User login
Cutis is a peer-reviewed clinical journal for the dermatologist, allergist, and general practitioner published monthly since 1965. Concise clinical articles present the practical side of dermatology, helping physicians to improve patient care. Cutis is referenced in Index Medicus/MEDLINE and is written and edited by industry leaders.
ass lick
assault rifle
balls
ballsac
black jack
bleach
Boko Haram
bondage
causas
cheap
child abuse
cocaine
compulsive behaviors
cost of miracles
cunt
Daech
display network stats
drug paraphernalia
explosion
fart
fda and death
fda AND warn
fda AND warning
fda AND warns
feom
fuck
gambling
gfc
gun
human trafficking
humira AND expensive
illegal
ISIL
ISIS
Islamic caliphate
Islamic state
madvocate
masturbation
mixed martial arts
MMA
molestation
national rifle association
NRA
nsfw
nuccitelli
pedophile
pedophilia
poker
porn
porn
pornography
psychedelic drug
recreational drug
sex slave rings
shit
slot machine
snort
substance abuse
terrorism
terrorist
texarkana
Texas hold 'em
UFC
section[contains(@class, 'nav-hidden')]
section[contains(@class, 'nav-hidden active')
A peer-reviewed, indexed journal for dermatologists with original research, image quizzes, cases and reviews, and columns.
Cemiplimab-Associated Eruption of Generalized Eruptive Keratoacanthoma of Grzybowski
To the Editor:
Treatment of cancer, including cutaneous malignancy, has been transformed by the use of immunotherapeutic agents such as immune checkpoint inhibitors (ICIs) that target cytotoxic T lymphocyte-associated antigen 4, programmed cell-death protein 1 (PD-1), or programmed cell-death ligand 1 (PD-L1). However, these drugs are associated with a distinct set of immune-related adverse events (IRAEs). We present a case of generalized eruptive keratoacanthoma of Grzybowski associated with the ICI cemiplimab.
A 94-year-old White woman presented to the dermatology clinic with acute onset of extensive, locally advanced cutaneous squamous cell carcinoma (cSCC) of the upper right posterolateral calf as well as multiple noninvasive cSCCs of the arms and legs. Her medical history was remarkable for widespread actinic keratoses and numerous cSCCs. The patient had no personal or family history of melanoma. Various cSCCs had required treatment with electrodesiccation and curettage, topical or intralesional 5-fluorouracil, and Mohs micrographic surgery. Approximately 1 year prior to presentation, oral acitretin was initiated to help control the cSCC. Given the extent of locally advanced disease, which was considered unresectable, she was referred to oncology but continued to follow up with dermatology. Positron emission tomography was remarkable for hypermetabolic cutaneous thickening in the upper right posterolateral calf with no evidence of visceral disease.

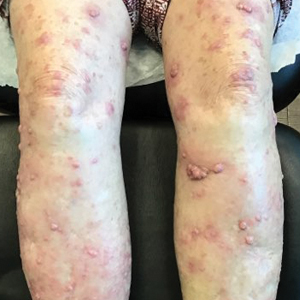
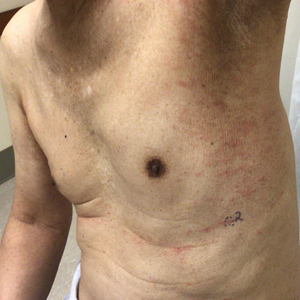
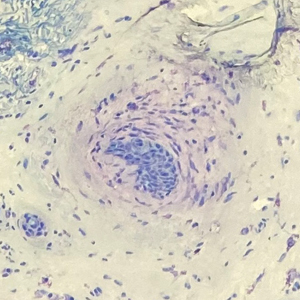
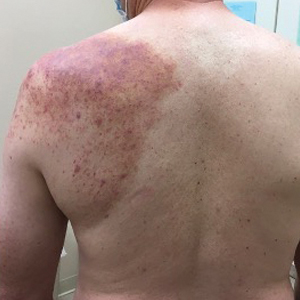
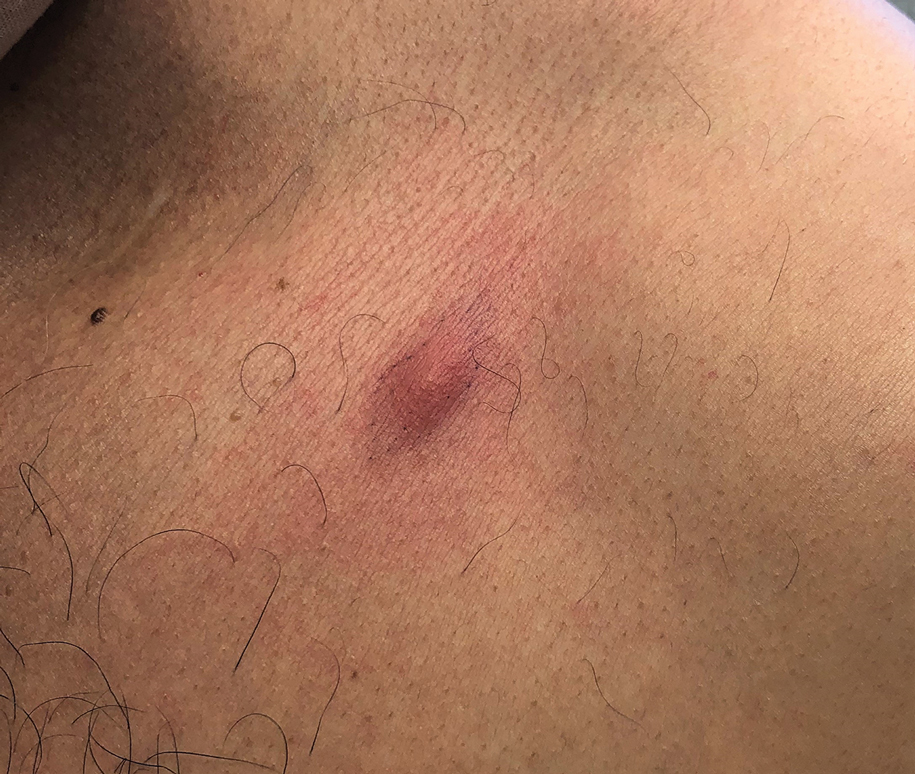
The patient was started on cemiplimab, an anti-PD-1 monoclonal antibody ICI indicated for the treatment of both metastatic and advanced cSCC. After 4 cycles of intravenous cemiplimab, the patient developed widespread nodules covering the arms and legs (Figure 1) as well as associated tenderness and pruritus. Biopsies of nodules revealed superficially invasive, well-differentiated cSCC consistent with keratoacanthoma. Although a lymphocytic infiltrate was present, no other specific reaction pattern, such as a lichenoid infiltrate, was present (Figure 2).

Positron emission tomography was repeated, demonstrating resolution of the right calf lesion; however, new diffuse cutaneous lesions and inguinal lymph node involvement were present, again without evidence of visceral disease. Given the clinical and histologic findings, a diagnosis of generalized eruptive keratoacanthoma of Grzybowski was made. Cemiplimab was discontinued after the fifth cycle. The patient declined further systemic treatment, instead choosing a regimen of topical steroids and an emollient.
Immunotherapeutics have transformed cancer therapy, which includes ICIs that target cytotoxic T lymphocyte-associated antigen 4, PD-1, or PD-L1. Increased activity of these checkpoints allows tumor cells to downregulate T-cell activation, thereby evading immune destruction. When PD-1 on T cells binds PD-L1 on tumor cells, T lymphocytes are inhibited from cytotoxic-mediated killing. Therefore, anti-PD-1 ICIs such as cemiplimab permit T-lymphocyte activation and destruction of malignant cells. However, this unique mechanism of immunotherapy is associated with an array of IRAEs, which often manifest in a delayed and prolonged fashion.1 Immune-related adverse events most commonly affect the gastrointestinal tract as well as the endocrine and dermatologic systems.2 Notably, patients with certain tumors who experience these adverse effects might be more likely to have superior overall survival; therefore, IRAEs are sometimes used as an indicator of favorable treatment response.2,3
Dermatologic IRAEs associated with the use of a PD-1 inhibitor include lichenoid reactions, pruritus, morbilliform eruptions, vitiligo, and bullous pemphigoid.4,5 Eruptions of keratoacanthoma rarely have been reported following treatment with the PD-1 inhibitors nivolumab and pembrolizumab.3,6,7 In our patient, we believe the profound and generalized eruptive keratoacanthoma—a well-differentiated cSCC variant—was related to treatment of locally advanced cSCC with cemiplimab. The mechanism underlying the formation of anti-PD-1 eruptive keratoacanthoma is not well understood. In susceptible patients, it is plausible that the inflammatory environment permitted by ICIs paradoxically induces regression of tumors such as locally invasive cSCC and simultaneously promotes formation of keratoacanthoma.
The role of inflammation in the pathogenesis and progression of cSCC is complex and possibly involves contrasting roles of leukocyte subpopulations.8 The increased incidence of cSCC in the immunocompromised population,8 PD-L1 overexpression in cSCC,9,10 and successful treatment of cSCC with PD-1 inhibition10 all suggest that inhibition of specific inflammatory pathways is pivotal in tumor pathogenesis. However, increased inflammation, particularly inflammation driven by T lymphocytes and Langerhans cells, also is believed to play a key role in the formation of cSCCs, including the degeneration of actinic keratosis into cSCC. Moreover, because keratoacanthomas are believed to be a cSCC variant and also are associated with PD-L1 overexpression,9 it is perplexing that PD-1 blockade may result in eruptive keratoacanthoma in some patients while also treating locally advanced cSCC, as seen in our patient. Successful treatment of keratoacanthoma with anti-inflammatory intralesional or topical corticosteroids adds to this complicated picture.3
We hypothesize that the pathogenesis of invasive cSCC and keratoacanthoma shares certain immune-mediated mechanisms but also differs in distinct manners. To understand the relationship between systemic treatment of cSCC and eruptive keratoacanthoma, further research is required.
In addition, the RAS/BRAF/MEK oncogenic pathway may be involved in the development of cSCCs associated with anti-PD-1. It is hypothesized that BRAF and MEK inhibition increases T-cell infiltration and increases PD-L1 expression on tumor cells,11 thus increasing the susceptibility of those cells to PD-1 blockade. Further supporting a relationship between the RAS/BRAF/MEK and PD-1 pathways, BRAF inhibitors are associated with development of SCCs and verrucal keratosis by upregulation of the RAS pathway.12,13 Perhaps a common mechanism underlying these pathways results in their shared association for an increased risk for cSCC upon blockade. More research is needed to fully elucidate the underlying biochemical mechanism of immunotherapy and formation of SCCs, such as keratoacanthoma.
Treatment of solitary keratoacanthoma often involves surgical excision; however, the sheer number of lesions in eruptive keratoacanthoma presents a larger dilemma. Because oral systemic retinoids have been shown to be most effective for treating eruptive keratoacanthoma, they are considered first-line therapy as monotherapy or in combination with surgical excision.3 Other treatment options include intralesional or topical corticosteroids, cyclosporine, 5-fluorouracil, imiquimod, and cryotherapy.3,6
The development of ICIs has revolutionized the treatment of cutaneous malignancy, yet we have a great deal more to comprehend on the systemic effects of these medications. Although IRAEs may signal a better response to therapy, some of these effects regrettably can be dose limiting. In our patient, cemiplimab was successful in treating locally advanced cSCC, but treatment also resulted in devastating widespread eruptive keratoacanthoma. The mechanism of this kind of eruption has yet to be understood; we hypothesize that it likely involves T lymphocyte–driven inflammation and the interplay of molecular and immune-mediated pathways.
- Ramos-Casals M, Brahmer JR, Callahan MK, et al. Immune-related adverse events of checkpoint inhibitors. Nat Rev Dis Primers. 2020;6:38. doi:10.1038/s41572-020-0160-6
- Das S, Johnson DB. Immune-related adverse events and anti-tumor efficacy of immune checkpoint inhibitors. J Immunother Cancer. 2019;7:306. doi:10.1186/s40425-019-0805-8
- Freites-Martinez A, Kwong BY, Rieger KE, et al. Eruptive keratoacanthomas associated with pembrolizumab therapy. JAMA Dermatol. 2017;153:694-697. doi:10.1001/jamadermatol.2017.0989
- Shen J, Chang J, Mendenhall M, et al. Diverse cutaneous adverse eruptions caused by anti-programmed cell death-1 (PD-1) and anti-programmed cell death ligand-1 (PD-L1) immunotherapies: clinicalfeatures and management. Ther Adv Med Oncol. 2018;10:1758834017751634. doi:10.1177/1758834017751634
- Bandino JP, Perry DM, Clarke CE, et al. Two cases of anti-programmed cell death 1-associated bullous pemphigoid-like disease and eruptive keratoacanthomas featuring combined histopathology. J Eur Acad Dermatol Venereol. 2017;31:E378-E380. doi:10.1111/jdv.14179
- Marsh RL, Kolodney JA, Iyengar S, et al. Formation of eruptive cutaneous squamous cell carcinomas after programmed cell death protein-1 blockade. JAAD Case Rep. 2020;6:390-393. doi:10.1016/j.jdcr.2020.02.024
- Antonov NK, Nair KG, Halasz CL. Transient eruptive keratoacanthomas associated with nivolumab. JAAD Case Rep. 2019;5:342-345. doi:10.1016/j.jdcr.2019.01.025
- Bottomley MJ, Thomson J, Harwood C, et al. The role of the immune system in cutaneous squamous cell carcinoma. Int J Mol Sci. 2019;20:2009. doi:10.3390/ijms20082009
- Gambichler T, Gnielka M, Rüddel I, et al. Expression of PD-L1 in keratoacanthoma and different stages of progression in cutaneous squamous cell carcinoma. Cancer Immunol Immunother. 2017;66:1199-1204. doi:10.1007/s00262-017-2015-x
- Patel R, Chang ALS. Immune checkpoint inhibitors for treating advanced cutaneous squamous cell carcinoma. Am J Clin Dermatol. 2019;20:477-482. doi:10.1007/s40257-019-00426-w
- Rozeman EA, Blank CU. Combining checkpoint inhibition and targeted therapy in melanoma. Nat Med. 2019;25:879-882. doi:10.1038/s41591-019-0482-7
- Dubauskas Z, Kunishige J, Prieto VG, Jonasch E, Hwu P, Tannir NM. Cutaneous squamous cell carcinoma and inflammation of actinic keratoses associated with sorafenib. Clin Genitourin Cancer. 2009;7:20-23. doi:10.3816/CGC.2009.n.003
- Chen P, Chen F, Zhou B. Systematic review and meta-analysis of prevalence of dermatological toxicities associated with vemurafenib treatment in patients with melanoma. Clin Exp Dermatol. 2019;44:243-251. doi:10.1111/ced.13751
To the Editor:
Treatment of cancer, including cutaneous malignancy, has been transformed by the use of immunotherapeutic agents such as immune checkpoint inhibitors (ICIs) that target cytotoxic T lymphocyte-associated antigen 4, programmed cell-death protein 1 (PD-1), or programmed cell-death ligand 1 (PD-L1). However, these drugs are associated with a distinct set of immune-related adverse events (IRAEs). We present a case of generalized eruptive keratoacanthoma of Grzybowski associated with the ICI cemiplimab.
A 94-year-old White woman presented to the dermatology clinic with acute onset of extensive, locally advanced cutaneous squamous cell carcinoma (cSCC) of the upper right posterolateral calf as well as multiple noninvasive cSCCs of the arms and legs. Her medical history was remarkable for widespread actinic keratoses and numerous cSCCs. The patient had no personal or family history of melanoma. Various cSCCs had required treatment with electrodesiccation and curettage, topical or intralesional 5-fluorouracil, and Mohs micrographic surgery. Approximately 1 year prior to presentation, oral acitretin was initiated to help control the cSCC. Given the extent of locally advanced disease, which was considered unresectable, she was referred to oncology but continued to follow up with dermatology. Positron emission tomography was remarkable for hypermetabolic cutaneous thickening in the upper right posterolateral calf with no evidence of visceral disease.

The patient was started on cemiplimab, an anti-PD-1 monoclonal antibody ICI indicated for the treatment of both metastatic and advanced cSCC. After 4 cycles of intravenous cemiplimab, the patient developed widespread nodules covering the arms and legs (Figure 1) as well as associated tenderness and pruritus. Biopsies of nodules revealed superficially invasive, well-differentiated cSCC consistent with keratoacanthoma. Although a lymphocytic infiltrate was present, no other specific reaction pattern, such as a lichenoid infiltrate, was present (Figure 2).

Positron emission tomography was repeated, demonstrating resolution of the right calf lesion; however, new diffuse cutaneous lesions and inguinal lymph node involvement were present, again without evidence of visceral disease. Given the clinical and histologic findings, a diagnosis of generalized eruptive keratoacanthoma of Grzybowski was made. Cemiplimab was discontinued after the fifth cycle. The patient declined further systemic treatment, instead choosing a regimen of topical steroids and an emollient.
Immunotherapeutics have transformed cancer therapy, which includes ICIs that target cytotoxic T lymphocyte-associated antigen 4, PD-1, or PD-L1. Increased activity of these checkpoints allows tumor cells to downregulate T-cell activation, thereby evading immune destruction. When PD-1 on T cells binds PD-L1 on tumor cells, T lymphocytes are inhibited from cytotoxic-mediated killing. Therefore, anti-PD-1 ICIs such as cemiplimab permit T-lymphocyte activation and destruction of malignant cells. However, this unique mechanism of immunotherapy is associated with an array of IRAEs, which often manifest in a delayed and prolonged fashion.1 Immune-related adverse events most commonly affect the gastrointestinal tract as well as the endocrine and dermatologic systems.2 Notably, patients with certain tumors who experience these adverse effects might be more likely to have superior overall survival; therefore, IRAEs are sometimes used as an indicator of favorable treatment response.2,3
Dermatologic IRAEs associated with the use of a PD-1 inhibitor include lichenoid reactions, pruritus, morbilliform eruptions, vitiligo, and bullous pemphigoid.4,5 Eruptions of keratoacanthoma rarely have been reported following treatment with the PD-1 inhibitors nivolumab and pembrolizumab.3,6,7 In our patient, we believe the profound and generalized eruptive keratoacanthoma—a well-differentiated cSCC variant—was related to treatment of locally advanced cSCC with cemiplimab. The mechanism underlying the formation of anti-PD-1 eruptive keratoacanthoma is not well understood. In susceptible patients, it is plausible that the inflammatory environment permitted by ICIs paradoxically induces regression of tumors such as locally invasive cSCC and simultaneously promotes formation of keratoacanthoma.
The role of inflammation in the pathogenesis and progression of cSCC is complex and possibly involves contrasting roles of leukocyte subpopulations.8 The increased incidence of cSCC in the immunocompromised population,8 PD-L1 overexpression in cSCC,9,10 and successful treatment of cSCC with PD-1 inhibition10 all suggest that inhibition of specific inflammatory pathways is pivotal in tumor pathogenesis. However, increased inflammation, particularly inflammation driven by T lymphocytes and Langerhans cells, also is believed to play a key role in the formation of cSCCs, including the degeneration of actinic keratosis into cSCC. Moreover, because keratoacanthomas are believed to be a cSCC variant and also are associated with PD-L1 overexpression,9 it is perplexing that PD-1 blockade may result in eruptive keratoacanthoma in some patients while also treating locally advanced cSCC, as seen in our patient. Successful treatment of keratoacanthoma with anti-inflammatory intralesional or topical corticosteroids adds to this complicated picture.3
We hypothesize that the pathogenesis of invasive cSCC and keratoacanthoma shares certain immune-mediated mechanisms but also differs in distinct manners. To understand the relationship between systemic treatment of cSCC and eruptive keratoacanthoma, further research is required.
In addition, the RAS/BRAF/MEK oncogenic pathway may be involved in the development of cSCCs associated with anti-PD-1. It is hypothesized that BRAF and MEK inhibition increases T-cell infiltration and increases PD-L1 expression on tumor cells,11 thus increasing the susceptibility of those cells to PD-1 blockade. Further supporting a relationship between the RAS/BRAF/MEK and PD-1 pathways, BRAF inhibitors are associated with development of SCCs and verrucal keratosis by upregulation of the RAS pathway.12,13 Perhaps a common mechanism underlying these pathways results in their shared association for an increased risk for cSCC upon blockade. More research is needed to fully elucidate the underlying biochemical mechanism of immunotherapy and formation of SCCs, such as keratoacanthoma.
Treatment of solitary keratoacanthoma often involves surgical excision; however, the sheer number of lesions in eruptive keratoacanthoma presents a larger dilemma. Because oral systemic retinoids have been shown to be most effective for treating eruptive keratoacanthoma, they are considered first-line therapy as monotherapy or in combination with surgical excision.3 Other treatment options include intralesional or topical corticosteroids, cyclosporine, 5-fluorouracil, imiquimod, and cryotherapy.3,6
The development of ICIs has revolutionized the treatment of cutaneous malignancy, yet we have a great deal more to comprehend on the systemic effects of these medications. Although IRAEs may signal a better response to therapy, some of these effects regrettably can be dose limiting. In our patient, cemiplimab was successful in treating locally advanced cSCC, but treatment also resulted in devastating widespread eruptive keratoacanthoma. The mechanism of this kind of eruption has yet to be understood; we hypothesize that it likely involves T lymphocyte–driven inflammation and the interplay of molecular and immune-mediated pathways.
To the Editor:
Treatment of cancer, including cutaneous malignancy, has been transformed by the use of immunotherapeutic agents such as immune checkpoint inhibitors (ICIs) that target cytotoxic T lymphocyte-associated antigen 4, programmed cell-death protein 1 (PD-1), or programmed cell-death ligand 1 (PD-L1). However, these drugs are associated with a distinct set of immune-related adverse events (IRAEs). We present a case of generalized eruptive keratoacanthoma of Grzybowski associated with the ICI cemiplimab.
A 94-year-old White woman presented to the dermatology clinic with acute onset of extensive, locally advanced cutaneous squamous cell carcinoma (cSCC) of the upper right posterolateral calf as well as multiple noninvasive cSCCs of the arms and legs. Her medical history was remarkable for widespread actinic keratoses and numerous cSCCs. The patient had no personal or family history of melanoma. Various cSCCs had required treatment with electrodesiccation and curettage, topical or intralesional 5-fluorouracil, and Mohs micrographic surgery. Approximately 1 year prior to presentation, oral acitretin was initiated to help control the cSCC. Given the extent of locally advanced disease, which was considered unresectable, she was referred to oncology but continued to follow up with dermatology. Positron emission tomography was remarkable for hypermetabolic cutaneous thickening in the upper right posterolateral calf with no evidence of visceral disease.

The patient was started on cemiplimab, an anti-PD-1 monoclonal antibody ICI indicated for the treatment of both metastatic and advanced cSCC. After 4 cycles of intravenous cemiplimab, the patient developed widespread nodules covering the arms and legs (Figure 1) as well as associated tenderness and pruritus. Biopsies of nodules revealed superficially invasive, well-differentiated cSCC consistent with keratoacanthoma. Although a lymphocytic infiltrate was present, no other specific reaction pattern, such as a lichenoid infiltrate, was present (Figure 2).

Positron emission tomography was repeated, demonstrating resolution of the right calf lesion; however, new diffuse cutaneous lesions and inguinal lymph node involvement were present, again without evidence of visceral disease. Given the clinical and histologic findings, a diagnosis of generalized eruptive keratoacanthoma of Grzybowski was made. Cemiplimab was discontinued after the fifth cycle. The patient declined further systemic treatment, instead choosing a regimen of topical steroids and an emollient.
Immunotherapeutics have transformed cancer therapy, which includes ICIs that target cytotoxic T lymphocyte-associated antigen 4, PD-1, or PD-L1. Increased activity of these checkpoints allows tumor cells to downregulate T-cell activation, thereby evading immune destruction. When PD-1 on T cells binds PD-L1 on tumor cells, T lymphocytes are inhibited from cytotoxic-mediated killing. Therefore, anti-PD-1 ICIs such as cemiplimab permit T-lymphocyte activation and destruction of malignant cells. However, this unique mechanism of immunotherapy is associated with an array of IRAEs, which often manifest in a delayed and prolonged fashion.1 Immune-related adverse events most commonly affect the gastrointestinal tract as well as the endocrine and dermatologic systems.2 Notably, patients with certain tumors who experience these adverse effects might be more likely to have superior overall survival; therefore, IRAEs are sometimes used as an indicator of favorable treatment response.2,3
Dermatologic IRAEs associated with the use of a PD-1 inhibitor include lichenoid reactions, pruritus, morbilliform eruptions, vitiligo, and bullous pemphigoid.4,5 Eruptions of keratoacanthoma rarely have been reported following treatment with the PD-1 inhibitors nivolumab and pembrolizumab.3,6,7 In our patient, we believe the profound and generalized eruptive keratoacanthoma—a well-differentiated cSCC variant—was related to treatment of locally advanced cSCC with cemiplimab. The mechanism underlying the formation of anti-PD-1 eruptive keratoacanthoma is not well understood. In susceptible patients, it is plausible that the inflammatory environment permitted by ICIs paradoxically induces regression of tumors such as locally invasive cSCC and simultaneously promotes formation of keratoacanthoma.
The role of inflammation in the pathogenesis and progression of cSCC is complex and possibly involves contrasting roles of leukocyte subpopulations.8 The increased incidence of cSCC in the immunocompromised population,8 PD-L1 overexpression in cSCC,9,10 and successful treatment of cSCC with PD-1 inhibition10 all suggest that inhibition of specific inflammatory pathways is pivotal in tumor pathogenesis. However, increased inflammation, particularly inflammation driven by T lymphocytes and Langerhans cells, also is believed to play a key role in the formation of cSCCs, including the degeneration of actinic keratosis into cSCC. Moreover, because keratoacanthomas are believed to be a cSCC variant and also are associated with PD-L1 overexpression,9 it is perplexing that PD-1 blockade may result in eruptive keratoacanthoma in some patients while also treating locally advanced cSCC, as seen in our patient. Successful treatment of keratoacanthoma with anti-inflammatory intralesional or topical corticosteroids adds to this complicated picture.3
We hypothesize that the pathogenesis of invasive cSCC and keratoacanthoma shares certain immune-mediated mechanisms but also differs in distinct manners. To understand the relationship between systemic treatment of cSCC and eruptive keratoacanthoma, further research is required.
In addition, the RAS/BRAF/MEK oncogenic pathway may be involved in the development of cSCCs associated with anti-PD-1. It is hypothesized that BRAF and MEK inhibition increases T-cell infiltration and increases PD-L1 expression on tumor cells,11 thus increasing the susceptibility of those cells to PD-1 blockade. Further supporting a relationship between the RAS/BRAF/MEK and PD-1 pathways, BRAF inhibitors are associated with development of SCCs and verrucal keratosis by upregulation of the RAS pathway.12,13 Perhaps a common mechanism underlying these pathways results in their shared association for an increased risk for cSCC upon blockade. More research is needed to fully elucidate the underlying biochemical mechanism of immunotherapy and formation of SCCs, such as keratoacanthoma.
Treatment of solitary keratoacanthoma often involves surgical excision; however, the sheer number of lesions in eruptive keratoacanthoma presents a larger dilemma. Because oral systemic retinoids have been shown to be most effective for treating eruptive keratoacanthoma, they are considered first-line therapy as monotherapy or in combination with surgical excision.3 Other treatment options include intralesional or topical corticosteroids, cyclosporine, 5-fluorouracil, imiquimod, and cryotherapy.3,6
The development of ICIs has revolutionized the treatment of cutaneous malignancy, yet we have a great deal more to comprehend on the systemic effects of these medications. Although IRAEs may signal a better response to therapy, some of these effects regrettably can be dose limiting. In our patient, cemiplimab was successful in treating locally advanced cSCC, but treatment also resulted in devastating widespread eruptive keratoacanthoma. The mechanism of this kind of eruption has yet to be understood; we hypothesize that it likely involves T lymphocyte–driven inflammation and the interplay of molecular and immune-mediated pathways.
- Ramos-Casals M, Brahmer JR, Callahan MK, et al. Immune-related adverse events of checkpoint inhibitors. Nat Rev Dis Primers. 2020;6:38. doi:10.1038/s41572-020-0160-6
- Das S, Johnson DB. Immune-related adverse events and anti-tumor efficacy of immune checkpoint inhibitors. J Immunother Cancer. 2019;7:306. doi:10.1186/s40425-019-0805-8
- Freites-Martinez A, Kwong BY, Rieger KE, et al. Eruptive keratoacanthomas associated with pembrolizumab therapy. JAMA Dermatol. 2017;153:694-697. doi:10.1001/jamadermatol.2017.0989
- Shen J, Chang J, Mendenhall M, et al. Diverse cutaneous adverse eruptions caused by anti-programmed cell death-1 (PD-1) and anti-programmed cell death ligand-1 (PD-L1) immunotherapies: clinicalfeatures and management. Ther Adv Med Oncol. 2018;10:1758834017751634. doi:10.1177/1758834017751634
- Bandino JP, Perry DM, Clarke CE, et al. Two cases of anti-programmed cell death 1-associated bullous pemphigoid-like disease and eruptive keratoacanthomas featuring combined histopathology. J Eur Acad Dermatol Venereol. 2017;31:E378-E380. doi:10.1111/jdv.14179
- Marsh RL, Kolodney JA, Iyengar S, et al. Formation of eruptive cutaneous squamous cell carcinomas after programmed cell death protein-1 blockade. JAAD Case Rep. 2020;6:390-393. doi:10.1016/j.jdcr.2020.02.024
- Antonov NK, Nair KG, Halasz CL. Transient eruptive keratoacanthomas associated with nivolumab. JAAD Case Rep. 2019;5:342-345. doi:10.1016/j.jdcr.2019.01.025
- Bottomley MJ, Thomson J, Harwood C, et al. The role of the immune system in cutaneous squamous cell carcinoma. Int J Mol Sci. 2019;20:2009. doi:10.3390/ijms20082009
- Gambichler T, Gnielka M, Rüddel I, et al. Expression of PD-L1 in keratoacanthoma and different stages of progression in cutaneous squamous cell carcinoma. Cancer Immunol Immunother. 2017;66:1199-1204. doi:10.1007/s00262-017-2015-x
- Patel R, Chang ALS. Immune checkpoint inhibitors for treating advanced cutaneous squamous cell carcinoma. Am J Clin Dermatol. 2019;20:477-482. doi:10.1007/s40257-019-00426-w
- Rozeman EA, Blank CU. Combining checkpoint inhibition and targeted therapy in melanoma. Nat Med. 2019;25:879-882. doi:10.1038/s41591-019-0482-7
- Dubauskas Z, Kunishige J, Prieto VG, Jonasch E, Hwu P, Tannir NM. Cutaneous squamous cell carcinoma and inflammation of actinic keratoses associated with sorafenib. Clin Genitourin Cancer. 2009;7:20-23. doi:10.3816/CGC.2009.n.003
- Chen P, Chen F, Zhou B. Systematic review and meta-analysis of prevalence of dermatological toxicities associated with vemurafenib treatment in patients with melanoma. Clin Exp Dermatol. 2019;44:243-251. doi:10.1111/ced.13751
- Ramos-Casals M, Brahmer JR, Callahan MK, et al. Immune-related adverse events of checkpoint inhibitors. Nat Rev Dis Primers. 2020;6:38. doi:10.1038/s41572-020-0160-6
- Das S, Johnson DB. Immune-related adverse events and anti-tumor efficacy of immune checkpoint inhibitors. J Immunother Cancer. 2019;7:306. doi:10.1186/s40425-019-0805-8
- Freites-Martinez A, Kwong BY, Rieger KE, et al. Eruptive keratoacanthomas associated with pembrolizumab therapy. JAMA Dermatol. 2017;153:694-697. doi:10.1001/jamadermatol.2017.0989
- Shen J, Chang J, Mendenhall M, et al. Diverse cutaneous adverse eruptions caused by anti-programmed cell death-1 (PD-1) and anti-programmed cell death ligand-1 (PD-L1) immunotherapies: clinicalfeatures and management. Ther Adv Med Oncol. 2018;10:1758834017751634. doi:10.1177/1758834017751634
- Bandino JP, Perry DM, Clarke CE, et al. Two cases of anti-programmed cell death 1-associated bullous pemphigoid-like disease and eruptive keratoacanthomas featuring combined histopathology. J Eur Acad Dermatol Venereol. 2017;31:E378-E380. doi:10.1111/jdv.14179
- Marsh RL, Kolodney JA, Iyengar S, et al. Formation of eruptive cutaneous squamous cell carcinomas after programmed cell death protein-1 blockade. JAAD Case Rep. 2020;6:390-393. doi:10.1016/j.jdcr.2020.02.024
- Antonov NK, Nair KG, Halasz CL. Transient eruptive keratoacanthomas associated with nivolumab. JAAD Case Rep. 2019;5:342-345. doi:10.1016/j.jdcr.2019.01.025
- Bottomley MJ, Thomson J, Harwood C, et al. The role of the immune system in cutaneous squamous cell carcinoma. Int J Mol Sci. 2019;20:2009. doi:10.3390/ijms20082009
- Gambichler T, Gnielka M, Rüddel I, et al. Expression of PD-L1 in keratoacanthoma and different stages of progression in cutaneous squamous cell carcinoma. Cancer Immunol Immunother. 2017;66:1199-1204. doi:10.1007/s00262-017-2015-x
- Patel R, Chang ALS. Immune checkpoint inhibitors for treating advanced cutaneous squamous cell carcinoma. Am J Clin Dermatol. 2019;20:477-482. doi:10.1007/s40257-019-00426-w
- Rozeman EA, Blank CU. Combining checkpoint inhibition and targeted therapy in melanoma. Nat Med. 2019;25:879-882. doi:10.1038/s41591-019-0482-7
- Dubauskas Z, Kunishige J, Prieto VG, Jonasch E, Hwu P, Tannir NM. Cutaneous squamous cell carcinoma and inflammation of actinic keratoses associated with sorafenib. Clin Genitourin Cancer. 2009;7:20-23. doi:10.3816/CGC.2009.n.003
- Chen P, Chen F, Zhou B. Systematic review and meta-analysis of prevalence of dermatological toxicities associated with vemurafenib treatment in patients with melanoma. Clin Exp Dermatol. 2019;44:243-251. doi:10.1111/ced.13751
Practice Points
- Immunotherapy, including immune checkpoint inhibitors such as programmed cell-death protein 1 (PD-1) inhibitors, is associated with an array of immune-related adverse events that often manifest in a delayed and prolonged manner. They most commonly affect the gastrointestinal tract as well as the endocrine and dermatologic systems.
- Dermatologic adverse effects associated with PD-1 inhibitors include lichenoid reactions, pruritus, morbilliform eruptions, vitiligo, and bullous pemphigoid.
- Eruptions of keratoacanthoma rarely have been reported following treatment with PD-1 inhibitors such as cemiplimab, nivolumab, and pembrolizumab.
Nasal Tanning Sprays: Illuminating the Risks of a Popular TikTok Trend
Nasal tanning spray is a recent phenomenon that has been gaining popularity among consumers on TikTok and other social media platforms. The active ingredient in the tanning spray is melanotan II—a synthetic analog of α‒melanocyte-stimulating hormone,1,2 a naturally occurring hormone responsible for skin pigmentation. α‒Melanocyte-stimulating hormone is a derivative of the precursor proopiomelanocortin, an agonist on the melanocortin-1 receptor that promotes formation of eumelanin.1,3 Eumelanin then provides pigmentation to the skin.3 Apart from its use for tanning, melanotan II has been reported to increase sexual function and aid in weight loss.1
Melanotan II is not approved by the US Food and Drug Administration; however, injectable formulations can be obtained illegally on the Internet as well as at some tanning salons and beauty parlors.4 Although injectable forms of melanotan II have been used for years to artificially increase skin pigmentation, the newly hyped nasal tanning sprays are drawing the attention of consumers. The synthetic chemical spray is inhaled into the nasal mucosae, where it is readily absorbed into the bloodstream to act on melanocortin receptors throughout the body, thus enhancing skin pigmentation.2 Because melanotan II is not approved, there is no guarantee that the product purchased from those sources is pure; therefore, consumers risk inhaling or injecting contaminated chemicals.5
In a 2017 study, Kirk and Greenfield6 cited self-image as a common concern among participants who expressed a preference for appearing tanned.6 Societal influence and standards to which young adults, particularly young women, often are accustomed drive some to take steps to achieve tanned skin, which they view as more attractive and healthier than untanned skin.7,8
Social media consumption is a significant risk factor for developing or exacerbating body dissatisfaction among impressionable teenagers and young adults, who may be less risk averse and therefore choose to embrace trends such as nasal tanning sprays to enhance their appearance, without considering possible consequences. Most young adults, and even teens, are aware of the risks associated with tanning beds, which may propel them to seek out what they perceive as a less-risky tanning alternative such as a tanner delivered via a nasal route, but it is unlikely that this group is fully informed about the possible dangers of nasal tanning sprays.
It is crucial for dermatologists and other clinicians to provide awareness and education about the potential harm of nasal tanning sprays. Along with the general risks of using an unregulated substance, common adverse effects include acne, facial flushing, gastrointestinal tract upset, and sensitivity to sunlight (Table).1,9,10 Several case reports have linked melanotan II to cutaneous changes, including dysplastic nevi and even melanoma.1 Less common complications, such as renal infarction and priapism, also have been observed with melanotan II use.9,10

Even with the known risks involving tanning beds and skin cancer, an analysis by Kream et al11 in 2020 showed that 90% (441/488) of tanning-related videos on TikTok promoted a positive view of tanning. Of these TikTok videos involving pro-tanning trends, 3% (12/441) were specifically about melanotan II nasal spray, injection, or both, which has only become more popular since this study was published.11
Dermatologists should be aware of the impact that tanning trends, such as nasal tanning spray, can have on all patients and initiate discussions regarding the risks of using these products with patients as appropriate. Alternatives to nasal tanning sprays such as spray-on tans and self-tanning lotions are safer ways for patients to achieve a tanned look without the health risks associated with melanotan II.
- Habbema L, Halk AB, Neumann M, et al. Risks of unregulated use of alpha-melanocyte-stimulating hormone analogues: a review. Int J Dermatol. 2017;56:975-980. doi:10.1111/ijd.13585
- Why you should never use nasal tanning spray. Cleveland Clinic Health Essentials [Internet]. November 1, 2022. Accessed December 18, 2023. https://health.clevelandclinic.org/nasal-tanning-spray
- Hjuler KF, Lorentzen HF. Melanoma associated with the use of melanotan-II. Dermatology. 2014;228:34-36. doi:10.1159/000356389
- Evans-Brown M, Dawson RT, Chandler M, et al. Use of melanotan I and II in the general population. BMJ. 2009;338:b566. doi:10.116/bmj.b566
- Callaghan DJ III. A glimpse into the underground market of melanotan. Dermatol Online J. 2018;24:1-5. doi:10.5070/D3245040036
- Kirk L, Greenfield S. Knowledge and attitudes of UK university students in relation to ultraviolet radiation (UVR) exposure and their sun-related behaviours: a qualitative study. BMJ Open. 2017;7:e014388. doi:10.1136/bmjopen-2016-014388
- Hay JL, Geller AC, Schoenhammer M, et al. Tanning and beauty: mother and teenage daughters in discussion. J Health Psychol. 2016;21:1261-1270. doi:10.1177/1359105314551621
- Gillen MM, Markey CN. The role of body image and depression in tanning behaviors and attitudes. Behav Med. 2017;38:74-82.
- Peters B, Hadimeri H, Wahlberg R, et al. Melanotan II: a possible cause of renal infarction: review of the literature and case report. CEN Case Rep. 2020;9:159-161. doi:10.1007/s13730-020-00447-z
- Mallory CW, Lopategui DM, Cordon BH. Melanotan tanning injection: a rare cause of priapism. Sex Med. 2021;9:100298. doi:10.1016/j.esxm.2020.100298
- Kream E, Watchmaker JD, Dover JS. TikTok sheds light on tanning: tanning is still popular and emerging trends pose new risks. Dermatol Surg. 2022;48:1018-1021. doi:10.1097/DSS.0000000000003549
Nasal tanning spray is a recent phenomenon that has been gaining popularity among consumers on TikTok and other social media platforms. The active ingredient in the tanning spray is melanotan II—a synthetic analog of α‒melanocyte-stimulating hormone,1,2 a naturally occurring hormone responsible for skin pigmentation. α‒Melanocyte-stimulating hormone is a derivative of the precursor proopiomelanocortin, an agonist on the melanocortin-1 receptor that promotes formation of eumelanin.1,3 Eumelanin then provides pigmentation to the skin.3 Apart from its use for tanning, melanotan II has been reported to increase sexual function and aid in weight loss.1
Melanotan II is not approved by the US Food and Drug Administration; however, injectable formulations can be obtained illegally on the Internet as well as at some tanning salons and beauty parlors.4 Although injectable forms of melanotan II have been used for years to artificially increase skin pigmentation, the newly hyped nasal tanning sprays are drawing the attention of consumers. The synthetic chemical spray is inhaled into the nasal mucosae, where it is readily absorbed into the bloodstream to act on melanocortin receptors throughout the body, thus enhancing skin pigmentation.2 Because melanotan II is not approved, there is no guarantee that the product purchased from those sources is pure; therefore, consumers risk inhaling or injecting contaminated chemicals.5
In a 2017 study, Kirk and Greenfield6 cited self-image as a common concern among participants who expressed a preference for appearing tanned.6 Societal influence and standards to which young adults, particularly young women, often are accustomed drive some to take steps to achieve tanned skin, which they view as more attractive and healthier than untanned skin.7,8
Social media consumption is a significant risk factor for developing or exacerbating body dissatisfaction among impressionable teenagers and young adults, who may be less risk averse and therefore choose to embrace trends such as nasal tanning sprays to enhance their appearance, without considering possible consequences. Most young adults, and even teens, are aware of the risks associated with tanning beds, which may propel them to seek out what they perceive as a less-risky tanning alternative such as a tanner delivered via a nasal route, but it is unlikely that this group is fully informed about the possible dangers of nasal tanning sprays.
It is crucial for dermatologists and other clinicians to provide awareness and education about the potential harm of nasal tanning sprays. Along with the general risks of using an unregulated substance, common adverse effects include acne, facial flushing, gastrointestinal tract upset, and sensitivity to sunlight (Table).1,9,10 Several case reports have linked melanotan II to cutaneous changes, including dysplastic nevi and even melanoma.1 Less common complications, such as renal infarction and priapism, also have been observed with melanotan II use.9,10

Even with the known risks involving tanning beds and skin cancer, an analysis by Kream et al11 in 2020 showed that 90% (441/488) of tanning-related videos on TikTok promoted a positive view of tanning. Of these TikTok videos involving pro-tanning trends, 3% (12/441) were specifically about melanotan II nasal spray, injection, or both, which has only become more popular since this study was published.11
Dermatologists should be aware of the impact that tanning trends, such as nasal tanning spray, can have on all patients and initiate discussions regarding the risks of using these products with patients as appropriate. Alternatives to nasal tanning sprays such as spray-on tans and self-tanning lotions are safer ways for patients to achieve a tanned look without the health risks associated with melanotan II.
Nasal tanning spray is a recent phenomenon that has been gaining popularity among consumers on TikTok and other social media platforms. The active ingredient in the tanning spray is melanotan II—a synthetic analog of α‒melanocyte-stimulating hormone,1,2 a naturally occurring hormone responsible for skin pigmentation. α‒Melanocyte-stimulating hormone is a derivative of the precursor proopiomelanocortin, an agonist on the melanocortin-1 receptor that promotes formation of eumelanin.1,3 Eumelanin then provides pigmentation to the skin.3 Apart from its use for tanning, melanotan II has been reported to increase sexual function and aid in weight loss.1
Melanotan II is not approved by the US Food and Drug Administration; however, injectable formulations can be obtained illegally on the Internet as well as at some tanning salons and beauty parlors.4 Although injectable forms of melanotan II have been used for years to artificially increase skin pigmentation, the newly hyped nasal tanning sprays are drawing the attention of consumers. The synthetic chemical spray is inhaled into the nasal mucosae, where it is readily absorbed into the bloodstream to act on melanocortin receptors throughout the body, thus enhancing skin pigmentation.2 Because melanotan II is not approved, there is no guarantee that the product purchased from those sources is pure; therefore, consumers risk inhaling or injecting contaminated chemicals.5
In a 2017 study, Kirk and Greenfield6 cited self-image as a common concern among participants who expressed a preference for appearing tanned.6 Societal influence and standards to which young adults, particularly young women, often are accustomed drive some to take steps to achieve tanned skin, which they view as more attractive and healthier than untanned skin.7,8
Social media consumption is a significant risk factor for developing or exacerbating body dissatisfaction among impressionable teenagers and young adults, who may be less risk averse and therefore choose to embrace trends such as nasal tanning sprays to enhance their appearance, without considering possible consequences. Most young adults, and even teens, are aware of the risks associated with tanning beds, which may propel them to seek out what they perceive as a less-risky tanning alternative such as a tanner delivered via a nasal route, but it is unlikely that this group is fully informed about the possible dangers of nasal tanning sprays.
It is crucial for dermatologists and other clinicians to provide awareness and education about the potential harm of nasal tanning sprays. Along with the general risks of using an unregulated substance, common adverse effects include acne, facial flushing, gastrointestinal tract upset, and sensitivity to sunlight (Table).1,9,10 Several case reports have linked melanotan II to cutaneous changes, including dysplastic nevi and even melanoma.1 Less common complications, such as renal infarction and priapism, also have been observed with melanotan II use.9,10

Even with the known risks involving tanning beds and skin cancer, an analysis by Kream et al11 in 2020 showed that 90% (441/488) of tanning-related videos on TikTok promoted a positive view of tanning. Of these TikTok videos involving pro-tanning trends, 3% (12/441) were specifically about melanotan II nasal spray, injection, or both, which has only become more popular since this study was published.11
Dermatologists should be aware of the impact that tanning trends, such as nasal tanning spray, can have on all patients and initiate discussions regarding the risks of using these products with patients as appropriate. Alternatives to nasal tanning sprays such as spray-on tans and self-tanning lotions are safer ways for patients to achieve a tanned look without the health risks associated with melanotan II.
- Habbema L, Halk AB, Neumann M, et al. Risks of unregulated use of alpha-melanocyte-stimulating hormone analogues: a review. Int J Dermatol. 2017;56:975-980. doi:10.1111/ijd.13585
- Why you should never use nasal tanning spray. Cleveland Clinic Health Essentials [Internet]. November 1, 2022. Accessed December 18, 2023. https://health.clevelandclinic.org/nasal-tanning-spray
- Hjuler KF, Lorentzen HF. Melanoma associated with the use of melanotan-II. Dermatology. 2014;228:34-36. doi:10.1159/000356389
- Evans-Brown M, Dawson RT, Chandler M, et al. Use of melanotan I and II in the general population. BMJ. 2009;338:b566. doi:10.116/bmj.b566
- Callaghan DJ III. A glimpse into the underground market of melanotan. Dermatol Online J. 2018;24:1-5. doi:10.5070/D3245040036
- Kirk L, Greenfield S. Knowledge and attitudes of UK university students in relation to ultraviolet radiation (UVR) exposure and their sun-related behaviours: a qualitative study. BMJ Open. 2017;7:e014388. doi:10.1136/bmjopen-2016-014388
- Hay JL, Geller AC, Schoenhammer M, et al. Tanning and beauty: mother and teenage daughters in discussion. J Health Psychol. 2016;21:1261-1270. doi:10.1177/1359105314551621
- Gillen MM, Markey CN. The role of body image and depression in tanning behaviors and attitudes. Behav Med. 2017;38:74-82.
- Peters B, Hadimeri H, Wahlberg R, et al. Melanotan II: a possible cause of renal infarction: review of the literature and case report. CEN Case Rep. 2020;9:159-161. doi:10.1007/s13730-020-00447-z
- Mallory CW, Lopategui DM, Cordon BH. Melanotan tanning injection: a rare cause of priapism. Sex Med. 2021;9:100298. doi:10.1016/j.esxm.2020.100298
- Kream E, Watchmaker JD, Dover JS. TikTok sheds light on tanning: tanning is still popular and emerging trends pose new risks. Dermatol Surg. 2022;48:1018-1021. doi:10.1097/DSS.0000000000003549
- Habbema L, Halk AB, Neumann M, et al. Risks of unregulated use of alpha-melanocyte-stimulating hormone analogues: a review. Int J Dermatol. 2017;56:975-980. doi:10.1111/ijd.13585
- Why you should never use nasal tanning spray. Cleveland Clinic Health Essentials [Internet]. November 1, 2022. Accessed December 18, 2023. https://health.clevelandclinic.org/nasal-tanning-spray
- Hjuler KF, Lorentzen HF. Melanoma associated with the use of melanotan-II. Dermatology. 2014;228:34-36. doi:10.1159/000356389
- Evans-Brown M, Dawson RT, Chandler M, et al. Use of melanotan I and II in the general population. BMJ. 2009;338:b566. doi:10.116/bmj.b566
- Callaghan DJ III. A glimpse into the underground market of melanotan. Dermatol Online J. 2018;24:1-5. doi:10.5070/D3245040036
- Kirk L, Greenfield S. Knowledge and attitudes of UK university students in relation to ultraviolet radiation (UVR) exposure and their sun-related behaviours: a qualitative study. BMJ Open. 2017;7:e014388. doi:10.1136/bmjopen-2016-014388
- Hay JL, Geller AC, Schoenhammer M, et al. Tanning and beauty: mother and teenage daughters in discussion. J Health Psychol. 2016;21:1261-1270. doi:10.1177/1359105314551621
- Gillen MM, Markey CN. The role of body image and depression in tanning behaviors and attitudes. Behav Med. 2017;38:74-82.
- Peters B, Hadimeri H, Wahlberg R, et al. Melanotan II: a possible cause of renal infarction: review of the literature and case report. CEN Case Rep. 2020;9:159-161. doi:10.1007/s13730-020-00447-z
- Mallory CW, Lopategui DM, Cordon BH. Melanotan tanning injection: a rare cause of priapism. Sex Med. 2021;9:100298. doi:10.1016/j.esxm.2020.100298
- Kream E, Watchmaker JD, Dover JS. TikTok sheds light on tanning: tanning is still popular and emerging trends pose new risks. Dermatol Surg. 2022;48:1018-1021. doi:10.1097/DSS.0000000000003549
PRACTICE POINTS
- Although tanning beds are arguably the most common and dangerous method used by patients to tan their skin, dermatologists should be aware of the other means by which patients may artificially increase skin pigmentation and the risks imposed by undertaking such practices.
- We challenge dermatologists to note the influence of social media on tanning trends and consider creating a platform on these mediums to combat misinformation and promote sun safety and skin health.
- We encourage dermatologists to diligently stay informed about the popular societal trends related to the skin such as the use of nasal tanning products (eg, melanotan I and II) and be proactive in discussing their risks with patients as deemed appropriate.
Migratory Nodules in a Traveler
The Diagnosis: Gnathostomiasis
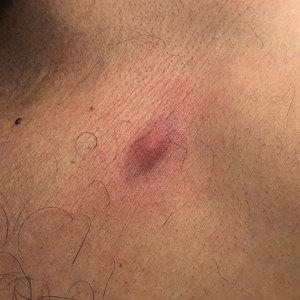
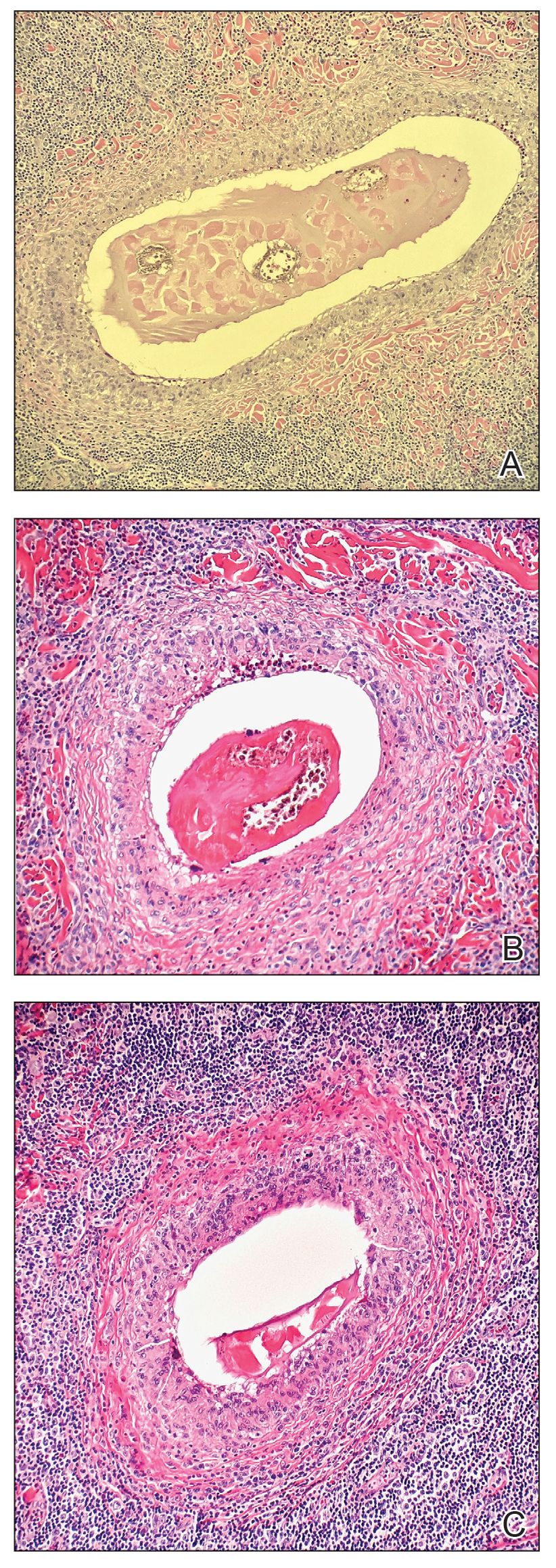
The biopsy demonstrated a dense, eosinophilic, granulomatous infiltrate surrounding sections of a parasite with skeletal muscle bundles and intestines containing a brush border and luminal debris (Figure), which was consistent with a diagnosis of gnathostomiasis. Upon further questioning, he revealed that while in Peru he frequently consumed ceviche, which is a dish typically made from fresh raw fish cured in lemon or lime juice. He subsequently was treated with oral ivermectin 0.2 mg/kg once daily for 2 days with no evidence of recurrence 12 months later.
Cutaneous gnathostomiasis is the most common manifestation of infection caused by the third-stage larvae of the genus Gnathostoma. The nematode is endemic to tropical and subtropical regions of Japan and Southeast Asia, particularly Thailand. The disease has been increasingly observed in Central and South America. Humans can become infected through ingestion of undercooked meats, particularly freshwater fish but also poultry, snakes, or frogs. Few cases have been reported in North America and Europe presumably due to more stringent regulations governing the sourcing and storage of fish for consumption.1-3 Restaurants in endemic regions also may use cheaper local freshwater or brackish fish compared to restaurants in the West, which use more expensive saltwater fish that do not harbor Gnathostoma species.1 There is a false belief among restauranteurs and consumers that the larvae can be reliably killed by marinating meat in citrus juice or with concurrent consumption of alcohol or hot spices.2 Adequately cooking or freezing meat to 20 °C for 3 to 5 days are the only effective ways to ensure that the larvae are killed.1-3
The parasite requires its natural definitive hosts—fish-eating mammals such as pigs, cats, and dogs—to complete its life cycle and reproduce. Humans are accidental hosts in whom the parasite fails to reach sexual maturity.1-3 Consequently, symptoms commonly are due to the migration of only 1 larva, but occasionally infection with 2 or more has been observed.1,4
Human infection initially may result in malaise, fever, anorexia, abdominal pain, nausea, vomiting, and diarrhea as the parasite migrates through the stomach, intestines, and liver. After 2 to 4 weeks, larvae may reach the skin where they most commonly create ill-defined, erythematous, indurated, round or oval plaques or nodules described as nodular migratory panniculitis. These lesions tend to develop on the trunk or arms and correspond to the location of the migrating worm.1,3,5 The larvae have been observed to migrate at 1 cm/h.6 Symptoms often wax and wane, with individual nodules lasting approximately 1 to 2 weeks. Uniquely, larval migration can result in a trail of subcutaneous hemorrhage that is considered pathognomonic and helps to differentiate gnathostomiasis from other forms of parasitosis such as strongyloidiasis and sparganosis.1,3 Larvae are highly motile and invasive, and they are capable of producing a wide range of symptoms affecting virtually any part of the body.1,2 Depending on the anatomic location of the migrating worm, infection also may result in neurologic, gastrointestinal, pulmonary, or ocular symptoms.1-3,7 Eosinophilia is common but can subside in the chronic stage, as seen in our patient.1
The classic triad of intermittent migratory nodules, eosinophilia, and a history of travel to Southeast Asia or another endemic region should raise suspicion for gnathostomiasis.1-3,5,7 Unfortunately, confirmatory testing such as Gnathostoma serology is not readily available in the United States, and available serologic tests demonstrate frequent false positives and incomplete crossreactivity.1,2,8 Accordingly, the diagnosis most commonly is solidified by combining cardinal clinical features with histologic findings of a dense eosinophilic inflammatory infiltrate involving the dermis and hypodermis.2,5 In one study, the larva itself was only found in 12 of 66 (18%) skin biopsy specimens from patients with gnathostomiasis.5 If the larva is detected within the sections, it ranges from 2.5 to 12.5 mm in length and 0.4 to 1.2 mm in width and can exhibit cuticular spines, intestinal cells, and characteristic large lateral chords.1,5
The treatment of choice is surgical removal of the worm. Oral albendazole (400–800 mg/d for 21 days) also is considered a first-line treatment and results in clinical cure in approximately 90% of cases. Two doses of oral ivermectin (0.2 mg/kg) spaced 24 to 48 hours apart is an acceptable alternative with comparable efficacy.1-3 Care should be taken if involvement of the central nervous system is suspected, as antihelminthic treatment theoretically could be deleterious due to an inflammatory response to the dying larvae.1,2,9
In the differential diagnosis, loiasis can resemble gnathostomiasis, but the former is endemic to Africa.3 Cutaneous larva migrans most frequently is caused by hookworms from the genus Ancylostoma, which classically leads to superficial serpiginous linear plaques that migrate at a rate of several millimeters per day. However, the larvae are believed to lack the collagenase enzyme required to penetrate the epidermal basement membrane and thus are not capable of producing deep-seated nodules or visceral symptoms.3Strongyloidiasis (larva currens) generally exhibits a more linear morphology, and infection would result in positive Strongyloides serology.7 Erythema nodosum is a septal panniculitis that can be triggered by infection, pregnancy, medications, connective tissue diseases, inflammatory conditions, and underlying malignancy.10
- Herman JS, Chiodini PL. Gnathostomiasis, another emerging imported disease. Clin Microbiol Rev. 2009;22:484-492.
- Liu GH, Sun MM, Elsheikha HM, et al. Human gnathostomiasis: a neglected food-borne zoonosis. Parasit Vectors. 2020;13:616.
- Tyring SK. Gnathostomiasis. In: Tyring SK, Lupi O, Hengge UR, eds. Tropical Dermatology. 2nd ed. Elsevier; 2017:77-78.
- Rusnak JM, Lucey DR. Clinical gnathostomiasis: case report and review of the English-language literature. Clin Infect Dis. 1993;16:33-50.
- Magaña M, Messina M, Bustamante F, et al. Gnathostomiasis: clinicopathologic study. Am J Dermatopathol. 2004;26:91-95.
- Chandenier J, Husson J, Canaple S, et al. Medullary gnathostomiasis in a white patient: use of immunodiagnosis and magnetic resonance imaging. Clin Infect Dis. 2001;32:E154-E157.
- Hamilton WL, Agranoff D. Imported gnathostomiasis manifesting as cutaneous larva migrans and Löffler’s syndrome. BMJ Case Rep. 2018;2018:bcr2017223132.
- Neumayr A, Ollague J, Bravo F, et al. Cross-reactivity pattern of Asian and American human gnathostomiasis in western blot assays using crude antigens prepared from Gnathostoma spinigerum and Gnathostoma binucleatum third-stage larvae. Am J Trop Med Hyg. 2016;95:413-416.
- Kraivichian K, Nuchprayoon S, Sitichalernchai P, et al. Treatment of cutaneous gnathostomiasis with ivermectin. Am J Trop Med Hyg. 2004;71:623-628.
- Pérez-Garza DM, Chavez-Alvarez S, Ocampo-Candiani J, et al. Erythema nodosum: a practical approach and diagnostic algorithm. Am J Clin Dermatol. 2021;22:367-378.
The Diagnosis: Gnathostomiasis
The biopsy demonstrated a dense, eosinophilic, granulomatous infiltrate surrounding sections of a parasite with skeletal muscle bundles and intestines containing a brush border and luminal debris (Figure), which was consistent with a diagnosis of gnathostomiasis. Upon further questioning, he revealed that while in Peru he frequently consumed ceviche, which is a dish typically made from fresh raw fish cured in lemon or lime juice. He subsequently was treated with oral ivermectin 0.2 mg/kg once daily for 2 days with no evidence of recurrence 12 months later.

Cutaneous gnathostomiasis is the most common manifestation of infection caused by the third-stage larvae of the genus Gnathostoma. The nematode is endemic to tropical and subtropical regions of Japan and Southeast Asia, particularly Thailand. The disease has been increasingly observed in Central and South America. Humans can become infected through ingestion of undercooked meats, particularly freshwater fish but also poultry, snakes, or frogs. Few cases have been reported in North America and Europe presumably due to more stringent regulations governing the sourcing and storage of fish for consumption.1-3 Restaurants in endemic regions also may use cheaper local freshwater or brackish fish compared to restaurants in the West, which use more expensive saltwater fish that do not harbor Gnathostoma species.1 There is a false belief among restauranteurs and consumers that the larvae can be reliably killed by marinating meat in citrus juice or with concurrent consumption of alcohol or hot spices.2 Adequately cooking or freezing meat to 20 °C for 3 to 5 days are the only effective ways to ensure that the larvae are killed.1-3
The parasite requires its natural definitive hosts—fish-eating mammals such as pigs, cats, and dogs—to complete its life cycle and reproduce. Humans are accidental hosts in whom the parasite fails to reach sexual maturity.1-3 Consequently, symptoms commonly are due to the migration of only 1 larva, but occasionally infection with 2 or more has been observed.1,4
Human infection initially may result in malaise, fever, anorexia, abdominal pain, nausea, vomiting, and diarrhea as the parasite migrates through the stomach, intestines, and liver. After 2 to 4 weeks, larvae may reach the skin where they most commonly create ill-defined, erythematous, indurated, round or oval plaques or nodules described as nodular migratory panniculitis. These lesions tend to develop on the trunk or arms and correspond to the location of the migrating worm.1,3,5 The larvae have been observed to migrate at 1 cm/h.6 Symptoms often wax and wane, with individual nodules lasting approximately 1 to 2 weeks. Uniquely, larval migration can result in a trail of subcutaneous hemorrhage that is considered pathognomonic and helps to differentiate gnathostomiasis from other forms of parasitosis such as strongyloidiasis and sparganosis.1,3 Larvae are highly motile and invasive, and they are capable of producing a wide range of symptoms affecting virtually any part of the body.1,2 Depending on the anatomic location of the migrating worm, infection also may result in neurologic, gastrointestinal, pulmonary, or ocular symptoms.1-3,7 Eosinophilia is common but can subside in the chronic stage, as seen in our patient.1
The classic triad of intermittent migratory nodules, eosinophilia, and a history of travel to Southeast Asia or another endemic region should raise suspicion for gnathostomiasis.1-3,5,7 Unfortunately, confirmatory testing such as Gnathostoma serology is not readily available in the United States, and available serologic tests demonstrate frequent false positives and incomplete crossreactivity.1,2,8 Accordingly, the diagnosis most commonly is solidified by combining cardinal clinical features with histologic findings of a dense eosinophilic inflammatory infiltrate involving the dermis and hypodermis.2,5 In one study, the larva itself was only found in 12 of 66 (18%) skin biopsy specimens from patients with gnathostomiasis.5 If the larva is detected within the sections, it ranges from 2.5 to 12.5 mm in length and 0.4 to 1.2 mm in width and can exhibit cuticular spines, intestinal cells, and characteristic large lateral chords.1,5
The treatment of choice is surgical removal of the worm. Oral albendazole (400–800 mg/d for 21 days) also is considered a first-line treatment and results in clinical cure in approximately 90% of cases. Two doses of oral ivermectin (0.2 mg/kg) spaced 24 to 48 hours apart is an acceptable alternative with comparable efficacy.1-3 Care should be taken if involvement of the central nervous system is suspected, as antihelminthic treatment theoretically could be deleterious due to an inflammatory response to the dying larvae.1,2,9
In the differential diagnosis, loiasis can resemble gnathostomiasis, but the former is endemic to Africa.3 Cutaneous larva migrans most frequently is caused by hookworms from the genus Ancylostoma, which classically leads to superficial serpiginous linear plaques that migrate at a rate of several millimeters per day. However, the larvae are believed to lack the collagenase enzyme required to penetrate the epidermal basement membrane and thus are not capable of producing deep-seated nodules or visceral symptoms.3Strongyloidiasis (larva currens) generally exhibits a more linear morphology, and infection would result in positive Strongyloides serology.7 Erythema nodosum is a septal panniculitis that can be triggered by infection, pregnancy, medications, connective tissue diseases, inflammatory conditions, and underlying malignancy.10
The Diagnosis: Gnathostomiasis
The biopsy demonstrated a dense, eosinophilic, granulomatous infiltrate surrounding sections of a parasite with skeletal muscle bundles and intestines containing a brush border and luminal debris (Figure), which was consistent with a diagnosis of gnathostomiasis. Upon further questioning, he revealed that while in Peru he frequently consumed ceviche, which is a dish typically made from fresh raw fish cured in lemon or lime juice. He subsequently was treated with oral ivermectin 0.2 mg/kg once daily for 2 days with no evidence of recurrence 12 months later.

Cutaneous gnathostomiasis is the most common manifestation of infection caused by the third-stage larvae of the genus Gnathostoma. The nematode is endemic to tropical and subtropical regions of Japan and Southeast Asia, particularly Thailand. The disease has been increasingly observed in Central and South America. Humans can become infected through ingestion of undercooked meats, particularly freshwater fish but also poultry, snakes, or frogs. Few cases have been reported in North America and Europe presumably due to more stringent regulations governing the sourcing and storage of fish for consumption.1-3 Restaurants in endemic regions also may use cheaper local freshwater or brackish fish compared to restaurants in the West, which use more expensive saltwater fish that do not harbor Gnathostoma species.1 There is a false belief among restauranteurs and consumers that the larvae can be reliably killed by marinating meat in citrus juice or with concurrent consumption of alcohol or hot spices.2 Adequately cooking or freezing meat to 20 °C for 3 to 5 days are the only effective ways to ensure that the larvae are killed.1-3
The parasite requires its natural definitive hosts—fish-eating mammals such as pigs, cats, and dogs—to complete its life cycle and reproduce. Humans are accidental hosts in whom the parasite fails to reach sexual maturity.1-3 Consequently, symptoms commonly are due to the migration of only 1 larva, but occasionally infection with 2 or more has been observed.1,4
Human infection initially may result in malaise, fever, anorexia, abdominal pain, nausea, vomiting, and diarrhea as the parasite migrates through the stomach, intestines, and liver. After 2 to 4 weeks, larvae may reach the skin where they most commonly create ill-defined, erythematous, indurated, round or oval plaques or nodules described as nodular migratory panniculitis. These lesions tend to develop on the trunk or arms and correspond to the location of the migrating worm.1,3,5 The larvae have been observed to migrate at 1 cm/h.6 Symptoms often wax and wane, with individual nodules lasting approximately 1 to 2 weeks. Uniquely, larval migration can result in a trail of subcutaneous hemorrhage that is considered pathognomonic and helps to differentiate gnathostomiasis from other forms of parasitosis such as strongyloidiasis and sparganosis.1,3 Larvae are highly motile and invasive, and they are capable of producing a wide range of symptoms affecting virtually any part of the body.1,2 Depending on the anatomic location of the migrating worm, infection also may result in neurologic, gastrointestinal, pulmonary, or ocular symptoms.1-3,7 Eosinophilia is common but can subside in the chronic stage, as seen in our patient.1
The classic triad of intermittent migratory nodules, eosinophilia, and a history of travel to Southeast Asia or another endemic region should raise suspicion for gnathostomiasis.1-3,5,7 Unfortunately, confirmatory testing such as Gnathostoma serology is not readily available in the United States, and available serologic tests demonstrate frequent false positives and incomplete crossreactivity.1,2,8 Accordingly, the diagnosis most commonly is solidified by combining cardinal clinical features with histologic findings of a dense eosinophilic inflammatory infiltrate involving the dermis and hypodermis.2,5 In one study, the larva itself was only found in 12 of 66 (18%) skin biopsy specimens from patients with gnathostomiasis.5 If the larva is detected within the sections, it ranges from 2.5 to 12.5 mm in length and 0.4 to 1.2 mm in width and can exhibit cuticular spines, intestinal cells, and characteristic large lateral chords.1,5
The treatment of choice is surgical removal of the worm. Oral albendazole (400–800 mg/d for 21 days) also is considered a first-line treatment and results in clinical cure in approximately 90% of cases. Two doses of oral ivermectin (0.2 mg/kg) spaced 24 to 48 hours apart is an acceptable alternative with comparable efficacy.1-3 Care should be taken if involvement of the central nervous system is suspected, as antihelminthic treatment theoretically could be deleterious due to an inflammatory response to the dying larvae.1,2,9
In the differential diagnosis, loiasis can resemble gnathostomiasis, but the former is endemic to Africa.3 Cutaneous larva migrans most frequently is caused by hookworms from the genus Ancylostoma, which classically leads to superficial serpiginous linear plaques that migrate at a rate of several millimeters per day. However, the larvae are believed to lack the collagenase enzyme required to penetrate the epidermal basement membrane and thus are not capable of producing deep-seated nodules or visceral symptoms.3Strongyloidiasis (larva currens) generally exhibits a more linear morphology, and infection would result in positive Strongyloides serology.7 Erythema nodosum is a septal panniculitis that can be triggered by infection, pregnancy, medications, connective tissue diseases, inflammatory conditions, and underlying malignancy.10
- Herman JS, Chiodini PL. Gnathostomiasis, another emerging imported disease. Clin Microbiol Rev. 2009;22:484-492.
- Liu GH, Sun MM, Elsheikha HM, et al. Human gnathostomiasis: a neglected food-borne zoonosis. Parasit Vectors. 2020;13:616.
- Tyring SK. Gnathostomiasis. In: Tyring SK, Lupi O, Hengge UR, eds. Tropical Dermatology. 2nd ed. Elsevier; 2017:77-78.
- Rusnak JM, Lucey DR. Clinical gnathostomiasis: case report and review of the English-language literature. Clin Infect Dis. 1993;16:33-50.
- Magaña M, Messina M, Bustamante F, et al. Gnathostomiasis: clinicopathologic study. Am J Dermatopathol. 2004;26:91-95.
- Chandenier J, Husson J, Canaple S, et al. Medullary gnathostomiasis in a white patient: use of immunodiagnosis and magnetic resonance imaging. Clin Infect Dis. 2001;32:E154-E157.
- Hamilton WL, Agranoff D. Imported gnathostomiasis manifesting as cutaneous larva migrans and Löffler’s syndrome. BMJ Case Rep. 2018;2018:bcr2017223132.
- Neumayr A, Ollague J, Bravo F, et al. Cross-reactivity pattern of Asian and American human gnathostomiasis in western blot assays using crude antigens prepared from Gnathostoma spinigerum and Gnathostoma binucleatum third-stage larvae. Am J Trop Med Hyg. 2016;95:413-416.
- Kraivichian K, Nuchprayoon S, Sitichalernchai P, et al. Treatment of cutaneous gnathostomiasis with ivermectin. Am J Trop Med Hyg. 2004;71:623-628.
- Pérez-Garza DM, Chavez-Alvarez S, Ocampo-Candiani J, et al. Erythema nodosum: a practical approach and diagnostic algorithm. Am J Clin Dermatol. 2021;22:367-378.
- Herman JS, Chiodini PL. Gnathostomiasis, another emerging imported disease. Clin Microbiol Rev. 2009;22:484-492.
- Liu GH, Sun MM, Elsheikha HM, et al. Human gnathostomiasis: a neglected food-borne zoonosis. Parasit Vectors. 2020;13:616.
- Tyring SK. Gnathostomiasis. In: Tyring SK, Lupi O, Hengge UR, eds. Tropical Dermatology. 2nd ed. Elsevier; 2017:77-78.
- Rusnak JM, Lucey DR. Clinical gnathostomiasis: case report and review of the English-language literature. Clin Infect Dis. 1993;16:33-50.
- Magaña M, Messina M, Bustamante F, et al. Gnathostomiasis: clinicopathologic study. Am J Dermatopathol. 2004;26:91-95.
- Chandenier J, Husson J, Canaple S, et al. Medullary gnathostomiasis in a white patient: use of immunodiagnosis and magnetic resonance imaging. Clin Infect Dis. 2001;32:E154-E157.
- Hamilton WL, Agranoff D. Imported gnathostomiasis manifesting as cutaneous larva migrans and Löffler’s syndrome. BMJ Case Rep. 2018;2018:bcr2017223132.
- Neumayr A, Ollague J, Bravo F, et al. Cross-reactivity pattern of Asian and American human gnathostomiasis in western blot assays using crude antigens prepared from Gnathostoma spinigerum and Gnathostoma binucleatum third-stage larvae. Am J Trop Med Hyg. 2016;95:413-416.
- Kraivichian K, Nuchprayoon S, Sitichalernchai P, et al. Treatment of cutaneous gnathostomiasis with ivermectin. Am J Trop Med Hyg. 2004;71:623-628.
- Pérez-Garza DM, Chavez-Alvarez S, Ocampo-Candiani J, et al. Erythema nodosum: a practical approach and diagnostic algorithm. Am J Clin Dermatol. 2021;22:367-378.
A 41-year-old man presented to a dermatology clinic in the United States with a migratory subcutaneous nodule overlying the left upper chest that initially developed 12 months prior and continued to migrate along the trunk and proximal aspect of the arms. The patient had spent the last 3 years residing in Peru. He never observed more than 1 nodule at a time and denied associated fever, headache, visual changes, chest pain, cough, abdominal pain, and diarrhea. Laboratory studies including a blood eosinophil count and serum Strongyloides immunoglobulins were within reference range. An excisional biopsy was performed.
Navigating Hair Loss in Medical School: Experiences of 2 Young Black Women
As medical students, we often assume we are exempt from the diagnoses we learn about. During the first 2 years of medical school, we learn about alopecia as a condition that may be associated with stress, hormonal imbalances, nutrient deficiencies, and aging. However, our curricula do not explore the subtypes, psychosocial impact, or even the overwhelming number of Black women who are disproportionately affected by alopecia. For Black women, hair is a colossal part of their cultural identity, learning from a young age how to nurture and style natural coils. It becomes devastating when women begin to lose them.
The diagnosis of alopecia subtypes in Black women has been explored in the literature; however, understanding the unique experiences of young Black women is an important part of patient care, as alopecia often is destructive to the patient’s self-image. Therefore, it is important to shed light on these experiences so others feel empowered and supported in their journeys. Herein, we share the experiences of 2 authors (J.D. and C.A.V.O.)—both young Black women—who navigated unexpected hair loss in medical school.
Jewell’s Story
During my first year of medical school, I noticed my hair was shedding more than usual, and my ponytail was not as thick as it once was. I also had an area in my crown that was abnormally thin. My parents suggested that it was a consequence of stress, but I knew something was not right. With only 1 Black dermatologist within 2 hours of Nashville, Tennessee, I remember worrying about seeing a dermatologist who did not understand Black hair. I still scheduled an appointment, but I remember debating if I should straighten my hair or wear my naturally curly Afro. The first dermatologist I saw diagnosed me with seborrheic dermatitis—without even examining my scalp. She told me that I had a “full head of hair” and that I had nothing to worry about. I was unconvinced. Weeks later, I met with another dermatologist who took the time to listen to my concerns. After a scalp biopsy and laboratory work, she diagnosed me with telogen effluvium and androgenetic alopecia. Months later, I had the opportunity to visit the Black dermatologist, and she diagnosed me with central centrifugal cicatricial alopecia. I am grateful for the earlier dermatologists I saw, but I finally feel at ease with my diagnosis and treatment plan after being seen by the latter.
Chidubem’s Story
From a young age, I was conditioned to think my hair was thick, unmanageable, and a nuisance. I grew accustomed to people yanking on my hair, and my gentle whispers of “this hurts” and “the braid is too tight” being ignored. That continued into adulthood. While studying for the US Medical Licensing Examination, I noticed a burning sensation on my scalp. I decided to ignore it. However, as the days progressed, the slight burning sensation turned into intense burning and itching. I still ignored it. Not only did I lack the funds for a dermatology appointment, but my licensing examination was approaching, and it was more important than anything related to my hair. After the examination, I eventually made an appointment with my primary care physician, who attributed my symptoms to the stressors of medical school. “I think you are having migraines,” she told me. So, I continued to ignore my symptoms. A year passed, and a hair braider pointed out that I had 2 well-defined bald patches on my scalp. I remember feeling angry and confused as to how I missed those findings. I could no longer ignore it—it bothered me less when no one else knew about it. I quickly made a dermatology appointment. Although I opted out of a biopsy, we decided to treat my hair loss empirically, and I have experienced drastic improvement.
Final Thoughts
We are 2 Black women living more than 500 miles away from each other at different medical institutions, yet we share the same experience, which many other women unfortunately face alone. It is not uncommon for us to feel unheard, dismissed, or misdiagnosed. We write this for the Black woman sorting through the feelings of confusion and shock as she traces the hairless spot on her scalp. We write this for the medical student ignoring their symptoms until after their examination. We even write this for any nondermatologists uncomfortable with diagnosing and treating textured hair. To improve patient satisfaction and overall health outcomes, physicians must approach patients with both knowledge and cultural competency. Most importantly, dermatologists (and other physicians) should be appropriately trained in not only the structural differences of textured hair but also the unique practices and beliefs among Black women in relation to their hair.
Acknowledgments—Jewell Dinkins is the inaugural recipient of the Janssen–Skin of Color Research Fellowship at Howard University (Washington, DC), and Chidubem A.V. Okeke is the inaugural recipient of the Women’s Dermatologic Society–La Roche-Posay dermatology fellowship at Howard University.
As medical students, we often assume we are exempt from the diagnoses we learn about. During the first 2 years of medical school, we learn about alopecia as a condition that may be associated with stress, hormonal imbalances, nutrient deficiencies, and aging. However, our curricula do not explore the subtypes, psychosocial impact, or even the overwhelming number of Black women who are disproportionately affected by alopecia. For Black women, hair is a colossal part of their cultural identity, learning from a young age how to nurture and style natural coils. It becomes devastating when women begin to lose them.
The diagnosis of alopecia subtypes in Black women has been explored in the literature; however, understanding the unique experiences of young Black women is an important part of patient care, as alopecia often is destructive to the patient’s self-image. Therefore, it is important to shed light on these experiences so others feel empowered and supported in their journeys. Herein, we share the experiences of 2 authors (J.D. and C.A.V.O.)—both young Black women—who navigated unexpected hair loss in medical school.
Jewell’s Story
During my first year of medical school, I noticed my hair was shedding more than usual, and my ponytail was not as thick as it once was. I also had an area in my crown that was abnormally thin. My parents suggested that it was a consequence of stress, but I knew something was not right. With only 1 Black dermatologist within 2 hours of Nashville, Tennessee, I remember worrying about seeing a dermatologist who did not understand Black hair. I still scheduled an appointment, but I remember debating if I should straighten my hair or wear my naturally curly Afro. The first dermatologist I saw diagnosed me with seborrheic dermatitis—without even examining my scalp. She told me that I had a “full head of hair” and that I had nothing to worry about. I was unconvinced. Weeks later, I met with another dermatologist who took the time to listen to my concerns. After a scalp biopsy and laboratory work, she diagnosed me with telogen effluvium and androgenetic alopecia. Months later, I had the opportunity to visit the Black dermatologist, and she diagnosed me with central centrifugal cicatricial alopecia. I am grateful for the earlier dermatologists I saw, but I finally feel at ease with my diagnosis and treatment plan after being seen by the latter.
Chidubem’s Story
From a young age, I was conditioned to think my hair was thick, unmanageable, and a nuisance. I grew accustomed to people yanking on my hair, and my gentle whispers of “this hurts” and “the braid is too tight” being ignored. That continued into adulthood. While studying for the US Medical Licensing Examination, I noticed a burning sensation on my scalp. I decided to ignore it. However, as the days progressed, the slight burning sensation turned into intense burning and itching. I still ignored it. Not only did I lack the funds for a dermatology appointment, but my licensing examination was approaching, and it was more important than anything related to my hair. After the examination, I eventually made an appointment with my primary care physician, who attributed my symptoms to the stressors of medical school. “I think you are having migraines,” she told me. So, I continued to ignore my symptoms. A year passed, and a hair braider pointed out that I had 2 well-defined bald patches on my scalp. I remember feeling angry and confused as to how I missed those findings. I could no longer ignore it—it bothered me less when no one else knew about it. I quickly made a dermatology appointment. Although I opted out of a biopsy, we decided to treat my hair loss empirically, and I have experienced drastic improvement.
Final Thoughts
We are 2 Black women living more than 500 miles away from each other at different medical institutions, yet we share the same experience, which many other women unfortunately face alone. It is not uncommon for us to feel unheard, dismissed, or misdiagnosed. We write this for the Black woman sorting through the feelings of confusion and shock as she traces the hairless spot on her scalp. We write this for the medical student ignoring their symptoms until after their examination. We even write this for any nondermatologists uncomfortable with diagnosing and treating textured hair. To improve patient satisfaction and overall health outcomes, physicians must approach patients with both knowledge and cultural competency. Most importantly, dermatologists (and other physicians) should be appropriately trained in not only the structural differences of textured hair but also the unique practices and beliefs among Black women in relation to their hair.
Acknowledgments—Jewell Dinkins is the inaugural recipient of the Janssen–Skin of Color Research Fellowship at Howard University (Washington, DC), and Chidubem A.V. Okeke is the inaugural recipient of the Women’s Dermatologic Society–La Roche-Posay dermatology fellowship at Howard University.
As medical students, we often assume we are exempt from the diagnoses we learn about. During the first 2 years of medical school, we learn about alopecia as a condition that may be associated with stress, hormonal imbalances, nutrient deficiencies, and aging. However, our curricula do not explore the subtypes, psychosocial impact, or even the overwhelming number of Black women who are disproportionately affected by alopecia. For Black women, hair is a colossal part of their cultural identity, learning from a young age how to nurture and style natural coils. It becomes devastating when women begin to lose them.
The diagnosis of alopecia subtypes in Black women has been explored in the literature; however, understanding the unique experiences of young Black women is an important part of patient care, as alopecia often is destructive to the patient’s self-image. Therefore, it is important to shed light on these experiences so others feel empowered and supported in their journeys. Herein, we share the experiences of 2 authors (J.D. and C.A.V.O.)—both young Black women—who navigated unexpected hair loss in medical school.
Jewell’s Story
During my first year of medical school, I noticed my hair was shedding more than usual, and my ponytail was not as thick as it once was. I also had an area in my crown that was abnormally thin. My parents suggested that it was a consequence of stress, but I knew something was not right. With only 1 Black dermatologist within 2 hours of Nashville, Tennessee, I remember worrying about seeing a dermatologist who did not understand Black hair. I still scheduled an appointment, but I remember debating if I should straighten my hair or wear my naturally curly Afro. The first dermatologist I saw diagnosed me with seborrheic dermatitis—without even examining my scalp. She told me that I had a “full head of hair” and that I had nothing to worry about. I was unconvinced. Weeks later, I met with another dermatologist who took the time to listen to my concerns. After a scalp biopsy and laboratory work, she diagnosed me with telogen effluvium and androgenetic alopecia. Months later, I had the opportunity to visit the Black dermatologist, and she diagnosed me with central centrifugal cicatricial alopecia. I am grateful for the earlier dermatologists I saw, but I finally feel at ease with my diagnosis and treatment plan after being seen by the latter.
Chidubem’s Story
From a young age, I was conditioned to think my hair was thick, unmanageable, and a nuisance. I grew accustomed to people yanking on my hair, and my gentle whispers of “this hurts” and “the braid is too tight” being ignored. That continued into adulthood. While studying for the US Medical Licensing Examination, I noticed a burning sensation on my scalp. I decided to ignore it. However, as the days progressed, the slight burning sensation turned into intense burning and itching. I still ignored it. Not only did I lack the funds for a dermatology appointment, but my licensing examination was approaching, and it was more important than anything related to my hair. After the examination, I eventually made an appointment with my primary care physician, who attributed my symptoms to the stressors of medical school. “I think you are having migraines,” she told me. So, I continued to ignore my symptoms. A year passed, and a hair braider pointed out that I had 2 well-defined bald patches on my scalp. I remember feeling angry and confused as to how I missed those findings. I could no longer ignore it—it bothered me less when no one else knew about it. I quickly made a dermatology appointment. Although I opted out of a biopsy, we decided to treat my hair loss empirically, and I have experienced drastic improvement.
Final Thoughts
We are 2 Black women living more than 500 miles away from each other at different medical institutions, yet we share the same experience, which many other women unfortunately face alone. It is not uncommon for us to feel unheard, dismissed, or misdiagnosed. We write this for the Black woman sorting through the feelings of confusion and shock as she traces the hairless spot on her scalp. We write this for the medical student ignoring their symptoms until after their examination. We even write this for any nondermatologists uncomfortable with diagnosing and treating textured hair. To improve patient satisfaction and overall health outcomes, physicians must approach patients with both knowledge and cultural competency. Most importantly, dermatologists (and other physicians) should be appropriately trained in not only the structural differences of textured hair but also the unique practices and beliefs among Black women in relation to their hair.
Acknowledgments—Jewell Dinkins is the inaugural recipient of the Janssen–Skin of Color Research Fellowship at Howard University (Washington, DC), and Chidubem A.V. Okeke is the inaugural recipient of the Women’s Dermatologic Society–La Roche-Posay dermatology fellowship at Howard University.
Practice Points
- Hair loss is a common dermatologic concern among Black women and can represent a diagnostic challenge to dermatologists who may not be familiar with textured hair.
- Dermatologists should practice cultural sensitivity and provide relevant recommendations to Black patients dealing with hair loss.
Thalidomide Analogue Drug Eruption Along the Lines of Blaschko
To the Editor:
Lenalidomide is a thalidomide analogue used to treat various hematologic malignancies, including non-Hodgkin lymphoma, myelodysplastic syndrome, and multiple myeloma (MM).1 Lenalidomide is referred to as a degrader therapeutic because it induces targeted protein degradation of disease-relevant proteins (eg, Ikaros family zinc finger protein 1 [IKZF1], Ikaros family zinc finger protein 3 [IKZF3], and casein kinase I isoform-α [CK1α]) as its primary mechanism of action.1,2 Although cutaneous adverse events are relatively common among thalidomide analogues, the morphologic and histopathologic descriptions of these drug eruptions have not been fully elucidated.3,4 We report a novel pityriasiform drug eruption followed by a clinical eruption suggestive of blaschkitis in a patient with MM who was being treated with lenalidomide.
A 76-year-old man presented to the dermatology clinic with a progressive, mildly pruritic eruption on the chest and axillae of 1 year’s duration. He had a medical history of chronic hepatitis B, malignant carcinoid tumor of the colon, prostate cancer, and MM. The eruption emerged 1 to 2 weeks after the patient started oral lenalidomide 10 mg/d and oral dexamethasone40 mg/wk following autologous stem cell transplantation for MM. The patient had not received any other therapy for MM.
Physical examination revealed multiple erythematous, hyperpigmented, scaly papules and plaques on the lateral chest and within the axillae (Figure 1). A skin biopsy from the left axilla demonstrated a mild lichenoid and perivascular lymphocytic infiltrate with scattered eosinophils, neutrophils, and extravasated erythrocytes. The overlying epidermis showed spongiosis with parakeratosis in addition to lymphocytic exocytosis (Figure 2). No fungal organisms were highlighted on periodic acid–Schiff staining. After this evaluation, we recommended that the patient discontinue lenalidomide and start taking a topical over-the-counter corticosteroid for 2 weeks. Over time, he noted marked improvement in the eruption and associated pruritus.
After a drug holiday of 2 months, the patient resumed a maintenance dosage of oral lenalidomide 10 mg/d. Four or 5 days after restarting lenalidomide, a pruritic eruption appeared that involved the axillae and the left lower abdomen, circling around to the left lower back. The axillary eruption resolved with a topical over-the-counter corticosteroid; the abdominal eruption persisted.
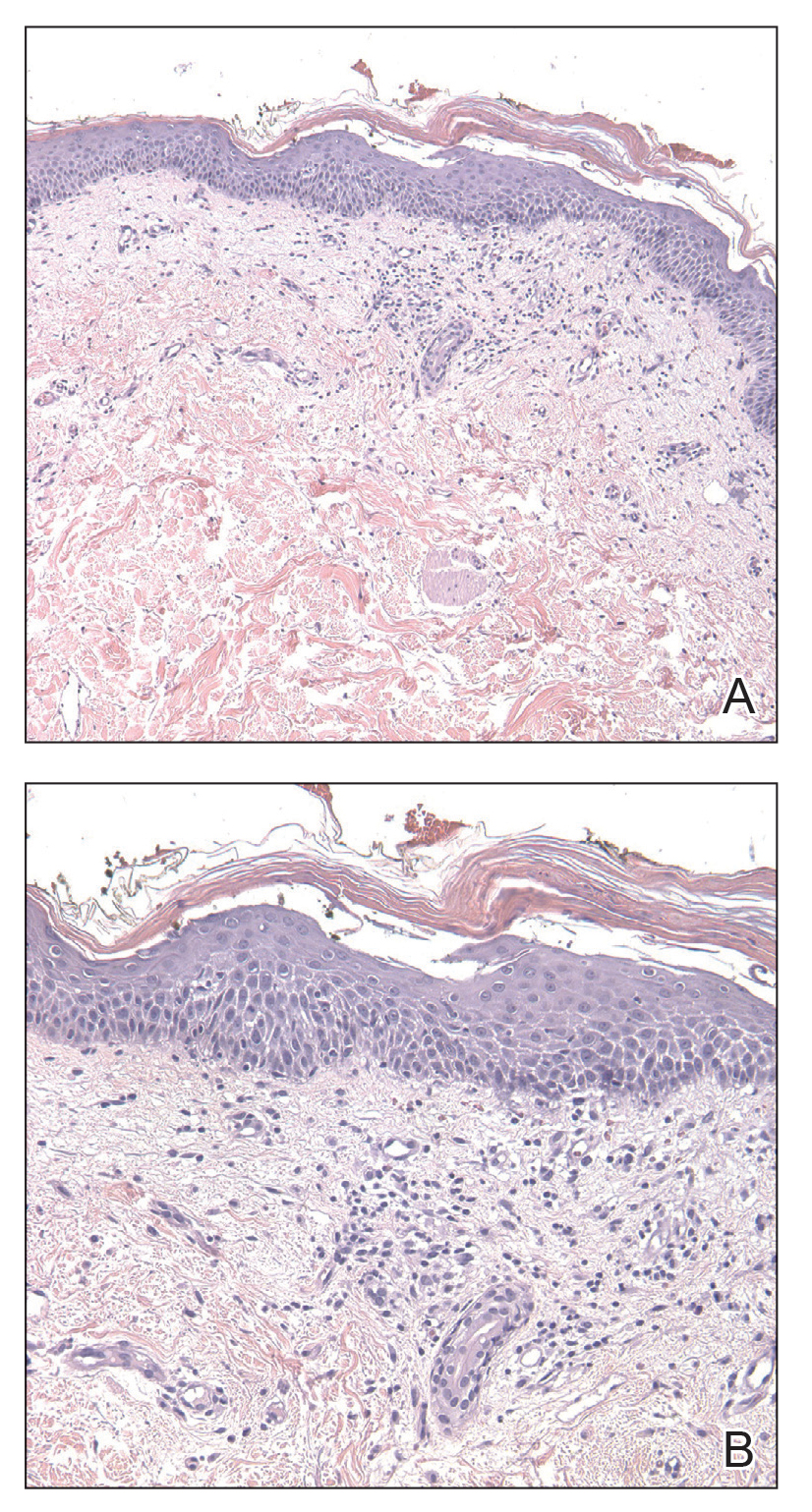
At the 3-month follow-up visit, physical examination revealed erythematous macules and papules that coalesced over a salmon-colored base along the lines of Blaschko extending from the left lower abdominal quadrant, crossing the left flank, and continuing to the left lower back without crossing the midline (Figure 3).
We recommended that the patient continue treatment through this eruption; he was instructed to apply a corticosteroid cream and resume lenalidomide at the maintenance dosage. A month later, he reported that the eruption and associated pruritus resolved with the corticosteroid cream and resumption of the maintenance dose of lenalidomide. The patient noted no further spread of the eruption.
Cutaneous adverse events are common following lenalidomide. In prior trials, the overall incidence of any-grade rash following lenalidomide exposure was 22% to 33%.5 A meta-analysis of 10 trials determined the overall incidence of all-grade and high-grade cutaneous adverse events after exposure to lenalidomide was 27.2% and 3.6%, respectively.6 Our case represents a pityriasiform eruption due to lenalidomide followed by a secondary eruption suggestive of blaschkitis.
The rash due to lenalidomide has been described as morbilliform, urticarial, dermatitic, acneform, and undefined.7 Lenalidomide-induced rash typically develops during the first month of therapy, similar to our patient’s presentation. It has even been observed in the first week of therapy.8 Severe reactions such as Stevens-Johnson syndrome and toxic epidermal necrolysis have been reported.5,6 Risk factors associated with rash secondary to lenalidomide include advanced age (≥70 years), presence of Bence-Jones protein-type MM in urine, and no prior chemotherapy.8 Our patient had 2 of these risk factors: advanced age and no prior chemotherapy for MM. The exact pathogenesis by which lenalidomide leads to a pityriasiform eruption, as in our patient, or to a rash in general is unclear. Studies have hypothesized that a lenalidomide-induced rash could be attributable to a delayed hypersensitivity type IV reaction or to a reaction related to the molecular mechanism of action of the drug.9
At the molecular level, the antimyeloma effects of lenalidomide include promoting degradation of transcription factors IKZF1 and IKZF3, which subsequently increases production of IL-2.1,2,9 Recombinant IL-2 has been associated with an increased incidence of rash in other cancers.9 Overexpression of programmed death 1(PD-1) and its ligand (PD-L1) has been demonstrated in MM; lenalidomide has been shown to downregulate both PD-1 and PD-L1. Patients receiving PD-1 and PD-L1 inhibitors commonly have developed rash.9 However, the association between lenalidomide and its downregulation of PD-1 and PD-L1 leading to rash has not been fully elucidated. Given the multiple malignancies in our patient—MM, prostate cancer, malignant carcinoid tumor—an underlying paraneoplastic phenomenon may be possible. Additionally, because our patient initially received dexamethasone along with lenalidomide, the manifestation of the initial pityriasiform rash may have been less severe due to the steroid use. Although our patient underwent a 2-month drug holiday following the initial pityriasiform eruption, most lenalidomide-induced rashes do not necessitate discontinuation of the drug.5,7
Our patient’s secondary drug eruption was clinically suggestive of lenalidomide-induced blaschkitis. A report of a German patient with plasmacytoma described a unilateral papular exanthem that developed 4 months after lenalidomide was initiated.10 The papular exanthem following the lines of Blaschko lines extended from that patient’s posterior left foot to the calf and on to the thigh and flank,10 which was more extensive than our patient’s eruption. Blaschkitis in this patient resolved with a corticosteroid cream and UV light therapy10; lenalidomide was not discontinued, similar to our patient.
The pathogenesis of our patient’s secondary eruption that preferentially involved the lines of Blaschko is unclear. After the initial pityriasiform eruption, the secondary eruption was blaschkitis. Distinguishing dermatomes from the lines of Blaschko, which are thought to represent pathways of epidermal cell migration and proliferation during embryologic development, is important. Genodermatoses such as incontinentia pigmenti and hypomelanosis of Ito involve the lines of Blaschko11; other disorders in the differential diagnosis of linear configurations include linear lichen planus, linear cutaneous lupus erythematosus, linear morphea, and lichen striatus.11 Notably, drug-induced blaschkitis is rare.
Cutaneous adverse reactions from thalidomide analogues are relatively common. Our case of lenalidomide-associated blaschkitis that developed following an initial pityriasiform drug eruption in a patient with MM highlights that dermatologists need to collaborate with the oncologist regarding the severity of drug eruptions to determine if the patient should continue treatment through the cutaneous eruptions or discontinue a vital medication.
- Jan M, Sperling AS, Ebert BL. Cancer therapies based on targeted protein degradation—lessons learned with lenalidomide. Nat Rev Clin Oncol. 2021;18:401-417. doi:10.1038/s41571-021-00479-z
- Shah UA, Mailankody S. Emerging immunotherapies in multiple myeloma. BMJ. 2020;370:3176. doi:10.1136/BMJ.M3176
- Richardson PG, Blood E, Mitsiades CS, et al. A randomized phase 2 study of lenalidomide therapy for patients with relapsed or relapsed and refractory multiple myeloma. Blood. 2006;108:3458-3464. doi:10.1182/BLOOD-2006-04-015909
- Benboubker L, Dimopoulos MA, Dispenzieri A, et al. Lenalidomide and dexamethasone in transplant-ineligible patients with myeloma. N Engl J Med. 2014;371:906-917. doi:10.1056/NEJMOA1402551
- Tinsley SM, Kurtin SE, Ridgeway JA. Practical management of lenalidomide-related rash. Clin Lymphoma Myeloma Leuk. 2015;15(suppl):S64-S69. doi:10.1016/J.CLML.2015.02.008
- Nardone B, Wu S, Garden BC, et al. Risk of rash associated with lenalidomide in cancer patients: a systematic review of the literature and meta-analysis. Clin Lymphoma Myeloma Leuk. 2013;13:424-429. doi:10.1016/J.CLML.2013.03.006
- Sviggum HP, Davis MDP, Rajkumar SV, et al. Dermatologic adverse effects of lenalidomide therapy for amyloidosis and multiple myeloma. Arch Dermatol. 2006;142:1298-1302. doi:10.1001/ARCHDERM.142.10.1298
- Sugi T, Nishigami Y, Saigo H, et al. Analysis of risk factors for lenalidomide-associated skin rash in patients with multiple myeloma. Leuk Lymphoma. 2021;62:1405-1410. doi:10.1080/10428194.2021.1876867
- Barley K, He W, Agarwal S, et al. Outcomes and management of lenalidomide-associated rash in patients with multiple myeloma. Leuk Lymphoma. 2016;57:2510-2515. doi:10.3109/10428194.2016.1151507
- Grape J, Frosch P. Papular drug eruption along the lines of Blaschko caused by lenalidomide [in German]. Hautarzt. 2011;62:618-620. doi:10.1007/S00105-010-2121-6
- Bolognia JL, Orlow SJ, Glick SA. Lines of Blaschko. J Am Acad Dermatol. 1994;31(2 pt 1):157-190. doi:10.1016/S0190-9622(94)70143-1
To the Editor:
Lenalidomide is a thalidomide analogue used to treat various hematologic malignancies, including non-Hodgkin lymphoma, myelodysplastic syndrome, and multiple myeloma (MM).1 Lenalidomide is referred to as a degrader therapeutic because it induces targeted protein degradation of disease-relevant proteins (eg, Ikaros family zinc finger protein 1 [IKZF1], Ikaros family zinc finger protein 3 [IKZF3], and casein kinase I isoform-α [CK1α]) as its primary mechanism of action.1,2 Although cutaneous adverse events are relatively common among thalidomide analogues, the morphologic and histopathologic descriptions of these drug eruptions have not been fully elucidated.3,4 We report a novel pityriasiform drug eruption followed by a clinical eruption suggestive of blaschkitis in a patient with MM who was being treated with lenalidomide.
A 76-year-old man presented to the dermatology clinic with a progressive, mildly pruritic eruption on the chest and axillae of 1 year’s duration. He had a medical history of chronic hepatitis B, malignant carcinoid tumor of the colon, prostate cancer, and MM. The eruption emerged 1 to 2 weeks after the patient started oral lenalidomide 10 mg/d and oral dexamethasone40 mg/wk following autologous stem cell transplantation for MM. The patient had not received any other therapy for MM.
Physical examination revealed multiple erythematous, hyperpigmented, scaly papules and plaques on the lateral chest and within the axillae (Figure 1). A skin biopsy from the left axilla demonstrated a mild lichenoid and perivascular lymphocytic infiltrate with scattered eosinophils, neutrophils, and extravasated erythrocytes. The overlying epidermis showed spongiosis with parakeratosis in addition to lymphocytic exocytosis (Figure 2). No fungal organisms were highlighted on periodic acid–Schiff staining. After this evaluation, we recommended that the patient discontinue lenalidomide and start taking a topical over-the-counter corticosteroid for 2 weeks. Over time, he noted marked improvement in the eruption and associated pruritus.

After a drug holiday of 2 months, the patient resumed a maintenance dosage of oral lenalidomide 10 mg/d. Four or 5 days after restarting lenalidomide, a pruritic eruption appeared that involved the axillae and the left lower abdomen, circling around to the left lower back. The axillary eruption resolved with a topical over-the-counter corticosteroid; the abdominal eruption persisted.

At the 3-month follow-up visit, physical examination revealed erythematous macules and papules that coalesced over a salmon-colored base along the lines of Blaschko extending from the left lower abdominal quadrant, crossing the left flank, and continuing to the left lower back without crossing the midline (Figure 3).

We recommended that the patient continue treatment through this eruption; he was instructed to apply a corticosteroid cream and resume lenalidomide at the maintenance dosage. A month later, he reported that the eruption and associated pruritus resolved with the corticosteroid cream and resumption of the maintenance dose of lenalidomide. The patient noted no further spread of the eruption.
Cutaneous adverse events are common following lenalidomide. In prior trials, the overall incidence of any-grade rash following lenalidomide exposure was 22% to 33%.5 A meta-analysis of 10 trials determined the overall incidence of all-grade and high-grade cutaneous adverse events after exposure to lenalidomide was 27.2% and 3.6%, respectively.6 Our case represents a pityriasiform eruption due to lenalidomide followed by a secondary eruption suggestive of blaschkitis.
The rash due to lenalidomide has been described as morbilliform, urticarial, dermatitic, acneform, and undefined.7 Lenalidomide-induced rash typically develops during the first month of therapy, similar to our patient’s presentation. It has even been observed in the first week of therapy.8 Severe reactions such as Stevens-Johnson syndrome and toxic epidermal necrolysis have been reported.5,6 Risk factors associated with rash secondary to lenalidomide include advanced age (≥70 years), presence of Bence-Jones protein-type MM in urine, and no prior chemotherapy.8 Our patient had 2 of these risk factors: advanced age and no prior chemotherapy for MM. The exact pathogenesis by which lenalidomide leads to a pityriasiform eruption, as in our patient, or to a rash in general is unclear. Studies have hypothesized that a lenalidomide-induced rash could be attributable to a delayed hypersensitivity type IV reaction or to a reaction related to the molecular mechanism of action of the drug.9
At the molecular level, the antimyeloma effects of lenalidomide include promoting degradation of transcription factors IKZF1 and IKZF3, which subsequently increases production of IL-2.1,2,9 Recombinant IL-2 has been associated with an increased incidence of rash in other cancers.9 Overexpression of programmed death 1(PD-1) and its ligand (PD-L1) has been demonstrated in MM; lenalidomide has been shown to downregulate both PD-1 and PD-L1. Patients receiving PD-1 and PD-L1 inhibitors commonly have developed rash.9 However, the association between lenalidomide and its downregulation of PD-1 and PD-L1 leading to rash has not been fully elucidated. Given the multiple malignancies in our patient—MM, prostate cancer, malignant carcinoid tumor—an underlying paraneoplastic phenomenon may be possible. Additionally, because our patient initially received dexamethasone along with lenalidomide, the manifestation of the initial pityriasiform rash may have been less severe due to the steroid use. Although our patient underwent a 2-month drug holiday following the initial pityriasiform eruption, most lenalidomide-induced rashes do not necessitate discontinuation of the drug.5,7
Our patient’s secondary drug eruption was clinically suggestive of lenalidomide-induced blaschkitis. A report of a German patient with plasmacytoma described a unilateral papular exanthem that developed 4 months after lenalidomide was initiated.10 The papular exanthem following the lines of Blaschko lines extended from that patient’s posterior left foot to the calf and on to the thigh and flank,10 which was more extensive than our patient’s eruption. Blaschkitis in this patient resolved with a corticosteroid cream and UV light therapy10; lenalidomide was not discontinued, similar to our patient.
The pathogenesis of our patient’s secondary eruption that preferentially involved the lines of Blaschko is unclear. After the initial pityriasiform eruption, the secondary eruption was blaschkitis. Distinguishing dermatomes from the lines of Blaschko, which are thought to represent pathways of epidermal cell migration and proliferation during embryologic development, is important. Genodermatoses such as incontinentia pigmenti and hypomelanosis of Ito involve the lines of Blaschko11; other disorders in the differential diagnosis of linear configurations include linear lichen planus, linear cutaneous lupus erythematosus, linear morphea, and lichen striatus.11 Notably, drug-induced blaschkitis is rare.
Cutaneous adverse reactions from thalidomide analogues are relatively common. Our case of lenalidomide-associated blaschkitis that developed following an initial pityriasiform drug eruption in a patient with MM highlights that dermatologists need to collaborate with the oncologist regarding the severity of drug eruptions to determine if the patient should continue treatment through the cutaneous eruptions or discontinue a vital medication.
To the Editor:
Lenalidomide is a thalidomide analogue used to treat various hematologic malignancies, including non-Hodgkin lymphoma, myelodysplastic syndrome, and multiple myeloma (MM).1 Lenalidomide is referred to as a degrader therapeutic because it induces targeted protein degradation of disease-relevant proteins (eg, Ikaros family zinc finger protein 1 [IKZF1], Ikaros family zinc finger protein 3 [IKZF3], and casein kinase I isoform-α [CK1α]) as its primary mechanism of action.1,2 Although cutaneous adverse events are relatively common among thalidomide analogues, the morphologic and histopathologic descriptions of these drug eruptions have not been fully elucidated.3,4 We report a novel pityriasiform drug eruption followed by a clinical eruption suggestive of blaschkitis in a patient with MM who was being treated with lenalidomide.
A 76-year-old man presented to the dermatology clinic with a progressive, mildly pruritic eruption on the chest and axillae of 1 year’s duration. He had a medical history of chronic hepatitis B, malignant carcinoid tumor of the colon, prostate cancer, and MM. The eruption emerged 1 to 2 weeks after the patient started oral lenalidomide 10 mg/d and oral dexamethasone40 mg/wk following autologous stem cell transplantation for MM. The patient had not received any other therapy for MM.
Physical examination revealed multiple erythematous, hyperpigmented, scaly papules and plaques on the lateral chest and within the axillae (Figure 1). A skin biopsy from the left axilla demonstrated a mild lichenoid and perivascular lymphocytic infiltrate with scattered eosinophils, neutrophils, and extravasated erythrocytes. The overlying epidermis showed spongiosis with parakeratosis in addition to lymphocytic exocytosis (Figure 2). No fungal organisms were highlighted on periodic acid–Schiff staining. After this evaluation, we recommended that the patient discontinue lenalidomide and start taking a topical over-the-counter corticosteroid for 2 weeks. Over time, he noted marked improvement in the eruption and associated pruritus.

After a drug holiday of 2 months, the patient resumed a maintenance dosage of oral lenalidomide 10 mg/d. Four or 5 days after restarting lenalidomide, a pruritic eruption appeared that involved the axillae and the left lower abdomen, circling around to the left lower back. The axillary eruption resolved with a topical over-the-counter corticosteroid; the abdominal eruption persisted.

At the 3-month follow-up visit, physical examination revealed erythematous macules and papules that coalesced over a salmon-colored base along the lines of Blaschko extending from the left lower abdominal quadrant, crossing the left flank, and continuing to the left lower back without crossing the midline (Figure 3).

We recommended that the patient continue treatment through this eruption; he was instructed to apply a corticosteroid cream and resume lenalidomide at the maintenance dosage. A month later, he reported that the eruption and associated pruritus resolved with the corticosteroid cream and resumption of the maintenance dose of lenalidomide. The patient noted no further spread of the eruption.
Cutaneous adverse events are common following lenalidomide. In prior trials, the overall incidence of any-grade rash following lenalidomide exposure was 22% to 33%.5 A meta-analysis of 10 trials determined the overall incidence of all-grade and high-grade cutaneous adverse events after exposure to lenalidomide was 27.2% and 3.6%, respectively.6 Our case represents a pityriasiform eruption due to lenalidomide followed by a secondary eruption suggestive of blaschkitis.
The rash due to lenalidomide has been described as morbilliform, urticarial, dermatitic, acneform, and undefined.7 Lenalidomide-induced rash typically develops during the first month of therapy, similar to our patient’s presentation. It has even been observed in the first week of therapy.8 Severe reactions such as Stevens-Johnson syndrome and toxic epidermal necrolysis have been reported.5,6 Risk factors associated with rash secondary to lenalidomide include advanced age (≥70 years), presence of Bence-Jones protein-type MM in urine, and no prior chemotherapy.8 Our patient had 2 of these risk factors: advanced age and no prior chemotherapy for MM. The exact pathogenesis by which lenalidomide leads to a pityriasiform eruption, as in our patient, or to a rash in general is unclear. Studies have hypothesized that a lenalidomide-induced rash could be attributable to a delayed hypersensitivity type IV reaction or to a reaction related to the molecular mechanism of action of the drug.9
At the molecular level, the antimyeloma effects of lenalidomide include promoting degradation of transcription factors IKZF1 and IKZF3, which subsequently increases production of IL-2.1,2,9 Recombinant IL-2 has been associated with an increased incidence of rash in other cancers.9 Overexpression of programmed death 1(PD-1) and its ligand (PD-L1) has been demonstrated in MM; lenalidomide has been shown to downregulate both PD-1 and PD-L1. Patients receiving PD-1 and PD-L1 inhibitors commonly have developed rash.9 However, the association between lenalidomide and its downregulation of PD-1 and PD-L1 leading to rash has not been fully elucidated. Given the multiple malignancies in our patient—MM, prostate cancer, malignant carcinoid tumor—an underlying paraneoplastic phenomenon may be possible. Additionally, because our patient initially received dexamethasone along with lenalidomide, the manifestation of the initial pityriasiform rash may have been less severe due to the steroid use. Although our patient underwent a 2-month drug holiday following the initial pityriasiform eruption, most lenalidomide-induced rashes do not necessitate discontinuation of the drug.5,7
Our patient’s secondary drug eruption was clinically suggestive of lenalidomide-induced blaschkitis. A report of a German patient with plasmacytoma described a unilateral papular exanthem that developed 4 months after lenalidomide was initiated.10 The papular exanthem following the lines of Blaschko lines extended from that patient’s posterior left foot to the calf and on to the thigh and flank,10 which was more extensive than our patient’s eruption. Blaschkitis in this patient resolved with a corticosteroid cream and UV light therapy10; lenalidomide was not discontinued, similar to our patient.
The pathogenesis of our patient’s secondary eruption that preferentially involved the lines of Blaschko is unclear. After the initial pityriasiform eruption, the secondary eruption was blaschkitis. Distinguishing dermatomes from the lines of Blaschko, which are thought to represent pathways of epidermal cell migration and proliferation during embryologic development, is important. Genodermatoses such as incontinentia pigmenti and hypomelanosis of Ito involve the lines of Blaschko11; other disorders in the differential diagnosis of linear configurations include linear lichen planus, linear cutaneous lupus erythematosus, linear morphea, and lichen striatus.11 Notably, drug-induced blaschkitis is rare.
Cutaneous adverse reactions from thalidomide analogues are relatively common. Our case of lenalidomide-associated blaschkitis that developed following an initial pityriasiform drug eruption in a patient with MM highlights that dermatologists need to collaborate with the oncologist regarding the severity of drug eruptions to determine if the patient should continue treatment through the cutaneous eruptions or discontinue a vital medication.
- Jan M, Sperling AS, Ebert BL. Cancer therapies based on targeted protein degradation—lessons learned with lenalidomide. Nat Rev Clin Oncol. 2021;18:401-417. doi:10.1038/s41571-021-00479-z
- Shah UA, Mailankody S. Emerging immunotherapies in multiple myeloma. BMJ. 2020;370:3176. doi:10.1136/BMJ.M3176
- Richardson PG, Blood E, Mitsiades CS, et al. A randomized phase 2 study of lenalidomide therapy for patients with relapsed or relapsed and refractory multiple myeloma. Blood. 2006;108:3458-3464. doi:10.1182/BLOOD-2006-04-015909
- Benboubker L, Dimopoulos MA, Dispenzieri A, et al. Lenalidomide and dexamethasone in transplant-ineligible patients with myeloma. N Engl J Med. 2014;371:906-917. doi:10.1056/NEJMOA1402551
- Tinsley SM, Kurtin SE, Ridgeway JA. Practical management of lenalidomide-related rash. Clin Lymphoma Myeloma Leuk. 2015;15(suppl):S64-S69. doi:10.1016/J.CLML.2015.02.008
- Nardone B, Wu S, Garden BC, et al. Risk of rash associated with lenalidomide in cancer patients: a systematic review of the literature and meta-analysis. Clin Lymphoma Myeloma Leuk. 2013;13:424-429. doi:10.1016/J.CLML.2013.03.006
- Sviggum HP, Davis MDP, Rajkumar SV, et al. Dermatologic adverse effects of lenalidomide therapy for amyloidosis and multiple myeloma. Arch Dermatol. 2006;142:1298-1302. doi:10.1001/ARCHDERM.142.10.1298
- Sugi T, Nishigami Y, Saigo H, et al. Analysis of risk factors for lenalidomide-associated skin rash in patients with multiple myeloma. Leuk Lymphoma. 2021;62:1405-1410. doi:10.1080/10428194.2021.1876867
- Barley K, He W, Agarwal S, et al. Outcomes and management of lenalidomide-associated rash in patients with multiple myeloma. Leuk Lymphoma. 2016;57:2510-2515. doi:10.3109/10428194.2016.1151507
- Grape J, Frosch P. Papular drug eruption along the lines of Blaschko caused by lenalidomide [in German]. Hautarzt. 2011;62:618-620. doi:10.1007/S00105-010-2121-6
- Bolognia JL, Orlow SJ, Glick SA. Lines of Blaschko. J Am Acad Dermatol. 1994;31(2 pt 1):157-190. doi:10.1016/S0190-9622(94)70143-1
- Jan M, Sperling AS, Ebert BL. Cancer therapies based on targeted protein degradation—lessons learned with lenalidomide. Nat Rev Clin Oncol. 2021;18:401-417. doi:10.1038/s41571-021-00479-z
- Shah UA, Mailankody S. Emerging immunotherapies in multiple myeloma. BMJ. 2020;370:3176. doi:10.1136/BMJ.M3176
- Richardson PG, Blood E, Mitsiades CS, et al. A randomized phase 2 study of lenalidomide therapy for patients with relapsed or relapsed and refractory multiple myeloma. Blood. 2006;108:3458-3464. doi:10.1182/BLOOD-2006-04-015909
- Benboubker L, Dimopoulos MA, Dispenzieri A, et al. Lenalidomide and dexamethasone in transplant-ineligible patients with myeloma. N Engl J Med. 2014;371:906-917. doi:10.1056/NEJMOA1402551
- Tinsley SM, Kurtin SE, Ridgeway JA. Practical management of lenalidomide-related rash. Clin Lymphoma Myeloma Leuk. 2015;15(suppl):S64-S69. doi:10.1016/J.CLML.2015.02.008
- Nardone B, Wu S, Garden BC, et al. Risk of rash associated with lenalidomide in cancer patients: a systematic review of the literature and meta-analysis. Clin Lymphoma Myeloma Leuk. 2013;13:424-429. doi:10.1016/J.CLML.2013.03.006
- Sviggum HP, Davis MDP, Rajkumar SV, et al. Dermatologic adverse effects of lenalidomide therapy for amyloidosis and multiple myeloma. Arch Dermatol. 2006;142:1298-1302. doi:10.1001/ARCHDERM.142.10.1298
- Sugi T, Nishigami Y, Saigo H, et al. Analysis of risk factors for lenalidomide-associated skin rash in patients with multiple myeloma. Leuk Lymphoma. 2021;62:1405-1410. doi:10.1080/10428194.2021.1876867
- Barley K, He W, Agarwal S, et al. Outcomes and management of lenalidomide-associated rash in patients with multiple myeloma. Leuk Lymphoma. 2016;57:2510-2515. doi:10.3109/10428194.2016.1151507
- Grape J, Frosch P. Papular drug eruption along the lines of Blaschko caused by lenalidomide [in German]. Hautarzt. 2011;62:618-620. doi:10.1007/S00105-010-2121-6
- Bolognia JL, Orlow SJ, Glick SA. Lines of Blaschko. J Am Acad Dermatol. 1994;31(2 pt 1):157-190. doi:10.1016/S0190-9622(94)70143-1
Practice Points
- Dermatologists should be aware of the variety of cutaneous adverse events that can arise from the use of immunotherapeutic agents for hematologic malignancies.
- Some cutaneous reactions to immunotherapeutic medications, such as pityriasiform eruption and blaschkitis, generally are benign and may not necessitate halting an important therapy.
The Role of Toluidine Blue in Mohs Micrographic Surgery: A Systematic Review
Toluidine blue (TB), a dye with metachromatic staining properties, was developed in 1856 by William Henry Perkin.1 Metachromasia is a perceptible change in the color of staining of living tissue due to the electrochemical properties of the tissue. Tissues that contain high concentrations of ionized sulfate and phosphate groups (high concentrations of free electronegative groups) form polymeric aggregates of the basic dye solution that alter the absorbed wavelengths of light.2 The function of this characteristic is to use a single dye to highlight different structures in tissue based on their relative chemical differences.3
Toluidine blue primarily was used within the dye industry until the 1960s, when it was first used in vital staining of the oral mucosa.2 Because of the tissue absorption potential, this technique was used to detect the location of oral malignancies.4 Since then, TB has progressively been used for staining fresh frozen sections in Mohs micrographic surgery (MMS). In a 2003 survey study (N=310), 16.8% of surgeons performing MMS reported using TB in their laboratory.5 We sought to systematically review the published literature describing the uses of TB in the setting of fresh frozen sections and MMS.
Methods
We conducted a systematic search of the PubMed and Cochrane databases for articles published before December 1, 2019, to identify any relevant studies in English. Electronic searches were performed using the terms toluidine blue and Mohs or Mohs micrographic surgery. We manually checked the bibliographies of the identified articles to further identify eligible studies.
Eligibility Criteria—The inclusion criteria were articles that (1) considered TB in the context of MMS, (2) were published in peer-reviewed journals, (3) were published in English, and (4) were available as full text. Systematic reviews were excluded.
Data Extraction and Outcomes—All relevant information regarding the study characteristics, including design, level of evidence, methodologic quality of evidence, pathology examined, and outcome measures, were collected by 2 independent reviewers (T.L. and A.D.) using a predetermined data sheet. The same 2 reviewers were used for all steps of the review process, data were independently obtained, and any discrepancy was introduced for a third opinion (D.H.) and agreed upon by the majority.
Quality Assessment—The level of evidence was evaluated based on the criteria of the Oxford Centre for Evidence-Based Medicine. Two reviewers (T.L. and A.D.) graded each article included in the review.
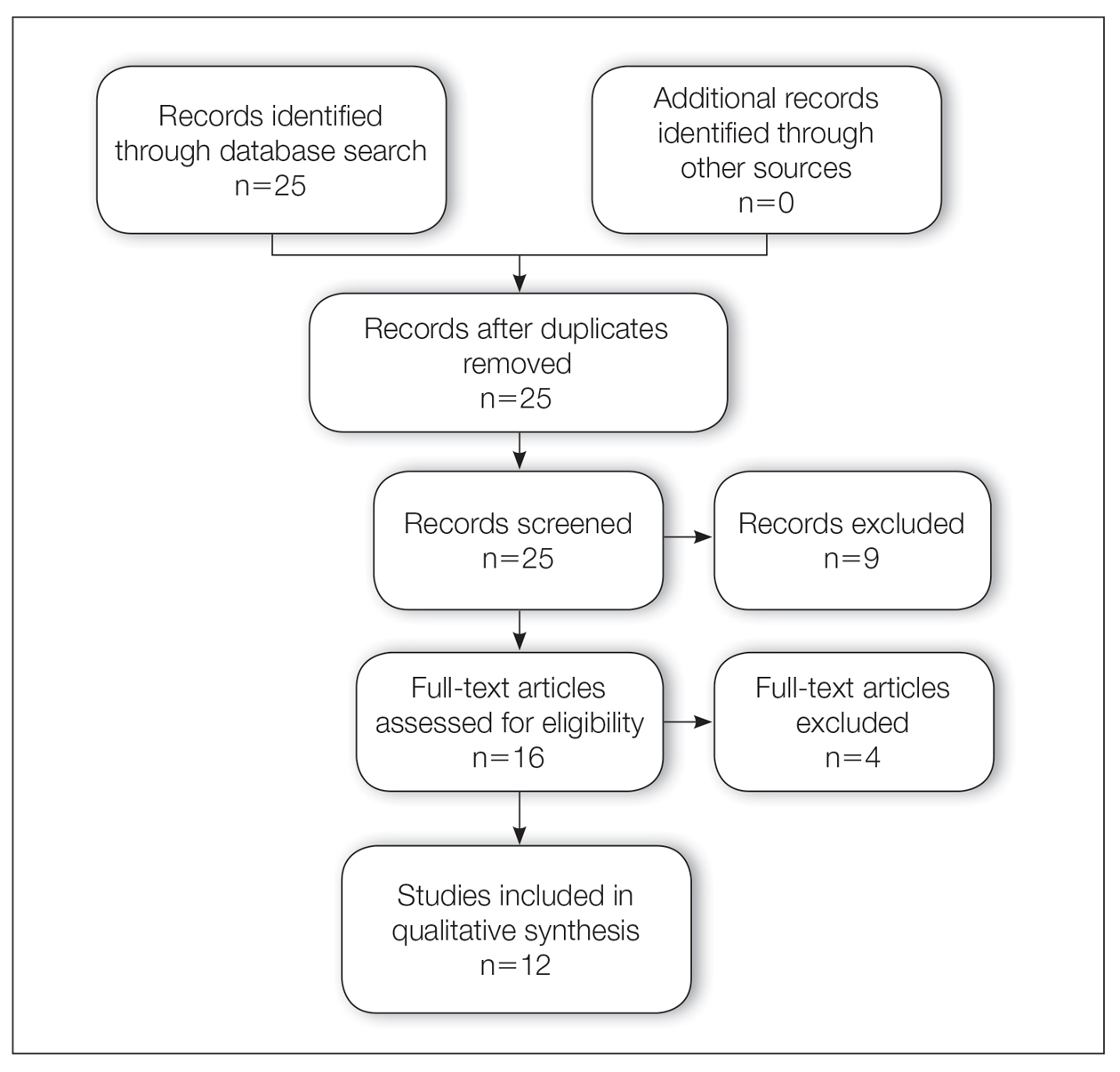
Results
A total of 25 articles were reviewed. After the titles and abstracts were screened for relevance, 12 articles remained (Figure 1). Of these, 1 compared basal cell carcinoma (BCC) and squamous cell carcinoma (SCC), 4 were related to BCC, 3 were related to SCC, 1 was related to microcystic adnexal carcinoma (MAC), 1 was related to primary cutaneous adenoid cystic carcinoma (PCACC), and 2 were related to technical aspects of the staining process (Table 1).
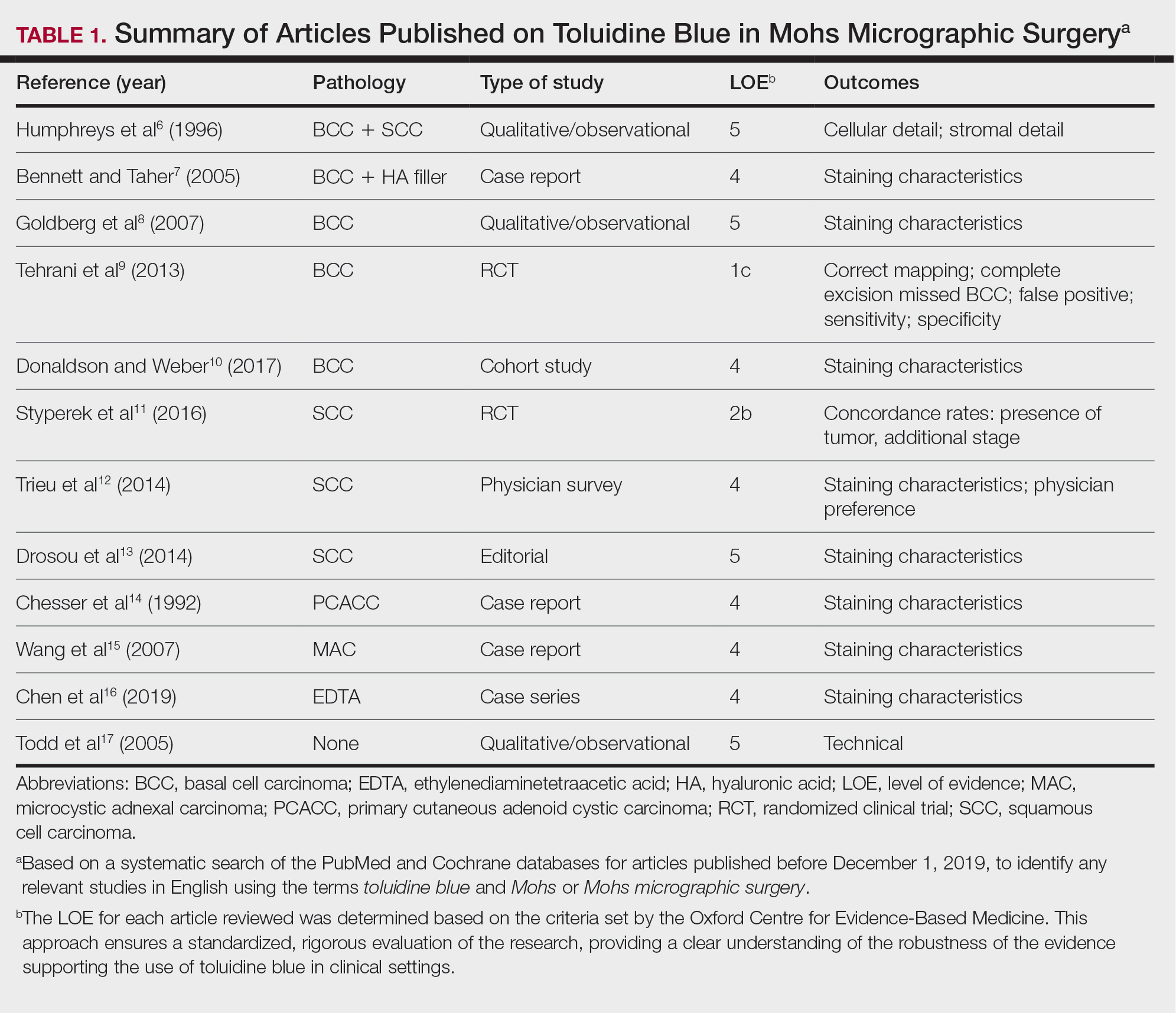
A majority of the articles included in this review were qualitative and observational in nature, describing the staining characteristics of TB. Study characteristics are summarized in Table 1.
Comment
Basal Cell Carcinoma—Toluidine blue staining characteristics help to identify BCC nests by differentiating them from hair follicles in frozen sections. The metachromatic characteristic of TB stains the inner root sheath deep blue and highlights the surrounding stromal mucin of BCC a magenta color.18,19 In hematoxylin and eosin (H&E) stains, these 2 distinct structures can be differentiated by cleft formation around tumor nests, mitotic figures, and the lack of a fibrous sheath present in BCC tumors.20 The advantages and limitations of TB staining of BCC are presented in Table 2.
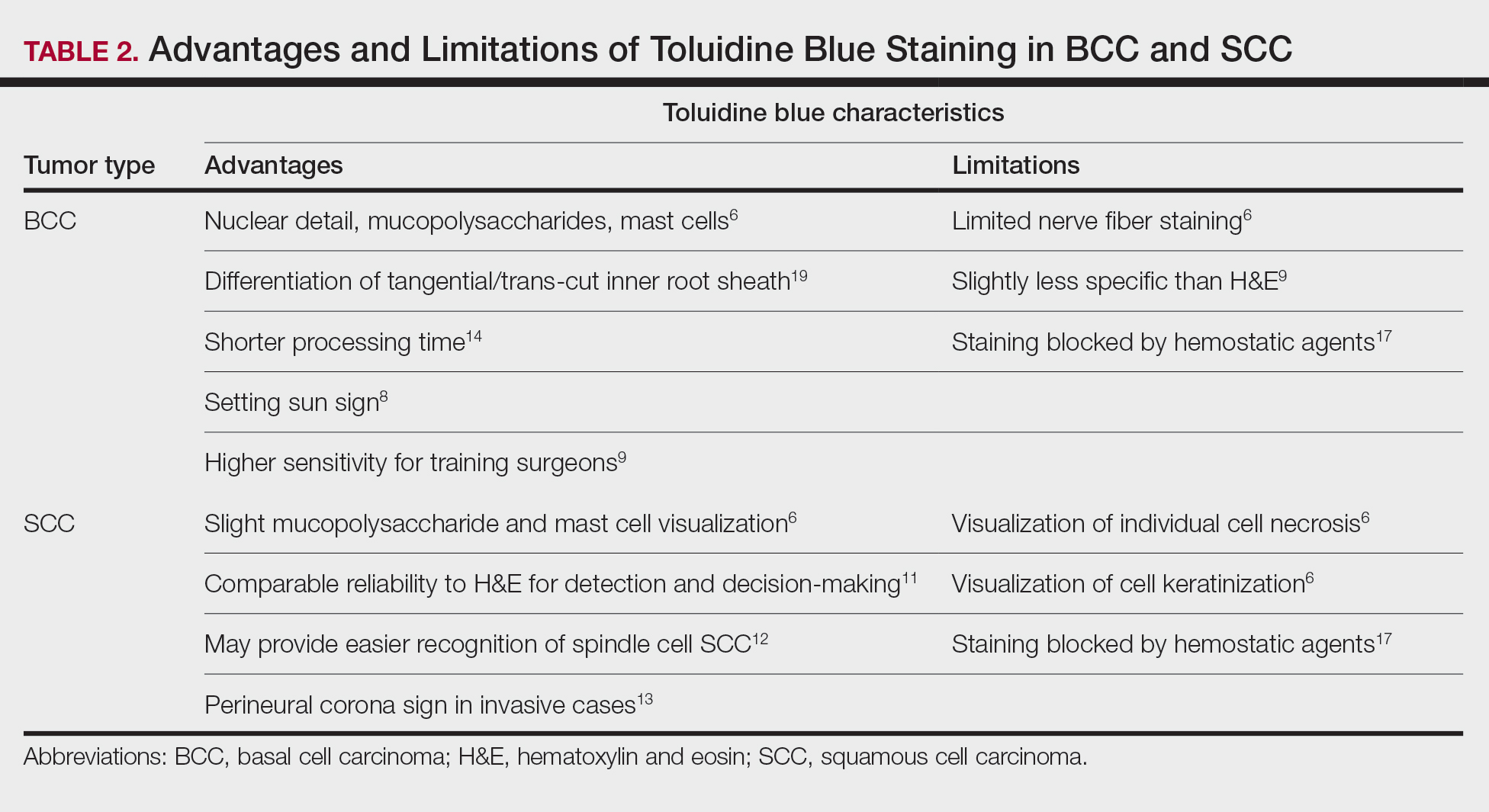
Humphreys et al6 suggested a noticeable difference between H&E and TB in the staining of cellular and stromal components. The nuclear detail of tumor cells was subjectively sharper and clearer with TB staining. The staining of stromal components may provide the most assistance in locating BCC islands. Mucopolysaccharide staining may be absent in H&E but stain a deep magenta with TB. Although the presence of mucopolysaccharides does not specifically indicate a tumor, it may prompt further attention and provide an indicator for sparse and infiltrative tumor cells.6 The metachromatic stromal change may indicate a narrow tumor-free margin where additional deeper sections often reveal tumor that may warrant additional resection margin in more aggressive malignancies. In particular, sclerosing/morpheaform BCCs have been shown to induce glycosaminoglycan synthesis and are highlighted more readily with TB than with H&E when compared to surrounding tissue.21 This differentiation in staining has remained a popular reason to routinely incorporate TB into the staining of infiltrative and morpheaform variants of BCC. Additionally, stromal mast cells are believed to be more abundant in the stroma of BCC and are more readily visualized in tissue specimens stained with TB, appearing as bright purple metachromatic granules. These granules are larger than normal and are increased in number.6
The margin behavior of BCC stained with TB was further characterized by Goldberg et al,8 who coined the term setting sun sign, which may be present in sequential sections of a disappearing nodule of a BCC tumor. Stroma, inflammatory infiltrate, and mast cells produce a magenta glow surrounding BCC tumors that is reminiscent of a setting sun (Figure 2). Invasive BCC is considered variable in this presentation, primarily because of zones of cell-free fluid and edema or the second area of inflammatory cells. This unique sign may benefit the inspecting Mohs surgeon by providing a clue to an underlying process that may have residual BCC tumors. The setting sun sign also may assist in identifying exact surgical margins.8

The nasal surface has a predilection for BCC.22 The skin of the nose has numerous look-alike structures to consider for complete tumor removal and avoidance of unnecessary removal. One challenge is distinguishing follicular basaloid proliferations (FBP) from BCC, a scenario that is more common on the nose.22 When TB staining was used, the sensitivity for detecting FBP reached 100% in 34 cases reviewed by Donaldson and Weber.10 None of the cases examined showed TB metachromasia surrounding FBP, thus indicating that TB can dependably identify this benign entity. Conversely, 5% (N=279) of BCCs confirmed on H&E did not exhibit surrounding TB metachromasia. This finding is concerning regarding the specificity of TB staining for BCC, but the authors of this study suggested the possibility that these exceptions were benign “simulants” (ie, trichoepithelioma) of BCC.10
The use of TB also has been shown to be statistically beneficial in Mohs training. In a single-center, single-fellow experiment, the sensitivity and specificity of using TB for BCC were extrapolated.9 Using TB as an adjunct in deep sections showed superior sensitivity to H&E alone in identifying BCC, increasing sensitivity from 96.3% to 99.7%. In a cohort of 352 BCC excisions and frozen sections, only 1 BCC was not completely excised. If H&E only had been performed, the fellow would have missed 13 residual BCC tumors.9
Bennett and Taher7 described a case in which hyaluronic acid (HA) from a filler injection was confused with the HA surrounding BCC tumor nests. They found that when TB is used as an adjunct, the HA filler is easier to differentiate from the HA surrounding the BCC tumor nests. In frozen sections stained with TB, the HA filler appeared as an amorphous, metachromatic, reddish-purple, whereas the HA surrounding the BCC tumor nests appeared as a well-defined red. These findings were less obvious in the same sections stained with H&E alone.7
Squamous Cell Carcinoma—In early investigations, the utility of TB in identifying SCC in frozen sections was thought to be limited. The description by Humphreys and colleagues6 of staining characteristics in SCC suggested that the nuclear detail that H&E provides is more easily recognized. The deep aqua nuclear staining produced with TB was considered more difficult to observe than the cytoplasmic eosinophilia of pyknotic and keratinizing cells in H&E.6
Toluidine blue may be beneficial in providing unique staining characteristics to further detail tumors that are difficult to interpret, such as spindle cell SCC and perineural invasion of aggressive SCC. In H&E, squamous cells of spindle cell SCC (scSCC) blend into the background of inflammatory cells and can be perceptibly difficult to locate. A small cohort of 3 Mohs surgeons who routinely use H&E were surveyed on their ability to detect a proven scSCC in H&E or TB by photograph.12 All 3 were able to detect the scSCC in the TB photographs, but only 2 of 3 were able to detect it in H&E photographs. All 3 surgeons agreed that TB was preferable to H&E for this tumor type. These findings suggested that TB may be superior and preferred over H&E for visualizing tumor cells of scSCC.12 The TB staining characteristics of perineural invasion of aggressive SCC have been referred to as the perineural corona sign because of the bright magenta stain that forms around affected nerves.13 Drosou et al13 suggested that TB may enhance the diagnostic accuracy for perineural SCC.
Rare Tumors—The adjunctive use of TB with H&E has been examined in rare tumors. Published reports have highlighted its use in MMS for treating MAC and PCACC. Toluidine blue exhibits staining advantages for these tumors. It may render isolated nests and perineural invasion of MAC more easily visible on frozen section.15
Although PCACC is rare, the recurrence rate is high.23 Toluidine blue has been used with MMS to ensure complete removal and higher cure rates. The metachromatic nature of TB is advantageous in staining the HA present in these tumors. Those who have reported the use of TB for PCACC prefer it to H&E for frozen sections.14
Technical Aspects—The staining time for TB-treated slides is reduced compared to H&E staining; staining can be efficiently done in frozen sections in less than 2.5 minutes using the method shown in Table 3.17 In comparison, typical H&E staining takes 9 minutes, and older TB techniques take 7 minutes.6
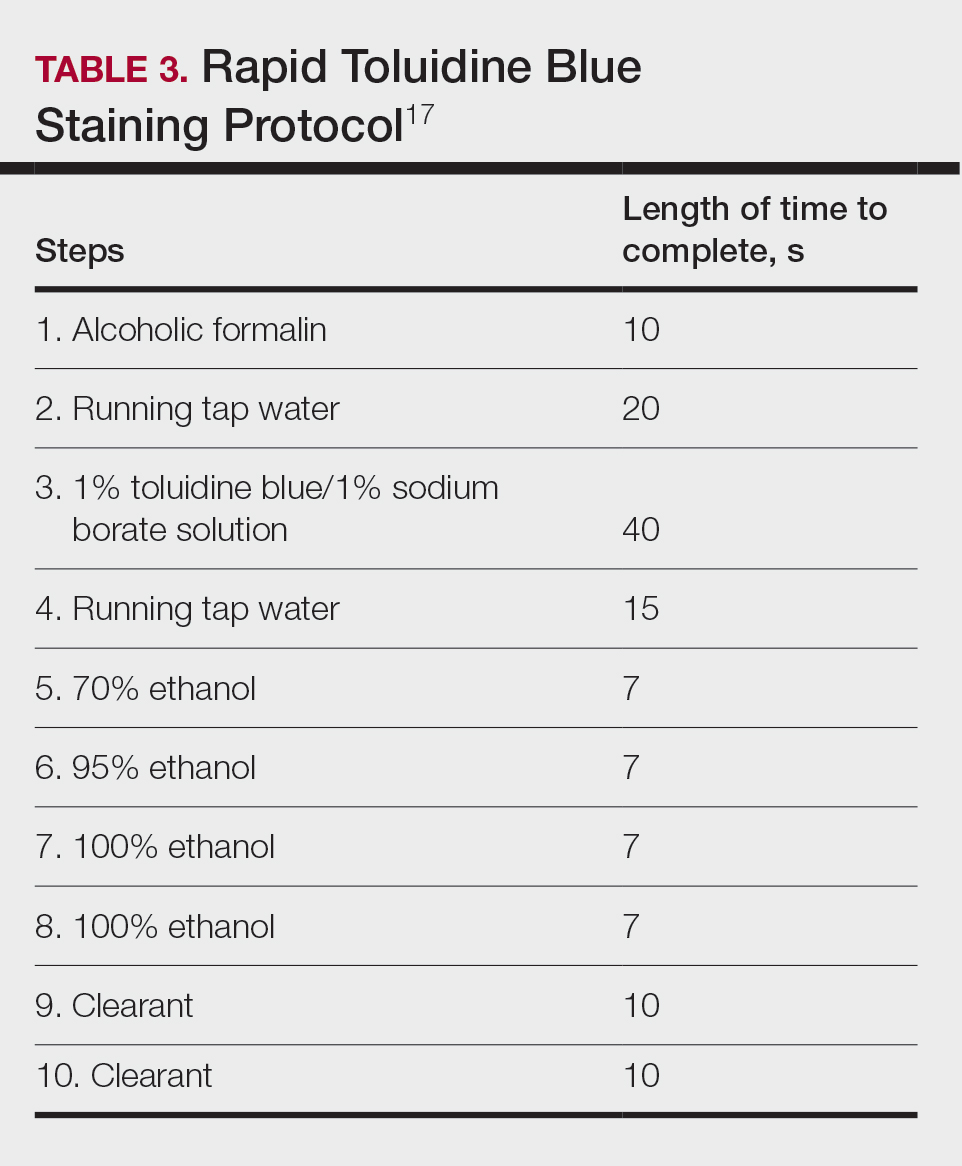
Conclusion
Toluidine blue may play an important and helpful role in the successful diagnosis and treatment of particular cutaneous tumors by providing additional diagnostic information. Although surgeons performing MMS will continue using the staining protocols with which they are most comfortable, adjunctive use of TB over time may provide an additional benefit at low risk for disrupting practice efficiency or workflow. Many Mohs surgeons are accustomed to using this stain, even preferring to interpret only TB-stained slides for cutaneous malignancy. Most published studies on this topic have been observational in nature, and additional controlled trials may be warranted to determine the effects on outcomes in real-world practice.
- Culling CF, Allison TR. Cellular Pathology Technique. 4th ed. Butterworths; 1985.
- Bergeron JA, Singer M. Metachromasy: an experimental and theoretical reevaluation. J Biophys Biochem Cytol. 1958;4:433-457. doi:10.1083/jcb.4.4.433
- Epstein JB, Scully C, Spinelli J. Toluidine blue and Lugol’s iodine application in the assessment of oral malignant disease and lesions at risk of malignancy. J Oral Pathol Med. 1992;21:160-163. doi:10.1111/j.1600-0714.1992.tb00094.x
- Warnakulasuriya KA, Johnson NW. Sensitivity and specificity of OraScan (R) toluidine blue mouthrinse in the detection of oral cancer and precancer. J Oral Pathol Med. 1996;25:97-103. doi:10.1111/j.1600-0714.1996.tb00201.x
- Silapunt S, Peterson SR, Alcalay J, et al. Mohs tissue mapping and processing: a survey study. Dermatol Surg. 2003;29:1109-1112; discussion 1112.
- Humphreys TR, Nemeth A, McCrevey S, et al. A pilot study comparing toluidine blue and hematoxylin and eosin staining of basal cell and squamous cell carcinoma during Mohs surgery. Dermatol Surg. 1996;22:693-697. doi:10.1111/j.1524-4725.1996.tb00619.x
- Bennett R, Taher M. Restylane persistent for 23 months found during Mohs micrographic surgery: a source of confusion with hyaluronic acid surrounding basal cell carcinoma. Dermatol Surg. 2005;31:1366-1369. doi:10.1111/j.1524-4725.2005.31223
- Goldberg LH, Wang SQ, Kimyai-Asadi A. The setting sun sign: visualizing the margins of a basal cell carcinoma on serial frozen sections stained with toluidine blue. Dermatol Surg. 2007;33:761-763. doi:10.1111/j.1524-4725.2007.33158.x
- Tehrani H, May K, Morris A, et al. Does the dual use of toluidine blue and hematoxylin and eosin staining improve basal cell carcinoma detection by Mohs surgery trainees? Dermatol Surg. 2013;39:995-1000. doi:10.1111/dsu.12180
- Donaldson MR, Weber LA. Toluidine blue supports differentiation of folliculocentric basaloid proliferation from basal cell carcinoma on frozen sections in a small single-practice cohort. Dermatol Surg. 2017;43:1303-1306. doi:10.1097/DSS.0000000000001107
- Styperek AR, Goldberg LH, Goldschmidt LE, et al. Toluidine blue and hematoxylin and eosin stains are comparable in evaluating squamous cell carcinoma during Mohs. Dermatol Surg. 2016;42:1279-1284. doi:10.1097/DSS.0000000000000872
- Trieu D, Drosou A, Goldberg LH, et al. Detecting spindle cell squamous cell carcinomas with toluidine blue on frozen sections. Dermatol Surg. 2014;40:1259-1260. doi:10.1097/DSS.0000000000000147
- Drosou A, Trieu D, Goldberg LH, et al. The perineural corona sign: enhancing detection of perineural squamous cell carcinoma during Mohs micrographic surgery with toluidine blue stain. J Am Acad Dermatol. 2014;71:826-827. doi:10.1016/j.jaad.2014.04.076
- Chesser RS, Bertler DE, Fitzpatrick JE, et al. Primary cutaneous adenoid cystic carcinoma treated with Mohs micrographic surgery toluidine blue technique. J Dermatol Surg Oncol. 1992;18:175-176. doi:10.1111/j.1524-4725.1992.tb02794.x
- Wang SQ, Goldberg LH, Nemeth A. The merits of adding toluidine blue-stained slides in Mohs surgery in the treatment of a microcystic adnexal carcinoma. J Am Acad Dermatol. 2007;56:1067-1069. doi:10.1016/j.jaad.2007.01.008
- Chen CL, Wilson S, Afzalneia R, et al. Topical aluminum chloride and Monsel’s solution block toluidine blue staining in Mohs frozen sections: mechanism and solution. Dermatol Surg. 2019;45:1019-1025. doi:10.1097/DSS.0000000000001761
- Todd MM, Lee JW, Marks VJ. Rapid toluidine blue stain for Mohs’ micrographic surgery. Dermatol Surg. 2005;31:244-245. doi:10.1111/j.1524-4725.2005.31053
- Picoto AM, Picoto A. Technical procedures for Mohs fresh tissue surgery. J Derm Surg Oncol. 1986;12:134-138. doi:10.1111/j.1524-4725.1986.tb01442.x
- Sperling LC, Winton GB. The transverse anatomy of androgenic alopecia. J Derm Surg Oncol. 1990;16:1127-1133. doi:10.1111/j.1524 -4725.1990.tb00024.x
- Smith-Zagone MJ, Schwartz MR. Frozen section of skin specimens. Arch Pathol Lab Med. 2005;129:1536-1543. doi:10.5858/2005-129-1536-FSOSS
- Moy RL, Potter TS, Uitto J. Increased glycosaminoglycans production in sclerosing basal cell carcinoma–derived fibroblasts and stimulation of normal skin fibroblast glycosaminoglycans production by a cytokine-derived from sclerosing basal cell carcinoma. Dermatol Surg. 2000;26:1029-1036. doi:10.1046/j.1524-4725.2000.0260111029.x
- Leshin B, White WL. Folliculocentric basaloid proliferation. The bulge (der Wulst) revisited. Arch Dermatol. 1990;126:900-906. doi:10.1001/archderm.126.7.900
- Seab JA, Graham JH. Primary cutaneous adenoid cystic carcinoma.J Am Acad Dermatol. 1987;17:113-118. doi:10.1016/s0190 -9622(87)70182-0
Toluidine blue (TB), a dye with metachromatic staining properties, was developed in 1856 by William Henry Perkin.1 Metachromasia is a perceptible change in the color of staining of living tissue due to the electrochemical properties of the tissue. Tissues that contain high concentrations of ionized sulfate and phosphate groups (high concentrations of free electronegative groups) form polymeric aggregates of the basic dye solution that alter the absorbed wavelengths of light.2 The function of this characteristic is to use a single dye to highlight different structures in tissue based on their relative chemical differences.3
Toluidine blue primarily was used within the dye industry until the 1960s, when it was first used in vital staining of the oral mucosa.2 Because of the tissue absorption potential, this technique was used to detect the location of oral malignancies.4 Since then, TB has progressively been used for staining fresh frozen sections in Mohs micrographic surgery (MMS). In a 2003 survey study (N=310), 16.8% of surgeons performing MMS reported using TB in their laboratory.5 We sought to systematically review the published literature describing the uses of TB in the setting of fresh frozen sections and MMS.
Methods
We conducted a systematic search of the PubMed and Cochrane databases for articles published before December 1, 2019, to identify any relevant studies in English. Electronic searches were performed using the terms toluidine blue and Mohs or Mohs micrographic surgery. We manually checked the bibliographies of the identified articles to further identify eligible studies.
Eligibility Criteria—The inclusion criteria were articles that (1) considered TB in the context of MMS, (2) were published in peer-reviewed journals, (3) were published in English, and (4) were available as full text. Systematic reviews were excluded.
Data Extraction and Outcomes—All relevant information regarding the study characteristics, including design, level of evidence, methodologic quality of evidence, pathology examined, and outcome measures, were collected by 2 independent reviewers (T.L. and A.D.) using a predetermined data sheet. The same 2 reviewers were used for all steps of the review process, data were independently obtained, and any discrepancy was introduced for a third opinion (D.H.) and agreed upon by the majority.
Quality Assessment—The level of evidence was evaluated based on the criteria of the Oxford Centre for Evidence-Based Medicine. Two reviewers (T.L. and A.D.) graded each article included in the review.

Results
A total of 25 articles were reviewed. After the titles and abstracts were screened for relevance, 12 articles remained (Figure 1). Of these, 1 compared basal cell carcinoma (BCC) and squamous cell carcinoma (SCC), 4 were related to BCC, 3 were related to SCC, 1 was related to microcystic adnexal carcinoma (MAC), 1 was related to primary cutaneous adenoid cystic carcinoma (PCACC), and 2 were related to technical aspects of the staining process (Table 1).

A majority of the articles included in this review were qualitative and observational in nature, describing the staining characteristics of TB. Study characteristics are summarized in Table 1.
Comment
Basal Cell Carcinoma—Toluidine blue staining characteristics help to identify BCC nests by differentiating them from hair follicles in frozen sections. The metachromatic characteristic of TB stains the inner root sheath deep blue and highlights the surrounding stromal mucin of BCC a magenta color.18,19 In hematoxylin and eosin (H&E) stains, these 2 distinct structures can be differentiated by cleft formation around tumor nests, mitotic figures, and the lack of a fibrous sheath present in BCC tumors.20 The advantages and limitations of TB staining of BCC are presented in Table 2.

Humphreys et al6 suggested a noticeable difference between H&E and TB in the staining of cellular and stromal components. The nuclear detail of tumor cells was subjectively sharper and clearer with TB staining. The staining of stromal components may provide the most assistance in locating BCC islands. Mucopolysaccharide staining may be absent in H&E but stain a deep magenta with TB. Although the presence of mucopolysaccharides does not specifically indicate a tumor, it may prompt further attention and provide an indicator for sparse and infiltrative tumor cells.6 The metachromatic stromal change may indicate a narrow tumor-free margin where additional deeper sections often reveal tumor that may warrant additional resection margin in more aggressive malignancies. In particular, sclerosing/morpheaform BCCs have been shown to induce glycosaminoglycan synthesis and are highlighted more readily with TB than with H&E when compared to surrounding tissue.21 This differentiation in staining has remained a popular reason to routinely incorporate TB into the staining of infiltrative and morpheaform variants of BCC. Additionally, stromal mast cells are believed to be more abundant in the stroma of BCC and are more readily visualized in tissue specimens stained with TB, appearing as bright purple metachromatic granules. These granules are larger than normal and are increased in number.6
The margin behavior of BCC stained with TB was further characterized by Goldberg et al,8 who coined the term setting sun sign, which may be present in sequential sections of a disappearing nodule of a BCC tumor. Stroma, inflammatory infiltrate, and mast cells produce a magenta glow surrounding BCC tumors that is reminiscent of a setting sun (Figure 2). Invasive BCC is considered variable in this presentation, primarily because of zones of cell-free fluid and edema or the second area of inflammatory cells. This unique sign may benefit the inspecting Mohs surgeon by providing a clue to an underlying process that may have residual BCC tumors. The setting sun sign also may assist in identifying exact surgical margins.8

The nasal surface has a predilection for BCC.22 The skin of the nose has numerous look-alike structures to consider for complete tumor removal and avoidance of unnecessary removal. One challenge is distinguishing follicular basaloid proliferations (FBP) from BCC, a scenario that is more common on the nose.22 When TB staining was used, the sensitivity for detecting FBP reached 100% in 34 cases reviewed by Donaldson and Weber.10 None of the cases examined showed TB metachromasia surrounding FBP, thus indicating that TB can dependably identify this benign entity. Conversely, 5% (N=279) of BCCs confirmed on H&E did not exhibit surrounding TB metachromasia. This finding is concerning regarding the specificity of TB staining for BCC, but the authors of this study suggested the possibility that these exceptions were benign “simulants” (ie, trichoepithelioma) of BCC.10
The use of TB also has been shown to be statistically beneficial in Mohs training. In a single-center, single-fellow experiment, the sensitivity and specificity of using TB for BCC were extrapolated.9 Using TB as an adjunct in deep sections showed superior sensitivity to H&E alone in identifying BCC, increasing sensitivity from 96.3% to 99.7%. In a cohort of 352 BCC excisions and frozen sections, only 1 BCC was not completely excised. If H&E only had been performed, the fellow would have missed 13 residual BCC tumors.9
Bennett and Taher7 described a case in which hyaluronic acid (HA) from a filler injection was confused with the HA surrounding BCC tumor nests. They found that when TB is used as an adjunct, the HA filler is easier to differentiate from the HA surrounding the BCC tumor nests. In frozen sections stained with TB, the HA filler appeared as an amorphous, metachromatic, reddish-purple, whereas the HA surrounding the BCC tumor nests appeared as a well-defined red. These findings were less obvious in the same sections stained with H&E alone.7
Squamous Cell Carcinoma—In early investigations, the utility of TB in identifying SCC in frozen sections was thought to be limited. The description by Humphreys and colleagues6 of staining characteristics in SCC suggested that the nuclear detail that H&E provides is more easily recognized. The deep aqua nuclear staining produced with TB was considered more difficult to observe than the cytoplasmic eosinophilia of pyknotic and keratinizing cells in H&E.6
Toluidine blue may be beneficial in providing unique staining characteristics to further detail tumors that are difficult to interpret, such as spindle cell SCC and perineural invasion of aggressive SCC. In H&E, squamous cells of spindle cell SCC (scSCC) blend into the background of inflammatory cells and can be perceptibly difficult to locate. A small cohort of 3 Mohs surgeons who routinely use H&E were surveyed on their ability to detect a proven scSCC in H&E or TB by photograph.12 All 3 were able to detect the scSCC in the TB photographs, but only 2 of 3 were able to detect it in H&E photographs. All 3 surgeons agreed that TB was preferable to H&E for this tumor type. These findings suggested that TB may be superior and preferred over H&E for visualizing tumor cells of scSCC.12 The TB staining characteristics of perineural invasion of aggressive SCC have been referred to as the perineural corona sign because of the bright magenta stain that forms around affected nerves.13 Drosou et al13 suggested that TB may enhance the diagnostic accuracy for perineural SCC.
Rare Tumors—The adjunctive use of TB with H&E has been examined in rare tumors. Published reports have highlighted its use in MMS for treating MAC and PCACC. Toluidine blue exhibits staining advantages for these tumors. It may render isolated nests and perineural invasion of MAC more easily visible on frozen section.15
Although PCACC is rare, the recurrence rate is high.23 Toluidine blue has been used with MMS to ensure complete removal and higher cure rates. The metachromatic nature of TB is advantageous in staining the HA present in these tumors. Those who have reported the use of TB for PCACC prefer it to H&E for frozen sections.14
Technical Aspects—The staining time for TB-treated slides is reduced compared to H&E staining; staining can be efficiently done in frozen sections in less than 2.5 minutes using the method shown in Table 3.17 In comparison, typical H&E staining takes 9 minutes, and older TB techniques take 7 minutes.6

Conclusion
Toluidine blue may play an important and helpful role in the successful diagnosis and treatment of particular cutaneous tumors by providing additional diagnostic information. Although surgeons performing MMS will continue using the staining protocols with which they are most comfortable, adjunctive use of TB over time may provide an additional benefit at low risk for disrupting practice efficiency or workflow. Many Mohs surgeons are accustomed to using this stain, even preferring to interpret only TB-stained slides for cutaneous malignancy. Most published studies on this topic have been observational in nature, and additional controlled trials may be warranted to determine the effects on outcomes in real-world practice.
Toluidine blue (TB), a dye with metachromatic staining properties, was developed in 1856 by William Henry Perkin.1 Metachromasia is a perceptible change in the color of staining of living tissue due to the electrochemical properties of the tissue. Tissues that contain high concentrations of ionized sulfate and phosphate groups (high concentrations of free electronegative groups) form polymeric aggregates of the basic dye solution that alter the absorbed wavelengths of light.2 The function of this characteristic is to use a single dye to highlight different structures in tissue based on their relative chemical differences.3
Toluidine blue primarily was used within the dye industry until the 1960s, when it was first used in vital staining of the oral mucosa.2 Because of the tissue absorption potential, this technique was used to detect the location of oral malignancies.4 Since then, TB has progressively been used for staining fresh frozen sections in Mohs micrographic surgery (MMS). In a 2003 survey study (N=310), 16.8% of surgeons performing MMS reported using TB in their laboratory.5 We sought to systematically review the published literature describing the uses of TB in the setting of fresh frozen sections and MMS.
Methods
We conducted a systematic search of the PubMed and Cochrane databases for articles published before December 1, 2019, to identify any relevant studies in English. Electronic searches were performed using the terms toluidine blue and Mohs or Mohs micrographic surgery. We manually checked the bibliographies of the identified articles to further identify eligible studies.
Eligibility Criteria—The inclusion criteria were articles that (1) considered TB in the context of MMS, (2) were published in peer-reviewed journals, (3) were published in English, and (4) were available as full text. Systematic reviews were excluded.
Data Extraction and Outcomes—All relevant information regarding the study characteristics, including design, level of evidence, methodologic quality of evidence, pathology examined, and outcome measures, were collected by 2 independent reviewers (T.L. and A.D.) using a predetermined data sheet. The same 2 reviewers were used for all steps of the review process, data were independently obtained, and any discrepancy was introduced for a third opinion (D.H.) and agreed upon by the majority.
Quality Assessment—The level of evidence was evaluated based on the criteria of the Oxford Centre for Evidence-Based Medicine. Two reviewers (T.L. and A.D.) graded each article included in the review.

Results
A total of 25 articles were reviewed. After the titles and abstracts were screened for relevance, 12 articles remained (Figure 1). Of these, 1 compared basal cell carcinoma (BCC) and squamous cell carcinoma (SCC), 4 were related to BCC, 3 were related to SCC, 1 was related to microcystic adnexal carcinoma (MAC), 1 was related to primary cutaneous adenoid cystic carcinoma (PCACC), and 2 were related to technical aspects of the staining process (Table 1).

A majority of the articles included in this review were qualitative and observational in nature, describing the staining characteristics of TB. Study characteristics are summarized in Table 1.
Comment
Basal Cell Carcinoma—Toluidine blue staining characteristics help to identify BCC nests by differentiating them from hair follicles in frozen sections. The metachromatic characteristic of TB stains the inner root sheath deep blue and highlights the surrounding stromal mucin of BCC a magenta color.18,19 In hematoxylin and eosin (H&E) stains, these 2 distinct structures can be differentiated by cleft formation around tumor nests, mitotic figures, and the lack of a fibrous sheath present in BCC tumors.20 The advantages and limitations of TB staining of BCC are presented in Table 2.

Humphreys et al6 suggested a noticeable difference between H&E and TB in the staining of cellular and stromal components. The nuclear detail of tumor cells was subjectively sharper and clearer with TB staining. The staining of stromal components may provide the most assistance in locating BCC islands. Mucopolysaccharide staining may be absent in H&E but stain a deep magenta with TB. Although the presence of mucopolysaccharides does not specifically indicate a tumor, it may prompt further attention and provide an indicator for sparse and infiltrative tumor cells.6 The metachromatic stromal change may indicate a narrow tumor-free margin where additional deeper sections often reveal tumor that may warrant additional resection margin in more aggressive malignancies. In particular, sclerosing/morpheaform BCCs have been shown to induce glycosaminoglycan synthesis and are highlighted more readily with TB than with H&E when compared to surrounding tissue.21 This differentiation in staining has remained a popular reason to routinely incorporate TB into the staining of infiltrative and morpheaform variants of BCC. Additionally, stromal mast cells are believed to be more abundant in the stroma of BCC and are more readily visualized in tissue specimens stained with TB, appearing as bright purple metachromatic granules. These granules are larger than normal and are increased in number.6
The margin behavior of BCC stained with TB was further characterized by Goldberg et al,8 who coined the term setting sun sign, which may be present in sequential sections of a disappearing nodule of a BCC tumor. Stroma, inflammatory infiltrate, and mast cells produce a magenta glow surrounding BCC tumors that is reminiscent of a setting sun (Figure 2). Invasive BCC is considered variable in this presentation, primarily because of zones of cell-free fluid and edema or the second area of inflammatory cells. This unique sign may benefit the inspecting Mohs surgeon by providing a clue to an underlying process that may have residual BCC tumors. The setting sun sign also may assist in identifying exact surgical margins.8

The nasal surface has a predilection for BCC.22 The skin of the nose has numerous look-alike structures to consider for complete tumor removal and avoidance of unnecessary removal. One challenge is distinguishing follicular basaloid proliferations (FBP) from BCC, a scenario that is more common on the nose.22 When TB staining was used, the sensitivity for detecting FBP reached 100% in 34 cases reviewed by Donaldson and Weber.10 None of the cases examined showed TB metachromasia surrounding FBP, thus indicating that TB can dependably identify this benign entity. Conversely, 5% (N=279) of BCCs confirmed on H&E did not exhibit surrounding TB metachromasia. This finding is concerning regarding the specificity of TB staining for BCC, but the authors of this study suggested the possibility that these exceptions were benign “simulants” (ie, trichoepithelioma) of BCC.10
The use of TB also has been shown to be statistically beneficial in Mohs training. In a single-center, single-fellow experiment, the sensitivity and specificity of using TB for BCC were extrapolated.9 Using TB as an adjunct in deep sections showed superior sensitivity to H&E alone in identifying BCC, increasing sensitivity from 96.3% to 99.7%. In a cohort of 352 BCC excisions and frozen sections, only 1 BCC was not completely excised. If H&E only had been performed, the fellow would have missed 13 residual BCC tumors.9
Bennett and Taher7 described a case in which hyaluronic acid (HA) from a filler injection was confused with the HA surrounding BCC tumor nests. They found that when TB is used as an adjunct, the HA filler is easier to differentiate from the HA surrounding the BCC tumor nests. In frozen sections stained with TB, the HA filler appeared as an amorphous, metachromatic, reddish-purple, whereas the HA surrounding the BCC tumor nests appeared as a well-defined red. These findings were less obvious in the same sections stained with H&E alone.7
Squamous Cell Carcinoma—In early investigations, the utility of TB in identifying SCC in frozen sections was thought to be limited. The description by Humphreys and colleagues6 of staining characteristics in SCC suggested that the nuclear detail that H&E provides is more easily recognized. The deep aqua nuclear staining produced with TB was considered more difficult to observe than the cytoplasmic eosinophilia of pyknotic and keratinizing cells in H&E.6
Toluidine blue may be beneficial in providing unique staining characteristics to further detail tumors that are difficult to interpret, such as spindle cell SCC and perineural invasion of aggressive SCC. In H&E, squamous cells of spindle cell SCC (scSCC) blend into the background of inflammatory cells and can be perceptibly difficult to locate. A small cohort of 3 Mohs surgeons who routinely use H&E were surveyed on their ability to detect a proven scSCC in H&E or TB by photograph.12 All 3 were able to detect the scSCC in the TB photographs, but only 2 of 3 were able to detect it in H&E photographs. All 3 surgeons agreed that TB was preferable to H&E for this tumor type. These findings suggested that TB may be superior and preferred over H&E for visualizing tumor cells of scSCC.12 The TB staining characteristics of perineural invasion of aggressive SCC have been referred to as the perineural corona sign because of the bright magenta stain that forms around affected nerves.13 Drosou et al13 suggested that TB may enhance the diagnostic accuracy for perineural SCC.
Rare Tumors—The adjunctive use of TB with H&E has been examined in rare tumors. Published reports have highlighted its use in MMS for treating MAC and PCACC. Toluidine blue exhibits staining advantages for these tumors. It may render isolated nests and perineural invasion of MAC more easily visible on frozen section.15
Although PCACC is rare, the recurrence rate is high.23 Toluidine blue has been used with MMS to ensure complete removal and higher cure rates. The metachromatic nature of TB is advantageous in staining the HA present in these tumors. Those who have reported the use of TB for PCACC prefer it to H&E for frozen sections.14
Technical Aspects—The staining time for TB-treated slides is reduced compared to H&E staining; staining can be efficiently done in frozen sections in less than 2.5 minutes using the method shown in Table 3.17 In comparison, typical H&E staining takes 9 minutes, and older TB techniques take 7 minutes.6

Conclusion
Toluidine blue may play an important and helpful role in the successful diagnosis and treatment of particular cutaneous tumors by providing additional diagnostic information. Although surgeons performing MMS will continue using the staining protocols with which they are most comfortable, adjunctive use of TB over time may provide an additional benefit at low risk for disrupting practice efficiency or workflow. Many Mohs surgeons are accustomed to using this stain, even preferring to interpret only TB-stained slides for cutaneous malignancy. Most published studies on this topic have been observational in nature, and additional controlled trials may be warranted to determine the effects on outcomes in real-world practice.
- Culling CF, Allison TR. Cellular Pathology Technique. 4th ed. Butterworths; 1985.
- Bergeron JA, Singer M. Metachromasy: an experimental and theoretical reevaluation. J Biophys Biochem Cytol. 1958;4:433-457. doi:10.1083/jcb.4.4.433
- Epstein JB, Scully C, Spinelli J. Toluidine blue and Lugol’s iodine application in the assessment of oral malignant disease and lesions at risk of malignancy. J Oral Pathol Med. 1992;21:160-163. doi:10.1111/j.1600-0714.1992.tb00094.x
- Warnakulasuriya KA, Johnson NW. Sensitivity and specificity of OraScan (R) toluidine blue mouthrinse in the detection of oral cancer and precancer. J Oral Pathol Med. 1996;25:97-103. doi:10.1111/j.1600-0714.1996.tb00201.x
- Silapunt S, Peterson SR, Alcalay J, et al. Mohs tissue mapping and processing: a survey study. Dermatol Surg. 2003;29:1109-1112; discussion 1112.
- Humphreys TR, Nemeth A, McCrevey S, et al. A pilot study comparing toluidine blue and hematoxylin and eosin staining of basal cell and squamous cell carcinoma during Mohs surgery. Dermatol Surg. 1996;22:693-697. doi:10.1111/j.1524-4725.1996.tb00619.x
- Bennett R, Taher M. Restylane persistent for 23 months found during Mohs micrographic surgery: a source of confusion with hyaluronic acid surrounding basal cell carcinoma. Dermatol Surg. 2005;31:1366-1369. doi:10.1111/j.1524-4725.2005.31223
- Goldberg LH, Wang SQ, Kimyai-Asadi A. The setting sun sign: visualizing the margins of a basal cell carcinoma on serial frozen sections stained with toluidine blue. Dermatol Surg. 2007;33:761-763. doi:10.1111/j.1524-4725.2007.33158.x
- Tehrani H, May K, Morris A, et al. Does the dual use of toluidine blue and hematoxylin and eosin staining improve basal cell carcinoma detection by Mohs surgery trainees? Dermatol Surg. 2013;39:995-1000. doi:10.1111/dsu.12180
- Donaldson MR, Weber LA. Toluidine blue supports differentiation of folliculocentric basaloid proliferation from basal cell carcinoma on frozen sections in a small single-practice cohort. Dermatol Surg. 2017;43:1303-1306. doi:10.1097/DSS.0000000000001107
- Styperek AR, Goldberg LH, Goldschmidt LE, et al. Toluidine blue and hematoxylin and eosin stains are comparable in evaluating squamous cell carcinoma during Mohs. Dermatol Surg. 2016;42:1279-1284. doi:10.1097/DSS.0000000000000872
- Trieu D, Drosou A, Goldberg LH, et al. Detecting spindle cell squamous cell carcinomas with toluidine blue on frozen sections. Dermatol Surg. 2014;40:1259-1260. doi:10.1097/DSS.0000000000000147
- Drosou A, Trieu D, Goldberg LH, et al. The perineural corona sign: enhancing detection of perineural squamous cell carcinoma during Mohs micrographic surgery with toluidine blue stain. J Am Acad Dermatol. 2014;71:826-827. doi:10.1016/j.jaad.2014.04.076
- Chesser RS, Bertler DE, Fitzpatrick JE, et al. Primary cutaneous adenoid cystic carcinoma treated with Mohs micrographic surgery toluidine blue technique. J Dermatol Surg Oncol. 1992;18:175-176. doi:10.1111/j.1524-4725.1992.tb02794.x
- Wang SQ, Goldberg LH, Nemeth A. The merits of adding toluidine blue-stained slides in Mohs surgery in the treatment of a microcystic adnexal carcinoma. J Am Acad Dermatol. 2007;56:1067-1069. doi:10.1016/j.jaad.2007.01.008
- Chen CL, Wilson S, Afzalneia R, et al. Topical aluminum chloride and Monsel’s solution block toluidine blue staining in Mohs frozen sections: mechanism and solution. Dermatol Surg. 2019;45:1019-1025. doi:10.1097/DSS.0000000000001761
- Todd MM, Lee JW, Marks VJ. Rapid toluidine blue stain for Mohs’ micrographic surgery. Dermatol Surg. 2005;31:244-245. doi:10.1111/j.1524-4725.2005.31053
- Picoto AM, Picoto A. Technical procedures for Mohs fresh tissue surgery. J Derm Surg Oncol. 1986;12:134-138. doi:10.1111/j.1524-4725.1986.tb01442.x
- Sperling LC, Winton GB. The transverse anatomy of androgenic alopecia. J Derm Surg Oncol. 1990;16:1127-1133. doi:10.1111/j.1524 -4725.1990.tb00024.x
- Smith-Zagone MJ, Schwartz MR. Frozen section of skin specimens. Arch Pathol Lab Med. 2005;129:1536-1543. doi:10.5858/2005-129-1536-FSOSS
- Moy RL, Potter TS, Uitto J. Increased glycosaminoglycans production in sclerosing basal cell carcinoma–derived fibroblasts and stimulation of normal skin fibroblast glycosaminoglycans production by a cytokine-derived from sclerosing basal cell carcinoma. Dermatol Surg. 2000;26:1029-1036. doi:10.1046/j.1524-4725.2000.0260111029.x
- Leshin B, White WL. Folliculocentric basaloid proliferation. The bulge (der Wulst) revisited. Arch Dermatol. 1990;126:900-906. doi:10.1001/archderm.126.7.900
- Seab JA, Graham JH. Primary cutaneous adenoid cystic carcinoma.J Am Acad Dermatol. 1987;17:113-118. doi:10.1016/s0190 -9622(87)70182-0
- Culling CF, Allison TR. Cellular Pathology Technique. 4th ed. Butterworths; 1985.
- Bergeron JA, Singer M. Metachromasy: an experimental and theoretical reevaluation. J Biophys Biochem Cytol. 1958;4:433-457. doi:10.1083/jcb.4.4.433
- Epstein JB, Scully C, Spinelli J. Toluidine blue and Lugol’s iodine application in the assessment of oral malignant disease and lesions at risk of malignancy. J Oral Pathol Med. 1992;21:160-163. doi:10.1111/j.1600-0714.1992.tb00094.x
- Warnakulasuriya KA, Johnson NW. Sensitivity and specificity of OraScan (R) toluidine blue mouthrinse in the detection of oral cancer and precancer. J Oral Pathol Med. 1996;25:97-103. doi:10.1111/j.1600-0714.1996.tb00201.x
- Silapunt S, Peterson SR, Alcalay J, et al. Mohs tissue mapping and processing: a survey study. Dermatol Surg. 2003;29:1109-1112; discussion 1112.
- Humphreys TR, Nemeth A, McCrevey S, et al. A pilot study comparing toluidine blue and hematoxylin and eosin staining of basal cell and squamous cell carcinoma during Mohs surgery. Dermatol Surg. 1996;22:693-697. doi:10.1111/j.1524-4725.1996.tb00619.x
- Bennett R, Taher M. Restylane persistent for 23 months found during Mohs micrographic surgery: a source of confusion with hyaluronic acid surrounding basal cell carcinoma. Dermatol Surg. 2005;31:1366-1369. doi:10.1111/j.1524-4725.2005.31223
- Goldberg LH, Wang SQ, Kimyai-Asadi A. The setting sun sign: visualizing the margins of a basal cell carcinoma on serial frozen sections stained with toluidine blue. Dermatol Surg. 2007;33:761-763. doi:10.1111/j.1524-4725.2007.33158.x
- Tehrani H, May K, Morris A, et al. Does the dual use of toluidine blue and hematoxylin and eosin staining improve basal cell carcinoma detection by Mohs surgery trainees? Dermatol Surg. 2013;39:995-1000. doi:10.1111/dsu.12180
- Donaldson MR, Weber LA. Toluidine blue supports differentiation of folliculocentric basaloid proliferation from basal cell carcinoma on frozen sections in a small single-practice cohort. Dermatol Surg. 2017;43:1303-1306. doi:10.1097/DSS.0000000000001107
- Styperek AR, Goldberg LH, Goldschmidt LE, et al. Toluidine blue and hematoxylin and eosin stains are comparable in evaluating squamous cell carcinoma during Mohs. Dermatol Surg. 2016;42:1279-1284. doi:10.1097/DSS.0000000000000872
- Trieu D, Drosou A, Goldberg LH, et al. Detecting spindle cell squamous cell carcinomas with toluidine blue on frozen sections. Dermatol Surg. 2014;40:1259-1260. doi:10.1097/DSS.0000000000000147
- Drosou A, Trieu D, Goldberg LH, et al. The perineural corona sign: enhancing detection of perineural squamous cell carcinoma during Mohs micrographic surgery with toluidine blue stain. J Am Acad Dermatol. 2014;71:826-827. doi:10.1016/j.jaad.2014.04.076
- Chesser RS, Bertler DE, Fitzpatrick JE, et al. Primary cutaneous adenoid cystic carcinoma treated with Mohs micrographic surgery toluidine blue technique. J Dermatol Surg Oncol. 1992;18:175-176. doi:10.1111/j.1524-4725.1992.tb02794.x
- Wang SQ, Goldberg LH, Nemeth A. The merits of adding toluidine blue-stained slides in Mohs surgery in the treatment of a microcystic adnexal carcinoma. J Am Acad Dermatol. 2007;56:1067-1069. doi:10.1016/j.jaad.2007.01.008
- Chen CL, Wilson S, Afzalneia R, et al. Topical aluminum chloride and Monsel’s solution block toluidine blue staining in Mohs frozen sections: mechanism and solution. Dermatol Surg. 2019;45:1019-1025. doi:10.1097/DSS.0000000000001761
- Todd MM, Lee JW, Marks VJ. Rapid toluidine blue stain for Mohs’ micrographic surgery. Dermatol Surg. 2005;31:244-245. doi:10.1111/j.1524-4725.2005.31053
- Picoto AM, Picoto A. Technical procedures for Mohs fresh tissue surgery. J Derm Surg Oncol. 1986;12:134-138. doi:10.1111/j.1524-4725.1986.tb01442.x
- Sperling LC, Winton GB. The transverse anatomy of androgenic alopecia. J Derm Surg Oncol. 1990;16:1127-1133. doi:10.1111/j.1524 -4725.1990.tb00024.x
- Smith-Zagone MJ, Schwartz MR. Frozen section of skin specimens. Arch Pathol Lab Med. 2005;129:1536-1543. doi:10.5858/2005-129-1536-FSOSS
- Moy RL, Potter TS, Uitto J. Increased glycosaminoglycans production in sclerosing basal cell carcinoma–derived fibroblasts and stimulation of normal skin fibroblast glycosaminoglycans production by a cytokine-derived from sclerosing basal cell carcinoma. Dermatol Surg. 2000;26:1029-1036. doi:10.1046/j.1524-4725.2000.0260111029.x
- Leshin B, White WL. Folliculocentric basaloid proliferation. The bulge (der Wulst) revisited. Arch Dermatol. 1990;126:900-906. doi:10.1001/archderm.126.7.900
- Seab JA, Graham JH. Primary cutaneous adenoid cystic carcinoma.J Am Acad Dermatol. 1987;17:113-118. doi:10.1016/s0190 -9622(87)70182-0
Practice Points
- Toluidine blue (TB) staining can be integrated into Mohs micrographic surgery (MMS) for enhanced diagnosis of cutaneous tumors. Its metachromatic properties can aid in differentiating tumor cells from surrounding tissues, especially in basal cell carcinomas and squamous cell carcinomas.
- It is important to develop expertise in interpreting TB-stained sections, as it may offer clearer visualization of nuclear details and stromal components, potentially leading to more accurate diagnosis and effective tumor margin identification.
- Toluidine blue staining can be incorporated into routine MMS practice considering its quick staining process and low disruption to workflow. This can potentially improve diagnostic efficiency without significantly lengthening surgery time.
Reactive Angioendotheliomatosis Following Ad26.COV2.S Vaccination
To the Editor:
Reactive angioendotheliomatosis (RAE) is a rare self-limited cutaneous vascular proliferation of endothelial cells within blood vessels that manifests clinically as infiltrated red-blue patches and plaques with purpura that can progress to occlude vascular lumina. The etiology of RAE is mostly idiopathic; however, the disorder typically occurs in association with a range of systemic diseases, including infection, cryoglobulinemia, leukemia, antiphospholipid syndrome, peripheral vascular disease, and arteriovenous fistula. Histopathologic examination of these lesions shows marked proliferation of endothelial cells, including occlusion of the lumen of blood vessels over wide areas.
After ruling out malignancy, treatment of RAE focuses on targeting the underlying cause or disease, if any is present; 75% of reported cases occur in association with systemic disease.1 Onset can occur at any age without predilection for sex. Reactive angioendotheliomatosis commonly manifests on the extremities but may occur on the head and neck in rare instances.2
The rarity of the condition and its poorly defined clinical characteristics make it difficult to develop a treatment plan. There are no standardized treatment guidelines for the reactive form of angiomatosis. We report a case of RAE that developed 2 weeks after vaccination with the Ad26.COV2.S vaccine (Johnson & Johnson Innovative Medicine [formerly Janssen Pharmaceutical Companies of Johnson & Johnson]) that improved following 2 weeks of treatment with a topical corticosteroid and an oral antihistamine.
A 58-year-old man presented to an outpatient dermatology clinic with pruritus and occasional paresthesia associated with a rash over the left arm of 1 month’s duration. The patient suspected that the rash may have formed secondary to the bite of oak mites on the arms and chest while he was carrying milled wood. Further inquiry into the patient’s history revealed that he received the Ad26.COV2.S vaccine 2 weeks prior to the appearance of the rash. He denied mechanical trauma. His medical history included hypercholesterolemia and a mild COVID-19 infection 8 months prior to the appearance of the rash that did not require hospitalization. He denied fever or chills during the 2 weeks following vaccination. The pruritus was minimally relieved for short periods with over-the-counter calamine lotion. The patient’s medication regimen included daily pravastatin and loratadine at the time of the initial visit. He used acetaminophen as needed for knee pain.
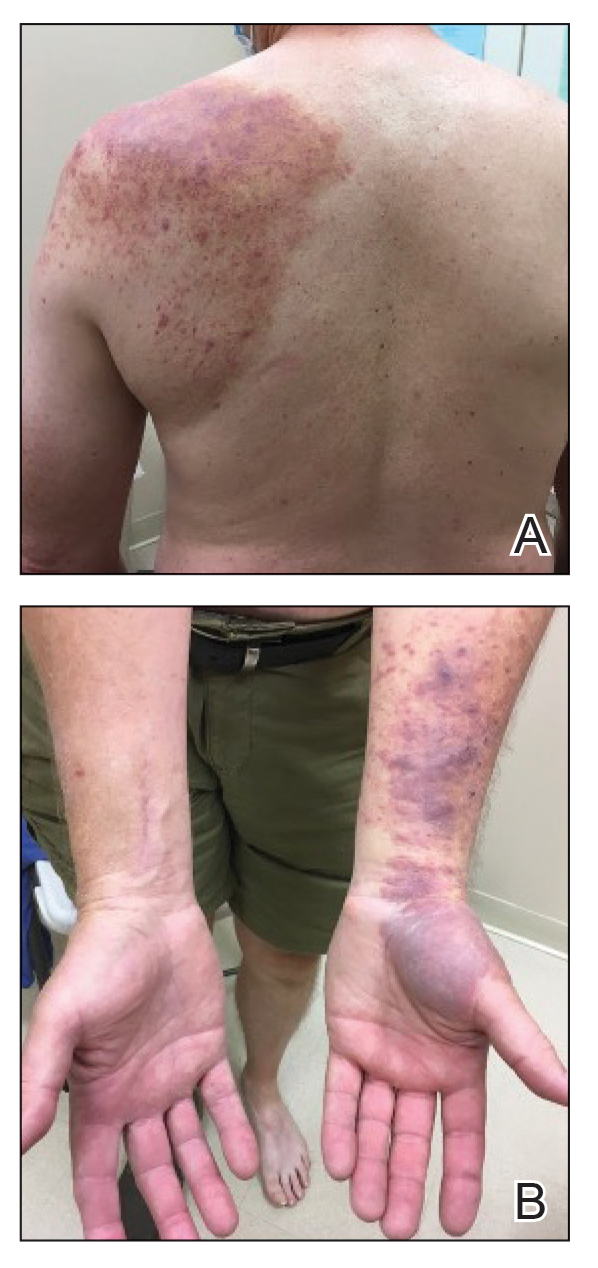
Physical examination revealed palpable purpura in a dermatomal distribution with nonpitting edema over the left scapula (Figure 1A), left anterolateral shoulder, left lateral volar forearm, and thenar eminence of the left hand (Figure 1B). Notably, the entire right arm, conjunctivae, tongue, lips, and bilateral fingernails were clear. Three 4-mm punch biopsies were performed at the initial presentation: 1 perilesional biopsy for direct immunofluorescence testing and 2 lesional biopsies for routine histologic evaluation. An extensive serologic workup failed to reveal abnormalities. An activated partial thromboplastin time, dilute Russell viper venom time, serum protein electrophoresis, and levels of rheumatoid factor and angiotensin-converting enzyme were within reference range. Anticardiolipin antibodies IgA, IgM, and IgG were negative. A cryoglobulin test was negative.
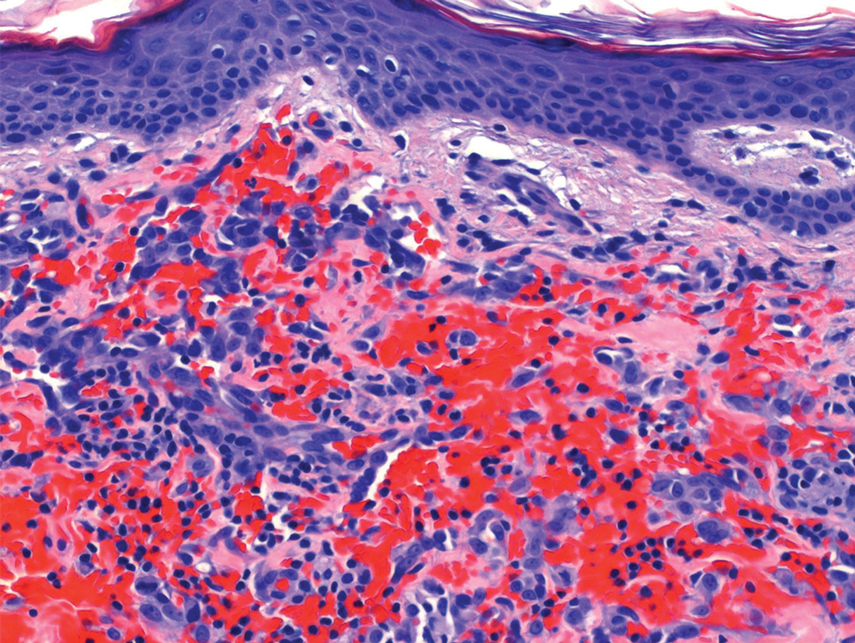
Histopathology revealed a proliferation of irregularly shaped vascular spaces with plump endothelium in the papillary dermis (Figure 2). Scattered leukocyte common antigen-positive lymphocytes were noted within lesions. The epidermis appeared normal, without evidence of spongiosis or alteration of the stratum corneum. Immunohistochemical studies of the perilesional skin biopsy revealed positivity for CD31 and D2-40 (Figure 3). Specimens were negative for CD20 and human herpesvirus 8. Direct immunofluorescence of the perilesional biopsy was negative.
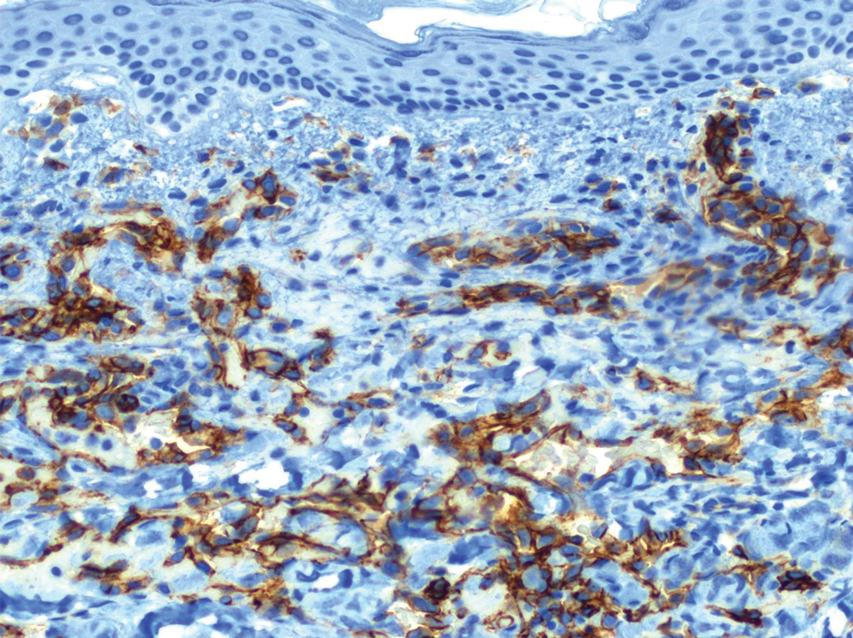
A diagnosis of RAE was made based on clinical and histologic findings. Treatment with triamcinolone ointment 0.1% twice daily and oral cetirizine 10 mg twice daily was initiated. Re-evaluation 2 weeks later revealed notable improvement in the affected areas, including decreased edema, improvement of the purpura, and absence of pruritus. The patient noted no further spread or blister formation while the active areas were being treated with the topical steroid. The treatment regimen was modified to triamcinolone ointment 0.1% once daily, and cetirizine was discontinued. At 3-month follow-up, active areas had completely resolved (Figure 4) and triamcinolone was discontinued. To date, the patient has not had recurrence of symptoms and remains healthy.
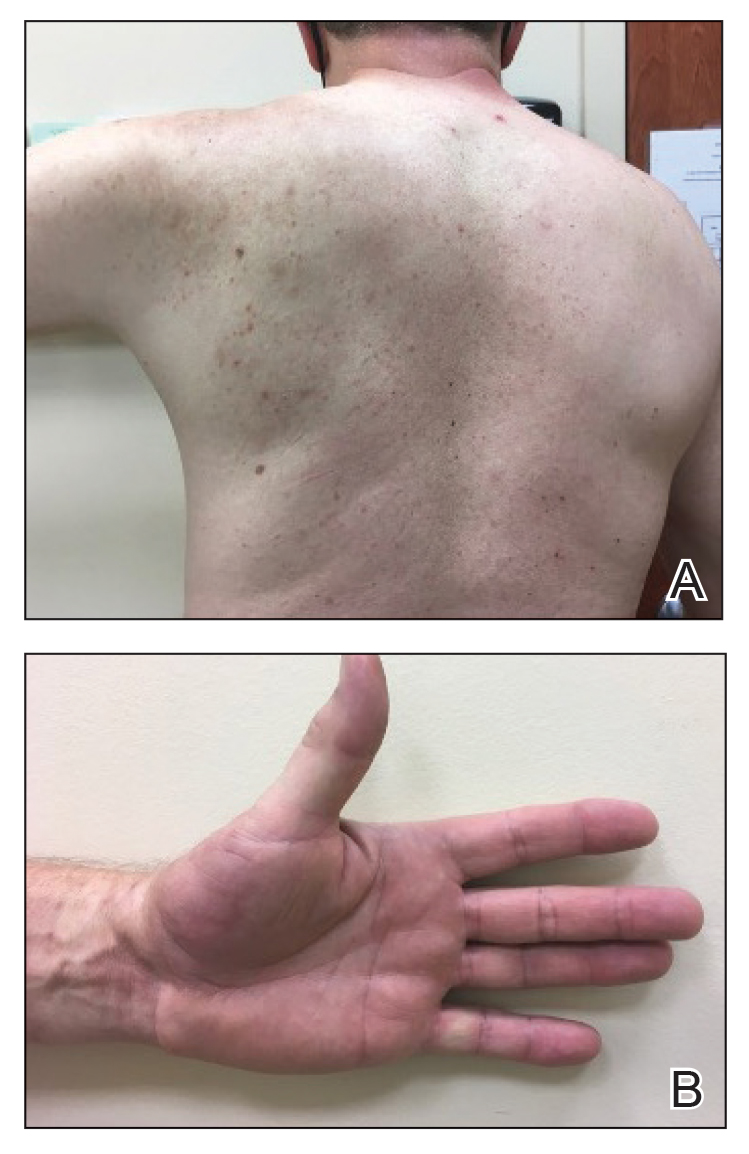
Gottron and Nikolowski3 reported the first case of RAE in an adult patient who presented with purpuric patches secondary to skin infarction. Current definitions use the umbrella term cutaneous reactive angiomatosis to cover 3 major subtypes: reactive angioendotheliomatosis, diffuse dermal angioendotheliomatosis, and acroangiodermatitis (pseudo-Kaposi sarcoma [KS]). The manifestation of these subgroups is clinically similar, and they must be differentiated through histologic evaluation.4
Reactive angioendotheliomatosis has an unknown pathogenesis and is poorly defined clinically. The exact pathophysiology is unknown but likely is linked to vaso-occlusion and hypoxia.1 A PubMed search of articles indexed for MEDLINE, as well as a review of Science Direct, Google Scholar, and Cochrane Library, using the terms reactive angioendotheliomatosis, COVID, vaccine, Ad26.COV2.S, and RAE in any combination revealed no prior cases of RAE in association with Ad26.COV2.S vaccination.
By the late 1980s, systemic angioendotheliomatosis was segregated into 2 distinct entities: malignant and reactive.4 The differential diagnosis of malignant systemic angioendotheliomatosis includes KS and angiosarcoma; nonmalignant causes are the variants of cutaneous reactive angiomatosis. It is important to rule out KS because of its malignant and deceptive nature. It is unknown if KS originates in blood vessels or lymphatic endothelial cells; however, evidence is strongly in favor of blood vessel origin using CD31 and CD34 endothelial markers.5 CD34 positivity is more reliable than CD31 in diagnosing KS, but the absence of both markers does not offer enough evidence to rule out KS on its own.6
In our patient, histopathology revealed cells positive for CD31 and D2-40; the latter is a lymphatic endothelial cell marker that stains the endothelium of lymphatic channels but not blood vessels.7 Positive D2-40 can be indicative of KS and non-KS lesions, each with a distinct staining pattern. D2-40 staining on non-KS lesions is confined to lymphatic vessels, as it was in our patient; in contrast, spindle-shaped cells also will be stained in KS lesions.8
Another cell marker, CD20, is a B cell–specific protein that can be measured to help diagnose malignant diseases such as B-cell lymphoma and leukemia. Human herpesvirus 8 (also known as KS-associated herpesvirus) is the infectious cause of KS and traditionally has been detected using methods such as the polymerase chain reaction.9,10
Most cases of RAE are idiopathic and occur in association with systemic disease, which was not the case in our patient. We speculated that his reaction was most likely triggered by vascular transfection of endothelial cells secondary to Ad26.COV2.S vaccination. Alternatively, vaccination may have caused vascular occlusion, though the lack of cyanosis, nail changes, and route of inoculant make this less likely.
All approved COVID-19 vaccines are designed solely for intramuscular injection. In comparison to other types of tissue, muscles have superior vascularity, allowing for enhanced mobilization of compounds, which results in faster systemic circulation.11 Alternative methods of injection, including intravascular, subcutaneous, and intradermal, may lead to decreased efficacy or adverse events, or both.
Prior cases of RAE have been treated with laser therapy, topical or systemic corticosteroids, excisional removal, or topical β-blockers, such as timolol.12β-Blocking agents act on β-adrenergic receptors on endothelial cells to inhibit angiogenesis by reducing release of blood vessel growth-signaling molecules and triggering apoptosis. In this patient, topical steroids and oral antihistamines were sufficient treatment.
Vaccine-related adverse events have been reported but remain rare. The benefits of Ad26.COV2.S vaccination for protection against COVID-19 outweigh the extremely low risk for adverse events.13 For that reason, the Centers for Disease Control and Prevention recommends a booster for individuals who are eligible to maximize protection. Intramuscular injection of Ad26.COV2.S resulted in a lower incidence of moderate to severe COVID-19 cases in all age groups vs the placebo group. Hypersensitivity adverse events were reported in 0.4% of Ad26.COV2.S-vaccinated patients vs 0.4% of patients who received a placebo; the more common reactions were nonanaphylactic.13
There have been 12 reports of cerebral venous sinus thrombosis with thrombocytopenia after Ad26.COV2.S vaccination, which sparked nationwide controversy over the safety of the Ad26.COV2.S vaccine.14 After further investigation into those reports, the US Food and Drug Administration and the Centers for Disease Control and Prevention concluded that the benefits of the Ad26.COV2.S vaccine outweigh the low risk for associated thrombosis.15
Although adverse reactions are rare, it is important that health care providers take proper safety measures before and while administering any COVID-19 vaccine. Patients should be screened for contraindications to the COVID-19 vaccine to mitigate adverse effects seen in the small percentage of patients who may need to take alternative precautions.
The broad tissue tropism and high transmissibility of SARS-CoV-2 are the main contributors to its infection having reached pandemic scale. The spike (S) protein on SARS-CoV-2 binds to ACE2, the most thoroughly studied SARS-CoV-2 receptor, which is found in a range of tissues, including arterial endothelial cells, leading to its transfection. Several studies have proposed that expression of the S protein causes endothelial dysfunction through cytokine release, activation of complement, and ultimately microvascular occlusion.16
Recent developments in the use of viral-like particles, such as vesicular stomatitis virus, may mitigate future cases of RAE that are associated with endothelial cell transfection. Vesicular stomatitis virus is a popular model virus for research applications due to its glycoprotein and matrix protein contributing to its broad tropism. Recent efforts to alter these proteins have successfully limited the broad tropism of vesicular stomatitis virus.17
The SARS-CoV-2 virus must be handled in a Biosafety Level 3 laboratory. Conversely, pseudoviruses can be handled in lower containment facilities due to their safe and efficacious nature, offering an avenue to expedite vaccine development against many viral outbreaks, including SARS-CoV-2.18
An increasing number of cutaneous manifestations have been associated with COVID-19 infection and vaccination. Eruptive pseudoangiomatosis, a rare self-limiting exanthem, has been reported in association with COVID-19 vaccination.19 Eruptive pseudoangiomatosis manifests as erythematous blanchable papules that resemble angiomas, typically in a widespread distribution. Eruptive pseudoangiomatosis has striking similarities to RAE histologically; both manifest as dilated dermal blood vessels with plump endothelial cells.
Our case is unique because of the vasculitic palpable nature of the lesions, which were localized to the left arm. Eruptive pseudoangiomatosis formation after COVID-19 infection or SARS-CoV-2 vaccination may suggest alteration of ACE2 by binding of S protein.20 Such alteration of the ACE2 pathway would lead to inflammation of angiotensin II, causing proliferation of endothelial cells in the formation of angiomalike lesions. This hypothesis suggests a paraviral eruption secondary to an immunologic reaction, not a classical virtual eruption from direct contact of the virus on blood vessels. Although EPA and RAE are harmless and self-limiting, these reports will spread awareness of the increasing number of skin manifestations related to COVID-19 and SARS-CoV-2 virus vaccination.
Acknowledgment—Thoughtful insights and comments on this manuscript were provided by Christine J. Ko, MD (New Haven, Connecticut); Christine L. Egan, MD (Glen Mills, Pennsylvania); Howard A. Bueller, MD (Delray Beach, Florida); and Juan Pablo Robles, PhD (Juriquilla, Mexico).
- McMenamin ME, Fletcher CDM. Reactive angioendotheliomatosis: a study of 15 cases demonstrating a wide clinicopathologic spectrum. Am J Surg Pathol. 2002;26:686-697. doi:10.1097/00000478-200206000-00001
- Khan S, Pujani M, Jetley S, et al. Angiomatosis: a rare vascular proliferation of head and neck region. J Cutan Aesthet Surg. 2015;8:108-110. doi:10.4103/0974-2077.158448
- Gottron HA, Nikolowski W. Extrarenal Lohlein focal nephritis of the skin in endocarditis. Arch Klin Exp Dermatol. 1958;207:156-176.
- Cooper PH. Angioendotheliomatosis: two separate diseases. J Cutan Pathol. 1988;15:259. doi:10.1111/j.1600-0560.1988.tb00556.x
- Cancian L, Hansen A, Boshoff C. Cellular origin of Kaposi’s sarcoma and Kaposi’s sarcoma-associated herpesvirus-induced cell reprogramming. Trends Cell Biol. Sep 2013;23:421-32. doi:10.1016/j.tcb.2013.04.001
- Russell Jones R, Orchard G, Zelger B, et al. Immunostaining for CD31 and CD34 in Kaposi sarcoma. J Clin Pathol. 1995;48:1011-1016. doi:10.1136/jcp.48.11.1011
- Kahn HJ, Bailey D, Marks A. Monoclonal antibody D2-40, a new marker of lymphatic endothelium, reacts with Kaposi’s sarcoma and a subset of angiosarcomas. Mod Pathol. 2002;15:434-440. doi:10.1038/modpathol.3880543
- Genedy RM, Hamza AM, Abdel Latef AA, et al. Sensitivity and specificity of D2-40 in differentiating Kaposi sarcoma from its mimickers. J Egyptian Womens Dermatolog Soc. 2021;18:67-74. doi:10.4103/jewd.jewd_61_20
- Mesri EA, Cesarman E, Boshoff C. Kaposi’s sarcoma and its associated herpesvirus. Nat Rev Cancer. 2010;10:707-719. doi:10.1038/nrc2888
- Patel RM, Goldblum JR, Hsi ED. Immunohistochemical detection of human herpes virus-8 latent nuclear antigen-1 is useful in the diagnosis of Kaposi sarcoma. Mod Pathol. 2004;17:456-460. doi:10.1038/modpathol.3800061
- Zuckerman JN. The importance of injecting vaccines into muscle. Different patients need different needle sizes. BMJ. 2000;321:1237-1238. doi:10.1136/bmj.321.7271.1237
- Bhatia R, Hazarika N, Chandrasekaran D, et al. Treatment of posttraumatic reactive angioendotheliomatosis with topical timolol maleate. JAMA Dermatol. 2021;157:1002-1004. doi:10.1001/jamadermatol.2021.1770
- Sadoff J, Gray G, Vandebosch A, et al; ENSEMBLE Study Group. Safety and efficacy of single-dose Ad26.COV2.S vaccine against Covid-19. N Engl J Med. 2021;384:2187-2201. doi:10.1056/NEJMoa2101544
- See I, Su JR, Lale A, et al. US case reports of cerebral venous sinus thrombosis with thrombocytopenia after Ad26.COV2.S vaccination, March 2 to April 21, 2021. JAMA. 2021;325:2448-2456. doi:10.1001/jama.2021.7517
- Berry CT, Eliliwi M, Gallagher S, et al. Cutaneous small vessel vasculitis following single-dose Janssen Ad26.COV2.S vaccination. JAAD Case Rep. 2021;15:11-14. doi:10.1016/j.jdcr.2021.07.002
- Flaumenhaft R, Enjyoji K, Schmaier AA. Vasculopathy in COVID-19. Blood. 2022;140:222-235. doi:10.1182/blood.2021012250
- Hastie E, Cataldi M, Marriott I, et al. Understanding and altering cell tropism of vesicular stomatitis virus. Virus Res. 2013;176:16-32. doi:10.1016/j.virusres.2013.06.003
- Xiong H-L, Wu Y-T, Cao J-L, et al. Robust neutralization assay based on SARS-CoV-2 S-protein-bearing vesicular stomatitis virus (VSV) pseudovirus and ACE2-overexpressing BHK21 cells. Emerg Microbes Infect. 2020;9:2105-2113. doi:10.1080/22221751.2020.1815589
- Mohta A, Jain SK, Mehta RD, et al. Development of eruptive pseudoangiomatosis following COVID-19 immunization – apropos of 5 cases. J Eur Acad Dermatol Venereol. 2021;35:e722-e725. doi:10.1111/jdv.17499
- Angeli F, Spanevello A, Reboldi G, et al. SARS-CoV-2 vaccines: lights and shadows. Eur J Intern Med. 2021;88:1-8. doi:10.1016/j.ejim.2021.04.019
To the Editor:
Reactive angioendotheliomatosis (RAE) is a rare self-limited cutaneous vascular proliferation of endothelial cells within blood vessels that manifests clinically as infiltrated red-blue patches and plaques with purpura that can progress to occlude vascular lumina. The etiology of RAE is mostly idiopathic; however, the disorder typically occurs in association with a range of systemic diseases, including infection, cryoglobulinemia, leukemia, antiphospholipid syndrome, peripheral vascular disease, and arteriovenous fistula. Histopathologic examination of these lesions shows marked proliferation of endothelial cells, including occlusion of the lumen of blood vessels over wide areas.
After ruling out malignancy, treatment of RAE focuses on targeting the underlying cause or disease, if any is present; 75% of reported cases occur in association with systemic disease.1 Onset can occur at any age without predilection for sex. Reactive angioendotheliomatosis commonly manifests on the extremities but may occur on the head and neck in rare instances.2
The rarity of the condition and its poorly defined clinical characteristics make it difficult to develop a treatment plan. There are no standardized treatment guidelines for the reactive form of angiomatosis. We report a case of RAE that developed 2 weeks after vaccination with the Ad26.COV2.S vaccine (Johnson & Johnson Innovative Medicine [formerly Janssen Pharmaceutical Companies of Johnson & Johnson]) that improved following 2 weeks of treatment with a topical corticosteroid and an oral antihistamine.
A 58-year-old man presented to an outpatient dermatology clinic with pruritus and occasional paresthesia associated with a rash over the left arm of 1 month’s duration. The patient suspected that the rash may have formed secondary to the bite of oak mites on the arms and chest while he was carrying milled wood. Further inquiry into the patient’s history revealed that he received the Ad26.COV2.S vaccine 2 weeks prior to the appearance of the rash. He denied mechanical trauma. His medical history included hypercholesterolemia and a mild COVID-19 infection 8 months prior to the appearance of the rash that did not require hospitalization. He denied fever or chills during the 2 weeks following vaccination. The pruritus was minimally relieved for short periods with over-the-counter calamine lotion. The patient’s medication regimen included daily pravastatin and loratadine at the time of the initial visit. He used acetaminophen as needed for knee pain.

Physical examination revealed palpable purpura in a dermatomal distribution with nonpitting edema over the left scapula (Figure 1A), left anterolateral shoulder, left lateral volar forearm, and thenar eminence of the left hand (Figure 1B). Notably, the entire right arm, conjunctivae, tongue, lips, and bilateral fingernails were clear. Three 4-mm punch biopsies were performed at the initial presentation: 1 perilesional biopsy for direct immunofluorescence testing and 2 lesional biopsies for routine histologic evaluation. An extensive serologic workup failed to reveal abnormalities. An activated partial thromboplastin time, dilute Russell viper venom time, serum protein electrophoresis, and levels of rheumatoid factor and angiotensin-converting enzyme were within reference range. Anticardiolipin antibodies IgA, IgM, and IgG were negative. A cryoglobulin test was negative.

Histopathology revealed a proliferation of irregularly shaped vascular spaces with plump endothelium in the papillary dermis (Figure 2). Scattered leukocyte common antigen-positive lymphocytes were noted within lesions. The epidermis appeared normal, without evidence of spongiosis or alteration of the stratum corneum. Immunohistochemical studies of the perilesional skin biopsy revealed positivity for CD31 and D2-40 (Figure 3). Specimens were negative for CD20 and human herpesvirus 8. Direct immunofluorescence of the perilesional biopsy was negative.

A diagnosis of RAE was made based on clinical and histologic findings. Treatment with triamcinolone ointment 0.1% twice daily and oral cetirizine 10 mg twice daily was initiated. Re-evaluation 2 weeks later revealed notable improvement in the affected areas, including decreased edema, improvement of the purpura, and absence of pruritus. The patient noted no further spread or blister formation while the active areas were being treated with the topical steroid. The treatment regimen was modified to triamcinolone ointment 0.1% once daily, and cetirizine was discontinued. At 3-month follow-up, active areas had completely resolved (Figure 4) and triamcinolone was discontinued. To date, the patient has not had recurrence of symptoms and remains healthy.

Gottron and Nikolowski3 reported the first case of RAE in an adult patient who presented with purpuric patches secondary to skin infarction. Current definitions use the umbrella term cutaneous reactive angiomatosis to cover 3 major subtypes: reactive angioendotheliomatosis, diffuse dermal angioendotheliomatosis, and acroangiodermatitis (pseudo-Kaposi sarcoma [KS]). The manifestation of these subgroups is clinically similar, and they must be differentiated through histologic evaluation.4
Reactive angioendotheliomatosis has an unknown pathogenesis and is poorly defined clinically. The exact pathophysiology is unknown but likely is linked to vaso-occlusion and hypoxia.1 A PubMed search of articles indexed for MEDLINE, as well as a review of Science Direct, Google Scholar, and Cochrane Library, using the terms reactive angioendotheliomatosis, COVID, vaccine, Ad26.COV2.S, and RAE in any combination revealed no prior cases of RAE in association with Ad26.COV2.S vaccination.
By the late 1980s, systemic angioendotheliomatosis was segregated into 2 distinct entities: malignant and reactive.4 The differential diagnosis of malignant systemic angioendotheliomatosis includes KS and angiosarcoma; nonmalignant causes are the variants of cutaneous reactive angiomatosis. It is important to rule out KS because of its malignant and deceptive nature. It is unknown if KS originates in blood vessels or lymphatic endothelial cells; however, evidence is strongly in favor of blood vessel origin using CD31 and CD34 endothelial markers.5 CD34 positivity is more reliable than CD31 in diagnosing KS, but the absence of both markers does not offer enough evidence to rule out KS on its own.6
In our patient, histopathology revealed cells positive for CD31 and D2-40; the latter is a lymphatic endothelial cell marker that stains the endothelium of lymphatic channels but not blood vessels.7 Positive D2-40 can be indicative of KS and non-KS lesions, each with a distinct staining pattern. D2-40 staining on non-KS lesions is confined to lymphatic vessels, as it was in our patient; in contrast, spindle-shaped cells also will be stained in KS lesions.8
Another cell marker, CD20, is a B cell–specific protein that can be measured to help diagnose malignant diseases such as B-cell lymphoma and leukemia. Human herpesvirus 8 (also known as KS-associated herpesvirus) is the infectious cause of KS and traditionally has been detected using methods such as the polymerase chain reaction.9,10
Most cases of RAE are idiopathic and occur in association with systemic disease, which was not the case in our patient. We speculated that his reaction was most likely triggered by vascular transfection of endothelial cells secondary to Ad26.COV2.S vaccination. Alternatively, vaccination may have caused vascular occlusion, though the lack of cyanosis, nail changes, and route of inoculant make this less likely.
All approved COVID-19 vaccines are designed solely for intramuscular injection. In comparison to other types of tissue, muscles have superior vascularity, allowing for enhanced mobilization of compounds, which results in faster systemic circulation.11 Alternative methods of injection, including intravascular, subcutaneous, and intradermal, may lead to decreased efficacy or adverse events, or both.
Prior cases of RAE have been treated with laser therapy, topical or systemic corticosteroids, excisional removal, or topical β-blockers, such as timolol.12β-Blocking agents act on β-adrenergic receptors on endothelial cells to inhibit angiogenesis by reducing release of blood vessel growth-signaling molecules and triggering apoptosis. In this patient, topical steroids and oral antihistamines were sufficient treatment.
Vaccine-related adverse events have been reported but remain rare. The benefits of Ad26.COV2.S vaccination for protection against COVID-19 outweigh the extremely low risk for adverse events.13 For that reason, the Centers for Disease Control and Prevention recommends a booster for individuals who are eligible to maximize protection. Intramuscular injection of Ad26.COV2.S resulted in a lower incidence of moderate to severe COVID-19 cases in all age groups vs the placebo group. Hypersensitivity adverse events were reported in 0.4% of Ad26.COV2.S-vaccinated patients vs 0.4% of patients who received a placebo; the more common reactions were nonanaphylactic.13
There have been 12 reports of cerebral venous sinus thrombosis with thrombocytopenia after Ad26.COV2.S vaccination, which sparked nationwide controversy over the safety of the Ad26.COV2.S vaccine.14 After further investigation into those reports, the US Food and Drug Administration and the Centers for Disease Control and Prevention concluded that the benefits of the Ad26.COV2.S vaccine outweigh the low risk for associated thrombosis.15
Although adverse reactions are rare, it is important that health care providers take proper safety measures before and while administering any COVID-19 vaccine. Patients should be screened for contraindications to the COVID-19 vaccine to mitigate adverse effects seen in the small percentage of patients who may need to take alternative precautions.
The broad tissue tropism and high transmissibility of SARS-CoV-2 are the main contributors to its infection having reached pandemic scale. The spike (S) protein on SARS-CoV-2 binds to ACE2, the most thoroughly studied SARS-CoV-2 receptor, which is found in a range of tissues, including arterial endothelial cells, leading to its transfection. Several studies have proposed that expression of the S protein causes endothelial dysfunction through cytokine release, activation of complement, and ultimately microvascular occlusion.16
Recent developments in the use of viral-like particles, such as vesicular stomatitis virus, may mitigate future cases of RAE that are associated with endothelial cell transfection. Vesicular stomatitis virus is a popular model virus for research applications due to its glycoprotein and matrix protein contributing to its broad tropism. Recent efforts to alter these proteins have successfully limited the broad tropism of vesicular stomatitis virus.17
The SARS-CoV-2 virus must be handled in a Biosafety Level 3 laboratory. Conversely, pseudoviruses can be handled in lower containment facilities due to their safe and efficacious nature, offering an avenue to expedite vaccine development against many viral outbreaks, including SARS-CoV-2.18
An increasing number of cutaneous manifestations have been associated with COVID-19 infection and vaccination. Eruptive pseudoangiomatosis, a rare self-limiting exanthem, has been reported in association with COVID-19 vaccination.19 Eruptive pseudoangiomatosis manifests as erythematous blanchable papules that resemble angiomas, typically in a widespread distribution. Eruptive pseudoangiomatosis has striking similarities to RAE histologically; both manifest as dilated dermal blood vessels with plump endothelial cells.
Our case is unique because of the vasculitic palpable nature of the lesions, which were localized to the left arm. Eruptive pseudoangiomatosis formation after COVID-19 infection or SARS-CoV-2 vaccination may suggest alteration of ACE2 by binding of S protein.20 Such alteration of the ACE2 pathway would lead to inflammation of angiotensin II, causing proliferation of endothelial cells in the formation of angiomalike lesions. This hypothesis suggests a paraviral eruption secondary to an immunologic reaction, not a classical virtual eruption from direct contact of the virus on blood vessels. Although EPA and RAE are harmless and self-limiting, these reports will spread awareness of the increasing number of skin manifestations related to COVID-19 and SARS-CoV-2 virus vaccination.
Acknowledgment—Thoughtful insights and comments on this manuscript were provided by Christine J. Ko, MD (New Haven, Connecticut); Christine L. Egan, MD (Glen Mills, Pennsylvania); Howard A. Bueller, MD (Delray Beach, Florida); and Juan Pablo Robles, PhD (Juriquilla, Mexico).
To the Editor:
Reactive angioendotheliomatosis (RAE) is a rare self-limited cutaneous vascular proliferation of endothelial cells within blood vessels that manifests clinically as infiltrated red-blue patches and plaques with purpura that can progress to occlude vascular lumina. The etiology of RAE is mostly idiopathic; however, the disorder typically occurs in association with a range of systemic diseases, including infection, cryoglobulinemia, leukemia, antiphospholipid syndrome, peripheral vascular disease, and arteriovenous fistula. Histopathologic examination of these lesions shows marked proliferation of endothelial cells, including occlusion of the lumen of blood vessels over wide areas.
After ruling out malignancy, treatment of RAE focuses on targeting the underlying cause or disease, if any is present; 75% of reported cases occur in association with systemic disease.1 Onset can occur at any age without predilection for sex. Reactive angioendotheliomatosis commonly manifests on the extremities but may occur on the head and neck in rare instances.2
The rarity of the condition and its poorly defined clinical characteristics make it difficult to develop a treatment plan. There are no standardized treatment guidelines for the reactive form of angiomatosis. We report a case of RAE that developed 2 weeks after vaccination with the Ad26.COV2.S vaccine (Johnson & Johnson Innovative Medicine [formerly Janssen Pharmaceutical Companies of Johnson & Johnson]) that improved following 2 weeks of treatment with a topical corticosteroid and an oral antihistamine.
A 58-year-old man presented to an outpatient dermatology clinic with pruritus and occasional paresthesia associated with a rash over the left arm of 1 month’s duration. The patient suspected that the rash may have formed secondary to the bite of oak mites on the arms and chest while he was carrying milled wood. Further inquiry into the patient’s history revealed that he received the Ad26.COV2.S vaccine 2 weeks prior to the appearance of the rash. He denied mechanical trauma. His medical history included hypercholesterolemia and a mild COVID-19 infection 8 months prior to the appearance of the rash that did not require hospitalization. He denied fever or chills during the 2 weeks following vaccination. The pruritus was minimally relieved for short periods with over-the-counter calamine lotion. The patient’s medication regimen included daily pravastatin and loratadine at the time of the initial visit. He used acetaminophen as needed for knee pain.

Physical examination revealed palpable purpura in a dermatomal distribution with nonpitting edema over the left scapula (Figure 1A), left anterolateral shoulder, left lateral volar forearm, and thenar eminence of the left hand (Figure 1B). Notably, the entire right arm, conjunctivae, tongue, lips, and bilateral fingernails were clear. Three 4-mm punch biopsies were performed at the initial presentation: 1 perilesional biopsy for direct immunofluorescence testing and 2 lesional biopsies for routine histologic evaluation. An extensive serologic workup failed to reveal abnormalities. An activated partial thromboplastin time, dilute Russell viper venom time, serum protein electrophoresis, and levels of rheumatoid factor and angiotensin-converting enzyme were within reference range. Anticardiolipin antibodies IgA, IgM, and IgG were negative. A cryoglobulin test was negative.

Histopathology revealed a proliferation of irregularly shaped vascular spaces with plump endothelium in the papillary dermis (Figure 2). Scattered leukocyte common antigen-positive lymphocytes were noted within lesions. The epidermis appeared normal, without evidence of spongiosis or alteration of the stratum corneum. Immunohistochemical studies of the perilesional skin biopsy revealed positivity for CD31 and D2-40 (Figure 3). Specimens were negative for CD20 and human herpesvirus 8. Direct immunofluorescence of the perilesional biopsy was negative.

A diagnosis of RAE was made based on clinical and histologic findings. Treatment with triamcinolone ointment 0.1% twice daily and oral cetirizine 10 mg twice daily was initiated. Re-evaluation 2 weeks later revealed notable improvement in the affected areas, including decreased edema, improvement of the purpura, and absence of pruritus. The patient noted no further spread or blister formation while the active areas were being treated with the topical steroid. The treatment regimen was modified to triamcinolone ointment 0.1% once daily, and cetirizine was discontinued. At 3-month follow-up, active areas had completely resolved (Figure 4) and triamcinolone was discontinued. To date, the patient has not had recurrence of symptoms and remains healthy.

Gottron and Nikolowski3 reported the first case of RAE in an adult patient who presented with purpuric patches secondary to skin infarction. Current definitions use the umbrella term cutaneous reactive angiomatosis to cover 3 major subtypes: reactive angioendotheliomatosis, diffuse dermal angioendotheliomatosis, and acroangiodermatitis (pseudo-Kaposi sarcoma [KS]). The manifestation of these subgroups is clinically similar, and they must be differentiated through histologic evaluation.4
Reactive angioendotheliomatosis has an unknown pathogenesis and is poorly defined clinically. The exact pathophysiology is unknown but likely is linked to vaso-occlusion and hypoxia.1 A PubMed search of articles indexed for MEDLINE, as well as a review of Science Direct, Google Scholar, and Cochrane Library, using the terms reactive angioendotheliomatosis, COVID, vaccine, Ad26.COV2.S, and RAE in any combination revealed no prior cases of RAE in association with Ad26.COV2.S vaccination.
By the late 1980s, systemic angioendotheliomatosis was segregated into 2 distinct entities: malignant and reactive.4 The differential diagnosis of malignant systemic angioendotheliomatosis includes KS and angiosarcoma; nonmalignant causes are the variants of cutaneous reactive angiomatosis. It is important to rule out KS because of its malignant and deceptive nature. It is unknown if KS originates in blood vessels or lymphatic endothelial cells; however, evidence is strongly in favor of blood vessel origin using CD31 and CD34 endothelial markers.5 CD34 positivity is more reliable than CD31 in diagnosing KS, but the absence of both markers does not offer enough evidence to rule out KS on its own.6
In our patient, histopathology revealed cells positive for CD31 and D2-40; the latter is a lymphatic endothelial cell marker that stains the endothelium of lymphatic channels but not blood vessels.7 Positive D2-40 can be indicative of KS and non-KS lesions, each with a distinct staining pattern. D2-40 staining on non-KS lesions is confined to lymphatic vessels, as it was in our patient; in contrast, spindle-shaped cells also will be stained in KS lesions.8
Another cell marker, CD20, is a B cell–specific protein that can be measured to help diagnose malignant diseases such as B-cell lymphoma and leukemia. Human herpesvirus 8 (also known as KS-associated herpesvirus) is the infectious cause of KS and traditionally has been detected using methods such as the polymerase chain reaction.9,10
Most cases of RAE are idiopathic and occur in association with systemic disease, which was not the case in our patient. We speculated that his reaction was most likely triggered by vascular transfection of endothelial cells secondary to Ad26.COV2.S vaccination. Alternatively, vaccination may have caused vascular occlusion, though the lack of cyanosis, nail changes, and route of inoculant make this less likely.
All approved COVID-19 vaccines are designed solely for intramuscular injection. In comparison to other types of tissue, muscles have superior vascularity, allowing for enhanced mobilization of compounds, which results in faster systemic circulation.11 Alternative methods of injection, including intravascular, subcutaneous, and intradermal, may lead to decreased efficacy or adverse events, or both.
Prior cases of RAE have been treated with laser therapy, topical or systemic corticosteroids, excisional removal, or topical β-blockers, such as timolol.12β-Blocking agents act on β-adrenergic receptors on endothelial cells to inhibit angiogenesis by reducing release of blood vessel growth-signaling molecules and triggering apoptosis. In this patient, topical steroids and oral antihistamines were sufficient treatment.
Vaccine-related adverse events have been reported but remain rare. The benefits of Ad26.COV2.S vaccination for protection against COVID-19 outweigh the extremely low risk for adverse events.13 For that reason, the Centers for Disease Control and Prevention recommends a booster for individuals who are eligible to maximize protection. Intramuscular injection of Ad26.COV2.S resulted in a lower incidence of moderate to severe COVID-19 cases in all age groups vs the placebo group. Hypersensitivity adverse events were reported in 0.4% of Ad26.COV2.S-vaccinated patients vs 0.4% of patients who received a placebo; the more common reactions were nonanaphylactic.13
There have been 12 reports of cerebral venous sinus thrombosis with thrombocytopenia after Ad26.COV2.S vaccination, which sparked nationwide controversy over the safety of the Ad26.COV2.S vaccine.14 After further investigation into those reports, the US Food and Drug Administration and the Centers for Disease Control and Prevention concluded that the benefits of the Ad26.COV2.S vaccine outweigh the low risk for associated thrombosis.15
Although adverse reactions are rare, it is important that health care providers take proper safety measures before and while administering any COVID-19 vaccine. Patients should be screened for contraindications to the COVID-19 vaccine to mitigate adverse effects seen in the small percentage of patients who may need to take alternative precautions.
The broad tissue tropism and high transmissibility of SARS-CoV-2 are the main contributors to its infection having reached pandemic scale. The spike (S) protein on SARS-CoV-2 binds to ACE2, the most thoroughly studied SARS-CoV-2 receptor, which is found in a range of tissues, including arterial endothelial cells, leading to its transfection. Several studies have proposed that expression of the S protein causes endothelial dysfunction through cytokine release, activation of complement, and ultimately microvascular occlusion.16
Recent developments in the use of viral-like particles, such as vesicular stomatitis virus, may mitigate future cases of RAE that are associated with endothelial cell transfection. Vesicular stomatitis virus is a popular model virus for research applications due to its glycoprotein and matrix protein contributing to its broad tropism. Recent efforts to alter these proteins have successfully limited the broad tropism of vesicular stomatitis virus.17
The SARS-CoV-2 virus must be handled in a Biosafety Level 3 laboratory. Conversely, pseudoviruses can be handled in lower containment facilities due to their safe and efficacious nature, offering an avenue to expedite vaccine development against many viral outbreaks, including SARS-CoV-2.18
An increasing number of cutaneous manifestations have been associated with COVID-19 infection and vaccination. Eruptive pseudoangiomatosis, a rare self-limiting exanthem, has been reported in association with COVID-19 vaccination.19 Eruptive pseudoangiomatosis manifests as erythematous blanchable papules that resemble angiomas, typically in a widespread distribution. Eruptive pseudoangiomatosis has striking similarities to RAE histologically; both manifest as dilated dermal blood vessels with plump endothelial cells.
Our case is unique because of the vasculitic palpable nature of the lesions, which were localized to the left arm. Eruptive pseudoangiomatosis formation after COVID-19 infection or SARS-CoV-2 vaccination may suggest alteration of ACE2 by binding of S protein.20 Such alteration of the ACE2 pathway would lead to inflammation of angiotensin II, causing proliferation of endothelial cells in the formation of angiomalike lesions. This hypothesis suggests a paraviral eruption secondary to an immunologic reaction, not a classical virtual eruption from direct contact of the virus on blood vessels. Although EPA and RAE are harmless and self-limiting, these reports will spread awareness of the increasing number of skin manifestations related to COVID-19 and SARS-CoV-2 virus vaccination.
Acknowledgment—Thoughtful insights and comments on this manuscript were provided by Christine J. Ko, MD (New Haven, Connecticut); Christine L. Egan, MD (Glen Mills, Pennsylvania); Howard A. Bueller, MD (Delray Beach, Florida); and Juan Pablo Robles, PhD (Juriquilla, Mexico).
- McMenamin ME, Fletcher CDM. Reactive angioendotheliomatosis: a study of 15 cases demonstrating a wide clinicopathologic spectrum. Am J Surg Pathol. 2002;26:686-697. doi:10.1097/00000478-200206000-00001
- Khan S, Pujani M, Jetley S, et al. Angiomatosis: a rare vascular proliferation of head and neck region. J Cutan Aesthet Surg. 2015;8:108-110. doi:10.4103/0974-2077.158448
- Gottron HA, Nikolowski W. Extrarenal Lohlein focal nephritis of the skin in endocarditis. Arch Klin Exp Dermatol. 1958;207:156-176.
- Cooper PH. Angioendotheliomatosis: two separate diseases. J Cutan Pathol. 1988;15:259. doi:10.1111/j.1600-0560.1988.tb00556.x
- Cancian L, Hansen A, Boshoff C. Cellular origin of Kaposi’s sarcoma and Kaposi’s sarcoma-associated herpesvirus-induced cell reprogramming. Trends Cell Biol. Sep 2013;23:421-32. doi:10.1016/j.tcb.2013.04.001
- Russell Jones R, Orchard G, Zelger B, et al. Immunostaining for CD31 and CD34 in Kaposi sarcoma. J Clin Pathol. 1995;48:1011-1016. doi:10.1136/jcp.48.11.1011
- Kahn HJ, Bailey D, Marks A. Monoclonal antibody D2-40, a new marker of lymphatic endothelium, reacts with Kaposi’s sarcoma and a subset of angiosarcomas. Mod Pathol. 2002;15:434-440. doi:10.1038/modpathol.3880543
- Genedy RM, Hamza AM, Abdel Latef AA, et al. Sensitivity and specificity of D2-40 in differentiating Kaposi sarcoma from its mimickers. J Egyptian Womens Dermatolog Soc. 2021;18:67-74. doi:10.4103/jewd.jewd_61_20
- Mesri EA, Cesarman E, Boshoff C. Kaposi’s sarcoma and its associated herpesvirus. Nat Rev Cancer. 2010;10:707-719. doi:10.1038/nrc2888
- Patel RM, Goldblum JR, Hsi ED. Immunohistochemical detection of human herpes virus-8 latent nuclear antigen-1 is useful in the diagnosis of Kaposi sarcoma. Mod Pathol. 2004;17:456-460. doi:10.1038/modpathol.3800061
- Zuckerman JN. The importance of injecting vaccines into muscle. Different patients need different needle sizes. BMJ. 2000;321:1237-1238. doi:10.1136/bmj.321.7271.1237
- Bhatia R, Hazarika N, Chandrasekaran D, et al. Treatment of posttraumatic reactive angioendotheliomatosis with topical timolol maleate. JAMA Dermatol. 2021;157:1002-1004. doi:10.1001/jamadermatol.2021.1770
- Sadoff J, Gray G, Vandebosch A, et al; ENSEMBLE Study Group. Safety and efficacy of single-dose Ad26.COV2.S vaccine against Covid-19. N Engl J Med. 2021;384:2187-2201. doi:10.1056/NEJMoa2101544
- See I, Su JR, Lale A, et al. US case reports of cerebral venous sinus thrombosis with thrombocytopenia after Ad26.COV2.S vaccination, March 2 to April 21, 2021. JAMA. 2021;325:2448-2456. doi:10.1001/jama.2021.7517
- Berry CT, Eliliwi M, Gallagher S, et al. Cutaneous small vessel vasculitis following single-dose Janssen Ad26.COV2.S vaccination. JAAD Case Rep. 2021;15:11-14. doi:10.1016/j.jdcr.2021.07.002
- Flaumenhaft R, Enjyoji K, Schmaier AA. Vasculopathy in COVID-19. Blood. 2022;140:222-235. doi:10.1182/blood.2021012250
- Hastie E, Cataldi M, Marriott I, et al. Understanding and altering cell tropism of vesicular stomatitis virus. Virus Res. 2013;176:16-32. doi:10.1016/j.virusres.2013.06.003
- Xiong H-L, Wu Y-T, Cao J-L, et al. Robust neutralization assay based on SARS-CoV-2 S-protein-bearing vesicular stomatitis virus (VSV) pseudovirus and ACE2-overexpressing BHK21 cells. Emerg Microbes Infect. 2020;9:2105-2113. doi:10.1080/22221751.2020.1815589
- Mohta A, Jain SK, Mehta RD, et al. Development of eruptive pseudoangiomatosis following COVID-19 immunization – apropos of 5 cases. J Eur Acad Dermatol Venereol. 2021;35:e722-e725. doi:10.1111/jdv.17499
- Angeli F, Spanevello A, Reboldi G, et al. SARS-CoV-2 vaccines: lights and shadows. Eur J Intern Med. 2021;88:1-8. doi:10.1016/j.ejim.2021.04.019
- McMenamin ME, Fletcher CDM. Reactive angioendotheliomatosis: a study of 15 cases demonstrating a wide clinicopathologic spectrum. Am J Surg Pathol. 2002;26:686-697. doi:10.1097/00000478-200206000-00001
- Khan S, Pujani M, Jetley S, et al. Angiomatosis: a rare vascular proliferation of head and neck region. J Cutan Aesthet Surg. 2015;8:108-110. doi:10.4103/0974-2077.158448
- Gottron HA, Nikolowski W. Extrarenal Lohlein focal nephritis of the skin in endocarditis. Arch Klin Exp Dermatol. 1958;207:156-176.
- Cooper PH. Angioendotheliomatosis: two separate diseases. J Cutan Pathol. 1988;15:259. doi:10.1111/j.1600-0560.1988.tb00556.x
- Cancian L, Hansen A, Boshoff C. Cellular origin of Kaposi’s sarcoma and Kaposi’s sarcoma-associated herpesvirus-induced cell reprogramming. Trends Cell Biol. Sep 2013;23:421-32. doi:10.1016/j.tcb.2013.04.001
- Russell Jones R, Orchard G, Zelger B, et al. Immunostaining for CD31 and CD34 in Kaposi sarcoma. J Clin Pathol. 1995;48:1011-1016. doi:10.1136/jcp.48.11.1011
- Kahn HJ, Bailey D, Marks A. Monoclonal antibody D2-40, a new marker of lymphatic endothelium, reacts with Kaposi’s sarcoma and a subset of angiosarcomas. Mod Pathol. 2002;15:434-440. doi:10.1038/modpathol.3880543
- Genedy RM, Hamza AM, Abdel Latef AA, et al. Sensitivity and specificity of D2-40 in differentiating Kaposi sarcoma from its mimickers. J Egyptian Womens Dermatolog Soc. 2021;18:67-74. doi:10.4103/jewd.jewd_61_20
- Mesri EA, Cesarman E, Boshoff C. Kaposi’s sarcoma and its associated herpesvirus. Nat Rev Cancer. 2010;10:707-719. doi:10.1038/nrc2888
- Patel RM, Goldblum JR, Hsi ED. Immunohistochemical detection of human herpes virus-8 latent nuclear antigen-1 is useful in the diagnosis of Kaposi sarcoma. Mod Pathol. 2004;17:456-460. doi:10.1038/modpathol.3800061
- Zuckerman JN. The importance of injecting vaccines into muscle. Different patients need different needle sizes. BMJ. 2000;321:1237-1238. doi:10.1136/bmj.321.7271.1237
- Bhatia R, Hazarika N, Chandrasekaran D, et al. Treatment of posttraumatic reactive angioendotheliomatosis with topical timolol maleate. JAMA Dermatol. 2021;157:1002-1004. doi:10.1001/jamadermatol.2021.1770
- Sadoff J, Gray G, Vandebosch A, et al; ENSEMBLE Study Group. Safety and efficacy of single-dose Ad26.COV2.S vaccine against Covid-19. N Engl J Med. 2021;384:2187-2201. doi:10.1056/NEJMoa2101544
- See I, Su JR, Lale A, et al. US case reports of cerebral venous sinus thrombosis with thrombocytopenia after Ad26.COV2.S vaccination, March 2 to April 21, 2021. JAMA. 2021;325:2448-2456. doi:10.1001/jama.2021.7517
- Berry CT, Eliliwi M, Gallagher S, et al. Cutaneous small vessel vasculitis following single-dose Janssen Ad26.COV2.S vaccination. JAAD Case Rep. 2021;15:11-14. doi:10.1016/j.jdcr.2021.07.002
- Flaumenhaft R, Enjyoji K, Schmaier AA. Vasculopathy in COVID-19. Blood. 2022;140:222-235. doi:10.1182/blood.2021012250
- Hastie E, Cataldi M, Marriott I, et al. Understanding and altering cell tropism of vesicular stomatitis virus. Virus Res. 2013;176:16-32. doi:10.1016/j.virusres.2013.06.003
- Xiong H-L, Wu Y-T, Cao J-L, et al. Robust neutralization assay based on SARS-CoV-2 S-protein-bearing vesicular stomatitis virus (VSV) pseudovirus and ACE2-overexpressing BHK21 cells. Emerg Microbes Infect. 2020;9:2105-2113. doi:10.1080/22221751.2020.1815589
- Mohta A, Jain SK, Mehta RD, et al. Development of eruptive pseudoangiomatosis following COVID-19 immunization – apropos of 5 cases. J Eur Acad Dermatol Venereol. 2021;35:e722-e725. doi:10.1111/jdv.17499
- Angeli F, Spanevello A, Reboldi G, et al. SARS-CoV-2 vaccines: lights and shadows. Eur J Intern Med. 2021;88:1-8. doi:10.1016/j.ejim.2021.04.019
Practice points
- Reactive angioendotheliomatosis (RAE) is a rare benign vascular proliferation of endothelial cells lining blood vessels that clinically appears similar to Kaposi sarcoma and must be differentiated by microscopic evaluation.
- An increasing number of reports link SARS-CoV-2 viral infection or vaccination against this virus with various cutaneous manifestations. Our case offers a link between RAE and Ad26.COV2.S vaccination.
Bilateral Burning Palmoplantar Lesions
The Diagnosis: Lichen Sclerosus
Histopathology revealed a thin epidermis with homogenization of the upper dermal collagen. By contrast, the lower dermis was sclerotic with patchy chronic dermal infiltrate (Figure). Ultimately, the patient’s clinical presentation and histopathologic findings led to a diagnosis of lichen sclerosus (LS).
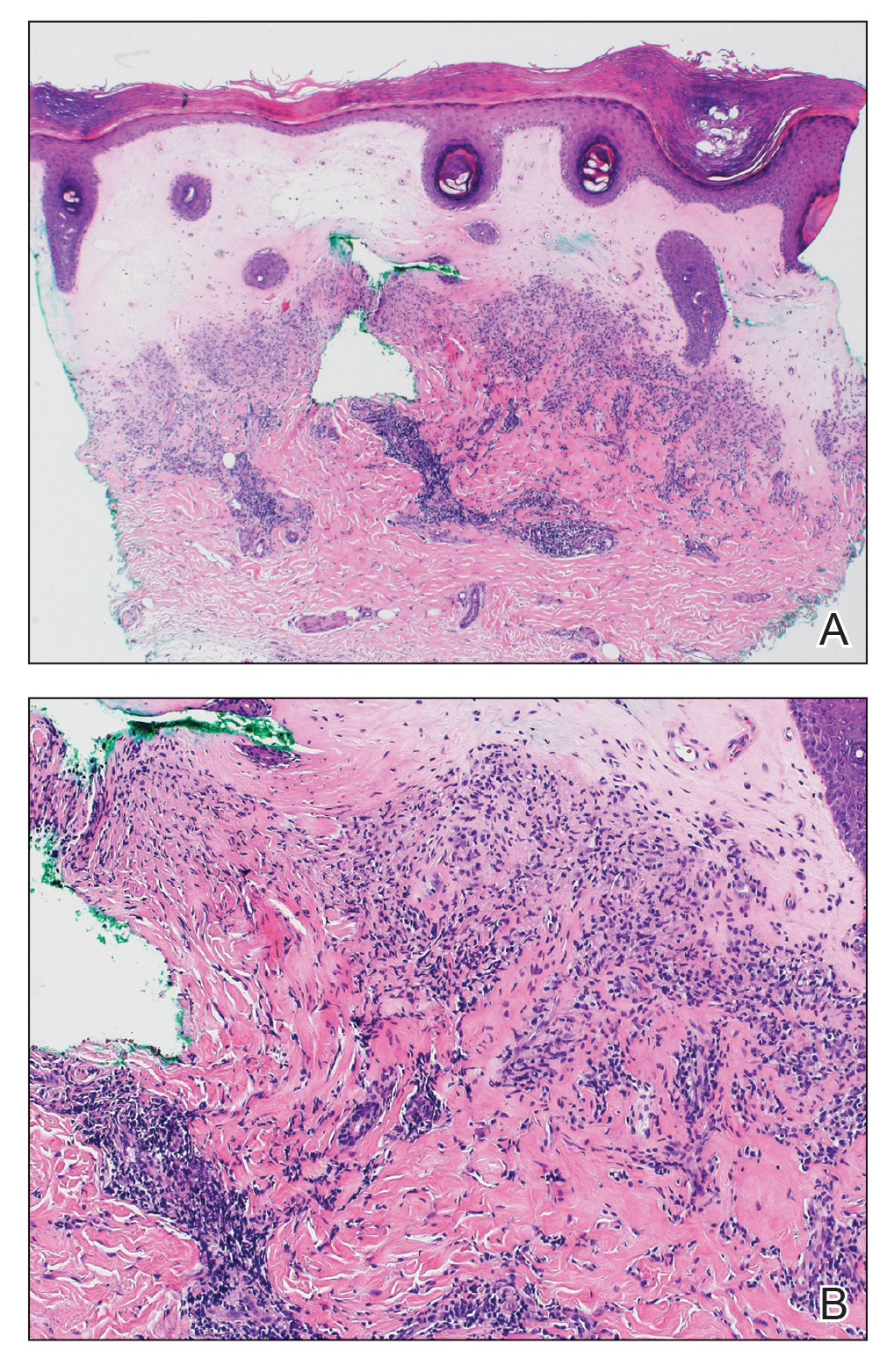
Lichen sclerosus is a rare chronic inflammatory skin condition that typically is characterized by porcelainwhite atrophic plaques on the skin, most often involving the external female genitalia including the vulva and perianal area.1 It is thought to be underdiagnosed and underreported.2 Extragenital manifestations may occur, though some cases are characterized by concomitant genital involvement.3,4 Our patient presented with palmoplantar distribution of plaques without genitalia involvement. Approximately 6% to 10% of patients with extragenital LS do not have genital involvement at the time of diagnosis.3,5 Furthermore, LS involving the palms and soles is exceedingly rare.2 Although extragenital LS may be asymptomatic, patients can experience debilitating pruritus; bullae with hemorrhage and erosion; plaque thickening with repeated excoriations; and painful fissuring, especially if lesions are in areas that are susceptible to friction or tension.3,6 New lesions on previously unaffected skin also may develop secondary to trauma through the Koebner phenomenon.1,6
Histologically, LS is characterized by epidermal hyperkeratosis accompanied by follicular plugging, epidermal atrophy with flattened rete ridges, vacuolization of the basal epidermis, marked edema in the superficial dermis (in early lesions) or homogenized collagen in the upper dermis (in established lesions), and a lymphohistiocytic infiltrate beneath the homogenized collagen. Although the pathogenesis of LS is unclear, purported etiologic factors from studies in genital disease include immune dysfunction, genetic predisposition, infection, and trauma.6 Lichen sclerosus is associated strongly with autoimmune diseases including alopecia areata, vitiligo, autoimmune thyroiditis, diabetes mellitus, and pernicious anemia, indicating its potential multifactorial etiology and linkage to T-lymphocyte dysfunction.1 Early LS lesions often appear as flat-topped and slightly scaly, hypopigmented, white or mildly erythematous, polygonal papules that coalesce to form larger plaques with peripheral erythema. With time, the inflammation subsides, and lesions become porcelain-white with varying degrees of palpable sclerosis, resembling thin paperlike wrinkles indicative of epidermal atrophy.6
The differential diagnosis of LS includes lichen planus (LP), morphea, discoid lupus erythematosus (DLE), and vitiligo.3 Lesions of LP commonly are described as flat-topped, polygonal, pink-purple papules localized mostly along the volar wrists, shins, presacral area, and hands.7 Lichen planus is considered to be more pruritic3 than LS and can be further distinguished by biopsy through identifying a well-formed granular layer and numerous cytoid bodies. Unlike LS, LP is not characterized by basement membrane thickening or epidermal atrophy.8
Skin lesions seen in morphea may resemble the classic atrophic white lesions of extragenital LS; however, it is unclear if the appearance of LS-like lesions with morphea is a simultaneous occurrence of 2 separate disorders or the development of clinical findings resembling LS in lesions of morphea.6 Furthermore, morphea involves deep inflammation and sclerosis of the dermis that may extend into subcutaneous fat without follicular plugging of the epidermis.3,9 In contrast, LS primarily affects the epidermis and dermis with the presence of epidermal follicular plugging.6
Lesions seen in DLE are characterized as well-defined, annular, erythematous patches and plaques followed by follicular hyperkeratosis with adherent scaling. Upon removal of the scale, follicle-sized keratotic spikes (carpet tacks) are present.10 Scaling of lesions and the carpet tack sign were absent in our patient. In addition, DLE typically reveals surrounding pigmentation and scarring over plaques,3 which were not observed in our patient.
Vitiligo commonly is associated with extragenital LS. As with LS, vitiligo can be explained by mechanisms of immune checkpoint inhibitor–induced cytotoxicity as well as perforin and granzyme-B expression.11 Although vitiligo resembles the late hypopigmented lesions of extragenital LS, there are no plaques or surface changes, and a larger, more generalized area of the skin typically is involved.3
- Chamli A, Souissi A. Lichen sclerosus. StatPearls [Internet]. StatPearls Publishing; 2022. http://www.ncbi.nlm.nih.gov/books/NBK538246/
- Gaddis KJ, Huang J, Haun PL. An atrophic and spiny eruption of the palms. JAMA Dermatol. 2018;154:1344-1345. doi:10.1001 /jamadermatol.2018.1265
- Arif T, Fatima R, Sami M. Extragenital lichen sclerosus: a comprehensive review [published online August 11, 2022]. Australas J Dermatol. doi:10.1111/ajd.13890
- Heibel HD, Styles AR, Cockerell CJ. A case of acral lichen sclerosus et atrophicus. JAAD Case Rep. 2020;8:26-27. doi:10.1016/j.jdcr.2020.12.008
- Seyffert J, Bibliowicz N, Harding T, et al. Palmar lichen sclerosus et atrophicus. JAAD Case Rep. 2020;6:697-699. doi:10.1016/j.jdcr.2020.06.005
- Jacobe H. Extragenital lichen sclerosus: clinical features and diagnosis. UpToDate. Updated July 11, 2023. Accessed December 14, 2023. https://www.uptodate.com/contents/extragenital-lichen-sclerosus?search=Lichen%20sclerosus&source =search_result&selectedTitle=2~66&usage_type=default&display_ rank=2
- Goldstein BG, Goldstein AO, Mostow E. Lichen planus. UpToDate. Updated October 25, 2021. Accessed December 14, 2023. https://www.uptodate.com/contents/lichen-planus?search=lichen%20 sclerosus&topicRef=15838&source=see_link
- Tallon B. Lichen sclerosus pathology. DermNet NZ website. Accessed December 5, 2023. https://dermnetnz.org/topics/lichen-sclerosus-pathology
- Jacobe H. Pathogenesis, clinical manifestations, and diagnosis of morphea (localized scleroderma) in adults. UpToDate. Updated November 15, 2021. Accessed December 14, 2023. https://medilib.ir/uptodate/show/13776
- McDaniel B, Sukumaran S, Koritala T, et al. Discoid lupus erythematosus. StatPearls [Internet]. StatPearls Publishing; 2022. Updated August 28, 2023. Accessed December 14, 2023. http://www.ncbi.nlm.nih.gov/books/NBK493145/
- Veronesi G, Scarfì F, Misciali C, et al. An unusual skin reaction in uveal melanoma during treatment with nivolumab: extragenital lichen sclerosus. Anticancer Drugs. 2019;30:969-972. doi:10.1097/ CAD.0000000000000819
The Diagnosis: Lichen Sclerosus
Histopathology revealed a thin epidermis with homogenization of the upper dermal collagen. By contrast, the lower dermis was sclerotic with patchy chronic dermal infiltrate (Figure). Ultimately, the patient’s clinical presentation and histopathologic findings led to a diagnosis of lichen sclerosus (LS).

Lichen sclerosus is a rare chronic inflammatory skin condition that typically is characterized by porcelainwhite atrophic plaques on the skin, most often involving the external female genitalia including the vulva and perianal area.1 It is thought to be underdiagnosed and underreported.2 Extragenital manifestations may occur, though some cases are characterized by concomitant genital involvement.3,4 Our patient presented with palmoplantar distribution of plaques without genitalia involvement. Approximately 6% to 10% of patients with extragenital LS do not have genital involvement at the time of diagnosis.3,5 Furthermore, LS involving the palms and soles is exceedingly rare.2 Although extragenital LS may be asymptomatic, patients can experience debilitating pruritus; bullae with hemorrhage and erosion; plaque thickening with repeated excoriations; and painful fissuring, especially if lesions are in areas that are susceptible to friction or tension.3,6 New lesions on previously unaffected skin also may develop secondary to trauma through the Koebner phenomenon.1,6
Histologically, LS is characterized by epidermal hyperkeratosis accompanied by follicular plugging, epidermal atrophy with flattened rete ridges, vacuolization of the basal epidermis, marked edema in the superficial dermis (in early lesions) or homogenized collagen in the upper dermis (in established lesions), and a lymphohistiocytic infiltrate beneath the homogenized collagen. Although the pathogenesis of LS is unclear, purported etiologic factors from studies in genital disease include immune dysfunction, genetic predisposition, infection, and trauma.6 Lichen sclerosus is associated strongly with autoimmune diseases including alopecia areata, vitiligo, autoimmune thyroiditis, diabetes mellitus, and pernicious anemia, indicating its potential multifactorial etiology and linkage to T-lymphocyte dysfunction.1 Early LS lesions often appear as flat-topped and slightly scaly, hypopigmented, white or mildly erythematous, polygonal papules that coalesce to form larger plaques with peripheral erythema. With time, the inflammation subsides, and lesions become porcelain-white with varying degrees of palpable sclerosis, resembling thin paperlike wrinkles indicative of epidermal atrophy.6
The differential diagnosis of LS includes lichen planus (LP), morphea, discoid lupus erythematosus (DLE), and vitiligo.3 Lesions of LP commonly are described as flat-topped, polygonal, pink-purple papules localized mostly along the volar wrists, shins, presacral area, and hands.7 Lichen planus is considered to be more pruritic3 than LS and can be further distinguished by biopsy through identifying a well-formed granular layer and numerous cytoid bodies. Unlike LS, LP is not characterized by basement membrane thickening or epidermal atrophy.8
Skin lesions seen in morphea may resemble the classic atrophic white lesions of extragenital LS; however, it is unclear if the appearance of LS-like lesions with morphea is a simultaneous occurrence of 2 separate disorders or the development of clinical findings resembling LS in lesions of morphea.6 Furthermore, morphea involves deep inflammation and sclerosis of the dermis that may extend into subcutaneous fat without follicular plugging of the epidermis.3,9 In contrast, LS primarily affects the epidermis and dermis with the presence of epidermal follicular plugging.6
Lesions seen in DLE are characterized as well-defined, annular, erythematous patches and plaques followed by follicular hyperkeratosis with adherent scaling. Upon removal of the scale, follicle-sized keratotic spikes (carpet tacks) are present.10 Scaling of lesions and the carpet tack sign were absent in our patient. In addition, DLE typically reveals surrounding pigmentation and scarring over plaques,3 which were not observed in our patient.
Vitiligo commonly is associated with extragenital LS. As with LS, vitiligo can be explained by mechanisms of immune checkpoint inhibitor–induced cytotoxicity as well as perforin and granzyme-B expression.11 Although vitiligo resembles the late hypopigmented lesions of extragenital LS, there are no plaques or surface changes, and a larger, more generalized area of the skin typically is involved.3
The Diagnosis: Lichen Sclerosus
Histopathology revealed a thin epidermis with homogenization of the upper dermal collagen. By contrast, the lower dermis was sclerotic with patchy chronic dermal infiltrate (Figure). Ultimately, the patient’s clinical presentation and histopathologic findings led to a diagnosis of lichen sclerosus (LS).

Lichen sclerosus is a rare chronic inflammatory skin condition that typically is characterized by porcelainwhite atrophic plaques on the skin, most often involving the external female genitalia including the vulva and perianal area.1 It is thought to be underdiagnosed and underreported.2 Extragenital manifestations may occur, though some cases are characterized by concomitant genital involvement.3,4 Our patient presented with palmoplantar distribution of plaques without genitalia involvement. Approximately 6% to 10% of patients with extragenital LS do not have genital involvement at the time of diagnosis.3,5 Furthermore, LS involving the palms and soles is exceedingly rare.2 Although extragenital LS may be asymptomatic, patients can experience debilitating pruritus; bullae with hemorrhage and erosion; plaque thickening with repeated excoriations; and painful fissuring, especially if lesions are in areas that are susceptible to friction or tension.3,6 New lesions on previously unaffected skin also may develop secondary to trauma through the Koebner phenomenon.1,6
Histologically, LS is characterized by epidermal hyperkeratosis accompanied by follicular plugging, epidermal atrophy with flattened rete ridges, vacuolization of the basal epidermis, marked edema in the superficial dermis (in early lesions) or homogenized collagen in the upper dermis (in established lesions), and a lymphohistiocytic infiltrate beneath the homogenized collagen. Although the pathogenesis of LS is unclear, purported etiologic factors from studies in genital disease include immune dysfunction, genetic predisposition, infection, and trauma.6 Lichen sclerosus is associated strongly with autoimmune diseases including alopecia areata, vitiligo, autoimmune thyroiditis, diabetes mellitus, and pernicious anemia, indicating its potential multifactorial etiology and linkage to T-lymphocyte dysfunction.1 Early LS lesions often appear as flat-topped and slightly scaly, hypopigmented, white or mildly erythematous, polygonal papules that coalesce to form larger plaques with peripheral erythema. With time, the inflammation subsides, and lesions become porcelain-white with varying degrees of palpable sclerosis, resembling thin paperlike wrinkles indicative of epidermal atrophy.6
The differential diagnosis of LS includes lichen planus (LP), morphea, discoid lupus erythematosus (DLE), and vitiligo.3 Lesions of LP commonly are described as flat-topped, polygonal, pink-purple papules localized mostly along the volar wrists, shins, presacral area, and hands.7 Lichen planus is considered to be more pruritic3 than LS and can be further distinguished by biopsy through identifying a well-formed granular layer and numerous cytoid bodies. Unlike LS, LP is not characterized by basement membrane thickening or epidermal atrophy.8
Skin lesions seen in morphea may resemble the classic atrophic white lesions of extragenital LS; however, it is unclear if the appearance of LS-like lesions with morphea is a simultaneous occurrence of 2 separate disorders or the development of clinical findings resembling LS in lesions of morphea.6 Furthermore, morphea involves deep inflammation and sclerosis of the dermis that may extend into subcutaneous fat without follicular plugging of the epidermis.3,9 In contrast, LS primarily affects the epidermis and dermis with the presence of epidermal follicular plugging.6
Lesions seen in DLE are characterized as well-defined, annular, erythematous patches and plaques followed by follicular hyperkeratosis with adherent scaling. Upon removal of the scale, follicle-sized keratotic spikes (carpet tacks) are present.10 Scaling of lesions and the carpet tack sign were absent in our patient. In addition, DLE typically reveals surrounding pigmentation and scarring over plaques,3 which were not observed in our patient.
Vitiligo commonly is associated with extragenital LS. As with LS, vitiligo can be explained by mechanisms of immune checkpoint inhibitor–induced cytotoxicity as well as perforin and granzyme-B expression.11 Although vitiligo resembles the late hypopigmented lesions of extragenital LS, there are no plaques or surface changes, and a larger, more generalized area of the skin typically is involved.3
- Chamli A, Souissi A. Lichen sclerosus. StatPearls [Internet]. StatPearls Publishing; 2022. http://www.ncbi.nlm.nih.gov/books/NBK538246/
- Gaddis KJ, Huang J, Haun PL. An atrophic and spiny eruption of the palms. JAMA Dermatol. 2018;154:1344-1345. doi:10.1001 /jamadermatol.2018.1265
- Arif T, Fatima R, Sami M. Extragenital lichen sclerosus: a comprehensive review [published online August 11, 2022]. Australas J Dermatol. doi:10.1111/ajd.13890
- Heibel HD, Styles AR, Cockerell CJ. A case of acral lichen sclerosus et atrophicus. JAAD Case Rep. 2020;8:26-27. doi:10.1016/j.jdcr.2020.12.008
- Seyffert J, Bibliowicz N, Harding T, et al. Palmar lichen sclerosus et atrophicus. JAAD Case Rep. 2020;6:697-699. doi:10.1016/j.jdcr.2020.06.005
- Jacobe H. Extragenital lichen sclerosus: clinical features and diagnosis. UpToDate. Updated July 11, 2023. Accessed December 14, 2023. https://www.uptodate.com/contents/extragenital-lichen-sclerosus?search=Lichen%20sclerosus&source =search_result&selectedTitle=2~66&usage_type=default&display_ rank=2
- Goldstein BG, Goldstein AO, Mostow E. Lichen planus. UpToDate. Updated October 25, 2021. Accessed December 14, 2023. https://www.uptodate.com/contents/lichen-planus?search=lichen%20 sclerosus&topicRef=15838&source=see_link
- Tallon B. Lichen sclerosus pathology. DermNet NZ website. Accessed December 5, 2023. https://dermnetnz.org/topics/lichen-sclerosus-pathology
- Jacobe H. Pathogenesis, clinical manifestations, and diagnosis of morphea (localized scleroderma) in adults. UpToDate. Updated November 15, 2021. Accessed December 14, 2023. https://medilib.ir/uptodate/show/13776
- McDaniel B, Sukumaran S, Koritala T, et al. Discoid lupus erythematosus. StatPearls [Internet]. StatPearls Publishing; 2022. Updated August 28, 2023. Accessed December 14, 2023. http://www.ncbi.nlm.nih.gov/books/NBK493145/
- Veronesi G, Scarfì F, Misciali C, et al. An unusual skin reaction in uveal melanoma during treatment with nivolumab: extragenital lichen sclerosus. Anticancer Drugs. 2019;30:969-972. doi:10.1097/ CAD.0000000000000819
- Chamli A, Souissi A. Lichen sclerosus. StatPearls [Internet]. StatPearls Publishing; 2022. http://www.ncbi.nlm.nih.gov/books/NBK538246/
- Gaddis KJ, Huang J, Haun PL. An atrophic and spiny eruption of the palms. JAMA Dermatol. 2018;154:1344-1345. doi:10.1001 /jamadermatol.2018.1265
- Arif T, Fatima R, Sami M. Extragenital lichen sclerosus: a comprehensive review [published online August 11, 2022]. Australas J Dermatol. doi:10.1111/ajd.13890
- Heibel HD, Styles AR, Cockerell CJ. A case of acral lichen sclerosus et atrophicus. JAAD Case Rep. 2020;8:26-27. doi:10.1016/j.jdcr.2020.12.008
- Seyffert J, Bibliowicz N, Harding T, et al. Palmar lichen sclerosus et atrophicus. JAAD Case Rep. 2020;6:697-699. doi:10.1016/j.jdcr.2020.06.005
- Jacobe H. Extragenital lichen sclerosus: clinical features and diagnosis. UpToDate. Updated July 11, 2023. Accessed December 14, 2023. https://www.uptodate.com/contents/extragenital-lichen-sclerosus?search=Lichen%20sclerosus&source =search_result&selectedTitle=2~66&usage_type=default&display_ rank=2
- Goldstein BG, Goldstein AO, Mostow E. Lichen planus. UpToDate. Updated October 25, 2021. Accessed December 14, 2023. https://www.uptodate.com/contents/lichen-planus?search=lichen%20 sclerosus&topicRef=15838&source=see_link
- Tallon B. Lichen sclerosus pathology. DermNet NZ website. Accessed December 5, 2023. https://dermnetnz.org/topics/lichen-sclerosus-pathology
- Jacobe H. Pathogenesis, clinical manifestations, and diagnosis of morphea (localized scleroderma) in adults. UpToDate. Updated November 15, 2021. Accessed December 14, 2023. https://medilib.ir/uptodate/show/13776
- McDaniel B, Sukumaran S, Koritala T, et al. Discoid lupus erythematosus. StatPearls [Internet]. StatPearls Publishing; 2022. Updated August 28, 2023. Accessed December 14, 2023. http://www.ncbi.nlm.nih.gov/books/NBK493145/
- Veronesi G, Scarfì F, Misciali C, et al. An unusual skin reaction in uveal melanoma during treatment with nivolumab: extragenital lichen sclerosus. Anticancer Drugs. 2019;30:969-972. doi:10.1097/ CAD.0000000000000819
A 59-year-old woman presented with atrophic, hypopigmented, ivory papules and plaques localized to the central palms and soles of 3 years’ duration. The lesions were associated with burning that was most notable after extended periods of ambulation. The lesions initially were diagnosed as plaque psoriasis by an external dermatology clinic. At the time of presentation to our clinic, treatment with several highpotency topical steroids and biologics approved for plaque psoriasis had failed. Her medical history and concurrent medical workup were notable for type 2 diabetes mellitus, liver dysfunction, thyroid nodules overseen by an endocrinologist, vitamin B12 and vitamin D deficiencies managed with supplementation, and diffuse androgenic alopecia with suspected telogen effluvium. Physical examination revealed no plaque fissuring, pruritus, or scaling. She had no history of radiation therapy or organ transplantation. A punch biopsy of the left palm was performed.
Diffuse Capillary Malformation With Undergrowth of a Limb in a Boy
To the Editor:
Capillary malformations (CMs), the most common vascular malformations that can affect the skin,1 present clinically as macules and patches of various colors, shapes, and sizes. Congenital structural abnormalities are associated with conditions such as Klippel-Trenaunay syndrome (KTS), cutis marmorata telangiectatica congenita (CMTC), and megalencephaly–capillary malformation syndrome.2 Diffuse CM with overgrowth (DCMO) of the soft tissue and bones is an established association of CMs; however, diffuse capillary malformation with undergrowth (DCMU) is a more recent term that describes the lesser-recognized counterpart to DCMO.3 Herein, we describe a case of CM with left-sided undergrowth.
An 11-year-old boy presented to our clinic with asymptomatic vascular patterning on the left side of the body that had been present since birth. He previously was diagnosed with congenital right hemihypertrophy. He reported that the areas gradually lightened over time, and he denied any history of ulceration or venous or lymphatic malformations. Additionally, he explained how the left arm and leg have been noticeably smaller than the right extremities throughout his life. Physical examination revealed superficial, violaceous, reticulated patches along the left upper back tracking down the arm, abdomen (Figure 1A), and anterior thigh (Figure 1B) without crossing the midline. A few dilated veins were noted in the same region as the patches. There was no evidence of scarring or depression found in the skin. The right arms and legs were visibly larger compared to the left side (Figure 2A), and there also was macrodactyly of the third digit of the left hand (Figure 2B). Radiography confirmed the limb length discrepancy and showed the right and left legs to measure 73.2 cm and 71.3 cm, respectively. Given the patient’s multifocal reticulated CMs and ipsilateral undergrowth, a diagnosis of DCMU was rendered. The superficial vascular pattern is likely to fade over time, which will partially be hidden by his darker complexion. He also was advised to continue to see an orthopedist to monitor the limb length incongruity. Surgical intervention was not recommended.
It ordinarily is thought that vascular anomalies of a limb may result in hypertrophy due to increased blood flow such as in KTS, but there are occasions where the affected limb(s) are inexplicably smaller.2,4 Cubiró et al3 observed that in 6 patients with unilateral CMs, all had ipsilateral limb undergrowth. They proposed the term diffuse capillary malformation with undergrowth as a distinct counterpart to DCMO. Diffuse capillary malformation with undergrowth is most similar to CMTC, as both can present with patchy or reticulated capillary staining with ipsilateral limb hypotrophy, but girth more often is affected than length; CMTC also may be associated with dermal atrophy and ulceration.2 The lesions of CMTC typically diminish within the first few years of life whereas those in DCMU tend to persist. Patients with KTS also can exhibit soft-tissue and bony undergrowth, which is termed inverse Klippel-Trenaunay syndrome3; however, the lack of the triad of capillary-lymphatic-venous malformation in our patient made this condition less likely. Additionally, it appears that our patient had left-sided undergrowth rather than the previously diagnosed right hemihypertrophy. The ipsilateral macrodactyly of the third digit of the left hand was an interesting observation and contrasted the undergrowth apparent in the rest of the left limb, which could be caused by increased blood flow specifically to the third digit resembling DCMO.4
Of note, genetic mutations have been implicated as a cause of vascular malformations and growth abnormalities. Specifically, mutations in the phosphoinositide-3-kinase–AKT pathway have been reported in these cases likely due its role in cell growth, proliferation, and angiogenesis.3,4 Future studies should investigate genetic associations in patients with DCMU to determine if there is a robust genotypic-phenotypic link.
Although CMs are a common occurrence in pediatric dermatology, CMs with concurrent limb undergrowth are rare. Our patient’s unique features included involvement of both an arm and leg as well as the presence of macrodactyly. We agree with the terminology for DCMU to describe multifocal reticulated vascular patterning with ipsilateral undergrowth.3
- Huang JT, Liang MG. Vascular malformations. Pediatr Clin North Am. 2010;57:1091-1110. doi:10.1016/j.pcl.2010.08.003
- Lee MS, Liang MG, Mulliken JB. Diffuse capillary malformation with overgrowth: a clinical subtype of vascular anomalies with hypertrophy. J Am Acad Dermatol. 2013;69:589-594. doi:10.1016/j.jaad.2013.05.030
- Cubiró X, Rozas‐Muñoz E, Castel P, et al. Clinical and genetic evaluation of six children with diffuse capillary malformation and undergrowth. Pediatr Dermatol. 2020;37:833-838. doi:10.1111/pde.14252
- Uihlein LC, Liang MG, Fishman SJ, et al. Capillary-venous malformation in the lower limb. Pediatr Dermatol. 2013;30:541-548. doi:10.1111/pde.12186
To the Editor:
Capillary malformations (CMs), the most common vascular malformations that can affect the skin,1 present clinically as macules and patches of various colors, shapes, and sizes. Congenital structural abnormalities are associated with conditions such as Klippel-Trenaunay syndrome (KTS), cutis marmorata telangiectatica congenita (CMTC), and megalencephaly–capillary malformation syndrome.2 Diffuse CM with overgrowth (DCMO) of the soft tissue and bones is an established association of CMs; however, diffuse capillary malformation with undergrowth (DCMU) is a more recent term that describes the lesser-recognized counterpart to DCMO.3 Herein, we describe a case of CM with left-sided undergrowth.

An 11-year-old boy presented to our clinic with asymptomatic vascular patterning on the left side of the body that had been present since birth. He previously was diagnosed with congenital right hemihypertrophy. He reported that the areas gradually lightened over time, and he denied any history of ulceration or venous or lymphatic malformations. Additionally, he explained how the left arm and leg have been noticeably smaller than the right extremities throughout his life. Physical examination revealed superficial, violaceous, reticulated patches along the left upper back tracking down the arm, abdomen (Figure 1A), and anterior thigh (Figure 1B) without crossing the midline. A few dilated veins were noted in the same region as the patches. There was no evidence of scarring or depression found in the skin. The right arms and legs were visibly larger compared to the left side (Figure 2A), and there also was macrodactyly of the third digit of the left hand (Figure 2B). Radiography confirmed the limb length discrepancy and showed the right and left legs to measure 73.2 cm and 71.3 cm, respectively. Given the patient’s multifocal reticulated CMs and ipsilateral undergrowth, a diagnosis of DCMU was rendered. The superficial vascular pattern is likely to fade over time, which will partially be hidden by his darker complexion. He also was advised to continue to see an orthopedist to monitor the limb length incongruity. Surgical intervention was not recommended.

It ordinarily is thought that vascular anomalies of a limb may result in hypertrophy due to increased blood flow such as in KTS, but there are occasions where the affected limb(s) are inexplicably smaller.2,4 Cubiró et al3 observed that in 6 patients with unilateral CMs, all had ipsilateral limb undergrowth. They proposed the term diffuse capillary malformation with undergrowth as a distinct counterpart to DCMO. Diffuse capillary malformation with undergrowth is most similar to CMTC, as both can present with patchy or reticulated capillary staining with ipsilateral limb hypotrophy, but girth more often is affected than length; CMTC also may be associated with dermal atrophy and ulceration.2 The lesions of CMTC typically diminish within the first few years of life whereas those in DCMU tend to persist. Patients with KTS also can exhibit soft-tissue and bony undergrowth, which is termed inverse Klippel-Trenaunay syndrome3; however, the lack of the triad of capillary-lymphatic-venous malformation in our patient made this condition less likely. Additionally, it appears that our patient had left-sided undergrowth rather than the previously diagnosed right hemihypertrophy. The ipsilateral macrodactyly of the third digit of the left hand was an interesting observation and contrasted the undergrowth apparent in the rest of the left limb, which could be caused by increased blood flow specifically to the third digit resembling DCMO.4
Of note, genetic mutations have been implicated as a cause of vascular malformations and growth abnormalities. Specifically, mutations in the phosphoinositide-3-kinase–AKT pathway have been reported in these cases likely due its role in cell growth, proliferation, and angiogenesis.3,4 Future studies should investigate genetic associations in patients with DCMU to determine if there is a robust genotypic-phenotypic link.
Although CMs are a common occurrence in pediatric dermatology, CMs with concurrent limb undergrowth are rare. Our patient’s unique features included involvement of both an arm and leg as well as the presence of macrodactyly. We agree with the terminology for DCMU to describe multifocal reticulated vascular patterning with ipsilateral undergrowth.3
To the Editor:
Capillary malformations (CMs), the most common vascular malformations that can affect the skin,1 present clinically as macules and patches of various colors, shapes, and sizes. Congenital structural abnormalities are associated with conditions such as Klippel-Trenaunay syndrome (KTS), cutis marmorata telangiectatica congenita (CMTC), and megalencephaly–capillary malformation syndrome.2 Diffuse CM with overgrowth (DCMO) of the soft tissue and bones is an established association of CMs; however, diffuse capillary malformation with undergrowth (DCMU) is a more recent term that describes the lesser-recognized counterpart to DCMO.3 Herein, we describe a case of CM with left-sided undergrowth.

An 11-year-old boy presented to our clinic with asymptomatic vascular patterning on the left side of the body that had been present since birth. He previously was diagnosed with congenital right hemihypertrophy. He reported that the areas gradually lightened over time, and he denied any history of ulceration or venous or lymphatic malformations. Additionally, he explained how the left arm and leg have been noticeably smaller than the right extremities throughout his life. Physical examination revealed superficial, violaceous, reticulated patches along the left upper back tracking down the arm, abdomen (Figure 1A), and anterior thigh (Figure 1B) without crossing the midline. A few dilated veins were noted in the same region as the patches. There was no evidence of scarring or depression found in the skin. The right arms and legs were visibly larger compared to the left side (Figure 2A), and there also was macrodactyly of the third digit of the left hand (Figure 2B). Radiography confirmed the limb length discrepancy and showed the right and left legs to measure 73.2 cm and 71.3 cm, respectively. Given the patient’s multifocal reticulated CMs and ipsilateral undergrowth, a diagnosis of DCMU was rendered. The superficial vascular pattern is likely to fade over time, which will partially be hidden by his darker complexion. He also was advised to continue to see an orthopedist to monitor the limb length incongruity. Surgical intervention was not recommended.

It ordinarily is thought that vascular anomalies of a limb may result in hypertrophy due to increased blood flow such as in KTS, but there are occasions where the affected limb(s) are inexplicably smaller.2,4 Cubiró et al3 observed that in 6 patients with unilateral CMs, all had ipsilateral limb undergrowth. They proposed the term diffuse capillary malformation with undergrowth as a distinct counterpart to DCMO. Diffuse capillary malformation with undergrowth is most similar to CMTC, as both can present with patchy or reticulated capillary staining with ipsilateral limb hypotrophy, but girth more often is affected than length; CMTC also may be associated with dermal atrophy and ulceration.2 The lesions of CMTC typically diminish within the first few years of life whereas those in DCMU tend to persist. Patients with KTS also can exhibit soft-tissue and bony undergrowth, which is termed inverse Klippel-Trenaunay syndrome3; however, the lack of the triad of capillary-lymphatic-venous malformation in our patient made this condition less likely. Additionally, it appears that our patient had left-sided undergrowth rather than the previously diagnosed right hemihypertrophy. The ipsilateral macrodactyly of the third digit of the left hand was an interesting observation and contrasted the undergrowth apparent in the rest of the left limb, which could be caused by increased blood flow specifically to the third digit resembling DCMO.4
Of note, genetic mutations have been implicated as a cause of vascular malformations and growth abnormalities. Specifically, mutations in the phosphoinositide-3-kinase–AKT pathway have been reported in these cases likely due its role in cell growth, proliferation, and angiogenesis.3,4 Future studies should investigate genetic associations in patients with DCMU to determine if there is a robust genotypic-phenotypic link.
Although CMs are a common occurrence in pediatric dermatology, CMs with concurrent limb undergrowth are rare. Our patient’s unique features included involvement of both an arm and leg as well as the presence of macrodactyly. We agree with the terminology for DCMU to describe multifocal reticulated vascular patterning with ipsilateral undergrowth.3
- Huang JT, Liang MG. Vascular malformations. Pediatr Clin North Am. 2010;57:1091-1110. doi:10.1016/j.pcl.2010.08.003
- Lee MS, Liang MG, Mulliken JB. Diffuse capillary malformation with overgrowth: a clinical subtype of vascular anomalies with hypertrophy. J Am Acad Dermatol. 2013;69:589-594. doi:10.1016/j.jaad.2013.05.030
- Cubiró X, Rozas‐Muñoz E, Castel P, et al. Clinical and genetic evaluation of six children with diffuse capillary malformation and undergrowth. Pediatr Dermatol. 2020;37:833-838. doi:10.1111/pde.14252
- Uihlein LC, Liang MG, Fishman SJ, et al. Capillary-venous malformation in the lower limb. Pediatr Dermatol. 2013;30:541-548. doi:10.1111/pde.12186
- Huang JT, Liang MG. Vascular malformations. Pediatr Clin North Am. 2010;57:1091-1110. doi:10.1016/j.pcl.2010.08.003
- Lee MS, Liang MG, Mulliken JB. Diffuse capillary malformation with overgrowth: a clinical subtype of vascular anomalies with hypertrophy. J Am Acad Dermatol. 2013;69:589-594. doi:10.1016/j.jaad.2013.05.030
- Cubiró X, Rozas‐Muñoz E, Castel P, et al. Clinical and genetic evaluation of six children with diffuse capillary malformation and undergrowth. Pediatr Dermatol. 2020;37:833-838. doi:10.1111/pde.14252
- Uihlein LC, Liang MG, Fishman SJ, et al. Capillary-venous malformation in the lower limb. Pediatr Dermatol. 2013;30:541-548. doi:10.1111/pde.12186
Practice Points
- The term diffuse capillary malformation with undergrowth (DCMU) describes a distinct counterpart to diffuse capillary malformation with overgrowth. It can be challenging to distinguish from other vascular malformations associated with congenital structural abnormalities.
- The vascular patterning of DCMU may fade over time, but patients should continue to be monitored for their structural incongruity.