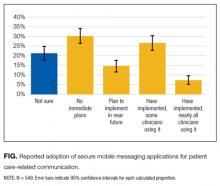
User login
Adjuvant Chemotherapy in the Treatment of Colon Cancer
INTRODUCTION
Colorectal cancer (CRC) is one of the most prevalent malignancies and is the fourth most common cancer in the United States, with an estimated 133,490 new cases diagnosed in 2016. Of these, approximately 95,520 are located in the colon and 39,970 are in the rectum.1 CRC is the third leading cause of cancer death in women and the second leading cause of cancer death in men, with an estimated 49,190 total deaths in 2016.2 The incidence appears to be increasing,3 especially in patients younger than 55 years of age;4 the reason for this increase remains uncertain.
A number of risk factors for the development of CRC have been identified. Numerous hered-itary CRC syndromes have been described, including familial adenomatous polyposis,5 hereditary non-polyposis colorectal cancer (HNPCC) or Lynch syndrome,6 and MUTYH-associated polyposis.7,8 A family history of CRC doubles the risk of developing CRC,9 and current guidelines support lowering the age of screening in individuals with a family history of CRC to 10 years younger than the age of diagnosis of the family member or 40 years of age, whichever is lower.10 Patients with a personal history of adenomatous polyps are at increased risk for developing CRC, as are patients with a personal history of CRC, with a relative risk ranging from 3 to 6.11 Ulcerative colitis and Crohn’s disease are associated with the development of CRC and also influence screening, though evidence suggests good control of these diseases may mitigate risk.12 Finally, modifiable risk factors for the development of CRC include high red meat consumption,13 diets low in fiber,14 obesity,13 smoking, alcohol use,15 and physical inactivity16; lifestyle modification targeting these factors has been shown to decrease rates of CRC.17 The majority of colon cancers present with clinical symptoms, often with rectal bleeding, abdominal pain, change in bowel habits, or obstructive symptoms. More rarely, these tumors are detected during screening colonoscopy, in which case they tend to be at an early stage.
SURGICAL MANAGEMENT
A critical goal in the resection of early-stage colon cancer is attaining R0 resection. Patients who achieve R0 resection as compared to R1 (microscopic residual tumor) and R2 (macroscopic residual tumor)18 have significantly improved long-term overall survival.19 Traditionally, open resection of the involved colonic segment was employed, with end-end anastomosis of the uninvolved free margins. Laparoscopic resection for early-stage disease has been utilized in attempts to decrease morbidity of open procedures, with similar outcomes and node sampling.20 Laparoscopic resection appears to provide similar outcomes even in locally advanced disease.21 Right-sided lesions are treated with right colectomy and primary ileocolic anastomosis.22 For patients presenting with obstructing masses, the Hartmann procedure is the most commonly performed operation. This involves creation of an ostomy with subtotal colectomy and subsequent ostomy reversal in a 2- or 3-stage protocol.23 Patients with locally advanced disease and invasion into surrounding structures require multivisceral resection, which involves resection en bloc with secondarily involved organs.24 Intestinal perforation presents a unique challenge and is associated with surgical complications, infection, and lower overall survival (OS) and 5-year disease-free survival (DFS). Complete mesocolic excision is a newer technique that has been performed with reports of better oncologic outcome at some centers; however, this approach is not currently considered standard of care.25
STAGING
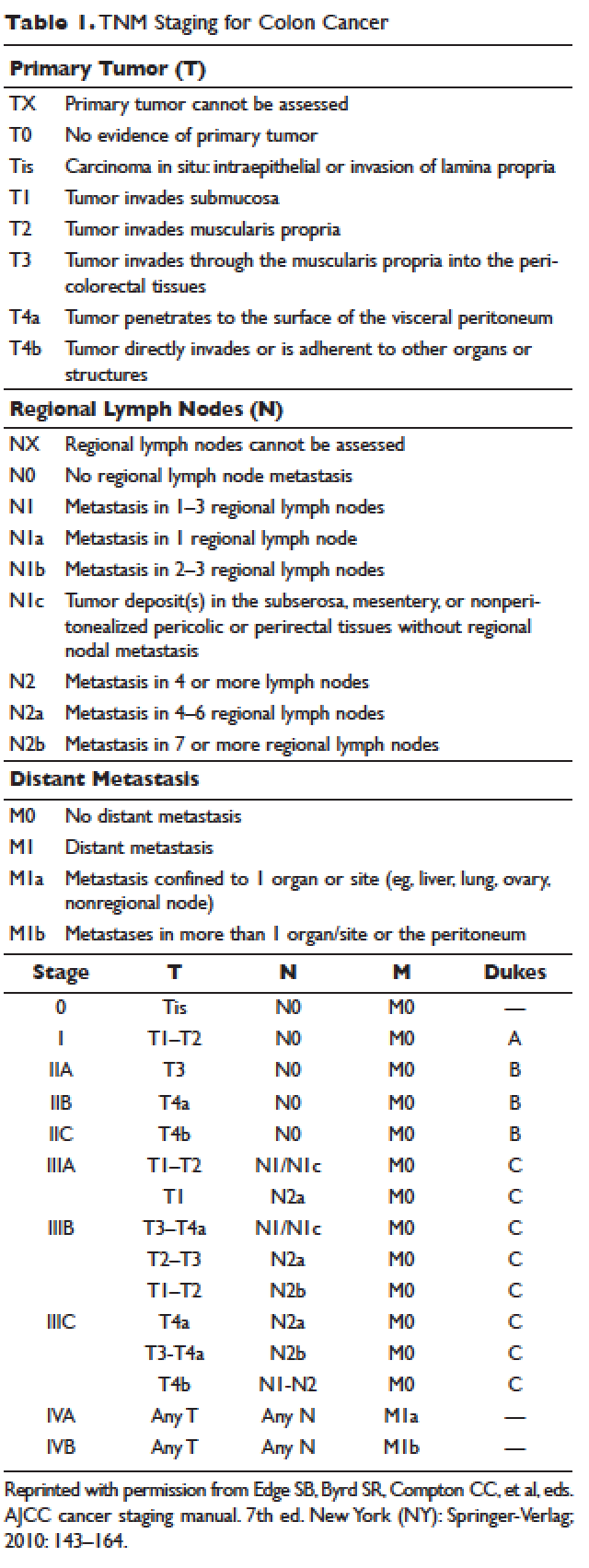
According to a report by the National Cancer Institute, the estimated 5-year relative survival rates for localized colon cancer (lymph node negative), regional (lymph node positive) disease, and distant (metastatic) disease are 89.9%, 71.3%, and 13.9%, respectively.1 However, efforts have been made to further classify patients into distinct categories to allow fine-tuning of prognostication. In the current system, staging of colon cancer utilizes the American Joint Committee on Cancer tumor/node/metastasis (TNM) system.20 Clinical and pathologic features include depth of invasion, local invasion of other organs, nodal involvement, and presence of distant metastasis (Table 1). Studies completed prior to the adoption of the TNM system used the Dukes criteria, which divided colon cancer into A, B, and C, corresponding to TNM stage I, stage IIA–IIC, and stage IIIA-IIIC. This classification is rarely used in more contemporary studies.
APPROACH TO ADJUVANT CHEMOTHERAPY
Adjuvant chemotherapy seeks to eliminate micrometastatic disease present following curative surgical resection. When stage 0 cancer is discovered incidentally during colonoscopy, endoscopic resection alone is the management of choice, as presence of micrometastatic disease is exceedingly unlikely.26 Stage I–III CRCs are treated with surgical resection withcurative intent. The 5-year survival rate for stage I and early-stage II CRC is estimated at 97% with surgery alone.27,28 The survival rate drops to about 60% for high-risk stage II tumors (T4aN0), and down to 50% or less for stage II-T4N0 or stage III cancers. Adjuvant chemotherapy is generally recommended to further decrease the rates of distant recurrence in certain cases of stage II and in all stage III tumors.
DETERMINATION OF BENEFIT FROM CHEMOTHERAPY: PROGNOSTIC MARKERS
Prior to administration of adjuvant chemotherapy, a clinical evaluation by the medical oncologist to determine appropriateness and safety of treatment is paramount. Poor performance status and comorbid conditions may indicate risk for excessive toxicity and minimal benefit from chemotherapy. CRC commonly presents in older individuals, with the median age at diagnosis of 69 years for men and 73 years for women.29 In this patient population, comorbidities such as cardiovascular disease, diabetes, and renal dysfunction are more prevalent.30 Decisions regarding adjuvant chemotherapy in this patient population have to take into consideration the fact that older patients may experience higher rates of toxicity with chemotherapy, including gastrointestinal toxicities and marrow suppression.31 Though some reports indicate patients older than 70 years derive similar benefit from adjuvant chemotherapy,32,33 a large pooled analysis of the ACCENT database, which included 7 adjuvant therapy trials and 14,528 patients, suggested limited benefit from the addition of oxaliplatin to fluorouracil in elderly patients.32 Other factors that weigh on the decision include stage, pathology, and presence of high-risk features. A common concern in the postoperative setting is delaying initiation of chemotherapy to allow adequate wound healing; however, evidence suggests that delays longer than 8 weeks leads to worse overall survival, with hazard ratios (HR) ranging from 1.4 to 1.7.34,35 Thus, the start of adjuvant therapy should ideally be within this time frame.
HIGH-RISK FEATURES
Multiple factors have been found to predict worse outcome and are classified as high-risk features (Table 2). Histologically, high-grade or poorly differentiated tumors are associated with higher recurrence rate and worse outcome.36 Certain histological subtypes, including mucinous and signet-ring, both appear to have more aggressive biology.37 Presence of microscopic invasion into surrounding blood vessels (vascular invasion) and nerves (perineural invasion) is associated with lower survival.38 Penetration of the cancer through the visceral peritoneum (T4a) or into surrounding structures (T4b) is associated with lower survival.36 During surgical resection, multiple lymph nodes are removed along with the primary tumor to evaluate for metastasis to the regional nodes. Multiple analyses have demonstrated that removal and pathologic assessment of fewer than 12 lymph nodes is associated with high risk of missing a positive node, and is thus equated with high risk.39–41 In addition, extension of tumor beyond the capsules of any single lymph node, termed extracapsular extension, is associated with an increased risk of all-cause mortality.42 Tumor deposits, or focal aggregates of adenocarcinoma in the pericolic fat that are not contiguous with the primary tumor and are not associated with lymph nodes, are currently classified as lymph nodes as N1c in the current TNM staging system. Presence of these deposits has been found to predict poor outcome stage for stage.43 Obstruction and/or perforation secondary to the tumor are also considered high-risk features that predict poor outcome.

SIDEDNESS
As reported at the 2016 American Society of Clinical Oncology annual meeting, tumor location predicts outcome in the metastatic setting. A report by Venook and colleagues based on a post-hoc analysis found that in the metastatic setting, location of the tumor primary in the left side is associated with longer OS (33.3 months) when compared to the right side of the colon (19.4 months).44 A retrospective analysis of multiple databases presented by Schrag and colleagues similarly reported inferior outcomes in patients with stage III and IV disease who had right-sided primary tumors.45 However, the prognostic implications for stage II disease remain uncertain.
BIOMARKERS
Given the controversy regarding adjuvant therapy of patients with stage II colon cancer, multiple biomarkers have been evaluated as possible predictive markers that can assist in this decision. The mismatch repair (MMR) system is a complex cellular enzymatic mechanism that identifies and corrects DNA errors during cell division and prevents mutagenesis.46 The familial cancer syndrome HNPCC is linked to alteration in a variety of MMR genes, leading to deficient mismatch repair (dMMR), also termed microsatellite instability-high (MSI-high).47,48 Epigenetic modification can also lead to silencing of the same implicated genes and accounts for 15% to 20% of sporadic colorectal cancer.49 These epigenetic modifications lead to hypermethylation of the promotor region of MLH1 in 70% of cases.50 The 4 MMR genes most commonly tested are MLH-1, MSH2, MSH6, and PMS2. Testing can be performed by immunohistochemistry or polymerase chain reaction.51 Across tumor histology and stage, MSI status is prognostic. Patients with MSI-high tumors have been shown to have improved prognosis and longer OS both in stage II and III disease52–54 and in the metastatic setting.55 However, despite this survival benefit, there is conflicting data as to whether patients with stage II, MSI-high colon cancer may benefit less from adjuvant chemotherapy. One early retrospective study compared outcomes of 70 patients with stage II and III disease and dMMR to those of 387 patients with stage II and III disease and proficient mismatch repair (pMMR). Adjuvant fluorouracil with leucovorin improved DFS for patients with pMMR (HR 0.67) but not for those with dMMR (HR 1.10). In addition, for patients with stage II disease and dMMR, the HR for OS was inferior at 2.95.56 Data collected from randomized clinical trials using fluorouracil-based adjuvant chemotherapy were analyzed in an attempt to predict benefit based on MSI status. Benefit was only seen in pMMR patients, with a HR of 0.72; this was not seen in the dMMR patients.57 Subsequent studies have had different findings and did not demonstrate a detrimental effect of fluorouracil in dMMR.58,59 For stage III patients, MSI status does not appear to affect benefit from chemotherapy, as analysis of data from the NSABP C-07 trial (Table 3) demonstrated benefit of FOLFOX (leucovorin, fluorouracil, oxaliplatin) in patients with dMMR status and stage III disease.59
Another genetic abnormality identified in colon cancers is chromosome 18q loss of heterozygosity (LOH). The presence of 18q LOH appears to be inversely associated with MSI-high status. Some reports have linked presence of 18q with worse outcome,60 but others question this, arguing the finding may simply be related to MSI status.61,62 This biomarker has not been established as a clear prognostic marker that can aid clinical decisions.
Most recently, expression of caudal-type homeobox transcription factor 2 (CDX2) has been reported as a novel prognostic and predictive tool. A 2015 report linked lack of expression of CDX2 to worse outcome; in this study, 5-year DFS was 41% in patients with CDX2-negative tumors versus 74% in the CDX2-positive tumors, with a HR of disease recurrence of 2.73 for CDX2-negative tumors.63 Similar numbers were observed in patients with stage II disease, with 5-year OS of 40% in patients with CDX2-negative tumors versus 70% in those with CDX2-positive tumors. Treatment of CDX2-negative patients with adjuvant chemotherapy improved outcomes: 5-year DFS in the stage II subgroup was 91% with chemotherapy versus 56% without, and in the stage III subgroup, 74% with chemotherapy versus 37% without. The authors concluded that patients with stage II and III colon cancer that is CDX2-negative may benefit from adjuvant chemotherapy. Importantly, CDX2-negativity is a rare event, occurring in only 6.9% of evaluable tumors.
RISK ASSESSMENT TOOLS
Several risk assessment tools have been developed in an attempt to aid clinical decision making regarding adjuvant chemotherapy for patients with stage II colon cancer. The Oncotype DX Colon Assay analyses a 12-gene signature in the pathologic sample and was developed with the goal to improve prognostication and aid in treatment decision making. The test utilizes reverse transcription-PCR on RNA extracted from the tumor.64 After evaluating 12 genes, a recurrence score is generated that predicts the risk of disease recurrence. This score was validated using data from 3 large clinical trials.65–67 Unlike the Oncotype Dx score used in breast cancer, the test in colon cancer has not been found to predict the benefit from chemotherapy and has not been incorporated widely into clinical practice.
Adjuvant! Online (available at www.adjuvantonline.com) is a web-based tool that combines clinical and histological features to estimate outcome. Calculations are based on US SEER tumor registry-reported outcomes.68 A second web-based tool, Numeracy (available at www.mayoclinic.com/calcs), was developed by the Mayo Clinic using pooled data from 7 randomized clinical trials including 3341 patients.68 Both tools seek to predict absolute benefit for patients treated with fluorouracil, though data suggests Adjuvant! Online may be more reliable in its predictive ability.69 Adjuvant! Online has also been validated in an Asian population70 and patients older than 70 years.71
MUTATIONAL ANALYSIS
Multiple mutations in proto-oncogenes have been found in colon cancer cells. One such proto-oncogene is BRAF, which encodes a serine-threonine kinase in the rapidly accelerated fibrosarcoma (RAF). Mutations in BRAF have been found in 5% to 10% of colon cancers and are associated with right-sided tumors.72 As a prognostic marker, some studies have associated BRAF mutations with worse prognosis, including shorter time to relapse and shorter OS.73,74 Two other proto-oncogenes are Kristen rat sarcoma viral oncogene homolog (KRAS) and neuroblastoma rat sarcoma viral oncogene homolog (NRAS), both of which encode proteins downstream of epidermal growth factor receptor (EGFR). KRAS and NRAS mutations have been shown to be predictive in the metastatic setting where they predict resistance to the EGFR inhibitors cetuximab and panitumumab.75,76 The effect of KRAS and NRAS mutations on outcome in stage II and III colon cancer is uncertain. Some studies suggest worse outcome in KRAS-mutated cancers,77 while others failed to demonstrate this finding.73
CASE PRESENTATION 1
A 53-year-old man with no past medical history presents to the emergency department with early satiety and generalized abdominal pain. Laboratory evaluation shows a microcytic anemia with normal white blood cell count, platelet count, renal function, and liver function tests. Computed tomography (CT) scan of the abdomen and pelvis show a 4-cm mass in the transverse colon without obstruction and without abnormality in the liver. CT scan of the chest does not demonstrate pathologic lymphadenopathy or other findings. He undergoes robotic laparoscopic transverse colon resection and appendectomy. Pathology confirms a 3.5-cm focus of adenocarcinoma of the colon with invasion through the muscularis propria and 5 of 27 regional lymph nodes positive for adenocarcinoma and uninvolved proximal, distal, and radial margins. He is given a stage of IIIB pT3 pN2a M0 and referred to medical oncology for further management, where 6 months of adjuvant FOLFOX chemotherapy is recommended.
ADJUVANT CHEMOTHERAPY IN STAGE III COLON CANCER
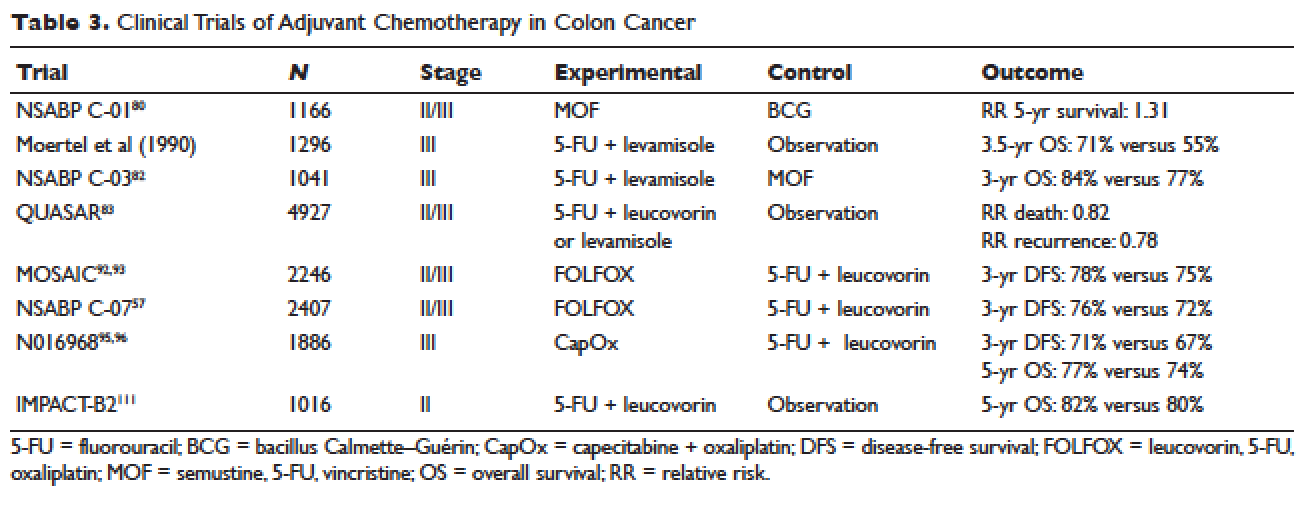
Postoperative adjuvant chemotherapy is the standard of care for patients with stage III disease. In the 1960s, infusional fluorouracil was first used to treat inoperable colon cancer.78,79 After encouraging results, the agent was used both intraluminally and intravenously as an adjuvant therapy for patients undergoing resection with curative intent; however, only modest benefits were described.80,81 The National Surgical Adjuvant Breast and Bowel Project (NSABP) C-01 trial (Table 3) was the first study to demonstrate a benefit from adjuvant chemotherapy in colon cancer. This study randomly assigned patients with stage II and III colon cancer to surgery alone, postoperative chemotherapy with fluorouracil, semustine, and vincristine (MOF), or postoperative bacillus Calmette-Guérin (BCG). DFS and OS were significantly improved with MOF chemotherapy.82 In 1990, a landmark study reported on outcomes after treatment of 1296 patients with stage III colon cancer with adjuvant fluorouracil and levamisole for 12 months. The combination was associated with a 41% reduction in risk of cancer recurrence and a 33% reduction in risk of death.83 The NSABP C-03 trial (Table 3) compared MOF to the combination of fluorouracil and leucovorin and demonstrated improved 3-year DFS (69% versus 73%) and 3-year OS (77% versus 84%) in patients with stage III disease.84 Building on these outcomes, the QUASAR study (Table 3) compared fluorouracil in combination with one of levamisole, low-dose leucovorin, or high-dose leucovorin. The study enrolled 4927 patients and found worse outcomes with fluorouracil plus levamisole and no difference in low-doseversus high-dose leucovorin.85 Levamisole fell out of use after associations with development of multifocal leukoencephalopathy,86 and was later shown to have inferior outcomes versus leucovorin when combined with fluorouracil.87,88 Intravenous fluorouracil has shown similar benefit when administered by bolus or infusion,89 although continuous infusion has been associated with lower incidence of severe toxicity.90 The efficacy of the oral fluoropyrimidine capecitabine has been shown to be equivalent to that of fluorouracil.91
Fluorouracil-based treatment remained the standard of care until the introduction of oxaliplatin in the mid-1990s. After encouraging results in the metastatic setting,92,93 the agent was moved to the adjuvant setting. The MOSAIC trial (Table 3) randomly assigned patients with stage II and III colon cancer to fluorouracil with leucovorin (FULV) versus FOLFOX given once every 2 weeks for 12 cycles. Analysis with respect to stage III patients showed a clear survival benefit, with a 10-year OS of 67.1% with FOLFOX chemotherapy versus 59% with fluorouracil and leucovorin.94,95 The NSABP C-07 (Table 3) trial used a similar trial design but employed bolus fluorouracil. More than 2400 patients with stage II and III colon cancer were randomly assigned to bolus FULV or bolus fluorouracil, leucovorin, and oxaliplatin (FLOX). The addition of oxaliplatin significantly improved outcomes, with 4-year DFS of 67% versus 71.8% for FULV and FLOX, respectively, and a HR of death of 0.80 with FLOX.59,96 The multicenter N016968 trial (Table 3) randomly assigned 1886 patients with stage III colon cancer to adjuvant capecitabine plus oxaliplatin (XELOX) or bolus fluorouracil plus leucovorin (FU/FA). The 3-year DFS was 70.9% versus 66.5% with XELOX and FU/FA, respectively, and 5-year OS was 77.6% versus 74.2%, respectively.97,98
In the metastatic setting, additional agents have shown efficacy, including irinotecan,99,100 bevacizumab,101,102 cetuximab,103,104 and regorafenib.105 This observation led to testing of these agents in earlier stage disease. The CALGB 89803 trial compared fluorouracil, leucovorin, and irinotecan to fluorouracil with leucovorin alone. No benefit in 5-year DFS or OS was seen.106 Similarly, infusional fluorouracil, leucovorin, and irinotecan (FOLFIRI) was not found to improve 5-year DFS as compared to fluorouracil with leucovorin alone in the PETACC-3 trial.107 The NSABP C-08 trial considered the addition of bevacizumab to FOLFOX. When compared to FOLFOX alone, the combination of bevacizumab to FOLFOX had similar 3-year DFS (77.9% versus 75.1%) and 5-year OS (82.5% versus 80.7%).108 This finding was confirmed in the Avant trial.109 The addition of cetuximab to FOLFOX was equally disappointing, as shown in the N0147 trial110 and PETACC-8 trial.111 Data on regorafenib in the adjuvant setting for stage III colon cancer is lacking; however, 2 ongoing clinical trials, NCT02425683 and NCT02664077, are each studying the use of regorafenib following completion of FOLFOX for patients with stage III disease.
Thus, after multiple trials comparing various regimens and despite attempts to improve outcomes by the addition of a third agent, the standard of care per National Comprehensive Cancer Network (NCCN) guidelines for management of stage III colon cancer remains 12 cycles of FOLFOX chemotherapy. Therapy should be initiated within 8 weeks of surgery. Data are emerging to support a short duration of therapy for patients with low-risk stage III tumors, as shown in an abstract presented at the 2017 American Society of Clinical Oncology annual meeting. The IDEA trial was a pooled analysis of 6 randomized clinical trials across multiple countries, all of which evaluated 3 versus 6 months of FOLFOX or capecitabine and oxaliplatin in the treatment of stage III colon cancer. The analysis was designed to test non-inferiority of 3 months of therapy as compared to 6 months. The analysis included 6088 patients across 244 centers in 6 countries. The overall analysis failed to establish noninferiority. The 3-year DFS rate was 74.6% for 3 months and 75.5% for 6 months, with a DFS HR of 1.07 and a confidence interval that did not meet the prespecified endpoint. Subgroup analysis suggested noninferiority for lower stage disease (T1–3 or N1) but not for higher stage disease (T4 or N2). Given the high rates of neuropathy with 6 months of oxaliplatin, these results suggest that 3 months of adjuvant therapy can be considered for patients with T1–3 or N1 disease in an attempt to limit toxicity.112
CASE PRESENTATION 2
A 57-year-old woman presents to the emergency department with fever and abdominal pain. CT of the abdomen and pelvis demonstrates a left-sided colonic mass with surrounding fat stranding and pelvic abscess. She is taken emergently for left hemicolectomy, cholecystectomy, and evacuation of pelvic abscess. Pathology reveals a 5-cm adenocarcinoma with invasion through the visceral peritoneum; 0/22 lymph nodes are involved. She is given a diagnosis of stage IIC and referred to medical oncology for further management. Due to her young age and presence of high-risk features, she is recommended adjuvant therapy with FOLFOX for 6 months.
ADJUVANT CHEMOTHERAPY IN STAGE II COLON CANCER
Because of excellent outcomes with surgical resection alone for stage II cancers, the use of adjuvant chemotherapy for patients with stage II disease is controversial. Limited prospective data is available to guide adjuvant treatment decisions for stage II patients. The QUASAR trial, which compared observation to adjuvant fluorouracil and leucovorin in patients with early-stage colon cancer, included 2963 patients with stage II disease and found a relative risk (RR) of death or recurrence of 0.82 and 0.78, respectively. Importantly, the absolute benefit of therapy was less than 5%.113 The IMPACT-B2 trial (Table 3) combined data from 5 separate trials and analyzed 1016 patients with stage II colon cancer who received fluorouracil with leucovorin or observation. Event-free survival was 0.86 versus 0.83 and 5-year OS was 82% versus 80%, suggesting no benefit.114 The benefit of addition of oxaliplatin to fluorouracil in stage II disease appears to be less than the benefit of adding this agent in the treatment of stage III CRC. As noted above, the MOSAIC trial randomly assigned patients with stage II and III colon cancer to receive adjuvant fluorouracil and leucovorin with or without oxaliplatin for 12 cycles. After a median follow-up of 9.5 years, 10-year OS rates for patients with stage II disease were 78.4% versus 79.5%. For patients with high-risk stage II disease (defined as T4, bowel perforation, or fewer than 10 lymph nodes examined), 10-year OS was 71.7% and 75.4% respectively, but these differences were not statistically significant.94
Because of conflicting data as to the benefit of adding oxaliplatin in stage II disease, oxaliplatin is not recommended for standard-risk stage II patients. The use of oxaliplatin in high-risk stage II tumors should be weighed carefully given the toxicity risk. Oxaliplatin is recognized to cause sensory neuropathy in many patients, which can become painful and debilitating.115 Two types of neuropathy are associated with oxaliplatin: acute and chronic. Acute neuropathy manifests most often as cold-induced paresthesias in the fingers and toes and is quite common, affecting up to 90% of patients. These symptoms are self-limited and resolve usually within 1 week of each treatment.116 Some patients, with reports ranging from 10% to 79%, develop chronic neuropathy that persists for 1 year or more and causes significant decrements in quality of life.117 Patients older than age 70 may be at greater risk for oxaliplatin-induced neuropathy, which would increase risk of falls in this population.118 In addition to neuropathy, oxaliplatin is associated with hypersensitivity reactions that can be severe and even fatal.119 In a single institution series, the incidence of severe reactions was 2%.120 Desensitization following hypersensitivity reactions is possible but requires a time-intensive protocol.121
Based on the inconclusive efficacy findings and due to concerns over toxicity, each decision must be individualized to fit patient characteristics and preferences. In general, for patients with stage II disease without high-risk features, an individualized discussion should be held as to the risks and benefits of single-agent fluorouracil, and this treatment should be offered in cases where the patient or provider would like to be aggressive. Patients with stage II cancer who have 1 or more high-risk features are often recommended adjuvant chemotherapy. Whether treatment with fluorouracil plus leucovorin or FOLFOX is preferred remains uncertain, and thus the risks and the potential gains of oxaliplatin must be discussed with the individual patient. MMR status can also influence the treatment recommendation for patients with stage II disease. In general, patients with standard-risk stage II tumors that are pMMR are offered MMR with leucovorin or oral capecitabine for 12 cycles. FOLFOX is considered for patients with MSI-high disease and those with multiple high-risk features.
MONITORING AFTER THERAPY
After completion of adjuvant chemotherapy, patients enter a period of survivorship. Patients are seen in clinic for symptom and laboratory monitoring of the complete blood count, liver function tests, and carcinoembryonic antigen (CEA). NCCN guidelines support history and physical examination with CEA testing every 3 to 6 months for the first 2 years, then every 6 months for the next 3 years, after which many patients continue to be seen annually. CT imaging of the chest, abdomen, and pelvis for monitoring of disease recurrence is recommended every 6 to 12 months for a total of 5 years. New elevations in CEA or liver function tests should prompt early imaging. Colonoscopy should be performed 1 year after completion of therapy; however, if no preoperative colonoscopy was performed, this should be done 3 to 6 months after completion. Colonoscopy is then repeated in 3 years and then every 5 years unless advanced adenomas are present.122
SUMMARY
The addition of chemotherapy to surgical management of colon cancer has lowered the rate of disease recurrence and improved long-term survival. Adjuvant FOLFOX for 12 cycles is the standard of care for patients with stage III colon cancer and for patients with stage II disease with certain high-risk features. Use of adjuvant chemotherapy in stage II disease without high-risk features is controversial, and treatment decisions should be individualized. Biologic markers such as MSI and CDX2 status as well as patient-related factors including age, overall health, and personal preferences can inform treatment decisions. If chemotherapy is recommended in this setting, it would be with single-agent fluorouracil in an infusional or oral formulation, unless the tumor has the MSI-high feature. Following completion of adjuvant therapy, patients should be followed with clinical evaluation, laboratory testing, and imaging for a total of 5 years as per recommended guidelines.
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA Cancer J Clin 2017;67(1):7–30.
- United States Cancer Statistics. 1999–2013 incidence and mortality web-based report. Atlanta: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention and National Cancer Institute, 2016. www.cdc.gov/uscs. Accessed June 12, 2017.
- Ahnen DJ, Wade SW, Jones WF, et al. The increasing incidence of young-onset colorectal cancer: a call to action. Mayo Clin Proc 2014;89:216–24.
- Jemal A, Fedewa SA, Anderson WF, et al. Colorectal cancer incidence patterns in the United States, 1974–2013. J Natl Cancer Inst 2017;109(8).
- Boursi B, Sella T, Liberman E, et al. The APC p.I1307K polymorphism is a significant risk factor for CRC in average risk Ashkenazi Jews. Eur J Cancer 2013;49:3680–5.
- Parry S, Win AK, Parry B, et al. Metachronous colorectal cancer risk for mismatch repair gene mutation carriers: the advantage of more extensive colon surgery. Gut 2011;60: 950–7.
- van Puijenbroek M, Nielsen M, Tops CM, et al. Identification of patients with (atypical) MUTYH-associated polyposis by KRAS2 c.34G > T prescreening followed by MUTYH hotspot analysis in formalin-fixed paraffin-embedded tissue. Clin Cancer Res 2008;14:139–42.
- Aretz S, Uhlhaas S, Goergens H, et al. MUTYH-associated polyposis: 70 of 71 patients with biallelic mutations present with an attenuated or atypical phenotype. Int J Cancer 2006;119:807–14.
- Tuohy TM, Rowe KG, Mineau GP, et al. Risk of colorectal cancer and adenomas in the families of patients with adenomas: a population-based study in Utah. Cancer 2014;120:35–42.
- Choi Y, Sateia HF, Peairs KS, Stewart RW. Screening for colorectal cancer. Semin Oncol 2017; 44:34–44.
- Atkin WS, Morson BC, Cuzick J. Long-term risk of colorectal cancer after excision of rectosigmoid adenomas. N Engl J Med 1992;326:658–62.
- Rutter MD. Surveillance programmes for neoplasia in colitis. J Gastroenterol 2011;46 Suppl 1:1–5.
- Giovannucci E. Modifiable risk factors for colon cancer. Gastroenterol Clin North Am 2002;31:925–43.
- Michels KB, Fuchs GS, Giovannucci E, et al. Fiber intake and incidence of colorectal cancer among 76,947 women and 47,279 men. Cancer Epidemiol Biomarkers Prev 2005;14:842–9.
- Omata F, Brown WR, Tokuda Y, et al. Modifiable risk factors for colorectal neoplasms and hyperplastic polyps. Intern Med 2009;48:123–8.
- Friedenreich CM, Neilson HK, Lynch BM. State of the epidemiological evidence on physical activity and cancer prevention. Eur J Cancer 2010;46:2593–604.
- Aleksandrova K, Pischon T, Jenab M, et al. Combined impact of healthy lifestyle factors on colorectal cancer: a large European cohort study. BMC Med 2014;12:168.
- Hermanek P, Wittekind C. The pathologist and the residual tumor (R) classification. Pathol Res Pract 1994;190:115–23.
- Lehnert T, Methner M, Pollok A, et al. Multivisceral resection for locally advanced primary colon and rectal cancer: an analysis of prognostic factors in 201 patients. Ann Surg 2002;235:217–25.
- Feinberg AE, et al. Oncologic outcomes following laparoscopic versus open resection of pT4 colon cancer: a systematic review and meta-analysis. Dis Colon Rectum 2017;60:116–125.
- Vignali A, et al. Laparoscopic treatment of advanced colonic cancer: a case-matched control with open surgery. Colorectal Dis 2013;15:944–8.
- Gainant A. Emergency management of acute colonic cancer obstruction. J Visc Surg 2012;149: e3–e10.
- Rosenman LD. Hartmann’s operation. Am J Surg 1994;168:283–4.
- Lee-Kong S, Lisle D. Surgical management of complicated colon cancer. Clin Colon Rectal Surg 2015;28:228–33.
- Bertelsen CA. Complete mesocolic excision an assessment of feasibility and outcome. Dan Med J 2017;64(2).
- Wolff WI SH. Definitive treatment of “malignant” polyps of the colon. Ann Surg 1975;182:516–25.
- Clinical Outcomes of Surgical Therapy Study Group, Nelson H, Sargent DJ, Wieand HS, et al. A comparison of laparoscopically assisted and open colectomy for colon cancer. N Engl J Med 2004;350:2050–9.
- Gunderson LL, Jessup JM, Sarjent DJ, et al. Revised tumor and node categorization for rectal cancer based on surveillance, epidemiology, and end results and rectal pooled analysis outcomes. J Clin Oncol 2010;28:256–63.
- Noone AM, Cronin KA, Altekruse SF, et al. Cancer incidence and survival trends by subtype using data from the Surveillance Epidemiology and End Results Program, 1992-2013. Cancer Epidemiol Biomarkers Prev 2017;26:632–41.
- Alves A, Panis Y, Mathieu P, et al. Postoperative mortality and morbidity in French patients undergoing colorectal surgery: results of a prospective multicenter study. Arch Surg 2005;140:278–83.
- Popescu RA, Norman A, Ross PJ, et al, Adjuvant or palliative chemotherapy for colorectal cancer in patients 70 years or older. J Clin Oncol 1999;17:2412–8.
- McCleary NJ, Meyerhardt JA, Green E, et al. Impact of age on the efficacy of newer adjuvant therapies in patients with stage II/III colon cancer: findings from the ACCENT database. J Clin Oncol 2013;31:2600–6.
- Tominaga T, Nonaka T, Sumida Y, et al. Effectiveness of adjuvant chemotherapy for elderly patients with lymph node-positive colorectal cancer. World J Surg Oncol 2016;14:197.
- Bos AC, van Erning FN, van Gestel YR, et al. Timing of adjuvant chemotherapy and its relation to survival among patients with stage III colon cancer. Eur J Cancer 2015;51:2553–61.
- Peixoto RD, Kumar A, Speers C, et al. Effect of delay in adjuvant oxaliplatin-based chemotherapy for stage III colon cancer. Clin Colorectal Cancer 2015;14:25–30.
- Compton CC, Fielding LP, Burgart LJ, et al. Prognostic factors in colorectal cancer. College of American Pathologists Consensus Statement 1999. Arch Pathol Lab Med 2000;124:979–94.
- Lieu CH, Lambert LA, Wolff RA, et al. Systemic chemotherapy and surgical cytoreduction for poorly differentiated and signet ring cell adenocarcinomas of the appendix. Ann Oncol 2012;23:652–8.
- Krasna MJ, Flancbaum L, Cody RP, et al. Vascular and neural invasion in colorectal carcinoma. Incidence and prognostic significance. Cancer 1988;61:1018–23.
- Cianchi F, Palomba A, Boddi V, et al. Lymph node recovery from colorectal tumor specimens: recommendation for a minimum number of lymph nodes to be examined. World J Surg 2002;26:384–9.
- Yoshimatsu K, et al. How many lymph nodes should be examined in Dukes’ B colorectal cancer? Determination on the basis of cumulative survival rate. Hepatogastroenterology 2005;52:1703–6.
- Caplin S, Cerottini JP, Bosman FT, et al. For patients with Dukes’ B (TNM Stage II) colorectal carcinoma, examination of six or fewer lymph nodes is related to poor prognosis. Cancer 1998;83:666–72.
- Veronese N, Nottegar A, Pea A, et al. Prognostic impact and implications of extracapsular lymph node involvement in colorectal cancer: a systematic review with meta-analysis. Ann Oncol 2016;27:42–8.
- Li J, Yang S, Hu J, et al. Tumor deposits counted as positive lymph nodes in TNM staging for advanced colorectal cancer: a retrospective multicenter study. Oncotarget 2016;7:18269–79.
- Venook A, Niedzwiecki D, Innocenti Fet al. Impact of primary (1º) tumor location on overall survival (OS) and progression-free survival (PFS) in patients (pts) with metastatic colorectal cancer (mCRC): Analysis of CALGB/SWOG 80405 (Alliance). J Clin Oncol 2016;34 no. 15 suppl. Abstract 3504.
- Schrag D, Brooks G, Meyerhardt JA ,et al. The relationship between primary tumor sidedness and prognosis in colorectal cancer. J Clin Oncol 2016;34 no. 15 suppl. Abstract 3505.
- Larrea AA, Lujan SA, Kunkel TA. SnapShot: DNA mismatch repair. Cell 2010;141:730 e1.
- Jass JR. Pathology of hereditary nonpolyposis colorectal cancer. Ann N Y Acad Sci 2000;910:62–73.
- Lynch HT, Smyrk T. Hereditary nonpolyposis colorectal cancer (Lynch syndrome). An updated review. Cancer 1996;78:1149–67.
- Aaltonen LA, Peltomäki P, Leach FS, et al. Clues to the pathogenesis of familial colorectal cancer. Science 1993;260:812–6.
- Chen W, Swanson BJ, Frankel WL. Molecular genetics of microsatellite-unstable colorectal cancer for pathologists. Diagn Pathol 2017;12:24.
- Bupathi M, Wu C. Biomarkers for immune therapy in colorectal cancer: mismatch-repair deficiency and others. J Gastrointest Oncol 2016;7:713–20.
- Popat S, Hubner R, Houlston RS. Systematic review of microsatellite instability and colorectal cancer prognosis. J Clin Oncol 2005;23:609–18.
- Gryfe R, Kim H, Hsieh ET, et al. Tumor microsatellite instability and clinical outcome in young patients with colorectal cancer. N Engl J Med 2000;342:69–77.
- Ogino S, Kuchiba A, Qian ZR, et al. Prognostic significance and molecular associations of 18q loss of heterozygosity: a cohort study of microsatellite stable colorectal cancers. J Clin Oncol 2009; 27:4591–8.
- Kim ST, Lee J, Park SH, et al. The effect of DNA mismatch repair (MMR) status on oxaliplatin-based first-line chemotherapy as in recurrent or metastatic colon cancer. Med Oncol 2010;27:1277–85.
- Sargent DJ, Monges G, Thibodeau SN, et al. Therapy in colon cancer. J Clin Oncol 2010;28:4664.
- Ribic CM, Sargent DJ, Moore MJ, et al. Tumor microsatellite-instability status as a predictor of benefit from fluorouracil-based adjuvant chemotherapy for colon cancer. N Engl J Med 2003;349:247–57.
- Hutchins G, Southward K, Handley K, et al. Value of mismatch repair, KRAS, and BRAF mutations in predicting recurrence and benefits from chemotherapy in colorectal cancer. J Clin Oncol 2011;29:1261–270.
- Yothers G, O’Connell MJ, Allegra CJ, et al. Oxaliplatin as adjuvant therapy for colon cancer: updated results of NSABP C-07 trial, including survival and subset analyses J Clin Oncol 2011;29:3768–74.
- Chang SC, Lin JK, Lin TC, Liang WY. Loss of heterozygosity: an independent prognostic factor of colorectal cancer. World J Gastroenterol 2005;11:778–84.
- Bertagnolli MM, Niedzwiecki D, Compton CC, et al. Microsatellite instability predicts improved response to adjuvant therapy with irinotecan, fluorouracil, and leucovorin in stage III colon cancer: Cancer and Leukemia Group B Protocol 89803. J Clin Oncol 2009;27:1814–21.
- Bertagnolli MM, Redston M, Compton CC, et al. Microsatellite instability and loss of heterozygosity at chromosomal location 18q: prospective evaluation of biomarkers for stages II and III colon cancer--a study of CALGB 9581 and 89803. J Clin Oncol 2011;29:3153–62.
- Dalerba P, et al. CDX2 as a prognostic biomarker in stage II and stage III colon cancer. N Engl J Med 2016;374: 211–22.
- Clark-Langone KM, Wu JY, Sangli C, et al. Biomarker discovery for colon cancer using a 761 gene RT-PCR assay. BMC Genomics 2007;8:279.
- Gray RG, Quirke P, Handley K, et al. Validation study of a quantitative multigene reverse transcriptase-polymerase chain reaction assay for assessment of recurrence risk in patients with stage II colon cancer. J Clin Oncol 2011;29:4611–9.
- Niedzwiecki D, Bertagnolli MM, Warren RS, et al. Documenting the natural history of patients with resected stage II adenocarcinoma of the colon after random assignment to adjuvant treatment with edrecolomab or observation: results from CALGB 9581. J Clin Oncol 2011;29:3146–52.
- Yothers G, O’Connell MJ, Lee M, et al. Validation of the 12-gene colon cancer recurrence score in NSABP C-07 as a predictor of recurrence in patients with stage II and III colon cancer treated with fluorouracil and leucovorin (FU/LV) and FU/LV plus oxaliplatin. J Clin Oncol 2013;31:4512–9.
- Gill S, Loprinzi CL, Sargent DJ, et al. Pooled analysis of fluorouracil-based adjuvant therapy for stage II and III colon cancer: who benefits and by how much? J Clin Oncol 2004;22:1797–806.
- Gill S, Loprinzi C, Kennecke H, et al. Prognostic web-based models for stage II and III colon cancer: A population and clinical trials-based validation of numeracy and adjuvant! online. Cancer 2011;117:4155–65.
- Jung M, Kim GW, Jung I, et al. Application of the Western-based adjuvant online model to Korean colon cancer patients; a single institution experience. BMC Cancer 2012;12:471.
- Papamichael D, Renfro LA, Matthaiou C, et al. Validity of Adjuvant! Online in older patients with stage III colon cancer based on 2967 patients from the ACCENT database. J Geriatr Oncol 2016;7:422–9.
- Tran B, Kopetz S, Tie J, et al. Impact of BRAF mutation and microsatellite instability on the pattern of metastatic spread and prognosis in metastatic colorectal cancer. Cancer 2011;117:4623–32.
- Roth AD, Tejpar S, Delorenzi M, et al. Prognostic role of KRAS and BRAF in stage II and III resected colon cancer: results of the translational study on the PETACC-3, EORTC 40993, SAKK 60-00 trial. J Clin Oncol 2010;28:466–74.
- Lochhead P, Kuchiba A, Imamura Y, et al. Microsatellite instability and BRAF mutation testing in colorectal cancer prognostication. J Natl Cancer Inst 2013;105:1151–6.
- Benvenuti S, Sartore-Bianchi A, Di Nicolantonio F, et al. Oncogenic activation of the RAS/RAF signaling pathway impairs the response of metastatic colorectal cancers to anti-epidermal growth factor receptor antibody therapies. Cancer Res 2007;67:2643–8.
- Therkildsen C, Bergmann TK, Henrichsen-Schnack T, et al. The predictive value of KRAS, NRAS, BRAF, PIK3CA and PTEN for anti-EGFR treatment in metastatic colorectal cancer: A systematic review and meta-analysis. Acta Oncol 2014;53:852–64.
- Taieb J, Le Malicot K, Shi Q, et al. Prognostic value of BRAF and KRAS mutations in MSI and MSS stage III colon cancer. J Natl Cancer Inst 2017;109(5).
- Palumbo LT, Sharpe WS, Henry JS. Cancer of the colon and rectum; analysis of 300 cases. Am J Surg 1965;109:439–44.
- Sharp GS, Benefiel WW. 5-Fluorouracil in the treatment of inoperable carcinoma of the colon and rectum. Cancer Chemother Rep 1962;20:97–101.
- Lawrence W Jr, Terz JJ, Horsley JS 3rd, et al. Chemotherapy as an adjuvant to surgery for colorectal cancer. Ann Surg 1975;181:616–23.
- Grage TD, et al. Adjuvant chemotherapy with 5-fluorouracil after surgical resection of colorectal carcinoma (COG protocol 7041). A preliminary report. Am J Surg 1977;133:59–66.
- Wolmark N, Fisher B, Rockette H, et al. Postoperative adjuvant chemotherapy or BCG for colon cancer: results from NSABP protocol C-01. J Natl Cancer Inst 1988;80:30–6.
- Moertel CG, Fleming TR, Macdonald JS, et al. Levamisole and fluorouracil for adjuvant therapy of resected colon carcinoma. N Engl J Med 1990;322:352–8.
- Wolmark N, Rockette H, Fisher B, et al. The benefit of leucovorin-modulated fluorouracil as postoperative adjuvant therapy for primary colon cancer: results from National Surgical Adjuvant Breast and Bowel Project protocol C-03. J Clin Oncol 1993;11:1879–87.
- Comparison of fluorouracil with additional levamisole, higher-dose folinic acid, or both, as adjuvant chemotherapy for colorectal cancer: a randomised trial. QUASAR Collaborative Group. Lancet 2000;355(9215):1588–96.
- Chen TC, Hinton DR, Leichman L, et al. Multifocal inflammatory leukoencephalopathy associated with levamisole and 5-fluorouracil: case report. Neurosurgery 1994;35:1138-42.
- Porschen R, Bermann A, Löffler T, et al. Fluorouracil plus leucovorin as effective adjuvant chemotherapy in curatively resected stage III colon cancer: results of the trial adjCCA-01. J Clin Oncol 2001;19:1787–94.
- Arkenau HT, Bermann A, Rettig K, et al. 5-Fluorouracil plus leucovorin is an effective adjuvant chemotherapy in curatively resected stage III colon cancer: long-term follow-up results of the adjCCA-01 trial. Ann Oncol 2003;14:395–9.
- Weinerman B, Shah A, Fields A, et al. Systemic infusion versus bolus chemotherapy with 5-fluorouracil in measurable metastatic colorectal cancer. Am J Clin Oncol 1992;15:518–23.
- Poplin EA, Benedetti JK, Estes NC, et al. Phase III Southwest Oncology Group 9415/Intergroup 0153 randomized trial of fluorouracil, leucovorin, and levamisole versus fluorouracil continuous infusion and levamisole for adjuvant treatment of stage III and high-risk stage II colon cancer. J Clin Oncol 2005;23:1819–25.
- Twelves C, Wong A, Nowacki MP, et al. Capecitabine as adjuvant treatment for stage III colon cancer. N Engl J Med 2005;352:2696–704.
- de Gramont A, Vignoud J, Tournigand C, et al. Oxaliplatin with high-dose leucovorin and 5-fluorouracil 48-hour continuous infusion in pretreated metastatic colorectal cancer. Eur J Cancer 1997;33:214–9.
- Diaz-Rubio E, Sastre J, Zaniboni A, et al. Oxaliplatin as single agent in previously untreated colorectal carcinoma patients: a phase II multicentric study. Ann Oncol 1998;9:105–8.
- André T, de Gramont A, Vernerey D, et al. Adjuvant fluorouracil, leucovorin, and oxaliplatin in Stage II to III Colon Cancer: Updated 10-Year Survival and Outcomes According to BRAF mutation and mismatch repair status of the MOSAIC Study. J Clin Oncol 2015;33:4176–87.
- Andre T, Boni C, Mounedji-Boudiaf L, et al. Oxaliplatin, fluorouracil, and leucovorin as adjuvant treatment for colon cancer. N Engl J Med 2004;350:2343–51.
- Kuebler JP, Wieand HS, O’Connell MJ, et al. Oxaliplatin combined with weekly bolus fluorouracil and leucovorin as surgical adjuvant chemotherapy for stage II and III colon cancer: results from NSABP C-07. J Clin Oncol 2007;25:2198–204.
- Haller DG, Tabernero J, Maroun J, et al. Capecitabine plus oxaliplatin compared with fluorouracil and folinic acid as adjuvant therapy for stage III colon cancer. J Clin Oncol 2011;29:1465–71.
- Schmoll HJ, et al. Capecitabine plus oxaliplatin compared with fluorouracil/folinic acid as adjuvant therapy for stage III colon cancer: final results of the NO16968 randomized controlled phase III trial. J Clin Oncol 2015;33:3733–40.
- Colucci G, Gebbia V, Paoletti G, et al. Phase III randomized trial of FOLFIRI versus FOLFOX4 in the treatment of advanced colorectal cancer: a multicenter study of the Gruppo Oncologico Dell’Italia Meridionale. J Clin Oncol 2005;23:4866–75.
- Tournigand C, André T, Achille E, et al. FOLFIRI followed by FOLFOX6 or the reverse sequence in advanced colorectal cancer: a randomized GERCOR study. J Clin Oncol 2004;22:229–37.
- Hurwitz H, Fehrenbacher L, Novotny W, et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med 2004;350:2335–42.
- Saltz LB, et al. Bevacizumab in combination with oxaliplatin-based chemotherapy as first-line therapy in metastatic colorectal cancer: a randomized phase III study. J Clin Oncol 2008;26:2013–9.
- Cremolini C, Loupakis F, Ruzzo A, et al. Predictors of benefit in colorectal cancer treated with cetuximab: are we getting “Lost in TranslationAL”? J Clin Oncol 2010;28:e173–4.
- Sorich MJ, Wiese MD, Rowland D, et al. Extended RAS mutations and anti-EGFR monoclonal antibody survival benefit in metastatic colorectal cancer: a meta-analysis of randomized, controlled trials. Ann Oncol 2015;26:13–21.
- Grothey A, van Cutsem E, Sobrero A, et al. Regorafenib monotherapy for previously treated metastatic colorectal cancer (CORRECT): an international, multicentre, randomised, placebo-controlled, phase 3 trial. Lancet 2013;381(9863):303–12.
- Saltz LB, Niedzwiecki D, Hollis D, et al. Irinotecan fluorouracil plus leucovorin is not superior to fluorouracil plus leucovorin alone as adjuvant treatment for stage III colon cancer: results of CALGB 89803. J Clin Oncol 2007;25:3456–61.
- Van Cutsem E, et al. Randomized phase III trial comparing biweekly infusional fluorouracil/leucovorin alone or with irinotecan in the adjuvant treatment of stage III colon cancer: PETACC-3. J Clin Oncol 2009;27:3117–25.
- Allegra CJ, et al. Bevacizumab in stage II-III colon cancer: 5-year update of the National Surgical Adjuvant Breast and Bowel Project C-08 trial. J Clin Oncol 2013;31:359–64.
- de Gramont A, et al. Bevacizumab plus oxaliplatin-based chemotherapy as adjuvant treatment for colon cancer (AVANT): a phase 3 randomised controlled trial. Lancet Oncol 2012;13:1225–33.
- Alberts SR, et al. Effect of oxaliplatin, fluorouracil, and leucovorin with or without cetuximab on survival among patients with resected stage III colon cancer: a randomized trial. JAMA 2012;307:1383–93.
- Taieb J, et al. Oxaliplatin, fluorouracil, and leucovorin with or without cetuximab in patients with resected stage III colon cancer (PETACC-8): an open-label, randomised phase 3 trial. Lancet Oncol 2014;15:862–73.
- Shi Q, Sobrero AF, Shields AF, et al. Prospective pooled analysis of six phase III trials investigating duration of adjuvant (adjuvant) oxaliplatin-based therapy (3 vs 6 months) for patients (pts) with stage III colon cancer (CC): The IDEA (International Duration Evaluation of Adjuvant chemotherapy) collaboration. In: Proceedings from the American Society of Clinical Oncology; June 1–5, 2017; Chicago. Abstract LBA1.
- Quasar Collaborative Group; Gray R, Barnwell J, McConkey C, et al. Adjuvant chemotherapy versus observation in patients with colorectal cancer: a randomised study. Lancet 2007;370(9604):2020–9.
- Efficacy of adjuvant fluorouracil and folinic acid in B2 colon cancer. International Multicentre Pooled Analysis of B2 Colon Cancer Trials (IMPACT B2) Investigators. J Clin Oncol 1999;17:1356–63.
- Kidwell KM, et al. Long-term neurotoxicity effects of oxaliplatin added to fluorouracil and leucovorin as adjuvant therapy for colon cancer: results from National Surgical Adjuvant Breast and Bowel Project trials C-07 and LTS-01. Cancer 2012;118:5614–22.
- Beijers AJ, Mols F, Vreugdenhil G. A systematic review on chronic oxaliplatin-induced peripheral neuropathy and the relation with oxaliplatin administration. Support Care Cancer 2014;22:1999–2007.
- Mols F, Beijers T, Lemmens V, et al. Chemotherapy-induced neuropathy and its association with quality of life among 2- to 11-year colorectal cancer survivors: results from the population-based PROFILES registry. J Clin Oncol 2013;31:2699–707.
- Raphael MJ, Fischer HD, Fung K, et al. Neurotoxicity outcomes in a population-based cohort of elderly patients treated with adjuvant oxaliplatin for colorectal cancer. Clin Colorectal Cancer 2017 March 24.
- Toki MI, Saif MW, Syrigos KN. Hypersensitivity reactions associated with oxaliplatin and their clinical management. Expert Opin Drug Saf 2014;13:1545–54.
- Siu SW, Chan RT, Au GK. Hypersensitivity reactions to oxaliplatin: experience in a single institute. Ann Oncol 2006;17:259–61.
- Wong JT, Ling M, Patil S, et al. Oxaliplatin hypersensitivity: evaluation, implications of skin testing, and desensitization. J Allergy Clin Immunol Pract 2014;2:40–5.
- Benson AB 3rd, Venook AP, Cederquist L, et al. NCCN Guidelines Colon Cancer Version 2.2017. www.nccn.org/professionals/physician_gls/pdf/colon.pdf. Accessed May 8, 2017.
- Wolmark N, Rockette H, Mamounas E, et al. Clinical trial to assess the relative efficacy of fluorouracil and leucovorin, fluorouracil and levamisole, and fluorouracil, leucovorin, and levamisole in patients with Dukes’ B and C carcinoma of the colon: results from National Surgical Adjuvant Breast and Bowel Project C-04. J Clin Oncol 1999;17:3553–9.
INTRODUCTION
Colorectal cancer (CRC) is one of the most prevalent malignancies and is the fourth most common cancer in the United States, with an estimated 133,490 new cases diagnosed in 2016. Of these, approximately 95,520 are located in the colon and 39,970 are in the rectum.1 CRC is the third leading cause of cancer death in women and the second leading cause of cancer death in men, with an estimated 49,190 total deaths in 2016.2 The incidence appears to be increasing,3 especially in patients younger than 55 years of age;4 the reason for this increase remains uncertain.
A number of risk factors for the development of CRC have been identified. Numerous hered-itary CRC syndromes have been described, including familial adenomatous polyposis,5 hereditary non-polyposis colorectal cancer (HNPCC) or Lynch syndrome,6 and MUTYH-associated polyposis.7,8 A family history of CRC doubles the risk of developing CRC,9 and current guidelines support lowering the age of screening in individuals with a family history of CRC to 10 years younger than the age of diagnosis of the family member or 40 years of age, whichever is lower.10 Patients with a personal history of adenomatous polyps are at increased risk for developing CRC, as are patients with a personal history of CRC, with a relative risk ranging from 3 to 6.11 Ulcerative colitis and Crohn’s disease are associated with the development of CRC and also influence screening, though evidence suggests good control of these diseases may mitigate risk.12 Finally, modifiable risk factors for the development of CRC include high red meat consumption,13 diets low in fiber,14 obesity,13 smoking, alcohol use,15 and physical inactivity16; lifestyle modification targeting these factors has been shown to decrease rates of CRC.17 The majority of colon cancers present with clinical symptoms, often with rectal bleeding, abdominal pain, change in bowel habits, or obstructive symptoms. More rarely, these tumors are detected during screening colonoscopy, in which case they tend to be at an early stage.
SURGICAL MANAGEMENT
A critical goal in the resection of early-stage colon cancer is attaining R0 resection. Patients who achieve R0 resection as compared to R1 (microscopic residual tumor) and R2 (macroscopic residual tumor)18 have significantly improved long-term overall survival.19 Traditionally, open resection of the involved colonic segment was employed, with end-end anastomosis of the uninvolved free margins. Laparoscopic resection for early-stage disease has been utilized in attempts to decrease morbidity of open procedures, with similar outcomes and node sampling.20 Laparoscopic resection appears to provide similar outcomes even in locally advanced disease.21 Right-sided lesions are treated with right colectomy and primary ileocolic anastomosis.22 For patients presenting with obstructing masses, the Hartmann procedure is the most commonly performed operation. This involves creation of an ostomy with subtotal colectomy and subsequent ostomy reversal in a 2- or 3-stage protocol.23 Patients with locally advanced disease and invasion into surrounding structures require multivisceral resection, which involves resection en bloc with secondarily involved organs.24 Intestinal perforation presents a unique challenge and is associated with surgical complications, infection, and lower overall survival (OS) and 5-year disease-free survival (DFS). Complete mesocolic excision is a newer technique that has been performed with reports of better oncologic outcome at some centers; however, this approach is not currently considered standard of care.25
STAGING
According to a report by the National Cancer Institute, the estimated 5-year relative survival rates for localized colon cancer (lymph node negative), regional (lymph node positive) disease, and distant (metastatic) disease are 89.9%, 71.3%, and 13.9%, respectively.1 However, efforts have been made to further classify patients into distinct categories to allow fine-tuning of prognostication. In the current system, staging of colon cancer utilizes the American Joint Committee on Cancer tumor/node/metastasis (TNM) system.20 Clinical and pathologic features include depth of invasion, local invasion of other organs, nodal involvement, and presence of distant metastasis (Table 1). Studies completed prior to the adoption of the TNM system used the Dukes criteria, which divided colon cancer into A, B, and C, corresponding to TNM stage I, stage IIA–IIC, and stage IIIA-IIIC. This classification is rarely used in more contemporary studies.

APPROACH TO ADJUVANT CHEMOTHERAPY
Adjuvant chemotherapy seeks to eliminate micrometastatic disease present following curative surgical resection. When stage 0 cancer is discovered incidentally during colonoscopy, endoscopic resection alone is the management of choice, as presence of micrometastatic disease is exceedingly unlikely.26 Stage I–III CRCs are treated with surgical resection withcurative intent. The 5-year survival rate for stage I and early-stage II CRC is estimated at 97% with surgery alone.27,28 The survival rate drops to about 60% for high-risk stage II tumors (T4aN0), and down to 50% or less for stage II-T4N0 or stage III cancers. Adjuvant chemotherapy is generally recommended to further decrease the rates of distant recurrence in certain cases of stage II and in all stage III tumors.
DETERMINATION OF BENEFIT FROM CHEMOTHERAPY: PROGNOSTIC MARKERS
Prior to administration of adjuvant chemotherapy, a clinical evaluation by the medical oncologist to determine appropriateness and safety of treatment is paramount. Poor performance status and comorbid conditions may indicate risk for excessive toxicity and minimal benefit from chemotherapy. CRC commonly presents in older individuals, with the median age at diagnosis of 69 years for men and 73 years for women.29 In this patient population, comorbidities such as cardiovascular disease, diabetes, and renal dysfunction are more prevalent.30 Decisions regarding adjuvant chemotherapy in this patient population have to take into consideration the fact that older patients may experience higher rates of toxicity with chemotherapy, including gastrointestinal toxicities and marrow suppression.31 Though some reports indicate patients older than 70 years derive similar benefit from adjuvant chemotherapy,32,33 a large pooled analysis of the ACCENT database, which included 7 adjuvant therapy trials and 14,528 patients, suggested limited benefit from the addition of oxaliplatin to fluorouracil in elderly patients.32 Other factors that weigh on the decision include stage, pathology, and presence of high-risk features. A common concern in the postoperative setting is delaying initiation of chemotherapy to allow adequate wound healing; however, evidence suggests that delays longer than 8 weeks leads to worse overall survival, with hazard ratios (HR) ranging from 1.4 to 1.7.34,35 Thus, the start of adjuvant therapy should ideally be within this time frame.
HIGH-RISK FEATURES
Multiple factors have been found to predict worse outcome and are classified as high-risk features (Table 2). Histologically, high-grade or poorly differentiated tumors are associated with higher recurrence rate and worse outcome.36 Certain histological subtypes, including mucinous and signet-ring, both appear to have more aggressive biology.37 Presence of microscopic invasion into surrounding blood vessels (vascular invasion) and nerves (perineural invasion) is associated with lower survival.38 Penetration of the cancer through the visceral peritoneum (T4a) or into surrounding structures (T4b) is associated with lower survival.36 During surgical resection, multiple lymph nodes are removed along with the primary tumor to evaluate for metastasis to the regional nodes. Multiple analyses have demonstrated that removal and pathologic assessment of fewer than 12 lymph nodes is associated with high risk of missing a positive node, and is thus equated with high risk.39–41 In addition, extension of tumor beyond the capsules of any single lymph node, termed extracapsular extension, is associated with an increased risk of all-cause mortality.42 Tumor deposits, or focal aggregates of adenocarcinoma in the pericolic fat that are not contiguous with the primary tumor and are not associated with lymph nodes, are currently classified as lymph nodes as N1c in the current TNM staging system. Presence of these deposits has been found to predict poor outcome stage for stage.43 Obstruction and/or perforation secondary to the tumor are also considered high-risk features that predict poor outcome.

SIDEDNESS
As reported at the 2016 American Society of Clinical Oncology annual meeting, tumor location predicts outcome in the metastatic setting. A report by Venook and colleagues based on a post-hoc analysis found that in the metastatic setting, location of the tumor primary in the left side is associated with longer OS (33.3 months) when compared to the right side of the colon (19.4 months).44 A retrospective analysis of multiple databases presented by Schrag and colleagues similarly reported inferior outcomes in patients with stage III and IV disease who had right-sided primary tumors.45 However, the prognostic implications for stage II disease remain uncertain.
BIOMARKERS
Given the controversy regarding adjuvant therapy of patients with stage II colon cancer, multiple biomarkers have been evaluated as possible predictive markers that can assist in this decision. The mismatch repair (MMR) system is a complex cellular enzymatic mechanism that identifies and corrects DNA errors during cell division and prevents mutagenesis.46 The familial cancer syndrome HNPCC is linked to alteration in a variety of MMR genes, leading to deficient mismatch repair (dMMR), also termed microsatellite instability-high (MSI-high).47,48 Epigenetic modification can also lead to silencing of the same implicated genes and accounts for 15% to 20% of sporadic colorectal cancer.49 These epigenetic modifications lead to hypermethylation of the promotor region of MLH1 in 70% of cases.50 The 4 MMR genes most commonly tested are MLH-1, MSH2, MSH6, and PMS2. Testing can be performed by immunohistochemistry or polymerase chain reaction.51 Across tumor histology and stage, MSI status is prognostic. Patients with MSI-high tumors have been shown to have improved prognosis and longer OS both in stage II and III disease52–54 and in the metastatic setting.55 However, despite this survival benefit, there is conflicting data as to whether patients with stage II, MSI-high colon cancer may benefit less from adjuvant chemotherapy. One early retrospective study compared outcomes of 70 patients with stage II and III disease and dMMR to those of 387 patients with stage II and III disease and proficient mismatch repair (pMMR). Adjuvant fluorouracil with leucovorin improved DFS for patients with pMMR (HR 0.67) but not for those with dMMR (HR 1.10). In addition, for patients with stage II disease and dMMR, the HR for OS was inferior at 2.95.56 Data collected from randomized clinical trials using fluorouracil-based adjuvant chemotherapy were analyzed in an attempt to predict benefit based on MSI status. Benefit was only seen in pMMR patients, with a HR of 0.72; this was not seen in the dMMR patients.57 Subsequent studies have had different findings and did not demonstrate a detrimental effect of fluorouracil in dMMR.58,59 For stage III patients, MSI status does not appear to affect benefit from chemotherapy, as analysis of data from the NSABP C-07 trial (Table 3) demonstrated benefit of FOLFOX (leucovorin, fluorouracil, oxaliplatin) in patients with dMMR status and stage III disease.59

Another genetic abnormality identified in colon cancers is chromosome 18q loss of heterozygosity (LOH). The presence of 18q LOH appears to be inversely associated with MSI-high status. Some reports have linked presence of 18q with worse outcome,60 but others question this, arguing the finding may simply be related to MSI status.61,62 This biomarker has not been established as a clear prognostic marker that can aid clinical decisions.
Most recently, expression of caudal-type homeobox transcription factor 2 (CDX2) has been reported as a novel prognostic and predictive tool. A 2015 report linked lack of expression of CDX2 to worse outcome; in this study, 5-year DFS was 41% in patients with CDX2-negative tumors versus 74% in the CDX2-positive tumors, with a HR of disease recurrence of 2.73 for CDX2-negative tumors.63 Similar numbers were observed in patients with stage II disease, with 5-year OS of 40% in patients with CDX2-negative tumors versus 70% in those with CDX2-positive tumors. Treatment of CDX2-negative patients with adjuvant chemotherapy improved outcomes: 5-year DFS in the stage II subgroup was 91% with chemotherapy versus 56% without, and in the stage III subgroup, 74% with chemotherapy versus 37% without. The authors concluded that patients with stage II and III colon cancer that is CDX2-negative may benefit from adjuvant chemotherapy. Importantly, CDX2-negativity is a rare event, occurring in only 6.9% of evaluable tumors.
RISK ASSESSMENT TOOLS
Several risk assessment tools have been developed in an attempt to aid clinical decision making regarding adjuvant chemotherapy for patients with stage II colon cancer. The Oncotype DX Colon Assay analyses a 12-gene signature in the pathologic sample and was developed with the goal to improve prognostication and aid in treatment decision making. The test utilizes reverse transcription-PCR on RNA extracted from the tumor.64 After evaluating 12 genes, a recurrence score is generated that predicts the risk of disease recurrence. This score was validated using data from 3 large clinical trials.65–67 Unlike the Oncotype Dx score used in breast cancer, the test in colon cancer has not been found to predict the benefit from chemotherapy and has not been incorporated widely into clinical practice.
Adjuvant! Online (available at www.adjuvantonline.com) is a web-based tool that combines clinical and histological features to estimate outcome. Calculations are based on US SEER tumor registry-reported outcomes.68 A second web-based tool, Numeracy (available at www.mayoclinic.com/calcs), was developed by the Mayo Clinic using pooled data from 7 randomized clinical trials including 3341 patients.68 Both tools seek to predict absolute benefit for patients treated with fluorouracil, though data suggests Adjuvant! Online may be more reliable in its predictive ability.69 Adjuvant! Online has also been validated in an Asian population70 and patients older than 70 years.71
MUTATIONAL ANALYSIS
Multiple mutations in proto-oncogenes have been found in colon cancer cells. One such proto-oncogene is BRAF, which encodes a serine-threonine kinase in the rapidly accelerated fibrosarcoma (RAF). Mutations in BRAF have been found in 5% to 10% of colon cancers and are associated with right-sided tumors.72 As a prognostic marker, some studies have associated BRAF mutations with worse prognosis, including shorter time to relapse and shorter OS.73,74 Two other proto-oncogenes are Kristen rat sarcoma viral oncogene homolog (KRAS) and neuroblastoma rat sarcoma viral oncogene homolog (NRAS), both of which encode proteins downstream of epidermal growth factor receptor (EGFR). KRAS and NRAS mutations have been shown to be predictive in the metastatic setting where they predict resistance to the EGFR inhibitors cetuximab and panitumumab.75,76 The effect of KRAS and NRAS mutations on outcome in stage II and III colon cancer is uncertain. Some studies suggest worse outcome in KRAS-mutated cancers,77 while others failed to demonstrate this finding.73
CASE PRESENTATION 1
A 53-year-old man with no past medical history presents to the emergency department with early satiety and generalized abdominal pain. Laboratory evaluation shows a microcytic anemia with normal white blood cell count, platelet count, renal function, and liver function tests. Computed tomography (CT) scan of the abdomen and pelvis show a 4-cm mass in the transverse colon without obstruction and without abnormality in the liver. CT scan of the chest does not demonstrate pathologic lymphadenopathy or other findings. He undergoes robotic laparoscopic transverse colon resection and appendectomy. Pathology confirms a 3.5-cm focus of adenocarcinoma of the colon with invasion through the muscularis propria and 5 of 27 regional lymph nodes positive for adenocarcinoma and uninvolved proximal, distal, and radial margins. He is given a stage of IIIB pT3 pN2a M0 and referred to medical oncology for further management, where 6 months of adjuvant FOLFOX chemotherapy is recommended.
ADJUVANT CHEMOTHERAPY IN STAGE III COLON CANCER
Postoperative adjuvant chemotherapy is the standard of care for patients with stage III disease. In the 1960s, infusional fluorouracil was first used to treat inoperable colon cancer.78,79 After encouraging results, the agent was used both intraluminally and intravenously as an adjuvant therapy for patients undergoing resection with curative intent; however, only modest benefits were described.80,81 The National Surgical Adjuvant Breast and Bowel Project (NSABP) C-01 trial (Table 3) was the first study to demonstrate a benefit from adjuvant chemotherapy in colon cancer. This study randomly assigned patients with stage II and III colon cancer to surgery alone, postoperative chemotherapy with fluorouracil, semustine, and vincristine (MOF), or postoperative bacillus Calmette-Guérin (BCG). DFS and OS were significantly improved with MOF chemotherapy.82 In 1990, a landmark study reported on outcomes after treatment of 1296 patients with stage III colon cancer with adjuvant fluorouracil and levamisole for 12 months. The combination was associated with a 41% reduction in risk of cancer recurrence and a 33% reduction in risk of death.83 The NSABP C-03 trial (Table 3) compared MOF to the combination of fluorouracil and leucovorin and demonstrated improved 3-year DFS (69% versus 73%) and 3-year OS (77% versus 84%) in patients with stage III disease.84 Building on these outcomes, the QUASAR study (Table 3) compared fluorouracil in combination with one of levamisole, low-dose leucovorin, or high-dose leucovorin. The study enrolled 4927 patients and found worse outcomes with fluorouracil plus levamisole and no difference in low-doseversus high-dose leucovorin.85 Levamisole fell out of use after associations with development of multifocal leukoencephalopathy,86 and was later shown to have inferior outcomes versus leucovorin when combined with fluorouracil.87,88 Intravenous fluorouracil has shown similar benefit when administered by bolus or infusion,89 although continuous infusion has been associated with lower incidence of severe toxicity.90 The efficacy of the oral fluoropyrimidine capecitabine has been shown to be equivalent to that of fluorouracil.91
Fluorouracil-based treatment remained the standard of care until the introduction of oxaliplatin in the mid-1990s. After encouraging results in the metastatic setting,92,93 the agent was moved to the adjuvant setting. The MOSAIC trial (Table 3) randomly assigned patients with stage II and III colon cancer to fluorouracil with leucovorin (FULV) versus FOLFOX given once every 2 weeks for 12 cycles. Analysis with respect to stage III patients showed a clear survival benefit, with a 10-year OS of 67.1% with FOLFOX chemotherapy versus 59% with fluorouracil and leucovorin.94,95 The NSABP C-07 (Table 3) trial used a similar trial design but employed bolus fluorouracil. More than 2400 patients with stage II and III colon cancer were randomly assigned to bolus FULV or bolus fluorouracil, leucovorin, and oxaliplatin (FLOX). The addition of oxaliplatin significantly improved outcomes, with 4-year DFS of 67% versus 71.8% for FULV and FLOX, respectively, and a HR of death of 0.80 with FLOX.59,96 The multicenter N016968 trial (Table 3) randomly assigned 1886 patients with stage III colon cancer to adjuvant capecitabine plus oxaliplatin (XELOX) or bolus fluorouracil plus leucovorin (FU/FA). The 3-year DFS was 70.9% versus 66.5% with XELOX and FU/FA, respectively, and 5-year OS was 77.6% versus 74.2%, respectively.97,98
In the metastatic setting, additional agents have shown efficacy, including irinotecan,99,100 bevacizumab,101,102 cetuximab,103,104 and regorafenib.105 This observation led to testing of these agents in earlier stage disease. The CALGB 89803 trial compared fluorouracil, leucovorin, and irinotecan to fluorouracil with leucovorin alone. No benefit in 5-year DFS or OS was seen.106 Similarly, infusional fluorouracil, leucovorin, and irinotecan (FOLFIRI) was not found to improve 5-year DFS as compared to fluorouracil with leucovorin alone in the PETACC-3 trial.107 The NSABP C-08 trial considered the addition of bevacizumab to FOLFOX. When compared to FOLFOX alone, the combination of bevacizumab to FOLFOX had similar 3-year DFS (77.9% versus 75.1%) and 5-year OS (82.5% versus 80.7%).108 This finding was confirmed in the Avant trial.109 The addition of cetuximab to FOLFOX was equally disappointing, as shown in the N0147 trial110 and PETACC-8 trial.111 Data on regorafenib in the adjuvant setting for stage III colon cancer is lacking; however, 2 ongoing clinical trials, NCT02425683 and NCT02664077, are each studying the use of regorafenib following completion of FOLFOX for patients with stage III disease.
Thus, after multiple trials comparing various regimens and despite attempts to improve outcomes by the addition of a third agent, the standard of care per National Comprehensive Cancer Network (NCCN) guidelines for management of stage III colon cancer remains 12 cycles of FOLFOX chemotherapy. Therapy should be initiated within 8 weeks of surgery. Data are emerging to support a short duration of therapy for patients with low-risk stage III tumors, as shown in an abstract presented at the 2017 American Society of Clinical Oncology annual meeting. The IDEA trial was a pooled analysis of 6 randomized clinical trials across multiple countries, all of which evaluated 3 versus 6 months of FOLFOX or capecitabine and oxaliplatin in the treatment of stage III colon cancer. The analysis was designed to test non-inferiority of 3 months of therapy as compared to 6 months. The analysis included 6088 patients across 244 centers in 6 countries. The overall analysis failed to establish noninferiority. The 3-year DFS rate was 74.6% for 3 months and 75.5% for 6 months, with a DFS HR of 1.07 and a confidence interval that did not meet the prespecified endpoint. Subgroup analysis suggested noninferiority for lower stage disease (T1–3 or N1) but not for higher stage disease (T4 or N2). Given the high rates of neuropathy with 6 months of oxaliplatin, these results suggest that 3 months of adjuvant therapy can be considered for patients with T1–3 or N1 disease in an attempt to limit toxicity.112
CASE PRESENTATION 2
A 57-year-old woman presents to the emergency department with fever and abdominal pain. CT of the abdomen and pelvis demonstrates a left-sided colonic mass with surrounding fat stranding and pelvic abscess. She is taken emergently for left hemicolectomy, cholecystectomy, and evacuation of pelvic abscess. Pathology reveals a 5-cm adenocarcinoma with invasion through the visceral peritoneum; 0/22 lymph nodes are involved. She is given a diagnosis of stage IIC and referred to medical oncology for further management. Due to her young age and presence of high-risk features, she is recommended adjuvant therapy with FOLFOX for 6 months.
ADJUVANT CHEMOTHERAPY IN STAGE II COLON CANCER
Because of excellent outcomes with surgical resection alone for stage II cancers, the use of adjuvant chemotherapy for patients with stage II disease is controversial. Limited prospective data is available to guide adjuvant treatment decisions for stage II patients. The QUASAR trial, which compared observation to adjuvant fluorouracil and leucovorin in patients with early-stage colon cancer, included 2963 patients with stage II disease and found a relative risk (RR) of death or recurrence of 0.82 and 0.78, respectively. Importantly, the absolute benefit of therapy was less than 5%.113 The IMPACT-B2 trial (Table 3) combined data from 5 separate trials and analyzed 1016 patients with stage II colon cancer who received fluorouracil with leucovorin or observation. Event-free survival was 0.86 versus 0.83 and 5-year OS was 82% versus 80%, suggesting no benefit.114 The benefit of addition of oxaliplatin to fluorouracil in stage II disease appears to be less than the benefit of adding this agent in the treatment of stage III CRC. As noted above, the MOSAIC trial randomly assigned patients with stage II and III colon cancer to receive adjuvant fluorouracil and leucovorin with or without oxaliplatin for 12 cycles. After a median follow-up of 9.5 years, 10-year OS rates for patients with stage II disease were 78.4% versus 79.5%. For patients with high-risk stage II disease (defined as T4, bowel perforation, or fewer than 10 lymph nodes examined), 10-year OS was 71.7% and 75.4% respectively, but these differences were not statistically significant.94
Because of conflicting data as to the benefit of adding oxaliplatin in stage II disease, oxaliplatin is not recommended for standard-risk stage II patients. The use of oxaliplatin in high-risk stage II tumors should be weighed carefully given the toxicity risk. Oxaliplatin is recognized to cause sensory neuropathy in many patients, which can become painful and debilitating.115 Two types of neuropathy are associated with oxaliplatin: acute and chronic. Acute neuropathy manifests most often as cold-induced paresthesias in the fingers and toes and is quite common, affecting up to 90% of patients. These symptoms are self-limited and resolve usually within 1 week of each treatment.116 Some patients, with reports ranging from 10% to 79%, develop chronic neuropathy that persists for 1 year or more and causes significant decrements in quality of life.117 Patients older than age 70 may be at greater risk for oxaliplatin-induced neuropathy, which would increase risk of falls in this population.118 In addition to neuropathy, oxaliplatin is associated with hypersensitivity reactions that can be severe and even fatal.119 In a single institution series, the incidence of severe reactions was 2%.120 Desensitization following hypersensitivity reactions is possible but requires a time-intensive protocol.121
Based on the inconclusive efficacy findings and due to concerns over toxicity, each decision must be individualized to fit patient characteristics and preferences. In general, for patients with stage II disease without high-risk features, an individualized discussion should be held as to the risks and benefits of single-agent fluorouracil, and this treatment should be offered in cases where the patient or provider would like to be aggressive. Patients with stage II cancer who have 1 or more high-risk features are often recommended adjuvant chemotherapy. Whether treatment with fluorouracil plus leucovorin or FOLFOX is preferred remains uncertain, and thus the risks and the potential gains of oxaliplatin must be discussed with the individual patient. MMR status can also influence the treatment recommendation for patients with stage II disease. In general, patients with standard-risk stage II tumors that are pMMR are offered MMR with leucovorin or oral capecitabine for 12 cycles. FOLFOX is considered for patients with MSI-high disease and those with multiple high-risk features.
MONITORING AFTER THERAPY
After completion of adjuvant chemotherapy, patients enter a period of survivorship. Patients are seen in clinic for symptom and laboratory monitoring of the complete blood count, liver function tests, and carcinoembryonic antigen (CEA). NCCN guidelines support history and physical examination with CEA testing every 3 to 6 months for the first 2 years, then every 6 months for the next 3 years, after which many patients continue to be seen annually. CT imaging of the chest, abdomen, and pelvis for monitoring of disease recurrence is recommended every 6 to 12 months for a total of 5 years. New elevations in CEA or liver function tests should prompt early imaging. Colonoscopy should be performed 1 year after completion of therapy; however, if no preoperative colonoscopy was performed, this should be done 3 to 6 months after completion. Colonoscopy is then repeated in 3 years and then every 5 years unless advanced adenomas are present.122
SUMMARY
The addition of chemotherapy to surgical management of colon cancer has lowered the rate of disease recurrence and improved long-term survival. Adjuvant FOLFOX for 12 cycles is the standard of care for patients with stage III colon cancer and for patients with stage II disease with certain high-risk features. Use of adjuvant chemotherapy in stage II disease without high-risk features is controversial, and treatment decisions should be individualized. Biologic markers such as MSI and CDX2 status as well as patient-related factors including age, overall health, and personal preferences can inform treatment decisions. If chemotherapy is recommended in this setting, it would be with single-agent fluorouracil in an infusional or oral formulation, unless the tumor has the MSI-high feature. Following completion of adjuvant therapy, patients should be followed with clinical evaluation, laboratory testing, and imaging for a total of 5 years as per recommended guidelines.
INTRODUCTION
Colorectal cancer (CRC) is one of the most prevalent malignancies and is the fourth most common cancer in the United States, with an estimated 133,490 new cases diagnosed in 2016. Of these, approximately 95,520 are located in the colon and 39,970 are in the rectum.1 CRC is the third leading cause of cancer death in women and the second leading cause of cancer death in men, with an estimated 49,190 total deaths in 2016.2 The incidence appears to be increasing,3 especially in patients younger than 55 years of age;4 the reason for this increase remains uncertain.
A number of risk factors for the development of CRC have been identified. Numerous hered-itary CRC syndromes have been described, including familial adenomatous polyposis,5 hereditary non-polyposis colorectal cancer (HNPCC) or Lynch syndrome,6 and MUTYH-associated polyposis.7,8 A family history of CRC doubles the risk of developing CRC,9 and current guidelines support lowering the age of screening in individuals with a family history of CRC to 10 years younger than the age of diagnosis of the family member or 40 years of age, whichever is lower.10 Patients with a personal history of adenomatous polyps are at increased risk for developing CRC, as are patients with a personal history of CRC, with a relative risk ranging from 3 to 6.11 Ulcerative colitis and Crohn’s disease are associated with the development of CRC and also influence screening, though evidence suggests good control of these diseases may mitigate risk.12 Finally, modifiable risk factors for the development of CRC include high red meat consumption,13 diets low in fiber,14 obesity,13 smoking, alcohol use,15 and physical inactivity16; lifestyle modification targeting these factors has been shown to decrease rates of CRC.17 The majority of colon cancers present with clinical symptoms, often with rectal bleeding, abdominal pain, change in bowel habits, or obstructive symptoms. More rarely, these tumors are detected during screening colonoscopy, in which case they tend to be at an early stage.
SURGICAL MANAGEMENT
A critical goal in the resection of early-stage colon cancer is attaining R0 resection. Patients who achieve R0 resection as compared to R1 (microscopic residual tumor) and R2 (macroscopic residual tumor)18 have significantly improved long-term overall survival.19 Traditionally, open resection of the involved colonic segment was employed, with end-end anastomosis of the uninvolved free margins. Laparoscopic resection for early-stage disease has been utilized in attempts to decrease morbidity of open procedures, with similar outcomes and node sampling.20 Laparoscopic resection appears to provide similar outcomes even in locally advanced disease.21 Right-sided lesions are treated with right colectomy and primary ileocolic anastomosis.22 For patients presenting with obstructing masses, the Hartmann procedure is the most commonly performed operation. This involves creation of an ostomy with subtotal colectomy and subsequent ostomy reversal in a 2- or 3-stage protocol.23 Patients with locally advanced disease and invasion into surrounding structures require multivisceral resection, which involves resection en bloc with secondarily involved organs.24 Intestinal perforation presents a unique challenge and is associated with surgical complications, infection, and lower overall survival (OS) and 5-year disease-free survival (DFS). Complete mesocolic excision is a newer technique that has been performed with reports of better oncologic outcome at some centers; however, this approach is not currently considered standard of care.25
STAGING
According to a report by the National Cancer Institute, the estimated 5-year relative survival rates for localized colon cancer (lymph node negative), regional (lymph node positive) disease, and distant (metastatic) disease are 89.9%, 71.3%, and 13.9%, respectively.1 However, efforts have been made to further classify patients into distinct categories to allow fine-tuning of prognostication. In the current system, staging of colon cancer utilizes the American Joint Committee on Cancer tumor/node/metastasis (TNM) system.20 Clinical and pathologic features include depth of invasion, local invasion of other organs, nodal involvement, and presence of distant metastasis (Table 1). Studies completed prior to the adoption of the TNM system used the Dukes criteria, which divided colon cancer into A, B, and C, corresponding to TNM stage I, stage IIA–IIC, and stage IIIA-IIIC. This classification is rarely used in more contemporary studies.

APPROACH TO ADJUVANT CHEMOTHERAPY
Adjuvant chemotherapy seeks to eliminate micrometastatic disease present following curative surgical resection. When stage 0 cancer is discovered incidentally during colonoscopy, endoscopic resection alone is the management of choice, as presence of micrometastatic disease is exceedingly unlikely.26 Stage I–III CRCs are treated with surgical resection withcurative intent. The 5-year survival rate for stage I and early-stage II CRC is estimated at 97% with surgery alone.27,28 The survival rate drops to about 60% for high-risk stage II tumors (T4aN0), and down to 50% or less for stage II-T4N0 or stage III cancers. Adjuvant chemotherapy is generally recommended to further decrease the rates of distant recurrence in certain cases of stage II and in all stage III tumors.
DETERMINATION OF BENEFIT FROM CHEMOTHERAPY: PROGNOSTIC MARKERS
Prior to administration of adjuvant chemotherapy, a clinical evaluation by the medical oncologist to determine appropriateness and safety of treatment is paramount. Poor performance status and comorbid conditions may indicate risk for excessive toxicity and minimal benefit from chemotherapy. CRC commonly presents in older individuals, with the median age at diagnosis of 69 years for men and 73 years for women.29 In this patient population, comorbidities such as cardiovascular disease, diabetes, and renal dysfunction are more prevalent.30 Decisions regarding adjuvant chemotherapy in this patient population have to take into consideration the fact that older patients may experience higher rates of toxicity with chemotherapy, including gastrointestinal toxicities and marrow suppression.31 Though some reports indicate patients older than 70 years derive similar benefit from adjuvant chemotherapy,32,33 a large pooled analysis of the ACCENT database, which included 7 adjuvant therapy trials and 14,528 patients, suggested limited benefit from the addition of oxaliplatin to fluorouracil in elderly patients.32 Other factors that weigh on the decision include stage, pathology, and presence of high-risk features. A common concern in the postoperative setting is delaying initiation of chemotherapy to allow adequate wound healing; however, evidence suggests that delays longer than 8 weeks leads to worse overall survival, with hazard ratios (HR) ranging from 1.4 to 1.7.34,35 Thus, the start of adjuvant therapy should ideally be within this time frame.
HIGH-RISK FEATURES
Multiple factors have been found to predict worse outcome and are classified as high-risk features (Table 2). Histologically, high-grade or poorly differentiated tumors are associated with higher recurrence rate and worse outcome.36 Certain histological subtypes, including mucinous and signet-ring, both appear to have more aggressive biology.37 Presence of microscopic invasion into surrounding blood vessels (vascular invasion) and nerves (perineural invasion) is associated with lower survival.38 Penetration of the cancer through the visceral peritoneum (T4a) or into surrounding structures (T4b) is associated with lower survival.36 During surgical resection, multiple lymph nodes are removed along with the primary tumor to evaluate for metastasis to the regional nodes. Multiple analyses have demonstrated that removal and pathologic assessment of fewer than 12 lymph nodes is associated with high risk of missing a positive node, and is thus equated with high risk.39–41 In addition, extension of tumor beyond the capsules of any single lymph node, termed extracapsular extension, is associated with an increased risk of all-cause mortality.42 Tumor deposits, or focal aggregates of adenocarcinoma in the pericolic fat that are not contiguous with the primary tumor and are not associated with lymph nodes, are currently classified as lymph nodes as N1c in the current TNM staging system. Presence of these deposits has been found to predict poor outcome stage for stage.43 Obstruction and/or perforation secondary to the tumor are also considered high-risk features that predict poor outcome.

SIDEDNESS
As reported at the 2016 American Society of Clinical Oncology annual meeting, tumor location predicts outcome in the metastatic setting. A report by Venook and colleagues based on a post-hoc analysis found that in the metastatic setting, location of the tumor primary in the left side is associated with longer OS (33.3 months) when compared to the right side of the colon (19.4 months).44 A retrospective analysis of multiple databases presented by Schrag and colleagues similarly reported inferior outcomes in patients with stage III and IV disease who had right-sided primary tumors.45 However, the prognostic implications for stage II disease remain uncertain.
BIOMARKERS
Given the controversy regarding adjuvant therapy of patients with stage II colon cancer, multiple biomarkers have been evaluated as possible predictive markers that can assist in this decision. The mismatch repair (MMR) system is a complex cellular enzymatic mechanism that identifies and corrects DNA errors during cell division and prevents mutagenesis.46 The familial cancer syndrome HNPCC is linked to alteration in a variety of MMR genes, leading to deficient mismatch repair (dMMR), also termed microsatellite instability-high (MSI-high).47,48 Epigenetic modification can also lead to silencing of the same implicated genes and accounts for 15% to 20% of sporadic colorectal cancer.49 These epigenetic modifications lead to hypermethylation of the promotor region of MLH1 in 70% of cases.50 The 4 MMR genes most commonly tested are MLH-1, MSH2, MSH6, and PMS2. Testing can be performed by immunohistochemistry or polymerase chain reaction.51 Across tumor histology and stage, MSI status is prognostic. Patients with MSI-high tumors have been shown to have improved prognosis and longer OS both in stage II and III disease52–54 and in the metastatic setting.55 However, despite this survival benefit, there is conflicting data as to whether patients with stage II, MSI-high colon cancer may benefit less from adjuvant chemotherapy. One early retrospective study compared outcomes of 70 patients with stage II and III disease and dMMR to those of 387 patients with stage II and III disease and proficient mismatch repair (pMMR). Adjuvant fluorouracil with leucovorin improved DFS for patients with pMMR (HR 0.67) but not for those with dMMR (HR 1.10). In addition, for patients with stage II disease and dMMR, the HR for OS was inferior at 2.95.56 Data collected from randomized clinical trials using fluorouracil-based adjuvant chemotherapy were analyzed in an attempt to predict benefit based on MSI status. Benefit was only seen in pMMR patients, with a HR of 0.72; this was not seen in the dMMR patients.57 Subsequent studies have had different findings and did not demonstrate a detrimental effect of fluorouracil in dMMR.58,59 For stage III patients, MSI status does not appear to affect benefit from chemotherapy, as analysis of data from the NSABP C-07 trial (Table 3) demonstrated benefit of FOLFOX (leucovorin, fluorouracil, oxaliplatin) in patients with dMMR status and stage III disease.59

Another genetic abnormality identified in colon cancers is chromosome 18q loss of heterozygosity (LOH). The presence of 18q LOH appears to be inversely associated with MSI-high status. Some reports have linked presence of 18q with worse outcome,60 but others question this, arguing the finding may simply be related to MSI status.61,62 This biomarker has not been established as a clear prognostic marker that can aid clinical decisions.
Most recently, expression of caudal-type homeobox transcription factor 2 (CDX2) has been reported as a novel prognostic and predictive tool. A 2015 report linked lack of expression of CDX2 to worse outcome; in this study, 5-year DFS was 41% in patients with CDX2-negative tumors versus 74% in the CDX2-positive tumors, with a HR of disease recurrence of 2.73 for CDX2-negative tumors.63 Similar numbers were observed in patients with stage II disease, with 5-year OS of 40% in patients with CDX2-negative tumors versus 70% in those with CDX2-positive tumors. Treatment of CDX2-negative patients with adjuvant chemotherapy improved outcomes: 5-year DFS in the stage II subgroup was 91% with chemotherapy versus 56% without, and in the stage III subgroup, 74% with chemotherapy versus 37% without. The authors concluded that patients with stage II and III colon cancer that is CDX2-negative may benefit from adjuvant chemotherapy. Importantly, CDX2-negativity is a rare event, occurring in only 6.9% of evaluable tumors.
RISK ASSESSMENT TOOLS
Several risk assessment tools have been developed in an attempt to aid clinical decision making regarding adjuvant chemotherapy for patients with stage II colon cancer. The Oncotype DX Colon Assay analyses a 12-gene signature in the pathologic sample and was developed with the goal to improve prognostication and aid in treatment decision making. The test utilizes reverse transcription-PCR on RNA extracted from the tumor.64 After evaluating 12 genes, a recurrence score is generated that predicts the risk of disease recurrence. This score was validated using data from 3 large clinical trials.65–67 Unlike the Oncotype Dx score used in breast cancer, the test in colon cancer has not been found to predict the benefit from chemotherapy and has not been incorporated widely into clinical practice.
Adjuvant! Online (available at www.adjuvantonline.com) is a web-based tool that combines clinical and histological features to estimate outcome. Calculations are based on US SEER tumor registry-reported outcomes.68 A second web-based tool, Numeracy (available at www.mayoclinic.com/calcs), was developed by the Mayo Clinic using pooled data from 7 randomized clinical trials including 3341 patients.68 Both tools seek to predict absolute benefit for patients treated with fluorouracil, though data suggests Adjuvant! Online may be more reliable in its predictive ability.69 Adjuvant! Online has also been validated in an Asian population70 and patients older than 70 years.71
MUTATIONAL ANALYSIS
Multiple mutations in proto-oncogenes have been found in colon cancer cells. One such proto-oncogene is BRAF, which encodes a serine-threonine kinase in the rapidly accelerated fibrosarcoma (RAF). Mutations in BRAF have been found in 5% to 10% of colon cancers and are associated with right-sided tumors.72 As a prognostic marker, some studies have associated BRAF mutations with worse prognosis, including shorter time to relapse and shorter OS.73,74 Two other proto-oncogenes are Kristen rat sarcoma viral oncogene homolog (KRAS) and neuroblastoma rat sarcoma viral oncogene homolog (NRAS), both of which encode proteins downstream of epidermal growth factor receptor (EGFR). KRAS and NRAS mutations have been shown to be predictive in the metastatic setting where they predict resistance to the EGFR inhibitors cetuximab and panitumumab.75,76 The effect of KRAS and NRAS mutations on outcome in stage II and III colon cancer is uncertain. Some studies suggest worse outcome in KRAS-mutated cancers,77 while others failed to demonstrate this finding.73
CASE PRESENTATION 1
A 53-year-old man with no past medical history presents to the emergency department with early satiety and generalized abdominal pain. Laboratory evaluation shows a microcytic anemia with normal white blood cell count, platelet count, renal function, and liver function tests. Computed tomography (CT) scan of the abdomen and pelvis show a 4-cm mass in the transverse colon without obstruction and without abnormality in the liver. CT scan of the chest does not demonstrate pathologic lymphadenopathy or other findings. He undergoes robotic laparoscopic transverse colon resection and appendectomy. Pathology confirms a 3.5-cm focus of adenocarcinoma of the colon with invasion through the muscularis propria and 5 of 27 regional lymph nodes positive for adenocarcinoma and uninvolved proximal, distal, and radial margins. He is given a stage of IIIB pT3 pN2a M0 and referred to medical oncology for further management, where 6 months of adjuvant FOLFOX chemotherapy is recommended.
ADJUVANT CHEMOTHERAPY IN STAGE III COLON CANCER
Postoperative adjuvant chemotherapy is the standard of care for patients with stage III disease. In the 1960s, infusional fluorouracil was first used to treat inoperable colon cancer.78,79 After encouraging results, the agent was used both intraluminally and intravenously as an adjuvant therapy for patients undergoing resection with curative intent; however, only modest benefits were described.80,81 The National Surgical Adjuvant Breast and Bowel Project (NSABP) C-01 trial (Table 3) was the first study to demonstrate a benefit from adjuvant chemotherapy in colon cancer. This study randomly assigned patients with stage II and III colon cancer to surgery alone, postoperative chemotherapy with fluorouracil, semustine, and vincristine (MOF), or postoperative bacillus Calmette-Guérin (BCG). DFS and OS were significantly improved with MOF chemotherapy.82 In 1990, a landmark study reported on outcomes after treatment of 1296 patients with stage III colon cancer with adjuvant fluorouracil and levamisole for 12 months. The combination was associated with a 41% reduction in risk of cancer recurrence and a 33% reduction in risk of death.83 The NSABP C-03 trial (Table 3) compared MOF to the combination of fluorouracil and leucovorin and demonstrated improved 3-year DFS (69% versus 73%) and 3-year OS (77% versus 84%) in patients with stage III disease.84 Building on these outcomes, the QUASAR study (Table 3) compared fluorouracil in combination with one of levamisole, low-dose leucovorin, or high-dose leucovorin. The study enrolled 4927 patients and found worse outcomes with fluorouracil plus levamisole and no difference in low-doseversus high-dose leucovorin.85 Levamisole fell out of use after associations with development of multifocal leukoencephalopathy,86 and was later shown to have inferior outcomes versus leucovorin when combined with fluorouracil.87,88 Intravenous fluorouracil has shown similar benefit when administered by bolus or infusion,89 although continuous infusion has been associated with lower incidence of severe toxicity.90 The efficacy of the oral fluoropyrimidine capecitabine has been shown to be equivalent to that of fluorouracil.91
Fluorouracil-based treatment remained the standard of care until the introduction of oxaliplatin in the mid-1990s. After encouraging results in the metastatic setting,92,93 the agent was moved to the adjuvant setting. The MOSAIC trial (Table 3) randomly assigned patients with stage II and III colon cancer to fluorouracil with leucovorin (FULV) versus FOLFOX given once every 2 weeks for 12 cycles. Analysis with respect to stage III patients showed a clear survival benefit, with a 10-year OS of 67.1% with FOLFOX chemotherapy versus 59% with fluorouracil and leucovorin.94,95 The NSABP C-07 (Table 3) trial used a similar trial design but employed bolus fluorouracil. More than 2400 patients with stage II and III colon cancer were randomly assigned to bolus FULV or bolus fluorouracil, leucovorin, and oxaliplatin (FLOX). The addition of oxaliplatin significantly improved outcomes, with 4-year DFS of 67% versus 71.8% for FULV and FLOX, respectively, and a HR of death of 0.80 with FLOX.59,96 The multicenter N016968 trial (Table 3) randomly assigned 1886 patients with stage III colon cancer to adjuvant capecitabine plus oxaliplatin (XELOX) or bolus fluorouracil plus leucovorin (FU/FA). The 3-year DFS was 70.9% versus 66.5% with XELOX and FU/FA, respectively, and 5-year OS was 77.6% versus 74.2%, respectively.97,98
In the metastatic setting, additional agents have shown efficacy, including irinotecan,99,100 bevacizumab,101,102 cetuximab,103,104 and regorafenib.105 This observation led to testing of these agents in earlier stage disease. The CALGB 89803 trial compared fluorouracil, leucovorin, and irinotecan to fluorouracil with leucovorin alone. No benefit in 5-year DFS or OS was seen.106 Similarly, infusional fluorouracil, leucovorin, and irinotecan (FOLFIRI) was not found to improve 5-year DFS as compared to fluorouracil with leucovorin alone in the PETACC-3 trial.107 The NSABP C-08 trial considered the addition of bevacizumab to FOLFOX. When compared to FOLFOX alone, the combination of bevacizumab to FOLFOX had similar 3-year DFS (77.9% versus 75.1%) and 5-year OS (82.5% versus 80.7%).108 This finding was confirmed in the Avant trial.109 The addition of cetuximab to FOLFOX was equally disappointing, as shown in the N0147 trial110 and PETACC-8 trial.111 Data on regorafenib in the adjuvant setting for stage III colon cancer is lacking; however, 2 ongoing clinical trials, NCT02425683 and NCT02664077, are each studying the use of regorafenib following completion of FOLFOX for patients with stage III disease.
Thus, after multiple trials comparing various regimens and despite attempts to improve outcomes by the addition of a third agent, the standard of care per National Comprehensive Cancer Network (NCCN) guidelines for management of stage III colon cancer remains 12 cycles of FOLFOX chemotherapy. Therapy should be initiated within 8 weeks of surgery. Data are emerging to support a short duration of therapy for patients with low-risk stage III tumors, as shown in an abstract presented at the 2017 American Society of Clinical Oncology annual meeting. The IDEA trial was a pooled analysis of 6 randomized clinical trials across multiple countries, all of which evaluated 3 versus 6 months of FOLFOX or capecitabine and oxaliplatin in the treatment of stage III colon cancer. The analysis was designed to test non-inferiority of 3 months of therapy as compared to 6 months. The analysis included 6088 patients across 244 centers in 6 countries. The overall analysis failed to establish noninferiority. The 3-year DFS rate was 74.6% for 3 months and 75.5% for 6 months, with a DFS HR of 1.07 and a confidence interval that did not meet the prespecified endpoint. Subgroup analysis suggested noninferiority for lower stage disease (T1–3 or N1) but not for higher stage disease (T4 or N2). Given the high rates of neuropathy with 6 months of oxaliplatin, these results suggest that 3 months of adjuvant therapy can be considered for patients with T1–3 or N1 disease in an attempt to limit toxicity.112
CASE PRESENTATION 2
A 57-year-old woman presents to the emergency department with fever and abdominal pain. CT of the abdomen and pelvis demonstrates a left-sided colonic mass with surrounding fat stranding and pelvic abscess. She is taken emergently for left hemicolectomy, cholecystectomy, and evacuation of pelvic abscess. Pathology reveals a 5-cm adenocarcinoma with invasion through the visceral peritoneum; 0/22 lymph nodes are involved. She is given a diagnosis of stage IIC and referred to medical oncology for further management. Due to her young age and presence of high-risk features, she is recommended adjuvant therapy with FOLFOX for 6 months.
ADJUVANT CHEMOTHERAPY IN STAGE II COLON CANCER
Because of excellent outcomes with surgical resection alone for stage II cancers, the use of adjuvant chemotherapy for patients with stage II disease is controversial. Limited prospective data is available to guide adjuvant treatment decisions for stage II patients. The QUASAR trial, which compared observation to adjuvant fluorouracil and leucovorin in patients with early-stage colon cancer, included 2963 patients with stage II disease and found a relative risk (RR) of death or recurrence of 0.82 and 0.78, respectively. Importantly, the absolute benefit of therapy was less than 5%.113 The IMPACT-B2 trial (Table 3) combined data from 5 separate trials and analyzed 1016 patients with stage II colon cancer who received fluorouracil with leucovorin or observation. Event-free survival was 0.86 versus 0.83 and 5-year OS was 82% versus 80%, suggesting no benefit.114 The benefit of addition of oxaliplatin to fluorouracil in stage II disease appears to be less than the benefit of adding this agent in the treatment of stage III CRC. As noted above, the MOSAIC trial randomly assigned patients with stage II and III colon cancer to receive adjuvant fluorouracil and leucovorin with or without oxaliplatin for 12 cycles. After a median follow-up of 9.5 years, 10-year OS rates for patients with stage II disease were 78.4% versus 79.5%. For patients with high-risk stage II disease (defined as T4, bowel perforation, or fewer than 10 lymph nodes examined), 10-year OS was 71.7% and 75.4% respectively, but these differences were not statistically significant.94
Because of conflicting data as to the benefit of adding oxaliplatin in stage II disease, oxaliplatin is not recommended for standard-risk stage II patients. The use of oxaliplatin in high-risk stage II tumors should be weighed carefully given the toxicity risk. Oxaliplatin is recognized to cause sensory neuropathy in many patients, which can become painful and debilitating.115 Two types of neuropathy are associated with oxaliplatin: acute and chronic. Acute neuropathy manifests most often as cold-induced paresthesias in the fingers and toes and is quite common, affecting up to 90% of patients. These symptoms are self-limited and resolve usually within 1 week of each treatment.116 Some patients, with reports ranging from 10% to 79%, develop chronic neuropathy that persists for 1 year or more and causes significant decrements in quality of life.117 Patients older than age 70 may be at greater risk for oxaliplatin-induced neuropathy, which would increase risk of falls in this population.118 In addition to neuropathy, oxaliplatin is associated with hypersensitivity reactions that can be severe and even fatal.119 In a single institution series, the incidence of severe reactions was 2%.120 Desensitization following hypersensitivity reactions is possible but requires a time-intensive protocol.121
Based on the inconclusive efficacy findings and due to concerns over toxicity, each decision must be individualized to fit patient characteristics and preferences. In general, for patients with stage II disease without high-risk features, an individualized discussion should be held as to the risks and benefits of single-agent fluorouracil, and this treatment should be offered in cases where the patient or provider would like to be aggressive. Patients with stage II cancer who have 1 or more high-risk features are often recommended adjuvant chemotherapy. Whether treatment with fluorouracil plus leucovorin or FOLFOX is preferred remains uncertain, and thus the risks and the potential gains of oxaliplatin must be discussed with the individual patient. MMR status can also influence the treatment recommendation for patients with stage II disease. In general, patients with standard-risk stage II tumors that are pMMR are offered MMR with leucovorin or oral capecitabine for 12 cycles. FOLFOX is considered for patients with MSI-high disease and those with multiple high-risk features.
MONITORING AFTER THERAPY
After completion of adjuvant chemotherapy, patients enter a period of survivorship. Patients are seen in clinic for symptom and laboratory monitoring of the complete blood count, liver function tests, and carcinoembryonic antigen (CEA). NCCN guidelines support history and physical examination with CEA testing every 3 to 6 months for the first 2 years, then every 6 months for the next 3 years, after which many patients continue to be seen annually. CT imaging of the chest, abdomen, and pelvis for monitoring of disease recurrence is recommended every 6 to 12 months for a total of 5 years. New elevations in CEA or liver function tests should prompt early imaging. Colonoscopy should be performed 1 year after completion of therapy; however, if no preoperative colonoscopy was performed, this should be done 3 to 6 months after completion. Colonoscopy is then repeated in 3 years and then every 5 years unless advanced adenomas are present.122
SUMMARY
The addition of chemotherapy to surgical management of colon cancer has lowered the rate of disease recurrence and improved long-term survival. Adjuvant FOLFOX for 12 cycles is the standard of care for patients with stage III colon cancer and for patients with stage II disease with certain high-risk features. Use of adjuvant chemotherapy in stage II disease without high-risk features is controversial, and treatment decisions should be individualized. Biologic markers such as MSI and CDX2 status as well as patient-related factors including age, overall health, and personal preferences can inform treatment decisions. If chemotherapy is recommended in this setting, it would be with single-agent fluorouracil in an infusional or oral formulation, unless the tumor has the MSI-high feature. Following completion of adjuvant therapy, patients should be followed with clinical evaluation, laboratory testing, and imaging for a total of 5 years as per recommended guidelines.
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA Cancer J Clin 2017;67(1):7–30.
- United States Cancer Statistics. 1999–2013 incidence and mortality web-based report. Atlanta: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention and National Cancer Institute, 2016. www.cdc.gov/uscs. Accessed June 12, 2017.
- Ahnen DJ, Wade SW, Jones WF, et al. The increasing incidence of young-onset colorectal cancer: a call to action. Mayo Clin Proc 2014;89:216–24.
- Jemal A, Fedewa SA, Anderson WF, et al. Colorectal cancer incidence patterns in the United States, 1974–2013. J Natl Cancer Inst 2017;109(8).
- Boursi B, Sella T, Liberman E, et al. The APC p.I1307K polymorphism is a significant risk factor for CRC in average risk Ashkenazi Jews. Eur J Cancer 2013;49:3680–5.
- Parry S, Win AK, Parry B, et al. Metachronous colorectal cancer risk for mismatch repair gene mutation carriers: the advantage of more extensive colon surgery. Gut 2011;60: 950–7.
- van Puijenbroek M, Nielsen M, Tops CM, et al. Identification of patients with (atypical) MUTYH-associated polyposis by KRAS2 c.34G > T prescreening followed by MUTYH hotspot analysis in formalin-fixed paraffin-embedded tissue. Clin Cancer Res 2008;14:139–42.
- Aretz S, Uhlhaas S, Goergens H, et al. MUTYH-associated polyposis: 70 of 71 patients with biallelic mutations present with an attenuated or atypical phenotype. Int J Cancer 2006;119:807–14.
- Tuohy TM, Rowe KG, Mineau GP, et al. Risk of colorectal cancer and adenomas in the families of patients with adenomas: a population-based study in Utah. Cancer 2014;120:35–42.
- Choi Y, Sateia HF, Peairs KS, Stewart RW. Screening for colorectal cancer. Semin Oncol 2017; 44:34–44.
- Atkin WS, Morson BC, Cuzick J. Long-term risk of colorectal cancer after excision of rectosigmoid adenomas. N Engl J Med 1992;326:658–62.
- Rutter MD. Surveillance programmes for neoplasia in colitis. J Gastroenterol 2011;46 Suppl 1:1–5.
- Giovannucci E. Modifiable risk factors for colon cancer. Gastroenterol Clin North Am 2002;31:925–43.
- Michels KB, Fuchs GS, Giovannucci E, et al. Fiber intake and incidence of colorectal cancer among 76,947 women and 47,279 men. Cancer Epidemiol Biomarkers Prev 2005;14:842–9.
- Omata F, Brown WR, Tokuda Y, et al. Modifiable risk factors for colorectal neoplasms and hyperplastic polyps. Intern Med 2009;48:123–8.
- Friedenreich CM, Neilson HK, Lynch BM. State of the epidemiological evidence on physical activity and cancer prevention. Eur J Cancer 2010;46:2593–604.
- Aleksandrova K, Pischon T, Jenab M, et al. Combined impact of healthy lifestyle factors on colorectal cancer: a large European cohort study. BMC Med 2014;12:168.
- Hermanek P, Wittekind C. The pathologist and the residual tumor (R) classification. Pathol Res Pract 1994;190:115–23.
- Lehnert T, Methner M, Pollok A, et al. Multivisceral resection for locally advanced primary colon and rectal cancer: an analysis of prognostic factors in 201 patients. Ann Surg 2002;235:217–25.
- Feinberg AE, et al. Oncologic outcomes following laparoscopic versus open resection of pT4 colon cancer: a systematic review and meta-analysis. Dis Colon Rectum 2017;60:116–125.
- Vignali A, et al. Laparoscopic treatment of advanced colonic cancer: a case-matched control with open surgery. Colorectal Dis 2013;15:944–8.
- Gainant A. Emergency management of acute colonic cancer obstruction. J Visc Surg 2012;149: e3–e10.
- Rosenman LD. Hartmann’s operation. Am J Surg 1994;168:283–4.
- Lee-Kong S, Lisle D. Surgical management of complicated colon cancer. Clin Colon Rectal Surg 2015;28:228–33.
- Bertelsen CA. Complete mesocolic excision an assessment of feasibility and outcome. Dan Med J 2017;64(2).
- Wolff WI SH. Definitive treatment of “malignant” polyps of the colon. Ann Surg 1975;182:516–25.
- Clinical Outcomes of Surgical Therapy Study Group, Nelson H, Sargent DJ, Wieand HS, et al. A comparison of laparoscopically assisted and open colectomy for colon cancer. N Engl J Med 2004;350:2050–9.
- Gunderson LL, Jessup JM, Sarjent DJ, et al. Revised tumor and node categorization for rectal cancer based on surveillance, epidemiology, and end results and rectal pooled analysis outcomes. J Clin Oncol 2010;28:256–63.
- Noone AM, Cronin KA, Altekruse SF, et al. Cancer incidence and survival trends by subtype using data from the Surveillance Epidemiology and End Results Program, 1992-2013. Cancer Epidemiol Biomarkers Prev 2017;26:632–41.
- Alves A, Panis Y, Mathieu P, et al. Postoperative mortality and morbidity in French patients undergoing colorectal surgery: results of a prospective multicenter study. Arch Surg 2005;140:278–83.
- Popescu RA, Norman A, Ross PJ, et al, Adjuvant or palliative chemotherapy for colorectal cancer in patients 70 years or older. J Clin Oncol 1999;17:2412–8.
- McCleary NJ, Meyerhardt JA, Green E, et al. Impact of age on the efficacy of newer adjuvant therapies in patients with stage II/III colon cancer: findings from the ACCENT database. J Clin Oncol 2013;31:2600–6.
- Tominaga T, Nonaka T, Sumida Y, et al. Effectiveness of adjuvant chemotherapy for elderly patients with lymph node-positive colorectal cancer. World J Surg Oncol 2016;14:197.
- Bos AC, van Erning FN, van Gestel YR, et al. Timing of adjuvant chemotherapy and its relation to survival among patients with stage III colon cancer. Eur J Cancer 2015;51:2553–61.
- Peixoto RD, Kumar A, Speers C, et al. Effect of delay in adjuvant oxaliplatin-based chemotherapy for stage III colon cancer. Clin Colorectal Cancer 2015;14:25–30.
- Compton CC, Fielding LP, Burgart LJ, et al. Prognostic factors in colorectal cancer. College of American Pathologists Consensus Statement 1999. Arch Pathol Lab Med 2000;124:979–94.
- Lieu CH, Lambert LA, Wolff RA, et al. Systemic chemotherapy and surgical cytoreduction for poorly differentiated and signet ring cell adenocarcinomas of the appendix. Ann Oncol 2012;23:652–8.
- Krasna MJ, Flancbaum L, Cody RP, et al. Vascular and neural invasion in colorectal carcinoma. Incidence and prognostic significance. Cancer 1988;61:1018–23.
- Cianchi F, Palomba A, Boddi V, et al. Lymph node recovery from colorectal tumor specimens: recommendation for a minimum number of lymph nodes to be examined. World J Surg 2002;26:384–9.
- Yoshimatsu K, et al. How many lymph nodes should be examined in Dukes’ B colorectal cancer? Determination on the basis of cumulative survival rate. Hepatogastroenterology 2005;52:1703–6.
- Caplin S, Cerottini JP, Bosman FT, et al. For patients with Dukes’ B (TNM Stage II) colorectal carcinoma, examination of six or fewer lymph nodes is related to poor prognosis. Cancer 1998;83:666–72.
- Veronese N, Nottegar A, Pea A, et al. Prognostic impact and implications of extracapsular lymph node involvement in colorectal cancer: a systematic review with meta-analysis. Ann Oncol 2016;27:42–8.
- Li J, Yang S, Hu J, et al. Tumor deposits counted as positive lymph nodes in TNM staging for advanced colorectal cancer: a retrospective multicenter study. Oncotarget 2016;7:18269–79.
- Venook A, Niedzwiecki D, Innocenti Fet al. Impact of primary (1º) tumor location on overall survival (OS) and progression-free survival (PFS) in patients (pts) with metastatic colorectal cancer (mCRC): Analysis of CALGB/SWOG 80405 (Alliance). J Clin Oncol 2016;34 no. 15 suppl. Abstract 3504.
- Schrag D, Brooks G, Meyerhardt JA ,et al. The relationship between primary tumor sidedness and prognosis in colorectal cancer. J Clin Oncol 2016;34 no. 15 suppl. Abstract 3505.
- Larrea AA, Lujan SA, Kunkel TA. SnapShot: DNA mismatch repair. Cell 2010;141:730 e1.
- Jass JR. Pathology of hereditary nonpolyposis colorectal cancer. Ann N Y Acad Sci 2000;910:62–73.
- Lynch HT, Smyrk T. Hereditary nonpolyposis colorectal cancer (Lynch syndrome). An updated review. Cancer 1996;78:1149–67.
- Aaltonen LA, Peltomäki P, Leach FS, et al. Clues to the pathogenesis of familial colorectal cancer. Science 1993;260:812–6.
- Chen W, Swanson BJ, Frankel WL. Molecular genetics of microsatellite-unstable colorectal cancer for pathologists. Diagn Pathol 2017;12:24.
- Bupathi M, Wu C. Biomarkers for immune therapy in colorectal cancer: mismatch-repair deficiency and others. J Gastrointest Oncol 2016;7:713–20.
- Popat S, Hubner R, Houlston RS. Systematic review of microsatellite instability and colorectal cancer prognosis. J Clin Oncol 2005;23:609–18.
- Gryfe R, Kim H, Hsieh ET, et al. Tumor microsatellite instability and clinical outcome in young patients with colorectal cancer. N Engl J Med 2000;342:69–77.
- Ogino S, Kuchiba A, Qian ZR, et al. Prognostic significance and molecular associations of 18q loss of heterozygosity: a cohort study of microsatellite stable colorectal cancers. J Clin Oncol 2009; 27:4591–8.
- Kim ST, Lee J, Park SH, et al. The effect of DNA mismatch repair (MMR) status on oxaliplatin-based first-line chemotherapy as in recurrent or metastatic colon cancer. Med Oncol 2010;27:1277–85.
- Sargent DJ, Monges G, Thibodeau SN, et al. Therapy in colon cancer. J Clin Oncol 2010;28:4664.
- Ribic CM, Sargent DJ, Moore MJ, et al. Tumor microsatellite-instability status as a predictor of benefit from fluorouracil-based adjuvant chemotherapy for colon cancer. N Engl J Med 2003;349:247–57.
- Hutchins G, Southward K, Handley K, et al. Value of mismatch repair, KRAS, and BRAF mutations in predicting recurrence and benefits from chemotherapy in colorectal cancer. J Clin Oncol 2011;29:1261–270.
- Yothers G, O’Connell MJ, Allegra CJ, et al. Oxaliplatin as adjuvant therapy for colon cancer: updated results of NSABP C-07 trial, including survival and subset analyses J Clin Oncol 2011;29:3768–74.
- Chang SC, Lin JK, Lin TC, Liang WY. Loss of heterozygosity: an independent prognostic factor of colorectal cancer. World J Gastroenterol 2005;11:778–84.
- Bertagnolli MM, Niedzwiecki D, Compton CC, et al. Microsatellite instability predicts improved response to adjuvant therapy with irinotecan, fluorouracil, and leucovorin in stage III colon cancer: Cancer and Leukemia Group B Protocol 89803. J Clin Oncol 2009;27:1814–21.
- Bertagnolli MM, Redston M, Compton CC, et al. Microsatellite instability and loss of heterozygosity at chromosomal location 18q: prospective evaluation of biomarkers for stages II and III colon cancer--a study of CALGB 9581 and 89803. J Clin Oncol 2011;29:3153–62.
- Dalerba P, et al. CDX2 as a prognostic biomarker in stage II and stage III colon cancer. N Engl J Med 2016;374: 211–22.
- Clark-Langone KM, Wu JY, Sangli C, et al. Biomarker discovery for colon cancer using a 761 gene RT-PCR assay. BMC Genomics 2007;8:279.
- Gray RG, Quirke P, Handley K, et al. Validation study of a quantitative multigene reverse transcriptase-polymerase chain reaction assay for assessment of recurrence risk in patients with stage II colon cancer. J Clin Oncol 2011;29:4611–9.
- Niedzwiecki D, Bertagnolli MM, Warren RS, et al. Documenting the natural history of patients with resected stage II adenocarcinoma of the colon after random assignment to adjuvant treatment with edrecolomab or observation: results from CALGB 9581. J Clin Oncol 2011;29:3146–52.
- Yothers G, O’Connell MJ, Lee M, et al. Validation of the 12-gene colon cancer recurrence score in NSABP C-07 as a predictor of recurrence in patients with stage II and III colon cancer treated with fluorouracil and leucovorin (FU/LV) and FU/LV plus oxaliplatin. J Clin Oncol 2013;31:4512–9.
- Gill S, Loprinzi CL, Sargent DJ, et al. Pooled analysis of fluorouracil-based adjuvant therapy for stage II and III colon cancer: who benefits and by how much? J Clin Oncol 2004;22:1797–806.
- Gill S, Loprinzi C, Kennecke H, et al. Prognostic web-based models for stage II and III colon cancer: A population and clinical trials-based validation of numeracy and adjuvant! online. Cancer 2011;117:4155–65.
- Jung M, Kim GW, Jung I, et al. Application of the Western-based adjuvant online model to Korean colon cancer patients; a single institution experience. BMC Cancer 2012;12:471.
- Papamichael D, Renfro LA, Matthaiou C, et al. Validity of Adjuvant! Online in older patients with stage III colon cancer based on 2967 patients from the ACCENT database. J Geriatr Oncol 2016;7:422–9.
- Tran B, Kopetz S, Tie J, et al. Impact of BRAF mutation and microsatellite instability on the pattern of metastatic spread and prognosis in metastatic colorectal cancer. Cancer 2011;117:4623–32.
- Roth AD, Tejpar S, Delorenzi M, et al. Prognostic role of KRAS and BRAF in stage II and III resected colon cancer: results of the translational study on the PETACC-3, EORTC 40993, SAKK 60-00 trial. J Clin Oncol 2010;28:466–74.
- Lochhead P, Kuchiba A, Imamura Y, et al. Microsatellite instability and BRAF mutation testing in colorectal cancer prognostication. J Natl Cancer Inst 2013;105:1151–6.
- Benvenuti S, Sartore-Bianchi A, Di Nicolantonio F, et al. Oncogenic activation of the RAS/RAF signaling pathway impairs the response of metastatic colorectal cancers to anti-epidermal growth factor receptor antibody therapies. Cancer Res 2007;67:2643–8.
- Therkildsen C, Bergmann TK, Henrichsen-Schnack T, et al. The predictive value of KRAS, NRAS, BRAF, PIK3CA and PTEN for anti-EGFR treatment in metastatic colorectal cancer: A systematic review and meta-analysis. Acta Oncol 2014;53:852–64.
- Taieb J, Le Malicot K, Shi Q, et al. Prognostic value of BRAF and KRAS mutations in MSI and MSS stage III colon cancer. J Natl Cancer Inst 2017;109(5).
- Palumbo LT, Sharpe WS, Henry JS. Cancer of the colon and rectum; analysis of 300 cases. Am J Surg 1965;109:439–44.
- Sharp GS, Benefiel WW. 5-Fluorouracil in the treatment of inoperable carcinoma of the colon and rectum. Cancer Chemother Rep 1962;20:97–101.
- Lawrence W Jr, Terz JJ, Horsley JS 3rd, et al. Chemotherapy as an adjuvant to surgery for colorectal cancer. Ann Surg 1975;181:616–23.
- Grage TD, et al. Adjuvant chemotherapy with 5-fluorouracil after surgical resection of colorectal carcinoma (COG protocol 7041). A preliminary report. Am J Surg 1977;133:59–66.
- Wolmark N, Fisher B, Rockette H, et al. Postoperative adjuvant chemotherapy or BCG for colon cancer: results from NSABP protocol C-01. J Natl Cancer Inst 1988;80:30–6.
- Moertel CG, Fleming TR, Macdonald JS, et al. Levamisole and fluorouracil for adjuvant therapy of resected colon carcinoma. N Engl J Med 1990;322:352–8.
- Wolmark N, Rockette H, Fisher B, et al. The benefit of leucovorin-modulated fluorouracil as postoperative adjuvant therapy for primary colon cancer: results from National Surgical Adjuvant Breast and Bowel Project protocol C-03. J Clin Oncol 1993;11:1879–87.
- Comparison of fluorouracil with additional levamisole, higher-dose folinic acid, or both, as adjuvant chemotherapy for colorectal cancer: a randomised trial. QUASAR Collaborative Group. Lancet 2000;355(9215):1588–96.
- Chen TC, Hinton DR, Leichman L, et al. Multifocal inflammatory leukoencephalopathy associated with levamisole and 5-fluorouracil: case report. Neurosurgery 1994;35:1138-42.
- Porschen R, Bermann A, Löffler T, et al. Fluorouracil plus leucovorin as effective adjuvant chemotherapy in curatively resected stage III colon cancer: results of the trial adjCCA-01. J Clin Oncol 2001;19:1787–94.
- Arkenau HT, Bermann A, Rettig K, et al. 5-Fluorouracil plus leucovorin is an effective adjuvant chemotherapy in curatively resected stage III colon cancer: long-term follow-up results of the adjCCA-01 trial. Ann Oncol 2003;14:395–9.
- Weinerman B, Shah A, Fields A, et al. Systemic infusion versus bolus chemotherapy with 5-fluorouracil in measurable metastatic colorectal cancer. Am J Clin Oncol 1992;15:518–23.
- Poplin EA, Benedetti JK, Estes NC, et al. Phase III Southwest Oncology Group 9415/Intergroup 0153 randomized trial of fluorouracil, leucovorin, and levamisole versus fluorouracil continuous infusion and levamisole for adjuvant treatment of stage III and high-risk stage II colon cancer. J Clin Oncol 2005;23:1819–25.
- Twelves C, Wong A, Nowacki MP, et al. Capecitabine as adjuvant treatment for stage III colon cancer. N Engl J Med 2005;352:2696–704.
- de Gramont A, Vignoud J, Tournigand C, et al. Oxaliplatin with high-dose leucovorin and 5-fluorouracil 48-hour continuous infusion in pretreated metastatic colorectal cancer. Eur J Cancer 1997;33:214–9.
- Diaz-Rubio E, Sastre J, Zaniboni A, et al. Oxaliplatin as single agent in previously untreated colorectal carcinoma patients: a phase II multicentric study. Ann Oncol 1998;9:105–8.
- André T, de Gramont A, Vernerey D, et al. Adjuvant fluorouracil, leucovorin, and oxaliplatin in Stage II to III Colon Cancer: Updated 10-Year Survival and Outcomes According to BRAF mutation and mismatch repair status of the MOSAIC Study. J Clin Oncol 2015;33:4176–87.
- Andre T, Boni C, Mounedji-Boudiaf L, et al. Oxaliplatin, fluorouracil, and leucovorin as adjuvant treatment for colon cancer. N Engl J Med 2004;350:2343–51.
- Kuebler JP, Wieand HS, O’Connell MJ, et al. Oxaliplatin combined with weekly bolus fluorouracil and leucovorin as surgical adjuvant chemotherapy for stage II and III colon cancer: results from NSABP C-07. J Clin Oncol 2007;25:2198–204.
- Haller DG, Tabernero J, Maroun J, et al. Capecitabine plus oxaliplatin compared with fluorouracil and folinic acid as adjuvant therapy for stage III colon cancer. J Clin Oncol 2011;29:1465–71.
- Schmoll HJ, et al. Capecitabine plus oxaliplatin compared with fluorouracil/folinic acid as adjuvant therapy for stage III colon cancer: final results of the NO16968 randomized controlled phase III trial. J Clin Oncol 2015;33:3733–40.
- Colucci G, Gebbia V, Paoletti G, et al. Phase III randomized trial of FOLFIRI versus FOLFOX4 in the treatment of advanced colorectal cancer: a multicenter study of the Gruppo Oncologico Dell’Italia Meridionale. J Clin Oncol 2005;23:4866–75.
- Tournigand C, André T, Achille E, et al. FOLFIRI followed by FOLFOX6 or the reverse sequence in advanced colorectal cancer: a randomized GERCOR study. J Clin Oncol 2004;22:229–37.
- Hurwitz H, Fehrenbacher L, Novotny W, et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med 2004;350:2335–42.
- Saltz LB, et al. Bevacizumab in combination with oxaliplatin-based chemotherapy as first-line therapy in metastatic colorectal cancer: a randomized phase III study. J Clin Oncol 2008;26:2013–9.
- Cremolini C, Loupakis F, Ruzzo A, et al. Predictors of benefit in colorectal cancer treated with cetuximab: are we getting “Lost in TranslationAL”? J Clin Oncol 2010;28:e173–4.
- Sorich MJ, Wiese MD, Rowland D, et al. Extended RAS mutations and anti-EGFR monoclonal antibody survival benefit in metastatic colorectal cancer: a meta-analysis of randomized, controlled trials. Ann Oncol 2015;26:13–21.
- Grothey A, van Cutsem E, Sobrero A, et al. Regorafenib monotherapy for previously treated metastatic colorectal cancer (CORRECT): an international, multicentre, randomised, placebo-controlled, phase 3 trial. Lancet 2013;381(9863):303–12.
- Saltz LB, Niedzwiecki D, Hollis D, et al. Irinotecan fluorouracil plus leucovorin is not superior to fluorouracil plus leucovorin alone as adjuvant treatment for stage III colon cancer: results of CALGB 89803. J Clin Oncol 2007;25:3456–61.
- Van Cutsem E, et al. Randomized phase III trial comparing biweekly infusional fluorouracil/leucovorin alone or with irinotecan in the adjuvant treatment of stage III colon cancer: PETACC-3. J Clin Oncol 2009;27:3117–25.
- Allegra CJ, et al. Bevacizumab in stage II-III colon cancer: 5-year update of the National Surgical Adjuvant Breast and Bowel Project C-08 trial. J Clin Oncol 2013;31:359–64.
- de Gramont A, et al. Bevacizumab plus oxaliplatin-based chemotherapy as adjuvant treatment for colon cancer (AVANT): a phase 3 randomised controlled trial. Lancet Oncol 2012;13:1225–33.
- Alberts SR, et al. Effect of oxaliplatin, fluorouracil, and leucovorin with or without cetuximab on survival among patients with resected stage III colon cancer: a randomized trial. JAMA 2012;307:1383–93.
- Taieb J, et al. Oxaliplatin, fluorouracil, and leucovorin with or without cetuximab in patients with resected stage III colon cancer (PETACC-8): an open-label, randomised phase 3 trial. Lancet Oncol 2014;15:862–73.
- Shi Q, Sobrero AF, Shields AF, et al. Prospective pooled analysis of six phase III trials investigating duration of adjuvant (adjuvant) oxaliplatin-based therapy (3 vs 6 months) for patients (pts) with stage III colon cancer (CC): The IDEA (International Duration Evaluation of Adjuvant chemotherapy) collaboration. In: Proceedings from the American Society of Clinical Oncology; June 1–5, 2017; Chicago. Abstract LBA1.
- Quasar Collaborative Group; Gray R, Barnwell J, McConkey C, et al. Adjuvant chemotherapy versus observation in patients with colorectal cancer: a randomised study. Lancet 2007;370(9604):2020–9.
- Efficacy of adjuvant fluorouracil and folinic acid in B2 colon cancer. International Multicentre Pooled Analysis of B2 Colon Cancer Trials (IMPACT B2) Investigators. J Clin Oncol 1999;17:1356–63.
- Kidwell KM, et al. Long-term neurotoxicity effects of oxaliplatin added to fluorouracil and leucovorin as adjuvant therapy for colon cancer: results from National Surgical Adjuvant Breast and Bowel Project trials C-07 and LTS-01. Cancer 2012;118:5614–22.
- Beijers AJ, Mols F, Vreugdenhil G. A systematic review on chronic oxaliplatin-induced peripheral neuropathy and the relation with oxaliplatin administration. Support Care Cancer 2014;22:1999–2007.
- Mols F, Beijers T, Lemmens V, et al. Chemotherapy-induced neuropathy and its association with quality of life among 2- to 11-year colorectal cancer survivors: results from the population-based PROFILES registry. J Clin Oncol 2013;31:2699–707.
- Raphael MJ, Fischer HD, Fung K, et al. Neurotoxicity outcomes in a population-based cohort of elderly patients treated with adjuvant oxaliplatin for colorectal cancer. Clin Colorectal Cancer 2017 March 24.
- Toki MI, Saif MW, Syrigos KN. Hypersensitivity reactions associated with oxaliplatin and their clinical management. Expert Opin Drug Saf 2014;13:1545–54.
- Siu SW, Chan RT, Au GK. Hypersensitivity reactions to oxaliplatin: experience in a single institute. Ann Oncol 2006;17:259–61.
- Wong JT, Ling M, Patil S, et al. Oxaliplatin hypersensitivity: evaluation, implications of skin testing, and desensitization. J Allergy Clin Immunol Pract 2014;2:40–5.
- Benson AB 3rd, Venook AP, Cederquist L, et al. NCCN Guidelines Colon Cancer Version 2.2017. www.nccn.org/professionals/physician_gls/pdf/colon.pdf. Accessed May 8, 2017.
- Wolmark N, Rockette H, Mamounas E, et al. Clinical trial to assess the relative efficacy of fluorouracil and leucovorin, fluorouracil and levamisole, and fluorouracil, leucovorin, and levamisole in patients with Dukes’ B and C carcinoma of the colon: results from National Surgical Adjuvant Breast and Bowel Project C-04. J Clin Oncol 1999;17:3553–9.
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA Cancer J Clin 2017;67(1):7–30.
- United States Cancer Statistics. 1999–2013 incidence and mortality web-based report. Atlanta: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention and National Cancer Institute, 2016. www.cdc.gov/uscs. Accessed June 12, 2017.
- Ahnen DJ, Wade SW, Jones WF, et al. The increasing incidence of young-onset colorectal cancer: a call to action. Mayo Clin Proc 2014;89:216–24.
- Jemal A, Fedewa SA, Anderson WF, et al. Colorectal cancer incidence patterns in the United States, 1974–2013. J Natl Cancer Inst 2017;109(8).
- Boursi B, Sella T, Liberman E, et al. The APC p.I1307K polymorphism is a significant risk factor for CRC in average risk Ashkenazi Jews. Eur J Cancer 2013;49:3680–5.
- Parry S, Win AK, Parry B, et al. Metachronous colorectal cancer risk for mismatch repair gene mutation carriers: the advantage of more extensive colon surgery. Gut 2011;60: 950–7.
- van Puijenbroek M, Nielsen M, Tops CM, et al. Identification of patients with (atypical) MUTYH-associated polyposis by KRAS2 c.34G > T prescreening followed by MUTYH hotspot analysis in formalin-fixed paraffin-embedded tissue. Clin Cancer Res 2008;14:139–42.
- Aretz S, Uhlhaas S, Goergens H, et al. MUTYH-associated polyposis: 70 of 71 patients with biallelic mutations present with an attenuated or atypical phenotype. Int J Cancer 2006;119:807–14.
- Tuohy TM, Rowe KG, Mineau GP, et al. Risk of colorectal cancer and adenomas in the families of patients with adenomas: a population-based study in Utah. Cancer 2014;120:35–42.
- Choi Y, Sateia HF, Peairs KS, Stewart RW. Screening for colorectal cancer. Semin Oncol 2017; 44:34–44.
- Atkin WS, Morson BC, Cuzick J. Long-term risk of colorectal cancer after excision of rectosigmoid adenomas. N Engl J Med 1992;326:658–62.
- Rutter MD. Surveillance programmes for neoplasia in colitis. J Gastroenterol 2011;46 Suppl 1:1–5.
- Giovannucci E. Modifiable risk factors for colon cancer. Gastroenterol Clin North Am 2002;31:925–43.
- Michels KB, Fuchs GS, Giovannucci E, et al. Fiber intake and incidence of colorectal cancer among 76,947 women and 47,279 men. Cancer Epidemiol Biomarkers Prev 2005;14:842–9.
- Omata F, Brown WR, Tokuda Y, et al. Modifiable risk factors for colorectal neoplasms and hyperplastic polyps. Intern Med 2009;48:123–8.
- Friedenreich CM, Neilson HK, Lynch BM. State of the epidemiological evidence on physical activity and cancer prevention. Eur J Cancer 2010;46:2593–604.
- Aleksandrova K, Pischon T, Jenab M, et al. Combined impact of healthy lifestyle factors on colorectal cancer: a large European cohort study. BMC Med 2014;12:168.
- Hermanek P, Wittekind C. The pathologist and the residual tumor (R) classification. Pathol Res Pract 1994;190:115–23.
- Lehnert T, Methner M, Pollok A, et al. Multivisceral resection for locally advanced primary colon and rectal cancer: an analysis of prognostic factors in 201 patients. Ann Surg 2002;235:217–25.
- Feinberg AE, et al. Oncologic outcomes following laparoscopic versus open resection of pT4 colon cancer: a systematic review and meta-analysis. Dis Colon Rectum 2017;60:116–125.
- Vignali A, et al. Laparoscopic treatment of advanced colonic cancer: a case-matched control with open surgery. Colorectal Dis 2013;15:944–8.
- Gainant A. Emergency management of acute colonic cancer obstruction. J Visc Surg 2012;149: e3–e10.
- Rosenman LD. Hartmann’s operation. Am J Surg 1994;168:283–4.
- Lee-Kong S, Lisle D. Surgical management of complicated colon cancer. Clin Colon Rectal Surg 2015;28:228–33.
- Bertelsen CA. Complete mesocolic excision an assessment of feasibility and outcome. Dan Med J 2017;64(2).
- Wolff WI SH. Definitive treatment of “malignant” polyps of the colon. Ann Surg 1975;182:516–25.
- Clinical Outcomes of Surgical Therapy Study Group, Nelson H, Sargent DJ, Wieand HS, et al. A comparison of laparoscopically assisted and open colectomy for colon cancer. N Engl J Med 2004;350:2050–9.
- Gunderson LL, Jessup JM, Sarjent DJ, et al. Revised tumor and node categorization for rectal cancer based on surveillance, epidemiology, and end results and rectal pooled analysis outcomes. J Clin Oncol 2010;28:256–63.
- Noone AM, Cronin KA, Altekruse SF, et al. Cancer incidence and survival trends by subtype using data from the Surveillance Epidemiology and End Results Program, 1992-2013. Cancer Epidemiol Biomarkers Prev 2017;26:632–41.
- Alves A, Panis Y, Mathieu P, et al. Postoperative mortality and morbidity in French patients undergoing colorectal surgery: results of a prospective multicenter study. Arch Surg 2005;140:278–83.
- Popescu RA, Norman A, Ross PJ, et al, Adjuvant or palliative chemotherapy for colorectal cancer in patients 70 years or older. J Clin Oncol 1999;17:2412–8.
- McCleary NJ, Meyerhardt JA, Green E, et al. Impact of age on the efficacy of newer adjuvant therapies in patients with stage II/III colon cancer: findings from the ACCENT database. J Clin Oncol 2013;31:2600–6.
- Tominaga T, Nonaka T, Sumida Y, et al. Effectiveness of adjuvant chemotherapy for elderly patients with lymph node-positive colorectal cancer. World J Surg Oncol 2016;14:197.
- Bos AC, van Erning FN, van Gestel YR, et al. Timing of adjuvant chemotherapy and its relation to survival among patients with stage III colon cancer. Eur J Cancer 2015;51:2553–61.
- Peixoto RD, Kumar A, Speers C, et al. Effect of delay in adjuvant oxaliplatin-based chemotherapy for stage III colon cancer. Clin Colorectal Cancer 2015;14:25–30.
- Compton CC, Fielding LP, Burgart LJ, et al. Prognostic factors in colorectal cancer. College of American Pathologists Consensus Statement 1999. Arch Pathol Lab Med 2000;124:979–94.
- Lieu CH, Lambert LA, Wolff RA, et al. Systemic chemotherapy and surgical cytoreduction for poorly differentiated and signet ring cell adenocarcinomas of the appendix. Ann Oncol 2012;23:652–8.
- Krasna MJ, Flancbaum L, Cody RP, et al. Vascular and neural invasion in colorectal carcinoma. Incidence and prognostic significance. Cancer 1988;61:1018–23.
- Cianchi F, Palomba A, Boddi V, et al. Lymph node recovery from colorectal tumor specimens: recommendation for a minimum number of lymph nodes to be examined. World J Surg 2002;26:384–9.
- Yoshimatsu K, et al. How many lymph nodes should be examined in Dukes’ B colorectal cancer? Determination on the basis of cumulative survival rate. Hepatogastroenterology 2005;52:1703–6.
- Caplin S, Cerottini JP, Bosman FT, et al. For patients with Dukes’ B (TNM Stage II) colorectal carcinoma, examination of six or fewer lymph nodes is related to poor prognosis. Cancer 1998;83:666–72.
- Veronese N, Nottegar A, Pea A, et al. Prognostic impact and implications of extracapsular lymph node involvement in colorectal cancer: a systematic review with meta-analysis. Ann Oncol 2016;27:42–8.
- Li J, Yang S, Hu J, et al. Tumor deposits counted as positive lymph nodes in TNM staging for advanced colorectal cancer: a retrospective multicenter study. Oncotarget 2016;7:18269–79.
- Venook A, Niedzwiecki D, Innocenti Fet al. Impact of primary (1º) tumor location on overall survival (OS) and progression-free survival (PFS) in patients (pts) with metastatic colorectal cancer (mCRC): Analysis of CALGB/SWOG 80405 (Alliance). J Clin Oncol 2016;34 no. 15 suppl. Abstract 3504.
- Schrag D, Brooks G, Meyerhardt JA ,et al. The relationship between primary tumor sidedness and prognosis in colorectal cancer. J Clin Oncol 2016;34 no. 15 suppl. Abstract 3505.
- Larrea AA, Lujan SA, Kunkel TA. SnapShot: DNA mismatch repair. Cell 2010;141:730 e1.
- Jass JR. Pathology of hereditary nonpolyposis colorectal cancer. Ann N Y Acad Sci 2000;910:62–73.
- Lynch HT, Smyrk T. Hereditary nonpolyposis colorectal cancer (Lynch syndrome). An updated review. Cancer 1996;78:1149–67.
- Aaltonen LA, Peltomäki P, Leach FS, et al. Clues to the pathogenesis of familial colorectal cancer. Science 1993;260:812–6.
- Chen W, Swanson BJ, Frankel WL. Molecular genetics of microsatellite-unstable colorectal cancer for pathologists. Diagn Pathol 2017;12:24.
- Bupathi M, Wu C. Biomarkers for immune therapy in colorectal cancer: mismatch-repair deficiency and others. J Gastrointest Oncol 2016;7:713–20.
- Popat S, Hubner R, Houlston RS. Systematic review of microsatellite instability and colorectal cancer prognosis. J Clin Oncol 2005;23:609–18.
- Gryfe R, Kim H, Hsieh ET, et al. Tumor microsatellite instability and clinical outcome in young patients with colorectal cancer. N Engl J Med 2000;342:69–77.
- Ogino S, Kuchiba A, Qian ZR, et al. Prognostic significance and molecular associations of 18q loss of heterozygosity: a cohort study of microsatellite stable colorectal cancers. J Clin Oncol 2009; 27:4591–8.
- Kim ST, Lee J, Park SH, et al. The effect of DNA mismatch repair (MMR) status on oxaliplatin-based first-line chemotherapy as in recurrent or metastatic colon cancer. Med Oncol 2010;27:1277–85.
- Sargent DJ, Monges G, Thibodeau SN, et al. Therapy in colon cancer. J Clin Oncol 2010;28:4664.
- Ribic CM, Sargent DJ, Moore MJ, et al. Tumor microsatellite-instability status as a predictor of benefit from fluorouracil-based adjuvant chemotherapy for colon cancer. N Engl J Med 2003;349:247–57.
- Hutchins G, Southward K, Handley K, et al. Value of mismatch repair, KRAS, and BRAF mutations in predicting recurrence and benefits from chemotherapy in colorectal cancer. J Clin Oncol 2011;29:1261–270.
- Yothers G, O’Connell MJ, Allegra CJ, et al. Oxaliplatin as adjuvant therapy for colon cancer: updated results of NSABP C-07 trial, including survival and subset analyses J Clin Oncol 2011;29:3768–74.
- Chang SC, Lin JK, Lin TC, Liang WY. Loss of heterozygosity: an independent prognostic factor of colorectal cancer. World J Gastroenterol 2005;11:778–84.
- Bertagnolli MM, Niedzwiecki D, Compton CC, et al. Microsatellite instability predicts improved response to adjuvant therapy with irinotecan, fluorouracil, and leucovorin in stage III colon cancer: Cancer and Leukemia Group B Protocol 89803. J Clin Oncol 2009;27:1814–21.
- Bertagnolli MM, Redston M, Compton CC, et al. Microsatellite instability and loss of heterozygosity at chromosomal location 18q: prospective evaluation of biomarkers for stages II and III colon cancer--a study of CALGB 9581 and 89803. J Clin Oncol 2011;29:3153–62.
- Dalerba P, et al. CDX2 as a prognostic biomarker in stage II and stage III colon cancer. N Engl J Med 2016;374: 211–22.
- Clark-Langone KM, Wu JY, Sangli C, et al. Biomarker discovery for colon cancer using a 761 gene RT-PCR assay. BMC Genomics 2007;8:279.
- Gray RG, Quirke P, Handley K, et al. Validation study of a quantitative multigene reverse transcriptase-polymerase chain reaction assay for assessment of recurrence risk in patients with stage II colon cancer. J Clin Oncol 2011;29:4611–9.
- Niedzwiecki D, Bertagnolli MM, Warren RS, et al. Documenting the natural history of patients with resected stage II adenocarcinoma of the colon after random assignment to adjuvant treatment with edrecolomab or observation: results from CALGB 9581. J Clin Oncol 2011;29:3146–52.
- Yothers G, O’Connell MJ, Lee M, et al. Validation of the 12-gene colon cancer recurrence score in NSABP C-07 as a predictor of recurrence in patients with stage II and III colon cancer treated with fluorouracil and leucovorin (FU/LV) and FU/LV plus oxaliplatin. J Clin Oncol 2013;31:4512–9.
- Gill S, Loprinzi CL, Sargent DJ, et al. Pooled analysis of fluorouracil-based adjuvant therapy for stage II and III colon cancer: who benefits and by how much? J Clin Oncol 2004;22:1797–806.
- Gill S, Loprinzi C, Kennecke H, et al. Prognostic web-based models for stage II and III colon cancer: A population and clinical trials-based validation of numeracy and adjuvant! online. Cancer 2011;117:4155–65.
- Jung M, Kim GW, Jung I, et al. Application of the Western-based adjuvant online model to Korean colon cancer patients; a single institution experience. BMC Cancer 2012;12:471.
- Papamichael D, Renfro LA, Matthaiou C, et al. Validity of Adjuvant! Online in older patients with stage III colon cancer based on 2967 patients from the ACCENT database. J Geriatr Oncol 2016;7:422–9.
- Tran B, Kopetz S, Tie J, et al. Impact of BRAF mutation and microsatellite instability on the pattern of metastatic spread and prognosis in metastatic colorectal cancer. Cancer 2011;117:4623–32.
- Roth AD, Tejpar S, Delorenzi M, et al. Prognostic role of KRAS and BRAF in stage II and III resected colon cancer: results of the translational study on the PETACC-3, EORTC 40993, SAKK 60-00 trial. J Clin Oncol 2010;28:466–74.
- Lochhead P, Kuchiba A, Imamura Y, et al. Microsatellite instability and BRAF mutation testing in colorectal cancer prognostication. J Natl Cancer Inst 2013;105:1151–6.
- Benvenuti S, Sartore-Bianchi A, Di Nicolantonio F, et al. Oncogenic activation of the RAS/RAF signaling pathway impairs the response of metastatic colorectal cancers to anti-epidermal growth factor receptor antibody therapies. Cancer Res 2007;67:2643–8.
- Therkildsen C, Bergmann TK, Henrichsen-Schnack T, et al. The predictive value of KRAS, NRAS, BRAF, PIK3CA and PTEN for anti-EGFR treatment in metastatic colorectal cancer: A systematic review and meta-analysis. Acta Oncol 2014;53:852–64.
- Taieb J, Le Malicot K, Shi Q, et al. Prognostic value of BRAF and KRAS mutations in MSI and MSS stage III colon cancer. J Natl Cancer Inst 2017;109(5).
- Palumbo LT, Sharpe WS, Henry JS. Cancer of the colon and rectum; analysis of 300 cases. Am J Surg 1965;109:439–44.
- Sharp GS, Benefiel WW. 5-Fluorouracil in the treatment of inoperable carcinoma of the colon and rectum. Cancer Chemother Rep 1962;20:97–101.
- Lawrence W Jr, Terz JJ, Horsley JS 3rd, et al. Chemotherapy as an adjuvant to surgery for colorectal cancer. Ann Surg 1975;181:616–23.
- Grage TD, et al. Adjuvant chemotherapy with 5-fluorouracil after surgical resection of colorectal carcinoma (COG protocol 7041). A preliminary report. Am J Surg 1977;133:59–66.
- Wolmark N, Fisher B, Rockette H, et al. Postoperative adjuvant chemotherapy or BCG for colon cancer: results from NSABP protocol C-01. J Natl Cancer Inst 1988;80:30–6.
- Moertel CG, Fleming TR, Macdonald JS, et al. Levamisole and fluorouracil for adjuvant therapy of resected colon carcinoma. N Engl J Med 1990;322:352–8.
- Wolmark N, Rockette H, Fisher B, et al. The benefit of leucovorin-modulated fluorouracil as postoperative adjuvant therapy for primary colon cancer: results from National Surgical Adjuvant Breast and Bowel Project protocol C-03. J Clin Oncol 1993;11:1879–87.
- Comparison of fluorouracil with additional levamisole, higher-dose folinic acid, or both, as adjuvant chemotherapy for colorectal cancer: a randomised trial. QUASAR Collaborative Group. Lancet 2000;355(9215):1588–96.
- Chen TC, Hinton DR, Leichman L, et al. Multifocal inflammatory leukoencephalopathy associated with levamisole and 5-fluorouracil: case report. Neurosurgery 1994;35:1138-42.
- Porschen R, Bermann A, Löffler T, et al. Fluorouracil plus leucovorin as effective adjuvant chemotherapy in curatively resected stage III colon cancer: results of the trial adjCCA-01. J Clin Oncol 2001;19:1787–94.
- Arkenau HT, Bermann A, Rettig K, et al. 5-Fluorouracil plus leucovorin is an effective adjuvant chemotherapy in curatively resected stage III colon cancer: long-term follow-up results of the adjCCA-01 trial. Ann Oncol 2003;14:395–9.
- Weinerman B, Shah A, Fields A, et al. Systemic infusion versus bolus chemotherapy with 5-fluorouracil in measurable metastatic colorectal cancer. Am J Clin Oncol 1992;15:518–23.
- Poplin EA, Benedetti JK, Estes NC, et al. Phase III Southwest Oncology Group 9415/Intergroup 0153 randomized trial of fluorouracil, leucovorin, and levamisole versus fluorouracil continuous infusion and levamisole for adjuvant treatment of stage III and high-risk stage II colon cancer. J Clin Oncol 2005;23:1819–25.
- Twelves C, Wong A, Nowacki MP, et al. Capecitabine as adjuvant treatment for stage III colon cancer. N Engl J Med 2005;352:2696–704.
- de Gramont A, Vignoud J, Tournigand C, et al. Oxaliplatin with high-dose leucovorin and 5-fluorouracil 48-hour continuous infusion in pretreated metastatic colorectal cancer. Eur J Cancer 1997;33:214–9.
- Diaz-Rubio E, Sastre J, Zaniboni A, et al. Oxaliplatin as single agent in previously untreated colorectal carcinoma patients: a phase II multicentric study. Ann Oncol 1998;9:105–8.
- André T, de Gramont A, Vernerey D, et al. Adjuvant fluorouracil, leucovorin, and oxaliplatin in Stage II to III Colon Cancer: Updated 10-Year Survival and Outcomes According to BRAF mutation and mismatch repair status of the MOSAIC Study. J Clin Oncol 2015;33:4176–87.
- Andre T, Boni C, Mounedji-Boudiaf L, et al. Oxaliplatin, fluorouracil, and leucovorin as adjuvant treatment for colon cancer. N Engl J Med 2004;350:2343–51.
- Kuebler JP, Wieand HS, O’Connell MJ, et al. Oxaliplatin combined with weekly bolus fluorouracil and leucovorin as surgical adjuvant chemotherapy for stage II and III colon cancer: results from NSABP C-07. J Clin Oncol 2007;25:2198–204.
- Haller DG, Tabernero J, Maroun J, et al. Capecitabine plus oxaliplatin compared with fluorouracil and folinic acid as adjuvant therapy for stage III colon cancer. J Clin Oncol 2011;29:1465–71.
- Schmoll HJ, et al. Capecitabine plus oxaliplatin compared with fluorouracil/folinic acid as adjuvant therapy for stage III colon cancer: final results of the NO16968 randomized controlled phase III trial. J Clin Oncol 2015;33:3733–40.
- Colucci G, Gebbia V, Paoletti G, et al. Phase III randomized trial of FOLFIRI versus FOLFOX4 in the treatment of advanced colorectal cancer: a multicenter study of the Gruppo Oncologico Dell’Italia Meridionale. J Clin Oncol 2005;23:4866–75.
- Tournigand C, André T, Achille E, et al. FOLFIRI followed by FOLFOX6 or the reverse sequence in advanced colorectal cancer: a randomized GERCOR study. J Clin Oncol 2004;22:229–37.
- Hurwitz H, Fehrenbacher L, Novotny W, et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med 2004;350:2335–42.
- Saltz LB, et al. Bevacizumab in combination with oxaliplatin-based chemotherapy as first-line therapy in metastatic colorectal cancer: a randomized phase III study. J Clin Oncol 2008;26:2013–9.
- Cremolini C, Loupakis F, Ruzzo A, et al. Predictors of benefit in colorectal cancer treated with cetuximab: are we getting “Lost in TranslationAL”? J Clin Oncol 2010;28:e173–4.
- Sorich MJ, Wiese MD, Rowland D, et al. Extended RAS mutations and anti-EGFR monoclonal antibody survival benefit in metastatic colorectal cancer: a meta-analysis of randomized, controlled trials. Ann Oncol 2015;26:13–21.
- Grothey A, van Cutsem E, Sobrero A, et al. Regorafenib monotherapy for previously treated metastatic colorectal cancer (CORRECT): an international, multicentre, randomised, placebo-controlled, phase 3 trial. Lancet 2013;381(9863):303–12.
- Saltz LB, Niedzwiecki D, Hollis D, et al. Irinotecan fluorouracil plus leucovorin is not superior to fluorouracil plus leucovorin alone as adjuvant treatment for stage III colon cancer: results of CALGB 89803. J Clin Oncol 2007;25:3456–61.
- Van Cutsem E, et al. Randomized phase III trial comparing biweekly infusional fluorouracil/leucovorin alone or with irinotecan in the adjuvant treatment of stage III colon cancer: PETACC-3. J Clin Oncol 2009;27:3117–25.
- Allegra CJ, et al. Bevacizumab in stage II-III colon cancer: 5-year update of the National Surgical Adjuvant Breast and Bowel Project C-08 trial. J Clin Oncol 2013;31:359–64.
- de Gramont A, et al. Bevacizumab plus oxaliplatin-based chemotherapy as adjuvant treatment for colon cancer (AVANT): a phase 3 randomised controlled trial. Lancet Oncol 2012;13:1225–33.
- Alberts SR, et al. Effect of oxaliplatin, fluorouracil, and leucovorin with or without cetuximab on survival among patients with resected stage III colon cancer: a randomized trial. JAMA 2012;307:1383–93.
- Taieb J, et al. Oxaliplatin, fluorouracil, and leucovorin with or without cetuximab in patients with resected stage III colon cancer (PETACC-8): an open-label, randomised phase 3 trial. Lancet Oncol 2014;15:862–73.
- Shi Q, Sobrero AF, Shields AF, et al. Prospective pooled analysis of six phase III trials investigating duration of adjuvant (adjuvant) oxaliplatin-based therapy (3 vs 6 months) for patients (pts) with stage III colon cancer (CC): The IDEA (International Duration Evaluation of Adjuvant chemotherapy) collaboration. In: Proceedings from the American Society of Clinical Oncology; June 1–5, 2017; Chicago. Abstract LBA1.
- Quasar Collaborative Group; Gray R, Barnwell J, McConkey C, et al. Adjuvant chemotherapy versus observation in patients with colorectal cancer: a randomised study. Lancet 2007;370(9604):2020–9.
- Efficacy of adjuvant fluorouracil and folinic acid in B2 colon cancer. International Multicentre Pooled Analysis of B2 Colon Cancer Trials (IMPACT B2) Investigators. J Clin Oncol 1999;17:1356–63.
- Kidwell KM, et al. Long-term neurotoxicity effects of oxaliplatin added to fluorouracil and leucovorin as adjuvant therapy for colon cancer: results from National Surgical Adjuvant Breast and Bowel Project trials C-07 and LTS-01. Cancer 2012;118:5614–22.
- Beijers AJ, Mols F, Vreugdenhil G. A systematic review on chronic oxaliplatin-induced peripheral neuropathy and the relation with oxaliplatin administration. Support Care Cancer 2014;22:1999–2007.
- Mols F, Beijers T, Lemmens V, et al. Chemotherapy-induced neuropathy and its association with quality of life among 2- to 11-year colorectal cancer survivors: results from the population-based PROFILES registry. J Clin Oncol 2013;31:2699–707.
- Raphael MJ, Fischer HD, Fung K, et al. Neurotoxicity outcomes in a population-based cohort of elderly patients treated with adjuvant oxaliplatin for colorectal cancer. Clin Colorectal Cancer 2017 March 24.
- Toki MI, Saif MW, Syrigos KN. Hypersensitivity reactions associated with oxaliplatin and their clinical management. Expert Opin Drug Saf 2014;13:1545–54.
- Siu SW, Chan RT, Au GK. Hypersensitivity reactions to oxaliplatin: experience in a single institute. Ann Oncol 2006;17:259–61.
- Wong JT, Ling M, Patil S, et al. Oxaliplatin hypersensitivity: evaluation, implications of skin testing, and desensitization. J Allergy Clin Immunol Pract 2014;2:40–5.
- Benson AB 3rd, Venook AP, Cederquist L, et al. NCCN Guidelines Colon Cancer Version 2.2017. www.nccn.org/professionals/physician_gls/pdf/colon.pdf. Accessed May 8, 2017.
- Wolmark N, Rockette H, Mamounas E, et al. Clinical trial to assess the relative efficacy of fluorouracil and leucovorin, fluorouracil and levamisole, and fluorouracil, leucovorin, and levamisole in patients with Dukes’ B and C carcinoma of the colon: results from National Surgical Adjuvant Breast and Bowel Project C-04. J Clin Oncol 1999;17:3553–9.
Pre- and postprocedure skin care guide for your surgical patients
Whether patients are having a biopsy, surgical excision, or Mohs surgery, the outcome will be improved when the proper skin care is used before and after the procedure. This is a guide that you can use to educate your patients about pre- and postprocedure skin care needs.
Presurgery skin care and supplements
The goal is to speed healing and minimize infection, scarring, and hyperpigmentation. For 2 weeks prior to surgery, recommend products that have been shown to speed wound healing by increasing keratinization and/or collagen production. Ingredients that should be used prior to wounding include retinoids such as tretinoin and retinol. Several studies have convincingly shown that pretreatment with tretinoin speeds wound healing.1,2,3 Kligman and associates evaluated healing after punch biopsy and found the wounds on arms pretreated with tretinoin cream 0.05% to 0.1% were significantly smaller – by 35% to 37% – on days 1 and 4, and were 47% to 50% smaller on days 6, 8, and 11, compared with the untreated arms.4 Most studies suggest a 2- to 4-week tretinoin pretreatment regimen5 because peak epidermal hypertrophy occurs after 7 days of tretinoin application and normalizes after 14 days of continued treatment.6 This approach allows the skin to recover from any retinoid dermatitis prior to surgery. Adapalene should be started 5-6 weeks prior to procedures because it has a longer half-life and requires an earlier initiation period.7
Although wound healing studies have not been conducted in this area, pretreating skin with topical ascorbic acid8 and hydroxyacids9 might help speed wound healing by increasing collagen synthesis.
Ingredients and activities to avoid presurgery
Patients should avoid using ingredients that could promote skin tumor growth. Although there are no studies evaluating the effects of growth factors on promoting the growth of skin cancer, caution is prudent. To reduce bruising, patients should avoid aspirin, ibuprofen, naproxen, St. John’s Wort, vitamin E, omega-3 fatty acids supplements, flax seed oil, ginseng, salmon, and alcohol. Most physicians agree that these should be avoided for 10 days prior to the procedure. Smoking should be avoided 4 weeks prior to the procedure.
Postsurgery skin care and supplements
Oral vitamin C and zinc supplements have been shown to speed wound healing in rats when taken immediately after a procedure.10 Oral Arnica tablets and tinctures are often used prior to and after surgery to reduce bruising and inflammation. There is much anecdotal support for the use of Arnica, but clinical trial evidence substantiating its efficacy to prevent bruising and reduce swelling is scant.
A protein important in wound repair, defensin, is available in a topical formulation. Defensin14 has been shown to activate the leucine-rich repeat-containing G-protein–coupled receptors 5 and 6 (also known as LGR5 and LGR6) stem cells. It speeds wound healing by increasing LGR stem cell migration into wound beds. Wounds should be covered to provide protection from sun exposure until reepithelialization occurs. Which occlusive ointments and wound repair products to use are beyond the scope of this article. Once epithelized, zinc oxide sunscreens can be used. These have been shown to be safe with minimal penetration into the skin.15
Ingredients to avoid post surgery
Topical retinoids should not be used post skin cancer surgery until epithelialization is complete. A study by Hung et al.16 in a porcine model used 0.05% tretinoin cream daily for 10 days prior to partial-thickness skin wounding demonstrated that use of tretinoin 10 days prior to wounding sped reepithelialization while use after the procedure slowed wound healing.
Acidic products will sting wounded skin. For this reason, benzoic acid, hydroxy acids, and ascorbic acid should be avoided until the skin has completely reepithelialized. Products with preservatives and fragrance should be avoided if possible.
Vitamin E derived from oral supplement capsules slowed healing after skin cancer surgery and had a high rate of contact dermatitis.17 Chemical sunscreens are more likely to cause an allergic contact dermatitis and should be avoided for 4 weeks after skin surgery. Organic products with essential oils and botanical ingredients may present a higher risk of contact dermatitis due to allergen exposure.
Conclusion
To ensure the best outcome from surgical treatments, patient education is a must! The more that patients know and understand about the ways in which they can prepare for their procedure and treat their skin after the procedure, the better the outcomes will be. Providers should give this type of information in an easy-to-follow printed instruction sheet because studies show that patients cannot remember most of the oral instructions offered by practitioners.
Encourage your patients to ask questions during their consultation and procedure and to get in touch with your office should they have any concerns when they leave. These steps help improve patient compliance and satisfaction, which will help you maintain a trusting relationship with established patients and attract new ones through word-of-mouth referrals.
Please email me at DrB@skintypesolutions.com if you have any other pre- and postprocedure skin care advice.
Dr. Leslie S. Baumann is a private practice dermatologist, researcher, author, and entrepreneur who practices in Miami. She founded the Cosmetic Dermatology Center at the University of Miami in 1997. Dr. Baumann wrote two textbooks: “Cosmetic Dermatology: Principles and Practice” (New York: McGraw-Hill, 2002), and “Cosmeceuticals and Cosmetic Ingredients,” (New York: McGraw-Hill, 2014), and a New York Times Best Sellers book for consumers, “The Skin Type Solution” (New York: Bantam Dell, 2006). Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Evolus, Galderma, and Revance. She is the founder and CEO of Skin Type Solutions Franchise Systems LLC.
References
1. Aesthetic Plast Surg. 1995 May-Jun;19(3):243-6.
2. Plast Reconstr Surg. 2011 Mar;127(3):1343-5.
3. J Am Acad Dermatol. 1998 Aug;39(2 Pt 3):S79-81.
4. Br J Dermatol. 1995 Jan;132(1):46-53.
5. J Am Acad Dermatol. 2004 Dec;51(6):940-6.
6. J Korean Med Sci. 1996 Aug;11(4):335-41.
7. Eur J Dermatol. 2002 Mar-Apr;12(2):145-8.
8. Proc Natl Acad Sci U S A. 1981 May;78(5):2879-82.
9. Exp Dermatol. 2003;12 Suppl 2:57-63.
10. Surg Today. 2004;34(9):747-51.
11. Ophthal Plast Reconstr Surg. 2017 Jan/Feb;33(1):47-52.
12. Wound Repair Regen. 1998 Mar-Apr;6(2):167-77.
13. Dermatol Surg. 1998 Jun;24(6):661-4.
14. Plast Reconstr Surg. 2013 Nov;132(5):1159-71.
15. ACS Nano. 2016 Feb 23;10(2):1810-9.
16. Arch Dermatol. 1989 Jan;125(1):65-9.
17. Dermatol Surg. 1999 Apr;25(4):311-5.
Retinoids should be used 2-3 times prior to procedures to speed healing.
Retinoids should not be used after the procedure until reepithelization has occurred.
Vitamin C and zinc supplements taken post procedure might speed wound healing.
Retinoids should be used 2-3 times prior to procedures to speed healing.
Retinoids should not be used after the procedure until reepithelization has occurred.
Vitamin C and zinc supplements taken post procedure might speed wound healing.
Retinoids should be used 2-3 times prior to procedures to speed healing.
Retinoids should not be used after the procedure until reepithelization has occurred.
Vitamin C and zinc supplements taken post procedure might speed wound healing.
Whether patients are having a biopsy, surgical excision, or Mohs surgery, the outcome will be improved when the proper skin care is used before and after the procedure. This is a guide that you can use to educate your patients about pre- and postprocedure skin care needs.
Presurgery skin care and supplements
The goal is to speed healing and minimize infection, scarring, and hyperpigmentation. For 2 weeks prior to surgery, recommend products that have been shown to speed wound healing by increasing keratinization and/or collagen production. Ingredients that should be used prior to wounding include retinoids such as tretinoin and retinol. Several studies have convincingly shown that pretreatment with tretinoin speeds wound healing.1,2,3 Kligman and associates evaluated healing after punch biopsy and found the wounds on arms pretreated with tretinoin cream 0.05% to 0.1% were significantly smaller – by 35% to 37% – on days 1 and 4, and were 47% to 50% smaller on days 6, 8, and 11, compared with the untreated arms.4 Most studies suggest a 2- to 4-week tretinoin pretreatment regimen5 because peak epidermal hypertrophy occurs after 7 days of tretinoin application and normalizes after 14 days of continued treatment.6 This approach allows the skin to recover from any retinoid dermatitis prior to surgery. Adapalene should be started 5-6 weeks prior to procedures because it has a longer half-life and requires an earlier initiation period.7
Although wound healing studies have not been conducted in this area, pretreating skin with topical ascorbic acid8 and hydroxyacids9 might help speed wound healing by increasing collagen synthesis.
Ingredients and activities to avoid presurgery
Patients should avoid using ingredients that could promote skin tumor growth. Although there are no studies evaluating the effects of growth factors on promoting the growth of skin cancer, caution is prudent. To reduce bruising, patients should avoid aspirin, ibuprofen, naproxen, St. John’s Wort, vitamin E, omega-3 fatty acids supplements, flax seed oil, ginseng, salmon, and alcohol. Most physicians agree that these should be avoided for 10 days prior to the procedure. Smoking should be avoided 4 weeks prior to the procedure.
Postsurgery skin care and supplements
Oral vitamin C and zinc supplements have been shown to speed wound healing in rats when taken immediately after a procedure.10 Oral Arnica tablets and tinctures are often used prior to and after surgery to reduce bruising and inflammation. There is much anecdotal support for the use of Arnica, but clinical trial evidence substantiating its efficacy to prevent bruising and reduce swelling is scant.
A protein important in wound repair, defensin, is available in a topical formulation. Defensin14 has been shown to activate the leucine-rich repeat-containing G-protein–coupled receptors 5 and 6 (also known as LGR5 and LGR6) stem cells. It speeds wound healing by increasing LGR stem cell migration into wound beds. Wounds should be covered to provide protection from sun exposure until reepithelialization occurs. Which occlusive ointments and wound repair products to use are beyond the scope of this article. Once epithelized, zinc oxide sunscreens can be used. These have been shown to be safe with minimal penetration into the skin.15
Ingredients to avoid post surgery
Topical retinoids should not be used post skin cancer surgery until epithelialization is complete. A study by Hung et al.16 in a porcine model used 0.05% tretinoin cream daily for 10 days prior to partial-thickness skin wounding demonstrated that use of tretinoin 10 days prior to wounding sped reepithelialization while use after the procedure slowed wound healing.
Acidic products will sting wounded skin. For this reason, benzoic acid, hydroxy acids, and ascorbic acid should be avoided until the skin has completely reepithelialized. Products with preservatives and fragrance should be avoided if possible.
Vitamin E derived from oral supplement capsules slowed healing after skin cancer surgery and had a high rate of contact dermatitis.17 Chemical sunscreens are more likely to cause an allergic contact dermatitis and should be avoided for 4 weeks after skin surgery. Organic products with essential oils and botanical ingredients may present a higher risk of contact dermatitis due to allergen exposure.
Conclusion
To ensure the best outcome from surgical treatments, patient education is a must! The more that patients know and understand about the ways in which they can prepare for their procedure and treat their skin after the procedure, the better the outcomes will be. Providers should give this type of information in an easy-to-follow printed instruction sheet because studies show that patients cannot remember most of the oral instructions offered by practitioners.
Encourage your patients to ask questions during their consultation and procedure and to get in touch with your office should they have any concerns when they leave. These steps help improve patient compliance and satisfaction, which will help you maintain a trusting relationship with established patients and attract new ones through word-of-mouth referrals.
Please email me at DrB@skintypesolutions.com if you have any other pre- and postprocedure skin care advice.
Dr. Leslie S. Baumann is a private practice dermatologist, researcher, author, and entrepreneur who practices in Miami. She founded the Cosmetic Dermatology Center at the University of Miami in 1997. Dr. Baumann wrote two textbooks: “Cosmetic Dermatology: Principles and Practice” (New York: McGraw-Hill, 2002), and “Cosmeceuticals and Cosmetic Ingredients,” (New York: McGraw-Hill, 2014), and a New York Times Best Sellers book for consumers, “The Skin Type Solution” (New York: Bantam Dell, 2006). Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Evolus, Galderma, and Revance. She is the founder and CEO of Skin Type Solutions Franchise Systems LLC.
References
1. Aesthetic Plast Surg. 1995 May-Jun;19(3):243-6.
2. Plast Reconstr Surg. 2011 Mar;127(3):1343-5.
3. J Am Acad Dermatol. 1998 Aug;39(2 Pt 3):S79-81.
4. Br J Dermatol. 1995 Jan;132(1):46-53.
5. J Am Acad Dermatol. 2004 Dec;51(6):940-6.
6. J Korean Med Sci. 1996 Aug;11(4):335-41.
7. Eur J Dermatol. 2002 Mar-Apr;12(2):145-8.
8. Proc Natl Acad Sci U S A. 1981 May;78(5):2879-82.
9. Exp Dermatol. 2003;12 Suppl 2:57-63.
10. Surg Today. 2004;34(9):747-51.
11. Ophthal Plast Reconstr Surg. 2017 Jan/Feb;33(1):47-52.
12. Wound Repair Regen. 1998 Mar-Apr;6(2):167-77.
13. Dermatol Surg. 1998 Jun;24(6):661-4.
14. Plast Reconstr Surg. 2013 Nov;132(5):1159-71.
15. ACS Nano. 2016 Feb 23;10(2):1810-9.
16. Arch Dermatol. 1989 Jan;125(1):65-9.
17. Dermatol Surg. 1999 Apr;25(4):311-5.
Whether patients are having a biopsy, surgical excision, or Mohs surgery, the outcome will be improved when the proper skin care is used before and after the procedure. This is a guide that you can use to educate your patients about pre- and postprocedure skin care needs.
Presurgery skin care and supplements
The goal is to speed healing and minimize infection, scarring, and hyperpigmentation. For 2 weeks prior to surgery, recommend products that have been shown to speed wound healing by increasing keratinization and/or collagen production. Ingredients that should be used prior to wounding include retinoids such as tretinoin and retinol. Several studies have convincingly shown that pretreatment with tretinoin speeds wound healing.1,2,3 Kligman and associates evaluated healing after punch biopsy and found the wounds on arms pretreated with tretinoin cream 0.05% to 0.1% were significantly smaller – by 35% to 37% – on days 1 and 4, and were 47% to 50% smaller on days 6, 8, and 11, compared with the untreated arms.4 Most studies suggest a 2- to 4-week tretinoin pretreatment regimen5 because peak epidermal hypertrophy occurs after 7 days of tretinoin application and normalizes after 14 days of continued treatment.6 This approach allows the skin to recover from any retinoid dermatitis prior to surgery. Adapalene should be started 5-6 weeks prior to procedures because it has a longer half-life and requires an earlier initiation period.7
Although wound healing studies have not been conducted in this area, pretreating skin with topical ascorbic acid8 and hydroxyacids9 might help speed wound healing by increasing collagen synthesis.
Ingredients and activities to avoid presurgery
Patients should avoid using ingredients that could promote skin tumor growth. Although there are no studies evaluating the effects of growth factors on promoting the growth of skin cancer, caution is prudent. To reduce bruising, patients should avoid aspirin, ibuprofen, naproxen, St. John’s Wort, vitamin E, omega-3 fatty acids supplements, flax seed oil, ginseng, salmon, and alcohol. Most physicians agree that these should be avoided for 10 days prior to the procedure. Smoking should be avoided 4 weeks prior to the procedure.
Postsurgery skin care and supplements
Oral vitamin C and zinc supplements have been shown to speed wound healing in rats when taken immediately after a procedure.10 Oral Arnica tablets and tinctures are often used prior to and after surgery to reduce bruising and inflammation. There is much anecdotal support for the use of Arnica, but clinical trial evidence substantiating its efficacy to prevent bruising and reduce swelling is scant.
A protein important in wound repair, defensin, is available in a topical formulation. Defensin14 has been shown to activate the leucine-rich repeat-containing G-protein–coupled receptors 5 and 6 (also known as LGR5 and LGR6) stem cells. It speeds wound healing by increasing LGR stem cell migration into wound beds. Wounds should be covered to provide protection from sun exposure until reepithelialization occurs. Which occlusive ointments and wound repair products to use are beyond the scope of this article. Once epithelized, zinc oxide sunscreens can be used. These have been shown to be safe with minimal penetration into the skin.15
Ingredients to avoid post surgery
Topical retinoids should not be used post skin cancer surgery until epithelialization is complete. A study by Hung et al.16 in a porcine model used 0.05% tretinoin cream daily for 10 days prior to partial-thickness skin wounding demonstrated that use of tretinoin 10 days prior to wounding sped reepithelialization while use after the procedure slowed wound healing.
Acidic products will sting wounded skin. For this reason, benzoic acid, hydroxy acids, and ascorbic acid should be avoided until the skin has completely reepithelialized. Products with preservatives and fragrance should be avoided if possible.
Vitamin E derived from oral supplement capsules slowed healing after skin cancer surgery and had a high rate of contact dermatitis.17 Chemical sunscreens are more likely to cause an allergic contact dermatitis and should be avoided for 4 weeks after skin surgery. Organic products with essential oils and botanical ingredients may present a higher risk of contact dermatitis due to allergen exposure.
Conclusion
To ensure the best outcome from surgical treatments, patient education is a must! The more that patients know and understand about the ways in which they can prepare for their procedure and treat their skin after the procedure, the better the outcomes will be. Providers should give this type of information in an easy-to-follow printed instruction sheet because studies show that patients cannot remember most of the oral instructions offered by practitioners.
Encourage your patients to ask questions during their consultation and procedure and to get in touch with your office should they have any concerns when they leave. These steps help improve patient compliance and satisfaction, which will help you maintain a trusting relationship with established patients and attract new ones through word-of-mouth referrals.
Please email me at DrB@skintypesolutions.com if you have any other pre- and postprocedure skin care advice.
Dr. Leslie S. Baumann is a private practice dermatologist, researcher, author, and entrepreneur who practices in Miami. She founded the Cosmetic Dermatology Center at the University of Miami in 1997. Dr. Baumann wrote two textbooks: “Cosmetic Dermatology: Principles and Practice” (New York: McGraw-Hill, 2002), and “Cosmeceuticals and Cosmetic Ingredients,” (New York: McGraw-Hill, 2014), and a New York Times Best Sellers book for consumers, “The Skin Type Solution” (New York: Bantam Dell, 2006). Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Evolus, Galderma, and Revance. She is the founder and CEO of Skin Type Solutions Franchise Systems LLC.
References
1. Aesthetic Plast Surg. 1995 May-Jun;19(3):243-6.
2. Plast Reconstr Surg. 2011 Mar;127(3):1343-5.
3. J Am Acad Dermatol. 1998 Aug;39(2 Pt 3):S79-81.
4. Br J Dermatol. 1995 Jan;132(1):46-53.
5. J Am Acad Dermatol. 2004 Dec;51(6):940-6.
6. J Korean Med Sci. 1996 Aug;11(4):335-41.
7. Eur J Dermatol. 2002 Mar-Apr;12(2):145-8.
8. Proc Natl Acad Sci U S A. 1981 May;78(5):2879-82.
9. Exp Dermatol. 2003;12 Suppl 2:57-63.
10. Surg Today. 2004;34(9):747-51.
11. Ophthal Plast Reconstr Surg. 2017 Jan/Feb;33(1):47-52.
12. Wound Repair Regen. 1998 Mar-Apr;6(2):167-77.
13. Dermatol Surg. 1998 Jun;24(6):661-4.
14. Plast Reconstr Surg. 2013 Nov;132(5):1159-71.
15. ACS Nano. 2016 Feb 23;10(2):1810-9.
16. Arch Dermatol. 1989 Jan;125(1):65-9.
17. Dermatol Surg. 1999 Apr;25(4):311-5.
Hospital-level factors associated with pediatric emergency department return visits
Return visit (RV) rate is a quality measure commonly used in the emergency department (ED) setting. This metric may represent suboptimal care at the index ED visit.1-5 Although patient- and visit-level factors affecting ED RVs have been evaluated,1,3,4,6-9 hospital-level factors and factors of a hospital’s patient population that may play roles in ED RV rates have not been examined. Identifying the factors associated with increased RVs may allow resources to be designated to areas that improve emergent care for children.10
Hospital readmission rates are a closely followed quality measure and are linked to reimbursement by the federal government, but a recent study found the influence a hospital can have on this marker may be mitigated by the impact of the social determinates of health (SDHs) of the hospital’s patient population.11 That study and others have prompted an ongoing debate about adjusting quality measures for SDHs.12,13 A clearer understanding of these interactions may permit us to focus on factors that can truly lead to improvement in care instead of penalizing practitioners or hospitals that provide care to those most in need.
Prior work has identified several SDHs associated with higher ED RV rates in patient- or visit-level analyses.3,11,14 We conducted a study of hospital-level characteristics and characteristics of a hospital’s patient population to identify potentially mutable factors associated with increased ED RV rates that, once recognized, may allow for improvement in this quality measure.
PATIENTS AND METHODS
This study was not considered human subjects research in accordance with Common Rule 45 CFR§46.104(f) and was evaluated by the Ann and Robert H. Lurie Children’s Hospital and Northwestern University Feinberg School of Medicine Institutional Review Boards and deemed exempt from review.
Study Population and Protocol
Our study had 2 data sources (to be described in detail): the Pediatric Health Information System (PHIS) and a survey of ED medical directors of the hospitals represented within PHIS. Hospitals were eligible for inclusion in the study if their data (1) met PHIS quality control standards for ED patient visits as determined by internal data assurance processes incorporated in PHIS,3,14,15 (2) included data only from an identifiable single main ED, and (3) completed the ED medical director’s survey.
PHIS Database
PHIS, an administrative database managed by Truven Health Analytics, includes data from ED, ambulatory surgery, observation, and inpatient encounters across Children’s Hospital Association member children’s hospitals in North America. Data are subjected to validity checks before being included in the database.16 PHIS assigns unique patient identifiers to track individual patient visits within participating institutions over time.
Hospitals were described by percentages of ED patients in several groups: age (<1, 1-4, 5-9, 10-14, and 15-18 years)17; sex; race/ethnicity; insurance type (commercial, government, other); ED International Classification of Diseases, Ninth Edition (ICD-9) diagnosis code–based severity classification system score (1-2, low severity; 3-5, high severity)18; complex
ED Medical Director Survey
A web-based survey was constructed in an iterative process based on literature review and expert opinion to assess hospital-level factors that may impact ED RV rates.3,7,24-26 The survey was piloted at 3 institutions to refine its structure and content.
The survey included 15 close-ended or multiple-choice questions on ED environment and operations and 2 open-ended questions, “What is the largest barrier to reducing the number of return visits within 72 hours of discharge from a previous ED visit?” and “In your opinion, what is the best way of reducing the number of the return visits within 72 hours of previous ED visit ?” (questionnaire in Supplemental material). Hospital characteristics from the survey included total clinical time allotment, or full-time equivalent (FTE), among all physicians, pediatric emergency medicine (PEM) fellowship-trained physicians, and all other (non-PEM) physicians. The data were standardized across sites by calculating FTE-per-10,000-visits values for each hospital; median duration of ED visit for admitted and discharged patients; median time from arrival to ED physician evaluation; rate of leaving without being seen; discharge educational material authorship and age specificity; follow-up visit scheduling procedure; and percentage of ED patients for whom English was a second language.
Responses to the 2 open-ended questions were independently categorized by Drs. Pittsenbarger and Alpern. Responses could be placed in more than 1 category if multiple answers to the question were included in the response. Categorizations were compared for consistency, and any inconsistencies were resolved by the consensus of the study investigators.
Outcome Measures From PHIS Database
All ED visits within a 12-month period (July 1, 2013–June 30, 2014) by patients younger than 18 years at time of index ED visit were eligible for inclusion in the study. An index visit was defined as any ED visit without another ED visit within the preceding 72 hours. The 72-hour time frame was used because it is the most widely studied time frame for ED RVs.5 Index ED visits that led to admission, observation status, death, or transfer were excluded.
The 2 primary outcomes of interest were (1) RVs within 72 hours of index ED visit discharge and (2) RVs within 72 hours that resulted in hospital admission or observation status at the next ED visit (RVA).7,9,27-30 For patients with multiple ED revisits within 72 hours, only the first was assessed. There was a 72-hour minimum between index visits for the same patient.
Statistical Analyses
To determine hospital groups based on RV and RVA rates, we adjusted RV and RVA rates using generalized linear mixed-effects models, controlling for clustering and allowing for correlated data (within hospitals), nonconstant variability (across hospitals), and non-normally distributed data, as we did in a study of patient-level factors associated with ED RV and RVA.3 For each calculated rate (RV, RVA), the hospitals were then classified into 3 groups based on whether the hospital’s adjusted RV and RVA rates were outside 2 SDs from the mean, below the 5th or above the 95th percentile, or within that range. These groups were labeled lowest outliers, highest outliers, and average-performing hospitals.
After the groups of hospitals were determined, we returned to using unadjusted data to statistically analyze them. We summarized continuous variables using minimum and maximum values, medians, and interquartile ranges (IQRs). We present categorical variables using counts and percentages. To identify hospital characteristics with the most potential to gain from improvement, we also analyzed associations using 2 collapsed groups: hospitals with RV (or RVA) rates included in the average-performing and lowest outlier groups and hospitals within the highest outlier group. Hospital characteristics and hospital’s patient population characteristics from the surveys are summarized based on RV and RVA rate groups. Differences in distributions among continuous variables were assessed by Kruskal-Wallis 1-way analysis of variance. Chi-square tests were used to evaluate differences in proportions among categorical variables. All statistical analyses were performed with SAS Version 9.4 (SAS Institute); 2-sided P < 0.05 was considered statistically significant.
RESULTS
Return Visit Rates and Hospital ED Site Population Characteristics
Twenty-four of 35 (68%) eligible hospitals that met PHIS quality control standards for ED patient visits responded to the ED medical director survey. The included hospitals that both met quality control standards and completed the survey had a total of 1,456,377 patient visits during the study period. Individual sites had annual volumes ranging from 26,627 to 96,637 ED encounters. The mean RV rate across the institutions was 3.7% (range, 3.0%-4.8%), and the mean RVA rate across the hospitals was 0.7% (range, 0.5%-1.1%) (Figure).
There were 5 hospitals with RV rates less than 2 SDs of the mean rate, placing them in the lowest outlier group for RV; 13 hospitals with RV rates within 2 SDs of the mean RV rate, placing them in the average-performing group; and 6 hospitals with RV rates above 2 SDs of the mean, placing them in the highest outlier group. Table 1 lists the hospital ED site population characteristics among the 3 RV rate groups. Hospitals in the highest outlier group served populations with higher proportions of patients with insurance from a government payer, lower proportions of patients covered by a commercial insurance plan, and higher proportion of patients with lower median household incomes.
In the RVA analysis, there were 6 hospitals with RVA rates less than 2 SDs of the mean RVA rate (lowest outliers); 14 hospitals with RVA rates within 2 SDs of the mean RVA rate (average performers); and 4 hospitals with RVA rates above 2 SDs of the mean RVA rate (highest outliers). When using these groups based on RVA rate, there were no statistically significant differences in hospital ED site population characteristics (Supplemental Table 1).
RV Rates and Hospital-Level Factors Survey Characteristics
Table 2 lists the ED medical director survey hospital-level data among the 3 RV rate groups. There were fewer FTEs by PEM fellowship-trained physicians per 10,000 patient visits at sites with higher RV rates (Table 2). Hospital-level characteristics assessed by the survey were not associated with RVA rates (Supplemental Table 2).
Evaluating characteristics of hospitals with the most potential to gain from improvement, hospitals with the highest RV rates (highest outlier group), compared with hospitals in the lowest outlier and average-performing groups collapsed together, persisted in having fewer PEM fellowship-trained physician FTEs per patient visit (Table 3). A similar collapsed analysis of RVA rates demonstrated that hospitals in the highest outlier group had longer-wait-to-physician time (81 minutes; IQR, 51-105 minutes) compared with hospitals in the other 2 groups (30 minutes; IQR, 19-42.5 minutes) (Table 3).
In response to the first qualitative question on the ED medial director survey, “In your opinion, what is the largest barrier to reducing the number of return visits within 72 hours of discharge from a previous ED visit?”, 15 directors (62.5%) reported limited access to primary care, 4 (16.6%) reported inadequate discharge instructions and/or education provided, and 3 (12.5%) reported lack of access to specialist care. To the second question, “In your opinion, what is the best way of reducing the number of the return visits within 72 hours of previous ED visit for the same condition?”, they responded that RVs could be reduced by innovations in scheduling primary care or specialty follow-up visits (19, 79%), improving discharge education and instructions (6, 25%), and identifying more case management or care coordination (4, 16.6%).
DISCUSSION
Other studies have identified patient- and visit-level characteristics associated with higher ED RV and RVA rates.3,8,9,31 However, as our goal was to identify possible modifiable institutional features, our study examined factors at hospital and population-served levels (instead of patient or visit level) that may impact ED RV and RVA rates. Interestingly, our sample of tertiary-care pediatric center EDs provided evidence of variability in RV and RVA rates. We identified factors associated with RV rates related to the SDHs of the populations served by the ED, which suggests these factors are not modifiable at an institution level. In addition, we found that the increased availability of PEM providers per patient visit correlated with fewer ED RVs.
Hospitals serving ED populations with more government-insured and fewer commercially insured patients had higher rates of return to the ED. Similarly, hospitals with larger proportions of patients from areas with lower median household incomes had higher RV rates. These factors may indicate that patients with limited resources may have more frequent ED RVs,3,6,32,33 and hospitals that serve them have higher ED RV rates. Our findings complement those of a recent study by Sills et al.,11 who evaluated hospital readmissions and proposed risk adjustment for performance reimbursement. This study found that hospital population-level race, ethnicity, insurance status, and household income were predictors of hospital readmission after discharge.
Of note, our data did not identify similar site-level attributes related to the population served that correlated with RVA rates. We postulate that the need for admission on RV may indicate an inherent clinical urgency or medical need associated with the return to the ED, whereas RV without admission may be related more to patient- or population-level sociodemographic factors than to quality of care and clinical course, which influence ED utilization.1,3,30 EDs treating higher proportions of patients of minority race or ethnicity, those with fewer financial resources, and those in more need of government health insurance are at higher risk for ED revisits.
We observed that increased PEM fellowship-trained physician staffing was associated with decreased RV rates. The availability of specialty-trained physicians in PEM may allow a larger proportion of patients treated by physicians with honed clinical skills for the patient population. Data from a single pediatric center showed PEM fellowship-trained physicians had admission rates lower than those of their counterparts without subspecialty fellowship training.34 The lower RV rate for this group in our study is especially interesting in light of previously reported lower admission rates at index visit in PEM trained physicians. With lower index admission rates, it may have been assumed that visits associated with PEM trained physician care would have an increased (rather than decreased) chance of RV. In addition, we noted the increased RVA rates were associated with longer waits to see a physician. These measures may indicate the effect of institutional access to robust resources (the ability to hire and support more specialty-trained physicians). These novel findings warrant further evaluation, particularly as our sample included only pediatric centers.
Our survey data demonstrated the impact that access to care has on ED RV rates. The ED medical directors indicated that limited access to outpatient appointments with PCPs and specialists was an important factor increasing ED RVs and a potential avenue for interventions. As the 2 open-ended questions addressed barriers and potential solutions, it is interesting that the respondents cited access to care and discharge instructions as the largest barriers and identified innovations in access to care and discharge education as important potential remedies.
This study demonstrated that, at the hospital level, ED RV quality measures are influenced by complex and varied SDHs that primarily reflect the characteristics of the patient populations served. Prior work has similarly highlighted the importance of gaining a rigorous understanding of other quality measures before widespread use, reporting, and dissemination of results.11,35-38 With this in mind, as quality measures are developed and implemented, care should be taken to ensure they accurately and appropriately reflect the quality of care provided to the patient and are not more representative of other factors not directly within institutional control. These findings call into question the usefulness of ED RVs as a quality measure for comparing institutions.
Study Limitations
This study had several limitations. The PHIS dataset tracks only patients within each institution and does not include RVs to other EDs, which may account for a proportion of RVs.39 Our survey response rate was 68% among medical directors, excluding 11 hospitals from analysis, which decreased the study’s power to detect differences that may be present between groups. In addition, the generalizability of our findings may be limited to tertiary-care children’s hospitals, as the PHIS dataset included only these types of healthcare facilities. We also included data only from the sites’ main EDs, and therefore cannot know if our results are applicable to satellite EDs. ED staffing of PEM physicians was analyzed using FTEs. However, number of clinical hours in 1 FTE may vary among sites, leading to imprecision in this hospital characteristic.
CONCLUSION
Hospitals with the highest RV rates served populations with a larger proportion of patients with government insurance and lower household income, and these hospitals had fewer PEM trained physicians. Variation in RV rates among hospitals may be indicative of the SDHs of their unique patient populations. ED revisit rates should be used cautiously in determining the quality of care of hospitals providing care to differing populations.
Disclosure
Nothing to report.
1. Goldman RD, Kapoor A, Mehta S. Children admitted to the hospital after returning to the emergency department within 72 hours. Pediatr Emerg Care. 2011;27(9):808-811. PubMed
2. Cho CS, Shapiro DJ, Cabana MD, Maselli JH, Hersh AL. A national depiction of children with return visits to the emergency department within 72 hours, 2001–2007. Pediatr Emerg Care. 2012;28(7):606-610. PubMed
3. Akenroye AT, Thurm CW, Neuman MI, et al. Prevalence and predictors of return visits to pediatric emergency departments. J Hosp Med. 2014;9(12):779-787. PubMed
4. Gallagher RA, Porter S, Monuteaux MC, Stack AM. Unscheduled return visits to the emergency department: the impact of language. Pediatr Emerg Care. 2013;29(5):579-583. PubMed
5. Sørup CM, Jacobsen P, Forberg JL. Evaluation of emergency department performance—a systematic review on recommended performance and quality-in-care measures. Scand J Trauma Resusc Emerg Med. 2013;21:62. PubMed
6. Gabayan GZ, Asch SM, Hsia RY, et al. Factors associated with short-term bounce-back admissions after emergency department discharge. Ann Emerg Med. 2013;62(2):136-144.e1. PubMed
7. Ali AB, Place R, Howell J, Malubay SM. Early pediatric emergency department return visits: a prospective patient-centric assessment. Clin Pediatr (Phila). 2012;51(7):651-658. PubMed
8. Alessandrini EA, Lavelle JM, Grenfell SM, Jacobstein CR, Shaw KN. Return visits to a pediatric emergency department. Pediatr Emerg Care. 2004;20(3):166-171. PubMed
9. Goldman RD, Ong M, Macpherson A. Unscheduled return visits to the pediatric emergency department—one-year experience. Pediatr Emerg Care. 2006;22(8):545-549. PubMed
10. Berry JG, Toomey SL, Zaslavsky AM, et al. Pediatric readmission prevalence and variability across hospitals. JAMA. 2013;309(4):372-380. PubMed
11. Sills MR, Hall M, Colvin JD, et al. Association of social determinants with children’s hospitals’ preventable readmissions performance. JAMA Pediatr. 2016;170(4):350-358. PubMed
12. Fiscella K, Burstin HR, Nerenz DR. Quality measures and sociodemographic risk factors: to adjust or not to adjust. JAMA. 2014;312(24):2615-2616. PubMed
13. Lipstein SH, Dunagan WC. The risks of not adjusting performance measures for sociodemographic factors. Ann Intern Med. 2014;161(8):594-596. PubMed
14. Berry JG, Hall DE, Kuo DZ, et al. Hospital utilization and characteristics of patients experiencing recurrent readmissions within children’s hospitals. JAMA. 2011;305(7):682-690. PubMed
15. Bourgeois FT, Monuteaux MC, Stack AM, Neuman MI. Variation in emergency department admission rates in US children’s hospitals. Pediatrics. 2014;134(3):539-545. PubMed
16. Fletcher DM. Achieving data quality. How data from a pediatric health information system earns the trust of its users. J AHIMA. 2004;75(10):22-26. PubMed
17. US Census Bureau. US Census current estimates data. 2014. https://www.census.gov/programs-surveys/popest/data/data-sets.2014.html. Accessed June 2015.
18. Alessandrini EA, Alpern ER, Chamberlain JM, Shea JA, Gorelick MH. A new diagnosis grouping system for child emergency department visits. Acad Emerg Med. 2010;17(2):204-213. PubMed
19. Feudtner C, Levin JE, Srivastava R, et al. How well can hospital readmission be predicted in a cohort of hospitalized children? A retrospective, multicenter study. Pediatrics. 2009;123(1):286-293. PubMed
20. Feinstein JA, Feudtner C, Kempe A. Adverse drug event–related emergency department visits associated with complex chronic conditions. Pediatrics. 2014;133(6):e1575-e1585. PubMed
21. Simon TD, Berry J, Feudtner C, et al. Children with complex chronic conditions in inpatient hospital settings in the United States. Pediatrics. 2010;126(4):647-655. PubMed
22. Dartmouth Medical School, Center for Evaluative Clinical Sciences. The Dartmouth Atlas of Health Care. Chicago, IL: American Hospital Publishing; 2015.
23. Kansagara D, Englander H, Salanitro A, et al. Risk prediction models for hospital readmission: a systematic review. JAMA. 2011;306(15):1688-1698. PubMed
24. Lawrence LM, Jenkins CA, Zhou C, Givens TG. The effect of diagnosis-specific computerized discharge instructions on 72-hour return visits to the pediatric emergency department. Pediatr Emerg Care. 2009;25(11):733-738. PubMed
25. National Quality Forum. National Quality Forum issue brief: strengthening pediatric quality measurement and reporting. J Healthc Qual. 2008;30(3):51-55. PubMed
26. Rising KL, Victor TW, Hollander JE, Carr BG. Patient returns to the emergency department: the time-to-return curve. Acad Emerg Med. 2014;21(8):864-871. PubMed
27. Cho CS, Shapiro DJ, Cabana MD, Maselli JH, Hersh AL. A national depiction of children with return visits to the emergency department within 72 hours, 2001–2007. Pediatr Emerg Care. 2012;28(7):606-610. PubMed
28. Adekoya N. Patients seen in emergency departments who had a prior visit within the previous 72 h—National Hospital Ambulatory Medical Care Survey, 2002. Public Health. 2005;119(10):914-918. PubMed
29. Mittal MK, Zorc JJ, Garcia-Espana JF, Shaw KN. An assessment of clinical performance measures for pediatric emergency physicians. Am J Med Qual. 2013;28(1):33-39. PubMed
30. Depiero AD, Ochsenschlager DW, Chamberlain JM. Analysis of pediatric hospitalizations after emergency department release as a quality improvement tool. Ann Emerg Med. 2002;39(2):159-163. PubMed
31. Sung SF, Liu KE, Chen SC, Lo CL, Lin KC, Hu YH. Predicting factors and risk stratification for return visits to the emergency department within 72 hours in pediatric patients. Pediatr Emerg Care. 2015;31(12):819-824. PubMed
32. Jacobstein CR, Alessandrini EA, Lavelle JM, Shaw KN. Unscheduled revisits to a pediatric emergency department: risk factors for children with fever or infection-related complaints. Pediatr Emerg Care. 2005;21(12):816-821. PubMed
33. Barnett ML, Hsu J, McWilliams J. Patient characteristics and differences in hospital readmission rates. JAMA Intern Med. 2015;175(11):1803-1812. PubMed
34. Gaucher N, Bailey B, Gravel J. Impact of physicians’ characteristics on the admission risk among children visiting a pediatric emergency department. Pediatr Emerg Care. 2012;28(2):120-124. PubMed
35. McHugh M, Neimeyer J, Powell E, Khare RK, Adams JG. An early look at performance on the emergency care measures included in Medicare’s hospital inpatient Value-Based Purchasing Program. Ann Emerg Med. 2013;61(6):616-623.e2. PubMed
36. Axon RN, Williams MV. Hospital readmission as an accountability measure. JAMA. 2011;305(5):504-505. PubMed
37. Adams JG. Ensuring the quality of quality metrics for emergency care. JAMA. 2016;315(7):659-660. PubMed
38. Payne NR, Flood A. Preventing pediatric readmissions: which ones and how? J Pediatr. 2015;166(3):519-520. PubMed
39. Khan A, Nakamura MM, Zaslavsky AM, et al. Same-hospital readmission rates as a measure of pediatric quality of care. JAMA Pediatr. 2015;169(10):905-912. PubMed
Return visit (RV) rate is a quality measure commonly used in the emergency department (ED) setting. This metric may represent suboptimal care at the index ED visit.1-5 Although patient- and visit-level factors affecting ED RVs have been evaluated,1,3,4,6-9 hospital-level factors and factors of a hospital’s patient population that may play roles in ED RV rates have not been examined. Identifying the factors associated with increased RVs may allow resources to be designated to areas that improve emergent care for children.10
Hospital readmission rates are a closely followed quality measure and are linked to reimbursement by the federal government, but a recent study found the influence a hospital can have on this marker may be mitigated by the impact of the social determinates of health (SDHs) of the hospital’s patient population.11 That study and others have prompted an ongoing debate about adjusting quality measures for SDHs.12,13 A clearer understanding of these interactions may permit us to focus on factors that can truly lead to improvement in care instead of penalizing practitioners or hospitals that provide care to those most in need.
Prior work has identified several SDHs associated with higher ED RV rates in patient- or visit-level analyses.3,11,14 We conducted a study of hospital-level characteristics and characteristics of a hospital’s patient population to identify potentially mutable factors associated with increased ED RV rates that, once recognized, may allow for improvement in this quality measure.
PATIENTS AND METHODS
This study was not considered human subjects research in accordance with Common Rule 45 CFR§46.104(f) and was evaluated by the Ann and Robert H. Lurie Children’s Hospital and Northwestern University Feinberg School of Medicine Institutional Review Boards and deemed exempt from review.
Study Population and Protocol
Our study had 2 data sources (to be described in detail): the Pediatric Health Information System (PHIS) and a survey of ED medical directors of the hospitals represented within PHIS. Hospitals were eligible for inclusion in the study if their data (1) met PHIS quality control standards for ED patient visits as determined by internal data assurance processes incorporated in PHIS,3,14,15 (2) included data only from an identifiable single main ED, and (3) completed the ED medical director’s survey.
PHIS Database
PHIS, an administrative database managed by Truven Health Analytics, includes data from ED, ambulatory surgery, observation, and inpatient encounters across Children’s Hospital Association member children’s hospitals in North America. Data are subjected to validity checks before being included in the database.16 PHIS assigns unique patient identifiers to track individual patient visits within participating institutions over time.
Hospitals were described by percentages of ED patients in several groups: age (<1, 1-4, 5-9, 10-14, and 15-18 years)17; sex; race/ethnicity; insurance type (commercial, government, other); ED International Classification of Diseases, Ninth Edition (ICD-9) diagnosis code–based severity classification system score (1-2, low severity; 3-5, high severity)18; complex
ED Medical Director Survey
A web-based survey was constructed in an iterative process based on literature review and expert opinion to assess hospital-level factors that may impact ED RV rates.3,7,24-26 The survey was piloted at 3 institutions to refine its structure and content.
The survey included 15 close-ended or multiple-choice questions on ED environment and operations and 2 open-ended questions, “What is the largest barrier to reducing the number of return visits within 72 hours of discharge from a previous ED visit?” and “In your opinion, what is the best way of reducing the number of the return visits within 72 hours of previous ED visit ?” (questionnaire in Supplemental material). Hospital characteristics from the survey included total clinical time allotment, or full-time equivalent (FTE), among all physicians, pediatric emergency medicine (PEM) fellowship-trained physicians, and all other (non-PEM) physicians. The data were standardized across sites by calculating FTE-per-10,000-visits values for each hospital; median duration of ED visit for admitted and discharged patients; median time from arrival to ED physician evaluation; rate of leaving without being seen; discharge educational material authorship and age specificity; follow-up visit scheduling procedure; and percentage of ED patients for whom English was a second language.
Responses to the 2 open-ended questions were independently categorized by Drs. Pittsenbarger and Alpern. Responses could be placed in more than 1 category if multiple answers to the question were included in the response. Categorizations were compared for consistency, and any inconsistencies were resolved by the consensus of the study investigators.
Outcome Measures From PHIS Database
All ED visits within a 12-month period (July 1, 2013–June 30, 2014) by patients younger than 18 years at time of index ED visit were eligible for inclusion in the study. An index visit was defined as any ED visit without another ED visit within the preceding 72 hours. The 72-hour time frame was used because it is the most widely studied time frame for ED RVs.5 Index ED visits that led to admission, observation status, death, or transfer were excluded.
The 2 primary outcomes of interest were (1) RVs within 72 hours of index ED visit discharge and (2) RVs within 72 hours that resulted in hospital admission or observation status at the next ED visit (RVA).7,9,27-30 For patients with multiple ED revisits within 72 hours, only the first was assessed. There was a 72-hour minimum between index visits for the same patient.
Statistical Analyses
To determine hospital groups based on RV and RVA rates, we adjusted RV and RVA rates using generalized linear mixed-effects models, controlling for clustering and allowing for correlated data (within hospitals), nonconstant variability (across hospitals), and non-normally distributed data, as we did in a study of patient-level factors associated with ED RV and RVA.3 For each calculated rate (RV, RVA), the hospitals were then classified into 3 groups based on whether the hospital’s adjusted RV and RVA rates were outside 2 SDs from the mean, below the 5th or above the 95th percentile, or within that range. These groups were labeled lowest outliers, highest outliers, and average-performing hospitals.
After the groups of hospitals were determined, we returned to using unadjusted data to statistically analyze them. We summarized continuous variables using minimum and maximum values, medians, and interquartile ranges (IQRs). We present categorical variables using counts and percentages. To identify hospital characteristics with the most potential to gain from improvement, we also analyzed associations using 2 collapsed groups: hospitals with RV (or RVA) rates included in the average-performing and lowest outlier groups and hospitals within the highest outlier group. Hospital characteristics and hospital’s patient population characteristics from the surveys are summarized based on RV and RVA rate groups. Differences in distributions among continuous variables were assessed by Kruskal-Wallis 1-way analysis of variance. Chi-square tests were used to evaluate differences in proportions among categorical variables. All statistical analyses were performed with SAS Version 9.4 (SAS Institute); 2-sided P < 0.05 was considered statistically significant.
RESULTS
Return Visit Rates and Hospital ED Site Population Characteristics
Twenty-four of 35 (68%) eligible hospitals that met PHIS quality control standards for ED patient visits responded to the ED medical director survey. The included hospitals that both met quality control standards and completed the survey had a total of 1,456,377 patient visits during the study period. Individual sites had annual volumes ranging from 26,627 to 96,637 ED encounters. The mean RV rate across the institutions was 3.7% (range, 3.0%-4.8%), and the mean RVA rate across the hospitals was 0.7% (range, 0.5%-1.1%) (Figure).
There were 5 hospitals with RV rates less than 2 SDs of the mean rate, placing them in the lowest outlier group for RV; 13 hospitals with RV rates within 2 SDs of the mean RV rate, placing them in the average-performing group; and 6 hospitals with RV rates above 2 SDs of the mean, placing them in the highest outlier group. Table 1 lists the hospital ED site population characteristics among the 3 RV rate groups. Hospitals in the highest outlier group served populations with higher proportions of patients with insurance from a government payer, lower proportions of patients covered by a commercial insurance plan, and higher proportion of patients with lower median household incomes.
In the RVA analysis, there were 6 hospitals with RVA rates less than 2 SDs of the mean RVA rate (lowest outliers); 14 hospitals with RVA rates within 2 SDs of the mean RVA rate (average performers); and 4 hospitals with RVA rates above 2 SDs of the mean RVA rate (highest outliers). When using these groups based on RVA rate, there were no statistically significant differences in hospital ED site population characteristics (Supplemental Table 1).
RV Rates and Hospital-Level Factors Survey Characteristics
Table 2 lists the ED medical director survey hospital-level data among the 3 RV rate groups. There were fewer FTEs by PEM fellowship-trained physicians per 10,000 patient visits at sites with higher RV rates (Table 2). Hospital-level characteristics assessed by the survey were not associated with RVA rates (Supplemental Table 2).
Evaluating characteristics of hospitals with the most potential to gain from improvement, hospitals with the highest RV rates (highest outlier group), compared with hospitals in the lowest outlier and average-performing groups collapsed together, persisted in having fewer PEM fellowship-trained physician FTEs per patient visit (Table 3). A similar collapsed analysis of RVA rates demonstrated that hospitals in the highest outlier group had longer-wait-to-physician time (81 minutes; IQR, 51-105 minutes) compared with hospitals in the other 2 groups (30 minutes; IQR, 19-42.5 minutes) (Table 3).
In response to the first qualitative question on the ED medial director survey, “In your opinion, what is the largest barrier to reducing the number of return visits within 72 hours of discharge from a previous ED visit?”, 15 directors (62.5%) reported limited access to primary care, 4 (16.6%) reported inadequate discharge instructions and/or education provided, and 3 (12.5%) reported lack of access to specialist care. To the second question, “In your opinion, what is the best way of reducing the number of the return visits within 72 hours of previous ED visit for the same condition?”, they responded that RVs could be reduced by innovations in scheduling primary care or specialty follow-up visits (19, 79%), improving discharge education and instructions (6, 25%), and identifying more case management or care coordination (4, 16.6%).
DISCUSSION
Other studies have identified patient- and visit-level characteristics associated with higher ED RV and RVA rates.3,8,9,31 However, as our goal was to identify possible modifiable institutional features, our study examined factors at hospital and population-served levels (instead of patient or visit level) that may impact ED RV and RVA rates. Interestingly, our sample of tertiary-care pediatric center EDs provided evidence of variability in RV and RVA rates. We identified factors associated with RV rates related to the SDHs of the populations served by the ED, which suggests these factors are not modifiable at an institution level. In addition, we found that the increased availability of PEM providers per patient visit correlated with fewer ED RVs.
Hospitals serving ED populations with more government-insured and fewer commercially insured patients had higher rates of return to the ED. Similarly, hospitals with larger proportions of patients from areas with lower median household incomes had higher RV rates. These factors may indicate that patients with limited resources may have more frequent ED RVs,3,6,32,33 and hospitals that serve them have higher ED RV rates. Our findings complement those of a recent study by Sills et al.,11 who evaluated hospital readmissions and proposed risk adjustment for performance reimbursement. This study found that hospital population-level race, ethnicity, insurance status, and household income were predictors of hospital readmission after discharge.
Of note, our data did not identify similar site-level attributes related to the population served that correlated with RVA rates. We postulate that the need for admission on RV may indicate an inherent clinical urgency or medical need associated with the return to the ED, whereas RV without admission may be related more to patient- or population-level sociodemographic factors than to quality of care and clinical course, which influence ED utilization.1,3,30 EDs treating higher proportions of patients of minority race or ethnicity, those with fewer financial resources, and those in more need of government health insurance are at higher risk for ED revisits.
We observed that increased PEM fellowship-trained physician staffing was associated with decreased RV rates. The availability of specialty-trained physicians in PEM may allow a larger proportion of patients treated by physicians with honed clinical skills for the patient population. Data from a single pediatric center showed PEM fellowship-trained physicians had admission rates lower than those of their counterparts without subspecialty fellowship training.34 The lower RV rate for this group in our study is especially interesting in light of previously reported lower admission rates at index visit in PEM trained physicians. With lower index admission rates, it may have been assumed that visits associated with PEM trained physician care would have an increased (rather than decreased) chance of RV. In addition, we noted the increased RVA rates were associated with longer waits to see a physician. These measures may indicate the effect of institutional access to robust resources (the ability to hire and support more specialty-trained physicians). These novel findings warrant further evaluation, particularly as our sample included only pediatric centers.
Our survey data demonstrated the impact that access to care has on ED RV rates. The ED medical directors indicated that limited access to outpatient appointments with PCPs and specialists was an important factor increasing ED RVs and a potential avenue for interventions. As the 2 open-ended questions addressed barriers and potential solutions, it is interesting that the respondents cited access to care and discharge instructions as the largest barriers and identified innovations in access to care and discharge education as important potential remedies.
This study demonstrated that, at the hospital level, ED RV quality measures are influenced by complex and varied SDHs that primarily reflect the characteristics of the patient populations served. Prior work has similarly highlighted the importance of gaining a rigorous understanding of other quality measures before widespread use, reporting, and dissemination of results.11,35-38 With this in mind, as quality measures are developed and implemented, care should be taken to ensure they accurately and appropriately reflect the quality of care provided to the patient and are not more representative of other factors not directly within institutional control. These findings call into question the usefulness of ED RVs as a quality measure for comparing institutions.
Study Limitations
This study had several limitations. The PHIS dataset tracks only patients within each institution and does not include RVs to other EDs, which may account for a proportion of RVs.39 Our survey response rate was 68% among medical directors, excluding 11 hospitals from analysis, which decreased the study’s power to detect differences that may be present between groups. In addition, the generalizability of our findings may be limited to tertiary-care children’s hospitals, as the PHIS dataset included only these types of healthcare facilities. We also included data only from the sites’ main EDs, and therefore cannot know if our results are applicable to satellite EDs. ED staffing of PEM physicians was analyzed using FTEs. However, number of clinical hours in 1 FTE may vary among sites, leading to imprecision in this hospital characteristic.
CONCLUSION
Hospitals with the highest RV rates served populations with a larger proportion of patients with government insurance and lower household income, and these hospitals had fewer PEM trained physicians. Variation in RV rates among hospitals may be indicative of the SDHs of their unique patient populations. ED revisit rates should be used cautiously in determining the quality of care of hospitals providing care to differing populations.
Disclosure
Nothing to report.
Return visit (RV) rate is a quality measure commonly used in the emergency department (ED) setting. This metric may represent suboptimal care at the index ED visit.1-5 Although patient- and visit-level factors affecting ED RVs have been evaluated,1,3,4,6-9 hospital-level factors and factors of a hospital’s patient population that may play roles in ED RV rates have not been examined. Identifying the factors associated with increased RVs may allow resources to be designated to areas that improve emergent care for children.10
Hospital readmission rates are a closely followed quality measure and are linked to reimbursement by the federal government, but a recent study found the influence a hospital can have on this marker may be mitigated by the impact of the social determinates of health (SDHs) of the hospital’s patient population.11 That study and others have prompted an ongoing debate about adjusting quality measures for SDHs.12,13 A clearer understanding of these interactions may permit us to focus on factors that can truly lead to improvement in care instead of penalizing practitioners or hospitals that provide care to those most in need.
Prior work has identified several SDHs associated with higher ED RV rates in patient- or visit-level analyses.3,11,14 We conducted a study of hospital-level characteristics and characteristics of a hospital’s patient population to identify potentially mutable factors associated with increased ED RV rates that, once recognized, may allow for improvement in this quality measure.
PATIENTS AND METHODS
This study was not considered human subjects research in accordance with Common Rule 45 CFR§46.104(f) and was evaluated by the Ann and Robert H. Lurie Children’s Hospital and Northwestern University Feinberg School of Medicine Institutional Review Boards and deemed exempt from review.
Study Population and Protocol
Our study had 2 data sources (to be described in detail): the Pediatric Health Information System (PHIS) and a survey of ED medical directors of the hospitals represented within PHIS. Hospitals were eligible for inclusion in the study if their data (1) met PHIS quality control standards for ED patient visits as determined by internal data assurance processes incorporated in PHIS,3,14,15 (2) included data only from an identifiable single main ED, and (3) completed the ED medical director’s survey.
PHIS Database
PHIS, an administrative database managed by Truven Health Analytics, includes data from ED, ambulatory surgery, observation, and inpatient encounters across Children’s Hospital Association member children’s hospitals in North America. Data are subjected to validity checks before being included in the database.16 PHIS assigns unique patient identifiers to track individual patient visits within participating institutions over time.
Hospitals were described by percentages of ED patients in several groups: age (<1, 1-4, 5-9, 10-14, and 15-18 years)17; sex; race/ethnicity; insurance type (commercial, government, other); ED International Classification of Diseases, Ninth Edition (ICD-9) diagnosis code–based severity classification system score (1-2, low severity; 3-5, high severity)18; complex
ED Medical Director Survey
A web-based survey was constructed in an iterative process based on literature review and expert opinion to assess hospital-level factors that may impact ED RV rates.3,7,24-26 The survey was piloted at 3 institutions to refine its structure and content.
The survey included 15 close-ended or multiple-choice questions on ED environment and operations and 2 open-ended questions, “What is the largest barrier to reducing the number of return visits within 72 hours of discharge from a previous ED visit?” and “In your opinion, what is the best way of reducing the number of the return visits within 72 hours of previous ED visit ?” (questionnaire in Supplemental material). Hospital characteristics from the survey included total clinical time allotment, or full-time equivalent (FTE), among all physicians, pediatric emergency medicine (PEM) fellowship-trained physicians, and all other (non-PEM) physicians. The data were standardized across sites by calculating FTE-per-10,000-visits values for each hospital; median duration of ED visit for admitted and discharged patients; median time from arrival to ED physician evaluation; rate of leaving without being seen; discharge educational material authorship and age specificity; follow-up visit scheduling procedure; and percentage of ED patients for whom English was a second language.
Responses to the 2 open-ended questions were independently categorized by Drs. Pittsenbarger and Alpern. Responses could be placed in more than 1 category if multiple answers to the question were included in the response. Categorizations were compared for consistency, and any inconsistencies were resolved by the consensus of the study investigators.
Outcome Measures From PHIS Database
All ED visits within a 12-month period (July 1, 2013–June 30, 2014) by patients younger than 18 years at time of index ED visit were eligible for inclusion in the study. An index visit was defined as any ED visit without another ED visit within the preceding 72 hours. The 72-hour time frame was used because it is the most widely studied time frame for ED RVs.5 Index ED visits that led to admission, observation status, death, or transfer were excluded.
The 2 primary outcomes of interest were (1) RVs within 72 hours of index ED visit discharge and (2) RVs within 72 hours that resulted in hospital admission or observation status at the next ED visit (RVA).7,9,27-30 For patients with multiple ED revisits within 72 hours, only the first was assessed. There was a 72-hour minimum between index visits for the same patient.
Statistical Analyses
To determine hospital groups based on RV and RVA rates, we adjusted RV and RVA rates using generalized linear mixed-effects models, controlling for clustering and allowing for correlated data (within hospitals), nonconstant variability (across hospitals), and non-normally distributed data, as we did in a study of patient-level factors associated with ED RV and RVA.3 For each calculated rate (RV, RVA), the hospitals were then classified into 3 groups based on whether the hospital’s adjusted RV and RVA rates were outside 2 SDs from the mean, below the 5th or above the 95th percentile, or within that range. These groups were labeled lowest outliers, highest outliers, and average-performing hospitals.
After the groups of hospitals were determined, we returned to using unadjusted data to statistically analyze them. We summarized continuous variables using minimum and maximum values, medians, and interquartile ranges (IQRs). We present categorical variables using counts and percentages. To identify hospital characteristics with the most potential to gain from improvement, we also analyzed associations using 2 collapsed groups: hospitals with RV (or RVA) rates included in the average-performing and lowest outlier groups and hospitals within the highest outlier group. Hospital characteristics and hospital’s patient population characteristics from the surveys are summarized based on RV and RVA rate groups. Differences in distributions among continuous variables were assessed by Kruskal-Wallis 1-way analysis of variance. Chi-square tests were used to evaluate differences in proportions among categorical variables. All statistical analyses were performed with SAS Version 9.4 (SAS Institute); 2-sided P < 0.05 was considered statistically significant.
RESULTS
Return Visit Rates and Hospital ED Site Population Characteristics
Twenty-four of 35 (68%) eligible hospitals that met PHIS quality control standards for ED patient visits responded to the ED medical director survey. The included hospitals that both met quality control standards and completed the survey had a total of 1,456,377 patient visits during the study period. Individual sites had annual volumes ranging from 26,627 to 96,637 ED encounters. The mean RV rate across the institutions was 3.7% (range, 3.0%-4.8%), and the mean RVA rate across the hospitals was 0.7% (range, 0.5%-1.1%) (Figure).
There were 5 hospitals with RV rates less than 2 SDs of the mean rate, placing them in the lowest outlier group for RV; 13 hospitals with RV rates within 2 SDs of the mean RV rate, placing them in the average-performing group; and 6 hospitals with RV rates above 2 SDs of the mean, placing them in the highest outlier group. Table 1 lists the hospital ED site population characteristics among the 3 RV rate groups. Hospitals in the highest outlier group served populations with higher proportions of patients with insurance from a government payer, lower proportions of patients covered by a commercial insurance plan, and higher proportion of patients with lower median household incomes.
In the RVA analysis, there were 6 hospitals with RVA rates less than 2 SDs of the mean RVA rate (lowest outliers); 14 hospitals with RVA rates within 2 SDs of the mean RVA rate (average performers); and 4 hospitals with RVA rates above 2 SDs of the mean RVA rate (highest outliers). When using these groups based on RVA rate, there were no statistically significant differences in hospital ED site population characteristics (Supplemental Table 1).
RV Rates and Hospital-Level Factors Survey Characteristics
Table 2 lists the ED medical director survey hospital-level data among the 3 RV rate groups. There were fewer FTEs by PEM fellowship-trained physicians per 10,000 patient visits at sites with higher RV rates (Table 2). Hospital-level characteristics assessed by the survey were not associated with RVA rates (Supplemental Table 2).
Evaluating characteristics of hospitals with the most potential to gain from improvement, hospitals with the highest RV rates (highest outlier group), compared with hospitals in the lowest outlier and average-performing groups collapsed together, persisted in having fewer PEM fellowship-trained physician FTEs per patient visit (Table 3). A similar collapsed analysis of RVA rates demonstrated that hospitals in the highest outlier group had longer-wait-to-physician time (81 minutes; IQR, 51-105 minutes) compared with hospitals in the other 2 groups (30 minutes; IQR, 19-42.5 minutes) (Table 3).
In response to the first qualitative question on the ED medial director survey, “In your opinion, what is the largest barrier to reducing the number of return visits within 72 hours of discharge from a previous ED visit?”, 15 directors (62.5%) reported limited access to primary care, 4 (16.6%) reported inadequate discharge instructions and/or education provided, and 3 (12.5%) reported lack of access to specialist care. To the second question, “In your opinion, what is the best way of reducing the number of the return visits within 72 hours of previous ED visit for the same condition?”, they responded that RVs could be reduced by innovations in scheduling primary care or specialty follow-up visits (19, 79%), improving discharge education and instructions (6, 25%), and identifying more case management or care coordination (4, 16.6%).
DISCUSSION
Other studies have identified patient- and visit-level characteristics associated with higher ED RV and RVA rates.3,8,9,31 However, as our goal was to identify possible modifiable institutional features, our study examined factors at hospital and population-served levels (instead of patient or visit level) that may impact ED RV and RVA rates. Interestingly, our sample of tertiary-care pediatric center EDs provided evidence of variability in RV and RVA rates. We identified factors associated with RV rates related to the SDHs of the populations served by the ED, which suggests these factors are not modifiable at an institution level. In addition, we found that the increased availability of PEM providers per patient visit correlated with fewer ED RVs.
Hospitals serving ED populations with more government-insured and fewer commercially insured patients had higher rates of return to the ED. Similarly, hospitals with larger proportions of patients from areas with lower median household incomes had higher RV rates. These factors may indicate that patients with limited resources may have more frequent ED RVs,3,6,32,33 and hospitals that serve them have higher ED RV rates. Our findings complement those of a recent study by Sills et al.,11 who evaluated hospital readmissions and proposed risk adjustment for performance reimbursement. This study found that hospital population-level race, ethnicity, insurance status, and household income were predictors of hospital readmission after discharge.
Of note, our data did not identify similar site-level attributes related to the population served that correlated with RVA rates. We postulate that the need for admission on RV may indicate an inherent clinical urgency or medical need associated with the return to the ED, whereas RV without admission may be related more to patient- or population-level sociodemographic factors than to quality of care and clinical course, which influence ED utilization.1,3,30 EDs treating higher proportions of patients of minority race or ethnicity, those with fewer financial resources, and those in more need of government health insurance are at higher risk for ED revisits.
We observed that increased PEM fellowship-trained physician staffing was associated with decreased RV rates. The availability of specialty-trained physicians in PEM may allow a larger proportion of patients treated by physicians with honed clinical skills for the patient population. Data from a single pediatric center showed PEM fellowship-trained physicians had admission rates lower than those of their counterparts without subspecialty fellowship training.34 The lower RV rate for this group in our study is especially interesting in light of previously reported lower admission rates at index visit in PEM trained physicians. With lower index admission rates, it may have been assumed that visits associated with PEM trained physician care would have an increased (rather than decreased) chance of RV. In addition, we noted the increased RVA rates were associated with longer waits to see a physician. These measures may indicate the effect of institutional access to robust resources (the ability to hire and support more specialty-trained physicians). These novel findings warrant further evaluation, particularly as our sample included only pediatric centers.
Our survey data demonstrated the impact that access to care has on ED RV rates. The ED medical directors indicated that limited access to outpatient appointments with PCPs and specialists was an important factor increasing ED RVs and a potential avenue for interventions. As the 2 open-ended questions addressed barriers and potential solutions, it is interesting that the respondents cited access to care and discharge instructions as the largest barriers and identified innovations in access to care and discharge education as important potential remedies.
This study demonstrated that, at the hospital level, ED RV quality measures are influenced by complex and varied SDHs that primarily reflect the characteristics of the patient populations served. Prior work has similarly highlighted the importance of gaining a rigorous understanding of other quality measures before widespread use, reporting, and dissemination of results.11,35-38 With this in mind, as quality measures are developed and implemented, care should be taken to ensure they accurately and appropriately reflect the quality of care provided to the patient and are not more representative of other factors not directly within institutional control. These findings call into question the usefulness of ED RVs as a quality measure for comparing institutions.
Study Limitations
This study had several limitations. The PHIS dataset tracks only patients within each institution and does not include RVs to other EDs, which may account for a proportion of RVs.39 Our survey response rate was 68% among medical directors, excluding 11 hospitals from analysis, which decreased the study’s power to detect differences that may be present between groups. In addition, the generalizability of our findings may be limited to tertiary-care children’s hospitals, as the PHIS dataset included only these types of healthcare facilities. We also included data only from the sites’ main EDs, and therefore cannot know if our results are applicable to satellite EDs. ED staffing of PEM physicians was analyzed using FTEs. However, number of clinical hours in 1 FTE may vary among sites, leading to imprecision in this hospital characteristic.
CONCLUSION
Hospitals with the highest RV rates served populations with a larger proportion of patients with government insurance and lower household income, and these hospitals had fewer PEM trained physicians. Variation in RV rates among hospitals may be indicative of the SDHs of their unique patient populations. ED revisit rates should be used cautiously in determining the quality of care of hospitals providing care to differing populations.
Disclosure
Nothing to report.
1. Goldman RD, Kapoor A, Mehta S. Children admitted to the hospital after returning to the emergency department within 72 hours. Pediatr Emerg Care. 2011;27(9):808-811. PubMed
2. Cho CS, Shapiro DJ, Cabana MD, Maselli JH, Hersh AL. A national depiction of children with return visits to the emergency department within 72 hours, 2001–2007. Pediatr Emerg Care. 2012;28(7):606-610. PubMed
3. Akenroye AT, Thurm CW, Neuman MI, et al. Prevalence and predictors of return visits to pediatric emergency departments. J Hosp Med. 2014;9(12):779-787. PubMed
4. Gallagher RA, Porter S, Monuteaux MC, Stack AM. Unscheduled return visits to the emergency department: the impact of language. Pediatr Emerg Care. 2013;29(5):579-583. PubMed
5. Sørup CM, Jacobsen P, Forberg JL. Evaluation of emergency department performance—a systematic review on recommended performance and quality-in-care measures. Scand J Trauma Resusc Emerg Med. 2013;21:62. PubMed
6. Gabayan GZ, Asch SM, Hsia RY, et al. Factors associated with short-term bounce-back admissions after emergency department discharge. Ann Emerg Med. 2013;62(2):136-144.e1. PubMed
7. Ali AB, Place R, Howell J, Malubay SM. Early pediatric emergency department return visits: a prospective patient-centric assessment. Clin Pediatr (Phila). 2012;51(7):651-658. PubMed
8. Alessandrini EA, Lavelle JM, Grenfell SM, Jacobstein CR, Shaw KN. Return visits to a pediatric emergency department. Pediatr Emerg Care. 2004;20(3):166-171. PubMed
9. Goldman RD, Ong M, Macpherson A. Unscheduled return visits to the pediatric emergency department—one-year experience. Pediatr Emerg Care. 2006;22(8):545-549. PubMed
10. Berry JG, Toomey SL, Zaslavsky AM, et al. Pediatric readmission prevalence and variability across hospitals. JAMA. 2013;309(4):372-380. PubMed
11. Sills MR, Hall M, Colvin JD, et al. Association of social determinants with children’s hospitals’ preventable readmissions performance. JAMA Pediatr. 2016;170(4):350-358. PubMed
12. Fiscella K, Burstin HR, Nerenz DR. Quality measures and sociodemographic risk factors: to adjust or not to adjust. JAMA. 2014;312(24):2615-2616. PubMed
13. Lipstein SH, Dunagan WC. The risks of not adjusting performance measures for sociodemographic factors. Ann Intern Med. 2014;161(8):594-596. PubMed
14. Berry JG, Hall DE, Kuo DZ, et al. Hospital utilization and characteristics of patients experiencing recurrent readmissions within children’s hospitals. JAMA. 2011;305(7):682-690. PubMed
15. Bourgeois FT, Monuteaux MC, Stack AM, Neuman MI. Variation in emergency department admission rates in US children’s hospitals. Pediatrics. 2014;134(3):539-545. PubMed
16. Fletcher DM. Achieving data quality. How data from a pediatric health information system earns the trust of its users. J AHIMA. 2004;75(10):22-26. PubMed
17. US Census Bureau. US Census current estimates data. 2014. https://www.census.gov/programs-surveys/popest/data/data-sets.2014.html. Accessed June 2015.
18. Alessandrini EA, Alpern ER, Chamberlain JM, Shea JA, Gorelick MH. A new diagnosis grouping system for child emergency department visits. Acad Emerg Med. 2010;17(2):204-213. PubMed
19. Feudtner C, Levin JE, Srivastava R, et al. How well can hospital readmission be predicted in a cohort of hospitalized children? A retrospective, multicenter study. Pediatrics. 2009;123(1):286-293. PubMed
20. Feinstein JA, Feudtner C, Kempe A. Adverse drug event–related emergency department visits associated with complex chronic conditions. Pediatrics. 2014;133(6):e1575-e1585. PubMed
21. Simon TD, Berry J, Feudtner C, et al. Children with complex chronic conditions in inpatient hospital settings in the United States. Pediatrics. 2010;126(4):647-655. PubMed
22. Dartmouth Medical School, Center for Evaluative Clinical Sciences. The Dartmouth Atlas of Health Care. Chicago, IL: American Hospital Publishing; 2015.
23. Kansagara D, Englander H, Salanitro A, et al. Risk prediction models for hospital readmission: a systematic review. JAMA. 2011;306(15):1688-1698. PubMed
24. Lawrence LM, Jenkins CA, Zhou C, Givens TG. The effect of diagnosis-specific computerized discharge instructions on 72-hour return visits to the pediatric emergency department. Pediatr Emerg Care. 2009;25(11):733-738. PubMed
25. National Quality Forum. National Quality Forum issue brief: strengthening pediatric quality measurement and reporting. J Healthc Qual. 2008;30(3):51-55. PubMed
26. Rising KL, Victor TW, Hollander JE, Carr BG. Patient returns to the emergency department: the time-to-return curve. Acad Emerg Med. 2014;21(8):864-871. PubMed
27. Cho CS, Shapiro DJ, Cabana MD, Maselli JH, Hersh AL. A national depiction of children with return visits to the emergency department within 72 hours, 2001–2007. Pediatr Emerg Care. 2012;28(7):606-610. PubMed
28. Adekoya N. Patients seen in emergency departments who had a prior visit within the previous 72 h—National Hospital Ambulatory Medical Care Survey, 2002. Public Health. 2005;119(10):914-918. PubMed
29. Mittal MK, Zorc JJ, Garcia-Espana JF, Shaw KN. An assessment of clinical performance measures for pediatric emergency physicians. Am J Med Qual. 2013;28(1):33-39. PubMed
30. Depiero AD, Ochsenschlager DW, Chamberlain JM. Analysis of pediatric hospitalizations after emergency department release as a quality improvement tool. Ann Emerg Med. 2002;39(2):159-163. PubMed
31. Sung SF, Liu KE, Chen SC, Lo CL, Lin KC, Hu YH. Predicting factors and risk stratification for return visits to the emergency department within 72 hours in pediatric patients. Pediatr Emerg Care. 2015;31(12):819-824. PubMed
32. Jacobstein CR, Alessandrini EA, Lavelle JM, Shaw KN. Unscheduled revisits to a pediatric emergency department: risk factors for children with fever or infection-related complaints. Pediatr Emerg Care. 2005;21(12):816-821. PubMed
33. Barnett ML, Hsu J, McWilliams J. Patient characteristics and differences in hospital readmission rates. JAMA Intern Med. 2015;175(11):1803-1812. PubMed
34. Gaucher N, Bailey B, Gravel J. Impact of physicians’ characteristics on the admission risk among children visiting a pediatric emergency department. Pediatr Emerg Care. 2012;28(2):120-124. PubMed
35. McHugh M, Neimeyer J, Powell E, Khare RK, Adams JG. An early look at performance on the emergency care measures included in Medicare’s hospital inpatient Value-Based Purchasing Program. Ann Emerg Med. 2013;61(6):616-623.e2. PubMed
36. Axon RN, Williams MV. Hospital readmission as an accountability measure. JAMA. 2011;305(5):504-505. PubMed
37. Adams JG. Ensuring the quality of quality metrics for emergency care. JAMA. 2016;315(7):659-660. PubMed
38. Payne NR, Flood A. Preventing pediatric readmissions: which ones and how? J Pediatr. 2015;166(3):519-520. PubMed
39. Khan A, Nakamura MM, Zaslavsky AM, et al. Same-hospital readmission rates as a measure of pediatric quality of care. JAMA Pediatr. 2015;169(10):905-912. PubMed
1. Goldman RD, Kapoor A, Mehta S. Children admitted to the hospital after returning to the emergency department within 72 hours. Pediatr Emerg Care. 2011;27(9):808-811. PubMed
2. Cho CS, Shapiro DJ, Cabana MD, Maselli JH, Hersh AL. A national depiction of children with return visits to the emergency department within 72 hours, 2001–2007. Pediatr Emerg Care. 2012;28(7):606-610. PubMed
3. Akenroye AT, Thurm CW, Neuman MI, et al. Prevalence and predictors of return visits to pediatric emergency departments. J Hosp Med. 2014;9(12):779-787. PubMed
4. Gallagher RA, Porter S, Monuteaux MC, Stack AM. Unscheduled return visits to the emergency department: the impact of language. Pediatr Emerg Care. 2013;29(5):579-583. PubMed
5. Sørup CM, Jacobsen P, Forberg JL. Evaluation of emergency department performance—a systematic review on recommended performance and quality-in-care measures. Scand J Trauma Resusc Emerg Med. 2013;21:62. PubMed
6. Gabayan GZ, Asch SM, Hsia RY, et al. Factors associated with short-term bounce-back admissions after emergency department discharge. Ann Emerg Med. 2013;62(2):136-144.e1. PubMed
7. Ali AB, Place R, Howell J, Malubay SM. Early pediatric emergency department return visits: a prospective patient-centric assessment. Clin Pediatr (Phila). 2012;51(7):651-658. PubMed
8. Alessandrini EA, Lavelle JM, Grenfell SM, Jacobstein CR, Shaw KN. Return visits to a pediatric emergency department. Pediatr Emerg Care. 2004;20(3):166-171. PubMed
9. Goldman RD, Ong M, Macpherson A. Unscheduled return visits to the pediatric emergency department—one-year experience. Pediatr Emerg Care. 2006;22(8):545-549. PubMed
10. Berry JG, Toomey SL, Zaslavsky AM, et al. Pediatric readmission prevalence and variability across hospitals. JAMA. 2013;309(4):372-380. PubMed
11. Sills MR, Hall M, Colvin JD, et al. Association of social determinants with children’s hospitals’ preventable readmissions performance. JAMA Pediatr. 2016;170(4):350-358. PubMed
12. Fiscella K, Burstin HR, Nerenz DR. Quality measures and sociodemographic risk factors: to adjust or not to adjust. JAMA. 2014;312(24):2615-2616. PubMed
13. Lipstein SH, Dunagan WC. The risks of not adjusting performance measures for sociodemographic factors. Ann Intern Med. 2014;161(8):594-596. PubMed
14. Berry JG, Hall DE, Kuo DZ, et al. Hospital utilization and characteristics of patients experiencing recurrent readmissions within children’s hospitals. JAMA. 2011;305(7):682-690. PubMed
15. Bourgeois FT, Monuteaux MC, Stack AM, Neuman MI. Variation in emergency department admission rates in US children’s hospitals. Pediatrics. 2014;134(3):539-545. PubMed
16. Fletcher DM. Achieving data quality. How data from a pediatric health information system earns the trust of its users. J AHIMA. 2004;75(10):22-26. PubMed
17. US Census Bureau. US Census current estimates data. 2014. https://www.census.gov/programs-surveys/popest/data/data-sets.2014.html. Accessed June 2015.
18. Alessandrini EA, Alpern ER, Chamberlain JM, Shea JA, Gorelick MH. A new diagnosis grouping system for child emergency department visits. Acad Emerg Med. 2010;17(2):204-213. PubMed
19. Feudtner C, Levin JE, Srivastava R, et al. How well can hospital readmission be predicted in a cohort of hospitalized children? A retrospective, multicenter study. Pediatrics. 2009;123(1):286-293. PubMed
20. Feinstein JA, Feudtner C, Kempe A. Adverse drug event–related emergency department visits associated with complex chronic conditions. Pediatrics. 2014;133(6):e1575-e1585. PubMed
21. Simon TD, Berry J, Feudtner C, et al. Children with complex chronic conditions in inpatient hospital settings in the United States. Pediatrics. 2010;126(4):647-655. PubMed
22. Dartmouth Medical School, Center for Evaluative Clinical Sciences. The Dartmouth Atlas of Health Care. Chicago, IL: American Hospital Publishing; 2015.
23. Kansagara D, Englander H, Salanitro A, et al. Risk prediction models for hospital readmission: a systematic review. JAMA. 2011;306(15):1688-1698. PubMed
24. Lawrence LM, Jenkins CA, Zhou C, Givens TG. The effect of diagnosis-specific computerized discharge instructions on 72-hour return visits to the pediatric emergency department. Pediatr Emerg Care. 2009;25(11):733-738. PubMed
25. National Quality Forum. National Quality Forum issue brief: strengthening pediatric quality measurement and reporting. J Healthc Qual. 2008;30(3):51-55. PubMed
26. Rising KL, Victor TW, Hollander JE, Carr BG. Patient returns to the emergency department: the time-to-return curve. Acad Emerg Med. 2014;21(8):864-871. PubMed
27. Cho CS, Shapiro DJ, Cabana MD, Maselli JH, Hersh AL. A national depiction of children with return visits to the emergency department within 72 hours, 2001–2007. Pediatr Emerg Care. 2012;28(7):606-610. PubMed
28. Adekoya N. Patients seen in emergency departments who had a prior visit within the previous 72 h—National Hospital Ambulatory Medical Care Survey, 2002. Public Health. 2005;119(10):914-918. PubMed
29. Mittal MK, Zorc JJ, Garcia-Espana JF, Shaw KN. An assessment of clinical performance measures for pediatric emergency physicians. Am J Med Qual. 2013;28(1):33-39. PubMed
30. Depiero AD, Ochsenschlager DW, Chamberlain JM. Analysis of pediatric hospitalizations after emergency department release as a quality improvement tool. Ann Emerg Med. 2002;39(2):159-163. PubMed
31. Sung SF, Liu KE, Chen SC, Lo CL, Lin KC, Hu YH. Predicting factors and risk stratification for return visits to the emergency department within 72 hours in pediatric patients. Pediatr Emerg Care. 2015;31(12):819-824. PubMed
32. Jacobstein CR, Alessandrini EA, Lavelle JM, Shaw KN. Unscheduled revisits to a pediatric emergency department: risk factors for children with fever or infection-related complaints. Pediatr Emerg Care. 2005;21(12):816-821. PubMed
33. Barnett ML, Hsu J, McWilliams J. Patient characteristics and differences in hospital readmission rates. JAMA Intern Med. 2015;175(11):1803-1812. PubMed
34. Gaucher N, Bailey B, Gravel J. Impact of physicians’ characteristics on the admission risk among children visiting a pediatric emergency department. Pediatr Emerg Care. 2012;28(2):120-124. PubMed
35. McHugh M, Neimeyer J, Powell E, Khare RK, Adams JG. An early look at performance on the emergency care measures included in Medicare’s hospital inpatient Value-Based Purchasing Program. Ann Emerg Med. 2013;61(6):616-623.e2. PubMed
36. Axon RN, Williams MV. Hospital readmission as an accountability measure. JAMA. 2011;305(5):504-505. PubMed
37. Adams JG. Ensuring the quality of quality metrics for emergency care. JAMA. 2016;315(7):659-660. PubMed
38. Payne NR, Flood A. Preventing pediatric readmissions: which ones and how? J Pediatr. 2015;166(3):519-520. PubMed
39. Khan A, Nakamura MM, Zaslavsky AM, et al. Same-hospital readmission rates as a measure of pediatric quality of care. JAMA Pediatr. 2015;169(10):905-912. PubMed
© 2017 Society of Hospital Medicine
Association of stress biomarkers with 30-day unplanned readmission and death
It has been theorized that the physiologic stress that hospitalized patients experience accounts for their transient vulnerability after discharge, or posthospital syndrome.1 Their acute illness and life-habit changes during hospitalization result in continued impairment of physiologic systems after discharge, and this impairment might leave them more susceptible to new health threats.1 However, the theory that the stress experienced after a hospitalization might be associated with readmission has never been investigated.
Four biomarkers of the hypothalamic-pituitary-adrenal (HPA) axis may help quantify posthospitalization stress: (1) midregional pro-adrenomedullin (ADM), a precursor reflecting adrenomedullin activity2; (2) copeptin (the C-terminal part of prepro-vasopressin, produced by the hypothalamus in response to stress3,4), the level of which closely correlates to the vasopressin level but is more stable and lacks circadian rhythm fluctuations5-7; (3) cortisol, released by the adrenal cortex in response to stress; and (4) prolactin, an indicator of HPA axis activity. These 4 stress biomarkers have been related to the severity, complications, or mortality of several diseases.3,5,8-17 Besides explaining the hypothetical association between posthospitalization stress and readmission and death, these biomarkers might be valuable in predicting which patients are at higher risk for readmission. Indeed, many prediction models have been developed to identify those patients, but most of these models underperform, target only very specific populations, or have not been externally validated.18
We hypothesized that the hospitalization stress measured by biomarkers is associated with readmission or death after discharge. In a prospective cohort study, we evaluated the association between 4 stress biomarkers (ADM, copeptin, cortisol, prolactin) and 30-day unplanned readmissions and deaths after an acute-care medical hospitalization, and assessed their additive value to validated readmission prediction scores.
METHODS
Study Design and Population
Our prospective cohort study included all consecutive patients aged ≥50 years and admitted to the department of general internal medicine at Fribourg Cantonal Hospital in Switzerland between April 8, 2013 and September 23, 2013. Exclusion criteria were discharge on day of admission; death before discharge; discharge to another division, another acute-care hospital, a rehabilitation clinic, or a palliative-care clinic; and refusal or inability to give informed consent. In this hypothesis-generating observational study, we collected data on a convenience sample of patients and did not calculate sample size before data collection. The study was approved by the local ethics committee, and all patients gave informed consent.
Outcomes
The primary outcome was the composite of first unplanned readmission (to any division of any acute-care hospital) or death within 30 days after discharge from index admission. We also included deaths that occurred after discharge, hypothesizing that patients who died may have been readmitted had they lived. The secondary outcome was the same as the primary, but the period was 90 days. Planned readmission was defined as scheduled hospitalization for nonemergent treatment (eg, chemotherapy) or investigation (eg, elective coronarography). All patients were called 6 months after discharge, and readmissions and deaths recorded. If a patient could not be reached directly, we called his or her next of kin, primary care physician, or nursing home, depending on availability. Furthermore, we checked electronic health records for any readmission or death recorded within the Fribourg hospital network, which includes all 3 acute-care hospitals (Fribourg, Riaz, Tavel) in the same canton (state).
Independent Variables
Stress biomarkers. We measured serum levels of 4 stress biomarkers (ADM, copeptin, cortisol, prolactin) at 8 am on an empty stomach on both day of admission and day of discharge. For a patient whose discharge decision was made after 8 hours for the same day, a blood sample was collected as soon as discharge was planned.
Clinical data. Collected data included demographics, history of hospitalization within 6 months before index admission, hospitalization diagnosis, and Charlson Comorbidity Index (CCI), which includes a list of medical conditions that are assigned a number of 1, 2, 3, or 6 points, according to their severity, and which has been associated with mortality.19
Causes of Admission, Unplanned Readmission, and Death
Causes of index admission, unplanned readmission, and death were obtained from medical records. We used our consensus opinion and a previous analysis20 to classify these causes by body system, and added 2 categories, cancer and infection (both associated with readmission20). The resulting 9 categories were (1) cancer, (2) respiratory disorder, (3) infectious disorder, (4) neurologic disorder (including dementia, psychiatric disorder, alcohol disorder, and intoxication), (5) gastrointestinal disorder, (6) osteoarticular disorder, (7) renal disorder, (8) cardiovascular disorder (including ischemic disease and heart failure), and (9) other.
Additional Performance With Existing Predictive Models
To better define the explanatory power of biomarkers to predict our outcome, we assessed the performance improvement of 2 validated readmission prediction scores by adding the stress biomarkers. As large effect sizes from additional predictors are needed to increase the power discrimination of a model, a significant performance improvement would further support the biomarkers’ important explanatory power. The 2 prediction scores tested were the LACE index (Length of stay, Admission Acuity, CCI, number of Emergency department visits within preceding 6 months21) and the HOSPITAL score (Hemoglobin level at discharge, discharge from Oncology service, Sodium level at discharge, any Procedure performed during index hospitalization, Index admission Type, number of Admissions within preceding 12 months, Length of stay). As we did not have an oncology service, we replaced “discharge from oncology service” with “active diagnosis of cancer.” “Length of stay” was tailored to the median in Switzerland (8 days instead of 5 days; Supplement Table 1).22,23
Data Analysis
Continuous variables were presented as medians with interquartile ranges (IQRs) because of their non-normal distribution, and categorical variables were presented as frequencies and percentages. We compared medians using the nonparametric K-sample test on the equality of medians, and compared frequencies using the Pearson χ2 test. The discriminatory power of each biomarker in predicting readmission and death was calculated with the area under the receiver operating characteristic (ROC) curve (AUROC), using serum levels at discharge to better reflect the postdischarge period. Cutoff levels were selected by taking the best compromise between sensitivity and specificity according to the ROC curves (point nearest top left corner).24
Univariate logistic regression analysis was used to test the prediction of 30-day and 90-day unplanned readmission or death by each biomarker. We built 2 different multivariate models: one adjusting for age and LACE index points21 and the other adjusting for age and HOSPITAL score.22,23
To explore any association between reduction of stress during hospitalization and postdischarge outcome, we additionally calculated for each biomarker the difference between admission and discharge serum levels and assessed its association with readmission or death by logistic regression analysis. Because of the modification of cortisol serum levels during corticosteroid therapy, we excluded patients who underwent systemic corticosteroid therapy before or during hospitalization for the cortisol analysis (n = 105/346). Patients with a missing biomarker level were excluded from the respective analyses: discharge (ADM, 28 patients; copeptin, 27; cortisol, 24; prolactin, 24) and admission (ADM, 12 patients; copeptin, 15; cortisol, 8; prolactin, 8).
To assess an additional value of the biomarkers to prediction scores, we assessed the accuracy of the HOSPITAL score and LACE index in their original versions21,22 and after adding each biomarker. We used AUROC to assess the discriminatory power and used the method of DeLong et al.25 to compare results with and without adding each biomarker. Calibration was evaluated by comparing Hosmer-Lemeshow goodness-of-fit tests (P > 0.05 indicates good fit). Risk reclassification was assessed by Net Reclassification Improvement (NRI),26 quantifying how appropriately a new model reclassifies patients, compared with an old model. Basically, patients without outcome are assigned +1 if correctly reclassified to a lower risk category or –1 if incorrectly reclassified to a higher risk category. NRInonevent is the sum of all points/numbers of patients. Conversely, patients with outcome are assigned +1 if correctly reclassified to a higher risk category or –1 if incorrectly reclassified to a lower risk category. NRIevent is the sum of all points/numbers of patients. NRIoverall is the sum of NRIevent and NRInonevent ranging from –2 to 2, with a positive value indicating better classification with the new model.
Two-sided P < 0.05 was used for statistical significance. All statistical analyses were performed with Stata Release 13.0 (StataCorp).
RESULTS
Among the 530 patients admitted to the ward, 184 were excluded (120 meeting exclusion criteria, 64 unable to give consent, Figure). Among the 346 patients included, 11.6% (n = 40) had a 30-day unplanned readmission or death (37 were readmitted, 2 died during readmission, 3 died without readmission). Within 90 days, 26.6% (n = 92) had a readmission or death (84 were readmitted, 10 died during or after readmission, 8 died without readmission).
Clinical Characteristics
Table 1 lists the patients’ baseline characteristics. Median age was 73 years (IQR, 64-82 years). Of the 346 patients included, 172 (49.7%) were men. Median CCI was 7 (IQR, 5-9); according to this index, 310 patients (89.6%) had at least 2 comorbidities. Median length of stay was 7 days (IQR, 4-12 days).
Primary Diagnoses of Admission, Unplanned Readmission, and Death
The 3 main causes of index admission were cardiovascular disorder (n = 92), infectious disorder (n = 70), and neurologic disorder (n = 66). Table 2 lists the causes of readmissions and deaths. A same-diagnosis category between index admission and readmission was found in 17 (45.9%) of the 37 readmitted patients and in 3 (60%) of the 5 patients who died.
Biomarkers and 30-Day Unplanned Readmission or Death
AUROC was 0.53 (95% confidence interval [CI], 0.43-0.63) for ADM, 0.60 (95% CI, 0.50-0.70) for copeptin, 0.59 (95% CI, 0.44-0.73) for cortisol, and 0.56 (95% CI, 0.45-0.66) for prolactin. The difference between admission and discharge levels was not associated with unplanned readmission or death for any of the biomarkers (Supplemental Table 2).
ADM and readmission or death. Median ADM level was not different between patients with and without readmission or death (1.0 nmol/L in each case; P = 1.00). The best cutoff level for ADM was 2 nmol/L (sensitivity, 16.7%; specificity, 91.8%). At this level, ADM was associated with a nonstatistically significant 130% increased odds of 30-day readmission or death (P = 0.09; Table 3, Supplemental Table 3). Conversely, the association with the 90-day outcome was significant (P = 0.02; Table 3, Supplemental Table 4).
Copeptin and readmission or death. Patients with 30-day readmission or death had a higher median copeptin level at discharge than patients without (10.4 pmol/L vs 7.3 pmol/L; P = 0.03). At a copeptin level higher than 9 pmol/L (to convert to pg/mL, divide by 0.249; sensitivity, 66.7%; specificity, 59.7%), both 30-day readmission or death (adjusted odds ratio [OR], 2.69; 95% CI, 1.29-5.64; P = 0.009) and 90-day readmission or death (adjusted OR, 2.76; 95% CI, 1.56-4.88; P < 0.001) were nearly 3 times as likely (Table 3, Supplemental Tables 3 and 4).
Cortisol and readmission or death. Median cortisol was not statistically different between patients with and without the primary outcome (431 nmol/L vs 465 nmol/L; P = 0.72). At a cortisol level higher than 590 nmol/L (to convert to μg/dL, divide by 27.59; sensitivity, 54.6%; specificity, 76.4%), 30-day outcome was more than 3 times as likely (adjusted OR, 3.43; 95% CI, 1.36-8.65; P = 0.009; Table 3, Supplemental Table 3). At 90 days, only the model that adjusted for age and LACE index points remained statistically significant (P = 0.02; Table 3, Supplemental Table 4).
Prolactin and readmission or death. Median prolactin was not statistically different between patients with and without the primary outcome (15.1 μg/L vs 14.1 μg/L; P = 0.24). The best cutoff level for prolactin was 23 μg/L (to convert to mIU/L, divide by 0.05; sensitivity, 27.8%; specificity, 82.9%). Prolactin was associated with a nonstatistically significant increased odds of 30-day (P = 0.16) and 90-day (P = 0.24) readmission or death (Table 3, Supplemental Tables 3 and 4).
Additive Value of Biomarkers to HOSPITAL Score and LACE Index
The AUROC for the original HOSPITAL score, 0.70 (95% CI, 0.60-0.80), nonsignificantly increased to 0.76 after adding the biomarkers (P > 0.14). For the LACE index, AUROC was 0.59 (95% CI, 0.49-0.68), with a significant 0.10 increase with cortisol (P = 0.04) and a near significant increase with copeptin (P = 0.08). Calibration remained almost unchanged after adding the biomarkers to both models (Supplemental Table 5). NRIoverall was positive for all biomarkers, with statistical significance for copeptin added to the HOSPITAL score (0.47; 95% CI, 0.13-0.79) and for cortisol added to the LACE index (0.62; 95% CI, 0.15-1.06).
DISCUSSION
In this prospective cohort study, 30-day and 90-day unplanned readmission or death was nearly 3 times as likely for patients with high copeptin levels on discharge from an acute-care medical hospitalization, and 30-day readmission or death was more than 3 times as likely for patients with high cortisol levels. High ADM and prolactin levels were not consistently associated with readmission or death. Adding such biomarkers to readmission prediction models improved their performance.
These findings support the theory of posthospital syndrome,1 which describes a period of vulnerability with increased stress after discharge from an acute-care hospitalization, and which may be associated with adverse outcome. The hormones cortisol and copeptin are strongly related to the stress response in humans.4,5 As copeptin level has been associated with adverse prognosis for several disorders affecting a wide range of physiologic systems,3,5,15,27 it may be a valuable biomarker of a stressful condition, even independent of the system affected by the acute illness, and its use may be widely generalizable, in contrast to predictive factors identified in other studies.18,28,29
Although cortisol was independently associated with 30-day readmission or death, and may be an interesting biomarker and less expensive than copeptin, its measurement is limited in patients treated with systemic corticosteroids. Compared with cortisol, copeptin does not undergo diurnal variation, is less affected by corticosteroid therapy, and mirrors stress levels better.5,7,30,31 Our results showed that, contrary to cortisol, copeptin was also associated with longer term outcome. High ADM level was associated with readmission or death at 90 days only; lack of a significant association at 30 days might be attributable to a lack of power (fewer outcomes at 30 days). Conversely, prolactin level was consistently not associated with outcome. Prolactin may be affected by many drugs that act on the dopaminergic system (eg, domperidone), and therefore its levels may be more difficult to interpret.
Levels of biomarkers were similar to those measured in patients without previously studied conditions (eg, myocardial infarction).5,8,10,13,14,16,17 In most previous analyses, levels were measured during a stressful event, whereas we measured them at discharge. Therefore, these biomarkers may constitute sensitive markers of remaining stress at discharge.
Our finding that copeptin level was independently associated with readmission or death supports its relevance as a possible simple measure of the risk of adverse postdischarge outcome, independently of disease type and independently of known predictors. Stress biomarkers may therefore be valuable predictors of which patients are at high risk. All these biomarkers can be measured within 30 minutes, extending their use beyond everyday practice, except for the possible need of an extra blood draw.
The most accurate and validated models are the HOSPITAL score (AUROC range, 0.68-0.7723,32-36) and the LACE index (AUROC range, 0.56-0.6823,34,35,37). Adding biomarkers to these models improved overall performance (up to 0.10 increase in AUROC), which is remarkable given that, once a particular level of discriminatory power is reached, extremely large effect sizes from additional markers are needed to increase AUROC.26 Incremental improvement is objectively supported by positive NRI. Our results suggest biomarkers added to prediction models may improve identification of high-risk patients.
We found that less than 50% of the primary diagnoses belonged to the same diagnosis category at readmission and at index admission. This result is in line with previous findings that readmissions were related to the primary diagnosis at index admission in only 22% to 46% of cases,20,38 and supports our study hypothesis that readmission is related to underlying stress factors often independent of the underlying illness.1
Study Limitations and Strengths
Our study had some limitations. First, it was a single-center study with a limited sample size. However, we found significant results within the sample. Second, we could not adjust for drugs that were acting on the dopaminergic system and might have affected prolactin levels. However, such interactions would limit the use of this biomarker in clinical practice anyway. Third, we used specific cutoffs, which might decrease analytical power, in comparison with continuous analyses. However, we followed a recognized method24 and found a significant association even with categorized levels. Furthermore, the distribution of biomarkers could not be normalized by logarithmic transformation, and cutoff values have the advantage of being integrable into score point systems (eg, HOSPITAL score, LACE index). Fourth, although in 2 models we found consistent associations with several potential confounders, we could not exclude residual confounding. Fifth, this study was not powered to assess the biomarkers’ predictive value for readmission and death, which might explain the lack of significant differences between AUROC with and without the biomarkers. For all these reasons, this study should be considered hypothesis-generating.
Our study also had its strengths. First, to our knowledge, this is the first study of the association between stress biomarkers at discharge and unplanned readmission or death. Second, the quality of our data was high, with a low percentage of missing biomarker levels. Third, we excluded planned readmissions. Fourth, we used an unselected medical patient population, which had the noteworthy advantage of widening the generalizability of results.
CONCLUSION
In this prospective cohort study, high copeptin and cortisol levels at discharge were significantly associated with increased odds, ranging from 2-fold to more than 3-fold, of unplanned readmission or death within 30 days after discharge from an internal medicine ward. This finding supports the theory that a physiologic stress that patients experience during hospitalization makes them more susceptible to new health threats (posthospital syndrome). These biomarkers, copeptin in particular, may help us better identify patients at high risk of early unplanned readmission or death.
Acknowledgment
Biomarker measurement was funded by the research fund of the Department of General Internal Medicine, Fribourg Cantonal Hospital, Fribourg, Switzerland.
Disclosure
Nothing to report.
1. Krumholz HM. Post-hospital syndrome—an acquired, transient condition of generalized risk. N Engl J Med. 2013;368(2):100-102. PubMed
2. Morgenthaler NG, Struck J, Alonso C, Bergmann A. Measurement of midregional proadrenomedullin in plasma with an immunoluminometric assay. Clin Chem. 2005;51(10):1823-1829. PubMed
3. Dobsa L, Edozien KC. Copeptin and its potential role in diagnosis and prognosis of various diseases. Biochem Med. 2013;23(2):172-190. PubMed
4. Yilman M, Erenler AK, Baydin A. Copeptin: a diagnostic factor for critical patients. Eur Rev Med Pharmacol Sci. 2015;19(16):3030-3036. PubMed
5. Katan M, Christ-Crain M. The stress hormone copeptin: a new prognostic biomarker in acute illness. Swiss Med Wkly. 2010;140:w13101. PubMed
6. Struck J, Morgenthaler NG, Bergmann A. Copeptin, a stable peptide derived from the vasopressin precursor, is elevated in serum of sepsis patients. Peptides. 2005;26(12):2500-2504. PubMed
7. Darzy KH, Dixit KC, Shalet SM, Morgenthaler NG, Brabant G. Circadian secretion pattern of copeptin, the C-terminal vasopressin precursor fragment. Clin Chem. 2010;56(7):1190-1191. PubMed
8. Labad J, Stojanovic-Pérez A, Montalvo I, et al. Stress biomarkers as predictors of transition to psychosis in at-risk mental states: roles for cortisol, prolactin and albumin. J Psychiatr Res. 2015;60:163-169. PubMed
9. Olsson T, Asplund K, Hagg E. Pituitary-thyroid axis, prolactin and growth hormone in patients with acute stroke. J Intern Med. 1990;228(3):287-290. PubMed
10. Parissis JT, Farmakis D, Fountoulaki K, et al. Clinical and neurohormonal correlates and prognostic value of serum prolactin levels in patients with chronic heart failure. Eur J Heart Fail. 2013;15(10):1122-1130. PubMed
11. Theodoropoulou A, Metallinos IC, Elloul J, et al. Prolactin, cortisol secretion and thyroid function in patients with stroke of mild severity. Horm Metab Res. 2006;38(9):587-591. PubMed
12. Vardas K, Apostolou K, Briassouli E, et al. Early response roles for prolactin cortisol and circulating and cellular levels of heat shock proteins 72 and 90α in severe sepsis and SIRS. Biomed Res Int. 2014;2014:803561. PubMed
13. Bahrmann P, Christ M, Hofner B, et al. Prognostic value of different biomarkers for cardiovascular death in unselected older patients in the emergency department. Eur Heart J Acute Cardiovasc Care. 2016;5(8):568-578. PubMed
14. Christ-Crain M, Morgenthaler NG, Stolz D, et al. Pro-adrenomedullin to predict severity and outcome in community-acquired pneumonia [ISRCTN04176397]. Crit Care. 2006;10(3):R96. PubMed
15. Artunc F, Nowak A, Mueller C, et al. Plasma concentrations of the vasoactive peptide fragments mid-regional pro-adrenomedullin, C-terminal pro-endothelin 1 and copeptin in hemodialysis patients: associated factors and prediction of mortality. PLoS One. 2014;9(1):e86148. PubMed
16. Rotman-Pikielny P, Roash V, Chen O, Limor R, Stern N, Gur HG. Serum cortisol levels in patients admitted to the department of medicine: prognostic correlations and effects of age, infection, and comorbidity. Am J Med Sci. 2006;332(2):61-67. PubMed
17. Yamaji M, Tsutamoto T, Kawahara C, et al. Serum cortisol as a useful predictor of cardiac events in patients with chronic heart failure: the impact of oxidative stress. Circ Heart Fail. 2009;2(6):608-615. PubMed
18. Kansagara D, Englander H, Salanitro A, et al. Risk prediction models for hospital readmission: a systematic review. JAMA. 2011;306(15):1688-1698. PubMed
19. Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373-383. PubMed
20. Donzé J, Lipsitz S, Bates DW, Schnipper JL. Causes and patterns of readmissions in patients with common comorbidities: retrospective cohort study. BMJ. 2013;347:f7171. PubMed
21. van Walraven C, Dhalla IA, Bell C, et al. Derivation and validation of an index to predict early death or unplanned readmission after discharge from hospital to the community. CMAJ. 2010;182(6):551-557. PubMed
22. Donzé J, Aujesky D, Williams D, Schnipper JL. Potentially avoidable 30-day hospital readmissions in medical patients: derivation and validation of a prediction model. JAMA Intern Med. 2013;173(8):632-638. PubMed
23. Aubert CE, Folly A, Mancinetti M, Hayoz D, Donzé J. Prospective validation and adaptation of the HOSPITAL score to predict high risk of unplanned readmission of medical patients. Swiss Med Wkly. 2016;146:w14335. PubMed
24. Zweig MH, Campbell G. Receiver-operating characteristic (ROC) plots: a fundamental evaluation tool in clinical medicine. Clin Chem. 1993;39(4):561-577. PubMed
25. DeLong ER, DeLong DM, Clarke-Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics. 1988;44(3):837-845. PubMed
26. Pencina MJ, D’Agostino RB Sr, Demler OV. Novel metrics for evaluating improvement in discrimination: net reclassification and integrated discrimination improvement for normal variables and nested models. Stat Med. 2012;31(2):101-113. PubMed
27. Folli C, Consonni D, Spessot M, et al. Diagnostic role of copeptin in patients presenting with chest pain in the emergency room. Eur J Intern Med. 2013;24(2):189-193. PubMed
28. Aujesky D, Mor MK, Geng M, Stone RA, Fine MJ, Ibrahim SA. Predictors of early hospital readmission after acute pulmonary embolism. Arch Intern Med. 2009;169(3):287-293. PubMed
29. Hammill BG, Curtis LH, Fonarow GC, et al. Incremental value of clinical data beyond claims data in predicting 30-day outcomes after heart failure hospitalization. Circ Cardiovasc Qual Outcomes. 2011;4(1):60-67. PubMed
30. Nickel CH, Bingisser R, Morgenthaler NG. The role of copeptin as a diagnostic and prognostic biomarker for risk stratification in the emergency department. BMC Med. 2012;10:7. PubMed
31. Katan M, Morgenthaler N, Widmer I, et al. Copeptin, a stable peptide derived from the vasopressin precursor, correlates with the individual stress level. Neuro Endocrinol Lett. 2008;29(3):341-346. PubMed
32. Donzé JD, Williams MV, Robinson EJ, et al. International validity of the HOSPITAL score to predict 30-day potentially avoidable hospital readmissions. JAMA Intern Med. 2016;176(4):496-502.PubMed
33. Burke RE, Schnipper JL, Williams MV, et al. The HOSPITAL score predicts potentially preventable 30-day readmissions in conditions targeted by the Hospital Readmissions Reduction Program. Med Care. 2017;55(3):285-290. PubMed
34. Garrison GM, Robelia PM, Pecina JL, Dawson NL. Comparing performance of 30-day readmission risk classifiers among hospitalized primary care patients. J Eval Clin Pract. 2017;23(3):524-529. PubMed
35. Cooksley T, Nanayakkara PW, Nickel CH, et al. Readmissions of medical patients: an external validation of two existing prediction scores. QJM. 2016;109(4):245-248. PubMed
36. Robinson R. The HOSPITAL score as a predictor of 30 day readmission in a retrospective study at a university affiliated community hospital. PeerJ. 2016;4:e2441. PubMed
37. Wang H, Robinson RD, Johnson C, et al. Using the LACE index to predict hospital readmissions in congestive heart failure patients. BMC Cardiovasc Disord. 2014;14:97. PubMed
38. Dunlay SM, Weston SA, Killian JM, Bell MR, Jaffe AS, Roger VL. Thirty-day rehospitalizations after acute myocardial infarction: a cohort study. Ann Intern Med. 2012;157(1):11-18. PubMed
It has been theorized that the physiologic stress that hospitalized patients experience accounts for their transient vulnerability after discharge, or posthospital syndrome.1 Their acute illness and life-habit changes during hospitalization result in continued impairment of physiologic systems after discharge, and this impairment might leave them more susceptible to new health threats.1 However, the theory that the stress experienced after a hospitalization might be associated with readmission has never been investigated.
Four biomarkers of the hypothalamic-pituitary-adrenal (HPA) axis may help quantify posthospitalization stress: (1) midregional pro-adrenomedullin (ADM), a precursor reflecting adrenomedullin activity2; (2) copeptin (the C-terminal part of prepro-vasopressin, produced by the hypothalamus in response to stress3,4), the level of which closely correlates to the vasopressin level but is more stable and lacks circadian rhythm fluctuations5-7; (3) cortisol, released by the adrenal cortex in response to stress; and (4) prolactin, an indicator of HPA axis activity. These 4 stress biomarkers have been related to the severity, complications, or mortality of several diseases.3,5,8-17 Besides explaining the hypothetical association between posthospitalization stress and readmission and death, these biomarkers might be valuable in predicting which patients are at higher risk for readmission. Indeed, many prediction models have been developed to identify those patients, but most of these models underperform, target only very specific populations, or have not been externally validated.18
We hypothesized that the hospitalization stress measured by biomarkers is associated with readmission or death after discharge. In a prospective cohort study, we evaluated the association between 4 stress biomarkers (ADM, copeptin, cortisol, prolactin) and 30-day unplanned readmissions and deaths after an acute-care medical hospitalization, and assessed their additive value to validated readmission prediction scores.
METHODS
Study Design and Population
Our prospective cohort study included all consecutive patients aged ≥50 years and admitted to the department of general internal medicine at Fribourg Cantonal Hospital in Switzerland between April 8, 2013 and September 23, 2013. Exclusion criteria were discharge on day of admission; death before discharge; discharge to another division, another acute-care hospital, a rehabilitation clinic, or a palliative-care clinic; and refusal or inability to give informed consent. In this hypothesis-generating observational study, we collected data on a convenience sample of patients and did not calculate sample size before data collection. The study was approved by the local ethics committee, and all patients gave informed consent.
Outcomes
The primary outcome was the composite of first unplanned readmission (to any division of any acute-care hospital) or death within 30 days after discharge from index admission. We also included deaths that occurred after discharge, hypothesizing that patients who died may have been readmitted had they lived. The secondary outcome was the same as the primary, but the period was 90 days. Planned readmission was defined as scheduled hospitalization for nonemergent treatment (eg, chemotherapy) or investigation (eg, elective coronarography). All patients were called 6 months after discharge, and readmissions and deaths recorded. If a patient could not be reached directly, we called his or her next of kin, primary care physician, or nursing home, depending on availability. Furthermore, we checked electronic health records for any readmission or death recorded within the Fribourg hospital network, which includes all 3 acute-care hospitals (Fribourg, Riaz, Tavel) in the same canton (state).
Independent Variables
Stress biomarkers. We measured serum levels of 4 stress biomarkers (ADM, copeptin, cortisol, prolactin) at 8 am on an empty stomach on both day of admission and day of discharge. For a patient whose discharge decision was made after 8 hours for the same day, a blood sample was collected as soon as discharge was planned.
Clinical data. Collected data included demographics, history of hospitalization within 6 months before index admission, hospitalization diagnosis, and Charlson Comorbidity Index (CCI), which includes a list of medical conditions that are assigned a number of 1, 2, 3, or 6 points, according to their severity, and which has been associated with mortality.19
Causes of Admission, Unplanned Readmission, and Death
Causes of index admission, unplanned readmission, and death were obtained from medical records. We used our consensus opinion and a previous analysis20 to classify these causes by body system, and added 2 categories, cancer and infection (both associated with readmission20). The resulting 9 categories were (1) cancer, (2) respiratory disorder, (3) infectious disorder, (4) neurologic disorder (including dementia, psychiatric disorder, alcohol disorder, and intoxication), (5) gastrointestinal disorder, (6) osteoarticular disorder, (7) renal disorder, (8) cardiovascular disorder (including ischemic disease and heart failure), and (9) other.
Additional Performance With Existing Predictive Models
To better define the explanatory power of biomarkers to predict our outcome, we assessed the performance improvement of 2 validated readmission prediction scores by adding the stress biomarkers. As large effect sizes from additional predictors are needed to increase the power discrimination of a model, a significant performance improvement would further support the biomarkers’ important explanatory power. The 2 prediction scores tested were the LACE index (Length of stay, Admission Acuity, CCI, number of Emergency department visits within preceding 6 months21) and the HOSPITAL score (Hemoglobin level at discharge, discharge from Oncology service, Sodium level at discharge, any Procedure performed during index hospitalization, Index admission Type, number of Admissions within preceding 12 months, Length of stay). As we did not have an oncology service, we replaced “discharge from oncology service” with “active diagnosis of cancer.” “Length of stay” was tailored to the median in Switzerland (8 days instead of 5 days; Supplement Table 1).22,23
Data Analysis
Continuous variables were presented as medians with interquartile ranges (IQRs) because of their non-normal distribution, and categorical variables were presented as frequencies and percentages. We compared medians using the nonparametric K-sample test on the equality of medians, and compared frequencies using the Pearson χ2 test. The discriminatory power of each biomarker in predicting readmission and death was calculated with the area under the receiver operating characteristic (ROC) curve (AUROC), using serum levels at discharge to better reflect the postdischarge period. Cutoff levels were selected by taking the best compromise between sensitivity and specificity according to the ROC curves (point nearest top left corner).24
Univariate logistic regression analysis was used to test the prediction of 30-day and 90-day unplanned readmission or death by each biomarker. We built 2 different multivariate models: one adjusting for age and LACE index points21 and the other adjusting for age and HOSPITAL score.22,23
To explore any association between reduction of stress during hospitalization and postdischarge outcome, we additionally calculated for each biomarker the difference between admission and discharge serum levels and assessed its association with readmission or death by logistic regression analysis. Because of the modification of cortisol serum levels during corticosteroid therapy, we excluded patients who underwent systemic corticosteroid therapy before or during hospitalization for the cortisol analysis (n = 105/346). Patients with a missing biomarker level were excluded from the respective analyses: discharge (ADM, 28 patients; copeptin, 27; cortisol, 24; prolactin, 24) and admission (ADM, 12 patients; copeptin, 15; cortisol, 8; prolactin, 8).
To assess an additional value of the biomarkers to prediction scores, we assessed the accuracy of the HOSPITAL score and LACE index in their original versions21,22 and after adding each biomarker. We used AUROC to assess the discriminatory power and used the method of DeLong et al.25 to compare results with and without adding each biomarker. Calibration was evaluated by comparing Hosmer-Lemeshow goodness-of-fit tests (P > 0.05 indicates good fit). Risk reclassification was assessed by Net Reclassification Improvement (NRI),26 quantifying how appropriately a new model reclassifies patients, compared with an old model. Basically, patients without outcome are assigned +1 if correctly reclassified to a lower risk category or –1 if incorrectly reclassified to a higher risk category. NRInonevent is the sum of all points/numbers of patients. Conversely, patients with outcome are assigned +1 if correctly reclassified to a higher risk category or –1 if incorrectly reclassified to a lower risk category. NRIevent is the sum of all points/numbers of patients. NRIoverall is the sum of NRIevent and NRInonevent ranging from –2 to 2, with a positive value indicating better classification with the new model.
Two-sided P < 0.05 was used for statistical significance. All statistical analyses were performed with Stata Release 13.0 (StataCorp).
RESULTS
Among the 530 patients admitted to the ward, 184 were excluded (120 meeting exclusion criteria, 64 unable to give consent, Figure). Among the 346 patients included, 11.6% (n = 40) had a 30-day unplanned readmission or death (37 were readmitted, 2 died during readmission, 3 died without readmission). Within 90 days, 26.6% (n = 92) had a readmission or death (84 were readmitted, 10 died during or after readmission, 8 died without readmission).
Clinical Characteristics
Table 1 lists the patients’ baseline characteristics. Median age was 73 years (IQR, 64-82 years). Of the 346 patients included, 172 (49.7%) were men. Median CCI was 7 (IQR, 5-9); according to this index, 310 patients (89.6%) had at least 2 comorbidities. Median length of stay was 7 days (IQR, 4-12 days).
Primary Diagnoses of Admission, Unplanned Readmission, and Death
The 3 main causes of index admission were cardiovascular disorder (n = 92), infectious disorder (n = 70), and neurologic disorder (n = 66). Table 2 lists the causes of readmissions and deaths. A same-diagnosis category between index admission and readmission was found in 17 (45.9%) of the 37 readmitted patients and in 3 (60%) of the 5 patients who died.
Biomarkers and 30-Day Unplanned Readmission or Death
AUROC was 0.53 (95% confidence interval [CI], 0.43-0.63) for ADM, 0.60 (95% CI, 0.50-0.70) for copeptin, 0.59 (95% CI, 0.44-0.73) for cortisol, and 0.56 (95% CI, 0.45-0.66) for prolactin. The difference between admission and discharge levels was not associated with unplanned readmission or death for any of the biomarkers (Supplemental Table 2).
ADM and readmission or death. Median ADM level was not different between patients with and without readmission or death (1.0 nmol/L in each case; P = 1.00). The best cutoff level for ADM was 2 nmol/L (sensitivity, 16.7%; specificity, 91.8%). At this level, ADM was associated with a nonstatistically significant 130% increased odds of 30-day readmission or death (P = 0.09; Table 3, Supplemental Table 3). Conversely, the association with the 90-day outcome was significant (P = 0.02; Table 3, Supplemental Table 4).
Copeptin and readmission or death. Patients with 30-day readmission or death had a higher median copeptin level at discharge than patients without (10.4 pmol/L vs 7.3 pmol/L; P = 0.03). At a copeptin level higher than 9 pmol/L (to convert to pg/mL, divide by 0.249; sensitivity, 66.7%; specificity, 59.7%), both 30-day readmission or death (adjusted odds ratio [OR], 2.69; 95% CI, 1.29-5.64; P = 0.009) and 90-day readmission or death (adjusted OR, 2.76; 95% CI, 1.56-4.88; P < 0.001) were nearly 3 times as likely (Table 3, Supplemental Tables 3 and 4).
Cortisol and readmission or death. Median cortisol was not statistically different between patients with and without the primary outcome (431 nmol/L vs 465 nmol/L; P = 0.72). At a cortisol level higher than 590 nmol/L (to convert to μg/dL, divide by 27.59; sensitivity, 54.6%; specificity, 76.4%), 30-day outcome was more than 3 times as likely (adjusted OR, 3.43; 95% CI, 1.36-8.65; P = 0.009; Table 3, Supplemental Table 3). At 90 days, only the model that adjusted for age and LACE index points remained statistically significant (P = 0.02; Table 3, Supplemental Table 4).
Prolactin and readmission or death. Median prolactin was not statistically different between patients with and without the primary outcome (15.1 μg/L vs 14.1 μg/L; P = 0.24). The best cutoff level for prolactin was 23 μg/L (to convert to mIU/L, divide by 0.05; sensitivity, 27.8%; specificity, 82.9%). Prolactin was associated with a nonstatistically significant increased odds of 30-day (P = 0.16) and 90-day (P = 0.24) readmission or death (Table 3, Supplemental Tables 3 and 4).
Additive Value of Biomarkers to HOSPITAL Score and LACE Index
The AUROC for the original HOSPITAL score, 0.70 (95% CI, 0.60-0.80), nonsignificantly increased to 0.76 after adding the biomarkers (P > 0.14). For the LACE index, AUROC was 0.59 (95% CI, 0.49-0.68), with a significant 0.10 increase with cortisol (P = 0.04) and a near significant increase with copeptin (P = 0.08). Calibration remained almost unchanged after adding the biomarkers to both models (Supplemental Table 5). NRIoverall was positive for all biomarkers, with statistical significance for copeptin added to the HOSPITAL score (0.47; 95% CI, 0.13-0.79) and for cortisol added to the LACE index (0.62; 95% CI, 0.15-1.06).
DISCUSSION
In this prospective cohort study, 30-day and 90-day unplanned readmission or death was nearly 3 times as likely for patients with high copeptin levels on discharge from an acute-care medical hospitalization, and 30-day readmission or death was more than 3 times as likely for patients with high cortisol levels. High ADM and prolactin levels were not consistently associated with readmission or death. Adding such biomarkers to readmission prediction models improved their performance.
These findings support the theory of posthospital syndrome,1 which describes a period of vulnerability with increased stress after discharge from an acute-care hospitalization, and which may be associated with adverse outcome. The hormones cortisol and copeptin are strongly related to the stress response in humans.4,5 As copeptin level has been associated with adverse prognosis for several disorders affecting a wide range of physiologic systems,3,5,15,27 it may be a valuable biomarker of a stressful condition, even independent of the system affected by the acute illness, and its use may be widely generalizable, in contrast to predictive factors identified in other studies.18,28,29
Although cortisol was independently associated with 30-day readmission or death, and may be an interesting biomarker and less expensive than copeptin, its measurement is limited in patients treated with systemic corticosteroids. Compared with cortisol, copeptin does not undergo diurnal variation, is less affected by corticosteroid therapy, and mirrors stress levels better.5,7,30,31 Our results showed that, contrary to cortisol, copeptin was also associated with longer term outcome. High ADM level was associated with readmission or death at 90 days only; lack of a significant association at 30 days might be attributable to a lack of power (fewer outcomes at 30 days). Conversely, prolactin level was consistently not associated with outcome. Prolactin may be affected by many drugs that act on the dopaminergic system (eg, domperidone), and therefore its levels may be more difficult to interpret.
Levels of biomarkers were similar to those measured in patients without previously studied conditions (eg, myocardial infarction).5,8,10,13,14,16,17 In most previous analyses, levels were measured during a stressful event, whereas we measured them at discharge. Therefore, these biomarkers may constitute sensitive markers of remaining stress at discharge.
Our finding that copeptin level was independently associated with readmission or death supports its relevance as a possible simple measure of the risk of adverse postdischarge outcome, independently of disease type and independently of known predictors. Stress biomarkers may therefore be valuable predictors of which patients are at high risk. All these biomarkers can be measured within 30 minutes, extending their use beyond everyday practice, except for the possible need of an extra blood draw.
The most accurate and validated models are the HOSPITAL score (AUROC range, 0.68-0.7723,32-36) and the LACE index (AUROC range, 0.56-0.6823,34,35,37). Adding biomarkers to these models improved overall performance (up to 0.10 increase in AUROC), which is remarkable given that, once a particular level of discriminatory power is reached, extremely large effect sizes from additional markers are needed to increase AUROC.26 Incremental improvement is objectively supported by positive NRI. Our results suggest biomarkers added to prediction models may improve identification of high-risk patients.
We found that less than 50% of the primary diagnoses belonged to the same diagnosis category at readmission and at index admission. This result is in line with previous findings that readmissions were related to the primary diagnosis at index admission in only 22% to 46% of cases,20,38 and supports our study hypothesis that readmission is related to underlying stress factors often independent of the underlying illness.1
Study Limitations and Strengths
Our study had some limitations. First, it was a single-center study with a limited sample size. However, we found significant results within the sample. Second, we could not adjust for drugs that were acting on the dopaminergic system and might have affected prolactin levels. However, such interactions would limit the use of this biomarker in clinical practice anyway. Third, we used specific cutoffs, which might decrease analytical power, in comparison with continuous analyses. However, we followed a recognized method24 and found a significant association even with categorized levels. Furthermore, the distribution of biomarkers could not be normalized by logarithmic transformation, and cutoff values have the advantage of being integrable into score point systems (eg, HOSPITAL score, LACE index). Fourth, although in 2 models we found consistent associations with several potential confounders, we could not exclude residual confounding. Fifth, this study was not powered to assess the biomarkers’ predictive value for readmission and death, which might explain the lack of significant differences between AUROC with and without the biomarkers. For all these reasons, this study should be considered hypothesis-generating.
Our study also had its strengths. First, to our knowledge, this is the first study of the association between stress biomarkers at discharge and unplanned readmission or death. Second, the quality of our data was high, with a low percentage of missing biomarker levels. Third, we excluded planned readmissions. Fourth, we used an unselected medical patient population, which had the noteworthy advantage of widening the generalizability of results.
CONCLUSION
In this prospective cohort study, high copeptin and cortisol levels at discharge were significantly associated with increased odds, ranging from 2-fold to more than 3-fold, of unplanned readmission or death within 30 days after discharge from an internal medicine ward. This finding supports the theory that a physiologic stress that patients experience during hospitalization makes them more susceptible to new health threats (posthospital syndrome). These biomarkers, copeptin in particular, may help us better identify patients at high risk of early unplanned readmission or death.
Acknowledgment
Biomarker measurement was funded by the research fund of the Department of General Internal Medicine, Fribourg Cantonal Hospital, Fribourg, Switzerland.
Disclosure
Nothing to report.
It has been theorized that the physiologic stress that hospitalized patients experience accounts for their transient vulnerability after discharge, or posthospital syndrome.1 Their acute illness and life-habit changes during hospitalization result in continued impairment of physiologic systems after discharge, and this impairment might leave them more susceptible to new health threats.1 However, the theory that the stress experienced after a hospitalization might be associated with readmission has never been investigated.
Four biomarkers of the hypothalamic-pituitary-adrenal (HPA) axis may help quantify posthospitalization stress: (1) midregional pro-adrenomedullin (ADM), a precursor reflecting adrenomedullin activity2; (2) copeptin (the C-terminal part of prepro-vasopressin, produced by the hypothalamus in response to stress3,4), the level of which closely correlates to the vasopressin level but is more stable and lacks circadian rhythm fluctuations5-7; (3) cortisol, released by the adrenal cortex in response to stress; and (4) prolactin, an indicator of HPA axis activity. These 4 stress biomarkers have been related to the severity, complications, or mortality of several diseases.3,5,8-17 Besides explaining the hypothetical association between posthospitalization stress and readmission and death, these biomarkers might be valuable in predicting which patients are at higher risk for readmission. Indeed, many prediction models have been developed to identify those patients, but most of these models underperform, target only very specific populations, or have not been externally validated.18
We hypothesized that the hospitalization stress measured by biomarkers is associated with readmission or death after discharge. In a prospective cohort study, we evaluated the association between 4 stress biomarkers (ADM, copeptin, cortisol, prolactin) and 30-day unplanned readmissions and deaths after an acute-care medical hospitalization, and assessed their additive value to validated readmission prediction scores.
METHODS
Study Design and Population
Our prospective cohort study included all consecutive patients aged ≥50 years and admitted to the department of general internal medicine at Fribourg Cantonal Hospital in Switzerland between April 8, 2013 and September 23, 2013. Exclusion criteria were discharge on day of admission; death before discharge; discharge to another division, another acute-care hospital, a rehabilitation clinic, or a palliative-care clinic; and refusal or inability to give informed consent. In this hypothesis-generating observational study, we collected data on a convenience sample of patients and did not calculate sample size before data collection. The study was approved by the local ethics committee, and all patients gave informed consent.
Outcomes
The primary outcome was the composite of first unplanned readmission (to any division of any acute-care hospital) or death within 30 days after discharge from index admission. We also included deaths that occurred after discharge, hypothesizing that patients who died may have been readmitted had they lived. The secondary outcome was the same as the primary, but the period was 90 days. Planned readmission was defined as scheduled hospitalization for nonemergent treatment (eg, chemotherapy) or investigation (eg, elective coronarography). All patients were called 6 months after discharge, and readmissions and deaths recorded. If a patient could not be reached directly, we called his or her next of kin, primary care physician, or nursing home, depending on availability. Furthermore, we checked electronic health records for any readmission or death recorded within the Fribourg hospital network, which includes all 3 acute-care hospitals (Fribourg, Riaz, Tavel) in the same canton (state).
Independent Variables
Stress biomarkers. We measured serum levels of 4 stress biomarkers (ADM, copeptin, cortisol, prolactin) at 8 am on an empty stomach on both day of admission and day of discharge. For a patient whose discharge decision was made after 8 hours for the same day, a blood sample was collected as soon as discharge was planned.
Clinical data. Collected data included demographics, history of hospitalization within 6 months before index admission, hospitalization diagnosis, and Charlson Comorbidity Index (CCI), which includes a list of medical conditions that are assigned a number of 1, 2, 3, or 6 points, according to their severity, and which has been associated with mortality.19
Causes of Admission, Unplanned Readmission, and Death
Causes of index admission, unplanned readmission, and death were obtained from medical records. We used our consensus opinion and a previous analysis20 to classify these causes by body system, and added 2 categories, cancer and infection (both associated with readmission20). The resulting 9 categories were (1) cancer, (2) respiratory disorder, (3) infectious disorder, (4) neurologic disorder (including dementia, psychiatric disorder, alcohol disorder, and intoxication), (5) gastrointestinal disorder, (6) osteoarticular disorder, (7) renal disorder, (8) cardiovascular disorder (including ischemic disease and heart failure), and (9) other.
Additional Performance With Existing Predictive Models
To better define the explanatory power of biomarkers to predict our outcome, we assessed the performance improvement of 2 validated readmission prediction scores by adding the stress biomarkers. As large effect sizes from additional predictors are needed to increase the power discrimination of a model, a significant performance improvement would further support the biomarkers’ important explanatory power. The 2 prediction scores tested were the LACE index (Length of stay, Admission Acuity, CCI, number of Emergency department visits within preceding 6 months21) and the HOSPITAL score (Hemoglobin level at discharge, discharge from Oncology service, Sodium level at discharge, any Procedure performed during index hospitalization, Index admission Type, number of Admissions within preceding 12 months, Length of stay). As we did not have an oncology service, we replaced “discharge from oncology service” with “active diagnosis of cancer.” “Length of stay” was tailored to the median in Switzerland (8 days instead of 5 days; Supplement Table 1).22,23
Data Analysis
Continuous variables were presented as medians with interquartile ranges (IQRs) because of their non-normal distribution, and categorical variables were presented as frequencies and percentages. We compared medians using the nonparametric K-sample test on the equality of medians, and compared frequencies using the Pearson χ2 test. The discriminatory power of each biomarker in predicting readmission and death was calculated with the area under the receiver operating characteristic (ROC) curve (AUROC), using serum levels at discharge to better reflect the postdischarge period. Cutoff levels were selected by taking the best compromise between sensitivity and specificity according to the ROC curves (point nearest top left corner).24
Univariate logistic regression analysis was used to test the prediction of 30-day and 90-day unplanned readmission or death by each biomarker. We built 2 different multivariate models: one adjusting for age and LACE index points21 and the other adjusting for age and HOSPITAL score.22,23
To explore any association between reduction of stress during hospitalization and postdischarge outcome, we additionally calculated for each biomarker the difference between admission and discharge serum levels and assessed its association with readmission or death by logistic regression analysis. Because of the modification of cortisol serum levels during corticosteroid therapy, we excluded patients who underwent systemic corticosteroid therapy before or during hospitalization for the cortisol analysis (n = 105/346). Patients with a missing biomarker level were excluded from the respective analyses: discharge (ADM, 28 patients; copeptin, 27; cortisol, 24; prolactin, 24) and admission (ADM, 12 patients; copeptin, 15; cortisol, 8; prolactin, 8).
To assess an additional value of the biomarkers to prediction scores, we assessed the accuracy of the HOSPITAL score and LACE index in their original versions21,22 and after adding each biomarker. We used AUROC to assess the discriminatory power and used the method of DeLong et al.25 to compare results with and without adding each biomarker. Calibration was evaluated by comparing Hosmer-Lemeshow goodness-of-fit tests (P > 0.05 indicates good fit). Risk reclassification was assessed by Net Reclassification Improvement (NRI),26 quantifying how appropriately a new model reclassifies patients, compared with an old model. Basically, patients without outcome are assigned +1 if correctly reclassified to a lower risk category or –1 if incorrectly reclassified to a higher risk category. NRInonevent is the sum of all points/numbers of patients. Conversely, patients with outcome are assigned +1 if correctly reclassified to a higher risk category or –1 if incorrectly reclassified to a lower risk category. NRIevent is the sum of all points/numbers of patients. NRIoverall is the sum of NRIevent and NRInonevent ranging from –2 to 2, with a positive value indicating better classification with the new model.
Two-sided P < 0.05 was used for statistical significance. All statistical analyses were performed with Stata Release 13.0 (StataCorp).
RESULTS
Among the 530 patients admitted to the ward, 184 were excluded (120 meeting exclusion criteria, 64 unable to give consent, Figure). Among the 346 patients included, 11.6% (n = 40) had a 30-day unplanned readmission or death (37 were readmitted, 2 died during readmission, 3 died without readmission). Within 90 days, 26.6% (n = 92) had a readmission or death (84 were readmitted, 10 died during or after readmission, 8 died without readmission).
Clinical Characteristics
Table 1 lists the patients’ baseline characteristics. Median age was 73 years (IQR, 64-82 years). Of the 346 patients included, 172 (49.7%) were men. Median CCI was 7 (IQR, 5-9); according to this index, 310 patients (89.6%) had at least 2 comorbidities. Median length of stay was 7 days (IQR, 4-12 days).
Primary Diagnoses of Admission, Unplanned Readmission, and Death
The 3 main causes of index admission were cardiovascular disorder (n = 92), infectious disorder (n = 70), and neurologic disorder (n = 66). Table 2 lists the causes of readmissions and deaths. A same-diagnosis category between index admission and readmission was found in 17 (45.9%) of the 37 readmitted patients and in 3 (60%) of the 5 patients who died.
Biomarkers and 30-Day Unplanned Readmission or Death
AUROC was 0.53 (95% confidence interval [CI], 0.43-0.63) for ADM, 0.60 (95% CI, 0.50-0.70) for copeptin, 0.59 (95% CI, 0.44-0.73) for cortisol, and 0.56 (95% CI, 0.45-0.66) for prolactin. The difference between admission and discharge levels was not associated with unplanned readmission or death for any of the biomarkers (Supplemental Table 2).
ADM and readmission or death. Median ADM level was not different between patients with and without readmission or death (1.0 nmol/L in each case; P = 1.00). The best cutoff level for ADM was 2 nmol/L (sensitivity, 16.7%; specificity, 91.8%). At this level, ADM was associated with a nonstatistically significant 130% increased odds of 30-day readmission or death (P = 0.09; Table 3, Supplemental Table 3). Conversely, the association with the 90-day outcome was significant (P = 0.02; Table 3, Supplemental Table 4).
Copeptin and readmission or death. Patients with 30-day readmission or death had a higher median copeptin level at discharge than patients without (10.4 pmol/L vs 7.3 pmol/L; P = 0.03). At a copeptin level higher than 9 pmol/L (to convert to pg/mL, divide by 0.249; sensitivity, 66.7%; specificity, 59.7%), both 30-day readmission or death (adjusted odds ratio [OR], 2.69; 95% CI, 1.29-5.64; P = 0.009) and 90-day readmission or death (adjusted OR, 2.76; 95% CI, 1.56-4.88; P < 0.001) were nearly 3 times as likely (Table 3, Supplemental Tables 3 and 4).
Cortisol and readmission or death. Median cortisol was not statistically different between patients with and without the primary outcome (431 nmol/L vs 465 nmol/L; P = 0.72). At a cortisol level higher than 590 nmol/L (to convert to μg/dL, divide by 27.59; sensitivity, 54.6%; specificity, 76.4%), 30-day outcome was more than 3 times as likely (adjusted OR, 3.43; 95% CI, 1.36-8.65; P = 0.009; Table 3, Supplemental Table 3). At 90 days, only the model that adjusted for age and LACE index points remained statistically significant (P = 0.02; Table 3, Supplemental Table 4).
Prolactin and readmission or death. Median prolactin was not statistically different between patients with and without the primary outcome (15.1 μg/L vs 14.1 μg/L; P = 0.24). The best cutoff level for prolactin was 23 μg/L (to convert to mIU/L, divide by 0.05; sensitivity, 27.8%; specificity, 82.9%). Prolactin was associated with a nonstatistically significant increased odds of 30-day (P = 0.16) and 90-day (P = 0.24) readmission or death (Table 3, Supplemental Tables 3 and 4).
Additive Value of Biomarkers to HOSPITAL Score and LACE Index
The AUROC for the original HOSPITAL score, 0.70 (95% CI, 0.60-0.80), nonsignificantly increased to 0.76 after adding the biomarkers (P > 0.14). For the LACE index, AUROC was 0.59 (95% CI, 0.49-0.68), with a significant 0.10 increase with cortisol (P = 0.04) and a near significant increase with copeptin (P = 0.08). Calibration remained almost unchanged after adding the biomarkers to both models (Supplemental Table 5). NRIoverall was positive for all biomarkers, with statistical significance for copeptin added to the HOSPITAL score (0.47; 95% CI, 0.13-0.79) and for cortisol added to the LACE index (0.62; 95% CI, 0.15-1.06).
DISCUSSION
In this prospective cohort study, 30-day and 90-day unplanned readmission or death was nearly 3 times as likely for patients with high copeptin levels on discharge from an acute-care medical hospitalization, and 30-day readmission or death was more than 3 times as likely for patients with high cortisol levels. High ADM and prolactin levels were not consistently associated with readmission or death. Adding such biomarkers to readmission prediction models improved their performance.
These findings support the theory of posthospital syndrome,1 which describes a period of vulnerability with increased stress after discharge from an acute-care hospitalization, and which may be associated with adverse outcome. The hormones cortisol and copeptin are strongly related to the stress response in humans.4,5 As copeptin level has been associated with adverse prognosis for several disorders affecting a wide range of physiologic systems,3,5,15,27 it may be a valuable biomarker of a stressful condition, even independent of the system affected by the acute illness, and its use may be widely generalizable, in contrast to predictive factors identified in other studies.18,28,29
Although cortisol was independently associated with 30-day readmission or death, and may be an interesting biomarker and less expensive than copeptin, its measurement is limited in patients treated with systemic corticosteroids. Compared with cortisol, copeptin does not undergo diurnal variation, is less affected by corticosteroid therapy, and mirrors stress levels better.5,7,30,31 Our results showed that, contrary to cortisol, copeptin was also associated with longer term outcome. High ADM level was associated with readmission or death at 90 days only; lack of a significant association at 30 days might be attributable to a lack of power (fewer outcomes at 30 days). Conversely, prolactin level was consistently not associated with outcome. Prolactin may be affected by many drugs that act on the dopaminergic system (eg, domperidone), and therefore its levels may be more difficult to interpret.
Levels of biomarkers were similar to those measured in patients without previously studied conditions (eg, myocardial infarction).5,8,10,13,14,16,17 In most previous analyses, levels were measured during a stressful event, whereas we measured them at discharge. Therefore, these biomarkers may constitute sensitive markers of remaining stress at discharge.
Our finding that copeptin level was independently associated with readmission or death supports its relevance as a possible simple measure of the risk of adverse postdischarge outcome, independently of disease type and independently of known predictors. Stress biomarkers may therefore be valuable predictors of which patients are at high risk. All these biomarkers can be measured within 30 minutes, extending their use beyond everyday practice, except for the possible need of an extra blood draw.
The most accurate and validated models are the HOSPITAL score (AUROC range, 0.68-0.7723,32-36) and the LACE index (AUROC range, 0.56-0.6823,34,35,37). Adding biomarkers to these models improved overall performance (up to 0.10 increase in AUROC), which is remarkable given that, once a particular level of discriminatory power is reached, extremely large effect sizes from additional markers are needed to increase AUROC.26 Incremental improvement is objectively supported by positive NRI. Our results suggest biomarkers added to prediction models may improve identification of high-risk patients.
We found that less than 50% of the primary diagnoses belonged to the same diagnosis category at readmission and at index admission. This result is in line with previous findings that readmissions were related to the primary diagnosis at index admission in only 22% to 46% of cases,20,38 and supports our study hypothesis that readmission is related to underlying stress factors often independent of the underlying illness.1
Study Limitations and Strengths
Our study had some limitations. First, it was a single-center study with a limited sample size. However, we found significant results within the sample. Second, we could not adjust for drugs that were acting on the dopaminergic system and might have affected prolactin levels. However, such interactions would limit the use of this biomarker in clinical practice anyway. Third, we used specific cutoffs, which might decrease analytical power, in comparison with continuous analyses. However, we followed a recognized method24 and found a significant association even with categorized levels. Furthermore, the distribution of biomarkers could not be normalized by logarithmic transformation, and cutoff values have the advantage of being integrable into score point systems (eg, HOSPITAL score, LACE index). Fourth, although in 2 models we found consistent associations with several potential confounders, we could not exclude residual confounding. Fifth, this study was not powered to assess the biomarkers’ predictive value for readmission and death, which might explain the lack of significant differences between AUROC with and without the biomarkers. For all these reasons, this study should be considered hypothesis-generating.
Our study also had its strengths. First, to our knowledge, this is the first study of the association between stress biomarkers at discharge and unplanned readmission or death. Second, the quality of our data was high, with a low percentage of missing biomarker levels. Third, we excluded planned readmissions. Fourth, we used an unselected medical patient population, which had the noteworthy advantage of widening the generalizability of results.
CONCLUSION
In this prospective cohort study, high copeptin and cortisol levels at discharge were significantly associated with increased odds, ranging from 2-fold to more than 3-fold, of unplanned readmission or death within 30 days after discharge from an internal medicine ward. This finding supports the theory that a physiologic stress that patients experience during hospitalization makes them more susceptible to new health threats (posthospital syndrome). These biomarkers, copeptin in particular, may help us better identify patients at high risk of early unplanned readmission or death.
Acknowledgment
Biomarker measurement was funded by the research fund of the Department of General Internal Medicine, Fribourg Cantonal Hospital, Fribourg, Switzerland.
Disclosure
Nothing to report.
1. Krumholz HM. Post-hospital syndrome—an acquired, transient condition of generalized risk. N Engl J Med. 2013;368(2):100-102. PubMed
2. Morgenthaler NG, Struck J, Alonso C, Bergmann A. Measurement of midregional proadrenomedullin in plasma with an immunoluminometric assay. Clin Chem. 2005;51(10):1823-1829. PubMed
3. Dobsa L, Edozien KC. Copeptin and its potential role in diagnosis and prognosis of various diseases. Biochem Med. 2013;23(2):172-190. PubMed
4. Yilman M, Erenler AK, Baydin A. Copeptin: a diagnostic factor for critical patients. Eur Rev Med Pharmacol Sci. 2015;19(16):3030-3036. PubMed
5. Katan M, Christ-Crain M. The stress hormone copeptin: a new prognostic biomarker in acute illness. Swiss Med Wkly. 2010;140:w13101. PubMed
6. Struck J, Morgenthaler NG, Bergmann A. Copeptin, a stable peptide derived from the vasopressin precursor, is elevated in serum of sepsis patients. Peptides. 2005;26(12):2500-2504. PubMed
7. Darzy KH, Dixit KC, Shalet SM, Morgenthaler NG, Brabant G. Circadian secretion pattern of copeptin, the C-terminal vasopressin precursor fragment. Clin Chem. 2010;56(7):1190-1191. PubMed
8. Labad J, Stojanovic-Pérez A, Montalvo I, et al. Stress biomarkers as predictors of transition to psychosis in at-risk mental states: roles for cortisol, prolactin and albumin. J Psychiatr Res. 2015;60:163-169. PubMed
9. Olsson T, Asplund K, Hagg E. Pituitary-thyroid axis, prolactin and growth hormone in patients with acute stroke. J Intern Med. 1990;228(3):287-290. PubMed
10. Parissis JT, Farmakis D, Fountoulaki K, et al. Clinical and neurohormonal correlates and prognostic value of serum prolactin levels in patients with chronic heart failure. Eur J Heart Fail. 2013;15(10):1122-1130. PubMed
11. Theodoropoulou A, Metallinos IC, Elloul J, et al. Prolactin, cortisol secretion and thyroid function in patients with stroke of mild severity. Horm Metab Res. 2006;38(9):587-591. PubMed
12. Vardas K, Apostolou K, Briassouli E, et al. Early response roles for prolactin cortisol and circulating and cellular levels of heat shock proteins 72 and 90α in severe sepsis and SIRS. Biomed Res Int. 2014;2014:803561. PubMed
13. Bahrmann P, Christ M, Hofner B, et al. Prognostic value of different biomarkers for cardiovascular death in unselected older patients in the emergency department. Eur Heart J Acute Cardiovasc Care. 2016;5(8):568-578. PubMed
14. Christ-Crain M, Morgenthaler NG, Stolz D, et al. Pro-adrenomedullin to predict severity and outcome in community-acquired pneumonia [ISRCTN04176397]. Crit Care. 2006;10(3):R96. PubMed
15. Artunc F, Nowak A, Mueller C, et al. Plasma concentrations of the vasoactive peptide fragments mid-regional pro-adrenomedullin, C-terminal pro-endothelin 1 and copeptin in hemodialysis patients: associated factors and prediction of mortality. PLoS One. 2014;9(1):e86148. PubMed
16. Rotman-Pikielny P, Roash V, Chen O, Limor R, Stern N, Gur HG. Serum cortisol levels in patients admitted to the department of medicine: prognostic correlations and effects of age, infection, and comorbidity. Am J Med Sci. 2006;332(2):61-67. PubMed
17. Yamaji M, Tsutamoto T, Kawahara C, et al. Serum cortisol as a useful predictor of cardiac events in patients with chronic heart failure: the impact of oxidative stress. Circ Heart Fail. 2009;2(6):608-615. PubMed
18. Kansagara D, Englander H, Salanitro A, et al. Risk prediction models for hospital readmission: a systematic review. JAMA. 2011;306(15):1688-1698. PubMed
19. Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373-383. PubMed
20. Donzé J, Lipsitz S, Bates DW, Schnipper JL. Causes and patterns of readmissions in patients with common comorbidities: retrospective cohort study. BMJ. 2013;347:f7171. PubMed
21. van Walraven C, Dhalla IA, Bell C, et al. Derivation and validation of an index to predict early death or unplanned readmission after discharge from hospital to the community. CMAJ. 2010;182(6):551-557. PubMed
22. Donzé J, Aujesky D, Williams D, Schnipper JL. Potentially avoidable 30-day hospital readmissions in medical patients: derivation and validation of a prediction model. JAMA Intern Med. 2013;173(8):632-638. PubMed
23. Aubert CE, Folly A, Mancinetti M, Hayoz D, Donzé J. Prospective validation and adaptation of the HOSPITAL score to predict high risk of unplanned readmission of medical patients. Swiss Med Wkly. 2016;146:w14335. PubMed
24. Zweig MH, Campbell G. Receiver-operating characteristic (ROC) plots: a fundamental evaluation tool in clinical medicine. Clin Chem. 1993;39(4):561-577. PubMed
25. DeLong ER, DeLong DM, Clarke-Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics. 1988;44(3):837-845. PubMed
26. Pencina MJ, D’Agostino RB Sr, Demler OV. Novel metrics for evaluating improvement in discrimination: net reclassification and integrated discrimination improvement for normal variables and nested models. Stat Med. 2012;31(2):101-113. PubMed
27. Folli C, Consonni D, Spessot M, et al. Diagnostic role of copeptin in patients presenting with chest pain in the emergency room. Eur J Intern Med. 2013;24(2):189-193. PubMed
28. Aujesky D, Mor MK, Geng M, Stone RA, Fine MJ, Ibrahim SA. Predictors of early hospital readmission after acute pulmonary embolism. Arch Intern Med. 2009;169(3):287-293. PubMed
29. Hammill BG, Curtis LH, Fonarow GC, et al. Incremental value of clinical data beyond claims data in predicting 30-day outcomes after heart failure hospitalization. Circ Cardiovasc Qual Outcomes. 2011;4(1):60-67. PubMed
30. Nickel CH, Bingisser R, Morgenthaler NG. The role of copeptin as a diagnostic and prognostic biomarker for risk stratification in the emergency department. BMC Med. 2012;10:7. PubMed
31. Katan M, Morgenthaler N, Widmer I, et al. Copeptin, a stable peptide derived from the vasopressin precursor, correlates with the individual stress level. Neuro Endocrinol Lett. 2008;29(3):341-346. PubMed
32. Donzé JD, Williams MV, Robinson EJ, et al. International validity of the HOSPITAL score to predict 30-day potentially avoidable hospital readmissions. JAMA Intern Med. 2016;176(4):496-502.PubMed
33. Burke RE, Schnipper JL, Williams MV, et al. The HOSPITAL score predicts potentially preventable 30-day readmissions in conditions targeted by the Hospital Readmissions Reduction Program. Med Care. 2017;55(3):285-290. PubMed
34. Garrison GM, Robelia PM, Pecina JL, Dawson NL. Comparing performance of 30-day readmission risk classifiers among hospitalized primary care patients. J Eval Clin Pract. 2017;23(3):524-529. PubMed
35. Cooksley T, Nanayakkara PW, Nickel CH, et al. Readmissions of medical patients: an external validation of two existing prediction scores. QJM. 2016;109(4):245-248. PubMed
36. Robinson R. The HOSPITAL score as a predictor of 30 day readmission in a retrospective study at a university affiliated community hospital. PeerJ. 2016;4:e2441. PubMed
37. Wang H, Robinson RD, Johnson C, et al. Using the LACE index to predict hospital readmissions in congestive heart failure patients. BMC Cardiovasc Disord. 2014;14:97. PubMed
38. Dunlay SM, Weston SA, Killian JM, Bell MR, Jaffe AS, Roger VL. Thirty-day rehospitalizations after acute myocardial infarction: a cohort study. Ann Intern Med. 2012;157(1):11-18. PubMed
1. Krumholz HM. Post-hospital syndrome—an acquired, transient condition of generalized risk. N Engl J Med. 2013;368(2):100-102. PubMed
2. Morgenthaler NG, Struck J, Alonso C, Bergmann A. Measurement of midregional proadrenomedullin in plasma with an immunoluminometric assay. Clin Chem. 2005;51(10):1823-1829. PubMed
3. Dobsa L, Edozien KC. Copeptin and its potential role in diagnosis and prognosis of various diseases. Biochem Med. 2013;23(2):172-190. PubMed
4. Yilman M, Erenler AK, Baydin A. Copeptin: a diagnostic factor for critical patients. Eur Rev Med Pharmacol Sci. 2015;19(16):3030-3036. PubMed
5. Katan M, Christ-Crain M. The stress hormone copeptin: a new prognostic biomarker in acute illness. Swiss Med Wkly. 2010;140:w13101. PubMed
6. Struck J, Morgenthaler NG, Bergmann A. Copeptin, a stable peptide derived from the vasopressin precursor, is elevated in serum of sepsis patients. Peptides. 2005;26(12):2500-2504. PubMed
7. Darzy KH, Dixit KC, Shalet SM, Morgenthaler NG, Brabant G. Circadian secretion pattern of copeptin, the C-terminal vasopressin precursor fragment. Clin Chem. 2010;56(7):1190-1191. PubMed
8. Labad J, Stojanovic-Pérez A, Montalvo I, et al. Stress biomarkers as predictors of transition to psychosis in at-risk mental states: roles for cortisol, prolactin and albumin. J Psychiatr Res. 2015;60:163-169. PubMed
9. Olsson T, Asplund K, Hagg E. Pituitary-thyroid axis, prolactin and growth hormone in patients with acute stroke. J Intern Med. 1990;228(3):287-290. PubMed
10. Parissis JT, Farmakis D, Fountoulaki K, et al. Clinical and neurohormonal correlates and prognostic value of serum prolactin levels in patients with chronic heart failure. Eur J Heart Fail. 2013;15(10):1122-1130. PubMed
11. Theodoropoulou A, Metallinos IC, Elloul J, et al. Prolactin, cortisol secretion and thyroid function in patients with stroke of mild severity. Horm Metab Res. 2006;38(9):587-591. PubMed
12. Vardas K, Apostolou K, Briassouli E, et al. Early response roles for prolactin cortisol and circulating and cellular levels of heat shock proteins 72 and 90α in severe sepsis and SIRS. Biomed Res Int. 2014;2014:803561. PubMed
13. Bahrmann P, Christ M, Hofner B, et al. Prognostic value of different biomarkers for cardiovascular death in unselected older patients in the emergency department. Eur Heart J Acute Cardiovasc Care. 2016;5(8):568-578. PubMed
14. Christ-Crain M, Morgenthaler NG, Stolz D, et al. Pro-adrenomedullin to predict severity and outcome in community-acquired pneumonia [ISRCTN04176397]. Crit Care. 2006;10(3):R96. PubMed
15. Artunc F, Nowak A, Mueller C, et al. Plasma concentrations of the vasoactive peptide fragments mid-regional pro-adrenomedullin, C-terminal pro-endothelin 1 and copeptin in hemodialysis patients: associated factors and prediction of mortality. PLoS One. 2014;9(1):e86148. PubMed
16. Rotman-Pikielny P, Roash V, Chen O, Limor R, Stern N, Gur HG. Serum cortisol levels in patients admitted to the department of medicine: prognostic correlations and effects of age, infection, and comorbidity. Am J Med Sci. 2006;332(2):61-67. PubMed
17. Yamaji M, Tsutamoto T, Kawahara C, et al. Serum cortisol as a useful predictor of cardiac events in patients with chronic heart failure: the impact of oxidative stress. Circ Heart Fail. 2009;2(6):608-615. PubMed
18. Kansagara D, Englander H, Salanitro A, et al. Risk prediction models for hospital readmission: a systematic review. JAMA. 2011;306(15):1688-1698. PubMed
19. Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373-383. PubMed
20. Donzé J, Lipsitz S, Bates DW, Schnipper JL. Causes and patterns of readmissions in patients with common comorbidities: retrospective cohort study. BMJ. 2013;347:f7171. PubMed
21. van Walraven C, Dhalla IA, Bell C, et al. Derivation and validation of an index to predict early death or unplanned readmission after discharge from hospital to the community. CMAJ. 2010;182(6):551-557. PubMed
22. Donzé J, Aujesky D, Williams D, Schnipper JL. Potentially avoidable 30-day hospital readmissions in medical patients: derivation and validation of a prediction model. JAMA Intern Med. 2013;173(8):632-638. PubMed
23. Aubert CE, Folly A, Mancinetti M, Hayoz D, Donzé J. Prospective validation and adaptation of the HOSPITAL score to predict high risk of unplanned readmission of medical patients. Swiss Med Wkly. 2016;146:w14335. PubMed
24. Zweig MH, Campbell G. Receiver-operating characteristic (ROC) plots: a fundamental evaluation tool in clinical medicine. Clin Chem. 1993;39(4):561-577. PubMed
25. DeLong ER, DeLong DM, Clarke-Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics. 1988;44(3):837-845. PubMed
26. Pencina MJ, D’Agostino RB Sr, Demler OV. Novel metrics for evaluating improvement in discrimination: net reclassification and integrated discrimination improvement for normal variables and nested models. Stat Med. 2012;31(2):101-113. PubMed
27. Folli C, Consonni D, Spessot M, et al. Diagnostic role of copeptin in patients presenting with chest pain in the emergency room. Eur J Intern Med. 2013;24(2):189-193. PubMed
28. Aujesky D, Mor MK, Geng M, Stone RA, Fine MJ, Ibrahim SA. Predictors of early hospital readmission after acute pulmonary embolism. Arch Intern Med. 2009;169(3):287-293. PubMed
29. Hammill BG, Curtis LH, Fonarow GC, et al. Incremental value of clinical data beyond claims data in predicting 30-day outcomes after heart failure hospitalization. Circ Cardiovasc Qual Outcomes. 2011;4(1):60-67. PubMed
30. Nickel CH, Bingisser R, Morgenthaler NG. The role of copeptin as a diagnostic and prognostic biomarker for risk stratification in the emergency department. BMC Med. 2012;10:7. PubMed
31. Katan M, Morgenthaler N, Widmer I, et al. Copeptin, a stable peptide derived from the vasopressin precursor, correlates with the individual stress level. Neuro Endocrinol Lett. 2008;29(3):341-346. PubMed
32. Donzé JD, Williams MV, Robinson EJ, et al. International validity of the HOSPITAL score to predict 30-day potentially avoidable hospital readmissions. JAMA Intern Med. 2016;176(4):496-502.PubMed
33. Burke RE, Schnipper JL, Williams MV, et al. The HOSPITAL score predicts potentially preventable 30-day readmissions in conditions targeted by the Hospital Readmissions Reduction Program. Med Care. 2017;55(3):285-290. PubMed
34. Garrison GM, Robelia PM, Pecina JL, Dawson NL. Comparing performance of 30-day readmission risk classifiers among hospitalized primary care patients. J Eval Clin Pract. 2017;23(3):524-529. PubMed
35. Cooksley T, Nanayakkara PW, Nickel CH, et al. Readmissions of medical patients: an external validation of two existing prediction scores. QJM. 2016;109(4):245-248. PubMed
36. Robinson R. The HOSPITAL score as a predictor of 30 day readmission in a retrospective study at a university affiliated community hospital. PeerJ. 2016;4:e2441. PubMed
37. Wang H, Robinson RD, Johnson C, et al. Using the LACE index to predict hospital readmissions in congestive heart failure patients. BMC Cardiovasc Disord. 2014;14:97. PubMed
38. Dunlay SM, Weston SA, Killian JM, Bell MR, Jaffe AS, Roger VL. Thirty-day rehospitalizations after acute myocardial infarction: a cohort study. Ann Intern Med. 2012;157(1):11-18. PubMed
© 2017 Society of Hospital Medicine
TB meningitis cases in U.S. are fewer but more complicated
BOSTON – The number of cases of meningitis caused by tuberculosis has fallen dramatically in the United States in recent decades as TB itself has become less common, according to findings from a study presented at the annual meeting of the American Academy of Neurology.
However, these findings from patient hospitalizations during 1993-2013 in the Nationwide Inpatient Sample database also indicate that neurologic complications from TB meningitis are on the rise.
The findings suggest that neurologists need to become involved whenever a patient with TB shows signs of neurologic problems, said study lead author Alexander E. Merkler, MD, of Cornell University, New York, in an interview. “They’re at high risk, and some complications can be life threatening.”
According to Dr. Merkler, TB meningitis occurs when a patient’s case of TB invades the meninges surrounding the brain. “It can lead to seizures, stroke, hydrocephalus, and death,” he said at the meeting.
TB meningitis can affect anyone with TB, he said, but those who are immunocompromised and those with diabetes are especially vulnerable.
For their current study, Dr. Merkler and his associates used the Nationwide Inpatient Sample database to track patients hospitalized in the United States with TB meningitis from 1993 to 2013. They found 16,196 new cases over the 20-year period and uncovered a dramatic decrease in the rate of hospitalizations: The incidence fell from 6.2 to 1.9 hospitalizations per million people (rate difference, 4.3; 95% confidence interval, 2.1-6.5; P less than .001), and mortality during index hospitalization fell from 17.6% (95% CI, 12.0%-23.2%) to 7.6%, (95% CI, 2.2%-13.0%).
Dr. Merkler said that mortality appears to have declined as TB itself has become less common. According to the Centers for Disease Control and Prevention, the number of reported TB cases nationally was 9,557 in 2015, a rate of 3.0 cases per 100,000 persons. The total number of annual cases fell each year from 1993 to 2014, the CDC reported, although the rate leveled off at around 3.0/100,000 from 2013 to 2015.
“The fewer people have lung TB, the less they’ll have it going into meningitis and the brain,” Dr. Merkler said. “In terms of mortality, it is going down because we have better supportive care. We’re better at keeping these patients alive and giving them antibiotics sooner.”
However, the study found that the rates of the following complications in hospitalized TB meningitis patients rose over the 20-year period:
• Hydrocephalus, from 2.3% (95% confidence interval, 0.5%-4.2%) to 5.4% (95% CI, 2.3%-10.0%).
• Seizure, from 2.9% (95% CI, 0.3%-5.4%) to 14.1% (95% CI, 7.3%-21.0%).
• Stroke, from 2.9% (95% CI, 0.6%-5.3%) to 13.0% (95% CI, 6.3%-19.8%).
• Vision and hearing impairment, from 8.2% (95% CI, 4.8%-11.6%) to 10.9% (95% CI, 4.1%-17.6%), and from 1.1% (95% CI, 0.0%-2.3%) to 3.3% (95% CI, 0.0%-6.9%), respectively.
Dr. Merkler said it’s not clear why these rates are going up, but it may be because patients have more complications as a result of living longer. Another theory is that a form of drug-resistant TB is boosting the level of these complications, Dr. Merkler said, but he’s skeptical of that idea: “I don’t know why drug resistance would lead to more neurological complications.”
The study was funded by the National Institute of Neurological Disorders and Stroke and the Michael Goldberg Stroke Research Fund. Dr. Merkler reported no relevant financial disclosures.
BOSTON – The number of cases of meningitis caused by tuberculosis has fallen dramatically in the United States in recent decades as TB itself has become less common, according to findings from a study presented at the annual meeting of the American Academy of Neurology.
However, these findings from patient hospitalizations during 1993-2013 in the Nationwide Inpatient Sample database also indicate that neurologic complications from TB meningitis are on the rise.
The findings suggest that neurologists need to become involved whenever a patient with TB shows signs of neurologic problems, said study lead author Alexander E. Merkler, MD, of Cornell University, New York, in an interview. “They’re at high risk, and some complications can be life threatening.”
According to Dr. Merkler, TB meningitis occurs when a patient’s case of TB invades the meninges surrounding the brain. “It can lead to seizures, stroke, hydrocephalus, and death,” he said at the meeting.
TB meningitis can affect anyone with TB, he said, but those who are immunocompromised and those with diabetes are especially vulnerable.
For their current study, Dr. Merkler and his associates used the Nationwide Inpatient Sample database to track patients hospitalized in the United States with TB meningitis from 1993 to 2013. They found 16,196 new cases over the 20-year period and uncovered a dramatic decrease in the rate of hospitalizations: The incidence fell from 6.2 to 1.9 hospitalizations per million people (rate difference, 4.3; 95% confidence interval, 2.1-6.5; P less than .001), and mortality during index hospitalization fell from 17.6% (95% CI, 12.0%-23.2%) to 7.6%, (95% CI, 2.2%-13.0%).
Dr. Merkler said that mortality appears to have declined as TB itself has become less common. According to the Centers for Disease Control and Prevention, the number of reported TB cases nationally was 9,557 in 2015, a rate of 3.0 cases per 100,000 persons. The total number of annual cases fell each year from 1993 to 2014, the CDC reported, although the rate leveled off at around 3.0/100,000 from 2013 to 2015.
“The fewer people have lung TB, the less they’ll have it going into meningitis and the brain,” Dr. Merkler said. “In terms of mortality, it is going down because we have better supportive care. We’re better at keeping these patients alive and giving them antibiotics sooner.”
However, the study found that the rates of the following complications in hospitalized TB meningitis patients rose over the 20-year period:
• Hydrocephalus, from 2.3% (95% confidence interval, 0.5%-4.2%) to 5.4% (95% CI, 2.3%-10.0%).
• Seizure, from 2.9% (95% CI, 0.3%-5.4%) to 14.1% (95% CI, 7.3%-21.0%).
• Stroke, from 2.9% (95% CI, 0.6%-5.3%) to 13.0% (95% CI, 6.3%-19.8%).
• Vision and hearing impairment, from 8.2% (95% CI, 4.8%-11.6%) to 10.9% (95% CI, 4.1%-17.6%), and from 1.1% (95% CI, 0.0%-2.3%) to 3.3% (95% CI, 0.0%-6.9%), respectively.
Dr. Merkler said it’s not clear why these rates are going up, but it may be because patients have more complications as a result of living longer. Another theory is that a form of drug-resistant TB is boosting the level of these complications, Dr. Merkler said, but he’s skeptical of that idea: “I don’t know why drug resistance would lead to more neurological complications.”
The study was funded by the National Institute of Neurological Disorders and Stroke and the Michael Goldberg Stroke Research Fund. Dr. Merkler reported no relevant financial disclosures.
BOSTON – The number of cases of meningitis caused by tuberculosis has fallen dramatically in the United States in recent decades as TB itself has become less common, according to findings from a study presented at the annual meeting of the American Academy of Neurology.
However, these findings from patient hospitalizations during 1993-2013 in the Nationwide Inpatient Sample database also indicate that neurologic complications from TB meningitis are on the rise.
The findings suggest that neurologists need to become involved whenever a patient with TB shows signs of neurologic problems, said study lead author Alexander E. Merkler, MD, of Cornell University, New York, in an interview. “They’re at high risk, and some complications can be life threatening.”
According to Dr. Merkler, TB meningitis occurs when a patient’s case of TB invades the meninges surrounding the brain. “It can lead to seizures, stroke, hydrocephalus, and death,” he said at the meeting.
TB meningitis can affect anyone with TB, he said, but those who are immunocompromised and those with diabetes are especially vulnerable.
For their current study, Dr. Merkler and his associates used the Nationwide Inpatient Sample database to track patients hospitalized in the United States with TB meningitis from 1993 to 2013. They found 16,196 new cases over the 20-year period and uncovered a dramatic decrease in the rate of hospitalizations: The incidence fell from 6.2 to 1.9 hospitalizations per million people (rate difference, 4.3; 95% confidence interval, 2.1-6.5; P less than .001), and mortality during index hospitalization fell from 17.6% (95% CI, 12.0%-23.2%) to 7.6%, (95% CI, 2.2%-13.0%).
Dr. Merkler said that mortality appears to have declined as TB itself has become less common. According to the Centers for Disease Control and Prevention, the number of reported TB cases nationally was 9,557 in 2015, a rate of 3.0 cases per 100,000 persons. The total number of annual cases fell each year from 1993 to 2014, the CDC reported, although the rate leveled off at around 3.0/100,000 from 2013 to 2015.
“The fewer people have lung TB, the less they’ll have it going into meningitis and the brain,” Dr. Merkler said. “In terms of mortality, it is going down because we have better supportive care. We’re better at keeping these patients alive and giving them antibiotics sooner.”
However, the study found that the rates of the following complications in hospitalized TB meningitis patients rose over the 20-year period:
• Hydrocephalus, from 2.3% (95% confidence interval, 0.5%-4.2%) to 5.4% (95% CI, 2.3%-10.0%).
• Seizure, from 2.9% (95% CI, 0.3%-5.4%) to 14.1% (95% CI, 7.3%-21.0%).
• Stroke, from 2.9% (95% CI, 0.6%-5.3%) to 13.0% (95% CI, 6.3%-19.8%).
• Vision and hearing impairment, from 8.2% (95% CI, 4.8%-11.6%) to 10.9% (95% CI, 4.1%-17.6%), and from 1.1% (95% CI, 0.0%-2.3%) to 3.3% (95% CI, 0.0%-6.9%), respectively.
Dr. Merkler said it’s not clear why these rates are going up, but it may be because patients have more complications as a result of living longer. Another theory is that a form of drug-resistant TB is boosting the level of these complications, Dr. Merkler said, but he’s skeptical of that idea: “I don’t know why drug resistance would lead to more neurological complications.”
The study was funded by the National Institute of Neurological Disorders and Stroke and the Michael Goldberg Stroke Research Fund. Dr. Merkler reported no relevant financial disclosures.
AT AAN 2017
Key clinical point:
Major finding: The rate of TB meningitis hospitalizations fell from 6.2 to 1.9 per million people (rate difference, 4.3; 95% CI, 2.1-6.5; P less than .001).
Data source: The Nationwide Inpatient Sample database, which revealed 16,196 new cases of TB meningitis from 1993 to 2013.
Disclosures: The study was funded by the National Institute of Neurological Disorders and Stroke and the Michael Goldberg Stroke Research Fund. Dr. Merkler reported no relevant financial disclosures.
Hospital-based clinicians’ use of technology for patient care-related communication: a national survey
Communication among healthcare professionals is essential for high-quality patient care. However, communication is difficult in hospitals because of heavy workloads, rapidly evolving plans of care, and geographic dispersion of team members. When hospital-based professionals are not in the same place at the same time, they rely on technology to communicate. Pagers have historically been used to support communication in hospitals, but are limited in their capabilities. Several recent small studies have shown that some physicians have started using standard text messaging on smartphones for patient care–related (PCR) messages.1-3 Although potentially enhancing clinician efficiency, use of standard text messaging for PCR messages raises concern about security risks related to transmission of protected health information. Addressing these concerns are emerging secure mobile messaging applications designed for PCR communication. Although recent studies suggest these applications are well received by users, the adoption rate is largely unknown.4,5
We conducted a study to see if there was a shift in use of hospital-based communication technologies under way. We surveyed a national sample of hospital-based clinicians to characterize current use of communication technologies, assess potential risks and perceptions related to use of standard text messaging for PCR messages, and characterize the adoption of secure mobile messaging applications designed for PCR communication.
METHODS
Study Design
The study was a cross-sectional survey of hospitalists—physicians and advanced practice providers whose primary professional focus is care of hospitalized patients. We studied hospitalists because of their role in coordinating care for complex medical patients and because prior studies identified communication as a major component of their work.6,7 The Northwestern University Institutional Review Board deemed this study exempt.
Survey Instrument
Four investigators (Drs. O’Leary, Liebovitz, Wu, and Reddy) with expertise in interprofessional communication and information technology created a draft survey based in part on results of prior studies assessing clinicians’ use of smartphones and standard text messaging for PCR communication.1,3 In the first section of the survey, we asked respondents which technologies were provided by their organization and which technologies they used for PCR communication. In the second section, we asked respondents about their use and perceptions of standard text messaging for PCR communication. In the third section, we asked about implementation and adoption of secure mobile messaging applications at their hospital. In the fourth and final section, we asked for demographic information.
We randomly selected 8 attendees of the 2015 Midwest Hospital Medicine Conference and invited them to participate in a focus group that would review a paper version of the draft survey and recommend revisions. Using the group’s feedback, we revised the ordinal response scale for questions related to standard text messaging and made other minor edits. We then created an Internet-based version of the survey and pilot-tested it with 8 hospitalists from 4 diverse hospitalist groups within the Northwestern Medicine Health System. We made additional minor edits based on pilot-test feedback.
Sampling Strategy
We used the largest hospitalist database maintained by the Society of Hospital Medicine (SHM). This database includes information on more than 28,000 individuals, representing SHM members and nonmembers who had participated in organizational events. In addition to clinically active hospitalists, the database includes non-hospitalists and clinically inactive hospitalists. We used this database to try to capture the largest possible number of potentially eligible hospitalists.
Survey Administration
We administered the survey in collaboration with SHM staff. E-mails that included a link to the survey on the Survey Monkey website were sent by SHM staff to individuals within the database. These e-mails were sent through Real Magnet, an e-mail marketing platform8 that allowed the SHM staff to determine the number of individuals who received and opened the e-mail and the number who clicked on the survey link. To try to promote participation, we offered respondents the chance to enter a lottery to win one of four $50 gift certificates. The initial e-mail was sent in April 2016, a reminder in May 2016, and a final reminder in July 2016.
Data Analysis
We calculated descriptive statistics of participants’ demographic characteristics. We estimated nonresponse bias by comparing demographic characteristics across waves of respondents using analysis of variance, t tests, and χ2 tests. This method is based on the finding that characteristics of late respondents often resemble those of nonrespondents.9 We collapsed response categories for communication technologies to simplify interpretation. For example, numeric pagers, alphanumeric pagers, and 2-way pagers were collapsed into a pagers category. We used t tests and χ2 tests to assess for associations between receipt of standard text messages for PCR communication and respondents’ age, sex, race, professional type, hospital size, practice location, and hospital teaching status. Similarly, we used t tests and χ2 tests to explore associations between implementation of secure mobile messaging application and respondents’ age, sex, race, professional type, hospital size, practice location, and hospital teaching status. All statistical analyses were performed with Stata Release 11.2 (StataCorp).
RESULTS
Participant Characteristics
Overall, the survey link was sent to 28,870 e-mail addresses. Addresses for which e-mails were undeliverable or for which the e-mail was never opened were excluded, yielding a total of 5,786 eligible respondents in the sample. After rejecting 42 clinically inactive individuals, 70 individuals who responded to only the initial item, and 27 duplicates, a total of 620 participant surveys were included in the final analysis. The adjusted response rate was 11.0%.
As shown in Table 1, mean (SD) respondent age was 42.9 (10.0) years, nearly half of the respondents were female, nearly a third were of nonwhite race, an overwhelming majority were physicians, and workplaces were in a variety of hospital settings. The sample size used to calculate demographic characteristics varied from 538 to 549 because of missing data for these items. We found no significant differences in demographic characteristics of respondents across the 3 survey waves, suggesting a lack of survey response bias (Supplemental Table).
Provision and Use of Communication Technologies for PCR Communication
Pagers were provided to the majority of respondents by their hospitals (79.8%, 495/620). Other devices were provided much less frequently, with 21.0% (130/620) reporting their organization provided a smartphone, 20.2% (125/620) a mobile phone, and 4.4% (27/620) a hands-free communication device. Organizations provided no device to 8.2% (51/620) of respondents and an “other” device to 5.5% (34/620).
An overwhelming majority used multiple technologies to receive PCR communication, with 17.7
Participants’ Experiences With Standard Text Messaging for PCR Communication
Participants’ experiences with standard text messaging for PCR communication are summarized in Table 3. Overall, 65.1% (369/ 567) of respondents reported receiving standard text messages for PCR communication at least once per week when on clinical duty, and 52.9% (300/567) received standard text messages at least once per day.
Overall, 21.5% (122/567) of respondents received standard text messages that included individually identifiable information at least once per day, and 41.3% (234/567) received messages that included some identifiable information (eg, patient initials, room number) at least once per day. About one-fifth of respondents (21.0%, 119/567) indicated receiving standard text messages for urgent clinical issues at least once per day. Receipt of standard text messages for a patient for whom the respondent was no longer providing care, delays in receipt of messages, messages missed because smartphones were set to vibrate, and receipt of messages when not on clinical duty occurred, but less frequently. We found no significant associations between receipt of PCR standard text messages once or more per day and respondents’ age, sex, race, professional type, hospital size, or hospital teaching status. A higher percentage of respondents in the South (63.2%, 96/152) and West (57.9%, 70/121) reported receipt of at least 1 PCR standard text message per day, compared with respondents in the Northeast (51.9%, 54/104), Midwest (45.2%, 61/135), and other (25.0%, 4/16) (P = 0.003).
Senders of PCR standard text messages. Of respondents who received standard text messages for PCR communication at least once per week, a majority reported receiving messages from physicians in the same specialty (88.6%, 327/369) and from physicians in other specialties (71.3%, 263/369). A minority of respondents reported receiving messages from nurses (35.0%, 129/369), social workers (30.6%, 113/369), and pharmacists (27.9%, 103/369).
Perceptions among users. Of respondents who received standard text messages for PCR communication at least once per week, an overwhelming majority agreed or strongly agreed that use of standard text messaging allowed them to provide better care (81.7%, 295/361) and made them more efficient (87.3%, 315/361). A majority also agreed or strongly agreed that standard text messaging posed a risk to the privacy and confidentiality of patient information (56.4%, 203/360), and nearly a third indicated that standard text messaging posed a risk to the timely receipt of messages by the correct individual (27.6%, 100/362). Overall, a large majority agreed or strongly agreed that the benefits of using standard text messaging for PCR communication outweighed the risks (85.0%, 306/360).
Adoption of Organization-Approved Secure Mobile Messaging Applications
Participants’ reported adoption of organization-approved secure mobile messaging applications is shown in the Figure. About one-fourth (26.6%, 146/549) of respondents reported that their organization had implemented a secure messaging application and that some clinicians were using it, whereas relatively few (7.3%, 40/549) reported that their organization had implemented an application that was being used by most clinicians. A substantial portion of respondents (21.3%, 117/549) were not sure whether their organization was planning to implement a secure mobile messaging application for PCR communication. We found no significant associations between partial or nearly full implementation of a secure mobile messaging application and respondents’ age, sex, race, professional type, hospital size, or practice location. A lower percentage of respondents in major teaching hospitals (28.0%, 67/239) reported partial or nearly full implementation of a secure mobile messaging application, compared with respondents from teaching hospitals (39.6%, 74/187) and nonteaching hospitals (39.2%, 40/102) (P = 0.02).
DISCUSSION
We found that pagers were the technology most commonly used by hospital-based clinicians, but also that a majority have used standard text messaging for PCR communication, and that relatively few hospitals had fully implemented secure mobile messaging applications. Our findings reveal a wide range of technology use and suggest an evolution to support communication among healthcare professionals.
The persistence of pagers as the technology most commonly provided by hospitals and used by clinicians for communication is noteworthy in that pagers are limited in their capabilities, typically not allowing a response to the message sender or the ability to forward a message, and often not allowing the ability to send messages to multiple recipients. The continued heavy use of pagers may be explained by their relatively low cost, especially compared with investment in new technologies, and reliable receipt of messages, even in areas with no cell phone service or WiFi signal. Furthermore, hospitals’ providing pagers allows for oversight, directory creation, and the potential for integration into other information systems. In 2 recent studies, inpatient paging communication was evaluated in depth. Carlile et al.10 found that the majority of pages requested a response, requiring an interruption in physician workflow to initiate a callback. Kummerow Broman et al.11 similarly found that a majority of pages requested a callback; they also found a high volume of nonurgent messages. With pager use, a high volume of messages, many of which require a response but are nonurgent, makes for a highly interruptive workflow.
That more than half of our hospital-based clinicians received standard text messages for PCR communication once or more per day is consistent with other, smaller studies. Kuhlmann et al.1 surveyed 97 pediatric hospitalists and found that a majority sent and received work-related text messages. Prochaska et al.2 surveyed 131 residents and found that standard text messaging was the communication method preferred by the majority of residents. Similar to these studies, our study found that receipt of standard text messages that included protected health information was fairly common. However, we identified additional risks related to standard text messaging. One-fifth of our respondents received standard text messages for urgent clinical issues once or more per day, and many respondents reported occasional receipt of messages regarding a patient for whom they were no longer providing care and receipt of messages when not on clinical duty. The usual inability to automate forwarding of standard text messages to another clinician creates the potential for clinically important messages to be delayed or missed. These risks have not been reported in the literature, and we think healthcare systems may not be fully aware of them. Our findings suggest that many clinicians have migrated from pagers to standard text messaging for the enhanced efficiency, and they perceive that the benefit of improved efficiency outweighs the risks to protected health information and the delay in receipt of clinically important messages by the correct individual.
Secure mobile messaging applications seem to address the limitations of both pagers and standard text messaging. Secure mobile messaging applications typically allow message response, message forwarding, multiple recipients, directory creation, the potential to create escalation schemes for nonresponse, and integration with other information systems, including electronic health records. Although several hospitals have developed their own systems,4,12,13 most hospitals likely will purchase a vendor-based system. We found that a minority of hospitals had implemented a secure messaging application, and even fewer had most of their clinicians using it. Although little research has been conducted on these applications, studies suggest they are well received by users.4,5 Given that paging communication studies have found a large portion of pages are sent by nurses and other non-physician team members, secure mobile messaging applications should allow for direct message exchange with all professionals caring for a patient.10,11 Furthermore, hospitals will need to ensure adequate cell phone and WiFi signal strength throughout their facilities to ensure reliable and timely delivery of messages.
Our study had several limitations. We used a large database to conduct a national survey but had a low response rate and some drop-off of responses within surveys. Our sample reflected respondent diversity, and our analyses of demographic characteristics found no significant differences across survey response waves. Unfortunately, we did not have nonrespondents’ characteristics and therefore could not compare them with respondents’. It is possible that nonrespondents may have had different practices related to use of communication technology, especially in light of the fact that the survey was conducted by e-mail. However, given our finding that use of standard text messaging was comparable to that in other studies,1,2 and given the similarity of respondents’ characteristics across response waves, our findings likely were not affected by nonresponse bias.9 Last, we used a survey that had not been validated. However, this survey was created by experts in interprofessional collaboration and information technology, was informed by prior studies, and was iteratively refined during pretesting and pilot testing.
CONCLUSION
Pagers remain the technology most commonly used by hospital-based clinicians, but a majority also use standard text messaging for PCR communication, and relatively few hospitals have fully implemented secure mobile messaging applications. The wide range of technologies used suggests an evolution of methods to support communication among healthcare professionals. An optimized system will improve communication efficiency while ensuring the security of their patients’ information and the timely receipt of that information by the intended clinician.
Acknowledgment
The authors thank the Society of Hospital Medicine and the society staff who helped administer the survey, especially Mr. Ethan Gray.
Disclosure
Nothing to report.
1. Kuhlmann S, Ahlers-Schmidt CR, Steinberger E. TXT@WORK: pediatric hospitalists and text messaging. Telemed J E Health. 2014;20(7):647-652. PubMed
2. Prochaska MT, Bird AN, Chadaga A, Arora VM. Resident use of text messaging for patient care: ease of use or breach of privacy? JMIR Med Inform. 2015;3(4):e37. PubMed
3. Tran K, Morra D, Lo V, Quan SD, Abrams H, Wu RC. Medical students and personal smartphones in the clinical environment: the impact on confidentiality of personal health information and professionalism. J Med Internet Res. 2014;16(5):e132. PubMed
4. Patel N, Siegler JE, Stromberg N, Ravitz N, Hanson CW. Perfect storm of inpatient communication needs and an innovative solution utilizing smartphones and secured messaging. Appl Clin Inform. 2016;7(3):777-789. PubMed
5. Przybylo JA, Wang A, Loftus P, Evans KH, Chu I, Shieh L. Smarter hospital communication: secure smartphone text messaging improves provider satisfaction and perception of efficacy, workflow. J Hosp Med. 2014;9(9):573-578. PubMed
6. O’Leary KJ, Liebovitz DM, Baker DW. How hospitalists spend their time: insights on efficiency and safety. J Hosp Med. 2006;1(2):88-93. PubMed
7. Tipping MD, Forth VE, O’Leary KJ, et al. Where did the day go?—a time-motion study of hospitalists. J Hosp Med. 2010;5(6):323-328. PubMed
8. Real Magnet. http://www.realmagnet.com. Accessed December 20, 2016.
9. Armstrong JS, Overton T. Estimating nonresponse bias in mail surveys. J Mark Res. 1977;14(3):396-402.
10. Carlile N, Rhatigan JJ, Bates DW. Why do we still page each other? Examining the frequency, types and senders of pages in academic medical services. BMJ Qual Saf. 2017;26(1):24-29. PubMed
11. Kummerow Broman K, Kensinger C, Phillips C, et al. Characterizing the clamor: an in-depth analysis of inpatient paging communication. Acad Med. 2016;91(7):1015-1021. PubMed
12. Dalal AK, Schnipper J, Massaro A, et al. A web-based and mobile patient-centered “microblog” messaging platform to improve care team communication in acute care. J Am Med Inform Assoc. 2017;24(e1):e178-e184. PubMed
13. Wu R, Lo V, Morra D, et al. A smartphone-enabled communication system to improve hospital communication: usage and perceptions of medical trainees and nurses on general internal medicine wards. J Hosp Med. 2015;10(2):83-89. PubMed
Communication among healthcare professionals is essential for high-quality patient care. However, communication is difficult in hospitals because of heavy workloads, rapidly evolving plans of care, and geographic dispersion of team members. When hospital-based professionals are not in the same place at the same time, they rely on technology to communicate. Pagers have historically been used to support communication in hospitals, but are limited in their capabilities. Several recent small studies have shown that some physicians have started using standard text messaging on smartphones for patient care–related (PCR) messages.1-3 Although potentially enhancing clinician efficiency, use of standard text messaging for PCR messages raises concern about security risks related to transmission of protected health information. Addressing these concerns are emerging secure mobile messaging applications designed for PCR communication. Although recent studies suggest these applications are well received by users, the adoption rate is largely unknown.4,5
We conducted a study to see if there was a shift in use of hospital-based communication technologies under way. We surveyed a national sample of hospital-based clinicians to characterize current use of communication technologies, assess potential risks and perceptions related to use of standard text messaging for PCR messages, and characterize the adoption of secure mobile messaging applications designed for PCR communication.
METHODS
Study Design
The study was a cross-sectional survey of hospitalists—physicians and advanced practice providers whose primary professional focus is care of hospitalized patients. We studied hospitalists because of their role in coordinating care for complex medical patients and because prior studies identified communication as a major component of their work.6,7 The Northwestern University Institutional Review Board deemed this study exempt.
Survey Instrument
Four investigators (Drs. O’Leary, Liebovitz, Wu, and Reddy) with expertise in interprofessional communication and information technology created a draft survey based in part on results of prior studies assessing clinicians’ use of smartphones and standard text messaging for PCR communication.1,3 In the first section of the survey, we asked respondents which technologies were provided by their organization and which technologies they used for PCR communication. In the second section, we asked respondents about their use and perceptions of standard text messaging for PCR communication. In the third section, we asked about implementation and adoption of secure mobile messaging applications at their hospital. In the fourth and final section, we asked for demographic information.
We randomly selected 8 attendees of the 2015 Midwest Hospital Medicine Conference and invited them to participate in a focus group that would review a paper version of the draft survey and recommend revisions. Using the group’s feedback, we revised the ordinal response scale for questions related to standard text messaging and made other minor edits. We then created an Internet-based version of the survey and pilot-tested it with 8 hospitalists from 4 diverse hospitalist groups within the Northwestern Medicine Health System. We made additional minor edits based on pilot-test feedback.
Sampling Strategy
We used the largest hospitalist database maintained by the Society of Hospital Medicine (SHM). This database includes information on more than 28,000 individuals, representing SHM members and nonmembers who had participated in organizational events. In addition to clinically active hospitalists, the database includes non-hospitalists and clinically inactive hospitalists. We used this database to try to capture the largest possible number of potentially eligible hospitalists.
Survey Administration
We administered the survey in collaboration with SHM staff. E-mails that included a link to the survey on the Survey Monkey website were sent by SHM staff to individuals within the database. These e-mails were sent through Real Magnet, an e-mail marketing platform8 that allowed the SHM staff to determine the number of individuals who received and opened the e-mail and the number who clicked on the survey link. To try to promote participation, we offered respondents the chance to enter a lottery to win one of four $50 gift certificates. The initial e-mail was sent in April 2016, a reminder in May 2016, and a final reminder in July 2016.
Data Analysis
We calculated descriptive statistics of participants’ demographic characteristics. We estimated nonresponse bias by comparing demographic characteristics across waves of respondents using analysis of variance, t tests, and χ2 tests. This method is based on the finding that characteristics of late respondents often resemble those of nonrespondents.9 We collapsed response categories for communication technologies to simplify interpretation. For example, numeric pagers, alphanumeric pagers, and 2-way pagers were collapsed into a pagers category. We used t tests and χ2 tests to assess for associations between receipt of standard text messages for PCR communication and respondents’ age, sex, race, professional type, hospital size, practice location, and hospital teaching status. Similarly, we used t tests and χ2 tests to explore associations between implementation of secure mobile messaging application and respondents’ age, sex, race, professional type, hospital size, practice location, and hospital teaching status. All statistical analyses were performed with Stata Release 11.2 (StataCorp).
RESULTS
Participant Characteristics
Overall, the survey link was sent to 28,870 e-mail addresses. Addresses for which e-mails were undeliverable or for which the e-mail was never opened were excluded, yielding a total of 5,786 eligible respondents in the sample. After rejecting 42 clinically inactive individuals, 70 individuals who responded to only the initial item, and 27 duplicates, a total of 620 participant surveys were included in the final analysis. The adjusted response rate was 11.0%.
As shown in Table 1, mean (SD) respondent age was 42.9 (10.0) years, nearly half of the respondents were female, nearly a third were of nonwhite race, an overwhelming majority were physicians, and workplaces were in a variety of hospital settings. The sample size used to calculate demographic characteristics varied from 538 to 549 because of missing data for these items. We found no significant differences in demographic characteristics of respondents across the 3 survey waves, suggesting a lack of survey response bias (Supplemental Table).
Provision and Use of Communication Technologies for PCR Communication
Pagers were provided to the majority of respondents by their hospitals (79.8%, 495/620). Other devices were provided much less frequently, with 21.0% (130/620) reporting their organization provided a smartphone, 20.2% (125/620) a mobile phone, and 4.4% (27/620) a hands-free communication device. Organizations provided no device to 8.2% (51/620) of respondents and an “other” device to 5.5% (34/620).
An overwhelming majority used multiple technologies to receive PCR communication, with 17.7
Participants’ Experiences With Standard Text Messaging for PCR Communication
Participants’ experiences with standard text messaging for PCR communication are summarized in Table 3. Overall, 65.1% (369/ 567) of respondents reported receiving standard text messages for PCR communication at least once per week when on clinical duty, and 52.9% (300/567) received standard text messages at least once per day.
Overall, 21.5% (122/567) of respondents received standard text messages that included individually identifiable information at least once per day, and 41.3% (234/567) received messages that included some identifiable information (eg, patient initials, room number) at least once per day. About one-fifth of respondents (21.0%, 119/567) indicated receiving standard text messages for urgent clinical issues at least once per day. Receipt of standard text messages for a patient for whom the respondent was no longer providing care, delays in receipt of messages, messages missed because smartphones were set to vibrate, and receipt of messages when not on clinical duty occurred, but less frequently. We found no significant associations between receipt of PCR standard text messages once or more per day and respondents’ age, sex, race, professional type, hospital size, or hospital teaching status. A higher percentage of respondents in the South (63.2%, 96/152) and West (57.9%, 70/121) reported receipt of at least 1 PCR standard text message per day, compared with respondents in the Northeast (51.9%, 54/104), Midwest (45.2%, 61/135), and other (25.0%, 4/16) (P = 0.003).
Senders of PCR standard text messages. Of respondents who received standard text messages for PCR communication at least once per week, a majority reported receiving messages from physicians in the same specialty (88.6%, 327/369) and from physicians in other specialties (71.3%, 263/369). A minority of respondents reported receiving messages from nurses (35.0%, 129/369), social workers (30.6%, 113/369), and pharmacists (27.9%, 103/369).
Perceptions among users. Of respondents who received standard text messages for PCR communication at least once per week, an overwhelming majority agreed or strongly agreed that use of standard text messaging allowed them to provide better care (81.7%, 295/361) and made them more efficient (87.3%, 315/361). A majority also agreed or strongly agreed that standard text messaging posed a risk to the privacy and confidentiality of patient information (56.4%, 203/360), and nearly a third indicated that standard text messaging posed a risk to the timely receipt of messages by the correct individual (27.6%, 100/362). Overall, a large majority agreed or strongly agreed that the benefits of using standard text messaging for PCR communication outweighed the risks (85.0%, 306/360).
Adoption of Organization-Approved Secure Mobile Messaging Applications
Participants’ reported adoption of organization-approved secure mobile messaging applications is shown in the Figure. About one-fourth (26.6%, 146/549) of respondents reported that their organization had implemented a secure messaging application and that some clinicians were using it, whereas relatively few (7.3%, 40/549) reported that their organization had implemented an application that was being used by most clinicians. A substantial portion of respondents (21.3%, 117/549) were not sure whether their organization was planning to implement a secure mobile messaging application for PCR communication. We found no significant associations between partial or nearly full implementation of a secure mobile messaging application and respondents’ age, sex, race, professional type, hospital size, or practice location. A lower percentage of respondents in major teaching hospitals (28.0%, 67/239) reported partial or nearly full implementation of a secure mobile messaging application, compared with respondents from teaching hospitals (39.6%, 74/187) and nonteaching hospitals (39.2%, 40/102) (P = 0.02).
DISCUSSION
We found that pagers were the technology most commonly used by hospital-based clinicians, but also that a majority have used standard text messaging for PCR communication, and that relatively few hospitals had fully implemented secure mobile messaging applications. Our findings reveal a wide range of technology use and suggest an evolution to support communication among healthcare professionals.
The persistence of pagers as the technology most commonly provided by hospitals and used by clinicians for communication is noteworthy in that pagers are limited in their capabilities, typically not allowing a response to the message sender or the ability to forward a message, and often not allowing the ability to send messages to multiple recipients. The continued heavy use of pagers may be explained by their relatively low cost, especially compared with investment in new technologies, and reliable receipt of messages, even in areas with no cell phone service or WiFi signal. Furthermore, hospitals’ providing pagers allows for oversight, directory creation, and the potential for integration into other information systems. In 2 recent studies, inpatient paging communication was evaluated in depth. Carlile et al.10 found that the majority of pages requested a response, requiring an interruption in physician workflow to initiate a callback. Kummerow Broman et al.11 similarly found that a majority of pages requested a callback; they also found a high volume of nonurgent messages. With pager use, a high volume of messages, many of which require a response but are nonurgent, makes for a highly interruptive workflow.
That more than half of our hospital-based clinicians received standard text messages for PCR communication once or more per day is consistent with other, smaller studies. Kuhlmann et al.1 surveyed 97 pediatric hospitalists and found that a majority sent and received work-related text messages. Prochaska et al.2 surveyed 131 residents and found that standard text messaging was the communication method preferred by the majority of residents. Similar to these studies, our study found that receipt of standard text messages that included protected health information was fairly common. However, we identified additional risks related to standard text messaging. One-fifth of our respondents received standard text messages for urgent clinical issues once or more per day, and many respondents reported occasional receipt of messages regarding a patient for whom they were no longer providing care and receipt of messages when not on clinical duty. The usual inability to automate forwarding of standard text messages to another clinician creates the potential for clinically important messages to be delayed or missed. These risks have not been reported in the literature, and we think healthcare systems may not be fully aware of them. Our findings suggest that many clinicians have migrated from pagers to standard text messaging for the enhanced efficiency, and they perceive that the benefit of improved efficiency outweighs the risks to protected health information and the delay in receipt of clinically important messages by the correct individual.
Secure mobile messaging applications seem to address the limitations of both pagers and standard text messaging. Secure mobile messaging applications typically allow message response, message forwarding, multiple recipients, directory creation, the potential to create escalation schemes for nonresponse, and integration with other information systems, including electronic health records. Although several hospitals have developed their own systems,4,12,13 most hospitals likely will purchase a vendor-based system. We found that a minority of hospitals had implemented a secure messaging application, and even fewer had most of their clinicians using it. Although little research has been conducted on these applications, studies suggest they are well received by users.4,5 Given that paging communication studies have found a large portion of pages are sent by nurses and other non-physician team members, secure mobile messaging applications should allow for direct message exchange with all professionals caring for a patient.10,11 Furthermore, hospitals will need to ensure adequate cell phone and WiFi signal strength throughout their facilities to ensure reliable and timely delivery of messages.
Our study had several limitations. We used a large database to conduct a national survey but had a low response rate and some drop-off of responses within surveys. Our sample reflected respondent diversity, and our analyses of demographic characteristics found no significant differences across survey response waves. Unfortunately, we did not have nonrespondents’ characteristics and therefore could not compare them with respondents’. It is possible that nonrespondents may have had different practices related to use of communication technology, especially in light of the fact that the survey was conducted by e-mail. However, given our finding that use of standard text messaging was comparable to that in other studies,1,2 and given the similarity of respondents’ characteristics across response waves, our findings likely were not affected by nonresponse bias.9 Last, we used a survey that had not been validated. However, this survey was created by experts in interprofessional collaboration and information technology, was informed by prior studies, and was iteratively refined during pretesting and pilot testing.
CONCLUSION
Pagers remain the technology most commonly used by hospital-based clinicians, but a majority also use standard text messaging for PCR communication, and relatively few hospitals have fully implemented secure mobile messaging applications. The wide range of technologies used suggests an evolution of methods to support communication among healthcare professionals. An optimized system will improve communication efficiency while ensuring the security of their patients’ information and the timely receipt of that information by the intended clinician.
Acknowledgment
The authors thank the Society of Hospital Medicine and the society staff who helped administer the survey, especially Mr. Ethan Gray.
Disclosure
Nothing to report.
Communication among healthcare professionals is essential for high-quality patient care. However, communication is difficult in hospitals because of heavy workloads, rapidly evolving plans of care, and geographic dispersion of team members. When hospital-based professionals are not in the same place at the same time, they rely on technology to communicate. Pagers have historically been used to support communication in hospitals, but are limited in their capabilities. Several recent small studies have shown that some physicians have started using standard text messaging on smartphones for patient care–related (PCR) messages.1-3 Although potentially enhancing clinician efficiency, use of standard text messaging for PCR messages raises concern about security risks related to transmission of protected health information. Addressing these concerns are emerging secure mobile messaging applications designed for PCR communication. Although recent studies suggest these applications are well received by users, the adoption rate is largely unknown.4,5
We conducted a study to see if there was a shift in use of hospital-based communication technologies under way. We surveyed a national sample of hospital-based clinicians to characterize current use of communication technologies, assess potential risks and perceptions related to use of standard text messaging for PCR messages, and characterize the adoption of secure mobile messaging applications designed for PCR communication.
METHODS
Study Design
The study was a cross-sectional survey of hospitalists—physicians and advanced practice providers whose primary professional focus is care of hospitalized patients. We studied hospitalists because of their role in coordinating care for complex medical patients and because prior studies identified communication as a major component of their work.6,7 The Northwestern University Institutional Review Board deemed this study exempt.
Survey Instrument
Four investigators (Drs. O’Leary, Liebovitz, Wu, and Reddy) with expertise in interprofessional communication and information technology created a draft survey based in part on results of prior studies assessing clinicians’ use of smartphones and standard text messaging for PCR communication.1,3 In the first section of the survey, we asked respondents which technologies were provided by their organization and which technologies they used for PCR communication. In the second section, we asked respondents about their use and perceptions of standard text messaging for PCR communication. In the third section, we asked about implementation and adoption of secure mobile messaging applications at their hospital. In the fourth and final section, we asked for demographic information.
We randomly selected 8 attendees of the 2015 Midwest Hospital Medicine Conference and invited them to participate in a focus group that would review a paper version of the draft survey and recommend revisions. Using the group’s feedback, we revised the ordinal response scale for questions related to standard text messaging and made other minor edits. We then created an Internet-based version of the survey and pilot-tested it with 8 hospitalists from 4 diverse hospitalist groups within the Northwestern Medicine Health System. We made additional minor edits based on pilot-test feedback.
Sampling Strategy
We used the largest hospitalist database maintained by the Society of Hospital Medicine (SHM). This database includes information on more than 28,000 individuals, representing SHM members and nonmembers who had participated in organizational events. In addition to clinically active hospitalists, the database includes non-hospitalists and clinically inactive hospitalists. We used this database to try to capture the largest possible number of potentially eligible hospitalists.
Survey Administration
We administered the survey in collaboration with SHM staff. E-mails that included a link to the survey on the Survey Monkey website were sent by SHM staff to individuals within the database. These e-mails were sent through Real Magnet, an e-mail marketing platform8 that allowed the SHM staff to determine the number of individuals who received and opened the e-mail and the number who clicked on the survey link. To try to promote participation, we offered respondents the chance to enter a lottery to win one of four $50 gift certificates. The initial e-mail was sent in April 2016, a reminder in May 2016, and a final reminder in July 2016.
Data Analysis
We calculated descriptive statistics of participants’ demographic characteristics. We estimated nonresponse bias by comparing demographic characteristics across waves of respondents using analysis of variance, t tests, and χ2 tests. This method is based on the finding that characteristics of late respondents often resemble those of nonrespondents.9 We collapsed response categories for communication technologies to simplify interpretation. For example, numeric pagers, alphanumeric pagers, and 2-way pagers were collapsed into a pagers category. We used t tests and χ2 tests to assess for associations between receipt of standard text messages for PCR communication and respondents’ age, sex, race, professional type, hospital size, practice location, and hospital teaching status. Similarly, we used t tests and χ2 tests to explore associations between implementation of secure mobile messaging application and respondents’ age, sex, race, professional type, hospital size, practice location, and hospital teaching status. All statistical analyses were performed with Stata Release 11.2 (StataCorp).
RESULTS
Participant Characteristics
Overall, the survey link was sent to 28,870 e-mail addresses. Addresses for which e-mails were undeliverable or for which the e-mail was never opened were excluded, yielding a total of 5,786 eligible respondents in the sample. After rejecting 42 clinically inactive individuals, 70 individuals who responded to only the initial item, and 27 duplicates, a total of 620 participant surveys were included in the final analysis. The adjusted response rate was 11.0%.
As shown in Table 1, mean (SD) respondent age was 42.9 (10.0) years, nearly half of the respondents were female, nearly a third were of nonwhite race, an overwhelming majority were physicians, and workplaces were in a variety of hospital settings. The sample size used to calculate demographic characteristics varied from 538 to 549 because of missing data for these items. We found no significant differences in demographic characteristics of respondents across the 3 survey waves, suggesting a lack of survey response bias (Supplemental Table).
Provision and Use of Communication Technologies for PCR Communication
Pagers were provided to the majority of respondents by their hospitals (79.8%, 495/620). Other devices were provided much less frequently, with 21.0% (130/620) reporting their organization provided a smartphone, 20.2% (125/620) a mobile phone, and 4.4% (27/620) a hands-free communication device. Organizations provided no device to 8.2% (51/620) of respondents and an “other” device to 5.5% (34/620).
An overwhelming majority used multiple technologies to receive PCR communication, with 17.7
Participants’ Experiences With Standard Text Messaging for PCR Communication
Participants’ experiences with standard text messaging for PCR communication are summarized in Table 3. Overall, 65.1% (369/ 567) of respondents reported receiving standard text messages for PCR communication at least once per week when on clinical duty, and 52.9% (300/567) received standard text messages at least once per day.
Overall, 21.5% (122/567) of respondents received standard text messages that included individually identifiable information at least once per day, and 41.3% (234/567) received messages that included some identifiable information (eg, patient initials, room number) at least once per day. About one-fifth of respondents (21.0%, 119/567) indicated receiving standard text messages for urgent clinical issues at least once per day. Receipt of standard text messages for a patient for whom the respondent was no longer providing care, delays in receipt of messages, messages missed because smartphones were set to vibrate, and receipt of messages when not on clinical duty occurred, but less frequently. We found no significant associations between receipt of PCR standard text messages once or more per day and respondents’ age, sex, race, professional type, hospital size, or hospital teaching status. A higher percentage of respondents in the South (63.2%, 96/152) and West (57.9%, 70/121) reported receipt of at least 1 PCR standard text message per day, compared with respondents in the Northeast (51.9%, 54/104), Midwest (45.2%, 61/135), and other (25.0%, 4/16) (P = 0.003).
Senders of PCR standard text messages. Of respondents who received standard text messages for PCR communication at least once per week, a majority reported receiving messages from physicians in the same specialty (88.6%, 327/369) and from physicians in other specialties (71.3%, 263/369). A minority of respondents reported receiving messages from nurses (35.0%, 129/369), social workers (30.6%, 113/369), and pharmacists (27.9%, 103/369).
Perceptions among users. Of respondents who received standard text messages for PCR communication at least once per week, an overwhelming majority agreed or strongly agreed that use of standard text messaging allowed them to provide better care (81.7%, 295/361) and made them more efficient (87.3%, 315/361). A majority also agreed or strongly agreed that standard text messaging posed a risk to the privacy and confidentiality of patient information (56.4%, 203/360), and nearly a third indicated that standard text messaging posed a risk to the timely receipt of messages by the correct individual (27.6%, 100/362). Overall, a large majority agreed or strongly agreed that the benefits of using standard text messaging for PCR communication outweighed the risks (85.0%, 306/360).
Adoption of Organization-Approved Secure Mobile Messaging Applications
Participants’ reported adoption of organization-approved secure mobile messaging applications is shown in the Figure. About one-fourth (26.6%, 146/549) of respondents reported that their organization had implemented a secure messaging application and that some clinicians were using it, whereas relatively few (7.3%, 40/549) reported that their organization had implemented an application that was being used by most clinicians. A substantial portion of respondents (21.3%, 117/549) were not sure whether their organization was planning to implement a secure mobile messaging application for PCR communication. We found no significant associations between partial or nearly full implementation of a secure mobile messaging application and respondents’ age, sex, race, professional type, hospital size, or practice location. A lower percentage of respondents in major teaching hospitals (28.0%, 67/239) reported partial or nearly full implementation of a secure mobile messaging application, compared with respondents from teaching hospitals (39.6%, 74/187) and nonteaching hospitals (39.2%, 40/102) (P = 0.02).
DISCUSSION
We found that pagers were the technology most commonly used by hospital-based clinicians, but also that a majority have used standard text messaging for PCR communication, and that relatively few hospitals had fully implemented secure mobile messaging applications. Our findings reveal a wide range of technology use and suggest an evolution to support communication among healthcare professionals.
The persistence of pagers as the technology most commonly provided by hospitals and used by clinicians for communication is noteworthy in that pagers are limited in their capabilities, typically not allowing a response to the message sender or the ability to forward a message, and often not allowing the ability to send messages to multiple recipients. The continued heavy use of pagers may be explained by their relatively low cost, especially compared with investment in new technologies, and reliable receipt of messages, even in areas with no cell phone service or WiFi signal. Furthermore, hospitals’ providing pagers allows for oversight, directory creation, and the potential for integration into other information systems. In 2 recent studies, inpatient paging communication was evaluated in depth. Carlile et al.10 found that the majority of pages requested a response, requiring an interruption in physician workflow to initiate a callback. Kummerow Broman et al.11 similarly found that a majority of pages requested a callback; they also found a high volume of nonurgent messages. With pager use, a high volume of messages, many of which require a response but are nonurgent, makes for a highly interruptive workflow.
That more than half of our hospital-based clinicians received standard text messages for PCR communication once or more per day is consistent with other, smaller studies. Kuhlmann et al.1 surveyed 97 pediatric hospitalists and found that a majority sent and received work-related text messages. Prochaska et al.2 surveyed 131 residents and found that standard text messaging was the communication method preferred by the majority of residents. Similar to these studies, our study found that receipt of standard text messages that included protected health information was fairly common. However, we identified additional risks related to standard text messaging. One-fifth of our respondents received standard text messages for urgent clinical issues once or more per day, and many respondents reported occasional receipt of messages regarding a patient for whom they were no longer providing care and receipt of messages when not on clinical duty. The usual inability to automate forwarding of standard text messages to another clinician creates the potential for clinically important messages to be delayed or missed. These risks have not been reported in the literature, and we think healthcare systems may not be fully aware of them. Our findings suggest that many clinicians have migrated from pagers to standard text messaging for the enhanced efficiency, and they perceive that the benefit of improved efficiency outweighs the risks to protected health information and the delay in receipt of clinically important messages by the correct individual.
Secure mobile messaging applications seem to address the limitations of both pagers and standard text messaging. Secure mobile messaging applications typically allow message response, message forwarding, multiple recipients, directory creation, the potential to create escalation schemes for nonresponse, and integration with other information systems, including electronic health records. Although several hospitals have developed their own systems,4,12,13 most hospitals likely will purchase a vendor-based system. We found that a minority of hospitals had implemented a secure messaging application, and even fewer had most of their clinicians using it. Although little research has been conducted on these applications, studies suggest they are well received by users.4,5 Given that paging communication studies have found a large portion of pages are sent by nurses and other non-physician team members, secure mobile messaging applications should allow for direct message exchange with all professionals caring for a patient.10,11 Furthermore, hospitals will need to ensure adequate cell phone and WiFi signal strength throughout their facilities to ensure reliable and timely delivery of messages.
Our study had several limitations. We used a large database to conduct a national survey but had a low response rate and some drop-off of responses within surveys. Our sample reflected respondent diversity, and our analyses of demographic characteristics found no significant differences across survey response waves. Unfortunately, we did not have nonrespondents’ characteristics and therefore could not compare them with respondents’. It is possible that nonrespondents may have had different practices related to use of communication technology, especially in light of the fact that the survey was conducted by e-mail. However, given our finding that use of standard text messaging was comparable to that in other studies,1,2 and given the similarity of respondents’ characteristics across response waves, our findings likely were not affected by nonresponse bias.9 Last, we used a survey that had not been validated. However, this survey was created by experts in interprofessional collaboration and information technology, was informed by prior studies, and was iteratively refined during pretesting and pilot testing.
CONCLUSION
Pagers remain the technology most commonly used by hospital-based clinicians, but a majority also use standard text messaging for PCR communication, and relatively few hospitals have fully implemented secure mobile messaging applications. The wide range of technologies used suggests an evolution of methods to support communication among healthcare professionals. An optimized system will improve communication efficiency while ensuring the security of their patients’ information and the timely receipt of that information by the intended clinician.
Acknowledgment
The authors thank the Society of Hospital Medicine and the society staff who helped administer the survey, especially Mr. Ethan Gray.
Disclosure
Nothing to report.
1. Kuhlmann S, Ahlers-Schmidt CR, Steinberger E. TXT@WORK: pediatric hospitalists and text messaging. Telemed J E Health. 2014;20(7):647-652. PubMed
2. Prochaska MT, Bird AN, Chadaga A, Arora VM. Resident use of text messaging for patient care: ease of use or breach of privacy? JMIR Med Inform. 2015;3(4):e37. PubMed
3. Tran K, Morra D, Lo V, Quan SD, Abrams H, Wu RC. Medical students and personal smartphones in the clinical environment: the impact on confidentiality of personal health information and professionalism. J Med Internet Res. 2014;16(5):e132. PubMed
4. Patel N, Siegler JE, Stromberg N, Ravitz N, Hanson CW. Perfect storm of inpatient communication needs and an innovative solution utilizing smartphones and secured messaging. Appl Clin Inform. 2016;7(3):777-789. PubMed
5. Przybylo JA, Wang A, Loftus P, Evans KH, Chu I, Shieh L. Smarter hospital communication: secure smartphone text messaging improves provider satisfaction and perception of efficacy, workflow. J Hosp Med. 2014;9(9):573-578. PubMed
6. O’Leary KJ, Liebovitz DM, Baker DW. How hospitalists spend their time: insights on efficiency and safety. J Hosp Med. 2006;1(2):88-93. PubMed
7. Tipping MD, Forth VE, O’Leary KJ, et al. Where did the day go?—a time-motion study of hospitalists. J Hosp Med. 2010;5(6):323-328. PubMed
8. Real Magnet. http://www.realmagnet.com. Accessed December 20, 2016.
9. Armstrong JS, Overton T. Estimating nonresponse bias in mail surveys. J Mark Res. 1977;14(3):396-402.
10. Carlile N, Rhatigan JJ, Bates DW. Why do we still page each other? Examining the frequency, types and senders of pages in academic medical services. BMJ Qual Saf. 2017;26(1):24-29. PubMed
11. Kummerow Broman K, Kensinger C, Phillips C, et al. Characterizing the clamor: an in-depth analysis of inpatient paging communication. Acad Med. 2016;91(7):1015-1021. PubMed
12. Dalal AK, Schnipper J, Massaro A, et al. A web-based and mobile patient-centered “microblog” messaging platform to improve care team communication in acute care. J Am Med Inform Assoc. 2017;24(e1):e178-e184. PubMed
13. Wu R, Lo V, Morra D, et al. A smartphone-enabled communication system to improve hospital communication: usage and perceptions of medical trainees and nurses on general internal medicine wards. J Hosp Med. 2015;10(2):83-89. PubMed
1. Kuhlmann S, Ahlers-Schmidt CR, Steinberger E. TXT@WORK: pediatric hospitalists and text messaging. Telemed J E Health. 2014;20(7):647-652. PubMed
2. Prochaska MT, Bird AN, Chadaga A, Arora VM. Resident use of text messaging for patient care: ease of use or breach of privacy? JMIR Med Inform. 2015;3(4):e37. PubMed
3. Tran K, Morra D, Lo V, Quan SD, Abrams H, Wu RC. Medical students and personal smartphones in the clinical environment: the impact on confidentiality of personal health information and professionalism. J Med Internet Res. 2014;16(5):e132. PubMed
4. Patel N, Siegler JE, Stromberg N, Ravitz N, Hanson CW. Perfect storm of inpatient communication needs and an innovative solution utilizing smartphones and secured messaging. Appl Clin Inform. 2016;7(3):777-789. PubMed
5. Przybylo JA, Wang A, Loftus P, Evans KH, Chu I, Shieh L. Smarter hospital communication: secure smartphone text messaging improves provider satisfaction and perception of efficacy, workflow. J Hosp Med. 2014;9(9):573-578. PubMed
6. O’Leary KJ, Liebovitz DM, Baker DW. How hospitalists spend their time: insights on efficiency and safety. J Hosp Med. 2006;1(2):88-93. PubMed
7. Tipping MD, Forth VE, O’Leary KJ, et al. Where did the day go?—a time-motion study of hospitalists. J Hosp Med. 2010;5(6):323-328. PubMed
8. Real Magnet. http://www.realmagnet.com. Accessed December 20, 2016.
9. Armstrong JS, Overton T. Estimating nonresponse bias in mail surveys. J Mark Res. 1977;14(3):396-402.
10. Carlile N, Rhatigan JJ, Bates DW. Why do we still page each other? Examining the frequency, types and senders of pages in academic medical services. BMJ Qual Saf. 2017;26(1):24-29. PubMed
11. Kummerow Broman K, Kensinger C, Phillips C, et al. Characterizing the clamor: an in-depth analysis of inpatient paging communication. Acad Med. 2016;91(7):1015-1021. PubMed
12. Dalal AK, Schnipper J, Massaro A, et al. A web-based and mobile patient-centered “microblog” messaging platform to improve care team communication in acute care. J Am Med Inform Assoc. 2017;24(e1):e178-e184. PubMed
13. Wu R, Lo V, Morra D, et al. A smartphone-enabled communication system to improve hospital communication: usage and perceptions of medical trainees and nurses on general internal medicine wards. J Hosp Med. 2015;10(2):83-89. PubMed
© 2017 Society of Hospital Medicine
Childhood vaccine trauma decreases adolescent HPV immunization uptake
The more vaccines children get at any one office visit between the ages of 4 and 6 years, the more fearful they are of needles later on and the less likely they are to start human papillomavirus vaccine (HPV) as adolescents, according to an investigation of 120 children.
Shot-stacking between ages 4 and 6 years is not uncommon, especially if children might not be back in the office any time soon. Although it’s convenient and often better reimbursed to give all the recommended 4- to 6-year-old shots – MMR, DTaP, varicella, IPV, and maybe flu and hepatitis B vaccines – in one visit, the investigators found a hidden cost.
“We don’t need to change the vaccine schedule,” but we do have to be careful because 4- to 6-year-olds are especially vulnerable to phobias. “I think the best thing would be to give one vaccine at age 4, two at age 5, and two at age 6,” years, said lead investigator and pediatric emergency physician Amy Baxter, MD, a clinical associate professor at the Medical College of Georgia, Augusta.
“I’ve dealt with needle phobia in the ED. These kids come in and they are already freaked out about needles. If they get diagnosed with diabetes or leukemia, parents don’t think they are going to be able to handle it,” she said in an interview.
Expanded school vaccination programs might help by making it more convenient for parents to space out shots. The development of patch, microneedle, or effective sublingual or intranasal options also would help.
Dr. Baxter and her colleagues asked 120 children aged 10 to 12 years old to rate their fear of needles on a visual analogue scale (VAS), from 0 points meaning no fear to 100. The investigators matched the scores against the children’s immunization records (Vaccine. 2017 Jun 20. doi: 10.1016/j.vaccine.2017.06.029).
There was no significant relationship between needle fear and the total number of injections. However, fear did correlate with the number of shots children received in 1 day from ages 4 to 6 years old. Six children (50%) who had four same-day injections during that period scored in the upper quartile of anxiety as adolescents (VAS greater than 83), versus 22 (27%) who had three, and 2 (10%) who had two. None of the children who had just one shot per office visit as preschoolers were very worried about needles (P = .0387).
The investigators found that the likelihood of being in the upper quartile of fear as adolescents increased with each additional same-day injection between the ages of 4 and 6 years (odds ratio, 3.108; 95% confidence interval, 1.311-7.367; P = .01).
The team checked back with the children a few years later to see who had started HPV immunizations by age 14 years. Just 27% of children in the upper quartile of needle fear had done so, versus 48% in the least anxious quartile (VAS less than 32). The study wasn’t powered to detect a statistically significant difference in HPV vaccine uptake, but decreased uptake with higher needle fear came close (OR, 2.57; 95% CI, 0.864-7.621; P = .0889).
“It’s the stacking that causes fear. There was no difference in the uptake of vaccines when they were spread out, but more shots” at one time “causes more distress, so there was a difference in fear 5 years later,” Dr. Baxter said.
The investigators also assessed parental concerns about immunizations. “What was interesting was that whether the parents were in the upper or lower quartile of anxiety didn’t impact HPV initiation at all. The children’s anxiety 2 years earlier seemed much more relevant,” Dr. Baxter said.
In a literature review of 15 studies from 1958-2016 that included over 8,000 subjects, the investigators also found that needle fear tracked neatly with the sixfold increase in scheduled vaccines since the 1970s, with more than 60% of people now endorsing some degree of injection anxiety – more than ever before. “The curve of increasing needle fear correlated strongly with increasing vaccine number,” they found (Kendall’s tau b = 0.747; 95% CI, 0.513-0.982; P = .0003).
As two-thirds of the literature review sample were adults, “these results imply that fear acquired in childhood persists,” they concluded.
The majority of children in the study were white. Their mothers were a mean of 44 years, with a mean education level of 18 years. Demographic differences didn’t affect needle fear.
Dr. Baxter is a pediatric pain researcher and the inventor of Buzzy, a bumblebee shaped vibrator to distract children and relieve pain during shots. When asked if readers could trust her study findings given her interest in selling the device, she noted that, in a previous study, “Buzzy for 15 seconds did not work well for 4 to 6 year olds” getting multiple injections at one office visit. “Buzzy is not the solution for giving four shots at once.”
The more vaccines children get at any one office visit between the ages of 4 and 6 years, the more fearful they are of needles later on and the less likely they are to start human papillomavirus vaccine (HPV) as adolescents, according to an investigation of 120 children.
Shot-stacking between ages 4 and 6 years is not uncommon, especially if children might not be back in the office any time soon. Although it’s convenient and often better reimbursed to give all the recommended 4- to 6-year-old shots – MMR, DTaP, varicella, IPV, and maybe flu and hepatitis B vaccines – in one visit, the investigators found a hidden cost.
“We don’t need to change the vaccine schedule,” but we do have to be careful because 4- to 6-year-olds are especially vulnerable to phobias. “I think the best thing would be to give one vaccine at age 4, two at age 5, and two at age 6,” years, said lead investigator and pediatric emergency physician Amy Baxter, MD, a clinical associate professor at the Medical College of Georgia, Augusta.
“I’ve dealt with needle phobia in the ED. These kids come in and they are already freaked out about needles. If they get diagnosed with diabetes or leukemia, parents don’t think they are going to be able to handle it,” she said in an interview.
Expanded school vaccination programs might help by making it more convenient for parents to space out shots. The development of patch, microneedle, or effective sublingual or intranasal options also would help.
Dr. Baxter and her colleagues asked 120 children aged 10 to 12 years old to rate their fear of needles on a visual analogue scale (VAS), from 0 points meaning no fear to 100. The investigators matched the scores against the children’s immunization records (Vaccine. 2017 Jun 20. doi: 10.1016/j.vaccine.2017.06.029).
There was no significant relationship between needle fear and the total number of injections. However, fear did correlate with the number of shots children received in 1 day from ages 4 to 6 years old. Six children (50%) who had four same-day injections during that period scored in the upper quartile of anxiety as adolescents (VAS greater than 83), versus 22 (27%) who had three, and 2 (10%) who had two. None of the children who had just one shot per office visit as preschoolers were very worried about needles (P = .0387).
The investigators found that the likelihood of being in the upper quartile of fear as adolescents increased with each additional same-day injection between the ages of 4 and 6 years (odds ratio, 3.108; 95% confidence interval, 1.311-7.367; P = .01).
The team checked back with the children a few years later to see who had started HPV immunizations by age 14 years. Just 27% of children in the upper quartile of needle fear had done so, versus 48% in the least anxious quartile (VAS less than 32). The study wasn’t powered to detect a statistically significant difference in HPV vaccine uptake, but decreased uptake with higher needle fear came close (OR, 2.57; 95% CI, 0.864-7.621; P = .0889).
“It’s the stacking that causes fear. There was no difference in the uptake of vaccines when they were spread out, but more shots” at one time “causes more distress, so there was a difference in fear 5 years later,” Dr. Baxter said.
The investigators also assessed parental concerns about immunizations. “What was interesting was that whether the parents were in the upper or lower quartile of anxiety didn’t impact HPV initiation at all. The children’s anxiety 2 years earlier seemed much more relevant,” Dr. Baxter said.
In a literature review of 15 studies from 1958-2016 that included over 8,000 subjects, the investigators also found that needle fear tracked neatly with the sixfold increase in scheduled vaccines since the 1970s, with more than 60% of people now endorsing some degree of injection anxiety – more than ever before. “The curve of increasing needle fear correlated strongly with increasing vaccine number,” they found (Kendall’s tau b = 0.747; 95% CI, 0.513-0.982; P = .0003).
As two-thirds of the literature review sample were adults, “these results imply that fear acquired in childhood persists,” they concluded.
The majority of children in the study were white. Their mothers were a mean of 44 years, with a mean education level of 18 years. Demographic differences didn’t affect needle fear.
Dr. Baxter is a pediatric pain researcher and the inventor of Buzzy, a bumblebee shaped vibrator to distract children and relieve pain during shots. When asked if readers could trust her study findings given her interest in selling the device, she noted that, in a previous study, “Buzzy for 15 seconds did not work well for 4 to 6 year olds” getting multiple injections at one office visit. “Buzzy is not the solution for giving four shots at once.”
The more vaccines children get at any one office visit between the ages of 4 and 6 years, the more fearful they are of needles later on and the less likely they are to start human papillomavirus vaccine (HPV) as adolescents, according to an investigation of 120 children.
Shot-stacking between ages 4 and 6 years is not uncommon, especially if children might not be back in the office any time soon. Although it’s convenient and often better reimbursed to give all the recommended 4- to 6-year-old shots – MMR, DTaP, varicella, IPV, and maybe flu and hepatitis B vaccines – in one visit, the investigators found a hidden cost.
“We don’t need to change the vaccine schedule,” but we do have to be careful because 4- to 6-year-olds are especially vulnerable to phobias. “I think the best thing would be to give one vaccine at age 4, two at age 5, and two at age 6,” years, said lead investigator and pediatric emergency physician Amy Baxter, MD, a clinical associate professor at the Medical College of Georgia, Augusta.
“I’ve dealt with needle phobia in the ED. These kids come in and they are already freaked out about needles. If they get diagnosed with diabetes or leukemia, parents don’t think they are going to be able to handle it,” she said in an interview.
Expanded school vaccination programs might help by making it more convenient for parents to space out shots. The development of patch, microneedle, or effective sublingual or intranasal options also would help.
Dr. Baxter and her colleagues asked 120 children aged 10 to 12 years old to rate their fear of needles on a visual analogue scale (VAS), from 0 points meaning no fear to 100. The investigators matched the scores against the children’s immunization records (Vaccine. 2017 Jun 20. doi: 10.1016/j.vaccine.2017.06.029).
There was no significant relationship between needle fear and the total number of injections. However, fear did correlate with the number of shots children received in 1 day from ages 4 to 6 years old. Six children (50%) who had four same-day injections during that period scored in the upper quartile of anxiety as adolescents (VAS greater than 83), versus 22 (27%) who had three, and 2 (10%) who had two. None of the children who had just one shot per office visit as preschoolers were very worried about needles (P = .0387).
The investigators found that the likelihood of being in the upper quartile of fear as adolescents increased with each additional same-day injection between the ages of 4 and 6 years (odds ratio, 3.108; 95% confidence interval, 1.311-7.367; P = .01).
The team checked back with the children a few years later to see who had started HPV immunizations by age 14 years. Just 27% of children in the upper quartile of needle fear had done so, versus 48% in the least anxious quartile (VAS less than 32). The study wasn’t powered to detect a statistically significant difference in HPV vaccine uptake, but decreased uptake with higher needle fear came close (OR, 2.57; 95% CI, 0.864-7.621; P = .0889).
“It’s the stacking that causes fear. There was no difference in the uptake of vaccines when they were spread out, but more shots” at one time “causes more distress, so there was a difference in fear 5 years later,” Dr. Baxter said.
The investigators also assessed parental concerns about immunizations. “What was interesting was that whether the parents were in the upper or lower quartile of anxiety didn’t impact HPV initiation at all. The children’s anxiety 2 years earlier seemed much more relevant,” Dr. Baxter said.
In a literature review of 15 studies from 1958-2016 that included over 8,000 subjects, the investigators also found that needle fear tracked neatly with the sixfold increase in scheduled vaccines since the 1970s, with more than 60% of people now endorsing some degree of injection anxiety – more than ever before. “The curve of increasing needle fear correlated strongly with increasing vaccine number,” they found (Kendall’s tau b = 0.747; 95% CI, 0.513-0.982; P = .0003).
As two-thirds of the literature review sample were adults, “these results imply that fear acquired in childhood persists,” they concluded.
The majority of children in the study were white. Their mothers were a mean of 44 years, with a mean education level of 18 years. Demographic differences didn’t affect needle fear.
Dr. Baxter is a pediatric pain researcher and the inventor of Buzzy, a bumblebee shaped vibrator to distract children and relieve pain during shots. When asked if readers could trust her study findings given her interest in selling the device, she noted that, in a previous study, “Buzzy for 15 seconds did not work well for 4 to 6 year olds” getting multiple injections at one office visit. “Buzzy is not the solution for giving four shots at once.”
FROM VACCINE
Key clinical point:
Major finding: Six children (50%) who had four same-day injections during that period scored in the upper quartile of anxiety as adolescents (VAS greater than 83), versus 22 (27%) who had three, and 2 (10%) who had two same-day injections. Just 27% of children in the upper quartile of needle fear had started HPV immunization at age 14 years, versus 48% in the least anxious quartile.
Data source: Survey and follow-up of 120 children and their parents.
Disclosures: The lead investigator is a pediatric pain researcher and the inventor of Buzzy, a bumblebee shaped vibrator to distract children and relieve pain during shots. When asked if readers could trust her findings given her interest in selling the device, she noted that, in a previous study, “Buzzy for 15 seconds did not work well for 4-6 year olds” getting multiple injections in one office visit. “Buzzy is not the solution for giving four shots at once.”
Nurses help more rheumatic disease patients get vaccinated
MADRID – A nurse-led program successfully increased the uptake of pneumococcal vaccination among patients with chronic inflammatory rheumatic diseases in a single-center study.
From the start to the end of a 4-month evaluation period, the rate of vaccination of at-risk patients increased from 17.1% (13/76) to 77.6% (59/76; P less than .001).
It is well known that patients with inflammatory rheumatic diseases, such as systemic lupus erythematous (SLE) and systemic vasculitis, are at high risk for contracting pneumococcal disease, reported Tiphaine Goulenok, MD, of Bichat Hospital, Paris, at the European Congress of Rheumatology. This is particularly the case if they are receiving immunosuppressive treatments.
Although French national guidelines were introduced in 2011 that recommend that such patients routinely receive pneumococcal vaccination, the vaccination rate is often lower than is desirable, Dr. Goulenok observed at a press briefing. Her prior research suggests that only 16.2% of patients with an indication for the PCV13 vaccine actually received it.
In the current study, 126 patients with inflammatory rheumatic diseases were consecutively recruited and seen at the day unit of Bichat Hospital. Of these patients, 76 were candidates for pneumococcal vaccination because they were receiving steroids or other immunosuppressive drugs. Of these patients, 13 were already vaccinated, and, of the 63 who were not, nurses correctly identified 56 (88.9%) who needed to be vaccinated, of whom 46 agreed and 10 refused.
“We found a low rate of pneumococcal vaccination among patients,” said Dr. Goulenok, “but, thanks to high screening by the nurses, vaccination coverage was increased and the nurse-led vaccination program was very efficient”.
Robert Landewé, MD, who chaired the press briefing, observed that, despite being in an “era of guidelines,” ways of successfully implementing them in practice remained a challenge. The nurse-led program appeared to be one way to increase pneumococcal vaccination uptake, but perhaps other ways need to be sought, especially as there may be substantial resistance to vaccination among patients, he said.
“Patients are sometimes more afraid of the consequences of vaccination than [of] the disease that is prevented by vaccination,” said Dr. Landewé, who is professor of rheumatology at the Academic Medical Center in Amsterdam (the Netherlands).
Dr. Goulenok and Dr. Landewé reported no disclosures.
MADRID – A nurse-led program successfully increased the uptake of pneumococcal vaccination among patients with chronic inflammatory rheumatic diseases in a single-center study.
From the start to the end of a 4-month evaluation period, the rate of vaccination of at-risk patients increased from 17.1% (13/76) to 77.6% (59/76; P less than .001).
It is well known that patients with inflammatory rheumatic diseases, such as systemic lupus erythematous (SLE) and systemic vasculitis, are at high risk for contracting pneumococcal disease, reported Tiphaine Goulenok, MD, of Bichat Hospital, Paris, at the European Congress of Rheumatology. This is particularly the case if they are receiving immunosuppressive treatments.
Although French national guidelines were introduced in 2011 that recommend that such patients routinely receive pneumococcal vaccination, the vaccination rate is often lower than is desirable, Dr. Goulenok observed at a press briefing. Her prior research suggests that only 16.2% of patients with an indication for the PCV13 vaccine actually received it.
In the current study, 126 patients with inflammatory rheumatic diseases were consecutively recruited and seen at the day unit of Bichat Hospital. Of these patients, 76 were candidates for pneumococcal vaccination because they were receiving steroids or other immunosuppressive drugs. Of these patients, 13 were already vaccinated, and, of the 63 who were not, nurses correctly identified 56 (88.9%) who needed to be vaccinated, of whom 46 agreed and 10 refused.
“We found a low rate of pneumococcal vaccination among patients,” said Dr. Goulenok, “but, thanks to high screening by the nurses, vaccination coverage was increased and the nurse-led vaccination program was very efficient”.
Robert Landewé, MD, who chaired the press briefing, observed that, despite being in an “era of guidelines,” ways of successfully implementing them in practice remained a challenge. The nurse-led program appeared to be one way to increase pneumococcal vaccination uptake, but perhaps other ways need to be sought, especially as there may be substantial resistance to vaccination among patients, he said.
“Patients are sometimes more afraid of the consequences of vaccination than [of] the disease that is prevented by vaccination,” said Dr. Landewé, who is professor of rheumatology at the Academic Medical Center in Amsterdam (the Netherlands).
Dr. Goulenok and Dr. Landewé reported no disclosures.
MADRID – A nurse-led program successfully increased the uptake of pneumococcal vaccination among patients with chronic inflammatory rheumatic diseases in a single-center study.
From the start to the end of a 4-month evaluation period, the rate of vaccination of at-risk patients increased from 17.1% (13/76) to 77.6% (59/76; P less than .001).
It is well known that patients with inflammatory rheumatic diseases, such as systemic lupus erythematous (SLE) and systemic vasculitis, are at high risk for contracting pneumococcal disease, reported Tiphaine Goulenok, MD, of Bichat Hospital, Paris, at the European Congress of Rheumatology. This is particularly the case if they are receiving immunosuppressive treatments.
Although French national guidelines were introduced in 2011 that recommend that such patients routinely receive pneumococcal vaccination, the vaccination rate is often lower than is desirable, Dr. Goulenok observed at a press briefing. Her prior research suggests that only 16.2% of patients with an indication for the PCV13 vaccine actually received it.
In the current study, 126 patients with inflammatory rheumatic diseases were consecutively recruited and seen at the day unit of Bichat Hospital. Of these patients, 76 were candidates for pneumococcal vaccination because they were receiving steroids or other immunosuppressive drugs. Of these patients, 13 were already vaccinated, and, of the 63 who were not, nurses correctly identified 56 (88.9%) who needed to be vaccinated, of whom 46 agreed and 10 refused.
“We found a low rate of pneumococcal vaccination among patients,” said Dr. Goulenok, “but, thanks to high screening by the nurses, vaccination coverage was increased and the nurse-led vaccination program was very efficient”.
Robert Landewé, MD, who chaired the press briefing, observed that, despite being in an “era of guidelines,” ways of successfully implementing them in practice remained a challenge. The nurse-led program appeared to be one way to increase pneumococcal vaccination uptake, but perhaps other ways need to be sought, especially as there may be substantial resistance to vaccination among patients, he said.
“Patients are sometimes more afraid of the consequences of vaccination than [of] the disease that is prevented by vaccination,” said Dr. Landewé, who is professor of rheumatology at the Academic Medical Center in Amsterdam (the Netherlands).
Dr. Goulenok and Dr. Landewé reported no disclosures.
AT THE EULAR 2017 CONGRESS
Key clinical point: A nurse-led program increased the uptake of a guideline-recommended vaccination in patients with chronic inflammatory rheumatic diseases.
Major finding: The pre- and postintervention pneumococcal vaccination rates were 17.1% (13/76) and 77.6% (59/76) of at-risk patients (P less than .001).
Data source: A 4-month, prospective pilot study of 126 consecutively recruited patients with chronic inflammatory rheumatic diseases.
Disclosures: The presenter and commentator had no disclosures to report.
English infant quadravalent group B meningococcal vaccine pays off
MADRID – Cases of invasive meningococcal disease in English 1 year olds dropped fourfold in the first year after the multicomponent group B meningococcal (MenB) vaccine (4CMenB, Bexsero) was added to the national infant immunization list, Shamez Ladhani, MD, Ph.D, reported at the annual meeting of the European Society for Paediatric Infectious Diseases.
“In 1 year olds, we have seen a significant decrease in the number of cases, compared with the number of cases predicted. I suspect the vaccine effectiveness is close to 94% against meningococcal group B,” said Dr. Ladhani of Public Health England, London.
Meningococcal meningitis and septicemia remain the leading cause of death for children under 5 in the UK. Public awareness of this threat is high, so 4CMenB has been well accepted. Of eligible infants, 96% have received the first dose and 89% the second. The first cohort became available for the 12-month booster dose in May 2016.
Between May and December of 2016, the Public Health England intensive surveillance program confirmed six cases of invasive meningococcal disease. During the same time frame in 2015, there were 24 cases, whereas, during the 4 years prior to introduction of 4CMenB, the average was 18 cases per year.
Three of the six 1-year-olds had meningitis, and three had septicemia. One was admitted to an ICU. None died. None had comorbid conditions placing them at increased risk. Five of the six children with invasive meningococcal disease had received two doses of the vaccine, and one child became ill 3 months after receiving the third dose.
The vaccine is licensed to be given as a four-dose series: three primary doses plus one booster dose. UK health officials deemed that excessive and not cost effective. Based on data from the vaccine trials, they determined that three doses are sufficient. This cut the cost of the national program by 25% while maintaining protection (Lancet. 2016 Dec 3;388(10061):2775-82).
Pediatricians from other countries were extremely curious about this innovative immunization program. What about vaccine side effects in infants? they asked.
Dr. Ladhani replied that studies in Australia, Northern Ireland, and Scotland have all shown a small uptick in mild fever and irritability in the first 3 days after the first dose of 4CMenB. It’s less of an issue with the second dose.
“One dose is not protective. You need two. Parents understand that, while these side effects are annoying, the risks associated with meningitis are far greater. They are very, very accepting of the vaccine,” according to Dr. Ladhani.
What about using 4CMenB in teenagers? physicians asked.
Dr. Ladhani predicted that the infant immunization program will have zero effect in adolescents. Teens are the main carriers of meningococcal bacteria. So, in theory, vaccinating them could not only protect the adolescents themselves but could benefit the whole population through herd immunity.
“The problem is we don’t have solid evidence that Bexsero protects against carriage. A massive carriage study is underway in Australia. It should provide data in 2 or 3 years. If we do see a carriage effect, then vaccinating teenagers becomes a very attractive option because you protect them and others around them,” he explained.
Dr. Ladhani reported having no financial conflicts regarding his study, funded by Public Health England.
MADRID – Cases of invasive meningococcal disease in English 1 year olds dropped fourfold in the first year after the multicomponent group B meningococcal (MenB) vaccine (4CMenB, Bexsero) was added to the national infant immunization list, Shamez Ladhani, MD, Ph.D, reported at the annual meeting of the European Society for Paediatric Infectious Diseases.
“In 1 year olds, we have seen a significant decrease in the number of cases, compared with the number of cases predicted. I suspect the vaccine effectiveness is close to 94% against meningococcal group B,” said Dr. Ladhani of Public Health England, London.
Meningococcal meningitis and septicemia remain the leading cause of death for children under 5 in the UK. Public awareness of this threat is high, so 4CMenB has been well accepted. Of eligible infants, 96% have received the first dose and 89% the second. The first cohort became available for the 12-month booster dose in May 2016.
Between May and December of 2016, the Public Health England intensive surveillance program confirmed six cases of invasive meningococcal disease. During the same time frame in 2015, there were 24 cases, whereas, during the 4 years prior to introduction of 4CMenB, the average was 18 cases per year.
Three of the six 1-year-olds had meningitis, and three had septicemia. One was admitted to an ICU. None died. None had comorbid conditions placing them at increased risk. Five of the six children with invasive meningococcal disease had received two doses of the vaccine, and one child became ill 3 months after receiving the third dose.
The vaccine is licensed to be given as a four-dose series: three primary doses plus one booster dose. UK health officials deemed that excessive and not cost effective. Based on data from the vaccine trials, they determined that three doses are sufficient. This cut the cost of the national program by 25% while maintaining protection (Lancet. 2016 Dec 3;388(10061):2775-82).
Pediatricians from other countries were extremely curious about this innovative immunization program. What about vaccine side effects in infants? they asked.
Dr. Ladhani replied that studies in Australia, Northern Ireland, and Scotland have all shown a small uptick in mild fever and irritability in the first 3 days after the first dose of 4CMenB. It’s less of an issue with the second dose.
“One dose is not protective. You need two. Parents understand that, while these side effects are annoying, the risks associated with meningitis are far greater. They are very, very accepting of the vaccine,” according to Dr. Ladhani.
What about using 4CMenB in teenagers? physicians asked.
Dr. Ladhani predicted that the infant immunization program will have zero effect in adolescents. Teens are the main carriers of meningococcal bacteria. So, in theory, vaccinating them could not only protect the adolescents themselves but could benefit the whole population through herd immunity.
“The problem is we don’t have solid evidence that Bexsero protects against carriage. A massive carriage study is underway in Australia. It should provide data in 2 or 3 years. If we do see a carriage effect, then vaccinating teenagers becomes a very attractive option because you protect them and others around them,” he explained.
Dr. Ladhani reported having no financial conflicts regarding his study, funded by Public Health England.
MADRID – Cases of invasive meningococcal disease in English 1 year olds dropped fourfold in the first year after the multicomponent group B meningococcal (MenB) vaccine (4CMenB, Bexsero) was added to the national infant immunization list, Shamez Ladhani, MD, Ph.D, reported at the annual meeting of the European Society for Paediatric Infectious Diseases.
“In 1 year olds, we have seen a significant decrease in the number of cases, compared with the number of cases predicted. I suspect the vaccine effectiveness is close to 94% against meningococcal group B,” said Dr. Ladhani of Public Health England, London.
Meningococcal meningitis and septicemia remain the leading cause of death for children under 5 in the UK. Public awareness of this threat is high, so 4CMenB has been well accepted. Of eligible infants, 96% have received the first dose and 89% the second. The first cohort became available for the 12-month booster dose in May 2016.
Between May and December of 2016, the Public Health England intensive surveillance program confirmed six cases of invasive meningococcal disease. During the same time frame in 2015, there were 24 cases, whereas, during the 4 years prior to introduction of 4CMenB, the average was 18 cases per year.
Three of the six 1-year-olds had meningitis, and three had septicemia. One was admitted to an ICU. None died. None had comorbid conditions placing them at increased risk. Five of the six children with invasive meningococcal disease had received two doses of the vaccine, and one child became ill 3 months after receiving the third dose.
The vaccine is licensed to be given as a four-dose series: three primary doses plus one booster dose. UK health officials deemed that excessive and not cost effective. Based on data from the vaccine trials, they determined that three doses are sufficient. This cut the cost of the national program by 25% while maintaining protection (Lancet. 2016 Dec 3;388(10061):2775-82).
Pediatricians from other countries were extremely curious about this innovative immunization program. What about vaccine side effects in infants? they asked.
Dr. Ladhani replied that studies in Australia, Northern Ireland, and Scotland have all shown a small uptick in mild fever and irritability in the first 3 days after the first dose of 4CMenB. It’s less of an issue with the second dose.
“One dose is not protective. You need two. Parents understand that, while these side effects are annoying, the risks associated with meningitis are far greater. They are very, very accepting of the vaccine,” according to Dr. Ladhani.
What about using 4CMenB in teenagers? physicians asked.
Dr. Ladhani predicted that the infant immunization program will have zero effect in adolescents. Teens are the main carriers of meningococcal bacteria. So, in theory, vaccinating them could not only protect the adolescents themselves but could benefit the whole population through herd immunity.
“The problem is we don’t have solid evidence that Bexsero protects against carriage. A massive carriage study is underway in Australia. It should provide data in 2 or 3 years. If we do see a carriage effect, then vaccinating teenagers becomes a very attractive option because you protect them and others around them,” he explained.
Dr. Ladhani reported having no financial conflicts regarding his study, funded by Public Health England.
AT ESPID 2017
Key clinical point:
Major finding: The number of confirmed cases of invasive meningococcal disease in English 1-year-olds dropped to six the year after introduction of the 4CMenB vaccine in the infant immunization schedule, down from 24 cases the previous year.
Data source: An enhanced surveillance study utilizing a combination of laboratory, public health, and clinical reporting to track and confirm all cases of invasive meningococcal disease in English 1-year-olds.
Disclosures: The study was funded by Public Health England. The presenter, who is employed by the agency, reported having no financial conflicts.
Transient tachypnea of newborn increases bronchiolitis risk
MADRID – A new Finnish study raises a provocative question: Is transient tachypnea of the newborn really transient?
Transient tachypnea of the newborn (TTN) has traditionally been viewed as a benign, self-limited condition involving 1-3 days of respiratory distress. But data from Finland’s comprehensive national health registries indicate that TTN in term babies is associated with significantly increased risk of subsequent bronchiolitis during infancy, Otto Helve, MD, reported at the annual meeting of the European Society for Paediatric Infectious Diseases.
“This association suggests similar pathogenic mechanisms in transient tachypnea of the newborn and bronchiolitis. We suggest that an intrinsic defect in sodium ion–driven pulmonary fluid transport may predispose to clinically significant bronchiolitis during the first year of life,” said Dr. Helve, a pediatrician at the National Institute for Health and Welfare, Helsinki, and the University of Helsinki.
Of more than 1 million term babies born in Finland during 1996-2015, 17,569 were diagnosed with TTN. During the same period, 40,338 infants were hospitalized with a diagnosis of bronchiolitis attributable to respiratory syncytial virus infection.
In a multivariate analysis adjusted for birth year, gender, delivery method, gestational age, and parity, TTN was independently associated with a 1.2-fold increased risk of bronchiolitis during the first year of life.
Dr. Helve reported having no financial conflicts of interest regarding his study.
MADRID – A new Finnish study raises a provocative question: Is transient tachypnea of the newborn really transient?
Transient tachypnea of the newborn (TTN) has traditionally been viewed as a benign, self-limited condition involving 1-3 days of respiratory distress. But data from Finland’s comprehensive national health registries indicate that TTN in term babies is associated with significantly increased risk of subsequent bronchiolitis during infancy, Otto Helve, MD, reported at the annual meeting of the European Society for Paediatric Infectious Diseases.
“This association suggests similar pathogenic mechanisms in transient tachypnea of the newborn and bronchiolitis. We suggest that an intrinsic defect in sodium ion–driven pulmonary fluid transport may predispose to clinically significant bronchiolitis during the first year of life,” said Dr. Helve, a pediatrician at the National Institute for Health and Welfare, Helsinki, and the University of Helsinki.
Of more than 1 million term babies born in Finland during 1996-2015, 17,569 were diagnosed with TTN. During the same period, 40,338 infants were hospitalized with a diagnosis of bronchiolitis attributable to respiratory syncytial virus infection.
In a multivariate analysis adjusted for birth year, gender, delivery method, gestational age, and parity, TTN was independently associated with a 1.2-fold increased risk of bronchiolitis during the first year of life.
Dr. Helve reported having no financial conflicts of interest regarding his study.
MADRID – A new Finnish study raises a provocative question: Is transient tachypnea of the newborn really transient?
Transient tachypnea of the newborn (TTN) has traditionally been viewed as a benign, self-limited condition involving 1-3 days of respiratory distress. But data from Finland’s comprehensive national health registries indicate that TTN in term babies is associated with significantly increased risk of subsequent bronchiolitis during infancy, Otto Helve, MD, reported at the annual meeting of the European Society for Paediatric Infectious Diseases.
“This association suggests similar pathogenic mechanisms in transient tachypnea of the newborn and bronchiolitis. We suggest that an intrinsic defect in sodium ion–driven pulmonary fluid transport may predispose to clinically significant bronchiolitis during the first year of life,” said Dr. Helve, a pediatrician at the National Institute for Health and Welfare, Helsinki, and the University of Helsinki.
Of more than 1 million term babies born in Finland during 1996-2015, 17,569 were diagnosed with TTN. During the same period, 40,338 infants were hospitalized with a diagnosis of bronchiolitis attributable to respiratory syncytial virus infection.
In a multivariate analysis adjusted for birth year, gender, delivery method, gestational age, and parity, TTN was independently associated with a 1.2-fold increased risk of bronchiolitis during the first year of life.
Dr. Helve reported having no financial conflicts of interest regarding his study.
AT ESPID 2017
Key clinical point:
Major finding: Transient tachypnea of the newborn was independently associated with a 1.2-fold increased risk of bronchiolitis during the first year of life of Finnish term babies.
Data source: This population-based case-control study included 17,569 Finnish term babies diagnosed with transient tachypnea of the newborn and 40,338 diagnosed with bronchiolitis.
Disclosures: Dr. Helve reported having no financial conflicts of interest.