User login
NCCN unveils 'Evidence Blocks' to facilitate treatment discussions
SAN FRANCISCO – The National Comprehensive Cancer Network (NCCN) has introduced an easy-to-use visual tool called Evidence Blocks to help physicians and patients compare various treatment options and individualize the selection among them.
“This information can serve as a starting point for shared decision making between the patient and health care team based on individual patients’ value systems,” chief executive officer Dr. Robert W. Carlson said at the NCCN Annual Congress: Hematologic Malignancies, where the tool was unveiled in a session and related press conference.
The first two NCCN guidelines to incorporate the Evidence Blocks – those for multiple myeloma and chronic myelogenous leukemia – were released at the same time. The organization hopes to incorporate them into all of its guidelines by early 2017, he said.
Development of the Evidence Blocks
The NCCN developed the Evidence Blocks to address requests from various stakeholders, according to Dr. Carlson. Guideline users wanted to know more about the rationale behind recommended therapies, asked for inclusion of information on costs, and sought an aid that would allow patients to make decisions based on their individual values.
“The patient perception of value is what should be most important to us,” he commented. “But even among patients, the concept of value differs greatly from patient to patient,” based on factors such as age, comorbidities, treatment goals, and health insurance coverage.
Each Evidence Block graphically displays five measures of information on a recommended therapy: efficacy, safety, quality of the evidence supporting the recommendation, consistency of the evidence supporting the recommendation, and affordability. Each column in the block represents one measure.
Blue shading indicates panelists’ average numeric score for the therapy on that measure rounded to the nearest integer, ranging from 1 (least favorable) to 5 (most favorable). Therefore, the more shading a therapy has, the more favorable its score.
The Evidence Blocks are added to the guidelines and aligned vertically on pages. “This display of the information graphically allows for very efficient scanning of multiple options for therapy. Comparisons across several regimens can be done very quickly and intuitively,” Dr. Carlson noted.
“We believe that the presentation of this type of information allows the health care provider and patient to make their own judgments of the value of specific interventions,” he added.
The affordability measure has generated the most discussion among panelists and stakeholders, according to Dr. Carlson. For this measure, panelists estimated the total cost of care for a therapy, including the costs of drugs, administration, required supportive care, toxicity monitoring, and care associated with management of toxicity. Scores range from very expensive to very inexpensive.
“We don’t use a dollar amount. Rather, it’s sort of what’s the total cost to society, if you will, of the medical intervention part of this,” he explained. “It’s important to understand that these estimates are not necessarily what a patient would pay because many patients have insurance programs that cover all of this cost.… However, we felt it was important to give patients as well as providers an estimate of what the overall magnitude of expense is because there are patients who have huge deductibles, there’s the doughnut hole within Medicare, and there are patients who have no insurance.”
The Evidence Blocks may help address a “conspiracy of silence” between physicians and patients when it comes to discussing treatment costs, whereby neither party wants to bring up this thorny issue, according to Dr. Carlson. “The Evidence Blocks demystify the discussion of cost because the affordability issue is there in front of you. So it gives people permission to talk about cost and affordability.”
It should be relatively easy to teach patients to use the Evidence Blocks. “I think you’ll find your patients will actually be interested in this and that they will not have as much difficulty interpreting this as you think they will, because the patient advocacy groups and the patient advocates that we have spoken with about this, they get this almost instantly,” he said.
Oncologist perspective
There is a critical need for tools such as the Evidence Blocks in making treatment decisions today, according to Dr. George Somlo, professor in the department of medical oncology and therapeutics research at the City of Hope Comprehensive Cancer Center in Duarte, Calif., and also a member of the NCCN multiple myeloma and breast cancer guideline panels.
Treatment options for multiple myeloma, as for many cancers, have exploded in the past few decades, he noted. “How do you go from making sense of having two drugs with a very poor outcome predicted to having literally dozens of agents approved and used in combination, and in essence being at the verge of curing patients with multiple myeloma?”
Dr. Somlo agreed that inclusion of costs in the Evidence Blocks would likely be beneficial as a conversation starter, recalling, “I’ve had patients who did not fill their prescription for a potentially curative medication because they were worried about the $2,500 or $3,500 copay.”
Patient-physician discussion will be important when it comes to using information from the new tool, he said. For example, in the NCCN guideline for multiple myeloma, some of the first-line regimens have identical Evidence Blocks; thus, consideration of factors such as comorbidities will become important.
“This kind of evidence-based scoring system can guide that kind of discussion with the patient and can tailor the individual therapeutic regimens,” he concluded.
Patient perspective
Breast cancer survivor Marta Nichols, who is vice president of investor relations at GoDaddy and a member of the California Breast Cancer Research Council based in San Francisco, welcomed the Evidence Blocks as a tool that will allow patients to make more informed decisions according to what matters most to them.
Only 33 years old at diagnosis, she and her husband had just begun to think about starting a family. “So my primary concern coming into my physician’s office was my fertility and what impact the treatment would have on my fertility. Certainly most physicians are concerned with efficacy – they want to see you survive. My concern was not just surviving, but also thriving and being able to give birth to children down the line,” she explained.
Patients today are overwhelmed not only by their cancer diagnosis, but also by the many treatment options and the new emphasis on shared decision making, Ms. Nichols noted. And that’s where the Evidence Blocks can make a difference.
“When I was diagnosed, it would have been hugely helpful for me to have information laid out in this very clear and systematic way… It would have given us the ability to make a much more informed decision,” she commented.
Multiple myeloma survivor Donald B. Orosco, who is president and chief financial officer of Orosco & Associates and owner of Monterey (Calif.) Speed and Sport, agreed, noting that his priorities when given the diagnosis more than two decades ago at age 47 differed somewhat.
“I adopted the feeling early on that I probably wasn’t going to see a cure for the disease in my lifetime, but I could accept that,” he elaborated. “I just said ‘Really, I’m interested in quality-of-life issues. I’d like to see my kids go into high school or possibly college.’ So I adopted [an approach of] trying to find something for me that would keep me alive and give me a relatively comfortable quality of life, that would allow me to continue to race cars or do whatever I had to do.”
Dr. Carlson, Dr. Somlo, Ms. Nichols, and Mr. Orosco disclosed no relevant conflicts of interest.
SAN FRANCISCO – The National Comprehensive Cancer Network (NCCN) has introduced an easy-to-use visual tool called Evidence Blocks to help physicians and patients compare various treatment options and individualize the selection among them.
“This information can serve as a starting point for shared decision making between the patient and health care team based on individual patients’ value systems,” chief executive officer Dr. Robert W. Carlson said at the NCCN Annual Congress: Hematologic Malignancies, where the tool was unveiled in a session and related press conference.
The first two NCCN guidelines to incorporate the Evidence Blocks – those for multiple myeloma and chronic myelogenous leukemia – were released at the same time. The organization hopes to incorporate them into all of its guidelines by early 2017, he said.
Development of the Evidence Blocks
The NCCN developed the Evidence Blocks to address requests from various stakeholders, according to Dr. Carlson. Guideline users wanted to know more about the rationale behind recommended therapies, asked for inclusion of information on costs, and sought an aid that would allow patients to make decisions based on their individual values.
“The patient perception of value is what should be most important to us,” he commented. “But even among patients, the concept of value differs greatly from patient to patient,” based on factors such as age, comorbidities, treatment goals, and health insurance coverage.
Each Evidence Block graphically displays five measures of information on a recommended therapy: efficacy, safety, quality of the evidence supporting the recommendation, consistency of the evidence supporting the recommendation, and affordability. Each column in the block represents one measure.
Blue shading indicates panelists’ average numeric score for the therapy on that measure rounded to the nearest integer, ranging from 1 (least favorable) to 5 (most favorable). Therefore, the more shading a therapy has, the more favorable its score.
The Evidence Blocks are added to the guidelines and aligned vertically on pages. “This display of the information graphically allows for very efficient scanning of multiple options for therapy. Comparisons across several regimens can be done very quickly and intuitively,” Dr. Carlson noted.
“We believe that the presentation of this type of information allows the health care provider and patient to make their own judgments of the value of specific interventions,” he added.
The affordability measure has generated the most discussion among panelists and stakeholders, according to Dr. Carlson. For this measure, panelists estimated the total cost of care for a therapy, including the costs of drugs, administration, required supportive care, toxicity monitoring, and care associated with management of toxicity. Scores range from very expensive to very inexpensive.
“We don’t use a dollar amount. Rather, it’s sort of what’s the total cost to society, if you will, of the medical intervention part of this,” he explained. “It’s important to understand that these estimates are not necessarily what a patient would pay because many patients have insurance programs that cover all of this cost.… However, we felt it was important to give patients as well as providers an estimate of what the overall magnitude of expense is because there are patients who have huge deductibles, there’s the doughnut hole within Medicare, and there are patients who have no insurance.”
The Evidence Blocks may help address a “conspiracy of silence” between physicians and patients when it comes to discussing treatment costs, whereby neither party wants to bring up this thorny issue, according to Dr. Carlson. “The Evidence Blocks demystify the discussion of cost because the affordability issue is there in front of you. So it gives people permission to talk about cost and affordability.”
It should be relatively easy to teach patients to use the Evidence Blocks. “I think you’ll find your patients will actually be interested in this and that they will not have as much difficulty interpreting this as you think they will, because the patient advocacy groups and the patient advocates that we have spoken with about this, they get this almost instantly,” he said.
Oncologist perspective
There is a critical need for tools such as the Evidence Blocks in making treatment decisions today, according to Dr. George Somlo, professor in the department of medical oncology and therapeutics research at the City of Hope Comprehensive Cancer Center in Duarte, Calif., and also a member of the NCCN multiple myeloma and breast cancer guideline panels.
Treatment options for multiple myeloma, as for many cancers, have exploded in the past few decades, he noted. “How do you go from making sense of having two drugs with a very poor outcome predicted to having literally dozens of agents approved and used in combination, and in essence being at the verge of curing patients with multiple myeloma?”
Dr. Somlo agreed that inclusion of costs in the Evidence Blocks would likely be beneficial as a conversation starter, recalling, “I’ve had patients who did not fill their prescription for a potentially curative medication because they were worried about the $2,500 or $3,500 copay.”
Patient-physician discussion will be important when it comes to using information from the new tool, he said. For example, in the NCCN guideline for multiple myeloma, some of the first-line regimens have identical Evidence Blocks; thus, consideration of factors such as comorbidities will become important.
“This kind of evidence-based scoring system can guide that kind of discussion with the patient and can tailor the individual therapeutic regimens,” he concluded.
Patient perspective
Breast cancer survivor Marta Nichols, who is vice president of investor relations at GoDaddy and a member of the California Breast Cancer Research Council based in San Francisco, welcomed the Evidence Blocks as a tool that will allow patients to make more informed decisions according to what matters most to them.
Only 33 years old at diagnosis, she and her husband had just begun to think about starting a family. “So my primary concern coming into my physician’s office was my fertility and what impact the treatment would have on my fertility. Certainly most physicians are concerned with efficacy – they want to see you survive. My concern was not just surviving, but also thriving and being able to give birth to children down the line,” she explained.
Patients today are overwhelmed not only by their cancer diagnosis, but also by the many treatment options and the new emphasis on shared decision making, Ms. Nichols noted. And that’s where the Evidence Blocks can make a difference.
“When I was diagnosed, it would have been hugely helpful for me to have information laid out in this very clear and systematic way… It would have given us the ability to make a much more informed decision,” she commented.
Multiple myeloma survivor Donald B. Orosco, who is president and chief financial officer of Orosco & Associates and owner of Monterey (Calif.) Speed and Sport, agreed, noting that his priorities when given the diagnosis more than two decades ago at age 47 differed somewhat.
“I adopted the feeling early on that I probably wasn’t going to see a cure for the disease in my lifetime, but I could accept that,” he elaborated. “I just said ‘Really, I’m interested in quality-of-life issues. I’d like to see my kids go into high school or possibly college.’ So I adopted [an approach of] trying to find something for me that would keep me alive and give me a relatively comfortable quality of life, that would allow me to continue to race cars or do whatever I had to do.”
Dr. Carlson, Dr. Somlo, Ms. Nichols, and Mr. Orosco disclosed no relevant conflicts of interest.
SAN FRANCISCO – The National Comprehensive Cancer Network (NCCN) has introduced an easy-to-use visual tool called Evidence Blocks to help physicians and patients compare various treatment options and individualize the selection among them.
“This information can serve as a starting point for shared decision making between the patient and health care team based on individual patients’ value systems,” chief executive officer Dr. Robert W. Carlson said at the NCCN Annual Congress: Hematologic Malignancies, where the tool was unveiled in a session and related press conference.
The first two NCCN guidelines to incorporate the Evidence Blocks – those for multiple myeloma and chronic myelogenous leukemia – were released at the same time. The organization hopes to incorporate them into all of its guidelines by early 2017, he said.
Development of the Evidence Blocks
The NCCN developed the Evidence Blocks to address requests from various stakeholders, according to Dr. Carlson. Guideline users wanted to know more about the rationale behind recommended therapies, asked for inclusion of information on costs, and sought an aid that would allow patients to make decisions based on their individual values.
“The patient perception of value is what should be most important to us,” he commented. “But even among patients, the concept of value differs greatly from patient to patient,” based on factors such as age, comorbidities, treatment goals, and health insurance coverage.
Each Evidence Block graphically displays five measures of information on a recommended therapy: efficacy, safety, quality of the evidence supporting the recommendation, consistency of the evidence supporting the recommendation, and affordability. Each column in the block represents one measure.
Blue shading indicates panelists’ average numeric score for the therapy on that measure rounded to the nearest integer, ranging from 1 (least favorable) to 5 (most favorable). Therefore, the more shading a therapy has, the more favorable its score.
The Evidence Blocks are added to the guidelines and aligned vertically on pages. “This display of the information graphically allows for very efficient scanning of multiple options for therapy. Comparisons across several regimens can be done very quickly and intuitively,” Dr. Carlson noted.
“We believe that the presentation of this type of information allows the health care provider and patient to make their own judgments of the value of specific interventions,” he added.
The affordability measure has generated the most discussion among panelists and stakeholders, according to Dr. Carlson. For this measure, panelists estimated the total cost of care for a therapy, including the costs of drugs, administration, required supportive care, toxicity monitoring, and care associated with management of toxicity. Scores range from very expensive to very inexpensive.
“We don’t use a dollar amount. Rather, it’s sort of what’s the total cost to society, if you will, of the medical intervention part of this,” he explained. “It’s important to understand that these estimates are not necessarily what a patient would pay because many patients have insurance programs that cover all of this cost.… However, we felt it was important to give patients as well as providers an estimate of what the overall magnitude of expense is because there are patients who have huge deductibles, there’s the doughnut hole within Medicare, and there are patients who have no insurance.”
The Evidence Blocks may help address a “conspiracy of silence” between physicians and patients when it comes to discussing treatment costs, whereby neither party wants to bring up this thorny issue, according to Dr. Carlson. “The Evidence Blocks demystify the discussion of cost because the affordability issue is there in front of you. So it gives people permission to talk about cost and affordability.”
It should be relatively easy to teach patients to use the Evidence Blocks. “I think you’ll find your patients will actually be interested in this and that they will not have as much difficulty interpreting this as you think they will, because the patient advocacy groups and the patient advocates that we have spoken with about this, they get this almost instantly,” he said.
Oncologist perspective
There is a critical need for tools such as the Evidence Blocks in making treatment decisions today, according to Dr. George Somlo, professor in the department of medical oncology and therapeutics research at the City of Hope Comprehensive Cancer Center in Duarte, Calif., and also a member of the NCCN multiple myeloma and breast cancer guideline panels.
Treatment options for multiple myeloma, as for many cancers, have exploded in the past few decades, he noted. “How do you go from making sense of having two drugs with a very poor outcome predicted to having literally dozens of agents approved and used in combination, and in essence being at the verge of curing patients with multiple myeloma?”
Dr. Somlo agreed that inclusion of costs in the Evidence Blocks would likely be beneficial as a conversation starter, recalling, “I’ve had patients who did not fill their prescription for a potentially curative medication because they were worried about the $2,500 or $3,500 copay.”
Patient-physician discussion will be important when it comes to using information from the new tool, he said. For example, in the NCCN guideline for multiple myeloma, some of the first-line regimens have identical Evidence Blocks; thus, consideration of factors such as comorbidities will become important.
“This kind of evidence-based scoring system can guide that kind of discussion with the patient and can tailor the individual therapeutic regimens,” he concluded.
Patient perspective
Breast cancer survivor Marta Nichols, who is vice president of investor relations at GoDaddy and a member of the California Breast Cancer Research Council based in San Francisco, welcomed the Evidence Blocks as a tool that will allow patients to make more informed decisions according to what matters most to them.
Only 33 years old at diagnosis, she and her husband had just begun to think about starting a family. “So my primary concern coming into my physician’s office was my fertility and what impact the treatment would have on my fertility. Certainly most physicians are concerned with efficacy – they want to see you survive. My concern was not just surviving, but also thriving and being able to give birth to children down the line,” she explained.
Patients today are overwhelmed not only by their cancer diagnosis, but also by the many treatment options and the new emphasis on shared decision making, Ms. Nichols noted. And that’s where the Evidence Blocks can make a difference.
“When I was diagnosed, it would have been hugely helpful for me to have information laid out in this very clear and systematic way… It would have given us the ability to make a much more informed decision,” she commented.
Multiple myeloma survivor Donald B. Orosco, who is president and chief financial officer of Orosco & Associates and owner of Monterey (Calif.) Speed and Sport, agreed, noting that his priorities when given the diagnosis more than two decades ago at age 47 differed somewhat.
“I adopted the feeling early on that I probably wasn’t going to see a cure for the disease in my lifetime, but I could accept that,” he elaborated. “I just said ‘Really, I’m interested in quality-of-life issues. I’d like to see my kids go into high school or possibly college.’ So I adopted [an approach of] trying to find something for me that would keep me alive and give me a relatively comfortable quality of life, that would allow me to continue to race cars or do whatever I had to do.”
Dr. Carlson, Dr. Somlo, Ms. Nichols, and Mr. Orosco disclosed no relevant conflicts of interest.
AT NCCN ANNUAL CONGRESS: HEMATOLOGIC MALIGNANCIES
ORBIT Score Predicts Bleeding Risk in AF Patients
NEW YORK - The five-factor ORBIT bleeding score accurately predicts major bleeding risk in patients with atrial fibrillation (AF) who are taking oral anticoagulants (OACs), researchers report.
"The ORBIT score highlights modifiable factors that increase bleeding risk and can help providers identify high-risk AF patients for closer monitoring," Dr. Emily C. O'Brien, from Duke Clinical Research Institute, Durham, North Carolina, said by email. "Along with clinical judgment, the ORBIT score can be used to give an estimate of bleeding risk for any AF patient considering OAC treatment."
Two existing bleeding scores - HAS-BLED and ATRIA - are based on small numbers of events and have shown inconsistent performance. They also may require elements that are not available for all OAC users, the researchers wrote.
Dr. O'Brien's team developed a five-element bleeding score and compared its performance with those of HAS-BLED and ATRIA using data from the ORBIT-AF and ROCKET-AF studies.
The numerical score included the five strongest predictors of bleeding:
-Older age (75 years and above): one point
-Reduced hemoglobin, hematocrit, or history of anemia: two points
-Bleeding history: two points
-Insufficient kidney function (eGFR below 60 mL/min/1.73 m2): one point
-Treatment with an antiplatelet agent: one point
Observed bleeding rates in the ORBIT-AF participants increased with increasing ORBIT bleeding score: from 2.4 per 100 patient-years in the low-risk group (scores 0-2) to 4.7 per 100 patient-years in the medium-risk group (score 3) to 8.1 per 100 patient-years in the high-risk group (scores 4-7), according to the Sept. 30 European Heart Journal online report.
In both the ORBIT-AF and ROCKET-AF cohorts, the ORBIT bleeding score showed better discrimination than the HAS-BLED and ATRIA scores.
Model calibration analysis also showed superior calibration for the ORBIT bleeding score. The HAS-BLED score showed relatively poor calibration for low-risk score strata, whereas the ATRIA score showed poor calibration for most risk groups.
"The ORBIT score is a simple, useful tool that predicts bleeding as well as other, more complicated scores and can be used in any AF patient regardless of the type of OAC he or she is taking," Dr. O'Brien said.
"For chronic conditions like AF, periodic assessment of risk for adverse events is important to support clinical decision-making," Dr. O'Brien explained. "Risk factors may
change over time particularly as patients get older. Therefore, incorporating new data on these factors into longitudinal risk assessment provides an optimal framework for ongoing AF management."
"While bleeding risk estimation can be helpful in identifying high-risk AF patients for closer monitoring, it is important to note that prior work has demonstrated a net clinical benefit of OAC even in patients with high estimated bleeding risk," the researchers wrote. "Further, while risk scores provide important information to the clinician for estimating risk of adverse events, they represent only one consideration relevant to therapeutic decision making."
Janssen Scientific Affairs sponsors ORBIT-AF; the Agency for Healthcare Research and Quality partially supported this research. Ten coauthors reported relevant relationships.
NEW YORK - The five-factor ORBIT bleeding score accurately predicts major bleeding risk in patients with atrial fibrillation (AF) who are taking oral anticoagulants (OACs), researchers report.
"The ORBIT score highlights modifiable factors that increase bleeding risk and can help providers identify high-risk AF patients for closer monitoring," Dr. Emily C. O'Brien, from Duke Clinical Research Institute, Durham, North Carolina, said by email. "Along with clinical judgment, the ORBIT score can be used to give an estimate of bleeding risk for any AF patient considering OAC treatment."
Two existing bleeding scores - HAS-BLED and ATRIA - are based on small numbers of events and have shown inconsistent performance. They also may require elements that are not available for all OAC users, the researchers wrote.
Dr. O'Brien's team developed a five-element bleeding score and compared its performance with those of HAS-BLED and ATRIA using data from the ORBIT-AF and ROCKET-AF studies.
The numerical score included the five strongest predictors of bleeding:
-Older age (75 years and above): one point
-Reduced hemoglobin, hematocrit, or history of anemia: two points
-Bleeding history: two points
-Insufficient kidney function (eGFR below 60 mL/min/1.73 m2): one point
-Treatment with an antiplatelet agent: one point
Observed bleeding rates in the ORBIT-AF participants increased with increasing ORBIT bleeding score: from 2.4 per 100 patient-years in the low-risk group (scores 0-2) to 4.7 per 100 patient-years in the medium-risk group (score 3) to 8.1 per 100 patient-years in the high-risk group (scores 4-7), according to the Sept. 30 European Heart Journal online report.
In both the ORBIT-AF and ROCKET-AF cohorts, the ORBIT bleeding score showed better discrimination than the HAS-BLED and ATRIA scores.
Model calibration analysis also showed superior calibration for the ORBIT bleeding score. The HAS-BLED score showed relatively poor calibration for low-risk score strata, whereas the ATRIA score showed poor calibration for most risk groups.
"The ORBIT score is a simple, useful tool that predicts bleeding as well as other, more complicated scores and can be used in any AF patient regardless of the type of OAC he or she is taking," Dr. O'Brien said.
"For chronic conditions like AF, periodic assessment of risk for adverse events is important to support clinical decision-making," Dr. O'Brien explained. "Risk factors may
change over time particularly as patients get older. Therefore, incorporating new data on these factors into longitudinal risk assessment provides an optimal framework for ongoing AF management."
"While bleeding risk estimation can be helpful in identifying high-risk AF patients for closer monitoring, it is important to note that prior work has demonstrated a net clinical benefit of OAC even in patients with high estimated bleeding risk," the researchers wrote. "Further, while risk scores provide important information to the clinician for estimating risk of adverse events, they represent only one consideration relevant to therapeutic decision making."
Janssen Scientific Affairs sponsors ORBIT-AF; the Agency for Healthcare Research and Quality partially supported this research. Ten coauthors reported relevant relationships.
NEW YORK - The five-factor ORBIT bleeding score accurately predicts major bleeding risk in patients with atrial fibrillation (AF) who are taking oral anticoagulants (OACs), researchers report.
"The ORBIT score highlights modifiable factors that increase bleeding risk and can help providers identify high-risk AF patients for closer monitoring," Dr. Emily C. O'Brien, from Duke Clinical Research Institute, Durham, North Carolina, said by email. "Along with clinical judgment, the ORBIT score can be used to give an estimate of bleeding risk for any AF patient considering OAC treatment."
Two existing bleeding scores - HAS-BLED and ATRIA - are based on small numbers of events and have shown inconsistent performance. They also may require elements that are not available for all OAC users, the researchers wrote.
Dr. O'Brien's team developed a five-element bleeding score and compared its performance with those of HAS-BLED and ATRIA using data from the ORBIT-AF and ROCKET-AF studies.
The numerical score included the five strongest predictors of bleeding:
-Older age (75 years and above): one point
-Reduced hemoglobin, hematocrit, or history of anemia: two points
-Bleeding history: two points
-Insufficient kidney function (eGFR below 60 mL/min/1.73 m2): one point
-Treatment with an antiplatelet agent: one point
Observed bleeding rates in the ORBIT-AF participants increased with increasing ORBIT bleeding score: from 2.4 per 100 patient-years in the low-risk group (scores 0-2) to 4.7 per 100 patient-years in the medium-risk group (score 3) to 8.1 per 100 patient-years in the high-risk group (scores 4-7), according to the Sept. 30 European Heart Journal online report.
In both the ORBIT-AF and ROCKET-AF cohorts, the ORBIT bleeding score showed better discrimination than the HAS-BLED and ATRIA scores.
Model calibration analysis also showed superior calibration for the ORBIT bleeding score. The HAS-BLED score showed relatively poor calibration for low-risk score strata, whereas the ATRIA score showed poor calibration for most risk groups.
"The ORBIT score is a simple, useful tool that predicts bleeding as well as other, more complicated scores and can be used in any AF patient regardless of the type of OAC he or she is taking," Dr. O'Brien said.
"For chronic conditions like AF, periodic assessment of risk for adverse events is important to support clinical decision-making," Dr. O'Brien explained. "Risk factors may
change over time particularly as patients get older. Therefore, incorporating new data on these factors into longitudinal risk assessment provides an optimal framework for ongoing AF management."
"While bleeding risk estimation can be helpful in identifying high-risk AF patients for closer monitoring, it is important to note that prior work has demonstrated a net clinical benefit of OAC even in patients with high estimated bleeding risk," the researchers wrote. "Further, while risk scores provide important information to the clinician for estimating risk of adverse events, they represent only one consideration relevant to therapeutic decision making."
Janssen Scientific Affairs sponsors ORBIT-AF; the Agency for Healthcare Research and Quality partially supported this research. Ten coauthors reported relevant relationships.
Emerging evidence is resolving questions in CML management
SAN FRANCISCO – “We really have an embarrassment of riches in chronic myelogenous leukemia, and the question is, which patients get which drugs? That’s really the major question that drives clinical care right now,” Dr. Jerald P. Radich, chair of the NCCN guidelines panel for chronic myelogenous leukemia (CML), told attendees of the National Comprehensive Cancer Network 10th Annual Congress: Hematologic Malignancies.
He discussed a variety of setting-specific quandaries in disease management and recent evidence from trials that is helping to provide some answers. He also reviewed some of the finer points of the NCCN’s CML guidelines, a new version of which was released at the congress.
How do you choose front-line therapy?
Tyrosine kinase inhibitors (TKIs) have dramatically improved the prognosis of CML, according to Dr. Radich, who is a member of the clinical research division, Fred Hutchinson Cancer Research Center, and an associate professor in the medical oncology division, University of Washington, both in Seattle.
Today, three TKIs—imatinib (Gleevec) and the second-generation agents nilotinib (Tasigna) and dasatinib (Sprycel)—are approved by the Food and Drug Administration as front-line therapy.
The 8-year data from the IRIS trial (International Randomized Study of Interferon Vs STI571), which tested imatinib in patients with newly diagnosed chronic-phase CML, showed that event-free survival was 81% and overall survival was 85% (Blood. 2009;114:462. Abstract 1126). “No matter how you slice it, these patients have done fantastically well,” Dr. Radich said.
The two other, newer therapies, nilotinib and dasatinib, have yielded significantly better short-term cytogenetic response and major molecular response rates in numerous trials. “But curiously enough, if you look at overall survival, there is no difference,” he commented. “We don’t know why the short-term efficacy of the second generations hasn’t translated into long-term efficacy yet.”
In the guidelines, the three front-line TKIs are nearly identical with respect to their Evidence Blocks, a new tool added to facilitate treatment comparisons and decision making.
But specific toxicities differ across the agents, which may tilt the decision one way or another. “If somebody has a history of really bad atherosclerotic events previously, then imatinib is probably not the best drug for them. If somebody has a history of pulmonary issues, then dasatinib may not be the best drug for them. And if somebody has diabetes, nilotinib probably isn’t the best drug for them,” Dr. Radich elaborated.
Costs for the three drugs are the same at present, he said. But imatinib will likely become available as a generic next year, which may make that drug the most attractive from the financial perspective.
When do you switch therapies?
There is some debate about how long to persevere with a front-line therapy when monitoring suggests the response is suboptimal, according to Dr. Radich.
Data have identified the BCR-ABL1 transcript level at 3 months to be a good predictor of long-term survival (J Clin Oncol. 2012;30:232-8). “We in the NCCN have said at 3 months or 6 months, you should consider changing [if the response is suboptimal]. You don’t have to, but you should consider changing because there are some other [therapies] that get rid of that,” he said.
However, he cautioned, a true assessment of response hinges critically on patients’ compliance with therapy. Studies using electronic monitors hidden in the caps of pill bottles suggest that even though the majority of patients say they take their TKI daily, only about 15% actually do.
Studies out of Europe and Australia have shown that some patients with a suboptimal response will have progression to accelerated phase/blast crisis in the interim between 3 months and 6 months, Dr. Radich noted. “So if you think that your patient is really, really religiously taking their drug, and if they still have not responded very well at 3 months, that might be an indication that you can consider changing therapy.”
Which second-line TKI should patients get?
The options for second-line TKI therapy include nilotinib and dasatinib, as well as bosutinib (Bosulif) and ponatinib (Iclusig).
About half of patients who experience progression on or become resistant to their first-line TKI have mutations, according to Dr. Radich. Here, a mutational analysis is warranted as the second-line agents differ somewhat with respect to the mutations they act against, which may guide the choice of agent. And toxicity profile may again dictate drugs that can and can’t be used for a specific patient.
Patients who have genuine resistance are unlikely to achieve a cure although they may have a cytogenetic response. “Most of us think that resistance might be forever and if we have a patient who has genuinely become resistant, you don’t need to transplant them necessarily [right away]. But that’s the time you have to have a conversation about doing transplantation and start doing HLA typing and the like,” he said.
Data suggest that the cytogenetic response to second-line therapy at 3 months predicts how patients will fare longer term (ASH 2008. Abstract 332).
“That ends up being a pretty good time point because if you have a patient who’s resistant and you start the search for a donor, for an HLA match, the median time to finding a donor is about 4 months,” Dr. Radich commented. “So a practical point is if you have to switch to a second-generation drug, you start a search. You find a donor, you evaluate again. If the patient had a great response, you say fine, we’re going to follow you; if they don’t, that’s a good time to move to transplant.”
In the guidelines, the Evidence Blocks for second-line (and later-line) therapies generally show what one might expect from clinical practice, Dr. Radich said. “As you march from primary therapy to secondary therapy to tertiary therapy, what happens? You all know, toxicity increases in those settings, efficacy goes down. And that’s exactly what happens here. And if you match up all these drugs, we say there is no difference in which one you pick.”
Can you discontinue therapy?
“The other issue that is really driving a lot of CML strategy these days is the issue of discontinuation,” Dr. Radich commented. Conventional belief has been that TKIs do not eliminate CML stem cells; therefore, patients will need to be on therapy lifelong.
“Turns out, like many things, we were wrong,” he said. “There have been a number of studies now showing that if you are PCR negative for a number of years and get off therapy, about 60% of patients will relapse usually within the first 3 months, but about 40% of patients will actually remain negative for BCR-ABL up to 2-3 years.”
“Now the good part is that all the patients pretty much who have relapse and get put back on drug go back to getting a response; they don’t go into complete molecular remission, however. And the reason that we worry that all these patients should still be [discontinued] on a clinical trial as opposed to doing it at home is we don’t know the long-term consequences of this,” Dr. Radich said.
A period of unopposed BCR-ABL activity may allow emergence of resistant clones that take years to become clinically evident, he noted. In fact, data from Hiroshima survivors show that CML can have “an amazing dormancy,” possibly 70 years.
“So I think you have to approach any discontinuation with some caution. The risk is probably very low, but it’s actually probably a number,” he said. The guidelines therefore recommend that in patients whose disease is responding to TKIs, discontinuation should be undertaken only in a clinical trial.
“How this weighs in, I think, is if you look at CMR [complete molecular response] rates, they are higher with second-generations than with imatinib. So if you have a patient who you some day want to get to a discontinuation trial, a younger person who wants to have children and the like, then second-generations might be your best option for getting them there,” Dr. Radich proposed.
Take-home message
The guidelines, with all of their algorithms and the new Evidence Blocks, are helpful but do not replace clinical wisdom and experience, Dr. Radich asserted in closing.
“You have to kind of use your clinical judgment, and that trumps everything else,” he said. “The other thing that’s important is all of you have different experiences with using these drugs, and nothing replaces that. If you are someone who uses drug A all the time, you know how to anticipate the complications, you know what to look for. It’s a lot better [to use that drug] than just jumping to drug C when you have never used it or have little experience, because really experience is the main thing.”
Dr. Radich disclosed that he has consulting and/or research funding relationships with Ariad Pharmaceuticals, Gilead Sciences, and Novartis.
SAN FRANCISCO – “We really have an embarrassment of riches in chronic myelogenous leukemia, and the question is, which patients get which drugs? That’s really the major question that drives clinical care right now,” Dr. Jerald P. Radich, chair of the NCCN guidelines panel for chronic myelogenous leukemia (CML), told attendees of the National Comprehensive Cancer Network 10th Annual Congress: Hematologic Malignancies.
He discussed a variety of setting-specific quandaries in disease management and recent evidence from trials that is helping to provide some answers. He also reviewed some of the finer points of the NCCN’s CML guidelines, a new version of which was released at the congress.
How do you choose front-line therapy?
Tyrosine kinase inhibitors (TKIs) have dramatically improved the prognosis of CML, according to Dr. Radich, who is a member of the clinical research division, Fred Hutchinson Cancer Research Center, and an associate professor in the medical oncology division, University of Washington, both in Seattle.
Today, three TKIs—imatinib (Gleevec) and the second-generation agents nilotinib (Tasigna) and dasatinib (Sprycel)—are approved by the Food and Drug Administration as front-line therapy.
The 8-year data from the IRIS trial (International Randomized Study of Interferon Vs STI571), which tested imatinib in patients with newly diagnosed chronic-phase CML, showed that event-free survival was 81% and overall survival was 85% (Blood. 2009;114:462. Abstract 1126). “No matter how you slice it, these patients have done fantastically well,” Dr. Radich said.
The two other, newer therapies, nilotinib and dasatinib, have yielded significantly better short-term cytogenetic response and major molecular response rates in numerous trials. “But curiously enough, if you look at overall survival, there is no difference,” he commented. “We don’t know why the short-term efficacy of the second generations hasn’t translated into long-term efficacy yet.”
In the guidelines, the three front-line TKIs are nearly identical with respect to their Evidence Blocks, a new tool added to facilitate treatment comparisons and decision making.
But specific toxicities differ across the agents, which may tilt the decision one way or another. “If somebody has a history of really bad atherosclerotic events previously, then imatinib is probably not the best drug for them. If somebody has a history of pulmonary issues, then dasatinib may not be the best drug for them. And if somebody has diabetes, nilotinib probably isn’t the best drug for them,” Dr. Radich elaborated.
Costs for the three drugs are the same at present, he said. But imatinib will likely become available as a generic next year, which may make that drug the most attractive from the financial perspective.
When do you switch therapies?
There is some debate about how long to persevere with a front-line therapy when monitoring suggests the response is suboptimal, according to Dr. Radich.
Data have identified the BCR-ABL1 transcript level at 3 months to be a good predictor of long-term survival (J Clin Oncol. 2012;30:232-8). “We in the NCCN have said at 3 months or 6 months, you should consider changing [if the response is suboptimal]. You don’t have to, but you should consider changing because there are some other [therapies] that get rid of that,” he said.
However, he cautioned, a true assessment of response hinges critically on patients’ compliance with therapy. Studies using electronic monitors hidden in the caps of pill bottles suggest that even though the majority of patients say they take their TKI daily, only about 15% actually do.
Studies out of Europe and Australia have shown that some patients with a suboptimal response will have progression to accelerated phase/blast crisis in the interim between 3 months and 6 months, Dr. Radich noted. “So if you think that your patient is really, really religiously taking their drug, and if they still have not responded very well at 3 months, that might be an indication that you can consider changing therapy.”
Which second-line TKI should patients get?
The options for second-line TKI therapy include nilotinib and dasatinib, as well as bosutinib (Bosulif) and ponatinib (Iclusig).
About half of patients who experience progression on or become resistant to their first-line TKI have mutations, according to Dr. Radich. Here, a mutational analysis is warranted as the second-line agents differ somewhat with respect to the mutations they act against, which may guide the choice of agent. And toxicity profile may again dictate drugs that can and can’t be used for a specific patient.
Patients who have genuine resistance are unlikely to achieve a cure although they may have a cytogenetic response. “Most of us think that resistance might be forever and if we have a patient who has genuinely become resistant, you don’t need to transplant them necessarily [right away]. But that’s the time you have to have a conversation about doing transplantation and start doing HLA typing and the like,” he said.
Data suggest that the cytogenetic response to second-line therapy at 3 months predicts how patients will fare longer term (ASH 2008. Abstract 332).
“That ends up being a pretty good time point because if you have a patient who’s resistant and you start the search for a donor, for an HLA match, the median time to finding a donor is about 4 months,” Dr. Radich commented. “So a practical point is if you have to switch to a second-generation drug, you start a search. You find a donor, you evaluate again. If the patient had a great response, you say fine, we’re going to follow you; if they don’t, that’s a good time to move to transplant.”
In the guidelines, the Evidence Blocks for second-line (and later-line) therapies generally show what one might expect from clinical practice, Dr. Radich said. “As you march from primary therapy to secondary therapy to tertiary therapy, what happens? You all know, toxicity increases in those settings, efficacy goes down. And that’s exactly what happens here. And if you match up all these drugs, we say there is no difference in which one you pick.”
Can you discontinue therapy?
“The other issue that is really driving a lot of CML strategy these days is the issue of discontinuation,” Dr. Radich commented. Conventional belief has been that TKIs do not eliminate CML stem cells; therefore, patients will need to be on therapy lifelong.
“Turns out, like many things, we were wrong,” he said. “There have been a number of studies now showing that if you are PCR negative for a number of years and get off therapy, about 60% of patients will relapse usually within the first 3 months, but about 40% of patients will actually remain negative for BCR-ABL up to 2-3 years.”
“Now the good part is that all the patients pretty much who have relapse and get put back on drug go back to getting a response; they don’t go into complete molecular remission, however. And the reason that we worry that all these patients should still be [discontinued] on a clinical trial as opposed to doing it at home is we don’t know the long-term consequences of this,” Dr. Radich said.
A period of unopposed BCR-ABL activity may allow emergence of resistant clones that take years to become clinically evident, he noted. In fact, data from Hiroshima survivors show that CML can have “an amazing dormancy,” possibly 70 years.
“So I think you have to approach any discontinuation with some caution. The risk is probably very low, but it’s actually probably a number,” he said. The guidelines therefore recommend that in patients whose disease is responding to TKIs, discontinuation should be undertaken only in a clinical trial.
“How this weighs in, I think, is if you look at CMR [complete molecular response] rates, they are higher with second-generations than with imatinib. So if you have a patient who you some day want to get to a discontinuation trial, a younger person who wants to have children and the like, then second-generations might be your best option for getting them there,” Dr. Radich proposed.
Take-home message
The guidelines, with all of their algorithms and the new Evidence Blocks, are helpful but do not replace clinical wisdom and experience, Dr. Radich asserted in closing.
“You have to kind of use your clinical judgment, and that trumps everything else,” he said. “The other thing that’s important is all of you have different experiences with using these drugs, and nothing replaces that. If you are someone who uses drug A all the time, you know how to anticipate the complications, you know what to look for. It’s a lot better [to use that drug] than just jumping to drug C when you have never used it or have little experience, because really experience is the main thing.”
Dr. Radich disclosed that he has consulting and/or research funding relationships with Ariad Pharmaceuticals, Gilead Sciences, and Novartis.
SAN FRANCISCO – “We really have an embarrassment of riches in chronic myelogenous leukemia, and the question is, which patients get which drugs? That’s really the major question that drives clinical care right now,” Dr. Jerald P. Radich, chair of the NCCN guidelines panel for chronic myelogenous leukemia (CML), told attendees of the National Comprehensive Cancer Network 10th Annual Congress: Hematologic Malignancies.
He discussed a variety of setting-specific quandaries in disease management and recent evidence from trials that is helping to provide some answers. He also reviewed some of the finer points of the NCCN’s CML guidelines, a new version of which was released at the congress.
How do you choose front-line therapy?
Tyrosine kinase inhibitors (TKIs) have dramatically improved the prognosis of CML, according to Dr. Radich, who is a member of the clinical research division, Fred Hutchinson Cancer Research Center, and an associate professor in the medical oncology division, University of Washington, both in Seattle.
Today, three TKIs—imatinib (Gleevec) and the second-generation agents nilotinib (Tasigna) and dasatinib (Sprycel)—are approved by the Food and Drug Administration as front-line therapy.
The 8-year data from the IRIS trial (International Randomized Study of Interferon Vs STI571), which tested imatinib in patients with newly diagnosed chronic-phase CML, showed that event-free survival was 81% and overall survival was 85% (Blood. 2009;114:462. Abstract 1126). “No matter how you slice it, these patients have done fantastically well,” Dr. Radich said.
The two other, newer therapies, nilotinib and dasatinib, have yielded significantly better short-term cytogenetic response and major molecular response rates in numerous trials. “But curiously enough, if you look at overall survival, there is no difference,” he commented. “We don’t know why the short-term efficacy of the second generations hasn’t translated into long-term efficacy yet.”
In the guidelines, the three front-line TKIs are nearly identical with respect to their Evidence Blocks, a new tool added to facilitate treatment comparisons and decision making.
But specific toxicities differ across the agents, which may tilt the decision one way or another. “If somebody has a history of really bad atherosclerotic events previously, then imatinib is probably not the best drug for them. If somebody has a history of pulmonary issues, then dasatinib may not be the best drug for them. And if somebody has diabetes, nilotinib probably isn’t the best drug for them,” Dr. Radich elaborated.
Costs for the three drugs are the same at present, he said. But imatinib will likely become available as a generic next year, which may make that drug the most attractive from the financial perspective.
When do you switch therapies?
There is some debate about how long to persevere with a front-line therapy when monitoring suggests the response is suboptimal, according to Dr. Radich.
Data have identified the BCR-ABL1 transcript level at 3 months to be a good predictor of long-term survival (J Clin Oncol. 2012;30:232-8). “We in the NCCN have said at 3 months or 6 months, you should consider changing [if the response is suboptimal]. You don’t have to, but you should consider changing because there are some other [therapies] that get rid of that,” he said.
However, he cautioned, a true assessment of response hinges critically on patients’ compliance with therapy. Studies using electronic monitors hidden in the caps of pill bottles suggest that even though the majority of patients say they take their TKI daily, only about 15% actually do.
Studies out of Europe and Australia have shown that some patients with a suboptimal response will have progression to accelerated phase/blast crisis in the interim between 3 months and 6 months, Dr. Radich noted. “So if you think that your patient is really, really religiously taking their drug, and if they still have not responded very well at 3 months, that might be an indication that you can consider changing therapy.”
Which second-line TKI should patients get?
The options for second-line TKI therapy include nilotinib and dasatinib, as well as bosutinib (Bosulif) and ponatinib (Iclusig).
About half of patients who experience progression on or become resistant to their first-line TKI have mutations, according to Dr. Radich. Here, a mutational analysis is warranted as the second-line agents differ somewhat with respect to the mutations they act against, which may guide the choice of agent. And toxicity profile may again dictate drugs that can and can’t be used for a specific patient.
Patients who have genuine resistance are unlikely to achieve a cure although they may have a cytogenetic response. “Most of us think that resistance might be forever and if we have a patient who has genuinely become resistant, you don’t need to transplant them necessarily [right away]. But that’s the time you have to have a conversation about doing transplantation and start doing HLA typing and the like,” he said.
Data suggest that the cytogenetic response to second-line therapy at 3 months predicts how patients will fare longer term (ASH 2008. Abstract 332).
“That ends up being a pretty good time point because if you have a patient who’s resistant and you start the search for a donor, for an HLA match, the median time to finding a donor is about 4 months,” Dr. Radich commented. “So a practical point is if you have to switch to a second-generation drug, you start a search. You find a donor, you evaluate again. If the patient had a great response, you say fine, we’re going to follow you; if they don’t, that’s a good time to move to transplant.”
In the guidelines, the Evidence Blocks for second-line (and later-line) therapies generally show what one might expect from clinical practice, Dr. Radich said. “As you march from primary therapy to secondary therapy to tertiary therapy, what happens? You all know, toxicity increases in those settings, efficacy goes down. And that’s exactly what happens here. And if you match up all these drugs, we say there is no difference in which one you pick.”
Can you discontinue therapy?
“The other issue that is really driving a lot of CML strategy these days is the issue of discontinuation,” Dr. Radich commented. Conventional belief has been that TKIs do not eliminate CML stem cells; therefore, patients will need to be on therapy lifelong.
“Turns out, like many things, we were wrong,” he said. “There have been a number of studies now showing that if you are PCR negative for a number of years and get off therapy, about 60% of patients will relapse usually within the first 3 months, but about 40% of patients will actually remain negative for BCR-ABL up to 2-3 years.”
“Now the good part is that all the patients pretty much who have relapse and get put back on drug go back to getting a response; they don’t go into complete molecular remission, however. And the reason that we worry that all these patients should still be [discontinued] on a clinical trial as opposed to doing it at home is we don’t know the long-term consequences of this,” Dr. Radich said.
A period of unopposed BCR-ABL activity may allow emergence of resistant clones that take years to become clinically evident, he noted. In fact, data from Hiroshima survivors show that CML can have “an amazing dormancy,” possibly 70 years.
“So I think you have to approach any discontinuation with some caution. The risk is probably very low, but it’s actually probably a number,” he said. The guidelines therefore recommend that in patients whose disease is responding to TKIs, discontinuation should be undertaken only in a clinical trial.
“How this weighs in, I think, is if you look at CMR [complete molecular response] rates, they are higher with second-generations than with imatinib. So if you have a patient who you some day want to get to a discontinuation trial, a younger person who wants to have children and the like, then second-generations might be your best option for getting them there,” Dr. Radich proposed.
Take-home message
The guidelines, with all of their algorithms and the new Evidence Blocks, are helpful but do not replace clinical wisdom and experience, Dr. Radich asserted in closing.
“You have to kind of use your clinical judgment, and that trumps everything else,” he said. “The other thing that’s important is all of you have different experiences with using these drugs, and nothing replaces that. If you are someone who uses drug A all the time, you know how to anticipate the complications, you know what to look for. It’s a lot better [to use that drug] than just jumping to drug C when you have never used it or have little experience, because really experience is the main thing.”
Dr. Radich disclosed that he has consulting and/or research funding relationships with Ariad Pharmaceuticals, Gilead Sciences, and Novartis.
EXPERT ANALYSIS FROM NCCN ANNUAL CONGRESS: HEMATOLOGIC MALIGNANCIES
Mental health care is ‘Code Black’
The initial explanation for why the teenager needed to be admitted seemed very flimsy to me. Finally, the office pediatrician came clean. The girl is an honor roll student, high achieving but currently overwhelmed by events in her life. She cannot handle being at home, but there is nowhere else for her to go for the weekend. Come Monday morning, the primary care physician (PCP) has arranged for her acceptance into a local mental health facility where she could get the care she needs. The prognosis was excellent. Her entire future could be markedly improved with just a little help getting through these current troubles. I thought about the patient down the hall. My partner had admitted her 2 days ago for acute-on-chronic abdominal pain, shortly after her third normal CT scan in 6 months. I strongly doubted that that patient had any more business being in a hospital than this latest admission – except, of course, for all the profit the hospital was making on the imaging. On reflection, I decided the PCP’s request no longer seemed so out of place.
The latest emergency department drama on TV has a tagline, “In the ER when there are more patients than resources, it’s called Code Black.” It contains scenes of mopping up bloody floors in the trauma bay. I’ve worked in an ED that was a major portal into a nearby pediatric mental health facility. The major traumas I cared for didn’t bleed from their life-threatening emotional wounds. Multiple times per week, children from across the city were brought in for evaluation. A few needed a toxicology work-up for ingestions. A few more needed some glue or sutures for very superficial self-inflicted forearm lacerations. Mostly I provided a medical screening before getting those teenagers moved as quickly as possible to a team of specialists who could help them. In some parts of this country that can take days.
On Oct. 5, 2015, California’s governor signed a state law permitting physician-assisted suicide. It is now the fifth state allowing that option. The pros and cons have been endlessly debated by ethicists. A brief Google search can find the philosophical arguments. I recommend a June 22, 2015, New Yorker article entitled, “The Death Treatment” by Rachel Aviv to provide a broad narrative perspective. Oregon’s Death With Dignity Act was passed 20 years ago, so it provides some scientific data. There are now 100 deaths per year under that Oregon act. The state also has 700 suicides yearly. So the leading method of suicide in Oregon, by a wide margin, continues to be used by people who are not terminally ill. That was the method of choice recently for my cousin. There was nothing dignified about it. My favorite actor, Robin Williams, in the movie “World’s Greatest Dad,” had the line, “If you’re that depressed, reach out to someone, and remember suicide is a permanent solution to temporary problems.” Five years later he took his own life.
I do enjoy debating the nuances of physician-assisted suicide with other ethicists, but I don’t confuse those academic exercises with addressing the real world problem of endemic suicide. Nationwide, there are 41,000 suicides each year, with about 5,000 in the 15- to 24-year age group. In comparison, 10,000 children annually will get cancer, but only 1,250 children will die of it. There will be about 900 pediatric recipients of lifesaving heart or liver transplants. With all this wealth, knowledge, and technology, the United States should be able to provide better treatment of mental illness. Parity of mental health services became law under President Clinton in 1996, repeated as law under President Bush in 2008, and affirmed again under President Obama. But those political promises have yet to bear fruit in real life. The system remains overloaded. For the sake of the children and young adults, pediatricians must promote expansion of mental health services.
Dr. Powell is a pediatric hospitalist and clinical ethics consultant living in St. Louis. Dr. Powell said he had no relevant financial disclosures or conflicts of interest.
The initial explanation for why the teenager needed to be admitted seemed very flimsy to me. Finally, the office pediatrician came clean. The girl is an honor roll student, high achieving but currently overwhelmed by events in her life. She cannot handle being at home, but there is nowhere else for her to go for the weekend. Come Monday morning, the primary care physician (PCP) has arranged for her acceptance into a local mental health facility where she could get the care she needs. The prognosis was excellent. Her entire future could be markedly improved with just a little help getting through these current troubles. I thought about the patient down the hall. My partner had admitted her 2 days ago for acute-on-chronic abdominal pain, shortly after her third normal CT scan in 6 months. I strongly doubted that that patient had any more business being in a hospital than this latest admission – except, of course, for all the profit the hospital was making on the imaging. On reflection, I decided the PCP’s request no longer seemed so out of place.
The latest emergency department drama on TV has a tagline, “In the ER when there are more patients than resources, it’s called Code Black.” It contains scenes of mopping up bloody floors in the trauma bay. I’ve worked in an ED that was a major portal into a nearby pediatric mental health facility. The major traumas I cared for didn’t bleed from their life-threatening emotional wounds. Multiple times per week, children from across the city were brought in for evaluation. A few needed a toxicology work-up for ingestions. A few more needed some glue or sutures for very superficial self-inflicted forearm lacerations. Mostly I provided a medical screening before getting those teenagers moved as quickly as possible to a team of specialists who could help them. In some parts of this country that can take days.
On Oct. 5, 2015, California’s governor signed a state law permitting physician-assisted suicide. It is now the fifth state allowing that option. The pros and cons have been endlessly debated by ethicists. A brief Google search can find the philosophical arguments. I recommend a June 22, 2015, New Yorker article entitled, “The Death Treatment” by Rachel Aviv to provide a broad narrative perspective. Oregon’s Death With Dignity Act was passed 20 years ago, so it provides some scientific data. There are now 100 deaths per year under that Oregon act. The state also has 700 suicides yearly. So the leading method of suicide in Oregon, by a wide margin, continues to be used by people who are not terminally ill. That was the method of choice recently for my cousin. There was nothing dignified about it. My favorite actor, Robin Williams, in the movie “World’s Greatest Dad,” had the line, “If you’re that depressed, reach out to someone, and remember suicide is a permanent solution to temporary problems.” Five years later he took his own life.
I do enjoy debating the nuances of physician-assisted suicide with other ethicists, but I don’t confuse those academic exercises with addressing the real world problem of endemic suicide. Nationwide, there are 41,000 suicides each year, with about 5,000 in the 15- to 24-year age group. In comparison, 10,000 children annually will get cancer, but only 1,250 children will die of it. There will be about 900 pediatric recipients of lifesaving heart or liver transplants. With all this wealth, knowledge, and technology, the United States should be able to provide better treatment of mental illness. Parity of mental health services became law under President Clinton in 1996, repeated as law under President Bush in 2008, and affirmed again under President Obama. But those political promises have yet to bear fruit in real life. The system remains overloaded. For the sake of the children and young adults, pediatricians must promote expansion of mental health services.
Dr. Powell is a pediatric hospitalist and clinical ethics consultant living in St. Louis. Dr. Powell said he had no relevant financial disclosures or conflicts of interest.
The initial explanation for why the teenager needed to be admitted seemed very flimsy to me. Finally, the office pediatrician came clean. The girl is an honor roll student, high achieving but currently overwhelmed by events in her life. She cannot handle being at home, but there is nowhere else for her to go for the weekend. Come Monday morning, the primary care physician (PCP) has arranged for her acceptance into a local mental health facility where she could get the care she needs. The prognosis was excellent. Her entire future could be markedly improved with just a little help getting through these current troubles. I thought about the patient down the hall. My partner had admitted her 2 days ago for acute-on-chronic abdominal pain, shortly after her third normal CT scan in 6 months. I strongly doubted that that patient had any more business being in a hospital than this latest admission – except, of course, for all the profit the hospital was making on the imaging. On reflection, I decided the PCP’s request no longer seemed so out of place.
The latest emergency department drama on TV has a tagline, “In the ER when there are more patients than resources, it’s called Code Black.” It contains scenes of mopping up bloody floors in the trauma bay. I’ve worked in an ED that was a major portal into a nearby pediatric mental health facility. The major traumas I cared for didn’t bleed from their life-threatening emotional wounds. Multiple times per week, children from across the city were brought in for evaluation. A few needed a toxicology work-up for ingestions. A few more needed some glue or sutures for very superficial self-inflicted forearm lacerations. Mostly I provided a medical screening before getting those teenagers moved as quickly as possible to a team of specialists who could help them. In some parts of this country that can take days.
On Oct. 5, 2015, California’s governor signed a state law permitting physician-assisted suicide. It is now the fifth state allowing that option. The pros and cons have been endlessly debated by ethicists. A brief Google search can find the philosophical arguments. I recommend a June 22, 2015, New Yorker article entitled, “The Death Treatment” by Rachel Aviv to provide a broad narrative perspective. Oregon’s Death With Dignity Act was passed 20 years ago, so it provides some scientific data. There are now 100 deaths per year under that Oregon act. The state also has 700 suicides yearly. So the leading method of suicide in Oregon, by a wide margin, continues to be used by people who are not terminally ill. That was the method of choice recently for my cousin. There was nothing dignified about it. My favorite actor, Robin Williams, in the movie “World’s Greatest Dad,” had the line, “If you’re that depressed, reach out to someone, and remember suicide is a permanent solution to temporary problems.” Five years later he took his own life.
I do enjoy debating the nuances of physician-assisted suicide with other ethicists, but I don’t confuse those academic exercises with addressing the real world problem of endemic suicide. Nationwide, there are 41,000 suicides each year, with about 5,000 in the 15- to 24-year age group. In comparison, 10,000 children annually will get cancer, but only 1,250 children will die of it. There will be about 900 pediatric recipients of lifesaving heart or liver transplants. With all this wealth, knowledge, and technology, the United States should be able to provide better treatment of mental illness. Parity of mental health services became law under President Clinton in 1996, repeated as law under President Bush in 2008, and affirmed again under President Obama. But those political promises have yet to bear fruit in real life. The system remains overloaded. For the sake of the children and young adults, pediatricians must promote expansion of mental health services.
Dr. Powell is a pediatric hospitalist and clinical ethics consultant living in St. Louis. Dr. Powell said he had no relevant financial disclosures or conflicts of interest.
ADHD treatment – beyond medications
While it is well established that medications can be an important aspect of treatment for youth who meet the criteria for attention-deficit/hyperactivity disorder (ADHD), too often clinicians neglect to address important nonpharmacologic interventions that have increasingly been shown to be effective. This column is devoted to reviewing some of the many components of a treatment plan other than medications that could be utilized to provide a more comprehensive and wellness-informed approach to children who struggle with ADHD symptoms.
Case summary
Ethan is a 7-year-old boy who presents with his parents for an ADHD evaluation. The pediatrician conducts the evaluation according to American Academy of Pediatrics guidelines, which include the use of rating scales from multiple sources. The outcome of the assessment is that Ethan does indeed meet criteria for ADHD and his symptoms are causing impairment in his school work, home environment, and interactions with his peers. Treatment is recommended.
Discussion
It is easy to rely exclusively on medications when the focus of the evaluation is solely about symptoms. When clinicians expand their view to assess various domains of wellness and health promotion, however, several other potential avenues for intervention often become apparent. Asking about sleep routines, nutrition, participation in the arts and music, physical activity, reading, and screen time – among other things – can reveal the following specific areas that require guidance and support:
• Exercise. Children today are increasingly sedentary, and there is increasing evidence that physical activity is inversely related to several ADHD behaviors (J Am Acad Child Adolesc Psychiatry. 2015 Jul;54:565-70). Counsel families about the importance of exercise and try to help the family develop a plan that includes the provision for regular physical activity. Joining sports teams may be particularly useful as it ensures that regular exercise takes place and offers some additional benefits inherent in playing with a team.
• Screen time. Although there has been active discussion lately about what constitutes “too much” screen time, it is clear that many children well exceed even the most liberal thresholds. Furthermore, there is increasing evidence that excessive screen time can lead to worsening attention problems over time (Pediatrics. 2004;113:708-13). One technique that can be effective, especially for younger children, is to have them “earn” their screen time by engaging in other activities such as reading or exercise.
• Nutrition. Apart from any specific deficiency states, research shows that a healthier diet in general is associated with lower levels of behavioral problems. With regard to ADHD, one aspect that is often worth investigating specifically is whether the child gets a nutritious breakfast each morning that can help keep attentional skills optimal.
• Musical training. Some intriguing new research is showing links between brain maturation and musical training, and in some of the very regions of the brain that have been implicated in ADHD (J Am Acad Child Adolesc Psychiatry. 2014;53:1153-61).
• Omega-3s. A meta-analysis demonstrated that omega-3 supplementation can improve ADHD symptoms (J Am Acad Child Adolesc Psychiatry. 2011 Oct;50:991-1000). While the optimal dose remains under investigation, there is some evidence that improved response was related to higher eicosapentaenoic acid doses.
• Skills training. To some degree, many skills associated with ADHD (disorganization, forgetfulness, distractibility) can be specifically taught with techniques such as mindfulness (J Atten Disord. 2015 Feb;19[2]:147-57). Having families work with counselors who have specific training in ADHD can be a very useful part of treatment and can help teach important lifelong skills. Parent behavioral therapy also can be effective around many behaviors such as defiance and aggression that accompany ADHD.
Case follow-up
The pediatrician decides to enhance her assessment by inquiring about many domains of wellness, and she discovers that Ethan has chronic problems getting to sleep, and he spends many hours each day playing video games to the exclusion of physical activity. She offers some strategies to improve these areas while the family investigates working with a counselor who has specific expertise in enhancing cognitive skills. Initial improvements are encouraging, and the family decides to pursue these avenues further while delaying medication treatment, at least for now.
By keeping in mind these important other treatment domains, pediatricians can avoid the trap of overrelying on medications as the sole method of treatment while encouraging techniques that will provide long-term benefits in overall health and wellness.
Dr. Rettew is an associate professor of psychiatry and pediatrics at the University of Vermont, Burlington. Dr. Rettew said he has no relevant financial disclosures. Follow him on Twitter @pedipsych. E-mail him at pdnews@frontlinemedcom.com.
While it is well established that medications can be an important aspect of treatment for youth who meet the criteria for attention-deficit/hyperactivity disorder (ADHD), too often clinicians neglect to address important nonpharmacologic interventions that have increasingly been shown to be effective. This column is devoted to reviewing some of the many components of a treatment plan other than medications that could be utilized to provide a more comprehensive and wellness-informed approach to children who struggle with ADHD symptoms.
Case summary
Ethan is a 7-year-old boy who presents with his parents for an ADHD evaluation. The pediatrician conducts the evaluation according to American Academy of Pediatrics guidelines, which include the use of rating scales from multiple sources. The outcome of the assessment is that Ethan does indeed meet criteria for ADHD and his symptoms are causing impairment in his school work, home environment, and interactions with his peers. Treatment is recommended.
Discussion
It is easy to rely exclusively on medications when the focus of the evaluation is solely about symptoms. When clinicians expand their view to assess various domains of wellness and health promotion, however, several other potential avenues for intervention often become apparent. Asking about sleep routines, nutrition, participation in the arts and music, physical activity, reading, and screen time – among other things – can reveal the following specific areas that require guidance and support:
• Exercise. Children today are increasingly sedentary, and there is increasing evidence that physical activity is inversely related to several ADHD behaviors (J Am Acad Child Adolesc Psychiatry. 2015 Jul;54:565-70). Counsel families about the importance of exercise and try to help the family develop a plan that includes the provision for regular physical activity. Joining sports teams may be particularly useful as it ensures that regular exercise takes place and offers some additional benefits inherent in playing with a team.
• Screen time. Although there has been active discussion lately about what constitutes “too much” screen time, it is clear that many children well exceed even the most liberal thresholds. Furthermore, there is increasing evidence that excessive screen time can lead to worsening attention problems over time (Pediatrics. 2004;113:708-13). One technique that can be effective, especially for younger children, is to have them “earn” their screen time by engaging in other activities such as reading or exercise.
• Nutrition. Apart from any specific deficiency states, research shows that a healthier diet in general is associated with lower levels of behavioral problems. With regard to ADHD, one aspect that is often worth investigating specifically is whether the child gets a nutritious breakfast each morning that can help keep attentional skills optimal.
• Musical training. Some intriguing new research is showing links between brain maturation and musical training, and in some of the very regions of the brain that have been implicated in ADHD (J Am Acad Child Adolesc Psychiatry. 2014;53:1153-61).
• Omega-3s. A meta-analysis demonstrated that omega-3 supplementation can improve ADHD symptoms (J Am Acad Child Adolesc Psychiatry. 2011 Oct;50:991-1000). While the optimal dose remains under investigation, there is some evidence that improved response was related to higher eicosapentaenoic acid doses.
• Skills training. To some degree, many skills associated with ADHD (disorganization, forgetfulness, distractibility) can be specifically taught with techniques such as mindfulness (J Atten Disord. 2015 Feb;19[2]:147-57). Having families work with counselors who have specific training in ADHD can be a very useful part of treatment and can help teach important lifelong skills. Parent behavioral therapy also can be effective around many behaviors such as defiance and aggression that accompany ADHD.
Case follow-up
The pediatrician decides to enhance her assessment by inquiring about many domains of wellness, and she discovers that Ethan has chronic problems getting to sleep, and he spends many hours each day playing video games to the exclusion of physical activity. She offers some strategies to improve these areas while the family investigates working with a counselor who has specific expertise in enhancing cognitive skills. Initial improvements are encouraging, and the family decides to pursue these avenues further while delaying medication treatment, at least for now.
By keeping in mind these important other treatment domains, pediatricians can avoid the trap of overrelying on medications as the sole method of treatment while encouraging techniques that will provide long-term benefits in overall health and wellness.
Dr. Rettew is an associate professor of psychiatry and pediatrics at the University of Vermont, Burlington. Dr. Rettew said he has no relevant financial disclosures. Follow him on Twitter @pedipsych. E-mail him at pdnews@frontlinemedcom.com.
While it is well established that medications can be an important aspect of treatment for youth who meet the criteria for attention-deficit/hyperactivity disorder (ADHD), too often clinicians neglect to address important nonpharmacologic interventions that have increasingly been shown to be effective. This column is devoted to reviewing some of the many components of a treatment plan other than medications that could be utilized to provide a more comprehensive and wellness-informed approach to children who struggle with ADHD symptoms.
Case summary
Ethan is a 7-year-old boy who presents with his parents for an ADHD evaluation. The pediatrician conducts the evaluation according to American Academy of Pediatrics guidelines, which include the use of rating scales from multiple sources. The outcome of the assessment is that Ethan does indeed meet criteria for ADHD and his symptoms are causing impairment in his school work, home environment, and interactions with his peers. Treatment is recommended.
Discussion
It is easy to rely exclusively on medications when the focus of the evaluation is solely about symptoms. When clinicians expand their view to assess various domains of wellness and health promotion, however, several other potential avenues for intervention often become apparent. Asking about sleep routines, nutrition, participation in the arts and music, physical activity, reading, and screen time – among other things – can reveal the following specific areas that require guidance and support:
• Exercise. Children today are increasingly sedentary, and there is increasing evidence that physical activity is inversely related to several ADHD behaviors (J Am Acad Child Adolesc Psychiatry. 2015 Jul;54:565-70). Counsel families about the importance of exercise and try to help the family develop a plan that includes the provision for regular physical activity. Joining sports teams may be particularly useful as it ensures that regular exercise takes place and offers some additional benefits inherent in playing with a team.
• Screen time. Although there has been active discussion lately about what constitutes “too much” screen time, it is clear that many children well exceed even the most liberal thresholds. Furthermore, there is increasing evidence that excessive screen time can lead to worsening attention problems over time (Pediatrics. 2004;113:708-13). One technique that can be effective, especially for younger children, is to have them “earn” their screen time by engaging in other activities such as reading or exercise.
• Nutrition. Apart from any specific deficiency states, research shows that a healthier diet in general is associated with lower levels of behavioral problems. With regard to ADHD, one aspect that is often worth investigating specifically is whether the child gets a nutritious breakfast each morning that can help keep attentional skills optimal.
• Musical training. Some intriguing new research is showing links between brain maturation and musical training, and in some of the very regions of the brain that have been implicated in ADHD (J Am Acad Child Adolesc Psychiatry. 2014;53:1153-61).
• Omega-3s. A meta-analysis demonstrated that omega-3 supplementation can improve ADHD symptoms (J Am Acad Child Adolesc Psychiatry. 2011 Oct;50:991-1000). While the optimal dose remains under investigation, there is some evidence that improved response was related to higher eicosapentaenoic acid doses.
• Skills training. To some degree, many skills associated with ADHD (disorganization, forgetfulness, distractibility) can be specifically taught with techniques such as mindfulness (J Atten Disord. 2015 Feb;19[2]:147-57). Having families work with counselors who have specific training in ADHD can be a very useful part of treatment and can help teach important lifelong skills. Parent behavioral therapy also can be effective around many behaviors such as defiance and aggression that accompany ADHD.
Case follow-up
The pediatrician decides to enhance her assessment by inquiring about many domains of wellness, and she discovers that Ethan has chronic problems getting to sleep, and he spends many hours each day playing video games to the exclusion of physical activity. She offers some strategies to improve these areas while the family investigates working with a counselor who has specific expertise in enhancing cognitive skills. Initial improvements are encouraging, and the family decides to pursue these avenues further while delaying medication treatment, at least for now.
By keeping in mind these important other treatment domains, pediatricians can avoid the trap of overrelying on medications as the sole method of treatment while encouraging techniques that will provide long-term benefits in overall health and wellness.
Dr. Rettew is an associate professor of psychiatry and pediatrics at the University of Vermont, Burlington. Dr. Rettew said he has no relevant financial disclosures. Follow him on Twitter @pedipsych. E-mail him at pdnews@frontlinemedcom.com.
FDA approves trabectedin for some advanced soft tissue sarcomas
The Food and Drug Administration has approved trabectedin for the treatment of advanced or unresectable liposarcomas and leiomyosarcomas that have been previously treated with anthracycline-based regimens.
Approval is based on improvements in progression-free survival in a trial of 518 participants with metastatic or recurrent leiomyosarcoma or liposarcoma, randomly assigned to receive either trabectedin (345 patients) or dacarbazine (173 patients). Median progression-free survival was 4.2 months for those in the trabectedin arm, compared with 1.5 months for those in the dacarbazine arm, according to an Oct. 23 statement issued by the FDA.
The most common side effects for those in the trabectedin arm were nausea, fatigue, vomiting, diarrhea, constipation, decreased appetite, dyspnea, headache, peripheral edema, neutropenia, thrombocytopenia, anemia, elevated liver enzymes, and decreases in albumin.
The drug label carries a warning of the risk of neutropenic sepsis, rhabdomyolysis, hepatotoxicity, extravasation, tissue necrosis, and cardiomyopathy. Women should be advised of potential risks to a developing fetus, and those who are breastfeeding should not take trabectedin, the FDA said.
Trabectedin, an alkylating drug, is marketed as Yondelis by Janssen Products of Raritan, N.J.
The label is available on the FDA website at drugsatfda.
lnikolaides@frontlinemedcom.com
On Twitter @NikolaidesLaura
The Food and Drug Administration has approved trabectedin for the treatment of advanced or unresectable liposarcomas and leiomyosarcomas that have been previously treated with anthracycline-based regimens.
Approval is based on improvements in progression-free survival in a trial of 518 participants with metastatic or recurrent leiomyosarcoma or liposarcoma, randomly assigned to receive either trabectedin (345 patients) or dacarbazine (173 patients). Median progression-free survival was 4.2 months for those in the trabectedin arm, compared with 1.5 months for those in the dacarbazine arm, according to an Oct. 23 statement issued by the FDA.
The most common side effects for those in the trabectedin arm were nausea, fatigue, vomiting, diarrhea, constipation, decreased appetite, dyspnea, headache, peripheral edema, neutropenia, thrombocytopenia, anemia, elevated liver enzymes, and decreases in albumin.
The drug label carries a warning of the risk of neutropenic sepsis, rhabdomyolysis, hepatotoxicity, extravasation, tissue necrosis, and cardiomyopathy. Women should be advised of potential risks to a developing fetus, and those who are breastfeeding should not take trabectedin, the FDA said.
Trabectedin, an alkylating drug, is marketed as Yondelis by Janssen Products of Raritan, N.J.
The label is available on the FDA website at drugsatfda.
lnikolaides@frontlinemedcom.com
On Twitter @NikolaidesLaura
The Food and Drug Administration has approved trabectedin for the treatment of advanced or unresectable liposarcomas and leiomyosarcomas that have been previously treated with anthracycline-based regimens.
Approval is based on improvements in progression-free survival in a trial of 518 participants with metastatic or recurrent leiomyosarcoma or liposarcoma, randomly assigned to receive either trabectedin (345 patients) or dacarbazine (173 patients). Median progression-free survival was 4.2 months for those in the trabectedin arm, compared with 1.5 months for those in the dacarbazine arm, according to an Oct. 23 statement issued by the FDA.
The most common side effects for those in the trabectedin arm were nausea, fatigue, vomiting, diarrhea, constipation, decreased appetite, dyspnea, headache, peripheral edema, neutropenia, thrombocytopenia, anemia, elevated liver enzymes, and decreases in albumin.
The drug label carries a warning of the risk of neutropenic sepsis, rhabdomyolysis, hepatotoxicity, extravasation, tissue necrosis, and cardiomyopathy. Women should be advised of potential risks to a developing fetus, and those who are breastfeeding should not take trabectedin, the FDA said.
Trabectedin, an alkylating drug, is marketed as Yondelis by Janssen Products of Raritan, N.J.
The label is available on the FDA website at drugsatfda.
lnikolaides@frontlinemedcom.com
On Twitter @NikolaidesLaura
Louisiana goes two for one on controlled substance prescriptions
In 2013, enough controlled substances were prescribed in Louisiana – 2006.2 prescriptions per 1,000 population – that each and every person in the state could have received two, according to a report from the Centers for Disease Control and Prevention.
The combined prescribing rate for opioids, benzodiazepines, and stimulants in Louisiana topped the eight states included in the CDC report, with West Virginia in second at 1,695.7 prescriptions per 1,000. At the low end, California was the only one of the eight states where the average controlled substance prescription rate was less than one per person, but just barely at 994.8 per 1,000 people, followed by Idaho at 1,292.3 per 1,000, according to the CDC investigators in a Morbidity and Mortality Weekly Report Surveillance Summary (2015 Oct;64[SS09]:1-14).
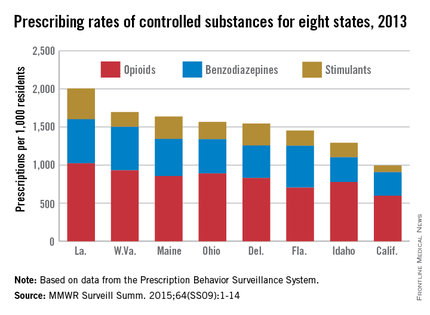
Of the three categories of controlled substances, opioids were by far the most commonly prescribed. At 1,021.7 prescriptions per 1,000 people, the opioid prescription rate in Louisiana was greater than the total controlled substance prescription rate in California. West Virginia had the second highest rate at 929.3, and California had the lowest at 596.3.
Prescription rates for stimulants and benzodiazepines were highest in Louisiana at 403.9 and 580.6 per 1,000 people, respectively. Maine had the next-highest stimulant prescription rate at 293.9, and West Virginia had the second-highest benzodiazepine prescription rate at 572.1. California had the lowest rate in both categories at 87.7 and 310.8, respectively.
Women received opioids and benzodiazepines at much higher rates than did men in every state, but stimulant-prescribing rates were higher for men in five states.
Opioid use was highest in people aged 55-64 years, though use spiked dramatically past the age of 25. People in Louisiana aged 55-64 receiving opioids had the highest controlled substance prescription rate of any measured age group, at 1,715.7 per 1,000 people. Benzodiazepine use was most common in people over 65 years, and stimulant prescriptions were highest in people younger than 18, likely because of the prevalence of childhood attention-deficit/hyperactivity disorder, the CDC investigators said.
The MMWR report used data collected by the Prescription Behavior Surveillance System. The eight states were included because they submitted data to the Prescription Behavior Surveillance System in time for the report, and they represent about one-quarter of the U.S. population.
In 2013, enough controlled substances were prescribed in Louisiana – 2006.2 prescriptions per 1,000 population – that each and every person in the state could have received two, according to a report from the Centers for Disease Control and Prevention.
The combined prescribing rate for opioids, benzodiazepines, and stimulants in Louisiana topped the eight states included in the CDC report, with West Virginia in second at 1,695.7 prescriptions per 1,000. At the low end, California was the only one of the eight states where the average controlled substance prescription rate was less than one per person, but just barely at 994.8 per 1,000 people, followed by Idaho at 1,292.3 per 1,000, according to the CDC investigators in a Morbidity and Mortality Weekly Report Surveillance Summary (2015 Oct;64[SS09]:1-14).

Of the three categories of controlled substances, opioids were by far the most commonly prescribed. At 1,021.7 prescriptions per 1,000 people, the opioid prescription rate in Louisiana was greater than the total controlled substance prescription rate in California. West Virginia had the second highest rate at 929.3, and California had the lowest at 596.3.
Prescription rates for stimulants and benzodiazepines were highest in Louisiana at 403.9 and 580.6 per 1,000 people, respectively. Maine had the next-highest stimulant prescription rate at 293.9, and West Virginia had the second-highest benzodiazepine prescription rate at 572.1. California had the lowest rate in both categories at 87.7 and 310.8, respectively.
Women received opioids and benzodiazepines at much higher rates than did men in every state, but stimulant-prescribing rates were higher for men in five states.
Opioid use was highest in people aged 55-64 years, though use spiked dramatically past the age of 25. People in Louisiana aged 55-64 receiving opioids had the highest controlled substance prescription rate of any measured age group, at 1,715.7 per 1,000 people. Benzodiazepine use was most common in people over 65 years, and stimulant prescriptions were highest in people younger than 18, likely because of the prevalence of childhood attention-deficit/hyperactivity disorder, the CDC investigators said.
The MMWR report used data collected by the Prescription Behavior Surveillance System. The eight states were included because they submitted data to the Prescription Behavior Surveillance System in time for the report, and they represent about one-quarter of the U.S. population.
In 2013, enough controlled substances were prescribed in Louisiana – 2006.2 prescriptions per 1,000 population – that each and every person in the state could have received two, according to a report from the Centers for Disease Control and Prevention.
The combined prescribing rate for opioids, benzodiazepines, and stimulants in Louisiana topped the eight states included in the CDC report, with West Virginia in second at 1,695.7 prescriptions per 1,000. At the low end, California was the only one of the eight states where the average controlled substance prescription rate was less than one per person, but just barely at 994.8 per 1,000 people, followed by Idaho at 1,292.3 per 1,000, according to the CDC investigators in a Morbidity and Mortality Weekly Report Surveillance Summary (2015 Oct;64[SS09]:1-14).

Of the three categories of controlled substances, opioids were by far the most commonly prescribed. At 1,021.7 prescriptions per 1,000 people, the opioid prescription rate in Louisiana was greater than the total controlled substance prescription rate in California. West Virginia had the second highest rate at 929.3, and California had the lowest at 596.3.
Prescription rates for stimulants and benzodiazepines were highest in Louisiana at 403.9 and 580.6 per 1,000 people, respectively. Maine had the next-highest stimulant prescription rate at 293.9, and West Virginia had the second-highest benzodiazepine prescription rate at 572.1. California had the lowest rate in both categories at 87.7 and 310.8, respectively.
Women received opioids and benzodiazepines at much higher rates than did men in every state, but stimulant-prescribing rates were higher for men in five states.
Opioid use was highest in people aged 55-64 years, though use spiked dramatically past the age of 25. People in Louisiana aged 55-64 receiving opioids had the highest controlled substance prescription rate of any measured age group, at 1,715.7 per 1,000 people. Benzodiazepine use was most common in people over 65 years, and stimulant prescriptions were highest in people younger than 18, likely because of the prevalence of childhood attention-deficit/hyperactivity disorder, the CDC investigators said.
The MMWR report used data collected by the Prescription Behavior Surveillance System. The eight states were included because they submitted data to the Prescription Behavior Surveillance System in time for the report, and they represent about one-quarter of the U.S. population.
FROM MORBIDITY AND MORTALITY WEEKLY REPORT
Lessons learned from the history of VBAC
In December 2014, The Wall Street Journal ran an article about a young mother who wanted a vaginal birth after C-section (VBAC) for her second child. After her hospital stopped offering VBACs, the woman had to find another place to deliver. She did have a successful VBAC, but her story is not unique – many women may not receive adequate consultations about or provider support for VBAC as a delivery option.
According to the article, a lack of clinical support was the reason the hospital discontinued VBACs. Although the hospital’s decision may have frustrated the mother, this ensured that she would not be promised a birthing option that the hospital could not deliver – in all senses of this word. Successful VBAC requires proper patient selection, appropriate consent and adequate provisions in case of emergencies.
Not every hospital has made such a choice. Based on studies of a trial of labor after cesarean, conducted after the 1960s, the rate of VBACs increased. As VBACs became more common, the approach to the procedure became more relaxed. VBACs went from only being performed in tertiary care hospitals with appropriate support for emergencies, to community hospitals with no backup. Patient selection became less rigorous, and the rate of complications went up, which, in turn, caused the number of associated legal claims to rise. Hospitals started discouraging VBACs, and ob.gyns. no longer counseled their patients about this option. The VBAC rate decreased, and the C-section rate increased.
Today, many women want to pursue a trial of labor after cesarean. Data from large clinical studies have demonstrated the safety and success of VBAC with proper care. Because of the storied history and a revival of interest in VBACs, we have invited Dr. Mark Landon, the Richard L. Meiling Professor and chairman of the department of obstetrics and gynecology at the Ohio State University, and the lead on one of the recent seminal VBAC studies, to address this topic.
Dr. Reece, who specializes in maternal-fetal medicine, is vice president for medical affairs at the University of Maryland, Baltimore, as well as the John Z. and Akiko K. Bowers Distinguished Professor and dean of the school of medicine. Dr. Reece reported having no relevant financial disclosures. He is the medical editor of this column. Contact him at obnews@frontlinemedcom.com.
In December 2014, The Wall Street Journal ran an article about a young mother who wanted a vaginal birth after C-section (VBAC) for her second child. After her hospital stopped offering VBACs, the woman had to find another place to deliver. She did have a successful VBAC, but her story is not unique – many women may not receive adequate consultations about or provider support for VBAC as a delivery option.
According to the article, a lack of clinical support was the reason the hospital discontinued VBACs. Although the hospital’s decision may have frustrated the mother, this ensured that she would not be promised a birthing option that the hospital could not deliver – in all senses of this word. Successful VBAC requires proper patient selection, appropriate consent and adequate provisions in case of emergencies.
Not every hospital has made such a choice. Based on studies of a trial of labor after cesarean, conducted after the 1960s, the rate of VBACs increased. As VBACs became more common, the approach to the procedure became more relaxed. VBACs went from only being performed in tertiary care hospitals with appropriate support for emergencies, to community hospitals with no backup. Patient selection became less rigorous, and the rate of complications went up, which, in turn, caused the number of associated legal claims to rise. Hospitals started discouraging VBACs, and ob.gyns. no longer counseled their patients about this option. The VBAC rate decreased, and the C-section rate increased.
Today, many women want to pursue a trial of labor after cesarean. Data from large clinical studies have demonstrated the safety and success of VBAC with proper care. Because of the storied history and a revival of interest in VBACs, we have invited Dr. Mark Landon, the Richard L. Meiling Professor and chairman of the department of obstetrics and gynecology at the Ohio State University, and the lead on one of the recent seminal VBAC studies, to address this topic.
Dr. Reece, who specializes in maternal-fetal medicine, is vice president for medical affairs at the University of Maryland, Baltimore, as well as the John Z. and Akiko K. Bowers Distinguished Professor and dean of the school of medicine. Dr. Reece reported having no relevant financial disclosures. He is the medical editor of this column. Contact him at obnews@frontlinemedcom.com.
In December 2014, The Wall Street Journal ran an article about a young mother who wanted a vaginal birth after C-section (VBAC) for her second child. After her hospital stopped offering VBACs, the woman had to find another place to deliver. She did have a successful VBAC, but her story is not unique – many women may not receive adequate consultations about or provider support for VBAC as a delivery option.
According to the article, a lack of clinical support was the reason the hospital discontinued VBACs. Although the hospital’s decision may have frustrated the mother, this ensured that she would not be promised a birthing option that the hospital could not deliver – in all senses of this word. Successful VBAC requires proper patient selection, appropriate consent and adequate provisions in case of emergencies.
Not every hospital has made such a choice. Based on studies of a trial of labor after cesarean, conducted after the 1960s, the rate of VBACs increased. As VBACs became more common, the approach to the procedure became more relaxed. VBACs went from only being performed in tertiary care hospitals with appropriate support for emergencies, to community hospitals with no backup. Patient selection became less rigorous, and the rate of complications went up, which, in turn, caused the number of associated legal claims to rise. Hospitals started discouraging VBACs, and ob.gyns. no longer counseled their patients about this option. The VBAC rate decreased, and the C-section rate increased.
Today, many women want to pursue a trial of labor after cesarean. Data from large clinical studies have demonstrated the safety and success of VBAC with proper care. Because of the storied history and a revival of interest in VBACs, we have invited Dr. Mark Landon, the Richard L. Meiling Professor and chairman of the department of obstetrics and gynecology at the Ohio State University, and the lead on one of the recent seminal VBAC studies, to address this topic.
Dr. Reece, who specializes in maternal-fetal medicine, is vice president for medical affairs at the University of Maryland, Baltimore, as well as the John Z. and Akiko K. Bowers Distinguished Professor and dean of the school of medicine. Dr. Reece reported having no relevant financial disclosures. He is the medical editor of this column. Contact him at obnews@frontlinemedcom.com.
Barriers to VBAC remain in spite of evidence
The relative safety of vaginal birth after cesarean (VBAC) has been documented in several large-scale studies in the past 15 years, and was affirmed in 2010 through a National Institutes of Health consensus development conference and a practice bulletin from the American College of Obstetricians and Gynecologists. Yet, despite all this research and review, rates of a trial of labor after cesarean (TOLAC) have increased only modestly in the last several years.
Approximately 20% of all births in 2013 in women with a history of one cesarean section involved a trial of labor, according to a recent report from the Centers for Disease Control and Prevention. This represents only a small increase from 2006, when the TOLAC rate had plummeted to approximately 15%.
The limited change is concerning because up to two-thirds of women with a prior cesarean delivery are candidates for a trial of labor, and many of them are excellent candidates. In total, 70% of the women who attempted labor in 2013 after a previous cesarean had successful VBACs, the CDC data shows.
Several European countries have TOLAC rates between 50% and 70%, but in the United States, as evidenced by the recent CDC data, there continues to be an underutilization of attempted VBAC. We must ask ourselves, are women truly able to choose TOLAC, or are they being dissuaded by the health care system?
I believe that the barriers are still pervasive. Too often, women who are TOLAC candidates are not receiving appropriate counseling – and too often, women are not even being presented the option of a trial of labor, even when staff are immediately available to provide emergency care if needed.
Rupture concerns in perspective
When the NIH consensus development panel reviewed VBAC in 2010, it concluded that TOLAC is a reasonable option for many women with a prior cesarean. The panel found that restricted access to VBAC/TOLAC stemmed from existing practice guidelines and the medical liability climate, and it called upon providers and others to “mitigate or even eliminate” the barriers that women face in finding clinicians and facilities able and willing to offer TOLAC.
ACOG’s 2010 practice bulletin also acknowledged the problem of limited access. ACOG recommended, as it had in an earlier bulletin, that TOLAC-VBAC be undertaken in facilities where staff are immediately available for emergency care. It added, however, that when such resources are not available, the best alternative may be to refer patients to a facility with available resources. Health care providers and insurance carriers “should do all they can to facilitate transfer of care or comanagement in support of a desired TOLAC,” ACOG’s document states.
Why, given such recommendations, are we still falling so short of where we should be?
A number of nonclinical factors are involved, but clearly, as both the NIH and ACOG have stated, the fear of litigation in cases of uterine rupture is a contributing factor. A ruptured uterus is indeed the principal risk associated with TOLAC, and it can have serious sequelae including perinatal death, hypoxic ischemic encephalopathy (HIE), and hysterectomy.
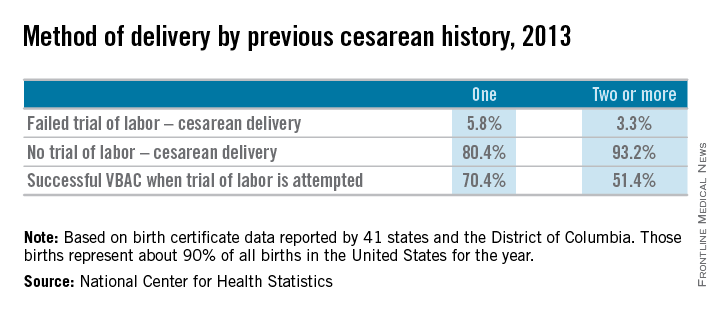
We must appreciate, however, that the absolute rates of uterine rupture and of serious adverse outcomes are quite low. The rupture rate in 2013 among women who underwent TOLAC but ultimately had a repeat cesarean section – the highest-risk group – was 495 per 100,000 live births, according to the CDC. This rate of approximately 0.5% is consistent with the level of risk reported in the literature for several decades.
In one of the two large observational studies done in the United States that have shed light on TOLAC outcomes, the rate of uterine rupture among women who underwent TOLAC was 0.7% for women with a prior low transverse incision, 2.0% for those with a prior low vertical incision, and 0.5% for those with an unknown type of prior incision. Overall, the rate of uterine rupture in this study’s cohort of 17,898 women who underwent TOLAC was 0.7% (N Engl J Med. 2004 Dec 16;351[25]:2581-9). The study was conducted at 19 medical centers belonging to the Eunice Kennedy Shriver National Institute of Child Health and Human Development’s Maternal-Fetal Medical Units (MFMU) Network.
The second large study conducted in the United States – a multicenter observational study in which records of approximately 25,000 women with a prior low-transverse cesarean section were reviewed – also showed rates of uterine rupture less than 1% (Am J Obstet Gynecol. 2005 Nov;193[5]:1656-62).
The attributable risk for perinatal death or HIE at term appears to be 1 per 2,000 TOLAC, according to the MFMU Network study.
Failed trials of labor resulting in repeat cesarean deliveries have consistently been associated with higher morbidity than scheduled repeat cesarean deliveries, with the greatest difference in rates for ruptured uterus. In the first MFMU Network study, there were no cases of uterine rupture among a cohort of 15,801 women who underwent elective repeat cesarean delivery, and in the second multicenter study of 25,000 women, this patient group had a rupture rate of 0.004%.
Yet, as ACOG points out, neither elective repeat cesarean deliveries nor TOLAC are without maternal or neonatal risk. Women who have successful VBAC delivery, on the other hand, have significantly lower morbidity and better outcomes than women who do not attempt labor. Women who undergo VBAC also avoid exposure to the significant risks of repeat cesarean deliveries in the long term.
Research unequivocally shows that the risk of placenta accreta, hysterectomy, hemorrhage, and other serious maternal morbidity increases progressively with each repeat cesarean delivery. Rates of placenta accreta have, in fact, been rising in the United States – a trend that should prompt us to think more about TOLAC.
Moreover, TOLAC is being shown to be a cost-effective strategy. In one analysis, TOLAC in a second pregnancy was cost-effective as long as the chance of VBAC exceeded approximately 74% (Obstet Gynecol. 2001 Jun;97[6]:932-41). More recently, TOLAC was found to be cost-effective across a wide variety of circumstances, including when a woman had a probability of VBAC as low as 43%. The model in this analysis, which used probability estimates from the MFMU Cesarean Registry, took a longer-term view by including probabilities of outcomes throughout a woman’s reproductive life that were contingent upon her initial choice regarding TOLAC (Am J Perinatol. 2013 Jan;30[1]:11-20).
Likelihood of success
Evaluating and discussing the likelihood of success with TOLAC is therefore key to the counseling process. The higher the likelihood of achieving VBAC, the more favorable the risk-benefit ratio will be and the more appealing it will be to consider.
According to one analysis, if a woman undergoing a TOLAC has at least a 60%-70% chance of VBAC, her chance of having major or minor morbidity is no greater than a woman undergoing a planned repeat cesarean delivery (Am J Obstet Gynecol 2009;200:56.e1-e6).
There are several prediction tools available that can be used at the first prenatal visit and in early labor to give a reasonably good estimate of success. One of these tools is available at the MFMU Network website (http://mfmu.bsc.gwu.edu). The tools take into account factors such as prior indication for cesarean delivery; history of vaginal delivery; demographic characteristics such as maternal age and body mass index; the occurrence of spontaneous labor; and cervical status at admission.
Prior vaginal delivery is one of the strongest predictors of a successful TOLAC. Research has consistently shown that women with a prior vaginal delivery – including a vaginal delivery predating an unsuccessful TOLAC – have significantly higher TOLAC success rates than women who did not have any prior vaginal delivery.
The indication for a prior cesarean delivery also clearly affects the likelihood of a successful TOLAC. Women whose first cesarean delivery was performed for a nonrecurring indication, such as breech presentation or low intolerance of labor, have TOLAC success rates that are similar to vaginal delivery rates for nulliparous women. Success rates for these women may exceed 85%. On the other hand, women who had a prior cesarean delivery for cephalopelvic disproportion or failure to progress have been shown to have lower TOLAC success rates ranging from 50%-67%.
Labor induction should be approached cautiously, as women who undergo induction of labor in TOLAC have an increased risk of repeat cesarean delivery. Still, success rates with induction are high. Data from the MFMU Cesarean Registry showed that about 66% of women undergoing induction after one prior cesarean delivery achieved VBAC versus 76% of women entering TOLAC spontaneously (Obstet Gynecol. 2007 Feb;109[2 Pt 1]:262-9). Another study of women undergoing induction after one prior cesarean reported an overall success rate of 78% (Obstet Gynecol. 2004 Mar;103[3]:534-8).
Whether induction specifically increases the risk for uterine rupture in TOLAC, compared with expectant management, is unclear. There also are conflicting data as to whether particular induction methods increase this risk.
Based on available data, ACOG considers induction of labor for either maternal or fetal indications to be an option for women undergoing TOLAC. Oxytocin may be used for induction as well as augmentation, but caution should be exercised at higher doses. While there is no clear dosing threshold for increased risk of rupture, research has suggested that higher doses of oxytocin are best avoided.
The use of prostaglandins is more controversial: Based on evidence from several small studies, ACOG concluded in its 2010 bulletin that misoprostol (prostaglandin E1) for cervical ripening is contraindicated in women undergoing TOLAC. It appears likely that rupture risk increases in patients who received both prostaglandins and oxytocin, so ACOG has advised avoiding their sequential use when prostaglandin E2 is used. This of course limits the options for the practitioner. Therefore, utilizing a Foley catheter followed by pitocin has been an approach advocated in some cases.
Uterine rupture is not predictable, and it is far more difficult to assess an individual’s risk of this complication than it is to assess the likelihood of VBAC. Still, there is value to discussing with the patient whether there are any other modifiers that could potentially influence the risk of rupture.
Since rates of uterine rupture are highest in women with previous classical or T-shaped incision, for example, it is important to try to ascertain what type of incision was previously used. It is widely appreciated that low-transverse uterine incisions are most favorable, but findings are mixed in regard to low-vertical incisions. Some research shows that women with a previous low-vertical incision do not have significantly lower VBAC success rates or higher risks of uterine rupture. TOLAC should therefore not be ruled out in these cases.
Additionally, TOLAC should not be ruled out for women who have had more than one cesarean delivery. Several studies have shown an increased risk of uterine rupture after two prior cesarean deliveries, compared with one, and one meta-analysis suggested a more than twofold increased risk (BJOG. 2010 Jan;117(1):5-19.).
In contrast, an analysis of the MFMU Cesarean Registry found no significant difference in rupture rates in women with one prior cesarean versus multiple prior cesareans (Obstet Gynecol. 2006 Jul;108[1]:12-20.).
It appears, therefore, that even if having more than one prior cesarean section is associated with an increased risk of rupture, the magnitude of this increase is small.
Just as women with a prior vaginal delivery have the highest chance of VBAC success, they also have the lowest rates of rupture among all women undergoing TOLAC.
Patient counseling
We must inform our patients who have had a cesarean section in the past of their options for childbirth in an unbiased manner.
The complications of both TOLAC and elective repeat cesarean section should be discussed, and every attempt should be made to individually assess both the likelihood of a successful VBAC and the comparative risk of maternal and perinatal morbidity. A shared decision-making process should be adopted, and whenever possible, the patient’s preference should be respected. In the end, a woman undergoing TOLAC should be truly motivated to pursue a trial of labor, because there are inherent risks.
One thing I’ve learned from my clinical practice and research on this issue is that the desire to undergo a vaginal delivery is powerful for some women. Many of my patients have self-referred for consultation about TOLAC after their ob.gyn. informed them that their hospital is not equipped, and they should therefore have a scheduled repeat operation. In many cases they discover that TOLAC is an option if they are willing to travel a half-hour or so.
We need to honor this desire and inform our patients of the option, and help facilitate delivery at another nearby hospital when our own facility is not equipped for TOLAC.
Dr. Landon is the Richard L. Meiling Professor and chairman of the department of obstetrics and gynecology at the Ohio State University, Columbus. He served for more than 25 years as Ohio State’s coinvestigator for the National Institutes of Child Health and Human Development Maternal Fetal Medicine Units Network. He reported having no relevant financial disclosures.
The relative safety of vaginal birth after cesarean (VBAC) has been documented in several large-scale studies in the past 15 years, and was affirmed in 2010 through a National Institutes of Health consensus development conference and a practice bulletin from the American College of Obstetricians and Gynecologists. Yet, despite all this research and review, rates of a trial of labor after cesarean (TOLAC) have increased only modestly in the last several years.
Approximately 20% of all births in 2013 in women with a history of one cesarean section involved a trial of labor, according to a recent report from the Centers for Disease Control and Prevention. This represents only a small increase from 2006, when the TOLAC rate had plummeted to approximately 15%.
The limited change is concerning because up to two-thirds of women with a prior cesarean delivery are candidates for a trial of labor, and many of them are excellent candidates. In total, 70% of the women who attempted labor in 2013 after a previous cesarean had successful VBACs, the CDC data shows.
Several European countries have TOLAC rates between 50% and 70%, but in the United States, as evidenced by the recent CDC data, there continues to be an underutilization of attempted VBAC. We must ask ourselves, are women truly able to choose TOLAC, or are they being dissuaded by the health care system?
I believe that the barriers are still pervasive. Too often, women who are TOLAC candidates are not receiving appropriate counseling – and too often, women are not even being presented the option of a trial of labor, even when staff are immediately available to provide emergency care if needed.
Rupture concerns in perspective
When the NIH consensus development panel reviewed VBAC in 2010, it concluded that TOLAC is a reasonable option for many women with a prior cesarean. The panel found that restricted access to VBAC/TOLAC stemmed from existing practice guidelines and the medical liability climate, and it called upon providers and others to “mitigate or even eliminate” the barriers that women face in finding clinicians and facilities able and willing to offer TOLAC.
ACOG’s 2010 practice bulletin also acknowledged the problem of limited access. ACOG recommended, as it had in an earlier bulletin, that TOLAC-VBAC be undertaken in facilities where staff are immediately available for emergency care. It added, however, that when such resources are not available, the best alternative may be to refer patients to a facility with available resources. Health care providers and insurance carriers “should do all they can to facilitate transfer of care or comanagement in support of a desired TOLAC,” ACOG’s document states.
Why, given such recommendations, are we still falling so short of where we should be?
A number of nonclinical factors are involved, but clearly, as both the NIH and ACOG have stated, the fear of litigation in cases of uterine rupture is a contributing factor. A ruptured uterus is indeed the principal risk associated with TOLAC, and it can have serious sequelae including perinatal death, hypoxic ischemic encephalopathy (HIE), and hysterectomy.

We must appreciate, however, that the absolute rates of uterine rupture and of serious adverse outcomes are quite low. The rupture rate in 2013 among women who underwent TOLAC but ultimately had a repeat cesarean section – the highest-risk group – was 495 per 100,000 live births, according to the CDC. This rate of approximately 0.5% is consistent with the level of risk reported in the literature for several decades.
In one of the two large observational studies done in the United States that have shed light on TOLAC outcomes, the rate of uterine rupture among women who underwent TOLAC was 0.7% for women with a prior low transverse incision, 2.0% for those with a prior low vertical incision, and 0.5% for those with an unknown type of prior incision. Overall, the rate of uterine rupture in this study’s cohort of 17,898 women who underwent TOLAC was 0.7% (N Engl J Med. 2004 Dec 16;351[25]:2581-9). The study was conducted at 19 medical centers belonging to the Eunice Kennedy Shriver National Institute of Child Health and Human Development’s Maternal-Fetal Medical Units (MFMU) Network.
The second large study conducted in the United States – a multicenter observational study in which records of approximately 25,000 women with a prior low-transverse cesarean section were reviewed – also showed rates of uterine rupture less than 1% (Am J Obstet Gynecol. 2005 Nov;193[5]:1656-62).
The attributable risk for perinatal death or HIE at term appears to be 1 per 2,000 TOLAC, according to the MFMU Network study.
Failed trials of labor resulting in repeat cesarean deliveries have consistently been associated with higher morbidity than scheduled repeat cesarean deliveries, with the greatest difference in rates for ruptured uterus. In the first MFMU Network study, there were no cases of uterine rupture among a cohort of 15,801 women who underwent elective repeat cesarean delivery, and in the second multicenter study of 25,000 women, this patient group had a rupture rate of 0.004%.
Yet, as ACOG points out, neither elective repeat cesarean deliveries nor TOLAC are without maternal or neonatal risk. Women who have successful VBAC delivery, on the other hand, have significantly lower morbidity and better outcomes than women who do not attempt labor. Women who undergo VBAC also avoid exposure to the significant risks of repeat cesarean deliveries in the long term.
Research unequivocally shows that the risk of placenta accreta, hysterectomy, hemorrhage, and other serious maternal morbidity increases progressively with each repeat cesarean delivery. Rates of placenta accreta have, in fact, been rising in the United States – a trend that should prompt us to think more about TOLAC.
Moreover, TOLAC is being shown to be a cost-effective strategy. In one analysis, TOLAC in a second pregnancy was cost-effective as long as the chance of VBAC exceeded approximately 74% (Obstet Gynecol. 2001 Jun;97[6]:932-41). More recently, TOLAC was found to be cost-effective across a wide variety of circumstances, including when a woman had a probability of VBAC as low as 43%. The model in this analysis, which used probability estimates from the MFMU Cesarean Registry, took a longer-term view by including probabilities of outcomes throughout a woman’s reproductive life that were contingent upon her initial choice regarding TOLAC (Am J Perinatol. 2013 Jan;30[1]:11-20).
Likelihood of success
Evaluating and discussing the likelihood of success with TOLAC is therefore key to the counseling process. The higher the likelihood of achieving VBAC, the more favorable the risk-benefit ratio will be and the more appealing it will be to consider.
According to one analysis, if a woman undergoing a TOLAC has at least a 60%-70% chance of VBAC, her chance of having major or minor morbidity is no greater than a woman undergoing a planned repeat cesarean delivery (Am J Obstet Gynecol 2009;200:56.e1-e6).
There are several prediction tools available that can be used at the first prenatal visit and in early labor to give a reasonably good estimate of success. One of these tools is available at the MFMU Network website (http://mfmu.bsc.gwu.edu). The tools take into account factors such as prior indication for cesarean delivery; history of vaginal delivery; demographic characteristics such as maternal age and body mass index; the occurrence of spontaneous labor; and cervical status at admission.
Prior vaginal delivery is one of the strongest predictors of a successful TOLAC. Research has consistently shown that women with a prior vaginal delivery – including a vaginal delivery predating an unsuccessful TOLAC – have significantly higher TOLAC success rates than women who did not have any prior vaginal delivery.
The indication for a prior cesarean delivery also clearly affects the likelihood of a successful TOLAC. Women whose first cesarean delivery was performed for a nonrecurring indication, such as breech presentation or low intolerance of labor, have TOLAC success rates that are similar to vaginal delivery rates for nulliparous women. Success rates for these women may exceed 85%. On the other hand, women who had a prior cesarean delivery for cephalopelvic disproportion or failure to progress have been shown to have lower TOLAC success rates ranging from 50%-67%.
Labor induction should be approached cautiously, as women who undergo induction of labor in TOLAC have an increased risk of repeat cesarean delivery. Still, success rates with induction are high. Data from the MFMU Cesarean Registry showed that about 66% of women undergoing induction after one prior cesarean delivery achieved VBAC versus 76% of women entering TOLAC spontaneously (Obstet Gynecol. 2007 Feb;109[2 Pt 1]:262-9). Another study of women undergoing induction after one prior cesarean reported an overall success rate of 78% (Obstet Gynecol. 2004 Mar;103[3]:534-8).
Whether induction specifically increases the risk for uterine rupture in TOLAC, compared with expectant management, is unclear. There also are conflicting data as to whether particular induction methods increase this risk.
Based on available data, ACOG considers induction of labor for either maternal or fetal indications to be an option for women undergoing TOLAC. Oxytocin may be used for induction as well as augmentation, but caution should be exercised at higher doses. While there is no clear dosing threshold for increased risk of rupture, research has suggested that higher doses of oxytocin are best avoided.
The use of prostaglandins is more controversial: Based on evidence from several small studies, ACOG concluded in its 2010 bulletin that misoprostol (prostaglandin E1) for cervical ripening is contraindicated in women undergoing TOLAC. It appears likely that rupture risk increases in patients who received both prostaglandins and oxytocin, so ACOG has advised avoiding their sequential use when prostaglandin E2 is used. This of course limits the options for the practitioner. Therefore, utilizing a Foley catheter followed by pitocin has been an approach advocated in some cases.
Uterine rupture is not predictable, and it is far more difficult to assess an individual’s risk of this complication than it is to assess the likelihood of VBAC. Still, there is value to discussing with the patient whether there are any other modifiers that could potentially influence the risk of rupture.
Since rates of uterine rupture are highest in women with previous classical or T-shaped incision, for example, it is important to try to ascertain what type of incision was previously used. It is widely appreciated that low-transverse uterine incisions are most favorable, but findings are mixed in regard to low-vertical incisions. Some research shows that women with a previous low-vertical incision do not have significantly lower VBAC success rates or higher risks of uterine rupture. TOLAC should therefore not be ruled out in these cases.
Additionally, TOLAC should not be ruled out for women who have had more than one cesarean delivery. Several studies have shown an increased risk of uterine rupture after two prior cesarean deliveries, compared with one, and one meta-analysis suggested a more than twofold increased risk (BJOG. 2010 Jan;117(1):5-19.).
In contrast, an analysis of the MFMU Cesarean Registry found no significant difference in rupture rates in women with one prior cesarean versus multiple prior cesareans (Obstet Gynecol. 2006 Jul;108[1]:12-20.).
It appears, therefore, that even if having more than one prior cesarean section is associated with an increased risk of rupture, the magnitude of this increase is small.
Just as women with a prior vaginal delivery have the highest chance of VBAC success, they also have the lowest rates of rupture among all women undergoing TOLAC.
Patient counseling
We must inform our patients who have had a cesarean section in the past of their options for childbirth in an unbiased manner.
The complications of both TOLAC and elective repeat cesarean section should be discussed, and every attempt should be made to individually assess both the likelihood of a successful VBAC and the comparative risk of maternal and perinatal morbidity. A shared decision-making process should be adopted, and whenever possible, the patient’s preference should be respected. In the end, a woman undergoing TOLAC should be truly motivated to pursue a trial of labor, because there are inherent risks.
One thing I’ve learned from my clinical practice and research on this issue is that the desire to undergo a vaginal delivery is powerful for some women. Many of my patients have self-referred for consultation about TOLAC after their ob.gyn. informed them that their hospital is not equipped, and they should therefore have a scheduled repeat operation. In many cases they discover that TOLAC is an option if they are willing to travel a half-hour or so.
We need to honor this desire and inform our patients of the option, and help facilitate delivery at another nearby hospital when our own facility is not equipped for TOLAC.
Dr. Landon is the Richard L. Meiling Professor and chairman of the department of obstetrics and gynecology at the Ohio State University, Columbus. He served for more than 25 years as Ohio State’s coinvestigator for the National Institutes of Child Health and Human Development Maternal Fetal Medicine Units Network. He reported having no relevant financial disclosures.
The relative safety of vaginal birth after cesarean (VBAC) has been documented in several large-scale studies in the past 15 years, and was affirmed in 2010 through a National Institutes of Health consensus development conference and a practice bulletin from the American College of Obstetricians and Gynecologists. Yet, despite all this research and review, rates of a trial of labor after cesarean (TOLAC) have increased only modestly in the last several years.
Approximately 20% of all births in 2013 in women with a history of one cesarean section involved a trial of labor, according to a recent report from the Centers for Disease Control and Prevention. This represents only a small increase from 2006, when the TOLAC rate had plummeted to approximately 15%.
The limited change is concerning because up to two-thirds of women with a prior cesarean delivery are candidates for a trial of labor, and many of them are excellent candidates. In total, 70% of the women who attempted labor in 2013 after a previous cesarean had successful VBACs, the CDC data shows.
Several European countries have TOLAC rates between 50% and 70%, but in the United States, as evidenced by the recent CDC data, there continues to be an underutilization of attempted VBAC. We must ask ourselves, are women truly able to choose TOLAC, or are they being dissuaded by the health care system?
I believe that the barriers are still pervasive. Too often, women who are TOLAC candidates are not receiving appropriate counseling – and too often, women are not even being presented the option of a trial of labor, even when staff are immediately available to provide emergency care if needed.
Rupture concerns in perspective
When the NIH consensus development panel reviewed VBAC in 2010, it concluded that TOLAC is a reasonable option for many women with a prior cesarean. The panel found that restricted access to VBAC/TOLAC stemmed from existing practice guidelines and the medical liability climate, and it called upon providers and others to “mitigate or even eliminate” the barriers that women face in finding clinicians and facilities able and willing to offer TOLAC.
ACOG’s 2010 practice bulletin also acknowledged the problem of limited access. ACOG recommended, as it had in an earlier bulletin, that TOLAC-VBAC be undertaken in facilities where staff are immediately available for emergency care. It added, however, that when such resources are not available, the best alternative may be to refer patients to a facility with available resources. Health care providers and insurance carriers “should do all they can to facilitate transfer of care or comanagement in support of a desired TOLAC,” ACOG’s document states.
Why, given such recommendations, are we still falling so short of where we should be?
A number of nonclinical factors are involved, but clearly, as both the NIH and ACOG have stated, the fear of litigation in cases of uterine rupture is a contributing factor. A ruptured uterus is indeed the principal risk associated with TOLAC, and it can have serious sequelae including perinatal death, hypoxic ischemic encephalopathy (HIE), and hysterectomy.

We must appreciate, however, that the absolute rates of uterine rupture and of serious adverse outcomes are quite low. The rupture rate in 2013 among women who underwent TOLAC but ultimately had a repeat cesarean section – the highest-risk group – was 495 per 100,000 live births, according to the CDC. This rate of approximately 0.5% is consistent with the level of risk reported in the literature for several decades.
In one of the two large observational studies done in the United States that have shed light on TOLAC outcomes, the rate of uterine rupture among women who underwent TOLAC was 0.7% for women with a prior low transverse incision, 2.0% for those with a prior low vertical incision, and 0.5% for those with an unknown type of prior incision. Overall, the rate of uterine rupture in this study’s cohort of 17,898 women who underwent TOLAC was 0.7% (N Engl J Med. 2004 Dec 16;351[25]:2581-9). The study was conducted at 19 medical centers belonging to the Eunice Kennedy Shriver National Institute of Child Health and Human Development’s Maternal-Fetal Medical Units (MFMU) Network.
The second large study conducted in the United States – a multicenter observational study in which records of approximately 25,000 women with a prior low-transverse cesarean section were reviewed – also showed rates of uterine rupture less than 1% (Am J Obstet Gynecol. 2005 Nov;193[5]:1656-62).
The attributable risk for perinatal death or HIE at term appears to be 1 per 2,000 TOLAC, according to the MFMU Network study.
Failed trials of labor resulting in repeat cesarean deliveries have consistently been associated with higher morbidity than scheduled repeat cesarean deliveries, with the greatest difference in rates for ruptured uterus. In the first MFMU Network study, there were no cases of uterine rupture among a cohort of 15,801 women who underwent elective repeat cesarean delivery, and in the second multicenter study of 25,000 women, this patient group had a rupture rate of 0.004%.
Yet, as ACOG points out, neither elective repeat cesarean deliveries nor TOLAC are without maternal or neonatal risk. Women who have successful VBAC delivery, on the other hand, have significantly lower morbidity and better outcomes than women who do not attempt labor. Women who undergo VBAC also avoid exposure to the significant risks of repeat cesarean deliveries in the long term.
Research unequivocally shows that the risk of placenta accreta, hysterectomy, hemorrhage, and other serious maternal morbidity increases progressively with each repeat cesarean delivery. Rates of placenta accreta have, in fact, been rising in the United States – a trend that should prompt us to think more about TOLAC.
Moreover, TOLAC is being shown to be a cost-effective strategy. In one analysis, TOLAC in a second pregnancy was cost-effective as long as the chance of VBAC exceeded approximately 74% (Obstet Gynecol. 2001 Jun;97[6]:932-41). More recently, TOLAC was found to be cost-effective across a wide variety of circumstances, including when a woman had a probability of VBAC as low as 43%. The model in this analysis, which used probability estimates from the MFMU Cesarean Registry, took a longer-term view by including probabilities of outcomes throughout a woman’s reproductive life that were contingent upon her initial choice regarding TOLAC (Am J Perinatol. 2013 Jan;30[1]:11-20).
Likelihood of success
Evaluating and discussing the likelihood of success with TOLAC is therefore key to the counseling process. The higher the likelihood of achieving VBAC, the more favorable the risk-benefit ratio will be and the more appealing it will be to consider.
According to one analysis, if a woman undergoing a TOLAC has at least a 60%-70% chance of VBAC, her chance of having major or minor morbidity is no greater than a woman undergoing a planned repeat cesarean delivery (Am J Obstet Gynecol 2009;200:56.e1-e6).
There are several prediction tools available that can be used at the first prenatal visit and in early labor to give a reasonably good estimate of success. One of these tools is available at the MFMU Network website (http://mfmu.bsc.gwu.edu). The tools take into account factors such as prior indication for cesarean delivery; history of vaginal delivery; demographic characteristics such as maternal age and body mass index; the occurrence of spontaneous labor; and cervical status at admission.
Prior vaginal delivery is one of the strongest predictors of a successful TOLAC. Research has consistently shown that women with a prior vaginal delivery – including a vaginal delivery predating an unsuccessful TOLAC – have significantly higher TOLAC success rates than women who did not have any prior vaginal delivery.
The indication for a prior cesarean delivery also clearly affects the likelihood of a successful TOLAC. Women whose first cesarean delivery was performed for a nonrecurring indication, such as breech presentation or low intolerance of labor, have TOLAC success rates that are similar to vaginal delivery rates for nulliparous women. Success rates for these women may exceed 85%. On the other hand, women who had a prior cesarean delivery for cephalopelvic disproportion or failure to progress have been shown to have lower TOLAC success rates ranging from 50%-67%.
Labor induction should be approached cautiously, as women who undergo induction of labor in TOLAC have an increased risk of repeat cesarean delivery. Still, success rates with induction are high. Data from the MFMU Cesarean Registry showed that about 66% of women undergoing induction after one prior cesarean delivery achieved VBAC versus 76% of women entering TOLAC spontaneously (Obstet Gynecol. 2007 Feb;109[2 Pt 1]:262-9). Another study of women undergoing induction after one prior cesarean reported an overall success rate of 78% (Obstet Gynecol. 2004 Mar;103[3]:534-8).
Whether induction specifically increases the risk for uterine rupture in TOLAC, compared with expectant management, is unclear. There also are conflicting data as to whether particular induction methods increase this risk.
Based on available data, ACOG considers induction of labor for either maternal or fetal indications to be an option for women undergoing TOLAC. Oxytocin may be used for induction as well as augmentation, but caution should be exercised at higher doses. While there is no clear dosing threshold for increased risk of rupture, research has suggested that higher doses of oxytocin are best avoided.
The use of prostaglandins is more controversial: Based on evidence from several small studies, ACOG concluded in its 2010 bulletin that misoprostol (prostaglandin E1) for cervical ripening is contraindicated in women undergoing TOLAC. It appears likely that rupture risk increases in patients who received both prostaglandins and oxytocin, so ACOG has advised avoiding their sequential use when prostaglandin E2 is used. This of course limits the options for the practitioner. Therefore, utilizing a Foley catheter followed by pitocin has been an approach advocated in some cases.
Uterine rupture is not predictable, and it is far more difficult to assess an individual’s risk of this complication than it is to assess the likelihood of VBAC. Still, there is value to discussing with the patient whether there are any other modifiers that could potentially influence the risk of rupture.
Since rates of uterine rupture are highest in women with previous classical or T-shaped incision, for example, it is important to try to ascertain what type of incision was previously used. It is widely appreciated that low-transverse uterine incisions are most favorable, but findings are mixed in regard to low-vertical incisions. Some research shows that women with a previous low-vertical incision do not have significantly lower VBAC success rates or higher risks of uterine rupture. TOLAC should therefore not be ruled out in these cases.
Additionally, TOLAC should not be ruled out for women who have had more than one cesarean delivery. Several studies have shown an increased risk of uterine rupture after two prior cesarean deliveries, compared with one, and one meta-analysis suggested a more than twofold increased risk (BJOG. 2010 Jan;117(1):5-19.).
In contrast, an analysis of the MFMU Cesarean Registry found no significant difference in rupture rates in women with one prior cesarean versus multiple prior cesareans (Obstet Gynecol. 2006 Jul;108[1]:12-20.).
It appears, therefore, that even if having more than one prior cesarean section is associated with an increased risk of rupture, the magnitude of this increase is small.
Just as women with a prior vaginal delivery have the highest chance of VBAC success, they also have the lowest rates of rupture among all women undergoing TOLAC.
Patient counseling
We must inform our patients who have had a cesarean section in the past of their options for childbirth in an unbiased manner.
The complications of both TOLAC and elective repeat cesarean section should be discussed, and every attempt should be made to individually assess both the likelihood of a successful VBAC and the comparative risk of maternal and perinatal morbidity. A shared decision-making process should be adopted, and whenever possible, the patient’s preference should be respected. In the end, a woman undergoing TOLAC should be truly motivated to pursue a trial of labor, because there are inherent risks.
One thing I’ve learned from my clinical practice and research on this issue is that the desire to undergo a vaginal delivery is powerful for some women. Many of my patients have self-referred for consultation about TOLAC after their ob.gyn. informed them that their hospital is not equipped, and they should therefore have a scheduled repeat operation. In many cases they discover that TOLAC is an option if they are willing to travel a half-hour or so.
We need to honor this desire and inform our patients of the option, and help facilitate delivery at another nearby hospital when our own facility is not equipped for TOLAC.
Dr. Landon is the Richard L. Meiling Professor and chairman of the department of obstetrics and gynecology at the Ohio State University, Columbus. He served for more than 25 years as Ohio State’s coinvestigator for the National Institutes of Child Health and Human Development Maternal Fetal Medicine Units Network. He reported having no relevant financial disclosures.
MDQ screen useful tool for bipolar on inpatient units
When screening for bipolar disorders, Mood Disorders Questionnaire scores proved more sensitive – but showed less specificity – in an inpatient mood disorders setting than an outpatient psychiatric population, a retrospective study shows. The results suggest that the MDQ can be used effectively on an inpatient psychiatry mood disorders unit, reported Dr. Simon Kung and his associates.
Dr. Kung of the Mayo Clinic in Rochester, Minn., and his associates evaluated 1,330 patients who checked into a mood disorders unit and administered the MDQ upon entry. After excluding patients with diagnoses that were neither unipolar or bipolar, 860 MDQs were ultimately used. Sensitivity and specificity were calculated for each number of questionnaire items checked positive.
The researchers determined that the optimal cutoff score for MDQs was 8, resulting in a sensitivity/specificity of 86%/71%, compared with 92%/64% using the recommended outpatient cutoff of 7.
Read the full article here: (J Affect Disord. 201515;188:97-100. doi:10.1016/j.jad.2015.08.060)
When screening for bipolar disorders, Mood Disorders Questionnaire scores proved more sensitive – but showed less specificity – in an inpatient mood disorders setting than an outpatient psychiatric population, a retrospective study shows. The results suggest that the MDQ can be used effectively on an inpatient psychiatry mood disorders unit, reported Dr. Simon Kung and his associates.
Dr. Kung of the Mayo Clinic in Rochester, Minn., and his associates evaluated 1,330 patients who checked into a mood disorders unit and administered the MDQ upon entry. After excluding patients with diagnoses that were neither unipolar or bipolar, 860 MDQs were ultimately used. Sensitivity and specificity were calculated for each number of questionnaire items checked positive.
The researchers determined that the optimal cutoff score for MDQs was 8, resulting in a sensitivity/specificity of 86%/71%, compared with 92%/64% using the recommended outpatient cutoff of 7.
Read the full article here: (J Affect Disord. 201515;188:97-100. doi:10.1016/j.jad.2015.08.060)
When screening for bipolar disorders, Mood Disorders Questionnaire scores proved more sensitive – but showed less specificity – in an inpatient mood disorders setting than an outpatient psychiatric population, a retrospective study shows. The results suggest that the MDQ can be used effectively on an inpatient psychiatry mood disorders unit, reported Dr. Simon Kung and his associates.
Dr. Kung of the Mayo Clinic in Rochester, Minn., and his associates evaluated 1,330 patients who checked into a mood disorders unit and administered the MDQ upon entry. After excluding patients with diagnoses that were neither unipolar or bipolar, 860 MDQs were ultimately used. Sensitivity and specificity were calculated for each number of questionnaire items checked positive.
The researchers determined that the optimal cutoff score for MDQs was 8, resulting in a sensitivity/specificity of 86%/71%, compared with 92%/64% using the recommended outpatient cutoff of 7.
Read the full article here: (J Affect Disord. 201515;188:97-100. doi:10.1016/j.jad.2015.08.060)
FROM THE JOURNAL OF AFFECTIVE DISORDERS