User login
Simplifying Allergic Contact Dermatitis Management with the Contact Allergen Management Program 2.0
Simplifying Allergic Contact Dermatitis Management with the Contact Allergen Management Program 2.0
While patch testing is the gold standard to diagnose type IV cutaneous hypersensitivity reactions, interpreting results can feel like trying to decipher a secret code, leaving patients feeling disempowered in avoiding their triggers. To truly manage allergic contact dermatitis (ACD), patients need comprehensive education on which allergens to avoid and ways to spot potential sources of exposure, including counseling, written guidelines, and lists of product alternatives.1 Patients who can recall and avoid their triggers experience greater improvement in clinical and quality-of-life scores.2 However, several studies have demonstrated that patients have difficulty recalling their allergens, even with longitudinal reminders.2-5 Quality-of-life and clinical outcomes also are not necessarily improved by successful allergen recall alone, as patients have reported limited success in actually avoiding allergens, highlighting the complexity of navigating exposures in daily life.2,6 To address these challenges, we examine common pitfalls patients encounter when avoiding allergens, highlight the benefits of utilizing safe lists and databases for allergen management, and introduce the updated Contact Allergen Management Program (CAMP) 2.0 as an optimal tool for long-term management of ACD.
Allergen Avoidance Pitfalls
Simply reading ingredient labels to avoid allergens is only marginally effective, as patients need to identify and interpret multiple chemical names as well as cross-reactors and related compounds to achieve success. Some allergens, such as fragrances or manufacturing impurities, are not explicitly identified on product labels. Even patients who can practice diligent label reading may struggle to find information on household or occupational products when full ingredient disclosure is not required.
Many of the allergens included in the American Contact Dermatitis Society (ACDS) Core 90 Series have alternative chemical aliases, and many have related compounds.6 For example, individuals with contact allergy to formaldehyde or a formaldehyde releaser usually need to avoid multiple other formaldehyde-releasing chemicals. Patients who test positive to amidoamine or dimethylaminopropylamine also must avoid the surfactant cocamidopropyl betaine—not because it is a cross-reactor, but because it is an impurity in the synthetic pathway.
Fragrance is one of the most common causes of ACD but can be challenging to avoid. Patients with allergies to fragrance or specific compounds (eg, limonene, linalool hydroperoxides) need to be savvy enough to navigate a broad spectrum of synthetic and botanical fragrance additives. Avoiding products that contain “fragrance” or “parfum” is simple enough, but patients also may need to recognize more than 3000 chemical names to identify individual fragrance ingredients that may be listed separately.7 Further, some fragrances are added for alternative purposes—preservative, medicinal, or emulsification—in which case products may deceptively tout themselves as being “fragrance free” yet still contain a fragrance allergen. This is made even more complex considering additional additives that commonly may cross-react with individual fragrance compounds; balsam of Peru, for example, is a botanical amalgam containing more than 250 compounds, including several fragrance components, making it an excellent indicator of fragrance allergy.8 While balsam of Peru and its fragrance constituents will almost never be listed on a product label, it cross-reacts with several benzyl derivatives commonly used in cosmetic formulations, such as benzyl alcohol, benzyl acetate, benzoic acid, benzyl benzoate, and benzyl cinnamate.9,10
Given that ACD is a common reason for patients to seek dermatologic care, it is crucial for clinicians to equip themselves with effective strategies to support patients after patch testing.11 This includes efficient translation of patch test results into practical advice while avoiding the oversimplified suggestion to read product labels; however, education alone cannot address the complexities of managing ACD, which is where contact allergen databases come into play.
An Essential Tool: Patient Allergen Databases and Safe Lists
Contact allergen databases are like a trusty sidekick for patients and clinicians, providing easily accessible information and tools to support allergen avoidance and improve ACD outcomes. While there are several existing resources, the ACDS launched its CAMP database in 2011 for ACDS members and their patients.12 The CAMP allows clinicians to easily generate personalized safe lists for household, medicament, and personal care products, facilitating seamless patient access both online and via a mobile application. The database also includes allergen-specific handouts to guide patient education.13 A key highlight of the CAMP is automated management of cross-reactors, which allows patients to choose products without having to memorize complex cross-reactor algorithms and helps avoid overly restrictive safe lists (Table).12-15
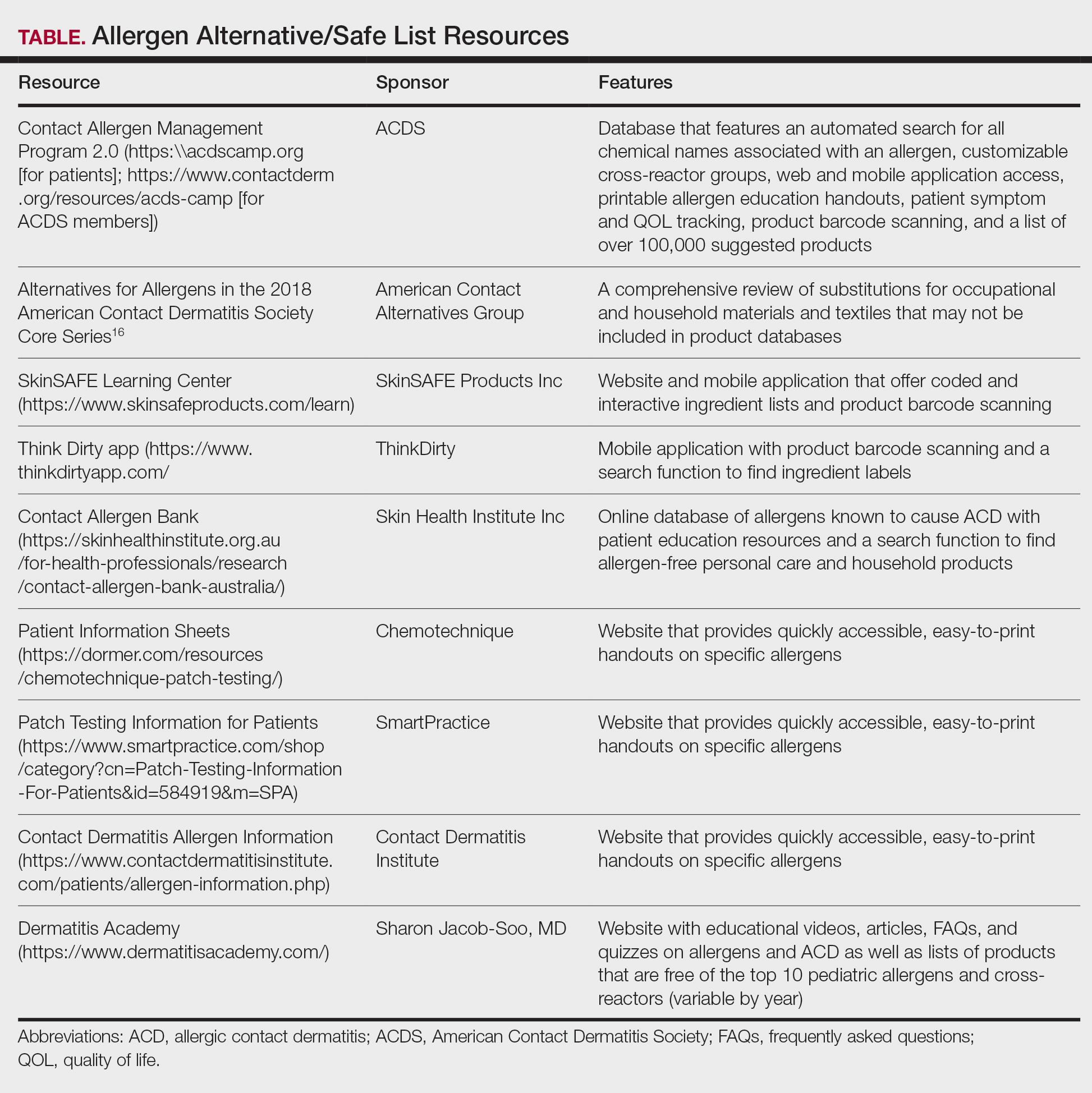
Other databases and online resources provide similar features, such as resources for patient education or finding safe products. The 2018 Alternatives for Allergens report is a vital adjunctive resource for guiding patients to suitable allergen-free products not included in commonly accessible product databases such as occupational materials, medical adhesives, shoes, or textiles.16
Introduction of CAMP 2.0
The latest version, CAMP 2.0, was launched in late 2024. The fully revamped database has a catalog of more than 100,000 products and comes packed with features that address many of the limitations found in the original CAMP. How does CAMP 2.0 work? The clinician inputs the patient’s allergens and makes choices about cross-reactor groups, and CAMP 2.0 outputs a list of allergen-free products that the patient can use when shopping for personal care products and the clinician can use for prescribing medicaments. The new user experience is intended to be more informative and engaging for all parties.
The CAMP 2.0 interface offers frequent product updates and streamlined database navigation, including enhanced search functions, barcode scanning, and a new mobile application for Apple and Android users. The mobile application also allows patients to track their symptoms and quality of life over time. With this additional functionality, there also is an extensive section for frequently asked questions and tutorials to help patients understand and utilize these features effectively.
Patients no longer have to wonder if a product that is not listed on their safe list is actually unsafe or just missing from the database. Several new features, including color-coded ingredient lists and organization of search codes into “safe” and “unsafe” product lists (Figure 1), help increase product transparency. These features can facilitate patient recognition of allergen names and cross-reactors in selected products. Future updates will include product purchasing through the mobile application and more educational handouts, including Spanish translations and dietary guidelines for systemic contact dermatitis.
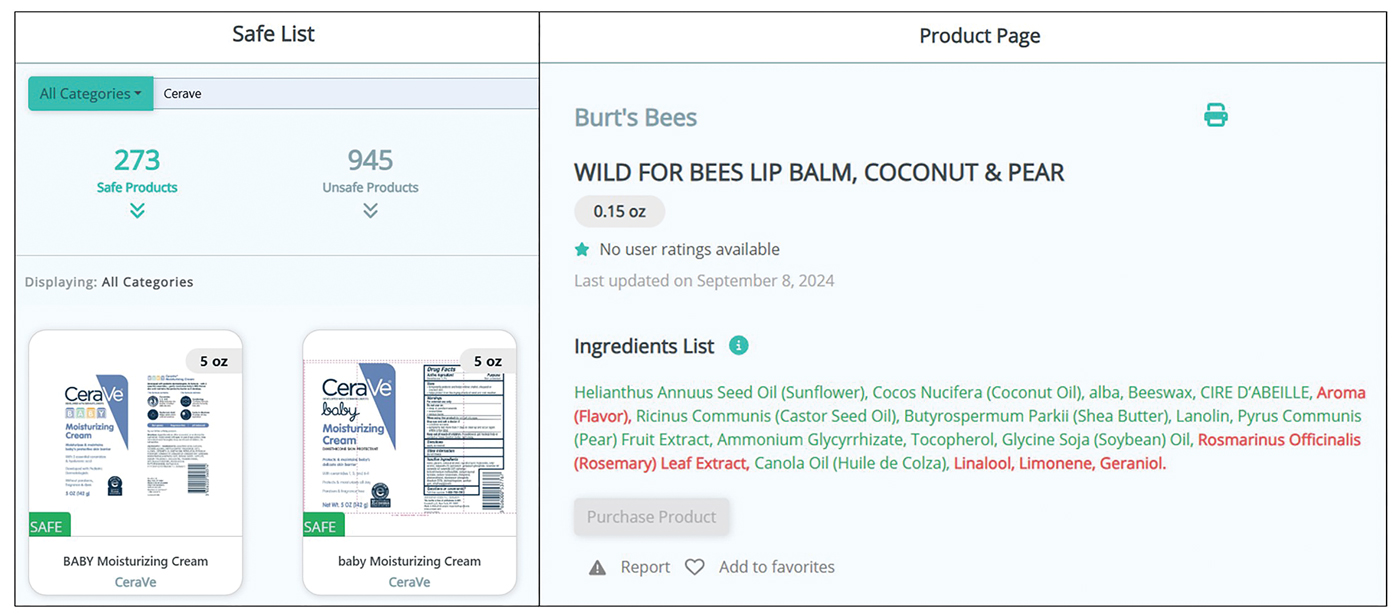
Patient Experience—Once patients complete patch testing with an ACDS member, they can access the CAMP 2.0 database for free via web-based or a mobile application. After setting up an account, patients gain immediate access to their allergen information, product database, and educational resources about ACD and CAMP 2.0. Patients can search for specific products using text or barcode scanning or browse through categorized lists of medical, household, and personal care items. Each product page contains the product name and brand along with a color-coded ingredient list to help patients identify safe and unsafe ingredients at a glance (Figure 1). Products not currently included in the database can be requested using the “Add Product” feature. Additional patient engagement features include options to mark favorite products, write reviews, and track quality of life over time.
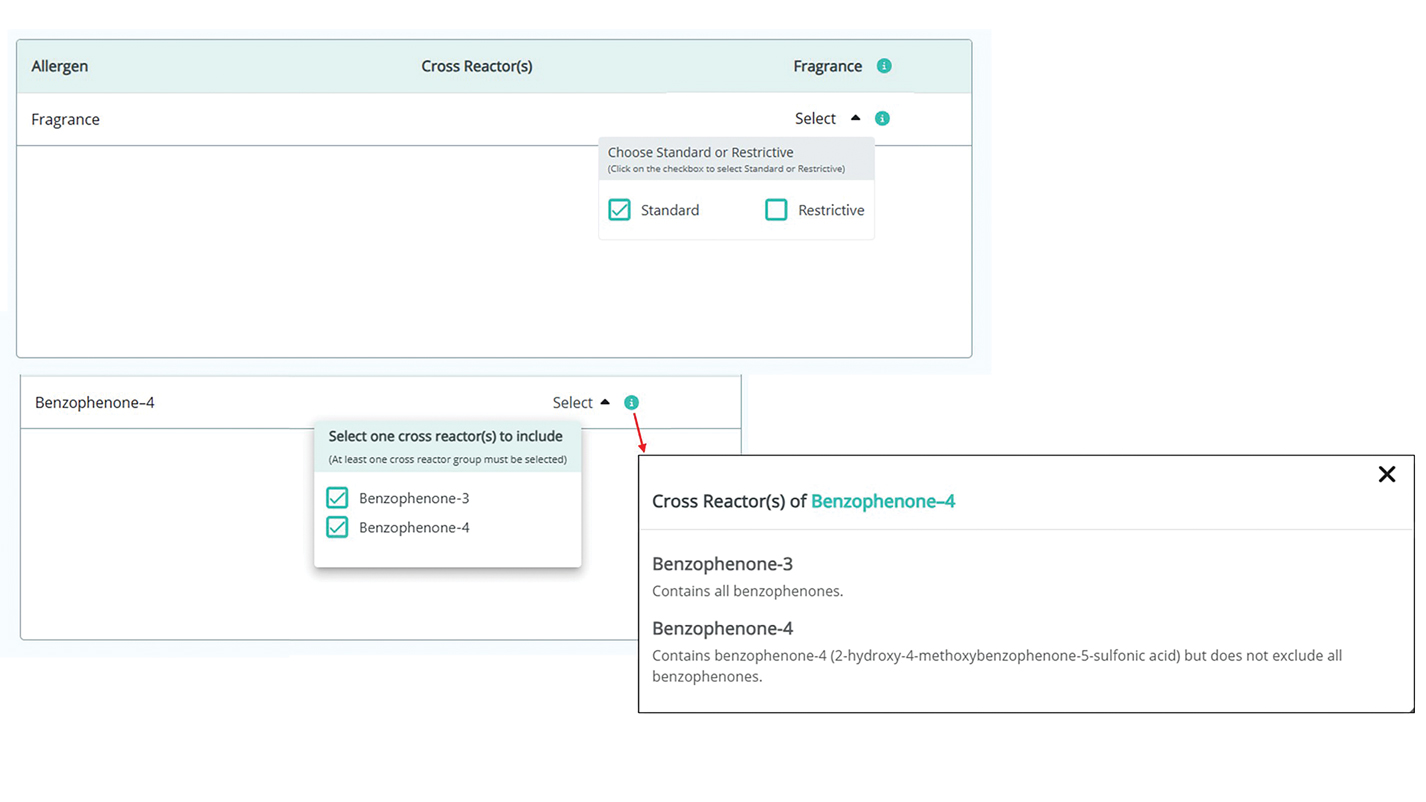
Physician Experience—The updated version includes several tutorials and frequently asked questions on how to improve ACD management and make the most of the new CAMP 2.0 tools and features. Generating patient allergen codes has been streamlined with an “Allergen Search” feature, allowing providers to quickly search and add or remove allergens to patients’ safe lists. Cross-reactor groups may be selectively added or removed for greater transparency and specificity in creating a patient safe list (Figure 2). Allergen codes now can be edited over time and are available for patient use via alphanumeric text or QR code format, which easily can be printed on a handout with instructions to help patients get acquainted with the system. For patient counseling, updated education handouts are available in the patient’s app and may be printed to provide supportive written educational material.
Approach to Long-Term Follow-up
When it comes to getting the most from patch testing, ongoing allergen avoidance is crucial. Patients may not see improvement unless they understand what ACD is and what needs to be done to improve it as well as become familiar with the names and common sources of their triggers.17 Clinicians can use CAMP 2.0 to facilitate patient improvement after patch testing, focusing on 3 key areas: continued patient education, patients’ ongoing progress in avoiding allergens, and monitored clinical improvement.
A solid understanding of ACD, such as its delayed (ie, 24-72 hours) onset after exposure, the need for allergen avoidance for at least 4 to 6 weeks before seeing improvement, and correlation of identified allergens with daily exposures, plays a major role in patient success. The CAMP 2.0 patch testing basics section is an excellent resource for patient-friendly explanations on patch testing and ACD. This resource, as well as allergen education handouts, may be reviewed at follow-up visits to continue to solidify patient learning.
Patients often have questions about allergen avoidance, such as occupational exposures, the suitability of specific products, or specific allergen names. These discussions are helpful for gauging how well patients are equipped to avoid their triggers as well as any hurdles they may be facing. If a patient still is experiencing flares after 6 to 8 weeks of safe-list adherence, it is important to take a thorough history of product use, daily exposures, and the patterns of distribution on the skin. Possible allergen exposures via topical medications also should be considered.18,19 Cross-checking products with a patient’s CAMP 2.0 safe list and correlating exposures with the continued ACD distribution are effective strategies to troubleshoot for unknown exposures to allergens.
Final Thoughts
Helping patients avoid allergens is essential to long-term management of ACD. The CAMP 2.0 safe list is an essential tool and a comprehensive reference for both patients and clinicians. With CAMP 2.0, allergen avoidance has never been more interactive or accessible.
- Tam I, Yu J. Allergic contact dermatitis in children: recommendations for patch testing. Curr Allergy Asthma Rep. 2020;20:41. doi:10.1007 /s11882-020-00939-z
- Dizdarevic A, Troensegaard W, Uldahl A, et al. Intervention study to evaluate the importance of information given to patients with contact allergy: a randomized, investigator-blinded clinical trial. Br J Dermatol. 2021;184:43-49. doi:10.1111/bjd.19119
- Jamil WN, Erikssohn I, Lindberg M. How well is the outcome of patch testing remembered by the patients? a 10-year follow-up of testing with the Swedish baseline series at the Department of Dermatology in Örebro, Sweden. Contact Dermatitis. 2012;66:215-220. doi:10.1111/j.1600-0536.2011.02039.x
- Scalf LA, Genebriera J, Davis MDP, et al. Patients’ perceptions of the usefulness and outcome of patch testing. J Am Acad Dermatol. 2007;56:928-932. doi:10.1016/j.jaad.2006.11.034
- Mossing K, Dizdarevic A, Svensson Å, et al. Impact on quality of life of an intervention providing additional information to patients with allergic contact dermatitis; a randomized clinical trial. J Eur Acad Dermatol Venereol. 2022;36:2166-2171. doi:10.1111/jdv.18412
- Schalock PC, Dunnick CA, Nedorost S, et al. American Contact Dermatitis Society Core Allergen Series: 2020 Update. Dermatitis. 2020;31:279-282. doi:10.1097/DER.0000000000000621
- Ingredient Breakdown: Fragrance. Think Dirty® Shop Clean. Accessed January 9, 2025. https://www.thinkdirtyapp.com/ingredient-breakdown-fragrance-3a8ef28f296a/
- Guarneri F, Corazza M, Stingeni L, et al. Myroxylon pereirae (balsam of Peru): still worth testing? Contact Dermatitis. 2021;85:269-273. doi:10.1111/cod.13839
- de Groot AC. Myroxylon pereirae resin (balsam of Peru)—a critical review of the literature and assessment of the significance of positive patch test reactions and the usefulness of restrictive diets. Contact Dermatitis. 2019;80:335-353. doi:10.1111/cod.13263
- Balsam of Peru: past and future. Allergic Contact Dermatitis Society; 2024. https://www.contactderm.org/UserFiles/members/Balsam_of_Peru___Past_and_Future.2.pdf
- Tramontana M, Hansel K, Bianchi L, et al. Advancing the understanding of allergic contact dermatitis: from pathophysiology to novel therapeutic approaches. Front Med. 2023;10. doi:10.3389 /fmed.2023.1184289
- McNamara D. ACDS launches Contact Allergen Management Program (CAMP). Internal Med News. March 7, 2011. Accessed December 31, 2024. https://www.mdedge.com/content/acds-launches-contact-allergen-management-program-camp-0
- Haque MZ, Rehman R, Guan L, et al. Recommendations to optimize patient education for allergic contact dermatitis: our approach. Contact Dermatitis. 2023;88:423-424. doi:10.1111/cod.14269
- Kist JM, el-Azhary RA, Hentz JG, et al. The Contact Allergen Replacement Database and treatment of allergic contact dermatitis. Arch Dermatol. 2004;140:1448-1450. doi:0.1001/archderm.140.12.1448
- El-Azhary RA, Yiannias JA. A new patient education approach in contact allergic dermatitis: the Contact Allergen Replacement Database (CARD). Int J Dermatol. 2004;43:278-280. doi:10.1111 /j.1365-4632.2004.01843.x
- Scheman A, Hylwa-Deufel S, Jacob SE, et al. Alternatives for allergens in the 2018 American Contact Dermatitis Society Core Series: report by the American Contact Alternatives Group. Dermatitis. 2019;30:87-105. doi:10.1097/DER.0000000000000453
- Mowad CM, Anderson B, Scheinman P, et al. Allergic contact dermatitis: patient management and education. J Am Acad Dermatol. 2016;74:1043-1054. doi:10.1016/j.jaad.2015.02.1144
- Ng A, Atwater AR, Reeder M. Contact allergy to topical medicaments, part 1: a double-edged sword. Cutis. 2021;108:271-275. doi:10.12788 /cutis.0390
- Nardelli A, D’Hooghe E, Drieghe J, et al. Allergic contact dermatitis from fragrance components in specific topical pharmaceutical products in Belgium. Contact Dermatitis. 2009;60:303-313. doi:10.1111 /j.1600-0536.2009.01542.x
While patch testing is the gold standard to diagnose type IV cutaneous hypersensitivity reactions, interpreting results can feel like trying to decipher a secret code, leaving patients feeling disempowered in avoiding their triggers. To truly manage allergic contact dermatitis (ACD), patients need comprehensive education on which allergens to avoid and ways to spot potential sources of exposure, including counseling, written guidelines, and lists of product alternatives.1 Patients who can recall and avoid their triggers experience greater improvement in clinical and quality-of-life scores.2 However, several studies have demonstrated that patients have difficulty recalling their allergens, even with longitudinal reminders.2-5 Quality-of-life and clinical outcomes also are not necessarily improved by successful allergen recall alone, as patients have reported limited success in actually avoiding allergens, highlighting the complexity of navigating exposures in daily life.2,6 To address these challenges, we examine common pitfalls patients encounter when avoiding allergens, highlight the benefits of utilizing safe lists and databases for allergen management, and introduce the updated Contact Allergen Management Program (CAMP) 2.0 as an optimal tool for long-term management of ACD.
Allergen Avoidance Pitfalls
Simply reading ingredient labels to avoid allergens is only marginally effective, as patients need to identify and interpret multiple chemical names as well as cross-reactors and related compounds to achieve success. Some allergens, such as fragrances or manufacturing impurities, are not explicitly identified on product labels. Even patients who can practice diligent label reading may struggle to find information on household or occupational products when full ingredient disclosure is not required.
Many of the allergens included in the American Contact Dermatitis Society (ACDS) Core 90 Series have alternative chemical aliases, and many have related compounds.6 For example, individuals with contact allergy to formaldehyde or a formaldehyde releaser usually need to avoid multiple other formaldehyde-releasing chemicals. Patients who test positive to amidoamine or dimethylaminopropylamine also must avoid the surfactant cocamidopropyl betaine—not because it is a cross-reactor, but because it is an impurity in the synthetic pathway.
Fragrance is one of the most common causes of ACD but can be challenging to avoid. Patients with allergies to fragrance or specific compounds (eg, limonene, linalool hydroperoxides) need to be savvy enough to navigate a broad spectrum of synthetic and botanical fragrance additives. Avoiding products that contain “fragrance” or “parfum” is simple enough, but patients also may need to recognize more than 3000 chemical names to identify individual fragrance ingredients that may be listed separately.7 Further, some fragrances are added for alternative purposes—preservative, medicinal, or emulsification—in which case products may deceptively tout themselves as being “fragrance free” yet still contain a fragrance allergen. This is made even more complex considering additional additives that commonly may cross-react with individual fragrance compounds; balsam of Peru, for example, is a botanical amalgam containing more than 250 compounds, including several fragrance components, making it an excellent indicator of fragrance allergy.8 While balsam of Peru and its fragrance constituents will almost never be listed on a product label, it cross-reacts with several benzyl derivatives commonly used in cosmetic formulations, such as benzyl alcohol, benzyl acetate, benzoic acid, benzyl benzoate, and benzyl cinnamate.9,10
Given that ACD is a common reason for patients to seek dermatologic care, it is crucial for clinicians to equip themselves with effective strategies to support patients after patch testing.11 This includes efficient translation of patch test results into practical advice while avoiding the oversimplified suggestion to read product labels; however, education alone cannot address the complexities of managing ACD, which is where contact allergen databases come into play.
An Essential Tool: Patient Allergen Databases and Safe Lists
Contact allergen databases are like a trusty sidekick for patients and clinicians, providing easily accessible information and tools to support allergen avoidance and improve ACD outcomes. While there are several existing resources, the ACDS launched its CAMP database in 2011 for ACDS members and their patients.12 The CAMP allows clinicians to easily generate personalized safe lists for household, medicament, and personal care products, facilitating seamless patient access both online and via a mobile application. The database also includes allergen-specific handouts to guide patient education.13 A key highlight of the CAMP is automated management of cross-reactors, which allows patients to choose products without having to memorize complex cross-reactor algorithms and helps avoid overly restrictive safe lists (Table).12-15

Other databases and online resources provide similar features, such as resources for patient education or finding safe products. The 2018 Alternatives for Allergens report is a vital adjunctive resource for guiding patients to suitable allergen-free products not included in commonly accessible product databases such as occupational materials, medical adhesives, shoes, or textiles.16
Introduction of CAMP 2.0
The latest version, CAMP 2.0, was launched in late 2024. The fully revamped database has a catalog of more than 100,000 products and comes packed with features that address many of the limitations found in the original CAMP. How does CAMP 2.0 work? The clinician inputs the patient’s allergens and makes choices about cross-reactor groups, and CAMP 2.0 outputs a list of allergen-free products that the patient can use when shopping for personal care products and the clinician can use for prescribing medicaments. The new user experience is intended to be more informative and engaging for all parties.
The CAMP 2.0 interface offers frequent product updates and streamlined database navigation, including enhanced search functions, barcode scanning, and a new mobile application for Apple and Android users. The mobile application also allows patients to track their symptoms and quality of life over time. With this additional functionality, there also is an extensive section for frequently asked questions and tutorials to help patients understand and utilize these features effectively.
Patients no longer have to wonder if a product that is not listed on their safe list is actually unsafe or just missing from the database. Several new features, including color-coded ingredient lists and organization of search codes into “safe” and “unsafe” product lists (Figure 1), help increase product transparency. These features can facilitate patient recognition of allergen names and cross-reactors in selected products. Future updates will include product purchasing through the mobile application and more educational handouts, including Spanish translations and dietary guidelines for systemic contact dermatitis.

Patient Experience—Once patients complete patch testing with an ACDS member, they can access the CAMP 2.0 database for free via web-based or a mobile application. After setting up an account, patients gain immediate access to their allergen information, product database, and educational resources about ACD and CAMP 2.0. Patients can search for specific products using text or barcode scanning or browse through categorized lists of medical, household, and personal care items. Each product page contains the product name and brand along with a color-coded ingredient list to help patients identify safe and unsafe ingredients at a glance (Figure 1). Products not currently included in the database can be requested using the “Add Product” feature. Additional patient engagement features include options to mark favorite products, write reviews, and track quality of life over time.
Physician Experience—The updated version includes several tutorials and frequently asked questions on how to improve ACD management and make the most of the new CAMP 2.0 tools and features. Generating patient allergen codes has been streamlined with an “Allergen Search” feature, allowing providers to quickly search and add or remove allergens to patients’ safe lists. Cross-reactor groups may be selectively added or removed for greater transparency and specificity in creating a patient safe list (Figure 2). Allergen codes now can be edited over time and are available for patient use via alphanumeric text or QR code format, which easily can be printed on a handout with instructions to help patients get acquainted with the system. For patient counseling, updated education handouts are available in the patient’s app and may be printed to provide supportive written educational material.

Approach to Long-Term Follow-up
When it comes to getting the most from patch testing, ongoing allergen avoidance is crucial. Patients may not see improvement unless they understand what ACD is and what needs to be done to improve it as well as become familiar with the names and common sources of their triggers.17 Clinicians can use CAMP 2.0 to facilitate patient improvement after patch testing, focusing on 3 key areas: continued patient education, patients’ ongoing progress in avoiding allergens, and monitored clinical improvement.
A solid understanding of ACD, such as its delayed (ie, 24-72 hours) onset after exposure, the need for allergen avoidance for at least 4 to 6 weeks before seeing improvement, and correlation of identified allergens with daily exposures, plays a major role in patient success. The CAMP 2.0 patch testing basics section is an excellent resource for patient-friendly explanations on patch testing and ACD. This resource, as well as allergen education handouts, may be reviewed at follow-up visits to continue to solidify patient learning.
Patients often have questions about allergen avoidance, such as occupational exposures, the suitability of specific products, or specific allergen names. These discussions are helpful for gauging how well patients are equipped to avoid their triggers as well as any hurdles they may be facing. If a patient still is experiencing flares after 6 to 8 weeks of safe-list adherence, it is important to take a thorough history of product use, daily exposures, and the patterns of distribution on the skin. Possible allergen exposures via topical medications also should be considered.18,19 Cross-checking products with a patient’s CAMP 2.0 safe list and correlating exposures with the continued ACD distribution are effective strategies to troubleshoot for unknown exposures to allergens.
Final Thoughts
Helping patients avoid allergens is essential to long-term management of ACD. The CAMP 2.0 safe list is an essential tool and a comprehensive reference for both patients and clinicians. With CAMP 2.0, allergen avoidance has never been more interactive or accessible.
While patch testing is the gold standard to diagnose type IV cutaneous hypersensitivity reactions, interpreting results can feel like trying to decipher a secret code, leaving patients feeling disempowered in avoiding their triggers. To truly manage allergic contact dermatitis (ACD), patients need comprehensive education on which allergens to avoid and ways to spot potential sources of exposure, including counseling, written guidelines, and lists of product alternatives.1 Patients who can recall and avoid their triggers experience greater improvement in clinical and quality-of-life scores.2 However, several studies have demonstrated that patients have difficulty recalling their allergens, even with longitudinal reminders.2-5 Quality-of-life and clinical outcomes also are not necessarily improved by successful allergen recall alone, as patients have reported limited success in actually avoiding allergens, highlighting the complexity of navigating exposures in daily life.2,6 To address these challenges, we examine common pitfalls patients encounter when avoiding allergens, highlight the benefits of utilizing safe lists and databases for allergen management, and introduce the updated Contact Allergen Management Program (CAMP) 2.0 as an optimal tool for long-term management of ACD.
Allergen Avoidance Pitfalls
Simply reading ingredient labels to avoid allergens is only marginally effective, as patients need to identify and interpret multiple chemical names as well as cross-reactors and related compounds to achieve success. Some allergens, such as fragrances or manufacturing impurities, are not explicitly identified on product labels. Even patients who can practice diligent label reading may struggle to find information on household or occupational products when full ingredient disclosure is not required.
Many of the allergens included in the American Contact Dermatitis Society (ACDS) Core 90 Series have alternative chemical aliases, and many have related compounds.6 For example, individuals with contact allergy to formaldehyde or a formaldehyde releaser usually need to avoid multiple other formaldehyde-releasing chemicals. Patients who test positive to amidoamine or dimethylaminopropylamine also must avoid the surfactant cocamidopropyl betaine—not because it is a cross-reactor, but because it is an impurity in the synthetic pathway.
Fragrance is one of the most common causes of ACD but can be challenging to avoid. Patients with allergies to fragrance or specific compounds (eg, limonene, linalool hydroperoxides) need to be savvy enough to navigate a broad spectrum of synthetic and botanical fragrance additives. Avoiding products that contain “fragrance” or “parfum” is simple enough, but patients also may need to recognize more than 3000 chemical names to identify individual fragrance ingredients that may be listed separately.7 Further, some fragrances are added for alternative purposes—preservative, medicinal, or emulsification—in which case products may deceptively tout themselves as being “fragrance free” yet still contain a fragrance allergen. This is made even more complex considering additional additives that commonly may cross-react with individual fragrance compounds; balsam of Peru, for example, is a botanical amalgam containing more than 250 compounds, including several fragrance components, making it an excellent indicator of fragrance allergy.8 While balsam of Peru and its fragrance constituents will almost never be listed on a product label, it cross-reacts with several benzyl derivatives commonly used in cosmetic formulations, such as benzyl alcohol, benzyl acetate, benzoic acid, benzyl benzoate, and benzyl cinnamate.9,10
Given that ACD is a common reason for patients to seek dermatologic care, it is crucial for clinicians to equip themselves with effective strategies to support patients after patch testing.11 This includes efficient translation of patch test results into practical advice while avoiding the oversimplified suggestion to read product labels; however, education alone cannot address the complexities of managing ACD, which is where contact allergen databases come into play.
An Essential Tool: Patient Allergen Databases and Safe Lists
Contact allergen databases are like a trusty sidekick for patients and clinicians, providing easily accessible information and tools to support allergen avoidance and improve ACD outcomes. While there are several existing resources, the ACDS launched its CAMP database in 2011 for ACDS members and their patients.12 The CAMP allows clinicians to easily generate personalized safe lists for household, medicament, and personal care products, facilitating seamless patient access both online and via a mobile application. The database also includes allergen-specific handouts to guide patient education.13 A key highlight of the CAMP is automated management of cross-reactors, which allows patients to choose products without having to memorize complex cross-reactor algorithms and helps avoid overly restrictive safe lists (Table).12-15

Other databases and online resources provide similar features, such as resources for patient education or finding safe products. The 2018 Alternatives for Allergens report is a vital adjunctive resource for guiding patients to suitable allergen-free products not included in commonly accessible product databases such as occupational materials, medical adhesives, shoes, or textiles.16
Introduction of CAMP 2.0
The latest version, CAMP 2.0, was launched in late 2024. The fully revamped database has a catalog of more than 100,000 products and comes packed with features that address many of the limitations found in the original CAMP. How does CAMP 2.0 work? The clinician inputs the patient’s allergens and makes choices about cross-reactor groups, and CAMP 2.0 outputs a list of allergen-free products that the patient can use when shopping for personal care products and the clinician can use for prescribing medicaments. The new user experience is intended to be more informative and engaging for all parties.
The CAMP 2.0 interface offers frequent product updates and streamlined database navigation, including enhanced search functions, barcode scanning, and a new mobile application for Apple and Android users. The mobile application also allows patients to track their symptoms and quality of life over time. With this additional functionality, there also is an extensive section for frequently asked questions and tutorials to help patients understand and utilize these features effectively.
Patients no longer have to wonder if a product that is not listed on their safe list is actually unsafe or just missing from the database. Several new features, including color-coded ingredient lists and organization of search codes into “safe” and “unsafe” product lists (Figure 1), help increase product transparency. These features can facilitate patient recognition of allergen names and cross-reactors in selected products. Future updates will include product purchasing through the mobile application and more educational handouts, including Spanish translations and dietary guidelines for systemic contact dermatitis.

Patient Experience—Once patients complete patch testing with an ACDS member, they can access the CAMP 2.0 database for free via web-based or a mobile application. After setting up an account, patients gain immediate access to their allergen information, product database, and educational resources about ACD and CAMP 2.0. Patients can search for specific products using text or barcode scanning or browse through categorized lists of medical, household, and personal care items. Each product page contains the product name and brand along with a color-coded ingredient list to help patients identify safe and unsafe ingredients at a glance (Figure 1). Products not currently included in the database can be requested using the “Add Product” feature. Additional patient engagement features include options to mark favorite products, write reviews, and track quality of life over time.
Physician Experience—The updated version includes several tutorials and frequently asked questions on how to improve ACD management and make the most of the new CAMP 2.0 tools and features. Generating patient allergen codes has been streamlined with an “Allergen Search” feature, allowing providers to quickly search and add or remove allergens to patients’ safe lists. Cross-reactor groups may be selectively added or removed for greater transparency and specificity in creating a patient safe list (Figure 2). Allergen codes now can be edited over time and are available for patient use via alphanumeric text or QR code format, which easily can be printed on a handout with instructions to help patients get acquainted with the system. For patient counseling, updated education handouts are available in the patient’s app and may be printed to provide supportive written educational material.

Approach to Long-Term Follow-up
When it comes to getting the most from patch testing, ongoing allergen avoidance is crucial. Patients may not see improvement unless they understand what ACD is and what needs to be done to improve it as well as become familiar with the names and common sources of their triggers.17 Clinicians can use CAMP 2.0 to facilitate patient improvement after patch testing, focusing on 3 key areas: continued patient education, patients’ ongoing progress in avoiding allergens, and monitored clinical improvement.
A solid understanding of ACD, such as its delayed (ie, 24-72 hours) onset after exposure, the need for allergen avoidance for at least 4 to 6 weeks before seeing improvement, and correlation of identified allergens with daily exposures, plays a major role in patient success. The CAMP 2.0 patch testing basics section is an excellent resource for patient-friendly explanations on patch testing and ACD. This resource, as well as allergen education handouts, may be reviewed at follow-up visits to continue to solidify patient learning.
Patients often have questions about allergen avoidance, such as occupational exposures, the suitability of specific products, or specific allergen names. These discussions are helpful for gauging how well patients are equipped to avoid their triggers as well as any hurdles they may be facing. If a patient still is experiencing flares after 6 to 8 weeks of safe-list adherence, it is important to take a thorough history of product use, daily exposures, and the patterns of distribution on the skin. Possible allergen exposures via topical medications also should be considered.18,19 Cross-checking products with a patient’s CAMP 2.0 safe list and correlating exposures with the continued ACD distribution are effective strategies to troubleshoot for unknown exposures to allergens.
Final Thoughts
Helping patients avoid allergens is essential to long-term management of ACD. The CAMP 2.0 safe list is an essential tool and a comprehensive reference for both patients and clinicians. With CAMP 2.0, allergen avoidance has never been more interactive or accessible.
- Tam I, Yu J. Allergic contact dermatitis in children: recommendations for patch testing. Curr Allergy Asthma Rep. 2020;20:41. doi:10.1007 /s11882-020-00939-z
- Dizdarevic A, Troensegaard W, Uldahl A, et al. Intervention study to evaluate the importance of information given to patients with contact allergy: a randomized, investigator-blinded clinical trial. Br J Dermatol. 2021;184:43-49. doi:10.1111/bjd.19119
- Jamil WN, Erikssohn I, Lindberg M. How well is the outcome of patch testing remembered by the patients? a 10-year follow-up of testing with the Swedish baseline series at the Department of Dermatology in Örebro, Sweden. Contact Dermatitis. 2012;66:215-220. doi:10.1111/j.1600-0536.2011.02039.x
- Scalf LA, Genebriera J, Davis MDP, et al. Patients’ perceptions of the usefulness and outcome of patch testing. J Am Acad Dermatol. 2007;56:928-932. doi:10.1016/j.jaad.2006.11.034
- Mossing K, Dizdarevic A, Svensson Å, et al. Impact on quality of life of an intervention providing additional information to patients with allergic contact dermatitis; a randomized clinical trial. J Eur Acad Dermatol Venereol. 2022;36:2166-2171. doi:10.1111/jdv.18412
- Schalock PC, Dunnick CA, Nedorost S, et al. American Contact Dermatitis Society Core Allergen Series: 2020 Update. Dermatitis. 2020;31:279-282. doi:10.1097/DER.0000000000000621
- Ingredient Breakdown: Fragrance. Think Dirty® Shop Clean. Accessed January 9, 2025. https://www.thinkdirtyapp.com/ingredient-breakdown-fragrance-3a8ef28f296a/
- Guarneri F, Corazza M, Stingeni L, et al. Myroxylon pereirae (balsam of Peru): still worth testing? Contact Dermatitis. 2021;85:269-273. doi:10.1111/cod.13839
- de Groot AC. Myroxylon pereirae resin (balsam of Peru)—a critical review of the literature and assessment of the significance of positive patch test reactions and the usefulness of restrictive diets. Contact Dermatitis. 2019;80:335-353. doi:10.1111/cod.13263
- Balsam of Peru: past and future. Allergic Contact Dermatitis Society; 2024. https://www.contactderm.org/UserFiles/members/Balsam_of_Peru___Past_and_Future.2.pdf
- Tramontana M, Hansel K, Bianchi L, et al. Advancing the understanding of allergic contact dermatitis: from pathophysiology to novel therapeutic approaches. Front Med. 2023;10. doi:10.3389 /fmed.2023.1184289
- McNamara D. ACDS launches Contact Allergen Management Program (CAMP). Internal Med News. March 7, 2011. Accessed December 31, 2024. https://www.mdedge.com/content/acds-launches-contact-allergen-management-program-camp-0
- Haque MZ, Rehman R, Guan L, et al. Recommendations to optimize patient education for allergic contact dermatitis: our approach. Contact Dermatitis. 2023;88:423-424. doi:10.1111/cod.14269
- Kist JM, el-Azhary RA, Hentz JG, et al. The Contact Allergen Replacement Database and treatment of allergic contact dermatitis. Arch Dermatol. 2004;140:1448-1450. doi:0.1001/archderm.140.12.1448
- El-Azhary RA, Yiannias JA. A new patient education approach in contact allergic dermatitis: the Contact Allergen Replacement Database (CARD). Int J Dermatol. 2004;43:278-280. doi:10.1111 /j.1365-4632.2004.01843.x
- Scheman A, Hylwa-Deufel S, Jacob SE, et al. Alternatives for allergens in the 2018 American Contact Dermatitis Society Core Series: report by the American Contact Alternatives Group. Dermatitis. 2019;30:87-105. doi:10.1097/DER.0000000000000453
- Mowad CM, Anderson B, Scheinman P, et al. Allergic contact dermatitis: patient management and education. J Am Acad Dermatol. 2016;74:1043-1054. doi:10.1016/j.jaad.2015.02.1144
- Ng A, Atwater AR, Reeder M. Contact allergy to topical medicaments, part 1: a double-edged sword. Cutis. 2021;108:271-275. doi:10.12788 /cutis.0390
- Nardelli A, D’Hooghe E, Drieghe J, et al. Allergic contact dermatitis from fragrance components in specific topical pharmaceutical products in Belgium. Contact Dermatitis. 2009;60:303-313. doi:10.1111 /j.1600-0536.2009.01542.x
- Tam I, Yu J. Allergic contact dermatitis in children: recommendations for patch testing. Curr Allergy Asthma Rep. 2020;20:41. doi:10.1007 /s11882-020-00939-z
- Dizdarevic A, Troensegaard W, Uldahl A, et al. Intervention study to evaluate the importance of information given to patients with contact allergy: a randomized, investigator-blinded clinical trial. Br J Dermatol. 2021;184:43-49. doi:10.1111/bjd.19119
- Jamil WN, Erikssohn I, Lindberg M. How well is the outcome of patch testing remembered by the patients? a 10-year follow-up of testing with the Swedish baseline series at the Department of Dermatology in Örebro, Sweden. Contact Dermatitis. 2012;66:215-220. doi:10.1111/j.1600-0536.2011.02039.x
- Scalf LA, Genebriera J, Davis MDP, et al. Patients’ perceptions of the usefulness and outcome of patch testing. J Am Acad Dermatol. 2007;56:928-932. doi:10.1016/j.jaad.2006.11.034
- Mossing K, Dizdarevic A, Svensson Å, et al. Impact on quality of life of an intervention providing additional information to patients with allergic contact dermatitis; a randomized clinical trial. J Eur Acad Dermatol Venereol. 2022;36:2166-2171. doi:10.1111/jdv.18412
- Schalock PC, Dunnick CA, Nedorost S, et al. American Contact Dermatitis Society Core Allergen Series: 2020 Update. Dermatitis. 2020;31:279-282. doi:10.1097/DER.0000000000000621
- Ingredient Breakdown: Fragrance. Think Dirty® Shop Clean. Accessed January 9, 2025. https://www.thinkdirtyapp.com/ingredient-breakdown-fragrance-3a8ef28f296a/
- Guarneri F, Corazza M, Stingeni L, et al. Myroxylon pereirae (balsam of Peru): still worth testing? Contact Dermatitis. 2021;85:269-273. doi:10.1111/cod.13839
- de Groot AC. Myroxylon pereirae resin (balsam of Peru)—a critical review of the literature and assessment of the significance of positive patch test reactions and the usefulness of restrictive diets. Contact Dermatitis. 2019;80:335-353. doi:10.1111/cod.13263
- Balsam of Peru: past and future. Allergic Contact Dermatitis Society; 2024. https://www.contactderm.org/UserFiles/members/Balsam_of_Peru___Past_and_Future.2.pdf
- Tramontana M, Hansel K, Bianchi L, et al. Advancing the understanding of allergic contact dermatitis: from pathophysiology to novel therapeutic approaches. Front Med. 2023;10. doi:10.3389 /fmed.2023.1184289
- McNamara D. ACDS launches Contact Allergen Management Program (CAMP). Internal Med News. March 7, 2011. Accessed December 31, 2024. https://www.mdedge.com/content/acds-launches-contact-allergen-management-program-camp-0
- Haque MZ, Rehman R, Guan L, et al. Recommendations to optimize patient education for allergic contact dermatitis: our approach. Contact Dermatitis. 2023;88:423-424. doi:10.1111/cod.14269
- Kist JM, el-Azhary RA, Hentz JG, et al. The Contact Allergen Replacement Database and treatment of allergic contact dermatitis. Arch Dermatol. 2004;140:1448-1450. doi:0.1001/archderm.140.12.1448
- El-Azhary RA, Yiannias JA. A new patient education approach in contact allergic dermatitis: the Contact Allergen Replacement Database (CARD). Int J Dermatol. 2004;43:278-280. doi:10.1111 /j.1365-4632.2004.01843.x
- Scheman A, Hylwa-Deufel S, Jacob SE, et al. Alternatives for allergens in the 2018 American Contact Dermatitis Society Core Series: report by the American Contact Alternatives Group. Dermatitis. 2019;30:87-105. doi:10.1097/DER.0000000000000453
- Mowad CM, Anderson B, Scheinman P, et al. Allergic contact dermatitis: patient management and education. J Am Acad Dermatol. 2016;74:1043-1054. doi:10.1016/j.jaad.2015.02.1144
- Ng A, Atwater AR, Reeder M. Contact allergy to topical medicaments, part 1: a double-edged sword. Cutis. 2021;108:271-275. doi:10.12788 /cutis.0390
- Nardelli A, D’Hooghe E, Drieghe J, et al. Allergic contact dermatitis from fragrance components in specific topical pharmaceutical products in Belgium. Contact Dermatitis. 2009;60:303-313. doi:10.1111 /j.1600-0536.2009.01542.x
Simplifying Allergic Contact Dermatitis Management with the Contact Allergen Management Program 2.0
Simplifying Allergic Contact Dermatitis Management with the Contact Allergen Management Program 2.0
PRACTICE POINTS
- Comprehensive patient education is critical for appropriate allergen avoidance after patch testing, and allergen databases and product safe lists are invaluable tools to complement clinical guidance.
- The updated Contact Allergen Management Program 2.0 offers an updated approach to patient guidance, including a database of more than 100,000 products and an easy-to-use platform to find safe, allergen-free products.
- Interactive learning resources, product pages, and quality-of-life tracking tools can help equip patients with information to encourage further autonomy in allergen avoidance.
Sulfites: The 2024 American Contact Dermatitis Society Allergen of the Year
The American Contact Dermatitis Society (ACDS) selected sulfites as the 2024 Allergen of the Year.1 Due to their preservative and antioxidant properties, sulfites are prevalent in a variety of foods, beverages, medications, and personal care products; however, sulfites also have been implicated as a potential contact allergen. In this article, we review common sources of sulfite exposure, clinical manifestations of allergic contact dermatitis (ACD) to sulfites, and patch testing considerations for this emerging allergen.
What Are Sulfites?
Sulfiting agents are compounds that contain the sulfite ion SO32-, including sulfur dioxide, sodium disulfite (sodium metabisulfite), and potassium metabisulfite.2 Sulfites occur naturally in the environment and commonly are used as preservatives, antibrowning agents, and antioxidants in various foods, beverages, medications, cosmetics, and skin care products. As antibrowning agents and antioxidants, sulfites help maintain the natural appearance of foods and other products and prevent premature spoiling by inactivating oxidative enzymes.3 It should be noted that sulfites and sulfates are distinct and unrelated compounds that do not cross-react.1
Common Sources of Sulfite Exposure
From a morning glass of juice to an evening shower, in the pharmacy and at the hair salon, sulfite exposure is ubiquitous in most daily routines. Sulfites are present in many foods and beverages, either as a byproduct of natural fermentation or as an additive to prevent spoiling and color change. The Table provides examples of foods with high sulfite content.1,4-6 In particular, dried fruit, bottled lemon juice, wine, grape juice, sauerkraut juice, and pickled onions have high sulfite content.

Topical medications and personal care products represent other potential sources of sulfite exposure. A number of reports have shown that sulfites may be included in topical steroids,7 antibiotics,8 antifungals,9 hemorrhoidal preparations,10 local anesthetics,11 and urinary catheterization gel,12 highlighting their many potential applications. In addition, a comprehensive ingredient analysis of 264 ophthalmic medications found that 3.8% of the products contained sodium disulfite.13 Sulfites may be found in personal care products, including facial and hand cleansers, shampoos, moisturizers, and toothpastes. Hair dyes also commonly contain sulfites,7 which are listed in as many as 90% of hair dye kits in the ACDS Contact Allergen Management Program database.1
Occupational exposures also are widespread, as sulfites are extensively utilized across diverse industries such as pharmaceuticals, health care, leather manufacturing, mineral extraction, food preparation, chemical manufacturing, textiles, alcohol brewing, and wine production.1
Sulfites also are used in the rubber industry—particularly in gloves—due to their anticoagulant and preservative properties.4 This is relevant to health care providers, who may use dozens of disposable gloves in a single day. In an experimental pilot study, researchers detected sulfites in 83% (5/6) of natural rubber latex gloves, 96% (23/24) of synthetic (nitrile) gloves, and 0% (0/5) of polyvinyl chloride gloves.14 While this study was limited to a small sample size, it demonstrates the common use of sulfites in certain rubber gloves and encourages future studies to determine whether there is a quantitative threshold to elicit allergic reactions.
Sulfite Allergy
In 1968, an early case report of ACD to sulfites was published involving a pharmaceutical worker who developed hand eczema after working at a factory for 3 months and had a positive patch test to potassium metabisulfite.15 There have been other cases published in the literature since then, including localized ACD as well as less common cases of systemic contact dermatitis following oral, injectable, and rectal sulfite exposures.16
The North American Contact Dermatitis Group found that, among 132 (2.7%) of 4885 patients with positive patch tests to sodium disulfite from 2017 to 2018, the most commonly involved body sites were the face (28.8%) and hands (20.5%) followed by a scattered/generalized distribution (13.6%). Involvement of the face and hands may correlate with the most frequent sources of exposure that were identified, including personal care products (particularly hair dyes)(18.9%), medications (9.1%), and foods (7.6%).17 A multicenter analysis of patch test results from Germany, Austria, and Switzerland from 1999 to 2013 showed that 357 (2.9%) of 12,156 patients had positive reactions to sodium disulfite, with the most commonly identified exposure sources being topical pharmaceutical agents (59.3%); cosmetics, creams, and sunscreens (13.6%); and systemic drugs (6.8%).18 However, it is not always possible to determine the clinical relevance of a positive patch test to sulfites.1
Other than the face and hands, there have been other unexpected anatomic locations for sulfite ACD (eg, the lower back), and systemic contact dermatitis has manifested with widespread rashes due to oral, rectal, and parenteral exposure.4,16,19 There is no definitive link between sulfite contact allergy and patient sex, but there seems to be a higher prevalence in patients older than 40 years, perhaps related to overall lifetime exposure.1
Immediate hypersensitivity reactions to sulfites also have been reported, including urticaria, angioedema, and anaphylaxis.4 Due to multiple cases of severe dermatologic and respiratory reactions to food products containing sulfites,20 the US Food and Drug Administration prohibited their use in fresh fruit and vegetables as antibrowning agents in 1986 and required labels on packaged foods that contained sulfites at more than 10 parts per million.21 However, food and drinks produced in restaurants, bakeries, and cafes as well as those that are distributed directly to consumers from the preparation site are exempt from these rules.17
In addition, consuming high amounts of dietary sulfites has been linked to headaches through unclear (ie, not necessarily allergic) mechanisms.4,22 One study found that wine with a higher sulfite concentration was associated with increased risk for headaches in participants who had a history of headaches related to wine consumption.22
Patch Testing to Sulfites
The North American Contact Dermatitis Group has tested sodium disulfite since 2017 and found an increased frequency of positive patch tests from 2.7% (N=4885) in 2017 and 201817 to 3.3% (N=4115) in 2019 and 202023 among patients referred for testing. Similarly, patch testing to sodium disulfite in nearly 40,000 patients in 9 European countries showed a pooled prevalence of reactions of 3.1%.17 However, this contact allergy may go unrecognized, as sulfites are not included in common patch test series, including the thin-layer rapid use epicutaneous test and the ACDS Core Allergen Series.24,25 The relatively high patch test positivity to sulfites along with the prevalence of daily exposures supports the addition of sulfites to more patch test screening series.
The recommended patch test concentration for sodium disulfite is 1% in petrolatum.5 Testing in aqueous solutions is not recommended because they can cause sulfites to break down, potentially producing false-positive or irritant patch test reactions.7,26,27
Recommendations for Patients With Sulfite Allergies
Individuals with contact allergies to sulfites should be counseled on exposure sources and should be given resources providing a list of safe products, such as the ACDS Contact Allergen Management Program (https://www.acdscamp.org/login) or SkinSAFE (https://www.skinsafeproducts.com/). Prescribers should be cognizant of sulfites that are present in prescription medications. Just because a patient has a positive patch test to sulfites does not automatically imply that they will need to modify their diet to avoid sulfite-containing foods; in the absence of cheilitis or a distribution suggestive of systemic contact dermatitis (eg, vesicular hand/foot dermatitis, intertriginous eruptions), this step may be unnecessary. On the other hand, individuals who have experienced immediate hypersensitivity reactions to sulfites should avoid sulfite-containing foods and carry an epinephrine autoinjector.
Final Interpretation
Sulfites are ubiquitous compounds found in various foods, beverages, medications, and personal care products in addition to a range of occupational exposures. The face and hands are the most common sites of sulfite ACD. Despite patch test positivity in as many as 3% of tested patients,17,23 sulfite allergy may be missed due to lack of routine testing on standard screening series.
- Ekstein SF, Warshaw EM. Sulfites: allergen of the year 2024. Dermatitis. 2024;35:6-12. doi:10.1089/derm.2023.0154
- Gunnison AF, Jacobsen DW. Sulfite hypersensitivity. a critical review. CRC Crit Rev Toxicol. 1987;17:185-214. doi:10.3109/10408448709071208
- Clough SR. Sodium sulfite. In: Wexler P, ed. Encyclopedia of Toxicology. 3rd ed. Academic Press; 2014: 341-343.
- Vally H, Misso NL, Madan V. Clinical effects of sulphite additives. Clin Exp Allergy. 2009;39:1643-1651. doi:10.1111/j.1365-2222.2009.03362.x
- Ralph N, Verma S, Merry S, et al. What is the relevance of contact allergy to sodium metabisulfite and which concentration of the allergen should we use? Dermatitis. 2015;26:162-165. doi:10.1097/der.0000000000000120
- Madan V, Walker SL, Beck MH. Sodium metabisulfite allergy is common but is it relevant? Contact Dermatitis. 2007;57:173-176. doi:10.1111/j.1600-0536.2007.01188.x
- García-Gavín J, Parente J, Goossens A. Allergic contact dermatitis caused by sodium metabisulfite: a challenging allergen. a case series and literature review. Contact Dermatitis. 2012;67:260-269. doi:10.1111/j.1600-0536.2012.02135.x
- Milpied B, van Wassenhove L, Larousse C, et al. Contact dermatitis from rifamycin. Contact Dermatitis. 1986;14:252-253. doi:10.1111/j.1600-0536.1986.tb01240.x
- Lodi A, Chiarelli G, Mancini LL, et al. Contact allergy to sodium sulfite contained in an antifungal preparation. Contact Dermatitis. 1993;29:97. doi:10.1111/j.1600-0536.1993.tb03493.x
- Sánchez-Pérez J, Abajo P, Córdoba S, et al. Allergic contact dermatitis from sodium metabisulfite in an antihemorrhoidal cream. Contact Dermatitis. 2000;42:176-177.
- Boyd AH, Warshaw EM. Sulfites: no longer a zebra? Dermatitis. 2017;28:364-366. doi:10.1097/der.0000000000000312
- Grosch E, Mahler V. Allergic contact dermatitis caused by a catheter system containing sodium metabisulfite. Contact Dermatitis. 2017;76:186-187. doi:10.1111/cod.12675
- Shaver RL, Warshaw EM. Contact allergens in prescription topical ophthalmic medications. Dermatitis. 2022;33:135-143. doi:10.1097/der.0000000000000751
- Dendooven E, Darrigade AS, Foubert K, et al. The presence of sulfites in ‘natural rubber latex’ and ‘synthetic’ rubber gloves: an experimental pilot study. Br J Dermatol. 2020;182:1054-1055. doi:10.1111/bjd.18608
- Nater JP. Allergic contact dermatitis caused by potassium metabisulfite. Dermatologica. 1968;136:477-478. doi:10.1159/000254143
- Borges AS, Valejo Coelho MM, Fernandes C, et al. Systemic allergic dermatitis caused by sodium metabisulfite in rectal enemas. Contact Dermatitis. 2018;78:429-430. doi:10.1111/cod.12971
- Warshaw EM, Buonomo M, DeKoven JG, et al. Patch testing with sodium disulfite: North American Contact Dermatitis Group experience, 2017 to 2018. Contact Dermatitis. 2021;85:285-296. doi:10.1111/cod.13860
- Häberle M, Geier J, Mahler V. Contact allergy to sulfites: clinical and occupational relevance—new data from the German Contact Dermatitis Research Group and the Information Network of Departments of Dermatology (IVDK). J Dtsch Dermatol Ges. 2016;14:938-941. doi:10.1111/ddg.13009
- Tan MG, Li HO, Pratt MD. Systemic allergic dermatitis to sodium metabisulfite in local anesthetic solution. Contact Dermatitis. 2022;86:120-121. doi:10.1111/cod.13978
- D’Amore T, Di Taranto A, Berardi G, et al. Sulfites in meat: occurrence, activity, toxicity, regulation, and detection. a comprehensive review. Compr Rev Food Sci Food Saf. 2020;19:2701-2720. doi:10.1111/1541-4337.12607
- Grotheer P, Marshall M, Simonne A. Sulfites: separating fact from fiction. May 11, 2022. UF IFAS Extension. University of Florida. Accessed October 4, 2024. https://edis.ifas.ufl.edu/publication/FY731
- Silva M, Gama J, Pinto N, et al. Sulfite concentration and the occurrence of headache in young adults: a prospective study. Eur J Clin Nutr. 2019;73:1316-1322. doi:10.1038/s41430-019-0420-2
- DeKoven JG, Warshaw EM, Reeder MJ, et al. North American Contact Dermatitis Group patch test results: 2019-2020. Dermatitis. 2023;34:90-104. doi:10.1089/derm.2022.29017.jdk
- T.R.U.E. Test. Thin-layer rapid use epicutaneous patch test. SmartPractice Dermatology Allergy. Accessed October 4, 2024. https://www.smartpractice.com/shop/category?id=581719&m=SPA
- Schalock PC, Dunnick CA, Nedorost, et al; American Contact Dermatitis Society Core Allergen Series Committee. American Contact Dermatitis Society Core Allergen Series: 2020 update. Dermatitis. 2020;31:279-282.
- Kaaman AC, Boman A, Wrangsjö K, et al. Contact allergy to sodium metabisulfite: an occupational problem. Contact Dermatitis. 2010;63:110-112. doi:10.1111/j.1600-0536.2010.01756.x
- Vena GA, Foti C, Angelini G. Sulfite contact allergy. Contact Dermatitis. 1994;31:172-175. doi:10.1111/j.1600-0536.1994.tb01959.x
The American Contact Dermatitis Society (ACDS) selected sulfites as the 2024 Allergen of the Year.1 Due to their preservative and antioxidant properties, sulfites are prevalent in a variety of foods, beverages, medications, and personal care products; however, sulfites also have been implicated as a potential contact allergen. In this article, we review common sources of sulfite exposure, clinical manifestations of allergic contact dermatitis (ACD) to sulfites, and patch testing considerations for this emerging allergen.
What Are Sulfites?
Sulfiting agents are compounds that contain the sulfite ion SO32-, including sulfur dioxide, sodium disulfite (sodium metabisulfite), and potassium metabisulfite.2 Sulfites occur naturally in the environment and commonly are used as preservatives, antibrowning agents, and antioxidants in various foods, beverages, medications, cosmetics, and skin care products. As antibrowning agents and antioxidants, sulfites help maintain the natural appearance of foods and other products and prevent premature spoiling by inactivating oxidative enzymes.3 It should be noted that sulfites and sulfates are distinct and unrelated compounds that do not cross-react.1
Common Sources of Sulfite Exposure
From a morning glass of juice to an evening shower, in the pharmacy and at the hair salon, sulfite exposure is ubiquitous in most daily routines. Sulfites are present in many foods and beverages, either as a byproduct of natural fermentation or as an additive to prevent spoiling and color change. The Table provides examples of foods with high sulfite content.1,4-6 In particular, dried fruit, bottled lemon juice, wine, grape juice, sauerkraut juice, and pickled onions have high sulfite content.

Topical medications and personal care products represent other potential sources of sulfite exposure. A number of reports have shown that sulfites may be included in topical steroids,7 antibiotics,8 antifungals,9 hemorrhoidal preparations,10 local anesthetics,11 and urinary catheterization gel,12 highlighting their many potential applications. In addition, a comprehensive ingredient analysis of 264 ophthalmic medications found that 3.8% of the products contained sodium disulfite.13 Sulfites may be found in personal care products, including facial and hand cleansers, shampoos, moisturizers, and toothpastes. Hair dyes also commonly contain sulfites,7 which are listed in as many as 90% of hair dye kits in the ACDS Contact Allergen Management Program database.1
Occupational exposures also are widespread, as sulfites are extensively utilized across diverse industries such as pharmaceuticals, health care, leather manufacturing, mineral extraction, food preparation, chemical manufacturing, textiles, alcohol brewing, and wine production.1
Sulfites also are used in the rubber industry—particularly in gloves—due to their anticoagulant and preservative properties.4 This is relevant to health care providers, who may use dozens of disposable gloves in a single day. In an experimental pilot study, researchers detected sulfites in 83% (5/6) of natural rubber latex gloves, 96% (23/24) of synthetic (nitrile) gloves, and 0% (0/5) of polyvinyl chloride gloves.14 While this study was limited to a small sample size, it demonstrates the common use of sulfites in certain rubber gloves and encourages future studies to determine whether there is a quantitative threshold to elicit allergic reactions.
Sulfite Allergy
In 1968, an early case report of ACD to sulfites was published involving a pharmaceutical worker who developed hand eczema after working at a factory for 3 months and had a positive patch test to potassium metabisulfite.15 There have been other cases published in the literature since then, including localized ACD as well as less common cases of systemic contact dermatitis following oral, injectable, and rectal sulfite exposures.16
The North American Contact Dermatitis Group found that, among 132 (2.7%) of 4885 patients with positive patch tests to sodium disulfite from 2017 to 2018, the most commonly involved body sites were the face (28.8%) and hands (20.5%) followed by a scattered/generalized distribution (13.6%). Involvement of the face and hands may correlate with the most frequent sources of exposure that were identified, including personal care products (particularly hair dyes)(18.9%), medications (9.1%), and foods (7.6%).17 A multicenter analysis of patch test results from Germany, Austria, and Switzerland from 1999 to 2013 showed that 357 (2.9%) of 12,156 patients had positive reactions to sodium disulfite, with the most commonly identified exposure sources being topical pharmaceutical agents (59.3%); cosmetics, creams, and sunscreens (13.6%); and systemic drugs (6.8%).18 However, it is not always possible to determine the clinical relevance of a positive patch test to sulfites.1
Other than the face and hands, there have been other unexpected anatomic locations for sulfite ACD (eg, the lower back), and systemic contact dermatitis has manifested with widespread rashes due to oral, rectal, and parenteral exposure.4,16,19 There is no definitive link between sulfite contact allergy and patient sex, but there seems to be a higher prevalence in patients older than 40 years, perhaps related to overall lifetime exposure.1
Immediate hypersensitivity reactions to sulfites also have been reported, including urticaria, angioedema, and anaphylaxis.4 Due to multiple cases of severe dermatologic and respiratory reactions to food products containing sulfites,20 the US Food and Drug Administration prohibited their use in fresh fruit and vegetables as antibrowning agents in 1986 and required labels on packaged foods that contained sulfites at more than 10 parts per million.21 However, food and drinks produced in restaurants, bakeries, and cafes as well as those that are distributed directly to consumers from the preparation site are exempt from these rules.17
In addition, consuming high amounts of dietary sulfites has been linked to headaches through unclear (ie, not necessarily allergic) mechanisms.4,22 One study found that wine with a higher sulfite concentration was associated with increased risk for headaches in participants who had a history of headaches related to wine consumption.22
Patch Testing to Sulfites
The North American Contact Dermatitis Group has tested sodium disulfite since 2017 and found an increased frequency of positive patch tests from 2.7% (N=4885) in 2017 and 201817 to 3.3% (N=4115) in 2019 and 202023 among patients referred for testing. Similarly, patch testing to sodium disulfite in nearly 40,000 patients in 9 European countries showed a pooled prevalence of reactions of 3.1%.17 However, this contact allergy may go unrecognized, as sulfites are not included in common patch test series, including the thin-layer rapid use epicutaneous test and the ACDS Core Allergen Series.24,25 The relatively high patch test positivity to sulfites along with the prevalence of daily exposures supports the addition of sulfites to more patch test screening series.
The recommended patch test concentration for sodium disulfite is 1% in petrolatum.5 Testing in aqueous solutions is not recommended because they can cause sulfites to break down, potentially producing false-positive or irritant patch test reactions.7,26,27
Recommendations for Patients With Sulfite Allergies
Individuals with contact allergies to sulfites should be counseled on exposure sources and should be given resources providing a list of safe products, such as the ACDS Contact Allergen Management Program (https://www.acdscamp.org/login) or SkinSAFE (https://www.skinsafeproducts.com/). Prescribers should be cognizant of sulfites that are present in prescription medications. Just because a patient has a positive patch test to sulfites does not automatically imply that they will need to modify their diet to avoid sulfite-containing foods; in the absence of cheilitis or a distribution suggestive of systemic contact dermatitis (eg, vesicular hand/foot dermatitis, intertriginous eruptions), this step may be unnecessary. On the other hand, individuals who have experienced immediate hypersensitivity reactions to sulfites should avoid sulfite-containing foods and carry an epinephrine autoinjector.
Final Interpretation
Sulfites are ubiquitous compounds found in various foods, beverages, medications, and personal care products in addition to a range of occupational exposures. The face and hands are the most common sites of sulfite ACD. Despite patch test positivity in as many as 3% of tested patients,17,23 sulfite allergy may be missed due to lack of routine testing on standard screening series.
The American Contact Dermatitis Society (ACDS) selected sulfites as the 2024 Allergen of the Year.1 Due to their preservative and antioxidant properties, sulfites are prevalent in a variety of foods, beverages, medications, and personal care products; however, sulfites also have been implicated as a potential contact allergen. In this article, we review common sources of sulfite exposure, clinical manifestations of allergic contact dermatitis (ACD) to sulfites, and patch testing considerations for this emerging allergen.
What Are Sulfites?
Sulfiting agents are compounds that contain the sulfite ion SO32-, including sulfur dioxide, sodium disulfite (sodium metabisulfite), and potassium metabisulfite.2 Sulfites occur naturally in the environment and commonly are used as preservatives, antibrowning agents, and antioxidants in various foods, beverages, medications, cosmetics, and skin care products. As antibrowning agents and antioxidants, sulfites help maintain the natural appearance of foods and other products and prevent premature spoiling by inactivating oxidative enzymes.3 It should be noted that sulfites and sulfates are distinct and unrelated compounds that do not cross-react.1
Common Sources of Sulfite Exposure
From a morning glass of juice to an evening shower, in the pharmacy and at the hair salon, sulfite exposure is ubiquitous in most daily routines. Sulfites are present in many foods and beverages, either as a byproduct of natural fermentation or as an additive to prevent spoiling and color change. The Table provides examples of foods with high sulfite content.1,4-6 In particular, dried fruit, bottled lemon juice, wine, grape juice, sauerkraut juice, and pickled onions have high sulfite content.

Topical medications and personal care products represent other potential sources of sulfite exposure. A number of reports have shown that sulfites may be included in topical steroids,7 antibiotics,8 antifungals,9 hemorrhoidal preparations,10 local anesthetics,11 and urinary catheterization gel,12 highlighting their many potential applications. In addition, a comprehensive ingredient analysis of 264 ophthalmic medications found that 3.8% of the products contained sodium disulfite.13 Sulfites may be found in personal care products, including facial and hand cleansers, shampoos, moisturizers, and toothpastes. Hair dyes also commonly contain sulfites,7 which are listed in as many as 90% of hair dye kits in the ACDS Contact Allergen Management Program database.1
Occupational exposures also are widespread, as sulfites are extensively utilized across diverse industries such as pharmaceuticals, health care, leather manufacturing, mineral extraction, food preparation, chemical manufacturing, textiles, alcohol brewing, and wine production.1
Sulfites also are used in the rubber industry—particularly in gloves—due to their anticoagulant and preservative properties.4 This is relevant to health care providers, who may use dozens of disposable gloves in a single day. In an experimental pilot study, researchers detected sulfites in 83% (5/6) of natural rubber latex gloves, 96% (23/24) of synthetic (nitrile) gloves, and 0% (0/5) of polyvinyl chloride gloves.14 While this study was limited to a small sample size, it demonstrates the common use of sulfites in certain rubber gloves and encourages future studies to determine whether there is a quantitative threshold to elicit allergic reactions.
Sulfite Allergy
In 1968, an early case report of ACD to sulfites was published involving a pharmaceutical worker who developed hand eczema after working at a factory for 3 months and had a positive patch test to potassium metabisulfite.15 There have been other cases published in the literature since then, including localized ACD as well as less common cases of systemic contact dermatitis following oral, injectable, and rectal sulfite exposures.16
The North American Contact Dermatitis Group found that, among 132 (2.7%) of 4885 patients with positive patch tests to sodium disulfite from 2017 to 2018, the most commonly involved body sites were the face (28.8%) and hands (20.5%) followed by a scattered/generalized distribution (13.6%). Involvement of the face and hands may correlate with the most frequent sources of exposure that were identified, including personal care products (particularly hair dyes)(18.9%), medications (9.1%), and foods (7.6%).17 A multicenter analysis of patch test results from Germany, Austria, and Switzerland from 1999 to 2013 showed that 357 (2.9%) of 12,156 patients had positive reactions to sodium disulfite, with the most commonly identified exposure sources being topical pharmaceutical agents (59.3%); cosmetics, creams, and sunscreens (13.6%); and systemic drugs (6.8%).18 However, it is not always possible to determine the clinical relevance of a positive patch test to sulfites.1
Other than the face and hands, there have been other unexpected anatomic locations for sulfite ACD (eg, the lower back), and systemic contact dermatitis has manifested with widespread rashes due to oral, rectal, and parenteral exposure.4,16,19 There is no definitive link between sulfite contact allergy and patient sex, but there seems to be a higher prevalence in patients older than 40 years, perhaps related to overall lifetime exposure.1
Immediate hypersensitivity reactions to sulfites also have been reported, including urticaria, angioedema, and anaphylaxis.4 Due to multiple cases of severe dermatologic and respiratory reactions to food products containing sulfites,20 the US Food and Drug Administration prohibited their use in fresh fruit and vegetables as antibrowning agents in 1986 and required labels on packaged foods that contained sulfites at more than 10 parts per million.21 However, food and drinks produced in restaurants, bakeries, and cafes as well as those that are distributed directly to consumers from the preparation site are exempt from these rules.17
In addition, consuming high amounts of dietary sulfites has been linked to headaches through unclear (ie, not necessarily allergic) mechanisms.4,22 One study found that wine with a higher sulfite concentration was associated with increased risk for headaches in participants who had a history of headaches related to wine consumption.22
Patch Testing to Sulfites
The North American Contact Dermatitis Group has tested sodium disulfite since 2017 and found an increased frequency of positive patch tests from 2.7% (N=4885) in 2017 and 201817 to 3.3% (N=4115) in 2019 and 202023 among patients referred for testing. Similarly, patch testing to sodium disulfite in nearly 40,000 patients in 9 European countries showed a pooled prevalence of reactions of 3.1%.17 However, this contact allergy may go unrecognized, as sulfites are not included in common patch test series, including the thin-layer rapid use epicutaneous test and the ACDS Core Allergen Series.24,25 The relatively high patch test positivity to sulfites along with the prevalence of daily exposures supports the addition of sulfites to more patch test screening series.
The recommended patch test concentration for sodium disulfite is 1% in petrolatum.5 Testing in aqueous solutions is not recommended because they can cause sulfites to break down, potentially producing false-positive or irritant patch test reactions.7,26,27
Recommendations for Patients With Sulfite Allergies
Individuals with contact allergies to sulfites should be counseled on exposure sources and should be given resources providing a list of safe products, such as the ACDS Contact Allergen Management Program (https://www.acdscamp.org/login) or SkinSAFE (https://www.skinsafeproducts.com/). Prescribers should be cognizant of sulfites that are present in prescription medications. Just because a patient has a positive patch test to sulfites does not automatically imply that they will need to modify their diet to avoid sulfite-containing foods; in the absence of cheilitis or a distribution suggestive of systemic contact dermatitis (eg, vesicular hand/foot dermatitis, intertriginous eruptions), this step may be unnecessary. On the other hand, individuals who have experienced immediate hypersensitivity reactions to sulfites should avoid sulfite-containing foods and carry an epinephrine autoinjector.
Final Interpretation
Sulfites are ubiquitous compounds found in various foods, beverages, medications, and personal care products in addition to a range of occupational exposures. The face and hands are the most common sites of sulfite ACD. Despite patch test positivity in as many as 3% of tested patients,17,23 sulfite allergy may be missed due to lack of routine testing on standard screening series.
- Ekstein SF, Warshaw EM. Sulfites: allergen of the year 2024. Dermatitis. 2024;35:6-12. doi:10.1089/derm.2023.0154
- Gunnison AF, Jacobsen DW. Sulfite hypersensitivity. a critical review. CRC Crit Rev Toxicol. 1987;17:185-214. doi:10.3109/10408448709071208
- Clough SR. Sodium sulfite. In: Wexler P, ed. Encyclopedia of Toxicology. 3rd ed. Academic Press; 2014: 341-343.
- Vally H, Misso NL, Madan V. Clinical effects of sulphite additives. Clin Exp Allergy. 2009;39:1643-1651. doi:10.1111/j.1365-2222.2009.03362.x
- Ralph N, Verma S, Merry S, et al. What is the relevance of contact allergy to sodium metabisulfite and which concentration of the allergen should we use? Dermatitis. 2015;26:162-165. doi:10.1097/der.0000000000000120
- Madan V, Walker SL, Beck MH. Sodium metabisulfite allergy is common but is it relevant? Contact Dermatitis. 2007;57:173-176. doi:10.1111/j.1600-0536.2007.01188.x
- García-Gavín J, Parente J, Goossens A. Allergic contact dermatitis caused by sodium metabisulfite: a challenging allergen. a case series and literature review. Contact Dermatitis. 2012;67:260-269. doi:10.1111/j.1600-0536.2012.02135.x
- Milpied B, van Wassenhove L, Larousse C, et al. Contact dermatitis from rifamycin. Contact Dermatitis. 1986;14:252-253. doi:10.1111/j.1600-0536.1986.tb01240.x
- Lodi A, Chiarelli G, Mancini LL, et al. Contact allergy to sodium sulfite contained in an antifungal preparation. Contact Dermatitis. 1993;29:97. doi:10.1111/j.1600-0536.1993.tb03493.x
- Sánchez-Pérez J, Abajo P, Córdoba S, et al. Allergic contact dermatitis from sodium metabisulfite in an antihemorrhoidal cream. Contact Dermatitis. 2000;42:176-177.
- Boyd AH, Warshaw EM. Sulfites: no longer a zebra? Dermatitis. 2017;28:364-366. doi:10.1097/der.0000000000000312
- Grosch E, Mahler V. Allergic contact dermatitis caused by a catheter system containing sodium metabisulfite. Contact Dermatitis. 2017;76:186-187. doi:10.1111/cod.12675
- Shaver RL, Warshaw EM. Contact allergens in prescription topical ophthalmic medications. Dermatitis. 2022;33:135-143. doi:10.1097/der.0000000000000751
- Dendooven E, Darrigade AS, Foubert K, et al. The presence of sulfites in ‘natural rubber latex’ and ‘synthetic’ rubber gloves: an experimental pilot study. Br J Dermatol. 2020;182:1054-1055. doi:10.1111/bjd.18608
- Nater JP. Allergic contact dermatitis caused by potassium metabisulfite. Dermatologica. 1968;136:477-478. doi:10.1159/000254143
- Borges AS, Valejo Coelho MM, Fernandes C, et al. Systemic allergic dermatitis caused by sodium metabisulfite in rectal enemas. Contact Dermatitis. 2018;78:429-430. doi:10.1111/cod.12971
- Warshaw EM, Buonomo M, DeKoven JG, et al. Patch testing with sodium disulfite: North American Contact Dermatitis Group experience, 2017 to 2018. Contact Dermatitis. 2021;85:285-296. doi:10.1111/cod.13860
- Häberle M, Geier J, Mahler V. Contact allergy to sulfites: clinical and occupational relevance—new data from the German Contact Dermatitis Research Group and the Information Network of Departments of Dermatology (IVDK). J Dtsch Dermatol Ges. 2016;14:938-941. doi:10.1111/ddg.13009
- Tan MG, Li HO, Pratt MD. Systemic allergic dermatitis to sodium metabisulfite in local anesthetic solution. Contact Dermatitis. 2022;86:120-121. doi:10.1111/cod.13978
- D’Amore T, Di Taranto A, Berardi G, et al. Sulfites in meat: occurrence, activity, toxicity, regulation, and detection. a comprehensive review. Compr Rev Food Sci Food Saf. 2020;19:2701-2720. doi:10.1111/1541-4337.12607
- Grotheer P, Marshall M, Simonne A. Sulfites: separating fact from fiction. May 11, 2022. UF IFAS Extension. University of Florida. Accessed October 4, 2024. https://edis.ifas.ufl.edu/publication/FY731
- Silva M, Gama J, Pinto N, et al. Sulfite concentration and the occurrence of headache in young adults: a prospective study. Eur J Clin Nutr. 2019;73:1316-1322. doi:10.1038/s41430-019-0420-2
- DeKoven JG, Warshaw EM, Reeder MJ, et al. North American Contact Dermatitis Group patch test results: 2019-2020. Dermatitis. 2023;34:90-104. doi:10.1089/derm.2022.29017.jdk
- T.R.U.E. Test. Thin-layer rapid use epicutaneous patch test. SmartPractice Dermatology Allergy. Accessed October 4, 2024. https://www.smartpractice.com/shop/category?id=581719&m=SPA
- Schalock PC, Dunnick CA, Nedorost, et al; American Contact Dermatitis Society Core Allergen Series Committee. American Contact Dermatitis Society Core Allergen Series: 2020 update. Dermatitis. 2020;31:279-282.
- Kaaman AC, Boman A, Wrangsjö K, et al. Contact allergy to sodium metabisulfite: an occupational problem. Contact Dermatitis. 2010;63:110-112. doi:10.1111/j.1600-0536.2010.01756.x
- Vena GA, Foti C, Angelini G. Sulfite contact allergy. Contact Dermatitis. 1994;31:172-175. doi:10.1111/j.1600-0536.1994.tb01959.x
- Ekstein SF, Warshaw EM. Sulfites: allergen of the year 2024. Dermatitis. 2024;35:6-12. doi:10.1089/derm.2023.0154
- Gunnison AF, Jacobsen DW. Sulfite hypersensitivity. a critical review. CRC Crit Rev Toxicol. 1987;17:185-214. doi:10.3109/10408448709071208
- Clough SR. Sodium sulfite. In: Wexler P, ed. Encyclopedia of Toxicology. 3rd ed. Academic Press; 2014: 341-343.
- Vally H, Misso NL, Madan V. Clinical effects of sulphite additives. Clin Exp Allergy. 2009;39:1643-1651. doi:10.1111/j.1365-2222.2009.03362.x
- Ralph N, Verma S, Merry S, et al. What is the relevance of contact allergy to sodium metabisulfite and which concentration of the allergen should we use? Dermatitis. 2015;26:162-165. doi:10.1097/der.0000000000000120
- Madan V, Walker SL, Beck MH. Sodium metabisulfite allergy is common but is it relevant? Contact Dermatitis. 2007;57:173-176. doi:10.1111/j.1600-0536.2007.01188.x
- García-Gavín J, Parente J, Goossens A. Allergic contact dermatitis caused by sodium metabisulfite: a challenging allergen. a case series and literature review. Contact Dermatitis. 2012;67:260-269. doi:10.1111/j.1600-0536.2012.02135.x
- Milpied B, van Wassenhove L, Larousse C, et al. Contact dermatitis from rifamycin. Contact Dermatitis. 1986;14:252-253. doi:10.1111/j.1600-0536.1986.tb01240.x
- Lodi A, Chiarelli G, Mancini LL, et al. Contact allergy to sodium sulfite contained in an antifungal preparation. Contact Dermatitis. 1993;29:97. doi:10.1111/j.1600-0536.1993.tb03493.x
- Sánchez-Pérez J, Abajo P, Córdoba S, et al. Allergic contact dermatitis from sodium metabisulfite in an antihemorrhoidal cream. Contact Dermatitis. 2000;42:176-177.
- Boyd AH, Warshaw EM. Sulfites: no longer a zebra? Dermatitis. 2017;28:364-366. doi:10.1097/der.0000000000000312
- Grosch E, Mahler V. Allergic contact dermatitis caused by a catheter system containing sodium metabisulfite. Contact Dermatitis. 2017;76:186-187. doi:10.1111/cod.12675
- Shaver RL, Warshaw EM. Contact allergens in prescription topical ophthalmic medications. Dermatitis. 2022;33:135-143. doi:10.1097/der.0000000000000751
- Dendooven E, Darrigade AS, Foubert K, et al. The presence of sulfites in ‘natural rubber latex’ and ‘synthetic’ rubber gloves: an experimental pilot study. Br J Dermatol. 2020;182:1054-1055. doi:10.1111/bjd.18608
- Nater JP. Allergic contact dermatitis caused by potassium metabisulfite. Dermatologica. 1968;136:477-478. doi:10.1159/000254143
- Borges AS, Valejo Coelho MM, Fernandes C, et al. Systemic allergic dermatitis caused by sodium metabisulfite in rectal enemas. Contact Dermatitis. 2018;78:429-430. doi:10.1111/cod.12971
- Warshaw EM, Buonomo M, DeKoven JG, et al. Patch testing with sodium disulfite: North American Contact Dermatitis Group experience, 2017 to 2018. Contact Dermatitis. 2021;85:285-296. doi:10.1111/cod.13860
- Häberle M, Geier J, Mahler V. Contact allergy to sulfites: clinical and occupational relevance—new data from the German Contact Dermatitis Research Group and the Information Network of Departments of Dermatology (IVDK). J Dtsch Dermatol Ges. 2016;14:938-941. doi:10.1111/ddg.13009
- Tan MG, Li HO, Pratt MD. Systemic allergic dermatitis to sodium metabisulfite in local anesthetic solution. Contact Dermatitis. 2022;86:120-121. doi:10.1111/cod.13978
- D’Amore T, Di Taranto A, Berardi G, et al. Sulfites in meat: occurrence, activity, toxicity, regulation, and detection. a comprehensive review. Compr Rev Food Sci Food Saf. 2020;19:2701-2720. doi:10.1111/1541-4337.12607
- Grotheer P, Marshall M, Simonne A. Sulfites: separating fact from fiction. May 11, 2022. UF IFAS Extension. University of Florida. Accessed October 4, 2024. https://edis.ifas.ufl.edu/publication/FY731
- Silva M, Gama J, Pinto N, et al. Sulfite concentration and the occurrence of headache in young adults: a prospective study. Eur J Clin Nutr. 2019;73:1316-1322. doi:10.1038/s41430-019-0420-2
- DeKoven JG, Warshaw EM, Reeder MJ, et al. North American Contact Dermatitis Group patch test results: 2019-2020. Dermatitis. 2023;34:90-104. doi:10.1089/derm.2022.29017.jdk
- T.R.U.E. Test. Thin-layer rapid use epicutaneous patch test. SmartPractice Dermatology Allergy. Accessed October 4, 2024. https://www.smartpractice.com/shop/category?id=581719&m=SPA
- Schalock PC, Dunnick CA, Nedorost, et al; American Contact Dermatitis Society Core Allergen Series Committee. American Contact Dermatitis Society Core Allergen Series: 2020 update. Dermatitis. 2020;31:279-282.
- Kaaman AC, Boman A, Wrangsjö K, et al. Contact allergy to sodium metabisulfite: an occupational problem. Contact Dermatitis. 2010;63:110-112. doi:10.1111/j.1600-0536.2010.01756.x
- Vena GA, Foti C, Angelini G. Sulfite contact allergy. Contact Dermatitis. 1994;31:172-175. doi:10.1111/j.1600-0536.1994.tb01959.x
Practice Points
- Sulfites are ubiquitous compounds that serve as preservatives and antioxidants in various foods, beverages, medications, and personal care products.
- Allergic contact dermatitis to sulfites most commonly affects the face and hands.
- Because sulfites are not included in most patch test screening series, contact allergy to sulfites may be missed unless expanded testing is performed.
