User login
Optimizing diabetes treatment in the presence of obesity
Diabesity was a term coined by Sims et al1 in the 1970s to describe diabetes occurring in the setting of obesity. Today, the link between type 2 diabetes mellitus (DM), obesity, and insulin resistance is well recognized, and 80% of people with type 2 DM are overweight or obese.2,3 Unfortunately, weight gain is a known side effect of most agents used to treat type 2 DM (eg, insulin, sulfonylureas, thiazolidinediones), and this often leads to nonadherence, poor glycemic control, and further weight gain.
During the past several years, evidence has emerged of a neurophysiologic mechanism that involves hormones from adipocytes, pancreatic islet cells, and the gastrointestinal tract implicated in both obesity and diabetes.2 This has led to research for drugs that not only either target obesity and diabetes or reduce hemoglobin A1c (HbA1c), but also have weight loss as a potential side effect.
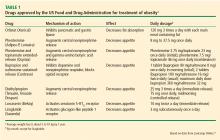
In this paper, we review medications approved for the treatment of type 2 DM (including pramlintide, also approved for type 1 DM) that also have weight loss as a side effect. Drugs we will discuss include glucagon-like peptide-1 (GLP-1) receptor agonists, sodium-glucose cotransporter-2 (SGLT-2) inhibitors, neuroendocrine peptide hormones, alpha-glucosidase inhibitors, and metformin. Where appropriate, we also comment on the effects of the drugs on cardiovascular outcomes.
GLP-1 RECEPTOR AGONISTS
Mechanism of action
GLP-1 is a hormone produced from the proglucagon gene in the alpha cells of the pancreas, in the L cells of intestinal mucosa (predominantly in the ileum and distal colon), and in structures of the nervous system including the brainstem, hypothalamus, and vagal afferent nerves.4 Food in the gastrointestinal tract, especially if high in fats and carbohydrates, stimulates secretion of GLP-1 in the L cells, which in turn amplifies insulin secretion in a glucose-dependent manner (the incretin effect).4 Glucagon secretion is inhibited by GLP-1 during times of hyperglycemia but not hypoglycemia, thereby preventing inappropriately high levels of the hormone.5 Peripheral GLP-1 activates a cascade of centrally mediated signals that ultimately result in secretion of insulin by the pancreas and slowing of gastrointestinal motility.5 Lastly, GLP-1 exerts an anorexic effect by acting on central pathways that mediate satiation.6
Bioactive forms of GLP-1 are rapidly degraded in the circulation by the dipeptidyl peptidase-4 enzyme. GLP-1 receptor agonists have slightly altered molecular structure and longer duration of action than native GLP-1. Short-acting GLP-1 agonists (eg, exenatide, lixisenatide) have more effect on gastric emptying and lower postprandial blood glucose levels, whereas long-acting GLP-1 agonists (eg, liraglutide, albiglutide, dulaglutide, semaglutide, exenatide) have a greater effect on fasting glucose levels.4
Effects on HbA1c and weight loss
Of the current GLP-1 agonists, exenatide and liraglutide have been on the market the longest, thus studied more in terms of weight reduction.
Exenatide. Exenatide BID was the first GLP-1 agonist, approved by the US Food and Drug Administration (FDA) in 2005 for the treatment of type 2 DM. In a 30-week triple-blind, placebo-controlled study of 336 patients already on background therapy with metformin, progressive weight loss was noted with exenatide 5 μg (−1.6 ± 0.4 kg) and exenatide 10 μg (−2.8 ± 0.5 kg) compared with placebo (−0.3 ± 0.3 kg; P < .001).15 A meta-analysis of 14 trials with 2,583 patients showed significant weight reduction with both exenatide 5 μg twice daily (a difference of −0.56 kg, 95% confidence interval [CI] −1.07 to −0.06, P = .0002) in 8 trials and exenatide 10 μg twice daily (a difference of −1.24 kg, 95% CI −1.69 to −0.78, P < .001) in 12 trials, after treatment for more than 16 weeks.16
Liraglutide. Liraglutide has a longer half-life than exenatide and is administered once daily. It is not a first-line therapy for type 2 DM and is recommended as an add-on. Approved daily doses for type 2 DM are 1.2 mg and 1.8 mg.
Multiple studies of glycemic control and weight loss with liraglutide have been conducted since its introduction to the US market in 2010. In the Liraglutide Effect and Action in Diabetes (LEAD) series of trials, liraglutide use as monotherapy or in combination with oral agents was associated with significant dose-dependent weight loss.17 Liraglutide monotherapy (at 1.2 mg and 1.8 mg) compared with glimepiride in the LEAD-3 trial led to significant weight reduction (2.1 kg and 2.5 kg, respectively, P < .001) after 16 weeks, and was sustained up to 52 weeks.18 Addition of liraglutide (at 1.2 mg and 1.8 mg) to metformin plus rosiglitazone resulted in significant weight loss (1.02 kg and 2.02 kg, respectively) whereas the addition of placebo caused a 0.6-kg weight gain (P < .001).19 The SCALE study randomized 846 adults with type 2 DM who were overweight to obese (body mass index [BMI] ≥ 27 kg/m2), were taking 0 to 3 oral antihyperglycemic agents (metformin, thiazolidinedione, and a sulfonylurea), and had stable body weight and an HbA1c of 7% to 10% to liraglutide 1.8 mg, liraglutide 3.0 mg, or placebo. Mean weight loss after 56 weeks was 6.0% (6.4 kg) with liraglutide 1.8 mg, 4.7% (5.0 kg) with liraglutide 3.0 mg, and 2.0% (2.2 kg) with placebo.20
In 2016, high-dose once-daily liraglutide 3.0 mg (Saxenda) was approved by the FDA for weight loss. In a double-blind randomized trial of liraglutide 3.0 mg vs placebo in patients who had a BMI of at least 30 or who had a BMI of at least 27 plus treated or untreated dyslipidemia or hypertension, Pi-Sunyer et al21 reported a mean weight reduction of 8.4 ± 7.3 kg with liraglutide vs 2.8 ± 6.5 kg with placebo (a difference of −5.6 kg, 95% CI −6.0 to −5.1, P < .001) after 56 weeks. Furthermore, 63.2% of patients in the liraglutide group lost at least 5% of body weight vs 27.1% with placebo, and 33.1% in the liraglutide group lost 10% or more of body weight vs 10.6% in the placebo group (P < .001).21 Of note, liraglutide 3.0 mg is not indicated for type 2 DM per se.
In a 2012 meta-analysis of randomized controlled trials of adults with and without type 2 DM, with a BMI of 25 or greater, and who received GLP-1 receptor agonists at clinically relevant doses (exenatide ≥ 10 μg/day, exenatide ≥ 2 mg/week, or liraglutide ≥ 1.2 mg/day), those taking GLP-1 receptor agonists had more weight loss than those on a control intervention (oral antihyperglycemic, insulin, or placebo) at a minimum of 20 weeks, with a weighted mean difference −2.9 kg (95% CI −3.6 to −2.2) in 21 trials and 6,411 participants.22
GLP-1 agonists currently being investigated for obesity treatment are lixisenatide, albiglutide, taspoglutide, and oxyntomodulin.23
Cardiovascular outcomes
The presence of GLP-1 receptors in blood vessels and myocardium has led to the hypothesis that GLP-1 receptor agonists can improve cardiovascular disease outcomes.24 In the pivotal Liraglutide Effect and Action in Diabetes: Evaluation of Cardiovascular Outcome Results (LEADER) trial, 9,340 patients with type 2 DM and increased cardiovascular disease risk were randomized to liraglutide vs placebo.25 The hazard ratio (HR) for time to the primary end point of cardiovascular death, nonfatal myocardial infarction, or nonfatal stroke was 0.87 (P = .01 for superiority, P < .001 for noninferiority) for liraglutide compared with placebo after 3.8 years. The incidence of death from any cause or cardiovascular cause was also lower with liraglutide.25
Adverse effects
Tolerable transient nausea and vomiting are reported adverse effects; these symptoms occur early in therapy, usually resolve in 4 to 8 weeks, and appear to be associated with greater weight loss.26 Although no causal relationship between GLP-1 receptor agonist use and pancreatitis or pancreatic cancer has been established to date, several cases of acute pancreatitis have been reported.25 Alternative therapies should be considered in patients with a history of or risk factors for pancreatitis.
Combined with insulin
A product that combines insulin glargine and lixisenatide (Soliqua) is FDA-approved for patients with type 2 DM. In a 30-week randomized controlled trial of the combination product vs insulin glargine alone in patients with type 2 DM not controlled on basal insulin with or without up to 2 oral agents, the combination product resulted in an HbA1c reduction from baseline of 1.1% vs 0.6% for insulin glargine alone (P < .001).27 Mean body weight decreased by 0.7 kg with the combination product and increased by 0.7 kg with insulin glargine (P < .001).27 In a 24-week study of a lixisenatide-insulin glargine combination vs insulin glargine in insulin-naïve patients taking metformin, there was a reduction in HbA1c of about −1.7% from baseline in both groups, while the combination group had a 1-kg weight reduction compared with a 0.5-kg weight increase in the insulin glargine group (P < .001).28
SGLT-2 INHIBITORS
Mechanism of action
In a healthy normoglycemic person, about 180 g of glucose per day is filtered into the glomerular filtrate and reabsorbed into the circulation.29 SGLT-2 facilitates the reabsorption of glucose in the proximal convoluted tubule of the kidneys. Approximately 90% of glucose reabsorption is mediated by SGLT-2 found in the S1 and S2 segments of the proximal convoluted tubule, and the remaining 10% by SGLT-1 in the S3 segment. At serum glucose levels above 180 g, the reabsorptive capacity of the nephron is overwhelmed, resulting in glycosuria.30 SGLT-2 expression is also increased in patients with diabetes, thus leading to increased glucose reabsorption into the circulation, further contributing to hyperglycemia.30 Inhibition of SGLT-2 alleviates hyperglycemia by decreasing glucose reabsorption (30% to 50% of filtered glucose) in the kidneys and by increasing excretion (50 mg to 80 mg of glucose) in the urine.31 SGLT-2 inhibitors currently FDA-approved are canagliflozin (Invokana), dapagliflozin (Farxiga), and empagliflozin (Jardiance).
HbA1c
SGLT-2 inhibitors have relatively weak glycemic efficacy. A meta-analysis of SGLT-2 inhibitors vs other antidiabetic medications or placebo found that SGLT-2 inhibitors appeared to have a “favorable effect” on HbA1c, with a mean difference vs placebo of −0.66% (95% CI −0.73% to −0.58%) and a mean difference vs other antihyperglycemic medications of −0.06% (95% CI 0.18% to 0.05%).32
Weight loss
The same meta-analysis found that SGLT-2 inhibitors reduced body weight (mean difference −1.8 kg, 95% CI −3.50 kg to −0.11 kg).32 And in a randomized controlled trial, monotherapy with canagliflozin 100 mg/day and 300 mg/day resulted in body weight reduction of 2.2% (1.9 kg) and 3.3% (−2.9 kg), respectively, after 26 weeks.33 A Japanese study showed a dose-related total body weight loss with empagliflozin vs placebo ranging from 2.5 ± 0.2 kg (5-mg dose) to 3.1 ± 0.2 kg (50-mg dose) after 12 weeks.34 Bolinder et al35 reported that adding dapagliflozin 10 mg to metformin in patients with type 2 DM reduced total body weight by −2.96 kg (95% CI −3.51 to −2.41, P < .001) at week 24. Whole-body dual-energy x-ray absorptiometry and magnetic resonance imaging findings in this study revealed a decrease in fat mass and visceral and subcutaneous adipose tissue after treatment with dapagliflozin, thus suggesting urinary loss of glucose (and hence caloric loss) contributing to weight reduction in addition to initial weight loss from fluid loss due to osmotic diuresis.35 A continuous decline in total body weight was observed in a 78-week extension study resulting in −4.54 kg (95% CI −5.43 to −3.66 kg) at week 102, along with further reduction in total body fat mass as measured by dual-energy x-ray absorptiometry.36
Cardiovascular outcomes
The landmark study Empagliflozin, Cardiovascular Outcomes and Mortality in Type 2 Diabetes (EMPA-REG) involving 7,020 patients was the first large cardiovascular outcomes trial in patients with type 2 DM and overt cardiovascular disease. A relative risk reduction of 14% (12.1% to 10.5%, HR 0.86, 95% CI 0.74 to 0.99) in major adverse cardiovascular events (cardiovascular death, nonfatal myocardial infarction, and nonfatal stroke) was observed with empagliflozin.37 Rates of all-cause mortality and hospitalization for heart failure relative risk reductions were 32% (8.3% to 5.7%; HR 0.68 [0.57, 0.8]) and 35% (4.1% to 2.7%; HR 0.65 [0.50, 0.85]), respectively, with empagliflozin. The mechanism behind this cardiovascular benefit is unknown but is currently being explored.37
Adverse effects
Increased risk of urinary tract and genital infections are known adverse effects of SGLT-2s. Other effects noted include postural hypotension from volume depletion and a transient increase in serum creatinine and decrease in glomerular filtration.29
NEUROENDOCRINE PEPTIDE HORMONE: AMYLIN ANALOGUES
Mechanism of action
Amylin is a 37-amino-acid neuroendocrine peptide hormone secreted primarily by pancreatic beta cells. It promotes early satiety, and its anorexigenic effects are mediated by its action on the neurons of the area postrema in the brain.38 After a meal, amylin decreases gastric acid secretion and slows gastric emptying. It is co-secreted with insulin in a 1:20 amylin-to-insulin ratio and inhibits glucagon secretion via a centrally mediated mechanism.39
Pramlintide (Symlin) is an amylin analogue administered subcutaneously immediately before major meals. It decreases postprandial glucose levels and has been approved by the FDA as an adjunct to prandial insulin in patients with type 1 and type 2 DM.40
HbA1c
Amylin secretion is impaired in type 1 and type 2 DM, and small but significant reductions in HbA1c have been observed with addition of pramlintide to usual insulin regimens. In patients with type 1 DM, HbA1c levels were reduced by 0.4% to 0.6% after 26 weeks on 30 μg 3 times daily to 60 μg 4 times daily of pramlintide added to insulin.41,42 And pramlintide 120 μg added to usual antihyperglycemic therapy in patients with type 2 DM has been reported to decrease HbA1c by 0.7% at week 16 or 26.43,44
Weight loss
A meta-analysis of 8 randomized controlled trials assessed the effects of pramlintide on glycemic control and weight in patients with type 2 DM treated with insulin and in obese patients without diabetes.45 In these trials, patients took at least 120 μg of pramlintide before 2 to 3 meals for at least 12 weeks; a total of 1,616 participants were included. In the type 2 DM group, pramlintide reduced body weight by 2.57 kg (95% CI −3.44 to −1.70 kg, P < .001) vs control, over 16 to 52 weeks.45 The nondiabetic obese group had a weight loss of −2.27 kg (95%CI −2.88 to −1.66 kg, P < .001) vs control.45
Pramlintide and a pramlintide-phentermine combination are currently under investigation for treatment of obesity.23
Cardiovascular outcomes
Cardiovascular outcomes in patients treated with pramlintide have not been studied to date, but reductions have been observed in markers of cardiovascular risk including high-sensitivity C-reactive protein and triglycerides.46
Adverse effects
Transient mild-to-moderate nausea is the most common adverse effect of pramlintide. Hypoglycemia has also been reported, more frequently in patients with type 1 DM, which is possibly associated with inadequate reduction in insulin.
ALPHA-GLUCOSIDASE INHIBITORS
Mechanism of action
Alpha-glucosidase inhibitors competitively inhibit the alpha-glucosidase enzymes at the brush border of the small intestine. Taken orally before meals, these drugs mitigate postprandial hyperglycemia by preventing the breakdown of complex carbohydrates into simpler monosaccharides, thus delaying their absorption.47 These agents may be used as monotherapy or in combination with other antihyperglycemic agents. They work independently from insulin, although they have been shown to potentiate GLP-1 secretion.48 Acarbose and miglitol are currently approved in the United States. Acarbose has been more extensively studied worldwide.
HbA1c
Alpha-glucosidase inhibitors have been reported to reduce mean HbA1c by 0.8% (95% CI −0.9% to −0.7%), as well as fasting and postprandial glucose, and postprandial insulin levels.49
Weight loss
There is conflicting evidence on whether alpha-glucosidase inhibitor therapy has a neutral or beneficial effect on body weight. A Cochrane meta-analysis observed significant BMI reduction with acarbose, although no effect on body weight was noted,49 whereas in another meta-analysis, body weight was significantly reduced by 0.96 kg (95% CI −1.80 to −0.12 kg) when acarbose was added to metformin.50 A review of pooled data from worldwide post-marketing studies for acarbose reported a weight reduction after 3 months of 0.98 ± 2.11 kg in overweight patients and 1.67 ± 3.02 kg in obese patients.51
Cardiovascular outcomes
In the Study to Prevent Non-Insulin-Dependent Diabetes Mellitus (STOP-NIDDM), when compared with placebo, treatment of patients with impaired glucose tolerance with acarbose significantly reduced the incidence of cardiovascular events (HR 0.51, 95% CI 0.28 to 0.95, P = .03), myocardial infarction (HR 0.09, 95% CI 0.01 to 0.72, P = .02), and newly diagnosed hypertension (HR 0.66, 95% CI 0.49 to 0.89, P = .006).52
Adverse effects
Although mild, gastrointestinal effects of flatulence and diarrhea can be bothersome and result in discontinuation of the drug in most patients.
METFORMIN
Mechanism of action
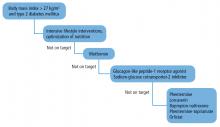
Metformin is the first-line antihyperglycemic agent for type 2 DM recommended by the American Diabetes Association and European Association for the Study of Diabetes.53,54 The main action of metformin is to decrease glucose production in the liver. In the small intestine, metformin stimulates the L cells to produce GLP-1, and in skeletal muscle, it increases glucose uptake and disposal.55
HbA1c
As monotherapy, metformin has resulted in HbA1c reductions of 0.88% to 1.2%.55
Weight loss
Reduced food intake56,57 and gastrointestinal intolerance58 occurring early in therapy have been noted to account for weight loss in short-term studies of non-diabetic obese patients treated with metformin.59 Long-term trials of patients with and without diabetes have yielded mixed results on weight reduction from metformin as monotherapy or adjunct therapy. In the United Kingdom Prospective Diabetes Study (UKPDS), metformin had resulted in approximately 1.5 kg of weight gain (slightly less than the 4-kg weight gain in the glibenclamide group).60 Improved antihyperglycemic efficacy of other antihyperglycemic agents (insulin, sulfonylureas, and thiazolidinediones) with addition of metformin led to dose-lowering of the antihyperglycemic agents, ultimately resulting in amelioration of weight gain; this has also led to small weight reductions in some studies.59 In the Diabetes Prevention Program study of patients with impaired glucose tolerance, metformin treatment resulted in an average weight loss of 2.1 kg compared with placebo (−0.1 kg) and lifestyle intervention (−5.6 kg; P <.001).61
Cardiovascular outcomes
Metformin has been observed to decrease micro- and macrovascular complications. Compared with diet alone, metformin was associated with a 39% reduction in the risk of myocardial infarction, and a 30% lower risk of a composite of macrovascular diseases (myocardial infarction, sudden death, angina, stroke, and peripheral disease).60
Adverse effects
The most common adverse effect of metformin is gastrointestinal intolerance from abdominal pain, flatulence, and diarrhea.62 Metformin-associated lactic acidosis is a serious and potentially life-threatening effect; and vitamin B12 deficiency may occur with long-term treatment.62
TAKE-HOME POINTS
As more medications and interventions are being developed to counter obesity, it also makes sense to select diabetes medications that do not contribute to weight gain in patients who are already overweight or obese. The effects of available medications can be maximized and treatment regimens individualized (based on patients’ needs and preferences, within the limitations of drug costs and side effects), along with lifestyle modification, to target diabesity.
- Sims EAH, Danforth E Jr, Horton ES, Bray GA, Glennon JA, Salans LB. Endocrine and metabolic effects of experimental obesity in man. Recent Prog Horm Res 1973; 29:457–496.
- Scheen AJ, Van Gaal LF. Combating the dual burden: therapeutic targeting of common pathways in obesity and type 2 diabetes. Lancet Diabetes Endocrinol 2014; 2:911–922.
- Kahn SE, Hull RL, Utzschneider KM. Mechanisms linking obesity to insulin resistance and type 2 diabetes. Nature 2006; 444:840–846.
- Iepsen EW, Torekov SS, Holst JJ. Therapies for inter-relating diabetes and obesity—GLP-1 and obesity. Expert Opin Pharmacother 2014; 15:2487–2500.
- Meier JJ, Nauck MA. Glucagon-like peptide 1(GLP-1) in biology and pathology. Diabetes Metab Res Rev 2005; 21:91–117.
- Turton MD, O’Shea D, Gunn I, et al. A role for glucagon-like peptide-1 in the central regulation of feeding. Nature 1996; 379:69–72.
- Drucker DJ. Glucagon-like peptides: regulators of cell proliferation, differentiation, and apoptosis. Mol Endocrinol 2003; 17:161–171.
- Shyangdan DS, Royle P, Clar C, Sharma P, Waugh N, Snaith A. Glucagon-like peptide analogues for type 2 diabetes mellitus. Cochrane Database Syst Rev 2011; 10:CD006423. doi:10.1002/14651858.CD006423.pub2.
- Byetta [package insert]. Wilmington, DE: AstraZeneca Pharmaceuticals LP; 2015. Available at http://www.azpicentral.com/byetta/pi_byetta.pdf. Accessed June 22, 2017.
- Victoza [package insert]. Bagsvaerd, Denmark: Novo Nordisk A/S; 2016. Available at http://www.novo-pi.com/victoza.pdf. Accessed June 22, 2017.
- Bydureon [package insert]. Wilmington, DE: AstraZeneca Pharmaceuticals LP; 2015. Available at http://www.azpicentral.com/bydureon/pi_bydureon.pdf. Accessed June 22, 2017.
- Tanzeum [package insert]. Wilmington, DE: GlaxoSmithKline; 2016. Available at https://www.gsksource.com/pharma/content/dam/GlaxoSmithKline/US/en/Prescribing_Information/Tanzeum/pdf/TANZEUM-PI-MG-IFU-COMBINED.PDF. Accessed June 22, 2017.
- Trulicity [package insert]. Indianapolis, IN: Eli Lilly and Company 2014. Available at http://pi.lilly.com/us/trulicity-uspi.pdf. Accessed June 22, 2017.
- Adlyxin [package insert]. Bridgewater, NJ: Sanofi-Aventis U.S. LLC; 2016. Available at http://products.sanofi.us/adlyxin/adlyxin.pdf. Accessed June 22, 2017.
- DeFronzo RA, Ratner RE, Han J, Kim DD, Fineman MS, Baron AD. Effects of exenatide (exendin-4) on glycemic control and weight over 30 weeks in metformin-treated patients with type 2 diabetes. Diabetes Care 2005; 28:1092–1100.
- Nikfar S, Abdollahi M, Salari P. The efficacy and tolerability of exenatide in comparison to placebo; a systematic review and meta-analysis of randomized clinical trials. J Pharm Pharm Sci 2012; 15:1–30.
- Blonde L, Russell-Jones D. The safety and efficacy of liraglutide with or without oral antidiabetic drug therapy in type 2 diabetes: an overview of the LEAD 1-5 studies. Diabetes Obes Metab 2009; 11(suppl 3):26–34.
- Garber A, Henry R, Ratner R, et al; LEAD-3 (Mono) Study Group. Liraglutide versus glimepiride monotherapy for type 2 diabetes (LEAD-3 Mono): a randomised, 52-week, phase III, double-blind, parallel-treatment trial. Lancet 2009; 373:473–481.
- Zinman B, Gerich J, Buse JB, et al; LEAD-4 Study Investigators. Efficacy and safety of the human glucagon-like peptide-1 analog liraglutide in combination with metformin and thiazolidinedione in patients with type 2 diabetes (LEAD-4 Met+TZD). Diabetes Care 2009; 32:1224–1230.
- Davies MJ, Bergenstal R, Bode B, et al. Efficacy of liraglutide for weight loss among patients with type 2 diabetes: the SCALE Diabetes Randomized Clinical Trial. JAMA 2015; 314:687–699.
- Pi-Sunyer X, Astrup A, Fujioka K, et al; SCALE Obesity and Prediabetes NN8022-1839 Study Group. A randomized, controlled trial of 3.0 mg of liraglutide in weight management. N Engl J Med 2015; 373:11–22.
- Vilsboll T, Christensen M, Junker AE, Knop FK, Gluud LL. Effects of glucagon-like peptide-1 receptor agonists on weight loss: systematic review and meta-analyses of randomised controlled trials. BMJ 2012; 344:d7771.
- Valsamakis G, Konstantakou P, Mastorakos G. New targets for drug treatment of obesity. Annu Rev Pharmacol Toxicol 2017; 57:585–605.
- Sivertsen J, Rosenmeier J, Holst JJ, Vilsboll T. The effect of glucagon-like peptide 1 on cardiovascular risk. Nat Rev Cardiol 2012; 9:209–222.
- Marso SP, Daniels GH, Brown-Frandsen K, et al; LEADER Steering Committee; LEADER Trial Investigators. Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med 2016; 375:311–322.
- Lean ME, Carraro R, Finer N, et al; NN8022-1807 Investigators. Tolerability of nausea and vomiting and associations with weight loss in a randomized trial of liraglutide in obese, non-diabetic adults. Int J Obes (Lond) 2014; 38:689–697.
- Aroda VR, Rosenstock J, Wysham C, et al; LixiLan-L Trial Investigators. Efficacy and safety of lixilan, a titratable fixed-ratio combination of insulin glargine plus lixisenatide in type 2 diabetes inadequately controlled on basal insulin and metformin: the LixiLan-L Randomized Trial. Diabetes Care 2016; 39:1972–1980.
- Rosenstock J, Diamant M, Aroda VR, et al; LixiLan PoC Study Group. Efficacy and safety of LixiLan, a titratable fixed-ratio combination of lixisenatide and insulin glargine, versus insulin glargine in type 2 diabetes inadequately controlled on metformin monotherapy: the LixiLan Proof-of-Concept Randomized Trial. Diabetes Care 2016; 39:1579–1586.
- Monica Reddy RP, Inzucchi SE. SGLT2 inhibitors in the management of type 2 diabetes. Endocrine 2016; 53:364–372.
- DeFronzo RA, Davidson JA, Del Prato S. The role of the kidneys in glucose homeostasis: a new path towards normalizing glycaemia. Diabetes Obes Metab 2012; 14:5–14.
- Liu JJ, Lee T, DeFronzo RA. Why do SGLT2 inhibitors inhibit only 30-50% of renal glucose reabsorption in humans? Diabetes 2012; 61:2199–2204.
- Vasilakou D, Karagiannis T, Athanasiadou E, et al. Sodium-glucose cotransporter 2 inhibitors for type 2 diabetes: a systematic review and meta-analysis. Ann Intern Med 2013; 159:262–274.
- Stenlof K, Cefalu WT, Kim KA, et al. Efficacy and safety of canagliflozin monotherapy in subjects with type 2 diabetes mellitus inadequately controlled with diet and exercise. Diabetes Obes Metab 2013; 15:372–382.
- Kadowaki T, Haneda M, Inagaki N, et al. Empagliflozin monotherapy in Japanese patients with type 2 diabetes mellitus: a randomized, 12-week, double-blind, placebo-controlled, phase II trial. Adv Ther 2014; 31:621–638.
- Bolinder J, Ljunggren O, Kullberg J, et al. Effects of dapagliflozin on body weight, total fat mass, and regional adipose tissue distribution in patients with type 2 diabetes mellitus with inadequate glycemic control on metformin. J Clin Endocrinol Metab 2012; 97:1020–1031.
- Bolinder J, Ljunggren O, Johansson L, et al. Dapagliflozin maintains glycaemic control while reducing weight and body fat mass over 2 years in patients with type 2 diabetes mellitus inadequately controlled on metformin. Diabetes Obes Metab 2014; 16:159–169.
- Zinman B, Wanner C, Lachin JM, et al; EMPA-REG OUTCOME Investigators. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med 2015; 373:2117–2128.
- Lutz TA. Effects of amylin on eating and adiposity. Handb Exp Pharmacol 2012; (209):231–250.
- Hieronymus L, Griffin S. Role of amylin in type 1 and type 2 diabetes. Diabetes Educ 2015; 41(suppl 1):47S–56S.
- Aronoff SL. Rationale for treatment options for mealtime glucose control in patients with type 2 diabetes. Postgrad Med 2017; 129:231–241.
- Ratner RE, Dickey R, Fineman M, et al. Amylin replacement with pramlintide as an adjunct to insulin therapy improves long-term glycaemic and weight control in type 1 diabetes mellitus: a 1-year, randomized controlled trial. Diabet Med 2004; 21:1204–1212.
- Edelman S, Garg S, Frias J, et al. A double-blind, placebo-controlled trial assessing pramlintide treatment in the setting of intensive insulin therapy in type 1 diabetes. Diabetes Care 2006; 29:2189–2195.
- Riddle M, Frias J, Zhang B, et al. Pramlintide improved glycemic control and reduced weight in patients with type 2 diabetes using basal insulin. Diabetes Care 2007; 30:2794–2799.
- Hollander PA, Levy P, Fineman MS, et al. Pramlintide as an adjunct to insulin therapy improves long-term glycemic and weight control in patients with type 2 diabetes: a 1-year randomized controlled trial. Diabetes Care 2003; 26:784–790.
- Singh-Franco D, Perez A, Harrington C. The effect of pramlintide acetate on glycemic control and weight in patients with type 2 diabetes mellitus and in obese patients without diabetes: a systematic review and meta-analysis. Diabetes Obes Metab 2011; 13:169–180.
- Wysham C, Lush C, Zhang B, Maier H, Wilhelm K. Effect of pramlintide as an adjunct to basal insulin on markers of cardiovascular risk in patients with type 2 diabetes. Curr Med Res Opin 2008; 24:79–85.
- Bischoff H. Pharmacology of alpha-glucosidase inhibition. Eur J Clin Invest 1994; 24(suppl 3):3–10.
- Lee A, Patrick P, Wishart J, Horowitz M, Morley JE. The effects of miglitol on glucagon-like peptide-1 secretion and appetite sensations in obese type 2 diabetics. Diabetes Obes Metab 2002; 4:329–335.
- van de Laar FA, Lucassen PL, Akkermans RP, van de Lisdonk EH, Rutten GE, van Weel C. Alpha-glucosidase inhibitors for patients with type 2 diabetes: results from a Cochrane systematic review and meta-analysis. Diabetes Care 2005; 28:154–163.
- Gross JL, Kramer CK, Leitao CB, et al; Diabetes and Endocrinology Meta-analysis Group (DEMA). Effect of antihyperglycemic agents added to metformin and a sulfonylurea on glycemic control and weight gain in type 2 diabetes: a network meta-analysis. Ann Intern Med 2011; 154:672–679.
- Schnell O, Weng J, Sheu WH, et al. Acarbose reduces body weight irrespective of glycemic control in patients with diabetes: results of a worldwide, non-interventional, observational study data pool. J Diabetes Complications 2016; 30:628–637.
- Chiasson JL, Josse RG, Gomis R, Hanefeld M, Karasik A, Laakso M; STOP-NIDDM Trial Research Group. Acarbose treatment and the risk of cardiovascular disease and hypertension in patients with impaired glucose tolerance: the STOP-NIDDM trial. JAMA 2003; 290:486–494.
- Inzucchi SE, Bergenstal RM, Buse JB, et al. Management of hyperglycemia in type 2 diabetes, 2015: a patient-centered approach: update to a position statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care 2015; 38:140–149.
- American Diabetes Association. Pharmacologic approaches to glycemic treatment. Diabetes Care 2017; 40:S64–S74.
- Tan MH, Alquraini H, Mizokami-Stout K, MacEachern M. Metformin: From research to clinical practice. Endocrinol Metab Clin North Am 2016; 45:819–843.
- Paolisso G, Amato L, Eccellente R, et al. Effect of metformin on food intake in obese subjects. Eur J Clin Invest 1998; 28: 441–446.
- Lee A, Morley JE. Metformin decreases food consumption and induces weight loss in subjects with obesity with type II noninsulin-dependent diabetes. Obes Res 1998; 6: 47–53.
- Scarpello JH. Optimal dosing strategies for maximising the clinical response to metformin in type 2 diabetes. Br J Diabetes Vasc Dis 2001; 1: 28–36.
- Golay A. Metformin and body weight. Int J Obes (Lond) 2008; 32:61–72.
- UK Prospective Diabetes Study (UKPDS) Group. Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34). Lancet 1998; 352:854–865.
- Knowler WC, Barrett-Connor E, Fowler SE, et al; Diabetes Prevention Program Research Group. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med 2002; 346:393–403.
- Fujita Y, Inagaki N. Metformin: new preparations and nonglycemic benefits. Curr Diab Rep 2017; 17:5.
Diabesity was a term coined by Sims et al1 in the 1970s to describe diabetes occurring in the setting of obesity. Today, the link between type 2 diabetes mellitus (DM), obesity, and insulin resistance is well recognized, and 80% of people with type 2 DM are overweight or obese.2,3 Unfortunately, weight gain is a known side effect of most agents used to treat type 2 DM (eg, insulin, sulfonylureas, thiazolidinediones), and this often leads to nonadherence, poor glycemic control, and further weight gain.
During the past several years, evidence has emerged of a neurophysiologic mechanism that involves hormones from adipocytes, pancreatic islet cells, and the gastrointestinal tract implicated in both obesity and diabetes.2 This has led to research for drugs that not only either target obesity and diabetes or reduce hemoglobin A1c (HbA1c), but also have weight loss as a potential side effect.
In this paper, we review medications approved for the treatment of type 2 DM (including pramlintide, also approved for type 1 DM) that also have weight loss as a side effect. Drugs we will discuss include glucagon-like peptide-1 (GLP-1) receptor agonists, sodium-glucose cotransporter-2 (SGLT-2) inhibitors, neuroendocrine peptide hormones, alpha-glucosidase inhibitors, and metformin. Where appropriate, we also comment on the effects of the drugs on cardiovascular outcomes.
GLP-1 RECEPTOR AGONISTS
Mechanism of action
GLP-1 is a hormone produced from the proglucagon gene in the alpha cells of the pancreas, in the L cells of intestinal mucosa (predominantly in the ileum and distal colon), and in structures of the nervous system including the brainstem, hypothalamus, and vagal afferent nerves.4 Food in the gastrointestinal tract, especially if high in fats and carbohydrates, stimulates secretion of GLP-1 in the L cells, which in turn amplifies insulin secretion in a glucose-dependent manner (the incretin effect).4 Glucagon secretion is inhibited by GLP-1 during times of hyperglycemia but not hypoglycemia, thereby preventing inappropriately high levels of the hormone.5 Peripheral GLP-1 activates a cascade of centrally mediated signals that ultimately result in secretion of insulin by the pancreas and slowing of gastrointestinal motility.5 Lastly, GLP-1 exerts an anorexic effect by acting on central pathways that mediate satiation.6
Bioactive forms of GLP-1 are rapidly degraded in the circulation by the dipeptidyl peptidase-4 enzyme. GLP-1 receptor agonists have slightly altered molecular structure and longer duration of action than native GLP-1. Short-acting GLP-1 agonists (eg, exenatide, lixisenatide) have more effect on gastric emptying and lower postprandial blood glucose levels, whereas long-acting GLP-1 agonists (eg, liraglutide, albiglutide, dulaglutide, semaglutide, exenatide) have a greater effect on fasting glucose levels.4
Effects on HbA1c and weight loss
Of the current GLP-1 agonists, exenatide and liraglutide have been on the market the longest, thus studied more in terms of weight reduction.
Exenatide. Exenatide BID was the first GLP-1 agonist, approved by the US Food and Drug Administration (FDA) in 2005 for the treatment of type 2 DM. In a 30-week triple-blind, placebo-controlled study of 336 patients already on background therapy with metformin, progressive weight loss was noted with exenatide 5 μg (−1.6 ± 0.4 kg) and exenatide 10 μg (−2.8 ± 0.5 kg) compared with placebo (−0.3 ± 0.3 kg; P < .001).15 A meta-analysis of 14 trials with 2,583 patients showed significant weight reduction with both exenatide 5 μg twice daily (a difference of −0.56 kg, 95% confidence interval [CI] −1.07 to −0.06, P = .0002) in 8 trials and exenatide 10 μg twice daily (a difference of −1.24 kg, 95% CI −1.69 to −0.78, P < .001) in 12 trials, after treatment for more than 16 weeks.16
Liraglutide. Liraglutide has a longer half-life than exenatide and is administered once daily. It is not a first-line therapy for type 2 DM and is recommended as an add-on. Approved daily doses for type 2 DM are 1.2 mg and 1.8 mg.
Multiple studies of glycemic control and weight loss with liraglutide have been conducted since its introduction to the US market in 2010. In the Liraglutide Effect and Action in Diabetes (LEAD) series of trials, liraglutide use as monotherapy or in combination with oral agents was associated with significant dose-dependent weight loss.17 Liraglutide monotherapy (at 1.2 mg and 1.8 mg) compared with glimepiride in the LEAD-3 trial led to significant weight reduction (2.1 kg and 2.5 kg, respectively, P < .001) after 16 weeks, and was sustained up to 52 weeks.18 Addition of liraglutide (at 1.2 mg and 1.8 mg) to metformin plus rosiglitazone resulted in significant weight loss (1.02 kg and 2.02 kg, respectively) whereas the addition of placebo caused a 0.6-kg weight gain (P < .001).19 The SCALE study randomized 846 adults with type 2 DM who were overweight to obese (body mass index [BMI] ≥ 27 kg/m2), were taking 0 to 3 oral antihyperglycemic agents (metformin, thiazolidinedione, and a sulfonylurea), and had stable body weight and an HbA1c of 7% to 10% to liraglutide 1.8 mg, liraglutide 3.0 mg, or placebo. Mean weight loss after 56 weeks was 6.0% (6.4 kg) with liraglutide 1.8 mg, 4.7% (5.0 kg) with liraglutide 3.0 mg, and 2.0% (2.2 kg) with placebo.20
In 2016, high-dose once-daily liraglutide 3.0 mg (Saxenda) was approved by the FDA for weight loss. In a double-blind randomized trial of liraglutide 3.0 mg vs placebo in patients who had a BMI of at least 30 or who had a BMI of at least 27 plus treated or untreated dyslipidemia or hypertension, Pi-Sunyer et al21 reported a mean weight reduction of 8.4 ± 7.3 kg with liraglutide vs 2.8 ± 6.5 kg with placebo (a difference of −5.6 kg, 95% CI −6.0 to −5.1, P < .001) after 56 weeks. Furthermore, 63.2% of patients in the liraglutide group lost at least 5% of body weight vs 27.1% with placebo, and 33.1% in the liraglutide group lost 10% or more of body weight vs 10.6% in the placebo group (P < .001).21 Of note, liraglutide 3.0 mg is not indicated for type 2 DM per se.
In a 2012 meta-analysis of randomized controlled trials of adults with and without type 2 DM, with a BMI of 25 or greater, and who received GLP-1 receptor agonists at clinically relevant doses (exenatide ≥ 10 μg/day, exenatide ≥ 2 mg/week, or liraglutide ≥ 1.2 mg/day), those taking GLP-1 receptor agonists had more weight loss than those on a control intervention (oral antihyperglycemic, insulin, or placebo) at a minimum of 20 weeks, with a weighted mean difference −2.9 kg (95% CI −3.6 to −2.2) in 21 trials and 6,411 participants.22
GLP-1 agonists currently being investigated for obesity treatment are lixisenatide, albiglutide, taspoglutide, and oxyntomodulin.23
Cardiovascular outcomes
The presence of GLP-1 receptors in blood vessels and myocardium has led to the hypothesis that GLP-1 receptor agonists can improve cardiovascular disease outcomes.24 In the pivotal Liraglutide Effect and Action in Diabetes: Evaluation of Cardiovascular Outcome Results (LEADER) trial, 9,340 patients with type 2 DM and increased cardiovascular disease risk were randomized to liraglutide vs placebo.25 The hazard ratio (HR) for time to the primary end point of cardiovascular death, nonfatal myocardial infarction, or nonfatal stroke was 0.87 (P = .01 for superiority, P < .001 for noninferiority) for liraglutide compared with placebo after 3.8 years. The incidence of death from any cause or cardiovascular cause was also lower with liraglutide.25
Adverse effects
Tolerable transient nausea and vomiting are reported adverse effects; these symptoms occur early in therapy, usually resolve in 4 to 8 weeks, and appear to be associated with greater weight loss.26 Although no causal relationship between GLP-1 receptor agonist use and pancreatitis or pancreatic cancer has been established to date, several cases of acute pancreatitis have been reported.25 Alternative therapies should be considered in patients with a history of or risk factors for pancreatitis.
Combined with insulin
A product that combines insulin glargine and lixisenatide (Soliqua) is FDA-approved for patients with type 2 DM. In a 30-week randomized controlled trial of the combination product vs insulin glargine alone in patients with type 2 DM not controlled on basal insulin with or without up to 2 oral agents, the combination product resulted in an HbA1c reduction from baseline of 1.1% vs 0.6% for insulin glargine alone (P < .001).27 Mean body weight decreased by 0.7 kg with the combination product and increased by 0.7 kg with insulin glargine (P < .001).27 In a 24-week study of a lixisenatide-insulin glargine combination vs insulin glargine in insulin-naïve patients taking metformin, there was a reduction in HbA1c of about −1.7% from baseline in both groups, while the combination group had a 1-kg weight reduction compared with a 0.5-kg weight increase in the insulin glargine group (P < .001).28
SGLT-2 INHIBITORS
Mechanism of action
In a healthy normoglycemic person, about 180 g of glucose per day is filtered into the glomerular filtrate and reabsorbed into the circulation.29 SGLT-2 facilitates the reabsorption of glucose in the proximal convoluted tubule of the kidneys. Approximately 90% of glucose reabsorption is mediated by SGLT-2 found in the S1 and S2 segments of the proximal convoluted tubule, and the remaining 10% by SGLT-1 in the S3 segment. At serum glucose levels above 180 g, the reabsorptive capacity of the nephron is overwhelmed, resulting in glycosuria.30 SGLT-2 expression is also increased in patients with diabetes, thus leading to increased glucose reabsorption into the circulation, further contributing to hyperglycemia.30 Inhibition of SGLT-2 alleviates hyperglycemia by decreasing glucose reabsorption (30% to 50% of filtered glucose) in the kidneys and by increasing excretion (50 mg to 80 mg of glucose) in the urine.31 SGLT-2 inhibitors currently FDA-approved are canagliflozin (Invokana), dapagliflozin (Farxiga), and empagliflozin (Jardiance).
HbA1c
SGLT-2 inhibitors have relatively weak glycemic efficacy. A meta-analysis of SGLT-2 inhibitors vs other antidiabetic medications or placebo found that SGLT-2 inhibitors appeared to have a “favorable effect” on HbA1c, with a mean difference vs placebo of −0.66% (95% CI −0.73% to −0.58%) and a mean difference vs other antihyperglycemic medications of −0.06% (95% CI 0.18% to 0.05%).32
Weight loss
The same meta-analysis found that SGLT-2 inhibitors reduced body weight (mean difference −1.8 kg, 95% CI −3.50 kg to −0.11 kg).32 And in a randomized controlled trial, monotherapy with canagliflozin 100 mg/day and 300 mg/day resulted in body weight reduction of 2.2% (1.9 kg) and 3.3% (−2.9 kg), respectively, after 26 weeks.33 A Japanese study showed a dose-related total body weight loss with empagliflozin vs placebo ranging from 2.5 ± 0.2 kg (5-mg dose) to 3.1 ± 0.2 kg (50-mg dose) after 12 weeks.34 Bolinder et al35 reported that adding dapagliflozin 10 mg to metformin in patients with type 2 DM reduced total body weight by −2.96 kg (95% CI −3.51 to −2.41, P < .001) at week 24. Whole-body dual-energy x-ray absorptiometry and magnetic resonance imaging findings in this study revealed a decrease in fat mass and visceral and subcutaneous adipose tissue after treatment with dapagliflozin, thus suggesting urinary loss of glucose (and hence caloric loss) contributing to weight reduction in addition to initial weight loss from fluid loss due to osmotic diuresis.35 A continuous decline in total body weight was observed in a 78-week extension study resulting in −4.54 kg (95% CI −5.43 to −3.66 kg) at week 102, along with further reduction in total body fat mass as measured by dual-energy x-ray absorptiometry.36
Cardiovascular outcomes
The landmark study Empagliflozin, Cardiovascular Outcomes and Mortality in Type 2 Diabetes (EMPA-REG) involving 7,020 patients was the first large cardiovascular outcomes trial in patients with type 2 DM and overt cardiovascular disease. A relative risk reduction of 14% (12.1% to 10.5%, HR 0.86, 95% CI 0.74 to 0.99) in major adverse cardiovascular events (cardiovascular death, nonfatal myocardial infarction, and nonfatal stroke) was observed with empagliflozin.37 Rates of all-cause mortality and hospitalization for heart failure relative risk reductions were 32% (8.3% to 5.7%; HR 0.68 [0.57, 0.8]) and 35% (4.1% to 2.7%; HR 0.65 [0.50, 0.85]), respectively, with empagliflozin. The mechanism behind this cardiovascular benefit is unknown but is currently being explored.37
Adverse effects
Increased risk of urinary tract and genital infections are known adverse effects of SGLT-2s. Other effects noted include postural hypotension from volume depletion and a transient increase in serum creatinine and decrease in glomerular filtration.29
NEUROENDOCRINE PEPTIDE HORMONE: AMYLIN ANALOGUES
Mechanism of action
Amylin is a 37-amino-acid neuroendocrine peptide hormone secreted primarily by pancreatic beta cells. It promotes early satiety, and its anorexigenic effects are mediated by its action on the neurons of the area postrema in the brain.38 After a meal, amylin decreases gastric acid secretion and slows gastric emptying. It is co-secreted with insulin in a 1:20 amylin-to-insulin ratio and inhibits glucagon secretion via a centrally mediated mechanism.39
Pramlintide (Symlin) is an amylin analogue administered subcutaneously immediately before major meals. It decreases postprandial glucose levels and has been approved by the FDA as an adjunct to prandial insulin in patients with type 1 and type 2 DM.40
HbA1c
Amylin secretion is impaired in type 1 and type 2 DM, and small but significant reductions in HbA1c have been observed with addition of pramlintide to usual insulin regimens. In patients with type 1 DM, HbA1c levels were reduced by 0.4% to 0.6% after 26 weeks on 30 μg 3 times daily to 60 μg 4 times daily of pramlintide added to insulin.41,42 And pramlintide 120 μg added to usual antihyperglycemic therapy in patients with type 2 DM has been reported to decrease HbA1c by 0.7% at week 16 or 26.43,44
Weight loss
A meta-analysis of 8 randomized controlled trials assessed the effects of pramlintide on glycemic control and weight in patients with type 2 DM treated with insulin and in obese patients without diabetes.45 In these trials, patients took at least 120 μg of pramlintide before 2 to 3 meals for at least 12 weeks; a total of 1,616 participants were included. In the type 2 DM group, pramlintide reduced body weight by 2.57 kg (95% CI −3.44 to −1.70 kg, P < .001) vs control, over 16 to 52 weeks.45 The nondiabetic obese group had a weight loss of −2.27 kg (95%CI −2.88 to −1.66 kg, P < .001) vs control.45
Pramlintide and a pramlintide-phentermine combination are currently under investigation for treatment of obesity.23
Cardiovascular outcomes
Cardiovascular outcomes in patients treated with pramlintide have not been studied to date, but reductions have been observed in markers of cardiovascular risk including high-sensitivity C-reactive protein and triglycerides.46
Adverse effects
Transient mild-to-moderate nausea is the most common adverse effect of pramlintide. Hypoglycemia has also been reported, more frequently in patients with type 1 DM, which is possibly associated with inadequate reduction in insulin.
ALPHA-GLUCOSIDASE INHIBITORS
Mechanism of action
Alpha-glucosidase inhibitors competitively inhibit the alpha-glucosidase enzymes at the brush border of the small intestine. Taken orally before meals, these drugs mitigate postprandial hyperglycemia by preventing the breakdown of complex carbohydrates into simpler monosaccharides, thus delaying their absorption.47 These agents may be used as monotherapy or in combination with other antihyperglycemic agents. They work independently from insulin, although they have been shown to potentiate GLP-1 secretion.48 Acarbose and miglitol are currently approved in the United States. Acarbose has been more extensively studied worldwide.
HbA1c
Alpha-glucosidase inhibitors have been reported to reduce mean HbA1c by 0.8% (95% CI −0.9% to −0.7%), as well as fasting and postprandial glucose, and postprandial insulin levels.49
Weight loss
There is conflicting evidence on whether alpha-glucosidase inhibitor therapy has a neutral or beneficial effect on body weight. A Cochrane meta-analysis observed significant BMI reduction with acarbose, although no effect on body weight was noted,49 whereas in another meta-analysis, body weight was significantly reduced by 0.96 kg (95% CI −1.80 to −0.12 kg) when acarbose was added to metformin.50 A review of pooled data from worldwide post-marketing studies for acarbose reported a weight reduction after 3 months of 0.98 ± 2.11 kg in overweight patients and 1.67 ± 3.02 kg in obese patients.51
Cardiovascular outcomes
In the Study to Prevent Non-Insulin-Dependent Diabetes Mellitus (STOP-NIDDM), when compared with placebo, treatment of patients with impaired glucose tolerance with acarbose significantly reduced the incidence of cardiovascular events (HR 0.51, 95% CI 0.28 to 0.95, P = .03), myocardial infarction (HR 0.09, 95% CI 0.01 to 0.72, P = .02), and newly diagnosed hypertension (HR 0.66, 95% CI 0.49 to 0.89, P = .006).52
Adverse effects
Although mild, gastrointestinal effects of flatulence and diarrhea can be bothersome and result in discontinuation of the drug in most patients.
METFORMIN
Mechanism of action
Metformin is the first-line antihyperglycemic agent for type 2 DM recommended by the American Diabetes Association and European Association for the Study of Diabetes.53,54 The main action of metformin is to decrease glucose production in the liver. In the small intestine, metformin stimulates the L cells to produce GLP-1, and in skeletal muscle, it increases glucose uptake and disposal.55
HbA1c
As monotherapy, metformin has resulted in HbA1c reductions of 0.88% to 1.2%.55
Weight loss
Reduced food intake56,57 and gastrointestinal intolerance58 occurring early in therapy have been noted to account for weight loss in short-term studies of non-diabetic obese patients treated with metformin.59 Long-term trials of patients with and without diabetes have yielded mixed results on weight reduction from metformin as monotherapy or adjunct therapy. In the United Kingdom Prospective Diabetes Study (UKPDS), metformin had resulted in approximately 1.5 kg of weight gain (slightly less than the 4-kg weight gain in the glibenclamide group).60 Improved antihyperglycemic efficacy of other antihyperglycemic agents (insulin, sulfonylureas, and thiazolidinediones) with addition of metformin led to dose-lowering of the antihyperglycemic agents, ultimately resulting in amelioration of weight gain; this has also led to small weight reductions in some studies.59 In the Diabetes Prevention Program study of patients with impaired glucose tolerance, metformin treatment resulted in an average weight loss of 2.1 kg compared with placebo (−0.1 kg) and lifestyle intervention (−5.6 kg; P <.001).61
Cardiovascular outcomes
Metformin has been observed to decrease micro- and macrovascular complications. Compared with diet alone, metformin was associated with a 39% reduction in the risk of myocardial infarction, and a 30% lower risk of a composite of macrovascular diseases (myocardial infarction, sudden death, angina, stroke, and peripheral disease).60
Adverse effects
The most common adverse effect of metformin is gastrointestinal intolerance from abdominal pain, flatulence, and diarrhea.62 Metformin-associated lactic acidosis is a serious and potentially life-threatening effect; and vitamin B12 deficiency may occur with long-term treatment.62
TAKE-HOME POINTS
As more medications and interventions are being developed to counter obesity, it also makes sense to select diabetes medications that do not contribute to weight gain in patients who are already overweight or obese. The effects of available medications can be maximized and treatment regimens individualized (based on patients’ needs and preferences, within the limitations of drug costs and side effects), along with lifestyle modification, to target diabesity.
Diabesity was a term coined by Sims et al1 in the 1970s to describe diabetes occurring in the setting of obesity. Today, the link between type 2 diabetes mellitus (DM), obesity, and insulin resistance is well recognized, and 80% of people with type 2 DM are overweight or obese.2,3 Unfortunately, weight gain is a known side effect of most agents used to treat type 2 DM (eg, insulin, sulfonylureas, thiazolidinediones), and this often leads to nonadherence, poor glycemic control, and further weight gain.
During the past several years, evidence has emerged of a neurophysiologic mechanism that involves hormones from adipocytes, pancreatic islet cells, and the gastrointestinal tract implicated in both obesity and diabetes.2 This has led to research for drugs that not only either target obesity and diabetes or reduce hemoglobin A1c (HbA1c), but also have weight loss as a potential side effect.
In this paper, we review medications approved for the treatment of type 2 DM (including pramlintide, also approved for type 1 DM) that also have weight loss as a side effect. Drugs we will discuss include glucagon-like peptide-1 (GLP-1) receptor agonists, sodium-glucose cotransporter-2 (SGLT-2) inhibitors, neuroendocrine peptide hormones, alpha-glucosidase inhibitors, and metformin. Where appropriate, we also comment on the effects of the drugs on cardiovascular outcomes.
GLP-1 RECEPTOR AGONISTS
Mechanism of action
GLP-1 is a hormone produced from the proglucagon gene in the alpha cells of the pancreas, in the L cells of intestinal mucosa (predominantly in the ileum and distal colon), and in structures of the nervous system including the brainstem, hypothalamus, and vagal afferent nerves.4 Food in the gastrointestinal tract, especially if high in fats and carbohydrates, stimulates secretion of GLP-1 in the L cells, which in turn amplifies insulin secretion in a glucose-dependent manner (the incretin effect).4 Glucagon secretion is inhibited by GLP-1 during times of hyperglycemia but not hypoglycemia, thereby preventing inappropriately high levels of the hormone.5 Peripheral GLP-1 activates a cascade of centrally mediated signals that ultimately result in secretion of insulin by the pancreas and slowing of gastrointestinal motility.5 Lastly, GLP-1 exerts an anorexic effect by acting on central pathways that mediate satiation.6
Bioactive forms of GLP-1 are rapidly degraded in the circulation by the dipeptidyl peptidase-4 enzyme. GLP-1 receptor agonists have slightly altered molecular structure and longer duration of action than native GLP-1. Short-acting GLP-1 agonists (eg, exenatide, lixisenatide) have more effect on gastric emptying and lower postprandial blood glucose levels, whereas long-acting GLP-1 agonists (eg, liraglutide, albiglutide, dulaglutide, semaglutide, exenatide) have a greater effect on fasting glucose levels.4
Effects on HbA1c and weight loss
Of the current GLP-1 agonists, exenatide and liraglutide have been on the market the longest, thus studied more in terms of weight reduction.
Exenatide. Exenatide BID was the first GLP-1 agonist, approved by the US Food and Drug Administration (FDA) in 2005 for the treatment of type 2 DM. In a 30-week triple-blind, placebo-controlled study of 336 patients already on background therapy with metformin, progressive weight loss was noted with exenatide 5 μg (−1.6 ± 0.4 kg) and exenatide 10 μg (−2.8 ± 0.5 kg) compared with placebo (−0.3 ± 0.3 kg; P < .001).15 A meta-analysis of 14 trials with 2,583 patients showed significant weight reduction with both exenatide 5 μg twice daily (a difference of −0.56 kg, 95% confidence interval [CI] −1.07 to −0.06, P = .0002) in 8 trials and exenatide 10 μg twice daily (a difference of −1.24 kg, 95% CI −1.69 to −0.78, P < .001) in 12 trials, after treatment for more than 16 weeks.16
Liraglutide. Liraglutide has a longer half-life than exenatide and is administered once daily. It is not a first-line therapy for type 2 DM and is recommended as an add-on. Approved daily doses for type 2 DM are 1.2 mg and 1.8 mg.
Multiple studies of glycemic control and weight loss with liraglutide have been conducted since its introduction to the US market in 2010. In the Liraglutide Effect and Action in Diabetes (LEAD) series of trials, liraglutide use as monotherapy or in combination with oral agents was associated with significant dose-dependent weight loss.17 Liraglutide monotherapy (at 1.2 mg and 1.8 mg) compared with glimepiride in the LEAD-3 trial led to significant weight reduction (2.1 kg and 2.5 kg, respectively, P < .001) after 16 weeks, and was sustained up to 52 weeks.18 Addition of liraglutide (at 1.2 mg and 1.8 mg) to metformin plus rosiglitazone resulted in significant weight loss (1.02 kg and 2.02 kg, respectively) whereas the addition of placebo caused a 0.6-kg weight gain (P < .001).19 The SCALE study randomized 846 adults with type 2 DM who were overweight to obese (body mass index [BMI] ≥ 27 kg/m2), were taking 0 to 3 oral antihyperglycemic agents (metformin, thiazolidinedione, and a sulfonylurea), and had stable body weight and an HbA1c of 7% to 10% to liraglutide 1.8 mg, liraglutide 3.0 mg, or placebo. Mean weight loss after 56 weeks was 6.0% (6.4 kg) with liraglutide 1.8 mg, 4.7% (5.0 kg) with liraglutide 3.0 mg, and 2.0% (2.2 kg) with placebo.20
In 2016, high-dose once-daily liraglutide 3.0 mg (Saxenda) was approved by the FDA for weight loss. In a double-blind randomized trial of liraglutide 3.0 mg vs placebo in patients who had a BMI of at least 30 or who had a BMI of at least 27 plus treated or untreated dyslipidemia or hypertension, Pi-Sunyer et al21 reported a mean weight reduction of 8.4 ± 7.3 kg with liraglutide vs 2.8 ± 6.5 kg with placebo (a difference of −5.6 kg, 95% CI −6.0 to −5.1, P < .001) after 56 weeks. Furthermore, 63.2% of patients in the liraglutide group lost at least 5% of body weight vs 27.1% with placebo, and 33.1% in the liraglutide group lost 10% or more of body weight vs 10.6% in the placebo group (P < .001).21 Of note, liraglutide 3.0 mg is not indicated for type 2 DM per se.
In a 2012 meta-analysis of randomized controlled trials of adults with and without type 2 DM, with a BMI of 25 or greater, and who received GLP-1 receptor agonists at clinically relevant doses (exenatide ≥ 10 μg/day, exenatide ≥ 2 mg/week, or liraglutide ≥ 1.2 mg/day), those taking GLP-1 receptor agonists had more weight loss than those on a control intervention (oral antihyperglycemic, insulin, or placebo) at a minimum of 20 weeks, with a weighted mean difference −2.9 kg (95% CI −3.6 to −2.2) in 21 trials and 6,411 participants.22
GLP-1 agonists currently being investigated for obesity treatment are lixisenatide, albiglutide, taspoglutide, and oxyntomodulin.23
Cardiovascular outcomes
The presence of GLP-1 receptors in blood vessels and myocardium has led to the hypothesis that GLP-1 receptor agonists can improve cardiovascular disease outcomes.24 In the pivotal Liraglutide Effect and Action in Diabetes: Evaluation of Cardiovascular Outcome Results (LEADER) trial, 9,340 patients with type 2 DM and increased cardiovascular disease risk were randomized to liraglutide vs placebo.25 The hazard ratio (HR) for time to the primary end point of cardiovascular death, nonfatal myocardial infarction, or nonfatal stroke was 0.87 (P = .01 for superiority, P < .001 for noninferiority) for liraglutide compared with placebo after 3.8 years. The incidence of death from any cause or cardiovascular cause was also lower with liraglutide.25
Adverse effects
Tolerable transient nausea and vomiting are reported adverse effects; these symptoms occur early in therapy, usually resolve in 4 to 8 weeks, and appear to be associated with greater weight loss.26 Although no causal relationship between GLP-1 receptor agonist use and pancreatitis or pancreatic cancer has been established to date, several cases of acute pancreatitis have been reported.25 Alternative therapies should be considered in patients with a history of or risk factors for pancreatitis.
Combined with insulin
A product that combines insulin glargine and lixisenatide (Soliqua) is FDA-approved for patients with type 2 DM. In a 30-week randomized controlled trial of the combination product vs insulin glargine alone in patients with type 2 DM not controlled on basal insulin with or without up to 2 oral agents, the combination product resulted in an HbA1c reduction from baseline of 1.1% vs 0.6% for insulin glargine alone (P < .001).27 Mean body weight decreased by 0.7 kg with the combination product and increased by 0.7 kg with insulin glargine (P < .001).27 In a 24-week study of a lixisenatide-insulin glargine combination vs insulin glargine in insulin-naïve patients taking metformin, there was a reduction in HbA1c of about −1.7% from baseline in both groups, while the combination group had a 1-kg weight reduction compared with a 0.5-kg weight increase in the insulin glargine group (P < .001).28
SGLT-2 INHIBITORS
Mechanism of action
In a healthy normoglycemic person, about 180 g of glucose per day is filtered into the glomerular filtrate and reabsorbed into the circulation.29 SGLT-2 facilitates the reabsorption of glucose in the proximal convoluted tubule of the kidneys. Approximately 90% of glucose reabsorption is mediated by SGLT-2 found in the S1 and S2 segments of the proximal convoluted tubule, and the remaining 10% by SGLT-1 in the S3 segment. At serum glucose levels above 180 g, the reabsorptive capacity of the nephron is overwhelmed, resulting in glycosuria.30 SGLT-2 expression is also increased in patients with diabetes, thus leading to increased glucose reabsorption into the circulation, further contributing to hyperglycemia.30 Inhibition of SGLT-2 alleviates hyperglycemia by decreasing glucose reabsorption (30% to 50% of filtered glucose) in the kidneys and by increasing excretion (50 mg to 80 mg of glucose) in the urine.31 SGLT-2 inhibitors currently FDA-approved are canagliflozin (Invokana), dapagliflozin (Farxiga), and empagliflozin (Jardiance).
HbA1c
SGLT-2 inhibitors have relatively weak glycemic efficacy. A meta-analysis of SGLT-2 inhibitors vs other antidiabetic medications or placebo found that SGLT-2 inhibitors appeared to have a “favorable effect” on HbA1c, with a mean difference vs placebo of −0.66% (95% CI −0.73% to −0.58%) and a mean difference vs other antihyperglycemic medications of −0.06% (95% CI 0.18% to 0.05%).32
Weight loss
The same meta-analysis found that SGLT-2 inhibitors reduced body weight (mean difference −1.8 kg, 95% CI −3.50 kg to −0.11 kg).32 And in a randomized controlled trial, monotherapy with canagliflozin 100 mg/day and 300 mg/day resulted in body weight reduction of 2.2% (1.9 kg) and 3.3% (−2.9 kg), respectively, after 26 weeks.33 A Japanese study showed a dose-related total body weight loss with empagliflozin vs placebo ranging from 2.5 ± 0.2 kg (5-mg dose) to 3.1 ± 0.2 kg (50-mg dose) after 12 weeks.34 Bolinder et al35 reported that adding dapagliflozin 10 mg to metformin in patients with type 2 DM reduced total body weight by −2.96 kg (95% CI −3.51 to −2.41, P < .001) at week 24. Whole-body dual-energy x-ray absorptiometry and magnetic resonance imaging findings in this study revealed a decrease in fat mass and visceral and subcutaneous adipose tissue after treatment with dapagliflozin, thus suggesting urinary loss of glucose (and hence caloric loss) contributing to weight reduction in addition to initial weight loss from fluid loss due to osmotic diuresis.35 A continuous decline in total body weight was observed in a 78-week extension study resulting in −4.54 kg (95% CI −5.43 to −3.66 kg) at week 102, along with further reduction in total body fat mass as measured by dual-energy x-ray absorptiometry.36
Cardiovascular outcomes
The landmark study Empagliflozin, Cardiovascular Outcomes and Mortality in Type 2 Diabetes (EMPA-REG) involving 7,020 patients was the first large cardiovascular outcomes trial in patients with type 2 DM and overt cardiovascular disease. A relative risk reduction of 14% (12.1% to 10.5%, HR 0.86, 95% CI 0.74 to 0.99) in major adverse cardiovascular events (cardiovascular death, nonfatal myocardial infarction, and nonfatal stroke) was observed with empagliflozin.37 Rates of all-cause mortality and hospitalization for heart failure relative risk reductions were 32% (8.3% to 5.7%; HR 0.68 [0.57, 0.8]) and 35% (4.1% to 2.7%; HR 0.65 [0.50, 0.85]), respectively, with empagliflozin. The mechanism behind this cardiovascular benefit is unknown but is currently being explored.37
Adverse effects
Increased risk of urinary tract and genital infections are known adverse effects of SGLT-2s. Other effects noted include postural hypotension from volume depletion and a transient increase in serum creatinine and decrease in glomerular filtration.29
NEUROENDOCRINE PEPTIDE HORMONE: AMYLIN ANALOGUES
Mechanism of action
Amylin is a 37-amino-acid neuroendocrine peptide hormone secreted primarily by pancreatic beta cells. It promotes early satiety, and its anorexigenic effects are mediated by its action on the neurons of the area postrema in the brain.38 After a meal, amylin decreases gastric acid secretion and slows gastric emptying. It is co-secreted with insulin in a 1:20 amylin-to-insulin ratio and inhibits glucagon secretion via a centrally mediated mechanism.39
Pramlintide (Symlin) is an amylin analogue administered subcutaneously immediately before major meals. It decreases postprandial glucose levels and has been approved by the FDA as an adjunct to prandial insulin in patients with type 1 and type 2 DM.40
HbA1c
Amylin secretion is impaired in type 1 and type 2 DM, and small but significant reductions in HbA1c have been observed with addition of pramlintide to usual insulin regimens. In patients with type 1 DM, HbA1c levels were reduced by 0.4% to 0.6% after 26 weeks on 30 μg 3 times daily to 60 μg 4 times daily of pramlintide added to insulin.41,42 And pramlintide 120 μg added to usual antihyperglycemic therapy in patients with type 2 DM has been reported to decrease HbA1c by 0.7% at week 16 or 26.43,44
Weight loss
A meta-analysis of 8 randomized controlled trials assessed the effects of pramlintide on glycemic control and weight in patients with type 2 DM treated with insulin and in obese patients without diabetes.45 In these trials, patients took at least 120 μg of pramlintide before 2 to 3 meals for at least 12 weeks; a total of 1,616 participants were included. In the type 2 DM group, pramlintide reduced body weight by 2.57 kg (95% CI −3.44 to −1.70 kg, P < .001) vs control, over 16 to 52 weeks.45 The nondiabetic obese group had a weight loss of −2.27 kg (95%CI −2.88 to −1.66 kg, P < .001) vs control.45
Pramlintide and a pramlintide-phentermine combination are currently under investigation for treatment of obesity.23
Cardiovascular outcomes
Cardiovascular outcomes in patients treated with pramlintide have not been studied to date, but reductions have been observed in markers of cardiovascular risk including high-sensitivity C-reactive protein and triglycerides.46
Adverse effects
Transient mild-to-moderate nausea is the most common adverse effect of pramlintide. Hypoglycemia has also been reported, more frequently in patients with type 1 DM, which is possibly associated with inadequate reduction in insulin.
ALPHA-GLUCOSIDASE INHIBITORS
Mechanism of action
Alpha-glucosidase inhibitors competitively inhibit the alpha-glucosidase enzymes at the brush border of the small intestine. Taken orally before meals, these drugs mitigate postprandial hyperglycemia by preventing the breakdown of complex carbohydrates into simpler monosaccharides, thus delaying their absorption.47 These agents may be used as monotherapy or in combination with other antihyperglycemic agents. They work independently from insulin, although they have been shown to potentiate GLP-1 secretion.48 Acarbose and miglitol are currently approved in the United States. Acarbose has been more extensively studied worldwide.
HbA1c
Alpha-glucosidase inhibitors have been reported to reduce mean HbA1c by 0.8% (95% CI −0.9% to −0.7%), as well as fasting and postprandial glucose, and postprandial insulin levels.49
Weight loss
There is conflicting evidence on whether alpha-glucosidase inhibitor therapy has a neutral or beneficial effect on body weight. A Cochrane meta-analysis observed significant BMI reduction with acarbose, although no effect on body weight was noted,49 whereas in another meta-analysis, body weight was significantly reduced by 0.96 kg (95% CI −1.80 to −0.12 kg) when acarbose was added to metformin.50 A review of pooled data from worldwide post-marketing studies for acarbose reported a weight reduction after 3 months of 0.98 ± 2.11 kg in overweight patients and 1.67 ± 3.02 kg in obese patients.51
Cardiovascular outcomes
In the Study to Prevent Non-Insulin-Dependent Diabetes Mellitus (STOP-NIDDM), when compared with placebo, treatment of patients with impaired glucose tolerance with acarbose significantly reduced the incidence of cardiovascular events (HR 0.51, 95% CI 0.28 to 0.95, P = .03), myocardial infarction (HR 0.09, 95% CI 0.01 to 0.72, P = .02), and newly diagnosed hypertension (HR 0.66, 95% CI 0.49 to 0.89, P = .006).52
Adverse effects
Although mild, gastrointestinal effects of flatulence and diarrhea can be bothersome and result in discontinuation of the drug in most patients.
METFORMIN
Mechanism of action
Metformin is the first-line antihyperglycemic agent for type 2 DM recommended by the American Diabetes Association and European Association for the Study of Diabetes.53,54 The main action of metformin is to decrease glucose production in the liver. In the small intestine, metformin stimulates the L cells to produce GLP-1, and in skeletal muscle, it increases glucose uptake and disposal.55
HbA1c
As monotherapy, metformin has resulted in HbA1c reductions of 0.88% to 1.2%.55
Weight loss
Reduced food intake56,57 and gastrointestinal intolerance58 occurring early in therapy have been noted to account for weight loss in short-term studies of non-diabetic obese patients treated with metformin.59 Long-term trials of patients with and without diabetes have yielded mixed results on weight reduction from metformin as monotherapy or adjunct therapy. In the United Kingdom Prospective Diabetes Study (UKPDS), metformin had resulted in approximately 1.5 kg of weight gain (slightly less than the 4-kg weight gain in the glibenclamide group).60 Improved antihyperglycemic efficacy of other antihyperglycemic agents (insulin, sulfonylureas, and thiazolidinediones) with addition of metformin led to dose-lowering of the antihyperglycemic agents, ultimately resulting in amelioration of weight gain; this has also led to small weight reductions in some studies.59 In the Diabetes Prevention Program study of patients with impaired glucose tolerance, metformin treatment resulted in an average weight loss of 2.1 kg compared with placebo (−0.1 kg) and lifestyle intervention (−5.6 kg; P <.001).61
Cardiovascular outcomes
Metformin has been observed to decrease micro- and macrovascular complications. Compared with diet alone, metformin was associated with a 39% reduction in the risk of myocardial infarction, and a 30% lower risk of a composite of macrovascular diseases (myocardial infarction, sudden death, angina, stroke, and peripheral disease).60
Adverse effects
The most common adverse effect of metformin is gastrointestinal intolerance from abdominal pain, flatulence, and diarrhea.62 Metformin-associated lactic acidosis is a serious and potentially life-threatening effect; and vitamin B12 deficiency may occur with long-term treatment.62
TAKE-HOME POINTS
As more medications and interventions are being developed to counter obesity, it also makes sense to select diabetes medications that do not contribute to weight gain in patients who are already overweight or obese. The effects of available medications can be maximized and treatment regimens individualized (based on patients’ needs and preferences, within the limitations of drug costs and side effects), along with lifestyle modification, to target diabesity.
- Sims EAH, Danforth E Jr, Horton ES, Bray GA, Glennon JA, Salans LB. Endocrine and metabolic effects of experimental obesity in man. Recent Prog Horm Res 1973; 29:457–496.
- Scheen AJ, Van Gaal LF. Combating the dual burden: therapeutic targeting of common pathways in obesity and type 2 diabetes. Lancet Diabetes Endocrinol 2014; 2:911–922.
- Kahn SE, Hull RL, Utzschneider KM. Mechanisms linking obesity to insulin resistance and type 2 diabetes. Nature 2006; 444:840–846.
- Iepsen EW, Torekov SS, Holst JJ. Therapies for inter-relating diabetes and obesity—GLP-1 and obesity. Expert Opin Pharmacother 2014; 15:2487–2500.
- Meier JJ, Nauck MA. Glucagon-like peptide 1(GLP-1) in biology and pathology. Diabetes Metab Res Rev 2005; 21:91–117.
- Turton MD, O’Shea D, Gunn I, et al. A role for glucagon-like peptide-1 in the central regulation of feeding. Nature 1996; 379:69–72.
- Drucker DJ. Glucagon-like peptides: regulators of cell proliferation, differentiation, and apoptosis. Mol Endocrinol 2003; 17:161–171.
- Shyangdan DS, Royle P, Clar C, Sharma P, Waugh N, Snaith A. Glucagon-like peptide analogues for type 2 diabetes mellitus. Cochrane Database Syst Rev 2011; 10:CD006423. doi:10.1002/14651858.CD006423.pub2.
- Byetta [package insert]. Wilmington, DE: AstraZeneca Pharmaceuticals LP; 2015. Available at http://www.azpicentral.com/byetta/pi_byetta.pdf. Accessed June 22, 2017.
- Victoza [package insert]. Bagsvaerd, Denmark: Novo Nordisk A/S; 2016. Available at http://www.novo-pi.com/victoza.pdf. Accessed June 22, 2017.
- Bydureon [package insert]. Wilmington, DE: AstraZeneca Pharmaceuticals LP; 2015. Available at http://www.azpicentral.com/bydureon/pi_bydureon.pdf. Accessed June 22, 2017.
- Tanzeum [package insert]. Wilmington, DE: GlaxoSmithKline; 2016. Available at https://www.gsksource.com/pharma/content/dam/GlaxoSmithKline/US/en/Prescribing_Information/Tanzeum/pdf/TANZEUM-PI-MG-IFU-COMBINED.PDF. Accessed June 22, 2017.
- Trulicity [package insert]. Indianapolis, IN: Eli Lilly and Company 2014. Available at http://pi.lilly.com/us/trulicity-uspi.pdf. Accessed June 22, 2017.
- Adlyxin [package insert]. Bridgewater, NJ: Sanofi-Aventis U.S. LLC; 2016. Available at http://products.sanofi.us/adlyxin/adlyxin.pdf. Accessed June 22, 2017.
- DeFronzo RA, Ratner RE, Han J, Kim DD, Fineman MS, Baron AD. Effects of exenatide (exendin-4) on glycemic control and weight over 30 weeks in metformin-treated patients with type 2 diabetes. Diabetes Care 2005; 28:1092–1100.
- Nikfar S, Abdollahi M, Salari P. The efficacy and tolerability of exenatide in comparison to placebo; a systematic review and meta-analysis of randomized clinical trials. J Pharm Pharm Sci 2012; 15:1–30.
- Blonde L, Russell-Jones D. The safety and efficacy of liraglutide with or without oral antidiabetic drug therapy in type 2 diabetes: an overview of the LEAD 1-5 studies. Diabetes Obes Metab 2009; 11(suppl 3):26–34.
- Garber A, Henry R, Ratner R, et al; LEAD-3 (Mono) Study Group. Liraglutide versus glimepiride monotherapy for type 2 diabetes (LEAD-3 Mono): a randomised, 52-week, phase III, double-blind, parallel-treatment trial. Lancet 2009; 373:473–481.
- Zinman B, Gerich J, Buse JB, et al; LEAD-4 Study Investigators. Efficacy and safety of the human glucagon-like peptide-1 analog liraglutide in combination with metformin and thiazolidinedione in patients with type 2 diabetes (LEAD-4 Met+TZD). Diabetes Care 2009; 32:1224–1230.
- Davies MJ, Bergenstal R, Bode B, et al. Efficacy of liraglutide for weight loss among patients with type 2 diabetes: the SCALE Diabetes Randomized Clinical Trial. JAMA 2015; 314:687–699.
- Pi-Sunyer X, Astrup A, Fujioka K, et al; SCALE Obesity and Prediabetes NN8022-1839 Study Group. A randomized, controlled trial of 3.0 mg of liraglutide in weight management. N Engl J Med 2015; 373:11–22.
- Vilsboll T, Christensen M, Junker AE, Knop FK, Gluud LL. Effects of glucagon-like peptide-1 receptor agonists on weight loss: systematic review and meta-analyses of randomised controlled trials. BMJ 2012; 344:d7771.
- Valsamakis G, Konstantakou P, Mastorakos G. New targets for drug treatment of obesity. Annu Rev Pharmacol Toxicol 2017; 57:585–605.
- Sivertsen J, Rosenmeier J, Holst JJ, Vilsboll T. The effect of glucagon-like peptide 1 on cardiovascular risk. Nat Rev Cardiol 2012; 9:209–222.
- Marso SP, Daniels GH, Brown-Frandsen K, et al; LEADER Steering Committee; LEADER Trial Investigators. Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med 2016; 375:311–322.
- Lean ME, Carraro R, Finer N, et al; NN8022-1807 Investigators. Tolerability of nausea and vomiting and associations with weight loss in a randomized trial of liraglutide in obese, non-diabetic adults. Int J Obes (Lond) 2014; 38:689–697.
- Aroda VR, Rosenstock J, Wysham C, et al; LixiLan-L Trial Investigators. Efficacy and safety of lixilan, a titratable fixed-ratio combination of insulin glargine plus lixisenatide in type 2 diabetes inadequately controlled on basal insulin and metformin: the LixiLan-L Randomized Trial. Diabetes Care 2016; 39:1972–1980.
- Rosenstock J, Diamant M, Aroda VR, et al; LixiLan PoC Study Group. Efficacy and safety of LixiLan, a titratable fixed-ratio combination of lixisenatide and insulin glargine, versus insulin glargine in type 2 diabetes inadequately controlled on metformin monotherapy: the LixiLan Proof-of-Concept Randomized Trial. Diabetes Care 2016; 39:1579–1586.
- Monica Reddy RP, Inzucchi SE. SGLT2 inhibitors in the management of type 2 diabetes. Endocrine 2016; 53:364–372.
- DeFronzo RA, Davidson JA, Del Prato S. The role of the kidneys in glucose homeostasis: a new path towards normalizing glycaemia. Diabetes Obes Metab 2012; 14:5–14.
- Liu JJ, Lee T, DeFronzo RA. Why do SGLT2 inhibitors inhibit only 30-50% of renal glucose reabsorption in humans? Diabetes 2012; 61:2199–2204.
- Vasilakou D, Karagiannis T, Athanasiadou E, et al. Sodium-glucose cotransporter 2 inhibitors for type 2 diabetes: a systematic review and meta-analysis. Ann Intern Med 2013; 159:262–274.
- Stenlof K, Cefalu WT, Kim KA, et al. Efficacy and safety of canagliflozin monotherapy in subjects with type 2 diabetes mellitus inadequately controlled with diet and exercise. Diabetes Obes Metab 2013; 15:372–382.
- Kadowaki T, Haneda M, Inagaki N, et al. Empagliflozin monotherapy in Japanese patients with type 2 diabetes mellitus: a randomized, 12-week, double-blind, placebo-controlled, phase II trial. Adv Ther 2014; 31:621–638.
- Bolinder J, Ljunggren O, Kullberg J, et al. Effects of dapagliflozin on body weight, total fat mass, and regional adipose tissue distribution in patients with type 2 diabetes mellitus with inadequate glycemic control on metformin. J Clin Endocrinol Metab 2012; 97:1020–1031.
- Bolinder J, Ljunggren O, Johansson L, et al. Dapagliflozin maintains glycaemic control while reducing weight and body fat mass over 2 years in patients with type 2 diabetes mellitus inadequately controlled on metformin. Diabetes Obes Metab 2014; 16:159–169.
- Zinman B, Wanner C, Lachin JM, et al; EMPA-REG OUTCOME Investigators. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med 2015; 373:2117–2128.
- Lutz TA. Effects of amylin on eating and adiposity. Handb Exp Pharmacol 2012; (209):231–250.
- Hieronymus L, Griffin S. Role of amylin in type 1 and type 2 diabetes. Diabetes Educ 2015; 41(suppl 1):47S–56S.
- Aronoff SL. Rationale for treatment options for mealtime glucose control in patients with type 2 diabetes. Postgrad Med 2017; 129:231–241.
- Ratner RE, Dickey R, Fineman M, et al. Amylin replacement with pramlintide as an adjunct to insulin therapy improves long-term glycaemic and weight control in type 1 diabetes mellitus: a 1-year, randomized controlled trial. Diabet Med 2004; 21:1204–1212.
- Edelman S, Garg S, Frias J, et al. A double-blind, placebo-controlled trial assessing pramlintide treatment in the setting of intensive insulin therapy in type 1 diabetes. Diabetes Care 2006; 29:2189–2195.
- Riddle M, Frias J, Zhang B, et al. Pramlintide improved glycemic control and reduced weight in patients with type 2 diabetes using basal insulin. Diabetes Care 2007; 30:2794–2799.
- Hollander PA, Levy P, Fineman MS, et al. Pramlintide as an adjunct to insulin therapy improves long-term glycemic and weight control in patients with type 2 diabetes: a 1-year randomized controlled trial. Diabetes Care 2003; 26:784–790.
- Singh-Franco D, Perez A, Harrington C. The effect of pramlintide acetate on glycemic control and weight in patients with type 2 diabetes mellitus and in obese patients without diabetes: a systematic review and meta-analysis. Diabetes Obes Metab 2011; 13:169–180.
- Wysham C, Lush C, Zhang B, Maier H, Wilhelm K. Effect of pramlintide as an adjunct to basal insulin on markers of cardiovascular risk in patients with type 2 diabetes. Curr Med Res Opin 2008; 24:79–85.
- Bischoff H. Pharmacology of alpha-glucosidase inhibition. Eur J Clin Invest 1994; 24(suppl 3):3–10.
- Lee A, Patrick P, Wishart J, Horowitz M, Morley JE. The effects of miglitol on glucagon-like peptide-1 secretion and appetite sensations in obese type 2 diabetics. Diabetes Obes Metab 2002; 4:329–335.
- van de Laar FA, Lucassen PL, Akkermans RP, van de Lisdonk EH, Rutten GE, van Weel C. Alpha-glucosidase inhibitors for patients with type 2 diabetes: results from a Cochrane systematic review and meta-analysis. Diabetes Care 2005; 28:154–163.
- Gross JL, Kramer CK, Leitao CB, et al; Diabetes and Endocrinology Meta-analysis Group (DEMA). Effect of antihyperglycemic agents added to metformin and a sulfonylurea on glycemic control and weight gain in type 2 diabetes: a network meta-analysis. Ann Intern Med 2011; 154:672–679.
- Schnell O, Weng J, Sheu WH, et al. Acarbose reduces body weight irrespective of glycemic control in patients with diabetes: results of a worldwide, non-interventional, observational study data pool. J Diabetes Complications 2016; 30:628–637.
- Chiasson JL, Josse RG, Gomis R, Hanefeld M, Karasik A, Laakso M; STOP-NIDDM Trial Research Group. Acarbose treatment and the risk of cardiovascular disease and hypertension in patients with impaired glucose tolerance: the STOP-NIDDM trial. JAMA 2003; 290:486–494.
- Inzucchi SE, Bergenstal RM, Buse JB, et al. Management of hyperglycemia in type 2 diabetes, 2015: a patient-centered approach: update to a position statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care 2015; 38:140–149.
- American Diabetes Association. Pharmacologic approaches to glycemic treatment. Diabetes Care 2017; 40:S64–S74.
- Tan MH, Alquraini H, Mizokami-Stout K, MacEachern M. Metformin: From research to clinical practice. Endocrinol Metab Clin North Am 2016; 45:819–843.
- Paolisso G, Amato L, Eccellente R, et al. Effect of metformin on food intake in obese subjects. Eur J Clin Invest 1998; 28: 441–446.
- Lee A, Morley JE. Metformin decreases food consumption and induces weight loss in subjects with obesity with type II noninsulin-dependent diabetes. Obes Res 1998; 6: 47–53.
- Scarpello JH. Optimal dosing strategies for maximising the clinical response to metformin in type 2 diabetes. Br J Diabetes Vasc Dis 2001; 1: 28–36.
- Golay A. Metformin and body weight. Int J Obes (Lond) 2008; 32:61–72.
- UK Prospective Diabetes Study (UKPDS) Group. Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34). Lancet 1998; 352:854–865.
- Knowler WC, Barrett-Connor E, Fowler SE, et al; Diabetes Prevention Program Research Group. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med 2002; 346:393–403.
- Fujita Y, Inagaki N. Metformin: new preparations and nonglycemic benefits. Curr Diab Rep 2017; 17:5.
- Sims EAH, Danforth E Jr, Horton ES, Bray GA, Glennon JA, Salans LB. Endocrine and metabolic effects of experimental obesity in man. Recent Prog Horm Res 1973; 29:457–496.
- Scheen AJ, Van Gaal LF. Combating the dual burden: therapeutic targeting of common pathways in obesity and type 2 diabetes. Lancet Diabetes Endocrinol 2014; 2:911–922.
- Kahn SE, Hull RL, Utzschneider KM. Mechanisms linking obesity to insulin resistance and type 2 diabetes. Nature 2006; 444:840–846.
- Iepsen EW, Torekov SS, Holst JJ. Therapies for inter-relating diabetes and obesity—GLP-1 and obesity. Expert Opin Pharmacother 2014; 15:2487–2500.
- Meier JJ, Nauck MA. Glucagon-like peptide 1(GLP-1) in biology and pathology. Diabetes Metab Res Rev 2005; 21:91–117.
- Turton MD, O’Shea D, Gunn I, et al. A role for glucagon-like peptide-1 in the central regulation of feeding. Nature 1996; 379:69–72.
- Drucker DJ. Glucagon-like peptides: regulators of cell proliferation, differentiation, and apoptosis. Mol Endocrinol 2003; 17:161–171.
- Shyangdan DS, Royle P, Clar C, Sharma P, Waugh N, Snaith A. Glucagon-like peptide analogues for type 2 diabetes mellitus. Cochrane Database Syst Rev 2011; 10:CD006423. doi:10.1002/14651858.CD006423.pub2.
- Byetta [package insert]. Wilmington, DE: AstraZeneca Pharmaceuticals LP; 2015. Available at http://www.azpicentral.com/byetta/pi_byetta.pdf. Accessed June 22, 2017.
- Victoza [package insert]. Bagsvaerd, Denmark: Novo Nordisk A/S; 2016. Available at http://www.novo-pi.com/victoza.pdf. Accessed June 22, 2017.
- Bydureon [package insert]. Wilmington, DE: AstraZeneca Pharmaceuticals LP; 2015. Available at http://www.azpicentral.com/bydureon/pi_bydureon.pdf. Accessed June 22, 2017.
- Tanzeum [package insert]. Wilmington, DE: GlaxoSmithKline; 2016. Available at https://www.gsksource.com/pharma/content/dam/GlaxoSmithKline/US/en/Prescribing_Information/Tanzeum/pdf/TANZEUM-PI-MG-IFU-COMBINED.PDF. Accessed June 22, 2017.
- Trulicity [package insert]. Indianapolis, IN: Eli Lilly and Company 2014. Available at http://pi.lilly.com/us/trulicity-uspi.pdf. Accessed June 22, 2017.
- Adlyxin [package insert]. Bridgewater, NJ: Sanofi-Aventis U.S. LLC; 2016. Available at http://products.sanofi.us/adlyxin/adlyxin.pdf. Accessed June 22, 2017.
- DeFronzo RA, Ratner RE, Han J, Kim DD, Fineman MS, Baron AD. Effects of exenatide (exendin-4) on glycemic control and weight over 30 weeks in metformin-treated patients with type 2 diabetes. Diabetes Care 2005; 28:1092–1100.
- Nikfar S, Abdollahi M, Salari P. The efficacy and tolerability of exenatide in comparison to placebo; a systematic review and meta-analysis of randomized clinical trials. J Pharm Pharm Sci 2012; 15:1–30.
- Blonde L, Russell-Jones D. The safety and efficacy of liraglutide with or without oral antidiabetic drug therapy in type 2 diabetes: an overview of the LEAD 1-5 studies. Diabetes Obes Metab 2009; 11(suppl 3):26–34.
- Garber A, Henry R, Ratner R, et al; LEAD-3 (Mono) Study Group. Liraglutide versus glimepiride monotherapy for type 2 diabetes (LEAD-3 Mono): a randomised, 52-week, phase III, double-blind, parallel-treatment trial. Lancet 2009; 373:473–481.
- Zinman B, Gerich J, Buse JB, et al; LEAD-4 Study Investigators. Efficacy and safety of the human glucagon-like peptide-1 analog liraglutide in combination with metformin and thiazolidinedione in patients with type 2 diabetes (LEAD-4 Met+TZD). Diabetes Care 2009; 32:1224–1230.
- Davies MJ, Bergenstal R, Bode B, et al. Efficacy of liraglutide for weight loss among patients with type 2 diabetes: the SCALE Diabetes Randomized Clinical Trial. JAMA 2015; 314:687–699.
- Pi-Sunyer X, Astrup A, Fujioka K, et al; SCALE Obesity and Prediabetes NN8022-1839 Study Group. A randomized, controlled trial of 3.0 mg of liraglutide in weight management. N Engl J Med 2015; 373:11–22.
- Vilsboll T, Christensen M, Junker AE, Knop FK, Gluud LL. Effects of glucagon-like peptide-1 receptor agonists on weight loss: systematic review and meta-analyses of randomised controlled trials. BMJ 2012; 344:d7771.
- Valsamakis G, Konstantakou P, Mastorakos G. New targets for drug treatment of obesity. Annu Rev Pharmacol Toxicol 2017; 57:585–605.
- Sivertsen J, Rosenmeier J, Holst JJ, Vilsboll T. The effect of glucagon-like peptide 1 on cardiovascular risk. Nat Rev Cardiol 2012; 9:209–222.
- Marso SP, Daniels GH, Brown-Frandsen K, et al; LEADER Steering Committee; LEADER Trial Investigators. Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med 2016; 375:311–322.
- Lean ME, Carraro R, Finer N, et al; NN8022-1807 Investigators. Tolerability of nausea and vomiting and associations with weight loss in a randomized trial of liraglutide in obese, non-diabetic adults. Int J Obes (Lond) 2014; 38:689–697.
- Aroda VR, Rosenstock J, Wysham C, et al; LixiLan-L Trial Investigators. Efficacy and safety of lixilan, a titratable fixed-ratio combination of insulin glargine plus lixisenatide in type 2 diabetes inadequately controlled on basal insulin and metformin: the LixiLan-L Randomized Trial. Diabetes Care 2016; 39:1972–1980.
- Rosenstock J, Diamant M, Aroda VR, et al; LixiLan PoC Study Group. Efficacy and safety of LixiLan, a titratable fixed-ratio combination of lixisenatide and insulin glargine, versus insulin glargine in type 2 diabetes inadequately controlled on metformin monotherapy: the LixiLan Proof-of-Concept Randomized Trial. Diabetes Care 2016; 39:1579–1586.
- Monica Reddy RP, Inzucchi SE. SGLT2 inhibitors in the management of type 2 diabetes. Endocrine 2016; 53:364–372.
- DeFronzo RA, Davidson JA, Del Prato S. The role of the kidneys in glucose homeostasis: a new path towards normalizing glycaemia. Diabetes Obes Metab 2012; 14:5–14.
- Liu JJ, Lee T, DeFronzo RA. Why do SGLT2 inhibitors inhibit only 30-50% of renal glucose reabsorption in humans? Diabetes 2012; 61:2199–2204.
- Vasilakou D, Karagiannis T, Athanasiadou E, et al. Sodium-glucose cotransporter 2 inhibitors for type 2 diabetes: a systematic review and meta-analysis. Ann Intern Med 2013; 159:262–274.
- Stenlof K, Cefalu WT, Kim KA, et al. Efficacy and safety of canagliflozin monotherapy in subjects with type 2 diabetes mellitus inadequately controlled with diet and exercise. Diabetes Obes Metab 2013; 15:372–382.
- Kadowaki T, Haneda M, Inagaki N, et al. Empagliflozin monotherapy in Japanese patients with type 2 diabetes mellitus: a randomized, 12-week, double-blind, placebo-controlled, phase II trial. Adv Ther 2014; 31:621–638.
- Bolinder J, Ljunggren O, Kullberg J, et al. Effects of dapagliflozin on body weight, total fat mass, and regional adipose tissue distribution in patients with type 2 diabetes mellitus with inadequate glycemic control on metformin. J Clin Endocrinol Metab 2012; 97:1020–1031.
- Bolinder J, Ljunggren O, Johansson L, et al. Dapagliflozin maintains glycaemic control while reducing weight and body fat mass over 2 years in patients with type 2 diabetes mellitus inadequately controlled on metformin. Diabetes Obes Metab 2014; 16:159–169.
- Zinman B, Wanner C, Lachin JM, et al; EMPA-REG OUTCOME Investigators. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med 2015; 373:2117–2128.
- Lutz TA. Effects of amylin on eating and adiposity. Handb Exp Pharmacol 2012; (209):231–250.
- Hieronymus L, Griffin S. Role of amylin in type 1 and type 2 diabetes. Diabetes Educ 2015; 41(suppl 1):47S–56S.
- Aronoff SL. Rationale for treatment options for mealtime glucose control in patients with type 2 diabetes. Postgrad Med 2017; 129:231–241.
- Ratner RE, Dickey R, Fineman M, et al. Amylin replacement with pramlintide as an adjunct to insulin therapy improves long-term glycaemic and weight control in type 1 diabetes mellitus: a 1-year, randomized controlled trial. Diabet Med 2004; 21:1204–1212.
- Edelman S, Garg S, Frias J, et al. A double-blind, placebo-controlled trial assessing pramlintide treatment in the setting of intensive insulin therapy in type 1 diabetes. Diabetes Care 2006; 29:2189–2195.
- Riddle M, Frias J, Zhang B, et al. Pramlintide improved glycemic control and reduced weight in patients with type 2 diabetes using basal insulin. Diabetes Care 2007; 30:2794–2799.
- Hollander PA, Levy P, Fineman MS, et al. Pramlintide as an adjunct to insulin therapy improves long-term glycemic and weight control in patients with type 2 diabetes: a 1-year randomized controlled trial. Diabetes Care 2003; 26:784–790.
- Singh-Franco D, Perez A, Harrington C. The effect of pramlintide acetate on glycemic control and weight in patients with type 2 diabetes mellitus and in obese patients without diabetes: a systematic review and meta-analysis. Diabetes Obes Metab 2011; 13:169–180.
- Wysham C, Lush C, Zhang B, Maier H, Wilhelm K. Effect of pramlintide as an adjunct to basal insulin on markers of cardiovascular risk in patients with type 2 diabetes. Curr Med Res Opin 2008; 24:79–85.
- Bischoff H. Pharmacology of alpha-glucosidase inhibition. Eur J Clin Invest 1994; 24(suppl 3):3–10.
- Lee A, Patrick P, Wishart J, Horowitz M, Morley JE. The effects of miglitol on glucagon-like peptide-1 secretion and appetite sensations in obese type 2 diabetics. Diabetes Obes Metab 2002; 4:329–335.
- van de Laar FA, Lucassen PL, Akkermans RP, van de Lisdonk EH, Rutten GE, van Weel C. Alpha-glucosidase inhibitors for patients with type 2 diabetes: results from a Cochrane systematic review and meta-analysis. Diabetes Care 2005; 28:154–163.
- Gross JL, Kramer CK, Leitao CB, et al; Diabetes and Endocrinology Meta-analysis Group (DEMA). Effect of antihyperglycemic agents added to metformin and a sulfonylurea on glycemic control and weight gain in type 2 diabetes: a network meta-analysis. Ann Intern Med 2011; 154:672–679.
- Schnell O, Weng J, Sheu WH, et al. Acarbose reduces body weight irrespective of glycemic control in patients with diabetes: results of a worldwide, non-interventional, observational study data pool. J Diabetes Complications 2016; 30:628–637.
- Chiasson JL, Josse RG, Gomis R, Hanefeld M, Karasik A, Laakso M; STOP-NIDDM Trial Research Group. Acarbose treatment and the risk of cardiovascular disease and hypertension in patients with impaired glucose tolerance: the STOP-NIDDM trial. JAMA 2003; 290:486–494.
- Inzucchi SE, Bergenstal RM, Buse JB, et al. Management of hyperglycemia in type 2 diabetes, 2015: a patient-centered approach: update to a position statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care 2015; 38:140–149.
- American Diabetes Association. Pharmacologic approaches to glycemic treatment. Diabetes Care 2017; 40:S64–S74.
- Tan MH, Alquraini H, Mizokami-Stout K, MacEachern M. Metformin: From research to clinical practice. Endocrinol Metab Clin North Am 2016; 45:819–843.
- Paolisso G, Amato L, Eccellente R, et al. Effect of metformin on food intake in obese subjects. Eur J Clin Invest 1998; 28: 441–446.
- Lee A, Morley JE. Metformin decreases food consumption and induces weight loss in subjects with obesity with type II noninsulin-dependent diabetes. Obes Res 1998; 6: 47–53.
- Scarpello JH. Optimal dosing strategies for maximising the clinical response to metformin in type 2 diabetes. Br J Diabetes Vasc Dis 2001; 1: 28–36.
- Golay A. Metformin and body weight. Int J Obes (Lond) 2008; 32:61–72.
- UK Prospective Diabetes Study (UKPDS) Group. Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34). Lancet 1998; 352:854–865.
- Knowler WC, Barrett-Connor E, Fowler SE, et al; Diabetes Prevention Program Research Group. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med 2002; 346:393–403.
- Fujita Y, Inagaki N. Metformin: new preparations and nonglycemic benefits. Curr Diab Rep 2017; 17:5.
KEY POINTS
- The rationale for GLP-1 receptor agonists is that peripheral GLP-1 activates a cascade of centrally mediated signals that ultimately result in secretion of insulin by the pancreas and slowing of gastrointestinal motility. It also exerts an anorexic effect by acting on central pathways that mediate satiation.
- SGLT-2 inhibitors have relatively weak glycemic efficacy. Inhibition of SGLT-2 alleviates hyperglycemia by decreasing glucose reabsorption in the kidneys and by increasing excretion in the urine, suggesting urinary loss of glucose (and hence caloric loss). This is thought to contribute to weight reduction in addition to initial weight loss from fluid loss due to osmotic diuresis.
- Meta-analyses so far have shown that alpha-glucosidase inhibitors have either a neutral or a beneficial effect on body weight.
Antiobesity drugs in the management of type 2 diabetes: A shift in thinking?
Obesity is a leading public health concern, affecting nearly 60 million adult Americans.1 It is a major risk factor for the development of insulin resistance and type 2 diabetes mellitus (DM).2 More than 90% of patients with type 2 DM have obesity, and obesity is a major obstacle to achieving long-term glycemic control.3
Clinical studies have demonstrated that a 6- to 7-kg increase in body weight increases the risk of developing type 2 DM by 50%, while a 5-kg loss reduces the risk by a similar amount.4 As a result, most patients who have a body mass index greater than 40 kg/m2 suffer from type 2 DM.5 Strong evidence exists that bariatric surgery and its resulting weight loss has positive effects on fasting blood sugar, hemoglobin A1c (HbA1c), lipid profiles, and other metabolic variables.6
When combined, obesity and type 2 DM carry a significant burden of micro- and macrovascular complications such as retinopathy, nephropathy, neuropathy, and cardiovascular disease. As a result, a high prevalence of morbidity and mortality is seen among patients with obesity and type 2 DM; those between the ages of 51 and 61 have a 7-times higher mortality rate compared with nonobese normoglycemic people, and patients with diabetes alone have a 2.6-times higher mortality rate.7
A DILEMMA IN THE CLINIC: FOCUS ON THE SUGAR OR THE WEIGHT?
Although type 2 DM and obesity go hand in hand, clinicians tend to focus on the sugar and neglect the weight, concentrating their efforts on improving blood glucose indices, and prescribing in many instances medications that cause weight gain. As a result, we are faced with a rising epidemic of obesity, perpetuating a preexisting epidemic of diabetes.
An optimal, comprehensive approach to managing patients with type 2 DM should encompass both the control of dysglycemia and its associated comorbidities, obesity being the key player.8 However, clinical practice is often misaligned with the evidence. For instance, many of our first-line oral treatments for type 2 DM (except for metformin) are associated with weight gain.9 With time, control of glycemia becomes more and more ineffective, at which point therapy is intensified with insulin, further exacerbating the weight gain.10
Therefore, it seems counterintuitive to treat a disease for which obesity is one of the main risk factors with medications that promote weight gain. Yet healthcare providers are faced with a therapeutic dilemma: should they focus their efforts on improving patients’ glycemic control, or should they invest in helping these patients lose weight? Although an ideal approach would incorporate both aspects, the reality is that it is far from practical.
A few issues impinge on integrating weight loss in the care of type 2 DM. Although the American Medical Association recognized obesity as a disease in 2013,11 some providers still perceive obesity as a self-inflicted condition that is due to bad lifestyle and behavior.11 Many clinicians may also have low expectations for patients’ success, and often lack the time and knowledge to intervene regarding nutrition, physical activity, and psychological issues pertinent to the management of obesity in type 2 DM. Therefore, in many cases, it seems less complicated and more rewarding for both patients and physicians to concentrate on improving the HbA1c value rather than investing efforts in weight loss. For diabetic patients with obesity, this could mean that clinicians may prescribe glucose-lowering therapies, such as insulin and sulfonylureas, at the expense of weight gain. Additionally, clinicians often experience the need to provide recommendations more aligned with metrics that dictate reimbursement (eg, HbA1c targets) within healthcare systems that still raise concerns regarding obesity visit reimbursements.
Lastly, the lack of trustworthy or pertinent evidence (lack of comparative effectiveness research) for antiobesity medications may limit their use in daily practice. Physicians have had little confidence in the efficacy of antiobesity drugs, and often raise significant safety concerns, especially after witnessing important fiascos in this field, eg, dexfenfluramine, rimonabant, and sibutramine.2,12,13
As a result, many of our patients with obesity and type 2 DM may not consider the need for weight loss, and may not even be aware that type 2 DM is caused by obesity and physical inactivity in the first place. Others have accumulated a significant degree of frustration, and have “thrown in the towel” already after unsuccessful weight-loss efforts, many of which were not medically supervised.
For all of the above reasons, both clinicians and patients often concentrate their efforts on treating blood glucose numbers rather than the “obesity-diabetes” as a whole.14 And as a result, our practices are slowly filling up with patients with obesity and type 2 DM who are treated primarily with insulin, resulting in a progressive (and untreated) obesity and diabetes epidemic.
DRUGS FOR TREATING OBESITY AND TYPE 2 DM
Orlistat
Orlistat (Xenical) is the only weight-loss drug approved by the US Food and Drug Administration (FDA) that acts outside the brain. It inhibits pancreatic lipases, resulting in up to 30% less fat absorption in the gut. Orlistat has been approved for long-term use by the FDA.
Benefits. In the XENical in the Prevention of Diabetes in Obese Subjects study, treatment with orlistat resulted in a significant reduction in the cumulative incidence of type 2 DM after 4 years of treatment (9.0% with placebo vs 6.2% with orlistat), corresponding to a risk reduction of 37.3%.16 Mean weight loss after 4 years was significantly greater in the orlistat group (5.8 vs 3.0 kg with placebo; P < .001).16 Other benefits of orlistat included a reduction in low-density lipoprotein cholesterol independent of that expected from change in body weight.16
Adverse effects include flatulence with discharge and fecal urgency after high-fat dietary indiscretions. Serum levels of fat-soluble vitamins (A, D, E, and K) were lower with orlistat than with placebo,16 and a fat-soluble vitamin supplement should be taken 2 hours before or after taking orlistat. Serious but very uncommon adverse events such as kidney damage have been reported.17 Kidney and liver function should be monitored while taking orlistat.
Phentermine
Phentermine (Adipex-P, Lomaira), a sympathomimetic amine, is the most commonly prescribed antiobesity drug in the United States. A schedule IV controlled substance, it is FDA-approved for short-term use (up to 12 weeks). Its primary mechanism of action is mediated by reduction in hunger perception. It was first developed in the 1970s and is available in doses ranging from 8 mg to 37.5 mg daily.18
Benefits. In a randomized trial, at 28 weeks, weight loss was 1.5 kg with placebo and 5.3 kg with phentermine.19 No long-term (> 1 year) randomized controlled trials of the effectiveness of phentermine monotherapy in weight loss have been conducted.
Adverse effects. Dizziness, dry mouth, insomnia, constipation, and increase in heart rate were most common.19
Phentermine is contraindicated in patients with coronary artery disease, congestive heart failure, stroke, and uncontrolled hypertension. Currently, no data exist on the long-term cardiovascular effects of phentermine. We believe phentermine, used in patients at low to intermediate cardiovascular risk, is a useful “jumpstart” tool, in combination with lifestyle changes, to achieve weight loss and improve metabolic values for those with type 2 DM and obesity.
Phentermine is a controlled substance per Ohio law. Patients must be seen once a month by the prescribing provider and prescriptions are limited to a 30-day supply, which must be filled within 7 days of the date of the prescription. Phentermine can only be prescribed for a maximum of 3 months and must be discontinued for 6 months before patients are eligible for a new prescription.
Phentermine and topiramate extended-release
Obesity is a product of complex interactions between several neurohormonal pathways. Approaches simultaneously targeting more than one regulatory pathway have become popular and quite efficient strategies in treating patients with obesity.20 Stemming from such approaches, antiobesity drug combinations such as phentermine and topiramate extended-release (Qsymia) have become increasingly recognized and used in clinical practice. The combination of these 2 medications has been approved for long-term use by the FDA.
Phentermine and topiramate extended-release is a fixed-dose combination that was approved for weight loss in 2012. Topiramate, an anticonvulsant, and phentermine exert their anorexigenic effects through regulating various brain neurotransmitters and result in more weight loss when used together than when either is used alone. Several clinical trials evaluated the efficacy of low doses of this combination in weight loss.
Benefits. In a randomized trial in patients with obesity and cardiometabolic diseases, at 56 weeks, the mean weight loss was:
- 1.2% in the placebo group
- 7.8% in the group receiving phentermine 7.5 mg and topiramate 46 mg
- 9.8% in the group receiving phentermine 15 mg and topiramate 92 mg.21
Patients in the active treatment groups also had significant improvements in cardiovascular and metabolic risk factors such as waist circumference, systolic blood pressure, and total cholesterol/high-density lipoprotein cholesterol ratio. At 56 weeks, patients with diabetes and prediabetes taking this preparation had greater reductions in HbA1c values, and fewer prediabetes patients progressed to type 2 DM.21
Adverse effects most commonly seen were dry mouth, paresthesia, and constipation.21
This combination is contraindicated in pregnancy, patients with recent stroke, uncontrolled hypertension, coronary artery disease, glaucoma, hyperthyroidism, or in patients taking monoamine oxidase inhibitors. Women of childbearing age should be tested for pregnancy before starting therapy, and monthly thereafter, and also be advised to use effective methods of contraception while taking the medication. Topiramate has been associated with the development of renal stones and thus should be used with caution in patients with a history of kidney stones.
Bupropion and naltrexone sustained-release
Bupropion and naltrexone sustained-release (Contrave) is another FDA-approved combination drug for chronic weight management. Bupropion is a dopamine and norepinephrine reuptake inhibitor approved for depression and smoking cessation, and naltrexone is an opioid receptor antagonist approved for treating alcohol and opioid dependence. The combination of these 2 medications has been approved for long-term use by the FDA.
Benefits. In a randomized trial in patients with obesity and type 2 DM, weight loss at 56 weeks was:
- 1.8% with placebo
- 5.0% with naltrexone 32 mg and bupropion 360 mg daily.
Absolute reductions in HbA1c were:
- 0.1% with placebo
- 0.6% with naltrexone-bupropion.
Improvements were also seen in other cardiometabolic risk factors such as triglyceride and high-density lipoprotein cholesterol levels.22
Adverse effects. The most common adverse effect leading to drug discontinuation was nausea. Other adverse effects reported were constipation, headache, vomiting, and dizziness.22
Naltrexone-bupropion is contraindicated in patients with a history of seizure disorder or a diagnosis of anorexia nervosa or bulimia, or who are on chronic opioid therapy.
Diethylpropion
Diethylpropion (Tenuate, Tenuate Dospan) is a central nervous system stimulant similar to bupropion in its structure. It was approved by the FDA for treating obesity in 1959. It should be used as part of a short-term weight-loss plan, along with a low-calorie diet. Diethylpropion is also a controlled substance and, as with phentermine therapy, patients are required to be seen once a month by their prescriber. Diethylpropion cannot be prescribed for more than 3 months.
Benefits. Weight loss in a randomized trial at 6 months:
- 3.2% with placebo
- 9.8% with diethylpropion 50 mg twice a day.23
After 6 months, all participants received diethylpropion in an open-label extension for an additional 6 months. At 12 months, the mean weight loss produced by diethylpropion was 10.6%.23 No differences in heart rate, blood pressure, electrocardiographic results, or psychiatric evaluations were observed.
Adverse effects. As with phentermine, common side effects of diethylpropion include insomnia, dry mouth, dizziness, headache, mild increases in blood pressure, and palpitations.23
Lorcaserin
Lorcaserin (Belviq) was approved by the FDA for chronic weight management in June 2012. It exerts its effects through binding selectively to central 5-HT2C serotonin receptors, with poor affinity for 5-HT2A and 5-HT2B receptors. Nonselective serotoninergic agents, including fenfluramine and dexfenfluramine, were withdrawn from the market in 1997 after being reported to be associated with valvular heart abnormalities.24 Lorcaserin has been approved for long-term use by the FDA.
Benefits. Mean weight loss at 1 year in the Behavioral Modification and Lorcaserin for Overweight and Obesity Management in Diabetes Mellitus trial25 was:
- 1.5% with placebo
- 5.0% with lorcaserin 10 mg once daily
- 4.5% with lorcaserin 10 mg twice daily.
Absolute reductions in HbA1c values were:
- 0.4% with placebo
- 0.9% with lorcaserin 10 mg once daily
- 1.0% with lorcaserin 10 mg twice daily.
Absolute reductions in fasting plasma glucose values were:
- 11.9 mg/dL with placebo
- 27.4 mg/dL with lorcaserin 10 mg once daily
- 28.4 mg/dL with lorcaserin 10 mg twice daily.25
Adverse effects. The most common adverse effects were headache, dizziness, and fatigue. There was no significant increase in valvulopathy on echocardiography of participants receiving lorcaserin compared with placebo.25
Liraglutide
Liraglutide (Saxenda, Victoza) is a glucagon-like peptide-1 (GLP-1) receptor agonist. Native GLP-1 is a hormone secreted by intestinal L cells in response to consumption of fat and carbohydrate-rich foods. It stimulates the release of insulin and suppresses any inappropriately elevated postprandial glucagon levels. In addition to its effect on glucose metabolism, GLP-1 also reduces appetite and delays gastric emptying in humans.26 Unlike the extremely short half-life of native GLP-1 (estimated at 1 to 2 minutes), liraglutide has a half-life of 13 hours, allowing it to be given once daily.26 Liraglutide medication has been approved for long-term use by the FDA.
Benefits. The Liraglutide Effect and Action in Diabetes 1–5 studies compared the effects of liraglutide monotherapy with antidiabetic oral medications or insulin, as well as in combination with antidiabetic oral agents. Liraglutide (Victoza) at doses approved for type 2 DM of 1.2 mg and 1.8 mg daily had significant effects in reducing HbA1c by 0.48% to 1.84% and weight by 2.5 kg to 4 kg.27,28 At a dose of 3.0 mg, liraglutide (Saxenda) is approved for chronic weight management. This dose of liraglutide has been shown to be effective and safe in patients with type 2 DM and obesity.
In the 56-week SCALE Diabetes trial,29 liraglutide at a dose of 3.0 mg resulted in 6.0% weight reduction, compared with 2.0% in the placebo group. Of participants receiving 3.0 mg of liraglutide, 54.3% achieved more than 5% weight loss at 56 weeks compared with 21.4% with placebo. Liraglutide also resulted in significant improvements in HbA1c (mean change −1.3% vs −0.3% with placebo), fasting and postprandial glucose levels, and fasting glucagon levels.29
The Liraglutide Effect and Action in Diabetes: Evaluation of Cardiovascular Outcome Results trial has shown liraglutide to significantly reduce rates of major cardiovascular events (first occurrence of cardiovascular death, nonfatal myocardial infarction, or nonfatal stroke) in patients with elevated cardiovascular risk factors.30 These findings make liraglutide a favorable choice for high-risk patients with type 2 DM, obesity, and cardiovascular disease.
It is important to indicate that if a 5% weight loss is not achieved by 3 months with any of these weight-loss medications, it would be reasonable to stop the medication and consider switching to a different medication. These medications work best when combined with diet and increased physical activity. Weight-loss medications should never be used during pregnancy.
Women of childbearing age should be advised to use effective contraception methods while taking any of the above antiobesity medications.
Diabetes medications associated with weight loss: Metformin and SGLT-2 inhibitors
Although not FDA-approved for weight management, metformin has anorexigenic effects that aid in weight loss. It also inhibits hepatic glucose production and improves peripheral insulin sensitivity, making it a useful agent in patients with type 2 DM and obesity.
A meta-analysis of 31 trials showed that metformin reduced body mass index by 5.3% compared with placebo.31 Metformin should be considered as a first-line agent in obese patients with type 2 DM.
In healthy people, nearly all glucose is filtered in the glomerulus, but then 98% of it is reabsorbed in the proximal tubule by sodium-glucose cotransporter-2 (SGLT-2). Drugs that inhibit SGLT-2 increase urinary glucose excretion and, as a result, help control hyperglycemia. Another, off-label effect of excreting more glucose is weight loss: a sustained weight loss of about 3 kg to 5 kg in clinical studies.32 Although they can be used as monotherapies, SGLT-2 inhibitors are usually used as add-on therapies in patients with type 2 DM.
AN ALGORITHM FOR TREATMENT
First, we believe that lifestyle interventions by optimization of nutrition and physical activity should be the cornerstone therapy in the management plan of any patient with type 2 DM and obesity. These interventions are best implemented through a comprehensive, multidisciplinary approach that integrates the care of dietitians, physical therapists, exercise physiologists, psychologists, and social workers.33 Patients need also to be seen frequently, ie, at least once every 3 months. The possibility of seeing patients in group-shared medical appointments on a monthly basis could also be considered.
We also believe that metformin should be added early in the course of treatment for its known benefits of improving insulin sensitivity and suppressing appetite. Target HbA1c goals and body weight in patients with type 2 diabetes and obesity should be tailored to the individual based on age, general health status, risk of hypoglycemia, capacity to do physical activity, and associated comorbidities. If no improvements are seen (HbA1c > 7% and < 3 % weight loss) despite lifestyle changes and the addition of metformin, the possibility of adding a GLP-1 receptor agonist or an SGLT-2 inhibitor as a second-line therapy should be considered. Both classes of medications aid in lowering HbA1c and promote further weight loss.
If no clinical progress is achieved at 3 months, the possibility of adding an FDA-approved weight-loss medication, as discussed above, should be strongly considered. Of note, this algorithm targets different endogenous pathways for weight loss and thus minimizes weight regain through compensatory mechanisms.
THE NEED FOR PATIENT-CENTERED WEIGHT-LOSS CONVERSATIONS
Patient-centered care has become a core quality measure in our healthcare systems and a key to our patients’ success. The decision to start an antiobesity drug should therefore reflect careful consideration of medical and personal patient issues, all of which are valued differently by patients.34
Individualized therapy is even more relevant among patients suffering from a significant burden of disease. About 80% of patients with diabetes live with at least 1 other medical condition,35 and each of these patients spends over 2 hours a day, on average, following doctors’ recommendations.36 If antiobesity medications are prescribed without careful consideration of the patient’s preexisting workload, they will be destined to fail. Therefore, it becomes crucial to first account for the patient’s ability to cope with therapy intensification. This requires careful deliberation between healthcare providers and patients, in aims of targeting a weight-loss plan that fits patients’ goals and is aligned with providers’ expectations.
Healthcare systems also play a key role in supporting better conversations about obesity in type 2 DM patients. They could implement multifaceted initiatives to promote shared decision-making and the use of decision aids to advance patient-centered obesity practices.37 Policymakers could redesign quality measures aimed at capturing the quality of obesity conversations, and develop policies that support better education for clinicians regarding the importance of addressing obesity with adequate communication and patient-centered skills. Guidelines are often too disease-specific and do not consider comorbidities in their context when providing recommendations.38 Thus, diabetes societies should respond to the need to guide care for patients with diabetes and its comorbidities, particularly obesity.
CONCLUSIONS
Obesity is a serious global health issue and a leading risk factor for type 2 DM. Lifestyle measures are the cornerstone of preventing and treating obesity and type 2 DM. Emerging data support the effectiveness of intensive, interdisciplinary weight-loss programs in patients with diabetes. The use of antiobesity drugs should be considered in patients who have not achieved adequate responses to lifestyle interventions. Medications should be tailored to the individual’s health risks and metabolic and psychobehavioral characteristics. In many cases, the addition of weight-loss drugs will help accomplish and maintain the recommended 10% weight reduction, resulting in improvement in glycemic control and significant reduction in cardiovascular risk factors. New studies combining antiobesity and antidiabetes medications in the context of lifestyle interventions will help define the optimal therapeutic approach for patients with type 2 DM and obesity.
- Ogden CL, Carroll MD, Fryar CD, Flegal KM. Prevalence of obesity among adults and youth: United States, 2011–2014. NCHS Data Brief 2015; (219):1–8.
- Lyznicki JM, Young DC, Riggs JA, Davis RM; for the Council on Scientific Affairs, American Medical Association. Obesity: assessment and management in primary care. Am Fam Physician 2001; 63:2185–2196.
- World Health Organization (WHO). Obesity and overweight fact sheet. www.who.int/mediacentre/factsheets/fs311/en. Updated June 2016. Accessed June 22, 2017.
- Daniels J. Obesity: America’s epidemic. Am J Nurs 2006; 106:40–49.
- Colditz GA, Willett WC, Rotnitzky A, Manson JE. Weight gain as a risk factor for clinical diabetes mellitus in women. Ann Intern Med 1995; 122:481–486.
- Schauer PR, Bhatt DL, Kirwan JP, et al; STAMPEDE Investigators. Bariatric surgery versus intensive medical therapy for diabetes-5-year outcomes. N Engl J Med 2017; 376:641–651.
- Oldridge NB, Stump TE, Nothwehr FK, Clark DO. Prevalence and outcomes of comorbid metabolic and cardiovascular conditions in middle- and older-age adults. J Clin Epidemiol 2001; 54:928–934.
- Garber AJ, Abrahamson MJ, Barzilay JI, et al. Consensus statement by the American Association of Clinical Endocrinologists and American College of Endocrinology on the comprehensive type 2 diabetes management algorithm—2016 executive summary. Endocr Pract 2016; 22:84–113.
- McFarlane SI. Antidiabetic medications and weight gain: implications for the practicing physician. Curr Diab Rep 2009; 9:249–254.
- UK Prospective Diabetes Study (UKPDS) Group. Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34). Lancet 1998; 352:854–865.
- Beal E. The pros and cons of designating obesity a disease: the new AMA designation stirs debate. Am J Nurs 2013; 113:18–19.
- Kraschnewski JL, Sciamanna CN, Stuckey HL, et al. A silent response to the obesity epidemic: decline in US physician weight counseling. Med Care 2013; 51:186–192.
- Potter MB, Vu JD, Croughan-Minihane M. Weight management: what patients want from their primary care physicians. J Fam Pract 2001; 50:513–518.
- Pappachan JM, Viswanath AK. Medical management of diabesity: do we have realistic targets? Curr Diab Rep 2017; 17:4.
- Lexicomp Online, Lexi-Drugs, Hudson, OH: Wolters Kluwer Clinical Drug Information, Inc., 2017.
- Torgerson JS, Hauptman J, Boldrin MN, Sjöström L. XENical in the prevention of diabetes in obese subjects (XENDOS) study: a randomized study of orlistat as an adjunct to lifestyle changes for the prevention of type 2 diabetes in obese patients. Diabetes Care 2004; 27:155–161.
- Buysschaert B, Aydin S, Morelle J, Hermans MP, Jadoul M, Demoulin N. Weight loss at a high cost: orlistat-induced late-onset severe kidney disease. Diabetes Metab 2016; 42:62–64.
- Colman E. Anorectics on trial: a half century of federal regulation of prescription appetite suppressants. Ann Intern Med 2005; 143:380–385.
- Aronne LJ, Wadden TA, Peterson C, Winslow D, Odeh S, Gadde KM. Evaluation of phentermine and topiramate versus phentermine/topiramate extended-release in obese adults. Obesity (Silver Spring) 2013; 21:2163–2171.
- Solas M, Milagro FI, Martínez-Urbistondo D, Ramirez MJ, Martínez JA. Precision obesity treatments including pharmacogenetic and nutrigenetic approaches. Trends Pharmacol Sci 2016; 37:575–593.
- Gadde KM, Allison DB, Ryan DH, et al. Effects of low-dose, controlled-release, phentermine plus topiramate combination on weight and associated comorbidities in overweight and obese adults (CONQUER): a randomised, placebo-controlled, phase 3 trial. Lancet 2011; 377:1341–1352.
- Hollander P, Gupta AK, Plodkowski R, et al; for the COR-Diabetes Study Group. Effects of naltrexone sustained-release/bupropion sustained-release combination therapy on body weight and glycemic parameters in overweight and obese patients with type 2 diabetes. Diabetes Care 2013; 36:4022–4029.
- Cercato C, Roizenblatt VA, Leança CC, et al. A randomized double-blind placebo-controlled study of the long-term efficacy and safety of diethylpropion in the treatment of obese subjects. Int J Obes (Lond) 2009; 33:857–865.
- Gardin JM, Schumacher D, Constantine G, Davis KD, Leung C, Reid CL. Valvular abnormalities and cardiovascular status following exposure to dexfenfluramine or phentermine/fenfluramine. JAMA 2000; 283:1703–1709.
- O’Neil PM, Smith SR, Weissman NJ, et al. Randomized placebo-controlled clinical trial of lorcaserin for weight loss in type 2 diabetes mellitus: the BLOOM-DM study. Obesity (Silver Spring) 2012; 20:1426–1436.
- Amori RE, Lau J, Pittas AG. Efficacy and safety of incretin therapy in type 2 diabetes: systematic review and meta-analysis. JAMA 2007; 298:194–206.
- Blonde L, Russell-Jones D. The safety and efficacy of liraglutide with or without oral antidiabetic drug therapy in type 2 diabetes: an overview of the LEAD 1–5 studies. Diabetes Obes Metab 2009; 11(suppl 3):26–34.
- Buse JB, Rosenstock J, Sesti G, et al; for the LEAD-6 Study Group. Liraglutide once a day versus exenatide twice a day for type 2 diabetes: a 26-week randomised, parallel-group, multinational, open-label trial (LEAD-6). Lancet 2009; 374:39–47.
- Davies MJ, Bergenstal R, Bode B, et al; for the NN8022-1922 Study Group. Efficacy of liraglutide for weight loss among patients with type 2 diabetes: the SCALE diabetes randomized clinical trial. JAMA 2015; 314:687–699.
- Marso SP, Daniels GH, Brown-Frandsen K, et al; for the LEADER Steering Committee on behalf of the LEADER Trial Investigators. Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med 2016; 375:311–322.
- Salpeter SR, Buckley NS, Kahn JA, Salpeter EE. Meta-analysis: metformin treatment in persons at risk for diabetes mellitus. Am J Med 2008; 121:149–157.
- Vasilakou D, Karagiannis T, Athanasiadou E, et al. Sodium-glucose cotransporter 2 inhibitors for type 2 diabetes: a systematic review and meta-analysis. Ann Intern Med 2013; 159:262–274.
- Burguera B, Jesus Tur J; Escudero AJ, et al. An intensive lifestyle intervention is an effective treatment of morbid obesity: the TRAMOMTANA study—a two-year randomized controlled clinical trial. Int J Endocrinol 2015; 2015:194696.
- Hargraves I, LeBlanc A, Shah ND, Montori VM. Shared decision making: the need for patient-clinician conversation, not just information. Health Aff (Millwood) 2016; 35:627–629.
- Lin P-J, Kent DM, Winn AN, Cohen JT, Neumann PJ. Multiple chronic conditions in type 2 diabetes mellitus: prevalence and consequences. Am J Manag Care 2015; 21:e23–e34.
- Russell LB, Suh D-C, Safford MA. Time requirements for diabetes self-management: too much for many? J Fam Pract 2005; 54:52–56.
- Serrano V, Rodriguez-Gutierrez R, Hargraves I, Gionfriddo MR, Tamhane S, Montori VM. Shared decision-making in the care of individuals with diabetes. Diabet Med 2016; 33:742–751.
- Wyatt KD, Stuart LM, Brito JP, et al. Out of context: clinical practice guidelines and patients with multiple chronic conditions: a systematic review. Med Care 2014; 52(suppl 3):S92–S100.
Obesity is a leading public health concern, affecting nearly 60 million adult Americans.1 It is a major risk factor for the development of insulin resistance and type 2 diabetes mellitus (DM).2 More than 90% of patients with type 2 DM have obesity, and obesity is a major obstacle to achieving long-term glycemic control.3
Clinical studies have demonstrated that a 6- to 7-kg increase in body weight increases the risk of developing type 2 DM by 50%, while a 5-kg loss reduces the risk by a similar amount.4 As a result, most patients who have a body mass index greater than 40 kg/m2 suffer from type 2 DM.5 Strong evidence exists that bariatric surgery and its resulting weight loss has positive effects on fasting blood sugar, hemoglobin A1c (HbA1c), lipid profiles, and other metabolic variables.6
When combined, obesity and type 2 DM carry a significant burden of micro- and macrovascular complications such as retinopathy, nephropathy, neuropathy, and cardiovascular disease. As a result, a high prevalence of morbidity and mortality is seen among patients with obesity and type 2 DM; those between the ages of 51 and 61 have a 7-times higher mortality rate compared with nonobese normoglycemic people, and patients with diabetes alone have a 2.6-times higher mortality rate.7
A DILEMMA IN THE CLINIC: FOCUS ON THE SUGAR OR THE WEIGHT?
Although type 2 DM and obesity go hand in hand, clinicians tend to focus on the sugar and neglect the weight, concentrating their efforts on improving blood glucose indices, and prescribing in many instances medications that cause weight gain. As a result, we are faced with a rising epidemic of obesity, perpetuating a preexisting epidemic of diabetes.
An optimal, comprehensive approach to managing patients with type 2 DM should encompass both the control of dysglycemia and its associated comorbidities, obesity being the key player.8 However, clinical practice is often misaligned with the evidence. For instance, many of our first-line oral treatments for type 2 DM (except for metformin) are associated with weight gain.9 With time, control of glycemia becomes more and more ineffective, at which point therapy is intensified with insulin, further exacerbating the weight gain.10
Therefore, it seems counterintuitive to treat a disease for which obesity is one of the main risk factors with medications that promote weight gain. Yet healthcare providers are faced with a therapeutic dilemma: should they focus their efforts on improving patients’ glycemic control, or should they invest in helping these patients lose weight? Although an ideal approach would incorporate both aspects, the reality is that it is far from practical.
A few issues impinge on integrating weight loss in the care of type 2 DM. Although the American Medical Association recognized obesity as a disease in 2013,11 some providers still perceive obesity as a self-inflicted condition that is due to bad lifestyle and behavior.11 Many clinicians may also have low expectations for patients’ success, and often lack the time and knowledge to intervene regarding nutrition, physical activity, and psychological issues pertinent to the management of obesity in type 2 DM. Therefore, in many cases, it seems less complicated and more rewarding for both patients and physicians to concentrate on improving the HbA1c value rather than investing efforts in weight loss. For diabetic patients with obesity, this could mean that clinicians may prescribe glucose-lowering therapies, such as insulin and sulfonylureas, at the expense of weight gain. Additionally, clinicians often experience the need to provide recommendations more aligned with metrics that dictate reimbursement (eg, HbA1c targets) within healthcare systems that still raise concerns regarding obesity visit reimbursements.
Lastly, the lack of trustworthy or pertinent evidence (lack of comparative effectiveness research) for antiobesity medications may limit their use in daily practice. Physicians have had little confidence in the efficacy of antiobesity drugs, and often raise significant safety concerns, especially after witnessing important fiascos in this field, eg, dexfenfluramine, rimonabant, and sibutramine.2,12,13
As a result, many of our patients with obesity and type 2 DM may not consider the need for weight loss, and may not even be aware that type 2 DM is caused by obesity and physical inactivity in the first place. Others have accumulated a significant degree of frustration, and have “thrown in the towel” already after unsuccessful weight-loss efforts, many of which were not medically supervised.
For all of the above reasons, both clinicians and patients often concentrate their efforts on treating blood glucose numbers rather than the “obesity-diabetes” as a whole.14 And as a result, our practices are slowly filling up with patients with obesity and type 2 DM who are treated primarily with insulin, resulting in a progressive (and untreated) obesity and diabetes epidemic.
DRUGS FOR TREATING OBESITY AND TYPE 2 DM
Orlistat
Orlistat (Xenical) is the only weight-loss drug approved by the US Food and Drug Administration (FDA) that acts outside the brain. It inhibits pancreatic lipases, resulting in up to 30% less fat absorption in the gut. Orlistat has been approved for long-term use by the FDA.
Benefits. In the XENical in the Prevention of Diabetes in Obese Subjects study, treatment with orlistat resulted in a significant reduction in the cumulative incidence of type 2 DM after 4 years of treatment (9.0% with placebo vs 6.2% with orlistat), corresponding to a risk reduction of 37.3%.16 Mean weight loss after 4 years was significantly greater in the orlistat group (5.8 vs 3.0 kg with placebo; P < .001).16 Other benefits of orlistat included a reduction in low-density lipoprotein cholesterol independent of that expected from change in body weight.16
Adverse effects include flatulence with discharge and fecal urgency after high-fat dietary indiscretions. Serum levels of fat-soluble vitamins (A, D, E, and K) were lower with orlistat than with placebo,16 and a fat-soluble vitamin supplement should be taken 2 hours before or after taking orlistat. Serious but very uncommon adverse events such as kidney damage have been reported.17 Kidney and liver function should be monitored while taking orlistat.
Phentermine
Phentermine (Adipex-P, Lomaira), a sympathomimetic amine, is the most commonly prescribed antiobesity drug in the United States. A schedule IV controlled substance, it is FDA-approved for short-term use (up to 12 weeks). Its primary mechanism of action is mediated by reduction in hunger perception. It was first developed in the 1970s and is available in doses ranging from 8 mg to 37.5 mg daily.18
Benefits. In a randomized trial, at 28 weeks, weight loss was 1.5 kg with placebo and 5.3 kg with phentermine.19 No long-term (> 1 year) randomized controlled trials of the effectiveness of phentermine monotherapy in weight loss have been conducted.
Adverse effects. Dizziness, dry mouth, insomnia, constipation, and increase in heart rate were most common.19
Phentermine is contraindicated in patients with coronary artery disease, congestive heart failure, stroke, and uncontrolled hypertension. Currently, no data exist on the long-term cardiovascular effects of phentermine. We believe phentermine, used in patients at low to intermediate cardiovascular risk, is a useful “jumpstart” tool, in combination with lifestyle changes, to achieve weight loss and improve metabolic values for those with type 2 DM and obesity.
Phentermine is a controlled substance per Ohio law. Patients must be seen once a month by the prescribing provider and prescriptions are limited to a 30-day supply, which must be filled within 7 days of the date of the prescription. Phentermine can only be prescribed for a maximum of 3 months and must be discontinued for 6 months before patients are eligible for a new prescription.
Phentermine and topiramate extended-release
Obesity is a product of complex interactions between several neurohormonal pathways. Approaches simultaneously targeting more than one regulatory pathway have become popular and quite efficient strategies in treating patients with obesity.20 Stemming from such approaches, antiobesity drug combinations such as phentermine and topiramate extended-release (Qsymia) have become increasingly recognized and used in clinical practice. The combination of these 2 medications has been approved for long-term use by the FDA.
Phentermine and topiramate extended-release is a fixed-dose combination that was approved for weight loss in 2012. Topiramate, an anticonvulsant, and phentermine exert their anorexigenic effects through regulating various brain neurotransmitters and result in more weight loss when used together than when either is used alone. Several clinical trials evaluated the efficacy of low doses of this combination in weight loss.
Benefits. In a randomized trial in patients with obesity and cardiometabolic diseases, at 56 weeks, the mean weight loss was:
- 1.2% in the placebo group
- 7.8% in the group receiving phentermine 7.5 mg and topiramate 46 mg
- 9.8% in the group receiving phentermine 15 mg and topiramate 92 mg.21
Patients in the active treatment groups also had significant improvements in cardiovascular and metabolic risk factors such as waist circumference, systolic blood pressure, and total cholesterol/high-density lipoprotein cholesterol ratio. At 56 weeks, patients with diabetes and prediabetes taking this preparation had greater reductions in HbA1c values, and fewer prediabetes patients progressed to type 2 DM.21
Adverse effects most commonly seen were dry mouth, paresthesia, and constipation.21
This combination is contraindicated in pregnancy, patients with recent stroke, uncontrolled hypertension, coronary artery disease, glaucoma, hyperthyroidism, or in patients taking monoamine oxidase inhibitors. Women of childbearing age should be tested for pregnancy before starting therapy, and monthly thereafter, and also be advised to use effective methods of contraception while taking the medication. Topiramate has been associated with the development of renal stones and thus should be used with caution in patients with a history of kidney stones.
Bupropion and naltrexone sustained-release
Bupropion and naltrexone sustained-release (Contrave) is another FDA-approved combination drug for chronic weight management. Bupropion is a dopamine and norepinephrine reuptake inhibitor approved for depression and smoking cessation, and naltrexone is an opioid receptor antagonist approved for treating alcohol and opioid dependence. The combination of these 2 medications has been approved for long-term use by the FDA.
Benefits. In a randomized trial in patients with obesity and type 2 DM, weight loss at 56 weeks was:
- 1.8% with placebo
- 5.0% with naltrexone 32 mg and bupropion 360 mg daily.
Absolute reductions in HbA1c were:
- 0.1% with placebo
- 0.6% with naltrexone-bupropion.
Improvements were also seen in other cardiometabolic risk factors such as triglyceride and high-density lipoprotein cholesterol levels.22
Adverse effects. The most common adverse effect leading to drug discontinuation was nausea. Other adverse effects reported were constipation, headache, vomiting, and dizziness.22
Naltrexone-bupropion is contraindicated in patients with a history of seizure disorder or a diagnosis of anorexia nervosa or bulimia, or who are on chronic opioid therapy.
Diethylpropion
Diethylpropion (Tenuate, Tenuate Dospan) is a central nervous system stimulant similar to bupropion in its structure. It was approved by the FDA for treating obesity in 1959. It should be used as part of a short-term weight-loss plan, along with a low-calorie diet. Diethylpropion is also a controlled substance and, as with phentermine therapy, patients are required to be seen once a month by their prescriber. Diethylpropion cannot be prescribed for more than 3 months.
Benefits. Weight loss in a randomized trial at 6 months:
- 3.2% with placebo
- 9.8% with diethylpropion 50 mg twice a day.23
After 6 months, all participants received diethylpropion in an open-label extension for an additional 6 months. At 12 months, the mean weight loss produced by diethylpropion was 10.6%.23 No differences in heart rate, blood pressure, electrocardiographic results, or psychiatric evaluations were observed.
Adverse effects. As with phentermine, common side effects of diethylpropion include insomnia, dry mouth, dizziness, headache, mild increases in blood pressure, and palpitations.23
Lorcaserin
Lorcaserin (Belviq) was approved by the FDA for chronic weight management in June 2012. It exerts its effects through binding selectively to central 5-HT2C serotonin receptors, with poor affinity for 5-HT2A and 5-HT2B receptors. Nonselective serotoninergic agents, including fenfluramine and dexfenfluramine, were withdrawn from the market in 1997 after being reported to be associated with valvular heart abnormalities.24 Lorcaserin has been approved for long-term use by the FDA.
Benefits. Mean weight loss at 1 year in the Behavioral Modification and Lorcaserin for Overweight and Obesity Management in Diabetes Mellitus trial25 was:
- 1.5% with placebo
- 5.0% with lorcaserin 10 mg once daily
- 4.5% with lorcaserin 10 mg twice daily.
Absolute reductions in HbA1c values were:
- 0.4% with placebo
- 0.9% with lorcaserin 10 mg once daily
- 1.0% with lorcaserin 10 mg twice daily.
Absolute reductions in fasting plasma glucose values were:
- 11.9 mg/dL with placebo
- 27.4 mg/dL with lorcaserin 10 mg once daily
- 28.4 mg/dL with lorcaserin 10 mg twice daily.25
Adverse effects. The most common adverse effects were headache, dizziness, and fatigue. There was no significant increase in valvulopathy on echocardiography of participants receiving lorcaserin compared with placebo.25
Liraglutide
Liraglutide (Saxenda, Victoza) is a glucagon-like peptide-1 (GLP-1) receptor agonist. Native GLP-1 is a hormone secreted by intestinal L cells in response to consumption of fat and carbohydrate-rich foods. It stimulates the release of insulin and suppresses any inappropriately elevated postprandial glucagon levels. In addition to its effect on glucose metabolism, GLP-1 also reduces appetite and delays gastric emptying in humans.26 Unlike the extremely short half-life of native GLP-1 (estimated at 1 to 2 minutes), liraglutide has a half-life of 13 hours, allowing it to be given once daily.26 Liraglutide medication has been approved for long-term use by the FDA.
Benefits. The Liraglutide Effect and Action in Diabetes 1–5 studies compared the effects of liraglutide monotherapy with antidiabetic oral medications or insulin, as well as in combination with antidiabetic oral agents. Liraglutide (Victoza) at doses approved for type 2 DM of 1.2 mg and 1.8 mg daily had significant effects in reducing HbA1c by 0.48% to 1.84% and weight by 2.5 kg to 4 kg.27,28 At a dose of 3.0 mg, liraglutide (Saxenda) is approved for chronic weight management. This dose of liraglutide has been shown to be effective and safe in patients with type 2 DM and obesity.
In the 56-week SCALE Diabetes trial,29 liraglutide at a dose of 3.0 mg resulted in 6.0% weight reduction, compared with 2.0% in the placebo group. Of participants receiving 3.0 mg of liraglutide, 54.3% achieved more than 5% weight loss at 56 weeks compared with 21.4% with placebo. Liraglutide also resulted in significant improvements in HbA1c (mean change −1.3% vs −0.3% with placebo), fasting and postprandial glucose levels, and fasting glucagon levels.29
The Liraglutide Effect and Action in Diabetes: Evaluation of Cardiovascular Outcome Results trial has shown liraglutide to significantly reduce rates of major cardiovascular events (first occurrence of cardiovascular death, nonfatal myocardial infarction, or nonfatal stroke) in patients with elevated cardiovascular risk factors.30 These findings make liraglutide a favorable choice for high-risk patients with type 2 DM, obesity, and cardiovascular disease.
It is important to indicate that if a 5% weight loss is not achieved by 3 months with any of these weight-loss medications, it would be reasonable to stop the medication and consider switching to a different medication. These medications work best when combined with diet and increased physical activity. Weight-loss medications should never be used during pregnancy.
Women of childbearing age should be advised to use effective contraception methods while taking any of the above antiobesity medications.
Diabetes medications associated with weight loss: Metformin and SGLT-2 inhibitors
Although not FDA-approved for weight management, metformin has anorexigenic effects that aid in weight loss. It also inhibits hepatic glucose production and improves peripheral insulin sensitivity, making it a useful agent in patients with type 2 DM and obesity.
A meta-analysis of 31 trials showed that metformin reduced body mass index by 5.3% compared with placebo.31 Metformin should be considered as a first-line agent in obese patients with type 2 DM.
In healthy people, nearly all glucose is filtered in the glomerulus, but then 98% of it is reabsorbed in the proximal tubule by sodium-glucose cotransporter-2 (SGLT-2). Drugs that inhibit SGLT-2 increase urinary glucose excretion and, as a result, help control hyperglycemia. Another, off-label effect of excreting more glucose is weight loss: a sustained weight loss of about 3 kg to 5 kg in clinical studies.32 Although they can be used as monotherapies, SGLT-2 inhibitors are usually used as add-on therapies in patients with type 2 DM.
AN ALGORITHM FOR TREATMENT
First, we believe that lifestyle interventions by optimization of nutrition and physical activity should be the cornerstone therapy in the management plan of any patient with type 2 DM and obesity. These interventions are best implemented through a comprehensive, multidisciplinary approach that integrates the care of dietitians, physical therapists, exercise physiologists, psychologists, and social workers.33 Patients need also to be seen frequently, ie, at least once every 3 months. The possibility of seeing patients in group-shared medical appointments on a monthly basis could also be considered.
We also believe that metformin should be added early in the course of treatment for its known benefits of improving insulin sensitivity and suppressing appetite. Target HbA1c goals and body weight in patients with type 2 diabetes and obesity should be tailored to the individual based on age, general health status, risk of hypoglycemia, capacity to do physical activity, and associated comorbidities. If no improvements are seen (HbA1c > 7% and < 3 % weight loss) despite lifestyle changes and the addition of metformin, the possibility of adding a GLP-1 receptor agonist or an SGLT-2 inhibitor as a second-line therapy should be considered. Both classes of medications aid in lowering HbA1c and promote further weight loss.
If no clinical progress is achieved at 3 months, the possibility of adding an FDA-approved weight-loss medication, as discussed above, should be strongly considered. Of note, this algorithm targets different endogenous pathways for weight loss and thus minimizes weight regain through compensatory mechanisms.
THE NEED FOR PATIENT-CENTERED WEIGHT-LOSS CONVERSATIONS
Patient-centered care has become a core quality measure in our healthcare systems and a key to our patients’ success. The decision to start an antiobesity drug should therefore reflect careful consideration of medical and personal patient issues, all of which are valued differently by patients.34
Individualized therapy is even more relevant among patients suffering from a significant burden of disease. About 80% of patients with diabetes live with at least 1 other medical condition,35 and each of these patients spends over 2 hours a day, on average, following doctors’ recommendations.36 If antiobesity medications are prescribed without careful consideration of the patient’s preexisting workload, they will be destined to fail. Therefore, it becomes crucial to first account for the patient’s ability to cope with therapy intensification. This requires careful deliberation between healthcare providers and patients, in aims of targeting a weight-loss plan that fits patients’ goals and is aligned with providers’ expectations.
Healthcare systems also play a key role in supporting better conversations about obesity in type 2 DM patients. They could implement multifaceted initiatives to promote shared decision-making and the use of decision aids to advance patient-centered obesity practices.37 Policymakers could redesign quality measures aimed at capturing the quality of obesity conversations, and develop policies that support better education for clinicians regarding the importance of addressing obesity with adequate communication and patient-centered skills. Guidelines are often too disease-specific and do not consider comorbidities in their context when providing recommendations.38 Thus, diabetes societies should respond to the need to guide care for patients with diabetes and its comorbidities, particularly obesity.
CONCLUSIONS
Obesity is a serious global health issue and a leading risk factor for type 2 DM. Lifestyle measures are the cornerstone of preventing and treating obesity and type 2 DM. Emerging data support the effectiveness of intensive, interdisciplinary weight-loss programs in patients with diabetes. The use of antiobesity drugs should be considered in patients who have not achieved adequate responses to lifestyle interventions. Medications should be tailored to the individual’s health risks and metabolic and psychobehavioral characteristics. In many cases, the addition of weight-loss drugs will help accomplish and maintain the recommended 10% weight reduction, resulting in improvement in glycemic control and significant reduction in cardiovascular risk factors. New studies combining antiobesity and antidiabetes medications in the context of lifestyle interventions will help define the optimal therapeutic approach for patients with type 2 DM and obesity.
Obesity is a leading public health concern, affecting nearly 60 million adult Americans.1 It is a major risk factor for the development of insulin resistance and type 2 diabetes mellitus (DM).2 More than 90% of patients with type 2 DM have obesity, and obesity is a major obstacle to achieving long-term glycemic control.3
Clinical studies have demonstrated that a 6- to 7-kg increase in body weight increases the risk of developing type 2 DM by 50%, while a 5-kg loss reduces the risk by a similar amount.4 As a result, most patients who have a body mass index greater than 40 kg/m2 suffer from type 2 DM.5 Strong evidence exists that bariatric surgery and its resulting weight loss has positive effects on fasting blood sugar, hemoglobin A1c (HbA1c), lipid profiles, and other metabolic variables.6
When combined, obesity and type 2 DM carry a significant burden of micro- and macrovascular complications such as retinopathy, nephropathy, neuropathy, and cardiovascular disease. As a result, a high prevalence of morbidity and mortality is seen among patients with obesity and type 2 DM; those between the ages of 51 and 61 have a 7-times higher mortality rate compared with nonobese normoglycemic people, and patients with diabetes alone have a 2.6-times higher mortality rate.7
A DILEMMA IN THE CLINIC: FOCUS ON THE SUGAR OR THE WEIGHT?
Although type 2 DM and obesity go hand in hand, clinicians tend to focus on the sugar and neglect the weight, concentrating their efforts on improving blood glucose indices, and prescribing in many instances medications that cause weight gain. As a result, we are faced with a rising epidemic of obesity, perpetuating a preexisting epidemic of diabetes.
An optimal, comprehensive approach to managing patients with type 2 DM should encompass both the control of dysglycemia and its associated comorbidities, obesity being the key player.8 However, clinical practice is often misaligned with the evidence. For instance, many of our first-line oral treatments for type 2 DM (except for metformin) are associated with weight gain.9 With time, control of glycemia becomes more and more ineffective, at which point therapy is intensified with insulin, further exacerbating the weight gain.10
Therefore, it seems counterintuitive to treat a disease for which obesity is one of the main risk factors with medications that promote weight gain. Yet healthcare providers are faced with a therapeutic dilemma: should they focus their efforts on improving patients’ glycemic control, or should they invest in helping these patients lose weight? Although an ideal approach would incorporate both aspects, the reality is that it is far from practical.
A few issues impinge on integrating weight loss in the care of type 2 DM. Although the American Medical Association recognized obesity as a disease in 2013,11 some providers still perceive obesity as a self-inflicted condition that is due to bad lifestyle and behavior.11 Many clinicians may also have low expectations for patients’ success, and often lack the time and knowledge to intervene regarding nutrition, physical activity, and psychological issues pertinent to the management of obesity in type 2 DM. Therefore, in many cases, it seems less complicated and more rewarding for both patients and physicians to concentrate on improving the HbA1c value rather than investing efforts in weight loss. For diabetic patients with obesity, this could mean that clinicians may prescribe glucose-lowering therapies, such as insulin and sulfonylureas, at the expense of weight gain. Additionally, clinicians often experience the need to provide recommendations more aligned with metrics that dictate reimbursement (eg, HbA1c targets) within healthcare systems that still raise concerns regarding obesity visit reimbursements.
Lastly, the lack of trustworthy or pertinent evidence (lack of comparative effectiveness research) for antiobesity medications may limit their use in daily practice. Physicians have had little confidence in the efficacy of antiobesity drugs, and often raise significant safety concerns, especially after witnessing important fiascos in this field, eg, dexfenfluramine, rimonabant, and sibutramine.2,12,13
As a result, many of our patients with obesity and type 2 DM may not consider the need for weight loss, and may not even be aware that type 2 DM is caused by obesity and physical inactivity in the first place. Others have accumulated a significant degree of frustration, and have “thrown in the towel” already after unsuccessful weight-loss efforts, many of which were not medically supervised.
For all of the above reasons, both clinicians and patients often concentrate their efforts on treating blood glucose numbers rather than the “obesity-diabetes” as a whole.14 And as a result, our practices are slowly filling up with patients with obesity and type 2 DM who are treated primarily with insulin, resulting in a progressive (and untreated) obesity and diabetes epidemic.
DRUGS FOR TREATING OBESITY AND TYPE 2 DM
Orlistat
Orlistat (Xenical) is the only weight-loss drug approved by the US Food and Drug Administration (FDA) that acts outside the brain. It inhibits pancreatic lipases, resulting in up to 30% less fat absorption in the gut. Orlistat has been approved for long-term use by the FDA.
Benefits. In the XENical in the Prevention of Diabetes in Obese Subjects study, treatment with orlistat resulted in a significant reduction in the cumulative incidence of type 2 DM after 4 years of treatment (9.0% with placebo vs 6.2% with orlistat), corresponding to a risk reduction of 37.3%.16 Mean weight loss after 4 years was significantly greater in the orlistat group (5.8 vs 3.0 kg with placebo; P < .001).16 Other benefits of orlistat included a reduction in low-density lipoprotein cholesterol independent of that expected from change in body weight.16
Adverse effects include flatulence with discharge and fecal urgency after high-fat dietary indiscretions. Serum levels of fat-soluble vitamins (A, D, E, and K) were lower with orlistat than with placebo,16 and a fat-soluble vitamin supplement should be taken 2 hours before or after taking orlistat. Serious but very uncommon adverse events such as kidney damage have been reported.17 Kidney and liver function should be monitored while taking orlistat.
Phentermine
Phentermine (Adipex-P, Lomaira), a sympathomimetic amine, is the most commonly prescribed antiobesity drug in the United States. A schedule IV controlled substance, it is FDA-approved for short-term use (up to 12 weeks). Its primary mechanism of action is mediated by reduction in hunger perception. It was first developed in the 1970s and is available in doses ranging from 8 mg to 37.5 mg daily.18
Benefits. In a randomized trial, at 28 weeks, weight loss was 1.5 kg with placebo and 5.3 kg with phentermine.19 No long-term (> 1 year) randomized controlled trials of the effectiveness of phentermine monotherapy in weight loss have been conducted.
Adverse effects. Dizziness, dry mouth, insomnia, constipation, and increase in heart rate were most common.19
Phentermine is contraindicated in patients with coronary artery disease, congestive heart failure, stroke, and uncontrolled hypertension. Currently, no data exist on the long-term cardiovascular effects of phentermine. We believe phentermine, used in patients at low to intermediate cardiovascular risk, is a useful “jumpstart” tool, in combination with lifestyle changes, to achieve weight loss and improve metabolic values for those with type 2 DM and obesity.
Phentermine is a controlled substance per Ohio law. Patients must be seen once a month by the prescribing provider and prescriptions are limited to a 30-day supply, which must be filled within 7 days of the date of the prescription. Phentermine can only be prescribed for a maximum of 3 months and must be discontinued for 6 months before patients are eligible for a new prescription.
Phentermine and topiramate extended-release
Obesity is a product of complex interactions between several neurohormonal pathways. Approaches simultaneously targeting more than one regulatory pathway have become popular and quite efficient strategies in treating patients with obesity.20 Stemming from such approaches, antiobesity drug combinations such as phentermine and topiramate extended-release (Qsymia) have become increasingly recognized and used in clinical practice. The combination of these 2 medications has been approved for long-term use by the FDA.
Phentermine and topiramate extended-release is a fixed-dose combination that was approved for weight loss in 2012. Topiramate, an anticonvulsant, and phentermine exert their anorexigenic effects through regulating various brain neurotransmitters and result in more weight loss when used together than when either is used alone. Several clinical trials evaluated the efficacy of low doses of this combination in weight loss.
Benefits. In a randomized trial in patients with obesity and cardiometabolic diseases, at 56 weeks, the mean weight loss was:
- 1.2% in the placebo group
- 7.8% in the group receiving phentermine 7.5 mg and topiramate 46 mg
- 9.8% in the group receiving phentermine 15 mg and topiramate 92 mg.21
Patients in the active treatment groups also had significant improvements in cardiovascular and metabolic risk factors such as waist circumference, systolic blood pressure, and total cholesterol/high-density lipoprotein cholesterol ratio. At 56 weeks, patients with diabetes and prediabetes taking this preparation had greater reductions in HbA1c values, and fewer prediabetes patients progressed to type 2 DM.21
Adverse effects most commonly seen were dry mouth, paresthesia, and constipation.21
This combination is contraindicated in pregnancy, patients with recent stroke, uncontrolled hypertension, coronary artery disease, glaucoma, hyperthyroidism, or in patients taking monoamine oxidase inhibitors. Women of childbearing age should be tested for pregnancy before starting therapy, and monthly thereafter, and also be advised to use effective methods of contraception while taking the medication. Topiramate has been associated with the development of renal stones and thus should be used with caution in patients with a history of kidney stones.
Bupropion and naltrexone sustained-release
Bupropion and naltrexone sustained-release (Contrave) is another FDA-approved combination drug for chronic weight management. Bupropion is a dopamine and norepinephrine reuptake inhibitor approved for depression and smoking cessation, and naltrexone is an opioid receptor antagonist approved for treating alcohol and opioid dependence. The combination of these 2 medications has been approved for long-term use by the FDA.
Benefits. In a randomized trial in patients with obesity and type 2 DM, weight loss at 56 weeks was:
- 1.8% with placebo
- 5.0% with naltrexone 32 mg and bupropion 360 mg daily.
Absolute reductions in HbA1c were:
- 0.1% with placebo
- 0.6% with naltrexone-bupropion.
Improvements were also seen in other cardiometabolic risk factors such as triglyceride and high-density lipoprotein cholesterol levels.22
Adverse effects. The most common adverse effect leading to drug discontinuation was nausea. Other adverse effects reported were constipation, headache, vomiting, and dizziness.22
Naltrexone-bupropion is contraindicated in patients with a history of seizure disorder or a diagnosis of anorexia nervosa or bulimia, or who are on chronic opioid therapy.
Diethylpropion
Diethylpropion (Tenuate, Tenuate Dospan) is a central nervous system stimulant similar to bupropion in its structure. It was approved by the FDA for treating obesity in 1959. It should be used as part of a short-term weight-loss plan, along with a low-calorie diet. Diethylpropion is also a controlled substance and, as with phentermine therapy, patients are required to be seen once a month by their prescriber. Diethylpropion cannot be prescribed for more than 3 months.
Benefits. Weight loss in a randomized trial at 6 months:
- 3.2% with placebo
- 9.8% with diethylpropion 50 mg twice a day.23
After 6 months, all participants received diethylpropion in an open-label extension for an additional 6 months. At 12 months, the mean weight loss produced by diethylpropion was 10.6%.23 No differences in heart rate, blood pressure, electrocardiographic results, or psychiatric evaluations were observed.
Adverse effects. As with phentermine, common side effects of diethylpropion include insomnia, dry mouth, dizziness, headache, mild increases in blood pressure, and palpitations.23
Lorcaserin
Lorcaserin (Belviq) was approved by the FDA for chronic weight management in June 2012. It exerts its effects through binding selectively to central 5-HT2C serotonin receptors, with poor affinity for 5-HT2A and 5-HT2B receptors. Nonselective serotoninergic agents, including fenfluramine and dexfenfluramine, were withdrawn from the market in 1997 after being reported to be associated with valvular heart abnormalities.24 Lorcaserin has been approved for long-term use by the FDA.
Benefits. Mean weight loss at 1 year in the Behavioral Modification and Lorcaserin for Overweight and Obesity Management in Diabetes Mellitus trial25 was:
- 1.5% with placebo
- 5.0% with lorcaserin 10 mg once daily
- 4.5% with lorcaserin 10 mg twice daily.
Absolute reductions in HbA1c values were:
- 0.4% with placebo
- 0.9% with lorcaserin 10 mg once daily
- 1.0% with lorcaserin 10 mg twice daily.
Absolute reductions in fasting plasma glucose values were:
- 11.9 mg/dL with placebo
- 27.4 mg/dL with lorcaserin 10 mg once daily
- 28.4 mg/dL with lorcaserin 10 mg twice daily.25
Adverse effects. The most common adverse effects were headache, dizziness, and fatigue. There was no significant increase in valvulopathy on echocardiography of participants receiving lorcaserin compared with placebo.25
Liraglutide
Liraglutide (Saxenda, Victoza) is a glucagon-like peptide-1 (GLP-1) receptor agonist. Native GLP-1 is a hormone secreted by intestinal L cells in response to consumption of fat and carbohydrate-rich foods. It stimulates the release of insulin and suppresses any inappropriately elevated postprandial glucagon levels. In addition to its effect on glucose metabolism, GLP-1 also reduces appetite and delays gastric emptying in humans.26 Unlike the extremely short half-life of native GLP-1 (estimated at 1 to 2 minutes), liraglutide has a half-life of 13 hours, allowing it to be given once daily.26 Liraglutide medication has been approved for long-term use by the FDA.
Benefits. The Liraglutide Effect and Action in Diabetes 1–5 studies compared the effects of liraglutide monotherapy with antidiabetic oral medications or insulin, as well as in combination with antidiabetic oral agents. Liraglutide (Victoza) at doses approved for type 2 DM of 1.2 mg and 1.8 mg daily had significant effects in reducing HbA1c by 0.48% to 1.84% and weight by 2.5 kg to 4 kg.27,28 At a dose of 3.0 mg, liraglutide (Saxenda) is approved for chronic weight management. This dose of liraglutide has been shown to be effective and safe in patients with type 2 DM and obesity.
In the 56-week SCALE Diabetes trial,29 liraglutide at a dose of 3.0 mg resulted in 6.0% weight reduction, compared with 2.0% in the placebo group. Of participants receiving 3.0 mg of liraglutide, 54.3% achieved more than 5% weight loss at 56 weeks compared with 21.4% with placebo. Liraglutide also resulted in significant improvements in HbA1c (mean change −1.3% vs −0.3% with placebo), fasting and postprandial glucose levels, and fasting glucagon levels.29
The Liraglutide Effect and Action in Diabetes: Evaluation of Cardiovascular Outcome Results trial has shown liraglutide to significantly reduce rates of major cardiovascular events (first occurrence of cardiovascular death, nonfatal myocardial infarction, or nonfatal stroke) in patients with elevated cardiovascular risk factors.30 These findings make liraglutide a favorable choice for high-risk patients with type 2 DM, obesity, and cardiovascular disease.
It is important to indicate that if a 5% weight loss is not achieved by 3 months with any of these weight-loss medications, it would be reasonable to stop the medication and consider switching to a different medication. These medications work best when combined with diet and increased physical activity. Weight-loss medications should never be used during pregnancy.
Women of childbearing age should be advised to use effective contraception methods while taking any of the above antiobesity medications.
Diabetes medications associated with weight loss: Metformin and SGLT-2 inhibitors
Although not FDA-approved for weight management, metformin has anorexigenic effects that aid in weight loss. It also inhibits hepatic glucose production and improves peripheral insulin sensitivity, making it a useful agent in patients with type 2 DM and obesity.
A meta-analysis of 31 trials showed that metformin reduced body mass index by 5.3% compared with placebo.31 Metformin should be considered as a first-line agent in obese patients with type 2 DM.
In healthy people, nearly all glucose is filtered in the glomerulus, but then 98% of it is reabsorbed in the proximal tubule by sodium-glucose cotransporter-2 (SGLT-2). Drugs that inhibit SGLT-2 increase urinary glucose excretion and, as a result, help control hyperglycemia. Another, off-label effect of excreting more glucose is weight loss: a sustained weight loss of about 3 kg to 5 kg in clinical studies.32 Although they can be used as monotherapies, SGLT-2 inhibitors are usually used as add-on therapies in patients with type 2 DM.
AN ALGORITHM FOR TREATMENT
First, we believe that lifestyle interventions by optimization of nutrition and physical activity should be the cornerstone therapy in the management plan of any patient with type 2 DM and obesity. These interventions are best implemented through a comprehensive, multidisciplinary approach that integrates the care of dietitians, physical therapists, exercise physiologists, psychologists, and social workers.33 Patients need also to be seen frequently, ie, at least once every 3 months. The possibility of seeing patients in group-shared medical appointments on a monthly basis could also be considered.
We also believe that metformin should be added early in the course of treatment for its known benefits of improving insulin sensitivity and suppressing appetite. Target HbA1c goals and body weight in patients with type 2 diabetes and obesity should be tailored to the individual based on age, general health status, risk of hypoglycemia, capacity to do physical activity, and associated comorbidities. If no improvements are seen (HbA1c > 7% and < 3 % weight loss) despite lifestyle changes and the addition of metformin, the possibility of adding a GLP-1 receptor agonist or an SGLT-2 inhibitor as a second-line therapy should be considered. Both classes of medications aid in lowering HbA1c and promote further weight loss.
If no clinical progress is achieved at 3 months, the possibility of adding an FDA-approved weight-loss medication, as discussed above, should be strongly considered. Of note, this algorithm targets different endogenous pathways for weight loss and thus minimizes weight regain through compensatory mechanisms.
THE NEED FOR PATIENT-CENTERED WEIGHT-LOSS CONVERSATIONS
Patient-centered care has become a core quality measure in our healthcare systems and a key to our patients’ success. The decision to start an antiobesity drug should therefore reflect careful consideration of medical and personal patient issues, all of which are valued differently by patients.34
Individualized therapy is even more relevant among patients suffering from a significant burden of disease. About 80% of patients with diabetes live with at least 1 other medical condition,35 and each of these patients spends over 2 hours a day, on average, following doctors’ recommendations.36 If antiobesity medications are prescribed without careful consideration of the patient’s preexisting workload, they will be destined to fail. Therefore, it becomes crucial to first account for the patient’s ability to cope with therapy intensification. This requires careful deliberation between healthcare providers and patients, in aims of targeting a weight-loss plan that fits patients’ goals and is aligned with providers’ expectations.
Healthcare systems also play a key role in supporting better conversations about obesity in type 2 DM patients. They could implement multifaceted initiatives to promote shared decision-making and the use of decision aids to advance patient-centered obesity practices.37 Policymakers could redesign quality measures aimed at capturing the quality of obesity conversations, and develop policies that support better education for clinicians regarding the importance of addressing obesity with adequate communication and patient-centered skills. Guidelines are often too disease-specific and do not consider comorbidities in their context when providing recommendations.38 Thus, diabetes societies should respond to the need to guide care for patients with diabetes and its comorbidities, particularly obesity.
CONCLUSIONS
Obesity is a serious global health issue and a leading risk factor for type 2 DM. Lifestyle measures are the cornerstone of preventing and treating obesity and type 2 DM. Emerging data support the effectiveness of intensive, interdisciplinary weight-loss programs in patients with diabetes. The use of antiobesity drugs should be considered in patients who have not achieved adequate responses to lifestyle interventions. Medications should be tailored to the individual’s health risks and metabolic and psychobehavioral characteristics. In many cases, the addition of weight-loss drugs will help accomplish and maintain the recommended 10% weight reduction, resulting in improvement in glycemic control and significant reduction in cardiovascular risk factors. New studies combining antiobesity and antidiabetes medications in the context of lifestyle interventions will help define the optimal therapeutic approach for patients with type 2 DM and obesity.
- Ogden CL, Carroll MD, Fryar CD, Flegal KM. Prevalence of obesity among adults and youth: United States, 2011–2014. NCHS Data Brief 2015; (219):1–8.
- Lyznicki JM, Young DC, Riggs JA, Davis RM; for the Council on Scientific Affairs, American Medical Association. Obesity: assessment and management in primary care. Am Fam Physician 2001; 63:2185–2196.
- World Health Organization (WHO). Obesity and overweight fact sheet. www.who.int/mediacentre/factsheets/fs311/en. Updated June 2016. Accessed June 22, 2017.
- Daniels J. Obesity: America’s epidemic. Am J Nurs 2006; 106:40–49.
- Colditz GA, Willett WC, Rotnitzky A, Manson JE. Weight gain as a risk factor for clinical diabetes mellitus in women. Ann Intern Med 1995; 122:481–486.
- Schauer PR, Bhatt DL, Kirwan JP, et al; STAMPEDE Investigators. Bariatric surgery versus intensive medical therapy for diabetes-5-year outcomes. N Engl J Med 2017; 376:641–651.
- Oldridge NB, Stump TE, Nothwehr FK, Clark DO. Prevalence and outcomes of comorbid metabolic and cardiovascular conditions in middle- and older-age adults. J Clin Epidemiol 2001; 54:928–934.
- Garber AJ, Abrahamson MJ, Barzilay JI, et al. Consensus statement by the American Association of Clinical Endocrinologists and American College of Endocrinology on the comprehensive type 2 diabetes management algorithm—2016 executive summary. Endocr Pract 2016; 22:84–113.
- McFarlane SI. Antidiabetic medications and weight gain: implications for the practicing physician. Curr Diab Rep 2009; 9:249–254.
- UK Prospective Diabetes Study (UKPDS) Group. Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34). Lancet 1998; 352:854–865.
- Beal E. The pros and cons of designating obesity a disease: the new AMA designation stirs debate. Am J Nurs 2013; 113:18–19.
- Kraschnewski JL, Sciamanna CN, Stuckey HL, et al. A silent response to the obesity epidemic: decline in US physician weight counseling. Med Care 2013; 51:186–192.
- Potter MB, Vu JD, Croughan-Minihane M. Weight management: what patients want from their primary care physicians. J Fam Pract 2001; 50:513–518.
- Pappachan JM, Viswanath AK. Medical management of diabesity: do we have realistic targets? Curr Diab Rep 2017; 17:4.
- Lexicomp Online, Lexi-Drugs, Hudson, OH: Wolters Kluwer Clinical Drug Information, Inc., 2017.
- Torgerson JS, Hauptman J, Boldrin MN, Sjöström L. XENical in the prevention of diabetes in obese subjects (XENDOS) study: a randomized study of orlistat as an adjunct to lifestyle changes for the prevention of type 2 diabetes in obese patients. Diabetes Care 2004; 27:155–161.
- Buysschaert B, Aydin S, Morelle J, Hermans MP, Jadoul M, Demoulin N. Weight loss at a high cost: orlistat-induced late-onset severe kidney disease. Diabetes Metab 2016; 42:62–64.
- Colman E. Anorectics on trial: a half century of federal regulation of prescription appetite suppressants. Ann Intern Med 2005; 143:380–385.
- Aronne LJ, Wadden TA, Peterson C, Winslow D, Odeh S, Gadde KM. Evaluation of phentermine and topiramate versus phentermine/topiramate extended-release in obese adults. Obesity (Silver Spring) 2013; 21:2163–2171.
- Solas M, Milagro FI, Martínez-Urbistondo D, Ramirez MJ, Martínez JA. Precision obesity treatments including pharmacogenetic and nutrigenetic approaches. Trends Pharmacol Sci 2016; 37:575–593.
- Gadde KM, Allison DB, Ryan DH, et al. Effects of low-dose, controlled-release, phentermine plus topiramate combination on weight and associated comorbidities in overweight and obese adults (CONQUER): a randomised, placebo-controlled, phase 3 trial. Lancet 2011; 377:1341–1352.
- Hollander P, Gupta AK, Plodkowski R, et al; for the COR-Diabetes Study Group. Effects of naltrexone sustained-release/bupropion sustained-release combination therapy on body weight and glycemic parameters in overweight and obese patients with type 2 diabetes. Diabetes Care 2013; 36:4022–4029.
- Cercato C, Roizenblatt VA, Leança CC, et al. A randomized double-blind placebo-controlled study of the long-term efficacy and safety of diethylpropion in the treatment of obese subjects. Int J Obes (Lond) 2009; 33:857–865.
- Gardin JM, Schumacher D, Constantine G, Davis KD, Leung C, Reid CL. Valvular abnormalities and cardiovascular status following exposure to dexfenfluramine or phentermine/fenfluramine. JAMA 2000; 283:1703–1709.
- O’Neil PM, Smith SR, Weissman NJ, et al. Randomized placebo-controlled clinical trial of lorcaserin for weight loss in type 2 diabetes mellitus: the BLOOM-DM study. Obesity (Silver Spring) 2012; 20:1426–1436.
- Amori RE, Lau J, Pittas AG. Efficacy and safety of incretin therapy in type 2 diabetes: systematic review and meta-analysis. JAMA 2007; 298:194–206.
- Blonde L, Russell-Jones D. The safety and efficacy of liraglutide with or without oral antidiabetic drug therapy in type 2 diabetes: an overview of the LEAD 1–5 studies. Diabetes Obes Metab 2009; 11(suppl 3):26–34.
- Buse JB, Rosenstock J, Sesti G, et al; for the LEAD-6 Study Group. Liraglutide once a day versus exenatide twice a day for type 2 diabetes: a 26-week randomised, parallel-group, multinational, open-label trial (LEAD-6). Lancet 2009; 374:39–47.
- Davies MJ, Bergenstal R, Bode B, et al; for the NN8022-1922 Study Group. Efficacy of liraglutide for weight loss among patients with type 2 diabetes: the SCALE diabetes randomized clinical trial. JAMA 2015; 314:687–699.
- Marso SP, Daniels GH, Brown-Frandsen K, et al; for the LEADER Steering Committee on behalf of the LEADER Trial Investigators. Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med 2016; 375:311–322.
- Salpeter SR, Buckley NS, Kahn JA, Salpeter EE. Meta-analysis: metformin treatment in persons at risk for diabetes mellitus. Am J Med 2008; 121:149–157.
- Vasilakou D, Karagiannis T, Athanasiadou E, et al. Sodium-glucose cotransporter 2 inhibitors for type 2 diabetes: a systematic review and meta-analysis. Ann Intern Med 2013; 159:262–274.
- Burguera B, Jesus Tur J; Escudero AJ, et al. An intensive lifestyle intervention is an effective treatment of morbid obesity: the TRAMOMTANA study—a two-year randomized controlled clinical trial. Int J Endocrinol 2015; 2015:194696.
- Hargraves I, LeBlanc A, Shah ND, Montori VM. Shared decision making: the need for patient-clinician conversation, not just information. Health Aff (Millwood) 2016; 35:627–629.
- Lin P-J, Kent DM, Winn AN, Cohen JT, Neumann PJ. Multiple chronic conditions in type 2 diabetes mellitus: prevalence and consequences. Am J Manag Care 2015; 21:e23–e34.
- Russell LB, Suh D-C, Safford MA. Time requirements for diabetes self-management: too much for many? J Fam Pract 2005; 54:52–56.
- Serrano V, Rodriguez-Gutierrez R, Hargraves I, Gionfriddo MR, Tamhane S, Montori VM. Shared decision-making in the care of individuals with diabetes. Diabet Med 2016; 33:742–751.
- Wyatt KD, Stuart LM, Brito JP, et al. Out of context: clinical practice guidelines and patients with multiple chronic conditions: a systematic review. Med Care 2014; 52(suppl 3):S92–S100.
- Ogden CL, Carroll MD, Fryar CD, Flegal KM. Prevalence of obesity among adults and youth: United States, 2011–2014. NCHS Data Brief 2015; (219):1–8.
- Lyznicki JM, Young DC, Riggs JA, Davis RM; for the Council on Scientific Affairs, American Medical Association. Obesity: assessment and management in primary care. Am Fam Physician 2001; 63:2185–2196.
- World Health Organization (WHO). Obesity and overweight fact sheet. www.who.int/mediacentre/factsheets/fs311/en. Updated June 2016. Accessed June 22, 2017.
- Daniels J. Obesity: America’s epidemic. Am J Nurs 2006; 106:40–49.
- Colditz GA, Willett WC, Rotnitzky A, Manson JE. Weight gain as a risk factor for clinical diabetes mellitus in women. Ann Intern Med 1995; 122:481–486.
- Schauer PR, Bhatt DL, Kirwan JP, et al; STAMPEDE Investigators. Bariatric surgery versus intensive medical therapy for diabetes-5-year outcomes. N Engl J Med 2017; 376:641–651.
- Oldridge NB, Stump TE, Nothwehr FK, Clark DO. Prevalence and outcomes of comorbid metabolic and cardiovascular conditions in middle- and older-age adults. J Clin Epidemiol 2001; 54:928–934.
- Garber AJ, Abrahamson MJ, Barzilay JI, et al. Consensus statement by the American Association of Clinical Endocrinologists and American College of Endocrinology on the comprehensive type 2 diabetes management algorithm—2016 executive summary. Endocr Pract 2016; 22:84–113.
- McFarlane SI. Antidiabetic medications and weight gain: implications for the practicing physician. Curr Diab Rep 2009; 9:249–254.
- UK Prospective Diabetes Study (UKPDS) Group. Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34). Lancet 1998; 352:854–865.
- Beal E. The pros and cons of designating obesity a disease: the new AMA designation stirs debate. Am J Nurs 2013; 113:18–19.
- Kraschnewski JL, Sciamanna CN, Stuckey HL, et al. A silent response to the obesity epidemic: decline in US physician weight counseling. Med Care 2013; 51:186–192.
- Potter MB, Vu JD, Croughan-Minihane M. Weight management: what patients want from their primary care physicians. J Fam Pract 2001; 50:513–518.
- Pappachan JM, Viswanath AK. Medical management of diabesity: do we have realistic targets? Curr Diab Rep 2017; 17:4.
- Lexicomp Online, Lexi-Drugs, Hudson, OH: Wolters Kluwer Clinical Drug Information, Inc., 2017.
- Torgerson JS, Hauptman J, Boldrin MN, Sjöström L. XENical in the prevention of diabetes in obese subjects (XENDOS) study: a randomized study of orlistat as an adjunct to lifestyle changes for the prevention of type 2 diabetes in obese patients. Diabetes Care 2004; 27:155–161.
- Buysschaert B, Aydin S, Morelle J, Hermans MP, Jadoul M, Demoulin N. Weight loss at a high cost: orlistat-induced late-onset severe kidney disease. Diabetes Metab 2016; 42:62–64.
- Colman E. Anorectics on trial: a half century of federal regulation of prescription appetite suppressants. Ann Intern Med 2005; 143:380–385.
- Aronne LJ, Wadden TA, Peterson C, Winslow D, Odeh S, Gadde KM. Evaluation of phentermine and topiramate versus phentermine/topiramate extended-release in obese adults. Obesity (Silver Spring) 2013; 21:2163–2171.
- Solas M, Milagro FI, Martínez-Urbistondo D, Ramirez MJ, Martínez JA. Precision obesity treatments including pharmacogenetic and nutrigenetic approaches. Trends Pharmacol Sci 2016; 37:575–593.
- Gadde KM, Allison DB, Ryan DH, et al. Effects of low-dose, controlled-release, phentermine plus topiramate combination on weight and associated comorbidities in overweight and obese adults (CONQUER): a randomised, placebo-controlled, phase 3 trial. Lancet 2011; 377:1341–1352.
- Hollander P, Gupta AK, Plodkowski R, et al; for the COR-Diabetes Study Group. Effects of naltrexone sustained-release/bupropion sustained-release combination therapy on body weight and glycemic parameters in overweight and obese patients with type 2 diabetes. Diabetes Care 2013; 36:4022–4029.
- Cercato C, Roizenblatt VA, Leança CC, et al. A randomized double-blind placebo-controlled study of the long-term efficacy and safety of diethylpropion in the treatment of obese subjects. Int J Obes (Lond) 2009; 33:857–865.
- Gardin JM, Schumacher D, Constantine G, Davis KD, Leung C, Reid CL. Valvular abnormalities and cardiovascular status following exposure to dexfenfluramine or phentermine/fenfluramine. JAMA 2000; 283:1703–1709.
- O’Neil PM, Smith SR, Weissman NJ, et al. Randomized placebo-controlled clinical trial of lorcaserin for weight loss in type 2 diabetes mellitus: the BLOOM-DM study. Obesity (Silver Spring) 2012; 20:1426–1436.
- Amori RE, Lau J, Pittas AG. Efficacy and safety of incretin therapy in type 2 diabetes: systematic review and meta-analysis. JAMA 2007; 298:194–206.
- Blonde L, Russell-Jones D. The safety and efficacy of liraglutide with or without oral antidiabetic drug therapy in type 2 diabetes: an overview of the LEAD 1–5 studies. Diabetes Obes Metab 2009; 11(suppl 3):26–34.
- Buse JB, Rosenstock J, Sesti G, et al; for the LEAD-6 Study Group. Liraglutide once a day versus exenatide twice a day for type 2 diabetes: a 26-week randomised, parallel-group, multinational, open-label trial (LEAD-6). Lancet 2009; 374:39–47.
- Davies MJ, Bergenstal R, Bode B, et al; for the NN8022-1922 Study Group. Efficacy of liraglutide for weight loss among patients with type 2 diabetes: the SCALE diabetes randomized clinical trial. JAMA 2015; 314:687–699.
- Marso SP, Daniels GH, Brown-Frandsen K, et al; for the LEADER Steering Committee on behalf of the LEADER Trial Investigators. Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med 2016; 375:311–322.
- Salpeter SR, Buckley NS, Kahn JA, Salpeter EE. Meta-analysis: metformin treatment in persons at risk for diabetes mellitus. Am J Med 2008; 121:149–157.
- Vasilakou D, Karagiannis T, Athanasiadou E, et al. Sodium-glucose cotransporter 2 inhibitors for type 2 diabetes: a systematic review and meta-analysis. Ann Intern Med 2013; 159:262–274.
- Burguera B, Jesus Tur J; Escudero AJ, et al. An intensive lifestyle intervention is an effective treatment of morbid obesity: the TRAMOMTANA study—a two-year randomized controlled clinical trial. Int J Endocrinol 2015; 2015:194696.
- Hargraves I, LeBlanc A, Shah ND, Montori VM. Shared decision making: the need for patient-clinician conversation, not just information. Health Aff (Millwood) 2016; 35:627–629.
- Lin P-J, Kent DM, Winn AN, Cohen JT, Neumann PJ. Multiple chronic conditions in type 2 diabetes mellitus: prevalence and consequences. Am J Manag Care 2015; 21:e23–e34.
- Russell LB, Suh D-C, Safford MA. Time requirements for diabetes self-management: too much for many? J Fam Pract 2005; 54:52–56.
- Serrano V, Rodriguez-Gutierrez R, Hargraves I, Gionfriddo MR, Tamhane S, Montori VM. Shared decision-making in the care of individuals with diabetes. Diabet Med 2016; 33:742–751.
- Wyatt KD, Stuart LM, Brito JP, et al. Out of context: clinical practice guidelines and patients with multiple chronic conditions: a systematic review. Med Care 2014; 52(suppl 3):S92–S100.
KEY POINTS
- Obesity contributes to type 2 DM and worsens its control. Yet insulin therapy and most first-line diabetes drugs cause weight gain as a side effect.
- We believe that physicians should include body weight along with blood glucose levels as targets of therapy in patients with type 2 DM.
- Several drugs are approved for weight loss, and although their effect on weight tends to be moderate, some have been shown to reduce the incidence of type 2 DM and improve diabetic control.
- A stepwise approach to managing type 2 DM and obesity starts with lifestyle interventions and advances to adding (1) metformin, (2) a glucagon-like peptide-1 receptor agonist or a sodium-glucose cotransporter-2 inhibitor, and (3) one of the approved weight-loss drugs.
Metabolic surgery for treating type 2 diabetes mellitus: Now supported by the world's leading diabetes organizations
LIMITATIONS OF LIFESTYLE MANAGEMENT AND MEDICATIONS
First-line therapy with lifestyle management and second-line therapy with medications, including oral agents and insulin, are the mainstays of type 2 DM therapy. Although these approaches have reduced hyperglycemia and cardiovascular mortality, many patients have poor glycemic control and develop severe diabetes-related complications. A study using data from the National Health and Nutrition Examination Survey (N = 4,926) to evaluate success rates of lifestyle management plus drug therapy found that just 53% of patients with type 2 DM maintained a hemoglobin A1c (HbA1c) below 7%.6 Similarly, only 51% of those patients achieved a systolic and diastolic blood pressure less than 130/80 mm Hg, and only 56% achieved a low-density lipoprotein cholesterol level less than 100 mg/dL. Altogether, only 19% of the study cohort achieved all 3 therapy targets. Documented limitations of lifestyle counseling and drug therapy include behavior maladaptation, limitations in drug potency, nonadherence to medications, adverse effects, and economic deterrents.7
METABOLIC SURGERY FOR TYPE 2 DM
For patients with obesity and type 2 DM in whom lifestyle management and medications do not achieve desired treatment goals, bariatric surgery has emerged as the most effective treatment for attaining significant and durable weight loss. These gastrointestinal (GI) procedures, which reduce gastric volume with or without rerouting nutrient flow through the small intestine, were developed to yield long-term weight loss in patients with severe obesity. It is now known that they also cause dramatic improvement or remission of obesity-related comorbidities, especially type 2 DM. Research has shown that these effects are not only secondary to weight loss but also depend on neuroendocrine mechanisms secondary to changes in GI physiology. For these reasons, bariatric surgery is increasingly used with the primary intent to treat type 2 DM or metabolic disease, a practice referred to as metabolic surgery.
For more than 2 decades, indications for metabolic surgery reflected guidelines from a 1991 National Institutes of Health (NIH) consensus conference, which suggested considering surgery only in patients with a BMI of 40 kg/m2 or greater or a BMI of 35 kg/m2 or greater and significant obesity-related comorbidities.11 Guidelines published in 2013 expanded the recommendations to include adults with a BMI of at least 35 kg/m2 and an obesity-related comorbidity, such as diabetes, who are motivated to lose weight.4 These recommendations were primarily designed to guide the use of surgery as a weight-loss intervention for severe obesity. However, guidelines published in 2016 support use of metabolic surgery as a specific treatment for type 2 DM.5
Potential mechanisms resolving type 2 DM: More than weight loss
Bariatric surgery has been shown to have profound glucoregulatory effects. These include rapid improvement in hyperglycemia and reduction in exogenous insulin requirements that occur early after surgery and before the patient has any significant weight loss.12,13 Additionally, experiments in rodents showed that changes to GI anatomy can directly influence glucose homeostasis, independently of weight loss and caloric restriction.14
Although the exact molecular mechanisms underlying the effects of metabolic surgery on diabetes are not fully understood, many factors appear to play a role, including changes in bile acid metabolism, GI tract nutrient sensing, glucose utilization, insulin resistance, and intestinal microbiomes.15 These changes, acting through peripheral or central pathways, or perhaps both, lead to reduced hepatic glucose production, increased tissue glucose uptake, improved insulin sensitivity, and enhanced beta-cell function. A constellation of gut-derived neuroendocrine changes, rather than a single overarching mechanism, is the likely mediator of postoperative glycemic improvement, with the contributing factors varying according to the surgical procedure.
METABOLIC SURGERY OUTCOMES
Weight loss
Long-term reduction of excess body fat is a major goal of metabolic and bariatric surgery. Weight loss is usually expressed as either the percent of weight loss or the percent of excess weight loss (ie, weight loss above ideal weight). A meta-analysis of mostly short-term weight-loss outcomes (ie, < 5 years) from more than 22,000 procedures found an overall mean excess weight loss of 47.5% for patients who underwent LAGB, 61.6% for RYGB, 68.2% for vertical-banded gastroplasty, and 70.1% for BPD-DS.16 Vertical-banded gastroplasty differs from LAGB in that both a band and staples are used to create a small stomach pouch. Excess weight loss for SG generally averages 50% to 55%, which is intermediate between LAGB and RYGB.17,18
The Swedish Obese Subjects study (N = 4,047), a prospective study of bariatric surgery vs nonsurgical weight management of severely obese patients (BMI > 34), is the largest weight-loss study with the longest follow-up.19 At 20 years, the mean weight loss was 26% for gastric bypass, 18% for vertical-banded gastroplasty, 13% for gastric banding, and 1% for controls. A 10-year study in 1,787 severely obese patients (BMI ≥ 35) who underwent RYGB had 21% more weight loss from their baseline weight than the nonsurgical match.20 At 4-year follow-up in 2,410 patients, there were significant variations in weight loss depending on the procedure: 27.5% for RYGB, 17.8% for SG, and 10.6% in LAGB. Between 2% and 31% regained weight back to baseline: 30.5% for LAGB, 14.6% for SG, and 2.5% for RYGB.20 In contrast, long-term medical (nonsurgical) weight loss rarely exceeds 5%, even with intensive lifestyle intervention.21
Diabetes remission, cardiovascular risk factors, glycemic control
A meta-analysis of 19 mostly observational studies (N = 4,070 patients) reported an overall type 2 DM remission rate of 78% after bariatric surgery with 1 to 3 years of follow-up.22 Resolution or remission was typically defined as becoming “nondiabetic” with normal HbA1c without medications. In the Swedish Obese Subjects study, the remission rate was 72% at 2 years and 36% at 10 years compared with 21% and 13%, respectively, for the nonsurgical controls (P < .001).23 Bariatric surgery was also markedly more effective than nonsurgical treatment in preventing type 2 DM, with a relative risk reduction of 78%.
A systematic review published in 2012 evaluated long-term cardiovascular risk reduction after bariatric surgery in 73 studies and 19,543 patients.24 At a mean follow-up of 57.8 months, the average excess weight loss for all procedures was 54% and rates of remission or improvement were 63% for hypertension, 73% for type 2 DM, and 65% for hyperlipidemia. Results from 12 cohort-matched, nonrandomized studies comparing bariatric surgery vs nonsurgical controls suggest that improvements in surrogate disease markers such as HbA1c, blood pressure, lipids, and body weight after surgery translate to reduced macrovascular and microvascular events and death.25 One of these studies involving male veterans who were mostly at high cardiovascular risk reported a 42% reduction in mortality at 10 years compared with medical therapy.26
In the Swedish Obese Subjects study, the mortality rate from cardiovascular disease in the bariatric surgical group was lower than for control patients (adjusted hazard ratio, 0.47; P = .002) despite a greater prevalence of smoking and higher baseline weights and blood pressures in the surgical cohort.19 For patients with type 2 DM in this study, surgery was associated with a 50% reduction in microvascular complications.27 After 15 years of follow-up, the cumulative incidence of microvascular complications was 41.8 per 1,000 person-years for control patients and 20.6 per 1,000 person-years in the surgery group (hazard ratio, 0.44; P < .001).
These observational, nonrandomized study data suggest that in patients with type 2 DM, bariatric surgery is significantly better than medical management alone in improving glycemic control, reducing cardiovascular risk factors, and lowering long-term morbidity and mortality associated with type 2 DM.
METABOLIC SURGERY: CLINICAL TRIALS
Collectively, these RCTs showed that surgery was significantly superior to medical treatment in reaching the designated glycemic target (P < .05 for all). The one exception showed that diabetes remission for LAGB vs medical treatment was 33% and 23%, respectively.41 This result might be due to patients in this study having advanced type 2 DM (HbA1c 8.2% ± 1.2%, with 40% on insulin), and they likely had reduced beta-cell function. Overall, surgery decreased HbA1c by 2% to 3.5%, whereas medical treatment lowered it by only 1% to 1.5%. Most of these studies also showed superiority of surgery over medical treatment in achieving secondary end points such as weight loss, remission of metabolic syndrome, reduction in diabetes and cardiovascular medications, and improvement in triglycerides, lipids, and quality of life. Results were mixed in terms of improvements in systolic and diastolic blood pressure or low-density lipoproteins after surgery vs medical treatment, but many studies did show a corresponding reduction in medication usage.
Durability of the effects of surgery was demonstrated in a 5-year study that showed superior and durable weight loss and glycemic control (remission) with both RYGB and BPD in severely obese patients (BMI ≥ 35) vs medical therapy.32 Similarly, Schauer et al43 showed that RYGB and SG were more effective than intensive medical therapy in improving or, in some cases, resolving hyperglycemia for 5 years. In the RCTs, patients who preoperatively had shorter duration of diabetes, lower HbA1c levels, no insulin requirement, and more postoperative weight loss were more likely to achieve diabetes remission.
Although previous guidelines and payer coverage policies had limited metabolic surgery to severely obese patients (BMI ≥ 35 kg/m2), nearly all RCTs showed that the surgical procedures, especially RYGB and SG, were equally effective in patients with BMI 30 to 35 kg/m2. This is particularly important given that most patients with type 2 DM have a BMI less than 35 kg/m2. The effect of surgery in these patients with mild obesity is also durable out to at least 5 years.43
No RCT was sufficiently powered to detect differences in macrovascular or microvascular complications or death, especially at the relatively short follow-up, and no such differences have been detected thus far. The STAMPEDE (Surgical Therapy and Medications Potentially Eradicate Diabetes Efficiently) trial43 showed that bariatric surgery (RYGB or SG) did not appear to worsen or improve retinopathy outcomes at 5 years compared with intensive medical management.
METABOLIC SURGERY: ADVERSE EVENTS
Surgical complications
Nutritional deficiencies
Postoperative nutritional deficiencies are typically associated with diminished nutrient intake or the malabsorptive effect of bariatric procedures. They are more common after RYGB and BPD-DS and less common after SG and LAGB. In addition, there is a high prevalence of nutritional deficiencies (35%–80%) in patients seeking bariatric surgery; thus, poor preoperative nutrition may be a factor in the development of postoperative deficiencies. Common preoperative nutrient deficiencies are vitamin A (11%), vitamin B12 (13%), vitamin D (40%), zinc (30%), iron (16%), ferritin (9%), selenium (58%), and folate (6%).51 Recommendations are to assess for these deficiencies and correct any identified before surgery.
Mild anemia after bariatric procedures is common, occurring in 15% to 20% of cases, and it is believed to result from reduced absorption of iron and B12, as well from pre-existing iron deficiency anemia in premenopausal patients.52 Deficiencies in trace minerals (selenium, zinc, and copper) and vitamins (B12, B1, A, E, D, and K) can occur after bariatric procedures, especially after BPD-DS.53 Nutrient deficiencies can be prevented or corrected with appropriate vitamin, iron, and calcium supplementation.54
Bone mineral density may decrease after bariatric surgery (14% in the proximal femur).55 Reduced mechanical loading after weight loss, reduced consumption and malabsorption of micronutrients (calcium, vitamin D), and neurohormonal alterations are potential underlying mechanisms of bone mineral density reduction after bariatric surgery. Rates of bone fracture and osteoporosis are not well delineated, raising questions about whether bone loss after bariatric surgery is clinically relevant or a functional adaptation to skeletal unloading. However, the extreme malabsorptive procedures of BPD-DS have been associated with severe calcium and vitamin D deficiencies, leading to decreased bone mineral density and osteoporosis.
Protein malnutrition also can occur after these extreme malabsorptive procedures. Patients require postoperative oral protein supplementation (80–100 g/day) and lifelong monitoring for nutritional complications after these procedures.56
Additional complications
Other late complications of bariatric surgery that are less clear in incidence and cause include kidney stones, alcohol abuse, depression, and suicide. One study of patients after RYGB (N = 4,690) reported a significantly higher prevalence of kidney stones than in obese controls: 7.5% vs 4.6%, respectively.57 Proposed causes of kidney stone formation following bariatric surgery include hyperoxaluria, hypocitraturia, and elevated urine acidity.58
The prevalence of alcohol-use disorder after bariatric surgery ranges from 7.6% to 11.8% and appears to be higher in patients with a history of alcohol use.59 Paradoxically, while bariatric surgery has been shown to significantly decrease depression,60 some studies suggest that a slight increase in the risk of suicide may occur,61 while others do not.62 A recent review concluded that accurate rates of suicide after bariatric surgery are not known, but practitioners should be aware of this concern and appropriately screen and counsel their patients.63
Although the 12 RCTs reported in Table 1 were not powered to detect differences in treatment-related complications, the overall rates of complications were consistent with those in observational studies.9 The most common surgical complications were anemia (15%), need for reoperation (8%), and GI (5%–10%). The 30-day surgical mortality rate was 0.2% (1 death) among the 465 surgical patients. Complications were not limited to the surgical patients. In the medical-treatment control group of the STAMPEDE trial,30 anemia (16%) and weight gain (16%) were common. Investigators reported challenges with medication compliance, including adverse effects leading to discontinuation of medications. Mild hypoglycemia was common, with no significant differences between the surgical and medical treatment groups.
METABOLIC SURGERY: COST EFFECTIVENESS
The cost of bariatric procedures varies considerably but, in general, ranges from $20,000 to $30,000, similar to the cost of cholecystectomy, hysterectomy, and colectomy. Retrospective analyses and modeling studies indicate that metabolic surgery is cost-effective and may present a cost savings in patients with type 2 DM, with a break-even time between 5 and 10 years.64,65 The cost savings, largely based on assumptions of long-term effectiveness and safety, result from reductions in medication use, outpatient care costs, and long-term complications of type 2 DM.
WHO SHOULD HAVE METABOLIC SURGERY?
Until recently, there was no clear national or international consensus on the role of metabolic surgery in treating type 2 DM. In 2015, the 2nd Diabetes Surgery Summit (DSS-II) Consensus Conference published guidelines that were endorsed by more than 50 diabetes and medical organizations.5 The recommendations cover many clinically relevant issues, including patient selection, preoperative evaluation, choice of procedure, and postoperative follow-up. The consensus conference delegates concluded that there is sufficient evidence demonstrating that metabolic surgery achieves excellent glycemic control and reduces cardiovascular risk factors.
The treatment algorithm from DSS-II incorporates appropriate use of all 3 treatment modalities: lifestyle intervention, drug therapy, and surgery (Figure 5).5 The 2017 Standards of Care for Diabetes from the American Diabetes Association include those key indications in the recommendations for metabolic surgery (Table 3).2
SUMMARY
The safety of metabolic surgery has significantly improved with the advent of laparoscopic surgery and recent national quality improvement initiatives that have made gastric bypass and SG as safe as cholecystectomy and appendectomy. Although observational studies suggest that metabolic surgery is associated with a reduction in cardiovascular and diabetes complications and mortality, these observations have not been confirmed in long-term RCTs.
Based on the published evidence, metabolic surgery is now endorsed as a standard treatment option, which provides patients and practitioners with a powerful tool to help combat the life-impairing effects of type 2 DM.
- Bays HE, Chapman RH, Grandy S; for the SHIELD Investigators Group. The relationship of body mass index to diabetes mellitus, hypertension and dyslipidaemia: comparison of data from two national surveys. Int J Clin Pract May 2007; 61:737–747.
- Marathe PH, Gao HX, Close KL. American Diabetes Association standards of medical care in diabetes—2017. Diabetes Care 2017; 40(suppl 1):S1–S135.
- Fox CS, Golden SH, Anderson C, et al; American Heart Association; American Diabetes Association. Update on prevention of cardiovascular disease in adults with type 2 diabetes mellitus in light of recent evidence: a scientific statement from the American Heart Association and the American Diabetes Association. Circulation 2015; 132:691–718.
- Jensen MD, Ryan DH, Apovian CM, et al; American College of Cardiology/American Heart Association Task Force on Practice Guidelines; Obesity Society. 2013 AHA/ACC/TOS guideline for the management of overweight and obesity in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and The Obesity Society. J Am Coll Cardiol 2014; 63:2985–3023.
- Rubino F, Nathan DM, Eckel RH, et al; Delegates of the 2nd Diabetes Surgery Summit. Metabolic surgery in the treatment algorithm for type 2 diabetes: a joint statement by international diabetes organizations. Diabetes Care 2016; 39:861–877.
- Stark Casagrande S, Fradkin JE, Saydah SH, Rust KF, Cowie CC. The prevalence of meeting A1C, blood pressure, and LDL goals among people with diabetes, 1988–2010. Diabetes Care 2013; 36:2271–2279.
- Kolandaivelu K, Leiden BB, O’Gara PT, Bhatt DL. Non-adherence to cardiovascular medications. Eur Heart J 2014; 35:3267–3276.
- Angrisani L, Santonicola A, Iovino P, Formisano G, Buchwald H, Scopinaro N. Bariatric surgery worldwide 2013. Obes Surg 2015; 25:1822–1832.
- Schauer PR, Mingrone G, Ikramuddin S, Wolfe B. Clinical outcomes of metabolic surgery: efficacy of glycemic control, weight loss, and remission of diabetes. Diabetes Care 2016; 39:902–911.
- Khorgami Z, Andalib A, Corcelles R, Aminian A, Brethauer S, Schauer P. Recent national trends in the surgical treatment of obesity: sleeve gastrectomy dominates. Surg Obes Relat Dis 2015; 11(suppl):S1–S34 [Abstract A111].
- Consensus Development Conference Panel. NIH conference. Gastrointestinal surgery for severe obesity. Ann Intern Med 1991; 115:956–961.
- Pories WJ, MacDonald KG Jr, Flickinger EG, et al. Is type II diabetes mellitus (NIDDM) a surgical disease? Ann Surg 1992; 215:633–642.
- Schauer PR, Burguera B, Ikramuddin S, et al. Effect of laparoscopic Roux-en Y gastric bypass on type 2 diabetes mellitus. Ann Surg 2003; 238:467–484.
- Rubino F, Marescaux J. Effect of duodenal-jejunal exclusion in a non-obese animal model of type 2 diabetes: a new perspective for an old disease. Ann Surg 2004; 239:1–11.
- Batterham RL, Cummings DE. Mechanisms of diabetes improvement following bariatric/metabolic surgery. Diabetes Care 2016; 39:893–901.
- Buchwald H, Avidor Y, Braunwald E, et al. Bariatric surgery: a systematic review and meta-analysis. JAMA 2004; 292:1724–1737.
- Brethauer SA, Hammel JP, Schauer PR. Systematic review of sleeve gastrectomy as staging and primary bariatric procedure. Surg Obes Relat Dis 2009; 5:469–475.
- Eid GM, Brethauer S, Mattar SG, Titchner RL, Gourash W, Schauer PR. Laparoscopic sleeve gastrectomy for super obese patients: forty-eight percent excess weight loss after 6 to 8 years with 93% follow-up. Ann Surg 2012; 256:262–265.
- Sjöström L, Peltonen M, Jacobson P, et al. Bariatric surgery and long-term cardiovascular events. JAMA 2012; 307:56–65.
- Maciejewski ML, Arterburn DE, Van Scoyoc L, et al. Bariatric surgery and long-term durability of weight loss. JAMA Surg 2016; 151:1046–1055.
- Wing RR, Bolin P, Brancati FL, et al; for the Look AHEAD Research Group. Cardiovascular effects of intensive lifestyle intervention in type 2 diabetes. N Engl J Med 2013; 369:145–154.
- Buchwald H, Estok R, Fahrbach K, et al. Weight and type 2 diabetes after bariatric surgery: systematic review and meta-analysis. Am J Med 2009; 122:248–256.
- Sjöström L, Lindroos AK, Peltonen M, et al; Swedish Obese Subjects Study Scientific Group. Lifestyle, diabetes, and cardiovascular risk factors 10 years after bariatric surgery. N Engl J Med 2004; 351:2683–2693.
- Vest AR, Heneghan HM, Agarwal S, Schauer PR, Young JB. Bariatric surgery and cardiovascular outcomes: a systematic review. Heart 2012; 98:1763–1777.
- Vest AR, Heneghan HM, Schauer PR, Young JB. Surgical management of obesity and the relationship to cardiovascular disease. Circulation 2013; 127:945–959.
- Arterburn DE, Olsen MK, Smith VA, et al. Association between bariatric surgery and long-term survival. JAMA 2015; 313:62–70.
- Sjöström L, Peltonen M, Jacobson P, et al. Association of bariatric surgery with long-term remission of type 2 diabetes and with microvascular and macrovascular complications. JAMA 2014; 311:2297–2304.
- Dixon JB, O’Brien PE, Playfair J, et al. Adjustable gastric banding and conventional therapy for type 2 diabetes: a randomized controlled trial. JAMA 2008; 299:316–323.
- Schauer PR, Kashyap SR, Wolski K, et al. Bariatric surgery versus intensive medical therapy in obese patients with diabetes. N Engl J Med 2012; 366:1567–1576.
- Schauer PR, Bhatt DL, Kirwan JP, et al; STAMPEDE Investigators. Bariatric surgery versus intensive medical therapy for diabetes—3-year outcomes. N Engl J Med 2014; 370:2002–2013.
- Mingrone G, Panunzi S, De Gaetano A, et al. Bariatric surgery versus conventional medical therapy for type 2 diabetes. N Engl J Med 2012; 366:1577–1585.
- Mingrone G, Panunzi S, De Gaetano A, et al. Bariatric-metabolic surgery versus conventional medical treatment in obese patients with type 2 diabetes: 5 year follow-up of an open-label, single-centre, randomized controlled trial. Lancet 2015; 386:964–973.
- Ikramuddin S, Korner J, Lee WJ, et al. Roux-en-Y gastric bypass vs intensive medical management for the control of type 2 diabetes, hypertension, and hyperlipidemia: the Diabetes Surgery Study randomized clinical trial. JAMA 2013; 309:2240–2249.
- Ikramuddin S, Billington CJ, Lee WJ, et al. Roux-en-Y gastric bypass for diabetes (the Diabetes Surgery Study): 2-year outcomes of a 5-year, randomized, controlled trial. Lancet Diabetes Endocrinol 2015; 3:413–422.
- Liang Z, Wu Q, Chen B, Yu P, Zhao H, Ouyang X. Effect of laparoscopic Roux-en-Y gastric bypass surgery on type 2 diabetes mellitus with hypertension: a randomized controlled trial. Diabetes Res Clin Pract 2013; 101:50–56.
- Halperin F, Ding SA, Simonson DC, et al. Roux-en-Y gastric bypass surgery or lifestyle with intensive medical management in patients with type 2 diabetes: feasibility and 1-year results of a randomized clinical trial. JAMA Surg 2014; 149:716–726.
- Courcoulas AP, Goodpaster BH, Eagleton JK, et al. Surgical vs medical treatments for type 2 diabetes mellitus: a randomized clinical trial. JAMA Surg 2014; 149:707–715.
- Courcoulas AP, Belle SH, Neiberg RH, et al. Three-year outcomes of bariatric surgery vs. lifestyle intervention for type 2 diabetes mellitus treatment: a randomized clinical trial. JAMA Surg 2015; 150:931–940.
- Wentworth JM, Playfair J, Laurie C, et al. Multidisciplinary diabetes care with and without bariatric surgery in overweight people: a randomised controlled trial. Lancet Diabetes Endocrinol 2014; 2:545–552.
- Parikh M, Chung M, Sheth S, et al. Randomized pilot trial of bariatric surgery versus intensive medical weight management on diabetes remission in type 2 diabetic patients who do not meet NIH criteria for surgery and the role of soluble RAGE as a novel biomarker of success. Ann Surg 2014; 260:617–622.
- Ding SA, Simonson DC, Wewalka M, et al. Adjustable gastric band surgery or medical management in patients with type 2 diabetes: a randomized clinical trial. J Clin Endocrinol Metab 2015; 100:2546–2556.
- Cummings DE, Arterburn DE, Westbrook EO, et al. Gastric bypass surgery vs. intensive lifestyle and medical intervention for type 2 diabetes: the CROSSROADS randomized controlled trial. Diabetologia 2016; 59:945–953.
- Schauer PR, Bhatt DL, Kirwan JP, et al; STAMPEDE Investigators. Metabolic surgery vs. intensive medical therapy for diabetes: 5-year outcomes. N Engl J Med 2017; 376:641–651.
- Shah SS, Todkar J, Phadake U, et al. Gastric bypass vs. medical/lifestyle care for type 2 diabetes in South Asians with BMI 25-40 kg/m2: the COSMID randomized trial [261-OR]. Presented at the American Diabetes Association’s 76th Scientific Session; June 10–14, 2016; New Orleans, LA.
- Flum DR, Belle SH, King WC, et al; Longitudinal Assessment of Bariatric Surgery (LABS) Consortium. Perioperative safety in the longitudinal assessment of bariatric surgery. N Engl J Med 2009; 361:445–454.
- Aminian A, Brethauer SA, Kirwan JP, Kashyap SR, Burguera B, Schauer PR. How safe is metabolic/diabetes surgery? Diabetes Obes Metab 2015; 17:198–201.
- Thodiyil PA, Yenumula P, Rogula T, et al. Selective non operative management of leaks after gastric bypass: lessons learned from 2675 consecutive patients. Ann Surg 2008; 248:782–792.
- Rogula T, Yenumula PR, Schauer PR. A complication of Roux-en-Y gastric bypass: intestinal obstruction. Surg Endosc 2007; 21:1914–1918.
- Thornton CM, Rozen WM, So D, Kaplan ED, Wilkinson S. Reducing band slippage in laparoscopic adjustable gastric banding: the mesh plication pars flaccida technique. Obes Surg 2009; 19:1702–1706.
- Himpens J, Cadière G-B, Bazi M, Vouche M, Cadière B, Dapri G. Long-term outcomes of laparoscopic adjustable gastric banding. Arch Surg 2011; 146:802–807.
- Madan AK, Orth WS, Tichansky DS, Ternovits CA. Vitamin and trace mineral levels after laparoscopic gastric bypass. Obes Surg 2006; 16:603–606.
- Love AL, Billett HH. Obesity, bariatric surgery, and iron deficiency: true, true, true and related. Am J Hematol 2008; 83:403–409.
- Shankar P, Boylan M, Sriram K. Micronutrient deficiencies after bariatric surgery. Nutrition 2010; 26:1031–1037.
- Gong K, Gagner M, Pomp A, Almahmeed T, Bardaro SJ. Micronutrient deficiencies after laparoscopic gastric bypass: recommendations. Obes Surg 2008; 18:1062–1066.
- Scibora LM. Skeletal effects of bariatric surgery: examining bone loss, potential mechanisms and clinical relevance. Diabetes Obes Metab 2014; 16:1204–1213.
- Baptista V, Wassef W. Bariatric procedures: an update on techniques, outcomes and complications. Curr Opin Gastroenterol 2013; 29:684–693.
- Matlaga BR, Shore AD, Magnuson T, Clark JM, Johns R, Makary MA. Effect of gastric bypass surgery on kidney stone disease. J Urol 2009; 181:2573–2577.
- Sakhaee K, Poindexter J, Aguirre C. The effects of bariatric surgery on bone and nephrolithiasis. Bone 2016; 84:1–8.
- Li L, Wu LT. Substance use after bariatric surgery: a review. J Psychiatr Res 2016; 76:16–29.
- Ayloo S, Thompson K, Choudhury N, Sheriffdeen R. Correlation between the Beck Depression Inventory and bariatric surgical procedures. Surg Obes Relat Dis 2015; 11:637–342.
- Adams TD, Gress RE, Smith SC, et al. Long-term mortality after gastric bypass surgery. N Engl J Med 2007; 357:753–761.
- Sjöström L, Narbro K, Sjöström CD, et al; Swedish Obese Subjects Study. Effects of bariatric surgery on mortality in Swedish obese subjects. N Engl J Med 2007; 357:741–752.
- Mitchell JE, Crosby R, de Zwaan M, et al. Possible risk factors for increased suicide following bariatric surgery. Obesity (Silver Spring) 2013; 21:665–672.
- Fouse T, Schauer P. The socioeconomic impact of morbid obesity and factors affecting access to obesity surgery. Surg Clin North Am 2016; 96:669–679.
- Rubin JK, Hinrichs-Krapels S, Hesketh R, Martin A, Herman WH, Rubino F. Identifying barriers to appropriate use of metabolic/bariatric surgery for type 2 diabetes treatment: policy lab results. Diabetes Care 2016; 39:954–963.
LIMITATIONS OF LIFESTYLE MANAGEMENT AND MEDICATIONS
First-line therapy with lifestyle management and second-line therapy with medications, including oral agents and insulin, are the mainstays of type 2 DM therapy. Although these approaches have reduced hyperglycemia and cardiovascular mortality, many patients have poor glycemic control and develop severe diabetes-related complications. A study using data from the National Health and Nutrition Examination Survey (N = 4,926) to evaluate success rates of lifestyle management plus drug therapy found that just 53% of patients with type 2 DM maintained a hemoglobin A1c (HbA1c) below 7%.6 Similarly, only 51% of those patients achieved a systolic and diastolic blood pressure less than 130/80 mm Hg, and only 56% achieved a low-density lipoprotein cholesterol level less than 100 mg/dL. Altogether, only 19% of the study cohort achieved all 3 therapy targets. Documented limitations of lifestyle counseling and drug therapy include behavior maladaptation, limitations in drug potency, nonadherence to medications, adverse effects, and economic deterrents.7
METABOLIC SURGERY FOR TYPE 2 DM
For patients with obesity and type 2 DM in whom lifestyle management and medications do not achieve desired treatment goals, bariatric surgery has emerged as the most effective treatment for attaining significant and durable weight loss. These gastrointestinal (GI) procedures, which reduce gastric volume with or without rerouting nutrient flow through the small intestine, were developed to yield long-term weight loss in patients with severe obesity. It is now known that they also cause dramatic improvement or remission of obesity-related comorbidities, especially type 2 DM. Research has shown that these effects are not only secondary to weight loss but also depend on neuroendocrine mechanisms secondary to changes in GI physiology. For these reasons, bariatric surgery is increasingly used with the primary intent to treat type 2 DM or metabolic disease, a practice referred to as metabolic surgery.
For more than 2 decades, indications for metabolic surgery reflected guidelines from a 1991 National Institutes of Health (NIH) consensus conference, which suggested considering surgery only in patients with a BMI of 40 kg/m2 or greater or a BMI of 35 kg/m2 or greater and significant obesity-related comorbidities.11 Guidelines published in 2013 expanded the recommendations to include adults with a BMI of at least 35 kg/m2 and an obesity-related comorbidity, such as diabetes, who are motivated to lose weight.4 These recommendations were primarily designed to guide the use of surgery as a weight-loss intervention for severe obesity. However, guidelines published in 2016 support use of metabolic surgery as a specific treatment for type 2 DM.5
Potential mechanisms resolving type 2 DM: More than weight loss
Bariatric surgery has been shown to have profound glucoregulatory effects. These include rapid improvement in hyperglycemia and reduction in exogenous insulin requirements that occur early after surgery and before the patient has any significant weight loss.12,13 Additionally, experiments in rodents showed that changes to GI anatomy can directly influence glucose homeostasis, independently of weight loss and caloric restriction.14
Although the exact molecular mechanisms underlying the effects of metabolic surgery on diabetes are not fully understood, many factors appear to play a role, including changes in bile acid metabolism, GI tract nutrient sensing, glucose utilization, insulin resistance, and intestinal microbiomes.15 These changes, acting through peripheral or central pathways, or perhaps both, lead to reduced hepatic glucose production, increased tissue glucose uptake, improved insulin sensitivity, and enhanced beta-cell function. A constellation of gut-derived neuroendocrine changes, rather than a single overarching mechanism, is the likely mediator of postoperative glycemic improvement, with the contributing factors varying according to the surgical procedure.
METABOLIC SURGERY OUTCOMES
Weight loss
Long-term reduction of excess body fat is a major goal of metabolic and bariatric surgery. Weight loss is usually expressed as either the percent of weight loss or the percent of excess weight loss (ie, weight loss above ideal weight). A meta-analysis of mostly short-term weight-loss outcomes (ie, < 5 years) from more than 22,000 procedures found an overall mean excess weight loss of 47.5% for patients who underwent LAGB, 61.6% for RYGB, 68.2% for vertical-banded gastroplasty, and 70.1% for BPD-DS.16 Vertical-banded gastroplasty differs from LAGB in that both a band and staples are used to create a small stomach pouch. Excess weight loss for SG generally averages 50% to 55%, which is intermediate between LAGB and RYGB.17,18
The Swedish Obese Subjects study (N = 4,047), a prospective study of bariatric surgery vs nonsurgical weight management of severely obese patients (BMI > 34), is the largest weight-loss study with the longest follow-up.19 At 20 years, the mean weight loss was 26% for gastric bypass, 18% for vertical-banded gastroplasty, 13% for gastric banding, and 1% for controls. A 10-year study in 1,787 severely obese patients (BMI ≥ 35) who underwent RYGB had 21% more weight loss from their baseline weight than the nonsurgical match.20 At 4-year follow-up in 2,410 patients, there were significant variations in weight loss depending on the procedure: 27.5% for RYGB, 17.8% for SG, and 10.6% in LAGB. Between 2% and 31% regained weight back to baseline: 30.5% for LAGB, 14.6% for SG, and 2.5% for RYGB.20 In contrast, long-term medical (nonsurgical) weight loss rarely exceeds 5%, even with intensive lifestyle intervention.21
Diabetes remission, cardiovascular risk factors, glycemic control
A meta-analysis of 19 mostly observational studies (N = 4,070 patients) reported an overall type 2 DM remission rate of 78% after bariatric surgery with 1 to 3 years of follow-up.22 Resolution or remission was typically defined as becoming “nondiabetic” with normal HbA1c without medications. In the Swedish Obese Subjects study, the remission rate was 72% at 2 years and 36% at 10 years compared with 21% and 13%, respectively, for the nonsurgical controls (P < .001).23 Bariatric surgery was also markedly more effective than nonsurgical treatment in preventing type 2 DM, with a relative risk reduction of 78%.
A systematic review published in 2012 evaluated long-term cardiovascular risk reduction after bariatric surgery in 73 studies and 19,543 patients.24 At a mean follow-up of 57.8 months, the average excess weight loss for all procedures was 54% and rates of remission or improvement were 63% for hypertension, 73% for type 2 DM, and 65% for hyperlipidemia. Results from 12 cohort-matched, nonrandomized studies comparing bariatric surgery vs nonsurgical controls suggest that improvements in surrogate disease markers such as HbA1c, blood pressure, lipids, and body weight after surgery translate to reduced macrovascular and microvascular events and death.25 One of these studies involving male veterans who were mostly at high cardiovascular risk reported a 42% reduction in mortality at 10 years compared with medical therapy.26
In the Swedish Obese Subjects study, the mortality rate from cardiovascular disease in the bariatric surgical group was lower than for control patients (adjusted hazard ratio, 0.47; P = .002) despite a greater prevalence of smoking and higher baseline weights and blood pressures in the surgical cohort.19 For patients with type 2 DM in this study, surgery was associated with a 50% reduction in microvascular complications.27 After 15 years of follow-up, the cumulative incidence of microvascular complications was 41.8 per 1,000 person-years for control patients and 20.6 per 1,000 person-years in the surgery group (hazard ratio, 0.44; P < .001).
These observational, nonrandomized study data suggest that in patients with type 2 DM, bariatric surgery is significantly better than medical management alone in improving glycemic control, reducing cardiovascular risk factors, and lowering long-term morbidity and mortality associated with type 2 DM.
METABOLIC SURGERY: CLINICAL TRIALS
Collectively, these RCTs showed that surgery was significantly superior to medical treatment in reaching the designated glycemic target (P < .05 for all). The one exception showed that diabetes remission for LAGB vs medical treatment was 33% and 23%, respectively.41 This result might be due to patients in this study having advanced type 2 DM (HbA1c 8.2% ± 1.2%, with 40% on insulin), and they likely had reduced beta-cell function. Overall, surgery decreased HbA1c by 2% to 3.5%, whereas medical treatment lowered it by only 1% to 1.5%. Most of these studies also showed superiority of surgery over medical treatment in achieving secondary end points such as weight loss, remission of metabolic syndrome, reduction in diabetes and cardiovascular medications, and improvement in triglycerides, lipids, and quality of life. Results were mixed in terms of improvements in systolic and diastolic blood pressure or low-density lipoproteins after surgery vs medical treatment, but many studies did show a corresponding reduction in medication usage.
Durability of the effects of surgery was demonstrated in a 5-year study that showed superior and durable weight loss and glycemic control (remission) with both RYGB and BPD in severely obese patients (BMI ≥ 35) vs medical therapy.32 Similarly, Schauer et al43 showed that RYGB and SG were more effective than intensive medical therapy in improving or, in some cases, resolving hyperglycemia for 5 years. In the RCTs, patients who preoperatively had shorter duration of diabetes, lower HbA1c levels, no insulin requirement, and more postoperative weight loss were more likely to achieve diabetes remission.
Although previous guidelines and payer coverage policies had limited metabolic surgery to severely obese patients (BMI ≥ 35 kg/m2), nearly all RCTs showed that the surgical procedures, especially RYGB and SG, were equally effective in patients with BMI 30 to 35 kg/m2. This is particularly important given that most patients with type 2 DM have a BMI less than 35 kg/m2. The effect of surgery in these patients with mild obesity is also durable out to at least 5 years.43
No RCT was sufficiently powered to detect differences in macrovascular or microvascular complications or death, especially at the relatively short follow-up, and no such differences have been detected thus far. The STAMPEDE (Surgical Therapy and Medications Potentially Eradicate Diabetes Efficiently) trial43 showed that bariatric surgery (RYGB or SG) did not appear to worsen or improve retinopathy outcomes at 5 years compared with intensive medical management.
METABOLIC SURGERY: ADVERSE EVENTS
Surgical complications
Nutritional deficiencies
Postoperative nutritional deficiencies are typically associated with diminished nutrient intake or the malabsorptive effect of bariatric procedures. They are more common after RYGB and BPD-DS and less common after SG and LAGB. In addition, there is a high prevalence of nutritional deficiencies (35%–80%) in patients seeking bariatric surgery; thus, poor preoperative nutrition may be a factor in the development of postoperative deficiencies. Common preoperative nutrient deficiencies are vitamin A (11%), vitamin B12 (13%), vitamin D (40%), zinc (30%), iron (16%), ferritin (9%), selenium (58%), and folate (6%).51 Recommendations are to assess for these deficiencies and correct any identified before surgery.
Mild anemia after bariatric procedures is common, occurring in 15% to 20% of cases, and it is believed to result from reduced absorption of iron and B12, as well from pre-existing iron deficiency anemia in premenopausal patients.52 Deficiencies in trace minerals (selenium, zinc, and copper) and vitamins (B12, B1, A, E, D, and K) can occur after bariatric procedures, especially after BPD-DS.53 Nutrient deficiencies can be prevented or corrected with appropriate vitamin, iron, and calcium supplementation.54
Bone mineral density may decrease after bariatric surgery (14% in the proximal femur).55 Reduced mechanical loading after weight loss, reduced consumption and malabsorption of micronutrients (calcium, vitamin D), and neurohormonal alterations are potential underlying mechanisms of bone mineral density reduction after bariatric surgery. Rates of bone fracture and osteoporosis are not well delineated, raising questions about whether bone loss after bariatric surgery is clinically relevant or a functional adaptation to skeletal unloading. However, the extreme malabsorptive procedures of BPD-DS have been associated with severe calcium and vitamin D deficiencies, leading to decreased bone mineral density and osteoporosis.
Protein malnutrition also can occur after these extreme malabsorptive procedures. Patients require postoperative oral protein supplementation (80–100 g/day) and lifelong monitoring for nutritional complications after these procedures.56
Additional complications
Other late complications of bariatric surgery that are less clear in incidence and cause include kidney stones, alcohol abuse, depression, and suicide. One study of patients after RYGB (N = 4,690) reported a significantly higher prevalence of kidney stones than in obese controls: 7.5% vs 4.6%, respectively.57 Proposed causes of kidney stone formation following bariatric surgery include hyperoxaluria, hypocitraturia, and elevated urine acidity.58
The prevalence of alcohol-use disorder after bariatric surgery ranges from 7.6% to 11.8% and appears to be higher in patients with a history of alcohol use.59 Paradoxically, while bariatric surgery has been shown to significantly decrease depression,60 some studies suggest that a slight increase in the risk of suicide may occur,61 while others do not.62 A recent review concluded that accurate rates of suicide after bariatric surgery are not known, but practitioners should be aware of this concern and appropriately screen and counsel their patients.63
Although the 12 RCTs reported in Table 1 were not powered to detect differences in treatment-related complications, the overall rates of complications were consistent with those in observational studies.9 The most common surgical complications were anemia (15%), need for reoperation (8%), and GI (5%–10%). The 30-day surgical mortality rate was 0.2% (1 death) among the 465 surgical patients. Complications were not limited to the surgical patients. In the medical-treatment control group of the STAMPEDE trial,30 anemia (16%) and weight gain (16%) were common. Investigators reported challenges with medication compliance, including adverse effects leading to discontinuation of medications. Mild hypoglycemia was common, with no significant differences between the surgical and medical treatment groups.
METABOLIC SURGERY: COST EFFECTIVENESS
The cost of bariatric procedures varies considerably but, in general, ranges from $20,000 to $30,000, similar to the cost of cholecystectomy, hysterectomy, and colectomy. Retrospective analyses and modeling studies indicate that metabolic surgery is cost-effective and may present a cost savings in patients with type 2 DM, with a break-even time between 5 and 10 years.64,65 The cost savings, largely based on assumptions of long-term effectiveness and safety, result from reductions in medication use, outpatient care costs, and long-term complications of type 2 DM.
WHO SHOULD HAVE METABOLIC SURGERY?
Until recently, there was no clear national or international consensus on the role of metabolic surgery in treating type 2 DM. In 2015, the 2nd Diabetes Surgery Summit (DSS-II) Consensus Conference published guidelines that were endorsed by more than 50 diabetes and medical organizations.5 The recommendations cover many clinically relevant issues, including patient selection, preoperative evaluation, choice of procedure, and postoperative follow-up. The consensus conference delegates concluded that there is sufficient evidence demonstrating that metabolic surgery achieves excellent glycemic control and reduces cardiovascular risk factors.
The treatment algorithm from DSS-II incorporates appropriate use of all 3 treatment modalities: lifestyle intervention, drug therapy, and surgery (Figure 5).5 The 2017 Standards of Care for Diabetes from the American Diabetes Association include those key indications in the recommendations for metabolic surgery (Table 3).2
SUMMARY
The safety of metabolic surgery has significantly improved with the advent of laparoscopic surgery and recent national quality improvement initiatives that have made gastric bypass and SG as safe as cholecystectomy and appendectomy. Although observational studies suggest that metabolic surgery is associated with a reduction in cardiovascular and diabetes complications and mortality, these observations have not been confirmed in long-term RCTs.
Based on the published evidence, metabolic surgery is now endorsed as a standard treatment option, which provides patients and practitioners with a powerful tool to help combat the life-impairing effects of type 2 DM.
LIMITATIONS OF LIFESTYLE MANAGEMENT AND MEDICATIONS
First-line therapy with lifestyle management and second-line therapy with medications, including oral agents and insulin, are the mainstays of type 2 DM therapy. Although these approaches have reduced hyperglycemia and cardiovascular mortality, many patients have poor glycemic control and develop severe diabetes-related complications. A study using data from the National Health and Nutrition Examination Survey (N = 4,926) to evaluate success rates of lifestyle management plus drug therapy found that just 53% of patients with type 2 DM maintained a hemoglobin A1c (HbA1c) below 7%.6 Similarly, only 51% of those patients achieved a systolic and diastolic blood pressure less than 130/80 mm Hg, and only 56% achieved a low-density lipoprotein cholesterol level less than 100 mg/dL. Altogether, only 19% of the study cohort achieved all 3 therapy targets. Documented limitations of lifestyle counseling and drug therapy include behavior maladaptation, limitations in drug potency, nonadherence to medications, adverse effects, and economic deterrents.7
METABOLIC SURGERY FOR TYPE 2 DM
For patients with obesity and type 2 DM in whom lifestyle management and medications do not achieve desired treatment goals, bariatric surgery has emerged as the most effective treatment for attaining significant and durable weight loss. These gastrointestinal (GI) procedures, which reduce gastric volume with or without rerouting nutrient flow through the small intestine, were developed to yield long-term weight loss in patients with severe obesity. It is now known that they also cause dramatic improvement or remission of obesity-related comorbidities, especially type 2 DM. Research has shown that these effects are not only secondary to weight loss but also depend on neuroendocrine mechanisms secondary to changes in GI physiology. For these reasons, bariatric surgery is increasingly used with the primary intent to treat type 2 DM or metabolic disease, a practice referred to as metabolic surgery.
For more than 2 decades, indications for metabolic surgery reflected guidelines from a 1991 National Institutes of Health (NIH) consensus conference, which suggested considering surgery only in patients with a BMI of 40 kg/m2 or greater or a BMI of 35 kg/m2 or greater and significant obesity-related comorbidities.11 Guidelines published in 2013 expanded the recommendations to include adults with a BMI of at least 35 kg/m2 and an obesity-related comorbidity, such as diabetes, who are motivated to lose weight.4 These recommendations were primarily designed to guide the use of surgery as a weight-loss intervention for severe obesity. However, guidelines published in 2016 support use of metabolic surgery as a specific treatment for type 2 DM.5
Potential mechanisms resolving type 2 DM: More than weight loss
Bariatric surgery has been shown to have profound glucoregulatory effects. These include rapid improvement in hyperglycemia and reduction in exogenous insulin requirements that occur early after surgery and before the patient has any significant weight loss.12,13 Additionally, experiments in rodents showed that changes to GI anatomy can directly influence glucose homeostasis, independently of weight loss and caloric restriction.14
Although the exact molecular mechanisms underlying the effects of metabolic surgery on diabetes are not fully understood, many factors appear to play a role, including changes in bile acid metabolism, GI tract nutrient sensing, glucose utilization, insulin resistance, and intestinal microbiomes.15 These changes, acting through peripheral or central pathways, or perhaps both, lead to reduced hepatic glucose production, increased tissue glucose uptake, improved insulin sensitivity, and enhanced beta-cell function. A constellation of gut-derived neuroendocrine changes, rather than a single overarching mechanism, is the likely mediator of postoperative glycemic improvement, with the contributing factors varying according to the surgical procedure.
METABOLIC SURGERY OUTCOMES
Weight loss
Long-term reduction of excess body fat is a major goal of metabolic and bariatric surgery. Weight loss is usually expressed as either the percent of weight loss or the percent of excess weight loss (ie, weight loss above ideal weight). A meta-analysis of mostly short-term weight-loss outcomes (ie, < 5 years) from more than 22,000 procedures found an overall mean excess weight loss of 47.5% for patients who underwent LAGB, 61.6% for RYGB, 68.2% for vertical-banded gastroplasty, and 70.1% for BPD-DS.16 Vertical-banded gastroplasty differs from LAGB in that both a band and staples are used to create a small stomach pouch. Excess weight loss for SG generally averages 50% to 55%, which is intermediate between LAGB and RYGB.17,18
The Swedish Obese Subjects study (N = 4,047), a prospective study of bariatric surgery vs nonsurgical weight management of severely obese patients (BMI > 34), is the largest weight-loss study with the longest follow-up.19 At 20 years, the mean weight loss was 26% for gastric bypass, 18% for vertical-banded gastroplasty, 13% for gastric banding, and 1% for controls. A 10-year study in 1,787 severely obese patients (BMI ≥ 35) who underwent RYGB had 21% more weight loss from their baseline weight than the nonsurgical match.20 At 4-year follow-up in 2,410 patients, there were significant variations in weight loss depending on the procedure: 27.5% for RYGB, 17.8% for SG, and 10.6% in LAGB. Between 2% and 31% regained weight back to baseline: 30.5% for LAGB, 14.6% for SG, and 2.5% for RYGB.20 In contrast, long-term medical (nonsurgical) weight loss rarely exceeds 5%, even with intensive lifestyle intervention.21
Diabetes remission, cardiovascular risk factors, glycemic control
A meta-analysis of 19 mostly observational studies (N = 4,070 patients) reported an overall type 2 DM remission rate of 78% after bariatric surgery with 1 to 3 years of follow-up.22 Resolution or remission was typically defined as becoming “nondiabetic” with normal HbA1c without medications. In the Swedish Obese Subjects study, the remission rate was 72% at 2 years and 36% at 10 years compared with 21% and 13%, respectively, for the nonsurgical controls (P < .001).23 Bariatric surgery was also markedly more effective than nonsurgical treatment in preventing type 2 DM, with a relative risk reduction of 78%.
A systematic review published in 2012 evaluated long-term cardiovascular risk reduction after bariatric surgery in 73 studies and 19,543 patients.24 At a mean follow-up of 57.8 months, the average excess weight loss for all procedures was 54% and rates of remission or improvement were 63% for hypertension, 73% for type 2 DM, and 65% for hyperlipidemia. Results from 12 cohort-matched, nonrandomized studies comparing bariatric surgery vs nonsurgical controls suggest that improvements in surrogate disease markers such as HbA1c, blood pressure, lipids, and body weight after surgery translate to reduced macrovascular and microvascular events and death.25 One of these studies involving male veterans who were mostly at high cardiovascular risk reported a 42% reduction in mortality at 10 years compared with medical therapy.26
In the Swedish Obese Subjects study, the mortality rate from cardiovascular disease in the bariatric surgical group was lower than for control patients (adjusted hazard ratio, 0.47; P = .002) despite a greater prevalence of smoking and higher baseline weights and blood pressures in the surgical cohort.19 For patients with type 2 DM in this study, surgery was associated with a 50% reduction in microvascular complications.27 After 15 years of follow-up, the cumulative incidence of microvascular complications was 41.8 per 1,000 person-years for control patients and 20.6 per 1,000 person-years in the surgery group (hazard ratio, 0.44; P < .001).
These observational, nonrandomized study data suggest that in patients with type 2 DM, bariatric surgery is significantly better than medical management alone in improving glycemic control, reducing cardiovascular risk factors, and lowering long-term morbidity and mortality associated with type 2 DM.
METABOLIC SURGERY: CLINICAL TRIALS
Collectively, these RCTs showed that surgery was significantly superior to medical treatment in reaching the designated glycemic target (P < .05 for all). The one exception showed that diabetes remission for LAGB vs medical treatment was 33% and 23%, respectively.41 This result might be due to patients in this study having advanced type 2 DM (HbA1c 8.2% ± 1.2%, with 40% on insulin), and they likely had reduced beta-cell function. Overall, surgery decreased HbA1c by 2% to 3.5%, whereas medical treatment lowered it by only 1% to 1.5%. Most of these studies also showed superiority of surgery over medical treatment in achieving secondary end points such as weight loss, remission of metabolic syndrome, reduction in diabetes and cardiovascular medications, and improvement in triglycerides, lipids, and quality of life. Results were mixed in terms of improvements in systolic and diastolic blood pressure or low-density lipoproteins after surgery vs medical treatment, but many studies did show a corresponding reduction in medication usage.
Durability of the effects of surgery was demonstrated in a 5-year study that showed superior and durable weight loss and glycemic control (remission) with both RYGB and BPD in severely obese patients (BMI ≥ 35) vs medical therapy.32 Similarly, Schauer et al43 showed that RYGB and SG were more effective than intensive medical therapy in improving or, in some cases, resolving hyperglycemia for 5 years. In the RCTs, patients who preoperatively had shorter duration of diabetes, lower HbA1c levels, no insulin requirement, and more postoperative weight loss were more likely to achieve diabetes remission.
Although previous guidelines and payer coverage policies had limited metabolic surgery to severely obese patients (BMI ≥ 35 kg/m2), nearly all RCTs showed that the surgical procedures, especially RYGB and SG, were equally effective in patients with BMI 30 to 35 kg/m2. This is particularly important given that most patients with type 2 DM have a BMI less than 35 kg/m2. The effect of surgery in these patients with mild obesity is also durable out to at least 5 years.43
No RCT was sufficiently powered to detect differences in macrovascular or microvascular complications or death, especially at the relatively short follow-up, and no such differences have been detected thus far. The STAMPEDE (Surgical Therapy and Medications Potentially Eradicate Diabetes Efficiently) trial43 showed that bariatric surgery (RYGB or SG) did not appear to worsen or improve retinopathy outcomes at 5 years compared with intensive medical management.
METABOLIC SURGERY: ADVERSE EVENTS
Surgical complications
Nutritional deficiencies
Postoperative nutritional deficiencies are typically associated with diminished nutrient intake or the malabsorptive effect of bariatric procedures. They are more common after RYGB and BPD-DS and less common after SG and LAGB. In addition, there is a high prevalence of nutritional deficiencies (35%–80%) in patients seeking bariatric surgery; thus, poor preoperative nutrition may be a factor in the development of postoperative deficiencies. Common preoperative nutrient deficiencies are vitamin A (11%), vitamin B12 (13%), vitamin D (40%), zinc (30%), iron (16%), ferritin (9%), selenium (58%), and folate (6%).51 Recommendations are to assess for these deficiencies and correct any identified before surgery.
Mild anemia after bariatric procedures is common, occurring in 15% to 20% of cases, and it is believed to result from reduced absorption of iron and B12, as well from pre-existing iron deficiency anemia in premenopausal patients.52 Deficiencies in trace minerals (selenium, zinc, and copper) and vitamins (B12, B1, A, E, D, and K) can occur after bariatric procedures, especially after BPD-DS.53 Nutrient deficiencies can be prevented or corrected with appropriate vitamin, iron, and calcium supplementation.54
Bone mineral density may decrease after bariatric surgery (14% in the proximal femur).55 Reduced mechanical loading after weight loss, reduced consumption and malabsorption of micronutrients (calcium, vitamin D), and neurohormonal alterations are potential underlying mechanisms of bone mineral density reduction after bariatric surgery. Rates of bone fracture and osteoporosis are not well delineated, raising questions about whether bone loss after bariatric surgery is clinically relevant or a functional adaptation to skeletal unloading. However, the extreme malabsorptive procedures of BPD-DS have been associated with severe calcium and vitamin D deficiencies, leading to decreased bone mineral density and osteoporosis.
Protein malnutrition also can occur after these extreme malabsorptive procedures. Patients require postoperative oral protein supplementation (80–100 g/day) and lifelong monitoring for nutritional complications after these procedures.56
Additional complications
Other late complications of bariatric surgery that are less clear in incidence and cause include kidney stones, alcohol abuse, depression, and suicide. One study of patients after RYGB (N = 4,690) reported a significantly higher prevalence of kidney stones than in obese controls: 7.5% vs 4.6%, respectively.57 Proposed causes of kidney stone formation following bariatric surgery include hyperoxaluria, hypocitraturia, and elevated urine acidity.58
The prevalence of alcohol-use disorder after bariatric surgery ranges from 7.6% to 11.8% and appears to be higher in patients with a history of alcohol use.59 Paradoxically, while bariatric surgery has been shown to significantly decrease depression,60 some studies suggest that a slight increase in the risk of suicide may occur,61 while others do not.62 A recent review concluded that accurate rates of suicide after bariatric surgery are not known, but practitioners should be aware of this concern and appropriately screen and counsel their patients.63
Although the 12 RCTs reported in Table 1 were not powered to detect differences in treatment-related complications, the overall rates of complications were consistent with those in observational studies.9 The most common surgical complications were anemia (15%), need for reoperation (8%), and GI (5%–10%). The 30-day surgical mortality rate was 0.2% (1 death) among the 465 surgical patients. Complications were not limited to the surgical patients. In the medical-treatment control group of the STAMPEDE trial,30 anemia (16%) and weight gain (16%) were common. Investigators reported challenges with medication compliance, including adverse effects leading to discontinuation of medications. Mild hypoglycemia was common, with no significant differences between the surgical and medical treatment groups.
METABOLIC SURGERY: COST EFFECTIVENESS
The cost of bariatric procedures varies considerably but, in general, ranges from $20,000 to $30,000, similar to the cost of cholecystectomy, hysterectomy, and colectomy. Retrospective analyses and modeling studies indicate that metabolic surgery is cost-effective and may present a cost savings in patients with type 2 DM, with a break-even time between 5 and 10 years.64,65 The cost savings, largely based on assumptions of long-term effectiveness and safety, result from reductions in medication use, outpatient care costs, and long-term complications of type 2 DM.
WHO SHOULD HAVE METABOLIC SURGERY?
Until recently, there was no clear national or international consensus on the role of metabolic surgery in treating type 2 DM. In 2015, the 2nd Diabetes Surgery Summit (DSS-II) Consensus Conference published guidelines that were endorsed by more than 50 diabetes and medical organizations.5 The recommendations cover many clinically relevant issues, including patient selection, preoperative evaluation, choice of procedure, and postoperative follow-up. The consensus conference delegates concluded that there is sufficient evidence demonstrating that metabolic surgery achieves excellent glycemic control and reduces cardiovascular risk factors.
The treatment algorithm from DSS-II incorporates appropriate use of all 3 treatment modalities: lifestyle intervention, drug therapy, and surgery (Figure 5).5 The 2017 Standards of Care for Diabetes from the American Diabetes Association include those key indications in the recommendations for metabolic surgery (Table 3).2
SUMMARY
The safety of metabolic surgery has significantly improved with the advent of laparoscopic surgery and recent national quality improvement initiatives that have made gastric bypass and SG as safe as cholecystectomy and appendectomy. Although observational studies suggest that metabolic surgery is associated with a reduction in cardiovascular and diabetes complications and mortality, these observations have not been confirmed in long-term RCTs.
Based on the published evidence, metabolic surgery is now endorsed as a standard treatment option, which provides patients and practitioners with a powerful tool to help combat the life-impairing effects of type 2 DM.
- Bays HE, Chapman RH, Grandy S; for the SHIELD Investigators Group. The relationship of body mass index to diabetes mellitus, hypertension and dyslipidaemia: comparison of data from two national surveys. Int J Clin Pract May 2007; 61:737–747.
- Marathe PH, Gao HX, Close KL. American Diabetes Association standards of medical care in diabetes—2017. Diabetes Care 2017; 40(suppl 1):S1–S135.
- Fox CS, Golden SH, Anderson C, et al; American Heart Association; American Diabetes Association. Update on prevention of cardiovascular disease in adults with type 2 diabetes mellitus in light of recent evidence: a scientific statement from the American Heart Association and the American Diabetes Association. Circulation 2015; 132:691–718.
- Jensen MD, Ryan DH, Apovian CM, et al; American College of Cardiology/American Heart Association Task Force on Practice Guidelines; Obesity Society. 2013 AHA/ACC/TOS guideline for the management of overweight and obesity in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and The Obesity Society. J Am Coll Cardiol 2014; 63:2985–3023.
- Rubino F, Nathan DM, Eckel RH, et al; Delegates of the 2nd Diabetes Surgery Summit. Metabolic surgery in the treatment algorithm for type 2 diabetes: a joint statement by international diabetes organizations. Diabetes Care 2016; 39:861–877.
- Stark Casagrande S, Fradkin JE, Saydah SH, Rust KF, Cowie CC. The prevalence of meeting A1C, blood pressure, and LDL goals among people with diabetes, 1988–2010. Diabetes Care 2013; 36:2271–2279.
- Kolandaivelu K, Leiden BB, O’Gara PT, Bhatt DL. Non-adherence to cardiovascular medications. Eur Heart J 2014; 35:3267–3276.
- Angrisani L, Santonicola A, Iovino P, Formisano G, Buchwald H, Scopinaro N. Bariatric surgery worldwide 2013. Obes Surg 2015; 25:1822–1832.
- Schauer PR, Mingrone G, Ikramuddin S, Wolfe B. Clinical outcomes of metabolic surgery: efficacy of glycemic control, weight loss, and remission of diabetes. Diabetes Care 2016; 39:902–911.
- Khorgami Z, Andalib A, Corcelles R, Aminian A, Brethauer S, Schauer P. Recent national trends in the surgical treatment of obesity: sleeve gastrectomy dominates. Surg Obes Relat Dis 2015; 11(suppl):S1–S34 [Abstract A111].
- Consensus Development Conference Panel. NIH conference. Gastrointestinal surgery for severe obesity. Ann Intern Med 1991; 115:956–961.
- Pories WJ, MacDonald KG Jr, Flickinger EG, et al. Is type II diabetes mellitus (NIDDM) a surgical disease? Ann Surg 1992; 215:633–642.
- Schauer PR, Burguera B, Ikramuddin S, et al. Effect of laparoscopic Roux-en Y gastric bypass on type 2 diabetes mellitus. Ann Surg 2003; 238:467–484.
- Rubino F, Marescaux J. Effect of duodenal-jejunal exclusion in a non-obese animal model of type 2 diabetes: a new perspective for an old disease. Ann Surg 2004; 239:1–11.
- Batterham RL, Cummings DE. Mechanisms of diabetes improvement following bariatric/metabolic surgery. Diabetes Care 2016; 39:893–901.
- Buchwald H, Avidor Y, Braunwald E, et al. Bariatric surgery: a systematic review and meta-analysis. JAMA 2004; 292:1724–1737.
- Brethauer SA, Hammel JP, Schauer PR. Systematic review of sleeve gastrectomy as staging and primary bariatric procedure. Surg Obes Relat Dis 2009; 5:469–475.
- Eid GM, Brethauer S, Mattar SG, Titchner RL, Gourash W, Schauer PR. Laparoscopic sleeve gastrectomy for super obese patients: forty-eight percent excess weight loss after 6 to 8 years with 93% follow-up. Ann Surg 2012; 256:262–265.
- Sjöström L, Peltonen M, Jacobson P, et al. Bariatric surgery and long-term cardiovascular events. JAMA 2012; 307:56–65.
- Maciejewski ML, Arterburn DE, Van Scoyoc L, et al. Bariatric surgery and long-term durability of weight loss. JAMA Surg 2016; 151:1046–1055.
- Wing RR, Bolin P, Brancati FL, et al; for the Look AHEAD Research Group. Cardiovascular effects of intensive lifestyle intervention in type 2 diabetes. N Engl J Med 2013; 369:145–154.
- Buchwald H, Estok R, Fahrbach K, et al. Weight and type 2 diabetes after bariatric surgery: systematic review and meta-analysis. Am J Med 2009; 122:248–256.
- Sjöström L, Lindroos AK, Peltonen M, et al; Swedish Obese Subjects Study Scientific Group. Lifestyle, diabetes, and cardiovascular risk factors 10 years after bariatric surgery. N Engl J Med 2004; 351:2683–2693.
- Vest AR, Heneghan HM, Agarwal S, Schauer PR, Young JB. Bariatric surgery and cardiovascular outcomes: a systematic review. Heart 2012; 98:1763–1777.
- Vest AR, Heneghan HM, Schauer PR, Young JB. Surgical management of obesity and the relationship to cardiovascular disease. Circulation 2013; 127:945–959.
- Arterburn DE, Olsen MK, Smith VA, et al. Association between bariatric surgery and long-term survival. JAMA 2015; 313:62–70.
- Sjöström L, Peltonen M, Jacobson P, et al. Association of bariatric surgery with long-term remission of type 2 diabetes and with microvascular and macrovascular complications. JAMA 2014; 311:2297–2304.
- Dixon JB, O’Brien PE, Playfair J, et al. Adjustable gastric banding and conventional therapy for type 2 diabetes: a randomized controlled trial. JAMA 2008; 299:316–323.
- Schauer PR, Kashyap SR, Wolski K, et al. Bariatric surgery versus intensive medical therapy in obese patients with diabetes. N Engl J Med 2012; 366:1567–1576.
- Schauer PR, Bhatt DL, Kirwan JP, et al; STAMPEDE Investigators. Bariatric surgery versus intensive medical therapy for diabetes—3-year outcomes. N Engl J Med 2014; 370:2002–2013.
- Mingrone G, Panunzi S, De Gaetano A, et al. Bariatric surgery versus conventional medical therapy for type 2 diabetes. N Engl J Med 2012; 366:1577–1585.
- Mingrone G, Panunzi S, De Gaetano A, et al. Bariatric-metabolic surgery versus conventional medical treatment in obese patients with type 2 diabetes: 5 year follow-up of an open-label, single-centre, randomized controlled trial. Lancet 2015; 386:964–973.
- Ikramuddin S, Korner J, Lee WJ, et al. Roux-en-Y gastric bypass vs intensive medical management for the control of type 2 diabetes, hypertension, and hyperlipidemia: the Diabetes Surgery Study randomized clinical trial. JAMA 2013; 309:2240–2249.
- Ikramuddin S, Billington CJ, Lee WJ, et al. Roux-en-Y gastric bypass for diabetes (the Diabetes Surgery Study): 2-year outcomes of a 5-year, randomized, controlled trial. Lancet Diabetes Endocrinol 2015; 3:413–422.
- Liang Z, Wu Q, Chen B, Yu P, Zhao H, Ouyang X. Effect of laparoscopic Roux-en-Y gastric bypass surgery on type 2 diabetes mellitus with hypertension: a randomized controlled trial. Diabetes Res Clin Pract 2013; 101:50–56.
- Halperin F, Ding SA, Simonson DC, et al. Roux-en-Y gastric bypass surgery or lifestyle with intensive medical management in patients with type 2 diabetes: feasibility and 1-year results of a randomized clinical trial. JAMA Surg 2014; 149:716–726.
- Courcoulas AP, Goodpaster BH, Eagleton JK, et al. Surgical vs medical treatments for type 2 diabetes mellitus: a randomized clinical trial. JAMA Surg 2014; 149:707–715.
- Courcoulas AP, Belle SH, Neiberg RH, et al. Three-year outcomes of bariatric surgery vs. lifestyle intervention for type 2 diabetes mellitus treatment: a randomized clinical trial. JAMA Surg 2015; 150:931–940.
- Wentworth JM, Playfair J, Laurie C, et al. Multidisciplinary diabetes care with and without bariatric surgery in overweight people: a randomised controlled trial. Lancet Diabetes Endocrinol 2014; 2:545–552.
- Parikh M, Chung M, Sheth S, et al. Randomized pilot trial of bariatric surgery versus intensive medical weight management on diabetes remission in type 2 diabetic patients who do not meet NIH criteria for surgery and the role of soluble RAGE as a novel biomarker of success. Ann Surg 2014; 260:617–622.
- Ding SA, Simonson DC, Wewalka M, et al. Adjustable gastric band surgery or medical management in patients with type 2 diabetes: a randomized clinical trial. J Clin Endocrinol Metab 2015; 100:2546–2556.
- Cummings DE, Arterburn DE, Westbrook EO, et al. Gastric bypass surgery vs. intensive lifestyle and medical intervention for type 2 diabetes: the CROSSROADS randomized controlled trial. Diabetologia 2016; 59:945–953.
- Schauer PR, Bhatt DL, Kirwan JP, et al; STAMPEDE Investigators. Metabolic surgery vs. intensive medical therapy for diabetes: 5-year outcomes. N Engl J Med 2017; 376:641–651.
- Shah SS, Todkar J, Phadake U, et al. Gastric bypass vs. medical/lifestyle care for type 2 diabetes in South Asians with BMI 25-40 kg/m2: the COSMID randomized trial [261-OR]. Presented at the American Diabetes Association’s 76th Scientific Session; June 10–14, 2016; New Orleans, LA.
- Flum DR, Belle SH, King WC, et al; Longitudinal Assessment of Bariatric Surgery (LABS) Consortium. Perioperative safety in the longitudinal assessment of bariatric surgery. N Engl J Med 2009; 361:445–454.
- Aminian A, Brethauer SA, Kirwan JP, Kashyap SR, Burguera B, Schauer PR. How safe is metabolic/diabetes surgery? Diabetes Obes Metab 2015; 17:198–201.
- Thodiyil PA, Yenumula P, Rogula T, et al. Selective non operative management of leaks after gastric bypass: lessons learned from 2675 consecutive patients. Ann Surg 2008; 248:782–792.
- Rogula T, Yenumula PR, Schauer PR. A complication of Roux-en-Y gastric bypass: intestinal obstruction. Surg Endosc 2007; 21:1914–1918.
- Thornton CM, Rozen WM, So D, Kaplan ED, Wilkinson S. Reducing band slippage in laparoscopic adjustable gastric banding: the mesh plication pars flaccida technique. Obes Surg 2009; 19:1702–1706.
- Himpens J, Cadière G-B, Bazi M, Vouche M, Cadière B, Dapri G. Long-term outcomes of laparoscopic adjustable gastric banding. Arch Surg 2011; 146:802–807.
- Madan AK, Orth WS, Tichansky DS, Ternovits CA. Vitamin and trace mineral levels after laparoscopic gastric bypass. Obes Surg 2006; 16:603–606.
- Love AL, Billett HH. Obesity, bariatric surgery, and iron deficiency: true, true, true and related. Am J Hematol 2008; 83:403–409.
- Shankar P, Boylan M, Sriram K. Micronutrient deficiencies after bariatric surgery. Nutrition 2010; 26:1031–1037.
- Gong K, Gagner M, Pomp A, Almahmeed T, Bardaro SJ. Micronutrient deficiencies after laparoscopic gastric bypass: recommendations. Obes Surg 2008; 18:1062–1066.
- Scibora LM. Skeletal effects of bariatric surgery: examining bone loss, potential mechanisms and clinical relevance. Diabetes Obes Metab 2014; 16:1204–1213.
- Baptista V, Wassef W. Bariatric procedures: an update on techniques, outcomes and complications. Curr Opin Gastroenterol 2013; 29:684–693.
- Matlaga BR, Shore AD, Magnuson T, Clark JM, Johns R, Makary MA. Effect of gastric bypass surgery on kidney stone disease. J Urol 2009; 181:2573–2577.
- Sakhaee K, Poindexter J, Aguirre C. The effects of bariatric surgery on bone and nephrolithiasis. Bone 2016; 84:1–8.
- Li L, Wu LT. Substance use after bariatric surgery: a review. J Psychiatr Res 2016; 76:16–29.
- Ayloo S, Thompson K, Choudhury N, Sheriffdeen R. Correlation between the Beck Depression Inventory and bariatric surgical procedures. Surg Obes Relat Dis 2015; 11:637–342.
- Adams TD, Gress RE, Smith SC, et al. Long-term mortality after gastric bypass surgery. N Engl J Med 2007; 357:753–761.
- Sjöström L, Narbro K, Sjöström CD, et al; Swedish Obese Subjects Study. Effects of bariatric surgery on mortality in Swedish obese subjects. N Engl J Med 2007; 357:741–752.
- Mitchell JE, Crosby R, de Zwaan M, et al. Possible risk factors for increased suicide following bariatric surgery. Obesity (Silver Spring) 2013; 21:665–672.
- Fouse T, Schauer P. The socioeconomic impact of morbid obesity and factors affecting access to obesity surgery. Surg Clin North Am 2016; 96:669–679.
- Rubin JK, Hinrichs-Krapels S, Hesketh R, Martin A, Herman WH, Rubino F. Identifying barriers to appropriate use of metabolic/bariatric surgery for type 2 diabetes treatment: policy lab results. Diabetes Care 2016; 39:954–963.
- Bays HE, Chapman RH, Grandy S; for the SHIELD Investigators Group. The relationship of body mass index to diabetes mellitus, hypertension and dyslipidaemia: comparison of data from two national surveys. Int J Clin Pract May 2007; 61:737–747.
- Marathe PH, Gao HX, Close KL. American Diabetes Association standards of medical care in diabetes—2017. Diabetes Care 2017; 40(suppl 1):S1–S135.
- Fox CS, Golden SH, Anderson C, et al; American Heart Association; American Diabetes Association. Update on prevention of cardiovascular disease in adults with type 2 diabetes mellitus in light of recent evidence: a scientific statement from the American Heart Association and the American Diabetes Association. Circulation 2015; 132:691–718.
- Jensen MD, Ryan DH, Apovian CM, et al; American College of Cardiology/American Heart Association Task Force on Practice Guidelines; Obesity Society. 2013 AHA/ACC/TOS guideline for the management of overweight and obesity in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and The Obesity Society. J Am Coll Cardiol 2014; 63:2985–3023.
- Rubino F, Nathan DM, Eckel RH, et al; Delegates of the 2nd Diabetes Surgery Summit. Metabolic surgery in the treatment algorithm for type 2 diabetes: a joint statement by international diabetes organizations. Diabetes Care 2016; 39:861–877.
- Stark Casagrande S, Fradkin JE, Saydah SH, Rust KF, Cowie CC. The prevalence of meeting A1C, blood pressure, and LDL goals among people with diabetes, 1988–2010. Diabetes Care 2013; 36:2271–2279.
- Kolandaivelu K, Leiden BB, O’Gara PT, Bhatt DL. Non-adherence to cardiovascular medications. Eur Heart J 2014; 35:3267–3276.
- Angrisani L, Santonicola A, Iovino P, Formisano G, Buchwald H, Scopinaro N. Bariatric surgery worldwide 2013. Obes Surg 2015; 25:1822–1832.
- Schauer PR, Mingrone G, Ikramuddin S, Wolfe B. Clinical outcomes of metabolic surgery: efficacy of glycemic control, weight loss, and remission of diabetes. Diabetes Care 2016; 39:902–911.
- Khorgami Z, Andalib A, Corcelles R, Aminian A, Brethauer S, Schauer P. Recent national trends in the surgical treatment of obesity: sleeve gastrectomy dominates. Surg Obes Relat Dis 2015; 11(suppl):S1–S34 [Abstract A111].
- Consensus Development Conference Panel. NIH conference. Gastrointestinal surgery for severe obesity. Ann Intern Med 1991; 115:956–961.
- Pories WJ, MacDonald KG Jr, Flickinger EG, et al. Is type II diabetes mellitus (NIDDM) a surgical disease? Ann Surg 1992; 215:633–642.
- Schauer PR, Burguera B, Ikramuddin S, et al. Effect of laparoscopic Roux-en Y gastric bypass on type 2 diabetes mellitus. Ann Surg 2003; 238:467–484.
- Rubino F, Marescaux J. Effect of duodenal-jejunal exclusion in a non-obese animal model of type 2 diabetes: a new perspective for an old disease. Ann Surg 2004; 239:1–11.
- Batterham RL, Cummings DE. Mechanisms of diabetes improvement following bariatric/metabolic surgery. Diabetes Care 2016; 39:893–901.
- Buchwald H, Avidor Y, Braunwald E, et al. Bariatric surgery: a systematic review and meta-analysis. JAMA 2004; 292:1724–1737.
- Brethauer SA, Hammel JP, Schauer PR. Systematic review of sleeve gastrectomy as staging and primary bariatric procedure. Surg Obes Relat Dis 2009; 5:469–475.
- Eid GM, Brethauer S, Mattar SG, Titchner RL, Gourash W, Schauer PR. Laparoscopic sleeve gastrectomy for super obese patients: forty-eight percent excess weight loss after 6 to 8 years with 93% follow-up. Ann Surg 2012; 256:262–265.
- Sjöström L, Peltonen M, Jacobson P, et al. Bariatric surgery and long-term cardiovascular events. JAMA 2012; 307:56–65.
- Maciejewski ML, Arterburn DE, Van Scoyoc L, et al. Bariatric surgery and long-term durability of weight loss. JAMA Surg 2016; 151:1046–1055.
- Wing RR, Bolin P, Brancati FL, et al; for the Look AHEAD Research Group. Cardiovascular effects of intensive lifestyle intervention in type 2 diabetes. N Engl J Med 2013; 369:145–154.
- Buchwald H, Estok R, Fahrbach K, et al. Weight and type 2 diabetes after bariatric surgery: systematic review and meta-analysis. Am J Med 2009; 122:248–256.
- Sjöström L, Lindroos AK, Peltonen M, et al; Swedish Obese Subjects Study Scientific Group. Lifestyle, diabetes, and cardiovascular risk factors 10 years after bariatric surgery. N Engl J Med 2004; 351:2683–2693.
- Vest AR, Heneghan HM, Agarwal S, Schauer PR, Young JB. Bariatric surgery and cardiovascular outcomes: a systematic review. Heart 2012; 98:1763–1777.
- Vest AR, Heneghan HM, Schauer PR, Young JB. Surgical management of obesity and the relationship to cardiovascular disease. Circulation 2013; 127:945–959.
- Arterburn DE, Olsen MK, Smith VA, et al. Association between bariatric surgery and long-term survival. JAMA 2015; 313:62–70.
- Sjöström L, Peltonen M, Jacobson P, et al. Association of bariatric surgery with long-term remission of type 2 diabetes and with microvascular and macrovascular complications. JAMA 2014; 311:2297–2304.
- Dixon JB, O’Brien PE, Playfair J, et al. Adjustable gastric banding and conventional therapy for type 2 diabetes: a randomized controlled trial. JAMA 2008; 299:316–323.
- Schauer PR, Kashyap SR, Wolski K, et al. Bariatric surgery versus intensive medical therapy in obese patients with diabetes. N Engl J Med 2012; 366:1567–1576.
- Schauer PR, Bhatt DL, Kirwan JP, et al; STAMPEDE Investigators. Bariatric surgery versus intensive medical therapy for diabetes—3-year outcomes. N Engl J Med 2014; 370:2002–2013.
- Mingrone G, Panunzi S, De Gaetano A, et al. Bariatric surgery versus conventional medical therapy for type 2 diabetes. N Engl J Med 2012; 366:1577–1585.
- Mingrone G, Panunzi S, De Gaetano A, et al. Bariatric-metabolic surgery versus conventional medical treatment in obese patients with type 2 diabetes: 5 year follow-up of an open-label, single-centre, randomized controlled trial. Lancet 2015; 386:964–973.
- Ikramuddin S, Korner J, Lee WJ, et al. Roux-en-Y gastric bypass vs intensive medical management for the control of type 2 diabetes, hypertension, and hyperlipidemia: the Diabetes Surgery Study randomized clinical trial. JAMA 2013; 309:2240–2249.
- Ikramuddin S, Billington CJ, Lee WJ, et al. Roux-en-Y gastric bypass for diabetes (the Diabetes Surgery Study): 2-year outcomes of a 5-year, randomized, controlled trial. Lancet Diabetes Endocrinol 2015; 3:413–422.
- Liang Z, Wu Q, Chen B, Yu P, Zhao H, Ouyang X. Effect of laparoscopic Roux-en-Y gastric bypass surgery on type 2 diabetes mellitus with hypertension: a randomized controlled trial. Diabetes Res Clin Pract 2013; 101:50–56.
- Halperin F, Ding SA, Simonson DC, et al. Roux-en-Y gastric bypass surgery or lifestyle with intensive medical management in patients with type 2 diabetes: feasibility and 1-year results of a randomized clinical trial. JAMA Surg 2014; 149:716–726.
- Courcoulas AP, Goodpaster BH, Eagleton JK, et al. Surgical vs medical treatments for type 2 diabetes mellitus: a randomized clinical trial. JAMA Surg 2014; 149:707–715.
- Courcoulas AP, Belle SH, Neiberg RH, et al. Three-year outcomes of bariatric surgery vs. lifestyle intervention for type 2 diabetes mellitus treatment: a randomized clinical trial. JAMA Surg 2015; 150:931–940.
- Wentworth JM, Playfair J, Laurie C, et al. Multidisciplinary diabetes care with and without bariatric surgery in overweight people: a randomised controlled trial. Lancet Diabetes Endocrinol 2014; 2:545–552.
- Parikh M, Chung M, Sheth S, et al. Randomized pilot trial of bariatric surgery versus intensive medical weight management on diabetes remission in type 2 diabetic patients who do not meet NIH criteria for surgery and the role of soluble RAGE as a novel biomarker of success. Ann Surg 2014; 260:617–622.
- Ding SA, Simonson DC, Wewalka M, et al. Adjustable gastric band surgery or medical management in patients with type 2 diabetes: a randomized clinical trial. J Clin Endocrinol Metab 2015; 100:2546–2556.
- Cummings DE, Arterburn DE, Westbrook EO, et al. Gastric bypass surgery vs. intensive lifestyle and medical intervention for type 2 diabetes: the CROSSROADS randomized controlled trial. Diabetologia 2016; 59:945–953.
- Schauer PR, Bhatt DL, Kirwan JP, et al; STAMPEDE Investigators. Metabolic surgery vs. intensive medical therapy for diabetes: 5-year outcomes. N Engl J Med 2017; 376:641–651.
- Shah SS, Todkar J, Phadake U, et al. Gastric bypass vs. medical/lifestyle care for type 2 diabetes in South Asians with BMI 25-40 kg/m2: the COSMID randomized trial [261-OR]. Presented at the American Diabetes Association’s 76th Scientific Session; June 10–14, 2016; New Orleans, LA.
- Flum DR, Belle SH, King WC, et al; Longitudinal Assessment of Bariatric Surgery (LABS) Consortium. Perioperative safety in the longitudinal assessment of bariatric surgery. N Engl J Med 2009; 361:445–454.
- Aminian A, Brethauer SA, Kirwan JP, Kashyap SR, Burguera B, Schauer PR. How safe is metabolic/diabetes surgery? Diabetes Obes Metab 2015; 17:198–201.
- Thodiyil PA, Yenumula P, Rogula T, et al. Selective non operative management of leaks after gastric bypass: lessons learned from 2675 consecutive patients. Ann Surg 2008; 248:782–792.
- Rogula T, Yenumula PR, Schauer PR. A complication of Roux-en-Y gastric bypass: intestinal obstruction. Surg Endosc 2007; 21:1914–1918.
- Thornton CM, Rozen WM, So D, Kaplan ED, Wilkinson S. Reducing band slippage in laparoscopic adjustable gastric banding: the mesh plication pars flaccida technique. Obes Surg 2009; 19:1702–1706.
- Himpens J, Cadière G-B, Bazi M, Vouche M, Cadière B, Dapri G. Long-term outcomes of laparoscopic adjustable gastric banding. Arch Surg 2011; 146:802–807.
- Madan AK, Orth WS, Tichansky DS, Ternovits CA. Vitamin and trace mineral levels after laparoscopic gastric bypass. Obes Surg 2006; 16:603–606.
- Love AL, Billett HH. Obesity, bariatric surgery, and iron deficiency: true, true, true and related. Am J Hematol 2008; 83:403–409.
- Shankar P, Boylan M, Sriram K. Micronutrient deficiencies after bariatric surgery. Nutrition 2010; 26:1031–1037.
- Gong K, Gagner M, Pomp A, Almahmeed T, Bardaro SJ. Micronutrient deficiencies after laparoscopic gastric bypass: recommendations. Obes Surg 2008; 18:1062–1066.
- Scibora LM. Skeletal effects of bariatric surgery: examining bone loss, potential mechanisms and clinical relevance. Diabetes Obes Metab 2014; 16:1204–1213.
- Baptista V, Wassef W. Bariatric procedures: an update on techniques, outcomes and complications. Curr Opin Gastroenterol 2013; 29:684–693.
- Matlaga BR, Shore AD, Magnuson T, Clark JM, Johns R, Makary MA. Effect of gastric bypass surgery on kidney stone disease. J Urol 2009; 181:2573–2577.
- Sakhaee K, Poindexter J, Aguirre C. The effects of bariatric surgery on bone and nephrolithiasis. Bone 2016; 84:1–8.
- Li L, Wu LT. Substance use after bariatric surgery: a review. J Psychiatr Res 2016; 76:16–29.
- Ayloo S, Thompson K, Choudhury N, Sheriffdeen R. Correlation between the Beck Depression Inventory and bariatric surgical procedures. Surg Obes Relat Dis 2015; 11:637–342.
- Adams TD, Gress RE, Smith SC, et al. Long-term mortality after gastric bypass surgery. N Engl J Med 2007; 357:753–761.
- Sjöström L, Narbro K, Sjöström CD, et al; Swedish Obese Subjects Study. Effects of bariatric surgery on mortality in Swedish obese subjects. N Engl J Med 2007; 357:741–752.
- Mitchell JE, Crosby R, de Zwaan M, et al. Possible risk factors for increased suicide following bariatric surgery. Obesity (Silver Spring) 2013; 21:665–672.
- Fouse T, Schauer P. The socioeconomic impact of morbid obesity and factors affecting access to obesity surgery. Surg Clin North Am 2016; 96:669–679.
- Rubin JK, Hinrichs-Krapels S, Hesketh R, Martin A, Herman WH, Rubino F. Identifying barriers to appropriate use of metabolic/bariatric surgery for type 2 diabetes treatment: policy lab results. Diabetes Care 2016; 39:954–963.
KEY POINTS
- Randomized clinical trials have shown that metabolic surgery is statistically superior to medical treatment in achieving targeted glycemic levels along with improvements in weight loss, remission of metabolic syndrome, reduction in medications, and improvements in lipid levels.
- The safety of metabolic and bariatric surgery has significantly improved with the advent of laparoscopic surgery, resulting in complication profiles similar to those of cholecystectomy and appendectomy.
- Metabolic surgery is now recommended as standard treatment option for type 2 diabetes in patients with body mass index levels as low as 30 kg/m2.
— Bonus Article — Medical Treatment of Diabetes Mellitus
Medical Treatment of Diabetes Mellitus
In the United States, 57.9% of patients with diabetes mellitus (DM) have at least 1 diabetes-related complication and 14.3% of patients with diabetes have 3 or more diabetes-related complications.1 Achieving glycemic control in patients with DM reduces the development and progression of retinopathy, nephropathy, and neuropathy. Aggressive treatment of dyslipidemia and hypertension decreases macrovascular complications.2–4 The techniques for monitoring blood glucose and the various treatment options available to manage glycemic control in patients with diabetes are reviewed below.
Measuring Glycemic Control
The primary techniques available to assess the quality of a patient’s glycemic control are self-monitoring of blood glucose and interval measurement of hemoglobin A1c (HbA1c). Continuous glucose monitoring is also available and may be appropriate for select patients, such as patients with brittle diabetes and those using insulin pumps.
Self-monitoring of blood glucose
For patients with type 1 DM and patients with insulin-dependent type 2 DM, self-monitoring of blood glucose allows patients to adjust insulin dosing to prevent hypoglycemia and hyperglycemia.2,5–7 The American Diabetes Association (ADA) guidelines recommend that patients with type 1 DM self-monitor their glucose:
- Before eating
- At bedtime
- Before exercise
- If hypoglycemia is suspected
- Until hypoglycemia is corrected
- Postprandially upon occasion
- And before critical tasks (ie, driving).8
Patients should be educated about how to use real-time blood glucose values to adjust their food intake and medical therapy.
It is commonly recommended that patients with type 2 DM self-monitor their blood glucose levels, but the evidence to support the effectiveness of this practice is inconclusive. Initial studies showed reductions in HbA1c with self-monitoring; however, the inclusion of beneficial health behaviors such as diet and exercise in the analyses makes it difficult to assess the effectiveness of self-monitor blood glucose alone.2,9
The ADA recommends that nonpregnant adults maintain blood glucose levels of 80 mg/dL to 130 mg/dL preprandial and less than 180 mg/dL postprandial.8 The blood glucose goals for patients with gestational diabetes are 95 mg/dL or less preprandial and either 140 mg/dL or less 1-hour postprandial or 120 mg/dL or less 2-hours postprandial.
HbA1c
HbA1c tests reflect the mean blood glucose values over a 3-month period and can predict patients’ risk of microvascular complications.10,11 The ADA recommends that patients with stable glycemic control have an HbA1c test at least twice a year. Quarterly HbA1c testing is suggested for patients with a recent change in therapy or for patients not meeting their glycemic goals.8
Measurement of HbA1c is influenced by the red blood cell turnover rate; therefore, anemia, transfusions, and hemoglobinopathies can cause inaccurate test values. The ADA recommends that nonpregnant adults maintain HbA1c levels near 7%. For patients with diabetes who become pregnant, the goal is HbA1c levels less than 6.0%.8 The ADA also recommends that select patients, especially those with a long life expectancy and little comorbidity, adopt glycemic targets near normal levels (HbA1c < 6.5%), providing the target can be achieved without significant hypoglycemia.8
Glycemic Treatment
Insulin sensitizers
Biguanides (metformin)
Metformin is the only available biguanide. Metformin should be used as a first-line therapy in patients with type 2 DM whenever possible.13 Metformin suppresses hepatic glucose output and primarily affects fasting glycemia; however, reduced postprandial glucose concentrations also occur.
The most common side effects of metformin are diarrhea, nausea, and abdominal discomfort. Metformin has the potential to produce very rare but life-threatening lactic acidosis (< 1 in 100,000). The use of metformin is contraindicated in patients with a glomerular filtration rate less than 30 mL/min, with acidosis, hypoxia, or dehydration.8
Metformin usually does not lead to hypoglycemia when used as monotherapy. It can lead to weight loss (3%–5% of body weight), and it has been shown to decrease plasma triglyceride concentrations (10%–20%).8,14,15
Thiazolidinediones
Thiazolidinediones (TZDs) primarily enhance the insulin sensitivity of muscle and fat tissue and mildly enhance insulin sensitivity of the liver. TZDs lower fasting and postprandial blood glucose levels.
Major side effects of TZDs include weight gain, with an increase in subcutaneous adiposity, and fluid retention. Fluid retention typically manifests as peripheral edema, but heart failure can occur on occasion. These agents should be avoided in patients with functional class III or IV heart failure. The PROactive trial of the TZD pioglitazone found that pioglitazone did not increase cardiovascular risk compared with placebo.16 TZDs have been associated with an increased risk of fractures, particularly in women. When used as monotherapy, TZDs do not cause hypoglycemia. Pioglitazone lowers triglyceride levels, increases high-density lipoprotein cholesterol, and increases the low-density lipoprotein cholesterol particle size.8,16–18
Insulin secretagogues
Insulin secretagogues such as sulfonylureas and glinides stimulate secretion of insulin from the pancreas regardless of the ambient glucose concentration.
Sulfonylureas
Sulfonylureas lower fasting and postprandial glucose levels. The main side effects include weight gain (about 2 kg upon initiation) and hypoglycemia. The UK Prospective Diabetes Study (UKPDS) trial showed a decrease in microvascular complications with the use of sulfonylureas.19 Caution should be used in patients with liver or kidney dysfunction or patients who frequently skip meals. Newer, second-generation sulfonylureas (ie, glipizide and glimepiride) may have less risk of hypoglycemia because their action is somewhat glucose dependent.8,17,19
Glinides
Glinides, which include repaglinide and nateglenide, have a rapid onset of action and a short duration of action, so they are a good option for patients with erratically timed meals. Glinides have a lower risk of hypoglycemia than sulfonylureas. Caution must be used with glinides in patients with liver dysfunction. Dosing is immediately before meals.8,17
Alpha-glucosidase inhibitors
Alpha-glucosidase inhibitors such as acarbose, miglitol, and voglibose block the enzyme alpha-glucosidase in the cells of the brush border of the small intestine, which delays absorption of carbohydrates. Alpha-glucosidase inhibitors primarily affect postprandial hyperglycemia without causing hypoglycemia. Abdominal cramps, bloating, flatulence, and diarrhea are the most common side effects. Use of alpha-glucosidase inhibitors should be avoided in patients with severe hepatic or renal impairment. Dosing is prior to carbohydrate-containing meals.8,20
Incretin-based therapies
Therapies that target the incretin hormones to increase insulin production include glucagon-like peptide-1 (GLP-1) receptor agonists and dipeptidyl peptidase-4 (DPP-4) inhibitors.
GLP-1 agonists
Exenatide, liraglutide, albiglutide, and dulaglutide are synthetic analogs of the GLP-1 hormone. GLP-1 is produced in the small intestine; it stimulates insulin secretion and inhibits glucagon secretion in a glucose-dependent manner. It also delays gastric emptying and suppresses appetite through central pathways. GLP-1 agonists primarily decrease postprandial blood glucose levels; however, a moderate reduction in fasting blood glucose and some weight loss can also occur.
The major side effects are gastrointestinal complaints such as nausea, vomiting, and diarrhea. Hypoglycemia does not occur unless GLP-1 analogues are combined with a sulfonylurea or insulin. There is a slightly increased risk of acute pancreatitis in patients using GLP-1 agonist medications, and patients must be warned to discontinue use of these medications if abdominal pain occurs.
Dosing of GLP-1 agonist medications is either twice daily, daily, or weekly by subcutaneous injection.8,21
DPP-4 inhibitors
DPP-4 is an enzyme that rapidly degrades GLP-1. Suppression of DPP-4 leads to higher levels of insulin secretion and suppression of glucagon secretion in a glucose-dependent manner.
The DPP-4 inhibitors such as linagliptin, sitagliptin, saxagliptin, and alogliptin are given orally once daily. An increased risk of acute pancreatitis has been reported in some patients. Dose reduction is needed in patients with renal impairment for most of these medications.8,22
SLGT-2 inhibitors
SGLT-2 inhibitors include canagliflozin, dapagliflozin, and empagliflozin and are the newest group of antidiabetic medications. These medications inhibit glucose reabsorption in proximal tubule of the kidney leading to glycosuria, which lowers the blood glucose concentration, lowers blood pressure, and leads to some weight loss. Empagliflozin was shown to be cardioprotective in some patients.23
SGLT-2 inhibitors are given once a day in the morning and the primary side effects are polyuria and genital yeast infections. These medications are contraindicated in patients with severe end-stage renal disease and those who are on dialysis.8,24
Pramlintide (amylinomimetics)
Pramlintide, an amylinomimetic, is a synthetic drug that acts like amylin, a hormone secreted by beta cells that suppresses glucagon secretion, slows gastric emptying, and suppresses appetite through central pathways. Pramlintide acts primarily on postprandial blood glucose levels.
The side effects of pramlintide are gastrointestinal complaints, especially nausea. Currently, pramlintide is approved only as an adjunctive therapy with insulin, and it can be used in patients with type 1 DM or type 2 DM. The dose for type 1 DM is 15 µg before each meal subcutaneously, and for type 2 DM it is generally 60 µg before meals.25
Dopamine-receptor agonist (bromocriptine)
Bromocriptine is a central dopamine-receptor agonist, and when given in rapid-release form within 2 hours of awakening in the morning, it improves glycemic control for patients with type 2 DM. The mechanism of action resulting in improved glycemic control is unknown. Studies have demonstrated the cardiovascular safety of bromocriptine.26
Side effects of bromocriptine include hypotension, somnolence, and nausea. Individuals with psychiatric disorders may experience exacerbation while taking bromocriptine. Bromocriptine is taken with food to diminish nausea.27
Insulin
Insulin and insulin analogues remain the most direct method of reducing hyperglycemia. There is no upper limit in dosing for therapeutic effect, so it can be used to bring any HbA1c down to near-normal levels. Other benefits of insulin include reducing triglyceride levels and increasing high-density lipoprotein cholesterol.
Hypoglycemia is a concern with use of insulin, and studies have shown that episodes for which the patient required assistance due to the hypoglycemia occurred between 1 and 3 times per 100 patient-years.13 Weight gain can occur after initiation of insulin therapy, and patients typically gain 2 kg to 4 kg.8
Initiation and Titration of Therapy
All patients with type 1 DM require insulin therapy. There are 2 regimens available: basal-bolus and insulin-pump therapy. Patients with type 2 DM often require insulin, which can be combined with oral hypoglycemic agents. Regimens include basal insulin only, twice-daily premixed insulin, basal-bolus therapy, and insulin-pump therapy.28
Basal-bolus therapy
The basal-bolus regimen combines a long-acting agent for basal-insulin needs that is used once or twice daily and a rapid-acting agent for prandial coverage. Traditionally, 50% of the total daily dose is given as basal insulin (detemir, glargine, degludec) and the remaining dose as prandial insulin divided equally before meals (regular, lispro, glulisine, or aspart).
The meal dose of insulin can be fixed, but it is better to determine the dose based on the carbohydrate content of the meal. To do so, patients should be educated about carbohydrate counting and the dose of insulin required to cover the carbohydrate content of the meal. Consultation with a diabetes educator is needed for patients to effectively dose insulin based on the carbohydrate content of meals. Patients are also provided with a sliding scale of supplemental insulin to use as a third component of therapy when the blood glucose level is higher than desired.
The starting total daily insulin dose is typically 0.3 U/kg for patients with type 1 DM and 0.5 U/kg for patients with type 2 DM if no other medications are used. The ADA recommends adding basal insulin at 0.1 to 0.2 U/kg for patients with type 2 DM once they need it. The key to good glycemic control is self-monitoring of blood glucose by the patient and frequent adjustment of the regimen until control is achieved.8
Insulin-pump therapy
The insulin pump allows the use of different basal insulin rates at different periods of the day for greater flexibility with daily dosing. The insulin pump also allows administration of the meal bolus as a single discrete bolus or as an extended bolus (square bolus) over a certain period of time, which allows a better match between insulin delivery and glucose absorption from the meal in patients with abnormalities of gastric emptying. Use of an insulin pump should be considered in the following patients:
- Patients unable to achieve target goals with basal-bolus regimens
- Patients with frequent hypoglycemia, dawn phenomenon, or brittle diabetes
- Pregnant patients
- Patients with insulin sensitivity or those requiring more intense monitoring due to complications.
Recently, continuous glucose monitors have been developed that measure interstitial glucose levels. Continuous glucose monitoring has been shown to lower HbA1c in adult patients with type 1 DM.29
Gestational diabetes
In patients with gestational diabetes, insulin therapy is indicated when exercise and nutritional therapy are ineffective in controlling prandial and fasting blood glucose levels. Basal therapy alone may be sufficient, but a basal-bolus regimen is often required.8
Summary
- Glycemic control reduces the development and progression of complications of diabetes such as retinopathy, nephropathy, and neuropathy.
- The primary techniques available to assess the quality of a patient’s glycemic control are self-monitoring of blood glucose and interval measurement of HbA1c.
- Available treatment options to control blood glucose include insulin sensitizers, insulin secretagogues, alpha-glucosidase inhibitors, incretin-based therapies, SGLT-2 inhibitors, amylinomimetics (pramlintide), dopamine-receptor agonist (bromocriptine), and insulin.
- Mitka M. Report quantifies diabetes complications. JAMA 2007; 297:2337–2338.
- Welschen LM, Bloemendal E, Nijpels G, et al. Self-monitoring of blood glucose in patients with type 2 diabetes who are not using insulin: a systematic review. Diabetes Care 2005; 28:1510–1517.
- UK Prospective Diabetes Study (UKPDS) Group. Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34) [published erratum appears in Lancet 1998; 352:1558]. Lancet 1998; 352:854–865.
- Chase HP, Jackson WE, Hoops SL, Cockerham RS, Archer PG, O’Brien D. Glucose control and the renal and retinal complications of insulin-dependent diabetes. JAMA 1989; 261:1155–1160.
- The Diabetes Control and Complications Trial Research Group. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med 1993; 329:977–986.
- Evans JM, Newton RW, Ruta DA, MacDonald TM, Stevenson RJ, Morris AD. Frequency of blood glucose monitoring in relation to glycaemic control: observational study with diabetes database. BMJ 1999; 319:83–86.
- Bergenstal, RM, James GR III; Global Consensus Conference on Glucose Monitoring Panel. The role of self-monitoring of blood glucose in the care of people with diabetes: report of a global consensus conference. Am J Med 2005; 118(suppl 9A):1S–6S.
- American Diabetes Association. Standards of medical care in diabetes—2017: summary of revisions. Diabetes Care 2017; 40(suppl 1):S1–S135.
- Schwedes U, Siebolds M, Mertes G; for the SMBG Study Group. Meal-related structured self-monitoring of blood glucose: effect on diabetes control in non-insulin-treated type 2 diabetic patients. Diabetes Care 2002; 25:1928–1932.
- Saudek CD, Derr RL, Kalyani RR. Assessing glycemia in diabetes using self-monitoring blood glucose and hemoglobin A1c. JAMA 2006; 295:1688–1697.
- Delamater A. Clinical use of hemoglobin A1c to improve diabetes management. Clinical Diabetes 2006; 24:6–8.
- Inzucchi SE, Bergenstal RM, Buse JB, et al. Management of hyperglycemia in type 2 diabetes, 2015: a patient-centered approach: update to a position statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care 2015; 38:140–149.
- Nathan DM, Buse JB, Davidson MB, et al; American Diabetes Association; European Association for Study of Diabetes. Medical management of hyperglycemia in type 2 diabetes: a consensus algorithm for the initiation and adjustment of therapy: a consensus statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care 2009; 32:193–203.
- Bailey CJ, Turner RC. Metformin. N Engl J Med 1996; 334:574–579.
- Bailey CJ. Biguanides and NIDDM. Diabetes Care 1992; 15:755–772.
- Dormandy JA, Charbonnel C, Eckland DJ, et al; PROactive investigators. Secondary prevention of macrovascular events in patients with type 2 diabetes in the PROactive Study (PROspective pioglitAzone Clinical Trial In macroVascular Events): a randomised controlled trial. Lancet 2005; 366:1279–1289.
- Fonseca VA, Kulkarni KD. Management of type 2 diabetes: oral agents, insulin, and injectables. J Am Diet Assoc 2008; 108(4 suppl 1):S29–S33.
- Nathan DM, Buse JB, Davidson MB, et al. Management of hyperglycemia in type 2 diabetes: a consensus algorithm for the initiation and adjustment of therapy—update regarding thiazolidinediones: a consensus statement from the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care 2008; 31:173–175.
- UK Prospective Diabetes Study (UKPDS) Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33) [erratum published in Lancet 1999; 354:602]. Lancet 1998; 352:837–853.
- Chiasson J-L, Josse RG, Gomis R, Hanefeld M, Karsik A, Laakso M; for the STOP-NIDDM Trial Research Group. Acarbose treatment and the risk of cardiovascular disease and hypertension in patients with impaired glucose tolerance: The STOP-NIDDM Trial. JAMA 2003; 290:486–494.
- Victoza [package insert]. Bagsvaerd, Denmark: Novo Nordisk; 2010. https://www.accessdata.fda.gov/drugsatfda_docs/label/2010/022341lbl.pdf. Accessed June 26, 2017.
- Nauck MA, Vilsbøll T, Gallwitz B, Garber A, Madsbad S. Incretin-based therapies viewpoints on the way to consensus. Diabetes Care 2009; 32(suppl 2):S223–S231.
- ZinmanB, Wanner C, Larchin JM; EMPA-REG OUTCOME Investigators. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med 2015; 373:2117–2128.
- Abdul-Ghani MA, Norton L, DeFronzo RA. Role of sodium-glucose cotransporter 2 (SGLT 2) inhibitors in the treatment of type 2 diabetes. Endocr Rev 2011; 32:515–531.
- Symlin (Pramlintide acetate) [package insert]. Wilmington DE: AstraZeneca; 2015. Pharmaceuticals LP. http://www.azpicentral.com/symlin/pi_symlin.pdf#page=1. Accessed June 26, 2017.
- Gaziano JM, Cincotta AH, O’Connor CM, et al. Randomized clinical trial of quick-release bromocriptine among patients with type 2 diabetes on overall safety and cardiovascular outcomes. Diabetes Care 2010; 33:1503–1508.
- Cycloset [package insert]. Tiverton, RI: VeroScience LLC; 2016. http://www.veroscience.com/documents/CyclosetPackageInsertFeb062017.pdf. Accessed June 26, 2017.
- Hirsch IB, Bergenstal RM, Parkin CG, Wright Jr, E, Buse JB. A real-world approach to insulin therapy in primary care practice. Clinical Diabetes 2005; 23:78–86.
- Juvenile Diabetes Research Foundation Continuous Glucose Monitoring Study Group; Tamborlane WV, Beck RW, Bode BW, et al. Continuous glucose monitoring and intensive treatment of type 1 diabetes. N Engl J Med 2008; 359:1464–1476.
In the United States, 57.9% of patients with diabetes mellitus (DM) have at least 1 diabetes-related complication and 14.3% of patients with diabetes have 3 or more diabetes-related complications.1 Achieving glycemic control in patients with DM reduces the development and progression of retinopathy, nephropathy, and neuropathy. Aggressive treatment of dyslipidemia and hypertension decreases macrovascular complications.2–4 The techniques for monitoring blood glucose and the various treatment options available to manage glycemic control in patients with diabetes are reviewed below.
Measuring Glycemic Control
The primary techniques available to assess the quality of a patient’s glycemic control are self-monitoring of blood glucose and interval measurement of hemoglobin A1c (HbA1c). Continuous glucose monitoring is also available and may be appropriate for select patients, such as patients with brittle diabetes and those using insulin pumps.
Self-monitoring of blood glucose
For patients with type 1 DM and patients with insulin-dependent type 2 DM, self-monitoring of blood glucose allows patients to adjust insulin dosing to prevent hypoglycemia and hyperglycemia.2,5–7 The American Diabetes Association (ADA) guidelines recommend that patients with type 1 DM self-monitor their glucose:
- Before eating
- At bedtime
- Before exercise
- If hypoglycemia is suspected
- Until hypoglycemia is corrected
- Postprandially upon occasion
- And before critical tasks (ie, driving).8
Patients should be educated about how to use real-time blood glucose values to adjust their food intake and medical therapy.
It is commonly recommended that patients with type 2 DM self-monitor their blood glucose levels, but the evidence to support the effectiveness of this practice is inconclusive. Initial studies showed reductions in HbA1c with self-monitoring; however, the inclusion of beneficial health behaviors such as diet and exercise in the analyses makes it difficult to assess the effectiveness of self-monitor blood glucose alone.2,9
The ADA recommends that nonpregnant adults maintain blood glucose levels of 80 mg/dL to 130 mg/dL preprandial and less than 180 mg/dL postprandial.8 The blood glucose goals for patients with gestational diabetes are 95 mg/dL or less preprandial and either 140 mg/dL or less 1-hour postprandial or 120 mg/dL or less 2-hours postprandial.
HbA1c
HbA1c tests reflect the mean blood glucose values over a 3-month period and can predict patients’ risk of microvascular complications.10,11 The ADA recommends that patients with stable glycemic control have an HbA1c test at least twice a year. Quarterly HbA1c testing is suggested for patients with a recent change in therapy or for patients not meeting their glycemic goals.8
Measurement of HbA1c is influenced by the red blood cell turnover rate; therefore, anemia, transfusions, and hemoglobinopathies can cause inaccurate test values. The ADA recommends that nonpregnant adults maintain HbA1c levels near 7%. For patients with diabetes who become pregnant, the goal is HbA1c levels less than 6.0%.8 The ADA also recommends that select patients, especially those with a long life expectancy and little comorbidity, adopt glycemic targets near normal levels (HbA1c < 6.5%), providing the target can be achieved without significant hypoglycemia.8
Glycemic Treatment
Insulin sensitizers
Biguanides (metformin)
Metformin is the only available biguanide. Metformin should be used as a first-line therapy in patients with type 2 DM whenever possible.13 Metformin suppresses hepatic glucose output and primarily affects fasting glycemia; however, reduced postprandial glucose concentrations also occur.
The most common side effects of metformin are diarrhea, nausea, and abdominal discomfort. Metformin has the potential to produce very rare but life-threatening lactic acidosis (< 1 in 100,000). The use of metformin is contraindicated in patients with a glomerular filtration rate less than 30 mL/min, with acidosis, hypoxia, or dehydration.8
Metformin usually does not lead to hypoglycemia when used as monotherapy. It can lead to weight loss (3%–5% of body weight), and it has been shown to decrease plasma triglyceride concentrations (10%–20%).8,14,15
Thiazolidinediones
Thiazolidinediones (TZDs) primarily enhance the insulin sensitivity of muscle and fat tissue and mildly enhance insulin sensitivity of the liver. TZDs lower fasting and postprandial blood glucose levels.
Major side effects of TZDs include weight gain, with an increase in subcutaneous adiposity, and fluid retention. Fluid retention typically manifests as peripheral edema, but heart failure can occur on occasion. These agents should be avoided in patients with functional class III or IV heart failure. The PROactive trial of the TZD pioglitazone found that pioglitazone did not increase cardiovascular risk compared with placebo.16 TZDs have been associated with an increased risk of fractures, particularly in women. When used as monotherapy, TZDs do not cause hypoglycemia. Pioglitazone lowers triglyceride levels, increases high-density lipoprotein cholesterol, and increases the low-density lipoprotein cholesterol particle size.8,16–18
Insulin secretagogues
Insulin secretagogues such as sulfonylureas and glinides stimulate secretion of insulin from the pancreas regardless of the ambient glucose concentration.
Sulfonylureas
Sulfonylureas lower fasting and postprandial glucose levels. The main side effects include weight gain (about 2 kg upon initiation) and hypoglycemia. The UK Prospective Diabetes Study (UKPDS) trial showed a decrease in microvascular complications with the use of sulfonylureas.19 Caution should be used in patients with liver or kidney dysfunction or patients who frequently skip meals. Newer, second-generation sulfonylureas (ie, glipizide and glimepiride) may have less risk of hypoglycemia because their action is somewhat glucose dependent.8,17,19
Glinides
Glinides, which include repaglinide and nateglenide, have a rapid onset of action and a short duration of action, so they are a good option for patients with erratically timed meals. Glinides have a lower risk of hypoglycemia than sulfonylureas. Caution must be used with glinides in patients with liver dysfunction. Dosing is immediately before meals.8,17
Alpha-glucosidase inhibitors
Alpha-glucosidase inhibitors such as acarbose, miglitol, and voglibose block the enzyme alpha-glucosidase in the cells of the brush border of the small intestine, which delays absorption of carbohydrates. Alpha-glucosidase inhibitors primarily affect postprandial hyperglycemia without causing hypoglycemia. Abdominal cramps, bloating, flatulence, and diarrhea are the most common side effects. Use of alpha-glucosidase inhibitors should be avoided in patients with severe hepatic or renal impairment. Dosing is prior to carbohydrate-containing meals.8,20
Incretin-based therapies
Therapies that target the incretin hormones to increase insulin production include glucagon-like peptide-1 (GLP-1) receptor agonists and dipeptidyl peptidase-4 (DPP-4) inhibitors.
GLP-1 agonists
Exenatide, liraglutide, albiglutide, and dulaglutide are synthetic analogs of the GLP-1 hormone. GLP-1 is produced in the small intestine; it stimulates insulin secretion and inhibits glucagon secretion in a glucose-dependent manner. It also delays gastric emptying and suppresses appetite through central pathways. GLP-1 agonists primarily decrease postprandial blood glucose levels; however, a moderate reduction in fasting blood glucose and some weight loss can also occur.
The major side effects are gastrointestinal complaints such as nausea, vomiting, and diarrhea. Hypoglycemia does not occur unless GLP-1 analogues are combined with a sulfonylurea or insulin. There is a slightly increased risk of acute pancreatitis in patients using GLP-1 agonist medications, and patients must be warned to discontinue use of these medications if abdominal pain occurs.
Dosing of GLP-1 agonist medications is either twice daily, daily, or weekly by subcutaneous injection.8,21
DPP-4 inhibitors
DPP-4 is an enzyme that rapidly degrades GLP-1. Suppression of DPP-4 leads to higher levels of insulin secretion and suppression of glucagon secretion in a glucose-dependent manner.
The DPP-4 inhibitors such as linagliptin, sitagliptin, saxagliptin, and alogliptin are given orally once daily. An increased risk of acute pancreatitis has been reported in some patients. Dose reduction is needed in patients with renal impairment for most of these medications.8,22
SLGT-2 inhibitors
SGLT-2 inhibitors include canagliflozin, dapagliflozin, and empagliflozin and are the newest group of antidiabetic medications. These medications inhibit glucose reabsorption in proximal tubule of the kidney leading to glycosuria, which lowers the blood glucose concentration, lowers blood pressure, and leads to some weight loss. Empagliflozin was shown to be cardioprotective in some patients.23
SGLT-2 inhibitors are given once a day in the morning and the primary side effects are polyuria and genital yeast infections. These medications are contraindicated in patients with severe end-stage renal disease and those who are on dialysis.8,24
Pramlintide (amylinomimetics)
Pramlintide, an amylinomimetic, is a synthetic drug that acts like amylin, a hormone secreted by beta cells that suppresses glucagon secretion, slows gastric emptying, and suppresses appetite through central pathways. Pramlintide acts primarily on postprandial blood glucose levels.
The side effects of pramlintide are gastrointestinal complaints, especially nausea. Currently, pramlintide is approved only as an adjunctive therapy with insulin, and it can be used in patients with type 1 DM or type 2 DM. The dose for type 1 DM is 15 µg before each meal subcutaneously, and for type 2 DM it is generally 60 µg before meals.25
Dopamine-receptor agonist (bromocriptine)
Bromocriptine is a central dopamine-receptor agonist, and when given in rapid-release form within 2 hours of awakening in the morning, it improves glycemic control for patients with type 2 DM. The mechanism of action resulting in improved glycemic control is unknown. Studies have demonstrated the cardiovascular safety of bromocriptine.26
Side effects of bromocriptine include hypotension, somnolence, and nausea. Individuals with psychiatric disorders may experience exacerbation while taking bromocriptine. Bromocriptine is taken with food to diminish nausea.27
Insulin
Insulin and insulin analogues remain the most direct method of reducing hyperglycemia. There is no upper limit in dosing for therapeutic effect, so it can be used to bring any HbA1c down to near-normal levels. Other benefits of insulin include reducing triglyceride levels and increasing high-density lipoprotein cholesterol.
Hypoglycemia is a concern with use of insulin, and studies have shown that episodes for which the patient required assistance due to the hypoglycemia occurred between 1 and 3 times per 100 patient-years.13 Weight gain can occur after initiation of insulin therapy, and patients typically gain 2 kg to 4 kg.8
Initiation and Titration of Therapy
All patients with type 1 DM require insulin therapy. There are 2 regimens available: basal-bolus and insulin-pump therapy. Patients with type 2 DM often require insulin, which can be combined with oral hypoglycemic agents. Regimens include basal insulin only, twice-daily premixed insulin, basal-bolus therapy, and insulin-pump therapy.28
Basal-bolus therapy
The basal-bolus regimen combines a long-acting agent for basal-insulin needs that is used once or twice daily and a rapid-acting agent for prandial coverage. Traditionally, 50% of the total daily dose is given as basal insulin (detemir, glargine, degludec) and the remaining dose as prandial insulin divided equally before meals (regular, lispro, glulisine, or aspart).
The meal dose of insulin can be fixed, but it is better to determine the dose based on the carbohydrate content of the meal. To do so, patients should be educated about carbohydrate counting and the dose of insulin required to cover the carbohydrate content of the meal. Consultation with a diabetes educator is needed for patients to effectively dose insulin based on the carbohydrate content of meals. Patients are also provided with a sliding scale of supplemental insulin to use as a third component of therapy when the blood glucose level is higher than desired.
The starting total daily insulin dose is typically 0.3 U/kg for patients with type 1 DM and 0.5 U/kg for patients with type 2 DM if no other medications are used. The ADA recommends adding basal insulin at 0.1 to 0.2 U/kg for patients with type 2 DM once they need it. The key to good glycemic control is self-monitoring of blood glucose by the patient and frequent adjustment of the regimen until control is achieved.8
Insulin-pump therapy
The insulin pump allows the use of different basal insulin rates at different periods of the day for greater flexibility with daily dosing. The insulin pump also allows administration of the meal bolus as a single discrete bolus or as an extended bolus (square bolus) over a certain period of time, which allows a better match between insulin delivery and glucose absorption from the meal in patients with abnormalities of gastric emptying. Use of an insulin pump should be considered in the following patients:
- Patients unable to achieve target goals with basal-bolus regimens
- Patients with frequent hypoglycemia, dawn phenomenon, or brittle diabetes
- Pregnant patients
- Patients with insulin sensitivity or those requiring more intense monitoring due to complications.
Recently, continuous glucose monitors have been developed that measure interstitial glucose levels. Continuous glucose monitoring has been shown to lower HbA1c in adult patients with type 1 DM.29
Gestational diabetes
In patients with gestational diabetes, insulin therapy is indicated when exercise and nutritional therapy are ineffective in controlling prandial and fasting blood glucose levels. Basal therapy alone may be sufficient, but a basal-bolus regimen is often required.8
Summary
- Glycemic control reduces the development and progression of complications of diabetes such as retinopathy, nephropathy, and neuropathy.
- The primary techniques available to assess the quality of a patient’s glycemic control are self-monitoring of blood glucose and interval measurement of HbA1c.
- Available treatment options to control blood glucose include insulin sensitizers, insulin secretagogues, alpha-glucosidase inhibitors, incretin-based therapies, SGLT-2 inhibitors, amylinomimetics (pramlintide), dopamine-receptor agonist (bromocriptine), and insulin.
In the United States, 57.9% of patients with diabetes mellitus (DM) have at least 1 diabetes-related complication and 14.3% of patients with diabetes have 3 or more diabetes-related complications.1 Achieving glycemic control in patients with DM reduces the development and progression of retinopathy, nephropathy, and neuropathy. Aggressive treatment of dyslipidemia and hypertension decreases macrovascular complications.2–4 The techniques for monitoring blood glucose and the various treatment options available to manage glycemic control in patients with diabetes are reviewed below.
Measuring Glycemic Control
The primary techniques available to assess the quality of a patient’s glycemic control are self-monitoring of blood glucose and interval measurement of hemoglobin A1c (HbA1c). Continuous glucose monitoring is also available and may be appropriate for select patients, such as patients with brittle diabetes and those using insulin pumps.
Self-monitoring of blood glucose
For patients with type 1 DM and patients with insulin-dependent type 2 DM, self-monitoring of blood glucose allows patients to adjust insulin dosing to prevent hypoglycemia and hyperglycemia.2,5–7 The American Diabetes Association (ADA) guidelines recommend that patients with type 1 DM self-monitor their glucose:
- Before eating
- At bedtime
- Before exercise
- If hypoglycemia is suspected
- Until hypoglycemia is corrected
- Postprandially upon occasion
- And before critical tasks (ie, driving).8
Patients should be educated about how to use real-time blood glucose values to adjust their food intake and medical therapy.
It is commonly recommended that patients with type 2 DM self-monitor their blood glucose levels, but the evidence to support the effectiveness of this practice is inconclusive. Initial studies showed reductions in HbA1c with self-monitoring; however, the inclusion of beneficial health behaviors such as diet and exercise in the analyses makes it difficult to assess the effectiveness of self-monitor blood glucose alone.2,9
The ADA recommends that nonpregnant adults maintain blood glucose levels of 80 mg/dL to 130 mg/dL preprandial and less than 180 mg/dL postprandial.8 The blood glucose goals for patients with gestational diabetes are 95 mg/dL or less preprandial and either 140 mg/dL or less 1-hour postprandial or 120 mg/dL or less 2-hours postprandial.
HbA1c
HbA1c tests reflect the mean blood glucose values over a 3-month period and can predict patients’ risk of microvascular complications.10,11 The ADA recommends that patients with stable glycemic control have an HbA1c test at least twice a year. Quarterly HbA1c testing is suggested for patients with a recent change in therapy or for patients not meeting their glycemic goals.8
Measurement of HbA1c is influenced by the red blood cell turnover rate; therefore, anemia, transfusions, and hemoglobinopathies can cause inaccurate test values. The ADA recommends that nonpregnant adults maintain HbA1c levels near 7%. For patients with diabetes who become pregnant, the goal is HbA1c levels less than 6.0%.8 The ADA also recommends that select patients, especially those with a long life expectancy and little comorbidity, adopt glycemic targets near normal levels (HbA1c < 6.5%), providing the target can be achieved without significant hypoglycemia.8
Glycemic Treatment
Insulin sensitizers
Biguanides (metformin)
Metformin is the only available biguanide. Metformin should be used as a first-line therapy in patients with type 2 DM whenever possible.13 Metformin suppresses hepatic glucose output and primarily affects fasting glycemia; however, reduced postprandial glucose concentrations also occur.
The most common side effects of metformin are diarrhea, nausea, and abdominal discomfort. Metformin has the potential to produce very rare but life-threatening lactic acidosis (< 1 in 100,000). The use of metformin is contraindicated in patients with a glomerular filtration rate less than 30 mL/min, with acidosis, hypoxia, or dehydration.8
Metformin usually does not lead to hypoglycemia when used as monotherapy. It can lead to weight loss (3%–5% of body weight), and it has been shown to decrease plasma triglyceride concentrations (10%–20%).8,14,15
Thiazolidinediones
Thiazolidinediones (TZDs) primarily enhance the insulin sensitivity of muscle and fat tissue and mildly enhance insulin sensitivity of the liver. TZDs lower fasting and postprandial blood glucose levels.
Major side effects of TZDs include weight gain, with an increase in subcutaneous adiposity, and fluid retention. Fluid retention typically manifests as peripheral edema, but heart failure can occur on occasion. These agents should be avoided in patients with functional class III or IV heart failure. The PROactive trial of the TZD pioglitazone found that pioglitazone did not increase cardiovascular risk compared with placebo.16 TZDs have been associated with an increased risk of fractures, particularly in women. When used as monotherapy, TZDs do not cause hypoglycemia. Pioglitazone lowers triglyceride levels, increases high-density lipoprotein cholesterol, and increases the low-density lipoprotein cholesterol particle size.8,16–18
Insulin secretagogues
Insulin secretagogues such as sulfonylureas and glinides stimulate secretion of insulin from the pancreas regardless of the ambient glucose concentration.
Sulfonylureas
Sulfonylureas lower fasting and postprandial glucose levels. The main side effects include weight gain (about 2 kg upon initiation) and hypoglycemia. The UK Prospective Diabetes Study (UKPDS) trial showed a decrease in microvascular complications with the use of sulfonylureas.19 Caution should be used in patients with liver or kidney dysfunction or patients who frequently skip meals. Newer, second-generation sulfonylureas (ie, glipizide and glimepiride) may have less risk of hypoglycemia because their action is somewhat glucose dependent.8,17,19
Glinides
Glinides, which include repaglinide and nateglenide, have a rapid onset of action and a short duration of action, so they are a good option for patients with erratically timed meals. Glinides have a lower risk of hypoglycemia than sulfonylureas. Caution must be used with glinides in patients with liver dysfunction. Dosing is immediately before meals.8,17
Alpha-glucosidase inhibitors
Alpha-glucosidase inhibitors such as acarbose, miglitol, and voglibose block the enzyme alpha-glucosidase in the cells of the brush border of the small intestine, which delays absorption of carbohydrates. Alpha-glucosidase inhibitors primarily affect postprandial hyperglycemia without causing hypoglycemia. Abdominal cramps, bloating, flatulence, and diarrhea are the most common side effects. Use of alpha-glucosidase inhibitors should be avoided in patients with severe hepatic or renal impairment. Dosing is prior to carbohydrate-containing meals.8,20
Incretin-based therapies
Therapies that target the incretin hormones to increase insulin production include glucagon-like peptide-1 (GLP-1) receptor agonists and dipeptidyl peptidase-4 (DPP-4) inhibitors.
GLP-1 agonists
Exenatide, liraglutide, albiglutide, and dulaglutide are synthetic analogs of the GLP-1 hormone. GLP-1 is produced in the small intestine; it stimulates insulin secretion and inhibits glucagon secretion in a glucose-dependent manner. It also delays gastric emptying and suppresses appetite through central pathways. GLP-1 agonists primarily decrease postprandial blood glucose levels; however, a moderate reduction in fasting blood glucose and some weight loss can also occur.
The major side effects are gastrointestinal complaints such as nausea, vomiting, and diarrhea. Hypoglycemia does not occur unless GLP-1 analogues are combined with a sulfonylurea or insulin. There is a slightly increased risk of acute pancreatitis in patients using GLP-1 agonist medications, and patients must be warned to discontinue use of these medications if abdominal pain occurs.
Dosing of GLP-1 agonist medications is either twice daily, daily, or weekly by subcutaneous injection.8,21
DPP-4 inhibitors
DPP-4 is an enzyme that rapidly degrades GLP-1. Suppression of DPP-4 leads to higher levels of insulin secretion and suppression of glucagon secretion in a glucose-dependent manner.
The DPP-4 inhibitors such as linagliptin, sitagliptin, saxagliptin, and alogliptin are given orally once daily. An increased risk of acute pancreatitis has been reported in some patients. Dose reduction is needed in patients with renal impairment for most of these medications.8,22
SLGT-2 inhibitors
SGLT-2 inhibitors include canagliflozin, dapagliflozin, and empagliflozin and are the newest group of antidiabetic medications. These medications inhibit glucose reabsorption in proximal tubule of the kidney leading to glycosuria, which lowers the blood glucose concentration, lowers blood pressure, and leads to some weight loss. Empagliflozin was shown to be cardioprotective in some patients.23
SGLT-2 inhibitors are given once a day in the morning and the primary side effects are polyuria and genital yeast infections. These medications are contraindicated in patients with severe end-stage renal disease and those who are on dialysis.8,24
Pramlintide (amylinomimetics)
Pramlintide, an amylinomimetic, is a synthetic drug that acts like amylin, a hormone secreted by beta cells that suppresses glucagon secretion, slows gastric emptying, and suppresses appetite through central pathways. Pramlintide acts primarily on postprandial blood glucose levels.
The side effects of pramlintide are gastrointestinal complaints, especially nausea. Currently, pramlintide is approved only as an adjunctive therapy with insulin, and it can be used in patients with type 1 DM or type 2 DM. The dose for type 1 DM is 15 µg before each meal subcutaneously, and for type 2 DM it is generally 60 µg before meals.25
Dopamine-receptor agonist (bromocriptine)
Bromocriptine is a central dopamine-receptor agonist, and when given in rapid-release form within 2 hours of awakening in the morning, it improves glycemic control for patients with type 2 DM. The mechanism of action resulting in improved glycemic control is unknown. Studies have demonstrated the cardiovascular safety of bromocriptine.26
Side effects of bromocriptine include hypotension, somnolence, and nausea. Individuals with psychiatric disorders may experience exacerbation while taking bromocriptine. Bromocriptine is taken with food to diminish nausea.27
Insulin
Insulin and insulin analogues remain the most direct method of reducing hyperglycemia. There is no upper limit in dosing for therapeutic effect, so it can be used to bring any HbA1c down to near-normal levels. Other benefits of insulin include reducing triglyceride levels and increasing high-density lipoprotein cholesterol.
Hypoglycemia is a concern with use of insulin, and studies have shown that episodes for which the patient required assistance due to the hypoglycemia occurred between 1 and 3 times per 100 patient-years.13 Weight gain can occur after initiation of insulin therapy, and patients typically gain 2 kg to 4 kg.8
Initiation and Titration of Therapy
All patients with type 1 DM require insulin therapy. There are 2 regimens available: basal-bolus and insulin-pump therapy. Patients with type 2 DM often require insulin, which can be combined with oral hypoglycemic agents. Regimens include basal insulin only, twice-daily premixed insulin, basal-bolus therapy, and insulin-pump therapy.28
Basal-bolus therapy
The basal-bolus regimen combines a long-acting agent for basal-insulin needs that is used once or twice daily and a rapid-acting agent for prandial coverage. Traditionally, 50% of the total daily dose is given as basal insulin (detemir, glargine, degludec) and the remaining dose as prandial insulin divided equally before meals (regular, lispro, glulisine, or aspart).
The meal dose of insulin can be fixed, but it is better to determine the dose based on the carbohydrate content of the meal. To do so, patients should be educated about carbohydrate counting and the dose of insulin required to cover the carbohydrate content of the meal. Consultation with a diabetes educator is needed for patients to effectively dose insulin based on the carbohydrate content of meals. Patients are also provided with a sliding scale of supplemental insulin to use as a third component of therapy when the blood glucose level is higher than desired.
The starting total daily insulin dose is typically 0.3 U/kg for patients with type 1 DM and 0.5 U/kg for patients with type 2 DM if no other medications are used. The ADA recommends adding basal insulin at 0.1 to 0.2 U/kg for patients with type 2 DM once they need it. The key to good glycemic control is self-monitoring of blood glucose by the patient and frequent adjustment of the regimen until control is achieved.8
Insulin-pump therapy
The insulin pump allows the use of different basal insulin rates at different periods of the day for greater flexibility with daily dosing. The insulin pump also allows administration of the meal bolus as a single discrete bolus or as an extended bolus (square bolus) over a certain period of time, which allows a better match between insulin delivery and glucose absorption from the meal in patients with abnormalities of gastric emptying. Use of an insulin pump should be considered in the following patients:
- Patients unable to achieve target goals with basal-bolus regimens
- Patients with frequent hypoglycemia, dawn phenomenon, or brittle diabetes
- Pregnant patients
- Patients with insulin sensitivity or those requiring more intense monitoring due to complications.
Recently, continuous glucose monitors have been developed that measure interstitial glucose levels. Continuous glucose monitoring has been shown to lower HbA1c in adult patients with type 1 DM.29
Gestational diabetes
In patients with gestational diabetes, insulin therapy is indicated when exercise and nutritional therapy are ineffective in controlling prandial and fasting blood glucose levels. Basal therapy alone may be sufficient, but a basal-bolus regimen is often required.8
Summary
- Glycemic control reduces the development and progression of complications of diabetes such as retinopathy, nephropathy, and neuropathy.
- The primary techniques available to assess the quality of a patient’s glycemic control are self-monitoring of blood glucose and interval measurement of HbA1c.
- Available treatment options to control blood glucose include insulin sensitizers, insulin secretagogues, alpha-glucosidase inhibitors, incretin-based therapies, SGLT-2 inhibitors, amylinomimetics (pramlintide), dopamine-receptor agonist (bromocriptine), and insulin.
- Mitka M. Report quantifies diabetes complications. JAMA 2007; 297:2337–2338.
- Welschen LM, Bloemendal E, Nijpels G, et al. Self-monitoring of blood glucose in patients with type 2 diabetes who are not using insulin: a systematic review. Diabetes Care 2005; 28:1510–1517.
- UK Prospective Diabetes Study (UKPDS) Group. Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34) [published erratum appears in Lancet 1998; 352:1558]. Lancet 1998; 352:854–865.
- Chase HP, Jackson WE, Hoops SL, Cockerham RS, Archer PG, O’Brien D. Glucose control and the renal and retinal complications of insulin-dependent diabetes. JAMA 1989; 261:1155–1160.
- The Diabetes Control and Complications Trial Research Group. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med 1993; 329:977–986.
- Evans JM, Newton RW, Ruta DA, MacDonald TM, Stevenson RJ, Morris AD. Frequency of blood glucose monitoring in relation to glycaemic control: observational study with diabetes database. BMJ 1999; 319:83–86.
- Bergenstal, RM, James GR III; Global Consensus Conference on Glucose Monitoring Panel. The role of self-monitoring of blood glucose in the care of people with diabetes: report of a global consensus conference. Am J Med 2005; 118(suppl 9A):1S–6S.
- American Diabetes Association. Standards of medical care in diabetes—2017: summary of revisions. Diabetes Care 2017; 40(suppl 1):S1–S135.
- Schwedes U, Siebolds M, Mertes G; for the SMBG Study Group. Meal-related structured self-monitoring of blood glucose: effect on diabetes control in non-insulin-treated type 2 diabetic patients. Diabetes Care 2002; 25:1928–1932.
- Saudek CD, Derr RL, Kalyani RR. Assessing glycemia in diabetes using self-monitoring blood glucose and hemoglobin A1c. JAMA 2006; 295:1688–1697.
- Delamater A. Clinical use of hemoglobin A1c to improve diabetes management. Clinical Diabetes 2006; 24:6–8.
- Inzucchi SE, Bergenstal RM, Buse JB, et al. Management of hyperglycemia in type 2 diabetes, 2015: a patient-centered approach: update to a position statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care 2015; 38:140–149.
- Nathan DM, Buse JB, Davidson MB, et al; American Diabetes Association; European Association for Study of Diabetes. Medical management of hyperglycemia in type 2 diabetes: a consensus algorithm for the initiation and adjustment of therapy: a consensus statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care 2009; 32:193–203.
- Bailey CJ, Turner RC. Metformin. N Engl J Med 1996; 334:574–579.
- Bailey CJ. Biguanides and NIDDM. Diabetes Care 1992; 15:755–772.
- Dormandy JA, Charbonnel C, Eckland DJ, et al; PROactive investigators. Secondary prevention of macrovascular events in patients with type 2 diabetes in the PROactive Study (PROspective pioglitAzone Clinical Trial In macroVascular Events): a randomised controlled trial. Lancet 2005; 366:1279–1289.
- Fonseca VA, Kulkarni KD. Management of type 2 diabetes: oral agents, insulin, and injectables. J Am Diet Assoc 2008; 108(4 suppl 1):S29–S33.
- Nathan DM, Buse JB, Davidson MB, et al. Management of hyperglycemia in type 2 diabetes: a consensus algorithm for the initiation and adjustment of therapy—update regarding thiazolidinediones: a consensus statement from the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care 2008; 31:173–175.
- UK Prospective Diabetes Study (UKPDS) Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33) [erratum published in Lancet 1999; 354:602]. Lancet 1998; 352:837–853.
- Chiasson J-L, Josse RG, Gomis R, Hanefeld M, Karsik A, Laakso M; for the STOP-NIDDM Trial Research Group. Acarbose treatment and the risk of cardiovascular disease and hypertension in patients with impaired glucose tolerance: The STOP-NIDDM Trial. JAMA 2003; 290:486–494.
- Victoza [package insert]. Bagsvaerd, Denmark: Novo Nordisk; 2010. https://www.accessdata.fda.gov/drugsatfda_docs/label/2010/022341lbl.pdf. Accessed June 26, 2017.
- Nauck MA, Vilsbøll T, Gallwitz B, Garber A, Madsbad S. Incretin-based therapies viewpoints on the way to consensus. Diabetes Care 2009; 32(suppl 2):S223–S231.
- ZinmanB, Wanner C, Larchin JM; EMPA-REG OUTCOME Investigators. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med 2015; 373:2117–2128.
- Abdul-Ghani MA, Norton L, DeFronzo RA. Role of sodium-glucose cotransporter 2 (SGLT 2) inhibitors in the treatment of type 2 diabetes. Endocr Rev 2011; 32:515–531.
- Symlin (Pramlintide acetate) [package insert]. Wilmington DE: AstraZeneca; 2015. Pharmaceuticals LP. http://www.azpicentral.com/symlin/pi_symlin.pdf#page=1. Accessed June 26, 2017.
- Gaziano JM, Cincotta AH, O’Connor CM, et al. Randomized clinical trial of quick-release bromocriptine among patients with type 2 diabetes on overall safety and cardiovascular outcomes. Diabetes Care 2010; 33:1503–1508.
- Cycloset [package insert]. Tiverton, RI: VeroScience LLC; 2016. http://www.veroscience.com/documents/CyclosetPackageInsertFeb062017.pdf. Accessed June 26, 2017.
- Hirsch IB, Bergenstal RM, Parkin CG, Wright Jr, E, Buse JB. A real-world approach to insulin therapy in primary care practice. Clinical Diabetes 2005; 23:78–86.
- Juvenile Diabetes Research Foundation Continuous Glucose Monitoring Study Group; Tamborlane WV, Beck RW, Bode BW, et al. Continuous glucose monitoring and intensive treatment of type 1 diabetes. N Engl J Med 2008; 359:1464–1476.
- Mitka M. Report quantifies diabetes complications. JAMA 2007; 297:2337–2338.
- Welschen LM, Bloemendal E, Nijpels G, et al. Self-monitoring of blood glucose in patients with type 2 diabetes who are not using insulin: a systematic review. Diabetes Care 2005; 28:1510–1517.
- UK Prospective Diabetes Study (UKPDS) Group. Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34) [published erratum appears in Lancet 1998; 352:1558]. Lancet 1998; 352:854–865.
- Chase HP, Jackson WE, Hoops SL, Cockerham RS, Archer PG, O’Brien D. Glucose control and the renal and retinal complications of insulin-dependent diabetes. JAMA 1989; 261:1155–1160.
- The Diabetes Control and Complications Trial Research Group. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med 1993; 329:977–986.
- Evans JM, Newton RW, Ruta DA, MacDonald TM, Stevenson RJ, Morris AD. Frequency of blood glucose monitoring in relation to glycaemic control: observational study with diabetes database. BMJ 1999; 319:83–86.
- Bergenstal, RM, James GR III; Global Consensus Conference on Glucose Monitoring Panel. The role of self-monitoring of blood glucose in the care of people with diabetes: report of a global consensus conference. Am J Med 2005; 118(suppl 9A):1S–6S.
- American Diabetes Association. Standards of medical care in diabetes—2017: summary of revisions. Diabetes Care 2017; 40(suppl 1):S1–S135.
- Schwedes U, Siebolds M, Mertes G; for the SMBG Study Group. Meal-related structured self-monitoring of blood glucose: effect on diabetes control in non-insulin-treated type 2 diabetic patients. Diabetes Care 2002; 25:1928–1932.
- Saudek CD, Derr RL, Kalyani RR. Assessing glycemia in diabetes using self-monitoring blood glucose and hemoglobin A1c. JAMA 2006; 295:1688–1697.
- Delamater A. Clinical use of hemoglobin A1c to improve diabetes management. Clinical Diabetes 2006; 24:6–8.
- Inzucchi SE, Bergenstal RM, Buse JB, et al. Management of hyperglycemia in type 2 diabetes, 2015: a patient-centered approach: update to a position statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care 2015; 38:140–149.
- Nathan DM, Buse JB, Davidson MB, et al; American Diabetes Association; European Association for Study of Diabetes. Medical management of hyperglycemia in type 2 diabetes: a consensus algorithm for the initiation and adjustment of therapy: a consensus statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care 2009; 32:193–203.
- Bailey CJ, Turner RC. Metformin. N Engl J Med 1996; 334:574–579.
- Bailey CJ. Biguanides and NIDDM. Diabetes Care 1992; 15:755–772.
- Dormandy JA, Charbonnel C, Eckland DJ, et al; PROactive investigators. Secondary prevention of macrovascular events in patients with type 2 diabetes in the PROactive Study (PROspective pioglitAzone Clinical Trial In macroVascular Events): a randomised controlled trial. Lancet 2005; 366:1279–1289.
- Fonseca VA, Kulkarni KD. Management of type 2 diabetes: oral agents, insulin, and injectables. J Am Diet Assoc 2008; 108(4 suppl 1):S29–S33.
- Nathan DM, Buse JB, Davidson MB, et al. Management of hyperglycemia in type 2 diabetes: a consensus algorithm for the initiation and adjustment of therapy—update regarding thiazolidinediones: a consensus statement from the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care 2008; 31:173–175.
- UK Prospective Diabetes Study (UKPDS) Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33) [erratum published in Lancet 1999; 354:602]. Lancet 1998; 352:837–853.
- Chiasson J-L, Josse RG, Gomis R, Hanefeld M, Karsik A, Laakso M; for the STOP-NIDDM Trial Research Group. Acarbose treatment and the risk of cardiovascular disease and hypertension in patients with impaired glucose tolerance: The STOP-NIDDM Trial. JAMA 2003; 290:486–494.
- Victoza [package insert]. Bagsvaerd, Denmark: Novo Nordisk; 2010. https://www.accessdata.fda.gov/drugsatfda_docs/label/2010/022341lbl.pdf. Accessed June 26, 2017.
- Nauck MA, Vilsbøll T, Gallwitz B, Garber A, Madsbad S. Incretin-based therapies viewpoints on the way to consensus. Diabetes Care 2009; 32(suppl 2):S223–S231.
- ZinmanB, Wanner C, Larchin JM; EMPA-REG OUTCOME Investigators. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med 2015; 373:2117–2128.
- Abdul-Ghani MA, Norton L, DeFronzo RA. Role of sodium-glucose cotransporter 2 (SGLT 2) inhibitors in the treatment of type 2 diabetes. Endocr Rev 2011; 32:515–531.
- Symlin (Pramlintide acetate) [package insert]. Wilmington DE: AstraZeneca; 2015. Pharmaceuticals LP. http://www.azpicentral.com/symlin/pi_symlin.pdf#page=1. Accessed June 26, 2017.
- Gaziano JM, Cincotta AH, O’Connor CM, et al. Randomized clinical trial of quick-release bromocriptine among patients with type 2 diabetes on overall safety and cardiovascular outcomes. Diabetes Care 2010; 33:1503–1508.
- Cycloset [package insert]. Tiverton, RI: VeroScience LLC; 2016. http://www.veroscience.com/documents/CyclosetPackageInsertFeb062017.pdf. Accessed June 26, 2017.
- Hirsch IB, Bergenstal RM, Parkin CG, Wright Jr, E, Buse JB. A real-world approach to insulin therapy in primary care practice. Clinical Diabetes 2005; 23:78–86.
- Juvenile Diabetes Research Foundation Continuous Glucose Monitoring Study Group; Tamborlane WV, Beck RW, Bode BW, et al. Continuous glucose monitoring and intensive treatment of type 1 diabetes. N Engl J Med 2008; 359:1464–1476.
Medical Treatment of Diabetes Mellitus
Medical Treatment of Diabetes Mellitus
Dosing accuracy of direct oral anticoagulants in an academic medical center
Direct-acting oral anticoagulants (DOACs) have been introduced into clinical use for stroke prevention in patients with nonvalvular atrial fibrillation (NVAF), prevention of venous thrombosis after hip or knee surgery, and treatment of deep vein thrombosis (DVT) and pulmonary embolism (PE).1-7 Advantages of DOACs over warfarin are often stated as fixed dosing, minor drug and food interactions, wider therapeutic index, and no need for laboratory test monitoring.1,8 Yet, recommended DOAC dosages vary by renal function and therapeutic indications. Dosing recommendations for prevention of stroke in patients with NVAF are based on estimated creatinine clearance (dabigatran, rivaroxaban, edoxaban), age (apixaban), weight (apixaban, edoxaban), serum creatinine level (apixaban, edoxaban), and presence of cirrhosis by Child-Pugh class9,10 (apixaban, edoxaban).4-6,11,12 Dosing recommendations based on coadministration of strong CYP34A and P-glycoprotein inhibitors or inducers vary by DOAC. In addition, dabigatran cannot be crushed and must be stored in its original packaging, and rivaroxaban should be taken with food when the dose is over 10 mg.
We studied DOAC prescribing in adults admitted to a large academic medical center by comparing initial prescribed dosing with FDA-approved prescribing information. We hypothesized that the complexity of DOAC dosing may not be recognized by prescribers.
METHODS
Our study protocol was approved by the Committee on Human Research (Institutional Review Board) of the University of California San Francisco.
Data Collection
We used electronic medical records (EMRs) to identify adult inpatients who were prescribed a DOAC (apixaban, dabigatran, edoxaban, or rivaroxaban) at the University of California San Francisco Medical Center, a large academic hospital, between July 1, 2014 and June 30, 2015. Demographic and medical information related to therapeutic indications, contraindications, and indications for dose adjustments were collected and included diagnoses classified by International Classification of Diseases, Ninth Revision (ICD-9) and Tenth Revision (ICD-10) for venous thromboses; phlebitis or thrombophlebitis; PE or venous embolism; atrial arrhythmias; surgical procedures; cirrhosis and/or ascites or liver disease; coagulopathies; artificial heart valves or implanted devices; prior use of medications including parenteral anticoagulants; and laboratory data obtained before the first DOAC order (serum creatinine level, estimated glomerular filtration rate [eGFR] determined by Chronic Kidney Disease Epidemiology Collaboration,13 international normalized ratio, or, if available, activated partial thromboplastin time and bilirubin level). Creatinine clearance was calculated with the Cockcroft-Gault method14 using total body weight, per drug label recommendation. Child-Pugh class was calculated if cirrhosis was diagnosed.10 DOAC dose, frequency, dosing directions, and prescriber medical specialty were determined.
Accuracy of search results was confirmed by review of the first 200 patients’ records. Records were manually reviewed for encounters lacking ICD-9/10 codes and approved DOAC indications (30%) and encounters having multiple coded diagnostic indications (to identify the indication). ICD-9 codes for venous thrombosis were reviewed to differentiate acute from chronic events.
Data Analysis
The main outcome was concordance or discordance between the first DOAC prescribing order and the FDA-approved prescribing information at the time. Initial classification, performed by 2 independent reviewers (a pharmacist and a physician, or 2 pharmacists), was followed by adjudication and individual record review (by 2 independent reviewers) of all initial prescribing orders classified as discordant. A third reviewer adjudicated any disagreement. Records and notes were reviewed to identify stated or potential reasons for dosing variation and pre-admission prescriptions. Data are presented as means and standard deviations (SDs) and as raw numbers and percentages. Differences in patient characteristics by DOAC or therapeutic indication were determined by analysis of variance (ANOVA) with Bonferroni correction for post hoc comparisons. Dosing information was categorized as the same as recommended, lower than recommended, higher than recommended, or avoid drug use (drug–drug or drug–disease interaction), per FDA-approved prescribing information, and χ2 tests were used to determine whether variation in dosing occurred by individual DOAC, therapeutic indication, or prescriber specialty. Relationships between dosing variation and age or renal function were tested by ANOVA with Bonferroni correction for post hoc comparisons.
RESULTS
Patients with AF were older with lower creatinine clearance compared to patients with other diagnoses. Mean (SD) patient age was 72.1 (12.7) years for AF, 53.1 (10.9) years for chronic PE, 55.5 (14) years for acute PE, 56.4 (15.9) years for chronic DVT, 57.9 (18.4) years for acute DVT, and 61.4 (11.6) years for DVT prevention after hip or knee surgery (P < 0.0001 for all comparisons). Mean (SD) estimated creatinine clearance was 76.8 (43.5) mL/min for AF, 92.4 (44.4) mL/min for DVT prevention after hip or knee surgery, 111 (53) mL/min for chronic DVT, 118 (55) mL/min for acute DVT, 126 (60) mL/min for chronic PE, and 127 (54) mL/min for acute PE (P < 0.0001 for all comparisons). Differences between patient groups by therapeutic indication were not detected for weight, body mass index, or serum creatinine level.
The most frequent deviation from prescribing recommendations was omission of directions to administer rivaroxaban with food—93% (248/268) of orders—but not for DVT prevention after hip or knee surgery, for which the 10-mg dose is appropriately administered without food. Doses were the same as recommended for 82% of apixaban orders, 84% of rivaroxaban orders, and 93% of initial dabigatran orders (P < 0.05 for differences; Table 3). Dosages not concordant with FDA recommendations were prescribed in 44 (18.1%) of 243 apixaban orders, 41 (14.3%) of 286 rivaroxaban orders, and 7 (7.2%) of 89 initial dabigatran orders. Lower than recommended doses were more common than higher than recommended doses (Table 3, Figure 1): 15.2% versus 2.1% of apixaban orders, 9.4% versus 3.5% of rivaroxaban orders, and 4.2% versus 1.0% of initial dabigatran orders (P < 0.05). Failure to avoid drug use (for potential drug–drug or drug–disease interactions) was uncommon (1%-2%). There were more deviations from recommended doses for patients with AF or DVT prevention after hip or knee surgery than for patients with acute or chronic PE or acute DVT (Table 3). No significant differences were detected between prescribed and recommended doses by prescriber specialty.
For apixaban, patients who were prescribed lower than recommended doses were older than those prescribed recommended doses: mean (SD), 78.1 (12.2) years versus 71 (13.6) years (P = 0.003). Seventy-six percent of those prescribed lower than recommended doses were older than 75. Prescriptions for apixaban at lower than recommended doses were continuations of prior outpatient prescriptions in 20 of 37 cases (almost half), and in 12 cases (one-fourth) antiplatelet drugs were coprescribed (aspirin in 10 cases, clopidogrel in 1, prasugrel in 1). For rivaroxaban, older age was associated with both lower than recommended dosing (P = 0.003) and higher than recommended dosing (P < 0.001). Variations from prescribing recommendations were continuations of outpatient rivaroxaban doses in about two-thirds (26 of 41; 63.4 %) with 13 receiving antiplatelet drugs. For dabigatran, 6 of 7 orders not in agreement with recommendations were continuations of outpatient dosing.
The specific equation used to estimate renal function also had the potential to lead to dosing errors. Among the 41 rivaroxaban patients categorized as receiving doses discordant with recommendations, 8 would have had an inappropriate DOAC dose if eGFR were used instead of eCrCL as recommended. No relationships were detected for other patient variables/measures and dosing deviations from recommendations.
DISCUSSION
We examined initial hospital orders for DOACs in adults admitted to a single academic medical center during 2014-2015. Dabigatran, apixaban and rivaroxaban were prescribed for prevention of stroke in patients with atrial fibrillation/flutter (AF) in three quarters of the encounters similar to national patterns. (15) Prescribing departures from FDA-approved recommendations ranged from failure to prescribe rivaroxaban with food to failure to recognize drug-drug interactions in 1% to 2%. Unexpectedly, lower than recommended dosing was more common than higher than recommended dosing of the three DOACs.
Rivaroxaban bioavailability is dose dependent with the presence of food required to enhance absorption for doses over 10 mg that are used for prevention of stroke in patients with non-valvular AF or treatment of DVT or PE.5,16 Peak rivaroxaban concentrations are 75% higher and the total area under the concentration vs. time curve after dosing is 40% higher when rivaroxaban is administered with high fat high calorie meals compared to the fasting state.16 If rivaroxaban is not administered with food, drug concentrations and pharmacologic effects may be less than in clinical trials that specified co-administration with food.17-19 A small survey of outpatients receiving rivaroxaban found that 23% reported taking it without food.20 With electronic pharmacy systems in almost all hospitals and electronic prescriber order entry in most, automated addition of directions for rivaroxaban administration with food for doses over 10 mg to labels or dispensing instructions could easily correct this deviation from recommended practice.
Lower than recommended doses were prescribed in 9.4% of orders for rivaroxaban and 15.2% of orders for apixaban, with dose-deviations often appearing to be a continuation of outpatient doses. Patients 75 years or older were more likely to receive lower than recommended dosing of apixaban. Reductions in apixaban doses from 5 mg twice daily to 2.5 mg twice daily are recommended in patients with non-valvular AF with two of the following criteria: age ≥80 y, weight ≤60 kg, serum creatinine ≥1.5 mg/dL or co-administration of a strong PgP inhibitor to a patient without 2 of the 3 dose reduction criteria. Our study was not designed to determine reasons for under-dosing, but we speculate that clinicians may have considered patients aged 75-79 years to be similar to those 80 years of age or older, or, older and not as healthy as those enrolled in randomized trials.21-25 The median age of our patients with AF receiving apixaban was 75y (interquartile range of 16) vs 70y ( interquartile range 63-76) in the pivotal trial comparing warfarin to apixaban.21 Renal function was also lower with 37% having eCrCL below 50 mL/min compared to 17% in ARISTOTLE. (21). Twenty-six percent of our apixaban-treated AF patients qualified for the lower 2.5 mg twice daily compared to only 5% of ARISTOTLE participants,21 further suggesting differences between patients in our sample compared to randomized trial participants.
Concerns regarding bleeding or falls in older patients, may also have contributed to lower than recommended doses. Recent analyses of patients at risk for falls confirmed that increased risk of falling was associated with more bone fractures, bleeding and all-cause death but not stroke or systemic emboli, and with less severe bleeding with the DOAC edoxaban compared to warfarin.26 While a rationale for personalized or lower than recommended dosing of apixaban may exist in very old patients and those at risk of falls and bleeding, more data are needed to determine outcomes of lower than recommended doses of DOACs before such an approach can be endorsed. Monitoring of anticoagulant effect in patients who receive doses lower than those investigated in clinical trials could provide important information. The assays that measure DOAC effects are likely to be more available because of the use of reversal agents in the setting of bleeding with DOACs.27
We had anticipated higher than recommended dosing for rivaroxaban as recommendations are based on creatinine clearance while laboratories routinely report estimated glomerular filtration rate (eGFR) that can provide higher estimates of renal clearance and estimated DOAC doses in older and smaller individuals.28 Higher than recommended dosing was found in only 3.5% of our sample. In half, eGFR estimates were higher than creatinine clearance estimates. An international postmarketing registry of rivaroxaban use for the prevention of stroke in patients with NVAF, which included outpatients, found that 36% of those with creatinine clearances below 50 mL/min received a dose higher than recommended, and 15% received a dose lower than expected.29 A more recent outpatient registry report on patients with NVAF, in which apixaban, dabigatran, or rivaroxaban was administered, found that overall 9.4% received a dose lower than recommended, and 3.4% were overdosed, with a similar percentage (34%) of rivaroxaban patients with creatinine clearance of 15 to 50 mL/min receiving higher than recommended dosing.30 The lower rate of higher-than-recommended doses that we observed may have been related to the routine measurement of serum creatinine and attention to dosing adjustments for renal function in the inpatient setting compared to the outpatient setting. In addition, renal function data may not be available to outpatient pharmacies, limiting potential input on dosing recommendations. At least one cardiac society recommends monitoring of renal function in patients treated with DOACs, annually in patients with normal estimated creatinine clearance and more frequently (at intervals in months equal to the creatinine clearance divided by 10) in patients with abnormal creatinine clearance.11 A hospital encounter provides an opportunity to assess or reassess renal status to optimize DOAC dosing.
Dabigatran was the first DOAC introduced into use in the United States with the same dose recommended for prevention of stroke in patients with AF or venous thromboembolic disease with reductions for creatinine clearance below 30 mL/min or creatinine clearance between 30 and 50 mL/min and concomitant use of the potent P-glycoprotein inhibitor dronedarone or systemic ketoconazole. The relative simplicity of dosing may have been responsible for the lowest rate of prescribing outside of recommendations observed in this study, but the low dabigatran use limits analyses of contributing factors.
Failure to avoid drug use in combination with use of strong P-glycoprotein inducers or inhibitors was infrequent but should be preventable. Current prescribing recommendations refer to “strong” P-glycoprotein inhibitors and list different specific agents that interact with each DOAC without a standardized definition or classification. Standardized classifications or reference sources would be helpful.
Our primary goal in this study was to compare initial prescribed dosing of DOACs with FDA-approved prescribing directions. However, therapeutic indication data warrant discussion. In our sample, 7.5% of patients with AF had bioprosthetic valves or recent mitral valve repair or replacement. Using the NVAF definition found in the 2014 AHA/ACC/HRS (American Heart Association, American College of Cardiology, Heart Rhythm Society) AF guidelines1—“absence of rheumatic mitral valve disease, a prosthetic heart valve, or mitral valve repair”—these patients would not appear to be candidates for DOACs. However, arguments have been made that a bioprosthetic heart valve or native valve after valve repair does not have a risk profile for thromboembolism that differs from other forms of NVAF and would be equally responsive to DOAC therapy.31 Data are sparse, but retrospective subanalyses of limited numbers of patients with valvular disease (including bioprosthesis and mitral repair patients but excluding mechanical valve patients) enrolled in the pivotal DOAC studies support this conclusion.32 For the first months after biological valve replacement (including catheter-based valve replacement), recent European guidelines recommend vitamin K antagonists but also state, “NOACs probably deliver the same protection.”8 DOACs were also used for management of venous thromboembolic disease (both acute and chronic) in patients with active cancer. Our data predate the most recent American College of Chest Physician guidelines on treatment of venous thromboembolism in patients with cancer, which provide grade 2B recommendations for use of low-molecular-weight heparin (LMWH) over vitamin K antagonists and grade 2C recommendations for use of LMWH over dabigatran, rivaroxaban, apixaban, or edoxaban.33
Our study had several limitations. First, data were from a single US academic medical center, though similar rates of prescribing deviation from recommendations have been reported for rivaroxaban and dabigatran in NVAF patients in other countries.29,34 Second, therapeutic indications may have been misclassified because of errors, incomplete EMR data, or multiple indications. Third, we analyzed the first DOAC order and not dispensing information or subsequent corrections. Therefore, deviations from recommendations should not be interpreted as errors that reached patients. We evaluated dosing based on the measures used at the time of hospital admission, noting that, in a significant fraction of deviations from recommended doses, they represented continuations of outpatient doses when renal function or weight may have differed, and it is unknown whether patients were counseled to take rivaroxaban with food in the outpatient setting. Fourth, the number of patients with acute DVT was small, so firm conclusions cannot be drawn for this specific population. Fifth, our estimates of off-label dosing may have been underestimates, as data on cancer and cancer activity or cardiac valvular disease may not have been complete.
CONCLUSION
Healthcare professionals are prescribing DOACs in ways that differ from recommendations. These differences may reflect the older ages and reduced renal function of clinical populations relative to randomized clinical trial groups, but they could also potentially alter clinical efficacy. Our findings support the need to evaluate the appropriateness and dosing of DOACs at each encounter and to determine the outcomes of patients treated with lower than recommended doses of DOACs and the outcomes of DOAC-treated patients with bioprostheses or active malignancies.
Acknowledgment
The authors thank Tobias Schmelzinger for electronic data extraction and compilation and University of California San Francisco students Eduardo De La Torre Cruz (School of Pharmacy) and Carlos Mikell (School of Medicine) for assistance with data review.
Disclosure
Dr. Schwartz reports receiving personal fees from Bristol-Myers Squibb and Amgen and grants from Bristol-Myers Squibb and Pfizer, outside the submitted work. The other authors have nothing to report.
1. January CT, Wann LS, Alpert JS, et al. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: executive summary. A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the Heart Rhythm Society. J Am Coll Cardiol. 2014;64(21):2246-2280. PubMed
2. Saraf K, Morris PD, Garg P, Sheridan P, Storey R. Non–vitamin K antagonist oral anticoagulants (NOACs): clinical evidence and therapeutic considerations. Postgrad Med J. 2014;90(1067):520-528. PubMed
3. Yeh CH, Gross PL, Weitz JI. Evolving use of new oral anticoagulants for treatment of venous thromboembolism. Blood. 2014;124(7):1020-1028. PubMed
4. Pradaxa website. https://www.pradaxa.com. Accessed June 1, 2017.
5. Xarelto website. https://www.xarelto-us.com. Accessed June 1, 2017.
6. Eliquis website. http://www.eliquis.com. Accessed June 1, 2017.
7. Savaysa [prescribing information]. Tokyo, Japan: Daiichi Sankyo; 2015.
8. Kirchhof P, Benussi S, Kotecha D, et al. 2016 ESC guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Eur Heart J. 2016;37(38):2893-2962. PubMed
9. Child C, Turcotte J. Surgery and portal hypertension. In: Child CG, ed. The Liver and Portal Hypertension. Philadelphia, PA: Saunders; 1964:50-64. PubMed
10. Pugh RN, Murray-Lyon IM, Dawson JL, Pietroni MC, Williams R. Transection of the oesophagus for bleeding oesophageal varices. Br J Surg. 1973;60(8):646-649. PubMed
11. Heidbuchel H, Verhamme P, Alings M, et al. Updated European Heart Rhythm Association practical guide on the use of non–vitamin K antagonist anticoagulants in patients with non-valvular atrial fibrillation. Europace. 2015;17(10):1467-1507. PubMed
12. Savaysa website. https://savaysahcp.com. Accessed June 1, 2017.
13. Levey AS, Stevens LA, Schmid CH, et al; CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration). A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150(9):604-612. PubMed
14. Cockcroft DW, Gault MH. Prediction of creatinine clearance from serum creatinine. Nephron. 1976;16(1):31-41. PubMed
15. Rose AJ, Reisman JI, Allen AL, Miller DR. Potentially inappropriate prescribing of direct-acting oral anticoagulants in the Veterans Health Administration. Am J Pharm Benefits. 2016;4(4):e75-e80.
16. Stampfuss J, Kubitza D, Becka M, Mueck W. The effect of food on the absorption and pharmacokinetics of rivaroxaban. Int J Clin Pharmacol Ther. 2013;51(7):549-561. PubMed
17. Patel MR, Mahaffey KW, Garg J, et al; ROCKET AF Investigators. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med. 2011;365(10):883-891. PubMed
18. EINSTEIN Investigators, Bauersachs R, Berkowitz SD, et al. Oral rivaroxaban for symptomatic venous thromboembolism. N Engl J Med. 2010;363(26):2499-2510. PubMed
19. EINSTEIN-PE Investigators, Büller HR, Prins MH, et al. Oral rivaroxaban for the treatment of symptomatic pulmonary embolism. N Engl J Med. 2012;366(14):1287-1297. PubMed
20. Simon J, Hawes E, Deyo Z, Bryant-Shilliday B. Evaluation of prescribing and patient use of target-specific oral anticoagulants in the outpatient setting. J Clin Pharm Ther. 2015;40(5):525-530. PubMed
21. Granger CB, Alexander JH, McMurray JJ, et al; ARISTOTLE Committees and Investigators. Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2011;365(11):981-992. PubMed
22. Ruff CT, Giugliano RP, Braunwald E, et al. Comparison of the efficacy and safety of new oral anticoagulants with warfarin in patients with atrial fibrillation: a meta-analysis of randomised trials. Lancet. 2014;383(9921):955-962. PubMed
23. van der Hulle T, Kooiman J, den Exter PL, Dekkers OM, Klok FA, Huisman MV. Effectiveness and safety of novel oral anticoagulants as compared with vitamin K antagonists in the treatment of acute symptomatic venous thromboembolism: a systematic review and meta-analysis. J Thromb Haemost. 2014;12(3):320-328. PubMed
24. Schuh T, Reichardt B, Finsterer J, Stöllberger C. Age-dependency of prescribing patterns of oral anticoagulant drugs in Austria during 2011–2014. J Thromb Thrombolysis. 2016;42(3):447-451. PubMed
25. Stöllberger C, Brooks R, Finsterer J, Pachofszky T. Use of direct-acting oral anticoagulants in nonagenarians: a call for more data. Drugs Aging. 2016;33(5):315-320. PubMed
26. Steffel J, Giugliano RP, Braunwald E, et al. Edoxaban versus warfarin in atrial fibrillation patients at risk of falling: ENGAGE AF-TIMI 48 analysis. J Am Coll Cardiol. 2016;68(11):1169-1178. PubMed
27. Ruff CT, Giugliano RP, Antman EM. Management of bleeding with non–vitamin K antagonist oral anticoagulants in the era of specific reversal agents. Circulation. 2016;134(3):248-261. PubMed
28. Schwartz JB. Potential impact of substituting estimated glomerular filtration rate for estimated creatinine clearance for dosing of direct oral anticoagulants. J Am Geriatr Soc. 2016;64(10):1996-2002. PubMed
29. Camm AJ, Amarenco P, Haas S, et al; XANTUS Investigators. XANTUS: a real-world, prospective, observational study of patients treated with rivaroxaban for stroke prevention in atrial fibrillation. Eur Heart J. 2016;37(14):1145-1153. PubMed
30. Steinberg BA, Shrader P, Thomas L, et al; ORBIT-AF Investigators and Patients. Off-label dosing of non–vitamin K antagonist oral anticoagulants and adverse outcomes: the ORBIT-AF II Registry. J Am Coll Cardiol. 2016;68(24):2597-2604. PubMed
31. Fauchier L, Philippart R, Clementy N, et al. How to define valvular atrial fibrillation? Arch Cardiovasc Dis. 2015;108(10):530-539. PubMed
32. Di Biase L. Use of direct oral anticoagulants in patients with atrial fibrillation and valvular heart lesions. J Am Heart Assoc. 2016;5(2). PubMed
33. Kearon C, Akl EA, Ornelas J, et al. Antithrombotic therapy for VTE disease:
CHEST guideline and expert panel report. Chest. 2016;149(2):315-352. PubMed
34. Larock AS, Mullier F, Sennesael AL, et al. Appropriateness of prescribing dabigatran
etexilate and rivaroxaban in patients with nonvalvular atrial fibrillation: a prospective
study. Ann Pharmacother. 2014;48(10):1258-1268. PubMed
Direct-acting oral anticoagulants (DOACs) have been introduced into clinical use for stroke prevention in patients with nonvalvular atrial fibrillation (NVAF), prevention of venous thrombosis after hip or knee surgery, and treatment of deep vein thrombosis (DVT) and pulmonary embolism (PE).1-7 Advantages of DOACs over warfarin are often stated as fixed dosing, minor drug and food interactions, wider therapeutic index, and no need for laboratory test monitoring.1,8 Yet, recommended DOAC dosages vary by renal function and therapeutic indications. Dosing recommendations for prevention of stroke in patients with NVAF are based on estimated creatinine clearance (dabigatran, rivaroxaban, edoxaban), age (apixaban), weight (apixaban, edoxaban), serum creatinine level (apixaban, edoxaban), and presence of cirrhosis by Child-Pugh class9,10 (apixaban, edoxaban).4-6,11,12 Dosing recommendations based on coadministration of strong CYP34A and P-glycoprotein inhibitors or inducers vary by DOAC. In addition, dabigatran cannot be crushed and must be stored in its original packaging, and rivaroxaban should be taken with food when the dose is over 10 mg.
We studied DOAC prescribing in adults admitted to a large academic medical center by comparing initial prescribed dosing with FDA-approved prescribing information. We hypothesized that the complexity of DOAC dosing may not be recognized by prescribers.
METHODS
Our study protocol was approved by the Committee on Human Research (Institutional Review Board) of the University of California San Francisco.
Data Collection
We used electronic medical records (EMRs) to identify adult inpatients who were prescribed a DOAC (apixaban, dabigatran, edoxaban, or rivaroxaban) at the University of California San Francisco Medical Center, a large academic hospital, between July 1, 2014 and June 30, 2015. Demographic and medical information related to therapeutic indications, contraindications, and indications for dose adjustments were collected and included diagnoses classified by International Classification of Diseases, Ninth Revision (ICD-9) and Tenth Revision (ICD-10) for venous thromboses; phlebitis or thrombophlebitis; PE or venous embolism; atrial arrhythmias; surgical procedures; cirrhosis and/or ascites or liver disease; coagulopathies; artificial heart valves or implanted devices; prior use of medications including parenteral anticoagulants; and laboratory data obtained before the first DOAC order (serum creatinine level, estimated glomerular filtration rate [eGFR] determined by Chronic Kidney Disease Epidemiology Collaboration,13 international normalized ratio, or, if available, activated partial thromboplastin time and bilirubin level). Creatinine clearance was calculated with the Cockcroft-Gault method14 using total body weight, per drug label recommendation. Child-Pugh class was calculated if cirrhosis was diagnosed.10 DOAC dose, frequency, dosing directions, and prescriber medical specialty were determined.
Accuracy of search results was confirmed by review of the first 200 patients’ records. Records were manually reviewed for encounters lacking ICD-9/10 codes and approved DOAC indications (30%) and encounters having multiple coded diagnostic indications (to identify the indication). ICD-9 codes for venous thrombosis were reviewed to differentiate acute from chronic events.
Data Analysis
The main outcome was concordance or discordance between the first DOAC prescribing order and the FDA-approved prescribing information at the time. Initial classification, performed by 2 independent reviewers (a pharmacist and a physician, or 2 pharmacists), was followed by adjudication and individual record review (by 2 independent reviewers) of all initial prescribing orders classified as discordant. A third reviewer adjudicated any disagreement. Records and notes were reviewed to identify stated or potential reasons for dosing variation and pre-admission prescriptions. Data are presented as means and standard deviations (SDs) and as raw numbers and percentages. Differences in patient characteristics by DOAC or therapeutic indication were determined by analysis of variance (ANOVA) with Bonferroni correction for post hoc comparisons. Dosing information was categorized as the same as recommended, lower than recommended, higher than recommended, or avoid drug use (drug–drug or drug–disease interaction), per FDA-approved prescribing information, and χ2 tests were used to determine whether variation in dosing occurred by individual DOAC, therapeutic indication, or prescriber specialty. Relationships between dosing variation and age or renal function were tested by ANOVA with Bonferroni correction for post hoc comparisons.
RESULTS
Patients with AF were older with lower creatinine clearance compared to patients with other diagnoses. Mean (SD) patient age was 72.1 (12.7) years for AF, 53.1 (10.9) years for chronic PE, 55.5 (14) years for acute PE, 56.4 (15.9) years for chronic DVT, 57.9 (18.4) years for acute DVT, and 61.4 (11.6) years for DVT prevention after hip or knee surgery (P < 0.0001 for all comparisons). Mean (SD) estimated creatinine clearance was 76.8 (43.5) mL/min for AF, 92.4 (44.4) mL/min for DVT prevention after hip or knee surgery, 111 (53) mL/min for chronic DVT, 118 (55) mL/min for acute DVT, 126 (60) mL/min for chronic PE, and 127 (54) mL/min for acute PE (P < 0.0001 for all comparisons). Differences between patient groups by therapeutic indication were not detected for weight, body mass index, or serum creatinine level.
The most frequent deviation from prescribing recommendations was omission of directions to administer rivaroxaban with food—93% (248/268) of orders—but not for DVT prevention after hip or knee surgery, for which the 10-mg dose is appropriately administered without food. Doses were the same as recommended for 82% of apixaban orders, 84% of rivaroxaban orders, and 93% of initial dabigatran orders (P < 0.05 for differences; Table 3). Dosages not concordant with FDA recommendations were prescribed in 44 (18.1%) of 243 apixaban orders, 41 (14.3%) of 286 rivaroxaban orders, and 7 (7.2%) of 89 initial dabigatran orders. Lower than recommended doses were more common than higher than recommended doses (Table 3, Figure 1): 15.2% versus 2.1% of apixaban orders, 9.4% versus 3.5% of rivaroxaban orders, and 4.2% versus 1.0% of initial dabigatran orders (P < 0.05). Failure to avoid drug use (for potential drug–drug or drug–disease interactions) was uncommon (1%-2%). There were more deviations from recommended doses for patients with AF or DVT prevention after hip or knee surgery than for patients with acute or chronic PE or acute DVT (Table 3). No significant differences were detected between prescribed and recommended doses by prescriber specialty.
For apixaban, patients who were prescribed lower than recommended doses were older than those prescribed recommended doses: mean (SD), 78.1 (12.2) years versus 71 (13.6) years (P = 0.003). Seventy-six percent of those prescribed lower than recommended doses were older than 75. Prescriptions for apixaban at lower than recommended doses were continuations of prior outpatient prescriptions in 20 of 37 cases (almost half), and in 12 cases (one-fourth) antiplatelet drugs were coprescribed (aspirin in 10 cases, clopidogrel in 1, prasugrel in 1). For rivaroxaban, older age was associated with both lower than recommended dosing (P = 0.003) and higher than recommended dosing (P < 0.001). Variations from prescribing recommendations were continuations of outpatient rivaroxaban doses in about two-thirds (26 of 41; 63.4 %) with 13 receiving antiplatelet drugs. For dabigatran, 6 of 7 orders not in agreement with recommendations were continuations of outpatient dosing.
The specific equation used to estimate renal function also had the potential to lead to dosing errors. Among the 41 rivaroxaban patients categorized as receiving doses discordant with recommendations, 8 would have had an inappropriate DOAC dose if eGFR were used instead of eCrCL as recommended. No relationships were detected for other patient variables/measures and dosing deviations from recommendations.
DISCUSSION
We examined initial hospital orders for DOACs in adults admitted to a single academic medical center during 2014-2015. Dabigatran, apixaban and rivaroxaban were prescribed for prevention of stroke in patients with atrial fibrillation/flutter (AF) in three quarters of the encounters similar to national patterns. (15) Prescribing departures from FDA-approved recommendations ranged from failure to prescribe rivaroxaban with food to failure to recognize drug-drug interactions in 1% to 2%. Unexpectedly, lower than recommended dosing was more common than higher than recommended dosing of the three DOACs.
Rivaroxaban bioavailability is dose dependent with the presence of food required to enhance absorption for doses over 10 mg that are used for prevention of stroke in patients with non-valvular AF or treatment of DVT or PE.5,16 Peak rivaroxaban concentrations are 75% higher and the total area under the concentration vs. time curve after dosing is 40% higher when rivaroxaban is administered with high fat high calorie meals compared to the fasting state.16 If rivaroxaban is not administered with food, drug concentrations and pharmacologic effects may be less than in clinical trials that specified co-administration with food.17-19 A small survey of outpatients receiving rivaroxaban found that 23% reported taking it without food.20 With electronic pharmacy systems in almost all hospitals and electronic prescriber order entry in most, automated addition of directions for rivaroxaban administration with food for doses over 10 mg to labels or dispensing instructions could easily correct this deviation from recommended practice.
Lower than recommended doses were prescribed in 9.4% of orders for rivaroxaban and 15.2% of orders for apixaban, with dose-deviations often appearing to be a continuation of outpatient doses. Patients 75 years or older were more likely to receive lower than recommended dosing of apixaban. Reductions in apixaban doses from 5 mg twice daily to 2.5 mg twice daily are recommended in patients with non-valvular AF with two of the following criteria: age ≥80 y, weight ≤60 kg, serum creatinine ≥1.5 mg/dL or co-administration of a strong PgP inhibitor to a patient without 2 of the 3 dose reduction criteria. Our study was not designed to determine reasons for under-dosing, but we speculate that clinicians may have considered patients aged 75-79 years to be similar to those 80 years of age or older, or, older and not as healthy as those enrolled in randomized trials.21-25 The median age of our patients with AF receiving apixaban was 75y (interquartile range of 16) vs 70y ( interquartile range 63-76) in the pivotal trial comparing warfarin to apixaban.21 Renal function was also lower with 37% having eCrCL below 50 mL/min compared to 17% in ARISTOTLE. (21). Twenty-six percent of our apixaban-treated AF patients qualified for the lower 2.5 mg twice daily compared to only 5% of ARISTOTLE participants,21 further suggesting differences between patients in our sample compared to randomized trial participants.
Concerns regarding bleeding or falls in older patients, may also have contributed to lower than recommended doses. Recent analyses of patients at risk for falls confirmed that increased risk of falling was associated with more bone fractures, bleeding and all-cause death but not stroke or systemic emboli, and with less severe bleeding with the DOAC edoxaban compared to warfarin.26 While a rationale for personalized or lower than recommended dosing of apixaban may exist in very old patients and those at risk of falls and bleeding, more data are needed to determine outcomes of lower than recommended doses of DOACs before such an approach can be endorsed. Monitoring of anticoagulant effect in patients who receive doses lower than those investigated in clinical trials could provide important information. The assays that measure DOAC effects are likely to be more available because of the use of reversal agents in the setting of bleeding with DOACs.27
We had anticipated higher than recommended dosing for rivaroxaban as recommendations are based on creatinine clearance while laboratories routinely report estimated glomerular filtration rate (eGFR) that can provide higher estimates of renal clearance and estimated DOAC doses in older and smaller individuals.28 Higher than recommended dosing was found in only 3.5% of our sample. In half, eGFR estimates were higher than creatinine clearance estimates. An international postmarketing registry of rivaroxaban use for the prevention of stroke in patients with NVAF, which included outpatients, found that 36% of those with creatinine clearances below 50 mL/min received a dose higher than recommended, and 15% received a dose lower than expected.29 A more recent outpatient registry report on patients with NVAF, in which apixaban, dabigatran, or rivaroxaban was administered, found that overall 9.4% received a dose lower than recommended, and 3.4% were overdosed, with a similar percentage (34%) of rivaroxaban patients with creatinine clearance of 15 to 50 mL/min receiving higher than recommended dosing.30 The lower rate of higher-than-recommended doses that we observed may have been related to the routine measurement of serum creatinine and attention to dosing adjustments for renal function in the inpatient setting compared to the outpatient setting. In addition, renal function data may not be available to outpatient pharmacies, limiting potential input on dosing recommendations. At least one cardiac society recommends monitoring of renal function in patients treated with DOACs, annually in patients with normal estimated creatinine clearance and more frequently (at intervals in months equal to the creatinine clearance divided by 10) in patients with abnormal creatinine clearance.11 A hospital encounter provides an opportunity to assess or reassess renal status to optimize DOAC dosing.
Dabigatran was the first DOAC introduced into use in the United States with the same dose recommended for prevention of stroke in patients with AF or venous thromboembolic disease with reductions for creatinine clearance below 30 mL/min or creatinine clearance between 30 and 50 mL/min and concomitant use of the potent P-glycoprotein inhibitor dronedarone or systemic ketoconazole. The relative simplicity of dosing may have been responsible for the lowest rate of prescribing outside of recommendations observed in this study, but the low dabigatran use limits analyses of contributing factors.
Failure to avoid drug use in combination with use of strong P-glycoprotein inducers or inhibitors was infrequent but should be preventable. Current prescribing recommendations refer to “strong” P-glycoprotein inhibitors and list different specific agents that interact with each DOAC without a standardized definition or classification. Standardized classifications or reference sources would be helpful.
Our primary goal in this study was to compare initial prescribed dosing of DOACs with FDA-approved prescribing directions. However, therapeutic indication data warrant discussion. In our sample, 7.5% of patients with AF had bioprosthetic valves or recent mitral valve repair or replacement. Using the NVAF definition found in the 2014 AHA/ACC/HRS (American Heart Association, American College of Cardiology, Heart Rhythm Society) AF guidelines1—“absence of rheumatic mitral valve disease, a prosthetic heart valve, or mitral valve repair”—these patients would not appear to be candidates for DOACs. However, arguments have been made that a bioprosthetic heart valve or native valve after valve repair does not have a risk profile for thromboembolism that differs from other forms of NVAF and would be equally responsive to DOAC therapy.31 Data are sparse, but retrospective subanalyses of limited numbers of patients with valvular disease (including bioprosthesis and mitral repair patients but excluding mechanical valve patients) enrolled in the pivotal DOAC studies support this conclusion.32 For the first months after biological valve replacement (including catheter-based valve replacement), recent European guidelines recommend vitamin K antagonists but also state, “NOACs probably deliver the same protection.”8 DOACs were also used for management of venous thromboembolic disease (both acute and chronic) in patients with active cancer. Our data predate the most recent American College of Chest Physician guidelines on treatment of venous thromboembolism in patients with cancer, which provide grade 2B recommendations for use of low-molecular-weight heparin (LMWH) over vitamin K antagonists and grade 2C recommendations for use of LMWH over dabigatran, rivaroxaban, apixaban, or edoxaban.33
Our study had several limitations. First, data were from a single US academic medical center, though similar rates of prescribing deviation from recommendations have been reported for rivaroxaban and dabigatran in NVAF patients in other countries.29,34 Second, therapeutic indications may have been misclassified because of errors, incomplete EMR data, or multiple indications. Third, we analyzed the first DOAC order and not dispensing information or subsequent corrections. Therefore, deviations from recommendations should not be interpreted as errors that reached patients. We evaluated dosing based on the measures used at the time of hospital admission, noting that, in a significant fraction of deviations from recommended doses, they represented continuations of outpatient doses when renal function or weight may have differed, and it is unknown whether patients were counseled to take rivaroxaban with food in the outpatient setting. Fourth, the number of patients with acute DVT was small, so firm conclusions cannot be drawn for this specific population. Fifth, our estimates of off-label dosing may have been underestimates, as data on cancer and cancer activity or cardiac valvular disease may not have been complete.
CONCLUSION
Healthcare professionals are prescribing DOACs in ways that differ from recommendations. These differences may reflect the older ages and reduced renal function of clinical populations relative to randomized clinical trial groups, but they could also potentially alter clinical efficacy. Our findings support the need to evaluate the appropriateness and dosing of DOACs at each encounter and to determine the outcomes of patients treated with lower than recommended doses of DOACs and the outcomes of DOAC-treated patients with bioprostheses or active malignancies.
Acknowledgment
The authors thank Tobias Schmelzinger for electronic data extraction and compilation and University of California San Francisco students Eduardo De La Torre Cruz (School of Pharmacy) and Carlos Mikell (School of Medicine) for assistance with data review.
Disclosure
Dr. Schwartz reports receiving personal fees from Bristol-Myers Squibb and Amgen and grants from Bristol-Myers Squibb and Pfizer, outside the submitted work. The other authors have nothing to report.
Direct-acting oral anticoagulants (DOACs) have been introduced into clinical use for stroke prevention in patients with nonvalvular atrial fibrillation (NVAF), prevention of venous thrombosis after hip or knee surgery, and treatment of deep vein thrombosis (DVT) and pulmonary embolism (PE).1-7 Advantages of DOACs over warfarin are often stated as fixed dosing, minor drug and food interactions, wider therapeutic index, and no need for laboratory test monitoring.1,8 Yet, recommended DOAC dosages vary by renal function and therapeutic indications. Dosing recommendations for prevention of stroke in patients with NVAF are based on estimated creatinine clearance (dabigatran, rivaroxaban, edoxaban), age (apixaban), weight (apixaban, edoxaban), serum creatinine level (apixaban, edoxaban), and presence of cirrhosis by Child-Pugh class9,10 (apixaban, edoxaban).4-6,11,12 Dosing recommendations based on coadministration of strong CYP34A and P-glycoprotein inhibitors or inducers vary by DOAC. In addition, dabigatran cannot be crushed and must be stored in its original packaging, and rivaroxaban should be taken with food when the dose is over 10 mg.
We studied DOAC prescribing in adults admitted to a large academic medical center by comparing initial prescribed dosing with FDA-approved prescribing information. We hypothesized that the complexity of DOAC dosing may not be recognized by prescribers.
METHODS
Our study protocol was approved by the Committee on Human Research (Institutional Review Board) of the University of California San Francisco.
Data Collection
We used electronic medical records (EMRs) to identify adult inpatients who were prescribed a DOAC (apixaban, dabigatran, edoxaban, or rivaroxaban) at the University of California San Francisco Medical Center, a large academic hospital, between July 1, 2014 and June 30, 2015. Demographic and medical information related to therapeutic indications, contraindications, and indications for dose adjustments were collected and included diagnoses classified by International Classification of Diseases, Ninth Revision (ICD-9) and Tenth Revision (ICD-10) for venous thromboses; phlebitis or thrombophlebitis; PE or venous embolism; atrial arrhythmias; surgical procedures; cirrhosis and/or ascites or liver disease; coagulopathies; artificial heart valves or implanted devices; prior use of medications including parenteral anticoagulants; and laboratory data obtained before the first DOAC order (serum creatinine level, estimated glomerular filtration rate [eGFR] determined by Chronic Kidney Disease Epidemiology Collaboration,13 international normalized ratio, or, if available, activated partial thromboplastin time and bilirubin level). Creatinine clearance was calculated with the Cockcroft-Gault method14 using total body weight, per drug label recommendation. Child-Pugh class was calculated if cirrhosis was diagnosed.10 DOAC dose, frequency, dosing directions, and prescriber medical specialty were determined.
Accuracy of search results was confirmed by review of the first 200 patients’ records. Records were manually reviewed for encounters lacking ICD-9/10 codes and approved DOAC indications (30%) and encounters having multiple coded diagnostic indications (to identify the indication). ICD-9 codes for venous thrombosis were reviewed to differentiate acute from chronic events.
Data Analysis
The main outcome was concordance or discordance between the first DOAC prescribing order and the FDA-approved prescribing information at the time. Initial classification, performed by 2 independent reviewers (a pharmacist and a physician, or 2 pharmacists), was followed by adjudication and individual record review (by 2 independent reviewers) of all initial prescribing orders classified as discordant. A third reviewer adjudicated any disagreement. Records and notes were reviewed to identify stated or potential reasons for dosing variation and pre-admission prescriptions. Data are presented as means and standard deviations (SDs) and as raw numbers and percentages. Differences in patient characteristics by DOAC or therapeutic indication were determined by analysis of variance (ANOVA) with Bonferroni correction for post hoc comparisons. Dosing information was categorized as the same as recommended, lower than recommended, higher than recommended, or avoid drug use (drug–drug or drug–disease interaction), per FDA-approved prescribing information, and χ2 tests were used to determine whether variation in dosing occurred by individual DOAC, therapeutic indication, or prescriber specialty. Relationships between dosing variation and age or renal function were tested by ANOVA with Bonferroni correction for post hoc comparisons.
RESULTS
Patients with AF were older with lower creatinine clearance compared to patients with other diagnoses. Mean (SD) patient age was 72.1 (12.7) years for AF, 53.1 (10.9) years for chronic PE, 55.5 (14) years for acute PE, 56.4 (15.9) years for chronic DVT, 57.9 (18.4) years for acute DVT, and 61.4 (11.6) years for DVT prevention after hip or knee surgery (P < 0.0001 for all comparisons). Mean (SD) estimated creatinine clearance was 76.8 (43.5) mL/min for AF, 92.4 (44.4) mL/min for DVT prevention after hip or knee surgery, 111 (53) mL/min for chronic DVT, 118 (55) mL/min for acute DVT, 126 (60) mL/min for chronic PE, and 127 (54) mL/min for acute PE (P < 0.0001 for all comparisons). Differences between patient groups by therapeutic indication were not detected for weight, body mass index, or serum creatinine level.
The most frequent deviation from prescribing recommendations was omission of directions to administer rivaroxaban with food—93% (248/268) of orders—but not for DVT prevention after hip or knee surgery, for which the 10-mg dose is appropriately administered without food. Doses were the same as recommended for 82% of apixaban orders, 84% of rivaroxaban orders, and 93% of initial dabigatran orders (P < 0.05 for differences; Table 3). Dosages not concordant with FDA recommendations were prescribed in 44 (18.1%) of 243 apixaban orders, 41 (14.3%) of 286 rivaroxaban orders, and 7 (7.2%) of 89 initial dabigatran orders. Lower than recommended doses were more common than higher than recommended doses (Table 3, Figure 1): 15.2% versus 2.1% of apixaban orders, 9.4% versus 3.5% of rivaroxaban orders, and 4.2% versus 1.0% of initial dabigatran orders (P < 0.05). Failure to avoid drug use (for potential drug–drug or drug–disease interactions) was uncommon (1%-2%). There were more deviations from recommended doses for patients with AF or DVT prevention after hip or knee surgery than for patients with acute or chronic PE or acute DVT (Table 3). No significant differences were detected between prescribed and recommended doses by prescriber specialty.
For apixaban, patients who were prescribed lower than recommended doses were older than those prescribed recommended doses: mean (SD), 78.1 (12.2) years versus 71 (13.6) years (P = 0.003). Seventy-six percent of those prescribed lower than recommended doses were older than 75. Prescriptions for apixaban at lower than recommended doses were continuations of prior outpatient prescriptions in 20 of 37 cases (almost half), and in 12 cases (one-fourth) antiplatelet drugs were coprescribed (aspirin in 10 cases, clopidogrel in 1, prasugrel in 1). For rivaroxaban, older age was associated with both lower than recommended dosing (P = 0.003) and higher than recommended dosing (P < 0.001). Variations from prescribing recommendations were continuations of outpatient rivaroxaban doses in about two-thirds (26 of 41; 63.4 %) with 13 receiving antiplatelet drugs. For dabigatran, 6 of 7 orders not in agreement with recommendations were continuations of outpatient dosing.
The specific equation used to estimate renal function also had the potential to lead to dosing errors. Among the 41 rivaroxaban patients categorized as receiving doses discordant with recommendations, 8 would have had an inappropriate DOAC dose if eGFR were used instead of eCrCL as recommended. No relationships were detected for other patient variables/measures and dosing deviations from recommendations.
DISCUSSION
We examined initial hospital orders for DOACs in adults admitted to a single academic medical center during 2014-2015. Dabigatran, apixaban and rivaroxaban were prescribed for prevention of stroke in patients with atrial fibrillation/flutter (AF) in three quarters of the encounters similar to national patterns. (15) Prescribing departures from FDA-approved recommendations ranged from failure to prescribe rivaroxaban with food to failure to recognize drug-drug interactions in 1% to 2%. Unexpectedly, lower than recommended dosing was more common than higher than recommended dosing of the three DOACs.
Rivaroxaban bioavailability is dose dependent with the presence of food required to enhance absorption for doses over 10 mg that are used for prevention of stroke in patients with non-valvular AF or treatment of DVT or PE.5,16 Peak rivaroxaban concentrations are 75% higher and the total area under the concentration vs. time curve after dosing is 40% higher when rivaroxaban is administered with high fat high calorie meals compared to the fasting state.16 If rivaroxaban is not administered with food, drug concentrations and pharmacologic effects may be less than in clinical trials that specified co-administration with food.17-19 A small survey of outpatients receiving rivaroxaban found that 23% reported taking it without food.20 With electronic pharmacy systems in almost all hospitals and electronic prescriber order entry in most, automated addition of directions for rivaroxaban administration with food for doses over 10 mg to labels or dispensing instructions could easily correct this deviation from recommended practice.
Lower than recommended doses were prescribed in 9.4% of orders for rivaroxaban and 15.2% of orders for apixaban, with dose-deviations often appearing to be a continuation of outpatient doses. Patients 75 years or older were more likely to receive lower than recommended dosing of apixaban. Reductions in apixaban doses from 5 mg twice daily to 2.5 mg twice daily are recommended in patients with non-valvular AF with two of the following criteria: age ≥80 y, weight ≤60 kg, serum creatinine ≥1.5 mg/dL or co-administration of a strong PgP inhibitor to a patient without 2 of the 3 dose reduction criteria. Our study was not designed to determine reasons for under-dosing, but we speculate that clinicians may have considered patients aged 75-79 years to be similar to those 80 years of age or older, or, older and not as healthy as those enrolled in randomized trials.21-25 The median age of our patients with AF receiving apixaban was 75y (interquartile range of 16) vs 70y ( interquartile range 63-76) in the pivotal trial comparing warfarin to apixaban.21 Renal function was also lower with 37% having eCrCL below 50 mL/min compared to 17% in ARISTOTLE. (21). Twenty-six percent of our apixaban-treated AF patients qualified for the lower 2.5 mg twice daily compared to only 5% of ARISTOTLE participants,21 further suggesting differences between patients in our sample compared to randomized trial participants.
Concerns regarding bleeding or falls in older patients, may also have contributed to lower than recommended doses. Recent analyses of patients at risk for falls confirmed that increased risk of falling was associated with more bone fractures, bleeding and all-cause death but not stroke or systemic emboli, and with less severe bleeding with the DOAC edoxaban compared to warfarin.26 While a rationale for personalized or lower than recommended dosing of apixaban may exist in very old patients and those at risk of falls and bleeding, more data are needed to determine outcomes of lower than recommended doses of DOACs before such an approach can be endorsed. Monitoring of anticoagulant effect in patients who receive doses lower than those investigated in clinical trials could provide important information. The assays that measure DOAC effects are likely to be more available because of the use of reversal agents in the setting of bleeding with DOACs.27
We had anticipated higher than recommended dosing for rivaroxaban as recommendations are based on creatinine clearance while laboratories routinely report estimated glomerular filtration rate (eGFR) that can provide higher estimates of renal clearance and estimated DOAC doses in older and smaller individuals.28 Higher than recommended dosing was found in only 3.5% of our sample. In half, eGFR estimates were higher than creatinine clearance estimates. An international postmarketing registry of rivaroxaban use for the prevention of stroke in patients with NVAF, which included outpatients, found that 36% of those with creatinine clearances below 50 mL/min received a dose higher than recommended, and 15% received a dose lower than expected.29 A more recent outpatient registry report on patients with NVAF, in which apixaban, dabigatran, or rivaroxaban was administered, found that overall 9.4% received a dose lower than recommended, and 3.4% were overdosed, with a similar percentage (34%) of rivaroxaban patients with creatinine clearance of 15 to 50 mL/min receiving higher than recommended dosing.30 The lower rate of higher-than-recommended doses that we observed may have been related to the routine measurement of serum creatinine and attention to dosing adjustments for renal function in the inpatient setting compared to the outpatient setting. In addition, renal function data may not be available to outpatient pharmacies, limiting potential input on dosing recommendations. At least one cardiac society recommends monitoring of renal function in patients treated with DOACs, annually in patients with normal estimated creatinine clearance and more frequently (at intervals in months equal to the creatinine clearance divided by 10) in patients with abnormal creatinine clearance.11 A hospital encounter provides an opportunity to assess or reassess renal status to optimize DOAC dosing.
Dabigatran was the first DOAC introduced into use in the United States with the same dose recommended for prevention of stroke in patients with AF or venous thromboembolic disease with reductions for creatinine clearance below 30 mL/min or creatinine clearance between 30 and 50 mL/min and concomitant use of the potent P-glycoprotein inhibitor dronedarone or systemic ketoconazole. The relative simplicity of dosing may have been responsible for the lowest rate of prescribing outside of recommendations observed in this study, but the low dabigatran use limits analyses of contributing factors.
Failure to avoid drug use in combination with use of strong P-glycoprotein inducers or inhibitors was infrequent but should be preventable. Current prescribing recommendations refer to “strong” P-glycoprotein inhibitors and list different specific agents that interact with each DOAC without a standardized definition or classification. Standardized classifications or reference sources would be helpful.
Our primary goal in this study was to compare initial prescribed dosing of DOACs with FDA-approved prescribing directions. However, therapeutic indication data warrant discussion. In our sample, 7.5% of patients with AF had bioprosthetic valves or recent mitral valve repair or replacement. Using the NVAF definition found in the 2014 AHA/ACC/HRS (American Heart Association, American College of Cardiology, Heart Rhythm Society) AF guidelines1—“absence of rheumatic mitral valve disease, a prosthetic heart valve, or mitral valve repair”—these patients would not appear to be candidates for DOACs. However, arguments have been made that a bioprosthetic heart valve or native valve after valve repair does not have a risk profile for thromboembolism that differs from other forms of NVAF and would be equally responsive to DOAC therapy.31 Data are sparse, but retrospective subanalyses of limited numbers of patients with valvular disease (including bioprosthesis and mitral repair patients but excluding mechanical valve patients) enrolled in the pivotal DOAC studies support this conclusion.32 For the first months after biological valve replacement (including catheter-based valve replacement), recent European guidelines recommend vitamin K antagonists but also state, “NOACs probably deliver the same protection.”8 DOACs were also used for management of venous thromboembolic disease (both acute and chronic) in patients with active cancer. Our data predate the most recent American College of Chest Physician guidelines on treatment of venous thromboembolism in patients with cancer, which provide grade 2B recommendations for use of low-molecular-weight heparin (LMWH) over vitamin K antagonists and grade 2C recommendations for use of LMWH over dabigatran, rivaroxaban, apixaban, or edoxaban.33
Our study had several limitations. First, data were from a single US academic medical center, though similar rates of prescribing deviation from recommendations have been reported for rivaroxaban and dabigatran in NVAF patients in other countries.29,34 Second, therapeutic indications may have been misclassified because of errors, incomplete EMR data, or multiple indications. Third, we analyzed the first DOAC order and not dispensing information or subsequent corrections. Therefore, deviations from recommendations should not be interpreted as errors that reached patients. We evaluated dosing based on the measures used at the time of hospital admission, noting that, in a significant fraction of deviations from recommended doses, they represented continuations of outpatient doses when renal function or weight may have differed, and it is unknown whether patients were counseled to take rivaroxaban with food in the outpatient setting. Fourth, the number of patients with acute DVT was small, so firm conclusions cannot be drawn for this specific population. Fifth, our estimates of off-label dosing may have been underestimates, as data on cancer and cancer activity or cardiac valvular disease may not have been complete.
CONCLUSION
Healthcare professionals are prescribing DOACs in ways that differ from recommendations. These differences may reflect the older ages and reduced renal function of clinical populations relative to randomized clinical trial groups, but they could also potentially alter clinical efficacy. Our findings support the need to evaluate the appropriateness and dosing of DOACs at each encounter and to determine the outcomes of patients treated with lower than recommended doses of DOACs and the outcomes of DOAC-treated patients with bioprostheses or active malignancies.
Acknowledgment
The authors thank Tobias Schmelzinger for electronic data extraction and compilation and University of California San Francisco students Eduardo De La Torre Cruz (School of Pharmacy) and Carlos Mikell (School of Medicine) for assistance with data review.
Disclosure
Dr. Schwartz reports receiving personal fees from Bristol-Myers Squibb and Amgen and grants from Bristol-Myers Squibb and Pfizer, outside the submitted work. The other authors have nothing to report.
1. January CT, Wann LS, Alpert JS, et al. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: executive summary. A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the Heart Rhythm Society. J Am Coll Cardiol. 2014;64(21):2246-2280. PubMed
2. Saraf K, Morris PD, Garg P, Sheridan P, Storey R. Non–vitamin K antagonist oral anticoagulants (NOACs): clinical evidence and therapeutic considerations. Postgrad Med J. 2014;90(1067):520-528. PubMed
3. Yeh CH, Gross PL, Weitz JI. Evolving use of new oral anticoagulants for treatment of venous thromboembolism. Blood. 2014;124(7):1020-1028. PubMed
4. Pradaxa website. https://www.pradaxa.com. Accessed June 1, 2017.
5. Xarelto website. https://www.xarelto-us.com. Accessed June 1, 2017.
6. Eliquis website. http://www.eliquis.com. Accessed June 1, 2017.
7. Savaysa [prescribing information]. Tokyo, Japan: Daiichi Sankyo; 2015.
8. Kirchhof P, Benussi S, Kotecha D, et al. 2016 ESC guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Eur Heart J. 2016;37(38):2893-2962. PubMed
9. Child C, Turcotte J. Surgery and portal hypertension. In: Child CG, ed. The Liver and Portal Hypertension. Philadelphia, PA: Saunders; 1964:50-64. PubMed
10. Pugh RN, Murray-Lyon IM, Dawson JL, Pietroni MC, Williams R. Transection of the oesophagus for bleeding oesophageal varices. Br J Surg. 1973;60(8):646-649. PubMed
11. Heidbuchel H, Verhamme P, Alings M, et al. Updated European Heart Rhythm Association practical guide on the use of non–vitamin K antagonist anticoagulants in patients with non-valvular atrial fibrillation. Europace. 2015;17(10):1467-1507. PubMed
12. Savaysa website. https://savaysahcp.com. Accessed June 1, 2017.
13. Levey AS, Stevens LA, Schmid CH, et al; CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration). A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150(9):604-612. PubMed
14. Cockcroft DW, Gault MH. Prediction of creatinine clearance from serum creatinine. Nephron. 1976;16(1):31-41. PubMed
15. Rose AJ, Reisman JI, Allen AL, Miller DR. Potentially inappropriate prescribing of direct-acting oral anticoagulants in the Veterans Health Administration. Am J Pharm Benefits. 2016;4(4):e75-e80.
16. Stampfuss J, Kubitza D, Becka M, Mueck W. The effect of food on the absorption and pharmacokinetics of rivaroxaban. Int J Clin Pharmacol Ther. 2013;51(7):549-561. PubMed
17. Patel MR, Mahaffey KW, Garg J, et al; ROCKET AF Investigators. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med. 2011;365(10):883-891. PubMed
18. EINSTEIN Investigators, Bauersachs R, Berkowitz SD, et al. Oral rivaroxaban for symptomatic venous thromboembolism. N Engl J Med. 2010;363(26):2499-2510. PubMed
19. EINSTEIN-PE Investigators, Büller HR, Prins MH, et al. Oral rivaroxaban for the treatment of symptomatic pulmonary embolism. N Engl J Med. 2012;366(14):1287-1297. PubMed
20. Simon J, Hawes E, Deyo Z, Bryant-Shilliday B. Evaluation of prescribing and patient use of target-specific oral anticoagulants in the outpatient setting. J Clin Pharm Ther. 2015;40(5):525-530. PubMed
21. Granger CB, Alexander JH, McMurray JJ, et al; ARISTOTLE Committees and Investigators. Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2011;365(11):981-992. PubMed
22. Ruff CT, Giugliano RP, Braunwald E, et al. Comparison of the efficacy and safety of new oral anticoagulants with warfarin in patients with atrial fibrillation: a meta-analysis of randomised trials. Lancet. 2014;383(9921):955-962. PubMed
23. van der Hulle T, Kooiman J, den Exter PL, Dekkers OM, Klok FA, Huisman MV. Effectiveness and safety of novel oral anticoagulants as compared with vitamin K antagonists in the treatment of acute symptomatic venous thromboembolism: a systematic review and meta-analysis. J Thromb Haemost. 2014;12(3):320-328. PubMed
24. Schuh T, Reichardt B, Finsterer J, Stöllberger C. Age-dependency of prescribing patterns of oral anticoagulant drugs in Austria during 2011–2014. J Thromb Thrombolysis. 2016;42(3):447-451. PubMed
25. Stöllberger C, Brooks R, Finsterer J, Pachofszky T. Use of direct-acting oral anticoagulants in nonagenarians: a call for more data. Drugs Aging. 2016;33(5):315-320. PubMed
26. Steffel J, Giugliano RP, Braunwald E, et al. Edoxaban versus warfarin in atrial fibrillation patients at risk of falling: ENGAGE AF-TIMI 48 analysis. J Am Coll Cardiol. 2016;68(11):1169-1178. PubMed
27. Ruff CT, Giugliano RP, Antman EM. Management of bleeding with non–vitamin K antagonist oral anticoagulants in the era of specific reversal agents. Circulation. 2016;134(3):248-261. PubMed
28. Schwartz JB. Potential impact of substituting estimated glomerular filtration rate for estimated creatinine clearance for dosing of direct oral anticoagulants. J Am Geriatr Soc. 2016;64(10):1996-2002. PubMed
29. Camm AJ, Amarenco P, Haas S, et al; XANTUS Investigators. XANTUS: a real-world, prospective, observational study of patients treated with rivaroxaban for stroke prevention in atrial fibrillation. Eur Heart J. 2016;37(14):1145-1153. PubMed
30. Steinberg BA, Shrader P, Thomas L, et al; ORBIT-AF Investigators and Patients. Off-label dosing of non–vitamin K antagonist oral anticoagulants and adverse outcomes: the ORBIT-AF II Registry. J Am Coll Cardiol. 2016;68(24):2597-2604. PubMed
31. Fauchier L, Philippart R, Clementy N, et al. How to define valvular atrial fibrillation? Arch Cardiovasc Dis. 2015;108(10):530-539. PubMed
32. Di Biase L. Use of direct oral anticoagulants in patients with atrial fibrillation and valvular heart lesions. J Am Heart Assoc. 2016;5(2). PubMed
33. Kearon C, Akl EA, Ornelas J, et al. Antithrombotic therapy for VTE disease:
CHEST guideline and expert panel report. Chest. 2016;149(2):315-352. PubMed
34. Larock AS, Mullier F, Sennesael AL, et al. Appropriateness of prescribing dabigatran
etexilate and rivaroxaban in patients with nonvalvular atrial fibrillation: a prospective
study. Ann Pharmacother. 2014;48(10):1258-1268. PubMed
1. January CT, Wann LS, Alpert JS, et al. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: executive summary. A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the Heart Rhythm Society. J Am Coll Cardiol. 2014;64(21):2246-2280. PubMed
2. Saraf K, Morris PD, Garg P, Sheridan P, Storey R. Non–vitamin K antagonist oral anticoagulants (NOACs): clinical evidence and therapeutic considerations. Postgrad Med J. 2014;90(1067):520-528. PubMed
3. Yeh CH, Gross PL, Weitz JI. Evolving use of new oral anticoagulants for treatment of venous thromboembolism. Blood. 2014;124(7):1020-1028. PubMed
4. Pradaxa website. https://www.pradaxa.com. Accessed June 1, 2017.
5. Xarelto website. https://www.xarelto-us.com. Accessed June 1, 2017.
6. Eliquis website. http://www.eliquis.com. Accessed June 1, 2017.
7. Savaysa [prescribing information]. Tokyo, Japan: Daiichi Sankyo; 2015.
8. Kirchhof P, Benussi S, Kotecha D, et al. 2016 ESC guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Eur Heart J. 2016;37(38):2893-2962. PubMed
9. Child C, Turcotte J. Surgery and portal hypertension. In: Child CG, ed. The Liver and Portal Hypertension. Philadelphia, PA: Saunders; 1964:50-64. PubMed
10. Pugh RN, Murray-Lyon IM, Dawson JL, Pietroni MC, Williams R. Transection of the oesophagus for bleeding oesophageal varices. Br J Surg. 1973;60(8):646-649. PubMed
11. Heidbuchel H, Verhamme P, Alings M, et al. Updated European Heart Rhythm Association practical guide on the use of non–vitamin K antagonist anticoagulants in patients with non-valvular atrial fibrillation. Europace. 2015;17(10):1467-1507. PubMed
12. Savaysa website. https://savaysahcp.com. Accessed June 1, 2017.
13. Levey AS, Stevens LA, Schmid CH, et al; CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration). A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150(9):604-612. PubMed
14. Cockcroft DW, Gault MH. Prediction of creatinine clearance from serum creatinine. Nephron. 1976;16(1):31-41. PubMed
15. Rose AJ, Reisman JI, Allen AL, Miller DR. Potentially inappropriate prescribing of direct-acting oral anticoagulants in the Veterans Health Administration. Am J Pharm Benefits. 2016;4(4):e75-e80.
16. Stampfuss J, Kubitza D, Becka M, Mueck W. The effect of food on the absorption and pharmacokinetics of rivaroxaban. Int J Clin Pharmacol Ther. 2013;51(7):549-561. PubMed
17. Patel MR, Mahaffey KW, Garg J, et al; ROCKET AF Investigators. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med. 2011;365(10):883-891. PubMed
18. EINSTEIN Investigators, Bauersachs R, Berkowitz SD, et al. Oral rivaroxaban for symptomatic venous thromboembolism. N Engl J Med. 2010;363(26):2499-2510. PubMed
19. EINSTEIN-PE Investigators, Büller HR, Prins MH, et al. Oral rivaroxaban for the treatment of symptomatic pulmonary embolism. N Engl J Med. 2012;366(14):1287-1297. PubMed
20. Simon J, Hawes E, Deyo Z, Bryant-Shilliday B. Evaluation of prescribing and patient use of target-specific oral anticoagulants in the outpatient setting. J Clin Pharm Ther. 2015;40(5):525-530. PubMed
21. Granger CB, Alexander JH, McMurray JJ, et al; ARISTOTLE Committees and Investigators. Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2011;365(11):981-992. PubMed
22. Ruff CT, Giugliano RP, Braunwald E, et al. Comparison of the efficacy and safety of new oral anticoagulants with warfarin in patients with atrial fibrillation: a meta-analysis of randomised trials. Lancet. 2014;383(9921):955-962. PubMed
23. van der Hulle T, Kooiman J, den Exter PL, Dekkers OM, Klok FA, Huisman MV. Effectiveness and safety of novel oral anticoagulants as compared with vitamin K antagonists in the treatment of acute symptomatic venous thromboembolism: a systematic review and meta-analysis. J Thromb Haemost. 2014;12(3):320-328. PubMed
24. Schuh T, Reichardt B, Finsterer J, Stöllberger C. Age-dependency of prescribing patterns of oral anticoagulant drugs in Austria during 2011–2014. J Thromb Thrombolysis. 2016;42(3):447-451. PubMed
25. Stöllberger C, Brooks R, Finsterer J, Pachofszky T. Use of direct-acting oral anticoagulants in nonagenarians: a call for more data. Drugs Aging. 2016;33(5):315-320. PubMed
26. Steffel J, Giugliano RP, Braunwald E, et al. Edoxaban versus warfarin in atrial fibrillation patients at risk of falling: ENGAGE AF-TIMI 48 analysis. J Am Coll Cardiol. 2016;68(11):1169-1178. PubMed
27. Ruff CT, Giugliano RP, Antman EM. Management of bleeding with non–vitamin K antagonist oral anticoagulants in the era of specific reversal agents. Circulation. 2016;134(3):248-261. PubMed
28. Schwartz JB. Potential impact of substituting estimated glomerular filtration rate for estimated creatinine clearance for dosing of direct oral anticoagulants. J Am Geriatr Soc. 2016;64(10):1996-2002. PubMed
29. Camm AJ, Amarenco P, Haas S, et al; XANTUS Investigators. XANTUS: a real-world, prospective, observational study of patients treated with rivaroxaban for stroke prevention in atrial fibrillation. Eur Heart J. 2016;37(14):1145-1153. PubMed
30. Steinberg BA, Shrader P, Thomas L, et al; ORBIT-AF Investigators and Patients. Off-label dosing of non–vitamin K antagonist oral anticoagulants and adverse outcomes: the ORBIT-AF II Registry. J Am Coll Cardiol. 2016;68(24):2597-2604. PubMed
31. Fauchier L, Philippart R, Clementy N, et al. How to define valvular atrial fibrillation? Arch Cardiovasc Dis. 2015;108(10):530-539. PubMed
32. Di Biase L. Use of direct oral anticoagulants in patients with atrial fibrillation and valvular heart lesions. J Am Heart Assoc. 2016;5(2). PubMed
33. Kearon C, Akl EA, Ornelas J, et al. Antithrombotic therapy for VTE disease:
CHEST guideline and expert panel report. Chest. 2016;149(2):315-352. PubMed
34. Larock AS, Mullier F, Sennesael AL, et al. Appropriateness of prescribing dabigatran
etexilate and rivaroxaban in patients with nonvalvular atrial fibrillation: a prospective
study. Ann Pharmacother. 2014;48(10):1258-1268. PubMed
© 2017 Society of Hospital Medicine
July 2017 Digital Edition
Click here to access the July 2017 Digital Edition.
Table of Contents
- Nonpathologic Postdeployment Transition Symptoms in Combat National Guard Members and Reservists
- Sarcopenia and the New ICD-10-CM Code: Screening, Staging, and Diagnosis Considerations
- Cerebral Venous Thrombosis
- Peer Support for Whistleblowers
- Symptoms Mimicking Those of Hypokalemic Periodic Paralysis Induced by Soluble Barium Poisoning
- Implementation of a Patient Medication Disposal Program at a VA Medical Center
- The VA Is in Critical Condition, but What Is the Prognosis?
- Leonard Wood: Advocate of Military Preparedness

Click here to access the July 2017 Digital Edition.
Table of Contents
- Nonpathologic Postdeployment Transition Symptoms in Combat National Guard Members and Reservists
- Sarcopenia and the New ICD-10-CM Code: Screening, Staging, and Diagnosis Considerations
- Cerebral Venous Thrombosis
- Peer Support for Whistleblowers
- Symptoms Mimicking Those of Hypokalemic Periodic Paralysis Induced by Soluble Barium Poisoning
- Implementation of a Patient Medication Disposal Program at a VA Medical Center
- The VA Is in Critical Condition, but What Is the Prognosis?
- Leonard Wood: Advocate of Military Preparedness

Click here to access the July 2017 Digital Edition.
Table of Contents
- Nonpathologic Postdeployment Transition Symptoms in Combat National Guard Members and Reservists
- Sarcopenia and the New ICD-10-CM Code: Screening, Staging, and Diagnosis Considerations
- Cerebral Venous Thrombosis
- Peer Support for Whistleblowers
- Symptoms Mimicking Those of Hypokalemic Periodic Paralysis Induced by Soluble Barium Poisoning
- Implementation of a Patient Medication Disposal Program at a VA Medical Center
- The VA Is in Critical Condition, but What Is the Prognosis?
- Leonard Wood: Advocate of Military Preparedness

Federal Health Care Data Trends 2017
Dozing off: Examining excessive daytime sleepiness in psychiatric patients
Excessive daytime sleepiness (EDS) is “the inability to maintain wakefulness and alertness during the major waking periods of the day, with sleep occurring unintentionally or at inappropriate times, almost daily for at least 3 months,” according to the American Academy of Sleep Medicine.1 EDS is common, with a prevalence up to 25% to 30% in the general population.1-4 The prevalence rate varies in different studies, primarily because of inconsistent definitions of EDS, and therefore differences in diagnosis and assessment.1,2,4 In a study of 300 psychiatric outpatients, 34% had EDS.3 However, studies and evidence reviewing EDS in psychiatric patients are limited.
The causes of EDS are many and varied,1,8 including medical and psychiatric etiologies. A thorough history, screening at-risk patients, and timely sleep center referral are vital to detect and appropriately manage the cause of EDS.5
This article reviews the literature on EDS, with a focus on the risks of untreated EDS, common etiologies of the condition, as well as a brief description of screening and treatment strategies.
EDS vs fatigue
Many patients describe EDS as “fatigue”1; however, a patient’s report of fatigue could be mistaken for EDS.4 Although there is overlap, it is important for physicians to distinguish between these 2 entities for accurate identification and treatment.1,4
Risk of inadequate screening
A study of 117 patients with symptomatic coronary artery disease showed that EDS is associated with significantly greater incidence of cardiovascular adverse events at 16-month follow up.2 This study had limitations such as small sample size; therefore, more studies are needed. Because of these risks, timely and accurate diagnosis not only improves the patient’s quality of life and reduces polypharmacy but also can be life-saving.
Common causes of EDS in psychiatric patients
Because of the high prevalence and severity of impairments caused by EDS, it is essential for psychiatrists to be informed about causes of EDS and thoroughly assess for the potential underlying etiology before concluding that the sleep problem is a manifestation of the psychiatric disorder and prescribing psychotropic medication for it.
Some common causes of EDS in psychiatric patients include:
Sleep-disordered breathing.8 Obstructive sleep apnea (OSA) is often underdiagnosed,6,7 and considering how common it is,6 psychiatrists likely will see many patients with OSA in their practice.5 OSA has a higher prevalence among patients with psychiatric disorders such as depression6,9 and schizophrenia. Additionally, there is evidence suggesting that patients with OSA are more likely to suffer from depression and EDS than healthy controls6,9,10; some of the proposed mechanisms are sleep fragmentation and hypoxemia.6,9-11 OSA is the most common form of sleep-disordered breathing and is a common cause of EDS.1,2,12 Also, undiagnosed and untreated OSA in patients with depression could cause refractoriness to pharmacological treatment of depression.6,9,10
When unrecognized and untreated, OSA can be life-threatening. Despite this, OSA is not regularly screened for in clinical psychiatric practice.6,10 Therefore, it is imperative that psychiatrists be well-acquainted with measures to identify at-risk patients and refer to a sleep specialist when appropriate.
OSA is accompanied by irritability, cognitive difficulties, and poor sleep, creating an overlap with symptoms of depressive disorders.6,10 Use of sedative hypnotic medications, such as benzodiazepines, which further reduces muscle tone in the airway and suppresses respiratory effort, can worsen OSA symptoms5,6,10 and pose cerebrovascular, cardiovascular, and potentially life-threatening risks, and therefore is not indicated in this population.9,13
Obesity is a risk factor for OSA.6 Patients with mood disorders or schizophrenia or other psychotic disorders are at higher risk of obesity because of psychotropic-induced weight gain, stress-induced mechanisms, and/or lower levels of self-care. When these patients have unrecognized or untreated OSA and are prescribed sedative medications at night or stimulant medications during the day, they could be at increased cardiac or respiratory risks without resolving their underlying condition. A diligent psychiatrist can dramatically reduce the risks by referring a patient for nocturnal polysomnography,1 helping the patient implement lifestyle modifications (eg, exercise, weight loss, and healthy nutrition), prescribing judiciously, and monitoring closely for such risks. An accurate diagnosis of and treatment for OSA can improve sleep6 dramatically and help depressive symptoms through better sleep, more daytime energy and concentration, and adequate oxygenation of the brain while sleeping.
Psychiatrists can screen for OSA using the STOP-Bang (Snoring, Tired, Observed apnea, Pressure, Body mass index, Age, Neck circumference, Gender) Questionnaire, which is a quick, 8-item screening scale that helps to categorize OSA risk as mild, moderate, or severe.12 Hypertension, snoring, and/or gasping for breath (“observed apnea”)—a history which often is provided by spouses or significant others—daytime dozing and/or tiredness, having a large neck circumference or volume, body mass index, male sex, and age are items on the STOP-Bang Questionnaire and also are features that should raise high clinical suspicion of OSA.12 Referral for nocturnal polysomnography in at-risk patients should be the next step1,5 in any sleep-related breathing disorder.
Treatment for OSA involves continuous positive airway pressure (CPAP) therapy, which has been shown to relieve OSA and decrease related EDS.5,6 Other treatment modalities, such as oral appliances and surgery, may be used5 in some cases, but more studies are needed for conclusive results.
Several studies have shown improved depression, mood, and cognition after administering treatment such as CPAP6,9,14 in patients with OSA and depression. Considering the significant risks of cardiovascular,8 cerebrovascular,8 and overall morbidity and mortality associated with untreated OSA,12 it is important to routinely screen for sleep-disordered breathing in patients with depression9 or other psychiatric disorders and refer for specialized sleep evaluation and treatment, when indicated.
Medications. EDS can result from some prescription and over-the-counter medications.1,2,5,7 Sedating antidepressants, antihistamines, antipsychotics, anticonvulsants,1,8 and beta blockers2 could cause sedation, which can persist during daytime, although a few studies did not find an association between antipsychotic use and EDS.3 Benzodiazepines and other sedative-hypnotics,1,7 especially long-acting agents or higher dosages,5 can lead to EDS and decreased alertness. Non-psychotropics, such as opioid pain medications,1,7 antitussives, and skeletal muscle relaxants, also can contribute to or cause daytime sedation.7 When using these agents, psychiatrists should monitor and routinely assess patients while aiming for the lowest effective dosage when feasible.
This strategy creates a framework for psychiatrists to routinely educate patients about these commonly encountered side effects, reduce polypharmacy when possible, and help patients effectively manage or prevent these adverse effects.
Depression.1 Some studies found >45% patients with depression had EDS.3,13,15 Besides an association between depression and EDS,13,16 Chellappa and Araújo13 also found a significant association between EDS and suicidal ideation. The causes of EDS in patients with depression may be varied, ranging from restless legs syndrome, residual depressive symptoms,15 to OSA. Depression is often comorbid with OSA,6 with up to 20% of patients with depression suffering from OSA,10 creating higher risk for EDS. Depressive disorders are routinely assessed during an evaluation of OSA at sleep centers, but OSA often is not screened in psychiatric practice.10
There is a strong need for regular screening for OSA in patients with depression, particularly because most studies show a link between the 2 conditions.10 Both depression and OSA have some common risk factors, such as obesity, hypertension, and metabolic syndrome.10 Patients with these conditions are at greater risk for OSA, and therefore a psychiatrist should proactively screen and refer such patients for nocturnal polysomnography when they suspect OSA. Patients with OSA and depression often present to the psychiatrist with depressive symptoms that appear to be resistant to pharmacological treatment,10 therefore underscoring the importance of screening and ruling out OSA in patients with depression.
Circadian rhythm disorders, restless legs syndrome, alcohol and other substance use, and use of prescription sedative-hypnotics are more common in patients with depression; therefore, this population is at high risk for EDS.
Circadian rhythm disorders and insufficient sleep syndrome. Insufficient sleep syndrome1,2,8 frequently causes EDS and occurs more commonly in busy people who try to get by with less sleep.8 Over time, the effect of sleep loss is cumulative and can be accompanied by mood symptoms, such as irritability, fatigue, and problems with concentration.8 Shift workers1,8 commonly experience insufficient sleep as well as circadian rhythm disorders and EDS. Modafinil is FDA-approved for EDS in shift work sleep disorder.
Geriatric patients may experience advanced sleep phase syndrome involving early awakenings.8 Adolescents, on the other hand, often suffer from delayed sleep phase syndrome, which is a type of circadian rhythm disorder, related to increasing academic and social pressures, natural pubertal shift to later sleep onset, pervading technology use, and often nebulous bedtime routines. This can be a cause of sleep persisting into daytime.8 Taking a careful history and a sleep diary may be useful because this disorder might be confused for insomnia. Treatment involves gradual shifting of the time of sleep onset through bright light exposure and other modalities.8
Adolescents might not be forthcoming about the severity of their sleep problems; therefore, psychiatrists should screen proactively through clinical interviews of patients and parents and consider this possibility when encountering an adolescent with recent-onset attention or cognitive difficulties.
Treatment for circadian rhythm disorders usually includes planned or prescribed sleep scheduling, timed light exposure,8 and occasional use of melatonin or other sedative agents.17
Hypersomnia of central origin, which includes narcolepsy, idiopathic hypersomnia, and recurrent hypersomnia, can present with EDS.1,18,19 Narcolepsy is a rare, debilitating sleep disorder that manifests as EDS or sleep attacks, with or without cataplexy, and sleep paralysis.5,8,18,19 The Multiple Sleep Latency Test and polysomnography are used for diagnosis.1,5 Shortened REM latency is a classic finding often noted on polysomnography. Treatment involves pharmacologic and behavioral strategies and education.5,8 Modafinil is FDA-approved for EDS associated with narcolepsy. Stimulant medications have been used for narcolepsy in the past; further studies are needed to establish benefit–risk ratio of use in this population.18
Kleine-Levin syndrome is a form of recurrent hypersomnia, a less common sleep disorder, characterized by episodes of excessive sleepiness accompanied by hyperphagia and hypersexuality.5,18,19
Other medical conditions,1 such as the rare familial fatal insomnia, neurological conditions1 such as encephalitis,8 epilepsy,8 Alzheimer’s disease or other types of dementia,8 Parkinson’s disease,1 or multiple sclerosis,1,18 can cause excessive daytime fatigue by causing secondary insomnia or hypersomnia.
Treating the underlying disorder is an important first step in these cases. In addition, coordinating with neurologists or other specialists involved in caring for patients with these conditions is important. Regularly reviewing and simplifying the often complex medication regimen, when possible, can go a long way in mitigating EDS in this population.
Other disorders affecting sleep. Restless legs syndrome and periodic limb movement disorder are other causes of EDS.3 Treatment involves lifestyle modifications, iron supplementation in certain patients, and use of dopaminergic agents such as ropinirole, pramipexole, and other medications, depending on severity of the condition, comorbidities, and other factors.20
Alcohol or substance use. Substance use or withdrawal can be associated with sleep disorders, such as hypersomnia,19 insomnia,19 and related EDS.5 For example, alcohol use disorder affects REM sleep, and can cause EDS. Secondary central apnea can be the result of long-standing opioid use19 and can present like EDS.
Insomnia. Primary insomnia rarely causes EDS.5 Insomnia due to a medical or psychiatric condition may be an indirect cause of EDS by causing sleep deprivation.
Steps for timely and accurate diagnosis
Utilize the following steps for facilitating timely diagnosis and treatment of EDS:
Thorough history. Patients often describe “tiredness” instead of sleepiness.8 Therefore, the astute psychiatrist should explore further when patients are presenting with this concern, especially by asking more specific questions such as the tendency to doze off during daytime.8
Family members can be vital sources for obtaining a complete history,5 especially because patients might deny,8 minimize, or not be fully aware1 of the extent of their symptoms. Asking family members about patient’s snoring, irregular breathing, or gasping at night can be particularly valuable.5 Obtaining a family history of sleep disorders can be particularly important, especially in conditions such as OSA and narcolepsy.
Asking about any history of safety issues,8 including sleepiness during driving, cooking, or other activities, is also important.
Use of scales and other screening measures. Psychiatrists can use initial screening measures in the office setting. Epworth Sleepiness Scale15,21 is a validated,2 short, self-administered measure to assess the level of daytime sleepiness; however, it has some limitations such as not being able to measure changes in sleepiness from hour to hour or day to day. Because of its limitations, the Epworth Sleepiness Scale should not be used by itself as a diagnostic tool.3 It has been commonly used for detecting OSA2 and narcolepsy. The Stanford Sleepiness Scale is a self-rating scale that measures the subjective degree of sleepiness and alertness; it has limitations as well, such as having little correlation with chronic sleep loss.8 Other tools such as visual analogue scales also could be helpful.8 For more specialized testing, such as Multiple Sleep Latency Test or polysomnography, referral to a sleep specialist is ideal.8
Education. The assessment is an opportunity for the psychiatrist to educate patients about sleep hygiene, the importance of regular bedtimes, and getting adequate sleep to avoid accumulating a sleep deficit.
Urgent referral of at-risk populations. Prompt or urgent referral of at-risk populations, such as geriatric patients or those with a history of dozing off during driving, is invaluable in preventing morbidity and mortality from untreated sleep disorders.
Patients with severe daytime sleepiness should be advised to not drive or operate heavy machinery until this condition is adequately controlled.18
Bottom Line
1. Chervin RD. Approach to the patient with excessive daytime sleepiness. http://www.uptodate.com/contents/approach-to-the-patient-with-excessive-daytime-sleepiness. Updated January 2016. Accessed June 5, 2017.
2. Lee CH, Ng WY, Hau W, et al. Excessive daytime sleepiness is associated with longer culprit lesion and adverse outcomes in patients with coronary artery disease. J Clin Sleep Med. 2013;9(12):1267-1272.
3. Hawley CJ, Gale TM, Sivakumaran T, et al. Excessive daytime sleepiness in psychiatric disorders: prevalence, correlates and clinical significance. Psychiatry Res. 2010;175(1-2):138-141.
4. Pigeon WR, Sateia MJ, Ferguson RJ. Distinguishing between excessive daytime sleepiness and fatigue: toward improved detection and treatment. J Psychosom Res. 2003;54(1):61-69.
5. Krahn LE. Excessive daytime sleepiness: diagnosing the causes. Current Psychiatry. 2002;1(1):49-57.
6. Ejaz SM, Khawaja IS, Bhatia S, et al. Obstructive sleep apnea and depression: a review. Innov Clin Neurosci. 2011;8(8):17-25.
7. Pagel JF. Excessive daytime sleepiness. Am Fam Physician. 2009;79(5):391-396.
8. Guilleminault C, Brooks SN. Excessive daytime sleepiness: a challenge for the practising neurologist. Brain. 2001;124(pt 8):1482-1491.
9. Cheng P, Casement M, Chen CF, et al. Sleep disordered breathing in major depressive disorder. J Sleep Res. 2013;22(4):459-462.
10. Schröder CM, O’Hara R. Depression and obstructive sleep apnea (OSA). Ann Gen Psychiatry. 2005;4:13.
11. Bardwell WA, Berry CC, Ancoli-Israel S, et al. Psychological correlates of sleep apnea. J Psychosom Res. 1999;47(6):583-596.
12. Chung F, Abdullah HR, Liao P. STOP-Bang Questionnaire: a practical approach to screen for obstructive sleep apnea. Chest. 2016;149(3):631-638.
13. Chellappa SL, Araújo JF. Excessive daytime sleepiness in patients with depressive disorder. Rev Bras Psiquiatr. 2006;28(2):126-129.
14. Habukawa M, Uchimura N, Kakuma T, et al. Effect of CPAP treatment on residual depressive symptoms in patients with major depression and coexisting sleep apnea: contribution of daytime sleepiness to residual depressive symptoms. Sleep Med. 2010;11(6):552-557.
15. Lundt L. Use of the Epworth Sleepiness Scale to evaluate the symptom of excessive sleepiness in major depressive disorder. Gen Hosp Psychiatry. 2005;27(2):146-148.
16. Hawley CJ. Excessive daytime sleepiness in psychiatry: a relevant focus for clinical attention and treatment? Int J Psychiatry Clin Pract. 2006;10(2):117-123.
17. Dodson ER, Zee PC. Therapeutics for circadian rhythm sleep disorders. Sleep Med Clin. 2010;5(4):701-715.
18. Morgenthaler TI, Kapur VK, Brown TM, et al; Standards of Practice Committee of the American Academy of Sleep Medicine. Practice parameters for the treatment of narcolepsy and other hypersomnias of central origin. Sleep. 2007;30(12):1705-1711.
19. Thorpy MJ. Classification of sleep disorders. Neurotherapeutics. 2012;9(4):687-701.
20. National Institute of Neurological Disorders and Stroke. Restless legs syndrome information page. https://www.ninds.nih.gov/Disorders/All-Disorders/Restless-Legs-Syndrome-Information-Page. Accessed June 2, 2017.
21. Johns MW. Reliability and factor analysis of the Epworth Sleepiness Scale. Sleep. 1992;15(4):376-381.
Excessive daytime sleepiness (EDS) is “the inability to maintain wakefulness and alertness during the major waking periods of the day, with sleep occurring unintentionally or at inappropriate times, almost daily for at least 3 months,” according to the American Academy of Sleep Medicine.1 EDS is common, with a prevalence up to 25% to 30% in the general population.1-4 The prevalence rate varies in different studies, primarily because of inconsistent definitions of EDS, and therefore differences in diagnosis and assessment.1,2,4 In a study of 300 psychiatric outpatients, 34% had EDS.3 However, studies and evidence reviewing EDS in psychiatric patients are limited.
The causes of EDS are many and varied,1,8 including medical and psychiatric etiologies. A thorough history, screening at-risk patients, and timely sleep center referral are vital to detect and appropriately manage the cause of EDS.5
This article reviews the literature on EDS, with a focus on the risks of untreated EDS, common etiologies of the condition, as well as a brief description of screening and treatment strategies.
EDS vs fatigue
Many patients describe EDS as “fatigue”1; however, a patient’s report of fatigue could be mistaken for EDS.4 Although there is overlap, it is important for physicians to distinguish between these 2 entities for accurate identification and treatment.1,4
Risk of inadequate screening
A study of 117 patients with symptomatic coronary artery disease showed that EDS is associated with significantly greater incidence of cardiovascular adverse events at 16-month follow up.2 This study had limitations such as small sample size; therefore, more studies are needed. Because of these risks, timely and accurate diagnosis not only improves the patient’s quality of life and reduces polypharmacy but also can be life-saving.
Common causes of EDS in psychiatric patients
Because of the high prevalence and severity of impairments caused by EDS, it is essential for psychiatrists to be informed about causes of EDS and thoroughly assess for the potential underlying etiology before concluding that the sleep problem is a manifestation of the psychiatric disorder and prescribing psychotropic medication for it.
Some common causes of EDS in psychiatric patients include:
Sleep-disordered breathing.8 Obstructive sleep apnea (OSA) is often underdiagnosed,6,7 and considering how common it is,6 psychiatrists likely will see many patients with OSA in their practice.5 OSA has a higher prevalence among patients with psychiatric disorders such as depression6,9 and schizophrenia. Additionally, there is evidence suggesting that patients with OSA are more likely to suffer from depression and EDS than healthy controls6,9,10; some of the proposed mechanisms are sleep fragmentation and hypoxemia.6,9-11 OSA is the most common form of sleep-disordered breathing and is a common cause of EDS.1,2,12 Also, undiagnosed and untreated OSA in patients with depression could cause refractoriness to pharmacological treatment of depression.6,9,10
When unrecognized and untreated, OSA can be life-threatening. Despite this, OSA is not regularly screened for in clinical psychiatric practice.6,10 Therefore, it is imperative that psychiatrists be well-acquainted with measures to identify at-risk patients and refer to a sleep specialist when appropriate.
OSA is accompanied by irritability, cognitive difficulties, and poor sleep, creating an overlap with symptoms of depressive disorders.6,10 Use of sedative hypnotic medications, such as benzodiazepines, which further reduces muscle tone in the airway and suppresses respiratory effort, can worsen OSA symptoms5,6,10 and pose cerebrovascular, cardiovascular, and potentially life-threatening risks, and therefore is not indicated in this population.9,13
Obesity is a risk factor for OSA.6 Patients with mood disorders or schizophrenia or other psychotic disorders are at higher risk of obesity because of psychotropic-induced weight gain, stress-induced mechanisms, and/or lower levels of self-care. When these patients have unrecognized or untreated OSA and are prescribed sedative medications at night or stimulant medications during the day, they could be at increased cardiac or respiratory risks without resolving their underlying condition. A diligent psychiatrist can dramatically reduce the risks by referring a patient for nocturnal polysomnography,1 helping the patient implement lifestyle modifications (eg, exercise, weight loss, and healthy nutrition), prescribing judiciously, and monitoring closely for such risks. An accurate diagnosis of and treatment for OSA can improve sleep6 dramatically and help depressive symptoms through better sleep, more daytime energy and concentration, and adequate oxygenation of the brain while sleeping.
Psychiatrists can screen for OSA using the STOP-Bang (Snoring, Tired, Observed apnea, Pressure, Body mass index, Age, Neck circumference, Gender) Questionnaire, which is a quick, 8-item screening scale that helps to categorize OSA risk as mild, moderate, or severe.12 Hypertension, snoring, and/or gasping for breath (“observed apnea”)—a history which often is provided by spouses or significant others—daytime dozing and/or tiredness, having a large neck circumference or volume, body mass index, male sex, and age are items on the STOP-Bang Questionnaire and also are features that should raise high clinical suspicion of OSA.12 Referral for nocturnal polysomnography in at-risk patients should be the next step1,5 in any sleep-related breathing disorder.
Treatment for OSA involves continuous positive airway pressure (CPAP) therapy, which has been shown to relieve OSA and decrease related EDS.5,6 Other treatment modalities, such as oral appliances and surgery, may be used5 in some cases, but more studies are needed for conclusive results.
Several studies have shown improved depression, mood, and cognition after administering treatment such as CPAP6,9,14 in patients with OSA and depression. Considering the significant risks of cardiovascular,8 cerebrovascular,8 and overall morbidity and mortality associated with untreated OSA,12 it is important to routinely screen for sleep-disordered breathing in patients with depression9 or other psychiatric disorders and refer for specialized sleep evaluation and treatment, when indicated.
Medications. EDS can result from some prescription and over-the-counter medications.1,2,5,7 Sedating antidepressants, antihistamines, antipsychotics, anticonvulsants,1,8 and beta blockers2 could cause sedation, which can persist during daytime, although a few studies did not find an association between antipsychotic use and EDS.3 Benzodiazepines and other sedative-hypnotics,1,7 especially long-acting agents or higher dosages,5 can lead to EDS and decreased alertness. Non-psychotropics, such as opioid pain medications,1,7 antitussives, and skeletal muscle relaxants, also can contribute to or cause daytime sedation.7 When using these agents, psychiatrists should monitor and routinely assess patients while aiming for the lowest effective dosage when feasible.
This strategy creates a framework for psychiatrists to routinely educate patients about these commonly encountered side effects, reduce polypharmacy when possible, and help patients effectively manage or prevent these adverse effects.
Depression.1 Some studies found >45% patients with depression had EDS.3,13,15 Besides an association between depression and EDS,13,16 Chellappa and Araújo13 also found a significant association between EDS and suicidal ideation. The causes of EDS in patients with depression may be varied, ranging from restless legs syndrome, residual depressive symptoms,15 to OSA. Depression is often comorbid with OSA,6 with up to 20% of patients with depression suffering from OSA,10 creating higher risk for EDS. Depressive disorders are routinely assessed during an evaluation of OSA at sleep centers, but OSA often is not screened in psychiatric practice.10
There is a strong need for regular screening for OSA in patients with depression, particularly because most studies show a link between the 2 conditions.10 Both depression and OSA have some common risk factors, such as obesity, hypertension, and metabolic syndrome.10 Patients with these conditions are at greater risk for OSA, and therefore a psychiatrist should proactively screen and refer such patients for nocturnal polysomnography when they suspect OSA. Patients with OSA and depression often present to the psychiatrist with depressive symptoms that appear to be resistant to pharmacological treatment,10 therefore underscoring the importance of screening and ruling out OSA in patients with depression.
Circadian rhythm disorders, restless legs syndrome, alcohol and other substance use, and use of prescription sedative-hypnotics are more common in patients with depression; therefore, this population is at high risk for EDS.
Circadian rhythm disorders and insufficient sleep syndrome. Insufficient sleep syndrome1,2,8 frequently causes EDS and occurs more commonly in busy people who try to get by with less sleep.8 Over time, the effect of sleep loss is cumulative and can be accompanied by mood symptoms, such as irritability, fatigue, and problems with concentration.8 Shift workers1,8 commonly experience insufficient sleep as well as circadian rhythm disorders and EDS. Modafinil is FDA-approved for EDS in shift work sleep disorder.
Geriatric patients may experience advanced sleep phase syndrome involving early awakenings.8 Adolescents, on the other hand, often suffer from delayed sleep phase syndrome, which is a type of circadian rhythm disorder, related to increasing academic and social pressures, natural pubertal shift to later sleep onset, pervading technology use, and often nebulous bedtime routines. This can be a cause of sleep persisting into daytime.8 Taking a careful history and a sleep diary may be useful because this disorder might be confused for insomnia. Treatment involves gradual shifting of the time of sleep onset through bright light exposure and other modalities.8
Adolescents might not be forthcoming about the severity of their sleep problems; therefore, psychiatrists should screen proactively through clinical interviews of patients and parents and consider this possibility when encountering an adolescent with recent-onset attention or cognitive difficulties.
Treatment for circadian rhythm disorders usually includes planned or prescribed sleep scheduling, timed light exposure,8 and occasional use of melatonin or other sedative agents.17
Hypersomnia of central origin, which includes narcolepsy, idiopathic hypersomnia, and recurrent hypersomnia, can present with EDS.1,18,19 Narcolepsy is a rare, debilitating sleep disorder that manifests as EDS or sleep attacks, with or without cataplexy, and sleep paralysis.5,8,18,19 The Multiple Sleep Latency Test and polysomnography are used for diagnosis.1,5 Shortened REM latency is a classic finding often noted on polysomnography. Treatment involves pharmacologic and behavioral strategies and education.5,8 Modafinil is FDA-approved for EDS associated with narcolepsy. Stimulant medications have been used for narcolepsy in the past; further studies are needed to establish benefit–risk ratio of use in this population.18
Kleine-Levin syndrome is a form of recurrent hypersomnia, a less common sleep disorder, characterized by episodes of excessive sleepiness accompanied by hyperphagia and hypersexuality.5,18,19
Other medical conditions,1 such as the rare familial fatal insomnia, neurological conditions1 such as encephalitis,8 epilepsy,8 Alzheimer’s disease or other types of dementia,8 Parkinson’s disease,1 or multiple sclerosis,1,18 can cause excessive daytime fatigue by causing secondary insomnia or hypersomnia.
Treating the underlying disorder is an important first step in these cases. In addition, coordinating with neurologists or other specialists involved in caring for patients with these conditions is important. Regularly reviewing and simplifying the often complex medication regimen, when possible, can go a long way in mitigating EDS in this population.
Other disorders affecting sleep. Restless legs syndrome and periodic limb movement disorder are other causes of EDS.3 Treatment involves lifestyle modifications, iron supplementation in certain patients, and use of dopaminergic agents such as ropinirole, pramipexole, and other medications, depending on severity of the condition, comorbidities, and other factors.20
Alcohol or substance use. Substance use or withdrawal can be associated with sleep disorders, such as hypersomnia,19 insomnia,19 and related EDS.5 For example, alcohol use disorder affects REM sleep, and can cause EDS. Secondary central apnea can be the result of long-standing opioid use19 and can present like EDS.
Insomnia. Primary insomnia rarely causes EDS.5 Insomnia due to a medical or psychiatric condition may be an indirect cause of EDS by causing sleep deprivation.
Steps for timely and accurate diagnosis
Utilize the following steps for facilitating timely diagnosis and treatment of EDS:
Thorough history. Patients often describe “tiredness” instead of sleepiness.8 Therefore, the astute psychiatrist should explore further when patients are presenting with this concern, especially by asking more specific questions such as the tendency to doze off during daytime.8
Family members can be vital sources for obtaining a complete history,5 especially because patients might deny,8 minimize, or not be fully aware1 of the extent of their symptoms. Asking family members about patient’s snoring, irregular breathing, or gasping at night can be particularly valuable.5 Obtaining a family history of sleep disorders can be particularly important, especially in conditions such as OSA and narcolepsy.
Asking about any history of safety issues,8 including sleepiness during driving, cooking, or other activities, is also important.
Use of scales and other screening measures. Psychiatrists can use initial screening measures in the office setting. Epworth Sleepiness Scale15,21 is a validated,2 short, self-administered measure to assess the level of daytime sleepiness; however, it has some limitations such as not being able to measure changes in sleepiness from hour to hour or day to day. Because of its limitations, the Epworth Sleepiness Scale should not be used by itself as a diagnostic tool.3 It has been commonly used for detecting OSA2 and narcolepsy. The Stanford Sleepiness Scale is a self-rating scale that measures the subjective degree of sleepiness and alertness; it has limitations as well, such as having little correlation with chronic sleep loss.8 Other tools such as visual analogue scales also could be helpful.8 For more specialized testing, such as Multiple Sleep Latency Test or polysomnography, referral to a sleep specialist is ideal.8
Education. The assessment is an opportunity for the psychiatrist to educate patients about sleep hygiene, the importance of regular bedtimes, and getting adequate sleep to avoid accumulating a sleep deficit.
Urgent referral of at-risk populations. Prompt or urgent referral of at-risk populations, such as geriatric patients or those with a history of dozing off during driving, is invaluable in preventing morbidity and mortality from untreated sleep disorders.
Patients with severe daytime sleepiness should be advised to not drive or operate heavy machinery until this condition is adequately controlled.18
Bottom Line
Excessive daytime sleepiness (EDS) is “the inability to maintain wakefulness and alertness during the major waking periods of the day, with sleep occurring unintentionally or at inappropriate times, almost daily for at least 3 months,” according to the American Academy of Sleep Medicine.1 EDS is common, with a prevalence up to 25% to 30% in the general population.1-4 The prevalence rate varies in different studies, primarily because of inconsistent definitions of EDS, and therefore differences in diagnosis and assessment.1,2,4 In a study of 300 psychiatric outpatients, 34% had EDS.3 However, studies and evidence reviewing EDS in psychiatric patients are limited.
The causes of EDS are many and varied,1,8 including medical and psychiatric etiologies. A thorough history, screening at-risk patients, and timely sleep center referral are vital to detect and appropriately manage the cause of EDS.5
This article reviews the literature on EDS, with a focus on the risks of untreated EDS, common etiologies of the condition, as well as a brief description of screening and treatment strategies.
EDS vs fatigue
Many patients describe EDS as “fatigue”1; however, a patient’s report of fatigue could be mistaken for EDS.4 Although there is overlap, it is important for physicians to distinguish between these 2 entities for accurate identification and treatment.1,4
Risk of inadequate screening
A study of 117 patients with symptomatic coronary artery disease showed that EDS is associated with significantly greater incidence of cardiovascular adverse events at 16-month follow up.2 This study had limitations such as small sample size; therefore, more studies are needed. Because of these risks, timely and accurate diagnosis not only improves the patient’s quality of life and reduces polypharmacy but also can be life-saving.
Common causes of EDS in psychiatric patients
Because of the high prevalence and severity of impairments caused by EDS, it is essential for psychiatrists to be informed about causes of EDS and thoroughly assess for the potential underlying etiology before concluding that the sleep problem is a manifestation of the psychiatric disorder and prescribing psychotropic medication for it.
Some common causes of EDS in psychiatric patients include:
Sleep-disordered breathing.8 Obstructive sleep apnea (OSA) is often underdiagnosed,6,7 and considering how common it is,6 psychiatrists likely will see many patients with OSA in their practice.5 OSA has a higher prevalence among patients with psychiatric disorders such as depression6,9 and schizophrenia. Additionally, there is evidence suggesting that patients with OSA are more likely to suffer from depression and EDS than healthy controls6,9,10; some of the proposed mechanisms are sleep fragmentation and hypoxemia.6,9-11 OSA is the most common form of sleep-disordered breathing and is a common cause of EDS.1,2,12 Also, undiagnosed and untreated OSA in patients with depression could cause refractoriness to pharmacological treatment of depression.6,9,10
When unrecognized and untreated, OSA can be life-threatening. Despite this, OSA is not regularly screened for in clinical psychiatric practice.6,10 Therefore, it is imperative that psychiatrists be well-acquainted with measures to identify at-risk patients and refer to a sleep specialist when appropriate.
OSA is accompanied by irritability, cognitive difficulties, and poor sleep, creating an overlap with symptoms of depressive disorders.6,10 Use of sedative hypnotic medications, such as benzodiazepines, which further reduces muscle tone in the airway and suppresses respiratory effort, can worsen OSA symptoms5,6,10 and pose cerebrovascular, cardiovascular, and potentially life-threatening risks, and therefore is not indicated in this population.9,13
Obesity is a risk factor for OSA.6 Patients with mood disorders or schizophrenia or other psychotic disorders are at higher risk of obesity because of psychotropic-induced weight gain, stress-induced mechanisms, and/or lower levels of self-care. When these patients have unrecognized or untreated OSA and are prescribed sedative medications at night or stimulant medications during the day, they could be at increased cardiac or respiratory risks without resolving their underlying condition. A diligent psychiatrist can dramatically reduce the risks by referring a patient for nocturnal polysomnography,1 helping the patient implement lifestyle modifications (eg, exercise, weight loss, and healthy nutrition), prescribing judiciously, and monitoring closely for such risks. An accurate diagnosis of and treatment for OSA can improve sleep6 dramatically and help depressive symptoms through better sleep, more daytime energy and concentration, and adequate oxygenation of the brain while sleeping.
Psychiatrists can screen for OSA using the STOP-Bang (Snoring, Tired, Observed apnea, Pressure, Body mass index, Age, Neck circumference, Gender) Questionnaire, which is a quick, 8-item screening scale that helps to categorize OSA risk as mild, moderate, or severe.12 Hypertension, snoring, and/or gasping for breath (“observed apnea”)—a history which often is provided by spouses or significant others—daytime dozing and/or tiredness, having a large neck circumference or volume, body mass index, male sex, and age are items on the STOP-Bang Questionnaire and also are features that should raise high clinical suspicion of OSA.12 Referral for nocturnal polysomnography in at-risk patients should be the next step1,5 in any sleep-related breathing disorder.
Treatment for OSA involves continuous positive airway pressure (CPAP) therapy, which has been shown to relieve OSA and decrease related EDS.5,6 Other treatment modalities, such as oral appliances and surgery, may be used5 in some cases, but more studies are needed for conclusive results.
Several studies have shown improved depression, mood, and cognition after administering treatment such as CPAP6,9,14 in patients with OSA and depression. Considering the significant risks of cardiovascular,8 cerebrovascular,8 and overall morbidity and mortality associated with untreated OSA,12 it is important to routinely screen for sleep-disordered breathing in patients with depression9 or other psychiatric disorders and refer for specialized sleep evaluation and treatment, when indicated.
Medications. EDS can result from some prescription and over-the-counter medications.1,2,5,7 Sedating antidepressants, antihistamines, antipsychotics, anticonvulsants,1,8 and beta blockers2 could cause sedation, which can persist during daytime, although a few studies did not find an association between antipsychotic use and EDS.3 Benzodiazepines and other sedative-hypnotics,1,7 especially long-acting agents or higher dosages,5 can lead to EDS and decreased alertness. Non-psychotropics, such as opioid pain medications,1,7 antitussives, and skeletal muscle relaxants, also can contribute to or cause daytime sedation.7 When using these agents, psychiatrists should monitor and routinely assess patients while aiming for the lowest effective dosage when feasible.
This strategy creates a framework for psychiatrists to routinely educate patients about these commonly encountered side effects, reduce polypharmacy when possible, and help patients effectively manage or prevent these adverse effects.
Depression.1 Some studies found >45% patients with depression had EDS.3,13,15 Besides an association between depression and EDS,13,16 Chellappa and Araújo13 also found a significant association between EDS and suicidal ideation. The causes of EDS in patients with depression may be varied, ranging from restless legs syndrome, residual depressive symptoms,15 to OSA. Depression is often comorbid with OSA,6 with up to 20% of patients with depression suffering from OSA,10 creating higher risk for EDS. Depressive disorders are routinely assessed during an evaluation of OSA at sleep centers, but OSA often is not screened in psychiatric practice.10
There is a strong need for regular screening for OSA in patients with depression, particularly because most studies show a link between the 2 conditions.10 Both depression and OSA have some common risk factors, such as obesity, hypertension, and metabolic syndrome.10 Patients with these conditions are at greater risk for OSA, and therefore a psychiatrist should proactively screen and refer such patients for nocturnal polysomnography when they suspect OSA. Patients with OSA and depression often present to the psychiatrist with depressive symptoms that appear to be resistant to pharmacological treatment,10 therefore underscoring the importance of screening and ruling out OSA in patients with depression.
Circadian rhythm disorders, restless legs syndrome, alcohol and other substance use, and use of prescription sedative-hypnotics are more common in patients with depression; therefore, this population is at high risk for EDS.
Circadian rhythm disorders and insufficient sleep syndrome. Insufficient sleep syndrome1,2,8 frequently causes EDS and occurs more commonly in busy people who try to get by with less sleep.8 Over time, the effect of sleep loss is cumulative and can be accompanied by mood symptoms, such as irritability, fatigue, and problems with concentration.8 Shift workers1,8 commonly experience insufficient sleep as well as circadian rhythm disorders and EDS. Modafinil is FDA-approved for EDS in shift work sleep disorder.
Geriatric patients may experience advanced sleep phase syndrome involving early awakenings.8 Adolescents, on the other hand, often suffer from delayed sleep phase syndrome, which is a type of circadian rhythm disorder, related to increasing academic and social pressures, natural pubertal shift to later sleep onset, pervading technology use, and often nebulous bedtime routines. This can be a cause of sleep persisting into daytime.8 Taking a careful history and a sleep diary may be useful because this disorder might be confused for insomnia. Treatment involves gradual shifting of the time of sleep onset through bright light exposure and other modalities.8
Adolescents might not be forthcoming about the severity of their sleep problems; therefore, psychiatrists should screen proactively through clinical interviews of patients and parents and consider this possibility when encountering an adolescent with recent-onset attention or cognitive difficulties.
Treatment for circadian rhythm disorders usually includes planned or prescribed sleep scheduling, timed light exposure,8 and occasional use of melatonin or other sedative agents.17
Hypersomnia of central origin, which includes narcolepsy, idiopathic hypersomnia, and recurrent hypersomnia, can present with EDS.1,18,19 Narcolepsy is a rare, debilitating sleep disorder that manifests as EDS or sleep attacks, with or without cataplexy, and sleep paralysis.5,8,18,19 The Multiple Sleep Latency Test and polysomnography are used for diagnosis.1,5 Shortened REM latency is a classic finding often noted on polysomnography. Treatment involves pharmacologic and behavioral strategies and education.5,8 Modafinil is FDA-approved for EDS associated with narcolepsy. Stimulant medications have been used for narcolepsy in the past; further studies are needed to establish benefit–risk ratio of use in this population.18
Kleine-Levin syndrome is a form of recurrent hypersomnia, a less common sleep disorder, characterized by episodes of excessive sleepiness accompanied by hyperphagia and hypersexuality.5,18,19
Other medical conditions,1 such as the rare familial fatal insomnia, neurological conditions1 such as encephalitis,8 epilepsy,8 Alzheimer’s disease or other types of dementia,8 Parkinson’s disease,1 or multiple sclerosis,1,18 can cause excessive daytime fatigue by causing secondary insomnia or hypersomnia.
Treating the underlying disorder is an important first step in these cases. In addition, coordinating with neurologists or other specialists involved in caring for patients with these conditions is important. Regularly reviewing and simplifying the often complex medication regimen, when possible, can go a long way in mitigating EDS in this population.
Other disorders affecting sleep. Restless legs syndrome and periodic limb movement disorder are other causes of EDS.3 Treatment involves lifestyle modifications, iron supplementation in certain patients, and use of dopaminergic agents such as ropinirole, pramipexole, and other medications, depending on severity of the condition, comorbidities, and other factors.20
Alcohol or substance use. Substance use or withdrawal can be associated with sleep disorders, such as hypersomnia,19 insomnia,19 and related EDS.5 For example, alcohol use disorder affects REM sleep, and can cause EDS. Secondary central apnea can be the result of long-standing opioid use19 and can present like EDS.
Insomnia. Primary insomnia rarely causes EDS.5 Insomnia due to a medical or psychiatric condition may be an indirect cause of EDS by causing sleep deprivation.
Steps for timely and accurate diagnosis
Utilize the following steps for facilitating timely diagnosis and treatment of EDS:
Thorough history. Patients often describe “tiredness” instead of sleepiness.8 Therefore, the astute psychiatrist should explore further when patients are presenting with this concern, especially by asking more specific questions such as the tendency to doze off during daytime.8
Family members can be vital sources for obtaining a complete history,5 especially because patients might deny,8 minimize, or not be fully aware1 of the extent of their symptoms. Asking family members about patient’s snoring, irregular breathing, or gasping at night can be particularly valuable.5 Obtaining a family history of sleep disorders can be particularly important, especially in conditions such as OSA and narcolepsy.
Asking about any history of safety issues,8 including sleepiness during driving, cooking, or other activities, is also important.
Use of scales and other screening measures. Psychiatrists can use initial screening measures in the office setting. Epworth Sleepiness Scale15,21 is a validated,2 short, self-administered measure to assess the level of daytime sleepiness; however, it has some limitations such as not being able to measure changes in sleepiness from hour to hour or day to day. Because of its limitations, the Epworth Sleepiness Scale should not be used by itself as a diagnostic tool.3 It has been commonly used for detecting OSA2 and narcolepsy. The Stanford Sleepiness Scale is a self-rating scale that measures the subjective degree of sleepiness and alertness; it has limitations as well, such as having little correlation with chronic sleep loss.8 Other tools such as visual analogue scales also could be helpful.8 For more specialized testing, such as Multiple Sleep Latency Test or polysomnography, referral to a sleep specialist is ideal.8
Education. The assessment is an opportunity for the psychiatrist to educate patients about sleep hygiene, the importance of regular bedtimes, and getting adequate sleep to avoid accumulating a sleep deficit.
Urgent referral of at-risk populations. Prompt or urgent referral of at-risk populations, such as geriatric patients or those with a history of dozing off during driving, is invaluable in preventing morbidity and mortality from untreated sleep disorders.
Patients with severe daytime sleepiness should be advised to not drive or operate heavy machinery until this condition is adequately controlled.18
Bottom Line
1. Chervin RD. Approach to the patient with excessive daytime sleepiness. http://www.uptodate.com/contents/approach-to-the-patient-with-excessive-daytime-sleepiness. Updated January 2016. Accessed June 5, 2017.
2. Lee CH, Ng WY, Hau W, et al. Excessive daytime sleepiness is associated with longer culprit lesion and adverse outcomes in patients with coronary artery disease. J Clin Sleep Med. 2013;9(12):1267-1272.
3. Hawley CJ, Gale TM, Sivakumaran T, et al. Excessive daytime sleepiness in psychiatric disorders: prevalence, correlates and clinical significance. Psychiatry Res. 2010;175(1-2):138-141.
4. Pigeon WR, Sateia MJ, Ferguson RJ. Distinguishing between excessive daytime sleepiness and fatigue: toward improved detection and treatment. J Psychosom Res. 2003;54(1):61-69.
5. Krahn LE. Excessive daytime sleepiness: diagnosing the causes. Current Psychiatry. 2002;1(1):49-57.
6. Ejaz SM, Khawaja IS, Bhatia S, et al. Obstructive sleep apnea and depression: a review. Innov Clin Neurosci. 2011;8(8):17-25.
7. Pagel JF. Excessive daytime sleepiness. Am Fam Physician. 2009;79(5):391-396.
8. Guilleminault C, Brooks SN. Excessive daytime sleepiness: a challenge for the practising neurologist. Brain. 2001;124(pt 8):1482-1491.
9. Cheng P, Casement M, Chen CF, et al. Sleep disordered breathing in major depressive disorder. J Sleep Res. 2013;22(4):459-462.
10. Schröder CM, O’Hara R. Depression and obstructive sleep apnea (OSA). Ann Gen Psychiatry. 2005;4:13.
11. Bardwell WA, Berry CC, Ancoli-Israel S, et al. Psychological correlates of sleep apnea. J Psychosom Res. 1999;47(6):583-596.
12. Chung F, Abdullah HR, Liao P. STOP-Bang Questionnaire: a practical approach to screen for obstructive sleep apnea. Chest. 2016;149(3):631-638.
13. Chellappa SL, Araújo JF. Excessive daytime sleepiness in patients with depressive disorder. Rev Bras Psiquiatr. 2006;28(2):126-129.
14. Habukawa M, Uchimura N, Kakuma T, et al. Effect of CPAP treatment on residual depressive symptoms in patients with major depression and coexisting sleep apnea: contribution of daytime sleepiness to residual depressive symptoms. Sleep Med. 2010;11(6):552-557.
15. Lundt L. Use of the Epworth Sleepiness Scale to evaluate the symptom of excessive sleepiness in major depressive disorder. Gen Hosp Psychiatry. 2005;27(2):146-148.
16. Hawley CJ. Excessive daytime sleepiness in psychiatry: a relevant focus for clinical attention and treatment? Int J Psychiatry Clin Pract. 2006;10(2):117-123.
17. Dodson ER, Zee PC. Therapeutics for circadian rhythm sleep disorders. Sleep Med Clin. 2010;5(4):701-715.
18. Morgenthaler TI, Kapur VK, Brown TM, et al; Standards of Practice Committee of the American Academy of Sleep Medicine. Practice parameters for the treatment of narcolepsy and other hypersomnias of central origin. Sleep. 2007;30(12):1705-1711.
19. Thorpy MJ. Classification of sleep disorders. Neurotherapeutics. 2012;9(4):687-701.
20. National Institute of Neurological Disorders and Stroke. Restless legs syndrome information page. https://www.ninds.nih.gov/Disorders/All-Disorders/Restless-Legs-Syndrome-Information-Page. Accessed June 2, 2017.
21. Johns MW. Reliability and factor analysis of the Epworth Sleepiness Scale. Sleep. 1992;15(4):376-381.
1. Chervin RD. Approach to the patient with excessive daytime sleepiness. http://www.uptodate.com/contents/approach-to-the-patient-with-excessive-daytime-sleepiness. Updated January 2016. Accessed June 5, 2017.
2. Lee CH, Ng WY, Hau W, et al. Excessive daytime sleepiness is associated with longer culprit lesion and adverse outcomes in patients with coronary artery disease. J Clin Sleep Med. 2013;9(12):1267-1272.
3. Hawley CJ, Gale TM, Sivakumaran T, et al. Excessive daytime sleepiness in psychiatric disorders: prevalence, correlates and clinical significance. Psychiatry Res. 2010;175(1-2):138-141.
4. Pigeon WR, Sateia MJ, Ferguson RJ. Distinguishing between excessive daytime sleepiness and fatigue: toward improved detection and treatment. J Psychosom Res. 2003;54(1):61-69.
5. Krahn LE. Excessive daytime sleepiness: diagnosing the causes. Current Psychiatry. 2002;1(1):49-57.
6. Ejaz SM, Khawaja IS, Bhatia S, et al. Obstructive sleep apnea and depression: a review. Innov Clin Neurosci. 2011;8(8):17-25.
7. Pagel JF. Excessive daytime sleepiness. Am Fam Physician. 2009;79(5):391-396.
8. Guilleminault C, Brooks SN. Excessive daytime sleepiness: a challenge for the practising neurologist. Brain. 2001;124(pt 8):1482-1491.
9. Cheng P, Casement M, Chen CF, et al. Sleep disordered breathing in major depressive disorder. J Sleep Res. 2013;22(4):459-462.
10. Schröder CM, O’Hara R. Depression and obstructive sleep apnea (OSA). Ann Gen Psychiatry. 2005;4:13.
11. Bardwell WA, Berry CC, Ancoli-Israel S, et al. Psychological correlates of sleep apnea. J Psychosom Res. 1999;47(6):583-596.
12. Chung F, Abdullah HR, Liao P. STOP-Bang Questionnaire: a practical approach to screen for obstructive sleep apnea. Chest. 2016;149(3):631-638.
13. Chellappa SL, Araújo JF. Excessive daytime sleepiness in patients with depressive disorder. Rev Bras Psiquiatr. 2006;28(2):126-129.
14. Habukawa M, Uchimura N, Kakuma T, et al. Effect of CPAP treatment on residual depressive symptoms in patients with major depression and coexisting sleep apnea: contribution of daytime sleepiness to residual depressive symptoms. Sleep Med. 2010;11(6):552-557.
15. Lundt L. Use of the Epworth Sleepiness Scale to evaluate the symptom of excessive sleepiness in major depressive disorder. Gen Hosp Psychiatry. 2005;27(2):146-148.
16. Hawley CJ. Excessive daytime sleepiness in psychiatry: a relevant focus for clinical attention and treatment? Int J Psychiatry Clin Pract. 2006;10(2):117-123.
17. Dodson ER, Zee PC. Therapeutics for circadian rhythm sleep disorders. Sleep Med Clin. 2010;5(4):701-715.
18. Morgenthaler TI, Kapur VK, Brown TM, et al; Standards of Practice Committee of the American Academy of Sleep Medicine. Practice parameters for the treatment of narcolepsy and other hypersomnias of central origin. Sleep. 2007;30(12):1705-1711.
19. Thorpy MJ. Classification of sleep disorders. Neurotherapeutics. 2012;9(4):687-701.
20. National Institute of Neurological Disorders and Stroke. Restless legs syndrome information page. https://www.ninds.nih.gov/Disorders/All-Disorders/Restless-Legs-Syndrome-Information-Page. Accessed June 2, 2017.
21. Johns MW. Reliability and factor analysis of the Epworth Sleepiness Scale. Sleep. 1992;15(4):376-381.