User login
Cutis is a peer-reviewed clinical journal for the dermatologist, allergist, and general practitioner published monthly since 1965. Concise clinical articles present the practical side of dermatology, helping physicians to improve patient care. Cutis is referenced in Index Medicus/MEDLINE and is written and edited by industry leaders.
ass lick
assault rifle
balls
ballsac
black jack
bleach
Boko Haram
bondage
causas
cheap
child abuse
cocaine
compulsive behaviors
cost of miracles
cunt
Daech
display network stats
drug paraphernalia
explosion
fart
fda and death
fda AND warn
fda AND warning
fda AND warns
feom
fuck
gambling
gfc
gun
human trafficking
humira AND expensive
illegal
ISIL
ISIS
Islamic caliphate
Islamic state
madvocate
masturbation
mixed martial arts
MMA
molestation
national rifle association
NRA
nsfw
nuccitelli
pedophile
pedophilia
poker
porn
porn
pornography
psychedelic drug
recreational drug
sex slave rings
shit
slot machine
snort
substance abuse
terrorism
terrorist
texarkana
Texas hold 'em
UFC
section[contains(@class, 'nav-hidden')]
section[contains(@class, 'nav-hidden active')
A peer-reviewed, indexed journal for dermatologists with original research, image quizzes, cases and reviews, and columns.
Association of BRAF V600E Status of Incident Melanoma and Risk for a Second Primary Malignancy: A Population-Based Study
The incidence of cutaneous melanoma in the United States has increased in the last 30 years, with the American Cancer Society estimating that 99,780 new melanomas will be diagnosed and 7650 melanoma-related deaths will occur in 2022.1 Patients with melanoma have an increased risk for developing a second primary melanoma or other malignancy, such as salivary gland, small intestine, breast, prostate, renal, or thyroid cancer, but most commonly nonmelanoma skin cancer.2,3 The incidence rate of melanoma among residents of Olmsted County, Minnesota, from 1970 through 2009 has already been described for various age groups4-7; however, the incidence of a second primary malignancy, including melanoma, within these incident cohorts remains unknown.
Mutations in the BRAF oncogene occur in approximately 50% of melanomas.8,9
Although the BRAF mutation event in melanoma is sporadic and should not necessarily affect the development of an unrelated malignancy, we hypothesized that the exposures that may have predisposed a particular individual to a BRAF-mutated melanoma also may have a higher chance of predisposing that individual to the development of another primary malignancy. In this population-based study, we aimed to determine whether the specific melanoma feature of mutant BRAF V600E expression was associated with the development of a second primary malignancy.
Methods
This study was approved by the institutional review boards of the Mayo Clinic and Olmsted Medical Center (both in Rochester, Minnesota). The reporting of this study is compliant with the Strengthening the Reporting of Observational Studies in Epidemiology statement.15
Patient Selection and BRAF Assessment—The Rochester Epidemiology Project (REP) links comprehensive health care records for virtually all residents of Olmsted County, Minnesota, across different medical providers. The REP provides an index of diagnostic and therapeutic procedures, tracks timelines and outcomes of individuals and their medical conditions, and is ideal for population-based studies.
We obtained a list of all residents of Olmsted County aged 18 to 60 years who had a melanoma diagnosed according to the International Classification of Diseases, Ninth Revision, from January 1, 1970, through December 30, 2009; these cohorts have been analyzed previously.4-7 Of the 638 individuals identified, 380 had a melanoma tissue block on file at Mayo Clinic with enough tumor present in available tissue blocks for BRAF assessment. All specimens were reviewed by a board-certified dermatopathologist (J.S.L.) to confirm the diagnosis of melanoma. Tissue blocks were recut, and formalin-fixed, paraffin-embedded tissue sections were stained for BRAF V600E (Spring Bioscience Corporation). BRAF-stained specimens and the associated hematoxylin and eosin−stained slides were reviewed. Melanocyte cytoplasmic staining for BRAF was graded as negative if no staining was evident. BRAF was graded as positive if focal or partial staining was observed (<50% of tumor or low BRAF expression) or if diffuse staining was evident (>50% of tumor or high BRAF expression).
Using resources of the REP, we confirmed patients’ residency status in Olmsted County at the time of diagnosis of the incident melanoma. Patients who denied access to their medical records for research purposes were excluded. We used the complete record of each patient to confirm the date of diagnosis of the incident melanoma. Baseline characteristics of patients and their incident melanomas (eg, anatomic site and pathologic stage according to the American Joint Committee on Cancer classification) were obtained. When only the Clark level was included in the dermatopathology report, the corresponding Breslow thickness was extrapolated from the Clark level,18 and the pathologic stage according to the American Joint Committee on Cancer classification (7th edition) was determined.
For our study, specific diagnostic codes—International Classification of Diseases, Ninth and Tenth Revisions; Hospital International Classification of Diseases Adaptation19; and Berkson16—were applied across individual records to identify all second primary malignancies using the resources of the REP. The diagnosis date, morphology, and anatomic location of second primary malignancies were confirmed from examination of the clinical records.
Statistical Analysis—Baseline characteristics were compared by BRAF V600E expression using Wilcoxon rank sum and χ2 tests. The rate of developing a second primary malignancy at 5, 10, 15, and 20 years after the incident malignant melanoma was estimated with the Kaplan-Meier method. The duration of follow-up was calculated from the incident melanoma date to the second primary malignancy date or the last follow-up date. Patients with a history of the malignancy of interest, except skin cancers, before the incident melanoma date were excluded because it was not possible to distinguish between recurrence of a prior malignancy and a second primary malignancy. Associations of BRAF V600E expression with the development of a second primary malignancy were evaluated with Cox proportional hazards regression models and summarized with hazard ratios (HRs) and 95% CIs; all associations were adjusted for potential confounders such as age at the incident melanoma, year of the incident melanoma, and sex.
Results
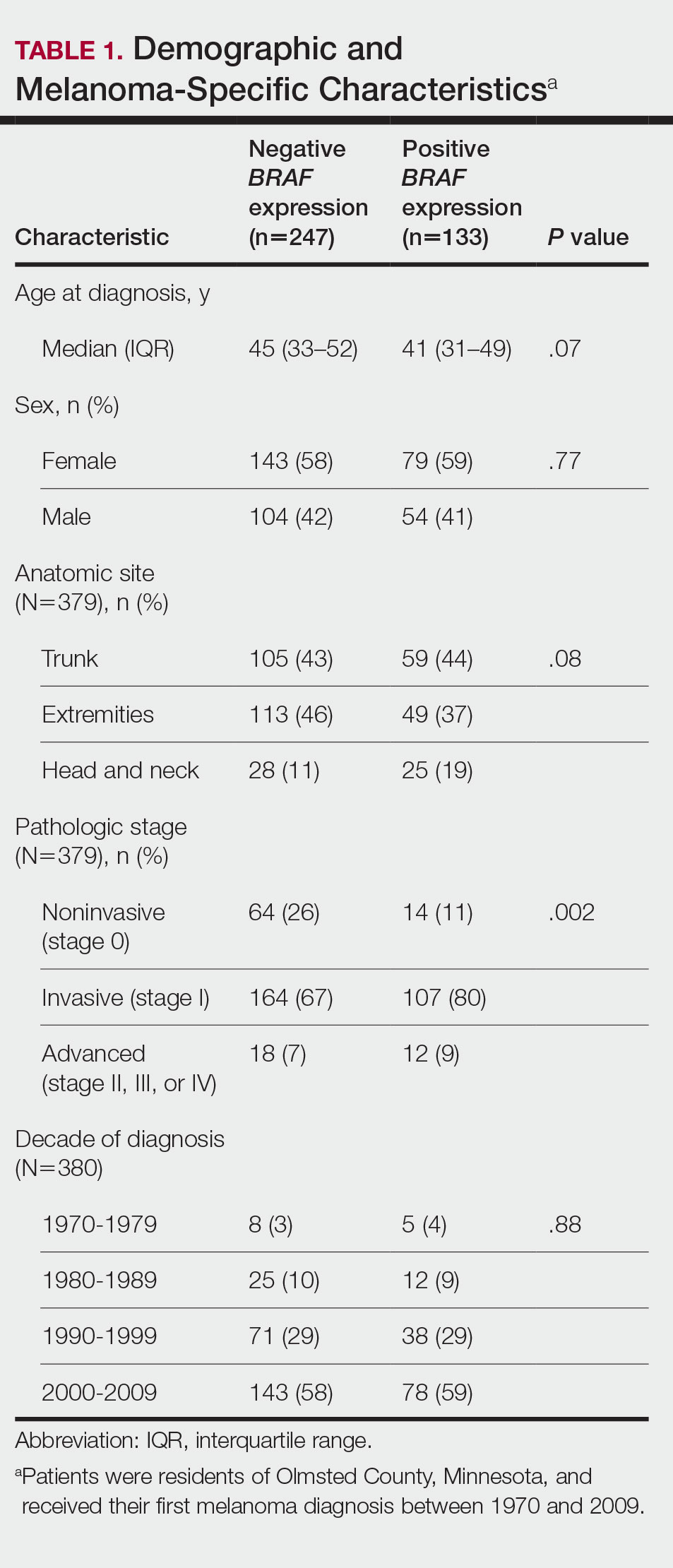
Cumulative Incidence of Second Primary Melanoma—Of 133 patients with positive BRAF V600E expression, we identified 14 (10.5%), 1 (0.8%), and 1 (0.8%) who had 1, 2, and 4 subsequent melanomas, respectively. Of the 247 patients with negative BRAF V600E expression, we identified 15 (6%), 4 (1.6%), 2 (0.8%), and 1 (0.4%) patients who had 1, 2, 3, and 4 subsequent melanomas, respectively; BRAF V600E expression was not associated with the number of subsequent melanomas (P=.37; Wilcoxon rank sum test). The cumulative incidences of developing a second primary melanoma (n=38 among the 380 patients studied) at 5, 10, 15, and 20 years after the incident melanoma were 5.3%, 7.6%, 8.1%, and 14.6%, respectively.
Cumulative Incidence of All Second Primary Malignancies—Of the 380 patients studied, 60 (16%) had at least 1 malignancy diagnosed before the incident melanoma. Of the remaining 320 patients, 104 later had at least 1 malignancy develop, including a second primary melanoma, at a median (IQR) of 8.0 (2.7–16.2) years after the incident melanoma; the 104 patients with at least 1 subsequent malignancy included 40 with BRAF-positive and 64 with BRAF-negative melanomas. The cumulative incidences of developing at least 1 malignancy of any kind at 5, 10, 15, and 20 years after the incident melanoma were 15.0%, 20.5%, 31.2%, and 47.0%, respectively. Table 2 shows the number of patients with at least 1 second primary malignancy after the incident melanoma stratified by BRAF status.
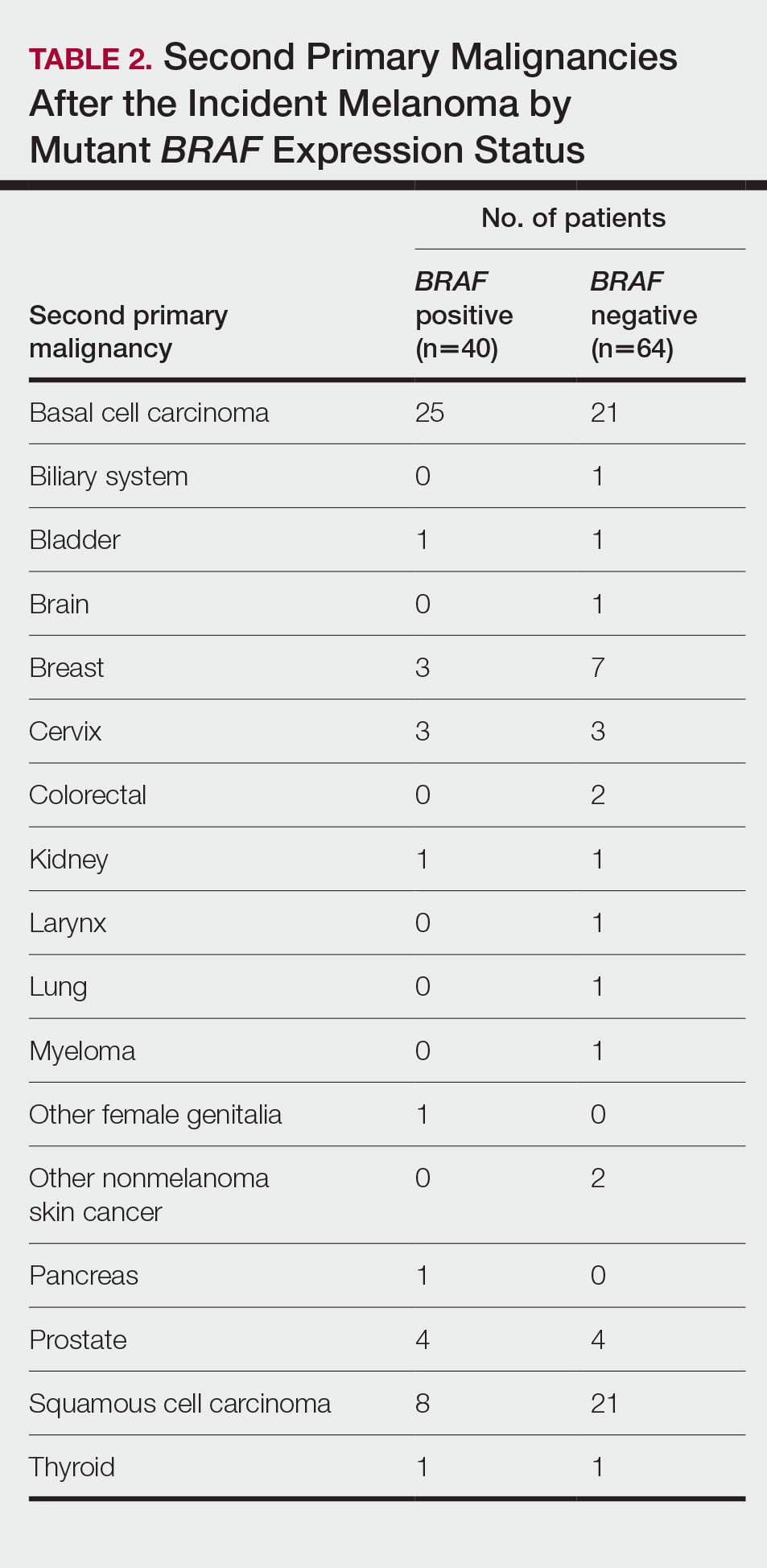
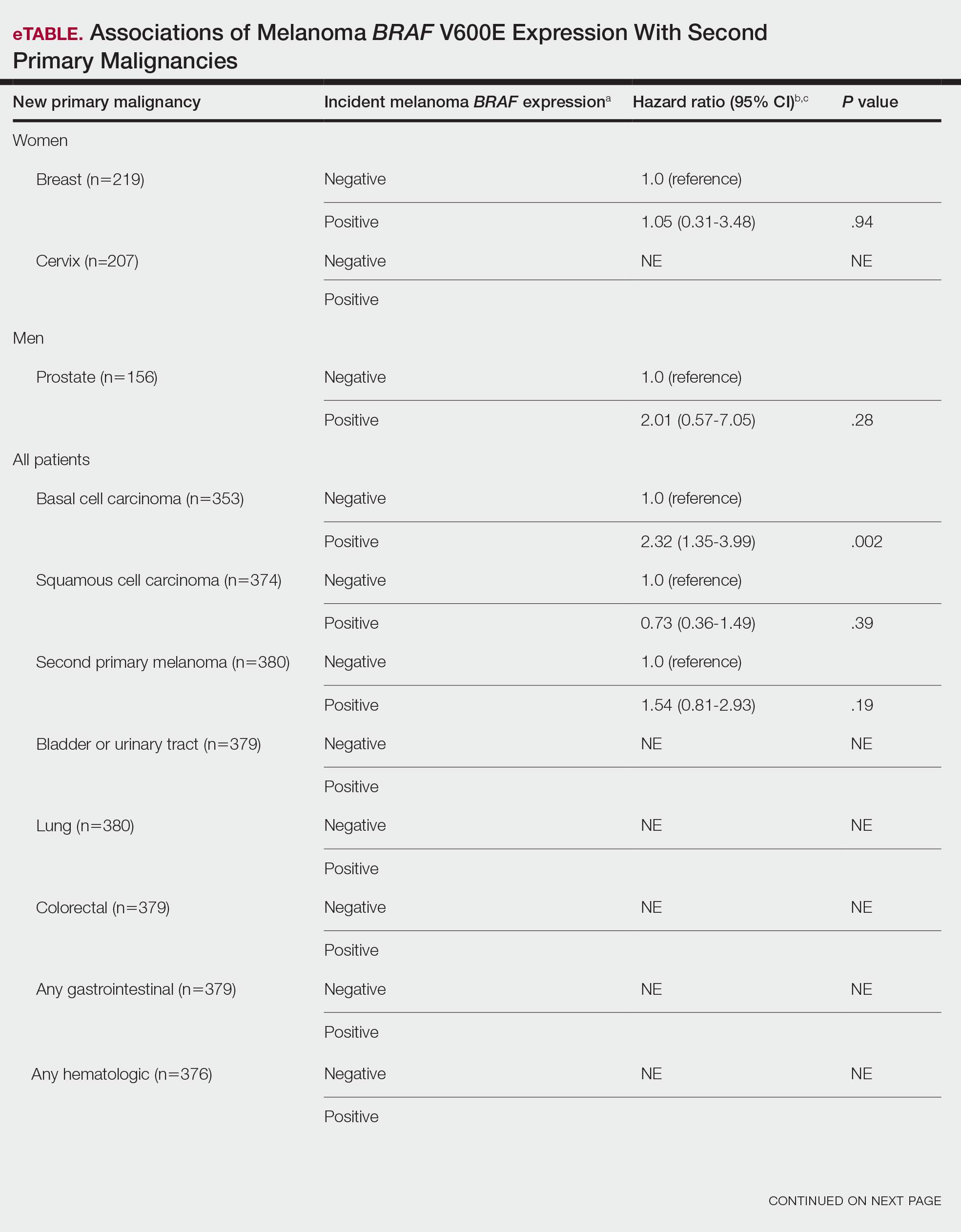
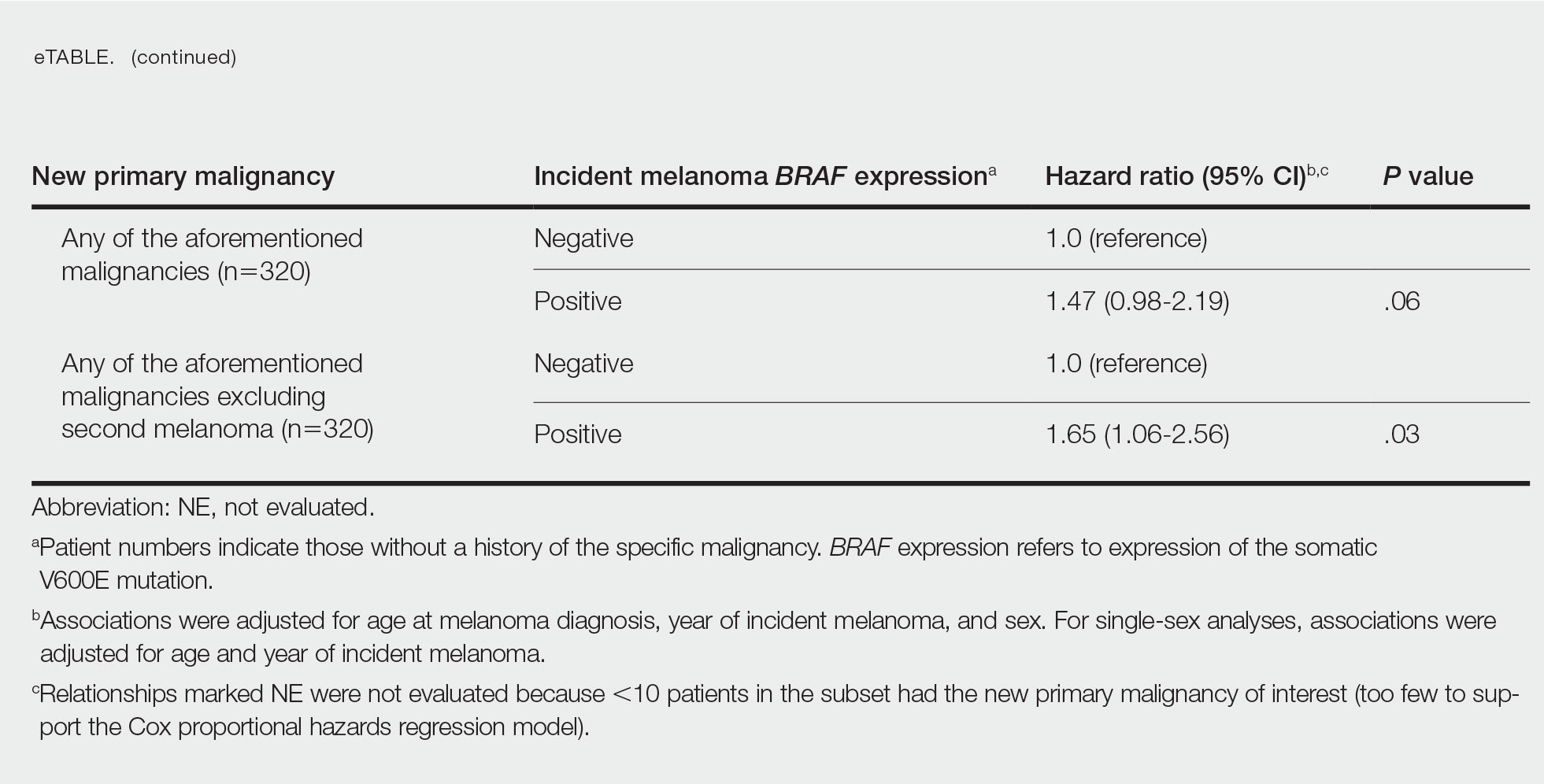
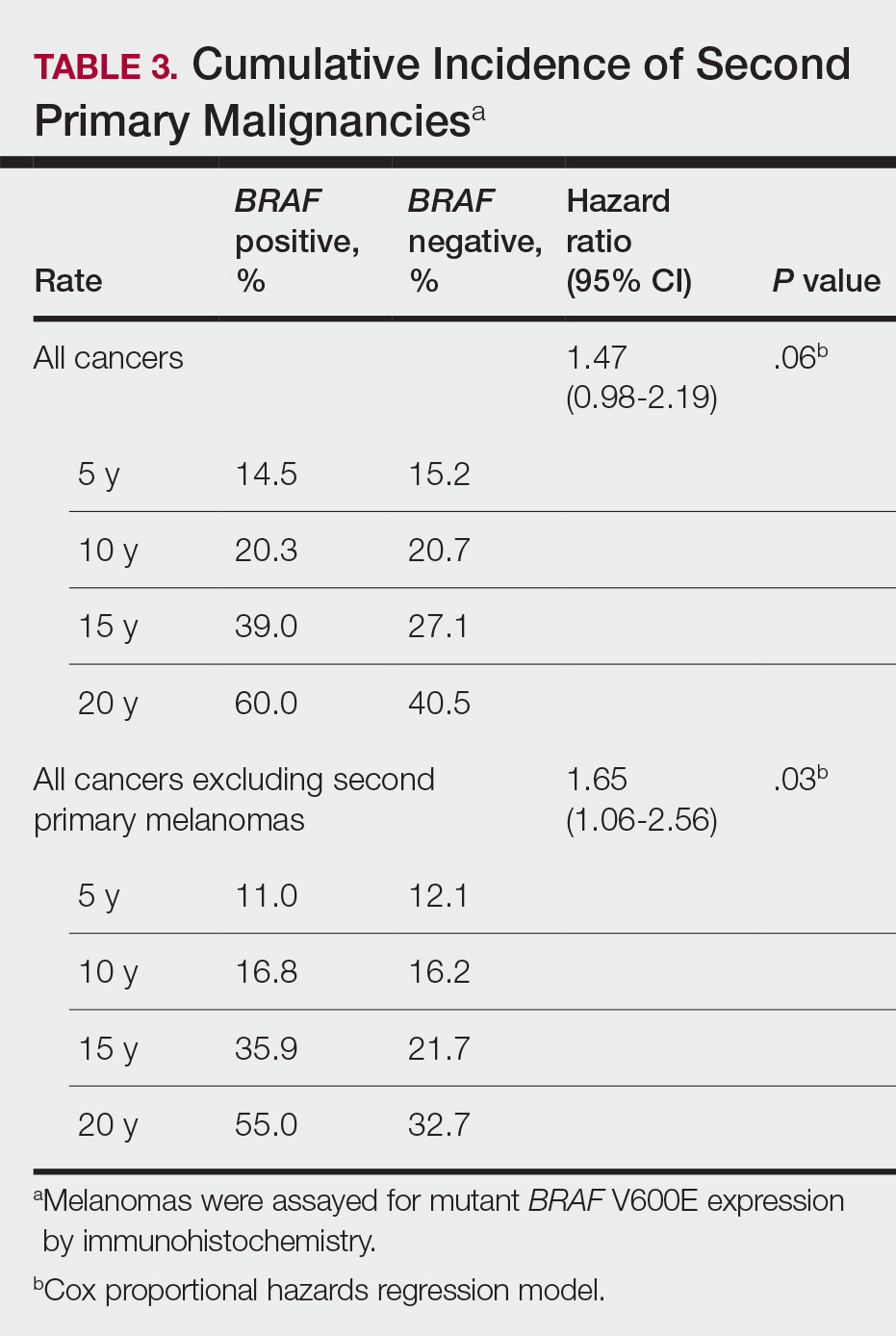
BRAF V600E Expression and Association With Second Primary Malignancy—The eTable shows the associations of mutant BRAF V600E expression status with the development of a new primary malignancy. Malignancies affecting fewer than 10 patients were excluded from the analysis because there were too few events to support the Cox model. Positive BRAF V600E expression was associated with subsequent development of BCCs (HR, 2.32; 95% CI, 1.35-3.99; P=.002) and the development of all combined second primary malignancies excluding melanoma (HR, 1.65; 95% CI, 1.06-2.56; P=.03). However, BRAF V600E status was no longer a significant factor when all second primary malignancies, including second melanomas, were considered (P=.06). Table 3 shows the 5-, 10-, 15-, and 20-year cumulative incidences of all second primary malignancies according to mutant BRAF status.
Comment
Association of BRAF V600E Expression With Second Primary Malignancies—BRAF V600E expression of an incident melanoma was associated with the development of all combined second primary malignancies excluding melanoma; however, this association was not statistically significant when second primary melanomas were included. A possible explanation is that individuals with more than 1 primary melanoma possess additional genetic risk—CDKN2A or CDKN4 gene mutations or MC1R variation—that outweighed the effect of BRAF expression in the statistical analysis.
The 5- and 10-year cumulative incidences of all second primary malignancies excluding second primary melanoma were similar between BRAF-positive and BRAF-negative melanoma, but the 15- and 20-year cumulative incidences were greater for the BRAF-positive cohort. This could reflect the association of BRAF expression with BCCs and the increased likelihood of their occurrence with cumulative sun exposure and advancing age. BRAF expression was associated with the development of BCCs, but the reason for this association was unclear. BRAF-mutated melanoma occurs more frequently on sun-protected sites,20 whereas sporadic BCC generally occurs on sun-exposed sites. However, BRAF-mutated melanoma is associated with high levels of ambient UV exposure early in life, particularly birth through 20 years of age,21 and we speculate that such early UV exposure influences the later development of BCCs.
Development of BRAF-Mutated Cancers—It currently is not understood why the same somatic mutation can cause different types of cancer. A recent translational research study showed that in mice models, precursor cells of the pancreas and bile duct responded differently when exposed to PIK3CA and KRAS oncogenes, and tumorigenesis is influenced by specific cooperating genetic events in the tissue microenvironment. Future research investigating these molecular interactions may lead to better understanding of cancer pathogenesis and direct the design of new targeted therapies.22,23
Regarding environmental influences on the development of BRAF-mutated cancers, we found 1 population-based study that identified an association between high iodine content of drinking water and the prevalence of T1799A BRAF papillary thyroid carcinoma in 5 regions in China.24 Another study identified an increased risk for colorectal cancer and nonmelanoma skin cancer in the first-degree relatives of index patients with BRAF V600E colorectal cancer.25 Two studies by institutions in China and Sweden reported the frequency of BRAF mutations in cohorts of patients with melanoma.26,27
Additional studies investigating a possible association between BRAF-mutated melanoma and other cancers with larger numbers of participants than in our study may become more feasible in the future with increased routine genetic testing of biopsied cancers.
Study Limitations—Limitations of this retrospective epidemiologic study include the possibility of ascertainment bias during data collection. We did not account for known risk factors for cancer (eg, excessive sun exposure, smoking).
The main clinical implications from this study are that we do not have enough evidence to recommend BRAF testing for all incident melanomas, and BRAF-mutated melanomas cannot be associated with increased risk for developing other forms of cancer, with the possible exception of BCCs
Conclusion
Physicians should be aware of the risk for a second primary malignancy after an incident melanoma, and we emphasize the importance of long-term cancer surveillance.
Acknowledgment—We thank Ms. Jayne H. Feind (Rochester, Minnesota) for assistance with study coordination.
- American Cancer Society. Key statistics for melanoma skin cancer. Updated January 12, 2022. Accessed August 15, 2022.https://www.cancer.org/cancer/melanoma-skin-cancer/about/key-statistics.html
- American Cancer Society. Second Cancers After Melanoma Skin Cancer. Accessed August 19, 2022. https://www.cancer.org/cancer/melanoma-skin-cancer/after-treatment/second-cancers.html
- Spanogle JP, Clarke CA, Aroner S, et al. Risk of second primary malignancies following cutaneous melanoma diagnosis: a population-based study. J Am Acad Dermatol. 2010;62:757-767.
- Olazagasti Lourido JM, Ma JE, Lohse CM, et al. Increasing incidence of melanoma in the elderly: an epidemiological study in Olmsted County, Minnesota. Mayo Clin Proc. 2016;91:1555-1562.
- Reed KB, Brewer JD, Lohse CM, et al. Increasing incidence of melanoma among young adults: an epidemiological study in Olmsted County, Minnesota. Mayo Clin Proc. 2012;87:328-334.
- Lowe GC, Brewer JD, Peters MS, et al. Incidence of melanoma in the pediatric population: a population-based study in Olmsted County, Minnesota. Pediatr Derm. 2015;32:618-620.
- Lowe GC, Saavedra A, Reed KB, et al. Increasing incidence of melanoma among middle-aged adults: an epidemiologic study in Olmsted County, Minnesota. Mayo Clin Proc. 2014;89:52-59.
- Ascierto PA, Kirkwood JM, Grob JJ, et al. The role of BRAF V600 mutation in melanoma [editorial]. J Transl Med. 2012;10:85.
- Davies H, Bignell GR, Cox C, et al. Mutations of the BRAF gene in human cancer. Nature. 2002;417:949-954.
- Miller AJ, Mihm MC Jr. Melanoma. N Engl J Med. 2006;355:51-65.
- Tiacci E, Trifonov V, Schiavoni G, et al. BRAF mutations in hairy-cell leukemia. N Engl J Med. 2011;364:2305-2315.
- Xing M. BRAF mutation in thyroid cancer. Endocr Relat Cancer. 2005;12:245-262.
- Moreau S, Saiag P, Aegerter P, et al. Prognostic value of BRAF(V600) mutations in melanoma patients after resection of metastatic lymph nodes. Ann Surg Oncol. 2012;19:4314-4321.
- Flaherty KT, Robert C, Hersey P, et al. Improved survival with MEK inhibition in BRAF-mutated melanoma. N Engl J Med. 2012;367:107-114.
- von Elm E, Altman DG, Egger M, et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. J Clin Epidemiol. 2008;61:344-349.
- Rocca WA, Yawn BP, St Sauver JL, et al. History of the Rochester Epidemiology Project: half a century of medical records linkage in a US population. Mayo Clin Proc. 2012;87:1202-1213.
- St. Sauver JL, Grossardt BR, Yawn BP, et al. Data resource profile: the Rochester Epidemiology Project (REP) medical records-linkage system. Int J Epidemiol. 2012;41:1614-1624.
- National Cancer Institute. Staging: melanoma of the skin, vulva, penis and scrotum staging. Accessed August 15, 2022. https://training.seer.cancer.gov/melanoma/abstract-code-stage/staging.html
- Pakhomov SV, Buntrock JD, Chute CG. Automating the assignment of diagnosis codes to patient encounters using example-based and machine learning techniques. J Am Med Inform Assoc. 2006;13:516-525.
- Curtin JA, Fridlyand J, Kageshita T, et al. Distinct sets of genetic alterations in melanoma. N Engl J Med. 2005;353:2135-2147.
- Thomas NE, Edmiston SN, Alexander A, et al. Number of nevi and early-life ambient UV exposure are associated with BRAF-mutant melanoma. Cancer Epidemiol Biomarkers Prev. 2007;16:991-997.
- German Cancer Research Center. Why identical mutations cause different types of cancer. July 19, 2021. Accessed August 15, 2022. https://www.dkfz.de/en/presse/pressemitteilungen/2021/dkfz-pm-21-41-Why-identical-mutations-cause-different-types-of-cancer.php
- Falcomatà C, Bärthel S, Ulrich A, et al. Genetic screens identify a context-specific PI3K/p27Kip1 node driving extrahepatic biliary cancer. Cancer Discov. 2021;11:3158-3177.
- Guan H, Ji M, Bao R, et al. Association of high iodine intake with the T1799A BRAF mutation in papillary thyroid cancer. J Clin Endocrinol Metab. 2009;94:1612-1617.
- Wish TA, Hyde AJ, Parfrey PS, et al. Increased cancer predisposition in family members of colorectal cancer patients harboring the p.V600E BRAF mutation: a population-based study. Cancer Epidemiol Biomarkers Prev. 2010;19:1831-1839.
- Zebary A, Omholt K, Vassilaki I, et al. KIT, NRAS, BRAF and PTEN mutations in a sample of Swedish patients with acral lentiginous melanoma. J Dermatol Sci. 2013;72:284-289.
- Si L, Kong Y, Xu X, et al. Prevalence of BRAF V600E mutation in Chinese melanoma patients: large scale analysis of BRAF and NRAS mutations in a 432-case cohort. Eur J Cancer. 2012;48:94-100.
- Safaee Ardekani G, Jafarnejad SM, Khosravi S, et al. Disease progression and patient survival are significantly influenced by BRAF protein expression in primary melanoma. Br J Dermatol. 2013;169:320-328.
The incidence of cutaneous melanoma in the United States has increased in the last 30 years, with the American Cancer Society estimating that 99,780 new melanomas will be diagnosed and 7650 melanoma-related deaths will occur in 2022.1 Patients with melanoma have an increased risk for developing a second primary melanoma or other malignancy, such as salivary gland, small intestine, breast, prostate, renal, or thyroid cancer, but most commonly nonmelanoma skin cancer.2,3 The incidence rate of melanoma among residents of Olmsted County, Minnesota, from 1970 through 2009 has already been described for various age groups4-7; however, the incidence of a second primary malignancy, including melanoma, within these incident cohorts remains unknown.
Mutations in the BRAF oncogene occur in approximately 50% of melanomas.8,9
Although the BRAF mutation event in melanoma is sporadic and should not necessarily affect the development of an unrelated malignancy, we hypothesized that the exposures that may have predisposed a particular individual to a BRAF-mutated melanoma also may have a higher chance of predisposing that individual to the development of another primary malignancy. In this population-based study, we aimed to determine whether the specific melanoma feature of mutant BRAF V600E expression was associated with the development of a second primary malignancy.
Methods
This study was approved by the institutional review boards of the Mayo Clinic and Olmsted Medical Center (both in Rochester, Minnesota). The reporting of this study is compliant with the Strengthening the Reporting of Observational Studies in Epidemiology statement.15
Patient Selection and BRAF Assessment—The Rochester Epidemiology Project (REP) links comprehensive health care records for virtually all residents of Olmsted County, Minnesota, across different medical providers. The REP provides an index of diagnostic and therapeutic procedures, tracks timelines and outcomes of individuals and their medical conditions, and is ideal for population-based studies.
We obtained a list of all residents of Olmsted County aged 18 to 60 years who had a melanoma diagnosed according to the International Classification of Diseases, Ninth Revision, from January 1, 1970, through December 30, 2009; these cohorts have been analyzed previously.4-7 Of the 638 individuals identified, 380 had a melanoma tissue block on file at Mayo Clinic with enough tumor present in available tissue blocks for BRAF assessment. All specimens were reviewed by a board-certified dermatopathologist (J.S.L.) to confirm the diagnosis of melanoma. Tissue blocks were recut, and formalin-fixed, paraffin-embedded tissue sections were stained for BRAF V600E (Spring Bioscience Corporation). BRAF-stained specimens and the associated hematoxylin and eosin−stained slides were reviewed. Melanocyte cytoplasmic staining for BRAF was graded as negative if no staining was evident. BRAF was graded as positive if focal or partial staining was observed (<50% of tumor or low BRAF expression) or if diffuse staining was evident (>50% of tumor or high BRAF expression).
Using resources of the REP, we confirmed patients’ residency status in Olmsted County at the time of diagnosis of the incident melanoma. Patients who denied access to their medical records for research purposes were excluded. We used the complete record of each patient to confirm the date of diagnosis of the incident melanoma. Baseline characteristics of patients and their incident melanomas (eg, anatomic site and pathologic stage according to the American Joint Committee on Cancer classification) were obtained. When only the Clark level was included in the dermatopathology report, the corresponding Breslow thickness was extrapolated from the Clark level,18 and the pathologic stage according to the American Joint Committee on Cancer classification (7th edition) was determined.
For our study, specific diagnostic codes—International Classification of Diseases, Ninth and Tenth Revisions; Hospital International Classification of Diseases Adaptation19; and Berkson16—were applied across individual records to identify all second primary malignancies using the resources of the REP. The diagnosis date, morphology, and anatomic location of second primary malignancies were confirmed from examination of the clinical records.
Statistical Analysis—Baseline characteristics were compared by BRAF V600E expression using Wilcoxon rank sum and χ2 tests. The rate of developing a second primary malignancy at 5, 10, 15, and 20 years after the incident malignant melanoma was estimated with the Kaplan-Meier method. The duration of follow-up was calculated from the incident melanoma date to the second primary malignancy date or the last follow-up date. Patients with a history of the malignancy of interest, except skin cancers, before the incident melanoma date were excluded because it was not possible to distinguish between recurrence of a prior malignancy and a second primary malignancy. Associations of BRAF V600E expression with the development of a second primary malignancy were evaluated with Cox proportional hazards regression models and summarized with hazard ratios (HRs) and 95% CIs; all associations were adjusted for potential confounders such as age at the incident melanoma, year of the incident melanoma, and sex.
Results

Cumulative Incidence of Second Primary Melanoma—Of 133 patients with positive BRAF V600E expression, we identified 14 (10.5%), 1 (0.8%), and 1 (0.8%) who had 1, 2, and 4 subsequent melanomas, respectively. Of the 247 patients with negative BRAF V600E expression, we identified 15 (6%), 4 (1.6%), 2 (0.8%), and 1 (0.4%) patients who had 1, 2, 3, and 4 subsequent melanomas, respectively; BRAF V600E expression was not associated with the number of subsequent melanomas (P=.37; Wilcoxon rank sum test). The cumulative incidences of developing a second primary melanoma (n=38 among the 380 patients studied) at 5, 10, 15, and 20 years after the incident melanoma were 5.3%, 7.6%, 8.1%, and 14.6%, respectively.
Cumulative Incidence of All Second Primary Malignancies—Of the 380 patients studied, 60 (16%) had at least 1 malignancy diagnosed before the incident melanoma. Of the remaining 320 patients, 104 later had at least 1 malignancy develop, including a second primary melanoma, at a median (IQR) of 8.0 (2.7–16.2) years after the incident melanoma; the 104 patients with at least 1 subsequent malignancy included 40 with BRAF-positive and 64 with BRAF-negative melanomas. The cumulative incidences of developing at least 1 malignancy of any kind at 5, 10, 15, and 20 years after the incident melanoma were 15.0%, 20.5%, 31.2%, and 47.0%, respectively. Table 2 shows the number of patients with at least 1 second primary malignancy after the incident melanoma stratified by BRAF status.

BRAF V600E Expression and Association With Second Primary Malignancy—The eTable shows the associations of mutant BRAF V600E expression status with the development of a new primary malignancy. Malignancies affecting fewer than 10 patients were excluded from the analysis because there were too few events to support the Cox model. Positive BRAF V600E expression was associated with subsequent development of BCCs (HR, 2.32; 95% CI, 1.35-3.99; P=.002) and the development of all combined second primary malignancies excluding melanoma (HR, 1.65; 95% CI, 1.06-2.56; P=.03). However, BRAF V600E status was no longer a significant factor when all second primary malignancies, including second melanomas, were considered (P=.06). Table 3 shows the 5-, 10-, 15-, and 20-year cumulative incidences of all second primary malignancies according to mutant BRAF status.


Comment
Association of BRAF V600E Expression With Second Primary Malignancies—BRAF V600E expression of an incident melanoma was associated with the development of all combined second primary malignancies excluding melanoma; however, this association was not statistically significant when second primary melanomas were included. A possible explanation is that individuals with more than 1 primary melanoma possess additional genetic risk—CDKN2A or CDKN4 gene mutations or MC1R variation—that outweighed the effect of BRAF expression in the statistical analysis.

The 5- and 10-year cumulative incidences of all second primary malignancies excluding second primary melanoma were similar between BRAF-positive and BRAF-negative melanoma, but the 15- and 20-year cumulative incidences were greater for the BRAF-positive cohort. This could reflect the association of BRAF expression with BCCs and the increased likelihood of their occurrence with cumulative sun exposure and advancing age. BRAF expression was associated with the development of BCCs, but the reason for this association was unclear. BRAF-mutated melanoma occurs more frequently on sun-protected sites,20 whereas sporadic BCC generally occurs on sun-exposed sites. However, BRAF-mutated melanoma is associated with high levels of ambient UV exposure early in life, particularly birth through 20 years of age,21 and we speculate that such early UV exposure influences the later development of BCCs.
Development of BRAF-Mutated Cancers—It currently is not understood why the same somatic mutation can cause different types of cancer. A recent translational research study showed that in mice models, precursor cells of the pancreas and bile duct responded differently when exposed to PIK3CA and KRAS oncogenes, and tumorigenesis is influenced by specific cooperating genetic events in the tissue microenvironment. Future research investigating these molecular interactions may lead to better understanding of cancer pathogenesis and direct the design of new targeted therapies.22,23
Regarding environmental influences on the development of BRAF-mutated cancers, we found 1 population-based study that identified an association between high iodine content of drinking water and the prevalence of T1799A BRAF papillary thyroid carcinoma in 5 regions in China.24 Another study identified an increased risk for colorectal cancer and nonmelanoma skin cancer in the first-degree relatives of index patients with BRAF V600E colorectal cancer.25 Two studies by institutions in China and Sweden reported the frequency of BRAF mutations in cohorts of patients with melanoma.26,27
Additional studies investigating a possible association between BRAF-mutated melanoma and other cancers with larger numbers of participants than in our study may become more feasible in the future with increased routine genetic testing of biopsied cancers.
Study Limitations—Limitations of this retrospective epidemiologic study include the possibility of ascertainment bias during data collection. We did not account for known risk factors for cancer (eg, excessive sun exposure, smoking).
The main clinical implications from this study are that we do not have enough evidence to recommend BRAF testing for all incident melanomas, and BRAF-mutated melanomas cannot be associated with increased risk for developing other forms of cancer, with the possible exception of BCCs
Conclusion
Physicians should be aware of the risk for a second primary malignancy after an incident melanoma, and we emphasize the importance of long-term cancer surveillance.
Acknowledgment—We thank Ms. Jayne H. Feind (Rochester, Minnesota) for assistance with study coordination.
The incidence of cutaneous melanoma in the United States has increased in the last 30 years, with the American Cancer Society estimating that 99,780 new melanomas will be diagnosed and 7650 melanoma-related deaths will occur in 2022.1 Patients with melanoma have an increased risk for developing a second primary melanoma or other malignancy, such as salivary gland, small intestine, breast, prostate, renal, or thyroid cancer, but most commonly nonmelanoma skin cancer.2,3 The incidence rate of melanoma among residents of Olmsted County, Minnesota, from 1970 through 2009 has already been described for various age groups4-7; however, the incidence of a second primary malignancy, including melanoma, within these incident cohorts remains unknown.
Mutations in the BRAF oncogene occur in approximately 50% of melanomas.8,9
Although the BRAF mutation event in melanoma is sporadic and should not necessarily affect the development of an unrelated malignancy, we hypothesized that the exposures that may have predisposed a particular individual to a BRAF-mutated melanoma also may have a higher chance of predisposing that individual to the development of another primary malignancy. In this population-based study, we aimed to determine whether the specific melanoma feature of mutant BRAF V600E expression was associated with the development of a second primary malignancy.
Methods
This study was approved by the institutional review boards of the Mayo Clinic and Olmsted Medical Center (both in Rochester, Minnesota). The reporting of this study is compliant with the Strengthening the Reporting of Observational Studies in Epidemiology statement.15
Patient Selection and BRAF Assessment—The Rochester Epidemiology Project (REP) links comprehensive health care records for virtually all residents of Olmsted County, Minnesota, across different medical providers. The REP provides an index of diagnostic and therapeutic procedures, tracks timelines and outcomes of individuals and their medical conditions, and is ideal for population-based studies.
We obtained a list of all residents of Olmsted County aged 18 to 60 years who had a melanoma diagnosed according to the International Classification of Diseases, Ninth Revision, from January 1, 1970, through December 30, 2009; these cohorts have been analyzed previously.4-7 Of the 638 individuals identified, 380 had a melanoma tissue block on file at Mayo Clinic with enough tumor present in available tissue blocks for BRAF assessment. All specimens were reviewed by a board-certified dermatopathologist (J.S.L.) to confirm the diagnosis of melanoma. Tissue blocks were recut, and formalin-fixed, paraffin-embedded tissue sections were stained for BRAF V600E (Spring Bioscience Corporation). BRAF-stained specimens and the associated hematoxylin and eosin−stained slides were reviewed. Melanocyte cytoplasmic staining for BRAF was graded as negative if no staining was evident. BRAF was graded as positive if focal or partial staining was observed (<50% of tumor or low BRAF expression) or if diffuse staining was evident (>50% of tumor or high BRAF expression).
Using resources of the REP, we confirmed patients’ residency status in Olmsted County at the time of diagnosis of the incident melanoma. Patients who denied access to their medical records for research purposes were excluded. We used the complete record of each patient to confirm the date of diagnosis of the incident melanoma. Baseline characteristics of patients and their incident melanomas (eg, anatomic site and pathologic stage according to the American Joint Committee on Cancer classification) were obtained. When only the Clark level was included in the dermatopathology report, the corresponding Breslow thickness was extrapolated from the Clark level,18 and the pathologic stage according to the American Joint Committee on Cancer classification (7th edition) was determined.
For our study, specific diagnostic codes—International Classification of Diseases, Ninth and Tenth Revisions; Hospital International Classification of Diseases Adaptation19; and Berkson16—were applied across individual records to identify all second primary malignancies using the resources of the REP. The diagnosis date, morphology, and anatomic location of second primary malignancies were confirmed from examination of the clinical records.
Statistical Analysis—Baseline characteristics were compared by BRAF V600E expression using Wilcoxon rank sum and χ2 tests. The rate of developing a second primary malignancy at 5, 10, 15, and 20 years after the incident malignant melanoma was estimated with the Kaplan-Meier method. The duration of follow-up was calculated from the incident melanoma date to the second primary malignancy date or the last follow-up date. Patients with a history of the malignancy of interest, except skin cancers, before the incident melanoma date were excluded because it was not possible to distinguish between recurrence of a prior malignancy and a second primary malignancy. Associations of BRAF V600E expression with the development of a second primary malignancy were evaluated with Cox proportional hazards regression models and summarized with hazard ratios (HRs) and 95% CIs; all associations were adjusted for potential confounders such as age at the incident melanoma, year of the incident melanoma, and sex.
Results

Cumulative Incidence of Second Primary Melanoma—Of 133 patients with positive BRAF V600E expression, we identified 14 (10.5%), 1 (0.8%), and 1 (0.8%) who had 1, 2, and 4 subsequent melanomas, respectively. Of the 247 patients with negative BRAF V600E expression, we identified 15 (6%), 4 (1.6%), 2 (0.8%), and 1 (0.4%) patients who had 1, 2, 3, and 4 subsequent melanomas, respectively; BRAF V600E expression was not associated with the number of subsequent melanomas (P=.37; Wilcoxon rank sum test). The cumulative incidences of developing a second primary melanoma (n=38 among the 380 patients studied) at 5, 10, 15, and 20 years after the incident melanoma were 5.3%, 7.6%, 8.1%, and 14.6%, respectively.
Cumulative Incidence of All Second Primary Malignancies—Of the 380 patients studied, 60 (16%) had at least 1 malignancy diagnosed before the incident melanoma. Of the remaining 320 patients, 104 later had at least 1 malignancy develop, including a second primary melanoma, at a median (IQR) of 8.0 (2.7–16.2) years after the incident melanoma; the 104 patients with at least 1 subsequent malignancy included 40 with BRAF-positive and 64 with BRAF-negative melanomas. The cumulative incidences of developing at least 1 malignancy of any kind at 5, 10, 15, and 20 years after the incident melanoma were 15.0%, 20.5%, 31.2%, and 47.0%, respectively. Table 2 shows the number of patients with at least 1 second primary malignancy after the incident melanoma stratified by BRAF status.

BRAF V600E Expression and Association With Second Primary Malignancy—The eTable shows the associations of mutant BRAF V600E expression status with the development of a new primary malignancy. Malignancies affecting fewer than 10 patients were excluded from the analysis because there were too few events to support the Cox model. Positive BRAF V600E expression was associated with subsequent development of BCCs (HR, 2.32; 95% CI, 1.35-3.99; P=.002) and the development of all combined second primary malignancies excluding melanoma (HR, 1.65; 95% CI, 1.06-2.56; P=.03). However, BRAF V600E status was no longer a significant factor when all second primary malignancies, including second melanomas, were considered (P=.06). Table 3 shows the 5-, 10-, 15-, and 20-year cumulative incidences of all second primary malignancies according to mutant BRAF status.


Comment
Association of BRAF V600E Expression With Second Primary Malignancies—BRAF V600E expression of an incident melanoma was associated with the development of all combined second primary malignancies excluding melanoma; however, this association was not statistically significant when second primary melanomas were included. A possible explanation is that individuals with more than 1 primary melanoma possess additional genetic risk—CDKN2A or CDKN4 gene mutations or MC1R variation—that outweighed the effect of BRAF expression in the statistical analysis.

The 5- and 10-year cumulative incidences of all second primary malignancies excluding second primary melanoma were similar between BRAF-positive and BRAF-negative melanoma, but the 15- and 20-year cumulative incidences were greater for the BRAF-positive cohort. This could reflect the association of BRAF expression with BCCs and the increased likelihood of their occurrence with cumulative sun exposure and advancing age. BRAF expression was associated with the development of BCCs, but the reason for this association was unclear. BRAF-mutated melanoma occurs more frequently on sun-protected sites,20 whereas sporadic BCC generally occurs on sun-exposed sites. However, BRAF-mutated melanoma is associated with high levels of ambient UV exposure early in life, particularly birth through 20 years of age,21 and we speculate that such early UV exposure influences the later development of BCCs.
Development of BRAF-Mutated Cancers—It currently is not understood why the same somatic mutation can cause different types of cancer. A recent translational research study showed that in mice models, precursor cells of the pancreas and bile duct responded differently when exposed to PIK3CA and KRAS oncogenes, and tumorigenesis is influenced by specific cooperating genetic events in the tissue microenvironment. Future research investigating these molecular interactions may lead to better understanding of cancer pathogenesis and direct the design of new targeted therapies.22,23
Regarding environmental influences on the development of BRAF-mutated cancers, we found 1 population-based study that identified an association between high iodine content of drinking water and the prevalence of T1799A BRAF papillary thyroid carcinoma in 5 regions in China.24 Another study identified an increased risk for colorectal cancer and nonmelanoma skin cancer in the first-degree relatives of index patients with BRAF V600E colorectal cancer.25 Two studies by institutions in China and Sweden reported the frequency of BRAF mutations in cohorts of patients with melanoma.26,27
Additional studies investigating a possible association between BRAF-mutated melanoma and other cancers with larger numbers of participants than in our study may become more feasible in the future with increased routine genetic testing of biopsied cancers.
Study Limitations—Limitations of this retrospective epidemiologic study include the possibility of ascertainment bias during data collection. We did not account for known risk factors for cancer (eg, excessive sun exposure, smoking).
The main clinical implications from this study are that we do not have enough evidence to recommend BRAF testing for all incident melanomas, and BRAF-mutated melanomas cannot be associated with increased risk for developing other forms of cancer, with the possible exception of BCCs
Conclusion
Physicians should be aware of the risk for a second primary malignancy after an incident melanoma, and we emphasize the importance of long-term cancer surveillance.
Acknowledgment—We thank Ms. Jayne H. Feind (Rochester, Minnesota) for assistance with study coordination.
- American Cancer Society. Key statistics for melanoma skin cancer. Updated January 12, 2022. Accessed August 15, 2022.https://www.cancer.org/cancer/melanoma-skin-cancer/about/key-statistics.html
- American Cancer Society. Second Cancers After Melanoma Skin Cancer. Accessed August 19, 2022. https://www.cancer.org/cancer/melanoma-skin-cancer/after-treatment/second-cancers.html
- Spanogle JP, Clarke CA, Aroner S, et al. Risk of second primary malignancies following cutaneous melanoma diagnosis: a population-based study. J Am Acad Dermatol. 2010;62:757-767.
- Olazagasti Lourido JM, Ma JE, Lohse CM, et al. Increasing incidence of melanoma in the elderly: an epidemiological study in Olmsted County, Minnesota. Mayo Clin Proc. 2016;91:1555-1562.
- Reed KB, Brewer JD, Lohse CM, et al. Increasing incidence of melanoma among young adults: an epidemiological study in Olmsted County, Minnesota. Mayo Clin Proc. 2012;87:328-334.
- Lowe GC, Brewer JD, Peters MS, et al. Incidence of melanoma in the pediatric population: a population-based study in Olmsted County, Minnesota. Pediatr Derm. 2015;32:618-620.
- Lowe GC, Saavedra A, Reed KB, et al. Increasing incidence of melanoma among middle-aged adults: an epidemiologic study in Olmsted County, Minnesota. Mayo Clin Proc. 2014;89:52-59.
- Ascierto PA, Kirkwood JM, Grob JJ, et al. The role of BRAF V600 mutation in melanoma [editorial]. J Transl Med. 2012;10:85.
- Davies H, Bignell GR, Cox C, et al. Mutations of the BRAF gene in human cancer. Nature. 2002;417:949-954.
- Miller AJ, Mihm MC Jr. Melanoma. N Engl J Med. 2006;355:51-65.
- Tiacci E, Trifonov V, Schiavoni G, et al. BRAF mutations in hairy-cell leukemia. N Engl J Med. 2011;364:2305-2315.
- Xing M. BRAF mutation in thyroid cancer. Endocr Relat Cancer. 2005;12:245-262.
- Moreau S, Saiag P, Aegerter P, et al. Prognostic value of BRAF(V600) mutations in melanoma patients after resection of metastatic lymph nodes. Ann Surg Oncol. 2012;19:4314-4321.
- Flaherty KT, Robert C, Hersey P, et al. Improved survival with MEK inhibition in BRAF-mutated melanoma. N Engl J Med. 2012;367:107-114.
- von Elm E, Altman DG, Egger M, et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. J Clin Epidemiol. 2008;61:344-349.
- Rocca WA, Yawn BP, St Sauver JL, et al. History of the Rochester Epidemiology Project: half a century of medical records linkage in a US population. Mayo Clin Proc. 2012;87:1202-1213.
- St. Sauver JL, Grossardt BR, Yawn BP, et al. Data resource profile: the Rochester Epidemiology Project (REP) medical records-linkage system. Int J Epidemiol. 2012;41:1614-1624.
- National Cancer Institute. Staging: melanoma of the skin, vulva, penis and scrotum staging. Accessed August 15, 2022. https://training.seer.cancer.gov/melanoma/abstract-code-stage/staging.html
- Pakhomov SV, Buntrock JD, Chute CG. Automating the assignment of diagnosis codes to patient encounters using example-based and machine learning techniques. J Am Med Inform Assoc. 2006;13:516-525.
- Curtin JA, Fridlyand J, Kageshita T, et al. Distinct sets of genetic alterations in melanoma. N Engl J Med. 2005;353:2135-2147.
- Thomas NE, Edmiston SN, Alexander A, et al. Number of nevi and early-life ambient UV exposure are associated with BRAF-mutant melanoma. Cancer Epidemiol Biomarkers Prev. 2007;16:991-997.
- German Cancer Research Center. Why identical mutations cause different types of cancer. July 19, 2021. Accessed August 15, 2022. https://www.dkfz.de/en/presse/pressemitteilungen/2021/dkfz-pm-21-41-Why-identical-mutations-cause-different-types-of-cancer.php
- Falcomatà C, Bärthel S, Ulrich A, et al. Genetic screens identify a context-specific PI3K/p27Kip1 node driving extrahepatic biliary cancer. Cancer Discov. 2021;11:3158-3177.
- Guan H, Ji M, Bao R, et al. Association of high iodine intake with the T1799A BRAF mutation in papillary thyroid cancer. J Clin Endocrinol Metab. 2009;94:1612-1617.
- Wish TA, Hyde AJ, Parfrey PS, et al. Increased cancer predisposition in family members of colorectal cancer patients harboring the p.V600E BRAF mutation: a population-based study. Cancer Epidemiol Biomarkers Prev. 2010;19:1831-1839.
- Zebary A, Omholt K, Vassilaki I, et al. KIT, NRAS, BRAF and PTEN mutations in a sample of Swedish patients with acral lentiginous melanoma. J Dermatol Sci. 2013;72:284-289.
- Si L, Kong Y, Xu X, et al. Prevalence of BRAF V600E mutation in Chinese melanoma patients: large scale analysis of BRAF and NRAS mutations in a 432-case cohort. Eur J Cancer. 2012;48:94-100.
- Safaee Ardekani G, Jafarnejad SM, Khosravi S, et al. Disease progression and patient survival are significantly influenced by BRAF protein expression in primary melanoma. Br J Dermatol. 2013;169:320-328.
- American Cancer Society. Key statistics for melanoma skin cancer. Updated January 12, 2022. Accessed August 15, 2022.https://www.cancer.org/cancer/melanoma-skin-cancer/about/key-statistics.html
- American Cancer Society. Second Cancers After Melanoma Skin Cancer. Accessed August 19, 2022. https://www.cancer.org/cancer/melanoma-skin-cancer/after-treatment/second-cancers.html
- Spanogle JP, Clarke CA, Aroner S, et al. Risk of second primary malignancies following cutaneous melanoma diagnosis: a population-based study. J Am Acad Dermatol. 2010;62:757-767.
- Olazagasti Lourido JM, Ma JE, Lohse CM, et al. Increasing incidence of melanoma in the elderly: an epidemiological study in Olmsted County, Minnesota. Mayo Clin Proc. 2016;91:1555-1562.
- Reed KB, Brewer JD, Lohse CM, et al. Increasing incidence of melanoma among young adults: an epidemiological study in Olmsted County, Minnesota. Mayo Clin Proc. 2012;87:328-334.
- Lowe GC, Brewer JD, Peters MS, et al. Incidence of melanoma in the pediatric population: a population-based study in Olmsted County, Minnesota. Pediatr Derm. 2015;32:618-620.
- Lowe GC, Saavedra A, Reed KB, et al. Increasing incidence of melanoma among middle-aged adults: an epidemiologic study in Olmsted County, Minnesota. Mayo Clin Proc. 2014;89:52-59.
- Ascierto PA, Kirkwood JM, Grob JJ, et al. The role of BRAF V600 mutation in melanoma [editorial]. J Transl Med. 2012;10:85.
- Davies H, Bignell GR, Cox C, et al. Mutations of the BRAF gene in human cancer. Nature. 2002;417:949-954.
- Miller AJ, Mihm MC Jr. Melanoma. N Engl J Med. 2006;355:51-65.
- Tiacci E, Trifonov V, Schiavoni G, et al. BRAF mutations in hairy-cell leukemia. N Engl J Med. 2011;364:2305-2315.
- Xing M. BRAF mutation in thyroid cancer. Endocr Relat Cancer. 2005;12:245-262.
- Moreau S, Saiag P, Aegerter P, et al. Prognostic value of BRAF(V600) mutations in melanoma patients after resection of metastatic lymph nodes. Ann Surg Oncol. 2012;19:4314-4321.
- Flaherty KT, Robert C, Hersey P, et al. Improved survival with MEK inhibition in BRAF-mutated melanoma. N Engl J Med. 2012;367:107-114.
- von Elm E, Altman DG, Egger M, et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. J Clin Epidemiol. 2008;61:344-349.
- Rocca WA, Yawn BP, St Sauver JL, et al. History of the Rochester Epidemiology Project: half a century of medical records linkage in a US population. Mayo Clin Proc. 2012;87:1202-1213.
- St. Sauver JL, Grossardt BR, Yawn BP, et al. Data resource profile: the Rochester Epidemiology Project (REP) medical records-linkage system. Int J Epidemiol. 2012;41:1614-1624.
- National Cancer Institute. Staging: melanoma of the skin, vulva, penis and scrotum staging. Accessed August 15, 2022. https://training.seer.cancer.gov/melanoma/abstract-code-stage/staging.html
- Pakhomov SV, Buntrock JD, Chute CG. Automating the assignment of diagnosis codes to patient encounters using example-based and machine learning techniques. J Am Med Inform Assoc. 2006;13:516-525.
- Curtin JA, Fridlyand J, Kageshita T, et al. Distinct sets of genetic alterations in melanoma. N Engl J Med. 2005;353:2135-2147.
- Thomas NE, Edmiston SN, Alexander A, et al. Number of nevi and early-life ambient UV exposure are associated with BRAF-mutant melanoma. Cancer Epidemiol Biomarkers Prev. 2007;16:991-997.
- German Cancer Research Center. Why identical mutations cause different types of cancer. July 19, 2021. Accessed August 15, 2022. https://www.dkfz.de/en/presse/pressemitteilungen/2021/dkfz-pm-21-41-Why-identical-mutations-cause-different-types-of-cancer.php
- Falcomatà C, Bärthel S, Ulrich A, et al. Genetic screens identify a context-specific PI3K/p27Kip1 node driving extrahepatic biliary cancer. Cancer Discov. 2021;11:3158-3177.
- Guan H, Ji M, Bao R, et al. Association of high iodine intake with the T1799A BRAF mutation in papillary thyroid cancer. J Clin Endocrinol Metab. 2009;94:1612-1617.
- Wish TA, Hyde AJ, Parfrey PS, et al. Increased cancer predisposition in family members of colorectal cancer patients harboring the p.V600E BRAF mutation: a population-based study. Cancer Epidemiol Biomarkers Prev. 2010;19:1831-1839.
- Zebary A, Omholt K, Vassilaki I, et al. KIT, NRAS, BRAF and PTEN mutations in a sample of Swedish patients with acral lentiginous melanoma. J Dermatol Sci. 2013;72:284-289.
- Si L, Kong Y, Xu X, et al. Prevalence of BRAF V600E mutation in Chinese melanoma patients: large scale analysis of BRAF and NRAS mutations in a 432-case cohort. Eur J Cancer. 2012;48:94-100.
- Safaee Ardekani G, Jafarnejad SM, Khosravi S, et al. Disease progression and patient survival are significantly influenced by BRAF protein expression in primary melanoma. Br J Dermatol. 2013;169:320-328.
Practice Points
- Dermatologists should be aware of the long-term risk of second primary malignancies after an incident melanoma.
- BRAF mutations occur in melanomas and several other cancers. Our study found that melanoma BRAF V600E expression is associated with an increased risk for basal cell carcinomas.
Risk Factors Predicting Cellulitis Diagnosis in a Prospective Cohort Undergoing Dermatology Consultation in the Emergency Department
Cellulitis is an infection of the skin and skin-associated structures characterized by redness, warmth, swelling, and pain of the affected area. Cellulitis most commonly occurs in middle-aged and older adults and frequently affects the lower extremities.1 Serious complications of cellulitis such as bacteremia, metastatic infection, and sepsis are rare, and most cases of cellulitis in patients with normal vital signs and mental status can be managed with outpatient treatment.2
Diagnosis of cellulitis can be confounded by a number of similarly presenting conditions collectively known as pseudocellulitis, such as venous stasis dermatitis and deep vein thrombosis.1 Misdiagnosis of cellulitis is common, with rates exceeding 30% among hospitalized patients initially diagnosed with cellulitis.3,4 Dermatology or infectious disease assessment is considered the diagnostic gold standard for cellulitis4,5 but is not always readily available, especially in resource-constrained settings.
Most cases of uncomplicated cellulitis can be managed with outpatient treatment, especially because serious complications are rare. Frequent misdiagnosis leads to repeat or unnecessary hospitalization and antibiosis. Exceptions necessitating hospitalization usually are predicated on signs of systemic infection, severe immunocompromised states, or failure of prior outpatient therapy.6 Such presentations can be distinguished by corresponding notable historical or examination factors, such as vital sign abnormalities suggesting systemic infection or history of malignancy leading to an immunocompromised state.
We sought to evaluate factors leading to the diagnosis of cellulitis in a cohort of patients with uncomplicated presentations receiving dermatology consultation to emphasize findings indicative of cellulitis in the absence of clinical or historical factors suggestive of other conditions necessitating hospitalization, such as systemic infection.
Methods
Study Participants—A prospective cohort study of patients presenting to an emergency department (ED) between October 2012 and January 2017 at an urban academic medical center in Boston, Massachusetts, was conducted with approval of study design and procedures by the relevant institutional review board. Patients older than 18 years were eligible for inclusion if given an initial diagnosis of cellulitis by an ED physician. Patients were excluded if incarcerated, pregnant, or unable to provide informed consent. Other exclusion criteria includedinfections overlying temporary or permanent indwelling hardware, animal or human bites, or sites of recent surgery (within the prior 4 weeks); preceding antibiotic treatment for more than 24 hours; or clinical or radiographic evidence of complications requiring alternative management such as osteomyelitis or abscess. Patients presenting with an elevated heart rate (>100 beats per minute) or body temperature (>100.5 °F [38.1 °C]) also were excluded. Eligible patients were enrolled upon providing written informed consent, and no remuneration was offered for participation.
Dermatology Consultation Intervention—A random subset of enrolled patients received dermatology consultation within 24 hours of presentation. Consultation consisted of a patient interview and physical examination with care recommendations to relevant ED and inpatient teams. Consultations confirmed the presence or absence of cellulitis as the primary outcome and also noted the presence of any pseudocellulitis diagnoses either occurring concomitantly with or mimicking cellulitis as a secondary outcome.
Statistical Analysis—Patient characteristics were analyzed to identify factors independently associated with the diagnosis of cellulitis in cases affecting the lower extremities. Factors were recorded with categorical variables reported as counts and percentages and continuous variables as means and standard deviations. Univariate analyses between categorical variables or discretized continuous variables and cellulitis diagnosis were conducted via Fisher exact test to identify a preliminary set of potential risk factors. Continuous variables were discretized at multiple incremental values with the discretization most significantly associated with cellulitis diagnosis selected as a preliminary risk factor. Multivariate analyses involved using any objective preliminary factor meeting a significance threshold of P<.1 in univariate comparisons in a multivariate logistic regression model for prediction of cellulitis diagnosis with corresponding calculation of odds ratios with confidence intervals and receiver operating characteristic. Factors with confidence intervals that excluded 1 were considered significant independent predictors of cellulitis. Analyses were performed using Python version 3.8 (Python Software Foundation).
Results
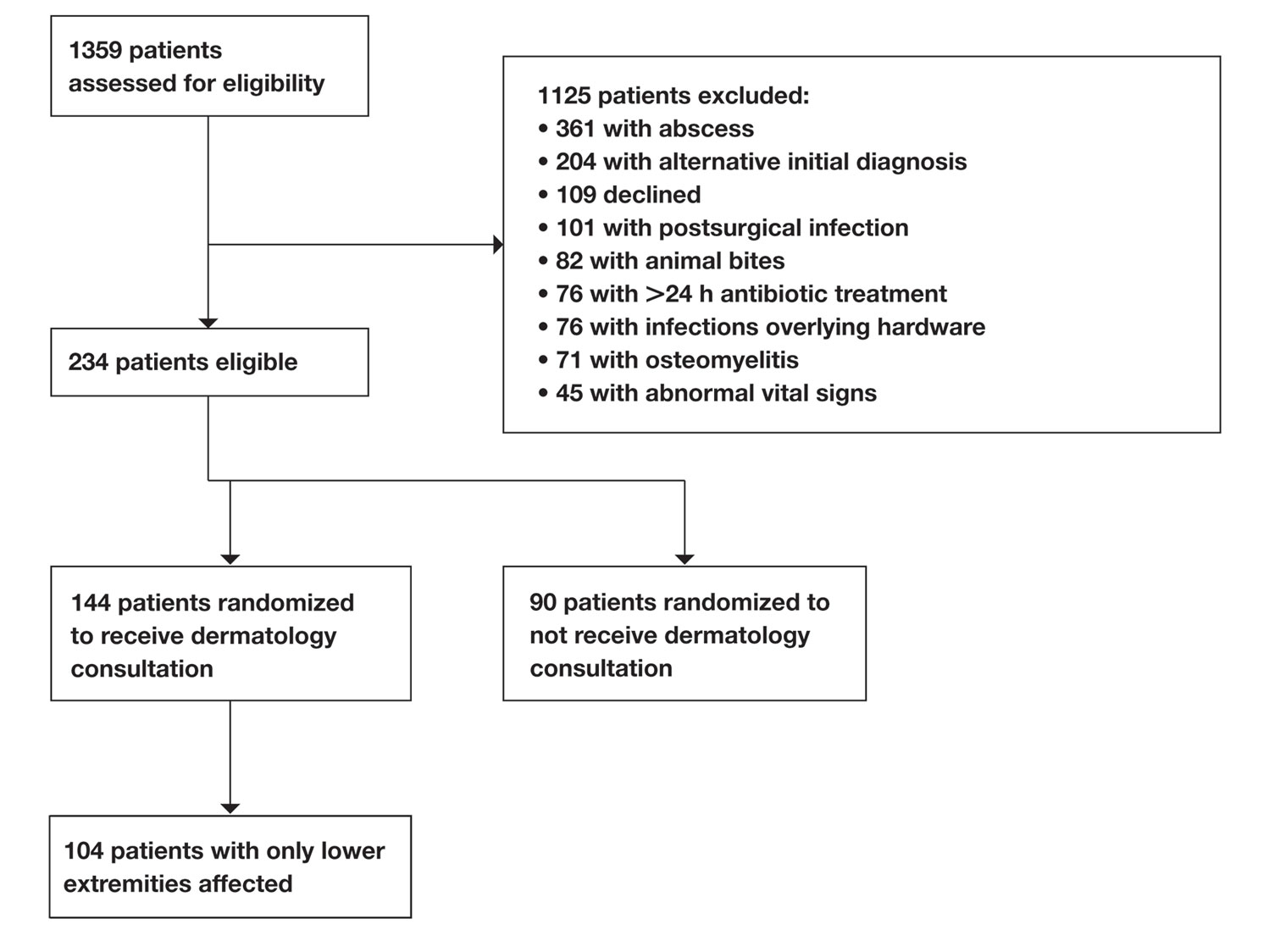
Of 1359 patients screened for eligibility, 104 patients with presumed lower extremity cellulitis undergoing dermatology consultation were included in this study (Figure). The mean patient age (SD) was 60.4 (19.2) years, and 63.5% of patients were male. In the study population, 63 (60.6%) patients received a final diagnosis of cellulitis. The most common pseudocellulitis diagnosis identified was venous stasis dermatitis, which occurred in 12 (11.5%) patients with concomitant cellulitis and in 12 (11.5%) patients mimicking cellulitis (Table).
Univariate comparisons revealed a diverse set of historical, examination, and laboratory factors associated with cellulitis diagnosis. Diagnosis of cellulitis was associated with unilateral presentation, recent trauma to the affected site, and history of cellulitis or onychomycosis. Diagnosis of cellulitis also was associated with elevated white blood cell count, absolute neutrophil count, C-reactive protein, body mass index, hematocrit, and platelet count; age less than 75 years; and lower serum sodium and serum chloride levels. These were the independent factors included in the multivariate analysis, which consisted of a logistic regression model for prediction of cellulitis (eTable).
Multivariate logistic regression on all preliminary factors significantly associated with cellulitis diagnosis in univariate comparisons demonstrated leukocytosis, which was defined as having a white blood cell count exceeding 11,000/μL, unilateral presentation, history of onychomycosis, and trauma to the affected site as significant independent predictors of cellulitis diagnosis; history of cellulitis approached significance (eTable). Unilateral presentation and leukocytosis were the strongest predictors; having either of these factors had a sensitivity of 93.7% and a negative predictive value of 76.5%.

Comment
Importance of Identifying Pseudocellulitis—Successful diagnosis of cellulitis can be confounded by pseudocellulitis that can present concomitantly with or in lieu of cellulitis itself. Although cellulitis mostly affects the lower extremities in adults, pseudocellulitis also was common in this study population of patients with suspected lower extremity cellulitis, occurring both as a mimicker and concomitantly with cellulitis with substantial frequency. Notably, among patients with both venous stasis dermatitis and cellulitis diagnosed, most patients (n=10/12; 83.3%) had unilateral presentations of cellulitis as evidenced by signs and symptoms more notably affecting one lower extremity than the other. These findings suggest that certain pseudocellulitis diagnoses may predispose patients to cellulitis by disrupting the skin barrier, leading to bacterial infiltration; however, these pseudocellulitis diagnoses typically affect both lower extremities equally,1 and asymmetric involvement suggests the presence of overlying cellulitis. Furthermore, the most common pseudocellulitis entities found, such as venous stasis dermatitis, hematoma, and eczema, do not benefit from antibiotic treatment and require alternative therapy.1 Successful discrimination of these pseudocellulitis entities is critical to bolster proper antibiotic stewardship and discourage unnecessary hospitalization.
Independent Predictors of Cellulitis—Unilateral presentation and leukocytosis each emerged as strong independent predictors of cellulitis diagnosis in this study. Having either of these factors furthermore demonstrated high sensitivity and negative predictive value for cellulitis diagnosis. Other notable risk factors were history of onychomycosis, cellulitis, and trauma to the affected site. Prior studies have identified similar historical factors as predisposing patients to cellulitis.7-9 Interestingly, warmth of the affected area on physical examination emerged as strongly associated with cellulitis but was not included in the final predictive model because of its subjective determination. These factors may be especially important in diagnosing cellulitis in patients without concerning vital signs and with concomitant or prior pseudocellulitis.
Study Limitations—This study was limited to patients with uncomplicated presentations to emphasize discrimination of factors associated with cellulitis in the absence of suggestive signs of infection, such as vital sign abnormalities. Signs such as fever and tachypnea have been previously correlated to outpatient treatment failure and necessity for hospitalization.10-12 This study instead focused on patients without concerning vital signs to reduce confounding by such factors in more severe presentations that heighten suspicion for infection and increase likelihood of additional treatment measures. For such patients, suggestive historical factors, such as those discovered in this study, should be considered instead. Interestingly, increased age did not emerge as a significant predictor in this population in contrast to other predictive models that included patients with vital sign abnormalities. Notably, older patients tend to have more variable vital signs, especially in response to physiologic stressors such as infection.13 As such, age may serve as a proxy for vital sign abnormalities to some degree in such predictive models, leading to heightened suspicion for infection in older patients. This study demonstrated that in the absence of concerning vital signs, historical rather than demographic factors are more predictive of cellulitis.
Conclusion
Unilateral presentation and leukocytosis emerged as strong independent predictors of lower extremity cellulitis in patients with uncomplicated presentations. Having either of these factors had a sensitivity of 93.7% and a negative predictive value of 76.5%. Other factors such as history of cellulitis, onychomycosis, and recent trauma to the affected site emerged as additional predictors. These historical, examination, and laboratory characteristics may be especially useful for successful diagnosis of cellulitis in varied practice settings, including outpatient clinics and EDs.
- Raff AB, Kroshinsky D. Cellulitis: a review. JAMA. 2016;316:325-337.
- Gunderson CG, Cherry BM, Fisher A. Do patients with cellulitis need to be hospitalized? a systematic review and meta-analysis of mortality rates of inpatients with cellulitis. J Gen Intern Med. 2018;33:1553-1560.
- Ko LN, Garza-Mayers AC, St. John J, et al. Effect of dermatology consultation on outcomes for patients with presumed cellulitis: a randomized clinical trial. JAMA Dermatol. 2018;154:529-536.
- David CV, Chira S, Eells SJ, et al. Diagnostic accuracy in patients admitted to hospitals with cellulitis. Dermatol Online J. 2011;17:1.
- Hughey LC. The impact dermatologists can have on misdiagnosis of cellulitis and overuse of antibiotics: closing the gap. JAMA Dermatol. 2014;150:1061-1062.
- Stevens DL, Bisno AL, Chambers HF, et al. Practice guidelines for the diagnosis and management of skin and soft tissue infections: 2014 update by the Infectious Diseases Society of America. Clin Infect Dis. 2014;59:147-159.
- Björnsdóttir S, Gottfredsson M, Thórisdóttir AS, et al. Risk factors for acute cellulitis of the lower limb: a prospective case-control study. Clin Infect Dis. 2005;41:1416-1422.
- Roujeau JC, Sigurgeirsson B, Korting HC, et al. Chronic dermatomycoses of the foot as risk factors for acute bacterial cellulitis of the leg: a case-control study. Dermatology. 2004;209:301-307.
- McNamara DR, Tleyjeh IM, Berbari EF, et al. A predictive model of recurrent lower extremity cellulitis in a population-based cohort. Arch Intern Med. 2007;167:709-715.
- Yadav K, Suh KN, Eagles D, et al. Predictors of oral antibiotic treatment failure for nonpurulent skin and soft tissue infections in the emergency department. Acad Emerg Med. 2019;26:51-59.
- Peterson D, McLeod S, Woolfrey K, et al. Predictors of failure of empiric outpatient antibiotic therapy in emergency department patients with uncomplicated cellulitis. Acad Emerg Med. 2014;21:526-531.
- Volz KA, Canham L, Kaplan E, et al. Identifying patients with cellulitis who are likely to require inpatient admission after a stay in an ED observation unit. Am J Emerg Med. 2013;31:360-364.
- Chester JG, Rudolph JL. Vital signs in older patients: age-related changes. J Am Med Dir Assoc. 2011;12:337-343.
Cellulitis is an infection of the skin and skin-associated structures characterized by redness, warmth, swelling, and pain of the affected area. Cellulitis most commonly occurs in middle-aged and older adults and frequently affects the lower extremities.1 Serious complications of cellulitis such as bacteremia, metastatic infection, and sepsis are rare, and most cases of cellulitis in patients with normal vital signs and mental status can be managed with outpatient treatment.2
Diagnosis of cellulitis can be confounded by a number of similarly presenting conditions collectively known as pseudocellulitis, such as venous stasis dermatitis and deep vein thrombosis.1 Misdiagnosis of cellulitis is common, with rates exceeding 30% among hospitalized patients initially diagnosed with cellulitis.3,4 Dermatology or infectious disease assessment is considered the diagnostic gold standard for cellulitis4,5 but is not always readily available, especially in resource-constrained settings.
Most cases of uncomplicated cellulitis can be managed with outpatient treatment, especially because serious complications are rare. Frequent misdiagnosis leads to repeat or unnecessary hospitalization and antibiosis. Exceptions necessitating hospitalization usually are predicated on signs of systemic infection, severe immunocompromised states, or failure of prior outpatient therapy.6 Such presentations can be distinguished by corresponding notable historical or examination factors, such as vital sign abnormalities suggesting systemic infection or history of malignancy leading to an immunocompromised state.
We sought to evaluate factors leading to the diagnosis of cellulitis in a cohort of patients with uncomplicated presentations receiving dermatology consultation to emphasize findings indicative of cellulitis in the absence of clinical or historical factors suggestive of other conditions necessitating hospitalization, such as systemic infection.
Methods
Study Participants—A prospective cohort study of patients presenting to an emergency department (ED) between October 2012 and January 2017 at an urban academic medical center in Boston, Massachusetts, was conducted with approval of study design and procedures by the relevant institutional review board. Patients older than 18 years were eligible for inclusion if given an initial diagnosis of cellulitis by an ED physician. Patients were excluded if incarcerated, pregnant, or unable to provide informed consent. Other exclusion criteria includedinfections overlying temporary or permanent indwelling hardware, animal or human bites, or sites of recent surgery (within the prior 4 weeks); preceding antibiotic treatment for more than 24 hours; or clinical or radiographic evidence of complications requiring alternative management such as osteomyelitis or abscess. Patients presenting with an elevated heart rate (>100 beats per minute) or body temperature (>100.5 °F [38.1 °C]) also were excluded. Eligible patients were enrolled upon providing written informed consent, and no remuneration was offered for participation.
Dermatology Consultation Intervention—A random subset of enrolled patients received dermatology consultation within 24 hours of presentation. Consultation consisted of a patient interview and physical examination with care recommendations to relevant ED and inpatient teams. Consultations confirmed the presence or absence of cellulitis as the primary outcome and also noted the presence of any pseudocellulitis diagnoses either occurring concomitantly with or mimicking cellulitis as a secondary outcome.
Statistical Analysis—Patient characteristics were analyzed to identify factors independently associated with the diagnosis of cellulitis in cases affecting the lower extremities. Factors were recorded with categorical variables reported as counts and percentages and continuous variables as means and standard deviations. Univariate analyses between categorical variables or discretized continuous variables and cellulitis diagnosis were conducted via Fisher exact test to identify a preliminary set of potential risk factors. Continuous variables were discretized at multiple incremental values with the discretization most significantly associated with cellulitis diagnosis selected as a preliminary risk factor. Multivariate analyses involved using any objective preliminary factor meeting a significance threshold of P<.1 in univariate comparisons in a multivariate logistic regression model for prediction of cellulitis diagnosis with corresponding calculation of odds ratios with confidence intervals and receiver operating characteristic. Factors with confidence intervals that excluded 1 were considered significant independent predictors of cellulitis. Analyses were performed using Python version 3.8 (Python Software Foundation).
Results
Of 1359 patients screened for eligibility, 104 patients with presumed lower extremity cellulitis undergoing dermatology consultation were included in this study (Figure). The mean patient age (SD) was 60.4 (19.2) years, and 63.5% of patients were male. In the study population, 63 (60.6%) patients received a final diagnosis of cellulitis. The most common pseudocellulitis diagnosis identified was venous stasis dermatitis, which occurred in 12 (11.5%) patients with concomitant cellulitis and in 12 (11.5%) patients mimicking cellulitis (Table).

Univariate comparisons revealed a diverse set of historical, examination, and laboratory factors associated with cellulitis diagnosis. Diagnosis of cellulitis was associated with unilateral presentation, recent trauma to the affected site, and history of cellulitis or onychomycosis. Diagnosis of cellulitis also was associated with elevated white blood cell count, absolute neutrophil count, C-reactive protein, body mass index, hematocrit, and platelet count; age less than 75 years; and lower serum sodium and serum chloride levels. These were the independent factors included in the multivariate analysis, which consisted of a logistic regression model for prediction of cellulitis (eTable).

Multivariate logistic regression on all preliminary factors significantly associated with cellulitis diagnosis in univariate comparisons demonstrated leukocytosis, which was defined as having a white blood cell count exceeding 11,000/μL, unilateral presentation, history of onychomycosis, and trauma to the affected site as significant independent predictors of cellulitis diagnosis; history of cellulitis approached significance (eTable). Unilateral presentation and leukocytosis were the strongest predictors; having either of these factors had a sensitivity of 93.7% and a negative predictive value of 76.5%.

Comment
Importance of Identifying Pseudocellulitis—Successful diagnosis of cellulitis can be confounded by pseudocellulitis that can present concomitantly with or in lieu of cellulitis itself. Although cellulitis mostly affects the lower extremities in adults, pseudocellulitis also was common in this study population of patients with suspected lower extremity cellulitis, occurring both as a mimicker and concomitantly with cellulitis with substantial frequency. Notably, among patients with both venous stasis dermatitis and cellulitis diagnosed, most patients (n=10/12; 83.3%) had unilateral presentations of cellulitis as evidenced by signs and symptoms more notably affecting one lower extremity than the other. These findings suggest that certain pseudocellulitis diagnoses may predispose patients to cellulitis by disrupting the skin barrier, leading to bacterial infiltration; however, these pseudocellulitis diagnoses typically affect both lower extremities equally,1 and asymmetric involvement suggests the presence of overlying cellulitis. Furthermore, the most common pseudocellulitis entities found, such as venous stasis dermatitis, hematoma, and eczema, do not benefit from antibiotic treatment and require alternative therapy.1 Successful discrimination of these pseudocellulitis entities is critical to bolster proper antibiotic stewardship and discourage unnecessary hospitalization.
Independent Predictors of Cellulitis—Unilateral presentation and leukocytosis each emerged as strong independent predictors of cellulitis diagnosis in this study. Having either of these factors furthermore demonstrated high sensitivity and negative predictive value for cellulitis diagnosis. Other notable risk factors were history of onychomycosis, cellulitis, and trauma to the affected site. Prior studies have identified similar historical factors as predisposing patients to cellulitis.7-9 Interestingly, warmth of the affected area on physical examination emerged as strongly associated with cellulitis but was not included in the final predictive model because of its subjective determination. These factors may be especially important in diagnosing cellulitis in patients without concerning vital signs and with concomitant or prior pseudocellulitis.
Study Limitations—This study was limited to patients with uncomplicated presentations to emphasize discrimination of factors associated with cellulitis in the absence of suggestive signs of infection, such as vital sign abnormalities. Signs such as fever and tachypnea have been previously correlated to outpatient treatment failure and necessity for hospitalization.10-12 This study instead focused on patients without concerning vital signs to reduce confounding by such factors in more severe presentations that heighten suspicion for infection and increase likelihood of additional treatment measures. For such patients, suggestive historical factors, such as those discovered in this study, should be considered instead. Interestingly, increased age did not emerge as a significant predictor in this population in contrast to other predictive models that included patients with vital sign abnormalities. Notably, older patients tend to have more variable vital signs, especially in response to physiologic stressors such as infection.13 As such, age may serve as a proxy for vital sign abnormalities to some degree in such predictive models, leading to heightened suspicion for infection in older patients. This study demonstrated that in the absence of concerning vital signs, historical rather than demographic factors are more predictive of cellulitis.
Conclusion
Unilateral presentation and leukocytosis emerged as strong independent predictors of lower extremity cellulitis in patients with uncomplicated presentations. Having either of these factors had a sensitivity of 93.7% and a negative predictive value of 76.5%. Other factors such as history of cellulitis, onychomycosis, and recent trauma to the affected site emerged as additional predictors. These historical, examination, and laboratory characteristics may be especially useful for successful diagnosis of cellulitis in varied practice settings, including outpatient clinics and EDs.
Cellulitis is an infection of the skin and skin-associated structures characterized by redness, warmth, swelling, and pain of the affected area. Cellulitis most commonly occurs in middle-aged and older adults and frequently affects the lower extremities.1 Serious complications of cellulitis such as bacteremia, metastatic infection, and sepsis are rare, and most cases of cellulitis in patients with normal vital signs and mental status can be managed with outpatient treatment.2
Diagnosis of cellulitis can be confounded by a number of similarly presenting conditions collectively known as pseudocellulitis, such as venous stasis dermatitis and deep vein thrombosis.1 Misdiagnosis of cellulitis is common, with rates exceeding 30% among hospitalized patients initially diagnosed with cellulitis.3,4 Dermatology or infectious disease assessment is considered the diagnostic gold standard for cellulitis4,5 but is not always readily available, especially in resource-constrained settings.
Most cases of uncomplicated cellulitis can be managed with outpatient treatment, especially because serious complications are rare. Frequent misdiagnosis leads to repeat or unnecessary hospitalization and antibiosis. Exceptions necessitating hospitalization usually are predicated on signs of systemic infection, severe immunocompromised states, or failure of prior outpatient therapy.6 Such presentations can be distinguished by corresponding notable historical or examination factors, such as vital sign abnormalities suggesting systemic infection or history of malignancy leading to an immunocompromised state.
We sought to evaluate factors leading to the diagnosis of cellulitis in a cohort of patients with uncomplicated presentations receiving dermatology consultation to emphasize findings indicative of cellulitis in the absence of clinical or historical factors suggestive of other conditions necessitating hospitalization, such as systemic infection.
Methods
Study Participants—A prospective cohort study of patients presenting to an emergency department (ED) between October 2012 and January 2017 at an urban academic medical center in Boston, Massachusetts, was conducted with approval of study design and procedures by the relevant institutional review board. Patients older than 18 years were eligible for inclusion if given an initial diagnosis of cellulitis by an ED physician. Patients were excluded if incarcerated, pregnant, or unable to provide informed consent. Other exclusion criteria includedinfections overlying temporary or permanent indwelling hardware, animal or human bites, or sites of recent surgery (within the prior 4 weeks); preceding antibiotic treatment for more than 24 hours; or clinical or radiographic evidence of complications requiring alternative management such as osteomyelitis or abscess. Patients presenting with an elevated heart rate (>100 beats per minute) or body temperature (>100.5 °F [38.1 °C]) also were excluded. Eligible patients were enrolled upon providing written informed consent, and no remuneration was offered for participation.
Dermatology Consultation Intervention—A random subset of enrolled patients received dermatology consultation within 24 hours of presentation. Consultation consisted of a patient interview and physical examination with care recommendations to relevant ED and inpatient teams. Consultations confirmed the presence or absence of cellulitis as the primary outcome and also noted the presence of any pseudocellulitis diagnoses either occurring concomitantly with or mimicking cellulitis as a secondary outcome.
Statistical Analysis—Patient characteristics were analyzed to identify factors independently associated with the diagnosis of cellulitis in cases affecting the lower extremities. Factors were recorded with categorical variables reported as counts and percentages and continuous variables as means and standard deviations. Univariate analyses between categorical variables or discretized continuous variables and cellulitis diagnosis were conducted via Fisher exact test to identify a preliminary set of potential risk factors. Continuous variables were discretized at multiple incremental values with the discretization most significantly associated with cellulitis diagnosis selected as a preliminary risk factor. Multivariate analyses involved using any objective preliminary factor meeting a significance threshold of P<.1 in univariate comparisons in a multivariate logistic regression model for prediction of cellulitis diagnosis with corresponding calculation of odds ratios with confidence intervals and receiver operating characteristic. Factors with confidence intervals that excluded 1 were considered significant independent predictors of cellulitis. Analyses were performed using Python version 3.8 (Python Software Foundation).
Results
Of 1359 patients screened for eligibility, 104 patients with presumed lower extremity cellulitis undergoing dermatology consultation were included in this study (Figure). The mean patient age (SD) was 60.4 (19.2) years, and 63.5% of patients were male. In the study population, 63 (60.6%) patients received a final diagnosis of cellulitis. The most common pseudocellulitis diagnosis identified was venous stasis dermatitis, which occurred in 12 (11.5%) patients with concomitant cellulitis and in 12 (11.5%) patients mimicking cellulitis (Table).

Univariate comparisons revealed a diverse set of historical, examination, and laboratory factors associated with cellulitis diagnosis. Diagnosis of cellulitis was associated with unilateral presentation, recent trauma to the affected site, and history of cellulitis or onychomycosis. Diagnosis of cellulitis also was associated with elevated white blood cell count, absolute neutrophil count, C-reactive protein, body mass index, hematocrit, and platelet count; age less than 75 years; and lower serum sodium and serum chloride levels. These were the independent factors included in the multivariate analysis, which consisted of a logistic regression model for prediction of cellulitis (eTable).

Multivariate logistic regression on all preliminary factors significantly associated with cellulitis diagnosis in univariate comparisons demonstrated leukocytosis, which was defined as having a white blood cell count exceeding 11,000/μL, unilateral presentation, history of onychomycosis, and trauma to the affected site as significant independent predictors of cellulitis diagnosis; history of cellulitis approached significance (eTable). Unilateral presentation and leukocytosis were the strongest predictors; having either of these factors had a sensitivity of 93.7% and a negative predictive value of 76.5%.

Comment
Importance of Identifying Pseudocellulitis—Successful diagnosis of cellulitis can be confounded by pseudocellulitis that can present concomitantly with or in lieu of cellulitis itself. Although cellulitis mostly affects the lower extremities in adults, pseudocellulitis also was common in this study population of patients with suspected lower extremity cellulitis, occurring both as a mimicker and concomitantly with cellulitis with substantial frequency. Notably, among patients with both venous stasis dermatitis and cellulitis diagnosed, most patients (n=10/12; 83.3%) had unilateral presentations of cellulitis as evidenced by signs and symptoms more notably affecting one lower extremity than the other. These findings suggest that certain pseudocellulitis diagnoses may predispose patients to cellulitis by disrupting the skin barrier, leading to bacterial infiltration; however, these pseudocellulitis diagnoses typically affect both lower extremities equally,1 and asymmetric involvement suggests the presence of overlying cellulitis. Furthermore, the most common pseudocellulitis entities found, such as venous stasis dermatitis, hematoma, and eczema, do not benefit from antibiotic treatment and require alternative therapy.1 Successful discrimination of these pseudocellulitis entities is critical to bolster proper antibiotic stewardship and discourage unnecessary hospitalization.
Independent Predictors of Cellulitis—Unilateral presentation and leukocytosis each emerged as strong independent predictors of cellulitis diagnosis in this study. Having either of these factors furthermore demonstrated high sensitivity and negative predictive value for cellulitis diagnosis. Other notable risk factors were history of onychomycosis, cellulitis, and trauma to the affected site. Prior studies have identified similar historical factors as predisposing patients to cellulitis.7-9 Interestingly, warmth of the affected area on physical examination emerged as strongly associated with cellulitis but was not included in the final predictive model because of its subjective determination. These factors may be especially important in diagnosing cellulitis in patients without concerning vital signs and with concomitant or prior pseudocellulitis.
Study Limitations—This study was limited to patients with uncomplicated presentations to emphasize discrimination of factors associated with cellulitis in the absence of suggestive signs of infection, such as vital sign abnormalities. Signs such as fever and tachypnea have been previously correlated to outpatient treatment failure and necessity for hospitalization.10-12 This study instead focused on patients without concerning vital signs to reduce confounding by such factors in more severe presentations that heighten suspicion for infection and increase likelihood of additional treatment measures. For such patients, suggestive historical factors, such as those discovered in this study, should be considered instead. Interestingly, increased age did not emerge as a significant predictor in this population in contrast to other predictive models that included patients with vital sign abnormalities. Notably, older patients tend to have more variable vital signs, especially in response to physiologic stressors such as infection.13 As such, age may serve as a proxy for vital sign abnormalities to some degree in such predictive models, leading to heightened suspicion for infection in older patients. This study demonstrated that in the absence of concerning vital signs, historical rather than demographic factors are more predictive of cellulitis.
Conclusion
Unilateral presentation and leukocytosis emerged as strong independent predictors of lower extremity cellulitis in patients with uncomplicated presentations. Having either of these factors had a sensitivity of 93.7% and a negative predictive value of 76.5%. Other factors such as history of cellulitis, onychomycosis, and recent trauma to the affected site emerged as additional predictors. These historical, examination, and laboratory characteristics may be especially useful for successful diagnosis of cellulitis in varied practice settings, including outpatient clinics and EDs.
- Raff AB, Kroshinsky D. Cellulitis: a review. JAMA. 2016;316:325-337.
- Gunderson CG, Cherry BM, Fisher A. Do patients with cellulitis need to be hospitalized? a systematic review and meta-analysis of mortality rates of inpatients with cellulitis. J Gen Intern Med. 2018;33:1553-1560.
- Ko LN, Garza-Mayers AC, St. John J, et al. Effect of dermatology consultation on outcomes for patients with presumed cellulitis: a randomized clinical trial. JAMA Dermatol. 2018;154:529-536.
- David CV, Chira S, Eells SJ, et al. Diagnostic accuracy in patients admitted to hospitals with cellulitis. Dermatol Online J. 2011;17:1.
- Hughey LC. The impact dermatologists can have on misdiagnosis of cellulitis and overuse of antibiotics: closing the gap. JAMA Dermatol. 2014;150:1061-1062.
- Stevens DL, Bisno AL, Chambers HF, et al. Practice guidelines for the diagnosis and management of skin and soft tissue infections: 2014 update by the Infectious Diseases Society of America. Clin Infect Dis. 2014;59:147-159.
- Björnsdóttir S, Gottfredsson M, Thórisdóttir AS, et al. Risk factors for acute cellulitis of the lower limb: a prospective case-control study. Clin Infect Dis. 2005;41:1416-1422.
- Roujeau JC, Sigurgeirsson B, Korting HC, et al. Chronic dermatomycoses of the foot as risk factors for acute bacterial cellulitis of the leg: a case-control study. Dermatology. 2004;209:301-307.
- McNamara DR, Tleyjeh IM, Berbari EF, et al. A predictive model of recurrent lower extremity cellulitis in a population-based cohort. Arch Intern Med. 2007;167:709-715.
- Yadav K, Suh KN, Eagles D, et al. Predictors of oral antibiotic treatment failure for nonpurulent skin and soft tissue infections in the emergency department. Acad Emerg Med. 2019;26:51-59.
- Peterson D, McLeod S, Woolfrey K, et al. Predictors of failure of empiric outpatient antibiotic therapy in emergency department patients with uncomplicated cellulitis. Acad Emerg Med. 2014;21:526-531.
- Volz KA, Canham L, Kaplan E, et al. Identifying patients with cellulitis who are likely to require inpatient admission after a stay in an ED observation unit. Am J Emerg Med. 2013;31:360-364.
- Chester JG, Rudolph JL. Vital signs in older patients: age-related changes. J Am Med Dir Assoc. 2011;12:337-343.
- Raff AB, Kroshinsky D. Cellulitis: a review. JAMA. 2016;316:325-337.
- Gunderson CG, Cherry BM, Fisher A. Do patients with cellulitis need to be hospitalized? a systematic review and meta-analysis of mortality rates of inpatients with cellulitis. J Gen Intern Med. 2018;33:1553-1560.
- Ko LN, Garza-Mayers AC, St. John J, et al. Effect of dermatology consultation on outcomes for patients with presumed cellulitis: a randomized clinical trial. JAMA Dermatol. 2018;154:529-536.
- David CV, Chira S, Eells SJ, et al. Diagnostic accuracy in patients admitted to hospitals with cellulitis. Dermatol Online J. 2011;17:1.
- Hughey LC. The impact dermatologists can have on misdiagnosis of cellulitis and overuse of antibiotics: closing the gap. JAMA Dermatol. 2014;150:1061-1062.
- Stevens DL, Bisno AL, Chambers HF, et al. Practice guidelines for the diagnosis and management of skin and soft tissue infections: 2014 update by the Infectious Diseases Society of America. Clin Infect Dis. 2014;59:147-159.
- Björnsdóttir S, Gottfredsson M, Thórisdóttir AS, et al. Risk factors for acute cellulitis of the lower limb: a prospective case-control study. Clin Infect Dis. 2005;41:1416-1422.
- Roujeau JC, Sigurgeirsson B, Korting HC, et al. Chronic dermatomycoses of the foot as risk factors for acute bacterial cellulitis of the leg: a case-control study. Dermatology. 2004;209:301-307.
- McNamara DR, Tleyjeh IM, Berbari EF, et al. A predictive model of recurrent lower extremity cellulitis in a population-based cohort. Arch Intern Med. 2007;167:709-715.
- Yadav K, Suh KN, Eagles D, et al. Predictors of oral antibiotic treatment failure for nonpurulent skin and soft tissue infections in the emergency department. Acad Emerg Med. 2019;26:51-59.
- Peterson D, McLeod S, Woolfrey K, et al. Predictors of failure of empiric outpatient antibiotic therapy in emergency department patients with uncomplicated cellulitis. Acad Emerg Med. 2014;21:526-531.
- Volz KA, Canham L, Kaplan E, et al. Identifying patients with cellulitis who are likely to require inpatient admission after a stay in an ED observation unit. Am J Emerg Med. 2013;31:360-364.
- Chester JG, Rudolph JL. Vital signs in older patients: age-related changes. J Am Med Dir Assoc. 2011;12:337-343.
Practice Points
- Unilateral involvement and leukocytosis are both highly predictive of lower extremity cellulitis in uncomplicated presentations.
- Historical factors such as history of onychomycosis and trauma to the affected site are more predictive of lower extremity cellulitis than demographic factors such as age in uncomplicated presentations of cellulitis.
Health Literacy in Dermatology Patients: How to Level the Playing Field
Health literacy is a multifaceted construct that encompasses the knowledge of health and health systems, utilization of information related to health, and ability to maintain health.1 Low health literacy impairs health outcomes, disproportionately affecting socioeconomically disadvantaged populations, including racial minorities and the older population. Consistently, it is associated with fewer vaccinations and screenings, higher health care utilization, and poorer ability to take medications or interpret health information.2
With growing utilization of the Internet for health information,3 much patient education now occurs outside the clinic. Differential utilization of the Internet can exacerbate disparities in health outcomes: people with a lower family income more frequently engage in health information and dialogue online.3 Despite opportunities to improve literacy and narrow gaps in care, a lack of awareness, advocacy, and funding limit patient- and community-based initiatives. Herein, we discuss health literacy challenges in dermatology, offer potential solutions, and propose ways that stakeholders can prioritize health literacy advocacy to improve outcomes.
The Importance of Health Literacy in Dermatology
Dermatology patients often face challenges that demand greater health literacy. Active participation in health promotion, protection, and maintenance can remarkably improve outcomes. When patients understand disease pathogenesis and the rationale behind treatment choices, adherence to a treatment regimen might improve.
However, understanding dermatologic diseases and disorders can be challenging. First, many are chronic inflammatory conditions that require intricate treatment regimens. Second, the complexity of those diseases and disorders continues to grow in the era of new research and unprecedented expansion of treatment options.
For chronic conditions that require ongoing complex management, researchers have developed advanced patient tools. For instance, the eczema action plan helps atopic dermatitis patients manage conditions from home.4 However, patients with greater literacy and the ability to participate will better utilize such tools and have fewer uncontrolled flares. Patient tools meant to improve outcomes might, instead, widen gaps in care. Even with nonchronic conditions, such as nonmelanoma skin cancer, continued awareness and the need for preventive care, timely diagnosis, and appropriate intervention remain critical.
Limited Accessibility of Patient Education Materials
Patient education in dermatology occurs through several formats. Because online health resources are more readily available to those with less access to health care, the potential for such resources to narrow health disparities is immense. However, online resources have not adequately taken advantage of the opportunity to make health information openly accessible to its users. The readability of online patient education materials on a large expanse of dermatologic conditions is far too advanced.5 The readability level of some resources is as high as 17th grade (graduate school), which is much higher than the American Medical Association recommendation6 that patient education materials be presented at a 6th-grade level or less. Furthermore, the quality and comprehensiveness of content is highly variable. Rather than serving as an equalizer, the Internet may widen the gap as low health literacy continues to impair the accessibility of health information.
Solutions to Level the Playing Field
What can be done to increase the readability of patient education materials? Leveling the playing field begins with creating materials at an appropriate readability level, including online content, printed handouts, and after-visit summaries in the clinic. Writers of patient education materials should be cognizant of their choice of language and routinely use a free readability checker (https://readabilityformulas.com). Patient education materials should reflect the American Medical Association’s recommended 6th-grade level. Creators should maintain a high standard of quality and comprehensiveness; prior studies note no inverse correlation between readability and quality.5 In the age of multimedia presentation, non–print-based materials can be explored, such as audio or video for online content, podcasts, and webinars. Providers also should take the opportunity to be mindful of health literacy in clinic. Beyond assessing the readability of written resources for a patient, assessing that patient’s health literacy and tailoring one’s language will maximize engagement.
Systemic Change Is Needed
Ultimately, systemic change is needed to address the root causes of health literacy disparity, requiring advocacy for social welfare, public health, and public policy initiatives. In recognizing existing efforts, such as community outreach teams and hospital committees to evaluate health literacy materials, numerous barriers remain. Despite the notable impact of health literacy on health outcomes, there is a lack of advocacy and funds to conduct health literacy–related work.7 Because dermatologists provide holistic care and remain mindful of patients’ health literacy in the clinic, they should continue to advocate for increased awareness, improved funding, and support for local and federal initiatives.
Final Thoughts
With more opportunities to narrow gaps in care, it is more pertinent than ever to acknowledge the impact of health literacy on dermatology outcomes. Leveling the playing field begins with (1) an awareness of health literacy and (2) creating readable and comprehensible patient education content. Greater advocacy from community and professional organizations; increased funding from nonprofit organizations, industry, and federal institutions; and increased involvement by dermatologists in bringing greater attention to health literacy will improve outcomes in dermatology.
- Liu C, Wang D, Liu C, et al. What is the meaning of health literacy? a systematic review and qualitative synthesis. Fam Med Community Health. 2020;8:e000351. doi:10.1136/fmch-2020-000351
- Berkman ND, Sheridan SL, Donahue KE, et al. Low health literacy and health outcomes: an updated systematic review. Ann Intern Med. 2011;155:97-107. doi:10.7326/0003-4819-155-2-201107190-00005
- Rice RE. Influences, usage, and outcomes of Internet health information searching: multivariate results from the Pew surveys. Int J Med Inform. 2006;75:8-28. doi:10.1016/j.ijmedinf.2005.07.032
- Brown J, Weitz NW, Liang A, et al. Does an eczema action plan improve atopic dermatitis? a single-site randomized controlled trial. Clin Pediatr (Phila). 2018;57:1624-1629. doi:10.1177/0009922818795906
- De DR, Shih T, Katta R, et al. Readability, quality, and timeliness of patient online health resources for contact dermatitis and patch testing. Dermatitis. 2022;33:155-160. doi:10.1097/DER.0000000000000789
- Weiss BD. Health Literacy: A Manual for Clinicians. American Medical Association, American Medical Foundation; 2003.
- Nutbeam D, McGill B, Premkumar P. Improving health literacy in community populations: a review of progress. Health Promot Int. 2018;33:901-911. doi:10.1093/heapro/dax015
Health literacy is a multifaceted construct that encompasses the knowledge of health and health systems, utilization of information related to health, and ability to maintain health.1 Low health literacy impairs health outcomes, disproportionately affecting socioeconomically disadvantaged populations, including racial minorities and the older population. Consistently, it is associated with fewer vaccinations and screenings, higher health care utilization, and poorer ability to take medications or interpret health information.2
With growing utilization of the Internet for health information,3 much patient education now occurs outside the clinic. Differential utilization of the Internet can exacerbate disparities in health outcomes: people with a lower family income more frequently engage in health information and dialogue online.3 Despite opportunities to improve literacy and narrow gaps in care, a lack of awareness, advocacy, and funding limit patient- and community-based initiatives. Herein, we discuss health literacy challenges in dermatology, offer potential solutions, and propose ways that stakeholders can prioritize health literacy advocacy to improve outcomes.
The Importance of Health Literacy in Dermatology
Dermatology patients often face challenges that demand greater health literacy. Active participation in health promotion, protection, and maintenance can remarkably improve outcomes. When patients understand disease pathogenesis and the rationale behind treatment choices, adherence to a treatment regimen might improve.
However, understanding dermatologic diseases and disorders can be challenging. First, many are chronic inflammatory conditions that require intricate treatment regimens. Second, the complexity of those diseases and disorders continues to grow in the era of new research and unprecedented expansion of treatment options.
For chronic conditions that require ongoing complex management, researchers have developed advanced patient tools. For instance, the eczema action plan helps atopic dermatitis patients manage conditions from home.4 However, patients with greater literacy and the ability to participate will better utilize such tools and have fewer uncontrolled flares. Patient tools meant to improve outcomes might, instead, widen gaps in care. Even with nonchronic conditions, such as nonmelanoma skin cancer, continued awareness and the need for preventive care, timely diagnosis, and appropriate intervention remain critical.
Limited Accessibility of Patient Education Materials
Patient education in dermatology occurs through several formats. Because online health resources are more readily available to those with less access to health care, the potential for such resources to narrow health disparities is immense. However, online resources have not adequately taken advantage of the opportunity to make health information openly accessible to its users. The readability of online patient education materials on a large expanse of dermatologic conditions is far too advanced.5 The readability level of some resources is as high as 17th grade (graduate school), which is much higher than the American Medical Association recommendation6 that patient education materials be presented at a 6th-grade level or less. Furthermore, the quality and comprehensiveness of content is highly variable. Rather than serving as an equalizer, the Internet may widen the gap as low health literacy continues to impair the accessibility of health information.
Solutions to Level the Playing Field
What can be done to increase the readability of patient education materials? Leveling the playing field begins with creating materials at an appropriate readability level, including online content, printed handouts, and after-visit summaries in the clinic. Writers of patient education materials should be cognizant of their choice of language and routinely use a free readability checker (https://readabilityformulas.com). Patient education materials should reflect the American Medical Association’s recommended 6th-grade level. Creators should maintain a high standard of quality and comprehensiveness; prior studies note no inverse correlation between readability and quality.5 In the age of multimedia presentation, non–print-based materials can be explored, such as audio or video for online content, podcasts, and webinars. Providers also should take the opportunity to be mindful of health literacy in clinic. Beyond assessing the readability of written resources for a patient, assessing that patient’s health literacy and tailoring one’s language will maximize engagement.
Systemic Change Is Needed
Ultimately, systemic change is needed to address the root causes of health literacy disparity, requiring advocacy for social welfare, public health, and public policy initiatives. In recognizing existing efforts, such as community outreach teams and hospital committees to evaluate health literacy materials, numerous barriers remain. Despite the notable impact of health literacy on health outcomes, there is a lack of advocacy and funds to conduct health literacy–related work.7 Because dermatologists provide holistic care and remain mindful of patients’ health literacy in the clinic, they should continue to advocate for increased awareness, improved funding, and support for local and federal initiatives.
Final Thoughts
With more opportunities to narrow gaps in care, it is more pertinent than ever to acknowledge the impact of health literacy on dermatology outcomes. Leveling the playing field begins with (1) an awareness of health literacy and (2) creating readable and comprehensible patient education content. Greater advocacy from community and professional organizations; increased funding from nonprofit organizations, industry, and federal institutions; and increased involvement by dermatologists in bringing greater attention to health literacy will improve outcomes in dermatology.
Health literacy is a multifaceted construct that encompasses the knowledge of health and health systems, utilization of information related to health, and ability to maintain health.1 Low health literacy impairs health outcomes, disproportionately affecting socioeconomically disadvantaged populations, including racial minorities and the older population. Consistently, it is associated with fewer vaccinations and screenings, higher health care utilization, and poorer ability to take medications or interpret health information.2
With growing utilization of the Internet for health information,3 much patient education now occurs outside the clinic. Differential utilization of the Internet can exacerbate disparities in health outcomes: people with a lower family income more frequently engage in health information and dialogue online.3 Despite opportunities to improve literacy and narrow gaps in care, a lack of awareness, advocacy, and funding limit patient- and community-based initiatives. Herein, we discuss health literacy challenges in dermatology, offer potential solutions, and propose ways that stakeholders can prioritize health literacy advocacy to improve outcomes.
The Importance of Health Literacy in Dermatology
Dermatology patients often face challenges that demand greater health literacy. Active participation in health promotion, protection, and maintenance can remarkably improve outcomes. When patients understand disease pathogenesis and the rationale behind treatment choices, adherence to a treatment regimen might improve.
However, understanding dermatologic diseases and disorders can be challenging. First, many are chronic inflammatory conditions that require intricate treatment regimens. Second, the complexity of those diseases and disorders continues to grow in the era of new research and unprecedented expansion of treatment options.
For chronic conditions that require ongoing complex management, researchers have developed advanced patient tools. For instance, the eczema action plan helps atopic dermatitis patients manage conditions from home.4 However, patients with greater literacy and the ability to participate will better utilize such tools and have fewer uncontrolled flares. Patient tools meant to improve outcomes might, instead, widen gaps in care. Even with nonchronic conditions, such as nonmelanoma skin cancer, continued awareness and the need for preventive care, timely diagnosis, and appropriate intervention remain critical.
Limited Accessibility of Patient Education Materials
Patient education in dermatology occurs through several formats. Because online health resources are more readily available to those with less access to health care, the potential for such resources to narrow health disparities is immense. However, online resources have not adequately taken advantage of the opportunity to make health information openly accessible to its users. The readability of online patient education materials on a large expanse of dermatologic conditions is far too advanced.5 The readability level of some resources is as high as 17th grade (graduate school), which is much higher than the American Medical Association recommendation6 that patient education materials be presented at a 6th-grade level or less. Furthermore, the quality and comprehensiveness of content is highly variable. Rather than serving as an equalizer, the Internet may widen the gap as low health literacy continues to impair the accessibility of health information.
Solutions to Level the Playing Field
What can be done to increase the readability of patient education materials? Leveling the playing field begins with creating materials at an appropriate readability level, including online content, printed handouts, and after-visit summaries in the clinic. Writers of patient education materials should be cognizant of their choice of language and routinely use a free readability checker (https://readabilityformulas.com). Patient education materials should reflect the American Medical Association’s recommended 6th-grade level. Creators should maintain a high standard of quality and comprehensiveness; prior studies note no inverse correlation between readability and quality.5 In the age of multimedia presentation, non–print-based materials can be explored, such as audio or video for online content, podcasts, and webinars. Providers also should take the opportunity to be mindful of health literacy in clinic. Beyond assessing the readability of written resources for a patient, assessing that patient’s health literacy and tailoring one’s language will maximize engagement.
Systemic Change Is Needed
Ultimately, systemic change is needed to address the root causes of health literacy disparity, requiring advocacy for social welfare, public health, and public policy initiatives. In recognizing existing efforts, such as community outreach teams and hospital committees to evaluate health literacy materials, numerous barriers remain. Despite the notable impact of health literacy on health outcomes, there is a lack of advocacy and funds to conduct health literacy–related work.7 Because dermatologists provide holistic care and remain mindful of patients’ health literacy in the clinic, they should continue to advocate for increased awareness, improved funding, and support for local and federal initiatives.
Final Thoughts
With more opportunities to narrow gaps in care, it is more pertinent than ever to acknowledge the impact of health literacy on dermatology outcomes. Leveling the playing field begins with (1) an awareness of health literacy and (2) creating readable and comprehensible patient education content. Greater advocacy from community and professional organizations; increased funding from nonprofit organizations, industry, and federal institutions; and increased involvement by dermatologists in bringing greater attention to health literacy will improve outcomes in dermatology.
- Liu C, Wang D, Liu C, et al. What is the meaning of health literacy? a systematic review and qualitative synthesis. Fam Med Community Health. 2020;8:e000351. doi:10.1136/fmch-2020-000351
- Berkman ND, Sheridan SL, Donahue KE, et al. Low health literacy and health outcomes: an updated systematic review. Ann Intern Med. 2011;155:97-107. doi:10.7326/0003-4819-155-2-201107190-00005
- Rice RE. Influences, usage, and outcomes of Internet health information searching: multivariate results from the Pew surveys. Int J Med Inform. 2006;75:8-28. doi:10.1016/j.ijmedinf.2005.07.032
- Brown J, Weitz NW, Liang A, et al. Does an eczema action plan improve atopic dermatitis? a single-site randomized controlled trial. Clin Pediatr (Phila). 2018;57:1624-1629. doi:10.1177/0009922818795906
- De DR, Shih T, Katta R, et al. Readability, quality, and timeliness of patient online health resources for contact dermatitis and patch testing. Dermatitis. 2022;33:155-160. doi:10.1097/DER.0000000000000789
- Weiss BD. Health Literacy: A Manual for Clinicians. American Medical Association, American Medical Foundation; 2003.
- Nutbeam D, McGill B, Premkumar P. Improving health literacy in community populations: a review of progress. Health Promot Int. 2018;33:901-911. doi:10.1093/heapro/dax015
- Liu C, Wang D, Liu C, et al. What is the meaning of health literacy? a systematic review and qualitative synthesis. Fam Med Community Health. 2020;8:e000351. doi:10.1136/fmch-2020-000351
- Berkman ND, Sheridan SL, Donahue KE, et al. Low health literacy and health outcomes: an updated systematic review. Ann Intern Med. 2011;155:97-107. doi:10.7326/0003-4819-155-2-201107190-00005
- Rice RE. Influences, usage, and outcomes of Internet health information searching: multivariate results from the Pew surveys. Int J Med Inform. 2006;75:8-28. doi:10.1016/j.ijmedinf.2005.07.032
- Brown J, Weitz NW, Liang A, et al. Does an eczema action plan improve atopic dermatitis? a single-site randomized controlled trial. Clin Pediatr (Phila). 2018;57:1624-1629. doi:10.1177/0009922818795906
- De DR, Shih T, Katta R, et al. Readability, quality, and timeliness of patient online health resources for contact dermatitis and patch testing. Dermatitis. 2022;33:155-160. doi:10.1097/DER.0000000000000789
- Weiss BD. Health Literacy: A Manual for Clinicians. American Medical Association, American Medical Foundation; 2003.
- Nutbeam D, McGill B, Premkumar P. Improving health literacy in community populations: a review of progress. Health Promot Int. 2018;33:901-911. doi:10.1093/heapro/dax015
Linear Hypopigmentation on the Right Arm
The Diagnosis: Chemical Leukoderma
A clinical diagnosis of chemical leukoderma was made. In our patient, the observed linear hypopigmentation likely resulted from the prior treatment for De Quervain tenosynovitis in which an intralesional corticosteroid entered the lymphatic channel causing a linear distribution of chemical leukoderma. The hypopigmentation self-resolved at 6-month follow-up, and the patient was counseled to continue steroid injections if indicated.
Chemical leukoderma is an acquired depigmenting dermatosis that displays vitiligolike patterning. Detailed personal and family history in addition to complete physical examination are crucial given the inability to distinguish chemical leukoderma from vitiligo on histopathology. A set of clinical criteria proposed by Ghosh and Mukhopadhyay1 includes the presence of acquired depigmented macules and patches resembling vitiligo, history of repeat exposure to certain chemical substances, hypopigmentation at the site of exposure, and/ or confettilike white macules. Three of these 4 clinical findings must be present to establish a diagnosis of chemical leukoderma. The extent of disease involvement may be graded as follows: Stage I is defined as leukoderma only at the site of contact to the offending agent. Stage II involvement is characterized by local spread beyond the exposure site via the lymphatic system. Stages IIIA and IIIB leukoderma entail hematogenous spread distant to the site of chemical exposure. Although stage IIIA leukoderma is limited to cutaneous involvement, stage IIIB findings are marked by systemic organ involvement. Stage IV disease is defined by the distant spread of hypopigmented macules and patches that continues following 1 year of strict avoidance of the causative agent.1
The pathogenesis behind chemical leukoderma is not completely understood. Studies have suggested that individuals with certain genetic susceptibilities are predisposed to developing the condition after being exposed to chemicals with melanocytotoxic properties.2,3 It has been proposed that the chemicals accelerate pre-existing cellular stress cascades within melanocytes to levels higher than what healthy cells can tolerate. Genetic factors can increase an individual’s total melanocytic stress or establish a lower cellular threshold for stress than what the immune system can manage.4 These influences culminate in an inflammatory response that results in melanocytic destruction and subsequent cutaneous hypopigmentation.
The most well-known offending chemical agents are phenol and catechol derivatives, such as hydroquinone, which is used in topical bleaching agents to treat diseases of hyperpigmentation, including melasma.2 Potent topical or intralesional corticosteroids also may precipitate chemical leukoderma, most notably in individuals with darker skin tones. Hypomelanosis induced by intralesional steroids frequently occurs weeks to months after administration and commonly is observed in a stellate or linear pattern with an irregular outline.5 Other offending chemical agents include sulfhydryls, mercurials, arsenic, benzoyl peroxide, azelaic acid, imiquimod, chloroquine, and tyrosine kinase inhibitors.2,5
Segmental vitiligo is characterized by unilateral hypopigmentation in a linear or blocklike distribution that does not cross the midline. However, onset of segmental vitiligo classically occurs prior to 30 years of age and frequently is related with early leukotrichia.6 Additionally, the hypomelanosis associated with segmental vitiligo more often presents as broad bands or patches that occasionally have a blaschkoid distribution and most commonly appear on the face.5 Lichen striatus is a lichenoid dermatosis that presents as asymptomatic pink or hypopigmented papules that follow the Blaschko lines, often favoring the extremities. Postinflammatory hypopigmentation also may occur as an associated sequela of resolved lichen striatus. Although the disease onset of lichen striatus may occur in adulthood, it typically appears in childhood and is triggered by factors such as trauma, hypersensitivity reactions, viral infections, and medications. Physical injuries such as trauma following surgical procedures also can lead to hypomelanosis; however, our patient denied any relevant surgical history. Progressive macular hypomelanosis is a skin condition presenting as ill-defined, nummular, hypopigmented macules or patches that commonly affects women with darker skin tones with an ethnic background from a tropical location or residing in a tropical environment.5 Lesions frequently appear on the trunk and rarely progress to the proximal extremities, making it an unlikely diagnosis for our patient.
In most cases of chemical leukoderma, spontaneous repigmentation often occurs within 12 months after the elimination of the offending substance; however, hypopigmented lesions may persist or continue to develop at sites distant from the initial site despite discontinuing the causative agent.1 Therapies for vitiligo, such as topical corticosteroids, topical immunosuppressants, narrowband UVB phototherapy, and psoralen plus UVA photochemotherapy, may be utilized for chemical leukoderma that does not self-resolve.
- Ghosh S, Mukhopadhyay S. Chemical leucoderma: a clinicoaetiological study of 864 cases in the perspective of a developing country [published online September 6, 2008]. Br J Dermatol. 2009;160:40-47.
- Ghosh S. Chemical leukoderma: what’s new on etiopathological and clinical aspects? Indian J Dermatol. 2010;55:255.
- Boissy RE, Manga P. On the etiology of contact/occupational vitiligo. Pigment Cell Res. 2004;17:208-214.
- Harris J. Chemical-induced vitiligo. Dermatol Clin. 2017; 35:151-161.
- Bolognia JL, Schaffer JV, Cerroni L, et al. Vitiligo and other disorders of hypopigmentation. In: Bolognia JL, Schaffer JV, Cerroni L, eds. Dermatology. 4th ed. Mosby/Elsevier; 2018:1087-1114.
- Rodrigues M, Ezzedine K, Hamzavi I, et al. New discoveries in the pathogenesis and classification of vitiligo. J Am Acad Dermatol. 2017;77:1-13.
The Diagnosis: Chemical Leukoderma
A clinical diagnosis of chemical leukoderma was made. In our patient, the observed linear hypopigmentation likely resulted from the prior treatment for De Quervain tenosynovitis in which an intralesional corticosteroid entered the lymphatic channel causing a linear distribution of chemical leukoderma. The hypopigmentation self-resolved at 6-month follow-up, and the patient was counseled to continue steroid injections if indicated.
Chemical leukoderma is an acquired depigmenting dermatosis that displays vitiligolike patterning. Detailed personal and family history in addition to complete physical examination are crucial given the inability to distinguish chemical leukoderma from vitiligo on histopathology. A set of clinical criteria proposed by Ghosh and Mukhopadhyay1 includes the presence of acquired depigmented macules and patches resembling vitiligo, history of repeat exposure to certain chemical substances, hypopigmentation at the site of exposure, and/ or confettilike white macules. Three of these 4 clinical findings must be present to establish a diagnosis of chemical leukoderma. The extent of disease involvement may be graded as follows: Stage I is defined as leukoderma only at the site of contact to the offending agent. Stage II involvement is characterized by local spread beyond the exposure site via the lymphatic system. Stages IIIA and IIIB leukoderma entail hematogenous spread distant to the site of chemical exposure. Although stage IIIA leukoderma is limited to cutaneous involvement, stage IIIB findings are marked by systemic organ involvement. Stage IV disease is defined by the distant spread of hypopigmented macules and patches that continues following 1 year of strict avoidance of the causative agent.1
The pathogenesis behind chemical leukoderma is not completely understood. Studies have suggested that individuals with certain genetic susceptibilities are predisposed to developing the condition after being exposed to chemicals with melanocytotoxic properties.2,3 It has been proposed that the chemicals accelerate pre-existing cellular stress cascades within melanocytes to levels higher than what healthy cells can tolerate. Genetic factors can increase an individual’s total melanocytic stress or establish a lower cellular threshold for stress than what the immune system can manage.4 These influences culminate in an inflammatory response that results in melanocytic destruction and subsequent cutaneous hypopigmentation.
The most well-known offending chemical agents are phenol and catechol derivatives, such as hydroquinone, which is used in topical bleaching agents to treat diseases of hyperpigmentation, including melasma.2 Potent topical or intralesional corticosteroids also may precipitate chemical leukoderma, most notably in individuals with darker skin tones. Hypomelanosis induced by intralesional steroids frequently occurs weeks to months after administration and commonly is observed in a stellate or linear pattern with an irregular outline.5 Other offending chemical agents include sulfhydryls, mercurials, arsenic, benzoyl peroxide, azelaic acid, imiquimod, chloroquine, and tyrosine kinase inhibitors.2,5
Segmental vitiligo is characterized by unilateral hypopigmentation in a linear or blocklike distribution that does not cross the midline. However, onset of segmental vitiligo classically occurs prior to 30 years of age and frequently is related with early leukotrichia.6 Additionally, the hypomelanosis associated with segmental vitiligo more often presents as broad bands or patches that occasionally have a blaschkoid distribution and most commonly appear on the face.5 Lichen striatus is a lichenoid dermatosis that presents as asymptomatic pink or hypopigmented papules that follow the Blaschko lines, often favoring the extremities. Postinflammatory hypopigmentation also may occur as an associated sequela of resolved lichen striatus. Although the disease onset of lichen striatus may occur in adulthood, it typically appears in childhood and is triggered by factors such as trauma, hypersensitivity reactions, viral infections, and medications. Physical injuries such as trauma following surgical procedures also can lead to hypomelanosis; however, our patient denied any relevant surgical history. Progressive macular hypomelanosis is a skin condition presenting as ill-defined, nummular, hypopigmented macules or patches that commonly affects women with darker skin tones with an ethnic background from a tropical location or residing in a tropical environment.5 Lesions frequently appear on the trunk and rarely progress to the proximal extremities, making it an unlikely diagnosis for our patient.
In most cases of chemical leukoderma, spontaneous repigmentation often occurs within 12 months after the elimination of the offending substance; however, hypopigmented lesions may persist or continue to develop at sites distant from the initial site despite discontinuing the causative agent.1 Therapies for vitiligo, such as topical corticosteroids, topical immunosuppressants, narrowband UVB phototherapy, and psoralen plus UVA photochemotherapy, may be utilized for chemical leukoderma that does not self-resolve.
The Diagnosis: Chemical Leukoderma
A clinical diagnosis of chemical leukoderma was made. In our patient, the observed linear hypopigmentation likely resulted from the prior treatment for De Quervain tenosynovitis in which an intralesional corticosteroid entered the lymphatic channel causing a linear distribution of chemical leukoderma. The hypopigmentation self-resolved at 6-month follow-up, and the patient was counseled to continue steroid injections if indicated.
Chemical leukoderma is an acquired depigmenting dermatosis that displays vitiligolike patterning. Detailed personal and family history in addition to complete physical examination are crucial given the inability to distinguish chemical leukoderma from vitiligo on histopathology. A set of clinical criteria proposed by Ghosh and Mukhopadhyay1 includes the presence of acquired depigmented macules and patches resembling vitiligo, history of repeat exposure to certain chemical substances, hypopigmentation at the site of exposure, and/ or confettilike white macules. Three of these 4 clinical findings must be present to establish a diagnosis of chemical leukoderma. The extent of disease involvement may be graded as follows: Stage I is defined as leukoderma only at the site of contact to the offending agent. Stage II involvement is characterized by local spread beyond the exposure site via the lymphatic system. Stages IIIA and IIIB leukoderma entail hematogenous spread distant to the site of chemical exposure. Although stage IIIA leukoderma is limited to cutaneous involvement, stage IIIB findings are marked by systemic organ involvement. Stage IV disease is defined by the distant spread of hypopigmented macules and patches that continues following 1 year of strict avoidance of the causative agent.1
The pathogenesis behind chemical leukoderma is not completely understood. Studies have suggested that individuals with certain genetic susceptibilities are predisposed to developing the condition after being exposed to chemicals with melanocytotoxic properties.2,3 It has been proposed that the chemicals accelerate pre-existing cellular stress cascades within melanocytes to levels higher than what healthy cells can tolerate. Genetic factors can increase an individual’s total melanocytic stress or establish a lower cellular threshold for stress than what the immune system can manage.4 These influences culminate in an inflammatory response that results in melanocytic destruction and subsequent cutaneous hypopigmentation.
The most well-known offending chemical agents are phenol and catechol derivatives, such as hydroquinone, which is used in topical bleaching agents to treat diseases of hyperpigmentation, including melasma.2 Potent topical or intralesional corticosteroids also may precipitate chemical leukoderma, most notably in individuals with darker skin tones. Hypomelanosis induced by intralesional steroids frequently occurs weeks to months after administration and commonly is observed in a stellate or linear pattern with an irregular outline.5 Other offending chemical agents include sulfhydryls, mercurials, arsenic, benzoyl peroxide, azelaic acid, imiquimod, chloroquine, and tyrosine kinase inhibitors.2,5
Segmental vitiligo is characterized by unilateral hypopigmentation in a linear or blocklike distribution that does not cross the midline. However, onset of segmental vitiligo classically occurs prior to 30 years of age and frequently is related with early leukotrichia.6 Additionally, the hypomelanosis associated with segmental vitiligo more often presents as broad bands or patches that occasionally have a blaschkoid distribution and most commonly appear on the face.5 Lichen striatus is a lichenoid dermatosis that presents as asymptomatic pink or hypopigmented papules that follow the Blaschko lines, often favoring the extremities. Postinflammatory hypopigmentation also may occur as an associated sequela of resolved lichen striatus. Although the disease onset of lichen striatus may occur in adulthood, it typically appears in childhood and is triggered by factors such as trauma, hypersensitivity reactions, viral infections, and medications. Physical injuries such as trauma following surgical procedures also can lead to hypomelanosis; however, our patient denied any relevant surgical history. Progressive macular hypomelanosis is a skin condition presenting as ill-defined, nummular, hypopigmented macules or patches that commonly affects women with darker skin tones with an ethnic background from a tropical location or residing in a tropical environment.5 Lesions frequently appear on the trunk and rarely progress to the proximal extremities, making it an unlikely diagnosis for our patient.
In most cases of chemical leukoderma, spontaneous repigmentation often occurs within 12 months after the elimination of the offending substance; however, hypopigmented lesions may persist or continue to develop at sites distant from the initial site despite discontinuing the causative agent.1 Therapies for vitiligo, such as topical corticosteroids, topical immunosuppressants, narrowband UVB phototherapy, and psoralen plus UVA photochemotherapy, may be utilized for chemical leukoderma that does not self-resolve.
- Ghosh S, Mukhopadhyay S. Chemical leucoderma: a clinicoaetiological study of 864 cases in the perspective of a developing country [published online September 6, 2008]. Br J Dermatol. 2009;160:40-47.
- Ghosh S. Chemical leukoderma: what’s new on etiopathological and clinical aspects? Indian J Dermatol. 2010;55:255.
- Boissy RE, Manga P. On the etiology of contact/occupational vitiligo. Pigment Cell Res. 2004;17:208-214.
- Harris J. Chemical-induced vitiligo. Dermatol Clin. 2017; 35:151-161.
- Bolognia JL, Schaffer JV, Cerroni L, et al. Vitiligo and other disorders of hypopigmentation. In: Bolognia JL, Schaffer JV, Cerroni L, eds. Dermatology. 4th ed. Mosby/Elsevier; 2018:1087-1114.
- Rodrigues M, Ezzedine K, Hamzavi I, et al. New discoveries in the pathogenesis and classification of vitiligo. J Am Acad Dermatol. 2017;77:1-13.
- Ghosh S, Mukhopadhyay S. Chemical leucoderma: a clinicoaetiological study of 864 cases in the perspective of a developing country [published online September 6, 2008]. Br J Dermatol. 2009;160:40-47.
- Ghosh S. Chemical leukoderma: what’s new on etiopathological and clinical aspects? Indian J Dermatol. 2010;55:255.
- Boissy RE, Manga P. On the etiology of contact/occupational vitiligo. Pigment Cell Res. 2004;17:208-214.
- Harris J. Chemical-induced vitiligo. Dermatol Clin. 2017; 35:151-161.
- Bolognia JL, Schaffer JV, Cerroni L, et al. Vitiligo and other disorders of hypopigmentation. In: Bolognia JL, Schaffer JV, Cerroni L, eds. Dermatology. 4th ed. Mosby/Elsevier; 2018:1087-1114.
- Rodrigues M, Ezzedine K, Hamzavi I, et al. New discoveries in the pathogenesis and classification of vitiligo. J Am Acad Dermatol. 2017;77:1-13.
A 73-year-old woman presented to the dermatology clinic with hypopigmentation along the right arm. Her medical history was notable for prior treatment with intralesional triamcinolone injections for De Quervain tenosynovitis. Two months after receiving the steroid injections she noted progressive spreading of an asymptomatic white discoloration originating on the right wrist. Physical examination revealed a hypopigmented atrophic patch on the medial aspect of the right wrist (left) with linear hypopigmented patches extending proximally up the forearm (right).
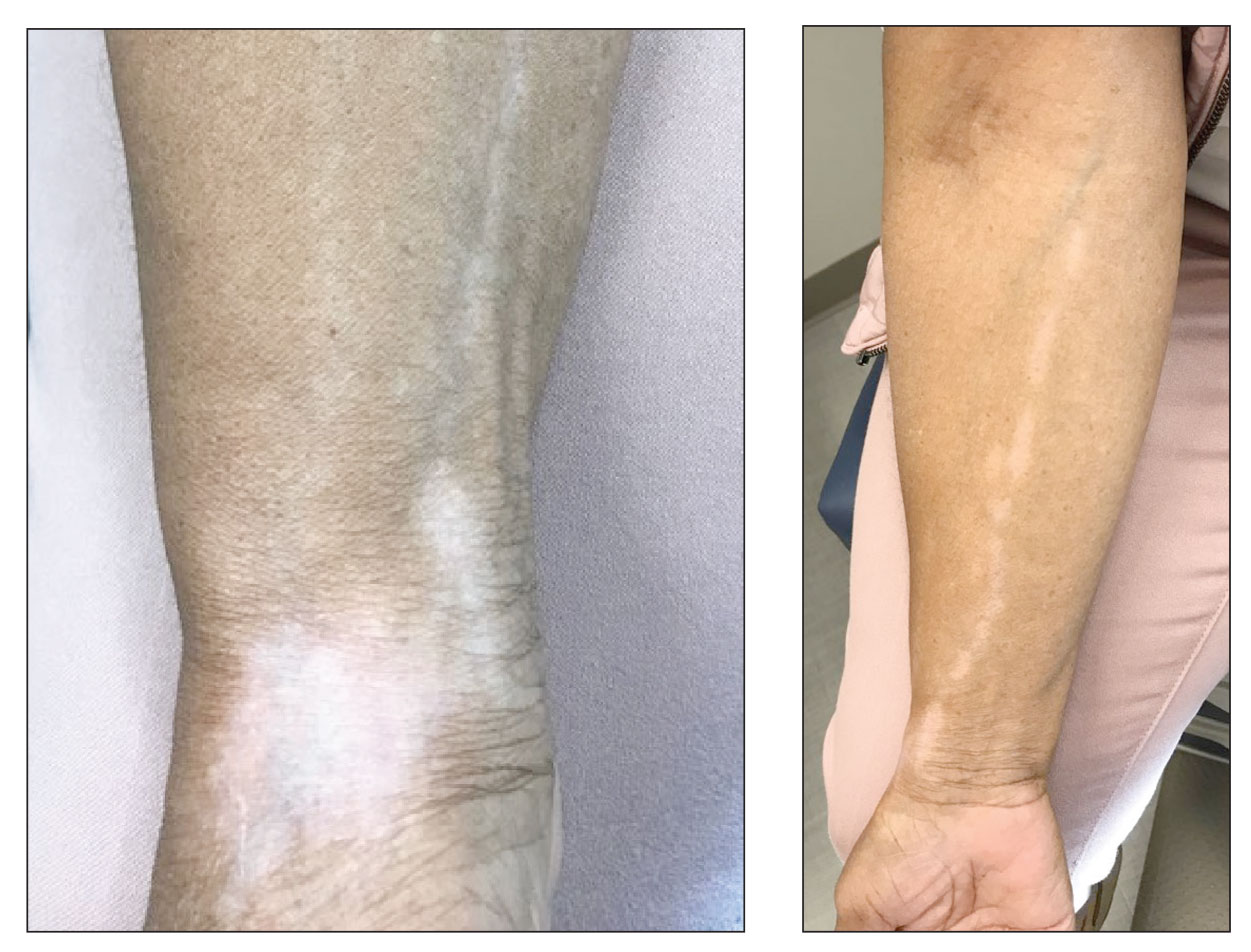
The Ethical Implications of Dermatology Residents Treating Attending Physicians
Residents are confronted daily with situations in clinic that require a foundation in medical ethics to assist in decision-making. Attending physicians require health care services and at times may seek care from resident physicians. If the attending physician has direct oversight over the resident, however, the ethics of the resident treating them need to be addressed. Although patients have autonomy to choose whoever they want as a physician, nonmaleficence dictates that the resident may forego treatment due to concerns for providing suboptimal care; however, this same attending may be treated under specific circumstances. This column explores the ethical implications of both situations.
The Ethical Dilemma of Treating an Attending
Imagine this scenario: You are in your resident general dermatology clinic seeing patients with an attending overseeing your clinical decisions following each encounter. You look on your schedule and see that the next patient is one of your pediatric dermatology attendings for a total-body skin examination (TBSE). You have never treated a physician that oversees you, and you ponder whether you should perform the examination or fetch your attending to perform the encounter alone.
This conundrum then brings other questions to mind: Would changing the reason for the appointment (ie, an acute problem vs a TBSE) alter your decision as to whether or not you would treat this attending? Would the situation be different if this was an attending in a different department?
Ethics Curriculum for Residents
Medical providers face ethical dilemmas daily, and dermatologists and dermatology residents are not excluded. Dermatoethics can provide a framework for the best approach to this hypothetical situation. To equip residents with resources on ethics and a cognitive framework to approach similar situations, the American Board of Dermatology has created an ethics curriculum for residents to learn over their 3 years of training.1
One study that analyzed the ethical themes portrayed in essays by fourth-year medical students showed that the most common themes included autonomy, social justice, nonmaleficence, beneficence, honesty, and respect.2 These themes must be considered in different permutations throughout ethical conundrums.
In the situation of an attending physician who supervises a resident in another clinic voluntarily attending the resident clinic, the physician is aware of the resident’s skills and qualifications and knows that supervision is being provided by an attending physician, which allows informed consent to be made, as a study by Unruh et al3 shows. The patient’s autonomy allows them to choose their treating provider.
However, there are several reasons why the resident may be hesitant to enter the room. One concern may be that during a TBSE the provider usually examines the patient’s genitals, rectum, and breasts.4 Because the resident knows the individual personally, the patient and/or the provider may be uncomfortable checking these areas, leaving a portion of the examination unperformed. This neglect may harm the patient (eg, a genital melanoma is missed), violating the tenant of nonmaleficence.
The effect of the medical hierarchy also should be considered. The de facto hierarchy of attendings supervising residents, interns, and medical students, with each group having some oversight over the next, can have positive effects on education and appropriate patient management but also can prove to be detrimental to the patient and provider in some circumstances. Studies have shown that residents may be less willing to disagree with their superior’s opinions for fear of negative reactions and harmful effects on their future careers.5-7 The hierarchy of medicine also can affect a resident’s moral judgement by intimidating the practitioner to perform tasks or make diagnoses they may not wish to make.5,6,8,9 For example, the resident may send a prescription for a medication that the attending requested despite no clear indication of need. This mingling of patient and supervisor roles can result in a resident treating their attending physician inconsistently with their standard of care.
Navigating the Ethics of Treating Family Members
The American Medical Association Code of Medical Ethics Opinions on Patient-Physician Relationships highlights treating family members as an important ethical topic. Although most residents and attendings are not biologically related, a familial-style relationship exists in many dermatology programs between attendings and residents due to the close-knit nature of dermatology programs. Diagnostic and treatment accuracy may be diminished by the discomfort or disbelief that a condition could affect someone the resident cares about.10
The American Medical Association also states that a physician can treat family members in an emergency situation or for short-term minor problems. If these 2 exceptions were to be extrapolated to apply to situations involving residents and attendings in addition to family, there would be situations where a dermatology resident could ethically treat their attending physician.10 If the attending physician was worried about a problem that was deemed potentially life-threatening, such as a rapidly progressive bullous eruption concerning for Stevens-Johnson syndrome following the initiation of a new medication, and they wanted an urgent evaluation and biopsy, an ethicist could argue that urgent treatment is medically indicated as deferring treatment could have negative consequences on the patient’s health. In addition, if the attending found a splinter in their finger following yardwork and needed assistance in removal, this also could be treated by their resident, as it is minimally invasive and has a finite conclusion.
Treating Nonsupervisory Attendings
In the case of performing a TBSE on an attending from another specialty, it would be acceptable and less ethically ambiguous if no close personal relationship existed between the two practitioners, as this patient would have no direct oversight over the resident physician.
Final Thoughts
Each situation that residents face may carry ethical implications with perspectives from the patient, provider, and bystanders. The above scenarios highlight specific instances that a dermatology resident may face and provide insight into how they may approach the situations. At the same time, it is important to remember that every situation is different and requires a unique approach. Fortunately,physicians—specifically dermatologists—are provided many resources to help navigate challenging scenarios.
Acknowledgments—The author thanks Jane M. Grant-Kels, MD (Farmington, Connecticut), for reviewing this paper and providing feedback to improve its content, as well as Warren R. Heymann, MD (Camden, New Jersey), for assisting in the creation of this topic and article.
- Dermatoethics. American Board of Dermatology website. Accessed August 9, 2022. https://www.abderm.org/residents-and-fellows/dermatoethics
- House JB, Theyyunni N, Barnosky AR, et al. Understanding ethical dilemmas in the emergency department: views from medical students’ essays. J Emerg Med. 2015;48:492-498.
- Unruh KP, Dhulipala SC, Holt GE. Patient understanding of the role of the orthopedic resident. J Surg Educ. 2013;70:345-349.
- Grandhi R, Grant-Kels JM. Naked and vulnerable: the ethics of chaperoning full-body skin examinations. J Am Acad Dermatol. 2017;76:1221-1223.
- Salehi PP, Jacobs D, Suhail-Sindhu T, et al. Consequences of medical hierarchy on medical students, residents, and medical education in otolaryngology. Otolaryngol Head Neck Surg. 2020;163:906-914.
- Lomis KD, Carpenter RO, Miller BM. Moral distress in the third year of medical school: a descriptive review of student case reflections. Am J Surg. 2009;197:107-112.
- Troughton R, Mariano V, Campbell A, et al. Understanding determinants of infection control practices in surgery: the role of shared ownership and team hierarchy. Antimicrob Resist Infect Control. 2019;8:116.
- Chiu PP, Hilliard RI, Azzie G, et al. Experience of moral distress among pediatric surgery trainees. J Pediatr Surg. 2008;43:986-993.
- Martinez W, Lo B. Medical students’ experiences with medical errors: an analysis of medical student essays. Med Educ. 2008;42:733-741.
- Chapter 1. opinions on patient-physician relationships. American Medical Association website. Accessed on August 9, 2022. https://www.ama-assn.org/system/files/code-of-medical-ethics-chapter-1.pdf
Residents are confronted daily with situations in clinic that require a foundation in medical ethics to assist in decision-making. Attending physicians require health care services and at times may seek care from resident physicians. If the attending physician has direct oversight over the resident, however, the ethics of the resident treating them need to be addressed. Although patients have autonomy to choose whoever they want as a physician, nonmaleficence dictates that the resident may forego treatment due to concerns for providing suboptimal care; however, this same attending may be treated under specific circumstances. This column explores the ethical implications of both situations.
The Ethical Dilemma of Treating an Attending
Imagine this scenario: You are in your resident general dermatology clinic seeing patients with an attending overseeing your clinical decisions following each encounter. You look on your schedule and see that the next patient is one of your pediatric dermatology attendings for a total-body skin examination (TBSE). You have never treated a physician that oversees you, and you ponder whether you should perform the examination or fetch your attending to perform the encounter alone.
This conundrum then brings other questions to mind: Would changing the reason for the appointment (ie, an acute problem vs a TBSE) alter your decision as to whether or not you would treat this attending? Would the situation be different if this was an attending in a different department?
Ethics Curriculum for Residents
Medical providers face ethical dilemmas daily, and dermatologists and dermatology residents are not excluded. Dermatoethics can provide a framework for the best approach to this hypothetical situation. To equip residents with resources on ethics and a cognitive framework to approach similar situations, the American Board of Dermatology has created an ethics curriculum for residents to learn over their 3 years of training.1
One study that analyzed the ethical themes portrayed in essays by fourth-year medical students showed that the most common themes included autonomy, social justice, nonmaleficence, beneficence, honesty, and respect.2 These themes must be considered in different permutations throughout ethical conundrums.
In the situation of an attending physician who supervises a resident in another clinic voluntarily attending the resident clinic, the physician is aware of the resident’s skills and qualifications and knows that supervision is being provided by an attending physician, which allows informed consent to be made, as a study by Unruh et al3 shows. The patient’s autonomy allows them to choose their treating provider.
However, there are several reasons why the resident may be hesitant to enter the room. One concern may be that during a TBSE the provider usually examines the patient’s genitals, rectum, and breasts.4 Because the resident knows the individual personally, the patient and/or the provider may be uncomfortable checking these areas, leaving a portion of the examination unperformed. This neglect may harm the patient (eg, a genital melanoma is missed), violating the tenant of nonmaleficence.
The effect of the medical hierarchy also should be considered. The de facto hierarchy of attendings supervising residents, interns, and medical students, with each group having some oversight over the next, can have positive effects on education and appropriate patient management but also can prove to be detrimental to the patient and provider in some circumstances. Studies have shown that residents may be less willing to disagree with their superior’s opinions for fear of negative reactions and harmful effects on their future careers.5-7 The hierarchy of medicine also can affect a resident’s moral judgement by intimidating the practitioner to perform tasks or make diagnoses they may not wish to make.5,6,8,9 For example, the resident may send a prescription for a medication that the attending requested despite no clear indication of need. This mingling of patient and supervisor roles can result in a resident treating their attending physician inconsistently with their standard of care.
Navigating the Ethics of Treating Family Members
The American Medical Association Code of Medical Ethics Opinions on Patient-Physician Relationships highlights treating family members as an important ethical topic. Although most residents and attendings are not biologically related, a familial-style relationship exists in many dermatology programs between attendings and residents due to the close-knit nature of dermatology programs. Diagnostic and treatment accuracy may be diminished by the discomfort or disbelief that a condition could affect someone the resident cares about.10
The American Medical Association also states that a physician can treat family members in an emergency situation or for short-term minor problems. If these 2 exceptions were to be extrapolated to apply to situations involving residents and attendings in addition to family, there would be situations where a dermatology resident could ethically treat their attending physician.10 If the attending physician was worried about a problem that was deemed potentially life-threatening, such as a rapidly progressive bullous eruption concerning for Stevens-Johnson syndrome following the initiation of a new medication, and they wanted an urgent evaluation and biopsy, an ethicist could argue that urgent treatment is medically indicated as deferring treatment could have negative consequences on the patient’s health. In addition, if the attending found a splinter in their finger following yardwork and needed assistance in removal, this also could be treated by their resident, as it is minimally invasive and has a finite conclusion.
Treating Nonsupervisory Attendings
In the case of performing a TBSE on an attending from another specialty, it would be acceptable and less ethically ambiguous if no close personal relationship existed between the two practitioners, as this patient would have no direct oversight over the resident physician.
Final Thoughts
Each situation that residents face may carry ethical implications with perspectives from the patient, provider, and bystanders. The above scenarios highlight specific instances that a dermatology resident may face and provide insight into how they may approach the situations. At the same time, it is important to remember that every situation is different and requires a unique approach. Fortunately,physicians—specifically dermatologists—are provided many resources to help navigate challenging scenarios.
Acknowledgments—The author thanks Jane M. Grant-Kels, MD (Farmington, Connecticut), for reviewing this paper and providing feedback to improve its content, as well as Warren R. Heymann, MD (Camden, New Jersey), for assisting in the creation of this topic and article.
Residents are confronted daily with situations in clinic that require a foundation in medical ethics to assist in decision-making. Attending physicians require health care services and at times may seek care from resident physicians. If the attending physician has direct oversight over the resident, however, the ethics of the resident treating them need to be addressed. Although patients have autonomy to choose whoever they want as a physician, nonmaleficence dictates that the resident may forego treatment due to concerns for providing suboptimal care; however, this same attending may be treated under specific circumstances. This column explores the ethical implications of both situations.
The Ethical Dilemma of Treating an Attending
Imagine this scenario: You are in your resident general dermatology clinic seeing patients with an attending overseeing your clinical decisions following each encounter. You look on your schedule and see that the next patient is one of your pediatric dermatology attendings for a total-body skin examination (TBSE). You have never treated a physician that oversees you, and you ponder whether you should perform the examination or fetch your attending to perform the encounter alone.
This conundrum then brings other questions to mind: Would changing the reason for the appointment (ie, an acute problem vs a TBSE) alter your decision as to whether or not you would treat this attending? Would the situation be different if this was an attending in a different department?
Ethics Curriculum for Residents
Medical providers face ethical dilemmas daily, and dermatologists and dermatology residents are not excluded. Dermatoethics can provide a framework for the best approach to this hypothetical situation. To equip residents with resources on ethics and a cognitive framework to approach similar situations, the American Board of Dermatology has created an ethics curriculum for residents to learn over their 3 years of training.1
One study that analyzed the ethical themes portrayed in essays by fourth-year medical students showed that the most common themes included autonomy, social justice, nonmaleficence, beneficence, honesty, and respect.2 These themes must be considered in different permutations throughout ethical conundrums.
In the situation of an attending physician who supervises a resident in another clinic voluntarily attending the resident clinic, the physician is aware of the resident’s skills and qualifications and knows that supervision is being provided by an attending physician, which allows informed consent to be made, as a study by Unruh et al3 shows. The patient’s autonomy allows them to choose their treating provider.
However, there are several reasons why the resident may be hesitant to enter the room. One concern may be that during a TBSE the provider usually examines the patient’s genitals, rectum, and breasts.4 Because the resident knows the individual personally, the patient and/or the provider may be uncomfortable checking these areas, leaving a portion of the examination unperformed. This neglect may harm the patient (eg, a genital melanoma is missed), violating the tenant of nonmaleficence.
The effect of the medical hierarchy also should be considered. The de facto hierarchy of attendings supervising residents, interns, and medical students, with each group having some oversight over the next, can have positive effects on education and appropriate patient management but also can prove to be detrimental to the patient and provider in some circumstances. Studies have shown that residents may be less willing to disagree with their superior’s opinions for fear of negative reactions and harmful effects on their future careers.5-7 The hierarchy of medicine also can affect a resident’s moral judgement by intimidating the practitioner to perform tasks or make diagnoses they may not wish to make.5,6,8,9 For example, the resident may send a prescription for a medication that the attending requested despite no clear indication of need. This mingling of patient and supervisor roles can result in a resident treating their attending physician inconsistently with their standard of care.
Navigating the Ethics of Treating Family Members
The American Medical Association Code of Medical Ethics Opinions on Patient-Physician Relationships highlights treating family members as an important ethical topic. Although most residents and attendings are not biologically related, a familial-style relationship exists in many dermatology programs between attendings and residents due to the close-knit nature of dermatology programs. Diagnostic and treatment accuracy may be diminished by the discomfort or disbelief that a condition could affect someone the resident cares about.10
The American Medical Association also states that a physician can treat family members in an emergency situation or for short-term minor problems. If these 2 exceptions were to be extrapolated to apply to situations involving residents and attendings in addition to family, there would be situations where a dermatology resident could ethically treat their attending physician.10 If the attending physician was worried about a problem that was deemed potentially life-threatening, such as a rapidly progressive bullous eruption concerning for Stevens-Johnson syndrome following the initiation of a new medication, and they wanted an urgent evaluation and biopsy, an ethicist could argue that urgent treatment is medically indicated as deferring treatment could have negative consequences on the patient’s health. In addition, if the attending found a splinter in their finger following yardwork and needed assistance in removal, this also could be treated by their resident, as it is minimally invasive and has a finite conclusion.
Treating Nonsupervisory Attendings
In the case of performing a TBSE on an attending from another specialty, it would be acceptable and less ethically ambiguous if no close personal relationship existed between the two practitioners, as this patient would have no direct oversight over the resident physician.
Final Thoughts
Each situation that residents face may carry ethical implications with perspectives from the patient, provider, and bystanders. The above scenarios highlight specific instances that a dermatology resident may face and provide insight into how they may approach the situations. At the same time, it is important to remember that every situation is different and requires a unique approach. Fortunately,physicians—specifically dermatologists—are provided many resources to help navigate challenging scenarios.
Acknowledgments—The author thanks Jane M. Grant-Kels, MD (Farmington, Connecticut), for reviewing this paper and providing feedback to improve its content, as well as Warren R. Heymann, MD (Camden, New Jersey), for assisting in the creation of this topic and article.
- Dermatoethics. American Board of Dermatology website. Accessed August 9, 2022. https://www.abderm.org/residents-and-fellows/dermatoethics
- House JB, Theyyunni N, Barnosky AR, et al. Understanding ethical dilemmas in the emergency department: views from medical students’ essays. J Emerg Med. 2015;48:492-498.
- Unruh KP, Dhulipala SC, Holt GE. Patient understanding of the role of the orthopedic resident. J Surg Educ. 2013;70:345-349.
- Grandhi R, Grant-Kels JM. Naked and vulnerable: the ethics of chaperoning full-body skin examinations. J Am Acad Dermatol. 2017;76:1221-1223.
- Salehi PP, Jacobs D, Suhail-Sindhu T, et al. Consequences of medical hierarchy on medical students, residents, and medical education in otolaryngology. Otolaryngol Head Neck Surg. 2020;163:906-914.
- Lomis KD, Carpenter RO, Miller BM. Moral distress in the third year of medical school: a descriptive review of student case reflections. Am J Surg. 2009;197:107-112.
- Troughton R, Mariano V, Campbell A, et al. Understanding determinants of infection control practices in surgery: the role of shared ownership and team hierarchy. Antimicrob Resist Infect Control. 2019;8:116.
- Chiu PP, Hilliard RI, Azzie G, et al. Experience of moral distress among pediatric surgery trainees. J Pediatr Surg. 2008;43:986-993.
- Martinez W, Lo B. Medical students’ experiences with medical errors: an analysis of medical student essays. Med Educ. 2008;42:733-741.
- Chapter 1. opinions on patient-physician relationships. American Medical Association website. Accessed on August 9, 2022. https://www.ama-assn.org/system/files/code-of-medical-ethics-chapter-1.pdf
- Dermatoethics. American Board of Dermatology website. Accessed August 9, 2022. https://www.abderm.org/residents-and-fellows/dermatoethics
- House JB, Theyyunni N, Barnosky AR, et al. Understanding ethical dilemmas in the emergency department: views from medical students’ essays. J Emerg Med. 2015;48:492-498.
- Unruh KP, Dhulipala SC, Holt GE. Patient understanding of the role of the orthopedic resident. J Surg Educ. 2013;70:345-349.
- Grandhi R, Grant-Kels JM. Naked and vulnerable: the ethics of chaperoning full-body skin examinations. J Am Acad Dermatol. 2017;76:1221-1223.
- Salehi PP, Jacobs D, Suhail-Sindhu T, et al. Consequences of medical hierarchy on medical students, residents, and medical education in otolaryngology. Otolaryngol Head Neck Surg. 2020;163:906-914.
- Lomis KD, Carpenter RO, Miller BM. Moral distress in the third year of medical school: a descriptive review of student case reflections. Am J Surg. 2009;197:107-112.
- Troughton R, Mariano V, Campbell A, et al. Understanding determinants of infection control practices in surgery: the role of shared ownership and team hierarchy. Antimicrob Resist Infect Control. 2019;8:116.
- Chiu PP, Hilliard RI, Azzie G, et al. Experience of moral distress among pediatric surgery trainees. J Pediatr Surg. 2008;43:986-993.
- Martinez W, Lo B. Medical students’ experiences with medical errors: an analysis of medical student essays. Med Educ. 2008;42:733-741.
- Chapter 1. opinions on patient-physician relationships. American Medical Association website. Accessed on August 9, 2022. https://www.ama-assn.org/system/files/code-of-medical-ethics-chapter-1.pdf
Resident Pearls
- Dermatology residents should not perform total-body skin examinations on or provide long-term care to attending physicians that directly oversee them.
- Residents should only provide care to their attending physicians if the attending’s life is in imminent danger from delay of treatment or if it is a self-limited, minor problem.
Scattered Flesh-Colored Papules in a Linear Array in the Setting of Diffuse Skin Thickening
The Diagnosis: Scleromyxedema
A punch biopsy of the upper back performed at an outside institution revealed increased histiocytes and abundant interstitial mucin confined to the papillary dermis (Figures 1 and 2), consistent with the lichen myxedematosus (LM) papules that may be seen in scleromyxedema. Serum protein electrophoresis revealed the presence of a protein of restricted mobility on the gamma region that occupied 5.3% of the total protein (0.3 g/dL). Urine protein electrophoresis showed free kappa light chain monoclonal protein in the gamma region. Immunofixation electrophoresis revealed the presence of IgG kappa monoclonal protein in the gamma region with 10% monotype kappa cells. The presence of Raynaud phenomenon and positive antinuclear antibody (1:320, speckled) was noted. Laboratory studies for thyroid-stimulating hormone, C-reactive protein, Scl-70 antibody, myositis panel, ribonucleoprotein antibody, Smith antibody, Sjögren syndrome–related antigens A and B antibodies, rheumatoid factor, and RNA polymerase III antibody all were within reference range. Our patient was treated with monthly intravenous immunoglobulin (IVIG), and he noted substantial improvement in skin findings after 3 months of IVIG.
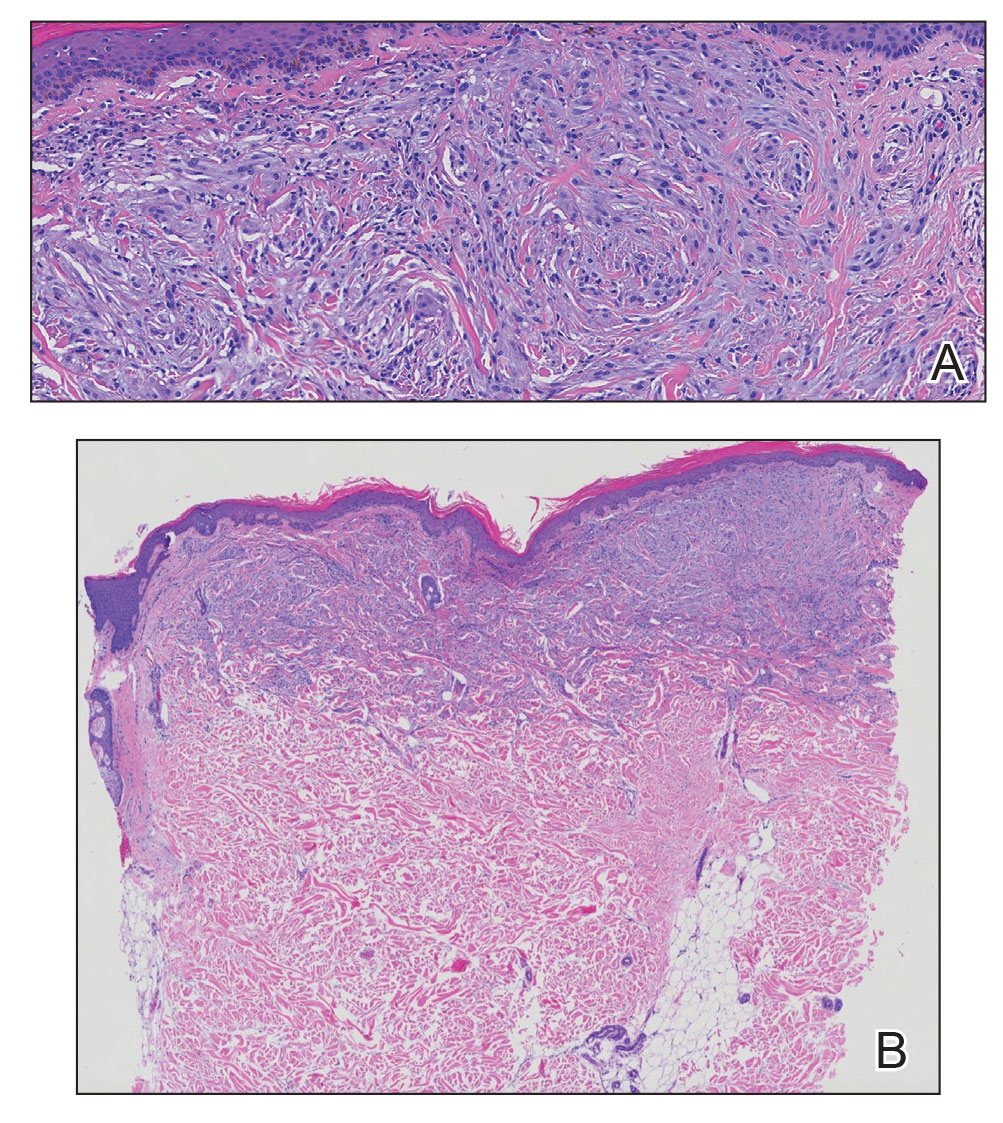
Localized lichen myxedematosus is a rare idiopathic cutaneous disease that clinically is characterized by waxy indurated papules and histologically is characterized by diffuse mucin deposition and fibroblast proliferation in the upper dermis.1 Scleromyxedema is a diffuse variant of LM in which the papules and plaques of LM are associated with skin thickening involving almost the entire body and associated systemic disease. The exact mechanism of this disease is unknown, but the most widely accepted hypothesis is that immunoglobulins and cytokines contribute to the synthesis of glycosaminoglycans and thereby the deposition of mucin in the dermis.2 Scleromyxedema has a chronic course and generally responds poorly to existing treatments.1 Partial improvement has been demonstrated in treatment with topical calcineurin inhibitors and topical steroids.2
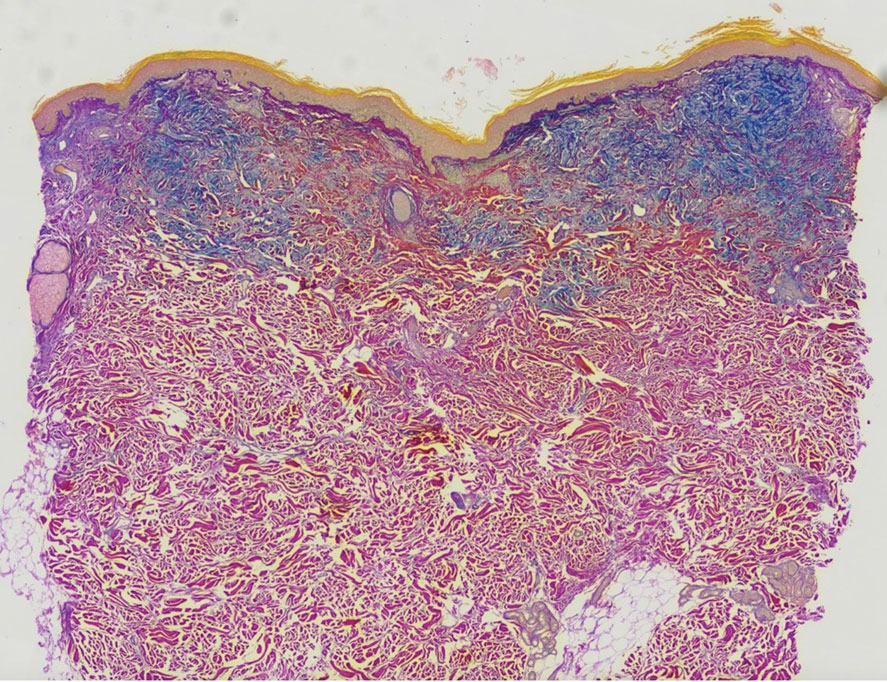
The differential diagnosis in our patient included scleromyxedema, scleredema, scleroderma, LM, and reticular erythematosus mucinosis. He was diagnosed with scleromyxedema with kappa monoclonal gammopathy. Scleromyxedema is a rare disorder involving the deposition of mucinous material in the papillary dermis that causes the formation of infiltrative skin lesions.3 The etiology is unknown, but the presence of a monoclonal protein is an important characteristic of this disorder. It is important to rule out thyroid disease as a possible etiology before concluding that the disease process is driven by the monoclonal gammopathy; this will help determine appropriate therapies.4,5 Usually the monoclonal protein is associated with the IgG lambda subtype. Intravenous immunoglobulin often is considered as a first-line treatment of scleromyxedema and usually is administered at a dosage of 2 g/kg divided over 2 to 5 consecutive days per month.3 Previously, our patient had been treated with IVIG for 3 years for chronic inflammatory demyelinating polyneuropathy and had stopped 1 to 2 years before his cutaneous symptoms started. Generally, scleromyxedema patients must stay on IVIG long-term to prevent relapse, typically every 6 to 8 weeks. Second-line treatments for scleromyxedema include systemic corticosteroids and thalidomide.6 Scleromyxedema and LM have several clinical and histopathologic features in common. Our patient’s biopsy revealed increased mucin deposition associated with fibroblast proliferation confined to the superficial dermis. These histologic changes can be seen in the setting of either LM or scleromyxedema. Our patient’s diffuse skin thickening and monoclonal gammopathy were more characteristic of scleromyxedema. In contrast, LM is a localized eruption with no internal organ manifestations and no associated systemic disease, such as monoclonal gammopathy and thyroid disease.
Scleredema adultorum of Buschke (also referred to as scleredema) is a rare idiopathic dermatologic condition characterized by thickening and tightening of the skin that leads to firm, nonpitting, woody edema that initially involves the upper back and neck but can spread to the face, scalp, and shoulders; importantly, scleredema spares the hands and feet.7 Scleredema has been associated with type 2 diabetes mellitus, streptococcal upper respiratory tract infections, and monoclonal gammopathy.8 Although our patient did have a monoclonal gammopathy, he also experienced prominent hand involvement with diffuse skin thickening, which is not typical of scleredema. Additionally, biopsy of scleredema would show increased mucin but would not show the proliferation of fibroblasts that was seen in our patient’s biopsy. Furthermore, scleredema has more profound diffuse superficial and deep mucin deposition compared to scleromyxedema. Scleroderma is an autoimmune cutaneous condition that is divided into 2 categories: localized scleroderma and systemic sclerosis (SSc).9 Localized scleroderma (also called morphea) often is characterized by indurated hyperpigmented or hypopigmented lesions. There is an absence of Raynaud phenomenon, telangiectasia, and systemic disease.9 Systemic sclerosis is further divided into 2 categories—limited cutaneous and diffuse cutaneous—which are differentiated by the extent of organ system involvement. Limited cutaneous SSc involves calcinosis, Raynaud phenomenon, esophageal dysmotility, skin sclerosis distal to the elbows and knees, and telangiectasia.9 Diffuse cutaneous SSc is characterized by Raynaud phenomenon; cutaneous sclerosis proximal to the elbows and knees; and fibrosis of the gastrointestinal, pulmonary, renal, and cardiac systems.9 Scl-70 antibodies are specific for diffuse cutaneous SSc, and centromere antibodies are specific for limited cutaneous SSc. Scleromyxedema shares many of the same clinical symptoms as scleroderma; therefore, histopathologic examination is important for differentiating these disorders. Histologically, scleroderma is characterized by thickened collagen bundles associated with a variable degree of perivascular and interstitial lymphoplasmacytic inflammation. No increased dermal mucin is present.9 Our patient did not have the clinical cutaneous features of localized scleroderma and lacked the signs of internal organ involvement that typically are found in SSc. He did have Raynaud phenomenon but did not have matlike telangiectases or Scl-70 or centromere antibodies.
Reticular erythematosus mucinosis (REM) is a rare inflammatory cutaneous disease that is characterized by diffuse reticular erythematous macules or papules that may be asymptomatic or associated with pruritus.10 Reticular erythematosus mucinosis most frequently affects middle-aged women and appears on the trunk.9 Our patient was not part of the demographic group most frequently affected by REM. More importantly, our patient’s lesions were not erythematous or reticular in appearance, making the diagnosis of REM unlikely. Furthermore, REM has no associated cutaneous sclerosis or induration.
- Nofal A, Amer H, Alakad R, et al. Lichen myxedematosus: diagnostic criteria, classification, and severity grading. Int J Dermatol. 2017;56:284-290.
- Christman MP, Sukhdeo K, Kim RH, et al. Papular mucinosis, or localized lichen myxedematosus (LM)(discrete papular type). Dermatol Online J. 2017;23:8.
- Haber R, Bachour J, El Gemayel M. Scleromyxedema treatment: a systematic review and update. Int J Dermatol. 2020;59:1191-1201.
- Hazan E, Griffin TD Jr, Jabbour SA, et al. Scleromyxedema in a patient with thyroid disease: an atypical case or a case for revised criteria? Cutis. 2020;105:E6-E10.
- Shenoy A, Steixner J, Beltrani V, et al. Discrete papular lichen myxedematosus and scleromyxedema with hypothyroidism: a report of two cases. Case Rep Dermatol. 2019;11:64-70.
- Hoffman JHO, Enk AH. Scleromyxedema. J Dtsch Dermatol Ges. 2020;18:1449-1467.
- Beers WH, Ince AI, Moore TL. Scleredema adultorum of Buschke: a case report and review of the literature. Semin Arthritis Rheum. 2006;35:355-359.
- Miguel D, Schliemann S, Elsner P. Treatment of scleroderma adultorum Buschke: a systematic review. Acta Derm Venereol. 2018;98:305-309.
- Rongioletti F, Ferreli C, Atzori L, et al. Scleroderma with an update about clinicopathological correlation. G Ital Dermatol Venereol. 2018;153:208-215.
- Ocanha-Xavier JP, Cola-Senra CO, Xavier-Junior JCC. Reticular erythematous mucinosis: literature review and case report of a 24-year-old patient with systemic erythematosus lupus. Lupus. 2021;30:325-335.
The Diagnosis: Scleromyxedema
A punch biopsy of the upper back performed at an outside institution revealed increased histiocytes and abundant interstitial mucin confined to the papillary dermis (Figures 1 and 2), consistent with the lichen myxedematosus (LM) papules that may be seen in scleromyxedema. Serum protein electrophoresis revealed the presence of a protein of restricted mobility on the gamma region that occupied 5.3% of the total protein (0.3 g/dL). Urine protein electrophoresis showed free kappa light chain monoclonal protein in the gamma region. Immunofixation electrophoresis revealed the presence of IgG kappa monoclonal protein in the gamma region with 10% monotype kappa cells. The presence of Raynaud phenomenon and positive antinuclear antibody (1:320, speckled) was noted. Laboratory studies for thyroid-stimulating hormone, C-reactive protein, Scl-70 antibody, myositis panel, ribonucleoprotein antibody, Smith antibody, Sjögren syndrome–related antigens A and B antibodies, rheumatoid factor, and RNA polymerase III antibody all were within reference range. Our patient was treated with monthly intravenous immunoglobulin (IVIG), and he noted substantial improvement in skin findings after 3 months of IVIG.

Localized lichen myxedematosus is a rare idiopathic cutaneous disease that clinically is characterized by waxy indurated papules and histologically is characterized by diffuse mucin deposition and fibroblast proliferation in the upper dermis.1 Scleromyxedema is a diffuse variant of LM in which the papules and plaques of LM are associated with skin thickening involving almost the entire body and associated systemic disease. The exact mechanism of this disease is unknown, but the most widely accepted hypothesis is that immunoglobulins and cytokines contribute to the synthesis of glycosaminoglycans and thereby the deposition of mucin in the dermis.2 Scleromyxedema has a chronic course and generally responds poorly to existing treatments.1 Partial improvement has been demonstrated in treatment with topical calcineurin inhibitors and topical steroids.2

The differential diagnosis in our patient included scleromyxedema, scleredema, scleroderma, LM, and reticular erythematosus mucinosis. He was diagnosed with scleromyxedema with kappa monoclonal gammopathy. Scleromyxedema is a rare disorder involving the deposition of mucinous material in the papillary dermis that causes the formation of infiltrative skin lesions.3 The etiology is unknown, but the presence of a monoclonal protein is an important characteristic of this disorder. It is important to rule out thyroid disease as a possible etiology before concluding that the disease process is driven by the monoclonal gammopathy; this will help determine appropriate therapies.4,5 Usually the monoclonal protein is associated with the IgG lambda subtype. Intravenous immunoglobulin often is considered as a first-line treatment of scleromyxedema and usually is administered at a dosage of 2 g/kg divided over 2 to 5 consecutive days per month.3 Previously, our patient had been treated with IVIG for 3 years for chronic inflammatory demyelinating polyneuropathy and had stopped 1 to 2 years before his cutaneous symptoms started. Generally, scleromyxedema patients must stay on IVIG long-term to prevent relapse, typically every 6 to 8 weeks. Second-line treatments for scleromyxedema include systemic corticosteroids and thalidomide.6 Scleromyxedema and LM have several clinical and histopathologic features in common. Our patient’s biopsy revealed increased mucin deposition associated with fibroblast proliferation confined to the superficial dermis. These histologic changes can be seen in the setting of either LM or scleromyxedema. Our patient’s diffuse skin thickening and monoclonal gammopathy were more characteristic of scleromyxedema. In contrast, LM is a localized eruption with no internal organ manifestations and no associated systemic disease, such as monoclonal gammopathy and thyroid disease.
Scleredema adultorum of Buschke (also referred to as scleredema) is a rare idiopathic dermatologic condition characterized by thickening and tightening of the skin that leads to firm, nonpitting, woody edema that initially involves the upper back and neck but can spread to the face, scalp, and shoulders; importantly, scleredema spares the hands and feet.7 Scleredema has been associated with type 2 diabetes mellitus, streptococcal upper respiratory tract infections, and monoclonal gammopathy.8 Although our patient did have a monoclonal gammopathy, he also experienced prominent hand involvement with diffuse skin thickening, which is not typical of scleredema. Additionally, biopsy of scleredema would show increased mucin but would not show the proliferation of fibroblasts that was seen in our patient’s biopsy. Furthermore, scleredema has more profound diffuse superficial and deep mucin deposition compared to scleromyxedema. Scleroderma is an autoimmune cutaneous condition that is divided into 2 categories: localized scleroderma and systemic sclerosis (SSc).9 Localized scleroderma (also called morphea) often is characterized by indurated hyperpigmented or hypopigmented lesions. There is an absence of Raynaud phenomenon, telangiectasia, and systemic disease.9 Systemic sclerosis is further divided into 2 categories—limited cutaneous and diffuse cutaneous—which are differentiated by the extent of organ system involvement. Limited cutaneous SSc involves calcinosis, Raynaud phenomenon, esophageal dysmotility, skin sclerosis distal to the elbows and knees, and telangiectasia.9 Diffuse cutaneous SSc is characterized by Raynaud phenomenon; cutaneous sclerosis proximal to the elbows and knees; and fibrosis of the gastrointestinal, pulmonary, renal, and cardiac systems.9 Scl-70 antibodies are specific for diffuse cutaneous SSc, and centromere antibodies are specific for limited cutaneous SSc. Scleromyxedema shares many of the same clinical symptoms as scleroderma; therefore, histopathologic examination is important for differentiating these disorders. Histologically, scleroderma is characterized by thickened collagen bundles associated with a variable degree of perivascular and interstitial lymphoplasmacytic inflammation. No increased dermal mucin is present.9 Our patient did not have the clinical cutaneous features of localized scleroderma and lacked the signs of internal organ involvement that typically are found in SSc. He did have Raynaud phenomenon but did not have matlike telangiectases or Scl-70 or centromere antibodies.
Reticular erythematosus mucinosis (REM) is a rare inflammatory cutaneous disease that is characterized by diffuse reticular erythematous macules or papules that may be asymptomatic or associated with pruritus.10 Reticular erythematosus mucinosis most frequently affects middle-aged women and appears on the trunk.9 Our patient was not part of the demographic group most frequently affected by REM. More importantly, our patient’s lesions were not erythematous or reticular in appearance, making the diagnosis of REM unlikely. Furthermore, REM has no associated cutaneous sclerosis or induration.
The Diagnosis: Scleromyxedema
A punch biopsy of the upper back performed at an outside institution revealed increased histiocytes and abundant interstitial mucin confined to the papillary dermis (Figures 1 and 2), consistent with the lichen myxedematosus (LM) papules that may be seen in scleromyxedema. Serum protein electrophoresis revealed the presence of a protein of restricted mobility on the gamma region that occupied 5.3% of the total protein (0.3 g/dL). Urine protein electrophoresis showed free kappa light chain monoclonal protein in the gamma region. Immunofixation electrophoresis revealed the presence of IgG kappa monoclonal protein in the gamma region with 10% monotype kappa cells. The presence of Raynaud phenomenon and positive antinuclear antibody (1:320, speckled) was noted. Laboratory studies for thyroid-stimulating hormone, C-reactive protein, Scl-70 antibody, myositis panel, ribonucleoprotein antibody, Smith antibody, Sjögren syndrome–related antigens A and B antibodies, rheumatoid factor, and RNA polymerase III antibody all were within reference range. Our patient was treated with monthly intravenous immunoglobulin (IVIG), and he noted substantial improvement in skin findings after 3 months of IVIG.

Localized lichen myxedematosus is a rare idiopathic cutaneous disease that clinically is characterized by waxy indurated papules and histologically is characterized by diffuse mucin deposition and fibroblast proliferation in the upper dermis.1 Scleromyxedema is a diffuse variant of LM in which the papules and plaques of LM are associated with skin thickening involving almost the entire body and associated systemic disease. The exact mechanism of this disease is unknown, but the most widely accepted hypothesis is that immunoglobulins and cytokines contribute to the synthesis of glycosaminoglycans and thereby the deposition of mucin in the dermis.2 Scleromyxedema has a chronic course and generally responds poorly to existing treatments.1 Partial improvement has been demonstrated in treatment with topical calcineurin inhibitors and topical steroids.2

The differential diagnosis in our patient included scleromyxedema, scleredema, scleroderma, LM, and reticular erythematosus mucinosis. He was diagnosed with scleromyxedema with kappa monoclonal gammopathy. Scleromyxedema is a rare disorder involving the deposition of mucinous material in the papillary dermis that causes the formation of infiltrative skin lesions.3 The etiology is unknown, but the presence of a monoclonal protein is an important characteristic of this disorder. It is important to rule out thyroid disease as a possible etiology before concluding that the disease process is driven by the monoclonal gammopathy; this will help determine appropriate therapies.4,5 Usually the monoclonal protein is associated with the IgG lambda subtype. Intravenous immunoglobulin often is considered as a first-line treatment of scleromyxedema and usually is administered at a dosage of 2 g/kg divided over 2 to 5 consecutive days per month.3 Previously, our patient had been treated with IVIG for 3 years for chronic inflammatory demyelinating polyneuropathy and had stopped 1 to 2 years before his cutaneous symptoms started. Generally, scleromyxedema patients must stay on IVIG long-term to prevent relapse, typically every 6 to 8 weeks. Second-line treatments for scleromyxedema include systemic corticosteroids and thalidomide.6 Scleromyxedema and LM have several clinical and histopathologic features in common. Our patient’s biopsy revealed increased mucin deposition associated with fibroblast proliferation confined to the superficial dermis. These histologic changes can be seen in the setting of either LM or scleromyxedema. Our patient’s diffuse skin thickening and monoclonal gammopathy were more characteristic of scleromyxedema. In contrast, LM is a localized eruption with no internal organ manifestations and no associated systemic disease, such as monoclonal gammopathy and thyroid disease.
Scleredema adultorum of Buschke (also referred to as scleredema) is a rare idiopathic dermatologic condition characterized by thickening and tightening of the skin that leads to firm, nonpitting, woody edema that initially involves the upper back and neck but can spread to the face, scalp, and shoulders; importantly, scleredema spares the hands and feet.7 Scleredema has been associated with type 2 diabetes mellitus, streptococcal upper respiratory tract infections, and monoclonal gammopathy.8 Although our patient did have a monoclonal gammopathy, he also experienced prominent hand involvement with diffuse skin thickening, which is not typical of scleredema. Additionally, biopsy of scleredema would show increased mucin but would not show the proliferation of fibroblasts that was seen in our patient’s biopsy. Furthermore, scleredema has more profound diffuse superficial and deep mucin deposition compared to scleromyxedema. Scleroderma is an autoimmune cutaneous condition that is divided into 2 categories: localized scleroderma and systemic sclerosis (SSc).9 Localized scleroderma (also called morphea) often is characterized by indurated hyperpigmented or hypopigmented lesions. There is an absence of Raynaud phenomenon, telangiectasia, and systemic disease.9 Systemic sclerosis is further divided into 2 categories—limited cutaneous and diffuse cutaneous—which are differentiated by the extent of organ system involvement. Limited cutaneous SSc involves calcinosis, Raynaud phenomenon, esophageal dysmotility, skin sclerosis distal to the elbows and knees, and telangiectasia.9 Diffuse cutaneous SSc is characterized by Raynaud phenomenon; cutaneous sclerosis proximal to the elbows and knees; and fibrosis of the gastrointestinal, pulmonary, renal, and cardiac systems.9 Scl-70 antibodies are specific for diffuse cutaneous SSc, and centromere antibodies are specific for limited cutaneous SSc. Scleromyxedema shares many of the same clinical symptoms as scleroderma; therefore, histopathologic examination is important for differentiating these disorders. Histologically, scleroderma is characterized by thickened collagen bundles associated with a variable degree of perivascular and interstitial lymphoplasmacytic inflammation. No increased dermal mucin is present.9 Our patient did not have the clinical cutaneous features of localized scleroderma and lacked the signs of internal organ involvement that typically are found in SSc. He did have Raynaud phenomenon but did not have matlike telangiectases or Scl-70 or centromere antibodies.
Reticular erythematosus mucinosis (REM) is a rare inflammatory cutaneous disease that is characterized by diffuse reticular erythematous macules or papules that may be asymptomatic or associated with pruritus.10 Reticular erythematosus mucinosis most frequently affects middle-aged women and appears on the trunk.9 Our patient was not part of the demographic group most frequently affected by REM. More importantly, our patient’s lesions were not erythematous or reticular in appearance, making the diagnosis of REM unlikely. Furthermore, REM has no associated cutaneous sclerosis or induration.
- Nofal A, Amer H, Alakad R, et al. Lichen myxedematosus: diagnostic criteria, classification, and severity grading. Int J Dermatol. 2017;56:284-290.
- Christman MP, Sukhdeo K, Kim RH, et al. Papular mucinosis, or localized lichen myxedematosus (LM)(discrete papular type). Dermatol Online J. 2017;23:8.
- Haber R, Bachour J, El Gemayel M. Scleromyxedema treatment: a systematic review and update. Int J Dermatol. 2020;59:1191-1201.
- Hazan E, Griffin TD Jr, Jabbour SA, et al. Scleromyxedema in a patient with thyroid disease: an atypical case or a case for revised criteria? Cutis. 2020;105:E6-E10.
- Shenoy A, Steixner J, Beltrani V, et al. Discrete papular lichen myxedematosus and scleromyxedema with hypothyroidism: a report of two cases. Case Rep Dermatol. 2019;11:64-70.
- Hoffman JHO, Enk AH. Scleromyxedema. J Dtsch Dermatol Ges. 2020;18:1449-1467.
- Beers WH, Ince AI, Moore TL. Scleredema adultorum of Buschke: a case report and review of the literature. Semin Arthritis Rheum. 2006;35:355-359.
- Miguel D, Schliemann S, Elsner P. Treatment of scleroderma adultorum Buschke: a systematic review. Acta Derm Venereol. 2018;98:305-309.
- Rongioletti F, Ferreli C, Atzori L, et al. Scleroderma with an update about clinicopathological correlation. G Ital Dermatol Venereol. 2018;153:208-215.
- Ocanha-Xavier JP, Cola-Senra CO, Xavier-Junior JCC. Reticular erythematous mucinosis: literature review and case report of a 24-year-old patient with systemic erythematosus lupus. Lupus. 2021;30:325-335.
- Nofal A, Amer H, Alakad R, et al. Lichen myxedematosus: diagnostic criteria, classification, and severity grading. Int J Dermatol. 2017;56:284-290.
- Christman MP, Sukhdeo K, Kim RH, et al. Papular mucinosis, or localized lichen myxedematosus (LM)(discrete papular type). Dermatol Online J. 2017;23:8.
- Haber R, Bachour J, El Gemayel M. Scleromyxedema treatment: a systematic review and update. Int J Dermatol. 2020;59:1191-1201.
- Hazan E, Griffin TD Jr, Jabbour SA, et al. Scleromyxedema in a patient with thyroid disease: an atypical case or a case for revised criteria? Cutis. 2020;105:E6-E10.
- Shenoy A, Steixner J, Beltrani V, et al. Discrete papular lichen myxedematosus and scleromyxedema with hypothyroidism: a report of two cases. Case Rep Dermatol. 2019;11:64-70.
- Hoffman JHO, Enk AH. Scleromyxedema. J Dtsch Dermatol Ges. 2020;18:1449-1467.
- Beers WH, Ince AI, Moore TL. Scleredema adultorum of Buschke: a case report and review of the literature. Semin Arthritis Rheum. 2006;35:355-359.
- Miguel D, Schliemann S, Elsner P. Treatment of scleroderma adultorum Buschke: a systematic review. Acta Derm Venereol. 2018;98:305-309.
- Rongioletti F, Ferreli C, Atzori L, et al. Scleroderma with an update about clinicopathological correlation. G Ital Dermatol Venereol. 2018;153:208-215.
- Ocanha-Xavier JP, Cola-Senra CO, Xavier-Junior JCC. Reticular erythematous mucinosis: literature review and case report of a 24-year-old patient with systemic erythematosus lupus. Lupus. 2021;30:325-335.
A 76-year-old man presented to our clinic with diffusely thickened and tightened skin that worsened over the course of 1 year, as well as numerous scattered small, firm, flesh-colored papules arranged in a linear pattern over the face, ears, neck, chest, abdomen, arms, hands, and knees. His symptoms progressed to include substantial skin thickening initially over the thighs followed by the arms, chest, back (top), and face. He developed confluent cobblestonelike plaques over the elbows and hands (bottom) and eventually developed decreased oral aperture limiting oral intake as well as decreased range of motion in the hands. The patient had a deep furrowed appearance of the brow accompanied by discrete, scattered, flesh-colored papules on the forehead and behind the ears. Deep furrows also were present on the back. When the proximal interphalangeal joints of the hands were extended, elevated rings with central depression were seen instead of horizontal folds.
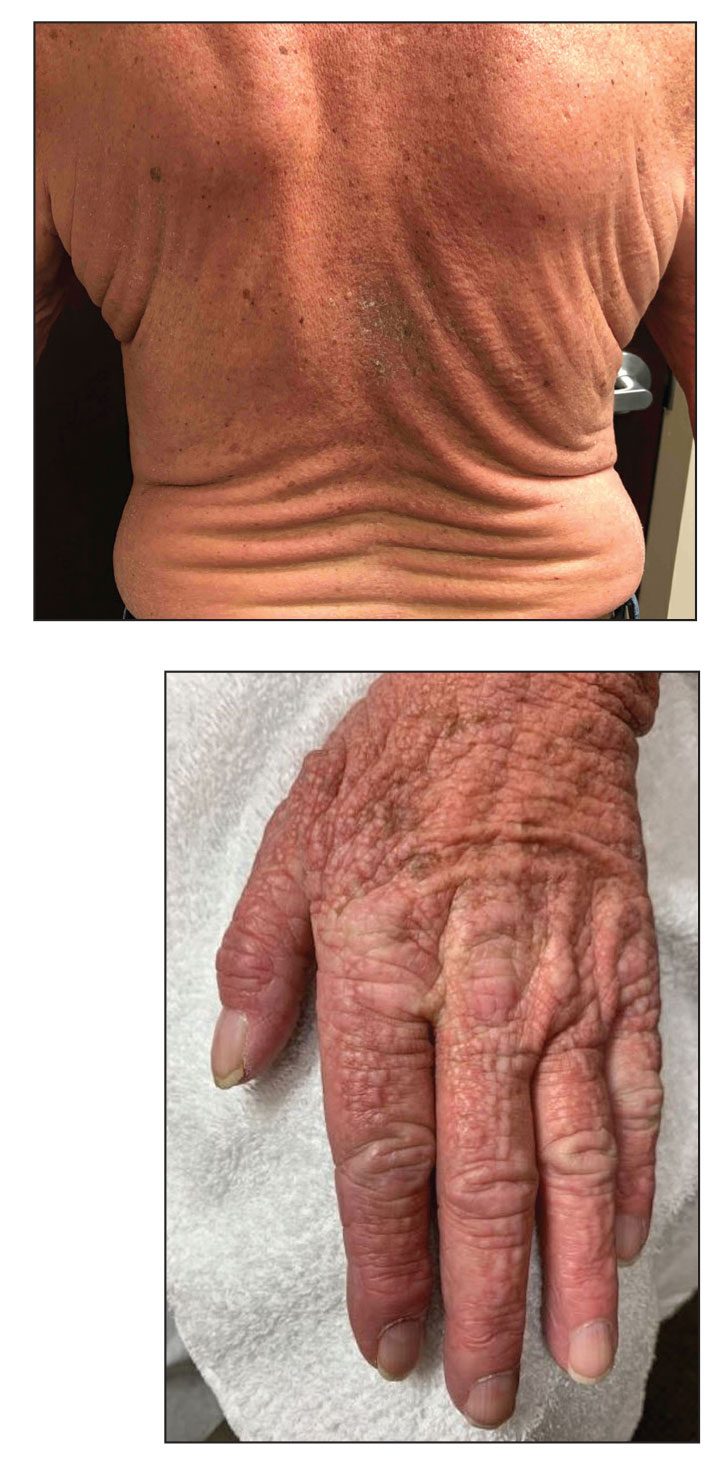
Angiolymphoid Hyperplasia with Eosinophilia in a Patient With Coccidioidomycosis
Angiolymphoid hyperplasia with eosinophilia (ALHE) is a rare nodular unencapsulated mass that is characterized by benign anomalous vascular hyperplasia of epithelioidlike endothelial cells attached to dilated blood vessels. The mass is surrounded by lymphocytes and eosinophils that can present clinically as papules, plaques, or nodules.1 The etiology of ALHE is unknown; it is hypothesized that it is a vascular neoplasm or a lymphoproliferative disorder.
Coccidioidomycosis (CM) is a prevalent deep fungal infection endemic to the southwestern United States caused by Coccidioides immitis and Coccidioides posadasii. Infection can occur from direct inoculation through abrasions or direct trauma but usually occurs through the inhalation of spores and can result in a reactive rash (eg, Sweet syndrome, erythema nodosum, interstitial granulomatous dermatitis).2 Coccidioidomycosis also can result in respiratory pneumonia and dissemination from pulmonary infection of the skin. As such, it is important to distinguish CM and its immunologically mediated eruptions for accurate diagnosis and treatment.
We report a novel case of ALHE as a reactive dermatologic presentation in a patient with CM.
Case Report
A 72-year-old woman presented to the dermatology clinic with itchy papules and plaques on the arms and legs of 17 years’ duration. Her medical history included coronary artery disease and hypercholesterolemia as well as a remote history of cutaneous marginal zone B-cell lymphoma of the nose, which was confirmed by histology and treated more than 10 years prior and has remained in remission for 6 years. Her current medications included aspirin, atorvastatin, lisinopril, and metoprolol succinate.
Our patient first presented to our dermatology clinic for itchy nodules and papules on the legs and arms. The patient previously had been seen by another dermatologist 2 months prior for the same condition. At that time, biopsies of the lesions were reported as prurigo nodules. Physical examination at the current presentation revealed round, pink to flesh-colored, raised papules and plaques scattered on the arms and legs (Figure 1). The differential diagnosis included lymphomatoid papulosis, cutaneous B-cell lymphoma, pseudolymphoma, cutaneous CM, and papular mucinosis.
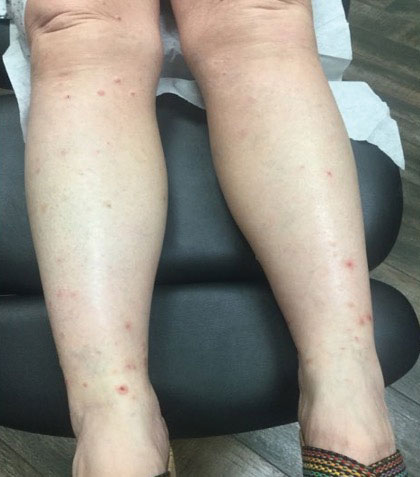
Four-mm punch biopsies of the right proximal pretibial region and left knee region were taken and sent for histologic analysis, direct immunofluorescence testing, and tissue culture. Testing for atypical mycobacteria and deep fungal infection was negative; bacterial cultures and sensitivity testing were negative. Direct immunofluorescence testing was negative. Microscopic examination of material from the right proximal pretibial region showed widely dilated, variously shaped, large blood vessels in a multinodular pattern; the vessels also were surrounded by an inflammatory cell infiltrate containing eosinophils. Histologic findings were consistent with ALHE.
Subsequent biopsies were completed 2 weeks and 1 month from the initial presentation. Both histology reports—from 2 different histopathology laboratories—were consistent with ALHE (Figure 2). Additional work-up during the patient’s initial visit to our clinic for the rash included CM serologic testing, which demonstrated IgM and IgG antibodies. Subsequently, chest radiography revealed a 2.2×2.3-cm mass in the right lower lobe of the lung. Follow-up computed tomography 1 month later confirmed the nodule in the same area to be 2.3×2.1×1.8 cm.
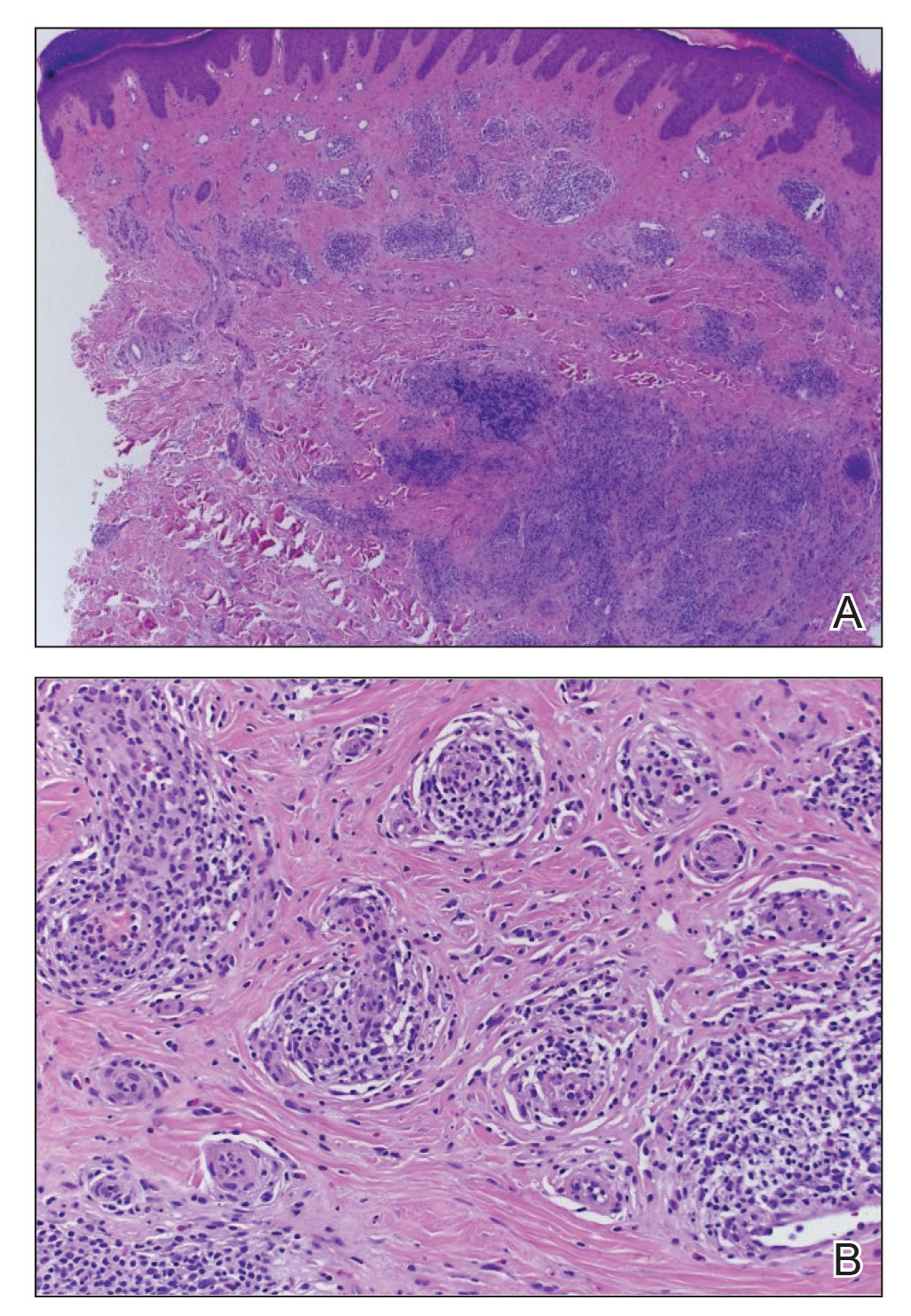
The patient was referred to pulmonology and was treated for pulmonary CM with oral fluconazole 200 mg twice daily for 4 months. Initial treatment also included clobetasol cream 0.05% applied twice daily, which did not produce marked improvement in pruritus. Narrowband UVB phototherapy was attempted, but the patient could not complete the course because of travel time to the office; however, the patient’s ALHE improved considerably with the fluconazole treatment for pulmonary CM.
Oral doxycycline 100 mg twice daily was added to the fluconazole 2 months after her initial visit to our office, which kept the ALHE at bay and helped with the pruritus (Figure 3). Pulmonology and primary care comanaged the pulmonary CM with oral fluconazole 200 mg twice daily. Repeat serologic testing for CM was negative for IgG and IgM after 14 months since the initial visit to the office.
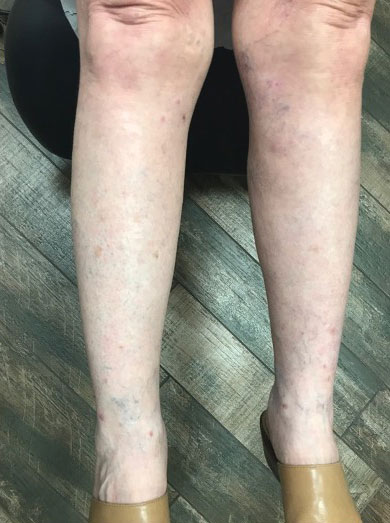
Comment
Pulmonary CM infection has varying dermatologic manifestations. A PubMed search of articles indexed for MEDLINE using the terms ALHE and coccidioidomycosis yielded no case reports; in fact, there have been few reported cases of ALHE at all. Notable conditions associated with ALHE include membranous nephropathy and arteriovenous malformations treated with corticosteroids and surgery, respectively.3,4 Our case is a rare presentation of CM infection manifesting with ALHE. Following treatment and remission for our patient’s CM infection, the ALHE lesion decreased in size.
Standard treatment of uncomplicated CM involves azole antifungals, typically oral fluconazole or itraconazole 400 to 600 mg/d. In more severe cases (eg, immunocompromised patients) amphotericin B can be used.5 Our patient was treated with oral fluconazole 200 mg twice daily for 4 months.
In the literature, treatment via surgical excision, steroid injection, pulsed-dye laser therapy, and radiotherapy also has been described.6-8 Antibiotics including clindamycin, doxycycline, and amoxicillin-clavulanate also have been shown to be effective.9
In our patient, ALHE improved when oral doxycycline 100 mg twice daily was added to the oral fluconazole. In fact, after 4 months of treatment, the CM infection and ALHE lesions both improved to a point at which the lesions were not visible. When those lesions recurred 15 months later, they responded with another course of doxycycline and fluconazole.
Upon recurrence, the patient was asked to have her care transferred to her pulmonologist, who then managed the fluconazole regimen. During the pulmonologist’s workup, no peripheral eosinophilia was found. This is important because eosinophils can be a marker for CM infection; in this case, however, the ALHE lesion was a reactive process to the infection. Classically known to play a reactive role in fungal infection, these white blood cells demonstrate reactivity to the environmental fungus Alternaria alternata by contact-dependent killing, utilizing β2 integrins and CD11b to recognize and adhere to β-glucan. Eosinophils react through contact-dependent killing, releasing cytotoxic granule proteins and proinflammatory mediators, and have been documented to occur in CM and Paracoccidioides brasiliensis infection, in which they deposit major basic protein on the organism.10 Most pertinent to our case with ALHE and CM is the ability of eosinophils to communicate with other immune cells. Eosinophils play a role in the active inflammation of CM through cytokine signaling, which may propagate formation of ALHE.
The function of eosinophils in ALHE is poorly understood; it is unclear whether they act as a primary driver of pathogenesis or are simply indicators of secondary infiltration or infection. Our review of the current literature suggests that eosinophils are unnecessary for progression of ALHE but might be involved at its onset. As reported, even monoclonal antibody therapy (eg, mepolizumab and benralizumab) that effectively depletes eosinophil levels by negating IL-5 signaling do not slow progression of ALHE.11 Symptomatic changes are modest at best (ie, simply softening the ALHE nodules).
Our patient had no peripheral eosinophilia, suggesting that the onset of ALHE might not be caused by eosinophilia but a different inflammatory process—in this patient, by CM. Because peripheral eosinophilia was not seen in our patient, the presence of eosinophils in the ALHE lesion likely is unnecessary for its onset or progression but is a secondary process that exacerbates the lesion. The pathogenesis is unknown but could be directed toward lymphocytes and plasma cells, with eosinophils as part of the dynamic process.11
Conclusion
Because reports of an association between CM and ALHE are limited, our case is distinguished by a unique clinical presentation of ALHE. When a patient is given a diagnosis of ALHE, it therefore is important to consider exposure to CM as a cause, especially in patients who reside in or travel to a region where CM is endemic.
- Wells GC, Whimster IW. Subcutaneous angiolymphoid hyperplasia with eosinophilia. Br J Dermatol. 1969;81:1-14. doi:10.1111/j.1365-2133.1969.tb15914.x
- DiCaudo D. Coccidioidomycosis. Semin Cutan Med Surg. 2014;33:140-145. doi:10.12788/j.sder.0111
- Onishi Y, Ohara K. Angiolymphoid hyperplasia with eosinophilia associated with arteriovenous malformation: a clinicopathological correlation with angiography and serial estimation of serum levels of renin, eosinophil cationic protein and interleukin 5. Br J Dermatol. 1999;140:1153-1156. doi:10.1046/j.1365-2133.1999.02880.x
- Matsumoto A, Matsui I, Namba T, et al. VEGF-A links angiolymphoid hyperplasia with eosinophilia (ALHE) to THSD7A membranous nephropathy: a report of 2 cases. Am J Kidney Dis. 2019;73:880-885. doi:10.1053/j.ajkd.2018.10.009
- Bercovitch RS, Catanzaro A, Schwartz BS, et al. Coccidioidomycosis during pregnancy: a review and recommendations for management. Clin Infect Dis. 2011;53:363-368. doi:10.1093/cid/cir410
- Youssef A, Hasan AR, Youssef Y, et al. Angiolymphoid hyperplasia with eosinophilia: a case report. J Med Case Rep. 2018;12:89. doi:10.1186/s13256-018-1599-x
- Abrahamson TG, Davis DA. Angiolymphoid hyperplasia witheosinophilia responsive to pulsed dye laser. J Am Acad Dermatol. 2003;49(2 suppl case reports):S195-S196. doi:10.1067/mjd.2003.314
- Lembo S, Balato A, Cirillo T, et al. A long-term follow-up of angiolymphoid hyperplasia with eosinophilia treated by corticosteroids: when a traditional therapy is still up-to-date. Case Rep Dermatol. 2011;3:64-67. doi:10.1159/000323182
- Cleveland E. Atypical presentation of angiolymphomatous hyperplasia with eosinophilia. J Am Acad Dermatol. 2018;79(3 suppl 1):AB53. doi:10.1016/j.jaad.2018.05.249
- Ravin KA, Loy M. The eosinophil in infection. Clin Rev Allergy Immunol. 2015;50:214-227. doi:10.1007/s12016-015-8525-4
- Grünewald M, Stölzl D, Wehkamp U, et al. Role of eosinophils in angiolymphoid hyperplasia with eosinophilia. JAMA Dermatol. 2021;157:1241-1243. doi:10.1001/jamadermatol.2021.2732
Angiolymphoid hyperplasia with eosinophilia (ALHE) is a rare nodular unencapsulated mass that is characterized by benign anomalous vascular hyperplasia of epithelioidlike endothelial cells attached to dilated blood vessels. The mass is surrounded by lymphocytes and eosinophils that can present clinically as papules, plaques, or nodules.1 The etiology of ALHE is unknown; it is hypothesized that it is a vascular neoplasm or a lymphoproliferative disorder.
Coccidioidomycosis (CM) is a prevalent deep fungal infection endemic to the southwestern United States caused by Coccidioides immitis and Coccidioides posadasii. Infection can occur from direct inoculation through abrasions or direct trauma but usually occurs through the inhalation of spores and can result in a reactive rash (eg, Sweet syndrome, erythema nodosum, interstitial granulomatous dermatitis).2 Coccidioidomycosis also can result in respiratory pneumonia and dissemination from pulmonary infection of the skin. As such, it is important to distinguish CM and its immunologically mediated eruptions for accurate diagnosis and treatment.
We report a novel case of ALHE as a reactive dermatologic presentation in a patient with CM.
Case Report
A 72-year-old woman presented to the dermatology clinic with itchy papules and plaques on the arms and legs of 17 years’ duration. Her medical history included coronary artery disease and hypercholesterolemia as well as a remote history of cutaneous marginal zone B-cell lymphoma of the nose, which was confirmed by histology and treated more than 10 years prior and has remained in remission for 6 years. Her current medications included aspirin, atorvastatin, lisinopril, and metoprolol succinate.
Our patient first presented to our dermatology clinic for itchy nodules and papules on the legs and arms. The patient previously had been seen by another dermatologist 2 months prior for the same condition. At that time, biopsies of the lesions were reported as prurigo nodules. Physical examination at the current presentation revealed round, pink to flesh-colored, raised papules and plaques scattered on the arms and legs (Figure 1). The differential diagnosis included lymphomatoid papulosis, cutaneous B-cell lymphoma, pseudolymphoma, cutaneous CM, and papular mucinosis.

Four-mm punch biopsies of the right proximal pretibial region and left knee region were taken and sent for histologic analysis, direct immunofluorescence testing, and tissue culture. Testing for atypical mycobacteria and deep fungal infection was negative; bacterial cultures and sensitivity testing were negative. Direct immunofluorescence testing was negative. Microscopic examination of material from the right proximal pretibial region showed widely dilated, variously shaped, large blood vessels in a multinodular pattern; the vessels also were surrounded by an inflammatory cell infiltrate containing eosinophils. Histologic findings were consistent with ALHE.
Subsequent biopsies were completed 2 weeks and 1 month from the initial presentation. Both histology reports—from 2 different histopathology laboratories—were consistent with ALHE (Figure 2). Additional work-up during the patient’s initial visit to our clinic for the rash included CM serologic testing, which demonstrated IgM and IgG antibodies. Subsequently, chest radiography revealed a 2.2×2.3-cm mass in the right lower lobe of the lung. Follow-up computed tomography 1 month later confirmed the nodule in the same area to be 2.3×2.1×1.8 cm.

The patient was referred to pulmonology and was treated for pulmonary CM with oral fluconazole 200 mg twice daily for 4 months. Initial treatment also included clobetasol cream 0.05% applied twice daily, which did not produce marked improvement in pruritus. Narrowband UVB phototherapy was attempted, but the patient could not complete the course because of travel time to the office; however, the patient’s ALHE improved considerably with the fluconazole treatment for pulmonary CM.
Oral doxycycline 100 mg twice daily was added to the fluconazole 2 months after her initial visit to our office, which kept the ALHE at bay and helped with the pruritus (Figure 3). Pulmonology and primary care comanaged the pulmonary CM with oral fluconazole 200 mg twice daily. Repeat serologic testing for CM was negative for IgG and IgM after 14 months since the initial visit to the office.

Comment
Pulmonary CM infection has varying dermatologic manifestations. A PubMed search of articles indexed for MEDLINE using the terms ALHE and coccidioidomycosis yielded no case reports; in fact, there have been few reported cases of ALHE at all. Notable conditions associated with ALHE include membranous nephropathy and arteriovenous malformations treated with corticosteroids and surgery, respectively.3,4 Our case is a rare presentation of CM infection manifesting with ALHE. Following treatment and remission for our patient’s CM infection, the ALHE lesion decreased in size.
Standard treatment of uncomplicated CM involves azole antifungals, typically oral fluconazole or itraconazole 400 to 600 mg/d. In more severe cases (eg, immunocompromised patients) amphotericin B can be used.5 Our patient was treated with oral fluconazole 200 mg twice daily for 4 months.
In the literature, treatment via surgical excision, steroid injection, pulsed-dye laser therapy, and radiotherapy also has been described.6-8 Antibiotics including clindamycin, doxycycline, and amoxicillin-clavulanate also have been shown to be effective.9
In our patient, ALHE improved when oral doxycycline 100 mg twice daily was added to the oral fluconazole. In fact, after 4 months of treatment, the CM infection and ALHE lesions both improved to a point at which the lesions were not visible. When those lesions recurred 15 months later, they responded with another course of doxycycline and fluconazole.
Upon recurrence, the patient was asked to have her care transferred to her pulmonologist, who then managed the fluconazole regimen. During the pulmonologist’s workup, no peripheral eosinophilia was found. This is important because eosinophils can be a marker for CM infection; in this case, however, the ALHE lesion was a reactive process to the infection. Classically known to play a reactive role in fungal infection, these white blood cells demonstrate reactivity to the environmental fungus Alternaria alternata by contact-dependent killing, utilizing β2 integrins and CD11b to recognize and adhere to β-glucan. Eosinophils react through contact-dependent killing, releasing cytotoxic granule proteins and proinflammatory mediators, and have been documented to occur in CM and Paracoccidioides brasiliensis infection, in which they deposit major basic protein on the organism.10 Most pertinent to our case with ALHE and CM is the ability of eosinophils to communicate with other immune cells. Eosinophils play a role in the active inflammation of CM through cytokine signaling, which may propagate formation of ALHE.
The function of eosinophils in ALHE is poorly understood; it is unclear whether they act as a primary driver of pathogenesis or are simply indicators of secondary infiltration or infection. Our review of the current literature suggests that eosinophils are unnecessary for progression of ALHE but might be involved at its onset. As reported, even monoclonal antibody therapy (eg, mepolizumab and benralizumab) that effectively depletes eosinophil levels by negating IL-5 signaling do not slow progression of ALHE.11 Symptomatic changes are modest at best (ie, simply softening the ALHE nodules).
Our patient had no peripheral eosinophilia, suggesting that the onset of ALHE might not be caused by eosinophilia but a different inflammatory process—in this patient, by CM. Because peripheral eosinophilia was not seen in our patient, the presence of eosinophils in the ALHE lesion likely is unnecessary for its onset or progression but is a secondary process that exacerbates the lesion. The pathogenesis is unknown but could be directed toward lymphocytes and plasma cells, with eosinophils as part of the dynamic process.11
Conclusion
Because reports of an association between CM and ALHE are limited, our case is distinguished by a unique clinical presentation of ALHE. When a patient is given a diagnosis of ALHE, it therefore is important to consider exposure to CM as a cause, especially in patients who reside in or travel to a region where CM is endemic.
Angiolymphoid hyperplasia with eosinophilia (ALHE) is a rare nodular unencapsulated mass that is characterized by benign anomalous vascular hyperplasia of epithelioidlike endothelial cells attached to dilated blood vessels. The mass is surrounded by lymphocytes and eosinophils that can present clinically as papules, plaques, or nodules.1 The etiology of ALHE is unknown; it is hypothesized that it is a vascular neoplasm or a lymphoproliferative disorder.
Coccidioidomycosis (CM) is a prevalent deep fungal infection endemic to the southwestern United States caused by Coccidioides immitis and Coccidioides posadasii. Infection can occur from direct inoculation through abrasions or direct trauma but usually occurs through the inhalation of spores and can result in a reactive rash (eg, Sweet syndrome, erythema nodosum, interstitial granulomatous dermatitis).2 Coccidioidomycosis also can result in respiratory pneumonia and dissemination from pulmonary infection of the skin. As such, it is important to distinguish CM and its immunologically mediated eruptions for accurate diagnosis and treatment.
We report a novel case of ALHE as a reactive dermatologic presentation in a patient with CM.
Case Report
A 72-year-old woman presented to the dermatology clinic with itchy papules and plaques on the arms and legs of 17 years’ duration. Her medical history included coronary artery disease and hypercholesterolemia as well as a remote history of cutaneous marginal zone B-cell lymphoma of the nose, which was confirmed by histology and treated more than 10 years prior and has remained in remission for 6 years. Her current medications included aspirin, atorvastatin, lisinopril, and metoprolol succinate.
Our patient first presented to our dermatology clinic for itchy nodules and papules on the legs and arms. The patient previously had been seen by another dermatologist 2 months prior for the same condition. At that time, biopsies of the lesions were reported as prurigo nodules. Physical examination at the current presentation revealed round, pink to flesh-colored, raised papules and plaques scattered on the arms and legs (Figure 1). The differential diagnosis included lymphomatoid papulosis, cutaneous B-cell lymphoma, pseudolymphoma, cutaneous CM, and papular mucinosis.

Four-mm punch biopsies of the right proximal pretibial region and left knee region were taken and sent for histologic analysis, direct immunofluorescence testing, and tissue culture. Testing for atypical mycobacteria and deep fungal infection was negative; bacterial cultures and sensitivity testing were negative. Direct immunofluorescence testing was negative. Microscopic examination of material from the right proximal pretibial region showed widely dilated, variously shaped, large blood vessels in a multinodular pattern; the vessels also were surrounded by an inflammatory cell infiltrate containing eosinophils. Histologic findings were consistent with ALHE.
Subsequent biopsies were completed 2 weeks and 1 month from the initial presentation. Both histology reports—from 2 different histopathology laboratories—were consistent with ALHE (Figure 2). Additional work-up during the patient’s initial visit to our clinic for the rash included CM serologic testing, which demonstrated IgM and IgG antibodies. Subsequently, chest radiography revealed a 2.2×2.3-cm mass in the right lower lobe of the lung. Follow-up computed tomography 1 month later confirmed the nodule in the same area to be 2.3×2.1×1.8 cm.

The patient was referred to pulmonology and was treated for pulmonary CM with oral fluconazole 200 mg twice daily for 4 months. Initial treatment also included clobetasol cream 0.05% applied twice daily, which did not produce marked improvement in pruritus. Narrowband UVB phototherapy was attempted, but the patient could not complete the course because of travel time to the office; however, the patient’s ALHE improved considerably with the fluconazole treatment for pulmonary CM.
Oral doxycycline 100 mg twice daily was added to the fluconazole 2 months after her initial visit to our office, which kept the ALHE at bay and helped with the pruritus (Figure 3). Pulmonology and primary care comanaged the pulmonary CM with oral fluconazole 200 mg twice daily. Repeat serologic testing for CM was negative for IgG and IgM after 14 months since the initial visit to the office.

Comment
Pulmonary CM infection has varying dermatologic manifestations. A PubMed search of articles indexed for MEDLINE using the terms ALHE and coccidioidomycosis yielded no case reports; in fact, there have been few reported cases of ALHE at all. Notable conditions associated with ALHE include membranous nephropathy and arteriovenous malformations treated with corticosteroids and surgery, respectively.3,4 Our case is a rare presentation of CM infection manifesting with ALHE. Following treatment and remission for our patient’s CM infection, the ALHE lesion decreased in size.
Standard treatment of uncomplicated CM involves azole antifungals, typically oral fluconazole or itraconazole 400 to 600 mg/d. In more severe cases (eg, immunocompromised patients) amphotericin B can be used.5 Our patient was treated with oral fluconazole 200 mg twice daily for 4 months.
In the literature, treatment via surgical excision, steroid injection, pulsed-dye laser therapy, and radiotherapy also has been described.6-8 Antibiotics including clindamycin, doxycycline, and amoxicillin-clavulanate also have been shown to be effective.9
In our patient, ALHE improved when oral doxycycline 100 mg twice daily was added to the oral fluconazole. In fact, after 4 months of treatment, the CM infection and ALHE lesions both improved to a point at which the lesions were not visible. When those lesions recurred 15 months later, they responded with another course of doxycycline and fluconazole.
Upon recurrence, the patient was asked to have her care transferred to her pulmonologist, who then managed the fluconazole regimen. During the pulmonologist’s workup, no peripheral eosinophilia was found. This is important because eosinophils can be a marker for CM infection; in this case, however, the ALHE lesion was a reactive process to the infection. Classically known to play a reactive role in fungal infection, these white blood cells demonstrate reactivity to the environmental fungus Alternaria alternata by contact-dependent killing, utilizing β2 integrins and CD11b to recognize and adhere to β-glucan. Eosinophils react through contact-dependent killing, releasing cytotoxic granule proteins and proinflammatory mediators, and have been documented to occur in CM and Paracoccidioides brasiliensis infection, in which they deposit major basic protein on the organism.10 Most pertinent to our case with ALHE and CM is the ability of eosinophils to communicate with other immune cells. Eosinophils play a role in the active inflammation of CM through cytokine signaling, which may propagate formation of ALHE.
The function of eosinophils in ALHE is poorly understood; it is unclear whether they act as a primary driver of pathogenesis or are simply indicators of secondary infiltration or infection. Our review of the current literature suggests that eosinophils are unnecessary for progression of ALHE but might be involved at its onset. As reported, even monoclonal antibody therapy (eg, mepolizumab and benralizumab) that effectively depletes eosinophil levels by negating IL-5 signaling do not slow progression of ALHE.11 Symptomatic changes are modest at best (ie, simply softening the ALHE nodules).
Our patient had no peripheral eosinophilia, suggesting that the onset of ALHE might not be caused by eosinophilia but a different inflammatory process—in this patient, by CM. Because peripheral eosinophilia was not seen in our patient, the presence of eosinophils in the ALHE lesion likely is unnecessary for its onset or progression but is a secondary process that exacerbates the lesion. The pathogenesis is unknown but could be directed toward lymphocytes and plasma cells, with eosinophils as part of the dynamic process.11
Conclusion
Because reports of an association between CM and ALHE are limited, our case is distinguished by a unique clinical presentation of ALHE. When a patient is given a diagnosis of ALHE, it therefore is important to consider exposure to CM as a cause, especially in patients who reside in or travel to a region where CM is endemic.
- Wells GC, Whimster IW. Subcutaneous angiolymphoid hyperplasia with eosinophilia. Br J Dermatol. 1969;81:1-14. doi:10.1111/j.1365-2133.1969.tb15914.x
- DiCaudo D. Coccidioidomycosis. Semin Cutan Med Surg. 2014;33:140-145. doi:10.12788/j.sder.0111
- Onishi Y, Ohara K. Angiolymphoid hyperplasia with eosinophilia associated with arteriovenous malformation: a clinicopathological correlation with angiography and serial estimation of serum levels of renin, eosinophil cationic protein and interleukin 5. Br J Dermatol. 1999;140:1153-1156. doi:10.1046/j.1365-2133.1999.02880.x
- Matsumoto A, Matsui I, Namba T, et al. VEGF-A links angiolymphoid hyperplasia with eosinophilia (ALHE) to THSD7A membranous nephropathy: a report of 2 cases. Am J Kidney Dis. 2019;73:880-885. doi:10.1053/j.ajkd.2018.10.009
- Bercovitch RS, Catanzaro A, Schwartz BS, et al. Coccidioidomycosis during pregnancy: a review and recommendations for management. Clin Infect Dis. 2011;53:363-368. doi:10.1093/cid/cir410
- Youssef A, Hasan AR, Youssef Y, et al. Angiolymphoid hyperplasia with eosinophilia: a case report. J Med Case Rep. 2018;12:89. doi:10.1186/s13256-018-1599-x
- Abrahamson TG, Davis DA. Angiolymphoid hyperplasia witheosinophilia responsive to pulsed dye laser. J Am Acad Dermatol. 2003;49(2 suppl case reports):S195-S196. doi:10.1067/mjd.2003.314
- Lembo S, Balato A, Cirillo T, et al. A long-term follow-up of angiolymphoid hyperplasia with eosinophilia treated by corticosteroids: when a traditional therapy is still up-to-date. Case Rep Dermatol. 2011;3:64-67. doi:10.1159/000323182
- Cleveland E. Atypical presentation of angiolymphomatous hyperplasia with eosinophilia. J Am Acad Dermatol. 2018;79(3 suppl 1):AB53. doi:10.1016/j.jaad.2018.05.249
- Ravin KA, Loy M. The eosinophil in infection. Clin Rev Allergy Immunol. 2015;50:214-227. doi:10.1007/s12016-015-8525-4
- Grünewald M, Stölzl D, Wehkamp U, et al. Role of eosinophils in angiolymphoid hyperplasia with eosinophilia. JAMA Dermatol. 2021;157:1241-1243. doi:10.1001/jamadermatol.2021.2732
- Wells GC, Whimster IW. Subcutaneous angiolymphoid hyperplasia with eosinophilia. Br J Dermatol. 1969;81:1-14. doi:10.1111/j.1365-2133.1969.tb15914.x
- DiCaudo D. Coccidioidomycosis. Semin Cutan Med Surg. 2014;33:140-145. doi:10.12788/j.sder.0111
- Onishi Y, Ohara K. Angiolymphoid hyperplasia with eosinophilia associated with arteriovenous malformation: a clinicopathological correlation with angiography and serial estimation of serum levels of renin, eosinophil cationic protein and interleukin 5. Br J Dermatol. 1999;140:1153-1156. doi:10.1046/j.1365-2133.1999.02880.x
- Matsumoto A, Matsui I, Namba T, et al. VEGF-A links angiolymphoid hyperplasia with eosinophilia (ALHE) to THSD7A membranous nephropathy: a report of 2 cases. Am J Kidney Dis. 2019;73:880-885. doi:10.1053/j.ajkd.2018.10.009
- Bercovitch RS, Catanzaro A, Schwartz BS, et al. Coccidioidomycosis during pregnancy: a review and recommendations for management. Clin Infect Dis. 2011;53:363-368. doi:10.1093/cid/cir410
- Youssef A, Hasan AR, Youssef Y, et al. Angiolymphoid hyperplasia with eosinophilia: a case report. J Med Case Rep. 2018;12:89. doi:10.1186/s13256-018-1599-x
- Abrahamson TG, Davis DA. Angiolymphoid hyperplasia witheosinophilia responsive to pulsed dye laser. J Am Acad Dermatol. 2003;49(2 suppl case reports):S195-S196. doi:10.1067/mjd.2003.314
- Lembo S, Balato A, Cirillo T, et al. A long-term follow-up of angiolymphoid hyperplasia with eosinophilia treated by corticosteroids: when a traditional therapy is still up-to-date. Case Rep Dermatol. 2011;3:64-67. doi:10.1159/000323182
- Cleveland E. Atypical presentation of angiolymphomatous hyperplasia with eosinophilia. J Am Acad Dermatol. 2018;79(3 suppl 1):AB53. doi:10.1016/j.jaad.2018.05.249
- Ravin KA, Loy M. The eosinophil in infection. Clin Rev Allergy Immunol. 2015;50:214-227. doi:10.1007/s12016-015-8525-4
- Grünewald M, Stölzl D, Wehkamp U, et al. Role of eosinophils in angiolymphoid hyperplasia with eosinophilia. JAMA Dermatol. 2021;157:1241-1243. doi:10.1001/jamadermatol.2021.2732
Practice Points
- Angiolymphoid hyperplasia with eosinophilia (ALHE) is a rare entity of unknown etiology.
- There is an association between ALHE and coccidioidomycosis (CM). Patients who present with ALHE and reside in a CM-endemic region should be examined for CM.
Dermatologists and the Aging Eye: Visual Performance in Physicians
The years start coming and they don’t stop coming.
Smash Mouth, “All Star”
Dermatologists, similar to everyone else, are subject to the inevitable: aging. More than 80% of the US population develops presbyopia, an age-related reduction in visual acuity, in their lifetime. The most common cause of refractive error in adults, presbyopia can contribute to reduced professional productivity, and individuals with uncorrected presbyopia face an estimated 8-fold increase in difficulty performing demanding near-vision tasks.1
As specialists who rely heavily on visual assessment, dermatologists likely are aware of presbyopia, seeking care as appropriate; however, visual correction is not one size fits all, and identifying effective job-specific adjustments may require considerable trial and error. To this end, if visual correction may be needed by a large majority of dermatologists at some point, why do we not have specialized recommendations to guide the corrective process according to the individual’s defect and type of practice within the specialty? Do we need resources for dermatologists concerning ophthalmologic wellness and key warning signs of visual acuity deficits and other ocular complications?
These matters are difficult to address, made more so by the lack of data examining correctable visual impairment (CVI) in dermatology. The basis for discussion is clear; however, visual skills are highly relevant to the practice of dermatology, and age-related visual changes often are inevitable. This article will provide an overview of CVI in related disciplines and the importance of understanding CVI and corrective options in dermatology.
CVI Across Medical Disciplines
Other predominantly visual medical specialties such as pathology, radiology, and surgery have initiated research evaluating the impact of CVI on their respective practices, although consistent data still are limited. Much of the work surrounding CVI in medicine can be identified in surgery and its subspecialties. A 2020 study by Tuna et al2 found that uncorrected myopia with greater than 1.75 diopter, hyperopia regardless of grade, and presbyopia with greater than 1.25 diopter correlated with reduced surgical performance when using the Da Vinci robotic system. A 2002 report by Wanzel et al3 was among the first of many studies to demonstrate the importance of visuospatial ability in surgical success. In radiology, Krupinski et al4 demonstrated reduced accuracy in detecting pulmonary nodules that correlated with increased myopia and decreased accommodation secondary to visual strain.
Most reports examining CVI across medical disciplines are primarily conversational or observational, with some utilizing surveys to assess the prevalence of CVI and the opinions of physicians in the field. For example, in a survey of 93 pathologists in Turkey, 93.5% (87/93) reported at least 1 type of refractive error. Eyeglasses were the most common form of correction (64.5% [60/93]); of those, 33.3% (31/93) reported using eyeglasses during microscopy.5
The importance of visual ability in other highly visual specialties suggests that parallels can be drawn to similar practices in dermatology. Detection of cutaneous lesions might be affected by changes in vision, similar to detection of pulmonary lesions in radiology. Likewise, dermatologic surgeons might experience a similar reduction in surgical performance due to impaired visual acuity or visuospatial ability.
The Importance of Visual Performancein Dermatology
With presbyopia often becoming clinically apparent at approximately 40 years of age,1,6 CVI has the potential to be present for much of a dermatologist’s career. Responsibility falls on the individual practitioner to recognize their visual deficit and seek appropriate optometric or ophthalmologic care. It should be emphasized that there are many effective avenues to correct refractive error, most of which can functionally restore an individual’s vision; however, each option prioritizes different visual attributes (eg, contrast, depth perception, clarity) that have varying degrees of importance in particular areas of dermatologic practice. For example, in addition to visual acuity, dermatologic surgeons might require optimized depth perception, whereas dermatologists performing detailed visual inspection or dermoscopy might instead require optimized contrast sensitivity and acuity. At present, the literature is silent on guiding dermatologists in selecting corrective approaches that enhance the visual characteristics most important for their practice. Lack of research and direction surrounding which visual correction techniques are best suited for individual tasks risks inaccurate and nonspecific conversations with our eye care providers. Focused educated dialogues about visual needs would streamline the process of finding appropriate correction, thereby reducing unnecessary trial and error. As each dermatologic subspecialty might require a unique subset of visual skills, the conceivable benefit of dermatology-specific visual correction resources is evident.
Additionally (although beyond the scope of this commentary), guidance on how a dermatologist should increase their awareness and approach to more serious ophthalmologic conditions—including retinal tear or detachment, age-related macular degeneration, and glaucoma—also would serve as a valuable resource. Overall, prompt identification of visual changes and educated discussions surrounding their correction would allow for optimization based on the required skill set and would improve overall outcomes.
Final Thoughts
Age-related visual changes are a highly prevalent and normal process that carry the potential to impact clinical practice. Fortunately, there are multiple corrective mechanisms that can functionally restore an individual’s eyesight. However, there are no resources to guide dermatologists in seeking specialty-specific correction centered on their daily tasks, which places the responsibility for such correction on the individual. This is a circumstance in which the task at hand is clear, yet we continue to individually reinvent the wheel. We should consider this an opportunity to work together with our optometry and ophthalmology colleagues to create centralized resources that assist dermatologists in navigating age-related visual changes.
Acknowledgments—The authors thank Delaney Stratton, DNP, FNP-BC (Tucson, Arizona); J. Daniel Twelker, OD, PhD (Tucson, Arizona); and Julia Freeman, MD (Pittsburgh, Pennsylvania), for their contributions to the manuscript, as well as Susan M. Swetter, MD (Palo Alto, California) for reviewing and providing feedback.
- Berdahl J, Bala C, Dhariwal M, et al. Patient and economic burden of presbyopia: a systematic literature review. Clin Ophthalmol. 2020;14:3439-3450. doi:10.2147/OPTH.S269597
- Tuna MB, Kilavuzoglu AE, Mourmouris P, et al. Impact of refractive errors on Da Vinci SI robotic system. JSLS. 2020;24:e2020.00031. doi:10.4293/JSLS.2020.00031
- Wanzel KR, Hamstra SJ, Anastakis DJ, et al. Effect of visual-spatial ability on learning of spatially-complex surgical skills. Lancet. 2002;359:230-231. doi:10.1016/S0140-6736(02)07441-X
- Krupinski EA, Berbaum KS, Caldwell RT, et al. Do long radiology workdays affect nodule detection in dynamic CT interpretation? J Am Coll Radiol. 2012;9:191-198. doi:10.1016/j.jacr.2011.11.013
- Akman O, Kösemehmetog˘lu K. Ocular diseases among pathologists and pathologists’ perceptions on ocular diseases: a survey study. Turk Patoloji Derg. 2015;31:194-199. doi:10.5146/tjpath.2015.01326
- Vitale S, Ellwein L, Cotch MF, et al. Prevalence of refractive error in the United States, 1999-2004. Arch Ophthalmol. 2008;126:1111-1119. doi:10.1001/archopht.126.8.1111
The years start coming and they don’t stop coming.
Smash Mouth, “All Star”
Dermatologists, similar to everyone else, are subject to the inevitable: aging. More than 80% of the US population develops presbyopia, an age-related reduction in visual acuity, in their lifetime. The most common cause of refractive error in adults, presbyopia can contribute to reduced professional productivity, and individuals with uncorrected presbyopia face an estimated 8-fold increase in difficulty performing demanding near-vision tasks.1
As specialists who rely heavily on visual assessment, dermatologists likely are aware of presbyopia, seeking care as appropriate; however, visual correction is not one size fits all, and identifying effective job-specific adjustments may require considerable trial and error. To this end, if visual correction may be needed by a large majority of dermatologists at some point, why do we not have specialized recommendations to guide the corrective process according to the individual’s defect and type of practice within the specialty? Do we need resources for dermatologists concerning ophthalmologic wellness and key warning signs of visual acuity deficits and other ocular complications?
These matters are difficult to address, made more so by the lack of data examining correctable visual impairment (CVI) in dermatology. The basis for discussion is clear; however, visual skills are highly relevant to the practice of dermatology, and age-related visual changes often are inevitable. This article will provide an overview of CVI in related disciplines and the importance of understanding CVI and corrective options in dermatology.
CVI Across Medical Disciplines
Other predominantly visual medical specialties such as pathology, radiology, and surgery have initiated research evaluating the impact of CVI on their respective practices, although consistent data still are limited. Much of the work surrounding CVI in medicine can be identified in surgery and its subspecialties. A 2020 study by Tuna et al2 found that uncorrected myopia with greater than 1.75 diopter, hyperopia regardless of grade, and presbyopia with greater than 1.25 diopter correlated with reduced surgical performance when using the Da Vinci robotic system. A 2002 report by Wanzel et al3 was among the first of many studies to demonstrate the importance of visuospatial ability in surgical success. In radiology, Krupinski et al4 demonstrated reduced accuracy in detecting pulmonary nodules that correlated with increased myopia and decreased accommodation secondary to visual strain.
Most reports examining CVI across medical disciplines are primarily conversational or observational, with some utilizing surveys to assess the prevalence of CVI and the opinions of physicians in the field. For example, in a survey of 93 pathologists in Turkey, 93.5% (87/93) reported at least 1 type of refractive error. Eyeglasses were the most common form of correction (64.5% [60/93]); of those, 33.3% (31/93) reported using eyeglasses during microscopy.5
The importance of visual ability in other highly visual specialties suggests that parallels can be drawn to similar practices in dermatology. Detection of cutaneous lesions might be affected by changes in vision, similar to detection of pulmonary lesions in radiology. Likewise, dermatologic surgeons might experience a similar reduction in surgical performance due to impaired visual acuity or visuospatial ability.
The Importance of Visual Performancein Dermatology
With presbyopia often becoming clinically apparent at approximately 40 years of age,1,6 CVI has the potential to be present for much of a dermatologist’s career. Responsibility falls on the individual practitioner to recognize their visual deficit and seek appropriate optometric or ophthalmologic care. It should be emphasized that there are many effective avenues to correct refractive error, most of which can functionally restore an individual’s vision; however, each option prioritizes different visual attributes (eg, contrast, depth perception, clarity) that have varying degrees of importance in particular areas of dermatologic practice. For example, in addition to visual acuity, dermatologic surgeons might require optimized depth perception, whereas dermatologists performing detailed visual inspection or dermoscopy might instead require optimized contrast sensitivity and acuity. At present, the literature is silent on guiding dermatologists in selecting corrective approaches that enhance the visual characteristics most important for their practice. Lack of research and direction surrounding which visual correction techniques are best suited for individual tasks risks inaccurate and nonspecific conversations with our eye care providers. Focused educated dialogues about visual needs would streamline the process of finding appropriate correction, thereby reducing unnecessary trial and error. As each dermatologic subspecialty might require a unique subset of visual skills, the conceivable benefit of dermatology-specific visual correction resources is evident.
Additionally (although beyond the scope of this commentary), guidance on how a dermatologist should increase their awareness and approach to more serious ophthalmologic conditions—including retinal tear or detachment, age-related macular degeneration, and glaucoma—also would serve as a valuable resource. Overall, prompt identification of visual changes and educated discussions surrounding their correction would allow for optimization based on the required skill set and would improve overall outcomes.
Final Thoughts
Age-related visual changes are a highly prevalent and normal process that carry the potential to impact clinical practice. Fortunately, there are multiple corrective mechanisms that can functionally restore an individual’s eyesight. However, there are no resources to guide dermatologists in seeking specialty-specific correction centered on their daily tasks, which places the responsibility for such correction on the individual. This is a circumstance in which the task at hand is clear, yet we continue to individually reinvent the wheel. We should consider this an opportunity to work together with our optometry and ophthalmology colleagues to create centralized resources that assist dermatologists in navigating age-related visual changes.
Acknowledgments—The authors thank Delaney Stratton, DNP, FNP-BC (Tucson, Arizona); J. Daniel Twelker, OD, PhD (Tucson, Arizona); and Julia Freeman, MD (Pittsburgh, Pennsylvania), for their contributions to the manuscript, as well as Susan M. Swetter, MD (Palo Alto, California) for reviewing and providing feedback.
The years start coming and they don’t stop coming.
Smash Mouth, “All Star”
Dermatologists, similar to everyone else, are subject to the inevitable: aging. More than 80% of the US population develops presbyopia, an age-related reduction in visual acuity, in their lifetime. The most common cause of refractive error in adults, presbyopia can contribute to reduced professional productivity, and individuals with uncorrected presbyopia face an estimated 8-fold increase in difficulty performing demanding near-vision tasks.1
As specialists who rely heavily on visual assessment, dermatologists likely are aware of presbyopia, seeking care as appropriate; however, visual correction is not one size fits all, and identifying effective job-specific adjustments may require considerable trial and error. To this end, if visual correction may be needed by a large majority of dermatologists at some point, why do we not have specialized recommendations to guide the corrective process according to the individual’s defect and type of practice within the specialty? Do we need resources for dermatologists concerning ophthalmologic wellness and key warning signs of visual acuity deficits and other ocular complications?
These matters are difficult to address, made more so by the lack of data examining correctable visual impairment (CVI) in dermatology. The basis for discussion is clear; however, visual skills are highly relevant to the practice of dermatology, and age-related visual changes often are inevitable. This article will provide an overview of CVI in related disciplines and the importance of understanding CVI and corrective options in dermatology.
CVI Across Medical Disciplines
Other predominantly visual medical specialties such as pathology, radiology, and surgery have initiated research evaluating the impact of CVI on their respective practices, although consistent data still are limited. Much of the work surrounding CVI in medicine can be identified in surgery and its subspecialties. A 2020 study by Tuna et al2 found that uncorrected myopia with greater than 1.75 diopter, hyperopia regardless of grade, and presbyopia with greater than 1.25 diopter correlated with reduced surgical performance when using the Da Vinci robotic system. A 2002 report by Wanzel et al3 was among the first of many studies to demonstrate the importance of visuospatial ability in surgical success. In radiology, Krupinski et al4 demonstrated reduced accuracy in detecting pulmonary nodules that correlated with increased myopia and decreased accommodation secondary to visual strain.
Most reports examining CVI across medical disciplines are primarily conversational or observational, with some utilizing surveys to assess the prevalence of CVI and the opinions of physicians in the field. For example, in a survey of 93 pathologists in Turkey, 93.5% (87/93) reported at least 1 type of refractive error. Eyeglasses were the most common form of correction (64.5% [60/93]); of those, 33.3% (31/93) reported using eyeglasses during microscopy.5
The importance of visual ability in other highly visual specialties suggests that parallels can be drawn to similar practices in dermatology. Detection of cutaneous lesions might be affected by changes in vision, similar to detection of pulmonary lesions in radiology. Likewise, dermatologic surgeons might experience a similar reduction in surgical performance due to impaired visual acuity or visuospatial ability.
The Importance of Visual Performancein Dermatology
With presbyopia often becoming clinically apparent at approximately 40 years of age,1,6 CVI has the potential to be present for much of a dermatologist’s career. Responsibility falls on the individual practitioner to recognize their visual deficit and seek appropriate optometric or ophthalmologic care. It should be emphasized that there are many effective avenues to correct refractive error, most of which can functionally restore an individual’s vision; however, each option prioritizes different visual attributes (eg, contrast, depth perception, clarity) that have varying degrees of importance in particular areas of dermatologic practice. For example, in addition to visual acuity, dermatologic surgeons might require optimized depth perception, whereas dermatologists performing detailed visual inspection or dermoscopy might instead require optimized contrast sensitivity and acuity. At present, the literature is silent on guiding dermatologists in selecting corrective approaches that enhance the visual characteristics most important for their practice. Lack of research and direction surrounding which visual correction techniques are best suited for individual tasks risks inaccurate and nonspecific conversations with our eye care providers. Focused educated dialogues about visual needs would streamline the process of finding appropriate correction, thereby reducing unnecessary trial and error. As each dermatologic subspecialty might require a unique subset of visual skills, the conceivable benefit of dermatology-specific visual correction resources is evident.
Additionally (although beyond the scope of this commentary), guidance on how a dermatologist should increase their awareness and approach to more serious ophthalmologic conditions—including retinal tear or detachment, age-related macular degeneration, and glaucoma—also would serve as a valuable resource. Overall, prompt identification of visual changes and educated discussions surrounding their correction would allow for optimization based on the required skill set and would improve overall outcomes.
Final Thoughts
Age-related visual changes are a highly prevalent and normal process that carry the potential to impact clinical practice. Fortunately, there are multiple corrective mechanisms that can functionally restore an individual’s eyesight. However, there are no resources to guide dermatologists in seeking specialty-specific correction centered on their daily tasks, which places the responsibility for such correction on the individual. This is a circumstance in which the task at hand is clear, yet we continue to individually reinvent the wheel. We should consider this an opportunity to work together with our optometry and ophthalmology colleagues to create centralized resources that assist dermatologists in navigating age-related visual changes.
Acknowledgments—The authors thank Delaney Stratton, DNP, FNP-BC (Tucson, Arizona); J. Daniel Twelker, OD, PhD (Tucson, Arizona); and Julia Freeman, MD (Pittsburgh, Pennsylvania), for their contributions to the manuscript, as well as Susan M. Swetter, MD (Palo Alto, California) for reviewing and providing feedback.
- Berdahl J, Bala C, Dhariwal M, et al. Patient and economic burden of presbyopia: a systematic literature review. Clin Ophthalmol. 2020;14:3439-3450. doi:10.2147/OPTH.S269597
- Tuna MB, Kilavuzoglu AE, Mourmouris P, et al. Impact of refractive errors on Da Vinci SI robotic system. JSLS. 2020;24:e2020.00031. doi:10.4293/JSLS.2020.00031
- Wanzel KR, Hamstra SJ, Anastakis DJ, et al. Effect of visual-spatial ability on learning of spatially-complex surgical skills. Lancet. 2002;359:230-231. doi:10.1016/S0140-6736(02)07441-X
- Krupinski EA, Berbaum KS, Caldwell RT, et al. Do long radiology workdays affect nodule detection in dynamic CT interpretation? J Am Coll Radiol. 2012;9:191-198. doi:10.1016/j.jacr.2011.11.013
- Akman O, Kösemehmetog˘lu K. Ocular diseases among pathologists and pathologists’ perceptions on ocular diseases: a survey study. Turk Patoloji Derg. 2015;31:194-199. doi:10.5146/tjpath.2015.01326
- Vitale S, Ellwein L, Cotch MF, et al. Prevalence of refractive error in the United States, 1999-2004. Arch Ophthalmol. 2008;126:1111-1119. doi:10.1001/archopht.126.8.1111
- Berdahl J, Bala C, Dhariwal M, et al. Patient and economic burden of presbyopia: a systematic literature review. Clin Ophthalmol. 2020;14:3439-3450. doi:10.2147/OPTH.S269597
- Tuna MB, Kilavuzoglu AE, Mourmouris P, et al. Impact of refractive errors on Da Vinci SI robotic system. JSLS. 2020;24:e2020.00031. doi:10.4293/JSLS.2020.00031
- Wanzel KR, Hamstra SJ, Anastakis DJ, et al. Effect of visual-spatial ability on learning of spatially-complex surgical skills. Lancet. 2002;359:230-231. doi:10.1016/S0140-6736(02)07441-X
- Krupinski EA, Berbaum KS, Caldwell RT, et al. Do long radiology workdays affect nodule detection in dynamic CT interpretation? J Am Coll Radiol. 2012;9:191-198. doi:10.1016/j.jacr.2011.11.013
- Akman O, Kösemehmetog˘lu K. Ocular diseases among pathologists and pathologists’ perceptions on ocular diseases: a survey study. Turk Patoloji Derg. 2015;31:194-199. doi:10.5146/tjpath.2015.01326
- Vitale S, Ellwein L, Cotch MF, et al. Prevalence of refractive error in the United States, 1999-2004. Arch Ophthalmol. 2008;126:1111-1119. doi:10.1001/archopht.126.8.1111
Practice Points
- With presbyopia becoming clinically apparent starting at 40 years of age, dermatologists should be vigilant for correctable visual impairment.
- Although many corrective options exist, more research is needed to understand whether dermatologic subspecialties are better suited to specific options.
- As a specialty, we should consider standardized visual correction guidance.
Transverse Leukonychia and Beau Lines Following COVID-19 Vaccination
To the Editor:
Nail abnormalities associated with SARS-CoV-2 infection that have been reported in the medical literature include nail psoriasis,1 Beau lines,2 onychomadesis,3 heterogeneous red-white discoloration of the nail bed,4 transverse orange nail lesions,3 and the red half‐moon nail sign.3,5 It has been hypothesized that these nail findings may be an indication of microvascular injury to the distal subungual arcade of the digit or may be indicative of a procoagulant state.5,6 Currently, there is limited knowledge of the effect of COVID-19 vaccines on nail changes. We report a patient who presented with transverse leukonychia (Mees lines) and Beau lines shortly after each dose of the Pfizer-BioNTech COVID-19 messenger RNA vaccine was administered (with a total of 2 doses administered on presentation).
A 64-year-old woman with a history of rheumatoid arthritis presented with peeling of the fingernails and proximal white discoloration of several fingernails of 2 months’ duration. The patient first noticed whitening of the nails 3 weeks after she recevied the first dose of the COVID-19 vaccine. Five days after receiving the second, she presented to the dermatology clinic and exhibited transverse leukonychia in most fingernails (Figure 1).
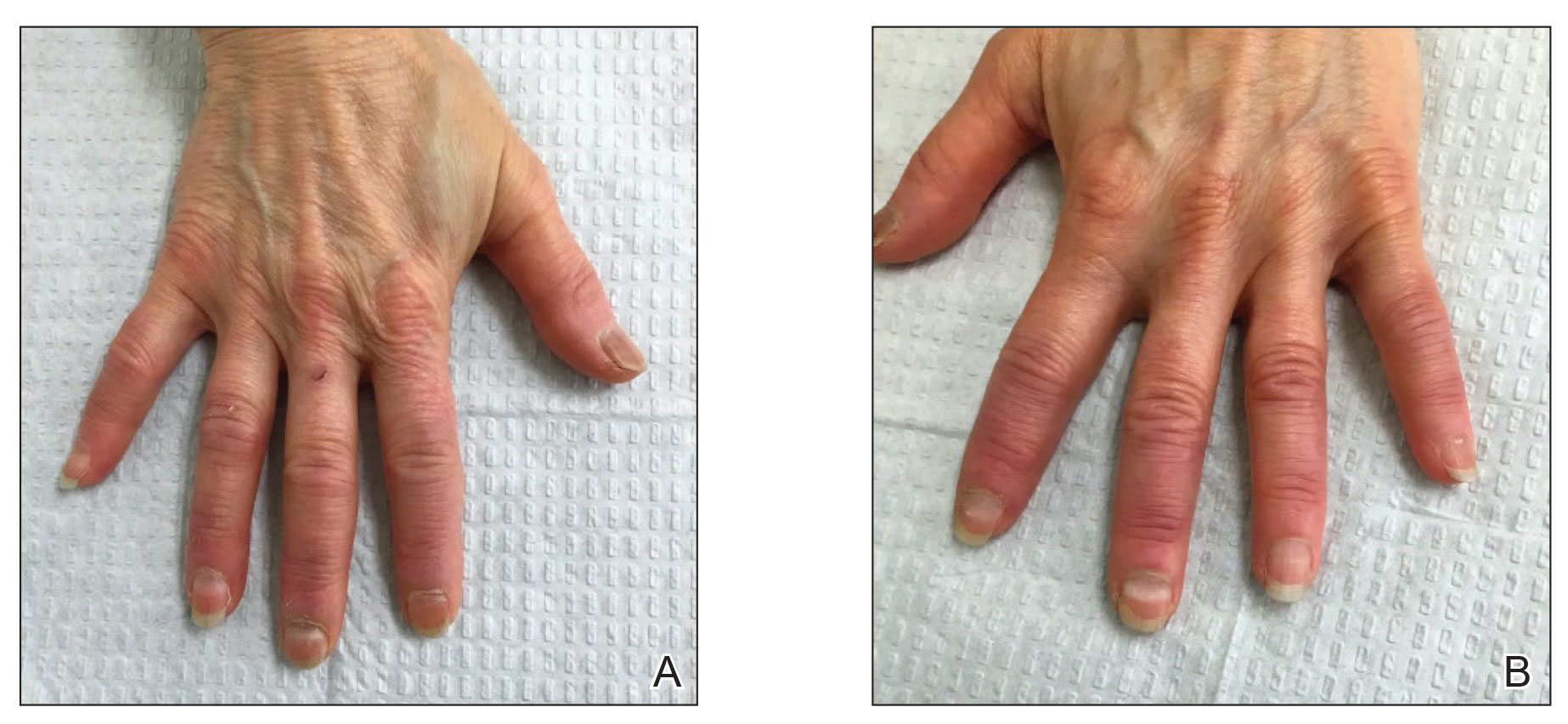
Six weeks following the second dose of the COVID-19 vaccine, the patient returned to the dermatology clinic with Beau lines on the second and third fingernails on the right hand (Figure 2A). Subtle erythema of the proximal nail folds and distal fingers was observed in both hands. The patient also exhibited mild onychorrhexis of the left thumbnail and mottled red-brown discoloration of the third finger on the left hand (Figure 2B). Splinter hemorrhages and melanonychia of several fingernails also were observed. Our patient denied any known history of infection with SARS-CoV-2, which was confirmed by a negative COVID-19 polymerase chain reaction test result. She also denied fevers, chills, nausea, and vomiting, she and reported feeling generally well in the context of these postvaccination nail changes.
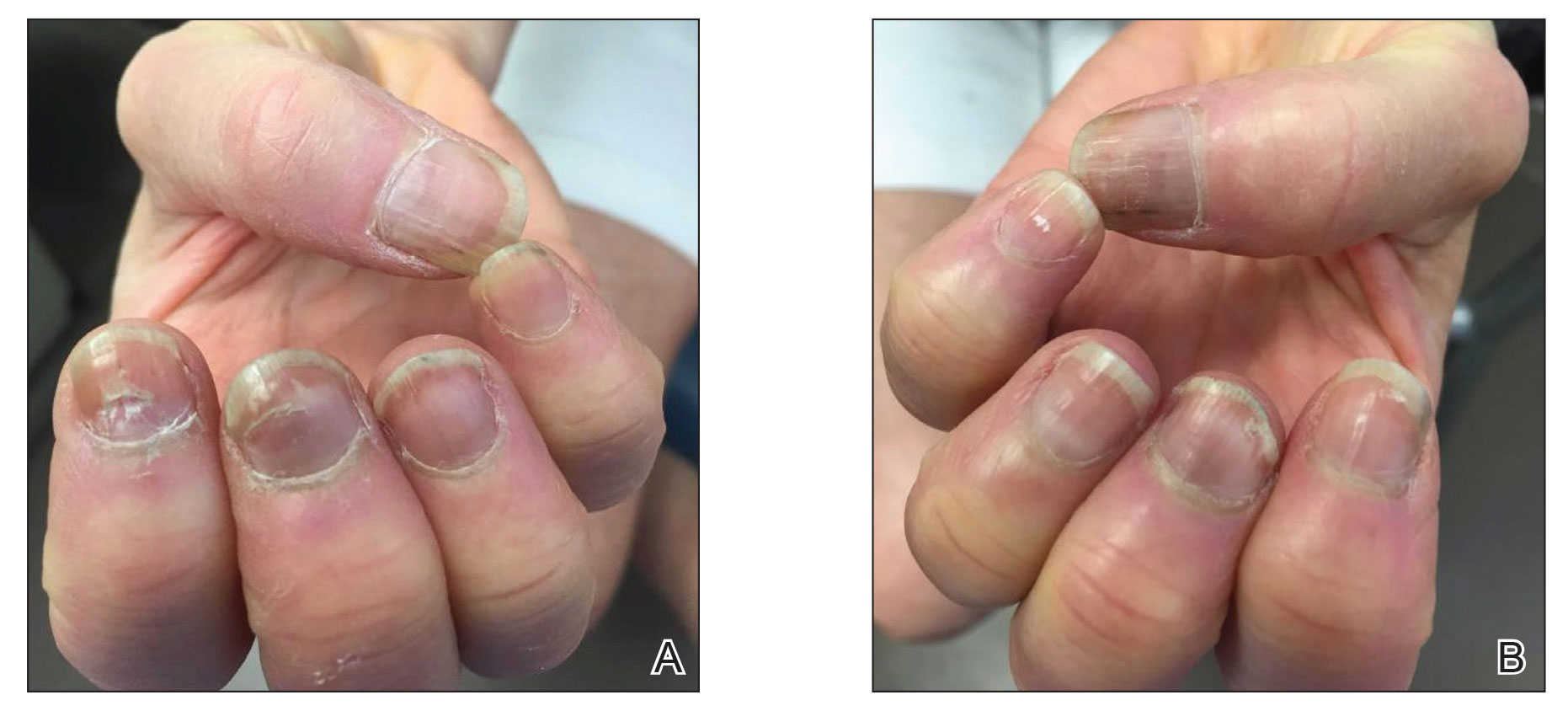
She reported no trauma or worsening of rheumatoid arthritis before or after COVID-19 vaccination. She was seronegative for rheumatoid arthritis and was being treated with hydroxychloroquine for the last year and methotrexate for the last 2 years. After each dose of the vaccine, methotrexate was withheld for 1 week and then resumed.
Subsequent follow-up examinations revealed the migration and resolution of transverse leukonychia and Beau lines. There also was interval improvement of the splinter hemorrhages. At 17 weeks following the second vaccine dose, all transverse leukonychia and Beau lines had resolved (Figure 3). The patient’s melanonychia remained unchanged.
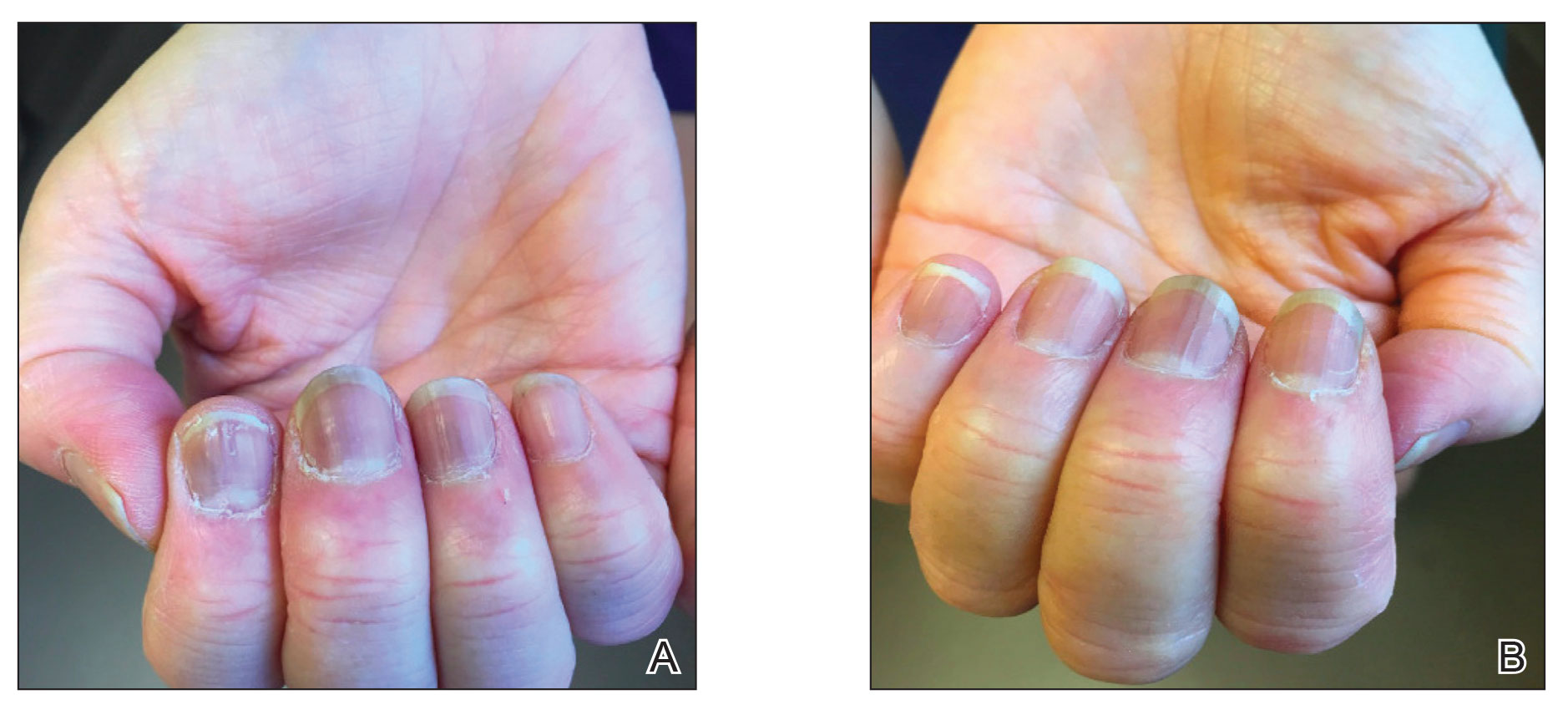
Laboratory evaluations drawn 1 month following the first dose of the COVID-19 vaccine, including comprehensive metabolic panel; erythrocyte sedimentation rate; C-reactive protein; and vitamin B12, ferritin, and iron levels were within reference range. The complete blood cell count only showed a mildly decreased white blood cell count (3.55×103/µL [reference range, 4.16–9.95×103/µL]) and mildly elevated mean corpuscular volume (101.9 fL [reference range, 79.3–98.6 fL), both near the patient’s baseline values prior to vaccination.
Documented cutaneous manifestations of SARS‐CoV‐2 infection have included perniolike lesions (known as COVID toes) and vesicular, urticarial, petechial, livedoid, or retiform purpura eruptions. Less frequently, nail findings in patients infected with COVID-19 have been reported, including Beau lines,2 onychomadesis,3 transverse leukonychia,3,7 and the red half‐moon nail sign.3,5 Single or multiple nails may be affected. Although the pathogenesis of nail manifestations related to COVID-19 remains unclear, complement-mediated microvascular injury and thrombosis as well as the procoagulant state, which have been associated with COVID-19, may offer possible explanations.5,6 The presence of microvascular abnormalities was observed in a nail fold video capillaroscopy study of the nails of 82 patients with COVID-19, revealing pericapillary edema, capillary ectasia, sludge flow, meandering capillaries and microvascular derangement, and low capillary density.8
Our patient exhibited transverse leukonychia of the fingernails, which is thought to result from abnormal keratinization of the nail plate due to systemic disorders that induce a temporary dysfunction of nail growth.9 Fernandez-Nieto et al7 reported transverse leukonychia in a patient with COVID-19 that was hypothesized to be due to a transitory nail matrix injury.
Beau lines and onychomadesis, which represent nail matrix arrest, commonly are seen with systemic drug treatments such as chemotherapy and in infectious diseases that precipitate systemic illness, such as hand, foot, and mouth disease. Although histologic examination was not performed in our patient due to cosmetic concerns, we believe that inflammation induced by the vaccine response also can trigger nail abnormalities such as transverse leukonychia and Beau lines. Both SARS-CoV-2 infections and the COVID-19 messenger RNA vaccines can induce systemic inflammation largely due a TH1-dominant response, and they also can trigger other inflammatory conditions. Reports of lichen planus and psoriasis triggered by vaccination—the hepatitis B vaccine,10 influenza vaccine,11 and even COVID-19 vaccines1,12—have been reported. Beau lines have been observed to spontaneously resolve in a self-limiting manner in asymptomatic patients with COVID-19.
Interestingly, our patient only showed 2 nails with Beau lines. We hypothesize that the immune response triggered by vaccination was more subdued than that caused by SARS-CoV-2 infection. Additionally, our patient was already being treated with immunosuppressants, which may have been associated with a reduced immune response despite being withheld right before vaccination. One may debate whether the nail abnormalities observed in our patient constituted an isolated finding from COVID-19 vaccination or were caused by reactivation of rheumatoid arthritis. We favor the former, as the rheumatoid arthritis remained stable before and after COVID-19 vaccination. Laboratory evaluations and physical examination revealed no evidence of flares, and our patient was otherwise healthy. Although the splinter hemorrhages also improved, it is difficult to comment as to whether they were caused by the vaccine or had existed prior to vaccination. However, we believe the melanonychia observed in the nails was unrelated to the vaccine and was likely a chronic manifestation due to long-term hydroxychloroquine and/or methotrexate use.
Given accelerated global vaccination efforts to control the COVID-19 pandemic, more cases of adverse nail manifestations associated with COVID-19 vaccines are expected. Dermatologists should be aware of and use the reported nail findings to educate patients and reassure them that ungual abnormalities are potential adverse effects of COVID-19 vaccines, but they should not discourage vaccination because they usually are temporary and self-resolving.
- Ricardo JW, Lipner SR. Case of de novo nail psoriasis triggered by the second dose of Pfizer-BioNTech BNT162b2 COVID-19 messenger RNA vaccine. JAAD Case Rep. 2021;17:18-20.
- Deng J, Ngo T, Zhu TH, et al. Telogen effluvium, Beau lines, and acral peeling associated with COVID-19 infection. JAAD Case Rep. 2021;13:138-140.
- Hadeler E, Morrison BW, Tosti A. A review of nail findings associated with COVID-19 infection. J Eur Acad Dermatol Venereol. 2021;35:E699-E709.
- Demir B, Yuksel EI, Cicek D, et al. Heterogeneous red-white discoloration of the nail bed and distal onycholysis in a patient with COVID-19. J Eur Acad Dermatol Venereol. 2021;35:E551-E553.
- Neri I, Guglielmo A, Virdi A, et al. The red half-moon nail sign: a novel manifestation of coronavirus infection. J Eur Acad Dermatol Venereol. 2020;34:E663-E665.
- Magro C, Mulvey JJ, Berlin D, et al. Complement associated microvascular injury and thrombosis in the pathogenesis of severe COVID-19 infection: a report of five cases. Transl Res. 2020;220:1-13.
- Fernandez-Nieto D, Jimenez-Cauhe J, Ortega-Quijano D, et al. Transverse leukonychia (Mees’ lines) nail alterations in a COVID-19 patient. Dermatol Ther. 2020;33:E13863.
- Natalello G, De Luca G, Gigante L, et al. Nailfold capillaroscopy findings in patients with coronavirus disease 2019: broadening the spectrum of COVID-19 microvascular involvement [published online September 17, 2020]. Microvasc Res. doi:10.1016/j.mvr.2020.104071
- Piccolo V, Corneli P, Zalaudek I, et al. Mees’ lines because of chemotherapy for Hodgkin’s lymphoma. Int J Dermatol. 2020;59:E38.
- Miteva L. Bullous lichen planus with nail involvement induced by hepatitis B vaccine in a child. Int J Dermatol. 2005;44:142-144.
- Gunes AT, Fetil E, Akarsu S, et al. Possible triggering effect of influenza vaccination on psoriasis [published online August 25, 2015]. J Immunol Res. doi:10.1155/2015/258430
- Hiltun I, Sarriugarte J, Martínez-de-Espronceda I, et al. Lichen planus arising after COVID-19 vaccination. J Eur Acad Dermatol Venereol. 2021;35:e414-e415.
To the Editor:
Nail abnormalities associated with SARS-CoV-2 infection that have been reported in the medical literature include nail psoriasis,1 Beau lines,2 onychomadesis,3 heterogeneous red-white discoloration of the nail bed,4 transverse orange nail lesions,3 and the red half‐moon nail sign.3,5 It has been hypothesized that these nail findings may be an indication of microvascular injury to the distal subungual arcade of the digit or may be indicative of a procoagulant state.5,6 Currently, there is limited knowledge of the effect of COVID-19 vaccines on nail changes. We report a patient who presented with transverse leukonychia (Mees lines) and Beau lines shortly after each dose of the Pfizer-BioNTech COVID-19 messenger RNA vaccine was administered (with a total of 2 doses administered on presentation).
A 64-year-old woman with a history of rheumatoid arthritis presented with peeling of the fingernails and proximal white discoloration of several fingernails of 2 months’ duration. The patient first noticed whitening of the nails 3 weeks after she recevied the first dose of the COVID-19 vaccine. Five days after receiving the second, she presented to the dermatology clinic and exhibited transverse leukonychia in most fingernails (Figure 1).

Six weeks following the second dose of the COVID-19 vaccine, the patient returned to the dermatology clinic with Beau lines on the second and third fingernails on the right hand (Figure 2A). Subtle erythema of the proximal nail folds and distal fingers was observed in both hands. The patient also exhibited mild onychorrhexis of the left thumbnail and mottled red-brown discoloration of the third finger on the left hand (Figure 2B). Splinter hemorrhages and melanonychia of several fingernails also were observed. Our patient denied any known history of infection with SARS-CoV-2, which was confirmed by a negative COVID-19 polymerase chain reaction test result. She also denied fevers, chills, nausea, and vomiting, she and reported feeling generally well in the context of these postvaccination nail changes.

She reported no trauma or worsening of rheumatoid arthritis before or after COVID-19 vaccination. She was seronegative for rheumatoid arthritis and was being treated with hydroxychloroquine for the last year and methotrexate for the last 2 years. After each dose of the vaccine, methotrexate was withheld for 1 week and then resumed.
Subsequent follow-up examinations revealed the migration and resolution of transverse leukonychia and Beau lines. There also was interval improvement of the splinter hemorrhages. At 17 weeks following the second vaccine dose, all transverse leukonychia and Beau lines had resolved (Figure 3). The patient’s melanonychia remained unchanged.

Laboratory evaluations drawn 1 month following the first dose of the COVID-19 vaccine, including comprehensive metabolic panel; erythrocyte sedimentation rate; C-reactive protein; and vitamin B12, ferritin, and iron levels were within reference range. The complete blood cell count only showed a mildly decreased white blood cell count (3.55×103/µL [reference range, 4.16–9.95×103/µL]) and mildly elevated mean corpuscular volume (101.9 fL [reference range, 79.3–98.6 fL), both near the patient’s baseline values prior to vaccination.
Documented cutaneous manifestations of SARS‐CoV‐2 infection have included perniolike lesions (known as COVID toes) and vesicular, urticarial, petechial, livedoid, or retiform purpura eruptions. Less frequently, nail findings in patients infected with COVID-19 have been reported, including Beau lines,2 onychomadesis,3 transverse leukonychia,3,7 and the red half‐moon nail sign.3,5 Single or multiple nails may be affected. Although the pathogenesis of nail manifestations related to COVID-19 remains unclear, complement-mediated microvascular injury and thrombosis as well as the procoagulant state, which have been associated with COVID-19, may offer possible explanations.5,6 The presence of microvascular abnormalities was observed in a nail fold video capillaroscopy study of the nails of 82 patients with COVID-19, revealing pericapillary edema, capillary ectasia, sludge flow, meandering capillaries and microvascular derangement, and low capillary density.8
Our patient exhibited transverse leukonychia of the fingernails, which is thought to result from abnormal keratinization of the nail plate due to systemic disorders that induce a temporary dysfunction of nail growth.9 Fernandez-Nieto et al7 reported transverse leukonychia in a patient with COVID-19 that was hypothesized to be due to a transitory nail matrix injury.
Beau lines and onychomadesis, which represent nail matrix arrest, commonly are seen with systemic drug treatments such as chemotherapy and in infectious diseases that precipitate systemic illness, such as hand, foot, and mouth disease. Although histologic examination was not performed in our patient due to cosmetic concerns, we believe that inflammation induced by the vaccine response also can trigger nail abnormalities such as transverse leukonychia and Beau lines. Both SARS-CoV-2 infections and the COVID-19 messenger RNA vaccines can induce systemic inflammation largely due a TH1-dominant response, and they also can trigger other inflammatory conditions. Reports of lichen planus and psoriasis triggered by vaccination—the hepatitis B vaccine,10 influenza vaccine,11 and even COVID-19 vaccines1,12—have been reported. Beau lines have been observed to spontaneously resolve in a self-limiting manner in asymptomatic patients with COVID-19.
Interestingly, our patient only showed 2 nails with Beau lines. We hypothesize that the immune response triggered by vaccination was more subdued than that caused by SARS-CoV-2 infection. Additionally, our patient was already being treated with immunosuppressants, which may have been associated with a reduced immune response despite being withheld right before vaccination. One may debate whether the nail abnormalities observed in our patient constituted an isolated finding from COVID-19 vaccination or were caused by reactivation of rheumatoid arthritis. We favor the former, as the rheumatoid arthritis remained stable before and after COVID-19 vaccination. Laboratory evaluations and physical examination revealed no evidence of flares, and our patient was otherwise healthy. Although the splinter hemorrhages also improved, it is difficult to comment as to whether they were caused by the vaccine or had existed prior to vaccination. However, we believe the melanonychia observed in the nails was unrelated to the vaccine and was likely a chronic manifestation due to long-term hydroxychloroquine and/or methotrexate use.
Given accelerated global vaccination efforts to control the COVID-19 pandemic, more cases of adverse nail manifestations associated with COVID-19 vaccines are expected. Dermatologists should be aware of and use the reported nail findings to educate patients and reassure them that ungual abnormalities are potential adverse effects of COVID-19 vaccines, but they should not discourage vaccination because they usually are temporary and self-resolving.
To the Editor:
Nail abnormalities associated with SARS-CoV-2 infection that have been reported in the medical literature include nail psoriasis,1 Beau lines,2 onychomadesis,3 heterogeneous red-white discoloration of the nail bed,4 transverse orange nail lesions,3 and the red half‐moon nail sign.3,5 It has been hypothesized that these nail findings may be an indication of microvascular injury to the distal subungual arcade of the digit or may be indicative of a procoagulant state.5,6 Currently, there is limited knowledge of the effect of COVID-19 vaccines on nail changes. We report a patient who presented with transverse leukonychia (Mees lines) and Beau lines shortly after each dose of the Pfizer-BioNTech COVID-19 messenger RNA vaccine was administered (with a total of 2 doses administered on presentation).
A 64-year-old woman with a history of rheumatoid arthritis presented with peeling of the fingernails and proximal white discoloration of several fingernails of 2 months’ duration. The patient first noticed whitening of the nails 3 weeks after she recevied the first dose of the COVID-19 vaccine. Five days after receiving the second, she presented to the dermatology clinic and exhibited transverse leukonychia in most fingernails (Figure 1).

Six weeks following the second dose of the COVID-19 vaccine, the patient returned to the dermatology clinic with Beau lines on the second and third fingernails on the right hand (Figure 2A). Subtle erythema of the proximal nail folds and distal fingers was observed in both hands. The patient also exhibited mild onychorrhexis of the left thumbnail and mottled red-brown discoloration of the third finger on the left hand (Figure 2B). Splinter hemorrhages and melanonychia of several fingernails also were observed. Our patient denied any known history of infection with SARS-CoV-2, which was confirmed by a negative COVID-19 polymerase chain reaction test result. She also denied fevers, chills, nausea, and vomiting, she and reported feeling generally well in the context of these postvaccination nail changes.

She reported no trauma or worsening of rheumatoid arthritis before or after COVID-19 vaccination. She was seronegative for rheumatoid arthritis and was being treated with hydroxychloroquine for the last year and methotrexate for the last 2 years. After each dose of the vaccine, methotrexate was withheld for 1 week and then resumed.
Subsequent follow-up examinations revealed the migration and resolution of transverse leukonychia and Beau lines. There also was interval improvement of the splinter hemorrhages. At 17 weeks following the second vaccine dose, all transverse leukonychia and Beau lines had resolved (Figure 3). The patient’s melanonychia remained unchanged.

Laboratory evaluations drawn 1 month following the first dose of the COVID-19 vaccine, including comprehensive metabolic panel; erythrocyte sedimentation rate; C-reactive protein; and vitamin B12, ferritin, and iron levels were within reference range. The complete blood cell count only showed a mildly decreased white blood cell count (3.55×103/µL [reference range, 4.16–9.95×103/µL]) and mildly elevated mean corpuscular volume (101.9 fL [reference range, 79.3–98.6 fL), both near the patient’s baseline values prior to vaccination.
Documented cutaneous manifestations of SARS‐CoV‐2 infection have included perniolike lesions (known as COVID toes) and vesicular, urticarial, petechial, livedoid, or retiform purpura eruptions. Less frequently, nail findings in patients infected with COVID-19 have been reported, including Beau lines,2 onychomadesis,3 transverse leukonychia,3,7 and the red half‐moon nail sign.3,5 Single or multiple nails may be affected. Although the pathogenesis of nail manifestations related to COVID-19 remains unclear, complement-mediated microvascular injury and thrombosis as well as the procoagulant state, which have been associated with COVID-19, may offer possible explanations.5,6 The presence of microvascular abnormalities was observed in a nail fold video capillaroscopy study of the nails of 82 patients with COVID-19, revealing pericapillary edema, capillary ectasia, sludge flow, meandering capillaries and microvascular derangement, and low capillary density.8
Our patient exhibited transverse leukonychia of the fingernails, which is thought to result from abnormal keratinization of the nail plate due to systemic disorders that induce a temporary dysfunction of nail growth.9 Fernandez-Nieto et al7 reported transverse leukonychia in a patient with COVID-19 that was hypothesized to be due to a transitory nail matrix injury.
Beau lines and onychomadesis, which represent nail matrix arrest, commonly are seen with systemic drug treatments such as chemotherapy and in infectious diseases that precipitate systemic illness, such as hand, foot, and mouth disease. Although histologic examination was not performed in our patient due to cosmetic concerns, we believe that inflammation induced by the vaccine response also can trigger nail abnormalities such as transverse leukonychia and Beau lines. Both SARS-CoV-2 infections and the COVID-19 messenger RNA vaccines can induce systemic inflammation largely due a TH1-dominant response, and they also can trigger other inflammatory conditions. Reports of lichen planus and psoriasis triggered by vaccination—the hepatitis B vaccine,10 influenza vaccine,11 and even COVID-19 vaccines1,12—have been reported. Beau lines have been observed to spontaneously resolve in a self-limiting manner in asymptomatic patients with COVID-19.
Interestingly, our patient only showed 2 nails with Beau lines. We hypothesize that the immune response triggered by vaccination was more subdued than that caused by SARS-CoV-2 infection. Additionally, our patient was already being treated with immunosuppressants, which may have been associated with a reduced immune response despite being withheld right before vaccination. One may debate whether the nail abnormalities observed in our patient constituted an isolated finding from COVID-19 vaccination or were caused by reactivation of rheumatoid arthritis. We favor the former, as the rheumatoid arthritis remained stable before and after COVID-19 vaccination. Laboratory evaluations and physical examination revealed no evidence of flares, and our patient was otherwise healthy. Although the splinter hemorrhages also improved, it is difficult to comment as to whether they were caused by the vaccine or had existed prior to vaccination. However, we believe the melanonychia observed in the nails was unrelated to the vaccine and was likely a chronic manifestation due to long-term hydroxychloroquine and/or methotrexate use.
Given accelerated global vaccination efforts to control the COVID-19 pandemic, more cases of adverse nail manifestations associated with COVID-19 vaccines are expected. Dermatologists should be aware of and use the reported nail findings to educate patients and reassure them that ungual abnormalities are potential adverse effects of COVID-19 vaccines, but they should not discourage vaccination because they usually are temporary and self-resolving.
- Ricardo JW, Lipner SR. Case of de novo nail psoriasis triggered by the second dose of Pfizer-BioNTech BNT162b2 COVID-19 messenger RNA vaccine. JAAD Case Rep. 2021;17:18-20.
- Deng J, Ngo T, Zhu TH, et al. Telogen effluvium, Beau lines, and acral peeling associated with COVID-19 infection. JAAD Case Rep. 2021;13:138-140.
- Hadeler E, Morrison BW, Tosti A. A review of nail findings associated with COVID-19 infection. J Eur Acad Dermatol Venereol. 2021;35:E699-E709.
- Demir B, Yuksel EI, Cicek D, et al. Heterogeneous red-white discoloration of the nail bed and distal onycholysis in a patient with COVID-19. J Eur Acad Dermatol Venereol. 2021;35:E551-E553.
- Neri I, Guglielmo A, Virdi A, et al. The red half-moon nail sign: a novel manifestation of coronavirus infection. J Eur Acad Dermatol Venereol. 2020;34:E663-E665.
- Magro C, Mulvey JJ, Berlin D, et al. Complement associated microvascular injury and thrombosis in the pathogenesis of severe COVID-19 infection: a report of five cases. Transl Res. 2020;220:1-13.
- Fernandez-Nieto D, Jimenez-Cauhe J, Ortega-Quijano D, et al. Transverse leukonychia (Mees’ lines) nail alterations in a COVID-19 patient. Dermatol Ther. 2020;33:E13863.
- Natalello G, De Luca G, Gigante L, et al. Nailfold capillaroscopy findings in patients with coronavirus disease 2019: broadening the spectrum of COVID-19 microvascular involvement [published online September 17, 2020]. Microvasc Res. doi:10.1016/j.mvr.2020.104071
- Piccolo V, Corneli P, Zalaudek I, et al. Mees’ lines because of chemotherapy for Hodgkin’s lymphoma. Int J Dermatol. 2020;59:E38.
- Miteva L. Bullous lichen planus with nail involvement induced by hepatitis B vaccine in a child. Int J Dermatol. 2005;44:142-144.
- Gunes AT, Fetil E, Akarsu S, et al. Possible triggering effect of influenza vaccination on psoriasis [published online August 25, 2015]. J Immunol Res. doi:10.1155/2015/258430
- Hiltun I, Sarriugarte J, Martínez-de-Espronceda I, et al. Lichen planus arising after COVID-19 vaccination. J Eur Acad Dermatol Venereol. 2021;35:e414-e415.
- Ricardo JW, Lipner SR. Case of de novo nail psoriasis triggered by the second dose of Pfizer-BioNTech BNT162b2 COVID-19 messenger RNA vaccine. JAAD Case Rep. 2021;17:18-20.
- Deng J, Ngo T, Zhu TH, et al. Telogen effluvium, Beau lines, and acral peeling associated with COVID-19 infection. JAAD Case Rep. 2021;13:138-140.
- Hadeler E, Morrison BW, Tosti A. A review of nail findings associated with COVID-19 infection. J Eur Acad Dermatol Venereol. 2021;35:E699-E709.
- Demir B, Yuksel EI, Cicek D, et al. Heterogeneous red-white discoloration of the nail bed and distal onycholysis in a patient with COVID-19. J Eur Acad Dermatol Venereol. 2021;35:E551-E553.
- Neri I, Guglielmo A, Virdi A, et al. The red half-moon nail sign: a novel manifestation of coronavirus infection. J Eur Acad Dermatol Venereol. 2020;34:E663-E665.
- Magro C, Mulvey JJ, Berlin D, et al. Complement associated microvascular injury and thrombosis in the pathogenesis of severe COVID-19 infection: a report of five cases. Transl Res. 2020;220:1-13.
- Fernandez-Nieto D, Jimenez-Cauhe J, Ortega-Quijano D, et al. Transverse leukonychia (Mees’ lines) nail alterations in a COVID-19 patient. Dermatol Ther. 2020;33:E13863.
- Natalello G, De Luca G, Gigante L, et al. Nailfold capillaroscopy findings in patients with coronavirus disease 2019: broadening the spectrum of COVID-19 microvascular involvement [published online September 17, 2020]. Microvasc Res. doi:10.1016/j.mvr.2020.104071
- Piccolo V, Corneli P, Zalaudek I, et al. Mees’ lines because of chemotherapy for Hodgkin’s lymphoma. Int J Dermatol. 2020;59:E38.
- Miteva L. Bullous lichen planus with nail involvement induced by hepatitis B vaccine in a child. Int J Dermatol. 2005;44:142-144.
- Gunes AT, Fetil E, Akarsu S, et al. Possible triggering effect of influenza vaccination on psoriasis [published online August 25, 2015]. J Immunol Res. doi:10.1155/2015/258430
- Hiltun I, Sarriugarte J, Martínez-de-Espronceda I, et al. Lichen planus arising after COVID-19 vaccination. J Eur Acad Dermatol Venereol. 2021;35:e414-e415.
Practice Points
- Given accelerated global vaccination efforts to control the COVID-19 pandemic, cases of nail changes associated with COVID-19 vaccines are expected.
- Nail abnormalities are a potential general, temporary, and self-limiting adverse effect of COVID-19 vaccines that should not discourage patients from getting vaccinated.
Pink Nodule Behind the Ear
The Diagnosis: Acanthoma Fissuratum
Acanthoma fissuratum is a skin lesion that results from consistent pressure, typically from ill-fitting eyeglass frames.1 The chronic irritation leads to collagen deposition and inflammation that gradually creates the lesion. Many patients never seek care, making incidence figures undeterminable.2 It usually presents as a firm, tender, flesh-colored or pink nodule or plaque with a central indentation from where the frame rests. This indentation splits the lesion in half and classically gives the appearance of a coffee bean.1 The repeated minor trauma at this point of contact also may lead to centralized ulceration, which further blurs the diagnosis to include basal cell carcinoma (BCC).3,4 Although the postauricular groove is the most cited location, lesions also may occur at other contact points of the glasses, such as the lateral aspect of the bridge of the nose and the superior auricular sulcus.5 Acanthoma fissuratum is not limited to the external head. Other etiologies of local trauma and pressure have led to its diagnosis in the upper labioalveolar fold, posterior fourchette of the vulva, penis, and external auditory canal.6-9
The diagnosis of acanthoma fissuratum mainly is clinical; however, due to its similar appearance to BCC and other lesions, a biopsy can be taken to support the diagnosis; a biopsy was not performed in our patient. The main features seen on histopathology include acanthosis, hyperkeratosis, variable parakeratosis, and perivascular nonspecific inflammatory infiltration. The epidermis may reflect the macroscopic frame indentation with central attenuation of the epidermis, which potentially is filled with inflammatory cells or keratin.5
Treatment normally encompasses removing the illfitting frames or fixing the fit, which gradually leads to reduction of the lesion.4,5 This occurred in our patient, who changed eyeglasses and saw an 80% resolution of the lesion in 8 months. Such improvement after removal of a trauma-inducing stimulus would not be seen in malignancies (eg, BCC, squamous cell carcinoma [SCC]), keloids, or cylindromas. If the granulation tissue does not regress or recurs, other potential treatments include excision, intralesional corticosteroids, and electrosurgery.5
Basal cell carcinoma is a common nonmelanoma skin cancer that most often presents on the sun-exposed areas of the head and neck, especially the cheeks, nasolabial folds, and forehead. Although the nodular subtype may clinically appear similar to acanthoma fissuratum, it more typically presents as a pearly papule or nodule with a sharp border, small telangiectases, and potential ulceration.10 Squamous cell carcinoma is another common nonmelanoma skin cancer that often arises in sun-exposed areas, which can include the postauricular area. Although the lesion can be associated with chronic wounds and also can grow vertically, SCC typically has a scalier and more hyperkeratotic surface that can ulcerate.1 A cylindroma is a benign sweat gland tumor that most commonly presents on the head and neck (also known as the turban tumor), though it can develop on the ear. It appears as solitary or multiple nodules that often are flesh colored, red, or blue with a shiny surface.1 Cylindromas are not known to be associated with chronic local trauma or irritation,11 such as wearing ill-fitting eyeglasses. Unlike acanthoma fissuratum, the treatment of cylindromas, BCC, and SCC most often involves excision.1 A keloid presents as a flesh-colored, red, or purple exophytic plaque that is composed of dense dermal tissue and progressively forms after local trauma. Although keloids can spontaneously develop, they commonly form on the ears in susceptible individuals after skin excisions including prior keloid removal, piercings, repairment of auricular traumas, or infections.1 The patient’s coffee bean–like lesion that coincided with wearing new eyeglasses better fits the diagnosis of acanthoma fissuratum than a keloid. Additionally, keloids typically do not regress without treatment. Keloid treatment consists of intralesional steroid injections, occlusive silicone dressings, compression, cryotherapy, radiation, and excisional surgery.1
- Sand M, Sand D, Brors D, et al. Cutaneous lesions of the external ear. Head Face Med. 2008;4. doi:10.1186/1746-160X-4-2
- Orengo I, Robbins K, Marsch A. Pathology of the ear. Semin Plast Surg. 2011;25:279-287. doi:10.1055/s-0031-1288920
- Ramroop S. Successful treatment of acanthoma fissuratum with intralesional triamcinolone acetonide. Clin Case Rep. 2020;8:702-703. doi:10.1002/ccr3.2708
- Delaney TJ, Stewart TW. Granuloma fissuratum. Br J Dermatol. 1971;84:373-375. doi:10.1111/j.1365-2133.1971.tb14235.x
- Deshpande NS, Sen A, Vasudevan B, et al. Acanthoma fissuratum: lest we forget. Indian Dermatol Online J. 2017;8:141-143. doi:10.4103/2229- 5178.202267
- Surron RL Jr. A fissured granulomatous lesion of the upper labioalveolar fold. Arch Dermatol Syph. 1932;26:425. doi:10.1001 /archderm.1932.01450030423004
- Kennedy CM, Dewdney S, Galask RP. Vulvar granuloma fissuratum: a description of fissuring of the posterior fourchette and the repair. Obstet Gynecol. 2005;105:1018-1023. doi:10.1097/01. AOG.0000158863.70819.53
- Lee JL, Lee YB, Cho BK, et al. Acanthoma fissuratum on the penis. Int J Dermatol. 2013;52:382-384. doi:10.1111/j.1365-4632.2011.04903.x
- Gonzalez SA, Moore AGN. Acanthoma fissuratum of the outer auditory canal from a hearing aid. J Cutan Pathol. 1989;16:304.
- Fania L, Didona D, Morese R, et al. Basal cell carcinoma: from pathophysiology to novel therapeutic approaches. Biomedicines. 2020;8:449. doi:10.3390/biomedicines8110449
- Chauhan DS, Guruprasad Y. Dermal cylindroma of the scalp. Natl J Maxillofac Surg. 2012;3:59-61. doi:10.4103/0975-5950.102163
The Diagnosis: Acanthoma Fissuratum
Acanthoma fissuratum is a skin lesion that results from consistent pressure, typically from ill-fitting eyeglass frames.1 The chronic irritation leads to collagen deposition and inflammation that gradually creates the lesion. Many patients never seek care, making incidence figures undeterminable.2 It usually presents as a firm, tender, flesh-colored or pink nodule or plaque with a central indentation from where the frame rests. This indentation splits the lesion in half and classically gives the appearance of a coffee bean.1 The repeated minor trauma at this point of contact also may lead to centralized ulceration, which further blurs the diagnosis to include basal cell carcinoma (BCC).3,4 Although the postauricular groove is the most cited location, lesions also may occur at other contact points of the glasses, such as the lateral aspect of the bridge of the nose and the superior auricular sulcus.5 Acanthoma fissuratum is not limited to the external head. Other etiologies of local trauma and pressure have led to its diagnosis in the upper labioalveolar fold, posterior fourchette of the vulva, penis, and external auditory canal.6-9
The diagnosis of acanthoma fissuratum mainly is clinical; however, due to its similar appearance to BCC and other lesions, a biopsy can be taken to support the diagnosis; a biopsy was not performed in our patient. The main features seen on histopathology include acanthosis, hyperkeratosis, variable parakeratosis, and perivascular nonspecific inflammatory infiltration. The epidermis may reflect the macroscopic frame indentation with central attenuation of the epidermis, which potentially is filled with inflammatory cells or keratin.5
Treatment normally encompasses removing the illfitting frames or fixing the fit, which gradually leads to reduction of the lesion.4,5 This occurred in our patient, who changed eyeglasses and saw an 80% resolution of the lesion in 8 months. Such improvement after removal of a trauma-inducing stimulus would not be seen in malignancies (eg, BCC, squamous cell carcinoma [SCC]), keloids, or cylindromas. If the granulation tissue does not regress or recurs, other potential treatments include excision, intralesional corticosteroids, and electrosurgery.5
Basal cell carcinoma is a common nonmelanoma skin cancer that most often presents on the sun-exposed areas of the head and neck, especially the cheeks, nasolabial folds, and forehead. Although the nodular subtype may clinically appear similar to acanthoma fissuratum, it more typically presents as a pearly papule or nodule with a sharp border, small telangiectases, and potential ulceration.10 Squamous cell carcinoma is another common nonmelanoma skin cancer that often arises in sun-exposed areas, which can include the postauricular area. Although the lesion can be associated with chronic wounds and also can grow vertically, SCC typically has a scalier and more hyperkeratotic surface that can ulcerate.1 A cylindroma is a benign sweat gland tumor that most commonly presents on the head and neck (also known as the turban tumor), though it can develop on the ear. It appears as solitary or multiple nodules that often are flesh colored, red, or blue with a shiny surface.1 Cylindromas are not known to be associated with chronic local trauma or irritation,11 such as wearing ill-fitting eyeglasses. Unlike acanthoma fissuratum, the treatment of cylindromas, BCC, and SCC most often involves excision.1 A keloid presents as a flesh-colored, red, or purple exophytic plaque that is composed of dense dermal tissue and progressively forms after local trauma. Although keloids can spontaneously develop, they commonly form on the ears in susceptible individuals after skin excisions including prior keloid removal, piercings, repairment of auricular traumas, or infections.1 The patient’s coffee bean–like lesion that coincided with wearing new eyeglasses better fits the diagnosis of acanthoma fissuratum than a keloid. Additionally, keloids typically do not regress without treatment. Keloid treatment consists of intralesional steroid injections, occlusive silicone dressings, compression, cryotherapy, radiation, and excisional surgery.1
The Diagnosis: Acanthoma Fissuratum
Acanthoma fissuratum is a skin lesion that results from consistent pressure, typically from ill-fitting eyeglass frames.1 The chronic irritation leads to collagen deposition and inflammation that gradually creates the lesion. Many patients never seek care, making incidence figures undeterminable.2 It usually presents as a firm, tender, flesh-colored or pink nodule or plaque with a central indentation from where the frame rests. This indentation splits the lesion in half and classically gives the appearance of a coffee bean.1 The repeated minor trauma at this point of contact also may lead to centralized ulceration, which further blurs the diagnosis to include basal cell carcinoma (BCC).3,4 Although the postauricular groove is the most cited location, lesions also may occur at other contact points of the glasses, such as the lateral aspect of the bridge of the nose and the superior auricular sulcus.5 Acanthoma fissuratum is not limited to the external head. Other etiologies of local trauma and pressure have led to its diagnosis in the upper labioalveolar fold, posterior fourchette of the vulva, penis, and external auditory canal.6-9
The diagnosis of acanthoma fissuratum mainly is clinical; however, due to its similar appearance to BCC and other lesions, a biopsy can be taken to support the diagnosis; a biopsy was not performed in our patient. The main features seen on histopathology include acanthosis, hyperkeratosis, variable parakeratosis, and perivascular nonspecific inflammatory infiltration. The epidermis may reflect the macroscopic frame indentation with central attenuation of the epidermis, which potentially is filled with inflammatory cells or keratin.5
Treatment normally encompasses removing the illfitting frames or fixing the fit, which gradually leads to reduction of the lesion.4,5 This occurred in our patient, who changed eyeglasses and saw an 80% resolution of the lesion in 8 months. Such improvement after removal of a trauma-inducing stimulus would not be seen in malignancies (eg, BCC, squamous cell carcinoma [SCC]), keloids, or cylindromas. If the granulation tissue does not regress or recurs, other potential treatments include excision, intralesional corticosteroids, and electrosurgery.5
Basal cell carcinoma is a common nonmelanoma skin cancer that most often presents on the sun-exposed areas of the head and neck, especially the cheeks, nasolabial folds, and forehead. Although the nodular subtype may clinically appear similar to acanthoma fissuratum, it more typically presents as a pearly papule or nodule with a sharp border, small telangiectases, and potential ulceration.10 Squamous cell carcinoma is another common nonmelanoma skin cancer that often arises in sun-exposed areas, which can include the postauricular area. Although the lesion can be associated with chronic wounds and also can grow vertically, SCC typically has a scalier and more hyperkeratotic surface that can ulcerate.1 A cylindroma is a benign sweat gland tumor that most commonly presents on the head and neck (also known as the turban tumor), though it can develop on the ear. It appears as solitary or multiple nodules that often are flesh colored, red, or blue with a shiny surface.1 Cylindromas are not known to be associated with chronic local trauma or irritation,11 such as wearing ill-fitting eyeglasses. Unlike acanthoma fissuratum, the treatment of cylindromas, BCC, and SCC most often involves excision.1 A keloid presents as a flesh-colored, red, or purple exophytic plaque that is composed of dense dermal tissue and progressively forms after local trauma. Although keloids can spontaneously develop, they commonly form on the ears in susceptible individuals after skin excisions including prior keloid removal, piercings, repairment of auricular traumas, or infections.1 The patient’s coffee bean–like lesion that coincided with wearing new eyeglasses better fits the diagnosis of acanthoma fissuratum than a keloid. Additionally, keloids typically do not regress without treatment. Keloid treatment consists of intralesional steroid injections, occlusive silicone dressings, compression, cryotherapy, radiation, and excisional surgery.1
- Sand M, Sand D, Brors D, et al. Cutaneous lesions of the external ear. Head Face Med. 2008;4. doi:10.1186/1746-160X-4-2
- Orengo I, Robbins K, Marsch A. Pathology of the ear. Semin Plast Surg. 2011;25:279-287. doi:10.1055/s-0031-1288920
- Ramroop S. Successful treatment of acanthoma fissuratum with intralesional triamcinolone acetonide. Clin Case Rep. 2020;8:702-703. doi:10.1002/ccr3.2708
- Delaney TJ, Stewart TW. Granuloma fissuratum. Br J Dermatol. 1971;84:373-375. doi:10.1111/j.1365-2133.1971.tb14235.x
- Deshpande NS, Sen A, Vasudevan B, et al. Acanthoma fissuratum: lest we forget. Indian Dermatol Online J. 2017;8:141-143. doi:10.4103/2229- 5178.202267
- Surron RL Jr. A fissured granulomatous lesion of the upper labioalveolar fold. Arch Dermatol Syph. 1932;26:425. doi:10.1001 /archderm.1932.01450030423004
- Kennedy CM, Dewdney S, Galask RP. Vulvar granuloma fissuratum: a description of fissuring of the posterior fourchette and the repair. Obstet Gynecol. 2005;105:1018-1023. doi:10.1097/01. AOG.0000158863.70819.53
- Lee JL, Lee YB, Cho BK, et al. Acanthoma fissuratum on the penis. Int J Dermatol. 2013;52:382-384. doi:10.1111/j.1365-4632.2011.04903.x
- Gonzalez SA, Moore AGN. Acanthoma fissuratum of the outer auditory canal from a hearing aid. J Cutan Pathol. 1989;16:304.
- Fania L, Didona D, Morese R, et al. Basal cell carcinoma: from pathophysiology to novel therapeutic approaches. Biomedicines. 2020;8:449. doi:10.3390/biomedicines8110449
- Chauhan DS, Guruprasad Y. Dermal cylindroma of the scalp. Natl J Maxillofac Surg. 2012;3:59-61. doi:10.4103/0975-5950.102163
- Sand M, Sand D, Brors D, et al. Cutaneous lesions of the external ear. Head Face Med. 2008;4. doi:10.1186/1746-160X-4-2
- Orengo I, Robbins K, Marsch A. Pathology of the ear. Semin Plast Surg. 2011;25:279-287. doi:10.1055/s-0031-1288920
- Ramroop S. Successful treatment of acanthoma fissuratum with intralesional triamcinolone acetonide. Clin Case Rep. 2020;8:702-703. doi:10.1002/ccr3.2708
- Delaney TJ, Stewart TW. Granuloma fissuratum. Br J Dermatol. 1971;84:373-375. doi:10.1111/j.1365-2133.1971.tb14235.x
- Deshpande NS, Sen A, Vasudevan B, et al. Acanthoma fissuratum: lest we forget. Indian Dermatol Online J. 2017;8:141-143. doi:10.4103/2229- 5178.202267
- Surron RL Jr. A fissured granulomatous lesion of the upper labioalveolar fold. Arch Dermatol Syph. 1932;26:425. doi:10.1001 /archderm.1932.01450030423004
- Kennedy CM, Dewdney S, Galask RP. Vulvar granuloma fissuratum: a description of fissuring of the posterior fourchette and the repair. Obstet Gynecol. 2005;105:1018-1023. doi:10.1097/01. AOG.0000158863.70819.53
- Lee JL, Lee YB, Cho BK, et al. Acanthoma fissuratum on the penis. Int J Dermatol. 2013;52:382-384. doi:10.1111/j.1365-4632.2011.04903.x
- Gonzalez SA, Moore AGN. Acanthoma fissuratum of the outer auditory canal from a hearing aid. J Cutan Pathol. 1989;16:304.
- Fania L, Didona D, Morese R, et al. Basal cell carcinoma: from pathophysiology to novel therapeutic approaches. Biomedicines. 2020;8:449. doi:10.3390/biomedicines8110449
- Chauhan DS, Guruprasad Y. Dermal cylindroma of the scalp. Natl J Maxillofac Surg. 2012;3:59-61. doi:10.4103/0975-5950.102163
A 62-year-old man presented to the dermatology office with a 1.5-cm, pink, rubbery nodule behind the left ear that sometimes was tender. He stated that the lesion gradually grew in size over the last 2 years, and it developed after he was fitted for new glasses.