User login
Skin Disease Education Foundation (SDEF): Las Vegas Dermatology Seminar
Consider phototherapy for certain psoriasis patients
LAS VEGAS – Psoriasis patients who are unresponsive to topical therapy, do not have psoriatic arthritis, and can make regular office visits may be candidates for successful treatment with phototherapy.
A patient with type II skin and extensive plaque psoriasis could be treated with a targeted NB-UVB laser, Dr. Kenneth B. Gordon said at Skin Disease Education Foundation’s annual Las Vegas Dermatology Seminar.
However, it’s important to evaluate each patient individually on phototherapy decisions, rather than relying on published protocols, noted Dr. Gordon of Northwestern University Feinberg School of Medicine in Chicago.
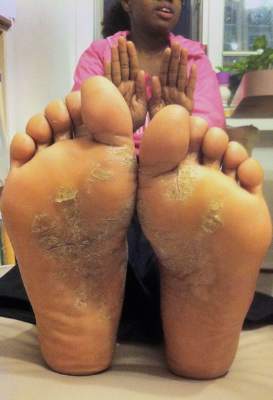
The advantages of the NB-UVB laser for psoriasis include a high rate of response (data show more than 75% of patients achieve PASI 75), the ability to avoid treating uninvolved skin, and potential long-term clearing of lesions, he said.
For someone with extensive plaque psoriasis, Dr. Gordon said he would start with 3 treatments per week, at 200 mJ, and increase the fluence by 25 mJ if necessary. “A good response can be expected in 4-6 weeks,” he said.
As for long-term treatment, “I leave it up to the patient but offer long-term maintenance,” said Dr. Gordon. Long-term efficacy of NB-UVB for plaque psoriasis has not been well studied, and safety data are unknown. “It is unclear whether there is a skin cancer risk,” he said. Reducing the number of exposures for a patient on maintenance therapy may be an option, he added.
Short-term treatment with NB-UVB can be effective in some psoriasis patients, such as in cases of an acute flare of guttate psoriasis, Dr. Gordon noted. Psoralen and UV light therapy (PUVA) is another option, he said.
Phototherapy also has a role in managing psoriasis in conjunction with retinoids, Dr. Gordon said. He cited an example of a 59-year-old woman with palmar psoriasis. For this patient, he said he would recommend starting with acitretin for approximately 1 month, if the patient tolerates it. “If the acitretin works by itself, no need to add phototherapy,” he said. However, if UVB is added, account for the photosensitizing agent, he emphasized. “Decrease the starting dose by 25-50 mJ or decrease the skin type by one,” he said.
Some psoriasis patients will not benefit from phototherapy, particularly those with erythroderma, said Dr. Gordon. “If skin is highly inflamed, phototherapy could induce easy burning, Koebnerization,” or other complications, he noted.
Dr. Gordon disclosed that his department at Northwestern derives income from phototherapy, but he personally does not. He also disclosed receiving research grants from and/or serving as a consultant to multiple companies including AbbVie, Amgen, Celgene, Eli Lilly, and Janssen.
LAS VEGAS – Psoriasis patients who are unresponsive to topical therapy, do not have psoriatic arthritis, and can make regular office visits may be candidates for successful treatment with phototherapy.
A patient with type II skin and extensive plaque psoriasis could be treated with a targeted NB-UVB laser, Dr. Kenneth B. Gordon said at Skin Disease Education Foundation’s annual Las Vegas Dermatology Seminar.
However, it’s important to evaluate each patient individually on phototherapy decisions, rather than relying on published protocols, noted Dr. Gordon of Northwestern University Feinberg School of Medicine in Chicago.
The advantages of the NB-UVB laser for psoriasis include a high rate of response (data show more than 75% of patients achieve PASI 75), the ability to avoid treating uninvolved skin, and potential long-term clearing of lesions, he said.
For someone with extensive plaque psoriasis, Dr. Gordon said he would start with 3 treatments per week, at 200 mJ, and increase the fluence by 25 mJ if necessary. “A good response can be expected in 4-6 weeks,” he said.
As for long-term treatment, “I leave it up to the patient but offer long-term maintenance,” said Dr. Gordon. Long-term efficacy of NB-UVB for plaque psoriasis has not been well studied, and safety data are unknown. “It is unclear whether there is a skin cancer risk,” he said. Reducing the number of exposures for a patient on maintenance therapy may be an option, he added.
Short-term treatment with NB-UVB can be effective in some psoriasis patients, such as in cases of an acute flare of guttate psoriasis, Dr. Gordon noted. Psoralen and UV light therapy (PUVA) is another option, he said.
Phototherapy also has a role in managing psoriasis in conjunction with retinoids, Dr. Gordon said. He cited an example of a 59-year-old woman with palmar psoriasis. For this patient, he said he would recommend starting with acitretin for approximately 1 month, if the patient tolerates it. “If the acitretin works by itself, no need to add phototherapy,” he said. However, if UVB is added, account for the photosensitizing agent, he emphasized. “Decrease the starting dose by 25-50 mJ or decrease the skin type by one,” he said.
Some psoriasis patients will not benefit from phototherapy, particularly those with erythroderma, said Dr. Gordon. “If skin is highly inflamed, phototherapy could induce easy burning, Koebnerization,” or other complications, he noted.
Dr. Gordon disclosed that his department at Northwestern derives income from phototherapy, but he personally does not. He also disclosed receiving research grants from and/or serving as a consultant to multiple companies including AbbVie, Amgen, Celgene, Eli Lilly, and Janssen.
LAS VEGAS – Psoriasis patients who are unresponsive to topical therapy, do not have psoriatic arthritis, and can make regular office visits may be candidates for successful treatment with phototherapy.
A patient with type II skin and extensive plaque psoriasis could be treated with a targeted NB-UVB laser, Dr. Kenneth B. Gordon said at Skin Disease Education Foundation’s annual Las Vegas Dermatology Seminar.
However, it’s important to evaluate each patient individually on phototherapy decisions, rather than relying on published protocols, noted Dr. Gordon of Northwestern University Feinberg School of Medicine in Chicago.
The advantages of the NB-UVB laser for psoriasis include a high rate of response (data show more than 75% of patients achieve PASI 75), the ability to avoid treating uninvolved skin, and potential long-term clearing of lesions, he said.
For someone with extensive plaque psoriasis, Dr. Gordon said he would start with 3 treatments per week, at 200 mJ, and increase the fluence by 25 mJ if necessary. “A good response can be expected in 4-6 weeks,” he said.
As for long-term treatment, “I leave it up to the patient but offer long-term maintenance,” said Dr. Gordon. Long-term efficacy of NB-UVB for plaque psoriasis has not been well studied, and safety data are unknown. “It is unclear whether there is a skin cancer risk,” he said. Reducing the number of exposures for a patient on maintenance therapy may be an option, he added.
Short-term treatment with NB-UVB can be effective in some psoriasis patients, such as in cases of an acute flare of guttate psoriasis, Dr. Gordon noted. Psoralen and UV light therapy (PUVA) is another option, he said.
Phototherapy also has a role in managing psoriasis in conjunction with retinoids, Dr. Gordon said. He cited an example of a 59-year-old woman with palmar psoriasis. For this patient, he said he would recommend starting with acitretin for approximately 1 month, if the patient tolerates it. “If the acitretin works by itself, no need to add phototherapy,” he said. However, if UVB is added, account for the photosensitizing agent, he emphasized. “Decrease the starting dose by 25-50 mJ or decrease the skin type by one,” he said.
Some psoriasis patients will not benefit from phototherapy, particularly those with erythroderma, said Dr. Gordon. “If skin is highly inflamed, phototherapy could induce easy burning, Koebnerization,” or other complications, he noted.
Dr. Gordon disclosed that his department at Northwestern derives income from phototherapy, but he personally does not. He also disclosed receiving research grants from and/or serving as a consultant to multiple companies including AbbVie, Amgen, Celgene, Eli Lilly, and Janssen.
EXPERT ANALYSIS FROM SDEF LAS VEGAS DERMATOLOGY SEMINAR
VIDEO: What Works Now and What’s Up Next for Laser Treatments of Vascular Malformations
LAS VEGAS – Pulsed dye lasers have stood the test of time for treating vascular malformations, rosacea, and telangectases, Dr. Christopher Zachary said at Skin Disease Education Foundation’s annual Las Vegas dermatology seminar.
However, Dr. Zachary noted in an interview at the meeting that the field of laser therapy is on the cusp of new innovation, not only in devices, but also in how the devices are used. Different wavelengths and combination treatments will provide more options for patients, he explained. “These are great times for those who are interested in treating vascular problems,” he said.
Dr. Zachary disclosed serving as a consultant for Cutera and Zeltiq, on the speakers bureau for Cynosure and Solta, and on the advisory board for Zeltiq.
SDEF and this news organization are owned by Frontline Medical Communications.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
LAS VEGAS – Pulsed dye lasers have stood the test of time for treating vascular malformations, rosacea, and telangectases, Dr. Christopher Zachary said at Skin Disease Education Foundation’s annual Las Vegas dermatology seminar.
However, Dr. Zachary noted in an interview at the meeting that the field of laser therapy is on the cusp of new innovation, not only in devices, but also in how the devices are used. Different wavelengths and combination treatments will provide more options for patients, he explained. “These are great times for those who are interested in treating vascular problems,” he said.
Dr. Zachary disclosed serving as a consultant for Cutera and Zeltiq, on the speakers bureau for Cynosure and Solta, and on the advisory board for Zeltiq.
SDEF and this news organization are owned by Frontline Medical Communications.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
LAS VEGAS – Pulsed dye lasers have stood the test of time for treating vascular malformations, rosacea, and telangectases, Dr. Christopher Zachary said at Skin Disease Education Foundation’s annual Las Vegas dermatology seminar.
However, Dr. Zachary noted in an interview at the meeting that the field of laser therapy is on the cusp of new innovation, not only in devices, but also in how the devices are used. Different wavelengths and combination treatments will provide more options for patients, he explained. “These are great times for those who are interested in treating vascular problems,” he said.
Dr. Zachary disclosed serving as a consultant for Cutera and Zeltiq, on the speakers bureau for Cynosure and Solta, and on the advisory board for Zeltiq.
SDEF and this news organization are owned by Frontline Medical Communications.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
AT SDEF LAS VEGAS DERMATOLOGY SEMINAR
VIDEO: What works now and what’s up next for laser treatments of vascular malformations
LAS VEGAS – Pulsed dye lasers have stood the test of time for treating vascular malformations, rosacea, and telangectases, Dr. Christopher Zachary said at Skin Disease Education Foundation’s annual Las Vegas dermatology seminar.
However, Dr. Zachary noted in an interview at the meeting that the field of laser therapy is on the cusp of new innovation, not only in devices, but also in how the devices are used. Different wavelengths and combination treatments will provide more options for patients, he explained. “These are great times for those who are interested in treating vascular problems,” he said.
Dr. Zachary disclosed serving as a consultant for Cutera and Zeltiq, on the speakers bureau for Cynosure and Solta, and on the advisory board for Zeltiq.
SDEF and this news organization are owned by Frontline Medical Communications.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
LAS VEGAS – Pulsed dye lasers have stood the test of time for treating vascular malformations, rosacea, and telangectases, Dr. Christopher Zachary said at Skin Disease Education Foundation’s annual Las Vegas dermatology seminar.
However, Dr. Zachary noted in an interview at the meeting that the field of laser therapy is on the cusp of new innovation, not only in devices, but also in how the devices are used. Different wavelengths and combination treatments will provide more options for patients, he explained. “These are great times for those who are interested in treating vascular problems,” he said.
Dr. Zachary disclosed serving as a consultant for Cutera and Zeltiq, on the speakers bureau for Cynosure and Solta, and on the advisory board for Zeltiq.
SDEF and this news organization are owned by Frontline Medical Communications.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
LAS VEGAS – Pulsed dye lasers have stood the test of time for treating vascular malformations, rosacea, and telangectases, Dr. Christopher Zachary said at Skin Disease Education Foundation’s annual Las Vegas dermatology seminar.
However, Dr. Zachary noted in an interview at the meeting that the field of laser therapy is on the cusp of new innovation, not only in devices, but also in how the devices are used. Different wavelengths and combination treatments will provide more options for patients, he explained. “These are great times for those who are interested in treating vascular problems,” he said.
Dr. Zachary disclosed serving as a consultant for Cutera and Zeltiq, on the speakers bureau for Cynosure and Solta, and on the advisory board for Zeltiq.
SDEF and this news organization are owned by Frontline Medical Communications.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
AT SDEF LAS VEGAS DERMATOLOGY SEMINAR
Apremilast succeeds against nail, scalp, palmoplantar psoriasis
LAS VEGAS – A twice-daily 30-mg dose of apremilast significantly improved moderate to severe nail, scalp, and palmoplantar psoriasis, compared with placebo, based on data from 411 adults in the ESTEEM 2 phase III study.
Overall, at 16 weeks, 29% of the patients randomized to apremilast achieved PASI-75, and 56% achieved PASI-50, compared with placebo patients (6% and 20%, respectively). The findings were presented in a poster at SDEF Las Vegas Dermatology Seminar by Dr. Jeffrey Crowley of Bakersfield (Calif.) Dermatology, and his colleagues.
In addition, patients with palmarplantar, nail, and scalp psoriasis who received apremilast showed significantly greater response rates, compared with placebo patients.
At baseline, 266 patients had scores of 1 or greater on the Nail Psoriasis Severity Index (NAPSI). Significantly more of these patients on apremilast achieved NAPSI-50 vs. placebo patients after 16 weeks (45% vs. 19%, respectively). The average percent change in NAPSI score from baseline was 29% for apremilast vs. 7% for placebo.
“Among patients initially randomized to apremilast and maintained on apremilast through week 32, nail psoriasis was further improved at week 32 (55% of patients achieved NAPSI-50)” and improvements were seen at week 32 in patients who switched from placebo to apremilast at 16 weeks, the researchers noted.
Response at 16 weeks was significantly greater on apremilast, compared with placebo, for 269 patients with scalp psoriasis (41% vs. 17%), and for 42 patients with palmoplantar psoriasis (65% vs. 31%).
Most adverse events were mild to moderate, and discontinuation rates because of adverse events were approximately 5% for placebo and treatment patients. The most common adverse events overall were nausea, diarrhea, nasopharyngitis, upper respiratory tract infection, headache, and vomiting.
The findings were limited by the short duration of the study, the researchers noted. However, apremilast was safe and well tolerated, and improvement was noted up to 32 weeks, they added.
Dr. Crowley reported receiving fees from Celgene during the study, and reported financial relationships with other companies, including AbbVie, Amgen, AstraZeneca, Eli Lilly, Janssen, Merck, and Pfizer.
LAS VEGAS – A twice-daily 30-mg dose of apremilast significantly improved moderate to severe nail, scalp, and palmoplantar psoriasis, compared with placebo, based on data from 411 adults in the ESTEEM 2 phase III study.
Overall, at 16 weeks, 29% of the patients randomized to apremilast achieved PASI-75, and 56% achieved PASI-50, compared with placebo patients (6% and 20%, respectively). The findings were presented in a poster at SDEF Las Vegas Dermatology Seminar by Dr. Jeffrey Crowley of Bakersfield (Calif.) Dermatology, and his colleagues.
In addition, patients with palmarplantar, nail, and scalp psoriasis who received apremilast showed significantly greater response rates, compared with placebo patients.
At baseline, 266 patients had scores of 1 or greater on the Nail Psoriasis Severity Index (NAPSI). Significantly more of these patients on apremilast achieved NAPSI-50 vs. placebo patients after 16 weeks (45% vs. 19%, respectively). The average percent change in NAPSI score from baseline was 29% for apremilast vs. 7% for placebo.
“Among patients initially randomized to apremilast and maintained on apremilast through week 32, nail psoriasis was further improved at week 32 (55% of patients achieved NAPSI-50)” and improvements were seen at week 32 in patients who switched from placebo to apremilast at 16 weeks, the researchers noted.
Response at 16 weeks was significantly greater on apremilast, compared with placebo, for 269 patients with scalp psoriasis (41% vs. 17%), and for 42 patients with palmoplantar psoriasis (65% vs. 31%).
Most adverse events were mild to moderate, and discontinuation rates because of adverse events were approximately 5% for placebo and treatment patients. The most common adverse events overall were nausea, diarrhea, nasopharyngitis, upper respiratory tract infection, headache, and vomiting.
The findings were limited by the short duration of the study, the researchers noted. However, apremilast was safe and well tolerated, and improvement was noted up to 32 weeks, they added.
Dr. Crowley reported receiving fees from Celgene during the study, and reported financial relationships with other companies, including AbbVie, Amgen, AstraZeneca, Eli Lilly, Janssen, Merck, and Pfizer.
LAS VEGAS – A twice-daily 30-mg dose of apremilast significantly improved moderate to severe nail, scalp, and palmoplantar psoriasis, compared with placebo, based on data from 411 adults in the ESTEEM 2 phase III study.
Overall, at 16 weeks, 29% of the patients randomized to apremilast achieved PASI-75, and 56% achieved PASI-50, compared with placebo patients (6% and 20%, respectively). The findings were presented in a poster at SDEF Las Vegas Dermatology Seminar by Dr. Jeffrey Crowley of Bakersfield (Calif.) Dermatology, and his colleagues.
In addition, patients with palmarplantar, nail, and scalp psoriasis who received apremilast showed significantly greater response rates, compared with placebo patients.
At baseline, 266 patients had scores of 1 or greater on the Nail Psoriasis Severity Index (NAPSI). Significantly more of these patients on apremilast achieved NAPSI-50 vs. placebo patients after 16 weeks (45% vs. 19%, respectively). The average percent change in NAPSI score from baseline was 29% for apremilast vs. 7% for placebo.
“Among patients initially randomized to apremilast and maintained on apremilast through week 32, nail psoriasis was further improved at week 32 (55% of patients achieved NAPSI-50)” and improvements were seen at week 32 in patients who switched from placebo to apremilast at 16 weeks, the researchers noted.
Response at 16 weeks was significantly greater on apremilast, compared with placebo, for 269 patients with scalp psoriasis (41% vs. 17%), and for 42 patients with palmoplantar psoriasis (65% vs. 31%).
Most adverse events were mild to moderate, and discontinuation rates because of adverse events were approximately 5% for placebo and treatment patients. The most common adverse events overall were nausea, diarrhea, nasopharyngitis, upper respiratory tract infection, headache, and vomiting.
The findings were limited by the short duration of the study, the researchers noted. However, apremilast was safe and well tolerated, and improvement was noted up to 32 weeks, they added.
Dr. Crowley reported receiving fees from Celgene during the study, and reported financial relationships with other companies, including AbbVie, Amgen, AstraZeneca, Eli Lilly, Janssen, Merck, and Pfizer.
AT SDEF LAS VEGAS DERMATOLOGY SEMINAR
Key clinical point: Apremilast significantly improved psoriasis in a subset of patient with nail, scalp, and palmoplantar involvement.
Major finding: After 16 weeks of twice-daily apremilast, significantly more patients with nail psoriasis achieved NAPSI-50, compared with placebo patients (45% vs. 19%, respectively).
Data source: Analysis of data from ESTEEM 2.
Disclosures:Dr. Crowley reported receiving fees from Celgene during the study and reported financial relationships with other companies, including AbbVie, Amgen, AstraZeneca, Eli Lilly, Janssen, Merck, and Pfizer.
VIDEO: Don’t discount topical therapy for psoriasis
LAS VEGAS– Don’t underestimate the value of topical therapy for treating psoriasis patients, Dr. Linda Stein Gold said at Skin Disease Education Foundation’s annual Las Vegas dermatology seminar.
Dr. Stein Gold explained how to overcome some of the compliance issues associated with topical therapies for psoriasis, and offered her suggestions for managing side effects in an interview at the meeting.
Dr. Stein Gold disclosed relationships with multiple pharmaceutical companies including Galderma, Stiefel, Allergan, Valeant, Ranbaxy, Promius, and Actavis.
SDEF and this news organization are owned by Frontline Medical Communications.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
LAS VEGAS– Don’t underestimate the value of topical therapy for treating psoriasis patients, Dr. Linda Stein Gold said at Skin Disease Education Foundation’s annual Las Vegas dermatology seminar.
Dr. Stein Gold explained how to overcome some of the compliance issues associated with topical therapies for psoriasis, and offered her suggestions for managing side effects in an interview at the meeting.
Dr. Stein Gold disclosed relationships with multiple pharmaceutical companies including Galderma, Stiefel, Allergan, Valeant, Ranbaxy, Promius, and Actavis.
SDEF and this news organization are owned by Frontline Medical Communications.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
LAS VEGAS– Don’t underestimate the value of topical therapy for treating psoriasis patients, Dr. Linda Stein Gold said at Skin Disease Education Foundation’s annual Las Vegas dermatology seminar.
Dr. Stein Gold explained how to overcome some of the compliance issues associated with topical therapies for psoriasis, and offered her suggestions for managing side effects in an interview at the meeting.
Dr. Stein Gold disclosed relationships with multiple pharmaceutical companies including Galderma, Stiefel, Allergan, Valeant, Ranbaxy, Promius, and Actavis.
SDEF and this news organization are owned by Frontline Medical Communications.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
AT SDEF LAS VEGAS DERMATOLOGY SEMINAR
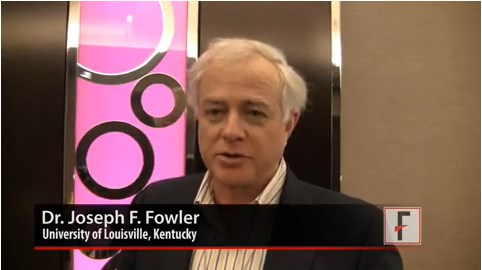
VIDEO: When atopic dermatitis and contact dermatitis collide
LAS VEGAS– Atopic dermatitis patients may be at increased risk for contact as well, Dr. Joseph Fowler said at Skin Disease Education Foundation’s annual Las Vegas dermatology seminar.
In an interview at the meeting, Dr. Fowler reviewed several emerging allergens, explained how contact dermatitis and atopic dermatitis interact, and shared some tips for identifying and managing both conditions when they occur simultaneously.
Dr. Fowler disclosed relationships with multiple pharmaceutical companies, none of which were relevant to the content of this video. SDEF and this news organization are owned by Frontline Medical Communications.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
LAS VEGAS– Atopic dermatitis patients may be at increased risk for contact as well, Dr. Joseph Fowler said at Skin Disease Education Foundation’s annual Las Vegas dermatology seminar.
In an interview at the meeting, Dr. Fowler reviewed several emerging allergens, explained how contact dermatitis and atopic dermatitis interact, and shared some tips for identifying and managing both conditions when they occur simultaneously.
Dr. Fowler disclosed relationships with multiple pharmaceutical companies, none of which were relevant to the content of this video. SDEF and this news organization are owned by Frontline Medical Communications.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
LAS VEGAS– Atopic dermatitis patients may be at increased risk for contact as well, Dr. Joseph Fowler said at Skin Disease Education Foundation’s annual Las Vegas dermatology seminar.
In an interview at the meeting, Dr. Fowler reviewed several emerging allergens, explained how contact dermatitis and atopic dermatitis interact, and shared some tips for identifying and managing both conditions when they occur simultaneously.
Dr. Fowler disclosed relationships with multiple pharmaceutical companies, none of which were relevant to the content of this video. SDEF and this news organization are owned by Frontline Medical Communications.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
AT SDEF LAS VEGAS DERMATOLOGY SEMINAR
Psoriasis patients’ QOL improved with calcipotriene/betamethasone
LAS VEGAS – Adults with localized psoriasis reported significant improvement in quality of life after 2 and 8 weeks of treatment with a combination topical suspension, based on data from 147 adults.
Calcipotriene 0.005% plus betamethasone diproprionate 0.064% (CBD) has shown safety and effectiveness in adults with psoriasis. Dr. Jerry Bagel, who is in group practice in East Windsor, N.J., and his colleagues documented real-life experiences with CBD and patient-reported outcomes. The findings were presented at the Skin Disease Education Foundation’s annual Las Vegas dermatology seminar.
The average change in quality of life scores from baseline was –4.2 at week 2 and –5.5 at week 8; both were statistically significant. In addition, 38% and 42% of patients at 2 weeks and 8 weeks, respectively, met the secondary endpoint of at least a 5-point improvement on the Dermatology Life Quality Index (DLQI).
The mean percent change in the patients’ perceptions of itching was –19% at week 2 and –29% at week 8, based on an itch visual analog scale, Dr. Bagel noted. Patient satisfaction with treatment also was measured using the Treatment Satisfaction Questionnaire for Medication–9 (TSQM-9), and the average scores for treatment effectiveness, convenience, and satisfaction were greater than 65 on a scale of 0-100 at week 2 and week 8.
Dr. Bagel and his colleagues studied 147 adults aged 18 years and older; patients’ average age was 49 years. More than half (57%) were women; 78% of patients were white. Approximately 56% were characterized as having moderate disease, and the average disease duration was 11 years. Only two patients reported treatment-emergent adverse events, but these were deemed unrelated to the study drug by the researchers.
The findings were limited by the use of self-reports. However, “patients with an extensive history of psoriasis and various levels of disease severity were satisfied with the product” in this real-life treatment profile, he noted.
Dr. Bagel has served as a consultant, speaker, and investigator for multiple companies, including Leo Pharma, which sponsored this study. SDEF and this news organization are owned by Frontline Medical Communications.
LAS VEGAS – Adults with localized psoriasis reported significant improvement in quality of life after 2 and 8 weeks of treatment with a combination topical suspension, based on data from 147 adults.
Calcipotriene 0.005% plus betamethasone diproprionate 0.064% (CBD) has shown safety and effectiveness in adults with psoriasis. Dr. Jerry Bagel, who is in group practice in East Windsor, N.J., and his colleagues documented real-life experiences with CBD and patient-reported outcomes. The findings were presented at the Skin Disease Education Foundation’s annual Las Vegas dermatology seminar.
The average change in quality of life scores from baseline was –4.2 at week 2 and –5.5 at week 8; both were statistically significant. In addition, 38% and 42% of patients at 2 weeks and 8 weeks, respectively, met the secondary endpoint of at least a 5-point improvement on the Dermatology Life Quality Index (DLQI).
The mean percent change in the patients’ perceptions of itching was –19% at week 2 and –29% at week 8, based on an itch visual analog scale, Dr. Bagel noted. Patient satisfaction with treatment also was measured using the Treatment Satisfaction Questionnaire for Medication–9 (TSQM-9), and the average scores for treatment effectiveness, convenience, and satisfaction were greater than 65 on a scale of 0-100 at week 2 and week 8.
Dr. Bagel and his colleagues studied 147 adults aged 18 years and older; patients’ average age was 49 years. More than half (57%) were women; 78% of patients were white. Approximately 56% were characterized as having moderate disease, and the average disease duration was 11 years. Only two patients reported treatment-emergent adverse events, but these were deemed unrelated to the study drug by the researchers.
The findings were limited by the use of self-reports. However, “patients with an extensive history of psoriasis and various levels of disease severity were satisfied with the product” in this real-life treatment profile, he noted.
Dr. Bagel has served as a consultant, speaker, and investigator for multiple companies, including Leo Pharma, which sponsored this study. SDEF and this news organization are owned by Frontline Medical Communications.
LAS VEGAS – Adults with localized psoriasis reported significant improvement in quality of life after 2 and 8 weeks of treatment with a combination topical suspension, based on data from 147 adults.
Calcipotriene 0.005% plus betamethasone diproprionate 0.064% (CBD) has shown safety and effectiveness in adults with psoriasis. Dr. Jerry Bagel, who is in group practice in East Windsor, N.J., and his colleagues documented real-life experiences with CBD and patient-reported outcomes. The findings were presented at the Skin Disease Education Foundation’s annual Las Vegas dermatology seminar.
The average change in quality of life scores from baseline was –4.2 at week 2 and –5.5 at week 8; both were statistically significant. In addition, 38% and 42% of patients at 2 weeks and 8 weeks, respectively, met the secondary endpoint of at least a 5-point improvement on the Dermatology Life Quality Index (DLQI).
The mean percent change in the patients’ perceptions of itching was –19% at week 2 and –29% at week 8, based on an itch visual analog scale, Dr. Bagel noted. Patient satisfaction with treatment also was measured using the Treatment Satisfaction Questionnaire for Medication–9 (TSQM-9), and the average scores for treatment effectiveness, convenience, and satisfaction were greater than 65 on a scale of 0-100 at week 2 and week 8.
Dr. Bagel and his colleagues studied 147 adults aged 18 years and older; patients’ average age was 49 years. More than half (57%) were women; 78% of patients were white. Approximately 56% were characterized as having moderate disease, and the average disease duration was 11 years. Only two patients reported treatment-emergent adverse events, but these were deemed unrelated to the study drug by the researchers.
The findings were limited by the use of self-reports. However, “patients with an extensive history of psoriasis and various levels of disease severity were satisfied with the product” in this real-life treatment profile, he noted.
Dr. Bagel has served as a consultant, speaker, and investigator for multiple companies, including Leo Pharma, which sponsored this study. SDEF and this news organization are owned by Frontline Medical Communications.
AT SDEF LAS VEGAS DERMATOLOGY SEMINAR
Key clinical point: Calcipotriene 0.005% plus betamethasone diproprionate 0.064% was safe and effective and improved patients’ perceived quality of life.
Major finding: The average change in quality of life scores from baseline was –4.2 at week 2 and –5.5 at week 8.
Data source: Data from 147 adults aged 18 years and older with psoraisis vulgaris of an average of 11 years’ duration.
Disclosures: Dr. Bagel has served as a consultant, speaker, and investigator for multiple companies, including Leo Pharma, which sponsored this study.
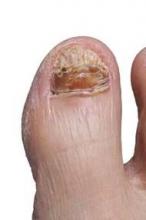
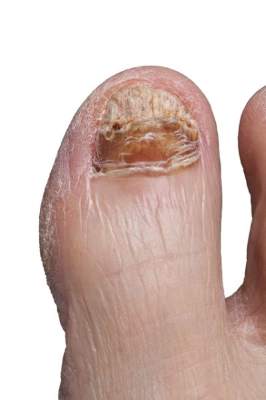
New onychomycosis drugs show similar success and side effects
LAS VEGAS – The latest topical therapies for onychomycosis are strong enough to penetrate nail polish, but patients report few side effects.
“New topical antifungals have improved the challenge of nail penetration and enhanced the efficacy of topical therapy,” Dr. David Pariser said at the Skin Disease Education Foundation’s annual Las Vegas dermatology seminar.
Two drugs, efinaconazole and tavaborole, have shown effectiveness against onychomycosis in clinical trials since 2013, and both have been approved by the Food and Drug Administration, said Dr. Pariser, a dermatologist in private practice in Norfolk, Va., and a professor at Eastern Virginia Medical School, Norfolk.
Significantly more patients in studies of both medications showed improvement after 52 weeks of treatment, compared with control patients, but the clinical trials of the two drugs aren’t directly comparable for several reasons, Dr. Pariser noted. The studies differed in their entry criteria, including patient age and nail involvement, and, in some cases, nails were clipped prior to clinical evaluation. In addition, the investigators in the studies used different laboratories, and the definitions of treatment success and investigators’ interpretations of clinical cure may vary, he noted.
Reports of adverse events related to the drugs were low for both. Although 65% of 801 patients in a pooled analysis of efinaconazole data reported at least one adverse event, only 8% of these events were deemed related to the study drug. No serious drug-related adverse events were reported in an analysis of 1,186 patients treated with tavaborole who reported at least one treatment-related adverse event.
The most common adverse events in efinaconazole patients included ingrown toenails, application-site dermatitis, and application-site vesicles, reported in approximately 2% of patients, and application-site pain reported by 1%. Similarly, the most common adverse events in tavaborole patients included application site exfoliation and ingrown toenail in approximately 3% of patients, application site erythema in 2%, and application site dermatitis in 1%.
In addition, early findings from separate studies of both medications suggest that their penetration ability is not affected by nail polish, although more research is needed, Dr. Pariser said.
Dr. Pariser has served as an investigator and consultant for Valeant and an investigator for Anacor. SDEF and this news organization are owned by Frontline Medical Communications.
LAS VEGAS – The latest topical therapies for onychomycosis are strong enough to penetrate nail polish, but patients report few side effects.
“New topical antifungals have improved the challenge of nail penetration and enhanced the efficacy of topical therapy,” Dr. David Pariser said at the Skin Disease Education Foundation’s annual Las Vegas dermatology seminar.
Two drugs, efinaconazole and tavaborole, have shown effectiveness against onychomycosis in clinical trials since 2013, and both have been approved by the Food and Drug Administration, said Dr. Pariser, a dermatologist in private practice in Norfolk, Va., and a professor at Eastern Virginia Medical School, Norfolk.
Significantly more patients in studies of both medications showed improvement after 52 weeks of treatment, compared with control patients, but the clinical trials of the two drugs aren’t directly comparable for several reasons, Dr. Pariser noted. The studies differed in their entry criteria, including patient age and nail involvement, and, in some cases, nails were clipped prior to clinical evaluation. In addition, the investigators in the studies used different laboratories, and the definitions of treatment success and investigators’ interpretations of clinical cure may vary, he noted.
Reports of adverse events related to the drugs were low for both. Although 65% of 801 patients in a pooled analysis of efinaconazole data reported at least one adverse event, only 8% of these events were deemed related to the study drug. No serious drug-related adverse events were reported in an analysis of 1,186 patients treated with tavaborole who reported at least one treatment-related adverse event.
The most common adverse events in efinaconazole patients included ingrown toenails, application-site dermatitis, and application-site vesicles, reported in approximately 2% of patients, and application-site pain reported by 1%. Similarly, the most common adverse events in tavaborole patients included application site exfoliation and ingrown toenail in approximately 3% of patients, application site erythema in 2%, and application site dermatitis in 1%.
In addition, early findings from separate studies of both medications suggest that their penetration ability is not affected by nail polish, although more research is needed, Dr. Pariser said.
Dr. Pariser has served as an investigator and consultant for Valeant and an investigator for Anacor. SDEF and this news organization are owned by Frontline Medical Communications.
LAS VEGAS – The latest topical therapies for onychomycosis are strong enough to penetrate nail polish, but patients report few side effects.
“New topical antifungals have improved the challenge of nail penetration and enhanced the efficacy of topical therapy,” Dr. David Pariser said at the Skin Disease Education Foundation’s annual Las Vegas dermatology seminar.
Two drugs, efinaconazole and tavaborole, have shown effectiveness against onychomycosis in clinical trials since 2013, and both have been approved by the Food and Drug Administration, said Dr. Pariser, a dermatologist in private practice in Norfolk, Va., and a professor at Eastern Virginia Medical School, Norfolk.
Significantly more patients in studies of both medications showed improvement after 52 weeks of treatment, compared with control patients, but the clinical trials of the two drugs aren’t directly comparable for several reasons, Dr. Pariser noted. The studies differed in their entry criteria, including patient age and nail involvement, and, in some cases, nails were clipped prior to clinical evaluation. In addition, the investigators in the studies used different laboratories, and the definitions of treatment success and investigators’ interpretations of clinical cure may vary, he noted.
Reports of adverse events related to the drugs were low for both. Although 65% of 801 patients in a pooled analysis of efinaconazole data reported at least one adverse event, only 8% of these events were deemed related to the study drug. No serious drug-related adverse events were reported in an analysis of 1,186 patients treated with tavaborole who reported at least one treatment-related adverse event.
The most common adverse events in efinaconazole patients included ingrown toenails, application-site dermatitis, and application-site vesicles, reported in approximately 2% of patients, and application-site pain reported by 1%. Similarly, the most common adverse events in tavaborole patients included application site exfoliation and ingrown toenail in approximately 3% of patients, application site erythema in 2%, and application site dermatitis in 1%.
In addition, early findings from separate studies of both medications suggest that their penetration ability is not affected by nail polish, although more research is needed, Dr. Pariser said.
Dr. Pariser has served as an investigator and consultant for Valeant and an investigator for Anacor. SDEF and this news organization are owned by Frontline Medical Communications.
EXPERT ANALYSIS FROM SDEF LAS VEGAS DERMATOLOGY SEMINAR