User login
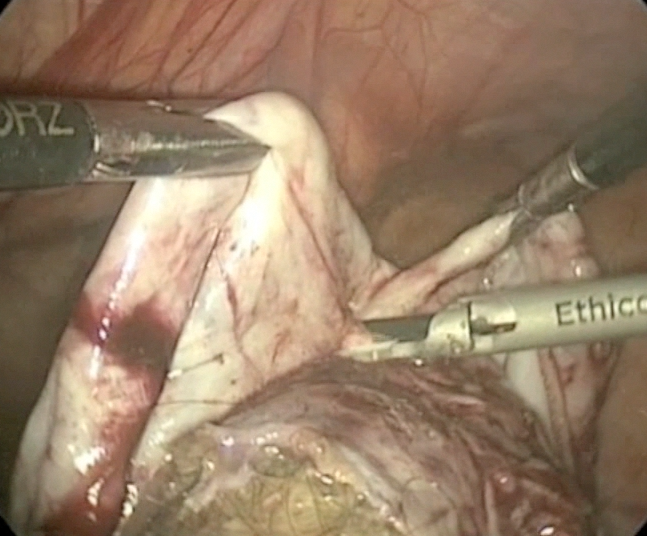
Laparoscopic cystectomy for large, bilateral ovarian dermoids
For more videos from the Society of Gynecologic Surgeons, click here
Visit the Society of Gynecologic Surgeons online: sgsonline.org
For more videos from the Society of Gynecologic Surgeons, click here
Visit the Society of Gynecologic Surgeons online: sgsonline.org
For more videos from the Society of Gynecologic Surgeons, click here
Visit the Society of Gynecologic Surgeons online: sgsonline.org
This video is brought to you by 
Prostate cancer’s future seen in molecular tests
HOLLYWOOD, FLA. – Current evidence suggests that molecular tests for prostate cancer are prognostic and can help clinicians and patients with difficult treatment decisions. In the not-too-distant future, gene tests could also guide choice of therapies.
“I think that the largest impact is going to come in areas of both the greatest treatment uncertainty and areas where we can be predictive about the response to treatment,” said Dr. Ashley Ross, a urologic oncologist and pathologist at the Sidney Kimmel Comprehensive Cancer Center at Johns Hopkins University, Baltimore.
Multigene panels may soon be able help identify which patients might benefit more from radical prostatectomy or radiation therapy, whether radiation therapy effects could be enhanced with the addition of androgen-deprivation therapy, and whether early use of docetaxel might add therapeutic benefit, he said at the annual conference of the National Comprehensive Cancer Network.
The 2016 iteration of the NCCN guidelines for the treatment of prostate cancer include a note stating that “men with clinically localized disease may consider the use of tumor-based molecular assays. Retrospective case cohort studies have shown that molecular assays performed on biopsy or prostatectomy specimens provide prognostic information independent of NCCN risk groups.”
The use of molecular assays may inform treatment decisions by helping to predict the likelihood of death if a patient is managed conservatively, risks for biochemical progression after radical prostatectomy or external beam therapy, and the likelihood that a patient could develop metastatic disease after radical prostatectomy or salvage radiotherapy, the guidelines say.
Dr. Ross reviewed the molecular biology of localized prostate cancer and the benefits and risks of currently available molecular tests.
“We’ve had an increased ability to get molecular information or genomic information from very limited amounts of routinely-stored pathologic tissue, and that’s resulted in the generation of many molecular-based tissue tests in prostate cancer. With the emergence of those tests and a lot of aggressive marketing, there has been a lot of confusion for patients and providers about whether we should use them or not and in what context,” he said.
Prostate cancer is genomically complex, even in the localized stage, with copy number alterations, deletions, and amplifications; chromosomal rearrangements; and point mutations, he said.
One of the best characterized genomic events is the early loss of the tumor suppressor gene PTEN (phosphatase and tensin homolog). This gene works within the PI3 kinase (PI3K)/AKT pathway. PI3K pathway mutations have been identified in up to 40% of all primary prostate cancers and 100% of mutations, Dr. Ross explained.
Loss of PTEN itself has been detected in about 15%-40% of primary prostate cancers and 50% of metastases, and the loss correlates with disease stage and tumor grade.
The NCCN guidelines list six available tissue-based tests for prostate cancer prognosis, including tests based on general cancer features such as cell-cycle proliferation, and those based on specific molecular features of cancer.
An example of the general type of test is the Ki-67 immunohistochemistry (IHC) test, which looks for a cellular marker of proliferation, and has been shown to have independent prognostic significance after radiation therapy or radical prostatectomy. This test is not currently recommended by the Medicare Molecular Diagnostic Services (MolDx) program, however.
Another general-type test is the Polaris quantitative reverse-transcriptase polymerase chain reaction (RT-PCR) panel, which tests for 31 cell-cycle-related genes and 15 “housekeeping” controls. This test is recommended for post-biopsy evaluation of men with very-low-risk or low-risk prostate cancer who at the time of diagnosis have at least 10 years of life expectancy. It has been shown to independently predict prostate cancer–specific mortality, biochemical failure/recurrence, and metastasis, the guidelines say.
Tests based on molecular features include:
• PTEN/ERG, an IHC or fluorescent in situ hybridization test that has been shown to predict prostate cancer–specific mortality, upgrading to Gleason pattern 4 on radical prostatectomy, and biochemical recurrence (not MolDx recommended).
• Decipher, a whole-transcriptome 1.4M RNA expression oligonucleotide microarray shown to predict biochemical failure, metastasis, and prostate cancer–specific mortality (recommended for postradical prostatectomy for patients with pT2 tumors with positive margins, and pT3 disease, and rising PSA above nadir);
• Oncotype DX, an RT-PCR assay for 12 prostate cancer genes and five housekeeping controls (recommended for post-biopsy evaluation of men with very-low-risk or low-risk prostate cancer who at the time of diagnosis have at least 10 years of life expectancy).
• ProMark, multiplex immunofluorescent staining of eight proteins, which has been shown to independently predict non–organ-confined pT3 disease or Gleason pattern 4 disease on radical prostatectomy (not reviewed).
Dr. Ross said that in his practice, he generally does not order molecular testing for surveillance of men older than 65 who have very-low-risk disease. For men with low-risk disease, however, molecular testing may help in clinical decision making to predict upgrading or disease progression.
“There’s limited data from surveillance populations, but these tests can be used in this context with retrospective data available, realizing that in most cases the tests will be confirmative, or another way of thinking about it is ‘noninformative,’ so there are some considerations about cost in that context,” he said. For men with intermediate or high-risk disease, however, currently available tests are not good at predicting what an individual patient’s response would be to a specific type of therapy, whether surgery, radiation, androgen deprivation, chemotherapy, or a combination.
“This is an area where predictive biomarkers would be very informative. There is ongoing research, and I think this is an area of potentially large advancement in how we risk-stratify our patients,” Dr. Ross said.
HOLLYWOOD, FLA. – Current evidence suggests that molecular tests for prostate cancer are prognostic and can help clinicians and patients with difficult treatment decisions. In the not-too-distant future, gene tests could also guide choice of therapies.
“I think that the largest impact is going to come in areas of both the greatest treatment uncertainty and areas where we can be predictive about the response to treatment,” said Dr. Ashley Ross, a urologic oncologist and pathologist at the Sidney Kimmel Comprehensive Cancer Center at Johns Hopkins University, Baltimore.
Multigene panels may soon be able help identify which patients might benefit more from radical prostatectomy or radiation therapy, whether radiation therapy effects could be enhanced with the addition of androgen-deprivation therapy, and whether early use of docetaxel might add therapeutic benefit, he said at the annual conference of the National Comprehensive Cancer Network.
The 2016 iteration of the NCCN guidelines for the treatment of prostate cancer include a note stating that “men with clinically localized disease may consider the use of tumor-based molecular assays. Retrospective case cohort studies have shown that molecular assays performed on biopsy or prostatectomy specimens provide prognostic information independent of NCCN risk groups.”
The use of molecular assays may inform treatment decisions by helping to predict the likelihood of death if a patient is managed conservatively, risks for biochemical progression after radical prostatectomy or external beam therapy, and the likelihood that a patient could develop metastatic disease after radical prostatectomy or salvage radiotherapy, the guidelines say.
Dr. Ross reviewed the molecular biology of localized prostate cancer and the benefits and risks of currently available molecular tests.
“We’ve had an increased ability to get molecular information or genomic information from very limited amounts of routinely-stored pathologic tissue, and that’s resulted in the generation of many molecular-based tissue tests in prostate cancer. With the emergence of those tests and a lot of aggressive marketing, there has been a lot of confusion for patients and providers about whether we should use them or not and in what context,” he said.
Prostate cancer is genomically complex, even in the localized stage, with copy number alterations, deletions, and amplifications; chromosomal rearrangements; and point mutations, he said.
One of the best characterized genomic events is the early loss of the tumor suppressor gene PTEN (phosphatase and tensin homolog). This gene works within the PI3 kinase (PI3K)/AKT pathway. PI3K pathway mutations have been identified in up to 40% of all primary prostate cancers and 100% of mutations, Dr. Ross explained.
Loss of PTEN itself has been detected in about 15%-40% of primary prostate cancers and 50% of metastases, and the loss correlates with disease stage and tumor grade.
The NCCN guidelines list six available tissue-based tests for prostate cancer prognosis, including tests based on general cancer features such as cell-cycle proliferation, and those based on specific molecular features of cancer.
An example of the general type of test is the Ki-67 immunohistochemistry (IHC) test, which looks for a cellular marker of proliferation, and has been shown to have independent prognostic significance after radiation therapy or radical prostatectomy. This test is not currently recommended by the Medicare Molecular Diagnostic Services (MolDx) program, however.
Another general-type test is the Polaris quantitative reverse-transcriptase polymerase chain reaction (RT-PCR) panel, which tests for 31 cell-cycle-related genes and 15 “housekeeping” controls. This test is recommended for post-biopsy evaluation of men with very-low-risk or low-risk prostate cancer who at the time of diagnosis have at least 10 years of life expectancy. It has been shown to independently predict prostate cancer–specific mortality, biochemical failure/recurrence, and metastasis, the guidelines say.
Tests based on molecular features include:
• PTEN/ERG, an IHC or fluorescent in situ hybridization test that has been shown to predict prostate cancer–specific mortality, upgrading to Gleason pattern 4 on radical prostatectomy, and biochemical recurrence (not MolDx recommended).
• Decipher, a whole-transcriptome 1.4M RNA expression oligonucleotide microarray shown to predict biochemical failure, metastasis, and prostate cancer–specific mortality (recommended for postradical prostatectomy for patients with pT2 tumors with positive margins, and pT3 disease, and rising PSA above nadir);
• Oncotype DX, an RT-PCR assay for 12 prostate cancer genes and five housekeeping controls (recommended for post-biopsy evaluation of men with very-low-risk or low-risk prostate cancer who at the time of diagnosis have at least 10 years of life expectancy).
• ProMark, multiplex immunofluorescent staining of eight proteins, which has been shown to independently predict non–organ-confined pT3 disease or Gleason pattern 4 disease on radical prostatectomy (not reviewed).
Dr. Ross said that in his practice, he generally does not order molecular testing for surveillance of men older than 65 who have very-low-risk disease. For men with low-risk disease, however, molecular testing may help in clinical decision making to predict upgrading or disease progression.
“There’s limited data from surveillance populations, but these tests can be used in this context with retrospective data available, realizing that in most cases the tests will be confirmative, or another way of thinking about it is ‘noninformative,’ so there are some considerations about cost in that context,” he said. For men with intermediate or high-risk disease, however, currently available tests are not good at predicting what an individual patient’s response would be to a specific type of therapy, whether surgery, radiation, androgen deprivation, chemotherapy, or a combination.
“This is an area where predictive biomarkers would be very informative. There is ongoing research, and I think this is an area of potentially large advancement in how we risk-stratify our patients,” Dr. Ross said.
HOLLYWOOD, FLA. – Current evidence suggests that molecular tests for prostate cancer are prognostic and can help clinicians and patients with difficult treatment decisions. In the not-too-distant future, gene tests could also guide choice of therapies.
“I think that the largest impact is going to come in areas of both the greatest treatment uncertainty and areas where we can be predictive about the response to treatment,” said Dr. Ashley Ross, a urologic oncologist and pathologist at the Sidney Kimmel Comprehensive Cancer Center at Johns Hopkins University, Baltimore.
Multigene panels may soon be able help identify which patients might benefit more from radical prostatectomy or radiation therapy, whether radiation therapy effects could be enhanced with the addition of androgen-deprivation therapy, and whether early use of docetaxel might add therapeutic benefit, he said at the annual conference of the National Comprehensive Cancer Network.
The 2016 iteration of the NCCN guidelines for the treatment of prostate cancer include a note stating that “men with clinically localized disease may consider the use of tumor-based molecular assays. Retrospective case cohort studies have shown that molecular assays performed on biopsy or prostatectomy specimens provide prognostic information independent of NCCN risk groups.”
The use of molecular assays may inform treatment decisions by helping to predict the likelihood of death if a patient is managed conservatively, risks for biochemical progression after radical prostatectomy or external beam therapy, and the likelihood that a patient could develop metastatic disease after radical prostatectomy or salvage radiotherapy, the guidelines say.
Dr. Ross reviewed the molecular biology of localized prostate cancer and the benefits and risks of currently available molecular tests.
“We’ve had an increased ability to get molecular information or genomic information from very limited amounts of routinely-stored pathologic tissue, and that’s resulted in the generation of many molecular-based tissue tests in prostate cancer. With the emergence of those tests and a lot of aggressive marketing, there has been a lot of confusion for patients and providers about whether we should use them or not and in what context,” he said.
Prostate cancer is genomically complex, even in the localized stage, with copy number alterations, deletions, and amplifications; chromosomal rearrangements; and point mutations, he said.
One of the best characterized genomic events is the early loss of the tumor suppressor gene PTEN (phosphatase and tensin homolog). This gene works within the PI3 kinase (PI3K)/AKT pathway. PI3K pathway mutations have been identified in up to 40% of all primary prostate cancers and 100% of mutations, Dr. Ross explained.
Loss of PTEN itself has been detected in about 15%-40% of primary prostate cancers and 50% of metastases, and the loss correlates with disease stage and tumor grade.
The NCCN guidelines list six available tissue-based tests for prostate cancer prognosis, including tests based on general cancer features such as cell-cycle proliferation, and those based on specific molecular features of cancer.
An example of the general type of test is the Ki-67 immunohistochemistry (IHC) test, which looks for a cellular marker of proliferation, and has been shown to have independent prognostic significance after radiation therapy or radical prostatectomy. This test is not currently recommended by the Medicare Molecular Diagnostic Services (MolDx) program, however.
Another general-type test is the Polaris quantitative reverse-transcriptase polymerase chain reaction (RT-PCR) panel, which tests for 31 cell-cycle-related genes and 15 “housekeeping” controls. This test is recommended for post-biopsy evaluation of men with very-low-risk or low-risk prostate cancer who at the time of diagnosis have at least 10 years of life expectancy. It has been shown to independently predict prostate cancer–specific mortality, biochemical failure/recurrence, and metastasis, the guidelines say.
Tests based on molecular features include:
• PTEN/ERG, an IHC or fluorescent in situ hybridization test that has been shown to predict prostate cancer–specific mortality, upgrading to Gleason pattern 4 on radical prostatectomy, and biochemical recurrence (not MolDx recommended).
• Decipher, a whole-transcriptome 1.4M RNA expression oligonucleotide microarray shown to predict biochemical failure, metastasis, and prostate cancer–specific mortality (recommended for postradical prostatectomy for patients with pT2 tumors with positive margins, and pT3 disease, and rising PSA above nadir);
• Oncotype DX, an RT-PCR assay for 12 prostate cancer genes and five housekeeping controls (recommended for post-biopsy evaluation of men with very-low-risk or low-risk prostate cancer who at the time of diagnosis have at least 10 years of life expectancy).
• ProMark, multiplex immunofluorescent staining of eight proteins, which has been shown to independently predict non–organ-confined pT3 disease or Gleason pattern 4 disease on radical prostatectomy (not reviewed).
Dr. Ross said that in his practice, he generally does not order molecular testing for surveillance of men older than 65 who have very-low-risk disease. For men with low-risk disease, however, molecular testing may help in clinical decision making to predict upgrading or disease progression.
“There’s limited data from surveillance populations, but these tests can be used in this context with retrospective data available, realizing that in most cases the tests will be confirmative, or another way of thinking about it is ‘noninformative,’ so there are some considerations about cost in that context,” he said. For men with intermediate or high-risk disease, however, currently available tests are not good at predicting what an individual patient’s response would be to a specific type of therapy, whether surgery, radiation, androgen deprivation, chemotherapy, or a combination.
“This is an area where predictive biomarkers would be very informative. There is ongoing research, and I think this is an area of potentially large advancement in how we risk-stratify our patients,” Dr. Ross said.
EXPERT ANALYSIS FROM THE NCCN ANNUAL CONFERENCE
Prostate cancer’s future seen in molecular tests
HOLLYWOOD, FLA. – Current evidence suggests that molecular tests for prostate cancer are prognostic and can help clinicians and patients with difficult treatment decisions. In the not-too-distant future, gene tests could also guide choice of therapies.
“I think that the largest impact is going to come in areas of both the greatest treatment uncertainty and areas where we can be predictive about the response to treatment,” said Dr. Ashley Ross, a urologic oncologist and pathologist at the Sidney Kimmel Comprehensive Cancer Center at Johns Hopkins University, Baltimore.
Multigene panels may soon be able help identify which patients might benefit more from radical prostatectomy or radiation therapy, whether radiation therapy effects could be enhanced with the addition of androgen-deprivation therapy, and whether early use of docetaxel might add therapeutic benefit, he said at the annual conference of the National Comprehensive Cancer Network.
The 2016 iteration of the NCCN guidelines for the treatment of prostate cancer include a note stating that “men with clinically localized disease may consider the use of tumor-based molecular assays. Retrospective case cohort studies have shown that molecular assays performed on biopsy or prostatectomy specimens provide prognostic information independent of NCCN risk groups.”
The use of molecular assays may inform treatment decisions by helping to predict the likelihood of death if a patient is managed conservatively, risks for biochemical progression after radical prostatectomy or external beam therapy, and the likelihood that a patient could develop metastatic disease after radical prostatectomy or salvage radiotherapy, the guidelines say.
Dr. Ross reviewed the molecular biology of localized prostate cancer and the benefits and risks of currently available molecular tests.
“We’ve had an increased ability to get molecular information or genomic information from very limited amounts of routinely-stored pathologic tissue, and that’s resulted in the generation of many molecular-based tissue tests in prostate cancer. With the emergence of those tests and a lot of aggressive marketing, there has been a lot of confusion for patients and providers about whether we should use them or not and in what context,” he said.
Prostate cancer is genomically complex, even in the localized stage, with copy number alterations, deletions, and amplifications; chromosomal rearrangements; and point mutations, he said.
One of the best characterized genomic events is the early loss of the tumor suppressor gene PTEN (phosphatase and tensin homolog). This gene works within the PI3 kinase (PI3K)/AKT pathway. PI3K pathway mutations have been identified in up to 40% of all primary prostate cancers and 100% of mutations, Dr. Ross explained.
Loss of PTEN itself has been detected in about 15%-40% of primary prostate cancers and 50% of metastases, and the loss correlates with disease stage and tumor grade.
The NCCN guidelines list six available tissue-based tests for prostate cancer prognosis, including tests based on general cancer features such as cell-cycle proliferation, and those based on specific molecular features of cancer.
An example of the general type of test is the Ki-67 immunohistochemistry (IHC) test, which looks for a cellular marker of proliferation, and has been shown to have independent prognostic significance after radiation therapy or radical prostatectomy. This test is not currently recommended by the Medicare Molecular Diagnostic Services (MolDx) program, however.
Another general-type test is the Polaris quantitative reverse-transcriptase polymerase chain reaction (RT-PCR) panel, which tests for 31 cell-cycle-related genes and 15 “housekeeping” controls. This test is recommended for post-biopsy evaluation of men with very-low-risk or low-risk prostate cancer who at the time of diagnosis have at least 10 years of life expectancy. It has been shown to independently predict prostate cancer–specific mortality, biochemical failure/recurrence, and metastasis, the guidelines say.
Tests based on molecular features include:
• PTEN/ERG, an IHC or fluorescent in situ hybridization test that has been shown to predict prostate cancer–specific mortality, upgrading to Gleason pattern 4 on radical prostatectomy, and biochemical recurrence (not MolDx recommended).
• Decipher, a whole-transcriptome 1.4M RNA expression oligonucleotide microarray shown to predict biochemical failure, metastasis, and prostate cancer–specific mortality (recommended for postradical prostatectomy for patients with pT2 tumors with positive margins, and pT3 disease, and rising PSA above nadir);
• Oncotype DX, an RT-PCR assay for 12 prostate cancer genes and five housekeeping controls (recommended for post-biopsy evaluation of men with very-low-risk or low-risk prostate cancer who at the time of diagnosis have at least 10 years of life expectancy).
• ProMark, multiplex immunofluorescent staining of eight proteins, which has been shown to independently predict non–organ-confined pT3 disease or Gleason pattern 4 disease on radical prostatectomy (not reviewed).
Dr. Ross said that in his practice, he generally does not order molecular testing for surveillance of men older than 65 who have very-low-risk disease. For men with low-risk disease, however, molecular testing may help in clinical decision making to predict upgrading or disease progression.
“There’s limited data from surveillance populations, but these tests can be used in this context with retrospective data available, realizing that in most cases the tests will be confirmative, or another way of thinking about it is ‘noninformative,’ so there are some considerations about cost in that context,” he said. For men with intermediate or high-risk disease, however, currently available tests are not good at predicting what an individual patient’s response would be to a specific type of therapy, whether surgery, radiation, androgen deprivation, chemotherapy, or a combination.
“This is an area where predictive biomarkers would be very informative. There is ongoing research, and I think this is an area of potentially large advancement in how we risk-stratify our patients,” Dr. Ross said.
HOLLYWOOD, FLA. – Current evidence suggests that molecular tests for prostate cancer are prognostic and can help clinicians and patients with difficult treatment decisions. In the not-too-distant future, gene tests could also guide choice of therapies.
“I think that the largest impact is going to come in areas of both the greatest treatment uncertainty and areas where we can be predictive about the response to treatment,” said Dr. Ashley Ross, a urologic oncologist and pathologist at the Sidney Kimmel Comprehensive Cancer Center at Johns Hopkins University, Baltimore.
Multigene panels may soon be able help identify which patients might benefit more from radical prostatectomy or radiation therapy, whether radiation therapy effects could be enhanced with the addition of androgen-deprivation therapy, and whether early use of docetaxel might add therapeutic benefit, he said at the annual conference of the National Comprehensive Cancer Network.
The 2016 iteration of the NCCN guidelines for the treatment of prostate cancer include a note stating that “men with clinically localized disease may consider the use of tumor-based molecular assays. Retrospective case cohort studies have shown that molecular assays performed on biopsy or prostatectomy specimens provide prognostic information independent of NCCN risk groups.”
The use of molecular assays may inform treatment decisions by helping to predict the likelihood of death if a patient is managed conservatively, risks for biochemical progression after radical prostatectomy or external beam therapy, and the likelihood that a patient could develop metastatic disease after radical prostatectomy or salvage radiotherapy, the guidelines say.
Dr. Ross reviewed the molecular biology of localized prostate cancer and the benefits and risks of currently available molecular tests.
“We’ve had an increased ability to get molecular information or genomic information from very limited amounts of routinely-stored pathologic tissue, and that’s resulted in the generation of many molecular-based tissue tests in prostate cancer. With the emergence of those tests and a lot of aggressive marketing, there has been a lot of confusion for patients and providers about whether we should use them or not and in what context,” he said.
Prostate cancer is genomically complex, even in the localized stage, with copy number alterations, deletions, and amplifications; chromosomal rearrangements; and point mutations, he said.
One of the best characterized genomic events is the early loss of the tumor suppressor gene PTEN (phosphatase and tensin homolog). This gene works within the PI3 kinase (PI3K)/AKT pathway. PI3K pathway mutations have been identified in up to 40% of all primary prostate cancers and 100% of mutations, Dr. Ross explained.
Loss of PTEN itself has been detected in about 15%-40% of primary prostate cancers and 50% of metastases, and the loss correlates with disease stage and tumor grade.
The NCCN guidelines list six available tissue-based tests for prostate cancer prognosis, including tests based on general cancer features such as cell-cycle proliferation, and those based on specific molecular features of cancer.
An example of the general type of test is the Ki-67 immunohistochemistry (IHC) test, which looks for a cellular marker of proliferation, and has been shown to have independent prognostic significance after radiation therapy or radical prostatectomy. This test is not currently recommended by the Medicare Molecular Diagnostic Services (MolDx) program, however.
Another general-type test is the Polaris quantitative reverse-transcriptase polymerase chain reaction (RT-PCR) panel, which tests for 31 cell-cycle-related genes and 15 “housekeeping” controls. This test is recommended for post-biopsy evaluation of men with very-low-risk or low-risk prostate cancer who at the time of diagnosis have at least 10 years of life expectancy. It has been shown to independently predict prostate cancer–specific mortality, biochemical failure/recurrence, and metastasis, the guidelines say.
Tests based on molecular features include:
• PTEN/ERG, an IHC or fluorescent in situ hybridization test that has been shown to predict prostate cancer–specific mortality, upgrading to Gleason pattern 4 on radical prostatectomy, and biochemical recurrence (not MolDx recommended).
• Decipher, a whole-transcriptome 1.4M RNA expression oligonucleotide microarray shown to predict biochemical failure, metastasis, and prostate cancer–specific mortality (recommended for postradical prostatectomy for patients with pT2 tumors with positive margins, and pT3 disease, and rising PSA above nadir);
• Oncotype DX, an RT-PCR assay for 12 prostate cancer genes and five housekeeping controls (recommended for post-biopsy evaluation of men with very-low-risk or low-risk prostate cancer who at the time of diagnosis have at least 10 years of life expectancy).
• ProMark, multiplex immunofluorescent staining of eight proteins, which has been shown to independently predict non–organ-confined pT3 disease or Gleason pattern 4 disease on radical prostatectomy (not reviewed).
Dr. Ross said that in his practice, he generally does not order molecular testing for surveillance of men older than 65 who have very-low-risk disease. For men with low-risk disease, however, molecular testing may help in clinical decision making to predict upgrading or disease progression.
“There’s limited data from surveillance populations, but these tests can be used in this context with retrospective data available, realizing that in most cases the tests will be confirmative, or another way of thinking about it is ‘noninformative,’ so there are some considerations about cost in that context,” he said. For men with intermediate or high-risk disease, however, currently available tests are not good at predicting what an individual patient’s response would be to a specific type of therapy, whether surgery, radiation, androgen deprivation, chemotherapy, or a combination.
“This is an area where predictive biomarkers would be very informative. There is ongoing research, and I think this is an area of potentially large advancement in how we risk-stratify our patients,” Dr. Ross said.
HOLLYWOOD, FLA. – Current evidence suggests that molecular tests for prostate cancer are prognostic and can help clinicians and patients with difficult treatment decisions. In the not-too-distant future, gene tests could also guide choice of therapies.
“I think that the largest impact is going to come in areas of both the greatest treatment uncertainty and areas where we can be predictive about the response to treatment,” said Dr. Ashley Ross, a urologic oncologist and pathologist at the Sidney Kimmel Comprehensive Cancer Center at Johns Hopkins University, Baltimore.
Multigene panels may soon be able help identify which patients might benefit more from radical prostatectomy or radiation therapy, whether radiation therapy effects could be enhanced with the addition of androgen-deprivation therapy, and whether early use of docetaxel might add therapeutic benefit, he said at the annual conference of the National Comprehensive Cancer Network.
The 2016 iteration of the NCCN guidelines for the treatment of prostate cancer include a note stating that “men with clinically localized disease may consider the use of tumor-based molecular assays. Retrospective case cohort studies have shown that molecular assays performed on biopsy or prostatectomy specimens provide prognostic information independent of NCCN risk groups.”
The use of molecular assays may inform treatment decisions by helping to predict the likelihood of death if a patient is managed conservatively, risks for biochemical progression after radical prostatectomy or external beam therapy, and the likelihood that a patient could develop metastatic disease after radical prostatectomy or salvage radiotherapy, the guidelines say.
Dr. Ross reviewed the molecular biology of localized prostate cancer and the benefits and risks of currently available molecular tests.
“We’ve had an increased ability to get molecular information or genomic information from very limited amounts of routinely-stored pathologic tissue, and that’s resulted in the generation of many molecular-based tissue tests in prostate cancer. With the emergence of those tests and a lot of aggressive marketing, there has been a lot of confusion for patients and providers about whether we should use them or not and in what context,” he said.
Prostate cancer is genomically complex, even in the localized stage, with copy number alterations, deletions, and amplifications; chromosomal rearrangements; and point mutations, he said.
One of the best characterized genomic events is the early loss of the tumor suppressor gene PTEN (phosphatase and tensin homolog). This gene works within the PI3 kinase (PI3K)/AKT pathway. PI3K pathway mutations have been identified in up to 40% of all primary prostate cancers and 100% of mutations, Dr. Ross explained.
Loss of PTEN itself has been detected in about 15%-40% of primary prostate cancers and 50% of metastases, and the loss correlates with disease stage and tumor grade.
The NCCN guidelines list six available tissue-based tests for prostate cancer prognosis, including tests based on general cancer features such as cell-cycle proliferation, and those based on specific molecular features of cancer.
An example of the general type of test is the Ki-67 immunohistochemistry (IHC) test, which looks for a cellular marker of proliferation, and has been shown to have independent prognostic significance after radiation therapy or radical prostatectomy. This test is not currently recommended by the Medicare Molecular Diagnostic Services (MolDx) program, however.
Another general-type test is the Polaris quantitative reverse-transcriptase polymerase chain reaction (RT-PCR) panel, which tests for 31 cell-cycle-related genes and 15 “housekeeping” controls. This test is recommended for post-biopsy evaluation of men with very-low-risk or low-risk prostate cancer who at the time of diagnosis have at least 10 years of life expectancy. It has been shown to independently predict prostate cancer–specific mortality, biochemical failure/recurrence, and metastasis, the guidelines say.
Tests based on molecular features include:
• PTEN/ERG, an IHC or fluorescent in situ hybridization test that has been shown to predict prostate cancer–specific mortality, upgrading to Gleason pattern 4 on radical prostatectomy, and biochemical recurrence (not MolDx recommended).
• Decipher, a whole-transcriptome 1.4M RNA expression oligonucleotide microarray shown to predict biochemical failure, metastasis, and prostate cancer–specific mortality (recommended for postradical prostatectomy for patients with pT2 tumors with positive margins, and pT3 disease, and rising PSA above nadir);
• Oncotype DX, an RT-PCR assay for 12 prostate cancer genes and five housekeeping controls (recommended for post-biopsy evaluation of men with very-low-risk or low-risk prostate cancer who at the time of diagnosis have at least 10 years of life expectancy).
• ProMark, multiplex immunofluorescent staining of eight proteins, which has been shown to independently predict non–organ-confined pT3 disease or Gleason pattern 4 disease on radical prostatectomy (not reviewed).
Dr. Ross said that in his practice, he generally does not order molecular testing for surveillance of men older than 65 who have very-low-risk disease. For men with low-risk disease, however, molecular testing may help in clinical decision making to predict upgrading or disease progression.
“There’s limited data from surveillance populations, but these tests can be used in this context with retrospective data available, realizing that in most cases the tests will be confirmative, or another way of thinking about it is ‘noninformative,’ so there are some considerations about cost in that context,” he said. For men with intermediate or high-risk disease, however, currently available tests are not good at predicting what an individual patient’s response would be to a specific type of therapy, whether surgery, radiation, androgen deprivation, chemotherapy, or a combination.
“This is an area where predictive biomarkers would be very informative. There is ongoing research, and I think this is an area of potentially large advancement in how we risk-stratify our patients,” Dr. Ross said.
EXPERT ANALYSIS FROM THE NCCN ANNUAL CONFERENCE
In newly diagnosed CLL, mutation tests are advised
Patients with newly diagnosed chronic lymphocytic leukemia should standardly undergo immunoglobulin heavy-chain variable region gene (IGHV) mutation status and interphase fluorescence in situ hybridization (FISH) tests, based on the results of a meta-analysis published in Blood.
“This change will help define the minimal standard initial prognostic evaluation for patients with CLL and help facilitate use of the powerful, recently developed, integrated prognostic indices, all of which are dependent on these 2 variables,” wrote Dr. Sameer A. Parikh of Mayo Clinic, Rochester, Minn., and associates.
IGHV and FISH have prognostic value independent of clinical stage in patients with newly diagnosed and previously untreated CLL, they said (Blood. 2016;127[14]:1752-60). Better understanding of the patient’s risk of disease progression at diagnosis can guide counseling and follow-up intervals, and could potentially influence the decision to treat high-risk patients on early intervention protocols.
IGHV and FISH also appear to provide additional information on progression-free and overall survival.
The researchers cautioned, however, that the results of these tests should not be used to initiate CLL-specific therapy. Only patients who meet indications for therapy based on the 2008 International Workshop on Chronic Lymphocytic Leukemia guidelines should receive treatment.
Further, they noted, the median age of patients included in studies that they analyzed was 64 years; the median age of patients with CLL is 72 years. The prognostic abilities of IGHV mutation and FISH may differ in these older individuals with CLL.
The researchers analyzed 31 studies that met the criteria for inclusion – full-length publications that included at least 200 patients and reported on the prognostic value of IGHV and/or FISH for predicting progression-free or overall survival in patients with newly diagnosed CLL.
They found that the median progression-free survival (range, about 1-5 years) was significantly shorter for patients with unmutated IGHV genes, than was the median progression-free survival (range, about 9-19 years) for those with mutated IGHV genes. Similarly, the median overall survival was significantly shorter for patients with unmutated IGHV (range, about 3-10 years) than for those with mutated IGHV (range, about 18-26 years).
For patients with high-risk FISH (including del17p13 and del11q23), the median progression-free survival was significantly shorter (range, about 0.1-5 years) than for those with low/intermediate-risk FISH (including del13q, normal, and trisomy 12; range, about 1.5-22 years). Median overall survival also significantly differed, ranging from about 3-10 years for patients with high-risk FISH and from about 7.5-20.5 years for those with low/intermediate-risk FISH.
In multivariable analyses, the hazard ratio for high-risk FISH ranged from 1.3 to 4.7 for progression-free survival and from 0.9 to 8.2 for overall survival. In studies reporting the results of multivariable analysis, high-risk FISH remained an independent predictor of progression-free survival in 8 of 17 studies and of overall survival in 10 of 14 studies, including in 10 of 13 studies adjusting for the prognostic impact of IGHV.
In multivariable analyses, IGHV remained an independent predictor of progression-free survival in 15 of 18 studies, including 12 of 15 studies adjusting for the prognostic impact of FISH. IGHV remained an independent predictor of overall survival in 11 of 15 studies reporting the results of multivariable analysis, including 10 of 14 studies adjusting for the prognostic impact of FISH.
Patients with newly diagnosed chronic lymphocytic leukemia should standardly undergo immunoglobulin heavy-chain variable region gene (IGHV) mutation status and interphase fluorescence in situ hybridization (FISH) tests, based on the results of a meta-analysis published in Blood.
“This change will help define the minimal standard initial prognostic evaluation for patients with CLL and help facilitate use of the powerful, recently developed, integrated prognostic indices, all of which are dependent on these 2 variables,” wrote Dr. Sameer A. Parikh of Mayo Clinic, Rochester, Minn., and associates.
IGHV and FISH have prognostic value independent of clinical stage in patients with newly diagnosed and previously untreated CLL, they said (Blood. 2016;127[14]:1752-60). Better understanding of the patient’s risk of disease progression at diagnosis can guide counseling and follow-up intervals, and could potentially influence the decision to treat high-risk patients on early intervention protocols.
IGHV and FISH also appear to provide additional information on progression-free and overall survival.
The researchers cautioned, however, that the results of these tests should not be used to initiate CLL-specific therapy. Only patients who meet indications for therapy based on the 2008 International Workshop on Chronic Lymphocytic Leukemia guidelines should receive treatment.
Further, they noted, the median age of patients included in studies that they analyzed was 64 years; the median age of patients with CLL is 72 years. The prognostic abilities of IGHV mutation and FISH may differ in these older individuals with CLL.
The researchers analyzed 31 studies that met the criteria for inclusion – full-length publications that included at least 200 patients and reported on the prognostic value of IGHV and/or FISH for predicting progression-free or overall survival in patients with newly diagnosed CLL.
They found that the median progression-free survival (range, about 1-5 years) was significantly shorter for patients with unmutated IGHV genes, than was the median progression-free survival (range, about 9-19 years) for those with mutated IGHV genes. Similarly, the median overall survival was significantly shorter for patients with unmutated IGHV (range, about 3-10 years) than for those with mutated IGHV (range, about 18-26 years).
For patients with high-risk FISH (including del17p13 and del11q23), the median progression-free survival was significantly shorter (range, about 0.1-5 years) than for those with low/intermediate-risk FISH (including del13q, normal, and trisomy 12; range, about 1.5-22 years). Median overall survival also significantly differed, ranging from about 3-10 years for patients with high-risk FISH and from about 7.5-20.5 years for those with low/intermediate-risk FISH.
In multivariable analyses, the hazard ratio for high-risk FISH ranged from 1.3 to 4.7 for progression-free survival and from 0.9 to 8.2 for overall survival. In studies reporting the results of multivariable analysis, high-risk FISH remained an independent predictor of progression-free survival in 8 of 17 studies and of overall survival in 10 of 14 studies, including in 10 of 13 studies adjusting for the prognostic impact of IGHV.
In multivariable analyses, IGHV remained an independent predictor of progression-free survival in 15 of 18 studies, including 12 of 15 studies adjusting for the prognostic impact of FISH. IGHV remained an independent predictor of overall survival in 11 of 15 studies reporting the results of multivariable analysis, including 10 of 14 studies adjusting for the prognostic impact of FISH.
Patients with newly diagnosed chronic lymphocytic leukemia should standardly undergo immunoglobulin heavy-chain variable region gene (IGHV) mutation status and interphase fluorescence in situ hybridization (FISH) tests, based on the results of a meta-analysis published in Blood.
“This change will help define the minimal standard initial prognostic evaluation for patients with CLL and help facilitate use of the powerful, recently developed, integrated prognostic indices, all of which are dependent on these 2 variables,” wrote Dr. Sameer A. Parikh of Mayo Clinic, Rochester, Minn., and associates.
IGHV and FISH have prognostic value independent of clinical stage in patients with newly diagnosed and previously untreated CLL, they said (Blood. 2016;127[14]:1752-60). Better understanding of the patient’s risk of disease progression at diagnosis can guide counseling and follow-up intervals, and could potentially influence the decision to treat high-risk patients on early intervention protocols.
IGHV and FISH also appear to provide additional information on progression-free and overall survival.
The researchers cautioned, however, that the results of these tests should not be used to initiate CLL-specific therapy. Only patients who meet indications for therapy based on the 2008 International Workshop on Chronic Lymphocytic Leukemia guidelines should receive treatment.
Further, they noted, the median age of patients included in studies that they analyzed was 64 years; the median age of patients with CLL is 72 years. The prognostic abilities of IGHV mutation and FISH may differ in these older individuals with CLL.
The researchers analyzed 31 studies that met the criteria for inclusion – full-length publications that included at least 200 patients and reported on the prognostic value of IGHV and/or FISH for predicting progression-free or overall survival in patients with newly diagnosed CLL.
They found that the median progression-free survival (range, about 1-5 years) was significantly shorter for patients with unmutated IGHV genes, than was the median progression-free survival (range, about 9-19 years) for those with mutated IGHV genes. Similarly, the median overall survival was significantly shorter for patients with unmutated IGHV (range, about 3-10 years) than for those with mutated IGHV (range, about 18-26 years).
For patients with high-risk FISH (including del17p13 and del11q23), the median progression-free survival was significantly shorter (range, about 0.1-5 years) than for those with low/intermediate-risk FISH (including del13q, normal, and trisomy 12; range, about 1.5-22 years). Median overall survival also significantly differed, ranging from about 3-10 years for patients with high-risk FISH and from about 7.5-20.5 years for those with low/intermediate-risk FISH.
In multivariable analyses, the hazard ratio for high-risk FISH ranged from 1.3 to 4.7 for progression-free survival and from 0.9 to 8.2 for overall survival. In studies reporting the results of multivariable analysis, high-risk FISH remained an independent predictor of progression-free survival in 8 of 17 studies and of overall survival in 10 of 14 studies, including in 10 of 13 studies adjusting for the prognostic impact of IGHV.
In multivariable analyses, IGHV remained an independent predictor of progression-free survival in 15 of 18 studies, including 12 of 15 studies adjusting for the prognostic impact of FISH. IGHV remained an independent predictor of overall survival in 11 of 15 studies reporting the results of multivariable analysis, including 10 of 14 studies adjusting for the prognostic impact of FISH.
FROM BLOOD
Rosacea linked to several autoimmune diseases in women
Rosacea in women is linked with an increased risk for a wide variety of autoimmune disorders including type 1 diabetes, celiac disease, multiple sclerosis, and rheumatoid arthritis, according to a large population-based case-control study.
The finding expands the association of rosacea and autoimmune diseases beyond the previously reported associations with type 1 diabetes and celiac disease, reported Dr. Alexander Egeberg of Herlev and Gentofte Hospital, University of Copenhagen, and colleagues (J Am Acad Dermatol. 2016;74:667-72.)
“Remember that the absolute risk is still low; having rosacea does not guarantee that patients will develop other autoimmune diseases, and vice versa,” Dr. Egeberg said in an interview. “The links may provide insight into the pathogenesis of these diseases, and it will be interesting to see if systemic treatment of rosacea also affects the autoimmune diseases and the other way around.”
Using Danish registered health records, the researchers found 6,759 patients with rosacea, and matched them by age and sex to 33,795 control subjects. About two-thirds of the patients were women.
Compared with their matched controls, people with rosacea were at least twice as likely to have type 1 diabetes, celiac disease, and rheumatoid arthritis (odds ratio 2.6 for type 1 diabetes, 2.0 for celiac disease, and 2.1 for rheumatoid arthritis) and 1.65 times as likely to have multiple sclerosis. The differences, with the exception of rheumatoid arthritis, were statistically significant only in women.
The researchers said the broader association with autoimmune comorbidities is “intriguing” and suggests the genetic component of rosacea could be stronger than previously assumed with autoimmune inflammatory pathways contributing to the disease course. Because distinct rosacea subtypes (erythematotelangiectatic, papulopustular, phymatous, and ocular) could be associated with specific comorbidities, future association studies should attempt to stratify for clinical subtypes of rosacea, they noted.
Rosacea in women is linked with an increased risk for a wide variety of autoimmune disorders including type 1 diabetes, celiac disease, multiple sclerosis, and rheumatoid arthritis, according to a large population-based case-control study.
The finding expands the association of rosacea and autoimmune diseases beyond the previously reported associations with type 1 diabetes and celiac disease, reported Dr. Alexander Egeberg of Herlev and Gentofte Hospital, University of Copenhagen, and colleagues (J Am Acad Dermatol. 2016;74:667-72.)
“Remember that the absolute risk is still low; having rosacea does not guarantee that patients will develop other autoimmune diseases, and vice versa,” Dr. Egeberg said in an interview. “The links may provide insight into the pathogenesis of these diseases, and it will be interesting to see if systemic treatment of rosacea also affects the autoimmune diseases and the other way around.”
Using Danish registered health records, the researchers found 6,759 patients with rosacea, and matched them by age and sex to 33,795 control subjects. About two-thirds of the patients were women.
Compared with their matched controls, people with rosacea were at least twice as likely to have type 1 diabetes, celiac disease, and rheumatoid arthritis (odds ratio 2.6 for type 1 diabetes, 2.0 for celiac disease, and 2.1 for rheumatoid arthritis) and 1.65 times as likely to have multiple sclerosis. The differences, with the exception of rheumatoid arthritis, were statistically significant only in women.
The researchers said the broader association with autoimmune comorbidities is “intriguing” and suggests the genetic component of rosacea could be stronger than previously assumed with autoimmune inflammatory pathways contributing to the disease course. Because distinct rosacea subtypes (erythematotelangiectatic, papulopustular, phymatous, and ocular) could be associated with specific comorbidities, future association studies should attempt to stratify for clinical subtypes of rosacea, they noted.
Rosacea in women is linked with an increased risk for a wide variety of autoimmune disorders including type 1 diabetes, celiac disease, multiple sclerosis, and rheumatoid arthritis, according to a large population-based case-control study.
The finding expands the association of rosacea and autoimmune diseases beyond the previously reported associations with type 1 diabetes and celiac disease, reported Dr. Alexander Egeberg of Herlev and Gentofte Hospital, University of Copenhagen, and colleagues (J Am Acad Dermatol. 2016;74:667-72.)
“Remember that the absolute risk is still low; having rosacea does not guarantee that patients will develop other autoimmune diseases, and vice versa,” Dr. Egeberg said in an interview. “The links may provide insight into the pathogenesis of these diseases, and it will be interesting to see if systemic treatment of rosacea also affects the autoimmune diseases and the other way around.”
Using Danish registered health records, the researchers found 6,759 patients with rosacea, and matched them by age and sex to 33,795 control subjects. About two-thirds of the patients were women.
Compared with their matched controls, people with rosacea were at least twice as likely to have type 1 diabetes, celiac disease, and rheumatoid arthritis (odds ratio 2.6 for type 1 diabetes, 2.0 for celiac disease, and 2.1 for rheumatoid arthritis) and 1.65 times as likely to have multiple sclerosis. The differences, with the exception of rheumatoid arthritis, were statistically significant only in women.
The researchers said the broader association with autoimmune comorbidities is “intriguing” and suggests the genetic component of rosacea could be stronger than previously assumed with autoimmune inflammatory pathways contributing to the disease course. Because distinct rosacea subtypes (erythematotelangiectatic, papulopustular, phymatous, and ocular) could be associated with specific comorbidities, future association studies should attempt to stratify for clinical subtypes of rosacea, they noted.
FROM JAAD
Key clinical point: Rosacea is linked with a broader spectrum of autoimmune diseases than previously recognized.
Major finding: Women with rosacea were more likely than were other women to have type 1 diabetes, celiac disease, multiple sclerosis, and rheumatoid arthritis.
Data source: A population-based case-control study of 6,759 patients with rosacea who were matched with 33,795 control subjects for age, sex, and calendar time.
Disclosures: Dr. Egeberg is employed by Pfizer. This research was performed independently through the authors’ academic university.
Marine ingredients and the skin
Just as we learned early in life that 70% of the human body is composed of water, water covers approximately the same percentage of the earth’s surface. While fishing and harvesting of algae have occurred throughout human history,1 it has only been since the 1970s that widespread scientific interest in the great biological and chemical diversity of the vast oceans of the world has led to investigations into medical and cosmetic applications of the rich life beneath the sea.2 During this period, the marine environment has been found to boast multiple organisms with unique metabolisms adapted for survival in challenging conditions, yielding secondary metabolites, some of which have become valuable in the pharmaceutical and cosmeceutical markets.3,4 Thus, the inclusion of bioactive substances from the sea in drugs and cosmetic products is primarily a recent phenomenon.1 In fact, marine ingredients in cosmetics are thought to confer various benefits to skin health, including antioxidant, anti-acne, anti-wrinkle, and anti-tyrosinase activity.
Chemistry and biologic activity
Several marine microbial natural products have been found to display antimicrobial, antitumor, and anti-inflammatory activity.2,5 And seaweed extracts (green, brown, and red algal compounds that include constituents such as phlorotannins, sulfated polysaccharides, and tyrosinase inhibitors) have been incorporated into cosmeceutical products, with a long history of traditional folk uses for various health – including skin – conditions.3,6,7 Kim and Li reviewed the beneficial health effects of marine fungi-derived terpenoids in 2012, reporting that hundreds of these compounds have been discovered in the last few decades, with many exhibiting anti-inflammatory, anticancer, antimicrobial, and antioxidant activity.8,9 Terpenoids, or isoprenoids, are a subclass of prenyllipids, which include prenylquinones, sterols, and terpenes, the largest class of natural substances.10
The terpenes are the largest group of biologically diverse marine compounds, and include the pseudopterosins, which are structurally discrete active metabolites of the Caribbean gorgonian soft coral Pseudopterogorgia elisabethae, which is native to the waters of the Caribbean Sea, Central Bahamas, Bermuda, the West Indies, and the Florida keys.11,12 The most common gorgonian corals are diterpenes.13 Twenty-six derivatives of the octocoral P. elisabethae (designated PsA-PsZ), also known as the sea whip, sea fan, or sea plume, have been isolated.11,12,14 Pseudopterosins were first isolated in 1986.14,15
Based on the identified biologic activities, particularly anti-inflammatory capacity, of pseudopterosins, researchers have investigated their potential for treatment of various conditions including asthma, cancer, contact dermatitis, dermatoheliosis, HIV, photodamage, psoriasis, and rheumatoid arthritis.1,11
After decades of extensive research of pseudopterosins, these tricyclic diterpene glycosides are thought to provide superior anti-inflammatory and analgesic properties, compared to standard anti-inflammatory treatments, without inducing adverse side effects; they also offer marked antimicrobial and wound-healing effects.3,11,14,16-19
Other marine diterpene glycosides include eleutherobins and fucosides, which also exhibit notable biologic activity.15 In particular, the anti-inflammatory and analgesic activities of pseudopterosins have been found to be concentration- and dose-dependently more potent than the standard-bearing indomethacin.11,14,17
Marine ingredients in topical formulations
The first product to include pseudopterosins was the skin formulation Resilience marketed by Estée Lauder over a decade ago.19,20 Natural marine ingredients have since been incorporated into a few more products, such as Imedeen, an oral skin care preparation that contains Marine Complex.21
In 2012, Rietveld et al. ascertained whether the Marine Complex from Imedeen could variously alter skin morphogenesis in female and male human skin equivalents. Cells were culled from female and male donors between the ages of 30 and 45 years for human skin equivalents that were cultured for 7 or 11 weeks with or without Marine Complex. The investigators found that the number of Ki67-positive epidermal cells was greatly augmented by Marine Complex in female human skin equivalents. The Marine Complex significantly spurred the level of secreted pro-collagen I and elevated the deposition of laminin 332 and collagen type VII in the dermis. Human skin equivalents treated with Marine Complex also exhibited more viable epidermal cell layers and a thicker dermal extracellular matrix, compared to controls, with these effects less salient in male human skin equivalents. The investigators concluded that supplementation with Marine Complex positively stimulated overall human skin equivalent tissue formation, with its effects on the basement membrane and dermal constituents suggestive of potential for use against human skin aging.21
Previously, Xhauflaire-Uhoda et al. evaluated the skin hydrating and firming dose-response effects of cosmetic preparations enriched in algae- and fish collagen–derived substances in randomized controlled double-blind medium-term (12 subjects aged 18-55 years) and short-term (3 subjects over the age of 50) trials. In the short term, serum formulations enriched in marine compounds manifested a superior moisturizing effect on the forearm compared with creams. In later stages, cream formulations were more active, especially after repeated applications. Investigators observed a sustained firming activity in association with both the lotion and cream during treatment, but such results did not persist after treatment was stopped.22
Product development
Technological advances, including sampling strategies, nanoscale nuclear magnetic resonance for structure determination, total chemical synthesis, fermentation, exploration of genomic and metagenomic resources, combinatorial biosynthesis, synthetic biology, and biotechnology represent important ways in which novel marine natural products are being developed, according to several authors.1,2,4
Conclusion
Marine ingredients are a relatively new and fascinating category of substances that can and are being harnessed for pharmaceutical, cosmeceutical, cosmetic, and nutritional uses. Beyond the challenges of obtaining sufficient raw materials and producing effective formulations, the continued viability of such resources may be threatened by human exploitation of the seas and climate change. That said, the oceans offer the greatest biodiversity on the planet and dermatologic preparations derived from such sources present intriguing possibilities, particularly the apparent anti-inflammatory activity of gorgonian and other terpenes. These compounds appear to have the potential to replace, or serve as desirable alternatives to, conventional therapies for inflammatory skin disorders.
References
1. Biotechnol Adv. 2011;29(5):468-82.
2. Mar Drugs. 2013;11(3):700-17.
3. Mar Drugs. 2014;12(2):1066-101.
4. Future Med Chem. 2011;3(12):1475-89.
5. Org Lett. 2000;2(4):507-10.
6. Mar Drugs. 2013;11(1):146-64.
7. J Cosmet Dermatol. 2014;13(1):56-67.
8. Adv Food Nutr Res. 2012;65:409-13.
9. Crit Rev Microbiol. 2011;37(3):245-9.
10. Nat Chem Biol. 2007;3(7):408-14.
11. J Drugs Dermatol. 2013;12(10):1177-9.
12. J Ind Microbiol Biotechnol. 2006;33(7):532-8.
13. Nat Prod Rep. 2009;26(5):681-710.
14. Proc Natl Acad Sci USA. 1986;83(17):6238-40.
15. Bioorg Med Chem. 2011;19(22):6702-19.
16. Arch Biochem Biophys. 2004;424(1):97-104.
17. Asia Pac J Clin Nutr. 2006;15(2):143-52.
18. J Nat Prod. 2004;67(10):1672-80.
19. Mar Drugs. 2004 May;2:73-82.
20. J Nat Prod. 2004;67(8):1216-38.
21. J Cosmet Dermatol. 2012;11(3):213-22.
22. Int J Cosmet Sci. 2008;30(2):131-8.
Dr. Baumann is chief executive officer of the Baumann Cosmetic & Research Institute in the Design District in Miami. She founded the Cosmetic Dermatology Center at the University of Miami in 1997. Dr. Baumann wrote the textbook, “Cosmetic Dermatology: Principles and Practice” (New York: McGraw-Hill, 2002), and a book for consumers, “The Skin Type Solution” (New York: Bantam Dell, 2006). She has contributed to the Cosmeceutical Critique column in Dermatology News since January 2001. Her latest book, “Cosmeceuticals and Cosmetic Ingredients,” was published in November 2014. Dr. Baumann has received funding for clinical grants from Allergan, Aveeno, Avon Products, Evolus, Galderma, GlaxoSmithKline, Kythera Biopharmaceuticals, Mary Kay, Medicis Pharmaceuticals, Neutrogena, Philosophy, Topix Pharmaceuticals, and Unilever.
Just as we learned early in life that 70% of the human body is composed of water, water covers approximately the same percentage of the earth’s surface. While fishing and harvesting of algae have occurred throughout human history,1 it has only been since the 1970s that widespread scientific interest in the great biological and chemical diversity of the vast oceans of the world has led to investigations into medical and cosmetic applications of the rich life beneath the sea.2 During this period, the marine environment has been found to boast multiple organisms with unique metabolisms adapted for survival in challenging conditions, yielding secondary metabolites, some of which have become valuable in the pharmaceutical and cosmeceutical markets.3,4 Thus, the inclusion of bioactive substances from the sea in drugs and cosmetic products is primarily a recent phenomenon.1 In fact, marine ingredients in cosmetics are thought to confer various benefits to skin health, including antioxidant, anti-acne, anti-wrinkle, and anti-tyrosinase activity.
Chemistry and biologic activity
Several marine microbial natural products have been found to display antimicrobial, antitumor, and anti-inflammatory activity.2,5 And seaweed extracts (green, brown, and red algal compounds that include constituents such as phlorotannins, sulfated polysaccharides, and tyrosinase inhibitors) have been incorporated into cosmeceutical products, with a long history of traditional folk uses for various health – including skin – conditions.3,6,7 Kim and Li reviewed the beneficial health effects of marine fungi-derived terpenoids in 2012, reporting that hundreds of these compounds have been discovered in the last few decades, with many exhibiting anti-inflammatory, anticancer, antimicrobial, and antioxidant activity.8,9 Terpenoids, or isoprenoids, are a subclass of prenyllipids, which include prenylquinones, sterols, and terpenes, the largest class of natural substances.10
The terpenes are the largest group of biologically diverse marine compounds, and include the pseudopterosins, which are structurally discrete active metabolites of the Caribbean gorgonian soft coral Pseudopterogorgia elisabethae, which is native to the waters of the Caribbean Sea, Central Bahamas, Bermuda, the West Indies, and the Florida keys.11,12 The most common gorgonian corals are diterpenes.13 Twenty-six derivatives of the octocoral P. elisabethae (designated PsA-PsZ), also known as the sea whip, sea fan, or sea plume, have been isolated.11,12,14 Pseudopterosins were first isolated in 1986.14,15
Based on the identified biologic activities, particularly anti-inflammatory capacity, of pseudopterosins, researchers have investigated their potential for treatment of various conditions including asthma, cancer, contact dermatitis, dermatoheliosis, HIV, photodamage, psoriasis, and rheumatoid arthritis.1,11
After decades of extensive research of pseudopterosins, these tricyclic diterpene glycosides are thought to provide superior anti-inflammatory and analgesic properties, compared to standard anti-inflammatory treatments, without inducing adverse side effects; they also offer marked antimicrobial and wound-healing effects.3,11,14,16-19
Other marine diterpene glycosides include eleutherobins and fucosides, which also exhibit notable biologic activity.15 In particular, the anti-inflammatory and analgesic activities of pseudopterosins have been found to be concentration- and dose-dependently more potent than the standard-bearing indomethacin.11,14,17
Marine ingredients in topical formulations
The first product to include pseudopterosins was the skin formulation Resilience marketed by Estée Lauder over a decade ago.19,20 Natural marine ingredients have since been incorporated into a few more products, such as Imedeen, an oral skin care preparation that contains Marine Complex.21
In 2012, Rietveld et al. ascertained whether the Marine Complex from Imedeen could variously alter skin morphogenesis in female and male human skin equivalents. Cells were culled from female and male donors between the ages of 30 and 45 years for human skin equivalents that were cultured for 7 or 11 weeks with or without Marine Complex. The investigators found that the number of Ki67-positive epidermal cells was greatly augmented by Marine Complex in female human skin equivalents. The Marine Complex significantly spurred the level of secreted pro-collagen I and elevated the deposition of laminin 332 and collagen type VII in the dermis. Human skin equivalents treated with Marine Complex also exhibited more viable epidermal cell layers and a thicker dermal extracellular matrix, compared to controls, with these effects less salient in male human skin equivalents. The investigators concluded that supplementation with Marine Complex positively stimulated overall human skin equivalent tissue formation, with its effects on the basement membrane and dermal constituents suggestive of potential for use against human skin aging.21
Previously, Xhauflaire-Uhoda et al. evaluated the skin hydrating and firming dose-response effects of cosmetic preparations enriched in algae- and fish collagen–derived substances in randomized controlled double-blind medium-term (12 subjects aged 18-55 years) and short-term (3 subjects over the age of 50) trials. In the short term, serum formulations enriched in marine compounds manifested a superior moisturizing effect on the forearm compared with creams. In later stages, cream formulations were more active, especially after repeated applications. Investigators observed a sustained firming activity in association with both the lotion and cream during treatment, but such results did not persist after treatment was stopped.22
Product development
Technological advances, including sampling strategies, nanoscale nuclear magnetic resonance for structure determination, total chemical synthesis, fermentation, exploration of genomic and metagenomic resources, combinatorial biosynthesis, synthetic biology, and biotechnology represent important ways in which novel marine natural products are being developed, according to several authors.1,2,4
Conclusion
Marine ingredients are a relatively new and fascinating category of substances that can and are being harnessed for pharmaceutical, cosmeceutical, cosmetic, and nutritional uses. Beyond the challenges of obtaining sufficient raw materials and producing effective formulations, the continued viability of such resources may be threatened by human exploitation of the seas and climate change. That said, the oceans offer the greatest biodiversity on the planet and dermatologic preparations derived from such sources present intriguing possibilities, particularly the apparent anti-inflammatory activity of gorgonian and other terpenes. These compounds appear to have the potential to replace, or serve as desirable alternatives to, conventional therapies for inflammatory skin disorders.
References
1. Biotechnol Adv. 2011;29(5):468-82.
2. Mar Drugs. 2013;11(3):700-17.
3. Mar Drugs. 2014;12(2):1066-101.
4. Future Med Chem. 2011;3(12):1475-89.
5. Org Lett. 2000;2(4):507-10.
6. Mar Drugs. 2013;11(1):146-64.
7. J Cosmet Dermatol. 2014;13(1):56-67.
8. Adv Food Nutr Res. 2012;65:409-13.
9. Crit Rev Microbiol. 2011;37(3):245-9.
10. Nat Chem Biol. 2007;3(7):408-14.
11. J Drugs Dermatol. 2013;12(10):1177-9.
12. J Ind Microbiol Biotechnol. 2006;33(7):532-8.
13. Nat Prod Rep. 2009;26(5):681-710.
14. Proc Natl Acad Sci USA. 1986;83(17):6238-40.
15. Bioorg Med Chem. 2011;19(22):6702-19.
16. Arch Biochem Biophys. 2004;424(1):97-104.
17. Asia Pac J Clin Nutr. 2006;15(2):143-52.
18. J Nat Prod. 2004;67(10):1672-80.
19. Mar Drugs. 2004 May;2:73-82.
20. J Nat Prod. 2004;67(8):1216-38.
21. J Cosmet Dermatol. 2012;11(3):213-22.
22. Int J Cosmet Sci. 2008;30(2):131-8.
Dr. Baumann is chief executive officer of the Baumann Cosmetic & Research Institute in the Design District in Miami. She founded the Cosmetic Dermatology Center at the University of Miami in 1997. Dr. Baumann wrote the textbook, “Cosmetic Dermatology: Principles and Practice” (New York: McGraw-Hill, 2002), and a book for consumers, “The Skin Type Solution” (New York: Bantam Dell, 2006). She has contributed to the Cosmeceutical Critique column in Dermatology News since January 2001. Her latest book, “Cosmeceuticals and Cosmetic Ingredients,” was published in November 2014. Dr. Baumann has received funding for clinical grants from Allergan, Aveeno, Avon Products, Evolus, Galderma, GlaxoSmithKline, Kythera Biopharmaceuticals, Mary Kay, Medicis Pharmaceuticals, Neutrogena, Philosophy, Topix Pharmaceuticals, and Unilever.
Just as we learned early in life that 70% of the human body is composed of water, water covers approximately the same percentage of the earth’s surface. While fishing and harvesting of algae have occurred throughout human history,1 it has only been since the 1970s that widespread scientific interest in the great biological and chemical diversity of the vast oceans of the world has led to investigations into medical and cosmetic applications of the rich life beneath the sea.2 During this period, the marine environment has been found to boast multiple organisms with unique metabolisms adapted for survival in challenging conditions, yielding secondary metabolites, some of which have become valuable in the pharmaceutical and cosmeceutical markets.3,4 Thus, the inclusion of bioactive substances from the sea in drugs and cosmetic products is primarily a recent phenomenon.1 In fact, marine ingredients in cosmetics are thought to confer various benefits to skin health, including antioxidant, anti-acne, anti-wrinkle, and anti-tyrosinase activity.
Chemistry and biologic activity
Several marine microbial natural products have been found to display antimicrobial, antitumor, and anti-inflammatory activity.2,5 And seaweed extracts (green, brown, and red algal compounds that include constituents such as phlorotannins, sulfated polysaccharides, and tyrosinase inhibitors) have been incorporated into cosmeceutical products, with a long history of traditional folk uses for various health – including skin – conditions.3,6,7 Kim and Li reviewed the beneficial health effects of marine fungi-derived terpenoids in 2012, reporting that hundreds of these compounds have been discovered in the last few decades, with many exhibiting anti-inflammatory, anticancer, antimicrobial, and antioxidant activity.8,9 Terpenoids, or isoprenoids, are a subclass of prenyllipids, which include prenylquinones, sterols, and terpenes, the largest class of natural substances.10
The terpenes are the largest group of biologically diverse marine compounds, and include the pseudopterosins, which are structurally discrete active metabolites of the Caribbean gorgonian soft coral Pseudopterogorgia elisabethae, which is native to the waters of the Caribbean Sea, Central Bahamas, Bermuda, the West Indies, and the Florida keys.11,12 The most common gorgonian corals are diterpenes.13 Twenty-six derivatives of the octocoral P. elisabethae (designated PsA-PsZ), also known as the sea whip, sea fan, or sea plume, have been isolated.11,12,14 Pseudopterosins were first isolated in 1986.14,15
Based on the identified biologic activities, particularly anti-inflammatory capacity, of pseudopterosins, researchers have investigated their potential for treatment of various conditions including asthma, cancer, contact dermatitis, dermatoheliosis, HIV, photodamage, psoriasis, and rheumatoid arthritis.1,11
After decades of extensive research of pseudopterosins, these tricyclic diterpene glycosides are thought to provide superior anti-inflammatory and analgesic properties, compared to standard anti-inflammatory treatments, without inducing adverse side effects; they also offer marked antimicrobial and wound-healing effects.3,11,14,16-19
Other marine diterpene glycosides include eleutherobins and fucosides, which also exhibit notable biologic activity.15 In particular, the anti-inflammatory and analgesic activities of pseudopterosins have been found to be concentration- and dose-dependently more potent than the standard-bearing indomethacin.11,14,17
Marine ingredients in topical formulations
The first product to include pseudopterosins was the skin formulation Resilience marketed by Estée Lauder over a decade ago.19,20 Natural marine ingredients have since been incorporated into a few more products, such as Imedeen, an oral skin care preparation that contains Marine Complex.21
In 2012, Rietveld et al. ascertained whether the Marine Complex from Imedeen could variously alter skin morphogenesis in female and male human skin equivalents. Cells were culled from female and male donors between the ages of 30 and 45 years for human skin equivalents that were cultured for 7 or 11 weeks with or without Marine Complex. The investigators found that the number of Ki67-positive epidermal cells was greatly augmented by Marine Complex in female human skin equivalents. The Marine Complex significantly spurred the level of secreted pro-collagen I and elevated the deposition of laminin 332 and collagen type VII in the dermis. Human skin equivalents treated with Marine Complex also exhibited more viable epidermal cell layers and a thicker dermal extracellular matrix, compared to controls, with these effects less salient in male human skin equivalents. The investigators concluded that supplementation with Marine Complex positively stimulated overall human skin equivalent tissue formation, with its effects on the basement membrane and dermal constituents suggestive of potential for use against human skin aging.21
Previously, Xhauflaire-Uhoda et al. evaluated the skin hydrating and firming dose-response effects of cosmetic preparations enriched in algae- and fish collagen–derived substances in randomized controlled double-blind medium-term (12 subjects aged 18-55 years) and short-term (3 subjects over the age of 50) trials. In the short term, serum formulations enriched in marine compounds manifested a superior moisturizing effect on the forearm compared with creams. In later stages, cream formulations were more active, especially after repeated applications. Investigators observed a sustained firming activity in association with both the lotion and cream during treatment, but such results did not persist after treatment was stopped.22
Product development
Technological advances, including sampling strategies, nanoscale nuclear magnetic resonance for structure determination, total chemical synthesis, fermentation, exploration of genomic and metagenomic resources, combinatorial biosynthesis, synthetic biology, and biotechnology represent important ways in which novel marine natural products are being developed, according to several authors.1,2,4
Conclusion
Marine ingredients are a relatively new and fascinating category of substances that can and are being harnessed for pharmaceutical, cosmeceutical, cosmetic, and nutritional uses. Beyond the challenges of obtaining sufficient raw materials and producing effective formulations, the continued viability of such resources may be threatened by human exploitation of the seas and climate change. That said, the oceans offer the greatest biodiversity on the planet and dermatologic preparations derived from such sources present intriguing possibilities, particularly the apparent anti-inflammatory activity of gorgonian and other terpenes. These compounds appear to have the potential to replace, or serve as desirable alternatives to, conventional therapies for inflammatory skin disorders.
References
1. Biotechnol Adv. 2011;29(5):468-82.
2. Mar Drugs. 2013;11(3):700-17.
3. Mar Drugs. 2014;12(2):1066-101.
4. Future Med Chem. 2011;3(12):1475-89.
5. Org Lett. 2000;2(4):507-10.
6. Mar Drugs. 2013;11(1):146-64.
7. J Cosmet Dermatol. 2014;13(1):56-67.
8. Adv Food Nutr Res. 2012;65:409-13.
9. Crit Rev Microbiol. 2011;37(3):245-9.
10. Nat Chem Biol. 2007;3(7):408-14.
11. J Drugs Dermatol. 2013;12(10):1177-9.
12. J Ind Microbiol Biotechnol. 2006;33(7):532-8.
13. Nat Prod Rep. 2009;26(5):681-710.
14. Proc Natl Acad Sci USA. 1986;83(17):6238-40.
15. Bioorg Med Chem. 2011;19(22):6702-19.
16. Arch Biochem Biophys. 2004;424(1):97-104.
17. Asia Pac J Clin Nutr. 2006;15(2):143-52.
18. J Nat Prod. 2004;67(10):1672-80.
19. Mar Drugs. 2004 May;2:73-82.
20. J Nat Prod. 2004;67(8):1216-38.
21. J Cosmet Dermatol. 2012;11(3):213-22.
22. Int J Cosmet Sci. 2008;30(2):131-8.
Dr. Baumann is chief executive officer of the Baumann Cosmetic & Research Institute in the Design District in Miami. She founded the Cosmetic Dermatology Center at the University of Miami in 1997. Dr. Baumann wrote the textbook, “Cosmetic Dermatology: Principles and Practice” (New York: McGraw-Hill, 2002), and a book for consumers, “The Skin Type Solution” (New York: Bantam Dell, 2006). She has contributed to the Cosmeceutical Critique column in Dermatology News since January 2001. Her latest book, “Cosmeceuticals and Cosmetic Ingredients,” was published in November 2014. Dr. Baumann has received funding for clinical grants from Allergan, Aveeno, Avon Products, Evolus, Galderma, GlaxoSmithKline, Kythera Biopharmaceuticals, Mary Kay, Medicis Pharmaceuticals, Neutrogena, Philosophy, Topix Pharmaceuticals, and Unilever.
Rigosertib falls short for high-risk myelodysplastic syndromes after failure on azacitidine or decitabine
Rigosertib failed to extend overall survival beyond that seen with best supportive care in a trial of patients who had myelodysplastic syndrome with excess blasts after failure of azacitidine or decitabine treatment.
A randomized phase III trial of rigosertib (NCT 02562443) is now underway in specific subgroups of high-risk patients, including patients with very high risk on the basis of the Revised International Prognostic Scoring System criteria, to determine whether the drug may benefit specific patient subgroups, according to Dr. Guillermo Garcia-Manero of the University of Texas MD Anderson Cancer Center, Houston, and his colleagues.
The ONTIME study (NCT01241500) was an open-label, randomized controlled trial at 74 medical centers in the US and Europe. Patients with refractory anemia with excess blasts ([RAEB]-1, RAEB-2, RAEB-t, or chronic myelomonocytic leukemia) were enrolled based on local site assessment and treatment failure with a hypomethylating drug in the past 2 years.
Patients were randomly assigned on a 2:1 basis to receive rigosertib or best supportive care with or without low-dose cytarabine. Randomization was stratified by pretreatment bone marrow blast percentage. The 199 patients given rigosertib received 1,800 mg per 24 hours via a 72-hour continuous intravenous infusion administered every other week. Another 100 patients were assigned to best supportive care.
Median follow-up was 19.5 months. Median overall survival was 8.2 months (95% confidence interval, 6.1-10.1) in the rigosertib group and 5.9 months (95% CI, 4.1-9.3) in the best supportive care group (hazard ratio, 0.87; 95% CI, 0.67-1.14; P = 0.33), the researchers reported (Lancet Oncol. 2016;17(4):496-508. doi: 10.1016/S1470-2045(16)00009-7).
The most common grade 3 or higher adverse events were anemia (18% of 184 patients in the rigosertib group and 8% of 91 patients in the best supportive care group), thrombocytopenia (19% vs 7%), neutropenia (17% vs. 8%), febrile neutropenia (12% vs 11%], and pneumonia (12% vs 11%). Adverse events led to death in 22% of 184 patients in the rigosertib group and 33% of 91 patients in the best supportive care group; three deaths were attributed to rigosertib treatment.
The study was funded by Onconova Therapeutics and the Leukemia and Lymphoma Society.
On Twitter @maryjodales
Rigosertib failed to extend overall survival beyond that seen with best supportive care in a trial of patients who had myelodysplastic syndrome with excess blasts after failure of azacitidine or decitabine treatment.
A randomized phase III trial of rigosertib (NCT 02562443) is now underway in specific subgroups of high-risk patients, including patients with very high risk on the basis of the Revised International Prognostic Scoring System criteria, to determine whether the drug may benefit specific patient subgroups, according to Dr. Guillermo Garcia-Manero of the University of Texas MD Anderson Cancer Center, Houston, and his colleagues.
The ONTIME study (NCT01241500) was an open-label, randomized controlled trial at 74 medical centers in the US and Europe. Patients with refractory anemia with excess blasts ([RAEB]-1, RAEB-2, RAEB-t, or chronic myelomonocytic leukemia) were enrolled based on local site assessment and treatment failure with a hypomethylating drug in the past 2 years.
Patients were randomly assigned on a 2:1 basis to receive rigosertib or best supportive care with or without low-dose cytarabine. Randomization was stratified by pretreatment bone marrow blast percentage. The 199 patients given rigosertib received 1,800 mg per 24 hours via a 72-hour continuous intravenous infusion administered every other week. Another 100 patients were assigned to best supportive care.
Median follow-up was 19.5 months. Median overall survival was 8.2 months (95% confidence interval, 6.1-10.1) in the rigosertib group and 5.9 months (95% CI, 4.1-9.3) in the best supportive care group (hazard ratio, 0.87; 95% CI, 0.67-1.14; P = 0.33), the researchers reported (Lancet Oncol. 2016;17(4):496-508. doi: 10.1016/S1470-2045(16)00009-7).
The most common grade 3 or higher adverse events were anemia (18% of 184 patients in the rigosertib group and 8% of 91 patients in the best supportive care group), thrombocytopenia (19% vs 7%), neutropenia (17% vs. 8%), febrile neutropenia (12% vs 11%], and pneumonia (12% vs 11%). Adverse events led to death in 22% of 184 patients in the rigosertib group and 33% of 91 patients in the best supportive care group; three deaths were attributed to rigosertib treatment.
The study was funded by Onconova Therapeutics and the Leukemia and Lymphoma Society.
On Twitter @maryjodales
Rigosertib failed to extend overall survival beyond that seen with best supportive care in a trial of patients who had myelodysplastic syndrome with excess blasts after failure of azacitidine or decitabine treatment.
A randomized phase III trial of rigosertib (NCT 02562443) is now underway in specific subgroups of high-risk patients, including patients with very high risk on the basis of the Revised International Prognostic Scoring System criteria, to determine whether the drug may benefit specific patient subgroups, according to Dr. Guillermo Garcia-Manero of the University of Texas MD Anderson Cancer Center, Houston, and his colleagues.
The ONTIME study (NCT01241500) was an open-label, randomized controlled trial at 74 medical centers in the US and Europe. Patients with refractory anemia with excess blasts ([RAEB]-1, RAEB-2, RAEB-t, or chronic myelomonocytic leukemia) were enrolled based on local site assessment and treatment failure with a hypomethylating drug in the past 2 years.
Patients were randomly assigned on a 2:1 basis to receive rigosertib or best supportive care with or without low-dose cytarabine. Randomization was stratified by pretreatment bone marrow blast percentage. The 199 patients given rigosertib received 1,800 mg per 24 hours via a 72-hour continuous intravenous infusion administered every other week. Another 100 patients were assigned to best supportive care.
Median follow-up was 19.5 months. Median overall survival was 8.2 months (95% confidence interval, 6.1-10.1) in the rigosertib group and 5.9 months (95% CI, 4.1-9.3) in the best supportive care group (hazard ratio, 0.87; 95% CI, 0.67-1.14; P = 0.33), the researchers reported (Lancet Oncol. 2016;17(4):496-508. doi: 10.1016/S1470-2045(16)00009-7).
The most common grade 3 or higher adverse events were anemia (18% of 184 patients in the rigosertib group and 8% of 91 patients in the best supportive care group), thrombocytopenia (19% vs 7%), neutropenia (17% vs. 8%), febrile neutropenia (12% vs 11%], and pneumonia (12% vs 11%). Adverse events led to death in 22% of 184 patients in the rigosertib group and 33% of 91 patients in the best supportive care group; three deaths were attributed to rigosertib treatment.
The study was funded by Onconova Therapeutics and the Leukemia and Lymphoma Society.
On Twitter @maryjodales
THE LANCET ONCOLOGY
Key clinical point: Rigosertib failed to extend overall survival beyond that seen with best supportive care in a trial of patients who had myelodysplastic syndrome with excess blasts after failure of azacitidine or decitabine treatment.
Major finding: Median overall survival was 8.2 months (95% CI, 6.1-10.1) in the rigosertib group and 5.9 months (95% CI, 4.1-9.3) in the best supportive care group.
Data source: An open-label, randomized controlled trial involving 299 patients at 74 medical centers.
Disclosures: The study was funded by Onconova Therapeutics and the Leukemia and Lymphoma Society..
Rigosertib falls short for high-risk myelodysplastic syndromes after failure on azacitidine or decitabine
Rigosertib failed to extend overall survival beyond that seen with best supportive care in a trial of patients who had myelodysplastic syndrome with excess blasts after failure of azacitidine or decitabine treatment.
A randomized phase III trial of rigosertib (NCT 02562443) is now underway in specific subgroups of high-risk patients, including patients with very high risk on the basis of the Revised International Prognostic Scoring System criteria, to determine whether the drug may benefit specific patient subgroups, according to Dr. Guillermo Garcia-Manero of the University of Texas MD Anderson Cancer Center, Houston, and his colleagues.
The ONTIME study (NCT01241500) was an open-label, randomized controlled trial at 74 medical centers in the US and Europe. Patients with refractory anemia with excess blasts ([RAEB]-1, RAEB-2, RAEB-t, or chronic myelomonocytic leukemia) were enrolled based on local site assessment and treatment failure with a hypomethylating drug in the past 2 years.
Patients were randomly assigned on a 2:1 basis to receive rigosertib or best supportive care with or without low-dose cytarabine. Randomization was stratified by pretreatment bone marrow blast percentage. The 199 patients given rigosertib received 1,800 mg per 24 hours via a 72-hour continuous intravenous infusion administered every other week. Another 100 patients were assigned to best supportive care.
Median follow-up was 19.5 months. Median overall survival was 8.2 months (95% confidence interval, 6.1-10.1) in the rigosertib group and 5.9 months (95% CI, 4.1-9.3) in the best supportive care group (hazard ratio, 0.87; 95% CI, 0.67-1.14; P = 0.33), the researchers reported (Lancet Oncol. 2016;17(4):496-508. doi: 10.1016/S1470-2045(16)00009-7).
The most common grade 3 or higher adverse events were anemia (18% of 184 patients in the rigosertib group and 8% of 91 patients in the best supportive care group), thrombocytopenia (19% vs 7%), neutropenia (17% vs. 8%), febrile neutropenia (12% vs 11%], and pneumonia (12% vs 11%). Adverse events led to death in 22% of 184 patients in the rigosertib group and 33% of 91 patients in the best supportive care group; three deaths were attributed to rigosertib treatment.
The study was funded by Onconova Therapeutics and the Leukemia and Lymphoma Society.
On Twitter @maryjodales
Rigosertib failed to extend overall survival beyond that seen with best supportive care in a trial of patients who had myelodysplastic syndrome with excess blasts after failure of azacitidine or decitabine treatment.
A randomized phase III trial of rigosertib (NCT 02562443) is now underway in specific subgroups of high-risk patients, including patients with very high risk on the basis of the Revised International Prognostic Scoring System criteria, to determine whether the drug may benefit specific patient subgroups, according to Dr. Guillermo Garcia-Manero of the University of Texas MD Anderson Cancer Center, Houston, and his colleagues.
The ONTIME study (NCT01241500) was an open-label, randomized controlled trial at 74 medical centers in the US and Europe. Patients with refractory anemia with excess blasts ([RAEB]-1, RAEB-2, RAEB-t, or chronic myelomonocytic leukemia) were enrolled based on local site assessment and treatment failure with a hypomethylating drug in the past 2 years.
Patients were randomly assigned on a 2:1 basis to receive rigosertib or best supportive care with or without low-dose cytarabine. Randomization was stratified by pretreatment bone marrow blast percentage. The 199 patients given rigosertib received 1,800 mg per 24 hours via a 72-hour continuous intravenous infusion administered every other week. Another 100 patients were assigned to best supportive care.
Median follow-up was 19.5 months. Median overall survival was 8.2 months (95% confidence interval, 6.1-10.1) in the rigosertib group and 5.9 months (95% CI, 4.1-9.3) in the best supportive care group (hazard ratio, 0.87; 95% CI, 0.67-1.14; P = 0.33), the researchers reported (Lancet Oncol. 2016;17(4):496-508. doi: 10.1016/S1470-2045(16)00009-7).
The most common grade 3 or higher adverse events were anemia (18% of 184 patients in the rigosertib group and 8% of 91 patients in the best supportive care group), thrombocytopenia (19% vs 7%), neutropenia (17% vs. 8%), febrile neutropenia (12% vs 11%], and pneumonia (12% vs 11%). Adverse events led to death in 22% of 184 patients in the rigosertib group and 33% of 91 patients in the best supportive care group; three deaths were attributed to rigosertib treatment.
The study was funded by Onconova Therapeutics and the Leukemia and Lymphoma Society.
On Twitter @maryjodales
Rigosertib failed to extend overall survival beyond that seen with best supportive care in a trial of patients who had myelodysplastic syndrome with excess blasts after failure of azacitidine or decitabine treatment.
A randomized phase III trial of rigosertib (NCT 02562443) is now underway in specific subgroups of high-risk patients, including patients with very high risk on the basis of the Revised International Prognostic Scoring System criteria, to determine whether the drug may benefit specific patient subgroups, according to Dr. Guillermo Garcia-Manero of the University of Texas MD Anderson Cancer Center, Houston, and his colleagues.
The ONTIME study (NCT01241500) was an open-label, randomized controlled trial at 74 medical centers in the US and Europe. Patients with refractory anemia with excess blasts ([RAEB]-1, RAEB-2, RAEB-t, or chronic myelomonocytic leukemia) were enrolled based on local site assessment and treatment failure with a hypomethylating drug in the past 2 years.
Patients were randomly assigned on a 2:1 basis to receive rigosertib or best supportive care with or without low-dose cytarabine. Randomization was stratified by pretreatment bone marrow blast percentage. The 199 patients given rigosertib received 1,800 mg per 24 hours via a 72-hour continuous intravenous infusion administered every other week. Another 100 patients were assigned to best supportive care.
Median follow-up was 19.5 months. Median overall survival was 8.2 months (95% confidence interval, 6.1-10.1) in the rigosertib group and 5.9 months (95% CI, 4.1-9.3) in the best supportive care group (hazard ratio, 0.87; 95% CI, 0.67-1.14; P = 0.33), the researchers reported (Lancet Oncol. 2016;17(4):496-508. doi: 10.1016/S1470-2045(16)00009-7).
The most common grade 3 or higher adverse events were anemia (18% of 184 patients in the rigosertib group and 8% of 91 patients in the best supportive care group), thrombocytopenia (19% vs 7%), neutropenia (17% vs. 8%), febrile neutropenia (12% vs 11%], and pneumonia (12% vs 11%). Adverse events led to death in 22% of 184 patients in the rigosertib group and 33% of 91 patients in the best supportive care group; three deaths were attributed to rigosertib treatment.
The study was funded by Onconova Therapeutics and the Leukemia and Lymphoma Society.
On Twitter @maryjodales
THE LANCET ONCOLOGY
Key clinical point: Rigosertib failed to extend overall survival beyond that seen with best supportive care in a trial of patients who had myelodysplastic syndrome with excess blasts after failure of azacitidine or decitabine treatment.
Major finding: Median overall survival was 8.2 months (95% CI, 6.1-10.1) in the rigosertib group and 5.9 months (95% CI, 4.1-9.3) in the best supportive care group.
Data source: An open-label, randomized controlled trial involving 299 patients at 74 medical centers.
Disclosures: The study was funded by Onconova Therapeutics and the Leukemia and Lymphoma Society..
Study Finds: Blood Pressure Medication and Stain Cholesterol Fighter Decreases the Risk of Heart Attack and Stroke in Patients with Hypertension and Moderate Risk of Heart Disease
(Reuters) - Patients with hypertension and moderate risk of heart disease slashed their long-term risk of heart attack and stroke 40 percent by taking a blood pressure medication as well as a statin cholesterol fighter, according to a large global study that could change medical practice.
Results from the trial, called HOPE-3, could prod far more doctors to add a statin to antihypertensive therapy for such patients who have no prior history of heart attack or stroke, researchers said.
The data was presented on Saturday at the annual scientific session of the American College of Cardiology in Chicago.
To enroll in the trial, patients had to have at least one risk factor for heart disease such as obesity or smoking, in addition to being over 60 for women and over 55 for men.
"Intermediate-risk people with hypertension had a clear benefit when taking both a statin and an agent that lowers blood pressure," Dr. Salim Yusuf, a professor of cardiology at McMaster University in Ontario, Canada who headed the 12,000-patient global study, said in an interview.
Patients with systolic blood pressure of 140 and higher were deemed in the study to have high blood pressure. They experienced a 40 percent reduced risk of heart attack and stroke over a six-year period when taking AstraZeneca Plc's statin Crestor (rosuvastatin) as well as a combination tablet containing blood pressure treatment candesartan and the diuretic hydrochlorothiazide.
Patients with normal or low systolic pressure had the same approximate 25 percent reduction in cardiovascular events as seen among patients in one arm of the study who took only statins.
Yusuf said the trial underscores that if a patient at moderate heart risk has high blood pressure, defined as 140 or higher, "give them both a statin and a blood pressure medication as a matter of course." He said statins are not automatically given now to patients with hypertension that are at only moderate risk of heart attack or stroke.
Yusuf's trial included research centers in China, India, Latin America, Africa and Canada, but not the United States because of far greater research costs there. The trial was funded by the Canadian Institutes of Health Research and AstraZeneca.
A separate study published in November found that lowering blood pressure to below 120 dramatically reduced heart failure and risk of death in adults aged 50 and older. But the five-year U.S. government-sponsored study of more than 9,300 patients showed a higher rate of adverse side effects, including kidney damage, in the aggressively treated patients.
(Reuters) - Patients with hypertension and moderate risk of heart disease slashed their long-term risk of heart attack and stroke 40 percent by taking a blood pressure medication as well as a statin cholesterol fighter, according to a large global study that could change medical practice.
Results from the trial, called HOPE-3, could prod far more doctors to add a statin to antihypertensive therapy for such patients who have no prior history of heart attack or stroke, researchers said.
The data was presented on Saturday at the annual scientific session of the American College of Cardiology in Chicago.
To enroll in the trial, patients had to have at least one risk factor for heart disease such as obesity or smoking, in addition to being over 60 for women and over 55 for men.
"Intermediate-risk people with hypertension had a clear benefit when taking both a statin and an agent that lowers blood pressure," Dr. Salim Yusuf, a professor of cardiology at McMaster University in Ontario, Canada who headed the 12,000-patient global study, said in an interview.
Patients with systolic blood pressure of 140 and higher were deemed in the study to have high blood pressure. They experienced a 40 percent reduced risk of heart attack and stroke over a six-year period when taking AstraZeneca Plc's statin Crestor (rosuvastatin) as well as a combination tablet containing blood pressure treatment candesartan and the diuretic hydrochlorothiazide.
Patients with normal or low systolic pressure had the same approximate 25 percent reduction in cardiovascular events as seen among patients in one arm of the study who took only statins.
Yusuf said the trial underscores that if a patient at moderate heart risk has high blood pressure, defined as 140 or higher, "give them both a statin and a blood pressure medication as a matter of course." He said statins are not automatically given now to patients with hypertension that are at only moderate risk of heart attack or stroke.
Yusuf's trial included research centers in China, India, Latin America, Africa and Canada, but not the United States because of far greater research costs there. The trial was funded by the Canadian Institutes of Health Research and AstraZeneca.
A separate study published in November found that lowering blood pressure to below 120 dramatically reduced heart failure and risk of death in adults aged 50 and older. But the five-year U.S. government-sponsored study of more than 9,300 patients showed a higher rate of adverse side effects, including kidney damage, in the aggressively treated patients.
(Reuters) - Patients with hypertension and moderate risk of heart disease slashed their long-term risk of heart attack and stroke 40 percent by taking a blood pressure medication as well as a statin cholesterol fighter, according to a large global study that could change medical practice.
Results from the trial, called HOPE-3, could prod far more doctors to add a statin to antihypertensive therapy for such patients who have no prior history of heart attack or stroke, researchers said.
The data was presented on Saturday at the annual scientific session of the American College of Cardiology in Chicago.
To enroll in the trial, patients had to have at least one risk factor for heart disease such as obesity or smoking, in addition to being over 60 for women and over 55 for men.
"Intermediate-risk people with hypertension had a clear benefit when taking both a statin and an agent that lowers blood pressure," Dr. Salim Yusuf, a professor of cardiology at McMaster University in Ontario, Canada who headed the 12,000-patient global study, said in an interview.
Patients with systolic blood pressure of 140 and higher were deemed in the study to have high blood pressure. They experienced a 40 percent reduced risk of heart attack and stroke over a six-year period when taking AstraZeneca Plc's statin Crestor (rosuvastatin) as well as a combination tablet containing blood pressure treatment candesartan and the diuretic hydrochlorothiazide.
Patients with normal or low systolic pressure had the same approximate 25 percent reduction in cardiovascular events as seen among patients in one arm of the study who took only statins.
Yusuf said the trial underscores that if a patient at moderate heart risk has high blood pressure, defined as 140 or higher, "give them both a statin and a blood pressure medication as a matter of course." He said statins are not automatically given now to patients with hypertension that are at only moderate risk of heart attack or stroke.
Yusuf's trial included research centers in China, India, Latin America, Africa and Canada, but not the United States because of far greater research costs there. The trial was funded by the Canadian Institutes of Health Research and AstraZeneca.
A separate study published in November found that lowering blood pressure to below 120 dramatically reduced heart failure and risk of death in adults aged 50 and older. But the five-year U.S. government-sponsored study of more than 9,300 patients showed a higher rate of adverse side effects, including kidney damage, in the aggressively treated patients.
TCD screening underused in sickle cell patients

with sickle cell anemia
Photo courtesy of St. Jude
Results of a large, retrospective study suggest the use of transcranial Doppler (TCD) screening is on the rise in US children and adolescents with sickle cell anemia.
However, the rate of TCD screening in these patients falls well below national recommendations.
In addition, TCD screening rates vary greatly by state, and the use of screening tends to decrease as patients grow older.
Sarah L. Reeves, PhD, of University of Michigan, Ann Arbor, and her colleagues reported these findings in JAMA Pediatrics.
The researchers noted that guidelines from the National Heart, Lung, and Blood Institute recommend that patients with sickle cell anemia receive annual TCD screenings from age 2 to 16 to identify those patients at the highest risk of stroke.
Dr Reeves and her colleagues wanted to determine if this recommendation is being followed. So they analyzed Medicaid claims data from 2005 through 2010 for Florida, Illinois, Louisiana, Michigan, South Carolina, and Texas.
The data included 4775 patients, ages 2 to 16, with sickle cell anemia. For these patients, TCD screening rates increased from 22% in 2005 to 44% in 2010 (P<0.001).
The researchers found that TCD screening rates varied significantly by state (P=0.004), and Texas had the lowest screening rate at any time point (7% in 2005).
The team also analyzed a subset of 2388 patients who were enrolled for 2 or more consecutive years to examine potential predictors of TCD screening.
This analysis revealed that, with each year of increasing age, a patient’s odds of receiving TCD screening decreased (odds ratio=0.97, P=0.002).
On the other hand, an increasing number of well-child visits was associated with higher odds of receiving TCD screening (odds ratio=1.10, P=0.007).
And the odds of receiving TCD screening were higher for patients who previously underwent TCD screening (odds ratio=2.44, P<0.001).
The researchers said these results suggest that, despite national recommendations, TCD screening rates remain low in young patients with sickle cell anemia in the US. ![]()

with sickle cell anemia
Photo courtesy of St. Jude
Results of a large, retrospective study suggest the use of transcranial Doppler (TCD) screening is on the rise in US children and adolescents with sickle cell anemia.
However, the rate of TCD screening in these patients falls well below national recommendations.
In addition, TCD screening rates vary greatly by state, and the use of screening tends to decrease as patients grow older.
Sarah L. Reeves, PhD, of University of Michigan, Ann Arbor, and her colleagues reported these findings in JAMA Pediatrics.
The researchers noted that guidelines from the National Heart, Lung, and Blood Institute recommend that patients with sickle cell anemia receive annual TCD screenings from age 2 to 16 to identify those patients at the highest risk of stroke.
Dr Reeves and her colleagues wanted to determine if this recommendation is being followed. So they analyzed Medicaid claims data from 2005 through 2010 for Florida, Illinois, Louisiana, Michigan, South Carolina, and Texas.
The data included 4775 patients, ages 2 to 16, with sickle cell anemia. For these patients, TCD screening rates increased from 22% in 2005 to 44% in 2010 (P<0.001).
The researchers found that TCD screening rates varied significantly by state (P=0.004), and Texas had the lowest screening rate at any time point (7% in 2005).
The team also analyzed a subset of 2388 patients who were enrolled for 2 or more consecutive years to examine potential predictors of TCD screening.
This analysis revealed that, with each year of increasing age, a patient’s odds of receiving TCD screening decreased (odds ratio=0.97, P=0.002).
On the other hand, an increasing number of well-child visits was associated with higher odds of receiving TCD screening (odds ratio=1.10, P=0.007).
And the odds of receiving TCD screening were higher for patients who previously underwent TCD screening (odds ratio=2.44, P<0.001).
The researchers said these results suggest that, despite national recommendations, TCD screening rates remain low in young patients with sickle cell anemia in the US. ![]()

with sickle cell anemia
Photo courtesy of St. Jude
Results of a large, retrospective study suggest the use of transcranial Doppler (TCD) screening is on the rise in US children and adolescents with sickle cell anemia.
However, the rate of TCD screening in these patients falls well below national recommendations.
In addition, TCD screening rates vary greatly by state, and the use of screening tends to decrease as patients grow older.
Sarah L. Reeves, PhD, of University of Michigan, Ann Arbor, and her colleagues reported these findings in JAMA Pediatrics.
The researchers noted that guidelines from the National Heart, Lung, and Blood Institute recommend that patients with sickle cell anemia receive annual TCD screenings from age 2 to 16 to identify those patients at the highest risk of stroke.
Dr Reeves and her colleagues wanted to determine if this recommendation is being followed. So they analyzed Medicaid claims data from 2005 through 2010 for Florida, Illinois, Louisiana, Michigan, South Carolina, and Texas.
The data included 4775 patients, ages 2 to 16, with sickle cell anemia. For these patients, TCD screening rates increased from 22% in 2005 to 44% in 2010 (P<0.001).
The researchers found that TCD screening rates varied significantly by state (P=0.004), and Texas had the lowest screening rate at any time point (7% in 2005).
The team also analyzed a subset of 2388 patients who were enrolled for 2 or more consecutive years to examine potential predictors of TCD screening.
This analysis revealed that, with each year of increasing age, a patient’s odds of receiving TCD screening decreased (odds ratio=0.97, P=0.002).
On the other hand, an increasing number of well-child visits was associated with higher odds of receiving TCD screening (odds ratio=1.10, P=0.007).
And the odds of receiving TCD screening were higher for patients who previously underwent TCD screening (odds ratio=2.44, P<0.001).
The researchers said these results suggest that, despite national recommendations, TCD screening rates remain low in young patients with sickle cell anemia in the US. ![]()