E/M visits within the 10- and 90-day global codes, as well as the maternity care bundle, were difficult to validate; therefore, the increased values would not be distributed to those procedural services.
Many professional societies expressed significant concerns about the resulting budget neutrality adjustments that would occur effective January 2021. The great news for ObGyns is that the American College of Obstetricians and Gynecologists (ACOG) was able to respond directly to CMS’s concerns with data to support the number of prenatal visits within the Obstetrical Bundle. Tapping into a de-identified, cloud-based data set of prenatal records—representing more than 1,100 obstetric providers with close to 30,000 recently completed pregnancies—ACOG was able to document both a mean and median number of prenatal visits across a broad geographic, payer, and patient demographic that supported the 13 prenatal visits in the Obstetrical Bundle.
With ACOG’s advocacy and ability to provide data to CMS, the proposed physician fee schedule rule for 2021 has proposed to incorporate the E/M increased reimbursement into the prenatal care codes. Now we urge the CMS to finalize this proposal. Although Medicare pays for a tiny number of pregnancies annually, we hope that all payers, including Medicaid and managed care plans, will agree with this acknowledgement of the increased work of evaluation and management that obstetricians provide during prenatal care. Join ACOG in telling CMS to finalize their proposal to increase the values of the global obstetric codes: https://acog.quorum.us/campaign/28579/.

- Centers for Medicare and Medicaid Services. https://www.cms.gov/. Accessed August 28, 2020.
- American Medical Association. CPT Evaluation and Management (E/M) Office or Other Outpatient (99202-99215) and Prolonged Services (99354, 99355, 99356, 99XXX) Code and Guideline Changes. 2019. https://www.ama-assn.org /system/files/2019-06/cpt-office-prolonged-svs -code-changes.pdf. Accessed August 28, 2020.
- The American Academy of Family Physicians. Family medicine updates. Ann Fam Med. 2020;18:84-85. doi: 10.1370/afm.2508.
- Centers for Medicare and Medicaid Services. Final policy, payment and quality provisions changes to the Medicare Physician Fee Schedule for calendar year 2019. November 1, 2018. https://www.cms.gov/newsroom/fact-sheets /final-policy-payment-and-quality-provisionschanges-medicare-physician-fee-schedulecalendar-year. Accessed August 28, 2020.
- Department of Health and Human Services; Centers for Medicare and Medicaid Services. 42 CFR Parts 410, 414, 415, 423, 424, and 425. Federal Register. 2020;85(159). https://www.govinfo.gov /content/pkg/FR-2020-08-17/pdf/2020-17127 .pdf. Accessed August 28, 2020.
- Centers for Medicare and Medicaid Services. https://www.cms.gov/. Accessed August 28, 2020.
- American Medical Association. CPT Evaluation and Management (E/M) Office or Other Outpatient (99202-99215) and Prolonged Services (99354, 99355, 99356, 99XXX) Code and Guideline Changes. 2019. https://www.ama-assn.org /system/files/2019-06/cpt-office-prolonged-svs -code-changes.pdf. Accessed August 28, 2020.
- The American Academy of Family Physicians. Family medicine updates. Ann Fam Med. 2020;18:84-85. doi: 10.1370/afm.2508.
- Centers for Medicare and Medicaid Services. Final policy, payment and quality provisions changes to the Medicare Physician Fee Schedule for calendar year 2019. November 1, 2018. https://www.cms.gov/newsroom/fact-sheets /final-policy-payment-and-quality-provisionschanges-medicare-physician-fee-schedulecalendar-year. Accessed August 28, 2020.
- Department of Health and Human Services; Centers for Medicare and Medicaid Services. 42 CFR Parts 410, 414, 415, 423, 424, and 425. Federal Register. 2020;85(159). https://www.govinfo.gov /content/pkg/FR-2020-08-17/pdf/2020-17127 .pdf. Accessed August 28, 2020.
New hormonal medical treatment is an important advance for AUB caused by uterine fibroids
Uterine leiomyomata (fibroids) are the most common pelvic tumor diagnosed in women.1 Women with symptomatic fibroids often report abnormal uterine bleeding (AUB) and pelvic cramping, fullness, or pain. Fibroids also may cause frequency of urination and contribute to fertility and pregnancy problems. Treatment options for the AUB caused by fibroids include, but are not limited to, hysterectomy, myomectomy, uterine artery embolization, endometrial ablation, insertion of a levonorgestrel intrauterine device, focused ultrasound surgery, radiofrequency ablation, leuprolide acetate, and elagolix plus low-dose hormone add-back (Oriahnn; AbbVie, North Chicago, Illinois).1 Oriahnn is the most recent addition to our treatment armamentarium for fibroids and represents the first US Food and Drug Administration (FDA)-approved long-term hormonal option for AUB caused by fibroids.
Gene dysregulation contributes to fibroid development
Most uterine fibroids are clonal tumors, which develop following a somatic mutation in a precursor uterine myocyte. The somatic mutation causes gene dysregulation that stimulates cell growth resulting in a benign tumor mass. The majority of fibroids contain a mutation in one of the following 6 genes: mediator complex subunit 12 (MED12), high mobility group AT-hook (HMGA2 or HMGA1), RAD51B, fumarate hydratase (FH), collagen type IV, alpha 5 chain (COL4A5), or collagen type IV alpha 6 chain (COL4A6).2
Gene dysregulation in fibroids may arise following chromothripsis of the uterine myocyte genome
Chromothripsis is a catastrophic intracellular genetic event in which one or more chromosomes are broken and reassemble in a new nucleic acid sequence, producing a derivative chromosome that contains complex genetic rearrangements.3 Chromothripsis is believed to occur frequently in uterine myocytes. It is unknown why uterine myocytes are susceptible to chromothripsis,3 or why a catastrophic intracellular event such as chromothripsis results in preferential mutations in the 6 genes that are associated with myoma formation.
Estrogen and progesterone influence fibroid size and cell activity
Although uterine fibroids are clonal tumors containing broken genes, they are also exquisitely responsive to estradiol and progesterone. Estradiol and progesterone play an important role in regulating fibroid size and function.4 Estrogen stimulates uterine fibroids to increase in size. In a hypoestrogenic state, uterine fibroids decrease in size. In addition, a hypoestrogenic state results in an atrophic endometrium and thereby reduces AUB. For women with uterine fibroids and AUB, a reversible hypoestrogenic state can be induced either with a parenteral GnRH-agonist analogue (leuprolide) or an oral GnRH-antagonist (elagolix). Both leuprolide and elagolix are approved for the treatment of uterine fibroids (see below).
Surprisingly, progesterone stimulates cell division in normal uterine myocytes and fibroid cells.5 In the luteal phase of the menstrual cycle, uterine myocyte mitoses are more frequent than in the follicular phase. In addition, synthetic progestins appear to maintain fibroid size in a hypoestrogenic environment. In one randomized trial, women with uterine fibroids treated with leuprolide acetate plus a placebo pill for 24 weeks had a 51% reduction in uterine volume as measured by ultrasound.6 Women with uterine fibroids treated with leuprolide acetate plus the synthetic progestin, oral medroxyprogesterone acetate 20 mg daily, had only a 15% reduction in uterine volume.6 This finding suggests that synthetic progestins partially block the decrease in uterine volume that occurs in a hypoestrogenic state.
Further evidence that progesterone plays a role in fibroid biology is the observation that treatment of women with uterine fibroids with the antiprogestin ulipristal decreases fibroid size and reduces AUB.7-9 Ulipristal was approved for the treatment of fibroids in many countries but not the United States. Reports of severe, life-threatening liver injury—some necessitating liver transplantation—among women using ulipristal prompted the European Medicines Agency (EMA) in 2020 to recommend that women stop taking ulipristal. In addition, the EMA recommended that no woman should initiate ulipristal treatment at this time.10
Continue to: Leuprolide acetate...
Leuprolide acetate
Leuprolide acetate is a peptide GnRH-agonist analogue. Initiation of leuprolide treatment stimulates gonadotropin release, but with chronic administration pituitary secretion of luteinizing hormone (LH) and follicle-stimulating hormone (FSH) decreases, resulting in reduced ovarian follicular activity, anovulation, and low serum concentration of estradiol and progesterone. Leuprolide treatment concomitant with iron therapy is approved by the FDA for improving red blood cell volume prior to surgery in women with fibroids, AUB, and anemia.11 Among women with fibroids, AUB, and anemia, after 12 weeks of treatment, the hemoglobin concentration was ≥12 g/dL in 79% treated with leuprolide plus iron and 56% treated with iron alone.11 The FDA recommends limiting preoperative leuprolide treatment to no more than 3 months. The approved leuprolide regimens are a maximum of 3 monthly injections of leuprolide 3.75 mg or a single injection of leuprolide 11.25 mg. Leuprolide treatment prior to hysterectomy surgery for uterine fibroids usually will result in a decrease in uterine size and may facilitate vaginal hysterectomy.
Elagolix plus estradiol plus norethindrone acetate (Oriahnn)
GnRH analogues cause a hypoestrogenic state resulting in adverse effects, including moderate to severe hot flashes and a reduction in bone mineral density. One approach to reducing the unwanted effects of hot flashes and decreased bone density is to combine a GnRH analogue with low-dose steroid hormone add-back therapy. Combining a GnRH analogue with low-dose steroid hormone add-back permits long-term treatment of AUB caused by fibroids, with few hot flashes and a minimal decrease in bone mineral density. The FDA recently has approved the combination of elagolix plus low-dose estradiol and norethindrone acetate (Oriahnn) for the long-term treatment of AUB caused by fibroids.
Elagolix is a nonpeptide oral GnRH antagonist that reduces pituitary secretion of LH and FSH, resulting in a decrease in ovarian follicular activity, anovulation, and low serum concentration of estradiol and progesterone. Unlike leuprolide, which causes an initial increase in LH and FSH secretion, the initiation of elagolix treatment causes an immediate and sustained reduction in LH and FSH secretion. Combining elagolix with a low dose of estradiol and norethindrone acetate reduces the side effects of hot flashes and decreased bone density. Clinical trials have reported that the combination of elagolix (300 mg) twice daily plus estradiol (1 mg) and norethindrone acetate (0.5 mg) once daily is an effective long-term treatment of AUB caused by uterine fibroids.
To study the efficacy of elagolix (alone or with estrogen-progestin add-back therapy) for the treatment of AUB caused by uterine fibroids, two identical trials were performed,12 in which 790 women participated. The participants had a mean age of 42 years and were documented to have heavy menstrual bleeding (>80 mL blood loss per cycle) and ultrasound-diagnosed uterine fibroids. The participants were randomized to one of 3 groups:
- elagolix (300 mg twice daily) plus low-dose steroid add-back (1 mg estradiol and 0.5 mg norethindrone acetate once daily),
- elagolix 300 mg twice daily with no steroid add-back (elagolix alone), or
- placebo for 6 months.12
Menstrual blood loss was quantified using the alkaline hematin method on collected sanitary products. The primary endpoint was menstrual blood loss <80 mL per cycle as well as a ≥50% reduction in quantified blood loss from baseline during the final month of treatment. At 6 months, the percentage of women achieving the primary endpoint in the first trial was 84% (elagolix alone), 69% (elagolix plus add-back), and 9% (placebo). Mean changes from baseline in lumbar spine bone density were −2.95% (elagolix alone), −0.76% (elagolix plus add-back), and −0.21% (placebo). The percentage of women reporting hot flashes was 64% in the elagolix group, 20% in the elagolix plus low-dose steroid add-back group, and 9% in the placebo group. Results were similar in the second trial.12
The initial trials were extended to 12 months with two groups: elagolix 300 mg twice daily plus low-dose hormone add-back with 1 mg estradiol and 0.5 mg norethindrone acetate once daily (n = 218) or elagolix 300 mg twice daily (elagolix alone) (n = 98).13 Following 12 months of treatment, heavy menstrual bleeding was controlled in 88% and 89% of women treated with elagolix plus add-back and elagolix alone, respectively. Amenorrhea was reported by 65% of the women in the elagolix plus add-back group. Compared with baseline bone density, at the end of 12 months of treatment, bone mineral density in the lumbar spine was reduced by -1.5% and -4.8% in the women treated with elagolix plus add-back and elagolix alone, respectively. Compared with baseline bone density, at 1 year following completion of treatment, bone mineral density in the lumbar spine was reduced by -0.6% and -2.0% in the women treated with elagolix plus add-back and elagolix alone, respectively. Similar trends were observed in total hip and femoral neck bone density. During treatment with elagolix plus add-back, adverse effects were modest, including hot flushes (6%), night sweats (3.2%), headache (5.5%), and nausea (4.1%). Two women developed liver transaminase levels >3 times the upper limit of normal, resulting in one woman discontinuing treatment.13
Continue to: Contraindications to Oriahnn include known allergies...
Contraindications to Oriahnn include known allergies to the components of the medication (including the yellow dye tartrazine); high risk of arterial, venous thrombotic or thromboembolic disorders; pregnancy; known osteoporosis; current breast cancer or other hormonally-sensitive malignancies; known liver disease; and concurrent use of organic anion transporting polypeptide 1B1 inhibitors, which includes many HIV antiviral medications.14 Undiagnosed AUB is a contraindication, and all women prescribed Oriahnn should have endometrial sampling before initiating treatment. Oriahnn should not be used for more than 24 months due to the risk of irreversible bone loss.14 Systemic estrogen and progestin combinations, a component of Oriahnn, increases the risk for pulmonary embolism, deep vein thrombosis, stroke, and myocardial infarction, especially in women at increased risk for these events (such as women >35 years who smoke cigarettes and women with uncontrolled hypertension).14 In two studies there was a higher incidence of depression, depressed mood, and/or tearfulness in women taking Oriahnn (3%) compared with those taking a placebo (1%).14 The FDA recommends promptly evaluating women with depressive symptoms to determine the risks of initiating and continuing Oriahnn therapy. In two studies there was a higher risk of reported alopecia among women taking Oriahnn (3.5%) compared with placebo (1%).14
It should be noted that elagolix is approved for the treatment of pelvic pain caused by endometriosis at a dose of 150 mg daily for 24 months or 200 mg twice daily for 6 months. The elagolix dose for the treatment of AUB caused by fibroids is 300 mg twice daily for up to 24 months, necessitating the addition of low-dose estradiol-norethindrone add-back to reduce the frequency and severity of hot flashes and minimize the loss of bone density. Norethindrone acetate also protects the endometrium from the stimulatory effect of estradiol, reducing the risk of developing endometrial hyperplasia and cancer. Oriahnn is formulated as two different capsules. A yellow and white capsule contains elagolix 300 mg plus estradiol 1 mg and norethindrone acetate 0.5 mg to be taken in the morning, and a blue and white capsule contains elagolix 300 mg to be taken in the evening.
AUB caused by fibroids is a common problem in gyn practice
There are many procedural interventions that are effective in reducing AUB caused by fibroids. However, prior to the approval of Oriahnn there were no hormonal medications that were FDA approved for the long-term treatment of AUB caused by fibroids. Hence, Oriahnn represents an important advance in the hormonal treatment of AUB caused by fibroids and expands the treatment options available to our patients. ●
Black women are more likely to develop fibroids and experience more severe fibroid symptoms. Obstetrician-gynecologists are experts in the diagnosis and treatment of fibroids. We play a key role in partnering with Black women to reduce fibroid disease burden.
Factors that increase the risk of developing fibroids include: increasing age, Black race, nulliparity, early menarche (<10 years of age), obesity, and consumption of red meat.1 The Nurses Health Study II is the largest prospective study of the factors that influence fibroid development.2 A total of 95,061 premenopausal nurses aged 25 to 44 years were followed from September 1989 through May 1993. Review of a sample of medical records demonstrated that the nurses participating in the study were reliable reporters of whether or not they had been diagnosed with fibroids. Based on a report of an ultrasound or hysterectomy diagnosis, the incidence rate for fibroids increased with age. Incidence rate per 1,000 women-years was 4.3 (age 25 to 29 years), 9.0 (30 to 34 years), 14.7 (age 35 to 39 years), and 22.5 (40 to 44 years). Compared with White race, Black race (but not Hispanic ethnicity or Asian race) was associated with an increased incidence of fibroids. Incidence rate per 1,000 women-years was 12.5 (White race), 37.9 (Black race), 14.5 (Hispanic ethnicity), and 10.4 (Asian race). The risk of developing fibroids was 3.25 times (95% CI, 2.71 to 3.88) greater among Black compared with White women after controlling for body mass index, age at first birth, years since last birth, history of infertility, age at first oral contraceptive use, marital status, and current alcohol use.2
Other epidemiology studies also report an increased incidence of fibroids among Black women.3,4 The size of the uterus, the size and number of fibroids, and the severity of fibroid symptoms are greater among Black versus White women.5,6 The molecular factors that increase fibroid incidence among Black women are unknown. Given the burden of fibroid disease among Black women, obstetrician-gynecologists are best positioned to ensure early diagnosis and to develop an effective follow-up and treatment plan for affected women.
References
1. Stewart EA, Laughlin-Tommaso SK, Catherino WH, et al. Uterine fibroids. Nat Rev Dis Primers. 2016;2:16043.
2. Marshall LM, Spiegelman D, Barbieri RL, et al. Variation in the incidence of uterine leiomyoma among premenopausal women by age and race. Obstet Gynecol. 1997;90:967-973.
3. Baird DD, Dunson DB, Hill MC, et al. High cumulative incidence of uterine leiomyoma in black and white women: ultrasound evidence. Am J Obstet Gynecol. 2003;188:100-107.
4. Brett KM, Marsh JV, Madans JH. Epidemiology of hysterectomy in the United States: demographic and reproductive factors in a nationally representative sample. J Womens Health. 1997;6:309-316.
5. Peddada SD, Laughlin SK, Miner K, et al. Growth of uterine leiomyomata among premenopausal black and white women. Proc Natl Acad Sci USA. 2008;105:1988719892.
6. Huyck KL, Panhuysen CI, Cuenco KT, et al. The impact of race as a risk factor for symptom severity and age at diagnosis of uterine leiomyomata among affected sisters. Am J Obstet Gynecol. 2008;198:168.e1-e9.
- Stewart EA. Uterine fibroids. N Engl J Med. 2015;372:1646-1655.
- Mehine M, Makinen N, Heinonen HR, et al. Genomics of uterine leiomyomas: insights from high-throughput sequencing. Fertil Steril. 2014;102:621-629.
- Mehine M, Kaasinen E, Makinen N, et al. Characterization of uterine leiomyomas by whole-genome sequencing. N Engl J Med. 2013;369:43-53.
- Moravek MB, Bulun SE. Endocrinology of uterine fibroids: steroid hormones, stem cells and genetic contribution. Curr Opin Obstet Gynecol. 2015;27:276-283.
- Rein MS. Advances in uterine leiomyoma research: the progesterone hypothesis. Environ Health Perspect. 2000;108(suppl 5):791-793.
- Friedman AJ, Barbieri RL, Doubilet PM, et al. A randomized double-blind trial of a gonadotropin-releasing hormone agonist (leuprolide) with or without medroxyprogesterone acetate in the treatment of leiomyomata uteri. Fertil Steril. 1988;49:404-409.
- Donnez J, Hudecek R, Donnez O, et al. Efficacy and safety of repeated use of ulipristal acetate in uterine fibroids. Fertil Steril. 2015;103:519-527.
- Donnez J, Tatarchuk TF, Bouchard P, et al. Ulipristal acetate versus placebo for fibroid treatment before surgery. N Engl J Med. 2012;366:409-420.
- Donnez J, Tomaszewski J, Vazquez F, et al. Ulipristal acetate versus leuprolide acetate for uterine fibroids. N Engl J Med. 2012;366:421-432.
- European Medicines Agency. Suspension of ulipristal acetate for uterine fibroids during ongoing EMA review of liver injury risk. March 13, 2020. https://www.ema.europa.eu/en/news/suspension-ulipristal-acetate-uterine-fibroids-during-ongoing-ema-review-liver-injury-risk#:~:text=EMA's%20safety%20committee%20(PRAC)%20has,the%20EU%20during%20the%20review. Accessed July 24, 2020.
- Lupron Depot [package insert]. Osaka, Japan: Takeda; Revised March 2012.
- Schlaff WD, Ackerman RT, Al-Hendy A, et al. Elagolix for heavy menstrual bleeding in women with uterine fibroids. N Engl J Med. 2020;382:328-340.
- Simon JA, Al-Hendy A, Archer DF, et al. Elagolix treatment for up to 12 months in women with heavy menstrual bleeding and uterine leiomyomas. Obstet Gynecol. 2020;135:1313-1326.
- Oriahnn [package insert]. North Chicago, IL: AbbVie; 2020.
Uterine leiomyomata (fibroids) are the most common pelvic tumor diagnosed in women.1 Women with symptomatic fibroids often report abnormal uterine bleeding (AUB) and pelvic cramping, fullness, or pain. Fibroids also may cause frequency of urination and contribute to fertility and pregnancy problems. Treatment options for the AUB caused by fibroids include, but are not limited to, hysterectomy, myomectomy, uterine artery embolization, endometrial ablation, insertion of a levonorgestrel intrauterine device, focused ultrasound surgery, radiofrequency ablation, leuprolide acetate, and elagolix plus low-dose hormone add-back (Oriahnn; AbbVie, North Chicago, Illinois).1 Oriahnn is the most recent addition to our treatment armamentarium for fibroids and represents the first US Food and Drug Administration (FDA)-approved long-term hormonal option for AUB caused by fibroids.
Gene dysregulation contributes to fibroid development
Most uterine fibroids are clonal tumors, which develop following a somatic mutation in a precursor uterine myocyte. The somatic mutation causes gene dysregulation that stimulates cell growth resulting in a benign tumor mass. The majority of fibroids contain a mutation in one of the following 6 genes: mediator complex subunit 12 (MED12), high mobility group AT-hook (HMGA2 or HMGA1), RAD51B, fumarate hydratase (FH), collagen type IV, alpha 5 chain (COL4A5), or collagen type IV alpha 6 chain (COL4A6).2
Gene dysregulation in fibroids may arise following chromothripsis of the uterine myocyte genome
Chromothripsis is a catastrophic intracellular genetic event in which one or more chromosomes are broken and reassemble in a new nucleic acid sequence, producing a derivative chromosome that contains complex genetic rearrangements.3 Chromothripsis is believed to occur frequently in uterine myocytes. It is unknown why uterine myocytes are susceptible to chromothripsis,3 or why a catastrophic intracellular event such as chromothripsis results in preferential mutations in the 6 genes that are associated with myoma formation.
Estrogen and progesterone influence fibroid size and cell activity
Although uterine fibroids are clonal tumors containing broken genes, they are also exquisitely responsive to estradiol and progesterone. Estradiol and progesterone play an important role in regulating fibroid size and function.4 Estrogen stimulates uterine fibroids to increase in size. In a hypoestrogenic state, uterine fibroids decrease in size. In addition, a hypoestrogenic state results in an atrophic endometrium and thereby reduces AUB. For women with uterine fibroids and AUB, a reversible hypoestrogenic state can be induced either with a parenteral GnRH-agonist analogue (leuprolide) or an oral GnRH-antagonist (elagolix). Both leuprolide and elagolix are approved for the treatment of uterine fibroids (see below).
Surprisingly, progesterone stimulates cell division in normal uterine myocytes and fibroid cells.5 In the luteal phase of the menstrual cycle, uterine myocyte mitoses are more frequent than in the follicular phase. In addition, synthetic progestins appear to maintain fibroid size in a hypoestrogenic environment. In one randomized trial, women with uterine fibroids treated with leuprolide acetate plus a placebo pill for 24 weeks had a 51% reduction in uterine volume as measured by ultrasound.6 Women with uterine fibroids treated with leuprolide acetate plus the synthetic progestin, oral medroxyprogesterone acetate 20 mg daily, had only a 15% reduction in uterine volume.6 This finding suggests that synthetic progestins partially block the decrease in uterine volume that occurs in a hypoestrogenic state.
Further evidence that progesterone plays a role in fibroid biology is the observation that treatment of women with uterine fibroids with the antiprogestin ulipristal decreases fibroid size and reduces AUB.7-9 Ulipristal was approved for the treatment of fibroids in many countries but not the United States. Reports of severe, life-threatening liver injury—some necessitating liver transplantation—among women using ulipristal prompted the European Medicines Agency (EMA) in 2020 to recommend that women stop taking ulipristal. In addition, the EMA recommended that no woman should initiate ulipristal treatment at this time.10
Continue to: Leuprolide acetate...
Leuprolide acetate
Leuprolide acetate is a peptide GnRH-agonist analogue. Initiation of leuprolide treatment stimulates gonadotropin release, but with chronic administration pituitary secretion of luteinizing hormone (LH) and follicle-stimulating hormone (FSH) decreases, resulting in reduced ovarian follicular activity, anovulation, and low serum concentration of estradiol and progesterone. Leuprolide treatment concomitant with iron therapy is approved by the FDA for improving red blood cell volume prior to surgery in women with fibroids, AUB, and anemia.11 Among women with fibroids, AUB, and anemia, after 12 weeks of treatment, the hemoglobin concentration was ≥12 g/dL in 79% treated with leuprolide plus iron and 56% treated with iron alone.11 The FDA recommends limiting preoperative leuprolide treatment to no more than 3 months. The approved leuprolide regimens are a maximum of 3 monthly injections of leuprolide 3.75 mg or a single injection of leuprolide 11.25 mg. Leuprolide treatment prior to hysterectomy surgery for uterine fibroids usually will result in a decrease in uterine size and may facilitate vaginal hysterectomy.
Elagolix plus estradiol plus norethindrone acetate (Oriahnn)
GnRH analogues cause a hypoestrogenic state resulting in adverse effects, including moderate to severe hot flashes and a reduction in bone mineral density. One approach to reducing the unwanted effects of hot flashes and decreased bone density is to combine a GnRH analogue with low-dose steroid hormone add-back therapy. Combining a GnRH analogue with low-dose steroid hormone add-back permits long-term treatment of AUB caused by fibroids, with few hot flashes and a minimal decrease in bone mineral density. The FDA recently has approved the combination of elagolix plus low-dose estradiol and norethindrone acetate (Oriahnn) for the long-term treatment of AUB caused by fibroids.
Elagolix is a nonpeptide oral GnRH antagonist that reduces pituitary secretion of LH and FSH, resulting in a decrease in ovarian follicular activity, anovulation, and low serum concentration of estradiol and progesterone. Unlike leuprolide, which causes an initial increase in LH and FSH secretion, the initiation of elagolix treatment causes an immediate and sustained reduction in LH and FSH secretion. Combining elagolix with a low dose of estradiol and norethindrone acetate reduces the side effects of hot flashes and decreased bone density. Clinical trials have reported that the combination of elagolix (300 mg) twice daily plus estradiol (1 mg) and norethindrone acetate (0.5 mg) once daily is an effective long-term treatment of AUB caused by uterine fibroids.
To study the efficacy of elagolix (alone or with estrogen-progestin add-back therapy) for the treatment of AUB caused by uterine fibroids, two identical trials were performed,12 in which 790 women participated. The participants had a mean age of 42 years and were documented to have heavy menstrual bleeding (>80 mL blood loss per cycle) and ultrasound-diagnosed uterine fibroids. The participants were randomized to one of 3 groups:
- elagolix (300 mg twice daily) plus low-dose steroid add-back (1 mg estradiol and 0.5 mg norethindrone acetate once daily),
- elagolix 300 mg twice daily with no steroid add-back (elagolix alone), or
- placebo for 6 months.12
Menstrual blood loss was quantified using the alkaline hematin method on collected sanitary products. The primary endpoint was menstrual blood loss <80 mL per cycle as well as a ≥50% reduction in quantified blood loss from baseline during the final month of treatment. At 6 months, the percentage of women achieving the primary endpoint in the first trial was 84% (elagolix alone), 69% (elagolix plus add-back), and 9% (placebo). Mean changes from baseline in lumbar spine bone density were −2.95% (elagolix alone), −0.76% (elagolix plus add-back), and −0.21% (placebo). The percentage of women reporting hot flashes was 64% in the elagolix group, 20% in the elagolix plus low-dose steroid add-back group, and 9% in the placebo group. Results were similar in the second trial.12
The initial trials were extended to 12 months with two groups: elagolix 300 mg twice daily plus low-dose hormone add-back with 1 mg estradiol and 0.5 mg norethindrone acetate once daily (n = 218) or elagolix 300 mg twice daily (elagolix alone) (n = 98).13 Following 12 months of treatment, heavy menstrual bleeding was controlled in 88% and 89% of women treated with elagolix plus add-back and elagolix alone, respectively. Amenorrhea was reported by 65% of the women in the elagolix plus add-back group. Compared with baseline bone density, at the end of 12 months of treatment, bone mineral density in the lumbar spine was reduced by -1.5% and -4.8% in the women treated with elagolix plus add-back and elagolix alone, respectively. Compared with baseline bone density, at 1 year following completion of treatment, bone mineral density in the lumbar spine was reduced by -0.6% and -2.0% in the women treated with elagolix plus add-back and elagolix alone, respectively. Similar trends were observed in total hip and femoral neck bone density. During treatment with elagolix plus add-back, adverse effects were modest, including hot flushes (6%), night sweats (3.2%), headache (5.5%), and nausea (4.1%). Two women developed liver transaminase levels >3 times the upper limit of normal, resulting in one woman discontinuing treatment.13
Continue to: Contraindications to Oriahnn include known allergies...
Contraindications to Oriahnn include known allergies to the components of the medication (including the yellow dye tartrazine); high risk of arterial, venous thrombotic or thromboembolic disorders; pregnancy; known osteoporosis; current breast cancer or other hormonally-sensitive malignancies; known liver disease; and concurrent use of organic anion transporting polypeptide 1B1 inhibitors, which includes many HIV antiviral medications.14 Undiagnosed AUB is a contraindication, and all women prescribed Oriahnn should have endometrial sampling before initiating treatment. Oriahnn should not be used for more than 24 months due to the risk of irreversible bone loss.14 Systemic estrogen and progestin combinations, a component of Oriahnn, increases the risk for pulmonary embolism, deep vein thrombosis, stroke, and myocardial infarction, especially in women at increased risk for these events (such as women >35 years who smoke cigarettes and women with uncontrolled hypertension).14 In two studies there was a higher incidence of depression, depressed mood, and/or tearfulness in women taking Oriahnn (3%) compared with those taking a placebo (1%).14 The FDA recommends promptly evaluating women with depressive symptoms to determine the risks of initiating and continuing Oriahnn therapy. In two studies there was a higher risk of reported alopecia among women taking Oriahnn (3.5%) compared with placebo (1%).14
It should be noted that elagolix is approved for the treatment of pelvic pain caused by endometriosis at a dose of 150 mg daily for 24 months or 200 mg twice daily for 6 months. The elagolix dose for the treatment of AUB caused by fibroids is 300 mg twice daily for up to 24 months, necessitating the addition of low-dose estradiol-norethindrone add-back to reduce the frequency and severity of hot flashes and minimize the loss of bone density. Norethindrone acetate also protects the endometrium from the stimulatory effect of estradiol, reducing the risk of developing endometrial hyperplasia and cancer. Oriahnn is formulated as two different capsules. A yellow and white capsule contains elagolix 300 mg plus estradiol 1 mg and norethindrone acetate 0.5 mg to be taken in the morning, and a blue and white capsule contains elagolix 300 mg to be taken in the evening.
AUB caused by fibroids is a common problem in gyn practice
There are many procedural interventions that are effective in reducing AUB caused by fibroids. However, prior to the approval of Oriahnn there were no hormonal medications that were FDA approved for the long-term treatment of AUB caused by fibroids. Hence, Oriahnn represents an important advance in the hormonal treatment of AUB caused by fibroids and expands the treatment options available to our patients. ●
Black women are more likely to develop fibroids and experience more severe fibroid symptoms. Obstetrician-gynecologists are experts in the diagnosis and treatment of fibroids. We play a key role in partnering with Black women to reduce fibroid disease burden.
Factors that increase the risk of developing fibroids include: increasing age, Black race, nulliparity, early menarche (<10 years of age), obesity, and consumption of red meat.1 The Nurses Health Study II is the largest prospective study of the factors that influence fibroid development.2 A total of 95,061 premenopausal nurses aged 25 to 44 years were followed from September 1989 through May 1993. Review of a sample of medical records demonstrated that the nurses participating in the study were reliable reporters of whether or not they had been diagnosed with fibroids. Based on a report of an ultrasound or hysterectomy diagnosis, the incidence rate for fibroids increased with age. Incidence rate per 1,000 women-years was 4.3 (age 25 to 29 years), 9.0 (30 to 34 years), 14.7 (age 35 to 39 years), and 22.5 (40 to 44 years). Compared with White race, Black race (but not Hispanic ethnicity or Asian race) was associated with an increased incidence of fibroids. Incidence rate per 1,000 women-years was 12.5 (White race), 37.9 (Black race), 14.5 (Hispanic ethnicity), and 10.4 (Asian race). The risk of developing fibroids was 3.25 times (95% CI, 2.71 to 3.88) greater among Black compared with White women after controlling for body mass index, age at first birth, years since last birth, history of infertility, age at first oral contraceptive use, marital status, and current alcohol use.2
Other epidemiology studies also report an increased incidence of fibroids among Black women.3,4 The size of the uterus, the size and number of fibroids, and the severity of fibroid symptoms are greater among Black versus White women.5,6 The molecular factors that increase fibroid incidence among Black women are unknown. Given the burden of fibroid disease among Black women, obstetrician-gynecologists are best positioned to ensure early diagnosis and to develop an effective follow-up and treatment plan for affected women.
References
1. Stewart EA, Laughlin-Tommaso SK, Catherino WH, et al. Uterine fibroids. Nat Rev Dis Primers. 2016;2:16043.
2. Marshall LM, Spiegelman D, Barbieri RL, et al. Variation in the incidence of uterine leiomyoma among premenopausal women by age and race. Obstet Gynecol. 1997;90:967-973.
3. Baird DD, Dunson DB, Hill MC, et al. High cumulative incidence of uterine leiomyoma in black and white women: ultrasound evidence. Am J Obstet Gynecol. 2003;188:100-107.
4. Brett KM, Marsh JV, Madans JH. Epidemiology of hysterectomy in the United States: demographic and reproductive factors in a nationally representative sample. J Womens Health. 1997;6:309-316.
5. Peddada SD, Laughlin SK, Miner K, et al. Growth of uterine leiomyomata among premenopausal black and white women. Proc Natl Acad Sci USA. 2008;105:1988719892.
6. Huyck KL, Panhuysen CI, Cuenco KT, et al. The impact of race as a risk factor for symptom severity and age at diagnosis of uterine leiomyomata among affected sisters. Am J Obstet Gynecol. 2008;198:168.e1-e9.
Uterine leiomyomata (fibroids) are the most common pelvic tumor diagnosed in women.1 Women with symptomatic fibroids often report abnormal uterine bleeding (AUB) and pelvic cramping, fullness, or pain. Fibroids also may cause frequency of urination and contribute to fertility and pregnancy problems. Treatment options for the AUB caused by fibroids include, but are not limited to, hysterectomy, myomectomy, uterine artery embolization, endometrial ablation, insertion of a levonorgestrel intrauterine device, focused ultrasound surgery, radiofrequency ablation, leuprolide acetate, and elagolix plus low-dose hormone add-back (Oriahnn; AbbVie, North Chicago, Illinois).1 Oriahnn is the most recent addition to our treatment armamentarium for fibroids and represents the first US Food and Drug Administration (FDA)-approved long-term hormonal option for AUB caused by fibroids.
Gene dysregulation contributes to fibroid development
Most uterine fibroids are clonal tumors, which develop following a somatic mutation in a precursor uterine myocyte. The somatic mutation causes gene dysregulation that stimulates cell growth resulting in a benign tumor mass. The majority of fibroids contain a mutation in one of the following 6 genes: mediator complex subunit 12 (MED12), high mobility group AT-hook (HMGA2 or HMGA1), RAD51B, fumarate hydratase (FH), collagen type IV, alpha 5 chain (COL4A5), or collagen type IV alpha 6 chain (COL4A6).2
Gene dysregulation in fibroids may arise following chromothripsis of the uterine myocyte genome
Chromothripsis is a catastrophic intracellular genetic event in which one or more chromosomes are broken and reassemble in a new nucleic acid sequence, producing a derivative chromosome that contains complex genetic rearrangements.3 Chromothripsis is believed to occur frequently in uterine myocytes. It is unknown why uterine myocytes are susceptible to chromothripsis,3 or why a catastrophic intracellular event such as chromothripsis results in preferential mutations in the 6 genes that are associated with myoma formation.
Estrogen and progesterone influence fibroid size and cell activity
Although uterine fibroids are clonal tumors containing broken genes, they are also exquisitely responsive to estradiol and progesterone. Estradiol and progesterone play an important role in regulating fibroid size and function.4 Estrogen stimulates uterine fibroids to increase in size. In a hypoestrogenic state, uterine fibroids decrease in size. In addition, a hypoestrogenic state results in an atrophic endometrium and thereby reduces AUB. For women with uterine fibroids and AUB, a reversible hypoestrogenic state can be induced either with a parenteral GnRH-agonist analogue (leuprolide) or an oral GnRH-antagonist (elagolix). Both leuprolide and elagolix are approved for the treatment of uterine fibroids (see below).
Surprisingly, progesterone stimulates cell division in normal uterine myocytes and fibroid cells.5 In the luteal phase of the menstrual cycle, uterine myocyte mitoses are more frequent than in the follicular phase. In addition, synthetic progestins appear to maintain fibroid size in a hypoestrogenic environment. In one randomized trial, women with uterine fibroids treated with leuprolide acetate plus a placebo pill for 24 weeks had a 51% reduction in uterine volume as measured by ultrasound.6 Women with uterine fibroids treated with leuprolide acetate plus the synthetic progestin, oral medroxyprogesterone acetate 20 mg daily, had only a 15% reduction in uterine volume.6 This finding suggests that synthetic progestins partially block the decrease in uterine volume that occurs in a hypoestrogenic state.
Further evidence that progesterone plays a role in fibroid biology is the observation that treatment of women with uterine fibroids with the antiprogestin ulipristal decreases fibroid size and reduces AUB.7-9 Ulipristal was approved for the treatment of fibroids in many countries but not the United States. Reports of severe, life-threatening liver injury—some necessitating liver transplantation—among women using ulipristal prompted the European Medicines Agency (EMA) in 2020 to recommend that women stop taking ulipristal. In addition, the EMA recommended that no woman should initiate ulipristal treatment at this time.10
Continue to: Leuprolide acetate...
Leuprolide acetate
Leuprolide acetate is a peptide GnRH-agonist analogue. Initiation of leuprolide treatment stimulates gonadotropin release, but with chronic administration pituitary secretion of luteinizing hormone (LH) and follicle-stimulating hormone (FSH) decreases, resulting in reduced ovarian follicular activity, anovulation, and low serum concentration of estradiol and progesterone. Leuprolide treatment concomitant with iron therapy is approved by the FDA for improving red blood cell volume prior to surgery in women with fibroids, AUB, and anemia.11 Among women with fibroids, AUB, and anemia, after 12 weeks of treatment, the hemoglobin concentration was ≥12 g/dL in 79% treated with leuprolide plus iron and 56% treated with iron alone.11 The FDA recommends limiting preoperative leuprolide treatment to no more than 3 months. The approved leuprolide regimens are a maximum of 3 monthly injections of leuprolide 3.75 mg or a single injection of leuprolide 11.25 mg. Leuprolide treatment prior to hysterectomy surgery for uterine fibroids usually will result in a decrease in uterine size and may facilitate vaginal hysterectomy.
Elagolix plus estradiol plus norethindrone acetate (Oriahnn)
GnRH analogues cause a hypoestrogenic state resulting in adverse effects, including moderate to severe hot flashes and a reduction in bone mineral density. One approach to reducing the unwanted effects of hot flashes and decreased bone density is to combine a GnRH analogue with low-dose steroid hormone add-back therapy. Combining a GnRH analogue with low-dose steroid hormone add-back permits long-term treatment of AUB caused by fibroids, with few hot flashes and a minimal decrease in bone mineral density. The FDA recently has approved the combination of elagolix plus low-dose estradiol and norethindrone acetate (Oriahnn) for the long-term treatment of AUB caused by fibroids.
Elagolix is a nonpeptide oral GnRH antagonist that reduces pituitary secretion of LH and FSH, resulting in a decrease in ovarian follicular activity, anovulation, and low serum concentration of estradiol and progesterone. Unlike leuprolide, which causes an initial increase in LH and FSH secretion, the initiation of elagolix treatment causes an immediate and sustained reduction in LH and FSH secretion. Combining elagolix with a low dose of estradiol and norethindrone acetate reduces the side effects of hot flashes and decreased bone density. Clinical trials have reported that the combination of elagolix (300 mg) twice daily plus estradiol (1 mg) and norethindrone acetate (0.5 mg) once daily is an effective long-term treatment of AUB caused by uterine fibroids.
To study the efficacy of elagolix (alone or with estrogen-progestin add-back therapy) for the treatment of AUB caused by uterine fibroids, two identical trials were performed,12 in which 790 women participated. The participants had a mean age of 42 years and were documented to have heavy menstrual bleeding (>80 mL blood loss per cycle) and ultrasound-diagnosed uterine fibroids. The participants were randomized to one of 3 groups:
- elagolix (300 mg twice daily) plus low-dose steroid add-back (1 mg estradiol and 0.5 mg norethindrone acetate once daily),
- elagolix 300 mg twice daily with no steroid add-back (elagolix alone), or
- placebo for 6 months.12
Menstrual blood loss was quantified using the alkaline hematin method on collected sanitary products. The primary endpoint was menstrual blood loss <80 mL per cycle as well as a ≥50% reduction in quantified blood loss from baseline during the final month of treatment. At 6 months, the percentage of women achieving the primary endpoint in the first trial was 84% (elagolix alone), 69% (elagolix plus add-back), and 9% (placebo). Mean changes from baseline in lumbar spine bone density were −2.95% (elagolix alone), −0.76% (elagolix plus add-back), and −0.21% (placebo). The percentage of women reporting hot flashes was 64% in the elagolix group, 20% in the elagolix plus low-dose steroid add-back group, and 9% in the placebo group. Results were similar in the second trial.12
The initial trials were extended to 12 months with two groups: elagolix 300 mg twice daily plus low-dose hormone add-back with 1 mg estradiol and 0.5 mg norethindrone acetate once daily (n = 218) or elagolix 300 mg twice daily (elagolix alone) (n = 98).13 Following 12 months of treatment, heavy menstrual bleeding was controlled in 88% and 89% of women treated with elagolix plus add-back and elagolix alone, respectively. Amenorrhea was reported by 65% of the women in the elagolix plus add-back group. Compared with baseline bone density, at the end of 12 months of treatment, bone mineral density in the lumbar spine was reduced by -1.5% and -4.8% in the women treated with elagolix plus add-back and elagolix alone, respectively. Compared with baseline bone density, at 1 year following completion of treatment, bone mineral density in the lumbar spine was reduced by -0.6% and -2.0% in the women treated with elagolix plus add-back and elagolix alone, respectively. Similar trends were observed in total hip and femoral neck bone density. During treatment with elagolix plus add-back, adverse effects were modest, including hot flushes (6%), night sweats (3.2%), headache (5.5%), and nausea (4.1%). Two women developed liver transaminase levels >3 times the upper limit of normal, resulting in one woman discontinuing treatment.13
Continue to: Contraindications to Oriahnn include known allergies...
Contraindications to Oriahnn include known allergies to the components of the medication (including the yellow dye tartrazine); high risk of arterial, venous thrombotic or thromboembolic disorders; pregnancy; known osteoporosis; current breast cancer or other hormonally-sensitive malignancies; known liver disease; and concurrent use of organic anion transporting polypeptide 1B1 inhibitors, which includes many HIV antiviral medications.14 Undiagnosed AUB is a contraindication, and all women prescribed Oriahnn should have endometrial sampling before initiating treatment. Oriahnn should not be used for more than 24 months due to the risk of irreversible bone loss.14 Systemic estrogen and progestin combinations, a component of Oriahnn, increases the risk for pulmonary embolism, deep vein thrombosis, stroke, and myocardial infarction, especially in women at increased risk for these events (such as women >35 years who smoke cigarettes and women with uncontrolled hypertension).14 In two studies there was a higher incidence of depression, depressed mood, and/or tearfulness in women taking Oriahnn (3%) compared with those taking a placebo (1%).14 The FDA recommends promptly evaluating women with depressive symptoms to determine the risks of initiating and continuing Oriahnn therapy. In two studies there was a higher risk of reported alopecia among women taking Oriahnn (3.5%) compared with placebo (1%).14
It should be noted that elagolix is approved for the treatment of pelvic pain caused by endometriosis at a dose of 150 mg daily for 24 months or 200 mg twice daily for 6 months. The elagolix dose for the treatment of AUB caused by fibroids is 300 mg twice daily for up to 24 months, necessitating the addition of low-dose estradiol-norethindrone add-back to reduce the frequency and severity of hot flashes and minimize the loss of bone density. Norethindrone acetate also protects the endometrium from the stimulatory effect of estradiol, reducing the risk of developing endometrial hyperplasia and cancer. Oriahnn is formulated as two different capsules. A yellow and white capsule contains elagolix 300 mg plus estradiol 1 mg and norethindrone acetate 0.5 mg to be taken in the morning, and a blue and white capsule contains elagolix 300 mg to be taken in the evening.
AUB caused by fibroids is a common problem in gyn practice
There are many procedural interventions that are effective in reducing AUB caused by fibroids. However, prior to the approval of Oriahnn there were no hormonal medications that were FDA approved for the long-term treatment of AUB caused by fibroids. Hence, Oriahnn represents an important advance in the hormonal treatment of AUB caused by fibroids and expands the treatment options available to our patients. ●
Black women are more likely to develop fibroids and experience more severe fibroid symptoms. Obstetrician-gynecologists are experts in the diagnosis and treatment of fibroids. We play a key role in partnering with Black women to reduce fibroid disease burden.
Factors that increase the risk of developing fibroids include: increasing age, Black race, nulliparity, early menarche (<10 years of age), obesity, and consumption of red meat.1 The Nurses Health Study II is the largest prospective study of the factors that influence fibroid development.2 A total of 95,061 premenopausal nurses aged 25 to 44 years were followed from September 1989 through May 1993. Review of a sample of medical records demonstrated that the nurses participating in the study were reliable reporters of whether or not they had been diagnosed with fibroids. Based on a report of an ultrasound or hysterectomy diagnosis, the incidence rate for fibroids increased with age. Incidence rate per 1,000 women-years was 4.3 (age 25 to 29 years), 9.0 (30 to 34 years), 14.7 (age 35 to 39 years), and 22.5 (40 to 44 years). Compared with White race, Black race (but not Hispanic ethnicity or Asian race) was associated with an increased incidence of fibroids. Incidence rate per 1,000 women-years was 12.5 (White race), 37.9 (Black race), 14.5 (Hispanic ethnicity), and 10.4 (Asian race). The risk of developing fibroids was 3.25 times (95% CI, 2.71 to 3.88) greater among Black compared with White women after controlling for body mass index, age at first birth, years since last birth, history of infertility, age at first oral contraceptive use, marital status, and current alcohol use.2
Other epidemiology studies also report an increased incidence of fibroids among Black women.3,4 The size of the uterus, the size and number of fibroids, and the severity of fibroid symptoms are greater among Black versus White women.5,6 The molecular factors that increase fibroid incidence among Black women are unknown. Given the burden of fibroid disease among Black women, obstetrician-gynecologists are best positioned to ensure early diagnosis and to develop an effective follow-up and treatment plan for affected women.
References
1. Stewart EA, Laughlin-Tommaso SK, Catherino WH, et al. Uterine fibroids. Nat Rev Dis Primers. 2016;2:16043.
2. Marshall LM, Spiegelman D, Barbieri RL, et al. Variation in the incidence of uterine leiomyoma among premenopausal women by age and race. Obstet Gynecol. 1997;90:967-973.
3. Baird DD, Dunson DB, Hill MC, et al. High cumulative incidence of uterine leiomyoma in black and white women: ultrasound evidence. Am J Obstet Gynecol. 2003;188:100-107.
4. Brett KM, Marsh JV, Madans JH. Epidemiology of hysterectomy in the United States: demographic and reproductive factors in a nationally representative sample. J Womens Health. 1997;6:309-316.
5. Peddada SD, Laughlin SK, Miner K, et al. Growth of uterine leiomyomata among premenopausal black and white women. Proc Natl Acad Sci USA. 2008;105:1988719892.
6. Huyck KL, Panhuysen CI, Cuenco KT, et al. The impact of race as a risk factor for symptom severity and age at diagnosis of uterine leiomyomata among affected sisters. Am J Obstet Gynecol. 2008;198:168.e1-e9.
- Stewart EA. Uterine fibroids. N Engl J Med. 2015;372:1646-1655.
- Mehine M, Makinen N, Heinonen HR, et al. Genomics of uterine leiomyomas: insights from high-throughput sequencing. Fertil Steril. 2014;102:621-629.
- Mehine M, Kaasinen E, Makinen N, et al. Characterization of uterine leiomyomas by whole-genome sequencing. N Engl J Med. 2013;369:43-53.
- Moravek MB, Bulun SE. Endocrinology of uterine fibroids: steroid hormones, stem cells and genetic contribution. Curr Opin Obstet Gynecol. 2015;27:276-283.
- Rein MS. Advances in uterine leiomyoma research: the progesterone hypothesis. Environ Health Perspect. 2000;108(suppl 5):791-793.
- Friedman AJ, Barbieri RL, Doubilet PM, et al. A randomized double-blind trial of a gonadotropin-releasing hormone agonist (leuprolide) with or without medroxyprogesterone acetate in the treatment of leiomyomata uteri. Fertil Steril. 1988;49:404-409.
- Donnez J, Hudecek R, Donnez O, et al. Efficacy and safety of repeated use of ulipristal acetate in uterine fibroids. Fertil Steril. 2015;103:519-527.
- Donnez J, Tatarchuk TF, Bouchard P, et al. Ulipristal acetate versus placebo for fibroid treatment before surgery. N Engl J Med. 2012;366:409-420.
- Donnez J, Tomaszewski J, Vazquez F, et al. Ulipristal acetate versus leuprolide acetate for uterine fibroids. N Engl J Med. 2012;366:421-432.
- European Medicines Agency. Suspension of ulipristal acetate for uterine fibroids during ongoing EMA review of liver injury risk. March 13, 2020. https://www.ema.europa.eu/en/news/suspension-ulipristal-acetate-uterine-fibroids-during-ongoing-ema-review-liver-injury-risk#:~:text=EMA's%20safety%20committee%20(PRAC)%20has,the%20EU%20during%20the%20review. Accessed July 24, 2020.
- Lupron Depot [package insert]. Osaka, Japan: Takeda; Revised March 2012.
- Schlaff WD, Ackerman RT, Al-Hendy A, et al. Elagolix for heavy menstrual bleeding in women with uterine fibroids. N Engl J Med. 2020;382:328-340.
- Simon JA, Al-Hendy A, Archer DF, et al. Elagolix treatment for up to 12 months in women with heavy menstrual bleeding and uterine leiomyomas. Obstet Gynecol. 2020;135:1313-1326.
- Oriahnn [package insert]. North Chicago, IL: AbbVie; 2020.
- Stewart EA. Uterine fibroids. N Engl J Med. 2015;372:1646-1655.
- Mehine M, Makinen N, Heinonen HR, et al. Genomics of uterine leiomyomas: insights from high-throughput sequencing. Fertil Steril. 2014;102:621-629.
- Mehine M, Kaasinen E, Makinen N, et al. Characterization of uterine leiomyomas by whole-genome sequencing. N Engl J Med. 2013;369:43-53.
- Moravek MB, Bulun SE. Endocrinology of uterine fibroids: steroid hormones, stem cells and genetic contribution. Curr Opin Obstet Gynecol. 2015;27:276-283.
- Rein MS. Advances in uterine leiomyoma research: the progesterone hypothesis. Environ Health Perspect. 2000;108(suppl 5):791-793.
- Friedman AJ, Barbieri RL, Doubilet PM, et al. A randomized double-blind trial of a gonadotropin-releasing hormone agonist (leuprolide) with or without medroxyprogesterone acetate in the treatment of leiomyomata uteri. Fertil Steril. 1988;49:404-409.
- Donnez J, Hudecek R, Donnez O, et al. Efficacy and safety of repeated use of ulipristal acetate in uterine fibroids. Fertil Steril. 2015;103:519-527.
- Donnez J, Tatarchuk TF, Bouchard P, et al. Ulipristal acetate versus placebo for fibroid treatment before surgery. N Engl J Med. 2012;366:409-420.
- Donnez J, Tomaszewski J, Vazquez F, et al. Ulipristal acetate versus leuprolide acetate for uterine fibroids. N Engl J Med. 2012;366:421-432.
- European Medicines Agency. Suspension of ulipristal acetate for uterine fibroids during ongoing EMA review of liver injury risk. March 13, 2020. https://www.ema.europa.eu/en/news/suspension-ulipristal-acetate-uterine-fibroids-during-ongoing-ema-review-liver-injury-risk#:~:text=EMA's%20safety%20committee%20(PRAC)%20has,the%20EU%20during%20the%20review. Accessed July 24, 2020.
- Lupron Depot [package insert]. Osaka, Japan: Takeda; Revised March 2012.
- Schlaff WD, Ackerman RT, Al-Hendy A, et al. Elagolix for heavy menstrual bleeding in women with uterine fibroids. N Engl J Med. 2020;382:328-340.
- Simon JA, Al-Hendy A, Archer DF, et al. Elagolix treatment for up to 12 months in women with heavy menstrual bleeding and uterine leiomyomas. Obstet Gynecol. 2020;135:1313-1326.
- Oriahnn [package insert]. North Chicago, IL: AbbVie; 2020.