User login
How your body language affects patient care
Patient surveys reveal communication to be one of the most important competencies a physician should possess.1 However, communication is not only what is spoken. A physician’s nonverbal communication or “body language” sets the trajectory for treatment from the moment the patient first sees the physician. Body language includes all forms of communication other than words,2 such as vocal tone, posture, and facial and body movements. Being mindful of such behaviors can provide physicians with greater access to their patients. Effective nonverbal communication can have significant effects on patient engagement, compliance, and outcome.
First impressions
The physician’s nonverbal behavior is crucial to the patient’s impression of his (her) physician.3 Appropriate eye gaze, proper distance or forward lean, direct body orientation, uncrossed legs and arms, and arm symmetry also have been associated with patient reports of satisfaction.3,4 A physician who displays these affiliative nonverbal behaviors is more likely to engage with the patient and be rated higher for patient satisfaction.5,6 Once a patient has developed rapport and an alliance with the physician and is satisfied with care, you likely will see improvements in patient adherence.
Adherence to treatment
The physician’s ability to verbally and nonverbally communicate a safe, encouraging, and efficient relationship is crucial for patient adherence to treatment. Patients report greater alliance with their physicians when they perceive genuine engagement and concern.7 The physician showing interest impacts the patient’s rating of the relationship6 and provides confidence that the physician is sensitive and understanding.8 As a result, the patient is more trusting and communicative, which allows for greater progress in the patient’s care because it often leads to attending appointments as well as medication adherence.9
Medication nonadherence is a complex issue that is influenced by several factors,10 but it is widely accepted that lack of communication and patient education are important factors.11 Nonverbal communication can help the clinician to distinguish patients who are unwilling to take medication from those who are willing but unable to do so.11
Overall adherence with care also can be affected by nonverbal behaviors. Positive perception of the physician’s tone of voice has been associated with greater attendance at appointments,12 greater referral rates to alcohol abuse treatment clinics,13 and lower rates of malpractice among surgeons.14 Such trends demonstrate the influence that effective nonverbal communication could have on health care costs by reducing doctor shopping and malpractice rates and increasing effective care.
Outcomes
Physician’s positive nonverbal communication has been linked to positive patient outcomes. Physical therapists who smile, nod, and maintain eye contact compared with those who do not smile or look away from the patient, have been shown to achieve greater short- and long-term improvements in functioning of their patients.15 Perceptions of physicians as distant or detached are associated with poorer patient outcomes.5,6,16 Pain patients with high nonverbal support from their physicians show increased pain tolerance and reduction in the amount of pain expressed, compared with those interacting with low nonverbal support physicians.17 Patients respond more to care if they feel their physician is engaged and sensitive to their needs.
There is much to gain if a physician is mindful of his body language. As Henry A. Nasrallah, MD, Editor-in-Chief of
1. McBride CA, Shugars DA, DiMatteo MR, et al. The physician’s role. Views of the public and the profession on seven aspects of patient care. Arch Fam Med. 1994;3(11):948-953.
2. Knapp ML, Hall JA, Horgan TG. Nonverbal communication in human interaction. 8th ed. Boston, MA: Wadsworth, Cengage Learning; 2014.
3. Beck RS, Daughtridge R, Sloane PD. Physician-patient communication in the primary care office: a systematic review. J Am Board Fam Pract. 2002;15(1):25-38.
4. Bensing J. Doctor-patient communication and the quality of care. Soc Sci Med. 1991;32(11):1301-1310.
5. Mast MS. On the importance of nonverbal communication in the physician-patient interaction. Patient Educ Couns. 2007;67(3):315-318.
6. Larsen KM, Smith CK. Assessment of nonverbal communication in the patient-physician interview. J Fam Pract. 1981;12(3):481-488.
7. Pinto RZ, Ferreira ML, Oliveira VC, et al. Patient-centred communication is associated with positive therapeutic alliance: a systematic review. J Physiother. 2012;58(2):77-87.
8. DiMatteo MR, Taranta A, Friedman HS, et al. Predicting patient satisfaction from physicians’ nonverbal communication skills. Med Care. 1980;18(4):376-387.
9. McCabe R, Bullenkamp J, Hansson L, et al. The therapeutic relationship and adherence to antipsychotic medication in schizophrenia. PLoS One. 2012;7(4):e36080.
10. Kardas P, Lewek P, Matyjaszczyk M. Determinants of patient adherence: a review of systematic reviews. Front Pharmacol. 2013;4:91.
11. Velligan DI, Weiden PJ, Sajatovic M, et al; Expert Consensus Panel on Adherence Problems in Serious and Persistent Mental Illness. The expert consensus guideline series: adherence problems in patients with serious and persistent mental illness. J Clin Psychiatry. 2009;70(suppl 4):1-46; quiz 47-48.
12. Cruz M, Roter DL, Weiland M, et al. Appointment length, psychiatrists’ communication behaviors, and medication management appointment adherence. Psychiatr Serv. 2013;64(9):886-892.
13. Milmoe S, Rosenthal R, Blane HT, et al. The doctor’s voice: postdictor of successful referral of alcoholic patients. J Abnorm Psychol. 1967;72(1):78-84.
14. Ambady N, Laplante D, Nguyen T, et al. Surgeons’ tone of voice: a clue to malpractice history. Surgery. 2002;132(1):5-9.
15. Ambady N, Koo J, Rosenthal R, et al. Physical therapists’ nonverbal communication predicts geriatric patients’ health outcomes. Psychol Aging. 2002;17(3):443-452.
16. Stewart MA. Effective physician-patient communication and health outcomes: a review. CMAJ. 1995;152(9):1423-1433.
17. Ruben MA, Blanch-Hartigan D, Hall JA. Nonverbal communication as a pain reliever: the impact of physician supportive nonverbal behavior on experimentally induced pain. Health Commun. 2016;1-7. doi: 10.1080/10410236.2016.1196418.
18. Nasrallah HA. The most powerful placebo is not a pill. Current Psychiatry. 2011;10(8):18-19.
Patient surveys reveal communication to be one of the most important competencies a physician should possess.1 However, communication is not only what is spoken. A physician’s nonverbal communication or “body language” sets the trajectory for treatment from the moment the patient first sees the physician. Body language includes all forms of communication other than words,2 such as vocal tone, posture, and facial and body movements. Being mindful of such behaviors can provide physicians with greater access to their patients. Effective nonverbal communication can have significant effects on patient engagement, compliance, and outcome.
First impressions
The physician’s nonverbal behavior is crucial to the patient’s impression of his (her) physician.3 Appropriate eye gaze, proper distance or forward lean, direct body orientation, uncrossed legs and arms, and arm symmetry also have been associated with patient reports of satisfaction.3,4 A physician who displays these affiliative nonverbal behaviors is more likely to engage with the patient and be rated higher for patient satisfaction.5,6 Once a patient has developed rapport and an alliance with the physician and is satisfied with care, you likely will see improvements in patient adherence.
Adherence to treatment
The physician’s ability to verbally and nonverbally communicate a safe, encouraging, and efficient relationship is crucial for patient adherence to treatment. Patients report greater alliance with their physicians when they perceive genuine engagement and concern.7 The physician showing interest impacts the patient’s rating of the relationship6 and provides confidence that the physician is sensitive and understanding.8 As a result, the patient is more trusting and communicative, which allows for greater progress in the patient’s care because it often leads to attending appointments as well as medication adherence.9
Medication nonadherence is a complex issue that is influenced by several factors,10 but it is widely accepted that lack of communication and patient education are important factors.11 Nonverbal communication can help the clinician to distinguish patients who are unwilling to take medication from those who are willing but unable to do so.11
Overall adherence with care also can be affected by nonverbal behaviors. Positive perception of the physician’s tone of voice has been associated with greater attendance at appointments,12 greater referral rates to alcohol abuse treatment clinics,13 and lower rates of malpractice among surgeons.14 Such trends demonstrate the influence that effective nonverbal communication could have on health care costs by reducing doctor shopping and malpractice rates and increasing effective care.
Outcomes
Physician’s positive nonverbal communication has been linked to positive patient outcomes. Physical therapists who smile, nod, and maintain eye contact compared with those who do not smile or look away from the patient, have been shown to achieve greater short- and long-term improvements in functioning of their patients.15 Perceptions of physicians as distant or detached are associated with poorer patient outcomes.5,6,16 Pain patients with high nonverbal support from their physicians show increased pain tolerance and reduction in the amount of pain expressed, compared with those interacting with low nonverbal support physicians.17 Patients respond more to care if they feel their physician is engaged and sensitive to their needs.
There is much to gain if a physician is mindful of his body language. As Henry A. Nasrallah, MD, Editor-in-Chief of
Patient surveys reveal communication to be one of the most important competencies a physician should possess.1 However, communication is not only what is spoken. A physician’s nonverbal communication or “body language” sets the trajectory for treatment from the moment the patient first sees the physician. Body language includes all forms of communication other than words,2 such as vocal tone, posture, and facial and body movements. Being mindful of such behaviors can provide physicians with greater access to their patients. Effective nonverbal communication can have significant effects on patient engagement, compliance, and outcome.
First impressions
The physician’s nonverbal behavior is crucial to the patient’s impression of his (her) physician.3 Appropriate eye gaze, proper distance or forward lean, direct body orientation, uncrossed legs and arms, and arm symmetry also have been associated with patient reports of satisfaction.3,4 A physician who displays these affiliative nonverbal behaviors is more likely to engage with the patient and be rated higher for patient satisfaction.5,6 Once a patient has developed rapport and an alliance with the physician and is satisfied with care, you likely will see improvements in patient adherence.
Adherence to treatment
The physician’s ability to verbally and nonverbally communicate a safe, encouraging, and efficient relationship is crucial for patient adherence to treatment. Patients report greater alliance with their physicians when they perceive genuine engagement and concern.7 The physician showing interest impacts the patient’s rating of the relationship6 and provides confidence that the physician is sensitive and understanding.8 As a result, the patient is more trusting and communicative, which allows for greater progress in the patient’s care because it often leads to attending appointments as well as medication adherence.9
Medication nonadherence is a complex issue that is influenced by several factors,10 but it is widely accepted that lack of communication and patient education are important factors.11 Nonverbal communication can help the clinician to distinguish patients who are unwilling to take medication from those who are willing but unable to do so.11
Overall adherence with care also can be affected by nonverbal behaviors. Positive perception of the physician’s tone of voice has been associated with greater attendance at appointments,12 greater referral rates to alcohol abuse treatment clinics,13 and lower rates of malpractice among surgeons.14 Such trends demonstrate the influence that effective nonverbal communication could have on health care costs by reducing doctor shopping and malpractice rates and increasing effective care.
Outcomes
Physician’s positive nonverbal communication has been linked to positive patient outcomes. Physical therapists who smile, nod, and maintain eye contact compared with those who do not smile or look away from the patient, have been shown to achieve greater short- and long-term improvements in functioning of their patients.15 Perceptions of physicians as distant or detached are associated with poorer patient outcomes.5,6,16 Pain patients with high nonverbal support from their physicians show increased pain tolerance and reduction in the amount of pain expressed, compared with those interacting with low nonverbal support physicians.17 Patients respond more to care if they feel their physician is engaged and sensitive to their needs.
There is much to gain if a physician is mindful of his body language. As Henry A. Nasrallah, MD, Editor-in-Chief of
1. McBride CA, Shugars DA, DiMatteo MR, et al. The physician’s role. Views of the public and the profession on seven aspects of patient care. Arch Fam Med. 1994;3(11):948-953.
2. Knapp ML, Hall JA, Horgan TG. Nonverbal communication in human interaction. 8th ed. Boston, MA: Wadsworth, Cengage Learning; 2014.
3. Beck RS, Daughtridge R, Sloane PD. Physician-patient communication in the primary care office: a systematic review. J Am Board Fam Pract. 2002;15(1):25-38.
4. Bensing J. Doctor-patient communication and the quality of care. Soc Sci Med. 1991;32(11):1301-1310.
5. Mast MS. On the importance of nonverbal communication in the physician-patient interaction. Patient Educ Couns. 2007;67(3):315-318.
6. Larsen KM, Smith CK. Assessment of nonverbal communication in the patient-physician interview. J Fam Pract. 1981;12(3):481-488.
7. Pinto RZ, Ferreira ML, Oliveira VC, et al. Patient-centred communication is associated with positive therapeutic alliance: a systematic review. J Physiother. 2012;58(2):77-87.
8. DiMatteo MR, Taranta A, Friedman HS, et al. Predicting patient satisfaction from physicians’ nonverbal communication skills. Med Care. 1980;18(4):376-387.
9. McCabe R, Bullenkamp J, Hansson L, et al. The therapeutic relationship and adherence to antipsychotic medication in schizophrenia. PLoS One. 2012;7(4):e36080.
10. Kardas P, Lewek P, Matyjaszczyk M. Determinants of patient adherence: a review of systematic reviews. Front Pharmacol. 2013;4:91.
11. Velligan DI, Weiden PJ, Sajatovic M, et al; Expert Consensus Panel on Adherence Problems in Serious and Persistent Mental Illness. The expert consensus guideline series: adherence problems in patients with serious and persistent mental illness. J Clin Psychiatry. 2009;70(suppl 4):1-46; quiz 47-48.
12. Cruz M, Roter DL, Weiland M, et al. Appointment length, psychiatrists’ communication behaviors, and medication management appointment adherence. Psychiatr Serv. 2013;64(9):886-892.
13. Milmoe S, Rosenthal R, Blane HT, et al. The doctor’s voice: postdictor of successful referral of alcoholic patients. J Abnorm Psychol. 1967;72(1):78-84.
14. Ambady N, Laplante D, Nguyen T, et al. Surgeons’ tone of voice: a clue to malpractice history. Surgery. 2002;132(1):5-9.
15. Ambady N, Koo J, Rosenthal R, et al. Physical therapists’ nonverbal communication predicts geriatric patients’ health outcomes. Psychol Aging. 2002;17(3):443-452.
16. Stewart MA. Effective physician-patient communication and health outcomes: a review. CMAJ. 1995;152(9):1423-1433.
17. Ruben MA, Blanch-Hartigan D, Hall JA. Nonverbal communication as a pain reliever: the impact of physician supportive nonverbal behavior on experimentally induced pain. Health Commun. 2016;1-7. doi: 10.1080/10410236.2016.1196418.
18. Nasrallah HA. The most powerful placebo is not a pill. Current Psychiatry. 2011;10(8):18-19.
1. McBride CA, Shugars DA, DiMatteo MR, et al. The physician’s role. Views of the public and the profession on seven aspects of patient care. Arch Fam Med. 1994;3(11):948-953.
2. Knapp ML, Hall JA, Horgan TG. Nonverbal communication in human interaction. 8th ed. Boston, MA: Wadsworth, Cengage Learning; 2014.
3. Beck RS, Daughtridge R, Sloane PD. Physician-patient communication in the primary care office: a systematic review. J Am Board Fam Pract. 2002;15(1):25-38.
4. Bensing J. Doctor-patient communication and the quality of care. Soc Sci Med. 1991;32(11):1301-1310.
5. Mast MS. On the importance of nonverbal communication in the physician-patient interaction. Patient Educ Couns. 2007;67(3):315-318.
6. Larsen KM, Smith CK. Assessment of nonverbal communication in the patient-physician interview. J Fam Pract. 1981;12(3):481-488.
7. Pinto RZ, Ferreira ML, Oliveira VC, et al. Patient-centred communication is associated with positive therapeutic alliance: a systematic review. J Physiother. 2012;58(2):77-87.
8. DiMatteo MR, Taranta A, Friedman HS, et al. Predicting patient satisfaction from physicians’ nonverbal communication skills. Med Care. 1980;18(4):376-387.
9. McCabe R, Bullenkamp J, Hansson L, et al. The therapeutic relationship and adherence to antipsychotic medication in schizophrenia. PLoS One. 2012;7(4):e36080.
10. Kardas P, Lewek P, Matyjaszczyk M. Determinants of patient adherence: a review of systematic reviews. Front Pharmacol. 2013;4:91.
11. Velligan DI, Weiden PJ, Sajatovic M, et al; Expert Consensus Panel on Adherence Problems in Serious and Persistent Mental Illness. The expert consensus guideline series: adherence problems in patients with serious and persistent mental illness. J Clin Psychiatry. 2009;70(suppl 4):1-46; quiz 47-48.
12. Cruz M, Roter DL, Weiland M, et al. Appointment length, psychiatrists’ communication behaviors, and medication management appointment adherence. Psychiatr Serv. 2013;64(9):886-892.
13. Milmoe S, Rosenthal R, Blane HT, et al. The doctor’s voice: postdictor of successful referral of alcoholic patients. J Abnorm Psychol. 1967;72(1):78-84.
14. Ambady N, Laplante D, Nguyen T, et al. Surgeons’ tone of voice: a clue to malpractice history. Surgery. 2002;132(1):5-9.
15. Ambady N, Koo J, Rosenthal R, et al. Physical therapists’ nonverbal communication predicts geriatric patients’ health outcomes. Psychol Aging. 2002;17(3):443-452.
16. Stewart MA. Effective physician-patient communication and health outcomes: a review. CMAJ. 1995;152(9):1423-1433.
17. Ruben MA, Blanch-Hartigan D, Hall JA. Nonverbal communication as a pain reliever: the impact of physician supportive nonverbal behavior on experimentally induced pain. Health Commun. 2016;1-7. doi: 10.1080/10410236.2016.1196418.
18. Nasrallah HA. The most powerful placebo is not a pill. Current Psychiatry. 2011;10(8):18-19.
Prescribing is the culmination of extensive medical training and psychologists don’t qualify
Practicing medicine without a license is a crime, but it seems to have become a hollow law. Politicians are now cynically legalizing it by granting prescribing privileges to individuals with no prior foundation of medical training. Perhaps it is because of serious ignorance of the difference between psychiatry and psychology or MD and PhD degrees. Or perhaps it is a quid pro quo to generous donors to their re-election campaigns who seek a convenient shortcut to the 28,000 hours it takes to become a psychiatrist in 8 years of medical school and psychiatric residency—and that comes after 4 years of college.
I recently consulted an attorney to discuss some legal documents. When he asked me what my line of work is, I then asked him if he knew the difference between a psychiatrist and a psychologist. He hesitated before admitting in an embarrassed tone that he did not really know and thought that they were all “shrinks” and very similar. I then informed him that both go through undergraduate college education, albeit taking very different courses, with pre-med scientific emphasis for future psychiatric physicians and predominately psychology emphasis for future psychologists.
However, psychiatrists then attend medical school for 4 years and rotate on multiple hospital-based medical specialties, such as internal medicine, surgery, pediatrics, obstetrics and gynecology, family medicine, neurology, pathology, psychiatry, ophthalmology, dermatology, anesthesia, radiology, otolaryngology, etc.
Psychologists, on the other hand, take additional advanced psychology courses in graduate school and write a dissertation that requires quite a bit of library time. After getting a MD, future psychiatrists spend 4 years in extensive training in residency programs across inpatient wards and outpatient clinics, assessing the physical and mental health of seriously sick patients with emphasis on both pharmacological and psychotherapeutic treatments for serious psychiatric disorders in patients, the majority of whom have comorbid medical conditions as well. Psychologists, on the other hand, spend 1 year of internship after getting their PhD or PsyD degree, essentially focused on developing counseling and psychotherapy skills. By the time they complete their training, psychologists and psychiatrists have disparate skills: heavily medical and pharmacological skills in psychiatrists and strong psychotherapeutic skills in psychologists.
After this long explanation, I asked the attorney what he thought about psychologists seeking prescription privileges. He was astounded that psychologists would attempt to expand this scope of practice through state legislations rather than going through medical training like all physicians. “That would be like practicing medicine without a license, which is a felony,” he said. He wryly added that his fellow malpractice and litigation lawyers will be the big winners while poorly treated patients will be the biggest losers. Being an avid runner, he also added that such a short-cut to prescribe without the requisite years of medial training reminded him of Rosie Ruiz, who snuck into the Boston marathon a couple of miles before the finish line and “won” the race, before she was caught and discredited.1
Psychology is a respected mental health discipline with strong psychotherapy training and orientation. For decades, psychologists have vigorously criticized the medical model of mental disorders that psychiatric physicians employ to diagnose and treat brain disorders that disrupt thinking, emotions, mood, cognition, and behavior. However, about 25 years ago, a small group of militant psychologists brazenly decided to lobby state legislatures to give them the right to prescribe psychotropics, although they have no formal medical training. Psychiatric physicians, represented by the American Psychiatric Association (APA), strongly opposed this initiative and regarded it as reckless disregard of the obvious need for extensive medical training to be able to prescribe drugs that affect every organ in the body, not only the brain. Psychiatric medications are associated with serious risks of morbidity and mortality.2 The ability to safely prescribe any medication represents the tip of a huge iceberg of 8 years of rigorous medical school education and specialty training. Yet, one of the early proponents of prescription privileges for psychologists, Patrick De Leon, sarcastically likened the ability to prescribe drugs to learning how to operate a desktop computer!
Not all psychologists agreed with the political campaign to lobby state legislatures to pass a law authorizing prescriptive privileges for psychologists.3-6 In fact, most academic psychologists oppose it.7 Most of the early supporters had a PsyD degree from professional schools of psychology, not a PhD degree in psychology, which is obtained from a university department of psychology. The National Alliance on Mental Illness is opposed to psychologists prescribing medications.8 Psychiatrists are outraged by this hazardous “solution” to the shortage of psychiatrists and point to the many potential dangers to patients. Some suggested that this is a quick way to enhance psychologists’ income and to generate more revenue for their professional journals and meetings with lucrative pharmaceutical ads and exhibit booths.
The campaign is ongoing, as Idaho became the fifth state to adopt such an ill-conceived “solution” to increasing access to mental health care, despite valiant effort by the APA to lobby against such laws. Although New Mexico (2002), Louisiana (2004), Illinois (2014), and Iowa (2016) have passed prescriptive authority for psychologists before Idaho, the APA has defeated such measures in numerous other states. But the painful truth is that this has been a lengthy political chess game in which psychologists have been gradually gaining ground and “capturing more pieces.”
Here is a brief, common sense rationale as to the need for full medical training necessary before safely and accurately prescribing medications, most of which are synthetic molecules, which are essentially foreign substances, with both benefits and risks detailed in the FDA-approved label of each drug that reaches the medical marketplace.
First: Making an accurate clinical diagnosis. If a patient presents with depression, the clinician must rule out other possible causes before diagnosing it as primary major depressive disorder for which an antidepressant can be prescribed. The panoply of secondary depressions, which are not treated with antidepressants, includes a variety of recreational drug-induced mood changes and dysphoria and depression induced by numerous prescription drugs (such as antihypertensives, hormonal contraceptives, steroids, interferon, proton pump inhibitors, H2 blockers, malaria drugs, etc.).
After drug-induced depression is ruled out, the clinician must rule out the possibility that an underlying medical condition might be causing the depression, which includes disorders such as hypothyroidism and other endocrinopathies, anemia, stroke, heart disease, hyperkalemia, lupus and other autoimmune disorders, cancer, Parkinsonism, etc. Therefore, a targeted exploration of past and current medical history, accompanied by a battery of lab tests (complete blood count, electrolytes, liver and kidney function tests, metabolic profile, thyroid-stimulating hormone, etc.) must be done to systematically arrive at the correct diagnosis. Only then can the proper treatment plan be determined, which may or may not include prescribing an antidepressant.
Conclusion: Medical training and psychiatric residency are required for an accurate diagnosis of a mental disorder. Even physicians with no psychiatric training might not have the full repertoire of knowledge needed to systematically rule out secondary depression.
Second: Drug selection. Psychiatric drugs can have various iatrogenic effects. Thus, the selection of an appropriate prescription medication from the available array of drugs approved for a given psychiatric indication must be safe and consistent with the patient’s medical history and must not potentially exacerbate ≥1 comorbid medical conditions.
Conclusion: Medical training and psychiatric residency are required.
Third: Knowledge of metabolic pathways of each psychiatric medication to be prescribed as well as the metabolic pathway of all other medications (psychiatric and non-psychiatric) the patient receives is essential to avoid adverse drug–drug interactions. This includes the hepatic enzymes (cytochromes), which often are responsible for metabolizing all the psychiatric and non-psychiatric drugs a patient is receiving. Knowledge of inhibitors and inducers of various cytochrome enzymes is vital for selecting a medication that does not cause a pharmacokinetic adverse reaction that can produce serious adverse effects (even death, such as with QTc prolongation) or can cause loss of efficacy of ≥1 medications that the patient is receiving, in addition to the antidepressant. Also, in addition to evaluating hepatic pathways, knowledge of renal excretion of the drug to be selected and the status of the patient’s kidney function or impairment must be evaluated.
Conclusion: Medical training is required.
Conclusion: Medical training is required.
Fifth: General medical treatment. Many patients might require combination drug therapy because of inadequate response to monotherapy. Clinicians must know what is rational and evidence-based polypharmacy and what is irrational, dangerous, or absurd polypharmacy.9 When possible, parsimonious pharmacotherapy should be employed to minimize the number of medications prescribed.10 A patient could experience severe drug–drug reactions that could lead to cardiopulmonary crises. The clinician must be able to examine, intervene, and manage the patient’s medical distress until help arrives.
Conclusion: Medical training is required.
Sixth: Pregnancy. Knowledge about the pharmacotherapeutic aspects of pregnant women with mental illness is critical. Full knowledge about what can or should not be prescribed during pregnancy (ie, avoiding teratogenic agents) is vital for physicians treating women with psychiatric illness who become pregnant.
Conclusion: Medical training is required.
Although I am against prescriptive privileges for psychologists, I want to emphasize how much I appreciate and respect what psychologists do for patients with mental illness. Their psychotherapy skills often are honed beyond those of psychiatrists who, by necessity, focus on medical diagnosis and pharmacotherapeutic management. Combination of pharmacotherapy and psychotherapy has been demonstrated to be superior to medications alone. In the 25 years since psychologists have been eagerly pursuing prescriptive privileges, neuroscience research has revealed the neurobiologic effects of psychotherapy. Many studies have shown that evidence-based psychotherapy can induce the same structural and functional brain changes as medications11,12 and can influence biomarkers that accompany psychiatric disorders just as medications do.13
Psychologists should reconsider the many potential hazards of prescription drugs compared with the relative safety and efficacy of psychotherapy. They should focus on their qualifications and main strength, which is psychotherapy, and collaborate with psychiatrists and nurse practitioners on a biopsychosocial approach to mental illness. They also should realize how physically ill most psychiatric patients are and the complex medical management they need for their myriad comorbidities.
Just as I began this editorial with an anecdote, I will end with an illustrative one as well. As an academic professor for the past 3 decades who has trained and supervised numerous psychiatric residents, I once closely supervised a former PhD psychologist who decided to become a psychiatrist by going to medical school, followed by a 4-year psychiatric residency. I asked her to compare her experience and functioning as a psychologist with her current work as a fourth-year psychiatric resident. Her response was enlightening: She said the 2 professions are vastly different in their knowledge base and in terms of how they conceptualize mental illness from a psychological vs medical model. As for prescribing medications, she added that even after 8 years of extensive medical training as a physician and a psychiatrist, she feels there is still much to learn about psychopharmacology to ensure not only efficacy but also safety, because a majority of psychiatric patients have ≥1 coexisting medical conditions and substance use as well. Based on her own experience as a psychologist who became a psychiatric physician, she was completely opposed to prescriptive privileges for psychologists unless they go to medical school and become eligible to prescribe safely.
This former resident is now a successful academic psychiatrist who continues to hone her psychopharmacology skills. State legislators should listen to professionals like her before they pass a law giving prescriptive authority to psychologists without having to go through the rigors of 28,000 hours of training in the 8 years of medical school and psychiatric residency. Legislators should also understand that like psychologists, social work counselors have hardly any medical training, yet they have never sought prescriptive privileges. That’s clearly rational and wise.
1. Rosie Ruiz tries to steal the Boston marathon. Runner’s World. http://www.runnersworld.com/running-times-info/rosie-ruiz-tries-to-steal-the-boston-marathon. Published July 1, 1980. Accessed May 15, 2017.
2. Nelson, JC, Spyker DA. Morbidity and mortality associated with medications used in the treatment of depression: an analysis of cases reported to U.S. Poison Control Centers, 2000-2014. Am J Psychiatry. 2017;174(5):438-450.
3. Robiner WN, Bearman DL, Berman M, et al. Prescriptive authority for psychologists: despite deficits in education and knowledge? J Clin Psychol Med Settings. 2003;10(3):211-221.
4. Robiner WN, Bearman DL, Berman M, et al. Prescriptive authority for psychologists: a looming health hazard? Clinical Psychology Science and Practice. 2002;9(3):231-248.
5. Kingsbury SJ. Some effects of prescribing privileges. Am Psychol. 1992;47(3):426-427.
6. Pollitt B. Fools gold: psychologists using disingenuous reasoning to mislead legislatures into granting psychologists prescriptive authority. Am J Law Med. 2003;29:489-524.
7. DeNelsky GY. The case against prescription privileges for psychologists. Am Psychol. 1996;51(3):207-212.
8. Walker K. An ethical dilemma: clinical psychologists prescribing psychotropic medications. Issues Ment Health Nurs. 2002;23(1):17-29.
9. Nasrallah HA. Polypharmacy subtypes: the necessary, the reasonable, the ridiculous and the hazardous. Current Psychiatry. 2011;10(4):10-12.
10. Nasrallah HA. Parsimonious pharmacotherapy. Current Psychiatry. 2011;10(5):12-16.
11. Shou H, Yang Z, Satterthwaite TD, et al. Cognitive behavioral therapy increases amygdala connectivity with the cognitive control network in both MDD and PTSD. Neuroimage Clin. 2017;14:464-470.
12. Månsson KN, Salami A, Frick A, et al. Neuroplasticity in response to cognitive behavior therapy for social anxiety disorder. Transl Psychiatry. 2015;5:e727.
13. Redei EE, Andrus BM, Kwasny MJ, et al. Blood transcriptomic biomarkers in adult primary care patients with major depressive disorder undergoing cognitive behavioral therapy. Transl Psychiatry. 2014;4:e442.
Practicing medicine without a license is a crime, but it seems to have become a hollow law. Politicians are now cynically legalizing it by granting prescribing privileges to individuals with no prior foundation of medical training. Perhaps it is because of serious ignorance of the difference between psychiatry and psychology or MD and PhD degrees. Or perhaps it is a quid pro quo to generous donors to their re-election campaigns who seek a convenient shortcut to the 28,000 hours it takes to become a psychiatrist in 8 years of medical school and psychiatric residency—and that comes after 4 years of college.
I recently consulted an attorney to discuss some legal documents. When he asked me what my line of work is, I then asked him if he knew the difference between a psychiatrist and a psychologist. He hesitated before admitting in an embarrassed tone that he did not really know and thought that they were all “shrinks” and very similar. I then informed him that both go through undergraduate college education, albeit taking very different courses, with pre-med scientific emphasis for future psychiatric physicians and predominately psychology emphasis for future psychologists.
However, psychiatrists then attend medical school for 4 years and rotate on multiple hospital-based medical specialties, such as internal medicine, surgery, pediatrics, obstetrics and gynecology, family medicine, neurology, pathology, psychiatry, ophthalmology, dermatology, anesthesia, radiology, otolaryngology, etc.
Psychologists, on the other hand, take additional advanced psychology courses in graduate school and write a dissertation that requires quite a bit of library time. After getting a MD, future psychiatrists spend 4 years in extensive training in residency programs across inpatient wards and outpatient clinics, assessing the physical and mental health of seriously sick patients with emphasis on both pharmacological and psychotherapeutic treatments for serious psychiatric disorders in patients, the majority of whom have comorbid medical conditions as well. Psychologists, on the other hand, spend 1 year of internship after getting their PhD or PsyD degree, essentially focused on developing counseling and psychotherapy skills. By the time they complete their training, psychologists and psychiatrists have disparate skills: heavily medical and pharmacological skills in psychiatrists and strong psychotherapeutic skills in psychologists.
After this long explanation, I asked the attorney what he thought about psychologists seeking prescription privileges. He was astounded that psychologists would attempt to expand this scope of practice through state legislations rather than going through medical training like all physicians. “That would be like practicing medicine without a license, which is a felony,” he said. He wryly added that his fellow malpractice and litigation lawyers will be the big winners while poorly treated patients will be the biggest losers. Being an avid runner, he also added that such a short-cut to prescribe without the requisite years of medial training reminded him of Rosie Ruiz, who snuck into the Boston marathon a couple of miles before the finish line and “won” the race, before she was caught and discredited.1
Psychology is a respected mental health discipline with strong psychotherapy training and orientation. For decades, psychologists have vigorously criticized the medical model of mental disorders that psychiatric physicians employ to diagnose and treat brain disorders that disrupt thinking, emotions, mood, cognition, and behavior. However, about 25 years ago, a small group of militant psychologists brazenly decided to lobby state legislatures to give them the right to prescribe psychotropics, although they have no formal medical training. Psychiatric physicians, represented by the American Psychiatric Association (APA), strongly opposed this initiative and regarded it as reckless disregard of the obvious need for extensive medical training to be able to prescribe drugs that affect every organ in the body, not only the brain. Psychiatric medications are associated with serious risks of morbidity and mortality.2 The ability to safely prescribe any medication represents the tip of a huge iceberg of 8 years of rigorous medical school education and specialty training. Yet, one of the early proponents of prescription privileges for psychologists, Patrick De Leon, sarcastically likened the ability to prescribe drugs to learning how to operate a desktop computer!
Not all psychologists agreed with the political campaign to lobby state legislatures to pass a law authorizing prescriptive privileges for psychologists.3-6 In fact, most academic psychologists oppose it.7 Most of the early supporters had a PsyD degree from professional schools of psychology, not a PhD degree in psychology, which is obtained from a university department of psychology. The National Alliance on Mental Illness is opposed to psychologists prescribing medications.8 Psychiatrists are outraged by this hazardous “solution” to the shortage of psychiatrists and point to the many potential dangers to patients. Some suggested that this is a quick way to enhance psychologists’ income and to generate more revenue for their professional journals and meetings with lucrative pharmaceutical ads and exhibit booths.
The campaign is ongoing, as Idaho became the fifth state to adopt such an ill-conceived “solution” to increasing access to mental health care, despite valiant effort by the APA to lobby against such laws. Although New Mexico (2002), Louisiana (2004), Illinois (2014), and Iowa (2016) have passed prescriptive authority for psychologists before Idaho, the APA has defeated such measures in numerous other states. But the painful truth is that this has been a lengthy political chess game in which psychologists have been gradually gaining ground and “capturing more pieces.”
Here is a brief, common sense rationale as to the need for full medical training necessary before safely and accurately prescribing medications, most of which are synthetic molecules, which are essentially foreign substances, with both benefits and risks detailed in the FDA-approved label of each drug that reaches the medical marketplace.
First: Making an accurate clinical diagnosis. If a patient presents with depression, the clinician must rule out other possible causes before diagnosing it as primary major depressive disorder for which an antidepressant can be prescribed. The panoply of secondary depressions, which are not treated with antidepressants, includes a variety of recreational drug-induced mood changes and dysphoria and depression induced by numerous prescription drugs (such as antihypertensives, hormonal contraceptives, steroids, interferon, proton pump inhibitors, H2 blockers, malaria drugs, etc.).
After drug-induced depression is ruled out, the clinician must rule out the possibility that an underlying medical condition might be causing the depression, which includes disorders such as hypothyroidism and other endocrinopathies, anemia, stroke, heart disease, hyperkalemia, lupus and other autoimmune disorders, cancer, Parkinsonism, etc. Therefore, a targeted exploration of past and current medical history, accompanied by a battery of lab tests (complete blood count, electrolytes, liver and kidney function tests, metabolic profile, thyroid-stimulating hormone, etc.) must be done to systematically arrive at the correct diagnosis. Only then can the proper treatment plan be determined, which may or may not include prescribing an antidepressant.
Conclusion: Medical training and psychiatric residency are required for an accurate diagnosis of a mental disorder. Even physicians with no psychiatric training might not have the full repertoire of knowledge needed to systematically rule out secondary depression.
Second: Drug selection. Psychiatric drugs can have various iatrogenic effects. Thus, the selection of an appropriate prescription medication from the available array of drugs approved for a given psychiatric indication must be safe and consistent with the patient’s medical history and must not potentially exacerbate ≥1 comorbid medical conditions.
Conclusion: Medical training and psychiatric residency are required.
Third: Knowledge of metabolic pathways of each psychiatric medication to be prescribed as well as the metabolic pathway of all other medications (psychiatric and non-psychiatric) the patient receives is essential to avoid adverse drug–drug interactions. This includes the hepatic enzymes (cytochromes), which often are responsible for metabolizing all the psychiatric and non-psychiatric drugs a patient is receiving. Knowledge of inhibitors and inducers of various cytochrome enzymes is vital for selecting a medication that does not cause a pharmacokinetic adverse reaction that can produce serious adverse effects (even death, such as with QTc prolongation) or can cause loss of efficacy of ≥1 medications that the patient is receiving, in addition to the antidepressant. Also, in addition to evaluating hepatic pathways, knowledge of renal excretion of the drug to be selected and the status of the patient’s kidney function or impairment must be evaluated.
Conclusion: Medical training is required.
Conclusion: Medical training is required.
Fifth: General medical treatment. Many patients might require combination drug therapy because of inadequate response to monotherapy. Clinicians must know what is rational and evidence-based polypharmacy and what is irrational, dangerous, or absurd polypharmacy.9 When possible, parsimonious pharmacotherapy should be employed to minimize the number of medications prescribed.10 A patient could experience severe drug–drug reactions that could lead to cardiopulmonary crises. The clinician must be able to examine, intervene, and manage the patient’s medical distress until help arrives.
Conclusion: Medical training is required.
Sixth: Pregnancy. Knowledge about the pharmacotherapeutic aspects of pregnant women with mental illness is critical. Full knowledge about what can or should not be prescribed during pregnancy (ie, avoiding teratogenic agents) is vital for physicians treating women with psychiatric illness who become pregnant.
Conclusion: Medical training is required.
Although I am against prescriptive privileges for psychologists, I want to emphasize how much I appreciate and respect what psychologists do for patients with mental illness. Their psychotherapy skills often are honed beyond those of psychiatrists who, by necessity, focus on medical diagnosis and pharmacotherapeutic management. Combination of pharmacotherapy and psychotherapy has been demonstrated to be superior to medications alone. In the 25 years since psychologists have been eagerly pursuing prescriptive privileges, neuroscience research has revealed the neurobiologic effects of psychotherapy. Many studies have shown that evidence-based psychotherapy can induce the same structural and functional brain changes as medications11,12 and can influence biomarkers that accompany psychiatric disorders just as medications do.13
Psychologists should reconsider the many potential hazards of prescription drugs compared with the relative safety and efficacy of psychotherapy. They should focus on their qualifications and main strength, which is psychotherapy, and collaborate with psychiatrists and nurse practitioners on a biopsychosocial approach to mental illness. They also should realize how physically ill most psychiatric patients are and the complex medical management they need for their myriad comorbidities.
Just as I began this editorial with an anecdote, I will end with an illustrative one as well. As an academic professor for the past 3 decades who has trained and supervised numerous psychiatric residents, I once closely supervised a former PhD psychologist who decided to become a psychiatrist by going to medical school, followed by a 4-year psychiatric residency. I asked her to compare her experience and functioning as a psychologist with her current work as a fourth-year psychiatric resident. Her response was enlightening: She said the 2 professions are vastly different in their knowledge base and in terms of how they conceptualize mental illness from a psychological vs medical model. As for prescribing medications, she added that even after 8 years of extensive medical training as a physician and a psychiatrist, she feels there is still much to learn about psychopharmacology to ensure not only efficacy but also safety, because a majority of psychiatric patients have ≥1 coexisting medical conditions and substance use as well. Based on her own experience as a psychologist who became a psychiatric physician, she was completely opposed to prescriptive privileges for psychologists unless they go to medical school and become eligible to prescribe safely.
This former resident is now a successful academic psychiatrist who continues to hone her psychopharmacology skills. State legislators should listen to professionals like her before they pass a law giving prescriptive authority to psychologists without having to go through the rigors of 28,000 hours of training in the 8 years of medical school and psychiatric residency. Legislators should also understand that like psychologists, social work counselors have hardly any medical training, yet they have never sought prescriptive privileges. That’s clearly rational and wise.
Practicing medicine without a license is a crime, but it seems to have become a hollow law. Politicians are now cynically legalizing it by granting prescribing privileges to individuals with no prior foundation of medical training. Perhaps it is because of serious ignorance of the difference between psychiatry and psychology or MD and PhD degrees. Or perhaps it is a quid pro quo to generous donors to their re-election campaigns who seek a convenient shortcut to the 28,000 hours it takes to become a psychiatrist in 8 years of medical school and psychiatric residency—and that comes after 4 years of college.
I recently consulted an attorney to discuss some legal documents. When he asked me what my line of work is, I then asked him if he knew the difference between a psychiatrist and a psychologist. He hesitated before admitting in an embarrassed tone that he did not really know and thought that they were all “shrinks” and very similar. I then informed him that both go through undergraduate college education, albeit taking very different courses, with pre-med scientific emphasis for future psychiatric physicians and predominately psychology emphasis for future psychologists.
However, psychiatrists then attend medical school for 4 years and rotate on multiple hospital-based medical specialties, such as internal medicine, surgery, pediatrics, obstetrics and gynecology, family medicine, neurology, pathology, psychiatry, ophthalmology, dermatology, anesthesia, radiology, otolaryngology, etc.
Psychologists, on the other hand, take additional advanced psychology courses in graduate school and write a dissertation that requires quite a bit of library time. After getting a MD, future psychiatrists spend 4 years in extensive training in residency programs across inpatient wards and outpatient clinics, assessing the physical and mental health of seriously sick patients with emphasis on both pharmacological and psychotherapeutic treatments for serious psychiatric disorders in patients, the majority of whom have comorbid medical conditions as well. Psychologists, on the other hand, spend 1 year of internship after getting their PhD or PsyD degree, essentially focused on developing counseling and psychotherapy skills. By the time they complete their training, psychologists and psychiatrists have disparate skills: heavily medical and pharmacological skills in psychiatrists and strong psychotherapeutic skills in psychologists.
After this long explanation, I asked the attorney what he thought about psychologists seeking prescription privileges. He was astounded that psychologists would attempt to expand this scope of practice through state legislations rather than going through medical training like all physicians. “That would be like practicing medicine without a license, which is a felony,” he said. He wryly added that his fellow malpractice and litigation lawyers will be the big winners while poorly treated patients will be the biggest losers. Being an avid runner, he also added that such a short-cut to prescribe without the requisite years of medial training reminded him of Rosie Ruiz, who snuck into the Boston marathon a couple of miles before the finish line and “won” the race, before she was caught and discredited.1
Psychology is a respected mental health discipline with strong psychotherapy training and orientation. For decades, psychologists have vigorously criticized the medical model of mental disorders that psychiatric physicians employ to diagnose and treat brain disorders that disrupt thinking, emotions, mood, cognition, and behavior. However, about 25 years ago, a small group of militant psychologists brazenly decided to lobby state legislatures to give them the right to prescribe psychotropics, although they have no formal medical training. Psychiatric physicians, represented by the American Psychiatric Association (APA), strongly opposed this initiative and regarded it as reckless disregard of the obvious need for extensive medical training to be able to prescribe drugs that affect every organ in the body, not only the brain. Psychiatric medications are associated with serious risks of morbidity and mortality.2 The ability to safely prescribe any medication represents the tip of a huge iceberg of 8 years of rigorous medical school education and specialty training. Yet, one of the early proponents of prescription privileges for psychologists, Patrick De Leon, sarcastically likened the ability to prescribe drugs to learning how to operate a desktop computer!
Not all psychologists agreed with the political campaign to lobby state legislatures to pass a law authorizing prescriptive privileges for psychologists.3-6 In fact, most academic psychologists oppose it.7 Most of the early supporters had a PsyD degree from professional schools of psychology, not a PhD degree in psychology, which is obtained from a university department of psychology. The National Alliance on Mental Illness is opposed to psychologists prescribing medications.8 Psychiatrists are outraged by this hazardous “solution” to the shortage of psychiatrists and point to the many potential dangers to patients. Some suggested that this is a quick way to enhance psychologists’ income and to generate more revenue for their professional journals and meetings with lucrative pharmaceutical ads and exhibit booths.
The campaign is ongoing, as Idaho became the fifth state to adopt such an ill-conceived “solution” to increasing access to mental health care, despite valiant effort by the APA to lobby against such laws. Although New Mexico (2002), Louisiana (2004), Illinois (2014), and Iowa (2016) have passed prescriptive authority for psychologists before Idaho, the APA has defeated such measures in numerous other states. But the painful truth is that this has been a lengthy political chess game in which psychologists have been gradually gaining ground and “capturing more pieces.”
Here is a brief, common sense rationale as to the need for full medical training necessary before safely and accurately prescribing medications, most of which are synthetic molecules, which are essentially foreign substances, with both benefits and risks detailed in the FDA-approved label of each drug that reaches the medical marketplace.
First: Making an accurate clinical diagnosis. If a patient presents with depression, the clinician must rule out other possible causes before diagnosing it as primary major depressive disorder for which an antidepressant can be prescribed. The panoply of secondary depressions, which are not treated with antidepressants, includes a variety of recreational drug-induced mood changes and dysphoria and depression induced by numerous prescription drugs (such as antihypertensives, hormonal contraceptives, steroids, interferon, proton pump inhibitors, H2 blockers, malaria drugs, etc.).
After drug-induced depression is ruled out, the clinician must rule out the possibility that an underlying medical condition might be causing the depression, which includes disorders such as hypothyroidism and other endocrinopathies, anemia, stroke, heart disease, hyperkalemia, lupus and other autoimmune disorders, cancer, Parkinsonism, etc. Therefore, a targeted exploration of past and current medical history, accompanied by a battery of lab tests (complete blood count, electrolytes, liver and kidney function tests, metabolic profile, thyroid-stimulating hormone, etc.) must be done to systematically arrive at the correct diagnosis. Only then can the proper treatment plan be determined, which may or may not include prescribing an antidepressant.
Conclusion: Medical training and psychiatric residency are required for an accurate diagnosis of a mental disorder. Even physicians with no psychiatric training might not have the full repertoire of knowledge needed to systematically rule out secondary depression.
Second: Drug selection. Psychiatric drugs can have various iatrogenic effects. Thus, the selection of an appropriate prescription medication from the available array of drugs approved for a given psychiatric indication must be safe and consistent with the patient’s medical history and must not potentially exacerbate ≥1 comorbid medical conditions.
Conclusion: Medical training and psychiatric residency are required.
Third: Knowledge of metabolic pathways of each psychiatric medication to be prescribed as well as the metabolic pathway of all other medications (psychiatric and non-psychiatric) the patient receives is essential to avoid adverse drug–drug interactions. This includes the hepatic enzymes (cytochromes), which often are responsible for metabolizing all the psychiatric and non-psychiatric drugs a patient is receiving. Knowledge of inhibitors and inducers of various cytochrome enzymes is vital for selecting a medication that does not cause a pharmacokinetic adverse reaction that can produce serious adverse effects (even death, such as with QTc prolongation) or can cause loss of efficacy of ≥1 medications that the patient is receiving, in addition to the antidepressant. Also, in addition to evaluating hepatic pathways, knowledge of renal excretion of the drug to be selected and the status of the patient’s kidney function or impairment must be evaluated.
Conclusion: Medical training is required.
Conclusion: Medical training is required.
Fifth: General medical treatment. Many patients might require combination drug therapy because of inadequate response to monotherapy. Clinicians must know what is rational and evidence-based polypharmacy and what is irrational, dangerous, or absurd polypharmacy.9 When possible, parsimonious pharmacotherapy should be employed to minimize the number of medications prescribed.10 A patient could experience severe drug–drug reactions that could lead to cardiopulmonary crises. The clinician must be able to examine, intervene, and manage the patient’s medical distress until help arrives.
Conclusion: Medical training is required.
Sixth: Pregnancy. Knowledge about the pharmacotherapeutic aspects of pregnant women with mental illness is critical. Full knowledge about what can or should not be prescribed during pregnancy (ie, avoiding teratogenic agents) is vital for physicians treating women with psychiatric illness who become pregnant.
Conclusion: Medical training is required.
Although I am against prescriptive privileges for psychologists, I want to emphasize how much I appreciate and respect what psychologists do for patients with mental illness. Their psychotherapy skills often are honed beyond those of psychiatrists who, by necessity, focus on medical diagnosis and pharmacotherapeutic management. Combination of pharmacotherapy and psychotherapy has been demonstrated to be superior to medications alone. In the 25 years since psychologists have been eagerly pursuing prescriptive privileges, neuroscience research has revealed the neurobiologic effects of psychotherapy. Many studies have shown that evidence-based psychotherapy can induce the same structural and functional brain changes as medications11,12 and can influence biomarkers that accompany psychiatric disorders just as medications do.13
Psychologists should reconsider the many potential hazards of prescription drugs compared with the relative safety and efficacy of psychotherapy. They should focus on their qualifications and main strength, which is psychotherapy, and collaborate with psychiatrists and nurse practitioners on a biopsychosocial approach to mental illness. They also should realize how physically ill most psychiatric patients are and the complex medical management they need for their myriad comorbidities.
Just as I began this editorial with an anecdote, I will end with an illustrative one as well. As an academic professor for the past 3 decades who has trained and supervised numerous psychiatric residents, I once closely supervised a former PhD psychologist who decided to become a psychiatrist by going to medical school, followed by a 4-year psychiatric residency. I asked her to compare her experience and functioning as a psychologist with her current work as a fourth-year psychiatric resident. Her response was enlightening: She said the 2 professions are vastly different in their knowledge base and in terms of how they conceptualize mental illness from a psychological vs medical model. As for prescribing medications, she added that even after 8 years of extensive medical training as a physician and a psychiatrist, she feels there is still much to learn about psychopharmacology to ensure not only efficacy but also safety, because a majority of psychiatric patients have ≥1 coexisting medical conditions and substance use as well. Based on her own experience as a psychologist who became a psychiatric physician, she was completely opposed to prescriptive privileges for psychologists unless they go to medical school and become eligible to prescribe safely.
This former resident is now a successful academic psychiatrist who continues to hone her psychopharmacology skills. State legislators should listen to professionals like her before they pass a law giving prescriptive authority to psychologists without having to go through the rigors of 28,000 hours of training in the 8 years of medical school and psychiatric residency. Legislators should also understand that like psychologists, social work counselors have hardly any medical training, yet they have never sought prescriptive privileges. That’s clearly rational and wise.
1. Rosie Ruiz tries to steal the Boston marathon. Runner’s World. http://www.runnersworld.com/running-times-info/rosie-ruiz-tries-to-steal-the-boston-marathon. Published July 1, 1980. Accessed May 15, 2017.
2. Nelson, JC, Spyker DA. Morbidity and mortality associated with medications used in the treatment of depression: an analysis of cases reported to U.S. Poison Control Centers, 2000-2014. Am J Psychiatry. 2017;174(5):438-450.
3. Robiner WN, Bearman DL, Berman M, et al. Prescriptive authority for psychologists: despite deficits in education and knowledge? J Clin Psychol Med Settings. 2003;10(3):211-221.
4. Robiner WN, Bearman DL, Berman M, et al. Prescriptive authority for psychologists: a looming health hazard? Clinical Psychology Science and Practice. 2002;9(3):231-248.
5. Kingsbury SJ. Some effects of prescribing privileges. Am Psychol. 1992;47(3):426-427.
6. Pollitt B. Fools gold: psychologists using disingenuous reasoning to mislead legislatures into granting psychologists prescriptive authority. Am J Law Med. 2003;29:489-524.
7. DeNelsky GY. The case against prescription privileges for psychologists. Am Psychol. 1996;51(3):207-212.
8. Walker K. An ethical dilemma: clinical psychologists prescribing psychotropic medications. Issues Ment Health Nurs. 2002;23(1):17-29.
9. Nasrallah HA. Polypharmacy subtypes: the necessary, the reasonable, the ridiculous and the hazardous. Current Psychiatry. 2011;10(4):10-12.
10. Nasrallah HA. Parsimonious pharmacotherapy. Current Psychiatry. 2011;10(5):12-16.
11. Shou H, Yang Z, Satterthwaite TD, et al. Cognitive behavioral therapy increases amygdala connectivity with the cognitive control network in both MDD and PTSD. Neuroimage Clin. 2017;14:464-470.
12. Månsson KN, Salami A, Frick A, et al. Neuroplasticity in response to cognitive behavior therapy for social anxiety disorder. Transl Psychiatry. 2015;5:e727.
13. Redei EE, Andrus BM, Kwasny MJ, et al. Blood transcriptomic biomarkers in adult primary care patients with major depressive disorder undergoing cognitive behavioral therapy. Transl Psychiatry. 2014;4:e442.
1. Rosie Ruiz tries to steal the Boston marathon. Runner’s World. http://www.runnersworld.com/running-times-info/rosie-ruiz-tries-to-steal-the-boston-marathon. Published July 1, 1980. Accessed May 15, 2017.
2. Nelson, JC, Spyker DA. Morbidity and mortality associated with medications used in the treatment of depression: an analysis of cases reported to U.S. Poison Control Centers, 2000-2014. Am J Psychiatry. 2017;174(5):438-450.
3. Robiner WN, Bearman DL, Berman M, et al. Prescriptive authority for psychologists: despite deficits in education and knowledge? J Clin Psychol Med Settings. 2003;10(3):211-221.
4. Robiner WN, Bearman DL, Berman M, et al. Prescriptive authority for psychologists: a looming health hazard? Clinical Psychology Science and Practice. 2002;9(3):231-248.
5. Kingsbury SJ. Some effects of prescribing privileges. Am Psychol. 1992;47(3):426-427.
6. Pollitt B. Fools gold: psychologists using disingenuous reasoning to mislead legislatures into granting psychologists prescriptive authority. Am J Law Med. 2003;29:489-524.
7. DeNelsky GY. The case against prescription privileges for psychologists. Am Psychol. 1996;51(3):207-212.
8. Walker K. An ethical dilemma: clinical psychologists prescribing psychotropic medications. Issues Ment Health Nurs. 2002;23(1):17-29.
9. Nasrallah HA. Polypharmacy subtypes: the necessary, the reasonable, the ridiculous and the hazardous. Current Psychiatry. 2011;10(4):10-12.
10. Nasrallah HA. Parsimonious pharmacotherapy. Current Psychiatry. 2011;10(5):12-16.
11. Shou H, Yang Z, Satterthwaite TD, et al. Cognitive behavioral therapy increases amygdala connectivity with the cognitive control network in both MDD and PTSD. Neuroimage Clin. 2017;14:464-470.
12. Månsson KN, Salami A, Frick A, et al. Neuroplasticity in response to cognitive behavior therapy for social anxiety disorder. Transl Psychiatry. 2015;5:e727.
13. Redei EE, Andrus BM, Kwasny MJ, et al. Blood transcriptomic biomarkers in adult primary care patients with major depressive disorder undergoing cognitive behavioral therapy. Transl Psychiatry. 2014;4:e442.
Preventing thrombosis without increasing bleeding risk
It may be possible to disrupt thrombosis without increasing the risk of bleeding, according to preclinical research published in Nature Communications.
“We have found a new thrombosis target that does not increase bleeding risk,” said study author Daniel I. Simon, MD, of University Hospitals Cleveland Medical Center in Cleveland, Ohio.
“Our discovery indicates that you can identify a new pathway and target that mediates blood clotting but does not affect our body’s natural processes to stop bleeding.”
The new pathway centers around a pair of protein receptors. One—Mac-1—is found on the surface of leukocytes recruited to sites of blood vessel injury, and the other—GPIbα—resides on the surface of platelets.
When the receptors interact, they trigger cascades of signals that amplify both inflammation and clotting.
The researchers found that genetically engineered mice, either without the Mac-1 receptor or with a mutant form of the receptor, could not bind GPIbα on platelets. As a result, the mice had delayed clot formation in response to artery injury.
However, these mice had similar platelet counts, platelet activation, plasma coagulation activity, and bleeding time as wild-type mice.
Additional experiments in mice showed that an antibody targeting Mac-1:GPIba inhibits thrombus formation.
And glucosamine, a small-molecule inhibitor of Mac-1:GPIba binding, inhibits thrombus formation without increasing bleeding risk.
Mice exposed to glucosamine were still able to successfully stop minor bleeding, like tail cuts, and maintain normal coagulation and platelet function.
The researchers believe these findings could lead to the development of better antithrombotic agents, as “the interaction between leukocyte Mac-1 and platelet GPIba is positioned as a novel and targetable mediator of thrombosis but not hemostasis.”
“Current anticlotting drugs and antiplatelet agents are effective in reducing heart attack and stroke but are associated with increased bleeding and transfusion,” Dr Simon said. “We have learned that bleeding and transfusion complications are equally as bad from a prognosis standpoint as heart attack or stroke.” ![]()
It may be possible to disrupt thrombosis without increasing the risk of bleeding, according to preclinical research published in Nature Communications.
“We have found a new thrombosis target that does not increase bleeding risk,” said study author Daniel I. Simon, MD, of University Hospitals Cleveland Medical Center in Cleveland, Ohio.
“Our discovery indicates that you can identify a new pathway and target that mediates blood clotting but does not affect our body’s natural processes to stop bleeding.”
The new pathway centers around a pair of protein receptors. One—Mac-1—is found on the surface of leukocytes recruited to sites of blood vessel injury, and the other—GPIbα—resides on the surface of platelets.
When the receptors interact, they trigger cascades of signals that amplify both inflammation and clotting.
The researchers found that genetically engineered mice, either without the Mac-1 receptor or with a mutant form of the receptor, could not bind GPIbα on platelets. As a result, the mice had delayed clot formation in response to artery injury.
However, these mice had similar platelet counts, platelet activation, plasma coagulation activity, and bleeding time as wild-type mice.
Additional experiments in mice showed that an antibody targeting Mac-1:GPIba inhibits thrombus formation.
And glucosamine, a small-molecule inhibitor of Mac-1:GPIba binding, inhibits thrombus formation without increasing bleeding risk.
Mice exposed to glucosamine were still able to successfully stop minor bleeding, like tail cuts, and maintain normal coagulation and platelet function.
The researchers believe these findings could lead to the development of better antithrombotic agents, as “the interaction between leukocyte Mac-1 and platelet GPIba is positioned as a novel and targetable mediator of thrombosis but not hemostasis.”
“Current anticlotting drugs and antiplatelet agents are effective in reducing heart attack and stroke but are associated with increased bleeding and transfusion,” Dr Simon said. “We have learned that bleeding and transfusion complications are equally as bad from a prognosis standpoint as heart attack or stroke.” ![]()
It may be possible to disrupt thrombosis without increasing the risk of bleeding, according to preclinical research published in Nature Communications.
“We have found a new thrombosis target that does not increase bleeding risk,” said study author Daniel I. Simon, MD, of University Hospitals Cleveland Medical Center in Cleveland, Ohio.
“Our discovery indicates that you can identify a new pathway and target that mediates blood clotting but does not affect our body’s natural processes to stop bleeding.”
The new pathway centers around a pair of protein receptors. One—Mac-1—is found on the surface of leukocytes recruited to sites of blood vessel injury, and the other—GPIbα—resides on the surface of platelets.
When the receptors interact, they trigger cascades of signals that amplify both inflammation and clotting.
The researchers found that genetically engineered mice, either without the Mac-1 receptor or with a mutant form of the receptor, could not bind GPIbα on platelets. As a result, the mice had delayed clot formation in response to artery injury.
However, these mice had similar platelet counts, platelet activation, plasma coagulation activity, and bleeding time as wild-type mice.
Additional experiments in mice showed that an antibody targeting Mac-1:GPIba inhibits thrombus formation.
And glucosamine, a small-molecule inhibitor of Mac-1:GPIba binding, inhibits thrombus formation without increasing bleeding risk.
Mice exposed to glucosamine were still able to successfully stop minor bleeding, like tail cuts, and maintain normal coagulation and platelet function.
The researchers believe these findings could lead to the development of better antithrombotic agents, as “the interaction between leukocyte Mac-1 and platelet GPIba is positioned as a novel and targetable mediator of thrombosis but not hemostasis.”
“Current anticlotting drugs and antiplatelet agents are effective in reducing heart attack and stroke but are associated with increased bleeding and transfusion,” Dr Simon said. “We have learned that bleeding and transfusion complications are equally as bad from a prognosis standpoint as heart attack or stroke.” ![]()
Gene plays key role in iron homeostasis
A gene known to prevent autoimmune diseases is a key regulator in iron uptake, according to research published in Cell Reports.
“We found previously that, when mice lack the gene Regnase-1, they suffer from severe autoimmune diseases and anemia,” said study author Masanori Yoshinaga, MD, of Kyoto University in Japan.
“At first, we assumed that anemia was a secondary effect, but, after detailed analysis, we found that the 2 symptoms develop independently.”
Continued study of mice with a Regnase-1 mutation revealed a functional defect in the principal site for iron absorption in the body, the duodenum.
“The next step was to find the role of Regnase-1 in iron-uptake maintenance,” Dr Yoshinaga said. “We started by looking at the most important iron-uptake gene, Transferrin Receptor 1, or TfR1.”
“Our results showed that Regnase-1 degrades the mRNA of TfR1, thereby inhibiting the synthesis of the TfR1 protein and, additionally, that it likely regulates other important iron-controlling genes.”
“Further analysis of Regnase-1 in iron-related homeostasis may provide insight into the mechanisms causing anemia and other iron-related disorders, perhaps eventually leading to new methods of treatment,” said study author Osamu Takeuchi, MD, PhD, of Kyoto University. ![]()
A gene known to prevent autoimmune diseases is a key regulator in iron uptake, according to research published in Cell Reports.
“We found previously that, when mice lack the gene Regnase-1, they suffer from severe autoimmune diseases and anemia,” said study author Masanori Yoshinaga, MD, of Kyoto University in Japan.
“At first, we assumed that anemia was a secondary effect, but, after detailed analysis, we found that the 2 symptoms develop independently.”
Continued study of mice with a Regnase-1 mutation revealed a functional defect in the principal site for iron absorption in the body, the duodenum.
“The next step was to find the role of Regnase-1 in iron-uptake maintenance,” Dr Yoshinaga said. “We started by looking at the most important iron-uptake gene, Transferrin Receptor 1, or TfR1.”
“Our results showed that Regnase-1 degrades the mRNA of TfR1, thereby inhibiting the synthesis of the TfR1 protein and, additionally, that it likely regulates other important iron-controlling genes.”
“Further analysis of Regnase-1 in iron-related homeostasis may provide insight into the mechanisms causing anemia and other iron-related disorders, perhaps eventually leading to new methods of treatment,” said study author Osamu Takeuchi, MD, PhD, of Kyoto University. ![]()
A gene known to prevent autoimmune diseases is a key regulator in iron uptake, according to research published in Cell Reports.
“We found previously that, when mice lack the gene Regnase-1, they suffer from severe autoimmune diseases and anemia,” said study author Masanori Yoshinaga, MD, of Kyoto University in Japan.
“At first, we assumed that anemia was a secondary effect, but, after detailed analysis, we found that the 2 symptoms develop independently.”
Continued study of mice with a Regnase-1 mutation revealed a functional defect in the principal site for iron absorption in the body, the duodenum.
“The next step was to find the role of Regnase-1 in iron-uptake maintenance,” Dr Yoshinaga said. “We started by looking at the most important iron-uptake gene, Transferrin Receptor 1, or TfR1.”
“Our results showed that Regnase-1 degrades the mRNA of TfR1, thereby inhibiting the synthesis of the TfR1 protein and, additionally, that it likely regulates other important iron-controlling genes.”
“Further analysis of Regnase-1 in iron-related homeostasis may provide insight into the mechanisms causing anemia and other iron-related disorders, perhaps eventually leading to new methods of treatment,” said study author Osamu Takeuchi, MD, PhD, of Kyoto University. ![]()
Stroke: A road map for subacute management
CASE › A 68-year-old woman with hypertension and hyperlipidemia comes into your office for evaluation of a 30-minute episode of sudden-onset right-hand weakness and difficulty speaking that occurred 4 days earlier. The patient, who is also a smoker, has come in at the insistence of her daughter. On examination, her blood pressure (BP) is 145/88 mm Hg and her heart rate is 76 beats/minute and regular. She appears well and her language function is normal. The rest of her examination is normal. How would you proceed?
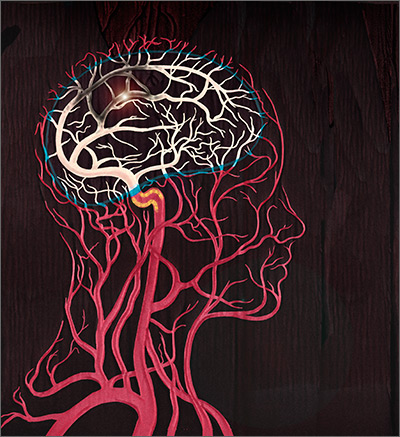
Stroke—the death of nerve cells due to a lack of blood supply from either infarction or hemorrhage—strikes nearly 800,000 people in the United States every year.1,2 Of these events, 130,000 are fatal, making stroke the fifth leading cause of death.3 Effective, early evaluation and cause-specific treatment are crucial parts of stroke care.
Research has helped to clarify the critical role primary care physicians play in recognizing, triaging, and managing stroke and transient ischemic attacks (TIA). This article reviews what we know about the different ways that a stroke and a TIA can present, the appropriate diagnostic work-up for patients presenting with symptoms of either event, and management strategies for subacute care (24 hours to up to 14 days after a stroke has occurred).4,5 Unless otherwise specified, this review will focus on ischemic stroke because 87% of strokes are attributable to ischemia.1
A follow-up to this article on secondary stroke prevention will appear in the journal next month.
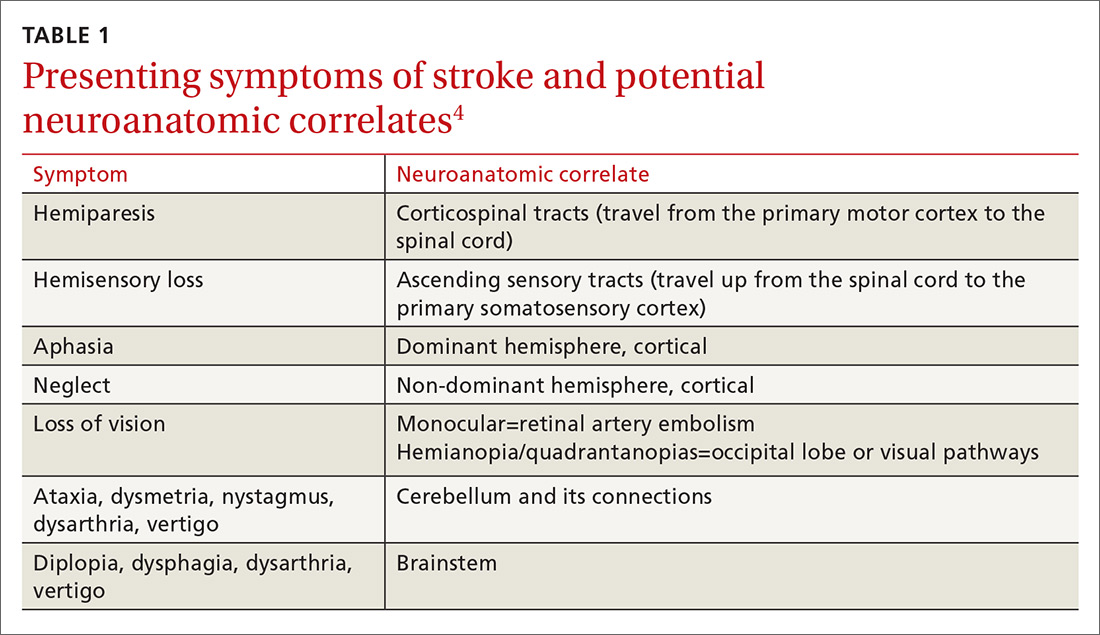
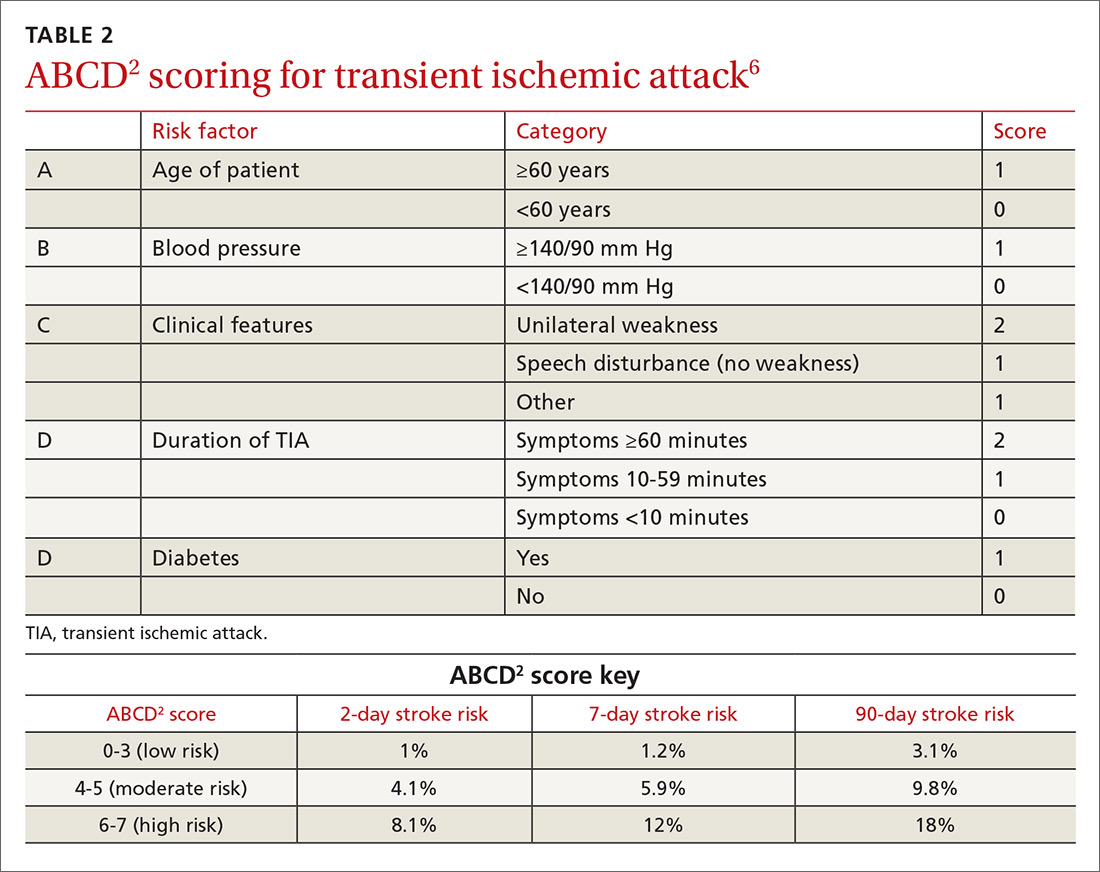
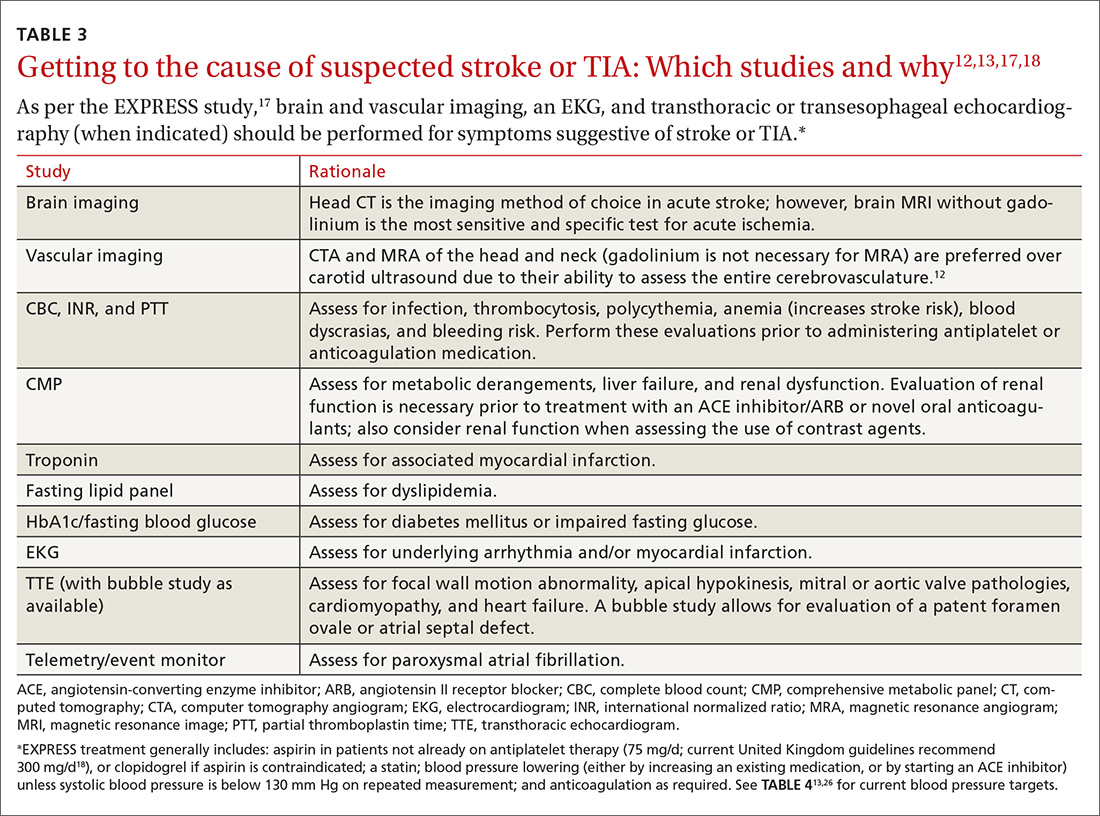
Look to onset more than type of symptoms for clues
Stroke presents as a sudden onset of neurologic deficits (language, motor, sensory, cerebellar, or brainstem functions) (TABLE 14). Because presenting symptoms can vary widely, sudden onset, rather than particular symptoms, should raise a red flag for potential stroke.
The differential diagnosis includes: seizure, complex migraine, medication effect (eg, slurred speech or confusion after taking a central nervous system [CNS] depressant), toxin exposure, electrolyte abnormalities (especially hypoglycemia), concussion/trauma, infection of the CNS, peripheral vertigo, demyelination, intracranial mass, Bell’s palsy, and psychogenic disorders. The history and physical, along with laboratory findings and brain imaging (detailed later in this article), will guide the FP toward (or away from) these various etiologies.
Optimal triage is a subject of ongoing interest and research
If stroke or TIA remains a possibility after an initial assessment, it’s time to stratify patients by risk.
One of the most widely accepted tools is the ABCD2 score (see TABLE 26). Clinicians can employ the ABCD2 risk stratification tool when trying to determine whether it is reasonable to pursue an expedited work-up (ie, <1 day) in the outpatient setting or recommend that the patient be evaluated in an emergency department (ED). The 90-day stroke rate following a TIA ranges from 3% with an ABCD2 score of 0 to 3 to 18% with a score of 6 or 7. A score of 0 to 3 is considered relatively low risk; in the absence of other compelling factors, rapid outpatient evaluation is appropriate. For patients with an ABCD2 score ≥4, referral to the ED or direct admission to the hospital is advised.
The validity of the ABCD2 score for risk stratification has been studied extensively with conflicting results.7-10 As with any assessment tool, it should be used as a guide, and should not supplant a full assessment of the patient or the judgment of the examining physician. In making the decision regarding inpatient or outpatient evaluation, it’s also important to consider available resources, access to specialists, and patient preference.
In a 2016 population-based study, the 30-day recurrent stroke/TIA rate for patients hospitalized for TIA was 3% compared with 10.7% for those discharged from the ED with referral to a stroke clinic and 10.6% for those discharged from the ED without a referral to a stroke clinic.11 These data suggest that only patients for whom you have a low clinical suspicion of stroke/TIA should be worked up as outpatients, and that hospital admission is advised in moderate- and high-risk cases. The findings also highlight the critical role that primary care physicians can play in triaging and managing these patients for secondary prevention.
CASE › This patient’s recent history of sudden-onset right-sided weakness and expressive language dysfunction is suspicious for left hemispheric ischemia. She has several risk factors for stroke, and her ABCD2 score is 5 (hypertension, age ≥60 years, unilateral weakness, and duration 10-59 min), which places her at moderate risk. Thus, the recommendation would be to have her go directly to an ED for rapid evaluation.
The diagnostic work-up
Even when a patient is sent to the ED, the FP plays a critical role in his or her continuing care. FPs will often coordinate with inpatient care and manage transition of care to the outpatient setting. (And in many communities, the ED or hospital physicians may themselves be family practitioners.)
In terms of care, not even an aspirin should be administered in a case like this because the patient has not yet had any neuroimaging, and differentiation of ischemic from hemorrhagic stroke cannot be made on clinical grounds alone. Once an ischemic stroke is confirmed, determining the etiology is critical given the significant management differences between the different types of stroke (atherosclerotic, cardioembolic, lacunar, or other).
Which imaging method, and when?
While a computerized tomography (CT) scan is the preferred initial imaging strategy for acute stroke to discern the ischemic type from the hemorrhagic, MRI is preferred for the evaluation of acute ischemic stroke because the method has a higher sensitivity for infarction and a greater ability to identify findings (such as demyelination) that would suggest an alternative diagnosis.
In addition to evaluating the brain parenchyma, physicians must also assess the cerebral vasculature. CT angiography (CTA) or MR angiography (MRA) of the head and neck are preferred over carotid ultrasound because they are capable of evaluating the entire cerebrovascular system12,13 and can be instrumental in identifying potential causes of stroke, as well as guiding therapeutic decisions. Carotid ultrasound is a reasonable alternative for patients presenting with symptoms indicative of anterior circulation involvement when CTA and MRA are unavailable or contraindicated, but it will not identify intracranial vascular disease, proximal common carotid disease, or vertebrobasilar disease.
Getting to the cause of suspected stroke: Labs and other diagnostic tests
A routine work-up includes BP checks, routine labs (complete blood count, complete metabolic panel, coagulation profile, and troponin), an electrocardiogram (EKG), a transthoracic echocardiogram (TTE) with bubble study if possible, and a minimum of 24 to 48 hours of cardiac rhythm monitoring. Cardiac rhythm monitoring should be extended in the setting of clinical concern for unidentified paroxysmal atrial fibrillation, such as an embolism without a proximal vascular source, multiple embolic infarcts in different vascular territories, a dilated left atrium, or other risk factors for atrial fibrillation that include smoking, systolic hypertension, diabetes, and heart failure (see TABLE 312,13,17,18).14-16 This standard diagnostic work-up will identify the cause of stroke in 70% to 80% of patients.19
Additional investigations to consider if the etiology is not yet elucidated include a transesophageal echocardiogram (TEE), cerebral angiography, a coagulopathy evaluation, a lumbar puncture, and a vasculitis work-up. If available, consultation with a neurologist is appropriate for any patient who has had a stroke or TIA. Patients with unclear etiologies or for whom there are questions concerning strategies for preventing secondary stroke should be referred to Neurology and preferably a stroke specialist.
Timing matters, even when symptoms have resolved (ie, TIA).11,20 The EXPRESS trial17 (the Early use of eXisting PREventive Strategies for Stroke) looked at the effect of urgent assessment and treatment (≤1 day) of patients presenting with a TIA or minor stroke on the risk of recurrent stroke within 90 days. The diagnostic work-up included brain and vascular imaging together with an EKG. This intensive approach led to an absolute risk reduction of 8.2% (from 10.3% to 2.1%) in the risk of recurrent stroke at 90 days (number needed to treat [NNT]=12).17
Expedited work-up and treatment was also recently evaluated in a non-trial, real-world setting and was associated with reducing recurrent stroke by more than half the rate reported in older studies.20 Overall, the data suggest that evaluation within 24 hours confers substantial benefit, and that this evaluation can happen in an outpatient setting.21-23
Acute management: Use of tPA
Once imaging rules out intracranial hemorrhage, patients should be treated with tissue plasminogen activator (tPA) or an endovascular intervention as per guidelines.24 For patients with ischemic stroke ineligible for tPA or endovascular treatments, the initial focus is to determine the etiology of the symptoms so that the best strategies for prevention of secondary stroke may be employed.
Aspirin should be provided within 24 to 48 hours to all patients after intracranial hemorrhage is ruled out. Aspirin should be delayed for 24 hours in those given thrombolytics. The initial recommended dose of aspirin is 325 mg with continued low-dose (81 mg) aspirin daily.13 The addition of clopidogrel to aspirin within 24 hours of an event and continued for 21 days, followed by aspirin alone, was shown to be beneficial in a Chinese population with high-risk TIA (ABCD2 score ≥4) or minor stroke (National Institutes of Health Stroke Scale [NIHSS] ≤3).25 Anticoagulation with heparin, warfarin, or a novel oral anticoagulant is generally not indicated in the acute setting due to the risk of hemorrhagic transformation.
Acute BP management depends upon the type of stroke (ischemic or hemorrhagic), eligibility for thrombolytics, timing of presentation, and possible comorbidities such as myocardial infarction or aortic dissection (see TABLE 413,26). In the absence of contraindications, high-intensity statins should be initiated in all patients able to take oral medications.
CASE › You appropriately referred your patient to the local ED. A head CT with head and neck CTA was performed. While the head CT did not show any abnormalities, the CTA demonstrated high-grade left internal carotid artery stenosis. The patient was given an initial dose of aspirin 325 mg and a high-intensity statin and admitted for further management. An MRI revealed a small shower of emboli in the left hemisphere, confirming the diagnosis of stroke over TIA. Labs were marginally remarkable with a low-density lipoprotein level of 115 mg/dL and an HbA1c of 6.2. Telemetry monitoring did not reveal any arrhythmias, and TTE was normal. BP remained in the high-normal to low-hypertensive range.
A Vascular Surgery consultation was obtained and the patient underwent a left carotid endarterectomy the following day. She did well without surgical complications. Her BP medications were adjusted; a combination of an angiotensin-converting enzyme inhibitor and a thiazide diuretic achieved a goal BP <140/90 mm Hg.
Permissive hypertension was not indicated due to her presentation >48 hours beyond the acute event. Low-dose aspirin and a high-intensity statin were continued, for secondary stroke prevention in the setting of atherosclerotic disease. She received smoking cessation counseling, which will continue.
CORRESPONDENCE
Stephen A. Martin, MD, EdM, Barre Family Health Center, 151 Worcester Road, Barre, MA 01005; stmartin@gmail.com.
1. Benjamin EJ, Blaha MJ, Chiuve SE, et al. Heart disease and stroke statistics-2017 update: a report from the American Heart Association. Circulation. 2017;135:e146-e603.
2. Sacco RL, Kasner SE, Broderick JP, et al. An updated definition of stroke for the 21st century: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2013;44:2064-2089.
3. Kochanek KD, Murphy SL, Xu J, et al. Mortality in the United States, 2013. NCHS Data Brief. 2014:1-8. Available at: https://www.cdc.gov/nchs/data/databriefs/db178.pdf. Accessed June 5, 2016.
4. Flossmann E, Redgrave JN, Briley D, et al. Reliability of clinical diagnosis of the symptomatic vascular territory in patients with recent transient ischemic attack or minor stroke. Stroke. 2008;39:2457-2460.
5. Josephson SA, Sidney S, Pham TN, et al. Higher ABCD2 score predicts patients most likely to have true transient ischemic attack. Stroke. 2008;39:3096-3098. Available at: http://www.ncbi.nlm.nih.gov/pubmed/18688003. Accessed June 5, 2016.
6. Hankey GJ. The ABCD, California, and unified ABCD2 risk scores predicted stroke within 2, 7, and 90 days after TIA. Evid Based Med. 2007;12:88.
7. Sheehan OC, Kyne L, Kelly LA, et al. Population-based study of ABCD2 score, carotid stenosis, and atrial fibrillation for early stroke prediction after transient ischemic attack: the North Dublin TIA study. Stroke. 2010;41:844-850.
8. Rothwell PM, Giles MF, Flossmann E, et al. A simple score (ABCD) to identify individuals at high early risk of stroke after transient ischaemic attack. Lancet. 2005; 366:29-36.
9. Tsivgoulis G, Spengos K, Manta P, et al. Validation of the ABCD score in identifying individuals at high early risk of stroke after a transient ischemic attack: a hospital-based case series study. Stroke. 2006;37:2892-2897.
10. Kiyohara T, Kamouchi M, Kumai Y, et al. ABCD3 and ABCD3-I scores are superior to ABCD2 score in the prediction of short- and long-term risks of stroke after transient ischemic attack. Stroke. 2014;45:418-425.
11. Sacco RL, Rundek T. The value of urgent specialized care for TIA and minor stroke. N Engl J Med. 2016;374:1577-1579.
12. Demchuk AM, Menon BK, Goyal M. Comparing vessel imaging: noncontrast computed tomography/computed tomographic angiography should be the new minimum standard in acute disabling stroke. Stroke. 2016;47:273-281.
13. Jauch EC, Saver JL, Adams HP, et al. Guidelines for the early management of patients with acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2013;44:870-947.
14. Gladstone DJ, Spring M, Dorian P, et al. Atrial fibrillation in patients with cryptogenic stroke. N Engl J Med. 2014;370:2467-2477.
15. Sanna T, Diener HC, Passman RS, et al. Cryptogenic stroke and underlying atrial fibrillation. N Engl J Med. 2014;370:2478-2486.
16. Christophersen IE, Yin X, Larson MG, et al. A comparison of the CHARGE-AF and the CHA2DS2-VASc risk scores for prediction of atrial fibrillation ni the Framingham Heart Study. Am Heart J. 2016;178:45-54.
17. Rothwell PM, Giles MF, Chandratheva A, et al. Effect of urgent treatment of transient ischaemic attack and minor stroke on early recurrent stroke (EXPRESS study): a prospective population-based sequential comparison. Lancet. 2007;370:1432-1442.
18. National Institute for Health and Care Excellence. Stroke and transient ischaemic attack in over 16s: diagnosis and initial management. Available at: https://www.nice.org.uk/guidance/cg68. Published 2008. Accessed February 5, 2017.
19. Hart RG, Diener HC, Coutts SB, et al. Embolic strokes of undetermined source: the case for a new clinical construct. Lancet Neurol. 2014;13:429-438.
20. Amarenco P, Lavallée PC, Labreuche J, et al. One-year risk of stroke after transient ischemic attack or minor stroke. N Engl J Med. 2016;374:1533-1542.
21. Joshi JK, Ouyang B, Prabhakaran S. Should TIA patients be hospitalized or referred to a same-day clinic? A decision analysis. Neurology. 2011;77:2082-2088.
22. Mijalski C, Silver B. TIA management: should TIA patients be admitted? should TIA patients get combination antiplatelet therapy? The Neurohospitalist. 2015;5:151-160.
23. Silver B, Adeoye O. Management of patients with transient ischemic attack in the emergency department. Neurology. 2016;86:1568-1569.
24. Demaerschalk BM, Kleindorfer DO, Adeoye OM, et al. Scientific rationale for the inclusion and exclusion criteria for intravenous alteplase in acute ischemic stroke: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2016;47:581-641.
25. Wang Y, Wang Y, Zhao X, et al. Clopidogrel with aspirin in acute minor stroke or transient ischemic attack. N Engl J Med. 2013;369:11-19.
26. Hemphill JC, Greenberg SM, Anderson CS, et al. Guidelines for the management of spontaneous intracerebral hemorrhage. Stroke. 2015;46:2032-2060.
CASE › A 68-year-old woman with hypertension and hyperlipidemia comes into your office for evaluation of a 30-minute episode of sudden-onset right-hand weakness and difficulty speaking that occurred 4 days earlier. The patient, who is also a smoker, has come in at the insistence of her daughter. On examination, her blood pressure (BP) is 145/88 mm Hg and her heart rate is 76 beats/minute and regular. She appears well and her language function is normal. The rest of her examination is normal. How would you proceed?
Stroke—the death of nerve cells due to a lack of blood supply from either infarction or hemorrhage—strikes nearly 800,000 people in the United States every year.1,2 Of these events, 130,000 are fatal, making stroke the fifth leading cause of death.3 Effective, early evaluation and cause-specific treatment are crucial parts of stroke care.
Research has helped to clarify the critical role primary care physicians play in recognizing, triaging, and managing stroke and transient ischemic attacks (TIA). This article reviews what we know about the different ways that a stroke and a TIA can present, the appropriate diagnostic work-up for patients presenting with symptoms of either event, and management strategies for subacute care (24 hours to up to 14 days after a stroke has occurred).4,5 Unless otherwise specified, this review will focus on ischemic stroke because 87% of strokes are attributable to ischemia.1
A follow-up to this article on secondary stroke prevention will appear in the journal next month.
Look to onset more than type of symptoms for clues
Stroke presents as a sudden onset of neurologic deficits (language, motor, sensory, cerebellar, or brainstem functions) (TABLE 14). Because presenting symptoms can vary widely, sudden onset, rather than particular symptoms, should raise a red flag for potential stroke.
The differential diagnosis includes: seizure, complex migraine, medication effect (eg, slurred speech or confusion after taking a central nervous system [CNS] depressant), toxin exposure, electrolyte abnormalities (especially hypoglycemia), concussion/trauma, infection of the CNS, peripheral vertigo, demyelination, intracranial mass, Bell’s palsy, and psychogenic disorders. The history and physical, along with laboratory findings and brain imaging (detailed later in this article), will guide the FP toward (or away from) these various etiologies.
Optimal triage is a subject of ongoing interest and research
If stroke or TIA remains a possibility after an initial assessment, it’s time to stratify patients by risk.
One of the most widely accepted tools is the ABCD2 score (see TABLE 26). Clinicians can employ the ABCD2 risk stratification tool when trying to determine whether it is reasonable to pursue an expedited work-up (ie, <1 day) in the outpatient setting or recommend that the patient be evaluated in an emergency department (ED). The 90-day stroke rate following a TIA ranges from 3% with an ABCD2 score of 0 to 3 to 18% with a score of 6 or 7. A score of 0 to 3 is considered relatively low risk; in the absence of other compelling factors, rapid outpatient evaluation is appropriate. For patients with an ABCD2 score ≥4, referral to the ED or direct admission to the hospital is advised.
The validity of the ABCD2 score for risk stratification has been studied extensively with conflicting results.7-10 As with any assessment tool, it should be used as a guide, and should not supplant a full assessment of the patient or the judgment of the examining physician. In making the decision regarding inpatient or outpatient evaluation, it’s also important to consider available resources, access to specialists, and patient preference.
In a 2016 population-based study, the 30-day recurrent stroke/TIA rate for patients hospitalized for TIA was 3% compared with 10.7% for those discharged from the ED with referral to a stroke clinic and 10.6% for those discharged from the ED without a referral to a stroke clinic.11 These data suggest that only patients for whom you have a low clinical suspicion of stroke/TIA should be worked up as outpatients, and that hospital admission is advised in moderate- and high-risk cases. The findings also highlight the critical role that primary care physicians can play in triaging and managing these patients for secondary prevention.
CASE › This patient’s recent history of sudden-onset right-sided weakness and expressive language dysfunction is suspicious for left hemispheric ischemia. She has several risk factors for stroke, and her ABCD2 score is 5 (hypertension, age ≥60 years, unilateral weakness, and duration 10-59 min), which places her at moderate risk. Thus, the recommendation would be to have her go directly to an ED for rapid evaluation.
The diagnostic work-up
Even when a patient is sent to the ED, the FP plays a critical role in his or her continuing care. FPs will often coordinate with inpatient care and manage transition of care to the outpatient setting. (And in many communities, the ED or hospital physicians may themselves be family practitioners.)
In terms of care, not even an aspirin should be administered in a case like this because the patient has not yet had any neuroimaging, and differentiation of ischemic from hemorrhagic stroke cannot be made on clinical grounds alone. Once an ischemic stroke is confirmed, determining the etiology is critical given the significant management differences between the different types of stroke (atherosclerotic, cardioembolic, lacunar, or other).
Which imaging method, and when?
While a computerized tomography (CT) scan is the preferred initial imaging strategy for acute stroke to discern the ischemic type from the hemorrhagic, MRI is preferred for the evaluation of acute ischemic stroke because the method has a higher sensitivity for infarction and a greater ability to identify findings (such as demyelination) that would suggest an alternative diagnosis.
In addition to evaluating the brain parenchyma, physicians must also assess the cerebral vasculature. CT angiography (CTA) or MR angiography (MRA) of the head and neck are preferred over carotid ultrasound because they are capable of evaluating the entire cerebrovascular system12,13 and can be instrumental in identifying potential causes of stroke, as well as guiding therapeutic decisions. Carotid ultrasound is a reasonable alternative for patients presenting with symptoms indicative of anterior circulation involvement when CTA and MRA are unavailable or contraindicated, but it will not identify intracranial vascular disease, proximal common carotid disease, or vertebrobasilar disease.
Getting to the cause of suspected stroke: Labs and other diagnostic tests
A routine work-up includes BP checks, routine labs (complete blood count, complete metabolic panel, coagulation profile, and troponin), an electrocardiogram (EKG), a transthoracic echocardiogram (TTE) with bubble study if possible, and a minimum of 24 to 48 hours of cardiac rhythm monitoring. Cardiac rhythm monitoring should be extended in the setting of clinical concern for unidentified paroxysmal atrial fibrillation, such as an embolism without a proximal vascular source, multiple embolic infarcts in different vascular territories, a dilated left atrium, or other risk factors for atrial fibrillation that include smoking, systolic hypertension, diabetes, and heart failure (see TABLE 312,13,17,18).14-16 This standard diagnostic work-up will identify the cause of stroke in 70% to 80% of patients.19
Additional investigations to consider if the etiology is not yet elucidated include a transesophageal echocardiogram (TEE), cerebral angiography, a coagulopathy evaluation, a lumbar puncture, and a vasculitis work-up. If available, consultation with a neurologist is appropriate for any patient who has had a stroke or TIA. Patients with unclear etiologies or for whom there are questions concerning strategies for preventing secondary stroke should be referred to Neurology and preferably a stroke specialist.
Timing matters, even when symptoms have resolved (ie, TIA).11,20 The EXPRESS trial17 (the Early use of eXisting PREventive Strategies for Stroke) looked at the effect of urgent assessment and treatment (≤1 day) of patients presenting with a TIA or minor stroke on the risk of recurrent stroke within 90 days. The diagnostic work-up included brain and vascular imaging together with an EKG. This intensive approach led to an absolute risk reduction of 8.2% (from 10.3% to 2.1%) in the risk of recurrent stroke at 90 days (number needed to treat [NNT]=12).17
Expedited work-up and treatment was also recently evaluated in a non-trial, real-world setting and was associated with reducing recurrent stroke by more than half the rate reported in older studies.20 Overall, the data suggest that evaluation within 24 hours confers substantial benefit, and that this evaluation can happen in an outpatient setting.21-23
Acute management: Use of tPA
Once imaging rules out intracranial hemorrhage, patients should be treated with tissue plasminogen activator (tPA) or an endovascular intervention as per guidelines.24 For patients with ischemic stroke ineligible for tPA or endovascular treatments, the initial focus is to determine the etiology of the symptoms so that the best strategies for prevention of secondary stroke may be employed.
Aspirin should be provided within 24 to 48 hours to all patients after intracranial hemorrhage is ruled out. Aspirin should be delayed for 24 hours in those given thrombolytics. The initial recommended dose of aspirin is 325 mg with continued low-dose (81 mg) aspirin daily.13 The addition of clopidogrel to aspirin within 24 hours of an event and continued for 21 days, followed by aspirin alone, was shown to be beneficial in a Chinese population with high-risk TIA (ABCD2 score ≥4) or minor stroke (National Institutes of Health Stroke Scale [NIHSS] ≤3).25 Anticoagulation with heparin, warfarin, or a novel oral anticoagulant is generally not indicated in the acute setting due to the risk of hemorrhagic transformation.
Acute BP management depends upon the type of stroke (ischemic or hemorrhagic), eligibility for thrombolytics, timing of presentation, and possible comorbidities such as myocardial infarction or aortic dissection (see TABLE 413,26). In the absence of contraindications, high-intensity statins should be initiated in all patients able to take oral medications.
CASE › You appropriately referred your patient to the local ED. A head CT with head and neck CTA was performed. While the head CT did not show any abnormalities, the CTA demonstrated high-grade left internal carotid artery stenosis. The patient was given an initial dose of aspirin 325 mg and a high-intensity statin and admitted for further management. An MRI revealed a small shower of emboli in the left hemisphere, confirming the diagnosis of stroke over TIA. Labs were marginally remarkable with a low-density lipoprotein level of 115 mg/dL and an HbA1c of 6.2. Telemetry monitoring did not reveal any arrhythmias, and TTE was normal. BP remained in the high-normal to low-hypertensive range.
A Vascular Surgery consultation was obtained and the patient underwent a left carotid endarterectomy the following day. She did well without surgical complications. Her BP medications were adjusted; a combination of an angiotensin-converting enzyme inhibitor and a thiazide diuretic achieved a goal BP <140/90 mm Hg.
Permissive hypertension was not indicated due to her presentation >48 hours beyond the acute event. Low-dose aspirin and a high-intensity statin were continued, for secondary stroke prevention in the setting of atherosclerotic disease. She received smoking cessation counseling, which will continue.
CORRESPONDENCE
Stephen A. Martin, MD, EdM, Barre Family Health Center, 151 Worcester Road, Barre, MA 01005; stmartin@gmail.com.
CASE › A 68-year-old woman with hypertension and hyperlipidemia comes into your office for evaluation of a 30-minute episode of sudden-onset right-hand weakness and difficulty speaking that occurred 4 days earlier. The patient, who is also a smoker, has come in at the insistence of her daughter. On examination, her blood pressure (BP) is 145/88 mm Hg and her heart rate is 76 beats/minute and regular. She appears well and her language function is normal. The rest of her examination is normal. How would you proceed?
Stroke—the death of nerve cells due to a lack of blood supply from either infarction or hemorrhage—strikes nearly 800,000 people in the United States every year.1,2 Of these events, 130,000 are fatal, making stroke the fifth leading cause of death.3 Effective, early evaluation and cause-specific treatment are crucial parts of stroke care.
Research has helped to clarify the critical role primary care physicians play in recognizing, triaging, and managing stroke and transient ischemic attacks (TIA). This article reviews what we know about the different ways that a stroke and a TIA can present, the appropriate diagnostic work-up for patients presenting with symptoms of either event, and management strategies for subacute care (24 hours to up to 14 days after a stroke has occurred).4,5 Unless otherwise specified, this review will focus on ischemic stroke because 87% of strokes are attributable to ischemia.1
A follow-up to this article on secondary stroke prevention will appear in the journal next month.
Look to onset more than type of symptoms for clues
Stroke presents as a sudden onset of neurologic deficits (language, motor, sensory, cerebellar, or brainstem functions) (TABLE 14). Because presenting symptoms can vary widely, sudden onset, rather than particular symptoms, should raise a red flag for potential stroke.
The differential diagnosis includes: seizure, complex migraine, medication effect (eg, slurred speech or confusion after taking a central nervous system [CNS] depressant), toxin exposure, electrolyte abnormalities (especially hypoglycemia), concussion/trauma, infection of the CNS, peripheral vertigo, demyelination, intracranial mass, Bell’s palsy, and psychogenic disorders. The history and physical, along with laboratory findings and brain imaging (detailed later in this article), will guide the FP toward (or away from) these various etiologies.
Optimal triage is a subject of ongoing interest and research
If stroke or TIA remains a possibility after an initial assessment, it’s time to stratify patients by risk.
One of the most widely accepted tools is the ABCD2 score (see TABLE 26). Clinicians can employ the ABCD2 risk stratification tool when trying to determine whether it is reasonable to pursue an expedited work-up (ie, <1 day) in the outpatient setting or recommend that the patient be evaluated in an emergency department (ED). The 90-day stroke rate following a TIA ranges from 3% with an ABCD2 score of 0 to 3 to 18% with a score of 6 or 7. A score of 0 to 3 is considered relatively low risk; in the absence of other compelling factors, rapid outpatient evaluation is appropriate. For patients with an ABCD2 score ≥4, referral to the ED or direct admission to the hospital is advised.
The validity of the ABCD2 score for risk stratification has been studied extensively with conflicting results.7-10 As with any assessment tool, it should be used as a guide, and should not supplant a full assessment of the patient or the judgment of the examining physician. In making the decision regarding inpatient or outpatient evaluation, it’s also important to consider available resources, access to specialists, and patient preference.
In a 2016 population-based study, the 30-day recurrent stroke/TIA rate for patients hospitalized for TIA was 3% compared with 10.7% for those discharged from the ED with referral to a stroke clinic and 10.6% for those discharged from the ED without a referral to a stroke clinic.11 These data suggest that only patients for whom you have a low clinical suspicion of stroke/TIA should be worked up as outpatients, and that hospital admission is advised in moderate- and high-risk cases. The findings also highlight the critical role that primary care physicians can play in triaging and managing these patients for secondary prevention.
CASE › This patient’s recent history of sudden-onset right-sided weakness and expressive language dysfunction is suspicious for left hemispheric ischemia. She has several risk factors for stroke, and her ABCD2 score is 5 (hypertension, age ≥60 years, unilateral weakness, and duration 10-59 min), which places her at moderate risk. Thus, the recommendation would be to have her go directly to an ED for rapid evaluation.
The diagnostic work-up
Even when a patient is sent to the ED, the FP plays a critical role in his or her continuing care. FPs will often coordinate with inpatient care and manage transition of care to the outpatient setting. (And in many communities, the ED or hospital physicians may themselves be family practitioners.)
In terms of care, not even an aspirin should be administered in a case like this because the patient has not yet had any neuroimaging, and differentiation of ischemic from hemorrhagic stroke cannot be made on clinical grounds alone. Once an ischemic stroke is confirmed, determining the etiology is critical given the significant management differences between the different types of stroke (atherosclerotic, cardioembolic, lacunar, or other).
Which imaging method, and when?
While a computerized tomography (CT) scan is the preferred initial imaging strategy for acute stroke to discern the ischemic type from the hemorrhagic, MRI is preferred for the evaluation of acute ischemic stroke because the method has a higher sensitivity for infarction and a greater ability to identify findings (such as demyelination) that would suggest an alternative diagnosis.
In addition to evaluating the brain parenchyma, physicians must also assess the cerebral vasculature. CT angiography (CTA) or MR angiography (MRA) of the head and neck are preferred over carotid ultrasound because they are capable of evaluating the entire cerebrovascular system12,13 and can be instrumental in identifying potential causes of stroke, as well as guiding therapeutic decisions. Carotid ultrasound is a reasonable alternative for patients presenting with symptoms indicative of anterior circulation involvement when CTA and MRA are unavailable or contraindicated, but it will not identify intracranial vascular disease, proximal common carotid disease, or vertebrobasilar disease.
Getting to the cause of suspected stroke: Labs and other diagnostic tests
A routine work-up includes BP checks, routine labs (complete blood count, complete metabolic panel, coagulation profile, and troponin), an electrocardiogram (EKG), a transthoracic echocardiogram (TTE) with bubble study if possible, and a minimum of 24 to 48 hours of cardiac rhythm monitoring. Cardiac rhythm monitoring should be extended in the setting of clinical concern for unidentified paroxysmal atrial fibrillation, such as an embolism without a proximal vascular source, multiple embolic infarcts in different vascular territories, a dilated left atrium, or other risk factors for atrial fibrillation that include smoking, systolic hypertension, diabetes, and heart failure (see TABLE 312,13,17,18).14-16 This standard diagnostic work-up will identify the cause of stroke in 70% to 80% of patients.19
Additional investigations to consider if the etiology is not yet elucidated include a transesophageal echocardiogram (TEE), cerebral angiography, a coagulopathy evaluation, a lumbar puncture, and a vasculitis work-up. If available, consultation with a neurologist is appropriate for any patient who has had a stroke or TIA. Patients with unclear etiologies or for whom there are questions concerning strategies for preventing secondary stroke should be referred to Neurology and preferably a stroke specialist.
Timing matters, even when symptoms have resolved (ie, TIA).11,20 The EXPRESS trial17 (the Early use of eXisting PREventive Strategies for Stroke) looked at the effect of urgent assessment and treatment (≤1 day) of patients presenting with a TIA or minor stroke on the risk of recurrent stroke within 90 days. The diagnostic work-up included brain and vascular imaging together with an EKG. This intensive approach led to an absolute risk reduction of 8.2% (from 10.3% to 2.1%) in the risk of recurrent stroke at 90 days (number needed to treat [NNT]=12).17
Expedited work-up and treatment was also recently evaluated in a non-trial, real-world setting and was associated with reducing recurrent stroke by more than half the rate reported in older studies.20 Overall, the data suggest that evaluation within 24 hours confers substantial benefit, and that this evaluation can happen in an outpatient setting.21-23
Acute management: Use of tPA
Once imaging rules out intracranial hemorrhage, patients should be treated with tissue plasminogen activator (tPA) or an endovascular intervention as per guidelines.24 For patients with ischemic stroke ineligible for tPA or endovascular treatments, the initial focus is to determine the etiology of the symptoms so that the best strategies for prevention of secondary stroke may be employed.
Aspirin should be provided within 24 to 48 hours to all patients after intracranial hemorrhage is ruled out. Aspirin should be delayed for 24 hours in those given thrombolytics. The initial recommended dose of aspirin is 325 mg with continued low-dose (81 mg) aspirin daily.13 The addition of clopidogrel to aspirin within 24 hours of an event and continued for 21 days, followed by aspirin alone, was shown to be beneficial in a Chinese population with high-risk TIA (ABCD2 score ≥4) or minor stroke (National Institutes of Health Stroke Scale [NIHSS] ≤3).25 Anticoagulation with heparin, warfarin, or a novel oral anticoagulant is generally not indicated in the acute setting due to the risk of hemorrhagic transformation.
Acute BP management depends upon the type of stroke (ischemic or hemorrhagic), eligibility for thrombolytics, timing of presentation, and possible comorbidities such as myocardial infarction or aortic dissection (see TABLE 413,26). In the absence of contraindications, high-intensity statins should be initiated in all patients able to take oral medications.
CASE › You appropriately referred your patient to the local ED. A head CT with head and neck CTA was performed. While the head CT did not show any abnormalities, the CTA demonstrated high-grade left internal carotid artery stenosis. The patient was given an initial dose of aspirin 325 mg and a high-intensity statin and admitted for further management. An MRI revealed a small shower of emboli in the left hemisphere, confirming the diagnosis of stroke over TIA. Labs were marginally remarkable with a low-density lipoprotein level of 115 mg/dL and an HbA1c of 6.2. Telemetry monitoring did not reveal any arrhythmias, and TTE was normal. BP remained in the high-normal to low-hypertensive range.
A Vascular Surgery consultation was obtained and the patient underwent a left carotid endarterectomy the following day. She did well without surgical complications. Her BP medications were adjusted; a combination of an angiotensin-converting enzyme inhibitor and a thiazide diuretic achieved a goal BP <140/90 mm Hg.
Permissive hypertension was not indicated due to her presentation >48 hours beyond the acute event. Low-dose aspirin and a high-intensity statin were continued, for secondary stroke prevention in the setting of atherosclerotic disease. She received smoking cessation counseling, which will continue.
CORRESPONDENCE
Stephen A. Martin, MD, EdM, Barre Family Health Center, 151 Worcester Road, Barre, MA 01005; stmartin@gmail.com.
1. Benjamin EJ, Blaha MJ, Chiuve SE, et al. Heart disease and stroke statistics-2017 update: a report from the American Heart Association. Circulation. 2017;135:e146-e603.
2. Sacco RL, Kasner SE, Broderick JP, et al. An updated definition of stroke for the 21st century: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2013;44:2064-2089.
3. Kochanek KD, Murphy SL, Xu J, et al. Mortality in the United States, 2013. NCHS Data Brief. 2014:1-8. Available at: https://www.cdc.gov/nchs/data/databriefs/db178.pdf. Accessed June 5, 2016.
4. Flossmann E, Redgrave JN, Briley D, et al. Reliability of clinical diagnosis of the symptomatic vascular territory in patients with recent transient ischemic attack or minor stroke. Stroke. 2008;39:2457-2460.
5. Josephson SA, Sidney S, Pham TN, et al. Higher ABCD2 score predicts patients most likely to have true transient ischemic attack. Stroke. 2008;39:3096-3098. Available at: http://www.ncbi.nlm.nih.gov/pubmed/18688003. Accessed June 5, 2016.
6. Hankey GJ. The ABCD, California, and unified ABCD2 risk scores predicted stroke within 2, 7, and 90 days after TIA. Evid Based Med. 2007;12:88.
7. Sheehan OC, Kyne L, Kelly LA, et al. Population-based study of ABCD2 score, carotid stenosis, and atrial fibrillation for early stroke prediction after transient ischemic attack: the North Dublin TIA study. Stroke. 2010;41:844-850.
8. Rothwell PM, Giles MF, Flossmann E, et al. A simple score (ABCD) to identify individuals at high early risk of stroke after transient ischaemic attack. Lancet. 2005; 366:29-36.
9. Tsivgoulis G, Spengos K, Manta P, et al. Validation of the ABCD score in identifying individuals at high early risk of stroke after a transient ischemic attack: a hospital-based case series study. Stroke. 2006;37:2892-2897.
10. Kiyohara T, Kamouchi M, Kumai Y, et al. ABCD3 and ABCD3-I scores are superior to ABCD2 score in the prediction of short- and long-term risks of stroke after transient ischemic attack. Stroke. 2014;45:418-425.
11. Sacco RL, Rundek T. The value of urgent specialized care for TIA and minor stroke. N Engl J Med. 2016;374:1577-1579.
12. Demchuk AM, Menon BK, Goyal M. Comparing vessel imaging: noncontrast computed tomography/computed tomographic angiography should be the new minimum standard in acute disabling stroke. Stroke. 2016;47:273-281.
13. Jauch EC, Saver JL, Adams HP, et al. Guidelines for the early management of patients with acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2013;44:870-947.
14. Gladstone DJ, Spring M, Dorian P, et al. Atrial fibrillation in patients with cryptogenic stroke. N Engl J Med. 2014;370:2467-2477.
15. Sanna T, Diener HC, Passman RS, et al. Cryptogenic stroke and underlying atrial fibrillation. N Engl J Med. 2014;370:2478-2486.
16. Christophersen IE, Yin X, Larson MG, et al. A comparison of the CHARGE-AF and the CHA2DS2-VASc risk scores for prediction of atrial fibrillation ni the Framingham Heart Study. Am Heart J. 2016;178:45-54.
17. Rothwell PM, Giles MF, Chandratheva A, et al. Effect of urgent treatment of transient ischaemic attack and minor stroke on early recurrent stroke (EXPRESS study): a prospective population-based sequential comparison. Lancet. 2007;370:1432-1442.
18. National Institute for Health and Care Excellence. Stroke and transient ischaemic attack in over 16s: diagnosis and initial management. Available at: https://www.nice.org.uk/guidance/cg68. Published 2008. Accessed February 5, 2017.
19. Hart RG, Diener HC, Coutts SB, et al. Embolic strokes of undetermined source: the case for a new clinical construct. Lancet Neurol. 2014;13:429-438.
20. Amarenco P, Lavallée PC, Labreuche J, et al. One-year risk of stroke after transient ischemic attack or minor stroke. N Engl J Med. 2016;374:1533-1542.
21. Joshi JK, Ouyang B, Prabhakaran S. Should TIA patients be hospitalized or referred to a same-day clinic? A decision analysis. Neurology. 2011;77:2082-2088.
22. Mijalski C, Silver B. TIA management: should TIA patients be admitted? should TIA patients get combination antiplatelet therapy? The Neurohospitalist. 2015;5:151-160.
23. Silver B, Adeoye O. Management of patients with transient ischemic attack in the emergency department. Neurology. 2016;86:1568-1569.
24. Demaerschalk BM, Kleindorfer DO, Adeoye OM, et al. Scientific rationale for the inclusion and exclusion criteria for intravenous alteplase in acute ischemic stroke: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2016;47:581-641.
25. Wang Y, Wang Y, Zhao X, et al. Clopidogrel with aspirin in acute minor stroke or transient ischemic attack. N Engl J Med. 2013;369:11-19.
26. Hemphill JC, Greenberg SM, Anderson CS, et al. Guidelines for the management of spontaneous intracerebral hemorrhage. Stroke. 2015;46:2032-2060.
1. Benjamin EJ, Blaha MJ, Chiuve SE, et al. Heart disease and stroke statistics-2017 update: a report from the American Heart Association. Circulation. 2017;135:e146-e603.
2. Sacco RL, Kasner SE, Broderick JP, et al. An updated definition of stroke for the 21st century: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2013;44:2064-2089.
3. Kochanek KD, Murphy SL, Xu J, et al. Mortality in the United States, 2013. NCHS Data Brief. 2014:1-8. Available at: https://www.cdc.gov/nchs/data/databriefs/db178.pdf. Accessed June 5, 2016.
4. Flossmann E, Redgrave JN, Briley D, et al. Reliability of clinical diagnosis of the symptomatic vascular territory in patients with recent transient ischemic attack or minor stroke. Stroke. 2008;39:2457-2460.
5. Josephson SA, Sidney S, Pham TN, et al. Higher ABCD2 score predicts patients most likely to have true transient ischemic attack. Stroke. 2008;39:3096-3098. Available at: http://www.ncbi.nlm.nih.gov/pubmed/18688003. Accessed June 5, 2016.
6. Hankey GJ. The ABCD, California, and unified ABCD2 risk scores predicted stroke within 2, 7, and 90 days after TIA. Evid Based Med. 2007;12:88.
7. Sheehan OC, Kyne L, Kelly LA, et al. Population-based study of ABCD2 score, carotid stenosis, and atrial fibrillation for early stroke prediction after transient ischemic attack: the North Dublin TIA study. Stroke. 2010;41:844-850.
8. Rothwell PM, Giles MF, Flossmann E, et al. A simple score (ABCD) to identify individuals at high early risk of stroke after transient ischaemic attack. Lancet. 2005; 366:29-36.
9. Tsivgoulis G, Spengos K, Manta P, et al. Validation of the ABCD score in identifying individuals at high early risk of stroke after a transient ischemic attack: a hospital-based case series study. Stroke. 2006;37:2892-2897.
10. Kiyohara T, Kamouchi M, Kumai Y, et al. ABCD3 and ABCD3-I scores are superior to ABCD2 score in the prediction of short- and long-term risks of stroke after transient ischemic attack. Stroke. 2014;45:418-425.
11. Sacco RL, Rundek T. The value of urgent specialized care for TIA and minor stroke. N Engl J Med. 2016;374:1577-1579.
12. Demchuk AM, Menon BK, Goyal M. Comparing vessel imaging: noncontrast computed tomography/computed tomographic angiography should be the new minimum standard in acute disabling stroke. Stroke. 2016;47:273-281.
13. Jauch EC, Saver JL, Adams HP, et al. Guidelines for the early management of patients with acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2013;44:870-947.
14. Gladstone DJ, Spring M, Dorian P, et al. Atrial fibrillation in patients with cryptogenic stroke. N Engl J Med. 2014;370:2467-2477.
15. Sanna T, Diener HC, Passman RS, et al. Cryptogenic stroke and underlying atrial fibrillation. N Engl J Med. 2014;370:2478-2486.
16. Christophersen IE, Yin X, Larson MG, et al. A comparison of the CHARGE-AF and the CHA2DS2-VASc risk scores for prediction of atrial fibrillation ni the Framingham Heart Study. Am Heart J. 2016;178:45-54.
17. Rothwell PM, Giles MF, Chandratheva A, et al. Effect of urgent treatment of transient ischaemic attack and minor stroke on early recurrent stroke (EXPRESS study): a prospective population-based sequential comparison. Lancet. 2007;370:1432-1442.
18. National Institute for Health and Care Excellence. Stroke and transient ischaemic attack in over 16s: diagnosis and initial management. Available at: https://www.nice.org.uk/guidance/cg68. Published 2008. Accessed February 5, 2017.
19. Hart RG, Diener HC, Coutts SB, et al. Embolic strokes of undetermined source: the case for a new clinical construct. Lancet Neurol. 2014;13:429-438.
20. Amarenco P, Lavallée PC, Labreuche J, et al. One-year risk of stroke after transient ischemic attack or minor stroke. N Engl J Med. 2016;374:1533-1542.
21. Joshi JK, Ouyang B, Prabhakaran S. Should TIA patients be hospitalized or referred to a same-day clinic? A decision analysis. Neurology. 2011;77:2082-2088.
22. Mijalski C, Silver B. TIA management: should TIA patients be admitted? should TIA patients get combination antiplatelet therapy? The Neurohospitalist. 2015;5:151-160.
23. Silver B, Adeoye O. Management of patients with transient ischemic attack in the emergency department. Neurology. 2016;86:1568-1569.
24. Demaerschalk BM, Kleindorfer DO, Adeoye OM, et al. Scientific rationale for the inclusion and exclusion criteria for intravenous alteplase in acute ischemic stroke: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2016;47:581-641.
25. Wang Y, Wang Y, Zhao X, et al. Clopidogrel with aspirin in acute minor stroke or transient ischemic attack. N Engl J Med. 2013;369:11-19.
26. Hemphill JC, Greenberg SM, Anderson CS, et al. Guidelines for the management of spontaneous intracerebral hemorrhage. Stroke. 2015;46:2032-2060.
PRACTICE RECOMMENDATIONS
› Perform an urgent work-up on patients presenting with symptoms of a transient ischemic attack or stroke. A
› Employ the ABCD2 risk stratification tool when determining whether it is reasonable to pursue an expedited work-up in the outpatient setting or recommend that a patient be evaluated in an emergency department. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
EMA recommends orphan designation for hemophilia B product
The European Medicines Agency’s (EMA’s) Committee for Orphan Medicinal Products has issued a positive opinion recommending orphan designation for CB 2679d/ISU304 for the treatment of hemophilia B.
CB 2679d is a coagulation factor IX variant that has demonstrated, in preclinical studies, the potential to normalize factor IX levels via a daily subcutaneous injection.
The product is being developed by Catalyst Biosciences and ISU Abxis. ISU Abxis plans to initiate a phase 1/2 study of CB 2679d in individuals with severe hemophilia B this month in South Korea.
Orphan designation provides regulatory and financial incentives for companies to develop and market therapies that treat life-threatening or chronically debilitating conditions affecting no more than 5 in 10,000 people in the European Union, and where no satisfactory treatment is available.
Orphan designation provides a 10-year period of marketing exclusivity if the drug receives regulatory approval.
The designation also provides incentives for companies seeking protocol assistance from the EMA during the product development phase and direct access to the centralized authorization procedure.
The EMA’s Committee for Orphan Medicinal Products adopts an opinion on the granting of orphan drug designation, and that opinion is submitted to the European Commission for a final decision. The commission typically makes a decision within 30 days of the submission. ![]()
The European Medicines Agency’s (EMA’s) Committee for Orphan Medicinal Products has issued a positive opinion recommending orphan designation for CB 2679d/ISU304 for the treatment of hemophilia B.
CB 2679d is a coagulation factor IX variant that has demonstrated, in preclinical studies, the potential to normalize factor IX levels via a daily subcutaneous injection.
The product is being developed by Catalyst Biosciences and ISU Abxis. ISU Abxis plans to initiate a phase 1/2 study of CB 2679d in individuals with severe hemophilia B this month in South Korea.
Orphan designation provides regulatory and financial incentives for companies to develop and market therapies that treat life-threatening or chronically debilitating conditions affecting no more than 5 in 10,000 people in the European Union, and where no satisfactory treatment is available.
Orphan designation provides a 10-year period of marketing exclusivity if the drug receives regulatory approval.
The designation also provides incentives for companies seeking protocol assistance from the EMA during the product development phase and direct access to the centralized authorization procedure.
The EMA’s Committee for Orphan Medicinal Products adopts an opinion on the granting of orphan drug designation, and that opinion is submitted to the European Commission for a final decision. The commission typically makes a decision within 30 days of the submission. ![]()
The European Medicines Agency’s (EMA’s) Committee for Orphan Medicinal Products has issued a positive opinion recommending orphan designation for CB 2679d/ISU304 for the treatment of hemophilia B.
CB 2679d is a coagulation factor IX variant that has demonstrated, in preclinical studies, the potential to normalize factor IX levels via a daily subcutaneous injection.
The product is being developed by Catalyst Biosciences and ISU Abxis. ISU Abxis plans to initiate a phase 1/2 study of CB 2679d in individuals with severe hemophilia B this month in South Korea.
Orphan designation provides regulatory and financial incentives for companies to develop and market therapies that treat life-threatening or chronically debilitating conditions affecting no more than 5 in 10,000 people in the European Union, and where no satisfactory treatment is available.
Orphan designation provides a 10-year period of marketing exclusivity if the drug receives regulatory approval.
The designation also provides incentives for companies seeking protocol assistance from the EMA during the product development phase and direct access to the centralized authorization procedure.
The EMA’s Committee for Orphan Medicinal Products adopts an opinion on the granting of orphan drug designation, and that opinion is submitted to the European Commission for a final decision. The commission typically makes a decision within 30 days of the submission. ![]()
Nodules on nose and tattoos
Based on the clinical presentation and skin biopsy results, the patient was given a diagnosis of cutaneous sarcoidosis. The biopsy from the right side of his nose demonstrated sarcoidal granulomas. A biopsy of one of the tattoo nodules showed sarcoidal granulomas, and close inspection revealed red tattoo pigment within the granulomatous inflammation. X-rays showed bilateral hilar lymphadenopathy, which was consistent with pulmonary sarcoidosis, and the lace-like appearance of the middle and distal phalanges was consistent with skeletal sarcoidosis.
Systemic sarcoidosis is an idiopathic, granulomatous disease that affects multiple organ systems, but primarily the lungs and lymphatic system. Cutaneous sarcoidosis can occur as a manifestation of systemic sarcoidosis. It may present as asymptomatic red or skin-colored papules and firm nodules within tattoos, old scars, or permanent makeup. Sarcoidosis usually occurs in red, black, or blue-black areas of tattoos, in which the pigment acts as a nidus for granuloma formation.
The first-line treatment for limited papules is a high-potency topical corticosteroid (eg, clobetasol 0.05% ointment applied twice weekly) and an intralesional corticosteroid (eg, triamcinolone, one 5-10 mg/mL injection every 4 weeks). Antimalarials such as hydroxychloroquine or methotrexate can also be helpful. Midpotency topical corticosteroids such as triamcinolone 0.1% cream and doxycycline hyclate have been reported to clear cutaneous lesions in tattoos. Oral corticosteroids are often effective for severe cutaneous sarcoidosis, but their multiple adverse effects (eg, diabetes and adrenal suppression) prevent prolonged use except in very low doses in conjunction with other therapies.
The nodules on this patient’s nose were successfully treated with intralesional triamcinolone 5 mg/mL. No treatment was initiated for the tattoo nodules because they were asymptomatic and the patient wasn’t bothered by their appearance. The patient’s hand swelling improved with a treatment of prednisone 10 mg/d. The rheumatologist considered a steroid-sparing immunosuppressive agent such as methotrexate; however, the patient was lost to follow-up.
Adapted from: Zhang J, Jansen R, Lim HW. Nodules on nose and tattoos. J Fam Pract. 2015;64:241-243.
Based on the clinical presentation and skin biopsy results, the patient was given a diagnosis of cutaneous sarcoidosis. The biopsy from the right side of his nose demonstrated sarcoidal granulomas. A biopsy of one of the tattoo nodules showed sarcoidal granulomas, and close inspection revealed red tattoo pigment within the granulomatous inflammation. X-rays showed bilateral hilar lymphadenopathy, which was consistent with pulmonary sarcoidosis, and the lace-like appearance of the middle and distal phalanges was consistent with skeletal sarcoidosis.
Systemic sarcoidosis is an idiopathic, granulomatous disease that affects multiple organ systems, but primarily the lungs and lymphatic system. Cutaneous sarcoidosis can occur as a manifestation of systemic sarcoidosis. It may present as asymptomatic red or skin-colored papules and firm nodules within tattoos, old scars, or permanent makeup. Sarcoidosis usually occurs in red, black, or blue-black areas of tattoos, in which the pigment acts as a nidus for granuloma formation.
The first-line treatment for limited papules is a high-potency topical corticosteroid (eg, clobetasol 0.05% ointment applied twice weekly) and an intralesional corticosteroid (eg, triamcinolone, one 5-10 mg/mL injection every 4 weeks). Antimalarials such as hydroxychloroquine or methotrexate can also be helpful. Midpotency topical corticosteroids such as triamcinolone 0.1% cream and doxycycline hyclate have been reported to clear cutaneous lesions in tattoos. Oral corticosteroids are often effective for severe cutaneous sarcoidosis, but their multiple adverse effects (eg, diabetes and adrenal suppression) prevent prolonged use except in very low doses in conjunction with other therapies.
The nodules on this patient’s nose were successfully treated with intralesional triamcinolone 5 mg/mL. No treatment was initiated for the tattoo nodules because they were asymptomatic and the patient wasn’t bothered by their appearance. The patient’s hand swelling improved with a treatment of prednisone 10 mg/d. The rheumatologist considered a steroid-sparing immunosuppressive agent such as methotrexate; however, the patient was lost to follow-up.
Adapted from: Zhang J, Jansen R, Lim HW. Nodules on nose and tattoos. J Fam Pract. 2015;64:241-243.
Based on the clinical presentation and skin biopsy results, the patient was given a diagnosis of cutaneous sarcoidosis. The biopsy from the right side of his nose demonstrated sarcoidal granulomas. A biopsy of one of the tattoo nodules showed sarcoidal granulomas, and close inspection revealed red tattoo pigment within the granulomatous inflammation. X-rays showed bilateral hilar lymphadenopathy, which was consistent with pulmonary sarcoidosis, and the lace-like appearance of the middle and distal phalanges was consistent with skeletal sarcoidosis.
Systemic sarcoidosis is an idiopathic, granulomatous disease that affects multiple organ systems, but primarily the lungs and lymphatic system. Cutaneous sarcoidosis can occur as a manifestation of systemic sarcoidosis. It may present as asymptomatic red or skin-colored papules and firm nodules within tattoos, old scars, or permanent makeup. Sarcoidosis usually occurs in red, black, or blue-black areas of tattoos, in which the pigment acts as a nidus for granuloma formation.
The first-line treatment for limited papules is a high-potency topical corticosteroid (eg, clobetasol 0.05% ointment applied twice weekly) and an intralesional corticosteroid (eg, triamcinolone, one 5-10 mg/mL injection every 4 weeks). Antimalarials such as hydroxychloroquine or methotrexate can also be helpful. Midpotency topical corticosteroids such as triamcinolone 0.1% cream and doxycycline hyclate have been reported to clear cutaneous lesions in tattoos. Oral corticosteroids are often effective for severe cutaneous sarcoidosis, but their multiple adverse effects (eg, diabetes and adrenal suppression) prevent prolonged use except in very low doses in conjunction with other therapies.
The nodules on this patient’s nose were successfully treated with intralesional triamcinolone 5 mg/mL. No treatment was initiated for the tattoo nodules because they were asymptomatic and the patient wasn’t bothered by their appearance. The patient’s hand swelling improved with a treatment of prednisone 10 mg/d. The rheumatologist considered a steroid-sparing immunosuppressive agent such as methotrexate; however, the patient was lost to follow-up.
Adapted from: Zhang J, Jansen R, Lim HW. Nodules on nose and tattoos. J Fam Pract. 2015;64:241-243.
Do oral decongestants have a clinically significant effect on BP in patients with hypertension?
EVIDENCE SUMMARY
A meta-analysis of 24 RCTs examined the effect of pseudoephedrine on BP and heart rate.1 Just 5 of the 24 studies specifically included hypertensive patients. In the population of patients with hypertension, the meta-analysis showed a small (1.2 mm Hg) rise in systolic BP with pseudoephedrine that was statistically significant (95% confidence interval [CI], 0.56-1.84 mm Hg), but the slight changes in diastolic BP and heart rate were not significant. No patient-oriented outcomes were measured.
The highest quality study within this group was a randomized, double-blind, placebo-controlled crossover study with 28 patients given sustained-release pseudoephedrine 120 mg twice daily for 72 hours, with BP measurements taken at 48 and 72 hours.2 The study was powered to identify an increase in systolic BP of 11 mm Hg, but the results showed just a 3.1 mm Hg rise in systolic BP at 48 hours (see TABLE1-7 for CI and other data).
In another double-blind, placebo-controlled RCT of 29 adults with hypertension (only 25 were included in the data analysis), there was no significant elevation in BP when oral pseudoephedrine was administered over the course of 3 days.3
Across the 5 studies in the meta-analysis, immediate-release and sustained-release forms of pseudoephedrine were included, hypertension was described as controlled but definitions of control were not always specified, and study length varied from 2 hours to 4 weeks.2-6 Patients on antihypertensive medications were included in some of the studies; patients who had active cardiovascular disease, peripheral vascular disease, and/or cerebrovascular disease were excluded.
One study specifically looked at the effects of a single dose of pseudoephedrine on BP in patients treated with 2 different beta-blockers and found no significant change from baseline, but this study was not powered to show differences less than 5 mm Hg.6 The study did show a change of 1 to 2 mm Hg in systolic BP, but this was not statistically significant.
An absence of information on older patients
There is a paucity of literature on treating older adults and medically complex patients (eg, those with uncontrolled or secondary causes of hypertension, cerebrovascular disease, coronary artery disease) with decongestants, as they were excluded in all studies. And the available evidence does not include reports of adverse events other than changes in BP.
1. Salerno SM, Jackson JL, Berbano EP. Effect of oral pseudoephedrine on blood pressure and heart rate. Arch Intern Med. 2005;165:1686-1694.
2. Beck RA, Mercado DL, Seguin SM, et al. Cardiovascular effects of pseudoephedrine in medically controlled hypertensive patients. Arch Int Med. 1992;152:1242-1245.
3. Bradley JG, Kallail KJ, Dorsch JN, et al. The effects of pseudoephedrine on blood pressure in patients with controlled, uncomplicated hypertension: a randomized, double-blind, placebo-controlled trial. J Am Board Fam Pract. 1991;4:201-206.
4. Chua SS, Benrimoj SI, Gordon RD, et al. A controlled clinical trial on the cardiovascular effects of single doses of pseudoephedrine in hypertensive patients. Br J Clin Pharmacol. 1989;28:369-372.
5. Coates ML, Rembold CM, Farr BM. Does pseudoephedrine increase blood pressure in patients with controlled hypertension? J Fam Pract. 1995;40:22-26.
6. Mores N, Campia U, Navarra P, et al. No cardiovascular effects of single-dose pseudoephedrine in patients with essential hypertension treated with beta-blockers. Eur J Clin Pharmacol. 1999;55:251-254.
7. Salerno SM, Jackson JL, Berbano EP. The impact of oral phenylpropanolamine on blood pressure: a meta-analysis and review of the literature. J Hum Hypertens. 2005;19:643-652.
EVIDENCE SUMMARY
A meta-analysis of 24 RCTs examined the effect of pseudoephedrine on BP and heart rate.1 Just 5 of the 24 studies specifically included hypertensive patients. In the population of patients with hypertension, the meta-analysis showed a small (1.2 mm Hg) rise in systolic BP with pseudoephedrine that was statistically significant (95% confidence interval [CI], 0.56-1.84 mm Hg), but the slight changes in diastolic BP and heart rate were not significant. No patient-oriented outcomes were measured.
The highest quality study within this group was a randomized, double-blind, placebo-controlled crossover study with 28 patients given sustained-release pseudoephedrine 120 mg twice daily for 72 hours, with BP measurements taken at 48 and 72 hours.2 The study was powered to identify an increase in systolic BP of 11 mm Hg, but the results showed just a 3.1 mm Hg rise in systolic BP at 48 hours (see TABLE1-7 for CI and other data).
In another double-blind, placebo-controlled RCT of 29 adults with hypertension (only 25 were included in the data analysis), there was no significant elevation in BP when oral pseudoephedrine was administered over the course of 3 days.3
Across the 5 studies in the meta-analysis, immediate-release and sustained-release forms of pseudoephedrine were included, hypertension was described as controlled but definitions of control were not always specified, and study length varied from 2 hours to 4 weeks.2-6 Patients on antihypertensive medications were included in some of the studies; patients who had active cardiovascular disease, peripheral vascular disease, and/or cerebrovascular disease were excluded.
One study specifically looked at the effects of a single dose of pseudoephedrine on BP in patients treated with 2 different beta-blockers and found no significant change from baseline, but this study was not powered to show differences less than 5 mm Hg.6 The study did show a change of 1 to 2 mm Hg in systolic BP, but this was not statistically significant.
An absence of information on older patients
There is a paucity of literature on treating older adults and medically complex patients (eg, those with uncontrolled or secondary causes of hypertension, cerebrovascular disease, coronary artery disease) with decongestants, as they were excluded in all studies. And the available evidence does not include reports of adverse events other than changes in BP.
EVIDENCE SUMMARY
A meta-analysis of 24 RCTs examined the effect of pseudoephedrine on BP and heart rate.1 Just 5 of the 24 studies specifically included hypertensive patients. In the population of patients with hypertension, the meta-analysis showed a small (1.2 mm Hg) rise in systolic BP with pseudoephedrine that was statistically significant (95% confidence interval [CI], 0.56-1.84 mm Hg), but the slight changes in diastolic BP and heart rate were not significant. No patient-oriented outcomes were measured.
The highest quality study within this group was a randomized, double-blind, placebo-controlled crossover study with 28 patients given sustained-release pseudoephedrine 120 mg twice daily for 72 hours, with BP measurements taken at 48 and 72 hours.2 The study was powered to identify an increase in systolic BP of 11 mm Hg, but the results showed just a 3.1 mm Hg rise in systolic BP at 48 hours (see TABLE1-7 for CI and other data).
In another double-blind, placebo-controlled RCT of 29 adults with hypertension (only 25 were included in the data analysis), there was no significant elevation in BP when oral pseudoephedrine was administered over the course of 3 days.3
Across the 5 studies in the meta-analysis, immediate-release and sustained-release forms of pseudoephedrine were included, hypertension was described as controlled but definitions of control were not always specified, and study length varied from 2 hours to 4 weeks.2-6 Patients on antihypertensive medications were included in some of the studies; patients who had active cardiovascular disease, peripheral vascular disease, and/or cerebrovascular disease were excluded.
One study specifically looked at the effects of a single dose of pseudoephedrine on BP in patients treated with 2 different beta-blockers and found no significant change from baseline, but this study was not powered to show differences less than 5 mm Hg.6 The study did show a change of 1 to 2 mm Hg in systolic BP, but this was not statistically significant.
An absence of information on older patients
There is a paucity of literature on treating older adults and medically complex patients (eg, those with uncontrolled or secondary causes of hypertension, cerebrovascular disease, coronary artery disease) with decongestants, as they were excluded in all studies. And the available evidence does not include reports of adverse events other than changes in BP.
1. Salerno SM, Jackson JL, Berbano EP. Effect of oral pseudoephedrine on blood pressure and heart rate. Arch Intern Med. 2005;165:1686-1694.
2. Beck RA, Mercado DL, Seguin SM, et al. Cardiovascular effects of pseudoephedrine in medically controlled hypertensive patients. Arch Int Med. 1992;152:1242-1245.
3. Bradley JG, Kallail KJ, Dorsch JN, et al. The effects of pseudoephedrine on blood pressure in patients with controlled, uncomplicated hypertension: a randomized, double-blind, placebo-controlled trial. J Am Board Fam Pract. 1991;4:201-206.
4. Chua SS, Benrimoj SI, Gordon RD, et al. A controlled clinical trial on the cardiovascular effects of single doses of pseudoephedrine in hypertensive patients. Br J Clin Pharmacol. 1989;28:369-372.
5. Coates ML, Rembold CM, Farr BM. Does pseudoephedrine increase blood pressure in patients with controlled hypertension? J Fam Pract. 1995;40:22-26.
6. Mores N, Campia U, Navarra P, et al. No cardiovascular effects of single-dose pseudoephedrine in patients with essential hypertension treated with beta-blockers. Eur J Clin Pharmacol. 1999;55:251-254.
7. Salerno SM, Jackson JL, Berbano EP. The impact of oral phenylpropanolamine on blood pressure: a meta-analysis and review of the literature. J Hum Hypertens. 2005;19:643-652.
1. Salerno SM, Jackson JL, Berbano EP. Effect of oral pseudoephedrine on blood pressure and heart rate. Arch Intern Med. 2005;165:1686-1694.
2. Beck RA, Mercado DL, Seguin SM, et al. Cardiovascular effects of pseudoephedrine in medically controlled hypertensive patients. Arch Int Med. 1992;152:1242-1245.
3. Bradley JG, Kallail KJ, Dorsch JN, et al. The effects of pseudoephedrine on blood pressure in patients with controlled, uncomplicated hypertension: a randomized, double-blind, placebo-controlled trial. J Am Board Fam Pract. 1991;4:201-206.
4. Chua SS, Benrimoj SI, Gordon RD, et al. A controlled clinical trial on the cardiovascular effects of single doses of pseudoephedrine in hypertensive patients. Br J Clin Pharmacol. 1989;28:369-372.
5. Coates ML, Rembold CM, Farr BM. Does pseudoephedrine increase blood pressure in patients with controlled hypertension? J Fam Pract. 1995;40:22-26.
6. Mores N, Campia U, Navarra P, et al. No cardiovascular effects of single-dose pseudoephedrine in patients with essential hypertension treated with beta-blockers. Eur J Clin Pharmacol. 1999;55:251-254.
7. Salerno SM, Jackson JL, Berbano EP. The impact of oral phenylpropanolamine on blood pressure: a meta-analysis and review of the literature. J Hum Hypertens. 2005;19:643-652.
Evidence-based answers from the Family Physicians Inquiries Network
EVIDENCE-BASED ANSWER:
It is unclear. Pseudoephedrine causes an average increase of 1.2 mm Hg in systolic blood pressure (BP) in patients with controlled hypertension. However, the studies are not adequately powered to provide evidence about whether this rise in systolic BP is linked to patient-oriented outcomes (strength of recommendation [SOR]: C, multiple randomized controlled trials [RCTs] supporting disease-oriented evidence). Significant variations in BP are defined differently among studies (TABLE1-7). In addition, we do not have data on chronic use of oral decongestants; the longest time on medication in these trials was 4 weeks.
Neuropathic pain treatment provides unexpected benefit
THE CASE
A 57-year-old African American woman was being treated at our clinic for neurogenic urinary incontinence (UI). The UI, which occurred day and night, began 2 years earlier following a laminectomy of vertebrae C3 to C6 with spinal fusion of C3 to C7 for cervical spinal stenosis. The UI persisted despite physical therapy and trials of oxybutynin and imipramine. Since the surgery, the patient had also been experiencing chronic (debilitating) neuropathic pain in both legs, and the sensation of incomplete bladder emptying. She denied bowel incontinence or saddle anesthesia. Her prescription medications included hydrocodone-acetaminophen 7.5/325 mg every 6 hours as needed for pain and lisinopril 20 mg/d for essential hypertension. The patient’s body mass index (BMI) was 23.3.
A urine culture initially grew Klebsiella pneumoniae, which we successfully treated with ciprofloxacin. A urinalysis was unremarkable, and blood urea nitrogen and creatinine levels were within normal limits.
We started the patient on oral duloxetine 30 mg/d for her neuropathic pain. The patient hadn’t undergone a urologic evaluation before starting duloxetine, so no urodynamic studies or measurements had been conducted. At that point, we sent the patient to a urologist for an evaluation.
At a follow-up visit with one of our clinic providers <3 months later, the patient reported that the duloxetine was providing her with some pain relief and that she was “waking up dry” in the mornings and having fewer UI symptoms throughout the day, as well as at night. The patient denied any adverse effects such as nausea, gastrointestinal upset, weight changes, xerostomia, fatigue, insomnia, headaches, or dizziness. Duloxetine was titrated up to 60 mg/d for better control of her neuropathic pain. At the next follow-up visit at our clinic 3 months later, her UI was 80% to 90% improved and she was able to stop her opioid pain medications.
DISCUSSION
UI is a significant problem in the United States and around the world. For women, the prevalence of UI ranges from 15% to 69%; among men, the prevalence is 5% to 24%.1-3 The economic burden of UI includes both medical and nonmedical (eg, pads, diapers, laundry, and dry cleaning) care. The total national cost was estimated at $66 billion in 2007: $49 billion for direct medical costs, $2 billion for direct nonmedical costs, and $15 billion for indirect costs.4 And those costs are expected to increase 25% by 2020, mainly because of the aging population.
Risk factors for UI other than gender include advancing age, obesity, non-Hispanic white race, depression, hypertension, type 2 diabetes mellitus, neurologic disease, and functional limitations/general poor health.5-7 Comorbid depression and BMI >30, as well as the presence and duration of diabetes, increase the odds for developing UI.7,8
Risk factors for women include hysterectomy,7 increasing parity, and delivery of at least one infant >9.5 pounds; the risk is the same for both vaginal and cesarean-section delivery.6 Specific risk factors for men include prostate cancer, prostate surgery, and prostate radiation.5
Significant, chronic comorbidities of UI include depression and chronic pain. While quality of life is negatively affected by UI alone, the coexistence of depression and UI produces an additive negative effect on quality of life.9
Types and treatment of UI
There are 5 types of UI: urge, stress, overflow, functional, and mixed.10
- Urge incontinence is the leakage of urine following a sensation of sudden urgency to void.
- Stress incontinence is urine leakage associated with increased intra-abdominal pressure such as with coughing or sneezing and is typically associated with weakened pelvic floor musculature.
- Overflow incontinence is more common in men, and is typically caused by prostatic disease. The urethral outlet is obstructed leading to increased pressure within the bladder and subsequent leakage of urine.
- Functional incontinence is caused by physical or cognitive impairment leading to a decreased ability to get to a bathroom quickly enough to void.
- Mixed incontinence is when symptoms of stress and urgency incontinence are present.
There are 3 broad categories of treatment methods for urinary incontinence: behavioral, pharmacologic, and surgical. Behavioral interventions are subdivided into caregiver-dependent (prompted voiding, habit retraining, and timed voiding) and patient-directed (bladder training, pelvic floor muscle training, strategies for bladder control, education, and self-monitoring) techniques. Pharmacologic treatment typically consists of antimuscarinics (eg, oxybutynin, tolterodine, solifenacin) and tricyclic antidepressants (eg, imipramine).11 Injections of onabotulinumtoxinA into the detrusor muscle have also been shown to reduce the symptoms of urinary incontinence.12 Surgical options for treatment of UI include retro-pubic suspension, slings, and, in some instances, artificial urethral sphincters.13
A novel treatment for neurogenic UI?
Despite the many treatments available for UI, none comprehensively addresses UI and its common comorbidities.
The role of duloxetine. Normal micturition is regulated by the somatic nervous system and an autonomic reflex arc; the neurotransmitters serotonin and norepinephrine play an important role in the neural regulation of micturition and urinary continence. Duloxetine, alone or as an adjunctive treatment, is a potential novel therapy that treats 2 common comorbidities of UI—chronic pain and depression.
As a selective serotonin norepinephrine reuptake inhibitor (SNRI), duloxetine acts at the molecular level to block the reuptake of serotonin and norepinephrine from synaptic clefts. Specifically, the medication blocks the 5-hydroxytryptamine (5-HT) reuptake transporters, as well as the norepinephrine transporters, of pre-synaptic neurons.14 Thus, the concentrations of 5-HT and norepinephrine increase in the synaptic cleft.
Functionally, the accumulation of norepinephrine inhibits micturition by relaxing the detrusor muscle and constricting the urethral smooth muscle. In addition, a higher concentration of 5-HT at the neuromuscular junction leads to constriction of the external urethral sphincter.
Duloxetine has been shown to be effective in the treatment of other types of UI, such as stress UI15 and mixed UI.16 Additionally, it was found to be effective when compared with placebo in women with overactive bladder syndrome17 and in women with multiple sclerosis and depression.18 However, we are not aware of any cases using duloxetine for the treatment of neurogenic UI.
THE TAKEAWAY
Duloxetine is a potential novel drug choice for the treatment of neurogenic UI. Its effects on serotonin and norepinephrine at the synaptic cleft and neuromuscular junction could provide relief for those who have not found relief from other therapies. Further research—particularly a prospective, randomized controlled trial—is needed to determine if duloxetine is, in fact, more than just a theoretical candidate to treat UI and, if so, the most effective dosing.
Offering duloxetine for the treatment of neurogenic UI would potentially address coexisting conditions—such as pain or depression—thus improving patient compliance and reducing health care spending. Before beginning therapy, urodynamic studies to identify the type of UI should be completed, or, at a minimum, post-void residual volume should be measured.
ACKNOWLEDGEMENTS
The authors would like to thank Julie Hughbanks, MLS, Library Manager, Parkview Health Resource Library, for her assistance with the library searches used for this case report.
1. Markland AD, Richter HE, Fwu CW, et al. Prevalence and trends of urinary incontinence in adults in the United States, 2001 to 2008. J Urol. 2011;186:589-593.
2. Buckley BS, Lapitan MC; Epidemiology Committee of the Fourth International Consultation on Incontinence, Paris, 2008. Prevalence of urinary incontinence in men, women, and children—current evidence: findings of the Fourth International Consultation on Incontinence. Urology. 2010;76:265-270.
3. Gorina Y, Schappert S, Bercovitz A, et al. Prevalence of incontinence among older Americans. Vital Health Stat 3. 2014;1-33.
4. Coyne KS, Wein A, Nicholson S, et al. Economic burden of urgency urinary incontinence in the United States: a systematic review. J Manag Care Pharm. 2014;20:130-140.
5. Shamliyan TA, Wyman JF, Ping R, et al. Male urinary incontinence: prevalence, risk factors, and preventive interventions. Rev Urol. 2009;11:145-165.
6. Matthews CA, Whitehead WE, Townsend MK, et al. Risk factors for urinary, fecal, or dual incontinence in the Nurses’ Health Study. Obstet Gynecol. 2013;122:539-545.
7. Danforth KN, Townsend MK, Lifford K, et al. Risk factors for urinary incontinence among middle-aged women. Am J Obstet Gynecol. 2006;194:339-345.
8. Lifford KL, Curhan GC, Hu FB, et al. Type 2 diabetes mellitus and risk of developing urinary incontinence. J Am Geriatr Soc. 2005;53:1851-1857.
9. Avery JC, Stocks NP, Duggan P, et al. Identifying the quality of life effects of urinary incontinence with depression in an Australian population. BMC Urol. 2013;13:11.
10. National Kidney and Urologic Diseases Information Clearinghouse. Urinary incontinence in women. Available at: http://kidney.niddk.nih.gov/KUDISEASES/pubs/uiwomen/UI-Women_508.pdf. Accessed January 2, 2015.
11. Ontario Medical Advisory Secretariat. Behavioural interventions for urinary incontinence in community-dwelling seniors: an evidence-based analysis. Ontario Health Technology Assessment Series. 2008:8. Available at: http://www.hqontario.ca/Portals/0/Documents/evidence/reports/rev_aic_ui_20081002.pdf. Accessed November 30, 2015.
12. Cox L, Cameron A. OnabotulinumtoxinA for the treatment of overactive bladder. Res Rep Urol. 2014;6:79-89.
13. Dmochowski RR, Blaivas JM, Gormley EA, et al. Update of AUA guideline on the surgical management of female stress urinary incontinence. J Urol. 2010;183:1906-1914.
14. Duloxetine. US National Library of Medicine: National Center for Biotechnology Information. 2015. Available at: http://pubchem.ncbi.nlm.nih.gov/compound/duloxetine. Accessed October 20, 2015.
15. Li J, Yang L, Pu C, et al. The role of duloxetine in stress urinary incontinence: a systematic review and meta-analysis. Int Urol Nephrol. 2013;45:679-686.
16. Bent AE, Gousse AE, Hendrix SL, et al. Duloxetine compared with placebo for the treatment of women with mixed urinary incontinence. Neurourol Urodyn. 2008;27:212-221.
17. Steers WD, Herschorn S, Kreder KJ, et al; Duloxetine OAB Study Group. Duloxetine compared with placebo for treating women with symptoms of overactive bladder. BJU Int. 2007;100:337-345.
18. Di Rezze S, Frasca V, Inghilleri M, et al. Duloxetine for the treatment of overactive bladder syndrome in multiple sclerosis: a pilot study. Clin Neuropharmacol. 2012;35:231-234.
THE CASE
A 57-year-old African American woman was being treated at our clinic for neurogenic urinary incontinence (UI). The UI, which occurred day and night, began 2 years earlier following a laminectomy of vertebrae C3 to C6 with spinal fusion of C3 to C7 for cervical spinal stenosis. The UI persisted despite physical therapy and trials of oxybutynin and imipramine. Since the surgery, the patient had also been experiencing chronic (debilitating) neuropathic pain in both legs, and the sensation of incomplete bladder emptying. She denied bowel incontinence or saddle anesthesia. Her prescription medications included hydrocodone-acetaminophen 7.5/325 mg every 6 hours as needed for pain and lisinopril 20 mg/d for essential hypertension. The patient’s body mass index (BMI) was 23.3.
A urine culture initially grew Klebsiella pneumoniae, which we successfully treated with ciprofloxacin. A urinalysis was unremarkable, and blood urea nitrogen and creatinine levels were within normal limits.
We started the patient on oral duloxetine 30 mg/d for her neuropathic pain. The patient hadn’t undergone a urologic evaluation before starting duloxetine, so no urodynamic studies or measurements had been conducted. At that point, we sent the patient to a urologist for an evaluation.
At a follow-up visit with one of our clinic providers <3 months later, the patient reported that the duloxetine was providing her with some pain relief and that she was “waking up dry” in the mornings and having fewer UI symptoms throughout the day, as well as at night. The patient denied any adverse effects such as nausea, gastrointestinal upset, weight changes, xerostomia, fatigue, insomnia, headaches, or dizziness. Duloxetine was titrated up to 60 mg/d for better control of her neuropathic pain. At the next follow-up visit at our clinic 3 months later, her UI was 80% to 90% improved and she was able to stop her opioid pain medications.
DISCUSSION
UI is a significant problem in the United States and around the world. For women, the prevalence of UI ranges from 15% to 69%; among men, the prevalence is 5% to 24%.1-3 The economic burden of UI includes both medical and nonmedical (eg, pads, diapers, laundry, and dry cleaning) care. The total national cost was estimated at $66 billion in 2007: $49 billion for direct medical costs, $2 billion for direct nonmedical costs, and $15 billion for indirect costs.4 And those costs are expected to increase 25% by 2020, mainly because of the aging population.
Risk factors for UI other than gender include advancing age, obesity, non-Hispanic white race, depression, hypertension, type 2 diabetes mellitus, neurologic disease, and functional limitations/general poor health.5-7 Comorbid depression and BMI >30, as well as the presence and duration of diabetes, increase the odds for developing UI.7,8
Risk factors for women include hysterectomy,7 increasing parity, and delivery of at least one infant >9.5 pounds; the risk is the same for both vaginal and cesarean-section delivery.6 Specific risk factors for men include prostate cancer, prostate surgery, and prostate radiation.5
Significant, chronic comorbidities of UI include depression and chronic pain. While quality of life is negatively affected by UI alone, the coexistence of depression and UI produces an additive negative effect on quality of life.9
Types and treatment of UI
There are 5 types of UI: urge, stress, overflow, functional, and mixed.10
- Urge incontinence is the leakage of urine following a sensation of sudden urgency to void.
- Stress incontinence is urine leakage associated with increased intra-abdominal pressure such as with coughing or sneezing and is typically associated with weakened pelvic floor musculature.
- Overflow incontinence is more common in men, and is typically caused by prostatic disease. The urethral outlet is obstructed leading to increased pressure within the bladder and subsequent leakage of urine.
- Functional incontinence is caused by physical or cognitive impairment leading to a decreased ability to get to a bathroom quickly enough to void.
- Mixed incontinence is when symptoms of stress and urgency incontinence are present.
There are 3 broad categories of treatment methods for urinary incontinence: behavioral, pharmacologic, and surgical. Behavioral interventions are subdivided into caregiver-dependent (prompted voiding, habit retraining, and timed voiding) and patient-directed (bladder training, pelvic floor muscle training, strategies for bladder control, education, and self-monitoring) techniques. Pharmacologic treatment typically consists of antimuscarinics (eg, oxybutynin, tolterodine, solifenacin) and tricyclic antidepressants (eg, imipramine).11 Injections of onabotulinumtoxinA into the detrusor muscle have also been shown to reduce the symptoms of urinary incontinence.12 Surgical options for treatment of UI include retro-pubic suspension, slings, and, in some instances, artificial urethral sphincters.13
A novel treatment for neurogenic UI?
Despite the many treatments available for UI, none comprehensively addresses UI and its common comorbidities.
The role of duloxetine. Normal micturition is regulated by the somatic nervous system and an autonomic reflex arc; the neurotransmitters serotonin and norepinephrine play an important role in the neural regulation of micturition and urinary continence. Duloxetine, alone or as an adjunctive treatment, is a potential novel therapy that treats 2 common comorbidities of UI—chronic pain and depression.
As a selective serotonin norepinephrine reuptake inhibitor (SNRI), duloxetine acts at the molecular level to block the reuptake of serotonin and norepinephrine from synaptic clefts. Specifically, the medication blocks the 5-hydroxytryptamine (5-HT) reuptake transporters, as well as the norepinephrine transporters, of pre-synaptic neurons.14 Thus, the concentrations of 5-HT and norepinephrine increase in the synaptic cleft.
Functionally, the accumulation of norepinephrine inhibits micturition by relaxing the detrusor muscle and constricting the urethral smooth muscle. In addition, a higher concentration of 5-HT at the neuromuscular junction leads to constriction of the external urethral sphincter.
Duloxetine has been shown to be effective in the treatment of other types of UI, such as stress UI15 and mixed UI.16 Additionally, it was found to be effective when compared with placebo in women with overactive bladder syndrome17 and in women with multiple sclerosis and depression.18 However, we are not aware of any cases using duloxetine for the treatment of neurogenic UI.
THE TAKEAWAY
Duloxetine is a potential novel drug choice for the treatment of neurogenic UI. Its effects on serotonin and norepinephrine at the synaptic cleft and neuromuscular junction could provide relief for those who have not found relief from other therapies. Further research—particularly a prospective, randomized controlled trial—is needed to determine if duloxetine is, in fact, more than just a theoretical candidate to treat UI and, if so, the most effective dosing.
Offering duloxetine for the treatment of neurogenic UI would potentially address coexisting conditions—such as pain or depression—thus improving patient compliance and reducing health care spending. Before beginning therapy, urodynamic studies to identify the type of UI should be completed, or, at a minimum, post-void residual volume should be measured.
ACKNOWLEDGEMENTS
The authors would like to thank Julie Hughbanks, MLS, Library Manager, Parkview Health Resource Library, for her assistance with the library searches used for this case report.
THE CASE
A 57-year-old African American woman was being treated at our clinic for neurogenic urinary incontinence (UI). The UI, which occurred day and night, began 2 years earlier following a laminectomy of vertebrae C3 to C6 with spinal fusion of C3 to C7 for cervical spinal stenosis. The UI persisted despite physical therapy and trials of oxybutynin and imipramine. Since the surgery, the patient had also been experiencing chronic (debilitating) neuropathic pain in both legs, and the sensation of incomplete bladder emptying. She denied bowel incontinence or saddle anesthesia. Her prescription medications included hydrocodone-acetaminophen 7.5/325 mg every 6 hours as needed for pain and lisinopril 20 mg/d for essential hypertension. The patient’s body mass index (BMI) was 23.3.
A urine culture initially grew Klebsiella pneumoniae, which we successfully treated with ciprofloxacin. A urinalysis was unremarkable, and blood urea nitrogen and creatinine levels were within normal limits.
We started the patient on oral duloxetine 30 mg/d for her neuropathic pain. The patient hadn’t undergone a urologic evaluation before starting duloxetine, so no urodynamic studies or measurements had been conducted. At that point, we sent the patient to a urologist for an evaluation.
At a follow-up visit with one of our clinic providers <3 months later, the patient reported that the duloxetine was providing her with some pain relief and that she was “waking up dry” in the mornings and having fewer UI symptoms throughout the day, as well as at night. The patient denied any adverse effects such as nausea, gastrointestinal upset, weight changes, xerostomia, fatigue, insomnia, headaches, or dizziness. Duloxetine was titrated up to 60 mg/d for better control of her neuropathic pain. At the next follow-up visit at our clinic 3 months later, her UI was 80% to 90% improved and she was able to stop her opioid pain medications.
DISCUSSION
UI is a significant problem in the United States and around the world. For women, the prevalence of UI ranges from 15% to 69%; among men, the prevalence is 5% to 24%.1-3 The economic burden of UI includes both medical and nonmedical (eg, pads, diapers, laundry, and dry cleaning) care. The total national cost was estimated at $66 billion in 2007: $49 billion for direct medical costs, $2 billion for direct nonmedical costs, and $15 billion for indirect costs.4 And those costs are expected to increase 25% by 2020, mainly because of the aging population.
Risk factors for UI other than gender include advancing age, obesity, non-Hispanic white race, depression, hypertension, type 2 diabetes mellitus, neurologic disease, and functional limitations/general poor health.5-7 Comorbid depression and BMI >30, as well as the presence and duration of diabetes, increase the odds for developing UI.7,8
Risk factors for women include hysterectomy,7 increasing parity, and delivery of at least one infant >9.5 pounds; the risk is the same for both vaginal and cesarean-section delivery.6 Specific risk factors for men include prostate cancer, prostate surgery, and prostate radiation.5
Significant, chronic comorbidities of UI include depression and chronic pain. While quality of life is negatively affected by UI alone, the coexistence of depression and UI produces an additive negative effect on quality of life.9
Types and treatment of UI
There are 5 types of UI: urge, stress, overflow, functional, and mixed.10
- Urge incontinence is the leakage of urine following a sensation of sudden urgency to void.
- Stress incontinence is urine leakage associated with increased intra-abdominal pressure such as with coughing or sneezing and is typically associated with weakened pelvic floor musculature.
- Overflow incontinence is more common in men, and is typically caused by prostatic disease. The urethral outlet is obstructed leading to increased pressure within the bladder and subsequent leakage of urine.
- Functional incontinence is caused by physical or cognitive impairment leading to a decreased ability to get to a bathroom quickly enough to void.
- Mixed incontinence is when symptoms of stress and urgency incontinence are present.
There are 3 broad categories of treatment methods for urinary incontinence: behavioral, pharmacologic, and surgical. Behavioral interventions are subdivided into caregiver-dependent (prompted voiding, habit retraining, and timed voiding) and patient-directed (bladder training, pelvic floor muscle training, strategies for bladder control, education, and self-monitoring) techniques. Pharmacologic treatment typically consists of antimuscarinics (eg, oxybutynin, tolterodine, solifenacin) and tricyclic antidepressants (eg, imipramine).11 Injections of onabotulinumtoxinA into the detrusor muscle have also been shown to reduce the symptoms of urinary incontinence.12 Surgical options for treatment of UI include retro-pubic suspension, slings, and, in some instances, artificial urethral sphincters.13
A novel treatment for neurogenic UI?
Despite the many treatments available for UI, none comprehensively addresses UI and its common comorbidities.
The role of duloxetine. Normal micturition is regulated by the somatic nervous system and an autonomic reflex arc; the neurotransmitters serotonin and norepinephrine play an important role in the neural regulation of micturition and urinary continence. Duloxetine, alone or as an adjunctive treatment, is a potential novel therapy that treats 2 common comorbidities of UI—chronic pain and depression.
As a selective serotonin norepinephrine reuptake inhibitor (SNRI), duloxetine acts at the molecular level to block the reuptake of serotonin and norepinephrine from synaptic clefts. Specifically, the medication blocks the 5-hydroxytryptamine (5-HT) reuptake transporters, as well as the norepinephrine transporters, of pre-synaptic neurons.14 Thus, the concentrations of 5-HT and norepinephrine increase in the synaptic cleft.
Functionally, the accumulation of norepinephrine inhibits micturition by relaxing the detrusor muscle and constricting the urethral smooth muscle. In addition, a higher concentration of 5-HT at the neuromuscular junction leads to constriction of the external urethral sphincter.
Duloxetine has been shown to be effective in the treatment of other types of UI, such as stress UI15 and mixed UI.16 Additionally, it was found to be effective when compared with placebo in women with overactive bladder syndrome17 and in women with multiple sclerosis and depression.18 However, we are not aware of any cases using duloxetine for the treatment of neurogenic UI.
THE TAKEAWAY
Duloxetine is a potential novel drug choice for the treatment of neurogenic UI. Its effects on serotonin and norepinephrine at the synaptic cleft and neuromuscular junction could provide relief for those who have not found relief from other therapies. Further research—particularly a prospective, randomized controlled trial—is needed to determine if duloxetine is, in fact, more than just a theoretical candidate to treat UI and, if so, the most effective dosing.
Offering duloxetine for the treatment of neurogenic UI would potentially address coexisting conditions—such as pain or depression—thus improving patient compliance and reducing health care spending. Before beginning therapy, urodynamic studies to identify the type of UI should be completed, or, at a minimum, post-void residual volume should be measured.
ACKNOWLEDGEMENTS
The authors would like to thank Julie Hughbanks, MLS, Library Manager, Parkview Health Resource Library, for her assistance with the library searches used for this case report.
1. Markland AD, Richter HE, Fwu CW, et al. Prevalence and trends of urinary incontinence in adults in the United States, 2001 to 2008. J Urol. 2011;186:589-593.
2. Buckley BS, Lapitan MC; Epidemiology Committee of the Fourth International Consultation on Incontinence, Paris, 2008. Prevalence of urinary incontinence in men, women, and children—current evidence: findings of the Fourth International Consultation on Incontinence. Urology. 2010;76:265-270.
3. Gorina Y, Schappert S, Bercovitz A, et al. Prevalence of incontinence among older Americans. Vital Health Stat 3. 2014;1-33.
4. Coyne KS, Wein A, Nicholson S, et al. Economic burden of urgency urinary incontinence in the United States: a systematic review. J Manag Care Pharm. 2014;20:130-140.
5. Shamliyan TA, Wyman JF, Ping R, et al. Male urinary incontinence: prevalence, risk factors, and preventive interventions. Rev Urol. 2009;11:145-165.
6. Matthews CA, Whitehead WE, Townsend MK, et al. Risk factors for urinary, fecal, or dual incontinence in the Nurses’ Health Study. Obstet Gynecol. 2013;122:539-545.
7. Danforth KN, Townsend MK, Lifford K, et al. Risk factors for urinary incontinence among middle-aged women. Am J Obstet Gynecol. 2006;194:339-345.
8. Lifford KL, Curhan GC, Hu FB, et al. Type 2 diabetes mellitus and risk of developing urinary incontinence. J Am Geriatr Soc. 2005;53:1851-1857.
9. Avery JC, Stocks NP, Duggan P, et al. Identifying the quality of life effects of urinary incontinence with depression in an Australian population. BMC Urol. 2013;13:11.
10. National Kidney and Urologic Diseases Information Clearinghouse. Urinary incontinence in women. Available at: http://kidney.niddk.nih.gov/KUDISEASES/pubs/uiwomen/UI-Women_508.pdf. Accessed January 2, 2015.
11. Ontario Medical Advisory Secretariat. Behavioural interventions for urinary incontinence in community-dwelling seniors: an evidence-based analysis. Ontario Health Technology Assessment Series. 2008:8. Available at: http://www.hqontario.ca/Portals/0/Documents/evidence/reports/rev_aic_ui_20081002.pdf. Accessed November 30, 2015.
12. Cox L, Cameron A. OnabotulinumtoxinA for the treatment of overactive bladder. Res Rep Urol. 2014;6:79-89.
13. Dmochowski RR, Blaivas JM, Gormley EA, et al. Update of AUA guideline on the surgical management of female stress urinary incontinence. J Urol. 2010;183:1906-1914.
14. Duloxetine. US National Library of Medicine: National Center for Biotechnology Information. 2015. Available at: http://pubchem.ncbi.nlm.nih.gov/compound/duloxetine. Accessed October 20, 2015.
15. Li J, Yang L, Pu C, et al. The role of duloxetine in stress urinary incontinence: a systematic review and meta-analysis. Int Urol Nephrol. 2013;45:679-686.
16. Bent AE, Gousse AE, Hendrix SL, et al. Duloxetine compared with placebo for the treatment of women with mixed urinary incontinence. Neurourol Urodyn. 2008;27:212-221.
17. Steers WD, Herschorn S, Kreder KJ, et al; Duloxetine OAB Study Group. Duloxetine compared with placebo for treating women with symptoms of overactive bladder. BJU Int. 2007;100:337-345.
18. Di Rezze S, Frasca V, Inghilleri M, et al. Duloxetine for the treatment of overactive bladder syndrome in multiple sclerosis: a pilot study. Clin Neuropharmacol. 2012;35:231-234.
1. Markland AD, Richter HE, Fwu CW, et al. Prevalence and trends of urinary incontinence in adults in the United States, 2001 to 2008. J Urol. 2011;186:589-593.
2. Buckley BS, Lapitan MC; Epidemiology Committee of the Fourth International Consultation on Incontinence, Paris, 2008. Prevalence of urinary incontinence in men, women, and children—current evidence: findings of the Fourth International Consultation on Incontinence. Urology. 2010;76:265-270.
3. Gorina Y, Schappert S, Bercovitz A, et al. Prevalence of incontinence among older Americans. Vital Health Stat 3. 2014;1-33.
4. Coyne KS, Wein A, Nicholson S, et al. Economic burden of urgency urinary incontinence in the United States: a systematic review. J Manag Care Pharm. 2014;20:130-140.
5. Shamliyan TA, Wyman JF, Ping R, et al. Male urinary incontinence: prevalence, risk factors, and preventive interventions. Rev Urol. 2009;11:145-165.
6. Matthews CA, Whitehead WE, Townsend MK, et al. Risk factors for urinary, fecal, or dual incontinence in the Nurses’ Health Study. Obstet Gynecol. 2013;122:539-545.
7. Danforth KN, Townsend MK, Lifford K, et al. Risk factors for urinary incontinence among middle-aged women. Am J Obstet Gynecol. 2006;194:339-345.
8. Lifford KL, Curhan GC, Hu FB, et al. Type 2 diabetes mellitus and risk of developing urinary incontinence. J Am Geriatr Soc. 2005;53:1851-1857.
9. Avery JC, Stocks NP, Duggan P, et al. Identifying the quality of life effects of urinary incontinence with depression in an Australian population. BMC Urol. 2013;13:11.
10. National Kidney and Urologic Diseases Information Clearinghouse. Urinary incontinence in women. Available at: http://kidney.niddk.nih.gov/KUDISEASES/pubs/uiwomen/UI-Women_508.pdf. Accessed January 2, 2015.
11. Ontario Medical Advisory Secretariat. Behavioural interventions for urinary incontinence in community-dwelling seniors: an evidence-based analysis. Ontario Health Technology Assessment Series. 2008:8. Available at: http://www.hqontario.ca/Portals/0/Documents/evidence/reports/rev_aic_ui_20081002.pdf. Accessed November 30, 2015.
12. Cox L, Cameron A. OnabotulinumtoxinA for the treatment of overactive bladder. Res Rep Urol. 2014;6:79-89.
13. Dmochowski RR, Blaivas JM, Gormley EA, et al. Update of AUA guideline on the surgical management of female stress urinary incontinence. J Urol. 2010;183:1906-1914.
14. Duloxetine. US National Library of Medicine: National Center for Biotechnology Information. 2015. Available at: http://pubchem.ncbi.nlm.nih.gov/compound/duloxetine. Accessed October 20, 2015.
15. Li J, Yang L, Pu C, et al. The role of duloxetine in stress urinary incontinence: a systematic review and meta-analysis. Int Urol Nephrol. 2013;45:679-686.
16. Bent AE, Gousse AE, Hendrix SL, et al. Duloxetine compared with placebo for the treatment of women with mixed urinary incontinence. Neurourol Urodyn. 2008;27:212-221.
17. Steers WD, Herschorn S, Kreder KJ, et al; Duloxetine OAB Study Group. Duloxetine compared with placebo for treating women with symptoms of overactive bladder. BJU Int. 2007;100:337-345.
18. Di Rezze S, Frasca V, Inghilleri M, et al. Duloxetine for the treatment of overactive bladder syndrome in multiple sclerosis: a pilot study. Clin Neuropharmacol. 2012;35:231-234.
The one thing that’s missing from the health care debate
The Affordable Care Act (aka Obamacare) may soon be out and the American Health Care Act (AHCA) may soon be in. Despite all of the rhetoric about making health care affordable by reducing insurance premiums, one thing has been conspicuously absent from the debate: how we are going to reduce the actual cost of health care. Yes, the AHCA may help reduce premiums, but what is most likely to result is not less expensive health care, but rather people paying less money on premiums and more out of their pockets for medicines and treatments. Especially troublesome is that older and sicker patients will be hit the hardest.
The American conundrum. Why do Americans pay twice what citizens of most other developed nations pay and get health care outcomes that are worse?1,2 Two reasons are that those who provide health care charge more in this country for services and medications, and physicians do a lot more testing and treatment here than their counterparts abroad.
One expert estimated that up to $700 billion could be saved by eliminating testing and treatments that provide marginal or no value to patients.3 For example, knee arthroscopy for moderate knee osteoarthritis produces no better outcomes than medical management.4 And many medications are much more expensive in the United States than in other countries. It seems that pharmaceutical companies are permitted greater profits here than elsewhere in the world, and these profits are at the expense of sick people and taxpayers.
How do we bend the cost curve downward? This is a tough question with no single correct answer, but we can all help. Some health care organizations have already reduced costs significantly without sacrificing quality by using team-based primary care as their foundation. Two examples are Nuka Health and Iora Health.5,6
As primary care physicians, we are in an ideal position to constrain unnecessary testing and treatments by establishing trusting relationships with patients, who will believe us when we tell them they don’t need an antibiotic for their chest cold or an MRI for their back pain.
If we control the cost of providing care, insurance premiums will follow suit.
1. The Commonwealth Fund. U.S. health care from a global perspective. Available at: http://www.commonwealthfund.org/publications/issue-briefs/2015/oct/us-health-care-from-a-global-perspective. Accessed May 14, 2017.
2. The Commonwealth Fund. US health system ranks last among eleven countries on measures of access, equity, quality, efficiency, and healthy lives. Available at: http://www.commonwealthfund.org/publications/press-releases/2014/jun/us-health-system-ranks-last. Accessed May 14, 2017.
3. Kelley R. Where can $700 billion in waste be cut annually from the U.S. healthcare system? Available at: http://www.hcca-info.org/Portals/0/PDFs/Resources/Conference_Handouts/Compliance_Institute/2010/P8handout6.pdf. Accessed May 14, 2017.
4. Kirkley A, Birmingham TB, Litchfield RB, et al. A randomized trial of arthroscopic surgery for osteoarthritis of the knee. N Engl J Med. 2008;359:1097-1107.
5. Gottlieb K. The Nuka System of Care: improving health through ownership and relationships. Int J Circumpolar Health. 2013;72. doi: 10.3402/ijch.v72i0.21118.
6. Iorahealth. Available at: www.iorahealth.com. Accessed May 14, 2017.
The Affordable Care Act (aka Obamacare) may soon be out and the American Health Care Act (AHCA) may soon be in. Despite all of the rhetoric about making health care affordable by reducing insurance premiums, one thing has been conspicuously absent from the debate: how we are going to reduce the actual cost of health care. Yes, the AHCA may help reduce premiums, but what is most likely to result is not less expensive health care, but rather people paying less money on premiums and more out of their pockets for medicines and treatments. Especially troublesome is that older and sicker patients will be hit the hardest.
The American conundrum. Why do Americans pay twice what citizens of most other developed nations pay and get health care outcomes that are worse?1,2 Two reasons are that those who provide health care charge more in this country for services and medications, and physicians do a lot more testing and treatment here than their counterparts abroad.
One expert estimated that up to $700 billion could be saved by eliminating testing and treatments that provide marginal or no value to patients.3 For example, knee arthroscopy for moderate knee osteoarthritis produces no better outcomes than medical management.4 And many medications are much more expensive in the United States than in other countries. It seems that pharmaceutical companies are permitted greater profits here than elsewhere in the world, and these profits are at the expense of sick people and taxpayers.
How do we bend the cost curve downward? This is a tough question with no single correct answer, but we can all help. Some health care organizations have already reduced costs significantly without sacrificing quality by using team-based primary care as their foundation. Two examples are Nuka Health and Iora Health.5,6
As primary care physicians, we are in an ideal position to constrain unnecessary testing and treatments by establishing trusting relationships with patients, who will believe us when we tell them they don’t need an antibiotic for their chest cold or an MRI for their back pain.
If we control the cost of providing care, insurance premiums will follow suit.
The Affordable Care Act (aka Obamacare) may soon be out and the American Health Care Act (AHCA) may soon be in. Despite all of the rhetoric about making health care affordable by reducing insurance premiums, one thing has been conspicuously absent from the debate: how we are going to reduce the actual cost of health care. Yes, the AHCA may help reduce premiums, but what is most likely to result is not less expensive health care, but rather people paying less money on premiums and more out of their pockets for medicines and treatments. Especially troublesome is that older and sicker patients will be hit the hardest.
The American conundrum. Why do Americans pay twice what citizens of most other developed nations pay and get health care outcomes that are worse?1,2 Two reasons are that those who provide health care charge more in this country for services and medications, and physicians do a lot more testing and treatment here than their counterparts abroad.
One expert estimated that up to $700 billion could be saved by eliminating testing and treatments that provide marginal or no value to patients.3 For example, knee arthroscopy for moderate knee osteoarthritis produces no better outcomes than medical management.4 And many medications are much more expensive in the United States than in other countries. It seems that pharmaceutical companies are permitted greater profits here than elsewhere in the world, and these profits are at the expense of sick people and taxpayers.
How do we bend the cost curve downward? This is a tough question with no single correct answer, but we can all help. Some health care organizations have already reduced costs significantly without sacrificing quality by using team-based primary care as their foundation. Two examples are Nuka Health and Iora Health.5,6
As primary care physicians, we are in an ideal position to constrain unnecessary testing and treatments by establishing trusting relationships with patients, who will believe us when we tell them they don’t need an antibiotic for their chest cold or an MRI for their back pain.
If we control the cost of providing care, insurance premiums will follow suit.
1. The Commonwealth Fund. U.S. health care from a global perspective. Available at: http://www.commonwealthfund.org/publications/issue-briefs/2015/oct/us-health-care-from-a-global-perspective. Accessed May 14, 2017.
2. The Commonwealth Fund. US health system ranks last among eleven countries on measures of access, equity, quality, efficiency, and healthy lives. Available at: http://www.commonwealthfund.org/publications/press-releases/2014/jun/us-health-system-ranks-last. Accessed May 14, 2017.
3. Kelley R. Where can $700 billion in waste be cut annually from the U.S. healthcare system? Available at: http://www.hcca-info.org/Portals/0/PDFs/Resources/Conference_Handouts/Compliance_Institute/2010/P8handout6.pdf. Accessed May 14, 2017.
4. Kirkley A, Birmingham TB, Litchfield RB, et al. A randomized trial of arthroscopic surgery for osteoarthritis of the knee. N Engl J Med. 2008;359:1097-1107.
5. Gottlieb K. The Nuka System of Care: improving health through ownership and relationships. Int J Circumpolar Health. 2013;72. doi: 10.3402/ijch.v72i0.21118.
6. Iorahealth. Available at: www.iorahealth.com. Accessed May 14, 2017.
1. The Commonwealth Fund. U.S. health care from a global perspective. Available at: http://www.commonwealthfund.org/publications/issue-briefs/2015/oct/us-health-care-from-a-global-perspective. Accessed May 14, 2017.
2. The Commonwealth Fund. US health system ranks last among eleven countries on measures of access, equity, quality, efficiency, and healthy lives. Available at: http://www.commonwealthfund.org/publications/press-releases/2014/jun/us-health-system-ranks-last. Accessed May 14, 2017.
3. Kelley R. Where can $700 billion in waste be cut annually from the U.S. healthcare system? Available at: http://www.hcca-info.org/Portals/0/PDFs/Resources/Conference_Handouts/Compliance_Institute/2010/P8handout6.pdf. Accessed May 14, 2017.
4. Kirkley A, Birmingham TB, Litchfield RB, et al. A randomized trial of arthroscopic surgery for osteoarthritis of the knee. N Engl J Med. 2008;359:1097-1107.
5. Gottlieb K. The Nuka System of Care: improving health through ownership and relationships. Int J Circumpolar Health. 2013;72. doi: 10.3402/ijch.v72i0.21118.
6. Iorahealth. Available at: www.iorahealth.com. Accessed May 14, 2017.