User login
How a genetic locus protects HSCs

in the bone marrow
The Dlk1-Gtl2 locus plays a critical role in protecting hematopoietic stem cells (HSCs), according to preclinical research.
The study suggests the mammalian imprinted gene Gtl2, located on mouse chromosome 12qF1, protects adult HSCs by restricting metabolic activity in the cells’ mitochondria.
This work indicates that Gtl2 may be useful as a biomarker to determine if cells are normal or potentially cancerous.
Linheng Li, PhD, of the Stowers Institute for Medical Research in Kansas City, Missouri, and his colleagues described this research in Cell Stem Cell.
The researchers knew that the Dlk1-Gtl2 locus produces multiple non-coding RNAs from the maternally inherited allele, including the largest microRNA cluster in the mammalian genome.
“Most of the non-coding RNAs at the Gtl2 locus have been documented to function as tumor suppressors to maintain normal cell function,” said study author Pengxu Qian, PhD, also from the Stowers Institute for Medical Research.
However, the role of this locus in HSCs was unclear. So the team studied HSCs in mice. They used transcriptome profiling to analyze 17 hematopoietic cell types.
The analyses revealed that non-coding RNAs expressed from the Gtl2 locus are predominantly enriched in fetal liver HSCs and adult long-term HSCs, and these non-coding RNAs sustain long-term HSC functionality.
Gtl2’s megacluster of microRNA suppresses the mTOR signaling pathway and downstream mitochondrial biogenesis and metabolism, thus blocking reactive oxygen species (ROS) that can damage adult stem cells.
When the researchers deleted the Dlk1-Gtl2 locus from the maternally inherited allele in HSCs, they observed increases in mitochondrial biogenesis, metabolic activity, and ROS levels, which led to cell death.
Dr Li said these findings suggest Gtl2 could be used as a biomarker because it could help label dormant (or reserve) stem cells in normal or potentially cancerous stem cell populations.
The addition of a fluorescent tag to the Gtl2 locus could allow researchers to mark other adult stem cells in the gut, hair follicle, muscle, and neural systems. ![]()

in the bone marrow
The Dlk1-Gtl2 locus plays a critical role in protecting hematopoietic stem cells (HSCs), according to preclinical research.
The study suggests the mammalian imprinted gene Gtl2, located on mouse chromosome 12qF1, protects adult HSCs by restricting metabolic activity in the cells’ mitochondria.
This work indicates that Gtl2 may be useful as a biomarker to determine if cells are normal or potentially cancerous.
Linheng Li, PhD, of the Stowers Institute for Medical Research in Kansas City, Missouri, and his colleagues described this research in Cell Stem Cell.
The researchers knew that the Dlk1-Gtl2 locus produces multiple non-coding RNAs from the maternally inherited allele, including the largest microRNA cluster in the mammalian genome.
“Most of the non-coding RNAs at the Gtl2 locus have been documented to function as tumor suppressors to maintain normal cell function,” said study author Pengxu Qian, PhD, also from the Stowers Institute for Medical Research.
However, the role of this locus in HSCs was unclear. So the team studied HSCs in mice. They used transcriptome profiling to analyze 17 hematopoietic cell types.
The analyses revealed that non-coding RNAs expressed from the Gtl2 locus are predominantly enriched in fetal liver HSCs and adult long-term HSCs, and these non-coding RNAs sustain long-term HSC functionality.
Gtl2’s megacluster of microRNA suppresses the mTOR signaling pathway and downstream mitochondrial biogenesis and metabolism, thus blocking reactive oxygen species (ROS) that can damage adult stem cells.
When the researchers deleted the Dlk1-Gtl2 locus from the maternally inherited allele in HSCs, they observed increases in mitochondrial biogenesis, metabolic activity, and ROS levels, which led to cell death.
Dr Li said these findings suggest Gtl2 could be used as a biomarker because it could help label dormant (or reserve) stem cells in normal or potentially cancerous stem cell populations.
The addition of a fluorescent tag to the Gtl2 locus could allow researchers to mark other adult stem cells in the gut, hair follicle, muscle, and neural systems. ![]()

in the bone marrow
The Dlk1-Gtl2 locus plays a critical role in protecting hematopoietic stem cells (HSCs), according to preclinical research.
The study suggests the mammalian imprinted gene Gtl2, located on mouse chromosome 12qF1, protects adult HSCs by restricting metabolic activity in the cells’ mitochondria.
This work indicates that Gtl2 may be useful as a biomarker to determine if cells are normal or potentially cancerous.
Linheng Li, PhD, of the Stowers Institute for Medical Research in Kansas City, Missouri, and his colleagues described this research in Cell Stem Cell.
The researchers knew that the Dlk1-Gtl2 locus produces multiple non-coding RNAs from the maternally inherited allele, including the largest microRNA cluster in the mammalian genome.
“Most of the non-coding RNAs at the Gtl2 locus have been documented to function as tumor suppressors to maintain normal cell function,” said study author Pengxu Qian, PhD, also from the Stowers Institute for Medical Research.
However, the role of this locus in HSCs was unclear. So the team studied HSCs in mice. They used transcriptome profiling to analyze 17 hematopoietic cell types.
The analyses revealed that non-coding RNAs expressed from the Gtl2 locus are predominantly enriched in fetal liver HSCs and adult long-term HSCs, and these non-coding RNAs sustain long-term HSC functionality.
Gtl2’s megacluster of microRNA suppresses the mTOR signaling pathway and downstream mitochondrial biogenesis and metabolism, thus blocking reactive oxygen species (ROS) that can damage adult stem cells.
When the researchers deleted the Dlk1-Gtl2 locus from the maternally inherited allele in HSCs, they observed increases in mitochondrial biogenesis, metabolic activity, and ROS levels, which led to cell death.
Dr Li said these findings suggest Gtl2 could be used as a biomarker because it could help label dormant (or reserve) stem cells in normal or potentially cancerous stem cell populations.
The addition of a fluorescent tag to the Gtl2 locus could allow researchers to mark other adult stem cells in the gut, hair follicle, muscle, and neural systems. ![]()
What is the optimal duration of PPI therapy for healing an ulcer?
It depends on the type of ulcer. For Helicobacter pylori-associated peptic ulcers, 7-day treatment with a proton pump inhibitor (PPI) plus 2 antibiotics heals more than 90% of ulcers and is as effective as the same regimen followed by 2 to 4 additional weeks of PPI therapy (strength of recommendation [SOR]: A, meta-analysis of randomized controlled trials [RCTs]).
For peptic ulcers associated with nonsteroidal anti-inflammatory drugs (NSAIDs), 8 weeks of PPI treatment is better than 4 weeks in the case of gastric ulcers, but no more effective than 4 weeks for duodenal ulcers. (SOR: A, meta-analysis of RCTs).
For gastric ulcers resulting from endoscopic submucosal dissection, 4 weeks of PPI therapy is as effective as 8 weeks, but both regimens leave nearly a third of ulcers unhealed (SOR: B, single RCT).
For H pylori ulcers, 7 days of therapy does the trick
A 2005 meta-analysis of 6 RCTs with 862 patients compared 7 days of triple therapy with a PPI and 2 antibiotics with the same regimen followed by 2 to 4 additional weeks of PPI therapy.1 One RCT studied both duodenal and gastric ulcers; the remaining 5 assessed only duodenal ulcers. Investigators included only studies that clearly identified both H pylori eradication and ulcer healing as treatment goals and specified the number of patients treated, the number who experienced successful healing, endoscopic ulcer confirmation, and no concurrent NSAID use.
Triple therapy regimens comprised either omeprazole or esomeprazole 20 mg twice daily plus clarithromycin and either metronidazole, amoxicillin, or tinidazole for 7 days. In all studies, patients randomly assigned to receive an additional 2 to 4 weeks of PPI treatment were given omeprazole 20 mg/d.
Mean ulcer healing rates were 91% (95% confidence interval [CI], 87%-95%) for 7 days of PPI triple therapy compared with 92% (95% CI, 89%-96%) when PPI treatment was extended for an additional 2 to 4 weeks (odds ratio=1.1; 95% CI, 0.71-1.7).
Longer PPI therapy works better for NSAID-associated gastric ulcers
A 1998 meta-analysis examined 2 large RCTs that evaluated healing rates of NSAID-associated ulcers at 4 weeks and 8 weeks in 656 patients with gastric or duodenal ulcers who were treated with omeprazole 20 mg/d or 40 mg/d.2 Patients had ulcers 3 mm or larger or more than 10 erosions in the stomach or duodenum. Gastric ulcers outnumbered duodenal ulcers 2 to 1. Patients had taken continuous therapeutic doses of NSAIDs for at least 5 days per week during 2 weeks in the month preceding PPI therapy; about half were H pylori-positive.
For gastric ulcers, treatment success at 8 weeks was significantly higher at both PPI doses than at 4 weeks. The 208 patients taking the 20-mg dose showed 67% treatment success at 4 weeks and 83% at 8 weeks (P=.001). The 212 patients taking 40 mg had 67% treatment success at 4 weeks and 82% at 8 weeks (P=.002).
Duodenal ulcers showed no difference in healing at 4 and 8 weeks at either PPI dose. The 20-mg dose (116 patients) produced 84% treatment success at 4 weeks compared with 93% at 8 weeks (P=.2), and the 40-mg dose (120 patients) showed 86% treatment success at 4 weeks compared with 88% at 8 weeks (P=.8).
Procedure-induced ulcers respond similarly to 4- and 8-week regimens
A 2014 RCT assessed the effect of 4 and 8 weeks of PPI treatment on healing of gastric ulcers resulting from endoscopic submucosal dissection (ESD), a procedure used to treat early gastric cancer or adenoma that leaves a large ulcer at the site.3 The study randomly assigned 84 patients to treatment with lansoprazole 30 mg/d for 4 or 8 weeks after undergoing ESD. Exclusion criteria included NSAID use or ingestion of mucosal protective agents within 4 weeks of the procedure, illness that might influence PPI effects, history of gastric surgery, and pregnancy or breastfeeding.
All patients underwent endoscopy the day after ESD and again at 8 weeks. Ulcer dimension (mm2) was determined by multiplying the longest diameter by the diameter perpendicular to the longest diameter. The ulcer reduction ratio, an assessment of healing, was determined by dividing the ulcer dimension at 8 weeks after ESD by the initial ulcer dimension.
No significant difference was observed in the 4-week and 8-week groups in terms of ulcer healing (68% vs 69%, respectively; P=.93) or the ulcer reduction ratio (0.0081 vs 0.0037, respectively; P=.15).
1. Gisbert JP, Pajares JM. Systematic review and meta-analysis: is 1-week proton pump inhibitor-based triple therapy sufficient to heal peptic ulcer? Aliment Pharmacol Ther. 2005;21:795-804.
2. Yeomans ND. New data on healing of nonsteroidal anti-inflammatory drug-associated ulcers and erosions. Omeprazole NSAID Steering Committee. Am J Med. 1998;104:56S-61S.
3. Park JH, Baek EK, Choi CH, et al. Comparison of the efficacy of 4- and 8-week lansoprazole treatment for ESD-induced gastric ulcers: a randomized, prospective, controlled study. Surg Endosc. 2014;28:235-241.
It depends on the type of ulcer. For Helicobacter pylori-associated peptic ulcers, 7-day treatment with a proton pump inhibitor (PPI) plus 2 antibiotics heals more than 90% of ulcers and is as effective as the same regimen followed by 2 to 4 additional weeks of PPI therapy (strength of recommendation [SOR]: A, meta-analysis of randomized controlled trials [RCTs]).
For peptic ulcers associated with nonsteroidal anti-inflammatory drugs (NSAIDs), 8 weeks of PPI treatment is better than 4 weeks in the case of gastric ulcers, but no more effective than 4 weeks for duodenal ulcers. (SOR: A, meta-analysis of RCTs).
For gastric ulcers resulting from endoscopic submucosal dissection, 4 weeks of PPI therapy is as effective as 8 weeks, but both regimens leave nearly a third of ulcers unhealed (SOR: B, single RCT).
For H pylori ulcers, 7 days of therapy does the trick
A 2005 meta-analysis of 6 RCTs with 862 patients compared 7 days of triple therapy with a PPI and 2 antibiotics with the same regimen followed by 2 to 4 additional weeks of PPI therapy.1 One RCT studied both duodenal and gastric ulcers; the remaining 5 assessed only duodenal ulcers. Investigators included only studies that clearly identified both H pylori eradication and ulcer healing as treatment goals and specified the number of patients treated, the number who experienced successful healing, endoscopic ulcer confirmation, and no concurrent NSAID use.
Triple therapy regimens comprised either omeprazole or esomeprazole 20 mg twice daily plus clarithromycin and either metronidazole, amoxicillin, or tinidazole for 7 days. In all studies, patients randomly assigned to receive an additional 2 to 4 weeks of PPI treatment were given omeprazole 20 mg/d.
Mean ulcer healing rates were 91% (95% confidence interval [CI], 87%-95%) for 7 days of PPI triple therapy compared with 92% (95% CI, 89%-96%) when PPI treatment was extended for an additional 2 to 4 weeks (odds ratio=1.1; 95% CI, 0.71-1.7).
Longer PPI therapy works better for NSAID-associated gastric ulcers
A 1998 meta-analysis examined 2 large RCTs that evaluated healing rates of NSAID-associated ulcers at 4 weeks and 8 weeks in 656 patients with gastric or duodenal ulcers who were treated with omeprazole 20 mg/d or 40 mg/d.2 Patients had ulcers 3 mm or larger or more than 10 erosions in the stomach or duodenum. Gastric ulcers outnumbered duodenal ulcers 2 to 1. Patients had taken continuous therapeutic doses of NSAIDs for at least 5 days per week during 2 weeks in the month preceding PPI therapy; about half were H pylori-positive.
For gastric ulcers, treatment success at 8 weeks was significantly higher at both PPI doses than at 4 weeks. The 208 patients taking the 20-mg dose showed 67% treatment success at 4 weeks and 83% at 8 weeks (P=.001). The 212 patients taking 40 mg had 67% treatment success at 4 weeks and 82% at 8 weeks (P=.002).
Duodenal ulcers showed no difference in healing at 4 and 8 weeks at either PPI dose. The 20-mg dose (116 patients) produced 84% treatment success at 4 weeks compared with 93% at 8 weeks (P=.2), and the 40-mg dose (120 patients) showed 86% treatment success at 4 weeks compared with 88% at 8 weeks (P=.8).
Procedure-induced ulcers respond similarly to 4- and 8-week regimens
A 2014 RCT assessed the effect of 4 and 8 weeks of PPI treatment on healing of gastric ulcers resulting from endoscopic submucosal dissection (ESD), a procedure used to treat early gastric cancer or adenoma that leaves a large ulcer at the site.3 The study randomly assigned 84 patients to treatment with lansoprazole 30 mg/d for 4 or 8 weeks after undergoing ESD. Exclusion criteria included NSAID use or ingestion of mucosal protective agents within 4 weeks of the procedure, illness that might influence PPI effects, history of gastric surgery, and pregnancy or breastfeeding.
All patients underwent endoscopy the day after ESD and again at 8 weeks. Ulcer dimension (mm2) was determined by multiplying the longest diameter by the diameter perpendicular to the longest diameter. The ulcer reduction ratio, an assessment of healing, was determined by dividing the ulcer dimension at 8 weeks after ESD by the initial ulcer dimension.
No significant difference was observed in the 4-week and 8-week groups in terms of ulcer healing (68% vs 69%, respectively; P=.93) or the ulcer reduction ratio (0.0081 vs 0.0037, respectively; P=.15).
It depends on the type of ulcer. For Helicobacter pylori-associated peptic ulcers, 7-day treatment with a proton pump inhibitor (PPI) plus 2 antibiotics heals more than 90% of ulcers and is as effective as the same regimen followed by 2 to 4 additional weeks of PPI therapy (strength of recommendation [SOR]: A, meta-analysis of randomized controlled trials [RCTs]).
For peptic ulcers associated with nonsteroidal anti-inflammatory drugs (NSAIDs), 8 weeks of PPI treatment is better than 4 weeks in the case of gastric ulcers, but no more effective than 4 weeks for duodenal ulcers. (SOR: A, meta-analysis of RCTs).
For gastric ulcers resulting from endoscopic submucosal dissection, 4 weeks of PPI therapy is as effective as 8 weeks, but both regimens leave nearly a third of ulcers unhealed (SOR: B, single RCT).
For H pylori ulcers, 7 days of therapy does the trick
A 2005 meta-analysis of 6 RCTs with 862 patients compared 7 days of triple therapy with a PPI and 2 antibiotics with the same regimen followed by 2 to 4 additional weeks of PPI therapy.1 One RCT studied both duodenal and gastric ulcers; the remaining 5 assessed only duodenal ulcers. Investigators included only studies that clearly identified both H pylori eradication and ulcer healing as treatment goals and specified the number of patients treated, the number who experienced successful healing, endoscopic ulcer confirmation, and no concurrent NSAID use.
Triple therapy regimens comprised either omeprazole or esomeprazole 20 mg twice daily plus clarithromycin and either metronidazole, amoxicillin, or tinidazole for 7 days. In all studies, patients randomly assigned to receive an additional 2 to 4 weeks of PPI treatment were given omeprazole 20 mg/d.
Mean ulcer healing rates were 91% (95% confidence interval [CI], 87%-95%) for 7 days of PPI triple therapy compared with 92% (95% CI, 89%-96%) when PPI treatment was extended for an additional 2 to 4 weeks (odds ratio=1.1; 95% CI, 0.71-1.7).
Longer PPI therapy works better for NSAID-associated gastric ulcers
A 1998 meta-analysis examined 2 large RCTs that evaluated healing rates of NSAID-associated ulcers at 4 weeks and 8 weeks in 656 patients with gastric or duodenal ulcers who were treated with omeprazole 20 mg/d or 40 mg/d.2 Patients had ulcers 3 mm or larger or more than 10 erosions in the stomach or duodenum. Gastric ulcers outnumbered duodenal ulcers 2 to 1. Patients had taken continuous therapeutic doses of NSAIDs for at least 5 days per week during 2 weeks in the month preceding PPI therapy; about half were H pylori-positive.
For gastric ulcers, treatment success at 8 weeks was significantly higher at both PPI doses than at 4 weeks. The 208 patients taking the 20-mg dose showed 67% treatment success at 4 weeks and 83% at 8 weeks (P=.001). The 212 patients taking 40 mg had 67% treatment success at 4 weeks and 82% at 8 weeks (P=.002).
Duodenal ulcers showed no difference in healing at 4 and 8 weeks at either PPI dose. The 20-mg dose (116 patients) produced 84% treatment success at 4 weeks compared with 93% at 8 weeks (P=.2), and the 40-mg dose (120 patients) showed 86% treatment success at 4 weeks compared with 88% at 8 weeks (P=.8).
Procedure-induced ulcers respond similarly to 4- and 8-week regimens
A 2014 RCT assessed the effect of 4 and 8 weeks of PPI treatment on healing of gastric ulcers resulting from endoscopic submucosal dissection (ESD), a procedure used to treat early gastric cancer or adenoma that leaves a large ulcer at the site.3 The study randomly assigned 84 patients to treatment with lansoprazole 30 mg/d for 4 or 8 weeks after undergoing ESD. Exclusion criteria included NSAID use or ingestion of mucosal protective agents within 4 weeks of the procedure, illness that might influence PPI effects, history of gastric surgery, and pregnancy or breastfeeding.
All patients underwent endoscopy the day after ESD and again at 8 weeks. Ulcer dimension (mm2) was determined by multiplying the longest diameter by the diameter perpendicular to the longest diameter. The ulcer reduction ratio, an assessment of healing, was determined by dividing the ulcer dimension at 8 weeks after ESD by the initial ulcer dimension.
No significant difference was observed in the 4-week and 8-week groups in terms of ulcer healing (68% vs 69%, respectively; P=.93) or the ulcer reduction ratio (0.0081 vs 0.0037, respectively; P=.15).
1. Gisbert JP, Pajares JM. Systematic review and meta-analysis: is 1-week proton pump inhibitor-based triple therapy sufficient to heal peptic ulcer? Aliment Pharmacol Ther. 2005;21:795-804.
2. Yeomans ND. New data on healing of nonsteroidal anti-inflammatory drug-associated ulcers and erosions. Omeprazole NSAID Steering Committee. Am J Med. 1998;104:56S-61S.
3. Park JH, Baek EK, Choi CH, et al. Comparison of the efficacy of 4- and 8-week lansoprazole treatment for ESD-induced gastric ulcers: a randomized, prospective, controlled study. Surg Endosc. 2014;28:235-241.
1. Gisbert JP, Pajares JM. Systematic review and meta-analysis: is 1-week proton pump inhibitor-based triple therapy sufficient to heal peptic ulcer? Aliment Pharmacol Ther. 2005;21:795-804.
2. Yeomans ND. New data on healing of nonsteroidal anti-inflammatory drug-associated ulcers and erosions. Omeprazole NSAID Steering Committee. Am J Med. 1998;104:56S-61S.
3. Park JH, Baek EK, Choi CH, et al. Comparison of the efficacy of 4- and 8-week lansoprazole treatment for ESD-induced gastric ulcers: a randomized, prospective, controlled study. Surg Endosc. 2014;28:235-241.
Evidence-based answers from the Family Physicians Inquiries Network
Is prazosin effective for PTSD-associated nightmares?
Yes. Prazosin has been shown to reduce both frequency and severity of nightmares in patients who meet diagnostic criteria for post-traumatic stress disorder (PTSD) (strength of recommendation: A, systematic review of randomized, controlled trials [RCTs]).
Patients who meet PTSD criteria show best response
A 2012 systematic review of prazosin (1-16 mg) for PTSD included 21 studies (4 RCTs, 4 open-label case series, 4 retrospective case series, and 9 case reports) with 285 patients, 85% of whom were combat veterans.1 All the studies were limited by small sample sizes and a lack of demographic diversity.
To measure prazosin’s effect on nightmares, the studies used the Clinician-Administered PTSD Scale (CAPS-B2), scored from 0 to 8, which sums the frequency of nightmares (0=none in the past week, 4=daily nightmares) and the intensity of distressing dreams (0=none, 4=incapacitating distress).
The 3 highest-quality RCTs used similar methods and included only 63 patients who met diagnostic criteria for PTSD. Each found statistically significant reductions in nightmares among patients taking prazosin compared with placebo (CAPS-B2 improvements of 3.3, 3.3, and 1.5 for prazosin vs 0.4, 0.9, and 0 for placebo; P<.05 for all comparisons).
In the fourth RCT, comprised of 50 patients, only 58% of participants met full clinical diagnostic criteria for PTSD. The primary outcome was the number of recalled nightmares, which didn’t show a statistically significant decrease in the prazosin group compared with placebo (decrease in mean weekly nightmares of 0.7 with prazosin vs an increase of 0.1 with placebo).
Prazosin provides significant relief in small study of combat veterans
A 2013 RCT evaluated the effect of prazosin on nightmares in 67 soldiers with combat PTSD.2 All patients met criteria for PTSD as outlined in the Diagnostic and Statistical Manual of Mental Disorders, 4th edition. Men received doses titrated to a mean of 4 mg in the morning and 15.6 mg at bedtime; women received a mean of 1.7 mg in the morning and 7 mg at bedtime.
After 15 weeks, the CAPS-B2 score decreased by 3.1 for prazosin compared with 1.2 for placebo (P<.05).
1. Kung S, Espinel Z, Lapid M. Treatment of nightmares with prazosin: a systematic review. Mayo Clin Proc. 2012;87:890-900.
2. Raskind MA, Peterson K, Williams T, et al. A trial of prazosin for combat trauma PTSD with nightmares in active-duty soldiers returned from Iraq and Afghanistan. Am J Psychiatry. 2013;170:1003-1010.
Yes. Prazosin has been shown to reduce both frequency and severity of nightmares in patients who meet diagnostic criteria for post-traumatic stress disorder (PTSD) (strength of recommendation: A, systematic review of randomized, controlled trials [RCTs]).
Patients who meet PTSD criteria show best response
A 2012 systematic review of prazosin (1-16 mg) for PTSD included 21 studies (4 RCTs, 4 open-label case series, 4 retrospective case series, and 9 case reports) with 285 patients, 85% of whom were combat veterans.1 All the studies were limited by small sample sizes and a lack of demographic diversity.
To measure prazosin’s effect on nightmares, the studies used the Clinician-Administered PTSD Scale (CAPS-B2), scored from 0 to 8, which sums the frequency of nightmares (0=none in the past week, 4=daily nightmares) and the intensity of distressing dreams (0=none, 4=incapacitating distress).
The 3 highest-quality RCTs used similar methods and included only 63 patients who met diagnostic criteria for PTSD. Each found statistically significant reductions in nightmares among patients taking prazosin compared with placebo (CAPS-B2 improvements of 3.3, 3.3, and 1.5 for prazosin vs 0.4, 0.9, and 0 for placebo; P<.05 for all comparisons).
In the fourth RCT, comprised of 50 patients, only 58% of participants met full clinical diagnostic criteria for PTSD. The primary outcome was the number of recalled nightmares, which didn’t show a statistically significant decrease in the prazosin group compared with placebo (decrease in mean weekly nightmares of 0.7 with prazosin vs an increase of 0.1 with placebo).
Prazosin provides significant relief in small study of combat veterans
A 2013 RCT evaluated the effect of prazosin on nightmares in 67 soldiers with combat PTSD.2 All patients met criteria for PTSD as outlined in the Diagnostic and Statistical Manual of Mental Disorders, 4th edition. Men received doses titrated to a mean of 4 mg in the morning and 15.6 mg at bedtime; women received a mean of 1.7 mg in the morning and 7 mg at bedtime.
After 15 weeks, the CAPS-B2 score decreased by 3.1 for prazosin compared with 1.2 for placebo (P<.05).
Yes. Prazosin has been shown to reduce both frequency and severity of nightmares in patients who meet diagnostic criteria for post-traumatic stress disorder (PTSD) (strength of recommendation: A, systematic review of randomized, controlled trials [RCTs]).
Patients who meet PTSD criteria show best response
A 2012 systematic review of prazosin (1-16 mg) for PTSD included 21 studies (4 RCTs, 4 open-label case series, 4 retrospective case series, and 9 case reports) with 285 patients, 85% of whom were combat veterans.1 All the studies were limited by small sample sizes and a lack of demographic diversity.
To measure prazosin’s effect on nightmares, the studies used the Clinician-Administered PTSD Scale (CAPS-B2), scored from 0 to 8, which sums the frequency of nightmares (0=none in the past week, 4=daily nightmares) and the intensity of distressing dreams (0=none, 4=incapacitating distress).
The 3 highest-quality RCTs used similar methods and included only 63 patients who met diagnostic criteria for PTSD. Each found statistically significant reductions in nightmares among patients taking prazosin compared with placebo (CAPS-B2 improvements of 3.3, 3.3, and 1.5 for prazosin vs 0.4, 0.9, and 0 for placebo; P<.05 for all comparisons).
In the fourth RCT, comprised of 50 patients, only 58% of participants met full clinical diagnostic criteria for PTSD. The primary outcome was the number of recalled nightmares, which didn’t show a statistically significant decrease in the prazosin group compared with placebo (decrease in mean weekly nightmares of 0.7 with prazosin vs an increase of 0.1 with placebo).
Prazosin provides significant relief in small study of combat veterans
A 2013 RCT evaluated the effect of prazosin on nightmares in 67 soldiers with combat PTSD.2 All patients met criteria for PTSD as outlined in the Diagnostic and Statistical Manual of Mental Disorders, 4th edition. Men received doses titrated to a mean of 4 mg in the morning and 15.6 mg at bedtime; women received a mean of 1.7 mg in the morning and 7 mg at bedtime.
After 15 weeks, the CAPS-B2 score decreased by 3.1 for prazosin compared with 1.2 for placebo (P<.05).
1. Kung S, Espinel Z, Lapid M. Treatment of nightmares with prazosin: a systematic review. Mayo Clin Proc. 2012;87:890-900.
2. Raskind MA, Peterson K, Williams T, et al. A trial of prazosin for combat trauma PTSD with nightmares in active-duty soldiers returned from Iraq and Afghanistan. Am J Psychiatry. 2013;170:1003-1010.
1. Kung S, Espinel Z, Lapid M. Treatment of nightmares with prazosin: a systematic review. Mayo Clin Proc. 2012;87:890-900.
2. Raskind MA, Peterson K, Williams T, et al. A trial of prazosin for combat trauma PTSD with nightmares in active-duty soldiers returned from Iraq and Afghanistan. Am J Psychiatry. 2013;170:1003-1010.
Evidence-based answers from the Family Physicians Inquiries Network
Should you bypass anticoagulant “bridging” before and after surgery?
Stop using low molecular weight heparin (LMWH) for surgical procedures to “bridge” low- to moderate-risk patients with atrial fibrillation (CHADS2 score ≤4) who are receiving warfarin. The risks outweigh the benefits.1
Strength of recommendation
B: Based on a single good-quality randomized control trial.
Douketis JD, Spyropoulos AC, Kaatz S, et al. Perioperative bridging anticoagulation in patients with atrial fibrillation. N Engl J Med. 2015;373:823-833.
Illustrative case
A 75-year-old man comes to your office for surgical clearance before right knee replacement surgery. He has diabetes and high blood pressure, and is taking warfarin for atrial fibrillation. He is scheduled for surgery in a week. What is the safest way to manage his warfarin in the perioperative period?
More than 2 million people are being treated with oral anticoagulation in North America to prevent stroke, or to prevent or treat venous thromboembolism.2 Since 2010, several new oral anticoagulants have been approved, including dabigatran, apixaban, and rivaroxaban. These new medications have a shorter half-life than older anticoagulants, which enables them to be stopped 1 to 2 days before surgery.
On the other hand, warfarin—which remains a common choice for anticoagulation—has a 3- to 7-day onset and elimination.3,4 This long clinical half-life presents a special challenge during the perioperative period. To reduce the risk of operative bleeding, the warfarin must be stopped days prior to the procedure, but physicians often worry that this will increase the risk of arterial or venous thromboembolism, including stroke.
An estimated 250,000 patients need perioperative management of their anticoagulation each year.5 As the US population continues to age and the incidence of conditions requiring anticoagulation (particularly atrial fibrillation) increases, this number is only going to rise.6
Current guidelines on bridging. American College of Chest Physicians (ACCP) guidelines recommend transition to “a short-acting anticoagulant, consisting of subcutaneous low molecular weight heparin (LMWH) or intravenous unfractionated heparin, for a 10- to 12-day period during interruption of vitamin K antagonist (VKA) therapy.”5 Furthermore, for an appropriate bridging regimen, the ACCP guidelines recommend stopping VKA therapy 5 days prior to the procedure and utilizing LMWH from within 24 to 48 hours of stopping VKA therapy until up to 24 hours before surgery.5 Postoperatively, VKA or LMWH therapy should be reinitiated within 24 hours and 24 to 72 hours, respectively, depending on the patient’s risk of bleeding during surgery.5
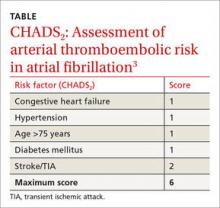
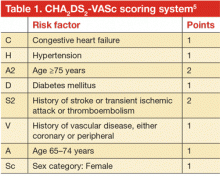
These guidelines recommend using CHADS2 scoring (TABLE3) to determine arterial thromboembolism (ATE) risk in atrial fibrillation.3,5 Patients at low risk for ATE (CHADS2 score 0-2) should not be bridged, and patients at high risk (CHADS2 score of 5-6) should always be bridged.5 These guidelines are less clear about bridging recommendations for moderate-risk patients (CHADS2 score 3-4).
Previous evidence on bridging. A 2012 meta-analysis of 34 studies evaluated the safety and efficacy of perioperative bridging with heparin in patients receiving VKA.7 Researchers found no difference in ATE events in 8 studies that compared groups that received bridging vs groups that simply stopped anticoagulation (odds ratio [OR]=0.80; 95% confidence interval [CI], 0.42–1.54).7 The group that received bridging had an increased risk of overall bleeding in 13 studies, and of major bleeding in 5 studies.7 This meta-analysis was limited by poor study quality and variation in the indication for VKA therapy.
A 2015 subgroup analysis of a larger cohort study of patients receiving anticoagulants for atrial fibrillation found an increased risk of bleeding when their anticoagulation was interrupted for procedures (OR for major bleeding=3.84; 95% CI, 2.07-7.14; P<.0001).8
Douketis et al1 conducted a randomized trial to clarify the need for and safety of bridging anticoagulation for ATE in patients with atrial fibrillation who were receiving warfarin.
STUDY SUMMARY: When it comes to stroke/TIA, there’s no advantage to bridging
This double blind, placebo-controlled trial compared bridging with dalteparin, a form of LMWH, to placebo among 1884 patients with atrial fibrillation on warfarin whose anticoagulation therapy needed to be interrupted for an elective procedure. Patients were included if they were receiving warfarin to prevent stroke, and had been on warfarin for at least 12 weeks, with a goal international normalized ratio (INR) of 2.0 to 3.0. Exclusion criteria included having a mechanical heart valve or having a stroke/transient ischemic attack (TIA; 12 weeks prior) or major bleeding (6 weeks prior). Cardiac, intracranial, and intraspinal surgeries were also excluded from the study.
The patients’ mean CHADS2 score was 2.3; 38.3% of patients had a CHADS2 score ≥3, and 9.4% of patients had a history of stroke. Forty-four percent of patients underwent a gastrointestinal procedure, 17.2% underwent a cardiothoracic procedure, and 9.2% underwent an orthopedic procedure.
Patients stopped taking warfarin 5 days before their procedure, and began subcutaneous dalteparin, 100 IU/kg, or an identical placebo 3 days before the procedure. The dalteparin/placebo was stopped 24 hours before the procedure and restarted after the procedure, until the patient’s INR was in the therapeutic range. Warfarin was resumed on the evening of the procedure or the following day.
The primary efficacy outcome was ATE, including stroke, TIA, or systemic embolism. The primary safety endpoint was major bleeding (defined as bleeding at a critical anatomic site, symptomatic or clinically overt bleeding, or a decrease in hemoglobin >2 g/dL). Secondary efficacy and safety outcomes included minor bleeding, acute myocardial infarction, deep vein thrombosis, pulmonary embolism, and death. Outcomes were assessed within 37 days of the procedure.
The incidence of ATE was 0.4% (4 events) in the no-bridging group vs 0.3% (3 events) in the bridging group (95% CI, -0.6 to 0.8; P=.01 for non-inferiority; P=.73 for superiority). Major bleeding occurred in 1.3% of the no-bridging group (12 events) and in 3.2% of the bridging group (29 events), indicating that no bridging was superior in terms of the major bleeding outcome (number needed to harm [NNH]=53; relative risk [RR]=0.41; 95% CI, 0.20-0.78; P=.005). The no-bridging group also had significantly fewer minor bleeds in comparison to the bridging group (NNH=11; 12% vs 20.9%; P<.001). There were no differences between groups in other secondary outcomes.
WHAT'S NEW: High-quality evidence suggests it’s OK to stop warfarin before surgery
This is the largest good-quality study to evaluate perioperative bridging in patients with atrial fibrillation who were at low or moderate risk for ATE (CHADS2 score 0-4). Previous studies suggested bridging increased bleeding and offered limited benefit for reducing the risk of ATE. However, this is the first study to include a large group of moderate-risk patients (CHADS2 score 3-4). This trial provides high-quality evidence to support the practice of simply stopping warfarin in the perioperative period, rather than bridging with LMWH.
CAVEATS: Findings might not apply to patients at highest risk
Most patients in this study had a CHADS2 score ≤3. About 3% had a CHADS2 score ≥5 or higher. It’s not clear whether these findings apply to patients with a CHADS2 score of 5 or 6.
This trial categorized ATE risk using the CHADS2 score, rather than the CHA2DS2-VASc, which includes additional risk factors and may more accurately predict stroke risk. Both patients who received bridging therapy and those who did not had a lower rate of stroke than predicted by CHADs2. This may reflect a limit of the predictive value of CHADS2, but should not have affected the rate of bleeding or ATE outcomes in this study.
CHALLENGES TO IMPLEMENTATION: Physicians may hesitate to disregard current guidelines
Strokes are devastating events for patients, families, and physicians, and they pose a greater risk of morbidity and mortality compared to bleeding. However, this study suggests patients who receive bridging have a higher risk of bleeding than stroke, which is in contrast to some physicians’ experience and current recommendations.
A physician caring for a patient who’s had a stroke may be inclined to recommend bridging despite the lack of efficacy and evidence of bleeding risk. Additionally, until guidelines reflect the most current research, physicians may be reluctant to provide care in contrast to these recommendations.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
1. Douketis JD, Spyropoulos AC, Kaatz S, et al. Perioperative bridging anticoagulation in patients with atrial fibrillation. N Engl J Med. 2015;373:823-833.
2. Guyatt GH, Akl EA, Crowther M, et al; American College of Chest Physicians Antithrombotic Therapy and Prevention of Thrombosis Panel. Executive summary: Antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2012;141:7S-47S.
3. Clark NP, Witt DM, Davies LE, et al. Bleeding, recurrent venous thromboembolism and mortality risks during warfarin interruption for invasive procedures. JAMA Intern Med. 2015;175:1163-1168.
4. Lip GY, Lane DA. Stroke prevention in atrial fibrillation: a systematic review. JAMA. 2015;313:1950-1962.
5. Douketis JD, Spyropoulos AC, Spencer FA, et al; American College of Chest Physicians. Perioperative management of antithrombotic therapy: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2012;141:e326S-e350S.
6. Miyasaka Y, Barnes ME, Gersh BJ, et al. Secular trends in incidence of atrial fibrillation in Olmsted County, Minnesota, 1980 to 2000, and implications on the projections for future prevalence. Circulation. 2006;114:119-125.
7. Siegal, D, Yudin J, Kaatz S, et al. Periprocedural heparin bridging in patients receiving vitamin k antagonists: systematic review and meta-analysis of bleeding and thromboembolic rates. Circulation. 2012;126:1630-1639.
8. Steinberg B, Peterson E, Kim S, et al; Outcomes Registry for Better Informed Treatment of Atrial Fibrillation Investigators and Patients. Use and outcomes associated with bridging during anticoagulation interruptions in patients with atrial fibrillation: findings from the Outcomes Registry for Better Informed Treatment of Atrial Fibrillation (ORBIT-AF). Circulation. 2015;131:488-494.
Stop using low molecular weight heparin (LMWH) for surgical procedures to “bridge” low- to moderate-risk patients with atrial fibrillation (CHADS2 score ≤4) who are receiving warfarin. The risks outweigh the benefits.1
Strength of recommendation
B: Based on a single good-quality randomized control trial.
Douketis JD, Spyropoulos AC, Kaatz S, et al. Perioperative bridging anticoagulation in patients with atrial fibrillation. N Engl J Med. 2015;373:823-833.
Illustrative case
A 75-year-old man comes to your office for surgical clearance before right knee replacement surgery. He has diabetes and high blood pressure, and is taking warfarin for atrial fibrillation. He is scheduled for surgery in a week. What is the safest way to manage his warfarin in the perioperative period?
More than 2 million people are being treated with oral anticoagulation in North America to prevent stroke, or to prevent or treat venous thromboembolism.2 Since 2010, several new oral anticoagulants have been approved, including dabigatran, apixaban, and rivaroxaban. These new medications have a shorter half-life than older anticoagulants, which enables them to be stopped 1 to 2 days before surgery.
On the other hand, warfarin—which remains a common choice for anticoagulation—has a 3- to 7-day onset and elimination.3,4 This long clinical half-life presents a special challenge during the perioperative period. To reduce the risk of operative bleeding, the warfarin must be stopped days prior to the procedure, but physicians often worry that this will increase the risk of arterial or venous thromboembolism, including stroke.
An estimated 250,000 patients need perioperative management of their anticoagulation each year.5 As the US population continues to age and the incidence of conditions requiring anticoagulation (particularly atrial fibrillation) increases, this number is only going to rise.6
Current guidelines on bridging. American College of Chest Physicians (ACCP) guidelines recommend transition to “a short-acting anticoagulant, consisting of subcutaneous low molecular weight heparin (LMWH) or intravenous unfractionated heparin, for a 10- to 12-day period during interruption of vitamin K antagonist (VKA) therapy.”5 Furthermore, for an appropriate bridging regimen, the ACCP guidelines recommend stopping VKA therapy 5 days prior to the procedure and utilizing LMWH from within 24 to 48 hours of stopping VKA therapy until up to 24 hours before surgery.5 Postoperatively, VKA or LMWH therapy should be reinitiated within 24 hours and 24 to 72 hours, respectively, depending on the patient’s risk of bleeding during surgery.5
These guidelines recommend using CHADS2 scoring (TABLE3) to determine arterial thromboembolism (ATE) risk in atrial fibrillation.3,5 Patients at low risk for ATE (CHADS2 score 0-2) should not be bridged, and patients at high risk (CHADS2 score of 5-6) should always be bridged.5 These guidelines are less clear about bridging recommendations for moderate-risk patients (CHADS2 score 3-4).
Previous evidence on bridging. A 2012 meta-analysis of 34 studies evaluated the safety and efficacy of perioperative bridging with heparin in patients receiving VKA.7 Researchers found no difference in ATE events in 8 studies that compared groups that received bridging vs groups that simply stopped anticoagulation (odds ratio [OR]=0.80; 95% confidence interval [CI], 0.42–1.54).7 The group that received bridging had an increased risk of overall bleeding in 13 studies, and of major bleeding in 5 studies.7 This meta-analysis was limited by poor study quality and variation in the indication for VKA therapy.
A 2015 subgroup analysis of a larger cohort study of patients receiving anticoagulants for atrial fibrillation found an increased risk of bleeding when their anticoagulation was interrupted for procedures (OR for major bleeding=3.84; 95% CI, 2.07-7.14; P<.0001).8
Douketis et al1 conducted a randomized trial to clarify the need for and safety of bridging anticoagulation for ATE in patients with atrial fibrillation who were receiving warfarin.
STUDY SUMMARY: When it comes to stroke/TIA, there’s no advantage to bridging
This double blind, placebo-controlled trial compared bridging with dalteparin, a form of LMWH, to placebo among 1884 patients with atrial fibrillation on warfarin whose anticoagulation therapy needed to be interrupted for an elective procedure. Patients were included if they were receiving warfarin to prevent stroke, and had been on warfarin for at least 12 weeks, with a goal international normalized ratio (INR) of 2.0 to 3.0. Exclusion criteria included having a mechanical heart valve or having a stroke/transient ischemic attack (TIA; 12 weeks prior) or major bleeding (6 weeks prior). Cardiac, intracranial, and intraspinal surgeries were also excluded from the study.
The patients’ mean CHADS2 score was 2.3; 38.3% of patients had a CHADS2 score ≥3, and 9.4% of patients had a history of stroke. Forty-four percent of patients underwent a gastrointestinal procedure, 17.2% underwent a cardiothoracic procedure, and 9.2% underwent an orthopedic procedure.
Patients stopped taking warfarin 5 days before their procedure, and began subcutaneous dalteparin, 100 IU/kg, or an identical placebo 3 days before the procedure. The dalteparin/placebo was stopped 24 hours before the procedure and restarted after the procedure, until the patient’s INR was in the therapeutic range. Warfarin was resumed on the evening of the procedure or the following day.
The primary efficacy outcome was ATE, including stroke, TIA, or systemic embolism. The primary safety endpoint was major bleeding (defined as bleeding at a critical anatomic site, symptomatic or clinically overt bleeding, or a decrease in hemoglobin >2 g/dL). Secondary efficacy and safety outcomes included minor bleeding, acute myocardial infarction, deep vein thrombosis, pulmonary embolism, and death. Outcomes were assessed within 37 days of the procedure.
The incidence of ATE was 0.4% (4 events) in the no-bridging group vs 0.3% (3 events) in the bridging group (95% CI, -0.6 to 0.8; P=.01 for non-inferiority; P=.73 for superiority). Major bleeding occurred in 1.3% of the no-bridging group (12 events) and in 3.2% of the bridging group (29 events), indicating that no bridging was superior in terms of the major bleeding outcome (number needed to harm [NNH]=53; relative risk [RR]=0.41; 95% CI, 0.20-0.78; P=.005). The no-bridging group also had significantly fewer minor bleeds in comparison to the bridging group (NNH=11; 12% vs 20.9%; P<.001). There were no differences between groups in other secondary outcomes.
WHAT'S NEW: High-quality evidence suggests it’s OK to stop warfarin before surgery
This is the largest good-quality study to evaluate perioperative bridging in patients with atrial fibrillation who were at low or moderate risk for ATE (CHADS2 score 0-4). Previous studies suggested bridging increased bleeding and offered limited benefit for reducing the risk of ATE. However, this is the first study to include a large group of moderate-risk patients (CHADS2 score 3-4). This trial provides high-quality evidence to support the practice of simply stopping warfarin in the perioperative period, rather than bridging with LMWH.
CAVEATS: Findings might not apply to patients at highest risk
Most patients in this study had a CHADS2 score ≤3. About 3% had a CHADS2 score ≥5 or higher. It’s not clear whether these findings apply to patients with a CHADS2 score of 5 or 6.
This trial categorized ATE risk using the CHADS2 score, rather than the CHA2DS2-VASc, which includes additional risk factors and may more accurately predict stroke risk. Both patients who received bridging therapy and those who did not had a lower rate of stroke than predicted by CHADs2. This may reflect a limit of the predictive value of CHADS2, but should not have affected the rate of bleeding or ATE outcomes in this study.
CHALLENGES TO IMPLEMENTATION: Physicians may hesitate to disregard current guidelines
Strokes are devastating events for patients, families, and physicians, and they pose a greater risk of morbidity and mortality compared to bleeding. However, this study suggests patients who receive bridging have a higher risk of bleeding than stroke, which is in contrast to some physicians’ experience and current recommendations.
A physician caring for a patient who’s had a stroke may be inclined to recommend bridging despite the lack of efficacy and evidence of bleeding risk. Additionally, until guidelines reflect the most current research, physicians may be reluctant to provide care in contrast to these recommendations.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
Stop using low molecular weight heparin (LMWH) for surgical procedures to “bridge” low- to moderate-risk patients with atrial fibrillation (CHADS2 score ≤4) who are receiving warfarin. The risks outweigh the benefits.1
Strength of recommendation
B: Based on a single good-quality randomized control trial.
Douketis JD, Spyropoulos AC, Kaatz S, et al. Perioperative bridging anticoagulation in patients with atrial fibrillation. N Engl J Med. 2015;373:823-833.
Illustrative case
A 75-year-old man comes to your office for surgical clearance before right knee replacement surgery. He has diabetes and high blood pressure, and is taking warfarin for atrial fibrillation. He is scheduled for surgery in a week. What is the safest way to manage his warfarin in the perioperative period?
More than 2 million people are being treated with oral anticoagulation in North America to prevent stroke, or to prevent or treat venous thromboembolism.2 Since 2010, several new oral anticoagulants have been approved, including dabigatran, apixaban, and rivaroxaban. These new medications have a shorter half-life than older anticoagulants, which enables them to be stopped 1 to 2 days before surgery.
On the other hand, warfarin—which remains a common choice for anticoagulation—has a 3- to 7-day onset and elimination.3,4 This long clinical half-life presents a special challenge during the perioperative period. To reduce the risk of operative bleeding, the warfarin must be stopped days prior to the procedure, but physicians often worry that this will increase the risk of arterial or venous thromboembolism, including stroke.
An estimated 250,000 patients need perioperative management of their anticoagulation each year.5 As the US population continues to age and the incidence of conditions requiring anticoagulation (particularly atrial fibrillation) increases, this number is only going to rise.6
Current guidelines on bridging. American College of Chest Physicians (ACCP) guidelines recommend transition to “a short-acting anticoagulant, consisting of subcutaneous low molecular weight heparin (LMWH) or intravenous unfractionated heparin, for a 10- to 12-day period during interruption of vitamin K antagonist (VKA) therapy.”5 Furthermore, for an appropriate bridging regimen, the ACCP guidelines recommend stopping VKA therapy 5 days prior to the procedure and utilizing LMWH from within 24 to 48 hours of stopping VKA therapy until up to 24 hours before surgery.5 Postoperatively, VKA or LMWH therapy should be reinitiated within 24 hours and 24 to 72 hours, respectively, depending on the patient’s risk of bleeding during surgery.5
These guidelines recommend using CHADS2 scoring (TABLE3) to determine arterial thromboembolism (ATE) risk in atrial fibrillation.3,5 Patients at low risk for ATE (CHADS2 score 0-2) should not be bridged, and patients at high risk (CHADS2 score of 5-6) should always be bridged.5 These guidelines are less clear about bridging recommendations for moderate-risk patients (CHADS2 score 3-4).
Previous evidence on bridging. A 2012 meta-analysis of 34 studies evaluated the safety and efficacy of perioperative bridging with heparin in patients receiving VKA.7 Researchers found no difference in ATE events in 8 studies that compared groups that received bridging vs groups that simply stopped anticoagulation (odds ratio [OR]=0.80; 95% confidence interval [CI], 0.42–1.54).7 The group that received bridging had an increased risk of overall bleeding in 13 studies, and of major bleeding in 5 studies.7 This meta-analysis was limited by poor study quality and variation in the indication for VKA therapy.
A 2015 subgroup analysis of a larger cohort study of patients receiving anticoagulants for atrial fibrillation found an increased risk of bleeding when their anticoagulation was interrupted for procedures (OR for major bleeding=3.84; 95% CI, 2.07-7.14; P<.0001).8
Douketis et al1 conducted a randomized trial to clarify the need for and safety of bridging anticoagulation for ATE in patients with atrial fibrillation who were receiving warfarin.
STUDY SUMMARY: When it comes to stroke/TIA, there’s no advantage to bridging
This double blind, placebo-controlled trial compared bridging with dalteparin, a form of LMWH, to placebo among 1884 patients with atrial fibrillation on warfarin whose anticoagulation therapy needed to be interrupted for an elective procedure. Patients were included if they were receiving warfarin to prevent stroke, and had been on warfarin for at least 12 weeks, with a goal international normalized ratio (INR) of 2.0 to 3.0. Exclusion criteria included having a mechanical heart valve or having a stroke/transient ischemic attack (TIA; 12 weeks prior) or major bleeding (6 weeks prior). Cardiac, intracranial, and intraspinal surgeries were also excluded from the study.
The patients’ mean CHADS2 score was 2.3; 38.3% of patients had a CHADS2 score ≥3, and 9.4% of patients had a history of stroke. Forty-four percent of patients underwent a gastrointestinal procedure, 17.2% underwent a cardiothoracic procedure, and 9.2% underwent an orthopedic procedure.
Patients stopped taking warfarin 5 days before their procedure, and began subcutaneous dalteparin, 100 IU/kg, or an identical placebo 3 days before the procedure. The dalteparin/placebo was stopped 24 hours before the procedure and restarted after the procedure, until the patient’s INR was in the therapeutic range. Warfarin was resumed on the evening of the procedure or the following day.
The primary efficacy outcome was ATE, including stroke, TIA, or systemic embolism. The primary safety endpoint was major bleeding (defined as bleeding at a critical anatomic site, symptomatic or clinically overt bleeding, or a decrease in hemoglobin >2 g/dL). Secondary efficacy and safety outcomes included minor bleeding, acute myocardial infarction, deep vein thrombosis, pulmonary embolism, and death. Outcomes were assessed within 37 days of the procedure.
The incidence of ATE was 0.4% (4 events) in the no-bridging group vs 0.3% (3 events) in the bridging group (95% CI, -0.6 to 0.8; P=.01 for non-inferiority; P=.73 for superiority). Major bleeding occurred in 1.3% of the no-bridging group (12 events) and in 3.2% of the bridging group (29 events), indicating that no bridging was superior in terms of the major bleeding outcome (number needed to harm [NNH]=53; relative risk [RR]=0.41; 95% CI, 0.20-0.78; P=.005). The no-bridging group also had significantly fewer minor bleeds in comparison to the bridging group (NNH=11; 12% vs 20.9%; P<.001). There were no differences between groups in other secondary outcomes.
WHAT'S NEW: High-quality evidence suggests it’s OK to stop warfarin before surgery
This is the largest good-quality study to evaluate perioperative bridging in patients with atrial fibrillation who were at low or moderate risk for ATE (CHADS2 score 0-4). Previous studies suggested bridging increased bleeding and offered limited benefit for reducing the risk of ATE. However, this is the first study to include a large group of moderate-risk patients (CHADS2 score 3-4). This trial provides high-quality evidence to support the practice of simply stopping warfarin in the perioperative period, rather than bridging with LMWH.
CAVEATS: Findings might not apply to patients at highest risk
Most patients in this study had a CHADS2 score ≤3. About 3% had a CHADS2 score ≥5 or higher. It’s not clear whether these findings apply to patients with a CHADS2 score of 5 or 6.
This trial categorized ATE risk using the CHADS2 score, rather than the CHA2DS2-VASc, which includes additional risk factors and may more accurately predict stroke risk. Both patients who received bridging therapy and those who did not had a lower rate of stroke than predicted by CHADs2. This may reflect a limit of the predictive value of CHADS2, but should not have affected the rate of bleeding or ATE outcomes in this study.
CHALLENGES TO IMPLEMENTATION: Physicians may hesitate to disregard current guidelines
Strokes are devastating events for patients, families, and physicians, and they pose a greater risk of morbidity and mortality compared to bleeding. However, this study suggests patients who receive bridging have a higher risk of bleeding than stroke, which is in contrast to some physicians’ experience and current recommendations.
A physician caring for a patient who’s had a stroke may be inclined to recommend bridging despite the lack of efficacy and evidence of bleeding risk. Additionally, until guidelines reflect the most current research, physicians may be reluctant to provide care in contrast to these recommendations.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
1. Douketis JD, Spyropoulos AC, Kaatz S, et al. Perioperative bridging anticoagulation in patients with atrial fibrillation. N Engl J Med. 2015;373:823-833.
2. Guyatt GH, Akl EA, Crowther M, et al; American College of Chest Physicians Antithrombotic Therapy and Prevention of Thrombosis Panel. Executive summary: Antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2012;141:7S-47S.
3. Clark NP, Witt DM, Davies LE, et al. Bleeding, recurrent venous thromboembolism and mortality risks during warfarin interruption for invasive procedures. JAMA Intern Med. 2015;175:1163-1168.
4. Lip GY, Lane DA. Stroke prevention in atrial fibrillation: a systematic review. JAMA. 2015;313:1950-1962.
5. Douketis JD, Spyropoulos AC, Spencer FA, et al; American College of Chest Physicians. Perioperative management of antithrombotic therapy: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2012;141:e326S-e350S.
6. Miyasaka Y, Barnes ME, Gersh BJ, et al. Secular trends in incidence of atrial fibrillation in Olmsted County, Minnesota, 1980 to 2000, and implications on the projections for future prevalence. Circulation. 2006;114:119-125.
7. Siegal, D, Yudin J, Kaatz S, et al. Periprocedural heparin bridging in patients receiving vitamin k antagonists: systematic review and meta-analysis of bleeding and thromboembolic rates. Circulation. 2012;126:1630-1639.
8. Steinberg B, Peterson E, Kim S, et al; Outcomes Registry for Better Informed Treatment of Atrial Fibrillation Investigators and Patients. Use and outcomes associated with bridging during anticoagulation interruptions in patients with atrial fibrillation: findings from the Outcomes Registry for Better Informed Treatment of Atrial Fibrillation (ORBIT-AF). Circulation. 2015;131:488-494.
1. Douketis JD, Spyropoulos AC, Kaatz S, et al. Perioperative bridging anticoagulation in patients with atrial fibrillation. N Engl J Med. 2015;373:823-833.
2. Guyatt GH, Akl EA, Crowther M, et al; American College of Chest Physicians Antithrombotic Therapy and Prevention of Thrombosis Panel. Executive summary: Antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2012;141:7S-47S.
3. Clark NP, Witt DM, Davies LE, et al. Bleeding, recurrent venous thromboembolism and mortality risks during warfarin interruption for invasive procedures. JAMA Intern Med. 2015;175:1163-1168.
4. Lip GY, Lane DA. Stroke prevention in atrial fibrillation: a systematic review. JAMA. 2015;313:1950-1962.
5. Douketis JD, Spyropoulos AC, Spencer FA, et al; American College of Chest Physicians. Perioperative management of antithrombotic therapy: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2012;141:e326S-e350S.
6. Miyasaka Y, Barnes ME, Gersh BJ, et al. Secular trends in incidence of atrial fibrillation in Olmsted County, Minnesota, 1980 to 2000, and implications on the projections for future prevalence. Circulation. 2006;114:119-125.
7. Siegal, D, Yudin J, Kaatz S, et al. Periprocedural heparin bridging in patients receiving vitamin k antagonists: systematic review and meta-analysis of bleeding and thromboembolic rates. Circulation. 2012;126:1630-1639.
8. Steinberg B, Peterson E, Kim S, et al; Outcomes Registry for Better Informed Treatment of Atrial Fibrillation Investigators and Patients. Use and outcomes associated with bridging during anticoagulation interruptions in patients with atrial fibrillation: findings from the Outcomes Registry for Better Informed Treatment of Atrial Fibrillation (ORBIT-AF). Circulation. 2015;131:488-494.
Copyright © 2015. The Family Physicians Inquiries Network. All rights reserved.
ACIP weighs in on meningococcal B vaccines
The Advisory Committee on Immunization Practices (ACIP) voted at its June 2015 meeting to make a “B” recommendation for the use of meningococcal B vaccine for individuals 16 through 23 years of age. The Committee felt that the vaccine can be used if one desires it, but at this time it should not be included in the category of a routinely recommended vaccine.
Meningococcal meningitis caused by serogroup B is a serious disease, but it is rare. From 2009 to 2013, the annual number of meningococcal B cases in individuals ages 11 to 24 years ranged from 54 to 67, with 5 to 10 deaths and 5 to 13 serious sequelae.1 Since 2009, there have been outbreaks on 7 university campuses with cases-per-outbreak numbering 2 to 13.1 These well publicized outbreaks created much disruption and an impression of increased risk among college students. But the surveillance system of the Centers for Disease Control and Prevention (CDC) demonstrates that the rate of infection among college students is actually lower than it is among individuals the same age who are not in college (TABLE 1).1
The combined incidence of 0.14/100,000 means that to prevent one case, 714,000 individuals need to be vaccinated; 5 million need to be vaccinated to prevent one death.1 These numbers are subject to yearly variation and would be more favorable should the incidence of the disease increase. (For a look at the historical incidence of meningococcal meningitis from all serotypes, see the FIGURE.1) The question facing ACIP was whether the current very low levels of meningococcal B disease merit widespread, routinely-recommended use of the vaccine.
A look at the 2 meningococcal B vaccines
Two meningococcal B vaccines are now licensed for use in the United States. MenB-FHbp (Trumenba, Pfizer) was licensed in October 2014 as a 3-dose series given at 0, 2, and 6 months.2 MenB-4C (Bexsero, Novartis/GSK) was licensed in January 2015 and requires 2 doses at 0 and ≥1 month.3 Both vaccines induce a level of antibody production that is considered immunogenic in a high proportion of those vaccinated, but the level of immunity wanes after 6 to 24 months. The clinical significance of this drop in immunity is unknown and cannot be tested currently because of the rarity of the disease. Unfortunately, the rate of asymptomatic carriage of meningococcal B does not appear to be affected by vaccination.1
Both vaccines produce local and systemic reactions at rates higher than other recommended vaccines for this age group: pain at the injection site (83%-85%), headache (33%-35%), myalgia (30%-48%), fatigue (35%-40%), induration (28%), nausea (18%), chills (15%), and arthralgia (13%).2,3 There is some theoretical concern about the potential for autoimmune disease from the use of meningococcal B vaccines that will be studied as the vaccines are used more widely.1 In addition, the CDC estimates that serious anaphylactic reactions can occur after administration of any vaccine, estimated at about one per every million doses.1
Meningococcal serotype B bacteria consist of different strains. The 2 approved vaccines cover today’s most frequently found strains in the United States, but it’s uncertain if this will hold true in the future.
USPSTF: Screen obese/overweight adults for type 2 diabetes
The United States Preventive Services Task Force (USPSTF) recently updated its recommendation for screening for type 2 diabetes in adults. USPSTF recommends screening adults, ages 40 to 70 years, who are obese or overweight and referring those who have abnormal blood glucose to intensive behavioral counseling to promote a healthful diet and physical activity.
The Task Force gave this recommendation a grade of B, meaning that it is likely to result in a moderate level of benefit from a reduction in progression to diabetes. The Task Force also emphasized that lifestyle modifications have a greater risk-reducing effect than metformin and other medications.
The recommendation rationale points out that screening might also benefit those at high risk of diabetes based on family history or race/ethnicity and does not apply to those with signs and symptoms of diabetes; testing in this latter group is considered diagnostic testing, not screening.
Screening can be done by measuring glycated hemoglobin A1c or fasting glucose or with a glucose tolerance test. The recommendation includes tables that list the cutoffs for abnormal glucose levels for impaired fasting glucose, impaired glucose tolerance, and increased average glucose level. Obesity is defined as a body mass index ≥30 kg/m2 and overweight as >25 kg/m2.
This new recommendation expands the list of those at risk and those who should be screened compared to the previous recommendation, but the Task Force found no evidence to support universal screening in adults as advocated by other organizations.
Source: USPSTF. Final recommendation statement. Abnormal blood glucose and type 2 diabetes mellitus: screening. Available at: http://www.uspreventiveservicestaskforce.org/Page/Document/RecommendationStatementFinal/screening-for-abnormal-blood-glucose-and-type-2-diabetes. Accessed November 13, 2015.
Recommendation considerations that came into play
A number of factors affected ACIP’s recommendation decision: the low incidence of the meningococcal B disease; the large number-needed-to-vaccinate to prevent a case and a death; uncertainties regarding the duration of protection; cost, lack of effect on carriage rates, and limited safety data with the potential for serious reactions to exceed the number of cases prevented; and the severity of the disease and the concern it elicits.
ACIP has multiple options when considering a vaccine: recommend it routinely for everyone or everyone in a defined group (A recommendation), recommend for individual decision making (B recommendation), recommend against use, and make no recommendation at all. Given that 2 meningococcal B vaccines are licensed in the United States and can be used by those who want them—and the Committee’s opinion that these vaccines should not (at this time) be included in the schedule of routinely-recommended vaccines—ACIP chose to make a B recommendation on their use (TABLE 2).1 Vaccines recommended by ACIP (both A and B recommendations) are mandated in the Affordable Care Act to be provided by commercial health insurance at no out-of-pocket expense to the patient.
A word about high-risk populations
At its February 2015 meeting, ACIP voted to recommend meningococcal B vaccine for use in high-risk populations and during outbreaks (TABLE 3).4 This recommendation—plus the most recent B recommendation for general use—comprise the totality of current recommendations for the prevention of meningococcal B disease in the United States.
1. MacNeil J. Considerations for the use of serogroup B meningococcal (MenB) vaccines in adolescents. Presented at: Advisory Committee on Immunization Practices; June 24, 2015; Atlanta, GA. Available at: http://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2015-06/mening-03-macneil.pdf. Accessed October 14, 2015.
2. Trumenba [package insert]. Philadelphia, PA: Wyeth Pharmaceuticals Inc. (Pfizer); 2014. Available at: http://www.fda.gov/downloads/BiologicsBloodVaccines/Vaccines/ApprovedProducts/UCM421139.pdf. Accessed October 14, 2015.
3. Bexsero [package insert]. Cambridge, MA: Novartis Vaccines and Diagnostics Inc; 2015. Available at: http://www.fda.gov/downloads/BiologicsBloodVaccines/Vaccines/ApprovedProducts/UCM431447.pdf. Accessed October 14, 2015.
4. Folaranmi T, Rubin L, Martin SW, et al. Use of serogroup B meningococcal vaccines in persons aged ≥10 years at increased risk for serogroup B meningococcal disease: recommendations of the Advisory Committee on Immunization Practices, 2015. MMWR Morb Mortal Wkly Rep. 2015;64:608-612.
The Advisory Committee on Immunization Practices (ACIP) voted at its June 2015 meeting to make a “B” recommendation for the use of meningococcal B vaccine for individuals 16 through 23 years of age. The Committee felt that the vaccine can be used if one desires it, but at this time it should not be included in the category of a routinely recommended vaccine.
Meningococcal meningitis caused by serogroup B is a serious disease, but it is rare. From 2009 to 2013, the annual number of meningococcal B cases in individuals ages 11 to 24 years ranged from 54 to 67, with 5 to 10 deaths and 5 to 13 serious sequelae.1 Since 2009, there have been outbreaks on 7 university campuses with cases-per-outbreak numbering 2 to 13.1 These well publicized outbreaks created much disruption and an impression of increased risk among college students. But the surveillance system of the Centers for Disease Control and Prevention (CDC) demonstrates that the rate of infection among college students is actually lower than it is among individuals the same age who are not in college (TABLE 1).1
The combined incidence of 0.14/100,000 means that to prevent one case, 714,000 individuals need to be vaccinated; 5 million need to be vaccinated to prevent one death.1 These numbers are subject to yearly variation and would be more favorable should the incidence of the disease increase. (For a look at the historical incidence of meningococcal meningitis from all serotypes, see the FIGURE.1) The question facing ACIP was whether the current very low levels of meningococcal B disease merit widespread, routinely-recommended use of the vaccine.
A look at the 2 meningococcal B vaccines
Two meningococcal B vaccines are now licensed for use in the United States. MenB-FHbp (Trumenba, Pfizer) was licensed in October 2014 as a 3-dose series given at 0, 2, and 6 months.2 MenB-4C (Bexsero, Novartis/GSK) was licensed in January 2015 and requires 2 doses at 0 and ≥1 month.3 Both vaccines induce a level of antibody production that is considered immunogenic in a high proportion of those vaccinated, but the level of immunity wanes after 6 to 24 months. The clinical significance of this drop in immunity is unknown and cannot be tested currently because of the rarity of the disease. Unfortunately, the rate of asymptomatic carriage of meningococcal B does not appear to be affected by vaccination.1
Both vaccines produce local and systemic reactions at rates higher than other recommended vaccines for this age group: pain at the injection site (83%-85%), headache (33%-35%), myalgia (30%-48%), fatigue (35%-40%), induration (28%), nausea (18%), chills (15%), and arthralgia (13%).2,3 There is some theoretical concern about the potential for autoimmune disease from the use of meningococcal B vaccines that will be studied as the vaccines are used more widely.1 In addition, the CDC estimates that serious anaphylactic reactions can occur after administration of any vaccine, estimated at about one per every million doses.1
Meningococcal serotype B bacteria consist of different strains. The 2 approved vaccines cover today’s most frequently found strains in the United States, but it’s uncertain if this will hold true in the future.
USPSTF: Screen obese/overweight adults for type 2 diabetes
The United States Preventive Services Task Force (USPSTF) recently updated its recommendation for screening for type 2 diabetes in adults. USPSTF recommends screening adults, ages 40 to 70 years, who are obese or overweight and referring those who have abnormal blood glucose to intensive behavioral counseling to promote a healthful diet and physical activity.
The Task Force gave this recommendation a grade of B, meaning that it is likely to result in a moderate level of benefit from a reduction in progression to diabetes. The Task Force also emphasized that lifestyle modifications have a greater risk-reducing effect than metformin and other medications.
The recommendation rationale points out that screening might also benefit those at high risk of diabetes based on family history or race/ethnicity and does not apply to those with signs and symptoms of diabetes; testing in this latter group is considered diagnostic testing, not screening.
Screening can be done by measuring glycated hemoglobin A1c or fasting glucose or with a glucose tolerance test. The recommendation includes tables that list the cutoffs for abnormal glucose levels for impaired fasting glucose, impaired glucose tolerance, and increased average glucose level. Obesity is defined as a body mass index ≥30 kg/m2 and overweight as >25 kg/m2.
This new recommendation expands the list of those at risk and those who should be screened compared to the previous recommendation, but the Task Force found no evidence to support universal screening in adults as advocated by other organizations.
Source: USPSTF. Final recommendation statement. Abnormal blood glucose and type 2 diabetes mellitus: screening. Available at: http://www.uspreventiveservicestaskforce.org/Page/Document/RecommendationStatementFinal/screening-for-abnormal-blood-glucose-and-type-2-diabetes. Accessed November 13, 2015.
Recommendation considerations that came into play
A number of factors affected ACIP’s recommendation decision: the low incidence of the meningococcal B disease; the large number-needed-to-vaccinate to prevent a case and a death; uncertainties regarding the duration of protection; cost, lack of effect on carriage rates, and limited safety data with the potential for serious reactions to exceed the number of cases prevented; and the severity of the disease and the concern it elicits.
ACIP has multiple options when considering a vaccine: recommend it routinely for everyone or everyone in a defined group (A recommendation), recommend for individual decision making (B recommendation), recommend against use, and make no recommendation at all. Given that 2 meningococcal B vaccines are licensed in the United States and can be used by those who want them—and the Committee’s opinion that these vaccines should not (at this time) be included in the schedule of routinely-recommended vaccines—ACIP chose to make a B recommendation on their use (TABLE 2).1 Vaccines recommended by ACIP (both A and B recommendations) are mandated in the Affordable Care Act to be provided by commercial health insurance at no out-of-pocket expense to the patient.
A word about high-risk populations
At its February 2015 meeting, ACIP voted to recommend meningococcal B vaccine for use in high-risk populations and during outbreaks (TABLE 3).4 This recommendation—plus the most recent B recommendation for general use—comprise the totality of current recommendations for the prevention of meningococcal B disease in the United States.
The Advisory Committee on Immunization Practices (ACIP) voted at its June 2015 meeting to make a “B” recommendation for the use of meningococcal B vaccine for individuals 16 through 23 years of age. The Committee felt that the vaccine can be used if one desires it, but at this time it should not be included in the category of a routinely recommended vaccine.
Meningococcal meningitis caused by serogroup B is a serious disease, but it is rare. From 2009 to 2013, the annual number of meningococcal B cases in individuals ages 11 to 24 years ranged from 54 to 67, with 5 to 10 deaths and 5 to 13 serious sequelae.1 Since 2009, there have been outbreaks on 7 university campuses with cases-per-outbreak numbering 2 to 13.1 These well publicized outbreaks created much disruption and an impression of increased risk among college students. But the surveillance system of the Centers for Disease Control and Prevention (CDC) demonstrates that the rate of infection among college students is actually lower than it is among individuals the same age who are not in college (TABLE 1).1
The combined incidence of 0.14/100,000 means that to prevent one case, 714,000 individuals need to be vaccinated; 5 million need to be vaccinated to prevent one death.1 These numbers are subject to yearly variation and would be more favorable should the incidence of the disease increase. (For a look at the historical incidence of meningococcal meningitis from all serotypes, see the FIGURE.1) The question facing ACIP was whether the current very low levels of meningococcal B disease merit widespread, routinely-recommended use of the vaccine.
A look at the 2 meningococcal B vaccines
Two meningococcal B vaccines are now licensed for use in the United States. MenB-FHbp (Trumenba, Pfizer) was licensed in October 2014 as a 3-dose series given at 0, 2, and 6 months.2 MenB-4C (Bexsero, Novartis/GSK) was licensed in January 2015 and requires 2 doses at 0 and ≥1 month.3 Both vaccines induce a level of antibody production that is considered immunogenic in a high proportion of those vaccinated, but the level of immunity wanes after 6 to 24 months. The clinical significance of this drop in immunity is unknown and cannot be tested currently because of the rarity of the disease. Unfortunately, the rate of asymptomatic carriage of meningococcal B does not appear to be affected by vaccination.1
Both vaccines produce local and systemic reactions at rates higher than other recommended vaccines for this age group: pain at the injection site (83%-85%), headache (33%-35%), myalgia (30%-48%), fatigue (35%-40%), induration (28%), nausea (18%), chills (15%), and arthralgia (13%).2,3 There is some theoretical concern about the potential for autoimmune disease from the use of meningococcal B vaccines that will be studied as the vaccines are used more widely.1 In addition, the CDC estimates that serious anaphylactic reactions can occur after administration of any vaccine, estimated at about one per every million doses.1
Meningococcal serotype B bacteria consist of different strains. The 2 approved vaccines cover today’s most frequently found strains in the United States, but it’s uncertain if this will hold true in the future.
USPSTF: Screen obese/overweight adults for type 2 diabetes
The United States Preventive Services Task Force (USPSTF) recently updated its recommendation for screening for type 2 diabetes in adults. USPSTF recommends screening adults, ages 40 to 70 years, who are obese or overweight and referring those who have abnormal blood glucose to intensive behavioral counseling to promote a healthful diet and physical activity.
The Task Force gave this recommendation a grade of B, meaning that it is likely to result in a moderate level of benefit from a reduction in progression to diabetes. The Task Force also emphasized that lifestyle modifications have a greater risk-reducing effect than metformin and other medications.
The recommendation rationale points out that screening might also benefit those at high risk of diabetes based on family history or race/ethnicity and does not apply to those with signs and symptoms of diabetes; testing in this latter group is considered diagnostic testing, not screening.
Screening can be done by measuring glycated hemoglobin A1c or fasting glucose or with a glucose tolerance test. The recommendation includes tables that list the cutoffs for abnormal glucose levels for impaired fasting glucose, impaired glucose tolerance, and increased average glucose level. Obesity is defined as a body mass index ≥30 kg/m2 and overweight as >25 kg/m2.
This new recommendation expands the list of those at risk and those who should be screened compared to the previous recommendation, but the Task Force found no evidence to support universal screening in adults as advocated by other organizations.
Source: USPSTF. Final recommendation statement. Abnormal blood glucose and type 2 diabetes mellitus: screening. Available at: http://www.uspreventiveservicestaskforce.org/Page/Document/RecommendationStatementFinal/screening-for-abnormal-blood-glucose-and-type-2-diabetes. Accessed November 13, 2015.
Recommendation considerations that came into play
A number of factors affected ACIP’s recommendation decision: the low incidence of the meningococcal B disease; the large number-needed-to-vaccinate to prevent a case and a death; uncertainties regarding the duration of protection; cost, lack of effect on carriage rates, and limited safety data with the potential for serious reactions to exceed the number of cases prevented; and the severity of the disease and the concern it elicits.
ACIP has multiple options when considering a vaccine: recommend it routinely for everyone or everyone in a defined group (A recommendation), recommend for individual decision making (B recommendation), recommend against use, and make no recommendation at all. Given that 2 meningococcal B vaccines are licensed in the United States and can be used by those who want them—and the Committee’s opinion that these vaccines should not (at this time) be included in the schedule of routinely-recommended vaccines—ACIP chose to make a B recommendation on their use (TABLE 2).1 Vaccines recommended by ACIP (both A and B recommendations) are mandated in the Affordable Care Act to be provided by commercial health insurance at no out-of-pocket expense to the patient.
A word about high-risk populations
At its February 2015 meeting, ACIP voted to recommend meningococcal B vaccine for use in high-risk populations and during outbreaks (TABLE 3).4 This recommendation—plus the most recent B recommendation for general use—comprise the totality of current recommendations for the prevention of meningococcal B disease in the United States.
1. MacNeil J. Considerations for the use of serogroup B meningococcal (MenB) vaccines in adolescents. Presented at: Advisory Committee on Immunization Practices; June 24, 2015; Atlanta, GA. Available at: http://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2015-06/mening-03-macneil.pdf. Accessed October 14, 2015.
2. Trumenba [package insert]. Philadelphia, PA: Wyeth Pharmaceuticals Inc. (Pfizer); 2014. Available at: http://www.fda.gov/downloads/BiologicsBloodVaccines/Vaccines/ApprovedProducts/UCM421139.pdf. Accessed October 14, 2015.
3. Bexsero [package insert]. Cambridge, MA: Novartis Vaccines and Diagnostics Inc; 2015. Available at: http://www.fda.gov/downloads/BiologicsBloodVaccines/Vaccines/ApprovedProducts/UCM431447.pdf. Accessed October 14, 2015.
4. Folaranmi T, Rubin L, Martin SW, et al. Use of serogroup B meningococcal vaccines in persons aged ≥10 years at increased risk for serogroup B meningococcal disease: recommendations of the Advisory Committee on Immunization Practices, 2015. MMWR Morb Mortal Wkly Rep. 2015;64:608-612.
1. MacNeil J. Considerations for the use of serogroup B meningococcal (MenB) vaccines in adolescents. Presented at: Advisory Committee on Immunization Practices; June 24, 2015; Atlanta, GA. Available at: http://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2015-06/mening-03-macneil.pdf. Accessed October 14, 2015.
2. Trumenba [package insert]. Philadelphia, PA: Wyeth Pharmaceuticals Inc. (Pfizer); 2014. Available at: http://www.fda.gov/downloads/BiologicsBloodVaccines/Vaccines/ApprovedProducts/UCM421139.pdf. Accessed October 14, 2015.
3. Bexsero [package insert]. Cambridge, MA: Novartis Vaccines and Diagnostics Inc; 2015. Available at: http://www.fda.gov/downloads/BiologicsBloodVaccines/Vaccines/ApprovedProducts/UCM431447.pdf. Accessed October 14, 2015.
4. Folaranmi T, Rubin L, Martin SW, et al. Use of serogroup B meningococcal vaccines in persons aged ≥10 years at increased risk for serogroup B meningococcal disease: recommendations of the Advisory Committee on Immunization Practices, 2015. MMWR Morb Mortal Wkly Rep. 2015;64:608-612.
Being honest about diagnostic uncertainty
Like everyone else’s grandmother, mine gave me all kinds of advice while I was growing up. Some tips I still remember.
One came when I was home for Thanksgiving during my first year of medical school. She was frustrated over her recent visit to an internist. He kept ordering more tests but wouldn’t answer questions about what might be causing her symptoms.
She told me that, if I didn’t know what was going on, to just say so. As a patient, she felt that an honest answer was better than silence.
Today, as a doctor, I agree with her. So, while I may still be doing tests to crack the case, I have no problem, when asked what I think is going on, with saying “I don’t know.”
This approach isn’t perfect for everyone. Some docs (and patients) may see it as a sign of incompetence or weakness, thinking that admitting fallibility is a breach of the relationship or that with some tests the doctor becomes omniscient. Of course, that’s far from the truth.
In my experience, patients prefer the honesty of my saying “I don’t know.” I’m not saying I’ll never know, I’m just saying that, at present, I’m still looking for the answer.
Nobody likes being in the dark about their health, but at the same time they don’t want to feel their doctor is keeping a secret from them. By making it clear that I’m not, I’m hoping to keep a strong therapeutic relationship. I promise them that when I know, they’ll know, and that I’m honest when stumped. If I need to refer elsewhere for an answer, I have no problem doing that. Medicine, and neurology in particular, is a complex field. If every diagnosis were a slam-dunk, we wouldn’t need specialists and subspecialists (and even subsubspecialists).
Most people know and understand that, recognize the inherent uncertainty of this job, and know that I don’t know. I promise them that “I don’t know” doesn’t mean I’m done looking, it just means I’m going to keep trying. That’s the best anyone can do. Right, Granny?
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
Like everyone else’s grandmother, mine gave me all kinds of advice while I was growing up. Some tips I still remember.
One came when I was home for Thanksgiving during my first year of medical school. She was frustrated over her recent visit to an internist. He kept ordering more tests but wouldn’t answer questions about what might be causing her symptoms.
She told me that, if I didn’t know what was going on, to just say so. As a patient, she felt that an honest answer was better than silence.
Today, as a doctor, I agree with her. So, while I may still be doing tests to crack the case, I have no problem, when asked what I think is going on, with saying “I don’t know.”
This approach isn’t perfect for everyone. Some docs (and patients) may see it as a sign of incompetence or weakness, thinking that admitting fallibility is a breach of the relationship or that with some tests the doctor becomes omniscient. Of course, that’s far from the truth.
In my experience, patients prefer the honesty of my saying “I don’t know.” I’m not saying I’ll never know, I’m just saying that, at present, I’m still looking for the answer.
Nobody likes being in the dark about their health, but at the same time they don’t want to feel their doctor is keeping a secret from them. By making it clear that I’m not, I’m hoping to keep a strong therapeutic relationship. I promise them that when I know, they’ll know, and that I’m honest when stumped. If I need to refer elsewhere for an answer, I have no problem doing that. Medicine, and neurology in particular, is a complex field. If every diagnosis were a slam-dunk, we wouldn’t need specialists and subspecialists (and even subsubspecialists).
Most people know and understand that, recognize the inherent uncertainty of this job, and know that I don’t know. I promise them that “I don’t know” doesn’t mean I’m done looking, it just means I’m going to keep trying. That’s the best anyone can do. Right, Granny?
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
Like everyone else’s grandmother, mine gave me all kinds of advice while I was growing up. Some tips I still remember.
One came when I was home for Thanksgiving during my first year of medical school. She was frustrated over her recent visit to an internist. He kept ordering more tests but wouldn’t answer questions about what might be causing her symptoms.
She told me that, if I didn’t know what was going on, to just say so. As a patient, she felt that an honest answer was better than silence.
Today, as a doctor, I agree with her. So, while I may still be doing tests to crack the case, I have no problem, when asked what I think is going on, with saying “I don’t know.”
This approach isn’t perfect for everyone. Some docs (and patients) may see it as a sign of incompetence or weakness, thinking that admitting fallibility is a breach of the relationship or that with some tests the doctor becomes omniscient. Of course, that’s far from the truth.
In my experience, patients prefer the honesty of my saying “I don’t know.” I’m not saying I’ll never know, I’m just saying that, at present, I’m still looking for the answer.
Nobody likes being in the dark about their health, but at the same time they don’t want to feel their doctor is keeping a secret from them. By making it clear that I’m not, I’m hoping to keep a strong therapeutic relationship. I promise them that when I know, they’ll know, and that I’m honest when stumped. If I need to refer elsewhere for an answer, I have no problem doing that. Medicine, and neurology in particular, is a complex field. If every diagnosis were a slam-dunk, we wouldn’t need specialists and subspecialists (and even subsubspecialists).
Most people know and understand that, recognize the inherent uncertainty of this job, and know that I don’t know. I promise them that “I don’t know” doesn’t mean I’m done looking, it just means I’m going to keep trying. That’s the best anyone can do. Right, Granny?
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
Cardiovascular Disease: From Milestones to Innovations
Supplement Editor:
Maan A. Fares, MD
Contents
Introduction: The transition from milestones to innovations
Maan a. Fares, MD
Transcatheter aortic valve replacement: History and current indications
Ahmad Zeeshan, MD; E. Murat Tuzcu, MD; Amar Krishnaswamy, MD; Samir Kapadia, MD; and Stephanie Mick, MD
Evolving strategies to prevent stroke and thromboembolism in nonvalvular atrial fibrillation
Ayman Hussein, MD; Walid Saliba, MD; and Oussama Wazni, MD
Stroke management and the impact of mobile stroke treatment units
Peter A. Rasmussen, MD
Biomarkers: Their potential in the diagnosis and treatment of heart failure
Barbara Heil, MD, and W.H. Wilson Tang, MD
Clinical challenges in diagnosing and managing adult hypertension
Joel Handler, MD
Supplement Editor:
Maan A. Fares, MD
Contents
Introduction: The transition from milestones to innovations
Maan a. Fares, MD
Transcatheter aortic valve replacement: History and current indications
Ahmad Zeeshan, MD; E. Murat Tuzcu, MD; Amar Krishnaswamy, MD; Samir Kapadia, MD; and Stephanie Mick, MD
Evolving strategies to prevent stroke and thromboembolism in nonvalvular atrial fibrillation
Ayman Hussein, MD; Walid Saliba, MD; and Oussama Wazni, MD
Stroke management and the impact of mobile stroke treatment units
Peter A. Rasmussen, MD
Biomarkers: Their potential in the diagnosis and treatment of heart failure
Barbara Heil, MD, and W.H. Wilson Tang, MD
Clinical challenges in diagnosing and managing adult hypertension
Joel Handler, MD
Supplement Editor:
Maan A. Fares, MD
Contents
Introduction: The transition from milestones to innovations
Maan a. Fares, MD
Transcatheter aortic valve replacement: History and current indications
Ahmad Zeeshan, MD; E. Murat Tuzcu, MD; Amar Krishnaswamy, MD; Samir Kapadia, MD; and Stephanie Mick, MD
Evolving strategies to prevent stroke and thromboembolism in nonvalvular atrial fibrillation
Ayman Hussein, MD; Walid Saliba, MD; and Oussama Wazni, MD
Stroke management and the impact of mobile stroke treatment units
Peter A. Rasmussen, MD
Biomarkers: Their potential in the diagnosis and treatment of heart failure
Barbara Heil, MD, and W.H. Wilson Tang, MD
Clinical challenges in diagnosing and managing adult hypertension
Joel Handler, MD
Introduction: The transition from milestones to innovations
Physicians who were educated and began practicing in the 20th century have witnessed some of the most significant innovations and discoveries in the history of healthcare. While major surgical and therapeutic milestones defined the previous century, our current century is defined by the high-speed pace of technological innovations that affect the practice of medicine. For example, the proliferation of hand-held communication devices now provides immediate access to a wealth of healthcare information. Ultimately, recollecting information will be less necessary and far less valuable than understanding the concepts behind it. The challenge for providers is to recognize how to incorporate these innovations into the traditional model of treating diseases with the goal of improving outcomes and containing costs.
With that objective in mind, the articles in this Cleveland Clinic Journal of Medicine supplement on cardiovascular disease aim to not only review traditional treatment models for cardiovascular disease but, more importantly, to address the broad implications of new innovations on day-to-day clinical practice.
Stephanie Mick, MD, and colleagues look at how the emergence of new devices and technologies has dramatically improved the treatment of severe aortic valve stenosis and expanded the patient population eligible for aortic valve replacement. The authors review the expanded array of surgical approaches to transcatheter aortic valve replacement and the development of new devices in light of their impact on reducing the risks and improving the outcomes associated with this therapy.
Oussama Wazni, MD, and colleagues present evidence underlying the evolving strategies to prevent serious complications of stroke and thromboembolism in patients with atrial fibrillation. Newer anticoagulants are changing the strategic picture. The article includes discussion of the safety and efficacy of the available anticoagulants, as well as nonpharmacologic approaches, and considers how the new data and medications affect traditional treatment models. The authors integrate the data into an evidence-based appraisal of how to best use these innovations to reduce stroke risk in this patient population.
Acute strokes have a significant impact on morbidity and mortality worldwide. Findings that stress the importance of reducing the “time to treatment”—the shorter the time, the better the outcomes—have pushed treatment approaches to center stage. A key factor is the time it takes for patients to arrive in the emergency department. One way to reduce this time is to take the treatment to the patient. Peter A. Rasmussen, MD, looks at how innovations in scanning technologies and wireless data transmissions have led to the development of spe- cially equipped mobile stroke units that can accurately differentiate the types of stroke and enable practitioners to more quickly begin appropriate thromboembolic therapy and reduce the time to therapy.
Barbara Heil, MD, and W. H. Wilson Tang, MD, review the use of cardiac biomarkers to diagnose and treat heart failure. Studies have shown the efficacy of using biomarkers to identify high-risk patients, but various factors limit their diagnostic accuracy and clinical adaptability. The authors summarize the data and explain how to incorporate biomarkers into clinical practice.
Hypertension control remains an elusive goal for practitioners. Joel Handler, MD, reviews how new evidence and innovations are revising the diagnostic guidelines and the recommended treatment strategies. He discusses innovations associated with out-of-office monitoring and new data from clinical trials that are changing the clinical practice model. He also addresses the controversy regarding systolic blood pressure goals in elderly patients and how these data have affected evidence-based guidelines.
We hope you find this supplement both informative and thought-provoking.
Physicians who were educated and began practicing in the 20th century have witnessed some of the most significant innovations and discoveries in the history of healthcare. While major surgical and therapeutic milestones defined the previous century, our current century is defined by the high-speed pace of technological innovations that affect the practice of medicine. For example, the proliferation of hand-held communication devices now provides immediate access to a wealth of healthcare information. Ultimately, recollecting information will be less necessary and far less valuable than understanding the concepts behind it. The challenge for providers is to recognize how to incorporate these innovations into the traditional model of treating diseases with the goal of improving outcomes and containing costs.
With that objective in mind, the articles in this Cleveland Clinic Journal of Medicine supplement on cardiovascular disease aim to not only review traditional treatment models for cardiovascular disease but, more importantly, to address the broad implications of new innovations on day-to-day clinical practice.
Stephanie Mick, MD, and colleagues look at how the emergence of new devices and technologies has dramatically improved the treatment of severe aortic valve stenosis and expanded the patient population eligible for aortic valve replacement. The authors review the expanded array of surgical approaches to transcatheter aortic valve replacement and the development of new devices in light of their impact on reducing the risks and improving the outcomes associated with this therapy.
Oussama Wazni, MD, and colleagues present evidence underlying the evolving strategies to prevent serious complications of stroke and thromboembolism in patients with atrial fibrillation. Newer anticoagulants are changing the strategic picture. The article includes discussion of the safety and efficacy of the available anticoagulants, as well as nonpharmacologic approaches, and considers how the new data and medications affect traditional treatment models. The authors integrate the data into an evidence-based appraisal of how to best use these innovations to reduce stroke risk in this patient population.
Acute strokes have a significant impact on morbidity and mortality worldwide. Findings that stress the importance of reducing the “time to treatment”—the shorter the time, the better the outcomes—have pushed treatment approaches to center stage. A key factor is the time it takes for patients to arrive in the emergency department. One way to reduce this time is to take the treatment to the patient. Peter A. Rasmussen, MD, looks at how innovations in scanning technologies and wireless data transmissions have led to the development of spe- cially equipped mobile stroke units that can accurately differentiate the types of stroke and enable practitioners to more quickly begin appropriate thromboembolic therapy and reduce the time to therapy.
Barbara Heil, MD, and W. H. Wilson Tang, MD, review the use of cardiac biomarkers to diagnose and treat heart failure. Studies have shown the efficacy of using biomarkers to identify high-risk patients, but various factors limit their diagnostic accuracy and clinical adaptability. The authors summarize the data and explain how to incorporate biomarkers into clinical practice.
Hypertension control remains an elusive goal for practitioners. Joel Handler, MD, reviews how new evidence and innovations are revising the diagnostic guidelines and the recommended treatment strategies. He discusses innovations associated with out-of-office monitoring and new data from clinical trials that are changing the clinical practice model. He also addresses the controversy regarding systolic blood pressure goals in elderly patients and how these data have affected evidence-based guidelines.
We hope you find this supplement both informative and thought-provoking.
Physicians who were educated and began practicing in the 20th century have witnessed some of the most significant innovations and discoveries in the history of healthcare. While major surgical and therapeutic milestones defined the previous century, our current century is defined by the high-speed pace of technological innovations that affect the practice of medicine. For example, the proliferation of hand-held communication devices now provides immediate access to a wealth of healthcare information. Ultimately, recollecting information will be less necessary and far less valuable than understanding the concepts behind it. The challenge for providers is to recognize how to incorporate these innovations into the traditional model of treating diseases with the goal of improving outcomes and containing costs.
With that objective in mind, the articles in this Cleveland Clinic Journal of Medicine supplement on cardiovascular disease aim to not only review traditional treatment models for cardiovascular disease but, more importantly, to address the broad implications of new innovations on day-to-day clinical practice.
Stephanie Mick, MD, and colleagues look at how the emergence of new devices and technologies has dramatically improved the treatment of severe aortic valve stenosis and expanded the patient population eligible for aortic valve replacement. The authors review the expanded array of surgical approaches to transcatheter aortic valve replacement and the development of new devices in light of their impact on reducing the risks and improving the outcomes associated with this therapy.
Oussama Wazni, MD, and colleagues present evidence underlying the evolving strategies to prevent serious complications of stroke and thromboembolism in patients with atrial fibrillation. Newer anticoagulants are changing the strategic picture. The article includes discussion of the safety and efficacy of the available anticoagulants, as well as nonpharmacologic approaches, and considers how the new data and medications affect traditional treatment models. The authors integrate the data into an evidence-based appraisal of how to best use these innovations to reduce stroke risk in this patient population.
Acute strokes have a significant impact on morbidity and mortality worldwide. Findings that stress the importance of reducing the “time to treatment”—the shorter the time, the better the outcomes—have pushed treatment approaches to center stage. A key factor is the time it takes for patients to arrive in the emergency department. One way to reduce this time is to take the treatment to the patient. Peter A. Rasmussen, MD, looks at how innovations in scanning technologies and wireless data transmissions have led to the development of spe- cially equipped mobile stroke units that can accurately differentiate the types of stroke and enable practitioners to more quickly begin appropriate thromboembolic therapy and reduce the time to therapy.
Barbara Heil, MD, and W. H. Wilson Tang, MD, review the use of cardiac biomarkers to diagnose and treat heart failure. Studies have shown the efficacy of using biomarkers to identify high-risk patients, but various factors limit their diagnostic accuracy and clinical adaptability. The authors summarize the data and explain how to incorporate biomarkers into clinical practice.
Hypertension control remains an elusive goal for practitioners. Joel Handler, MD, reviews how new evidence and innovations are revising the diagnostic guidelines and the recommended treatment strategies. He discusses innovations associated with out-of-office monitoring and new data from clinical trials that are changing the clinical practice model. He also addresses the controversy regarding systolic blood pressure goals in elderly patients and how these data have affected evidence-based guidelines.
We hope you find this supplement both informative and thought-provoking.
Transcatheter aortic valve replacement: History and current indications
Transcatheter aortic valve replacement (TAVR) has established itself as an effective way of treating high-risk patients with severe aortic valve stenosis. With new generations of existing valves and newer alternative devices, the procedure promises to become increasingly safer. The field is evolving rapidly and it will be important for interventional cardiologists and cardiac surgeons alike to stay abreast of developments. This article reviews the history of this promising procedure and examines its use in current practice.
HISTORICAL PERSPECTIVE
In 1980, Danish researcher H. R. Anderson reported developing and testing a balloon-expandable valve in animals.1 The technology was eventually acquired and further developed by Edwards Life Sciences (Irvine, California).
Alain Cribier started early work in humans in 2002 in France.2 He used a transfemoral arterial access to approach the aortic valve transseptally, but this procedure was associated with high rates of mortality and stroke.3 At the same time, in the United States, animal studies were being carried out by Lars G. Svensson, Todd Dewey, and Michael Mack to develop a transapical method of implantation,4,5 while John Webb and colleagues were also developing a transapical aortic valve implantation technique,6,7 and later went on to develop a retrograde transfemoral technique. This latter technique became feasible once Edwards developed a catheter that could be flexed to get around the aortic arch and across the aortic valve.
As the Edwards balloon-expandable valve (Sapien) was being developed, a nitinol-based self-expandable valve system was introduced by Medtronic: the CoreValve. Following feasibility studies,5,8 the safety and efficacy of these valves were established thorough the Placement of Aortic Transcatheter Valves (PARTNER) trial and the US Core Valve Pivotal Trial. These valves are currently approved by the US Food and Drug Administration (FDA) for patients for whom conventional surgery would pose an extreme or high risk.9–11
CLINICAL TRIALS OF TAVR
The two landmark prospective randomized trials of TAVR were the PARTNER trial and CoreValve Pivotal Trial.
The PARTNER trial consisted of two parts: PARTNER A, which compared the Sapien balloon-expandable transcatheter valve with surgical aortic valve replacement in patients at high surgical risk (Society of Thoracic Surgeons [STS] score > 10%), and PARTNER B, which compared TAVR with medical therapy in patients who could not undergo surgery (combined risk of serious morbidity or death of 50% or more, and two surgeons agreeing that the patient was inoperable).
Similarly, the CoreValve Pivotal Trial compared the self-expandable transcatheter valve with conventional medical and surgical treatment.
TAVR is comparable to surgery in outcomes, with caveats
In the PARTNER A trial, mortality rates were similar between patients who underwent Sapien TAVR and those who underwent surgical valve replacement at 30 days (3.4% and 6.5%, P = .07), 1 year (24.2% and 26.8%), and 2 years (33.9% and 35.0%). The patients in this group were randomized to either Sapien TAVR or surgery (Table 1).10,12
The combined rate of stroke and transient ischemic attack was higher in the patients assigned to TAVR at 30 days (5.5% with TAVR vs 2.4% with surgery, P = .04) and at 1 year (8.3% with TAVR vs 4.3% with surgery, P = .04). The difference was of small significance at 2 years (11.2% vs 6.5%, P = .05). At 30 days, the rate of major vascular complications was higher with TAVR (11.0% vs 3.2%), while surgery was associated with more frequent major bleeding episodes (19.5% vs 9.3%) and new-onset atrial fibrillation (16.0% vs 8.6%). The rate of new pacemaker requirement at 30 days was similar between the TAVR and surgical groups (3.8% vs 3.6%). Moderate or severe paravalvular aortic regurgitation was more common after TAVR at 30 days, 1 year, and 2 years. This aortic insufficiency was associated with increased late mortality.10,12
In the US CoreValve High Risk Study, no difference was found in the 30-day mortality rate in patients at high surgical risk randomized to CoreValve TAVR or surgery (3.3% and 4.5%) (Table 1). Surprisingly, the 1-year mortality rate was lower in the TAVR group than in the surgical group (14.1% vs 18.9%, respectively), a finding sustained at 2 years in data presented at the American College of Cardiology conference in March 2015.13–16
TAVR is superior to medical management, but the risk of stroke is higher
In the PARTNER B trial, inoperable patients were randomly assigned to undergo TAVR with a Sapien valve or medical management. TAVR resulted in lower mortality rates at 1 year (30.7% vs 50.7%) and 2 years (43.4% vs 68.0%) compared with medical management (Table 1).17 Of note, medical management included balloon valvuloplasty. The rate of the composite end point of death or repeat hospitalization was also lower with TAVR compared with medical therapy (44.1% vs 71.6%, respectively, at 1 year and 56.7% and 87.9%, respectively, at 2 years).17 The TAVR group had a higher stroke rate than the medical therapy group at 30 days (11.2% vs 5.5%, respectively) and at 2 years (13.8% vs 5.5%).17 Survival improved with TAVR in patients with an STS score of less than 15% but not in those with an STS score of 15% or higher.9
The very favorable results from the PARTNER trial rendered a randomized trial comparing self-expanding (CoreValve) TAVR and medical therapy unethical. Instead, a prospective single-arm study, the CoreValve Extreme Risk US Pivotal Trial, was used to compare the 12-month rate of death or major stroke with CoreValve TAVR vs a prespecified estimate of this rate with medical therapy.14 In about 500 patients who had a CoreValve attempt, the rate of all-cause mortality or major stroke at 1 year was significantly lower than the prespecified expected rate (26% vs 43%), reinforcing the results from the PARTNER Trial.14
Five-year outcomes
The 5-year PARTNER clinical and valve performance outcomes were published recently18 and continued to demonstrate equivalent outcomes for high-risk patients who underwent surgical aortic valve replacement or TAVR; there were no significant differences in all-cause mortality, cardiovascular mortality, stroke, or need for readmission to the hospital. The functional outcomes were similar as well, and no differences were demonstrated between surgical and TAVR valve performance.
Of note, moderate or severe aortic regurgitation occurred in 14% of patients in the TAVR group compared with 1% in the surgical aortic valve replacement group (P < .0001). This was associated with increased 5-year risk of death in the TAVR group (72.4% in those with moderate or severe aortic regurgitation vs 56.6% in those with mild aortic regurgitation or less; P = .003).
If the available randomized data are combined with observational reports, overall mortality and stroke rates are comparable between surgical aortic valve replacement and balloon-expandable or self-expandable TAVR in high-risk surgical candidates. Vascular complications, aortic regurgitation and permanent pacemaker insertion occur more frequently after TAVR, while major bleeding is more likely to occur after surgery.19 As newer generations of valves are developed, it is expected that aortic regurgitation and pacemaker rates will decrease over time. Indeed, trial data presented at the American College of Cardiology meeting in March 2015 for the third-generation Sapien valve (Sapien S3) showed only a 3.0% to 4.2% rate of significant paravalvular leak.
Contemporary valve comparison data
The valve used in the original PARTNER data was the first-generation Sapien valve. Since then, the second generation of this valve, the Sapien XT, has been introduced and is the model currently used in the United States (with the third-generation valve mentioned above, the Sapien S3, still available only through clinical trials). Thus, the two contemporary valves available for commercial use in the United States are the Edwards Sapien XT and Medtronic CoreValve. There are limited data comparing these valves head-to-head, but one recent trial attempted to do just that.
The Comparison of Transcatheter Heart Valves in High Risk Patients with Severe Aortic Stenosis: Medtronic CoreValve vs Edwards Sapien XT (CHOICE) trial compared the Edwards Sapien XT and CoreValve devices. Two hundred and forty-one patients were randomized. The primary end point of this trial was “device success” (a composite end point of four components: successful vascular access and deployment of the device with retrieval of the delivery system, correct position of the device, intended performance of the valve without moderate or severe insufficiency, and only one valve implanted in the correct anatomical location).
In this trial, the balloon-expandable Sapien XT valve showed a significantly higher device success rate than the self-expanding CoreValve, due to a significantly lower rate of aortic regurgitation (4.1% vs 18.3%, P < .001) and the less frequent need for implantation of more than one valve (0.8% vs 5.8%, P = .03). Placement of a permanent pacemaker was considerably less frequent in the balloon-expandable valve group (17.3% vs 37.6%, P = .001).20
PREOPERATIVE CONSIDERATIONS AND EVALUATION CRITERIA
Currently, TAVR is indicated for patients with symptomatic severe native aortic valve stenosis who are deemed at high risk or inoperable by a heart team including interventional cardiologists and cardiac surgeons. The CoreValve was also recently approved for valve-in-valve insertion in high-risk or inoperable patients with a prosthetic aortic valve in place.
The STS risk score is a reasonable preliminary risk assessment tool and is applicable to most patients being evaluated for aortic valve replacement. The STS risk score represents the percentage risk of unfavorable outcomes based on certain clinical variables. A calculator is available at riskcalc.sts.org. Patients considered at high risk are those with an STS operative risk score of 8% or higher or a postoperative 30-day risk of death of 15% or higher.
It is important to remember, though, that the STS score does not account for certain severe surgical risk factors. These include the presence of a "porcelain aorta" (heavy circumferential calcification of the ascending aorta precluding cross-clamping), history of mediastinal radiation, “hostile chest” (kyphoscoliosis, other deformities, previous coronary artery bypass grafting with adhesion of internal mammary artery to the back of sternum), severely compromised respiratory function (forced expiratory volume in 1 second < 1 L or < 40% predicted, diffusing capacity for carbon monoxide < 30%), severe pulmonary hypertension, severe liver disease (Model for End-stage Liver Disease score 8–20), severe dementia, severe cerebrovascular disease, and frailty.
With regard to this last risk factor, frailty is not simply old age but rather a measurable characteristic akin to weakness or disability. Several tests exist to measure frailty, including the “eyeball test” (the physician’s subjective assessment), Mini-Mental State Examination, gait speed/15-foot walk test, hand grip strength, serum albumin, and assessment of activities of daily living. Formal frailty testing is recommended during the course of a TAVR workup.
Risk assessment and patient suitability for TAVR is ultimately determined by the combined judgment of the heart valve team using both the STS score and consideration of these other factors.
Implantation approaches
Today, TAVR could be performed by several approaches: transfemoral arterial, transapical, transaortic via partial sternotomy or right anterior thoracotomy,21,22 transcarotid,23–25 and transaxillary or subclavian.26,27 Less commonly, transfemoral-venous routes have been performed utilizing either transseptal28 or caval-aortic puncture.29
The transfemoral approach is used most commonly by most institutions, including Cleveland Clinic. It allows for a completely percutaneous insertion and, in select cases, without endotracheal intubation and general anesthesia (Figure 1).
In patients with difficult femoral access due to severe calcification, extreme tortuosity, or small diameter, alternative access routes become a consideration. In this situation, at our institution, we favor the transaortic approach in patients who have not undergone cardiac surgery in the past, while the transapical approach is used in patients who had previous cardiac surgery. With the transapical approach, we have found the outcomes similar to those of transfemoral TAVR after propensity matching.30,31 Although there is a learning curve,32 transapical TAVR can be performed with very limited mortality and morbidity. In a recent series at Cleveland Clinic, the mortality rate with the transapical approach was 1.2%, renal failure occurred in 4.7%, and a pacemaker was placed in 5.9% of patients; there were no strokes.33 This approach can be utilized for simultaneous additional procedures like transcatheter mitral valve reimplantation and percutaneous coronary interventions.34–36
- Andersen HR, Knudsen LL, Hasenkam JM. Transluminal implantation of artificial heart valves. Description of a new expandable aortic valve and initial results with implantation by catheter technique in closed chest pigs. Eur Heart J 1992; 13:704– 708.
- Cribier A, Eltchaninoff H, Bash A, et al. Percutaneous transcatheter implantation of an aortic valve prosthesis for calcific aortic stenosis: first human case descrip- tion. Circulation 2002; 106:3006–3008.
- Cribier A, Eltchaninoff H, Tron C, et al. Early experience with percutaneous transcatheter implantation of heart valve prosthesis for the treatment of end-stage inoperable patients with calcific aortic stenosis. J Am Coll Cardiol 2004; 43:698– 703.
- Dewey TM, Walther T, Doss M, et al. Transapical aortic valve implantation: an animal feasibility study. Ann Thorac Surg 2006; 82:110–116.
- Svensson LG, Dewey T, Kapadia S, et al. United States feasibility study of trans- catheter insertion of a stented aortic valve by the left ventricular apex. Ann Thorac Surg 2008; 86:46–54.
- Lichtenstein SV, Cheung A, Ye J, et al. Transapical transcatheter aortic valve im- plantation in humans: initial clinical experience. Circulation 2006; 114:591–596.
- Webb JG, Pasupati S, Hyumphries K, et al. Percutaneous transarterial aortic valve replacement in selected high-risk patients with aortic stenosis. Circulation 2007; 116:755–763.
- Leon MB, Kodali S, Williams M, et al. Transcatheter aortic valve replacement in patients with critical aortic stenosis: rationale, device descriptions, early clinical experiences, and perspectives. Semin Thorac Cardiovasc Surg 2006; 18:165–174.
- Leon MB, Smith CR, Mack M, et al; PARTNER Trial Investigators. Transcatheter aortic-valve implantation for aortic stenosis in patients who cannot undergo sur- gery. N Engl J Med 2010; 363:1597–1607.
- Smith CR, Leon MB, Mack MJ, et al; PARTNER Trial Investigators. Transcatheter versus surgical aortic-valve replacement in high-risk patients. N Engl J Med 2011; 364:2187–2198.
- Adams DH, Popma JJ, Reardon MJ, et al; U.S. CoreValve Clinical Investigators. Transcatheter aortic-valve replacement with a self-expanding prosthesis. N Engl J Med 2014; 370:1790–1798.
- Kodali SK, Williams MR, Smith CR, et al; PARTNER Trial Investigators. Two- year outcomes after transcatheter or surgical aortic-valve replacement. N Engl J Med 2012; 366:1686–1695.
- Reardon M, et al. A randomized comparison of self-expanding
- Popma JJ, Adams DH, Reardon MJ, et al; CoreValve United States Clinical In- vestigators. Transcatheter aortic valve replacement using a self-expanding biopros- thesis in patients with severe aortic stenosis at extreme risk for surgery. J Am Coll Cardiol 2014; 63:1972–1981.
- Adams DH, Popma JJ, Reardon MJ. Transcatheter aortic-valve replacement with a self-expanding prosthesis (letter). N Engl J Med 2014; 371:967–968.
- Kaul S. Transcatheter aortic-valve replacement with a self-expanding prosthesis (letter). N Engl J Med 2014; 371:967.
- Makkar RR, Fontana GP, Jilaihawi H, et al. Transcathether aortic-valve re- placement for inoperable severe aortic stenosis. N Engl J Med 2012; 366: 1696–704.
- Mack MJ, Leon MB, Smith CR, et al; PARTNER 1 trial investigators. 5-year outcomes of transcatheter aortic valve replacement or surgical aortic valve re- placement for high surgical risk patients with aortic stenosis (PARTNER 1): a randomised controlled trial. Lancet 2015; 385:2477–2484.
- Cao C, Ang SC, Indraratna P, et al. Systematic review and meta-analysis of trans- catheter aortic valve implantation versus surgical aortic valve replacement for severe aortic stenosis. Ann Cardiothorac Surg 2013; 2:10–23.
- Abdel-Wahab M, Mehilli J, Frerker C, et al; CHOICE investigators. Comparison of balloon-expandable vs self-expandable valves in patients undergoing transcath- eter aortic valve replacement: the CHOICE randomized clinical trial. JAMA 2014; 311:1503–1514.
- Okuyama K, Jilaihawi H, Mirocha J, et al. Alternative access for balloon-ex- pandable transcatheter aortic valve replacement: comparison of the transaortic approach using right anterior thoracotomy to partial J-sternotomy. J Thorac Car- diovasc Surg 2014; 149:789–797.
- Lardizabal JA, O’Neill BP, Desai HV, et al. The transaortic approach for transcath- eter aortic valve replacement: initial clinical experience in the United States. J Am Coll Cardiol 2013; 61:2341–2345.
- Thourani VH, Gunter RL, Neravetla S, et al. Use of transaortic, transapical, and transcarotid transcatheter aortic valve replacement in inoperable patients. Ann Thorac Surg 2013; 96:1349–1357.
- Azmoun A, Amabile N, Ramadan R, et al. Transcatheter aortic valve implantation through carotid artery access under local anaesthesia. Eur J Cardiothorac Surg 2014; 46: 693–698.
- Rajagopal R, More RS, Roberts DH. Transcatheter aortic valve implantation through a transcarotid approach under local anesthesia. Catheter Cardiovasc In- terv 2014; 84:903–907.
- Fraccaro C, Napodano M, Tarantini G, et al. Expanding the eligibility for trans- catheter aortic valve implantation the trans-subclavian retrograde approach using: the III generation CoreValve revalving system. JACC Cardiovasc Interv 2009; 2:828–333.
- Petronio AS, De Carlo M, Bedogni F, et al. Safety and efficacy of the subclavian approach for transcatheter aortic valve implantation with the CoreValve revalving system. Circ Cardiovasc Interv 2010; 3:359–366.
- Cohen MG, Singh V, Martinez CA, et al. Transseptal antegrade transcatheter aor- tic valve replacement for patients with no other access approach—a contemporary experience. Catheter Cardiovasc Interv 2013; 82:987–993.
- Greenbaum AB, O’Neill WW, Paone G, et al. Caval-aortic access to allow trans- catheter aortic valve replacement in otherwise ineligible patients: initial human experience. J Am Coll Cardiol 2014; 63:2795–2804.
- D’Onofrio A, Salizzoni S, Agrifoglio M, et al. Medium term outcomes of trans- apical aortic valve implantation: results from the Italian Registry of Trans-Apical Aortic Valve Implantation. Ann Thorac Surg 2013; 96:830–835.
- Johansson M, Nozohoor S, Kimblad PO, Harnek J, Olivecrona GK, Sjögren J. Transapical versus transfemoral aortic valve implantation: a comparison of survival and safety. Ann Thorac Surg 2011; 91:57–63.
- Kempfert J, Rastan A, Holzhey D, et al. Transapical aortic valve implantation: analysis of risk factors and learning experience in 299 patients. Circulation 2011; 124(suppl):S124–S129.
- Aguirre J, Waskowski R, Poddar K, et al. Transcatheter aortic valve replacement: experience with the transapical approach, alternate access sites, and concomitant cardiac repairs. J Thorac Cardiovasc Surg 2014; 148:1417–1422.
- Al Kindi AH, Salhab KF, Roselli EE, Kapadia S, Tuzcu EM, Svensson LG. Alternative access options for transcatheter aortic valve replacement in patients with no conventional access and chest pathology. J Thorac Cardiovasc Surg 2014; 147:644–651.
- Salhab KF, Al Kindi AH, Lane JH, et al. Concomitant percutaneous coronary intervention and transcatheter aortic valve replacement: safe and feasible replace- ment alternative approaches in high-risk patients with severe aortic stenosis and coronary artery disease. J Card Surg 2013; 28:481–483.
- Al Kindi AH, Salhab KF, Kapadia S, et al. Simultaneous transapical transcatheter aortic and mitral valve replacement in a high-risk patient with a previous mitral bioprosthesis. J Thorac Cardiovasc Surg 2012; 144:e90–e91.
Transcatheter aortic valve replacement (TAVR) has established itself as an effective way of treating high-risk patients with severe aortic valve stenosis. With new generations of existing valves and newer alternative devices, the procedure promises to become increasingly safer. The field is evolving rapidly and it will be important for interventional cardiologists and cardiac surgeons alike to stay abreast of developments. This article reviews the history of this promising procedure and examines its use in current practice.
HISTORICAL PERSPECTIVE
In 1980, Danish researcher H. R. Anderson reported developing and testing a balloon-expandable valve in animals.1 The technology was eventually acquired and further developed by Edwards Life Sciences (Irvine, California).
Alain Cribier started early work in humans in 2002 in France.2 He used a transfemoral arterial access to approach the aortic valve transseptally, but this procedure was associated with high rates of mortality and stroke.3 At the same time, in the United States, animal studies were being carried out by Lars G. Svensson, Todd Dewey, and Michael Mack to develop a transapical method of implantation,4,5 while John Webb and colleagues were also developing a transapical aortic valve implantation technique,6,7 and later went on to develop a retrograde transfemoral technique. This latter technique became feasible once Edwards developed a catheter that could be flexed to get around the aortic arch and across the aortic valve.
As the Edwards balloon-expandable valve (Sapien) was being developed, a nitinol-based self-expandable valve system was introduced by Medtronic: the CoreValve. Following feasibility studies,5,8 the safety and efficacy of these valves were established thorough the Placement of Aortic Transcatheter Valves (PARTNER) trial and the US Core Valve Pivotal Trial. These valves are currently approved by the US Food and Drug Administration (FDA) for patients for whom conventional surgery would pose an extreme or high risk.9–11
CLINICAL TRIALS OF TAVR
The two landmark prospective randomized trials of TAVR were the PARTNER trial and CoreValve Pivotal Trial.
The PARTNER trial consisted of two parts: PARTNER A, which compared the Sapien balloon-expandable transcatheter valve with surgical aortic valve replacement in patients at high surgical risk (Society of Thoracic Surgeons [STS] score > 10%), and PARTNER B, which compared TAVR with medical therapy in patients who could not undergo surgery (combined risk of serious morbidity or death of 50% or more, and two surgeons agreeing that the patient was inoperable).
Similarly, the CoreValve Pivotal Trial compared the self-expandable transcatheter valve with conventional medical and surgical treatment.
TAVR is comparable to surgery in outcomes, with caveats
In the PARTNER A trial, mortality rates were similar between patients who underwent Sapien TAVR and those who underwent surgical valve replacement at 30 days (3.4% and 6.5%, P = .07), 1 year (24.2% and 26.8%), and 2 years (33.9% and 35.0%). The patients in this group were randomized to either Sapien TAVR or surgery (Table 1).10,12
The combined rate of stroke and transient ischemic attack was higher in the patients assigned to TAVR at 30 days (5.5% with TAVR vs 2.4% with surgery, P = .04) and at 1 year (8.3% with TAVR vs 4.3% with surgery, P = .04). The difference was of small significance at 2 years (11.2% vs 6.5%, P = .05). At 30 days, the rate of major vascular complications was higher with TAVR (11.0% vs 3.2%), while surgery was associated with more frequent major bleeding episodes (19.5% vs 9.3%) and new-onset atrial fibrillation (16.0% vs 8.6%). The rate of new pacemaker requirement at 30 days was similar between the TAVR and surgical groups (3.8% vs 3.6%). Moderate or severe paravalvular aortic regurgitation was more common after TAVR at 30 days, 1 year, and 2 years. This aortic insufficiency was associated with increased late mortality.10,12
In the US CoreValve High Risk Study, no difference was found in the 30-day mortality rate in patients at high surgical risk randomized to CoreValve TAVR or surgery (3.3% and 4.5%) (Table 1). Surprisingly, the 1-year mortality rate was lower in the TAVR group than in the surgical group (14.1% vs 18.9%, respectively), a finding sustained at 2 years in data presented at the American College of Cardiology conference in March 2015.13–16
TAVR is superior to medical management, but the risk of stroke is higher
In the PARTNER B trial, inoperable patients were randomly assigned to undergo TAVR with a Sapien valve or medical management. TAVR resulted in lower mortality rates at 1 year (30.7% vs 50.7%) and 2 years (43.4% vs 68.0%) compared with medical management (Table 1).17 Of note, medical management included balloon valvuloplasty. The rate of the composite end point of death or repeat hospitalization was also lower with TAVR compared with medical therapy (44.1% vs 71.6%, respectively, at 1 year and 56.7% and 87.9%, respectively, at 2 years).17 The TAVR group had a higher stroke rate than the medical therapy group at 30 days (11.2% vs 5.5%, respectively) and at 2 years (13.8% vs 5.5%).17 Survival improved with TAVR in patients with an STS score of less than 15% but not in those with an STS score of 15% or higher.9
The very favorable results from the PARTNER trial rendered a randomized trial comparing self-expanding (CoreValve) TAVR and medical therapy unethical. Instead, a prospective single-arm study, the CoreValve Extreme Risk US Pivotal Trial, was used to compare the 12-month rate of death or major stroke with CoreValve TAVR vs a prespecified estimate of this rate with medical therapy.14 In about 500 patients who had a CoreValve attempt, the rate of all-cause mortality or major stroke at 1 year was significantly lower than the prespecified expected rate (26% vs 43%), reinforcing the results from the PARTNER Trial.14
Five-year outcomes
The 5-year PARTNER clinical and valve performance outcomes were published recently18 and continued to demonstrate equivalent outcomes for high-risk patients who underwent surgical aortic valve replacement or TAVR; there were no significant differences in all-cause mortality, cardiovascular mortality, stroke, or need for readmission to the hospital. The functional outcomes were similar as well, and no differences were demonstrated between surgical and TAVR valve performance.
Of note, moderate or severe aortic regurgitation occurred in 14% of patients in the TAVR group compared with 1% in the surgical aortic valve replacement group (P < .0001). This was associated with increased 5-year risk of death in the TAVR group (72.4% in those with moderate or severe aortic regurgitation vs 56.6% in those with mild aortic regurgitation or less; P = .003).
If the available randomized data are combined with observational reports, overall mortality and stroke rates are comparable between surgical aortic valve replacement and balloon-expandable or self-expandable TAVR in high-risk surgical candidates. Vascular complications, aortic regurgitation and permanent pacemaker insertion occur more frequently after TAVR, while major bleeding is more likely to occur after surgery.19 As newer generations of valves are developed, it is expected that aortic regurgitation and pacemaker rates will decrease over time. Indeed, trial data presented at the American College of Cardiology meeting in March 2015 for the third-generation Sapien valve (Sapien S3) showed only a 3.0% to 4.2% rate of significant paravalvular leak.
Contemporary valve comparison data
The valve used in the original PARTNER data was the first-generation Sapien valve. Since then, the second generation of this valve, the Sapien XT, has been introduced and is the model currently used in the United States (with the third-generation valve mentioned above, the Sapien S3, still available only through clinical trials). Thus, the two contemporary valves available for commercial use in the United States are the Edwards Sapien XT and Medtronic CoreValve. There are limited data comparing these valves head-to-head, but one recent trial attempted to do just that.
The Comparison of Transcatheter Heart Valves in High Risk Patients with Severe Aortic Stenosis: Medtronic CoreValve vs Edwards Sapien XT (CHOICE) trial compared the Edwards Sapien XT and CoreValve devices. Two hundred and forty-one patients were randomized. The primary end point of this trial was “device success” (a composite end point of four components: successful vascular access and deployment of the device with retrieval of the delivery system, correct position of the device, intended performance of the valve without moderate or severe insufficiency, and only one valve implanted in the correct anatomical location).
In this trial, the balloon-expandable Sapien XT valve showed a significantly higher device success rate than the self-expanding CoreValve, due to a significantly lower rate of aortic regurgitation (4.1% vs 18.3%, P < .001) and the less frequent need for implantation of more than one valve (0.8% vs 5.8%, P = .03). Placement of a permanent pacemaker was considerably less frequent in the balloon-expandable valve group (17.3% vs 37.6%, P = .001).20
PREOPERATIVE CONSIDERATIONS AND EVALUATION CRITERIA
Currently, TAVR is indicated for patients with symptomatic severe native aortic valve stenosis who are deemed at high risk or inoperable by a heart team including interventional cardiologists and cardiac surgeons. The CoreValve was also recently approved for valve-in-valve insertion in high-risk or inoperable patients with a prosthetic aortic valve in place.
The STS risk score is a reasonable preliminary risk assessment tool and is applicable to most patients being evaluated for aortic valve replacement. The STS risk score represents the percentage risk of unfavorable outcomes based on certain clinical variables. A calculator is available at riskcalc.sts.org. Patients considered at high risk are those with an STS operative risk score of 8% or higher or a postoperative 30-day risk of death of 15% or higher.
It is important to remember, though, that the STS score does not account for certain severe surgical risk factors. These include the presence of a "porcelain aorta" (heavy circumferential calcification of the ascending aorta precluding cross-clamping), history of mediastinal radiation, “hostile chest” (kyphoscoliosis, other deformities, previous coronary artery bypass grafting with adhesion of internal mammary artery to the back of sternum), severely compromised respiratory function (forced expiratory volume in 1 second < 1 L or < 40% predicted, diffusing capacity for carbon monoxide < 30%), severe pulmonary hypertension, severe liver disease (Model for End-stage Liver Disease score 8–20), severe dementia, severe cerebrovascular disease, and frailty.
With regard to this last risk factor, frailty is not simply old age but rather a measurable characteristic akin to weakness or disability. Several tests exist to measure frailty, including the “eyeball test” (the physician’s subjective assessment), Mini-Mental State Examination, gait speed/15-foot walk test, hand grip strength, serum albumin, and assessment of activities of daily living. Formal frailty testing is recommended during the course of a TAVR workup.
Risk assessment and patient suitability for TAVR is ultimately determined by the combined judgment of the heart valve team using both the STS score and consideration of these other factors.
Implantation approaches
Today, TAVR could be performed by several approaches: transfemoral arterial, transapical, transaortic via partial sternotomy or right anterior thoracotomy,21,22 transcarotid,23–25 and transaxillary or subclavian.26,27 Less commonly, transfemoral-venous routes have been performed utilizing either transseptal28 or caval-aortic puncture.29
The transfemoral approach is used most commonly by most institutions, including Cleveland Clinic. It allows for a completely percutaneous insertion and, in select cases, without endotracheal intubation and general anesthesia (Figure 1).
In patients with difficult femoral access due to severe calcification, extreme tortuosity, or small diameter, alternative access routes become a consideration. In this situation, at our institution, we favor the transaortic approach in patients who have not undergone cardiac surgery in the past, while the transapical approach is used in patients who had previous cardiac surgery. With the transapical approach, we have found the outcomes similar to those of transfemoral TAVR after propensity matching.30,31 Although there is a learning curve,32 transapical TAVR can be performed with very limited mortality and morbidity. In a recent series at Cleveland Clinic, the mortality rate with the transapical approach was 1.2%, renal failure occurred in 4.7%, and a pacemaker was placed in 5.9% of patients; there were no strokes.33 This approach can be utilized for simultaneous additional procedures like transcatheter mitral valve reimplantation and percutaneous coronary interventions.34–36
Transcatheter aortic valve replacement (TAVR) has established itself as an effective way of treating high-risk patients with severe aortic valve stenosis. With new generations of existing valves and newer alternative devices, the procedure promises to become increasingly safer. The field is evolving rapidly and it will be important for interventional cardiologists and cardiac surgeons alike to stay abreast of developments. This article reviews the history of this promising procedure and examines its use in current practice.
HISTORICAL PERSPECTIVE
In 1980, Danish researcher H. R. Anderson reported developing and testing a balloon-expandable valve in animals.1 The technology was eventually acquired and further developed by Edwards Life Sciences (Irvine, California).
Alain Cribier started early work in humans in 2002 in France.2 He used a transfemoral arterial access to approach the aortic valve transseptally, but this procedure was associated with high rates of mortality and stroke.3 At the same time, in the United States, animal studies were being carried out by Lars G. Svensson, Todd Dewey, and Michael Mack to develop a transapical method of implantation,4,5 while John Webb and colleagues were also developing a transapical aortic valve implantation technique,6,7 and later went on to develop a retrograde transfemoral technique. This latter technique became feasible once Edwards developed a catheter that could be flexed to get around the aortic arch and across the aortic valve.
As the Edwards balloon-expandable valve (Sapien) was being developed, a nitinol-based self-expandable valve system was introduced by Medtronic: the CoreValve. Following feasibility studies,5,8 the safety and efficacy of these valves were established thorough the Placement of Aortic Transcatheter Valves (PARTNER) trial and the US Core Valve Pivotal Trial. These valves are currently approved by the US Food and Drug Administration (FDA) for patients for whom conventional surgery would pose an extreme or high risk.9–11
CLINICAL TRIALS OF TAVR
The two landmark prospective randomized trials of TAVR were the PARTNER trial and CoreValve Pivotal Trial.
The PARTNER trial consisted of two parts: PARTNER A, which compared the Sapien balloon-expandable transcatheter valve with surgical aortic valve replacement in patients at high surgical risk (Society of Thoracic Surgeons [STS] score > 10%), and PARTNER B, which compared TAVR with medical therapy in patients who could not undergo surgery (combined risk of serious morbidity or death of 50% or more, and two surgeons agreeing that the patient was inoperable).
Similarly, the CoreValve Pivotal Trial compared the self-expandable transcatheter valve with conventional medical and surgical treatment.
TAVR is comparable to surgery in outcomes, with caveats
In the PARTNER A trial, mortality rates were similar between patients who underwent Sapien TAVR and those who underwent surgical valve replacement at 30 days (3.4% and 6.5%, P = .07), 1 year (24.2% and 26.8%), and 2 years (33.9% and 35.0%). The patients in this group were randomized to either Sapien TAVR or surgery (Table 1).10,12
The combined rate of stroke and transient ischemic attack was higher in the patients assigned to TAVR at 30 days (5.5% with TAVR vs 2.4% with surgery, P = .04) and at 1 year (8.3% with TAVR vs 4.3% with surgery, P = .04). The difference was of small significance at 2 years (11.2% vs 6.5%, P = .05). At 30 days, the rate of major vascular complications was higher with TAVR (11.0% vs 3.2%), while surgery was associated with more frequent major bleeding episodes (19.5% vs 9.3%) and new-onset atrial fibrillation (16.0% vs 8.6%). The rate of new pacemaker requirement at 30 days was similar between the TAVR and surgical groups (3.8% vs 3.6%). Moderate or severe paravalvular aortic regurgitation was more common after TAVR at 30 days, 1 year, and 2 years. This aortic insufficiency was associated with increased late mortality.10,12
In the US CoreValve High Risk Study, no difference was found in the 30-day mortality rate in patients at high surgical risk randomized to CoreValve TAVR or surgery (3.3% and 4.5%) (Table 1). Surprisingly, the 1-year mortality rate was lower in the TAVR group than in the surgical group (14.1% vs 18.9%, respectively), a finding sustained at 2 years in data presented at the American College of Cardiology conference in March 2015.13–16
TAVR is superior to medical management, but the risk of stroke is higher
In the PARTNER B trial, inoperable patients were randomly assigned to undergo TAVR with a Sapien valve or medical management. TAVR resulted in lower mortality rates at 1 year (30.7% vs 50.7%) and 2 years (43.4% vs 68.0%) compared with medical management (Table 1).17 Of note, medical management included balloon valvuloplasty. The rate of the composite end point of death or repeat hospitalization was also lower with TAVR compared with medical therapy (44.1% vs 71.6%, respectively, at 1 year and 56.7% and 87.9%, respectively, at 2 years).17 The TAVR group had a higher stroke rate than the medical therapy group at 30 days (11.2% vs 5.5%, respectively) and at 2 years (13.8% vs 5.5%).17 Survival improved with TAVR in patients with an STS score of less than 15% but not in those with an STS score of 15% or higher.9
The very favorable results from the PARTNER trial rendered a randomized trial comparing self-expanding (CoreValve) TAVR and medical therapy unethical. Instead, a prospective single-arm study, the CoreValve Extreme Risk US Pivotal Trial, was used to compare the 12-month rate of death or major stroke with CoreValve TAVR vs a prespecified estimate of this rate with medical therapy.14 In about 500 patients who had a CoreValve attempt, the rate of all-cause mortality or major stroke at 1 year was significantly lower than the prespecified expected rate (26% vs 43%), reinforcing the results from the PARTNER Trial.14
Five-year outcomes
The 5-year PARTNER clinical and valve performance outcomes were published recently18 and continued to demonstrate equivalent outcomes for high-risk patients who underwent surgical aortic valve replacement or TAVR; there were no significant differences in all-cause mortality, cardiovascular mortality, stroke, or need for readmission to the hospital. The functional outcomes were similar as well, and no differences were demonstrated between surgical and TAVR valve performance.
Of note, moderate or severe aortic regurgitation occurred in 14% of patients in the TAVR group compared with 1% in the surgical aortic valve replacement group (P < .0001). This was associated with increased 5-year risk of death in the TAVR group (72.4% in those with moderate or severe aortic regurgitation vs 56.6% in those with mild aortic regurgitation or less; P = .003).
If the available randomized data are combined with observational reports, overall mortality and stroke rates are comparable between surgical aortic valve replacement and balloon-expandable or self-expandable TAVR in high-risk surgical candidates. Vascular complications, aortic regurgitation and permanent pacemaker insertion occur more frequently after TAVR, while major bleeding is more likely to occur after surgery.19 As newer generations of valves are developed, it is expected that aortic regurgitation and pacemaker rates will decrease over time. Indeed, trial data presented at the American College of Cardiology meeting in March 2015 for the third-generation Sapien valve (Sapien S3) showed only a 3.0% to 4.2% rate of significant paravalvular leak.
Contemporary valve comparison data
The valve used in the original PARTNER data was the first-generation Sapien valve. Since then, the second generation of this valve, the Sapien XT, has been introduced and is the model currently used in the United States (with the third-generation valve mentioned above, the Sapien S3, still available only through clinical trials). Thus, the two contemporary valves available for commercial use in the United States are the Edwards Sapien XT and Medtronic CoreValve. There are limited data comparing these valves head-to-head, but one recent trial attempted to do just that.
The Comparison of Transcatheter Heart Valves in High Risk Patients with Severe Aortic Stenosis: Medtronic CoreValve vs Edwards Sapien XT (CHOICE) trial compared the Edwards Sapien XT and CoreValve devices. Two hundred and forty-one patients were randomized. The primary end point of this trial was “device success” (a composite end point of four components: successful vascular access and deployment of the device with retrieval of the delivery system, correct position of the device, intended performance of the valve without moderate or severe insufficiency, and only one valve implanted in the correct anatomical location).
In this trial, the balloon-expandable Sapien XT valve showed a significantly higher device success rate than the self-expanding CoreValve, due to a significantly lower rate of aortic regurgitation (4.1% vs 18.3%, P < .001) and the less frequent need for implantation of more than one valve (0.8% vs 5.8%, P = .03). Placement of a permanent pacemaker was considerably less frequent in the balloon-expandable valve group (17.3% vs 37.6%, P = .001).20
PREOPERATIVE CONSIDERATIONS AND EVALUATION CRITERIA
Currently, TAVR is indicated for patients with symptomatic severe native aortic valve stenosis who are deemed at high risk or inoperable by a heart team including interventional cardiologists and cardiac surgeons. The CoreValve was also recently approved for valve-in-valve insertion in high-risk or inoperable patients with a prosthetic aortic valve in place.
The STS risk score is a reasonable preliminary risk assessment tool and is applicable to most patients being evaluated for aortic valve replacement. The STS risk score represents the percentage risk of unfavorable outcomes based on certain clinical variables. A calculator is available at riskcalc.sts.org. Patients considered at high risk are those with an STS operative risk score of 8% or higher or a postoperative 30-day risk of death of 15% or higher.
It is important to remember, though, that the STS score does not account for certain severe surgical risk factors. These include the presence of a "porcelain aorta" (heavy circumferential calcification of the ascending aorta precluding cross-clamping), history of mediastinal radiation, “hostile chest” (kyphoscoliosis, other deformities, previous coronary artery bypass grafting with adhesion of internal mammary artery to the back of sternum), severely compromised respiratory function (forced expiratory volume in 1 second < 1 L or < 40% predicted, diffusing capacity for carbon monoxide < 30%), severe pulmonary hypertension, severe liver disease (Model for End-stage Liver Disease score 8–20), severe dementia, severe cerebrovascular disease, and frailty.
With regard to this last risk factor, frailty is not simply old age but rather a measurable characteristic akin to weakness or disability. Several tests exist to measure frailty, including the “eyeball test” (the physician’s subjective assessment), Mini-Mental State Examination, gait speed/15-foot walk test, hand grip strength, serum albumin, and assessment of activities of daily living. Formal frailty testing is recommended during the course of a TAVR workup.
Risk assessment and patient suitability for TAVR is ultimately determined by the combined judgment of the heart valve team using both the STS score and consideration of these other factors.
Implantation approaches
Today, TAVR could be performed by several approaches: transfemoral arterial, transapical, transaortic via partial sternotomy or right anterior thoracotomy,21,22 transcarotid,23–25 and transaxillary or subclavian.26,27 Less commonly, transfemoral-venous routes have been performed utilizing either transseptal28 or caval-aortic puncture.29
The transfemoral approach is used most commonly by most institutions, including Cleveland Clinic. It allows for a completely percutaneous insertion and, in select cases, without endotracheal intubation and general anesthesia (Figure 1).
In patients with difficult femoral access due to severe calcification, extreme tortuosity, or small diameter, alternative access routes become a consideration. In this situation, at our institution, we favor the transaortic approach in patients who have not undergone cardiac surgery in the past, while the transapical approach is used in patients who had previous cardiac surgery. With the transapical approach, we have found the outcomes similar to those of transfemoral TAVR after propensity matching.30,31 Although there is a learning curve,32 transapical TAVR can be performed with very limited mortality and morbidity. In a recent series at Cleveland Clinic, the mortality rate with the transapical approach was 1.2%, renal failure occurred in 4.7%, and a pacemaker was placed in 5.9% of patients; there were no strokes.33 This approach can be utilized for simultaneous additional procedures like transcatheter mitral valve reimplantation and percutaneous coronary interventions.34–36
- Andersen HR, Knudsen LL, Hasenkam JM. Transluminal implantation of artificial heart valves. Description of a new expandable aortic valve and initial results with implantation by catheter technique in closed chest pigs. Eur Heart J 1992; 13:704– 708.
- Cribier A, Eltchaninoff H, Bash A, et al. Percutaneous transcatheter implantation of an aortic valve prosthesis for calcific aortic stenosis: first human case descrip- tion. Circulation 2002; 106:3006–3008.
- Cribier A, Eltchaninoff H, Tron C, et al. Early experience with percutaneous transcatheter implantation of heart valve prosthesis for the treatment of end-stage inoperable patients with calcific aortic stenosis. J Am Coll Cardiol 2004; 43:698– 703.
- Dewey TM, Walther T, Doss M, et al. Transapical aortic valve implantation: an animal feasibility study. Ann Thorac Surg 2006; 82:110–116.
- Svensson LG, Dewey T, Kapadia S, et al. United States feasibility study of trans- catheter insertion of a stented aortic valve by the left ventricular apex. Ann Thorac Surg 2008; 86:46–54.
- Lichtenstein SV, Cheung A, Ye J, et al. Transapical transcatheter aortic valve im- plantation in humans: initial clinical experience. Circulation 2006; 114:591–596.
- Webb JG, Pasupati S, Hyumphries K, et al. Percutaneous transarterial aortic valve replacement in selected high-risk patients with aortic stenosis. Circulation 2007; 116:755–763.
- Leon MB, Kodali S, Williams M, et al. Transcatheter aortic valve replacement in patients with critical aortic stenosis: rationale, device descriptions, early clinical experiences, and perspectives. Semin Thorac Cardiovasc Surg 2006; 18:165–174.
- Leon MB, Smith CR, Mack M, et al; PARTNER Trial Investigators. Transcatheter aortic-valve implantation for aortic stenosis in patients who cannot undergo sur- gery. N Engl J Med 2010; 363:1597–1607.
- Smith CR, Leon MB, Mack MJ, et al; PARTNER Trial Investigators. Transcatheter versus surgical aortic-valve replacement in high-risk patients. N Engl J Med 2011; 364:2187–2198.
- Adams DH, Popma JJ, Reardon MJ, et al; U.S. CoreValve Clinical Investigators. Transcatheter aortic-valve replacement with a self-expanding prosthesis. N Engl J Med 2014; 370:1790–1798.
- Kodali SK, Williams MR, Smith CR, et al; PARTNER Trial Investigators. Two- year outcomes after transcatheter or surgical aortic-valve replacement. N Engl J Med 2012; 366:1686–1695.
- Reardon M, et al. A randomized comparison of self-expanding
- Popma JJ, Adams DH, Reardon MJ, et al; CoreValve United States Clinical In- vestigators. Transcatheter aortic valve replacement using a self-expanding biopros- thesis in patients with severe aortic stenosis at extreme risk for surgery. J Am Coll Cardiol 2014; 63:1972–1981.
- Adams DH, Popma JJ, Reardon MJ. Transcatheter aortic-valve replacement with a self-expanding prosthesis (letter). N Engl J Med 2014; 371:967–968.
- Kaul S. Transcatheter aortic-valve replacement with a self-expanding prosthesis (letter). N Engl J Med 2014; 371:967.
- Makkar RR, Fontana GP, Jilaihawi H, et al. Transcathether aortic-valve re- placement for inoperable severe aortic stenosis. N Engl J Med 2012; 366: 1696–704.
- Mack MJ, Leon MB, Smith CR, et al; PARTNER 1 trial investigators. 5-year outcomes of transcatheter aortic valve replacement or surgical aortic valve re- placement for high surgical risk patients with aortic stenosis (PARTNER 1): a randomised controlled trial. Lancet 2015; 385:2477–2484.
- Cao C, Ang SC, Indraratna P, et al. Systematic review and meta-analysis of trans- catheter aortic valve implantation versus surgical aortic valve replacement for severe aortic stenosis. Ann Cardiothorac Surg 2013; 2:10–23.
- Abdel-Wahab M, Mehilli J, Frerker C, et al; CHOICE investigators. Comparison of balloon-expandable vs self-expandable valves in patients undergoing transcath- eter aortic valve replacement: the CHOICE randomized clinical trial. JAMA 2014; 311:1503–1514.
- Okuyama K, Jilaihawi H, Mirocha J, et al. Alternative access for balloon-ex- pandable transcatheter aortic valve replacement: comparison of the transaortic approach using right anterior thoracotomy to partial J-sternotomy. J Thorac Car- diovasc Surg 2014; 149:789–797.
- Lardizabal JA, O’Neill BP, Desai HV, et al. The transaortic approach for transcath- eter aortic valve replacement: initial clinical experience in the United States. J Am Coll Cardiol 2013; 61:2341–2345.
- Thourani VH, Gunter RL, Neravetla S, et al. Use of transaortic, transapical, and transcarotid transcatheter aortic valve replacement in inoperable patients. Ann Thorac Surg 2013; 96:1349–1357.
- Azmoun A, Amabile N, Ramadan R, et al. Transcatheter aortic valve implantation through carotid artery access under local anaesthesia. Eur J Cardiothorac Surg 2014; 46: 693–698.
- Rajagopal R, More RS, Roberts DH. Transcatheter aortic valve implantation through a transcarotid approach under local anesthesia. Catheter Cardiovasc In- terv 2014; 84:903–907.
- Fraccaro C, Napodano M, Tarantini G, et al. Expanding the eligibility for trans- catheter aortic valve implantation the trans-subclavian retrograde approach using: the III generation CoreValve revalving system. JACC Cardiovasc Interv 2009; 2:828–333.
- Petronio AS, De Carlo M, Bedogni F, et al. Safety and efficacy of the subclavian approach for transcatheter aortic valve implantation with the CoreValve revalving system. Circ Cardiovasc Interv 2010; 3:359–366.
- Cohen MG, Singh V, Martinez CA, et al. Transseptal antegrade transcatheter aor- tic valve replacement for patients with no other access approach—a contemporary experience. Catheter Cardiovasc Interv 2013; 82:987–993.
- Greenbaum AB, O’Neill WW, Paone G, et al. Caval-aortic access to allow trans- catheter aortic valve replacement in otherwise ineligible patients: initial human experience. J Am Coll Cardiol 2014; 63:2795–2804.
- D’Onofrio A, Salizzoni S, Agrifoglio M, et al. Medium term outcomes of trans- apical aortic valve implantation: results from the Italian Registry of Trans-Apical Aortic Valve Implantation. Ann Thorac Surg 2013; 96:830–835.
- Johansson M, Nozohoor S, Kimblad PO, Harnek J, Olivecrona GK, Sjögren J. Transapical versus transfemoral aortic valve implantation: a comparison of survival and safety. Ann Thorac Surg 2011; 91:57–63.
- Kempfert J, Rastan A, Holzhey D, et al. Transapical aortic valve implantation: analysis of risk factors and learning experience in 299 patients. Circulation 2011; 124(suppl):S124–S129.
- Aguirre J, Waskowski R, Poddar K, et al. Transcatheter aortic valve replacement: experience with the transapical approach, alternate access sites, and concomitant cardiac repairs. J Thorac Cardiovasc Surg 2014; 148:1417–1422.
- Al Kindi AH, Salhab KF, Roselli EE, Kapadia S, Tuzcu EM, Svensson LG. Alternative access options for transcatheter aortic valve replacement in patients with no conventional access and chest pathology. J Thorac Cardiovasc Surg 2014; 147:644–651.
- Salhab KF, Al Kindi AH, Lane JH, et al. Concomitant percutaneous coronary intervention and transcatheter aortic valve replacement: safe and feasible replace- ment alternative approaches in high-risk patients with severe aortic stenosis and coronary artery disease. J Card Surg 2013; 28:481–483.
- Al Kindi AH, Salhab KF, Kapadia S, et al. Simultaneous transapical transcatheter aortic and mitral valve replacement in a high-risk patient with a previous mitral bioprosthesis. J Thorac Cardiovasc Surg 2012; 144:e90–e91.
- Andersen HR, Knudsen LL, Hasenkam JM. Transluminal implantation of artificial heart valves. Description of a new expandable aortic valve and initial results with implantation by catheter technique in closed chest pigs. Eur Heart J 1992; 13:704– 708.
- Cribier A, Eltchaninoff H, Bash A, et al. Percutaneous transcatheter implantation of an aortic valve prosthesis for calcific aortic stenosis: first human case descrip- tion. Circulation 2002; 106:3006–3008.
- Cribier A, Eltchaninoff H, Tron C, et al. Early experience with percutaneous transcatheter implantation of heart valve prosthesis for the treatment of end-stage inoperable patients with calcific aortic stenosis. J Am Coll Cardiol 2004; 43:698– 703.
- Dewey TM, Walther T, Doss M, et al. Transapical aortic valve implantation: an animal feasibility study. Ann Thorac Surg 2006; 82:110–116.
- Svensson LG, Dewey T, Kapadia S, et al. United States feasibility study of trans- catheter insertion of a stented aortic valve by the left ventricular apex. Ann Thorac Surg 2008; 86:46–54.
- Lichtenstein SV, Cheung A, Ye J, et al. Transapical transcatheter aortic valve im- plantation in humans: initial clinical experience. Circulation 2006; 114:591–596.
- Webb JG, Pasupati S, Hyumphries K, et al. Percutaneous transarterial aortic valve replacement in selected high-risk patients with aortic stenosis. Circulation 2007; 116:755–763.
- Leon MB, Kodali S, Williams M, et al. Transcatheter aortic valve replacement in patients with critical aortic stenosis: rationale, device descriptions, early clinical experiences, and perspectives. Semin Thorac Cardiovasc Surg 2006; 18:165–174.
- Leon MB, Smith CR, Mack M, et al; PARTNER Trial Investigators. Transcatheter aortic-valve implantation for aortic stenosis in patients who cannot undergo sur- gery. N Engl J Med 2010; 363:1597–1607.
- Smith CR, Leon MB, Mack MJ, et al; PARTNER Trial Investigators. Transcatheter versus surgical aortic-valve replacement in high-risk patients. N Engl J Med 2011; 364:2187–2198.
- Adams DH, Popma JJ, Reardon MJ, et al; U.S. CoreValve Clinical Investigators. Transcatheter aortic-valve replacement with a self-expanding prosthesis. N Engl J Med 2014; 370:1790–1798.
- Kodali SK, Williams MR, Smith CR, et al; PARTNER Trial Investigators. Two- year outcomes after transcatheter or surgical aortic-valve replacement. N Engl J Med 2012; 366:1686–1695.
- Reardon M, et al. A randomized comparison of self-expanding
- Popma JJ, Adams DH, Reardon MJ, et al; CoreValve United States Clinical In- vestigators. Transcatheter aortic valve replacement using a self-expanding biopros- thesis in patients with severe aortic stenosis at extreme risk for surgery. J Am Coll Cardiol 2014; 63:1972–1981.
- Adams DH, Popma JJ, Reardon MJ. Transcatheter aortic-valve replacement with a self-expanding prosthesis (letter). N Engl J Med 2014; 371:967–968.
- Kaul S. Transcatheter aortic-valve replacement with a self-expanding prosthesis (letter). N Engl J Med 2014; 371:967.
- Makkar RR, Fontana GP, Jilaihawi H, et al. Transcathether aortic-valve re- placement for inoperable severe aortic stenosis. N Engl J Med 2012; 366: 1696–704.
- Mack MJ, Leon MB, Smith CR, et al; PARTNER 1 trial investigators. 5-year outcomes of transcatheter aortic valve replacement or surgical aortic valve re- placement for high surgical risk patients with aortic stenosis (PARTNER 1): a randomised controlled trial. Lancet 2015; 385:2477–2484.
- Cao C, Ang SC, Indraratna P, et al. Systematic review and meta-analysis of trans- catheter aortic valve implantation versus surgical aortic valve replacement for severe aortic stenosis. Ann Cardiothorac Surg 2013; 2:10–23.
- Abdel-Wahab M, Mehilli J, Frerker C, et al; CHOICE investigators. Comparison of balloon-expandable vs self-expandable valves in patients undergoing transcath- eter aortic valve replacement: the CHOICE randomized clinical trial. JAMA 2014; 311:1503–1514.
- Okuyama K, Jilaihawi H, Mirocha J, et al. Alternative access for balloon-ex- pandable transcatheter aortic valve replacement: comparison of the transaortic approach using right anterior thoracotomy to partial J-sternotomy. J Thorac Car- diovasc Surg 2014; 149:789–797.
- Lardizabal JA, O’Neill BP, Desai HV, et al. The transaortic approach for transcath- eter aortic valve replacement: initial clinical experience in the United States. J Am Coll Cardiol 2013; 61:2341–2345.
- Thourani VH, Gunter RL, Neravetla S, et al. Use of transaortic, transapical, and transcarotid transcatheter aortic valve replacement in inoperable patients. Ann Thorac Surg 2013; 96:1349–1357.
- Azmoun A, Amabile N, Ramadan R, et al. Transcatheter aortic valve implantation through carotid artery access under local anaesthesia. Eur J Cardiothorac Surg 2014; 46: 693–698.
- Rajagopal R, More RS, Roberts DH. Transcatheter aortic valve implantation through a transcarotid approach under local anesthesia. Catheter Cardiovasc In- terv 2014; 84:903–907.
- Fraccaro C, Napodano M, Tarantini G, et al. Expanding the eligibility for trans- catheter aortic valve implantation the trans-subclavian retrograde approach using: the III generation CoreValve revalving system. JACC Cardiovasc Interv 2009; 2:828–333.
- Petronio AS, De Carlo M, Bedogni F, et al. Safety and efficacy of the subclavian approach for transcatheter aortic valve implantation with the CoreValve revalving system. Circ Cardiovasc Interv 2010; 3:359–366.
- Cohen MG, Singh V, Martinez CA, et al. Transseptal antegrade transcatheter aor- tic valve replacement for patients with no other access approach—a contemporary experience. Catheter Cardiovasc Interv 2013; 82:987–993.
- Greenbaum AB, O’Neill WW, Paone G, et al. Caval-aortic access to allow trans- catheter aortic valve replacement in otherwise ineligible patients: initial human experience. J Am Coll Cardiol 2014; 63:2795–2804.
- D’Onofrio A, Salizzoni S, Agrifoglio M, et al. Medium term outcomes of trans- apical aortic valve implantation: results from the Italian Registry of Trans-Apical Aortic Valve Implantation. Ann Thorac Surg 2013; 96:830–835.
- Johansson M, Nozohoor S, Kimblad PO, Harnek J, Olivecrona GK, Sjögren J. Transapical versus transfemoral aortic valve implantation: a comparison of survival and safety. Ann Thorac Surg 2011; 91:57–63.
- Kempfert J, Rastan A, Holzhey D, et al. Transapical aortic valve implantation: analysis of risk factors and learning experience in 299 patients. Circulation 2011; 124(suppl):S124–S129.
- Aguirre J, Waskowski R, Poddar K, et al. Transcatheter aortic valve replacement: experience with the transapical approach, alternate access sites, and concomitant cardiac repairs. J Thorac Cardiovasc Surg 2014; 148:1417–1422.
- Al Kindi AH, Salhab KF, Roselli EE, Kapadia S, Tuzcu EM, Svensson LG. Alternative access options for transcatheter aortic valve replacement in patients with no conventional access and chest pathology. J Thorac Cardiovasc Surg 2014; 147:644–651.
- Salhab KF, Al Kindi AH, Lane JH, et al. Concomitant percutaneous coronary intervention and transcatheter aortic valve replacement: safe and feasible replace- ment alternative approaches in high-risk patients with severe aortic stenosis and coronary artery disease. J Card Surg 2013; 28:481–483.
- Al Kindi AH, Salhab KF, Kapadia S, et al. Simultaneous transapical transcatheter aortic and mitral valve replacement in a high-risk patient with a previous mitral bioprosthesis. J Thorac Cardiovasc Surg 2012; 144:e90–e91.
KEY POINTS
- In randomized trials, transcatheter aortic valve replacement (TAVR) has produced results that are comparable to surgical aortic valve replacement in high-risk patients. TAVR is superior to medical management in patients who cannot undergo surgery, although it is associated with higher rates of stroke.
- Risk assessment and suitability for TAVR is determined by a heart team composed of interventional cardiologists and cardiac surgeons. Society of Thoracic Surgeons Score and a number of other criteria mentioned below are considered during this process.
- The transfemoral arterial approach is the most common approach used by most institutions, but other approaches such as transaortic, transapical, transaxillary, and transcarotid are utilized if suitable in patients who have difficult femoral access.
Evolving strategies to prevent stroke and thromboembolism in nonvalvular atrial fibrillation
Atrial fibrillation (AF), the most common cardiac arrhythmia, has become a major public health problem. In the United States, the prevalence of AF was estimated at 2.7 to 6.1 million in 2010, and it is expected to rise to between 5.6 and 12 million by 2050.1 The arrhythmia is associated with impaired quality of life and increased morbidity and mortality.1,2 Stroke remains the most devastating consequence of AF.
The clinical management of patients with AF typically targets two main goals: prevention of stroke or thromboembolism and control of symptoms. This article addresses the evolving pharmacologic and nonpharmacologic strategies in stroke prevention in nonvalvular AF; reviews clinical trials evaluating medical and procedural strategies, including the novel oral anticoagulants and left atrial appendage (LAA) exclusion devices; and assesses the impact of these novel strategies on clinical practice.
RISK OF STROKE AND THROMBOEMBOLISM IN NONVALVULAR AF
Stroke occurrence from AF is primarily caused by thrombi formation in the left atrium, most commonly in the LAA. It is important to recognize that the cardiovascular risk factors for AF are also risk factors for atheroembolism; therefore, specific risk factor management is as important as anticoagulation when addressing stroke risk.
The incidence of all-cause stroke in patients with AF is 5%, and it is believed that AF causes approximately 15% of all strokes in the United States.1 This risk appears to be more significant in older patients who are more vulnerable to ischemic strokes. Estimates are that AF independently increases the risk of stroke by fivefold throughout all ages, with a steep increase in percentage of strokes attributed to AF from 1.5% at ages 50 to 59 to 23.5% at ages 80 to 89.1 Importantly, the clinical course of ischemic stroke associated with AF is often more severe than for strokes of other causes,3 further emphasizing the need for stroke prevention.
Assessment of stroke risk/thromboembolism
Multiple risk estimation scores have been developed based on epidemiologic data. Until recently, the CHADS2 score4 was the most commonly used, but it has been superseded by the CHA2DS2-VASc score.5 The point system for this scoring system is shown in Table 1. In contrast with CHADS2, this updated system assigns 2 points for age over 75 years and accounts for stroke risk in the relatively younger group of patients (age 65–75) and in females, neither of whom were included in CHADS2. The CHA2DS2-VASc score ranges between 0 and 9 with a respective estimated stroke risk of 0 to 15.2% per year. Note that for females who are younger than 65 years, no points are given for sex. The major advantage of the CHA2DS2-VASc score over the CHADS2 score is that it is more accurate for lower-risk categories. It has been adopted in most of the recent guidelines that address stroke risk in AF.
In clinical practice, practitioners use these scores to define three primary stroke risk categories: low, intermediate, or high. In our practice, we use a 2% per year cut-off to identify high-risk patients in whom the risk of stroke significantly outweighs the risk of bleeding on anticoagulants. In general, patients with a CHA2DS2-VASc score equal to or greater than 2 have a greater than 2% stroke risk per year and are most likely to benefit from antithrombotic therapies.
In male patients with a CHA2DS2-VASc score of 0 and in most patients with a score of 1, the stroke risk is less than 1% per year. These patients are not likely to derive benefit from anticoagulant therapy. They are usually approached on a case-by-case basis with careful assessment of bleeding risk and discussion of risks and benefits of anticoagulant strategies.
Assessment of bleeding risk
Any general approach to thromboembolism risk assessment in patients with AF should include an analysis that weighs the benefits of anticoagulant therapies against the risks of bleeding. Although no precise tools exist to predict bleeding risk, the HAS-BLED score is increasingly used.6 This score assigns 1 point to each of the following:
- systolic blood pressure greater than 160 mm Hg
- abnormal renal function
- abnormal liver function
- age older than 65
- prior cerebrovascular event
- prior bleeding
- history of labile international normalized ratios (INR)
- alcohol intake (> 8 U/week)
- drug use, especially antiplatelet agents or nonsteroidal anti-inflammatory drugs (NSAIDs).
In general, a HAS-BLED score of 3 or greater indicates increased 1-year risk of intracranial bleed, bleeding requiring hospitalization, drop in hemoglobin of at least 2 g/dL, or need for transfusion.
One problem with the bleeding risk scores is that they were derived from studies that included bleeding events of differing severity. Most bleeding events do not lead to death or severe disability with the exception of intracranial bleeding, which is, therefore, the primary concern when assessing bleeding risk.
The estimated bleeding risk with anticoagulant therapy ranges from 0.2% to 0.4% per year but could be much higher in patients with prior severe bleeding, intracranial hemorrhage, thrombocytopenia, coagulopathies, recent surgery, or ongoing bleeding, aortic dissection, malignant hypertension, and in those receiving a combination of anticoagulant and antiplatelet agents.
MEDICAL THERAPIES TO PREVENT STROKE AND THROMBOEMBOLISM IN AF
In general, anticoagulation reduces the risk of ischemic stroke and thromboembolic events by approximately two-thirds, regardless of baseline risk. Anticoagulant options have increased substantially in the past few years with the introduction of novel oral anticoagulants, including the direct thrombin-inhibitor dabigatran and the factor Xa inhibitors rivaroxaban, apixaban, and edoxaban.
Warfarin
Warfarin has been used for decades for stroke prevention. It remains the only acceptable anticoagulant in patients with valvular AF. Multiple randomized clinical trials have assessed the efficacy of warfarin for stroke prevention in patients with nonvalvular AF.7 These trials demonstrated that warfarin significantly reduces stroke risk, stroke severity, and 30-day mortality compared with no anticoagulant therapy.7,8
Although warfarin is one of the most efficacious drugs to prevent stroke in AF, it has several key limitations. The most important is the need for dose adjustment to keep the INR in a narrow window (2.0 to 3.0) in which net clinical benefit is achieved without increased bleeding risk. The need for continuous monitoring is an inconvenience to patients and often leads to drug discontinuation and nonadherence. A meta-analysis found that patients are only in the therapeutic INR about half of the time.9 Importantly, the time spent in therapeutic INR range cor- relates significantly with the reduction in stroke risk.10 Furthermore, patients who spend less than 40% of the time in the therapeutic INR range are at a higher stroke risk than those not taking warfarin.10
Another limitation with the medication is the dietary restriction on intake of vitamin K-rich green vegetables, which are emphasized as healthy food choices especially in patients with heart disease. Higher warfarin doses are required in patients who consume greens and salads. It is important that patients be consistent in their intake of vitamin K-rich foods to avoid labile INRs, a difficult task for most patients.
Finally, there are several drugs that might interact with warfarin and potentially interfere with its safety or efficacy. These drugs include amiodarone, statins including simvastatin and rosuvastatin (not atorvastatin or pravastatin), fibrates (fenofibrate, gemfibrozil), antibiotics (sulfamethoxazole/trimethoprim, metronidazole), and azole antifungals (fluconazole, miconazole, voriconazole). The use of drugs that induce the cytochrome P450 enzyme CYP2C9, such as rifampin, decrease warfarin effectiveness by reducing INR values. Other non-CYP2C9-dependent drug interactions exist as well.
Aspirin monotherapy or in combination with other agents
Aspirin monotherapy or aspirin plus clopidogrel both increase the risk of bleeding without appreciable benefit, and, as such, their use for stroke prevention in patients with AF is not well supported. The combination of aspirin plus low-dose warfarin was assessed in the SPAF-III trial11 that randomized AF patients at stroke risk to either aspirin plus low-dose warfarin or to dose-adjusted warfarin to target a therapeutic INR. In this trial, patients on aspirin plus low-dose warfarin had significantly higher morbidity and mortality than patients who took adjusted-dose warfarin alone. Thus, the combination of low-dose warfarin plus aspirin should not be used for stroke prevention in AF.
In contrast, the combination of aspirin plus full anticoagulation with warfarin has not been well studied. Limited post-hoc data from the SPORTIF trials suggest, however, that this combination does not reduce the risk of stroke or thromboembolism more than warfarin alone.12
Novel oral anticoagulants
Dabigatran. The value of dabigatran for prevention of stroke or thromboembolism in AF was tested in the RE-LY trial (Randomized Evaluation of Long-Term Anticoagulation Therapy).13 In this trial, 18,113 patients with AF at high risk for stroke were randomized to dabigatran (110 mg or 150 mg twice daily) or adjusted-dose warfarin (INR target 2.0–3.0). By intention-to-treat analysis, dabigatran 150 mg was superior to warfarin for stroke prevention. Importantly, the risk of intracranial or life-threatening bleeding was significantly lower for both dabigatran doses compared with warfarin. Of note, gastrointestinal bleeding was more common with dabigatran 150 mg than warfarin; rates were similar for dabigatran 110 mg versus warfarin.
Rivaroxaban. This factor-Xa inhibitor was assessed in ROCKET-AF (Rivaroxaban Once-daily oral direct factor Xa inhibition Compared with vitamin K antagonism for prevention of stroke and Embolism Trial in Atrial Fibrillation).14 In this trial, 14,264 patients with AF and at risk for stroke were randomized to rivaroxaban (20 mg once daily) or warfarin (INR target 2.5). In the warfarin arm, INR was in the therapeutic range only 55% of the time. Results showed rivaroxaban was noninferior, but not superior, to warfarin for the prevention of stroke or systemic thromboembolism, the primary end points. From a safety standpoint, the overall bleeding rates were similar in the treatment arms with less life-threatening (fatal or intracranial) hemorrhage events with rivaroxaban.
Apixaban. This factor-Xa inhibitor was tested for stroke prevention in AF in two separate clinical trials.15,16 In the AVERROES trial (Apixaban Versus Acetylsalicylic Acid to Prevent Strokes),15 5,599 patients with AF deemed “unsuitable” for warfarin were randomized to apixaban (5 mg twice daily) or aspirin (81–324 mg daily). The trial was terminated early due to superiority of apixaban in achieving the primary end point: occurrence of stroke or systemic embolism. Importantly, the risk of major bleeding appeared to be similar with apixaban versus aspirin.
The ARISTOTLE (Apixaban for Reduction in Stroke and Other Thromboembolic Events in Atrial Fibrillation) trial16 randomized 18,201 patients with AF and at least one additional risk factor for stroke to either apixaban 5 mg or warfarin (target INR 2.0–3.0). In this trial, apixaban was superior to warfarin for the prevention of stroke or systemic embolism, the primary efficacy end point. There also appeared to be a mortality benefit with apixaban versus warfarin. Importantly, the risk of major and intracranial bleeding, the primary safety outcomes, occurred at lower rates in the apixaban group.
All three of these oral anticoagulants require dose adjustment in patients with renal insufficiency and are contraindicated in patients with end-stage renal failure. They are not indicated in patients with valvular heart disease or a mechanical heart valves.
NONPHARMACOLOGIC INTERVENTIONS
Most atrial thrombi in patients with nonvalvular AF form in the LAA. Nonpharmacologic interventions have been developed to block the LAA to reduce the risk of stroke. These are especially valuable options for patients who are not candidates for chronic anticoagulation. In patients undergoing mitral valve surgery,17 ligation to close the LAA has become a standard practice at experienced centers. The introduction of less invasive catheter-based interventions to occlude the LAA has provided additional options.
Watchman device
The Watchman implant, a closure device that blocks the LAA, was recently approved by the US Food and Drug Administration (FDA) for stroke prevention in patients with nonvalvular AF who are at increased risk for stroke based on a CHA2DS2-VASc score of 2 or greater; candidates also must have an appropriate rationale for nonpharmacologic therapy. The expandable-cage device is surgically delivered into the LAA (Figure 1), which subsequently endothelializes and isolates the LAA. (Video 1 shows the delivery sheath positioned at the os of the appendage with a confirmatory contrast appendogram. Video 2 shows the delivery of the device into the appendage.) Therapeutic warfarin is required for a minimum of 45 days after implant followed by aspirin and clopidogrel for 6 months and then aspirin alone.
This device was initially tested in the PROTECT AF (Watchman Left Atrial Appendage System for Embolic PROTECTion in Patients with Atrial Fibrillation) trial, a noninferiority trial that randomized patients to either device implant or warfarin.18 Device implant was successful in 91% of patients in whom it was attempted. Overall, the study showed noninferiority of the device to warfarin in terms of the primary efficacy standpoint, which included stroke, systemic embolism, and cardiovascular death; however, this came at the expense of higher incidence of procedure-related complications, which seemed to be dependent on a learning curve with the device.
In the PREVAIL (Evaluation of the Watchman LAA Closure Device in Patients with Atrial Fibrillation Versus Long-Term Warfarin Therapy) trial,19 although noninferiority was not achieved, the event rates were low and the safety of the procedure was much improved. This was also demonstrated in registry data, which showed improved safety with device implantation with increased operator experience.
Of note, both of these trials included only patients who were eligible for warfarin. Another nonrandomized study20 assessed the use of this device in patients with a contraindication to long-term anticoagulation. Results showed a very low incidence of stroke, which was lower than CHADS2-matched controls taking either aspirin or clopidogrel. The FDA has approved this device for stroke prevention in AF.
Lariat system
The Lariat system is another percutaneous system for occlusion of the LAA. This device, which requires both atrial transseptal and epicardial access, ligates the appendage from the pericardial space. While some small studies have shown it safe and efficacious in patients with AF who cannot take anticoagulation,21 other reports have not been as encouraging.22 The device has been approved by the FDA for “soft tissue approximation”; it is not approved for stroke prevention.
SUMMARY
The most common serious complications of AF are stroke and thromboembolism. Medical and interventional therapies have been developed to prevent these complications. In patients with an estimated thromboembolic risk of greater than 1% to 2% per year, anticoagulation is warranted to reduce that risk. Warfarin remains one of the most studied and useful medications for this purpose, but its use is limited by the need for frequent monitoring, multiple drug interactions, dietary restrictions, and, most importantly, by the difficulty of consistently maintaining therapeutic INRs.
The recently introduced novel oral anticoagulants have been found to be at least noninferior to warfarin and for some agents to be superior to warfarin for the prevention of stroke and thromboembolism. Their main advantage is that they do not require monitoring and have fewer drug interactions and dietary restrictions. Most important, there appear to be fewer major and life-threatening bleeding events than with warfarin. However, use of these novel agents could be limited by patients’ renal function, which needs to be assessed when these agents are being considered. Another limitation with these agents (versus warfarin) is that patients are not therapeutically anticoagulated when they miss a dose, whereas missing a single dose of warfarin may not have the same effect.
In our practice, we have transitioned to use of these novel oral anticoagulants whenever possible using an approach that assesses the individual patient’s risks for both stroke and thromboembolism as well as the risk for bleeding. In patients who are at increased risk of bleeding but at risk of stroke in AF, percutaneous occlusion of the LAA using the Watchman device is offered. This device has been shown to be noninferior to warfarin for stroke prevention and may provide a survival benefit due to the reduction of life-threatening bleeding, which is an inherent risk with anticoagulants. One caveat is that there is a residual risk of stroke after undergoing LAA occlusion because the same risk factors for stroke in AF contribute to stroke from atherothrombosis and atheroembolism; also, thrombi can form in the body of the left atrium.
Clinical decision-making is often challenging in patients with AF who are at risk of stroke and bleeding. In fact, these risk factors often overlap. In our practice, we have established a multidisciplinary clinic for stroke prevention in AF that involves cardiologists, cardiac electrophysiologists, neurologists, gastroenterologists, and vascular medicine specialists. This model allows a multidisciplinary assessment of patients’ individual risks and ultimately facilitates clinical decision-making in terms of strategies to prevent stroke and thromboembolism in AF.
- Roger VL, Go AS, Lloyd-Jones DM, et al; American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics—2012 update: a report from the American Heart Association. Circulation 2012; 125:e2–e220.
- January CT, Wann LS, Alpert JS, et al; American College of Cardiology/American Heart Association Task Force on Practice Guidelines. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the Heart Rhythm Society. J Am Coll Cardiol 2014; 64(21):e1–76.
- Dulli DA, Stanko H, Levine RL. Atrial fibrillation is associated with severe acute ischemic stroke. Neuroepidemiology 2003; 22:118–123.
- Gage BF, Waterman AD, Shannon W, Boechler M, Rich MW, Radford MJ. Validation of clinical classification schemes for predicting stroke: results from the national registry of atrial fibrillation. JAMA 2001; 285:2864–2870.
- Lip GY, Nieuwlaat R, Pisters R, Lane DA, Crijns HJ. Refining clinical risk stratification for predicting stroke and thromboembolism in atrial fibrillation using a novel risk factor-based approach: the euro heart survey on atrial fibrillation. Chest 2010; 137:263-272.
- Pisters R, Lane DA, Nieuwlaat R, de Vos CB, Crijns HJ, Lip GY. A novel user-friendly score (HAS-BLED) to assess 1-year risk of major bleeding in patients with atrial fibrillation: the Euro Heart Survey. Chest 2010; 138:1093–1100.
- Hart RG, Pearce LA, Aguilar MI. Meta-analysis: antithrombotic therapy to prevent stroke in patients who have nonvalvular atrial fibrillation. Ann Intern Med 2007; 146:857–867.
- Johnsen SP, Svendsen ML, Hansen ML, Brandes A, Mehnert F, Husted SE. Preadmission oral anticoagulant treatment and clinical outcome among patients hospitalized with acute stroke and atrial fibrillation: a nationwide study. Stroke 2014; 45:168-175.
- Baker WL, Cios DA, Sander SD, Coleman CI. Meta-analysis to assess the quality of warfarin control in atrial fibrillation patients in the united states. J Manage Care Pharm 2009; 15:244-252.
- Morgan CL, McEwan P, Tukiendorf A, Robinson PA, Clemens A, Plumb JM. Warfarin treatment in patients with atrial fibrillation: observing outcomes associated with varying levels of INR control. Thromb Res 2009; 124:37–41.
- Adjusted-dose warfarin versus low-intensity, fixed-dose warfarin plus aspirin for high-risk patients with atrial fibrillation: Stroke prevention in atrial fibrillation III randomised clinical trial. Lancet 1996; 348(9028):633–638.
- Flaker GC, Gruber M, Connolly SJ, et al. Risks and benefits of combining aspirin with anticoagulant therapy in patients with atrial fibrillation: an exploratory analysis of stroke prevention using an oral thrombin inhibitor in atrial fibrillation (SPORTIF) trials. Am Heart J 2006; 152:967–973.
- Connolly SJ, Ezekowitz MD, Yusuf S, et al, and the RE-LY Steering Committee and Investigators. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med 2009; 361:1139–1151.
- Patel MR, Mahaffey KW, Garg J, et al. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med 2011; 365:883–891.
- Connolly SJ, Eikelboom J, Joyner C, et al. Apixaban in patients with atrial fibrillation. N Engl J Med 2011; 364:806–817.
- Granger CB, Alexander JH, McMurray JJ, et al. Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med 2011; 365:981–992.
- Garcia-Fernandez MA, Perez-David E, Quiles J, et al. Role of left atrial appendage obliteration in stroke reduction in patients with mitral valve prosthesis: a transesophageal echocardiographic study. J Am Coll Cardiol 2003; 42:1253–1258.
- Holmes DR, Reddy VY, Turi ZG, et al. Percutaneous closure of the left atrial appendage versus warfarin therapy for prevention of stroke in patients with atrial fibrillation: a randomised non-inferiority trial. Lancet 2009; 374(9689):534–542.
- Holmes DR Jr, Kar S, Price MJ, et al. Prospective randomized evaluation of the Watchman left atrial appendage closure device in patients with atrial fibrillation versus long-term warfarin therapy: The PREVAIL trial. J Am Coll Cardiol 2014; 64:1–12.
- Reddy VY, Mobius-Winkler S, Miller MA, et al. Left atrial appendage closure with the Watchman device in patients with a contraindication for oral anticoagulation: the ASAP study (ASA plavix feasibility study with watchman left atrial appendage closure technology). J Am Coll Cardiol 2013; 61:2551–2556.
- Bartus K, Han FT, Bednarek J, et al. Percutaneous left atrial appendage suture ligation using the LARIAT device in patients with atrial fibrillation: initial clinical experience. J Am Coll Cardiol 2013; 62:108–118.
- Chatterjee S, Herrmann HC, Wilensky RL, et al. Safety and procedural success of left atrial appendage exclusion with the Lariat device: a systematic review of published reports and analytic review of the FDA MAUDE database. JAMA Intern Med 2015; 175(7):1104–9.
Atrial fibrillation (AF), the most common cardiac arrhythmia, has become a major public health problem. In the United States, the prevalence of AF was estimated at 2.7 to 6.1 million in 2010, and it is expected to rise to between 5.6 and 12 million by 2050.1 The arrhythmia is associated with impaired quality of life and increased morbidity and mortality.1,2 Stroke remains the most devastating consequence of AF.
The clinical management of patients with AF typically targets two main goals: prevention of stroke or thromboembolism and control of symptoms. This article addresses the evolving pharmacologic and nonpharmacologic strategies in stroke prevention in nonvalvular AF; reviews clinical trials evaluating medical and procedural strategies, including the novel oral anticoagulants and left atrial appendage (LAA) exclusion devices; and assesses the impact of these novel strategies on clinical practice.
RISK OF STROKE AND THROMBOEMBOLISM IN NONVALVULAR AF
Stroke occurrence from AF is primarily caused by thrombi formation in the left atrium, most commonly in the LAA. It is important to recognize that the cardiovascular risk factors for AF are also risk factors for atheroembolism; therefore, specific risk factor management is as important as anticoagulation when addressing stroke risk.
The incidence of all-cause stroke in patients with AF is 5%, and it is believed that AF causes approximately 15% of all strokes in the United States.1 This risk appears to be more significant in older patients who are more vulnerable to ischemic strokes. Estimates are that AF independently increases the risk of stroke by fivefold throughout all ages, with a steep increase in percentage of strokes attributed to AF from 1.5% at ages 50 to 59 to 23.5% at ages 80 to 89.1 Importantly, the clinical course of ischemic stroke associated with AF is often more severe than for strokes of other causes,3 further emphasizing the need for stroke prevention.
Assessment of stroke risk/thromboembolism
Multiple risk estimation scores have been developed based on epidemiologic data. Until recently, the CHADS2 score4 was the most commonly used, but it has been superseded by the CHA2DS2-VASc score.5 The point system for this scoring system is shown in Table 1. In contrast with CHADS2, this updated system assigns 2 points for age over 75 years and accounts for stroke risk in the relatively younger group of patients (age 65–75) and in females, neither of whom were included in CHADS2. The CHA2DS2-VASc score ranges between 0 and 9 with a respective estimated stroke risk of 0 to 15.2% per year. Note that for females who are younger than 65 years, no points are given for sex. The major advantage of the CHA2DS2-VASc score over the CHADS2 score is that it is more accurate for lower-risk categories. It has been adopted in most of the recent guidelines that address stroke risk in AF.
In clinical practice, practitioners use these scores to define three primary stroke risk categories: low, intermediate, or high. In our practice, we use a 2% per year cut-off to identify high-risk patients in whom the risk of stroke significantly outweighs the risk of bleeding on anticoagulants. In general, patients with a CHA2DS2-VASc score equal to or greater than 2 have a greater than 2% stroke risk per year and are most likely to benefit from antithrombotic therapies.
In male patients with a CHA2DS2-VASc score of 0 and in most patients with a score of 1, the stroke risk is less than 1% per year. These patients are not likely to derive benefit from anticoagulant therapy. They are usually approached on a case-by-case basis with careful assessment of bleeding risk and discussion of risks and benefits of anticoagulant strategies.
Assessment of bleeding risk
Any general approach to thromboembolism risk assessment in patients with AF should include an analysis that weighs the benefits of anticoagulant therapies against the risks of bleeding. Although no precise tools exist to predict bleeding risk, the HAS-BLED score is increasingly used.6 This score assigns 1 point to each of the following:
- systolic blood pressure greater than 160 mm Hg
- abnormal renal function
- abnormal liver function
- age older than 65
- prior cerebrovascular event
- prior bleeding
- history of labile international normalized ratios (INR)
- alcohol intake (> 8 U/week)
- drug use, especially antiplatelet agents or nonsteroidal anti-inflammatory drugs (NSAIDs).
In general, a HAS-BLED score of 3 or greater indicates increased 1-year risk of intracranial bleed, bleeding requiring hospitalization, drop in hemoglobin of at least 2 g/dL, or need for transfusion.
One problem with the bleeding risk scores is that they were derived from studies that included bleeding events of differing severity. Most bleeding events do not lead to death or severe disability with the exception of intracranial bleeding, which is, therefore, the primary concern when assessing bleeding risk.
The estimated bleeding risk with anticoagulant therapy ranges from 0.2% to 0.4% per year but could be much higher in patients with prior severe bleeding, intracranial hemorrhage, thrombocytopenia, coagulopathies, recent surgery, or ongoing bleeding, aortic dissection, malignant hypertension, and in those receiving a combination of anticoagulant and antiplatelet agents.
MEDICAL THERAPIES TO PREVENT STROKE AND THROMBOEMBOLISM IN AF
In general, anticoagulation reduces the risk of ischemic stroke and thromboembolic events by approximately two-thirds, regardless of baseline risk. Anticoagulant options have increased substantially in the past few years with the introduction of novel oral anticoagulants, including the direct thrombin-inhibitor dabigatran and the factor Xa inhibitors rivaroxaban, apixaban, and edoxaban.
Warfarin
Warfarin has been used for decades for stroke prevention. It remains the only acceptable anticoagulant in patients with valvular AF. Multiple randomized clinical trials have assessed the efficacy of warfarin for stroke prevention in patients with nonvalvular AF.7 These trials demonstrated that warfarin significantly reduces stroke risk, stroke severity, and 30-day mortality compared with no anticoagulant therapy.7,8
Although warfarin is one of the most efficacious drugs to prevent stroke in AF, it has several key limitations. The most important is the need for dose adjustment to keep the INR in a narrow window (2.0 to 3.0) in which net clinical benefit is achieved without increased bleeding risk. The need for continuous monitoring is an inconvenience to patients and often leads to drug discontinuation and nonadherence. A meta-analysis found that patients are only in the therapeutic INR about half of the time.9 Importantly, the time spent in therapeutic INR range cor- relates significantly with the reduction in stroke risk.10 Furthermore, patients who spend less than 40% of the time in the therapeutic INR range are at a higher stroke risk than those not taking warfarin.10
Another limitation with the medication is the dietary restriction on intake of vitamin K-rich green vegetables, which are emphasized as healthy food choices especially in patients with heart disease. Higher warfarin doses are required in patients who consume greens and salads. It is important that patients be consistent in their intake of vitamin K-rich foods to avoid labile INRs, a difficult task for most patients.
Finally, there are several drugs that might interact with warfarin and potentially interfere with its safety or efficacy. These drugs include amiodarone, statins including simvastatin and rosuvastatin (not atorvastatin or pravastatin), fibrates (fenofibrate, gemfibrozil), antibiotics (sulfamethoxazole/trimethoprim, metronidazole), and azole antifungals (fluconazole, miconazole, voriconazole). The use of drugs that induce the cytochrome P450 enzyme CYP2C9, such as rifampin, decrease warfarin effectiveness by reducing INR values. Other non-CYP2C9-dependent drug interactions exist as well.
Aspirin monotherapy or in combination with other agents
Aspirin monotherapy or aspirin plus clopidogrel both increase the risk of bleeding without appreciable benefit, and, as such, their use for stroke prevention in patients with AF is not well supported. The combination of aspirin plus low-dose warfarin was assessed in the SPAF-III trial11 that randomized AF patients at stroke risk to either aspirin plus low-dose warfarin or to dose-adjusted warfarin to target a therapeutic INR. In this trial, patients on aspirin plus low-dose warfarin had significantly higher morbidity and mortality than patients who took adjusted-dose warfarin alone. Thus, the combination of low-dose warfarin plus aspirin should not be used for stroke prevention in AF.
In contrast, the combination of aspirin plus full anticoagulation with warfarin has not been well studied. Limited post-hoc data from the SPORTIF trials suggest, however, that this combination does not reduce the risk of stroke or thromboembolism more than warfarin alone.12
Novel oral anticoagulants
Dabigatran. The value of dabigatran for prevention of stroke or thromboembolism in AF was tested in the RE-LY trial (Randomized Evaluation of Long-Term Anticoagulation Therapy).13 In this trial, 18,113 patients with AF at high risk for stroke were randomized to dabigatran (110 mg or 150 mg twice daily) or adjusted-dose warfarin (INR target 2.0–3.0). By intention-to-treat analysis, dabigatran 150 mg was superior to warfarin for stroke prevention. Importantly, the risk of intracranial or life-threatening bleeding was significantly lower for both dabigatran doses compared with warfarin. Of note, gastrointestinal bleeding was more common with dabigatran 150 mg than warfarin; rates were similar for dabigatran 110 mg versus warfarin.
Rivaroxaban. This factor-Xa inhibitor was assessed in ROCKET-AF (Rivaroxaban Once-daily oral direct factor Xa inhibition Compared with vitamin K antagonism for prevention of stroke and Embolism Trial in Atrial Fibrillation).14 In this trial, 14,264 patients with AF and at risk for stroke were randomized to rivaroxaban (20 mg once daily) or warfarin (INR target 2.5). In the warfarin arm, INR was in the therapeutic range only 55% of the time. Results showed rivaroxaban was noninferior, but not superior, to warfarin for the prevention of stroke or systemic thromboembolism, the primary end points. From a safety standpoint, the overall bleeding rates were similar in the treatment arms with less life-threatening (fatal or intracranial) hemorrhage events with rivaroxaban.
Apixaban. This factor-Xa inhibitor was tested for stroke prevention in AF in two separate clinical trials.15,16 In the AVERROES trial (Apixaban Versus Acetylsalicylic Acid to Prevent Strokes),15 5,599 patients with AF deemed “unsuitable” for warfarin were randomized to apixaban (5 mg twice daily) or aspirin (81–324 mg daily). The trial was terminated early due to superiority of apixaban in achieving the primary end point: occurrence of stroke or systemic embolism. Importantly, the risk of major bleeding appeared to be similar with apixaban versus aspirin.
The ARISTOTLE (Apixaban for Reduction in Stroke and Other Thromboembolic Events in Atrial Fibrillation) trial16 randomized 18,201 patients with AF and at least one additional risk factor for stroke to either apixaban 5 mg or warfarin (target INR 2.0–3.0). In this trial, apixaban was superior to warfarin for the prevention of stroke or systemic embolism, the primary efficacy end point. There also appeared to be a mortality benefit with apixaban versus warfarin. Importantly, the risk of major and intracranial bleeding, the primary safety outcomes, occurred at lower rates in the apixaban group.
All three of these oral anticoagulants require dose adjustment in patients with renal insufficiency and are contraindicated in patients with end-stage renal failure. They are not indicated in patients with valvular heart disease or a mechanical heart valves.
NONPHARMACOLOGIC INTERVENTIONS
Most atrial thrombi in patients with nonvalvular AF form in the LAA. Nonpharmacologic interventions have been developed to block the LAA to reduce the risk of stroke. These are especially valuable options for patients who are not candidates for chronic anticoagulation. In patients undergoing mitral valve surgery,17 ligation to close the LAA has become a standard practice at experienced centers. The introduction of less invasive catheter-based interventions to occlude the LAA has provided additional options.
Watchman device
The Watchman implant, a closure device that blocks the LAA, was recently approved by the US Food and Drug Administration (FDA) for stroke prevention in patients with nonvalvular AF who are at increased risk for stroke based on a CHA2DS2-VASc score of 2 or greater; candidates also must have an appropriate rationale for nonpharmacologic therapy. The expandable-cage device is surgically delivered into the LAA (Figure 1), which subsequently endothelializes and isolates the LAA. (Video 1 shows the delivery sheath positioned at the os of the appendage with a confirmatory contrast appendogram. Video 2 shows the delivery of the device into the appendage.) Therapeutic warfarin is required for a minimum of 45 days after implant followed by aspirin and clopidogrel for 6 months and then aspirin alone.
This device was initially tested in the PROTECT AF (Watchman Left Atrial Appendage System for Embolic PROTECTion in Patients with Atrial Fibrillation) trial, a noninferiority trial that randomized patients to either device implant or warfarin.18 Device implant was successful in 91% of patients in whom it was attempted. Overall, the study showed noninferiority of the device to warfarin in terms of the primary efficacy standpoint, which included stroke, systemic embolism, and cardiovascular death; however, this came at the expense of higher incidence of procedure-related complications, which seemed to be dependent on a learning curve with the device.
In the PREVAIL (Evaluation of the Watchman LAA Closure Device in Patients with Atrial Fibrillation Versus Long-Term Warfarin Therapy) trial,19 although noninferiority was not achieved, the event rates were low and the safety of the procedure was much improved. This was also demonstrated in registry data, which showed improved safety with device implantation with increased operator experience.
Of note, both of these trials included only patients who were eligible for warfarin. Another nonrandomized study20 assessed the use of this device in patients with a contraindication to long-term anticoagulation. Results showed a very low incidence of stroke, which was lower than CHADS2-matched controls taking either aspirin or clopidogrel. The FDA has approved this device for stroke prevention in AF.
Lariat system
The Lariat system is another percutaneous system for occlusion of the LAA. This device, which requires both atrial transseptal and epicardial access, ligates the appendage from the pericardial space. While some small studies have shown it safe and efficacious in patients with AF who cannot take anticoagulation,21 other reports have not been as encouraging.22 The device has been approved by the FDA for “soft tissue approximation”; it is not approved for stroke prevention.
SUMMARY
The most common serious complications of AF are stroke and thromboembolism. Medical and interventional therapies have been developed to prevent these complications. In patients with an estimated thromboembolic risk of greater than 1% to 2% per year, anticoagulation is warranted to reduce that risk. Warfarin remains one of the most studied and useful medications for this purpose, but its use is limited by the need for frequent monitoring, multiple drug interactions, dietary restrictions, and, most importantly, by the difficulty of consistently maintaining therapeutic INRs.
The recently introduced novel oral anticoagulants have been found to be at least noninferior to warfarin and for some agents to be superior to warfarin for the prevention of stroke and thromboembolism. Their main advantage is that they do not require monitoring and have fewer drug interactions and dietary restrictions. Most important, there appear to be fewer major and life-threatening bleeding events than with warfarin. However, use of these novel agents could be limited by patients’ renal function, which needs to be assessed when these agents are being considered. Another limitation with these agents (versus warfarin) is that patients are not therapeutically anticoagulated when they miss a dose, whereas missing a single dose of warfarin may not have the same effect.
In our practice, we have transitioned to use of these novel oral anticoagulants whenever possible using an approach that assesses the individual patient’s risks for both stroke and thromboembolism as well as the risk for bleeding. In patients who are at increased risk of bleeding but at risk of stroke in AF, percutaneous occlusion of the LAA using the Watchman device is offered. This device has been shown to be noninferior to warfarin for stroke prevention and may provide a survival benefit due to the reduction of life-threatening bleeding, which is an inherent risk with anticoagulants. One caveat is that there is a residual risk of stroke after undergoing LAA occlusion because the same risk factors for stroke in AF contribute to stroke from atherothrombosis and atheroembolism; also, thrombi can form in the body of the left atrium.
Clinical decision-making is often challenging in patients with AF who are at risk of stroke and bleeding. In fact, these risk factors often overlap. In our practice, we have established a multidisciplinary clinic for stroke prevention in AF that involves cardiologists, cardiac electrophysiologists, neurologists, gastroenterologists, and vascular medicine specialists. This model allows a multidisciplinary assessment of patients’ individual risks and ultimately facilitates clinical decision-making in terms of strategies to prevent stroke and thromboembolism in AF.
Atrial fibrillation (AF), the most common cardiac arrhythmia, has become a major public health problem. In the United States, the prevalence of AF was estimated at 2.7 to 6.1 million in 2010, and it is expected to rise to between 5.6 and 12 million by 2050.1 The arrhythmia is associated with impaired quality of life and increased morbidity and mortality.1,2 Stroke remains the most devastating consequence of AF.
The clinical management of patients with AF typically targets two main goals: prevention of stroke or thromboembolism and control of symptoms. This article addresses the evolving pharmacologic and nonpharmacologic strategies in stroke prevention in nonvalvular AF; reviews clinical trials evaluating medical and procedural strategies, including the novel oral anticoagulants and left atrial appendage (LAA) exclusion devices; and assesses the impact of these novel strategies on clinical practice.
RISK OF STROKE AND THROMBOEMBOLISM IN NONVALVULAR AF
Stroke occurrence from AF is primarily caused by thrombi formation in the left atrium, most commonly in the LAA. It is important to recognize that the cardiovascular risk factors for AF are also risk factors for atheroembolism; therefore, specific risk factor management is as important as anticoagulation when addressing stroke risk.
The incidence of all-cause stroke in patients with AF is 5%, and it is believed that AF causes approximately 15% of all strokes in the United States.1 This risk appears to be more significant in older patients who are more vulnerable to ischemic strokes. Estimates are that AF independently increases the risk of stroke by fivefold throughout all ages, with a steep increase in percentage of strokes attributed to AF from 1.5% at ages 50 to 59 to 23.5% at ages 80 to 89.1 Importantly, the clinical course of ischemic stroke associated with AF is often more severe than for strokes of other causes,3 further emphasizing the need for stroke prevention.
Assessment of stroke risk/thromboembolism
Multiple risk estimation scores have been developed based on epidemiologic data. Until recently, the CHADS2 score4 was the most commonly used, but it has been superseded by the CHA2DS2-VASc score.5 The point system for this scoring system is shown in Table 1. In contrast with CHADS2, this updated system assigns 2 points for age over 75 years and accounts for stroke risk in the relatively younger group of patients (age 65–75) and in females, neither of whom were included in CHADS2. The CHA2DS2-VASc score ranges between 0 and 9 with a respective estimated stroke risk of 0 to 15.2% per year. Note that for females who are younger than 65 years, no points are given for sex. The major advantage of the CHA2DS2-VASc score over the CHADS2 score is that it is more accurate for lower-risk categories. It has been adopted in most of the recent guidelines that address stroke risk in AF.
In clinical practice, practitioners use these scores to define three primary stroke risk categories: low, intermediate, or high. In our practice, we use a 2% per year cut-off to identify high-risk patients in whom the risk of stroke significantly outweighs the risk of bleeding on anticoagulants. In general, patients with a CHA2DS2-VASc score equal to or greater than 2 have a greater than 2% stroke risk per year and are most likely to benefit from antithrombotic therapies.
In male patients with a CHA2DS2-VASc score of 0 and in most patients with a score of 1, the stroke risk is less than 1% per year. These patients are not likely to derive benefit from anticoagulant therapy. They are usually approached on a case-by-case basis with careful assessment of bleeding risk and discussion of risks and benefits of anticoagulant strategies.
Assessment of bleeding risk
Any general approach to thromboembolism risk assessment in patients with AF should include an analysis that weighs the benefits of anticoagulant therapies against the risks of bleeding. Although no precise tools exist to predict bleeding risk, the HAS-BLED score is increasingly used.6 This score assigns 1 point to each of the following:
- systolic blood pressure greater than 160 mm Hg
- abnormal renal function
- abnormal liver function
- age older than 65
- prior cerebrovascular event
- prior bleeding
- history of labile international normalized ratios (INR)
- alcohol intake (> 8 U/week)
- drug use, especially antiplatelet agents or nonsteroidal anti-inflammatory drugs (NSAIDs).
In general, a HAS-BLED score of 3 or greater indicates increased 1-year risk of intracranial bleed, bleeding requiring hospitalization, drop in hemoglobin of at least 2 g/dL, or need for transfusion.
One problem with the bleeding risk scores is that they were derived from studies that included bleeding events of differing severity. Most bleeding events do not lead to death or severe disability with the exception of intracranial bleeding, which is, therefore, the primary concern when assessing bleeding risk.
The estimated bleeding risk with anticoagulant therapy ranges from 0.2% to 0.4% per year but could be much higher in patients with prior severe bleeding, intracranial hemorrhage, thrombocytopenia, coagulopathies, recent surgery, or ongoing bleeding, aortic dissection, malignant hypertension, and in those receiving a combination of anticoagulant and antiplatelet agents.
MEDICAL THERAPIES TO PREVENT STROKE AND THROMBOEMBOLISM IN AF
In general, anticoagulation reduces the risk of ischemic stroke and thromboembolic events by approximately two-thirds, regardless of baseline risk. Anticoagulant options have increased substantially in the past few years with the introduction of novel oral anticoagulants, including the direct thrombin-inhibitor dabigatran and the factor Xa inhibitors rivaroxaban, apixaban, and edoxaban.
Warfarin
Warfarin has been used for decades for stroke prevention. It remains the only acceptable anticoagulant in patients with valvular AF. Multiple randomized clinical trials have assessed the efficacy of warfarin for stroke prevention in patients with nonvalvular AF.7 These trials demonstrated that warfarin significantly reduces stroke risk, stroke severity, and 30-day mortality compared with no anticoagulant therapy.7,8
Although warfarin is one of the most efficacious drugs to prevent stroke in AF, it has several key limitations. The most important is the need for dose adjustment to keep the INR in a narrow window (2.0 to 3.0) in which net clinical benefit is achieved without increased bleeding risk. The need for continuous monitoring is an inconvenience to patients and often leads to drug discontinuation and nonadherence. A meta-analysis found that patients are only in the therapeutic INR about half of the time.9 Importantly, the time spent in therapeutic INR range cor- relates significantly with the reduction in stroke risk.10 Furthermore, patients who spend less than 40% of the time in the therapeutic INR range are at a higher stroke risk than those not taking warfarin.10
Another limitation with the medication is the dietary restriction on intake of vitamin K-rich green vegetables, which are emphasized as healthy food choices especially in patients with heart disease. Higher warfarin doses are required in patients who consume greens and salads. It is important that patients be consistent in their intake of vitamin K-rich foods to avoid labile INRs, a difficult task for most patients.
Finally, there are several drugs that might interact with warfarin and potentially interfere with its safety or efficacy. These drugs include amiodarone, statins including simvastatin and rosuvastatin (not atorvastatin or pravastatin), fibrates (fenofibrate, gemfibrozil), antibiotics (sulfamethoxazole/trimethoprim, metronidazole), and azole antifungals (fluconazole, miconazole, voriconazole). The use of drugs that induce the cytochrome P450 enzyme CYP2C9, such as rifampin, decrease warfarin effectiveness by reducing INR values. Other non-CYP2C9-dependent drug interactions exist as well.
Aspirin monotherapy or in combination with other agents
Aspirin monotherapy or aspirin plus clopidogrel both increase the risk of bleeding without appreciable benefit, and, as such, their use for stroke prevention in patients with AF is not well supported. The combination of aspirin plus low-dose warfarin was assessed in the SPAF-III trial11 that randomized AF patients at stroke risk to either aspirin plus low-dose warfarin or to dose-adjusted warfarin to target a therapeutic INR. In this trial, patients on aspirin plus low-dose warfarin had significantly higher morbidity and mortality than patients who took adjusted-dose warfarin alone. Thus, the combination of low-dose warfarin plus aspirin should not be used for stroke prevention in AF.
In contrast, the combination of aspirin plus full anticoagulation with warfarin has not been well studied. Limited post-hoc data from the SPORTIF trials suggest, however, that this combination does not reduce the risk of stroke or thromboembolism more than warfarin alone.12
Novel oral anticoagulants
Dabigatran. The value of dabigatran for prevention of stroke or thromboembolism in AF was tested in the RE-LY trial (Randomized Evaluation of Long-Term Anticoagulation Therapy).13 In this trial, 18,113 patients with AF at high risk for stroke were randomized to dabigatran (110 mg or 150 mg twice daily) or adjusted-dose warfarin (INR target 2.0–3.0). By intention-to-treat analysis, dabigatran 150 mg was superior to warfarin for stroke prevention. Importantly, the risk of intracranial or life-threatening bleeding was significantly lower for both dabigatran doses compared with warfarin. Of note, gastrointestinal bleeding was more common with dabigatran 150 mg than warfarin; rates were similar for dabigatran 110 mg versus warfarin.
Rivaroxaban. This factor-Xa inhibitor was assessed in ROCKET-AF (Rivaroxaban Once-daily oral direct factor Xa inhibition Compared with vitamin K antagonism for prevention of stroke and Embolism Trial in Atrial Fibrillation).14 In this trial, 14,264 patients with AF and at risk for stroke were randomized to rivaroxaban (20 mg once daily) or warfarin (INR target 2.5). In the warfarin arm, INR was in the therapeutic range only 55% of the time. Results showed rivaroxaban was noninferior, but not superior, to warfarin for the prevention of stroke or systemic thromboembolism, the primary end points. From a safety standpoint, the overall bleeding rates were similar in the treatment arms with less life-threatening (fatal or intracranial) hemorrhage events with rivaroxaban.
Apixaban. This factor-Xa inhibitor was tested for stroke prevention in AF in two separate clinical trials.15,16 In the AVERROES trial (Apixaban Versus Acetylsalicylic Acid to Prevent Strokes),15 5,599 patients with AF deemed “unsuitable” for warfarin were randomized to apixaban (5 mg twice daily) or aspirin (81–324 mg daily). The trial was terminated early due to superiority of apixaban in achieving the primary end point: occurrence of stroke or systemic embolism. Importantly, the risk of major bleeding appeared to be similar with apixaban versus aspirin.
The ARISTOTLE (Apixaban for Reduction in Stroke and Other Thromboembolic Events in Atrial Fibrillation) trial16 randomized 18,201 patients with AF and at least one additional risk factor for stroke to either apixaban 5 mg or warfarin (target INR 2.0–3.0). In this trial, apixaban was superior to warfarin for the prevention of stroke or systemic embolism, the primary efficacy end point. There also appeared to be a mortality benefit with apixaban versus warfarin. Importantly, the risk of major and intracranial bleeding, the primary safety outcomes, occurred at lower rates in the apixaban group.
All three of these oral anticoagulants require dose adjustment in patients with renal insufficiency and are contraindicated in patients with end-stage renal failure. They are not indicated in patients with valvular heart disease or a mechanical heart valves.
NONPHARMACOLOGIC INTERVENTIONS
Most atrial thrombi in patients with nonvalvular AF form in the LAA. Nonpharmacologic interventions have been developed to block the LAA to reduce the risk of stroke. These are especially valuable options for patients who are not candidates for chronic anticoagulation. In patients undergoing mitral valve surgery,17 ligation to close the LAA has become a standard practice at experienced centers. The introduction of less invasive catheter-based interventions to occlude the LAA has provided additional options.
Watchman device
The Watchman implant, a closure device that blocks the LAA, was recently approved by the US Food and Drug Administration (FDA) for stroke prevention in patients with nonvalvular AF who are at increased risk for stroke based on a CHA2DS2-VASc score of 2 or greater; candidates also must have an appropriate rationale for nonpharmacologic therapy. The expandable-cage device is surgically delivered into the LAA (Figure 1), which subsequently endothelializes and isolates the LAA. (Video 1 shows the delivery sheath positioned at the os of the appendage with a confirmatory contrast appendogram. Video 2 shows the delivery of the device into the appendage.) Therapeutic warfarin is required for a minimum of 45 days after implant followed by aspirin and clopidogrel for 6 months and then aspirin alone.
This device was initially tested in the PROTECT AF (Watchman Left Atrial Appendage System for Embolic PROTECTion in Patients with Atrial Fibrillation) trial, a noninferiority trial that randomized patients to either device implant or warfarin.18 Device implant was successful in 91% of patients in whom it was attempted. Overall, the study showed noninferiority of the device to warfarin in terms of the primary efficacy standpoint, which included stroke, systemic embolism, and cardiovascular death; however, this came at the expense of higher incidence of procedure-related complications, which seemed to be dependent on a learning curve with the device.
In the PREVAIL (Evaluation of the Watchman LAA Closure Device in Patients with Atrial Fibrillation Versus Long-Term Warfarin Therapy) trial,19 although noninferiority was not achieved, the event rates were low and the safety of the procedure was much improved. This was also demonstrated in registry data, which showed improved safety with device implantation with increased operator experience.
Of note, both of these trials included only patients who were eligible for warfarin. Another nonrandomized study20 assessed the use of this device in patients with a contraindication to long-term anticoagulation. Results showed a very low incidence of stroke, which was lower than CHADS2-matched controls taking either aspirin or clopidogrel. The FDA has approved this device for stroke prevention in AF.
Lariat system
The Lariat system is another percutaneous system for occlusion of the LAA. This device, which requires both atrial transseptal and epicardial access, ligates the appendage from the pericardial space. While some small studies have shown it safe and efficacious in patients with AF who cannot take anticoagulation,21 other reports have not been as encouraging.22 The device has been approved by the FDA for “soft tissue approximation”; it is not approved for stroke prevention.
SUMMARY
The most common serious complications of AF are stroke and thromboembolism. Medical and interventional therapies have been developed to prevent these complications. In patients with an estimated thromboembolic risk of greater than 1% to 2% per year, anticoagulation is warranted to reduce that risk. Warfarin remains one of the most studied and useful medications for this purpose, but its use is limited by the need for frequent monitoring, multiple drug interactions, dietary restrictions, and, most importantly, by the difficulty of consistently maintaining therapeutic INRs.
The recently introduced novel oral anticoagulants have been found to be at least noninferior to warfarin and for some agents to be superior to warfarin for the prevention of stroke and thromboembolism. Their main advantage is that they do not require monitoring and have fewer drug interactions and dietary restrictions. Most important, there appear to be fewer major and life-threatening bleeding events than with warfarin. However, use of these novel agents could be limited by patients’ renal function, which needs to be assessed when these agents are being considered. Another limitation with these agents (versus warfarin) is that patients are not therapeutically anticoagulated when they miss a dose, whereas missing a single dose of warfarin may not have the same effect.
In our practice, we have transitioned to use of these novel oral anticoagulants whenever possible using an approach that assesses the individual patient’s risks for both stroke and thromboembolism as well as the risk for bleeding. In patients who are at increased risk of bleeding but at risk of stroke in AF, percutaneous occlusion of the LAA using the Watchman device is offered. This device has been shown to be noninferior to warfarin for stroke prevention and may provide a survival benefit due to the reduction of life-threatening bleeding, which is an inherent risk with anticoagulants. One caveat is that there is a residual risk of stroke after undergoing LAA occlusion because the same risk factors for stroke in AF contribute to stroke from atherothrombosis and atheroembolism; also, thrombi can form in the body of the left atrium.
Clinical decision-making is often challenging in patients with AF who are at risk of stroke and bleeding. In fact, these risk factors often overlap. In our practice, we have established a multidisciplinary clinic for stroke prevention in AF that involves cardiologists, cardiac electrophysiologists, neurologists, gastroenterologists, and vascular medicine specialists. This model allows a multidisciplinary assessment of patients’ individual risks and ultimately facilitates clinical decision-making in terms of strategies to prevent stroke and thromboembolism in AF.
- Roger VL, Go AS, Lloyd-Jones DM, et al; American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics—2012 update: a report from the American Heart Association. Circulation 2012; 125:e2–e220.
- January CT, Wann LS, Alpert JS, et al; American College of Cardiology/American Heart Association Task Force on Practice Guidelines. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the Heart Rhythm Society. J Am Coll Cardiol 2014; 64(21):e1–76.
- Dulli DA, Stanko H, Levine RL. Atrial fibrillation is associated with severe acute ischemic stroke. Neuroepidemiology 2003; 22:118–123.
- Gage BF, Waterman AD, Shannon W, Boechler M, Rich MW, Radford MJ. Validation of clinical classification schemes for predicting stroke: results from the national registry of atrial fibrillation. JAMA 2001; 285:2864–2870.
- Lip GY, Nieuwlaat R, Pisters R, Lane DA, Crijns HJ. Refining clinical risk stratification for predicting stroke and thromboembolism in atrial fibrillation using a novel risk factor-based approach: the euro heart survey on atrial fibrillation. Chest 2010; 137:263-272.
- Pisters R, Lane DA, Nieuwlaat R, de Vos CB, Crijns HJ, Lip GY. A novel user-friendly score (HAS-BLED) to assess 1-year risk of major bleeding in patients with atrial fibrillation: the Euro Heart Survey. Chest 2010; 138:1093–1100.
- Hart RG, Pearce LA, Aguilar MI. Meta-analysis: antithrombotic therapy to prevent stroke in patients who have nonvalvular atrial fibrillation. Ann Intern Med 2007; 146:857–867.
- Johnsen SP, Svendsen ML, Hansen ML, Brandes A, Mehnert F, Husted SE. Preadmission oral anticoagulant treatment and clinical outcome among patients hospitalized with acute stroke and atrial fibrillation: a nationwide study. Stroke 2014; 45:168-175.
- Baker WL, Cios DA, Sander SD, Coleman CI. Meta-analysis to assess the quality of warfarin control in atrial fibrillation patients in the united states. J Manage Care Pharm 2009; 15:244-252.
- Morgan CL, McEwan P, Tukiendorf A, Robinson PA, Clemens A, Plumb JM. Warfarin treatment in patients with atrial fibrillation: observing outcomes associated with varying levels of INR control. Thromb Res 2009; 124:37–41.
- Adjusted-dose warfarin versus low-intensity, fixed-dose warfarin plus aspirin for high-risk patients with atrial fibrillation: Stroke prevention in atrial fibrillation III randomised clinical trial. Lancet 1996; 348(9028):633–638.
- Flaker GC, Gruber M, Connolly SJ, et al. Risks and benefits of combining aspirin with anticoagulant therapy in patients with atrial fibrillation: an exploratory analysis of stroke prevention using an oral thrombin inhibitor in atrial fibrillation (SPORTIF) trials. Am Heart J 2006; 152:967–973.
- Connolly SJ, Ezekowitz MD, Yusuf S, et al, and the RE-LY Steering Committee and Investigators. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med 2009; 361:1139–1151.
- Patel MR, Mahaffey KW, Garg J, et al. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med 2011; 365:883–891.
- Connolly SJ, Eikelboom J, Joyner C, et al. Apixaban in patients with atrial fibrillation. N Engl J Med 2011; 364:806–817.
- Granger CB, Alexander JH, McMurray JJ, et al. Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med 2011; 365:981–992.
- Garcia-Fernandez MA, Perez-David E, Quiles J, et al. Role of left atrial appendage obliteration in stroke reduction in patients with mitral valve prosthesis: a transesophageal echocardiographic study. J Am Coll Cardiol 2003; 42:1253–1258.
- Holmes DR, Reddy VY, Turi ZG, et al. Percutaneous closure of the left atrial appendage versus warfarin therapy for prevention of stroke in patients with atrial fibrillation: a randomised non-inferiority trial. Lancet 2009; 374(9689):534–542.
- Holmes DR Jr, Kar S, Price MJ, et al. Prospective randomized evaluation of the Watchman left atrial appendage closure device in patients with atrial fibrillation versus long-term warfarin therapy: The PREVAIL trial. J Am Coll Cardiol 2014; 64:1–12.
- Reddy VY, Mobius-Winkler S, Miller MA, et al. Left atrial appendage closure with the Watchman device in patients with a contraindication for oral anticoagulation: the ASAP study (ASA plavix feasibility study with watchman left atrial appendage closure technology). J Am Coll Cardiol 2013; 61:2551–2556.
- Bartus K, Han FT, Bednarek J, et al. Percutaneous left atrial appendage suture ligation using the LARIAT device in patients with atrial fibrillation: initial clinical experience. J Am Coll Cardiol 2013; 62:108–118.
- Chatterjee S, Herrmann HC, Wilensky RL, et al. Safety and procedural success of left atrial appendage exclusion with the Lariat device: a systematic review of published reports and analytic review of the FDA MAUDE database. JAMA Intern Med 2015; 175(7):1104–9.
- Roger VL, Go AS, Lloyd-Jones DM, et al; American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics—2012 update: a report from the American Heart Association. Circulation 2012; 125:e2–e220.
- January CT, Wann LS, Alpert JS, et al; American College of Cardiology/American Heart Association Task Force on Practice Guidelines. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the Heart Rhythm Society. J Am Coll Cardiol 2014; 64(21):e1–76.
- Dulli DA, Stanko H, Levine RL. Atrial fibrillation is associated with severe acute ischemic stroke. Neuroepidemiology 2003; 22:118–123.
- Gage BF, Waterman AD, Shannon W, Boechler M, Rich MW, Radford MJ. Validation of clinical classification schemes for predicting stroke: results from the national registry of atrial fibrillation. JAMA 2001; 285:2864–2870.
- Lip GY, Nieuwlaat R, Pisters R, Lane DA, Crijns HJ. Refining clinical risk stratification for predicting stroke and thromboembolism in atrial fibrillation using a novel risk factor-based approach: the euro heart survey on atrial fibrillation. Chest 2010; 137:263-272.
- Pisters R, Lane DA, Nieuwlaat R, de Vos CB, Crijns HJ, Lip GY. A novel user-friendly score (HAS-BLED) to assess 1-year risk of major bleeding in patients with atrial fibrillation: the Euro Heart Survey. Chest 2010; 138:1093–1100.
- Hart RG, Pearce LA, Aguilar MI. Meta-analysis: antithrombotic therapy to prevent stroke in patients who have nonvalvular atrial fibrillation. Ann Intern Med 2007; 146:857–867.
- Johnsen SP, Svendsen ML, Hansen ML, Brandes A, Mehnert F, Husted SE. Preadmission oral anticoagulant treatment and clinical outcome among patients hospitalized with acute stroke and atrial fibrillation: a nationwide study. Stroke 2014; 45:168-175.
- Baker WL, Cios DA, Sander SD, Coleman CI. Meta-analysis to assess the quality of warfarin control in atrial fibrillation patients in the united states. J Manage Care Pharm 2009; 15:244-252.
- Morgan CL, McEwan P, Tukiendorf A, Robinson PA, Clemens A, Plumb JM. Warfarin treatment in patients with atrial fibrillation: observing outcomes associated with varying levels of INR control. Thromb Res 2009; 124:37–41.
- Adjusted-dose warfarin versus low-intensity, fixed-dose warfarin plus aspirin for high-risk patients with atrial fibrillation: Stroke prevention in atrial fibrillation III randomised clinical trial. Lancet 1996; 348(9028):633–638.
- Flaker GC, Gruber M, Connolly SJ, et al. Risks and benefits of combining aspirin with anticoagulant therapy in patients with atrial fibrillation: an exploratory analysis of stroke prevention using an oral thrombin inhibitor in atrial fibrillation (SPORTIF) trials. Am Heart J 2006; 152:967–973.
- Connolly SJ, Ezekowitz MD, Yusuf S, et al, and the RE-LY Steering Committee and Investigators. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med 2009; 361:1139–1151.
- Patel MR, Mahaffey KW, Garg J, et al. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med 2011; 365:883–891.
- Connolly SJ, Eikelboom J, Joyner C, et al. Apixaban in patients with atrial fibrillation. N Engl J Med 2011; 364:806–817.
- Granger CB, Alexander JH, McMurray JJ, et al. Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med 2011; 365:981–992.
- Garcia-Fernandez MA, Perez-David E, Quiles J, et al. Role of left atrial appendage obliteration in stroke reduction in patients with mitral valve prosthesis: a transesophageal echocardiographic study. J Am Coll Cardiol 2003; 42:1253–1258.
- Holmes DR, Reddy VY, Turi ZG, et al. Percutaneous closure of the left atrial appendage versus warfarin therapy for prevention of stroke in patients with atrial fibrillation: a randomised non-inferiority trial. Lancet 2009; 374(9689):534–542.
- Holmes DR Jr, Kar S, Price MJ, et al. Prospective randomized evaluation of the Watchman left atrial appendage closure device in patients with atrial fibrillation versus long-term warfarin therapy: The PREVAIL trial. J Am Coll Cardiol 2014; 64:1–12.
- Reddy VY, Mobius-Winkler S, Miller MA, et al. Left atrial appendage closure with the Watchman device in patients with a contraindication for oral anticoagulation: the ASAP study (ASA plavix feasibility study with watchman left atrial appendage closure technology). J Am Coll Cardiol 2013; 61:2551–2556.
- Bartus K, Han FT, Bednarek J, et al. Percutaneous left atrial appendage suture ligation using the LARIAT device in patients with atrial fibrillation: initial clinical experience. J Am Coll Cardiol 2013; 62:108–118.
- Chatterjee S, Herrmann HC, Wilensky RL, et al. Safety and procedural success of left atrial appendage exclusion with the Lariat device: a systematic review of published reports and analytic review of the FDA MAUDE database. JAMA Intern Med 2015; 175(7):1104–9.
KEY POINTS
- Specific risk factor management is as important as anticoagulation when addressing stroke risk.
- The CHADS2 score has been superseded by the CHA2DS2-VASc score, which is more accurate for lower-risk categories.
- Anticoagulant options have increased substantially in the past few years with the introduction of novel oral anticoagulants, including the direct thrombin-inhibitor dabigatran and the factor Xa inhibitors rivaroxaban, apixaban, and edoxaban.
- Most atrial thrombi in patients with nonvalvular atrial fibrillation form in the left atrial appendage (LAA); nonpharmacologic interventions have been developed to block the LAA and reduce the risk of stroke.