User login
Total Laparoscopic Segmental Resection With Transanal NOSE for Deep Colorectal Endometriosis
What to Know About NOSE
Traditionally, a laparoscopic approach to bowel resection secondary to endometriosis means enlargement of the port site or more commonly, a Pfannenstiel incision to introduce an anvil. Both incisions raise the risk of postoperative complications including pain and incisional hernia. Moreover, recovery time is prolonged.
Natural orifice specimen extraction (NOSE) makes use of a natural orifice, the vagina or anus, to extract the specimen and introduce the anvil. Transanal extraction for the management of endometriosis-related bowel disease was first described by the late David Redwine, MD, in his 1996 publication in Fertility and Sterility.1
The concern with use of vaginal NOSE surgery is rectovaginal fistula secondary to two incision lines in close opposition. Both transvaginal NOSE and transanal NOSE have been criticized for longer dissection of the mesorectum and thus longer resected specimen. Despite these concerns, over the past 10 years, articles have been published showing not only the feasibility of the NOSE technique, but excellent comparative outcomes, as well.2-4
A recent systematic review and meta-analysis by Kar et al. comparing NOSE extraction to minilaparotomy in bowel resection due to endometriosis revealed no significant difference in complication rate, but significantly shorter operation duration, reduced length of stay, and less blood loss in the NOSE surgical arm.5
The most recent study to be published in the Journal of Minimally Invasive Gynecology involving total laparoscopic segmental resection with transanal NOSE for the treatment of colorectal endometriosis was written by the guest author of this edition of the Master Class in Gynecologic Surgery, Professor Mario Malzoni, Scientific Director of the Malzoni Research Hospital, the Center for Advanced Pelvic Surgery, in Avellino, Italy. In the JMIG article, and in this Master Class, Professor Malzoni, a world-renowned endometriosis specialist recognized for his extraordinary skill and multi-organ surgical work, discusses the difference between the standard bowel resection technique with that of NOSE, and reports on his experience with NOSE.6
It is a pleasure and honor to welcome my friend and colleague, Professor Mario Malzoni, to this edition of the Master Class in Gynecologic Surgery.
Dr. Charles E. Miller is Professor, Obstetrics & Gynecology, Department of Clinical Sciences, Rosalind Franklin University of Medicine and Science, North Chicago, and Director, Minimally Invasive Gynecologic Surgery, Advocate Lutheran General Hospital, Park Ridge, both in Illinois. Email him at obnews@mdedge.com.
References
1. Redwine DB et al. Laparoscopically assisted transvaginal segmental resection of the rectosigmoid colon for endometriosis. Fertil Steril. 1996 Jan;65(1):193-7.
2. Akladios C et al. Totally laparoscopic intracorporeal anastomosis with natural orifice specimen extraction (NOSE) techniques, particularly suitable for bowel endometriosis. J Minim Invasive Gynecol. 2014 Nov-Dec;21(6):1095-102.
3. Bokor A et al. Natural orifice specimen extraction during laparoscopic bowel resection for colorectal endometriosis: technique and outcome. J Minim Invasive Gynecol. 2018 Sep-Oct;25(6):1065-74.
4. Grigoriadis G et al. Natural orifice specimen extraction colorectal resection for deep endometriosis: A 50 case series. J Minim Invasive Gynecol. 2022 Sep;29(9):1054-62.
5. Kar E et al. Natural orifice specimen extraction as a promising alternative for minilaparotomy in bowel resection due to endometriosis: A systematic review and meta-analysis. J Minim Invasive Gynecol. 2024 Jul;31(7):574-83.e1.
6. Malzoni M et al. Total laparoscopic segmental resection with transanal natural orifice specimen extraction for treatment of colorectal endometriosis: Descriptive analysis from the TrEnd study database. J Minim Invasive Gynecol. 2024 Oct;18:S1553-4650(24)01453-5.
Laparoscopic Resection With Transanal NOSE for Deep Colorectal Endometriosis
When segmental sigmoid colon/rectal resection is indicated for treatment of deep infiltrating bowel endometriosis, a totally laparoscopic resection with intracorporeal anastomosis and transanal natural orifice specimen extraction (NOSE) can be safe, effective, and advantageous compared with an abdominal mini-laparotomy for extracorporeal anastomosis and specimen retrieval.
With advanced laparoscopic surgical skills, accurate preoperative ultrasound evaluation, and a high-quality perioperative care protocol (including high-quality bowel preparation), segmental resection with NOSE can offer better preservation of vascularization, smaller resection sizes, lower rates of postoperative pain, a shorter time for gas passage after surgery, decreased hospital stays, and lower rates of wound infection and incisional hernia.
We have seen such improved results in our high-volume center since shifting in April 2021 from our classical technique for segmental rectosigmoid resection1 to a totally laparoscopic approach with NOSE. We first reported on this new technique in 2022 in a video article in the Journal of Minimally Invasive Gynecology,2and in October 2024, outcomes of 81 patients who underwent segmental sigmoid colon/rectal resection with intracorporeal anastomosis and transanal NOSE at our institution from April 2021 through March 2024 were reported in Journal of Minimally Invasive Gynecology.3Given our experience with this approach for deep endometriosis, I am convinced that NOSE should be preferred to transabdominal specimen extraction for segmental rectosigmoid resection. This is where the future lies for deep infiltrating endometriosis. Here, I share our outcomes and describe our technique.
Our Prior Approach With A Mini-Laparotomy
Our traditional laparoscopic approach for segmental colon/rectal resection of deep infiltrating endometriosis involved an abdominal mini-laparotomy (no more than 4 cm in length) for specimen retrieval, resection of the proximal rectum, and positioning of the head of a circular stapler for end-to-end anastomosis. (Connection of the two parts of the circular stapler for anastomosis was achieved via laparoscopy.)1
Several factors allowed us to minimize potential complications, including standardization of our technique, the use of three-dimensional endoscopes, and indocyanine green fluorescence angiography to assess the perfusion of the bowel after the completion of anastomosis. Still, an unpublished analysis of 1050 segmental bowel resections performed over more than 17 years using our traditional technique showed that hospital stays averaged 8 days, time to first defecation averaged 7 days, and time to first passage of flatus averaged 1 day.
Per the Clavien-Dindo classification system for surgical complications that was used at the time, 1.9% of these 1050 patients had grade 1 (minor) or grade 2 complications and 6% had grade 3 complications. Grade 2 complications were defined as needing intervention or a hospital stay more than twice the median for the procedure. Grade 3 complications were defined as leading to lasting disability or organ resection.
In the meantime, in colorectal cancer surgery, published studies on NOSE versus conventional laparoscopy with transabdominal specimen extraction have consistently pointed to the benefits of NOSE. A 2022 review 4 of 19 studies involving over 3400 patients and a 2022 meta-analysis 5 of 21 randomized controlled trials involving more than 2000 patients both showed significantly reduced postoperative morbidity and no differences in oncologic outcomes. Among the notable differences in the 2022 meta-analysis was a relative risk of postoperative infection of 0.34 for patients treated with NOSE compared with conventional laparoscopic techniques.
Among patients with deep rectal endometriosis, reported experience with intracorporeal anastomosis and NOSE for segmental resection is limited, with a few studies published between 2014 and 2022. Excluding anecdotal reports, only about 140 patients have been reported in the literature as having intracorporeal anastomosis and transanal NOSE for deep infiltrating bowel endometriosis. 3
Our Outcomes With A Totally Laparoscopic Approach
Our approach to evaluating our experience with totally laparoscopic resection with transanal NOSE has been to systematically collect data from consecutive patients and to analyze 1) complications of the technique, 2) conversion to the traditional technique/open surgery, and 3) endometriosis-free bowel resection margins and recurrence. Secondarily, we look at intraoperative blood loss, operative time, recovery of gastrointestinal function, hospital stay length, and reproductive outcomes.
Across 81 patients from our TrEnd study database (Surgical TReatment of women with deep ENDometriosis infiltrating the sigmoid colon and/or rectum) who received a totally laparoscopic standardized procedure involving intracorporeal anastomosis and transanal NOSE, we had no conversions even to mini-laparotomy, and no cases of protective colostomy or ileostomy.
Complete endometriosis removal was achieved in 100% of cases, with final pathology showing endometriosis-free resection margins, and patients remained free of bowel endometriosis at a median follow-up of 21 months.3Our analysis also shows improved gastrointestinal function recovery (median time to first defecation of 4 days, compared to 7 days previously, with the same 1-day median time to first passage of flatus) and a significantly improved hospital length of stay to a median of 3 days (interquartile range, 3-4.5 days); the latter reflects in part the absence of infection.3There were no intra-operative complications. Postoperative Clavien-Dindo grade 3 complications occurred in three patients (3.7%), two of whom required reoperation (one for hemoperitoneum and one for ureteral injury) and one who experienced rectal anastomotic bleeding on postoperative day 1. (The two requiring reoperation had significant right lateral parametrial involvement and underwent nerve-sparing parametrectomy at the time of bowel resection).3Notably, 7 of the 81 patients had totally laparoscopic double segmental colorectal resection — 6 with simultaneous ileocecal valve and rectal resection, and 1 with simultaneous ileal and rectal resection. None of these seven patients had postoperative complications. (A report on the six surgeries for deep endometriosis infiltrating the ileocecal valve and rectum was also published this year in the journal Colorectal Disease.6)
Median blood loss in the 81-patient cohort was 20 mL (IQR, 20-30 mL), and the median length of surgery was 160 minutes (IQR, 130-210 minutes).
Bowel endometriotic nodules had a mean maximum diameter of 4.3 cm (3.5-5.5 cm), a mean depth of invasion of 9 mm (7-9 mm), a mean estimated stenosis of 40% (35%-50%), and a mean distance from the anal verge of 13 cm (10-15 cm).3 (The cohort in this analysis did not include patients who received concomitant hysterectomy and transvaginal NOSE.)
Specifics of the New Technique, Including Totally Intracorporeal Anastomosis
As in our previous technique, the surgical procedure starts with left and dorsal rectal mobilization. The left pelvic sidewall dissection begins with incision of the parietal peritoneum along the pelvic portions of the psoas muscle. The retroperitoneal connective tissue is dissected in order to identify the ureter, the hypogastric nerve, and the presacral parietal pelvic fascia (PPPF). Dissection is carried downward in front of the PPPF and medially to the left hypogastric nerve.
Right and ventral rectal mobilization begins with incision of the peritoneum along the gray avascular line, which is seen with upward retraction of the rectum.
Again, for preservation of bowel and bladder function, the hypogastric nerve must be identified and preserved through dissection that displaces the nerve dorsally and laterally.
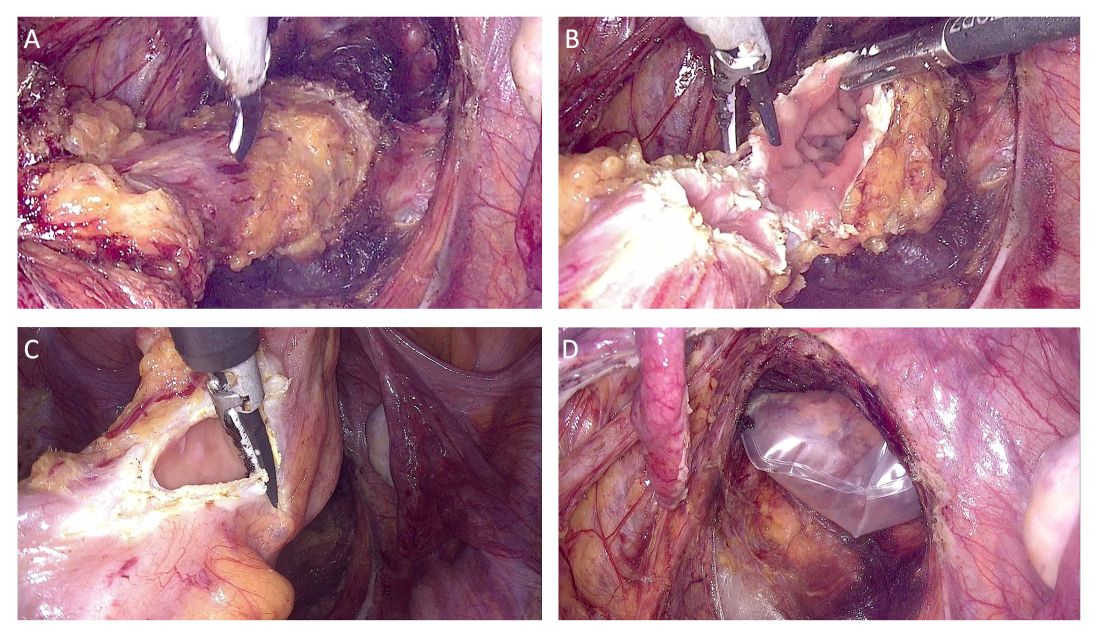
Dissection proceeds to sharply divide the nodule into two parts (rectal and rectocervical components). The caudal boundary of ventral dissection is reached by incising the septum and opening the rectovaginal space. The lymphovascular fatty tissue surrounding the rectum is then excised and the rectal tube is denuded at the level of the distal resection margin.
The distal and proximal resection margins are prepared and transected using a tissue-sealing device, and the resected rectal specimen is placed into a retrieval bag and pulled out through the anus (Figure 1).
The totally intracorporeal side-to-end anastomosis is performed using a traditional circular stapler. Its anvil is inserted through the proximal resection line and exteriorized forward on the antimesenteric side of the sigmoid colon/rectum. The proximal and distal resection lines are closed with a 60-mm linear endo-stapler, and finally, the shaft of the circular stapler is inserted into the rectum, and the two parts of the stapler are joined. The stapler is then closed and fired (Figure 2).
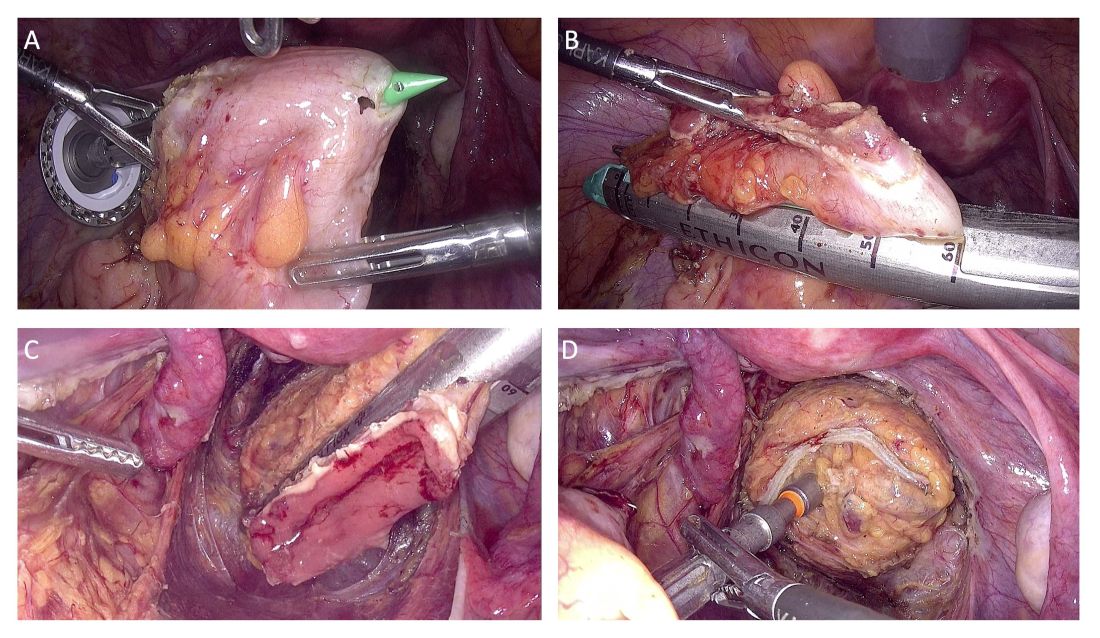
An air leak test is performed to check the quality of the rectal sutures. However, regardless of the results, we routinely apply interrupted stitches to the so-called “dog ears” in order to reduce tension along the anastomotic line.
We also evaluate the microvascularization of the two parts of the bowel at the level of the reanastomosis using indocyanine green fluorescence angiography. Doing so allows us to avoid complications caused by anastomotic leakage.
Patient Selection And Peri-Surgical Care
The decision to perform segmental resection as opposed to other more conservative laparoscopic excision techniques is made after skilled preoperative imaging reveals the number of lesions, the largest nodule diameter, the infiltration depth, and the circumference of bowel involvement.
A recently published international consensus statement7 on noninvasive imaging techniques for diagnosis of pelvic deep endometriosis and endometriosis classification systems concludes there is Level 1a evidence that preoperative imaging with transvaginal ultrasound (TVUS) can predict with good precision the size and degree of infiltration of deep endometriosis of the rectum (a Grade A statement, per the Oxford Centre for Evidence-Based Medicine levels of evidence).
The consensus document was published across seven different journals by international and European societies, and the American Association of Gynecologic Laparoscopists (AAGL), each of which contributed to a multidisciplinary panel of gynecological surgeons, sonographers, and radiologists.
At our center, we achieve precise preoperative evaluations with TVUS (other centers also employ MRI) and have long set clear preoperative indications for segmental resection. Based on our experience,8 a nodule ≥ 3 cm and infiltration of the tunica muscularis of the sigmoid colon/rectum ≥ 7 mm indicate the need for segmental resection. It is impossible to achieve surgical goals in such cases through shaving or discoid resection.
Our perioperative care protocol for total laparoscopic segmental resection with transanal NOSE, described in our new paper,3 includes several components: antibiotic therapy (metronidazole 500 mg 12 hours before surgery; cefazolin 2 g plus metronidazole 500 mg 1 hour before skin incision; and cefazolin 1 g plus metronidazole 500 mg 12 hours after surgery); good intraoperative bowel preparation (mechanical with oral polyethylene glycol solution preoperatively, and rectal irrigation with a mixed solution of povidine-iodine and normal saline intraoperatively); and venous thromboembolism prophylaxis postoperatively for 28 days.
Moving Forward, A Word On Classification
Multicenter trials and research with longer follow-up times will be helpful in advancing the use of totally laparoscopic segmental colorectal resection for deep endometriosis. To enable better sharing of diagnostic and therapeutic results — and to advance the credibility of research — my hope is that the field will reach further agreement on endometriosis classification systems. This would also help with the development of standards for postgraduate education.
We have made significant strides in recognizing imaging as a standard for diagnosis and treatment, and I believe we currently have two very good endometriosis classification systems — the #Enzian classification system issued in 2021 and the AAGL 2021 Endometriosis Classification — that can be used in combination with imaging to reliably and consistently describe deep endometriosis.
Mario Malzoni, MD, is scientific director of the Malzoni Research Hospital, the Center for Advanced Pelvic Surgery, in Avellino, Italy. He reported having no disclosures relevant to this Master Class.
References
1. Malzoni M et al. Surgical principles of segmental rectosigmoid resection and reanastomosis for deep infiltrating endometriosis. J Minim Invasive Gynecol. 2020;27(2):258.
2. Malzoni M et al. Totally laparoscopic resection with transanal natural orifice specimen extraction for deep endometriosis infiltrating the rectum. J Minim Invasive Gynecol. 2022;29(1):19.
3. Malzoni M et al. Total laparoscopic segmental resection with transanal natural orifice specimen extraction for treatment of colorectal endometriosis: Descriptive analysis from the TrEnd study database. J Minim Invasive Gynecol. 2024. (in press). 4. Brincat SD et al. Natural orifice versus transabdominal specimen extraction in laparoscopic surgery for colorectal cancer: meta-analysis. BJS Open 2022;6(3):zrac074.
5. Zhou Z et al. Laparoscopic natural orifice specimen extraction surgery versus conventional surgery in colorectal cancer: A meta-analysis of randomized controlled trials. Gastroenterol Res Pract. 2022 Jan 18;2022:6661651.
6. Malzoni M et al. Simultaneous total laparoscopic double segmental resection with transanal natural orifice specimen extraction for deep endometriosis infiltrating the ileocaecal valve and rectum—A video vignette. Colorectal Dis 2024 Jul 25.
7. Condous G et al. Noninvasive imaging techniques for diagnosis of pelvic deep endometriosis and endometriosis classification systems: An international consensus. J. Minim Invasive Gynecol. 2024;31(7):557-73.
8. Malzoni M et al. Preoperative ultrasound indications determine excision technique for bowel surgery for deep infiltrating endometriosis: A single, high-volume center. J Minim Invasive Gynecol. 2020;27(5):1141-7.
What to Know About NOSE
Traditionally, a laparoscopic approach to bowel resection secondary to endometriosis means enlargement of the port site or more commonly, a Pfannenstiel incision to introduce an anvil. Both incisions raise the risk of postoperative complications including pain and incisional hernia. Moreover, recovery time is prolonged.
Natural orifice specimen extraction (NOSE) makes use of a natural orifice, the vagina or anus, to extract the specimen and introduce the anvil. Transanal extraction for the management of endometriosis-related bowel disease was first described by the late David Redwine, MD, in his 1996 publication in Fertility and Sterility.1
The concern with use of vaginal NOSE surgery is rectovaginal fistula secondary to two incision lines in close opposition. Both transvaginal NOSE and transanal NOSE have been criticized for longer dissection of the mesorectum and thus longer resected specimen. Despite these concerns, over the past 10 years, articles have been published showing not only the feasibility of the NOSE technique, but excellent comparative outcomes, as well.2-4
A recent systematic review and meta-analysis by Kar et al. comparing NOSE extraction to minilaparotomy in bowel resection due to endometriosis revealed no significant difference in complication rate, but significantly shorter operation duration, reduced length of stay, and less blood loss in the NOSE surgical arm.5
The most recent study to be published in the Journal of Minimally Invasive Gynecology involving total laparoscopic segmental resection with transanal NOSE for the treatment of colorectal endometriosis was written by the guest author of this edition of the Master Class in Gynecologic Surgery, Professor Mario Malzoni, Scientific Director of the Malzoni Research Hospital, the Center for Advanced Pelvic Surgery, in Avellino, Italy. In the JMIG article, and in this Master Class, Professor Malzoni, a world-renowned endometriosis specialist recognized for his extraordinary skill and multi-organ surgical work, discusses the difference between the standard bowel resection technique with that of NOSE, and reports on his experience with NOSE.6
It is a pleasure and honor to welcome my friend and colleague, Professor Mario Malzoni, to this edition of the Master Class in Gynecologic Surgery.
Dr. Charles E. Miller is Professor, Obstetrics & Gynecology, Department of Clinical Sciences, Rosalind Franklin University of Medicine and Science, North Chicago, and Director, Minimally Invasive Gynecologic Surgery, Advocate Lutheran General Hospital, Park Ridge, both in Illinois. Email him at obnews@mdedge.com.
References
1. Redwine DB et al. Laparoscopically assisted transvaginal segmental resection of the rectosigmoid colon for endometriosis. Fertil Steril. 1996 Jan;65(1):193-7.
2. Akladios C et al. Totally laparoscopic intracorporeal anastomosis with natural orifice specimen extraction (NOSE) techniques, particularly suitable for bowel endometriosis. J Minim Invasive Gynecol. 2014 Nov-Dec;21(6):1095-102.
3. Bokor A et al. Natural orifice specimen extraction during laparoscopic bowel resection for colorectal endometriosis: technique and outcome. J Minim Invasive Gynecol. 2018 Sep-Oct;25(6):1065-74.
4. Grigoriadis G et al. Natural orifice specimen extraction colorectal resection for deep endometriosis: A 50 case series. J Minim Invasive Gynecol. 2022 Sep;29(9):1054-62.
5. Kar E et al. Natural orifice specimen extraction as a promising alternative for minilaparotomy in bowel resection due to endometriosis: A systematic review and meta-analysis. J Minim Invasive Gynecol. 2024 Jul;31(7):574-83.e1.
6. Malzoni M et al. Total laparoscopic segmental resection with transanal natural orifice specimen extraction for treatment of colorectal endometriosis: Descriptive analysis from the TrEnd study database. J Minim Invasive Gynecol. 2024 Oct;18:S1553-4650(24)01453-5.
Laparoscopic Resection With Transanal NOSE for Deep Colorectal Endometriosis
When segmental sigmoid colon/rectal resection is indicated for treatment of deep infiltrating bowel endometriosis, a totally laparoscopic resection with intracorporeal anastomosis and transanal natural orifice specimen extraction (NOSE) can be safe, effective, and advantageous compared with an abdominal mini-laparotomy for extracorporeal anastomosis and specimen retrieval.
With advanced laparoscopic surgical skills, accurate preoperative ultrasound evaluation, and a high-quality perioperative care protocol (including high-quality bowel preparation), segmental resection with NOSE can offer better preservation of vascularization, smaller resection sizes, lower rates of postoperative pain, a shorter time for gas passage after surgery, decreased hospital stays, and lower rates of wound infection and incisional hernia.
We have seen such improved results in our high-volume center since shifting in April 2021 from our classical technique for segmental rectosigmoid resection1 to a totally laparoscopic approach with NOSE. We first reported on this new technique in 2022 in a video article in the Journal of Minimally Invasive Gynecology,2and in October 2024, outcomes of 81 patients who underwent segmental sigmoid colon/rectal resection with intracorporeal anastomosis and transanal NOSE at our institution from April 2021 through March 2024 were reported in Journal of Minimally Invasive Gynecology.3Given our experience with this approach for deep endometriosis, I am convinced that NOSE should be preferred to transabdominal specimen extraction for segmental rectosigmoid resection. This is where the future lies for deep infiltrating endometriosis. Here, I share our outcomes and describe our technique.
Our Prior Approach With A Mini-Laparotomy
Our traditional laparoscopic approach for segmental colon/rectal resection of deep infiltrating endometriosis involved an abdominal mini-laparotomy (no more than 4 cm in length) for specimen retrieval, resection of the proximal rectum, and positioning of the head of a circular stapler for end-to-end anastomosis. (Connection of the two parts of the circular stapler for anastomosis was achieved via laparoscopy.)1
Several factors allowed us to minimize potential complications, including standardization of our technique, the use of three-dimensional endoscopes, and indocyanine green fluorescence angiography to assess the perfusion of the bowel after the completion of anastomosis. Still, an unpublished analysis of 1050 segmental bowel resections performed over more than 17 years using our traditional technique showed that hospital stays averaged 8 days, time to first defecation averaged 7 days, and time to first passage of flatus averaged 1 day.
Per the Clavien-Dindo classification system for surgical complications that was used at the time, 1.9% of these 1050 patients had grade 1 (minor) or grade 2 complications and 6% had grade 3 complications. Grade 2 complications were defined as needing intervention or a hospital stay more than twice the median for the procedure. Grade 3 complications were defined as leading to lasting disability or organ resection.
In the meantime, in colorectal cancer surgery, published studies on NOSE versus conventional laparoscopy with transabdominal specimen extraction have consistently pointed to the benefits of NOSE. A 2022 review 4 of 19 studies involving over 3400 patients and a 2022 meta-analysis 5 of 21 randomized controlled trials involving more than 2000 patients both showed significantly reduced postoperative morbidity and no differences in oncologic outcomes. Among the notable differences in the 2022 meta-analysis was a relative risk of postoperative infection of 0.34 for patients treated with NOSE compared with conventional laparoscopic techniques.
Among patients with deep rectal endometriosis, reported experience with intracorporeal anastomosis and NOSE for segmental resection is limited, with a few studies published between 2014 and 2022. Excluding anecdotal reports, only about 140 patients have been reported in the literature as having intracorporeal anastomosis and transanal NOSE for deep infiltrating bowel endometriosis. 3
Our Outcomes With A Totally Laparoscopic Approach
Our approach to evaluating our experience with totally laparoscopic resection with transanal NOSE has been to systematically collect data from consecutive patients and to analyze 1) complications of the technique, 2) conversion to the traditional technique/open surgery, and 3) endometriosis-free bowel resection margins and recurrence. Secondarily, we look at intraoperative blood loss, operative time, recovery of gastrointestinal function, hospital stay length, and reproductive outcomes.
Across 81 patients from our TrEnd study database (Surgical TReatment of women with deep ENDometriosis infiltrating the sigmoid colon and/or rectum) who received a totally laparoscopic standardized procedure involving intracorporeal anastomosis and transanal NOSE, we had no conversions even to mini-laparotomy, and no cases of protective colostomy or ileostomy.
Complete endometriosis removal was achieved in 100% of cases, with final pathology showing endometriosis-free resection margins, and patients remained free of bowel endometriosis at a median follow-up of 21 months.3Our analysis also shows improved gastrointestinal function recovery (median time to first defecation of 4 days, compared to 7 days previously, with the same 1-day median time to first passage of flatus) and a significantly improved hospital length of stay to a median of 3 days (interquartile range, 3-4.5 days); the latter reflects in part the absence of infection.3There were no intra-operative complications. Postoperative Clavien-Dindo grade 3 complications occurred in three patients (3.7%), two of whom required reoperation (one for hemoperitoneum and one for ureteral injury) and one who experienced rectal anastomotic bleeding on postoperative day 1. (The two requiring reoperation had significant right lateral parametrial involvement and underwent nerve-sparing parametrectomy at the time of bowel resection).3Notably, 7 of the 81 patients had totally laparoscopic double segmental colorectal resection — 6 with simultaneous ileocecal valve and rectal resection, and 1 with simultaneous ileal and rectal resection. None of these seven patients had postoperative complications. (A report on the six surgeries for deep endometriosis infiltrating the ileocecal valve and rectum was also published this year in the journal Colorectal Disease.6)
Median blood loss in the 81-patient cohort was 20 mL (IQR, 20-30 mL), and the median length of surgery was 160 minutes (IQR, 130-210 minutes).
Bowel endometriotic nodules had a mean maximum diameter of 4.3 cm (3.5-5.5 cm), a mean depth of invasion of 9 mm (7-9 mm), a mean estimated stenosis of 40% (35%-50%), and a mean distance from the anal verge of 13 cm (10-15 cm).3 (The cohort in this analysis did not include patients who received concomitant hysterectomy and transvaginal NOSE.)
Specifics of the New Technique, Including Totally Intracorporeal Anastomosis
As in our previous technique, the surgical procedure starts with left and dorsal rectal mobilization. The left pelvic sidewall dissection begins with incision of the parietal peritoneum along the pelvic portions of the psoas muscle. The retroperitoneal connective tissue is dissected in order to identify the ureter, the hypogastric nerve, and the presacral parietal pelvic fascia (PPPF). Dissection is carried downward in front of the PPPF and medially to the left hypogastric nerve.
Right and ventral rectal mobilization begins with incision of the peritoneum along the gray avascular line, which is seen with upward retraction of the rectum.
Again, for preservation of bowel and bladder function, the hypogastric nerve must be identified and preserved through dissection that displaces the nerve dorsally and laterally.
Dissection proceeds to sharply divide the nodule into two parts (rectal and rectocervical components). The caudal boundary of ventral dissection is reached by incising the septum and opening the rectovaginal space. The lymphovascular fatty tissue surrounding the rectum is then excised and the rectal tube is denuded at the level of the distal resection margin.
The distal and proximal resection margins are prepared and transected using a tissue-sealing device, and the resected rectal specimen is placed into a retrieval bag and pulled out through the anus (Figure 1).

The totally intracorporeal side-to-end anastomosis is performed using a traditional circular stapler. Its anvil is inserted through the proximal resection line and exteriorized forward on the antimesenteric side of the sigmoid colon/rectum. The proximal and distal resection lines are closed with a 60-mm linear endo-stapler, and finally, the shaft of the circular stapler is inserted into the rectum, and the two parts of the stapler are joined. The stapler is then closed and fired (Figure 2).

An air leak test is performed to check the quality of the rectal sutures. However, regardless of the results, we routinely apply interrupted stitches to the so-called “dog ears” in order to reduce tension along the anastomotic line.
We also evaluate the microvascularization of the two parts of the bowel at the level of the reanastomosis using indocyanine green fluorescence angiography. Doing so allows us to avoid complications caused by anastomotic leakage.
Patient Selection And Peri-Surgical Care
The decision to perform segmental resection as opposed to other more conservative laparoscopic excision techniques is made after skilled preoperative imaging reveals the number of lesions, the largest nodule diameter, the infiltration depth, and the circumference of bowel involvement.
A recently published international consensus statement7 on noninvasive imaging techniques for diagnosis of pelvic deep endometriosis and endometriosis classification systems concludes there is Level 1a evidence that preoperative imaging with transvaginal ultrasound (TVUS) can predict with good precision the size and degree of infiltration of deep endometriosis of the rectum (a Grade A statement, per the Oxford Centre for Evidence-Based Medicine levels of evidence).
The consensus document was published across seven different journals by international and European societies, and the American Association of Gynecologic Laparoscopists (AAGL), each of which contributed to a multidisciplinary panel of gynecological surgeons, sonographers, and radiologists.
At our center, we achieve precise preoperative evaluations with TVUS (other centers also employ MRI) and have long set clear preoperative indications for segmental resection. Based on our experience,8 a nodule ≥ 3 cm and infiltration of the tunica muscularis of the sigmoid colon/rectum ≥ 7 mm indicate the need for segmental resection. It is impossible to achieve surgical goals in such cases through shaving or discoid resection.
Our perioperative care protocol for total laparoscopic segmental resection with transanal NOSE, described in our new paper,3 includes several components: antibiotic therapy (metronidazole 500 mg 12 hours before surgery; cefazolin 2 g plus metronidazole 500 mg 1 hour before skin incision; and cefazolin 1 g plus metronidazole 500 mg 12 hours after surgery); good intraoperative bowel preparation (mechanical with oral polyethylene glycol solution preoperatively, and rectal irrigation with a mixed solution of povidine-iodine and normal saline intraoperatively); and venous thromboembolism prophylaxis postoperatively for 28 days.
Moving Forward, A Word On Classification
Multicenter trials and research with longer follow-up times will be helpful in advancing the use of totally laparoscopic segmental colorectal resection for deep endometriosis. To enable better sharing of diagnostic and therapeutic results — and to advance the credibility of research — my hope is that the field will reach further agreement on endometriosis classification systems. This would also help with the development of standards for postgraduate education.
We have made significant strides in recognizing imaging as a standard for diagnosis and treatment, and I believe we currently have two very good endometriosis classification systems — the #Enzian classification system issued in 2021 and the AAGL 2021 Endometriosis Classification — that can be used in combination with imaging to reliably and consistently describe deep endometriosis.
Mario Malzoni, MD, is scientific director of the Malzoni Research Hospital, the Center for Advanced Pelvic Surgery, in Avellino, Italy. He reported having no disclosures relevant to this Master Class.
References
1. Malzoni M et al. Surgical principles of segmental rectosigmoid resection and reanastomosis for deep infiltrating endometriosis. J Minim Invasive Gynecol. 2020;27(2):258.
2. Malzoni M et al. Totally laparoscopic resection with transanal natural orifice specimen extraction for deep endometriosis infiltrating the rectum. J Minim Invasive Gynecol. 2022;29(1):19.
3. Malzoni M et al. Total laparoscopic segmental resection with transanal natural orifice specimen extraction for treatment of colorectal endometriosis: Descriptive analysis from the TrEnd study database. J Minim Invasive Gynecol. 2024. (in press). 4. Brincat SD et al. Natural orifice versus transabdominal specimen extraction in laparoscopic surgery for colorectal cancer: meta-analysis. BJS Open 2022;6(3):zrac074.
5. Zhou Z et al. Laparoscopic natural orifice specimen extraction surgery versus conventional surgery in colorectal cancer: A meta-analysis of randomized controlled trials. Gastroenterol Res Pract. 2022 Jan 18;2022:6661651.
6. Malzoni M et al. Simultaneous total laparoscopic double segmental resection with transanal natural orifice specimen extraction for deep endometriosis infiltrating the ileocaecal valve and rectum—A video vignette. Colorectal Dis 2024 Jul 25.
7. Condous G et al. Noninvasive imaging techniques for diagnosis of pelvic deep endometriosis and endometriosis classification systems: An international consensus. J. Minim Invasive Gynecol. 2024;31(7):557-73.
8. Malzoni M et al. Preoperative ultrasound indications determine excision technique for bowel surgery for deep infiltrating endometriosis: A single, high-volume center. J Minim Invasive Gynecol. 2020;27(5):1141-7.
What to Know About NOSE
Traditionally, a laparoscopic approach to bowel resection secondary to endometriosis means enlargement of the port site or more commonly, a Pfannenstiel incision to introduce an anvil. Both incisions raise the risk of postoperative complications including pain and incisional hernia. Moreover, recovery time is prolonged.
Natural orifice specimen extraction (NOSE) makes use of a natural orifice, the vagina or anus, to extract the specimen and introduce the anvil. Transanal extraction for the management of endometriosis-related bowel disease was first described by the late David Redwine, MD, in his 1996 publication in Fertility and Sterility.1
The concern with use of vaginal NOSE surgery is rectovaginal fistula secondary to two incision lines in close opposition. Both transvaginal NOSE and transanal NOSE have been criticized for longer dissection of the mesorectum and thus longer resected specimen. Despite these concerns, over the past 10 years, articles have been published showing not only the feasibility of the NOSE technique, but excellent comparative outcomes, as well.2-4
A recent systematic review and meta-analysis by Kar et al. comparing NOSE extraction to minilaparotomy in bowel resection due to endometriosis revealed no significant difference in complication rate, but significantly shorter operation duration, reduced length of stay, and less blood loss in the NOSE surgical arm.5
The most recent study to be published in the Journal of Minimally Invasive Gynecology involving total laparoscopic segmental resection with transanal NOSE for the treatment of colorectal endometriosis was written by the guest author of this edition of the Master Class in Gynecologic Surgery, Professor Mario Malzoni, Scientific Director of the Malzoni Research Hospital, the Center for Advanced Pelvic Surgery, in Avellino, Italy. In the JMIG article, and in this Master Class, Professor Malzoni, a world-renowned endometriosis specialist recognized for his extraordinary skill and multi-organ surgical work, discusses the difference between the standard bowel resection technique with that of NOSE, and reports on his experience with NOSE.6
It is a pleasure and honor to welcome my friend and colleague, Professor Mario Malzoni, to this edition of the Master Class in Gynecologic Surgery.
Dr. Charles E. Miller is Professor, Obstetrics & Gynecology, Department of Clinical Sciences, Rosalind Franklin University of Medicine and Science, North Chicago, and Director, Minimally Invasive Gynecologic Surgery, Advocate Lutheran General Hospital, Park Ridge, both in Illinois. Email him at obnews@mdedge.com.
References
1. Redwine DB et al. Laparoscopically assisted transvaginal segmental resection of the rectosigmoid colon for endometriosis. Fertil Steril. 1996 Jan;65(1):193-7.
2. Akladios C et al. Totally laparoscopic intracorporeal anastomosis with natural orifice specimen extraction (NOSE) techniques, particularly suitable for bowel endometriosis. J Minim Invasive Gynecol. 2014 Nov-Dec;21(6):1095-102.
3. Bokor A et al. Natural orifice specimen extraction during laparoscopic bowel resection for colorectal endometriosis: technique and outcome. J Minim Invasive Gynecol. 2018 Sep-Oct;25(6):1065-74.
4. Grigoriadis G et al. Natural orifice specimen extraction colorectal resection for deep endometriosis: A 50 case series. J Minim Invasive Gynecol. 2022 Sep;29(9):1054-62.
5. Kar E et al. Natural orifice specimen extraction as a promising alternative for minilaparotomy in bowel resection due to endometriosis: A systematic review and meta-analysis. J Minim Invasive Gynecol. 2024 Jul;31(7):574-83.e1.
6. Malzoni M et al. Total laparoscopic segmental resection with transanal natural orifice specimen extraction for treatment of colorectal endometriosis: Descriptive analysis from the TrEnd study database. J Minim Invasive Gynecol. 2024 Oct;18:S1553-4650(24)01453-5.
Laparoscopic Resection With Transanal NOSE for Deep Colorectal Endometriosis
When segmental sigmoid colon/rectal resection is indicated for treatment of deep infiltrating bowel endometriosis, a totally laparoscopic resection with intracorporeal anastomosis and transanal natural orifice specimen extraction (NOSE) can be safe, effective, and advantageous compared with an abdominal mini-laparotomy for extracorporeal anastomosis and specimen retrieval.
With advanced laparoscopic surgical skills, accurate preoperative ultrasound evaluation, and a high-quality perioperative care protocol (including high-quality bowel preparation), segmental resection with NOSE can offer better preservation of vascularization, smaller resection sizes, lower rates of postoperative pain, a shorter time for gas passage after surgery, decreased hospital stays, and lower rates of wound infection and incisional hernia.
We have seen such improved results in our high-volume center since shifting in April 2021 from our classical technique for segmental rectosigmoid resection1 to a totally laparoscopic approach with NOSE. We first reported on this new technique in 2022 in a video article in the Journal of Minimally Invasive Gynecology,2and in October 2024, outcomes of 81 patients who underwent segmental sigmoid colon/rectal resection with intracorporeal anastomosis and transanal NOSE at our institution from April 2021 through March 2024 were reported in Journal of Minimally Invasive Gynecology.3Given our experience with this approach for deep endometriosis, I am convinced that NOSE should be preferred to transabdominal specimen extraction for segmental rectosigmoid resection. This is where the future lies for deep infiltrating endometriosis. Here, I share our outcomes and describe our technique.
Our Prior Approach With A Mini-Laparotomy
Our traditional laparoscopic approach for segmental colon/rectal resection of deep infiltrating endometriosis involved an abdominal mini-laparotomy (no more than 4 cm in length) for specimen retrieval, resection of the proximal rectum, and positioning of the head of a circular stapler for end-to-end anastomosis. (Connection of the two parts of the circular stapler for anastomosis was achieved via laparoscopy.)1
Several factors allowed us to minimize potential complications, including standardization of our technique, the use of three-dimensional endoscopes, and indocyanine green fluorescence angiography to assess the perfusion of the bowel after the completion of anastomosis. Still, an unpublished analysis of 1050 segmental bowel resections performed over more than 17 years using our traditional technique showed that hospital stays averaged 8 days, time to first defecation averaged 7 days, and time to first passage of flatus averaged 1 day.
Per the Clavien-Dindo classification system for surgical complications that was used at the time, 1.9% of these 1050 patients had grade 1 (minor) or grade 2 complications and 6% had grade 3 complications. Grade 2 complications were defined as needing intervention or a hospital stay more than twice the median for the procedure. Grade 3 complications were defined as leading to lasting disability or organ resection.
In the meantime, in colorectal cancer surgery, published studies on NOSE versus conventional laparoscopy with transabdominal specimen extraction have consistently pointed to the benefits of NOSE. A 2022 review 4 of 19 studies involving over 3400 patients and a 2022 meta-analysis 5 of 21 randomized controlled trials involving more than 2000 patients both showed significantly reduced postoperative morbidity and no differences in oncologic outcomes. Among the notable differences in the 2022 meta-analysis was a relative risk of postoperative infection of 0.34 for patients treated with NOSE compared with conventional laparoscopic techniques.
Among patients with deep rectal endometriosis, reported experience with intracorporeal anastomosis and NOSE for segmental resection is limited, with a few studies published between 2014 and 2022. Excluding anecdotal reports, only about 140 patients have been reported in the literature as having intracorporeal anastomosis and transanal NOSE for deep infiltrating bowel endometriosis. 3
Our Outcomes With A Totally Laparoscopic Approach
Our approach to evaluating our experience with totally laparoscopic resection with transanal NOSE has been to systematically collect data from consecutive patients and to analyze 1) complications of the technique, 2) conversion to the traditional technique/open surgery, and 3) endometriosis-free bowel resection margins and recurrence. Secondarily, we look at intraoperative blood loss, operative time, recovery of gastrointestinal function, hospital stay length, and reproductive outcomes.
Across 81 patients from our TrEnd study database (Surgical TReatment of women with deep ENDometriosis infiltrating the sigmoid colon and/or rectum) who received a totally laparoscopic standardized procedure involving intracorporeal anastomosis and transanal NOSE, we had no conversions even to mini-laparotomy, and no cases of protective colostomy or ileostomy.
Complete endometriosis removal was achieved in 100% of cases, with final pathology showing endometriosis-free resection margins, and patients remained free of bowel endometriosis at a median follow-up of 21 months.3Our analysis also shows improved gastrointestinal function recovery (median time to first defecation of 4 days, compared to 7 days previously, with the same 1-day median time to first passage of flatus) and a significantly improved hospital length of stay to a median of 3 days (interquartile range, 3-4.5 days); the latter reflects in part the absence of infection.3There were no intra-operative complications. Postoperative Clavien-Dindo grade 3 complications occurred in three patients (3.7%), two of whom required reoperation (one for hemoperitoneum and one for ureteral injury) and one who experienced rectal anastomotic bleeding on postoperative day 1. (The two requiring reoperation had significant right lateral parametrial involvement and underwent nerve-sparing parametrectomy at the time of bowel resection).3Notably, 7 of the 81 patients had totally laparoscopic double segmental colorectal resection — 6 with simultaneous ileocecal valve and rectal resection, and 1 with simultaneous ileal and rectal resection. None of these seven patients had postoperative complications. (A report on the six surgeries for deep endometriosis infiltrating the ileocecal valve and rectum was also published this year in the journal Colorectal Disease.6)
Median blood loss in the 81-patient cohort was 20 mL (IQR, 20-30 mL), and the median length of surgery was 160 minutes (IQR, 130-210 minutes).
Bowel endometriotic nodules had a mean maximum diameter of 4.3 cm (3.5-5.5 cm), a mean depth of invasion of 9 mm (7-9 mm), a mean estimated stenosis of 40% (35%-50%), and a mean distance from the anal verge of 13 cm (10-15 cm).3 (The cohort in this analysis did not include patients who received concomitant hysterectomy and transvaginal NOSE.)
Specifics of the New Technique, Including Totally Intracorporeal Anastomosis
As in our previous technique, the surgical procedure starts with left and dorsal rectal mobilization. The left pelvic sidewall dissection begins with incision of the parietal peritoneum along the pelvic portions of the psoas muscle. The retroperitoneal connective tissue is dissected in order to identify the ureter, the hypogastric nerve, and the presacral parietal pelvic fascia (PPPF). Dissection is carried downward in front of the PPPF and medially to the left hypogastric nerve.
Right and ventral rectal mobilization begins with incision of the peritoneum along the gray avascular line, which is seen with upward retraction of the rectum.
Again, for preservation of bowel and bladder function, the hypogastric nerve must be identified and preserved through dissection that displaces the nerve dorsally and laterally.
Dissection proceeds to sharply divide the nodule into two parts (rectal and rectocervical components). The caudal boundary of ventral dissection is reached by incising the septum and opening the rectovaginal space. The lymphovascular fatty tissue surrounding the rectum is then excised and the rectal tube is denuded at the level of the distal resection margin.
The distal and proximal resection margins are prepared and transected using a tissue-sealing device, and the resected rectal specimen is placed into a retrieval bag and pulled out through the anus (Figure 1).

The totally intracorporeal side-to-end anastomosis is performed using a traditional circular stapler. Its anvil is inserted through the proximal resection line and exteriorized forward on the antimesenteric side of the sigmoid colon/rectum. The proximal and distal resection lines are closed with a 60-mm linear endo-stapler, and finally, the shaft of the circular stapler is inserted into the rectum, and the two parts of the stapler are joined. The stapler is then closed and fired (Figure 2).

An air leak test is performed to check the quality of the rectal sutures. However, regardless of the results, we routinely apply interrupted stitches to the so-called “dog ears” in order to reduce tension along the anastomotic line.
We also evaluate the microvascularization of the two parts of the bowel at the level of the reanastomosis using indocyanine green fluorescence angiography. Doing so allows us to avoid complications caused by anastomotic leakage.
Patient Selection And Peri-Surgical Care
The decision to perform segmental resection as opposed to other more conservative laparoscopic excision techniques is made after skilled preoperative imaging reveals the number of lesions, the largest nodule diameter, the infiltration depth, and the circumference of bowel involvement.
A recently published international consensus statement7 on noninvasive imaging techniques for diagnosis of pelvic deep endometriosis and endometriosis classification systems concludes there is Level 1a evidence that preoperative imaging with transvaginal ultrasound (TVUS) can predict with good precision the size and degree of infiltration of deep endometriosis of the rectum (a Grade A statement, per the Oxford Centre for Evidence-Based Medicine levels of evidence).
The consensus document was published across seven different journals by international and European societies, and the American Association of Gynecologic Laparoscopists (AAGL), each of which contributed to a multidisciplinary panel of gynecological surgeons, sonographers, and radiologists.
At our center, we achieve precise preoperative evaluations with TVUS (other centers also employ MRI) and have long set clear preoperative indications for segmental resection. Based on our experience,8 a nodule ≥ 3 cm and infiltration of the tunica muscularis of the sigmoid colon/rectum ≥ 7 mm indicate the need for segmental resection. It is impossible to achieve surgical goals in such cases through shaving or discoid resection.
Our perioperative care protocol for total laparoscopic segmental resection with transanal NOSE, described in our new paper,3 includes several components: antibiotic therapy (metronidazole 500 mg 12 hours before surgery; cefazolin 2 g plus metronidazole 500 mg 1 hour before skin incision; and cefazolin 1 g plus metronidazole 500 mg 12 hours after surgery); good intraoperative bowel preparation (mechanical with oral polyethylene glycol solution preoperatively, and rectal irrigation with a mixed solution of povidine-iodine and normal saline intraoperatively); and venous thromboembolism prophylaxis postoperatively for 28 days.
Moving Forward, A Word On Classification
Multicenter trials and research with longer follow-up times will be helpful in advancing the use of totally laparoscopic segmental colorectal resection for deep endometriosis. To enable better sharing of diagnostic and therapeutic results — and to advance the credibility of research — my hope is that the field will reach further agreement on endometriosis classification systems. This would also help with the development of standards for postgraduate education.
We have made significant strides in recognizing imaging as a standard for diagnosis and treatment, and I believe we currently have two very good endometriosis classification systems — the #Enzian classification system issued in 2021 and the AAGL 2021 Endometriosis Classification — that can be used in combination with imaging to reliably and consistently describe deep endometriosis.
Mario Malzoni, MD, is scientific director of the Malzoni Research Hospital, the Center for Advanced Pelvic Surgery, in Avellino, Italy. He reported having no disclosures relevant to this Master Class.
References
1. Malzoni M et al. Surgical principles of segmental rectosigmoid resection and reanastomosis for deep infiltrating endometriosis. J Minim Invasive Gynecol. 2020;27(2):258.
2. Malzoni M et al. Totally laparoscopic resection with transanal natural orifice specimen extraction for deep endometriosis infiltrating the rectum. J Minim Invasive Gynecol. 2022;29(1):19.
3. Malzoni M et al. Total laparoscopic segmental resection with transanal natural orifice specimen extraction for treatment of colorectal endometriosis: Descriptive analysis from the TrEnd study database. J Minim Invasive Gynecol. 2024. (in press). 4. Brincat SD et al. Natural orifice versus transabdominal specimen extraction in laparoscopic surgery for colorectal cancer: meta-analysis. BJS Open 2022;6(3):zrac074.
5. Zhou Z et al. Laparoscopic natural orifice specimen extraction surgery versus conventional surgery in colorectal cancer: A meta-analysis of randomized controlled trials. Gastroenterol Res Pract. 2022 Jan 18;2022:6661651.
6. Malzoni M et al. Simultaneous total laparoscopic double segmental resection with transanal natural orifice specimen extraction for deep endometriosis infiltrating the ileocaecal valve and rectum—A video vignette. Colorectal Dis 2024 Jul 25.
7. Condous G et al. Noninvasive imaging techniques for diagnosis of pelvic deep endometriosis and endometriosis classification systems: An international consensus. J. Minim Invasive Gynecol. 2024;31(7):557-73.
8. Malzoni M et al. Preoperative ultrasound indications determine excision technique for bowel surgery for deep infiltrating endometriosis: A single, high-volume center. J Minim Invasive Gynecol. 2020;27(5):1141-7.
Fibroids: Medical Therapy Not Hysterectomy Should Be First Treatment Choice Interventional Options Case Study
Although hysterectomy remains the most common procedure for treating fibroids and fibroids are the leading indication for hysterectomy, its long-term sequelae make less invasive alternatives the better choice for managing most of these myometrial masses, an invited clinical practice paper in the New England Journal of Medicine (NEJM) asserts.
The practice summary also calls for earlier identification and treatment of fibroid disease and may raise awareness among general gynecologists and primary care physicians less familiar with newer treatments.
Based on a review of evidence and existing formal guidelines, the paper urges wider use of uterus-sparing approaches such as hormone therapy, uterine-artery embolization, focused ultrasound ablation, and radiofrequency ablation. Authored by ob.gyns. Elizabeth A. Stewart, MD, and Shannon K. Laughlin-Tommaso, MD, MPH, of the Mayo Clinic in Rochester, Minnesota, the document also features textbook-style diagrams illustrating procedures.
“To clarify, this is not a new guidance but an invited clinical practice paper,” Laughlin-Tommaso told this news organization. “I believe NEJM recognized the gap in knowledge among all providers, for early diagnosis of uterine fibroids, especially in young patients and those presenting with anemia.”
The less invasive treatments highlighted in the paper can help women recover faster and resume their normal activities more quickly, said Laughlin-Tommaso. “Additionally, many studies have now shown that there are health benefits to keeping the uterus and the ovaries.”
Despite multiple uterine-sparing options, however, a recent study in a commercially insured population found nearly 60% of fibroid patients undergoing hysterectomy had never received a prior conservative treatment.
Why hysterectomy for a benign condition? Hysterectomy, which is universally available in ob.gyn. practices, makes decision-making easier for medical providers and patients, Laughlin-Tommaso explained. “It’s the only treatment that is definitive in that patients will not have bleeding or fibroids in the future and providers don’t have to determine which fibroids to treat or remove.”
More common in Black women, fibroids affect up to 80% of persons with a uterus during their lifetime and up to 50% have symptoms such as heavy and prolonged menstrual bleeding, anemia-associated fatigue, pelvic pressure, and menstrual and nonmenstrual pain, the authors noted. These lesions can also compress nearby structures causing painful intercourse, constipation, and urinary frequency, urgency, or retention.
In 2021 the American College of Obstetricians and Gynecologists issued a practice bulletin on the management of symptomatic uterine leiomyomas, similarly endorsing individualized care that accounts for the desire to preserve fertility or the uterus, increase quality of life, and reduce symptoms. It, too, recommended medical management as first-line treatment for symptomatic fibroids.
“This paper will be helpful for clinicians by covering some of the newer options such as gonadotropin-releasing hormone antagonists introduced in the past 5 years and tailoring treatment to patients depending on whether they still want to conceive,” Sandra M. Hurtado, MD, an ob.gyn. and an assistant professor at UTHealth Houston Medical Center and McGovern Medical School in Houston, Texas, said in an interview. “And the illustrations will be useful to doctors who are not gynecologists and will help to explain the interventional options to patients,” added Hurtado, who was not involved in the paper.
Offering another outside perspective on the paper, Charles J. Ascher-Walsh, MD, senior system vice chair for gynecology and division director of urogynecology in the Raquel and Jaime Gilinski Department of Obstetrics, Gynecology and Reproductive Science at Mount Sinai in New York City, called it a useful though not new summary. Reaching the wider audience of NEJM may raise awareness of newer fibroid therapies among general, nonspecialist ob.gyns., whose practices may concentrate largely on obstetrics, he added, “and the excellent illustrations clarify the treatment options.” In his view, broader awareness may increase much-needed funding for this neglected area of research.
Among the paper’s recommendations:
Diagnosis
Pelvic ultrasonography is the most cost-effective imaging method, providing information on size, location, and number of fibroids and ruling out adnexal masses. It is limited, however, by less-accurate resolution if the uterine volume is greater than 375 mL or if fibroids number more than four.
Medical Alternatives to Hysterectomy
Early diagnosis and first-line medical therapies are recommended.
Contraceptive hormones to control heavy menstrual bleeding are the first step in most algorithms for treating fibroid-related bleeding, despite low-quality evidence.
Nonsteroidal anti-inflammatory agents and tranexamic acid during menstruation also limit heavy menses but have more evidence of efficacy for idiopathic heavy menses.
Gonadotropin-releasing hormone (GnRH) agonists in depot form are approved for short-term preoperative therapy. While they cause amenorrhea in nearly 90% of patients and reduce uterine volume by 30%-60%, they have a high incidence of hypogonadal symptoms, including bone loss and hot flushes. They also cause a “steroidal flare” when the stored gonadotropins are released and cause subsequent heavy menstrual bleeding with the rapid decrease in estrogen levels.
Oral GnRH antagonist combinations are a major therapeutic advance, pairing a GnRH antagonist (such as elagolix or relugolix, which rapidly inhibit ovarian steroidogenesis) with estradiol and progestin at doses equivalent to systemic levels in the early follicular phase of the menstrual cycle.
In clinical trials these combinations decreased heavy menstrual bleeding by 50%-75%, pain by 40%-50%, and bulk-related symptoms through a 10% decrease in uterine volume. Side effects are few, with hot flushes, headaches, and nausea occurring in fewer than 20% of participants.
Smaller fibroids in the submucosal to intramural spaces can be treated transcervically, while larger lesions of any type or smaller subserosa fibroids are treated abdominally.
Uterine-artery embolization uses minimally invasive radiologically guided catheterization to release embolic particles directly into both uterine arteries. This process causes ischemic infarction of the fibroids and decreases bleeding, pain, and bulk-related symptoms.
Other procedures shrink individual fibroids with energy that creates coagulative necrosis. These include focused ultrasound ablation (with MRI or ultrasound guidance) and radiofrequency ablation (with laparoscopic or transcervical ultrasound guidance).
Unlike uterine-artery embolization, which treats all fibroids concurrently, these therapies require individual targeting of fibroids.
Radiofrequency ablation can be done concurrently with other surgical therapies, such as laparoscopic excision of endometriosis or hysteroscopic myomectomy.
Myomectomy, or the surgical removal of fibroids, is most often used in persons actively seeking pregnancy or having very large fibroids in whom shrinkage would be inadequate. Most guidelines recommend surgical excision rather than shrinking procedures to optimize fertility. However, myomectomy often commits patients to future cesarean section, which increases pregnancy-related morbidity.
Although myomectomy is seen as superior to uterine-artery embolization for improving quality of life, both approaches provide substantial symptom relief.
Recurrence
Incidence of recurring fibroids is high, with, for example, new fibroids developing in approximately 50% of persons within 5 years of myomectomy.
Earlier this year, a large cohort study reported that myomectomy was best for avoiding reintervention after surgical leiomyoma management.
Reintervention rates vary according to procedure, patient age, disease extent, and symptoms and can be as high as 33% up to 5 years after treatment, with lower percentages seen among persons older than 45 years of age.
Hysterectomy
Minimally invasive hysterectomy is recommended. Drawbacks to hysterectomy include perioperative risk and concomitant oophorectomy, which was common until the early 2000s when large cohort studies showed elevated risks of death, cardiovascular disease, dementia, and other illnesses compared with hysterectomy plus ovarian conservation. Oophorectomy but not hysterectomy rates have since decreased.
Still needing study, according to Laughlin-Tommaso are the underlying reasons for health disparities in fibroids, especially among Black and Latina individuals. “Some studies have found associations with vitamin D deficiency and with stress and racism,” she said.
Looking ahead, the authors stressed the need for a fibroid risk-prediction model, a staging system, and large randomized trials of treatment effectiveness. Also needed are methods for primary and secondary prevention. “Earlier screening and medical treatment in primary care settings could potentially minimize morbidity and the incidence of unnecessary hysterectomies, and primary care–based screening trials are warranted,” they wrote.
In addition to procedural illustrations the practice document includes a vignette of a 33-year-old never-pregnant Black woman (but desiring motherhood) with heavy menstrual bleeding, abdominal bloating, and non–iron deficiency anemia. Evaluation for thalassemia and sickle cell anemia is negative, but ultrasonography reveals an enlarged uterus with multiple fibroids and normal ovaries.
In line with the clinical review, the authors prescribe oral GnRH agonist combination therapy, plus iron and multivitamin supplementation, and recommend annual reassessment — earlier if pregnancy is desired or if symptoms escalate. Since the patient prioritizes fertility, hysterectomy would be appropriate only if she had biopsy-proven cancer.
The authors received no external funding for this practice paper, but both have funding from the National Institutes of Health for fibroid research. Laughlin-Tommaso reported royalties from UpToDate. Stewart reported research support from the Agency for Healthcare Research and Quality, and speaking, data-monitoring, and consulting fees for various private companies, including AbbVie, Anylam Pharmaceuticals, ASKA Pharma, and Myovant Sciences. She holds a patent on treatment for abnormal uterine bleeding and has been involved in CME for various medical educational agencies. Hurtado and Ascher-Walsh had no relevant conflicts of interest to declare.
A version of this article first appeared on Medscape.com.
Although hysterectomy remains the most common procedure for treating fibroids and fibroids are the leading indication for hysterectomy, its long-term sequelae make less invasive alternatives the better choice for managing most of these myometrial masses, an invited clinical practice paper in the New England Journal of Medicine (NEJM) asserts.
The practice summary also calls for earlier identification and treatment of fibroid disease and may raise awareness among general gynecologists and primary care physicians less familiar with newer treatments.
Based on a review of evidence and existing formal guidelines, the paper urges wider use of uterus-sparing approaches such as hormone therapy, uterine-artery embolization, focused ultrasound ablation, and radiofrequency ablation. Authored by ob.gyns. Elizabeth A. Stewart, MD, and Shannon K. Laughlin-Tommaso, MD, MPH, of the Mayo Clinic in Rochester, Minnesota, the document also features textbook-style diagrams illustrating procedures.
“To clarify, this is not a new guidance but an invited clinical practice paper,” Laughlin-Tommaso told this news organization. “I believe NEJM recognized the gap in knowledge among all providers, for early diagnosis of uterine fibroids, especially in young patients and those presenting with anemia.”
The less invasive treatments highlighted in the paper can help women recover faster and resume their normal activities more quickly, said Laughlin-Tommaso. “Additionally, many studies have now shown that there are health benefits to keeping the uterus and the ovaries.”
Despite multiple uterine-sparing options, however, a recent study in a commercially insured population found nearly 60% of fibroid patients undergoing hysterectomy had never received a prior conservative treatment.
Why hysterectomy for a benign condition? Hysterectomy, which is universally available in ob.gyn. practices, makes decision-making easier for medical providers and patients, Laughlin-Tommaso explained. “It’s the only treatment that is definitive in that patients will not have bleeding or fibroids in the future and providers don’t have to determine which fibroids to treat or remove.”
More common in Black women, fibroids affect up to 80% of persons with a uterus during their lifetime and up to 50% have symptoms such as heavy and prolonged menstrual bleeding, anemia-associated fatigue, pelvic pressure, and menstrual and nonmenstrual pain, the authors noted. These lesions can also compress nearby structures causing painful intercourse, constipation, and urinary frequency, urgency, or retention.
In 2021 the American College of Obstetricians and Gynecologists issued a practice bulletin on the management of symptomatic uterine leiomyomas, similarly endorsing individualized care that accounts for the desire to preserve fertility or the uterus, increase quality of life, and reduce symptoms. It, too, recommended medical management as first-line treatment for symptomatic fibroids.
“This paper will be helpful for clinicians by covering some of the newer options such as gonadotropin-releasing hormone antagonists introduced in the past 5 years and tailoring treatment to patients depending on whether they still want to conceive,” Sandra M. Hurtado, MD, an ob.gyn. and an assistant professor at UTHealth Houston Medical Center and McGovern Medical School in Houston, Texas, said in an interview. “And the illustrations will be useful to doctors who are not gynecologists and will help to explain the interventional options to patients,” added Hurtado, who was not involved in the paper.
Offering another outside perspective on the paper, Charles J. Ascher-Walsh, MD, senior system vice chair for gynecology and division director of urogynecology in the Raquel and Jaime Gilinski Department of Obstetrics, Gynecology and Reproductive Science at Mount Sinai in New York City, called it a useful though not new summary. Reaching the wider audience of NEJM may raise awareness of newer fibroid therapies among general, nonspecialist ob.gyns., whose practices may concentrate largely on obstetrics, he added, “and the excellent illustrations clarify the treatment options.” In his view, broader awareness may increase much-needed funding for this neglected area of research.
Among the paper’s recommendations:
Diagnosis
Pelvic ultrasonography is the most cost-effective imaging method, providing information on size, location, and number of fibroids and ruling out adnexal masses. It is limited, however, by less-accurate resolution if the uterine volume is greater than 375 mL or if fibroids number more than four.
Medical Alternatives to Hysterectomy
Early diagnosis and first-line medical therapies are recommended.
Contraceptive hormones to control heavy menstrual bleeding are the first step in most algorithms for treating fibroid-related bleeding, despite low-quality evidence.
Nonsteroidal anti-inflammatory agents and tranexamic acid during menstruation also limit heavy menses but have more evidence of efficacy for idiopathic heavy menses.
Gonadotropin-releasing hormone (GnRH) agonists in depot form are approved for short-term preoperative therapy. While they cause amenorrhea in nearly 90% of patients and reduce uterine volume by 30%-60%, they have a high incidence of hypogonadal symptoms, including bone loss and hot flushes. They also cause a “steroidal flare” when the stored gonadotropins are released and cause subsequent heavy menstrual bleeding with the rapid decrease in estrogen levels.
Oral GnRH antagonist combinations are a major therapeutic advance, pairing a GnRH antagonist (such as elagolix or relugolix, which rapidly inhibit ovarian steroidogenesis) with estradiol and progestin at doses equivalent to systemic levels in the early follicular phase of the menstrual cycle.
In clinical trials these combinations decreased heavy menstrual bleeding by 50%-75%, pain by 40%-50%, and bulk-related symptoms through a 10% decrease in uterine volume. Side effects are few, with hot flushes, headaches, and nausea occurring in fewer than 20% of participants.
Smaller fibroids in the submucosal to intramural spaces can be treated transcervically, while larger lesions of any type or smaller subserosa fibroids are treated abdominally.
Uterine-artery embolization uses minimally invasive radiologically guided catheterization to release embolic particles directly into both uterine arteries. This process causes ischemic infarction of the fibroids and decreases bleeding, pain, and bulk-related symptoms.
Other procedures shrink individual fibroids with energy that creates coagulative necrosis. These include focused ultrasound ablation (with MRI or ultrasound guidance) and radiofrequency ablation (with laparoscopic or transcervical ultrasound guidance).
Unlike uterine-artery embolization, which treats all fibroids concurrently, these therapies require individual targeting of fibroids.
Radiofrequency ablation can be done concurrently with other surgical therapies, such as laparoscopic excision of endometriosis or hysteroscopic myomectomy.
Myomectomy, or the surgical removal of fibroids, is most often used in persons actively seeking pregnancy or having very large fibroids in whom shrinkage would be inadequate. Most guidelines recommend surgical excision rather than shrinking procedures to optimize fertility. However, myomectomy often commits patients to future cesarean section, which increases pregnancy-related morbidity.
Although myomectomy is seen as superior to uterine-artery embolization for improving quality of life, both approaches provide substantial symptom relief.
Recurrence
Incidence of recurring fibroids is high, with, for example, new fibroids developing in approximately 50% of persons within 5 years of myomectomy.
Earlier this year, a large cohort study reported that myomectomy was best for avoiding reintervention after surgical leiomyoma management.
Reintervention rates vary according to procedure, patient age, disease extent, and symptoms and can be as high as 33% up to 5 years after treatment, with lower percentages seen among persons older than 45 years of age.
Hysterectomy
Minimally invasive hysterectomy is recommended. Drawbacks to hysterectomy include perioperative risk and concomitant oophorectomy, which was common until the early 2000s when large cohort studies showed elevated risks of death, cardiovascular disease, dementia, and other illnesses compared with hysterectomy plus ovarian conservation. Oophorectomy but not hysterectomy rates have since decreased.
Still needing study, according to Laughlin-Tommaso are the underlying reasons for health disparities in fibroids, especially among Black and Latina individuals. “Some studies have found associations with vitamin D deficiency and with stress and racism,” she said.
Looking ahead, the authors stressed the need for a fibroid risk-prediction model, a staging system, and large randomized trials of treatment effectiveness. Also needed are methods for primary and secondary prevention. “Earlier screening and medical treatment in primary care settings could potentially minimize morbidity and the incidence of unnecessary hysterectomies, and primary care–based screening trials are warranted,” they wrote.
In addition to procedural illustrations the practice document includes a vignette of a 33-year-old never-pregnant Black woman (but desiring motherhood) with heavy menstrual bleeding, abdominal bloating, and non–iron deficiency anemia. Evaluation for thalassemia and sickle cell anemia is negative, but ultrasonography reveals an enlarged uterus with multiple fibroids and normal ovaries.
In line with the clinical review, the authors prescribe oral GnRH agonist combination therapy, plus iron and multivitamin supplementation, and recommend annual reassessment — earlier if pregnancy is desired or if symptoms escalate. Since the patient prioritizes fertility, hysterectomy would be appropriate only if she had biopsy-proven cancer.
The authors received no external funding for this practice paper, but both have funding from the National Institutes of Health for fibroid research. Laughlin-Tommaso reported royalties from UpToDate. Stewart reported research support from the Agency for Healthcare Research and Quality, and speaking, data-monitoring, and consulting fees for various private companies, including AbbVie, Anylam Pharmaceuticals, ASKA Pharma, and Myovant Sciences. She holds a patent on treatment for abnormal uterine bleeding and has been involved in CME for various medical educational agencies. Hurtado and Ascher-Walsh had no relevant conflicts of interest to declare.
A version of this article first appeared on Medscape.com.
Although hysterectomy remains the most common procedure for treating fibroids and fibroids are the leading indication for hysterectomy, its long-term sequelae make less invasive alternatives the better choice for managing most of these myometrial masses, an invited clinical practice paper in the New England Journal of Medicine (NEJM) asserts.
The practice summary also calls for earlier identification and treatment of fibroid disease and may raise awareness among general gynecologists and primary care physicians less familiar with newer treatments.
Based on a review of evidence and existing formal guidelines, the paper urges wider use of uterus-sparing approaches such as hormone therapy, uterine-artery embolization, focused ultrasound ablation, and radiofrequency ablation. Authored by ob.gyns. Elizabeth A. Stewart, MD, and Shannon K. Laughlin-Tommaso, MD, MPH, of the Mayo Clinic in Rochester, Minnesota, the document also features textbook-style diagrams illustrating procedures.
“To clarify, this is not a new guidance but an invited clinical practice paper,” Laughlin-Tommaso told this news organization. “I believe NEJM recognized the gap in knowledge among all providers, for early diagnosis of uterine fibroids, especially in young patients and those presenting with anemia.”
The less invasive treatments highlighted in the paper can help women recover faster and resume their normal activities more quickly, said Laughlin-Tommaso. “Additionally, many studies have now shown that there are health benefits to keeping the uterus and the ovaries.”
Despite multiple uterine-sparing options, however, a recent study in a commercially insured population found nearly 60% of fibroid patients undergoing hysterectomy had never received a prior conservative treatment.
Why hysterectomy for a benign condition? Hysterectomy, which is universally available in ob.gyn. practices, makes decision-making easier for medical providers and patients, Laughlin-Tommaso explained. “It’s the only treatment that is definitive in that patients will not have bleeding or fibroids in the future and providers don’t have to determine which fibroids to treat or remove.”
More common in Black women, fibroids affect up to 80% of persons with a uterus during their lifetime and up to 50% have symptoms such as heavy and prolonged menstrual bleeding, anemia-associated fatigue, pelvic pressure, and menstrual and nonmenstrual pain, the authors noted. These lesions can also compress nearby structures causing painful intercourse, constipation, and urinary frequency, urgency, or retention.
In 2021 the American College of Obstetricians and Gynecologists issued a practice bulletin on the management of symptomatic uterine leiomyomas, similarly endorsing individualized care that accounts for the desire to preserve fertility or the uterus, increase quality of life, and reduce symptoms. It, too, recommended medical management as first-line treatment for symptomatic fibroids.
“This paper will be helpful for clinicians by covering some of the newer options such as gonadotropin-releasing hormone antagonists introduced in the past 5 years and tailoring treatment to patients depending on whether they still want to conceive,” Sandra M. Hurtado, MD, an ob.gyn. and an assistant professor at UTHealth Houston Medical Center and McGovern Medical School in Houston, Texas, said in an interview. “And the illustrations will be useful to doctors who are not gynecologists and will help to explain the interventional options to patients,” added Hurtado, who was not involved in the paper.
Offering another outside perspective on the paper, Charles J. Ascher-Walsh, MD, senior system vice chair for gynecology and division director of urogynecology in the Raquel and Jaime Gilinski Department of Obstetrics, Gynecology and Reproductive Science at Mount Sinai in New York City, called it a useful though not new summary. Reaching the wider audience of NEJM may raise awareness of newer fibroid therapies among general, nonspecialist ob.gyns., whose practices may concentrate largely on obstetrics, he added, “and the excellent illustrations clarify the treatment options.” In his view, broader awareness may increase much-needed funding for this neglected area of research.
Among the paper’s recommendations:
Diagnosis
Pelvic ultrasonography is the most cost-effective imaging method, providing information on size, location, and number of fibroids and ruling out adnexal masses. It is limited, however, by less-accurate resolution if the uterine volume is greater than 375 mL or if fibroids number more than four.
Medical Alternatives to Hysterectomy
Early diagnosis and first-line medical therapies are recommended.
Contraceptive hormones to control heavy menstrual bleeding are the first step in most algorithms for treating fibroid-related bleeding, despite low-quality evidence.
Nonsteroidal anti-inflammatory agents and tranexamic acid during menstruation also limit heavy menses but have more evidence of efficacy for idiopathic heavy menses.
Gonadotropin-releasing hormone (GnRH) agonists in depot form are approved for short-term preoperative therapy. While they cause amenorrhea in nearly 90% of patients and reduce uterine volume by 30%-60%, they have a high incidence of hypogonadal symptoms, including bone loss and hot flushes. They also cause a “steroidal flare” when the stored gonadotropins are released and cause subsequent heavy menstrual bleeding with the rapid decrease in estrogen levels.
Oral GnRH antagonist combinations are a major therapeutic advance, pairing a GnRH antagonist (such as elagolix or relugolix, which rapidly inhibit ovarian steroidogenesis) with estradiol and progestin at doses equivalent to systemic levels in the early follicular phase of the menstrual cycle.
In clinical trials these combinations decreased heavy menstrual bleeding by 50%-75%, pain by 40%-50%, and bulk-related symptoms through a 10% decrease in uterine volume. Side effects are few, with hot flushes, headaches, and nausea occurring in fewer than 20% of participants.
Smaller fibroids in the submucosal to intramural spaces can be treated transcervically, while larger lesions of any type or smaller subserosa fibroids are treated abdominally.
Uterine-artery embolization uses minimally invasive radiologically guided catheterization to release embolic particles directly into both uterine arteries. This process causes ischemic infarction of the fibroids and decreases bleeding, pain, and bulk-related symptoms.
Other procedures shrink individual fibroids with energy that creates coagulative necrosis. These include focused ultrasound ablation (with MRI or ultrasound guidance) and radiofrequency ablation (with laparoscopic or transcervical ultrasound guidance).
Unlike uterine-artery embolization, which treats all fibroids concurrently, these therapies require individual targeting of fibroids.
Radiofrequency ablation can be done concurrently with other surgical therapies, such as laparoscopic excision of endometriosis or hysteroscopic myomectomy.
Myomectomy, or the surgical removal of fibroids, is most often used in persons actively seeking pregnancy or having very large fibroids in whom shrinkage would be inadequate. Most guidelines recommend surgical excision rather than shrinking procedures to optimize fertility. However, myomectomy often commits patients to future cesarean section, which increases pregnancy-related morbidity.
Although myomectomy is seen as superior to uterine-artery embolization for improving quality of life, both approaches provide substantial symptom relief.
Recurrence
Incidence of recurring fibroids is high, with, for example, new fibroids developing in approximately 50% of persons within 5 years of myomectomy.
Earlier this year, a large cohort study reported that myomectomy was best for avoiding reintervention after surgical leiomyoma management.
Reintervention rates vary according to procedure, patient age, disease extent, and symptoms and can be as high as 33% up to 5 years after treatment, with lower percentages seen among persons older than 45 years of age.
Hysterectomy
Minimally invasive hysterectomy is recommended. Drawbacks to hysterectomy include perioperative risk and concomitant oophorectomy, which was common until the early 2000s when large cohort studies showed elevated risks of death, cardiovascular disease, dementia, and other illnesses compared with hysterectomy plus ovarian conservation. Oophorectomy but not hysterectomy rates have since decreased.
Still needing study, according to Laughlin-Tommaso are the underlying reasons for health disparities in fibroids, especially among Black and Latina individuals. “Some studies have found associations with vitamin D deficiency and with stress and racism,” she said.
Looking ahead, the authors stressed the need for a fibroid risk-prediction model, a staging system, and large randomized trials of treatment effectiveness. Also needed are methods for primary and secondary prevention. “Earlier screening and medical treatment in primary care settings could potentially minimize morbidity and the incidence of unnecessary hysterectomies, and primary care–based screening trials are warranted,” they wrote.
In addition to procedural illustrations the practice document includes a vignette of a 33-year-old never-pregnant Black woman (but desiring motherhood) with heavy menstrual bleeding, abdominal bloating, and non–iron deficiency anemia. Evaluation for thalassemia and sickle cell anemia is negative, but ultrasonography reveals an enlarged uterus with multiple fibroids and normal ovaries.
In line with the clinical review, the authors prescribe oral GnRH agonist combination therapy, plus iron and multivitamin supplementation, and recommend annual reassessment — earlier if pregnancy is desired or if symptoms escalate. Since the patient prioritizes fertility, hysterectomy would be appropriate only if she had biopsy-proven cancer.
The authors received no external funding for this practice paper, but both have funding from the National Institutes of Health for fibroid research. Laughlin-Tommaso reported royalties from UpToDate. Stewart reported research support from the Agency for Healthcare Research and Quality, and speaking, data-monitoring, and consulting fees for various private companies, including AbbVie, Anylam Pharmaceuticals, ASKA Pharma, and Myovant Sciences. She holds a patent on treatment for abnormal uterine bleeding and has been involved in CME for various medical educational agencies. Hurtado and Ascher-Walsh had no relevant conflicts of interest to declare.
A version of this article first appeared on Medscape.com.
FROM NEW ENGLAND JOURNAL OF MEDICINE
Few Women Know Uterine Fibroid Risk, Treatment Options
Most women (72%) are not aware they are at risk for developing uterine fibroids, though up to 77% of women will develop them in their lifetime, results of a new survey indicate.
Data from The Harris Poll, conducted on behalf of the Society of Interventional Radiology, also found that 17% of women mistakenly think a hysterectomy is the only treatment option, including more than one in four women (27%) who are between the ages of 18 and 34. Results were shared in a press release. The survey included 1,122 US women, some who have been diagnosed with uterine fibroids.
Fibroids may not cause symptoms for some, but some women may have heavy, prolonged, debilitating bleeding. Some women experience pelvic pain, a diminished sex life, and declining energy. However, the growths do not spread to other body regions and typically are not dangerous.
Hysterectomy Is Only One Option
Among the women in the survey who had been diagnosed with fibroids, 53% were presented the option of hysterectomy and 20% were told about other, less-invasive options, including over-the-counter NSAIDs (19%); uterine fibroid embolization (UFE) (17%); oral contraceptives (17%); and endometrial ablation (17%).
“Women need to be informed about the complete range of options available for treating their uterine fibroids, not just the surgical options as is most commonly done by gynecologists,” John C. Lipman, MD, founder and medical director of the Atlanta Fibroid Center in Smyrna, Georgia, said in the press release.
The survey also found that:
- More than half of women ages 18-34 (56%) and women ages 35-44 (51%) were either not familiar with uterine fibroids or never heard of them.
- Awareness was particularly low among Hispanic women, as 50% of Hispanic women say they’ve never heard of or aren’t familiar with the condition, compared with 37% of Black women who answered that way.
- More than one third (36%) of Black women and 22% of Hispanic women mistakenly think they are not at risk for developing fibroids, yet research has shown that uterine fibroids are three times more common in Black women and two times more common in Hispanic women than in White women.
For this study, the full sample data is accurate to within +/– 3.2 percentage points using a 95% confidence level. The data are part of the report “The Fibroid Fix: What Women Need to Know,” published on July 9 by the Society of Interventional Radiology.
Linda Fan, MD, chief of gynecology at Yale University in New Haven, Connecticut, said she is not surprised by those numbers. She says many patients are referred to her department who have not been given the full array of medical options for their fibroids or have not had thorough discussions with their providers, such as whether they want to preserve their fertility, or how they feel about an incision, undergoing anesthesia, or having their uterus removed.
Sometimes the hysterectomy choice is clear, she said — for instance, if there are indications of the rare cancer leiomyosarcoma, or if a postmenopausal woman has rapid growth of fibroids or heavy bleeding. Fibroids should not start growing after menopause, she said.
Additional options include radiofrequency ablation, performed while a patient is under anesthesia, by laparoscopy or hysteroscopy. The procedure uses ultrasound to watch a probe as it shrinks the fibroids with heat.
Currently, if a woman wants large fibroids removed and wants to keep her fertility options open, Dr. Fan says, myomectomy or medication are best “because we have the most information or data on (those options).”
When treating patients who don’t prioritize fertility, she said, UFE is a good option that doesn’t need incisions or anesthesia. But patients sometimes require a lot of pain medication afterward, Dr. Fan said. With radiofrequency ablation, specifically the Acessa and Sonata procedures, she said, “patients don’t experience a lot of pain after the procedure because the shrinking happens when they’re asleep under anesthesia.”
Uterine Fibroid Embolization a Nonsurgical Option
The report describes how UFE works but the Harris Poll showed that 60% of women who have heard of UFE did not hear about it first from a healthcare provider.
“UFE is a nonsurgical treatment, performed by interventional radiologists, that has been proven to significantly reduce heavy menstrual bleeding, relieve uterine pain, and improve energy levels,” the authors write. “Through a tiny incision in the wrist or thigh, a catheter is guided via imaging to the vessels leading to the fibroids. Through this catheter, small clear particles are injected to block the blood flow leading to the fibroids causing them to shrink and disappear.”
After UFE, most women leave the hospital the day of or the day after treatment, according to the report authors, who add that many patients also report they can resume normal activity in about 2 weeks, more quickly than with surgical treatments.
In some cases, watchful waiting will be the best option, the report notes, and that may require repeated checkups and scans.
Dr. Lipman is an adviser on The Fibroid Fix report.
Most women (72%) are not aware they are at risk for developing uterine fibroids, though up to 77% of women will develop them in their lifetime, results of a new survey indicate.
Data from The Harris Poll, conducted on behalf of the Society of Interventional Radiology, also found that 17% of women mistakenly think a hysterectomy is the only treatment option, including more than one in four women (27%) who are between the ages of 18 and 34. Results were shared in a press release. The survey included 1,122 US women, some who have been diagnosed with uterine fibroids.
Fibroids may not cause symptoms for some, but some women may have heavy, prolonged, debilitating bleeding. Some women experience pelvic pain, a diminished sex life, and declining energy. However, the growths do not spread to other body regions and typically are not dangerous.
Hysterectomy Is Only One Option
Among the women in the survey who had been diagnosed with fibroids, 53% were presented the option of hysterectomy and 20% were told about other, less-invasive options, including over-the-counter NSAIDs (19%); uterine fibroid embolization (UFE) (17%); oral contraceptives (17%); and endometrial ablation (17%).
“Women need to be informed about the complete range of options available for treating their uterine fibroids, not just the surgical options as is most commonly done by gynecologists,” John C. Lipman, MD, founder and medical director of the Atlanta Fibroid Center in Smyrna, Georgia, said in the press release.
The survey also found that:
- More than half of women ages 18-34 (56%) and women ages 35-44 (51%) were either not familiar with uterine fibroids or never heard of them.
- Awareness was particularly low among Hispanic women, as 50% of Hispanic women say they’ve never heard of or aren’t familiar with the condition, compared with 37% of Black women who answered that way.
- More than one third (36%) of Black women and 22% of Hispanic women mistakenly think they are not at risk for developing fibroids, yet research has shown that uterine fibroids are three times more common in Black women and two times more common in Hispanic women than in White women.
For this study, the full sample data is accurate to within +/– 3.2 percentage points using a 95% confidence level. The data are part of the report “The Fibroid Fix: What Women Need to Know,” published on July 9 by the Society of Interventional Radiology.
Linda Fan, MD, chief of gynecology at Yale University in New Haven, Connecticut, said she is not surprised by those numbers. She says many patients are referred to her department who have not been given the full array of medical options for their fibroids or have not had thorough discussions with their providers, such as whether they want to preserve their fertility, or how they feel about an incision, undergoing anesthesia, or having their uterus removed.
Sometimes the hysterectomy choice is clear, she said — for instance, if there are indications of the rare cancer leiomyosarcoma, or if a postmenopausal woman has rapid growth of fibroids or heavy bleeding. Fibroids should not start growing after menopause, she said.
Additional options include radiofrequency ablation, performed while a patient is under anesthesia, by laparoscopy or hysteroscopy. The procedure uses ultrasound to watch a probe as it shrinks the fibroids with heat.
Currently, if a woman wants large fibroids removed and wants to keep her fertility options open, Dr. Fan says, myomectomy or medication are best “because we have the most information or data on (those options).”
When treating patients who don’t prioritize fertility, she said, UFE is a good option that doesn’t need incisions or anesthesia. But patients sometimes require a lot of pain medication afterward, Dr. Fan said. With radiofrequency ablation, specifically the Acessa and Sonata procedures, she said, “patients don’t experience a lot of pain after the procedure because the shrinking happens when they’re asleep under anesthesia.”
Uterine Fibroid Embolization a Nonsurgical Option
The report describes how UFE works but the Harris Poll showed that 60% of women who have heard of UFE did not hear about it first from a healthcare provider.
“UFE is a nonsurgical treatment, performed by interventional radiologists, that has been proven to significantly reduce heavy menstrual bleeding, relieve uterine pain, and improve energy levels,” the authors write. “Through a tiny incision in the wrist or thigh, a catheter is guided via imaging to the vessels leading to the fibroids. Through this catheter, small clear particles are injected to block the blood flow leading to the fibroids causing them to shrink and disappear.”
After UFE, most women leave the hospital the day of or the day after treatment, according to the report authors, who add that many patients also report they can resume normal activity in about 2 weeks, more quickly than with surgical treatments.
In some cases, watchful waiting will be the best option, the report notes, and that may require repeated checkups and scans.
Dr. Lipman is an adviser on The Fibroid Fix report.
Most women (72%) are not aware they are at risk for developing uterine fibroids, though up to 77% of women will develop them in their lifetime, results of a new survey indicate.
Data from The Harris Poll, conducted on behalf of the Society of Interventional Radiology, also found that 17% of women mistakenly think a hysterectomy is the only treatment option, including more than one in four women (27%) who are between the ages of 18 and 34. Results were shared in a press release. The survey included 1,122 US women, some who have been diagnosed with uterine fibroids.
Fibroids may not cause symptoms for some, but some women may have heavy, prolonged, debilitating bleeding. Some women experience pelvic pain, a diminished sex life, and declining energy. However, the growths do not spread to other body regions and typically are not dangerous.
Hysterectomy Is Only One Option
Among the women in the survey who had been diagnosed with fibroids, 53% were presented the option of hysterectomy and 20% were told about other, less-invasive options, including over-the-counter NSAIDs (19%); uterine fibroid embolization (UFE) (17%); oral contraceptives (17%); and endometrial ablation (17%).
“Women need to be informed about the complete range of options available for treating their uterine fibroids, not just the surgical options as is most commonly done by gynecologists,” John C. Lipman, MD, founder and medical director of the Atlanta Fibroid Center in Smyrna, Georgia, said in the press release.
The survey also found that:
- More than half of women ages 18-34 (56%) and women ages 35-44 (51%) were either not familiar with uterine fibroids or never heard of them.
- Awareness was particularly low among Hispanic women, as 50% of Hispanic women say they’ve never heard of or aren’t familiar with the condition, compared with 37% of Black women who answered that way.
- More than one third (36%) of Black women and 22% of Hispanic women mistakenly think they are not at risk for developing fibroids, yet research has shown that uterine fibroids are three times more common in Black women and two times more common in Hispanic women than in White women.
For this study, the full sample data is accurate to within +/– 3.2 percentage points using a 95% confidence level. The data are part of the report “The Fibroid Fix: What Women Need to Know,” published on July 9 by the Society of Interventional Radiology.
Linda Fan, MD, chief of gynecology at Yale University in New Haven, Connecticut, said she is not surprised by those numbers. She says many patients are referred to her department who have not been given the full array of medical options for their fibroids or have not had thorough discussions with their providers, such as whether they want to preserve their fertility, or how they feel about an incision, undergoing anesthesia, or having their uterus removed.
Sometimes the hysterectomy choice is clear, she said — for instance, if there are indications of the rare cancer leiomyosarcoma, or if a postmenopausal woman has rapid growth of fibroids or heavy bleeding. Fibroids should not start growing after menopause, she said.
Additional options include radiofrequency ablation, performed while a patient is under anesthesia, by laparoscopy or hysteroscopy. The procedure uses ultrasound to watch a probe as it shrinks the fibroids with heat.
Currently, if a woman wants large fibroids removed and wants to keep her fertility options open, Dr. Fan says, myomectomy or medication are best “because we have the most information or data on (those options).”
When treating patients who don’t prioritize fertility, she said, UFE is a good option that doesn’t need incisions or anesthesia. But patients sometimes require a lot of pain medication afterward, Dr. Fan said. With radiofrequency ablation, specifically the Acessa and Sonata procedures, she said, “patients don’t experience a lot of pain after the procedure because the shrinking happens when they’re asleep under anesthesia.”
Uterine Fibroid Embolization a Nonsurgical Option
The report describes how UFE works but the Harris Poll showed that 60% of women who have heard of UFE did not hear about it first from a healthcare provider.
“UFE is a nonsurgical treatment, performed by interventional radiologists, that has been proven to significantly reduce heavy menstrual bleeding, relieve uterine pain, and improve energy levels,” the authors write. “Through a tiny incision in the wrist or thigh, a catheter is guided via imaging to the vessels leading to the fibroids. Through this catheter, small clear particles are injected to block the blood flow leading to the fibroids causing them to shrink and disappear.”
After UFE, most women leave the hospital the day of or the day after treatment, according to the report authors, who add that many patients also report they can resume normal activity in about 2 weeks, more quickly than with surgical treatments.
In some cases, watchful waiting will be the best option, the report notes, and that may require repeated checkups and scans.
Dr. Lipman is an adviser on The Fibroid Fix report.
Myomectomy best for avoiding reintervention after fibroid procedures
Reintervention rates after uterus-preserving surgery for leiomyomata were lowest after vaginal myomectomy, the most frequent among four therapeutic approaches, a large cohort study reported.
Accounting for censoring, the 7-year reintervention risk for vaginal myomectomy was 20.6%, followed by uterine artery embolization (26%), endometrial ablation (35.5%), and hysteroscopic myomectomy (37%).
Hysterectomies accounted for 63.2% of reinterventions according to lead author Susanna D. Mitro, PhD, a research scientist in the Division of Research and Department of Obstetrics and Gynecology at Kaiser Permanente Northern California, Oakland, and colleagues.
Risk did not vary by body mass index, race/ethnicity, or Neighborhood Deprivation Index, but did vary for some procedures by age and parity,
These findings generally align with earlier research and “illustrate clinically meaningful long-term differences in reintervention rates after a first uterus-preserving treatment for leiomyomas,” the researchers wrote in Obstetrics & Gynecology.
The Study
In a cohort of 10,324 patients ages 18-50, 19.9% were Asian, 21.2% Black, 21.3% Hispanic, and 32.5% White, with 5.2% of other races and ethnicities. The most affected age groups were 41-45 and 46-50 years. All participants underwent a first uterus-preserving procedure after leiomyoma diagnosis according to 2009-2021 electronic health records at Kaiser Permanente Northern California.
Reintervention referred to a second uterus-preserving procedure or hysterectomy. Median follow-up was 3.8 years (interquartile range, 1.8-7.4 years), and the proportions of index procedures were as follows: 18% (1857) for hysteroscopic myomectomy; 16.2% (1669) for uterine artery embolization; 21.4% (2211) for endometrial ablations; and 44.4% (4,587) for myomectomy.
Reintervention rates were higher in younger patients after uterine artery embolization, with patients ages 18-35 at the index procedure having 1.4-3.7 times greater reintervention rates than patients ages 46-50 years. Reintervention rates for hysteroscopic myomectomy varied by parity, with multiparous patients at 35% greater risk than their nulliparous counterparts.
On the age issue, the authors note that symptom recurrence may be less common in older patients, perhaps because of the onset of menopause. “Alternatively, findings may be explained by age-specific care strategies: Older patients experiencing symptom recurrence may prefer to wait until the onset of menopause rather than pursuing another surgical treatment,” they wrote.
A recent study with 7 years’ follow-up reported a 2.4 times greater risk of hysterectomy after uterine artery embolization versus myomectomy. Reintervention rates may be lower after myomectomy because otherwise asymptomatic patients pursue myomectomy to treat infertility, the authors wrote. Alternatively, myomectomy may more completely remove leiomyomas.
These common benign tumors take a toll on healthcare resources, in 2012 costing up to $9.4 billion annually (in 2010 dollars) for related surgeries, medications, and procedures. Leiomyomas are reportedly the most frequent reason for hysterectomy.
Robust data on the optimal therapeutic approach to fibroids have been sparse, however, with a 2017 comparative-effectiveness review from the Agency for Healthcare Research and Quality reporting that evidence on leiomyoma treatments was insufficient to guide clinical care. Few well-conducted trials of leiomyoma treatment have directly compared different treatment options, the authors noted.
The rate of myomectomy is reported to be 9.2 per 10,000 woman-years in Black women and 1.3 per 10,000 woman years in White women, and the recurrence rate after myomectomy can be as great as 60% when patients are followed up to 5 years.
The authors said their findings “may be a reference to discuss expectations for treatment outcomes when choosing initial uterus-preserving treatment for leiomyomas, especially for patients receiving treatment years before the likely onset of menopause.”
This research was supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases of the National Institutes of Health. Coauthor Dr. Lauren Wise is a paid consultant for AbbVie and has received in-kind donations from Swiss Precision Diagnostics and Kindara.com; she has also received payment from the Gates Foundation.
Reintervention rates after uterus-preserving surgery for leiomyomata were lowest after vaginal myomectomy, the most frequent among four therapeutic approaches, a large cohort study reported.
Accounting for censoring, the 7-year reintervention risk for vaginal myomectomy was 20.6%, followed by uterine artery embolization (26%), endometrial ablation (35.5%), and hysteroscopic myomectomy (37%).
Hysterectomies accounted for 63.2% of reinterventions according to lead author Susanna D. Mitro, PhD, a research scientist in the Division of Research and Department of Obstetrics and Gynecology at Kaiser Permanente Northern California, Oakland, and colleagues.
Risk did not vary by body mass index, race/ethnicity, or Neighborhood Deprivation Index, but did vary for some procedures by age and parity,
These findings generally align with earlier research and “illustrate clinically meaningful long-term differences in reintervention rates after a first uterus-preserving treatment for leiomyomas,” the researchers wrote in Obstetrics & Gynecology.
The Study
In a cohort of 10,324 patients ages 18-50, 19.9% were Asian, 21.2% Black, 21.3% Hispanic, and 32.5% White, with 5.2% of other races and ethnicities. The most affected age groups were 41-45 and 46-50 years. All participants underwent a first uterus-preserving procedure after leiomyoma diagnosis according to 2009-2021 electronic health records at Kaiser Permanente Northern California.
Reintervention referred to a second uterus-preserving procedure or hysterectomy. Median follow-up was 3.8 years (interquartile range, 1.8-7.4 years), and the proportions of index procedures were as follows: 18% (1857) for hysteroscopic myomectomy; 16.2% (1669) for uterine artery embolization; 21.4% (2211) for endometrial ablations; and 44.4% (4,587) for myomectomy.
Reintervention rates were higher in younger patients after uterine artery embolization, with patients ages 18-35 at the index procedure having 1.4-3.7 times greater reintervention rates than patients ages 46-50 years. Reintervention rates for hysteroscopic myomectomy varied by parity, with multiparous patients at 35% greater risk than their nulliparous counterparts.
On the age issue, the authors note that symptom recurrence may be less common in older patients, perhaps because of the onset of menopause. “Alternatively, findings may be explained by age-specific care strategies: Older patients experiencing symptom recurrence may prefer to wait until the onset of menopause rather than pursuing another surgical treatment,” they wrote.
A recent study with 7 years’ follow-up reported a 2.4 times greater risk of hysterectomy after uterine artery embolization versus myomectomy. Reintervention rates may be lower after myomectomy because otherwise asymptomatic patients pursue myomectomy to treat infertility, the authors wrote. Alternatively, myomectomy may more completely remove leiomyomas.
These common benign tumors take a toll on healthcare resources, in 2012 costing up to $9.4 billion annually (in 2010 dollars) for related surgeries, medications, and procedures. Leiomyomas are reportedly the most frequent reason for hysterectomy.
Robust data on the optimal therapeutic approach to fibroids have been sparse, however, with a 2017 comparative-effectiveness review from the Agency for Healthcare Research and Quality reporting that evidence on leiomyoma treatments was insufficient to guide clinical care. Few well-conducted trials of leiomyoma treatment have directly compared different treatment options, the authors noted.
The rate of myomectomy is reported to be 9.2 per 10,000 woman-years in Black women and 1.3 per 10,000 woman years in White women, and the recurrence rate after myomectomy can be as great as 60% when patients are followed up to 5 years.
The authors said their findings “may be a reference to discuss expectations for treatment outcomes when choosing initial uterus-preserving treatment for leiomyomas, especially for patients receiving treatment years before the likely onset of menopause.”
This research was supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases of the National Institutes of Health. Coauthor Dr. Lauren Wise is a paid consultant for AbbVie and has received in-kind donations from Swiss Precision Diagnostics and Kindara.com; she has also received payment from the Gates Foundation.
Reintervention rates after uterus-preserving surgery for leiomyomata were lowest after vaginal myomectomy, the most frequent among four therapeutic approaches, a large cohort study reported.
Accounting for censoring, the 7-year reintervention risk for vaginal myomectomy was 20.6%, followed by uterine artery embolization (26%), endometrial ablation (35.5%), and hysteroscopic myomectomy (37%).
Hysterectomies accounted for 63.2% of reinterventions according to lead author Susanna D. Mitro, PhD, a research scientist in the Division of Research and Department of Obstetrics and Gynecology at Kaiser Permanente Northern California, Oakland, and colleagues.
Risk did not vary by body mass index, race/ethnicity, or Neighborhood Deprivation Index, but did vary for some procedures by age and parity,
These findings generally align with earlier research and “illustrate clinically meaningful long-term differences in reintervention rates after a first uterus-preserving treatment for leiomyomas,” the researchers wrote in Obstetrics & Gynecology.
The Study
In a cohort of 10,324 patients ages 18-50, 19.9% were Asian, 21.2% Black, 21.3% Hispanic, and 32.5% White, with 5.2% of other races and ethnicities. The most affected age groups were 41-45 and 46-50 years. All participants underwent a first uterus-preserving procedure after leiomyoma diagnosis according to 2009-2021 electronic health records at Kaiser Permanente Northern California.
Reintervention referred to a second uterus-preserving procedure or hysterectomy. Median follow-up was 3.8 years (interquartile range, 1.8-7.4 years), and the proportions of index procedures were as follows: 18% (1857) for hysteroscopic myomectomy; 16.2% (1669) for uterine artery embolization; 21.4% (2211) for endometrial ablations; and 44.4% (4,587) for myomectomy.
Reintervention rates were higher in younger patients after uterine artery embolization, with patients ages 18-35 at the index procedure having 1.4-3.7 times greater reintervention rates than patients ages 46-50 years. Reintervention rates for hysteroscopic myomectomy varied by parity, with multiparous patients at 35% greater risk than their nulliparous counterparts.
On the age issue, the authors note that symptom recurrence may be less common in older patients, perhaps because of the onset of menopause. “Alternatively, findings may be explained by age-specific care strategies: Older patients experiencing symptom recurrence may prefer to wait until the onset of menopause rather than pursuing another surgical treatment,” they wrote.
A recent study with 7 years’ follow-up reported a 2.4 times greater risk of hysterectomy after uterine artery embolization versus myomectomy. Reintervention rates may be lower after myomectomy because otherwise asymptomatic patients pursue myomectomy to treat infertility, the authors wrote. Alternatively, myomectomy may more completely remove leiomyomas.
These common benign tumors take a toll on healthcare resources, in 2012 costing up to $9.4 billion annually (in 2010 dollars) for related surgeries, medications, and procedures. Leiomyomas are reportedly the most frequent reason for hysterectomy.
Robust data on the optimal therapeutic approach to fibroids have been sparse, however, with a 2017 comparative-effectiveness review from the Agency for Healthcare Research and Quality reporting that evidence on leiomyoma treatments was insufficient to guide clinical care. Few well-conducted trials of leiomyoma treatment have directly compared different treatment options, the authors noted.
The rate of myomectomy is reported to be 9.2 per 10,000 woman-years in Black women and 1.3 per 10,000 woman years in White women, and the recurrence rate after myomectomy can be as great as 60% when patients are followed up to 5 years.
The authors said their findings “may be a reference to discuss expectations for treatment outcomes when choosing initial uterus-preserving treatment for leiomyomas, especially for patients receiving treatment years before the likely onset of menopause.”
This research was supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases of the National Institutes of Health. Coauthor Dr. Lauren Wise is a paid consultant for AbbVie and has received in-kind donations from Swiss Precision Diagnostics and Kindara.com; she has also received payment from the Gates Foundation.
FROM OBSTETRICS & GYNECOLOGY
Despite effective therapies, fibroid care still lacking
In 2022, two colleagues from Johns Hopkins University, Baltimore, Bhuchitra Singh, MD, MPH, MS, MBA, and James Segars Jr., MD, reviewed the available literature to evaluate the effectiveness of newer minimally invasive therapies in reducing bleeding and improving the quality of life and control of symptoms linked to uterine fibroids.
Their goal, according to Dr. Segars, a professor of obstetrics and gynecology and director of the division of women’s health research at Johns Hopkins, was to help guide clinicians and patients in making decisions about the use of the newer therapies, including radiofrequency ablation and ultrasound-guided removal of lesions.
But he and Dr. Singh, the director of clinical research at the Howard W. and Georgeanna Seegar Jones Laboratory of Reproductive Sciences and Women’s Health Research, were surprised by their findings. “The outcomes were relatively the same,” Dr. Segars said. “All of the modalities lead to significant reduction in bleeding and other fibroid-related symptoms.”
The data on long-term complications and risk for recurrence are sparse for some of the newer approaches, and not enough high-quality long-term studies have been conducted for the Food and Drug Administration to approve them as fertility-sparing treatments.
But perhaps, the biggest challenge now is to ensure that women can take advantage of these newer therapies, with large gaps in both the diagnosis of fibroids and geographic access to minimally invasive treatments.
A widespread condition widely underdiagnosed
Uterine fibroids occur in most women (the incidence rises with age) and can be found in up to 70% of women by the time they reach menopause. Risk factors include family history, increasing interval since last birth, hypertension, and obesity. Increasing parity and use of oral contraceptives are protective.
But as many as 50% of cases go undiagnosed, and one reason for this is the failure of clinicians to dig deeply enough into women’s menstrual histories to diagnose fibroids.
“The most common cause of anemia is heavy menstrual bleeding,” said Shannon Laughlin-Tommaso, MD, MPH, a professor of obstetrics and gynecology at Mayo Clinic in Rochester, Minn. She frequently sees patients who have already undergone colonoscopy to work-up the source of their anemia before anyone suspects that fibroids are the culprit.
“When women tell us about their periods, what they’ve been told is normal [bleeding] – or what they’ve always had and considered normal – is actually kind of on the heavier spectrum,” she said.
Ideally, treatment for uterine fibroids would fix abnormally prolonged or heavy menstrual bleeding, relieve pain, and ameliorate symptoms associated with an enlarged uterus, such as pelvic pressure, urinary frequency, and constipation. And the fibroids would never recur.
By those measures, hysterectomy fits the bill: Success rates in relieving symptoms are high, and the risk for recurrence is zero. But the procedure carries significant drawbacks: short-term complications of surgery, including infection, bleeding, and injury to the bowels and bladder along with potential long-term risks for cardiovascular disease, cancer, ovarian failure and premature menopause, depression, and decline in cognitive function. Those factors loom even larger for women who still hope to have children.
For that reason, the American College of Obstetricians and Gynecologists recommends myomectomy, or surgical removal of individual fibroids, for women who desire uterine preservation or future pregnancy. And the literature here is solid, according to Dr. Singh, who found that 95% of myomectomy patients achieved control of their bleeding symptoms, whether it was via laparoscopy, hysteroscopy, or laparotomy. Up to 40% of women may develop new fibroids, or leiomyomas, within 3 years, although only 12.2% required a second surgery up to after 5 years.
But myomectomy is invasive, requiring general anesthesia, incisions in the uterus, and stitches to close the organ.
Newer techniques have emerged that can effectively treat symptoms of fibroids without requiring surgery. Uterine artery embolization (UAE), which involves passing a catheter into the femoral artery, or laparoscopic uterine artery occlusion can be used to cut off the blood supply of the fibroid. Other techniques, including focused ultrasound surgery and radiofrequency ablation (RFA), use various forms of energy to heat and ablate fibroids. The latter two can be performed in outpatient settings and often without general anesthesia.
Approved for use in 1994, UAE has the most data available, with reduction in the volume of fibroids and uterine tissue lasting up to 5 years, and rates of reintervention of 19%-38% between 2 and 5 years after the procedure. Dr. Singh’s review found that 79%-98.5% of recipients of the procedure reported declines in bleeding that persisted for several years, which is comparable to myomectomy. Quality of life and pain scores also showed good improvement, with follow-up in the different studies ranging from 12 months to over 5 years, the analysis showed.
UAE does have its drawbacks. In rare cases, embolization can deprive the entire uterus and ovaries of blood, which can cause ovarian dysfunction and potentially result in premature menopause, although this outcome is most common in women who are older than 45 years. The procedure can often also be painful enough that overnight hospitalization is required.
Focused ultrasound surgeries, which include magnetic resonance–guided focused ultrasound surgery (MRgFUS) and high-intensity focused ultrasound (HIFU), were approved by the FDA in 2004. Focused ultrasound waves pass through the abdominal wall and produce significant heating, causing a burn that destroys the targeted tissue without damaging surrounding tissue. As with UAE, improvements in fibroid-associated bleeding and measures of quality of life were similar to those after myomectomy up to 3 years later.
But Dr. Singh noted that both focused ultrasound and RFA can damage the skin or internal organs. “[As] always with the thermal interventions, there is the probability of skin as well as internal organs that might get the thermal energy if it’s not focused correctly on to the fibroid itself,” he said. In addition, MRgFUS is not an option for women who are not good candidates to undergo an MRI, such as those with claustrophobia or pacemakers.
Also, with focus ultrasound and RFA, “we do worry about that fibroid getting blood flow back,” which can lead to recurrence of heavy menstrual bleeding, Dr. Laughlin-Tommaso noted.
Although data on RFA are limited to 12 months of follow-up, most women reported meaningful reductions in bleeding symptoms. Longer follow-up has been reported for bleeding symptoms after MRgFUS, with similar results up to 3 years later.
For Leslie Hansen-Lindner, MD, chief of obstetrics and gynecology at Atrium Health in Charlotte, N.C., choosing the right procedure starts with a patient-centered conversation weighing the pros and cons of the options and the woman’s goals.
“Is their goal to reduce the size and impact of their fibroid, bleed less, and have a better quality of life on their period?” Dr. Hansen-Lindner said. “Or is their goal to have the entire fibroid removed?”
If the former, an RFA is appealing to many women. If the latter, laparoscopic or mini-laparotomy myomectomy might be a better choice. Although fewer than 10% of patients require surgical reintervention at 3 years of follow-up for RFA, myomectomy has more consistent long-term evidence showing that fewer women require re-intervention and preserve their fertility, she added.
Age also plays a role in the decision: The closer a woman is to menopause, the less likely she is to experience a recurrence, so a less-invasive procedure is preferable. But for younger women hoping to become pregnant, the lower risk for recurrence and good prognosis for future fertility might sway the choice toward myomectomy.
The first laparoscopic RFA procedures were approved for uterine fibroids in 2012. Dr. Hansen-Lindner is a proponent of transcervical fibroid ablation (TFA), a newer RFA procedure that the FDA approved in 2018. Performed through the cervix, TFA requires no incisions and can generally be done without general anesthesia. Eligible candidates would be any woman with symptomatic fibroids, such as heavy menstrual bleeding, pain, or bulk symptoms. The contraindications are few.
“It’s going to come down to size and location of fibroids, and whether or not they would be accessible by the TFA,” Dr. Hansen-Lindner said. “I have to make sure that there isn’t a fibroid blocking their cervix and that the fibroids are accessible with this device.”
TFA also is not suitable for removing most submucosal lesions, which typically must be removed by hysteroscopic myomectomy. Dr. Hansen-Lindner said that she often uses TFA in conjunction with hysteroscopic myomectomy for this scenario. Although data on pregnancy after RFA (including TFA), MRgFUS, and HIFU are lacking, Gynesonics, the manufacturer of the Sonata System (the device that delivers radiofrequency energy to shrink the fibroid) has documented 79 pregnancies among the 2,200 women who have undergone TFA in the United States since 2018.
Disparities hampering care
Uterine fibroids are a particular problem for Black women, whose symptoms are more likely to be ignored by clinicians, according to Jodie Katon, PhD, a core investigator at the Veterans Affairs Greater Los Angeles Center for the Study of Healthcare Innovation, Implementation and Policy. Dr. Katon cited studies in which Black women interviewed about their experiences reported a consistent theme: Clinicians dismissed their symptoms, told them these were nothing to worry about, and advised them to lose weight. Those interactions not only delayed diagnosis among Black women but also led many of them to mistrust clinicians and avoid the health care system altogether.
The failure of clinicians to take their complaints seriously is just one of the disparities affecting Black women. In reviewing the literature, Dr. Laughlin-Tommaso, who also serves as the associate dean for Education Diversity, Equity, and Inclusion at the Mayo Clinic, found that African American women experience two to three times the risk for fibroids, compared with White women, as well as earlier onset and more severe disease, as measured by number and size of the lesions.
According to Dr. Katon, the etiology of fibroids is still poorly understood. “What we do know is that Black women are disproportionately exposed to a variety of factors that we have shown through observational studies are associated with increased risk of development of uterine fibroids.”
The list includes factors like stress; interpersonal racism; early age at menarche; various indicators of poor diets, such as vitamin D deficiency; the use of certain beauty products, specifically hair straighteners; as well as exposure to air pollution and other environmental toxins.
Laughlin-Tommaso also pointed to historical disparities in management, citing a doubled risk for hysterectomy for Black women in a study published in 2007 despite survey data suggesting that Black women report being more interested in uterine-preserving therapies rather than a hysterectomy.
Breaking down barriers of access to new treatments
Dr. Laughlin-Tommaso looked at more recent trends in the management of fibroids using data from the multicenter COMPARE-UF study, which enrolled women between 2015 and 2020 undergoing fibroid treatment into a longitudinal registry to track their outcomes. She found that Black women underwent hysterectomies at a lower rate than did White women and were instead more likely to undergo myomectomy or UAE.
Some of the change may reflect lack of approved minimally invasive procedures before 2000. “But now that we have expanded options, I think most women are opting not to have a hysterectomy,” Dr. Laughlin-Tommaso said.
Dr. Katon has research funding from the VA to look more closely at racial disparities in the treatment of fibroids. In a study published in April 2023, she reported some surprising trends.
During the period from 2010 to 2018, she found that Black veterans diagnosed with fibroids were less likely than White veterans were to receive treatment, regardless of their age or the severity of their symptoms. This finding held even among women with anemia, which should have been a clear indication for treatment.
But, as in the COMPARE-UF study, the subset of Black veterans who received an interventional treatment were less likely than their White peers were to undergo hysterectomy in favor of a fertility-sparing treatment as their initial procedure. Dr. Katon called it a “welcome but unexpected finding.”
But another significant barrier remains: The two newest types of procedures, RFA and guided focused ultrasound, are not commonly performed outside of tertiary care facilities. However, studies have found that all these procedures are cost effective (studies for myomectomy, UAE, MRgFUS, and TFA). The implementation of a category 1 billing code for laparoscopic RFA in 2017 has led more insurance companies to cover the service, and a category 1 code will be available for TFA effective January 2024.
Although RFA does require investment in specialized equipment, which limits facilities from offering the procedure, any gynecologist who routinely performs hysteroscopy can easily learn to do TFA. And the VA, which is committed to eliminating disparities in women’s health, established a 2-year advanced fellowship in minimally invasive gynecologic surgery in 2022 to help expand their capacity to offer these procedures.
The VA has been rapidly expanding their gynecology services, and Katon said that she is confident that ultrasound-guided procedures and RFA will become more available within the system. “I would say we’re keeping pace. And in some ways, you know, as a national system we may be positioned to actually outpace the rest of the U.S.”
Dr. Segars reported prior research funding for clinical trials from BioSpecifics Technologies, Bayer, Allergan, AbbVie, and ObsEva and currently receives funding from Myovant Sciences. Dr. Hansen-Lindner reported personal fees from Gynesonics. Dr. Singh, Dr. Laughlin-Tommaso, and Dr. Katon reported no financial conflicts of interest.
A version of this article first appeared on Medscape.com.
In 2022, two colleagues from Johns Hopkins University, Baltimore, Bhuchitra Singh, MD, MPH, MS, MBA, and James Segars Jr., MD, reviewed the available literature to evaluate the effectiveness of newer minimally invasive therapies in reducing bleeding and improving the quality of life and control of symptoms linked to uterine fibroids.
Their goal, according to Dr. Segars, a professor of obstetrics and gynecology and director of the division of women’s health research at Johns Hopkins, was to help guide clinicians and patients in making decisions about the use of the newer therapies, including radiofrequency ablation and ultrasound-guided removal of lesions.
But he and Dr. Singh, the director of clinical research at the Howard W. and Georgeanna Seegar Jones Laboratory of Reproductive Sciences and Women’s Health Research, were surprised by their findings. “The outcomes were relatively the same,” Dr. Segars said. “All of the modalities lead to significant reduction in bleeding and other fibroid-related symptoms.”
The data on long-term complications and risk for recurrence are sparse for some of the newer approaches, and not enough high-quality long-term studies have been conducted for the Food and Drug Administration to approve them as fertility-sparing treatments.
But perhaps, the biggest challenge now is to ensure that women can take advantage of these newer therapies, with large gaps in both the diagnosis of fibroids and geographic access to minimally invasive treatments.
A widespread condition widely underdiagnosed
Uterine fibroids occur in most women (the incidence rises with age) and can be found in up to 70% of women by the time they reach menopause. Risk factors include family history, increasing interval since last birth, hypertension, and obesity. Increasing parity and use of oral contraceptives are protective.
But as many as 50% of cases go undiagnosed, and one reason for this is the failure of clinicians to dig deeply enough into women’s menstrual histories to diagnose fibroids.
“The most common cause of anemia is heavy menstrual bleeding,” said Shannon Laughlin-Tommaso, MD, MPH, a professor of obstetrics and gynecology at Mayo Clinic in Rochester, Minn. She frequently sees patients who have already undergone colonoscopy to work-up the source of their anemia before anyone suspects that fibroids are the culprit.
“When women tell us about their periods, what they’ve been told is normal [bleeding] – or what they’ve always had and considered normal – is actually kind of on the heavier spectrum,” she said.
Ideally, treatment for uterine fibroids would fix abnormally prolonged or heavy menstrual bleeding, relieve pain, and ameliorate symptoms associated with an enlarged uterus, such as pelvic pressure, urinary frequency, and constipation. And the fibroids would never recur.
By those measures, hysterectomy fits the bill: Success rates in relieving symptoms are high, and the risk for recurrence is zero. But the procedure carries significant drawbacks: short-term complications of surgery, including infection, bleeding, and injury to the bowels and bladder along with potential long-term risks for cardiovascular disease, cancer, ovarian failure and premature menopause, depression, and decline in cognitive function. Those factors loom even larger for women who still hope to have children.
For that reason, the American College of Obstetricians and Gynecologists recommends myomectomy, or surgical removal of individual fibroids, for women who desire uterine preservation or future pregnancy. And the literature here is solid, according to Dr. Singh, who found that 95% of myomectomy patients achieved control of their bleeding symptoms, whether it was via laparoscopy, hysteroscopy, or laparotomy. Up to 40% of women may develop new fibroids, or leiomyomas, within 3 years, although only 12.2% required a second surgery up to after 5 years.
But myomectomy is invasive, requiring general anesthesia, incisions in the uterus, and stitches to close the organ.
Newer techniques have emerged that can effectively treat symptoms of fibroids without requiring surgery. Uterine artery embolization (UAE), which involves passing a catheter into the femoral artery, or laparoscopic uterine artery occlusion can be used to cut off the blood supply of the fibroid. Other techniques, including focused ultrasound surgery and radiofrequency ablation (RFA), use various forms of energy to heat and ablate fibroids. The latter two can be performed in outpatient settings and often without general anesthesia.
Approved for use in 1994, UAE has the most data available, with reduction in the volume of fibroids and uterine tissue lasting up to 5 years, and rates of reintervention of 19%-38% between 2 and 5 years after the procedure. Dr. Singh’s review found that 79%-98.5% of recipients of the procedure reported declines in bleeding that persisted for several years, which is comparable to myomectomy. Quality of life and pain scores also showed good improvement, with follow-up in the different studies ranging from 12 months to over 5 years, the analysis showed.
UAE does have its drawbacks. In rare cases, embolization can deprive the entire uterus and ovaries of blood, which can cause ovarian dysfunction and potentially result in premature menopause, although this outcome is most common in women who are older than 45 years. The procedure can often also be painful enough that overnight hospitalization is required.
Focused ultrasound surgeries, which include magnetic resonance–guided focused ultrasound surgery (MRgFUS) and high-intensity focused ultrasound (HIFU), were approved by the FDA in 2004. Focused ultrasound waves pass through the abdominal wall and produce significant heating, causing a burn that destroys the targeted tissue without damaging surrounding tissue. As with UAE, improvements in fibroid-associated bleeding and measures of quality of life were similar to those after myomectomy up to 3 years later.
But Dr. Singh noted that both focused ultrasound and RFA can damage the skin or internal organs. “[As] always with the thermal interventions, there is the probability of skin as well as internal organs that might get the thermal energy if it’s not focused correctly on to the fibroid itself,” he said. In addition, MRgFUS is not an option for women who are not good candidates to undergo an MRI, such as those with claustrophobia or pacemakers.
Also, with focus ultrasound and RFA, “we do worry about that fibroid getting blood flow back,” which can lead to recurrence of heavy menstrual bleeding, Dr. Laughlin-Tommaso noted.
Although data on RFA are limited to 12 months of follow-up, most women reported meaningful reductions in bleeding symptoms. Longer follow-up has been reported for bleeding symptoms after MRgFUS, with similar results up to 3 years later.
For Leslie Hansen-Lindner, MD, chief of obstetrics and gynecology at Atrium Health in Charlotte, N.C., choosing the right procedure starts with a patient-centered conversation weighing the pros and cons of the options and the woman’s goals.
“Is their goal to reduce the size and impact of their fibroid, bleed less, and have a better quality of life on their period?” Dr. Hansen-Lindner said. “Or is their goal to have the entire fibroid removed?”
If the former, an RFA is appealing to many women. If the latter, laparoscopic or mini-laparotomy myomectomy might be a better choice. Although fewer than 10% of patients require surgical reintervention at 3 years of follow-up for RFA, myomectomy has more consistent long-term evidence showing that fewer women require re-intervention and preserve their fertility, she added.
Age also plays a role in the decision: The closer a woman is to menopause, the less likely she is to experience a recurrence, so a less-invasive procedure is preferable. But for younger women hoping to become pregnant, the lower risk for recurrence and good prognosis for future fertility might sway the choice toward myomectomy.
The first laparoscopic RFA procedures were approved for uterine fibroids in 2012. Dr. Hansen-Lindner is a proponent of transcervical fibroid ablation (TFA), a newer RFA procedure that the FDA approved in 2018. Performed through the cervix, TFA requires no incisions and can generally be done without general anesthesia. Eligible candidates would be any woman with symptomatic fibroids, such as heavy menstrual bleeding, pain, or bulk symptoms. The contraindications are few.
“It’s going to come down to size and location of fibroids, and whether or not they would be accessible by the TFA,” Dr. Hansen-Lindner said. “I have to make sure that there isn’t a fibroid blocking their cervix and that the fibroids are accessible with this device.”
TFA also is not suitable for removing most submucosal lesions, which typically must be removed by hysteroscopic myomectomy. Dr. Hansen-Lindner said that she often uses TFA in conjunction with hysteroscopic myomectomy for this scenario. Although data on pregnancy after RFA (including TFA), MRgFUS, and HIFU are lacking, Gynesonics, the manufacturer of the Sonata System (the device that delivers radiofrequency energy to shrink the fibroid) has documented 79 pregnancies among the 2,200 women who have undergone TFA in the United States since 2018.
Disparities hampering care
Uterine fibroids are a particular problem for Black women, whose symptoms are more likely to be ignored by clinicians, according to Jodie Katon, PhD, a core investigator at the Veterans Affairs Greater Los Angeles Center for the Study of Healthcare Innovation, Implementation and Policy. Dr. Katon cited studies in which Black women interviewed about their experiences reported a consistent theme: Clinicians dismissed their symptoms, told them these were nothing to worry about, and advised them to lose weight. Those interactions not only delayed diagnosis among Black women but also led many of them to mistrust clinicians and avoid the health care system altogether.
The failure of clinicians to take their complaints seriously is just one of the disparities affecting Black women. In reviewing the literature, Dr. Laughlin-Tommaso, who also serves as the associate dean for Education Diversity, Equity, and Inclusion at the Mayo Clinic, found that African American women experience two to three times the risk for fibroids, compared with White women, as well as earlier onset and more severe disease, as measured by number and size of the lesions.
According to Dr. Katon, the etiology of fibroids is still poorly understood. “What we do know is that Black women are disproportionately exposed to a variety of factors that we have shown through observational studies are associated with increased risk of development of uterine fibroids.”
The list includes factors like stress; interpersonal racism; early age at menarche; various indicators of poor diets, such as vitamin D deficiency; the use of certain beauty products, specifically hair straighteners; as well as exposure to air pollution and other environmental toxins.
Laughlin-Tommaso also pointed to historical disparities in management, citing a doubled risk for hysterectomy for Black women in a study published in 2007 despite survey data suggesting that Black women report being more interested in uterine-preserving therapies rather than a hysterectomy.
Breaking down barriers of access to new treatments
Dr. Laughlin-Tommaso looked at more recent trends in the management of fibroids using data from the multicenter COMPARE-UF study, which enrolled women between 2015 and 2020 undergoing fibroid treatment into a longitudinal registry to track their outcomes. She found that Black women underwent hysterectomies at a lower rate than did White women and were instead more likely to undergo myomectomy or UAE.
Some of the change may reflect lack of approved minimally invasive procedures before 2000. “But now that we have expanded options, I think most women are opting not to have a hysterectomy,” Dr. Laughlin-Tommaso said.
Dr. Katon has research funding from the VA to look more closely at racial disparities in the treatment of fibroids. In a study published in April 2023, she reported some surprising trends.
During the period from 2010 to 2018, she found that Black veterans diagnosed with fibroids were less likely than White veterans were to receive treatment, regardless of their age or the severity of their symptoms. This finding held even among women with anemia, which should have been a clear indication for treatment.
But, as in the COMPARE-UF study, the subset of Black veterans who received an interventional treatment were less likely than their White peers were to undergo hysterectomy in favor of a fertility-sparing treatment as their initial procedure. Dr. Katon called it a “welcome but unexpected finding.”
But another significant barrier remains: The two newest types of procedures, RFA and guided focused ultrasound, are not commonly performed outside of tertiary care facilities. However, studies have found that all these procedures are cost effective (studies for myomectomy, UAE, MRgFUS, and TFA). The implementation of a category 1 billing code for laparoscopic RFA in 2017 has led more insurance companies to cover the service, and a category 1 code will be available for TFA effective January 2024.
Although RFA does require investment in specialized equipment, which limits facilities from offering the procedure, any gynecologist who routinely performs hysteroscopy can easily learn to do TFA. And the VA, which is committed to eliminating disparities in women’s health, established a 2-year advanced fellowship in minimally invasive gynecologic surgery in 2022 to help expand their capacity to offer these procedures.
The VA has been rapidly expanding their gynecology services, and Katon said that she is confident that ultrasound-guided procedures and RFA will become more available within the system. “I would say we’re keeping pace. And in some ways, you know, as a national system we may be positioned to actually outpace the rest of the U.S.”
Dr. Segars reported prior research funding for clinical trials from BioSpecifics Technologies, Bayer, Allergan, AbbVie, and ObsEva and currently receives funding from Myovant Sciences. Dr. Hansen-Lindner reported personal fees from Gynesonics. Dr. Singh, Dr. Laughlin-Tommaso, and Dr. Katon reported no financial conflicts of interest.
A version of this article first appeared on Medscape.com.
In 2022, two colleagues from Johns Hopkins University, Baltimore, Bhuchitra Singh, MD, MPH, MS, MBA, and James Segars Jr., MD, reviewed the available literature to evaluate the effectiveness of newer minimally invasive therapies in reducing bleeding and improving the quality of life and control of symptoms linked to uterine fibroids.
Their goal, according to Dr. Segars, a professor of obstetrics and gynecology and director of the division of women’s health research at Johns Hopkins, was to help guide clinicians and patients in making decisions about the use of the newer therapies, including radiofrequency ablation and ultrasound-guided removal of lesions.
But he and Dr. Singh, the director of clinical research at the Howard W. and Georgeanna Seegar Jones Laboratory of Reproductive Sciences and Women’s Health Research, were surprised by their findings. “The outcomes were relatively the same,” Dr. Segars said. “All of the modalities lead to significant reduction in bleeding and other fibroid-related symptoms.”
The data on long-term complications and risk for recurrence are sparse for some of the newer approaches, and not enough high-quality long-term studies have been conducted for the Food and Drug Administration to approve them as fertility-sparing treatments.
But perhaps, the biggest challenge now is to ensure that women can take advantage of these newer therapies, with large gaps in both the diagnosis of fibroids and geographic access to minimally invasive treatments.
A widespread condition widely underdiagnosed
Uterine fibroids occur in most women (the incidence rises with age) and can be found in up to 70% of women by the time they reach menopause. Risk factors include family history, increasing interval since last birth, hypertension, and obesity. Increasing parity and use of oral contraceptives are protective.
But as many as 50% of cases go undiagnosed, and one reason for this is the failure of clinicians to dig deeply enough into women’s menstrual histories to diagnose fibroids.
“The most common cause of anemia is heavy menstrual bleeding,” said Shannon Laughlin-Tommaso, MD, MPH, a professor of obstetrics and gynecology at Mayo Clinic in Rochester, Minn. She frequently sees patients who have already undergone colonoscopy to work-up the source of their anemia before anyone suspects that fibroids are the culprit.
“When women tell us about their periods, what they’ve been told is normal [bleeding] – or what they’ve always had and considered normal – is actually kind of on the heavier spectrum,” she said.
Ideally, treatment for uterine fibroids would fix abnormally prolonged or heavy menstrual bleeding, relieve pain, and ameliorate symptoms associated with an enlarged uterus, such as pelvic pressure, urinary frequency, and constipation. And the fibroids would never recur.
By those measures, hysterectomy fits the bill: Success rates in relieving symptoms are high, and the risk for recurrence is zero. But the procedure carries significant drawbacks: short-term complications of surgery, including infection, bleeding, and injury to the bowels and bladder along with potential long-term risks for cardiovascular disease, cancer, ovarian failure and premature menopause, depression, and decline in cognitive function. Those factors loom even larger for women who still hope to have children.
For that reason, the American College of Obstetricians and Gynecologists recommends myomectomy, or surgical removal of individual fibroids, for women who desire uterine preservation or future pregnancy. And the literature here is solid, according to Dr. Singh, who found that 95% of myomectomy patients achieved control of their bleeding symptoms, whether it was via laparoscopy, hysteroscopy, or laparotomy. Up to 40% of women may develop new fibroids, or leiomyomas, within 3 years, although only 12.2% required a second surgery up to after 5 years.
But myomectomy is invasive, requiring general anesthesia, incisions in the uterus, and stitches to close the organ.
Newer techniques have emerged that can effectively treat symptoms of fibroids without requiring surgery. Uterine artery embolization (UAE), which involves passing a catheter into the femoral artery, or laparoscopic uterine artery occlusion can be used to cut off the blood supply of the fibroid. Other techniques, including focused ultrasound surgery and radiofrequency ablation (RFA), use various forms of energy to heat and ablate fibroids. The latter two can be performed in outpatient settings and often without general anesthesia.
Approved for use in 1994, UAE has the most data available, with reduction in the volume of fibroids and uterine tissue lasting up to 5 years, and rates of reintervention of 19%-38% between 2 and 5 years after the procedure. Dr. Singh’s review found that 79%-98.5% of recipients of the procedure reported declines in bleeding that persisted for several years, which is comparable to myomectomy. Quality of life and pain scores also showed good improvement, with follow-up in the different studies ranging from 12 months to over 5 years, the analysis showed.
UAE does have its drawbacks. In rare cases, embolization can deprive the entire uterus and ovaries of blood, which can cause ovarian dysfunction and potentially result in premature menopause, although this outcome is most common in women who are older than 45 years. The procedure can often also be painful enough that overnight hospitalization is required.
Focused ultrasound surgeries, which include magnetic resonance–guided focused ultrasound surgery (MRgFUS) and high-intensity focused ultrasound (HIFU), were approved by the FDA in 2004. Focused ultrasound waves pass through the abdominal wall and produce significant heating, causing a burn that destroys the targeted tissue without damaging surrounding tissue. As with UAE, improvements in fibroid-associated bleeding and measures of quality of life were similar to those after myomectomy up to 3 years later.
But Dr. Singh noted that both focused ultrasound and RFA can damage the skin or internal organs. “[As] always with the thermal interventions, there is the probability of skin as well as internal organs that might get the thermal energy if it’s not focused correctly on to the fibroid itself,” he said. In addition, MRgFUS is not an option for women who are not good candidates to undergo an MRI, such as those with claustrophobia or pacemakers.
Also, with focus ultrasound and RFA, “we do worry about that fibroid getting blood flow back,” which can lead to recurrence of heavy menstrual bleeding, Dr. Laughlin-Tommaso noted.
Although data on RFA are limited to 12 months of follow-up, most women reported meaningful reductions in bleeding symptoms. Longer follow-up has been reported for bleeding symptoms after MRgFUS, with similar results up to 3 years later.
For Leslie Hansen-Lindner, MD, chief of obstetrics and gynecology at Atrium Health in Charlotte, N.C., choosing the right procedure starts with a patient-centered conversation weighing the pros and cons of the options and the woman’s goals.
“Is their goal to reduce the size and impact of their fibroid, bleed less, and have a better quality of life on their period?” Dr. Hansen-Lindner said. “Or is their goal to have the entire fibroid removed?”
If the former, an RFA is appealing to many women. If the latter, laparoscopic or mini-laparotomy myomectomy might be a better choice. Although fewer than 10% of patients require surgical reintervention at 3 years of follow-up for RFA, myomectomy has more consistent long-term evidence showing that fewer women require re-intervention and preserve their fertility, she added.
Age also plays a role in the decision: The closer a woman is to menopause, the less likely she is to experience a recurrence, so a less-invasive procedure is preferable. But for younger women hoping to become pregnant, the lower risk for recurrence and good prognosis for future fertility might sway the choice toward myomectomy.
The first laparoscopic RFA procedures were approved for uterine fibroids in 2012. Dr. Hansen-Lindner is a proponent of transcervical fibroid ablation (TFA), a newer RFA procedure that the FDA approved in 2018. Performed through the cervix, TFA requires no incisions and can generally be done without general anesthesia. Eligible candidates would be any woman with symptomatic fibroids, such as heavy menstrual bleeding, pain, or bulk symptoms. The contraindications are few.
“It’s going to come down to size and location of fibroids, and whether or not they would be accessible by the TFA,” Dr. Hansen-Lindner said. “I have to make sure that there isn’t a fibroid blocking their cervix and that the fibroids are accessible with this device.”
TFA also is not suitable for removing most submucosal lesions, which typically must be removed by hysteroscopic myomectomy. Dr. Hansen-Lindner said that she often uses TFA in conjunction with hysteroscopic myomectomy for this scenario. Although data on pregnancy after RFA (including TFA), MRgFUS, and HIFU are lacking, Gynesonics, the manufacturer of the Sonata System (the device that delivers radiofrequency energy to shrink the fibroid) has documented 79 pregnancies among the 2,200 women who have undergone TFA in the United States since 2018.
Disparities hampering care
Uterine fibroids are a particular problem for Black women, whose symptoms are more likely to be ignored by clinicians, according to Jodie Katon, PhD, a core investigator at the Veterans Affairs Greater Los Angeles Center for the Study of Healthcare Innovation, Implementation and Policy. Dr. Katon cited studies in which Black women interviewed about their experiences reported a consistent theme: Clinicians dismissed their symptoms, told them these were nothing to worry about, and advised them to lose weight. Those interactions not only delayed diagnosis among Black women but also led many of them to mistrust clinicians and avoid the health care system altogether.
The failure of clinicians to take their complaints seriously is just one of the disparities affecting Black women. In reviewing the literature, Dr. Laughlin-Tommaso, who also serves as the associate dean for Education Diversity, Equity, and Inclusion at the Mayo Clinic, found that African American women experience two to three times the risk for fibroids, compared with White women, as well as earlier onset and more severe disease, as measured by number and size of the lesions.
According to Dr. Katon, the etiology of fibroids is still poorly understood. “What we do know is that Black women are disproportionately exposed to a variety of factors that we have shown through observational studies are associated with increased risk of development of uterine fibroids.”
The list includes factors like stress; interpersonal racism; early age at menarche; various indicators of poor diets, such as vitamin D deficiency; the use of certain beauty products, specifically hair straighteners; as well as exposure to air pollution and other environmental toxins.
Laughlin-Tommaso also pointed to historical disparities in management, citing a doubled risk for hysterectomy for Black women in a study published in 2007 despite survey data suggesting that Black women report being more interested in uterine-preserving therapies rather than a hysterectomy.
Breaking down barriers of access to new treatments
Dr. Laughlin-Tommaso looked at more recent trends in the management of fibroids using data from the multicenter COMPARE-UF study, which enrolled women between 2015 and 2020 undergoing fibroid treatment into a longitudinal registry to track their outcomes. She found that Black women underwent hysterectomies at a lower rate than did White women and were instead more likely to undergo myomectomy or UAE.
Some of the change may reflect lack of approved minimally invasive procedures before 2000. “But now that we have expanded options, I think most women are opting not to have a hysterectomy,” Dr. Laughlin-Tommaso said.
Dr. Katon has research funding from the VA to look more closely at racial disparities in the treatment of fibroids. In a study published in April 2023, she reported some surprising trends.
During the period from 2010 to 2018, she found that Black veterans diagnosed with fibroids were less likely than White veterans were to receive treatment, regardless of their age or the severity of their symptoms. This finding held even among women with anemia, which should have been a clear indication for treatment.
But, as in the COMPARE-UF study, the subset of Black veterans who received an interventional treatment were less likely than their White peers were to undergo hysterectomy in favor of a fertility-sparing treatment as their initial procedure. Dr. Katon called it a “welcome but unexpected finding.”
But another significant barrier remains: The two newest types of procedures, RFA and guided focused ultrasound, are not commonly performed outside of tertiary care facilities. However, studies have found that all these procedures are cost effective (studies for myomectomy, UAE, MRgFUS, and TFA). The implementation of a category 1 billing code for laparoscopic RFA in 2017 has led more insurance companies to cover the service, and a category 1 code will be available for TFA effective January 2024.
Although RFA does require investment in specialized equipment, which limits facilities from offering the procedure, any gynecologist who routinely performs hysteroscopy can easily learn to do TFA. And the VA, which is committed to eliminating disparities in women’s health, established a 2-year advanced fellowship in minimally invasive gynecologic surgery in 2022 to help expand their capacity to offer these procedures.
The VA has been rapidly expanding their gynecology services, and Katon said that she is confident that ultrasound-guided procedures and RFA will become more available within the system. “I would say we’re keeping pace. And in some ways, you know, as a national system we may be positioned to actually outpace the rest of the U.S.”
Dr. Segars reported prior research funding for clinical trials from BioSpecifics Technologies, Bayer, Allergan, AbbVie, and ObsEva and currently receives funding from Myovant Sciences. Dr. Hansen-Lindner reported personal fees from Gynesonics. Dr. Singh, Dr. Laughlin-Tommaso, and Dr. Katon reported no financial conflicts of interest.
A version of this article first appeared on Medscape.com.
Fibroids: Growing management options for a prevalent problem
OBG Manag. 33(12). | doi 10.12788/obgm.0169
Fibroids: Is surgery the only management approach?
Two chronic gynecologic conditions notably affect a woman’s quality of life (QoL), including fertility – one is endometriosis, and the other is a fibroid uterus. For a benign tumor, fibroids have an impressive prevalence found in approximately 50%-60% of women during their reproductive years. By menopause, it is estimated that 70% of woman have a fibroid, yet the true incidence is unknown given that only 25% of women experience symptoms bothersome enough to warrant intervention. This month’s article reviews the burden of fibroids and the latest management options that may potentially avoid surgery.
Background
Fibroids are monoclonal tumors of uterine smooth muscle that originate from the myometrium. Risk factors include family history, being premenopausal, increasing time since last delivery, obesity, and hypertension (ACOG Practice Bulletin no. 228 Jun 2021: Obstet Gynecol. 2021 Jun 1;137[6]:e100-e15) but oral hormonal contraception, depot medroxyprogesterone acetate (MPA), and increased parity reduce the risk of fibroids. Compared with White women, Black women have a 2-3 times higher prevalence of fibroids, develop them at a younger age, and present with larger fibroids.
The FIGO leiomyoma classification is the agreed upon system for identifying fibroid location. Symptoms are all too familiar to gynecologists, with life-threatening hemorrhage with severe anemia being the most feared, particularly for FIGO types 1-5. Transvaginal ultrasound is the simplest imaging tool for evaluation.

Fibroids and fertility
Fibroids can impair fertility in several ways: alteration of local anatomy, including the detrimental effects of abnormal uterine bleeding; functional changes by increasing uterine contractions and impairing endometrium and myometrial blood supply; and changes to the local hormonal environment that could impair egg/sperm transport, or embryo implantation (Hum Reprod Update. 2017;22:665-86).
Prior to consideration of surgery, saline infusion sonogram can determine the degree of impact on the endometrium, which is most applicable to the infertility patient, but can also allow guidance toward the appropriate surgical approach.
Treatment options – medical
Management of fibroids is based on a woman’s age, desire for fertility, symptoms, and location of the fibroid(s). Expectant observation of a woman with fibroids may be a reasonable approach, provided the lack of symptoms impairing QoL and of anemia. Typically, there is no change in fibroid size during the short term, considered less than 1 year. Regarding fertility, studies are heterogeneous so there is no definitive conclusion that fibroids impair natural fertility (Reprod Biomed Online. 2021;43:100-10). Spontaneous regression, defined by a reduction in fibroid volume of greater than 20%, has been noted to occur in 7.0% of fibroids (Curr Obstet Gynecol Rep. 2018;7[3]:117-21).
When fertility is not desired, medical management of fibroids is the initial conservative approach. GnRH agonists have been utilized for temporary relief of menometrorrhagia because of fibroids and to reduce their volume, particularly preoperatively. However, extended treatment can induce bone mineral density loss. Add-back therapy (tibolone, raloxifene, estriol, and ipriflavone) is of value in reducing bone loss while MPA and tibolone may manage vasomotor symptoms. More recently, the use of a GnRH antagonist (elagolix) along with add-back therapy has been approved for up to 24 months by the Food and Drug Administration and has demonstrated a more than 50% amenorrhea rate at 12 months (Obstet Gynecol. 2020;135:1313-26).
Progesterone plays an important role in fibroid growth, but the mechanism is unclear. Although not FDA approved, selective progesterone receptor modulators (SPRM) act directly on fibroid size reduction at the level of the pituitary to induce amenorrhea through inhibition of ovulation. Also, more than one course of SPRMs can provide benefit for bleeding control and volume reduction. The SPRM ulipristal acetate for four courses of 3 months demonstrated 73.5% of patients experienced a fibroid volume reduction of greater than 25% and were amenorrheic (Fertil Steril. 2017;108:416-25). GnRH agonists or SPRMs may benefit women if the fibroid is larger than 3 cm or anemia exists, thereby precluding immediate surgery.
Other medication options include the levonorgestrel IUD, combined hormonal contraceptives, and tranexamic acid – all of which have limited data on effective results of treating abnormal uterine bleeding.
Treatment options – surgical
Fibroids are the most common reason for hysterectomy as they are the contributing indication in approximately one-third of surgeries. When future fertility is desired, current surgical options include hysteroscopic and laparoscopic (including robotic) myomectomy. Hysteroscopy is the standard approach for FIGO type 1 fibroids and can also manage some type 2 fibroids provided they are less than 3 cm and the latter is greater than 5 mm from the serosa. Type 2 fibroids may benefit from a “two-step” removal to allow the myometrium to contract and extrude the fibroid. In light of the risk of fluid overload with nonelectrolyte solutions that enable the use of monopolar cautery, many procedures are now performed with bipolar cautery or morcellators.
Laparoscopy (including robotic) has outcomes similar to those of laparotomy although the risk of uterine rupture with the former requires careful attention to thorough closure of the myometrial defect. Robotic myomectomy has outcomes similar to those of standard laparoscopy with less blood loss, but operating times may be prolonged (Best Pract Res Clin Obstet Gynaecol. 2018;46:113-9).
The rate of myomectomy is reported to be 9.2 per 10,000 woman-years in Black women and 1.3 per 10,000 woman years in White women (Fertil Steril 2017;108;416-25). The rate of recurrence after myomectomy can be as great as 60% when patients are followed up to 5 years. Intramural fibroids greater than 2.85 cm and not distorting the uterine cavity may decrease in vitro fertilization (IVF) success (Fertil Steril 2014;101:716-21).
Noninvasive treatment modalities
Uterine artery embolization (UAE) is the most popular minimally invasive alternative to surgical myomectomy. Risks include postembolization syndrome (pain, fever, nausea, leukocytosis, and occasionally malaise), infection, and damage to fertility. Rarely, loss of ovarian function can occur, particularly in women above age 45. Because of the disruption of uterine blood flow, UAE increases the risk of accelerating ovarian aging and infertility as well as atrophic endometrium. In addition, pregnancy complications are increased including miscarriage, preterm labor, and postpartum hemorrhage. There is debate regarding the need for cesarean section at time of delivery given the potential for weakening of the uterine wall following UAE.
High-intensity focused ultrasound (HIFU) is guided by ultrasound or MRI and involves a high-energy-density ultrasound wave passing through the skin. The wave is absorbed and transformed into heat, causing the tissue protein to coagulate, and to be absorbed by the body. The procedure is scarless, carries a minimal risk of infection, and offers less pain compared with traditional approaches. However, HIFU is time consuming, and skin burns and unintentional tissue injury are a risk. A meta-analysis demonstrated improved symptoms of fibroids at 6 and 12 months (J Min Invasive Gynecol. 2021 in press).
Ultrasound-guided microwave ablation (MWA) uses an ablative electrode that is directly inserted into the target tissue via transcutaneous or transcervical approach via ultrasound guidance using microwave to produce heat for tissue coagulation necrosis. The advantages of MWA compared with HIFU and RFA are a higher tissue temperature, larger ablation volume, shorter operating time, less pain and no adverse major events (J Min Invasive Gynecol. 2021, in press).
Conclusion
The current literature cannot conclude that fibroids reduce the likelihood of achieving pregnancy with or without fertility treatment, based on a specific size, number, or location (not including submucosal or cavity-distorting intramural fibroids). Definitive evidence on the efficacy of myomectomy to improve fertility remains limited. Hysteroscopic myomectomy presumably improves pregnancy rates, but there is uncertainty as to its role in reducing miscarriage. Novel nonsurgical modalities are available and are expected to continue being developed but clarity on fertility outcomes is needed.
Dr. Trolice is director of Fertility CARE – The IVF Center in Winter Park, Fla., and professor of obstetrics and gynecology at the University of Central Florida, Orlando. He has no conflicts of interests. Please contact him at obnews@mdedge.com.
Two chronic gynecologic conditions notably affect a woman’s quality of life (QoL), including fertility – one is endometriosis, and the other is a fibroid uterus. For a benign tumor, fibroids have an impressive prevalence found in approximately 50%-60% of women during their reproductive years. By menopause, it is estimated that 70% of woman have a fibroid, yet the true incidence is unknown given that only 25% of women experience symptoms bothersome enough to warrant intervention. This month’s article reviews the burden of fibroids and the latest management options that may potentially avoid surgery.
Background
Fibroids are monoclonal tumors of uterine smooth muscle that originate from the myometrium. Risk factors include family history, being premenopausal, increasing time since last delivery, obesity, and hypertension (ACOG Practice Bulletin no. 228 Jun 2021: Obstet Gynecol. 2021 Jun 1;137[6]:e100-e15) but oral hormonal contraception, depot medroxyprogesterone acetate (MPA), and increased parity reduce the risk of fibroids. Compared with White women, Black women have a 2-3 times higher prevalence of fibroids, develop them at a younger age, and present with larger fibroids.
The FIGO leiomyoma classification is the agreed upon system for identifying fibroid location. Symptoms are all too familiar to gynecologists, with life-threatening hemorrhage with severe anemia being the most feared, particularly for FIGO types 1-5. Transvaginal ultrasound is the simplest imaging tool for evaluation.

Fibroids and fertility
Fibroids can impair fertility in several ways: alteration of local anatomy, including the detrimental effects of abnormal uterine bleeding; functional changes by increasing uterine contractions and impairing endometrium and myometrial blood supply; and changes to the local hormonal environment that could impair egg/sperm transport, or embryo implantation (Hum Reprod Update. 2017;22:665-86).
Prior to consideration of surgery, saline infusion sonogram can determine the degree of impact on the endometrium, which is most applicable to the infertility patient, but can also allow guidance toward the appropriate surgical approach.
Treatment options – medical
Management of fibroids is based on a woman’s age, desire for fertility, symptoms, and location of the fibroid(s). Expectant observation of a woman with fibroids may be a reasonable approach, provided the lack of symptoms impairing QoL and of anemia. Typically, there is no change in fibroid size during the short term, considered less than 1 year. Regarding fertility, studies are heterogeneous so there is no definitive conclusion that fibroids impair natural fertility (Reprod Biomed Online. 2021;43:100-10). Spontaneous regression, defined by a reduction in fibroid volume of greater than 20%, has been noted to occur in 7.0% of fibroids (Curr Obstet Gynecol Rep. 2018;7[3]:117-21).
When fertility is not desired, medical management of fibroids is the initial conservative approach. GnRH agonists have been utilized for temporary relief of menometrorrhagia because of fibroids and to reduce their volume, particularly preoperatively. However, extended treatment can induce bone mineral density loss. Add-back therapy (tibolone, raloxifene, estriol, and ipriflavone) is of value in reducing bone loss while MPA and tibolone may manage vasomotor symptoms. More recently, the use of a GnRH antagonist (elagolix) along with add-back therapy has been approved for up to 24 months by the Food and Drug Administration and has demonstrated a more than 50% amenorrhea rate at 12 months (Obstet Gynecol. 2020;135:1313-26).
Progesterone plays an important role in fibroid growth, but the mechanism is unclear. Although not FDA approved, selective progesterone receptor modulators (SPRM) act directly on fibroid size reduction at the level of the pituitary to induce amenorrhea through inhibition of ovulation. Also, more than one course of SPRMs can provide benefit for bleeding control and volume reduction. The SPRM ulipristal acetate for four courses of 3 months demonstrated 73.5% of patients experienced a fibroid volume reduction of greater than 25% and were amenorrheic (Fertil Steril. 2017;108:416-25). GnRH agonists or SPRMs may benefit women if the fibroid is larger than 3 cm or anemia exists, thereby precluding immediate surgery.
Other medication options include the levonorgestrel IUD, combined hormonal contraceptives, and tranexamic acid – all of which have limited data on effective results of treating abnormal uterine bleeding.
Treatment options – surgical
Fibroids are the most common reason for hysterectomy as they are the contributing indication in approximately one-third of surgeries. When future fertility is desired, current surgical options include hysteroscopic and laparoscopic (including robotic) myomectomy. Hysteroscopy is the standard approach for FIGO type 1 fibroids and can also manage some type 2 fibroids provided they are less than 3 cm and the latter is greater than 5 mm from the serosa. Type 2 fibroids may benefit from a “two-step” removal to allow the myometrium to contract and extrude the fibroid. In light of the risk of fluid overload with nonelectrolyte solutions that enable the use of monopolar cautery, many procedures are now performed with bipolar cautery or morcellators.
Laparoscopy (including robotic) has outcomes similar to those of laparotomy although the risk of uterine rupture with the former requires careful attention to thorough closure of the myometrial defect. Robotic myomectomy has outcomes similar to those of standard laparoscopy with less blood loss, but operating times may be prolonged (Best Pract Res Clin Obstet Gynaecol. 2018;46:113-9).
The rate of myomectomy is reported to be 9.2 per 10,000 woman-years in Black women and 1.3 per 10,000 woman years in White women (Fertil Steril 2017;108;416-25). The rate of recurrence after myomectomy can be as great as 60% when patients are followed up to 5 years. Intramural fibroids greater than 2.85 cm and not distorting the uterine cavity may decrease in vitro fertilization (IVF) success (Fertil Steril 2014;101:716-21).
Noninvasive treatment modalities
Uterine artery embolization (UAE) is the most popular minimally invasive alternative to surgical myomectomy. Risks include postembolization syndrome (pain, fever, nausea, leukocytosis, and occasionally malaise), infection, and damage to fertility. Rarely, loss of ovarian function can occur, particularly in women above age 45. Because of the disruption of uterine blood flow, UAE increases the risk of accelerating ovarian aging and infertility as well as atrophic endometrium. In addition, pregnancy complications are increased including miscarriage, preterm labor, and postpartum hemorrhage. There is debate regarding the need for cesarean section at time of delivery given the potential for weakening of the uterine wall following UAE.
High-intensity focused ultrasound (HIFU) is guided by ultrasound or MRI and involves a high-energy-density ultrasound wave passing through the skin. The wave is absorbed and transformed into heat, causing the tissue protein to coagulate, and to be absorbed by the body. The procedure is scarless, carries a minimal risk of infection, and offers less pain compared with traditional approaches. However, HIFU is time consuming, and skin burns and unintentional tissue injury are a risk. A meta-analysis demonstrated improved symptoms of fibroids at 6 and 12 months (J Min Invasive Gynecol. 2021 in press).
Ultrasound-guided microwave ablation (MWA) uses an ablative electrode that is directly inserted into the target tissue via transcutaneous or transcervical approach via ultrasound guidance using microwave to produce heat for tissue coagulation necrosis. The advantages of MWA compared with HIFU and RFA are a higher tissue temperature, larger ablation volume, shorter operating time, less pain and no adverse major events (J Min Invasive Gynecol. 2021, in press).
Conclusion
The current literature cannot conclude that fibroids reduce the likelihood of achieving pregnancy with or without fertility treatment, based on a specific size, number, or location (not including submucosal or cavity-distorting intramural fibroids). Definitive evidence on the efficacy of myomectomy to improve fertility remains limited. Hysteroscopic myomectomy presumably improves pregnancy rates, but there is uncertainty as to its role in reducing miscarriage. Novel nonsurgical modalities are available and are expected to continue being developed but clarity on fertility outcomes is needed.
Dr. Trolice is director of Fertility CARE – The IVF Center in Winter Park, Fla., and professor of obstetrics and gynecology at the University of Central Florida, Orlando. He has no conflicts of interests. Please contact him at obnews@mdedge.com.
Two chronic gynecologic conditions notably affect a woman’s quality of life (QoL), including fertility – one is endometriosis, and the other is a fibroid uterus. For a benign tumor, fibroids have an impressive prevalence found in approximately 50%-60% of women during their reproductive years. By menopause, it is estimated that 70% of woman have a fibroid, yet the true incidence is unknown given that only 25% of women experience symptoms bothersome enough to warrant intervention. This month’s article reviews the burden of fibroids and the latest management options that may potentially avoid surgery.
Background
Fibroids are monoclonal tumors of uterine smooth muscle that originate from the myometrium. Risk factors include family history, being premenopausal, increasing time since last delivery, obesity, and hypertension (ACOG Practice Bulletin no. 228 Jun 2021: Obstet Gynecol. 2021 Jun 1;137[6]:e100-e15) but oral hormonal contraception, depot medroxyprogesterone acetate (MPA), and increased parity reduce the risk of fibroids. Compared with White women, Black women have a 2-3 times higher prevalence of fibroids, develop them at a younger age, and present with larger fibroids.
The FIGO leiomyoma classification is the agreed upon system for identifying fibroid location. Symptoms are all too familiar to gynecologists, with life-threatening hemorrhage with severe anemia being the most feared, particularly for FIGO types 1-5. Transvaginal ultrasound is the simplest imaging tool for evaluation.

Fibroids and fertility
Fibroids can impair fertility in several ways: alteration of local anatomy, including the detrimental effects of abnormal uterine bleeding; functional changes by increasing uterine contractions and impairing endometrium and myometrial blood supply; and changes to the local hormonal environment that could impair egg/sperm transport, or embryo implantation (Hum Reprod Update. 2017;22:665-86).
Prior to consideration of surgery, saline infusion sonogram can determine the degree of impact on the endometrium, which is most applicable to the infertility patient, but can also allow guidance toward the appropriate surgical approach.
Treatment options – medical
Management of fibroids is based on a woman’s age, desire for fertility, symptoms, and location of the fibroid(s). Expectant observation of a woman with fibroids may be a reasonable approach, provided the lack of symptoms impairing QoL and of anemia. Typically, there is no change in fibroid size during the short term, considered less than 1 year. Regarding fertility, studies are heterogeneous so there is no definitive conclusion that fibroids impair natural fertility (Reprod Biomed Online. 2021;43:100-10). Spontaneous regression, defined by a reduction in fibroid volume of greater than 20%, has been noted to occur in 7.0% of fibroids (Curr Obstet Gynecol Rep. 2018;7[3]:117-21).
When fertility is not desired, medical management of fibroids is the initial conservative approach. GnRH agonists have been utilized for temporary relief of menometrorrhagia because of fibroids and to reduce their volume, particularly preoperatively. However, extended treatment can induce bone mineral density loss. Add-back therapy (tibolone, raloxifene, estriol, and ipriflavone) is of value in reducing bone loss while MPA and tibolone may manage vasomotor symptoms. More recently, the use of a GnRH antagonist (elagolix) along with add-back therapy has been approved for up to 24 months by the Food and Drug Administration and has demonstrated a more than 50% amenorrhea rate at 12 months (Obstet Gynecol. 2020;135:1313-26).
Progesterone plays an important role in fibroid growth, but the mechanism is unclear. Although not FDA approved, selective progesterone receptor modulators (SPRM) act directly on fibroid size reduction at the level of the pituitary to induce amenorrhea through inhibition of ovulation. Also, more than one course of SPRMs can provide benefit for bleeding control and volume reduction. The SPRM ulipristal acetate for four courses of 3 months demonstrated 73.5% of patients experienced a fibroid volume reduction of greater than 25% and were amenorrheic (Fertil Steril. 2017;108:416-25). GnRH agonists or SPRMs may benefit women if the fibroid is larger than 3 cm or anemia exists, thereby precluding immediate surgery.
Other medication options include the levonorgestrel IUD, combined hormonal contraceptives, and tranexamic acid – all of which have limited data on effective results of treating abnormal uterine bleeding.
Treatment options – surgical
Fibroids are the most common reason for hysterectomy as they are the contributing indication in approximately one-third of surgeries. When future fertility is desired, current surgical options include hysteroscopic and laparoscopic (including robotic) myomectomy. Hysteroscopy is the standard approach for FIGO type 1 fibroids and can also manage some type 2 fibroids provided they are less than 3 cm and the latter is greater than 5 mm from the serosa. Type 2 fibroids may benefit from a “two-step” removal to allow the myometrium to contract and extrude the fibroid. In light of the risk of fluid overload with nonelectrolyte solutions that enable the use of monopolar cautery, many procedures are now performed with bipolar cautery or morcellators.
Laparoscopy (including robotic) has outcomes similar to those of laparotomy although the risk of uterine rupture with the former requires careful attention to thorough closure of the myometrial defect. Robotic myomectomy has outcomes similar to those of standard laparoscopy with less blood loss, but operating times may be prolonged (Best Pract Res Clin Obstet Gynaecol. 2018;46:113-9).
The rate of myomectomy is reported to be 9.2 per 10,000 woman-years in Black women and 1.3 per 10,000 woman years in White women (Fertil Steril 2017;108;416-25). The rate of recurrence after myomectomy can be as great as 60% when patients are followed up to 5 years. Intramural fibroids greater than 2.85 cm and not distorting the uterine cavity may decrease in vitro fertilization (IVF) success (Fertil Steril 2014;101:716-21).
Noninvasive treatment modalities
Uterine artery embolization (UAE) is the most popular minimally invasive alternative to surgical myomectomy. Risks include postembolization syndrome (pain, fever, nausea, leukocytosis, and occasionally malaise), infection, and damage to fertility. Rarely, loss of ovarian function can occur, particularly in women above age 45. Because of the disruption of uterine blood flow, UAE increases the risk of accelerating ovarian aging and infertility as well as atrophic endometrium. In addition, pregnancy complications are increased including miscarriage, preterm labor, and postpartum hemorrhage. There is debate regarding the need for cesarean section at time of delivery given the potential for weakening of the uterine wall following UAE.
High-intensity focused ultrasound (HIFU) is guided by ultrasound or MRI and involves a high-energy-density ultrasound wave passing through the skin. The wave is absorbed and transformed into heat, causing the tissue protein to coagulate, and to be absorbed by the body. The procedure is scarless, carries a minimal risk of infection, and offers less pain compared with traditional approaches. However, HIFU is time consuming, and skin burns and unintentional tissue injury are a risk. A meta-analysis demonstrated improved symptoms of fibroids at 6 and 12 months (J Min Invasive Gynecol. 2021 in press).
Ultrasound-guided microwave ablation (MWA) uses an ablative electrode that is directly inserted into the target tissue via transcutaneous or transcervical approach via ultrasound guidance using microwave to produce heat for tissue coagulation necrosis. The advantages of MWA compared with HIFU and RFA are a higher tissue temperature, larger ablation volume, shorter operating time, less pain and no adverse major events (J Min Invasive Gynecol. 2021, in press).
Conclusion
The current literature cannot conclude that fibroids reduce the likelihood of achieving pregnancy with or without fertility treatment, based on a specific size, number, or location (not including submucosal or cavity-distorting intramural fibroids). Definitive evidence on the efficacy of myomectomy to improve fertility remains limited. Hysteroscopic myomectomy presumably improves pregnancy rates, but there is uncertainty as to its role in reducing miscarriage. Novel nonsurgical modalities are available and are expected to continue being developed but clarity on fertility outcomes is needed.
Dr. Trolice is director of Fertility CARE – The IVF Center in Winter Park, Fla., and professor of obstetrics and gynecology at the University of Central Florida, Orlando. He has no conflicts of interests. Please contact him at obnews@mdedge.com.
Closing the racial gap in minimally invasive gyn hysterectomy and myomectomy
The historical mistreatment of Black bodies in gynecologic care has bled into present day inequities—from surgeries performed on enslaved Black women and sterilization of low-income Black women under federally funded programs, to higher rates of adverse health-related outcomes among Black women compared with their non-Black counterparts.1-3 Not only is the foundation of gynecology imperfect, so too is its current-day structure.
It is not enough to identify and describe racial inequities in health care; action plans to provide equitable care are called for. In this report, we aim to 1) contextualize the data on disparities in minimally invasive gynecologic surgery, specifically hysterectomy and myomectomy candidates and postsurgical outcomes, and 2) provide recommendations to close racial gaps in gynecologic treatment for more equitable experiences for minority women.
Black women and uterine fibroids
Uterine leiomyomas, or fibroids, are not only the most common benign pelvic tumor but they also cause a significant medical and financial burden in the United States, with estimated direct costs of $4.1 ̶ 9.4 billion.4 Fibroids can affect fertility and cause pain, bulk symptoms, heavy bleeding, anemia requiring blood transfusion, and poor pregnancy outcomes. The burden of disease for uterine fibroids is greatest for Black women.
The incidence of fibroids is 2 to 3 times higher in Black women compared with White women.5 According to ultrasound-based studies, the prevalence of fibroids among women aged 18 to 30 years was 26% among Black and 7% among White asymptomatic women.6 Earlier onset and more severe symptoms mean that there is a larger potential for impact on fertility for Black women. This coupled with the historical context of mistreatment of Black bodies makes the need for personalized medicine and culturally sensitive care critical.
Inequitable management of uterine fibroids
Although tumor size, location, and patient risk factors are used to determine the best treatment approach, the American College of Obstetricians and Gynecologists (ACOG) guidelines suggest that the use of alternative treatments to surgery should be first-line management instead of hysterectomy for most benign conditions.9 Conservative management will often help alleviate symptoms, slow the growth of fibroid(s), or bridge women to menopause, and treatment options include hormonal contraception, gonadotropin-releasing hormone agonists, hysteroscopic resection, uterine artery embolization, magnetic resonance-guided focused ultrasound, and myomectomy.
The rate of conservative management prior to hysterectomy varies by setting, reflecting potential bias in treatment decisions. Some medical settings have reported a 29% alternative management rate prior to hysterectomy, while others report much higher rates.10 A study using patient data from Kaiser Permanente Northern California (KPNC) showed that, within a large, diverse, and integrated health care system, more than 80% of patients received alternative treatments before undergoing hysterectomy; for those with symptomatic leiomyomas, 74.1% used alternative treatments prior to hysterectomy, and in logistic regression there was not a difference by race.11 Nationally, Black women are more likely to have hysterectomy or myomectomy compared with a nonsurgical uterine-sparing therapy.12,13
With about 600,000 cases per year within the United States, the hysterectomy is the most frequently performed benign gynecologic surgery.14 The most common indication is for “symptomatic fibroid uterus.” The approach to decision making for route of hysterectomy involves multiple patient and surgeon factors, including history of vaginal delivery, body mass index, history of previous surgery, uterine size, informed patient preference, and surgeon volume.15-17 ACOG recommends a minimally invasive hysterectomy (MIH) whenever feasible given its benefits in postoperative pain, recovery time, and blood loss. Myomectomy, particularly among women in their reproductive years desiring management of leiomyomas, is a uterine-sparing procedure versus hysterectomy. Minimally invasive myomectomy (MIM), compared with an open abdominal route, provides for lower drop in hemoglobin levels, shorter hospital stay, less adhesion formation, and decreased postoperative pain.18
Racial variations in hysterectomy rates persist overall and according to hysterectomy type. Black women are 2 to 3 times more likely to undergo hysterectomy for leiomyomas than other racial groups.19 These differences in rates have been shown to persist even when burden of disease is the same. One study found that Black women had increased odds of hysterectomy compared with their White counterparts even when there was no difference in mean fibroid volume by race,20 calling into question provider bias. Even in a universal insurance setting, Black patients have been found to have higher rates of open hysterectomies.21 Previous studies found that, despite growing frequency of laparoscopic and robotic-assisted hysterectomies, patients of a minority race had decreased odds of undergoing a MIH compared with their White counterparts.22
While little data exist on route of myomectomy by race, a recent study found minority women were more likely to undergo abdominal myomectomy compared with White women; Black women were twice as likely to undergo abdominal myomectomy (adjusted odds ratio [aOR], 1.9; 95% confidence interval [CI], 1.7–2.0), Asian American women were more than twice as likely (aOR, 2.3; 95% CI, 1.8–2.8), and Hispanic American women were 50% more likely to undergo abdominal myomectomy (aOR, 1.5; 95% CI, 1.2–1.9) when compared with White women.23 These differences remained after controlling for potential confounders, and there appeared to be an interaction between race and fibroid weight such that racial bias alone may not explain the differences.
Finally, Black women have higher perioperative complication rates compared with non-Black women. Postoperative complications including blood transfusion after myomectomy have been shown to be twice as high among Black women compared with White women. However, once uterine size, comorbidities, and fibroid number were controlled, race was not associated with higher complications. Black women, compared with White women, have been found to have 50% increased odds of morbidity after an abdominal myomectomy.24
Continue to: How to ensure that BIPOC women get the best management...
How to ensure that BIPOC women get the best management
Eliminating disparities and providing equitable and patient-centered care for Black, Indigenous, and people of color (BIPOC) women will require research, education, training, and targeted quality improvement initiatives.
Research into fibroids and comparative treatment outcomes
Uterine fibroids, despite their major public health impact, remain understudied. With Black women carrying the highest fibroid prevalence and severity burden, especially in their childbearing years, it is imperative that research efforts be focused on outcomes by race and ethnicity. Given the significant economic impact of fibroids, more efforts should be directed toward primary prevention of fibroid formation as well as secondary prevention and limitation of fibroid growth by affordable, effective, and safe means. For example, Bratka and colleagues researched the role of vitamin D in inhibiting growth of leiomyoma cells in animal models.25 Other innovative forms of management under investigation include aromatase inhibitors, green tea, cabergoline, elagolix, paricalcitol, and epigallocatechin gallate.26 Considerations such as stress, diet, and environmental risk factors have yet to be investigated in large studies.
Research contributing to evidence-based guidelines that address the needs of different patient populations affected by uterine fibroids is critical.8 Additionally, research conducted by Black women about Black women should be prioritized. In March 2021, the Stephanie Tubbs Jones Uterine Fibroid Research and Education Act of 2021 was introduced to fund $150 million in research supported by the National Institutes of Health (NIH). This is an opportunity to develop a research database to inform evidence-based culturally informed care regarding fertility counseling, medical management, and optimal surgical approach, as well as to award funding to minority researchers. There are disparities in distribution of funds from the NIH to minority researchers. Under-represented minorities are awarded fewer NIH grants compared with their counterparts despite initiatives to increase funding. Furthermore, in 2011, Black applicants for NIH funding were two-thirds as likely as White applicants to receive grants from 2000 ̶ 2006, even when accounting for publication record and training.27 Funding BIPOC researchers fuels diversity-driven investigation and can be useful in the charge to increase fibroid research.
Education and training: Changing the work force
Achieving equity requires change in provider work force. In a study of trends across multiple specialties including obstetrics and gynecology, Blacks and Latinx are more under-represented in 2016 than in 1990 across all specialties except for Black women in obstetrics and gynecology.28 It is well documented that under-represented minorities are more likely to engage in practice, research, service, and mentorship activities aligned with their identity.29 As a higher proportion of under-represented minority obstetricians and gynecologists practice in medically underserved areas,30 this presents a unique opportunity for gynecologists to improve care for and increase research involvement among BIPOC women.
Increasing BIPOC representation in medical and health care institutions and practices is not enough, however, to achieve health equity. Data from the Association of American Medical Colleges demonstrate that between 1978 and 2017 the total number of full-time obstetrics and gynecology faculty rose nearly fourfold from 1,688 to 6,347; however, the greatest rise in proportion of faculty who were nontenured was among women who were under-represented minorities.31 Additionally, there are disparities in wage by race even after controlling for hours worked and state of residence.32 Medical and academic centers and health care institutions and practices should proactively and systematically engage in the recruitment and retention of under-represented minority physicians and people in leadership roles. This will involve creating safe and inclusive work environments, with equal pay and promotion structures.
Quality initiatives to address provider bias
Provider bias should be addressed in clinical decision making and counseling of patients. Studies focused on ultrasonography have shown an estimated cumulative incidence of fibroids by age 50 of greater than 80% for Black women and nearly 70% for White women.5 Due to the prevalence and burden of fibroids among Black women there may be a provider bias in approach to management. Addressing this bias requires quality improvement efforts and investigation into patient and provider factors in management of fibroids. Black women have been a vulnerable population in medicine due to instances of mistreatment, and often times mistrust can play a role in how a patient views his or her care decisions. A patient-centered strategy allows patient factors such as age, uterine size, and cultural background to be considered such that a provider can tailor an approach that is best for the patient. Previous minority women focus groups have demonstrated that women have a strong desire for elective treatment;33 therefore, providers should listen openly to patients about their values and their perspectives on how fibroids affect their lives. Provider bias toward surgical volume, incentive for surgery, and implicit bias need to be addressed at every institution to work toward equitable and cost-effective care.
Integrated health care systems like Southern and Northern California Permanente Medical Group, using quality initiatives, have increased their minimally invasive surgery rates. Southern California Permanente Medical Group reached a 78% rate of MIH in a system of more than 350 surgeons performing benign indication hysterectomies as reported in 2011.34 Similarly, a study within KPNC, an institution with an MIH rate greater than 95%,35 found that racial disparities in route of MIH were eliminated through a quality improvement initiative described in detail in 2018 (FIGURE and TABLE).36
Conclusions
There are recognized successes in the gynecology field’s efforts to address racial disparities. Prior studies provide insight into opportunities to improve care in medical management of leiomyomas, minimally invasive route of hysterectomy and myomectomy, postsurgical outcomes, and institutional leadership. Particularly, when systemwide approaches are taken in the delivery of health care it is possible to significantly diminish racial disparities in gynecology.35 Much work remains to be done for our health care systems to provide equitable care.
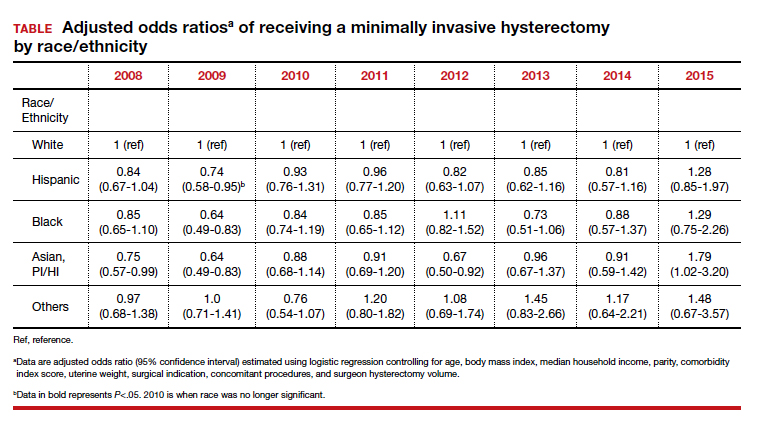
- Ojanuga D. The medical ethics of the ‘father of gynaecology,’ Dr J Marion Sims. J Med Ethics. 1993;19:28-31. doi: 10.1136/jme.19.1.28.
- Borrero S, Zite N, Creinin MD. Federally funded sterilization: time to rethink policy? Am J Public Health. 2012;102:1822-1825.
- Eaglehouse YL, Georg MW, Shriver CD, et al. Racial differences in time to breast cancer surgery and overall survival in the US Military Health System. JAMA Surg. 2019;154:e185113. doi: 10.1001/jamasurg.2018.5113.
- Soliman AM, Yang H, Du EX, et al. The direct and indirect costs of uterine fibroid tumors: a systematic review of the literature between 2000 and 2013. Am J Obstet Gynecol. 2015;213:141-160.
- Baird DD, Dunson DB, Hill MC, et al. High cumulative incidence of uterine leiomyoma in black and white women: ultrasound evidence. Am J Obstet Gynecol. 2003;188:100-107.
- Marshall LM, Spiegelman D, Barbieri RL, et al. Variation in the incidence of uterine leiomyoma among premenopausal women by age and race. Obstet Gynecol. 1997;90:967-973. doi: 10.1016/s0029-7844(97)00534-6.
- Styer AK, Rueda BR. The epidemiology and genetics of uterine leiomyoma. Best Pract Res Clin Obstet Gynaecol. 2016;34:3-12. doi: 10.1016/j.bpobgyn.2015.11.018.
- Al-Hendy A, Myers ER, Stewart E. Uterine fibroids: burden and unmet medical need. Semin Reprod Med. 2017;35:473-480. doi: 10.1055/s-0037-1607264.
- American College of Obstetricians and Gynecologists. ACOG practice bulletin. Alternatives to hysterectomy in the management of leiomyomas. Obstet Gynecol. 2008;112(2 pt 1):387-400.
- Corona LE, Swenson CW, Sheetz KH, et al. Use of other treatments before hysterectomy for benign conditions in a statewide hospital collaborative. Am J Obstet Gynecol. 2015;212:304.e1-e7. doi: 10.1016/j.ajog.2014.11.031.
- Nguyen NT, Merchant M, Ritterman Weintraub ML, et al. Alternative treatment utilization before hysterectomy for benign gynecologic conditions at a large integrated health system. J Minim Invasive Gynecol. 2019;26:847-855. doi: 10.1016/j.jmig.2018.08.013.
- Laughlin-Tommaso SK, Jacoby VL, Myers ER. Disparities in fibroid incidence, prognosis, and management. Obstet Gynecol Clin North Am. 2017;44:81-94. doi: 10.1016/j.ogc.2016.11.007.
- Borah BJ, Laughlin-Tommaso SK, Myers ER, et al. Association between patient characteristics and treatment procedure among patients with uterine leiomyomas. Obstet Gynecol. 2016;127:67-77.
- Whiteman MK, Hillis SD, Jamieson DJ, et al. Inpatient hysterectomy surveillance in the United States, 2000-2004. Am J Obstet Gynecol. 2008;198:34.e1-e7. doi:10.1016/j.ajog.2007.05.039.
- Bardens D, Solomayer E, Baum S, et al. The impact of the body mass index (BMI) on laparoscopic hysterectomy for benign disease. Arch Gynecol Obstet. 2014;289:803-807. doi: 10.1007/s00404-013-3050-2.
- Seracchioli R, Venturoli S, Vianello F, et al. Total laparoscopic hysterectomy compared with abdominal hysterectomy in the presence of a large uterus. J Am Assoc Gynecol Laparosc. 2002;9:333-338. doi: 10.1016/s1074-3804(05)60413.
- Boyd LR, Novetsky AP, Curtin JP. Effect of surgical volume on route of hysterectomy and short-term morbidity. Obstet Gynecol. 2010;116:909-915. doi: 10.1097/AOG.0b013e3181f395d9.
- Jin C, Hu Y, Chen XC, et al. Laparoscopic versus open myomectomy—a meta-analysis of randomized controlled trials. Eur J Obstet Gynecol Reprod Biol. 2009;145:14-21. doi: 10.1016/j.ejogrb.2009.03.009.
- Wechter ME, Stewart EA, Myers ER, et al. Leiomyoma-related hospitalization and surgery: prevalence and predicted growth based on population trends. Am J Obstet Gynecol. 2011;205:492.e1-e5. doi: 10.1016/j.ajog.2011.07.008.
- Bower JK, Schreiner PJ, Sternfeld B, et al. Black-White differences in hysterectomy prevalence: the CARDIA study. Am J Public Health. 2009;99:300-307. doi: 10.2105/AJPH.2008.133702.
- Ranjit A, Sharma M, Romano A, et al. Does universal insurance mitigate racial differences in minimally invasive hysterectomy? J Minim Invasive Gynecol. 2017;24. doi:10.1016/j.jmig.2017.03.016.
- Pollack LM, Olsen MA, Gehlert SJ, et al. Racial/ethnic disparities/differences in hysterectomy route in women likely eligible for minimally invasive surgery. J Minim Invasive Gynecol. 2020;27:1167-1177.e2. doi:10.1016/j.jmig.2019.09.003.
- Stentz NC, Cooney LG, Sammel MD, et al. Association of patient race with surgical practice and perioperative morbidity after myomectomy. Obstet Gynecol. 2018;132:291-297. doi: 10.1097/AOG.0000000000002738.
- Roth TM, Gustilo-Ashby T, Barber MD, et al. Effects of race and clinical factors on short-term outcomes of abdominal myomectomy. Obstet Gynecol. 2003;101(5 pt 1):881-884. doi: 10.1016/s0029-7844(03)00015-2.
- Bratka S, Diamond JS, Al-Hendy A, et al. The role of vitamin D in uterine fibroid biology. Fertil Steril. 2015;104:698-706. doi: 10.1016/j.fertnstert.2015.05.031.
- Ciebiera M, Łukaszuk K, Męczekalski B, et al. Alternative oral agents in prophylaxis and therapy of uterine fibroids—an up-to-date review. Int J Mol Sci. 2017;18:2586. doi:10.3390/ijms18122586.
- Hayden EC. Racial bias haunts NIH funding. Nature. 2015;527:145.
- Lett LA, Orji WU, Sebro R. Declining racial and ethnic representation in clinical academic medicine: a longitudinal study of 16 US medical specialties. PLoS One. 2018;13:e0207274. doi: 10.1371/journal.pone.0207274.
- Sánchez JP, Poll-Hunter N, Stern N, et al. Balancing two cultures: American Indian/Alaska Native medical students’ perceptions of academic medicine careers. J Community Health. 2016;41:871-880.
- Rayburn WF, Xierali IM, Castillo-Page L, et al. Racial and ethnic differences between obstetrician-gynecologists and other adult medical specialists. Obstet Gynecol. 2016;127:148-152. doi: 10.1097/AOG.0000000000001184.
- Esters D, Xierali IM, Nivet MA, et al. The rise of nontenured faculty in obstetrics and gynecology by sex and underrepresented in medicine status. Obstet Gynecol. 2019;134 suppl 1:34S-39S. doi: 10.1097/AOG.0000000000003484.
- Ly DP, Seabury SA, Jena AB. Differences in incomes of physicians in the United States by race and sex: observational study. BMJ. 2016;I2923. doi:10.1136/bmj.i2923.
- Groff JY, Mullen PD, Byrd T, et al. Decision making, beliefs, and attitudes toward hysterectomy: a focus group study with medically underserved women in Texas. J Womens Health Gend Based Med. 2000;9 suppl 2:S39-50. doi: 10.1089/152460900318759.
- Andryjowicz E, Wray T. Regional expansion of minimally invasive surgery for hysterectomy: implementation and methodology in a large multispecialty group. Perm J. 2011;15:42-46.
- Zaritsky E, Ojo A, Tucker LY, et al. Racial disparities in route of hysterectomy for benign indications within an integrated health care system. JAMA Netw Open. 2019;2:e1917004. doi: 10.1001/jamanetworkopen.2019.17004.
- Abel MK, Kho KA, Walter A, et al. Measuring quality in minimally invasive gynecologic surgery: what, how, and why? J Minim Invasive Gynecol. 2019;26:321-326. doi: 10.1016/j.jmig.2018.11.013.
The historical mistreatment of Black bodies in gynecologic care has bled into present day inequities—from surgeries performed on enslaved Black women and sterilization of low-income Black women under federally funded programs, to higher rates of adverse health-related outcomes among Black women compared with their non-Black counterparts.1-3 Not only is the foundation of gynecology imperfect, so too is its current-day structure.
It is not enough to identify and describe racial inequities in health care; action plans to provide equitable care are called for. In this report, we aim to 1) contextualize the data on disparities in minimally invasive gynecologic surgery, specifically hysterectomy and myomectomy candidates and postsurgical outcomes, and 2) provide recommendations to close racial gaps in gynecologic treatment for more equitable experiences for minority women.
Black women and uterine fibroids
Uterine leiomyomas, or fibroids, are not only the most common benign pelvic tumor but they also cause a significant medical and financial burden in the United States, with estimated direct costs of $4.1 ̶ 9.4 billion.4 Fibroids can affect fertility and cause pain, bulk symptoms, heavy bleeding, anemia requiring blood transfusion, and poor pregnancy outcomes. The burden of disease for uterine fibroids is greatest for Black women.
The incidence of fibroids is 2 to 3 times higher in Black women compared with White women.5 According to ultrasound-based studies, the prevalence of fibroids among women aged 18 to 30 years was 26% among Black and 7% among White asymptomatic women.6 Earlier onset and more severe symptoms mean that there is a larger potential for impact on fertility for Black women. This coupled with the historical context of mistreatment of Black bodies makes the need for personalized medicine and culturally sensitive care critical.
Inequitable management of uterine fibroids
Although tumor size, location, and patient risk factors are used to determine the best treatment approach, the American College of Obstetricians and Gynecologists (ACOG) guidelines suggest that the use of alternative treatments to surgery should be first-line management instead of hysterectomy for most benign conditions.9 Conservative management will often help alleviate symptoms, slow the growth of fibroid(s), or bridge women to menopause, and treatment options include hormonal contraception, gonadotropin-releasing hormone agonists, hysteroscopic resection, uterine artery embolization, magnetic resonance-guided focused ultrasound, and myomectomy.
The rate of conservative management prior to hysterectomy varies by setting, reflecting potential bias in treatment decisions. Some medical settings have reported a 29% alternative management rate prior to hysterectomy, while others report much higher rates.10 A study using patient data from Kaiser Permanente Northern California (KPNC) showed that, within a large, diverse, and integrated health care system, more than 80% of patients received alternative treatments before undergoing hysterectomy; for those with symptomatic leiomyomas, 74.1% used alternative treatments prior to hysterectomy, and in logistic regression there was not a difference by race.11 Nationally, Black women are more likely to have hysterectomy or myomectomy compared with a nonsurgical uterine-sparing therapy.12,13
With about 600,000 cases per year within the United States, the hysterectomy is the most frequently performed benign gynecologic surgery.14 The most common indication is for “symptomatic fibroid uterus.” The approach to decision making for route of hysterectomy involves multiple patient and surgeon factors, including history of vaginal delivery, body mass index, history of previous surgery, uterine size, informed patient preference, and surgeon volume.15-17 ACOG recommends a minimally invasive hysterectomy (MIH) whenever feasible given its benefits in postoperative pain, recovery time, and blood loss. Myomectomy, particularly among women in their reproductive years desiring management of leiomyomas, is a uterine-sparing procedure versus hysterectomy. Minimally invasive myomectomy (MIM), compared with an open abdominal route, provides for lower drop in hemoglobin levels, shorter hospital stay, less adhesion formation, and decreased postoperative pain.18
Racial variations in hysterectomy rates persist overall and according to hysterectomy type. Black women are 2 to 3 times more likely to undergo hysterectomy for leiomyomas than other racial groups.19 These differences in rates have been shown to persist even when burden of disease is the same. One study found that Black women had increased odds of hysterectomy compared with their White counterparts even when there was no difference in mean fibroid volume by race,20 calling into question provider bias. Even in a universal insurance setting, Black patients have been found to have higher rates of open hysterectomies.21 Previous studies found that, despite growing frequency of laparoscopic and robotic-assisted hysterectomies, patients of a minority race had decreased odds of undergoing a MIH compared with their White counterparts.22
While little data exist on route of myomectomy by race, a recent study found minority women were more likely to undergo abdominal myomectomy compared with White women; Black women were twice as likely to undergo abdominal myomectomy (adjusted odds ratio [aOR], 1.9; 95% confidence interval [CI], 1.7–2.0), Asian American women were more than twice as likely (aOR, 2.3; 95% CI, 1.8–2.8), and Hispanic American women were 50% more likely to undergo abdominal myomectomy (aOR, 1.5; 95% CI, 1.2–1.9) when compared with White women.23 These differences remained after controlling for potential confounders, and there appeared to be an interaction between race and fibroid weight such that racial bias alone may not explain the differences.
Finally, Black women have higher perioperative complication rates compared with non-Black women. Postoperative complications including blood transfusion after myomectomy have been shown to be twice as high among Black women compared with White women. However, once uterine size, comorbidities, and fibroid number were controlled, race was not associated with higher complications. Black women, compared with White women, have been found to have 50% increased odds of morbidity after an abdominal myomectomy.24
Continue to: How to ensure that BIPOC women get the best management...
How to ensure that BIPOC women get the best management
Eliminating disparities and providing equitable and patient-centered care for Black, Indigenous, and people of color (BIPOC) women will require research, education, training, and targeted quality improvement initiatives.
Research into fibroids and comparative treatment outcomes
Uterine fibroids, despite their major public health impact, remain understudied. With Black women carrying the highest fibroid prevalence and severity burden, especially in their childbearing years, it is imperative that research efforts be focused on outcomes by race and ethnicity. Given the significant economic impact of fibroids, more efforts should be directed toward primary prevention of fibroid formation as well as secondary prevention and limitation of fibroid growth by affordable, effective, and safe means. For example, Bratka and colleagues researched the role of vitamin D in inhibiting growth of leiomyoma cells in animal models.25 Other innovative forms of management under investigation include aromatase inhibitors, green tea, cabergoline, elagolix, paricalcitol, and epigallocatechin gallate.26 Considerations such as stress, diet, and environmental risk factors have yet to be investigated in large studies.
Research contributing to evidence-based guidelines that address the needs of different patient populations affected by uterine fibroids is critical.8 Additionally, research conducted by Black women about Black women should be prioritized. In March 2021, the Stephanie Tubbs Jones Uterine Fibroid Research and Education Act of 2021 was introduced to fund $150 million in research supported by the National Institutes of Health (NIH). This is an opportunity to develop a research database to inform evidence-based culturally informed care regarding fertility counseling, medical management, and optimal surgical approach, as well as to award funding to minority researchers. There are disparities in distribution of funds from the NIH to minority researchers. Under-represented minorities are awarded fewer NIH grants compared with their counterparts despite initiatives to increase funding. Furthermore, in 2011, Black applicants for NIH funding were two-thirds as likely as White applicants to receive grants from 2000 ̶ 2006, even when accounting for publication record and training.27 Funding BIPOC researchers fuels diversity-driven investigation and can be useful in the charge to increase fibroid research.
Education and training: Changing the work force
Achieving equity requires change in provider work force. In a study of trends across multiple specialties including obstetrics and gynecology, Blacks and Latinx are more under-represented in 2016 than in 1990 across all specialties except for Black women in obstetrics and gynecology.28 It is well documented that under-represented minorities are more likely to engage in practice, research, service, and mentorship activities aligned with their identity.29 As a higher proportion of under-represented minority obstetricians and gynecologists practice in medically underserved areas,30 this presents a unique opportunity for gynecologists to improve care for and increase research involvement among BIPOC women.
Increasing BIPOC representation in medical and health care institutions and practices is not enough, however, to achieve health equity. Data from the Association of American Medical Colleges demonstrate that between 1978 and 2017 the total number of full-time obstetrics and gynecology faculty rose nearly fourfold from 1,688 to 6,347; however, the greatest rise in proportion of faculty who were nontenured was among women who were under-represented minorities.31 Additionally, there are disparities in wage by race even after controlling for hours worked and state of residence.32 Medical and academic centers and health care institutions and practices should proactively and systematically engage in the recruitment and retention of under-represented minority physicians and people in leadership roles. This will involve creating safe and inclusive work environments, with equal pay and promotion structures.
Quality initiatives to address provider bias
Provider bias should be addressed in clinical decision making and counseling of patients. Studies focused on ultrasonography have shown an estimated cumulative incidence of fibroids by age 50 of greater than 80% for Black women and nearly 70% for White women.5 Due to the prevalence and burden of fibroids among Black women there may be a provider bias in approach to management. Addressing this bias requires quality improvement efforts and investigation into patient and provider factors in management of fibroids. Black women have been a vulnerable population in medicine due to instances of mistreatment, and often times mistrust can play a role in how a patient views his or her care decisions. A patient-centered strategy allows patient factors such as age, uterine size, and cultural background to be considered such that a provider can tailor an approach that is best for the patient. Previous minority women focus groups have demonstrated that women have a strong desire for elective treatment;33 therefore, providers should listen openly to patients about their values and their perspectives on how fibroids affect their lives. Provider bias toward surgical volume, incentive for surgery, and implicit bias need to be addressed at every institution to work toward equitable and cost-effective care.
Integrated health care systems like Southern and Northern California Permanente Medical Group, using quality initiatives, have increased their minimally invasive surgery rates. Southern California Permanente Medical Group reached a 78% rate of MIH in a system of more than 350 surgeons performing benign indication hysterectomies as reported in 2011.34 Similarly, a study within KPNC, an institution with an MIH rate greater than 95%,35 found that racial disparities in route of MIH were eliminated through a quality improvement initiative described in detail in 2018 (FIGURE and TABLE).36
Conclusions
There are recognized successes in the gynecology field’s efforts to address racial disparities. Prior studies provide insight into opportunities to improve care in medical management of leiomyomas, minimally invasive route of hysterectomy and myomectomy, postsurgical outcomes, and institutional leadership. Particularly, when systemwide approaches are taken in the delivery of health care it is possible to significantly diminish racial disparities in gynecology.35 Much work remains to be done for our health care systems to provide equitable care.


The historical mistreatment of Black bodies in gynecologic care has bled into present day inequities—from surgeries performed on enslaved Black women and sterilization of low-income Black women under federally funded programs, to higher rates of adverse health-related outcomes among Black women compared with their non-Black counterparts.1-3 Not only is the foundation of gynecology imperfect, so too is its current-day structure.
It is not enough to identify and describe racial inequities in health care; action plans to provide equitable care are called for. In this report, we aim to 1) contextualize the data on disparities in minimally invasive gynecologic surgery, specifically hysterectomy and myomectomy candidates and postsurgical outcomes, and 2) provide recommendations to close racial gaps in gynecologic treatment for more equitable experiences for minority women.
Black women and uterine fibroids
Uterine leiomyomas, or fibroids, are not only the most common benign pelvic tumor but they also cause a significant medical and financial burden in the United States, with estimated direct costs of $4.1 ̶ 9.4 billion.4 Fibroids can affect fertility and cause pain, bulk symptoms, heavy bleeding, anemia requiring blood transfusion, and poor pregnancy outcomes. The burden of disease for uterine fibroids is greatest for Black women.
The incidence of fibroids is 2 to 3 times higher in Black women compared with White women.5 According to ultrasound-based studies, the prevalence of fibroids among women aged 18 to 30 years was 26% among Black and 7% among White asymptomatic women.6 Earlier onset and more severe symptoms mean that there is a larger potential for impact on fertility for Black women. This coupled with the historical context of mistreatment of Black bodies makes the need for personalized medicine and culturally sensitive care critical.
Inequitable management of uterine fibroids
Although tumor size, location, and patient risk factors are used to determine the best treatment approach, the American College of Obstetricians and Gynecologists (ACOG) guidelines suggest that the use of alternative treatments to surgery should be first-line management instead of hysterectomy for most benign conditions.9 Conservative management will often help alleviate symptoms, slow the growth of fibroid(s), or bridge women to menopause, and treatment options include hormonal contraception, gonadotropin-releasing hormone agonists, hysteroscopic resection, uterine artery embolization, magnetic resonance-guided focused ultrasound, and myomectomy.
The rate of conservative management prior to hysterectomy varies by setting, reflecting potential bias in treatment decisions. Some medical settings have reported a 29% alternative management rate prior to hysterectomy, while others report much higher rates.10 A study using patient data from Kaiser Permanente Northern California (KPNC) showed that, within a large, diverse, and integrated health care system, more than 80% of patients received alternative treatments before undergoing hysterectomy; for those with symptomatic leiomyomas, 74.1% used alternative treatments prior to hysterectomy, and in logistic regression there was not a difference by race.11 Nationally, Black women are more likely to have hysterectomy or myomectomy compared with a nonsurgical uterine-sparing therapy.12,13
With about 600,000 cases per year within the United States, the hysterectomy is the most frequently performed benign gynecologic surgery.14 The most common indication is for “symptomatic fibroid uterus.” The approach to decision making for route of hysterectomy involves multiple patient and surgeon factors, including history of vaginal delivery, body mass index, history of previous surgery, uterine size, informed patient preference, and surgeon volume.15-17 ACOG recommends a minimally invasive hysterectomy (MIH) whenever feasible given its benefits in postoperative pain, recovery time, and blood loss. Myomectomy, particularly among women in their reproductive years desiring management of leiomyomas, is a uterine-sparing procedure versus hysterectomy. Minimally invasive myomectomy (MIM), compared with an open abdominal route, provides for lower drop in hemoglobin levels, shorter hospital stay, less adhesion formation, and decreased postoperative pain.18
Racial variations in hysterectomy rates persist overall and according to hysterectomy type. Black women are 2 to 3 times more likely to undergo hysterectomy for leiomyomas than other racial groups.19 These differences in rates have been shown to persist even when burden of disease is the same. One study found that Black women had increased odds of hysterectomy compared with their White counterparts even when there was no difference in mean fibroid volume by race,20 calling into question provider bias. Even in a universal insurance setting, Black patients have been found to have higher rates of open hysterectomies.21 Previous studies found that, despite growing frequency of laparoscopic and robotic-assisted hysterectomies, patients of a minority race had decreased odds of undergoing a MIH compared with their White counterparts.22
While little data exist on route of myomectomy by race, a recent study found minority women were more likely to undergo abdominal myomectomy compared with White women; Black women were twice as likely to undergo abdominal myomectomy (adjusted odds ratio [aOR], 1.9; 95% confidence interval [CI], 1.7–2.0), Asian American women were more than twice as likely (aOR, 2.3; 95% CI, 1.8–2.8), and Hispanic American women were 50% more likely to undergo abdominal myomectomy (aOR, 1.5; 95% CI, 1.2–1.9) when compared with White women.23 These differences remained after controlling for potential confounders, and there appeared to be an interaction between race and fibroid weight such that racial bias alone may not explain the differences.
Finally, Black women have higher perioperative complication rates compared with non-Black women. Postoperative complications including blood transfusion after myomectomy have been shown to be twice as high among Black women compared with White women. However, once uterine size, comorbidities, and fibroid number were controlled, race was not associated with higher complications. Black women, compared with White women, have been found to have 50% increased odds of morbidity after an abdominal myomectomy.24
Continue to: How to ensure that BIPOC women get the best management...
How to ensure that BIPOC women get the best management
Eliminating disparities and providing equitable and patient-centered care for Black, Indigenous, and people of color (BIPOC) women will require research, education, training, and targeted quality improvement initiatives.
Research into fibroids and comparative treatment outcomes
Uterine fibroids, despite their major public health impact, remain understudied. With Black women carrying the highest fibroid prevalence and severity burden, especially in their childbearing years, it is imperative that research efforts be focused on outcomes by race and ethnicity. Given the significant economic impact of fibroids, more efforts should be directed toward primary prevention of fibroid formation as well as secondary prevention and limitation of fibroid growth by affordable, effective, and safe means. For example, Bratka and colleagues researched the role of vitamin D in inhibiting growth of leiomyoma cells in animal models.25 Other innovative forms of management under investigation include aromatase inhibitors, green tea, cabergoline, elagolix, paricalcitol, and epigallocatechin gallate.26 Considerations such as stress, diet, and environmental risk factors have yet to be investigated in large studies.
Research contributing to evidence-based guidelines that address the needs of different patient populations affected by uterine fibroids is critical.8 Additionally, research conducted by Black women about Black women should be prioritized. In March 2021, the Stephanie Tubbs Jones Uterine Fibroid Research and Education Act of 2021 was introduced to fund $150 million in research supported by the National Institutes of Health (NIH). This is an opportunity to develop a research database to inform evidence-based culturally informed care regarding fertility counseling, medical management, and optimal surgical approach, as well as to award funding to minority researchers. There are disparities in distribution of funds from the NIH to minority researchers. Under-represented minorities are awarded fewer NIH grants compared with their counterparts despite initiatives to increase funding. Furthermore, in 2011, Black applicants for NIH funding were two-thirds as likely as White applicants to receive grants from 2000 ̶ 2006, even when accounting for publication record and training.27 Funding BIPOC researchers fuels diversity-driven investigation and can be useful in the charge to increase fibroid research.
Education and training: Changing the work force
Achieving equity requires change in provider work force. In a study of trends across multiple specialties including obstetrics and gynecology, Blacks and Latinx are more under-represented in 2016 than in 1990 across all specialties except for Black women in obstetrics and gynecology.28 It is well documented that under-represented minorities are more likely to engage in practice, research, service, and mentorship activities aligned with their identity.29 As a higher proportion of under-represented minority obstetricians and gynecologists practice in medically underserved areas,30 this presents a unique opportunity for gynecologists to improve care for and increase research involvement among BIPOC women.
Increasing BIPOC representation in medical and health care institutions and practices is not enough, however, to achieve health equity. Data from the Association of American Medical Colleges demonstrate that between 1978 and 2017 the total number of full-time obstetrics and gynecology faculty rose nearly fourfold from 1,688 to 6,347; however, the greatest rise in proportion of faculty who were nontenured was among women who were under-represented minorities.31 Additionally, there are disparities in wage by race even after controlling for hours worked and state of residence.32 Medical and academic centers and health care institutions and practices should proactively and systematically engage in the recruitment and retention of under-represented minority physicians and people in leadership roles. This will involve creating safe and inclusive work environments, with equal pay and promotion structures.
Quality initiatives to address provider bias
Provider bias should be addressed in clinical decision making and counseling of patients. Studies focused on ultrasonography have shown an estimated cumulative incidence of fibroids by age 50 of greater than 80% for Black women and nearly 70% for White women.5 Due to the prevalence and burden of fibroids among Black women there may be a provider bias in approach to management. Addressing this bias requires quality improvement efforts and investigation into patient and provider factors in management of fibroids. Black women have been a vulnerable population in medicine due to instances of mistreatment, and often times mistrust can play a role in how a patient views his or her care decisions. A patient-centered strategy allows patient factors such as age, uterine size, and cultural background to be considered such that a provider can tailor an approach that is best for the patient. Previous minority women focus groups have demonstrated that women have a strong desire for elective treatment;33 therefore, providers should listen openly to patients about their values and their perspectives on how fibroids affect their lives. Provider bias toward surgical volume, incentive for surgery, and implicit bias need to be addressed at every institution to work toward equitable and cost-effective care.
Integrated health care systems like Southern and Northern California Permanente Medical Group, using quality initiatives, have increased their minimally invasive surgery rates. Southern California Permanente Medical Group reached a 78% rate of MIH in a system of more than 350 surgeons performing benign indication hysterectomies as reported in 2011.34 Similarly, a study within KPNC, an institution with an MIH rate greater than 95%,35 found that racial disparities in route of MIH were eliminated through a quality improvement initiative described in detail in 2018 (FIGURE and TABLE).36
Conclusions
There are recognized successes in the gynecology field’s efforts to address racial disparities. Prior studies provide insight into opportunities to improve care in medical management of leiomyomas, minimally invasive route of hysterectomy and myomectomy, postsurgical outcomes, and institutional leadership. Particularly, when systemwide approaches are taken in the delivery of health care it is possible to significantly diminish racial disparities in gynecology.35 Much work remains to be done for our health care systems to provide equitable care.


- Ojanuga D. The medical ethics of the ‘father of gynaecology,’ Dr J Marion Sims. J Med Ethics. 1993;19:28-31. doi: 10.1136/jme.19.1.28.
- Borrero S, Zite N, Creinin MD. Federally funded sterilization: time to rethink policy? Am J Public Health. 2012;102:1822-1825.
- Eaglehouse YL, Georg MW, Shriver CD, et al. Racial differences in time to breast cancer surgery and overall survival in the US Military Health System. JAMA Surg. 2019;154:e185113. doi: 10.1001/jamasurg.2018.5113.
- Soliman AM, Yang H, Du EX, et al. The direct and indirect costs of uterine fibroid tumors: a systematic review of the literature between 2000 and 2013. Am J Obstet Gynecol. 2015;213:141-160.
- Baird DD, Dunson DB, Hill MC, et al. High cumulative incidence of uterine leiomyoma in black and white women: ultrasound evidence. Am J Obstet Gynecol. 2003;188:100-107.
- Marshall LM, Spiegelman D, Barbieri RL, et al. Variation in the incidence of uterine leiomyoma among premenopausal women by age and race. Obstet Gynecol. 1997;90:967-973. doi: 10.1016/s0029-7844(97)00534-6.
- Styer AK, Rueda BR. The epidemiology and genetics of uterine leiomyoma. Best Pract Res Clin Obstet Gynaecol. 2016;34:3-12. doi: 10.1016/j.bpobgyn.2015.11.018.
- Al-Hendy A, Myers ER, Stewart E. Uterine fibroids: burden and unmet medical need. Semin Reprod Med. 2017;35:473-480. doi: 10.1055/s-0037-1607264.
- American College of Obstetricians and Gynecologists. ACOG practice bulletin. Alternatives to hysterectomy in the management of leiomyomas. Obstet Gynecol. 2008;112(2 pt 1):387-400.
- Corona LE, Swenson CW, Sheetz KH, et al. Use of other treatments before hysterectomy for benign conditions in a statewide hospital collaborative. Am J Obstet Gynecol. 2015;212:304.e1-e7. doi: 10.1016/j.ajog.2014.11.031.
- Nguyen NT, Merchant M, Ritterman Weintraub ML, et al. Alternative treatment utilization before hysterectomy for benign gynecologic conditions at a large integrated health system. J Minim Invasive Gynecol. 2019;26:847-855. doi: 10.1016/j.jmig.2018.08.013.
- Laughlin-Tommaso SK, Jacoby VL, Myers ER. Disparities in fibroid incidence, prognosis, and management. Obstet Gynecol Clin North Am. 2017;44:81-94. doi: 10.1016/j.ogc.2016.11.007.
- Borah BJ, Laughlin-Tommaso SK, Myers ER, et al. Association between patient characteristics and treatment procedure among patients with uterine leiomyomas. Obstet Gynecol. 2016;127:67-77.
- Whiteman MK, Hillis SD, Jamieson DJ, et al. Inpatient hysterectomy surveillance in the United States, 2000-2004. Am J Obstet Gynecol. 2008;198:34.e1-e7. doi:10.1016/j.ajog.2007.05.039.
- Bardens D, Solomayer E, Baum S, et al. The impact of the body mass index (BMI) on laparoscopic hysterectomy for benign disease. Arch Gynecol Obstet. 2014;289:803-807. doi: 10.1007/s00404-013-3050-2.
- Seracchioli R, Venturoli S, Vianello F, et al. Total laparoscopic hysterectomy compared with abdominal hysterectomy in the presence of a large uterus. J Am Assoc Gynecol Laparosc. 2002;9:333-338. doi: 10.1016/s1074-3804(05)60413.
- Boyd LR, Novetsky AP, Curtin JP. Effect of surgical volume on route of hysterectomy and short-term morbidity. Obstet Gynecol. 2010;116:909-915. doi: 10.1097/AOG.0b013e3181f395d9.
- Jin C, Hu Y, Chen XC, et al. Laparoscopic versus open myomectomy—a meta-analysis of randomized controlled trials. Eur J Obstet Gynecol Reprod Biol. 2009;145:14-21. doi: 10.1016/j.ejogrb.2009.03.009.
- Wechter ME, Stewart EA, Myers ER, et al. Leiomyoma-related hospitalization and surgery: prevalence and predicted growth based on population trends. Am J Obstet Gynecol. 2011;205:492.e1-e5. doi: 10.1016/j.ajog.2011.07.008.
- Bower JK, Schreiner PJ, Sternfeld B, et al. Black-White differences in hysterectomy prevalence: the CARDIA study. Am J Public Health. 2009;99:300-307. doi: 10.2105/AJPH.2008.133702.
- Ranjit A, Sharma M, Romano A, et al. Does universal insurance mitigate racial differences in minimally invasive hysterectomy? J Minim Invasive Gynecol. 2017;24. doi:10.1016/j.jmig.2017.03.016.
- Pollack LM, Olsen MA, Gehlert SJ, et al. Racial/ethnic disparities/differences in hysterectomy route in women likely eligible for minimally invasive surgery. J Minim Invasive Gynecol. 2020;27:1167-1177.e2. doi:10.1016/j.jmig.2019.09.003.
- Stentz NC, Cooney LG, Sammel MD, et al. Association of patient race with surgical practice and perioperative morbidity after myomectomy. Obstet Gynecol. 2018;132:291-297. doi: 10.1097/AOG.0000000000002738.
- Roth TM, Gustilo-Ashby T, Barber MD, et al. Effects of race and clinical factors on short-term outcomes of abdominal myomectomy. Obstet Gynecol. 2003;101(5 pt 1):881-884. doi: 10.1016/s0029-7844(03)00015-2.
- Bratka S, Diamond JS, Al-Hendy A, et al. The role of vitamin D in uterine fibroid biology. Fertil Steril. 2015;104:698-706. doi: 10.1016/j.fertnstert.2015.05.031.
- Ciebiera M, Łukaszuk K, Męczekalski B, et al. Alternative oral agents in prophylaxis and therapy of uterine fibroids—an up-to-date review. Int J Mol Sci. 2017;18:2586. doi:10.3390/ijms18122586.
- Hayden EC. Racial bias haunts NIH funding. Nature. 2015;527:145.
- Lett LA, Orji WU, Sebro R. Declining racial and ethnic representation in clinical academic medicine: a longitudinal study of 16 US medical specialties. PLoS One. 2018;13:e0207274. doi: 10.1371/journal.pone.0207274.
- Sánchez JP, Poll-Hunter N, Stern N, et al. Balancing two cultures: American Indian/Alaska Native medical students’ perceptions of academic medicine careers. J Community Health. 2016;41:871-880.
- Rayburn WF, Xierali IM, Castillo-Page L, et al. Racial and ethnic differences between obstetrician-gynecologists and other adult medical specialists. Obstet Gynecol. 2016;127:148-152. doi: 10.1097/AOG.0000000000001184.
- Esters D, Xierali IM, Nivet MA, et al. The rise of nontenured faculty in obstetrics and gynecology by sex and underrepresented in medicine status. Obstet Gynecol. 2019;134 suppl 1:34S-39S. doi: 10.1097/AOG.0000000000003484.
- Ly DP, Seabury SA, Jena AB. Differences in incomes of physicians in the United States by race and sex: observational study. BMJ. 2016;I2923. doi:10.1136/bmj.i2923.
- Groff JY, Mullen PD, Byrd T, et al. Decision making, beliefs, and attitudes toward hysterectomy: a focus group study with medically underserved women in Texas. J Womens Health Gend Based Med. 2000;9 suppl 2:S39-50. doi: 10.1089/152460900318759.
- Andryjowicz E, Wray T. Regional expansion of minimally invasive surgery for hysterectomy: implementation and methodology in a large multispecialty group. Perm J. 2011;15:42-46.
- Zaritsky E, Ojo A, Tucker LY, et al. Racial disparities in route of hysterectomy for benign indications within an integrated health care system. JAMA Netw Open. 2019;2:e1917004. doi: 10.1001/jamanetworkopen.2019.17004.
- Abel MK, Kho KA, Walter A, et al. Measuring quality in minimally invasive gynecologic surgery: what, how, and why? J Minim Invasive Gynecol. 2019;26:321-326. doi: 10.1016/j.jmig.2018.11.013.
- Ojanuga D. The medical ethics of the ‘father of gynaecology,’ Dr J Marion Sims. J Med Ethics. 1993;19:28-31. doi: 10.1136/jme.19.1.28.
- Borrero S, Zite N, Creinin MD. Federally funded sterilization: time to rethink policy? Am J Public Health. 2012;102:1822-1825.
- Eaglehouse YL, Georg MW, Shriver CD, et al. Racial differences in time to breast cancer surgery and overall survival in the US Military Health System. JAMA Surg. 2019;154:e185113. doi: 10.1001/jamasurg.2018.5113.
- Soliman AM, Yang H, Du EX, et al. The direct and indirect costs of uterine fibroid tumors: a systematic review of the literature between 2000 and 2013. Am J Obstet Gynecol. 2015;213:141-160.
- Baird DD, Dunson DB, Hill MC, et al. High cumulative incidence of uterine leiomyoma in black and white women: ultrasound evidence. Am J Obstet Gynecol. 2003;188:100-107.
- Marshall LM, Spiegelman D, Barbieri RL, et al. Variation in the incidence of uterine leiomyoma among premenopausal women by age and race. Obstet Gynecol. 1997;90:967-973. doi: 10.1016/s0029-7844(97)00534-6.
- Styer AK, Rueda BR. The epidemiology and genetics of uterine leiomyoma. Best Pract Res Clin Obstet Gynaecol. 2016;34:3-12. doi: 10.1016/j.bpobgyn.2015.11.018.
- Al-Hendy A, Myers ER, Stewart E. Uterine fibroids: burden and unmet medical need. Semin Reprod Med. 2017;35:473-480. doi: 10.1055/s-0037-1607264.
- American College of Obstetricians and Gynecologists. ACOG practice bulletin. Alternatives to hysterectomy in the management of leiomyomas. Obstet Gynecol. 2008;112(2 pt 1):387-400.
- Corona LE, Swenson CW, Sheetz KH, et al. Use of other treatments before hysterectomy for benign conditions in a statewide hospital collaborative. Am J Obstet Gynecol. 2015;212:304.e1-e7. doi: 10.1016/j.ajog.2014.11.031.
- Nguyen NT, Merchant M, Ritterman Weintraub ML, et al. Alternative treatment utilization before hysterectomy for benign gynecologic conditions at a large integrated health system. J Minim Invasive Gynecol. 2019;26:847-855. doi: 10.1016/j.jmig.2018.08.013.
- Laughlin-Tommaso SK, Jacoby VL, Myers ER. Disparities in fibroid incidence, prognosis, and management. Obstet Gynecol Clin North Am. 2017;44:81-94. doi: 10.1016/j.ogc.2016.11.007.
- Borah BJ, Laughlin-Tommaso SK, Myers ER, et al. Association between patient characteristics and treatment procedure among patients with uterine leiomyomas. Obstet Gynecol. 2016;127:67-77.
- Whiteman MK, Hillis SD, Jamieson DJ, et al. Inpatient hysterectomy surveillance in the United States, 2000-2004. Am J Obstet Gynecol. 2008;198:34.e1-e7. doi:10.1016/j.ajog.2007.05.039.
- Bardens D, Solomayer E, Baum S, et al. The impact of the body mass index (BMI) on laparoscopic hysterectomy for benign disease. Arch Gynecol Obstet. 2014;289:803-807. doi: 10.1007/s00404-013-3050-2.
- Seracchioli R, Venturoli S, Vianello F, et al. Total laparoscopic hysterectomy compared with abdominal hysterectomy in the presence of a large uterus. J Am Assoc Gynecol Laparosc. 2002;9:333-338. doi: 10.1016/s1074-3804(05)60413.
- Boyd LR, Novetsky AP, Curtin JP. Effect of surgical volume on route of hysterectomy and short-term morbidity. Obstet Gynecol. 2010;116:909-915. doi: 10.1097/AOG.0b013e3181f395d9.
- Jin C, Hu Y, Chen XC, et al. Laparoscopic versus open myomectomy—a meta-analysis of randomized controlled trials. Eur J Obstet Gynecol Reprod Biol. 2009;145:14-21. doi: 10.1016/j.ejogrb.2009.03.009.
- Wechter ME, Stewart EA, Myers ER, et al. Leiomyoma-related hospitalization and surgery: prevalence and predicted growth based on population trends. Am J Obstet Gynecol. 2011;205:492.e1-e5. doi: 10.1016/j.ajog.2011.07.008.
- Bower JK, Schreiner PJ, Sternfeld B, et al. Black-White differences in hysterectomy prevalence: the CARDIA study. Am J Public Health. 2009;99:300-307. doi: 10.2105/AJPH.2008.133702.
- Ranjit A, Sharma M, Romano A, et al. Does universal insurance mitigate racial differences in minimally invasive hysterectomy? J Minim Invasive Gynecol. 2017;24. doi:10.1016/j.jmig.2017.03.016.
- Pollack LM, Olsen MA, Gehlert SJ, et al. Racial/ethnic disparities/differences in hysterectomy route in women likely eligible for minimally invasive surgery. J Minim Invasive Gynecol. 2020;27:1167-1177.e2. doi:10.1016/j.jmig.2019.09.003.
- Stentz NC, Cooney LG, Sammel MD, et al. Association of patient race with surgical practice and perioperative morbidity after myomectomy. Obstet Gynecol. 2018;132:291-297. doi: 10.1097/AOG.0000000000002738.
- Roth TM, Gustilo-Ashby T, Barber MD, et al. Effects of race and clinical factors on short-term outcomes of abdominal myomectomy. Obstet Gynecol. 2003;101(5 pt 1):881-884. doi: 10.1016/s0029-7844(03)00015-2.
- Bratka S, Diamond JS, Al-Hendy A, et al. The role of vitamin D in uterine fibroid biology. Fertil Steril. 2015;104:698-706. doi: 10.1016/j.fertnstert.2015.05.031.
- Ciebiera M, Łukaszuk K, Męczekalski B, et al. Alternative oral agents in prophylaxis and therapy of uterine fibroids—an up-to-date review. Int J Mol Sci. 2017;18:2586. doi:10.3390/ijms18122586.
- Hayden EC. Racial bias haunts NIH funding. Nature. 2015;527:145.
- Lett LA, Orji WU, Sebro R. Declining racial and ethnic representation in clinical academic medicine: a longitudinal study of 16 US medical specialties. PLoS One. 2018;13:e0207274. doi: 10.1371/journal.pone.0207274.
- Sánchez JP, Poll-Hunter N, Stern N, et al. Balancing two cultures: American Indian/Alaska Native medical students’ perceptions of academic medicine careers. J Community Health. 2016;41:871-880.
- Rayburn WF, Xierali IM, Castillo-Page L, et al. Racial and ethnic differences between obstetrician-gynecologists and other adult medical specialists. Obstet Gynecol. 2016;127:148-152. doi: 10.1097/AOG.0000000000001184.
- Esters D, Xierali IM, Nivet MA, et al. The rise of nontenured faculty in obstetrics and gynecology by sex and underrepresented in medicine status. Obstet Gynecol. 2019;134 suppl 1:34S-39S. doi: 10.1097/AOG.0000000000003484.
- Ly DP, Seabury SA, Jena AB. Differences in incomes of physicians in the United States by race and sex: observational study. BMJ. 2016;I2923. doi:10.1136/bmj.i2923.
- Groff JY, Mullen PD, Byrd T, et al. Decision making, beliefs, and attitudes toward hysterectomy: a focus group study with medically underserved women in Texas. J Womens Health Gend Based Med. 2000;9 suppl 2:S39-50. doi: 10.1089/152460900318759.
- Andryjowicz E, Wray T. Regional expansion of minimally invasive surgery for hysterectomy: implementation and methodology in a large multispecialty group. Perm J. 2011;15:42-46.
- Zaritsky E, Ojo A, Tucker LY, et al. Racial disparities in route of hysterectomy for benign indications within an integrated health care system. JAMA Netw Open. 2019;2:e1917004. doi: 10.1001/jamanetworkopen.2019.17004.
- Abel MK, Kho KA, Walter A, et al. Measuring quality in minimally invasive gynecologic surgery: what, how, and why? J Minim Invasive Gynecol. 2019;26:321-326. doi: 10.1016/j.jmig.2018.11.013.
FDA and power morcellation, gel for vaginal odor, and an intrauterine electrosurgery system
FDA guidance for power morcellation
“The FDA has granted marketing authorization for one containment system and continues to encourage innovation in this area” said the report. Olympus’ Pneumoliner is the only FDA cleared containment device to provide a laparoscopic option for appropriately identified patients undergoing myomectomy and hysterectomy. The containment system is sold with Olympus’ PK Morcellator, but the company says that it has made the Pneumoliner available to physicians choosing an alternate to the PK Morcellator, provided that there is device compatibility. The Pneumoliner “reduces the spread of benign tissue into the abdominal cavity, in which pathologies, like fibroids, may regrow when tissue or cells are inadvertently left behind,” according to Olympus.
Vaginal odor elimination gel
The gel is sold in 7 single-day applications, with a single tube used per day at bedtime to eliminate unwanted odor. To maintain freshness and comfort, a single tube of Relactagel can be used for 2 to 3 days after a woman’s menstrual cycle, says Kora Healthcare. The company warns that mild irritation can occur with product use during fungal infections or when small tears are present in the vaginal tissue and that use should be discontinued if irritation occurs. In addition, if trying to become pregnant Relatagel should not be used, advises Kora Healthcare, although the gel is not a contraceptive.
Intrauterine electrosurgery system
FDA guidance for power morcellation
“The FDA has granted marketing authorization for one containment system and continues to encourage innovation in this area” said the report. Olympus’ Pneumoliner is the only FDA cleared containment device to provide a laparoscopic option for appropriately identified patients undergoing myomectomy and hysterectomy. The containment system is sold with Olympus’ PK Morcellator, but the company says that it has made the Pneumoliner available to physicians choosing an alternate to the PK Morcellator, provided that there is device compatibility. The Pneumoliner “reduces the spread of benign tissue into the abdominal cavity, in which pathologies, like fibroids, may regrow when tissue or cells are inadvertently left behind,” according to Olympus.
Vaginal odor elimination gel
The gel is sold in 7 single-day applications, with a single tube used per day at bedtime to eliminate unwanted odor. To maintain freshness and comfort, a single tube of Relactagel can be used for 2 to 3 days after a woman’s menstrual cycle, says Kora Healthcare. The company warns that mild irritation can occur with product use during fungal infections or when small tears are present in the vaginal tissue and that use should be discontinued if irritation occurs. In addition, if trying to become pregnant Relatagel should not be used, advises Kora Healthcare, although the gel is not a contraceptive.
Intrauterine electrosurgery system
FDA guidance for power morcellation
“The FDA has granted marketing authorization for one containment system and continues to encourage innovation in this area” said the report. Olympus’ Pneumoliner is the only FDA cleared containment device to provide a laparoscopic option for appropriately identified patients undergoing myomectomy and hysterectomy. The containment system is sold with Olympus’ PK Morcellator, but the company says that it has made the Pneumoliner available to physicians choosing an alternate to the PK Morcellator, provided that there is device compatibility. The Pneumoliner “reduces the spread of benign tissue into the abdominal cavity, in which pathologies, like fibroids, may regrow when tissue or cells are inadvertently left behind,” according to Olympus.
Vaginal odor elimination gel
The gel is sold in 7 single-day applications, with a single tube used per day at bedtime to eliminate unwanted odor. To maintain freshness and comfort, a single tube of Relactagel can be used for 2 to 3 days after a woman’s menstrual cycle, says Kora Healthcare. The company warns that mild irritation can occur with product use during fungal infections or when small tears are present in the vaginal tissue and that use should be discontinued if irritation occurs. In addition, if trying to become pregnant Relatagel should not be used, advises Kora Healthcare, although the gel is not a contraceptive.
Intrauterine electrosurgery system
Cesarean myomectomy: Safe operation or surgical folly?
Uterine leiomyomata (fibroids) are the most common pelvic tumor of women. When women are planning to conceive, and their fibroid(s) are clinically significant, causing abnormal uterine bleeding or bulk symptoms, it is often optimal to remove the uterine tumor(s) before conception. Advances in minimally invasive surgery offer women the option of laparoscopic or robot-assisted myomectomy with a low rate of operative complications, including excessive blood loss and hysterectomy, and a low rate of postoperative complications, including major pelvic adhesions and uterine rupture during subsequent pregnancy.1-3 However, many women become pregnant when they have clinically significant fibroids, and at least one-third of these women will have a cesarean birth.
Important clinical issues are the relative benefits and risks of performing a myomectomy at the time of the cesarean birth, so called cesarean myomectomy. Cesarean myomectomy offers carefully selected women the opportunity to have a cesarean birth and myomectomy in one operation, thereby avoiding a second major operation. Over the past 6 decades, most experts in the United States and the United Kingdom have strongly recommended against myomectomy at the time of cesarean delivery because of the risk of excessive blood loss and hysterectomy. Recently, expert opinion has shifted, especially in continental Europe and Asia, and cesarean myomectomy is now viewed as an acceptable surgical option in a limited number of clinical situations, including removal of pedunculated fibroids, excision of large solitary subserosal fibroids, and to achieve optimal management of the hysterotomy incision.
Decades of expert guidance: Avoid cesarean myomectomy at all costs
Dr. K.S.J. Olah succinctly captured the standard teaching that cesarean myomectomy should be avoided in this personal vignette:
Many years ago as a trainee I removed a subserosal fibroid during a cesarean section that was hanging by a thin stalk on the back of the uterus. The berating I received was severe and disproportionate to the crime. The rule was that myomectomy performed at cesarean section was not just frowned upon but expressly forbidden. It has always been considered foolish to consider removing fibroids at cesarean section, mostly because of the associated morbidity and the risk of haemorrhage requiring hysterectomy.4
Dr. Olah quoted guidance from Shaw’s Textbook of Operative Gynaecology,5 “It should be stressed that myomectomy in pregnancy should be avoided at all costs, including at caesarean section.” However, large case series published over the past 10 years report that, in limited clinical situations, cesarean myomectomy is a viable surgical option, where benefit may outweigh risk.6-14 The current literature has many weaknesses, including failure to specifically identify the indication for the cesarean myomectomy and lack of controlled prospective clinical trials. In almost all cases, cesarean myomectomy is performed after delivery of the fetus and placenta.
Continue to: The pedunculated, FIGO type 7 fibroid...
The pedunculated, FIGO type 7 fibroid
The International Federation of Gynecology and Obstetrics (FIGO) leiomyoma classification system identifies subserosal pedunculated fibroids as type 7 (FIGURE).15 Pedunculated fibroids are attached to the uterus by a stalk that is ≤10% of the mean of the 3 diameters of the fibroid. When a clinically significant pedunculated fibroid, causing bulk symptoms, is encountered at cesarean birth, I recommend that it be removed. This will save many patients a second major operation to perform a myomectomy. The surgical risk of removing a pedunculated is low.
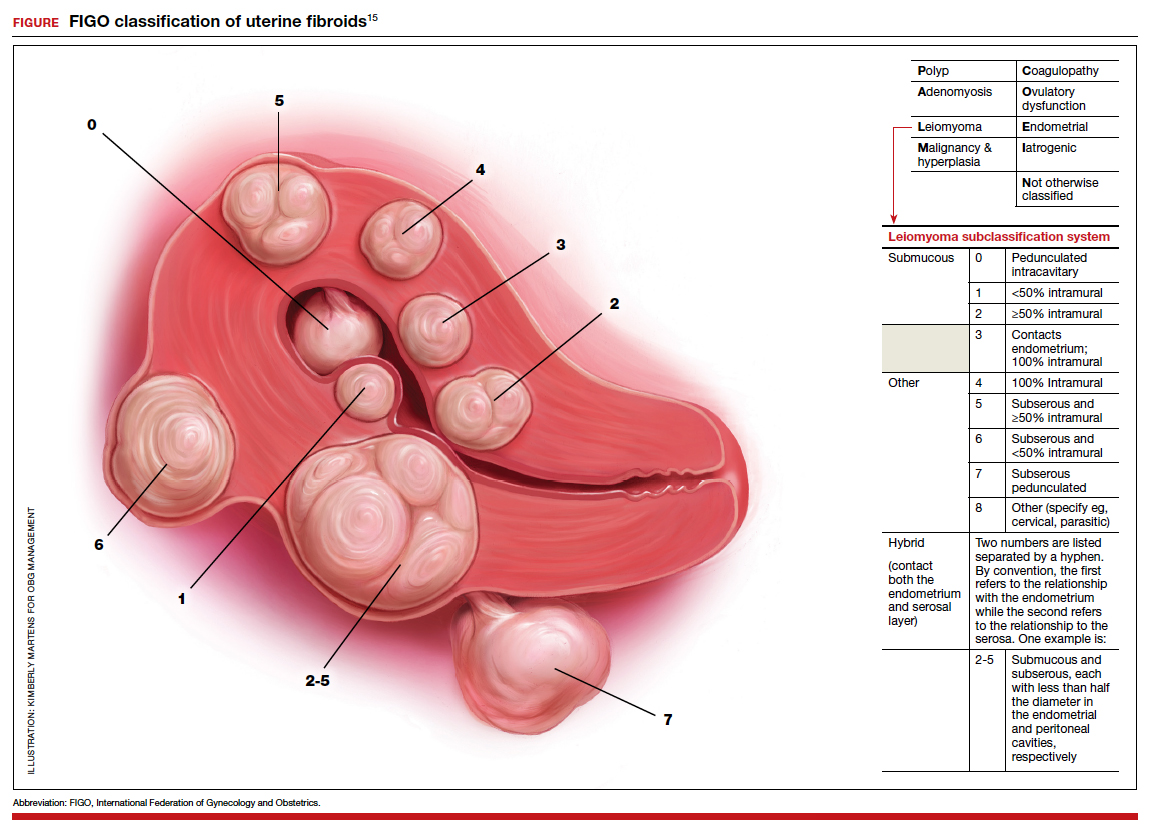
The solitary FIGO type 6 fibroid
Type 6 fibroids are subserosal fibroids with less than 50% of their mass being subserosal. The type 6 fibroid is relatively easy to enucleate from the uterus. Following removal of a type 6 fibroid, closure of the serosal defect is relatively straightforward. In carefully selected cases, if the type 6 fibroid is causing bulk symptoms, cesarean myomectomy may be indicated with a low risk of operative complications.
The FIGO type 2-5 fibroid
The type 2-5 fibroid is a transmural fibroid with significant mass abutting both the endometrial cavity and serosal surface. Excision of a type 2-5 fibroid is likely to result in a large transmyometrial defect that will be more difficult to close and could be associated with greater blood loss. Although data are limited, I would recommend against cesarean myomectomy for type 2-5 fibroids in most clinical situations.
Myomectomy to achieve optimal management of the cesarean hysterotomy incision
Many surgeons performing a cesarean birth for a woman with clinically significant fibroids will plan the hysterotomy incision to avoid the fibroids. However, following delivery and contraction of the uterus, proper closure of the hysterotomy incision may be very difficult without removing a fibroid that is abutting the hysterotomy incision. Surgeons have reported performing myomectomy on lower uterine segment fibroids before making the hysterotomy incision in order to facilitate the hysterotomy incision and closure.16 Myomectomy prior to delivery of the newborn must be associated with additional risks to the fetus. I would prefer to identify an optimal site to perform a hysterotomy, deliver the newborn and placenta, and then consider myomectomy.
Complications associated with cesarean myomectomy
The evidence concerning the complications of cesarean birth plus myomectomy compared with cesarean birth alone in women with fibroids is limited to case series. There are no reported controlled clinical trials to guide practice. The largest single case series reported on 1,242 women with fibroids who had a cesarean birth plus myomectomy compared with 3 control groups, including 200 women without fibroids who had a cesarean birth, 145 women with fibroids who had a cesarean birth and no myomectomy, and 51 women with fibroids who had a cesarean hysterectomy. The investigators reported no significant differences in preoperative to postoperative hemoglobin change, incidence of postoperative fever, or length of hospital stay among the 4 groups.8 The authors concluded that myomectomy during cesarean birth was a safe and effective procedure.
Continue to: A systematic review and meta-analysis reported...
A systematic review and meta-analysis reported on the results of 17 studies which included 4,702 women who had a cesarean myomectomy and 1,843 women with cesarean birth without myomectomy.17 The authors of the meta-analysis noted that most reported case series had excluded women with a high risk of bleeding, including women with placenta previa, placenta accreta, coagulation disorders, and a history of multiple myomectomy operations. The investigators reported that, compared with the control women, the women undergoing cesarean myomectomy had a statistically significant but clinically insignificant decrease in mean hemoglobin concentration (-0.27 g/dL), a significant increase in mean operative time (+15 minutes) and a significant increase in the length of hospital stay (+0.36 days). There was an increase in the need for blood transfusion (risk ratio, 1.45; 95% confidence interval, 1.05–1.99), but only 3% of women undergoing cesarean myomectomy received a blood transfusion. There was no significant difference between the two groups in the incidence of postoperative fever. The authors concluded that cesarean myomectomy is a safe procedure when performed by experienced surgeons with appropriate hemostatic techniques.
Techniques to reduce blood loss at the time of cesarean myomectomy
A detailed review of all the available techniques to reduce blood loss at the time of cesarean myomectomy is beyond the scope of this editorial. All gynecologists know that control of uterine blood flow through the uterine artery, infundibulopelvic vessels and internal iliac artery can help to reduce bleeding at the time of myomectomy. Tourniquets, vascular clamps, and artery ligation all have been reported to be useful at the time of cesarean myomectomy. In addition, intravenous infusion of oxytocin and tranexamic acid is often used at the time of cesarean myomectomy. Direct injection of uterotonics, including carbetocin, oxytocin, and vasopressin, into the uterus also has been reported. Cell saver blood salvage technology has been utilized in a limited number of cases of cesarean myomectomy.8,18,19
Medicine is not a static field
Discoveries and new data help guide advances in medical practice. After 6 decades of strict adherence to the advice that myomectomy in pregnancy should be avoided at all costs, including at caesarean delivery, new data indicate that in carefully selected cases cesarean myomectomy is an acceptable operation. ●
- Pitter MC, Gargiulo AR, Bonaventura LM, et al. Pregnancy outcomes following robot-assisted myomectomy. Hum Reprod. 2013;28:99-108.
- Pitter MC, Srouji SS, Gargiulo AR, et al. Fertility and symptom relief following robot-assisted laparoscopic myomectomy. Obstet Gynecol Int. 2015;2015:967568.
- Huberlant S, Lenot J, Neron M, et al. Fertility and obstetric outcomes after robot-assisted laparoscopic myomectomy. Int J Med Robot. 2020;16:e2059.
- Olah KSJ. Caesarean myomectomy: TE or not TE? BJOG. 2018;125:501.
- Shaw, et al. Textbook of Operative Gynaecology. Edinburgh: Churchill Livingston; 1977.
- Burton CA, Grimes DA, March CM. Surgical management of leiomyomata during pregnancy. Obstet Gynecol. 1989;74:707-709.
- Ortac F, Gungor M, Sonmezer M. Myomectomy during cesarean section. Int J Gynaecol Obstet. 1999;67:189-193.
- Li H, Du J, Jin L, et al. Myomectomy during cesarean section. Acta Obstetricia et Gynecologica. 2009;88:183-186.
- Kwon DH, Song JE, Yoon KR, et al. Obstet Gynecol Sci. 2014;57:367-372.
- Senturk MB, Polat M, Dogan O, et al. Outcome of cesarean myomectomy: is it a safe procedure? Geburtshilfe Frauenheilkd. 2017;77:1200-1206.
- Chauhan AR. Cesarean myomectomy: necessity or opportunity? J Obstet Gynecol India. 2018;68:432-436.
- Sparic R, Kadija S, Stefanovic A, et al. Cesarean myomectomy in modern obstetrics: more light and fewer shadows. J Obstet Gynaecol Res. 2017;43:798-804.
- Ramya T, Sabnis SS, Chitra TV, et al. Cesarean myomectomy: an experience from a tertiary care teaching hospital. J Obstet Gynaecol India. 2019;69:426-430.
- Zhao R, Wang X, Zou L, et al. Outcomes of myomectomy at the time of cesarean section among pregnant women with uterine fibroids: a retrospective cohort study. Biomed Res Int. 2019;7576934.
- Munro MG, Critchley HOD, Fraser IS; FIGO Menstrual Disorders Committee. The two FIGO systems for normal and abnormal uterine bleeding symptoms and classification of causes of abnormal uterine bleeding in the reproductive years: 2018 revisions. In J Gynaecol Obstet. 2018;143:393.
- Omar SZ, Sivanesaratnam V, Damodaran P. Large lower segment myoma—myomectomy at lower segment caesarean section—a report of two cases. Singapore Med J. 1999;40:109-110.
- Goyal M, Dawood AS, Elbohoty SB, et al. Cesarean myomectomy in the last ten years; A true shift from contraindication to indication: a systematic review and meta-analysis. Eur J Obstet Gynecol Reprod Biol. 2021;256:145-157.
- Lin JY, Lee WL, Wang PH, et al. Uterine artery occlusion and myomectomy for treatment of pregnant women with uterine leiomyomas who are undergoing caesarean section. J Obstet Gynecol Res. 2010;36:284-290.
- Alfred E, Joy G, Uduak O, et al. Cesarean myomectomy outcome in a Nigerian hospital district hospital. J Basic Clin Reprod Sci. 2013;2:115-118.

Uterine leiomyomata (fibroids) are the most common pelvic tumor of women. When women are planning to conceive, and their fibroid(s) are clinically significant, causing abnormal uterine bleeding or bulk symptoms, it is often optimal to remove the uterine tumor(s) before conception. Advances in minimally invasive surgery offer women the option of laparoscopic or robot-assisted myomectomy with a low rate of operative complications, including excessive blood loss and hysterectomy, and a low rate of postoperative complications, including major pelvic adhesions and uterine rupture during subsequent pregnancy.1-3 However, many women become pregnant when they have clinically significant fibroids, and at least one-third of these women will have a cesarean birth.
Important clinical issues are the relative benefits and risks of performing a myomectomy at the time of the cesarean birth, so called cesarean myomectomy. Cesarean myomectomy offers carefully selected women the opportunity to have a cesarean birth and myomectomy in one operation, thereby avoiding a second major operation. Over the past 6 decades, most experts in the United States and the United Kingdom have strongly recommended against myomectomy at the time of cesarean delivery because of the risk of excessive blood loss and hysterectomy. Recently, expert opinion has shifted, especially in continental Europe and Asia, and cesarean myomectomy is now viewed as an acceptable surgical option in a limited number of clinical situations, including removal of pedunculated fibroids, excision of large solitary subserosal fibroids, and to achieve optimal management of the hysterotomy incision.
Decades of expert guidance: Avoid cesarean myomectomy at all costs
Dr. K.S.J. Olah succinctly captured the standard teaching that cesarean myomectomy should be avoided in this personal vignette:
Many years ago as a trainee I removed a subserosal fibroid during a cesarean section that was hanging by a thin stalk on the back of the uterus. The berating I received was severe and disproportionate to the crime. The rule was that myomectomy performed at cesarean section was not just frowned upon but expressly forbidden. It has always been considered foolish to consider removing fibroids at cesarean section, mostly because of the associated morbidity and the risk of haemorrhage requiring hysterectomy.4
Dr. Olah quoted guidance from Shaw’s Textbook of Operative Gynaecology,5 “It should be stressed that myomectomy in pregnancy should be avoided at all costs, including at caesarean section.” However, large case series published over the past 10 years report that, in limited clinical situations, cesarean myomectomy is a viable surgical option, where benefit may outweigh risk.6-14 The current literature has many weaknesses, including failure to specifically identify the indication for the cesarean myomectomy and lack of controlled prospective clinical trials. In almost all cases, cesarean myomectomy is performed after delivery of the fetus and placenta.
Continue to: The pedunculated, FIGO type 7 fibroid...
The pedunculated, FIGO type 7 fibroid
The International Federation of Gynecology and Obstetrics (FIGO) leiomyoma classification system identifies subserosal pedunculated fibroids as type 7 (FIGURE).15 Pedunculated fibroids are attached to the uterus by a stalk that is ≤10% of the mean of the 3 diameters of the fibroid. When a clinically significant pedunculated fibroid, causing bulk symptoms, is encountered at cesarean birth, I recommend that it be removed. This will save many patients a second major operation to perform a myomectomy. The surgical risk of removing a pedunculated is low.

The solitary FIGO type 6 fibroid
Type 6 fibroids are subserosal fibroids with less than 50% of their mass being subserosal. The type 6 fibroid is relatively easy to enucleate from the uterus. Following removal of a type 6 fibroid, closure of the serosal defect is relatively straightforward. In carefully selected cases, if the type 6 fibroid is causing bulk symptoms, cesarean myomectomy may be indicated with a low risk of operative complications.
The FIGO type 2-5 fibroid
The type 2-5 fibroid is a transmural fibroid with significant mass abutting both the endometrial cavity and serosal surface. Excision of a type 2-5 fibroid is likely to result in a large transmyometrial defect that will be more difficult to close and could be associated with greater blood loss. Although data are limited, I would recommend against cesarean myomectomy for type 2-5 fibroids in most clinical situations.
Myomectomy to achieve optimal management of the cesarean hysterotomy incision
Many surgeons performing a cesarean birth for a woman with clinically significant fibroids will plan the hysterotomy incision to avoid the fibroids. However, following delivery and contraction of the uterus, proper closure of the hysterotomy incision may be very difficult without removing a fibroid that is abutting the hysterotomy incision. Surgeons have reported performing myomectomy on lower uterine segment fibroids before making the hysterotomy incision in order to facilitate the hysterotomy incision and closure.16 Myomectomy prior to delivery of the newborn must be associated with additional risks to the fetus. I would prefer to identify an optimal site to perform a hysterotomy, deliver the newborn and placenta, and then consider myomectomy.
Complications associated with cesarean myomectomy
The evidence concerning the complications of cesarean birth plus myomectomy compared with cesarean birth alone in women with fibroids is limited to case series. There are no reported controlled clinical trials to guide practice. The largest single case series reported on 1,242 women with fibroids who had a cesarean birth plus myomectomy compared with 3 control groups, including 200 women without fibroids who had a cesarean birth, 145 women with fibroids who had a cesarean birth and no myomectomy, and 51 women with fibroids who had a cesarean hysterectomy. The investigators reported no significant differences in preoperative to postoperative hemoglobin change, incidence of postoperative fever, or length of hospital stay among the 4 groups.8 The authors concluded that myomectomy during cesarean birth was a safe and effective procedure.
Continue to: A systematic review and meta-analysis reported...
A systematic review and meta-analysis reported on the results of 17 studies which included 4,702 women who had a cesarean myomectomy and 1,843 women with cesarean birth without myomectomy.17 The authors of the meta-analysis noted that most reported case series had excluded women with a high risk of bleeding, including women with placenta previa, placenta accreta, coagulation disorders, and a history of multiple myomectomy operations. The investigators reported that, compared with the control women, the women undergoing cesarean myomectomy had a statistically significant but clinically insignificant decrease in mean hemoglobin concentration (-0.27 g/dL), a significant increase in mean operative time (+15 minutes) and a significant increase in the length of hospital stay (+0.36 days). There was an increase in the need for blood transfusion (risk ratio, 1.45; 95% confidence interval, 1.05–1.99), but only 3% of women undergoing cesarean myomectomy received a blood transfusion. There was no significant difference between the two groups in the incidence of postoperative fever. The authors concluded that cesarean myomectomy is a safe procedure when performed by experienced surgeons with appropriate hemostatic techniques.
Techniques to reduce blood loss at the time of cesarean myomectomy
A detailed review of all the available techniques to reduce blood loss at the time of cesarean myomectomy is beyond the scope of this editorial. All gynecologists know that control of uterine blood flow through the uterine artery, infundibulopelvic vessels and internal iliac artery can help to reduce bleeding at the time of myomectomy. Tourniquets, vascular clamps, and artery ligation all have been reported to be useful at the time of cesarean myomectomy. In addition, intravenous infusion of oxytocin and tranexamic acid is often used at the time of cesarean myomectomy. Direct injection of uterotonics, including carbetocin, oxytocin, and vasopressin, into the uterus also has been reported. Cell saver blood salvage technology has been utilized in a limited number of cases of cesarean myomectomy.8,18,19
Medicine is not a static field
Discoveries and new data help guide advances in medical practice. After 6 decades of strict adherence to the advice that myomectomy in pregnancy should be avoided at all costs, including at caesarean delivery, new data indicate that in carefully selected cases cesarean myomectomy is an acceptable operation. ●

Uterine leiomyomata (fibroids) are the most common pelvic tumor of women. When women are planning to conceive, and their fibroid(s) are clinically significant, causing abnormal uterine bleeding or bulk symptoms, it is often optimal to remove the uterine tumor(s) before conception. Advances in minimally invasive surgery offer women the option of laparoscopic or robot-assisted myomectomy with a low rate of operative complications, including excessive blood loss and hysterectomy, and a low rate of postoperative complications, including major pelvic adhesions and uterine rupture during subsequent pregnancy.1-3 However, many women become pregnant when they have clinically significant fibroids, and at least one-third of these women will have a cesarean birth.
Important clinical issues are the relative benefits and risks of performing a myomectomy at the time of the cesarean birth, so called cesarean myomectomy. Cesarean myomectomy offers carefully selected women the opportunity to have a cesarean birth and myomectomy in one operation, thereby avoiding a second major operation. Over the past 6 decades, most experts in the United States and the United Kingdom have strongly recommended against myomectomy at the time of cesarean delivery because of the risk of excessive blood loss and hysterectomy. Recently, expert opinion has shifted, especially in continental Europe and Asia, and cesarean myomectomy is now viewed as an acceptable surgical option in a limited number of clinical situations, including removal of pedunculated fibroids, excision of large solitary subserosal fibroids, and to achieve optimal management of the hysterotomy incision.
Decades of expert guidance: Avoid cesarean myomectomy at all costs
Dr. K.S.J. Olah succinctly captured the standard teaching that cesarean myomectomy should be avoided in this personal vignette:
Many years ago as a trainee I removed a subserosal fibroid during a cesarean section that was hanging by a thin stalk on the back of the uterus. The berating I received was severe and disproportionate to the crime. The rule was that myomectomy performed at cesarean section was not just frowned upon but expressly forbidden. It has always been considered foolish to consider removing fibroids at cesarean section, mostly because of the associated morbidity and the risk of haemorrhage requiring hysterectomy.4
Dr. Olah quoted guidance from Shaw’s Textbook of Operative Gynaecology,5 “It should be stressed that myomectomy in pregnancy should be avoided at all costs, including at caesarean section.” However, large case series published over the past 10 years report that, in limited clinical situations, cesarean myomectomy is a viable surgical option, where benefit may outweigh risk.6-14 The current literature has many weaknesses, including failure to specifically identify the indication for the cesarean myomectomy and lack of controlled prospective clinical trials. In almost all cases, cesarean myomectomy is performed after delivery of the fetus and placenta.
Continue to: The pedunculated, FIGO type 7 fibroid...
The pedunculated, FIGO type 7 fibroid
The International Federation of Gynecology and Obstetrics (FIGO) leiomyoma classification system identifies subserosal pedunculated fibroids as type 7 (FIGURE).15 Pedunculated fibroids are attached to the uterus by a stalk that is ≤10% of the mean of the 3 diameters of the fibroid. When a clinically significant pedunculated fibroid, causing bulk symptoms, is encountered at cesarean birth, I recommend that it be removed. This will save many patients a second major operation to perform a myomectomy. The surgical risk of removing a pedunculated is low.

The solitary FIGO type 6 fibroid
Type 6 fibroids are subserosal fibroids with less than 50% of their mass being subserosal. The type 6 fibroid is relatively easy to enucleate from the uterus. Following removal of a type 6 fibroid, closure of the serosal defect is relatively straightforward. In carefully selected cases, if the type 6 fibroid is causing bulk symptoms, cesarean myomectomy may be indicated with a low risk of operative complications.
The FIGO type 2-5 fibroid
The type 2-5 fibroid is a transmural fibroid with significant mass abutting both the endometrial cavity and serosal surface. Excision of a type 2-5 fibroid is likely to result in a large transmyometrial defect that will be more difficult to close and could be associated with greater blood loss. Although data are limited, I would recommend against cesarean myomectomy for type 2-5 fibroids in most clinical situations.
Myomectomy to achieve optimal management of the cesarean hysterotomy incision
Many surgeons performing a cesarean birth for a woman with clinically significant fibroids will plan the hysterotomy incision to avoid the fibroids. However, following delivery and contraction of the uterus, proper closure of the hysterotomy incision may be very difficult without removing a fibroid that is abutting the hysterotomy incision. Surgeons have reported performing myomectomy on lower uterine segment fibroids before making the hysterotomy incision in order to facilitate the hysterotomy incision and closure.16 Myomectomy prior to delivery of the newborn must be associated with additional risks to the fetus. I would prefer to identify an optimal site to perform a hysterotomy, deliver the newborn and placenta, and then consider myomectomy.
Complications associated with cesarean myomectomy
The evidence concerning the complications of cesarean birth plus myomectomy compared with cesarean birth alone in women with fibroids is limited to case series. There are no reported controlled clinical trials to guide practice. The largest single case series reported on 1,242 women with fibroids who had a cesarean birth plus myomectomy compared with 3 control groups, including 200 women without fibroids who had a cesarean birth, 145 women with fibroids who had a cesarean birth and no myomectomy, and 51 women with fibroids who had a cesarean hysterectomy. The investigators reported no significant differences in preoperative to postoperative hemoglobin change, incidence of postoperative fever, or length of hospital stay among the 4 groups.8 The authors concluded that myomectomy during cesarean birth was a safe and effective procedure.
Continue to: A systematic review and meta-analysis reported...
A systematic review and meta-analysis reported on the results of 17 studies which included 4,702 women who had a cesarean myomectomy and 1,843 women with cesarean birth without myomectomy.17 The authors of the meta-analysis noted that most reported case series had excluded women with a high risk of bleeding, including women with placenta previa, placenta accreta, coagulation disorders, and a history of multiple myomectomy operations. The investigators reported that, compared with the control women, the women undergoing cesarean myomectomy had a statistically significant but clinically insignificant decrease in mean hemoglobin concentration (-0.27 g/dL), a significant increase in mean operative time (+15 minutes) and a significant increase in the length of hospital stay (+0.36 days). There was an increase in the need for blood transfusion (risk ratio, 1.45; 95% confidence interval, 1.05–1.99), but only 3% of women undergoing cesarean myomectomy received a blood transfusion. There was no significant difference between the two groups in the incidence of postoperative fever. The authors concluded that cesarean myomectomy is a safe procedure when performed by experienced surgeons with appropriate hemostatic techniques.
Techniques to reduce blood loss at the time of cesarean myomectomy
A detailed review of all the available techniques to reduce blood loss at the time of cesarean myomectomy is beyond the scope of this editorial. All gynecologists know that control of uterine blood flow through the uterine artery, infundibulopelvic vessels and internal iliac artery can help to reduce bleeding at the time of myomectomy. Tourniquets, vascular clamps, and artery ligation all have been reported to be useful at the time of cesarean myomectomy. In addition, intravenous infusion of oxytocin and tranexamic acid is often used at the time of cesarean myomectomy. Direct injection of uterotonics, including carbetocin, oxytocin, and vasopressin, into the uterus also has been reported. Cell saver blood salvage technology has been utilized in a limited number of cases of cesarean myomectomy.8,18,19
Medicine is not a static field
Discoveries and new data help guide advances in medical practice. After 6 decades of strict adherence to the advice that myomectomy in pregnancy should be avoided at all costs, including at caesarean delivery, new data indicate that in carefully selected cases cesarean myomectomy is an acceptable operation. ●
- Pitter MC, Gargiulo AR, Bonaventura LM, et al. Pregnancy outcomes following robot-assisted myomectomy. Hum Reprod. 2013;28:99-108.
- Pitter MC, Srouji SS, Gargiulo AR, et al. Fertility and symptom relief following robot-assisted laparoscopic myomectomy. Obstet Gynecol Int. 2015;2015:967568.
- Huberlant S, Lenot J, Neron M, et al. Fertility and obstetric outcomes after robot-assisted laparoscopic myomectomy. Int J Med Robot. 2020;16:e2059.
- Olah KSJ. Caesarean myomectomy: TE or not TE? BJOG. 2018;125:501.
- Shaw, et al. Textbook of Operative Gynaecology. Edinburgh: Churchill Livingston; 1977.
- Burton CA, Grimes DA, March CM. Surgical management of leiomyomata during pregnancy. Obstet Gynecol. 1989;74:707-709.
- Ortac F, Gungor M, Sonmezer M. Myomectomy during cesarean section. Int J Gynaecol Obstet. 1999;67:189-193.
- Li H, Du J, Jin L, et al. Myomectomy during cesarean section. Acta Obstetricia et Gynecologica. 2009;88:183-186.
- Kwon DH, Song JE, Yoon KR, et al. Obstet Gynecol Sci. 2014;57:367-372.
- Senturk MB, Polat M, Dogan O, et al. Outcome of cesarean myomectomy: is it a safe procedure? Geburtshilfe Frauenheilkd. 2017;77:1200-1206.
- Chauhan AR. Cesarean myomectomy: necessity or opportunity? J Obstet Gynecol India. 2018;68:432-436.
- Sparic R, Kadija S, Stefanovic A, et al. Cesarean myomectomy in modern obstetrics: more light and fewer shadows. J Obstet Gynaecol Res. 2017;43:798-804.
- Ramya T, Sabnis SS, Chitra TV, et al. Cesarean myomectomy: an experience from a tertiary care teaching hospital. J Obstet Gynaecol India. 2019;69:426-430.
- Zhao R, Wang X, Zou L, et al. Outcomes of myomectomy at the time of cesarean section among pregnant women with uterine fibroids: a retrospective cohort study. Biomed Res Int. 2019;7576934.
- Munro MG, Critchley HOD, Fraser IS; FIGO Menstrual Disorders Committee. The two FIGO systems for normal and abnormal uterine bleeding symptoms and classification of causes of abnormal uterine bleeding in the reproductive years: 2018 revisions. In J Gynaecol Obstet. 2018;143:393.
- Omar SZ, Sivanesaratnam V, Damodaran P. Large lower segment myoma—myomectomy at lower segment caesarean section—a report of two cases. Singapore Med J. 1999;40:109-110.
- Goyal M, Dawood AS, Elbohoty SB, et al. Cesarean myomectomy in the last ten years; A true shift from contraindication to indication: a systematic review and meta-analysis. Eur J Obstet Gynecol Reprod Biol. 2021;256:145-157.
- Lin JY, Lee WL, Wang PH, et al. Uterine artery occlusion and myomectomy for treatment of pregnant women with uterine leiomyomas who are undergoing caesarean section. J Obstet Gynecol Res. 2010;36:284-290.
- Alfred E, Joy G, Uduak O, et al. Cesarean myomectomy outcome in a Nigerian hospital district hospital. J Basic Clin Reprod Sci. 2013;2:115-118.
- Pitter MC, Gargiulo AR, Bonaventura LM, et al. Pregnancy outcomes following robot-assisted myomectomy. Hum Reprod. 2013;28:99-108.
- Pitter MC, Srouji SS, Gargiulo AR, et al. Fertility and symptom relief following robot-assisted laparoscopic myomectomy. Obstet Gynecol Int. 2015;2015:967568.
- Huberlant S, Lenot J, Neron M, et al. Fertility and obstetric outcomes after robot-assisted laparoscopic myomectomy. Int J Med Robot. 2020;16:e2059.
- Olah KSJ. Caesarean myomectomy: TE or not TE? BJOG. 2018;125:501.
- Shaw, et al. Textbook of Operative Gynaecology. Edinburgh: Churchill Livingston; 1977.
- Burton CA, Grimes DA, March CM. Surgical management of leiomyomata during pregnancy. Obstet Gynecol. 1989;74:707-709.
- Ortac F, Gungor M, Sonmezer M. Myomectomy during cesarean section. Int J Gynaecol Obstet. 1999;67:189-193.
- Li H, Du J, Jin L, et al. Myomectomy during cesarean section. Acta Obstetricia et Gynecologica. 2009;88:183-186.
- Kwon DH, Song JE, Yoon KR, et al. Obstet Gynecol Sci. 2014;57:367-372.
- Senturk MB, Polat M, Dogan O, et al. Outcome of cesarean myomectomy: is it a safe procedure? Geburtshilfe Frauenheilkd. 2017;77:1200-1206.
- Chauhan AR. Cesarean myomectomy: necessity or opportunity? J Obstet Gynecol India. 2018;68:432-436.
- Sparic R, Kadija S, Stefanovic A, et al. Cesarean myomectomy in modern obstetrics: more light and fewer shadows. J Obstet Gynaecol Res. 2017;43:798-804.
- Ramya T, Sabnis SS, Chitra TV, et al. Cesarean myomectomy: an experience from a tertiary care teaching hospital. J Obstet Gynaecol India. 2019;69:426-430.
- Zhao R, Wang X, Zou L, et al. Outcomes of myomectomy at the time of cesarean section among pregnant women with uterine fibroids: a retrospective cohort study. Biomed Res Int. 2019;7576934.
- Munro MG, Critchley HOD, Fraser IS; FIGO Menstrual Disorders Committee. The two FIGO systems for normal and abnormal uterine bleeding symptoms and classification of causes of abnormal uterine bleeding in the reproductive years: 2018 revisions. In J Gynaecol Obstet. 2018;143:393.
- Omar SZ, Sivanesaratnam V, Damodaran P. Large lower segment myoma—myomectomy at lower segment caesarean section—a report of two cases. Singapore Med J. 1999;40:109-110.
- Goyal M, Dawood AS, Elbohoty SB, et al. Cesarean myomectomy in the last ten years; A true shift from contraindication to indication: a systematic review and meta-analysis. Eur J Obstet Gynecol Reprod Biol. 2021;256:145-157.
- Lin JY, Lee WL, Wang PH, et al. Uterine artery occlusion and myomectomy for treatment of pregnant women with uterine leiomyomas who are undergoing caesarean section. J Obstet Gynecol Res. 2010;36:284-290.
- Alfred E, Joy G, Uduak O, et al. Cesarean myomectomy outcome in a Nigerian hospital district hospital. J Basic Clin Reprod Sci. 2013;2:115-118.