User login
Cutis is a peer-reviewed clinical journal for the dermatologist, allergist, and general practitioner published monthly since 1965. Concise clinical articles present the practical side of dermatology, helping physicians to improve patient care. Cutis is referenced in Index Medicus/MEDLINE and is written and edited by industry leaders.
ass lick
assault rifle
balls
ballsac
black jack
bleach
Boko Haram
bondage
causas
cheap
child abuse
cocaine
compulsive behaviors
cost of miracles
cunt
Daech
display network stats
drug paraphernalia
explosion
fart
fda and death
fda AND warn
fda AND warning
fda AND warns
feom
fuck
gambling
gfc
gun
human trafficking
humira AND expensive
illegal
ISIL
ISIS
Islamic caliphate
Islamic state
madvocate
masturbation
mixed martial arts
MMA
molestation
national rifle association
NRA
nsfw
nuccitelli
pedophile
pedophilia
poker
porn
porn
pornography
psychedelic drug
recreational drug
sex slave rings
shit
slot machine
snort
substance abuse
terrorism
terrorist
texarkana
Texas hold 'em
UFC
section[contains(@class, 'nav-hidden')]
section[contains(@class, 'nav-hidden active')
A peer-reviewed, indexed journal for dermatologists with original research, image quizzes, cases and reviews, and columns.
Telemedicine and Home Pregnancy Testing for iPLEDGE: A Survey of Clinician Perspectives
To the Editor:
In response to the challenges of the COVID-19 pandemic, iPLEDGE announced that they would accept results from home pregnancy tests and explicitly permit telemedicine.1 Given the financial and logistical burdens associated with iPLEDGE, these changes have the potential to increase access.2 However, it is unclear whether these modifications will be allowed to continue. We sought to evaluate clinician perspectives on the role of telemedicine and home pregnancy testing for iPLEDGE.
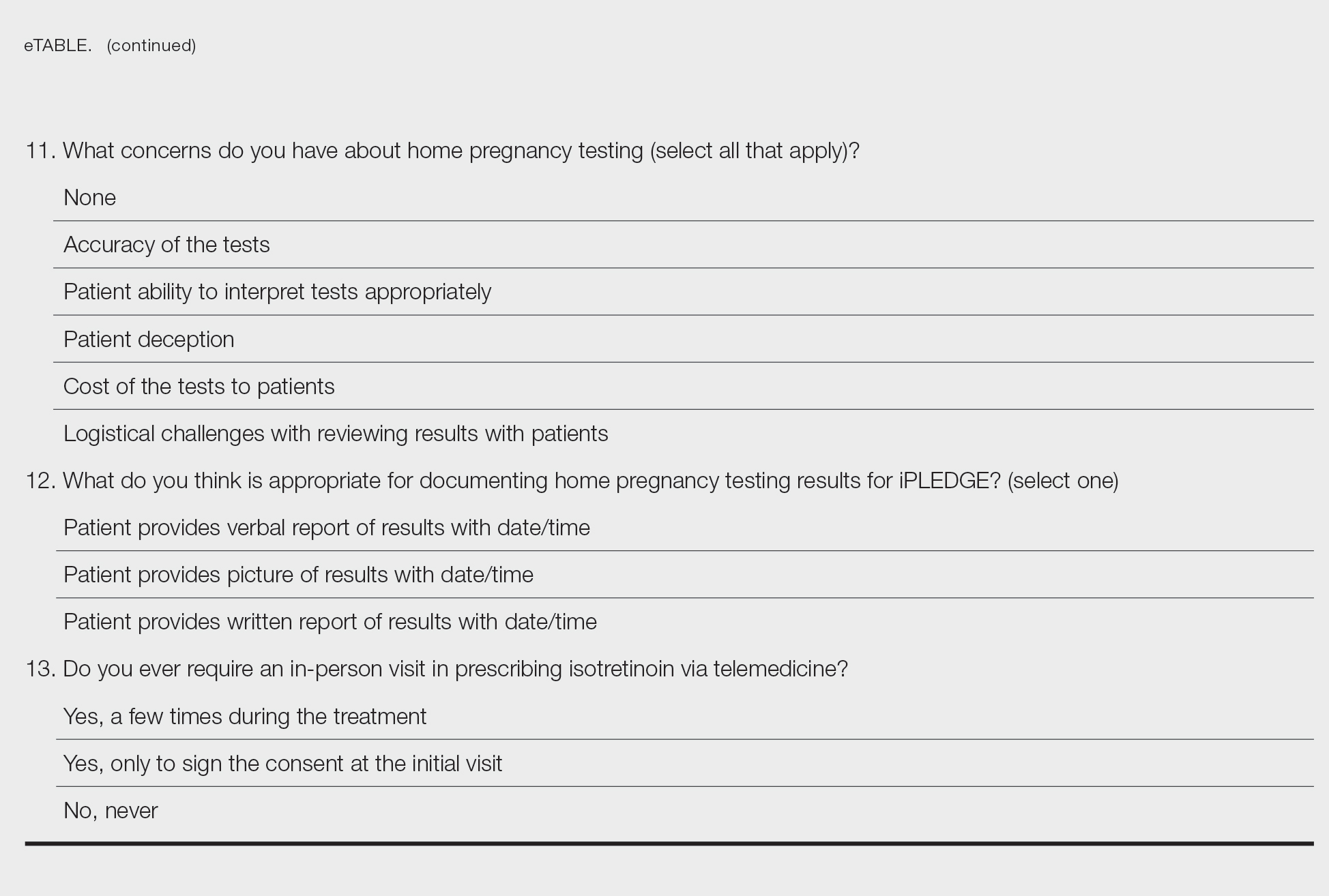
After piloting among several clinicians, a 13-question survey was distributed using the Qualtrics platform to members of the American Acne & Rosacea Society between April 14, 2021, and June 14, 2021. This survey consisted of items addressing provider practices and perspectives on telemedicine and home pregnancy testing for patients taking isotretinoin (eTable). Respondents were asked whether they think telemedicine and home pregnancy testing have improved access to care and whether they would like to continue these practices going forward. In addition, participants were asked about their concerns with home pregnancy testing and how comfortable they feel with home pregnancy testing for various contraceptive strategies (abstinence, condoms, combined oral contraceptives, and long-acting reversible contraception). This study was deemed exempt (category 2) by the University of Pennsylvania (Philadelphia, Pennsylvania) institutional review board (Protocol #844549).
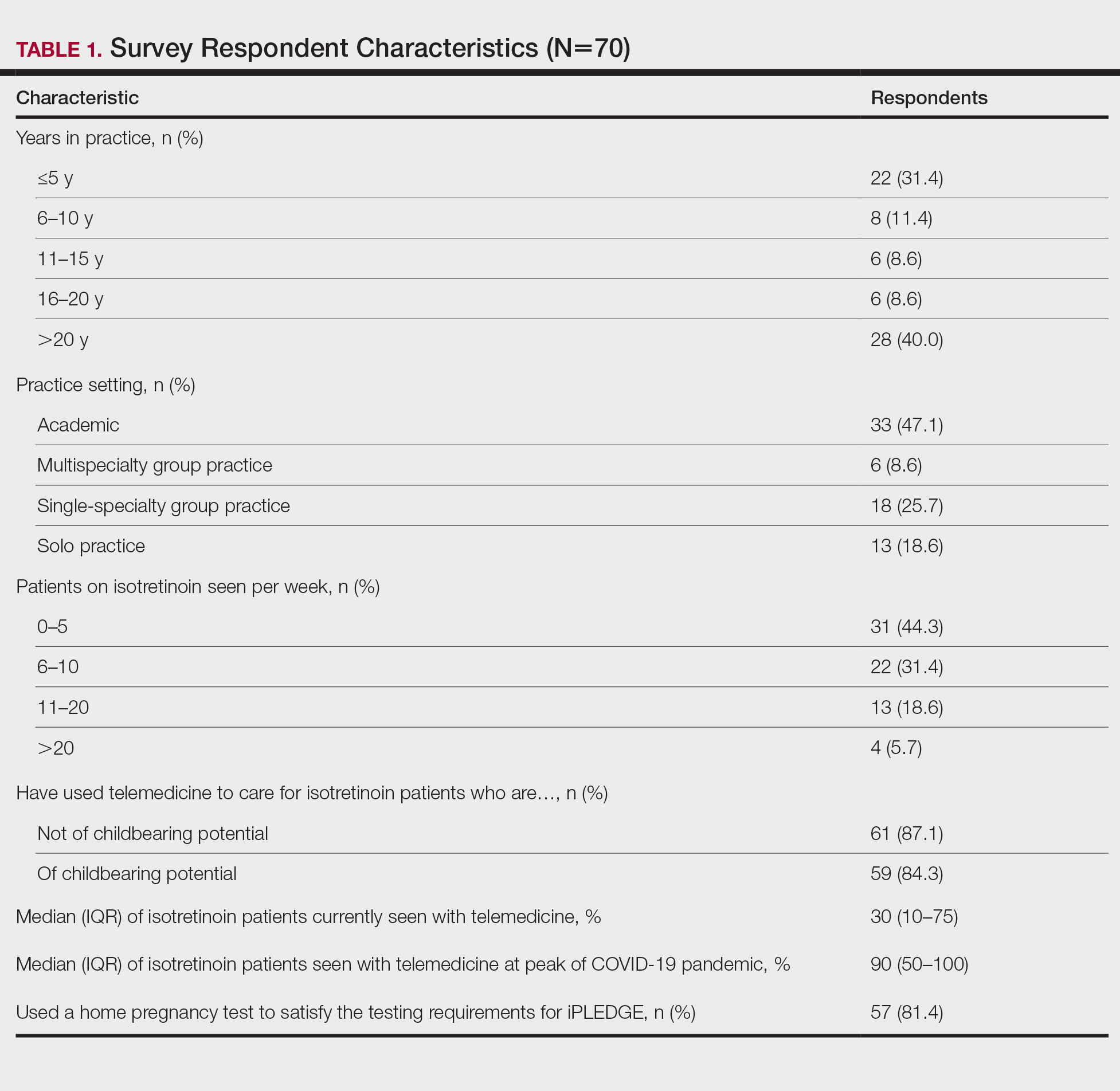
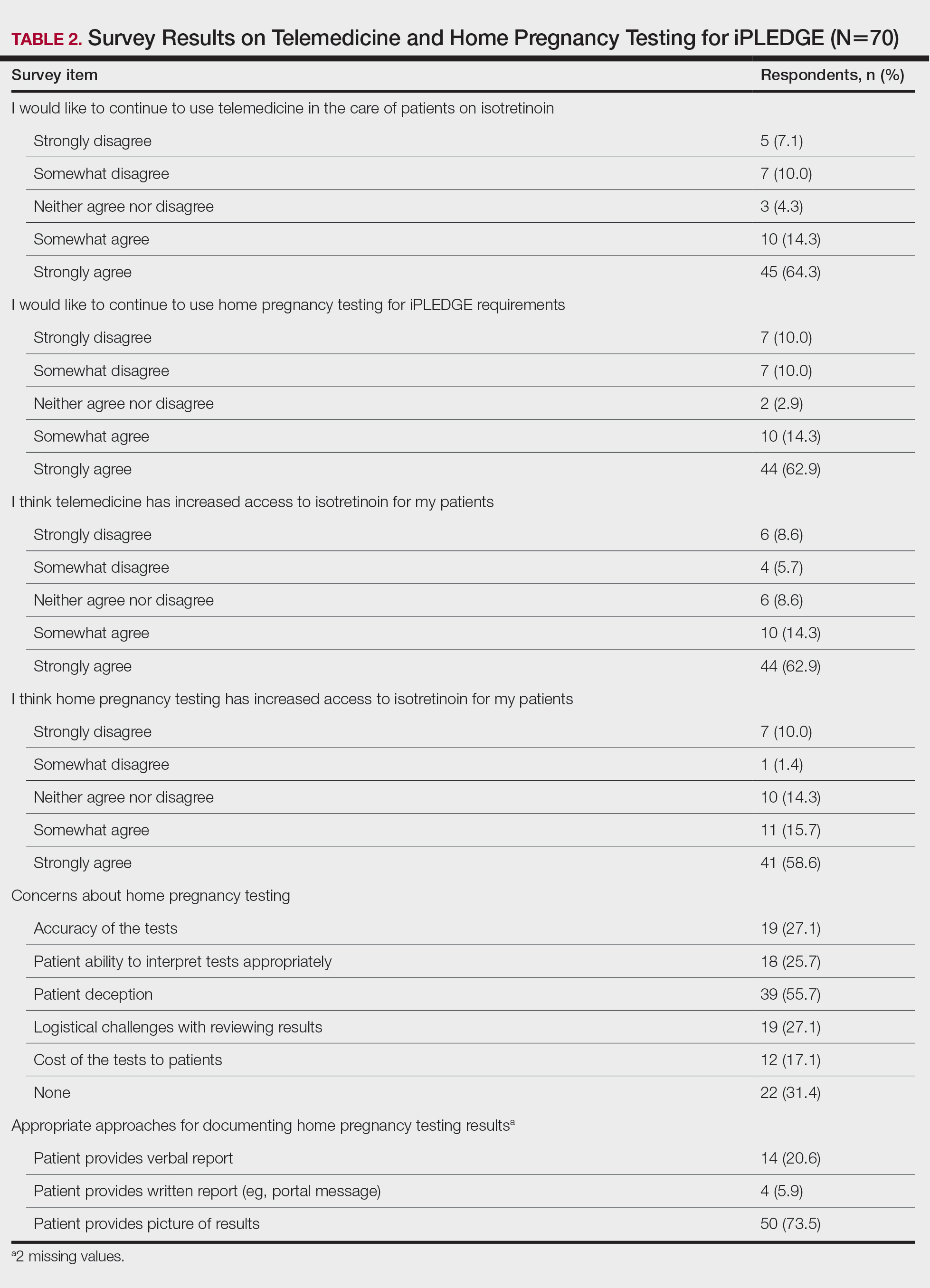
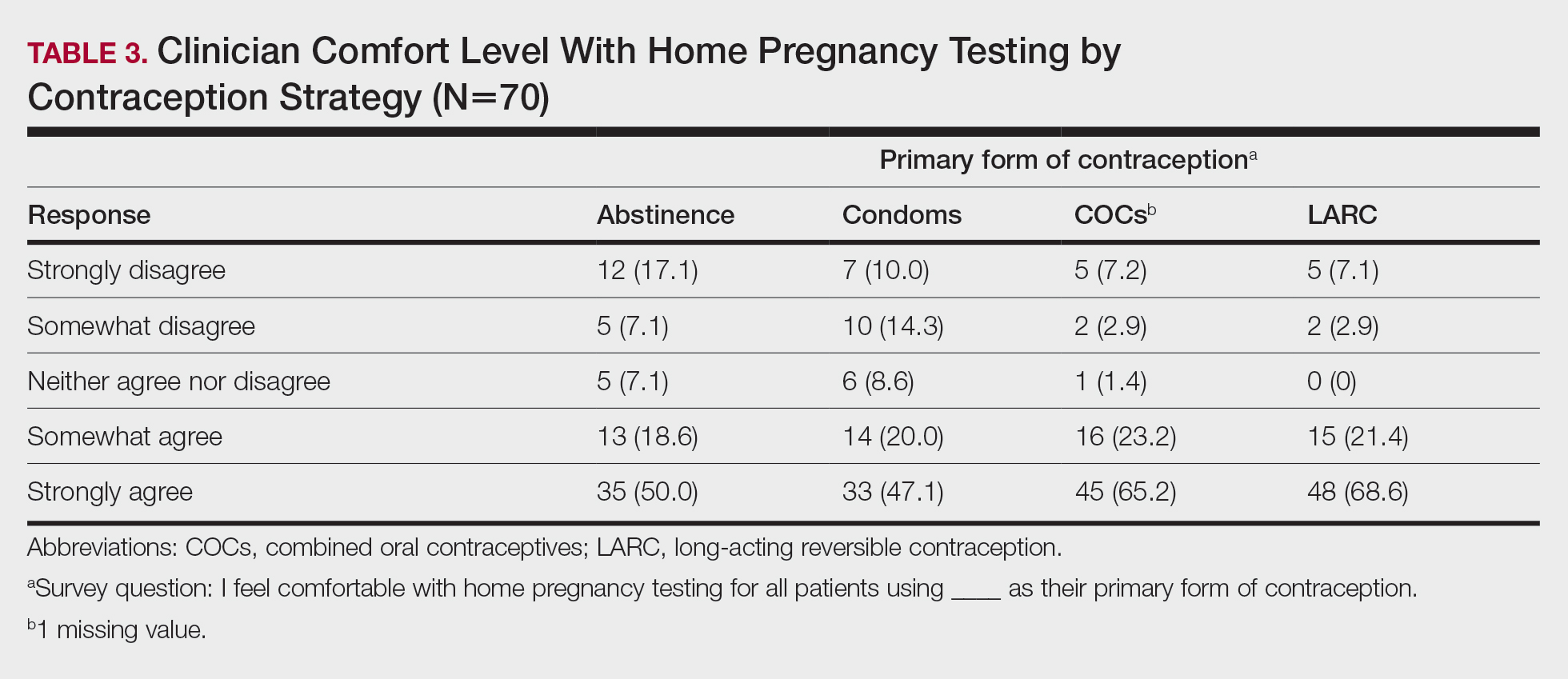
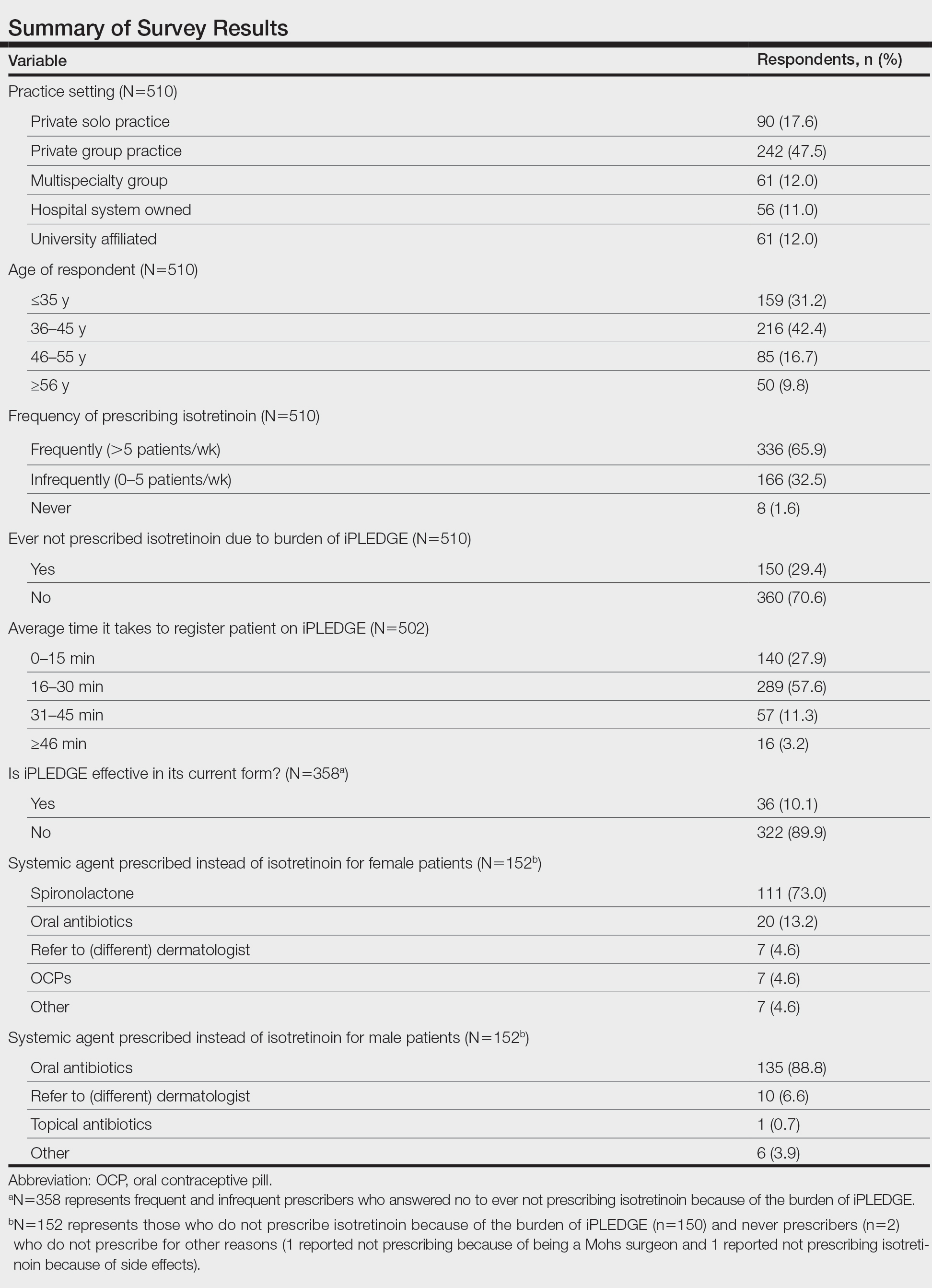
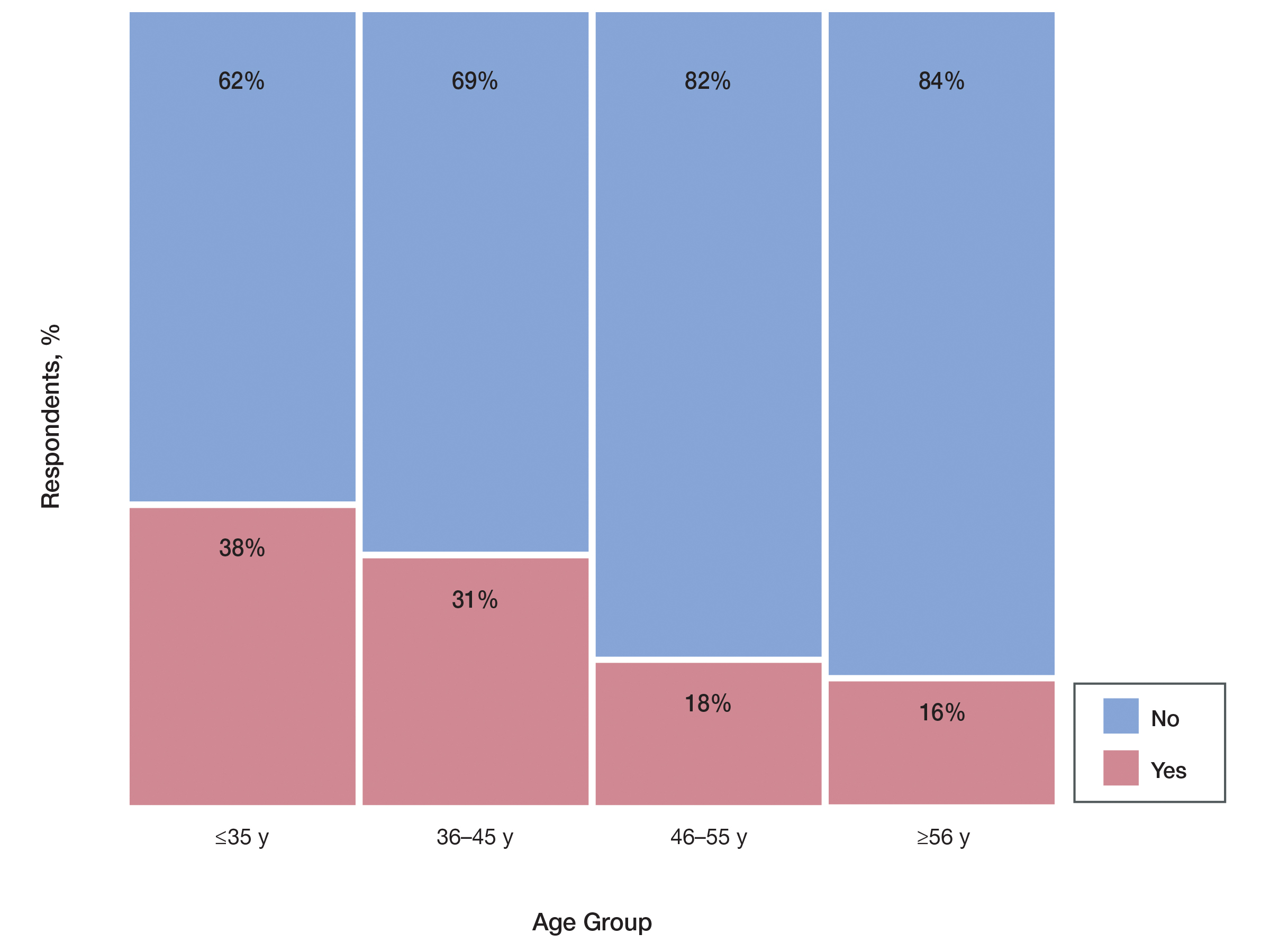
Among 70 clinicians who completed the survey (response rate, 6.4%), 33 (47.1%) practiced in an academic setting. At the peak of the COVID-19 pandemic, clinicians reported using telemedicine for a median of 90% (IQR=50%–100%) of their patients on isotretinoin, and 57 respondents (81.4%) reported having patients use a home pregnancy test for iPLEDGE (Table 1). More than 75% (55/70) agreed that they would like to continue to use telemedicine for patients on isotretinoin, and more than 75% (54/70) agreed that they would like to continue using home pregnancy testing for patients outside the setting of the COVID-19 pandemic. More than 75% (54/70) agreed that telemedicine has increased access for their patients, and more than 70% (52/70) agreed that home pregnancy testing has increased access (Table 2). Clinicians agreed that they would be comfortable using home pregnancy testing for patients choosing long-acting reversible contraception (63/70 [90.0%]), combined oral contraceptives (61/69 [88.4%]), condoms (47/70 [67.1%]), or abstinence (48/70 [68.6%])(Table 3).
The most common concerns about home pregnancy testing were patient deception (39/70 [55.7%]), logistical challenges with reviewing results (19/70 [27.1%]), accuracy of the tests (19/70 [27.1%]), and patient ability to interpret tests appropriately (18/70 [25.7%]). To document testing results, 50 respondents (73.5%) would require a picture of results, 4 (5.9%) would accept a written report from the patient, and 14 (20.6%) would accept a verbal report from the patient (Table 2).
In this survey, clinicians expressed interest in continuing to use telemedicine and home pregnancy testing to care for patients with acne treated with isotretinoin. More than 75% agreed that these changes have increased access, which is notable, as several studies have identified that female and minority patients may face iPLEDGE-associated access barriers.3,4 Continuing to allow home pregnancy testing and explicitly permitting telemedicine can enable clinicians to provide patient-centered care.2
Although clinicians felt comfortable with a variety of contraceptive strategies, particularly those with high reported effectiveness,5 there were concerns about deception and interpretation of test results. Future studies are needed to identify optimal workflows for home pregnancy testing and whether patients should be required to provide a photograph of the results.
This survey study is limited by the possibility of sampling and response bias due to the low response rate. Although the use of national listservs was employed to maximize the generalizability of the results, given the response rate, future studies are needed to evaluate whether these findings generalize to other settings. In addition, given iPLEDGE-associated access barriers, further research is needed to examine how changes such as telemedicine and home pregnancy testing influence both access to isotretinoin and pregnancy prevention.
Acknowledgments—We would like to thank Stacey Moore (Montclair, New Jersey) and the American Acne & Rosacea Society for their help distributing the survey.
- Kane S, Admani S. COVID-19 pandemic leading to the accelerated development of a virtual health model for isotretinoin. J Dermatol Nurses Assoc. 2021;13:54-57.
- Barbieri JS, Frieden IJ, Nagler AR. Isotretinoin, patient safety, and patient-centered care-time to reform iPLEDGE. JAMA Dermatol. 2020;156:21-22.
- Barbieri JS, Shin DB, Wang S, et al. Association of race/ethnicity and sex with differences in health care use and treatment for acne. JAMA Dermatol. 2020;156:312-319.
- Charrow A, Xia FD, Lu J, et al. Differences in isotretinoin start, interruption, and early termination across race and sex in the iPLEDGE era. PloS One. 2019;14:E0210445.
- Barbieri JS, Roe AH, Mostaghimi A. Simplifying contraception requirements for iPLEDGE: a decision analysis. J Am Acad Dermatol. 2020;83:104-108.
To the Editor:
In response to the challenges of the COVID-19 pandemic, iPLEDGE announced that they would accept results from home pregnancy tests and explicitly permit telemedicine.1 Given the financial and logistical burdens associated with iPLEDGE, these changes have the potential to increase access.2 However, it is unclear whether these modifications will be allowed to continue. We sought to evaluate clinician perspectives on the role of telemedicine and home pregnancy testing for iPLEDGE.
After piloting among several clinicians, a 13-question survey was distributed using the Qualtrics platform to members of the American Acne & Rosacea Society between April 14, 2021, and June 14, 2021. This survey consisted of items addressing provider practices and perspectives on telemedicine and home pregnancy testing for patients taking isotretinoin (eTable). Respondents were asked whether they think telemedicine and home pregnancy testing have improved access to care and whether they would like to continue these practices going forward. In addition, participants were asked about their concerns with home pregnancy testing and how comfortable they feel with home pregnancy testing for various contraceptive strategies (abstinence, condoms, combined oral contraceptives, and long-acting reversible contraception). This study was deemed exempt (category 2) by the University of Pennsylvania (Philadelphia, Pennsylvania) institutional review board (Protocol #844549).
Among 70 clinicians who completed the survey (response rate, 6.4%), 33 (47.1%) practiced in an academic setting. At the peak of the COVID-19 pandemic, clinicians reported using telemedicine for a median of 90% (IQR=50%–100%) of their patients on isotretinoin, and 57 respondents (81.4%) reported having patients use a home pregnancy test for iPLEDGE (Table 1). More than 75% (55/70) agreed that they would like to continue to use telemedicine for patients on isotretinoin, and more than 75% (54/70) agreed that they would like to continue using home pregnancy testing for patients outside the setting of the COVID-19 pandemic. More than 75% (54/70) agreed that telemedicine has increased access for their patients, and more than 70% (52/70) agreed that home pregnancy testing has increased access (Table 2). Clinicians agreed that they would be comfortable using home pregnancy testing for patients choosing long-acting reversible contraception (63/70 [90.0%]), combined oral contraceptives (61/69 [88.4%]), condoms (47/70 [67.1%]), or abstinence (48/70 [68.6%])(Table 3).
The most common concerns about home pregnancy testing were patient deception (39/70 [55.7%]), logistical challenges with reviewing results (19/70 [27.1%]), accuracy of the tests (19/70 [27.1%]), and patient ability to interpret tests appropriately (18/70 [25.7%]). To document testing results, 50 respondents (73.5%) would require a picture of results, 4 (5.9%) would accept a written report from the patient, and 14 (20.6%) would accept a verbal report from the patient (Table 2).
In this survey, clinicians expressed interest in continuing to use telemedicine and home pregnancy testing to care for patients with acne treated with isotretinoin. More than 75% agreed that these changes have increased access, which is notable, as several studies have identified that female and minority patients may face iPLEDGE-associated access barriers.3,4 Continuing to allow home pregnancy testing and explicitly permitting telemedicine can enable clinicians to provide patient-centered care.2
Although clinicians felt comfortable with a variety of contraceptive strategies, particularly those with high reported effectiveness,5 there were concerns about deception and interpretation of test results. Future studies are needed to identify optimal workflows for home pregnancy testing and whether patients should be required to provide a photograph of the results.
This survey study is limited by the possibility of sampling and response bias due to the low response rate. Although the use of national listservs was employed to maximize the generalizability of the results, given the response rate, future studies are needed to evaluate whether these findings generalize to other settings. In addition, given iPLEDGE-associated access barriers, further research is needed to examine how changes such as telemedicine and home pregnancy testing influence both access to isotretinoin and pregnancy prevention.
Acknowledgments—We would like to thank Stacey Moore (Montclair, New Jersey) and the American Acne & Rosacea Society for their help distributing the survey.
To the Editor:
In response to the challenges of the COVID-19 pandemic, iPLEDGE announced that they would accept results from home pregnancy tests and explicitly permit telemedicine.1 Given the financial and logistical burdens associated with iPLEDGE, these changes have the potential to increase access.2 However, it is unclear whether these modifications will be allowed to continue. We sought to evaluate clinician perspectives on the role of telemedicine and home pregnancy testing for iPLEDGE.
After piloting among several clinicians, a 13-question survey was distributed using the Qualtrics platform to members of the American Acne & Rosacea Society between April 14, 2021, and June 14, 2021. This survey consisted of items addressing provider practices and perspectives on telemedicine and home pregnancy testing for patients taking isotretinoin (eTable). Respondents were asked whether they think telemedicine and home pregnancy testing have improved access to care and whether they would like to continue these practices going forward. In addition, participants were asked about their concerns with home pregnancy testing and how comfortable they feel with home pregnancy testing for various contraceptive strategies (abstinence, condoms, combined oral contraceptives, and long-acting reversible contraception). This study was deemed exempt (category 2) by the University of Pennsylvania (Philadelphia, Pennsylvania) institutional review board (Protocol #844549).
Among 70 clinicians who completed the survey (response rate, 6.4%), 33 (47.1%) practiced in an academic setting. At the peak of the COVID-19 pandemic, clinicians reported using telemedicine for a median of 90% (IQR=50%–100%) of their patients on isotretinoin, and 57 respondents (81.4%) reported having patients use a home pregnancy test for iPLEDGE (Table 1). More than 75% (55/70) agreed that they would like to continue to use telemedicine for patients on isotretinoin, and more than 75% (54/70) agreed that they would like to continue using home pregnancy testing for patients outside the setting of the COVID-19 pandemic. More than 75% (54/70) agreed that telemedicine has increased access for their patients, and more than 70% (52/70) agreed that home pregnancy testing has increased access (Table 2). Clinicians agreed that they would be comfortable using home pregnancy testing for patients choosing long-acting reversible contraception (63/70 [90.0%]), combined oral contraceptives (61/69 [88.4%]), condoms (47/70 [67.1%]), or abstinence (48/70 [68.6%])(Table 3).
The most common concerns about home pregnancy testing were patient deception (39/70 [55.7%]), logistical challenges with reviewing results (19/70 [27.1%]), accuracy of the tests (19/70 [27.1%]), and patient ability to interpret tests appropriately (18/70 [25.7%]). To document testing results, 50 respondents (73.5%) would require a picture of results, 4 (5.9%) would accept a written report from the patient, and 14 (20.6%) would accept a verbal report from the patient (Table 2).
In this survey, clinicians expressed interest in continuing to use telemedicine and home pregnancy testing to care for patients with acne treated with isotretinoin. More than 75% agreed that these changes have increased access, which is notable, as several studies have identified that female and minority patients may face iPLEDGE-associated access barriers.3,4 Continuing to allow home pregnancy testing and explicitly permitting telemedicine can enable clinicians to provide patient-centered care.2
Although clinicians felt comfortable with a variety of contraceptive strategies, particularly those with high reported effectiveness,5 there were concerns about deception and interpretation of test results. Future studies are needed to identify optimal workflows for home pregnancy testing and whether patients should be required to provide a photograph of the results.
This survey study is limited by the possibility of sampling and response bias due to the low response rate. Although the use of national listservs was employed to maximize the generalizability of the results, given the response rate, future studies are needed to evaluate whether these findings generalize to other settings. In addition, given iPLEDGE-associated access barriers, further research is needed to examine how changes such as telemedicine and home pregnancy testing influence both access to isotretinoin and pregnancy prevention.
Acknowledgments—We would like to thank Stacey Moore (Montclair, New Jersey) and the American Acne & Rosacea Society for their help distributing the survey.
- Kane S, Admani S. COVID-19 pandemic leading to the accelerated development of a virtual health model for isotretinoin. J Dermatol Nurses Assoc. 2021;13:54-57.
- Barbieri JS, Frieden IJ, Nagler AR. Isotretinoin, patient safety, and patient-centered care-time to reform iPLEDGE. JAMA Dermatol. 2020;156:21-22.
- Barbieri JS, Shin DB, Wang S, et al. Association of race/ethnicity and sex with differences in health care use and treatment for acne. JAMA Dermatol. 2020;156:312-319.
- Charrow A, Xia FD, Lu J, et al. Differences in isotretinoin start, interruption, and early termination across race and sex in the iPLEDGE era. PloS One. 2019;14:E0210445.
- Barbieri JS, Roe AH, Mostaghimi A. Simplifying contraception requirements for iPLEDGE: a decision analysis. J Am Acad Dermatol. 2020;83:104-108.
- Kane S, Admani S. COVID-19 pandemic leading to the accelerated development of a virtual health model for isotretinoin. J Dermatol Nurses Assoc. 2021;13:54-57.
- Barbieri JS, Frieden IJ, Nagler AR. Isotretinoin, patient safety, and patient-centered care-time to reform iPLEDGE. JAMA Dermatol. 2020;156:21-22.
- Barbieri JS, Shin DB, Wang S, et al. Association of race/ethnicity and sex with differences in health care use and treatment for acne. JAMA Dermatol. 2020;156:312-319.
- Charrow A, Xia FD, Lu J, et al. Differences in isotretinoin start, interruption, and early termination across race and sex in the iPLEDGE era. PloS One. 2019;14:E0210445.
- Barbieri JS, Roe AH, Mostaghimi A. Simplifying contraception requirements for iPLEDGE: a decision analysis. J Am Acad Dermatol. 2020;83:104-108.
PRACTICE POINTS
- The majority of clinicians report that the use of telemedicine and home pregnancy testing for iPLEDGE has improved access to care and that they would like to continue these practices.
- Continuing to allow home pregnancy testing and explicitly permitting telemedicine can enable clinicians to provide patient-centered care for patients treated with isotretinoin.
What’s Diet Got to Do With It? Basic and Clinical Science Behind Diet and Acne
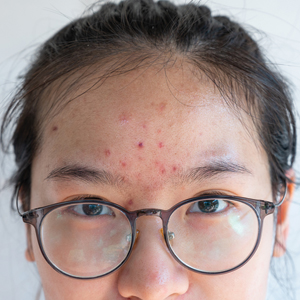
The current understanding of the pathogenesis of acne includes altered keratinization, follicular obstruction, overproduction of sebum, and microbial colonization ( Cutibacterium acnes ) of the pilosebaceous unit resulting in perifollicular inflammation. 1 A deeper dive into the hormonal and molecular drivers of acne have implicated insulin, insulinlike growth factor 1 (IGF-1), corticotropin-releasing hormone, the phosphoinositide 3 -kinase/Akt pathway, mitogen-activated protein kinase pathway, and the nuclear factor κ B pathway. 2-4 A Western diet comprised of high glycemic index foods, carbohydrates, and dairy enhances the production of insulin and IGF-1. A downstream effect of excess insulin and IGF-1 is overactivity of the mammalian target of rapamycin complex 1 (mTORC1), a major promoter of cellular growth and proliferation that primarily is regulated through nutrient availability. 5 This article will review our understanding of the impact of the Western diet on acne pathogenesis and highlight the existing evidence behind the contributions of the mTORC1 pathway in this process. Although quality randomized controlled trials analyzing these effects are limited, dermatologists should understand the existing evidence supporting the potential impacts of diet on acne.
The Western Diet
Glycemic Index—To assess the impact of a high glycemic index diet on acne, Kwon et al6 evaluated 32 patients with mild to moderate acne and placed them on a low or high glycemic index diet for 10 weeks. The low glycemic index diet group was found to have a 70% reduction in the mean number of inflammatory acne lesions from baseline (P<.05), while the high glycemic index diet group had no significant reduction. Noninflammatory lesion counts remained statistically unchanged.6 Smith et al7 studied 43 male patients with acne on either a low glycemic index diet or a self-directed high glycemic diet that was carbohydrate dense. The low glycemic index group showed greater improvement in lesion count as well as improved insulin sensitivity at 12 weeks. Specifically, the mean lesion count (SEM) decreased by 23.5 (3.9) in the low glycemic index group and by only 12.0 (3.5) in the control group (P=.03).7 Observational studies also have supported this hypothesis. After adjustment, an analysis of 24,452 participants in the NutriNet-Santé cohort found significant associations between current acne and the consumption of sugary beverages (adjusted OR, 1.18; 95% CI, 1.01-1.38) and the consumption of fatty and sugary products (adjusted OR, 1.54; 95% CI, 1.09-2.16).8 A Cochrane review that included only 2 studies (Kwon et al6 and Smith et al7) did not find evidence to suggest a low glycemic index diet for noninflammatory lesion count reduction but did note possible benefit for a reduction in inflammatory and total lesion counts; however, Kwon et al6 had incomplete data.9
Dairy—A large retrospective study including 47,355 nurses noted the frequency of milk intake was significantly associated with increased prevalence of acne in adolescence (prevalence ratio, 1.22; 95% CI, 1.03-1.44; P=.002).10 A 2019 meta-analysis further suggested a significant relationship between acne and milk in highest vs lowest intake groups (OR, 1.48; 95% CI, 1.31-1.66) with no significant heterogeneity between the studies (I2=23.6%, P=.24 for heterogeneity), as well as a positive relationship between the highest vs lowest intake of low-fat milk (OR, 1.25; 95% CI, 1.10-1.43) and skim milk (OR, 1.82; 95% CI, 1.34-2.47). In this meta-analysis, yogurt and cheese consumption were not significantly associated with acne (OR, 0.90; 95% CI, 0.73-1.11).11 One non–evidence-based explanation for this may be that fermented dairy products have different biological actions. Pasteurized milk allows microRNAs that directly activate mTORC1 to persist, whereas the bacteria present in the fermentation process may augment this.12 A separate meta-analysis from 2018 did find that yogurt consumption was positively associated with acne (OR, 1.36; 95% CI, 1.05-1.77; P=.022), highlighting the need for larger, more rigorous studies on this topic.13
Insulin and IGF-1—As reviewed above, acne has been considered a disease of Western society, with the Western diet at the center of this association.14 A typical Western diet consists of high glycemic index foods, carbohydrates, and dairy, all of which enhance the production of insulin and IGF-1. Insulin levels increase secondary to high blood glucose and to a lesser degree by protein intake.15 Insulinlike growth factor 1 production is most influenced by age and peaks during puberty; however, high protein diets also increase liver IGF-1 production and release.16 When present in excess, insulin can function as a growth factor. Insulin exerts its anabolic effects through the IGF-1 pathway; however, insulin and IGF-1 are produced in response to different signals.17 Endocrine production of IGF-1 represents 70% of blood levels, peaks at puberty, and rapidly declines in the third decade of life.18 Insulin is produced by the pancreas, and levels correspond to lifestyle and genetically induced insulin resistance.19
Adolescents have elevated levels of IGF-1 as a major driver of puberty-associated growth.20 Despite the natural decrease in IGF-1 following puberty, acne persists in many patients and can even develop for the first time in adulthood in a subset of patients. A study of 40 acne patients and 20 controls found that patients with acne who consumed a high glycemic–load diet was significantly higher than the number of controls consuming a similar diet (P=.008). Additionally, significantly higher levels of mean (SD) serum IGF-1 on quantitative sandwich enzyme-linked immunosorbent assay in acne patients vs controls (543.2 [174.7] ng/mL vs 316.9 [95.7] ng/mL; P<.001) was identified, and these levels correlated significantly with high glycemic–load diet consumption.21 In another study, Kartal et al22 found that basal and fasting insulin levels and homeostasis model assessment scores evaluating for insulin resistance were significantly higher in 36 women compared with 24 age/sex-matched controls (P<.05). This finding remained significant even after excluding women with hyperandrogenemia (P<.05).22
Highlighting the importance of IGF-1 in the pathogenesis of acne, patients with genetic disorders characterized by IGF-1 deficiency, such as Laron syndrome, do not develop acne despite having a functional androgen receptor. Treatment with IGF-1 in these patients induces acne, further supporting the role of IGF-1 in the pathogenesis of this condition.23
The mTORC1 Pathway
Comprised of mTOR in addition to other proteins, mTORC1 is a nutrient-sensitive regulator of cellular growth, proliferation, lipid synthesis, and protein translation.5 Increased activity of mTORC1 has been described in diabetes, neurodegenerative disease, and cancer,14,24 while decreased activity may promote longevity.25 Regulation of mTORC1 occurs through several mechanisms. Growth factors such as insulin and IGF-1 promote mTORC1 activation through the PI3K/Akt pathway. Several amino acids—specifically branched chain amino acids such as alanine, arginine, asparagine, glutamine, histidine, leucine, methionine, serine, threonine, and valine—also can activate mTORC1 independently.26 Excess glucose leads to decreased adenosine monophosphate–activated protein kinase and increased activity of mTORC1, which occurs separately from insulin or IGF-1.27 Starvation blocks mTORC1 via increased adenosine monophosphate–activated protein kinase and starvation-induced hypoxia.26,28 To activate mTORC1, both the IGF-1 or insulin signal and amino acid excess must be present.29 Although not studied in acne, altering the dietary protein content in obese mice has been shown to perturb the mTORC1 pathway, leading to pathologic changes in the mTORC1-autophagy signaling axis, increased amino acid release into the blood, and an acute elevation in mTORC1 signaling.30
Another major regulator of mTORC1 is Forkhead box protein O1 (FOXO1), which is a transcription factor that regulates mTORC1 through sestrin 3.31,32 Sestrin 3 is a stress-induced protein that helps regulate blood glucose and promote insulin sensitivity.33 When FOXO1 is translocated to the cell nucleus, it upregulates the expression of sestrin 3, resulting in mTORC1 inhibition.31,32 Insulin, IGF-1, and nutrient excess lead to FOXO1 translocation to the cell cytoplasm where it can no longer mitigate mTORC1 activity, while the fasted state leads to translocation to the nucleus.34 A single study evaluated the association between FOXO1, mTORC1, a high glycemic–load diet, and acne development. Immunohistochemical detection of mTORC1 assessed by digital image analysis revealed significantly greater expression in inflamed pilosebaceous units found in acne patients (P<.001). Immunohistochemical cytoplasmic expression of FOXO1 and mTOR (used as a proxy for mTORC1) was significantly higher in patients on a high glycemic–load diet (P=.021 and P=.009, respectively) as well as in patients with more severe forms of acne (P=.005 and P=.015, respectively) and elevated IGF-1 levels (P=.004 and P=.003, respectively).21
mTORC1 contributes to the proliferation of keratinocytes and excess sebum production, both independently and through androgen-mediated processes.35-40 Insulinlike growth factor 1 binding the IGF-1 receptor leads to proliferation of keratinocytes lining the sebaceous gland and hair follicle in vivo.35 In mice with epidermis-specific deletion of mTOR, keratinocyte proliferation was decreased and hair follicles were diminished both in number and development. Genetic loss of mTOR in the epidermis led to attenuated signaling pathways of mTORC1 and mTORC2.36
Androgen function is augmented by mTORC1, FOXO1, and IGF-1 through several mechanisms, which may partially explain the hormonal relationship to acne. Androgens increase IGF-1 within the hair follicle.37 In prostate cancer cells, IGF-1 then facilitates movement of FOXO1 to the cytoplasm, preventing it from blocking mTORC1. This effective inactivation of FOXO1 thus further augments the impact of androgens by both allowing unchecked mTORC1 pathway activity and increasing translocation of the androgen receptor (AR) to the nucleus where it exerts its effects.38 Interestingly, genetic polymorphisms of the AR have been shown to cause variable affinity of FOXO1 for the AR; specifically, shorter CAG (cytosine, adenine, guanine) repeat length may lead to decreased FOXO1 binding and is associated with an increased risk for acne.41-43 In addition to its effects on the hair follicle, IGF-1 stimulates production of testosterone and dehydroepiandrosterone as well as activates 5α-reductase, leading to higher dihydrotestosterone levels, which activate the AR with higher affinity than testosterone.44 In some tissues, androgens help regulate the mTORC1 pathway through positive feedback loops.45,46 At this time, we do not know if this occurs in the pathogenesis of acne.
Isotretinoin is the treatment of choice for refractory acne. It has been hypothesized that isotretinoin induces sebocyte apoptosis via the upregulation of FOXO transcription factors and p53.47 Elevated levels of nuclear FOXO1 have been found in the sebaceous glands of patients following initiation of treatment with isotretinoin and are hypothesized to play a major role in the drug’s effectiveness. Specifically, biopsies from 14 acne patients before and after 6 weeks of isotretinoin therapy were analyzed with immunohistochemical staining and found to have a significantly improved nuclear to cytoplasmic ratio of nonphosphorylated FOXO1 (P<.001).47
Practical Recommendations
Given the available evidence, it is important for dermatologists to address dietary recommendations in acne patients. Although large randomized controlled trials on diet and acne severity are challenging to conduct in this population, the existing literature suggests that patients should avoid high glycemic index simple sugars and processed grains, and patients should focus on eating more complex carbohydrates in the form of legumes, vegetables, fruits, and tubers.6-8 With regard to dairy, milk (especially skim) has been associated with increased risks for acne.11,13 Fermented dairy products may have less impact on acne severity and include cheese, yogurt (unsweetened to keep glycemic index low), and sour cream.12
- Zaenglein AL. Acne vulgaris. In: Bolognia JL, Schaffer JV, Cerroni L, eds. Dermatology. Elsevier; 2017:588-603.
- Ganceviciene R, Graziene V, Fimmel S, et al. Involvement of the corticotropin-releasing hormone system in the pathogenesis of acne vulgaris. Br J Dermatol. 2009;160:345-352.
- Kang S, Cho S, Chung JH, et al. Inflammation and extracellular matrix degradation mediated by activated transcription factors nuclear factor-kappaB and activator protein-1 in inflammatory acne lesions in vivo. Am J Pathol. 2005;166:1691-1699.
- Cong TX, Hao D, Wen X, et al. From pathogenesis of acne vulgaris to anti-acne agents. Arch Dermatol Res. 2019;311:337-349.
- Pópulo H, Lopes JM, Soares P. The mTOR signalling pathway in human cancer. Int J Mol Sci. 2012;13:1886-1918.
- Kwon HH, Yoon JY, Hong JS, et al. Clinical and histological effect of a low glycaemic load diet in treatment of acne vulgaris in Korean patients: a randomized, controlled trial. Acta Derm Venereol. 2012;92:241-246.
- Smith RN, Mann NJ, Braue A, et al. A low-glycemic-load diet improves symptoms in acne vulgaris patients: a randomized controlled trial. Am J Clin Nutr. 2007;86:107-115.
- Penso L, Touvier M, Deschasaux M, et al. Association between adult acne and dietary behaviors: findings from the NutriNet-Santé prospective cohort study. JAMA Dermatol. 2020;156:854-862.
- Cao H, Yang G, Wang Y, et al. Complementary therapies for acne vulgaris. Cochrane Database Syst Rev. 2015;1:CD009436.
- Adebamowo CA, Spiegelman D, Danby FW, et al. High school dietary dairy intake and teenage acne. J Am Acad Dermatol. 2005;52:207-214.
- Aghasi M, Golzarand M, Shab-Bidar S, et al. Dairy intake and acne development: a meta-analysis of observational studies. Clin Nutr. 2019;38:1067-1075.
- Melnik BC, Schmitz G. Pasteurized non-fermented cow’s milk but not fermented milk is a promoter of mTORC1-driven aging and increased mortality. Ageing Res Rev. 2021;67:101270.
- Juhl CR, Bergholdt HKM, Miller IM, et al. Dairy intake and acne vulgaris: a systematic review and meta-analysis of 78,529 children, adolescents, and young adults. Nutrients. 2018;10:1049. doi:10.3390/nu10081049
- Melnik BC. Linking diet to acne metabolomics, inflammation, and comedogenesis: an update. Clin Cosmet Investig Dermatol. 2015;8:371-388.
- Smart CEM, King BR, Lopez PE. Insulin dosing for fat and protein: is it time? Diabetes Care. 2020;43:13-15.
- Wan X, Wang S, Xu J, et al. Dietary protein-induced hepatic IGF-1 secretion mediated by PPARγ activation. PLoS One. 2017;12:E0173174.
- Bedinger DH, Adams SH. Metabolic, anabolic, and mitogenic insulin responses: a tissue-specific perspective for insulin receptor activators. Mol Cell Endocrinol. 2015;415:143-156.
- Gubbi S, Quipildor GF, Barzilai N, et al. 40 YEARS of IGF1: IGF1: the Jekyll and Hyde of the aging brain. J Mol Endocrinol. 2018;61:T171-T185.
- Kolb H, Kempf K, Röhling M, et al. Insulin: too much of a good thing is bad. BMC Med. 2020;18:224.
- Wood CL, Lane LC, Cheetham T. Puberty: normal physiology (brief overview). Best Pract Res Clin Endocrinol Metab. 2019;33:101265.
- Agamia NF, Abdallah DM, Sorour O, et al. Skin expression of mammalian target of rapamycin and forkhead box transcription factor O1, and serum insulin-like growth factor-1 in patients with acne vulgaris and their relationship with diet. Br J Dermatol. 2016;174:1299-1307.
- Kartal D, Yildiz H, Ertas R, et al. Association between isolated female acne and insulin resistance: a prospective study. G Ital Dermatol Venereol. 2016;151:353-357.
- Ben-Amitai D, Laron Z. Effect of insulin-like growth factor-1 deficiency or administration on the occurrence of acne. J Eur Acad Dermatol Venereol. 2011;25:950-954.
- Kim LC, Cook RS, Chen J. mTORC1 and mTORC2 in cancer and the tumor microenvironment. Oncogene. 2017;36:2191-2201.
- Weichhart T. mTOR as regulator of lifespan, aging, and cellular senescence: a mini-review. Gerontology. 2018;64:127-134.
- Melick CH, Jewell JL. Regulation of mTORC1 by upstream stimuli. Genes. 2020;11:989. doi:10.3390/genes11090989
- Li M, Zhang CS, Feng JW, et al. Aldolase is a sensor for both low and high glucose, linking to AMPK and mTORC1. Cell Res. 2021;31:478-481.
- Yan T, Zhang J, Tang D, et al. Hypoxia regulates mTORC1-mediated keratinocyte motility and migration via the AMPK pathway. PLoS One. 2017;12:E0169155.
- Dennis MD, Baum JI, Kimball SR, et al. Mechanisms involved in the coordinate regulation of mTORC1 by insulin and amino acids. J Biol Chem. 2011;286:8287-8296.
- Choi BSY, Daniel N, Houde VP, et al. Feeding diversified protein sources exacerbates hepatic insulin resistance via increased gut microbial branched-chain fatty acids and mTORC1 signaling in obese mice. Nat Commun. 2021;12:3377.
- Chen CC, Jeon SM, Bhaskar PT, et al. FoxOs inhibit mTORC1 and activate Akt by inducing the expression of Sestrin3 and Rictor. Dev Cell. 2010;18:592-604.
- Chen Y, Huang T, Yu Z, et al. The functions and roles of sestrins in regulating human diseases. Cell Mol Biol Lett. 2022;27:2.
- Tao R, Xiong X, Liangpunsakul S, et al. Sestrin 3 protein enhances hepatic insulin sensitivity by direct activation of the mTORC2-Akt signaling. Diabetes. 2015;64:1211-1223.
- Gross DN, Wan M, Birnbaum MJ. The role of FOXO in the regulation of metabolism. Curr Diab Rep. 2009;9:208-214.
- Gilhar A, Ish-Shalom S, Pillar T, et al. Effect of anti–insulin-like growth factor 1 on epidermal proliferation of human skin transplanted onto nude mice treated with growth hormone. Endocrinology. 1994;134:229-232.
- Ding X, Bloch W, Iden S, et al. mTORC1 and mTORC2 regulate skin morphogenesis and epidermal barrier formation. Nat Commun. 2016;7:13226.
- Inui S, Itami S. Androgen actions on the human hair follicle: perspectives. Exp Dermatol. 2013;22:168-171.
- Fan W, Yanase T, Morinaga H, et al. Insulin-like growth factor 1/insulin signaling activates androgen signaling through direct interactions of Foxo1 with androgen receptor. J Biol Chem. 2007;282:7329-7338.
- Alestas T, Ganceviciene R, Fimmel S, et al. Enzymes involved in the biosynthesis of leukotriene B4 and prostaglandin E2 are active in sebaceous glands. J Mol Med. 2006;84:75-87.
- Smith TM, Gilliland K, Clawson GA, et al. IGF-1 induces SREBP-1 expression and lipogenesis in SEB-1 sebocytes via activation of the phosphoinositide 3-kinase/Akt pathway. J Invest Dermatol. 2008;128:1286-1293.
- Furtado GV, Yang J, Wu D, et al. FOXO1 controls protein synthesis and transcript abundance of mutant polyglutamine proteins, preventing protein aggregation. Hum Mol Genet. 2021;30:996-1005.
- Melnik BC. Isotretinoin and FoxO1: a scientific hypothesis. Dermatoendocrinol. 2011;3:141-165.
- Heng AHS, Say YH, Sio YY, et al. Gene variants associated with acne vulgaris presentation and severity: a systematic review and meta-analysis. BMC Med Genomics. 2021;14:103.
- Li J, Al-Azzawi F. Mechanism of androgen receptor action. Maturitas. 2009;63:142-148.
- Zhao Y, Tindall DJ, Huang H. Modulation of androgen receptor by FOXA1 and FOXO1 factors in prostate cancer. Int J Biol Sci. 2014;10:614-619.
- Hamdi MM, Mutungi G. Dihydrotestosterone stimulates amino acid uptake and the expression of LAT2 in mouse skeletal muscle fibres through an ERK1/2-dependent mechanism. J Physiol. 2011;589(pt 14):3623-3640.
- Agamia NF, Hussein OM, Abdelmaksoud RE, et al. Effect of oral isotretinoin on the nucleocytoplasmic distribution of FoxO1 and FoxO3 proteins in sebaceous glands of patients with acne vulgaris. Exp Dermatol. 2018;27:1344-1351.
- Kolovou GD, Watts GF, Mikhailidis DP, et al. Postprandial hypertriglyceridaemia revisited in the era of non-fasting lipid profile testing: a 2019 expert panel statement, main text. Curr Vasc Pharmacol. 2019;17:498-514.
- Svoboda SA, Shields BE. Cutaneous manifestations of nutritional excess: pathophysiologic effects of hyperglycemia and hyperinsulinemia on the skin. Cutis. 2021;107:74-78.
- González-González JG, Mancillas-Adame LG, Fernández-Reyes M, et al. Androgenetic alopecia and insulin resistance in young men. Clin Endocrinol . 2009;71:494-499.
- Livadas S, Anagnostis P, Bosdou JK, et al. Polycystic ovary syndrome and type 2 diabetes mellitus: a state-of-the-art review. World J Diabetes. 2022;13:5-26.
The current understanding of the pathogenesis of acne includes altered keratinization, follicular obstruction, overproduction of sebum, and microbial colonization ( Cutibacterium acnes ) of the pilosebaceous unit resulting in perifollicular inflammation. 1 A deeper dive into the hormonal and molecular drivers of acne have implicated insulin, insulinlike growth factor 1 (IGF-1), corticotropin-releasing hormone, the phosphoinositide 3 -kinase/Akt pathway, mitogen-activated protein kinase pathway, and the nuclear factor κ B pathway. 2-4 A Western diet comprised of high glycemic index foods, carbohydrates, and dairy enhances the production of insulin and IGF-1. A downstream effect of excess insulin and IGF-1 is overactivity of the mammalian target of rapamycin complex 1 (mTORC1), a major promoter of cellular growth and proliferation that primarily is regulated through nutrient availability. 5 This article will review our understanding of the impact of the Western diet on acne pathogenesis and highlight the existing evidence behind the contributions of the mTORC1 pathway in this process. Although quality randomized controlled trials analyzing these effects are limited, dermatologists should understand the existing evidence supporting the potential impacts of diet on acne.
The Western Diet
Glycemic Index—To assess the impact of a high glycemic index diet on acne, Kwon et al6 evaluated 32 patients with mild to moderate acne and placed them on a low or high glycemic index diet for 10 weeks. The low glycemic index diet group was found to have a 70% reduction in the mean number of inflammatory acne lesions from baseline (P<.05), while the high glycemic index diet group had no significant reduction. Noninflammatory lesion counts remained statistically unchanged.6 Smith et al7 studied 43 male patients with acne on either a low glycemic index diet or a self-directed high glycemic diet that was carbohydrate dense. The low glycemic index group showed greater improvement in lesion count as well as improved insulin sensitivity at 12 weeks. Specifically, the mean lesion count (SEM) decreased by 23.5 (3.9) in the low glycemic index group and by only 12.0 (3.5) in the control group (P=.03).7 Observational studies also have supported this hypothesis. After adjustment, an analysis of 24,452 participants in the NutriNet-Santé cohort found significant associations between current acne and the consumption of sugary beverages (adjusted OR, 1.18; 95% CI, 1.01-1.38) and the consumption of fatty and sugary products (adjusted OR, 1.54; 95% CI, 1.09-2.16).8 A Cochrane review that included only 2 studies (Kwon et al6 and Smith et al7) did not find evidence to suggest a low glycemic index diet for noninflammatory lesion count reduction but did note possible benefit for a reduction in inflammatory and total lesion counts; however, Kwon et al6 had incomplete data.9
Dairy—A large retrospective study including 47,355 nurses noted the frequency of milk intake was significantly associated with increased prevalence of acne in adolescence (prevalence ratio, 1.22; 95% CI, 1.03-1.44; P=.002).10 A 2019 meta-analysis further suggested a significant relationship between acne and milk in highest vs lowest intake groups (OR, 1.48; 95% CI, 1.31-1.66) with no significant heterogeneity between the studies (I2=23.6%, P=.24 for heterogeneity), as well as a positive relationship between the highest vs lowest intake of low-fat milk (OR, 1.25; 95% CI, 1.10-1.43) and skim milk (OR, 1.82; 95% CI, 1.34-2.47). In this meta-analysis, yogurt and cheese consumption were not significantly associated with acne (OR, 0.90; 95% CI, 0.73-1.11).11 One non–evidence-based explanation for this may be that fermented dairy products have different biological actions. Pasteurized milk allows microRNAs that directly activate mTORC1 to persist, whereas the bacteria present in the fermentation process may augment this.12 A separate meta-analysis from 2018 did find that yogurt consumption was positively associated with acne (OR, 1.36; 95% CI, 1.05-1.77; P=.022), highlighting the need for larger, more rigorous studies on this topic.13
Insulin and IGF-1—As reviewed above, acne has been considered a disease of Western society, with the Western diet at the center of this association.14 A typical Western diet consists of high glycemic index foods, carbohydrates, and dairy, all of which enhance the production of insulin and IGF-1. Insulin levels increase secondary to high blood glucose and to a lesser degree by protein intake.15 Insulinlike growth factor 1 production is most influenced by age and peaks during puberty; however, high protein diets also increase liver IGF-1 production and release.16 When present in excess, insulin can function as a growth factor. Insulin exerts its anabolic effects through the IGF-1 pathway; however, insulin and IGF-1 are produced in response to different signals.17 Endocrine production of IGF-1 represents 70% of blood levels, peaks at puberty, and rapidly declines in the third decade of life.18 Insulin is produced by the pancreas, and levels correspond to lifestyle and genetically induced insulin resistance.19
Adolescents have elevated levels of IGF-1 as a major driver of puberty-associated growth.20 Despite the natural decrease in IGF-1 following puberty, acne persists in many patients and can even develop for the first time in adulthood in a subset of patients. A study of 40 acne patients and 20 controls found that patients with acne who consumed a high glycemic–load diet was significantly higher than the number of controls consuming a similar diet (P=.008). Additionally, significantly higher levels of mean (SD) serum IGF-1 on quantitative sandwich enzyme-linked immunosorbent assay in acne patients vs controls (543.2 [174.7] ng/mL vs 316.9 [95.7] ng/mL; P<.001) was identified, and these levels correlated significantly with high glycemic–load diet consumption.21 In another study, Kartal et al22 found that basal and fasting insulin levels and homeostasis model assessment scores evaluating for insulin resistance were significantly higher in 36 women compared with 24 age/sex-matched controls (P<.05). This finding remained significant even after excluding women with hyperandrogenemia (P<.05).22
Highlighting the importance of IGF-1 in the pathogenesis of acne, patients with genetic disorders characterized by IGF-1 deficiency, such as Laron syndrome, do not develop acne despite having a functional androgen receptor. Treatment with IGF-1 in these patients induces acne, further supporting the role of IGF-1 in the pathogenesis of this condition.23
The mTORC1 Pathway
Comprised of mTOR in addition to other proteins, mTORC1 is a nutrient-sensitive regulator of cellular growth, proliferation, lipid synthesis, and protein translation.5 Increased activity of mTORC1 has been described in diabetes, neurodegenerative disease, and cancer,14,24 while decreased activity may promote longevity.25 Regulation of mTORC1 occurs through several mechanisms. Growth factors such as insulin and IGF-1 promote mTORC1 activation through the PI3K/Akt pathway. Several amino acids—specifically branched chain amino acids such as alanine, arginine, asparagine, glutamine, histidine, leucine, methionine, serine, threonine, and valine—also can activate mTORC1 independently.26 Excess glucose leads to decreased adenosine monophosphate–activated protein kinase and increased activity of mTORC1, which occurs separately from insulin or IGF-1.27 Starvation blocks mTORC1 via increased adenosine monophosphate–activated protein kinase and starvation-induced hypoxia.26,28 To activate mTORC1, both the IGF-1 or insulin signal and amino acid excess must be present.29 Although not studied in acne, altering the dietary protein content in obese mice has been shown to perturb the mTORC1 pathway, leading to pathologic changes in the mTORC1-autophagy signaling axis, increased amino acid release into the blood, and an acute elevation in mTORC1 signaling.30
Another major regulator of mTORC1 is Forkhead box protein O1 (FOXO1), which is a transcription factor that regulates mTORC1 through sestrin 3.31,32 Sestrin 3 is a stress-induced protein that helps regulate blood glucose and promote insulin sensitivity.33 When FOXO1 is translocated to the cell nucleus, it upregulates the expression of sestrin 3, resulting in mTORC1 inhibition.31,32 Insulin, IGF-1, and nutrient excess lead to FOXO1 translocation to the cell cytoplasm where it can no longer mitigate mTORC1 activity, while the fasted state leads to translocation to the nucleus.34 A single study evaluated the association between FOXO1, mTORC1, a high glycemic–load diet, and acne development. Immunohistochemical detection of mTORC1 assessed by digital image analysis revealed significantly greater expression in inflamed pilosebaceous units found in acne patients (P<.001). Immunohistochemical cytoplasmic expression of FOXO1 and mTOR (used as a proxy for mTORC1) was significantly higher in patients on a high glycemic–load diet (P=.021 and P=.009, respectively) as well as in patients with more severe forms of acne (P=.005 and P=.015, respectively) and elevated IGF-1 levels (P=.004 and P=.003, respectively).21
mTORC1 contributes to the proliferation of keratinocytes and excess sebum production, both independently and through androgen-mediated processes.35-40 Insulinlike growth factor 1 binding the IGF-1 receptor leads to proliferation of keratinocytes lining the sebaceous gland and hair follicle in vivo.35 In mice with epidermis-specific deletion of mTOR, keratinocyte proliferation was decreased and hair follicles were diminished both in number and development. Genetic loss of mTOR in the epidermis led to attenuated signaling pathways of mTORC1 and mTORC2.36
Androgen function is augmented by mTORC1, FOXO1, and IGF-1 through several mechanisms, which may partially explain the hormonal relationship to acne. Androgens increase IGF-1 within the hair follicle.37 In prostate cancer cells, IGF-1 then facilitates movement of FOXO1 to the cytoplasm, preventing it from blocking mTORC1. This effective inactivation of FOXO1 thus further augments the impact of androgens by both allowing unchecked mTORC1 pathway activity and increasing translocation of the androgen receptor (AR) to the nucleus where it exerts its effects.38 Interestingly, genetic polymorphisms of the AR have been shown to cause variable affinity of FOXO1 for the AR; specifically, shorter CAG (cytosine, adenine, guanine) repeat length may lead to decreased FOXO1 binding and is associated with an increased risk for acne.41-43 In addition to its effects on the hair follicle, IGF-1 stimulates production of testosterone and dehydroepiandrosterone as well as activates 5α-reductase, leading to higher dihydrotestosterone levels, which activate the AR with higher affinity than testosterone.44 In some tissues, androgens help regulate the mTORC1 pathway through positive feedback loops.45,46 At this time, we do not know if this occurs in the pathogenesis of acne.
Isotretinoin is the treatment of choice for refractory acne. It has been hypothesized that isotretinoin induces sebocyte apoptosis via the upregulation of FOXO transcription factors and p53.47 Elevated levels of nuclear FOXO1 have been found in the sebaceous glands of patients following initiation of treatment with isotretinoin and are hypothesized to play a major role in the drug’s effectiveness. Specifically, biopsies from 14 acne patients before and after 6 weeks of isotretinoin therapy were analyzed with immunohistochemical staining and found to have a significantly improved nuclear to cytoplasmic ratio of nonphosphorylated FOXO1 (P<.001).47
Practical Recommendations
Given the available evidence, it is important for dermatologists to address dietary recommendations in acne patients. Although large randomized controlled trials on diet and acne severity are challenging to conduct in this population, the existing literature suggests that patients should avoid high glycemic index simple sugars and processed grains, and patients should focus on eating more complex carbohydrates in the form of legumes, vegetables, fruits, and tubers.6-8 With regard to dairy, milk (especially skim) has been associated with increased risks for acne.11,13 Fermented dairy products may have less impact on acne severity and include cheese, yogurt (unsweetened to keep glycemic index low), and sour cream.12
The current understanding of the pathogenesis of acne includes altered keratinization, follicular obstruction, overproduction of sebum, and microbial colonization ( Cutibacterium acnes ) of the pilosebaceous unit resulting in perifollicular inflammation. 1 A deeper dive into the hormonal and molecular drivers of acne have implicated insulin, insulinlike growth factor 1 (IGF-1), corticotropin-releasing hormone, the phosphoinositide 3 -kinase/Akt pathway, mitogen-activated protein kinase pathway, and the nuclear factor κ B pathway. 2-4 A Western diet comprised of high glycemic index foods, carbohydrates, and dairy enhances the production of insulin and IGF-1. A downstream effect of excess insulin and IGF-1 is overactivity of the mammalian target of rapamycin complex 1 (mTORC1), a major promoter of cellular growth and proliferation that primarily is regulated through nutrient availability. 5 This article will review our understanding of the impact of the Western diet on acne pathogenesis and highlight the existing evidence behind the contributions of the mTORC1 pathway in this process. Although quality randomized controlled trials analyzing these effects are limited, dermatologists should understand the existing evidence supporting the potential impacts of diet on acne.
The Western Diet
Glycemic Index—To assess the impact of a high glycemic index diet on acne, Kwon et al6 evaluated 32 patients with mild to moderate acne and placed them on a low or high glycemic index diet for 10 weeks. The low glycemic index diet group was found to have a 70% reduction in the mean number of inflammatory acne lesions from baseline (P<.05), while the high glycemic index diet group had no significant reduction. Noninflammatory lesion counts remained statistically unchanged.6 Smith et al7 studied 43 male patients with acne on either a low glycemic index diet or a self-directed high glycemic diet that was carbohydrate dense. The low glycemic index group showed greater improvement in lesion count as well as improved insulin sensitivity at 12 weeks. Specifically, the mean lesion count (SEM) decreased by 23.5 (3.9) in the low glycemic index group and by only 12.0 (3.5) in the control group (P=.03).7 Observational studies also have supported this hypothesis. After adjustment, an analysis of 24,452 participants in the NutriNet-Santé cohort found significant associations between current acne and the consumption of sugary beverages (adjusted OR, 1.18; 95% CI, 1.01-1.38) and the consumption of fatty and sugary products (adjusted OR, 1.54; 95% CI, 1.09-2.16).8 A Cochrane review that included only 2 studies (Kwon et al6 and Smith et al7) did not find evidence to suggest a low glycemic index diet for noninflammatory lesion count reduction but did note possible benefit for a reduction in inflammatory and total lesion counts; however, Kwon et al6 had incomplete data.9
Dairy—A large retrospective study including 47,355 nurses noted the frequency of milk intake was significantly associated with increased prevalence of acne in adolescence (prevalence ratio, 1.22; 95% CI, 1.03-1.44; P=.002).10 A 2019 meta-analysis further suggested a significant relationship between acne and milk in highest vs lowest intake groups (OR, 1.48; 95% CI, 1.31-1.66) with no significant heterogeneity between the studies (I2=23.6%, P=.24 for heterogeneity), as well as a positive relationship between the highest vs lowest intake of low-fat milk (OR, 1.25; 95% CI, 1.10-1.43) and skim milk (OR, 1.82; 95% CI, 1.34-2.47). In this meta-analysis, yogurt and cheese consumption were not significantly associated with acne (OR, 0.90; 95% CI, 0.73-1.11).11 One non–evidence-based explanation for this may be that fermented dairy products have different biological actions. Pasteurized milk allows microRNAs that directly activate mTORC1 to persist, whereas the bacteria present in the fermentation process may augment this.12 A separate meta-analysis from 2018 did find that yogurt consumption was positively associated with acne (OR, 1.36; 95% CI, 1.05-1.77; P=.022), highlighting the need for larger, more rigorous studies on this topic.13
Insulin and IGF-1—As reviewed above, acne has been considered a disease of Western society, with the Western diet at the center of this association.14 A typical Western diet consists of high glycemic index foods, carbohydrates, and dairy, all of which enhance the production of insulin and IGF-1. Insulin levels increase secondary to high blood glucose and to a lesser degree by protein intake.15 Insulinlike growth factor 1 production is most influenced by age and peaks during puberty; however, high protein diets also increase liver IGF-1 production and release.16 When present in excess, insulin can function as a growth factor. Insulin exerts its anabolic effects through the IGF-1 pathway; however, insulin and IGF-1 are produced in response to different signals.17 Endocrine production of IGF-1 represents 70% of blood levels, peaks at puberty, and rapidly declines in the third decade of life.18 Insulin is produced by the pancreas, and levels correspond to lifestyle and genetically induced insulin resistance.19
Adolescents have elevated levels of IGF-1 as a major driver of puberty-associated growth.20 Despite the natural decrease in IGF-1 following puberty, acne persists in many patients and can even develop for the first time in adulthood in a subset of patients. A study of 40 acne patients and 20 controls found that patients with acne who consumed a high glycemic–load diet was significantly higher than the number of controls consuming a similar diet (P=.008). Additionally, significantly higher levels of mean (SD) serum IGF-1 on quantitative sandwich enzyme-linked immunosorbent assay in acne patients vs controls (543.2 [174.7] ng/mL vs 316.9 [95.7] ng/mL; P<.001) was identified, and these levels correlated significantly with high glycemic–load diet consumption.21 In another study, Kartal et al22 found that basal and fasting insulin levels and homeostasis model assessment scores evaluating for insulin resistance were significantly higher in 36 women compared with 24 age/sex-matched controls (P<.05). This finding remained significant even after excluding women with hyperandrogenemia (P<.05).22
Highlighting the importance of IGF-1 in the pathogenesis of acne, patients with genetic disorders characterized by IGF-1 deficiency, such as Laron syndrome, do not develop acne despite having a functional androgen receptor. Treatment with IGF-1 in these patients induces acne, further supporting the role of IGF-1 in the pathogenesis of this condition.23
The mTORC1 Pathway
Comprised of mTOR in addition to other proteins, mTORC1 is a nutrient-sensitive regulator of cellular growth, proliferation, lipid synthesis, and protein translation.5 Increased activity of mTORC1 has been described in diabetes, neurodegenerative disease, and cancer,14,24 while decreased activity may promote longevity.25 Regulation of mTORC1 occurs through several mechanisms. Growth factors such as insulin and IGF-1 promote mTORC1 activation through the PI3K/Akt pathway. Several amino acids—specifically branched chain amino acids such as alanine, arginine, asparagine, glutamine, histidine, leucine, methionine, serine, threonine, and valine—also can activate mTORC1 independently.26 Excess glucose leads to decreased adenosine monophosphate–activated protein kinase and increased activity of mTORC1, which occurs separately from insulin or IGF-1.27 Starvation blocks mTORC1 via increased adenosine monophosphate–activated protein kinase and starvation-induced hypoxia.26,28 To activate mTORC1, both the IGF-1 or insulin signal and amino acid excess must be present.29 Although not studied in acne, altering the dietary protein content in obese mice has been shown to perturb the mTORC1 pathway, leading to pathologic changes in the mTORC1-autophagy signaling axis, increased amino acid release into the blood, and an acute elevation in mTORC1 signaling.30
Another major regulator of mTORC1 is Forkhead box protein O1 (FOXO1), which is a transcription factor that regulates mTORC1 through sestrin 3.31,32 Sestrin 3 is a stress-induced protein that helps regulate blood glucose and promote insulin sensitivity.33 When FOXO1 is translocated to the cell nucleus, it upregulates the expression of sestrin 3, resulting in mTORC1 inhibition.31,32 Insulin, IGF-1, and nutrient excess lead to FOXO1 translocation to the cell cytoplasm where it can no longer mitigate mTORC1 activity, while the fasted state leads to translocation to the nucleus.34 A single study evaluated the association between FOXO1, mTORC1, a high glycemic–load diet, and acne development. Immunohistochemical detection of mTORC1 assessed by digital image analysis revealed significantly greater expression in inflamed pilosebaceous units found in acne patients (P<.001). Immunohistochemical cytoplasmic expression of FOXO1 and mTOR (used as a proxy for mTORC1) was significantly higher in patients on a high glycemic–load diet (P=.021 and P=.009, respectively) as well as in patients with more severe forms of acne (P=.005 and P=.015, respectively) and elevated IGF-1 levels (P=.004 and P=.003, respectively).21
mTORC1 contributes to the proliferation of keratinocytes and excess sebum production, both independently and through androgen-mediated processes.35-40 Insulinlike growth factor 1 binding the IGF-1 receptor leads to proliferation of keratinocytes lining the sebaceous gland and hair follicle in vivo.35 In mice with epidermis-specific deletion of mTOR, keratinocyte proliferation was decreased and hair follicles were diminished both in number and development. Genetic loss of mTOR in the epidermis led to attenuated signaling pathways of mTORC1 and mTORC2.36
Androgen function is augmented by mTORC1, FOXO1, and IGF-1 through several mechanisms, which may partially explain the hormonal relationship to acne. Androgens increase IGF-1 within the hair follicle.37 In prostate cancer cells, IGF-1 then facilitates movement of FOXO1 to the cytoplasm, preventing it from blocking mTORC1. This effective inactivation of FOXO1 thus further augments the impact of androgens by both allowing unchecked mTORC1 pathway activity and increasing translocation of the androgen receptor (AR) to the nucleus where it exerts its effects.38 Interestingly, genetic polymorphisms of the AR have been shown to cause variable affinity of FOXO1 for the AR; specifically, shorter CAG (cytosine, adenine, guanine) repeat length may lead to decreased FOXO1 binding and is associated with an increased risk for acne.41-43 In addition to its effects on the hair follicle, IGF-1 stimulates production of testosterone and dehydroepiandrosterone as well as activates 5α-reductase, leading to higher dihydrotestosterone levels, which activate the AR with higher affinity than testosterone.44 In some tissues, androgens help regulate the mTORC1 pathway through positive feedback loops.45,46 At this time, we do not know if this occurs in the pathogenesis of acne.
Isotretinoin is the treatment of choice for refractory acne. It has been hypothesized that isotretinoin induces sebocyte apoptosis via the upregulation of FOXO transcription factors and p53.47 Elevated levels of nuclear FOXO1 have been found in the sebaceous glands of patients following initiation of treatment with isotretinoin and are hypothesized to play a major role in the drug’s effectiveness. Specifically, biopsies from 14 acne patients before and after 6 weeks of isotretinoin therapy were analyzed with immunohistochemical staining and found to have a significantly improved nuclear to cytoplasmic ratio of nonphosphorylated FOXO1 (P<.001).47
Practical Recommendations
Given the available evidence, it is important for dermatologists to address dietary recommendations in acne patients. Although large randomized controlled trials on diet and acne severity are challenging to conduct in this population, the existing literature suggests that patients should avoid high glycemic index simple sugars and processed grains, and patients should focus on eating more complex carbohydrates in the form of legumes, vegetables, fruits, and tubers.6-8 With regard to dairy, milk (especially skim) has been associated with increased risks for acne.11,13 Fermented dairy products may have less impact on acne severity and include cheese, yogurt (unsweetened to keep glycemic index low), and sour cream.12
- Zaenglein AL. Acne vulgaris. In: Bolognia JL, Schaffer JV, Cerroni L, eds. Dermatology. Elsevier; 2017:588-603.
- Ganceviciene R, Graziene V, Fimmel S, et al. Involvement of the corticotropin-releasing hormone system in the pathogenesis of acne vulgaris. Br J Dermatol. 2009;160:345-352.
- Kang S, Cho S, Chung JH, et al. Inflammation and extracellular matrix degradation mediated by activated transcription factors nuclear factor-kappaB and activator protein-1 in inflammatory acne lesions in vivo. Am J Pathol. 2005;166:1691-1699.
- Cong TX, Hao D, Wen X, et al. From pathogenesis of acne vulgaris to anti-acne agents. Arch Dermatol Res. 2019;311:337-349.
- Pópulo H, Lopes JM, Soares P. The mTOR signalling pathway in human cancer. Int J Mol Sci. 2012;13:1886-1918.
- Kwon HH, Yoon JY, Hong JS, et al. Clinical and histological effect of a low glycaemic load diet in treatment of acne vulgaris in Korean patients: a randomized, controlled trial. Acta Derm Venereol. 2012;92:241-246.
- Smith RN, Mann NJ, Braue A, et al. A low-glycemic-load diet improves symptoms in acne vulgaris patients: a randomized controlled trial. Am J Clin Nutr. 2007;86:107-115.
- Penso L, Touvier M, Deschasaux M, et al. Association between adult acne and dietary behaviors: findings from the NutriNet-Santé prospective cohort study. JAMA Dermatol. 2020;156:854-862.
- Cao H, Yang G, Wang Y, et al. Complementary therapies for acne vulgaris. Cochrane Database Syst Rev. 2015;1:CD009436.
- Adebamowo CA, Spiegelman D, Danby FW, et al. High school dietary dairy intake and teenage acne. J Am Acad Dermatol. 2005;52:207-214.
- Aghasi M, Golzarand M, Shab-Bidar S, et al. Dairy intake and acne development: a meta-analysis of observational studies. Clin Nutr. 2019;38:1067-1075.
- Melnik BC, Schmitz G. Pasteurized non-fermented cow’s milk but not fermented milk is a promoter of mTORC1-driven aging and increased mortality. Ageing Res Rev. 2021;67:101270.
- Juhl CR, Bergholdt HKM, Miller IM, et al. Dairy intake and acne vulgaris: a systematic review and meta-analysis of 78,529 children, adolescents, and young adults. Nutrients. 2018;10:1049. doi:10.3390/nu10081049
- Melnik BC. Linking diet to acne metabolomics, inflammation, and comedogenesis: an update. Clin Cosmet Investig Dermatol. 2015;8:371-388.
- Smart CEM, King BR, Lopez PE. Insulin dosing for fat and protein: is it time? Diabetes Care. 2020;43:13-15.
- Wan X, Wang S, Xu J, et al. Dietary protein-induced hepatic IGF-1 secretion mediated by PPARγ activation. PLoS One. 2017;12:E0173174.
- Bedinger DH, Adams SH. Metabolic, anabolic, and mitogenic insulin responses: a tissue-specific perspective for insulin receptor activators. Mol Cell Endocrinol. 2015;415:143-156.
- Gubbi S, Quipildor GF, Barzilai N, et al. 40 YEARS of IGF1: IGF1: the Jekyll and Hyde of the aging brain. J Mol Endocrinol. 2018;61:T171-T185.
- Kolb H, Kempf K, Röhling M, et al. Insulin: too much of a good thing is bad. BMC Med. 2020;18:224.
- Wood CL, Lane LC, Cheetham T. Puberty: normal physiology (brief overview). Best Pract Res Clin Endocrinol Metab. 2019;33:101265.
- Agamia NF, Abdallah DM, Sorour O, et al. Skin expression of mammalian target of rapamycin and forkhead box transcription factor O1, and serum insulin-like growth factor-1 in patients with acne vulgaris and their relationship with diet. Br J Dermatol. 2016;174:1299-1307.
- Kartal D, Yildiz H, Ertas R, et al. Association between isolated female acne and insulin resistance: a prospective study. G Ital Dermatol Venereol. 2016;151:353-357.
- Ben-Amitai D, Laron Z. Effect of insulin-like growth factor-1 deficiency or administration on the occurrence of acne. J Eur Acad Dermatol Venereol. 2011;25:950-954.
- Kim LC, Cook RS, Chen J. mTORC1 and mTORC2 in cancer and the tumor microenvironment. Oncogene. 2017;36:2191-2201.
- Weichhart T. mTOR as regulator of lifespan, aging, and cellular senescence: a mini-review. Gerontology. 2018;64:127-134.
- Melick CH, Jewell JL. Regulation of mTORC1 by upstream stimuli. Genes. 2020;11:989. doi:10.3390/genes11090989
- Li M, Zhang CS, Feng JW, et al. Aldolase is a sensor for both low and high glucose, linking to AMPK and mTORC1. Cell Res. 2021;31:478-481.
- Yan T, Zhang J, Tang D, et al. Hypoxia regulates mTORC1-mediated keratinocyte motility and migration via the AMPK pathway. PLoS One. 2017;12:E0169155.
- Dennis MD, Baum JI, Kimball SR, et al. Mechanisms involved in the coordinate regulation of mTORC1 by insulin and amino acids. J Biol Chem. 2011;286:8287-8296.
- Choi BSY, Daniel N, Houde VP, et al. Feeding diversified protein sources exacerbates hepatic insulin resistance via increased gut microbial branched-chain fatty acids and mTORC1 signaling in obese mice. Nat Commun. 2021;12:3377.
- Chen CC, Jeon SM, Bhaskar PT, et al. FoxOs inhibit mTORC1 and activate Akt by inducing the expression of Sestrin3 and Rictor. Dev Cell. 2010;18:592-604.
- Chen Y, Huang T, Yu Z, et al. The functions and roles of sestrins in regulating human diseases. Cell Mol Biol Lett. 2022;27:2.
- Tao R, Xiong X, Liangpunsakul S, et al. Sestrin 3 protein enhances hepatic insulin sensitivity by direct activation of the mTORC2-Akt signaling. Diabetes. 2015;64:1211-1223.
- Gross DN, Wan M, Birnbaum MJ. The role of FOXO in the regulation of metabolism. Curr Diab Rep. 2009;9:208-214.
- Gilhar A, Ish-Shalom S, Pillar T, et al. Effect of anti–insulin-like growth factor 1 on epidermal proliferation of human skin transplanted onto nude mice treated with growth hormone. Endocrinology. 1994;134:229-232.
- Ding X, Bloch W, Iden S, et al. mTORC1 and mTORC2 regulate skin morphogenesis and epidermal barrier formation. Nat Commun. 2016;7:13226.
- Inui S, Itami S. Androgen actions on the human hair follicle: perspectives. Exp Dermatol. 2013;22:168-171.
- Fan W, Yanase T, Morinaga H, et al. Insulin-like growth factor 1/insulin signaling activates androgen signaling through direct interactions of Foxo1 with androgen receptor. J Biol Chem. 2007;282:7329-7338.
- Alestas T, Ganceviciene R, Fimmel S, et al. Enzymes involved in the biosynthesis of leukotriene B4 and prostaglandin E2 are active in sebaceous glands. J Mol Med. 2006;84:75-87.
- Smith TM, Gilliland K, Clawson GA, et al. IGF-1 induces SREBP-1 expression and lipogenesis in SEB-1 sebocytes via activation of the phosphoinositide 3-kinase/Akt pathway. J Invest Dermatol. 2008;128:1286-1293.
- Furtado GV, Yang J, Wu D, et al. FOXO1 controls protein synthesis and transcript abundance of mutant polyglutamine proteins, preventing protein aggregation. Hum Mol Genet. 2021;30:996-1005.
- Melnik BC. Isotretinoin and FoxO1: a scientific hypothesis. Dermatoendocrinol. 2011;3:141-165.
- Heng AHS, Say YH, Sio YY, et al. Gene variants associated with acne vulgaris presentation and severity: a systematic review and meta-analysis. BMC Med Genomics. 2021;14:103.
- Li J, Al-Azzawi F. Mechanism of androgen receptor action. Maturitas. 2009;63:142-148.
- Zhao Y, Tindall DJ, Huang H. Modulation of androgen receptor by FOXA1 and FOXO1 factors in prostate cancer. Int J Biol Sci. 2014;10:614-619.
- Hamdi MM, Mutungi G. Dihydrotestosterone stimulates amino acid uptake and the expression of LAT2 in mouse skeletal muscle fibres through an ERK1/2-dependent mechanism. J Physiol. 2011;589(pt 14):3623-3640.
- Agamia NF, Hussein OM, Abdelmaksoud RE, et al. Effect of oral isotretinoin on the nucleocytoplasmic distribution of FoxO1 and FoxO3 proteins in sebaceous glands of patients with acne vulgaris. Exp Dermatol. 2018;27:1344-1351.
- Kolovou GD, Watts GF, Mikhailidis DP, et al. Postprandial hypertriglyceridaemia revisited in the era of non-fasting lipid profile testing: a 2019 expert panel statement, main text. Curr Vasc Pharmacol. 2019;17:498-514.
- Svoboda SA, Shields BE. Cutaneous manifestations of nutritional excess: pathophysiologic effects of hyperglycemia and hyperinsulinemia on the skin. Cutis. 2021;107:74-78.
- González-González JG, Mancillas-Adame LG, Fernández-Reyes M, et al. Androgenetic alopecia and insulin resistance in young men. Clin Endocrinol . 2009;71:494-499.
- Livadas S, Anagnostis P, Bosdou JK, et al. Polycystic ovary syndrome and type 2 diabetes mellitus: a state-of-the-art review. World J Diabetes. 2022;13:5-26.
- Zaenglein AL. Acne vulgaris. In: Bolognia JL, Schaffer JV, Cerroni L, eds. Dermatology. Elsevier; 2017:588-603.
- Ganceviciene R, Graziene V, Fimmel S, et al. Involvement of the corticotropin-releasing hormone system in the pathogenesis of acne vulgaris. Br J Dermatol. 2009;160:345-352.
- Kang S, Cho S, Chung JH, et al. Inflammation and extracellular matrix degradation mediated by activated transcription factors nuclear factor-kappaB and activator protein-1 in inflammatory acne lesions in vivo. Am J Pathol. 2005;166:1691-1699.
- Cong TX, Hao D, Wen X, et al. From pathogenesis of acne vulgaris to anti-acne agents. Arch Dermatol Res. 2019;311:337-349.
- Pópulo H, Lopes JM, Soares P. The mTOR signalling pathway in human cancer. Int J Mol Sci. 2012;13:1886-1918.
- Kwon HH, Yoon JY, Hong JS, et al. Clinical and histological effect of a low glycaemic load diet in treatment of acne vulgaris in Korean patients: a randomized, controlled trial. Acta Derm Venereol. 2012;92:241-246.
- Smith RN, Mann NJ, Braue A, et al. A low-glycemic-load diet improves symptoms in acne vulgaris patients: a randomized controlled trial. Am J Clin Nutr. 2007;86:107-115.
- Penso L, Touvier M, Deschasaux M, et al. Association between adult acne and dietary behaviors: findings from the NutriNet-Santé prospective cohort study. JAMA Dermatol. 2020;156:854-862.
- Cao H, Yang G, Wang Y, et al. Complementary therapies for acne vulgaris. Cochrane Database Syst Rev. 2015;1:CD009436.
- Adebamowo CA, Spiegelman D, Danby FW, et al. High school dietary dairy intake and teenage acne. J Am Acad Dermatol. 2005;52:207-214.
- Aghasi M, Golzarand M, Shab-Bidar S, et al. Dairy intake and acne development: a meta-analysis of observational studies. Clin Nutr. 2019;38:1067-1075.
- Melnik BC, Schmitz G. Pasteurized non-fermented cow’s milk but not fermented milk is a promoter of mTORC1-driven aging and increased mortality. Ageing Res Rev. 2021;67:101270.
- Juhl CR, Bergholdt HKM, Miller IM, et al. Dairy intake and acne vulgaris: a systematic review and meta-analysis of 78,529 children, adolescents, and young adults. Nutrients. 2018;10:1049. doi:10.3390/nu10081049
- Melnik BC. Linking diet to acne metabolomics, inflammation, and comedogenesis: an update. Clin Cosmet Investig Dermatol. 2015;8:371-388.
- Smart CEM, King BR, Lopez PE. Insulin dosing for fat and protein: is it time? Diabetes Care. 2020;43:13-15.
- Wan X, Wang S, Xu J, et al. Dietary protein-induced hepatic IGF-1 secretion mediated by PPARγ activation. PLoS One. 2017;12:E0173174.
- Bedinger DH, Adams SH. Metabolic, anabolic, and mitogenic insulin responses: a tissue-specific perspective for insulin receptor activators. Mol Cell Endocrinol. 2015;415:143-156.
- Gubbi S, Quipildor GF, Barzilai N, et al. 40 YEARS of IGF1: IGF1: the Jekyll and Hyde of the aging brain. J Mol Endocrinol. 2018;61:T171-T185.
- Kolb H, Kempf K, Röhling M, et al. Insulin: too much of a good thing is bad. BMC Med. 2020;18:224.
- Wood CL, Lane LC, Cheetham T. Puberty: normal physiology (brief overview). Best Pract Res Clin Endocrinol Metab. 2019;33:101265.
- Agamia NF, Abdallah DM, Sorour O, et al. Skin expression of mammalian target of rapamycin and forkhead box transcription factor O1, and serum insulin-like growth factor-1 in patients with acne vulgaris and their relationship with diet. Br J Dermatol. 2016;174:1299-1307.
- Kartal D, Yildiz H, Ertas R, et al. Association between isolated female acne and insulin resistance: a prospective study. G Ital Dermatol Venereol. 2016;151:353-357.
- Ben-Amitai D, Laron Z. Effect of insulin-like growth factor-1 deficiency or administration on the occurrence of acne. J Eur Acad Dermatol Venereol. 2011;25:950-954.
- Kim LC, Cook RS, Chen J. mTORC1 and mTORC2 in cancer and the tumor microenvironment. Oncogene. 2017;36:2191-2201.
- Weichhart T. mTOR as regulator of lifespan, aging, and cellular senescence: a mini-review. Gerontology. 2018;64:127-134.
- Melick CH, Jewell JL. Regulation of mTORC1 by upstream stimuli. Genes. 2020;11:989. doi:10.3390/genes11090989
- Li M, Zhang CS, Feng JW, et al. Aldolase is a sensor for both low and high glucose, linking to AMPK and mTORC1. Cell Res. 2021;31:478-481.
- Yan T, Zhang J, Tang D, et al. Hypoxia regulates mTORC1-mediated keratinocyte motility and migration via the AMPK pathway. PLoS One. 2017;12:E0169155.
- Dennis MD, Baum JI, Kimball SR, et al. Mechanisms involved in the coordinate regulation of mTORC1 by insulin and amino acids. J Biol Chem. 2011;286:8287-8296.
- Choi BSY, Daniel N, Houde VP, et al. Feeding diversified protein sources exacerbates hepatic insulin resistance via increased gut microbial branched-chain fatty acids and mTORC1 signaling in obese mice. Nat Commun. 2021;12:3377.
- Chen CC, Jeon SM, Bhaskar PT, et al. FoxOs inhibit mTORC1 and activate Akt by inducing the expression of Sestrin3 and Rictor. Dev Cell. 2010;18:592-604.
- Chen Y, Huang T, Yu Z, et al. The functions and roles of sestrins in regulating human diseases. Cell Mol Biol Lett. 2022;27:2.
- Tao R, Xiong X, Liangpunsakul S, et al. Sestrin 3 protein enhances hepatic insulin sensitivity by direct activation of the mTORC2-Akt signaling. Diabetes. 2015;64:1211-1223.
- Gross DN, Wan M, Birnbaum MJ. The role of FOXO in the regulation of metabolism. Curr Diab Rep. 2009;9:208-214.
- Gilhar A, Ish-Shalom S, Pillar T, et al. Effect of anti–insulin-like growth factor 1 on epidermal proliferation of human skin transplanted onto nude mice treated with growth hormone. Endocrinology. 1994;134:229-232.
- Ding X, Bloch W, Iden S, et al. mTORC1 and mTORC2 regulate skin morphogenesis and epidermal barrier formation. Nat Commun. 2016;7:13226.
- Inui S, Itami S. Androgen actions on the human hair follicle: perspectives. Exp Dermatol. 2013;22:168-171.
- Fan W, Yanase T, Morinaga H, et al. Insulin-like growth factor 1/insulin signaling activates androgen signaling through direct interactions of Foxo1 with androgen receptor. J Biol Chem. 2007;282:7329-7338.
- Alestas T, Ganceviciene R, Fimmel S, et al. Enzymes involved in the biosynthesis of leukotriene B4 and prostaglandin E2 are active in sebaceous glands. J Mol Med. 2006;84:75-87.
- Smith TM, Gilliland K, Clawson GA, et al. IGF-1 induces SREBP-1 expression and lipogenesis in SEB-1 sebocytes via activation of the phosphoinositide 3-kinase/Akt pathway. J Invest Dermatol. 2008;128:1286-1293.
- Furtado GV, Yang J, Wu D, et al. FOXO1 controls protein synthesis and transcript abundance of mutant polyglutamine proteins, preventing protein aggregation. Hum Mol Genet. 2021;30:996-1005.
- Melnik BC. Isotretinoin and FoxO1: a scientific hypothesis. Dermatoendocrinol. 2011;3:141-165.
- Heng AHS, Say YH, Sio YY, et al. Gene variants associated with acne vulgaris presentation and severity: a systematic review and meta-analysis. BMC Med Genomics. 2021;14:103.
- Li J, Al-Azzawi F. Mechanism of androgen receptor action. Maturitas. 2009;63:142-148.
- Zhao Y, Tindall DJ, Huang H. Modulation of androgen receptor by FOXA1 and FOXO1 factors in prostate cancer. Int J Biol Sci. 2014;10:614-619.
- Hamdi MM, Mutungi G. Dihydrotestosterone stimulates amino acid uptake and the expression of LAT2 in mouse skeletal muscle fibres through an ERK1/2-dependent mechanism. J Physiol. 2011;589(pt 14):3623-3640.
- Agamia NF, Hussein OM, Abdelmaksoud RE, et al. Effect of oral isotretinoin on the nucleocytoplasmic distribution of FoxO1 and FoxO3 proteins in sebaceous glands of patients with acne vulgaris. Exp Dermatol. 2018;27:1344-1351.
- Kolovou GD, Watts GF, Mikhailidis DP, et al. Postprandial hypertriglyceridaemia revisited in the era of non-fasting lipid profile testing: a 2019 expert panel statement, main text. Curr Vasc Pharmacol. 2019;17:498-514.
- Svoboda SA, Shields BE. Cutaneous manifestations of nutritional excess: pathophysiologic effects of hyperglycemia and hyperinsulinemia on the skin. Cutis. 2021;107:74-78.
- González-González JG, Mancillas-Adame LG, Fernández-Reyes M, et al. Androgenetic alopecia and insulin resistance in young men. Clin Endocrinol . 2009;71:494-499.
- Livadas S, Anagnostis P, Bosdou JK, et al. Polycystic ovary syndrome and type 2 diabetes mellitus: a state-of-the-art review. World J Diabetes. 2022;13:5-26.
Practice Points
- Patients are frequently interested in the role that diet plays in acne, and dermatologists should be aware of the current evidence to answer these questions effectively.
- One of the primary pathways in acne pathogenesis, mTORC1 (mammalian target of rapamycin complex 1), is partially regulated by nutrient availability, insulin, and insulinlike growth factor 1.
- Dietary recommendations for acne based on available evidence may include a low glycemic index diet and avoidance of certain dairy products.
- Insulin resistance may underlie the pathogenesis of acne in a subset of patients, and assessing insulin resistance in acne patients should be considered.
Adapting to Changes in Acne Management: Take One Step at a Time
After most dermatology residents graduate from their programs, they go out into practice and will often carry with them what they learned from their teachers, especially clinicians. Everyone else in their dermatology residency programs approaches disease management and the use of different therapies in the same way, right?
It does not take very long before these same dermatology residents realize that things are different in real-world clinical practice in many ways. Most clinicians develop a range of fairly predictable patterns in how they approach and treat common skin disorders such as acne, rosacea, psoriasis, atopic dermatitis/eczema, and seborrheic dermatitis. These patterns often include what testing is performed at baseline and at follow-up.
Recently, I have been giving thought to how clinicians—myself included—change their approaches to management of specific skin diseases over time, especially as new information and therapies emerge. Are we fast adopters, or are we slow adopters? How much evidence do we need to see before we consider adjusting our approach? Is the needle moving too fast or not fast enough?
I would like to use an example that relates to acne treatment, especially as this is one of the most common skin disorders encountered in outpatient dermatologic practice. Despite lack of US Food and Drug Administration (FDA) approval for use in acne, oral spironolactone commonly is used in females, especially adults, with acne vulgaris and has a long history as an acceptable approach in dermatology.1 Because spironolactone is a potassium-sparing diuretic, one question that commonly arises is: Do we monitor serum potassium levels at baseline and periodically during treatment with spironolactone? There has never been a definitive consensus on which approach to take. However, there has been evidence to suggest that such monitoring is not necessary in young healthy women due to a negligible risk for clinically relevant hyperkalemia.2,3
In fact, the suggestion that there is a very low risk for clinically significant hyperkalemia in healthy young women treated with spironolactone is accurate based on population-based studies. Nevertheless, the clinician is faced with confirming the patient is in fact healthy rather than assuming this is the case due to her “young” age. In addition, it is important to exclude potential drug-drug interactions that can increase the risk for hyperkalemia when coadministered with spironolactone and also to exclude an unknown underlying decrease in renal function.1 At the end of the day, I support the continued research that is being done to evaluate questions that can challenge the recycled dogma on how we manage patients, and I do not fault those who follow what they believe to be new cogent evidence. However, in the case of oral spironolactone use, I also could never fault a clinician for monitoring renal function and electrolytes including serum potassium levels in a female patient treated for acne, especially with a drug that has the known potential to cause hyperkalemia in certain clinical situations and is not FDA approved for the indication of acne (ie, the guidance that accompanies the level of investigation needed for such FDA approval is missing). The clinical judgment of the clinician who is responsible for the individual patient trumps the results from population-based studies completed thus far. Ultimately, it is the responsibility of that clinician to assure the safety of their patient in a manner that they are comfortable with.
It takes time to make changes in our approaches to patient management, and in the majority of cases, that is rightfully so. There are several potential limitations to how certain data are collected, and a reasonable verification of results over time is what tends to change behavior patterns. Ultimately, the common goal is to do what is in the best interest of our patients. No one can argue successfully against that.
- Kim GK, Del Rosso JQ. Oral spironolactone in post-teenage female patients with acne vulgaris: practical considerations for the clinician based on current data and clinical experience. J Clin Aesthet Dermatol. 2012;5:37-50.
- Plovanich M, Weng QY, Arash Mostaghimi A. Low usefulness of potassium monitoring among healthy young women taking spironolactone for acne. JAMA Dermatol. 2015;151:941-944.
- Barbieri JS, Margolis DJ, Mostaghimi A. Temporal trends and clinician variability in potassium monitoring of healthy young women treated for acne with spironolactone. JAMA Dermatol. 2021;157:296-300.
After most dermatology residents graduate from their programs, they go out into practice and will often carry with them what they learned from their teachers, especially clinicians. Everyone else in their dermatology residency programs approaches disease management and the use of different therapies in the same way, right?
It does not take very long before these same dermatology residents realize that things are different in real-world clinical practice in many ways. Most clinicians develop a range of fairly predictable patterns in how they approach and treat common skin disorders such as acne, rosacea, psoriasis, atopic dermatitis/eczema, and seborrheic dermatitis. These patterns often include what testing is performed at baseline and at follow-up.
Recently, I have been giving thought to how clinicians—myself included—change their approaches to management of specific skin diseases over time, especially as new information and therapies emerge. Are we fast adopters, or are we slow adopters? How much evidence do we need to see before we consider adjusting our approach? Is the needle moving too fast or not fast enough?
I would like to use an example that relates to acne treatment, especially as this is one of the most common skin disorders encountered in outpatient dermatologic practice. Despite lack of US Food and Drug Administration (FDA) approval for use in acne, oral spironolactone commonly is used in females, especially adults, with acne vulgaris and has a long history as an acceptable approach in dermatology.1 Because spironolactone is a potassium-sparing diuretic, one question that commonly arises is: Do we monitor serum potassium levels at baseline and periodically during treatment with spironolactone? There has never been a definitive consensus on which approach to take. However, there has been evidence to suggest that such monitoring is not necessary in young healthy women due to a negligible risk for clinically relevant hyperkalemia.2,3
In fact, the suggestion that there is a very low risk for clinically significant hyperkalemia in healthy young women treated with spironolactone is accurate based on population-based studies. Nevertheless, the clinician is faced with confirming the patient is in fact healthy rather than assuming this is the case due to her “young” age. In addition, it is important to exclude potential drug-drug interactions that can increase the risk for hyperkalemia when coadministered with spironolactone and also to exclude an unknown underlying decrease in renal function.1 At the end of the day, I support the continued research that is being done to evaluate questions that can challenge the recycled dogma on how we manage patients, and I do not fault those who follow what they believe to be new cogent evidence. However, in the case of oral spironolactone use, I also could never fault a clinician for monitoring renal function and electrolytes including serum potassium levels in a female patient treated for acne, especially with a drug that has the known potential to cause hyperkalemia in certain clinical situations and is not FDA approved for the indication of acne (ie, the guidance that accompanies the level of investigation needed for such FDA approval is missing). The clinical judgment of the clinician who is responsible for the individual patient trumps the results from population-based studies completed thus far. Ultimately, it is the responsibility of that clinician to assure the safety of their patient in a manner that they are comfortable with.
It takes time to make changes in our approaches to patient management, and in the majority of cases, that is rightfully so. There are several potential limitations to how certain data are collected, and a reasonable verification of results over time is what tends to change behavior patterns. Ultimately, the common goal is to do what is in the best interest of our patients. No one can argue successfully against that.
After most dermatology residents graduate from their programs, they go out into practice and will often carry with them what they learned from their teachers, especially clinicians. Everyone else in their dermatology residency programs approaches disease management and the use of different therapies in the same way, right?
It does not take very long before these same dermatology residents realize that things are different in real-world clinical practice in many ways. Most clinicians develop a range of fairly predictable patterns in how they approach and treat common skin disorders such as acne, rosacea, psoriasis, atopic dermatitis/eczema, and seborrheic dermatitis. These patterns often include what testing is performed at baseline and at follow-up.
Recently, I have been giving thought to how clinicians—myself included—change their approaches to management of specific skin diseases over time, especially as new information and therapies emerge. Are we fast adopters, or are we slow adopters? How much evidence do we need to see before we consider adjusting our approach? Is the needle moving too fast or not fast enough?
I would like to use an example that relates to acne treatment, especially as this is one of the most common skin disorders encountered in outpatient dermatologic practice. Despite lack of US Food and Drug Administration (FDA) approval for use in acne, oral spironolactone commonly is used in females, especially adults, with acne vulgaris and has a long history as an acceptable approach in dermatology.1 Because spironolactone is a potassium-sparing diuretic, one question that commonly arises is: Do we monitor serum potassium levels at baseline and periodically during treatment with spironolactone? There has never been a definitive consensus on which approach to take. However, there has been evidence to suggest that such monitoring is not necessary in young healthy women due to a negligible risk for clinically relevant hyperkalemia.2,3
In fact, the suggestion that there is a very low risk for clinically significant hyperkalemia in healthy young women treated with spironolactone is accurate based on population-based studies. Nevertheless, the clinician is faced with confirming the patient is in fact healthy rather than assuming this is the case due to her “young” age. In addition, it is important to exclude potential drug-drug interactions that can increase the risk for hyperkalemia when coadministered with spironolactone and also to exclude an unknown underlying decrease in renal function.1 At the end of the day, I support the continued research that is being done to evaluate questions that can challenge the recycled dogma on how we manage patients, and I do not fault those who follow what they believe to be new cogent evidence. However, in the case of oral spironolactone use, I also could never fault a clinician for monitoring renal function and electrolytes including serum potassium levels in a female patient treated for acne, especially with a drug that has the known potential to cause hyperkalemia in certain clinical situations and is not FDA approved for the indication of acne (ie, the guidance that accompanies the level of investigation needed for such FDA approval is missing). The clinical judgment of the clinician who is responsible for the individual patient trumps the results from population-based studies completed thus far. Ultimately, it is the responsibility of that clinician to assure the safety of their patient in a manner that they are comfortable with.
It takes time to make changes in our approaches to patient management, and in the majority of cases, that is rightfully so. There are several potential limitations to how certain data are collected, and a reasonable verification of results over time is what tends to change behavior patterns. Ultimately, the common goal is to do what is in the best interest of our patients. No one can argue successfully against that.
- Kim GK, Del Rosso JQ. Oral spironolactone in post-teenage female patients with acne vulgaris: practical considerations for the clinician based on current data and clinical experience. J Clin Aesthet Dermatol. 2012;5:37-50.
- Plovanich M, Weng QY, Arash Mostaghimi A. Low usefulness of potassium monitoring among healthy young women taking spironolactone for acne. JAMA Dermatol. 2015;151:941-944.
- Barbieri JS, Margolis DJ, Mostaghimi A. Temporal trends and clinician variability in potassium monitoring of healthy young women treated for acne with spironolactone. JAMA Dermatol. 2021;157:296-300.
- Kim GK, Del Rosso JQ. Oral spironolactone in post-teenage female patients with acne vulgaris: practical considerations for the clinician based on current data and clinical experience. J Clin Aesthet Dermatol. 2012;5:37-50.
- Plovanich M, Weng QY, Arash Mostaghimi A. Low usefulness of potassium monitoring among healthy young women taking spironolactone for acne. JAMA Dermatol. 2015;151:941-944.
- Barbieri JS, Margolis DJ, Mostaghimi A. Temporal trends and clinician variability in potassium monitoring of healthy young women treated for acne with spironolactone. JAMA Dermatol. 2021;157:296-300.
Administrative Burden of iPLEDGE Deters Isotretinoin Prescriptions: Results From a Survey of Dermatologists
Isotretinoin is the most effective treatment of recalcitrant acne, but because of its teratogenicity and potential association with psychiatric adverse effects, it has been heavily regulated by the US Food and Drug Administration (FDA) through the iPLEDGE program since 2006.1,2 To manage the risk of teratogenicity associated with isotretinoin, various pregnancy prevention programs have been developed, but none of these programs have demonstrated a zero fetal exposure rate. The FDA reported 122 isotretinoin-exposed pregnancies during the first year iPLEDGE was implemented, which was a slight increase from the 120 pregnancies reported the year after the implementation of the System to Manage Accutane-Related Teratogenicity program, iPLEDGE’s predecessor.3 The iPLEDGE program requires registration of all wholesalers distributing isotretinoin, all health care providers prescribing isotretinoin, all pharmacies dispensing isotretinoin, and all female and male patients prescribed isotretinoin to create a verifiable link that only enables patients who have met all criteria to pick up their prescriptions. For patients of reproductive potential, there are additional qualification criteria and monthly requirements; before receiving their prescription every month, patients of reproductive potential must undergo a urine or serum pregnancy test with negative results, and patients must be counseled by prescribers regarding the risks of the drug and verify they are using 2 methods of contraception (or practicing abstinence) each month before completing online questions that test their understanding of the drug’s side effects and their chosen methods of contraception.4 These requirements place burdens on both patients and prescribers. Studies have shown that in the 2 years after the implementation of iPLEDGE, there was a 29% decrease in isotretinoin prescriptions.1-3
We conducted a survey study to see if clinicians chose not to prescribe isotretinoin to appropriate candidates specifically because of the administrative burden of iPLEDGE. Secondarily, we investigated the medications these clinicians would prescribe instead of isotretinoin.
Methods
In March 2020, we administered an anonymous online survey consisting of 12 multiple-choice questions to verified board-certified dermatologists in the United States using a social media group. The University of Rochester’s (Rochester, New York) institutional review board determined that our protocol met criteria for exemption (IRB STUDY00004693).
Statistical Analysis—Primary analyses used Pearson χ2 tests to identify significant differences among respondent groups, practice settings, age of respondents, and time spent registering patients for iPLEDGE.
Results
Survey results from 510 respondents are summarized in the Table.
Burden of iPLEDGE—Of the respondents, 336 (65.9%) were frequent prescribers of isotretinoin, 166 (32.5%) were infrequent prescribers, and 8 (1.6%) were never prescribers. Significantly more isotretinoin prescribers estimated that their offices spend 16 to 30 minutes registering a new isotretinoin patient with the iPLEDGE program (289 [57.6%]) compared with 0 to 15 minutes (140 [27.9%]), 31 to 45 minutes (57 [11.3%]), and morethan 45 minutes (16 [3.2%])(χ23=22.09, P<.0001). Furthermore, 150 dermatologists reported sometimes not prescribing, and 2 reported never prescribing isotretinoin because of the burden of iPLEDGE.
Systemic Agents Prescribed Instead of Isotretinoin—Of the respondents, 73.0% (n=111) prescribed spironolactone to female patients and 88.8% (n=135) prescribed oral antibiotics to male patients instead of isotretinoin. Spironolactone typically is not prescribed to male patients with acne because of its feminizing side effects, such as gynecomastia.5 According to the American Academy of Dermatology guidelines on acne, systemic antibiotic usage should be limited to the shortest possible duration (ie, less than 3–4 months) because of potential bacterial resistance and reported associations with inflammatory bowel disease, Clostridium difficile infection, and candidiasis.6,7
Prescriber Demographics—The frequency of not prescribing isotretinoin did not vary by practice setting (χ 24=6.44, P=.1689) but did vary by age of the dermatologist (χ23=15.57, P=.0014). Dermatologists younger than 46 years were more likely (Figure) to report not prescribing isotretinoin because of the administrative burden of iPLEDGE. We speculate that this is because younger dermatologists are less established in their practices and therefore may have less support to complete registration without interruption of clinic workflow.
Comment
The results of our survey suggest that the administrative burden of iPLEDGE may be compelling prescribers to refrain from prescribing isotretinoin therapy to appropriate candidates when it would otherwise be the drug of choice.
Recent Changes to iPLEDGE—The FDA recently approved a modification to the iPLEDGE Risk Evaluation and Mitigation Strategy (REMS) program based on the advocacy efforts from the American Academy of Dermatology. Starting December 13, 2021, the 3 patient risk categories were consolidated into 2 gender-neutral categories: patients who can get pregnant and patients who cannot get pregnant.8 The iPLEDGE website was transitioned to a new system, and all iPLEDGE REMS users had to update their iPLEDGE accounts. After the implementation of the modified program, user access issues arose, leading to delayed treatment when patients, providers, and pharmacists were all locked out of the online system; users also experienced long hold times with the call center.8 This change highlights the ongoing critical need for a streamlined program that increases patient access to isotretinoin while maintaining safety.
Study Limitations—The main limitation of this study was the inability to calculate a true response rate to our survey. We distributed the survey via social media to maintain anonymity of the respondents. We could not track how many saw the link to compare with the number of respondents. Therefore, the only way we could calculate a response rate was with the total number of members in the group, which fluctuated around 4000 at the time we administered the survey. We calculated that we would need at least 351 responses to have a 5% margin of error at 95% confidence for our results to be generalizable and significant. We ultimately received 510 responses, which gave us a 4.05% margin of error at 95% confidence and an estimated 12.7% response rate. Since some members of the group are not active and did not see the survey link, our true response rate was likely higher. Therefore, we concluded that the survey was successful, and our significant responses were representative of US dermatologists.
Suggestions to Improve iPLEDGE Process—Our survey study should facilitate further discussions on the importance of simplifying iPLEDGE. One suggestion for improving iPLEDGE is to remove the initial registration month so care is not delayed. Currently, a patient who can get pregnant must be on 2 forms of contraception for 30 days after they register as a patient before they are eligible to fill their prescription.4 This process is unnecessarily long and arduous and could be eliminated as long as the patient has already been on an effective form of contraception and has a negative pregnancy test on the day of registration. The need to repeat contraception comprehension questions monthly is redundant and also could be removed. Another suggestion is to remove the category of patients who cannot become pregnant from the system entirely. Isotretinoin does not appear to be associated with adverse psychiatric effects as shown through the systematic review and meta-analysis of numerous studies.9 If anything, the treatment of acne with isotretinoin appears to mitigate depressive symptoms. The iPLEDGE program does not manage this largely debunked idea. Because the program’s sole goal is to manage the risk of isotretinoin’s teratogenicity, the category of those who cannot become pregnant should not be included.
Conclusion
This survey highlights the burdens of iPLEDGE for dermatologists and the need for a more streamlined risk management program. The burden was felt equally among all practice types but especially by younger dermatologists (<46 years). This time-consuming program is deterring some dermatologists from prescribing isotretinoin and ultimately limiting patient access to an effective medication.
Acknowledgment—The authors thank all of the responding clinicians who provided insight into the impact of iPLEDGE on their isotretinoin prescribing patterns.
- Prevost N, English JC. Isotretinoin: update on controversial issues. J Pediatr Adolesc Gynecol. 2013;26:290-293.
- Tkachenko E, Singer S, Sharma P, et al. US Food and Drug Administration reports of pregnancy and pregnancy-related adverse events associated with isotretinoin. JAMA Dermatol. 2019;155:1175-1179.
- Shin J, Cheetham TC, Wong L, et al. The impact of the IPLEDGE program on isotretinoin fetal exposure in an integrated health care system. J Am Acad Dermatol. 2011;65:1117-1125.
- iPLEDGE Program. About iPLEDGE. Accessed June 13, 2022. https://ipledgeprogram.com/#Main/AboutiPledge
- Marson JW, Baldwin HE. An overview of acne therapy, part 2: hormonal therapy and isotretinoin. Dermatol Clin. 2019;37:195-203.
- Margolis DJ, Fanelli M, Hoffstad O, et al. Potential association between the oral tetracycline class of antimicrobials used to treat acne and inflammatory bowel disease. Am J Gastroenterol. 2010;105:2610-2616.
- Zaenglein AL, Pathy AL, Schlosser BJ, et al. Guidelines of care for the management of acne vulgaris. J Am Acad Dermatol. 2016;74:945-973.e33.
- iPLEDGE Risk Evaluation and Mitigation Strategy (REMS). Updated January 14, 2022. Accessed June 13, 2022. https://www.fda.gov/drugs/postmarket-drug-safety-information-patients-and-providers/ipledge-risk-evaluation-and-mitigation-strategy-rems
- Huang YC, Cheng YC. Isotretinoin treatment for acne and risk of depression: a systematic review and meta-analysis. J Am Acad Dermatol. 2017;76:1068-1076.e9.
Isotretinoin is the most effective treatment of recalcitrant acne, but because of its teratogenicity and potential association with psychiatric adverse effects, it has been heavily regulated by the US Food and Drug Administration (FDA) through the iPLEDGE program since 2006.1,2 To manage the risk of teratogenicity associated with isotretinoin, various pregnancy prevention programs have been developed, but none of these programs have demonstrated a zero fetal exposure rate. The FDA reported 122 isotretinoin-exposed pregnancies during the first year iPLEDGE was implemented, which was a slight increase from the 120 pregnancies reported the year after the implementation of the System to Manage Accutane-Related Teratogenicity program, iPLEDGE’s predecessor.3 The iPLEDGE program requires registration of all wholesalers distributing isotretinoin, all health care providers prescribing isotretinoin, all pharmacies dispensing isotretinoin, and all female and male patients prescribed isotretinoin to create a verifiable link that only enables patients who have met all criteria to pick up their prescriptions. For patients of reproductive potential, there are additional qualification criteria and monthly requirements; before receiving their prescription every month, patients of reproductive potential must undergo a urine or serum pregnancy test with negative results, and patients must be counseled by prescribers regarding the risks of the drug and verify they are using 2 methods of contraception (or practicing abstinence) each month before completing online questions that test their understanding of the drug’s side effects and their chosen methods of contraception.4 These requirements place burdens on both patients and prescribers. Studies have shown that in the 2 years after the implementation of iPLEDGE, there was a 29% decrease in isotretinoin prescriptions.1-3
We conducted a survey study to see if clinicians chose not to prescribe isotretinoin to appropriate candidates specifically because of the administrative burden of iPLEDGE. Secondarily, we investigated the medications these clinicians would prescribe instead of isotretinoin.
Methods
In March 2020, we administered an anonymous online survey consisting of 12 multiple-choice questions to verified board-certified dermatologists in the United States using a social media group. The University of Rochester’s (Rochester, New York) institutional review board determined that our protocol met criteria for exemption (IRB STUDY00004693).
Statistical Analysis—Primary analyses used Pearson χ2 tests to identify significant differences among respondent groups, practice settings, age of respondents, and time spent registering patients for iPLEDGE.
Results
Survey results from 510 respondents are summarized in the Table.
Burden of iPLEDGE—Of the respondents, 336 (65.9%) were frequent prescribers of isotretinoin, 166 (32.5%) were infrequent prescribers, and 8 (1.6%) were never prescribers. Significantly more isotretinoin prescribers estimated that their offices spend 16 to 30 minutes registering a new isotretinoin patient with the iPLEDGE program (289 [57.6%]) compared with 0 to 15 minutes (140 [27.9%]), 31 to 45 minutes (57 [11.3%]), and morethan 45 minutes (16 [3.2%])(χ23=22.09, P<.0001). Furthermore, 150 dermatologists reported sometimes not prescribing, and 2 reported never prescribing isotretinoin because of the burden of iPLEDGE.
Systemic Agents Prescribed Instead of Isotretinoin—Of the respondents, 73.0% (n=111) prescribed spironolactone to female patients and 88.8% (n=135) prescribed oral antibiotics to male patients instead of isotretinoin. Spironolactone typically is not prescribed to male patients with acne because of its feminizing side effects, such as gynecomastia.5 According to the American Academy of Dermatology guidelines on acne, systemic antibiotic usage should be limited to the shortest possible duration (ie, less than 3–4 months) because of potential bacterial resistance and reported associations with inflammatory bowel disease, Clostridium difficile infection, and candidiasis.6,7
Prescriber Demographics—The frequency of not prescribing isotretinoin did not vary by practice setting (χ 24=6.44, P=.1689) but did vary by age of the dermatologist (χ23=15.57, P=.0014). Dermatologists younger than 46 years were more likely (Figure) to report not prescribing isotretinoin because of the administrative burden of iPLEDGE. We speculate that this is because younger dermatologists are less established in their practices and therefore may have less support to complete registration without interruption of clinic workflow.
Comment
The results of our survey suggest that the administrative burden of iPLEDGE may be compelling prescribers to refrain from prescribing isotretinoin therapy to appropriate candidates when it would otherwise be the drug of choice.
Recent Changes to iPLEDGE—The FDA recently approved a modification to the iPLEDGE Risk Evaluation and Mitigation Strategy (REMS) program based on the advocacy efforts from the American Academy of Dermatology. Starting December 13, 2021, the 3 patient risk categories were consolidated into 2 gender-neutral categories: patients who can get pregnant and patients who cannot get pregnant.8 The iPLEDGE website was transitioned to a new system, and all iPLEDGE REMS users had to update their iPLEDGE accounts. After the implementation of the modified program, user access issues arose, leading to delayed treatment when patients, providers, and pharmacists were all locked out of the online system; users also experienced long hold times with the call center.8 This change highlights the ongoing critical need for a streamlined program that increases patient access to isotretinoin while maintaining safety.
Study Limitations—The main limitation of this study was the inability to calculate a true response rate to our survey. We distributed the survey via social media to maintain anonymity of the respondents. We could not track how many saw the link to compare with the number of respondents. Therefore, the only way we could calculate a response rate was with the total number of members in the group, which fluctuated around 4000 at the time we administered the survey. We calculated that we would need at least 351 responses to have a 5% margin of error at 95% confidence for our results to be generalizable and significant. We ultimately received 510 responses, which gave us a 4.05% margin of error at 95% confidence and an estimated 12.7% response rate. Since some members of the group are not active and did not see the survey link, our true response rate was likely higher. Therefore, we concluded that the survey was successful, and our significant responses were representative of US dermatologists.
Suggestions to Improve iPLEDGE Process—Our survey study should facilitate further discussions on the importance of simplifying iPLEDGE. One suggestion for improving iPLEDGE is to remove the initial registration month so care is not delayed. Currently, a patient who can get pregnant must be on 2 forms of contraception for 30 days after they register as a patient before they are eligible to fill their prescription.4 This process is unnecessarily long and arduous and could be eliminated as long as the patient has already been on an effective form of contraception and has a negative pregnancy test on the day of registration. The need to repeat contraception comprehension questions monthly is redundant and also could be removed. Another suggestion is to remove the category of patients who cannot become pregnant from the system entirely. Isotretinoin does not appear to be associated with adverse psychiatric effects as shown through the systematic review and meta-analysis of numerous studies.9 If anything, the treatment of acne with isotretinoin appears to mitigate depressive symptoms. The iPLEDGE program does not manage this largely debunked idea. Because the program’s sole goal is to manage the risk of isotretinoin’s teratogenicity, the category of those who cannot become pregnant should not be included.
Conclusion
This survey highlights the burdens of iPLEDGE for dermatologists and the need for a more streamlined risk management program. The burden was felt equally among all practice types but especially by younger dermatologists (<46 years). This time-consuming program is deterring some dermatologists from prescribing isotretinoin and ultimately limiting patient access to an effective medication.
Acknowledgment—The authors thank all of the responding clinicians who provided insight into the impact of iPLEDGE on their isotretinoin prescribing patterns.
Isotretinoin is the most effective treatment of recalcitrant acne, but because of its teratogenicity and potential association with psychiatric adverse effects, it has been heavily regulated by the US Food and Drug Administration (FDA) through the iPLEDGE program since 2006.1,2 To manage the risk of teratogenicity associated with isotretinoin, various pregnancy prevention programs have been developed, but none of these programs have demonstrated a zero fetal exposure rate. The FDA reported 122 isotretinoin-exposed pregnancies during the first year iPLEDGE was implemented, which was a slight increase from the 120 pregnancies reported the year after the implementation of the System to Manage Accutane-Related Teratogenicity program, iPLEDGE’s predecessor.3 The iPLEDGE program requires registration of all wholesalers distributing isotretinoin, all health care providers prescribing isotretinoin, all pharmacies dispensing isotretinoin, and all female and male patients prescribed isotretinoin to create a verifiable link that only enables patients who have met all criteria to pick up their prescriptions. For patients of reproductive potential, there are additional qualification criteria and monthly requirements; before receiving their prescription every month, patients of reproductive potential must undergo a urine or serum pregnancy test with negative results, and patients must be counseled by prescribers regarding the risks of the drug and verify they are using 2 methods of contraception (or practicing abstinence) each month before completing online questions that test their understanding of the drug’s side effects and their chosen methods of contraception.4 These requirements place burdens on both patients and prescribers. Studies have shown that in the 2 years after the implementation of iPLEDGE, there was a 29% decrease in isotretinoin prescriptions.1-3
We conducted a survey study to see if clinicians chose not to prescribe isotretinoin to appropriate candidates specifically because of the administrative burden of iPLEDGE. Secondarily, we investigated the medications these clinicians would prescribe instead of isotretinoin.
Methods
In March 2020, we administered an anonymous online survey consisting of 12 multiple-choice questions to verified board-certified dermatologists in the United States using a social media group. The University of Rochester’s (Rochester, New York) institutional review board determined that our protocol met criteria for exemption (IRB STUDY00004693).
Statistical Analysis—Primary analyses used Pearson χ2 tests to identify significant differences among respondent groups, practice settings, age of respondents, and time spent registering patients for iPLEDGE.
Results
Survey results from 510 respondents are summarized in the Table.
Burden of iPLEDGE—Of the respondents, 336 (65.9%) were frequent prescribers of isotretinoin, 166 (32.5%) were infrequent prescribers, and 8 (1.6%) were never prescribers. Significantly more isotretinoin prescribers estimated that their offices spend 16 to 30 minutes registering a new isotretinoin patient with the iPLEDGE program (289 [57.6%]) compared with 0 to 15 minutes (140 [27.9%]), 31 to 45 minutes (57 [11.3%]), and morethan 45 minutes (16 [3.2%])(χ23=22.09, P<.0001). Furthermore, 150 dermatologists reported sometimes not prescribing, and 2 reported never prescribing isotretinoin because of the burden of iPLEDGE.
Systemic Agents Prescribed Instead of Isotretinoin—Of the respondents, 73.0% (n=111) prescribed spironolactone to female patients and 88.8% (n=135) prescribed oral antibiotics to male patients instead of isotretinoin. Spironolactone typically is not prescribed to male patients with acne because of its feminizing side effects, such as gynecomastia.5 According to the American Academy of Dermatology guidelines on acne, systemic antibiotic usage should be limited to the shortest possible duration (ie, less than 3–4 months) because of potential bacterial resistance and reported associations with inflammatory bowel disease, Clostridium difficile infection, and candidiasis.6,7
Prescriber Demographics—The frequency of not prescribing isotretinoin did not vary by practice setting (χ 24=6.44, P=.1689) but did vary by age of the dermatologist (χ23=15.57, P=.0014). Dermatologists younger than 46 years were more likely (Figure) to report not prescribing isotretinoin because of the administrative burden of iPLEDGE. We speculate that this is because younger dermatologists are less established in their practices and therefore may have less support to complete registration without interruption of clinic workflow.
Comment
The results of our survey suggest that the administrative burden of iPLEDGE may be compelling prescribers to refrain from prescribing isotretinoin therapy to appropriate candidates when it would otherwise be the drug of choice.
Recent Changes to iPLEDGE—The FDA recently approved a modification to the iPLEDGE Risk Evaluation and Mitigation Strategy (REMS) program based on the advocacy efforts from the American Academy of Dermatology. Starting December 13, 2021, the 3 patient risk categories were consolidated into 2 gender-neutral categories: patients who can get pregnant and patients who cannot get pregnant.8 The iPLEDGE website was transitioned to a new system, and all iPLEDGE REMS users had to update their iPLEDGE accounts. After the implementation of the modified program, user access issues arose, leading to delayed treatment when patients, providers, and pharmacists were all locked out of the online system; users also experienced long hold times with the call center.8 This change highlights the ongoing critical need for a streamlined program that increases patient access to isotretinoin while maintaining safety.
Study Limitations—The main limitation of this study was the inability to calculate a true response rate to our survey. We distributed the survey via social media to maintain anonymity of the respondents. We could not track how many saw the link to compare with the number of respondents. Therefore, the only way we could calculate a response rate was with the total number of members in the group, which fluctuated around 4000 at the time we administered the survey. We calculated that we would need at least 351 responses to have a 5% margin of error at 95% confidence for our results to be generalizable and significant. We ultimately received 510 responses, which gave us a 4.05% margin of error at 95% confidence and an estimated 12.7% response rate. Since some members of the group are not active and did not see the survey link, our true response rate was likely higher. Therefore, we concluded that the survey was successful, and our significant responses were representative of US dermatologists.
Suggestions to Improve iPLEDGE Process—Our survey study should facilitate further discussions on the importance of simplifying iPLEDGE. One suggestion for improving iPLEDGE is to remove the initial registration month so care is not delayed. Currently, a patient who can get pregnant must be on 2 forms of contraception for 30 days after they register as a patient before they are eligible to fill their prescription.4 This process is unnecessarily long and arduous and could be eliminated as long as the patient has already been on an effective form of contraception and has a negative pregnancy test on the day of registration. The need to repeat contraception comprehension questions monthly is redundant and also could be removed. Another suggestion is to remove the category of patients who cannot become pregnant from the system entirely. Isotretinoin does not appear to be associated with adverse psychiatric effects as shown through the systematic review and meta-analysis of numerous studies.9 If anything, the treatment of acne with isotretinoin appears to mitigate depressive symptoms. The iPLEDGE program does not manage this largely debunked idea. Because the program’s sole goal is to manage the risk of isotretinoin’s teratogenicity, the category of those who cannot become pregnant should not be included.
Conclusion
This survey highlights the burdens of iPLEDGE for dermatologists and the need for a more streamlined risk management program. The burden was felt equally among all practice types but especially by younger dermatologists (<46 years). This time-consuming program is deterring some dermatologists from prescribing isotretinoin and ultimately limiting patient access to an effective medication.
Acknowledgment—The authors thank all of the responding clinicians who provided insight into the impact of iPLEDGE on their isotretinoin prescribing patterns.
- Prevost N, English JC. Isotretinoin: update on controversial issues. J Pediatr Adolesc Gynecol. 2013;26:290-293.
- Tkachenko E, Singer S, Sharma P, et al. US Food and Drug Administration reports of pregnancy and pregnancy-related adverse events associated with isotretinoin. JAMA Dermatol. 2019;155:1175-1179.
- Shin J, Cheetham TC, Wong L, et al. The impact of the IPLEDGE program on isotretinoin fetal exposure in an integrated health care system. J Am Acad Dermatol. 2011;65:1117-1125.
- iPLEDGE Program. About iPLEDGE. Accessed June 13, 2022. https://ipledgeprogram.com/#Main/AboutiPledge
- Marson JW, Baldwin HE. An overview of acne therapy, part 2: hormonal therapy and isotretinoin. Dermatol Clin. 2019;37:195-203.
- Margolis DJ, Fanelli M, Hoffstad O, et al. Potential association between the oral tetracycline class of antimicrobials used to treat acne and inflammatory bowel disease. Am J Gastroenterol. 2010;105:2610-2616.
- Zaenglein AL, Pathy AL, Schlosser BJ, et al. Guidelines of care for the management of acne vulgaris. J Am Acad Dermatol. 2016;74:945-973.e33.
- iPLEDGE Risk Evaluation and Mitigation Strategy (REMS). Updated January 14, 2022. Accessed June 13, 2022. https://www.fda.gov/drugs/postmarket-drug-safety-information-patients-and-providers/ipledge-risk-evaluation-and-mitigation-strategy-rems
- Huang YC, Cheng YC. Isotretinoin treatment for acne and risk of depression: a systematic review and meta-analysis. J Am Acad Dermatol. 2017;76:1068-1076.e9.
- Prevost N, English JC. Isotretinoin: update on controversial issues. J Pediatr Adolesc Gynecol. 2013;26:290-293.
- Tkachenko E, Singer S, Sharma P, et al. US Food and Drug Administration reports of pregnancy and pregnancy-related adverse events associated with isotretinoin. JAMA Dermatol. 2019;155:1175-1179.
- Shin J, Cheetham TC, Wong L, et al. The impact of the IPLEDGE program on isotretinoin fetal exposure in an integrated health care system. J Am Acad Dermatol. 2011;65:1117-1125.
- iPLEDGE Program. About iPLEDGE. Accessed June 13, 2022. https://ipledgeprogram.com/#Main/AboutiPledge
- Marson JW, Baldwin HE. An overview of acne therapy, part 2: hormonal therapy and isotretinoin. Dermatol Clin. 2019;37:195-203.
- Margolis DJ, Fanelli M, Hoffstad O, et al. Potential association between the oral tetracycline class of antimicrobials used to treat acne and inflammatory bowel disease. Am J Gastroenterol. 2010;105:2610-2616.
- Zaenglein AL, Pathy AL, Schlosser BJ, et al. Guidelines of care for the management of acne vulgaris. J Am Acad Dermatol. 2016;74:945-973.e33.
- iPLEDGE Risk Evaluation and Mitigation Strategy (REMS). Updated January 14, 2022. Accessed June 13, 2022. https://www.fda.gov/drugs/postmarket-drug-safety-information-patients-and-providers/ipledge-risk-evaluation-and-mitigation-strategy-rems
- Huang YC, Cheng YC. Isotretinoin treatment for acne and risk of depression: a systematic review and meta-analysis. J Am Acad Dermatol. 2017;76:1068-1076.e9.
Practice Points
- Of clinicians who regularly prescribe isotretinoin, approximately 30% have at times chosen not to prescribe isotretinoin to patients with severe acne because of the burden of the iPLEDGE program.
- The US Food and Drug Administration should consider further streamlining the iPLEDGE program, as it is causing physician burden and therefore suboptimal treatment plans for patients.
Simple Intraoperative Technique to Improve Wound Edge Approximation for Residents
Practice Gap
Dermatology residents can struggle with surgical closure early in their training years. Although experienced dermatologic surgeons may intuitively be able to align edges for maximal cosmesis, doing so can prove challenging in the context of learning basic surgical techniques for early residents.
Furthermore, local anesthesia can distort cutaneous anatomy and surgical landmarks, requiring the surgeon to reexamine their closure technique. Patients may require position changes or may make involuntary movements, both of which require dynamic thinking and planning on the part of the dermatologic surgeon to achieve optimal outcomes.
The Technique
We propose the use of sutures to intraoperatively guide placement of the dermal needle. This technique can be used for various closure types; here, we demonstrate its use in a standard elliptical excision.
To begin, a standard length to width ellipse ratio of 3:1 is drawn with appropriate margins around a neoplasm.1 After excision and appropriate undermining of the ellipse, we typically use deep sutures to close the deep space. The first pass of the needle through tissue can be performed in a place of the surgeon’s preference but typically abides by the rule of halves or the zipper method (Figure 1A). To determine optimal placement of the second needle pass through tissue, we recommend applying gentle opposing traction forces to the wound apices to approximate the linear outcome of the wound edges. The surgeon can use a skin hook to guide placement of the needle to the contralateral wound edge in an unassisted method of this technique (Figure 1B). The surgeon’s assistant also can aid in applying cutaneous traction along the length of the excision if the surgeon wishes to free their hands (Figure 1C). Because the risk of needlestick injury at this step is small, it is prudent for the surgeon to advise the assistant to avoid needlestick injury by keeping their hands away from the needle path in the surgical site.
Although traction is being applied to the wound apices, the deep suture should extend across the wound with just enough pressure to leave a serosanguineous notched mark in the contralateral tissue edge (Figure 1D). After releasing traction on the wound edges, the surgeon can effortlessly visualize the target for needle placement and make a throw through the tissue accordingly.
This process can be continued until wound closure is complete (Figure 2). Top sutures or adhesive strips can be placed afterward for completing approximation of the wound edges superficially.
Practice Implications
By using this technique to align wound edges intraoperatively, the surgeon can have a functional guide for needle placement. The technique allows improvement of function and cosmesis of surgical wounds, while also accounting for topographical variations in the patient’s surgical site. Approximation of the wound edges is particularly important at the beginning of closure, as the wound edges align and approximate more with each subsequent stitch, with decreasing tension.2
In addition, when operating on a curvilinear or challenging topographical surface of the body, this technique can provide a clear template for guiding suture placement for approximating wound edges. Furthermore, local biodynamic anatomy might become distorted after excision of the tissue specimen due to release of centripetal tangential forces that were present in the pre-excised skin.1 Local change in biodynamic forces may be difficult to plan for preoperatively using other techniques.3
Although this technique can be utilized for all suture placements in closure, it is of greatest value when placing the first few sutures and when operating on nonplanar surfaces that might become distorted after excision. To ensure the best outcome, it is important to be certain that the area has been properly cleaned prior to surgery and a sterile technique is used.
- Paul SP. Biodynamic excisional skin tension lines for excisional surgery of the lower limb and the technique of using parallel relaxing incisions to further reduce wound tension. Plast Reconstr Surg Glob Open. 2017;5:E1614. doi:10.1097/GOX.0000000000001614
- Miller CJ, Antunes MB, Sobanko JF. Surgical technique for optimal outcomes: part II. repairing tissue: suturing. J Am Acad Dermatol. 2015;72:389-402. doi:10.1016/j.jaad.2014.08.006
- Parikh SA, Sloan B. Clinical pearl: a simple and effective technique for improving surgical closures for the early-learning resident. Cutis. 2017;100:338-339.
Practice Gap
Dermatology residents can struggle with surgical closure early in their training years. Although experienced dermatologic surgeons may intuitively be able to align edges for maximal cosmesis, doing so can prove challenging in the context of learning basic surgical techniques for early residents.
Furthermore, local anesthesia can distort cutaneous anatomy and surgical landmarks, requiring the surgeon to reexamine their closure technique. Patients may require position changes or may make involuntary movements, both of which require dynamic thinking and planning on the part of the dermatologic surgeon to achieve optimal outcomes.
The Technique
We propose the use of sutures to intraoperatively guide placement of the dermal needle. This technique can be used for various closure types; here, we demonstrate its use in a standard elliptical excision.
To begin, a standard length to width ellipse ratio of 3:1 is drawn with appropriate margins around a neoplasm.1 After excision and appropriate undermining of the ellipse, we typically use deep sutures to close the deep space. The first pass of the needle through tissue can be performed in a place of the surgeon’s preference but typically abides by the rule of halves or the zipper method (Figure 1A). To determine optimal placement of the second needle pass through tissue, we recommend applying gentle opposing traction forces to the wound apices to approximate the linear outcome of the wound edges. The surgeon can use a skin hook to guide placement of the needle to the contralateral wound edge in an unassisted method of this technique (Figure 1B). The surgeon’s assistant also can aid in applying cutaneous traction along the length of the excision if the surgeon wishes to free their hands (Figure 1C). Because the risk of needlestick injury at this step is small, it is prudent for the surgeon to advise the assistant to avoid needlestick injury by keeping their hands away from the needle path in the surgical site.
Although traction is being applied to the wound apices, the deep suture should extend across the wound with just enough pressure to leave a serosanguineous notched mark in the contralateral tissue edge (Figure 1D). After releasing traction on the wound edges, the surgeon can effortlessly visualize the target for needle placement and make a throw through the tissue accordingly.
This process can be continued until wound closure is complete (Figure 2). Top sutures or adhesive strips can be placed afterward for completing approximation of the wound edges superficially.
Practice Implications
By using this technique to align wound edges intraoperatively, the surgeon can have a functional guide for needle placement. The technique allows improvement of function and cosmesis of surgical wounds, while also accounting for topographical variations in the patient’s surgical site. Approximation of the wound edges is particularly important at the beginning of closure, as the wound edges align and approximate more with each subsequent stitch, with decreasing tension.2
In addition, when operating on a curvilinear or challenging topographical surface of the body, this technique can provide a clear template for guiding suture placement for approximating wound edges. Furthermore, local biodynamic anatomy might become distorted after excision of the tissue specimen due to release of centripetal tangential forces that were present in the pre-excised skin.1 Local change in biodynamic forces may be difficult to plan for preoperatively using other techniques.3
Although this technique can be utilized for all suture placements in closure, it is of greatest value when placing the first few sutures and when operating on nonplanar surfaces that might become distorted after excision. To ensure the best outcome, it is important to be certain that the area has been properly cleaned prior to surgery and a sterile technique is used.
Practice Gap
Dermatology residents can struggle with surgical closure early in their training years. Although experienced dermatologic surgeons may intuitively be able to align edges for maximal cosmesis, doing so can prove challenging in the context of learning basic surgical techniques for early residents.
Furthermore, local anesthesia can distort cutaneous anatomy and surgical landmarks, requiring the surgeon to reexamine their closure technique. Patients may require position changes or may make involuntary movements, both of which require dynamic thinking and planning on the part of the dermatologic surgeon to achieve optimal outcomes.
The Technique
We propose the use of sutures to intraoperatively guide placement of the dermal needle. This technique can be used for various closure types; here, we demonstrate its use in a standard elliptical excision.
To begin, a standard length to width ellipse ratio of 3:1 is drawn with appropriate margins around a neoplasm.1 After excision and appropriate undermining of the ellipse, we typically use deep sutures to close the deep space. The first pass of the needle through tissue can be performed in a place of the surgeon’s preference but typically abides by the rule of halves or the zipper method (Figure 1A). To determine optimal placement of the second needle pass through tissue, we recommend applying gentle opposing traction forces to the wound apices to approximate the linear outcome of the wound edges. The surgeon can use a skin hook to guide placement of the needle to the contralateral wound edge in an unassisted method of this technique (Figure 1B). The surgeon’s assistant also can aid in applying cutaneous traction along the length of the excision if the surgeon wishes to free their hands (Figure 1C). Because the risk of needlestick injury at this step is small, it is prudent for the surgeon to advise the assistant to avoid needlestick injury by keeping their hands away from the needle path in the surgical site.
Although traction is being applied to the wound apices, the deep suture should extend across the wound with just enough pressure to leave a serosanguineous notched mark in the contralateral tissue edge (Figure 1D). After releasing traction on the wound edges, the surgeon can effortlessly visualize the target for needle placement and make a throw through the tissue accordingly.
This process can be continued until wound closure is complete (Figure 2). Top sutures or adhesive strips can be placed afterward for completing approximation of the wound edges superficially.
Practice Implications
By using this technique to align wound edges intraoperatively, the surgeon can have a functional guide for needle placement. The technique allows improvement of function and cosmesis of surgical wounds, while also accounting for topographical variations in the patient’s surgical site. Approximation of the wound edges is particularly important at the beginning of closure, as the wound edges align and approximate more with each subsequent stitch, with decreasing tension.2
In addition, when operating on a curvilinear or challenging topographical surface of the body, this technique can provide a clear template for guiding suture placement for approximating wound edges. Furthermore, local biodynamic anatomy might become distorted after excision of the tissue specimen due to release of centripetal tangential forces that were present in the pre-excised skin.1 Local change in biodynamic forces may be difficult to plan for preoperatively using other techniques.3
Although this technique can be utilized for all suture placements in closure, it is of greatest value when placing the first few sutures and when operating on nonplanar surfaces that might become distorted after excision. To ensure the best outcome, it is important to be certain that the area has been properly cleaned prior to surgery and a sterile technique is used.
- Paul SP. Biodynamic excisional skin tension lines for excisional surgery of the lower limb and the technique of using parallel relaxing incisions to further reduce wound tension. Plast Reconstr Surg Glob Open. 2017;5:E1614. doi:10.1097/GOX.0000000000001614
- Miller CJ, Antunes MB, Sobanko JF. Surgical technique for optimal outcomes: part II. repairing tissue: suturing. J Am Acad Dermatol. 2015;72:389-402. doi:10.1016/j.jaad.2014.08.006
- Parikh SA, Sloan B. Clinical pearl: a simple and effective technique for improving surgical closures for the early-learning resident. Cutis. 2017;100:338-339.
- Paul SP. Biodynamic excisional skin tension lines for excisional surgery of the lower limb and the technique of using parallel relaxing incisions to further reduce wound tension. Plast Reconstr Surg Glob Open. 2017;5:E1614. doi:10.1097/GOX.0000000000001614
- Miller CJ, Antunes MB, Sobanko JF. Surgical technique for optimal outcomes: part II. repairing tissue: suturing. J Am Acad Dermatol. 2015;72:389-402. doi:10.1016/j.jaad.2014.08.006
- Parikh SA, Sloan B. Clinical pearl: a simple and effective technique for improving surgical closures for the early-learning resident. Cutis. 2017;100:338-339.
Itchy Vesicular Rash
The Diagnosis: Tinea Corporis Bullosa
At the time of presentation, a potassium hydroxide (KOH) preparation, fungal culture, and punch biopsy of the right ventral wrist was performed. The KOH preparation was positive for fungal hyphae characteristic of dermatophyte infections. Histologically, the biopsy showed intraepidermal and subepidermal blisters with neutrophil- and lymphocyte-rich contents (Figure 1). Fungal hyphae and spores were present within the stratum corneum and superficial epidermis (Figure 2), and fungal cultures grew Microsporum canis. The extent of the rash (upper and lower extremities, chest, and back), positive fungal culture, and KOH preparation all supported the diagnosis of tinea corporis bullosa, which was confirmed with biopsy. Oral prednisone use was discouraged and triamcinolone ointment was discontinued given that inappropriate treatment with steroids in the setting of fungal infection suppresses an inflammatory response and alters clinical appearance, obviating the persistent underlying infection.
Tinea corporis bullosa is a rare superficial dermatophyte fungal infection that often is acquired by close personto- person contact or contact with domestic animals. The infection begins as a circular pruritic plaque, generally with raised borders, which may be erythematous or hyperpigmented. By definition, tinea corporis occurs in sites other than the face, feet, hands, or groin area. Bullae formation is thought to be secondary to a delayed hypersensitivity reaction provoked by the presence of a dermatophyte antigen.1
Linear IgA bullous dermatosis is an immunemediated disease characterized by IgA deposition at the dermoepidermal junction. Linear IgA bullous dermatosis classically presents as widespread tense vesicles in an arciform or annular pattern. Mucosal involvement is common and typically presents with erosions, ulcerations, and scarring.2 Given the absence of mucosal involvement in our patient and a positive KOH preparation, linear IgA bullous dermatosis was an unlikely diagnosis.
Benign inoculation lymphoreticulosis, more commonly known as cat scratch disease (CSD), is a Bartonella henselae infection that results from a cat scratch or bite. Cat scratch disease can present as localized cutaneous and nodal involvement (lymphadenopathy) near the site of inoculation, or it may present as disseminated disease. Cutaneous lesions generally progress through vesicular, erythematous, and papular phases. Regional lymphadenopathy proximal to the inoculation site is the hallmark of CSD.3 Given the absence of lymphadenopathy in our patient as well as the sporadic distribution of lesions, CSD was an unlikely diagnosis.
Dermatitis herpetiformis (DH) is an autoimmune disorder with cutaneous manifestations of gluten sensitivity. Dermatitis herpetiformis presents as extremely pruritic papules and vesicles arranged in groups on areas such as the elbows, dorsal aspects of the forearms, knees, scalp, back, and buttocks. Most patients with DH have celiac disease or small bowel disease related to gluten sensitivity.4 Given our patient’s acute presentation in adulthood and lack of gluten sensitivity, DH was an unlikely diagnosis.
Bullous fixed drug reaction is a cutaneous eruption that typically presents in the setting of exposure to an offending drug/agent. Drug reactions can have various cutaneous presentations, with the most common being pigmented macules that progress into plaques.5 Given the isolated nature of our patient’s episode and apparent lack of association with medication, bullous fixed drug reaction was an unlikely diagnosis.
Tinea corporis bullosa is a rare clinical variant of tinea corporis that has only been reported in patients with a history of contact with different animals. There are many causative organisms related to tinea corporis; Trichophyton rubrum is the most common etiology of tinea corporis, while tinea corporis due to close contact with domesticated animals often is caused by M canis.6 The immunoinhibitory properties of the mannans in the fungal cell wall allow the organisms to adhere to the skin prior to invasion. Cutaneous invasion into dead cornified layers of the skin is credited to the proteases, subtilisinlike proteases (subtilases), and keratinases produced by the fungus.1 There are many different clinical presentations of tinea corporis due to the variability of causative organisms. An annular (ring-shaped) lesion with a central plaque and advancing border is the most typical presentation. Tinea corporis bullosa is characterized by the presence of bullae or vesicles in the borders of the scaly plaque. Rupture of the bullae subsequently leads to erosions and crusts over the plaque.
The diagnosis of tinea corporis bullosa often is clinical if the lesion is typical and can be confirmed using KOH preparation and fungal culture. Once the diagnosis is confirmed, topical antifungals are the standard treatment approach for localized superficial tinea corporis. Systemic antifungal treatment can be initiated if the lesion is extensive, recurrent, chronic, or unresponsive to topical treatment.1 Given our patient’s characteristic presentation, she was managed with an over-the-counter topical antifungal (terbinafine). The patient’s lesions dramatically improved, rendering oral therapy unnecessary. At 1-month follow-up, the rash had nearly resolved.
- Leung AK, Lam JM, Leong KF, et al. Tinea corporis: an updated review [published online July 20, 2020]. Drugs Context. doi:10.7573/dic.2020-5-6
- Guide SV, Marinkovich MP. Linear IgA bullous dermatosis. Clin Dermatol. 2001;19:719-727.
- Lamps LW, Scott MA. Cat-scratch disease: historic, clinical, and pathologic perspectives. Pathology Patterns Reviews. 2004;121(suppl):S71-S80.
- Caproni M, Antiga E, Melani L, et al. Guidelines for the diagnosis and treatment of dermatitis herpetiformis. J Eur Acad Dermatol Venereol. 2009;23:633-638.
- Patel S, John AM, Handler MZ, et al. Fixed drug eruptions: an update, emphasizing the potentially lethal generalized bullous fixed drug eruption. Am J Clin Dermatol. 2020;21:393-399.
- Ziemer M, Seyfarth F, Elsner P, et al. Atypical manifestations of tinea corporis. Mycoses. 2007;50:31-35.
The Diagnosis: Tinea Corporis Bullosa
At the time of presentation, a potassium hydroxide (KOH) preparation, fungal culture, and punch biopsy of the right ventral wrist was performed. The KOH preparation was positive for fungal hyphae characteristic of dermatophyte infections. Histologically, the biopsy showed intraepidermal and subepidermal blisters with neutrophil- and lymphocyte-rich contents (Figure 1). Fungal hyphae and spores were present within the stratum corneum and superficial epidermis (Figure 2), and fungal cultures grew Microsporum canis. The extent of the rash (upper and lower extremities, chest, and back), positive fungal culture, and KOH preparation all supported the diagnosis of tinea corporis bullosa, which was confirmed with biopsy. Oral prednisone use was discouraged and triamcinolone ointment was discontinued given that inappropriate treatment with steroids in the setting of fungal infection suppresses an inflammatory response and alters clinical appearance, obviating the persistent underlying infection.
Tinea corporis bullosa is a rare superficial dermatophyte fungal infection that often is acquired by close personto- person contact or contact with domestic animals. The infection begins as a circular pruritic plaque, generally with raised borders, which may be erythematous or hyperpigmented. By definition, tinea corporis occurs in sites other than the face, feet, hands, or groin area. Bullae formation is thought to be secondary to a delayed hypersensitivity reaction provoked by the presence of a dermatophyte antigen.1
Linear IgA bullous dermatosis is an immunemediated disease characterized by IgA deposition at the dermoepidermal junction. Linear IgA bullous dermatosis classically presents as widespread tense vesicles in an arciform or annular pattern. Mucosal involvement is common and typically presents with erosions, ulcerations, and scarring.2 Given the absence of mucosal involvement in our patient and a positive KOH preparation, linear IgA bullous dermatosis was an unlikely diagnosis.
Benign inoculation lymphoreticulosis, more commonly known as cat scratch disease (CSD), is a Bartonella henselae infection that results from a cat scratch or bite. Cat scratch disease can present as localized cutaneous and nodal involvement (lymphadenopathy) near the site of inoculation, or it may present as disseminated disease. Cutaneous lesions generally progress through vesicular, erythematous, and papular phases. Regional lymphadenopathy proximal to the inoculation site is the hallmark of CSD.3 Given the absence of lymphadenopathy in our patient as well as the sporadic distribution of lesions, CSD was an unlikely diagnosis.
Dermatitis herpetiformis (DH) is an autoimmune disorder with cutaneous manifestations of gluten sensitivity. Dermatitis herpetiformis presents as extremely pruritic papules and vesicles arranged in groups on areas such as the elbows, dorsal aspects of the forearms, knees, scalp, back, and buttocks. Most patients with DH have celiac disease or small bowel disease related to gluten sensitivity.4 Given our patient’s acute presentation in adulthood and lack of gluten sensitivity, DH was an unlikely diagnosis.
Bullous fixed drug reaction is a cutaneous eruption that typically presents in the setting of exposure to an offending drug/agent. Drug reactions can have various cutaneous presentations, with the most common being pigmented macules that progress into plaques.5 Given the isolated nature of our patient’s episode and apparent lack of association with medication, bullous fixed drug reaction was an unlikely diagnosis.
Tinea corporis bullosa is a rare clinical variant of tinea corporis that has only been reported in patients with a history of contact with different animals. There are many causative organisms related to tinea corporis; Trichophyton rubrum is the most common etiology of tinea corporis, while tinea corporis due to close contact with domesticated animals often is caused by M canis.6 The immunoinhibitory properties of the mannans in the fungal cell wall allow the organisms to adhere to the skin prior to invasion. Cutaneous invasion into dead cornified layers of the skin is credited to the proteases, subtilisinlike proteases (subtilases), and keratinases produced by the fungus.1 There are many different clinical presentations of tinea corporis due to the variability of causative organisms. An annular (ring-shaped) lesion with a central plaque and advancing border is the most typical presentation. Tinea corporis bullosa is characterized by the presence of bullae or vesicles in the borders of the scaly plaque. Rupture of the bullae subsequently leads to erosions and crusts over the plaque.
The diagnosis of tinea corporis bullosa often is clinical if the lesion is typical and can be confirmed using KOH preparation and fungal culture. Once the diagnosis is confirmed, topical antifungals are the standard treatment approach for localized superficial tinea corporis. Systemic antifungal treatment can be initiated if the lesion is extensive, recurrent, chronic, or unresponsive to topical treatment.1 Given our patient’s characteristic presentation, she was managed with an over-the-counter topical antifungal (terbinafine). The patient’s lesions dramatically improved, rendering oral therapy unnecessary. At 1-month follow-up, the rash had nearly resolved.
The Diagnosis: Tinea Corporis Bullosa
At the time of presentation, a potassium hydroxide (KOH) preparation, fungal culture, and punch biopsy of the right ventral wrist was performed. The KOH preparation was positive for fungal hyphae characteristic of dermatophyte infections. Histologically, the biopsy showed intraepidermal and subepidermal blisters with neutrophil- and lymphocyte-rich contents (Figure 1). Fungal hyphae and spores were present within the stratum corneum and superficial epidermis (Figure 2), and fungal cultures grew Microsporum canis. The extent of the rash (upper and lower extremities, chest, and back), positive fungal culture, and KOH preparation all supported the diagnosis of tinea corporis bullosa, which was confirmed with biopsy. Oral prednisone use was discouraged and triamcinolone ointment was discontinued given that inappropriate treatment with steroids in the setting of fungal infection suppresses an inflammatory response and alters clinical appearance, obviating the persistent underlying infection.
Tinea corporis bullosa is a rare superficial dermatophyte fungal infection that often is acquired by close personto- person contact or contact with domestic animals. The infection begins as a circular pruritic plaque, generally with raised borders, which may be erythematous or hyperpigmented. By definition, tinea corporis occurs in sites other than the face, feet, hands, or groin area. Bullae formation is thought to be secondary to a delayed hypersensitivity reaction provoked by the presence of a dermatophyte antigen.1
Linear IgA bullous dermatosis is an immunemediated disease characterized by IgA deposition at the dermoepidermal junction. Linear IgA bullous dermatosis classically presents as widespread tense vesicles in an arciform or annular pattern. Mucosal involvement is common and typically presents with erosions, ulcerations, and scarring.2 Given the absence of mucosal involvement in our patient and a positive KOH preparation, linear IgA bullous dermatosis was an unlikely diagnosis.
Benign inoculation lymphoreticulosis, more commonly known as cat scratch disease (CSD), is a Bartonella henselae infection that results from a cat scratch or bite. Cat scratch disease can present as localized cutaneous and nodal involvement (lymphadenopathy) near the site of inoculation, or it may present as disseminated disease. Cutaneous lesions generally progress through vesicular, erythematous, and papular phases. Regional lymphadenopathy proximal to the inoculation site is the hallmark of CSD.3 Given the absence of lymphadenopathy in our patient as well as the sporadic distribution of lesions, CSD was an unlikely diagnosis.
Dermatitis herpetiformis (DH) is an autoimmune disorder with cutaneous manifestations of gluten sensitivity. Dermatitis herpetiformis presents as extremely pruritic papules and vesicles arranged in groups on areas such as the elbows, dorsal aspects of the forearms, knees, scalp, back, and buttocks. Most patients with DH have celiac disease or small bowel disease related to gluten sensitivity.4 Given our patient’s acute presentation in adulthood and lack of gluten sensitivity, DH was an unlikely diagnosis.
Bullous fixed drug reaction is a cutaneous eruption that typically presents in the setting of exposure to an offending drug/agent. Drug reactions can have various cutaneous presentations, with the most common being pigmented macules that progress into plaques.5 Given the isolated nature of our patient’s episode and apparent lack of association with medication, bullous fixed drug reaction was an unlikely diagnosis.
Tinea corporis bullosa is a rare clinical variant of tinea corporis that has only been reported in patients with a history of contact with different animals. There are many causative organisms related to tinea corporis; Trichophyton rubrum is the most common etiology of tinea corporis, while tinea corporis due to close contact with domesticated animals often is caused by M canis.6 The immunoinhibitory properties of the mannans in the fungal cell wall allow the organisms to adhere to the skin prior to invasion. Cutaneous invasion into dead cornified layers of the skin is credited to the proteases, subtilisinlike proteases (subtilases), and keratinases produced by the fungus.1 There are many different clinical presentations of tinea corporis due to the variability of causative organisms. An annular (ring-shaped) lesion with a central plaque and advancing border is the most typical presentation. Tinea corporis bullosa is characterized by the presence of bullae or vesicles in the borders of the scaly plaque. Rupture of the bullae subsequently leads to erosions and crusts over the plaque.
The diagnosis of tinea corporis bullosa often is clinical if the lesion is typical and can be confirmed using KOH preparation and fungal culture. Once the diagnosis is confirmed, topical antifungals are the standard treatment approach for localized superficial tinea corporis. Systemic antifungal treatment can be initiated if the lesion is extensive, recurrent, chronic, or unresponsive to topical treatment.1 Given our patient’s characteristic presentation, she was managed with an over-the-counter topical antifungal (terbinafine). The patient’s lesions dramatically improved, rendering oral therapy unnecessary. At 1-month follow-up, the rash had nearly resolved.
- Leung AK, Lam JM, Leong KF, et al. Tinea corporis: an updated review [published online July 20, 2020]. Drugs Context. doi:10.7573/dic.2020-5-6
- Guide SV, Marinkovich MP. Linear IgA bullous dermatosis. Clin Dermatol. 2001;19:719-727.
- Lamps LW, Scott MA. Cat-scratch disease: historic, clinical, and pathologic perspectives. Pathology Patterns Reviews. 2004;121(suppl):S71-S80.
- Caproni M, Antiga E, Melani L, et al. Guidelines for the diagnosis and treatment of dermatitis herpetiformis. J Eur Acad Dermatol Venereol. 2009;23:633-638.
- Patel S, John AM, Handler MZ, et al. Fixed drug eruptions: an update, emphasizing the potentially lethal generalized bullous fixed drug eruption. Am J Clin Dermatol. 2020;21:393-399.
- Ziemer M, Seyfarth F, Elsner P, et al. Atypical manifestations of tinea corporis. Mycoses. 2007;50:31-35.
- Leung AK, Lam JM, Leong KF, et al. Tinea corporis: an updated review [published online July 20, 2020]. Drugs Context. doi:10.7573/dic.2020-5-6
- Guide SV, Marinkovich MP. Linear IgA bullous dermatosis. Clin Dermatol. 2001;19:719-727.
- Lamps LW, Scott MA. Cat-scratch disease: historic, clinical, and pathologic perspectives. Pathology Patterns Reviews. 2004;121(suppl):S71-S80.
- Caproni M, Antiga E, Melani L, et al. Guidelines for the diagnosis and treatment of dermatitis herpetiformis. J Eur Acad Dermatol Venereol. 2009;23:633-638.
- Patel S, John AM, Handler MZ, et al. Fixed drug eruptions: an update, emphasizing the potentially lethal generalized bullous fixed drug eruption. Am J Clin Dermatol. 2020;21:393-399.
- Ziemer M, Seyfarth F, Elsner P, et al. Atypical manifestations of tinea corporis. Mycoses. 2007;50:31-35.
A 38-year-old woman presented with a rash of 5 days’ duration that initially appeared on the wrists after playing with her kitten, with subsequent involvement of the chest, back, abdomen, and upper and lower extremities. Physical examination revealed multiple annular plaques with raised erythematous borders, rare peripheral vesicles, and superficial central scaling. Extreme pruritus accompanied the plaques, both of which developed after playing with her kitten. The patient noted that all lesions on the upper extremities evolved in areas subject to deep puncture while more superficially excoriated areas were unaffected. She denied any other prior skin conditions and had received a 5-day course of azithromycin without improvement prior to presentation; triamcinolone ointment 0.1% had provided only temporary relief. Primary care providers prescribed a short course of oral prednisone; however, she did not start it prior to presentation.
Erythematous Pedunculated Plaque on the Dorsal Aspect of the Foot
The Diagnosis: Molluscum Contagiosum
A tangential shave removal with electrocautery was performed. Histopathology demonstrated numerous eosinophilic intracytoplasmic inclusion bodies (Figure), confirming a diagnosis of molluscum contagiosum (MC).
Molluscum contagiosum is a common poxvirus infection that is transmitted through fomites, contact, or self-inoculation.1 This infection most frequently occurs in school-aged children younger than 8 years1-3; peak incidence is 6 years of age.2,3 The worldwide estimated prevalence in children is 5.1% to 11.5%.1,3 In children cohabitating with others infected by MC, approximately 40% of households experienced a spread of infection; the risk of transmission is not associated with greater number of lesions.4 In adults, infection most commonly occurs in the setting of immunodeficiency or as a sexually transmitted infection in immunocompetent patients.3 Molluscum contagiosum infection classically presents as 1- to 3-mm, flesh- or white-colored, dome-shaped, smooth papules with central umbilication.1 Lesions often occur in clusters or lines, indicating local spread. The trunk, extremities, and face are areas that frequently are involved.2,3
Atypical presentations of MC infection can occur, as demonstrated by our case. Involvement of hair follicles by the infection can result in follicular induction.1,5 Secondary infection can mimic abscess formation.1 Inflamed MC lesions demonstrating the “beginning of the end” sign often are mistaken for primary infection, which is thought to be an inflammatory immune response to the virus.6 Lesions located on the eye or eyelid can present as unilateral conjunctivitis, conjunctival or corneal nodules, eyelid abscesses, or chalazions.1 Giant MC is a nodular variant of this infection measuring larger than 1 cm in size that can present similar to epidermoid cysts, condyloma acuminatum, or verruca vulgaris.1,7 Other reported mimicked conditions include basal cell carcinoma, trichoepithelioma, appendageal tumors, keratoacanthoma, foreign body granulomas, nevus sebaceous, or ecthyma.1,3 Molluscum contagiosum also has been reported to present as large ulcerative growths.8 In immunocompromised patients, deep fungal infection is another mimicker.1 Lesions on the plantar surfaces of the feet often are misdiagnosed as plantar verruca and present with pain during ambulation.9
The diagnosis of MC is clinical, with additional diagnostic tools reserved for more challenging situations.1 In cases with atypical presentations, dermoscopy may aid diagnosis through visualization of orifices and vascular patterns including crown, radial, and punctiform vessels.10 Biopsy or fine-needle aspiration also can be utilized as a diagnostic tool. Histopathology often reveals pathognomonic intracytoplasmic inclusions or Henderson-Paterson bodies.8,10 The appearance of MC can mimic other conditions that should be included in the differential diagnosis. Pyogenic granuloma often presents as a benign red papule that may grow rapidly and become pedunculated, sometimes with bleeding and crusting, though histology reveals groups of proliferating capillaries.11 More than half of amelanotic melanomas present in the papulonodular form as vascular or ulcerated nodules, and others may appear as erythematous macules. Diagnosis of amelanotic melanoma is made through histologic examination, which reveals atypical melanocytes in nests or cords, in conjunction with immunohistochemical stains such as S-100.12 Spitz nevi often appear as round, dome-shaped papules that most commonly are red, pink, or fleshcolored. They appear histologically similar to melanoma with nests of atypical melanocytes and nuclear atypia.13
A variety of treatment modalities can be used for MC including cantharidin, curettage, and cryotherapy.14 Imiquimod no longer is recommended due to a lack of demonstrated superiority over placebo in recent studies as well as its adverse effects.3 Topical retinoids have been recommended; however, their use frequently is limited by local irritation.3,14 Cantharidin is the most frequently utilized treatment by pediatric dermatologists. Most health care providers report subjective satisfaction with its results and efficacy, though some side effects may occur including discomfort and temporary changes in pigmentation. Treatment for MC is not required, as the condition is self-limiting.14 Therapy often is reserved for those with extensive disease, complications from lesions, cosmetic or psychological concerns, or genital involvement given the potential for sexual transmission.3 Time to resolution without treatment varies and is more prolonged in immunocompromised patients. Mean time to resolution in immunocompetent hosts has been reported as 13.3 months, but most infections are noted to clear within 2 to 4 years.1,4 Although resolution without treatment occurs, transmission to others and negative impact on quality of life (QOL) can occur and support the need for treatment. Greater impact on QOL was observed in females, those with more lesions, and patients with a longer duration of symptoms. Moderate impact on QOL was reported in 28% of patients (n=301), and severe effects were reported in 11%.4
In conclusion, MC is a common, benign, treatable cutaneous viral infection that often presents as small, flesh-colored papules in children. Its appearance can mimic a variety of other conditions. In cases with abnormal presentations, definitive diagnosis with pathology can be important to differentiate MC from more dangerous etiologies that may require further treatment.
- Brown J, Janniger CK, Schwartz RA, et al. Childhood molluscum contagiosum. Int J Dermatol. 2006;45:93-99. doi:10.1111 /j.1365-4632.2006.02737.x
- Dohil MA, Lin P, Lee J, et al. The epidemiology of molluscum contagiosum in children. J Am Acad Dermatol. 2006;54:47-54. doi:10.1016/j.jaad.2005.08.035
- Robinson G, Townsend S, Jahnke MN. Molluscum contagiosum: review and update on clinical presentation, diagnosis, risk, prevention, and treatment. Curr Derm Rep. 2020;9:83-92.
- Olsen JR, Gallacher J, Finlay AY, et al. Time to resolution and effect on quality of life of molluscum contagiosum in children in the UK: a prospective community cohort study. Lancet Infect Dis. 2015;15:190-195. doi:10.1016/S1473-3099(14)71053-9
- Davey J, Biswas A. Follicular induction in a case of molluscum contagiosum: possible link with secondary anetoderma-like changes? Am J Dermatopathol. 2014;36:E19-E21. doi:10.1097/DAD.0b013e31828bc7c7
- Butala N, Siegfried E, Weissler A. Molluscum BOTE sign: a predictor of imminent resolution. Pediatrics. 2013;131:E1650-E1653. doi:10.1542/peds.2012-2933
- Uzuncakmak TK, Kuru BC, Zemheri EI, et al. Isolated giant molluscum contagiosum mimicking epidermoid cyst. Dermatol Pract Concept. 2016;6:71-73. doi:10.5826/dpc.0603a15
- Singh S, Swain M, Shukla S, et al. An unusual presentation of giant molluscum contagiosum diagnosed on cytology. Diagn Cytopathol. 2018;46:794-796. doi:10.1002/dc.23964
- Cohen PR, Tschen JA. Plantar molluscum contagiosum: a case report of molluscum contagiosum occurring on the sole of the foot and a review of the world literature. Cutis. 2012;90:35-41.
- Megalla M, Bronsnick T, Noor O, et al. Dermoscopic, confocal microscopic, and histologic characteristics of an atypical presentation of molluscum contagiosum. Ann Clin Pathol. 2014;2:1038.
- Patrice SJ, Wiss K, Mulliken JB. Pyogenic granuloma (lobular capillary hemangioma): a clinicopathologic study of 178 cases. Pediatr Dermatol. 1991;8:267-276. doi:10.1111/j.1525-1470.1991.tb00931.x
- Gong H-Z, Zheng H-Y, Li J. Amelanotic melanoma. Melanoma Res. 2019;29:221-230. doi:10.1097/CMR.0000000000000571
- Casso EM, Grin-Jorgensen CM, Grant-Kels JM. Spitz nevi. J Am Acad Dermatol. 1992;27(6 pt 1):901-913. doi:10.1016/0190-9622(92)70286-o
- Coloe J, Morrell DS. Cantharidin use among pediatric dermatologists in the treatment of molluscum contagiosum. Pediatr Dermatol. 2009;26:405-408.
The Diagnosis: Molluscum Contagiosum
A tangential shave removal with electrocautery was performed. Histopathology demonstrated numerous eosinophilic intracytoplasmic inclusion bodies (Figure), confirming a diagnosis of molluscum contagiosum (MC).
Molluscum contagiosum is a common poxvirus infection that is transmitted through fomites, contact, or self-inoculation.1 This infection most frequently occurs in school-aged children younger than 8 years1-3; peak incidence is 6 years of age.2,3 The worldwide estimated prevalence in children is 5.1% to 11.5%.1,3 In children cohabitating with others infected by MC, approximately 40% of households experienced a spread of infection; the risk of transmission is not associated with greater number of lesions.4 In adults, infection most commonly occurs in the setting of immunodeficiency or as a sexually transmitted infection in immunocompetent patients.3 Molluscum contagiosum infection classically presents as 1- to 3-mm, flesh- or white-colored, dome-shaped, smooth papules with central umbilication.1 Lesions often occur in clusters or lines, indicating local spread. The trunk, extremities, and face are areas that frequently are involved.2,3
Atypical presentations of MC infection can occur, as demonstrated by our case. Involvement of hair follicles by the infection can result in follicular induction.1,5 Secondary infection can mimic abscess formation.1 Inflamed MC lesions demonstrating the “beginning of the end” sign often are mistaken for primary infection, which is thought to be an inflammatory immune response to the virus.6 Lesions located on the eye or eyelid can present as unilateral conjunctivitis, conjunctival or corneal nodules, eyelid abscesses, or chalazions.1 Giant MC is a nodular variant of this infection measuring larger than 1 cm in size that can present similar to epidermoid cysts, condyloma acuminatum, or verruca vulgaris.1,7 Other reported mimicked conditions include basal cell carcinoma, trichoepithelioma, appendageal tumors, keratoacanthoma, foreign body granulomas, nevus sebaceous, or ecthyma.1,3 Molluscum contagiosum also has been reported to present as large ulcerative growths.8 In immunocompromised patients, deep fungal infection is another mimicker.1 Lesions on the plantar surfaces of the feet often are misdiagnosed as plantar verruca and present with pain during ambulation.9
The diagnosis of MC is clinical, with additional diagnostic tools reserved for more challenging situations.1 In cases with atypical presentations, dermoscopy may aid diagnosis through visualization of orifices and vascular patterns including crown, radial, and punctiform vessels.10 Biopsy or fine-needle aspiration also can be utilized as a diagnostic tool. Histopathology often reveals pathognomonic intracytoplasmic inclusions or Henderson-Paterson bodies.8,10 The appearance of MC can mimic other conditions that should be included in the differential diagnosis. Pyogenic granuloma often presents as a benign red papule that may grow rapidly and become pedunculated, sometimes with bleeding and crusting, though histology reveals groups of proliferating capillaries.11 More than half of amelanotic melanomas present in the papulonodular form as vascular or ulcerated nodules, and others may appear as erythematous macules. Diagnosis of amelanotic melanoma is made through histologic examination, which reveals atypical melanocytes in nests or cords, in conjunction with immunohistochemical stains such as S-100.12 Spitz nevi often appear as round, dome-shaped papules that most commonly are red, pink, or fleshcolored. They appear histologically similar to melanoma with nests of atypical melanocytes and nuclear atypia.13
A variety of treatment modalities can be used for MC including cantharidin, curettage, and cryotherapy.14 Imiquimod no longer is recommended due to a lack of demonstrated superiority over placebo in recent studies as well as its adverse effects.3 Topical retinoids have been recommended; however, their use frequently is limited by local irritation.3,14 Cantharidin is the most frequently utilized treatment by pediatric dermatologists. Most health care providers report subjective satisfaction with its results and efficacy, though some side effects may occur including discomfort and temporary changes in pigmentation. Treatment for MC is not required, as the condition is self-limiting.14 Therapy often is reserved for those with extensive disease, complications from lesions, cosmetic or psychological concerns, or genital involvement given the potential for sexual transmission.3 Time to resolution without treatment varies and is more prolonged in immunocompromised patients. Mean time to resolution in immunocompetent hosts has been reported as 13.3 months, but most infections are noted to clear within 2 to 4 years.1,4 Although resolution without treatment occurs, transmission to others and negative impact on quality of life (QOL) can occur and support the need for treatment. Greater impact on QOL was observed in females, those with more lesions, and patients with a longer duration of symptoms. Moderate impact on QOL was reported in 28% of patients (n=301), and severe effects were reported in 11%.4
In conclusion, MC is a common, benign, treatable cutaneous viral infection that often presents as small, flesh-colored papules in children. Its appearance can mimic a variety of other conditions. In cases with abnormal presentations, definitive diagnosis with pathology can be important to differentiate MC from more dangerous etiologies that may require further treatment.
The Diagnosis: Molluscum Contagiosum
A tangential shave removal with electrocautery was performed. Histopathology demonstrated numerous eosinophilic intracytoplasmic inclusion bodies (Figure), confirming a diagnosis of molluscum contagiosum (MC).
Molluscum contagiosum is a common poxvirus infection that is transmitted through fomites, contact, or self-inoculation.1 This infection most frequently occurs in school-aged children younger than 8 years1-3; peak incidence is 6 years of age.2,3 The worldwide estimated prevalence in children is 5.1% to 11.5%.1,3 In children cohabitating with others infected by MC, approximately 40% of households experienced a spread of infection; the risk of transmission is not associated with greater number of lesions.4 In adults, infection most commonly occurs in the setting of immunodeficiency or as a sexually transmitted infection in immunocompetent patients.3 Molluscum contagiosum infection classically presents as 1- to 3-mm, flesh- or white-colored, dome-shaped, smooth papules with central umbilication.1 Lesions often occur in clusters or lines, indicating local spread. The trunk, extremities, and face are areas that frequently are involved.2,3
Atypical presentations of MC infection can occur, as demonstrated by our case. Involvement of hair follicles by the infection can result in follicular induction.1,5 Secondary infection can mimic abscess formation.1 Inflamed MC lesions demonstrating the “beginning of the end” sign often are mistaken for primary infection, which is thought to be an inflammatory immune response to the virus.6 Lesions located on the eye or eyelid can present as unilateral conjunctivitis, conjunctival or corneal nodules, eyelid abscesses, or chalazions.1 Giant MC is a nodular variant of this infection measuring larger than 1 cm in size that can present similar to epidermoid cysts, condyloma acuminatum, or verruca vulgaris.1,7 Other reported mimicked conditions include basal cell carcinoma, trichoepithelioma, appendageal tumors, keratoacanthoma, foreign body granulomas, nevus sebaceous, or ecthyma.1,3 Molluscum contagiosum also has been reported to present as large ulcerative growths.8 In immunocompromised patients, deep fungal infection is another mimicker.1 Lesions on the plantar surfaces of the feet often are misdiagnosed as plantar verruca and present with pain during ambulation.9
The diagnosis of MC is clinical, with additional diagnostic tools reserved for more challenging situations.1 In cases with atypical presentations, dermoscopy may aid diagnosis through visualization of orifices and vascular patterns including crown, radial, and punctiform vessels.10 Biopsy or fine-needle aspiration also can be utilized as a diagnostic tool. Histopathology often reveals pathognomonic intracytoplasmic inclusions or Henderson-Paterson bodies.8,10 The appearance of MC can mimic other conditions that should be included in the differential diagnosis. Pyogenic granuloma often presents as a benign red papule that may grow rapidly and become pedunculated, sometimes with bleeding and crusting, though histology reveals groups of proliferating capillaries.11 More than half of amelanotic melanomas present in the papulonodular form as vascular or ulcerated nodules, and others may appear as erythematous macules. Diagnosis of amelanotic melanoma is made through histologic examination, which reveals atypical melanocytes in nests or cords, in conjunction with immunohistochemical stains such as S-100.12 Spitz nevi often appear as round, dome-shaped papules that most commonly are red, pink, or fleshcolored. They appear histologically similar to melanoma with nests of atypical melanocytes and nuclear atypia.13
A variety of treatment modalities can be used for MC including cantharidin, curettage, and cryotherapy.14 Imiquimod no longer is recommended due to a lack of demonstrated superiority over placebo in recent studies as well as its adverse effects.3 Topical retinoids have been recommended; however, their use frequently is limited by local irritation.3,14 Cantharidin is the most frequently utilized treatment by pediatric dermatologists. Most health care providers report subjective satisfaction with its results and efficacy, though some side effects may occur including discomfort and temporary changes in pigmentation. Treatment for MC is not required, as the condition is self-limiting.14 Therapy often is reserved for those with extensive disease, complications from lesions, cosmetic or psychological concerns, or genital involvement given the potential for sexual transmission.3 Time to resolution without treatment varies and is more prolonged in immunocompromised patients. Mean time to resolution in immunocompetent hosts has been reported as 13.3 months, but most infections are noted to clear within 2 to 4 years.1,4 Although resolution without treatment occurs, transmission to others and negative impact on quality of life (QOL) can occur and support the need for treatment. Greater impact on QOL was observed in females, those with more lesions, and patients with a longer duration of symptoms. Moderate impact on QOL was reported in 28% of patients (n=301), and severe effects were reported in 11%.4
In conclusion, MC is a common, benign, treatable cutaneous viral infection that often presents as small, flesh-colored papules in children. Its appearance can mimic a variety of other conditions. In cases with abnormal presentations, definitive diagnosis with pathology can be important to differentiate MC from more dangerous etiologies that may require further treatment.
- Brown J, Janniger CK, Schwartz RA, et al. Childhood molluscum contagiosum. Int J Dermatol. 2006;45:93-99. doi:10.1111 /j.1365-4632.2006.02737.x
- Dohil MA, Lin P, Lee J, et al. The epidemiology of molluscum contagiosum in children. J Am Acad Dermatol. 2006;54:47-54. doi:10.1016/j.jaad.2005.08.035
- Robinson G, Townsend S, Jahnke MN. Molluscum contagiosum: review and update on clinical presentation, diagnosis, risk, prevention, and treatment. Curr Derm Rep. 2020;9:83-92.
- Olsen JR, Gallacher J, Finlay AY, et al. Time to resolution and effect on quality of life of molluscum contagiosum in children in the UK: a prospective community cohort study. Lancet Infect Dis. 2015;15:190-195. doi:10.1016/S1473-3099(14)71053-9
- Davey J, Biswas A. Follicular induction in a case of molluscum contagiosum: possible link with secondary anetoderma-like changes? Am J Dermatopathol. 2014;36:E19-E21. doi:10.1097/DAD.0b013e31828bc7c7
- Butala N, Siegfried E, Weissler A. Molluscum BOTE sign: a predictor of imminent resolution. Pediatrics. 2013;131:E1650-E1653. doi:10.1542/peds.2012-2933
- Uzuncakmak TK, Kuru BC, Zemheri EI, et al. Isolated giant molluscum contagiosum mimicking epidermoid cyst. Dermatol Pract Concept. 2016;6:71-73. doi:10.5826/dpc.0603a15
- Singh S, Swain M, Shukla S, et al. An unusual presentation of giant molluscum contagiosum diagnosed on cytology. Diagn Cytopathol. 2018;46:794-796. doi:10.1002/dc.23964
- Cohen PR, Tschen JA. Plantar molluscum contagiosum: a case report of molluscum contagiosum occurring on the sole of the foot and a review of the world literature. Cutis. 2012;90:35-41.
- Megalla M, Bronsnick T, Noor O, et al. Dermoscopic, confocal microscopic, and histologic characteristics of an atypical presentation of molluscum contagiosum. Ann Clin Pathol. 2014;2:1038.
- Patrice SJ, Wiss K, Mulliken JB. Pyogenic granuloma (lobular capillary hemangioma): a clinicopathologic study of 178 cases. Pediatr Dermatol. 1991;8:267-276. doi:10.1111/j.1525-1470.1991.tb00931.x
- Gong H-Z, Zheng H-Y, Li J. Amelanotic melanoma. Melanoma Res. 2019;29:221-230. doi:10.1097/CMR.0000000000000571
- Casso EM, Grin-Jorgensen CM, Grant-Kels JM. Spitz nevi. J Am Acad Dermatol. 1992;27(6 pt 1):901-913. doi:10.1016/0190-9622(92)70286-o
- Coloe J, Morrell DS. Cantharidin use among pediatric dermatologists in the treatment of molluscum contagiosum. Pediatr Dermatol. 2009;26:405-408.
- Brown J, Janniger CK, Schwartz RA, et al. Childhood molluscum contagiosum. Int J Dermatol. 2006;45:93-99. doi:10.1111 /j.1365-4632.2006.02737.x
- Dohil MA, Lin P, Lee J, et al. The epidemiology of molluscum contagiosum in children. J Am Acad Dermatol. 2006;54:47-54. doi:10.1016/j.jaad.2005.08.035
- Robinson G, Townsend S, Jahnke MN. Molluscum contagiosum: review and update on clinical presentation, diagnosis, risk, prevention, and treatment. Curr Derm Rep. 2020;9:83-92.
- Olsen JR, Gallacher J, Finlay AY, et al. Time to resolution and effect on quality of life of molluscum contagiosum in children in the UK: a prospective community cohort study. Lancet Infect Dis. 2015;15:190-195. doi:10.1016/S1473-3099(14)71053-9
- Davey J, Biswas A. Follicular induction in a case of molluscum contagiosum: possible link with secondary anetoderma-like changes? Am J Dermatopathol. 2014;36:E19-E21. doi:10.1097/DAD.0b013e31828bc7c7
- Butala N, Siegfried E, Weissler A. Molluscum BOTE sign: a predictor of imminent resolution. Pediatrics. 2013;131:E1650-E1653. doi:10.1542/peds.2012-2933
- Uzuncakmak TK, Kuru BC, Zemheri EI, et al. Isolated giant molluscum contagiosum mimicking epidermoid cyst. Dermatol Pract Concept. 2016;6:71-73. doi:10.5826/dpc.0603a15
- Singh S, Swain M, Shukla S, et al. An unusual presentation of giant molluscum contagiosum diagnosed on cytology. Diagn Cytopathol. 2018;46:794-796. doi:10.1002/dc.23964
- Cohen PR, Tschen JA. Plantar molluscum contagiosum: a case report of molluscum contagiosum occurring on the sole of the foot and a review of the world literature. Cutis. 2012;90:35-41.
- Megalla M, Bronsnick T, Noor O, et al. Dermoscopic, confocal microscopic, and histologic characteristics of an atypical presentation of molluscum contagiosum. Ann Clin Pathol. 2014;2:1038.
- Patrice SJ, Wiss K, Mulliken JB. Pyogenic granuloma (lobular capillary hemangioma): a clinicopathologic study of 178 cases. Pediatr Dermatol. 1991;8:267-276. doi:10.1111/j.1525-1470.1991.tb00931.x
- Gong H-Z, Zheng H-Y, Li J. Amelanotic melanoma. Melanoma Res. 2019;29:221-230. doi:10.1097/CMR.0000000000000571
- Casso EM, Grin-Jorgensen CM, Grant-Kels JM. Spitz nevi. J Am Acad Dermatol. 1992;27(6 pt 1):901-913. doi:10.1016/0190-9622(92)70286-o
- Coloe J, Morrell DS. Cantharidin use among pediatric dermatologists in the treatment of molluscum contagiosum. Pediatr Dermatol. 2009;26:405-408.
A 13-year-old adolescent girl presented for evaluation of a lesion on the dorsal aspect of the right foot of 1 week’s duration. She had a history of acne vulgaris and seasonal allergic rhinitis. She previously had noticed a persistent, small, flesh-colored bump of unknown chronicity in the same location, which had been diagnosed as a skin tag at an outside clinic. She denied any prior treatment in this area. Approximately a week prior to presentation, the lesion became painful, larger, and darkened in color before draining yellowish fluid. Due to concern for superinfection, the patient was prescribed cephalexin by her pediatrician. Dermatologic examination revealed a 1-cm, violaceous, pedunculated plaque with hemorrhagic crust on the dorsal aspect of the right foot with surrounding erythema and tenderness.
Innovations in Dermatology Spring Abstract Compendium
Prioritizing Mental Health in Residency
The World Health Organization declared COVID-19 a pandemic on March 11, 2020, just 4 months before the start of a new residency cycle. Referred to as “COVID interns,” PGY-1 residents transitioning out of medical school in 2020 faced an unprecedented challenge of doctoring within a confused and ill-prepared health care system, while senior residents scrambled to adjust to their rapidly changing training programs. Each subsequent week brought more sobering news of increasing hospitalizations, intensive care unit admissions, and deaths; hospitals across the country resorted to the redeployment of residents across all specialties to buffer the growing need within their internal medicine and critical care units.1 And while the news and social media blurred into a collage of ventilator shortages, politicization of science, and “#healthcareheroes,” one study showed53.7% of medical interns (N=108) were struggling with mild to extremely severe depression, while 63.9% reported mild to severe anxiety.2
Many shortcomings of our health care system—ill preparedness, racial disparity, health illiteracy—were highlighted during the COVID-19 pandemic, and providers’ mental health was no exception.3 Classic psychosocial risk factors, such as high demands, lack of control, lack of institutional support, and absence of reward defined the workplace, leading Theorell4 to call it “a randomized trial for maximal worsening of the work environment.” Stress and burnout during residency are not novel concepts. A 2002 survey including 415 medical residency programs with a response from more than 4000 residents found depressive symptoms in 35% of respondents, paired with feelings of increased cynicism and decreased humanism despite major curricular reforms and duty hour limitations.5 Unfortunately, the statistics in the coming years hardly budged and, in the wake of the pandemic, culminated to more than 50% to 76% of physicians worldwide reporting burnout in 2020.6-8
As a COVID intern at Brigham and Women’s Hospital (Boston, Massachusetts), I also experienced the demanding workload and witnessed the struggle of my colleagues firsthand. Brigham and Women’s Hospital, similar to many of its peer institutions, implemented frequent mental health check-ins within its curriculum. Known as the Intern Humanistic Curriculum, these check-ins essentially were an echo chamber to unload the psychological burdens of our workdays, and we eagerly shared what made us angry, sad, hopeful, and hopeless. During one such session, I learned about moral injury, a term originating in the military defined as the psychological stress resulting from actions—or the lack of actions—that violates one’s moral or ethical code.9 With the onslaught of patient deaths for which most of us felt unprepared, we had all endured varying degrees of moral injury. Greenberg et al9 described 2 potential outcomes after moral injury: (1) the development of mental health disorders such as depression and posttraumatic stress disorder, or (2) posttraumatic growth, which is the bolstering of psychological resilience. Notably, the outcome is based on the way someone is supported before, during, and after the challenging incident.9
With the aim of psychological growth and developing resilience, residents should prioritize mental health throughout their training. To this end, several resources are readily available, many of which I actively use or frequently revisit, which are reviewed here.
Mindfulness Meditation App
Calm (https://www.calm.com/) is one of several popular mobile applications (apps) that delivers mindfulness mediation—the practice of attending to experiences, thoughts, and emotions without bias or judgment. With more than 100 million downloads, Calm includes meditation tutorials, breathing exercises, nature scenes and sounds, and audio programs taught by mindfulness experts for $69.99 a year or $14.99 a month. Systemic reviews have demonstrated reduced sleep disturbance, decreased ruminative thoughts and emotional reactivity, and increased awareness and acceptance in those practicing mindfulness meditation. Calm users have reported these benefits, with many able to forego the time- and cost-intensive cognitive behavioral therapy that requires highly trained therapists.10-12
Exercise to Relieve Stress
Both aerobic and anaerobic exercises are antidepressive and anxiolytic and also lower one’s overall sensitivity to stress. Whether it is governed by neurotransmitters such as the activation of the opioid systems or the release of endogenous endorphins or time spent focusing on a different task at hand, the benefits of exercise against mental stressors have been extensively studied and established.13 Consider obtaining a new gym membership at the start of residency or joining an intramural team. Both have the added benefit of expanding your social circle.
Socialize With Others
Social isolation and perceived loneliness are key stressors linked to neuroendocrine disturbances that underlie depression, anxiety, and even schizophrenia.14,15 Throughout residency there will be several social events and opportunities to gather with colleagues—inside or outside of the work environment—and residents should attend as time allows. Even virtual social interactions were found to reduce stress and help in the treatment of social anxiety disorder.14
Communicate About Stressors
Open up to your co-residents, friends, and family about any struggles that may be invisible on the outside. Even attendings can empathize with the struggles of residency, and the mentors in place are actively trained to prioritize resident wellness. If verbal communication is not your strength, try journaling. Writing helps to untangle and better define underlying stressors and is itself meditative.16,17 However, ensure that your journaling is focused on positive emotional responses and aims to determine the positive benefits within any stressful event; those solely expressing negative emotions were found to have higher levels of stress and anxiety afterward than they had before.17
Seek a Mental Health Specialist
As with all other human ailments, severe mental health disorders require specialists and proper medication. Unfortunately, substantial stigma accompanying mental health continues to permeate medicine, creating considerable barriers for residents in need of care.18 A 2016 survey of more than 2000 physicians found that those with mental illnesses did not seek treatment due to limited time, fear of being reported to a medical licensing board, concern over obtaining licensure, and shame or embarrassment at the diagnosis.19 Besides urging residents to seek care, more effort should be invested in addressing the stigma and ensuring confidentiality. In 2021, the internal medicine and medicine-pediatrics residency at the University of Colorado Anschutz Medical Campus (Aurora, Colorado) developed a confidential opt-out, rather than opt-in, mental health program, and appointments were made for all 80 interns in advance. In doing so, they found increased participation and self-reported wellness at a relatively low cost and simple implementation.20 For trainees without such access, online or mobile therapy platforms offering electronic mental health treatment or telepsychiatry also have been employed.21,22 The onus ultimately is still on the individual to seek the care they need. Although only an anecdotal piece of evidence, I have found the prevalence of physicians taking selective serotonin reuptake inhibitors such as escitalopram, sertraline, or fluoxetine to be strikingly common and quite beneficial.
Final Thoughts
Residency remains rife with financial, emotional, and physical stressors; even as the dust settles on the COVID-19 pandemic, the light shed on the importance of trainee mental health must remain illuminated. For the aforementioned resources to have an impact, residents need to be empowered to openly discuss mental health issues and to seek help if necessary. Finally, in 2018, the Journal of Graduate Medical Education published a 10-year prospective cohort study that found that emotional distress during residency persists in professional practice even 10 years after residency and is associated with future burnout.23 Trainees should consider prioritizing their mental health to not only improve their quality of life in the present but also as an investment for their future.
- Spiegelman J, Praiss A, Syeda S, et al. Preparation and redeployment of house staff during a pandemic. Semin Perinatol. 2020;44:151297.
- Debnath PR, Islam MS, Karmakar PK, et al. Mental health concerns, insomnia, and loneliness among intern doctors amidst the COVID-19 pandemic: evidence from a large tertiary care hospital in Bangladesh. Int J Ment Health Addict. 2021:1-21. doi:10.1007/s11469-021-00690-0
- O’Reilly-Shah VN, Gentry KR, Van Cleve W, et al. The COVID-19 pandemic highlights shortcomings in US health care informatics infrastructure: a call to action. Anesth Analg. 2020;131:340-344.
- Theorell T. COVID-19 and working conditions in health care. Psychother Psychosom. 2020;89:193-194.
- Collier VU, McCue JD, Markus A, et al. Stress in medical residency: status quo after a decade of reform? Ann Intern Med. 2002;136:384-390.
- AbuDujain NM, Almuhaideb QA, Alrumaihi NA, et al. The impact of the COVID-19 pandemic on medical interns’ education, training, and mental health: a cross-sectional study. Cureus. 2021;13:E19250.
- Amanullah S, Ramesh Shankar R. The impact of COVID-19 on physician burnout globally: a review. Healthcare (Basel). 2020;8:421.
- Lebares CC, Guvva EV, Ascher NL, et al. Burnout and stress among US surgery residents: psychological distress and resilience. J Am Coll Surg. 2018;226:80-90.
- Greenberg N, Docherty M, Gnanapragasam S, et al. Managing mental health challenges faced by healthcare workers during COVID-19 pandemic. BMJ. 2020;368:m1211.
- Gal E, Stefan S, Cristea IA. The efficacy of mindfulness meditation apps in enhancing users’ well-being and mental health related outcomes: a meta-analysis of randomized controlled trials. J Affect Disord. 2021;279:131-142.
- Huberty J, Green J, Glissmann C, et al. Efficacy of the mindfulness meditation mobile app “Calm” to reduce stress among college students: randomized controlled trial. JMIR Mhealth Uhealth. 2019;7:E14273.
- Huberty J, Puzia ME, Larkey L, et al. Can a meditation app help my sleep? a cross-sectional survey of Calm users. PLoS One. 2021;16:E0257518.
- Salmon P. Effects of physical exercise on anxiety, depression, and sensitivity to stress: a unifying theory. Clin Psychol Rev. 2001;21:33-61.
- Kampmann IL, Emmelkamp PM, Hartanto D, et al. Exposure to virtual social interactions in the treatment of social anxiety disorder: a randomized controlled trial. Behav Res Ther. 2016;77:147-156.
- Mumtaz F, Khan MI, Zubair M, et al. Neurobiology and consequences of social isolation stress in animal model-A comprehensive review. Biomed Pharmacother. 2018;105:1205-1222.
- Khanna P, Singh K. Stress management training and gratitude journaling in the classroom: an initial investigation in Indian context. Curr Psychol. 2021;40:5737-5748.
- Ullrich PM, Lutgendorf SK. Journaling about stressful events: effects of cognitive processing and emotional expression. Ann Behav Med. 2002;24:244-250.
- Outhoff K. Depression in doctors: a bitter pill to swallow. S Afr Fam Pract. 2019;61(suppl 1):S11-S14.
- Gold KJ, Andrew LB, Goldman EB, et al. “I would never want to have a mental health diagnosis on my record”: a survey of female physicians on mental health diagnosis, treatment, and reporting. Gen Hosp Psychiatry. 2016;43:51-57.
- Major A, Williams JG, McGuire WC, et al. Removing barriers: a confidential opt-out mental health pilot program for internal medicine interns. Acad Med. 2021;96:686-689.
- Greenhalgh T, Wherton J. Telepsychiatry: learning from the pandemic. Br J Psychiatry. 2022;220:1-5.
- Timakum T, Xie Q, Song M. Analysis of E-mental health research: mapping the relationship between information technology and mental healthcare. BMC Psychiatry. 2022;22:57.
- Raimo J, LaVine S, Spielmann K, et al. The correlation of stress in residency with future stress and burnout: a 10-year prospective cohort study. J Grad Med Educ. 2018;10:524-531.
The World Health Organization declared COVID-19 a pandemic on March 11, 2020, just 4 months before the start of a new residency cycle. Referred to as “COVID interns,” PGY-1 residents transitioning out of medical school in 2020 faced an unprecedented challenge of doctoring within a confused and ill-prepared health care system, while senior residents scrambled to adjust to their rapidly changing training programs. Each subsequent week brought more sobering news of increasing hospitalizations, intensive care unit admissions, and deaths; hospitals across the country resorted to the redeployment of residents across all specialties to buffer the growing need within their internal medicine and critical care units.1 And while the news and social media blurred into a collage of ventilator shortages, politicization of science, and “#healthcareheroes,” one study showed53.7% of medical interns (N=108) were struggling with mild to extremely severe depression, while 63.9% reported mild to severe anxiety.2
Many shortcomings of our health care system—ill preparedness, racial disparity, health illiteracy—were highlighted during the COVID-19 pandemic, and providers’ mental health was no exception.3 Classic psychosocial risk factors, such as high demands, lack of control, lack of institutional support, and absence of reward defined the workplace, leading Theorell4 to call it “a randomized trial for maximal worsening of the work environment.” Stress and burnout during residency are not novel concepts. A 2002 survey including 415 medical residency programs with a response from more than 4000 residents found depressive symptoms in 35% of respondents, paired with feelings of increased cynicism and decreased humanism despite major curricular reforms and duty hour limitations.5 Unfortunately, the statistics in the coming years hardly budged and, in the wake of the pandemic, culminated to more than 50% to 76% of physicians worldwide reporting burnout in 2020.6-8
As a COVID intern at Brigham and Women’s Hospital (Boston, Massachusetts), I also experienced the demanding workload and witnessed the struggle of my colleagues firsthand. Brigham and Women’s Hospital, similar to many of its peer institutions, implemented frequent mental health check-ins within its curriculum. Known as the Intern Humanistic Curriculum, these check-ins essentially were an echo chamber to unload the psychological burdens of our workdays, and we eagerly shared what made us angry, sad, hopeful, and hopeless. During one such session, I learned about moral injury, a term originating in the military defined as the psychological stress resulting from actions—or the lack of actions—that violates one’s moral or ethical code.9 With the onslaught of patient deaths for which most of us felt unprepared, we had all endured varying degrees of moral injury. Greenberg et al9 described 2 potential outcomes after moral injury: (1) the development of mental health disorders such as depression and posttraumatic stress disorder, or (2) posttraumatic growth, which is the bolstering of psychological resilience. Notably, the outcome is based on the way someone is supported before, during, and after the challenging incident.9
With the aim of psychological growth and developing resilience, residents should prioritize mental health throughout their training. To this end, several resources are readily available, many of which I actively use or frequently revisit, which are reviewed here.
Mindfulness Meditation App
Calm (https://www.calm.com/) is one of several popular mobile applications (apps) that delivers mindfulness mediation—the practice of attending to experiences, thoughts, and emotions without bias or judgment. With more than 100 million downloads, Calm includes meditation tutorials, breathing exercises, nature scenes and sounds, and audio programs taught by mindfulness experts for $69.99 a year or $14.99 a month. Systemic reviews have demonstrated reduced sleep disturbance, decreased ruminative thoughts and emotional reactivity, and increased awareness and acceptance in those practicing mindfulness meditation. Calm users have reported these benefits, with many able to forego the time- and cost-intensive cognitive behavioral therapy that requires highly trained therapists.10-12
Exercise to Relieve Stress
Both aerobic and anaerobic exercises are antidepressive and anxiolytic and also lower one’s overall sensitivity to stress. Whether it is governed by neurotransmitters such as the activation of the opioid systems or the release of endogenous endorphins or time spent focusing on a different task at hand, the benefits of exercise against mental stressors have been extensively studied and established.13 Consider obtaining a new gym membership at the start of residency or joining an intramural team. Both have the added benefit of expanding your social circle.
Socialize With Others
Social isolation and perceived loneliness are key stressors linked to neuroendocrine disturbances that underlie depression, anxiety, and even schizophrenia.14,15 Throughout residency there will be several social events and opportunities to gather with colleagues—inside or outside of the work environment—and residents should attend as time allows. Even virtual social interactions were found to reduce stress and help in the treatment of social anxiety disorder.14
Communicate About Stressors
Open up to your co-residents, friends, and family about any struggles that may be invisible on the outside. Even attendings can empathize with the struggles of residency, and the mentors in place are actively trained to prioritize resident wellness. If verbal communication is not your strength, try journaling. Writing helps to untangle and better define underlying stressors and is itself meditative.16,17 However, ensure that your journaling is focused on positive emotional responses and aims to determine the positive benefits within any stressful event; those solely expressing negative emotions were found to have higher levels of stress and anxiety afterward than they had before.17
Seek a Mental Health Specialist
As with all other human ailments, severe mental health disorders require specialists and proper medication. Unfortunately, substantial stigma accompanying mental health continues to permeate medicine, creating considerable barriers for residents in need of care.18 A 2016 survey of more than 2000 physicians found that those with mental illnesses did not seek treatment due to limited time, fear of being reported to a medical licensing board, concern over obtaining licensure, and shame or embarrassment at the diagnosis.19 Besides urging residents to seek care, more effort should be invested in addressing the stigma and ensuring confidentiality. In 2021, the internal medicine and medicine-pediatrics residency at the University of Colorado Anschutz Medical Campus (Aurora, Colorado) developed a confidential opt-out, rather than opt-in, mental health program, and appointments were made for all 80 interns in advance. In doing so, they found increased participation and self-reported wellness at a relatively low cost and simple implementation.20 For trainees without such access, online or mobile therapy platforms offering electronic mental health treatment or telepsychiatry also have been employed.21,22 The onus ultimately is still on the individual to seek the care they need. Although only an anecdotal piece of evidence, I have found the prevalence of physicians taking selective serotonin reuptake inhibitors such as escitalopram, sertraline, or fluoxetine to be strikingly common and quite beneficial.
Final Thoughts
Residency remains rife with financial, emotional, and physical stressors; even as the dust settles on the COVID-19 pandemic, the light shed on the importance of trainee mental health must remain illuminated. For the aforementioned resources to have an impact, residents need to be empowered to openly discuss mental health issues and to seek help if necessary. Finally, in 2018, the Journal of Graduate Medical Education published a 10-year prospective cohort study that found that emotional distress during residency persists in professional practice even 10 years after residency and is associated with future burnout.23 Trainees should consider prioritizing their mental health to not only improve their quality of life in the present but also as an investment for their future.
The World Health Organization declared COVID-19 a pandemic on March 11, 2020, just 4 months before the start of a new residency cycle. Referred to as “COVID interns,” PGY-1 residents transitioning out of medical school in 2020 faced an unprecedented challenge of doctoring within a confused and ill-prepared health care system, while senior residents scrambled to adjust to their rapidly changing training programs. Each subsequent week brought more sobering news of increasing hospitalizations, intensive care unit admissions, and deaths; hospitals across the country resorted to the redeployment of residents across all specialties to buffer the growing need within their internal medicine and critical care units.1 And while the news and social media blurred into a collage of ventilator shortages, politicization of science, and “#healthcareheroes,” one study showed53.7% of medical interns (N=108) were struggling with mild to extremely severe depression, while 63.9% reported mild to severe anxiety.2
Many shortcomings of our health care system—ill preparedness, racial disparity, health illiteracy—were highlighted during the COVID-19 pandemic, and providers’ mental health was no exception.3 Classic psychosocial risk factors, such as high demands, lack of control, lack of institutional support, and absence of reward defined the workplace, leading Theorell4 to call it “a randomized trial for maximal worsening of the work environment.” Stress and burnout during residency are not novel concepts. A 2002 survey including 415 medical residency programs with a response from more than 4000 residents found depressive symptoms in 35% of respondents, paired with feelings of increased cynicism and decreased humanism despite major curricular reforms and duty hour limitations.5 Unfortunately, the statistics in the coming years hardly budged and, in the wake of the pandemic, culminated to more than 50% to 76% of physicians worldwide reporting burnout in 2020.6-8
As a COVID intern at Brigham and Women’s Hospital (Boston, Massachusetts), I also experienced the demanding workload and witnessed the struggle of my colleagues firsthand. Brigham and Women’s Hospital, similar to many of its peer institutions, implemented frequent mental health check-ins within its curriculum. Known as the Intern Humanistic Curriculum, these check-ins essentially were an echo chamber to unload the psychological burdens of our workdays, and we eagerly shared what made us angry, sad, hopeful, and hopeless. During one such session, I learned about moral injury, a term originating in the military defined as the psychological stress resulting from actions—or the lack of actions—that violates one’s moral or ethical code.9 With the onslaught of patient deaths for which most of us felt unprepared, we had all endured varying degrees of moral injury. Greenberg et al9 described 2 potential outcomes after moral injury: (1) the development of mental health disorders such as depression and posttraumatic stress disorder, or (2) posttraumatic growth, which is the bolstering of psychological resilience. Notably, the outcome is based on the way someone is supported before, during, and after the challenging incident.9
With the aim of psychological growth and developing resilience, residents should prioritize mental health throughout their training. To this end, several resources are readily available, many of which I actively use or frequently revisit, which are reviewed here.
Mindfulness Meditation App
Calm (https://www.calm.com/) is one of several popular mobile applications (apps) that delivers mindfulness mediation—the practice of attending to experiences, thoughts, and emotions without bias or judgment. With more than 100 million downloads, Calm includes meditation tutorials, breathing exercises, nature scenes and sounds, and audio programs taught by mindfulness experts for $69.99 a year or $14.99 a month. Systemic reviews have demonstrated reduced sleep disturbance, decreased ruminative thoughts and emotional reactivity, and increased awareness and acceptance in those practicing mindfulness meditation. Calm users have reported these benefits, with many able to forego the time- and cost-intensive cognitive behavioral therapy that requires highly trained therapists.10-12
Exercise to Relieve Stress
Both aerobic and anaerobic exercises are antidepressive and anxiolytic and also lower one’s overall sensitivity to stress. Whether it is governed by neurotransmitters such as the activation of the opioid systems or the release of endogenous endorphins or time spent focusing on a different task at hand, the benefits of exercise against mental stressors have been extensively studied and established.13 Consider obtaining a new gym membership at the start of residency or joining an intramural team. Both have the added benefit of expanding your social circle.
Socialize With Others
Social isolation and perceived loneliness are key stressors linked to neuroendocrine disturbances that underlie depression, anxiety, and even schizophrenia.14,15 Throughout residency there will be several social events and opportunities to gather with colleagues—inside or outside of the work environment—and residents should attend as time allows. Even virtual social interactions were found to reduce stress and help in the treatment of social anxiety disorder.14
Communicate About Stressors
Open up to your co-residents, friends, and family about any struggles that may be invisible on the outside. Even attendings can empathize with the struggles of residency, and the mentors in place are actively trained to prioritize resident wellness. If verbal communication is not your strength, try journaling. Writing helps to untangle and better define underlying stressors and is itself meditative.16,17 However, ensure that your journaling is focused on positive emotional responses and aims to determine the positive benefits within any stressful event; those solely expressing negative emotions were found to have higher levels of stress and anxiety afterward than they had before.17
Seek a Mental Health Specialist
As with all other human ailments, severe mental health disorders require specialists and proper medication. Unfortunately, substantial stigma accompanying mental health continues to permeate medicine, creating considerable barriers for residents in need of care.18 A 2016 survey of more than 2000 physicians found that those with mental illnesses did not seek treatment due to limited time, fear of being reported to a medical licensing board, concern over obtaining licensure, and shame or embarrassment at the diagnosis.19 Besides urging residents to seek care, more effort should be invested in addressing the stigma and ensuring confidentiality. In 2021, the internal medicine and medicine-pediatrics residency at the University of Colorado Anschutz Medical Campus (Aurora, Colorado) developed a confidential opt-out, rather than opt-in, mental health program, and appointments were made for all 80 interns in advance. In doing so, they found increased participation and self-reported wellness at a relatively low cost and simple implementation.20 For trainees without such access, online or mobile therapy platforms offering electronic mental health treatment or telepsychiatry also have been employed.21,22 The onus ultimately is still on the individual to seek the care they need. Although only an anecdotal piece of evidence, I have found the prevalence of physicians taking selective serotonin reuptake inhibitors such as escitalopram, sertraline, or fluoxetine to be strikingly common and quite beneficial.
Final Thoughts
Residency remains rife with financial, emotional, and physical stressors; even as the dust settles on the COVID-19 pandemic, the light shed on the importance of trainee mental health must remain illuminated. For the aforementioned resources to have an impact, residents need to be empowered to openly discuss mental health issues and to seek help if necessary. Finally, in 2018, the Journal of Graduate Medical Education published a 10-year prospective cohort study that found that emotional distress during residency persists in professional practice even 10 years after residency and is associated with future burnout.23 Trainees should consider prioritizing their mental health to not only improve their quality of life in the present but also as an investment for their future.
- Spiegelman J, Praiss A, Syeda S, et al. Preparation and redeployment of house staff during a pandemic. Semin Perinatol. 2020;44:151297.
- Debnath PR, Islam MS, Karmakar PK, et al. Mental health concerns, insomnia, and loneliness among intern doctors amidst the COVID-19 pandemic: evidence from a large tertiary care hospital in Bangladesh. Int J Ment Health Addict. 2021:1-21. doi:10.1007/s11469-021-00690-0
- O’Reilly-Shah VN, Gentry KR, Van Cleve W, et al. The COVID-19 pandemic highlights shortcomings in US health care informatics infrastructure: a call to action. Anesth Analg. 2020;131:340-344.
- Theorell T. COVID-19 and working conditions in health care. Psychother Psychosom. 2020;89:193-194.
- Collier VU, McCue JD, Markus A, et al. Stress in medical residency: status quo after a decade of reform? Ann Intern Med. 2002;136:384-390.
- AbuDujain NM, Almuhaideb QA, Alrumaihi NA, et al. The impact of the COVID-19 pandemic on medical interns’ education, training, and mental health: a cross-sectional study. Cureus. 2021;13:E19250.
- Amanullah S, Ramesh Shankar R. The impact of COVID-19 on physician burnout globally: a review. Healthcare (Basel). 2020;8:421.
- Lebares CC, Guvva EV, Ascher NL, et al. Burnout and stress among US surgery residents: psychological distress and resilience. J Am Coll Surg. 2018;226:80-90.
- Greenberg N, Docherty M, Gnanapragasam S, et al. Managing mental health challenges faced by healthcare workers during COVID-19 pandemic. BMJ. 2020;368:m1211.
- Gal E, Stefan S, Cristea IA. The efficacy of mindfulness meditation apps in enhancing users’ well-being and mental health related outcomes: a meta-analysis of randomized controlled trials. J Affect Disord. 2021;279:131-142.
- Huberty J, Green J, Glissmann C, et al. Efficacy of the mindfulness meditation mobile app “Calm” to reduce stress among college students: randomized controlled trial. JMIR Mhealth Uhealth. 2019;7:E14273.
- Huberty J, Puzia ME, Larkey L, et al. Can a meditation app help my sleep? a cross-sectional survey of Calm users. PLoS One. 2021;16:E0257518.
- Salmon P. Effects of physical exercise on anxiety, depression, and sensitivity to stress: a unifying theory. Clin Psychol Rev. 2001;21:33-61.
- Kampmann IL, Emmelkamp PM, Hartanto D, et al. Exposure to virtual social interactions in the treatment of social anxiety disorder: a randomized controlled trial. Behav Res Ther. 2016;77:147-156.
- Mumtaz F, Khan MI, Zubair M, et al. Neurobiology and consequences of social isolation stress in animal model-A comprehensive review. Biomed Pharmacother. 2018;105:1205-1222.
- Khanna P, Singh K. Stress management training and gratitude journaling in the classroom: an initial investigation in Indian context. Curr Psychol. 2021;40:5737-5748.
- Ullrich PM, Lutgendorf SK. Journaling about stressful events: effects of cognitive processing and emotional expression. Ann Behav Med. 2002;24:244-250.
- Outhoff K. Depression in doctors: a bitter pill to swallow. S Afr Fam Pract. 2019;61(suppl 1):S11-S14.
- Gold KJ, Andrew LB, Goldman EB, et al. “I would never want to have a mental health diagnosis on my record”: a survey of female physicians on mental health diagnosis, treatment, and reporting. Gen Hosp Psychiatry. 2016;43:51-57.
- Major A, Williams JG, McGuire WC, et al. Removing barriers: a confidential opt-out mental health pilot program for internal medicine interns. Acad Med. 2021;96:686-689.
- Greenhalgh T, Wherton J. Telepsychiatry: learning from the pandemic. Br J Psychiatry. 2022;220:1-5.
- Timakum T, Xie Q, Song M. Analysis of E-mental health research: mapping the relationship between information technology and mental healthcare. BMC Psychiatry. 2022;22:57.
- Raimo J, LaVine S, Spielmann K, et al. The correlation of stress in residency with future stress and burnout: a 10-year prospective cohort study. J Grad Med Educ. 2018;10:524-531.
- Spiegelman J, Praiss A, Syeda S, et al. Preparation and redeployment of house staff during a pandemic. Semin Perinatol. 2020;44:151297.
- Debnath PR, Islam MS, Karmakar PK, et al. Mental health concerns, insomnia, and loneliness among intern doctors amidst the COVID-19 pandemic: evidence from a large tertiary care hospital in Bangladesh. Int J Ment Health Addict. 2021:1-21. doi:10.1007/s11469-021-00690-0
- O’Reilly-Shah VN, Gentry KR, Van Cleve W, et al. The COVID-19 pandemic highlights shortcomings in US health care informatics infrastructure: a call to action. Anesth Analg. 2020;131:340-344.
- Theorell T. COVID-19 and working conditions in health care. Psychother Psychosom. 2020;89:193-194.
- Collier VU, McCue JD, Markus A, et al. Stress in medical residency: status quo after a decade of reform? Ann Intern Med. 2002;136:384-390.
- AbuDujain NM, Almuhaideb QA, Alrumaihi NA, et al. The impact of the COVID-19 pandemic on medical interns’ education, training, and mental health: a cross-sectional study. Cureus. 2021;13:E19250.
- Amanullah S, Ramesh Shankar R. The impact of COVID-19 on physician burnout globally: a review. Healthcare (Basel). 2020;8:421.
- Lebares CC, Guvva EV, Ascher NL, et al. Burnout and stress among US surgery residents: psychological distress and resilience. J Am Coll Surg. 2018;226:80-90.
- Greenberg N, Docherty M, Gnanapragasam S, et al. Managing mental health challenges faced by healthcare workers during COVID-19 pandemic. BMJ. 2020;368:m1211.
- Gal E, Stefan S, Cristea IA. The efficacy of mindfulness meditation apps in enhancing users’ well-being and mental health related outcomes: a meta-analysis of randomized controlled trials. J Affect Disord. 2021;279:131-142.
- Huberty J, Green J, Glissmann C, et al. Efficacy of the mindfulness meditation mobile app “Calm” to reduce stress among college students: randomized controlled trial. JMIR Mhealth Uhealth. 2019;7:E14273.
- Huberty J, Puzia ME, Larkey L, et al. Can a meditation app help my sleep? a cross-sectional survey of Calm users. PLoS One. 2021;16:E0257518.
- Salmon P. Effects of physical exercise on anxiety, depression, and sensitivity to stress: a unifying theory. Clin Psychol Rev. 2001;21:33-61.
- Kampmann IL, Emmelkamp PM, Hartanto D, et al. Exposure to virtual social interactions in the treatment of social anxiety disorder: a randomized controlled trial. Behav Res Ther. 2016;77:147-156.
- Mumtaz F, Khan MI, Zubair M, et al. Neurobiology and consequences of social isolation stress in animal model-A comprehensive review. Biomed Pharmacother. 2018;105:1205-1222.
- Khanna P, Singh K. Stress management training and gratitude journaling in the classroom: an initial investigation in Indian context. Curr Psychol. 2021;40:5737-5748.
- Ullrich PM, Lutgendorf SK. Journaling about stressful events: effects of cognitive processing and emotional expression. Ann Behav Med. 2002;24:244-250.
- Outhoff K. Depression in doctors: a bitter pill to swallow. S Afr Fam Pract. 2019;61(suppl 1):S11-S14.
- Gold KJ, Andrew LB, Goldman EB, et al. “I would never want to have a mental health diagnosis on my record”: a survey of female physicians on mental health diagnosis, treatment, and reporting. Gen Hosp Psychiatry. 2016;43:51-57.
- Major A, Williams JG, McGuire WC, et al. Removing barriers: a confidential opt-out mental health pilot program for internal medicine interns. Acad Med. 2021;96:686-689.
- Greenhalgh T, Wherton J. Telepsychiatry: learning from the pandemic. Br J Psychiatry. 2022;220:1-5.
- Timakum T, Xie Q, Song M. Analysis of E-mental health research: mapping the relationship between information technology and mental healthcare. BMC Psychiatry. 2022;22:57.
- Raimo J, LaVine S, Spielmann K, et al. The correlation of stress in residency with future stress and burnout: a 10-year prospective cohort study. J Grad Med Educ. 2018;10:524-531.
Resident Pearl
- Although institution-sponsored wellness programs exist to promote the mental health of trainees, rates of anxiety and depression remain high among residents, which was further highlighted during the COVID-19 pandemic. Instead of passively engaging with wellness messages, residents must actively prioritize their own mental health to avoid stress and burnout.