User login
Practical Approaches in the Management of Bipolar Depression: Overcoming Challenges and Avoiding Pitfalls
In the past 2 decades, the burden of care for psychiatric complaints in primary care—including bipolar depression—has increased considerably. The prevalence of bipolar disorder (BPD) in primary care has been recently estimated to range up to 4.3%, and in studies with broader definitions of the disorder or in populations with higher-than-usual psychiatric disorders, the prevalence has been reported to be up to 11.4%. Even though BPD is seen commonly in primary care, there are still profound disparities in the delivery of care, including underdiagnosis, misdiagnosis, and inappropriate treatments. There is abundant evidence that BPD can be successfully managed in the primary care setting when adequate physician education, collaborative care teams, and patient education are employed. Efficacious and well-tolerated pharmacologic treatments for BPD are available, and evidence-based pharmacotherapy can be optimally managed by the primary care provider. In this supplement, experts in BPD discuss the recognition and management of bipolar depression and associated comorbidities in the primary care setting.
In the past 2 decades, the burden of care for psychiatric complaints in primary care—including bipolar depression—has increased considerably. The prevalence of bipolar disorder (BPD) in primary care has been recently estimated to range up to 4.3%, and in studies with broader definitions of the disorder or in populations with higher-than-usual psychiatric disorders, the prevalence has been reported to be up to 11.4%. Even though BPD is seen commonly in primary care, there are still profound disparities in the delivery of care, including underdiagnosis, misdiagnosis, and inappropriate treatments. There is abundant evidence that BPD can be successfully managed in the primary care setting when adequate physician education, collaborative care teams, and patient education are employed. Efficacious and well-tolerated pharmacologic treatments for BPD are available, and evidence-based pharmacotherapy can be optimally managed by the primary care provider. In this supplement, experts in BPD discuss the recognition and management of bipolar depression and associated comorbidities in the primary care setting.
In the past 2 decades, the burden of care for psychiatric complaints in primary care—including bipolar depression—has increased considerably. The prevalence of bipolar disorder (BPD) in primary care has been recently estimated to range up to 4.3%, and in studies with broader definitions of the disorder or in populations with higher-than-usual psychiatric disorders, the prevalence has been reported to be up to 11.4%. Even though BPD is seen commonly in primary care, there are still profound disparities in the delivery of care, including underdiagnosis, misdiagnosis, and inappropriate treatments. There is abundant evidence that BPD can be successfully managed in the primary care setting when adequate physician education, collaborative care teams, and patient education are employed. Efficacious and well-tolerated pharmacologic treatments for BPD are available, and evidence-based pharmacotherapy can be optimally managed by the primary care provider. In this supplement, experts in BPD discuss the recognition and management of bipolar depression and associated comorbidities in the primary care setting.
Standard Text Messaging for Smartphones Not HIPAA Compliant
Doctors were the first to begin using pagers and, along with drug dealers, appear to be the last to give them up. But we really need to get rid of them.
Sadly, for the foreseeable future, we will need a pager replacement, but, in the longer term, I’m hopeful that we can:
- Reduce the frequency of electronic interruptions—all forms of interruptions—and the adverse effects that reliably accompany them, and
- Ensure that each interruption has value—that is, reduce or eliminate the many low value and non-urgent messages we all get (e.g. the ones informing you of a lab result you’ve already seen).
Death to the Pager
I can’t imagine anyone who will be more pleased than I will if pagers go the way of now rare hospital-wide PA announcements. Some hospitals have eliminated these announcements entirely, and even critical messages like “code blue” announcements are sent directly to each responder via a pager or other personal device.
Around the time the first iPhone was born, hospital signs banning cell phones began coming down. It seems the fear that they would disrupt hospital electronics, such as telemetry and other monitoring devices, has proven largely unfounded (though, along with things like computer keyboards and stethoscopes, pagers and cell phones can serve as dangerous repositories of bacteria).
Now nearly everyone, from staff to patients, keeps a cell phone with them while in the hospital. I think that is the most important step toward getting rid of pagers. Many doctors already are using the standard text messaging apps that come with the phone to communicate with one another efficiently.
“Regular” Texting Won’t Cut It
Unfortunately, the standard text messaging that comes with every smartphone is not HIPAA compliant. Though I certainly don’t know how anyone would do it, it is apparently too easy for another person to intercept the message. So, if you’re texting information related to your clinical work, you need to make sure it doesn’t include anything that could be considered protected health information. It isn’t enough just to leave the patient’s name off the message. If you’re in the habit of regularly texting doctors, nurses, and other healthcare personnel about patient care, you are at high risk of violating HIPAA, even if you try hard to avoid it.
Another big drawback is that there isn’t a good way to turn off work-related texting when you’re off duty, while leaving your texting app open for communication with your friends and family. Hospital staff will sometimes fail to check whether you’re on duty before texting, and that will lead to your personal time being interrupted by work reminders.
I think these shortcomings mean that none of us should rely on the standard text messaging apps that come with our phones.
But in order for a different app or service to be of any value, we will need to ensure that most providers associated with our hospital are on the same messaging system. That is a tall order, but fortunately there are a lot of companies trying to produce an attractive product that makes it as easy as possible to attract a critical mass of users at your institution.
HIPAA-Compliant Texting Vendors
Many healthcare tech companies provide secure messaging, usually at no additional cost, as an add-on to their main products, such as charge capture software (e.g. IngeniousMed), or physician social networking (e.g. Doximity). Something like 30 companies now offer a dedicated HIPAA-compliant texting option, including IM Your Doc, Voalte, Telmediq, PerfectServe, Vocera, and TigerText. There are so many that it is awfully tough to understand all of their strengths and shortcomings in detail, but I’m having fun trying to do just that. And I anticipate there will be significant consolidation in vendors within the next two to three years.
The dedicated HIPAA-compliant texting services range in price from free for basic features to a monthly fee per user that varies depending on the features you choose to enable. Some offer integration with the hospital’s EHR, which can let a message sender who only knows the patient’s name to see which doctor, nurse, or other caregiver is currently responsible for the patient. Some offer integration with a call schedule and answering service, or even replace an answering service.
No pager replacement will be viable if there are sites in the hospital or elsewhere where it is out of contact; a solution that works on both cellular networks and Wi-Fi is essential. Some vendors offer the ability for messages not delivered to or acknowledged by the recipient to escalate to other forms of delivery after a specified period of time.
I would love to see a feature that I don’t think any vendor offers yet. It would be great if all messages the sender hasn’t marked “stat” or “urgent” first went to a queue in the EHR rather than immediately interrupting the recipient. That way a doctor or other caregiver could see messages while already working in the EHR, rather than glancing at each new message as it arrives, something that all too often needlessly interrupts another important task such as talking with a patient.
And, since most work in EHRs is done in front of a larger device with a full keyboard, it would be easier to type a quick reply message than it would be to rely on a smartphone keyboard for return messaging. Protocols could be established such that messages waiting in the EHR without a reply or dismissal after a specified time would then be sent to the recipient’s personal device.
A Texting Ecosystem
In nearly every case, the hospital will select the text messaging vendor, though hospitalists and nurses, who will typically be among the highest-volume users, should participate in the decision. But the real value of the system hinges on ensuring its wide adoption by most, or nearly all, hospital caregivers and affiliated ambulatory providers.
I would enjoy hearing from those who are already using a HIPAA-secure texting and pager replacement service now, as well as those still researching their options. This has the potential to meaningfully change the way hospitalists and others do their work.

Doctors were the first to begin using pagers and, along with drug dealers, appear to be the last to give them up. But we really need to get rid of them.
Sadly, for the foreseeable future, we will need a pager replacement, but, in the longer term, I’m hopeful that we can:
- Reduce the frequency of electronic interruptions—all forms of interruptions—and the adverse effects that reliably accompany them, and
- Ensure that each interruption has value—that is, reduce or eliminate the many low value and non-urgent messages we all get (e.g. the ones informing you of a lab result you’ve already seen).
Death to the Pager
I can’t imagine anyone who will be more pleased than I will if pagers go the way of now rare hospital-wide PA announcements. Some hospitals have eliminated these announcements entirely, and even critical messages like “code blue” announcements are sent directly to each responder via a pager or other personal device.
Around the time the first iPhone was born, hospital signs banning cell phones began coming down. It seems the fear that they would disrupt hospital electronics, such as telemetry and other monitoring devices, has proven largely unfounded (though, along with things like computer keyboards and stethoscopes, pagers and cell phones can serve as dangerous repositories of bacteria).
Now nearly everyone, from staff to patients, keeps a cell phone with them while in the hospital. I think that is the most important step toward getting rid of pagers. Many doctors already are using the standard text messaging apps that come with the phone to communicate with one another efficiently.
“Regular” Texting Won’t Cut It
Unfortunately, the standard text messaging that comes with every smartphone is not HIPAA compliant. Though I certainly don’t know how anyone would do it, it is apparently too easy for another person to intercept the message. So, if you’re texting information related to your clinical work, you need to make sure it doesn’t include anything that could be considered protected health information. It isn’t enough just to leave the patient’s name off the message. If you’re in the habit of regularly texting doctors, nurses, and other healthcare personnel about patient care, you are at high risk of violating HIPAA, even if you try hard to avoid it.
Another big drawback is that there isn’t a good way to turn off work-related texting when you’re off duty, while leaving your texting app open for communication with your friends and family. Hospital staff will sometimes fail to check whether you’re on duty before texting, and that will lead to your personal time being interrupted by work reminders.
I think these shortcomings mean that none of us should rely on the standard text messaging apps that come with our phones.
But in order for a different app or service to be of any value, we will need to ensure that most providers associated with our hospital are on the same messaging system. That is a tall order, but fortunately there are a lot of companies trying to produce an attractive product that makes it as easy as possible to attract a critical mass of users at your institution.
HIPAA-Compliant Texting Vendors
Many healthcare tech companies provide secure messaging, usually at no additional cost, as an add-on to their main products, such as charge capture software (e.g. IngeniousMed), or physician social networking (e.g. Doximity). Something like 30 companies now offer a dedicated HIPAA-compliant texting option, including IM Your Doc, Voalte, Telmediq, PerfectServe, Vocera, and TigerText. There are so many that it is awfully tough to understand all of their strengths and shortcomings in detail, but I’m having fun trying to do just that. And I anticipate there will be significant consolidation in vendors within the next two to three years.
The dedicated HIPAA-compliant texting services range in price from free for basic features to a monthly fee per user that varies depending on the features you choose to enable. Some offer integration with the hospital’s EHR, which can let a message sender who only knows the patient’s name to see which doctor, nurse, or other caregiver is currently responsible for the patient. Some offer integration with a call schedule and answering service, or even replace an answering service.
No pager replacement will be viable if there are sites in the hospital or elsewhere where it is out of contact; a solution that works on both cellular networks and Wi-Fi is essential. Some vendors offer the ability for messages not delivered to or acknowledged by the recipient to escalate to other forms of delivery after a specified period of time.
I would love to see a feature that I don’t think any vendor offers yet. It would be great if all messages the sender hasn’t marked “stat” or “urgent” first went to a queue in the EHR rather than immediately interrupting the recipient. That way a doctor or other caregiver could see messages while already working in the EHR, rather than glancing at each new message as it arrives, something that all too often needlessly interrupts another important task such as talking with a patient.
And, since most work in EHRs is done in front of a larger device with a full keyboard, it would be easier to type a quick reply message than it would be to rely on a smartphone keyboard for return messaging. Protocols could be established such that messages waiting in the EHR without a reply or dismissal after a specified time would then be sent to the recipient’s personal device.
A Texting Ecosystem
In nearly every case, the hospital will select the text messaging vendor, though hospitalists and nurses, who will typically be among the highest-volume users, should participate in the decision. But the real value of the system hinges on ensuring its wide adoption by most, or nearly all, hospital caregivers and affiliated ambulatory providers.
I would enjoy hearing from those who are already using a HIPAA-secure texting and pager replacement service now, as well as those still researching their options. This has the potential to meaningfully change the way hospitalists and others do their work.

Doctors were the first to begin using pagers and, along with drug dealers, appear to be the last to give them up. But we really need to get rid of them.
Sadly, for the foreseeable future, we will need a pager replacement, but, in the longer term, I’m hopeful that we can:
- Reduce the frequency of electronic interruptions—all forms of interruptions—and the adverse effects that reliably accompany them, and
- Ensure that each interruption has value—that is, reduce or eliminate the many low value and non-urgent messages we all get (e.g. the ones informing you of a lab result you’ve already seen).
Death to the Pager
I can’t imagine anyone who will be more pleased than I will if pagers go the way of now rare hospital-wide PA announcements. Some hospitals have eliminated these announcements entirely, and even critical messages like “code blue” announcements are sent directly to each responder via a pager or other personal device.
Around the time the first iPhone was born, hospital signs banning cell phones began coming down. It seems the fear that they would disrupt hospital electronics, such as telemetry and other monitoring devices, has proven largely unfounded (though, along with things like computer keyboards and stethoscopes, pagers and cell phones can serve as dangerous repositories of bacteria).
Now nearly everyone, from staff to patients, keeps a cell phone with them while in the hospital. I think that is the most important step toward getting rid of pagers. Many doctors already are using the standard text messaging apps that come with the phone to communicate with one another efficiently.
“Regular” Texting Won’t Cut It
Unfortunately, the standard text messaging that comes with every smartphone is not HIPAA compliant. Though I certainly don’t know how anyone would do it, it is apparently too easy for another person to intercept the message. So, if you’re texting information related to your clinical work, you need to make sure it doesn’t include anything that could be considered protected health information. It isn’t enough just to leave the patient’s name off the message. If you’re in the habit of regularly texting doctors, nurses, and other healthcare personnel about patient care, you are at high risk of violating HIPAA, even if you try hard to avoid it.
Another big drawback is that there isn’t a good way to turn off work-related texting when you’re off duty, while leaving your texting app open for communication with your friends and family. Hospital staff will sometimes fail to check whether you’re on duty before texting, and that will lead to your personal time being interrupted by work reminders.
I think these shortcomings mean that none of us should rely on the standard text messaging apps that come with our phones.
But in order for a different app or service to be of any value, we will need to ensure that most providers associated with our hospital are on the same messaging system. That is a tall order, but fortunately there are a lot of companies trying to produce an attractive product that makes it as easy as possible to attract a critical mass of users at your institution.
HIPAA-Compliant Texting Vendors
Many healthcare tech companies provide secure messaging, usually at no additional cost, as an add-on to their main products, such as charge capture software (e.g. IngeniousMed), or physician social networking (e.g. Doximity). Something like 30 companies now offer a dedicated HIPAA-compliant texting option, including IM Your Doc, Voalte, Telmediq, PerfectServe, Vocera, and TigerText. There are so many that it is awfully tough to understand all of their strengths and shortcomings in detail, but I’m having fun trying to do just that. And I anticipate there will be significant consolidation in vendors within the next two to three years.
The dedicated HIPAA-compliant texting services range in price from free for basic features to a monthly fee per user that varies depending on the features you choose to enable. Some offer integration with the hospital’s EHR, which can let a message sender who only knows the patient’s name to see which doctor, nurse, or other caregiver is currently responsible for the patient. Some offer integration with a call schedule and answering service, or even replace an answering service.
No pager replacement will be viable if there are sites in the hospital or elsewhere where it is out of contact; a solution that works on both cellular networks and Wi-Fi is essential. Some vendors offer the ability for messages not delivered to or acknowledged by the recipient to escalate to other forms of delivery after a specified period of time.
I would love to see a feature that I don’t think any vendor offers yet. It would be great if all messages the sender hasn’t marked “stat” or “urgent” first went to a queue in the EHR rather than immediately interrupting the recipient. That way a doctor or other caregiver could see messages while already working in the EHR, rather than glancing at each new message as it arrives, something that all too often needlessly interrupts another important task such as talking with a patient.
And, since most work in EHRs is done in front of a larger device with a full keyboard, it would be easier to type a quick reply message than it would be to rely on a smartphone keyboard for return messaging. Protocols could be established such that messages waiting in the EHR without a reply or dismissal after a specified time would then be sent to the recipient’s personal device.
A Texting Ecosystem
In nearly every case, the hospital will select the text messaging vendor, though hospitalists and nurses, who will typically be among the highest-volume users, should participate in the decision. But the real value of the system hinges on ensuring its wide adoption by most, or nearly all, hospital caregivers and affiliated ambulatory providers.
I would enjoy hearing from those who are already using a HIPAA-secure texting and pager replacement service now, as well as those still researching their options. This has the potential to meaningfully change the way hospitalists and others do their work.

Consider ACO Participation As Medicare Weighs Changes to Shared Savings Program
In December 2014, nearly three years since its launch, the Centers for Medicare and Medicaid Services (CMS) issued the first proposed rule changes to the Shared Savings Program. The changes, if approved, would take effect in the 2016 performance year and would focus on a host of alterations impacting participating accountable care organizations (ACOs), including reduced administrative burden, improved function and transparency, and enhanced incentives to participate in risk-based models.
Experts say the changes could address some of the biggest flaws in the program but also may not go far enough to incentivize more healthcare providers to participate—or protect them from the risk of financial loss. The rules are under review following a public comment period.
“Many features about the original rules weaken the incentives to participate in ACOs,” says Michael McWilliams, MD, PhD, associate professor of healthcare policy and medicine at Harvard Medical School and a practicing primary care physician at Brigham and Women’s Hospital in Boston.
The ACO model encourages providers to realize savings under fee-for-service Medicare through better-coordinated care and improvements in metrics related to utilization and quality. Any savings relative to a benchmark year are shared between the ACO and CMS.
This year, 424 ACOs are participating in the program nationwide, and while the number of Pioneer ACOs has fallen in recent years (Pioneer ACOs wager higher savings for participants at the risk of greater financial loss), a new independent report commissioned by CMS shows the program saved more than $300 annually per beneficiary in its first two years, achieving $384 million in savings.1 In a statement, CMS concluded this meets the criteria for expanding the Pioneer program; however, Dr. McWilliams says policy changes may still be needed to encourage participation in ACOs with downside risk.
In January, Dr. McWilliams and colleagues published a study in Health Affairs that demonstrated that existing benchmark rules may actually encourage higher Medicare spending as ACOs try to “fatten up” so they have more improvements to make and, therefore, more chance of success at realizing savings.2
Currently, providers’ performance is stacked against their performance and cost benchmarks established in the year prior to forming an ACO. As improvements are made, it becomes increasingly challenging for ACOs to do better. Dr. McWilliams says ACOs should instead be compared to other ACOs and providers.
It’s a “melting ice cube problem,” says Gregory Burke, MPA, director of innovation strategies for New York-based United Health Fund (UHF), a research and philanthropic organization focused on advancing healthcare.
—Gregory Burke, MPA, director of innovation Strategies, United Health Fund
“You are punishing the good, lean providers that are efficient,” he adds, “and rewarding people who are less efficient, in terms of cost of care and utilization of services.”
Burke and a colleague at UHF, health policy analyst Suzanne Brundage, recently completed qualitative and quantitative reports on ACOs in the state of New York, which currently make up 20% of Medicare fee-for-service beneficiaries.3,4
Through their analysis, which included structured interviews with 17 Pioneer ACO leaders, Burke and Brundage found ACO rules could change in the following ways to make the program sustainable and more attractive to providers:
- Patients should be attributed to PCPs within the ACO;
- Risk adjustment should be made for ACO providers serving a sicker population of patients; and
- Benchmark rules should be altered.
Additionally, Dr. McWilliams says the shared savings rate realized by ACOs should be higher than 50%, which is especially true for hospitals within an ACO, since the goals of the program are to reduce hospital visits, extensive specialist services, and testing services.
“For a hospital system, the bulk of the money comes from inpatient care,” Burke says. “We’re saying: ‘You stomp on your own air hose today, and a year from now I’ll give you 50% oxygen—and you have to share with your buddy.’”
The 2014 State of Hospital Medicine report indicates that 36% of adult hospitalist medicine groups are in hospitals either already involved in or considering involvement in an ACO; however, respondents in that report also reflect no clear role for hospitalists in the ACO model.
This is a point disputed by Val Akopov, MD, vice president and chief of hospital medicine at WellStar Health System, a not-for-profit organization in northwestern Atlanta and a participating ACO. Dr. Akopov highlights ways in which hospitals and hospitalists could take advantage of the model.
“Five measures fall into the domain of care coordination that are directly, unequivocally related to what hospitalists do, and these metrics are part of, in my opinion, what any hospital medicine program should have as a value proposition,” Dr. Akopov says.
At WellStar, for example, hospitalists have become part of the ACO structure by serving as medical directors and attending physicians at skilled nursing facilities (SNFs). They are “solely responsible to attend to patients in SNFs, and we have seen a dramatic improvement in readmission rates and quality metrics in nursing homes, such as incidence of falls, use of antipsychotics, and 30-day unplanned readmissions to acute care hospitals,” Dr. Akopov explains.
Additionally, WellStar hospitalists work with each inpatient to ensure they have primary care follow-up scheduled before discharge, Dr. Akopov says, noting that the model is a good opportunity to explore changes to the way hospitals and providers deliver care.
“There are roughly 38,000 Medicare patients in [our] ACO; it’s much easier to work out the kinks with innovations on a limited patient population and then extrapolate findings on 1.5 million annually, rather than trying to bite too much,” he says.
Despite the challenges, experts are optimistic the ACO model can—and will—work. In their reporting, Burke and Brundage found healthcare leaders participating in ACOs remain optimistic.
“It’s a post-Copernican universe, where the world no longer revolves around the hospital, so balancing the equation is a little different,” Burke says. “But they’re staying in the game, because that’s where the puck is going to be.”
Kelly April Tyrrell is a freelance writer in Madison, Wis.
References
- Affordable Care Act payment model saves more than $384 million in two years, meets criteria for first-ever expansion. Centers for Medicare & Medicaid Services website. Published May 4, 2015. Accessed May 11, 2015.
- Douven R, McGuire TG, McWilliams JM. Avoiding unintended incentives in ACO payment models. Health Aff. 2015;34(1):143-149.
- Burke, G, Brundage S. Accountable care in New York state: emerging themes and issues. United Hospital Fund. Accessed May 9, 2015.
- Burke, G and Brundage S. New York’s Medicare ACOs: participants and performance. United Hospital Fund. Accessed May 9, 2015.
In December 2014, nearly three years since its launch, the Centers for Medicare and Medicaid Services (CMS) issued the first proposed rule changes to the Shared Savings Program. The changes, if approved, would take effect in the 2016 performance year and would focus on a host of alterations impacting participating accountable care organizations (ACOs), including reduced administrative burden, improved function and transparency, and enhanced incentives to participate in risk-based models.
Experts say the changes could address some of the biggest flaws in the program but also may not go far enough to incentivize more healthcare providers to participate—or protect them from the risk of financial loss. The rules are under review following a public comment period.
“Many features about the original rules weaken the incentives to participate in ACOs,” says Michael McWilliams, MD, PhD, associate professor of healthcare policy and medicine at Harvard Medical School and a practicing primary care physician at Brigham and Women’s Hospital in Boston.
The ACO model encourages providers to realize savings under fee-for-service Medicare through better-coordinated care and improvements in metrics related to utilization and quality. Any savings relative to a benchmark year are shared between the ACO and CMS.
This year, 424 ACOs are participating in the program nationwide, and while the number of Pioneer ACOs has fallen in recent years (Pioneer ACOs wager higher savings for participants at the risk of greater financial loss), a new independent report commissioned by CMS shows the program saved more than $300 annually per beneficiary in its first two years, achieving $384 million in savings.1 In a statement, CMS concluded this meets the criteria for expanding the Pioneer program; however, Dr. McWilliams says policy changes may still be needed to encourage participation in ACOs with downside risk.
In January, Dr. McWilliams and colleagues published a study in Health Affairs that demonstrated that existing benchmark rules may actually encourage higher Medicare spending as ACOs try to “fatten up” so they have more improvements to make and, therefore, more chance of success at realizing savings.2
Currently, providers’ performance is stacked against their performance and cost benchmarks established in the year prior to forming an ACO. As improvements are made, it becomes increasingly challenging for ACOs to do better. Dr. McWilliams says ACOs should instead be compared to other ACOs and providers.
It’s a “melting ice cube problem,” says Gregory Burke, MPA, director of innovation strategies for New York-based United Health Fund (UHF), a research and philanthropic organization focused on advancing healthcare.
—Gregory Burke, MPA, director of innovation Strategies, United Health Fund
“You are punishing the good, lean providers that are efficient,” he adds, “and rewarding people who are less efficient, in terms of cost of care and utilization of services.”
Burke and a colleague at UHF, health policy analyst Suzanne Brundage, recently completed qualitative and quantitative reports on ACOs in the state of New York, which currently make up 20% of Medicare fee-for-service beneficiaries.3,4
Through their analysis, which included structured interviews with 17 Pioneer ACO leaders, Burke and Brundage found ACO rules could change in the following ways to make the program sustainable and more attractive to providers:
- Patients should be attributed to PCPs within the ACO;
- Risk adjustment should be made for ACO providers serving a sicker population of patients; and
- Benchmark rules should be altered.
Additionally, Dr. McWilliams says the shared savings rate realized by ACOs should be higher than 50%, which is especially true for hospitals within an ACO, since the goals of the program are to reduce hospital visits, extensive specialist services, and testing services.
“For a hospital system, the bulk of the money comes from inpatient care,” Burke says. “We’re saying: ‘You stomp on your own air hose today, and a year from now I’ll give you 50% oxygen—and you have to share with your buddy.’”
The 2014 State of Hospital Medicine report indicates that 36% of adult hospitalist medicine groups are in hospitals either already involved in or considering involvement in an ACO; however, respondents in that report also reflect no clear role for hospitalists in the ACO model.
This is a point disputed by Val Akopov, MD, vice president and chief of hospital medicine at WellStar Health System, a not-for-profit organization in northwestern Atlanta and a participating ACO. Dr. Akopov highlights ways in which hospitals and hospitalists could take advantage of the model.
“Five measures fall into the domain of care coordination that are directly, unequivocally related to what hospitalists do, and these metrics are part of, in my opinion, what any hospital medicine program should have as a value proposition,” Dr. Akopov says.
At WellStar, for example, hospitalists have become part of the ACO structure by serving as medical directors and attending physicians at skilled nursing facilities (SNFs). They are “solely responsible to attend to patients in SNFs, and we have seen a dramatic improvement in readmission rates and quality metrics in nursing homes, such as incidence of falls, use of antipsychotics, and 30-day unplanned readmissions to acute care hospitals,” Dr. Akopov explains.
Additionally, WellStar hospitalists work with each inpatient to ensure they have primary care follow-up scheduled before discharge, Dr. Akopov says, noting that the model is a good opportunity to explore changes to the way hospitals and providers deliver care.
“There are roughly 38,000 Medicare patients in [our] ACO; it’s much easier to work out the kinks with innovations on a limited patient population and then extrapolate findings on 1.5 million annually, rather than trying to bite too much,” he says.
Despite the challenges, experts are optimistic the ACO model can—and will—work. In their reporting, Burke and Brundage found healthcare leaders participating in ACOs remain optimistic.
“It’s a post-Copernican universe, where the world no longer revolves around the hospital, so balancing the equation is a little different,” Burke says. “But they’re staying in the game, because that’s where the puck is going to be.”
Kelly April Tyrrell is a freelance writer in Madison, Wis.
References
- Affordable Care Act payment model saves more than $384 million in two years, meets criteria for first-ever expansion. Centers for Medicare & Medicaid Services website. Published May 4, 2015. Accessed May 11, 2015.
- Douven R, McGuire TG, McWilliams JM. Avoiding unintended incentives in ACO payment models. Health Aff. 2015;34(1):143-149.
- Burke, G, Brundage S. Accountable care in New York state: emerging themes and issues. United Hospital Fund. Accessed May 9, 2015.
- Burke, G and Brundage S. New York’s Medicare ACOs: participants and performance. United Hospital Fund. Accessed May 9, 2015.
In December 2014, nearly three years since its launch, the Centers for Medicare and Medicaid Services (CMS) issued the first proposed rule changes to the Shared Savings Program. The changes, if approved, would take effect in the 2016 performance year and would focus on a host of alterations impacting participating accountable care organizations (ACOs), including reduced administrative burden, improved function and transparency, and enhanced incentives to participate in risk-based models.
Experts say the changes could address some of the biggest flaws in the program but also may not go far enough to incentivize more healthcare providers to participate—or protect them from the risk of financial loss. The rules are under review following a public comment period.
“Many features about the original rules weaken the incentives to participate in ACOs,” says Michael McWilliams, MD, PhD, associate professor of healthcare policy and medicine at Harvard Medical School and a practicing primary care physician at Brigham and Women’s Hospital in Boston.
The ACO model encourages providers to realize savings under fee-for-service Medicare through better-coordinated care and improvements in metrics related to utilization and quality. Any savings relative to a benchmark year are shared between the ACO and CMS.
This year, 424 ACOs are participating in the program nationwide, and while the number of Pioneer ACOs has fallen in recent years (Pioneer ACOs wager higher savings for participants at the risk of greater financial loss), a new independent report commissioned by CMS shows the program saved more than $300 annually per beneficiary in its first two years, achieving $384 million in savings.1 In a statement, CMS concluded this meets the criteria for expanding the Pioneer program; however, Dr. McWilliams says policy changes may still be needed to encourage participation in ACOs with downside risk.
In January, Dr. McWilliams and colleagues published a study in Health Affairs that demonstrated that existing benchmark rules may actually encourage higher Medicare spending as ACOs try to “fatten up” so they have more improvements to make and, therefore, more chance of success at realizing savings.2
Currently, providers’ performance is stacked against their performance and cost benchmarks established in the year prior to forming an ACO. As improvements are made, it becomes increasingly challenging for ACOs to do better. Dr. McWilliams says ACOs should instead be compared to other ACOs and providers.
It’s a “melting ice cube problem,” says Gregory Burke, MPA, director of innovation strategies for New York-based United Health Fund (UHF), a research and philanthropic organization focused on advancing healthcare.
—Gregory Burke, MPA, director of innovation Strategies, United Health Fund
“You are punishing the good, lean providers that are efficient,” he adds, “and rewarding people who are less efficient, in terms of cost of care and utilization of services.”
Burke and a colleague at UHF, health policy analyst Suzanne Brundage, recently completed qualitative and quantitative reports on ACOs in the state of New York, which currently make up 20% of Medicare fee-for-service beneficiaries.3,4
Through their analysis, which included structured interviews with 17 Pioneer ACO leaders, Burke and Brundage found ACO rules could change in the following ways to make the program sustainable and more attractive to providers:
- Patients should be attributed to PCPs within the ACO;
- Risk adjustment should be made for ACO providers serving a sicker population of patients; and
- Benchmark rules should be altered.
Additionally, Dr. McWilliams says the shared savings rate realized by ACOs should be higher than 50%, which is especially true for hospitals within an ACO, since the goals of the program are to reduce hospital visits, extensive specialist services, and testing services.
“For a hospital system, the bulk of the money comes from inpatient care,” Burke says. “We’re saying: ‘You stomp on your own air hose today, and a year from now I’ll give you 50% oxygen—and you have to share with your buddy.’”
The 2014 State of Hospital Medicine report indicates that 36% of adult hospitalist medicine groups are in hospitals either already involved in or considering involvement in an ACO; however, respondents in that report also reflect no clear role for hospitalists in the ACO model.
This is a point disputed by Val Akopov, MD, vice president and chief of hospital medicine at WellStar Health System, a not-for-profit organization in northwestern Atlanta and a participating ACO. Dr. Akopov highlights ways in which hospitals and hospitalists could take advantage of the model.
“Five measures fall into the domain of care coordination that are directly, unequivocally related to what hospitalists do, and these metrics are part of, in my opinion, what any hospital medicine program should have as a value proposition,” Dr. Akopov says.
At WellStar, for example, hospitalists have become part of the ACO structure by serving as medical directors and attending physicians at skilled nursing facilities (SNFs). They are “solely responsible to attend to patients in SNFs, and we have seen a dramatic improvement in readmission rates and quality metrics in nursing homes, such as incidence of falls, use of antipsychotics, and 30-day unplanned readmissions to acute care hospitals,” Dr. Akopov explains.
Additionally, WellStar hospitalists work with each inpatient to ensure they have primary care follow-up scheduled before discharge, Dr. Akopov says, noting that the model is a good opportunity to explore changes to the way hospitals and providers deliver care.
“There are roughly 38,000 Medicare patients in [our] ACO; it’s much easier to work out the kinks with innovations on a limited patient population and then extrapolate findings on 1.5 million annually, rather than trying to bite too much,” he says.
Despite the challenges, experts are optimistic the ACO model can—and will—work. In their reporting, Burke and Brundage found healthcare leaders participating in ACOs remain optimistic.
“It’s a post-Copernican universe, where the world no longer revolves around the hospital, so balancing the equation is a little different,” Burke says. “But they’re staying in the game, because that’s where the puck is going to be.”
Kelly April Tyrrell is a freelance writer in Madison, Wis.
References
- Affordable Care Act payment model saves more than $384 million in two years, meets criteria for first-ever expansion. Centers for Medicare & Medicaid Services website. Published May 4, 2015. Accessed May 11, 2015.
- Douven R, McGuire TG, McWilliams JM. Avoiding unintended incentives in ACO payment models. Health Aff. 2015;34(1):143-149.
- Burke, G, Brundage S. Accountable care in New York state: emerging themes and issues. United Hospital Fund. Accessed May 9, 2015.
- Burke, G and Brundage S. New York’s Medicare ACOs: participants and performance. United Hospital Fund. Accessed May 9, 2015.
Hospitals’ Uncompensated Costs Estimated at $27.3 Billion in 2014
The estimated total amount of uncompensated costs incurred by hospitals in 2014 was $27.3 billion, which is $7.4 billion, or 21 percent, less than uncompensated hospital care would have been in 2014 at 2013 levels, before Accountable Care Act Medicaid coverage provisions took effect. Federal data reported by CNBC on March 23 indicate most of the reduction came in the 28 states and the District of Columbia that expanded their Medicare programs under the act to cover nearly all poor people in their states, while those that did not could have seen their revenues decline by an additional $1.4 billion.
The estimated total amount of uncompensated costs incurred by hospitals in 2014 was $27.3 billion, which is $7.4 billion, or 21 percent, less than uncompensated hospital care would have been in 2014 at 2013 levels, before Accountable Care Act Medicaid coverage provisions took effect. Federal data reported by CNBC on March 23 indicate most of the reduction came in the 28 states and the District of Columbia that expanded their Medicare programs under the act to cover nearly all poor people in their states, while those that did not could have seen their revenues decline by an additional $1.4 billion.
The estimated total amount of uncompensated costs incurred by hospitals in 2014 was $27.3 billion, which is $7.4 billion, or 21 percent, less than uncompensated hospital care would have been in 2014 at 2013 levels, before Accountable Care Act Medicaid coverage provisions took effect. Federal data reported by CNBC on March 23 indicate most of the reduction came in the 28 states and the District of Columbia that expanded their Medicare programs under the act to cover nearly all poor people in their states, while those that did not could have seen their revenues decline by an additional $1.4 billion.
Quality Data Dashboards Provide Performance Feedback to Physicians
A best-of-research plenary presentation at HM15 in National Harbor, Md., described a project to link physicians’ schedules to the electronic health record (EHR) in order to provide real-time, individualized performance feedback on key quality improvement and value metrics.
The abstract’s lead author, Victoria Valencia, MPH, a research data and project manager at the University of California San Francisco (UCSF), explains that quality improvement priorities have driven feedback of quality metrics at the department level.
“Where I came in was to try to get the same quality metrics down to the level of the team,” she says. “We take data from our EPIC EHR, clean it up by removing outliers, merge it with our online scheduling program, and provide a robust visual presentation of individualized, real-time performance feedback to the clinical team.”
–Dr. Valencia
One example is counting the total number of phlebotomy “sticks” per day, per patient. Reporting this data helped to reduce the number of “sticks per day” by 20%, to 1.6 from 2.0. A similar approach is used for care transitions and the percentage of discharges with high-quality, after-visit summaries.
“The feedback is timely and actionable and allows the teams to address areas needing improvement,” Valencia says.
How have the doctors responded to this feedback?
“Our division is used to receiving quality feedback as part of an ongoing process that includes working meetings where the metrics are reviewed,” she says, adding that there hasn’t been pushback from the teams over these reports.
A best-of-research plenary presentation at HM15 in National Harbor, Md., described a project to link physicians’ schedules to the electronic health record (EHR) in order to provide real-time, individualized performance feedback on key quality improvement and value metrics.
The abstract’s lead author, Victoria Valencia, MPH, a research data and project manager at the University of California San Francisco (UCSF), explains that quality improvement priorities have driven feedback of quality metrics at the department level.
“Where I came in was to try to get the same quality metrics down to the level of the team,” she says. “We take data from our EPIC EHR, clean it up by removing outliers, merge it with our online scheduling program, and provide a robust visual presentation of individualized, real-time performance feedback to the clinical team.”
–Dr. Valencia
One example is counting the total number of phlebotomy “sticks” per day, per patient. Reporting this data helped to reduce the number of “sticks per day” by 20%, to 1.6 from 2.0. A similar approach is used for care transitions and the percentage of discharges with high-quality, after-visit summaries.
“The feedback is timely and actionable and allows the teams to address areas needing improvement,” Valencia says.
How have the doctors responded to this feedback?
“Our division is used to receiving quality feedback as part of an ongoing process that includes working meetings where the metrics are reviewed,” she says, adding that there hasn’t been pushback from the teams over these reports.
A best-of-research plenary presentation at HM15 in National Harbor, Md., described a project to link physicians’ schedules to the electronic health record (EHR) in order to provide real-time, individualized performance feedback on key quality improvement and value metrics.
The abstract’s lead author, Victoria Valencia, MPH, a research data and project manager at the University of California San Francisco (UCSF), explains that quality improvement priorities have driven feedback of quality metrics at the department level.
“Where I came in was to try to get the same quality metrics down to the level of the team,” she says. “We take data from our EPIC EHR, clean it up by removing outliers, merge it with our online scheduling program, and provide a robust visual presentation of individualized, real-time performance feedback to the clinical team.”
–Dr. Valencia
One example is counting the total number of phlebotomy “sticks” per day, per patient. Reporting this data helped to reduce the number of “sticks per day” by 20%, to 1.6 from 2.0. A similar approach is used for care transitions and the percentage of discharges with high-quality, after-visit summaries.
“The feedback is timely and actionable and allows the teams to address areas needing improvement,” Valencia says.
How have the doctors responded to this feedback?
“Our division is used to receiving quality feedback as part of an ongoing process that includes working meetings where the metrics are reviewed,” she says, adding that there hasn’t been pushback from the teams over these reports.
Why Physicians Override Best Practice Alerts
Research published earlier this year in the Journal of Hospital Medicine finds that rationales offered by physicians for overriding interruptive, computerized best practice alerts (BPAs) regarding whether or not to give blood transfusions vary widely, including specialty service protocolized behaviors, anticipation of surgical or procedural interventions, and imminent hospital transfers.
The electronic health record at Stanford University Medical Center in Palo Alto, Calif., has an automated alert function to check reported hemoglobin level and trigger a pop-up reminder when a doctor orders a transfusion for a patient with a hemoglobin level of 9 or above—outside of the recognized guidelines—prompting the doctor to either abort the transfusion or provide a reason for the override, explains co-author Lisa Shieh, MD, PhD, FHM, medical director of quality in the department of medicine at Stanford.
“Our study was trying to understand why providers still transfuse, even when we provide just-in-time education on transfusion recommendations,” she says. “We can’t say that all of these orders are inappropriate. But, for many reasons, blood has harms and is costly.
“We want to convey an overall understanding about why this issue is important.”
Although a substantial number of transfusions continue outside of the recommended guidelines, Stanford has reduced its numbers significantly.
“I’m a big believer in clinical decision support … if it’s designed well and doesn’t add to alert fatigue,” Dr. Shieh says. “I think this BPA was effective in education and making people stop and think why they were ordering transfusions. Our next step will be to look at the outlier practices and maybe have a conversation with them, doctor to doctor.”
Stanford is looking at sepsis treatment as a next target.
Research published earlier this year in the Journal of Hospital Medicine finds that rationales offered by physicians for overriding interruptive, computerized best practice alerts (BPAs) regarding whether or not to give blood transfusions vary widely, including specialty service protocolized behaviors, anticipation of surgical or procedural interventions, and imminent hospital transfers.
The electronic health record at Stanford University Medical Center in Palo Alto, Calif., has an automated alert function to check reported hemoglobin level and trigger a pop-up reminder when a doctor orders a transfusion for a patient with a hemoglobin level of 9 or above—outside of the recognized guidelines—prompting the doctor to either abort the transfusion or provide a reason for the override, explains co-author Lisa Shieh, MD, PhD, FHM, medical director of quality in the department of medicine at Stanford.
“Our study was trying to understand why providers still transfuse, even when we provide just-in-time education on transfusion recommendations,” she says. “We can’t say that all of these orders are inappropriate. But, for many reasons, blood has harms and is costly.
“We want to convey an overall understanding about why this issue is important.”
Although a substantial number of transfusions continue outside of the recommended guidelines, Stanford has reduced its numbers significantly.
“I’m a big believer in clinical decision support … if it’s designed well and doesn’t add to alert fatigue,” Dr. Shieh says. “I think this BPA was effective in education and making people stop and think why they were ordering transfusions. Our next step will be to look at the outlier practices and maybe have a conversation with them, doctor to doctor.”
Stanford is looking at sepsis treatment as a next target.
Research published earlier this year in the Journal of Hospital Medicine finds that rationales offered by physicians for overriding interruptive, computerized best practice alerts (BPAs) regarding whether or not to give blood transfusions vary widely, including specialty service protocolized behaviors, anticipation of surgical or procedural interventions, and imminent hospital transfers.
The electronic health record at Stanford University Medical Center in Palo Alto, Calif., has an automated alert function to check reported hemoglobin level and trigger a pop-up reminder when a doctor orders a transfusion for a patient with a hemoglobin level of 9 or above—outside of the recognized guidelines—prompting the doctor to either abort the transfusion or provide a reason for the override, explains co-author Lisa Shieh, MD, PhD, FHM, medical director of quality in the department of medicine at Stanford.
“Our study was trying to understand why providers still transfuse, even when we provide just-in-time education on transfusion recommendations,” she says. “We can’t say that all of these orders are inappropriate. But, for many reasons, blood has harms and is costly.
“We want to convey an overall understanding about why this issue is important.”
Although a substantial number of transfusions continue outside of the recommended guidelines, Stanford has reduced its numbers significantly.
“I’m a big believer in clinical decision support … if it’s designed well and doesn’t add to alert fatigue,” Dr. Shieh says. “I think this BPA was effective in education and making people stop and think why they were ordering transfusions. Our next step will be to look at the outlier practices and maybe have a conversation with them, doctor to doctor.”
Stanford is looking at sepsis treatment as a next target.
Hospitals with Hotel-Like Amenities Don’t Improve Satisfaction Scores
Hospital design may not contribute to patients’ satisfaction with the care given by their hospital professionals, according to new research from Johns Hopkins Hospital in Baltimore, published in the Journal of Hospital Medicine. Newly built hospitals often emphasize patient-centered features like reduced noise, natural light, visitor-friendly facilities, well-designed rooms, and hotel-like amenities, note the authors, led by Zishan Siddiqui, MD, attending physician and assistant professor of medicine at Johns Hopkins.
When Hopkins moved a number of its hospital units to the sleek new Sheikh Zayed Tower in 2012, researchers used a pre-post design experiment to compare patient satisfaction in the newer, more pleasing surroundings via Press Ganey and HCAHPS (Hospital Consumer Assessment of Healthcare Providers and Systems) survey scores. Patients responded positively to the new environment, with significant improvement in facility-related satisfaction, but were able to distinguish that satisfaction from their ratings of their doctors and nurses, which were not impacted by the new environment.
“It is more likely that provider-level interventions will have a greater impact on provider level and overall satisfaction,” the authors conclude. “Hospital administrators should not use outdated facilities as an excuse for suboptimal provider satisfaction scores.”
Hospital design may not contribute to patients’ satisfaction with the care given by their hospital professionals, according to new research from Johns Hopkins Hospital in Baltimore, published in the Journal of Hospital Medicine. Newly built hospitals often emphasize patient-centered features like reduced noise, natural light, visitor-friendly facilities, well-designed rooms, and hotel-like amenities, note the authors, led by Zishan Siddiqui, MD, attending physician and assistant professor of medicine at Johns Hopkins.
When Hopkins moved a number of its hospital units to the sleek new Sheikh Zayed Tower in 2012, researchers used a pre-post design experiment to compare patient satisfaction in the newer, more pleasing surroundings via Press Ganey and HCAHPS (Hospital Consumer Assessment of Healthcare Providers and Systems) survey scores. Patients responded positively to the new environment, with significant improvement in facility-related satisfaction, but were able to distinguish that satisfaction from their ratings of their doctors and nurses, which were not impacted by the new environment.
“It is more likely that provider-level interventions will have a greater impact on provider level and overall satisfaction,” the authors conclude. “Hospital administrators should not use outdated facilities as an excuse for suboptimal provider satisfaction scores.”
Hospital design may not contribute to patients’ satisfaction with the care given by their hospital professionals, according to new research from Johns Hopkins Hospital in Baltimore, published in the Journal of Hospital Medicine. Newly built hospitals often emphasize patient-centered features like reduced noise, natural light, visitor-friendly facilities, well-designed rooms, and hotel-like amenities, note the authors, led by Zishan Siddiqui, MD, attending physician and assistant professor of medicine at Johns Hopkins.
When Hopkins moved a number of its hospital units to the sleek new Sheikh Zayed Tower in 2012, researchers used a pre-post design experiment to compare patient satisfaction in the newer, more pleasing surroundings via Press Ganey and HCAHPS (Hospital Consumer Assessment of Healthcare Providers and Systems) survey scores. Patients responded positively to the new environment, with significant improvement in facility-related satisfaction, but were able to distinguish that satisfaction from their ratings of their doctors and nurses, which were not impacted by the new environment.
“It is more likely that provider-level interventions will have a greater impact on provider level and overall satisfaction,” the authors conclude. “Hospital administrators should not use outdated facilities as an excuse for suboptimal provider satisfaction scores.”
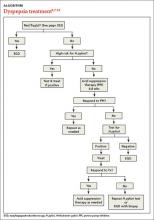
Managing dyspepsia
› Review the medications taken by patients who suffer from dyspepsia, as many drugs—bisphosphonates, antibiotics, steroids, and nonsteroidal anti-inflammatory drugs, among others—are associated with this condition. B
› Order an esophagogastroduodenoscopy for patients ages 55 years or older with new-onset dyspepsia and those who have red flags for more serious conditions, eg, a history of upper gastrointestinal (GI) cancer, unintended weight loss, GI bleeding, dysphagia, or a palpable mass. C
› Prescribe acid suppression therapy as first-line treatment for patients who have dyspepsia but are at low risk or have tested negative for Helicobacter pylori infection. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Each year, an estimated 25% to 30% of the US population suffers from dyspepsia.1 Most self-treat with home remedies and over-the-counter products, but others seek medical care. Dyspepsia accounts for an estimated 2% to 5% of primary care visits annually,2 mostly by patients who are found to have no organic, or structural, cause for their symptoms.1,3
Such patients are said to have functional dyspepsia (FD), a category that applies to about two-thirds of those with dyspepsia.1 A small number of cases are categorized as organic dyspepsia, indicating the presence of a clear structural or anatomic cause, such as an ulcer or mass. The remainder are said to have undifferentiated dyspepsia, which simply means that their signs and symptoms do not rise to the level for which further investigation is warranted and thus it is not known whether it is functional or organic.
There are many possible causes of FD, ranging from medications3,4 to abnormal gastroduodenal motility5,6 to Helicobacter pylori infection,7 and a comprehensive differential diagnosis. The first step in an investigation is to rule out red flags suggestive of gastrointestinal (GI) cancer or other serious disorders.
Patients with FD, like the vast majority of those you’ll treat in a primary care setting, suffer significant morbidity. Most have chronic symptoms, with intermittent flare-ups interspersed with periods of remission.8 In the text and dyspepsia treatment ALGORITHM5,7-12 that follow, you’ll find an evidence-based patient management approach.
Symptoms and causes: What to look for
The primary symptoms of dyspepsia include bothersome postprandial fullness, early satiety, and epigastric pain and burning. To meet the Rome criteria for dyspepsia, these symptoms must have been present for the last 3 months and have had an onset ≥6 months prior to diagnosis.2 Recurrent belching and nausea are also common, but are not included in the Rome diagnostic criteria.
Symptom severity is a poor predictor of the seriousness of the condition, however, and more intense symptoms are no more likely than milder cases to have an organic cause.13,14 Indeed, anxiety is a common comorbidity in patients with FD and a risk factor for the diagnosis. Compared with the general public, patients with FD have been found to have higher levels of anxiety, chronic tension, hostility, and hypochondriasis, and a tendency to be more pessimistic.15
Possible causes of FD
While the etiology of organic dyspepsia is clear, the cause of FD is often far more difficult to determine.
Medication use should always be considered, as many types of drugs—including bisphosphonates, antibiotics, narcotics, steroids, iron, metformin, and nonsteroidal anti-inflammatory drugs (NSAIDs)—are associated with dyspepsia.3,4
Gastroduodenal motility and accommodation, which has been found in numerous studies of patients with FD, is a proposed etiology.5,6
Visceral hypersensitivity also appears to play a role. In one study of patients with severe dyspepsia, 87% of those with FD had a reduced or altered GI pain threshold, compared with 20% of those with organic dyspepsia.16
H pylori, commonly linked to peptic ulcer disease (PUD), is also associated with both organic dyspepsia and FD.17,18 The gram-negative rod-shaped bacterium is present in approximately half of the population worldwide, but is more common in developing nations.7H pylori immunoglobulin G (IgG) is more prevalent in patients with dyspepsia, particularly in those younger than 30 years of age. The exact mechanism by which H pylori causes non-ulcerative dyspepsia is not clear, but inflammation, dysmotility, visceral hypersensitivity, and alteration of acid secretion have all been proposed.17
Dysfunctional intestinal epithelium is increasingly being considered in the pathophysiology of dyspepsia, among other conditions. Researchers theorize that certain foods, toxins, infections, and/or other stressors lead to changes in the structure and function of tight junctions, resulting in increased intestinal permeability.19 This in turn is thought to allow the outflow of antigens through the leaky epithelium and to stimulate an immune response—a process that may play a role in the increased GI inflammation or hypersensitivity associated with dyspepsia. The “leaky gut” theory may eventually lead to new ways to treat dyspepsia, but thus far, highquality evidence of the efficacy of treatments aimed at this mechanism is lacking.
A range of disorders included in the differential
The primary differential diagnosis for dyspepsia includes gastroesophageal reflux disease (GERD), esophagitis, chronic PUD (including both gastric and duodenal ulcers), and malignancy. The differential may also include biliary disorder, pancreatitis, hepatitis, or other liver disease; chronic abdominal wall pain, irritable bowel syndrome, motility disorders, or infiltrative diseases of the stomach (eosinophilic gastritis, Crohn’s disease, sarcoidosis); celiac disease and food sensitivities/allergies, including gluten, lactose, and other intolerances; cardiac disease, including acute coronary syndrome, myocardial infarction, and arrhythmias; intestinal angina; small intestine bacterial overgrowth; heavy metal toxicity; and hypercalcemia.8
Ulcers are found in approximately 10% of patients undergoing evaluation for dyspepsia.8 Previously, PUD was almost exclusively due to H pylori infection. In developed countries, however, chronic use of NSAIDs, including aspirin, has increased, and is now responsible for most ulcer diseases.20,21 The combination of H pylori infection and NSAID usage appears to be synergistic, with the risk of uncomplicated PUD estimated to be 17.5 times higher among those who test positive for H pylori and take NSAIDs vs a 3- to 4-fold increase in ulcer incidence among those with either of these risk factors alone.22
The work-up starts with a search for red flags
Symptom severity is a poor predictor of the seriousness of dyspepsia; more intense symptoms are no more likely than milder cases to have an organic cause.
Evaluation of a patient with dyspepsia begins with a thorough history. Start by determining whether the patient has any red flags, or alarm features, that may be associated with a more serious condition—particularly an underlying malignancy. One or more of the following is an indication for an esophagogastroduodenoscopy (EGD):5,8,12
• family and/or personal history of upper GI cancer
• unintended weight loss
• GI bleeding
• progressive dysphagia
• unexplained iron-deficiency anemia
• persistent vomiting
• palpable mass or lymphadenopathy
• jaundice.
While it is important to rule out these red flags, they are poor predictors of malignancy.23,24 With the exception of a single study, their positive predictive value was a mere 1%.8 Their usefulness lies in their ability to exclude malignancy, however; when none of these features is present, the negative predictive value for malignancy is >97%.8
Age is also a risk factor. In addition to red flags, EGD is recommended by the American Gastroenterological Association (AGA) for patients with new-onset dyspepsia who are 55 years or older—an age at which upper GI malignancy becomes more common. A repeat EGD is rarely indicated, unless Barrett’s esophagus or severe erosive esophagitis is found on the initial EGD.25
Physical exam, H pylori evaluation follow
A physical examination of all patients presenting with symptoms suggestive of dyspepsia is crucial. While the exam is usually normal, it may reveal epigastric tenderness on abdominal palpation. Rebound tenderness, guarding, or evidence of other abnormalities should raise the prospect of alternative diagnoses. GERD, for example, has many symptoms in common with dyspepsia, but is a more likely diagnosis in a patient who has retrosternal burning discomfort and regurgitation and reports that symptoms worsen at night and when lying down.
Lab work has limited value. Although laboratory work is not specifically addressed in the AGA guidelines (except for H pylori testing), a complete blood count is a reasonable part of an initial evaluation of dyspepsia to check for anemia. Other routine blood work is not needed, but further lab testing may be warranted based on the history, exam, and differential diagnosis.
H pylori risk. Because of the association between dyspepsia and H pylori, evaluating the patient’s risk for infection with this bacterium, based primarily on his or her current and previous living conditions (TABLE 1),9 is the next step. Although a test for H pylori could be included in the initial work-up of all patients with dyspepsia, a better—and more cost-effective—strategy is to initially test only those at high risk. (More on testing and treating H pylori in a bit.)
Initiate acid suppression therapy for low-risk patients
First-line treatment for patients with dyspepsia who have no red flags for malignancy or other serious conditions and either are not at high risk for H pylori or are at high risk but have been tested for it and had negative results is a 4- to 8-week course of acid suppression therapy. Patients at low risk for H pylori should be tested for the bacterium only if therapy fails to alleviate their symptoms.9
H2RAs or PPIs? A look at the evidence
In a Cochrane review, both H2 receptor antagonists (H2RAs) and proton pump inhibitors (PPIs) were significantly more effective than placebo for treating FD.26 However, H2RAs can lead to tachyphylaxis—an acute decrease in response to a drug—within 2 to 6 weeks, thus limiting their long-term efficacy.27
PPIs appear to be more effective than H2RAs, and are the AGA’s acid suppression drug of choice.11 The CADET study, a randomized controlled trial comparing PPIs (omeprazole 20 mg/d) with an H2RA (ranitidine 150 mg BID) and a prokinetic agent (cisapride 20 mg BID) as well as placebo for dyspepsia, found the PPI to be superior to the H2RA at 6 months.28 In a systematic review, the number needed to treat with PPI therapy for improvement of dyspepsia symptoms was 9.29
There is no specified time limit for the use of PPIs. AGA guidelines recommend that patients who respond to initial therapy stop treatment after 4 to 8 weeks.11 If symptoms recur, another course of the same treatment is justified; if necessary, therapy can continue long term. However, patients should be made aware of the risk for vitamin deficiency, osteoporosis, and fracture, as well as arrhythmias, Clostridium difficile infection, and rebound upon abrupt discontinuation of PPIs.
When to test for H pylori ...
Empiric treatment for H pylori is not recommended. Thus, testing is indicated for patients who have risk factors for the bacterium or who fail to respond to acid suppression therapy. There are various ways to test for the presence of H pylori. Which test you choose depends, in part, on patient-specific factors.
Serology. IgG serology testing is extremely useful in patients who have never been diagnosed with H pylori. It is best suited for those who are currently taking proton pump inhibitors (PPIs) or who recently completed a course of antibiotics, since neither medication affects the results of the serology test.
Serology testing should not be used, however, for any patient who was previously diagnosed with or treated for H pylori, because this type of test cannot distinguish between an active or past infection. The IgG serology test has a sensitivity of 87% and a specificity of 67%.30
Stool antigen. Stool tests using monoclonal antibodies to detect the presence of H pylori have a sensitivity of 87% to 92% and a specificity of 70%. Stool antigen is also an excellent post-treatment test to confirm that H pylori has been eradicated.31
Stool testing has some drawbacks, however. PPIs can decrease the sensitivity and should be discontinued at least 2 weeks prior to stool testing.32 In addition, a stool test for H pylori is not accurate if the patient has an acute GI bleed.
Urea breath testing. This is the most sensitive and specific test for active H pylori infection (90%-96% sensitivity and 88%-96% specificity).33 PPIs can lower the sensitivity of the test, however, and are typically discontinued at least 2 weeks prior to testing. Urea breath testing, like stool testing, is an excellent way to confirm that H pylori has been eradicated after treatment. However, it is more expensive than other tests for H pylori and often inconvenient to obtain.13
An EGD is indicated for a patient who has failed to respond to acid suppression therapy and has a negative serology, stool antigen, or urea breath test for H pylori.
Biopsy-based testing for H pylori is performed with EGD and is therefore reserved for patients who have red flags or other indications of a need for invasive testing. There are 3 types of biopsy-based tests: urease (sensitivity, 70%-90%; specificity, 95%); histology (87%-92% and 70%, respectively); and culture (85%-88% and 69%, respectively). Overall, the specificity is slightly better than that of noninvasive testing, but the sensitivity can be lowered by recent use of PPIs, bismuth, or antibiotics.12,34
... and how to treat it
H pylori infection is associated with an increased risk of noncardiac gastric adenocarcinoma, but a decreased risk of cardiac gastric adenocarcinoma and esophageal adenocarcinoma.35,36 Thus, the potential to reduce the risk of gastric cancer is not considered an indication for H pylori treatment. The possibility of improving dyspepsia symptoms is a reason to treat H pylori infection, although eradicating it does not always do so.
In a 2006 Cochrane Review, treating H pylori had a small but statistically significant benefit for patients with FD (NNT=14).37 A 2011 study on the effects of H pylori eradication on symptoms and quality of life in primary care patients with FD revealed a 12.5% improvement in quality of life and a 10.6% improvement in symptoms.38
The triple therapy regimen (a PPI + amoxicillin + clarithromycin) is the most common first-line H pylori treatment in the United States, and a good initial choice in regions in which clarithromycin resistance is low (TABLE 2).39-44 The standard duration is 7 days. A 2013 Cochrane Review showed that a longer duration (14 days) increased the rate of eradication (82% vs 73%), but this remains controversial.39 The addition of bismuth subsalicylate to the triple therapy regimen has been shown to increase the eradication rate of H pylori by approximately 10%.45 Adding probiotics (saccharomyces or lactobacillus) appears to increase eradication rates, as well.40
Sequential therapy consists of a 5-day course of treatment in which a PPI and amoxicillin are taken twice a day, followed by another 5-day course of a PPI, clarithromycin, and metronidazole. A recent meta-analysis of sequential therapy showed that it is superior to 7-day triple therapy but equivalent to 14-day triple therapy.40
LOAD (levofloxacin, omeprazole, nitazoxanide, and doxycycline) therapy for 7 to 10 days can be used in place of triple therapy in areas of high resistance or for persistent H pylori. In one study, the H pylori eradication rate for a 7-day course of LOAD therapy—levofloxacin and doxycycline taken once a day, omeprazole before breakfast, and nitazoxanide twice daily—was 90% vs 73.3% for a 7-day course of triple therapy.41
Quadruple therapy has 2 variations: bismuth-based and non-bismuth (concomitant) therapy. The latter uses the base triple therapy and adds either metronidazole or tinidazole for 7 to 14 days. In a multicenter randomized trial, this concomitant therapy was found to have similar efficacy to sequential therapy.42
Bismuth-based quad therapy includes a PPI, bismuth, metronidazole, and tetracycline. A meta-analysis found it to have a higher rate of eradication than triple therapy for patients with antibiotic resistance.43,44
For persistent H pylori, a PPI, levofloxacin, and amoxicillin for 10 days has been shown to be more effective and better tolerated than quadruple therapy.12
Confirmation is indicated when symptoms persist
If dyspepsia symptoms persist after H pylori treatment, it is reasonable to retest to confirm that the infection has in fact been eradicated. Confirmation is also indicated if the patient has an H pylori-associated ulcer or a prior history of gastric cancer.
Retesting should be performed at least 4 to 6 weeks after treatment is completed. If H pylori has not been eradicated, you can try another regimen. If retesting confirms eradication and symptoms persist, EGD with biopsy is indicated. Although EGD typically has a very low yield, even for patients with red flags, this invasive test often provides reassurance and increased satisfaction for patients with persistent symptoms.46
More options for challenging cases
Managing FD is challenging when both initial acid suppression therapy and H pylori eradication fail. Unproven but low-risk treatments include modification of eating habits (eg, eating slower, not gulping food), reducing stress, discontinuing medications that may be related to symptoms, avoiding foods that seem to exacerbate symptoms, and cutting down or eliminating tobacco, caffeine, alcohol, and carbonated beverages.8 Bismuth salts have been shown to be superior to placebo for the treatment of dyspepsia.25 Small studies have also demonstrated a favorable risk–benefit ratio for peppermint oil and caraway oil for the treatment of FD.47 Prokinetics have shown efficacy compared with placebo, although a Cochrane review questioned their efficacy based on publication bias.26
There is no good evidence of efficacy for over-the-counter antacids, such as TUMS, or for GI “cocktails” (antacid, antispasmotic, and lidocaine), sucralfate, psychological interventions (eg, cognitive behavioral therapy, relaxation therapy, or hypnosis), or antidepressants.48,49 Several recent randomized controlled trials have shown the efficacy of acupuncture for the treatment of dyspepsia.49,50 Ginger may also be helpful; it has been found to help with nausea in other GI conditions, but it’s uncertain whether it can help patients with dyspepsia.51
CORRESPONDENCE
Michael Malone, MD, 845 Fishburn Road, Hershey, PA 17053; mmalone@hmc.psu.edu
1. Shaib Y, El-Serag HB. The prevalence and risk factors of functional dyspepsia in a multiethnic population in the United States. Am J Gastroenterol. 2004;99:2210-2216.
2. Talley NJ. Dyspepsia: management guidelines for the millennium. Gut. 2002;50(suppl 4):iv72–iv78.
3. Harmon RC, Peura DA. Evaluation and management of dyspepsia. Therap Adv Gastroenterol. 2010;3:87–98.
4. Bazaldua OV, Schneider FD. Evaluation and management of dyspepsia. Am Fam Physician. 1999;60:1773-1784.
5. Tack J, Talley NJ, Camilleri M, et al. Functional gastroduodenal disorders. Gastroenterology. 2006;130:1466-1479.
6. Haag S, Talley NJ, Holtmann G. Symptom patterns in functional dyspepsia and irritable bowel syndrome: relationship to disturbances in gastric emptying and response to a nutrient challenge in consulters and non-consulters. Gut. 2004;53:1445-1451.
7. Malfertheiner P, Megraud F, O’Morain CA, et al; European Helicobacter Study Group. Management of Helicobacter pylori infection—the Maastricht IV/Florence Consensus Report. Gut. 2012;61:646-664.
8. Talley NJ, Vakil NB, Moayyedi P. American Gastroenterological Association technical review on the evaluation of dyspepsia. Gastroenterology. 2005;129:1756-1780.
9. Moayyedi P, Axon AT. The usefulness of the likelihood ratio in the diagnosis of dyspepsia and gastroesophageal reflux disease. Am J Gastroenterol. 1999;94:3122-3125.
10. McColl KE. Clinical practice. Helicobacter pylori infection. N Engl J Med. 2010;362:1597-1604.
11. Kahrilas PJ, Shaheen NJ, Vaezi MF, et al; American Gastroenterological Association. American Gastroenterological Association Medical Position Statement on the management of gastroesophageal reflux disease. Gastroenterology. 2008;135:1383-1391.
12. Chey WD, Wong BC; Practice Parameters Committee of the American College of Gastroenterology. American College of Gastroenterology guideline on the management of Helicobacter pylori Infection. Am J Gastroenterol. 2007;102:1808-1825.
13. Moayyedi P, Talley NJ, Fennerty MB, et al. Can the clinical history distinguish between organic and functional dyspepsia? JAMA. 2006;295:1566-1576.
14. Eslick GD, Howell SC, Hammer J, et al. Empirically derived symptom sub-groups correspond poorly with diagnostic criteria for functional dyspepsia and irritable bowel syndrome. A factor and cluster analysis of a patient sample. Aliment Pharmacol Ther. 2004;19:133-140.
15. Aro P, Talley NJ, Ronkainen J, et al. Anxiety is associated with uninvestigated and functional dyspepsia (Rome III criteria) in a Swedish population-based study. Gastroenterology. 2009;137:94-100.
16. Mertz H, Fullerton S, Naliboff B, et al. Symptoms and visceral perception in severe functional and organic dyspepsia. Gut. 1998;42:814-822.
17. O’Morain C. Role of Helicobacter pylori in functional dyspepsia. World J Gastroenterol. 2006;12:2677-2680.
18. Shmuely H, Obure S, Passaro DJ, et al. Dyspepsia symptoms and Helicobacter pylori infection, Nakuru, Kenya. Emerg Infect Dis. 2003;9:1103-1107.
19. Barbara G, Zecchi L, Barbaro R, et al. Mucosal permeability and immune activation as potential therapeutic targets of probiotics in irritable bowel syndrome. J Clin Gastroenterol. 2012;46(suppl):S52-S55.
20. Liu NJ, Lee CS, Tang JH, et al. Outcomes of bleeding peptic ulcers: a prospective study. J Gastroenterol Hepatol. 2008;23:e340-e347.
21. Ramsoekh D, van Leerdam ME, Rauws EA, et al. Outcome of peptic ulcer bleeding, nonsteroidal anti-inflammatory drug use, and Helicobacter pylori infection. Clin Gastroenterol Hepatol. 2005;3:859-864.
22. Papatheodoridis GV, Sougioultzis S, Archimandritis AJ. Effects of Helicobacter pylori and nonsteroidal anti-inflammatory drugs on peptic ulcer disease: a systematic review. Clin Gastroenterol Hepatol. 2006;4:130-142.
23. Bai Y, Li ZS, Zou DW, et al. Alarm features and age for predicting upper gastrointestinal malignancy in Chinese patients with dyspepsia with high background prevalence of Helicobacter pylori infection and upper gastrointestinal malignancy: an endoscopic database review of 102,665 patients from 1996 to 2006. Gut. 2010;59:722-728.
24. Vakil N. Dyspepsia, peptic ulcer, and H. pylori: a remembrance of things past. Am J Gastroenterol. 2010;105:572-574.
25. Shaheen NJ, Weinberg DS, Denberg TD, et al; Clinical Guidelines Committee of the American College of Physicians. Upper endoscopy for gastroesophageal reflux disease: best practice advice from the clinical guidelines committee of the American College of Physicians. Ann Intern Med. 2012;157:808-816.
26. Moayyedi P, Soo S, Deeks J, et al. Pharmacological interventions for non-ulcer dyspepsia. Cochrane Database Syst Rev. 2006;(4):CD001960.
27. Chiu CT, Hsu CM, Wang CC, et al. Randomised clinical trial: sodium alginate oral suspension is non-inferior to omeprazole in the treatment of patients with non-erosive gastroesophageal disease. Aliment Pharmacol Ther. 2013;38:1054-1064.
28. Veldhuyzen van Zanten SJ, Chiba N, Armstrong D, et al. A randomized trial comparing omeprazole, ranitidine, cisapride, or placebo in helicobacter pylori negative, primary care patients with dyspepsia: the CADET-HN Study. Am J Gastroenterol. 2005;100:1477-1488.
29. Moayyedi P, Delaney BC, Vakil N, et al. The efficacy of proton pump inhibitors in nonulcer dyspepsia: a systematic review and economic analysis. Gastroenterology. 2004;127:1329-1337.
30. Garza-González E, Bosques-Padilla FJ, Tijerina-Menchaca R, et al. Comparison of endoscopy-based and serum-based methods for the diagnosis of Helicobacter pylori. Can J Gastroenterol. 2003;17:101-106.
31. Kodama M, Murakami K, Okimoto T, et al. Influence of proton pump inhibitor treatment on Helicobacter pylori stool antigen test. World J Gastroenterol. 2012;18:44-48.
32. Shimoyama T. Stool antigen tests for the management of Helicobacter pylori infection. World J Gastroenterol. 2013;19:8188-8191.
33. Howden CW, Hunt RH. Guidelines for the management of Helicobacter pylori infection. Ad Hoc Committee on Practice Parameters of the American College of Gastroenterology. Am J Gastroenterol. 1998;93:2330-2338.
34. Gisbert J, Abraira V. Accuracy of Helicobacter pylori diagnostic tests in patients with bleeding peptic ulcer: a systematic review and meta-analysis. Am J Gastroenterol. 2006;101:848-863.
35. Kamangar F, Dawsey SM, Blaser MJ, et al. Opposing risks of gastric cardiac and noncardia gastric adenocarcinomas associated with Helicobacter pylori seropositivity. J Natl Cancer Inst. 2006;98:1445-1452.
36. Islami F, Kamangar F. Helicobacter pylori and esophageal cancer risk: a meta-analysis. Cancer Prevent Res (Phila). 2008;1:329-338.
37. Moayyedi P, Soo S, Deeks J, et al. Eradication of Helicobacter pylori for non-ulcer dyspepsia. Cochrane Database Syst Rev. 2006;(2):CD002096.
38. Mazzoleni LE, Sander GB, Francesconi CF, et al. Helicobacter pylori eradication in functional dyspepsia: HEROES trial. Arch Intern Med. 2011;171:1929-1936.
39. Yuan Y, Ford AC, Khan KJ, et al. Optimum duration of regimens for Helicobacter pylori eradication. Cochrane Database Syst Rev. 2013;(12):CD008337.
40. Zou J, Dong J, Yu X. Meta-analysis: Lactobacillus containing quadruple therapy versus standard triple first-line therapy for Helicobacter pylori eradication. Helicobacter. 2009;14:97-107.
41. Basu PP, Rayapudi K, Pacana T, et al. A randomized study comparing levofloxacin, omeprazole, nitazoxanide, and doxycycline versus triple therapy for the eradication of Helicobacter pylori. Am J Gastroenterol. 2011;106:1970-1975.
42. Wu DC, Hsu PI, Wu JY, et al. Sequential and concomitant therapy with 4 drugs are equally effective for eradication of H. pylori infection. Clin Gastroenterol Hepatol. 2010;8:36–41.
43. Osato R, Reddy R, Reddy SG, et al. Pattern of primary resistance of Helicobacter pylori to metronidazole or clarithromycin in the United States. Arch Intern Med. 2001;161:1217-1220.
44. Fischbach L, Evans EL. Meta-analysis: the effect of antibiotic resistance status on the efficacy of triple and quadruple firstline therapies for Helicobacter pylori. Aliment Pharmacol Ther. 2007;26:343-357.
45. Hinostroza Morales D, Díaz Ferrer J. Addition of bismuth subsalicylate to triple eradication therapy for Helicobacter pylori infection: efficiency and adverse events. Rev Gastroenterol Peru. 2014;34:315-320.
46. Rabeneck L, Wristers K, Souchek J, et al. Impact of upper endoscopy on satisfaction in patients with previously uninvestigated dyspepsia. Gastrointest Endosc. 2003;57:295-299.
47. Hojo M, Miwa H, Yokoyama T, et al. Treatment of functional dyspepsia with antianxiety or antidepressive agents: systematic review. J Gastroenterol. 2005;40:1036-1042.
48. Soo S, Moayyedi P, Deeks J, et al. Psychological interventions for non-ulcer dyspepsia. Cochrane Database Syst Rev. 2005;(2):CD002301.
49. Lima FA, Ferreira LE, Pace FH. Acupuncture effectiveness as a complementary therapy in functional dyspepsia patients. Arq Gastroenterol. 2013;50:202-207.
50. Ma TT, Yu SY, Li Y, et al. Randomised clinical trial: an assessment of acupuncture on specific meridian or specific acupoint vs. sham acupuncture for treating functional dyspepsia. Aliment Pharmacol Ther. 2012;35:552-561.
51. Koretz RL, Rotblatt M. Complementary and alternative medicine in gastroenterology: the good, the bad, and the ugly. Clin Gastroenterol Hepatol. 2004;2:957-967.
› Review the medications taken by patients who suffer from dyspepsia, as many drugs—bisphosphonates, antibiotics, steroids, and nonsteroidal anti-inflammatory drugs, among others—are associated with this condition. B
› Order an esophagogastroduodenoscopy for patients ages 55 years or older with new-onset dyspepsia and those who have red flags for more serious conditions, eg, a history of upper gastrointestinal (GI) cancer, unintended weight loss, GI bleeding, dysphagia, or a palpable mass. C
› Prescribe acid suppression therapy as first-line treatment for patients who have dyspepsia but are at low risk or have tested negative for Helicobacter pylori infection. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Each year, an estimated 25% to 30% of the US population suffers from dyspepsia.1 Most self-treat with home remedies and over-the-counter products, but others seek medical care. Dyspepsia accounts for an estimated 2% to 5% of primary care visits annually,2 mostly by patients who are found to have no organic, or structural, cause for their symptoms.1,3
Such patients are said to have functional dyspepsia (FD), a category that applies to about two-thirds of those with dyspepsia.1 A small number of cases are categorized as organic dyspepsia, indicating the presence of a clear structural or anatomic cause, such as an ulcer or mass. The remainder are said to have undifferentiated dyspepsia, which simply means that their signs and symptoms do not rise to the level for which further investigation is warranted and thus it is not known whether it is functional or organic.
There are many possible causes of FD, ranging from medications3,4 to abnormal gastroduodenal motility5,6 to Helicobacter pylori infection,7 and a comprehensive differential diagnosis. The first step in an investigation is to rule out red flags suggestive of gastrointestinal (GI) cancer or other serious disorders.
Patients with FD, like the vast majority of those you’ll treat in a primary care setting, suffer significant morbidity. Most have chronic symptoms, with intermittent flare-ups interspersed with periods of remission.8 In the text and dyspepsia treatment ALGORITHM5,7-12 that follow, you’ll find an evidence-based patient management approach.
Symptoms and causes: What to look for
The primary symptoms of dyspepsia include bothersome postprandial fullness, early satiety, and epigastric pain and burning. To meet the Rome criteria for dyspepsia, these symptoms must have been present for the last 3 months and have had an onset ≥6 months prior to diagnosis.2 Recurrent belching and nausea are also common, but are not included in the Rome diagnostic criteria.
Symptom severity is a poor predictor of the seriousness of the condition, however, and more intense symptoms are no more likely than milder cases to have an organic cause.13,14 Indeed, anxiety is a common comorbidity in patients with FD and a risk factor for the diagnosis. Compared with the general public, patients with FD have been found to have higher levels of anxiety, chronic tension, hostility, and hypochondriasis, and a tendency to be more pessimistic.15
Possible causes of FD
While the etiology of organic dyspepsia is clear, the cause of FD is often far more difficult to determine.
Medication use should always be considered, as many types of drugs—including bisphosphonates, antibiotics, narcotics, steroids, iron, metformin, and nonsteroidal anti-inflammatory drugs (NSAIDs)—are associated with dyspepsia.3,4
Gastroduodenal motility and accommodation, which has been found in numerous studies of patients with FD, is a proposed etiology.5,6
Visceral hypersensitivity also appears to play a role. In one study of patients with severe dyspepsia, 87% of those with FD had a reduced or altered GI pain threshold, compared with 20% of those with organic dyspepsia.16
H pylori, commonly linked to peptic ulcer disease (PUD), is also associated with both organic dyspepsia and FD.17,18 The gram-negative rod-shaped bacterium is present in approximately half of the population worldwide, but is more common in developing nations.7H pylori immunoglobulin G (IgG) is more prevalent in patients with dyspepsia, particularly in those younger than 30 years of age. The exact mechanism by which H pylori causes non-ulcerative dyspepsia is not clear, but inflammation, dysmotility, visceral hypersensitivity, and alteration of acid secretion have all been proposed.17
Dysfunctional intestinal epithelium is increasingly being considered in the pathophysiology of dyspepsia, among other conditions. Researchers theorize that certain foods, toxins, infections, and/or other stressors lead to changes in the structure and function of tight junctions, resulting in increased intestinal permeability.19 This in turn is thought to allow the outflow of antigens through the leaky epithelium and to stimulate an immune response—a process that may play a role in the increased GI inflammation or hypersensitivity associated with dyspepsia. The “leaky gut” theory may eventually lead to new ways to treat dyspepsia, but thus far, highquality evidence of the efficacy of treatments aimed at this mechanism is lacking.
A range of disorders included in the differential
The primary differential diagnosis for dyspepsia includes gastroesophageal reflux disease (GERD), esophagitis, chronic PUD (including both gastric and duodenal ulcers), and malignancy. The differential may also include biliary disorder, pancreatitis, hepatitis, or other liver disease; chronic abdominal wall pain, irritable bowel syndrome, motility disorders, or infiltrative diseases of the stomach (eosinophilic gastritis, Crohn’s disease, sarcoidosis); celiac disease and food sensitivities/allergies, including gluten, lactose, and other intolerances; cardiac disease, including acute coronary syndrome, myocardial infarction, and arrhythmias; intestinal angina; small intestine bacterial overgrowth; heavy metal toxicity; and hypercalcemia.8
Ulcers are found in approximately 10% of patients undergoing evaluation for dyspepsia.8 Previously, PUD was almost exclusively due to H pylori infection. In developed countries, however, chronic use of NSAIDs, including aspirin, has increased, and is now responsible for most ulcer diseases.20,21 The combination of H pylori infection and NSAID usage appears to be synergistic, with the risk of uncomplicated PUD estimated to be 17.5 times higher among those who test positive for H pylori and take NSAIDs vs a 3- to 4-fold increase in ulcer incidence among those with either of these risk factors alone.22
The work-up starts with a search for red flags
Symptom severity is a poor predictor of the seriousness of dyspepsia; more intense symptoms are no more likely than milder cases to have an organic cause.
Evaluation of a patient with dyspepsia begins with a thorough history. Start by determining whether the patient has any red flags, or alarm features, that may be associated with a more serious condition—particularly an underlying malignancy. One or more of the following is an indication for an esophagogastroduodenoscopy (EGD):5,8,12
• family and/or personal history of upper GI cancer
• unintended weight loss
• GI bleeding
• progressive dysphagia
• unexplained iron-deficiency anemia
• persistent vomiting
• palpable mass or lymphadenopathy
• jaundice.
While it is important to rule out these red flags, they are poor predictors of malignancy.23,24 With the exception of a single study, their positive predictive value was a mere 1%.8 Their usefulness lies in their ability to exclude malignancy, however; when none of these features is present, the negative predictive value for malignancy is >97%.8
Age is also a risk factor. In addition to red flags, EGD is recommended by the American Gastroenterological Association (AGA) for patients with new-onset dyspepsia who are 55 years or older—an age at which upper GI malignancy becomes more common. A repeat EGD is rarely indicated, unless Barrett’s esophagus or severe erosive esophagitis is found on the initial EGD.25
Physical exam, H pylori evaluation follow
A physical examination of all patients presenting with symptoms suggestive of dyspepsia is crucial. While the exam is usually normal, it may reveal epigastric tenderness on abdominal palpation. Rebound tenderness, guarding, or evidence of other abnormalities should raise the prospect of alternative diagnoses. GERD, for example, has many symptoms in common with dyspepsia, but is a more likely diagnosis in a patient who has retrosternal burning discomfort and regurgitation and reports that symptoms worsen at night and when lying down.
Lab work has limited value. Although laboratory work is not specifically addressed in the AGA guidelines (except for H pylori testing), a complete blood count is a reasonable part of an initial evaluation of dyspepsia to check for anemia. Other routine blood work is not needed, but further lab testing may be warranted based on the history, exam, and differential diagnosis.
H pylori risk. Because of the association between dyspepsia and H pylori, evaluating the patient’s risk for infection with this bacterium, based primarily on his or her current and previous living conditions (TABLE 1),9 is the next step. Although a test for H pylori could be included in the initial work-up of all patients with dyspepsia, a better—and more cost-effective—strategy is to initially test only those at high risk. (More on testing and treating H pylori in a bit.)
Initiate acid suppression therapy for low-risk patients
First-line treatment for patients with dyspepsia who have no red flags for malignancy or other serious conditions and either are not at high risk for H pylori or are at high risk but have been tested for it and had negative results is a 4- to 8-week course of acid suppression therapy. Patients at low risk for H pylori should be tested for the bacterium only if therapy fails to alleviate their symptoms.9
H2RAs or PPIs? A look at the evidence
In a Cochrane review, both H2 receptor antagonists (H2RAs) and proton pump inhibitors (PPIs) were significantly more effective than placebo for treating FD.26 However, H2RAs can lead to tachyphylaxis—an acute decrease in response to a drug—within 2 to 6 weeks, thus limiting their long-term efficacy.27
PPIs appear to be more effective than H2RAs, and are the AGA’s acid suppression drug of choice.11 The CADET study, a randomized controlled trial comparing PPIs (omeprazole 20 mg/d) with an H2RA (ranitidine 150 mg BID) and a prokinetic agent (cisapride 20 mg BID) as well as placebo for dyspepsia, found the PPI to be superior to the H2RA at 6 months.28 In a systematic review, the number needed to treat with PPI therapy for improvement of dyspepsia symptoms was 9.29
There is no specified time limit for the use of PPIs. AGA guidelines recommend that patients who respond to initial therapy stop treatment after 4 to 8 weeks.11 If symptoms recur, another course of the same treatment is justified; if necessary, therapy can continue long term. However, patients should be made aware of the risk for vitamin deficiency, osteoporosis, and fracture, as well as arrhythmias, Clostridium difficile infection, and rebound upon abrupt discontinuation of PPIs.
When to test for H pylori ...
Empiric treatment for H pylori is not recommended. Thus, testing is indicated for patients who have risk factors for the bacterium or who fail to respond to acid suppression therapy. There are various ways to test for the presence of H pylori. Which test you choose depends, in part, on patient-specific factors.
Serology. IgG serology testing is extremely useful in patients who have never been diagnosed with H pylori. It is best suited for those who are currently taking proton pump inhibitors (PPIs) or who recently completed a course of antibiotics, since neither medication affects the results of the serology test.
Serology testing should not be used, however, for any patient who was previously diagnosed with or treated for H pylori, because this type of test cannot distinguish between an active or past infection. The IgG serology test has a sensitivity of 87% and a specificity of 67%.30
Stool antigen. Stool tests using monoclonal antibodies to detect the presence of H pylori have a sensitivity of 87% to 92% and a specificity of 70%. Stool antigen is also an excellent post-treatment test to confirm that H pylori has been eradicated.31
Stool testing has some drawbacks, however. PPIs can decrease the sensitivity and should be discontinued at least 2 weeks prior to stool testing.32 In addition, a stool test for H pylori is not accurate if the patient has an acute GI bleed.
Urea breath testing. This is the most sensitive and specific test for active H pylori infection (90%-96% sensitivity and 88%-96% specificity).33 PPIs can lower the sensitivity of the test, however, and are typically discontinued at least 2 weeks prior to testing. Urea breath testing, like stool testing, is an excellent way to confirm that H pylori has been eradicated after treatment. However, it is more expensive than other tests for H pylori and often inconvenient to obtain.13
An EGD is indicated for a patient who has failed to respond to acid suppression therapy and has a negative serology, stool antigen, or urea breath test for H pylori.
Biopsy-based testing for H pylori is performed with EGD and is therefore reserved for patients who have red flags or other indications of a need for invasive testing. There are 3 types of biopsy-based tests: urease (sensitivity, 70%-90%; specificity, 95%); histology (87%-92% and 70%, respectively); and culture (85%-88% and 69%, respectively). Overall, the specificity is slightly better than that of noninvasive testing, but the sensitivity can be lowered by recent use of PPIs, bismuth, or antibiotics.12,34
... and how to treat it
H pylori infection is associated with an increased risk of noncardiac gastric adenocarcinoma, but a decreased risk of cardiac gastric adenocarcinoma and esophageal adenocarcinoma.35,36 Thus, the potential to reduce the risk of gastric cancer is not considered an indication for H pylori treatment. The possibility of improving dyspepsia symptoms is a reason to treat H pylori infection, although eradicating it does not always do so.
In a 2006 Cochrane Review, treating H pylori had a small but statistically significant benefit for patients with FD (NNT=14).37 A 2011 study on the effects of H pylori eradication on symptoms and quality of life in primary care patients with FD revealed a 12.5% improvement in quality of life and a 10.6% improvement in symptoms.38
The triple therapy regimen (a PPI + amoxicillin + clarithromycin) is the most common first-line H pylori treatment in the United States, and a good initial choice in regions in which clarithromycin resistance is low (TABLE 2).39-44 The standard duration is 7 days. A 2013 Cochrane Review showed that a longer duration (14 days) increased the rate of eradication (82% vs 73%), but this remains controversial.39 The addition of bismuth subsalicylate to the triple therapy regimen has been shown to increase the eradication rate of H pylori by approximately 10%.45 Adding probiotics (saccharomyces or lactobacillus) appears to increase eradication rates, as well.40
Sequential therapy consists of a 5-day course of treatment in which a PPI and amoxicillin are taken twice a day, followed by another 5-day course of a PPI, clarithromycin, and metronidazole. A recent meta-analysis of sequential therapy showed that it is superior to 7-day triple therapy but equivalent to 14-day triple therapy.40
LOAD (levofloxacin, omeprazole, nitazoxanide, and doxycycline) therapy for 7 to 10 days can be used in place of triple therapy in areas of high resistance or for persistent H pylori. In one study, the H pylori eradication rate for a 7-day course of LOAD therapy—levofloxacin and doxycycline taken once a day, omeprazole before breakfast, and nitazoxanide twice daily—was 90% vs 73.3% for a 7-day course of triple therapy.41
Quadruple therapy has 2 variations: bismuth-based and non-bismuth (concomitant) therapy. The latter uses the base triple therapy and adds either metronidazole or tinidazole for 7 to 14 days. In a multicenter randomized trial, this concomitant therapy was found to have similar efficacy to sequential therapy.42
Bismuth-based quad therapy includes a PPI, bismuth, metronidazole, and tetracycline. A meta-analysis found it to have a higher rate of eradication than triple therapy for patients with antibiotic resistance.43,44
For persistent H pylori, a PPI, levofloxacin, and amoxicillin for 10 days has been shown to be more effective and better tolerated than quadruple therapy.12
Confirmation is indicated when symptoms persist
If dyspepsia symptoms persist after H pylori treatment, it is reasonable to retest to confirm that the infection has in fact been eradicated. Confirmation is also indicated if the patient has an H pylori-associated ulcer or a prior history of gastric cancer.
Retesting should be performed at least 4 to 6 weeks after treatment is completed. If H pylori has not been eradicated, you can try another regimen. If retesting confirms eradication and symptoms persist, EGD with biopsy is indicated. Although EGD typically has a very low yield, even for patients with red flags, this invasive test often provides reassurance and increased satisfaction for patients with persistent symptoms.46
More options for challenging cases
Managing FD is challenging when both initial acid suppression therapy and H pylori eradication fail. Unproven but low-risk treatments include modification of eating habits (eg, eating slower, not gulping food), reducing stress, discontinuing medications that may be related to symptoms, avoiding foods that seem to exacerbate symptoms, and cutting down or eliminating tobacco, caffeine, alcohol, and carbonated beverages.8 Bismuth salts have been shown to be superior to placebo for the treatment of dyspepsia.25 Small studies have also demonstrated a favorable risk–benefit ratio for peppermint oil and caraway oil for the treatment of FD.47 Prokinetics have shown efficacy compared with placebo, although a Cochrane review questioned their efficacy based on publication bias.26
There is no good evidence of efficacy for over-the-counter antacids, such as TUMS, or for GI “cocktails” (antacid, antispasmotic, and lidocaine), sucralfate, psychological interventions (eg, cognitive behavioral therapy, relaxation therapy, or hypnosis), or antidepressants.48,49 Several recent randomized controlled trials have shown the efficacy of acupuncture for the treatment of dyspepsia.49,50 Ginger may also be helpful; it has been found to help with nausea in other GI conditions, but it’s uncertain whether it can help patients with dyspepsia.51
CORRESPONDENCE
Michael Malone, MD, 845 Fishburn Road, Hershey, PA 17053; mmalone@hmc.psu.edu
› Review the medications taken by patients who suffer from dyspepsia, as many drugs—bisphosphonates, antibiotics, steroids, and nonsteroidal anti-inflammatory drugs, among others—are associated with this condition. B
› Order an esophagogastroduodenoscopy for patients ages 55 years or older with new-onset dyspepsia and those who have red flags for more serious conditions, eg, a history of upper gastrointestinal (GI) cancer, unintended weight loss, GI bleeding, dysphagia, or a palpable mass. C
› Prescribe acid suppression therapy as first-line treatment for patients who have dyspepsia but are at low risk or have tested negative for Helicobacter pylori infection. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Each year, an estimated 25% to 30% of the US population suffers from dyspepsia.1 Most self-treat with home remedies and over-the-counter products, but others seek medical care. Dyspepsia accounts for an estimated 2% to 5% of primary care visits annually,2 mostly by patients who are found to have no organic, or structural, cause for their symptoms.1,3
Such patients are said to have functional dyspepsia (FD), a category that applies to about two-thirds of those with dyspepsia.1 A small number of cases are categorized as organic dyspepsia, indicating the presence of a clear structural or anatomic cause, such as an ulcer or mass. The remainder are said to have undifferentiated dyspepsia, which simply means that their signs and symptoms do not rise to the level for which further investigation is warranted and thus it is not known whether it is functional or organic.
There are many possible causes of FD, ranging from medications3,4 to abnormal gastroduodenal motility5,6 to Helicobacter pylori infection,7 and a comprehensive differential diagnosis. The first step in an investigation is to rule out red flags suggestive of gastrointestinal (GI) cancer or other serious disorders.
Patients with FD, like the vast majority of those you’ll treat in a primary care setting, suffer significant morbidity. Most have chronic symptoms, with intermittent flare-ups interspersed with periods of remission.8 In the text and dyspepsia treatment ALGORITHM5,7-12 that follow, you’ll find an evidence-based patient management approach.
Symptoms and causes: What to look for
The primary symptoms of dyspepsia include bothersome postprandial fullness, early satiety, and epigastric pain and burning. To meet the Rome criteria for dyspepsia, these symptoms must have been present for the last 3 months and have had an onset ≥6 months prior to diagnosis.2 Recurrent belching and nausea are also common, but are not included in the Rome diagnostic criteria.
Symptom severity is a poor predictor of the seriousness of the condition, however, and more intense symptoms are no more likely than milder cases to have an organic cause.13,14 Indeed, anxiety is a common comorbidity in patients with FD and a risk factor for the diagnosis. Compared with the general public, patients with FD have been found to have higher levels of anxiety, chronic tension, hostility, and hypochondriasis, and a tendency to be more pessimistic.15
Possible causes of FD
While the etiology of organic dyspepsia is clear, the cause of FD is often far more difficult to determine.
Medication use should always be considered, as many types of drugs—including bisphosphonates, antibiotics, narcotics, steroids, iron, metformin, and nonsteroidal anti-inflammatory drugs (NSAIDs)—are associated with dyspepsia.3,4
Gastroduodenal motility and accommodation, which has been found in numerous studies of patients with FD, is a proposed etiology.5,6
Visceral hypersensitivity also appears to play a role. In one study of patients with severe dyspepsia, 87% of those with FD had a reduced or altered GI pain threshold, compared with 20% of those with organic dyspepsia.16
H pylori, commonly linked to peptic ulcer disease (PUD), is also associated with both organic dyspepsia and FD.17,18 The gram-negative rod-shaped bacterium is present in approximately half of the population worldwide, but is more common in developing nations.7H pylori immunoglobulin G (IgG) is more prevalent in patients with dyspepsia, particularly in those younger than 30 years of age. The exact mechanism by which H pylori causes non-ulcerative dyspepsia is not clear, but inflammation, dysmotility, visceral hypersensitivity, and alteration of acid secretion have all been proposed.17
Dysfunctional intestinal epithelium is increasingly being considered in the pathophysiology of dyspepsia, among other conditions. Researchers theorize that certain foods, toxins, infections, and/or other stressors lead to changes in the structure and function of tight junctions, resulting in increased intestinal permeability.19 This in turn is thought to allow the outflow of antigens through the leaky epithelium and to stimulate an immune response—a process that may play a role in the increased GI inflammation or hypersensitivity associated with dyspepsia. The “leaky gut” theory may eventually lead to new ways to treat dyspepsia, but thus far, highquality evidence of the efficacy of treatments aimed at this mechanism is lacking.
A range of disorders included in the differential
The primary differential diagnosis for dyspepsia includes gastroesophageal reflux disease (GERD), esophagitis, chronic PUD (including both gastric and duodenal ulcers), and malignancy. The differential may also include biliary disorder, pancreatitis, hepatitis, or other liver disease; chronic abdominal wall pain, irritable bowel syndrome, motility disorders, or infiltrative diseases of the stomach (eosinophilic gastritis, Crohn’s disease, sarcoidosis); celiac disease and food sensitivities/allergies, including gluten, lactose, and other intolerances; cardiac disease, including acute coronary syndrome, myocardial infarction, and arrhythmias; intestinal angina; small intestine bacterial overgrowth; heavy metal toxicity; and hypercalcemia.8
Ulcers are found in approximately 10% of patients undergoing evaluation for dyspepsia.8 Previously, PUD was almost exclusively due to H pylori infection. In developed countries, however, chronic use of NSAIDs, including aspirin, has increased, and is now responsible for most ulcer diseases.20,21 The combination of H pylori infection and NSAID usage appears to be synergistic, with the risk of uncomplicated PUD estimated to be 17.5 times higher among those who test positive for H pylori and take NSAIDs vs a 3- to 4-fold increase in ulcer incidence among those with either of these risk factors alone.22
The work-up starts with a search for red flags
Symptom severity is a poor predictor of the seriousness of dyspepsia; more intense symptoms are no more likely than milder cases to have an organic cause.
Evaluation of a patient with dyspepsia begins with a thorough history. Start by determining whether the patient has any red flags, or alarm features, that may be associated with a more serious condition—particularly an underlying malignancy. One or more of the following is an indication for an esophagogastroduodenoscopy (EGD):5,8,12
• family and/or personal history of upper GI cancer
• unintended weight loss
• GI bleeding
• progressive dysphagia
• unexplained iron-deficiency anemia
• persistent vomiting
• palpable mass or lymphadenopathy
• jaundice.
While it is important to rule out these red flags, they are poor predictors of malignancy.23,24 With the exception of a single study, their positive predictive value was a mere 1%.8 Their usefulness lies in their ability to exclude malignancy, however; when none of these features is present, the negative predictive value for malignancy is >97%.8
Age is also a risk factor. In addition to red flags, EGD is recommended by the American Gastroenterological Association (AGA) for patients with new-onset dyspepsia who are 55 years or older—an age at which upper GI malignancy becomes more common. A repeat EGD is rarely indicated, unless Barrett’s esophagus or severe erosive esophagitis is found on the initial EGD.25
Physical exam, H pylori evaluation follow
A physical examination of all patients presenting with symptoms suggestive of dyspepsia is crucial. While the exam is usually normal, it may reveal epigastric tenderness on abdominal palpation. Rebound tenderness, guarding, or evidence of other abnormalities should raise the prospect of alternative diagnoses. GERD, for example, has many symptoms in common with dyspepsia, but is a more likely diagnosis in a patient who has retrosternal burning discomfort and regurgitation and reports that symptoms worsen at night and when lying down.
Lab work has limited value. Although laboratory work is not specifically addressed in the AGA guidelines (except for H pylori testing), a complete blood count is a reasonable part of an initial evaluation of dyspepsia to check for anemia. Other routine blood work is not needed, but further lab testing may be warranted based on the history, exam, and differential diagnosis.
H pylori risk. Because of the association between dyspepsia and H pylori, evaluating the patient’s risk for infection with this bacterium, based primarily on his or her current and previous living conditions (TABLE 1),9 is the next step. Although a test for H pylori could be included in the initial work-up of all patients with dyspepsia, a better—and more cost-effective—strategy is to initially test only those at high risk. (More on testing and treating H pylori in a bit.)
Initiate acid suppression therapy for low-risk patients
First-line treatment for patients with dyspepsia who have no red flags for malignancy or other serious conditions and either are not at high risk for H pylori or are at high risk but have been tested for it and had negative results is a 4- to 8-week course of acid suppression therapy. Patients at low risk for H pylori should be tested for the bacterium only if therapy fails to alleviate their symptoms.9
H2RAs or PPIs? A look at the evidence
In a Cochrane review, both H2 receptor antagonists (H2RAs) and proton pump inhibitors (PPIs) were significantly more effective than placebo for treating FD.26 However, H2RAs can lead to tachyphylaxis—an acute decrease in response to a drug—within 2 to 6 weeks, thus limiting their long-term efficacy.27
PPIs appear to be more effective than H2RAs, and are the AGA’s acid suppression drug of choice.11 The CADET study, a randomized controlled trial comparing PPIs (omeprazole 20 mg/d) with an H2RA (ranitidine 150 mg BID) and a prokinetic agent (cisapride 20 mg BID) as well as placebo for dyspepsia, found the PPI to be superior to the H2RA at 6 months.28 In a systematic review, the number needed to treat with PPI therapy for improvement of dyspepsia symptoms was 9.29
There is no specified time limit for the use of PPIs. AGA guidelines recommend that patients who respond to initial therapy stop treatment after 4 to 8 weeks.11 If symptoms recur, another course of the same treatment is justified; if necessary, therapy can continue long term. However, patients should be made aware of the risk for vitamin deficiency, osteoporosis, and fracture, as well as arrhythmias, Clostridium difficile infection, and rebound upon abrupt discontinuation of PPIs.
When to test for H pylori ...
Empiric treatment for H pylori is not recommended. Thus, testing is indicated for patients who have risk factors for the bacterium or who fail to respond to acid suppression therapy. There are various ways to test for the presence of H pylori. Which test you choose depends, in part, on patient-specific factors.
Serology. IgG serology testing is extremely useful in patients who have never been diagnosed with H pylori. It is best suited for those who are currently taking proton pump inhibitors (PPIs) or who recently completed a course of antibiotics, since neither medication affects the results of the serology test.
Serology testing should not be used, however, for any patient who was previously diagnosed with or treated for H pylori, because this type of test cannot distinguish between an active or past infection. The IgG serology test has a sensitivity of 87% and a specificity of 67%.30
Stool antigen. Stool tests using monoclonal antibodies to detect the presence of H pylori have a sensitivity of 87% to 92% and a specificity of 70%. Stool antigen is also an excellent post-treatment test to confirm that H pylori has been eradicated.31
Stool testing has some drawbacks, however. PPIs can decrease the sensitivity and should be discontinued at least 2 weeks prior to stool testing.32 In addition, a stool test for H pylori is not accurate if the patient has an acute GI bleed.
Urea breath testing. This is the most sensitive and specific test for active H pylori infection (90%-96% sensitivity and 88%-96% specificity).33 PPIs can lower the sensitivity of the test, however, and are typically discontinued at least 2 weeks prior to testing. Urea breath testing, like stool testing, is an excellent way to confirm that H pylori has been eradicated after treatment. However, it is more expensive than other tests for H pylori and often inconvenient to obtain.13
An EGD is indicated for a patient who has failed to respond to acid suppression therapy and has a negative serology, stool antigen, or urea breath test for H pylori.
Biopsy-based testing for H pylori is performed with EGD and is therefore reserved for patients who have red flags or other indications of a need for invasive testing. There are 3 types of biopsy-based tests: urease (sensitivity, 70%-90%; specificity, 95%); histology (87%-92% and 70%, respectively); and culture (85%-88% and 69%, respectively). Overall, the specificity is slightly better than that of noninvasive testing, but the sensitivity can be lowered by recent use of PPIs, bismuth, or antibiotics.12,34
... and how to treat it
H pylori infection is associated with an increased risk of noncardiac gastric adenocarcinoma, but a decreased risk of cardiac gastric adenocarcinoma and esophageal adenocarcinoma.35,36 Thus, the potential to reduce the risk of gastric cancer is not considered an indication for H pylori treatment. The possibility of improving dyspepsia symptoms is a reason to treat H pylori infection, although eradicating it does not always do so.
In a 2006 Cochrane Review, treating H pylori had a small but statistically significant benefit for patients with FD (NNT=14).37 A 2011 study on the effects of H pylori eradication on symptoms and quality of life in primary care patients with FD revealed a 12.5% improvement in quality of life and a 10.6% improvement in symptoms.38
The triple therapy regimen (a PPI + amoxicillin + clarithromycin) is the most common first-line H pylori treatment in the United States, and a good initial choice in regions in which clarithromycin resistance is low (TABLE 2).39-44 The standard duration is 7 days. A 2013 Cochrane Review showed that a longer duration (14 days) increased the rate of eradication (82% vs 73%), but this remains controversial.39 The addition of bismuth subsalicylate to the triple therapy regimen has been shown to increase the eradication rate of H pylori by approximately 10%.45 Adding probiotics (saccharomyces or lactobacillus) appears to increase eradication rates, as well.40
Sequential therapy consists of a 5-day course of treatment in which a PPI and amoxicillin are taken twice a day, followed by another 5-day course of a PPI, clarithromycin, and metronidazole. A recent meta-analysis of sequential therapy showed that it is superior to 7-day triple therapy but equivalent to 14-day triple therapy.40
LOAD (levofloxacin, omeprazole, nitazoxanide, and doxycycline) therapy for 7 to 10 days can be used in place of triple therapy in areas of high resistance or for persistent H pylori. In one study, the H pylori eradication rate for a 7-day course of LOAD therapy—levofloxacin and doxycycline taken once a day, omeprazole before breakfast, and nitazoxanide twice daily—was 90% vs 73.3% for a 7-day course of triple therapy.41
Quadruple therapy has 2 variations: bismuth-based and non-bismuth (concomitant) therapy. The latter uses the base triple therapy and adds either metronidazole or tinidazole for 7 to 14 days. In a multicenter randomized trial, this concomitant therapy was found to have similar efficacy to sequential therapy.42
Bismuth-based quad therapy includes a PPI, bismuth, metronidazole, and tetracycline. A meta-analysis found it to have a higher rate of eradication than triple therapy for patients with antibiotic resistance.43,44
For persistent H pylori, a PPI, levofloxacin, and amoxicillin for 10 days has been shown to be more effective and better tolerated than quadruple therapy.12
Confirmation is indicated when symptoms persist
If dyspepsia symptoms persist after H pylori treatment, it is reasonable to retest to confirm that the infection has in fact been eradicated. Confirmation is also indicated if the patient has an H pylori-associated ulcer or a prior history of gastric cancer.
Retesting should be performed at least 4 to 6 weeks after treatment is completed. If H pylori has not been eradicated, you can try another regimen. If retesting confirms eradication and symptoms persist, EGD with biopsy is indicated. Although EGD typically has a very low yield, even for patients with red flags, this invasive test often provides reassurance and increased satisfaction for patients with persistent symptoms.46
More options for challenging cases
Managing FD is challenging when both initial acid suppression therapy and H pylori eradication fail. Unproven but low-risk treatments include modification of eating habits (eg, eating slower, not gulping food), reducing stress, discontinuing medications that may be related to symptoms, avoiding foods that seem to exacerbate symptoms, and cutting down or eliminating tobacco, caffeine, alcohol, and carbonated beverages.8 Bismuth salts have been shown to be superior to placebo for the treatment of dyspepsia.25 Small studies have also demonstrated a favorable risk–benefit ratio for peppermint oil and caraway oil for the treatment of FD.47 Prokinetics have shown efficacy compared with placebo, although a Cochrane review questioned their efficacy based on publication bias.26
There is no good evidence of efficacy for over-the-counter antacids, such as TUMS, or for GI “cocktails” (antacid, antispasmotic, and lidocaine), sucralfate, psychological interventions (eg, cognitive behavioral therapy, relaxation therapy, or hypnosis), or antidepressants.48,49 Several recent randomized controlled trials have shown the efficacy of acupuncture for the treatment of dyspepsia.49,50 Ginger may also be helpful; it has been found to help with nausea in other GI conditions, but it’s uncertain whether it can help patients with dyspepsia.51
CORRESPONDENCE
Michael Malone, MD, 845 Fishburn Road, Hershey, PA 17053; mmalone@hmc.psu.edu
1. Shaib Y, El-Serag HB. The prevalence and risk factors of functional dyspepsia in a multiethnic population in the United States. Am J Gastroenterol. 2004;99:2210-2216.
2. Talley NJ. Dyspepsia: management guidelines for the millennium. Gut. 2002;50(suppl 4):iv72–iv78.
3. Harmon RC, Peura DA. Evaluation and management of dyspepsia. Therap Adv Gastroenterol. 2010;3:87–98.
4. Bazaldua OV, Schneider FD. Evaluation and management of dyspepsia. Am Fam Physician. 1999;60:1773-1784.
5. Tack J, Talley NJ, Camilleri M, et al. Functional gastroduodenal disorders. Gastroenterology. 2006;130:1466-1479.
6. Haag S, Talley NJ, Holtmann G. Symptom patterns in functional dyspepsia and irritable bowel syndrome: relationship to disturbances in gastric emptying and response to a nutrient challenge in consulters and non-consulters. Gut. 2004;53:1445-1451.
7. Malfertheiner P, Megraud F, O’Morain CA, et al; European Helicobacter Study Group. Management of Helicobacter pylori infection—the Maastricht IV/Florence Consensus Report. Gut. 2012;61:646-664.
8. Talley NJ, Vakil NB, Moayyedi P. American Gastroenterological Association technical review on the evaluation of dyspepsia. Gastroenterology. 2005;129:1756-1780.
9. Moayyedi P, Axon AT. The usefulness of the likelihood ratio in the diagnosis of dyspepsia and gastroesophageal reflux disease. Am J Gastroenterol. 1999;94:3122-3125.
10. McColl KE. Clinical practice. Helicobacter pylori infection. N Engl J Med. 2010;362:1597-1604.
11. Kahrilas PJ, Shaheen NJ, Vaezi MF, et al; American Gastroenterological Association. American Gastroenterological Association Medical Position Statement on the management of gastroesophageal reflux disease. Gastroenterology. 2008;135:1383-1391.
12. Chey WD, Wong BC; Practice Parameters Committee of the American College of Gastroenterology. American College of Gastroenterology guideline on the management of Helicobacter pylori Infection. Am J Gastroenterol. 2007;102:1808-1825.
13. Moayyedi P, Talley NJ, Fennerty MB, et al. Can the clinical history distinguish between organic and functional dyspepsia? JAMA. 2006;295:1566-1576.
14. Eslick GD, Howell SC, Hammer J, et al. Empirically derived symptom sub-groups correspond poorly with diagnostic criteria for functional dyspepsia and irritable bowel syndrome. A factor and cluster analysis of a patient sample. Aliment Pharmacol Ther. 2004;19:133-140.
15. Aro P, Talley NJ, Ronkainen J, et al. Anxiety is associated with uninvestigated and functional dyspepsia (Rome III criteria) in a Swedish population-based study. Gastroenterology. 2009;137:94-100.
16. Mertz H, Fullerton S, Naliboff B, et al. Symptoms and visceral perception in severe functional and organic dyspepsia. Gut. 1998;42:814-822.
17. O’Morain C. Role of Helicobacter pylori in functional dyspepsia. World J Gastroenterol. 2006;12:2677-2680.
18. Shmuely H, Obure S, Passaro DJ, et al. Dyspepsia symptoms and Helicobacter pylori infection, Nakuru, Kenya. Emerg Infect Dis. 2003;9:1103-1107.
19. Barbara G, Zecchi L, Barbaro R, et al. Mucosal permeability and immune activation as potential therapeutic targets of probiotics in irritable bowel syndrome. J Clin Gastroenterol. 2012;46(suppl):S52-S55.
20. Liu NJ, Lee CS, Tang JH, et al. Outcomes of bleeding peptic ulcers: a prospective study. J Gastroenterol Hepatol. 2008;23:e340-e347.
21. Ramsoekh D, van Leerdam ME, Rauws EA, et al. Outcome of peptic ulcer bleeding, nonsteroidal anti-inflammatory drug use, and Helicobacter pylori infection. Clin Gastroenterol Hepatol. 2005;3:859-864.
22. Papatheodoridis GV, Sougioultzis S, Archimandritis AJ. Effects of Helicobacter pylori and nonsteroidal anti-inflammatory drugs on peptic ulcer disease: a systematic review. Clin Gastroenterol Hepatol. 2006;4:130-142.
23. Bai Y, Li ZS, Zou DW, et al. Alarm features and age for predicting upper gastrointestinal malignancy in Chinese patients with dyspepsia with high background prevalence of Helicobacter pylori infection and upper gastrointestinal malignancy: an endoscopic database review of 102,665 patients from 1996 to 2006. Gut. 2010;59:722-728.
24. Vakil N. Dyspepsia, peptic ulcer, and H. pylori: a remembrance of things past. Am J Gastroenterol. 2010;105:572-574.
25. Shaheen NJ, Weinberg DS, Denberg TD, et al; Clinical Guidelines Committee of the American College of Physicians. Upper endoscopy for gastroesophageal reflux disease: best practice advice from the clinical guidelines committee of the American College of Physicians. Ann Intern Med. 2012;157:808-816.
26. Moayyedi P, Soo S, Deeks J, et al. Pharmacological interventions for non-ulcer dyspepsia. Cochrane Database Syst Rev. 2006;(4):CD001960.
27. Chiu CT, Hsu CM, Wang CC, et al. Randomised clinical trial: sodium alginate oral suspension is non-inferior to omeprazole in the treatment of patients with non-erosive gastroesophageal disease. Aliment Pharmacol Ther. 2013;38:1054-1064.
28. Veldhuyzen van Zanten SJ, Chiba N, Armstrong D, et al. A randomized trial comparing omeprazole, ranitidine, cisapride, or placebo in helicobacter pylori negative, primary care patients with dyspepsia: the CADET-HN Study. Am J Gastroenterol. 2005;100:1477-1488.
29. Moayyedi P, Delaney BC, Vakil N, et al. The efficacy of proton pump inhibitors in nonulcer dyspepsia: a systematic review and economic analysis. Gastroenterology. 2004;127:1329-1337.
30. Garza-González E, Bosques-Padilla FJ, Tijerina-Menchaca R, et al. Comparison of endoscopy-based and serum-based methods for the diagnosis of Helicobacter pylori. Can J Gastroenterol. 2003;17:101-106.
31. Kodama M, Murakami K, Okimoto T, et al. Influence of proton pump inhibitor treatment on Helicobacter pylori stool antigen test. World J Gastroenterol. 2012;18:44-48.
32. Shimoyama T. Stool antigen tests for the management of Helicobacter pylori infection. World J Gastroenterol. 2013;19:8188-8191.
33. Howden CW, Hunt RH. Guidelines for the management of Helicobacter pylori infection. Ad Hoc Committee on Practice Parameters of the American College of Gastroenterology. Am J Gastroenterol. 1998;93:2330-2338.
34. Gisbert J, Abraira V. Accuracy of Helicobacter pylori diagnostic tests in patients with bleeding peptic ulcer: a systematic review and meta-analysis. Am J Gastroenterol. 2006;101:848-863.
35. Kamangar F, Dawsey SM, Blaser MJ, et al. Opposing risks of gastric cardiac and noncardia gastric adenocarcinomas associated with Helicobacter pylori seropositivity. J Natl Cancer Inst. 2006;98:1445-1452.
36. Islami F, Kamangar F. Helicobacter pylori and esophageal cancer risk: a meta-analysis. Cancer Prevent Res (Phila). 2008;1:329-338.
37. Moayyedi P, Soo S, Deeks J, et al. Eradication of Helicobacter pylori for non-ulcer dyspepsia. Cochrane Database Syst Rev. 2006;(2):CD002096.
38. Mazzoleni LE, Sander GB, Francesconi CF, et al. Helicobacter pylori eradication in functional dyspepsia: HEROES trial. Arch Intern Med. 2011;171:1929-1936.
39. Yuan Y, Ford AC, Khan KJ, et al. Optimum duration of regimens for Helicobacter pylori eradication. Cochrane Database Syst Rev. 2013;(12):CD008337.
40. Zou J, Dong J, Yu X. Meta-analysis: Lactobacillus containing quadruple therapy versus standard triple first-line therapy for Helicobacter pylori eradication. Helicobacter. 2009;14:97-107.
41. Basu PP, Rayapudi K, Pacana T, et al. A randomized study comparing levofloxacin, omeprazole, nitazoxanide, and doxycycline versus triple therapy for the eradication of Helicobacter pylori. Am J Gastroenterol. 2011;106:1970-1975.
42. Wu DC, Hsu PI, Wu JY, et al. Sequential and concomitant therapy with 4 drugs are equally effective for eradication of H. pylori infection. Clin Gastroenterol Hepatol. 2010;8:36–41.
43. Osato R, Reddy R, Reddy SG, et al. Pattern of primary resistance of Helicobacter pylori to metronidazole or clarithromycin in the United States. Arch Intern Med. 2001;161:1217-1220.
44. Fischbach L, Evans EL. Meta-analysis: the effect of antibiotic resistance status on the efficacy of triple and quadruple firstline therapies for Helicobacter pylori. Aliment Pharmacol Ther. 2007;26:343-357.
45. Hinostroza Morales D, Díaz Ferrer J. Addition of bismuth subsalicylate to triple eradication therapy for Helicobacter pylori infection: efficiency and adverse events. Rev Gastroenterol Peru. 2014;34:315-320.
46. Rabeneck L, Wristers K, Souchek J, et al. Impact of upper endoscopy on satisfaction in patients with previously uninvestigated dyspepsia. Gastrointest Endosc. 2003;57:295-299.
47. Hojo M, Miwa H, Yokoyama T, et al. Treatment of functional dyspepsia with antianxiety or antidepressive agents: systematic review. J Gastroenterol. 2005;40:1036-1042.
48. Soo S, Moayyedi P, Deeks J, et al. Psychological interventions for non-ulcer dyspepsia. Cochrane Database Syst Rev. 2005;(2):CD002301.
49. Lima FA, Ferreira LE, Pace FH. Acupuncture effectiveness as a complementary therapy in functional dyspepsia patients. Arq Gastroenterol. 2013;50:202-207.
50. Ma TT, Yu SY, Li Y, et al. Randomised clinical trial: an assessment of acupuncture on specific meridian or specific acupoint vs. sham acupuncture for treating functional dyspepsia. Aliment Pharmacol Ther. 2012;35:552-561.
51. Koretz RL, Rotblatt M. Complementary and alternative medicine in gastroenterology: the good, the bad, and the ugly. Clin Gastroenterol Hepatol. 2004;2:957-967.
1. Shaib Y, El-Serag HB. The prevalence and risk factors of functional dyspepsia in a multiethnic population in the United States. Am J Gastroenterol. 2004;99:2210-2216.
2. Talley NJ. Dyspepsia: management guidelines for the millennium. Gut. 2002;50(suppl 4):iv72–iv78.
3. Harmon RC, Peura DA. Evaluation and management of dyspepsia. Therap Adv Gastroenterol. 2010;3:87–98.
4. Bazaldua OV, Schneider FD. Evaluation and management of dyspepsia. Am Fam Physician. 1999;60:1773-1784.
5. Tack J, Talley NJ, Camilleri M, et al. Functional gastroduodenal disorders. Gastroenterology. 2006;130:1466-1479.
6. Haag S, Talley NJ, Holtmann G. Symptom patterns in functional dyspepsia and irritable bowel syndrome: relationship to disturbances in gastric emptying and response to a nutrient challenge in consulters and non-consulters. Gut. 2004;53:1445-1451.
7. Malfertheiner P, Megraud F, O’Morain CA, et al; European Helicobacter Study Group. Management of Helicobacter pylori infection—the Maastricht IV/Florence Consensus Report. Gut. 2012;61:646-664.
8. Talley NJ, Vakil NB, Moayyedi P. American Gastroenterological Association technical review on the evaluation of dyspepsia. Gastroenterology. 2005;129:1756-1780.
9. Moayyedi P, Axon AT. The usefulness of the likelihood ratio in the diagnosis of dyspepsia and gastroesophageal reflux disease. Am J Gastroenterol. 1999;94:3122-3125.
10. McColl KE. Clinical practice. Helicobacter pylori infection. N Engl J Med. 2010;362:1597-1604.
11. Kahrilas PJ, Shaheen NJ, Vaezi MF, et al; American Gastroenterological Association. American Gastroenterological Association Medical Position Statement on the management of gastroesophageal reflux disease. Gastroenterology. 2008;135:1383-1391.
12. Chey WD, Wong BC; Practice Parameters Committee of the American College of Gastroenterology. American College of Gastroenterology guideline on the management of Helicobacter pylori Infection. Am J Gastroenterol. 2007;102:1808-1825.
13. Moayyedi P, Talley NJ, Fennerty MB, et al. Can the clinical history distinguish between organic and functional dyspepsia? JAMA. 2006;295:1566-1576.
14. Eslick GD, Howell SC, Hammer J, et al. Empirically derived symptom sub-groups correspond poorly with diagnostic criteria for functional dyspepsia and irritable bowel syndrome. A factor and cluster analysis of a patient sample. Aliment Pharmacol Ther. 2004;19:133-140.
15. Aro P, Talley NJ, Ronkainen J, et al. Anxiety is associated with uninvestigated and functional dyspepsia (Rome III criteria) in a Swedish population-based study. Gastroenterology. 2009;137:94-100.
16. Mertz H, Fullerton S, Naliboff B, et al. Symptoms and visceral perception in severe functional and organic dyspepsia. Gut. 1998;42:814-822.
17. O’Morain C. Role of Helicobacter pylori in functional dyspepsia. World J Gastroenterol. 2006;12:2677-2680.
18. Shmuely H, Obure S, Passaro DJ, et al. Dyspepsia symptoms and Helicobacter pylori infection, Nakuru, Kenya. Emerg Infect Dis. 2003;9:1103-1107.
19. Barbara G, Zecchi L, Barbaro R, et al. Mucosal permeability and immune activation as potential therapeutic targets of probiotics in irritable bowel syndrome. J Clin Gastroenterol. 2012;46(suppl):S52-S55.
20. Liu NJ, Lee CS, Tang JH, et al. Outcomes of bleeding peptic ulcers: a prospective study. J Gastroenterol Hepatol. 2008;23:e340-e347.
21. Ramsoekh D, van Leerdam ME, Rauws EA, et al. Outcome of peptic ulcer bleeding, nonsteroidal anti-inflammatory drug use, and Helicobacter pylori infection. Clin Gastroenterol Hepatol. 2005;3:859-864.
22. Papatheodoridis GV, Sougioultzis S, Archimandritis AJ. Effects of Helicobacter pylori and nonsteroidal anti-inflammatory drugs on peptic ulcer disease: a systematic review. Clin Gastroenterol Hepatol. 2006;4:130-142.
23. Bai Y, Li ZS, Zou DW, et al. Alarm features and age for predicting upper gastrointestinal malignancy in Chinese patients with dyspepsia with high background prevalence of Helicobacter pylori infection and upper gastrointestinal malignancy: an endoscopic database review of 102,665 patients from 1996 to 2006. Gut. 2010;59:722-728.
24. Vakil N. Dyspepsia, peptic ulcer, and H. pylori: a remembrance of things past. Am J Gastroenterol. 2010;105:572-574.
25. Shaheen NJ, Weinberg DS, Denberg TD, et al; Clinical Guidelines Committee of the American College of Physicians. Upper endoscopy for gastroesophageal reflux disease: best practice advice from the clinical guidelines committee of the American College of Physicians. Ann Intern Med. 2012;157:808-816.
26. Moayyedi P, Soo S, Deeks J, et al. Pharmacological interventions for non-ulcer dyspepsia. Cochrane Database Syst Rev. 2006;(4):CD001960.
27. Chiu CT, Hsu CM, Wang CC, et al. Randomised clinical trial: sodium alginate oral suspension is non-inferior to omeprazole in the treatment of patients with non-erosive gastroesophageal disease. Aliment Pharmacol Ther. 2013;38:1054-1064.
28. Veldhuyzen van Zanten SJ, Chiba N, Armstrong D, et al. A randomized trial comparing omeprazole, ranitidine, cisapride, or placebo in helicobacter pylori negative, primary care patients with dyspepsia: the CADET-HN Study. Am J Gastroenterol. 2005;100:1477-1488.
29. Moayyedi P, Delaney BC, Vakil N, et al. The efficacy of proton pump inhibitors in nonulcer dyspepsia: a systematic review and economic analysis. Gastroenterology. 2004;127:1329-1337.
30. Garza-González E, Bosques-Padilla FJ, Tijerina-Menchaca R, et al. Comparison of endoscopy-based and serum-based methods for the diagnosis of Helicobacter pylori. Can J Gastroenterol. 2003;17:101-106.
31. Kodama M, Murakami K, Okimoto T, et al. Influence of proton pump inhibitor treatment on Helicobacter pylori stool antigen test. World J Gastroenterol. 2012;18:44-48.
32. Shimoyama T. Stool antigen tests for the management of Helicobacter pylori infection. World J Gastroenterol. 2013;19:8188-8191.
33. Howden CW, Hunt RH. Guidelines for the management of Helicobacter pylori infection. Ad Hoc Committee on Practice Parameters of the American College of Gastroenterology. Am J Gastroenterol. 1998;93:2330-2338.
34. Gisbert J, Abraira V. Accuracy of Helicobacter pylori diagnostic tests in patients with bleeding peptic ulcer: a systematic review and meta-analysis. Am J Gastroenterol. 2006;101:848-863.
35. Kamangar F, Dawsey SM, Blaser MJ, et al. Opposing risks of gastric cardiac and noncardia gastric adenocarcinomas associated with Helicobacter pylori seropositivity. J Natl Cancer Inst. 2006;98:1445-1452.
36. Islami F, Kamangar F. Helicobacter pylori and esophageal cancer risk: a meta-analysis. Cancer Prevent Res (Phila). 2008;1:329-338.
37. Moayyedi P, Soo S, Deeks J, et al. Eradication of Helicobacter pylori for non-ulcer dyspepsia. Cochrane Database Syst Rev. 2006;(2):CD002096.
38. Mazzoleni LE, Sander GB, Francesconi CF, et al. Helicobacter pylori eradication in functional dyspepsia: HEROES trial. Arch Intern Med. 2011;171:1929-1936.
39. Yuan Y, Ford AC, Khan KJ, et al. Optimum duration of regimens for Helicobacter pylori eradication. Cochrane Database Syst Rev. 2013;(12):CD008337.
40. Zou J, Dong J, Yu X. Meta-analysis: Lactobacillus containing quadruple therapy versus standard triple first-line therapy for Helicobacter pylori eradication. Helicobacter. 2009;14:97-107.
41. Basu PP, Rayapudi K, Pacana T, et al. A randomized study comparing levofloxacin, omeprazole, nitazoxanide, and doxycycline versus triple therapy for the eradication of Helicobacter pylori. Am J Gastroenterol. 2011;106:1970-1975.
42. Wu DC, Hsu PI, Wu JY, et al. Sequential and concomitant therapy with 4 drugs are equally effective for eradication of H. pylori infection. Clin Gastroenterol Hepatol. 2010;8:36–41.
43. Osato R, Reddy R, Reddy SG, et al. Pattern of primary resistance of Helicobacter pylori to metronidazole or clarithromycin in the United States. Arch Intern Med. 2001;161:1217-1220.
44. Fischbach L, Evans EL. Meta-analysis: the effect of antibiotic resistance status on the efficacy of triple and quadruple firstline therapies for Helicobacter pylori. Aliment Pharmacol Ther. 2007;26:343-357.
45. Hinostroza Morales D, Díaz Ferrer J. Addition of bismuth subsalicylate to triple eradication therapy for Helicobacter pylori infection: efficiency and adverse events. Rev Gastroenterol Peru. 2014;34:315-320.
46. Rabeneck L, Wristers K, Souchek J, et al. Impact of upper endoscopy on satisfaction in patients with previously uninvestigated dyspepsia. Gastrointest Endosc. 2003;57:295-299.
47. Hojo M, Miwa H, Yokoyama T, et al. Treatment of functional dyspepsia with antianxiety or antidepressive agents: systematic review. J Gastroenterol. 2005;40:1036-1042.
48. Soo S, Moayyedi P, Deeks J, et al. Psychological interventions for non-ulcer dyspepsia. Cochrane Database Syst Rev. 2005;(2):CD002301.
49. Lima FA, Ferreira LE, Pace FH. Acupuncture effectiveness as a complementary therapy in functional dyspepsia patients. Arq Gastroenterol. 2013;50:202-207.
50. Ma TT, Yu SY, Li Y, et al. Randomised clinical trial: an assessment of acupuncture on specific meridian or specific acupoint vs. sham acupuncture for treating functional dyspepsia. Aliment Pharmacol Ther. 2012;35:552-561.
51. Koretz RL, Rotblatt M. Complementary and alternative medicine in gastroenterology: the good, the bad, and the ugly. Clin Gastroenterol Hepatol. 2004;2:957-967.
Improving our approach to preventive care
› Avoid scheduling annual visits exclusively for preventive care. A
› Institute simple practice changes to improve the preventive services you provide, such as implementing standing orders for influenza vaccines. A
› Adopt components of the chronic care model for preventive services wherever possible—using ancillary providers to remind patients to undergo colorectal cancer screening and recommending apps that support self-management, for example. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
For well over a century, the periodic health exam has been associated with the delivery of preventive services1—a model widely accepted by physicians and patients alike. Approximately 8% of ambulatory care visits are for check-ups, and more than 20% of US residents schedule a health exam annually.2
Periodic health exams, however, do not result in optimal preventive care. Evidence suggests that important preventive services, such as dietary counseling, occur at only 8% of such visits; that just 10 seconds, on average, is devoted to smoking cessation; and that 80% of the preventive services patients receive are delivered outside of scheduled health exams.2 Because physicians and patients alike understandably prioritize acute problems, any discussion of health maintenance issues during a periodic check-up is likely to occur despite the visit’s agenda, not as part of it.3-7
Primary care physicians and practices are increasingly being held accountable for their performance on preventive measures. With that in mind, being familiar with evidence-based guidelines relating to the delivery of preventive services in ambulatory care settings, such as those developed by the US Preventive Services Task Force (USPSTF),8 is crucial. Identifying elements of the chronic care model that can be effectively applied to preventive care—and recognizing that the reactive acute care model is ineffective for both chronic illness and preventive services—is essential, as well.
Preventive care that's evidence-based
Good practice guidelines should have the following features, according to the Institute of Medicine:9
• Validity. Application would lead to the desired health and cost outcomes.
• Reliability/reproducibility. Others using the same data/interpretation would reach the same conclusion.
• Clinical applicability. Patient populations are appropriately defined.
• Clinical flexibility. Known or generally expected exceptions are identified.
• Clarity. Guideline uses unambiguous language with precise definitions.
• Multidisciplinary process. Developed with the participation of all key stakeholders.
• Scheduled review. Periodically reviewed and revised to incorporate new evidence/changing consensus.
• Documentation. Procedures used in development are well documented.
The USPSTF guidelines are consistent with these attributes.8
Some brief interventions fail
Guidelines are useful as practice standards for establishing preventive care goals, but their existence alone does not ensure the delivery of high-quality preventive services in an office setting. One key factor is time. It is estimated that a primary care physician with an average-sized patient panel would require an additional 7.4 hours per day to achieve 100% compliance with all of the USPSTF recommendations.10
Counseling, in particular, is an intervention that may be more effective in theory than in practice. Evidence suggests that even when primary care providers are trained in preventive counseling (and many are not), many brief interventions are not effective at creating sustained behavior change or health improvement.11
Others are effective
That’s not to say that there’s little that can be done. Use of standing orders for influenza vaccination is one example of an effective and easily implemented preventive measure that requires little or no additional physician time. Yet some doctors are resistant, fearing loss of control or lawsuits. In fact, the National Vaccine Injury Compensation Program specifically provides protection against vaccinerelated malpractice claims.12 A standing order for office staff to arrange a screening mammogram is another effective intervention; it has been shown to improve screening rates by as much as 30%.13
Lessons from chronic illness management
Chronic illness care and preventive care have much in common.14 Both acknowledge the need for proactive screening and counseling to bring about behavior change. In addition, both require ongoing care and follow-up, as well as depend on links to community resources. And finally, both are resource-intensive—too resource-intensive, some say, to be delivered in a cost-effective manner.
The Chronic Care Model has been proposed as a framework for improving preventive services (TABLE).15,16 Evidence suggests adopting some key components of chronic care can lead to significant gains in the delivery of preventive care, with the largest improvement seen when multiple components are used simultaneously.17-20
Self-management support. A growing body of evidence shows that self-management activities are associated with improved outcomes.21 Instituting a peer support group for pregnant women at a federally qualified health center, for example, led to a 7% absolute risk reduction in preterm births.22
Not all efforts to encourage self-management are effective, however, and evaluation is crucial to determine what works. In one large-scale trial, the use of patient reminder cards to facilitate a reduction in health risk behaviors such as tobacco use, risky drinking, unhealthy dietary patterns, and physical inactivity led to fewer health risk assessments being performed, fewer individual counseling encounters, and no change in these behaviors.23
Decision support. Clinical decision support systems, which generate patient-specific evidence-based assessments and/or recommendations that are actionable as part of the workflow at the point of care, have been found to improve care.24,25 An example of this is a prompt that reminds the physician to discuss chemoprevention with a patient at high risk for breast cancer.
Delivery system design. The patient-centered medical home (PCMH) is a well-known example of a redesign of health care delivery.26 Conversion to this model is associated with a small positive effect on preventive interventions.27 However, the persistence of a fee-for-service payment system—which does not include physician reimbursement for some of the added services incorporated into the PCMH—limits the implementation of the PCMH model.28
Many practices are improving health care delivery by using nonphysicians for various tasks related to preventive care. Care managers, for example, typically have smaller caseloads and focus on reducing unnecessary treatment for patients with high-risk conditions, such as congestive heart failure, while patient navigators generally have less clinical expertise but more knowledge of community services.
In one study, practice-initiated phone conversations with nonphysicians increased colorectal cancer screening by up to 40%.29 And in one pilot program, the use of ancillary providers led to an increase in colorectal cancer screening by as much as 123%.30 The human touch seems to be key to the success of these interventions. Passive reminders, such as videos being shown on waiting room televisions, have not proven to be effective.31
Clinical information systems. Early on, the power of electronic health records (EHRs) to improve practices’ delivery of preventive services was recognized. As early as 1995, the use of a reminder system to highlight such services during acute care visits was linked to improvements in counseling about smoking cessation and higher rates of cervical cancer screening, among other preventive measures.32,33 Overall, the use of EHRs alone has been shown to improve rates of preventive services by as much as 66%, with most practices reporting improvements of at least 20%.34
Today, EHRs that are Meaningful Use Stage 2-compliant have the tools needed to improve care. Requirements include the ability to generate patient registries of all those with a given disease and to identify patients on the registry who have not received needed care.35 To improve preventive care, registries should focus on the mitigation of risk factors, such as identifying—and contacting—patients with diabetes who are in need of, or overdue for, an annual eye exam.
Trials using the registry function, in combination with automated messaging to deliver targeted information to various patient groups (identified by the demographic information available from the EHR), are ongoing.
Community resources. Many clinicians have informal referral relationships with community organizations, such as the YMCA. Physician practices that establish links to community resources have the potential to have a large effect on unhealthy behaviors. (See “Putting theory into practice: 2 cases”.) However, a systematic review found that, while evidence to support such connections is mainly positive, research is limited and further evaluation is needed.36
The Practical Playbook (practicalplaybook.org)37 developed by the deBeaumont Foundation, Duke Department of Community and Family Medicine, and the Centers for Disease Control and Prevention, offers concrete examples of how physician practices are linking with community resources to improve the health of the population. For example, Duke University’s “Just for Us” program provides in-home chronic illness care to 350 high-risk elderly individuals. The LATCH program connects thousands of Latino immigrants to health care services and culturally and linguistically appropriate health education classes.37
CASE 1
Dominic B, a 53-year-old patient, has scheduled a visit for a cough that has persisted for 4 weeks. The patient is a nonsmoker, is married, and has no first-degree relatives with cancer. But when you review his chart before the visit, you note that he missed his 6-month check-up for hypertension and hyperlipidemia. In addition, your electronic health record (EHR ) flags the fact that he has not undergone colorectal screening and that his immunizations are not current. Because you have standing orders in place, your medical assistant gives him a flu shot and a pamphlet providing information on colorectal cancer screening before you enter the room.
During the visit, Mr. B mentions that his father, age 82, recently had a heart attack. This event—reinforced by the postcards and phone messages he received from your office after he missed his 6-month follow-up—prompted him to reluctantly admit it was “time for a check-up.” You take the patient’s blood pressure (BP) and review his lipid panel (blood work was ordered prior to his visit) with him. He is relieved to know that he will not need to be on a statin and agrees to be screened for colorectal cancer using a sensitive stool study.
Before he leaves, the patient requests medication for erectile dysfunction—a problem he never reported before. You ask him to keep a diary and return in 2 months, and promise to discuss his erectile dysfunction at that time.
CASE 2
A review of your practice’s patient registry reveals that Gladys P, age 55, is behind on breast and cervical cancer screening. She has had only a few sporadic office visits, the last of which was for bronchitis 18 months ago. At that time, the patient’s systolic BP was 162 mm Hg. You told her you would recheck it in 6 weeks, but she failed to return for follow-up.
Ms. P smokes, but has no other chronic diseases and takes no medications. There is no record of a mammogram or Pap smear, and you don’t know whether she sees a gynecologist routinely. Your office contacts her and discovers that you are the only doctor she sees. The patient tells the medical assistant who placed the call that her car broke down but she has not had money to repair or replace it, so she has had no way to get to your office.
Your staff arranges for her to get a ride from a community volunteer group, first to the nearby hospital for a mammogram and then to your office, where you perform a Pap smear and address her elevated BP and smoking. You are rewarded for the counseling and preventive care with a letter and a bonus check from Ms. P’s insurance carrier, congratulating you on your quality improvement efforts.
Growing emphasis on quality
Systemic changes in the US health care system are occurring rapidly, with an emphasis on quality and improved outcomes. Many physicians are now required to submit data to external agencies for payment, and much of the data is grounded in preventive standards. Medicare’s Physicians Quality Reporting System requires that all Medicare providers provide data on preventive and chronic illness care. Rates of vaccination, obesity screening, and tobacco use screening are examples of preventive services that will be reported publicly on the Centers for Medicare & Medicaid Services’ Physician Compare Web site.38
Physicians who work in accountable care organizations are required to meet quality standards on the delivery of certain preventive services, including breast cancer screening, colorectal cancer screening, influenza and pneumonia immunization, body mass index screening and follow-up, tobacco use screening and cessation intervention, screening for high blood pressure and followup, and screening for clinical depression and follow-up.39 As patients discover that the Affordable Care Act mandates that preventive services be covered with no cost sharing, they are likely to become more receptive to physician attempts to provide them.40,41
CORRESPONDENCE
Gerald Liu, MD, 1504 Springhill Avenue, Suite 3414, Mobile, AL 36604-3207; gliu@health.southalabama.edu
1. Han P. Historical changes in the objectives of the periodic health exam. Ann Intern Med. 1997;127:910-917.
2. Mehrota A, Zaslavsky A, Anyanian J. Preventive health examinations and preventive gynecologic examinations in the United States. Arch Intern Med. 2007;167:1876-1883.
3. Jean CR, Stange KC, Nutting PA. Competing demands of primary care: a model for the delivery of clinical preventive services. J Fam Pract. 1994;38:166-171.
4. McGinnis JM, Foege WH. The immediate versus the important. JAMA. 2004;291:1263-1264.
5. Crabtree BF, Miller WL, Tallia AF, et al. Delivery of clinical preventive services in family medicine offices. Ann Fam Med. 2005;3:430-435.
6. US Department of Health and Human Services. August 28, 2013. Healthy People 2020. Available at: www.healthypeople.gov. Accessed September 13, 2013.
7. Pollak KI, Krause KM, Yarnall KS, et al. Estimated time spent on preventive services by primary care physicians. BMC Health Serv Res. 2008;8:245.
8. US Preventive Services Task Force. Guide to Clinical Preventive Services. 2nd ed. Washington, DC: Office of Disease Prevention and Health Promotion; 1996.
9. Field MJ, Lohr KN, Committee to Advise the Public Health Service on Clinical Practice Guidelines, Institute of Medicine. Clinical Practice Guidelines: Directions for a New Program. Washington, DC: National Academies Press; 1990.
10. Yarnell K, Pollak K, Ostbye T, et al. Primary care: is there enough time for prevention? Am J Public Health. 2003;93:635-641.
11. Butler CC, Simpson SA, Hood K, et al. Training practitioners to deliver opportunistic multiple behaviour change counselling in primary care: a cluster randomized trial. BMJ. 2013;346:f1191.
12. Yonas M, Nowalk M, Zimmerman R, et al. Examining structural and clinical factors associated with implementation of standing orders for adult immunization. J Healthcare Qual. 2012;34:34-42.
13. Donahue K, Plescia M, Stafford K. Do standing orders help with chronic disease care and health maintenance in ambulatory practice? J Fam Pract. 2010;59:226-227.
14. Glasgow R, Orleans T, Wagner E. Does the chronic care model serve also as a template for improving prevention? Milbank Q. 2002;79.
15. Wagner EH. Chronic disease management: What will it take to improve care for chronic illness? Effective Clin Pract. 1998;1:2-4.
16. Barr V, Robinson S, Marin-Link B, et al. Chronic care model: an integration of concepts and strategies from population health promotion and the chronic care model. Hosp Q. 2003;7:73-83.
17. Bodenheimer T, Wagner EH, Grumbach K. Improving primary care for patients with chronic illness. JAMA. 2002;288:1775-1779.
18. Moore LG. Escaping the tyranny of the urgent by delivering planned care. Fam Pract Manage. 2006;13:37-40.
19. Tsai AC, Morton SC, Mangione CM, et al. A meta-analysis of interventions to improve care for chronic illnesses. Am J Managed Care. 2005;11:478-488.
20. Coleman K, Austin BT, Brach C, et al. Evidence on the chronic care model in the new millenium. Health Affairs. 2009;28:75-85.
21. Pearson ML, Mattke S, Shaw R, et al. Patient Self-Management Support Programs: An Evaluation. Final Contract Report. Publication No. 08-0011. Rockville, MD: Agency for Healthcare Research and Quality. November 2007.
22. Feder J. Restructuring care in a federally qualified health center to better meet patients’ needs. Health Affairs. 2011. Available at: http://content.healthaffairs.org/content/30/3/419.full.html. Accessed February 13, 2014.
23. Hung D, Rundall T, Tallia A, et al. Rethinking prevention in primary care: applying the chronic care model to address health risk behaviors. Milbank Q. 2007;85:69-91.
24. Lobach D, Sanders GD, Bright TJ, et al. Enabling Health Care Decisionmaking Through Clinical Decision Support and Knowledge Management. Evidence Report No. 203. Publication No. 12-E001-EF. Rockville, MD: Agency for Healthcare Research and Quality. April 2012.
25. Kawamoto K, Houlihan C, Balas E, et al. Improving clinical practice using clinical decision support systems: a systemic review of trials to identify features critical to success. BMJ. 2005;330:765.
26. American Academy of Family Physicians, American Academy of Pediatrics, American College of Physicians, American Osteopathic Association. Joint Principles of the Patient-Centered Medical Home. March 2007. American Academy of Family Physicians Web site. Available at: http://www.aafp.org/dam/AAFP/documents/practice_management/pcmh/initiatives/PCMHJoint.pdf. Accessed May 7, 2015.
27. Agency for Healthcare Research and Quality. The Patient-Centered Medical Home. Closing the Quality Gap: Revisiting the State of the Science: Evidence Report/Technology Assessment Executive Summary No. 208. Agency for Healthcare Research and Quality Web site. Available at: http://www.effectivehealthcare.ahrq.gov/ehc/products/391/1178/EvidReport208_CQGPatientCenteredMedicalHome_FinalReport_20120703.pdf. Accessed May 7, 2015.
28. Peikes D, Zutshi A, Genevro J, et al. Early Evidence on the Patient-Centered Medical Home. Final Report. Publication No. 12-0020-EF. Rockville, MD: Agency for Healthcare Research and Quality. February 2012.
29. Liu G, Perkins A. Using a lay cancer screening navigator to increase colorectal cancer screening rates. J Am Board Fam Med. 2015;28:280-282.
30. Baker N, Parsons M, Donnelly S, et al. Improving colon cancer screening rates in primary care: a pilot study emphasising the role of the medical assistant. Qual Saf Health Care. 2009;18:355-359.
31. Holden D, Jonas D, Portersfield D. Systematic review: enhancing the use and quality of colorectal cancer screening. Ann Intern Med. 2010;152:668-676.
32. Dexheimer JW, Talbot TR, Sanders DL, et al. Prompting clinicians about preventive care measures: a systematic review of randomized controlled trials. J Am Med Inf Assoc. 2008;15:311-320.
33. Shea S, DuMouchel W, Bahamonde L. A meta-analysis of 16 randomized controlled trials to evaluate computer-based clinical reminder systems for preventive care in the ambulatory setting. J Am Med Inf Assoc. 1996;3:399-409.
34. Chaudhry B, Wang J, Wu S, et al. Systematic review: impact of health information technology on quality, efficiency, and costs of medical care. Ann Intern Med. 2006;144:742-752.
35. Future of Family Medicine Leadership Committee. The future of family medicine: a collaborative project of the family medicine community. Ann Fam Med. 2004;2(Suppl 1):S3-S32.
36. Porterfield D, Hinnant L, Kane H, et al. Linkages Between Clinical Practices and Community Organizations for Prevention: Final Report. Rockville, MD: Agency for Healthcare Research and Quality. October 2010.
37. deBeaumont Foundation, Duke Community and Family Medicine, Centers for Disease Control and Prevention. A Practical Playbook. Available at: https://practicalplaybook.org. Accessed March 29, 2015.
38. Physician Quality Reporting System. Centers for Medicare and Medicaid Services. Available at: http://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/PQRS. Accessed March 29, 2015.
39. RTI International. Accountable Care Organization 2014 Program Analysis Quality Performance Standards Narrative Measure Specifications. 2014. Centers for Medicare and Medicaid Services Web site. Available at: http://www.cms.gov/Medicare/Medicare-Feefor-Service-Payment/sharedsavingsprogram/Downloads/ACONarrativeMeasures-Specs.pdf. Accessed May 7, 2015.
40. Koh K, Sebelius K. Promoting prevention through the Affordable Care Act. N Engl J Med. 2010;363:1296-1299.
41. Rosenbaum S. The patient protection and affordable care act: Implications for public health policy and practice. Public Health Rep. 2011;126:130-135.
› Avoid scheduling annual visits exclusively for preventive care. A
› Institute simple practice changes to improve the preventive services you provide, such as implementing standing orders for influenza vaccines. A
› Adopt components of the chronic care model for preventive services wherever possible—using ancillary providers to remind patients to undergo colorectal cancer screening and recommending apps that support self-management, for example. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
For well over a century, the periodic health exam has been associated with the delivery of preventive services1—a model widely accepted by physicians and patients alike. Approximately 8% of ambulatory care visits are for check-ups, and more than 20% of US residents schedule a health exam annually.2
Periodic health exams, however, do not result in optimal preventive care. Evidence suggests that important preventive services, such as dietary counseling, occur at only 8% of such visits; that just 10 seconds, on average, is devoted to smoking cessation; and that 80% of the preventive services patients receive are delivered outside of scheduled health exams.2 Because physicians and patients alike understandably prioritize acute problems, any discussion of health maintenance issues during a periodic check-up is likely to occur despite the visit’s agenda, not as part of it.3-7
Primary care physicians and practices are increasingly being held accountable for their performance on preventive measures. With that in mind, being familiar with evidence-based guidelines relating to the delivery of preventive services in ambulatory care settings, such as those developed by the US Preventive Services Task Force (USPSTF),8 is crucial. Identifying elements of the chronic care model that can be effectively applied to preventive care—and recognizing that the reactive acute care model is ineffective for both chronic illness and preventive services—is essential, as well.
Preventive care that's evidence-based
Good practice guidelines should have the following features, according to the Institute of Medicine:9
• Validity. Application would lead to the desired health and cost outcomes.
• Reliability/reproducibility. Others using the same data/interpretation would reach the same conclusion.
• Clinical applicability. Patient populations are appropriately defined.
• Clinical flexibility. Known or generally expected exceptions are identified.
• Clarity. Guideline uses unambiguous language with precise definitions.
• Multidisciplinary process. Developed with the participation of all key stakeholders.
• Scheduled review. Periodically reviewed and revised to incorporate new evidence/changing consensus.
• Documentation. Procedures used in development are well documented.
The USPSTF guidelines are consistent with these attributes.8
Some brief interventions fail
Guidelines are useful as practice standards for establishing preventive care goals, but their existence alone does not ensure the delivery of high-quality preventive services in an office setting. One key factor is time. It is estimated that a primary care physician with an average-sized patient panel would require an additional 7.4 hours per day to achieve 100% compliance with all of the USPSTF recommendations.10
Counseling, in particular, is an intervention that may be more effective in theory than in practice. Evidence suggests that even when primary care providers are trained in preventive counseling (and many are not), many brief interventions are not effective at creating sustained behavior change or health improvement.11
Others are effective
That’s not to say that there’s little that can be done. Use of standing orders for influenza vaccination is one example of an effective and easily implemented preventive measure that requires little or no additional physician time. Yet some doctors are resistant, fearing loss of control or lawsuits. In fact, the National Vaccine Injury Compensation Program specifically provides protection against vaccinerelated malpractice claims.12 A standing order for office staff to arrange a screening mammogram is another effective intervention; it has been shown to improve screening rates by as much as 30%.13
Lessons from chronic illness management
Chronic illness care and preventive care have much in common.14 Both acknowledge the need for proactive screening and counseling to bring about behavior change. In addition, both require ongoing care and follow-up, as well as depend on links to community resources. And finally, both are resource-intensive—too resource-intensive, some say, to be delivered in a cost-effective manner.
The Chronic Care Model has been proposed as a framework for improving preventive services (TABLE).15,16 Evidence suggests adopting some key components of chronic care can lead to significant gains in the delivery of preventive care, with the largest improvement seen when multiple components are used simultaneously.17-20
Self-management support. A growing body of evidence shows that self-management activities are associated with improved outcomes.21 Instituting a peer support group for pregnant women at a federally qualified health center, for example, led to a 7% absolute risk reduction in preterm births.22
Not all efforts to encourage self-management are effective, however, and evaluation is crucial to determine what works. In one large-scale trial, the use of patient reminder cards to facilitate a reduction in health risk behaviors such as tobacco use, risky drinking, unhealthy dietary patterns, and physical inactivity led to fewer health risk assessments being performed, fewer individual counseling encounters, and no change in these behaviors.23
Decision support. Clinical decision support systems, which generate patient-specific evidence-based assessments and/or recommendations that are actionable as part of the workflow at the point of care, have been found to improve care.24,25 An example of this is a prompt that reminds the physician to discuss chemoprevention with a patient at high risk for breast cancer.
Delivery system design. The patient-centered medical home (PCMH) is a well-known example of a redesign of health care delivery.26 Conversion to this model is associated with a small positive effect on preventive interventions.27 However, the persistence of a fee-for-service payment system—which does not include physician reimbursement for some of the added services incorporated into the PCMH—limits the implementation of the PCMH model.28
Many practices are improving health care delivery by using nonphysicians for various tasks related to preventive care. Care managers, for example, typically have smaller caseloads and focus on reducing unnecessary treatment for patients with high-risk conditions, such as congestive heart failure, while patient navigators generally have less clinical expertise but more knowledge of community services.
In one study, practice-initiated phone conversations with nonphysicians increased colorectal cancer screening by up to 40%.29 And in one pilot program, the use of ancillary providers led to an increase in colorectal cancer screening by as much as 123%.30 The human touch seems to be key to the success of these interventions. Passive reminders, such as videos being shown on waiting room televisions, have not proven to be effective.31
Clinical information systems. Early on, the power of electronic health records (EHRs) to improve practices’ delivery of preventive services was recognized. As early as 1995, the use of a reminder system to highlight such services during acute care visits was linked to improvements in counseling about smoking cessation and higher rates of cervical cancer screening, among other preventive measures.32,33 Overall, the use of EHRs alone has been shown to improve rates of preventive services by as much as 66%, with most practices reporting improvements of at least 20%.34
Today, EHRs that are Meaningful Use Stage 2-compliant have the tools needed to improve care. Requirements include the ability to generate patient registries of all those with a given disease and to identify patients on the registry who have not received needed care.35 To improve preventive care, registries should focus on the mitigation of risk factors, such as identifying—and contacting—patients with diabetes who are in need of, or overdue for, an annual eye exam.
Trials using the registry function, in combination with automated messaging to deliver targeted information to various patient groups (identified by the demographic information available from the EHR), are ongoing.
Community resources. Many clinicians have informal referral relationships with community organizations, such as the YMCA. Physician practices that establish links to community resources have the potential to have a large effect on unhealthy behaviors. (See “Putting theory into practice: 2 cases”.) However, a systematic review found that, while evidence to support such connections is mainly positive, research is limited and further evaluation is needed.36
The Practical Playbook (practicalplaybook.org)37 developed by the deBeaumont Foundation, Duke Department of Community and Family Medicine, and the Centers for Disease Control and Prevention, offers concrete examples of how physician practices are linking with community resources to improve the health of the population. For example, Duke University’s “Just for Us” program provides in-home chronic illness care to 350 high-risk elderly individuals. The LATCH program connects thousands of Latino immigrants to health care services and culturally and linguistically appropriate health education classes.37
CASE 1
Dominic B, a 53-year-old patient, has scheduled a visit for a cough that has persisted for 4 weeks. The patient is a nonsmoker, is married, and has no first-degree relatives with cancer. But when you review his chart before the visit, you note that he missed his 6-month check-up for hypertension and hyperlipidemia. In addition, your electronic health record (EHR ) flags the fact that he has not undergone colorectal screening and that his immunizations are not current. Because you have standing orders in place, your medical assistant gives him a flu shot and a pamphlet providing information on colorectal cancer screening before you enter the room.
During the visit, Mr. B mentions that his father, age 82, recently had a heart attack. This event—reinforced by the postcards and phone messages he received from your office after he missed his 6-month follow-up—prompted him to reluctantly admit it was “time for a check-up.” You take the patient’s blood pressure (BP) and review his lipid panel (blood work was ordered prior to his visit) with him. He is relieved to know that he will not need to be on a statin and agrees to be screened for colorectal cancer using a sensitive stool study.
Before he leaves, the patient requests medication for erectile dysfunction—a problem he never reported before. You ask him to keep a diary and return in 2 months, and promise to discuss his erectile dysfunction at that time.
CASE 2
A review of your practice’s patient registry reveals that Gladys P, age 55, is behind on breast and cervical cancer screening. She has had only a few sporadic office visits, the last of which was for bronchitis 18 months ago. At that time, the patient’s systolic BP was 162 mm Hg. You told her you would recheck it in 6 weeks, but she failed to return for follow-up.
Ms. P smokes, but has no other chronic diseases and takes no medications. There is no record of a mammogram or Pap smear, and you don’t know whether she sees a gynecologist routinely. Your office contacts her and discovers that you are the only doctor she sees. The patient tells the medical assistant who placed the call that her car broke down but she has not had money to repair or replace it, so she has had no way to get to your office.
Your staff arranges for her to get a ride from a community volunteer group, first to the nearby hospital for a mammogram and then to your office, where you perform a Pap smear and address her elevated BP and smoking. You are rewarded for the counseling and preventive care with a letter and a bonus check from Ms. P’s insurance carrier, congratulating you on your quality improvement efforts.
Growing emphasis on quality
Systemic changes in the US health care system are occurring rapidly, with an emphasis on quality and improved outcomes. Many physicians are now required to submit data to external agencies for payment, and much of the data is grounded in preventive standards. Medicare’s Physicians Quality Reporting System requires that all Medicare providers provide data on preventive and chronic illness care. Rates of vaccination, obesity screening, and tobacco use screening are examples of preventive services that will be reported publicly on the Centers for Medicare & Medicaid Services’ Physician Compare Web site.38
Physicians who work in accountable care organizations are required to meet quality standards on the delivery of certain preventive services, including breast cancer screening, colorectal cancer screening, influenza and pneumonia immunization, body mass index screening and follow-up, tobacco use screening and cessation intervention, screening for high blood pressure and followup, and screening for clinical depression and follow-up.39 As patients discover that the Affordable Care Act mandates that preventive services be covered with no cost sharing, they are likely to become more receptive to physician attempts to provide them.40,41
CORRESPONDENCE
Gerald Liu, MD, 1504 Springhill Avenue, Suite 3414, Mobile, AL 36604-3207; gliu@health.southalabama.edu
› Avoid scheduling annual visits exclusively for preventive care. A
› Institute simple practice changes to improve the preventive services you provide, such as implementing standing orders for influenza vaccines. A
› Adopt components of the chronic care model for preventive services wherever possible—using ancillary providers to remind patients to undergo colorectal cancer screening and recommending apps that support self-management, for example. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
For well over a century, the periodic health exam has been associated with the delivery of preventive services1—a model widely accepted by physicians and patients alike. Approximately 8% of ambulatory care visits are for check-ups, and more than 20% of US residents schedule a health exam annually.2
Periodic health exams, however, do not result in optimal preventive care. Evidence suggests that important preventive services, such as dietary counseling, occur at only 8% of such visits; that just 10 seconds, on average, is devoted to smoking cessation; and that 80% of the preventive services patients receive are delivered outside of scheduled health exams.2 Because physicians and patients alike understandably prioritize acute problems, any discussion of health maintenance issues during a periodic check-up is likely to occur despite the visit’s agenda, not as part of it.3-7
Primary care physicians and practices are increasingly being held accountable for their performance on preventive measures. With that in mind, being familiar with evidence-based guidelines relating to the delivery of preventive services in ambulatory care settings, such as those developed by the US Preventive Services Task Force (USPSTF),8 is crucial. Identifying elements of the chronic care model that can be effectively applied to preventive care—and recognizing that the reactive acute care model is ineffective for both chronic illness and preventive services—is essential, as well.
Preventive care that's evidence-based
Good practice guidelines should have the following features, according to the Institute of Medicine:9
• Validity. Application would lead to the desired health and cost outcomes.
• Reliability/reproducibility. Others using the same data/interpretation would reach the same conclusion.
• Clinical applicability. Patient populations are appropriately defined.
• Clinical flexibility. Known or generally expected exceptions are identified.
• Clarity. Guideline uses unambiguous language with precise definitions.
• Multidisciplinary process. Developed with the participation of all key stakeholders.
• Scheduled review. Periodically reviewed and revised to incorporate new evidence/changing consensus.
• Documentation. Procedures used in development are well documented.
The USPSTF guidelines are consistent with these attributes.8
Some brief interventions fail
Guidelines are useful as practice standards for establishing preventive care goals, but their existence alone does not ensure the delivery of high-quality preventive services in an office setting. One key factor is time. It is estimated that a primary care physician with an average-sized patient panel would require an additional 7.4 hours per day to achieve 100% compliance with all of the USPSTF recommendations.10
Counseling, in particular, is an intervention that may be more effective in theory than in practice. Evidence suggests that even when primary care providers are trained in preventive counseling (and many are not), many brief interventions are not effective at creating sustained behavior change or health improvement.11
Others are effective
That’s not to say that there’s little that can be done. Use of standing orders for influenza vaccination is one example of an effective and easily implemented preventive measure that requires little or no additional physician time. Yet some doctors are resistant, fearing loss of control or lawsuits. In fact, the National Vaccine Injury Compensation Program specifically provides protection against vaccinerelated malpractice claims.12 A standing order for office staff to arrange a screening mammogram is another effective intervention; it has been shown to improve screening rates by as much as 30%.13
Lessons from chronic illness management
Chronic illness care and preventive care have much in common.14 Both acknowledge the need for proactive screening and counseling to bring about behavior change. In addition, both require ongoing care and follow-up, as well as depend on links to community resources. And finally, both are resource-intensive—too resource-intensive, some say, to be delivered in a cost-effective manner.
The Chronic Care Model has been proposed as a framework for improving preventive services (TABLE).15,16 Evidence suggests adopting some key components of chronic care can lead to significant gains in the delivery of preventive care, with the largest improvement seen when multiple components are used simultaneously.17-20
Self-management support. A growing body of evidence shows that self-management activities are associated with improved outcomes.21 Instituting a peer support group for pregnant women at a federally qualified health center, for example, led to a 7% absolute risk reduction in preterm births.22
Not all efforts to encourage self-management are effective, however, and evaluation is crucial to determine what works. In one large-scale trial, the use of patient reminder cards to facilitate a reduction in health risk behaviors such as tobacco use, risky drinking, unhealthy dietary patterns, and physical inactivity led to fewer health risk assessments being performed, fewer individual counseling encounters, and no change in these behaviors.23
Decision support. Clinical decision support systems, which generate patient-specific evidence-based assessments and/or recommendations that are actionable as part of the workflow at the point of care, have been found to improve care.24,25 An example of this is a prompt that reminds the physician to discuss chemoprevention with a patient at high risk for breast cancer.
Delivery system design. The patient-centered medical home (PCMH) is a well-known example of a redesign of health care delivery.26 Conversion to this model is associated with a small positive effect on preventive interventions.27 However, the persistence of a fee-for-service payment system—which does not include physician reimbursement for some of the added services incorporated into the PCMH—limits the implementation of the PCMH model.28
Many practices are improving health care delivery by using nonphysicians for various tasks related to preventive care. Care managers, for example, typically have smaller caseloads and focus on reducing unnecessary treatment for patients with high-risk conditions, such as congestive heart failure, while patient navigators generally have less clinical expertise but more knowledge of community services.
In one study, practice-initiated phone conversations with nonphysicians increased colorectal cancer screening by up to 40%.29 And in one pilot program, the use of ancillary providers led to an increase in colorectal cancer screening by as much as 123%.30 The human touch seems to be key to the success of these interventions. Passive reminders, such as videos being shown on waiting room televisions, have not proven to be effective.31
Clinical information systems. Early on, the power of electronic health records (EHRs) to improve practices’ delivery of preventive services was recognized. As early as 1995, the use of a reminder system to highlight such services during acute care visits was linked to improvements in counseling about smoking cessation and higher rates of cervical cancer screening, among other preventive measures.32,33 Overall, the use of EHRs alone has been shown to improve rates of preventive services by as much as 66%, with most practices reporting improvements of at least 20%.34
Today, EHRs that are Meaningful Use Stage 2-compliant have the tools needed to improve care. Requirements include the ability to generate patient registries of all those with a given disease and to identify patients on the registry who have not received needed care.35 To improve preventive care, registries should focus on the mitigation of risk factors, such as identifying—and contacting—patients with diabetes who are in need of, or overdue for, an annual eye exam.
Trials using the registry function, in combination with automated messaging to deliver targeted information to various patient groups (identified by the demographic information available from the EHR), are ongoing.
Community resources. Many clinicians have informal referral relationships with community organizations, such as the YMCA. Physician practices that establish links to community resources have the potential to have a large effect on unhealthy behaviors. (See “Putting theory into practice: 2 cases”.) However, a systematic review found that, while evidence to support such connections is mainly positive, research is limited and further evaluation is needed.36
The Practical Playbook (practicalplaybook.org)37 developed by the deBeaumont Foundation, Duke Department of Community and Family Medicine, and the Centers for Disease Control and Prevention, offers concrete examples of how physician practices are linking with community resources to improve the health of the population. For example, Duke University’s “Just for Us” program provides in-home chronic illness care to 350 high-risk elderly individuals. The LATCH program connects thousands of Latino immigrants to health care services and culturally and linguistically appropriate health education classes.37
CASE 1
Dominic B, a 53-year-old patient, has scheduled a visit for a cough that has persisted for 4 weeks. The patient is a nonsmoker, is married, and has no first-degree relatives with cancer. But when you review his chart before the visit, you note that he missed his 6-month check-up for hypertension and hyperlipidemia. In addition, your electronic health record (EHR ) flags the fact that he has not undergone colorectal screening and that his immunizations are not current. Because you have standing orders in place, your medical assistant gives him a flu shot and a pamphlet providing information on colorectal cancer screening before you enter the room.
During the visit, Mr. B mentions that his father, age 82, recently had a heart attack. This event—reinforced by the postcards and phone messages he received from your office after he missed his 6-month follow-up—prompted him to reluctantly admit it was “time for a check-up.” You take the patient’s blood pressure (BP) and review his lipid panel (blood work was ordered prior to his visit) with him. He is relieved to know that he will not need to be on a statin and agrees to be screened for colorectal cancer using a sensitive stool study.
Before he leaves, the patient requests medication for erectile dysfunction—a problem he never reported before. You ask him to keep a diary and return in 2 months, and promise to discuss his erectile dysfunction at that time.
CASE 2
A review of your practice’s patient registry reveals that Gladys P, age 55, is behind on breast and cervical cancer screening. She has had only a few sporadic office visits, the last of which was for bronchitis 18 months ago. At that time, the patient’s systolic BP was 162 mm Hg. You told her you would recheck it in 6 weeks, but she failed to return for follow-up.
Ms. P smokes, but has no other chronic diseases and takes no medications. There is no record of a mammogram or Pap smear, and you don’t know whether she sees a gynecologist routinely. Your office contacts her and discovers that you are the only doctor she sees. The patient tells the medical assistant who placed the call that her car broke down but she has not had money to repair or replace it, so she has had no way to get to your office.
Your staff arranges for her to get a ride from a community volunteer group, first to the nearby hospital for a mammogram and then to your office, where you perform a Pap smear and address her elevated BP and smoking. You are rewarded for the counseling and preventive care with a letter and a bonus check from Ms. P’s insurance carrier, congratulating you on your quality improvement efforts.
Growing emphasis on quality
Systemic changes in the US health care system are occurring rapidly, with an emphasis on quality and improved outcomes. Many physicians are now required to submit data to external agencies for payment, and much of the data is grounded in preventive standards. Medicare’s Physicians Quality Reporting System requires that all Medicare providers provide data on preventive and chronic illness care. Rates of vaccination, obesity screening, and tobacco use screening are examples of preventive services that will be reported publicly on the Centers for Medicare & Medicaid Services’ Physician Compare Web site.38
Physicians who work in accountable care organizations are required to meet quality standards on the delivery of certain preventive services, including breast cancer screening, colorectal cancer screening, influenza and pneumonia immunization, body mass index screening and follow-up, tobacco use screening and cessation intervention, screening for high blood pressure and followup, and screening for clinical depression and follow-up.39 As patients discover that the Affordable Care Act mandates that preventive services be covered with no cost sharing, they are likely to become more receptive to physician attempts to provide them.40,41
CORRESPONDENCE
Gerald Liu, MD, 1504 Springhill Avenue, Suite 3414, Mobile, AL 36604-3207; gliu@health.southalabama.edu
1. Han P. Historical changes in the objectives of the periodic health exam. Ann Intern Med. 1997;127:910-917.
2. Mehrota A, Zaslavsky A, Anyanian J. Preventive health examinations and preventive gynecologic examinations in the United States. Arch Intern Med. 2007;167:1876-1883.
3. Jean CR, Stange KC, Nutting PA. Competing demands of primary care: a model for the delivery of clinical preventive services. J Fam Pract. 1994;38:166-171.
4. McGinnis JM, Foege WH. The immediate versus the important. JAMA. 2004;291:1263-1264.
5. Crabtree BF, Miller WL, Tallia AF, et al. Delivery of clinical preventive services in family medicine offices. Ann Fam Med. 2005;3:430-435.
6. US Department of Health and Human Services. August 28, 2013. Healthy People 2020. Available at: www.healthypeople.gov. Accessed September 13, 2013.
7. Pollak KI, Krause KM, Yarnall KS, et al. Estimated time spent on preventive services by primary care physicians. BMC Health Serv Res. 2008;8:245.
8. US Preventive Services Task Force. Guide to Clinical Preventive Services. 2nd ed. Washington, DC: Office of Disease Prevention and Health Promotion; 1996.
9. Field MJ, Lohr KN, Committee to Advise the Public Health Service on Clinical Practice Guidelines, Institute of Medicine. Clinical Practice Guidelines: Directions for a New Program. Washington, DC: National Academies Press; 1990.
10. Yarnell K, Pollak K, Ostbye T, et al. Primary care: is there enough time for prevention? Am J Public Health. 2003;93:635-641.
11. Butler CC, Simpson SA, Hood K, et al. Training practitioners to deliver opportunistic multiple behaviour change counselling in primary care: a cluster randomized trial. BMJ. 2013;346:f1191.
12. Yonas M, Nowalk M, Zimmerman R, et al. Examining structural and clinical factors associated with implementation of standing orders for adult immunization. J Healthcare Qual. 2012;34:34-42.
13. Donahue K, Plescia M, Stafford K. Do standing orders help with chronic disease care and health maintenance in ambulatory practice? J Fam Pract. 2010;59:226-227.
14. Glasgow R, Orleans T, Wagner E. Does the chronic care model serve also as a template for improving prevention? Milbank Q. 2002;79.
15. Wagner EH. Chronic disease management: What will it take to improve care for chronic illness? Effective Clin Pract. 1998;1:2-4.
16. Barr V, Robinson S, Marin-Link B, et al. Chronic care model: an integration of concepts and strategies from population health promotion and the chronic care model. Hosp Q. 2003;7:73-83.
17. Bodenheimer T, Wagner EH, Grumbach K. Improving primary care for patients with chronic illness. JAMA. 2002;288:1775-1779.
18. Moore LG. Escaping the tyranny of the urgent by delivering planned care. Fam Pract Manage. 2006;13:37-40.
19. Tsai AC, Morton SC, Mangione CM, et al. A meta-analysis of interventions to improve care for chronic illnesses. Am J Managed Care. 2005;11:478-488.
20. Coleman K, Austin BT, Brach C, et al. Evidence on the chronic care model in the new millenium. Health Affairs. 2009;28:75-85.
21. Pearson ML, Mattke S, Shaw R, et al. Patient Self-Management Support Programs: An Evaluation. Final Contract Report. Publication No. 08-0011. Rockville, MD: Agency for Healthcare Research and Quality. November 2007.
22. Feder J. Restructuring care in a federally qualified health center to better meet patients’ needs. Health Affairs. 2011. Available at: http://content.healthaffairs.org/content/30/3/419.full.html. Accessed February 13, 2014.
23. Hung D, Rundall T, Tallia A, et al. Rethinking prevention in primary care: applying the chronic care model to address health risk behaviors. Milbank Q. 2007;85:69-91.
24. Lobach D, Sanders GD, Bright TJ, et al. Enabling Health Care Decisionmaking Through Clinical Decision Support and Knowledge Management. Evidence Report No. 203. Publication No. 12-E001-EF. Rockville, MD: Agency for Healthcare Research and Quality. April 2012.
25. Kawamoto K, Houlihan C, Balas E, et al. Improving clinical practice using clinical decision support systems: a systemic review of trials to identify features critical to success. BMJ. 2005;330:765.
26. American Academy of Family Physicians, American Academy of Pediatrics, American College of Physicians, American Osteopathic Association. Joint Principles of the Patient-Centered Medical Home. March 2007. American Academy of Family Physicians Web site. Available at: http://www.aafp.org/dam/AAFP/documents/practice_management/pcmh/initiatives/PCMHJoint.pdf. Accessed May 7, 2015.
27. Agency for Healthcare Research and Quality. The Patient-Centered Medical Home. Closing the Quality Gap: Revisiting the State of the Science: Evidence Report/Technology Assessment Executive Summary No. 208. Agency for Healthcare Research and Quality Web site. Available at: http://www.effectivehealthcare.ahrq.gov/ehc/products/391/1178/EvidReport208_CQGPatientCenteredMedicalHome_FinalReport_20120703.pdf. Accessed May 7, 2015.
28. Peikes D, Zutshi A, Genevro J, et al. Early Evidence on the Patient-Centered Medical Home. Final Report. Publication No. 12-0020-EF. Rockville, MD: Agency for Healthcare Research and Quality. February 2012.
29. Liu G, Perkins A. Using a lay cancer screening navigator to increase colorectal cancer screening rates. J Am Board Fam Med. 2015;28:280-282.
30. Baker N, Parsons M, Donnelly S, et al. Improving colon cancer screening rates in primary care: a pilot study emphasising the role of the medical assistant. Qual Saf Health Care. 2009;18:355-359.
31. Holden D, Jonas D, Portersfield D. Systematic review: enhancing the use and quality of colorectal cancer screening. Ann Intern Med. 2010;152:668-676.
32. Dexheimer JW, Talbot TR, Sanders DL, et al. Prompting clinicians about preventive care measures: a systematic review of randomized controlled trials. J Am Med Inf Assoc. 2008;15:311-320.
33. Shea S, DuMouchel W, Bahamonde L. A meta-analysis of 16 randomized controlled trials to evaluate computer-based clinical reminder systems for preventive care in the ambulatory setting. J Am Med Inf Assoc. 1996;3:399-409.
34. Chaudhry B, Wang J, Wu S, et al. Systematic review: impact of health information technology on quality, efficiency, and costs of medical care. Ann Intern Med. 2006;144:742-752.
35. Future of Family Medicine Leadership Committee. The future of family medicine: a collaborative project of the family medicine community. Ann Fam Med. 2004;2(Suppl 1):S3-S32.
36. Porterfield D, Hinnant L, Kane H, et al. Linkages Between Clinical Practices and Community Organizations for Prevention: Final Report. Rockville, MD: Agency for Healthcare Research and Quality. October 2010.
37. deBeaumont Foundation, Duke Community and Family Medicine, Centers for Disease Control and Prevention. A Practical Playbook. Available at: https://practicalplaybook.org. Accessed March 29, 2015.
38. Physician Quality Reporting System. Centers for Medicare and Medicaid Services. Available at: http://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/PQRS. Accessed March 29, 2015.
39. RTI International. Accountable Care Organization 2014 Program Analysis Quality Performance Standards Narrative Measure Specifications. 2014. Centers for Medicare and Medicaid Services Web site. Available at: http://www.cms.gov/Medicare/Medicare-Feefor-Service-Payment/sharedsavingsprogram/Downloads/ACONarrativeMeasures-Specs.pdf. Accessed May 7, 2015.
40. Koh K, Sebelius K. Promoting prevention through the Affordable Care Act. N Engl J Med. 2010;363:1296-1299.
41. Rosenbaum S. The patient protection and affordable care act: Implications for public health policy and practice. Public Health Rep. 2011;126:130-135.
1. Han P. Historical changes in the objectives of the periodic health exam. Ann Intern Med. 1997;127:910-917.
2. Mehrota A, Zaslavsky A, Anyanian J. Preventive health examinations and preventive gynecologic examinations in the United States. Arch Intern Med. 2007;167:1876-1883.
3. Jean CR, Stange KC, Nutting PA. Competing demands of primary care: a model for the delivery of clinical preventive services. J Fam Pract. 1994;38:166-171.
4. McGinnis JM, Foege WH. The immediate versus the important. JAMA. 2004;291:1263-1264.
5. Crabtree BF, Miller WL, Tallia AF, et al. Delivery of clinical preventive services in family medicine offices. Ann Fam Med. 2005;3:430-435.
6. US Department of Health and Human Services. August 28, 2013. Healthy People 2020. Available at: www.healthypeople.gov. Accessed September 13, 2013.
7. Pollak KI, Krause KM, Yarnall KS, et al. Estimated time spent on preventive services by primary care physicians. BMC Health Serv Res. 2008;8:245.
8. US Preventive Services Task Force. Guide to Clinical Preventive Services. 2nd ed. Washington, DC: Office of Disease Prevention and Health Promotion; 1996.
9. Field MJ, Lohr KN, Committee to Advise the Public Health Service on Clinical Practice Guidelines, Institute of Medicine. Clinical Practice Guidelines: Directions for a New Program. Washington, DC: National Academies Press; 1990.
10. Yarnell K, Pollak K, Ostbye T, et al. Primary care: is there enough time for prevention? Am J Public Health. 2003;93:635-641.
11. Butler CC, Simpson SA, Hood K, et al. Training practitioners to deliver opportunistic multiple behaviour change counselling in primary care: a cluster randomized trial. BMJ. 2013;346:f1191.
12. Yonas M, Nowalk M, Zimmerman R, et al. Examining structural and clinical factors associated with implementation of standing orders for adult immunization. J Healthcare Qual. 2012;34:34-42.
13. Donahue K, Plescia M, Stafford K. Do standing orders help with chronic disease care and health maintenance in ambulatory practice? J Fam Pract. 2010;59:226-227.
14. Glasgow R, Orleans T, Wagner E. Does the chronic care model serve also as a template for improving prevention? Milbank Q. 2002;79.
15. Wagner EH. Chronic disease management: What will it take to improve care for chronic illness? Effective Clin Pract. 1998;1:2-4.
16. Barr V, Robinson S, Marin-Link B, et al. Chronic care model: an integration of concepts and strategies from population health promotion and the chronic care model. Hosp Q. 2003;7:73-83.
17. Bodenheimer T, Wagner EH, Grumbach K. Improving primary care for patients with chronic illness. JAMA. 2002;288:1775-1779.
18. Moore LG. Escaping the tyranny of the urgent by delivering planned care. Fam Pract Manage. 2006;13:37-40.
19. Tsai AC, Morton SC, Mangione CM, et al. A meta-analysis of interventions to improve care for chronic illnesses. Am J Managed Care. 2005;11:478-488.
20. Coleman K, Austin BT, Brach C, et al. Evidence on the chronic care model in the new millenium. Health Affairs. 2009;28:75-85.
21. Pearson ML, Mattke S, Shaw R, et al. Patient Self-Management Support Programs: An Evaluation. Final Contract Report. Publication No. 08-0011. Rockville, MD: Agency for Healthcare Research and Quality. November 2007.
22. Feder J. Restructuring care in a federally qualified health center to better meet patients’ needs. Health Affairs. 2011. Available at: http://content.healthaffairs.org/content/30/3/419.full.html. Accessed February 13, 2014.
23. Hung D, Rundall T, Tallia A, et al. Rethinking prevention in primary care: applying the chronic care model to address health risk behaviors. Milbank Q. 2007;85:69-91.
24. Lobach D, Sanders GD, Bright TJ, et al. Enabling Health Care Decisionmaking Through Clinical Decision Support and Knowledge Management. Evidence Report No. 203. Publication No. 12-E001-EF. Rockville, MD: Agency for Healthcare Research and Quality. April 2012.
25. Kawamoto K, Houlihan C, Balas E, et al. Improving clinical practice using clinical decision support systems: a systemic review of trials to identify features critical to success. BMJ. 2005;330:765.
26. American Academy of Family Physicians, American Academy of Pediatrics, American College of Physicians, American Osteopathic Association. Joint Principles of the Patient-Centered Medical Home. March 2007. American Academy of Family Physicians Web site. Available at: http://www.aafp.org/dam/AAFP/documents/practice_management/pcmh/initiatives/PCMHJoint.pdf. Accessed May 7, 2015.
27. Agency for Healthcare Research and Quality. The Patient-Centered Medical Home. Closing the Quality Gap: Revisiting the State of the Science: Evidence Report/Technology Assessment Executive Summary No. 208. Agency for Healthcare Research and Quality Web site. Available at: http://www.effectivehealthcare.ahrq.gov/ehc/products/391/1178/EvidReport208_CQGPatientCenteredMedicalHome_FinalReport_20120703.pdf. Accessed May 7, 2015.
28. Peikes D, Zutshi A, Genevro J, et al. Early Evidence on the Patient-Centered Medical Home. Final Report. Publication No. 12-0020-EF. Rockville, MD: Agency for Healthcare Research and Quality. February 2012.
29. Liu G, Perkins A. Using a lay cancer screening navigator to increase colorectal cancer screening rates. J Am Board Fam Med. 2015;28:280-282.
30. Baker N, Parsons M, Donnelly S, et al. Improving colon cancer screening rates in primary care: a pilot study emphasising the role of the medical assistant. Qual Saf Health Care. 2009;18:355-359.
31. Holden D, Jonas D, Portersfield D. Systematic review: enhancing the use and quality of colorectal cancer screening. Ann Intern Med. 2010;152:668-676.
32. Dexheimer JW, Talbot TR, Sanders DL, et al. Prompting clinicians about preventive care measures: a systematic review of randomized controlled trials. J Am Med Inf Assoc. 2008;15:311-320.
33. Shea S, DuMouchel W, Bahamonde L. A meta-analysis of 16 randomized controlled trials to evaluate computer-based clinical reminder systems for preventive care in the ambulatory setting. J Am Med Inf Assoc. 1996;3:399-409.
34. Chaudhry B, Wang J, Wu S, et al. Systematic review: impact of health information technology on quality, efficiency, and costs of medical care. Ann Intern Med. 2006;144:742-752.
35. Future of Family Medicine Leadership Committee. The future of family medicine: a collaborative project of the family medicine community. Ann Fam Med. 2004;2(Suppl 1):S3-S32.
36. Porterfield D, Hinnant L, Kane H, et al. Linkages Between Clinical Practices and Community Organizations for Prevention: Final Report. Rockville, MD: Agency for Healthcare Research and Quality. October 2010.
37. deBeaumont Foundation, Duke Community and Family Medicine, Centers for Disease Control and Prevention. A Practical Playbook. Available at: https://practicalplaybook.org. Accessed March 29, 2015.
38. Physician Quality Reporting System. Centers for Medicare and Medicaid Services. Available at: http://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/PQRS. Accessed March 29, 2015.
39. RTI International. Accountable Care Organization 2014 Program Analysis Quality Performance Standards Narrative Measure Specifications. 2014. Centers for Medicare and Medicaid Services Web site. Available at: http://www.cms.gov/Medicare/Medicare-Feefor-Service-Payment/sharedsavingsprogram/Downloads/ACONarrativeMeasures-Specs.pdf. Accessed May 7, 2015.
40. Koh K, Sebelius K. Promoting prevention through the Affordable Care Act. N Engl J Med. 2010;363:1296-1299.
41. Rosenbaum S. The patient protection and affordable care act: Implications for public health policy and practice. Public Health Rep. 2011;126:130-135.
Tips for assessing, managing temper tantrums
Concerns about tantrums come up a lot in pediatric care. We all know about telling parents to ignore tantrums in toddlers and not to give in. But what about when this advice does not work?
I like to think of tantrums as emotions that go beyond the child’s control. This reframing helps families consider that not all tantrums are an attempt by the child to manipulate them. That is an important first step in avoiding a solely punitive response and instead encouraging parents to look for the source of the imbalance.
Temper tantrums are most common when a child is making developmental spurts in abilities or thinking that are typically unevenly matched with self-control. There is a lot of unevenness in children’s ability to do, say, or tolerate feelings between the early tantrums of the 9-month-old until the greater coping of the 6-year-old. For example, 87% of 18- to 24-month-olds have tantrums just as they acquire autonomy and some language, yet can’t really speak their feelings, while 91% of 30- to 36-month-olds have tantrums because they can imagine big things, but are only capable of or allowed small ones. Even at 42-48 months, more than half have tantrums, which often are associated with the stress and fatigue from dropping their nap.
Life is frustrating for kids. Young children want to try to use their new skills such as climbing, opening things, or scribbling, but parents – at first delighted – suddenly want them to stop! At first, every new word is celebrated, but then toddler talk gets routine, and toddlers may be ignored or even shushed. When the child has a strong desire, the words may not be there, or emotions may make it hard to talk at all, leading to frustration.
With the development of a sense of self, the song is “I want,” “mine,” and “no!” Sharing is not in the child’s repertoire until age 3 years or older. Temperamentally more intense children give up less easily or are not readily distracted.
The threshold for frustration depends on the child’s overall state. Is the child hungry? Tired? Stressed? In pain? Here is where the differential diagnosis of excessive tantrums needs to also include pain from a medical condition such as celiac disease, arthritis, migraine, or sickle cell disease. Children under age 7 years commonly have a low tolerance for sensations as simple as loud noises and elastic waistbands, but those with sensory integration disorder are at the extreme in what sounds, feelings, or motions they cannot bear at any age and may need specific intervention by occupational or physical therapists. Mental health conditions such as attention-deficit/hyperactivity disorder, depression, anxiety, and bipolar disorder also predispose to irritable responses to even normal stresses, often in combination with lagging skills and poor sleep. Consider these when tantrums are extreme.
An age period of tantrums may be expected and accepted by parents, thus the name “terrible twos,” but if tantrums persist, they can wear out even a patient parent. Signs that a child’s tantrums are beyond the usual range include a frequency of more than once a day, a duration of more than the typical 5 minutes, or persistence after age 6 years. When you are asked if a child’s tantrums are “normal,” these are useful parameters. It also helps to explain to parents the natural course of anger arousal that starts with a trigger, peaks within 3 minutes, then subsides rapidly (usually a total of 90 seconds), and although starting with anger, ends up with sadness. Asking parents to collect this information helps them avoid interfering with or reinforcing tantrums.
Understanding the child’s temperament and needs, and avoiding triggers, can prevent many tantrums. What was she doing just before the tantrum started? What were the triggers such as fatigue, hunger, inability to express herself, or a buildup of jealousy from repeated sibling intrusions? Are there skill deficits setting him off, especially fine motor or language delays? Management then needs to focus on avoiding these triggers, if possible, and diagnosing and treating developmental delays.
Next, parents can try to distract by jollying, making a joke, or singing. These are useful moments of modeling. Some parents are worried that distracting the child with something more fun to do will interfere with his learning to cope. If distraction works, they should use it!
Often nothing works, and the child has to explode and recover on her own. Talking, cajoling, or scolding during the fit is useless – like trying to squash dynamite after the flame has hit the powder.
While standing by silently ignoring tantrums is usually the fastest way to reduce them, some children calm down faster if held. This does not reinforce the fits as long as the child’s demand is not fulfilled. Instead, it lends adult “ego support” to reassure the child that all is well and life goes on. Children quickly go from angry to sad; older children are even embarrassed by their loss of control. Comfort is appropriate and kind, as long as at least one parent can do this authentically.
Point out that frustrations in small doses are crucial for learning frustration tolerance. Parents who overprotect their child from any little stress to avoid fits is doing him a disservice. Instead, attention, praise, or marks for little bits of self-control effort or for “using your words” builds self-control over time. Times of transitions such as coming for dinner or going to brush teeth are often times of tantrums; these deserve a 2-minute warning and praise or marks for success in “moving on.”(Stopping electronics without a fit is another . Hint: If the child has a fit, he gets no electronics the next day.)
Adult management may be reinforcing tantrums. When parents give the child what she was screaming for, or remove a demand – such as to take a bath – that had sparked a fit, they can count on having an even worse reaction the next time.
I coach parents to think together about the six main things that set off their child’s tantrums and decide in advance on which ones they will hold their ground. Then, when the child just begins to beg for that snack, the parent should decide instantly if this is a “yes” or a “no” (aiming for more yeses). Parental “giving in” before a tantrum starts models positive flexibility for the child and avoids reinforcement. When an event on the “no” list comes up, both parents are then better able to have an unequivocal response and then walk away.
“But when should we teach him a lesson?” parents often ask. If parents interpret a tantrum as manipulative, a moral failing, or an evil tendency, they tend to react with anger and even loss of control themselves. Be alert for risks of excessive punishment in these cases. Not only is their response a poor model and scary for the child, it can even become an exciting, reinforcing display. If parents are depressed or tend to ignore the child as a norm, it may be worth it to the child to throw a fit to bring them to life. You can emphasize that positive attention to good behavior and silent ignoring of fits is more effective and avoids these side effects.
Parents may experience tantrums as a battle of wills that they are not willing to lose, imagining a future rebellious teen. They need education on the normal imbalances of childhood and on both prevention and intervention strategies. What they can lose in the present is their child’s confidence in adult kindness, the opportunity to model flexibility and self-control, and a relationship with their child that conveys acceptance.
Dr. Howard is assistant professor of pediatrics at the Johns Hopkins University School of Medicine, Baltimore, and creator of CHADIS (www.CHADIS.com). She has no other relevant disclosures. Dr. Howard’s contribution to this publication was as a paid expert to Frontline Medical Communications.
Concerns about tantrums come up a lot in pediatric care. We all know about telling parents to ignore tantrums in toddlers and not to give in. But what about when this advice does not work?
I like to think of tantrums as emotions that go beyond the child’s control. This reframing helps families consider that not all tantrums are an attempt by the child to manipulate them. That is an important first step in avoiding a solely punitive response and instead encouraging parents to look for the source of the imbalance.
Temper tantrums are most common when a child is making developmental spurts in abilities or thinking that are typically unevenly matched with self-control. There is a lot of unevenness in children’s ability to do, say, or tolerate feelings between the early tantrums of the 9-month-old until the greater coping of the 6-year-old. For example, 87% of 18- to 24-month-olds have tantrums just as they acquire autonomy and some language, yet can’t really speak their feelings, while 91% of 30- to 36-month-olds have tantrums because they can imagine big things, but are only capable of or allowed small ones. Even at 42-48 months, more than half have tantrums, which often are associated with the stress and fatigue from dropping their nap.
Life is frustrating for kids. Young children want to try to use their new skills such as climbing, opening things, or scribbling, but parents – at first delighted – suddenly want them to stop! At first, every new word is celebrated, but then toddler talk gets routine, and toddlers may be ignored or even shushed. When the child has a strong desire, the words may not be there, or emotions may make it hard to talk at all, leading to frustration.
With the development of a sense of self, the song is “I want,” “mine,” and “no!” Sharing is not in the child’s repertoire until age 3 years or older. Temperamentally more intense children give up less easily or are not readily distracted.
The threshold for frustration depends on the child’s overall state. Is the child hungry? Tired? Stressed? In pain? Here is where the differential diagnosis of excessive tantrums needs to also include pain from a medical condition such as celiac disease, arthritis, migraine, or sickle cell disease. Children under age 7 years commonly have a low tolerance for sensations as simple as loud noises and elastic waistbands, but those with sensory integration disorder are at the extreme in what sounds, feelings, or motions they cannot bear at any age and may need specific intervention by occupational or physical therapists. Mental health conditions such as attention-deficit/hyperactivity disorder, depression, anxiety, and bipolar disorder also predispose to irritable responses to even normal stresses, often in combination with lagging skills and poor sleep. Consider these when tantrums are extreme.
An age period of tantrums may be expected and accepted by parents, thus the name “terrible twos,” but if tantrums persist, they can wear out even a patient parent. Signs that a child’s tantrums are beyond the usual range include a frequency of more than once a day, a duration of more than the typical 5 minutes, or persistence after age 6 years. When you are asked if a child’s tantrums are “normal,” these are useful parameters. It also helps to explain to parents the natural course of anger arousal that starts with a trigger, peaks within 3 minutes, then subsides rapidly (usually a total of 90 seconds), and although starting with anger, ends up with sadness. Asking parents to collect this information helps them avoid interfering with or reinforcing tantrums.
Understanding the child’s temperament and needs, and avoiding triggers, can prevent many tantrums. What was she doing just before the tantrum started? What were the triggers such as fatigue, hunger, inability to express herself, or a buildup of jealousy from repeated sibling intrusions? Are there skill deficits setting him off, especially fine motor or language delays? Management then needs to focus on avoiding these triggers, if possible, and diagnosing and treating developmental delays.
Next, parents can try to distract by jollying, making a joke, or singing. These are useful moments of modeling. Some parents are worried that distracting the child with something more fun to do will interfere with his learning to cope. If distraction works, they should use it!
Often nothing works, and the child has to explode and recover on her own. Talking, cajoling, or scolding during the fit is useless – like trying to squash dynamite after the flame has hit the powder.
While standing by silently ignoring tantrums is usually the fastest way to reduce them, some children calm down faster if held. This does not reinforce the fits as long as the child’s demand is not fulfilled. Instead, it lends adult “ego support” to reassure the child that all is well and life goes on. Children quickly go from angry to sad; older children are even embarrassed by their loss of control. Comfort is appropriate and kind, as long as at least one parent can do this authentically.
Point out that frustrations in small doses are crucial for learning frustration tolerance. Parents who overprotect their child from any little stress to avoid fits is doing him a disservice. Instead, attention, praise, or marks for little bits of self-control effort or for “using your words” builds self-control over time. Times of transitions such as coming for dinner or going to brush teeth are often times of tantrums; these deserve a 2-minute warning and praise or marks for success in “moving on.”(Stopping electronics without a fit is another . Hint: If the child has a fit, he gets no electronics the next day.)
Adult management may be reinforcing tantrums. When parents give the child what she was screaming for, or remove a demand – such as to take a bath – that had sparked a fit, they can count on having an even worse reaction the next time.
I coach parents to think together about the six main things that set off their child’s tantrums and decide in advance on which ones they will hold their ground. Then, when the child just begins to beg for that snack, the parent should decide instantly if this is a “yes” or a “no” (aiming for more yeses). Parental “giving in” before a tantrum starts models positive flexibility for the child and avoids reinforcement. When an event on the “no” list comes up, both parents are then better able to have an unequivocal response and then walk away.
“But when should we teach him a lesson?” parents often ask. If parents interpret a tantrum as manipulative, a moral failing, or an evil tendency, they tend to react with anger and even loss of control themselves. Be alert for risks of excessive punishment in these cases. Not only is their response a poor model and scary for the child, it can even become an exciting, reinforcing display. If parents are depressed or tend to ignore the child as a norm, it may be worth it to the child to throw a fit to bring them to life. You can emphasize that positive attention to good behavior and silent ignoring of fits is more effective and avoids these side effects.
Parents may experience tantrums as a battle of wills that they are not willing to lose, imagining a future rebellious teen. They need education on the normal imbalances of childhood and on both prevention and intervention strategies. What they can lose in the present is their child’s confidence in adult kindness, the opportunity to model flexibility and self-control, and a relationship with their child that conveys acceptance.
Dr. Howard is assistant professor of pediatrics at the Johns Hopkins University School of Medicine, Baltimore, and creator of CHADIS (www.CHADIS.com). She has no other relevant disclosures. Dr. Howard’s contribution to this publication was as a paid expert to Frontline Medical Communications.
Concerns about tantrums come up a lot in pediatric care. We all know about telling parents to ignore tantrums in toddlers and not to give in. But what about when this advice does not work?
I like to think of tantrums as emotions that go beyond the child’s control. This reframing helps families consider that not all tantrums are an attempt by the child to manipulate them. That is an important first step in avoiding a solely punitive response and instead encouraging parents to look for the source of the imbalance.
Temper tantrums are most common when a child is making developmental spurts in abilities or thinking that are typically unevenly matched with self-control. There is a lot of unevenness in children’s ability to do, say, or tolerate feelings between the early tantrums of the 9-month-old until the greater coping of the 6-year-old. For example, 87% of 18- to 24-month-olds have tantrums just as they acquire autonomy and some language, yet can’t really speak their feelings, while 91% of 30- to 36-month-olds have tantrums because they can imagine big things, but are only capable of or allowed small ones. Even at 42-48 months, more than half have tantrums, which often are associated with the stress and fatigue from dropping their nap.
Life is frustrating for kids. Young children want to try to use their new skills such as climbing, opening things, or scribbling, but parents – at first delighted – suddenly want them to stop! At first, every new word is celebrated, but then toddler talk gets routine, and toddlers may be ignored or even shushed. When the child has a strong desire, the words may not be there, or emotions may make it hard to talk at all, leading to frustration.
With the development of a sense of self, the song is “I want,” “mine,” and “no!” Sharing is not in the child’s repertoire until age 3 years or older. Temperamentally more intense children give up less easily or are not readily distracted.
The threshold for frustration depends on the child’s overall state. Is the child hungry? Tired? Stressed? In pain? Here is where the differential diagnosis of excessive tantrums needs to also include pain from a medical condition such as celiac disease, arthritis, migraine, or sickle cell disease. Children under age 7 years commonly have a low tolerance for sensations as simple as loud noises and elastic waistbands, but those with sensory integration disorder are at the extreme in what sounds, feelings, or motions they cannot bear at any age and may need specific intervention by occupational or physical therapists. Mental health conditions such as attention-deficit/hyperactivity disorder, depression, anxiety, and bipolar disorder also predispose to irritable responses to even normal stresses, often in combination with lagging skills and poor sleep. Consider these when tantrums are extreme.
An age period of tantrums may be expected and accepted by parents, thus the name “terrible twos,” but if tantrums persist, they can wear out even a patient parent. Signs that a child’s tantrums are beyond the usual range include a frequency of more than once a day, a duration of more than the typical 5 minutes, or persistence after age 6 years. When you are asked if a child’s tantrums are “normal,” these are useful parameters. It also helps to explain to parents the natural course of anger arousal that starts with a trigger, peaks within 3 minutes, then subsides rapidly (usually a total of 90 seconds), and although starting with anger, ends up with sadness. Asking parents to collect this information helps them avoid interfering with or reinforcing tantrums.
Understanding the child’s temperament and needs, and avoiding triggers, can prevent many tantrums. What was she doing just before the tantrum started? What were the triggers such as fatigue, hunger, inability to express herself, or a buildup of jealousy from repeated sibling intrusions? Are there skill deficits setting him off, especially fine motor or language delays? Management then needs to focus on avoiding these triggers, if possible, and diagnosing and treating developmental delays.
Next, parents can try to distract by jollying, making a joke, or singing. These are useful moments of modeling. Some parents are worried that distracting the child with something more fun to do will interfere with his learning to cope. If distraction works, they should use it!
Often nothing works, and the child has to explode and recover on her own. Talking, cajoling, or scolding during the fit is useless – like trying to squash dynamite after the flame has hit the powder.
While standing by silently ignoring tantrums is usually the fastest way to reduce them, some children calm down faster if held. This does not reinforce the fits as long as the child’s demand is not fulfilled. Instead, it lends adult “ego support” to reassure the child that all is well and life goes on. Children quickly go from angry to sad; older children are even embarrassed by their loss of control. Comfort is appropriate and kind, as long as at least one parent can do this authentically.
Point out that frustrations in small doses are crucial for learning frustration tolerance. Parents who overprotect their child from any little stress to avoid fits is doing him a disservice. Instead, attention, praise, or marks for little bits of self-control effort or for “using your words” builds self-control over time. Times of transitions such as coming for dinner or going to brush teeth are often times of tantrums; these deserve a 2-minute warning and praise or marks for success in “moving on.”(Stopping electronics without a fit is another . Hint: If the child has a fit, he gets no electronics the next day.)
Adult management may be reinforcing tantrums. When parents give the child what she was screaming for, or remove a demand – such as to take a bath – that had sparked a fit, they can count on having an even worse reaction the next time.
I coach parents to think together about the six main things that set off their child’s tantrums and decide in advance on which ones they will hold their ground. Then, when the child just begins to beg for that snack, the parent should decide instantly if this is a “yes” or a “no” (aiming for more yeses). Parental “giving in” before a tantrum starts models positive flexibility for the child and avoids reinforcement. When an event on the “no” list comes up, both parents are then better able to have an unequivocal response and then walk away.
“But when should we teach him a lesson?” parents often ask. If parents interpret a tantrum as manipulative, a moral failing, or an evil tendency, they tend to react with anger and even loss of control themselves. Be alert for risks of excessive punishment in these cases. Not only is their response a poor model and scary for the child, it can even become an exciting, reinforcing display. If parents are depressed or tend to ignore the child as a norm, it may be worth it to the child to throw a fit to bring them to life. You can emphasize that positive attention to good behavior and silent ignoring of fits is more effective and avoids these side effects.
Parents may experience tantrums as a battle of wills that they are not willing to lose, imagining a future rebellious teen. They need education on the normal imbalances of childhood and on both prevention and intervention strategies. What they can lose in the present is their child’s confidence in adult kindness, the opportunity to model flexibility and self-control, and a relationship with their child that conveys acceptance.
Dr. Howard is assistant professor of pediatrics at the Johns Hopkins University School of Medicine, Baltimore, and creator of CHADIS (www.CHADIS.com). She has no other relevant disclosures. Dr. Howard’s contribution to this publication was as a paid expert to Frontline Medical Communications.