User login
VIDEO: Attaining the tools to start your own quality improvement project
Quality improvement at the program level is a major concern for most hospitalists. That’s exactly why Venkata Dontaraju, MD, MRCP, FHM, attended a Tuesday afternoon HM17 workshop entitled “Adding to Your Toolbox: QI Methodologies.”
Dr. Dontaraju, a hospitalist for 7 years with Rockford Health Physicians in Loves Park, Ill., wants to begin QI projects of his own. He planned to attend a number of QI-focused sessions at the annual meeting, with the toolbox session laying the foundation for such work.
“There is a lot of emphasis on cutting down the waste in health care, and also improving the processes,” he said. “That is where the role of QI comes into place. I want to do QI projects at my own hospital, but I first want to get the tools necessary for a successful project.”
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Quality improvement at the program level is a major concern for most hospitalists. That’s exactly why Venkata Dontaraju, MD, MRCP, FHM, attended a Tuesday afternoon HM17 workshop entitled “Adding to Your Toolbox: QI Methodologies.”
Dr. Dontaraju, a hospitalist for 7 years with Rockford Health Physicians in Loves Park, Ill., wants to begin QI projects of his own. He planned to attend a number of QI-focused sessions at the annual meeting, with the toolbox session laying the foundation for such work.
“There is a lot of emphasis on cutting down the waste in health care, and also improving the processes,” he said. “That is where the role of QI comes into place. I want to do QI projects at my own hospital, but I first want to get the tools necessary for a successful project.”
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Quality improvement at the program level is a major concern for most hospitalists. That’s exactly why Venkata Dontaraju, MD, MRCP, FHM, attended a Tuesday afternoon HM17 workshop entitled “Adding to Your Toolbox: QI Methodologies.”
Dr. Dontaraju, a hospitalist for 7 years with Rockford Health Physicians in Loves Park, Ill., wants to begin QI projects of his own. He planned to attend a number of QI-focused sessions at the annual meeting, with the toolbox session laying the foundation for such work.
“There is a lot of emphasis on cutting down the waste in health care, and also improving the processes,” he said. “That is where the role of QI comes into place. I want to do QI projects at my own hospital, but I first want to get the tools necessary for a successful project.”
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Forum focuses on forging Q.I. connections
Scrounging for information that could help her with quality improvement (Q.I.) projects, Charmaine Lewis, MD, MPH, the quality director with New Hanover Hospitalists in Wilmington, N.C., said she finds herself reviewing posters at the Annual Meeting and squinting at the images to see whether the electronic health records involved match the system she uses back home.
There’s got to be a better way.
Mangla Gulati, MD, SFHM, associate professor of medicine and chief quality officer at the University of Maryland, and Jenna Goldstein, director of SHM’s Center for Hospital Innovation and Improvement, led what grew into a lively, thoughtful discussion about ways for hospitalists to exchange information and to get support with Q.I. efforts at their centers.
The hospitalists who attended brought their experiences and their questions from a diverse range of centers, from Anchorage, Alaska, to southern California to Poughkeepsie, New York. They emphasized the need for easier access to initiatives that mirror what they are trying to do at their own centers, for more mentoring opportunities, and for more SHM chapters outside major metropolitan areas to help with networking. And they offered lessons on what they have already learned.
Remus Popa, MD, FHM, associate professor of medicine at the University of California, Riverside, who has led Q.I. projects on the use of telemetry, said a successful project will likely be more than an idea that blooms inside the head of a hospitalist.
“We have to find something that the institution really needs,” Dr. Popa said. “You have to design a better fit with them. Otherwise, it’s going to be a tough conversation about resources, right?”
Esther Lee, MD, a hospitalist in Anchorage, Alaska, asked whether it might be possible for SHM to host a “mini-conference” specifically on quality improvement. At the Annual Meeting, she said, she often finds herself torn between attending Q.I. sessions and sessions on clinical topics.
SHM’s Goldstein said it was a possibility.
“It is something that we have talked about and will continue to talk about,” she said.
David Lucier, MD, MBA, MPH, Director of Quality and Safety for Hospital Medicine at Massachusetts General Hospital, said that emphasizing the safety aspect of a project can often help push it along.
“I find these [types of projects] to be quite compelling, at least in reordering the priority list,” he said.
Ms. Goldstein said the suggestions were helpful in SHM’s goal of creating a “culture of ‘quality enthusiasts.’”
“We want to be the home for quality for hospitalists,” she said. “So this input is really helpful for all of us in here.”
Scrounging for information that could help her with quality improvement (Q.I.) projects, Charmaine Lewis, MD, MPH, the quality director with New Hanover Hospitalists in Wilmington, N.C., said she finds herself reviewing posters at the Annual Meeting and squinting at the images to see whether the electronic health records involved match the system she uses back home.
There’s got to be a better way.
Mangla Gulati, MD, SFHM, associate professor of medicine and chief quality officer at the University of Maryland, and Jenna Goldstein, director of SHM’s Center for Hospital Innovation and Improvement, led what grew into a lively, thoughtful discussion about ways for hospitalists to exchange information and to get support with Q.I. efforts at their centers.
The hospitalists who attended brought their experiences and their questions from a diverse range of centers, from Anchorage, Alaska, to southern California to Poughkeepsie, New York. They emphasized the need for easier access to initiatives that mirror what they are trying to do at their own centers, for more mentoring opportunities, and for more SHM chapters outside major metropolitan areas to help with networking. And they offered lessons on what they have already learned.
Remus Popa, MD, FHM, associate professor of medicine at the University of California, Riverside, who has led Q.I. projects on the use of telemetry, said a successful project will likely be more than an idea that blooms inside the head of a hospitalist.
“We have to find something that the institution really needs,” Dr. Popa said. “You have to design a better fit with them. Otherwise, it’s going to be a tough conversation about resources, right?”
Esther Lee, MD, a hospitalist in Anchorage, Alaska, asked whether it might be possible for SHM to host a “mini-conference” specifically on quality improvement. At the Annual Meeting, she said, she often finds herself torn between attending Q.I. sessions and sessions on clinical topics.
SHM’s Goldstein said it was a possibility.
“It is something that we have talked about and will continue to talk about,” she said.
David Lucier, MD, MBA, MPH, Director of Quality and Safety for Hospital Medicine at Massachusetts General Hospital, said that emphasizing the safety aspect of a project can often help push it along.
“I find these [types of projects] to be quite compelling, at least in reordering the priority list,” he said.
Ms. Goldstein said the suggestions were helpful in SHM’s goal of creating a “culture of ‘quality enthusiasts.’”
“We want to be the home for quality for hospitalists,” she said. “So this input is really helpful for all of us in here.”
Scrounging for information that could help her with quality improvement (Q.I.) projects, Charmaine Lewis, MD, MPH, the quality director with New Hanover Hospitalists in Wilmington, N.C., said she finds herself reviewing posters at the Annual Meeting and squinting at the images to see whether the electronic health records involved match the system she uses back home.
There’s got to be a better way.
Mangla Gulati, MD, SFHM, associate professor of medicine and chief quality officer at the University of Maryland, and Jenna Goldstein, director of SHM’s Center for Hospital Innovation and Improvement, led what grew into a lively, thoughtful discussion about ways for hospitalists to exchange information and to get support with Q.I. efforts at their centers.
The hospitalists who attended brought their experiences and their questions from a diverse range of centers, from Anchorage, Alaska, to southern California to Poughkeepsie, New York. They emphasized the need for easier access to initiatives that mirror what they are trying to do at their own centers, for more mentoring opportunities, and for more SHM chapters outside major metropolitan areas to help with networking. And they offered lessons on what they have already learned.
Remus Popa, MD, FHM, associate professor of medicine at the University of California, Riverside, who has led Q.I. projects on the use of telemetry, said a successful project will likely be more than an idea that blooms inside the head of a hospitalist.
“We have to find something that the institution really needs,” Dr. Popa said. “You have to design a better fit with them. Otherwise, it’s going to be a tough conversation about resources, right?”
Esther Lee, MD, a hospitalist in Anchorage, Alaska, asked whether it might be possible for SHM to host a “mini-conference” specifically on quality improvement. At the Annual Meeting, she said, she often finds herself torn between attending Q.I. sessions and sessions on clinical topics.
SHM’s Goldstein said it was a possibility.
“It is something that we have talked about and will continue to talk about,” she said.
David Lucier, MD, MBA, MPH, Director of Quality and Safety for Hospital Medicine at Massachusetts General Hospital, said that emphasizing the safety aspect of a project can often help push it along.
“I find these [types of projects] to be quite compelling, at least in reordering the priority list,” he said.
Ms. Goldstein said the suggestions were helpful in SHM’s goal of creating a “culture of ‘quality enthusiasts.’”
“We want to be the home for quality for hospitalists,” she said. “So this input is really helpful for all of us in here.”
High CD86+pDC counts may predict CML relapses
Patients with chronic myeloid leukemia (CML) with high CD86+pDC counts had a higher risk of relapse after discontinuing tyrosine kinase inhibitor (TKI) therapy, according to new findings published in Leukemia.
Of patients who achieve a deep molecular remission, only a minority are able to sustain it and remain off therapy. Even when deep remission is achieved, TKI therapy fails to eradicate CML stem cells, which can perpetuate disease.
“This is clinically reflected by the long-term persistence of BCR-ABL messenger RNA (mRNA) in the majority of patients,” wrote C. Schütz, MD, of the University Hospital Marburg (Germany) and colleagues (Leukemia. 2017 Apr;31[4]:829-36). “Even with undetectable BCR-ABL mRNA levels, patients frequently relapse after TKI cessation.”
The researchers investigated whether the expression of the T-cell inhibitory receptor (CTLA-4)-ligand CD86 (B7.2) on plasmacytoid dendritic cells (pDC) could have an effect on the risk of relapse in CML patients who discontinue TKI therapy after achieving remission.
The frequency of CD86+pDC was analyzed in 14 CML patients who were in treatment-free remission, in 130 patients in molecular remission who were part of the CML-V study, and prospectively in 122 EURO-SKI patients right before they discontinued TKI therapy.
The authors found that CML patients in molecular remission had a significantly higher frequency of CD86+pDC expression, compared with normal donors (P less than .0024). In contrast, those who were in treatment-free remission had consistently low CD86+pDC.
These results suggest that low CD86+pDC could be predictive of treatment-free remission.
To test the hypothesis that low CD86+pDC frequencies during TKI-induced molecular remission were associated with a lower risk of molecular relapse after stopping TKI therapy, the study authors measured CD86+pDC levels in the 122 EURO-SKI patients before they stopped therapy, and then prospectively monitored them for relapse.
Findings showed that the 122 EURO-SKI patients had a significantly higher CD86+pDC frequency than did 8 healthy donors (median, 20.8% vs. 7.3%; P = .0024).
When matched with the treatment-free remission patients, the 73 patients in the EURO-SKI group who did not relapse within the first 12 months after stopping therapy had a significantly lower median frequency of CD86+pDC at baseline, compared with the 49 patients who did relapse (P = .014).
Patients who relapsed also demonstrated higher absolute CD86+pDC counts (CD86+pDC per 105 lymphocytes) at baseline (median, 86.1 vs. 50.6; P = .0147).
Based on the findings, the authors noted that they provided “for the first time evidence that relapse biology after TKI discontinuation depends on the quantity of activated pDC and a T-cell exhaustion phenotype, rather than TKI pretreatment duration per se.”
The Clinical Research Group of the German Research Foundation and the German José Carreras Leukaemia Foundation funded the study. Several of the authors report relationships with Ariad, Bristol-Myers Squibb, Novartis, and Pfizer.
Patients with chronic myeloid leukemia (CML) with high CD86+pDC counts had a higher risk of relapse after discontinuing tyrosine kinase inhibitor (TKI) therapy, according to new findings published in Leukemia.
Of patients who achieve a deep molecular remission, only a minority are able to sustain it and remain off therapy. Even when deep remission is achieved, TKI therapy fails to eradicate CML stem cells, which can perpetuate disease.
“This is clinically reflected by the long-term persistence of BCR-ABL messenger RNA (mRNA) in the majority of patients,” wrote C. Schütz, MD, of the University Hospital Marburg (Germany) and colleagues (Leukemia. 2017 Apr;31[4]:829-36). “Even with undetectable BCR-ABL mRNA levels, patients frequently relapse after TKI cessation.”
The researchers investigated whether the expression of the T-cell inhibitory receptor (CTLA-4)-ligand CD86 (B7.2) on plasmacytoid dendritic cells (pDC) could have an effect on the risk of relapse in CML patients who discontinue TKI therapy after achieving remission.
The frequency of CD86+pDC was analyzed in 14 CML patients who were in treatment-free remission, in 130 patients in molecular remission who were part of the CML-V study, and prospectively in 122 EURO-SKI patients right before they discontinued TKI therapy.
The authors found that CML patients in molecular remission had a significantly higher frequency of CD86+pDC expression, compared with normal donors (P less than .0024). In contrast, those who were in treatment-free remission had consistently low CD86+pDC.
These results suggest that low CD86+pDC could be predictive of treatment-free remission.
To test the hypothesis that low CD86+pDC frequencies during TKI-induced molecular remission were associated with a lower risk of molecular relapse after stopping TKI therapy, the study authors measured CD86+pDC levels in the 122 EURO-SKI patients before they stopped therapy, and then prospectively monitored them for relapse.
Findings showed that the 122 EURO-SKI patients had a significantly higher CD86+pDC frequency than did 8 healthy donors (median, 20.8% vs. 7.3%; P = .0024).
When matched with the treatment-free remission patients, the 73 patients in the EURO-SKI group who did not relapse within the first 12 months after stopping therapy had a significantly lower median frequency of CD86+pDC at baseline, compared with the 49 patients who did relapse (P = .014).
Patients who relapsed also demonstrated higher absolute CD86+pDC counts (CD86+pDC per 105 lymphocytes) at baseline (median, 86.1 vs. 50.6; P = .0147).
Based on the findings, the authors noted that they provided “for the first time evidence that relapse biology after TKI discontinuation depends on the quantity of activated pDC and a T-cell exhaustion phenotype, rather than TKI pretreatment duration per se.”
The Clinical Research Group of the German Research Foundation and the German José Carreras Leukaemia Foundation funded the study. Several of the authors report relationships with Ariad, Bristol-Myers Squibb, Novartis, and Pfizer.
Patients with chronic myeloid leukemia (CML) with high CD86+pDC counts had a higher risk of relapse after discontinuing tyrosine kinase inhibitor (TKI) therapy, according to new findings published in Leukemia.
Of patients who achieve a deep molecular remission, only a minority are able to sustain it and remain off therapy. Even when deep remission is achieved, TKI therapy fails to eradicate CML stem cells, which can perpetuate disease.
“This is clinically reflected by the long-term persistence of BCR-ABL messenger RNA (mRNA) in the majority of patients,” wrote C. Schütz, MD, of the University Hospital Marburg (Germany) and colleagues (Leukemia. 2017 Apr;31[4]:829-36). “Even with undetectable BCR-ABL mRNA levels, patients frequently relapse after TKI cessation.”
The researchers investigated whether the expression of the T-cell inhibitory receptor (CTLA-4)-ligand CD86 (B7.2) on plasmacytoid dendritic cells (pDC) could have an effect on the risk of relapse in CML patients who discontinue TKI therapy after achieving remission.
The frequency of CD86+pDC was analyzed in 14 CML patients who were in treatment-free remission, in 130 patients in molecular remission who were part of the CML-V study, and prospectively in 122 EURO-SKI patients right before they discontinued TKI therapy.
The authors found that CML patients in molecular remission had a significantly higher frequency of CD86+pDC expression, compared with normal donors (P less than .0024). In contrast, those who were in treatment-free remission had consistently low CD86+pDC.
These results suggest that low CD86+pDC could be predictive of treatment-free remission.
To test the hypothesis that low CD86+pDC frequencies during TKI-induced molecular remission were associated with a lower risk of molecular relapse after stopping TKI therapy, the study authors measured CD86+pDC levels in the 122 EURO-SKI patients before they stopped therapy, and then prospectively monitored them for relapse.
Findings showed that the 122 EURO-SKI patients had a significantly higher CD86+pDC frequency than did 8 healthy donors (median, 20.8% vs. 7.3%; P = .0024).
When matched with the treatment-free remission patients, the 73 patients in the EURO-SKI group who did not relapse within the first 12 months after stopping therapy had a significantly lower median frequency of CD86+pDC at baseline, compared with the 49 patients who did relapse (P = .014).
Patients who relapsed also demonstrated higher absolute CD86+pDC counts (CD86+pDC per 105 lymphocytes) at baseline (median, 86.1 vs. 50.6; P = .0147).
Based on the findings, the authors noted that they provided “for the first time evidence that relapse biology after TKI discontinuation depends on the quantity of activated pDC and a T-cell exhaustion phenotype, rather than TKI pretreatment duration per se.”
The Clinical Research Group of the German Research Foundation and the German José Carreras Leukaemia Foundation funded the study. Several of the authors report relationships with Ariad, Bristol-Myers Squibb, Novartis, and Pfizer.
FROM LEUKEMIA
Key clinical point: High CD86+pDC counts predicted relapses in CML patients who stopped TKI therapy.
Major finding: CML patients in molecular remission had significantly higher CD86+pDC frequencies, while patients in treatment-free remission had consistently low CD86+pDC.
Data source: A study that used patient cohorts (n = 14, n = 130, n = 122) at different stages of TKI discontinuation and remission.
Disclosures: The Clinical Research Group of the German Research Foundation and the German José Carreras Leukaemia Foundation funded the study. Several of the authors report relationships with Ariad, Bristol-Myers Squibb, Novartis, and Pfizer.
Proper UTI diagnosis, treatment relies on cautious approach
LAS VEGAS – Prudent use of catheters, cultures, and antibiotics are three keys to proper urinary tract diagnosis and management, according to a speaker at this year’s annual meeting of the Society of Hospital Medicine.
“We have not done very well with decreasing catheter-associated urinary tract infections,” said Jennifer Hanrahan, DO, an assistant professor of infectious disease medicine at Case Western Reserve University in Cleveland, Ohio, and an infectious disease physician at MetroHealth Medical Center, where she is the medical director for infection prevention. “The main reason is people don’t really think of i
The main way to reduce catheter-associated urinary tract infections is to avoid catheters, she said, noting “that’s obvious, but unnecessary catheters get put in all the time.”
Dr. Hanrahan recommended only putting them in when absolutely necessary, doing so in a sterile manner, and then continually monitoring whether the patient still needs the catheter.
Knowing when to obtain a urine culture is key to ensuring proper diagnosing and treatment for UTIs. A person who is asymptomatic does not need a culture, Dr. Hanrahan said during the well-attended, rapid-fire session. Those who do need a culture include septic patients with no apparent cause for their symptomatic presentation, despite a careful history taking and physical exam. Also, patients with pelvic pain, or flank tenderness for whom no cause can otherwise be determined should be cultured. It is also appropriate to screen for asymptomatic bacteriuria for pregnant patients, since it can be a sign of premature labor, and for patients about to undergo any invasive urologic procedure, Dr Hanrahan said.
“An awful lot of people have asymptomatic bacteriuria all the time,” she added, “and it doesn’t mean anything.”
Reasons to not culture include urine that smells “off” or that is cloudy or has sediment. “Anyone who has eaten asparagus knows that, after you eat it, your urine smells weird. It doesn’t mean you have a UTI,” she said.
She recommended against “pan culturing” in sepsis, and culturing “just because” when there is a clearly identifiable cause for the fever.
Once a diagnosis is made, Dr. Hanrahan urged physicians to avoid the overuse of antibiotics, suggesting that, whenever possible, the shortest possible course should be used. In order to help preserve antibiotic resistance, she also recommended using antibiotics that are not as prevalent, in order to help preserve antibiotic resistance. These could include nitrofurantoin and fosfomycin.
Dr. Hanrahan had no relevant financial disclosures.
LAS VEGAS – Prudent use of catheters, cultures, and antibiotics are three keys to proper urinary tract diagnosis and management, according to a speaker at this year’s annual meeting of the Society of Hospital Medicine.
“We have not done very well with decreasing catheter-associated urinary tract infections,” said Jennifer Hanrahan, DO, an assistant professor of infectious disease medicine at Case Western Reserve University in Cleveland, Ohio, and an infectious disease physician at MetroHealth Medical Center, where she is the medical director for infection prevention. “The main reason is people don’t really think of i
The main way to reduce catheter-associated urinary tract infections is to avoid catheters, she said, noting “that’s obvious, but unnecessary catheters get put in all the time.”
Dr. Hanrahan recommended only putting them in when absolutely necessary, doing so in a sterile manner, and then continually monitoring whether the patient still needs the catheter.
Knowing when to obtain a urine culture is key to ensuring proper diagnosing and treatment for UTIs. A person who is asymptomatic does not need a culture, Dr. Hanrahan said during the well-attended, rapid-fire session. Those who do need a culture include septic patients with no apparent cause for their symptomatic presentation, despite a careful history taking and physical exam. Also, patients with pelvic pain, or flank tenderness for whom no cause can otherwise be determined should be cultured. It is also appropriate to screen for asymptomatic bacteriuria for pregnant patients, since it can be a sign of premature labor, and for patients about to undergo any invasive urologic procedure, Dr Hanrahan said.
“An awful lot of people have asymptomatic bacteriuria all the time,” she added, “and it doesn’t mean anything.”
Reasons to not culture include urine that smells “off” or that is cloudy or has sediment. “Anyone who has eaten asparagus knows that, after you eat it, your urine smells weird. It doesn’t mean you have a UTI,” she said.
She recommended against “pan culturing” in sepsis, and culturing “just because” when there is a clearly identifiable cause for the fever.
Once a diagnosis is made, Dr. Hanrahan urged physicians to avoid the overuse of antibiotics, suggesting that, whenever possible, the shortest possible course should be used. In order to help preserve antibiotic resistance, she also recommended using antibiotics that are not as prevalent, in order to help preserve antibiotic resistance. These could include nitrofurantoin and fosfomycin.
Dr. Hanrahan had no relevant financial disclosures.
LAS VEGAS – Prudent use of catheters, cultures, and antibiotics are three keys to proper urinary tract diagnosis and management, according to a speaker at this year’s annual meeting of the Society of Hospital Medicine.
“We have not done very well with decreasing catheter-associated urinary tract infections,” said Jennifer Hanrahan, DO, an assistant professor of infectious disease medicine at Case Western Reserve University in Cleveland, Ohio, and an infectious disease physician at MetroHealth Medical Center, where she is the medical director for infection prevention. “The main reason is people don’t really think of i
The main way to reduce catheter-associated urinary tract infections is to avoid catheters, she said, noting “that’s obvious, but unnecessary catheters get put in all the time.”
Dr. Hanrahan recommended only putting them in when absolutely necessary, doing so in a sterile manner, and then continually monitoring whether the patient still needs the catheter.
Knowing when to obtain a urine culture is key to ensuring proper diagnosing and treatment for UTIs. A person who is asymptomatic does not need a culture, Dr. Hanrahan said during the well-attended, rapid-fire session. Those who do need a culture include septic patients with no apparent cause for their symptomatic presentation, despite a careful history taking and physical exam. Also, patients with pelvic pain, or flank tenderness for whom no cause can otherwise be determined should be cultured. It is also appropriate to screen for asymptomatic bacteriuria for pregnant patients, since it can be a sign of premature labor, and for patients about to undergo any invasive urologic procedure, Dr Hanrahan said.
“An awful lot of people have asymptomatic bacteriuria all the time,” she added, “and it doesn’t mean anything.”
Reasons to not culture include urine that smells “off” or that is cloudy or has sediment. “Anyone who has eaten asparagus knows that, after you eat it, your urine smells weird. It doesn’t mean you have a UTI,” she said.
She recommended against “pan culturing” in sepsis, and culturing “just because” when there is a clearly identifiable cause for the fever.
Once a diagnosis is made, Dr. Hanrahan urged physicians to avoid the overuse of antibiotics, suggesting that, whenever possible, the shortest possible course should be used. In order to help preserve antibiotic resistance, she also recommended using antibiotics that are not as prevalent, in order to help preserve antibiotic resistance. These could include nitrofurantoin and fosfomycin.
Dr. Hanrahan had no relevant financial disclosures.
Hospitalists offer tips on QI projects
Anjala Tess, MD, a hospitalist at Beth Israel Deaconess Medical Center and assistant professor of medicine at Harvard Medical School, Boston, asked the audience how many of them had done a quality improvement project that had failed. It was not a time to be bashful: Almost half the hospitalists in the room admitted that it had happened to them.
Dr. Tess and Darlene Tad-y, MD, chair of the Physicians-in-Training Committee and an assistant professor of medicine at the University of Colorado, were there to offer help in their session, “Adding to Your QI Toolbox.”
They highlighted three tools that they say are crucial to a project’s success: Creating a process map for clearly outlining how the project will work, interacting with stakeholders productively, and displaying data in a meaningful way.
Process mapping is a way of visualizing work or a process as distinct, ordered, and related steps, Dr. Tess said. Arranged on Post-It notes or written on a white board, the process should be one that can easily be updated. Seen objectively as a set of steps, it helps remove bias in how a process is viewed, she said.
“I would encourage you to do this on every QI project that you do where you are trying to accomplish something, because it makes a huge difference in understanding the work,” she said.
Stakeholder analysis – understanding the key people whose support could determine the success of the project – is also critical, she said. This can help get buy-in for the change, and it make a project stronger – and without it, you could doom your project, Dr. Tess said.
Doing this well involves understanding their financial and emotional interests, all the way down to whether they prefer face-to-face communication or e-mail, she said.
Then there’s the data. For it to matter, it must be presented well, and that requires context, Dr. Tad-y said.
“Data are just raw facts and figures,” she said. “Data are not the same as information.”
She suggested using run charts, in which data are plotted in some kind of order, usually chronological order. This kind of chart will typically include a median line, showing practice patterns before the QI project began, as well as a “goal line” to aim for, and notations on the timeline when changes were made, she said.
Others will be looking to the project manager for the meaning behind the data, she said.
“The story is going to come from you,” she said. “Otherwise, it’s just numbers.”
Anjala Tess, MD, a hospitalist at Beth Israel Deaconess Medical Center and assistant professor of medicine at Harvard Medical School, Boston, asked the audience how many of them had done a quality improvement project that had failed. It was not a time to be bashful: Almost half the hospitalists in the room admitted that it had happened to them.
Dr. Tess and Darlene Tad-y, MD, chair of the Physicians-in-Training Committee and an assistant professor of medicine at the University of Colorado, were there to offer help in their session, “Adding to Your QI Toolbox.”
They highlighted three tools that they say are crucial to a project’s success: Creating a process map for clearly outlining how the project will work, interacting with stakeholders productively, and displaying data in a meaningful way.
Process mapping is a way of visualizing work or a process as distinct, ordered, and related steps, Dr. Tess said. Arranged on Post-It notes or written on a white board, the process should be one that can easily be updated. Seen objectively as a set of steps, it helps remove bias in how a process is viewed, she said.
“I would encourage you to do this on every QI project that you do where you are trying to accomplish something, because it makes a huge difference in understanding the work,” she said.
Stakeholder analysis – understanding the key people whose support could determine the success of the project – is also critical, she said. This can help get buy-in for the change, and it make a project stronger – and without it, you could doom your project, Dr. Tess said.
Doing this well involves understanding their financial and emotional interests, all the way down to whether they prefer face-to-face communication or e-mail, she said.
Then there’s the data. For it to matter, it must be presented well, and that requires context, Dr. Tad-y said.
“Data are just raw facts and figures,” she said. “Data are not the same as information.”
She suggested using run charts, in which data are plotted in some kind of order, usually chronological order. This kind of chart will typically include a median line, showing practice patterns before the QI project began, as well as a “goal line” to aim for, and notations on the timeline when changes were made, she said.
Others will be looking to the project manager for the meaning behind the data, she said.
“The story is going to come from you,” she said. “Otherwise, it’s just numbers.”
Anjala Tess, MD, a hospitalist at Beth Israel Deaconess Medical Center and assistant professor of medicine at Harvard Medical School, Boston, asked the audience how many of them had done a quality improvement project that had failed. It was not a time to be bashful: Almost half the hospitalists in the room admitted that it had happened to them.
Dr. Tess and Darlene Tad-y, MD, chair of the Physicians-in-Training Committee and an assistant professor of medicine at the University of Colorado, were there to offer help in their session, “Adding to Your QI Toolbox.”
They highlighted three tools that they say are crucial to a project’s success: Creating a process map for clearly outlining how the project will work, interacting with stakeholders productively, and displaying data in a meaningful way.
Process mapping is a way of visualizing work or a process as distinct, ordered, and related steps, Dr. Tess said. Arranged on Post-It notes or written on a white board, the process should be one that can easily be updated. Seen objectively as a set of steps, it helps remove bias in how a process is viewed, she said.
“I would encourage you to do this on every QI project that you do where you are trying to accomplish something, because it makes a huge difference in understanding the work,” she said.
Stakeholder analysis – understanding the key people whose support could determine the success of the project – is also critical, she said. This can help get buy-in for the change, and it make a project stronger – and without it, you could doom your project, Dr. Tess said.
Doing this well involves understanding their financial and emotional interests, all the way down to whether they prefer face-to-face communication or e-mail, she said.
Then there’s the data. For it to matter, it must be presented well, and that requires context, Dr. Tad-y said.
“Data are just raw facts and figures,” she said. “Data are not the same as information.”
She suggested using run charts, in which data are plotted in some kind of order, usually chronological order. This kind of chart will typically include a median line, showing practice patterns before the QI project began, as well as a “goal line” to aim for, and notations on the timeline when changes were made, she said.
Others will be looking to the project manager for the meaning behind the data, she said.
“The story is going to come from you,” she said. “Otherwise, it’s just numbers.”
Work-life balance is not a ‘thing’ but alignment is
We are destined to fail at achieving work-life balance because it does not exist, nor has it ever existed, according to an expert on how people experience time.
The field of chronemics studies the space between people, namely how they communicate with others and with themselves as they experience life in a matrix, not just linearly.
“When we talk about time, we need to remember that context and communication really matter,” Dawna Ballard, PhD, a chronemics expert and associate professor of communication at the University of Texas at Austin, said during a session Tuesday. “We think of our concept of time as ‘truth,’ but when we experience other cultures, often we see they don’t function according to our idea of time.”
The modern concept of time as told by a clock was invented during the Industrial Age to control people and events by forcing a direct line between their lives outside the factory and the farm, according to Dr. Ballard. That is not to say that industrial time is bad. It is efficient and perhaps even necessary for organizing large groups of people, she said.
Time management as a concept evolved out of the ethos of “punching the clock” at the factory, but has carried over to the office where in fact, according to Dr. Ballard, people do not tend to be as effective if they are expected to work in a linear way, since that is not the experience in today’s digital world where interruptions are common. In a survey of 1,000 people, after researchers subtracted the time people spend on email, social media, and other digital interruptions, only 3½ hours of the typical 8-hour work day were left for actual work, and these remaining hours were not consecutive, Dr. Ballard noted.
“In reality, we operate in relationship to time more like we did in preindustrial times than we did during industrial times,” said Dr. Ballard. “Medicine has always had that approach, but the management of it is by people who are still being trained according to industrial [notions] of time.”
The resulting cognitive dissonance contributes to people experiencing guilt for not “balancing” their day properly, according to Dr. Ballard. Because to balance something means separating the pieces and quantifying them separately, people whose lives interrupt them throughout the work day find themselves thinking they have “failed” at achieving a work/life balance, when what they really have done is experience their life as it actually is. “No one likes to feel like a failure, particularly people who are high achievers and who expect to have agency over their lives.”
Dr. Ballard said that an alternative to seeing work and life as components that must be balanced is to instead view these things as being in an alignment that can shift over time. She also suggested challenging the accepted notions of what being productive really means in the context of how one’s life actually is, and to occasionally put down the smartphone and consciously practice experiencing time in an unstructured way.
We are destined to fail at achieving work-life balance because it does not exist, nor has it ever existed, according to an expert on how people experience time.
The field of chronemics studies the space between people, namely how they communicate with others and with themselves as they experience life in a matrix, not just linearly.
“When we talk about time, we need to remember that context and communication really matter,” Dawna Ballard, PhD, a chronemics expert and associate professor of communication at the University of Texas at Austin, said during a session Tuesday. “We think of our concept of time as ‘truth,’ but when we experience other cultures, often we see they don’t function according to our idea of time.”
The modern concept of time as told by a clock was invented during the Industrial Age to control people and events by forcing a direct line between their lives outside the factory and the farm, according to Dr. Ballard. That is not to say that industrial time is bad. It is efficient and perhaps even necessary for organizing large groups of people, she said.
Time management as a concept evolved out of the ethos of “punching the clock” at the factory, but has carried over to the office where in fact, according to Dr. Ballard, people do not tend to be as effective if they are expected to work in a linear way, since that is not the experience in today’s digital world where interruptions are common. In a survey of 1,000 people, after researchers subtracted the time people spend on email, social media, and other digital interruptions, only 3½ hours of the typical 8-hour work day were left for actual work, and these remaining hours were not consecutive, Dr. Ballard noted.
“In reality, we operate in relationship to time more like we did in preindustrial times than we did during industrial times,” said Dr. Ballard. “Medicine has always had that approach, but the management of it is by people who are still being trained according to industrial [notions] of time.”
The resulting cognitive dissonance contributes to people experiencing guilt for not “balancing” their day properly, according to Dr. Ballard. Because to balance something means separating the pieces and quantifying them separately, people whose lives interrupt them throughout the work day find themselves thinking they have “failed” at achieving a work/life balance, when what they really have done is experience their life as it actually is. “No one likes to feel like a failure, particularly people who are high achievers and who expect to have agency over their lives.”
Dr. Ballard said that an alternative to seeing work and life as components that must be balanced is to instead view these things as being in an alignment that can shift over time. She also suggested challenging the accepted notions of what being productive really means in the context of how one’s life actually is, and to occasionally put down the smartphone and consciously practice experiencing time in an unstructured way.
We are destined to fail at achieving work-life balance because it does not exist, nor has it ever existed, according to an expert on how people experience time.
The field of chronemics studies the space between people, namely how they communicate with others and with themselves as they experience life in a matrix, not just linearly.
“When we talk about time, we need to remember that context and communication really matter,” Dawna Ballard, PhD, a chronemics expert and associate professor of communication at the University of Texas at Austin, said during a session Tuesday. “We think of our concept of time as ‘truth,’ but when we experience other cultures, often we see they don’t function according to our idea of time.”
The modern concept of time as told by a clock was invented during the Industrial Age to control people and events by forcing a direct line between their lives outside the factory and the farm, according to Dr. Ballard. That is not to say that industrial time is bad. It is efficient and perhaps even necessary for organizing large groups of people, she said.
Time management as a concept evolved out of the ethos of “punching the clock” at the factory, but has carried over to the office where in fact, according to Dr. Ballard, people do not tend to be as effective if they are expected to work in a linear way, since that is not the experience in today’s digital world where interruptions are common. In a survey of 1,000 people, after researchers subtracted the time people spend on email, social media, and other digital interruptions, only 3½ hours of the typical 8-hour work day were left for actual work, and these remaining hours were not consecutive, Dr. Ballard noted.
“In reality, we operate in relationship to time more like we did in preindustrial times than we did during industrial times,” said Dr. Ballard. “Medicine has always had that approach, but the management of it is by people who are still being trained according to industrial [notions] of time.”
The resulting cognitive dissonance contributes to people experiencing guilt for not “balancing” their day properly, according to Dr. Ballard. Because to balance something means separating the pieces and quantifying them separately, people whose lives interrupt them throughout the work day find themselves thinking they have “failed” at achieving a work/life balance, when what they really have done is experience their life as it actually is. “No one likes to feel like a failure, particularly people who are high achievers and who expect to have agency over their lives.”
Dr. Ballard said that an alternative to seeing work and life as components that must be balanced is to instead view these things as being in an alignment that can shift over time. She also suggested challenging the accepted notions of what being productive really means in the context of how one’s life actually is, and to occasionally put down the smartphone and consciously practice experiencing time in an unstructured way.
VIDEO: SHM seeks sites to test pediatric transition tool
Would you like to help the Society of Hospital Medicine translate its award-winning Project BOOST® Mentored Implementation Program into the pediatric setting?
“We’re hoping to get six sites to help us implement this project so we can collect data and see how well it works for pediatrics,” James O’Callaghan, MD, medical director, EvergreenHealth, Seattle Children’s, said in an interview.
In this video, recorded during HM17 , Dr. O’Callaghan describes how Pedi-BOOST is intended to work, and what types of pediatric transition concerns it is intended to address.
For more information, please visit the SHM website.
Dr. O’Callaghan had no relevant disclosures.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Would you like to help the Society of Hospital Medicine translate its award-winning Project BOOST® Mentored Implementation Program into the pediatric setting?
“We’re hoping to get six sites to help us implement this project so we can collect data and see how well it works for pediatrics,” James O’Callaghan, MD, medical director, EvergreenHealth, Seattle Children’s, said in an interview.
In this video, recorded during HM17 , Dr. O’Callaghan describes how Pedi-BOOST is intended to work, and what types of pediatric transition concerns it is intended to address.
For more information, please visit the SHM website.
Dr. O’Callaghan had no relevant disclosures.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Would you like to help the Society of Hospital Medicine translate its award-winning Project BOOST® Mentored Implementation Program into the pediatric setting?
“We’re hoping to get six sites to help us implement this project so we can collect data and see how well it works for pediatrics,” James O’Callaghan, MD, medical director, EvergreenHealth, Seattle Children’s, said in an interview.
In this video, recorded during HM17 , Dr. O’Callaghan describes how Pedi-BOOST is intended to work, and what types of pediatric transition concerns it is intended to address.
For more information, please visit the SHM website.
Dr. O’Callaghan had no relevant disclosures.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Digestive Disease Week® – always the biggest GI meeting in the world
GI & Hepatology News will be in Chicago this week at the McCormick Place Convention Center reporting the latest news and perspective across gastroenterology. Studies at this year’s meeting have a decidedly genetic slant as the genetic bases for many GI and liver diseases are being determined and studied for their use in treatments.
Our reporters will cover comparative effectiveness studies and controversies in inflammatory bowel disease; Clostridium difficile colitis; and prevention and treatment of clinical hepatitis, among many other topics, as well as an NIH Consortium presentation on chronic pancreatitis, diabetes, and pancreatic cancer.
Highly anticipated presentations include:
- Genetic markers could improve treatment of hepatitis C.
- Nonsurgical weight-loss treatment could help patients with limited options.
- First-ever autonomously controlled “capsule robot” explores colon.
- Enzyme could be a game-changer for gluten-sensitive patients.
Our team will provide daily coverage, starting Saturday, May 6.
GI & Hepatology News will be in Chicago this week at the McCormick Place Convention Center reporting the latest news and perspective across gastroenterology. Studies at this year’s meeting have a decidedly genetic slant as the genetic bases for many GI and liver diseases are being determined and studied for their use in treatments.
Our reporters will cover comparative effectiveness studies and controversies in inflammatory bowel disease; Clostridium difficile colitis; and prevention and treatment of clinical hepatitis, among many other topics, as well as an NIH Consortium presentation on chronic pancreatitis, diabetes, and pancreatic cancer.
Highly anticipated presentations include:
- Genetic markers could improve treatment of hepatitis C.
- Nonsurgical weight-loss treatment could help patients with limited options.
- First-ever autonomously controlled “capsule robot” explores colon.
- Enzyme could be a game-changer for gluten-sensitive patients.
Our team will provide daily coverage, starting Saturday, May 6.
GI & Hepatology News will be in Chicago this week at the McCormick Place Convention Center reporting the latest news and perspective across gastroenterology. Studies at this year’s meeting have a decidedly genetic slant as the genetic bases for many GI and liver diseases are being determined and studied for their use in treatments.
Our reporters will cover comparative effectiveness studies and controversies in inflammatory bowel disease; Clostridium difficile colitis; and prevention and treatment of clinical hepatitis, among many other topics, as well as an NIH Consortium presentation on chronic pancreatitis, diabetes, and pancreatic cancer.
Highly anticipated presentations include:
- Genetic markers could improve treatment of hepatitis C.
- Nonsurgical weight-loss treatment could help patients with limited options.
- First-ever autonomously controlled “capsule robot” explores colon.
- Enzyme could be a game-changer for gluten-sensitive patients.
Our team will provide daily coverage, starting Saturday, May 6.
FROM DDW
Top Acthar prescribers reap hefty payments from drug maker
BOSTON – A new analysis finds that some of the biggest neurologist prescribers of repository corticotropin gel (Acthar) – the extraordinarily expensive multiple sclerosis (MS) relapse drug – reaped extensive payments from its manufacturer, with one taking in $130,307 in a single year.
Together, 51 neurologists accounted for 980 Medicare claims worth more than $39 million in Acthar spending in 2014, almost half of the entire estimated $83 million in Medicare spending on neurologist-prescribed Acthar that year.
“There is a small group of neurologists – less than 1% – who are prescribing Acthar at considerable cost to Medicare and may do this in part because of financial relationships with the company that sells Acthar,” said study lead author Dennis Bourdette, MD, chair and research professor of neurology at Oregon Health and Science University, Portland.
Dr. Bourdette acknowledges that the research doesn’t prove a causal relationship between payments and prescriptions. In response to questions, Acthar manufacturer Mallinckrodt Pharmaceuticals, already under fire for the $34,000-per-vial cost of the drug, questioned the study design and denied wrongdoing in a statement: “Mallinckrodt is committed to following the highest standards for integrity and compliance in all of our business practices, including our collaboration with physicians.”
Acthar, also known as H.P. Acthar, is the poster child for stunningly expensive medication. According to the Federal Trade Commission, the cost of the drug rose from $40 per vial in 2001 to more than $34,000 this year. The New York Times reports that Medicare spending on Acthar topped half a billion in 2015.
Earlier this year, Mallinckrodt ARD (formerly Questcor) and its parent company agreed to pay a $100 million fine to settle charges that it created an illegal monopoly over the drug.
Dr. Bourdette and colleagues released their findings at the annual meeting of the American Academy of Neurology.
In an interview, Dr. Bourdette said the study’s roots lie in his concern about the medication, whose transformation from an inexpensive 1950s-era medication for pulmonary sarcoidosis to high-priced MS treatment has drawn national media attention.
“I believe that Acthar is tremendously overpriced and a waste of health care money,” Dr. Bourdette said. “I wanted to find out how extensive an economic problem it was and how much money Medicare was spending on it since this data was easily accessible.”
According to the study, Medicare spent $1.3 billion from 2011 to 2015 on Acthar, with about 25% of the cost due to prescriptions from neurologists. “When I discovered that a relatively small number of neurologists was prescribing it commonly at a cost of $40-$50 million a year to Medicare, I was interested in determining why they were prescribing this expensive therapy and postulated that it might be related to financial support they were receiving from the manufacturer,” Dr. Bourdette said.
The researchers examined the Medicare Part D Public Use File to determine which neurologists prescribed Acthar frequently in 2014. They identified 51 who prescribed Acthar 10 or more times that year and were frequent prescribers of MS disease-modifying therapy, indicating they treated many people with MS.
The 51 neurologists accounted for a mean of 19 Acthar claims each in 2014 (range, 11-50) totaling a mean annual cost of $770,145 (range, $354,479-$3,623,509). Together, the neurologists accounted for total Medicare spending on Acthar of $39,277,380.
The researchers also chose a control group – 51 neurologists who prescribed glatiramer acetate (Copaxone) more than 10 times in 2014 and also prescribed a similar frequency of all MS disease-modifying therapies as the high-frequency Acthar group.
“Acthar is used episodically to treat MS relapses, and glatiramer acetate is taken chronically to prevent relapses and disability,” Dr. Bourdette said. He added that glatiramer acetate is now available in a generic, but it wasn’t in 2014.
The Acthar and Copaxone groups were nearly identical in terms of gender (about two-thirds men) and years since graduation (a mean of 26), but the Acthar prescribers were more likely to work in small practices (1-10 doctors), file more prescription claims, see more Medicare patients, and practice in the South or West. The demographic information came from CMS Physician Compare.
In terms of overall drug maker payments, neurologists in the Acthar group accepted much more (median, $54,270; range, $623-$369,847) than did the Copaxone group (median, $1,747, range, $0-$256,305, P less than .001). Payment information came from the federal Open Payments database.
As for payments directly from the manufacturers of the two drugs, the Acthar prescribers accepted a median of $5,344 (range, $0-$130,307) from its manufacturer, while the Copaxone prescribers accepted a median of $137 (range, $0-$168,373) from Teva (P = .003).
“The payments are primarily for giving lectures or serving on advisory boards,” Dr. Bourdette said. “These types of payments are commonly made by pharmaceutical companies to physicians who participate in these types of activities.”
In a statement, Mallinckrodt Pharmaceuticals contends that the study inappropriately compares Acthar, often a later-line therapy, to Copaxone, which it says is often a first-line therapy.
Dr. Bourdette responded that the researchers chose a comparison group of top prescribers of Copaxone “as a marker of neurologists who treated a significant number of patients with MS.”
Mallinckrodt also notes that “there may be unmeasured confounding factors in the matching process between the comparator physician groups. These could include differences in the patient characteristics managed by these physicians including disease severity affecting prescribing patterns.”
The researchers agree that there may be differences between the groups, Dr. Bourdette said. However, he added, “the fact remains that the two groups differed in the amount of money they received as open-source payments from pharmaceutical companies. We doubt that the severity of their case mixes should lead to one group receiving more pharmaceutical open-source payments than another.”
Finally, Mallinckrodt says there’s no proof of a causal connection between the payments and the prescriptions; the study authors agree. And, the company says, “this pattern of correlation would be expected in any scenario where a small number of prescribers are the experts in the use of a later-line drug to treat a limited subset of patients.”
Dr. Bourdette rejects this contention. Few of the high Acthar prescribers practice at academic centers, he said, and few are recognized for their MS expertise. “So to suggest that they are experts in the use of Acthar for the treatment of MS when they are not as a group recognized as being experts on the treatment of MS in general is incredible,” he said. “Why a small group of neurologists prescribe Acthar remains a mystery, but it is not because the majority are leaders in the field of MS therapeutics.”
In an interview, Eric G. Campbell, PhD, professor of medicine and director of research at the Mongan Institute Health Policy Center at Harvard Medical School, Boston, said the study findings fit in with previous research that has found that “the more money that people get, the more they use the drug.
“Any reasonable person looking at this data would assume or at least consider very strongly that there is a causal relationship here,” he said.
What should be done? “There are lots of ways to stop this,” Dr. Campbell said. “One could simply impose rules that forbid doctors who accept payments for marketing drugs from billing Medicare or private payers for the care they provide. Large provider organizations could pass rules that forbid this kind of behavior. Finally, the government could vigorously pursue stiff penalties against physicians who accept payments that are really nothing more than incentives to encourage or sustain prescribing practices.”
The study was supported by a National Multiple Sclerosis Society grant. Dr. Bourdette said that he provides consulting to Magellan Healthcare, a company that provides recommendations to insurance companies on the approval of high-cost therapies. Dr. Campbell disclosed that he serves as an expert witness on law cases related to conflicts of interest in medicine.
BOSTON – A new analysis finds that some of the biggest neurologist prescribers of repository corticotropin gel (Acthar) – the extraordinarily expensive multiple sclerosis (MS) relapse drug – reaped extensive payments from its manufacturer, with one taking in $130,307 in a single year.
Together, 51 neurologists accounted for 980 Medicare claims worth more than $39 million in Acthar spending in 2014, almost half of the entire estimated $83 million in Medicare spending on neurologist-prescribed Acthar that year.
“There is a small group of neurologists – less than 1% – who are prescribing Acthar at considerable cost to Medicare and may do this in part because of financial relationships with the company that sells Acthar,” said study lead author Dennis Bourdette, MD, chair and research professor of neurology at Oregon Health and Science University, Portland.
Dr. Bourdette acknowledges that the research doesn’t prove a causal relationship between payments and prescriptions. In response to questions, Acthar manufacturer Mallinckrodt Pharmaceuticals, already under fire for the $34,000-per-vial cost of the drug, questioned the study design and denied wrongdoing in a statement: “Mallinckrodt is committed to following the highest standards for integrity and compliance in all of our business practices, including our collaboration with physicians.”
Acthar, also known as H.P. Acthar, is the poster child for stunningly expensive medication. According to the Federal Trade Commission, the cost of the drug rose from $40 per vial in 2001 to more than $34,000 this year. The New York Times reports that Medicare spending on Acthar topped half a billion in 2015.
Earlier this year, Mallinckrodt ARD (formerly Questcor) and its parent company agreed to pay a $100 million fine to settle charges that it created an illegal monopoly over the drug.
Dr. Bourdette and colleagues released their findings at the annual meeting of the American Academy of Neurology.
In an interview, Dr. Bourdette said the study’s roots lie in his concern about the medication, whose transformation from an inexpensive 1950s-era medication for pulmonary sarcoidosis to high-priced MS treatment has drawn national media attention.
“I believe that Acthar is tremendously overpriced and a waste of health care money,” Dr. Bourdette said. “I wanted to find out how extensive an economic problem it was and how much money Medicare was spending on it since this data was easily accessible.”
According to the study, Medicare spent $1.3 billion from 2011 to 2015 on Acthar, with about 25% of the cost due to prescriptions from neurologists. “When I discovered that a relatively small number of neurologists was prescribing it commonly at a cost of $40-$50 million a year to Medicare, I was interested in determining why they were prescribing this expensive therapy and postulated that it might be related to financial support they were receiving from the manufacturer,” Dr. Bourdette said.
The researchers examined the Medicare Part D Public Use File to determine which neurologists prescribed Acthar frequently in 2014. They identified 51 who prescribed Acthar 10 or more times that year and were frequent prescribers of MS disease-modifying therapy, indicating they treated many people with MS.
The 51 neurologists accounted for a mean of 19 Acthar claims each in 2014 (range, 11-50) totaling a mean annual cost of $770,145 (range, $354,479-$3,623,509). Together, the neurologists accounted for total Medicare spending on Acthar of $39,277,380.
The researchers also chose a control group – 51 neurologists who prescribed glatiramer acetate (Copaxone) more than 10 times in 2014 and also prescribed a similar frequency of all MS disease-modifying therapies as the high-frequency Acthar group.
“Acthar is used episodically to treat MS relapses, and glatiramer acetate is taken chronically to prevent relapses and disability,” Dr. Bourdette said. He added that glatiramer acetate is now available in a generic, but it wasn’t in 2014.
The Acthar and Copaxone groups were nearly identical in terms of gender (about two-thirds men) and years since graduation (a mean of 26), but the Acthar prescribers were more likely to work in small practices (1-10 doctors), file more prescription claims, see more Medicare patients, and practice in the South or West. The demographic information came from CMS Physician Compare.
In terms of overall drug maker payments, neurologists in the Acthar group accepted much more (median, $54,270; range, $623-$369,847) than did the Copaxone group (median, $1,747, range, $0-$256,305, P less than .001). Payment information came from the federal Open Payments database.
As for payments directly from the manufacturers of the two drugs, the Acthar prescribers accepted a median of $5,344 (range, $0-$130,307) from its manufacturer, while the Copaxone prescribers accepted a median of $137 (range, $0-$168,373) from Teva (P = .003).
“The payments are primarily for giving lectures or serving on advisory boards,” Dr. Bourdette said. “These types of payments are commonly made by pharmaceutical companies to physicians who participate in these types of activities.”
In a statement, Mallinckrodt Pharmaceuticals contends that the study inappropriately compares Acthar, often a later-line therapy, to Copaxone, which it says is often a first-line therapy.
Dr. Bourdette responded that the researchers chose a comparison group of top prescribers of Copaxone “as a marker of neurologists who treated a significant number of patients with MS.”
Mallinckrodt also notes that “there may be unmeasured confounding factors in the matching process between the comparator physician groups. These could include differences in the patient characteristics managed by these physicians including disease severity affecting prescribing patterns.”
The researchers agree that there may be differences between the groups, Dr. Bourdette said. However, he added, “the fact remains that the two groups differed in the amount of money they received as open-source payments from pharmaceutical companies. We doubt that the severity of their case mixes should lead to one group receiving more pharmaceutical open-source payments than another.”
Finally, Mallinckrodt says there’s no proof of a causal connection between the payments and the prescriptions; the study authors agree. And, the company says, “this pattern of correlation would be expected in any scenario where a small number of prescribers are the experts in the use of a later-line drug to treat a limited subset of patients.”
Dr. Bourdette rejects this contention. Few of the high Acthar prescribers practice at academic centers, he said, and few are recognized for their MS expertise. “So to suggest that they are experts in the use of Acthar for the treatment of MS when they are not as a group recognized as being experts on the treatment of MS in general is incredible,” he said. “Why a small group of neurologists prescribe Acthar remains a mystery, but it is not because the majority are leaders in the field of MS therapeutics.”
In an interview, Eric G. Campbell, PhD, professor of medicine and director of research at the Mongan Institute Health Policy Center at Harvard Medical School, Boston, said the study findings fit in with previous research that has found that “the more money that people get, the more they use the drug.
“Any reasonable person looking at this data would assume or at least consider very strongly that there is a causal relationship here,” he said.
What should be done? “There are lots of ways to stop this,” Dr. Campbell said. “One could simply impose rules that forbid doctors who accept payments for marketing drugs from billing Medicare or private payers for the care they provide. Large provider organizations could pass rules that forbid this kind of behavior. Finally, the government could vigorously pursue stiff penalties against physicians who accept payments that are really nothing more than incentives to encourage or sustain prescribing practices.”
The study was supported by a National Multiple Sclerosis Society grant. Dr. Bourdette said that he provides consulting to Magellan Healthcare, a company that provides recommendations to insurance companies on the approval of high-cost therapies. Dr. Campbell disclosed that he serves as an expert witness on law cases related to conflicts of interest in medicine.
BOSTON – A new analysis finds that some of the biggest neurologist prescribers of repository corticotropin gel (Acthar) – the extraordinarily expensive multiple sclerosis (MS) relapse drug – reaped extensive payments from its manufacturer, with one taking in $130,307 in a single year.
Together, 51 neurologists accounted for 980 Medicare claims worth more than $39 million in Acthar spending in 2014, almost half of the entire estimated $83 million in Medicare spending on neurologist-prescribed Acthar that year.
“There is a small group of neurologists – less than 1% – who are prescribing Acthar at considerable cost to Medicare and may do this in part because of financial relationships with the company that sells Acthar,” said study lead author Dennis Bourdette, MD, chair and research professor of neurology at Oregon Health and Science University, Portland.
Dr. Bourdette acknowledges that the research doesn’t prove a causal relationship between payments and prescriptions. In response to questions, Acthar manufacturer Mallinckrodt Pharmaceuticals, already under fire for the $34,000-per-vial cost of the drug, questioned the study design and denied wrongdoing in a statement: “Mallinckrodt is committed to following the highest standards for integrity and compliance in all of our business practices, including our collaboration with physicians.”
Acthar, also known as H.P. Acthar, is the poster child for stunningly expensive medication. According to the Federal Trade Commission, the cost of the drug rose from $40 per vial in 2001 to more than $34,000 this year. The New York Times reports that Medicare spending on Acthar topped half a billion in 2015.
Earlier this year, Mallinckrodt ARD (formerly Questcor) and its parent company agreed to pay a $100 million fine to settle charges that it created an illegal monopoly over the drug.
Dr. Bourdette and colleagues released their findings at the annual meeting of the American Academy of Neurology.
In an interview, Dr. Bourdette said the study’s roots lie in his concern about the medication, whose transformation from an inexpensive 1950s-era medication for pulmonary sarcoidosis to high-priced MS treatment has drawn national media attention.
“I believe that Acthar is tremendously overpriced and a waste of health care money,” Dr. Bourdette said. “I wanted to find out how extensive an economic problem it was and how much money Medicare was spending on it since this data was easily accessible.”
According to the study, Medicare spent $1.3 billion from 2011 to 2015 on Acthar, with about 25% of the cost due to prescriptions from neurologists. “When I discovered that a relatively small number of neurologists was prescribing it commonly at a cost of $40-$50 million a year to Medicare, I was interested in determining why they were prescribing this expensive therapy and postulated that it might be related to financial support they were receiving from the manufacturer,” Dr. Bourdette said.
The researchers examined the Medicare Part D Public Use File to determine which neurologists prescribed Acthar frequently in 2014. They identified 51 who prescribed Acthar 10 or more times that year and were frequent prescribers of MS disease-modifying therapy, indicating they treated many people with MS.
The 51 neurologists accounted for a mean of 19 Acthar claims each in 2014 (range, 11-50) totaling a mean annual cost of $770,145 (range, $354,479-$3,623,509). Together, the neurologists accounted for total Medicare spending on Acthar of $39,277,380.
The researchers also chose a control group – 51 neurologists who prescribed glatiramer acetate (Copaxone) more than 10 times in 2014 and also prescribed a similar frequency of all MS disease-modifying therapies as the high-frequency Acthar group.
“Acthar is used episodically to treat MS relapses, and glatiramer acetate is taken chronically to prevent relapses and disability,” Dr. Bourdette said. He added that glatiramer acetate is now available in a generic, but it wasn’t in 2014.
The Acthar and Copaxone groups were nearly identical in terms of gender (about two-thirds men) and years since graduation (a mean of 26), but the Acthar prescribers were more likely to work in small practices (1-10 doctors), file more prescription claims, see more Medicare patients, and practice in the South or West. The demographic information came from CMS Physician Compare.
In terms of overall drug maker payments, neurologists in the Acthar group accepted much more (median, $54,270; range, $623-$369,847) than did the Copaxone group (median, $1,747, range, $0-$256,305, P less than .001). Payment information came from the federal Open Payments database.
As for payments directly from the manufacturers of the two drugs, the Acthar prescribers accepted a median of $5,344 (range, $0-$130,307) from its manufacturer, while the Copaxone prescribers accepted a median of $137 (range, $0-$168,373) from Teva (P = .003).
“The payments are primarily for giving lectures or serving on advisory boards,” Dr. Bourdette said. “These types of payments are commonly made by pharmaceutical companies to physicians who participate in these types of activities.”
In a statement, Mallinckrodt Pharmaceuticals contends that the study inappropriately compares Acthar, often a later-line therapy, to Copaxone, which it says is often a first-line therapy.
Dr. Bourdette responded that the researchers chose a comparison group of top prescribers of Copaxone “as a marker of neurologists who treated a significant number of patients with MS.”
Mallinckrodt also notes that “there may be unmeasured confounding factors in the matching process between the comparator physician groups. These could include differences in the patient characteristics managed by these physicians including disease severity affecting prescribing patterns.”
The researchers agree that there may be differences between the groups, Dr. Bourdette said. However, he added, “the fact remains that the two groups differed in the amount of money they received as open-source payments from pharmaceutical companies. We doubt that the severity of their case mixes should lead to one group receiving more pharmaceutical open-source payments than another.”
Finally, Mallinckrodt says there’s no proof of a causal connection between the payments and the prescriptions; the study authors agree. And, the company says, “this pattern of correlation would be expected in any scenario where a small number of prescribers are the experts in the use of a later-line drug to treat a limited subset of patients.”
Dr. Bourdette rejects this contention. Few of the high Acthar prescribers practice at academic centers, he said, and few are recognized for their MS expertise. “So to suggest that they are experts in the use of Acthar for the treatment of MS when they are not as a group recognized as being experts on the treatment of MS in general is incredible,” he said. “Why a small group of neurologists prescribe Acthar remains a mystery, but it is not because the majority are leaders in the field of MS therapeutics.”
In an interview, Eric G. Campbell, PhD, professor of medicine and director of research at the Mongan Institute Health Policy Center at Harvard Medical School, Boston, said the study findings fit in with previous research that has found that “the more money that people get, the more they use the drug.
“Any reasonable person looking at this data would assume or at least consider very strongly that there is a causal relationship here,” he said.
What should be done? “There are lots of ways to stop this,” Dr. Campbell said. “One could simply impose rules that forbid doctors who accept payments for marketing drugs from billing Medicare or private payers for the care they provide. Large provider organizations could pass rules that forbid this kind of behavior. Finally, the government could vigorously pursue stiff penalties against physicians who accept payments that are really nothing more than incentives to encourage or sustain prescribing practices.”
The study was supported by a National Multiple Sclerosis Society grant. Dr. Bourdette said that he provides consulting to Magellan Healthcare, a company that provides recommendations to insurance companies on the approval of high-cost therapies. Dr. Campbell disclosed that he serves as an expert witness on law cases related to conflicts of interest in medicine.
AT AAN 2017
Key clinical point:
Major finding: The top Acthar prescribers reaped much more in payments from drug makers overall in 2014 than did a control group of top prescribers of another MS drug (median of $54,270 vs. $1,747; P less than .001) and from the manufacturer of each drug (median of $5,344 vs. $137; P = .003).
Data source: Medicare Part D Public Use File, CMS Physician Compare, Open Payments database, 2014.
Disclosures: The work was supported by a National Multiple Sclerosis Society grant. Dr. Bourdette provides consulting to Magellan Healthcare, a company that provides recommendations to insurance companies on the approval of high-cost therapies.
Serpentine Supravenous Hyperpigmentation Following Cisplatin and Pemetrexed Chemotherapy
To the Editor:
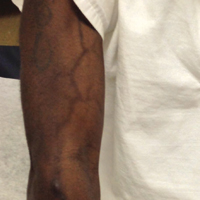
Serpentine supravenous hyperpigmentation (SSH) is a rare phenomenon characterized by linear hyperpigmentation of the skin overlying veins secondary to intravenous antineoplastic therapy. The term was first suggested by Hrushesky1 in 1976 as an uncommon side effect of administering intravenous 5-fluorouracil (5-FU). Although 5-FU is the most frequent offending agent, cases involving treatment with actinomycin, cyclophosphamide, docetaxel, fotemustine, nitrogen mustard, nitrosoureas, taxanes, and triazinate, as well as various combinations of chemotherapeutic agents, also have been observed.2,3 We present the case of SSH following a cisplatin and pemetrexed chemotherapy regimen.
A 52-year-old man with newly diagnosed inoperable adenocarcinoma in the left upper lung lobe received 2 cycles of treatment with cisplatin 138 mg and pemetrexed 920 mg 21 days apart. The first cycle of chemotherapy was delivered intravenously through the left forearm and the second cycle through the right forearm. Each infusion was followed by a 20-cc 0.9% saline flush. The patient developed nausea, vomiting, diarrhea, and hyperpigmentation tracing the path of infusion on the right arm as well as a slight darkness on the left arm that were noted by medical staff. At that time, cisplatin was discontinued from the chemotherapeutic regimen.
A port-a-cath was inserted into the patient’s right upper chest 4 weeks later and was used for subsequent infusions. Carboplatin 450 mg was initiated with pemetrexed thereafter. The patient was seen in the dermatology clinic 3 weeks after the insertion of the port-a-cath for evaluation of diffuse tinea versicolor of the trunk. Further examination of the arms revealed asymptomatic serpiginous hyperpigmentation overlying the superficial venous network tracing from the prior intravenous access points in the bilateral forearms to the upper arms (Figure). There was no evidence of extravasation or phlebitis prior to the hyperpigmentation. The patient was continued on pemetrexed and was subsequently lost to follow-up.
Cisplatin was the first member of the platinum-based chemotherapeutic agent class and is now one of the most potent and widely used in the treatment of solid malignancies. The cytotoxic mode of action is primarily mediated through interaction with DNA to form intrastrand cross-link adducts leading to aberrant mitosis and culminating in the activation of apoptosis. A variety of dermatologic complications have been reported with cisplatin chemotherapy including melanonychia, oral mucosal hyperpigmentation, hypersensitivity reactions, extravasation,4 Raynaud phenomenon, and flushing.5

Two cases of SSH have been reported following combination chemotherapy with cisplatin included in the regimen. A 61-year-old man with inoperable esophageal squamous cell carcinoma received cisplatin and 5-FU in addition to concurrent radiotherapy.6 After worsening renal function, cisplatin promptly was replaced with leucovorin. The patient developed SSH after the eighth infusion of 5-FU–leucovorin delivered through a peripheral catheter over a 24-hour period. The cutaneous side effect was attributed to the use of intravenous 5-FU.6 The second case involved a 48-year-old woman diagnosed with Paget disease of the breast who received adjuvant therapy with 12 courses of once-daily 5-FU and docetaxel for 5 years as well as 2 courses of vinorelbine and 1 course of cisplatin and etoposide for lung metastases.7 Serpentine supravenous hyperpigmentation lesions slowly developed over approximately 6 months. Based on the literature, the authors speculated that 5-FU and vinorelbine were most likely to be responsible. They noted, however, the inability to clarify the relationship between the onset of skin lesions and the time course of the chemotherapy.7 Although these cases do not directly implicate cisplatin as the cause of SSH, the possibility of a delayed reaction or augmentation of another drug’s effect cannot be excluded.
Pemetrexed, on the other hand, has not been associated with SSH. Several cutaneous adverse reactions have been reported, including acute generalized exanthematous pustulosis, alopecia, pityriasis lichenoides, radiation recall dermatitis, toxic epidermal necrolysis, and urticarial vasculitis.8 Three cases of pemetrexed-induced skin hyperpigmentation including the palms of the hands and soles of the feet as well as diffuse hyperpigmentation sparing only the palms and soles have been reported.8-10
Similar cases of SSH have demonstrated histopathologic findings with increased basal melanin synthesis and occasional melanophages in the papillary dermis without inflammatory changes.7,11 Although the unique serpentine pattern of hyperpigmentation is instantly recognizable, clinical differential diagnosis may include thrombophlebitis, cutis marmorata, erythema ab igne, livedo reticularis, and lichen planus.2,12
The exact mechanism of SSH has not been conclusively elucidated. Several studies postulate that direct cytotoxic damage causes loss of endothelial integrity permitting the extravasation of the agent to the overlying epidermis and interfering with melanogenesis.2,6,11 Other hypotheses include direct stimulation of melanocytes, depletion of reduced thioredoxin leading to tyrosinase stimulation, hyperthermia-related changes including reduced cytokine production and/or increased expression of melanocyte-stimulating hormone receptor, subclinical phlebitis leading to postinflammatory hyperpigmentation, or hyperpigmentation secondary to increased blood flow in certain areas and therefore increased drug deposition.12,13
Currently, there is no specific therapy recommended for SSH and the pigment may persist anywhere from a few months to more than a year after completing chemotherapy.2,7 Although discontinuing the offending agent would certainly prevent further development, due to the benign nature of the reaction, modifying therapy based on cutaneous findings alone is not recommended.12 Several authors have suggested avoiding peripheral infusions of chemotherapeutic agents known to cause SSH or have recommended using a permanent central venous catheter.6,7 Another option, which needs further investigation, is the administration of an abundant flush following chemotherapy. This technique was described in a case report of a 47-year-old man who developed persistent SSH in the right forearm following docetaxel injection.13 Copious venous washing with 1000 mL of isotonic saline solution following the second infusion in the unaffected arm prevented discoloration. The lack of subsequent reaction may support the theory that direct toxic effect on the vascular endothelium results in hyperpigmentation of the supravenous skin.13
Serpentine supravenous hyperpigmentation is an uncommon cutaneous reaction secondary to antineoplastic therapies. Given the widespread use of chemotherapeutic regimens, dermatologists should be aware of the reaction. Additional studies are warranted to better elucidate the pathogenesis and investigate how infusion techniques might aid in the prevention of skin discoloration. Although this side effect originally was described in relation to 5-FU, subsequent observations have included other chemotherapeutic agents. In light of the findings presented in this report, cisplatin and pemetrexed should be considered on the list of offending agents. Ultimately, patients should be reassured that the lesions are benign, self-limiting, and gradually resolve on their own in most cases.12
- Hrushesky WJ. Letter: serpentine supravenous fluorouracil hyperpigmentation. JAMA. 1976;236:138.
- Ghosh SK, Bandyopadhyay D, Ghoshal L, et al. Letter: docetaxel-induced supravenous serpentine dermatitis. Dermatol Online J. 2011;17:16.
- Pujol RM, Rocamora V, Lopez-Pousa A, et al. Persistent supravenous erythematous eruption: a rare local complication of intravenous 5-fluorouracil therapy. J Am Acad Dermatol. 1998;39:839-842.
- Kufe DW, Pollock RE, Weichsebaum RR, et al, eds. Holland-Frei Cancer Medicine. 6th ed. Hamilton, Ontario, Canada: BC Decker Inc; 2000.
- Mann MW, Berk DR, Popkin DL, et al. Handbook of Dermatology: A Practical Manual. Hoboken, NJ: Wiley-Blackwell; 2009.
- Chan CC, Lin SJ. Serpentine supravenous hyperpigmentation. N Engl J Med. 2010;29:363.
- Ouyang Y-H, Chu C-Y, Hu S-L. Linear hyperpigmentation of the left hand following chemotherapy. Dermatol Sinica. 2004;22:262-263.
- Piérard-Franchimont C, Quatresooz P, Reginster MA, et al. Revisiting cutaneous adverse reactions to pemetrexed. Oncol Lett. 2011;2:769-772.
- Buchinger K, Stahel R, Niggemeier V, et al. Pemetrexed-induced neutropenic enteritis and severe cutaneous hyperpigmentation in a patient with malignant pleural mesothelioma. Lung Cancer. 2013;80:347-349.
- Schallier D, Decoster L, De Greve J. Pemetrexed-induced hyperpigmentation of the skin. Anticancer Res. 2011;31:1753-1755.
- Rao R, Balachandran C. Serpentine supravenous pigmentation. a rare vasculocutaneous effect induced by systemic 5-fluoruracil. Indian J Dermatol Venereol Leprol. 2010;76:714-715.
- Geddes ER, Cohen PR. Antineoplastic agent-associated serpentine supravenous hyperpigmentation: superficial venous system hyperpigmentation following intravenous chemotherapy. South Med J. 2010;103:231-235.
- Ayodogan I, Kavak A, Parlak AH, et al. Persistent serpentine supravenous hyperpigmented eruption associated with docetaxel. J Eur Acad Dermatol Venereol. 2005;19:345-347.
To the Editor:
Serpentine supravenous hyperpigmentation (SSH) is a rare phenomenon characterized by linear hyperpigmentation of the skin overlying veins secondary to intravenous antineoplastic therapy. The term was first suggested by Hrushesky1 in 1976 as an uncommon side effect of administering intravenous 5-fluorouracil (5-FU). Although 5-FU is the most frequent offending agent, cases involving treatment with actinomycin, cyclophosphamide, docetaxel, fotemustine, nitrogen mustard, nitrosoureas, taxanes, and triazinate, as well as various combinations of chemotherapeutic agents, also have been observed.2,3 We present the case of SSH following a cisplatin and pemetrexed chemotherapy regimen.
A 52-year-old man with newly diagnosed inoperable adenocarcinoma in the left upper lung lobe received 2 cycles of treatment with cisplatin 138 mg and pemetrexed 920 mg 21 days apart. The first cycle of chemotherapy was delivered intravenously through the left forearm and the second cycle through the right forearm. Each infusion was followed by a 20-cc 0.9% saline flush. The patient developed nausea, vomiting, diarrhea, and hyperpigmentation tracing the path of infusion on the right arm as well as a slight darkness on the left arm that were noted by medical staff. At that time, cisplatin was discontinued from the chemotherapeutic regimen.
A port-a-cath was inserted into the patient’s right upper chest 4 weeks later and was used for subsequent infusions. Carboplatin 450 mg was initiated with pemetrexed thereafter. The patient was seen in the dermatology clinic 3 weeks after the insertion of the port-a-cath for evaluation of diffuse tinea versicolor of the trunk. Further examination of the arms revealed asymptomatic serpiginous hyperpigmentation overlying the superficial venous network tracing from the prior intravenous access points in the bilateral forearms to the upper arms (Figure). There was no evidence of extravasation or phlebitis prior to the hyperpigmentation. The patient was continued on pemetrexed and was subsequently lost to follow-up.
Cisplatin was the first member of the platinum-based chemotherapeutic agent class and is now one of the most potent and widely used in the treatment of solid malignancies. The cytotoxic mode of action is primarily mediated through interaction with DNA to form intrastrand cross-link adducts leading to aberrant mitosis and culminating in the activation of apoptosis. A variety of dermatologic complications have been reported with cisplatin chemotherapy including melanonychia, oral mucosal hyperpigmentation, hypersensitivity reactions, extravasation,4 Raynaud phenomenon, and flushing.5

Two cases of SSH have been reported following combination chemotherapy with cisplatin included in the regimen. A 61-year-old man with inoperable esophageal squamous cell carcinoma received cisplatin and 5-FU in addition to concurrent radiotherapy.6 After worsening renal function, cisplatin promptly was replaced with leucovorin. The patient developed SSH after the eighth infusion of 5-FU–leucovorin delivered through a peripheral catheter over a 24-hour period. The cutaneous side effect was attributed to the use of intravenous 5-FU.6 The second case involved a 48-year-old woman diagnosed with Paget disease of the breast who received adjuvant therapy with 12 courses of once-daily 5-FU and docetaxel for 5 years as well as 2 courses of vinorelbine and 1 course of cisplatin and etoposide for lung metastases.7 Serpentine supravenous hyperpigmentation lesions slowly developed over approximately 6 months. Based on the literature, the authors speculated that 5-FU and vinorelbine were most likely to be responsible. They noted, however, the inability to clarify the relationship between the onset of skin lesions and the time course of the chemotherapy.7 Although these cases do not directly implicate cisplatin as the cause of SSH, the possibility of a delayed reaction or augmentation of another drug’s effect cannot be excluded.
Pemetrexed, on the other hand, has not been associated with SSH. Several cutaneous adverse reactions have been reported, including acute generalized exanthematous pustulosis, alopecia, pityriasis lichenoides, radiation recall dermatitis, toxic epidermal necrolysis, and urticarial vasculitis.8 Three cases of pemetrexed-induced skin hyperpigmentation including the palms of the hands and soles of the feet as well as diffuse hyperpigmentation sparing only the palms and soles have been reported.8-10
Similar cases of SSH have demonstrated histopathologic findings with increased basal melanin synthesis and occasional melanophages in the papillary dermis without inflammatory changes.7,11 Although the unique serpentine pattern of hyperpigmentation is instantly recognizable, clinical differential diagnosis may include thrombophlebitis, cutis marmorata, erythema ab igne, livedo reticularis, and lichen planus.2,12
The exact mechanism of SSH has not been conclusively elucidated. Several studies postulate that direct cytotoxic damage causes loss of endothelial integrity permitting the extravasation of the agent to the overlying epidermis and interfering with melanogenesis.2,6,11 Other hypotheses include direct stimulation of melanocytes, depletion of reduced thioredoxin leading to tyrosinase stimulation, hyperthermia-related changes including reduced cytokine production and/or increased expression of melanocyte-stimulating hormone receptor, subclinical phlebitis leading to postinflammatory hyperpigmentation, or hyperpigmentation secondary to increased blood flow in certain areas and therefore increased drug deposition.12,13
Currently, there is no specific therapy recommended for SSH and the pigment may persist anywhere from a few months to more than a year after completing chemotherapy.2,7 Although discontinuing the offending agent would certainly prevent further development, due to the benign nature of the reaction, modifying therapy based on cutaneous findings alone is not recommended.12 Several authors have suggested avoiding peripheral infusions of chemotherapeutic agents known to cause SSH or have recommended using a permanent central venous catheter.6,7 Another option, which needs further investigation, is the administration of an abundant flush following chemotherapy. This technique was described in a case report of a 47-year-old man who developed persistent SSH in the right forearm following docetaxel injection.13 Copious venous washing with 1000 mL of isotonic saline solution following the second infusion in the unaffected arm prevented discoloration. The lack of subsequent reaction may support the theory that direct toxic effect on the vascular endothelium results in hyperpigmentation of the supravenous skin.13
Serpentine supravenous hyperpigmentation is an uncommon cutaneous reaction secondary to antineoplastic therapies. Given the widespread use of chemotherapeutic regimens, dermatologists should be aware of the reaction. Additional studies are warranted to better elucidate the pathogenesis and investigate how infusion techniques might aid in the prevention of skin discoloration. Although this side effect originally was described in relation to 5-FU, subsequent observations have included other chemotherapeutic agents. In light of the findings presented in this report, cisplatin and pemetrexed should be considered on the list of offending agents. Ultimately, patients should be reassured that the lesions are benign, self-limiting, and gradually resolve on their own in most cases.12
To the Editor:
Serpentine supravenous hyperpigmentation (SSH) is a rare phenomenon characterized by linear hyperpigmentation of the skin overlying veins secondary to intravenous antineoplastic therapy. The term was first suggested by Hrushesky1 in 1976 as an uncommon side effect of administering intravenous 5-fluorouracil (5-FU). Although 5-FU is the most frequent offending agent, cases involving treatment with actinomycin, cyclophosphamide, docetaxel, fotemustine, nitrogen mustard, nitrosoureas, taxanes, and triazinate, as well as various combinations of chemotherapeutic agents, also have been observed.2,3 We present the case of SSH following a cisplatin and pemetrexed chemotherapy regimen.
A 52-year-old man with newly diagnosed inoperable adenocarcinoma in the left upper lung lobe received 2 cycles of treatment with cisplatin 138 mg and pemetrexed 920 mg 21 days apart. The first cycle of chemotherapy was delivered intravenously through the left forearm and the second cycle through the right forearm. Each infusion was followed by a 20-cc 0.9% saline flush. The patient developed nausea, vomiting, diarrhea, and hyperpigmentation tracing the path of infusion on the right arm as well as a slight darkness on the left arm that were noted by medical staff. At that time, cisplatin was discontinued from the chemotherapeutic regimen.
A port-a-cath was inserted into the patient’s right upper chest 4 weeks later and was used for subsequent infusions. Carboplatin 450 mg was initiated with pemetrexed thereafter. The patient was seen in the dermatology clinic 3 weeks after the insertion of the port-a-cath for evaluation of diffuse tinea versicolor of the trunk. Further examination of the arms revealed asymptomatic serpiginous hyperpigmentation overlying the superficial venous network tracing from the prior intravenous access points in the bilateral forearms to the upper arms (Figure). There was no evidence of extravasation or phlebitis prior to the hyperpigmentation. The patient was continued on pemetrexed and was subsequently lost to follow-up.
Cisplatin was the first member of the platinum-based chemotherapeutic agent class and is now one of the most potent and widely used in the treatment of solid malignancies. The cytotoxic mode of action is primarily mediated through interaction with DNA to form intrastrand cross-link adducts leading to aberrant mitosis and culminating in the activation of apoptosis. A variety of dermatologic complications have been reported with cisplatin chemotherapy including melanonychia, oral mucosal hyperpigmentation, hypersensitivity reactions, extravasation,4 Raynaud phenomenon, and flushing.5

Two cases of SSH have been reported following combination chemotherapy with cisplatin included in the regimen. A 61-year-old man with inoperable esophageal squamous cell carcinoma received cisplatin and 5-FU in addition to concurrent radiotherapy.6 After worsening renal function, cisplatin promptly was replaced with leucovorin. The patient developed SSH after the eighth infusion of 5-FU–leucovorin delivered through a peripheral catheter over a 24-hour period. The cutaneous side effect was attributed to the use of intravenous 5-FU.6 The second case involved a 48-year-old woman diagnosed with Paget disease of the breast who received adjuvant therapy with 12 courses of once-daily 5-FU and docetaxel for 5 years as well as 2 courses of vinorelbine and 1 course of cisplatin and etoposide for lung metastases.7 Serpentine supravenous hyperpigmentation lesions slowly developed over approximately 6 months. Based on the literature, the authors speculated that 5-FU and vinorelbine were most likely to be responsible. They noted, however, the inability to clarify the relationship between the onset of skin lesions and the time course of the chemotherapy.7 Although these cases do not directly implicate cisplatin as the cause of SSH, the possibility of a delayed reaction or augmentation of another drug’s effect cannot be excluded.
Pemetrexed, on the other hand, has not been associated with SSH. Several cutaneous adverse reactions have been reported, including acute generalized exanthematous pustulosis, alopecia, pityriasis lichenoides, radiation recall dermatitis, toxic epidermal necrolysis, and urticarial vasculitis.8 Three cases of pemetrexed-induced skin hyperpigmentation including the palms of the hands and soles of the feet as well as diffuse hyperpigmentation sparing only the palms and soles have been reported.8-10
Similar cases of SSH have demonstrated histopathologic findings with increased basal melanin synthesis and occasional melanophages in the papillary dermis without inflammatory changes.7,11 Although the unique serpentine pattern of hyperpigmentation is instantly recognizable, clinical differential diagnosis may include thrombophlebitis, cutis marmorata, erythema ab igne, livedo reticularis, and lichen planus.2,12
The exact mechanism of SSH has not been conclusively elucidated. Several studies postulate that direct cytotoxic damage causes loss of endothelial integrity permitting the extravasation of the agent to the overlying epidermis and interfering with melanogenesis.2,6,11 Other hypotheses include direct stimulation of melanocytes, depletion of reduced thioredoxin leading to tyrosinase stimulation, hyperthermia-related changes including reduced cytokine production and/or increased expression of melanocyte-stimulating hormone receptor, subclinical phlebitis leading to postinflammatory hyperpigmentation, or hyperpigmentation secondary to increased blood flow in certain areas and therefore increased drug deposition.12,13
Currently, there is no specific therapy recommended for SSH and the pigment may persist anywhere from a few months to more than a year after completing chemotherapy.2,7 Although discontinuing the offending agent would certainly prevent further development, due to the benign nature of the reaction, modifying therapy based on cutaneous findings alone is not recommended.12 Several authors have suggested avoiding peripheral infusions of chemotherapeutic agents known to cause SSH or have recommended using a permanent central venous catheter.6,7 Another option, which needs further investigation, is the administration of an abundant flush following chemotherapy. This technique was described in a case report of a 47-year-old man who developed persistent SSH in the right forearm following docetaxel injection.13 Copious venous washing with 1000 mL of isotonic saline solution following the second infusion in the unaffected arm prevented discoloration. The lack of subsequent reaction may support the theory that direct toxic effect on the vascular endothelium results in hyperpigmentation of the supravenous skin.13
Serpentine supravenous hyperpigmentation is an uncommon cutaneous reaction secondary to antineoplastic therapies. Given the widespread use of chemotherapeutic regimens, dermatologists should be aware of the reaction. Additional studies are warranted to better elucidate the pathogenesis and investigate how infusion techniques might aid in the prevention of skin discoloration. Although this side effect originally was described in relation to 5-FU, subsequent observations have included other chemotherapeutic agents. In light of the findings presented in this report, cisplatin and pemetrexed should be considered on the list of offending agents. Ultimately, patients should be reassured that the lesions are benign, self-limiting, and gradually resolve on their own in most cases.12
- Hrushesky WJ. Letter: serpentine supravenous fluorouracil hyperpigmentation. JAMA. 1976;236:138.
- Ghosh SK, Bandyopadhyay D, Ghoshal L, et al. Letter: docetaxel-induced supravenous serpentine dermatitis. Dermatol Online J. 2011;17:16.
- Pujol RM, Rocamora V, Lopez-Pousa A, et al. Persistent supravenous erythematous eruption: a rare local complication of intravenous 5-fluorouracil therapy. J Am Acad Dermatol. 1998;39:839-842.
- Kufe DW, Pollock RE, Weichsebaum RR, et al, eds. Holland-Frei Cancer Medicine. 6th ed. Hamilton, Ontario, Canada: BC Decker Inc; 2000.
- Mann MW, Berk DR, Popkin DL, et al. Handbook of Dermatology: A Practical Manual. Hoboken, NJ: Wiley-Blackwell; 2009.
- Chan CC, Lin SJ. Serpentine supravenous hyperpigmentation. N Engl J Med. 2010;29:363.
- Ouyang Y-H, Chu C-Y, Hu S-L. Linear hyperpigmentation of the left hand following chemotherapy. Dermatol Sinica. 2004;22:262-263.
- Piérard-Franchimont C, Quatresooz P, Reginster MA, et al. Revisiting cutaneous adverse reactions to pemetrexed. Oncol Lett. 2011;2:769-772.
- Buchinger K, Stahel R, Niggemeier V, et al. Pemetrexed-induced neutropenic enteritis and severe cutaneous hyperpigmentation in a patient with malignant pleural mesothelioma. Lung Cancer. 2013;80:347-349.
- Schallier D, Decoster L, De Greve J. Pemetrexed-induced hyperpigmentation of the skin. Anticancer Res. 2011;31:1753-1755.
- Rao R, Balachandran C. Serpentine supravenous pigmentation. a rare vasculocutaneous effect induced by systemic 5-fluoruracil. Indian J Dermatol Venereol Leprol. 2010;76:714-715.
- Geddes ER, Cohen PR. Antineoplastic agent-associated serpentine supravenous hyperpigmentation: superficial venous system hyperpigmentation following intravenous chemotherapy. South Med J. 2010;103:231-235.
- Ayodogan I, Kavak A, Parlak AH, et al. Persistent serpentine supravenous hyperpigmented eruption associated with docetaxel. J Eur Acad Dermatol Venereol. 2005;19:345-347.
- Hrushesky WJ. Letter: serpentine supravenous fluorouracil hyperpigmentation. JAMA. 1976;236:138.
- Ghosh SK, Bandyopadhyay D, Ghoshal L, et al. Letter: docetaxel-induced supravenous serpentine dermatitis. Dermatol Online J. 2011;17:16.
- Pujol RM, Rocamora V, Lopez-Pousa A, et al. Persistent supravenous erythematous eruption: a rare local complication of intravenous 5-fluorouracil therapy. J Am Acad Dermatol. 1998;39:839-842.
- Kufe DW, Pollock RE, Weichsebaum RR, et al, eds. Holland-Frei Cancer Medicine. 6th ed. Hamilton, Ontario, Canada: BC Decker Inc; 2000.
- Mann MW, Berk DR, Popkin DL, et al. Handbook of Dermatology: A Practical Manual. Hoboken, NJ: Wiley-Blackwell; 2009.
- Chan CC, Lin SJ. Serpentine supravenous hyperpigmentation. N Engl J Med. 2010;29:363.
- Ouyang Y-H, Chu C-Y, Hu S-L. Linear hyperpigmentation of the left hand following chemotherapy. Dermatol Sinica. 2004;22:262-263.
- Piérard-Franchimont C, Quatresooz P, Reginster MA, et al. Revisiting cutaneous adverse reactions to pemetrexed. Oncol Lett. 2011;2:769-772.
- Buchinger K, Stahel R, Niggemeier V, et al. Pemetrexed-induced neutropenic enteritis and severe cutaneous hyperpigmentation in a patient with malignant pleural mesothelioma. Lung Cancer. 2013;80:347-349.
- Schallier D, Decoster L, De Greve J. Pemetrexed-induced hyperpigmentation of the skin. Anticancer Res. 2011;31:1753-1755.
- Rao R, Balachandran C. Serpentine supravenous pigmentation. a rare vasculocutaneous effect induced by systemic 5-fluoruracil. Indian J Dermatol Venereol Leprol. 2010;76:714-715.
- Geddes ER, Cohen PR. Antineoplastic agent-associated serpentine supravenous hyperpigmentation: superficial venous system hyperpigmentation following intravenous chemotherapy. South Med J. 2010;103:231-235.
- Ayodogan I, Kavak A, Parlak AH, et al. Persistent serpentine supravenous hyperpigmented eruption associated with docetaxel. J Eur Acad Dermatol Venereol. 2005;19:345-347.
Practice Points
- A variety of dermatologic complications have been reported with cisplatin chemotherapy, including serpentine supravenous hyperpigmentation (SSH); however, pemetrexed has not been associated with SSH.
- Although discontinuing the offending agent would certainly prevent further development, due to the benign nature of the reaction, modifying therapy based on cutaneous findings alone is not recommended.