User login
Helping your obese patient achieve a healthier weight
In 2015-2016, almost 40% of adults and 18.5% of children ages 2 to 19 years in the United States met the definition for obesity—a chronic, relapsing, multifactorial, neurobehavioral disease that results in adverse metabolic, biomechanical, and psychosocial health consequences.1,2
Tremendous resources have been invested in research, policy development, and public education to try to prevent obesity and its related complications. Despite this, the obesity epidemic has worsened. Here, we explore how to evaluate and treat obese patients in a primary care setting based on the evidence and our experience seeing patients specifically for weight management in a family medicine residency teaching clinic. Pharmacotherapy and surgery, while often helpful, are outside the scope of this article.
It begins withan obesity-friendly office
Patients may have reservations about health care interactions specific to obesity, so it is important to invite them into a setting that facilitates trust and encourages collaboration. Actively engage patients with unhealthy weight by creating an environment where they feel comfortable. Offer wide chairs without armrests, which will easily accommodate patients of all sizes, and ensure that scales have a weight capacity > 400 lb. Communicate a message to patients, via waiting room materials and videos, that focuses on health rather than on weight or body mass index (BMI).
Understand the patient’s goals and challenges
Most (although not all) family physicians will see obese patients in the context of a visit for diabetes, hypertension, or another condition. However, we feel that having visits specifically to address weight in the initial stages of weight management is helpful. The focus of an initial visit should be getting to know how obesity has affected the patient and what his or her motive is in attempting to lose weight. Explore previous attempts at weight loss and establish what the patient’s highest weight has been, as this will impact weight-loss goals. For example, if a patient has weighed > 300 lb all her adult life, it will be extremely difficult to maintain a weight loss of 150 lb.
What else to ask about. Discuss stressors that may be causing increased food intake or poor food choices, including hunger, anger, loneliness, and sleep difficulties. Multidisciplinary care including a psychologist can aid in addressing these issues. Ask patients if they keep a food diary (and if not, recommend that they start), as food diaries are often helpful in elucidating eating and drinking patterns. Determine a patient’s current and past levels of physical activity, as this will guide the fitness goals you develop for him or her.
Screen for psychosocial disorders
As noted earlier, the physical component of obesity is commonly associated with mood disorders such as anxiety and depression.2 This requires a multidisciplinary team effort to facilitate healing in the patient struggling with obesity.
Screening for depression and anxiety using standardized tools such as the Patient Health Questionnaire-9 or the Generalized Anxiety Disorder-7 is encouraged in patients who are overweight or obese. Positive screens should be addressed as part of the patient’s treatment plan, as untreated depression and anxiety can inhibit success with weight loss. Be mindful that many medications commonly used to treat these conditions can impair weight loss and even promote weight gain.
Continue to: Don't overlook binge-eating disorders
Don’t overlook binge-eating disorders. Screening specifically for binge-eating disorders is important, given the implications on treatment. The US Department of Veterans Affairs developed a single-item tool for this purpose, the VA Binge Eating Screener. The validated questionnaire asks, “On average, how often have you eaten extremely large amounts of food at one time and felt that your eating was out of control at that time?” Response options are: “Never,” “< 1 time/week,” “1 time/week,” “2-4 times/week,” and “5+ times/week.” A response of ≥ 2 times/week had a sensitivity of 88.9% and specificity of 83.2% for binge-eating disorder.3
Patients with positive screens should undergo psychotherapy and consider pharmacotherapy with lisdexamfetamine as part of their treatment plan. Caution should be used if recommending intermittent fasting for someone with binge-eating disorder.
Evaluate for underlying causes and assess for comorbidities
Review the patient’s current medication list and history. Many medications can cause weight gain, and weight loss can often be achieved by deprescribing such medications. When feasible, prescribe an alternative medication with a more favorable weight profile. A previous article in The Journal of Family Practice addresses this in more depth.4
Laboratory and other testing
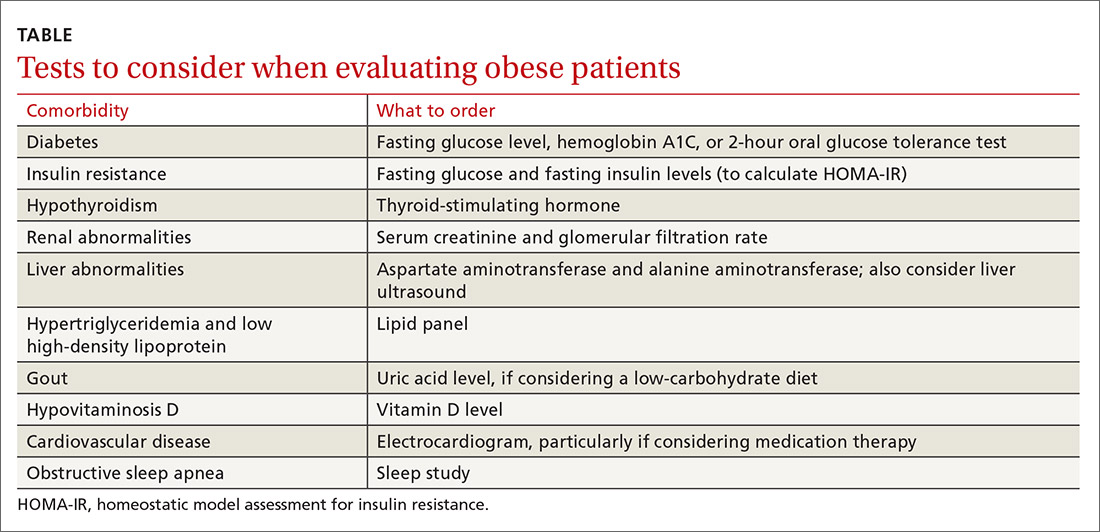
Laboratory analysis should primarily be focused on determining treatment alterations specific to underlying pathophysiology. Tests to consider ordering are outlined in the Table
Diabetes and insulin resistance. The American Diabetes Association recommends screening patients who are overweight or obese and have an additional risk factor for diabetes.5 This can be done by obtaining a fasting glucose level, hemoglobin A1C, or a 2-hour oral glucose tolerance test.
Continue to: Since it is known that...
Since it is known that insulin resistance increases the risk for coronary heart disease6 and can be treated effectively,7 we recommend testing for insulin resistance in patients who do not already have impaired fasting glucose, prediabetes, type 2 diabetes, or impaired glucose tolerance. The homeostatic model assessment for insulin resistance (HOMA-IR)8 is a measure of insulin resistance and can be calculated from the fasting insulin and fasting glucose levels. This measure should not be done in isolation, but it can be a useful adjunct in identifying patients with insulin resistance and directing treatment.
If there is evidence of diabetes or insulin resistance, consider treatment with metformin ± initiation of a low-carbohydrate diet.
Hypothyroidism. Consider screening for thyroid dysfunction with a thyroid-stimulating hormone level, if it has not been checked previously.
Renal abnormalities. When serum creatinine levels and glomerular filtration rate indicate chronic kidney disease, consider recommending a protein-restricted diet and adjust medications according to renal dosing protocols, as indicated.
Liver abnormalities, including nonalcoholic fatty liver disease (NAFLD). Monitor aspartate aminotransferase and alanine aminotransferase for resolution of elevations as weight loss is achieved. If abnormalities persist, consider ordering a liver ultrasound. Traditionally, low-calorie diets have been prescribed to treat NAFLD, but evidence shows that low-carbohydrate diets can also be effective.9
Continue to: Hypertriglyceridemia and low high-density lipoprotein (HDL) levels
Hypertriglyceridemia and low high-density lipoprotein (HDL) levels. Obtain a lipid panel if one has not been completed within the past several years, as hypertriglyceridemia and low HDL can improve dramatically with specific dietary changes.7 Observe trends to assess for resolution of lipid abnormalities as weight loss is achieved.
Gout. Consider checking a uric acid level if you are thinking about recommending a low-carbohydrate diet, particularly in patients with a history of gout, as this may temporarily increase the risk of gout flare.
Hypovitaminosis D. If the patient’s vitamin D level is low, consider appropriate supplementation to support the patient’s overall health. While vitamin D deficiency is common in obesity, the role of supplementation in this population is unclear.
Cardiovascular disease. Consider ordering an electrocardiogram, particularly if you are thinking of prescribing medication therapy. Use caution with initiation of certain medications, such as phentermine or diethylproprion, in the presence of arrhythmias or active cardiovascular disease.
Obstructive sleep apnea. Sleep health is important to address, since obesity is one of the most significant risk factors for obstructive sleep apnea.10 If your patient is given a diagnosis of OSA following a sleep study, consider treatment with continuous positive airway pressure (CPAP), although there are conflicting studies regarding the effects of CPAP therapy in OSA on weight.11,12
Continue to: Provide guidance on lifestyle changes
Provide guidance on lifestyle changes
Addressing obesity with patients can be challenging in a busy primary care clinic, but it is imperative to helping patients achieve overall health. Counseling on nutrition and physical activity is an important part of this process.
There is no one-size-fits-all approach to nutrition counseling. Focus on creating individualized plans through which patients can achieve success. Some guidance follows, but also beware of common pitfalls that we have observed in clinical practice which, when addressed, can enable significant weight loss (see “Common pitfalls inhibiting weight loss”).
SIDEBAR
Common pitfalls inhibiting weight loss
On the part of the patient:
- Continuing to consume substantial amounts of high-calorie drinks.
- Taking in excessive amounts of sugar-rich foods, including cough drops.
- Using non-nutritive sweeteners (eg, aspartame, saccharin, sucralose, and erythritol). Although the mechanism is not certain, some people are able to lose weight while consuming these substances, while others are not.
On the part of the provider:
- Prescribing a diet that the patient cannot sustain long term.
- Overlooking the issue of food availability for the patient.
Choose an approach that works for the patient. Commonly prescribed diets to address obesity include, but are not limited to, Atkins, Dietary Approaches to Stop Hypertension (DASH), Glycemic Index, Mediterranean, Ornish, Paleolithic, Zone, whole food plant-based, and ketogenic. We attempt to engage patients in making the decision on what food choices are appropriate for them considering their food availability, culture, and belief systems. For patients who prefer a vegan or vegetarian whole food diet, it is important to note that these diets are generally deficient in vitamin B12 and omega 3 fatty acids, so supplementing these should be considered.
Rather than focus on a specific diet, which may not be sustainable long term, encourage healthy eating habits. Low-carbohydrate diets have been shown to promote greater weight loss compared to low-fat diets.13,14 Low-calorie diets can also be quite effective in promoting short-term weight loss. In our clinic, when weight loss is the primary goal, patients are typically encouraged to focus on either calorie or carbohydrate restriction in the initial stages of weight loss.
Eliminate sugar and refined carbohydrates. While rigorous mortality data are not available, more recent trials have demonstrated significant improvements in atherosclerotic cardiovascular disease risk markers, including weight reduction and diabetes reversal, when following a diet that markedly decreases carbohydrate intake, especially sugar and refined carbohydrates.7,14-17
Continue to: We recommend that patients focus...
We recommend that patients focus on eliminating sweetened beverages, such as soft drinks, sports drinks, energy drinks, vitamin water, sweet tea, chocolate milk, and Frappuccinos. We also recommend substantially limiting or eliminating fruit juices and fruit smoothies due to their high sugar content. For example, 8 oz of orange juice contains 26 g of carbohydrates, which is almost as much as 8 oz of soda.
Compared with eating whole fruit, consuming fruit juice has demonstrated a small amount of weight gain in young children and adults.18,19 It also has shown a higher insulin response compared with eating the same amount of carbohydrates in whole fruit.20 Better options to drink include water, unsweetened tea, and black coffee. Also, avoid ultra-processed carbohydrates from foods such as breads, cereals, and pastries, as they have similar effects on blood glucose when compared to sugar.21
Greatly restrict highly processed foods. The evidence suggests that the availability of processed food is associated with increasing obesity.22 Simple advice to offer your patients is to encourage them to shop the perimeter of the grocery store, where fresh produce, meat, and dairy products are primarily located, and avoid the inner aisles, which contain primarily processed foods. Choosing food items with 5 or fewer ingredients is a starting point when teaching patients to read labels.
Consider limiting saturated fats. In 1977, the Dietary Guidelines for Americans recommended that Americans eat no more than 30% of total energy intake from fat and less than 10% of total energy intake from saturated fat; however, no randomized controlled trials had been done that supported this recommendation and epidemiologic data supporting it were weak.23
The 2015 Dietary Guidelines continue to recommend limiting total energy intake from saturated fats.24 While there may be a small decrease in cardiovascular risk with a reduction of saturated fat intake and replacement with unsaturated fats, no overall mortality benefit has been demonstrated.24,25 More research is needed in this area to guide patients in decisions regarding consumption of saturated fats and what types of unsaturated fats are best for their health.
Continue to: Eat only 3 meals per day
Eat only 3 meals per day, but aim for fewer than that. The prescription of fasting is a modality that can be used for weight loss and improved health. Fasting has been a prescribed healing practice for thousands of years.26 It is a practice that virtually every major religion in the world embraces. Studies have demonstrated fasting to be safe and effective in the setting of obesity without significant comorbidities, and it may promote weight loss and metabolic health.26-29
There are multiple types of intermittent fasting. A practical way for patients to start is by restricting the number of hours in which they eat or drink calorie-containing beverages to 8 hours per day. In our experience, this regimen is easier for most patients to follow than alternate-day or other longer fasts. While there has been caution in the prescription of intermittent fasting due to concerns about causing eating disorders, a recent small study did not demonstrate increased risk of eating disorders with daily intermittent fasting.30
Participate in healthy exercise. Nonpharmacologic office-based strategies for treating obesity have generally focused on increasing exercise and decreasing caloric intake.31 While exercise has significant health benefits, including preventing weight regain, evidence does not support monotherapy with exercise as an effective long-term weight-loss strategy.32 There are no studies available that adequately support prescribing an exact dose of exercise.33 Generally, less than 150 minutes of exercise per week is not effective and more than that does have a dose-related response.33
Follow up to help patients stay on target
There is no ideal interval for follow-up visits. However, frequent visits—anywhere from weekly to monthly—in the initial stages of weight loss increase the patient’s sense of accountability and, in our experience, seem to be helpful.
Patients may also choose to track their progress by weighing themselves regularly. A small study published in the International Journal of Obesity found that patients who weighed themselves daily had greater and more sustained weight loss than those who didn’t.34 But the decision of whether to weigh one’s self at home should be individualized for each patient.
CORRESPONDENCE
Wesley Eichorn, DO, 1000 Oakland Drive, Kalamazoo, MI 49008; wesley.eichorn@med.wmich.edu
1. Hales CM, Carroll MD, Fryar CD, et al. Prevalence of obesity among adults and youth: United States, 2015-2016 key findings data from the National Health and Nutrition Examination Survey. NCHS Data Brief. 2017;(288):1-8.
2. Seger JC, Horn DB, Westman EC, et al. Obesity Algorithm, presented by the Obesity Medicine Association. Accessed March 5, 2021. www.obesityalgorithm.org. 2016-2017
3. Dorflinger LM, Ruser CB, Masheb RM. A brief screening measure for binge eating in primary care. Eat Behav. 2017;26:163-166. https://doi.org/10.1016/j.eatbeh.2017.03.009
4. Saunders KH, Igel LI, Shukla AP, et al. Drug-induced weight gain: rethinking our choices. J Fam Pract. 2016;65:780-788.
5. American Diabetes Association. 2. Classification and Diagnosis of Diabetes: Standards of Medical Care in Diabetes—2019. Diabetes Care. 2019;42(suppl 1):S13-S28. https://doi.org/10.2337/dc19-S002
6. Reaven G. Insulin resistance and coronary heart disease in nondiabetic individuals. Arterioscler Thromb Vasc Biol. 2012;32:1754-1759. https://doi.org/10.1161/ATVBAHA.111.241885/-/DC1
7. Hallberg S, McKenzie A, Williams P, et al. Effectiveness and safety of a novel care model for the management of type 2 diabetes at 1 year: an open-label, non-randomized, controlled study. Diabetes Ther. 2018;9:583-612. https://doi.org/10.6084/m9.figshare
8. Wallace TM, Levy JC, Matthews DR. Use and abuse of HOMA modeling. Diabetes Care. 2004;27:1487-1495.
9. Vilar-Gomez E, Athinarayanan SJ, Adams RN, et al. Post hoc analyses of surrogate markers of non-alcoholic fatty liver disease (NAFLD) and liver fibrosis in patients with type 2 diabetes in a digitally supported continuous care intervention: an open-label, non-randomised controlled study. BMJ Open. 2019;9:e023597. https://doi.org/10.1136/bmjopen-2018-023597
10. Young T, Peppard PE, Gottlieb DJ. Epidemiology of obstructive sleep apnea. Am J Respir Crit Care Med. 2002;165:1217-1239. https://doi.org/10.1164/rccm.2109080
11. Drager LF, Brunoni AR, Jenner R, et al. Effects of CPAP on body weight in patients with obstructive sleep apnoea: a meta-analysis of randomised trials. Thorax. 2015;70:258-264. https://doi.org/10.1136/thoraxjnl-2014-205361
12. Bosworth T. CPAP use associated with greater weight loss in obese patients with sleep apnea. CHEST Physician. Published March 29, 2019. Accessed March 5, 2021. www.mdedge.com/chestphysician/article/197827/sleep-medicine/cpap-use-associated-greater-weight-loss-obese-patients
13. Tobias DK, Chen M, Manson JAE, et al. Effect of low-fat diet interventions versus other diet interventions on long-term weight change in adults: a systematic review and meta-analysis. Lancet Diabetes Endocrinol. 2015;3:968-979. https://doi.org/10.1016/S2213-8587(15)00367-8
14. Sackner-Bernstein J, Kanter D, Kaul S. Dietary intervention for overweight and obese adults: comparison of low-carbohydrate and low-fat diets: a meta-analysis. PLoS One. 2015;10:e0139817. https://doi.org/10.1371/journal.pone.0139817
15. Bezerra Bueno N, Vieira De Melo IS, Lima De Oliveira S, et al. Very-low-carbohydrate ketogenic diet v low-fat diet for long-term weight loss: a meta-analysis of randomised controlled trials. Br J Nutr. 2013;110:1178-1187. https://doi.org/10.1017/S0007114513000548
16. Santos FL, Esteves SS, da Costa Pereira A, et al. Systematic review and meta-analysis of clinical trials of the effects of low carbohydrate diets on cardiovascular risk factors. Obes Rev. 2012;13:1048-1066. https://doi.org/10.1111/j.1467-789X.2012.01021.x
17. Athinarayanan SJ, Adams RN, Hallberg SJ, et al. Long-term effects of a novel continuous remote care intervention including nutritional ketosis for the management of type 2 diabetes: a 2-year non-randomized clinical trial. bioRxiv. 2018;10:348. https://doi.org/10.1101/476275
18. Auerbach BJ, Dibey S, Vallila-Buchman P, et al. Review of 100% fruit juice and chronic health conditions: implications for sugar-sweetened beverage policy. Adv Nutr. 2018;9:78-85. https://doi.org/10.1093/advances/nmx006
19. Faith MS, Dennison BA, Edmunds LS, et al. Fruit juice intake predicts increased adiposity gain in children from low-income families: weight status-by-environment interaction. Pediatrics. 2006;118:2066-2075. https://doi.org/10.1542/peds.2006-1117
20. Bolton RP, Burroughs LF, Heaton KW. The role of dietary fiber in satiety, insulin: studies with fruit and fruit. Am J Clin Nutr. 1981;84:211-217. https://doi.org/10.1093/ajcn/34.2.211
21. Unwin D, Haslam D, Livesey G. It is the glycaemic response to, not the carbohydrate content of food that matters in diabetes and obesity: the glycaemic index revisited. J Insul Resist. 2016;1(1):a8. https://doi.org/10.4102/jir.v1i1.8
22. Monteiro CA, Moubarac JC, Levy RB, et al. Household availability of ultra-processed foods and obesity in nineteen European countries. Public Health Nutr. 2018;21:18-26. https://doi.org/10.1017/S1368980017001379
23. Harcombe Z, Baker JS, Cooper SM, et al. Evidence from randomised controlled trials did not support the introduction of dietary fat guidelines in 1977 and 1983: a systematic review and meta-analysis. Open Hear. 2015;2:e000196. https://doi.org/10.1136/openhrt-2014
24. US Department of Health and Human Services and US Department of Agriculture. 2015-2020 Dietary Guidelines for Americans. 8th edition. Published December 2015. Accessed March 5, 2021. http://health.gov/dietaryguidelines/2015/guidelines/
25. Harcombe Z, Baker JS, DiNicolantonio JJ, et al. Evidence from randomised controlled trials does not support current dietary fat guidelines: a systematic review and meta-analysis. Open Hear. 2016;3:e000409. https://doi.org/10.1136/openhrt-2016-000409
26. Fung J. The Obesity Code: Unlocking the Secrets of Weight Loss. Greystone Books; 2016.
27. Mattson MP, Longo VD, Harvie M. Impact of intermittent fasting on health and disease processes. Ageing Res Rev. 2017;39:46-58. https://doi.org/10.1016/j.arr.2016.10.005
28. Patterson RE, Sears DD. Metabolic Effects of Intermittent Fasting. Annu Rev Nutr. 2017; 37:371-393. https://doi.org/10.1146/annurev-nutr-071816-064634
29. Duncan GG. Intermittent fasts in the correction and control of intractable obesity. Trans Am Clin Climatol Assoc. 1962;74:121-129.
30. Gabel K, Hoddy KK, Varady KA. Safety of 8-h time restricted feeding in adults with obesity. Appl Physiol Nutr Metab. 2019;44:107-109. https://doi.org/10.1139/apnm-2018-0389
31. Erlandson M, Ivey LC, Seikel K. Update on office-based strategies for the management of obesity. Am Fam Physician. 2016;94:361-368.
32. Malhotra A, Noakes T, Phinney S. It is time to bust the myth of physical inactivity and obesity: you cannot outrun a bad diet. Br J Sports Med. 2015;49:967-968. https://doi.org/10.1136/bjsports-2015-094911
33. Donnelly JE, Blair SN, Jakicic JM, et al. Appropriate physical activity intervention strategies for weight loss and prevention of weight regain for adults. Med Sci Sports Exerc. 2009;41:459-471. https://doi.org/10.1249/MSS.0b013e3181949333
34. Zheng Y, Burke LE, Danford CA, et al. Patterns of self-weighing behavior and weight change in a weight loss trial. Int J Obes (Lond). 2016;40:1392-1396. https://doi.org/10.1038/ijo.2016.68
In 2015-2016, almost 40% of adults and 18.5% of children ages 2 to 19 years in the United States met the definition for obesity—a chronic, relapsing, multifactorial, neurobehavioral disease that results in adverse metabolic, biomechanical, and psychosocial health consequences.1,2
Tremendous resources have been invested in research, policy development, and public education to try to prevent obesity and its related complications. Despite this, the obesity epidemic has worsened. Here, we explore how to evaluate and treat obese patients in a primary care setting based on the evidence and our experience seeing patients specifically for weight management in a family medicine residency teaching clinic. Pharmacotherapy and surgery, while often helpful, are outside the scope of this article.
It begins withan obesity-friendly office
Patients may have reservations about health care interactions specific to obesity, so it is important to invite them into a setting that facilitates trust and encourages collaboration. Actively engage patients with unhealthy weight by creating an environment where they feel comfortable. Offer wide chairs without armrests, which will easily accommodate patients of all sizes, and ensure that scales have a weight capacity > 400 lb. Communicate a message to patients, via waiting room materials and videos, that focuses on health rather than on weight or body mass index (BMI).
Understand the patient’s goals and challenges
Most (although not all) family physicians will see obese patients in the context of a visit for diabetes, hypertension, or another condition. However, we feel that having visits specifically to address weight in the initial stages of weight management is helpful. The focus of an initial visit should be getting to know how obesity has affected the patient and what his or her motive is in attempting to lose weight. Explore previous attempts at weight loss and establish what the patient’s highest weight has been, as this will impact weight-loss goals. For example, if a patient has weighed > 300 lb all her adult life, it will be extremely difficult to maintain a weight loss of 150 lb.
What else to ask about. Discuss stressors that may be causing increased food intake or poor food choices, including hunger, anger, loneliness, and sleep difficulties. Multidisciplinary care including a psychologist can aid in addressing these issues. Ask patients if they keep a food diary (and if not, recommend that they start), as food diaries are often helpful in elucidating eating and drinking patterns. Determine a patient’s current and past levels of physical activity, as this will guide the fitness goals you develop for him or her.
Screen for psychosocial disorders
As noted earlier, the physical component of obesity is commonly associated with mood disorders such as anxiety and depression.2 This requires a multidisciplinary team effort to facilitate healing in the patient struggling with obesity.
Screening for depression and anxiety using standardized tools such as the Patient Health Questionnaire-9 or the Generalized Anxiety Disorder-7 is encouraged in patients who are overweight or obese. Positive screens should be addressed as part of the patient’s treatment plan, as untreated depression and anxiety can inhibit success with weight loss. Be mindful that many medications commonly used to treat these conditions can impair weight loss and even promote weight gain.
Continue to: Don't overlook binge-eating disorders
Don’t overlook binge-eating disorders. Screening specifically for binge-eating disorders is important, given the implications on treatment. The US Department of Veterans Affairs developed a single-item tool for this purpose, the VA Binge Eating Screener. The validated questionnaire asks, “On average, how often have you eaten extremely large amounts of food at one time and felt that your eating was out of control at that time?” Response options are: “Never,” “< 1 time/week,” “1 time/week,” “2-4 times/week,” and “5+ times/week.” A response of ≥ 2 times/week had a sensitivity of 88.9% and specificity of 83.2% for binge-eating disorder.3
Patients with positive screens should undergo psychotherapy and consider pharmacotherapy with lisdexamfetamine as part of their treatment plan. Caution should be used if recommending intermittent fasting for someone with binge-eating disorder.
Evaluate for underlying causes and assess for comorbidities
Review the patient’s current medication list and history. Many medications can cause weight gain, and weight loss can often be achieved by deprescribing such medications. When feasible, prescribe an alternative medication with a more favorable weight profile. A previous article in The Journal of Family Practice addresses this in more depth.4
Laboratory and other testing
Laboratory analysis should primarily be focused on determining treatment alterations specific to underlying pathophysiology. Tests to consider ordering are outlined in the Table
Diabetes and insulin resistance. The American Diabetes Association recommends screening patients who are overweight or obese and have an additional risk factor for diabetes.5 This can be done by obtaining a fasting glucose level, hemoglobin A1C, or a 2-hour oral glucose tolerance test.
Continue to: Since it is known that...
Since it is known that insulin resistance increases the risk for coronary heart disease6 and can be treated effectively,7 we recommend testing for insulin resistance in patients who do not already have impaired fasting glucose, prediabetes, type 2 diabetes, or impaired glucose tolerance. The homeostatic model assessment for insulin resistance (HOMA-IR)8 is a measure of insulin resistance and can be calculated from the fasting insulin and fasting glucose levels. This measure should not be done in isolation, but it can be a useful adjunct in identifying patients with insulin resistance and directing treatment.
If there is evidence of diabetes or insulin resistance, consider treatment with metformin ± initiation of a low-carbohydrate diet.
Hypothyroidism. Consider screening for thyroid dysfunction with a thyroid-stimulating hormone level, if it has not been checked previously.
Renal abnormalities. When serum creatinine levels and glomerular filtration rate indicate chronic kidney disease, consider recommending a protein-restricted diet and adjust medications according to renal dosing protocols, as indicated.
Liver abnormalities, including nonalcoholic fatty liver disease (NAFLD). Monitor aspartate aminotransferase and alanine aminotransferase for resolution of elevations as weight loss is achieved. If abnormalities persist, consider ordering a liver ultrasound. Traditionally, low-calorie diets have been prescribed to treat NAFLD, but evidence shows that low-carbohydrate diets can also be effective.9
Continue to: Hypertriglyceridemia and low high-density lipoprotein (HDL) levels
Hypertriglyceridemia and low high-density lipoprotein (HDL) levels. Obtain a lipid panel if one has not been completed within the past several years, as hypertriglyceridemia and low HDL can improve dramatically with specific dietary changes.7 Observe trends to assess for resolution of lipid abnormalities as weight loss is achieved.
Gout. Consider checking a uric acid level if you are thinking about recommending a low-carbohydrate diet, particularly in patients with a history of gout, as this may temporarily increase the risk of gout flare.
Hypovitaminosis D. If the patient’s vitamin D level is low, consider appropriate supplementation to support the patient’s overall health. While vitamin D deficiency is common in obesity, the role of supplementation in this population is unclear.
Cardiovascular disease. Consider ordering an electrocardiogram, particularly if you are thinking of prescribing medication therapy. Use caution with initiation of certain medications, such as phentermine or diethylproprion, in the presence of arrhythmias or active cardiovascular disease.
Obstructive sleep apnea. Sleep health is important to address, since obesity is one of the most significant risk factors for obstructive sleep apnea.10 If your patient is given a diagnosis of OSA following a sleep study, consider treatment with continuous positive airway pressure (CPAP), although there are conflicting studies regarding the effects of CPAP therapy in OSA on weight.11,12
Continue to: Provide guidance on lifestyle changes
Provide guidance on lifestyle changes
Addressing obesity with patients can be challenging in a busy primary care clinic, but it is imperative to helping patients achieve overall health. Counseling on nutrition and physical activity is an important part of this process.
There is no one-size-fits-all approach to nutrition counseling. Focus on creating individualized plans through which patients can achieve success. Some guidance follows, but also beware of common pitfalls that we have observed in clinical practice which, when addressed, can enable significant weight loss (see “Common pitfalls inhibiting weight loss”).
SIDEBAR
Common pitfalls inhibiting weight loss
On the part of the patient:
- Continuing to consume substantial amounts of high-calorie drinks.
- Taking in excessive amounts of sugar-rich foods, including cough drops.
- Using non-nutritive sweeteners (eg, aspartame, saccharin, sucralose, and erythritol). Although the mechanism is not certain, some people are able to lose weight while consuming these substances, while others are not.
On the part of the provider:
- Prescribing a diet that the patient cannot sustain long term.
- Overlooking the issue of food availability for the patient.
Choose an approach that works for the patient. Commonly prescribed diets to address obesity include, but are not limited to, Atkins, Dietary Approaches to Stop Hypertension (DASH), Glycemic Index, Mediterranean, Ornish, Paleolithic, Zone, whole food plant-based, and ketogenic. We attempt to engage patients in making the decision on what food choices are appropriate for them considering their food availability, culture, and belief systems. For patients who prefer a vegan or vegetarian whole food diet, it is important to note that these diets are generally deficient in vitamin B12 and omega 3 fatty acids, so supplementing these should be considered.
Rather than focus on a specific diet, which may not be sustainable long term, encourage healthy eating habits. Low-carbohydrate diets have been shown to promote greater weight loss compared to low-fat diets.13,14 Low-calorie diets can also be quite effective in promoting short-term weight loss. In our clinic, when weight loss is the primary goal, patients are typically encouraged to focus on either calorie or carbohydrate restriction in the initial stages of weight loss.
Eliminate sugar and refined carbohydrates. While rigorous mortality data are not available, more recent trials have demonstrated significant improvements in atherosclerotic cardiovascular disease risk markers, including weight reduction and diabetes reversal, when following a diet that markedly decreases carbohydrate intake, especially sugar and refined carbohydrates.7,14-17
Continue to: We recommend that patients focus...
We recommend that patients focus on eliminating sweetened beverages, such as soft drinks, sports drinks, energy drinks, vitamin water, sweet tea, chocolate milk, and Frappuccinos. We also recommend substantially limiting or eliminating fruit juices and fruit smoothies due to their high sugar content. For example, 8 oz of orange juice contains 26 g of carbohydrates, which is almost as much as 8 oz of soda.
Compared with eating whole fruit, consuming fruit juice has demonstrated a small amount of weight gain in young children and adults.18,19 It also has shown a higher insulin response compared with eating the same amount of carbohydrates in whole fruit.20 Better options to drink include water, unsweetened tea, and black coffee. Also, avoid ultra-processed carbohydrates from foods such as breads, cereals, and pastries, as they have similar effects on blood glucose when compared to sugar.21
Greatly restrict highly processed foods. The evidence suggests that the availability of processed food is associated with increasing obesity.22 Simple advice to offer your patients is to encourage them to shop the perimeter of the grocery store, where fresh produce, meat, and dairy products are primarily located, and avoid the inner aisles, which contain primarily processed foods. Choosing food items with 5 or fewer ingredients is a starting point when teaching patients to read labels.
Consider limiting saturated fats. In 1977, the Dietary Guidelines for Americans recommended that Americans eat no more than 30% of total energy intake from fat and less than 10% of total energy intake from saturated fat; however, no randomized controlled trials had been done that supported this recommendation and epidemiologic data supporting it were weak.23
The 2015 Dietary Guidelines continue to recommend limiting total energy intake from saturated fats.24 While there may be a small decrease in cardiovascular risk with a reduction of saturated fat intake and replacement with unsaturated fats, no overall mortality benefit has been demonstrated.24,25 More research is needed in this area to guide patients in decisions regarding consumption of saturated fats and what types of unsaturated fats are best for their health.
Continue to: Eat only 3 meals per day
Eat only 3 meals per day, but aim for fewer than that. The prescription of fasting is a modality that can be used for weight loss and improved health. Fasting has been a prescribed healing practice for thousands of years.26 It is a practice that virtually every major religion in the world embraces. Studies have demonstrated fasting to be safe and effective in the setting of obesity without significant comorbidities, and it may promote weight loss and metabolic health.26-29
There are multiple types of intermittent fasting. A practical way for patients to start is by restricting the number of hours in which they eat or drink calorie-containing beverages to 8 hours per day. In our experience, this regimen is easier for most patients to follow than alternate-day or other longer fasts. While there has been caution in the prescription of intermittent fasting due to concerns about causing eating disorders, a recent small study did not demonstrate increased risk of eating disorders with daily intermittent fasting.30
Participate in healthy exercise. Nonpharmacologic office-based strategies for treating obesity have generally focused on increasing exercise and decreasing caloric intake.31 While exercise has significant health benefits, including preventing weight regain, evidence does not support monotherapy with exercise as an effective long-term weight-loss strategy.32 There are no studies available that adequately support prescribing an exact dose of exercise.33 Generally, less than 150 minutes of exercise per week is not effective and more than that does have a dose-related response.33
Follow up to help patients stay on target
There is no ideal interval for follow-up visits. However, frequent visits—anywhere from weekly to monthly—in the initial stages of weight loss increase the patient’s sense of accountability and, in our experience, seem to be helpful.
Patients may also choose to track their progress by weighing themselves regularly. A small study published in the International Journal of Obesity found that patients who weighed themselves daily had greater and more sustained weight loss than those who didn’t.34 But the decision of whether to weigh one’s self at home should be individualized for each patient.
CORRESPONDENCE
Wesley Eichorn, DO, 1000 Oakland Drive, Kalamazoo, MI 49008; wesley.eichorn@med.wmich.edu
In 2015-2016, almost 40% of adults and 18.5% of children ages 2 to 19 years in the United States met the definition for obesity—a chronic, relapsing, multifactorial, neurobehavioral disease that results in adverse metabolic, biomechanical, and psychosocial health consequences.1,2
Tremendous resources have been invested in research, policy development, and public education to try to prevent obesity and its related complications. Despite this, the obesity epidemic has worsened. Here, we explore how to evaluate and treat obese patients in a primary care setting based on the evidence and our experience seeing patients specifically for weight management in a family medicine residency teaching clinic. Pharmacotherapy and surgery, while often helpful, are outside the scope of this article.
It begins withan obesity-friendly office
Patients may have reservations about health care interactions specific to obesity, so it is important to invite them into a setting that facilitates trust and encourages collaboration. Actively engage patients with unhealthy weight by creating an environment where they feel comfortable. Offer wide chairs without armrests, which will easily accommodate patients of all sizes, and ensure that scales have a weight capacity > 400 lb. Communicate a message to patients, via waiting room materials and videos, that focuses on health rather than on weight or body mass index (BMI).
Understand the patient’s goals and challenges
Most (although not all) family physicians will see obese patients in the context of a visit for diabetes, hypertension, or another condition. However, we feel that having visits specifically to address weight in the initial stages of weight management is helpful. The focus of an initial visit should be getting to know how obesity has affected the patient and what his or her motive is in attempting to lose weight. Explore previous attempts at weight loss and establish what the patient’s highest weight has been, as this will impact weight-loss goals. For example, if a patient has weighed > 300 lb all her adult life, it will be extremely difficult to maintain a weight loss of 150 lb.
What else to ask about. Discuss stressors that may be causing increased food intake or poor food choices, including hunger, anger, loneliness, and sleep difficulties. Multidisciplinary care including a psychologist can aid in addressing these issues. Ask patients if they keep a food diary (and if not, recommend that they start), as food diaries are often helpful in elucidating eating and drinking patterns. Determine a patient’s current and past levels of physical activity, as this will guide the fitness goals you develop for him or her.
Screen for psychosocial disorders
As noted earlier, the physical component of obesity is commonly associated with mood disorders such as anxiety and depression.2 This requires a multidisciplinary team effort to facilitate healing in the patient struggling with obesity.
Screening for depression and anxiety using standardized tools such as the Patient Health Questionnaire-9 or the Generalized Anxiety Disorder-7 is encouraged in patients who are overweight or obese. Positive screens should be addressed as part of the patient’s treatment plan, as untreated depression and anxiety can inhibit success with weight loss. Be mindful that many medications commonly used to treat these conditions can impair weight loss and even promote weight gain.
Continue to: Don't overlook binge-eating disorders
Don’t overlook binge-eating disorders. Screening specifically for binge-eating disorders is important, given the implications on treatment. The US Department of Veterans Affairs developed a single-item tool for this purpose, the VA Binge Eating Screener. The validated questionnaire asks, “On average, how often have you eaten extremely large amounts of food at one time and felt that your eating was out of control at that time?” Response options are: “Never,” “< 1 time/week,” “1 time/week,” “2-4 times/week,” and “5+ times/week.” A response of ≥ 2 times/week had a sensitivity of 88.9% and specificity of 83.2% for binge-eating disorder.3
Patients with positive screens should undergo psychotherapy and consider pharmacotherapy with lisdexamfetamine as part of their treatment plan. Caution should be used if recommending intermittent fasting for someone with binge-eating disorder.
Evaluate for underlying causes and assess for comorbidities
Review the patient’s current medication list and history. Many medications can cause weight gain, and weight loss can often be achieved by deprescribing such medications. When feasible, prescribe an alternative medication with a more favorable weight profile. A previous article in The Journal of Family Practice addresses this in more depth.4
Laboratory and other testing
Laboratory analysis should primarily be focused on determining treatment alterations specific to underlying pathophysiology. Tests to consider ordering are outlined in the Table
Diabetes and insulin resistance. The American Diabetes Association recommends screening patients who are overweight or obese and have an additional risk factor for diabetes.5 This can be done by obtaining a fasting glucose level, hemoglobin A1C, or a 2-hour oral glucose tolerance test.
Continue to: Since it is known that...
Since it is known that insulin resistance increases the risk for coronary heart disease6 and can be treated effectively,7 we recommend testing for insulin resistance in patients who do not already have impaired fasting glucose, prediabetes, type 2 diabetes, or impaired glucose tolerance. The homeostatic model assessment for insulin resistance (HOMA-IR)8 is a measure of insulin resistance and can be calculated from the fasting insulin and fasting glucose levels. This measure should not be done in isolation, but it can be a useful adjunct in identifying patients with insulin resistance and directing treatment.
If there is evidence of diabetes or insulin resistance, consider treatment with metformin ± initiation of a low-carbohydrate diet.
Hypothyroidism. Consider screening for thyroid dysfunction with a thyroid-stimulating hormone level, if it has not been checked previously.
Renal abnormalities. When serum creatinine levels and glomerular filtration rate indicate chronic kidney disease, consider recommending a protein-restricted diet and adjust medications according to renal dosing protocols, as indicated.
Liver abnormalities, including nonalcoholic fatty liver disease (NAFLD). Monitor aspartate aminotransferase and alanine aminotransferase for resolution of elevations as weight loss is achieved. If abnormalities persist, consider ordering a liver ultrasound. Traditionally, low-calorie diets have been prescribed to treat NAFLD, but evidence shows that low-carbohydrate diets can also be effective.9
Continue to: Hypertriglyceridemia and low high-density lipoprotein (HDL) levels
Hypertriglyceridemia and low high-density lipoprotein (HDL) levels. Obtain a lipid panel if one has not been completed within the past several years, as hypertriglyceridemia and low HDL can improve dramatically with specific dietary changes.7 Observe trends to assess for resolution of lipid abnormalities as weight loss is achieved.
Gout. Consider checking a uric acid level if you are thinking about recommending a low-carbohydrate diet, particularly in patients with a history of gout, as this may temporarily increase the risk of gout flare.
Hypovitaminosis D. If the patient’s vitamin D level is low, consider appropriate supplementation to support the patient’s overall health. While vitamin D deficiency is common in obesity, the role of supplementation in this population is unclear.
Cardiovascular disease. Consider ordering an electrocardiogram, particularly if you are thinking of prescribing medication therapy. Use caution with initiation of certain medications, such as phentermine or diethylproprion, in the presence of arrhythmias or active cardiovascular disease.
Obstructive sleep apnea. Sleep health is important to address, since obesity is one of the most significant risk factors for obstructive sleep apnea.10 If your patient is given a diagnosis of OSA following a sleep study, consider treatment with continuous positive airway pressure (CPAP), although there are conflicting studies regarding the effects of CPAP therapy in OSA on weight.11,12
Continue to: Provide guidance on lifestyle changes
Provide guidance on lifestyle changes
Addressing obesity with patients can be challenging in a busy primary care clinic, but it is imperative to helping patients achieve overall health. Counseling on nutrition and physical activity is an important part of this process.
There is no one-size-fits-all approach to nutrition counseling. Focus on creating individualized plans through which patients can achieve success. Some guidance follows, but also beware of common pitfalls that we have observed in clinical practice which, when addressed, can enable significant weight loss (see “Common pitfalls inhibiting weight loss”).
SIDEBAR
Common pitfalls inhibiting weight loss
On the part of the patient:
- Continuing to consume substantial amounts of high-calorie drinks.
- Taking in excessive amounts of sugar-rich foods, including cough drops.
- Using non-nutritive sweeteners (eg, aspartame, saccharin, sucralose, and erythritol). Although the mechanism is not certain, some people are able to lose weight while consuming these substances, while others are not.
On the part of the provider:
- Prescribing a diet that the patient cannot sustain long term.
- Overlooking the issue of food availability for the patient.
Choose an approach that works for the patient. Commonly prescribed diets to address obesity include, but are not limited to, Atkins, Dietary Approaches to Stop Hypertension (DASH), Glycemic Index, Mediterranean, Ornish, Paleolithic, Zone, whole food plant-based, and ketogenic. We attempt to engage patients in making the decision on what food choices are appropriate for them considering their food availability, culture, and belief systems. For patients who prefer a vegan or vegetarian whole food diet, it is important to note that these diets are generally deficient in vitamin B12 and omega 3 fatty acids, so supplementing these should be considered.
Rather than focus on a specific diet, which may not be sustainable long term, encourage healthy eating habits. Low-carbohydrate diets have been shown to promote greater weight loss compared to low-fat diets.13,14 Low-calorie diets can also be quite effective in promoting short-term weight loss. In our clinic, when weight loss is the primary goal, patients are typically encouraged to focus on either calorie or carbohydrate restriction in the initial stages of weight loss.
Eliminate sugar and refined carbohydrates. While rigorous mortality data are not available, more recent trials have demonstrated significant improvements in atherosclerotic cardiovascular disease risk markers, including weight reduction and diabetes reversal, when following a diet that markedly decreases carbohydrate intake, especially sugar and refined carbohydrates.7,14-17
Continue to: We recommend that patients focus...
We recommend that patients focus on eliminating sweetened beverages, such as soft drinks, sports drinks, energy drinks, vitamin water, sweet tea, chocolate milk, and Frappuccinos. We also recommend substantially limiting or eliminating fruit juices and fruit smoothies due to their high sugar content. For example, 8 oz of orange juice contains 26 g of carbohydrates, which is almost as much as 8 oz of soda.
Compared with eating whole fruit, consuming fruit juice has demonstrated a small amount of weight gain in young children and adults.18,19 It also has shown a higher insulin response compared with eating the same amount of carbohydrates in whole fruit.20 Better options to drink include water, unsweetened tea, and black coffee. Also, avoid ultra-processed carbohydrates from foods such as breads, cereals, and pastries, as they have similar effects on blood glucose when compared to sugar.21
Greatly restrict highly processed foods. The evidence suggests that the availability of processed food is associated with increasing obesity.22 Simple advice to offer your patients is to encourage them to shop the perimeter of the grocery store, where fresh produce, meat, and dairy products are primarily located, and avoid the inner aisles, which contain primarily processed foods. Choosing food items with 5 or fewer ingredients is a starting point when teaching patients to read labels.
Consider limiting saturated fats. In 1977, the Dietary Guidelines for Americans recommended that Americans eat no more than 30% of total energy intake from fat and less than 10% of total energy intake from saturated fat; however, no randomized controlled trials had been done that supported this recommendation and epidemiologic data supporting it were weak.23
The 2015 Dietary Guidelines continue to recommend limiting total energy intake from saturated fats.24 While there may be a small decrease in cardiovascular risk with a reduction of saturated fat intake and replacement with unsaturated fats, no overall mortality benefit has been demonstrated.24,25 More research is needed in this area to guide patients in decisions regarding consumption of saturated fats and what types of unsaturated fats are best for their health.
Continue to: Eat only 3 meals per day
Eat only 3 meals per day, but aim for fewer than that. The prescription of fasting is a modality that can be used for weight loss and improved health. Fasting has been a prescribed healing practice for thousands of years.26 It is a practice that virtually every major religion in the world embraces. Studies have demonstrated fasting to be safe and effective in the setting of obesity without significant comorbidities, and it may promote weight loss and metabolic health.26-29
There are multiple types of intermittent fasting. A practical way for patients to start is by restricting the number of hours in which they eat or drink calorie-containing beverages to 8 hours per day. In our experience, this regimen is easier for most patients to follow than alternate-day or other longer fasts. While there has been caution in the prescription of intermittent fasting due to concerns about causing eating disorders, a recent small study did not demonstrate increased risk of eating disorders with daily intermittent fasting.30
Participate in healthy exercise. Nonpharmacologic office-based strategies for treating obesity have generally focused on increasing exercise and decreasing caloric intake.31 While exercise has significant health benefits, including preventing weight regain, evidence does not support monotherapy with exercise as an effective long-term weight-loss strategy.32 There are no studies available that adequately support prescribing an exact dose of exercise.33 Generally, less than 150 minutes of exercise per week is not effective and more than that does have a dose-related response.33
Follow up to help patients stay on target
There is no ideal interval for follow-up visits. However, frequent visits—anywhere from weekly to monthly—in the initial stages of weight loss increase the patient’s sense of accountability and, in our experience, seem to be helpful.
Patients may also choose to track their progress by weighing themselves regularly. A small study published in the International Journal of Obesity found that patients who weighed themselves daily had greater and more sustained weight loss than those who didn’t.34 But the decision of whether to weigh one’s self at home should be individualized for each patient.
CORRESPONDENCE
Wesley Eichorn, DO, 1000 Oakland Drive, Kalamazoo, MI 49008; wesley.eichorn@med.wmich.edu
1. Hales CM, Carroll MD, Fryar CD, et al. Prevalence of obesity among adults and youth: United States, 2015-2016 key findings data from the National Health and Nutrition Examination Survey. NCHS Data Brief. 2017;(288):1-8.
2. Seger JC, Horn DB, Westman EC, et al. Obesity Algorithm, presented by the Obesity Medicine Association. Accessed March 5, 2021. www.obesityalgorithm.org. 2016-2017
3. Dorflinger LM, Ruser CB, Masheb RM. A brief screening measure for binge eating in primary care. Eat Behav. 2017;26:163-166. https://doi.org/10.1016/j.eatbeh.2017.03.009
4. Saunders KH, Igel LI, Shukla AP, et al. Drug-induced weight gain: rethinking our choices. J Fam Pract. 2016;65:780-788.
5. American Diabetes Association. 2. Classification and Diagnosis of Diabetes: Standards of Medical Care in Diabetes—2019. Diabetes Care. 2019;42(suppl 1):S13-S28. https://doi.org/10.2337/dc19-S002
6. Reaven G. Insulin resistance and coronary heart disease in nondiabetic individuals. Arterioscler Thromb Vasc Biol. 2012;32:1754-1759. https://doi.org/10.1161/ATVBAHA.111.241885/-/DC1
7. Hallberg S, McKenzie A, Williams P, et al. Effectiveness and safety of a novel care model for the management of type 2 diabetes at 1 year: an open-label, non-randomized, controlled study. Diabetes Ther. 2018;9:583-612. https://doi.org/10.6084/m9.figshare
8. Wallace TM, Levy JC, Matthews DR. Use and abuse of HOMA modeling. Diabetes Care. 2004;27:1487-1495.
9. Vilar-Gomez E, Athinarayanan SJ, Adams RN, et al. Post hoc analyses of surrogate markers of non-alcoholic fatty liver disease (NAFLD) and liver fibrosis in patients with type 2 diabetes in a digitally supported continuous care intervention: an open-label, non-randomised controlled study. BMJ Open. 2019;9:e023597. https://doi.org/10.1136/bmjopen-2018-023597
10. Young T, Peppard PE, Gottlieb DJ. Epidemiology of obstructive sleep apnea. Am J Respir Crit Care Med. 2002;165:1217-1239. https://doi.org/10.1164/rccm.2109080
11. Drager LF, Brunoni AR, Jenner R, et al. Effects of CPAP on body weight in patients with obstructive sleep apnoea: a meta-analysis of randomised trials. Thorax. 2015;70:258-264. https://doi.org/10.1136/thoraxjnl-2014-205361
12. Bosworth T. CPAP use associated with greater weight loss in obese patients with sleep apnea. CHEST Physician. Published March 29, 2019. Accessed March 5, 2021. www.mdedge.com/chestphysician/article/197827/sleep-medicine/cpap-use-associated-greater-weight-loss-obese-patients
13. Tobias DK, Chen M, Manson JAE, et al. Effect of low-fat diet interventions versus other diet interventions on long-term weight change in adults: a systematic review and meta-analysis. Lancet Diabetes Endocrinol. 2015;3:968-979. https://doi.org/10.1016/S2213-8587(15)00367-8
14. Sackner-Bernstein J, Kanter D, Kaul S. Dietary intervention for overweight and obese adults: comparison of low-carbohydrate and low-fat diets: a meta-analysis. PLoS One. 2015;10:e0139817. https://doi.org/10.1371/journal.pone.0139817
15. Bezerra Bueno N, Vieira De Melo IS, Lima De Oliveira S, et al. Very-low-carbohydrate ketogenic diet v low-fat diet for long-term weight loss: a meta-analysis of randomised controlled trials. Br J Nutr. 2013;110:1178-1187. https://doi.org/10.1017/S0007114513000548
16. Santos FL, Esteves SS, da Costa Pereira A, et al. Systematic review and meta-analysis of clinical trials of the effects of low carbohydrate diets on cardiovascular risk factors. Obes Rev. 2012;13:1048-1066. https://doi.org/10.1111/j.1467-789X.2012.01021.x
17. Athinarayanan SJ, Adams RN, Hallberg SJ, et al. Long-term effects of a novel continuous remote care intervention including nutritional ketosis for the management of type 2 diabetes: a 2-year non-randomized clinical trial. bioRxiv. 2018;10:348. https://doi.org/10.1101/476275
18. Auerbach BJ, Dibey S, Vallila-Buchman P, et al. Review of 100% fruit juice and chronic health conditions: implications for sugar-sweetened beverage policy. Adv Nutr. 2018;9:78-85. https://doi.org/10.1093/advances/nmx006
19. Faith MS, Dennison BA, Edmunds LS, et al. Fruit juice intake predicts increased adiposity gain in children from low-income families: weight status-by-environment interaction. Pediatrics. 2006;118:2066-2075. https://doi.org/10.1542/peds.2006-1117
20. Bolton RP, Burroughs LF, Heaton KW. The role of dietary fiber in satiety, insulin: studies with fruit and fruit. Am J Clin Nutr. 1981;84:211-217. https://doi.org/10.1093/ajcn/34.2.211
21. Unwin D, Haslam D, Livesey G. It is the glycaemic response to, not the carbohydrate content of food that matters in diabetes and obesity: the glycaemic index revisited. J Insul Resist. 2016;1(1):a8. https://doi.org/10.4102/jir.v1i1.8
22. Monteiro CA, Moubarac JC, Levy RB, et al. Household availability of ultra-processed foods and obesity in nineteen European countries. Public Health Nutr. 2018;21:18-26. https://doi.org/10.1017/S1368980017001379
23. Harcombe Z, Baker JS, Cooper SM, et al. Evidence from randomised controlled trials did not support the introduction of dietary fat guidelines in 1977 and 1983: a systematic review and meta-analysis. Open Hear. 2015;2:e000196. https://doi.org/10.1136/openhrt-2014
24. US Department of Health and Human Services and US Department of Agriculture. 2015-2020 Dietary Guidelines for Americans. 8th edition. Published December 2015. Accessed March 5, 2021. http://health.gov/dietaryguidelines/2015/guidelines/
25. Harcombe Z, Baker JS, DiNicolantonio JJ, et al. Evidence from randomised controlled trials does not support current dietary fat guidelines: a systematic review and meta-analysis. Open Hear. 2016;3:e000409. https://doi.org/10.1136/openhrt-2016-000409
26. Fung J. The Obesity Code: Unlocking the Secrets of Weight Loss. Greystone Books; 2016.
27. Mattson MP, Longo VD, Harvie M. Impact of intermittent fasting on health and disease processes. Ageing Res Rev. 2017;39:46-58. https://doi.org/10.1016/j.arr.2016.10.005
28. Patterson RE, Sears DD. Metabolic Effects of Intermittent Fasting. Annu Rev Nutr. 2017; 37:371-393. https://doi.org/10.1146/annurev-nutr-071816-064634
29. Duncan GG. Intermittent fasts in the correction and control of intractable obesity. Trans Am Clin Climatol Assoc. 1962;74:121-129.
30. Gabel K, Hoddy KK, Varady KA. Safety of 8-h time restricted feeding in adults with obesity. Appl Physiol Nutr Metab. 2019;44:107-109. https://doi.org/10.1139/apnm-2018-0389
31. Erlandson M, Ivey LC, Seikel K. Update on office-based strategies for the management of obesity. Am Fam Physician. 2016;94:361-368.
32. Malhotra A, Noakes T, Phinney S. It is time to bust the myth of physical inactivity and obesity: you cannot outrun a bad diet. Br J Sports Med. 2015;49:967-968. https://doi.org/10.1136/bjsports-2015-094911
33. Donnelly JE, Blair SN, Jakicic JM, et al. Appropriate physical activity intervention strategies for weight loss and prevention of weight regain for adults. Med Sci Sports Exerc. 2009;41:459-471. https://doi.org/10.1249/MSS.0b013e3181949333
34. Zheng Y, Burke LE, Danford CA, et al. Patterns of self-weighing behavior and weight change in a weight loss trial. Int J Obes (Lond). 2016;40:1392-1396. https://doi.org/10.1038/ijo.2016.68
1. Hales CM, Carroll MD, Fryar CD, et al. Prevalence of obesity among adults and youth: United States, 2015-2016 key findings data from the National Health and Nutrition Examination Survey. NCHS Data Brief. 2017;(288):1-8.
2. Seger JC, Horn DB, Westman EC, et al. Obesity Algorithm, presented by the Obesity Medicine Association. Accessed March 5, 2021. www.obesityalgorithm.org. 2016-2017
3. Dorflinger LM, Ruser CB, Masheb RM. A brief screening measure for binge eating in primary care. Eat Behav. 2017;26:163-166. https://doi.org/10.1016/j.eatbeh.2017.03.009
4. Saunders KH, Igel LI, Shukla AP, et al. Drug-induced weight gain: rethinking our choices. J Fam Pract. 2016;65:780-788.
5. American Diabetes Association. 2. Classification and Diagnosis of Diabetes: Standards of Medical Care in Diabetes—2019. Diabetes Care. 2019;42(suppl 1):S13-S28. https://doi.org/10.2337/dc19-S002
6. Reaven G. Insulin resistance and coronary heart disease in nondiabetic individuals. Arterioscler Thromb Vasc Biol. 2012;32:1754-1759. https://doi.org/10.1161/ATVBAHA.111.241885/-/DC1
7. Hallberg S, McKenzie A, Williams P, et al. Effectiveness and safety of a novel care model for the management of type 2 diabetes at 1 year: an open-label, non-randomized, controlled study. Diabetes Ther. 2018;9:583-612. https://doi.org/10.6084/m9.figshare
8. Wallace TM, Levy JC, Matthews DR. Use and abuse of HOMA modeling. Diabetes Care. 2004;27:1487-1495.
9. Vilar-Gomez E, Athinarayanan SJ, Adams RN, et al. Post hoc analyses of surrogate markers of non-alcoholic fatty liver disease (NAFLD) and liver fibrosis in patients with type 2 diabetes in a digitally supported continuous care intervention: an open-label, non-randomised controlled study. BMJ Open. 2019;9:e023597. https://doi.org/10.1136/bmjopen-2018-023597
10. Young T, Peppard PE, Gottlieb DJ. Epidemiology of obstructive sleep apnea. Am J Respir Crit Care Med. 2002;165:1217-1239. https://doi.org/10.1164/rccm.2109080
11. Drager LF, Brunoni AR, Jenner R, et al. Effects of CPAP on body weight in patients with obstructive sleep apnoea: a meta-analysis of randomised trials. Thorax. 2015;70:258-264. https://doi.org/10.1136/thoraxjnl-2014-205361
12. Bosworth T. CPAP use associated with greater weight loss in obese patients with sleep apnea. CHEST Physician. Published March 29, 2019. Accessed March 5, 2021. www.mdedge.com/chestphysician/article/197827/sleep-medicine/cpap-use-associated-greater-weight-loss-obese-patients
13. Tobias DK, Chen M, Manson JAE, et al. Effect of low-fat diet interventions versus other diet interventions on long-term weight change in adults: a systematic review and meta-analysis. Lancet Diabetes Endocrinol. 2015;3:968-979. https://doi.org/10.1016/S2213-8587(15)00367-8
14. Sackner-Bernstein J, Kanter D, Kaul S. Dietary intervention for overweight and obese adults: comparison of low-carbohydrate and low-fat diets: a meta-analysis. PLoS One. 2015;10:e0139817. https://doi.org/10.1371/journal.pone.0139817
15. Bezerra Bueno N, Vieira De Melo IS, Lima De Oliveira S, et al. Very-low-carbohydrate ketogenic diet v low-fat diet for long-term weight loss: a meta-analysis of randomised controlled trials. Br J Nutr. 2013;110:1178-1187. https://doi.org/10.1017/S0007114513000548
16. Santos FL, Esteves SS, da Costa Pereira A, et al. Systematic review and meta-analysis of clinical trials of the effects of low carbohydrate diets on cardiovascular risk factors. Obes Rev. 2012;13:1048-1066. https://doi.org/10.1111/j.1467-789X.2012.01021.x
17. Athinarayanan SJ, Adams RN, Hallberg SJ, et al. Long-term effects of a novel continuous remote care intervention including nutritional ketosis for the management of type 2 diabetes: a 2-year non-randomized clinical trial. bioRxiv. 2018;10:348. https://doi.org/10.1101/476275
18. Auerbach BJ, Dibey S, Vallila-Buchman P, et al. Review of 100% fruit juice and chronic health conditions: implications for sugar-sweetened beverage policy. Adv Nutr. 2018;9:78-85. https://doi.org/10.1093/advances/nmx006
19. Faith MS, Dennison BA, Edmunds LS, et al. Fruit juice intake predicts increased adiposity gain in children from low-income families: weight status-by-environment interaction. Pediatrics. 2006;118:2066-2075. https://doi.org/10.1542/peds.2006-1117
20. Bolton RP, Burroughs LF, Heaton KW. The role of dietary fiber in satiety, insulin: studies with fruit and fruit. Am J Clin Nutr. 1981;84:211-217. https://doi.org/10.1093/ajcn/34.2.211
21. Unwin D, Haslam D, Livesey G. It is the glycaemic response to, not the carbohydrate content of food that matters in diabetes and obesity: the glycaemic index revisited. J Insul Resist. 2016;1(1):a8. https://doi.org/10.4102/jir.v1i1.8
22. Monteiro CA, Moubarac JC, Levy RB, et al. Household availability of ultra-processed foods and obesity in nineteen European countries. Public Health Nutr. 2018;21:18-26. https://doi.org/10.1017/S1368980017001379
23. Harcombe Z, Baker JS, Cooper SM, et al. Evidence from randomised controlled trials did not support the introduction of dietary fat guidelines in 1977 and 1983: a systematic review and meta-analysis. Open Hear. 2015;2:e000196. https://doi.org/10.1136/openhrt-2014
24. US Department of Health and Human Services and US Department of Agriculture. 2015-2020 Dietary Guidelines for Americans. 8th edition. Published December 2015. Accessed March 5, 2021. http://health.gov/dietaryguidelines/2015/guidelines/
25. Harcombe Z, Baker JS, DiNicolantonio JJ, et al. Evidence from randomised controlled trials does not support current dietary fat guidelines: a systematic review and meta-analysis. Open Hear. 2016;3:e000409. https://doi.org/10.1136/openhrt-2016-000409
26. Fung J. The Obesity Code: Unlocking the Secrets of Weight Loss. Greystone Books; 2016.
27. Mattson MP, Longo VD, Harvie M. Impact of intermittent fasting on health and disease processes. Ageing Res Rev. 2017;39:46-58. https://doi.org/10.1016/j.arr.2016.10.005
28. Patterson RE, Sears DD. Metabolic Effects of Intermittent Fasting. Annu Rev Nutr. 2017; 37:371-393. https://doi.org/10.1146/annurev-nutr-071816-064634
29. Duncan GG. Intermittent fasts in the correction and control of intractable obesity. Trans Am Clin Climatol Assoc. 1962;74:121-129.
30. Gabel K, Hoddy KK, Varady KA. Safety of 8-h time restricted feeding in adults with obesity. Appl Physiol Nutr Metab. 2019;44:107-109. https://doi.org/10.1139/apnm-2018-0389
31. Erlandson M, Ivey LC, Seikel K. Update on office-based strategies for the management of obesity. Am Fam Physician. 2016;94:361-368.
32. Malhotra A, Noakes T, Phinney S. It is time to bust the myth of physical inactivity and obesity: you cannot outrun a bad diet. Br J Sports Med. 2015;49:967-968. https://doi.org/10.1136/bjsports-2015-094911
33. Donnelly JE, Blair SN, Jakicic JM, et al. Appropriate physical activity intervention strategies for weight loss and prevention of weight regain for adults. Med Sci Sports Exerc. 2009;41:459-471. https://doi.org/10.1249/MSS.0b013e3181949333
34. Zheng Y, Burke LE, Danford CA, et al. Patterns of self-weighing behavior and weight change in a weight loss trial. Int J Obes (Lond). 2016;40:1392-1396. https://doi.org/10.1038/ijo.2016.68
PRACTICE RECOMMENDATIONS
› Create an office environment where patients feel comfortable discussing their weight. C
› Screen overweight and obese patients for comorbidities. B
› Focus on nutritional changes more than exercise when working with patients who want to lose weight. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series