User login
Frail elders at high mortality risk in the year following surgery
SAN DIEGO – Frail elderly patients face a significantly increased risk of mortality in the year after undergoing major elective noncardiac surgery, a large study from Canada showed.
“The current literature on perioperative frailty clearly shows that being frail before surgery substantially increases your risk of adverse postoperative outcomes,” Dr. Daniel I. McIsaac said in an interview prior to the annual meeting of the American Society of Anesthesiologists, where the study was presented. “In fact, frailty may underlie a lot of the associations between advanced age and adverse postoperative outcomes. Frailty increases in prevalence with increasing age, and as we all know, the population is aging. Therefore, we expect to see an increasing number of frail patients coming for surgery.”
In an effort to determine the risk of 1-year mortality in frail elderly patients having major elective surgery, the researchers used population-based health administrative data in Ontario, to identify 202,811 patients over the age of 65 who had intermediate- to high-risk elective noncardiac surgery between 2002 and 2012. They used the Johns Hopkins Adjusted Clinical Groups (ACG) frailty indicator and captured all deaths that occurred within 1 year of surgery. Proportional hazards regression models adjusted for age, gender, and socioeconomic status were used to evaluate the impact of frailty on 1-year postoperative mortality.
Of the 202,811 patients, 6,289 (3.1%) were frail, reported Dr. McIsaac of the department of anesthesiology at the University of Ottawa. The 1-year postoperative mortality was 13.6% among frail patients, compared with 4.8% of nonfrail patients, for an adjusted hazard ratio of 2.23. Mortality was higher among frail patients for all types of surgery, compared with their nonfrail counterparts, with the exception of pancreaticoduodenectomy. Frailty had the strongest impact on the risk of mortality after total joint arthroplasty (adjusted hazard ratio of 3.79 for hip replacement and adjusted HR of 2.68 for knee replacement).
The risk of postoperative mortality for frail patients was much higher than for nonfrail patients in the early time period after surgery, especially during the first postoperative week. “Depending on how you control for other variables, a frail patient was 13-35 times more likely to die in the week after surgery than a nonfrail patient of the same age having the same surgery,” said Dr. McIsaac, who is also a staff anesthesiologist at the Ottawa Hospital. “This makes a lot of sense; frail patients are vulnerable to stressors, and surgery puts an enormous physiological stress on even healthy patients. Future work clearly needs to focus [on] addressing this high-risk time in the immediate postoperative period.”
He acknowledged certain limitations of the study, including its reliance on health administrative data and the fact that frailty “is a challenging exposure to study because there are a plethora of instruments that can be used to call someone frail. We used a validated set of frailty-defining diagnoses that have been shown to identify people with multidimensional frailty. That said, you can’t necessarily generalize our findings to patients identified as frail using other instruments.”
The findings, Dr. McIsaac concluded, suggest that clinicians should focus on identifying frail patients prior to surgery, “support them to ensure that they are more likely to derive benefit from surgery than harm, and focus on optimizing their care after surgery to address this early mortality risk.”
The study was funded by departments of anesthesiology at the University of Ottawa and at the Ottawa Hospital. Dr. McIsaac reported having no financial disclosures.
SAN DIEGO – Frail elderly patients face a significantly increased risk of mortality in the year after undergoing major elective noncardiac surgery, a large study from Canada showed.
“The current literature on perioperative frailty clearly shows that being frail before surgery substantially increases your risk of adverse postoperative outcomes,” Dr. Daniel I. McIsaac said in an interview prior to the annual meeting of the American Society of Anesthesiologists, where the study was presented. “In fact, frailty may underlie a lot of the associations between advanced age and adverse postoperative outcomes. Frailty increases in prevalence with increasing age, and as we all know, the population is aging. Therefore, we expect to see an increasing number of frail patients coming for surgery.”
In an effort to determine the risk of 1-year mortality in frail elderly patients having major elective surgery, the researchers used population-based health administrative data in Ontario, to identify 202,811 patients over the age of 65 who had intermediate- to high-risk elective noncardiac surgery between 2002 and 2012. They used the Johns Hopkins Adjusted Clinical Groups (ACG) frailty indicator and captured all deaths that occurred within 1 year of surgery. Proportional hazards regression models adjusted for age, gender, and socioeconomic status were used to evaluate the impact of frailty on 1-year postoperative mortality.
Of the 202,811 patients, 6,289 (3.1%) were frail, reported Dr. McIsaac of the department of anesthesiology at the University of Ottawa. The 1-year postoperative mortality was 13.6% among frail patients, compared with 4.8% of nonfrail patients, for an adjusted hazard ratio of 2.23. Mortality was higher among frail patients for all types of surgery, compared with their nonfrail counterparts, with the exception of pancreaticoduodenectomy. Frailty had the strongest impact on the risk of mortality after total joint arthroplasty (adjusted hazard ratio of 3.79 for hip replacement and adjusted HR of 2.68 for knee replacement).
The risk of postoperative mortality for frail patients was much higher than for nonfrail patients in the early time period after surgery, especially during the first postoperative week. “Depending on how you control for other variables, a frail patient was 13-35 times more likely to die in the week after surgery than a nonfrail patient of the same age having the same surgery,” said Dr. McIsaac, who is also a staff anesthesiologist at the Ottawa Hospital. “This makes a lot of sense; frail patients are vulnerable to stressors, and surgery puts an enormous physiological stress on even healthy patients. Future work clearly needs to focus [on] addressing this high-risk time in the immediate postoperative period.”
He acknowledged certain limitations of the study, including its reliance on health administrative data and the fact that frailty “is a challenging exposure to study because there are a plethora of instruments that can be used to call someone frail. We used a validated set of frailty-defining diagnoses that have been shown to identify people with multidimensional frailty. That said, you can’t necessarily generalize our findings to patients identified as frail using other instruments.”
The findings, Dr. McIsaac concluded, suggest that clinicians should focus on identifying frail patients prior to surgery, “support them to ensure that they are more likely to derive benefit from surgery than harm, and focus on optimizing their care after surgery to address this early mortality risk.”
The study was funded by departments of anesthesiology at the University of Ottawa and at the Ottawa Hospital. Dr. McIsaac reported having no financial disclosures.
SAN DIEGO – Frail elderly patients face a significantly increased risk of mortality in the year after undergoing major elective noncardiac surgery, a large study from Canada showed.
“The current literature on perioperative frailty clearly shows that being frail before surgery substantially increases your risk of adverse postoperative outcomes,” Dr. Daniel I. McIsaac said in an interview prior to the annual meeting of the American Society of Anesthesiologists, where the study was presented. “In fact, frailty may underlie a lot of the associations between advanced age and adverse postoperative outcomes. Frailty increases in prevalence with increasing age, and as we all know, the population is aging. Therefore, we expect to see an increasing number of frail patients coming for surgery.”
In an effort to determine the risk of 1-year mortality in frail elderly patients having major elective surgery, the researchers used population-based health administrative data in Ontario, to identify 202,811 patients over the age of 65 who had intermediate- to high-risk elective noncardiac surgery between 2002 and 2012. They used the Johns Hopkins Adjusted Clinical Groups (ACG) frailty indicator and captured all deaths that occurred within 1 year of surgery. Proportional hazards regression models adjusted for age, gender, and socioeconomic status were used to evaluate the impact of frailty on 1-year postoperative mortality.
Of the 202,811 patients, 6,289 (3.1%) were frail, reported Dr. McIsaac of the department of anesthesiology at the University of Ottawa. The 1-year postoperative mortality was 13.6% among frail patients, compared with 4.8% of nonfrail patients, for an adjusted hazard ratio of 2.23. Mortality was higher among frail patients for all types of surgery, compared with their nonfrail counterparts, with the exception of pancreaticoduodenectomy. Frailty had the strongest impact on the risk of mortality after total joint arthroplasty (adjusted hazard ratio of 3.79 for hip replacement and adjusted HR of 2.68 for knee replacement).
The risk of postoperative mortality for frail patients was much higher than for nonfrail patients in the early time period after surgery, especially during the first postoperative week. “Depending on how you control for other variables, a frail patient was 13-35 times more likely to die in the week after surgery than a nonfrail patient of the same age having the same surgery,” said Dr. McIsaac, who is also a staff anesthesiologist at the Ottawa Hospital. “This makes a lot of sense; frail patients are vulnerable to stressors, and surgery puts an enormous physiological stress on even healthy patients. Future work clearly needs to focus [on] addressing this high-risk time in the immediate postoperative period.”
He acknowledged certain limitations of the study, including its reliance on health administrative data and the fact that frailty “is a challenging exposure to study because there are a plethora of instruments that can be used to call someone frail. We used a validated set of frailty-defining diagnoses that have been shown to identify people with multidimensional frailty. That said, you can’t necessarily generalize our findings to patients identified as frail using other instruments.”
The findings, Dr. McIsaac concluded, suggest that clinicians should focus on identifying frail patients prior to surgery, “support them to ensure that they are more likely to derive benefit from surgery than harm, and focus on optimizing their care after surgery to address this early mortality risk.”
The study was funded by departments of anesthesiology at the University of Ottawa and at the Ottawa Hospital. Dr. McIsaac reported having no financial disclosures.
AT THE ASA ANNUAL MEETING
Key clinical point: Frail elderly patients face an increased risk of mortality within 1 year of undergoing noncardiac surgery.
Major finding: The 1-year postoperative mortality was 13.6% among frail patients, compared with 4.8% of nonfrail patients, for an adjusted hazard ratio of 2.23.
Data source: A study of 202,811 patients over the age of 65 years who underwent noncardiac surgery between 2002 and 2012.
Disclosures: The study was funded by departments of anesthesiology at the University of Ottawa and at The Ottawa Hospital. Dr. McIsaac reported having no financial disclosures.
TCT: Paclitaxel-coated balloon delivers durable SFA patency
SAN FRANCISCO – Treatment of femoropopliteal arterial disease with a paclitaxel-coated balloon produced durable, 2-year benefits compared with conventional balloon angioplasty during extended follow-up of the pivotal trial that led to U.S. approval of this drug-coated balloon.
The durability of the benefit first seen after 1 year when follow-up continued out to 2 years was an important finding that distinguishes the IN.PACT Admiral paclitaxel-covered balloon used in the current study from the first and only other drug-covered balloon (DCB) approved for U.S. practice, the Lutonix 035 DCB.
“Not all drug-coated balloons are the same,” Dr. John R. Laird said while reporting the IN.PACT Admiral DCB results at the Transcatheter Cardiovascular Therapeutics annual meeting.
Although both the IN.PACT Admiral and Lutonix 035 DCB have paclitaxel coatings, the two devices differ by paclitaxel dose density on the balloon’s surface (3.5 mcg/mm2 and 2.0 mcg/mm2, respectively), type of excipient (carrier) used, and the balloon coating, noted Dr. Laird, professor and medical director of the Vascular Center at the University of California, Davis in Sacramento.
After the first year, primary patency ran 82% among the 220 patients randomized to the DCB and 52% in patients treated with percutaneous transluminal angioplasty, a statistically significant 30 percentage point difference in favor of the DCB. After 2 years, the rates were 79% in the DCB arm and 50% with a conventional balloon. “We saw no late catch-up that reduced the patency rate,” said Dr. Laird.
The INPACT SFA I(Randomized Trial of IN.PACT Admiral Drug Coated Balloon vs. Standard PTA for the Treatment of SFA and Proximal Popliteal Arterial Disease) trial enrolled 331 patients at 57 centers in the United States and Europe. Researchers reported the study’s primary efficacy and safety endpoints with 1-year follow-up earlier this year (Circulation. 2015 Feb 3;131:495-502). Concurrent with Dr. Laird’s report at the meeting, the 2-year results appeared online (J Amer Coll Card. 2015.doi:10.1016/j.jacc.2015.09.063).
Dr. Laird acknowledged that some types of stents also have shown good efficacy for treating femoropopliteal disease, but he had reservations about placing a stent when the DCB option exists.
“A lot of people have the sense that if we can avoid placing a stent in a femoral artery it helps preserve future treatment options for the patient. The problem with a stent is that once in-stent restenosis occurs in a leg artery, then the chances of getting a good result with an intravascular approach are poor,” Dr. Laird said at the meeting, sponsored by the Cardiovascular Research Foundation.
One potentially concerning finding from the 2-year follow-up was a statistically significant excess of all-cause mortality in the patients who received the DCB, with 16 deaths in the DCB arm and 1 death in the control, angioplasty arm. Dr. Laird dismissed the clinical importance of the finding, noting that all the deaths in the DCB arm had been independently adjudicated with none judged related to the device or procedure. In addition, the deaths occurred an average of 560 days following the procedure.
On Twitter @mitchelzoler
The IN.PACT Admiral paclitaxel-covered balloon provides a powerful new tool for treating superficial femoral artery and popliteal artery disease that works better than does a conventional balloon and avoids using a stent.
Not all drug-coated balloons (DCBs) are alike, even if they use the same antiproliferative drug, paclitaxel. The evidence suggests that the IN.PACT Admiral drug-coated balloon is superior to the performance of the Lutonix 035 DCB, although this has only been assessed in separate studies and not as a head-to-head comparison.
 |
Dr. Gary Gershony |
Another option for treating superficial femoropopliteal disease is with any of a variety of stents. I think the general feeling among peripheral-artery specialists is that it’s better for patients to avoid having a stent permanently in their leg when other, equally-good options are available to try first. Sometimes placing a stent is unavoidable to produce a substantially better revascularization outcome, for example when a dissection occurs or for treating a significant residual stenosis.
The IN.PACT Admiral DCB has not yet been tested on complex or calcified lesions so its performance in those settings is not yet know. The basic message from this 2-year follow-up is that this paclitaxel-coated balloon had better results out to 2-years than a conventional balloon for lesions that were not especially complex and with an average length of 9 cm. For many patients with lesions like these a DCB is a good option because it may produce a durable result while maintaining the option to use a stent later if necessary.
Vascular specialists have been concerned about longer-term follow-up of the results from the IN.PACT SFA trial to see if a signal appeared of catchup restenosis between years 1 and 2. The results showed no evidence of this. It is reassuring to see this DCB technology can produce an effect that’s durable for 2 years without leaving behind a permanent implant. It strengthens the case for this particular DCB but should not be extrapolated to all drug-coated balloons or to all types of femoropopliteal lesions.
Dr. Gary Gershony is an interventional cardiologist and medical director of cardiovascular research, education and technology at John Muir Cardiovascular Institute of John Muir Health in Concord, Calif. He had no relevant disclosures. He made these comments as a discussant for the report and in an interview.
The IN.PACT Admiral paclitaxel-covered balloon provides a powerful new tool for treating superficial femoral artery and popliteal artery disease that works better than does a conventional balloon and avoids using a stent.
Not all drug-coated balloons (DCBs) are alike, even if they use the same antiproliferative drug, paclitaxel. The evidence suggests that the IN.PACT Admiral drug-coated balloon is superior to the performance of the Lutonix 035 DCB, although this has only been assessed in separate studies and not as a head-to-head comparison.
 |
Dr. Gary Gershony |
Another option for treating superficial femoropopliteal disease is with any of a variety of stents. I think the general feeling among peripheral-artery specialists is that it’s better for patients to avoid having a stent permanently in their leg when other, equally-good options are available to try first. Sometimes placing a stent is unavoidable to produce a substantially better revascularization outcome, for example when a dissection occurs or for treating a significant residual stenosis.
The IN.PACT Admiral DCB has not yet been tested on complex or calcified lesions so its performance in those settings is not yet know. The basic message from this 2-year follow-up is that this paclitaxel-coated balloon had better results out to 2-years than a conventional balloon for lesions that were not especially complex and with an average length of 9 cm. For many patients with lesions like these a DCB is a good option because it may produce a durable result while maintaining the option to use a stent later if necessary.
Vascular specialists have been concerned about longer-term follow-up of the results from the IN.PACT SFA trial to see if a signal appeared of catchup restenosis between years 1 and 2. The results showed no evidence of this. It is reassuring to see this DCB technology can produce an effect that’s durable for 2 years without leaving behind a permanent implant. It strengthens the case for this particular DCB but should not be extrapolated to all drug-coated balloons or to all types of femoropopliteal lesions.
Dr. Gary Gershony is an interventional cardiologist and medical director of cardiovascular research, education and technology at John Muir Cardiovascular Institute of John Muir Health in Concord, Calif. He had no relevant disclosures. He made these comments as a discussant for the report and in an interview.
The IN.PACT Admiral paclitaxel-covered balloon provides a powerful new tool for treating superficial femoral artery and popliteal artery disease that works better than does a conventional balloon and avoids using a stent.
Not all drug-coated balloons (DCBs) are alike, even if they use the same antiproliferative drug, paclitaxel. The evidence suggests that the IN.PACT Admiral drug-coated balloon is superior to the performance of the Lutonix 035 DCB, although this has only been assessed in separate studies and not as a head-to-head comparison.
 |
Dr. Gary Gershony |
Another option for treating superficial femoropopliteal disease is with any of a variety of stents. I think the general feeling among peripheral-artery specialists is that it’s better for patients to avoid having a stent permanently in their leg when other, equally-good options are available to try first. Sometimes placing a stent is unavoidable to produce a substantially better revascularization outcome, for example when a dissection occurs or for treating a significant residual stenosis.
The IN.PACT Admiral DCB has not yet been tested on complex or calcified lesions so its performance in those settings is not yet know. The basic message from this 2-year follow-up is that this paclitaxel-coated balloon had better results out to 2-years than a conventional balloon for lesions that were not especially complex and with an average length of 9 cm. For many patients with lesions like these a DCB is a good option because it may produce a durable result while maintaining the option to use a stent later if necessary.
Vascular specialists have been concerned about longer-term follow-up of the results from the IN.PACT SFA trial to see if a signal appeared of catchup restenosis between years 1 and 2. The results showed no evidence of this. It is reassuring to see this DCB technology can produce an effect that’s durable for 2 years without leaving behind a permanent implant. It strengthens the case for this particular DCB but should not be extrapolated to all drug-coated balloons or to all types of femoropopliteal lesions.
Dr. Gary Gershony is an interventional cardiologist and medical director of cardiovascular research, education and technology at John Muir Cardiovascular Institute of John Muir Health in Concord, Calif. He had no relevant disclosures. He made these comments as a discussant for the report and in an interview.
SAN FRANCISCO – Treatment of femoropopliteal arterial disease with a paclitaxel-coated balloon produced durable, 2-year benefits compared with conventional balloon angioplasty during extended follow-up of the pivotal trial that led to U.S. approval of this drug-coated balloon.
The durability of the benefit first seen after 1 year when follow-up continued out to 2 years was an important finding that distinguishes the IN.PACT Admiral paclitaxel-covered balloon used in the current study from the first and only other drug-covered balloon (DCB) approved for U.S. practice, the Lutonix 035 DCB.
“Not all drug-coated balloons are the same,” Dr. John R. Laird said while reporting the IN.PACT Admiral DCB results at the Transcatheter Cardiovascular Therapeutics annual meeting.
Although both the IN.PACT Admiral and Lutonix 035 DCB have paclitaxel coatings, the two devices differ by paclitaxel dose density on the balloon’s surface (3.5 mcg/mm2 and 2.0 mcg/mm2, respectively), type of excipient (carrier) used, and the balloon coating, noted Dr. Laird, professor and medical director of the Vascular Center at the University of California, Davis in Sacramento.
After the first year, primary patency ran 82% among the 220 patients randomized to the DCB and 52% in patients treated with percutaneous transluminal angioplasty, a statistically significant 30 percentage point difference in favor of the DCB. After 2 years, the rates were 79% in the DCB arm and 50% with a conventional balloon. “We saw no late catch-up that reduced the patency rate,” said Dr. Laird.
The INPACT SFA I(Randomized Trial of IN.PACT Admiral Drug Coated Balloon vs. Standard PTA for the Treatment of SFA and Proximal Popliteal Arterial Disease) trial enrolled 331 patients at 57 centers in the United States and Europe. Researchers reported the study’s primary efficacy and safety endpoints with 1-year follow-up earlier this year (Circulation. 2015 Feb 3;131:495-502). Concurrent with Dr. Laird’s report at the meeting, the 2-year results appeared online (J Amer Coll Card. 2015.doi:10.1016/j.jacc.2015.09.063).
Dr. Laird acknowledged that some types of stents also have shown good efficacy for treating femoropopliteal disease, but he had reservations about placing a stent when the DCB option exists.
“A lot of people have the sense that if we can avoid placing a stent in a femoral artery it helps preserve future treatment options for the patient. The problem with a stent is that once in-stent restenosis occurs in a leg artery, then the chances of getting a good result with an intravascular approach are poor,” Dr. Laird said at the meeting, sponsored by the Cardiovascular Research Foundation.
One potentially concerning finding from the 2-year follow-up was a statistically significant excess of all-cause mortality in the patients who received the DCB, with 16 deaths in the DCB arm and 1 death in the control, angioplasty arm. Dr. Laird dismissed the clinical importance of the finding, noting that all the deaths in the DCB arm had been independently adjudicated with none judged related to the device or procedure. In addition, the deaths occurred an average of 560 days following the procedure.
On Twitter @mitchelzoler
SAN FRANCISCO – Treatment of femoropopliteal arterial disease with a paclitaxel-coated balloon produced durable, 2-year benefits compared with conventional balloon angioplasty during extended follow-up of the pivotal trial that led to U.S. approval of this drug-coated balloon.
The durability of the benefit first seen after 1 year when follow-up continued out to 2 years was an important finding that distinguishes the IN.PACT Admiral paclitaxel-covered balloon used in the current study from the first and only other drug-covered balloon (DCB) approved for U.S. practice, the Lutonix 035 DCB.
“Not all drug-coated balloons are the same,” Dr. John R. Laird said while reporting the IN.PACT Admiral DCB results at the Transcatheter Cardiovascular Therapeutics annual meeting.
Although both the IN.PACT Admiral and Lutonix 035 DCB have paclitaxel coatings, the two devices differ by paclitaxel dose density on the balloon’s surface (3.5 mcg/mm2 and 2.0 mcg/mm2, respectively), type of excipient (carrier) used, and the balloon coating, noted Dr. Laird, professor and medical director of the Vascular Center at the University of California, Davis in Sacramento.
After the first year, primary patency ran 82% among the 220 patients randomized to the DCB and 52% in patients treated with percutaneous transluminal angioplasty, a statistically significant 30 percentage point difference in favor of the DCB. After 2 years, the rates were 79% in the DCB arm and 50% with a conventional balloon. “We saw no late catch-up that reduced the patency rate,” said Dr. Laird.
The INPACT SFA I(Randomized Trial of IN.PACT Admiral Drug Coated Balloon vs. Standard PTA for the Treatment of SFA and Proximal Popliteal Arterial Disease) trial enrolled 331 patients at 57 centers in the United States and Europe. Researchers reported the study’s primary efficacy and safety endpoints with 1-year follow-up earlier this year (Circulation. 2015 Feb 3;131:495-502). Concurrent with Dr. Laird’s report at the meeting, the 2-year results appeared online (J Amer Coll Card. 2015.doi:10.1016/j.jacc.2015.09.063).
Dr. Laird acknowledged that some types of stents also have shown good efficacy for treating femoropopliteal disease, but he had reservations about placing a stent when the DCB option exists.
“A lot of people have the sense that if we can avoid placing a stent in a femoral artery it helps preserve future treatment options for the patient. The problem with a stent is that once in-stent restenosis occurs in a leg artery, then the chances of getting a good result with an intravascular approach are poor,” Dr. Laird said at the meeting, sponsored by the Cardiovascular Research Foundation.
One potentially concerning finding from the 2-year follow-up was a statistically significant excess of all-cause mortality in the patients who received the DCB, with 16 deaths in the DCB arm and 1 death in the control, angioplasty arm. Dr. Laird dismissed the clinical importance of the finding, noting that all the deaths in the DCB arm had been independently adjudicated with none judged related to the device or procedure. In addition, the deaths occurred an average of 560 days following the procedure.
On Twitter @mitchelzoler
AT TCT 2015
Key clinical point: Two-year follow-up of paclitaxel-coated balloon treatment of femoropopliteal lesions showed durable and substantially better patency, compared with conventional balloon treatment.
Major finding: Two-year primary patency rate was 79% after treatment with the IN.PACT Admiral balloon and 50% with a conventional balloon.
Data source: INPACT SFA 1, a multicenter, randomized trial with 331 enrolled patients.
Disclosures: INPACT SFA I was sponsored by Medtronic, the company that markets the IN.PACT Admiral drug-coated balloon. Dr. Laird has been a consultant to Medtronic as well as to Bard, Abbott Vascular, Boston Scientific and Cordis. He also owns stock in several device companies.
Dose-intensive, multiagent regimen improves outcomes from low-risk rhabdomyosarcoma
A dose-intensive multiagent regimen including dose-compressed cycles of ifosfamide/etoposide and vincristine/doxorubicin/cyclophosphamide, irinotecan, and radiation resulted in improved outcomes for patients with low-risk stage IV rhabdomyosarcoma (RMS), but not for patients with high-risk disease, according to results from the Children’s Oncology Group study.
For all patients with stage IV rhabdomyosarcoma, the 3-year event-free and overall survival rates were 38% and 56%, respectively. Patients with stage IV RMS with one or fewer Oberlin risk factors had 3-year event-free survival and overall survival rates of 69% and 79%, respectively; patients with two or more Oberlin risk factors had rates of 20% and 14%, respectively (Jour Clin Oncol. 2015 Oct 26. doi: 10.1200/JCO.2015.63.4048).
The study identified an expanded group of patients with low-risk metastatic RMS that included patients with embryonal RMS aged 10 years and older but with an Oberlin score of less than 2. The results in this group represent an improvement over previous study results. However, for the remainder of high-risk patients with alveolar RMS, different approaches are needed, according to Dr. Brenda Weigel of the University of Minnesota, Minneapolis, and her colleagues.
“Unfortunately, alveolar RMS has fewer genetic aberrations than embryonal RMS and no known recurrently mutated cancer consensus genes, which limits genetic targets available for therapeutic approaches,” they wrote.
The Children’s Oncology Group study ARST0431 enrolled 109 patients with metastatic RMS who had no prior chemotherapy or radiation treatment from 2006 to 2008.
The study combined three treatment strategies: dose intensification by interval compression, use of active agents identified in previous phase II window studies, and use of irinotecan as a radiation sensitizer. The 54-week treatment schedule began with two cycles of vincristine/irinotecan followed by interval-compressed vincristine/doxorubicin/cyclophosphamide and ifosfamide/etoposide (cycles began every 14 days), and finished with four cycles of standard vincristine/actinomycin/cyclophosphamide (VAC) and two more cycles of vincristine/irinotecan. The treatment plan also included radiation of primary and metastatic sites at week 19.
The most common nonhematologic adverse event of grade 3 or higher was diarrhea, reported in 20% of patients during the time period when they received irinotecan. Febrile neutropenia occurred in 63% of patients.
Dr. Weigel reported financial relationships with Genentech and Eli Lilly/ImClone System. Several of her coauthors reported having ties to industry sources.
A dose-intensive multiagent regimen including dose-compressed cycles of ifosfamide/etoposide and vincristine/doxorubicin/cyclophosphamide, irinotecan, and radiation resulted in improved outcomes for patients with low-risk stage IV rhabdomyosarcoma (RMS), but not for patients with high-risk disease, according to results from the Children’s Oncology Group study.
For all patients with stage IV rhabdomyosarcoma, the 3-year event-free and overall survival rates were 38% and 56%, respectively. Patients with stage IV RMS with one or fewer Oberlin risk factors had 3-year event-free survival and overall survival rates of 69% and 79%, respectively; patients with two or more Oberlin risk factors had rates of 20% and 14%, respectively (Jour Clin Oncol. 2015 Oct 26. doi: 10.1200/JCO.2015.63.4048).
The study identified an expanded group of patients with low-risk metastatic RMS that included patients with embryonal RMS aged 10 years and older but with an Oberlin score of less than 2. The results in this group represent an improvement over previous study results. However, for the remainder of high-risk patients with alveolar RMS, different approaches are needed, according to Dr. Brenda Weigel of the University of Minnesota, Minneapolis, and her colleagues.
“Unfortunately, alveolar RMS has fewer genetic aberrations than embryonal RMS and no known recurrently mutated cancer consensus genes, which limits genetic targets available for therapeutic approaches,” they wrote.
The Children’s Oncology Group study ARST0431 enrolled 109 patients with metastatic RMS who had no prior chemotherapy or radiation treatment from 2006 to 2008.
The study combined three treatment strategies: dose intensification by interval compression, use of active agents identified in previous phase II window studies, and use of irinotecan as a radiation sensitizer. The 54-week treatment schedule began with two cycles of vincristine/irinotecan followed by interval-compressed vincristine/doxorubicin/cyclophosphamide and ifosfamide/etoposide (cycles began every 14 days), and finished with four cycles of standard vincristine/actinomycin/cyclophosphamide (VAC) and two more cycles of vincristine/irinotecan. The treatment plan also included radiation of primary and metastatic sites at week 19.
The most common nonhematologic adverse event of grade 3 or higher was diarrhea, reported in 20% of patients during the time period when they received irinotecan. Febrile neutropenia occurred in 63% of patients.
Dr. Weigel reported financial relationships with Genentech and Eli Lilly/ImClone System. Several of her coauthors reported having ties to industry sources.
A dose-intensive multiagent regimen including dose-compressed cycles of ifosfamide/etoposide and vincristine/doxorubicin/cyclophosphamide, irinotecan, and radiation resulted in improved outcomes for patients with low-risk stage IV rhabdomyosarcoma (RMS), but not for patients with high-risk disease, according to results from the Children’s Oncology Group study.
For all patients with stage IV rhabdomyosarcoma, the 3-year event-free and overall survival rates were 38% and 56%, respectively. Patients with stage IV RMS with one or fewer Oberlin risk factors had 3-year event-free survival and overall survival rates of 69% and 79%, respectively; patients with two or more Oberlin risk factors had rates of 20% and 14%, respectively (Jour Clin Oncol. 2015 Oct 26. doi: 10.1200/JCO.2015.63.4048).
The study identified an expanded group of patients with low-risk metastatic RMS that included patients with embryonal RMS aged 10 years and older but with an Oberlin score of less than 2. The results in this group represent an improvement over previous study results. However, for the remainder of high-risk patients with alveolar RMS, different approaches are needed, according to Dr. Brenda Weigel of the University of Minnesota, Minneapolis, and her colleagues.
“Unfortunately, alveolar RMS has fewer genetic aberrations than embryonal RMS and no known recurrently mutated cancer consensus genes, which limits genetic targets available for therapeutic approaches,” they wrote.
The Children’s Oncology Group study ARST0431 enrolled 109 patients with metastatic RMS who had no prior chemotherapy or radiation treatment from 2006 to 2008.
The study combined three treatment strategies: dose intensification by interval compression, use of active agents identified in previous phase II window studies, and use of irinotecan as a radiation sensitizer. The 54-week treatment schedule began with two cycles of vincristine/irinotecan followed by interval-compressed vincristine/doxorubicin/cyclophosphamide and ifosfamide/etoposide (cycles began every 14 days), and finished with four cycles of standard vincristine/actinomycin/cyclophosphamide (VAC) and two more cycles of vincristine/irinotecan. The treatment plan also included radiation of primary and metastatic sites at week 19.
The most common nonhematologic adverse event of grade 3 or higher was diarrhea, reported in 20% of patients during the time period when they received irinotecan. Febrile neutropenia occurred in 63% of patients.
Dr. Weigel reported financial relationships with Genentech and Eli Lilly/ImClone System. Several of her coauthors reported having ties to industry sources.
Key clinical point: A dose-intensive, multiagent regimen including dose-compressed cycles of ifosfamide/etoposide and vincristine/doxorubicin/cyclophosphamide, irinotecan, and radiation resulted in improved outcomes for patients with low-risk stage IV rhabdomyosarcoma (RMS), but not for patients with high-risk disease.
Major finding: Patients with stage IV rhabdomyosarcoma with one or fewer Oberlin risk factors had 3-year event-free survival and overall survival rates of 69% and 79%, respectively; patients with two or more Oberlin risk factors had rates of 20% and 14%, respectively.
Data source: The Children’s Oncology Group study ARST0431 involving 109 patients with metastatic RMS who had no prior chemotherapy or radiation treatment from 2006 to 2008.
Disclosures: Dr. Weigel reported financial relationships with Genentech and Eli Lilly/ImClone System. Several of her coauthors reported having ties to industry sources.
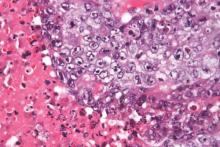
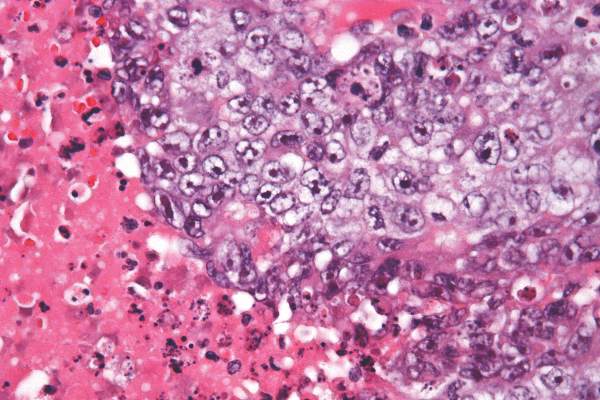
High-radiation doses improve survival with inoperable intrahepatic cholangiocarcinoma
Using recent advances in radiotherapy (RT) planning and delivery, high-dose radiation delivered to hepatic tumors produced major survival benefits in patients with inoperable intrahepatic cholangiocarcinoma (IHCC), investigators reported online in Journal of Clinical Oncology.
“Treatment with ablative doses of RT using high-quality daily CT image guidance with inspiration breath-hold gating can achieve survival times comparable to those achieved with resection,” wrote Dr. Randa Tao, radiation oncologist at the University of Texas MD Anderson Cancer Center, Houston, and her colleagues (Jour Clin Onc. 2015 Oct 26 [doi: 10.1200/JCO.2015.61.3778]).
From 2002 to 2014, 79 patients with inoperable IHCC were treated with definitive RT. The median survival time was 30 months; 1-, 2-, and 3-year overall survival rates were 87%, 61%, and 44%, respectively. Median progression-free survival was 30 months, and 1-, 2-, and 3-year progression-free survival rates were 88%, 61%, and 39%, respectively.
After completion of RT, 38 patients (48%) had primary tumor progression. Actuarial 1-, 2-, and 3-year local control rates were 81%, 45%, and 27%, respectively, with median duration of 23 months. The majority of patients (34) had recurrence within the high-dose radiation region, three had both in-field and marginal progression, and one had recurrence at the margin.
RT dose was the most important prognostic factor for overall survival and local control. Patients treated with doses higher than the conventional 50.4 Gy had a median survival of 43 months, compared with 23 months for patients treated with doses 50.4 Gy or less (P = .01).
Total biologically effective dose (BED) affected outcomes also. The 2- and 3-year overall survival rates for patients treated with BED greater than 80.5 Gy were both 73%, compared with 58% and 38% for those treated with BED of 80.5 Gy or less.
The treatment was well tolerated, with no cases of radiation induced liver disease observed.
The investigators recommend that higher total RT doses and higher doses delivered per fraction to achieve BED greater than 80.5 Gy should be considered for all patients as long as image guidance is used to ensure that the dose is delivered safely, and dose constraints to the liver, bile duct, stomach, and bowel can be met. The findings support the use of 67.5 Gy in 15 fractions (BED, 97.88 Gy).
Dr. Tao reported having no disclosures. Several of her coauthors reported having ties to industry sources.
Using recent advances in radiotherapy (RT) planning and delivery, high-dose radiation delivered to hepatic tumors produced major survival benefits in patients with inoperable intrahepatic cholangiocarcinoma (IHCC), investigators reported online in Journal of Clinical Oncology.
“Treatment with ablative doses of RT using high-quality daily CT image guidance with inspiration breath-hold gating can achieve survival times comparable to those achieved with resection,” wrote Dr. Randa Tao, radiation oncologist at the University of Texas MD Anderson Cancer Center, Houston, and her colleagues (Jour Clin Onc. 2015 Oct 26 [doi: 10.1200/JCO.2015.61.3778]).
From 2002 to 2014, 79 patients with inoperable IHCC were treated with definitive RT. The median survival time was 30 months; 1-, 2-, and 3-year overall survival rates were 87%, 61%, and 44%, respectively. Median progression-free survival was 30 months, and 1-, 2-, and 3-year progression-free survival rates were 88%, 61%, and 39%, respectively.
After completion of RT, 38 patients (48%) had primary tumor progression. Actuarial 1-, 2-, and 3-year local control rates were 81%, 45%, and 27%, respectively, with median duration of 23 months. The majority of patients (34) had recurrence within the high-dose radiation region, three had both in-field and marginal progression, and one had recurrence at the margin.
RT dose was the most important prognostic factor for overall survival and local control. Patients treated with doses higher than the conventional 50.4 Gy had a median survival of 43 months, compared with 23 months for patients treated with doses 50.4 Gy or less (P = .01).
Total biologically effective dose (BED) affected outcomes also. The 2- and 3-year overall survival rates for patients treated with BED greater than 80.5 Gy were both 73%, compared with 58% and 38% for those treated with BED of 80.5 Gy or less.
The treatment was well tolerated, with no cases of radiation induced liver disease observed.
The investigators recommend that higher total RT doses and higher doses delivered per fraction to achieve BED greater than 80.5 Gy should be considered for all patients as long as image guidance is used to ensure that the dose is delivered safely, and dose constraints to the liver, bile duct, stomach, and bowel can be met. The findings support the use of 67.5 Gy in 15 fractions (BED, 97.88 Gy).
Dr. Tao reported having no disclosures. Several of her coauthors reported having ties to industry sources.
Using recent advances in radiotherapy (RT) planning and delivery, high-dose radiation delivered to hepatic tumors produced major survival benefits in patients with inoperable intrahepatic cholangiocarcinoma (IHCC), investigators reported online in Journal of Clinical Oncology.
“Treatment with ablative doses of RT using high-quality daily CT image guidance with inspiration breath-hold gating can achieve survival times comparable to those achieved with resection,” wrote Dr. Randa Tao, radiation oncologist at the University of Texas MD Anderson Cancer Center, Houston, and her colleagues (Jour Clin Onc. 2015 Oct 26 [doi: 10.1200/JCO.2015.61.3778]).
From 2002 to 2014, 79 patients with inoperable IHCC were treated with definitive RT. The median survival time was 30 months; 1-, 2-, and 3-year overall survival rates were 87%, 61%, and 44%, respectively. Median progression-free survival was 30 months, and 1-, 2-, and 3-year progression-free survival rates were 88%, 61%, and 39%, respectively.
After completion of RT, 38 patients (48%) had primary tumor progression. Actuarial 1-, 2-, and 3-year local control rates were 81%, 45%, and 27%, respectively, with median duration of 23 months. The majority of patients (34) had recurrence within the high-dose radiation region, three had both in-field and marginal progression, and one had recurrence at the margin.
RT dose was the most important prognostic factor for overall survival and local control. Patients treated with doses higher than the conventional 50.4 Gy had a median survival of 43 months, compared with 23 months for patients treated with doses 50.4 Gy or less (P = .01).
Total biologically effective dose (BED) affected outcomes also. The 2- and 3-year overall survival rates for patients treated with BED greater than 80.5 Gy were both 73%, compared with 58% and 38% for those treated with BED of 80.5 Gy or less.
The treatment was well tolerated, with no cases of radiation induced liver disease observed.
The investigators recommend that higher total RT doses and higher doses delivered per fraction to achieve BED greater than 80.5 Gy should be considered for all patients as long as image guidance is used to ensure that the dose is delivered safely, and dose constraints to the liver, bile duct, stomach, and bowel can be met. The findings support the use of 67.5 Gy in 15 fractions (BED, 97.88 Gy).
Dr. Tao reported having no disclosures. Several of her coauthors reported having ties to industry sources.
Key clinical point: High-radiation doses delivered to hepatic tumors were well tolerated and survival outcomes were comparable to surgical resection.
Major finding: The median survival time was 30 months; 1-, 2-, and 3-year overall survival rates were 87%, 61%, and 44%, respectively.
Data source: From 2002 to 2014, 79 patients with IHCC were treated with definitive radiotherapy at the University of Texas MD Anderson Cancer Center.
Disclosures: Dr. Tao reported having no disclosures. Several of her coauthors reported having ties to industry sources.
Low BMI predicted worse survival in mCRC
In patients with metastatic colorectal cancer (mCRC), body mass index (BMI) was prognostic for overall survival (OS) and progression-free survival (PFS), investigators reported online in Journal of Clinical Oncology.
Risks were highest at the lowest BMI values, decreased as BMI increased to 28 kg/m2, and plateaued at higher BMI values.
By pooling data from more than 21,000 patients enrolled worldwide in 25 randomized trials for frontline treatment, “we have shown that BMI is prognostic for OS and PFS in this population, but with a shape of the risk curve across the BMI spectrum, different than that observed in the adjuvant setting,” wrote Lindsay Renfro, Ph.D., of the Mayo Clinic, Rochester, Minn., and her colleagues (Jour Clin Onc. 2015 Oct 26 [doi: 10.1200/JCO.2015.61.6441]).
Patients with a BMI of 18.5 kg/m2 had a 50% increased risk of death (95% confidence interval, 43%-56%). After researchers adjusted for age, sex, performance status, and clinical characteristics, the prognostic significance of BMI remained (P less than .001).
Previous studies showed that obese patients with stage II or III colon cancer were at increased risk for disease recurrence or death, but results of the current study showed obese patients with mCRC were not at increased risk.
Men with low BMIs had a greater risk of death than did women. Both men and women with moderate and higher BMIs had similar risks. Previous studies have shown that the prognosis for women with colorectal cancer is improved over men, possibly because of the protective effect of estrogen.
The results suggest that patients with mCRC and low BMI are likely cachectic, a condition that affects approximately 50% of patients with colon cancer and is associated with a 20% mortality rate, the authors noted.
In patients with metastatic colorectal cancer (mCRC), body mass index (BMI) was prognostic for overall survival (OS) and progression-free survival (PFS), investigators reported online in Journal of Clinical Oncology.
Risks were highest at the lowest BMI values, decreased as BMI increased to 28 kg/m2, and plateaued at higher BMI values.
By pooling data from more than 21,000 patients enrolled worldwide in 25 randomized trials for frontline treatment, “we have shown that BMI is prognostic for OS and PFS in this population, but with a shape of the risk curve across the BMI spectrum, different than that observed in the adjuvant setting,” wrote Lindsay Renfro, Ph.D., of the Mayo Clinic, Rochester, Minn., and her colleagues (Jour Clin Onc. 2015 Oct 26 [doi: 10.1200/JCO.2015.61.6441]).
Patients with a BMI of 18.5 kg/m2 had a 50% increased risk of death (95% confidence interval, 43%-56%). After researchers adjusted for age, sex, performance status, and clinical characteristics, the prognostic significance of BMI remained (P less than .001).
Previous studies showed that obese patients with stage II or III colon cancer were at increased risk for disease recurrence or death, but results of the current study showed obese patients with mCRC were not at increased risk.
Men with low BMIs had a greater risk of death than did women. Both men and women with moderate and higher BMIs had similar risks. Previous studies have shown that the prognosis for women with colorectal cancer is improved over men, possibly because of the protective effect of estrogen.
The results suggest that patients with mCRC and low BMI are likely cachectic, a condition that affects approximately 50% of patients with colon cancer and is associated with a 20% mortality rate, the authors noted.
In patients with metastatic colorectal cancer (mCRC), body mass index (BMI) was prognostic for overall survival (OS) and progression-free survival (PFS), investigators reported online in Journal of Clinical Oncology.
Risks were highest at the lowest BMI values, decreased as BMI increased to 28 kg/m2, and plateaued at higher BMI values.
By pooling data from more than 21,000 patients enrolled worldwide in 25 randomized trials for frontline treatment, “we have shown that BMI is prognostic for OS and PFS in this population, but with a shape of the risk curve across the BMI spectrum, different than that observed in the adjuvant setting,” wrote Lindsay Renfro, Ph.D., of the Mayo Clinic, Rochester, Minn., and her colleagues (Jour Clin Onc. 2015 Oct 26 [doi: 10.1200/JCO.2015.61.6441]).
Patients with a BMI of 18.5 kg/m2 had a 50% increased risk of death (95% confidence interval, 43%-56%). After researchers adjusted for age, sex, performance status, and clinical characteristics, the prognostic significance of BMI remained (P less than .001).
Previous studies showed that obese patients with stage II or III colon cancer were at increased risk for disease recurrence or death, but results of the current study showed obese patients with mCRC were not at increased risk.
Men with low BMIs had a greater risk of death than did women. Both men and women with moderate and higher BMIs had similar risks. Previous studies have shown that the prognosis for women with colorectal cancer is improved over men, possibly because of the protective effect of estrogen.
The results suggest that patients with mCRC and low BMI are likely cachectic, a condition that affects approximately 50% of patients with colon cancer and is associated with a 20% mortality rate, the authors noted.
FROM JOURNAL OF CLINICAL ONCOLOGY
Key clinical point: Low BMI predicted worse overall and progression-free survival in patients with mCRC.
Major finding: Risks of death and disease progression were highest for patients with the lowest BMI, decreased as BMI increased to 28 kg/m2, and plateaued at higher BMI values.
Data source: Retrospective analysis of data from 25 first-line clinical trials that included 21,149 patients with metastatic colorectal cancer.
Disclosures: Dr. Renfro reported having no disclosures. Several of her coauthors reported having ties to industry sources.
MINIDEP: A simple, self-administered depression screening tool
Depression is a debilitating illness, and many cases go unrecognized and untreated. There are several depression inventories and questionnaires available for practitioners’ use, but many are long or require a specially trained rater or administrator.1-10
One well-known depression screening questionnaire is the Patient Health Questionnaire (PHQ-9). This instrument is a combination of a 2-item questionnaire and, if the 2-item questionnaire is positive, a 7-item questionnaire.2,3 Even if the PHQ-9 is used, it requires a trained healthcare professional to administer it, limiting its use.
On the other hand, the MINIDEP depression screening tool that I developed can be self-administered by the patient either online or while he (she) is in the waiting room. It can be used by any health care specialist (psychiatrist, psychologist, family practitioner, etc.) as part of the patient’s evaluation.
Unlike most conventional screening questionnaires, MINIDEP has only 7 questions but covers most of the DSM-5 criteria for major depressive disorder. It also includes a question on unexplained pains or aches, which often is the only symptom that patients report, but is absent in the PHQ-9 and in other screening questionnaires.
Having a simple, easy-to-remember mnemonic means that this questionnaire can be used by medical students, residents, allied health and mental health professionals, and primary care physicians to screen for depression in the community.11
MINIDEP Categories/areas of concern addressed
Mood (lowered) and emotional lability.
Interest and desires (anhedonia).
Nutrition, poor appetite, and weight loss or gain.
Insomnia or hypersomnia.
Death or dying (thinking of), feeling worthless or guilty, or making suicidal plans.
Energy (decreased), impaired daily activities, and worsened cognitive ability.
Pains and aches (in absence of unexplained medical illnesses).
I propose rating scores for this questionnaire (Figure) as follows:
0 to 3 Points: Patient is not clinically depressed. Evaluation by a mental health professional might be unnecessary.
4 to 9 Pointsa: Depression is suspected. Further evaluation by a mental health professional (not necessarily a psychiatrist) is warranted.
aThorough psychiatric evaluation also is warranted if the patient has scored 4 to 9 points, with at least 1 point from Question 5.
≥10 points: Depression is confirmed. The patient should be evaluated by a psychiatrist for suicidal thoughts.
Note that this proposed rating scale is based on my experience, although I believe it could be useful. To increase this screening tool’s sensitivity, in my experience, evaluation by a mental health professional might be necessary when a patient scores only 3 points on MINIDEP. The optimal number of points for triggering a clinical decision and this questionnaire’s sensitivity and specificity, however, need to be studied.
Disclosure
The author reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Depression in adults: screening. U.S. Preventive Services Task Force. http://www.uspreventiveservicestaskforce.org/Page/Topic/recommendation-summary/depressionin-adults-screening. Updated July 2015. Accessed October 2, 2015.
2. Patient Health Questionnaire (PHQ-9). U.S. Preventive Services Task Force. http://www.integration.samhsa.gov/images/res/PHQ%20-%20Questions.pdf. Published October 4, 2005. Accessed September 30, 2015.
3. Patient Health Questionnaire (PHQ-9 & PHQ-2). American Psychological Association. http://www.apa.org/pi/about/publications/caregivers/practice-settings/assessment/tools/patient-health.aspx. Accessed October 2, 2015.
4. Online assessment measures. American Psychiatric Association. http://www.psychiatry.org/practice/dsm/dsm5/online-assessment-measures#Disorder. Accessed October 2, 2015.
5. Depression screening. Mental Health America. http://www.mentalhealthamerica.net/mental-health-screen/patient-health. Accessed October 2, 2015.
6. Major Depressive Disorder Diagnostic Criteria—SIGE CAPS. Family Medicine Reference. http://www.fammedref.org/mnemonic/major-depressive-disorder-
diagnostic-criteria-sigme-caps. Accessed October2, 2015.
7. Welcome to the Wakefield Self-Report Questionnaire, a screening test for depression. Counselling Resource. http://counsellingresource.com/lib/quizzes/depression-testing/wakefield. Accessed October 2, 2015.
8. Goldberg’s Depression and Mania Self-Rating Scales. Psy-World. http://www.psy-world.com/goldberg.htm. Published 1993. Accessed October 2, 2015.
9. Radloff LS. The CES-D Scale: a self-report depression scale for research in the general population. Appl Psychol Meas. 1977;1(3):385-401.
10. Zung WW. A self-rating depression scale. Arch Gen Psychiatry. 1965;12:63-70.
11. Graypel EA. MINIDEP. http://www.minidep.com. Accessed October 2, 2015.
Depression is a debilitating illness, and many cases go unrecognized and untreated. There are several depression inventories and questionnaires available for practitioners’ use, but many are long or require a specially trained rater or administrator.1-10
One well-known depression screening questionnaire is the Patient Health Questionnaire (PHQ-9). This instrument is a combination of a 2-item questionnaire and, if the 2-item questionnaire is positive, a 7-item questionnaire.2,3 Even if the PHQ-9 is used, it requires a trained healthcare professional to administer it, limiting its use.
On the other hand, the MINIDEP depression screening tool that I developed can be self-administered by the patient either online or while he (she) is in the waiting room. It can be used by any health care specialist (psychiatrist, psychologist, family practitioner, etc.) as part of the patient’s evaluation.
Unlike most conventional screening questionnaires, MINIDEP has only 7 questions but covers most of the DSM-5 criteria for major depressive disorder. It also includes a question on unexplained pains or aches, which often is the only symptom that patients report, but is absent in the PHQ-9 and in other screening questionnaires.
Having a simple, easy-to-remember mnemonic means that this questionnaire can be used by medical students, residents, allied health and mental health professionals, and primary care physicians to screen for depression in the community.11
MINIDEP Categories/areas of concern addressed
Mood (lowered) and emotional lability.
Interest and desires (anhedonia).
Nutrition, poor appetite, and weight loss or gain.
Insomnia or hypersomnia.
Death or dying (thinking of), feeling worthless or guilty, or making suicidal plans.
Energy (decreased), impaired daily activities, and worsened cognitive ability.
Pains and aches (in absence of unexplained medical illnesses).
I propose rating scores for this questionnaire (Figure) as follows:
0 to 3 Points: Patient is not clinically depressed. Evaluation by a mental health professional might be unnecessary.
4 to 9 Pointsa: Depression is suspected. Further evaluation by a mental health professional (not necessarily a psychiatrist) is warranted.
aThorough psychiatric evaluation also is warranted if the patient has scored 4 to 9 points, with at least 1 point from Question 5.
≥10 points: Depression is confirmed. The patient should be evaluated by a psychiatrist for suicidal thoughts.
Note that this proposed rating scale is based on my experience, although I believe it could be useful. To increase this screening tool’s sensitivity, in my experience, evaluation by a mental health professional might be necessary when a patient scores only 3 points on MINIDEP. The optimal number of points for triggering a clinical decision and this questionnaire’s sensitivity and specificity, however, need to be studied.
Disclosure
The author reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Depression is a debilitating illness, and many cases go unrecognized and untreated. There are several depression inventories and questionnaires available for practitioners’ use, but many are long or require a specially trained rater or administrator.1-10
One well-known depression screening questionnaire is the Patient Health Questionnaire (PHQ-9). This instrument is a combination of a 2-item questionnaire and, if the 2-item questionnaire is positive, a 7-item questionnaire.2,3 Even if the PHQ-9 is used, it requires a trained healthcare professional to administer it, limiting its use.
On the other hand, the MINIDEP depression screening tool that I developed can be self-administered by the patient either online or while he (she) is in the waiting room. It can be used by any health care specialist (psychiatrist, psychologist, family practitioner, etc.) as part of the patient’s evaluation.
Unlike most conventional screening questionnaires, MINIDEP has only 7 questions but covers most of the DSM-5 criteria for major depressive disorder. It also includes a question on unexplained pains or aches, which often is the only symptom that patients report, but is absent in the PHQ-9 and in other screening questionnaires.
Having a simple, easy-to-remember mnemonic means that this questionnaire can be used by medical students, residents, allied health and mental health professionals, and primary care physicians to screen for depression in the community.11
MINIDEP Categories/areas of concern addressed
Mood (lowered) and emotional lability.
Interest and desires (anhedonia).
Nutrition, poor appetite, and weight loss or gain.
Insomnia or hypersomnia.
Death or dying (thinking of), feeling worthless or guilty, or making suicidal plans.
Energy (decreased), impaired daily activities, and worsened cognitive ability.
Pains and aches (in absence of unexplained medical illnesses).
I propose rating scores for this questionnaire (Figure) as follows:
0 to 3 Points: Patient is not clinically depressed. Evaluation by a mental health professional might be unnecessary.
4 to 9 Pointsa: Depression is suspected. Further evaluation by a mental health professional (not necessarily a psychiatrist) is warranted.
aThorough psychiatric evaluation also is warranted if the patient has scored 4 to 9 points, with at least 1 point from Question 5.
≥10 points: Depression is confirmed. The patient should be evaluated by a psychiatrist for suicidal thoughts.
Note that this proposed rating scale is based on my experience, although I believe it could be useful. To increase this screening tool’s sensitivity, in my experience, evaluation by a mental health professional might be necessary when a patient scores only 3 points on MINIDEP. The optimal number of points for triggering a clinical decision and this questionnaire’s sensitivity and specificity, however, need to be studied.
Disclosure
The author reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Depression in adults: screening. U.S. Preventive Services Task Force. http://www.uspreventiveservicestaskforce.org/Page/Topic/recommendation-summary/depressionin-adults-screening. Updated July 2015. Accessed October 2, 2015.
2. Patient Health Questionnaire (PHQ-9). U.S. Preventive Services Task Force. http://www.integration.samhsa.gov/images/res/PHQ%20-%20Questions.pdf. Published October 4, 2005. Accessed September 30, 2015.
3. Patient Health Questionnaire (PHQ-9 & PHQ-2). American Psychological Association. http://www.apa.org/pi/about/publications/caregivers/practice-settings/assessment/tools/patient-health.aspx. Accessed October 2, 2015.
4. Online assessment measures. American Psychiatric Association. http://www.psychiatry.org/practice/dsm/dsm5/online-assessment-measures#Disorder. Accessed October 2, 2015.
5. Depression screening. Mental Health America. http://www.mentalhealthamerica.net/mental-health-screen/patient-health. Accessed October 2, 2015.
6. Major Depressive Disorder Diagnostic Criteria—SIGE CAPS. Family Medicine Reference. http://www.fammedref.org/mnemonic/major-depressive-disorder-
diagnostic-criteria-sigme-caps. Accessed October2, 2015.
7. Welcome to the Wakefield Self-Report Questionnaire, a screening test for depression. Counselling Resource. http://counsellingresource.com/lib/quizzes/depression-testing/wakefield. Accessed October 2, 2015.
8. Goldberg’s Depression and Mania Self-Rating Scales. Psy-World. http://www.psy-world.com/goldberg.htm. Published 1993. Accessed October 2, 2015.
9. Radloff LS. The CES-D Scale: a self-report depression scale for research in the general population. Appl Psychol Meas. 1977;1(3):385-401.
10. Zung WW. A self-rating depression scale. Arch Gen Psychiatry. 1965;12:63-70.
11. Graypel EA. MINIDEP. http://www.minidep.com. Accessed October 2, 2015.
1. Depression in adults: screening. U.S. Preventive Services Task Force. http://www.uspreventiveservicestaskforce.org/Page/Topic/recommendation-summary/depressionin-adults-screening. Updated July 2015. Accessed October 2, 2015.
2. Patient Health Questionnaire (PHQ-9). U.S. Preventive Services Task Force. http://www.integration.samhsa.gov/images/res/PHQ%20-%20Questions.pdf. Published October 4, 2005. Accessed September 30, 2015.
3. Patient Health Questionnaire (PHQ-9 & PHQ-2). American Psychological Association. http://www.apa.org/pi/about/publications/caregivers/practice-settings/assessment/tools/patient-health.aspx. Accessed October 2, 2015.
4. Online assessment measures. American Psychiatric Association. http://www.psychiatry.org/practice/dsm/dsm5/online-assessment-measures#Disorder. Accessed October 2, 2015.
5. Depression screening. Mental Health America. http://www.mentalhealthamerica.net/mental-health-screen/patient-health. Accessed October 2, 2015.
6. Major Depressive Disorder Diagnostic Criteria—SIGE CAPS. Family Medicine Reference. http://www.fammedref.org/mnemonic/major-depressive-disorder-
diagnostic-criteria-sigme-caps. Accessed October2, 2015.
7. Welcome to the Wakefield Self-Report Questionnaire, a screening test for depression. Counselling Resource. http://counsellingresource.com/lib/quizzes/depression-testing/wakefield. Accessed October 2, 2015.
8. Goldberg’s Depression and Mania Self-Rating Scales. Psy-World. http://www.psy-world.com/goldberg.htm. Published 1993. Accessed October 2, 2015.
9. Radloff LS. The CES-D Scale: a self-report depression scale for research in the general population. Appl Psychol Meas. 1977;1(3):385-401.
10. Zung WW. A self-rating depression scale. Arch Gen Psychiatry. 1965;12:63-70.
11. Graypel EA. MINIDEP. http://www.minidep.com. Accessed October 2, 2015.
Venlafaxine discontinuation syndrome: Prevention and management
Most antidepressants lead to adverse discontinuation symptoms when they are abruptly stopped or rapidly tapered. Antidepressants with a short half-life, such as paroxetine and venlafaxine, can cause significantly more severe discontinuation symptoms compared with antidepressants with a longer half-life.
One culprit in particular
Among serotonin-norepinephrine reuptake inhibitors (SNRIs), venlafaxine is notorious for severe discontinuation symptoms. Venlafaxine has a half-life of 3 to 7 hours, and its active metabolite, desvenlafaxine, possesses a half-life of 9 to 13 hours. Higher frequency of discontinuation symptoms is associated with the use of higher dosages of venlafaxine and longer duration of treatment.
Venlafaxine is available in immediate release (IR) and extended release (XR) formulations. Venlafaxine XR has a slower release, extending the time to peak plasma concentration and, therefore, has once daily dosing and fewer side effects; however, it offers no substantial advantage over IR formulation in terms of diminished withdrawal effects. Desvenlafaxine also is marketed as an antidepressant and, although one can speculate that the drug would have a lower rate of discontinuation symptoms than venlafaxine, no evidence supports this hypothesis.
A range of venlafaxine discontinuation symptoms have been reported (Table).1
Preventing discontinuation symptoms
Patients for whom venlafaxine is prescribed should be informed about discontinuation symptoms, especially those who have a history of noncompliance. Monitor patients closely for discontinuation symptoms when venlafaxine is stopped—even if the patient is switched to another antidepressant. A gradual dosage reduction is recommended rather than abrupt termination or rapid dosage reduction. Immediately switching from venlafaxine to a selective serotonin reuptake inhibitor (SSRI) generally is not recommended, although it could alleviate some discontinuation symptoms2; cross-taper medication over 2 to 3 weeks.
Switching from venlafaxine to another SNRI, such as duloxetine, is less well studied. At venlafaxine dosages of <150 mg/d, an immediate switch to another SNRI of equivalent dosage generally is well-tolerated. For higher dosages, a gradual cross-taper is advised.2
Most patients tolerate a venlafaxine dosage reduction by 75 mg/d, at 1-week intervals. For patients who experience severe discontinuation symptoms with a minor dosage reduction, venlafaxine can be tapered over 10 months with approximately 1% dosage reduction every 3 days. Stahl3 recommends dissolving the tablet in 100 mL of juice, discarding 1 mL, and drinking the rest. After 3 days, 2 mL can be discarded, etc.
Another strategy to prevent discontinuation syndrome is to initiate fluoxetine—an SSRI with a long half-life—before taper; maintain fluoxetine dosage while venlafaxine is tapered; and then taper fluoxetine.
Managing discontinuation symptoms
If your patient experiences significant discontinuation symptoms, resume the last prescribed venlafaxine dosage, with a plan for a more gradual taper. Acute discontinuation syndrome also can be treated by initiating fluoxetine, 10 to 20 mg/d; after symptoms resolve, fluoxetine can be tapered over 2 to 3 weeks.
Disclosure
The author reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Effexor (venlafaxine hydrochloride) [package insert]. Philadelphia, PA: Wyeth Pharmaceuticals Inc; 2012.
2. Hirsch M, Birnbaum RJ. Antidepressant medication in adults: switching and discontinuing medication. http://www.uptodate.com/contents/antidepressant-medicationin-adults-switching-and-discontinuing-medication. Updated January 16, 2015. Accessed October 8, 2015.
3. Stahl SM. Venlafaxine. In: Stahl SM. The prescriber’s guide (Stahl’s essential psychopharmacology). 4th ed. New York, NY: Cambridge University Press; 2011:637-638.
Most antidepressants lead to adverse discontinuation symptoms when they are abruptly stopped or rapidly tapered. Antidepressants with a short half-life, such as paroxetine and venlafaxine, can cause significantly more severe discontinuation symptoms compared with antidepressants with a longer half-life.
One culprit in particular
Among serotonin-norepinephrine reuptake inhibitors (SNRIs), venlafaxine is notorious for severe discontinuation symptoms. Venlafaxine has a half-life of 3 to 7 hours, and its active metabolite, desvenlafaxine, possesses a half-life of 9 to 13 hours. Higher frequency of discontinuation symptoms is associated with the use of higher dosages of venlafaxine and longer duration of treatment.
Venlafaxine is available in immediate release (IR) and extended release (XR) formulations. Venlafaxine XR has a slower release, extending the time to peak plasma concentration and, therefore, has once daily dosing and fewer side effects; however, it offers no substantial advantage over IR formulation in terms of diminished withdrawal effects. Desvenlafaxine also is marketed as an antidepressant and, although one can speculate that the drug would have a lower rate of discontinuation symptoms than venlafaxine, no evidence supports this hypothesis.
A range of venlafaxine discontinuation symptoms have been reported (Table).1
Preventing discontinuation symptoms
Patients for whom venlafaxine is prescribed should be informed about discontinuation symptoms, especially those who have a history of noncompliance. Monitor patients closely for discontinuation symptoms when venlafaxine is stopped—even if the patient is switched to another antidepressant. A gradual dosage reduction is recommended rather than abrupt termination or rapid dosage reduction. Immediately switching from venlafaxine to a selective serotonin reuptake inhibitor (SSRI) generally is not recommended, although it could alleviate some discontinuation symptoms2; cross-taper medication over 2 to 3 weeks.
Switching from venlafaxine to another SNRI, such as duloxetine, is less well studied. At venlafaxine dosages of <150 mg/d, an immediate switch to another SNRI of equivalent dosage generally is well-tolerated. For higher dosages, a gradual cross-taper is advised.2
Most patients tolerate a venlafaxine dosage reduction by 75 mg/d, at 1-week intervals. For patients who experience severe discontinuation symptoms with a minor dosage reduction, venlafaxine can be tapered over 10 months with approximately 1% dosage reduction every 3 days. Stahl3 recommends dissolving the tablet in 100 mL of juice, discarding 1 mL, and drinking the rest. After 3 days, 2 mL can be discarded, etc.
Another strategy to prevent discontinuation syndrome is to initiate fluoxetine—an SSRI with a long half-life—before taper; maintain fluoxetine dosage while venlafaxine is tapered; and then taper fluoxetine.
Managing discontinuation symptoms
If your patient experiences significant discontinuation symptoms, resume the last prescribed venlafaxine dosage, with a plan for a more gradual taper. Acute discontinuation syndrome also can be treated by initiating fluoxetine, 10 to 20 mg/d; after symptoms resolve, fluoxetine can be tapered over 2 to 3 weeks.
Disclosure
The author reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Most antidepressants lead to adverse discontinuation symptoms when they are abruptly stopped or rapidly tapered. Antidepressants with a short half-life, such as paroxetine and venlafaxine, can cause significantly more severe discontinuation symptoms compared with antidepressants with a longer half-life.
One culprit in particular
Among serotonin-norepinephrine reuptake inhibitors (SNRIs), venlafaxine is notorious for severe discontinuation symptoms. Venlafaxine has a half-life of 3 to 7 hours, and its active metabolite, desvenlafaxine, possesses a half-life of 9 to 13 hours. Higher frequency of discontinuation symptoms is associated with the use of higher dosages of venlafaxine and longer duration of treatment.
Venlafaxine is available in immediate release (IR) and extended release (XR) formulations. Venlafaxine XR has a slower release, extending the time to peak plasma concentration and, therefore, has once daily dosing and fewer side effects; however, it offers no substantial advantage over IR formulation in terms of diminished withdrawal effects. Desvenlafaxine also is marketed as an antidepressant and, although one can speculate that the drug would have a lower rate of discontinuation symptoms than venlafaxine, no evidence supports this hypothesis.
A range of venlafaxine discontinuation symptoms have been reported (Table).1
Preventing discontinuation symptoms
Patients for whom venlafaxine is prescribed should be informed about discontinuation symptoms, especially those who have a history of noncompliance. Monitor patients closely for discontinuation symptoms when venlafaxine is stopped—even if the patient is switched to another antidepressant. A gradual dosage reduction is recommended rather than abrupt termination or rapid dosage reduction. Immediately switching from venlafaxine to a selective serotonin reuptake inhibitor (SSRI) generally is not recommended, although it could alleviate some discontinuation symptoms2; cross-taper medication over 2 to 3 weeks.
Switching from venlafaxine to another SNRI, such as duloxetine, is less well studied. At venlafaxine dosages of <150 mg/d, an immediate switch to another SNRI of equivalent dosage generally is well-tolerated. For higher dosages, a gradual cross-taper is advised.2
Most patients tolerate a venlafaxine dosage reduction by 75 mg/d, at 1-week intervals. For patients who experience severe discontinuation symptoms with a minor dosage reduction, venlafaxine can be tapered over 10 months with approximately 1% dosage reduction every 3 days. Stahl3 recommends dissolving the tablet in 100 mL of juice, discarding 1 mL, and drinking the rest. After 3 days, 2 mL can be discarded, etc.
Another strategy to prevent discontinuation syndrome is to initiate fluoxetine—an SSRI with a long half-life—before taper; maintain fluoxetine dosage while venlafaxine is tapered; and then taper fluoxetine.
Managing discontinuation symptoms
If your patient experiences significant discontinuation symptoms, resume the last prescribed venlafaxine dosage, with a plan for a more gradual taper. Acute discontinuation syndrome also can be treated by initiating fluoxetine, 10 to 20 mg/d; after symptoms resolve, fluoxetine can be tapered over 2 to 3 weeks.
Disclosure
The author reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Effexor (venlafaxine hydrochloride) [package insert]. Philadelphia, PA: Wyeth Pharmaceuticals Inc; 2012.
2. Hirsch M, Birnbaum RJ. Antidepressant medication in adults: switching and discontinuing medication. http://www.uptodate.com/contents/antidepressant-medicationin-adults-switching-and-discontinuing-medication. Updated January 16, 2015. Accessed October 8, 2015.
3. Stahl SM. Venlafaxine. In: Stahl SM. The prescriber’s guide (Stahl’s essential psychopharmacology). 4th ed. New York, NY: Cambridge University Press; 2011:637-638.
1. Effexor (venlafaxine hydrochloride) [package insert]. Philadelphia, PA: Wyeth Pharmaceuticals Inc; 2012.
2. Hirsch M, Birnbaum RJ. Antidepressant medication in adults: switching and discontinuing medication. http://www.uptodate.com/contents/antidepressant-medicationin-adults-switching-and-discontinuing-medication. Updated January 16, 2015. Accessed October 8, 2015.
3. Stahl SM. Venlafaxine. In: Stahl SM. The prescriber’s guide (Stahl’s essential psychopharmacology). 4th ed. New York, NY: Cambridge University Press; 2011:637-638.
Psychosis and catatonia after dancing with a dangerous partner
CASE Rigid, frightened, and mute
Mr. D, age 23, presents for evaluation immediately after discharge from another hospital, where he had been treated for altered mental status.
Ten days earlier, Mr. D’s friends obtained 2C-B (2,5-dimethoxy-4-bromophenethylamine), from the “Darknet,” an underground niche of the Internet. He ingested 20 mg of 2C-B in powder form. Although his friends recovered from a “safe trip,” Mr. D decompensated rapidly over the next few days with persistent psychosis, experiencing both auditory and visual hallucinations. He is “acting strange“ at work, and trying to find “hidden codes” in data. Mr. D also has persistent thought disorganization. He speaks of “connections” between people and things, and says that he is an alien in a spaceship. His friends and family report that he is talking rapidly and is sleeping only 2 or 3 hours each night. Mr. D abruptly quit his job as an analyst a few days after taking the drug.
Mr. D is a single, Ivy League-educated man and is described as hardworking and analytical. His family denies any recent mood changes or life stressors. They report that 1 month ago, Mr. D began smoking marijuana daily. He has no significant medical or psychiatric history, and no family history of psychiatric disorders.
What is your most likely diagnosis for Mr. D?
a) delirium due to a general medical condition
b) substance-induced psychotic disorder
c) catatonia due to a general medical condition
d) schizophrenia
e) bipolar I disorder, currently manic, with psychosis
The authors’ observations
Ring-substituted phenethylamines, commonly known as 2Cs, are designer drugs that are emerging as new substances of abuse.1 2C-B belongs to the phenethylamine subclass of monoamine alkaloids that includes more familiar drugs such as amphetamines, methamphetamines, and 3,4-methylenedioxy-methamphetamine (MDMA).2 It was first synthesized in 1974 by Alexander Shulgin, later described in his book Phenethylamines I Have Known and Loved: A Chemical Love Story, and its hallucinogenic activity is reported to be similar to LSD, mescaline, and psilocybin.3 The literature is scant on the acute effects of 2C intoxication or long-term sequelae of 2C ingestion.1 Most available information regarding the pharmacology of 2C-B comes from users who have reported their drug experiences on blogs, Web sites and forums, and in the media.4
2C-B usually is taken orally in powder or tablet form, in a dose of 10 to 50 mg.4 After an onset period of 20 to 90 minutes, the drug’s effect reaches maximum effect in 15 to 30 minutes, then plateaus for 2 to 7 hours, and comes down within 1 to 2 hours.4 2C-B is known to be orally active, and its hallucinogenic effects are mediated by its actions as a partial serotonin 5HT-2A and 5HT-2C receptor agonist.5 Entactogenic-stimulating effects have been reported at low doses (4 to 10 mg), whereas visual hallucinations with intense colors and object distortion have been reported at moderate doses (10 to 20 mg).4
2C-B, which users often take at parties or raves, appeared on the drug market in the mid 1980s and early 1990s under the names Nexus, Erox, Performax, Toonies, Bromo, Spectrum, and Venus and marketed as a replacement for MDMA after it became a Schedule I drug in the United States.4,6 Some users consume 2C-B in combination with other illicit drugs, including MDMA (called a “party pack”) or LSD (referred to as a “banana split”).6
According to the U.S. Drug Enforcement Agency, law enforcement authorities first seized 2C-B laboratories in California in 1986 and Arizona in 1992.6 Distribution of the drug has been sporadic since it became Schedule I in 1995, and it has been seized from several states, including Virginia, Nevada, Maine, Illinois, Missouri, South Dakota, and Kansas.6
EXAMINATION Passive and mute
On examination, Mr. D is lying in bed with eyes closed and extremities extended in an odd, rigid posture. He is resistant to attempts at passive movement, is nonresponsive to verbal commands, and is mute. A review of vital signs shows tachycardia, 110 beats per minute, but the physical exam is otherwise unremarkable. His Bush-Francis Catatonia Rating Scale (BFCRS) score is 17, indicating a diagnosis of catatonia. Mini-Mental Status Examination cannot be completed because Mr. D is unable to participate.
Laboratory studies reveal an elevated creatinine kinase (CK) level of 356 U/L. Results of a complete blood count, comprehensive metabolic panel, urinalysis, and thyroid-stimulating hormone are normal. Blood alcohol level is <10 mg/dL. Acetaminophen and salicylate levels are normal (<5 mg/dL). Records from his recent hospitalization reveal normal head CT, chest radiography, EEG, and urinalysis, and a negative urine drug screen.
What is the next step in managing Mr. D’s catatonic symptoms?
a) IV normal saline
b) IV lorazepam
c) emergent electroconvulsive therapy (ECT)
d) IM haloperidol
e) IM olanzapine
TREATMENT Saline and psychotropics
While in the emergency room, Mr. D receives 2 L of IV saline. His CK level falls to 137 U/L. A challenge with IV lorazepam, 2 mg, also is performed. Mr. D becomes talkative and follows commands with fluid movements, but his disorganized, delusional thoughts persist. BFCRS score has improved to 9 (Table 1). He is admitted to the psychiatric unit and started on oral lorazepam, 2 mg, 3 times daily, for catatonia, and olanzapine, 10 mg/d, for psychosis.
The differential diagnosis for Mr. D’s psychosis includes substance-induced psychotic disorder, schizophrenia, bipolar disorder, and psychosis with another organic cause (Table 2).7 Further medical workup is completed, including a urine drug screen, testing for HIV, hepatitis B, syphilis, lead and heavy metals, ceruloplasmin, vitamin B12, folate, antinuclear antibody, sedimentation rate, and brain MRI. Cannabinoids are detected in his urine drug screen. Another urine sample is sent to an outside lab to test for several synthetic drugs, including MDMA, 3,4-methylenedioxy- N-ethyl-amphetamine, 2C-B, 2C-C, 2C-I, and 2C-P, results of which also are negative.
By the second day of hospitalization, Mr. D appears less disorganized but continues to complain of “scrambled thoughts” and appears guarded. Despite initial response to IV lorazepam and its continuation in oral form, over the next day Mr. D appears more psychomotor-slowed, with motor stiffness. His score on the BFCRS increases, with significant posturing; vital signs remain stable, however.
What is your next step in managing his catatonic symptoms?
a) increase olanzapine
b) decrease olanzapine
c) decrease lorazepam
d) emergent ECT
e) switch to haloperidol
The authors’ observations
Although catatonia can be associated with a mood or psychotic disorder, it also can be induced by a medication or general medical condition (Table 3).8 It is thought that catatonia is associated with decreased γ-aminobutyric acid (GABA) and dopamine D2 receptor activity, and increased N-methyl-d-aspartate (NMDA) receptor activity.9 Antipsychotics could worsen catatonia through D2 blockade. Benzodiazepines, however, improve catatonia by increasing GABA and decreasing NMDA receptor activity. In this case, Mr. D was naïve to antipsychotics and seemed to be sensitive to them, as evidenced by his worsening symptoms.
Which condition should be considered in the differential diagnosis?
a) parkinsonian-hyperpyrexia syndrome
b) neuroleptic malignant syndrome (NMS)
c) stiff person syndrome
d) serotonin syndrome
e) CNS infection
The authors’ observations
NMS, catatonia, and parkinsonian-hyperpyrexia syndrome are all related to diminished action of dopamine at the D2 receptor. Although the mechanism of catatonia is not completely understood, NMS is thought to be caused by blockade at the D2 receptors by antipsychotics, whereas parkinsonian-hyperpyrexia syndrome is related to withdrawal of dopamine agonists. Because of the similarity in symptoms and proposed mechanisms, some experts hypothesize that NMS is a drug-induced malignant catatonia.10,11 Interestingly, NMS and catatonia respond to withdrawal of antipsychotics, and addition of benzodiazepines and ECT.
Mr. D showed posturing and other behavioral abnormalities, which are less common in NMS. Furthermore, although he had episodes of mild tachycardia, autonomic dysregulation—a hallmark of NMS—was not found. Given the common shared deficiency of activity at the D2 receptor in both NMS and catatonia, antipsychotics could cause or worsen either condition.
TREATMENT ECT
Mr. D’s olanzapine dosage is decreased to 2.5 mg/d. His catatonic symptoms improve with each dosage of oral lorazepam; however, effects seem to lessen and last for shorter periods over the following day. Additionally, Mr. D again becomes more disorganized, stiff, and unable to feed or bathe himself, and develops episodes of mild tachycardia.
Given Mr. D’s partial and poorly sustained response to lorazepam, a trial of ECT is pursued. On the third day of hospitalization, he receives ECT with bi-frontal lead placement at 25% energy. Concurrently, olanzapine is discontinued because of worsening muscle stiffness and concern about neuroleptic sensitivity. His BFCRS score after ECT is 2, and he is noted to be more interactive on the inpatient unit. He continues to receive ECT 3 times a week, with notable improvement, but ongoing psychotic symptoms and catatonic symptoms partially reemerge between ECT treatments. Lead placement is changed to bi-temporal by the third treatment, and the energy setting is increased from 25% to 50%, and to 75% by the sixth treatment. An additional nighttime dose of oral lorazepam, 2 mg, is added after the sixth treatment, in an attempt to reduce “wearing off” by morning.
After the seventh treatment, Mr. D is able to maintain logical conversation without re-emergence of catatonic symptoms over 2 days, signifying a turning point in the treatment course. The ECT energy setting is decreased to 50% to minimize potential memory deficits. His insight into his illness and treatment dramatically improve over the next few days. ECT is discontinued after the tenth treatment and Mr. D is discharged home to the care of his family.
The authors’ observations
Randomized clinical trials studying the effectiveness of ECT for catatonia are limited. Much of what we know about ECT comes from case reports that describe excellent outcomes for a variety of treatment-resistant illnesses, including catatonia in mood disorders, schizophrenia, autism, and other organic brain disease.12
Although benzodiazepines often are the first-line treatment for catatonia caused by any underlying illness, one study showed only 1 of 41 patients achieved remission with benzodiazepines, compared with 100% of those treated with ECT13; another study supported these results with 8 of 9 lorazepam non-responders responding to ECT.14 There are few case reports of substance-induced catatonia in the absence of other chronic mental illness, although none report use of ECT. However, a study showed no significant difference in the effectiveness of ECT for catatonia caused by an affective disorder or schizophrenia.15
Mr. D’s case exemplifies complete remission of catatonia induced by a psychoactive substance.
OUTCOME Steady improvement
Mr. D is followed in the outpatient clinic for 1 month after discharge; lorazepam is tapered successfully. During this time frame, psychotic and catatonic symptoms do not re-emerge. He reports some initial working memory deficits that improve steadily. There is no evidence of any significant psychiatric signs or symptoms, including neurovegetative symptoms of depression, mania or hypomania, perceptual disturbances, or disorganized thoughts or behaviors. He remains abstinent from alcohol, tobacco, and all psychoactive substances.
Bottom Line
Persistent psychosis and catatonia after the use of newer designer drugs such as 2C-B are rare, but these drugs carry serious potential complications that clinicians should be aware of. Benzodiazepines and electroconvulsive therapy have been proved effective for catatonia that is related to a number of psychiatric illnesses, often resulting in good outcomes. However, current evidence on their use is limited, particularly regarding treatment of substance-induced psychosis and catatonia.
Related Resources
• Meyer MR, Maurer HH. Metabolism of designer drugs of abuse: an updated review. Curr Drug Metab. 2010;11(5):468-482.
• Rickli A, Luethi D, Reinisch J, et al. Receptor interaction profiles of novel N-2-methoxybenzyl (NBOMe) derivatives of 2,5-dimethoxy-substituted phenethylamines (2C drugs). Neuropharmacology. 2015;99:546-553.
Drug Brand Names
Haloperidol • Haldol
Lorazepam • Ativan
Olanzapine • Zyprexa
Disclosures
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Dean BV, Stellpflug SJ, Burnett AM, et al. 2C or not 2C: phenethylamine designer drug review. J Med Toxicol. 2013;9(2):172-178.
2. Hill SL, Thomas SH. Clinical toxicology of newer recreational drugs. Clin Toxicol (Phila). 2011;49(8):705-719.
3. Shulgin A, Shulgin A. PiHKAL: a chemical love story. Berkley, CA: Transform Press; 1991.
4. Papoutsis I, Nikolaou P, Stefanidou M, et al. 25B-NBOMe and its precursor 2C-B: modern trends and hidden dangers. Forensic Toxicology. 2015;3(1):1-11.
5. Caudevilla-Gálligo F, Riba J, Ventura M, et al. 4-Bromo-2, 5-dimethoxyphenethylamine (2C-B): presence in the recreational drug market in Spain, pattern of use and subjective effects. J Psychopharmacol. 2012;26(7):1026-1035.
6. National Drug Intelligence Center. Information bulletin: 2C-B (Nexus) reappears on the club drug scene. http:// www.Justice.gov/archive/ndic/pubs0/665. Published May 2001. Accessed June 12, 2015.
7. Freudenreich O, Schulz SC, Goff DC. Initial medical work-up of first episode psychosis: a conceptual review. Early Interv Psychiatry. 2009;3(1):10-18.
8. Masand PS, Levenson JL, et al. Mania, catatonia, and psychosis. In: Levenson JL, ed. The American Psychiatric Publishing textbook of psychosomatic medicine. Washington, DC: American Psychiatric Publishing; 2005: 239-241.
9. Carroll BT. The universal field of hypothesis of catatonia and neuroleptic malignant syndrome. CNS Spectr. 2000;5(7):26-33.
10. Lee JW. Neuroleptic-induced catatonia: clinical presentation, response to benzodiazepines, and relationship to neuroleptic malignant syndrome. J Clin Psychopharmacol. 2010;30(1):3-10.
11. Vancaester E, Santens P. Catatonia and neuroleptic malignant syndrome: two sides of a coin? Acta Neurol Belg. 2007;107(2):47-50.
12. Sienaert P, Dhossche DM, Vancampfort D, et al. A clinical review of the treatment of catatonia. Front Psychiatry. 2014;5:181.
13. Hatta K, Miyakawa K, Ota T, et al. Maximal response to electroconvulsive therapy for the treatment of catatonic symptoms. J ECT. 2007;23(4):233-235.
14. Payee H, Chandrasekaran R, Raju GV. Catatonic syndrome: treatment response to Lorazepam. Indian J Psychiatry. 1999;41(1):49-53.
15. Rohland BM, Carroll BT, Jacoby RG. ECT in the treatment of the catatonic syndrome. J Affect Disord. 1993;29(4):255-261.
3,4-methylenedioxy-methamphetamine, MDMA, Phenethylamines I Have Known and Loved: A Chemical Love Story, LSD, substance abuse, substance use
CASE Rigid, frightened, and mute
Mr. D, age 23, presents for evaluation immediately after discharge from another hospital, where he had been treated for altered mental status.
Ten days earlier, Mr. D’s friends obtained 2C-B (2,5-dimethoxy-4-bromophenethylamine), from the “Darknet,” an underground niche of the Internet. He ingested 20 mg of 2C-B in powder form. Although his friends recovered from a “safe trip,” Mr. D decompensated rapidly over the next few days with persistent psychosis, experiencing both auditory and visual hallucinations. He is “acting strange“ at work, and trying to find “hidden codes” in data. Mr. D also has persistent thought disorganization. He speaks of “connections” between people and things, and says that he is an alien in a spaceship. His friends and family report that he is talking rapidly and is sleeping only 2 or 3 hours each night. Mr. D abruptly quit his job as an analyst a few days after taking the drug.
Mr. D is a single, Ivy League-educated man and is described as hardworking and analytical. His family denies any recent mood changes or life stressors. They report that 1 month ago, Mr. D began smoking marijuana daily. He has no significant medical or psychiatric history, and no family history of psychiatric disorders.
What is your most likely diagnosis for Mr. D?
a) delirium due to a general medical condition
b) substance-induced psychotic disorder
c) catatonia due to a general medical condition
d) schizophrenia
e) bipolar I disorder, currently manic, with psychosis
The authors’ observations
Ring-substituted phenethylamines, commonly known as 2Cs, are designer drugs that are emerging as new substances of abuse.1 2C-B belongs to the phenethylamine subclass of monoamine alkaloids that includes more familiar drugs such as amphetamines, methamphetamines, and 3,4-methylenedioxy-methamphetamine (MDMA).2 It was first synthesized in 1974 by Alexander Shulgin, later described in his book Phenethylamines I Have Known and Loved: A Chemical Love Story, and its hallucinogenic activity is reported to be similar to LSD, mescaline, and psilocybin.3 The literature is scant on the acute effects of 2C intoxication or long-term sequelae of 2C ingestion.1 Most available information regarding the pharmacology of 2C-B comes from users who have reported their drug experiences on blogs, Web sites and forums, and in the media.4
2C-B usually is taken orally in powder or tablet form, in a dose of 10 to 50 mg.4 After an onset period of 20 to 90 minutes, the drug’s effect reaches maximum effect in 15 to 30 minutes, then plateaus for 2 to 7 hours, and comes down within 1 to 2 hours.4 2C-B is known to be orally active, and its hallucinogenic effects are mediated by its actions as a partial serotonin 5HT-2A and 5HT-2C receptor agonist.5 Entactogenic-stimulating effects have been reported at low doses (4 to 10 mg), whereas visual hallucinations with intense colors and object distortion have been reported at moderate doses (10 to 20 mg).4
2C-B, which users often take at parties or raves, appeared on the drug market in the mid 1980s and early 1990s under the names Nexus, Erox, Performax, Toonies, Bromo, Spectrum, and Venus and marketed as a replacement for MDMA after it became a Schedule I drug in the United States.4,6 Some users consume 2C-B in combination with other illicit drugs, including MDMA (called a “party pack”) or LSD (referred to as a “banana split”).6
According to the U.S. Drug Enforcement Agency, law enforcement authorities first seized 2C-B laboratories in California in 1986 and Arizona in 1992.6 Distribution of the drug has been sporadic since it became Schedule I in 1995, and it has been seized from several states, including Virginia, Nevada, Maine, Illinois, Missouri, South Dakota, and Kansas.6
EXAMINATION Passive and mute
On examination, Mr. D is lying in bed with eyes closed and extremities extended in an odd, rigid posture. He is resistant to attempts at passive movement, is nonresponsive to verbal commands, and is mute. A review of vital signs shows tachycardia, 110 beats per minute, but the physical exam is otherwise unremarkable. His Bush-Francis Catatonia Rating Scale (BFCRS) score is 17, indicating a diagnosis of catatonia. Mini-Mental Status Examination cannot be completed because Mr. D is unable to participate.
Laboratory studies reveal an elevated creatinine kinase (CK) level of 356 U/L. Results of a complete blood count, comprehensive metabolic panel, urinalysis, and thyroid-stimulating hormone are normal. Blood alcohol level is <10 mg/dL. Acetaminophen and salicylate levels are normal (<5 mg/dL). Records from his recent hospitalization reveal normal head CT, chest radiography, EEG, and urinalysis, and a negative urine drug screen.
What is the next step in managing Mr. D’s catatonic symptoms?
a) IV normal saline
b) IV lorazepam
c) emergent electroconvulsive therapy (ECT)
d) IM haloperidol
e) IM olanzapine
TREATMENT Saline and psychotropics
While in the emergency room, Mr. D receives 2 L of IV saline. His CK level falls to 137 U/L. A challenge with IV lorazepam, 2 mg, also is performed. Mr. D becomes talkative and follows commands with fluid movements, but his disorganized, delusional thoughts persist. BFCRS score has improved to 9 (Table 1). He is admitted to the psychiatric unit and started on oral lorazepam, 2 mg, 3 times daily, for catatonia, and olanzapine, 10 mg/d, for psychosis.
The differential diagnosis for Mr. D’s psychosis includes substance-induced psychotic disorder, schizophrenia, bipolar disorder, and psychosis with another organic cause (Table 2).7 Further medical workup is completed, including a urine drug screen, testing for HIV, hepatitis B, syphilis, lead and heavy metals, ceruloplasmin, vitamin B12, folate, antinuclear antibody, sedimentation rate, and brain MRI. Cannabinoids are detected in his urine drug screen. Another urine sample is sent to an outside lab to test for several synthetic drugs, including MDMA, 3,4-methylenedioxy- N-ethyl-amphetamine, 2C-B, 2C-C, 2C-I, and 2C-P, results of which also are negative.
By the second day of hospitalization, Mr. D appears less disorganized but continues to complain of “scrambled thoughts” and appears guarded. Despite initial response to IV lorazepam and its continuation in oral form, over the next day Mr. D appears more psychomotor-slowed, with motor stiffness. His score on the BFCRS increases, with significant posturing; vital signs remain stable, however.
What is your next step in managing his catatonic symptoms?
a) increase olanzapine
b) decrease olanzapine
c) decrease lorazepam
d) emergent ECT
e) switch to haloperidol
The authors’ observations
Although catatonia can be associated with a mood or psychotic disorder, it also can be induced by a medication or general medical condition (Table 3).8 It is thought that catatonia is associated with decreased γ-aminobutyric acid (GABA) and dopamine D2 receptor activity, and increased N-methyl-d-aspartate (NMDA) receptor activity.9 Antipsychotics could worsen catatonia through D2 blockade. Benzodiazepines, however, improve catatonia by increasing GABA and decreasing NMDA receptor activity. In this case, Mr. D was naïve to antipsychotics and seemed to be sensitive to them, as evidenced by his worsening symptoms.
Which condition should be considered in the differential diagnosis?
a) parkinsonian-hyperpyrexia syndrome
b) neuroleptic malignant syndrome (NMS)
c) stiff person syndrome
d) serotonin syndrome
e) CNS infection
The authors’ observations
NMS, catatonia, and parkinsonian-hyperpyrexia syndrome are all related to diminished action of dopamine at the D2 receptor. Although the mechanism of catatonia is not completely understood, NMS is thought to be caused by blockade at the D2 receptors by antipsychotics, whereas parkinsonian-hyperpyrexia syndrome is related to withdrawal of dopamine agonists. Because of the similarity in symptoms and proposed mechanisms, some experts hypothesize that NMS is a drug-induced malignant catatonia.10,11 Interestingly, NMS and catatonia respond to withdrawal of antipsychotics, and addition of benzodiazepines and ECT.
Mr. D showed posturing and other behavioral abnormalities, which are less common in NMS. Furthermore, although he had episodes of mild tachycardia, autonomic dysregulation—a hallmark of NMS—was not found. Given the common shared deficiency of activity at the D2 receptor in both NMS and catatonia, antipsychotics could cause or worsen either condition.
TREATMENT ECT
Mr. D’s olanzapine dosage is decreased to 2.5 mg/d. His catatonic symptoms improve with each dosage of oral lorazepam; however, effects seem to lessen and last for shorter periods over the following day. Additionally, Mr. D again becomes more disorganized, stiff, and unable to feed or bathe himself, and develops episodes of mild tachycardia.
Given Mr. D’s partial and poorly sustained response to lorazepam, a trial of ECT is pursued. On the third day of hospitalization, he receives ECT with bi-frontal lead placement at 25% energy. Concurrently, olanzapine is discontinued because of worsening muscle stiffness and concern about neuroleptic sensitivity. His BFCRS score after ECT is 2, and he is noted to be more interactive on the inpatient unit. He continues to receive ECT 3 times a week, with notable improvement, but ongoing psychotic symptoms and catatonic symptoms partially reemerge between ECT treatments. Lead placement is changed to bi-temporal by the third treatment, and the energy setting is increased from 25% to 50%, and to 75% by the sixth treatment. An additional nighttime dose of oral lorazepam, 2 mg, is added after the sixth treatment, in an attempt to reduce “wearing off” by morning.
After the seventh treatment, Mr. D is able to maintain logical conversation without re-emergence of catatonic symptoms over 2 days, signifying a turning point in the treatment course. The ECT energy setting is decreased to 50% to minimize potential memory deficits. His insight into his illness and treatment dramatically improve over the next few days. ECT is discontinued after the tenth treatment and Mr. D is discharged home to the care of his family.
The authors’ observations
Randomized clinical trials studying the effectiveness of ECT for catatonia are limited. Much of what we know about ECT comes from case reports that describe excellent outcomes for a variety of treatment-resistant illnesses, including catatonia in mood disorders, schizophrenia, autism, and other organic brain disease.12
Although benzodiazepines often are the first-line treatment for catatonia caused by any underlying illness, one study showed only 1 of 41 patients achieved remission with benzodiazepines, compared with 100% of those treated with ECT13; another study supported these results with 8 of 9 lorazepam non-responders responding to ECT.14 There are few case reports of substance-induced catatonia in the absence of other chronic mental illness, although none report use of ECT. However, a study showed no significant difference in the effectiveness of ECT for catatonia caused by an affective disorder or schizophrenia.15
Mr. D’s case exemplifies complete remission of catatonia induced by a psychoactive substance.
OUTCOME Steady improvement
Mr. D is followed in the outpatient clinic for 1 month after discharge; lorazepam is tapered successfully. During this time frame, psychotic and catatonic symptoms do not re-emerge. He reports some initial working memory deficits that improve steadily. There is no evidence of any significant psychiatric signs or symptoms, including neurovegetative symptoms of depression, mania or hypomania, perceptual disturbances, or disorganized thoughts or behaviors. He remains abstinent from alcohol, tobacco, and all psychoactive substances.
Bottom Line
Persistent psychosis and catatonia after the use of newer designer drugs such as 2C-B are rare, but these drugs carry serious potential complications that clinicians should be aware of. Benzodiazepines and electroconvulsive therapy have been proved effective for catatonia that is related to a number of psychiatric illnesses, often resulting in good outcomes. However, current evidence on their use is limited, particularly regarding treatment of substance-induced psychosis and catatonia.
Related Resources
• Meyer MR, Maurer HH. Metabolism of designer drugs of abuse: an updated review. Curr Drug Metab. 2010;11(5):468-482.
• Rickli A, Luethi D, Reinisch J, et al. Receptor interaction profiles of novel N-2-methoxybenzyl (NBOMe) derivatives of 2,5-dimethoxy-substituted phenethylamines (2C drugs). Neuropharmacology. 2015;99:546-553.
Drug Brand Names
Haloperidol • Haldol
Lorazepam • Ativan
Olanzapine • Zyprexa
Disclosures
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
CASE Rigid, frightened, and mute
Mr. D, age 23, presents for evaluation immediately after discharge from another hospital, where he had been treated for altered mental status.
Ten days earlier, Mr. D’s friends obtained 2C-B (2,5-dimethoxy-4-bromophenethylamine), from the “Darknet,” an underground niche of the Internet. He ingested 20 mg of 2C-B in powder form. Although his friends recovered from a “safe trip,” Mr. D decompensated rapidly over the next few days with persistent psychosis, experiencing both auditory and visual hallucinations. He is “acting strange“ at work, and trying to find “hidden codes” in data. Mr. D also has persistent thought disorganization. He speaks of “connections” between people and things, and says that he is an alien in a spaceship. His friends and family report that he is talking rapidly and is sleeping only 2 or 3 hours each night. Mr. D abruptly quit his job as an analyst a few days after taking the drug.
Mr. D is a single, Ivy League-educated man and is described as hardworking and analytical. His family denies any recent mood changes or life stressors. They report that 1 month ago, Mr. D began smoking marijuana daily. He has no significant medical or psychiatric history, and no family history of psychiatric disorders.
What is your most likely diagnosis for Mr. D?
a) delirium due to a general medical condition
b) substance-induced psychotic disorder
c) catatonia due to a general medical condition
d) schizophrenia
e) bipolar I disorder, currently manic, with psychosis
The authors’ observations
Ring-substituted phenethylamines, commonly known as 2Cs, are designer drugs that are emerging as new substances of abuse.1 2C-B belongs to the phenethylamine subclass of monoamine alkaloids that includes more familiar drugs such as amphetamines, methamphetamines, and 3,4-methylenedioxy-methamphetamine (MDMA).2 It was first synthesized in 1974 by Alexander Shulgin, later described in his book Phenethylamines I Have Known and Loved: A Chemical Love Story, and its hallucinogenic activity is reported to be similar to LSD, mescaline, and psilocybin.3 The literature is scant on the acute effects of 2C intoxication or long-term sequelae of 2C ingestion.1 Most available information regarding the pharmacology of 2C-B comes from users who have reported their drug experiences on blogs, Web sites and forums, and in the media.4
2C-B usually is taken orally in powder or tablet form, in a dose of 10 to 50 mg.4 After an onset period of 20 to 90 minutes, the drug’s effect reaches maximum effect in 15 to 30 minutes, then plateaus for 2 to 7 hours, and comes down within 1 to 2 hours.4 2C-B is known to be orally active, and its hallucinogenic effects are mediated by its actions as a partial serotonin 5HT-2A and 5HT-2C receptor agonist.5 Entactogenic-stimulating effects have been reported at low doses (4 to 10 mg), whereas visual hallucinations with intense colors and object distortion have been reported at moderate doses (10 to 20 mg).4
2C-B, which users often take at parties or raves, appeared on the drug market in the mid 1980s and early 1990s under the names Nexus, Erox, Performax, Toonies, Bromo, Spectrum, and Venus and marketed as a replacement for MDMA after it became a Schedule I drug in the United States.4,6 Some users consume 2C-B in combination with other illicit drugs, including MDMA (called a “party pack”) or LSD (referred to as a “banana split”).6
According to the U.S. Drug Enforcement Agency, law enforcement authorities first seized 2C-B laboratories in California in 1986 and Arizona in 1992.6 Distribution of the drug has been sporadic since it became Schedule I in 1995, and it has been seized from several states, including Virginia, Nevada, Maine, Illinois, Missouri, South Dakota, and Kansas.6
EXAMINATION Passive and mute
On examination, Mr. D is lying in bed with eyes closed and extremities extended in an odd, rigid posture. He is resistant to attempts at passive movement, is nonresponsive to verbal commands, and is mute. A review of vital signs shows tachycardia, 110 beats per minute, but the physical exam is otherwise unremarkable. His Bush-Francis Catatonia Rating Scale (BFCRS) score is 17, indicating a diagnosis of catatonia. Mini-Mental Status Examination cannot be completed because Mr. D is unable to participate.
Laboratory studies reveal an elevated creatinine kinase (CK) level of 356 U/L. Results of a complete blood count, comprehensive metabolic panel, urinalysis, and thyroid-stimulating hormone are normal. Blood alcohol level is <10 mg/dL. Acetaminophen and salicylate levels are normal (<5 mg/dL). Records from his recent hospitalization reveal normal head CT, chest radiography, EEG, and urinalysis, and a negative urine drug screen.
What is the next step in managing Mr. D’s catatonic symptoms?
a) IV normal saline
b) IV lorazepam
c) emergent electroconvulsive therapy (ECT)
d) IM haloperidol
e) IM olanzapine
TREATMENT Saline and psychotropics
While in the emergency room, Mr. D receives 2 L of IV saline. His CK level falls to 137 U/L. A challenge with IV lorazepam, 2 mg, also is performed. Mr. D becomes talkative and follows commands with fluid movements, but his disorganized, delusional thoughts persist. BFCRS score has improved to 9 (Table 1). He is admitted to the psychiatric unit and started on oral lorazepam, 2 mg, 3 times daily, for catatonia, and olanzapine, 10 mg/d, for psychosis.
The differential diagnosis for Mr. D’s psychosis includes substance-induced psychotic disorder, schizophrenia, bipolar disorder, and psychosis with another organic cause (Table 2).7 Further medical workup is completed, including a urine drug screen, testing for HIV, hepatitis B, syphilis, lead and heavy metals, ceruloplasmin, vitamin B12, folate, antinuclear antibody, sedimentation rate, and brain MRI. Cannabinoids are detected in his urine drug screen. Another urine sample is sent to an outside lab to test for several synthetic drugs, including MDMA, 3,4-methylenedioxy- N-ethyl-amphetamine, 2C-B, 2C-C, 2C-I, and 2C-P, results of which also are negative.
By the second day of hospitalization, Mr. D appears less disorganized but continues to complain of “scrambled thoughts” and appears guarded. Despite initial response to IV lorazepam and its continuation in oral form, over the next day Mr. D appears more psychomotor-slowed, with motor stiffness. His score on the BFCRS increases, with significant posturing; vital signs remain stable, however.
What is your next step in managing his catatonic symptoms?
a) increase olanzapine
b) decrease olanzapine
c) decrease lorazepam
d) emergent ECT
e) switch to haloperidol
The authors’ observations
Although catatonia can be associated with a mood or psychotic disorder, it also can be induced by a medication or general medical condition (Table 3).8 It is thought that catatonia is associated with decreased γ-aminobutyric acid (GABA) and dopamine D2 receptor activity, and increased N-methyl-d-aspartate (NMDA) receptor activity.9 Antipsychotics could worsen catatonia through D2 blockade. Benzodiazepines, however, improve catatonia by increasing GABA and decreasing NMDA receptor activity. In this case, Mr. D was naïve to antipsychotics and seemed to be sensitive to them, as evidenced by his worsening symptoms.
Which condition should be considered in the differential diagnosis?
a) parkinsonian-hyperpyrexia syndrome
b) neuroleptic malignant syndrome (NMS)
c) stiff person syndrome
d) serotonin syndrome
e) CNS infection
The authors’ observations
NMS, catatonia, and parkinsonian-hyperpyrexia syndrome are all related to diminished action of dopamine at the D2 receptor. Although the mechanism of catatonia is not completely understood, NMS is thought to be caused by blockade at the D2 receptors by antipsychotics, whereas parkinsonian-hyperpyrexia syndrome is related to withdrawal of dopamine agonists. Because of the similarity in symptoms and proposed mechanisms, some experts hypothesize that NMS is a drug-induced malignant catatonia.10,11 Interestingly, NMS and catatonia respond to withdrawal of antipsychotics, and addition of benzodiazepines and ECT.
Mr. D showed posturing and other behavioral abnormalities, which are less common in NMS. Furthermore, although he had episodes of mild tachycardia, autonomic dysregulation—a hallmark of NMS—was not found. Given the common shared deficiency of activity at the D2 receptor in both NMS and catatonia, antipsychotics could cause or worsen either condition.
TREATMENT ECT
Mr. D’s olanzapine dosage is decreased to 2.5 mg/d. His catatonic symptoms improve with each dosage of oral lorazepam; however, effects seem to lessen and last for shorter periods over the following day. Additionally, Mr. D again becomes more disorganized, stiff, and unable to feed or bathe himself, and develops episodes of mild tachycardia.
Given Mr. D’s partial and poorly sustained response to lorazepam, a trial of ECT is pursued. On the third day of hospitalization, he receives ECT with bi-frontal lead placement at 25% energy. Concurrently, olanzapine is discontinued because of worsening muscle stiffness and concern about neuroleptic sensitivity. His BFCRS score after ECT is 2, and he is noted to be more interactive on the inpatient unit. He continues to receive ECT 3 times a week, with notable improvement, but ongoing psychotic symptoms and catatonic symptoms partially reemerge between ECT treatments. Lead placement is changed to bi-temporal by the third treatment, and the energy setting is increased from 25% to 50%, and to 75% by the sixth treatment. An additional nighttime dose of oral lorazepam, 2 mg, is added after the sixth treatment, in an attempt to reduce “wearing off” by morning.
After the seventh treatment, Mr. D is able to maintain logical conversation without re-emergence of catatonic symptoms over 2 days, signifying a turning point in the treatment course. The ECT energy setting is decreased to 50% to minimize potential memory deficits. His insight into his illness and treatment dramatically improve over the next few days. ECT is discontinued after the tenth treatment and Mr. D is discharged home to the care of his family.
The authors’ observations
Randomized clinical trials studying the effectiveness of ECT for catatonia are limited. Much of what we know about ECT comes from case reports that describe excellent outcomes for a variety of treatment-resistant illnesses, including catatonia in mood disorders, schizophrenia, autism, and other organic brain disease.12
Although benzodiazepines often are the first-line treatment for catatonia caused by any underlying illness, one study showed only 1 of 41 patients achieved remission with benzodiazepines, compared with 100% of those treated with ECT13; another study supported these results with 8 of 9 lorazepam non-responders responding to ECT.14 There are few case reports of substance-induced catatonia in the absence of other chronic mental illness, although none report use of ECT. However, a study showed no significant difference in the effectiveness of ECT for catatonia caused by an affective disorder or schizophrenia.15
Mr. D’s case exemplifies complete remission of catatonia induced by a psychoactive substance.
OUTCOME Steady improvement
Mr. D is followed in the outpatient clinic for 1 month after discharge; lorazepam is tapered successfully. During this time frame, psychotic and catatonic symptoms do not re-emerge. He reports some initial working memory deficits that improve steadily. There is no evidence of any significant psychiatric signs or symptoms, including neurovegetative symptoms of depression, mania or hypomania, perceptual disturbances, or disorganized thoughts or behaviors. He remains abstinent from alcohol, tobacco, and all psychoactive substances.
Bottom Line
Persistent psychosis and catatonia after the use of newer designer drugs such as 2C-B are rare, but these drugs carry serious potential complications that clinicians should be aware of. Benzodiazepines and electroconvulsive therapy have been proved effective for catatonia that is related to a number of psychiatric illnesses, often resulting in good outcomes. However, current evidence on their use is limited, particularly regarding treatment of substance-induced psychosis and catatonia.
Related Resources
• Meyer MR, Maurer HH. Metabolism of designer drugs of abuse: an updated review. Curr Drug Metab. 2010;11(5):468-482.
• Rickli A, Luethi D, Reinisch J, et al. Receptor interaction profiles of novel N-2-methoxybenzyl (NBOMe) derivatives of 2,5-dimethoxy-substituted phenethylamines (2C drugs). Neuropharmacology. 2015;99:546-553.
Drug Brand Names
Haloperidol • Haldol
Lorazepam • Ativan
Olanzapine • Zyprexa
Disclosures
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Dean BV, Stellpflug SJ, Burnett AM, et al. 2C or not 2C: phenethylamine designer drug review. J Med Toxicol. 2013;9(2):172-178.
2. Hill SL, Thomas SH. Clinical toxicology of newer recreational drugs. Clin Toxicol (Phila). 2011;49(8):705-719.
3. Shulgin A, Shulgin A. PiHKAL: a chemical love story. Berkley, CA: Transform Press; 1991.
4. Papoutsis I, Nikolaou P, Stefanidou M, et al. 25B-NBOMe and its precursor 2C-B: modern trends and hidden dangers. Forensic Toxicology. 2015;3(1):1-11.
5. Caudevilla-Gálligo F, Riba J, Ventura M, et al. 4-Bromo-2, 5-dimethoxyphenethylamine (2C-B): presence in the recreational drug market in Spain, pattern of use and subjective effects. J Psychopharmacol. 2012;26(7):1026-1035.
6. National Drug Intelligence Center. Information bulletin: 2C-B (Nexus) reappears on the club drug scene. http:// www.Justice.gov/archive/ndic/pubs0/665. Published May 2001. Accessed June 12, 2015.
7. Freudenreich O, Schulz SC, Goff DC. Initial medical work-up of first episode psychosis: a conceptual review. Early Interv Psychiatry. 2009;3(1):10-18.
8. Masand PS, Levenson JL, et al. Mania, catatonia, and psychosis. In: Levenson JL, ed. The American Psychiatric Publishing textbook of psychosomatic medicine. Washington, DC: American Psychiatric Publishing; 2005: 239-241.
9. Carroll BT. The universal field of hypothesis of catatonia and neuroleptic malignant syndrome. CNS Spectr. 2000;5(7):26-33.
10. Lee JW. Neuroleptic-induced catatonia: clinical presentation, response to benzodiazepines, and relationship to neuroleptic malignant syndrome. J Clin Psychopharmacol. 2010;30(1):3-10.
11. Vancaester E, Santens P. Catatonia and neuroleptic malignant syndrome: two sides of a coin? Acta Neurol Belg. 2007;107(2):47-50.
12. Sienaert P, Dhossche DM, Vancampfort D, et al. A clinical review of the treatment of catatonia. Front Psychiatry. 2014;5:181.
13. Hatta K, Miyakawa K, Ota T, et al. Maximal response to electroconvulsive therapy for the treatment of catatonic symptoms. J ECT. 2007;23(4):233-235.
14. Payee H, Chandrasekaran R, Raju GV. Catatonic syndrome: treatment response to Lorazepam. Indian J Psychiatry. 1999;41(1):49-53.
15. Rohland BM, Carroll BT, Jacoby RG. ECT in the treatment of the catatonic syndrome. J Affect Disord. 1993;29(4):255-261.
1. Dean BV, Stellpflug SJ, Burnett AM, et al. 2C or not 2C: phenethylamine designer drug review. J Med Toxicol. 2013;9(2):172-178.
2. Hill SL, Thomas SH. Clinical toxicology of newer recreational drugs. Clin Toxicol (Phila). 2011;49(8):705-719.
3. Shulgin A, Shulgin A. PiHKAL: a chemical love story. Berkley, CA: Transform Press; 1991.
4. Papoutsis I, Nikolaou P, Stefanidou M, et al. 25B-NBOMe and its precursor 2C-B: modern trends and hidden dangers. Forensic Toxicology. 2015;3(1):1-11.
5. Caudevilla-Gálligo F, Riba J, Ventura M, et al. 4-Bromo-2, 5-dimethoxyphenethylamine (2C-B): presence in the recreational drug market in Spain, pattern of use and subjective effects. J Psychopharmacol. 2012;26(7):1026-1035.
6. National Drug Intelligence Center. Information bulletin: 2C-B (Nexus) reappears on the club drug scene. http:// www.Justice.gov/archive/ndic/pubs0/665. Published May 2001. Accessed June 12, 2015.
7. Freudenreich O, Schulz SC, Goff DC. Initial medical work-up of first episode psychosis: a conceptual review. Early Interv Psychiatry. 2009;3(1):10-18.
8. Masand PS, Levenson JL, et al. Mania, catatonia, and psychosis. In: Levenson JL, ed. The American Psychiatric Publishing textbook of psychosomatic medicine. Washington, DC: American Psychiatric Publishing; 2005: 239-241.
9. Carroll BT. The universal field of hypothesis of catatonia and neuroleptic malignant syndrome. CNS Spectr. 2000;5(7):26-33.
10. Lee JW. Neuroleptic-induced catatonia: clinical presentation, response to benzodiazepines, and relationship to neuroleptic malignant syndrome. J Clin Psychopharmacol. 2010;30(1):3-10.
11. Vancaester E, Santens P. Catatonia and neuroleptic malignant syndrome: two sides of a coin? Acta Neurol Belg. 2007;107(2):47-50.
12. Sienaert P, Dhossche DM, Vancampfort D, et al. A clinical review of the treatment of catatonia. Front Psychiatry. 2014;5:181.
13. Hatta K, Miyakawa K, Ota T, et al. Maximal response to electroconvulsive therapy for the treatment of catatonic symptoms. J ECT. 2007;23(4):233-235.
14. Payee H, Chandrasekaran R, Raju GV. Catatonic syndrome: treatment response to Lorazepam. Indian J Psychiatry. 1999;41(1):49-53.
15. Rohland BM, Carroll BT, Jacoby RG. ECT in the treatment of the catatonic syndrome. J Affect Disord. 1993;29(4):255-261.
3,4-methylenedioxy-methamphetamine, MDMA, Phenethylamines I Have Known and Loved: A Chemical Love Story, LSD, substance abuse, substance use
3,4-methylenedioxy-methamphetamine, MDMA, Phenethylamines I Have Known and Loved: A Chemical Love Story, LSD, substance abuse, substance use
A Streak of Trouble in Fingernail?
A 14-year-old girl is brought to dermatology by her mother, following referral from her pediatrician for evaluation of fingernail changes. Initially, a faint brown streak was observed in the nail of her left fourth finger. Over time, the spot darkened and widened, and the adjacent nail plate flattened.
A few weeks before presentation to dermatology, the adjacent cuticle darkened. The patient denies any sensitivity in the area. Her mother denies any significant or relevant medical history.
EXAMINATION
The dark brown streak measures 4.5 mm in width and runs longitudinally the entire length of the nail plate. The discolored area coincides exactly with flattening of the nail plate, which, when viewed on edge, also exhibits darkening. Significantly, the adjacent cuticle is similarly affected.
No changes are observed on other digits. The child is of Native American ancestry, with type IV skin.
What is the diagnosis?
DISCUSSION
Generically, this problem is termed melanonychia; this case represents the most common type, longitudinal melanonychia (LM). Typically, it originates in the nail matrix with increased melanin production; this causes darkening of the onchocytes, which migrate distally as the nail plate grows.
This benign version is an extremely common problem, especially in darker-skinned patients. Almost 100% of African-Americans older than 50 have it, often in several nails, as do 77% of African Americans older than 20 and 10% to 20% of Japanese persons. Comparatively, only 1% to 2% of white individuals are affected.
The main significance of LM, of course, is the fact that it can represent melanoma, which, for a variety of reasons, is often belatedly identified in subungual areas and is therefore associated with relatively poor survival. Five-year survival rates for subungual melanoma are around 30%, while 10-year survival is only 13%—much lower than for melanomas elsewhere on the body. For example, patients with melanomas removed from other areas of the hand demonstrate 100% five-year survival rates.
Subungual melanoma presenting in this manner is considered one of the so-called acrolentiginous melanomas, which present in peripheral “acral” locations (eg, scalp, soles, mucosal surfaces). While these are not inherently more dangerous in terms of biologic behavior, they tend to escape detection because of their location and (often) atypical appearance, thus having more time in which to become invasive.
Perhaps the most useful and puzzling conundrum associated with acrolentiginous melanomas is this: Darker-skinned patients are, in general, at low risk for melanoma compared to fair-skinned redheads and blondes. However, when dark-skinned patients do develop melanoma, it tends to manifest in an acral area (one reason why melanoma in these patients has a relatively poor prognosis).
Darkening under or in the nails can have other causes; it has been associated with Cushing disease, Addison disease, and alkaptonuria, to name just a few. It can also be associated with skin diseases, including psoriasis, Darier disease, lichen planus, and lichen striatus. A number of drugs, among them minocycline, can produce focal discoloration in or under the nails.
This subungual lesion was biopsied at its proximal origin, under digital block and using a tourniquet. The 3-mm punch specimen was sent for pathologic examination, which showed benign nevoid tissue—effectively ruling out melanoma. Even if the nail is permanently deformed, this was considered a small price to pay for the patient’s peace of mind.
TAKE-HOME LEARNING POINTS
• Dark streaks under fingernails are common in those with darker skin, in whom they often appear in multiple fingers. In the absence of change, these are usually safe.
• The related conundrum is that when those with darker skin develop melanoma, the subungual areas, or other light-skinned areas (sole, mouth, palms), are often where it manifests.
• New and/or changing areas of subungual pigmentation need to be referred to dermatology for evaluation and possible biopsy.
• When this discoloration also involves the adjacent cuticle or other perionychial skin, even more urgency is added to the referral.
A 14-year-old girl is brought to dermatology by her mother, following referral from her pediatrician for evaluation of fingernail changes. Initially, a faint brown streak was observed in the nail of her left fourth finger. Over time, the spot darkened and widened, and the adjacent nail plate flattened.
A few weeks before presentation to dermatology, the adjacent cuticle darkened. The patient denies any sensitivity in the area. Her mother denies any significant or relevant medical history.
EXAMINATION
The dark brown streak measures 4.5 mm in width and runs longitudinally the entire length of the nail plate. The discolored area coincides exactly with flattening of the nail plate, which, when viewed on edge, also exhibits darkening. Significantly, the adjacent cuticle is similarly affected.
No changes are observed on other digits. The child is of Native American ancestry, with type IV skin.
What is the diagnosis?
DISCUSSION
Generically, this problem is termed melanonychia; this case represents the most common type, longitudinal melanonychia (LM). Typically, it originates in the nail matrix with increased melanin production; this causes darkening of the onchocytes, which migrate distally as the nail plate grows.
This benign version is an extremely common problem, especially in darker-skinned patients. Almost 100% of African-Americans older than 50 have it, often in several nails, as do 77% of African Americans older than 20 and 10% to 20% of Japanese persons. Comparatively, only 1% to 2% of white individuals are affected.
The main significance of LM, of course, is the fact that it can represent melanoma, which, for a variety of reasons, is often belatedly identified in subungual areas and is therefore associated with relatively poor survival. Five-year survival rates for subungual melanoma are around 30%, while 10-year survival is only 13%—much lower than for melanomas elsewhere on the body. For example, patients with melanomas removed from other areas of the hand demonstrate 100% five-year survival rates.
Subungual melanoma presenting in this manner is considered one of the so-called acrolentiginous melanomas, which present in peripheral “acral” locations (eg, scalp, soles, mucosal surfaces). While these are not inherently more dangerous in terms of biologic behavior, they tend to escape detection because of their location and (often) atypical appearance, thus having more time in which to become invasive.
Perhaps the most useful and puzzling conundrum associated with acrolentiginous melanomas is this: Darker-skinned patients are, in general, at low risk for melanoma compared to fair-skinned redheads and blondes. However, when dark-skinned patients do develop melanoma, it tends to manifest in an acral area (one reason why melanoma in these patients has a relatively poor prognosis).
Darkening under or in the nails can have other causes; it has been associated with Cushing disease, Addison disease, and alkaptonuria, to name just a few. It can also be associated with skin diseases, including psoriasis, Darier disease, lichen planus, and lichen striatus. A number of drugs, among them minocycline, can produce focal discoloration in or under the nails.
This subungual lesion was biopsied at its proximal origin, under digital block and using a tourniquet. The 3-mm punch specimen was sent for pathologic examination, which showed benign nevoid tissue—effectively ruling out melanoma. Even if the nail is permanently deformed, this was considered a small price to pay for the patient’s peace of mind.
TAKE-HOME LEARNING POINTS
• Dark streaks under fingernails are common in those with darker skin, in whom they often appear in multiple fingers. In the absence of change, these are usually safe.
• The related conundrum is that when those with darker skin develop melanoma, the subungual areas, or other light-skinned areas (sole, mouth, palms), are often where it manifests.
• New and/or changing areas of subungual pigmentation need to be referred to dermatology for evaluation and possible biopsy.
• When this discoloration also involves the adjacent cuticle or other perionychial skin, even more urgency is added to the referral.
A 14-year-old girl is brought to dermatology by her mother, following referral from her pediatrician for evaluation of fingernail changes. Initially, a faint brown streak was observed in the nail of her left fourth finger. Over time, the spot darkened and widened, and the adjacent nail plate flattened.
A few weeks before presentation to dermatology, the adjacent cuticle darkened. The patient denies any sensitivity in the area. Her mother denies any significant or relevant medical history.
EXAMINATION
The dark brown streak measures 4.5 mm in width and runs longitudinally the entire length of the nail plate. The discolored area coincides exactly with flattening of the nail plate, which, when viewed on edge, also exhibits darkening. Significantly, the adjacent cuticle is similarly affected.
No changes are observed on other digits. The child is of Native American ancestry, with type IV skin.
What is the diagnosis?
DISCUSSION
Generically, this problem is termed melanonychia; this case represents the most common type, longitudinal melanonychia (LM). Typically, it originates in the nail matrix with increased melanin production; this causes darkening of the onchocytes, which migrate distally as the nail plate grows.
This benign version is an extremely common problem, especially in darker-skinned patients. Almost 100% of African-Americans older than 50 have it, often in several nails, as do 77% of African Americans older than 20 and 10% to 20% of Japanese persons. Comparatively, only 1% to 2% of white individuals are affected.
The main significance of LM, of course, is the fact that it can represent melanoma, which, for a variety of reasons, is often belatedly identified in subungual areas and is therefore associated with relatively poor survival. Five-year survival rates for subungual melanoma are around 30%, while 10-year survival is only 13%—much lower than for melanomas elsewhere on the body. For example, patients with melanomas removed from other areas of the hand demonstrate 100% five-year survival rates.
Subungual melanoma presenting in this manner is considered one of the so-called acrolentiginous melanomas, which present in peripheral “acral” locations (eg, scalp, soles, mucosal surfaces). While these are not inherently more dangerous in terms of biologic behavior, they tend to escape detection because of their location and (often) atypical appearance, thus having more time in which to become invasive.
Perhaps the most useful and puzzling conundrum associated with acrolentiginous melanomas is this: Darker-skinned patients are, in general, at low risk for melanoma compared to fair-skinned redheads and blondes. However, when dark-skinned patients do develop melanoma, it tends to manifest in an acral area (one reason why melanoma in these patients has a relatively poor prognosis).
Darkening under or in the nails can have other causes; it has been associated with Cushing disease, Addison disease, and alkaptonuria, to name just a few. It can also be associated with skin diseases, including psoriasis, Darier disease, lichen planus, and lichen striatus. A number of drugs, among them minocycline, can produce focal discoloration in or under the nails.
This subungual lesion was biopsied at its proximal origin, under digital block and using a tourniquet. The 3-mm punch specimen was sent for pathologic examination, which showed benign nevoid tissue—effectively ruling out melanoma. Even if the nail is permanently deformed, this was considered a small price to pay for the patient’s peace of mind.
TAKE-HOME LEARNING POINTS
• Dark streaks under fingernails are common in those with darker skin, in whom they often appear in multiple fingers. In the absence of change, these are usually safe.
• The related conundrum is that when those with darker skin develop melanoma, the subungual areas, or other light-skinned areas (sole, mouth, palms), are often where it manifests.
• New and/or changing areas of subungual pigmentation need to be referred to dermatology for evaluation and possible biopsy.
• When this discoloration also involves the adjacent cuticle or other perionychial skin, even more urgency is added to the referral.
Botulinum toxin for depression? An idea that’s raising some eyebrows
Psychiatry is experiencing a major paradigm shift.1 No longer is depression a disease of norepinephrine and serotonin deficiency. Today, we are exploring inflammation, methylation, epigenetics, and neuroplasticity as major players; we are using innovative treatment interventions such as ketamine, magnets, psilocin, anti-inflammatories, and even botulinum toxin.
In 2006, dermatologist Eric Finzi, MD, PhD, reported a case series of 10 depressed patients who were given a single course of botulinum toxin A (BTA, onabotulinum-toxinA) injections in the forehead.2 After 2 months, 9 out of the 10 patients were no longer depressed. The 10th patient, who reported improvement in symptoms but not remission, was the only patient with bipolar depression.
As a psychiatrist (M.M.) and a dermatologist (J.R.), we conducted a randomized controlled trial3 to challenge the difficult-to-swallow notion that a cosmetic intervention could help severely depressed patients. After reporting our positive findings and hearing numerous encouraging patient testimonials, we present a favorable review on the treatment of depression using BTA. We also present the top 10 questions we are asked at lectures about this novel treatment.
A deadly toxin used to treat medical conditions
Botulinum toxin is one of the deadliest substance known to man.4 It was named after the gram-positive bacterium Clostridium botulinum, which causes so-called floppy baby syndrome in infants who eat contaminated honey. Botulinum toxin prevents nerves from releasing acetylcholine, which causes muscle paralysis.
In the wrong hands, botulinum toxin can be exploited for chemical warfare.4 However, doctors are using it to treat >50 medical conditions, including migraine, cervical dystonia, strabismus, overactive bladder, urinary incontinence, excessive sweating, muscle spasm, and now depression.5,6 In 2014, BTA was the top cosmetic treatment in the United States, with >3 million procedures performed, generating more than 1 billion dollars in revenue.7
The most common site injected with BTA for cosmetic treatments is the glabellar region, which is the area directly above and in between the eyebrows (ie, the lower forehead). The glabella comprises 2 main muscles: the central procerus flanked by a pair of corrugators (Figure). When expressing fear, anger, sadness, or anguish, these muscles contract, causing the appearance of 2 vertical wrinkles, referred to as the “11s.” The wrinkles also can form the shape of an upside-down “U,” known as the omega sign.8 BTA prevents contraction of these muscles and therefore prevents the appearance of a furrowed brow. During cosmetic procedures, approximately 20 to 50 units of BTA are spread out over 5 glabellar injection sites.9 A similar technique is being used in studies of BTA for depression2,3,10,11 (Figure).
BTA for depression is new to the mental health world but, before psychiatrists caught on, dermatologists were aware that BTA could improve quality of life,12 reduce negative emotions,13 and increase feelings of well-being.14
The evidence
To date, there have been 2 case series,2,15 3 randomized control trials (RCTs),3,10,11 1 pooled analysis,16,17 and 1 meta-analysis18 looking at botulinum for depression (Table 1).2,10,11,15-17 In each trial, a single treatment of BTA (ie, 1 doctor’s visit; 29 to 40 units of BTA distributed into 5 glabellar injections sites), was the intervention studied.2
The first case series, by Finzi and Wasserman2 is described above. A second case series, published in 2013, describes 50 female patients, one-half depressed and one-half non-depressed, all of whom received 20 units of BTA into the glabella.15 At 12 weeks, depression scores in the depressed group had decreased by 54% (14.9 point drop on Beck Depression Inventory [BDI], P < .001) and self-esteem scores had increased significantly. In non-depressed participants, depression scores and self-esteem scores remained constant throughout the 12 weeks.
A pooled analysis reported results of 3 RCTs16,17 consisting of a total of 134 depressed patients, males and females age 18 to 65 who received BTA (n = 59) or placebo (n = 74) into the glabellar region. At the end of 6 weeks, BDI scores in the depressed group had decreased by 47.4% (14.3 points) compared with a 16.2% decrease (5.1 points) in the placebo group. This corresponds to a 52.5% vs 8.0% response rate and a 42.4% vs 8.0% remission rate, respectively (Table 1,1,2,10,11,15-17). There was no difference between the 2 groups in sex, age, depression severity, and number of antidepressants being taken. Females received 29 units and males received 10 to 11 units more to account for higher muscle mass (Figure).
Depression as measured by the physician-administered Hamilton Depression Rating Scale (HAM-D) and the Montgomery-Åsberg Depression Rating Scale showed similar reduction in overall scores (−45.7% vs −14.6%), response rates (54.2% vs 10.7%) and remission rates (30.5% vs 6.7%) with BTA.
Although these improvements in depression scores do not reach those seen with electroconvulsive therapy,19,20 they are comparable to placebo-controlled studies of antidepressants.21,22
Doesn’t this technique work because people who look better, feel better?
Aesthetic improvement alone is unlikely to explain the entire story. A recent study showed that improvement in wrinkle score did not correlate with improvement in mood.23 Furthermore, some patients in RCTs did not like the cosmetic effects of BTA but still reported feeling less depressed after treatment.10
How might it work?
Several theories about the mechanism of action have been proposed:
• The facial feedback hypothesis dates to Charles Darwin in 1872: Facial movements influence emotional states. Numerous studies have confirmed this. Strack et al24 found that patients asked to smile while reading comics found them to be funnier. Ekman et al25 found that imitating angry facial expressions made body temperature and heart rate rise. Dialectical behavioral therapy expert Marsha Linehan recognized the importance of modifying facial expressions (from grimacing to smiling) and posture (from clenched fists to open hands) when feeling distressed, because it is hard to feel “willful” when your “mind is going one way and your body is going another.”26 Accordingly, for a person who continuously “looks” depressed or distressed, reducing the anguished facial expression using botulinum toxin might diminish the entwined negative emotions.
• A more pleasant facial expression improves social interactions, which leads to improvement in self-esteem and mood. Social biologists argue that (1) we are attracted to those who have more pleasant facial expressions and (2) we steer clear of those who appear angry or depressed (a negative facial expression, such as a growling dog, is perceived as a threat). Therefore, anyone who looks depressed might have less rewarding interpersonal interactions, which can contribute to a poor mood.
On a similar note, mirror neurons are regions in the brain that are activated by witnessing another person’s emotional cues. When our mirror neurons light up, we can feel an observed experience, which is why we often feel nervous around anxious people, or cringe when we see others get hurt, or why we might prefer engaging with people who appear happier. It is possible that, after BTA injection, a person’s social connectivity is improved because of a more positive reciprocal firing of mirror neurons.
• BTA leads to direct and indirect neurochemical changes in the brain that can reduce depression. Functional MRI studies have shown that after glabellar BTA injections, the amygdala was less responsive to negative stimuli.27,28 For example, patients who were treated with BTA and then shown pictures of angry people had an attenuated amygdala response to the photos.
This is an important finding, especially for patients who have been traumatized. After a traumatic event, the amygdala “remembers” what happened, which is good, in some ways (it prevents us from getting into a similar dangerous situation), but bad in others (the traumatized amygdala may falsely perceive a non-threatening stimuli as threatening). A hypervigilant amygdala can lead to an out-of-proportion fear response, depression, and anxiety. Therefore, quelling an overactive amygdala with BTA could improve emotional dysregulation and posttraumatic disorders.
Many of our patients reported that, after BTA injection, “traumatic events didn’t feel as traumatizing,” as one said. The emotional pain and rumination that often follow a life stressor “does not overstay its welcome” and patients are able to “move on” more quickly.
It is unknown why the amygdala is quieted after BTA; researchers hypothesize that BTA suppresses facial feedback signals from the forehead branch of the trigeminal nerve to the brain. Another hypothesis is that BTA is directly transported by the trigeminal nerve into the brain and exerts central pharmacological effects on the amygdala.29 This theory has only been studied in rat models.30
When does it start working? How long does it last?
From what we know, BTA for depression could start working as early as 2 weeks and could last as long as 6 months. In one RCT, the earliest follow-up was 2 weeks,10 at which time the depressed patients had responded to botulinum toxin (P ≤ .05). In the other 2 controlled trials, the earliest follow-up was 3 weeks, at which time a more robust response was seen (P < .001). Aesthetically, BTA usually lasts 3 months. It is unclear how long the antidepressant effects last but, in the longest trial,3 depression symptoms continued to improve at 6 months, after cosmetic effects had worn off.
These findings raise a series of questions:
• Do mood effects outlast cosmetic effects? If so, why?
• Does botulinum toxin start to work sooner than 2 weeks?
• Will adherence improve if a patient has to be treated only every 6 months?
In our clinical experience, depressed patients who responded to BTA injection report a slow resurfacing of depressive symptoms 4 to 6 months after treatment, at which point they usually return for “maintenance treatment” (same dosing, same injection configuration).
Will psychiatrists administer the treatment?
Any physician or physician extender can, when properly trained, inject BTA. The question is: Do psychiatrists want to? Administrating botulinum toxin requires more labor and preparation than prescribing a drug (Table 2,31) and requires placing hands on patients. Depending on the type of psychiatric practice, this may be a “deal-breaker” for some providers, such as those in a psychoanalytic practice who might worry about boundaries.
As a basis for comparison, despite several indications for BTA for headache and neurologic conditions, few neurologists have added botulinum toxin to their practice. Dermatologists who are comfortable seeing psychiatric patients or family practitioners, who are already set up for injection procedures, could become custodians of this intervention.
Which patients are candidates for the treatment?
Patients with anxious or agitated depression might be ideal candidates for BTA injection. A recent study looked at predictors of response: Patients with a high agitation score (as measured on item 9 of the HAM-D) were more likely to respond, with a sensitivity of 100%, a specificity of 56%, and an overall precision of 78%.32 So far, no other predictors of response have been clearly identified. Higher baseline wrinkle scores do not predict better response.23 Sex and age do not have any predictive value. The treatment appears to be equally effective in males and females; because only a handful of males have been treated (n = 14), however, these patients need to be studied further.
Is botulinum toxin better as monotherapy or augmentation strategy?
So far, it appears to be equally effective as monotherapy or augmentation strategy,16 but more studies are needed.
How expensive is it?
Estimates of patient cost include the cost of the product and the professional fee for injection. As a point of reference, for cosmetic purposes, depending on practice location, dermatologists charge $11 to $20 per unit of BTA. Therefore, 1 treatment of BTA for depression (29 to 40 units) can cost a patient $319 to $800.
When treating a patient with BTA for medical indications, such as tension headache, insurance often reimburses the physician for the BTA at cost (paid with a J code: J0585) and pay an injection fee (a procedure code) of $150 to $200. A recent analysis of cost-effectiveness estimated that BTA for depression would cost a patient $1,200 to $1,600 annually.33 Compared with the price of branded medications (eg, $500 to $2,000 annually)33 plus weekly psychotherapy (eg, $2,000 to $5,000 annually), BTA may be a cost-effective option for patients who do not respond to conventional treatments. Of course, for patients who tolerate and respond to generic medications or have a therapist who charges on a sliding scale, BTA is not the most cost-effective option.
What about injecting other areas of the face?
We’ve thought about it but haven’t tried it. There are several muscles around the mouth that allow us to smile and frown. BTA injections in the depressor anguli oris, a muscle around the mouth that is largely responsible for frowning, could treat depression. However, if the mechanism of action is via amygdala desensitization through the trigeminal nerve, treating mouth frown muscles might not work.
Is it safe?
BTA in the glabella has an exceptionally good safety profile.9,31,34 Adverse reactions, which include eyelid droop, pain, bruising, and redness at the injection site, are minor and temporary.9 In addition, BTA has few drug–drug interactions. The biggest complaint for most patients is discomfort upon injection, which often is described as feeling like “an ant bite.”
In the pooled analysis of RCTs, apart from local irritation immediately after injection, temporary headache was the only relevant, and possibly treatment-related, adverse event. Headache occurred in 13.6% (n = 8) of the BTA group and 9.3% (n = 7) of the placebo group (P = .44). Compared with antidepressants such as citalopram, where approximately 38.6% of patients report a moderate or severe side-effect burden,21 BTA is well tolerated.
Are other studies underway?
Larger studies are being conducted,35 mainly to confirm what pilot studies have shown. It would be interesting to discover other predictors of response and if different dosing and injection configurations could strengthen the response rate and extend the duration of effect.
Because of the cosmetic effects of BTA, further studies are needed to address the problem of blinding. In earlier studies, raters were blinded during appointments because patients wore surgical caps that covered their glabellar region.3,10 Patients did not know their treatment intervention, but 52% to 90% of patients guessed correctly.3,10,11 Although unblinding is a common problem in “blinded” trials in which some researchers have reported >75% of participants and raters guessed the intervention correctly,36 it is a particularly sensitive area in studies that involve a change in appearance because it is almost impossible to prevent someone from looking in a mirror.
Summing up
Botulinum toxin for depression is not ready for prime time. The FDA has not approved its use for psychiatric indications, and Medicare and commercial insurance do not reimburse for this procedure as a treatment for depression. Patients who request BTA for depression must be informed that this use is off-label.
For now, we recommend psychotherapy or medication management, or both, for most patients with major depression. In addition, until larger studies are done, we recommend that patients who are interested in BTA for depression use it as an add-on to conventional treatment. However, if larger studies replicate the findings of the smaller studies we have described, botulinum toxin could become a novel therapeutic agent in the fight against depression.
Bottom Line
In pilot studies, botulinum toxin A (BTA) has shown efficacy in improving symptoms of depression. Although considered safe, BTA is not FDA-approved for psychiatric indications, and Medicare and commercial insurance do not reimburse for this procedure for depression. Larger studies are underway to determine if this novel treatment can be introduced into practice.
Related Resources
• Wollmer MA, Magid M, Kruger THC. Botulinum toxin treatment in depression. In: Bewley A, Taylor RE, Reichenberg JS, et al, eds. Practical psychodermatology. Hoboken, NJ: John Wiley & Sons; 2014:216-219.
• Botox for depression. www.botoxfordepression.com.
• Botox and depression. www.botoxanddepression.com.
Drug Brand Names
Botulinum toxin A • Botox
Citalopram • Celexa
Acknowledgments
We thank the Brain and Behavior Research Foundation for granting Dr. Magid a young investigator award and for continuing to invest in innovative research ideas. We thank Dr. Eric Finzi, MD, PhD, Axel Wollmer, MD, and Tillmann Krüger, MD, for their continued collaboration in this area of research.
Disclosures
In July 2011, Dr. Magid received a young investigator award from the Brain and Behavior Research Foundation for her study on treating depression using botulinum toxin (Grant number 17648). In November 2012, after completion and as a result of the study on treating depression using botulinum toxin, Dr. Magid became a consultant with Allergan to discuss study findings. In September 2015, Dr. Magid became a speaker for IPSEN Innovation. Dr. Reichenberg is married to Dr. Magid. Dr. Reichenberg has no other conflicts of interest to disclose.
1. Nasrallah HA. 10 Recent paradigm shifts in the neurobiology and treatment of depression. Current Psychiatry. 2015;14(2):10-13.
2. Finzi E, Wasserman E. Treatment of depression with botulinum toxin A: a case series. Dermatol Surg. 2006;32(5):645-649; discussion 649-650.
3. Magid M, Reichenberg JS, Poth PE, et al. Treatment of major depressive disorder using botulinum toxin A: a 24-week randomized, double-blind, placebo-controlled study. J Clin Psychiatry. 2014;75(8):837-844.
4. Koussoulakos S. Botulinum neurotoxin: the ugly duckling. Eur Neurol. 2008;61(6):331-342.
5. Chen S. Clinical uses of botulinum neurotoxins: current indications, limitations and future developments. Toxins (Basel). 2012;4(10):913-939.
6. Bhidayasiri R, Truong DD. Expanding use of botulinum toxin. J Neurol Sci. 2005;235(1-1):1-9.
7. Cosmetic surgery national data bank statistics. American Society for Asethetic Plastic Surgery. http://www.surgery. org/sites/default/files/2014-Stats.pdf. Published 2014. Accessed May 30, 2015.
8. Shorter E. Darwin’s contribution to psychiatry. Br J Psychiatry. 2009;195(6):473-474.
9. Winter L, Spiegel J. Botulinum toxin type-A in the treatment of glabellar lines. Clin Cosmet Investig Dermatol. 2009;3:1-4.
10. Wollmer MA, de Boer C, Kalak N, et al. Facing depression with botulinum toxin: a randomized controlled trial. J Psychiatr Res. 2012;46(5):574-581.
11. Finzi E, Rosenthal NE. Treatment of depression with onabotulinumtoxinA: a randomized, double-blind, placebo controlled trial. J Psychiatr Res. 2014;52:1-6.
12. Hexsel D, Brum C, Porto MD, et al. Quality of life and satisfaction of patients after full-face injections of abobotulinum toxin type A: a randomized, phase IV clinical trial. J Drugs Dermatol. 2013;12(12):1363-1367.
13. Lewis MB, Bowler PJ. Botulinum toxin cosmetic therapy correlates with a more positive mood. J Cosmet Dermatol. 2009;8(1):24-26.
14. Sommer B, Zschocke I, Bergfeld D, et al. Satisfaction of patients after treatment with botulinum toxin for dynamic facial lines. Dermatol Surg. 2003;29(5):456-460.
15. Hexsel D, Brum C, Siega C, et al. Evaluation of self‐esteem and depression symptoms in depressed and nondepressed subjects treated with onabotulinumtoxina for glabellar lines. Dermatol Surg. 2013;39(7):1088-1096.
16. Magid M, Reichenberg JS, Finzi E, et al. Treating depression with botulinum toxin: update and meta-analysis from clinic trials. Paper presented at: XVI World Congress of Psychiatry; September 14-18, 2014; Madrid, Spain.
17. Magid M, Finzi E, Kruger TH, et al. Treating depression with botulinum toxin: a pooled analysis of randomized controlled trials. Pharmacopsychiatry. 2015;48(6):205-210.
18. Parsaik A, Mascarenhas S, Hashmi A, et al. Role of botulinum toxin in depression: a systematic review and meta-analysis. J Psychiatr Pract. In press.
19. Scott AI, ed. The ECT handbook, 2nd ed. The third report of the Royal College of Psychiatrists’ Special Committee of ECT. London, United Kingdom: The Royal College of Psychiatrists; 2005.
20. Ren J, Li H, Palaniyappan L, et al. Repetitive transcranial magnetic stimulation versus electroconvulsive therapy for major depression: a systematic review and meta-analysis. Prog Neuropsychopharmacol Biol Psychiatry. 2014;51:181-189.
21. Trivedi MH, Rush AJ, Wisniewski SR, et al; STAR*D Study Team. Evaluation of outcomes with citalopram for depression using measurement-based care in STAR* D: implications for clinical practice. Am J Psychiatry. 2006;163(1):28-40.
22. Gibbons RD, Hur K, Brown CH, et al. Benefits from antidepressants: synthesis of 6-week patient-level outcomes from double-blind placebo-controlled randomized trials of fluoxetine and venlafaxine. Arch Gen Psychiatry. 2012;69(6):572-579.
23. Reichenberg JS, Magid M, Keeling B. Botulinum toxin for depression: does the presence of rhytids predict response? Presented at: Texas Dermatology Society; May 2015; Bastrop, Texas.
24. Strack F, Martin LL, Stepper S. Inhibiting and facilitating conditions of the human smile: a nonobtrusive test of the facial feedback hypothesis. J Pers Soc Psychol. 1988;54(5):768-777.
25. Ekman P, Levenson RW, Friesen WV. Autonomic nervous system activity distinguishes among emotions. Science. 1983;221(4616):1208-1210.
26. Linehan MM. DBT skills training manual, 2nd ed. New York, NY: Guilford Publications; 2014.
27. Hennenlotter A, Dresel C, Castrop F, et al. The link between facial feedback and neural activity within central circuitries of emotion—new insights from botulinum toxin-induced denervation of frown muscles. Cereb Cortex. 2009;19(3):537-542.
28. Kim MJ, Neta M, Davis FC, et al. Botulinum toxin-induced facial muscle paralysis affects amygdala responses to the perception of emotional expressions: preliminary findings from an A-B-A design. Biol Mood Anxiety Disord. 2014;4:11.
29. Mazzocchio R, Caleo M. More than at the neuromuscular synapse: actions of botulinum neurotoxin A in the central nervous system. Neuroscientist. 2015;21(1):44-61.
30. Antonucci F, Rossi C, Gianfranceschi L, et al. Long-distance retrograde effects of botulinum neurotoxin A. J Neurosci. 2008;28(14):3689-3696.
31. U.S. Food and Drug Administration. Medication guide: botox. http://www.fda.gov/downloads/drugs/drugsafety/ucm176360.pdf. Updated September 2013. Accessed June 7, 2015.
32. Wollmer MA, Kalak N, Jung S, et al. Agitation predicts response of depression to botulinum toxin treatment in a randomized controlled trial. Front Psychiatry. 2014;5:36.
33. Beer K. Cost effectiveness of botulinum toxins for the treatment of depression: preliminary observations. J Drugs Dermatol. 2010;9(1):27-30.
34. Brin MF, Boodhoo TI, Pogoda JM, et al. Safety and tolerability of onabotulinumtoxinA in the treatment of facial lines: a meta-analysis of individual patient data from global clinical registration studies in 1678 participants. J Am Acad Dermatol. 2009;61(6):961-970.e1-11.
35. Botulinum toxin and depression. ClinicalTrials.gov. https:// clinicaltrials.gov/ct2/results?term=botulinum+toxin+and+ depression&Search=Search. Accessed June 1, 2015.
36. Rabkin JG, Markowitz JS, Stewart J, et al. How blind is blind? Assessment of patient and doctor medication guesses in a placebo-controlled trial of imipramine and phenelzine. Psychiatry Res. 1986;19(1):75-86.
Psychiatry is experiencing a major paradigm shift.1 No longer is depression a disease of norepinephrine and serotonin deficiency. Today, we are exploring inflammation, methylation, epigenetics, and neuroplasticity as major players; we are using innovative treatment interventions such as ketamine, magnets, psilocin, anti-inflammatories, and even botulinum toxin.
In 2006, dermatologist Eric Finzi, MD, PhD, reported a case series of 10 depressed patients who were given a single course of botulinum toxin A (BTA, onabotulinum-toxinA) injections in the forehead.2 After 2 months, 9 out of the 10 patients were no longer depressed. The 10th patient, who reported improvement in symptoms but not remission, was the only patient with bipolar depression.
As a psychiatrist (M.M.) and a dermatologist (J.R.), we conducted a randomized controlled trial3 to challenge the difficult-to-swallow notion that a cosmetic intervention could help severely depressed patients. After reporting our positive findings and hearing numerous encouraging patient testimonials, we present a favorable review on the treatment of depression using BTA. We also present the top 10 questions we are asked at lectures about this novel treatment.
A deadly toxin used to treat medical conditions
Botulinum toxin is one of the deadliest substance known to man.4 It was named after the gram-positive bacterium Clostridium botulinum, which causes so-called floppy baby syndrome in infants who eat contaminated honey. Botulinum toxin prevents nerves from releasing acetylcholine, which causes muscle paralysis.
In the wrong hands, botulinum toxin can be exploited for chemical warfare.4 However, doctors are using it to treat >50 medical conditions, including migraine, cervical dystonia, strabismus, overactive bladder, urinary incontinence, excessive sweating, muscle spasm, and now depression.5,6 In 2014, BTA was the top cosmetic treatment in the United States, with >3 million procedures performed, generating more than 1 billion dollars in revenue.7
The most common site injected with BTA for cosmetic treatments is the glabellar region, which is the area directly above and in between the eyebrows (ie, the lower forehead). The glabella comprises 2 main muscles: the central procerus flanked by a pair of corrugators (Figure). When expressing fear, anger, sadness, or anguish, these muscles contract, causing the appearance of 2 vertical wrinkles, referred to as the “11s.” The wrinkles also can form the shape of an upside-down “U,” known as the omega sign.8 BTA prevents contraction of these muscles and therefore prevents the appearance of a furrowed brow. During cosmetic procedures, approximately 20 to 50 units of BTA are spread out over 5 glabellar injection sites.9 A similar technique is being used in studies of BTA for depression2,3,10,11 (Figure).
BTA for depression is new to the mental health world but, before psychiatrists caught on, dermatologists were aware that BTA could improve quality of life,12 reduce negative emotions,13 and increase feelings of well-being.14
The evidence
To date, there have been 2 case series,2,15 3 randomized control trials (RCTs),3,10,11 1 pooled analysis,16,17 and 1 meta-analysis18 looking at botulinum for depression (Table 1).2,10,11,15-17 In each trial, a single treatment of BTA (ie, 1 doctor’s visit; 29 to 40 units of BTA distributed into 5 glabellar injections sites), was the intervention studied.2
The first case series, by Finzi and Wasserman2 is described above. A second case series, published in 2013, describes 50 female patients, one-half depressed and one-half non-depressed, all of whom received 20 units of BTA into the glabella.15 At 12 weeks, depression scores in the depressed group had decreased by 54% (14.9 point drop on Beck Depression Inventory [BDI], P < .001) and self-esteem scores had increased significantly. In non-depressed participants, depression scores and self-esteem scores remained constant throughout the 12 weeks.
A pooled analysis reported results of 3 RCTs16,17 consisting of a total of 134 depressed patients, males and females age 18 to 65 who received BTA (n = 59) or placebo (n = 74) into the glabellar region. At the end of 6 weeks, BDI scores in the depressed group had decreased by 47.4% (14.3 points) compared with a 16.2% decrease (5.1 points) in the placebo group. This corresponds to a 52.5% vs 8.0% response rate and a 42.4% vs 8.0% remission rate, respectively (Table 1,1,2,10,11,15-17). There was no difference between the 2 groups in sex, age, depression severity, and number of antidepressants being taken. Females received 29 units and males received 10 to 11 units more to account for higher muscle mass (Figure).
Depression as measured by the physician-administered Hamilton Depression Rating Scale (HAM-D) and the Montgomery-Åsberg Depression Rating Scale showed similar reduction in overall scores (−45.7% vs −14.6%), response rates (54.2% vs 10.7%) and remission rates (30.5% vs 6.7%) with BTA.
Although these improvements in depression scores do not reach those seen with electroconvulsive therapy,19,20 they are comparable to placebo-controlled studies of antidepressants.21,22
Doesn’t this technique work because people who look better, feel better?
Aesthetic improvement alone is unlikely to explain the entire story. A recent study showed that improvement in wrinkle score did not correlate with improvement in mood.23 Furthermore, some patients in RCTs did not like the cosmetic effects of BTA but still reported feeling less depressed after treatment.10
How might it work?
Several theories about the mechanism of action have been proposed:
• The facial feedback hypothesis dates to Charles Darwin in 1872: Facial movements influence emotional states. Numerous studies have confirmed this. Strack et al24 found that patients asked to smile while reading comics found them to be funnier. Ekman et al25 found that imitating angry facial expressions made body temperature and heart rate rise. Dialectical behavioral therapy expert Marsha Linehan recognized the importance of modifying facial expressions (from grimacing to smiling) and posture (from clenched fists to open hands) when feeling distressed, because it is hard to feel “willful” when your “mind is going one way and your body is going another.”26 Accordingly, for a person who continuously “looks” depressed or distressed, reducing the anguished facial expression using botulinum toxin might diminish the entwined negative emotions.
• A more pleasant facial expression improves social interactions, which leads to improvement in self-esteem and mood. Social biologists argue that (1) we are attracted to those who have more pleasant facial expressions and (2) we steer clear of those who appear angry or depressed (a negative facial expression, such as a growling dog, is perceived as a threat). Therefore, anyone who looks depressed might have less rewarding interpersonal interactions, which can contribute to a poor mood.
On a similar note, mirror neurons are regions in the brain that are activated by witnessing another person’s emotional cues. When our mirror neurons light up, we can feel an observed experience, which is why we often feel nervous around anxious people, or cringe when we see others get hurt, or why we might prefer engaging with people who appear happier. It is possible that, after BTA injection, a person’s social connectivity is improved because of a more positive reciprocal firing of mirror neurons.
• BTA leads to direct and indirect neurochemical changes in the brain that can reduce depression. Functional MRI studies have shown that after glabellar BTA injections, the amygdala was less responsive to negative stimuli.27,28 For example, patients who were treated with BTA and then shown pictures of angry people had an attenuated amygdala response to the photos.
This is an important finding, especially for patients who have been traumatized. After a traumatic event, the amygdala “remembers” what happened, which is good, in some ways (it prevents us from getting into a similar dangerous situation), but bad in others (the traumatized amygdala may falsely perceive a non-threatening stimuli as threatening). A hypervigilant amygdala can lead to an out-of-proportion fear response, depression, and anxiety. Therefore, quelling an overactive amygdala with BTA could improve emotional dysregulation and posttraumatic disorders.
Many of our patients reported that, after BTA injection, “traumatic events didn’t feel as traumatizing,” as one said. The emotional pain and rumination that often follow a life stressor “does not overstay its welcome” and patients are able to “move on” more quickly.
It is unknown why the amygdala is quieted after BTA; researchers hypothesize that BTA suppresses facial feedback signals from the forehead branch of the trigeminal nerve to the brain. Another hypothesis is that BTA is directly transported by the trigeminal nerve into the brain and exerts central pharmacological effects on the amygdala.29 This theory has only been studied in rat models.30
When does it start working? How long does it last?
From what we know, BTA for depression could start working as early as 2 weeks and could last as long as 6 months. In one RCT, the earliest follow-up was 2 weeks,10 at which time the depressed patients had responded to botulinum toxin (P ≤ .05). In the other 2 controlled trials, the earliest follow-up was 3 weeks, at which time a more robust response was seen (P < .001). Aesthetically, BTA usually lasts 3 months. It is unclear how long the antidepressant effects last but, in the longest trial,3 depression symptoms continued to improve at 6 months, after cosmetic effects had worn off.
These findings raise a series of questions:
• Do mood effects outlast cosmetic effects? If so, why?
• Does botulinum toxin start to work sooner than 2 weeks?
• Will adherence improve if a patient has to be treated only every 6 months?
In our clinical experience, depressed patients who responded to BTA injection report a slow resurfacing of depressive symptoms 4 to 6 months after treatment, at which point they usually return for “maintenance treatment” (same dosing, same injection configuration).
Will psychiatrists administer the treatment?
Any physician or physician extender can, when properly trained, inject BTA. The question is: Do psychiatrists want to? Administrating botulinum toxin requires more labor and preparation than prescribing a drug (Table 2,31) and requires placing hands on patients. Depending on the type of psychiatric practice, this may be a “deal-breaker” for some providers, such as those in a psychoanalytic practice who might worry about boundaries.
As a basis for comparison, despite several indications for BTA for headache and neurologic conditions, few neurologists have added botulinum toxin to their practice. Dermatologists who are comfortable seeing psychiatric patients or family practitioners, who are already set up for injection procedures, could become custodians of this intervention.
Which patients are candidates for the treatment?
Patients with anxious or agitated depression might be ideal candidates for BTA injection. A recent study looked at predictors of response: Patients with a high agitation score (as measured on item 9 of the HAM-D) were more likely to respond, with a sensitivity of 100%, a specificity of 56%, and an overall precision of 78%.32 So far, no other predictors of response have been clearly identified. Higher baseline wrinkle scores do not predict better response.23 Sex and age do not have any predictive value. The treatment appears to be equally effective in males and females; because only a handful of males have been treated (n = 14), however, these patients need to be studied further.
Is botulinum toxin better as monotherapy or augmentation strategy?
So far, it appears to be equally effective as monotherapy or augmentation strategy,16 but more studies are needed.
How expensive is it?
Estimates of patient cost include the cost of the product and the professional fee for injection. As a point of reference, for cosmetic purposes, depending on practice location, dermatologists charge $11 to $20 per unit of BTA. Therefore, 1 treatment of BTA for depression (29 to 40 units) can cost a patient $319 to $800.
When treating a patient with BTA for medical indications, such as tension headache, insurance often reimburses the physician for the BTA at cost (paid with a J code: J0585) and pay an injection fee (a procedure code) of $150 to $200. A recent analysis of cost-effectiveness estimated that BTA for depression would cost a patient $1,200 to $1,600 annually.33 Compared with the price of branded medications (eg, $500 to $2,000 annually)33 plus weekly psychotherapy (eg, $2,000 to $5,000 annually), BTA may be a cost-effective option for patients who do not respond to conventional treatments. Of course, for patients who tolerate and respond to generic medications or have a therapist who charges on a sliding scale, BTA is not the most cost-effective option.
What about injecting other areas of the face?
We’ve thought about it but haven’t tried it. There are several muscles around the mouth that allow us to smile and frown. BTA injections in the depressor anguli oris, a muscle around the mouth that is largely responsible for frowning, could treat depression. However, if the mechanism of action is via amygdala desensitization through the trigeminal nerve, treating mouth frown muscles might not work.
Is it safe?
BTA in the glabella has an exceptionally good safety profile.9,31,34 Adverse reactions, which include eyelid droop, pain, bruising, and redness at the injection site, are minor and temporary.9 In addition, BTA has few drug–drug interactions. The biggest complaint for most patients is discomfort upon injection, which often is described as feeling like “an ant bite.”
In the pooled analysis of RCTs, apart from local irritation immediately after injection, temporary headache was the only relevant, and possibly treatment-related, adverse event. Headache occurred in 13.6% (n = 8) of the BTA group and 9.3% (n = 7) of the placebo group (P = .44). Compared with antidepressants such as citalopram, where approximately 38.6% of patients report a moderate or severe side-effect burden,21 BTA is well tolerated.
Are other studies underway?
Larger studies are being conducted,35 mainly to confirm what pilot studies have shown. It would be interesting to discover other predictors of response and if different dosing and injection configurations could strengthen the response rate and extend the duration of effect.
Because of the cosmetic effects of BTA, further studies are needed to address the problem of blinding. In earlier studies, raters were blinded during appointments because patients wore surgical caps that covered their glabellar region.3,10 Patients did not know their treatment intervention, but 52% to 90% of patients guessed correctly.3,10,11 Although unblinding is a common problem in “blinded” trials in which some researchers have reported >75% of participants and raters guessed the intervention correctly,36 it is a particularly sensitive area in studies that involve a change in appearance because it is almost impossible to prevent someone from looking in a mirror.
Summing up
Botulinum toxin for depression is not ready for prime time. The FDA has not approved its use for psychiatric indications, and Medicare and commercial insurance do not reimburse for this procedure as a treatment for depression. Patients who request BTA for depression must be informed that this use is off-label.
For now, we recommend psychotherapy or medication management, or both, for most patients with major depression. In addition, until larger studies are done, we recommend that patients who are interested in BTA for depression use it as an add-on to conventional treatment. However, if larger studies replicate the findings of the smaller studies we have described, botulinum toxin could become a novel therapeutic agent in the fight against depression.
Bottom Line
In pilot studies, botulinum toxin A (BTA) has shown efficacy in improving symptoms of depression. Although considered safe, BTA is not FDA-approved for psychiatric indications, and Medicare and commercial insurance do not reimburse for this procedure for depression. Larger studies are underway to determine if this novel treatment can be introduced into practice.
Related Resources
• Wollmer MA, Magid M, Kruger THC. Botulinum toxin treatment in depression. In: Bewley A, Taylor RE, Reichenberg JS, et al, eds. Practical psychodermatology. Hoboken, NJ: John Wiley & Sons; 2014:216-219.
• Botox for depression. www.botoxfordepression.com.
• Botox and depression. www.botoxanddepression.com.
Drug Brand Names
Botulinum toxin A • Botox
Citalopram • Celexa
Acknowledgments
We thank the Brain and Behavior Research Foundation for granting Dr. Magid a young investigator award and for continuing to invest in innovative research ideas. We thank Dr. Eric Finzi, MD, PhD, Axel Wollmer, MD, and Tillmann Krüger, MD, for their continued collaboration in this area of research.
Disclosures
In July 2011, Dr. Magid received a young investigator award from the Brain and Behavior Research Foundation for her study on treating depression using botulinum toxin (Grant number 17648). In November 2012, after completion and as a result of the study on treating depression using botulinum toxin, Dr. Magid became a consultant with Allergan to discuss study findings. In September 2015, Dr. Magid became a speaker for IPSEN Innovation. Dr. Reichenberg is married to Dr. Magid. Dr. Reichenberg has no other conflicts of interest to disclose.
Psychiatry is experiencing a major paradigm shift.1 No longer is depression a disease of norepinephrine and serotonin deficiency. Today, we are exploring inflammation, methylation, epigenetics, and neuroplasticity as major players; we are using innovative treatment interventions such as ketamine, magnets, psilocin, anti-inflammatories, and even botulinum toxin.
In 2006, dermatologist Eric Finzi, MD, PhD, reported a case series of 10 depressed patients who were given a single course of botulinum toxin A (BTA, onabotulinum-toxinA) injections in the forehead.2 After 2 months, 9 out of the 10 patients were no longer depressed. The 10th patient, who reported improvement in symptoms but not remission, was the only patient with bipolar depression.
As a psychiatrist (M.M.) and a dermatologist (J.R.), we conducted a randomized controlled trial3 to challenge the difficult-to-swallow notion that a cosmetic intervention could help severely depressed patients. After reporting our positive findings and hearing numerous encouraging patient testimonials, we present a favorable review on the treatment of depression using BTA. We also present the top 10 questions we are asked at lectures about this novel treatment.
A deadly toxin used to treat medical conditions
Botulinum toxin is one of the deadliest substance known to man.4 It was named after the gram-positive bacterium Clostridium botulinum, which causes so-called floppy baby syndrome in infants who eat contaminated honey. Botulinum toxin prevents nerves from releasing acetylcholine, which causes muscle paralysis.
In the wrong hands, botulinum toxin can be exploited for chemical warfare.4 However, doctors are using it to treat >50 medical conditions, including migraine, cervical dystonia, strabismus, overactive bladder, urinary incontinence, excessive sweating, muscle spasm, and now depression.5,6 In 2014, BTA was the top cosmetic treatment in the United States, with >3 million procedures performed, generating more than 1 billion dollars in revenue.7
The most common site injected with BTA for cosmetic treatments is the glabellar region, which is the area directly above and in between the eyebrows (ie, the lower forehead). The glabella comprises 2 main muscles: the central procerus flanked by a pair of corrugators (Figure). When expressing fear, anger, sadness, or anguish, these muscles contract, causing the appearance of 2 vertical wrinkles, referred to as the “11s.” The wrinkles also can form the shape of an upside-down “U,” known as the omega sign.8 BTA prevents contraction of these muscles and therefore prevents the appearance of a furrowed brow. During cosmetic procedures, approximately 20 to 50 units of BTA are spread out over 5 glabellar injection sites.9 A similar technique is being used in studies of BTA for depression2,3,10,11 (Figure).
BTA for depression is new to the mental health world but, before psychiatrists caught on, dermatologists were aware that BTA could improve quality of life,12 reduce negative emotions,13 and increase feelings of well-being.14
The evidence
To date, there have been 2 case series,2,15 3 randomized control trials (RCTs),3,10,11 1 pooled analysis,16,17 and 1 meta-analysis18 looking at botulinum for depression (Table 1).2,10,11,15-17 In each trial, a single treatment of BTA (ie, 1 doctor’s visit; 29 to 40 units of BTA distributed into 5 glabellar injections sites), was the intervention studied.2
The first case series, by Finzi and Wasserman2 is described above. A second case series, published in 2013, describes 50 female patients, one-half depressed and one-half non-depressed, all of whom received 20 units of BTA into the glabella.15 At 12 weeks, depression scores in the depressed group had decreased by 54% (14.9 point drop on Beck Depression Inventory [BDI], P < .001) and self-esteem scores had increased significantly. In non-depressed participants, depression scores and self-esteem scores remained constant throughout the 12 weeks.
A pooled analysis reported results of 3 RCTs16,17 consisting of a total of 134 depressed patients, males and females age 18 to 65 who received BTA (n = 59) or placebo (n = 74) into the glabellar region. At the end of 6 weeks, BDI scores in the depressed group had decreased by 47.4% (14.3 points) compared with a 16.2% decrease (5.1 points) in the placebo group. This corresponds to a 52.5% vs 8.0% response rate and a 42.4% vs 8.0% remission rate, respectively (Table 1,1,2,10,11,15-17). There was no difference between the 2 groups in sex, age, depression severity, and number of antidepressants being taken. Females received 29 units and males received 10 to 11 units more to account for higher muscle mass (Figure).
Depression as measured by the physician-administered Hamilton Depression Rating Scale (HAM-D) and the Montgomery-Åsberg Depression Rating Scale showed similar reduction in overall scores (−45.7% vs −14.6%), response rates (54.2% vs 10.7%) and remission rates (30.5% vs 6.7%) with BTA.
Although these improvements in depression scores do not reach those seen with electroconvulsive therapy,19,20 they are comparable to placebo-controlled studies of antidepressants.21,22
Doesn’t this technique work because people who look better, feel better?
Aesthetic improvement alone is unlikely to explain the entire story. A recent study showed that improvement in wrinkle score did not correlate with improvement in mood.23 Furthermore, some patients in RCTs did not like the cosmetic effects of BTA but still reported feeling less depressed after treatment.10
How might it work?
Several theories about the mechanism of action have been proposed:
• The facial feedback hypothesis dates to Charles Darwin in 1872: Facial movements influence emotional states. Numerous studies have confirmed this. Strack et al24 found that patients asked to smile while reading comics found them to be funnier. Ekman et al25 found that imitating angry facial expressions made body temperature and heart rate rise. Dialectical behavioral therapy expert Marsha Linehan recognized the importance of modifying facial expressions (from grimacing to smiling) and posture (from clenched fists to open hands) when feeling distressed, because it is hard to feel “willful” when your “mind is going one way and your body is going another.”26 Accordingly, for a person who continuously “looks” depressed or distressed, reducing the anguished facial expression using botulinum toxin might diminish the entwined negative emotions.
• A more pleasant facial expression improves social interactions, which leads to improvement in self-esteem and mood. Social biologists argue that (1) we are attracted to those who have more pleasant facial expressions and (2) we steer clear of those who appear angry or depressed (a negative facial expression, such as a growling dog, is perceived as a threat). Therefore, anyone who looks depressed might have less rewarding interpersonal interactions, which can contribute to a poor mood.
On a similar note, mirror neurons are regions in the brain that are activated by witnessing another person’s emotional cues. When our mirror neurons light up, we can feel an observed experience, which is why we often feel nervous around anxious people, or cringe when we see others get hurt, or why we might prefer engaging with people who appear happier. It is possible that, after BTA injection, a person’s social connectivity is improved because of a more positive reciprocal firing of mirror neurons.
• BTA leads to direct and indirect neurochemical changes in the brain that can reduce depression. Functional MRI studies have shown that after glabellar BTA injections, the amygdala was less responsive to negative stimuli.27,28 For example, patients who were treated with BTA and then shown pictures of angry people had an attenuated amygdala response to the photos.
This is an important finding, especially for patients who have been traumatized. After a traumatic event, the amygdala “remembers” what happened, which is good, in some ways (it prevents us from getting into a similar dangerous situation), but bad in others (the traumatized amygdala may falsely perceive a non-threatening stimuli as threatening). A hypervigilant amygdala can lead to an out-of-proportion fear response, depression, and anxiety. Therefore, quelling an overactive amygdala with BTA could improve emotional dysregulation and posttraumatic disorders.
Many of our patients reported that, after BTA injection, “traumatic events didn’t feel as traumatizing,” as one said. The emotional pain and rumination that often follow a life stressor “does not overstay its welcome” and patients are able to “move on” more quickly.
It is unknown why the amygdala is quieted after BTA; researchers hypothesize that BTA suppresses facial feedback signals from the forehead branch of the trigeminal nerve to the brain. Another hypothesis is that BTA is directly transported by the trigeminal nerve into the brain and exerts central pharmacological effects on the amygdala.29 This theory has only been studied in rat models.30
When does it start working? How long does it last?
From what we know, BTA for depression could start working as early as 2 weeks and could last as long as 6 months. In one RCT, the earliest follow-up was 2 weeks,10 at which time the depressed patients had responded to botulinum toxin (P ≤ .05). In the other 2 controlled trials, the earliest follow-up was 3 weeks, at which time a more robust response was seen (P < .001). Aesthetically, BTA usually lasts 3 months. It is unclear how long the antidepressant effects last but, in the longest trial,3 depression symptoms continued to improve at 6 months, after cosmetic effects had worn off.
These findings raise a series of questions:
• Do mood effects outlast cosmetic effects? If so, why?
• Does botulinum toxin start to work sooner than 2 weeks?
• Will adherence improve if a patient has to be treated only every 6 months?
In our clinical experience, depressed patients who responded to BTA injection report a slow resurfacing of depressive symptoms 4 to 6 months after treatment, at which point they usually return for “maintenance treatment” (same dosing, same injection configuration).
Will psychiatrists administer the treatment?
Any physician or physician extender can, when properly trained, inject BTA. The question is: Do psychiatrists want to? Administrating botulinum toxin requires more labor and preparation than prescribing a drug (Table 2,31) and requires placing hands on patients. Depending on the type of psychiatric practice, this may be a “deal-breaker” for some providers, such as those in a psychoanalytic practice who might worry about boundaries.
As a basis for comparison, despite several indications for BTA for headache and neurologic conditions, few neurologists have added botulinum toxin to their practice. Dermatologists who are comfortable seeing psychiatric patients or family practitioners, who are already set up for injection procedures, could become custodians of this intervention.
Which patients are candidates for the treatment?
Patients with anxious or agitated depression might be ideal candidates for BTA injection. A recent study looked at predictors of response: Patients with a high agitation score (as measured on item 9 of the HAM-D) were more likely to respond, with a sensitivity of 100%, a specificity of 56%, and an overall precision of 78%.32 So far, no other predictors of response have been clearly identified. Higher baseline wrinkle scores do not predict better response.23 Sex and age do not have any predictive value. The treatment appears to be equally effective in males and females; because only a handful of males have been treated (n = 14), however, these patients need to be studied further.
Is botulinum toxin better as monotherapy or augmentation strategy?
So far, it appears to be equally effective as monotherapy or augmentation strategy,16 but more studies are needed.
How expensive is it?
Estimates of patient cost include the cost of the product and the professional fee for injection. As a point of reference, for cosmetic purposes, depending on practice location, dermatologists charge $11 to $20 per unit of BTA. Therefore, 1 treatment of BTA for depression (29 to 40 units) can cost a patient $319 to $800.
When treating a patient with BTA for medical indications, such as tension headache, insurance often reimburses the physician for the BTA at cost (paid with a J code: J0585) and pay an injection fee (a procedure code) of $150 to $200. A recent analysis of cost-effectiveness estimated that BTA for depression would cost a patient $1,200 to $1,600 annually.33 Compared with the price of branded medications (eg, $500 to $2,000 annually)33 plus weekly psychotherapy (eg, $2,000 to $5,000 annually), BTA may be a cost-effective option for patients who do not respond to conventional treatments. Of course, for patients who tolerate and respond to generic medications or have a therapist who charges on a sliding scale, BTA is not the most cost-effective option.
What about injecting other areas of the face?
We’ve thought about it but haven’t tried it. There are several muscles around the mouth that allow us to smile and frown. BTA injections in the depressor anguli oris, a muscle around the mouth that is largely responsible for frowning, could treat depression. However, if the mechanism of action is via amygdala desensitization through the trigeminal nerve, treating mouth frown muscles might not work.
Is it safe?
BTA in the glabella has an exceptionally good safety profile.9,31,34 Adverse reactions, which include eyelid droop, pain, bruising, and redness at the injection site, are minor and temporary.9 In addition, BTA has few drug–drug interactions. The biggest complaint for most patients is discomfort upon injection, which often is described as feeling like “an ant bite.”
In the pooled analysis of RCTs, apart from local irritation immediately after injection, temporary headache was the only relevant, and possibly treatment-related, adverse event. Headache occurred in 13.6% (n = 8) of the BTA group and 9.3% (n = 7) of the placebo group (P = .44). Compared with antidepressants such as citalopram, where approximately 38.6% of patients report a moderate or severe side-effect burden,21 BTA is well tolerated.
Are other studies underway?
Larger studies are being conducted,35 mainly to confirm what pilot studies have shown. It would be interesting to discover other predictors of response and if different dosing and injection configurations could strengthen the response rate and extend the duration of effect.
Because of the cosmetic effects of BTA, further studies are needed to address the problem of blinding. In earlier studies, raters were blinded during appointments because patients wore surgical caps that covered their glabellar region.3,10 Patients did not know their treatment intervention, but 52% to 90% of patients guessed correctly.3,10,11 Although unblinding is a common problem in “blinded” trials in which some researchers have reported >75% of participants and raters guessed the intervention correctly,36 it is a particularly sensitive area in studies that involve a change in appearance because it is almost impossible to prevent someone from looking in a mirror.
Summing up
Botulinum toxin for depression is not ready for prime time. The FDA has not approved its use for psychiatric indications, and Medicare and commercial insurance do not reimburse for this procedure as a treatment for depression. Patients who request BTA for depression must be informed that this use is off-label.
For now, we recommend psychotherapy or medication management, or both, for most patients with major depression. In addition, until larger studies are done, we recommend that patients who are interested in BTA for depression use it as an add-on to conventional treatment. However, if larger studies replicate the findings of the smaller studies we have described, botulinum toxin could become a novel therapeutic agent in the fight against depression.
Bottom Line
In pilot studies, botulinum toxin A (BTA) has shown efficacy in improving symptoms of depression. Although considered safe, BTA is not FDA-approved for psychiatric indications, and Medicare and commercial insurance do not reimburse for this procedure for depression. Larger studies are underway to determine if this novel treatment can be introduced into practice.
Related Resources
• Wollmer MA, Magid M, Kruger THC. Botulinum toxin treatment in depression. In: Bewley A, Taylor RE, Reichenberg JS, et al, eds. Practical psychodermatology. Hoboken, NJ: John Wiley & Sons; 2014:216-219.
• Botox for depression. www.botoxfordepression.com.
• Botox and depression. www.botoxanddepression.com.
Drug Brand Names
Botulinum toxin A • Botox
Citalopram • Celexa
Acknowledgments
We thank the Brain and Behavior Research Foundation for granting Dr. Magid a young investigator award and for continuing to invest in innovative research ideas. We thank Dr. Eric Finzi, MD, PhD, Axel Wollmer, MD, and Tillmann Krüger, MD, for their continued collaboration in this area of research.
Disclosures
In July 2011, Dr. Magid received a young investigator award from the Brain and Behavior Research Foundation for her study on treating depression using botulinum toxin (Grant number 17648). In November 2012, after completion and as a result of the study on treating depression using botulinum toxin, Dr. Magid became a consultant with Allergan to discuss study findings. In September 2015, Dr. Magid became a speaker for IPSEN Innovation. Dr. Reichenberg is married to Dr. Magid. Dr. Reichenberg has no other conflicts of interest to disclose.
1. Nasrallah HA. 10 Recent paradigm shifts in the neurobiology and treatment of depression. Current Psychiatry. 2015;14(2):10-13.
2. Finzi E, Wasserman E. Treatment of depression with botulinum toxin A: a case series. Dermatol Surg. 2006;32(5):645-649; discussion 649-650.
3. Magid M, Reichenberg JS, Poth PE, et al. Treatment of major depressive disorder using botulinum toxin A: a 24-week randomized, double-blind, placebo-controlled study. J Clin Psychiatry. 2014;75(8):837-844.
4. Koussoulakos S. Botulinum neurotoxin: the ugly duckling. Eur Neurol. 2008;61(6):331-342.
5. Chen S. Clinical uses of botulinum neurotoxins: current indications, limitations and future developments. Toxins (Basel). 2012;4(10):913-939.
6. Bhidayasiri R, Truong DD. Expanding use of botulinum toxin. J Neurol Sci. 2005;235(1-1):1-9.
7. Cosmetic surgery national data bank statistics. American Society for Asethetic Plastic Surgery. http://www.surgery. org/sites/default/files/2014-Stats.pdf. Published 2014. Accessed May 30, 2015.
8. Shorter E. Darwin’s contribution to psychiatry. Br J Psychiatry. 2009;195(6):473-474.
9. Winter L, Spiegel J. Botulinum toxin type-A in the treatment of glabellar lines. Clin Cosmet Investig Dermatol. 2009;3:1-4.
10. Wollmer MA, de Boer C, Kalak N, et al. Facing depression with botulinum toxin: a randomized controlled trial. J Psychiatr Res. 2012;46(5):574-581.
11. Finzi E, Rosenthal NE. Treatment of depression with onabotulinumtoxinA: a randomized, double-blind, placebo controlled trial. J Psychiatr Res. 2014;52:1-6.
12. Hexsel D, Brum C, Porto MD, et al. Quality of life and satisfaction of patients after full-face injections of abobotulinum toxin type A: a randomized, phase IV clinical trial. J Drugs Dermatol. 2013;12(12):1363-1367.
13. Lewis MB, Bowler PJ. Botulinum toxin cosmetic therapy correlates with a more positive mood. J Cosmet Dermatol. 2009;8(1):24-26.
14. Sommer B, Zschocke I, Bergfeld D, et al. Satisfaction of patients after treatment with botulinum toxin for dynamic facial lines. Dermatol Surg. 2003;29(5):456-460.
15. Hexsel D, Brum C, Siega C, et al. Evaluation of self‐esteem and depression symptoms in depressed and nondepressed subjects treated with onabotulinumtoxina for glabellar lines. Dermatol Surg. 2013;39(7):1088-1096.
16. Magid M, Reichenberg JS, Finzi E, et al. Treating depression with botulinum toxin: update and meta-analysis from clinic trials. Paper presented at: XVI World Congress of Psychiatry; September 14-18, 2014; Madrid, Spain.
17. Magid M, Finzi E, Kruger TH, et al. Treating depression with botulinum toxin: a pooled analysis of randomized controlled trials. Pharmacopsychiatry. 2015;48(6):205-210.
18. Parsaik A, Mascarenhas S, Hashmi A, et al. Role of botulinum toxin in depression: a systematic review and meta-analysis. J Psychiatr Pract. In press.
19. Scott AI, ed. The ECT handbook, 2nd ed. The third report of the Royal College of Psychiatrists’ Special Committee of ECT. London, United Kingdom: The Royal College of Psychiatrists; 2005.
20. Ren J, Li H, Palaniyappan L, et al. Repetitive transcranial magnetic stimulation versus electroconvulsive therapy for major depression: a systematic review and meta-analysis. Prog Neuropsychopharmacol Biol Psychiatry. 2014;51:181-189.
21. Trivedi MH, Rush AJ, Wisniewski SR, et al; STAR*D Study Team. Evaluation of outcomes with citalopram for depression using measurement-based care in STAR* D: implications for clinical practice. Am J Psychiatry. 2006;163(1):28-40.
22. Gibbons RD, Hur K, Brown CH, et al. Benefits from antidepressants: synthesis of 6-week patient-level outcomes from double-blind placebo-controlled randomized trials of fluoxetine and venlafaxine. Arch Gen Psychiatry. 2012;69(6):572-579.
23. Reichenberg JS, Magid M, Keeling B. Botulinum toxin for depression: does the presence of rhytids predict response? Presented at: Texas Dermatology Society; May 2015; Bastrop, Texas.
24. Strack F, Martin LL, Stepper S. Inhibiting and facilitating conditions of the human smile: a nonobtrusive test of the facial feedback hypothesis. J Pers Soc Psychol. 1988;54(5):768-777.
25. Ekman P, Levenson RW, Friesen WV. Autonomic nervous system activity distinguishes among emotions. Science. 1983;221(4616):1208-1210.
26. Linehan MM. DBT skills training manual, 2nd ed. New York, NY: Guilford Publications; 2014.
27. Hennenlotter A, Dresel C, Castrop F, et al. The link between facial feedback and neural activity within central circuitries of emotion—new insights from botulinum toxin-induced denervation of frown muscles. Cereb Cortex. 2009;19(3):537-542.
28. Kim MJ, Neta M, Davis FC, et al. Botulinum toxin-induced facial muscle paralysis affects amygdala responses to the perception of emotional expressions: preliminary findings from an A-B-A design. Biol Mood Anxiety Disord. 2014;4:11.
29. Mazzocchio R, Caleo M. More than at the neuromuscular synapse: actions of botulinum neurotoxin A in the central nervous system. Neuroscientist. 2015;21(1):44-61.
30. Antonucci F, Rossi C, Gianfranceschi L, et al. Long-distance retrograde effects of botulinum neurotoxin A. J Neurosci. 2008;28(14):3689-3696.
31. U.S. Food and Drug Administration. Medication guide: botox. http://www.fda.gov/downloads/drugs/drugsafety/ucm176360.pdf. Updated September 2013. Accessed June 7, 2015.
32. Wollmer MA, Kalak N, Jung S, et al. Agitation predicts response of depression to botulinum toxin treatment in a randomized controlled trial. Front Psychiatry. 2014;5:36.
33. Beer K. Cost effectiveness of botulinum toxins for the treatment of depression: preliminary observations. J Drugs Dermatol. 2010;9(1):27-30.
34. Brin MF, Boodhoo TI, Pogoda JM, et al. Safety and tolerability of onabotulinumtoxinA in the treatment of facial lines: a meta-analysis of individual patient data from global clinical registration studies in 1678 participants. J Am Acad Dermatol. 2009;61(6):961-970.e1-11.
35. Botulinum toxin and depression. ClinicalTrials.gov. https:// clinicaltrials.gov/ct2/results?term=botulinum+toxin+and+ depression&Search=Search. Accessed June 1, 2015.
36. Rabkin JG, Markowitz JS, Stewart J, et al. How blind is blind? Assessment of patient and doctor medication guesses in a placebo-controlled trial of imipramine and phenelzine. Psychiatry Res. 1986;19(1):75-86.
1. Nasrallah HA. 10 Recent paradigm shifts in the neurobiology and treatment of depression. Current Psychiatry. 2015;14(2):10-13.
2. Finzi E, Wasserman E. Treatment of depression with botulinum toxin A: a case series. Dermatol Surg. 2006;32(5):645-649; discussion 649-650.
3. Magid M, Reichenberg JS, Poth PE, et al. Treatment of major depressive disorder using botulinum toxin A: a 24-week randomized, double-blind, placebo-controlled study. J Clin Psychiatry. 2014;75(8):837-844.
4. Koussoulakos S. Botulinum neurotoxin: the ugly duckling. Eur Neurol. 2008;61(6):331-342.
5. Chen S. Clinical uses of botulinum neurotoxins: current indications, limitations and future developments. Toxins (Basel). 2012;4(10):913-939.
6. Bhidayasiri R, Truong DD. Expanding use of botulinum toxin. J Neurol Sci. 2005;235(1-1):1-9.
7. Cosmetic surgery national data bank statistics. American Society for Asethetic Plastic Surgery. http://www.surgery. org/sites/default/files/2014-Stats.pdf. Published 2014. Accessed May 30, 2015.
8. Shorter E. Darwin’s contribution to psychiatry. Br J Psychiatry. 2009;195(6):473-474.
9. Winter L, Spiegel J. Botulinum toxin type-A in the treatment of glabellar lines. Clin Cosmet Investig Dermatol. 2009;3:1-4.
10. Wollmer MA, de Boer C, Kalak N, et al. Facing depression with botulinum toxin: a randomized controlled trial. J Psychiatr Res. 2012;46(5):574-581.
11. Finzi E, Rosenthal NE. Treatment of depression with onabotulinumtoxinA: a randomized, double-blind, placebo controlled trial. J Psychiatr Res. 2014;52:1-6.
12. Hexsel D, Brum C, Porto MD, et al. Quality of life and satisfaction of patients after full-face injections of abobotulinum toxin type A: a randomized, phase IV clinical trial. J Drugs Dermatol. 2013;12(12):1363-1367.
13. Lewis MB, Bowler PJ. Botulinum toxin cosmetic therapy correlates with a more positive mood. J Cosmet Dermatol. 2009;8(1):24-26.
14. Sommer B, Zschocke I, Bergfeld D, et al. Satisfaction of patients after treatment with botulinum toxin for dynamic facial lines. Dermatol Surg. 2003;29(5):456-460.
15. Hexsel D, Brum C, Siega C, et al. Evaluation of self‐esteem and depression symptoms in depressed and nondepressed subjects treated with onabotulinumtoxina for glabellar lines. Dermatol Surg. 2013;39(7):1088-1096.
16. Magid M, Reichenberg JS, Finzi E, et al. Treating depression with botulinum toxin: update and meta-analysis from clinic trials. Paper presented at: XVI World Congress of Psychiatry; September 14-18, 2014; Madrid, Spain.
17. Magid M, Finzi E, Kruger TH, et al. Treating depression with botulinum toxin: a pooled analysis of randomized controlled trials. Pharmacopsychiatry. 2015;48(6):205-210.
18. Parsaik A, Mascarenhas S, Hashmi A, et al. Role of botulinum toxin in depression: a systematic review and meta-analysis. J Psychiatr Pract. In press.
19. Scott AI, ed. The ECT handbook, 2nd ed. The third report of the Royal College of Psychiatrists’ Special Committee of ECT. London, United Kingdom: The Royal College of Psychiatrists; 2005.
20. Ren J, Li H, Palaniyappan L, et al. Repetitive transcranial magnetic stimulation versus electroconvulsive therapy for major depression: a systematic review and meta-analysis. Prog Neuropsychopharmacol Biol Psychiatry. 2014;51:181-189.
21. Trivedi MH, Rush AJ, Wisniewski SR, et al; STAR*D Study Team. Evaluation of outcomes with citalopram for depression using measurement-based care in STAR* D: implications for clinical practice. Am J Psychiatry. 2006;163(1):28-40.
22. Gibbons RD, Hur K, Brown CH, et al. Benefits from antidepressants: synthesis of 6-week patient-level outcomes from double-blind placebo-controlled randomized trials of fluoxetine and venlafaxine. Arch Gen Psychiatry. 2012;69(6):572-579.
23. Reichenberg JS, Magid M, Keeling B. Botulinum toxin for depression: does the presence of rhytids predict response? Presented at: Texas Dermatology Society; May 2015; Bastrop, Texas.
24. Strack F, Martin LL, Stepper S. Inhibiting and facilitating conditions of the human smile: a nonobtrusive test of the facial feedback hypothesis. J Pers Soc Psychol. 1988;54(5):768-777.
25. Ekman P, Levenson RW, Friesen WV. Autonomic nervous system activity distinguishes among emotions. Science. 1983;221(4616):1208-1210.
26. Linehan MM. DBT skills training manual, 2nd ed. New York, NY: Guilford Publications; 2014.
27. Hennenlotter A, Dresel C, Castrop F, et al. The link between facial feedback and neural activity within central circuitries of emotion—new insights from botulinum toxin-induced denervation of frown muscles. Cereb Cortex. 2009;19(3):537-542.
28. Kim MJ, Neta M, Davis FC, et al. Botulinum toxin-induced facial muscle paralysis affects amygdala responses to the perception of emotional expressions: preliminary findings from an A-B-A design. Biol Mood Anxiety Disord. 2014;4:11.
29. Mazzocchio R, Caleo M. More than at the neuromuscular synapse: actions of botulinum neurotoxin A in the central nervous system. Neuroscientist. 2015;21(1):44-61.
30. Antonucci F, Rossi C, Gianfranceschi L, et al. Long-distance retrograde effects of botulinum neurotoxin A. J Neurosci. 2008;28(14):3689-3696.
31. U.S. Food and Drug Administration. Medication guide: botox. http://www.fda.gov/downloads/drugs/drugsafety/ucm176360.pdf. Updated September 2013. Accessed June 7, 2015.
32. Wollmer MA, Kalak N, Jung S, et al. Agitation predicts response of depression to botulinum toxin treatment in a randomized controlled trial. Front Psychiatry. 2014;5:36.
33. Beer K. Cost effectiveness of botulinum toxins for the treatment of depression: preliminary observations. J Drugs Dermatol. 2010;9(1):27-30.
34. Brin MF, Boodhoo TI, Pogoda JM, et al. Safety and tolerability of onabotulinumtoxinA in the treatment of facial lines: a meta-analysis of individual patient data from global clinical registration studies in 1678 participants. J Am Acad Dermatol. 2009;61(6):961-970.e1-11.
35. Botulinum toxin and depression. ClinicalTrials.gov. https:// clinicaltrials.gov/ct2/results?term=botulinum+toxin+and+ depression&Search=Search. Accessed June 1, 2015.
36. Rabkin JG, Markowitz JS, Stewart J, et al. How blind is blind? Assessment of patient and doctor medication guesses in a placebo-controlled trial of imipramine and phenelzine. Psychiatry Res. 1986;19(1):75-86.