User login
Spreading Ulcerations and Lymphadenopathy in a Traveler Returning from Costa Rica
Spreading Ulcerations and Lymphadenopathy in a Traveler Returning from Costa Rica
THE DIAGNOSIS: Cutaneous Leishmaniasis
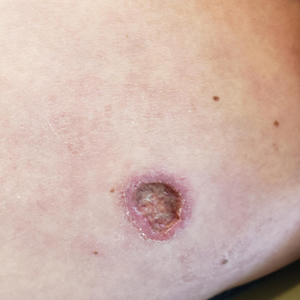
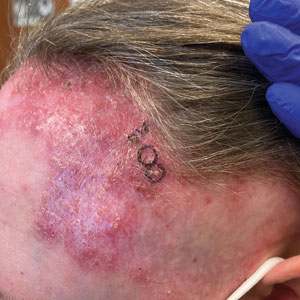
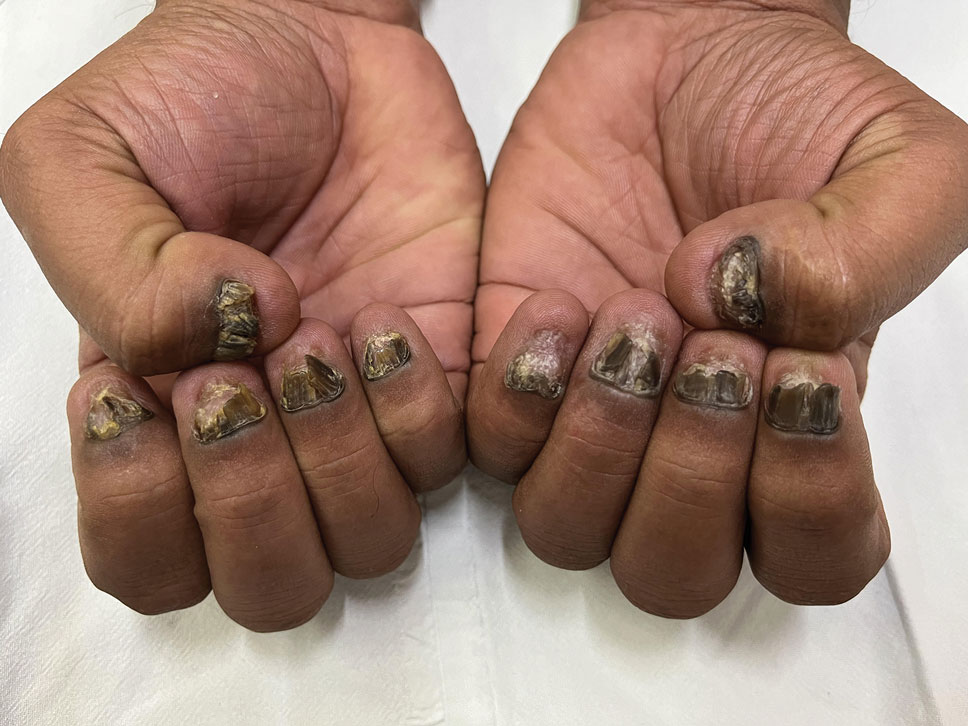
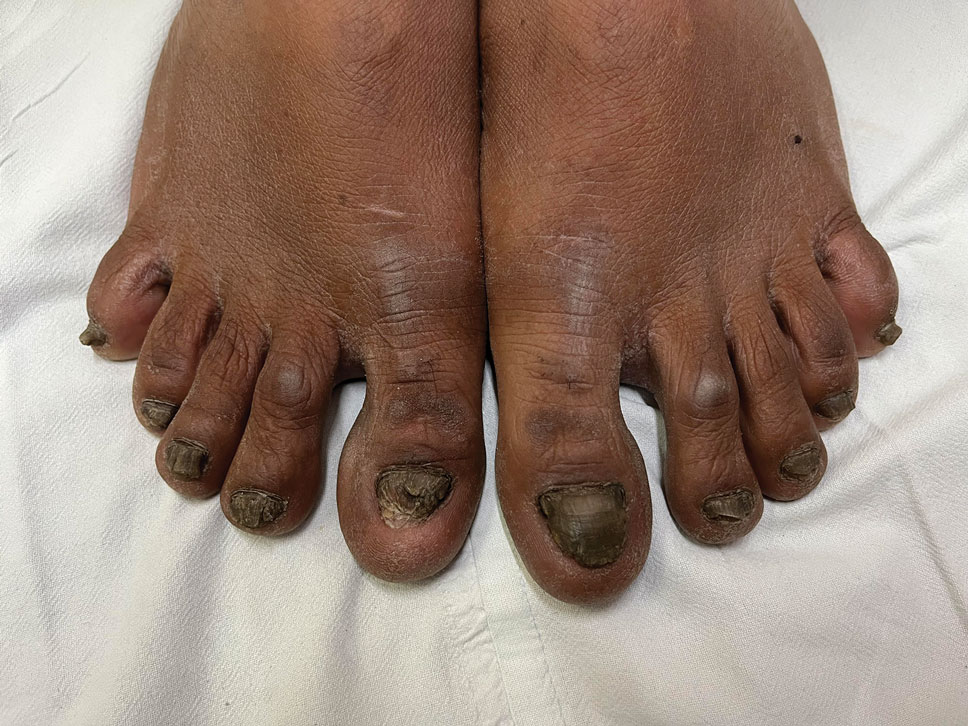
The biopsy results revealed amastigotes at the periphery of parasitized histiocytes, consistent with a diagnosis of cutaneous leishmaniasis. Polymerase chain reaction analysis revealed Leishmania guyanensis species complex, which includes both L guyanensis and Leishmania panamensis. In this case of disseminated cutaneous leishmaniasis (Figure 1), our patient received a prolonged course of systemic therapy with oral miltefosine 50 mg 3 times daily. At the most recent follow-up appointment, she showed ongoing resolution of ulcerations, subcutaneous plaques, and lymphadenopathy on the trunk and face, but development of subcutaneous nodules continued on the arms and legs. At the next follow-up, physical examination revealed that the lesions slowly started to fade.
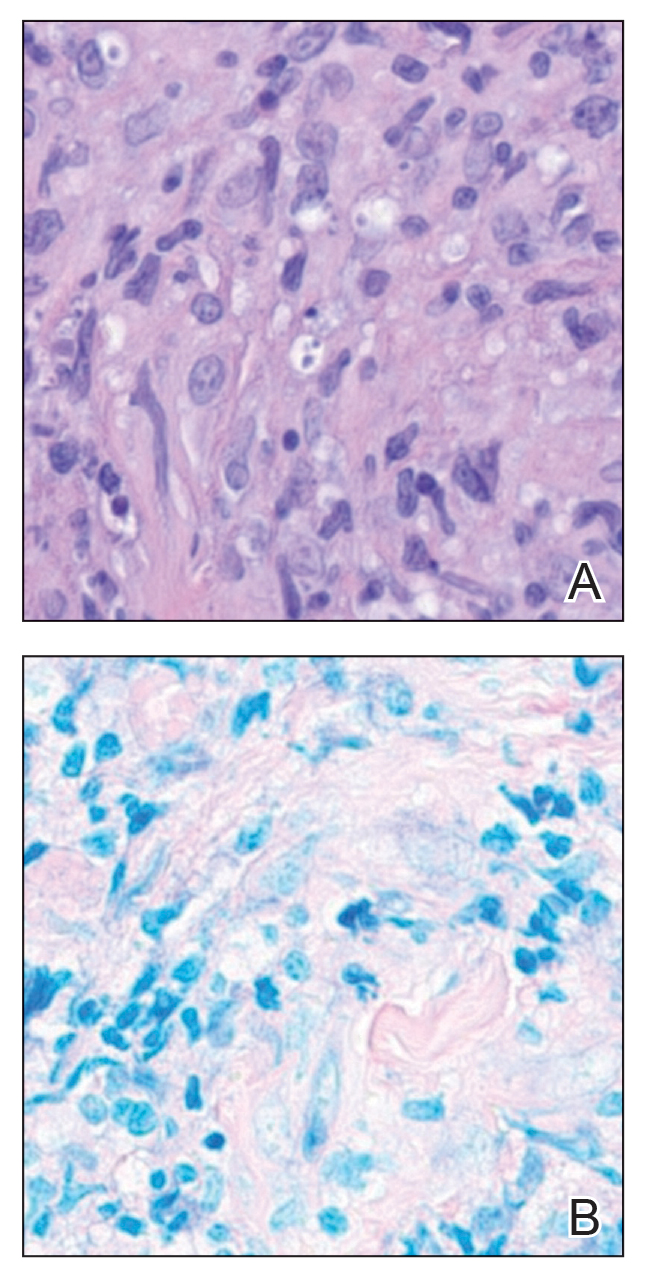
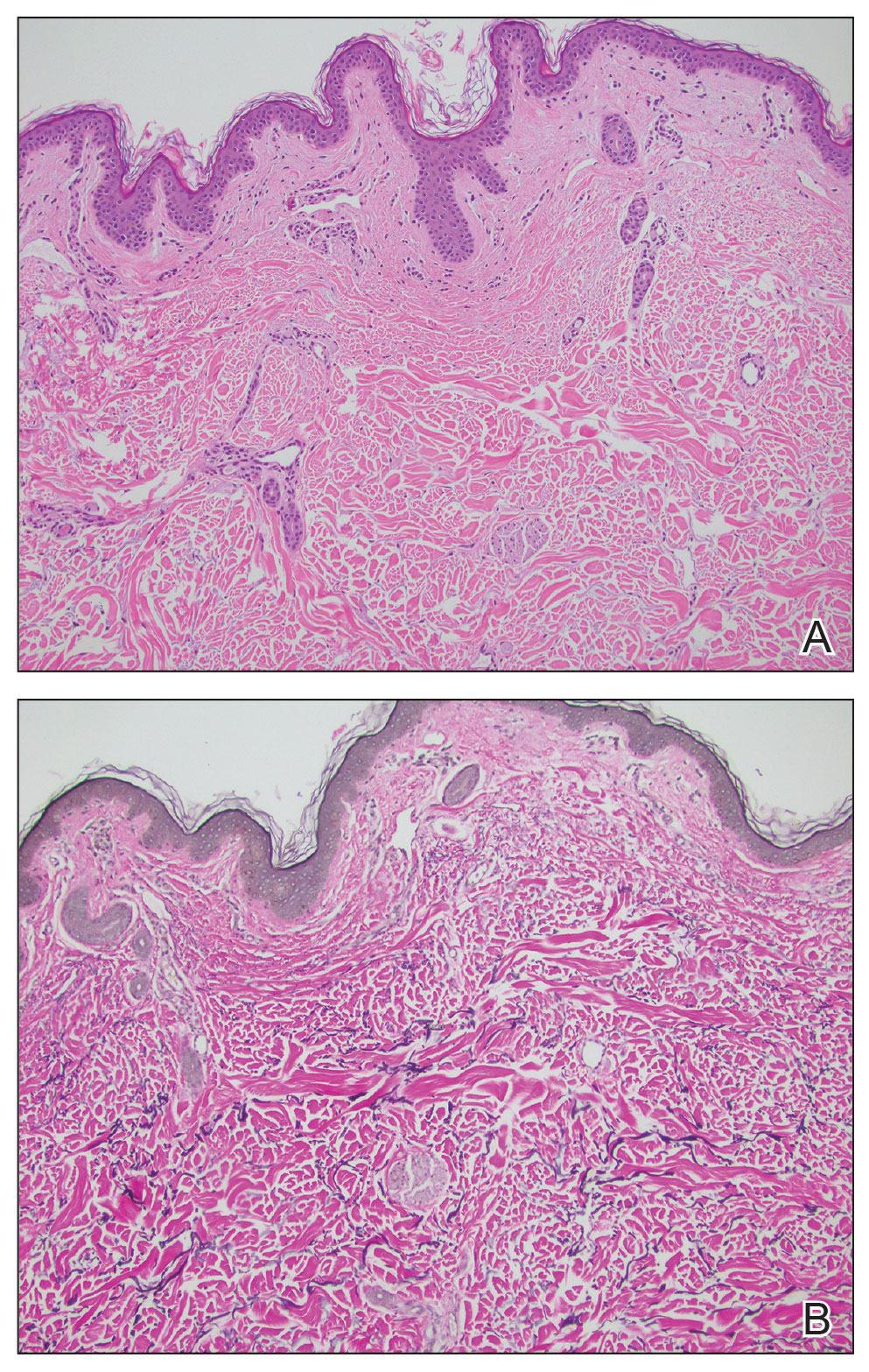
Leishmania species are parasites transmitted by bites of female sand flies, which belong to the genera Phlebotomus (Old World, Eastern Hemisphere) and Lutzomyia (New World, Western Hemisphere) genera.1 Leishmania species have a complex life cycle, propagating within human macrophages, ultimately leading to cutaneous, mucocutaneous, and visceral disease manifestations.2 Cutaneous leishmaniasis manifests classically as scattered, painless, slow-healing ulcers.3 A biopsy taken from the edge of a cutaneous ulcer for hematoxylin and eosin processing is recommended for initial diagnosis, and subsequent polymerase chain reaction of the sample is required for speciation, which guides therapeutic options.4,5 Classic hematoxylin and eosin and Giemsa stain findings include amastigotes lining the edges of parasitized histiocytes (Figure 2).
Systemic treatment options include sodium stibogluconate, amphotericin B, pentamidine, paromomycin, miltefosine, and azole antifungals.2,5 Geography often plays a critical role in selecting treatment options due to resistance rates of individual Leishmania species; for example, paromomycin compounds are more effective for cutaneous disease caused by Leishmania major than Leishmania tropica. Miltefosine is not effective for treating Leishmania braziliensis which can be acquired outside Guatemala, and higher doses of amphotericin B are recommended for visceral disease from East Africa.2,5 In patients with cutaneous leishmaniasis caused by L guyanensis, miltefosine remains a first-line option due to its oral formulation and long half-life within organisms, though there is a risk for teratogenicity.2 Amphotericin B remains the most effective treatment for visceral leishmaniasis and can be used off label to treat mucocutaneous disease or when cutaneous disease is refractory to other treatment options.3
Given the potential of L guyanensis to progress to mucocutaneous disease, monitoring for mucosal involvement should be performed at regular intervals for 6 months to 1 year.2 Treatment may be considered efficacious if no new skin lesions occur after 4 to 6 weeks of therapy; existing skin lesions should be re-epithelializing and reduced by 50% in size, with most cutaneous disease adequately controlled after 3 months of therapy.2
- Olivier M, Minguez-Menendez A, Fernandez-Prada C. Leishmania viannia guyanensis. Trends Parasitol. 2019;35:1018-1019. doi:10.1016 /j.pt.2019.06.008
- Singh R, Kashif M, Srivastava P, et al. Recent advances in chemotherapeutics for leishmaniasis: importance of the cellular biochemistry of the parasite and its molecular interaction with the host. Pathogens. 2023;12:706. doi:10.3390/pathogens12050706
- Aronson N, Herwaldt BL, Libman M, et al. Diagnosis and treatment of leishmaniasis: clinical practice guidelines by the Infectious Diseases Society of America (IDSA) and the American Society of Tropical Medicine and Hygiene (ASTMH). Clin Infect Dis. 2016;63: 1539-1557. doi:10.1093/cid/ciw742
- Specimen Collection Guide for Laboratory Diagnosis of Leishmaniasis. Centers for Disease Control and Prevention. Accessed October 14, 2025. https://www.cdc.gov/dpdx/diagnosticprocedures /other/leish.html
- Aronson NE, Joya CA. Cutaneous leishmaniasis: updates in diagnosis and management. Infect Dis Clin North Am. 2019;33:101-117. doi:10.1016/j.idc.2018.10.004
THE DIAGNOSIS: Cutaneous Leishmaniasis
The biopsy results revealed amastigotes at the periphery of parasitized histiocytes, consistent with a diagnosis of cutaneous leishmaniasis. Polymerase chain reaction analysis revealed Leishmania guyanensis species complex, which includes both L guyanensis and Leishmania panamensis. In this case of disseminated cutaneous leishmaniasis (Figure 1), our patient received a prolonged course of systemic therapy with oral miltefosine 50 mg 3 times daily. At the most recent follow-up appointment, she showed ongoing resolution of ulcerations, subcutaneous plaques, and lymphadenopathy on the trunk and face, but development of subcutaneous nodules continued on the arms and legs. At the next follow-up, physical examination revealed that the lesions slowly started to fade.

Leishmania species are parasites transmitted by bites of female sand flies, which belong to the genera Phlebotomus (Old World, Eastern Hemisphere) and Lutzomyia (New World, Western Hemisphere) genera.1 Leishmania species have a complex life cycle, propagating within human macrophages, ultimately leading to cutaneous, mucocutaneous, and visceral disease manifestations.2 Cutaneous leishmaniasis manifests classically as scattered, painless, slow-healing ulcers.3 A biopsy taken from the edge of a cutaneous ulcer for hematoxylin and eosin processing is recommended for initial diagnosis, and subsequent polymerase chain reaction of the sample is required for speciation, which guides therapeutic options.4,5 Classic hematoxylin and eosin and Giemsa stain findings include amastigotes lining the edges of parasitized histiocytes (Figure 2).

Systemic treatment options include sodium stibogluconate, amphotericin B, pentamidine, paromomycin, miltefosine, and azole antifungals.2,5 Geography often plays a critical role in selecting treatment options due to resistance rates of individual Leishmania species; for example, paromomycin compounds are more effective for cutaneous disease caused by Leishmania major than Leishmania tropica. Miltefosine is not effective for treating Leishmania braziliensis which can be acquired outside Guatemala, and higher doses of amphotericin B are recommended for visceral disease from East Africa.2,5 In patients with cutaneous leishmaniasis caused by L guyanensis, miltefosine remains a first-line option due to its oral formulation and long half-life within organisms, though there is a risk for teratogenicity.2 Amphotericin B remains the most effective treatment for visceral leishmaniasis and can be used off label to treat mucocutaneous disease or when cutaneous disease is refractory to other treatment options.3
Given the potential of L guyanensis to progress to mucocutaneous disease, monitoring for mucosal involvement should be performed at regular intervals for 6 months to 1 year.2 Treatment may be considered efficacious if no new skin lesions occur after 4 to 6 weeks of therapy; existing skin lesions should be re-epithelializing and reduced by 50% in size, with most cutaneous disease adequately controlled after 3 months of therapy.2
THE DIAGNOSIS: Cutaneous Leishmaniasis
The biopsy results revealed amastigotes at the periphery of parasitized histiocytes, consistent with a diagnosis of cutaneous leishmaniasis. Polymerase chain reaction analysis revealed Leishmania guyanensis species complex, which includes both L guyanensis and Leishmania panamensis. In this case of disseminated cutaneous leishmaniasis (Figure 1), our patient received a prolonged course of systemic therapy with oral miltefosine 50 mg 3 times daily. At the most recent follow-up appointment, she showed ongoing resolution of ulcerations, subcutaneous plaques, and lymphadenopathy on the trunk and face, but development of subcutaneous nodules continued on the arms and legs. At the next follow-up, physical examination revealed that the lesions slowly started to fade.

Leishmania species are parasites transmitted by bites of female sand flies, which belong to the genera Phlebotomus (Old World, Eastern Hemisphere) and Lutzomyia (New World, Western Hemisphere) genera.1 Leishmania species have a complex life cycle, propagating within human macrophages, ultimately leading to cutaneous, mucocutaneous, and visceral disease manifestations.2 Cutaneous leishmaniasis manifests classically as scattered, painless, slow-healing ulcers.3 A biopsy taken from the edge of a cutaneous ulcer for hematoxylin and eosin processing is recommended for initial diagnosis, and subsequent polymerase chain reaction of the sample is required for speciation, which guides therapeutic options.4,5 Classic hematoxylin and eosin and Giemsa stain findings include amastigotes lining the edges of parasitized histiocytes (Figure 2).

Systemic treatment options include sodium stibogluconate, amphotericin B, pentamidine, paromomycin, miltefosine, and azole antifungals.2,5 Geography often plays a critical role in selecting treatment options due to resistance rates of individual Leishmania species; for example, paromomycin compounds are more effective for cutaneous disease caused by Leishmania major than Leishmania tropica. Miltefosine is not effective for treating Leishmania braziliensis which can be acquired outside Guatemala, and higher doses of amphotericin B are recommended for visceral disease from East Africa.2,5 In patients with cutaneous leishmaniasis caused by L guyanensis, miltefosine remains a first-line option due to its oral formulation and long half-life within organisms, though there is a risk for teratogenicity.2 Amphotericin B remains the most effective treatment for visceral leishmaniasis and can be used off label to treat mucocutaneous disease or when cutaneous disease is refractory to other treatment options.3
Given the potential of L guyanensis to progress to mucocutaneous disease, monitoring for mucosal involvement should be performed at regular intervals for 6 months to 1 year.2 Treatment may be considered efficacious if no new skin lesions occur after 4 to 6 weeks of therapy; existing skin lesions should be re-epithelializing and reduced by 50% in size, with most cutaneous disease adequately controlled after 3 months of therapy.2
- Olivier M, Minguez-Menendez A, Fernandez-Prada C. Leishmania viannia guyanensis. Trends Parasitol. 2019;35:1018-1019. doi:10.1016 /j.pt.2019.06.008
- Singh R, Kashif M, Srivastava P, et al. Recent advances in chemotherapeutics for leishmaniasis: importance of the cellular biochemistry of the parasite and its molecular interaction with the host. Pathogens. 2023;12:706. doi:10.3390/pathogens12050706
- Aronson N, Herwaldt BL, Libman M, et al. Diagnosis and treatment of leishmaniasis: clinical practice guidelines by the Infectious Diseases Society of America (IDSA) and the American Society of Tropical Medicine and Hygiene (ASTMH). Clin Infect Dis. 2016;63: 1539-1557. doi:10.1093/cid/ciw742
- Specimen Collection Guide for Laboratory Diagnosis of Leishmaniasis. Centers for Disease Control and Prevention. Accessed October 14, 2025. https://www.cdc.gov/dpdx/diagnosticprocedures /other/leish.html
- Aronson NE, Joya CA. Cutaneous leishmaniasis: updates in diagnosis and management. Infect Dis Clin North Am. 2019;33:101-117. doi:10.1016/j.idc.2018.10.004
- Olivier M, Minguez-Menendez A, Fernandez-Prada C. Leishmania viannia guyanensis. Trends Parasitol. 2019;35:1018-1019. doi:10.1016 /j.pt.2019.06.008
- Singh R, Kashif M, Srivastava P, et al. Recent advances in chemotherapeutics for leishmaniasis: importance of the cellular biochemistry of the parasite and its molecular interaction with the host. Pathogens. 2023;12:706. doi:10.3390/pathogens12050706
- Aronson N, Herwaldt BL, Libman M, et al. Diagnosis and treatment of leishmaniasis: clinical practice guidelines by the Infectious Diseases Society of America (IDSA) and the American Society of Tropical Medicine and Hygiene (ASTMH). Clin Infect Dis. 2016;63: 1539-1557. doi:10.1093/cid/ciw742
- Specimen Collection Guide for Laboratory Diagnosis of Leishmaniasis. Centers for Disease Control and Prevention. Accessed October 14, 2025. https://www.cdc.gov/dpdx/diagnosticprocedures /other/leish.html
- Aronson NE, Joya CA. Cutaneous leishmaniasis: updates in diagnosis and management. Infect Dis Clin North Am. 2019;33:101-117. doi:10.1016/j.idc.2018.10.004
Spreading Ulcerations and Lymphadenopathy in a Traveler Returning from Costa Rica
Spreading Ulcerations and Lymphadenopathy in a Traveler Returning from Costa Rica
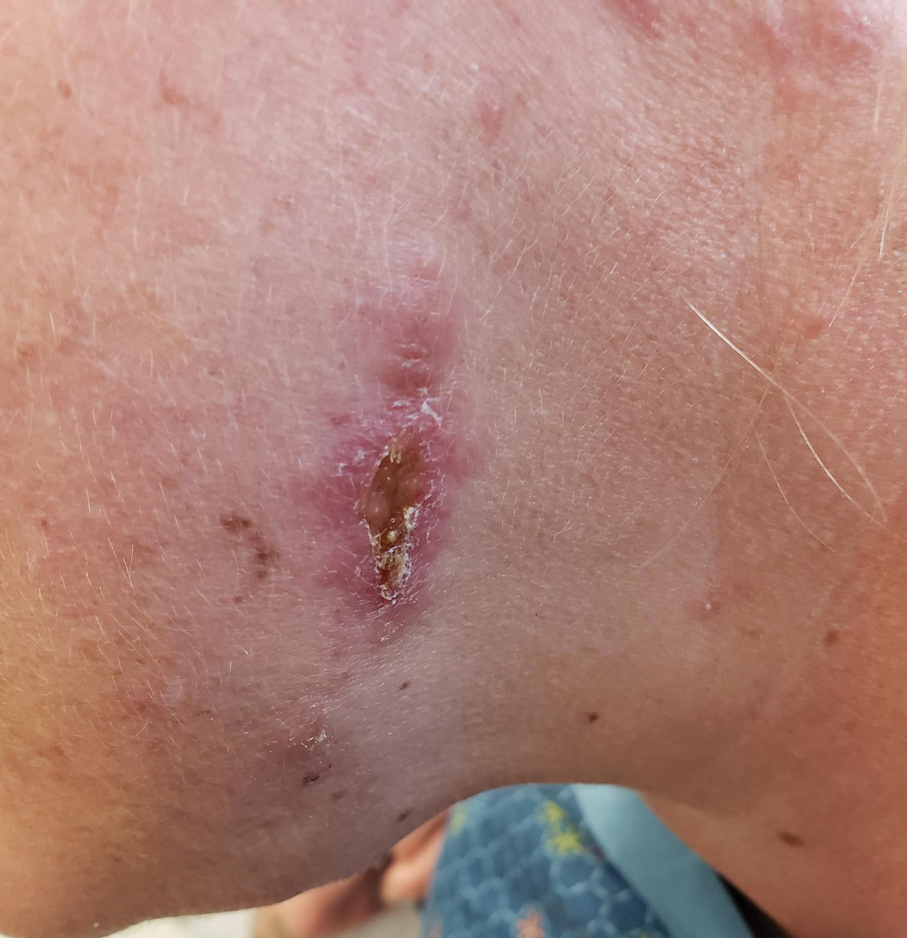
A 43-year-old woman presented to the dermatology clinic with widespread scaly plaques and ulcerations of 2 months’ duration. Her medical history was otherwise unremarkable. The patient reported that the eruption began after returning from a vacation to Costa Rica, during which she spent time on the beach and white-water rafting. She noted that she had been exposed to numerous insects during her trip, and that her roommate, who had accompanied her, had similar exposure history and lesions. The plaques were refractory to multiple oral antibiotics previously prescribed by primary care. Physical examination revealed submental lymphadenopathy and painless ulcerations with indurated borders without purulent drainage alongside scattered scaly papules and plaques on the face, neck, arms, and legs. A biopsy was taken from an ulceration edge on the left thigh.
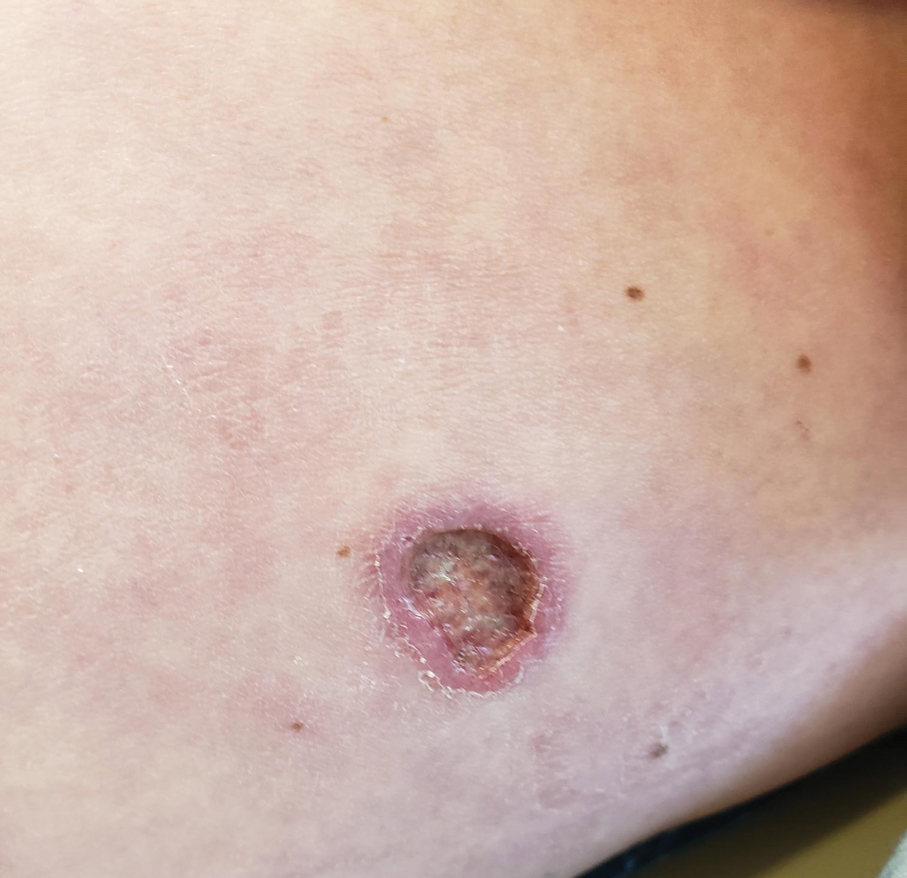
Crusted Lesion at the Implantation Site of a Pacemaker
Crusted Lesion at the Implantation Site of a Pacemaker
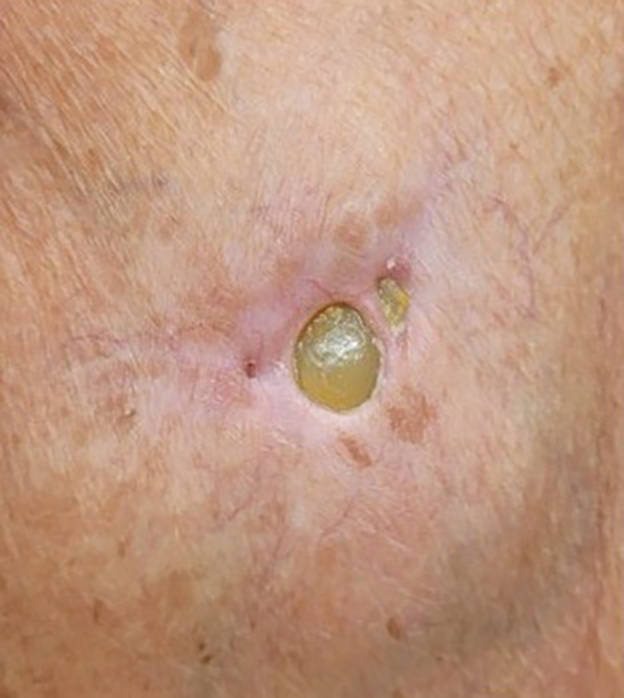
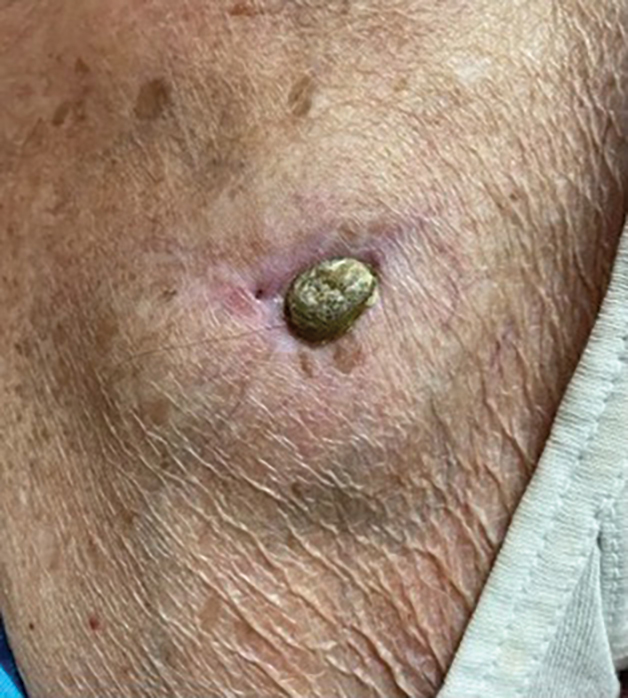
THE DIAGNOSIS: Pacemaker Extrusion
The lesion crust was easily scraped away to reveal extrusion of the permanent pacemaker (PPM) through the skin with a visible overlying gelatinous biofilm (Figure). The patient subsequently completed a 2-week course of clindamycin 300 mg 3 times daily followed by generator and lead removal, with reimplantation of the PPM into the right chest, as is the standard of care in the treatment of pacemaker extrusion.1
Ours is the first known reported case of pacemaker extrusion referred to dermatology with a primary concern for cutaneous malignancy. Pacemaker extrusion through the skin is not common, but it is the most common complication of PPM implantation, followed by infection.1 Pacemaker extrusion results from pressure necrosis and occurs when the PPM emerges through erythematous skin.1,2 Pacemaker extrusions generally are diagnosed by cardiology; however, it is important for dermatologists to recognize this phenomenon and differentiate it from other cutaneous pathologies, as the morphology of skin changes related to pacemaker extrusion through the skin can mimic cutaneous malignancy or other primary skin disease, especially if the outer layer of a biofilm that forms around the PPM hardens to form a crust. Our case emphasizes the importance of removing crusts when evaluating lesions.3
- Harcombe AA, Newell SA, Ludman PF, et al. Late complications following permanent pacemaker implantation or elective unit replacement. Heart. 1998;80:240-244. doi:10.1136/hrt.80.3.240
- Sanderson A, Hahn B. Pacemaker extrusion. Ann Emerg Med. 2013;62:648. doi:10.1016/j.annemergmed.2013.04.022
- Andrade AC, Hayashida MZ, Enokihara MMSES, et al. Dermoscopy of crusted lesion: diagnostic challenge and choice of technique for the analysis. An Bras Dermatol. 2021;96:387-388. doi:10.1016/j.abd.2020.06.016
THE DIAGNOSIS: Pacemaker Extrusion
The lesion crust was easily scraped away to reveal extrusion of the permanent pacemaker (PPM) through the skin with a visible overlying gelatinous biofilm (Figure). The patient subsequently completed a 2-week course of clindamycin 300 mg 3 times daily followed by generator and lead removal, with reimplantation of the PPM into the right chest, as is the standard of care in the treatment of pacemaker extrusion.1

Ours is the first known reported case of pacemaker extrusion referred to dermatology with a primary concern for cutaneous malignancy. Pacemaker extrusion through the skin is not common, but it is the most common complication of PPM implantation, followed by infection.1 Pacemaker extrusion results from pressure necrosis and occurs when the PPM emerges through erythematous skin.1,2 Pacemaker extrusions generally are diagnosed by cardiology; however, it is important for dermatologists to recognize this phenomenon and differentiate it from other cutaneous pathologies, as the morphology of skin changes related to pacemaker extrusion through the skin can mimic cutaneous malignancy or other primary skin disease, especially if the outer layer of a biofilm that forms around the PPM hardens to form a crust. Our case emphasizes the importance of removing crusts when evaluating lesions.3
THE DIAGNOSIS: Pacemaker Extrusion
The lesion crust was easily scraped away to reveal extrusion of the permanent pacemaker (PPM) through the skin with a visible overlying gelatinous biofilm (Figure). The patient subsequently completed a 2-week course of clindamycin 300 mg 3 times daily followed by generator and lead removal, with reimplantation of the PPM into the right chest, as is the standard of care in the treatment of pacemaker extrusion.1

Ours is the first known reported case of pacemaker extrusion referred to dermatology with a primary concern for cutaneous malignancy. Pacemaker extrusion through the skin is not common, but it is the most common complication of PPM implantation, followed by infection.1 Pacemaker extrusion results from pressure necrosis and occurs when the PPM emerges through erythematous skin.1,2 Pacemaker extrusions generally are diagnosed by cardiology; however, it is important for dermatologists to recognize this phenomenon and differentiate it from other cutaneous pathologies, as the morphology of skin changes related to pacemaker extrusion through the skin can mimic cutaneous malignancy or other primary skin disease, especially if the outer layer of a biofilm that forms around the PPM hardens to form a crust. Our case emphasizes the importance of removing crusts when evaluating lesions.3
- Harcombe AA, Newell SA, Ludman PF, et al. Late complications following permanent pacemaker implantation or elective unit replacement. Heart. 1998;80:240-244. doi:10.1136/hrt.80.3.240
- Sanderson A, Hahn B. Pacemaker extrusion. Ann Emerg Med. 2013;62:648. doi:10.1016/j.annemergmed.2013.04.022
- Andrade AC, Hayashida MZ, Enokihara MMSES, et al. Dermoscopy of crusted lesion: diagnostic challenge and choice of technique for the analysis. An Bras Dermatol. 2021;96:387-388. doi:10.1016/j.abd.2020.06.016
- Harcombe AA, Newell SA, Ludman PF, et al. Late complications following permanent pacemaker implantation or elective unit replacement. Heart. 1998;80:240-244. doi:10.1136/hrt.80.3.240
- Sanderson A, Hahn B. Pacemaker extrusion. Ann Emerg Med. 2013;62:648. doi:10.1016/j.annemergmed.2013.04.022
- Andrade AC, Hayashida MZ, Enokihara MMSES, et al. Dermoscopy of crusted lesion: diagnostic challenge and choice of technique for the analysis. An Bras Dermatol. 2021;96:387-388. doi:10.1016/j.abd.2020.06.016
Crusted Lesion at the Implantation Site of a Pacemaker
Crusted Lesion at the Implantation Site of a Pacemaker
A 78-year-old woman was referred to dermatology from the cardiology clinic with concerns of a nonhealing, scablike lesion on the left chest over the implantation site of a dual-chamber permanent pacemaker (PPM). Eight months prior, the patient underwent successful PPM implantation for symptomatic bradycardia and second-degree atrioventricular block. Her cardiologists subsequently noticed an oozing crusting scab at the site of implantation and eventually referred her to dermatology with concerns for squamous cell carcinoma. Physical examination at the current presentation revealed an exophytic serous crust overlying the PPM implantation site on the left chest.
Nonhealing Lesion on the Ear in a Child
Nonhealing Lesion on the Ear in a Child
THE DIAGNOSIS: Cutaneous Leishmaniasis
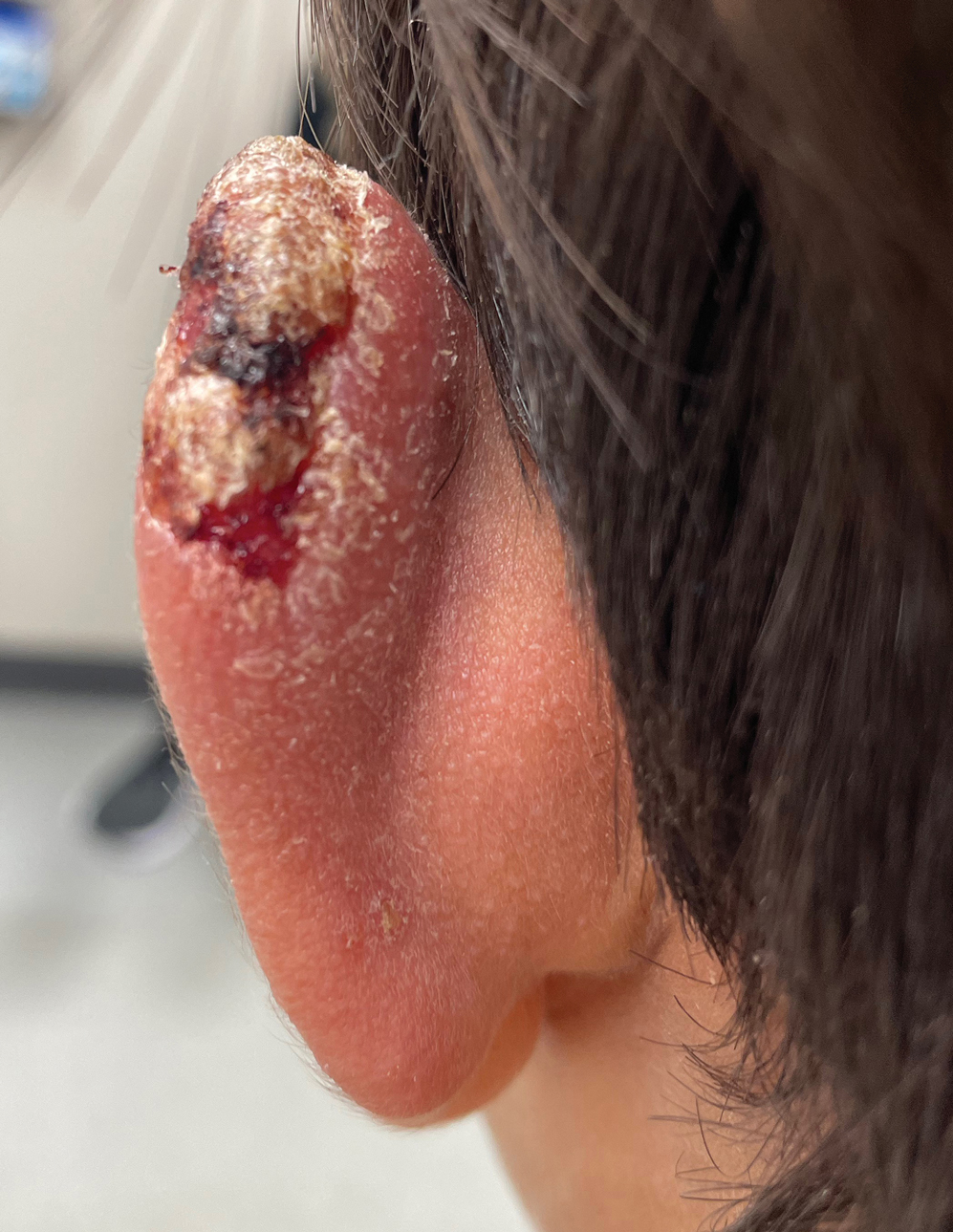
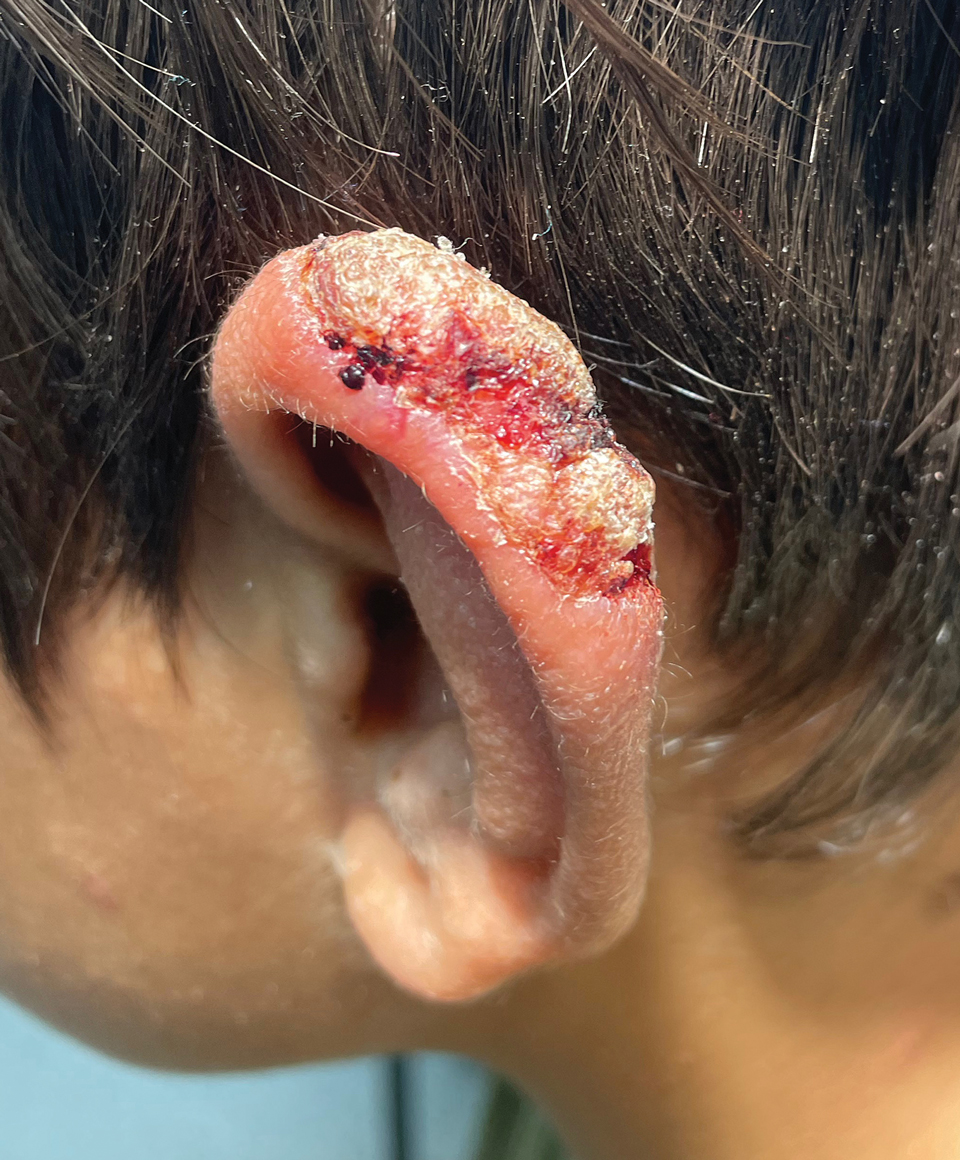
The biopsy results demonstrated a nonspecific chronic granulomatous inflammatory infiltrate, including few multinucleated histiocytes, a surrounding mixed inflammatory infiltrate, mostly mature lymphocytes, few plasma cells, and fragmented neutrophils. A special stain panel was negative for acid-fast bacilli (AFB), Fite, and periodic acid–Schiff for fungi. Bacterial cultures from biopsy tissue grew normal skin flora, and both fungal and AFB cultures were negative. A second punch biopsy was recommended by infectious disease due to clinical suspicion of cutaneous leishmaniasis (CL). Histopathology showed nonnecrotizing granulomas with dense lymphoplasmacytic inflammation and negative Giemsa staining for Leishmania amastigotes; however, it was concluded by pathology that the reason for the negative Leishmania staining was the late stage of the disease, indicated by the presence of granulomas, which can make visualization of organisms difficult. Nonetheless, universal polymerase chain reaction (PCR) testing was positive for Leishmania tropica. Thus, although microscopic analysis was negative for visualization of Leishmania amastigotes, molecular analysis via PCR ultimately demonstrated a positive result and confirmed the diagnosis of CL (Figure 1). The variance in diagnostic accuracy exemplified in our case reinforces the need for multimodal diagnosis.
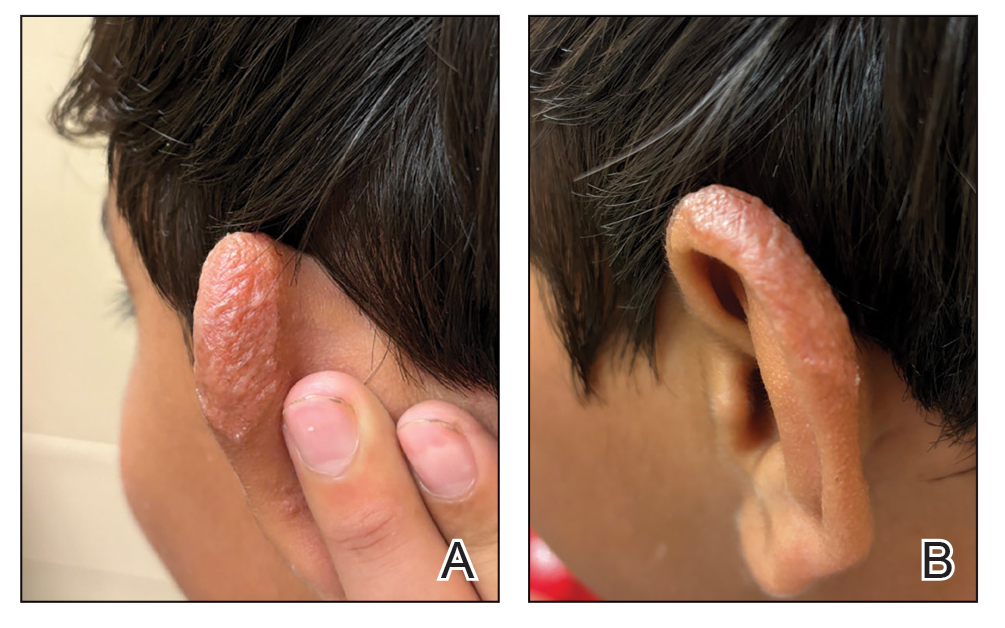
Multiple factors needed to be considered with regard to treatment in our patient, including but not limited to the location of the lesion on a slow-healing cartilaginous surface and the patient’s age. Considering the recalcitrant nature of the lesion and the L tropica strain exhibiting resistance to topical treatments, systemic therapies were the only option. Furthermore, parenteral routes of administration were confounded by the patient’s age, decreasing the likelihood of compliance with therapy. With these variables in mind and recommendations from the Infectious Diseases Society of America and the Centers for Disease Control and Prevention, the best treatment for our patient was deemed to be a 28-day course of oral miltefosine 50 mg twice daily. Compared to the initial presentation, a 1-month follow-up visit after completing the 28-day course of treatment demonstrated flattening of the lesion (Figure 2).
Leishmaniasis is a disease caused by a protozoan parasite of the Leishmania genus, spread via inoculation from the bite of sandfly vectors.1 Cutaneous leishmaniasis is the most common clinical manifestation of leishmaniasis. Other clinical manifestations include mucocutaneous leishmaniasis and visceral leishmaniasis.1,2 Cutaneous leishmaniasis typically manifests as open wounds on areas of skin that may have been exposed to sandfly bites.3 The lesion may not appear until weeks to months or even years after the initial inoculation.2 Initially, CL manifests as papules that may progress to nodular plaques, with eventual evolution to volcanic ulcerations with raised borders and central crateriform indentations covered by scabs or crusting.2,3 The infection may be localized or diffuse—in either case, development of satellite lesions, regional lymphadenopathy, and/or nodular lymphangitis is not uncommon. Generally, CL is not lethal, but the severity of the lesions may vary and can lead to permanent scarring and atrophy.2 Many cases of CL remain undiagnosed because of its appearance as a nonspecific ulcer that can mimic many other cutaneous lesions and because it generally heals spontaneously, leaving only scarring as an indicator of prior infection.4 Thus, CL requires a high diagnostic suspicion, as it can have a nonspecific presentation and is rare in nonendemic regions.
Diagnosis of CL is accomplished via microscopy, isoenzyme analysis, or serology or is made molecularly.3 Microscopic diagnosis includes visualization of Leishmania amastigotes, the stage of replication that occurs after the promastigote stage is phagocytosed by macrophages.3 Amastigote is the only stage that can be visualized in human tissue and is stained via Giemsa and/or hematoxylin and eosin.3 However, Leishmania amastigotes are morphologically indistinguishable from Trypanosoma cruzi amastigotes on microscopy, thus limiting diagnostic accuracy.3 Moreover, there is potential for missed diagnosis of persistent CL caused by L tropica due to fewer parasites being present, further complicating the diagnosis.5 In these cases, molecular diagnostics are helpful as they have higher sensitivity and quicker results. Additionally, DNA technologies can differentiate strains, which is beneficial for guiding treatment. Isoenzyme analysis also can help identify Leishmania species, although results can take weeks to return.3 Serologic testing is useful for suspected visceral leishmaniasis despite negative definitive diagnoses or conflicts with conducting definitive studies; however, there is not a strong antibody response in CL, thus serology is ineffective.3,5 Furthermore, serology can have cross-reactivity with T cruzi and cannot be used to assess for treatment response.3,5 The Infectious Diseases Society of America guidelines for diagnosis of leishmaniasis recommend using multiple methods to ensure a positive result, with molecular assays being the most sensitive.5
Differential diagnoses include any cause of cutaneous ulcerated lesions, including but not limited to mycobacterial or fungal infections. Leprosy often initially manifests with a hypopigmented macule with a raised border, although there often are associated neuropathic symptoms.6 Cutaneous tuberculosis is an extremely rare manifestation that occurs via direct inoculation of the mycobacterium, occurring primarily in children. Initially, it may manifest as a firm red papule that progresses to a painless shallow ulcer with a granular base.7 Cutaneous chromoblastomycosis is a fungal infection resulting from an initial cutaneous injury, similar to our patient, followed by a slow-developing warty lesion that may heal into ivory scars or spread as plaques on normal skin.8 The verrucous lesions seen in cutaneous chromoblastomycosis tend to manifest on the lower extremities and are unlikely to manifest on the head. Sarcoidosis is another granulomatous skin eruption that can be clinically nonspecific.9 Histologically, lesions may demonstrate noncaseating granulomatous inflammation, as seen with cutaneous leishmaniasis, with a broad presentation; for example, lupus pernio, a sarcoid variant, manifests as large blue-red dusky nodules/plaques on the face, ears, or digits.9 Other sarcoid lesions include red/brown, thickened, circular plaques; variably discolored papulonodular lesions; or mucosal involvement.9 Ultimately, it is important to differentiate these nonspecific and similarly appearing lesions through diagnostic techniques such as AFB culture and smear, fungal staining, tuberculosis testing, and PCR in more challenging cases.
Treatment of cutaneous leishmaniasis should be individualized to each case.5 A more than 50% reduction in lesion size within 4 to 6 weeks indicates successful treatment. Ulcerated lesions should be fully re-epithelialized and healed by 3 months posttreatment. Treatment failure is categorized by failure of reepithelization, incomplete healing by 3 months, or worsening of the lesion at any time, each necessitating additional treatment, such as a second course of miltefosine or a different medication regimen.5 Careful monitoring is required throughout treatment, assessing for treatment failure, adding to the challenges of leishmaniasis.
In conclusion, CL requires a high index of suspicion in nonendemic areas to ensure successful diagnosis and treatment. Our case highlights the importance of using multimodal diagnostic techniques for CL, as a single modality may not exhibit a positive result due to variations in diagnostic accuracy. Our case also exhibits the complex treatment of CL, and the considerations that should be made when choosing a treatment modality.
- Leishmaniasis. World Health Organization. Accessed September 14, 2024. https://www.who.int/news-room/fact-sheets/detail/leishmaniasis
- Clinical overview of leishmaniasis. Centers for Disease Control and Prevention. Accessed September 14, 2024. https://www.cdc.gov/leishmaniasis/hcp/clinical-overview/index.html
- CDC DPDx Leishmaniasis. Centers for Disease Control and Prevention. Accessed September 15, 2024. https://www.cdc.gov/dpdx/leishmaniasis/index.html
- Stark CG. Leishmaniasis differential diagnoses. Medscape. March 21, 2023. Accessed October 12, 2024. https://emedicine.medscape.com/article/220298-differential
- Aronson N, Herwaldt BL, Libman M, et al. Diagnosis and treatment of leishmaniasis: clinical practice guidelines by the Infectious Diseases Society of America (IDSA) and the American Society of Tropical Medicine and Hygiene (ASTMH). Clin Infect Dis. 2016;63:E202-E264. doi:10.1093/cid/ciw670
- Lewis FS. Dermatologic manifestations of leprosy. Medscape. June 19, 2023. Accessed October 12, 2024. https://emedicine.medscape.com/article/1104977-overview
- Ngan V. Cutaneous tuberculosis. DermNet. Accessed October 12, 2024. https://dermnetnz.org/topics/cutaneous-tuberculosis
- Schwartz RA. Chromoblastomycosis. Medscape. May 13, 2023. Accessed October 12, 2024. https://emedicine.medscape.com/article/1092695-overview#a4
- Elyoussfi S, Coulson I. Sarcoidosis. DermNet. May 31, 2024. Accessed October 25, 2024. https://dermnetnz.org/topics/sarcoidosis
THE DIAGNOSIS: Cutaneous Leishmaniasis
The biopsy results demonstrated a nonspecific chronic granulomatous inflammatory infiltrate, including few multinucleated histiocytes, a surrounding mixed inflammatory infiltrate, mostly mature lymphocytes, few plasma cells, and fragmented neutrophils. A special stain panel was negative for acid-fast bacilli (AFB), Fite, and periodic acid–Schiff for fungi. Bacterial cultures from biopsy tissue grew normal skin flora, and both fungal and AFB cultures were negative. A second punch biopsy was recommended by infectious disease due to clinical suspicion of cutaneous leishmaniasis (CL). Histopathology showed nonnecrotizing granulomas with dense lymphoplasmacytic inflammation and negative Giemsa staining for Leishmania amastigotes; however, it was concluded by pathology that the reason for the negative Leishmania staining was the late stage of the disease, indicated by the presence of granulomas, which can make visualization of organisms difficult. Nonetheless, universal polymerase chain reaction (PCR) testing was positive for Leishmania tropica. Thus, although microscopic analysis was negative for visualization of Leishmania amastigotes, molecular analysis via PCR ultimately demonstrated a positive result and confirmed the diagnosis of CL (Figure 1). The variance in diagnostic accuracy exemplified in our case reinforces the need for multimodal diagnosis.

Multiple factors needed to be considered with regard to treatment in our patient, including but not limited to the location of the lesion on a slow-healing cartilaginous surface and the patient’s age. Considering the recalcitrant nature of the lesion and the L tropica strain exhibiting resistance to topical treatments, systemic therapies were the only option. Furthermore, parenteral routes of administration were confounded by the patient’s age, decreasing the likelihood of compliance with therapy. With these variables in mind and recommendations from the Infectious Diseases Society of America and the Centers for Disease Control and Prevention, the best treatment for our patient was deemed to be a 28-day course of oral miltefosine 50 mg twice daily. Compared to the initial presentation, a 1-month follow-up visit after completing the 28-day course of treatment demonstrated flattening of the lesion (Figure 2).

Leishmaniasis is a disease caused by a protozoan parasite of the Leishmania genus, spread via inoculation from the bite of sandfly vectors.1 Cutaneous leishmaniasis is the most common clinical manifestation of leishmaniasis. Other clinical manifestations include mucocutaneous leishmaniasis and visceral leishmaniasis.1,2 Cutaneous leishmaniasis typically manifests as open wounds on areas of skin that may have been exposed to sandfly bites.3 The lesion may not appear until weeks to months or even years after the initial inoculation.2 Initially, CL manifests as papules that may progress to nodular plaques, with eventual evolution to volcanic ulcerations with raised borders and central crateriform indentations covered by scabs or crusting.2,3 The infection may be localized or diffuse—in either case, development of satellite lesions, regional lymphadenopathy, and/or nodular lymphangitis is not uncommon. Generally, CL is not lethal, but the severity of the lesions may vary and can lead to permanent scarring and atrophy.2 Many cases of CL remain undiagnosed because of its appearance as a nonspecific ulcer that can mimic many other cutaneous lesions and because it generally heals spontaneously, leaving only scarring as an indicator of prior infection.4 Thus, CL requires a high diagnostic suspicion, as it can have a nonspecific presentation and is rare in nonendemic regions.
Diagnosis of CL is accomplished via microscopy, isoenzyme analysis, or serology or is made molecularly.3 Microscopic diagnosis includes visualization of Leishmania amastigotes, the stage of replication that occurs after the promastigote stage is phagocytosed by macrophages.3 Amastigote is the only stage that can be visualized in human tissue and is stained via Giemsa and/or hematoxylin and eosin.3 However, Leishmania amastigotes are morphologically indistinguishable from Trypanosoma cruzi amastigotes on microscopy, thus limiting diagnostic accuracy.3 Moreover, there is potential for missed diagnosis of persistent CL caused by L tropica due to fewer parasites being present, further complicating the diagnosis.5 In these cases, molecular diagnostics are helpful as they have higher sensitivity and quicker results. Additionally, DNA technologies can differentiate strains, which is beneficial for guiding treatment. Isoenzyme analysis also can help identify Leishmania species, although results can take weeks to return.3 Serologic testing is useful for suspected visceral leishmaniasis despite negative definitive diagnoses or conflicts with conducting definitive studies; however, there is not a strong antibody response in CL, thus serology is ineffective.3,5 Furthermore, serology can have cross-reactivity with T cruzi and cannot be used to assess for treatment response.3,5 The Infectious Diseases Society of America guidelines for diagnosis of leishmaniasis recommend using multiple methods to ensure a positive result, with molecular assays being the most sensitive.5
Differential diagnoses include any cause of cutaneous ulcerated lesions, including but not limited to mycobacterial or fungal infections. Leprosy often initially manifests with a hypopigmented macule with a raised border, although there often are associated neuropathic symptoms.6 Cutaneous tuberculosis is an extremely rare manifestation that occurs via direct inoculation of the mycobacterium, occurring primarily in children. Initially, it may manifest as a firm red papule that progresses to a painless shallow ulcer with a granular base.7 Cutaneous chromoblastomycosis is a fungal infection resulting from an initial cutaneous injury, similar to our patient, followed by a slow-developing warty lesion that may heal into ivory scars or spread as plaques on normal skin.8 The verrucous lesions seen in cutaneous chromoblastomycosis tend to manifest on the lower extremities and are unlikely to manifest on the head. Sarcoidosis is another granulomatous skin eruption that can be clinically nonspecific.9 Histologically, lesions may demonstrate noncaseating granulomatous inflammation, as seen with cutaneous leishmaniasis, with a broad presentation; for example, lupus pernio, a sarcoid variant, manifests as large blue-red dusky nodules/plaques on the face, ears, or digits.9 Other sarcoid lesions include red/brown, thickened, circular plaques; variably discolored papulonodular lesions; or mucosal involvement.9 Ultimately, it is important to differentiate these nonspecific and similarly appearing lesions through diagnostic techniques such as AFB culture and smear, fungal staining, tuberculosis testing, and PCR in more challenging cases.
Treatment of cutaneous leishmaniasis should be individualized to each case.5 A more than 50% reduction in lesion size within 4 to 6 weeks indicates successful treatment. Ulcerated lesions should be fully re-epithelialized and healed by 3 months posttreatment. Treatment failure is categorized by failure of reepithelization, incomplete healing by 3 months, or worsening of the lesion at any time, each necessitating additional treatment, such as a second course of miltefosine or a different medication regimen.5 Careful monitoring is required throughout treatment, assessing for treatment failure, adding to the challenges of leishmaniasis.
In conclusion, CL requires a high index of suspicion in nonendemic areas to ensure successful diagnosis and treatment. Our case highlights the importance of using multimodal diagnostic techniques for CL, as a single modality may not exhibit a positive result due to variations in diagnostic accuracy. Our case also exhibits the complex treatment of CL, and the considerations that should be made when choosing a treatment modality.
THE DIAGNOSIS: Cutaneous Leishmaniasis
The biopsy results demonstrated a nonspecific chronic granulomatous inflammatory infiltrate, including few multinucleated histiocytes, a surrounding mixed inflammatory infiltrate, mostly mature lymphocytes, few plasma cells, and fragmented neutrophils. A special stain panel was negative for acid-fast bacilli (AFB), Fite, and periodic acid–Schiff for fungi. Bacterial cultures from biopsy tissue grew normal skin flora, and both fungal and AFB cultures were negative. A second punch biopsy was recommended by infectious disease due to clinical suspicion of cutaneous leishmaniasis (CL). Histopathology showed nonnecrotizing granulomas with dense lymphoplasmacytic inflammation and negative Giemsa staining for Leishmania amastigotes; however, it was concluded by pathology that the reason for the negative Leishmania staining was the late stage of the disease, indicated by the presence of granulomas, which can make visualization of organisms difficult. Nonetheless, universal polymerase chain reaction (PCR) testing was positive for Leishmania tropica. Thus, although microscopic analysis was negative for visualization of Leishmania amastigotes, molecular analysis via PCR ultimately demonstrated a positive result and confirmed the diagnosis of CL (Figure 1). The variance in diagnostic accuracy exemplified in our case reinforces the need for multimodal diagnosis.

Multiple factors needed to be considered with regard to treatment in our patient, including but not limited to the location of the lesion on a slow-healing cartilaginous surface and the patient’s age. Considering the recalcitrant nature of the lesion and the L tropica strain exhibiting resistance to topical treatments, systemic therapies were the only option. Furthermore, parenteral routes of administration were confounded by the patient’s age, decreasing the likelihood of compliance with therapy. With these variables in mind and recommendations from the Infectious Diseases Society of America and the Centers for Disease Control and Prevention, the best treatment for our patient was deemed to be a 28-day course of oral miltefosine 50 mg twice daily. Compared to the initial presentation, a 1-month follow-up visit after completing the 28-day course of treatment demonstrated flattening of the lesion (Figure 2).

Leishmaniasis is a disease caused by a protozoan parasite of the Leishmania genus, spread via inoculation from the bite of sandfly vectors.1 Cutaneous leishmaniasis is the most common clinical manifestation of leishmaniasis. Other clinical manifestations include mucocutaneous leishmaniasis and visceral leishmaniasis.1,2 Cutaneous leishmaniasis typically manifests as open wounds on areas of skin that may have been exposed to sandfly bites.3 The lesion may not appear until weeks to months or even years after the initial inoculation.2 Initially, CL manifests as papules that may progress to nodular plaques, with eventual evolution to volcanic ulcerations with raised borders and central crateriform indentations covered by scabs or crusting.2,3 The infection may be localized or diffuse—in either case, development of satellite lesions, regional lymphadenopathy, and/or nodular lymphangitis is not uncommon. Generally, CL is not lethal, but the severity of the lesions may vary and can lead to permanent scarring and atrophy.2 Many cases of CL remain undiagnosed because of its appearance as a nonspecific ulcer that can mimic many other cutaneous lesions and because it generally heals spontaneously, leaving only scarring as an indicator of prior infection.4 Thus, CL requires a high diagnostic suspicion, as it can have a nonspecific presentation and is rare in nonendemic regions.
Diagnosis of CL is accomplished via microscopy, isoenzyme analysis, or serology or is made molecularly.3 Microscopic diagnosis includes visualization of Leishmania amastigotes, the stage of replication that occurs after the promastigote stage is phagocytosed by macrophages.3 Amastigote is the only stage that can be visualized in human tissue and is stained via Giemsa and/or hematoxylin and eosin.3 However, Leishmania amastigotes are morphologically indistinguishable from Trypanosoma cruzi amastigotes on microscopy, thus limiting diagnostic accuracy.3 Moreover, there is potential for missed diagnosis of persistent CL caused by L tropica due to fewer parasites being present, further complicating the diagnosis.5 In these cases, molecular diagnostics are helpful as they have higher sensitivity and quicker results. Additionally, DNA technologies can differentiate strains, which is beneficial for guiding treatment. Isoenzyme analysis also can help identify Leishmania species, although results can take weeks to return.3 Serologic testing is useful for suspected visceral leishmaniasis despite negative definitive diagnoses or conflicts with conducting definitive studies; however, there is not a strong antibody response in CL, thus serology is ineffective.3,5 Furthermore, serology can have cross-reactivity with T cruzi and cannot be used to assess for treatment response.3,5 The Infectious Diseases Society of America guidelines for diagnosis of leishmaniasis recommend using multiple methods to ensure a positive result, with molecular assays being the most sensitive.5
Differential diagnoses include any cause of cutaneous ulcerated lesions, including but not limited to mycobacterial or fungal infections. Leprosy often initially manifests with a hypopigmented macule with a raised border, although there often are associated neuropathic symptoms.6 Cutaneous tuberculosis is an extremely rare manifestation that occurs via direct inoculation of the mycobacterium, occurring primarily in children. Initially, it may manifest as a firm red papule that progresses to a painless shallow ulcer with a granular base.7 Cutaneous chromoblastomycosis is a fungal infection resulting from an initial cutaneous injury, similar to our patient, followed by a slow-developing warty lesion that may heal into ivory scars or spread as plaques on normal skin.8 The verrucous lesions seen in cutaneous chromoblastomycosis tend to manifest on the lower extremities and are unlikely to manifest on the head. Sarcoidosis is another granulomatous skin eruption that can be clinically nonspecific.9 Histologically, lesions may demonstrate noncaseating granulomatous inflammation, as seen with cutaneous leishmaniasis, with a broad presentation; for example, lupus pernio, a sarcoid variant, manifests as large blue-red dusky nodules/plaques on the face, ears, or digits.9 Other sarcoid lesions include red/brown, thickened, circular plaques; variably discolored papulonodular lesions; or mucosal involvement.9 Ultimately, it is important to differentiate these nonspecific and similarly appearing lesions through diagnostic techniques such as AFB culture and smear, fungal staining, tuberculosis testing, and PCR in more challenging cases.
Treatment of cutaneous leishmaniasis should be individualized to each case.5 A more than 50% reduction in lesion size within 4 to 6 weeks indicates successful treatment. Ulcerated lesions should be fully re-epithelialized and healed by 3 months posttreatment. Treatment failure is categorized by failure of reepithelization, incomplete healing by 3 months, or worsening of the lesion at any time, each necessitating additional treatment, such as a second course of miltefosine or a different medication regimen.5 Careful monitoring is required throughout treatment, assessing for treatment failure, adding to the challenges of leishmaniasis.
In conclusion, CL requires a high index of suspicion in nonendemic areas to ensure successful diagnosis and treatment. Our case highlights the importance of using multimodal diagnostic techniques for CL, as a single modality may not exhibit a positive result due to variations in diagnostic accuracy. Our case also exhibits the complex treatment of CL, and the considerations that should be made when choosing a treatment modality.
- Leishmaniasis. World Health Organization. Accessed September 14, 2024. https://www.who.int/news-room/fact-sheets/detail/leishmaniasis
- Clinical overview of leishmaniasis. Centers for Disease Control and Prevention. Accessed September 14, 2024. https://www.cdc.gov/leishmaniasis/hcp/clinical-overview/index.html
- CDC DPDx Leishmaniasis. Centers for Disease Control and Prevention. Accessed September 15, 2024. https://www.cdc.gov/dpdx/leishmaniasis/index.html
- Stark CG. Leishmaniasis differential diagnoses. Medscape. March 21, 2023. Accessed October 12, 2024. https://emedicine.medscape.com/article/220298-differential
- Aronson N, Herwaldt BL, Libman M, et al. Diagnosis and treatment of leishmaniasis: clinical practice guidelines by the Infectious Diseases Society of America (IDSA) and the American Society of Tropical Medicine and Hygiene (ASTMH). Clin Infect Dis. 2016;63:E202-E264. doi:10.1093/cid/ciw670
- Lewis FS. Dermatologic manifestations of leprosy. Medscape. June 19, 2023. Accessed October 12, 2024. https://emedicine.medscape.com/article/1104977-overview
- Ngan V. Cutaneous tuberculosis. DermNet. Accessed October 12, 2024. https://dermnetnz.org/topics/cutaneous-tuberculosis
- Schwartz RA. Chromoblastomycosis. Medscape. May 13, 2023. Accessed October 12, 2024. https://emedicine.medscape.com/article/1092695-overview#a4
- Elyoussfi S, Coulson I. Sarcoidosis. DermNet. May 31, 2024. Accessed October 25, 2024. https://dermnetnz.org/topics/sarcoidosis
- Leishmaniasis. World Health Organization. Accessed September 14, 2024. https://www.who.int/news-room/fact-sheets/detail/leishmaniasis
- Clinical overview of leishmaniasis. Centers for Disease Control and Prevention. Accessed September 14, 2024. https://www.cdc.gov/leishmaniasis/hcp/clinical-overview/index.html
- CDC DPDx Leishmaniasis. Centers for Disease Control and Prevention. Accessed September 15, 2024. https://www.cdc.gov/dpdx/leishmaniasis/index.html
- Stark CG. Leishmaniasis differential diagnoses. Medscape. March 21, 2023. Accessed October 12, 2024. https://emedicine.medscape.com/article/220298-differential
- Aronson N, Herwaldt BL, Libman M, et al. Diagnosis and treatment of leishmaniasis: clinical practice guidelines by the Infectious Diseases Society of America (IDSA) and the American Society of Tropical Medicine and Hygiene (ASTMH). Clin Infect Dis. 2016;63:E202-E264. doi:10.1093/cid/ciw670
- Lewis FS. Dermatologic manifestations of leprosy. Medscape. June 19, 2023. Accessed October 12, 2024. https://emedicine.medscape.com/article/1104977-overview
- Ngan V. Cutaneous tuberculosis. DermNet. Accessed October 12, 2024. https://dermnetnz.org/topics/cutaneous-tuberculosis
- Schwartz RA. Chromoblastomycosis. Medscape. May 13, 2023. Accessed October 12, 2024. https://emedicine.medscape.com/article/1092695-overview#a4
- Elyoussfi S, Coulson I. Sarcoidosis. DermNet. May 31, 2024. Accessed October 25, 2024. https://dermnetnz.org/topics/sarcoidosis
Nonhealing Lesion on the Ear in a Child
Nonhealing Lesion on the Ear in a Child
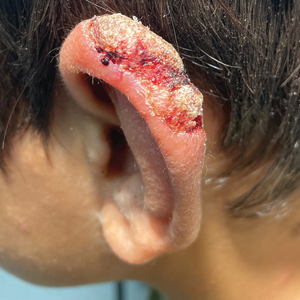
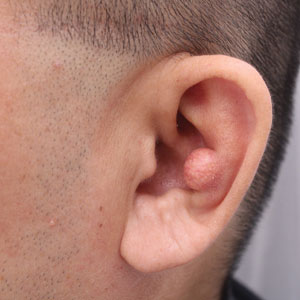
A 10-year-old boy who recently emigrated from Afghanistan presented to his pediatrician for evaluation of a painless nonhealing plaque on the posterior left pinna of more than 1 year's duration. The lesion reportedly started as a small scratch following an ear injury, initially improved with an unknown topical treatment administered in Afghanistan, and then recurred with no other associated lesions and no known insect bite. The lesion persisted for more than 1 year postemigration before the patient presented to his pediatrician, who noted signs of excoriation, which was confirmed by the patient's father. The patient was started on a 7-day course of cephalexin oral suspension and topical mupirocin 2%. After 2 months without improvement, a 2-week course of oral trimethoprim/sulfamethoxazole was initiated; however, the lesion continued to grow with no signs of healing, and he was referred to dermatology.
The patient presented to pediatric dermatology 3 months after the initial presentation to his pediatrician and 2 weeks after he completed the course of oral trimethoprim/sulfamethoxazole. Physical examination demonstrated a papulosquamous eruption with swelling and blistering on the helix of the left ear. Based on these findings, the patient was started on a 1-month trial of topical triamcinolone 1% followed by the addition of topical pimecrolimus 1%. Due to no improvement of the lesion and subsequent progression to ulceration, a punch biopsy was performed.
Progressive Dystrophy of the Fingernails and Toenails
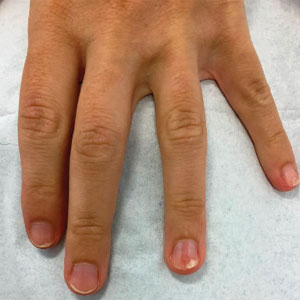
Progressive Dystrophy of the Fingernails and Toenails
THE DIAGNOSIS: Nail Lichen Planus
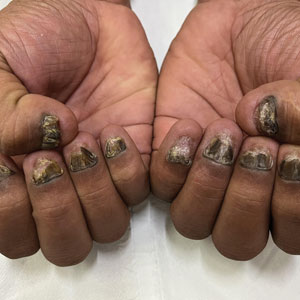
The biopsy results showed features of hypergranulosis of the matricial epithelium, irregular acanthosis, apoptotic keratinocytes along the basal layer, and a lichenoid infiltrate consistent with nail lichen planus. The patient was started on topical clobetasol propionate 0.05% applied once daily under overnight occlusion. Additionally, intramatricial triamcinolone acetonide (2.5 mg/mL; 0.1 mL per injection) was administered into the affected nail matrix at 4-week intervals for a total of 2 sessions. At the 2-month follow-up visit, the patient reported improvement in longitudinal ridging; however, he subsequently was lost to follow-up.
Nail lichen planus is a chronic inflammatory disorder that occurs in 10% to 15% of patients with lichen planus worldwide and is more common in adults than children.1 It can manifest independently or concurrently with cutaneous and/or oral mucosal involvement. The fingernails are more commonly affected than the toenails.2 The clinical features of nail lichen planus can be classified based on involvement of the nail matrix (longitudinal ridging, red lunula, thinning of the nail plate, koilonychia, trachyonychia, pterygium, and anonychia) or nail bed (onycholysis, subungual hyperkeratosis, and splinter hemorrhages).1
In our patient, who presented with chronic progressive nail dystrophy affecting all 20 nails, onychomycosis, nail psoriasis, onychotillomania, and idiopathic trachyonychia were included in the differential.1
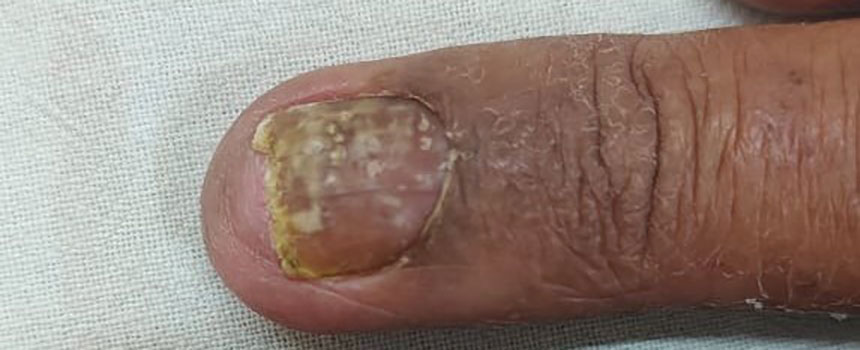
Onychomycosis manifests as white or yellow-brown discoloration of the nail, onycholysis, subungual hyperkeratosis, and thickening of the nail plate. Diagnosis is confirmed by the presence of septate hyphae (dermatophytes) or budding yeast cells (Candida species) on a potassium hydroxide mount. Other diagnostic modalities include dermoscopy, fungal culture, and histopathology of nail clippings, with demonstration of fungal elements identified on periodic acid-Schiff staining (eFigure 1).3
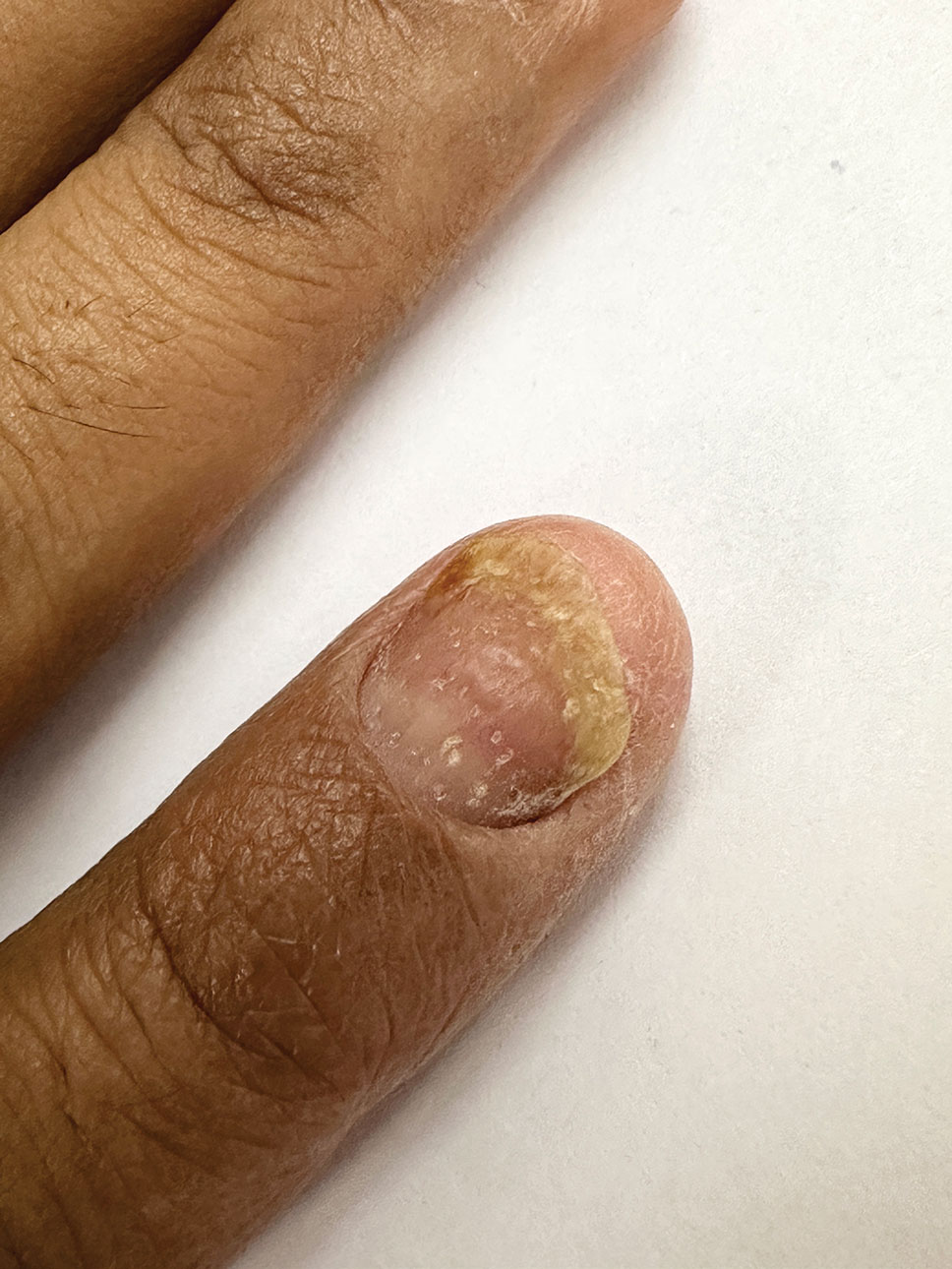
Nail psoriasis characteristically manifests as deep irregular pitting of the nails. Other features favoring psoriasis include involvement of the nail matrix manifesting as leukonychia, red lunula, and crumbling, as well as involvement of the nail bed manifesting as onycholysis, subungual hyperkeratosis, salmon patches/oil spots, and splinter hemorrhages (eFigure 2).4 Diagnosis primarily is clinical, supported by histopathology when uncertainty exists.
Onychotillomania is a behavioral disorder characterized by an irresistible urge or impulse in patients to either pick or pull at their fingernails and/or toenails. Clinicopathologic features of the involved nails are nonspecific and atypical, with possible involvement of periungual and digital skin. Diagnosis of onychotillomania is challenging.5 Dermoscopic features including anonychia with multiple obliquely arranged nail bed hemorrhages, gray pigmentation of the nail bed, and wavy lines, has been proposed to aid the diagnosis of onychotillomania.6
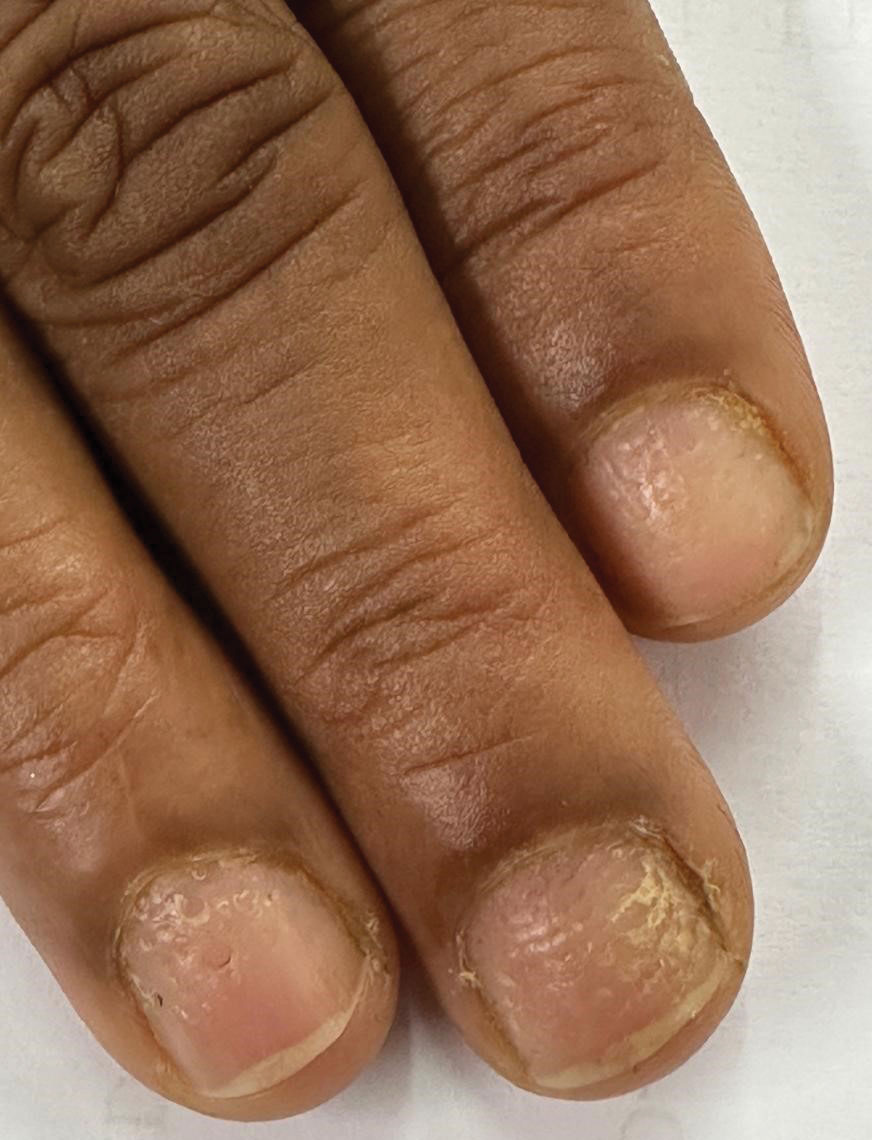
Idiopathic trachyonychia is isolated nail involvement characterized by rough, ridged, and thin nails affecting multiple or all of the fingernails and toenails without an underlying systemic or dermatologic condition (eFigure 3). The terms trachyonychia and 20-nail dystrophy have been used interchangeably in the literature; however, trachyonychia does not always involve all 20 nails. Other conditions causing widespread dystrophy of all 20 nails cannot be diagnosed as 20-nail dystrophy or trachyonychia without the distinct morphologic features of thin brittle nails with pronounced longitudinal ridging.7
Prompt diagnosis and early intervention in nail lichen planus is crucial due to the potential for irreversible scarring. First-line treatment options include intramatricial and intramuscular triamcinolone acetonide for 3 to 6 months.4 Second-line therapies include oral retinoids such as acitretin and alitretinoin and immunosuppressive agents such as azathioprine, mycophenolate mofetil, and cyclosporine. Other reported treatment options include clobetasol propionate, tacrolimus, dapsone, griseofulvin, etanercept, hydroxychloroquine, methotrexate, and UV therapy.4
- Gupta MK, Lipner SR. Review of nail lichen planus: epidemiology, pathogenesis, diagnosis, and treatment. Dermatol Clin. 2021;39:221-230. doi:10.1016/j.det.2020.12.002
- Iorizzo M, Tosti A, Starace M, et al. Isolated nail lichen planus: an expert consensus on treatment of the classical form. J Am Acad Dermatol. 2020;83:1717-1723. doi:10.1016/j.jaad.2020.02.056
- Leung AKC, Lam JM, Leong KF, et al. Onychomycosis: an updated review. Recent Pat Inflamm Allergy Drug Discov. 2020;14:32-45. doi:10.2174/1872213X13666191026090713
- Hwang JK, Grover C, Iorizzo M, et al. Nail psoriasis and nail lichen planus: updates on diagnosis and management. J Am Acad Dermatol. 2024;90:585-596. doi:10.1016/j.jaad.2023.11.024
- Sidiropoulou P, Sgouros D, Theodoropoulos K, et al. Onychotillomania: a chameleon-like disorder: case report and review of literature. Skin Appendage Disord. 2019;5:104-107. doi:10.1159/000489941
- Maddy AJ, Tosti A. Dermoscopic features of onychotillomania: a study of 36 cases. J Am Acad Dermatol. 2018;79:702-705. doi:10.1016 /j.jaad.2018.04.015
- Haber JS, Chairatchaneeboon M, Rubin AI. Trachyonychia: review and update on clinical aspects, histology, and therapy. Skin Appendage Disord. 2017;2:109-115. doi:10.1159/000449063
THE DIAGNOSIS: Nail Lichen Planus
The biopsy results showed features of hypergranulosis of the matricial epithelium, irregular acanthosis, apoptotic keratinocytes along the basal layer, and a lichenoid infiltrate consistent with nail lichen planus. The patient was started on topical clobetasol propionate 0.05% applied once daily under overnight occlusion. Additionally, intramatricial triamcinolone acetonide (2.5 mg/mL; 0.1 mL per injection) was administered into the affected nail matrix at 4-week intervals for a total of 2 sessions. At the 2-month follow-up visit, the patient reported improvement in longitudinal ridging; however, he subsequently was lost to follow-up.
Nail lichen planus is a chronic inflammatory disorder that occurs in 10% to 15% of patients with lichen planus worldwide and is more common in adults than children.1 It can manifest independently or concurrently with cutaneous and/or oral mucosal involvement. The fingernails are more commonly affected than the toenails.2 The clinical features of nail lichen planus can be classified based on involvement of the nail matrix (longitudinal ridging, red lunula, thinning of the nail plate, koilonychia, trachyonychia, pterygium, and anonychia) or nail bed (onycholysis, subungual hyperkeratosis, and splinter hemorrhages).1
In our patient, who presented with chronic progressive nail dystrophy affecting all 20 nails, onychomycosis, nail psoriasis, onychotillomania, and idiopathic trachyonychia were included in the differential.1
Onychomycosis manifests as white or yellow-brown discoloration of the nail, onycholysis, subungual hyperkeratosis, and thickening of the nail plate. Diagnosis is confirmed by the presence of septate hyphae (dermatophytes) or budding yeast cells (Candida species) on a potassium hydroxide mount. Other diagnostic modalities include dermoscopy, fungal culture, and histopathology of nail clippings, with demonstration of fungal elements identified on periodic acid-Schiff staining (eFigure 1).3

Nail psoriasis characteristically manifests as deep irregular pitting of the nails. Other features favoring psoriasis include involvement of the nail matrix manifesting as leukonychia, red lunula, and crumbling, as well as involvement of the nail bed manifesting as onycholysis, subungual hyperkeratosis, salmon patches/oil spots, and splinter hemorrhages (eFigure 2).4 Diagnosis primarily is clinical, supported by histopathology when uncertainty exists.

Onychotillomania is a behavioral disorder characterized by an irresistible urge or impulse in patients to either pick or pull at their fingernails and/or toenails. Clinicopathologic features of the involved nails are nonspecific and atypical, with possible involvement of periungual and digital skin. Diagnosis of onychotillomania is challenging.5 Dermoscopic features including anonychia with multiple obliquely arranged nail bed hemorrhages, gray pigmentation of the nail bed, and wavy lines, has been proposed to aid the diagnosis of onychotillomania.6
Idiopathic trachyonychia is isolated nail involvement characterized by rough, ridged, and thin nails affecting multiple or all of the fingernails and toenails without an underlying systemic or dermatologic condition (eFigure 3). The terms trachyonychia and 20-nail dystrophy have been used interchangeably in the literature; however, trachyonychia does not always involve all 20 nails. Other conditions causing widespread dystrophy of all 20 nails cannot be diagnosed as 20-nail dystrophy or trachyonychia without the distinct morphologic features of thin brittle nails with pronounced longitudinal ridging.7

Prompt diagnosis and early intervention in nail lichen planus is crucial due to the potential for irreversible scarring. First-line treatment options include intramatricial and intramuscular triamcinolone acetonide for 3 to 6 months.4 Second-line therapies include oral retinoids such as acitretin and alitretinoin and immunosuppressive agents such as azathioprine, mycophenolate mofetil, and cyclosporine. Other reported treatment options include clobetasol propionate, tacrolimus, dapsone, griseofulvin, etanercept, hydroxychloroquine, methotrexate, and UV therapy.4
THE DIAGNOSIS: Nail Lichen Planus
The biopsy results showed features of hypergranulosis of the matricial epithelium, irregular acanthosis, apoptotic keratinocytes along the basal layer, and a lichenoid infiltrate consistent with nail lichen planus. The patient was started on topical clobetasol propionate 0.05% applied once daily under overnight occlusion. Additionally, intramatricial triamcinolone acetonide (2.5 mg/mL; 0.1 mL per injection) was administered into the affected nail matrix at 4-week intervals for a total of 2 sessions. At the 2-month follow-up visit, the patient reported improvement in longitudinal ridging; however, he subsequently was lost to follow-up.
Nail lichen planus is a chronic inflammatory disorder that occurs in 10% to 15% of patients with lichen planus worldwide and is more common in adults than children.1 It can manifest independently or concurrently with cutaneous and/or oral mucosal involvement. The fingernails are more commonly affected than the toenails.2 The clinical features of nail lichen planus can be classified based on involvement of the nail matrix (longitudinal ridging, red lunula, thinning of the nail plate, koilonychia, trachyonychia, pterygium, and anonychia) or nail bed (onycholysis, subungual hyperkeratosis, and splinter hemorrhages).1
In our patient, who presented with chronic progressive nail dystrophy affecting all 20 nails, onychomycosis, nail psoriasis, onychotillomania, and idiopathic trachyonychia were included in the differential.1
Onychomycosis manifests as white or yellow-brown discoloration of the nail, onycholysis, subungual hyperkeratosis, and thickening of the nail plate. Diagnosis is confirmed by the presence of septate hyphae (dermatophytes) or budding yeast cells (Candida species) on a potassium hydroxide mount. Other diagnostic modalities include dermoscopy, fungal culture, and histopathology of nail clippings, with demonstration of fungal elements identified on periodic acid-Schiff staining (eFigure 1).3

Nail psoriasis characteristically manifests as deep irregular pitting of the nails. Other features favoring psoriasis include involvement of the nail matrix manifesting as leukonychia, red lunula, and crumbling, as well as involvement of the nail bed manifesting as onycholysis, subungual hyperkeratosis, salmon patches/oil spots, and splinter hemorrhages (eFigure 2).4 Diagnosis primarily is clinical, supported by histopathology when uncertainty exists.

Onychotillomania is a behavioral disorder characterized by an irresistible urge or impulse in patients to either pick or pull at their fingernails and/or toenails. Clinicopathologic features of the involved nails are nonspecific and atypical, with possible involvement of periungual and digital skin. Diagnosis of onychotillomania is challenging.5 Dermoscopic features including anonychia with multiple obliquely arranged nail bed hemorrhages, gray pigmentation of the nail bed, and wavy lines, has been proposed to aid the diagnosis of onychotillomania.6
Idiopathic trachyonychia is isolated nail involvement characterized by rough, ridged, and thin nails affecting multiple or all of the fingernails and toenails without an underlying systemic or dermatologic condition (eFigure 3). The terms trachyonychia and 20-nail dystrophy have been used interchangeably in the literature; however, trachyonychia does not always involve all 20 nails. Other conditions causing widespread dystrophy of all 20 nails cannot be diagnosed as 20-nail dystrophy or trachyonychia without the distinct morphologic features of thin brittle nails with pronounced longitudinal ridging.7

Prompt diagnosis and early intervention in nail lichen planus is crucial due to the potential for irreversible scarring. First-line treatment options include intramatricial and intramuscular triamcinolone acetonide for 3 to 6 months.4 Second-line therapies include oral retinoids such as acitretin and alitretinoin and immunosuppressive agents such as azathioprine, mycophenolate mofetil, and cyclosporine. Other reported treatment options include clobetasol propionate, tacrolimus, dapsone, griseofulvin, etanercept, hydroxychloroquine, methotrexate, and UV therapy.4
- Gupta MK, Lipner SR. Review of nail lichen planus: epidemiology, pathogenesis, diagnosis, and treatment. Dermatol Clin. 2021;39:221-230. doi:10.1016/j.det.2020.12.002
- Iorizzo M, Tosti A, Starace M, et al. Isolated nail lichen planus: an expert consensus on treatment of the classical form. J Am Acad Dermatol. 2020;83:1717-1723. doi:10.1016/j.jaad.2020.02.056
- Leung AKC, Lam JM, Leong KF, et al. Onychomycosis: an updated review. Recent Pat Inflamm Allergy Drug Discov. 2020;14:32-45. doi:10.2174/1872213X13666191026090713
- Hwang JK, Grover C, Iorizzo M, et al. Nail psoriasis and nail lichen planus: updates on diagnosis and management. J Am Acad Dermatol. 2024;90:585-596. doi:10.1016/j.jaad.2023.11.024
- Sidiropoulou P, Sgouros D, Theodoropoulos K, et al. Onychotillomania: a chameleon-like disorder: case report and review of literature. Skin Appendage Disord. 2019;5:104-107. doi:10.1159/000489941
- Maddy AJ, Tosti A. Dermoscopic features of onychotillomania: a study of 36 cases. J Am Acad Dermatol. 2018;79:702-705. doi:10.1016 /j.jaad.2018.04.015
- Haber JS, Chairatchaneeboon M, Rubin AI. Trachyonychia: review and update on clinical aspects, histology, and therapy. Skin Appendage Disord. 2017;2:109-115. doi:10.1159/000449063
- Gupta MK, Lipner SR. Review of nail lichen planus: epidemiology, pathogenesis, diagnosis, and treatment. Dermatol Clin. 2021;39:221-230. doi:10.1016/j.det.2020.12.002
- Iorizzo M, Tosti A, Starace M, et al. Isolated nail lichen planus: an expert consensus on treatment of the classical form. J Am Acad Dermatol. 2020;83:1717-1723. doi:10.1016/j.jaad.2020.02.056
- Leung AKC, Lam JM, Leong KF, et al. Onychomycosis: an updated review. Recent Pat Inflamm Allergy Drug Discov. 2020;14:32-45. doi:10.2174/1872213X13666191026090713
- Hwang JK, Grover C, Iorizzo M, et al. Nail psoriasis and nail lichen planus: updates on diagnosis and management. J Am Acad Dermatol. 2024;90:585-596. doi:10.1016/j.jaad.2023.11.024
- Sidiropoulou P, Sgouros D, Theodoropoulos K, et al. Onychotillomania: a chameleon-like disorder: case report and review of literature. Skin Appendage Disord. 2019;5:104-107. doi:10.1159/000489941
- Maddy AJ, Tosti A. Dermoscopic features of onychotillomania: a study of 36 cases. J Am Acad Dermatol. 2018;79:702-705. doi:10.1016 /j.jaad.2018.04.015
- Haber JS, Chairatchaneeboon M, Rubin AI. Trachyonychia: review and update on clinical aspects, histology, and therapy. Skin Appendage Disord. 2017;2:109-115. doi:10.1159/000449063
Progressive Dystrophy of the Fingernails and Toenails
Progressive Dystrophy of the Fingernails and Toenails
A 35-year-old man presented to the dermatology department with gradually progressive dystrophy of the fingernails and toenails of 20 years’ duration. The patient reported no history of other dermatologic conditions. Physical examination revealed longitudinal ridging of all 20 nails and discoloration of the nail plates, as well as a few nails showing pterygium and anonychia; the skin and mucosal surfaces were otherwise normal, and nail plate thinning was not observed. A potassium hydroxide mount was negative. A biopsy of the nail matrix on the left thumbnail was performed.
Cobblestonelike Papules on the Neck
The Diagnosis: Fibroelastolytic Papulosis
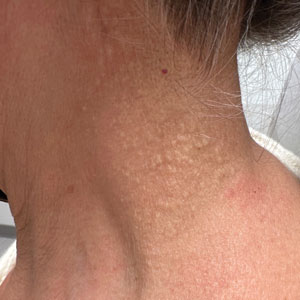
Histopathology demonstrated decreased density and fragmentation of elastic fibers in the superficial reticular and papillary dermis consistent with an elastolytic disease process (Figure). Of note, elastolysis typically is visualized with Verhoeff-van Gieson stain but cannot be visualized well with standard hematoxylin and eosin staining. Additional staining with Congo red was negative for amyloid, and colloidal iron did not show any increase in dermal mucin, ruling out amyloidosis and scleromyxedema, respectively. Based on the histopathologic findings and the clinical history, a diagnosis of fibroelastolytic papulosis (FP) was made. Given the benign nature of the condition, the patient was prescribed a topical steroid (clobetasol 0.05%) for symptomatic relief.
Cutaneous conditions can arise from abnormalities in the elastin composition of connective tissue due to abnormal elastin formation or degradation (elastolysis).1 Fibroelastolytic papulosis is a distinct elastolytic disorder diagnosed histologically by a notable loss of elastic fibers localized to the papillary dermis.2 Fibroelastolytic papulosis is an acquired condition linked to exposure to UV radiation, abnormal elastogenesis, and hormonal factors that commonly involves the neck, supraclavicular area, and upper back.1-3 Predominantly affecting elderly women, FP is characterized by soft white papules that often coalesce into a cobblestonelike plaque.2 Because the condition rarely is seen in men, there is speculation that it may involve genetic, hereditary, and hormonal factors that have yet to be identified.1
Fibroelastolytic papulosis can be classified as either pseudoxanthoma elasticum–like papillary dermal elastolysis or white fibrous papulosis.2,3 White fibrous papulosis manifests with haphazardly arranged collagen fibers in the reticular and deep dermis with papillary dermal elastolysis and most commonly develops on the neck.3 Although our patient’s lesion was on the neck, the absence of thickened collagen bands on histology supported classification as the pseudoxanthoma elasticum– like papillary dermal elastolysis subtype.
Fibroelastolytic papulosis can be distinguished from other elastic abnormalities by its characteristic clinical appearance, demographic distribution, and associated histopathologic findings. The differential diagnosis of FP includes pseudoxanthoma elasticum (PXE), anetoderma, scleromyxedema, and lichen amyloidosis.
Pseudoxanthoma elasticum is a hereditary or acquired multisystem disease characterized by fragmentation and calcification of elastic fibers in the mid dermis.1,4 Its clinical presentation resembles that of FP, appearing as small, asymptomatic, yellowish or flesh-colored papules in a reticular pattern that progressively coalesce into larger plaques with a cobblestonelike appearance.1 Like FP, PXE commonly affects the flexural creases in women but in contrast may manifest earlier (ie, second or third decades of life). Additionally, the pathogenesis of PXE is not related to UV radiation exposure. The hereditary form develops due to a gene variation, whereas the acquired form may be due to conditions associated with physiologic and/or mechanical stress.1
Anetoderma, also known as macular atrophy, is another condition that demonstrates elastic tissue loss in the dermis on histopathology.1 Anetoderma commonly is seen in younger patients and can be differentiated from FP by the antecedent presence of an inflammatory process. Anetoderma is classified as primary or secondary. Primary anetoderma is associated with prothrombotic abnormalities, while secondary anetoderma is associated with systemic disease including but not limited to sarcoidosis, systemic lupus erythematous, and Graves disease.1
Neither lichen myxedematosus (LM) nor lichen amyloidosis (LA) are true elastolytic conditions. Lichen myxedematosus is considered in the differential diagnosis of FP due to the associated loss of elastin observed with disease progression. An idiopathic cutaneous mucinosis, LM is a localized form of scleromyxedema, which is characterized by small, firm, waxy papules; mucin deposition in the skin; fibroblast proliferation; and fibrosis. On histologic analysis, typical findings of LM include irregularly arranged fibroblasts, diffuse mucin deposition within the upper and mid reticular dermis, increased collagen deposition, and a decrease in elastin fibers.5
Lichen amyloidosis is a subtype of primary localized cutaneous amyloidosis, a rare condition characterized by the extracellular deposition of amyloid proteins in the skin and a lack of systemic involvement. Although it is not an elastolytic condition, LA is clinically similar to FP, often manifesting as multiple localized, pruritic, hyperpigmented papules that can coalesce into larger plaques; it tends to develop on the shins, calves, ankles, and thighs.6,7 The condition commonly manifests in the fifth and sixth decades of life; however, in contrast to FP, LA is more prevalent in men and individuals from Central and South American as well as Middle Eastern and non-Chinese Asian populations.8 Lichen amyloidosis is a keratin-derived amyloidosis with cytokeratin-based amyloid precursors that only deposit in the dermis.6 Histopathology reveals colloid bodies due to the presence of apoptotic basal keratinocytes. The etiology of LA is unknown, but on rare occasions it has been associated with multiple endocrine neoplasia 2A rearranged during transfection mutations.6
In summary, FP is an uncommonly diagnosed elastolytic condition that often is asymptomatic or associated with mild pruritus. Biopsy is warranted to help differentiate it from mimicker conditions that may be associated with systemic disease. Currently, there is no established therapy that provides successful treatment. Research suggests unsatisfactory results with the use of topical tretinoin or topical antioxidants.3 More recently, nonablative fractional resurfacing lasers have been evaluated as a possible therapeutic strategy of promise for elastic disorders.9
- Andrés-Ramos I, Alegría-Landa V, Gimeno I, et al. Cutaneous elastic tissue anomalies. Am J Dermatopathol. 2019;41:85-117. doi:10.1097/DAD.0000000000001275
- Valbuena V, Assaad D, Yeung J. Pseudoxanthoma elasticum-like papillary dermal elastolysis: a single case report. J Cutan Med Surg. 2017;21:345-347. doi:10.1177/1203475417699407
- Dokic Y, Tschen J. White fibrous papulosis of the axillae and neck. Cureus. 2020;12:E7635. doi:10.7759/cureus.7635
- Recio-Monescillo M, Torre-Castro J, Manzanas C, et al. Papillary dermal elastolysis histopathology mimicking folliculotropic mycosis fungoides. J Cutan Pathol. 2023;50:430-433. doi:10.1111/cup.14402
- Cokonis Georgakis CD, Falasca G, Georgakis A, et al. Scleromyxedema. Clin Dermatol. 2006;24:493-497. doi:10.1016/j.clindermatol.2006.07.011
- Weidner T, Illing T, Elsner P. Primary localized cutaneous amyloidosis: a systematic treatment review. Am J Clin Dermatol. 2017;18:629-642. doi:10.1007/s40257-017-0278-9
- Ladizinski B, Lee KC. Lichen amyloidosis. CMAJ. 2014;186:532. doi:10.1503/cmaj.130698
- Chen JF, Chen YF. Answer: can you identify this condition? Can Fam Physician. 2012;58:1234-1235.
- Foering K, Torbeck RL, Frank MP, et al. Treatment of pseudoxanthoma elasticum-like papillary dermal elastolysis with nonablative fractional resurfacing laser resulting in clinical and histologic improvement in elastin and collagen. J Cosmet Laser Ther. 2018;20:382-384. doi:10.1080/14764172.2017.1358457
The Diagnosis: Fibroelastolytic Papulosis
Histopathology demonstrated decreased density and fragmentation of elastic fibers in the superficial reticular and papillary dermis consistent with an elastolytic disease process (Figure). Of note, elastolysis typically is visualized with Verhoeff-van Gieson stain but cannot be visualized well with standard hematoxylin and eosin staining. Additional staining with Congo red was negative for amyloid, and colloidal iron did not show any increase in dermal mucin, ruling out amyloidosis and scleromyxedema, respectively. Based on the histopathologic findings and the clinical history, a diagnosis of fibroelastolytic papulosis (FP) was made. Given the benign nature of the condition, the patient was prescribed a topical steroid (clobetasol 0.05%) for symptomatic relief.

Cutaneous conditions can arise from abnormalities in the elastin composition of connective tissue due to abnormal elastin formation or degradation (elastolysis).1 Fibroelastolytic papulosis is a distinct elastolytic disorder diagnosed histologically by a notable loss of elastic fibers localized to the papillary dermis.2 Fibroelastolytic papulosis is an acquired condition linked to exposure to UV radiation, abnormal elastogenesis, and hormonal factors that commonly involves the neck, supraclavicular area, and upper back.1-3 Predominantly affecting elderly women, FP is characterized by soft white papules that often coalesce into a cobblestonelike plaque.2 Because the condition rarely is seen in men, there is speculation that it may involve genetic, hereditary, and hormonal factors that have yet to be identified.1
Fibroelastolytic papulosis can be classified as either pseudoxanthoma elasticum–like papillary dermal elastolysis or white fibrous papulosis.2,3 White fibrous papulosis manifests with haphazardly arranged collagen fibers in the reticular and deep dermis with papillary dermal elastolysis and most commonly develops on the neck.3 Although our patient’s lesion was on the neck, the absence of thickened collagen bands on histology supported classification as the pseudoxanthoma elasticum– like papillary dermal elastolysis subtype.
Fibroelastolytic papulosis can be distinguished from other elastic abnormalities by its characteristic clinical appearance, demographic distribution, and associated histopathologic findings. The differential diagnosis of FP includes pseudoxanthoma elasticum (PXE), anetoderma, scleromyxedema, and lichen amyloidosis.
Pseudoxanthoma elasticum is a hereditary or acquired multisystem disease characterized by fragmentation and calcification of elastic fibers in the mid dermis.1,4 Its clinical presentation resembles that of FP, appearing as small, asymptomatic, yellowish or flesh-colored papules in a reticular pattern that progressively coalesce into larger plaques with a cobblestonelike appearance.1 Like FP, PXE commonly affects the flexural creases in women but in contrast may manifest earlier (ie, second or third decades of life). Additionally, the pathogenesis of PXE is not related to UV radiation exposure. The hereditary form develops due to a gene variation, whereas the acquired form may be due to conditions associated with physiologic and/or mechanical stress.1
Anetoderma, also known as macular atrophy, is another condition that demonstrates elastic tissue loss in the dermis on histopathology.1 Anetoderma commonly is seen in younger patients and can be differentiated from FP by the antecedent presence of an inflammatory process. Anetoderma is classified as primary or secondary. Primary anetoderma is associated with prothrombotic abnormalities, while secondary anetoderma is associated with systemic disease including but not limited to sarcoidosis, systemic lupus erythematous, and Graves disease.1
Neither lichen myxedematosus (LM) nor lichen amyloidosis (LA) are true elastolytic conditions. Lichen myxedematosus is considered in the differential diagnosis of FP due to the associated loss of elastin observed with disease progression. An idiopathic cutaneous mucinosis, LM is a localized form of scleromyxedema, which is characterized by small, firm, waxy papules; mucin deposition in the skin; fibroblast proliferation; and fibrosis. On histologic analysis, typical findings of LM include irregularly arranged fibroblasts, diffuse mucin deposition within the upper and mid reticular dermis, increased collagen deposition, and a decrease in elastin fibers.5
Lichen amyloidosis is a subtype of primary localized cutaneous amyloidosis, a rare condition characterized by the extracellular deposition of amyloid proteins in the skin and a lack of systemic involvement. Although it is not an elastolytic condition, LA is clinically similar to FP, often manifesting as multiple localized, pruritic, hyperpigmented papules that can coalesce into larger plaques; it tends to develop on the shins, calves, ankles, and thighs.6,7 The condition commonly manifests in the fifth and sixth decades of life; however, in contrast to FP, LA is more prevalent in men and individuals from Central and South American as well as Middle Eastern and non-Chinese Asian populations.8 Lichen amyloidosis is a keratin-derived amyloidosis with cytokeratin-based amyloid precursors that only deposit in the dermis.6 Histopathology reveals colloid bodies due to the presence of apoptotic basal keratinocytes. The etiology of LA is unknown, but on rare occasions it has been associated with multiple endocrine neoplasia 2A rearranged during transfection mutations.6
In summary, FP is an uncommonly diagnosed elastolytic condition that often is asymptomatic or associated with mild pruritus. Biopsy is warranted to help differentiate it from mimicker conditions that may be associated with systemic disease. Currently, there is no established therapy that provides successful treatment. Research suggests unsatisfactory results with the use of topical tretinoin or topical antioxidants.3 More recently, nonablative fractional resurfacing lasers have been evaluated as a possible therapeutic strategy of promise for elastic disorders.9
The Diagnosis: Fibroelastolytic Papulosis
Histopathology demonstrated decreased density and fragmentation of elastic fibers in the superficial reticular and papillary dermis consistent with an elastolytic disease process (Figure). Of note, elastolysis typically is visualized with Verhoeff-van Gieson stain but cannot be visualized well with standard hematoxylin and eosin staining. Additional staining with Congo red was negative for amyloid, and colloidal iron did not show any increase in dermal mucin, ruling out amyloidosis and scleromyxedema, respectively. Based on the histopathologic findings and the clinical history, a diagnosis of fibroelastolytic papulosis (FP) was made. Given the benign nature of the condition, the patient was prescribed a topical steroid (clobetasol 0.05%) for symptomatic relief.

Cutaneous conditions can arise from abnormalities in the elastin composition of connective tissue due to abnormal elastin formation or degradation (elastolysis).1 Fibroelastolytic papulosis is a distinct elastolytic disorder diagnosed histologically by a notable loss of elastic fibers localized to the papillary dermis.2 Fibroelastolytic papulosis is an acquired condition linked to exposure to UV radiation, abnormal elastogenesis, and hormonal factors that commonly involves the neck, supraclavicular area, and upper back.1-3 Predominantly affecting elderly women, FP is characterized by soft white papules that often coalesce into a cobblestonelike plaque.2 Because the condition rarely is seen in men, there is speculation that it may involve genetic, hereditary, and hormonal factors that have yet to be identified.1
Fibroelastolytic papulosis can be classified as either pseudoxanthoma elasticum–like papillary dermal elastolysis or white fibrous papulosis.2,3 White fibrous papulosis manifests with haphazardly arranged collagen fibers in the reticular and deep dermis with papillary dermal elastolysis and most commonly develops on the neck.3 Although our patient’s lesion was on the neck, the absence of thickened collagen bands on histology supported classification as the pseudoxanthoma elasticum– like papillary dermal elastolysis subtype.
Fibroelastolytic papulosis can be distinguished from other elastic abnormalities by its characteristic clinical appearance, demographic distribution, and associated histopathologic findings. The differential diagnosis of FP includes pseudoxanthoma elasticum (PXE), anetoderma, scleromyxedema, and lichen amyloidosis.
Pseudoxanthoma elasticum is a hereditary or acquired multisystem disease characterized by fragmentation and calcification of elastic fibers in the mid dermis.1,4 Its clinical presentation resembles that of FP, appearing as small, asymptomatic, yellowish or flesh-colored papules in a reticular pattern that progressively coalesce into larger plaques with a cobblestonelike appearance.1 Like FP, PXE commonly affects the flexural creases in women but in contrast may manifest earlier (ie, second or third decades of life). Additionally, the pathogenesis of PXE is not related to UV radiation exposure. The hereditary form develops due to a gene variation, whereas the acquired form may be due to conditions associated with physiologic and/or mechanical stress.1
Anetoderma, also known as macular atrophy, is another condition that demonstrates elastic tissue loss in the dermis on histopathology.1 Anetoderma commonly is seen in younger patients and can be differentiated from FP by the antecedent presence of an inflammatory process. Anetoderma is classified as primary or secondary. Primary anetoderma is associated with prothrombotic abnormalities, while secondary anetoderma is associated with systemic disease including but not limited to sarcoidosis, systemic lupus erythematous, and Graves disease.1
Neither lichen myxedematosus (LM) nor lichen amyloidosis (LA) are true elastolytic conditions. Lichen myxedematosus is considered in the differential diagnosis of FP due to the associated loss of elastin observed with disease progression. An idiopathic cutaneous mucinosis, LM is a localized form of scleromyxedema, which is characterized by small, firm, waxy papules; mucin deposition in the skin; fibroblast proliferation; and fibrosis. On histologic analysis, typical findings of LM include irregularly arranged fibroblasts, diffuse mucin deposition within the upper and mid reticular dermis, increased collagen deposition, and a decrease in elastin fibers.5
Lichen amyloidosis is a subtype of primary localized cutaneous amyloidosis, a rare condition characterized by the extracellular deposition of amyloid proteins in the skin and a lack of systemic involvement. Although it is not an elastolytic condition, LA is clinically similar to FP, often manifesting as multiple localized, pruritic, hyperpigmented papules that can coalesce into larger plaques; it tends to develop on the shins, calves, ankles, and thighs.6,7 The condition commonly manifests in the fifth and sixth decades of life; however, in contrast to FP, LA is more prevalent in men and individuals from Central and South American as well as Middle Eastern and non-Chinese Asian populations.8 Lichen amyloidosis is a keratin-derived amyloidosis with cytokeratin-based amyloid precursors that only deposit in the dermis.6 Histopathology reveals colloid bodies due to the presence of apoptotic basal keratinocytes. The etiology of LA is unknown, but on rare occasions it has been associated with multiple endocrine neoplasia 2A rearranged during transfection mutations.6
In summary, FP is an uncommonly diagnosed elastolytic condition that often is asymptomatic or associated with mild pruritus. Biopsy is warranted to help differentiate it from mimicker conditions that may be associated with systemic disease. Currently, there is no established therapy that provides successful treatment. Research suggests unsatisfactory results with the use of topical tretinoin or topical antioxidants.3 More recently, nonablative fractional resurfacing lasers have been evaluated as a possible therapeutic strategy of promise for elastic disorders.9
- Andrés-Ramos I, Alegría-Landa V, Gimeno I, et al. Cutaneous elastic tissue anomalies. Am J Dermatopathol. 2019;41:85-117. doi:10.1097/DAD.0000000000001275
- Valbuena V, Assaad D, Yeung J. Pseudoxanthoma elasticum-like papillary dermal elastolysis: a single case report. J Cutan Med Surg. 2017;21:345-347. doi:10.1177/1203475417699407
- Dokic Y, Tschen J. White fibrous papulosis of the axillae and neck. Cureus. 2020;12:E7635. doi:10.7759/cureus.7635
- Recio-Monescillo M, Torre-Castro J, Manzanas C, et al. Papillary dermal elastolysis histopathology mimicking folliculotropic mycosis fungoides. J Cutan Pathol. 2023;50:430-433. doi:10.1111/cup.14402
- Cokonis Georgakis CD, Falasca G, Georgakis A, et al. Scleromyxedema. Clin Dermatol. 2006;24:493-497. doi:10.1016/j.clindermatol.2006.07.011
- Weidner T, Illing T, Elsner P. Primary localized cutaneous amyloidosis: a systematic treatment review. Am J Clin Dermatol. 2017;18:629-642. doi:10.1007/s40257-017-0278-9
- Ladizinski B, Lee KC. Lichen amyloidosis. CMAJ. 2014;186:532. doi:10.1503/cmaj.130698
- Chen JF, Chen YF. Answer: can you identify this condition? Can Fam Physician. 2012;58:1234-1235.
- Foering K, Torbeck RL, Frank MP, et al. Treatment of pseudoxanthoma elasticum-like papillary dermal elastolysis with nonablative fractional resurfacing laser resulting in clinical and histologic improvement in elastin and collagen. J Cosmet Laser Ther. 2018;20:382-384. doi:10.1080/14764172.2017.1358457
- Andrés-Ramos I, Alegría-Landa V, Gimeno I, et al. Cutaneous elastic tissue anomalies. Am J Dermatopathol. 2019;41:85-117. doi:10.1097/DAD.0000000000001275
- Valbuena V, Assaad D, Yeung J. Pseudoxanthoma elasticum-like papillary dermal elastolysis: a single case report. J Cutan Med Surg. 2017;21:345-347. doi:10.1177/1203475417699407
- Dokic Y, Tschen J. White fibrous papulosis of the axillae and neck. Cureus. 2020;12:E7635. doi:10.7759/cureus.7635
- Recio-Monescillo M, Torre-Castro J, Manzanas C, et al. Papillary dermal elastolysis histopathology mimicking folliculotropic mycosis fungoides. J Cutan Pathol. 2023;50:430-433. doi:10.1111/cup.14402
- Cokonis Georgakis CD, Falasca G, Georgakis A, et al. Scleromyxedema. Clin Dermatol. 2006;24:493-497. doi:10.1016/j.clindermatol.2006.07.011
- Weidner T, Illing T, Elsner P. Primary localized cutaneous amyloidosis: a systematic treatment review. Am J Clin Dermatol. 2017;18:629-642. doi:10.1007/s40257-017-0278-9
- Ladizinski B, Lee KC. Lichen amyloidosis. CMAJ. 2014;186:532. doi:10.1503/cmaj.130698
- Chen JF, Chen YF. Answer: can you identify this condition? Can Fam Physician. 2012;58:1234-1235.
- Foering K, Torbeck RL, Frank MP, et al. Treatment of pseudoxanthoma elasticum-like papillary dermal elastolysis with nonablative fractional resurfacing laser resulting in clinical and histologic improvement in elastin and collagen. J Cosmet Laser Ther. 2018;20:382-384. doi:10.1080/14764172.2017.1358457
A 76-year-old woman presented to the dermatology clinic for evaluation of a pruritic rash on the posterior lateral neck of several years’ duration. The rash had been slowly worsening and was intermittently symptomatic. Physical examination revealed monomorphous flesh-colored papules coalescing on the neck, yielding a cobblestonelike texture. The patient had been treated previously by dermatology with topical steroids, but symptoms persisted. A punch biopsy of the left lateral neck was performed.
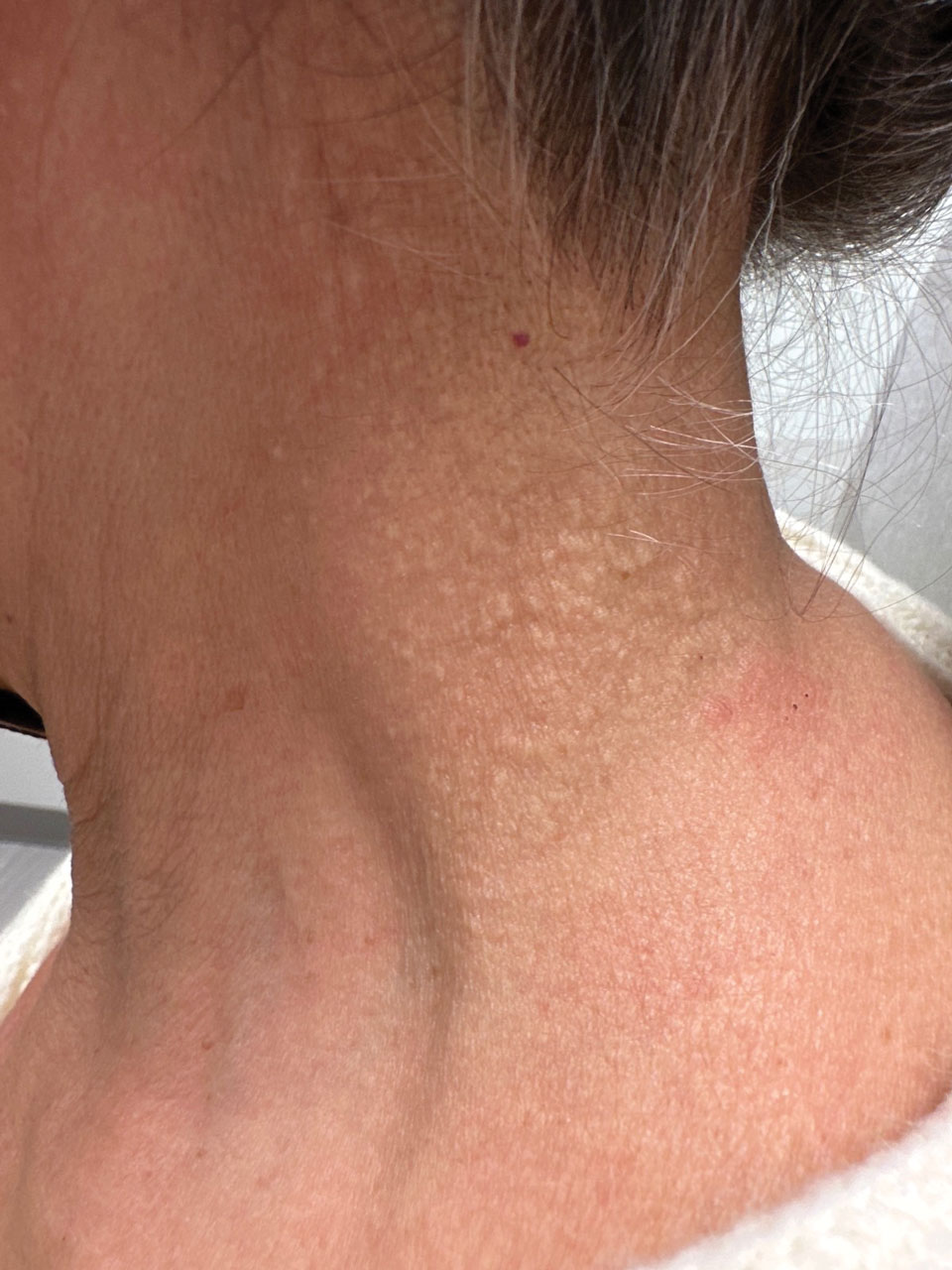
Alopecia and Pruritic Rash on the Forehead and Scalp
Alopecia and Pruritic Rash on the Forehead and Scalp
THE DIAGNOSIS: Folliculitis Decalvans
Biopsy results revealed a brisk perifollicular and intrafollicular mixed inflammatory infiltrate comprising lymphocytes, neutrophils, and plasma cells filling the upper dermis and encircling dilated hair follicles. Elastic stain (Verhoeff-van Gieson) demonstrated loss of elastic fibers in areas of scarring. Periodic acid–Schiff with diastase staining was negative for fungal elements, while Gram staining revealed colonies of bacterial cocci in the stratum corneum and within the hair follicles. Immunofluorescence was unremarkable, and culture revealed methicillin-sensitive Staphylococcus aureus, leading to a diagnosis of folliculitis decalvans (FD). The patient was treated with doxycycline 100 mg twice daily and received intralesional triamcinolone 2.5 mg/mL (total volume, 2 mL) every 6 weeks with considerable improvement in pustules, erythema, and scaling (Figure). While not yet in complete remission, our patient demonstrated short regrowing hairs in areas of incomplete scarring and focal remaining perifollicular erythema and scale along the midline frontal scalp 5 months after initial presentation.
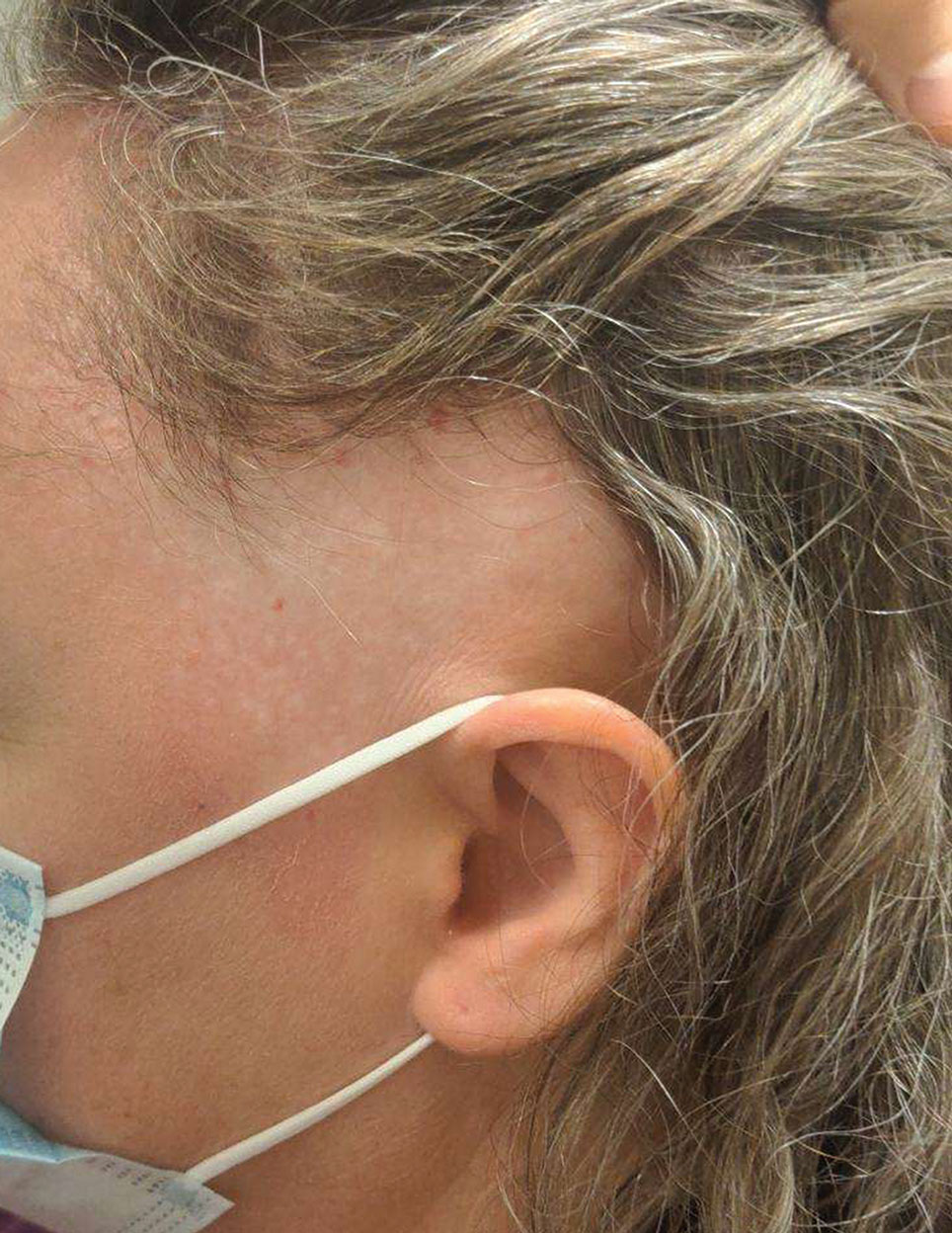
Folliculitis decalvans is an uncommon subtype of cicatricial alopecia that may mimic other forms of alopecia. Cicatricial alopecia often is difficult to diagnose due to its overlapping clinical characteristics, but early diagnosis is essential for appropriate management and prevention of further permanent hair loss. Traditionally classified as a primary neutrophilic cicatricial alopecia, lymphocyte-predominant variants of FD now are recognized.1
Patients with FD typically present with patchy scarring alopecia at the vertex scalp that gradually expands and may demonstrate secondary features of follicular tufting and pustules.1-3 While the epidemiology of FD is poorly characterized, Vañó-Galván et al4 reported that FD accounted for 2.8% of all alopecia cases and 10.5% of cicatricial alopecia cases in a multicenter study of 2835 patients. The pathophysiology of FD still is under investigation but is thought to result from a dysregulated immune response to a chronic bacterial infection (eg, S aureus), with resulting neutrophilpredominant inflammation in early stages.1-3 Vañó-Galván et al4 reported that, among 35 patients with FD cultured for bacteria, 74% (26/35) returned positive results, 96% (25/26) of which grew S aureus.5
A systematic review of 20 studies that included 263 patients found rifampin and clindamycin to be the most common treatments for FD; however, there is insufficient evidence to determine if this treatment is the most effective.6 In our patient, clindamycin was avoided due to its propensity to negatively alter the gut microbiome long term.7 Other therapies such as oral tetracyclines, high-potency topical steroids, and intralesional triamcinolone also can be used to achieve disease remission.5,6 Other treatments such as isotretinoin, red-light photodynamic therapy, tacrolimus, and external beam radiation have been reported in the literature but vary in efficacy.6 Our patient improved on a regimen of topical benzoyl peroxide wash, oral doxycycline, and intralesional triamcinolone.
Notably, FD may share clinical features with other causes of cicatricial alopecia. In our patient, FD mimicked other entities including discoid lupus erythematosus, frontal fibrosing alopecia, dissecting cellulitis, and erosive pustular dermatosis (Table).1-14 Discoid lupus erythematosus manifests as round hypopigmented and hyperpigmented plaques with associated atrophy, perifollicular erythema, and follicular plugging. Frontal fibrosing alopecia is a primary lymphocytic scarring alopecia that manifests in a bandlike linear distribution over the frontal scalp and may involve the temporal scalp, posterior hairline, and/or eyebrows. Isolated hairs (known as lonely hairs) often are seen. Dissecting cellulitis is characterized by boggy nodules associated with alopecia on the scalp without notable epidermal change, although pustules and sinus tracts may develop.9 Erosive pustular dermatosis is a diagnosis of exclusion but often is seen in older adults with chronic sun damage and clinically manifests with eroded plaques with adherent crusts.10
While our patient presented with several overlapping clinical features, including progressive hair loss along the frontal scalp in a bandlike pattern suspicious for frontal fibrosing alopecia as well as atrophic depigmented plaques with adherent peripheral scaling suspicious for discoid lupus erythematosus, the presence of pustules was an important clue. The biopsy demonstrating a mixed infiltrate inclusive of neutrophils confirmed the diagnosis of FD.
- Olsen EA, Bergfeld WF, Cotsarelis G, et al. Summary of North American Hair Research Society (NAHRS)-sponsored Workshop on Cicatricial Alopecia, Duke University Medical Center, February 10 and 11, 2001. J Am Acad Dermatol. 2003;48:103-110. doi:10.1067/mjd.2003.68
- Filbrandt R, Rufaut N, Jones L. Primary cicatricial alopecia: diagnosis and treatment. CMAJ. 2013;185:1579-1585. doi:10.1503/cmaj.111570
- Otberg N, Kang H, Alzolibani AA, et al. Folliculitis decalvans. Dermatol Ther. 2008;21:238-244. doi:10.1111/j.1529-8019.2008.00204.x
- Vañó-Galván S, Saceda-Corralo D, Blume-Peytavi U, et al. Frequency of the types of alopecia at twenty-two specialist hair clinics: a multicenter study. Skin Appendage Disord. 2019;5:309-315. doi:10.1159/000496708
- Vañó-Galván S, Molina-Ruiz AM, Fernández-Crehuet P, et al. Folliculitis decalvans: a multicentre review of 82 patients. J Eur Acad Dermatol Venereol. 2015;29:1750-1757. doi:10.1111/jdv.12993
- Rambhia PH, Conic RRZ, Murad A, et al. Updates in therapeutics for folliculitis decalvans: a systematic review with evidence-based analysis. J Am Acad Dermatol. 2019;80:794-801. doi:10.1016/j.jaad.2018.07.050
- Zimmermann P, Curtis N. The effect of antibiotics on the composition of the intestinal microbiota - a systematic review. J Infect. 2019;79:471-489. doi:10.1016/j.jinf.2019.10.008
- Kanti V, Röwert-Huber J, Vogt A, et al. Cicatricial alopecia. J Dtsch Dermatol Ges. 2018;16:435-461. doi:10.1111/ddg.13498
- Melo DF, Slaibi EB, Siqueira TMFM, et al. Trichoscopy findings in dissecting cellulitis. An Bras Dermatol. 2019;94:608-611. doi:10.1016/j.abd.2019.09.006
- Anzai A, Pirmez R, Vincenzi C, et al. Trichoscopy findings of frontal fibrosing alopecia on the eyebrows: a study of 151 cases. J Am Acad Dermatol. 2021;85:1130-1134. doi:10.1016/j.jaad.2019.12.023
- Starace M, Loi C, Bruni F, et al. Erosive pustular dermatosis of the scalp: clinical, trichoscopic, and histopathologic features of 20 cases. J Am Acad Dermatol. 2017;76:1109-1114. doi:10.1016/j.jaad.2016.12.016
- Rongioletti F, Christana K. Cicatricial (scarring) alopecias: an overview of pathogenesis, classification, diagnosis, and treatment. Am J Clin Dermatol. 2012;13:247-260. doi:10.2165/11596960-000000000-00000
- Badaoui A, Reygagne P, Cavelier-Balloy B, et al. Dissecting cellulitis of the scalp: a retrospective study of 51 patients and review of literature. Br J Dermatol. 2016;174:421-423. doi:10.1111/bjd.13999
- Michelerio A, Vassallo C, Fiandrino G, et al. Erosive pustular dermatosis of the scalp: a clinicopathologic study of fifty cases. Dermatopathology (Basel). 2021;8:450-462. doi:10.3390/dermatopathology8040048
THE DIAGNOSIS: Folliculitis Decalvans
Biopsy results revealed a brisk perifollicular and intrafollicular mixed inflammatory infiltrate comprising lymphocytes, neutrophils, and plasma cells filling the upper dermis and encircling dilated hair follicles. Elastic stain (Verhoeff-van Gieson) demonstrated loss of elastic fibers in areas of scarring. Periodic acid–Schiff with diastase staining was negative for fungal elements, while Gram staining revealed colonies of bacterial cocci in the stratum corneum and within the hair follicles. Immunofluorescence was unremarkable, and culture revealed methicillin-sensitive Staphylococcus aureus, leading to a diagnosis of folliculitis decalvans (FD). The patient was treated with doxycycline 100 mg twice daily and received intralesional triamcinolone 2.5 mg/mL (total volume, 2 mL) every 6 weeks with considerable improvement in pustules, erythema, and scaling (Figure). While not yet in complete remission, our patient demonstrated short regrowing hairs in areas of incomplete scarring and focal remaining perifollicular erythema and scale along the midline frontal scalp 5 months after initial presentation.

Folliculitis decalvans is an uncommon subtype of cicatricial alopecia that may mimic other forms of alopecia. Cicatricial alopecia often is difficult to diagnose due to its overlapping clinical characteristics, but early diagnosis is essential for appropriate management and prevention of further permanent hair loss. Traditionally classified as a primary neutrophilic cicatricial alopecia, lymphocyte-predominant variants of FD now are recognized.1
Patients with FD typically present with patchy scarring alopecia at the vertex scalp that gradually expands and may demonstrate secondary features of follicular tufting and pustules.1-3 While the epidemiology of FD is poorly characterized, Vañó-Galván et al4 reported that FD accounted for 2.8% of all alopecia cases and 10.5% of cicatricial alopecia cases in a multicenter study of 2835 patients. The pathophysiology of FD still is under investigation but is thought to result from a dysregulated immune response to a chronic bacterial infection (eg, S aureus), with resulting neutrophilpredominant inflammation in early stages.1-3 Vañó-Galván et al4 reported that, among 35 patients with FD cultured for bacteria, 74% (26/35) returned positive results, 96% (25/26) of which grew S aureus.5
A systematic review of 20 studies that included 263 patients found rifampin and clindamycin to be the most common treatments for FD; however, there is insufficient evidence to determine if this treatment is the most effective.6 In our patient, clindamycin was avoided due to its propensity to negatively alter the gut microbiome long term.7 Other therapies such as oral tetracyclines, high-potency topical steroids, and intralesional triamcinolone also can be used to achieve disease remission.5,6 Other treatments such as isotretinoin, red-light photodynamic therapy, tacrolimus, and external beam radiation have been reported in the literature but vary in efficacy.6 Our patient improved on a regimen of topical benzoyl peroxide wash, oral doxycycline, and intralesional triamcinolone.
Notably, FD may share clinical features with other causes of cicatricial alopecia. In our patient, FD mimicked other entities including discoid lupus erythematosus, frontal fibrosing alopecia, dissecting cellulitis, and erosive pustular dermatosis (Table).1-14 Discoid lupus erythematosus manifests as round hypopigmented and hyperpigmented plaques with associated atrophy, perifollicular erythema, and follicular plugging. Frontal fibrosing alopecia is a primary lymphocytic scarring alopecia that manifests in a bandlike linear distribution over the frontal scalp and may involve the temporal scalp, posterior hairline, and/or eyebrows. Isolated hairs (known as lonely hairs) often are seen. Dissecting cellulitis is characterized by boggy nodules associated with alopecia on the scalp without notable epidermal change, although pustules and sinus tracts may develop.9 Erosive pustular dermatosis is a diagnosis of exclusion but often is seen in older adults with chronic sun damage and clinically manifests with eroded plaques with adherent crusts.10

While our patient presented with several overlapping clinical features, including progressive hair loss along the frontal scalp in a bandlike pattern suspicious for frontal fibrosing alopecia as well as atrophic depigmented plaques with adherent peripheral scaling suspicious for discoid lupus erythematosus, the presence of pustules was an important clue. The biopsy demonstrating a mixed infiltrate inclusive of neutrophils confirmed the diagnosis of FD.
THE DIAGNOSIS: Folliculitis Decalvans
Biopsy results revealed a brisk perifollicular and intrafollicular mixed inflammatory infiltrate comprising lymphocytes, neutrophils, and plasma cells filling the upper dermis and encircling dilated hair follicles. Elastic stain (Verhoeff-van Gieson) demonstrated loss of elastic fibers in areas of scarring. Periodic acid–Schiff with diastase staining was negative for fungal elements, while Gram staining revealed colonies of bacterial cocci in the stratum corneum and within the hair follicles. Immunofluorescence was unremarkable, and culture revealed methicillin-sensitive Staphylococcus aureus, leading to a diagnosis of folliculitis decalvans (FD). The patient was treated with doxycycline 100 mg twice daily and received intralesional triamcinolone 2.5 mg/mL (total volume, 2 mL) every 6 weeks with considerable improvement in pustules, erythema, and scaling (Figure). While not yet in complete remission, our patient demonstrated short regrowing hairs in areas of incomplete scarring and focal remaining perifollicular erythema and scale along the midline frontal scalp 5 months after initial presentation.

Folliculitis decalvans is an uncommon subtype of cicatricial alopecia that may mimic other forms of alopecia. Cicatricial alopecia often is difficult to diagnose due to its overlapping clinical characteristics, but early diagnosis is essential for appropriate management and prevention of further permanent hair loss. Traditionally classified as a primary neutrophilic cicatricial alopecia, lymphocyte-predominant variants of FD now are recognized.1
Patients with FD typically present with patchy scarring alopecia at the vertex scalp that gradually expands and may demonstrate secondary features of follicular tufting and pustules.1-3 While the epidemiology of FD is poorly characterized, Vañó-Galván et al4 reported that FD accounted for 2.8% of all alopecia cases and 10.5% of cicatricial alopecia cases in a multicenter study of 2835 patients. The pathophysiology of FD still is under investigation but is thought to result from a dysregulated immune response to a chronic bacterial infection (eg, S aureus), with resulting neutrophilpredominant inflammation in early stages.1-3 Vañó-Galván et al4 reported that, among 35 patients with FD cultured for bacteria, 74% (26/35) returned positive results, 96% (25/26) of which grew S aureus.5
A systematic review of 20 studies that included 263 patients found rifampin and clindamycin to be the most common treatments for FD; however, there is insufficient evidence to determine if this treatment is the most effective.6 In our patient, clindamycin was avoided due to its propensity to negatively alter the gut microbiome long term.7 Other therapies such as oral tetracyclines, high-potency topical steroids, and intralesional triamcinolone also can be used to achieve disease remission.5,6 Other treatments such as isotretinoin, red-light photodynamic therapy, tacrolimus, and external beam radiation have been reported in the literature but vary in efficacy.6 Our patient improved on a regimen of topical benzoyl peroxide wash, oral doxycycline, and intralesional triamcinolone.
Notably, FD may share clinical features with other causes of cicatricial alopecia. In our patient, FD mimicked other entities including discoid lupus erythematosus, frontal fibrosing alopecia, dissecting cellulitis, and erosive pustular dermatosis (Table).1-14 Discoid lupus erythematosus manifests as round hypopigmented and hyperpigmented plaques with associated atrophy, perifollicular erythema, and follicular plugging. Frontal fibrosing alopecia is a primary lymphocytic scarring alopecia that manifests in a bandlike linear distribution over the frontal scalp and may involve the temporal scalp, posterior hairline, and/or eyebrows. Isolated hairs (known as lonely hairs) often are seen. Dissecting cellulitis is characterized by boggy nodules associated with alopecia on the scalp without notable epidermal change, although pustules and sinus tracts may develop.9 Erosive pustular dermatosis is a diagnosis of exclusion but often is seen in older adults with chronic sun damage and clinically manifests with eroded plaques with adherent crusts.10

While our patient presented with several overlapping clinical features, including progressive hair loss along the frontal scalp in a bandlike pattern suspicious for frontal fibrosing alopecia as well as atrophic depigmented plaques with adherent peripheral scaling suspicious for discoid lupus erythematosus, the presence of pustules was an important clue. The biopsy demonstrating a mixed infiltrate inclusive of neutrophils confirmed the diagnosis of FD.
- Olsen EA, Bergfeld WF, Cotsarelis G, et al. Summary of North American Hair Research Society (NAHRS)-sponsored Workshop on Cicatricial Alopecia, Duke University Medical Center, February 10 and 11, 2001. J Am Acad Dermatol. 2003;48:103-110. doi:10.1067/mjd.2003.68
- Filbrandt R, Rufaut N, Jones L. Primary cicatricial alopecia: diagnosis and treatment. CMAJ. 2013;185:1579-1585. doi:10.1503/cmaj.111570
- Otberg N, Kang H, Alzolibani AA, et al. Folliculitis decalvans. Dermatol Ther. 2008;21:238-244. doi:10.1111/j.1529-8019.2008.00204.x
- Vañó-Galván S, Saceda-Corralo D, Blume-Peytavi U, et al. Frequency of the types of alopecia at twenty-two specialist hair clinics: a multicenter study. Skin Appendage Disord. 2019;5:309-315. doi:10.1159/000496708
- Vañó-Galván S, Molina-Ruiz AM, Fernández-Crehuet P, et al. Folliculitis decalvans: a multicentre review of 82 patients. J Eur Acad Dermatol Venereol. 2015;29:1750-1757. doi:10.1111/jdv.12993
- Rambhia PH, Conic RRZ, Murad A, et al. Updates in therapeutics for folliculitis decalvans: a systematic review with evidence-based analysis. J Am Acad Dermatol. 2019;80:794-801. doi:10.1016/j.jaad.2018.07.050
- Zimmermann P, Curtis N. The effect of antibiotics on the composition of the intestinal microbiota - a systematic review. J Infect. 2019;79:471-489. doi:10.1016/j.jinf.2019.10.008
- Kanti V, Röwert-Huber J, Vogt A, et al. Cicatricial alopecia. J Dtsch Dermatol Ges. 2018;16:435-461. doi:10.1111/ddg.13498
- Melo DF, Slaibi EB, Siqueira TMFM, et al. Trichoscopy findings in dissecting cellulitis. An Bras Dermatol. 2019;94:608-611. doi:10.1016/j.abd.2019.09.006
- Anzai A, Pirmez R, Vincenzi C, et al. Trichoscopy findings of frontal fibrosing alopecia on the eyebrows: a study of 151 cases. J Am Acad Dermatol. 2021;85:1130-1134. doi:10.1016/j.jaad.2019.12.023
- Starace M, Loi C, Bruni F, et al. Erosive pustular dermatosis of the scalp: clinical, trichoscopic, and histopathologic features of 20 cases. J Am Acad Dermatol. 2017;76:1109-1114. doi:10.1016/j.jaad.2016.12.016
- Rongioletti F, Christana K. Cicatricial (scarring) alopecias: an overview of pathogenesis, classification, diagnosis, and treatment. Am J Clin Dermatol. 2012;13:247-260. doi:10.2165/11596960-000000000-00000
- Badaoui A, Reygagne P, Cavelier-Balloy B, et al. Dissecting cellulitis of the scalp: a retrospective study of 51 patients and review of literature. Br J Dermatol. 2016;174:421-423. doi:10.1111/bjd.13999
- Michelerio A, Vassallo C, Fiandrino G, et al. Erosive pustular dermatosis of the scalp: a clinicopathologic study of fifty cases. Dermatopathology (Basel). 2021;8:450-462. doi:10.3390/dermatopathology8040048
- Olsen EA, Bergfeld WF, Cotsarelis G, et al. Summary of North American Hair Research Society (NAHRS)-sponsored Workshop on Cicatricial Alopecia, Duke University Medical Center, February 10 and 11, 2001. J Am Acad Dermatol. 2003;48:103-110. doi:10.1067/mjd.2003.68
- Filbrandt R, Rufaut N, Jones L. Primary cicatricial alopecia: diagnosis and treatment. CMAJ. 2013;185:1579-1585. doi:10.1503/cmaj.111570
- Otberg N, Kang H, Alzolibani AA, et al. Folliculitis decalvans. Dermatol Ther. 2008;21:238-244. doi:10.1111/j.1529-8019.2008.00204.x
- Vañó-Galván S, Saceda-Corralo D, Blume-Peytavi U, et al. Frequency of the types of alopecia at twenty-two specialist hair clinics: a multicenter study. Skin Appendage Disord. 2019;5:309-315. doi:10.1159/000496708
- Vañó-Galván S, Molina-Ruiz AM, Fernández-Crehuet P, et al. Folliculitis decalvans: a multicentre review of 82 patients. J Eur Acad Dermatol Venereol. 2015;29:1750-1757. doi:10.1111/jdv.12993
- Rambhia PH, Conic RRZ, Murad A, et al. Updates in therapeutics for folliculitis decalvans: a systematic review with evidence-based analysis. J Am Acad Dermatol. 2019;80:794-801. doi:10.1016/j.jaad.2018.07.050
- Zimmermann P, Curtis N. The effect of antibiotics on the composition of the intestinal microbiota - a systematic review. J Infect. 2019;79:471-489. doi:10.1016/j.jinf.2019.10.008
- Kanti V, Röwert-Huber J, Vogt A, et al. Cicatricial alopecia. J Dtsch Dermatol Ges. 2018;16:435-461. doi:10.1111/ddg.13498
- Melo DF, Slaibi EB, Siqueira TMFM, et al. Trichoscopy findings in dissecting cellulitis. An Bras Dermatol. 2019;94:608-611. doi:10.1016/j.abd.2019.09.006
- Anzai A, Pirmez R, Vincenzi C, et al. Trichoscopy findings of frontal fibrosing alopecia on the eyebrows: a study of 151 cases. J Am Acad Dermatol. 2021;85:1130-1134. doi:10.1016/j.jaad.2019.12.023
- Starace M, Loi C, Bruni F, et al. Erosive pustular dermatosis of the scalp: clinical, trichoscopic, and histopathologic features of 20 cases. J Am Acad Dermatol. 2017;76:1109-1114. doi:10.1016/j.jaad.2016.12.016
- Rongioletti F, Christana K. Cicatricial (scarring) alopecias: an overview of pathogenesis, classification, diagnosis, and treatment. Am J Clin Dermatol. 2012;13:247-260. doi:10.2165/11596960-000000000-00000
- Badaoui A, Reygagne P, Cavelier-Balloy B, et al. Dissecting cellulitis of the scalp: a retrospective study of 51 patients and review of literature. Br J Dermatol. 2016;174:421-423. doi:10.1111/bjd.13999
- Michelerio A, Vassallo C, Fiandrino G, et al. Erosive pustular dermatosis of the scalp: a clinicopathologic study of fifty cases. Dermatopathology (Basel). 2021;8:450-462. doi:10.3390/dermatopathology8040048
Alopecia and Pruritic Rash on the Forehead and Scalp
Alopecia and Pruritic Rash on the Forehead and Scalp
A 52-year-old woman presented to the dermatology department with an intermittently pruritic rash in a bandlike distribution on the left upper forehead and the frontal and temporal scalp of 4 years’ duration. The rash initially was diagnosed as psoriasis at an outside facility. Treatment over the year prior to presentation included tildrakizumab-asmn; topical crisaborole 2%; and excimer laser, which was complicated by blistering. The patient reported no history of topical or injected steroid use in the involved areas. Physical examination at the current presentation revealed arcuate erythematous plaques with follicular prominence, perifollicular scaling, pustules, and lone hairs. There also were porcelain-white atrophic plaques with loss of follicular ostia that were most prominent over the temporal scalp. A biopsy of the left lateral forehead was performed.
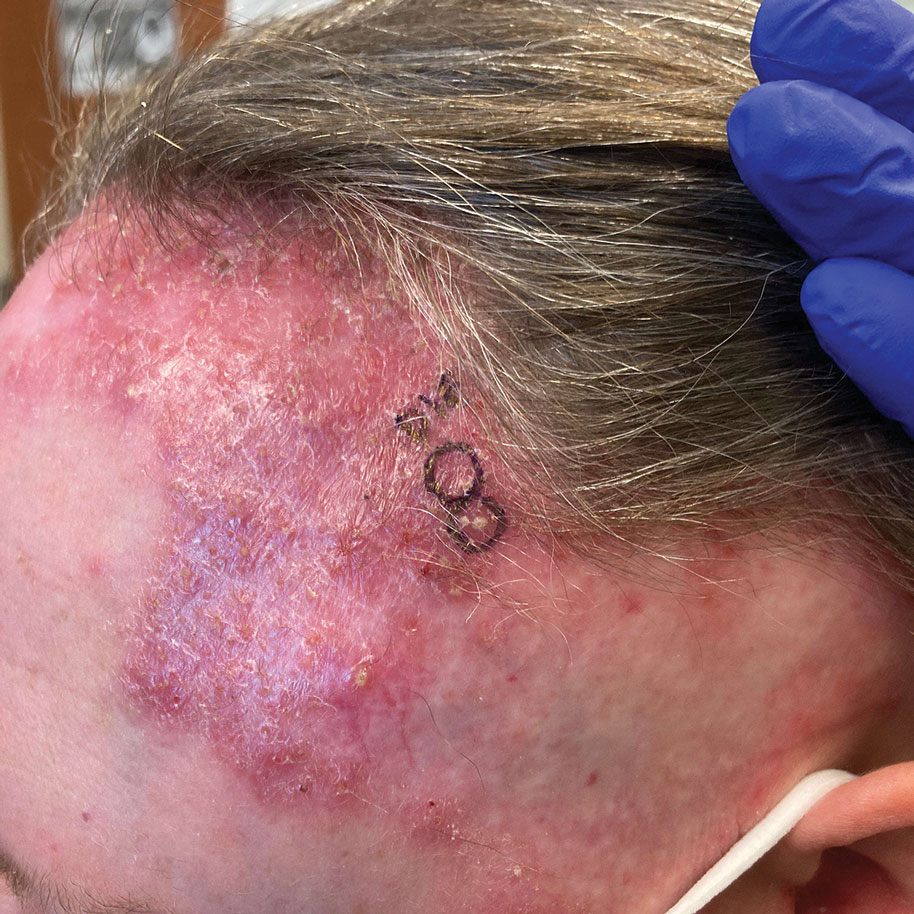
Solitary Yellow Papule on the Upper Back in an Infant
The Diagnosis: Juvenile Xanthogranuloma
Given the patient’s age, clinical features of the lesion, and characteristic setting-sun pattern on dermoscopy, a diagnosis of juvenile xanthogranuloma (JXG) was made. The patient showed no other signs of neurofibromatosis type 1 (NF1) or systemic disease and was managed conservatively with observation and routine follow-up. Minimal growth of the lesion was noted at 1-year follow-up, and he was meeting all age-appropriate developmental milestones and showed no other symptoms consistent with NF1.
Juvenile xanthogranuloma is the most common childhood non–Langerhans cell histiocytosis. While it typically manifests as an isolated condition, JXG also can be associated with NF1 as well as juvenile myelomonocytic leukemia.1-3 Neurofibromatosis type 1 is a multisystem disorder with variable clinical manifestations that commonly is associated with skin findings such as café au lait macules, intertriginous freckling, and neurofibromas, in addition to JXG.2,3 Diagnosis of JXG should prompt noninvasive evaluation for further signs and symptoms of NF1, including thorough patient and family history and physical examination to identify other characteristic cutaneous findings, and can include consideration of slit lamp eye examination and radiography for identification of osseous findings.
The pathogenesis of JXG is not fully known, though there is evidence that it may be associated with a mutation in the mitogen-activated protein kinase pathway.1 The majority of cases appear in the first year of life.4 Clinically, JXG can manifest with extracutaneous lesions, including on the eyes and lungs.5-7 Juvenile xanthogranuloma can be noninvasively diagnosed with dermoscopy. As seen in our patient, dermoscopic findings include a red-yellow or yellow-orange background with an erythematous border, typically described as a setting-sun pattern.4,8 Biopsy can confirm the diagnosis; however, given the usually benign course, this often is unnecessary. Most pediatric patients with cutaneous manifestations have a self-limited course with regression over several months to years. Generally, no treatment is required for cutaneous manifestations alone; however, lesions can be removed for aesthetic concerns. For those with systemic involvement, a range of other treatments have been used, including chemotherapy, radiotherapy, systemic corticosteroids, and cyclosporine.6,7
The differential diagnosis for JXG includes Brooke-Spiegler syndrome, Fabry disease, solitary cutaneous mastocytoma, and tuberous sclerosis complex. Brooke-Spiegler syndrome is an autosomal-dominant condition characterized by the growth of adnexal neoplasms, including trichoepitheliomas, cylindromas, and spiradenomas. These lesions usually manifest on the face but can include other areas such as the trunk.9 Fabry disease is an X-linked recessive lysosomal storage disorder with cutaneous manifestations such as angiokeratoma corporis diffusum and hypohidrosis. Patients also may present with systemic symptoms including hypertension and renal and cardiovascular disease.10 Mastocytosis encompasses several clinical disorders defined by mast cell hyperplasia and accumulation in various organ systems, and solitary cutaneous mastocytoma is the most common manifestation in children.11,12 Cutaneous mastocytoma can manifest as a single red-brown or yellow papule, usually located on the arms or legs.13 Solitary cutaneous mastocytomas in pediatric patients typically are diagnosed based on clinical appearance and the formation of a wheal upon firm palpation (Darier sign).11-13 Our patient did not demonstrate the Darier sign, and the lesion was asymptomatic. Tuberous sclerosis complex is an autosomal-dominant neurocutaneous disorder with neurologic and skin findings that occur early in the disease course and include facial angiofibromas, hypomelanotic macules, shagreen patches, and café-au-lait macules.14
- Durham BH, Lopez Rodrigo E, Picarsic J, et al. Activating mutations in CSF1R and additional receptor tyrosine kinases in histiocytic neoplasms. Nat Med. 2019;25:1839-1842.
- Friedman JM. Neurofibromatosis 1. In: Adam MP, Feldman J, Mirzaa GM, et al, eds. GeneReviews®. University of Washington, Seattle; 1998.
- Miraglia E, Laghi A, Moramarco A, et al. Juvenile xanthogranuloma in neurofibromatosis type 1. Prevalence and possible correlation with lymphoproliferative diseases: experience of a single center and review of the literature. Clin Ther. 2022;173:353-355.
- Collie JS, Harper CD, Fillman EP. Juvenile xanthogranuloma. StatPearls [Internet]. StatPearls Publishing; 2025. Updated August 8, 2023. Accessed November 4, 2025. https://www.ncbi.nlm.nih.gov/books/NBK526103/
- Newman B, Hu W, Nigro K, et al. Aggressive histiocytic disorders that can involve the skin. J Am Acad Dermatol. 2007;56:302-316.
- Freyer DR, Kennedy R, Bostrom BC, et al. Juvenile xanthogranuloma: forms of systemic disease and their clinical implications. J Pediatr. 1996;129:227-237.
- Murphy JT, Soeken T, Megison S, et al. Juvenile xanthogranuloma: diverse presentations of noncutaneous disease. J Pediatr Hematol Oncol.2014;36:641-645.
- Xu J, Ma L. Dermoscopic patterns in juvenile xanthogranuloma based on the histological classification. Front Med (Lausanne). 2021;7:618946.
- Kazakov DV. Brooke-Spiegler syndrome and phenotypic variants: an update. Head Neck Pathol. 2016;10:125-130.
- Bokhari SRA, Zulfiqar H, Hariz A. Fabry disease. StatPearls [Internet]. StatPearls Publishing; 2025. Update July 4, 2023. Accessed November 4, 2025. https://www.ncbi.nlm.nih.gov/books/NBK435996/
- Hartmann K, Escribano L, Grattan C, et al. Cutaneous manifestations in patients with mastocytosis: consensus report of the European Competence Network on Mastocytosis; the American Academy of Allergy, Asthma & Immunology; and the European Academy of Allergology and Clinical Immunology. J Allergy Clin Immunol. 2016;137:35-45.
- Klaiber N, Kumar S, Irani AM. Mastocytosis in children. Curr Allergy Asthma Rep. 2017;17:80.
- Sławin´ ska M, Kaszuba A, Lange M, et al. Dermoscopic features of different forms of cutaneous mastocytosis: a systematic review. J Clin Med. 2022;11:4649.
- Teng JM, Cowen EW, Wataya-Kaneda M, et al. Dermatologic and dental aspects of the 2012 International Tuberous Sclerosis Complex Consensus Statements. JAMA Dermatol. 2014;150:1095-1101.
The Diagnosis: Juvenile Xanthogranuloma
Given the patient’s age, clinical features of the lesion, and characteristic setting-sun pattern on dermoscopy, a diagnosis of juvenile xanthogranuloma (JXG) was made. The patient showed no other signs of neurofibromatosis type 1 (NF1) or systemic disease and was managed conservatively with observation and routine follow-up. Minimal growth of the lesion was noted at 1-year follow-up, and he was meeting all age-appropriate developmental milestones and showed no other symptoms consistent with NF1.
Juvenile xanthogranuloma is the most common childhood non–Langerhans cell histiocytosis. While it typically manifests as an isolated condition, JXG also can be associated with NF1 as well as juvenile myelomonocytic leukemia.1-3 Neurofibromatosis type 1 is a multisystem disorder with variable clinical manifestations that commonly is associated with skin findings such as café au lait macules, intertriginous freckling, and neurofibromas, in addition to JXG.2,3 Diagnosis of JXG should prompt noninvasive evaluation for further signs and symptoms of NF1, including thorough patient and family history and physical examination to identify other characteristic cutaneous findings, and can include consideration of slit lamp eye examination and radiography for identification of osseous findings.
The pathogenesis of JXG is not fully known, though there is evidence that it may be associated with a mutation in the mitogen-activated protein kinase pathway.1 The majority of cases appear in the first year of life.4 Clinically, JXG can manifest with extracutaneous lesions, including on the eyes and lungs.5-7 Juvenile xanthogranuloma can be noninvasively diagnosed with dermoscopy. As seen in our patient, dermoscopic findings include a red-yellow or yellow-orange background with an erythematous border, typically described as a setting-sun pattern.4,8 Biopsy can confirm the diagnosis; however, given the usually benign course, this often is unnecessary. Most pediatric patients with cutaneous manifestations have a self-limited course with regression over several months to years. Generally, no treatment is required for cutaneous manifestations alone; however, lesions can be removed for aesthetic concerns. For those with systemic involvement, a range of other treatments have been used, including chemotherapy, radiotherapy, systemic corticosteroids, and cyclosporine.6,7
The differential diagnosis for JXG includes Brooke-Spiegler syndrome, Fabry disease, solitary cutaneous mastocytoma, and tuberous sclerosis complex. Brooke-Spiegler syndrome is an autosomal-dominant condition characterized by the growth of adnexal neoplasms, including trichoepitheliomas, cylindromas, and spiradenomas. These lesions usually manifest on the face but can include other areas such as the trunk.9 Fabry disease is an X-linked recessive lysosomal storage disorder with cutaneous manifestations such as angiokeratoma corporis diffusum and hypohidrosis. Patients also may present with systemic symptoms including hypertension and renal and cardiovascular disease.10 Mastocytosis encompasses several clinical disorders defined by mast cell hyperplasia and accumulation in various organ systems, and solitary cutaneous mastocytoma is the most common manifestation in children.11,12 Cutaneous mastocytoma can manifest as a single red-brown or yellow papule, usually located on the arms or legs.13 Solitary cutaneous mastocytomas in pediatric patients typically are diagnosed based on clinical appearance and the formation of a wheal upon firm palpation (Darier sign).11-13 Our patient did not demonstrate the Darier sign, and the lesion was asymptomatic. Tuberous sclerosis complex is an autosomal-dominant neurocutaneous disorder with neurologic and skin findings that occur early in the disease course and include facial angiofibromas, hypomelanotic macules, shagreen patches, and café-au-lait macules.14
The Diagnosis: Juvenile Xanthogranuloma
Given the patient’s age, clinical features of the lesion, and characteristic setting-sun pattern on dermoscopy, a diagnosis of juvenile xanthogranuloma (JXG) was made. The patient showed no other signs of neurofibromatosis type 1 (NF1) or systemic disease and was managed conservatively with observation and routine follow-up. Minimal growth of the lesion was noted at 1-year follow-up, and he was meeting all age-appropriate developmental milestones and showed no other symptoms consistent with NF1.
Juvenile xanthogranuloma is the most common childhood non–Langerhans cell histiocytosis. While it typically manifests as an isolated condition, JXG also can be associated with NF1 as well as juvenile myelomonocytic leukemia.1-3 Neurofibromatosis type 1 is a multisystem disorder with variable clinical manifestations that commonly is associated with skin findings such as café au lait macules, intertriginous freckling, and neurofibromas, in addition to JXG.2,3 Diagnosis of JXG should prompt noninvasive evaluation for further signs and symptoms of NF1, including thorough patient and family history and physical examination to identify other characteristic cutaneous findings, and can include consideration of slit lamp eye examination and radiography for identification of osseous findings.
The pathogenesis of JXG is not fully known, though there is evidence that it may be associated with a mutation in the mitogen-activated protein kinase pathway.1 The majority of cases appear in the first year of life.4 Clinically, JXG can manifest with extracutaneous lesions, including on the eyes and lungs.5-7 Juvenile xanthogranuloma can be noninvasively diagnosed with dermoscopy. As seen in our patient, dermoscopic findings include a red-yellow or yellow-orange background with an erythematous border, typically described as a setting-sun pattern.4,8 Biopsy can confirm the diagnosis; however, given the usually benign course, this often is unnecessary. Most pediatric patients with cutaneous manifestations have a self-limited course with regression over several months to years. Generally, no treatment is required for cutaneous manifestations alone; however, lesions can be removed for aesthetic concerns. For those with systemic involvement, a range of other treatments have been used, including chemotherapy, radiotherapy, systemic corticosteroids, and cyclosporine.6,7
The differential diagnosis for JXG includes Brooke-Spiegler syndrome, Fabry disease, solitary cutaneous mastocytoma, and tuberous sclerosis complex. Brooke-Spiegler syndrome is an autosomal-dominant condition characterized by the growth of adnexal neoplasms, including trichoepitheliomas, cylindromas, and spiradenomas. These lesions usually manifest on the face but can include other areas such as the trunk.9 Fabry disease is an X-linked recessive lysosomal storage disorder with cutaneous manifestations such as angiokeratoma corporis diffusum and hypohidrosis. Patients also may present with systemic symptoms including hypertension and renal and cardiovascular disease.10 Mastocytosis encompasses several clinical disorders defined by mast cell hyperplasia and accumulation in various organ systems, and solitary cutaneous mastocytoma is the most common manifestation in children.11,12 Cutaneous mastocytoma can manifest as a single red-brown or yellow papule, usually located on the arms or legs.13 Solitary cutaneous mastocytomas in pediatric patients typically are diagnosed based on clinical appearance and the formation of a wheal upon firm palpation (Darier sign).11-13 Our patient did not demonstrate the Darier sign, and the lesion was asymptomatic. Tuberous sclerosis complex is an autosomal-dominant neurocutaneous disorder with neurologic and skin findings that occur early in the disease course and include facial angiofibromas, hypomelanotic macules, shagreen patches, and café-au-lait macules.14
- Durham BH, Lopez Rodrigo E, Picarsic J, et al. Activating mutations in CSF1R and additional receptor tyrosine kinases in histiocytic neoplasms. Nat Med. 2019;25:1839-1842.
- Friedman JM. Neurofibromatosis 1. In: Adam MP, Feldman J, Mirzaa GM, et al, eds. GeneReviews®. University of Washington, Seattle; 1998.
- Miraglia E, Laghi A, Moramarco A, et al. Juvenile xanthogranuloma in neurofibromatosis type 1. Prevalence and possible correlation with lymphoproliferative diseases: experience of a single center and review of the literature. Clin Ther. 2022;173:353-355.
- Collie JS, Harper CD, Fillman EP. Juvenile xanthogranuloma. StatPearls [Internet]. StatPearls Publishing; 2025. Updated August 8, 2023. Accessed November 4, 2025. https://www.ncbi.nlm.nih.gov/books/NBK526103/
- Newman B, Hu W, Nigro K, et al. Aggressive histiocytic disorders that can involve the skin. J Am Acad Dermatol. 2007;56:302-316.
- Freyer DR, Kennedy R, Bostrom BC, et al. Juvenile xanthogranuloma: forms of systemic disease and their clinical implications. J Pediatr. 1996;129:227-237.
- Murphy JT, Soeken T, Megison S, et al. Juvenile xanthogranuloma: diverse presentations of noncutaneous disease. J Pediatr Hematol Oncol.2014;36:641-645.
- Xu J, Ma L. Dermoscopic patterns in juvenile xanthogranuloma based on the histological classification. Front Med (Lausanne). 2021;7:618946.
- Kazakov DV. Brooke-Spiegler syndrome and phenotypic variants: an update. Head Neck Pathol. 2016;10:125-130.
- Bokhari SRA, Zulfiqar H, Hariz A. Fabry disease. StatPearls [Internet]. StatPearls Publishing; 2025. Update July 4, 2023. Accessed November 4, 2025. https://www.ncbi.nlm.nih.gov/books/NBK435996/
- Hartmann K, Escribano L, Grattan C, et al. Cutaneous manifestations in patients with mastocytosis: consensus report of the European Competence Network on Mastocytosis; the American Academy of Allergy, Asthma & Immunology; and the European Academy of Allergology and Clinical Immunology. J Allergy Clin Immunol. 2016;137:35-45.
- Klaiber N, Kumar S, Irani AM. Mastocytosis in children. Curr Allergy Asthma Rep. 2017;17:80.
- Sławin´ ska M, Kaszuba A, Lange M, et al. Dermoscopic features of different forms of cutaneous mastocytosis: a systematic review. J Clin Med. 2022;11:4649.
- Teng JM, Cowen EW, Wataya-Kaneda M, et al. Dermatologic and dental aspects of the 2012 International Tuberous Sclerosis Complex Consensus Statements. JAMA Dermatol. 2014;150:1095-1101.
- Durham BH, Lopez Rodrigo E, Picarsic J, et al. Activating mutations in CSF1R and additional receptor tyrosine kinases in histiocytic neoplasms. Nat Med. 2019;25:1839-1842.
- Friedman JM. Neurofibromatosis 1. In: Adam MP, Feldman J, Mirzaa GM, et al, eds. GeneReviews®. University of Washington, Seattle; 1998.
- Miraglia E, Laghi A, Moramarco A, et al. Juvenile xanthogranuloma in neurofibromatosis type 1. Prevalence and possible correlation with lymphoproliferative diseases: experience of a single center and review of the literature. Clin Ther. 2022;173:353-355.
- Collie JS, Harper CD, Fillman EP. Juvenile xanthogranuloma. StatPearls [Internet]. StatPearls Publishing; 2025. Updated August 8, 2023. Accessed November 4, 2025. https://www.ncbi.nlm.nih.gov/books/NBK526103/
- Newman B, Hu W, Nigro K, et al. Aggressive histiocytic disorders that can involve the skin. J Am Acad Dermatol. 2007;56:302-316.
- Freyer DR, Kennedy R, Bostrom BC, et al. Juvenile xanthogranuloma: forms of systemic disease and their clinical implications. J Pediatr. 1996;129:227-237.
- Murphy JT, Soeken T, Megison S, et al. Juvenile xanthogranuloma: diverse presentations of noncutaneous disease. J Pediatr Hematol Oncol.2014;36:641-645.
- Xu J, Ma L. Dermoscopic patterns in juvenile xanthogranuloma based on the histological classification. Front Med (Lausanne). 2021;7:618946.
- Kazakov DV. Brooke-Spiegler syndrome and phenotypic variants: an update. Head Neck Pathol. 2016;10:125-130.
- Bokhari SRA, Zulfiqar H, Hariz A. Fabry disease. StatPearls [Internet]. StatPearls Publishing; 2025. Update July 4, 2023. Accessed November 4, 2025. https://www.ncbi.nlm.nih.gov/books/NBK435996/
- Hartmann K, Escribano L, Grattan C, et al. Cutaneous manifestations in patients with mastocytosis: consensus report of the European Competence Network on Mastocytosis; the American Academy of Allergy, Asthma & Immunology; and the European Academy of Allergology and Clinical Immunology. J Allergy Clin Immunol. 2016;137:35-45.
- Klaiber N, Kumar S, Irani AM. Mastocytosis in children. Curr Allergy Asthma Rep. 2017;17:80.
- Sławin´ ska M, Kaszuba A, Lange M, et al. Dermoscopic features of different forms of cutaneous mastocytosis: a systematic review. J Clin Med. 2022;11:4649.
- Teng JM, Cowen EW, Wataya-Kaneda M, et al. Dermatologic and dental aspects of the 2012 International Tuberous Sclerosis Complex Consensus Statements. JAMA Dermatol. 2014;150:1095-1101.
A 6-month-old male infant with a history of cradle cap and an infantile hemangioma on the left shoulder presented to the dermatology clinic for evaluation of a slow-growing yellow papule on the upper back of 3 months’ duration. The lesion initially was noted 2 months prior to the current presentation by the patient’s pediatrician, who recommended follow-up with dermatology after an unsuccessful attempt at incision and drainage. Physical examination revealed a 7-mm, yellow, dome-shaped papule with a red collarette on the right upper back. No axillary freckling, ocular findings, or other skin findings were found. The patient was born at term with no complications, and his mother reported that he was otherwise healthy. There were no developmental concerns or known allergies, and his family history was negative for any similar lesions. Dermoscopic examination of the lesion revealed a well-circumscribed, circular, yellow-orange papule with an erythematous border and setting-sun appearance.
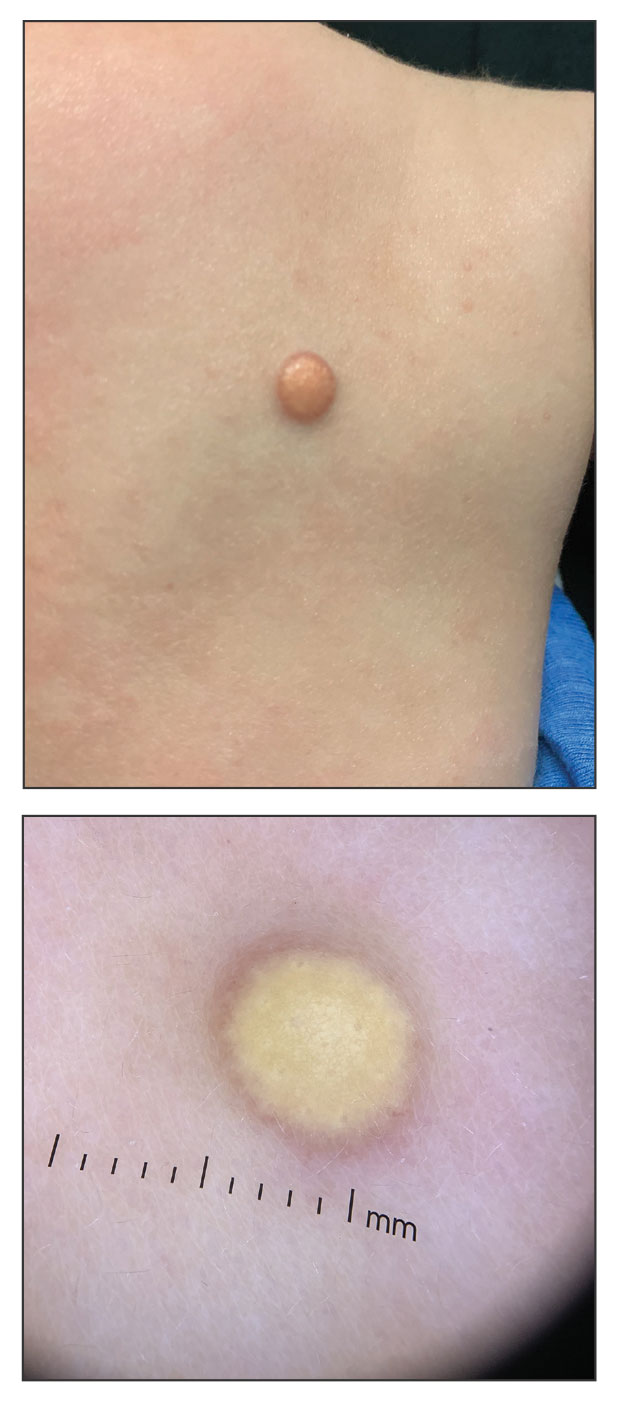
Reticulated Hyperpigmentation on the Knee and Thigh
Reticulated Hyperpigmentation on the Knee and Thigh
The patient was diagnosed with erythema ab igne based on characteristic skin findings on physical examination along with a convincing history of chronic localized heat exposure. Erythema ab igne manifests as a persistent reticulated, erythematous, or hyperpigmented rash at sites of chronic heat exposure.1 Commonplace items that emit heat such as electric heaters, car heaters, heating pads, hot water bottles, and, in our case, laptops also emit infrared radiation, which can lead to changes in the skin with long-term exposure.2 Because exposure to these sources often is limited to one area of the body, erythema ab igne usually manifests locally, as exemplified in this case. Chronic heat exposure and infrared radiation from these sources are thought to induce hyperthermia below the threshold for a thermal burn, and the cutaneous findings correspond with the dermal venous plexus.3
Diagnosis of erythema ab igne primarily is made clinically based on characteristic skin findings and exposure history. Relevant history may include occupations with prolonged heat exposure, such as baking, silversmithing, or foundry work. Heat exposure also may result from cultural practices such as cupping with moxibustion.4 Additionally, repeated use of heating pads or hot water bottles for pain relief by patients diagnosed with chronic pain or an underlying illness may contribute to development of erythema ab igne.1,4
Biopsy was not needed for diagnosis of this patient, but if the presentation is equivocal and history of potential exposures is unclear, a biopsy may be taken. A hematoxylin and eosin stain would reveal dilation of small vascular channels in the superficial dermis, contributing to the classic reticulated appearance. Biopsy findings also would reveal either an interface dermatitis or pigment incontinence containing melanin-laden macrophages correlating to either the erythema or hyperpigmentation, respectively.4
The prognosis for erythema ab igne is excellent, especially if diagnosed early. Treatment involves removal of the inciting heat source.1 The discoloration may resolve within a few months to years or may persist. If the hyperpigmentation is persistent, patients may consider laser treatments or lightening agents such as topical hydroquinone or topical tretinoin.4 However, if undiagnosed, patients may be at risk for development of a cutaneous malignancy, such as squamous cell carcinoma, Merkel cell carcinoma, poorly differentiated carcinoma, or cutaneous marginal zone lymphoma.2,4 Malignant transformation has been reported to occur decades after the initial skin eruption, although the risk is rare5; however, due to this risk, patients with erythema ab igne should be followed regularly and screened for new lesions in the affected areas.
- Tan S, Bertucci V. Erythema ab igne: an old condition new again. CMAJ. 2000;162:77-78.
- Miller K, Hunt R, Chu J, et al. Erythema ab igne. Dermatol Online J. 2011;17:28.
- Kesty K, Feldman SR. Erythema ab igne: evolving technology, evolving presentation. Dermatol Online J. 2014;20:13030.
- Harview CL, Krenitsky A. Erythema ab igne: a clinical review. Cutis. 2023;111:E33-E38. doi:10.12788/cutis.0771
- Wipf AJ, Brown MR. Malignant transformation of erythema ab igne. JAAD Case Rep. 2022;26:85-87. doi:10.1016/j.jdcr.2022.06.018
The patient was diagnosed with erythema ab igne based on characteristic skin findings on physical examination along with a convincing history of chronic localized heat exposure. Erythema ab igne manifests as a persistent reticulated, erythematous, or hyperpigmented rash at sites of chronic heat exposure.1 Commonplace items that emit heat such as electric heaters, car heaters, heating pads, hot water bottles, and, in our case, laptops also emit infrared radiation, which can lead to changes in the skin with long-term exposure.2 Because exposure to these sources often is limited to one area of the body, erythema ab igne usually manifests locally, as exemplified in this case. Chronic heat exposure and infrared radiation from these sources are thought to induce hyperthermia below the threshold for a thermal burn, and the cutaneous findings correspond with the dermal venous plexus.3
Diagnosis of erythema ab igne primarily is made clinically based on characteristic skin findings and exposure history. Relevant history may include occupations with prolonged heat exposure, such as baking, silversmithing, or foundry work. Heat exposure also may result from cultural practices such as cupping with moxibustion.4 Additionally, repeated use of heating pads or hot water bottles for pain relief by patients diagnosed with chronic pain or an underlying illness may contribute to development of erythema ab igne.1,4
Biopsy was not needed for diagnosis of this patient, but if the presentation is equivocal and history of potential exposures is unclear, a biopsy may be taken. A hematoxylin and eosin stain would reveal dilation of small vascular channels in the superficial dermis, contributing to the classic reticulated appearance. Biopsy findings also would reveal either an interface dermatitis or pigment incontinence containing melanin-laden macrophages correlating to either the erythema or hyperpigmentation, respectively.4
The prognosis for erythema ab igne is excellent, especially if diagnosed early. Treatment involves removal of the inciting heat source.1 The discoloration may resolve within a few months to years or may persist. If the hyperpigmentation is persistent, patients may consider laser treatments or lightening agents such as topical hydroquinone or topical tretinoin.4 However, if undiagnosed, patients may be at risk for development of a cutaneous malignancy, such as squamous cell carcinoma, Merkel cell carcinoma, poorly differentiated carcinoma, or cutaneous marginal zone lymphoma.2,4 Malignant transformation has been reported to occur decades after the initial skin eruption, although the risk is rare5; however, due to this risk, patients with erythema ab igne should be followed regularly and screened for new lesions in the affected areas.
The patient was diagnosed with erythema ab igne based on characteristic skin findings on physical examination along with a convincing history of chronic localized heat exposure. Erythema ab igne manifests as a persistent reticulated, erythematous, or hyperpigmented rash at sites of chronic heat exposure.1 Commonplace items that emit heat such as electric heaters, car heaters, heating pads, hot water bottles, and, in our case, laptops also emit infrared radiation, which can lead to changes in the skin with long-term exposure.2 Because exposure to these sources often is limited to one area of the body, erythema ab igne usually manifests locally, as exemplified in this case. Chronic heat exposure and infrared radiation from these sources are thought to induce hyperthermia below the threshold for a thermal burn, and the cutaneous findings correspond with the dermal venous plexus.3
Diagnosis of erythema ab igne primarily is made clinically based on characteristic skin findings and exposure history. Relevant history may include occupations with prolonged heat exposure, such as baking, silversmithing, or foundry work. Heat exposure also may result from cultural practices such as cupping with moxibustion.4 Additionally, repeated use of heating pads or hot water bottles for pain relief by patients diagnosed with chronic pain or an underlying illness may contribute to development of erythema ab igne.1,4
Biopsy was not needed for diagnosis of this patient, but if the presentation is equivocal and history of potential exposures is unclear, a biopsy may be taken. A hematoxylin and eosin stain would reveal dilation of small vascular channels in the superficial dermis, contributing to the classic reticulated appearance. Biopsy findings also would reveal either an interface dermatitis or pigment incontinence containing melanin-laden macrophages correlating to either the erythema or hyperpigmentation, respectively.4
The prognosis for erythema ab igne is excellent, especially if diagnosed early. Treatment involves removal of the inciting heat source.1 The discoloration may resolve within a few months to years or may persist. If the hyperpigmentation is persistent, patients may consider laser treatments or lightening agents such as topical hydroquinone or topical tretinoin.4 However, if undiagnosed, patients may be at risk for development of a cutaneous malignancy, such as squamous cell carcinoma, Merkel cell carcinoma, poorly differentiated carcinoma, or cutaneous marginal zone lymphoma.2,4 Malignant transformation has been reported to occur decades after the initial skin eruption, although the risk is rare5; however, due to this risk, patients with erythema ab igne should be followed regularly and screened for new lesions in the affected areas.
- Tan S, Bertucci V. Erythema ab igne: an old condition new again. CMAJ. 2000;162:77-78.
- Miller K, Hunt R, Chu J, et al. Erythema ab igne. Dermatol Online J. 2011;17:28.
- Kesty K, Feldman SR. Erythema ab igne: evolving technology, evolving presentation. Dermatol Online J. 2014;20:13030.
- Harview CL, Krenitsky A. Erythema ab igne: a clinical review. Cutis. 2023;111:E33-E38. doi:10.12788/cutis.0771
- Wipf AJ, Brown MR. Malignant transformation of erythema ab igne. JAAD Case Rep. 2022;26:85-87. doi:10.1016/j.jdcr.2022.06.018
- Tan S, Bertucci V. Erythema ab igne: an old condition new again. CMAJ. 2000;162:77-78.
- Miller K, Hunt R, Chu J, et al. Erythema ab igne. Dermatol Online J. 2011;17:28.
- Kesty K, Feldman SR. Erythema ab igne: evolving technology, evolving presentation. Dermatol Online J. 2014;20:13030.
- Harview CL, Krenitsky A. Erythema ab igne: a clinical review. Cutis. 2023;111:E33-E38. doi:10.12788/cutis.0771
- Wipf AJ, Brown MR. Malignant transformation of erythema ab igne. JAAD Case Rep. 2022;26:85-87. doi:10.1016/j.jdcr.2022.06.018
Reticulated Hyperpigmentation on the Knee and Thigh
Reticulated Hyperpigmentation on the Knee and Thigh
A 25-year-old woman with an unremarkable medical history presented to the dermatology clinic for evaluation of a persistent rash on the right knee and distal thigh of several months’ duration. The patient noted that the rash had been asymptomatic, and she denied any history of trauma to the area. She reported that she worked as a teacher and had repeatedly stayed up late using her laptop for months. Rather than use a desk, she often would work sitting with her laptop in her lap.
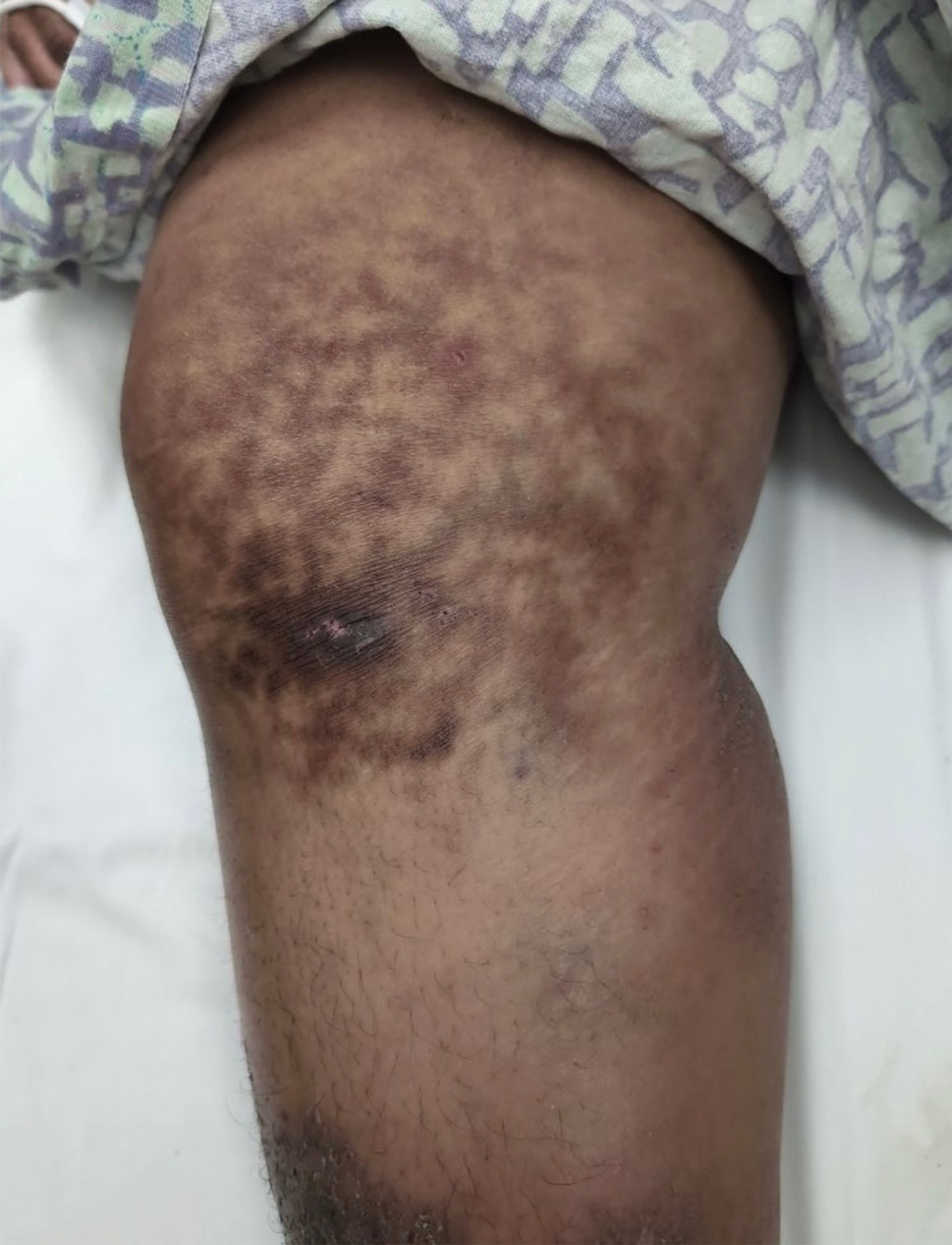
Flesh-Colored Lesion on the Ear
Flesh-Colored Lesion on the Ear
THE DIAGNOSIS: Gouty Tophus
The lesion was excised and sent for histopathologic examination (eFigures 1 and 2), revealing aggregates of feathery, amorphous, pale-pink material, which confirmed the diagnosis of gouty tophus. The surgical site was left to heal by secondary intention. Upon further evaluation, the patient reported recurrent monoarticular joint pain in the ankles and feet, and laboratory workup revealed elevated serum uric acid. He was advised to follow up with his primary care physician to discuss systemic treatment options for gout.
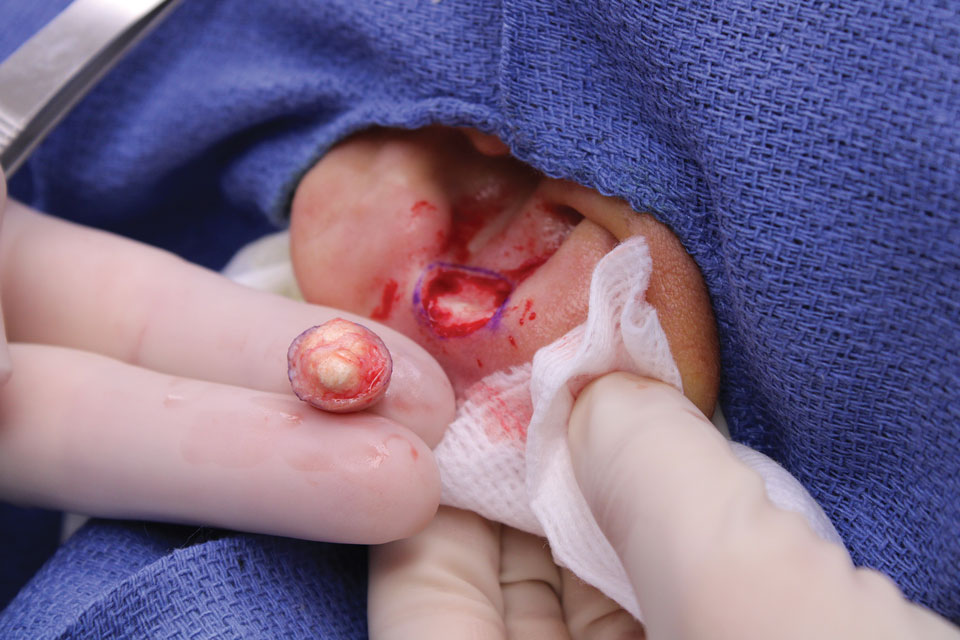
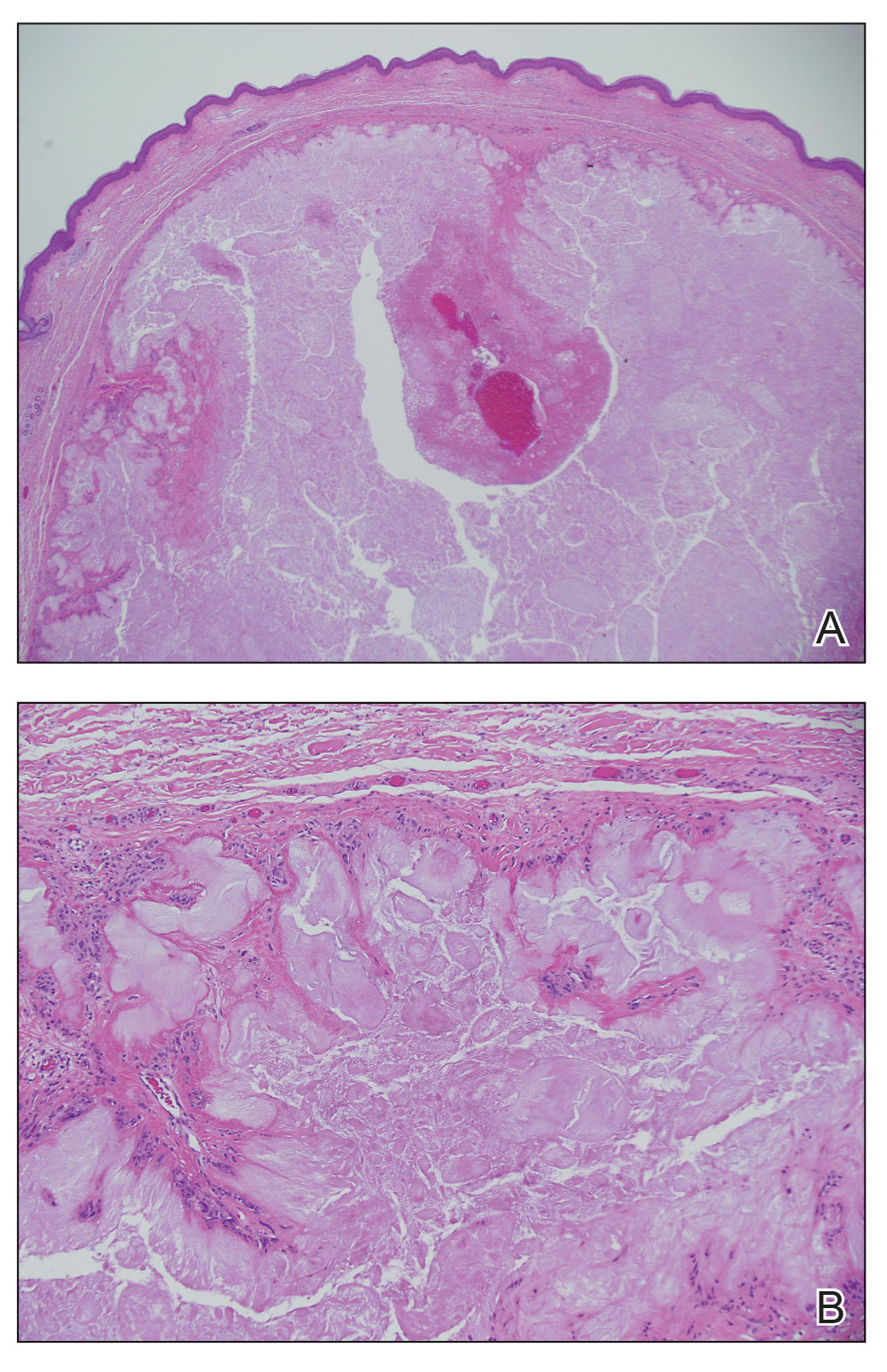
Gout is an inflammatory arthritis characterized by the deposition of monosodium urate monohydrate crystals in the joints, soft tissue, and bone due to elevated serum uric acid. Uric acid is the final product of purine metabolism, and serum levels may be elevated due to excess production or underexcretion. Multiple genetic, environmental, and metabolic factors influence these processes.1 Collections of monosodium urate crystals may develop intra- or extra-articularly, the latter of which are known as gouty tophi. These nodules have a classic chalklike consistency and typically are seen in patients with untreated gout starting approximately 10 years after the first flare. The most common locations for subcutaneous gouty tophi are acral sites (eg, fingertips, ears) as well as the wrists, knees, and elbows (olecranon bursae). Rarely, gouty panniculitis also may develop.2
Histopathology of gouty tophi reveals nodular aggregates of acellular, amorphous, pale-pink material surrounded by palisading histiocytes and multinucleated giant cells. The presence of needlelike monosodium urate crystals, which display negative birefringence, is diagnostic. Unfortunately, these crystals are destroyed in routine formalin processing.3
There are limited data regarding treatment of gouty tophi. Urate-lowering systemic medications such as pegloticase may be beneficial, but more data are needed.4 We pursued surgical excision in our case for definitive diagnosis; however, it is not a common treatment for gouty tophi. Typically, urate-lowering therapy is utilized to resolve or shrink lesions over time.5
The differential diagnosis for gouty tophi includes epidermal inclusion cyst (EIC), the most common type of cutaneous cyst. Though EICs can manifest anywhere on the body, they are not as common on the ears as gouty tophi. Epidermal inclusion cysts clinically manifest as soft subcutaneous nodules, and a central punctum often is noted. These lesions are derived from the follicular infundibulum and histologically are characterized by a cystic cavity lined by a stratified squamous epithelium with a granular layer. The cavity contains loose laminated keratin material.6
Pseudocyst of the auricle is a benign cystic swelling of the pinna that can develop spontaneously but most often manifests following trauma to the area, which is believed to separate the tissue planes in the cartilage, allowing fluid to accumulate. This lesion typically is asymptomatic, though some patients report mild tenderness.7 Histology shows a cystic structure within the cartilage without an epithelial lining, and a perivascular inflammatory response often is observed.8
Pilomatricoma, also known as pilomatrixoma, is a benign tumor derived from the hair follicle matrix that manifests as a firm, slow-growing, painless subcutaneous nodule. It most often is found on the head and neck, commonly in the periauricular area.9 Though rare, it has been found on the auricle and external auditory canal.10 Histologically, pilomatricomas are well-defined tumors containing internal trabeculae. They contain populations of basaloid and ghost cells and often calcify, sometimes with resultant bone formation.9
Dermoid cysts are benign tumors that develop along lines of embryonic closure and often are diagnosed at birth or in early childhood. They most commonly manifest on the head and neck, typically in the supraorbital area. Rarely, they have been reported on the ear.6 Dermoid cysts may resemble EICs clinically and histopathologically, except that the cyst wall contains mature adnexal structures such as hair follicles and sebaceous glands.
- Dalbeth N, Merriman TR, Stamp LK. Gout. Lancet. 2016;388:2039-2052. doi:10.1016/S0140-6736(16)00346-9
- Gaviria JL, Ortega VG, Gaona J, et al. Unusual dermatological manifestations of gout: review of literature and a case report. Plast Reconstr Surg Glob Open. 2015;3:E445. doi:10.1097/GOX.0000000000000420
- Towiwat P, Chhana A, Dalbeth N. The anatomical pathology of gout: a systematic literature review. BMC Musculoskelet Disord. 2019;20:140. doi:10.1186/s12891-019-2519-y
- Sriranganathan MK, Vinik O, Pardo Pardo J, et al. Interventions for tophi in gout. Cochrane Database Syst Rev. 2021;8:CD010069. doi:10.1002/14651858.CD010069.pub3
- Evidence review for surgical excision of tophi. Gout: diagnosis and management. National Institute for Health and Care Excellence (NICE). June 2022. Accessed October 8, 2025. https://www.ncbi.nlm.nih.gov/books/NBK583526/
- Cho Y, Lee DH. Clinical characteristics of idiopathic epidermoid and dermoid cysts of the ear. J Audiol Otol. 2017;21:77-80. doi:10.7874 /jao.2017.21.2.77
- Ballan A, Zogheib S, Hanna C, et al. Auricular pseudocysts: a systematic review of the literature. Int J Dermatol. 2022;61:109-117. doi:10.1111/ijd.15816
- Lim CM, Goh YH, Chao SS, et al. Pseudocyst of the auricle: a histologic perspective. Laryngoscope. 2004;114:1281-1284. doi:10.1097/00005537-200407000-00026
- Jones CD, Ho W, Robertson BF, et al. Pilomatrixoma: a comprehensive review of the literature. Am J Dermatopathol. 2018; 40:631-641. doi:10.1097/DAD.0000000000001118
- McInerney NJ, Nae A, Brennan S, et al. Pilomatricoma of the external auditory canal. Royal College of Surgeons in Ireland. 2023. doi:10.1016/j.xocr.2023.10053
THE DIAGNOSIS: Gouty Tophus
The lesion was excised and sent for histopathologic examination (eFigures 1 and 2), revealing aggregates of feathery, amorphous, pale-pink material, which confirmed the diagnosis of gouty tophus. The surgical site was left to heal by secondary intention. Upon further evaluation, the patient reported recurrent monoarticular joint pain in the ankles and feet, and laboratory workup revealed elevated serum uric acid. He was advised to follow up with his primary care physician to discuss systemic treatment options for gout.


Gout is an inflammatory arthritis characterized by the deposition of monosodium urate monohydrate crystals in the joints, soft tissue, and bone due to elevated serum uric acid. Uric acid is the final product of purine metabolism, and serum levels may be elevated due to excess production or underexcretion. Multiple genetic, environmental, and metabolic factors influence these processes.1 Collections of monosodium urate crystals may develop intra- or extra-articularly, the latter of which are known as gouty tophi. These nodules have a classic chalklike consistency and typically are seen in patients with untreated gout starting approximately 10 years after the first flare. The most common locations for subcutaneous gouty tophi are acral sites (eg, fingertips, ears) as well as the wrists, knees, and elbows (olecranon bursae). Rarely, gouty panniculitis also may develop.2
Histopathology of gouty tophi reveals nodular aggregates of acellular, amorphous, pale-pink material surrounded by palisading histiocytes and multinucleated giant cells. The presence of needlelike monosodium urate crystals, which display negative birefringence, is diagnostic. Unfortunately, these crystals are destroyed in routine formalin processing.3
There are limited data regarding treatment of gouty tophi. Urate-lowering systemic medications such as pegloticase may be beneficial, but more data are needed.4 We pursued surgical excision in our case for definitive diagnosis; however, it is not a common treatment for gouty tophi. Typically, urate-lowering therapy is utilized to resolve or shrink lesions over time.5
The differential diagnosis for gouty tophi includes epidermal inclusion cyst (EIC), the most common type of cutaneous cyst. Though EICs can manifest anywhere on the body, they are not as common on the ears as gouty tophi. Epidermal inclusion cysts clinically manifest as soft subcutaneous nodules, and a central punctum often is noted. These lesions are derived from the follicular infundibulum and histologically are characterized by a cystic cavity lined by a stratified squamous epithelium with a granular layer. The cavity contains loose laminated keratin material.6
Pseudocyst of the auricle is a benign cystic swelling of the pinna that can develop spontaneously but most often manifests following trauma to the area, which is believed to separate the tissue planes in the cartilage, allowing fluid to accumulate. This lesion typically is asymptomatic, though some patients report mild tenderness.7 Histology shows a cystic structure within the cartilage without an epithelial lining, and a perivascular inflammatory response often is observed.8
Pilomatricoma, also known as pilomatrixoma, is a benign tumor derived from the hair follicle matrix that manifests as a firm, slow-growing, painless subcutaneous nodule. It most often is found on the head and neck, commonly in the periauricular area.9 Though rare, it has been found on the auricle and external auditory canal.10 Histologically, pilomatricomas are well-defined tumors containing internal trabeculae. They contain populations of basaloid and ghost cells and often calcify, sometimes with resultant bone formation.9
Dermoid cysts are benign tumors that develop along lines of embryonic closure and often are diagnosed at birth or in early childhood. They most commonly manifest on the head and neck, typically in the supraorbital area. Rarely, they have been reported on the ear.6 Dermoid cysts may resemble EICs clinically and histopathologically, except that the cyst wall contains mature adnexal structures such as hair follicles and sebaceous glands.
THE DIAGNOSIS: Gouty Tophus
The lesion was excised and sent for histopathologic examination (eFigures 1 and 2), revealing aggregates of feathery, amorphous, pale-pink material, which confirmed the diagnosis of gouty tophus. The surgical site was left to heal by secondary intention. Upon further evaluation, the patient reported recurrent monoarticular joint pain in the ankles and feet, and laboratory workup revealed elevated serum uric acid. He was advised to follow up with his primary care physician to discuss systemic treatment options for gout.


Gout is an inflammatory arthritis characterized by the deposition of monosodium urate monohydrate crystals in the joints, soft tissue, and bone due to elevated serum uric acid. Uric acid is the final product of purine metabolism, and serum levels may be elevated due to excess production or underexcretion. Multiple genetic, environmental, and metabolic factors influence these processes.1 Collections of monosodium urate crystals may develop intra- or extra-articularly, the latter of which are known as gouty tophi. These nodules have a classic chalklike consistency and typically are seen in patients with untreated gout starting approximately 10 years after the first flare. The most common locations for subcutaneous gouty tophi are acral sites (eg, fingertips, ears) as well as the wrists, knees, and elbows (olecranon bursae). Rarely, gouty panniculitis also may develop.2
Histopathology of gouty tophi reveals nodular aggregates of acellular, amorphous, pale-pink material surrounded by palisading histiocytes and multinucleated giant cells. The presence of needlelike monosodium urate crystals, which display negative birefringence, is diagnostic. Unfortunately, these crystals are destroyed in routine formalin processing.3
There are limited data regarding treatment of gouty tophi. Urate-lowering systemic medications such as pegloticase may be beneficial, but more data are needed.4 We pursued surgical excision in our case for definitive diagnosis; however, it is not a common treatment for gouty tophi. Typically, urate-lowering therapy is utilized to resolve or shrink lesions over time.5
The differential diagnosis for gouty tophi includes epidermal inclusion cyst (EIC), the most common type of cutaneous cyst. Though EICs can manifest anywhere on the body, they are not as common on the ears as gouty tophi. Epidermal inclusion cysts clinically manifest as soft subcutaneous nodules, and a central punctum often is noted. These lesions are derived from the follicular infundibulum and histologically are characterized by a cystic cavity lined by a stratified squamous epithelium with a granular layer. The cavity contains loose laminated keratin material.6
Pseudocyst of the auricle is a benign cystic swelling of the pinna that can develop spontaneously but most often manifests following trauma to the area, which is believed to separate the tissue planes in the cartilage, allowing fluid to accumulate. This lesion typically is asymptomatic, though some patients report mild tenderness.7 Histology shows a cystic structure within the cartilage without an epithelial lining, and a perivascular inflammatory response often is observed.8
Pilomatricoma, also known as pilomatrixoma, is a benign tumor derived from the hair follicle matrix that manifests as a firm, slow-growing, painless subcutaneous nodule. It most often is found on the head and neck, commonly in the periauricular area.9 Though rare, it has been found on the auricle and external auditory canal.10 Histologically, pilomatricomas are well-defined tumors containing internal trabeculae. They contain populations of basaloid and ghost cells and often calcify, sometimes with resultant bone formation.9
Dermoid cysts are benign tumors that develop along lines of embryonic closure and often are diagnosed at birth or in early childhood. They most commonly manifest on the head and neck, typically in the supraorbital area. Rarely, they have been reported on the ear.6 Dermoid cysts may resemble EICs clinically and histopathologically, except that the cyst wall contains mature adnexal structures such as hair follicles and sebaceous glands.
- Dalbeth N, Merriman TR, Stamp LK. Gout. Lancet. 2016;388:2039-2052. doi:10.1016/S0140-6736(16)00346-9
- Gaviria JL, Ortega VG, Gaona J, et al. Unusual dermatological manifestations of gout: review of literature and a case report. Plast Reconstr Surg Glob Open. 2015;3:E445. doi:10.1097/GOX.0000000000000420
- Towiwat P, Chhana A, Dalbeth N. The anatomical pathology of gout: a systematic literature review. BMC Musculoskelet Disord. 2019;20:140. doi:10.1186/s12891-019-2519-y
- Sriranganathan MK, Vinik O, Pardo Pardo J, et al. Interventions for tophi in gout. Cochrane Database Syst Rev. 2021;8:CD010069. doi:10.1002/14651858.CD010069.pub3
- Evidence review for surgical excision of tophi. Gout: diagnosis and management. National Institute for Health and Care Excellence (NICE). June 2022. Accessed October 8, 2025. https://www.ncbi.nlm.nih.gov/books/NBK583526/
- Cho Y, Lee DH. Clinical characteristics of idiopathic epidermoid and dermoid cysts of the ear. J Audiol Otol. 2017;21:77-80. doi:10.7874 /jao.2017.21.2.77
- Ballan A, Zogheib S, Hanna C, et al. Auricular pseudocysts: a systematic review of the literature. Int J Dermatol. 2022;61:109-117. doi:10.1111/ijd.15816
- Lim CM, Goh YH, Chao SS, et al. Pseudocyst of the auricle: a histologic perspective. Laryngoscope. 2004;114:1281-1284. doi:10.1097/00005537-200407000-00026
- Jones CD, Ho W, Robertson BF, et al. Pilomatrixoma: a comprehensive review of the literature. Am J Dermatopathol. 2018; 40:631-641. doi:10.1097/DAD.0000000000001118
- McInerney NJ, Nae A, Brennan S, et al. Pilomatricoma of the external auditory canal. Royal College of Surgeons in Ireland. 2023. doi:10.1016/j.xocr.2023.10053
- Dalbeth N, Merriman TR, Stamp LK. Gout. Lancet. 2016;388:2039-2052. doi:10.1016/S0140-6736(16)00346-9
- Gaviria JL, Ortega VG, Gaona J, et al. Unusual dermatological manifestations of gout: review of literature and a case report. Plast Reconstr Surg Glob Open. 2015;3:E445. doi:10.1097/GOX.0000000000000420
- Towiwat P, Chhana A, Dalbeth N. The anatomical pathology of gout: a systematic literature review. BMC Musculoskelet Disord. 2019;20:140. doi:10.1186/s12891-019-2519-y
- Sriranganathan MK, Vinik O, Pardo Pardo J, et al. Interventions for tophi in gout. Cochrane Database Syst Rev. 2021;8:CD010069. doi:10.1002/14651858.CD010069.pub3
- Evidence review for surgical excision of tophi. Gout: diagnosis and management. National Institute for Health and Care Excellence (NICE). June 2022. Accessed October 8, 2025. https://www.ncbi.nlm.nih.gov/books/NBK583526/
- Cho Y, Lee DH. Clinical characteristics of idiopathic epidermoid and dermoid cysts of the ear. J Audiol Otol. 2017;21:77-80. doi:10.7874 /jao.2017.21.2.77
- Ballan A, Zogheib S, Hanna C, et al. Auricular pseudocysts: a systematic review of the literature. Int J Dermatol. 2022;61:109-117. doi:10.1111/ijd.15816
- Lim CM, Goh YH, Chao SS, et al. Pseudocyst of the auricle: a histologic perspective. Laryngoscope. 2004;114:1281-1284. doi:10.1097/00005537-200407000-00026
- Jones CD, Ho W, Robertson BF, et al. Pilomatrixoma: a comprehensive review of the literature. Am J Dermatopathol. 2018; 40:631-641. doi:10.1097/DAD.0000000000001118
- McInerney NJ, Nae A, Brennan S, et al. Pilomatricoma of the external auditory canal. Royal College of Surgeons in Ireland. 2023. doi:10.1016/j.xocr.2023.10053
Flesh-Colored Lesion on the Ear
Flesh-Colored Lesion on the Ear
A 46-year-old man with a history of hypertension, hyperlipidemia, and type 2 diabetes presented to the dermatology clinic with a painless nodule on the left ear of 2 years’ duration. The patient denied any bleeding, drainage, or prior trauma to the area. He noted that the lesion had grown slowly over time. Physical examination revealed a 1.5×1.5-cm, flesh-colored, subcutaneous nodule with overlying telangiectasias on the left antihelix.
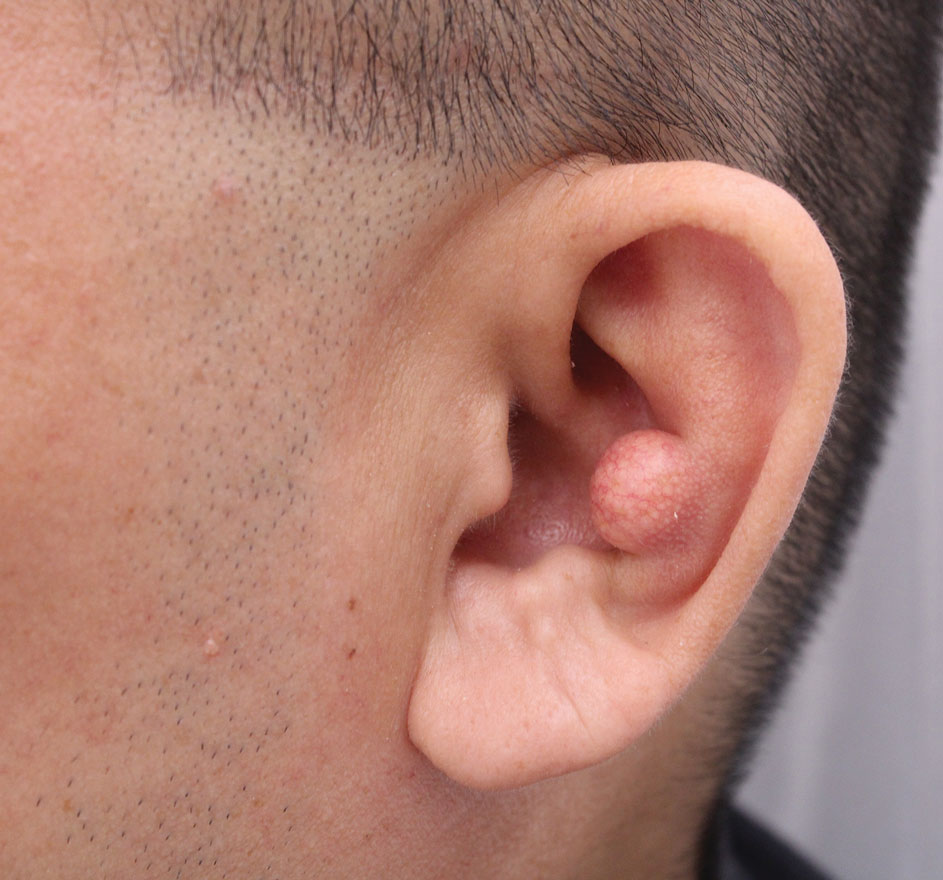
Longitudinal Erythronychia Manifesting With Pain and Cold Sensitivity
The Diagnosis: Glomangiomyoma
The nail unit excision specimen showed collections of cuboidal cells and spindled cells within the corium that were consistent with a diagnosis of a glomangiomyoma, a rare glomus tumor variant (Figure). Glomus tumors are benign neoplasms comprising glomus bodies, which are arteriovenous anastomoses involved in thermoregulation.1 They develop in areas densely populated by glomus bodies, including the fingers, toes, and subungual areas. Glomus tumors most commonly develop in middle-aged women.2 Clinically, they manifest with a characteristic triad of intense pain, point tenderness, and cold sensitivity and may appear as reddish-pink or blue macules under the nail plate and/or longitudinal erythronychia.2-6 The presence of multiple glomus tumors is associated with neurofibromatosis type 1.7
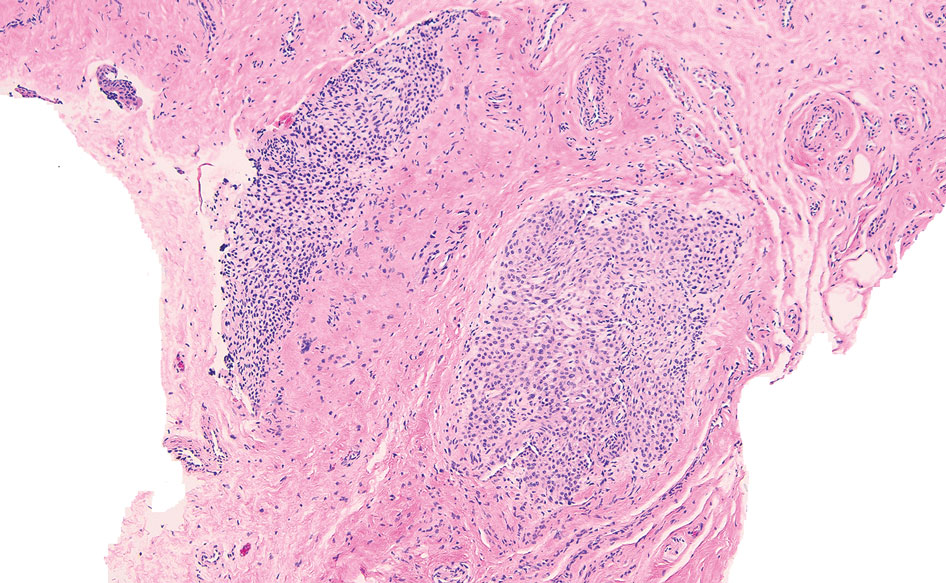
Advanced imaging including ultrasonography and magnetic resonance imaging (MRI) may help confirm the diagnosis but may not be cost effective, as excision with histopathology is needed to relieve symptoms and render a definitive diagnosis. Radiography is highly insensitive in identifying bone erosions associated with glomus tumors.8 With ultrasonography, glomus tumors appear hypoechoic; with Doppler ultrasonography, they appear hypervascular. With MRI, glomus tumors appear as well-defined nodular lesions with hypointense signal intensity on T1-weighted sequence and hyperintense signal intensity on T2-weighted sequence, with strong enhancement using gadolinium-based contrast.9,10 On histopathology, a glomus tumor appears as a nodular tumor with sheets of oval-nucleated cells arranged in multicellular layers surrounding blood vessels and are immunoreactive for α-smooth muscle actin, muscle-specific actin, and type IV collagen.11,12
There are several glomus tumor variants. The most common is a solid glomus tumor, which predominantly is composed of glomus cells, followed by glomangioma, which mainly is composed of blood vessels. Glomangiomyoma, which mostly is composed of smooth muscle cells, is the rarest variant.13
While glomus tumors are common in the subungual areas, it is an uncommon location for glomangiomyomas, which have been reported in the nail unit in only 7 prior case reports identified through searches of PubMed and Google Scholar using the terms glomangiomyoma, glomangiomyoma nail, and subungual glomangiomyoma (Table).13-19 Glomangiomyomas more commonly are described in solid organs, including the stomach, kidney, pancreas, and bladder.16 The mean age of patients with subungual glomangiomyomas, including our patient, was 40.4 years (range, 3-61 years), with the majority being female (75.0% [6/8]). Most patients presented with fingernail involvement (75.0% [6/8]), nail dystrophy (eg, nail plate thinning, longitudinal grooves, splinter hemorrhages, longitudinal erythronychia)(62.5% [5/8]), and intermittent pain and/or point tenderness in the affected nail (75.0% [6/8]).13-19 Notably, only our patient had longitudinal erythronychia as a clinical feature, and only one other case described MRI findings, which included a lobulated mass with intense contrast and distal phalanx destruction.18 One patient was a 3-year-old girl with a family history of generalized multiple glomangiomyomas. Although subungual glomangiomyoma was not confirmed on histopathology, the diagnosis in this patient was presumed based on her family history.13 On histopathology, glomangiomyomas are composed of oval-nucleated cells surrounding blood vessels. These oval-nucleated cells then gradually transition to smooth muscle cells.20
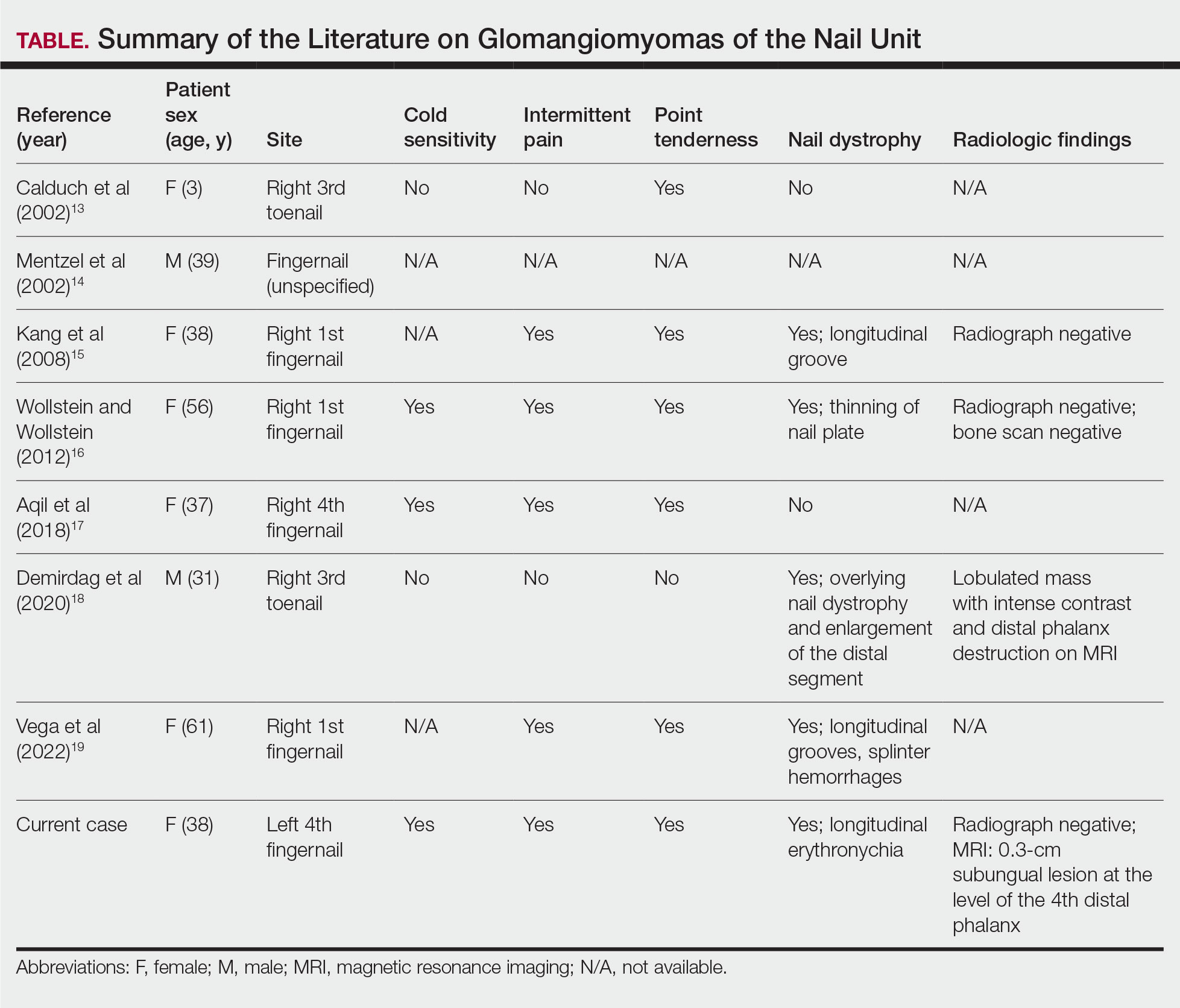
A myxoid cyst is composed of a pseudocyst, which lacks a cyst lining, and is a result of synovial fluid from the distal interphalangeal joint entering the pseudocyst space.2 It typically manifests with a longitudinal groove in the nail plate. A flesh-colored nodule may be appreciated between the cuticle and the distal interphalangeal joint.2 The depth of the longitudinal groove may vary depending on the volume of synovial fluid within the myxoid cyst.21 In a series of 35 cases of subungual myxoid cysts, none manifested with longitudinal erythronychia. Due to their composition, myxoid cysts can be distinguished easily from solid tumors of the nail unit via transillumination.22 Pain is a much less common with myxoid cysts vs glomus tumors, as the filling of the pseudocyst space with synovial fluid typically is gradual, allowing the surrounding tissue to accommodate and adapt over time.21 In equivocal cases, MRI or high-resolution ultrasonography may be used to distinguish myxoid cysts and glomus tumors.8 Histopathology shows accumulation of mucin in the dermis with surrounding fibrous stroma.23
Subungual neuromas are painful benign tumors that develop due to disorganized neural proliferation following disruption to peripheral nerves secondary to trauma or surgery. In 3 case reports, subungual neuromas manifested as painful subungual nodules, with proximal nail plate ridging, or onycholysis.24-26 Since neuromas have only rarely been described in the subungual region, reports of MRI and ultrasonography findings are unknown. Histopathology is needed to distinguish neuromas from glomus tumors. Histopathology shows an acapsular structure consisting of disorganized spindle-cell proliferation and nerve fibers arranged in a tangle of fascicles within fibrotic tissue.25 On immunochemistry, spindle cells typically are positive for cellular antigen protein S100.26
Leiomyomas are benign neoplasms derived from smooth muscle, typically localized to the uterus or gastrointestinal tract, and have been described rarely in the nail unit.27,28 It is hypothesized that subungual leiomyomas originate from the vascular smooth muscle in the subcutaneous layer of the nail unit.28 Like glomus tumors, leiomyomas of the subungual region often manifest with pain and longitudinal erythronychia.27-30 Subungual leiomyomas may be distinguished from glomus tumors via advanced imaging techniques, including ultrasonography and MRI. Cutaneous leiomyomas have been described with mild to moderate internal low flow vascularity on Doppler ultrasonography, while glomus tumors typically reveal high internal vascularity.28 Biopsy with histopathology is needed for definitive diagnosis. On histopathology, leiomyomas demonstrate bland-appearing spindle-shaped cells with elongated nuclei arranged in fascicles.27 They typically are positive for α-smooth muscle actin and caldesmon on immunostaining.
Eccrine spiradenomas are benign adnexal tumors likely of apocrine origin with limited case reports in the literature.31,32 Clinically, eccrine spiradenomas involving the nail unit may manifest with longitudinal nail splitting of the nail or as a papule on the proximal nail fold, with associated tenderness.31,32 In a report of a 50-year-old woman with a histopathologically confirmed eccrine spiradenoma manifesting with longitudinal splitting of the nail and pain in the proximal nail fold, the mass appeared hypoechoic on ultrasonography with increased intramass vascularity on Doppler, while MRI showed an intensely enhancing lesion.31 These imaging features, combined with a classically manifesting feature of pain, make eccrine spiradenomas difficult to distinguish from glomus tumors; therefore, histopathologic examination can provide a definitive diagnosis, and surgical excision is used for treatment.31 On histopathology, these tumors are well circumscribed and composed of both small dark basaloid cells with peripheral compact nuclei and larger cells with central pale nuclei, which may be arranged in tubules.31,32
- Gombos Z, Zhang PJ. Glomus tumor. Arch Pathol Lab Med. 2008;132: 1448-1452. doi:10.5858/2008-132-1448-gt
- Hare AQ, Rich P. Nail tumors. Dermatol Clin. 2021;39:281-292. doi:10.1016/j.det.2020.12.007
- Hazani R, Houle JM, Kasdan ML, et al. Glomus tumors of the hand. Eplasty. 2008;8:E48.
- Hwang JK, Lipner SR. Blue nail discoloration: literature review and diagnostic algorithms. Am J Clin Dermatol. 2023;24:419-441. doi:10.1007/s40257-023-00768-6
- Lipner SR, Scher RK. Longitudinal erythronychia of the fingernail. JAMA Dermatol. 2016;152:1271-1272. doi:10.1001/jamadermatol.2016.2747
- Jellinek NJ, Lipner SR. Longitudinal erythronychia: retrospective single-center study evaluating differential diagnosis and the likelihood of malignancy. Dermatol Surg. 2016;42:310-319. doi:10.1097 /DSS.0000000000000594
- Lipner SR, Scher RK. Subungual glomus tumors: underrecognized clinical findings in neurofibromatosis 1. J Am Acad Dermatol. 2021;84:E269. doi:10.1016/j.jaad.2020.08.129
- Dhami A, Vale SM, Richardson ML, et al. Comparing ultrasound with magnetic resonance imaging in the evaluation of subungual glomus tumors and subungual myxoid cysts. Skin Appendage Disord. 2023;9:262-267. doi:10.1159/000530397
- Baek HJ, Lee SJ, Cho KH, et al. Subungual tumors: clinicopathologic correlation with US and MR imaging findings. Radiographics. 2010;30:1621-1636. doi:10.1148/rg.306105514
- Patel T, Meena V, Meena P. Hand and foot glomus tumors: significance of MRI diagnosis followed by histopathological assessment. Cureus. 2022;14:E30038. doi:10.7759/cureus.30038
- Mravic M, LaChaud G, Nguyen A, et al. Clinical and histopathological diagnosis of glomus tumor: an institutional experience of 138 cases. Int J Surg Pathol. 2015;23:181-188. doi:10.1177/1066896914567330
- Folpe AL, Fanburg-Smith JC, Miettinen M, et al. Atypical and malignant glomus tumors: analysis of 52 cases, with a proposal for the reclassification of glomus tumors. Am J Surg Pathol. 2001;25:1-12. doi:10.1097/00000478-200101000-00001
- Calduch L, Monteagudo C, Martínez-Ruiz E, et al. Familial generalized multiple glomangiomyoma: report of a new family, with immunohistochemical and ultrastructural studies and review of the literature. Pediatr Dermatol. 2002;19:402-408. doi:10.1046/j.1525-1470.2002.00114.x
- Mentzel T, Hügel H, Kutzner H. CD34-positive glomus tumor: clinicopathologic and immunohistochemical analysis of six cases with myxoid stromal changes. J Cutan Pathol. 2002;29:421-425. doi:10.1034 /j.1600-0560.2002.290706.x
- Kang TW, Lee KH, Park CJ. A case of subungual glomangiomyoma with myxoid stromal change. Korean J Dermatol. 2008;46:550-553.
- Wollstein A, Wollstein R. Subungual glomangiomyoma—a case report. Hand Surg. 2012;17:271-273. doi:10.1142/S021881041272032X
- Aqil N, Gallouj S, Moustaide K, et al. Painful tumors in a patient with neurofibromatosis type 1: a case report. J Med Case Rep. 2018;12:319. doi:10.1186/s13256-018-1847-0
- Demirdag HG, Akay BN, Kirmizi A, et al. Subungual glomangiomyoma. J Am Podiatr Med Assoc. 2020;110:Article_13. doi:10.7547/19-051
- Vega SML, Ruiz SJA, Ramírez CS, et al. Subungual glomangiomyoma: a case report. Dermatol Cosmet Med Quir. 2022;20:258-262.
- Chalise S, Jha A, Neupane PR. Glomangiomyoma of uncertain malignant potential in the urinary bladder: a case report. JNMA J Nepal Med Assoc. 2021;59:719-722. doi:10.31729/jnma.5388
- de Berker D, Goettman S, Baran R. Subungual myxoid cysts: clinical manifestations and response to therapy. J Am Acad Dermatol. 2002;46:394-398. doi:10.1067/mjd.2002.119652
- Gupta MK, Lipner SR. Transillumination for improved diagnosis of digital myxoid cysts. Cutis. 2020;105:82.
- Fernandez-Flores A, Saeb-Lima M. Mucin as a diagnostic clue in dermatopathology. J Cutan Pathol. 2016;43:1005-1016. doi:10.1111/cup.12782
- Choi R, Kim SR, Glusac EJ, et al. Subungual neuroma masquerading as green nail syndrome. JAAD Case Rep. 2022;20:17-19. doi:10.1016 /j.jdcr.2021.11.025
- Rashid RM, Rashid RM, Thomas V. Subungal traumatic neuroma. J Am Acad Dermatol. 2010;63:E7-E8. doi:10.1016/j.jaad.2010.01.028
- Whitehouse HJ, Urwin R, Stables G. Traumatic subungual neuroma. Clin Exp Dermatol. 2018;43:65-66. doi:10.1111/ced.13247
- Lipner SR, Ko D, Husain S. Subungual leiyomyoma presenting as erythronychia: case report and review of the literature. J Drugs Dermatol. 2019;18:465-467.
- Taleb E, Saldías C, Gonzalez S, et al. Sonographic characteristics of leiomyomatous tumors of skin and nail: a case series. Dermatol Pract Concept. 2022;12:e2022082. doi:10.5826/dpc.1203a82
- Baran R, Requena L, Drapé JL. Subungual angioleiomyoma masquerading as a glomus tumour. Br J Dermatol. 2000;142:1239-1241. doi:10.1046/ j.1365-2133.2000.03560.x
- Watabe D, Sakurai E, Mori S, et al. Subungual angioleiomyoma. Indian J Dermatol Venereol Leprol. 2017;83:74-75. doi:10.4103/0378-6323 .185045
- Jha AK, Sinha R, Kumar A, et al. Spiradenoma causing longitudinal splitting of the nail. Clin Exp Dermatol. 2016;41:754-756. doi:10.1111 /ced.12886
- Leach BC, Graham BS. Papular lesion of the proximal nail fold. eccrine spiradenoma. Arch Dermatol. 2004;140:1003-1008. doi:10.1001 /archderm.140.8.1003-a
The Diagnosis: Glomangiomyoma
The nail unit excision specimen showed collections of cuboidal cells and spindled cells within the corium that were consistent with a diagnosis of a glomangiomyoma, a rare glomus tumor variant (Figure). Glomus tumors are benign neoplasms comprising glomus bodies, which are arteriovenous anastomoses involved in thermoregulation.1 They develop in areas densely populated by glomus bodies, including the fingers, toes, and subungual areas. Glomus tumors most commonly develop in middle-aged women.2 Clinically, they manifest with a characteristic triad of intense pain, point tenderness, and cold sensitivity and may appear as reddish-pink or blue macules under the nail plate and/or longitudinal erythronychia.2-6 The presence of multiple glomus tumors is associated with neurofibromatosis type 1.7

Advanced imaging including ultrasonography and magnetic resonance imaging (MRI) may help confirm the diagnosis but may not be cost effective, as excision with histopathology is needed to relieve symptoms and render a definitive diagnosis. Radiography is highly insensitive in identifying bone erosions associated with glomus tumors.8 With ultrasonography, glomus tumors appear hypoechoic; with Doppler ultrasonography, they appear hypervascular. With MRI, glomus tumors appear as well-defined nodular lesions with hypointense signal intensity on T1-weighted sequence and hyperintense signal intensity on T2-weighted sequence, with strong enhancement using gadolinium-based contrast.9,10 On histopathology, a glomus tumor appears as a nodular tumor with sheets of oval-nucleated cells arranged in multicellular layers surrounding blood vessels and are immunoreactive for α-smooth muscle actin, muscle-specific actin, and type IV collagen.11,12
There are several glomus tumor variants. The most common is a solid glomus tumor, which predominantly is composed of glomus cells, followed by glomangioma, which mainly is composed of blood vessels. Glomangiomyoma, which mostly is composed of smooth muscle cells, is the rarest variant.13
While glomus tumors are common in the subungual areas, it is an uncommon location for glomangiomyomas, which have been reported in the nail unit in only 7 prior case reports identified through searches of PubMed and Google Scholar using the terms glomangiomyoma, glomangiomyoma nail, and subungual glomangiomyoma (Table).13-19 Glomangiomyomas more commonly are described in solid organs, including the stomach, kidney, pancreas, and bladder.16 The mean age of patients with subungual glomangiomyomas, including our patient, was 40.4 years (range, 3-61 years), with the majority being female (75.0% [6/8]). Most patients presented with fingernail involvement (75.0% [6/8]), nail dystrophy (eg, nail plate thinning, longitudinal grooves, splinter hemorrhages, longitudinal erythronychia)(62.5% [5/8]), and intermittent pain and/or point tenderness in the affected nail (75.0% [6/8]).13-19 Notably, only our patient had longitudinal erythronychia as a clinical feature, and only one other case described MRI findings, which included a lobulated mass with intense contrast and distal phalanx destruction.18 One patient was a 3-year-old girl with a family history of generalized multiple glomangiomyomas. Although subungual glomangiomyoma was not confirmed on histopathology, the diagnosis in this patient was presumed based on her family history.13 On histopathology, glomangiomyomas are composed of oval-nucleated cells surrounding blood vessels. These oval-nucleated cells then gradually transition to smooth muscle cells.20

A myxoid cyst is composed of a pseudocyst, which lacks a cyst lining, and is a result of synovial fluid from the distal interphalangeal joint entering the pseudocyst space.2 It typically manifests with a longitudinal groove in the nail plate. A flesh-colored nodule may be appreciated between the cuticle and the distal interphalangeal joint.2 The depth of the longitudinal groove may vary depending on the volume of synovial fluid within the myxoid cyst.21 In a series of 35 cases of subungual myxoid cysts, none manifested with longitudinal erythronychia. Due to their composition, myxoid cysts can be distinguished easily from solid tumors of the nail unit via transillumination.22 Pain is a much less common with myxoid cysts vs glomus tumors, as the filling of the pseudocyst space with synovial fluid typically is gradual, allowing the surrounding tissue to accommodate and adapt over time.21 In equivocal cases, MRI or high-resolution ultrasonography may be used to distinguish myxoid cysts and glomus tumors.8 Histopathology shows accumulation of mucin in the dermis with surrounding fibrous stroma.23
Subungual neuromas are painful benign tumors that develop due to disorganized neural proliferation following disruption to peripheral nerves secondary to trauma or surgery. In 3 case reports, subungual neuromas manifested as painful subungual nodules, with proximal nail plate ridging, or onycholysis.24-26 Since neuromas have only rarely been described in the subungual region, reports of MRI and ultrasonography findings are unknown. Histopathology is needed to distinguish neuromas from glomus tumors. Histopathology shows an acapsular structure consisting of disorganized spindle-cell proliferation and nerve fibers arranged in a tangle of fascicles within fibrotic tissue.25 On immunochemistry, spindle cells typically are positive for cellular antigen protein S100.26
Leiomyomas are benign neoplasms derived from smooth muscle, typically localized to the uterus or gastrointestinal tract, and have been described rarely in the nail unit.27,28 It is hypothesized that subungual leiomyomas originate from the vascular smooth muscle in the subcutaneous layer of the nail unit.28 Like glomus tumors, leiomyomas of the subungual region often manifest with pain and longitudinal erythronychia.27-30 Subungual leiomyomas may be distinguished from glomus tumors via advanced imaging techniques, including ultrasonography and MRI. Cutaneous leiomyomas have been described with mild to moderate internal low flow vascularity on Doppler ultrasonography, while glomus tumors typically reveal high internal vascularity.28 Biopsy with histopathology is needed for definitive diagnosis. On histopathology, leiomyomas demonstrate bland-appearing spindle-shaped cells with elongated nuclei arranged in fascicles.27 They typically are positive for α-smooth muscle actin and caldesmon on immunostaining.
Eccrine spiradenomas are benign adnexal tumors likely of apocrine origin with limited case reports in the literature.31,32 Clinically, eccrine spiradenomas involving the nail unit may manifest with longitudinal nail splitting of the nail or as a papule on the proximal nail fold, with associated tenderness.31,32 In a report of a 50-year-old woman with a histopathologically confirmed eccrine spiradenoma manifesting with longitudinal splitting of the nail and pain in the proximal nail fold, the mass appeared hypoechoic on ultrasonography with increased intramass vascularity on Doppler, while MRI showed an intensely enhancing lesion.31 These imaging features, combined with a classically manifesting feature of pain, make eccrine spiradenomas difficult to distinguish from glomus tumors; therefore, histopathologic examination can provide a definitive diagnosis, and surgical excision is used for treatment.31 On histopathology, these tumors are well circumscribed and composed of both small dark basaloid cells with peripheral compact nuclei and larger cells with central pale nuclei, which may be arranged in tubules.31,32
The Diagnosis: Glomangiomyoma
The nail unit excision specimen showed collections of cuboidal cells and spindled cells within the corium that were consistent with a diagnosis of a glomangiomyoma, a rare glomus tumor variant (Figure). Glomus tumors are benign neoplasms comprising glomus bodies, which are arteriovenous anastomoses involved in thermoregulation.1 They develop in areas densely populated by glomus bodies, including the fingers, toes, and subungual areas. Glomus tumors most commonly develop in middle-aged women.2 Clinically, they manifest with a characteristic triad of intense pain, point tenderness, and cold sensitivity and may appear as reddish-pink or blue macules under the nail plate and/or longitudinal erythronychia.2-6 The presence of multiple glomus tumors is associated with neurofibromatosis type 1.7

Advanced imaging including ultrasonography and magnetic resonance imaging (MRI) may help confirm the diagnosis but may not be cost effective, as excision with histopathology is needed to relieve symptoms and render a definitive diagnosis. Radiography is highly insensitive in identifying bone erosions associated with glomus tumors.8 With ultrasonography, glomus tumors appear hypoechoic; with Doppler ultrasonography, they appear hypervascular. With MRI, glomus tumors appear as well-defined nodular lesions with hypointense signal intensity on T1-weighted sequence and hyperintense signal intensity on T2-weighted sequence, with strong enhancement using gadolinium-based contrast.9,10 On histopathology, a glomus tumor appears as a nodular tumor with sheets of oval-nucleated cells arranged in multicellular layers surrounding blood vessels and are immunoreactive for α-smooth muscle actin, muscle-specific actin, and type IV collagen.11,12
There are several glomus tumor variants. The most common is a solid glomus tumor, which predominantly is composed of glomus cells, followed by glomangioma, which mainly is composed of blood vessels. Glomangiomyoma, which mostly is composed of smooth muscle cells, is the rarest variant.13
While glomus tumors are common in the subungual areas, it is an uncommon location for glomangiomyomas, which have been reported in the nail unit in only 7 prior case reports identified through searches of PubMed and Google Scholar using the terms glomangiomyoma, glomangiomyoma nail, and subungual glomangiomyoma (Table).13-19 Glomangiomyomas more commonly are described in solid organs, including the stomach, kidney, pancreas, and bladder.16 The mean age of patients with subungual glomangiomyomas, including our patient, was 40.4 years (range, 3-61 years), with the majority being female (75.0% [6/8]). Most patients presented with fingernail involvement (75.0% [6/8]), nail dystrophy (eg, nail plate thinning, longitudinal grooves, splinter hemorrhages, longitudinal erythronychia)(62.5% [5/8]), and intermittent pain and/or point tenderness in the affected nail (75.0% [6/8]).13-19 Notably, only our patient had longitudinal erythronychia as a clinical feature, and only one other case described MRI findings, which included a lobulated mass with intense contrast and distal phalanx destruction.18 One patient was a 3-year-old girl with a family history of generalized multiple glomangiomyomas. Although subungual glomangiomyoma was not confirmed on histopathology, the diagnosis in this patient was presumed based on her family history.13 On histopathology, glomangiomyomas are composed of oval-nucleated cells surrounding blood vessels. These oval-nucleated cells then gradually transition to smooth muscle cells.20

A myxoid cyst is composed of a pseudocyst, which lacks a cyst lining, and is a result of synovial fluid from the distal interphalangeal joint entering the pseudocyst space.2 It typically manifests with a longitudinal groove in the nail plate. A flesh-colored nodule may be appreciated between the cuticle and the distal interphalangeal joint.2 The depth of the longitudinal groove may vary depending on the volume of synovial fluid within the myxoid cyst.21 In a series of 35 cases of subungual myxoid cysts, none manifested with longitudinal erythronychia. Due to their composition, myxoid cysts can be distinguished easily from solid tumors of the nail unit via transillumination.22 Pain is a much less common with myxoid cysts vs glomus tumors, as the filling of the pseudocyst space with synovial fluid typically is gradual, allowing the surrounding tissue to accommodate and adapt over time.21 In equivocal cases, MRI or high-resolution ultrasonography may be used to distinguish myxoid cysts and glomus tumors.8 Histopathology shows accumulation of mucin in the dermis with surrounding fibrous stroma.23
Subungual neuromas are painful benign tumors that develop due to disorganized neural proliferation following disruption to peripheral nerves secondary to trauma or surgery. In 3 case reports, subungual neuromas manifested as painful subungual nodules, with proximal nail plate ridging, or onycholysis.24-26 Since neuromas have only rarely been described in the subungual region, reports of MRI and ultrasonography findings are unknown. Histopathology is needed to distinguish neuromas from glomus tumors. Histopathology shows an acapsular structure consisting of disorganized spindle-cell proliferation and nerve fibers arranged in a tangle of fascicles within fibrotic tissue.25 On immunochemistry, spindle cells typically are positive for cellular antigen protein S100.26
Leiomyomas are benign neoplasms derived from smooth muscle, typically localized to the uterus or gastrointestinal tract, and have been described rarely in the nail unit.27,28 It is hypothesized that subungual leiomyomas originate from the vascular smooth muscle in the subcutaneous layer of the nail unit.28 Like glomus tumors, leiomyomas of the subungual region often manifest with pain and longitudinal erythronychia.27-30 Subungual leiomyomas may be distinguished from glomus tumors via advanced imaging techniques, including ultrasonography and MRI. Cutaneous leiomyomas have been described with mild to moderate internal low flow vascularity on Doppler ultrasonography, while glomus tumors typically reveal high internal vascularity.28 Biopsy with histopathology is needed for definitive diagnosis. On histopathology, leiomyomas demonstrate bland-appearing spindle-shaped cells with elongated nuclei arranged in fascicles.27 They typically are positive for α-smooth muscle actin and caldesmon on immunostaining.
Eccrine spiradenomas are benign adnexal tumors likely of apocrine origin with limited case reports in the literature.31,32 Clinically, eccrine spiradenomas involving the nail unit may manifest with longitudinal nail splitting of the nail or as a papule on the proximal nail fold, with associated tenderness.31,32 In a report of a 50-year-old woman with a histopathologically confirmed eccrine spiradenoma manifesting with longitudinal splitting of the nail and pain in the proximal nail fold, the mass appeared hypoechoic on ultrasonography with increased intramass vascularity on Doppler, while MRI showed an intensely enhancing lesion.31 These imaging features, combined with a classically manifesting feature of pain, make eccrine spiradenomas difficult to distinguish from glomus tumors; therefore, histopathologic examination can provide a definitive diagnosis, and surgical excision is used for treatment.31 On histopathology, these tumors are well circumscribed and composed of both small dark basaloid cells with peripheral compact nuclei and larger cells with central pale nuclei, which may be arranged in tubules.31,32
- Gombos Z, Zhang PJ. Glomus tumor. Arch Pathol Lab Med. 2008;132: 1448-1452. doi:10.5858/2008-132-1448-gt
- Hare AQ, Rich P. Nail tumors. Dermatol Clin. 2021;39:281-292. doi:10.1016/j.det.2020.12.007
- Hazani R, Houle JM, Kasdan ML, et al. Glomus tumors of the hand. Eplasty. 2008;8:E48.
- Hwang JK, Lipner SR. Blue nail discoloration: literature review and diagnostic algorithms. Am J Clin Dermatol. 2023;24:419-441. doi:10.1007/s40257-023-00768-6
- Lipner SR, Scher RK. Longitudinal erythronychia of the fingernail. JAMA Dermatol. 2016;152:1271-1272. doi:10.1001/jamadermatol.2016.2747
- Jellinek NJ, Lipner SR. Longitudinal erythronychia: retrospective single-center study evaluating differential diagnosis and the likelihood of malignancy. Dermatol Surg. 2016;42:310-319. doi:10.1097 /DSS.0000000000000594
- Lipner SR, Scher RK. Subungual glomus tumors: underrecognized clinical findings in neurofibromatosis 1. J Am Acad Dermatol. 2021;84:E269. doi:10.1016/j.jaad.2020.08.129
- Dhami A, Vale SM, Richardson ML, et al. Comparing ultrasound with magnetic resonance imaging in the evaluation of subungual glomus tumors and subungual myxoid cysts. Skin Appendage Disord. 2023;9:262-267. doi:10.1159/000530397
- Baek HJ, Lee SJ, Cho KH, et al. Subungual tumors: clinicopathologic correlation with US and MR imaging findings. Radiographics. 2010;30:1621-1636. doi:10.1148/rg.306105514
- Patel T, Meena V, Meena P. Hand and foot glomus tumors: significance of MRI diagnosis followed by histopathological assessment. Cureus. 2022;14:E30038. doi:10.7759/cureus.30038
- Mravic M, LaChaud G, Nguyen A, et al. Clinical and histopathological diagnosis of glomus tumor: an institutional experience of 138 cases. Int J Surg Pathol. 2015;23:181-188. doi:10.1177/1066896914567330
- Folpe AL, Fanburg-Smith JC, Miettinen M, et al. Atypical and malignant glomus tumors: analysis of 52 cases, with a proposal for the reclassification of glomus tumors. Am J Surg Pathol. 2001;25:1-12. doi:10.1097/00000478-200101000-00001
- Calduch L, Monteagudo C, Martínez-Ruiz E, et al. Familial generalized multiple glomangiomyoma: report of a new family, with immunohistochemical and ultrastructural studies and review of the literature. Pediatr Dermatol. 2002;19:402-408. doi:10.1046/j.1525-1470.2002.00114.x
- Mentzel T, Hügel H, Kutzner H. CD34-positive glomus tumor: clinicopathologic and immunohistochemical analysis of six cases with myxoid stromal changes. J Cutan Pathol. 2002;29:421-425. doi:10.1034 /j.1600-0560.2002.290706.x
- Kang TW, Lee KH, Park CJ. A case of subungual glomangiomyoma with myxoid stromal change. Korean J Dermatol. 2008;46:550-553.
- Wollstein A, Wollstein R. Subungual glomangiomyoma—a case report. Hand Surg. 2012;17:271-273. doi:10.1142/S021881041272032X
- Aqil N, Gallouj S, Moustaide K, et al. Painful tumors in a patient with neurofibromatosis type 1: a case report. J Med Case Rep. 2018;12:319. doi:10.1186/s13256-018-1847-0
- Demirdag HG, Akay BN, Kirmizi A, et al. Subungual glomangiomyoma. J Am Podiatr Med Assoc. 2020;110:Article_13. doi:10.7547/19-051
- Vega SML, Ruiz SJA, Ramírez CS, et al. Subungual glomangiomyoma: a case report. Dermatol Cosmet Med Quir. 2022;20:258-262.
- Chalise S, Jha A, Neupane PR. Glomangiomyoma of uncertain malignant potential in the urinary bladder: a case report. JNMA J Nepal Med Assoc. 2021;59:719-722. doi:10.31729/jnma.5388
- de Berker D, Goettman S, Baran R. Subungual myxoid cysts: clinical manifestations and response to therapy. J Am Acad Dermatol. 2002;46:394-398. doi:10.1067/mjd.2002.119652
- Gupta MK, Lipner SR. Transillumination for improved diagnosis of digital myxoid cysts. Cutis. 2020;105:82.
- Fernandez-Flores A, Saeb-Lima M. Mucin as a diagnostic clue in dermatopathology. J Cutan Pathol. 2016;43:1005-1016. doi:10.1111/cup.12782
- Choi R, Kim SR, Glusac EJ, et al. Subungual neuroma masquerading as green nail syndrome. JAAD Case Rep. 2022;20:17-19. doi:10.1016 /j.jdcr.2021.11.025
- Rashid RM, Rashid RM, Thomas V. Subungal traumatic neuroma. J Am Acad Dermatol. 2010;63:E7-E8. doi:10.1016/j.jaad.2010.01.028
- Whitehouse HJ, Urwin R, Stables G. Traumatic subungual neuroma. Clin Exp Dermatol. 2018;43:65-66. doi:10.1111/ced.13247
- Lipner SR, Ko D, Husain S. Subungual leiyomyoma presenting as erythronychia: case report and review of the literature. J Drugs Dermatol. 2019;18:465-467.
- Taleb E, Saldías C, Gonzalez S, et al. Sonographic characteristics of leiomyomatous tumors of skin and nail: a case series. Dermatol Pract Concept. 2022;12:e2022082. doi:10.5826/dpc.1203a82
- Baran R, Requena L, Drapé JL. Subungual angioleiomyoma masquerading as a glomus tumour. Br J Dermatol. 2000;142:1239-1241. doi:10.1046/ j.1365-2133.2000.03560.x
- Watabe D, Sakurai E, Mori S, et al. Subungual angioleiomyoma. Indian J Dermatol Venereol Leprol. 2017;83:74-75. doi:10.4103/0378-6323 .185045
- Jha AK, Sinha R, Kumar A, et al. Spiradenoma causing longitudinal splitting of the nail. Clin Exp Dermatol. 2016;41:754-756. doi:10.1111 /ced.12886
- Leach BC, Graham BS. Papular lesion of the proximal nail fold. eccrine spiradenoma. Arch Dermatol. 2004;140:1003-1008. doi:10.1001 /archderm.140.8.1003-a
- Gombos Z, Zhang PJ. Glomus tumor. Arch Pathol Lab Med. 2008;132: 1448-1452. doi:10.5858/2008-132-1448-gt
- Hare AQ, Rich P. Nail tumors. Dermatol Clin. 2021;39:281-292. doi:10.1016/j.det.2020.12.007
- Hazani R, Houle JM, Kasdan ML, et al. Glomus tumors of the hand. Eplasty. 2008;8:E48.
- Hwang JK, Lipner SR. Blue nail discoloration: literature review and diagnostic algorithms. Am J Clin Dermatol. 2023;24:419-441. doi:10.1007/s40257-023-00768-6
- Lipner SR, Scher RK. Longitudinal erythronychia of the fingernail. JAMA Dermatol. 2016;152:1271-1272. doi:10.1001/jamadermatol.2016.2747
- Jellinek NJ, Lipner SR. Longitudinal erythronychia: retrospective single-center study evaluating differential diagnosis and the likelihood of malignancy. Dermatol Surg. 2016;42:310-319. doi:10.1097 /DSS.0000000000000594
- Lipner SR, Scher RK. Subungual glomus tumors: underrecognized clinical findings in neurofibromatosis 1. J Am Acad Dermatol. 2021;84:E269. doi:10.1016/j.jaad.2020.08.129
- Dhami A, Vale SM, Richardson ML, et al. Comparing ultrasound with magnetic resonance imaging in the evaluation of subungual glomus tumors and subungual myxoid cysts. Skin Appendage Disord. 2023;9:262-267. doi:10.1159/000530397
- Baek HJ, Lee SJ, Cho KH, et al. Subungual tumors: clinicopathologic correlation with US and MR imaging findings. Radiographics. 2010;30:1621-1636. doi:10.1148/rg.306105514
- Patel T, Meena V, Meena P. Hand and foot glomus tumors: significance of MRI diagnosis followed by histopathological assessment. Cureus. 2022;14:E30038. doi:10.7759/cureus.30038
- Mravic M, LaChaud G, Nguyen A, et al. Clinical and histopathological diagnosis of glomus tumor: an institutional experience of 138 cases. Int J Surg Pathol. 2015;23:181-188. doi:10.1177/1066896914567330
- Folpe AL, Fanburg-Smith JC, Miettinen M, et al. Atypical and malignant glomus tumors: analysis of 52 cases, with a proposal for the reclassification of glomus tumors. Am J Surg Pathol. 2001;25:1-12. doi:10.1097/00000478-200101000-00001
- Calduch L, Monteagudo C, Martínez-Ruiz E, et al. Familial generalized multiple glomangiomyoma: report of a new family, with immunohistochemical and ultrastructural studies and review of the literature. Pediatr Dermatol. 2002;19:402-408. doi:10.1046/j.1525-1470.2002.00114.x
- Mentzel T, Hügel H, Kutzner H. CD34-positive glomus tumor: clinicopathologic and immunohistochemical analysis of six cases with myxoid stromal changes. J Cutan Pathol. 2002;29:421-425. doi:10.1034 /j.1600-0560.2002.290706.x
- Kang TW, Lee KH, Park CJ. A case of subungual glomangiomyoma with myxoid stromal change. Korean J Dermatol. 2008;46:550-553.
- Wollstein A, Wollstein R. Subungual glomangiomyoma—a case report. Hand Surg. 2012;17:271-273. doi:10.1142/S021881041272032X
- Aqil N, Gallouj S, Moustaide K, et al. Painful tumors in a patient with neurofibromatosis type 1: a case report. J Med Case Rep. 2018;12:319. doi:10.1186/s13256-018-1847-0
- Demirdag HG, Akay BN, Kirmizi A, et al. Subungual glomangiomyoma. J Am Podiatr Med Assoc. 2020;110:Article_13. doi:10.7547/19-051
- Vega SML, Ruiz SJA, Ramírez CS, et al. Subungual glomangiomyoma: a case report. Dermatol Cosmet Med Quir. 2022;20:258-262.
- Chalise S, Jha A, Neupane PR. Glomangiomyoma of uncertain malignant potential in the urinary bladder: a case report. JNMA J Nepal Med Assoc. 2021;59:719-722. doi:10.31729/jnma.5388
- de Berker D, Goettman S, Baran R. Subungual myxoid cysts: clinical manifestations and response to therapy. J Am Acad Dermatol. 2002;46:394-398. doi:10.1067/mjd.2002.119652
- Gupta MK, Lipner SR. Transillumination for improved diagnosis of digital myxoid cysts. Cutis. 2020;105:82.
- Fernandez-Flores A, Saeb-Lima M. Mucin as a diagnostic clue in dermatopathology. J Cutan Pathol. 2016;43:1005-1016. doi:10.1111/cup.12782
- Choi R, Kim SR, Glusac EJ, et al. Subungual neuroma masquerading as green nail syndrome. JAAD Case Rep. 2022;20:17-19. doi:10.1016 /j.jdcr.2021.11.025
- Rashid RM, Rashid RM, Thomas V. Subungal traumatic neuroma. J Am Acad Dermatol. 2010;63:E7-E8. doi:10.1016/j.jaad.2010.01.028
- Whitehouse HJ, Urwin R, Stables G. Traumatic subungual neuroma. Clin Exp Dermatol. 2018;43:65-66. doi:10.1111/ced.13247
- Lipner SR, Ko D, Husain S. Subungual leiyomyoma presenting as erythronychia: case report and review of the literature. J Drugs Dermatol. 2019;18:465-467.
- Taleb E, Saldías C, Gonzalez S, et al. Sonographic characteristics of leiomyomatous tumors of skin and nail: a case series. Dermatol Pract Concept. 2022;12:e2022082. doi:10.5826/dpc.1203a82
- Baran R, Requena L, Drapé JL. Subungual angioleiomyoma masquerading as a glomus tumour. Br J Dermatol. 2000;142:1239-1241. doi:10.1046/ j.1365-2133.2000.03560.x
- Watabe D, Sakurai E, Mori S, et al. Subungual angioleiomyoma. Indian J Dermatol Venereol Leprol. 2017;83:74-75. doi:10.4103/0378-6323 .185045
- Jha AK, Sinha R, Kumar A, et al. Spiradenoma causing longitudinal splitting of the nail. Clin Exp Dermatol. 2016;41:754-756. doi:10.1111 /ced.12886
- Leach BC, Graham BS. Papular lesion of the proximal nail fold. eccrine spiradenoma. Arch Dermatol. 2004;140:1003-1008. doi:10.1001 /archderm.140.8.1003-a
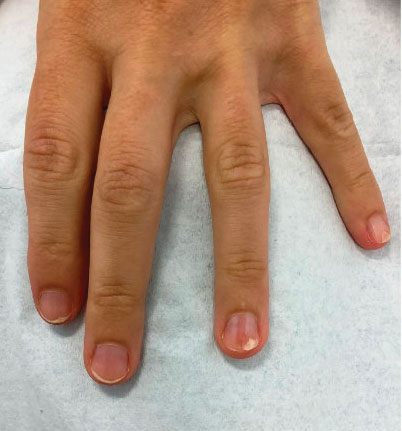
A 38-year-old woman presented to our nail specialty clinic with a red line and associated pain on the left fourth fingernail of 2 and 3 years’ duration, respectively. The patient described the pain as throbbing, with sensitivity to pressure and cold. She noted that the nail grew slowly and would sometimes split at the distal edge. She did not recall any discrete trauma to the digit or nail. The patient was right-handed, making the symptoms less likely to be due to overuse from daily activities. She had received no prior treatment for these symptoms.
The patient’s medical history included iron deficiency as well as acne and eczema. She had no personal or family history of skin cancer. Physical examination of the affected digit and nail revealed a longitudinal red line and distal onycholysis. With contact dermoscopy, the red line blanched. Pressure applied using a #11 scalpel blade elicited pinpoint tenderness (positive Love test), and application of an ice pack caused pain (positive cold test). A radiograph of the left hand was negative for bone erosions, and magnetic resonance imaging showed a 0.3-cm subungual lesion at the level of the fourth distal phalanx. An excision of the nail unit was performed.