User login
, in a multicenter, randomized trial.
In addition, in the study, those on 200-mg and 100-mg daily doses of abrocitinib experienced significantly greater reductions in signs and symptoms of AD at 12 and 16 weeks, than those on placebo, the authors reported.
The findings from the JADE COMPARE trial, published on March 25 in the New England Journal of Medicine, suggest abrocitinib will provide clinicians with another treatment option for patients who don’t get adequate relief from either topical medications or dupilumab. Abrocitinib is associated with a different set of adverse reactions than dupilumab, according to investigators.
In 2017, dupilumab (Dupixent) became the first systemic drug approved by the Food and Drug Administration specifically for AD, though systemic steroids and other immunosuppressant drugs are sometimes prescribed. A monoclonal antibody delivered by subcutaneous injection, dupilumab binds to interleukin-4 receptors to block signaling pathways involved in AD; it is now approved for treatment of patients with moderate to severe AD down to age 6 years.
“It is sort of the bar for efficacy and for safety in those patients, because that’s what we have right now,” said one of the JADE Compare investigators and study author, Jonathan I. Silverberg, MD, PhD, MPH, associate professor of dermatology and director of clinical research and contact dermatitis at George Washington University, Washington, said in an interview. “For any new therapy coming to market, we really do want to understand how it compares to what’s out there.”
Abrocitinib is a small molecule that inhibits JAK1, which is thought to modulate multiple cytokines involved in AD, including interleukin (IL)–4, IL-13, IL-31, IL-22, and thymic stromal lymphopoietin. Two other JAK1 inhibitors, baricitinib and upadacitinib, are also being investigated as systemic treatments for AD.
In JADE COMPARE, people with moderate to severe AD from 18 countries on four continents, entered a 28-day screening period during which they discontinued treatments. They began using emollients twice a day at least 7 days before being randomly assigned to a treatment group, and continued on topical medication once daily. Topical treatments included low- or medium-potency topical glucocorticoids, calcineurin inhibitors, and phosphodiesterase-4 inhibitors.
The researchers randomly assigned 838 to trial groups: 226 received 200 mg of abrocitinib orally once a day, 238 received 100 mg of abrocitinib once a day, 243 received a 300-mg dupilumab injection every other week, and 131 received placebo versions of both medications, for 16 weeks. The mean age of the patients overall was about 38 years; about two-thirds were White.
At 2 weeks, half of the patients on 200 mg of abrocitinib and 31.8% of those on the 100-mg dose had an itch response, defined as at least a 4-point improvement from baseline in the 0-10 Peak Pruritus Numerical Rating Scale. This was compared with 26.4% of those on dupilumab and 13.8% of those on placebo.
And at 12 weeks, more of the patients in the 200-mg abrocitinib group than in the other groups had an Investigator’s Global Assessment (IGA) response (defined as clear or almost clear) and more had an Eczema Area and Severity Index (EASI-75) response (defined as an improvement of at least 75%). (See Table) EASI-75 and IGA responses at week 12 were the primary outcomes of the study.
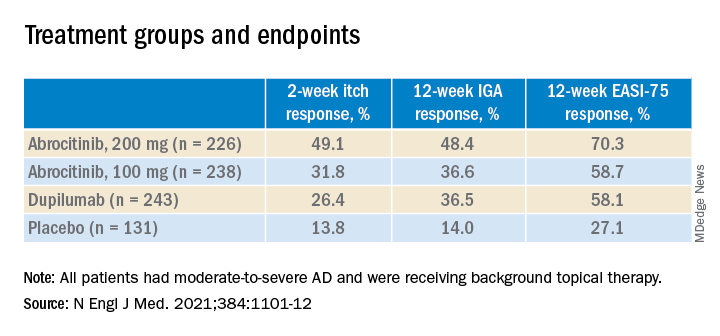
The differences between both abrocitinib groups and the placebo group were statistically significant by all these measures (P < .001). The difference between the 200-mg abrocitinib and the dupilumab group was only significant for itch at 2 weeks, and the difference in itch response between the 100-mg group and the dupilumab group at 2 weeks was not significant (P < .20).
At 16 weeks, the EASI-75 response (a secondary endpoint) among those on either dose of abrocitinib was not significantly different than among those on dupilumab (71% and 60.3% among those on 200 mg and 100 mg, respectively; and 65.5% among those on dupilumab, compared with 30.6% of those on placebo).
“The patients I have on this medicine [abrocitinib] are very happy,” said one of the study authors, Melinda Gooderham, MsC, MD, an assistant professor at Queen’s University, Kingston, Ont., an investigator in the trial. “It works very quickly for itch,” she said in an interview.
The study didn’t have sufficient statistical power to fully explore the comparison to dupilumab, and future trials will go deeper into the comparison, she added.
Still, in this trial, abrocitinib demonstrated a clear advantage in the speed and depth of efficacy, Dr. Silverberg noted. “The 100-mg dose of abrocitinib was about as effective as, or maybe slightly less effective than, dupilumab, and the 200-mg dose was more effective than dupilumab.”
The overall incidence of adverse events was higher in the 200-mg abrocitinib arm than in the other groups, but the incidence of serious or severe adverse events, and the incidence of adverse events that resulted in discontinuing the medication, were similar across the trial groups.
However, nausea affected 11.1% of the patients in the 200-mg abrocitinib group and 4.2% of those in the 100-mg abrocitinib group. Acne was also reported in these groups (6.6% and 2.9%, among those on 200 mg and 100 mg, respectively, compared with 1.2% of those on dupilumab and none of those on placebo). In a few of those on abrocitinib, herpes zoster flared up. And median platelet counts decreased among the patients taking abrocitinib, although none dropped below 75,000/mm3. Serious infections were reported in two patients on abrocitinib, but resolved.
By contrast, only 2.9% of the patients on dupilumab had nausea. But 6.2% in the dupilumab group had conjunctivitis, compared with 1.3% of patients in the 200-mg abrocitinib group and 0.8 in the 100-mg abrocitinib group.
As an oral medication, abrocitinib will appeal to patients who want to avoid injections, and dosing will be easier to adjust, Dr. Silverberg said. On the other hand, he added, dupilumab will have an advantage for patients who don’t want to take a daily medication, or who are concerned about the adverse events associated with abrocitinib, particularly those with blood-clotting disorders.
On the basis of two previous JADE phase 3 trials, Pfizer has submitted a new drug application for abrocitinib for treating moderate to severe AD in patients aged 12 and older to the FDA; a decision is expected in April, according to the company. The company has also applied to market the drug in Europe and the United Kingdom.
The study was funded by Pfizer. Dr. Silverberg’s disclosures included serving as a consultant to companies including AbbVie, Pfizer, and Regeneron. Several authors are Pfizer employees; other authors had disclosures related to Pfizer and other pharmaceutical companies.
, in a multicenter, randomized trial.
In addition, in the study, those on 200-mg and 100-mg daily doses of abrocitinib experienced significantly greater reductions in signs and symptoms of AD at 12 and 16 weeks, than those on placebo, the authors reported.
The findings from the JADE COMPARE trial, published on March 25 in the New England Journal of Medicine, suggest abrocitinib will provide clinicians with another treatment option for patients who don’t get adequate relief from either topical medications or dupilumab. Abrocitinib is associated with a different set of adverse reactions than dupilumab, according to investigators.
In 2017, dupilumab (Dupixent) became the first systemic drug approved by the Food and Drug Administration specifically for AD, though systemic steroids and other immunosuppressant drugs are sometimes prescribed. A monoclonal antibody delivered by subcutaneous injection, dupilumab binds to interleukin-4 receptors to block signaling pathways involved in AD; it is now approved for treatment of patients with moderate to severe AD down to age 6 years.
“It is sort of the bar for efficacy and for safety in those patients, because that’s what we have right now,” said one of the JADE Compare investigators and study author, Jonathan I. Silverberg, MD, PhD, MPH, associate professor of dermatology and director of clinical research and contact dermatitis at George Washington University, Washington, said in an interview. “For any new therapy coming to market, we really do want to understand how it compares to what’s out there.”
Abrocitinib is a small molecule that inhibits JAK1, which is thought to modulate multiple cytokines involved in AD, including interleukin (IL)–4, IL-13, IL-31, IL-22, and thymic stromal lymphopoietin. Two other JAK1 inhibitors, baricitinib and upadacitinib, are also being investigated as systemic treatments for AD.
In JADE COMPARE, people with moderate to severe AD from 18 countries on four continents, entered a 28-day screening period during which they discontinued treatments. They began using emollients twice a day at least 7 days before being randomly assigned to a treatment group, and continued on topical medication once daily. Topical treatments included low- or medium-potency topical glucocorticoids, calcineurin inhibitors, and phosphodiesterase-4 inhibitors.
The researchers randomly assigned 838 to trial groups: 226 received 200 mg of abrocitinib orally once a day, 238 received 100 mg of abrocitinib once a day, 243 received a 300-mg dupilumab injection every other week, and 131 received placebo versions of both medications, for 16 weeks. The mean age of the patients overall was about 38 years; about two-thirds were White.
At 2 weeks, half of the patients on 200 mg of abrocitinib and 31.8% of those on the 100-mg dose had an itch response, defined as at least a 4-point improvement from baseline in the 0-10 Peak Pruritus Numerical Rating Scale. This was compared with 26.4% of those on dupilumab and 13.8% of those on placebo.
And at 12 weeks, more of the patients in the 200-mg abrocitinib group than in the other groups had an Investigator’s Global Assessment (IGA) response (defined as clear or almost clear) and more had an Eczema Area and Severity Index (EASI-75) response (defined as an improvement of at least 75%). (See Table) EASI-75 and IGA responses at week 12 were the primary outcomes of the study.

The differences between both abrocitinib groups and the placebo group were statistically significant by all these measures (P < .001). The difference between the 200-mg abrocitinib and the dupilumab group was only significant for itch at 2 weeks, and the difference in itch response between the 100-mg group and the dupilumab group at 2 weeks was not significant (P < .20).
At 16 weeks, the EASI-75 response (a secondary endpoint) among those on either dose of abrocitinib was not significantly different than among those on dupilumab (71% and 60.3% among those on 200 mg and 100 mg, respectively; and 65.5% among those on dupilumab, compared with 30.6% of those on placebo).
“The patients I have on this medicine [abrocitinib] are very happy,” said one of the study authors, Melinda Gooderham, MsC, MD, an assistant professor at Queen’s University, Kingston, Ont., an investigator in the trial. “It works very quickly for itch,” she said in an interview.
The study didn’t have sufficient statistical power to fully explore the comparison to dupilumab, and future trials will go deeper into the comparison, she added.
Still, in this trial, abrocitinib demonstrated a clear advantage in the speed and depth of efficacy, Dr. Silverberg noted. “The 100-mg dose of abrocitinib was about as effective as, or maybe slightly less effective than, dupilumab, and the 200-mg dose was more effective than dupilumab.”
The overall incidence of adverse events was higher in the 200-mg abrocitinib arm than in the other groups, but the incidence of serious or severe adverse events, and the incidence of adverse events that resulted in discontinuing the medication, were similar across the trial groups.
However, nausea affected 11.1% of the patients in the 200-mg abrocitinib group and 4.2% of those in the 100-mg abrocitinib group. Acne was also reported in these groups (6.6% and 2.9%, among those on 200 mg and 100 mg, respectively, compared with 1.2% of those on dupilumab and none of those on placebo). In a few of those on abrocitinib, herpes zoster flared up. And median platelet counts decreased among the patients taking abrocitinib, although none dropped below 75,000/mm3. Serious infections were reported in two patients on abrocitinib, but resolved.
By contrast, only 2.9% of the patients on dupilumab had nausea. But 6.2% in the dupilumab group had conjunctivitis, compared with 1.3% of patients in the 200-mg abrocitinib group and 0.8 in the 100-mg abrocitinib group.
As an oral medication, abrocitinib will appeal to patients who want to avoid injections, and dosing will be easier to adjust, Dr. Silverberg said. On the other hand, he added, dupilumab will have an advantage for patients who don’t want to take a daily medication, or who are concerned about the adverse events associated with abrocitinib, particularly those with blood-clotting disorders.
On the basis of two previous JADE phase 3 trials, Pfizer has submitted a new drug application for abrocitinib for treating moderate to severe AD in patients aged 12 and older to the FDA; a decision is expected in April, according to the company. The company has also applied to market the drug in Europe and the United Kingdom.
The study was funded by Pfizer. Dr. Silverberg’s disclosures included serving as a consultant to companies including AbbVie, Pfizer, and Regeneron. Several authors are Pfizer employees; other authors had disclosures related to Pfizer and other pharmaceutical companies.
, in a multicenter, randomized trial.
In addition, in the study, those on 200-mg and 100-mg daily doses of abrocitinib experienced significantly greater reductions in signs and symptoms of AD at 12 and 16 weeks, than those on placebo, the authors reported.
The findings from the JADE COMPARE trial, published on March 25 in the New England Journal of Medicine, suggest abrocitinib will provide clinicians with another treatment option for patients who don’t get adequate relief from either topical medications or dupilumab. Abrocitinib is associated with a different set of adverse reactions than dupilumab, according to investigators.
In 2017, dupilumab (Dupixent) became the first systemic drug approved by the Food and Drug Administration specifically for AD, though systemic steroids and other immunosuppressant drugs are sometimes prescribed. A monoclonal antibody delivered by subcutaneous injection, dupilumab binds to interleukin-4 receptors to block signaling pathways involved in AD; it is now approved for treatment of patients with moderate to severe AD down to age 6 years.
“It is sort of the bar for efficacy and for safety in those patients, because that’s what we have right now,” said one of the JADE Compare investigators and study author, Jonathan I. Silverberg, MD, PhD, MPH, associate professor of dermatology and director of clinical research and contact dermatitis at George Washington University, Washington, said in an interview. “For any new therapy coming to market, we really do want to understand how it compares to what’s out there.”
Abrocitinib is a small molecule that inhibits JAK1, which is thought to modulate multiple cytokines involved in AD, including interleukin (IL)–4, IL-13, IL-31, IL-22, and thymic stromal lymphopoietin. Two other JAK1 inhibitors, baricitinib and upadacitinib, are also being investigated as systemic treatments for AD.
In JADE COMPARE, people with moderate to severe AD from 18 countries on four continents, entered a 28-day screening period during which they discontinued treatments. They began using emollients twice a day at least 7 days before being randomly assigned to a treatment group, and continued on topical medication once daily. Topical treatments included low- or medium-potency topical glucocorticoids, calcineurin inhibitors, and phosphodiesterase-4 inhibitors.
The researchers randomly assigned 838 to trial groups: 226 received 200 mg of abrocitinib orally once a day, 238 received 100 mg of abrocitinib once a day, 243 received a 300-mg dupilumab injection every other week, and 131 received placebo versions of both medications, for 16 weeks. The mean age of the patients overall was about 38 years; about two-thirds were White.
At 2 weeks, half of the patients on 200 mg of abrocitinib and 31.8% of those on the 100-mg dose had an itch response, defined as at least a 4-point improvement from baseline in the 0-10 Peak Pruritus Numerical Rating Scale. This was compared with 26.4% of those on dupilumab and 13.8% of those on placebo.
And at 12 weeks, more of the patients in the 200-mg abrocitinib group than in the other groups had an Investigator’s Global Assessment (IGA) response (defined as clear or almost clear) and more had an Eczema Area and Severity Index (EASI-75) response (defined as an improvement of at least 75%). (See Table) EASI-75 and IGA responses at week 12 were the primary outcomes of the study.

The differences between both abrocitinib groups and the placebo group were statistically significant by all these measures (P < .001). The difference between the 200-mg abrocitinib and the dupilumab group was only significant for itch at 2 weeks, and the difference in itch response between the 100-mg group and the dupilumab group at 2 weeks was not significant (P < .20).
At 16 weeks, the EASI-75 response (a secondary endpoint) among those on either dose of abrocitinib was not significantly different than among those on dupilumab (71% and 60.3% among those on 200 mg and 100 mg, respectively; and 65.5% among those on dupilumab, compared with 30.6% of those on placebo).
“The patients I have on this medicine [abrocitinib] are very happy,” said one of the study authors, Melinda Gooderham, MsC, MD, an assistant professor at Queen’s University, Kingston, Ont., an investigator in the trial. “It works very quickly for itch,” she said in an interview.
The study didn’t have sufficient statistical power to fully explore the comparison to dupilumab, and future trials will go deeper into the comparison, she added.
Still, in this trial, abrocitinib demonstrated a clear advantage in the speed and depth of efficacy, Dr. Silverberg noted. “The 100-mg dose of abrocitinib was about as effective as, or maybe slightly less effective than, dupilumab, and the 200-mg dose was more effective than dupilumab.”
The overall incidence of adverse events was higher in the 200-mg abrocitinib arm than in the other groups, but the incidence of serious or severe adverse events, and the incidence of adverse events that resulted in discontinuing the medication, were similar across the trial groups.
However, nausea affected 11.1% of the patients in the 200-mg abrocitinib group and 4.2% of those in the 100-mg abrocitinib group. Acne was also reported in these groups (6.6% and 2.9%, among those on 200 mg and 100 mg, respectively, compared with 1.2% of those on dupilumab and none of those on placebo). In a few of those on abrocitinib, herpes zoster flared up. And median platelet counts decreased among the patients taking abrocitinib, although none dropped below 75,000/mm3. Serious infections were reported in two patients on abrocitinib, but resolved.
By contrast, only 2.9% of the patients on dupilumab had nausea. But 6.2% in the dupilumab group had conjunctivitis, compared with 1.3% of patients in the 200-mg abrocitinib group and 0.8 in the 100-mg abrocitinib group.
As an oral medication, abrocitinib will appeal to patients who want to avoid injections, and dosing will be easier to adjust, Dr. Silverberg said. On the other hand, he added, dupilumab will have an advantage for patients who don’t want to take a daily medication, or who are concerned about the adverse events associated with abrocitinib, particularly those with blood-clotting disorders.
On the basis of two previous JADE phase 3 trials, Pfizer has submitted a new drug application for abrocitinib for treating moderate to severe AD in patients aged 12 and older to the FDA; a decision is expected in April, according to the company. The company has also applied to market the drug in Europe and the United Kingdom.
The study was funded by Pfizer. Dr. Silverberg’s disclosures included serving as a consultant to companies including AbbVie, Pfizer, and Regeneron. Several authors are Pfizer employees; other authors had disclosures related to Pfizer and other pharmaceutical companies.

