User login
Duodenal resurfacing achieves metabolic benefits in type 2 diabetes
SAN DIEGO – A first-in-human study suggests that a novel technique holds promise for the treatment of diabetes, reducing the complications, associated morbidity, and economic burden of this disease. Duodenal mucosal resurfacing (DMR) achieved a significant reduction in hemoglobin A1c (HbA1c) levels as well as a robust reduction in the liver enzymes aspartate aminotransferase and alanine aminotransferase in patients with poorly controlled type 2 diabetes.
DMR entails thermal ablation of the duodenal mucosa via minimally invasive endoscopy. According to the pioneer behind this technique, Dr. Harith Rajagopalan, who is the founder and CEO of Fractyl Laboratories, in Waltham, Mass., DMR can be compared to laser resurfacing of the skin to remove actinic keratosis. The procedure removes the surface cells of the duodenum, which are insulin resistant, and they heal and become insulin sensitive.
The procedure does not involve surgery or implants, and takes about 60 minutes.
“We hope that once the procedure is done, patients will adopt lifestyle interventions. In the future, we plan to study whether DMR will have even greater potential if patients adapt to a healthy lifestyle,” he said.
During the annual Digestive Disease Week, Dr. Rajagopalan detailed experience with the first 39 humans treated with DMR. All patients had poorly controlled type 2 diabetes and were taking at least one antidiabetic medication. The average age was 53 years, mean body mass index was 31 kg/m2, and 50% were taking metformin. DMR was performed after 2 weeks of a low-calorie diet.
In this single-arm study, DMR reduced HbA1c by 1.2%, reduced ALT from 40 to 27 IU/L, and reduced AST from 32 to 22 IU/L over a period of 6 months. Patients with the highest entry levels of AST and ALT had the greatest reductions.
Of the 39 patients, 28 had a 9-cm ablation of the duodenum, and in these patients, the metabolic benefits were even more robust. Thus, 9 cm was identified as the optimal surface area for ablation in future studies.
The procedure was well tolerated, with minimal gastrointestinal symptoms. Adverse events were mostly mild and mainly abdominal pain due to air for the first 2 days following DMR. Three episodes of duodenal stenosis were reported over the first 6 weeks post procedure.
“These resolved with endoscopic balloon dilatation. We have since improved the procedure, and have not seen subsequent events,” Dr Rajagopalan said.
“We know that bariatric surgeries, particularly those that prevent contact of nutrients with mucosa, improve metabolic measures of metabolism in type 2 diabetes, including indicators of fatty liver disease. Revita resurfaces the duodenal mucosa. We have shown in this first-in-human study that the procedure appears to be safe, that the optimal length of the segment to be resurfaced is 9 cm, and that the procedure is associated with metabolic benefits that persist through 6 months after the mucosa heals,” Dr Rajagopalan said.
Longer-term data are needed beyond 6 months, he noted. The investigators will have 12-month data from this study in a few months, and a multicenter trial is now being conducted in Europe in patients with type 2 diabetes treated with DMR and 9-cm resurfacing of the duodenal mucosa.
“These early results raise the intriguing possibility that our intervention might be used not only in type 2 diabetes, but also in patients with metabolic liver disease and other insulin resistance–mediated diseases,” he said.
Insulin resistance plays a central role across a range of disorders, affecting many different organs, including the liver and gastrointestinal tract. Insulin resistance leads to inherent complications and associated syndromes. Just as polycystic ovarian syndrome is now called metabolic reproductive syndrome, nonalcoholic fatty liver disease and nonalcoholic steatohepatitis could be called metabolic liver disease, he suggested.
“Although some patients treated with DMR lost a modest amount of weight, the liver enzyme levels were still reduced. The weight really didn’t budge, yet the liver enzymes were reduced, which was surprising. This forces the question whether obesity really causes type 2 diabetes, or is the cause of insulin resistance independent of weight and are there other contributing factors than obesity,” he said.
“This study shows the potential for a single-point upper GI intervention that can exert broad metabolic effects in glycemia and fatty liver disease,” he commented.
“Even though the duodenal mucosa regenerates over months 1-3, there appears to be a sustained effect of DMR. However, we need controlled studies that prevent medication adjustments that affect glycemic signals,” Dr Rajagopalan noted.
SAN DIEGO – A first-in-human study suggests that a novel technique holds promise for the treatment of diabetes, reducing the complications, associated morbidity, and economic burden of this disease. Duodenal mucosal resurfacing (DMR) achieved a significant reduction in hemoglobin A1c (HbA1c) levels as well as a robust reduction in the liver enzymes aspartate aminotransferase and alanine aminotransferase in patients with poorly controlled type 2 diabetes.
DMR entails thermal ablation of the duodenal mucosa via minimally invasive endoscopy. According to the pioneer behind this technique, Dr. Harith Rajagopalan, who is the founder and CEO of Fractyl Laboratories, in Waltham, Mass., DMR can be compared to laser resurfacing of the skin to remove actinic keratosis. The procedure removes the surface cells of the duodenum, which are insulin resistant, and they heal and become insulin sensitive.
The procedure does not involve surgery or implants, and takes about 60 minutes.
“We hope that once the procedure is done, patients will adopt lifestyle interventions. In the future, we plan to study whether DMR will have even greater potential if patients adapt to a healthy lifestyle,” he said.
During the annual Digestive Disease Week, Dr. Rajagopalan detailed experience with the first 39 humans treated with DMR. All patients had poorly controlled type 2 diabetes and were taking at least one antidiabetic medication. The average age was 53 years, mean body mass index was 31 kg/m2, and 50% were taking metformin. DMR was performed after 2 weeks of a low-calorie diet.
In this single-arm study, DMR reduced HbA1c by 1.2%, reduced ALT from 40 to 27 IU/L, and reduced AST from 32 to 22 IU/L over a period of 6 months. Patients with the highest entry levels of AST and ALT had the greatest reductions.
Of the 39 patients, 28 had a 9-cm ablation of the duodenum, and in these patients, the metabolic benefits were even more robust. Thus, 9 cm was identified as the optimal surface area for ablation in future studies.
The procedure was well tolerated, with minimal gastrointestinal symptoms. Adverse events were mostly mild and mainly abdominal pain due to air for the first 2 days following DMR. Three episodes of duodenal stenosis were reported over the first 6 weeks post procedure.
“These resolved with endoscopic balloon dilatation. We have since improved the procedure, and have not seen subsequent events,” Dr Rajagopalan said.
“We know that bariatric surgeries, particularly those that prevent contact of nutrients with mucosa, improve metabolic measures of metabolism in type 2 diabetes, including indicators of fatty liver disease. Revita resurfaces the duodenal mucosa. We have shown in this first-in-human study that the procedure appears to be safe, that the optimal length of the segment to be resurfaced is 9 cm, and that the procedure is associated with metabolic benefits that persist through 6 months after the mucosa heals,” Dr Rajagopalan said.
Longer-term data are needed beyond 6 months, he noted. The investigators will have 12-month data from this study in a few months, and a multicenter trial is now being conducted in Europe in patients with type 2 diabetes treated with DMR and 9-cm resurfacing of the duodenal mucosa.
“These early results raise the intriguing possibility that our intervention might be used not only in type 2 diabetes, but also in patients with metabolic liver disease and other insulin resistance–mediated diseases,” he said.
Insulin resistance plays a central role across a range of disorders, affecting many different organs, including the liver and gastrointestinal tract. Insulin resistance leads to inherent complications and associated syndromes. Just as polycystic ovarian syndrome is now called metabolic reproductive syndrome, nonalcoholic fatty liver disease and nonalcoholic steatohepatitis could be called metabolic liver disease, he suggested.
“Although some patients treated with DMR lost a modest amount of weight, the liver enzyme levels were still reduced. The weight really didn’t budge, yet the liver enzymes were reduced, which was surprising. This forces the question whether obesity really causes type 2 diabetes, or is the cause of insulin resistance independent of weight and are there other contributing factors than obesity,” he said.
“This study shows the potential for a single-point upper GI intervention that can exert broad metabolic effects in glycemia and fatty liver disease,” he commented.
“Even though the duodenal mucosa regenerates over months 1-3, there appears to be a sustained effect of DMR. However, we need controlled studies that prevent medication adjustments that affect glycemic signals,” Dr Rajagopalan noted.
SAN DIEGO – A first-in-human study suggests that a novel technique holds promise for the treatment of diabetes, reducing the complications, associated morbidity, and economic burden of this disease. Duodenal mucosal resurfacing (DMR) achieved a significant reduction in hemoglobin A1c (HbA1c) levels as well as a robust reduction in the liver enzymes aspartate aminotransferase and alanine aminotransferase in patients with poorly controlled type 2 diabetes.
DMR entails thermal ablation of the duodenal mucosa via minimally invasive endoscopy. According to the pioneer behind this technique, Dr. Harith Rajagopalan, who is the founder and CEO of Fractyl Laboratories, in Waltham, Mass., DMR can be compared to laser resurfacing of the skin to remove actinic keratosis. The procedure removes the surface cells of the duodenum, which are insulin resistant, and they heal and become insulin sensitive.
The procedure does not involve surgery or implants, and takes about 60 minutes.
“We hope that once the procedure is done, patients will adopt lifestyle interventions. In the future, we plan to study whether DMR will have even greater potential if patients adapt to a healthy lifestyle,” he said.
During the annual Digestive Disease Week, Dr. Rajagopalan detailed experience with the first 39 humans treated with DMR. All patients had poorly controlled type 2 diabetes and were taking at least one antidiabetic medication. The average age was 53 years, mean body mass index was 31 kg/m2, and 50% were taking metformin. DMR was performed after 2 weeks of a low-calorie diet.
In this single-arm study, DMR reduced HbA1c by 1.2%, reduced ALT from 40 to 27 IU/L, and reduced AST from 32 to 22 IU/L over a period of 6 months. Patients with the highest entry levels of AST and ALT had the greatest reductions.
Of the 39 patients, 28 had a 9-cm ablation of the duodenum, and in these patients, the metabolic benefits were even more robust. Thus, 9 cm was identified as the optimal surface area for ablation in future studies.
The procedure was well tolerated, with minimal gastrointestinal symptoms. Adverse events were mostly mild and mainly abdominal pain due to air for the first 2 days following DMR. Three episodes of duodenal stenosis were reported over the first 6 weeks post procedure.
“These resolved with endoscopic balloon dilatation. We have since improved the procedure, and have not seen subsequent events,” Dr Rajagopalan said.
“We know that bariatric surgeries, particularly those that prevent contact of nutrients with mucosa, improve metabolic measures of metabolism in type 2 diabetes, including indicators of fatty liver disease. Revita resurfaces the duodenal mucosa. We have shown in this first-in-human study that the procedure appears to be safe, that the optimal length of the segment to be resurfaced is 9 cm, and that the procedure is associated with metabolic benefits that persist through 6 months after the mucosa heals,” Dr Rajagopalan said.
Longer-term data are needed beyond 6 months, he noted. The investigators will have 12-month data from this study in a few months, and a multicenter trial is now being conducted in Europe in patients with type 2 diabetes treated with DMR and 9-cm resurfacing of the duodenal mucosa.
“These early results raise the intriguing possibility that our intervention might be used not only in type 2 diabetes, but also in patients with metabolic liver disease and other insulin resistance–mediated diseases,” he said.
Insulin resistance plays a central role across a range of disorders, affecting many different organs, including the liver and gastrointestinal tract. Insulin resistance leads to inherent complications and associated syndromes. Just as polycystic ovarian syndrome is now called metabolic reproductive syndrome, nonalcoholic fatty liver disease and nonalcoholic steatohepatitis could be called metabolic liver disease, he suggested.
“Although some patients treated with DMR lost a modest amount of weight, the liver enzyme levels were still reduced. The weight really didn’t budge, yet the liver enzymes were reduced, which was surprising. This forces the question whether obesity really causes type 2 diabetes, or is the cause of insulin resistance independent of weight and are there other contributing factors than obesity,” he said.
“This study shows the potential for a single-point upper GI intervention that can exert broad metabolic effects in glycemia and fatty liver disease,” he commented.
“Even though the duodenal mucosa regenerates over months 1-3, there appears to be a sustained effect of DMR. However, we need controlled studies that prevent medication adjustments that affect glycemic signals,” Dr Rajagopalan noted.
AT DDW® 2016
Key clinical point: Duodenal mucosal resurfacing appears to convert insulin-resistant cells to insulin-sensitive cells.
Major finding: DMR reduced HbA1c by 1.2%, reduced ALT from 40 to 27 IU/L, and reduced AST from 32 to 22 IU/L over a period of 6 months.
Data source: A single-arm study in 39 patients with type 2 diabetes.
Disclosures: The study was sponsored by Fractyl.
A Case of Cold Feet Hands
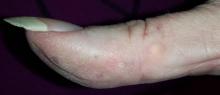
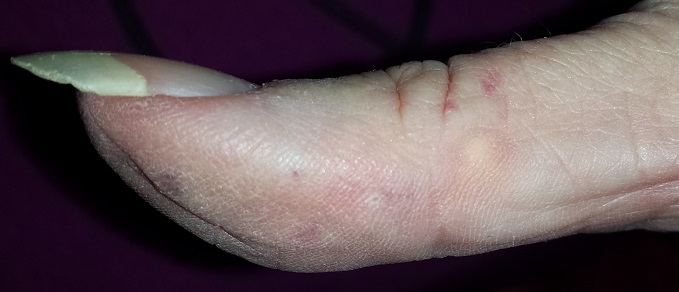
A 51-year-old woman is referred to dermatology for multiple problems she has had for several months—and, in some cases, years. The most immediate is a new nodule on the side of one thumb. Although it is asymptomatic, the fact that it simply “appeared” worries her, because her favorite uncle recently died of melanoma.
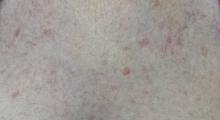
Several new lesions also materialized on her finger pads and chest. These do not cause symptoms either, but their newness makes them worrisome.
When asked about her health in general, the patient initially states it is “fine”—until her husband corrects her. She then recalls that she is being seen by various specialists, including a gastroenterologist for dysphagia-like symptoms and a rheumatologist for vague joint pains and what sounds like Raynaud disease.
EXAMINATION
On the lateral right thumb is a shallow, intradermal, white lesion. It is firm and round and measures 5 mm. No overlying skin changes (eg, redness or disruption of the skin surface) are observed. Under local anesthesia, a small incision is made in the lesion’s surface, allowing for exploration with small forceps; granular, gritty, white material is extracted.
On the affected thumb and two other fingers, telangiectasias can be seen. There are additional patches on the patient’s chest, but none around her mouth.
No sign of Raynaud disease is seen. However, the patient is adamant that this only appears when her hands are quite cold.
What is the diagnosis?
DISCUSSION
Connecting these dots leads to the likelihood of CREST syndrome, a variant of systemic sclerosis (SS); the acronym stands for calcinosis, Raynaud disease, esophageal dysmotility, sclerodactyly, and telangiectasia. The classification of CREST in the spectrum of SS is still open to discussion, but it is widely recognized as a real and distinct condition.
SS shares with CREST the involvement of anticentromere antibodies, but there are significant differences in the clinical course of each condition. Raynaud disease is often the first sign of CREST and can precede the other findings by years. Unlike SS, CREST typically spares the kidneys, and if it affects the lungs at all, it is through pulmonary artery hypertension and not fibrosis (the latter of which is seen in SS). Both conditions, however, can present with swelling of the hands and dysphagia.
Interpretation of these disparate findings requires the skill of a rheumatologist, who often shares management with other specialists.
As for differential diagnoses: Any one of these components can be a stand-alone diagnosis or can manifest with other diseases or syndromes. But when found together, they are highly suggestive of CREST. In this patient’s case, more testing needs to be done before a definitive diagnosis can be made.
TAKE-HOME LEARNING POINTS
• CREST syndrome is considered a variant of systemic sclerosis but has significantly different features and clinical course.
• The components of CREST (calcinosis, Raynaud disease, esophageal dysmotility, sclerodactyly, and telangiectasia) are often viewed as isolated phenomena by those unfamiliar with the condition.
• Raynaud disease is often the initial sign of CREST, preceding the rest by years.
• The telangiectasias seen with CREST often manifest on the hands and chest but can also be found on mucosal surfaces.
A 51-year-old woman is referred to dermatology for multiple problems she has had for several months—and, in some cases, years. The most immediate is a new nodule on the side of one thumb. Although it is asymptomatic, the fact that it simply “appeared” worries her, because her favorite uncle recently died of melanoma.
Several new lesions also materialized on her finger pads and chest. These do not cause symptoms either, but their newness makes them worrisome.
When asked about her health in general, the patient initially states it is “fine”—until her husband corrects her. She then recalls that she is being seen by various specialists, including a gastroenterologist for dysphagia-like symptoms and a rheumatologist for vague joint pains and what sounds like Raynaud disease.
EXAMINATION
On the lateral right thumb is a shallow, intradermal, white lesion. It is firm and round and measures 5 mm. No overlying skin changes (eg, redness or disruption of the skin surface) are observed. Under local anesthesia, a small incision is made in the lesion’s surface, allowing for exploration with small forceps; granular, gritty, white material is extracted.
On the affected thumb and two other fingers, telangiectasias can be seen. There are additional patches on the patient’s chest, but none around her mouth.
No sign of Raynaud disease is seen. However, the patient is adamant that this only appears when her hands are quite cold.
What is the diagnosis?
DISCUSSION
Connecting these dots leads to the likelihood of CREST syndrome, a variant of systemic sclerosis (SS); the acronym stands for calcinosis, Raynaud disease, esophageal dysmotility, sclerodactyly, and telangiectasia. The classification of CREST in the spectrum of SS is still open to discussion, but it is widely recognized as a real and distinct condition.
SS shares with CREST the involvement of anticentromere antibodies, but there are significant differences in the clinical course of each condition. Raynaud disease is often the first sign of CREST and can precede the other findings by years. Unlike SS, CREST typically spares the kidneys, and if it affects the lungs at all, it is through pulmonary artery hypertension and not fibrosis (the latter of which is seen in SS). Both conditions, however, can present with swelling of the hands and dysphagia.
Interpretation of these disparate findings requires the skill of a rheumatologist, who often shares management with other specialists.
As for differential diagnoses: Any one of these components can be a stand-alone diagnosis or can manifest with other diseases or syndromes. But when found together, they are highly suggestive of CREST. In this patient’s case, more testing needs to be done before a definitive diagnosis can be made.
TAKE-HOME LEARNING POINTS
• CREST syndrome is considered a variant of systemic sclerosis but has significantly different features and clinical course.
• The components of CREST (calcinosis, Raynaud disease, esophageal dysmotility, sclerodactyly, and telangiectasia) are often viewed as isolated phenomena by those unfamiliar with the condition.
• Raynaud disease is often the initial sign of CREST, preceding the rest by years.
• The telangiectasias seen with CREST often manifest on the hands and chest but can also be found on mucosal surfaces.
A 51-year-old woman is referred to dermatology for multiple problems she has had for several months—and, in some cases, years. The most immediate is a new nodule on the side of one thumb. Although it is asymptomatic, the fact that it simply “appeared” worries her, because her favorite uncle recently died of melanoma.
Several new lesions also materialized on her finger pads and chest. These do not cause symptoms either, but their newness makes them worrisome.
When asked about her health in general, the patient initially states it is “fine”—until her husband corrects her. She then recalls that she is being seen by various specialists, including a gastroenterologist for dysphagia-like symptoms and a rheumatologist for vague joint pains and what sounds like Raynaud disease.
EXAMINATION
On the lateral right thumb is a shallow, intradermal, white lesion. It is firm and round and measures 5 mm. No overlying skin changes (eg, redness or disruption of the skin surface) are observed. Under local anesthesia, a small incision is made in the lesion’s surface, allowing for exploration with small forceps; granular, gritty, white material is extracted.
On the affected thumb and two other fingers, telangiectasias can be seen. There are additional patches on the patient’s chest, but none around her mouth.
No sign of Raynaud disease is seen. However, the patient is adamant that this only appears when her hands are quite cold.
What is the diagnosis?
DISCUSSION
Connecting these dots leads to the likelihood of CREST syndrome, a variant of systemic sclerosis (SS); the acronym stands for calcinosis, Raynaud disease, esophageal dysmotility, sclerodactyly, and telangiectasia. The classification of CREST in the spectrum of SS is still open to discussion, but it is widely recognized as a real and distinct condition.
SS shares with CREST the involvement of anticentromere antibodies, but there are significant differences in the clinical course of each condition. Raynaud disease is often the first sign of CREST and can precede the other findings by years. Unlike SS, CREST typically spares the kidneys, and if it affects the lungs at all, it is through pulmonary artery hypertension and not fibrosis (the latter of which is seen in SS). Both conditions, however, can present with swelling of the hands and dysphagia.
Interpretation of these disparate findings requires the skill of a rheumatologist, who often shares management with other specialists.
As for differential diagnoses: Any one of these components can be a stand-alone diagnosis or can manifest with other diseases or syndromes. But when found together, they are highly suggestive of CREST. In this patient’s case, more testing needs to be done before a definitive diagnosis can be made.
TAKE-HOME LEARNING POINTS
• CREST syndrome is considered a variant of systemic sclerosis but has significantly different features and clinical course.
• The components of CREST (calcinosis, Raynaud disease, esophageal dysmotility, sclerodactyly, and telangiectasia) are often viewed as isolated phenomena by those unfamiliar with the condition.
• Raynaud disease is often the initial sign of CREST, preceding the rest by years.
• The telangiectasias seen with CREST often manifest on the hands and chest but can also be found on mucosal surfaces.
New heart failure guidelines
Recently published guidelines for the pharmacologic management of heart failure in patients with reduced ejection fraction (HFrEF) focus on two drugs – both of which have been approved by the Food and Drug Administration – that have shown promise in managing heart failure patients.
The guideline committee has deemed that the combination drug sacubitril-valsartan (Entresto, Novartis) and ivabradine (Corlanor, Amgen) as “milestone” achievements. In this reader’s mind, compared with the mortality and morbidity effect of beta-blockers and ACE inhibitors, they really don’t make it to that status. They do, however, reach the threshold of important adjuncts to the care for heart failure treatment.
Ivabradine was shown to improve the combination of rehospitalization and mortality when added to conventional therapy, including beta-blockers, by decreasing heart rate. Unfortunately, many of the patients in the trial were not adequately treated with beta-blockers. Sacubitril-valsartan, a drug we have commented about in previous columns, is interesting and adds an additional effect on mortality and morbidity when compared with enalapril alone. In the PARADIGM-HF trial, it showed a significant 20% decrease in rehospitalization and total mortality. A strong case can be made that it should be used instead of an ACE inhibitor, although it is associated with some increased hypotension and angioneurotic edema.
Sacubitril-valsartan poses a significant social issue in regard to its pricing. Most of the drugs we use for the treatment of heart failure cost less than a dollar a day when introduced, and pennies now, and they are immensely effective. Sacubitril-valsartan on average moves the mortality needle a bit, but at a cost of 20 times its competitor, enalapril. It doesn’t do it in all patients, so that it might be reasonable to use it just in those patients who are failing with an ACE inhibitor alone. This is the first time heart failure doctors have had to grapple with the problem of drug pricing, whereas the oncologist and the lipid specialists have been facing this issue for some time. Is it worth $500 a month, compared to $25 a month, to possibly move the mortality index, which has already been modified with the previous ACE inhibitors, down a bit? I suppose that the decision is up to all of us to make.
Reading the guidelines, however, provided me with some insight that I have missed in the past. Of the 17 members of the guideline writing team representing the American College of Cardiology, American Heart Association, and the Heart Failure Society of America, eight had “significant relationships” with the two pharmaceutical companies in question. Committee members were advised not to vote if they had a relevant relationship with industry. How they functioned in the committee proceeding it’s impossible to tell, but the potential for bias is there. I would imagine that eight other heart failure specialists who did not have a conflict could have been found to serve on the writing committee. As guidelines have become such a major part of the construct of the pharmacopoeia, potential bias like this should and easily can be avoided.
Dr. Goldstein, medical editor of Cardiology News, is professor of medicine at Wayne State University and division head emeritus of cardiovascular medicine at Henry Ford Hospital, both in Detroit. He is on data safety monitoring committees for the National Institutes of Health and several pharmaceutical companies.
Recently published guidelines for the pharmacologic management of heart failure in patients with reduced ejection fraction (HFrEF) focus on two drugs – both of which have been approved by the Food and Drug Administration – that have shown promise in managing heart failure patients.
The guideline committee has deemed that the combination drug sacubitril-valsartan (Entresto, Novartis) and ivabradine (Corlanor, Amgen) as “milestone” achievements. In this reader’s mind, compared with the mortality and morbidity effect of beta-blockers and ACE inhibitors, they really don’t make it to that status. They do, however, reach the threshold of important adjuncts to the care for heart failure treatment.
Ivabradine was shown to improve the combination of rehospitalization and mortality when added to conventional therapy, including beta-blockers, by decreasing heart rate. Unfortunately, many of the patients in the trial were not adequately treated with beta-blockers. Sacubitril-valsartan, a drug we have commented about in previous columns, is interesting and adds an additional effect on mortality and morbidity when compared with enalapril alone. In the PARADIGM-HF trial, it showed a significant 20% decrease in rehospitalization and total mortality. A strong case can be made that it should be used instead of an ACE inhibitor, although it is associated with some increased hypotension and angioneurotic edema.
Sacubitril-valsartan poses a significant social issue in regard to its pricing. Most of the drugs we use for the treatment of heart failure cost less than a dollar a day when introduced, and pennies now, and they are immensely effective. Sacubitril-valsartan on average moves the mortality needle a bit, but at a cost of 20 times its competitor, enalapril. It doesn’t do it in all patients, so that it might be reasonable to use it just in those patients who are failing with an ACE inhibitor alone. This is the first time heart failure doctors have had to grapple with the problem of drug pricing, whereas the oncologist and the lipid specialists have been facing this issue for some time. Is it worth $500 a month, compared to $25 a month, to possibly move the mortality index, which has already been modified with the previous ACE inhibitors, down a bit? I suppose that the decision is up to all of us to make.
Reading the guidelines, however, provided me with some insight that I have missed in the past. Of the 17 members of the guideline writing team representing the American College of Cardiology, American Heart Association, and the Heart Failure Society of America, eight had “significant relationships” with the two pharmaceutical companies in question. Committee members were advised not to vote if they had a relevant relationship with industry. How they functioned in the committee proceeding it’s impossible to tell, but the potential for bias is there. I would imagine that eight other heart failure specialists who did not have a conflict could have been found to serve on the writing committee. As guidelines have become such a major part of the construct of the pharmacopoeia, potential bias like this should and easily can be avoided.
Dr. Goldstein, medical editor of Cardiology News, is professor of medicine at Wayne State University and division head emeritus of cardiovascular medicine at Henry Ford Hospital, both in Detroit. He is on data safety monitoring committees for the National Institutes of Health and several pharmaceutical companies.
Recently published guidelines for the pharmacologic management of heart failure in patients with reduced ejection fraction (HFrEF) focus on two drugs – both of which have been approved by the Food and Drug Administration – that have shown promise in managing heart failure patients.
The guideline committee has deemed that the combination drug sacubitril-valsartan (Entresto, Novartis) and ivabradine (Corlanor, Amgen) as “milestone” achievements. In this reader’s mind, compared with the mortality and morbidity effect of beta-blockers and ACE inhibitors, they really don’t make it to that status. They do, however, reach the threshold of important adjuncts to the care for heart failure treatment.
Ivabradine was shown to improve the combination of rehospitalization and mortality when added to conventional therapy, including beta-blockers, by decreasing heart rate. Unfortunately, many of the patients in the trial were not adequately treated with beta-blockers. Sacubitril-valsartan, a drug we have commented about in previous columns, is interesting and adds an additional effect on mortality and morbidity when compared with enalapril alone. In the PARADIGM-HF trial, it showed a significant 20% decrease in rehospitalization and total mortality. A strong case can be made that it should be used instead of an ACE inhibitor, although it is associated with some increased hypotension and angioneurotic edema.
Sacubitril-valsartan poses a significant social issue in regard to its pricing. Most of the drugs we use for the treatment of heart failure cost less than a dollar a day when introduced, and pennies now, and they are immensely effective. Sacubitril-valsartan on average moves the mortality needle a bit, but at a cost of 20 times its competitor, enalapril. It doesn’t do it in all patients, so that it might be reasonable to use it just in those patients who are failing with an ACE inhibitor alone. This is the first time heart failure doctors have had to grapple with the problem of drug pricing, whereas the oncologist and the lipid specialists have been facing this issue for some time. Is it worth $500 a month, compared to $25 a month, to possibly move the mortality index, which has already been modified with the previous ACE inhibitors, down a bit? I suppose that the decision is up to all of us to make.
Reading the guidelines, however, provided me with some insight that I have missed in the past. Of the 17 members of the guideline writing team representing the American College of Cardiology, American Heart Association, and the Heart Failure Society of America, eight had “significant relationships” with the two pharmaceutical companies in question. Committee members were advised not to vote if they had a relevant relationship with industry. How they functioned in the committee proceeding it’s impossible to tell, but the potential for bias is there. I would imagine that eight other heart failure specialists who did not have a conflict could have been found to serve on the writing committee. As guidelines have become such a major part of the construct of the pharmacopoeia, potential bias like this should and easily can be avoided.
Dr. Goldstein, medical editor of Cardiology News, is professor of medicine at Wayne State University and division head emeritus of cardiovascular medicine at Henry Ford Hospital, both in Detroit. He is on data safety monitoring committees for the National Institutes of Health and several pharmaceutical companies.
The Association Between Sleep and Seizure Types
Patients with non-acquired focal epilepsy are more likely to experience seizures while asleep, when compared to patients with generalized epilepsy. An analysis of nearly 1400 patients enrolled in the Epilepsy Phenome/Genome Project also revealed that these sleep/wake patterns applied to both convulsive and nonconvulsive seizures. The study further found that seizures occurring within an hour of awakening were more likely to happen in patients with generalized epilepsy, for both convulsive and nonconvulsive seizures. The researchers also discovered that the timing of seizures in first degree relatives predicted the timing of seizures in the proband, suggesting a genetic underpinning to the correlations.
Winawer MR, Shih J, Beck ES, Hunter JE, Epstein MP; EPGP Investigators. Genetic effects on sleep/wake variation on seizures. Epilepsia. 2016;57(4):557-665.
Patients with non-acquired focal epilepsy are more likely to experience seizures while asleep, when compared to patients with generalized epilepsy. An analysis of nearly 1400 patients enrolled in the Epilepsy Phenome/Genome Project also revealed that these sleep/wake patterns applied to both convulsive and nonconvulsive seizures. The study further found that seizures occurring within an hour of awakening were more likely to happen in patients with generalized epilepsy, for both convulsive and nonconvulsive seizures. The researchers also discovered that the timing of seizures in first degree relatives predicted the timing of seizures in the proband, suggesting a genetic underpinning to the correlations.
Winawer MR, Shih J, Beck ES, Hunter JE, Epstein MP; EPGP Investigators. Genetic effects on sleep/wake variation on seizures. Epilepsia. 2016;57(4):557-665.
Patients with non-acquired focal epilepsy are more likely to experience seizures while asleep, when compared to patients with generalized epilepsy. An analysis of nearly 1400 patients enrolled in the Epilepsy Phenome/Genome Project also revealed that these sleep/wake patterns applied to both convulsive and nonconvulsive seizures. The study further found that seizures occurring within an hour of awakening were more likely to happen in patients with generalized epilepsy, for both convulsive and nonconvulsive seizures. The researchers also discovered that the timing of seizures in first degree relatives predicted the timing of seizures in the proband, suggesting a genetic underpinning to the correlations.
Winawer MR, Shih J, Beck ES, Hunter JE, Epstein MP; EPGP Investigators. Genetic effects on sleep/wake variation on seizures. Epilepsia. 2016;57(4):557-665.
The Stigma Attached to Epilepsy is Alive and Unwell
Misconceptions about epilepsy abound in the Western world. An analysis of English language publications revealed that many people have “socially exclusionary attitudes” toward persons with epilepsy, are ignorant about proper treatment, and tend to overgeneralize about people with epilepsy in a way that stigmatizes them. The literature review also found that intervention studies have been effective in improving attitudes about the disease but concluded that “many were targeted to healthcare and education settings, were time intensive, and impractical for broad general population implementation.”
Herrman LK, Welter E, Berg AT, et al. Epilepsy misconceptions and stigma reduction: current status in Western countries. Epilepsy Behav. 2016;60:165-173.
Misconceptions about epilepsy abound in the Western world. An analysis of English language publications revealed that many people have “socially exclusionary attitudes” toward persons with epilepsy, are ignorant about proper treatment, and tend to overgeneralize about people with epilepsy in a way that stigmatizes them. The literature review also found that intervention studies have been effective in improving attitudes about the disease but concluded that “many were targeted to healthcare and education settings, were time intensive, and impractical for broad general population implementation.”
Herrman LK, Welter E, Berg AT, et al. Epilepsy misconceptions and stigma reduction: current status in Western countries. Epilepsy Behav. 2016;60:165-173.
Misconceptions about epilepsy abound in the Western world. An analysis of English language publications revealed that many people have “socially exclusionary attitudes” toward persons with epilepsy, are ignorant about proper treatment, and tend to overgeneralize about people with epilepsy in a way that stigmatizes them. The literature review also found that intervention studies have been effective in improving attitudes about the disease but concluded that “many were targeted to healthcare and education settings, were time intensive, and impractical for broad general population implementation.”
Herrman LK, Welter E, Berg AT, et al. Epilepsy misconceptions and stigma reduction: current status in Western countries. Epilepsy Behav. 2016;60:165-173.
Wilson disease
To the Editor: We read the IM Board Review article by Hanouneh et al in the February issue of the Journal with great interest.1 The authors described an interesting case of a young woman presenting with what initially seemed to be jaundice of acute onset, with rapid progression to acute encephalopathy and worsening liver failure. The patient was eventually diagnosed with fulminant Wilson disease and, thankfully, underwent successful liver transplant. We thank the authors for their in-depth review of the common causes of acute liver failure, the general approach to management, and the tailored treatment of Wilson disease in such settings.
However, we believe that several aspects merit further attention. First, on initial presentation and investigation, it would have been important to consider cholestatic hepatobiliary pathologic processes (eg, choledocholithiasis, cholangitis, primary biliary cirrhosis, primary sclerosing cholangitis), given the characteristic liver panel results.
Second, the authors rightly pointed out that hemolytic anemia is common in patients with acute liver failure secondary to Wilson disease. However, it is important to keep in mind that additional testing should include Coombs testing (typically negative in Wilson disease) and examination of the peripheral smear to exclude other etiologies, since such conditions as thrombotic thrombocytopenic purpura may present with multiorgan failure as well.2
Third, the authors report that Kayser-Fleischer rings are pathognomonic for Wilson disease. However, many reports in peer-reviewed medical journals suggest that this may not be the case and the overall clinical picture should be
considered.3
Fourth, while the authors focus their attention on liver transplant, several other treatments deserve mentioning. We agree that liver transplant is considered the only lifesaving treatment. But in certain situations, molecular absorbent recirculation systems and hemodialysis may provide temporary support while awaiting transportation to a liver transplant center or actual liver transplant.4
- Hanouneh MA, Garber A, Tavill AS, Zein NN, Hanouneh IA. A tale of two sisters with liver disease. Cleve Clin J Med 2016; 83:109–115.
- Nguyen TC, Cruz MA, Carcillo JA. Thrombocytopenia-associated multiple organ failure and acute kidney injury. Crit Care Clin 2015; 31:661–674.
- Frommer D, Morris J, Sherlock S, Abrams J, Newman S. Kayser-Fleischer-like rings in patients without Wilson’s disease. Gastroenterology 1977; 72:1331–1335.
- Hamlyn AN, Gollan JL, Douglas AP, Sherlock S. Fulminant Wilson’s disease with haemolysis and renal failure: copper studies and assessment of dialysis regimens. Br Med J 1977; 2:660–663.
To the Editor: We read the IM Board Review article by Hanouneh et al in the February issue of the Journal with great interest.1 The authors described an interesting case of a young woman presenting with what initially seemed to be jaundice of acute onset, with rapid progression to acute encephalopathy and worsening liver failure. The patient was eventually diagnosed with fulminant Wilson disease and, thankfully, underwent successful liver transplant. We thank the authors for their in-depth review of the common causes of acute liver failure, the general approach to management, and the tailored treatment of Wilson disease in such settings.
However, we believe that several aspects merit further attention. First, on initial presentation and investigation, it would have been important to consider cholestatic hepatobiliary pathologic processes (eg, choledocholithiasis, cholangitis, primary biliary cirrhosis, primary sclerosing cholangitis), given the characteristic liver panel results.
Second, the authors rightly pointed out that hemolytic anemia is common in patients with acute liver failure secondary to Wilson disease. However, it is important to keep in mind that additional testing should include Coombs testing (typically negative in Wilson disease) and examination of the peripheral smear to exclude other etiologies, since such conditions as thrombotic thrombocytopenic purpura may present with multiorgan failure as well.2
Third, the authors report that Kayser-Fleischer rings are pathognomonic for Wilson disease. However, many reports in peer-reviewed medical journals suggest that this may not be the case and the overall clinical picture should be
considered.3
Fourth, while the authors focus their attention on liver transplant, several other treatments deserve mentioning. We agree that liver transplant is considered the only lifesaving treatment. But in certain situations, molecular absorbent recirculation systems and hemodialysis may provide temporary support while awaiting transportation to a liver transplant center or actual liver transplant.4
To the Editor: We read the IM Board Review article by Hanouneh et al in the February issue of the Journal with great interest.1 The authors described an interesting case of a young woman presenting with what initially seemed to be jaundice of acute onset, with rapid progression to acute encephalopathy and worsening liver failure. The patient was eventually diagnosed with fulminant Wilson disease and, thankfully, underwent successful liver transplant. We thank the authors for their in-depth review of the common causes of acute liver failure, the general approach to management, and the tailored treatment of Wilson disease in such settings.
However, we believe that several aspects merit further attention. First, on initial presentation and investigation, it would have been important to consider cholestatic hepatobiliary pathologic processes (eg, choledocholithiasis, cholangitis, primary biliary cirrhosis, primary sclerosing cholangitis), given the characteristic liver panel results.
Second, the authors rightly pointed out that hemolytic anemia is common in patients with acute liver failure secondary to Wilson disease. However, it is important to keep in mind that additional testing should include Coombs testing (typically negative in Wilson disease) and examination of the peripheral smear to exclude other etiologies, since such conditions as thrombotic thrombocytopenic purpura may present with multiorgan failure as well.2
Third, the authors report that Kayser-Fleischer rings are pathognomonic for Wilson disease. However, many reports in peer-reviewed medical journals suggest that this may not be the case and the overall clinical picture should be
considered.3
Fourth, while the authors focus their attention on liver transplant, several other treatments deserve mentioning. We agree that liver transplant is considered the only lifesaving treatment. But in certain situations, molecular absorbent recirculation systems and hemodialysis may provide temporary support while awaiting transportation to a liver transplant center or actual liver transplant.4
- Hanouneh MA, Garber A, Tavill AS, Zein NN, Hanouneh IA. A tale of two sisters with liver disease. Cleve Clin J Med 2016; 83:109–115.
- Nguyen TC, Cruz MA, Carcillo JA. Thrombocytopenia-associated multiple organ failure and acute kidney injury. Crit Care Clin 2015; 31:661–674.
- Frommer D, Morris J, Sherlock S, Abrams J, Newman S. Kayser-Fleischer-like rings in patients without Wilson’s disease. Gastroenterology 1977; 72:1331–1335.
- Hamlyn AN, Gollan JL, Douglas AP, Sherlock S. Fulminant Wilson’s disease with haemolysis and renal failure: copper studies and assessment of dialysis regimens. Br Med J 1977; 2:660–663.
- Hanouneh MA, Garber A, Tavill AS, Zein NN, Hanouneh IA. A tale of two sisters with liver disease. Cleve Clin J Med 2016; 83:109–115.
- Nguyen TC, Cruz MA, Carcillo JA. Thrombocytopenia-associated multiple organ failure and acute kidney injury. Crit Care Clin 2015; 31:661–674.
- Frommer D, Morris J, Sherlock S, Abrams J, Newman S. Kayser-Fleischer-like rings in patients without Wilson’s disease. Gastroenterology 1977; 72:1331–1335.
- Hamlyn AN, Gollan JL, Douglas AP, Sherlock S. Fulminant Wilson’s disease with haemolysis and renal failure: copper studies and assessment of dialysis regimens. Br Med J 1977; 2:660–663.
In reply: Wilson disease
In Reply: We thank Dr. Mirrakhimov and colleagues for bringing important questions to our attention.
In terms of the differential diagnosis of cholestatic liver injury, we agree that pathologic processes such choledocholithiasis, cholangitis, primary biliary cirrhosis, and primary sclerosing cholangitis should be generally considered. However, in the case we described, the patient had no abdominal pain or fever, which makes choledocholithiasis or cholangitis very unlikely. Primary biliary cirrhosis and primary sclerosing cholangitis can cause chronic liver disease but should not be considered in the differential diagnosis of acute liver injury (acute hepatitis), such as in the case we described.
We agree that the hemolytic anemia typically seen in patients with Wilson disease is Coombs-negative, and that Coombs testing and a peripheral smear should be performed. Both were negative in our patient.
We also agree with Dr. Mirrakhimov and colleagues that Kayser-Fleischer rings are not necessarily specific for Wilson disease and can be seen in patients with other forms of cholestatic liver disease such as primary biliary cirrhosis. However, Kayser-Fleischer rings are pathognomonic for acute liver failure from Wilson disease. In other words, when Kayser-Fleischer rings are seen in a patient with acute liver failure, the diagnosis is Wilson disease until proven otherwise.
We discussed on page 112 of our article other treatments such as plasmapheresis as adjunctive therapy to bridge patients with acute liver failure secondary to Wilson disease to transplant. However, liver transplant is still the only definitive and potentially curative treatment.
In Reply: We thank Dr. Mirrakhimov and colleagues for bringing important questions to our attention.
In terms of the differential diagnosis of cholestatic liver injury, we agree that pathologic processes such choledocholithiasis, cholangitis, primary biliary cirrhosis, and primary sclerosing cholangitis should be generally considered. However, in the case we described, the patient had no abdominal pain or fever, which makes choledocholithiasis or cholangitis very unlikely. Primary biliary cirrhosis and primary sclerosing cholangitis can cause chronic liver disease but should not be considered in the differential diagnosis of acute liver injury (acute hepatitis), such as in the case we described.
We agree that the hemolytic anemia typically seen in patients with Wilson disease is Coombs-negative, and that Coombs testing and a peripheral smear should be performed. Both were negative in our patient.
We also agree with Dr. Mirrakhimov and colleagues that Kayser-Fleischer rings are not necessarily specific for Wilson disease and can be seen in patients with other forms of cholestatic liver disease such as primary biliary cirrhosis. However, Kayser-Fleischer rings are pathognomonic for acute liver failure from Wilson disease. In other words, when Kayser-Fleischer rings are seen in a patient with acute liver failure, the diagnosis is Wilson disease until proven otherwise.
We discussed on page 112 of our article other treatments such as plasmapheresis as adjunctive therapy to bridge patients with acute liver failure secondary to Wilson disease to transplant. However, liver transplant is still the only definitive and potentially curative treatment.
In Reply: We thank Dr. Mirrakhimov and colleagues for bringing important questions to our attention.
In terms of the differential diagnosis of cholestatic liver injury, we agree that pathologic processes such choledocholithiasis, cholangitis, primary biliary cirrhosis, and primary sclerosing cholangitis should be generally considered. However, in the case we described, the patient had no abdominal pain or fever, which makes choledocholithiasis or cholangitis very unlikely. Primary biliary cirrhosis and primary sclerosing cholangitis can cause chronic liver disease but should not be considered in the differential diagnosis of acute liver injury (acute hepatitis), such as in the case we described.
We agree that the hemolytic anemia typically seen in patients with Wilson disease is Coombs-negative, and that Coombs testing and a peripheral smear should be performed. Both were negative in our patient.
We also agree with Dr. Mirrakhimov and colleagues that Kayser-Fleischer rings are not necessarily specific for Wilson disease and can be seen in patients with other forms of cholestatic liver disease such as primary biliary cirrhosis. However, Kayser-Fleischer rings are pathognomonic for acute liver failure from Wilson disease. In other words, when Kayser-Fleischer rings are seen in a patient with acute liver failure, the diagnosis is Wilson disease until proven otherwise.
We discussed on page 112 of our article other treatments such as plasmapheresis as adjunctive therapy to bridge patients with acute liver failure secondary to Wilson disease to transplant. However, liver transplant is still the only definitive and potentially curative treatment.
What’s the most effective topical Tx for scalp psoriasis?
Single-agent therapy with a very potent or potent topical corticosteroid appears more effective than other topical agents, including vitamin D3 analogues, for treating scalp psoriasis (strength of recommendation [SOR]: A, systematic reviews of randomized controlled trials [RCTs]).
Combined therapy with a vitamin D3 analogue and a potent topical corticosteroid may be slightly more effective than monotherapy with either agent (SOR: B, systematic reviews of RCTs with inconsistent results).
Evidence summary
A 2013 meta-analysis of 26 RCTs with 8020 patients evaluated topical treatments for scalp psoriasis as part of a subanalysis of a larger Cochrane review of psoriasis therapy.1 Only 20 studies reported the severity of disease: 13 studies looked at moderate to severe scalp psoriasis and the others examined mild to severe disease.
Results were reported as standardized mean differences (SMD) and also converted to a 6-point global improvement scale created by the authors to provide a combined endpoint of provider- or patient-assessed improvement in symptoms such as redness, thickness, and scaling. Higher scores indicate more improvement.
Compared with placebo, the very potent corticosteroid clobetasol propionate improved psoriasis by 1.9 points on the 6-point scale (4 trials, 788 patients; SMD= −1.6; 95% confidence interval [CI], −1.8 to −1.3). The potent steroid betamethasone diproprionate improved symptoms by 1.3 points compared with placebo (2 trials, 712 patients; SMD= −1.1; 95% CI, −1.3 to −0.90).
The topical corticosteroids clobetasol, betamethasone diproprionate, and betamethasone valerate improved symptoms more than the vitamin D3 analogue calcipotriol in head-to-head trials. The corticosteroid improvement scores exceeded calcipotriol scores by 0.5 points (1 trial, 151 patients; SMD=0.37; 95% CI, 0.05-0.69), 0.6 points (1 trial, 1676 patients; SMD=0.48; 95% CI, 0.32-0.64), and 0.5 points (1 trial, 510 patients; SMD=0.37; 95% CI, 0.20-0.55), respectively.
Combination therapy with a vitamin D3 analogue and a corticosteroid yielded approximately 0.2 points of improvement over corticosteroid alone (6 trials, 2444 patients; SMD= −0.18; 95% CI, −0.26 to −0.10). Four trials of combination therapy (2581 patients) resulted in 0.5 to 1.2 points of improvement compared with vitamin D3 analogues alone (SMD=0.64; 95% CI, 0.44-0.84). Specific strengths and dosing regimens weren’t reported.
The Cochrane systematic review, using the same outcome reporting methods, provided data on the vitamin D3 analogue calcipotriol compared with placebo for treating scalp psoriasis.2 Calcipotriol resulted in 0.9 points of improvement on the 6-point global improvement scale (2 trials, 457 patients; SMD= −0.72; 95% CI, −1.3 to −0.16).
Very potent corticosteroids show a better response than potent agents
In 2013, a meta-analysis of 13 placebo-controlled RCTs (5640 patients) evaluated topical therapies for scalp psoriasis licensed in the United Kingdom. This meta-analysis included the same placebo-controlled studies as the Cochrane review but added one study published after the search date of the review.3
The outcome reporting was different from the Cochrane review. The primary outcome was percentage of patients with at least moderate scalp psoriasis who achieved clear or nearly clear status on provider assessment scales. All treatments were compared to twice-daily placebo with a response rate of 11%.
Very potent steroids had response rates of 78% for twice-daily application (risk ratio [RR]=7.0; 95% CI, 5.6-8.0) and 69% for once-daily application (RR=6.2; 95% CI, 3.0-8.3). The combination of a vitamin D3 analogue and a potent corticosteroid showed a response rate of 64% (RR=5.7; 95% CI, 2.4-8.0) whereas response rates for potent corticosteroids alone were 57% (RR=5.0; 95% CI, 1.6-7.8) for once-daily application and 49% (RR=4.4; 95% CI, 2.2-6.7) for twice-daily administration. The authors suggested patient satisfaction at using once daily vs twice daily application as a possible explanation for the difference in response rate.
Vitamin D3 analogues showed response rates of approximately 34%, which is nonsignificant for once-daily application (RR=3.1; 95% CI, 0.71-6.6) but significant for twice-daily administration (RR=3.1; 95% CI, 1.3-5.9). Exact numbers of studies and participants, as well as specific agents and preparation information, were not included.
1. Mason AR, Mason JM, Cork MJ, et al. Topical treatments for chronic plaque psoriasis of the scalp: a systematic review. Br J Dermatol. 2013;169:519-527.
2. Mason A, Mason J, Cork M, et al. Topical treatments for chronic plaque psoriasis: an abridged Cochrane systematic review. J Am Acad Dermatol. 2013; 69:799-807.
3. Samarasekera EJ, Sawyer L, Wonderling D, et al. Topical therapies for the treatment of plaque psoriasis: systematic review and network meta-analyses. Br J Dermatol. 2013;168:954-967.
Single-agent therapy with a very potent or potent topical corticosteroid appears more effective than other topical agents, including vitamin D3 analogues, for treating scalp psoriasis (strength of recommendation [SOR]: A, systematic reviews of randomized controlled trials [RCTs]).
Combined therapy with a vitamin D3 analogue and a potent topical corticosteroid may be slightly more effective than monotherapy with either agent (SOR: B, systematic reviews of RCTs with inconsistent results).
Evidence summary
A 2013 meta-analysis of 26 RCTs with 8020 patients evaluated topical treatments for scalp psoriasis as part of a subanalysis of a larger Cochrane review of psoriasis therapy.1 Only 20 studies reported the severity of disease: 13 studies looked at moderate to severe scalp psoriasis and the others examined mild to severe disease.
Results were reported as standardized mean differences (SMD) and also converted to a 6-point global improvement scale created by the authors to provide a combined endpoint of provider- or patient-assessed improvement in symptoms such as redness, thickness, and scaling. Higher scores indicate more improvement.
Compared with placebo, the very potent corticosteroid clobetasol propionate improved psoriasis by 1.9 points on the 6-point scale (4 trials, 788 patients; SMD= −1.6; 95% confidence interval [CI], −1.8 to −1.3). The potent steroid betamethasone diproprionate improved symptoms by 1.3 points compared with placebo (2 trials, 712 patients; SMD= −1.1; 95% CI, −1.3 to −0.90).
The topical corticosteroids clobetasol, betamethasone diproprionate, and betamethasone valerate improved symptoms more than the vitamin D3 analogue calcipotriol in head-to-head trials. The corticosteroid improvement scores exceeded calcipotriol scores by 0.5 points (1 trial, 151 patients; SMD=0.37; 95% CI, 0.05-0.69), 0.6 points (1 trial, 1676 patients; SMD=0.48; 95% CI, 0.32-0.64), and 0.5 points (1 trial, 510 patients; SMD=0.37; 95% CI, 0.20-0.55), respectively.
Combination therapy with a vitamin D3 analogue and a corticosteroid yielded approximately 0.2 points of improvement over corticosteroid alone (6 trials, 2444 patients; SMD= −0.18; 95% CI, −0.26 to −0.10). Four trials of combination therapy (2581 patients) resulted in 0.5 to 1.2 points of improvement compared with vitamin D3 analogues alone (SMD=0.64; 95% CI, 0.44-0.84). Specific strengths and dosing regimens weren’t reported.
The Cochrane systematic review, using the same outcome reporting methods, provided data on the vitamin D3 analogue calcipotriol compared with placebo for treating scalp psoriasis.2 Calcipotriol resulted in 0.9 points of improvement on the 6-point global improvement scale (2 trials, 457 patients; SMD= −0.72; 95% CI, −1.3 to −0.16).
Very potent corticosteroids show a better response than potent agents
In 2013, a meta-analysis of 13 placebo-controlled RCTs (5640 patients) evaluated topical therapies for scalp psoriasis licensed in the United Kingdom. This meta-analysis included the same placebo-controlled studies as the Cochrane review but added one study published after the search date of the review.3
The outcome reporting was different from the Cochrane review. The primary outcome was percentage of patients with at least moderate scalp psoriasis who achieved clear or nearly clear status on provider assessment scales. All treatments were compared to twice-daily placebo with a response rate of 11%.
Very potent steroids had response rates of 78% for twice-daily application (risk ratio [RR]=7.0; 95% CI, 5.6-8.0) and 69% for once-daily application (RR=6.2; 95% CI, 3.0-8.3). The combination of a vitamin D3 analogue and a potent corticosteroid showed a response rate of 64% (RR=5.7; 95% CI, 2.4-8.0) whereas response rates for potent corticosteroids alone were 57% (RR=5.0; 95% CI, 1.6-7.8) for once-daily application and 49% (RR=4.4; 95% CI, 2.2-6.7) for twice-daily administration. The authors suggested patient satisfaction at using once daily vs twice daily application as a possible explanation for the difference in response rate.
Vitamin D3 analogues showed response rates of approximately 34%, which is nonsignificant for once-daily application (RR=3.1; 95% CI, 0.71-6.6) but significant for twice-daily administration (RR=3.1; 95% CI, 1.3-5.9). Exact numbers of studies and participants, as well as specific agents and preparation information, were not included.
Single-agent therapy with a very potent or potent topical corticosteroid appears more effective than other topical agents, including vitamin D3 analogues, for treating scalp psoriasis (strength of recommendation [SOR]: A, systematic reviews of randomized controlled trials [RCTs]).
Combined therapy with a vitamin D3 analogue and a potent topical corticosteroid may be slightly more effective than monotherapy with either agent (SOR: B, systematic reviews of RCTs with inconsistent results).
Evidence summary
A 2013 meta-analysis of 26 RCTs with 8020 patients evaluated topical treatments for scalp psoriasis as part of a subanalysis of a larger Cochrane review of psoriasis therapy.1 Only 20 studies reported the severity of disease: 13 studies looked at moderate to severe scalp psoriasis and the others examined mild to severe disease.
Results were reported as standardized mean differences (SMD) and also converted to a 6-point global improvement scale created by the authors to provide a combined endpoint of provider- or patient-assessed improvement in symptoms such as redness, thickness, and scaling. Higher scores indicate more improvement.
Compared with placebo, the very potent corticosteroid clobetasol propionate improved psoriasis by 1.9 points on the 6-point scale (4 trials, 788 patients; SMD= −1.6; 95% confidence interval [CI], −1.8 to −1.3). The potent steroid betamethasone diproprionate improved symptoms by 1.3 points compared with placebo (2 trials, 712 patients; SMD= −1.1; 95% CI, −1.3 to −0.90).
The topical corticosteroids clobetasol, betamethasone diproprionate, and betamethasone valerate improved symptoms more than the vitamin D3 analogue calcipotriol in head-to-head trials. The corticosteroid improvement scores exceeded calcipotriol scores by 0.5 points (1 trial, 151 patients; SMD=0.37; 95% CI, 0.05-0.69), 0.6 points (1 trial, 1676 patients; SMD=0.48; 95% CI, 0.32-0.64), and 0.5 points (1 trial, 510 patients; SMD=0.37; 95% CI, 0.20-0.55), respectively.
Combination therapy with a vitamin D3 analogue and a corticosteroid yielded approximately 0.2 points of improvement over corticosteroid alone (6 trials, 2444 patients; SMD= −0.18; 95% CI, −0.26 to −0.10). Four trials of combination therapy (2581 patients) resulted in 0.5 to 1.2 points of improvement compared with vitamin D3 analogues alone (SMD=0.64; 95% CI, 0.44-0.84). Specific strengths and dosing regimens weren’t reported.
The Cochrane systematic review, using the same outcome reporting methods, provided data on the vitamin D3 analogue calcipotriol compared with placebo for treating scalp psoriasis.2 Calcipotriol resulted in 0.9 points of improvement on the 6-point global improvement scale (2 trials, 457 patients; SMD= −0.72; 95% CI, −1.3 to −0.16).
Very potent corticosteroids show a better response than potent agents
In 2013, a meta-analysis of 13 placebo-controlled RCTs (5640 patients) evaluated topical therapies for scalp psoriasis licensed in the United Kingdom. This meta-analysis included the same placebo-controlled studies as the Cochrane review but added one study published after the search date of the review.3
The outcome reporting was different from the Cochrane review. The primary outcome was percentage of patients with at least moderate scalp psoriasis who achieved clear or nearly clear status on provider assessment scales. All treatments were compared to twice-daily placebo with a response rate of 11%.
Very potent steroids had response rates of 78% for twice-daily application (risk ratio [RR]=7.0; 95% CI, 5.6-8.0) and 69% for once-daily application (RR=6.2; 95% CI, 3.0-8.3). The combination of a vitamin D3 analogue and a potent corticosteroid showed a response rate of 64% (RR=5.7; 95% CI, 2.4-8.0) whereas response rates for potent corticosteroids alone were 57% (RR=5.0; 95% CI, 1.6-7.8) for once-daily application and 49% (RR=4.4; 95% CI, 2.2-6.7) for twice-daily administration. The authors suggested patient satisfaction at using once daily vs twice daily application as a possible explanation for the difference in response rate.
Vitamin D3 analogues showed response rates of approximately 34%, which is nonsignificant for once-daily application (RR=3.1; 95% CI, 0.71-6.6) but significant for twice-daily administration (RR=3.1; 95% CI, 1.3-5.9). Exact numbers of studies and participants, as well as specific agents and preparation information, were not included.
1. Mason AR, Mason JM, Cork MJ, et al. Topical treatments for chronic plaque psoriasis of the scalp: a systematic review. Br J Dermatol. 2013;169:519-527.
2. Mason A, Mason J, Cork M, et al. Topical treatments for chronic plaque psoriasis: an abridged Cochrane systematic review. J Am Acad Dermatol. 2013; 69:799-807.
3. Samarasekera EJ, Sawyer L, Wonderling D, et al. Topical therapies for the treatment of plaque psoriasis: systematic review and network meta-analyses. Br J Dermatol. 2013;168:954-967.
1. Mason AR, Mason JM, Cork MJ, et al. Topical treatments for chronic plaque psoriasis of the scalp: a systematic review. Br J Dermatol. 2013;169:519-527.
2. Mason A, Mason J, Cork M, et al. Topical treatments for chronic plaque psoriasis: an abridged Cochrane systematic review. J Am Acad Dermatol. 2013; 69:799-807.
3. Samarasekera EJ, Sawyer L, Wonderling D, et al. Topical therapies for the treatment of plaque psoriasis: systematic review and network meta-analyses. Br J Dermatol. 2013;168:954-967.
Evidence-based answers from the Family Physicians Inquiries Network
Patients With Epilepsy Have an Internet Disadvantage That May Impede Self-Management
Persons with epilepsy are less likely to use the Internet compared with the general public. The recent CDC study that arrived at that conclusion suggested that this disparity may put patients with epilepsy at a disadvantage because it limits their access to online tools that can optimize their self-care and improve their quality of life. The study was based on data from the 2013 National Health Interview Survey, which confirmed that the disparity existed in all three age groups analyzed: 18-44 years, 45-59 years, 60 years and older.
US Centers for Disease Control and Prevention Epilepsy Program. Internet use and looking up information online in adults with epilepsy varies by epilepsy status — 2013 National Health Interview Survey. Epilepsy Behav. 2016;54:47-49.
Persons with epilepsy are less likely to use the Internet compared with the general public. The recent CDC study that arrived at that conclusion suggested that this disparity may put patients with epilepsy at a disadvantage because it limits their access to online tools that can optimize their self-care and improve their quality of life. The study was based on data from the 2013 National Health Interview Survey, which confirmed that the disparity existed in all three age groups analyzed: 18-44 years, 45-59 years, 60 years and older.
US Centers for Disease Control and Prevention Epilepsy Program. Internet use and looking up information online in adults with epilepsy varies by epilepsy status — 2013 National Health Interview Survey. Epilepsy Behav. 2016;54:47-49.
Persons with epilepsy are less likely to use the Internet compared with the general public. The recent CDC study that arrived at that conclusion suggested that this disparity may put patients with epilepsy at a disadvantage because it limits their access to online tools that can optimize their self-care and improve their quality of life. The study was based on data from the 2013 National Health Interview Survey, which confirmed that the disparity existed in all three age groups analyzed: 18-44 years, 45-59 years, 60 years and older.
US Centers for Disease Control and Prevention Epilepsy Program. Internet use and looking up information online in adults with epilepsy varies by epilepsy status — 2013 National Health Interview Survey. Epilepsy Behav. 2016;54:47-49.
Do novel oral anticoagulants safely prevent stroke in patients with nonvalvular A-fib?
Yes. Dabigatran, rivaroxaban, and apixaban are safe and effective compared with warfarin for preventing stroke in patients with nonvalvular atrial fibrillation. These novel oral anticoagulants (NOACs) are noninferior in reducing the number of strokes and systemic emboli and in lowering all-cause mortality while not increasing major bleeding complications and hemorrhagic events (strength of recommendation: A, consistent meta-analyses of randomized controlled trials [RCTs]).
Evidence summary
A 2014 meta-analysis of 4 RCTs including 71,683 patients with nonvalvular atrial fibrillation evaluated the NOACs dabigatran, rivaroxaban, apixaban, and edoxaban, for efficacy and safety compared with warfarin.1 The RCTs analyzed 42,411 patients receiving NOACs and 29,272 patients receiving warfarin. All trials were designed to show noninferiority. Selection criteria for RCTs included all phase 3 trials of available NOACs (edoxaban isn’t available in the United States). Median follow-up was 1.8 to 2.8 years.
Pooled data demonstrated that NOACs were noninferior to warfarin in preventing stroke or systemic embolism (relative risk [RR]=0.81; 95% confidence interval [CI], 0.73-0.91; number needed to treat [NNT]=147). The main benefit was derived from the relatively large decrease in the rate of hemorrhagic stroke (RR=0.49; 95% CI, 0.38-0.64; NNT=97) compared with warfarin. All-cause mortality was lower with NOACs as well (RR=0.90; 95% CI, 0.85-0.95; NNT=128).
A significant increase in gastrointestinal bleeding occurred with NOACs compared with warfarin (RR=1.3; 95% CI, 1.1-1.6; number needed to harm=185), but NOACs were associated with a decrease in intracranial hemorrhage similar to the reduction in hemorrhagic stroke (RR=0.48; 95% CI, 0.39-0.59; NNT=132).
NOACs show no significant difference in bleeding complications vs warfarin
A 2013 meta-analysis of 5 RCTs including 51,895 patients with nonvalvular atrial fibrillation compared the efficacy and safety of the NOACs dabigatran, rivaroxaban, apixaban, and ximelagatran, with the efficacy and safety of warfarin.2 This review included the 3 studies of dabigatran, rivaroxaban, and apixaban from the previously described review, as well as 2 trials of ximelagatran that were not included in the other review (presumably because ximelagatran was no longer available owing to liver toxicity). This review didn’t include the study of edoxaban that was published after the search dates of the literature review.
All trials were designed to show noninferiority. Selection criteria included a study population of at least 3000 patients and use of intention-to-treat analysis. Only 3 of the trials were double-blinded, and 2 were open-label. Mean follow-up was 16 months; median was 24 months.
NOACs were noninferior to vitamin K antagonists in the rate of stroke or systemic embolism (RR=0.82; 95% CI, 0.69-0.98; NNT=200), the rate of death from any cause (RR=0.91; 95% CI, 0.85-0.96; NNT=145), and the rate of hemorrhagic strokes (RR=0.51; 95% CI, 0.41-0.64). NOACs showed no significant difference in major bleeding compared with warfarin (RR=0.83; 95% CI, 0.69-1.0), and were noninferior for minor bleeding (RR=0.88; 95% CI, 0.80-0.97). There was no difference in ischemic stroke (RR=0.87; 95% CI, 0.75-1.06) and major noncerebral bleeding (RR=0.88; 95% CI, 0.73-1.08).
The ACCP weighs in
The American College of Chest Physicians’ 2012 clinical practice guidelines for antithrombotic therapy for atrial fibrillation recommend dabigatran 150 mg twice daily rather than adjusted-dose warfarin therapy for patients with nonvalvular atrial fibrillation requiring thromboembolism prophylaxis (Grade 2B, weak recommendation based on RCTs with important limitations).3
1. Ruff CT, Giugliano RP, Braunwald E, et al. Comparison of the efficacy and safety of new oral anticoagulants with warfarin in patients with atrial fibrillation: a meta-analysis of randomised trials. Lancet. 2014;383:955-962.
2. Dogliotti A, Paolasso E, Giugliano RP, et al. Novel oral anticoagulants in atrial fibrillation: a meta-analysis of large, randomized, controlled trials vs warfarin. Clin Cardiol. 2013;36:61-67.
3. You JJ, Singer DE, Howard PA, et al. Antithrombotic therapy for atrial fibrillation: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(2 Suppl):e531S-e575S.
Yes. Dabigatran, rivaroxaban, and apixaban are safe and effective compared with warfarin for preventing stroke in patients with nonvalvular atrial fibrillation. These novel oral anticoagulants (NOACs) are noninferior in reducing the number of strokes and systemic emboli and in lowering all-cause mortality while not increasing major bleeding complications and hemorrhagic events (strength of recommendation: A, consistent meta-analyses of randomized controlled trials [RCTs]).
Evidence summary
A 2014 meta-analysis of 4 RCTs including 71,683 patients with nonvalvular atrial fibrillation evaluated the NOACs dabigatran, rivaroxaban, apixaban, and edoxaban, for efficacy and safety compared with warfarin.1 The RCTs analyzed 42,411 patients receiving NOACs and 29,272 patients receiving warfarin. All trials were designed to show noninferiority. Selection criteria for RCTs included all phase 3 trials of available NOACs (edoxaban isn’t available in the United States). Median follow-up was 1.8 to 2.8 years.
Pooled data demonstrated that NOACs were noninferior to warfarin in preventing stroke or systemic embolism (relative risk [RR]=0.81; 95% confidence interval [CI], 0.73-0.91; number needed to treat [NNT]=147). The main benefit was derived from the relatively large decrease in the rate of hemorrhagic stroke (RR=0.49; 95% CI, 0.38-0.64; NNT=97) compared with warfarin. All-cause mortality was lower with NOACs as well (RR=0.90; 95% CI, 0.85-0.95; NNT=128).
A significant increase in gastrointestinal bleeding occurred with NOACs compared with warfarin (RR=1.3; 95% CI, 1.1-1.6; number needed to harm=185), but NOACs were associated with a decrease in intracranial hemorrhage similar to the reduction in hemorrhagic stroke (RR=0.48; 95% CI, 0.39-0.59; NNT=132).
NOACs show no significant difference in bleeding complications vs warfarin
A 2013 meta-analysis of 5 RCTs including 51,895 patients with nonvalvular atrial fibrillation compared the efficacy and safety of the NOACs dabigatran, rivaroxaban, apixaban, and ximelagatran, with the efficacy and safety of warfarin.2 This review included the 3 studies of dabigatran, rivaroxaban, and apixaban from the previously described review, as well as 2 trials of ximelagatran that were not included in the other review (presumably because ximelagatran was no longer available owing to liver toxicity). This review didn’t include the study of edoxaban that was published after the search dates of the literature review.
All trials were designed to show noninferiority. Selection criteria included a study population of at least 3000 patients and use of intention-to-treat analysis. Only 3 of the trials were double-blinded, and 2 were open-label. Mean follow-up was 16 months; median was 24 months.
NOACs were noninferior to vitamin K antagonists in the rate of stroke or systemic embolism (RR=0.82; 95% CI, 0.69-0.98; NNT=200), the rate of death from any cause (RR=0.91; 95% CI, 0.85-0.96; NNT=145), and the rate of hemorrhagic strokes (RR=0.51; 95% CI, 0.41-0.64). NOACs showed no significant difference in major bleeding compared with warfarin (RR=0.83; 95% CI, 0.69-1.0), and were noninferior for minor bleeding (RR=0.88; 95% CI, 0.80-0.97). There was no difference in ischemic stroke (RR=0.87; 95% CI, 0.75-1.06) and major noncerebral bleeding (RR=0.88; 95% CI, 0.73-1.08).
The ACCP weighs in
The American College of Chest Physicians’ 2012 clinical practice guidelines for antithrombotic therapy for atrial fibrillation recommend dabigatran 150 mg twice daily rather than adjusted-dose warfarin therapy for patients with nonvalvular atrial fibrillation requiring thromboembolism prophylaxis (Grade 2B, weak recommendation based on RCTs with important limitations).3
Yes. Dabigatran, rivaroxaban, and apixaban are safe and effective compared with warfarin for preventing stroke in patients with nonvalvular atrial fibrillation. These novel oral anticoagulants (NOACs) are noninferior in reducing the number of strokes and systemic emboli and in lowering all-cause mortality while not increasing major bleeding complications and hemorrhagic events (strength of recommendation: A, consistent meta-analyses of randomized controlled trials [RCTs]).
Evidence summary
A 2014 meta-analysis of 4 RCTs including 71,683 patients with nonvalvular atrial fibrillation evaluated the NOACs dabigatran, rivaroxaban, apixaban, and edoxaban, for efficacy and safety compared with warfarin.1 The RCTs analyzed 42,411 patients receiving NOACs and 29,272 patients receiving warfarin. All trials were designed to show noninferiority. Selection criteria for RCTs included all phase 3 trials of available NOACs (edoxaban isn’t available in the United States). Median follow-up was 1.8 to 2.8 years.
Pooled data demonstrated that NOACs were noninferior to warfarin in preventing stroke or systemic embolism (relative risk [RR]=0.81; 95% confidence interval [CI], 0.73-0.91; number needed to treat [NNT]=147). The main benefit was derived from the relatively large decrease in the rate of hemorrhagic stroke (RR=0.49; 95% CI, 0.38-0.64; NNT=97) compared with warfarin. All-cause mortality was lower with NOACs as well (RR=0.90; 95% CI, 0.85-0.95; NNT=128).
A significant increase in gastrointestinal bleeding occurred with NOACs compared with warfarin (RR=1.3; 95% CI, 1.1-1.6; number needed to harm=185), but NOACs were associated with a decrease in intracranial hemorrhage similar to the reduction in hemorrhagic stroke (RR=0.48; 95% CI, 0.39-0.59; NNT=132).
NOACs show no significant difference in bleeding complications vs warfarin
A 2013 meta-analysis of 5 RCTs including 51,895 patients with nonvalvular atrial fibrillation compared the efficacy and safety of the NOACs dabigatran, rivaroxaban, apixaban, and ximelagatran, with the efficacy and safety of warfarin.2 This review included the 3 studies of dabigatran, rivaroxaban, and apixaban from the previously described review, as well as 2 trials of ximelagatran that were not included in the other review (presumably because ximelagatran was no longer available owing to liver toxicity). This review didn’t include the study of edoxaban that was published after the search dates of the literature review.
All trials were designed to show noninferiority. Selection criteria included a study population of at least 3000 patients and use of intention-to-treat analysis. Only 3 of the trials were double-blinded, and 2 were open-label. Mean follow-up was 16 months; median was 24 months.
NOACs were noninferior to vitamin K antagonists in the rate of stroke or systemic embolism (RR=0.82; 95% CI, 0.69-0.98; NNT=200), the rate of death from any cause (RR=0.91; 95% CI, 0.85-0.96; NNT=145), and the rate of hemorrhagic strokes (RR=0.51; 95% CI, 0.41-0.64). NOACs showed no significant difference in major bleeding compared with warfarin (RR=0.83; 95% CI, 0.69-1.0), and were noninferior for minor bleeding (RR=0.88; 95% CI, 0.80-0.97). There was no difference in ischemic stroke (RR=0.87; 95% CI, 0.75-1.06) and major noncerebral bleeding (RR=0.88; 95% CI, 0.73-1.08).
The ACCP weighs in
The American College of Chest Physicians’ 2012 clinical practice guidelines for antithrombotic therapy for atrial fibrillation recommend dabigatran 150 mg twice daily rather than adjusted-dose warfarin therapy for patients with nonvalvular atrial fibrillation requiring thromboembolism prophylaxis (Grade 2B, weak recommendation based on RCTs with important limitations).3
1. Ruff CT, Giugliano RP, Braunwald E, et al. Comparison of the efficacy and safety of new oral anticoagulants with warfarin in patients with atrial fibrillation: a meta-analysis of randomised trials. Lancet. 2014;383:955-962.
2. Dogliotti A, Paolasso E, Giugliano RP, et al. Novel oral anticoagulants in atrial fibrillation: a meta-analysis of large, randomized, controlled trials vs warfarin. Clin Cardiol. 2013;36:61-67.
3. You JJ, Singer DE, Howard PA, et al. Antithrombotic therapy for atrial fibrillation: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(2 Suppl):e531S-e575S.
1. Ruff CT, Giugliano RP, Braunwald E, et al. Comparison of the efficacy and safety of new oral anticoagulants with warfarin in patients with atrial fibrillation: a meta-analysis of randomised trials. Lancet. 2014;383:955-962.
2. Dogliotti A, Paolasso E, Giugliano RP, et al. Novel oral anticoagulants in atrial fibrillation: a meta-analysis of large, randomized, controlled trials vs warfarin. Clin Cardiol. 2013;36:61-67.
3. You JJ, Singer DE, Howard PA, et al. Antithrombotic therapy for atrial fibrillation: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(2 Suppl):e531S-e575S.
Evidence-based answers from the Family Physicians Inquiries Network