User login
LAS VEGAS – A twice-daily 30-mg dose of apremilast significantly improved moderate to severe nail, scalp, and palmoplantar psoriasis, compared with placebo, based on data from 411 adults in the ESTEEM 2 phase III study.
Overall, at 16 weeks, 29% of the patients randomized to apremilast achieved PASI-75, and 56% achieved PASI-50, compared with placebo patients (6% and 20%, respectively). The findings were presented in a poster at SDEF Las Vegas Dermatology Seminar by Dr. Jeffrey Crowley of Bakersfield (Calif.) Dermatology, and his colleagues.
In addition, patients with palmarplantar, nail, and scalp psoriasis who received apremilast showed significantly greater response rates, compared with placebo patients.
At baseline, 266 patients had scores of 1 or greater on the Nail Psoriasis Severity Index (NAPSI). Significantly more of these patients on apremilast achieved NAPSI-50 vs. placebo patients after 16 weeks (45% vs. 19%, respectively). The average percent change in NAPSI score from baseline was 29% for apremilast vs. 7% for placebo.
“Among patients initially randomized to apremilast and maintained on apremilast through week 32, nail psoriasis was further improved at week 32 (55% of patients achieved NAPSI-50)” and improvements were seen at week 32 in patients who switched from placebo to apremilast at 16 weeks, the researchers noted.
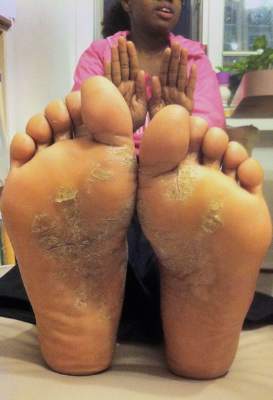
Response at 16 weeks was significantly greater on apremilast, compared with placebo, for 269 patients with scalp psoriasis (41% vs. 17%), and for 42 patients with palmoplantar psoriasis (65% vs. 31%).
Most adverse events were mild to moderate, and discontinuation rates because of adverse events were approximately 5% for placebo and treatment patients. The most common adverse events overall were nausea, diarrhea, nasopharyngitis, upper respiratory tract infection, headache, and vomiting.
The findings were limited by the short duration of the study, the researchers noted. However, apremilast was safe and well tolerated, and improvement was noted up to 32 weeks, they added.
Dr. Crowley reported receiving fees from Celgene during the study, and reported financial relationships with other companies, including AbbVie, Amgen, AstraZeneca, Eli Lilly, Janssen, Merck, and Pfizer.
LAS VEGAS – A twice-daily 30-mg dose of apremilast significantly improved moderate to severe nail, scalp, and palmoplantar psoriasis, compared with placebo, based on data from 411 adults in the ESTEEM 2 phase III study.
Overall, at 16 weeks, 29% of the patients randomized to apremilast achieved PASI-75, and 56% achieved PASI-50, compared with placebo patients (6% and 20%, respectively). The findings were presented in a poster at SDEF Las Vegas Dermatology Seminar by Dr. Jeffrey Crowley of Bakersfield (Calif.) Dermatology, and his colleagues.
In addition, patients with palmarplantar, nail, and scalp psoriasis who received apremilast showed significantly greater response rates, compared with placebo patients.
At baseline, 266 patients had scores of 1 or greater on the Nail Psoriasis Severity Index (NAPSI). Significantly more of these patients on apremilast achieved NAPSI-50 vs. placebo patients after 16 weeks (45% vs. 19%, respectively). The average percent change in NAPSI score from baseline was 29% for apremilast vs. 7% for placebo.
“Among patients initially randomized to apremilast and maintained on apremilast through week 32, nail psoriasis was further improved at week 32 (55% of patients achieved NAPSI-50)” and improvements were seen at week 32 in patients who switched from placebo to apremilast at 16 weeks, the researchers noted.
Response at 16 weeks was significantly greater on apremilast, compared with placebo, for 269 patients with scalp psoriasis (41% vs. 17%), and for 42 patients with palmoplantar psoriasis (65% vs. 31%).
Most adverse events were mild to moderate, and discontinuation rates because of adverse events were approximately 5% for placebo and treatment patients. The most common adverse events overall were nausea, diarrhea, nasopharyngitis, upper respiratory tract infection, headache, and vomiting.
The findings were limited by the short duration of the study, the researchers noted. However, apremilast was safe and well tolerated, and improvement was noted up to 32 weeks, they added.
Dr. Crowley reported receiving fees from Celgene during the study, and reported financial relationships with other companies, including AbbVie, Amgen, AstraZeneca, Eli Lilly, Janssen, Merck, and Pfizer.
LAS VEGAS – A twice-daily 30-mg dose of apremilast significantly improved moderate to severe nail, scalp, and palmoplantar psoriasis, compared with placebo, based on data from 411 adults in the ESTEEM 2 phase III study.
Overall, at 16 weeks, 29% of the patients randomized to apremilast achieved PASI-75, and 56% achieved PASI-50, compared with placebo patients (6% and 20%, respectively). The findings were presented in a poster at SDEF Las Vegas Dermatology Seminar by Dr. Jeffrey Crowley of Bakersfield (Calif.) Dermatology, and his colleagues.
In addition, patients with palmarplantar, nail, and scalp psoriasis who received apremilast showed significantly greater response rates, compared with placebo patients.
At baseline, 266 patients had scores of 1 or greater on the Nail Psoriasis Severity Index (NAPSI). Significantly more of these patients on apremilast achieved NAPSI-50 vs. placebo patients after 16 weeks (45% vs. 19%, respectively). The average percent change in NAPSI score from baseline was 29% for apremilast vs. 7% for placebo.
“Among patients initially randomized to apremilast and maintained on apremilast through week 32, nail psoriasis was further improved at week 32 (55% of patients achieved NAPSI-50)” and improvements were seen at week 32 in patients who switched from placebo to apremilast at 16 weeks, the researchers noted.
Response at 16 weeks was significantly greater on apremilast, compared with placebo, for 269 patients with scalp psoriasis (41% vs. 17%), and for 42 patients with palmoplantar psoriasis (65% vs. 31%).
Most adverse events were mild to moderate, and discontinuation rates because of adverse events were approximately 5% for placebo and treatment patients. The most common adverse events overall were nausea, diarrhea, nasopharyngitis, upper respiratory tract infection, headache, and vomiting.
The findings were limited by the short duration of the study, the researchers noted. However, apremilast was safe and well tolerated, and improvement was noted up to 32 weeks, they added.
Dr. Crowley reported receiving fees from Celgene during the study, and reported financial relationships with other companies, including AbbVie, Amgen, AstraZeneca, Eli Lilly, Janssen, Merck, and Pfizer.
AT SDEF LAS VEGAS DERMATOLOGY SEMINAR
Key clinical point: Apremilast significantly improved psoriasis in a subset of patient with nail, scalp, and palmoplantar involvement.
Major finding: After 16 weeks of twice-daily apremilast, significantly more patients with nail psoriasis achieved NAPSI-50, compared with placebo patients (45% vs. 19%, respectively).
Data source: Analysis of data from ESTEEM 2.
Disclosures:Dr. Crowley reported receiving fees from Celgene during the study and reported financial relationships with other companies, including AbbVie, Amgen, AstraZeneca, Eli Lilly, Janssen, Merck, and Pfizer.