User login
Which psychotropics carry the greatest risk of QTc prolongation?
- Screen patients for risk factors for prolonged QTc interval, such as congenital long QT syndrome, family history of cardiac conduction abnormalities, and previous occurrences of medication-mediated QTc prolongation.
- Obtain baseline and steady state ECG when initiating high-risk agents, particularly when administering combination therapy.
- Use the lowest effective dose of antidepressants and antipsychotics and monitor symptoms closely.
Mrs. A, age 68, has a 40-year history of schizoaffective disorder with comorbid anxiety disorder not otherwise specified, type 2 diabetes mellitus, and hypertension. She takes furosemide, 40 mg/d, lisinopril, 20 mg/d, and metformin, 2,000 mg/d, for hypertension and diabetes; lorazepam, 1.5 mg/d, and paroxetine, 40 mg/d, for anxiety; and quetiapine extended release, 800 mg/d, for psychotic features and mood dysregulation with schizoaffective disorder. Mrs. A’s husband died 5 years ago and she lives alone in a senior care facility. Mrs. A uses a weekly pill reminder box because her residential facility does not monitor medication adherence. She sees her psychiatrist once a month and her primary care provider every 3 months. She has no history of illicit drug, alcohol, or tobacco use.
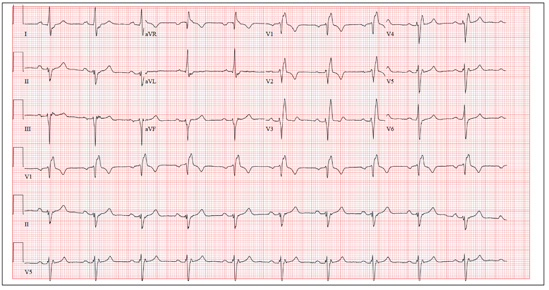
Two weeks ago, Mrs. A was found leaning against the wall in a hallway, complaining of dizziness and disorientation, and unable to find her way back to her apartment. In the emergency department, her serum potassium is low (3.0 mEq/L; normal range: 3.5 to 5.0), fasting glucose is elevated (110 mg/dL; range: 65 to 99), and ECG reveals a prolonged QTc interval of 530 milliseconds. Before this episode, Mrs. A had been medically stable without mood or psychotic symptoms, although her daughter reported medication self-administration was becoming difficult.
Exposure to psychotropics carries a risk of QTc prolongation. The QT interval is an ECG measure of ventricular depolarization and repolarization. The QTc designation indicates a correction for heart rate with increasing heart rate correlating with a shorter QT interval. Readings of 440 milliseconds are considered normal.1 QTc prolongation is defined as >450 milliseconds for men and >470 milliseconds for women.2 An increase in the QT interval is a predictor of serious cardiac events.3
Antidepressants and antipsychotics have been associated with QTc prolongation. When identifying agents that could disrupt cardiac conduction, clinicians need to consider whether the drug’s molecular structure, receptor affinity, or pharmacologic effects are most critical.2 Although these may be important, patient-specific variables that increase the risk of QTc prolongation may have greater impact. These include:
- age >65
- female sex
- electrolyte imbalances (specifically low serum potassium and magnesium levels)
- high or toxic serum levels of the suspected drug
- preexisting cardiovascular impairment, such as bradycardia.4,5
Other risk factors include concurrent use of an agent with similar cardiovascular effects or one that competes for metabolism (either enzymatic or at the binding site), physiologic limitations such as renal insufficiency, and medication changes that may increase or decrease psychotropic clearance.4,6 Geriatric patients with dementia have an increased risk for cardiovascular-related death.7,8
Antidepressants
Among tricyclic antidepressants, most reports of QTc prolongation involve amitriptyline and maprotiline.9 Risk factors include demographics (eg, female sex, age), personal or family history (congenital long QT syndrome, cardiovascular disease), and concurrent conditions or drug use, particularly those associated with QTc prolongation.3 Desipramine and nortriptyline also have been identified as high-risk agents.10
QTc prolongation has been reported with all selective serotonin reuptake inhibitors at plasma concentrations above the therapeutic level.11 Fluoxetine-associated QTc prolongation was limited to cases of overdose or when additional risk factors were reported.4 QTc prolongation from psychotropics could increase the risk of torsades de pointes, according to an analysis of the FDA Adverse Event Reporting System.12 In 2011, the FDA reported an increased risk of abnormal heart rhythms—including QTc prolongation—with citalopram doses >40 mg/d.13 Although cases of QTc prolongation with paroxetine have not been reported,11 the Arizona Center for Education and Research on Therapeutics lists paroxetine with other agents that may increase the risk for QTc prolongation with concurrent use of medications that may prolong QTc interval.14 Venlafaxine doses >300 mg/d may require additional cardiac monitoring.5,12 Data from venlafaxine poisoning case reports found a positive correlation between dose and QTc prolongation.15 In a review of toxicology database information, Wenzel-Seifert et al4 found extended QT interval with citalopram, fluoxetine, and venlafaxine at toxic doses or in the presence of additional risk factors such as sex, older age, or personal or family history of congenital long QT syndrome or cardiovascular disease.
Antipsychotics
Case reports, case series, and research trials have evaluated the risk of QTc prolongation with antipsychotics (Table).1,2,4,16,17 The first-generation antipsychotics thioridazine,4,16,18 mesoridazine,16,18 chlorpromazine,19 and haloperidol3 warrant cardiac monitoring. The QTc prolongation effects of thioridazine and its active metabolite mesoridazine are well-documented and thioridazine-mediated QTc prolongation increases are dose-dependent.4,18 ECG monitoring is recommended with IV haloperidol, which is used for delirium in adults.20 QTc prolongation has been associated with long-term ziprasidone use more often than with risperidone, olanzapine, or quetiapine.19 Ziprasidone prolongs the QTc interval an average of 20 milliseconds,21 which could represent a clinically significant change. QTc prolongation for iloperidone is comparable to ziprasidone and haloperidol.22 There is some evidence that aripiprazole may shorten, rather than prolong, the QTc interval.4,17
Cardiovascular adverse effects associated with clozapine—including QTc prolongation—are dose-dependent.3 Olanzapine prolongs QTc interval, although the mean change is less than with other agents unless other variables were present, such as:
- concomitant use of medications that may prolong QTc interval (ie, amantadine, hydroxyzine, or tamoxifen2)
- preexisting cardiovascular conduction disorders
- higher doses (>40 mg/d).3,23
In 17 case reports of cardiac changes associated with quetiapine use, doses ranged from 100 mg/d24 to an overdose of 36 g/d.25 Only 1 patient death was reported secondary to overdose and preexisting dysrhythmia and hypertension.26 QTc prolongation associated with risperidone was minor1 based on oral doses in the normal therapeutic range and incidences of overdose.10 Paliperidone27 and lurasidone28 are associated with clinically insignificant QTc prolongation. Changes in QTc interval were positively correlated with asenapine dose, although at the highest dose of 40 mg/d, the increase was <5 milliseconds.29
Mrs. A presents with a number of risk factors for QTc prolongation, including older age, female sex, and psychiatric and medical comorbidities that require medication. A pill count revealed that she was taking more than the prescribed daily doses of her medications. During the interview, Mrs. A said that if she missed her medication time, she would take them when she remembered. If she could not remember if she took her pills, she would take them again. Her physicians will explore strategies to increase medication adherence.
Table
Examples of QTc prolongation associated with select antipsychoticsa
| Antipsychotic | Approximate QTc interval prolongation in millisecondsb |
|---|---|
| Aripiprazole4,17 | -1 to -4 |
| Clozapine4 | 10 |
| Haloperidol1,2 | 7 to 15 |
| Mesoridazine16 | 39 to 53 |
| Olanzapine1 | 2 to 6.5 |
| Paliperidone4 | 2 to 4 |
| Pimozide2 | 19 |
| Quetiapine1,2 | 6 to 15 |
| Risperidone1,2 | 3.5 to 10 |
| Sertindole1 | 30 |
| Thioridazine2,16 | 33 to 41 |
| Ziprasidone1,2 | 16 to 21 |
| aList is not comprehensive. Other antipsychotics may be associated with QTc prolongation bQTc prolongation interval may depend on the route of administration | |
Related Resources
- De Hert M, Detraux J, van Winkel R, et al. Metabolic and cardiovascular adverse effects associated with antipsychotic drugs. Nat Rev Endocrinol. 2011;8(2):114-126.
- Vieweg WV, Wood MA, Fernandez A, et al. Proarrhythmic risk with antipsychotic and antidepressant drugs: implications in the elderly. Drugs Aging. 2009;26(12):997-1012.
- Sandson NB, Armstrong SC, Cozza KL. An overview of psychotropic drug-drug interactions. Psychosomatics. 2005;46(5):464-494.
Drug Brand Names
- Amantadine • Symmetrel
- Amitriptyline • Elavil
- Aripiprazole • Abilify
- Asenapine • Saphris
- Chlorpromazine • Thorazine
- Citalopram • Celexa
- Clozapine • Clozaril
- Desipramine • Norpramin
- Fluoxetine • Prozac
- Furosemide • Lasix
- Haloperidol • Haldol
- Hydroxyzine • Atarax, Vistaril
- Iloperidone • Fanapt
- Lisinopril • Prinivil, Zestril
- Lorazepam • Ativan
- Lurasidone • Latuda
- Maprotiline • Ludiomil
- Mesoridazine • Serentil
- Metformin • Glucophage
- Nortriptyline • Pamelor
- Olanzapine • Zyprexa
- Paliperidone • Invega
- Paroxetine • Paxil
- Pimozide • Orap
- Quetiapine • Seroquel
- Risperidone • Risperdal
- Tamoxifen • Nolvadex, Soltamox
- Thioridazine • Mellaril
- Venlafaxine • Effexor
- Ziprasidone • Geodon
Disclosures
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products. No similar work by the authors is under review or in press. No funding was requested or received in conjunction with this manuscript.
1. Muscatello MR, Bruno A, Pandolfo G, et al. Emerging treatments in the management of schizophrenia - focus on sertindole. Drug Des Devel Ther. 2010;4:187-201.
2. Taylor DM. Antipsychotics and QT prolongation. Acta Psychiatr Scand. 2003;107(2):85-95.
3. Alvarez PA, Pahissa J. QT alterations in psychopharmacology: proven candidates and suspects. Curr Drug Saf. 2010;5(1):97-104.
4. Wenzel-Seifert K, Wittmann M, Haen E. QTc prolongation by psychotropic drugs and the risk of torsade de pointes. Dtsch Arztebl Int. 2011;108(41):687-693.
5. Vieweg WV. New generation antipsychotic drugs and QTc interval prolongation. Prim Care Companion J Clin Psychiatry. 2003;5(5):205-215.
6. Nielsen J, Graff C, Kanters JK, et al. Assessing QT interval prolongation and its associated risks with antipsychotics. CNS Drugs. 2011;25(6):473-490.
7. Gill SS, Bronskill SE, Normand SL, et al. Antipsychotic drug use and mortality in older adults with dementia. Ann Intern Med. 2007;146(11):775-786.
8. Schneeweiss S, Setoguchi S, Brookhart A, et al. Risk of death associated with the use of conventional versus atypical antipsychotic drugs among elderly patients. CMAJ. 2007;176(5):627-632.
9. Vieweg WV, Wood MA. Tricyclic antidepressants QT interval prolongation, and torsade de pointes. Psychosomatics. 2004;45(5):371-377.
10. Jeon SH, Jaekal J, Lee SH, et al. Effects of nortriptyline on QT prolongation: a safety pharmacology study. Hum Exp Toxicol. 2011;30(10):1649-1656.
11. Wenzel-Seifert K, Wittmann M, Haen E. Torsade de pointes episodes under treatment with selective serotonin reuptake inhibitors. Pharmacopsychiatry. 2010;43(7):279-281.
12. Poluzzi E, Raschi E, Moretti U, et al. Drug-induced torsades de pointes: data mining of the public version of the FDA Adverse Event Reporting System (AERS). Pharmacoepidemiol Drug Saf. 2009;18(6):512-518.
13. U.S. Food and Drug Administration. FDA drug safety communication: revised recommendations for Celexa (citalopram hydrobromide) related to a potential risk of abnormal heart rhythms with high doses. http://www.fda.gov/Drugs/DrugSafety/ucm297391.htm. Published March 28, 2012. Accessed June 26, 2012.
14. Arizona CERT-QT Center for Education and Research on Therapeutics. QT drug lists by risk groups. http://www.azcert.org/medical-pros/drug-lists/drug-lists.cfm. Accessed June 26 2012.
15. Howell C, Wilson AD, Waring WS. Cardiovascular toxicity due to venlafaxine poisoning in adults: a review of 235 consecutive cases. Br J Clin Pharmacol. 2007;64(2):192-197.
16. Salih IS, Thanacoody RH, McKay GA, et al. Comparison of the effects of thioridazine and mesoridazine on the QT interval in healthy adults after single oral doses. Clin Pharmacol Ther. 2007;82(5):548-554.
17. Goodnick PJ, Jerry J, Parra F. Psychotropic drugs and the ECG: focus on the QTc interval. Expert Opin Pharmacother. 2002;3(5):479-498.
18. Dallaire S. Thioridazine (Mellaril) and mesoridazine (Serentil): prolongation of the QTc interval. CMAJ. 2001;164(1):91,95.-
19. Haddad PM, Anderson IM. Antipsychotic-related QTc prolongation torsade de pointes and sudden death. Drugs. 2002;62(11):1649-1671.
20. Shapiro BA, Warren J, Egol AB, et al. Practice parameters for intravenous analgesia and sedation for adult patients in the intensive care unit: an executive summary. Crit Care Med. 1995;23(9):1596-1600.
21. Vieweg WV, Hasnain M. Question regarding ziprasidone and QTc interval prolongation in the ZODIAC Study. Am J Psychiatry. 2011;168(6):650-651.
22. Caccia S, Pasina L, Nobili A. New atypical antipsychotics for schizophrenia: iloperidone. Drug Des Devel Ther. 2010;4:33-48.
23. Dineen S, Withrow K, Voronovitch L, et al. QTc prolongation and high-dose olanzapine. Psychosomatics. 2003;44(2):174-175.
24. Vieweg WV, Schneider RK, Wood MA. Torsade de pointes in a patient with complex medical and psychiatric conditions receiving low-dose quetiapine. Acta Psychiatr Scand. 2005;112(4):318-322.
25. Capuano A, Ruggiero S, Vestini F, et al. Survival from coma induced by an intentional 36-g overdose of extended-release quetiapine. Drug Chem Toxicol. 2011;34(4):475-477.
26. Fernandes PP, Marcil WA. Death associated with quetiapine overdose. Am J Psychiatry. 2002;159(12):2114.-
27. Sedky K, Nazir R, Lindenmayer JP, et al. Paliperidone palmitate: once-monthly treatment option for schizophrenia. Current Psychiatry. 2010;9(3):48-50.
28. Citrome L. Lurasidone for schizophrenia: a review of the efficacy and safety profile for this newly approved second-generation antipsychotic. Int J Clin Pract. 2011;65(2):189-210.
29. Chapel S, Hutmacher MM, Haig G, et al. Exposure-response analysis in patients with schizophrenia to assess the effect of asenapine on QTc prolongation. J Clin Pharmacol. 2009;49(11):1297-1308.
- Screen patients for risk factors for prolonged QTc interval, such as congenital long QT syndrome, family history of cardiac conduction abnormalities, and previous occurrences of medication-mediated QTc prolongation.
- Obtain baseline and steady state ECG when initiating high-risk agents, particularly when administering combination therapy.
- Use the lowest effective dose of antidepressants and antipsychotics and monitor symptoms closely.
Mrs. A, age 68, has a 40-year history of schizoaffective disorder with comorbid anxiety disorder not otherwise specified, type 2 diabetes mellitus, and hypertension. She takes furosemide, 40 mg/d, lisinopril, 20 mg/d, and metformin, 2,000 mg/d, for hypertension and diabetes; lorazepam, 1.5 mg/d, and paroxetine, 40 mg/d, for anxiety; and quetiapine extended release, 800 mg/d, for psychotic features and mood dysregulation with schizoaffective disorder. Mrs. A’s husband died 5 years ago and she lives alone in a senior care facility. Mrs. A uses a weekly pill reminder box because her residential facility does not monitor medication adherence. She sees her psychiatrist once a month and her primary care provider every 3 months. She has no history of illicit drug, alcohol, or tobacco use.
Two weeks ago, Mrs. A was found leaning against the wall in a hallway, complaining of dizziness and disorientation, and unable to find her way back to her apartment. In the emergency department, her serum potassium is low (3.0 mEq/L; normal range: 3.5 to 5.0), fasting glucose is elevated (110 mg/dL; range: 65 to 99), and ECG reveals a prolonged QTc interval of 530 milliseconds. Before this episode, Mrs. A had been medically stable without mood or psychotic symptoms, although her daughter reported medication self-administration was becoming difficult.
Exposure to psychotropics carries a risk of QTc prolongation. The QT interval is an ECG measure of ventricular depolarization and repolarization. The QTc designation indicates a correction for heart rate with increasing heart rate correlating with a shorter QT interval. Readings of 440 milliseconds are considered normal.1 QTc prolongation is defined as >450 milliseconds for men and >470 milliseconds for women.2 An increase in the QT interval is a predictor of serious cardiac events.3
Antidepressants and antipsychotics have been associated with QTc prolongation. When identifying agents that could disrupt cardiac conduction, clinicians need to consider whether the drug’s molecular structure, receptor affinity, or pharmacologic effects are most critical.2 Although these may be important, patient-specific variables that increase the risk of QTc prolongation may have greater impact. These include:
- age >65
- female sex
- electrolyte imbalances (specifically low serum potassium and magnesium levels)
- high or toxic serum levels of the suspected drug
- preexisting cardiovascular impairment, such as bradycardia.4,5
Other risk factors include concurrent use of an agent with similar cardiovascular effects or one that competes for metabolism (either enzymatic or at the binding site), physiologic limitations such as renal insufficiency, and medication changes that may increase or decrease psychotropic clearance.4,6 Geriatric patients with dementia have an increased risk for cardiovascular-related death.7,8
Antidepressants
Among tricyclic antidepressants, most reports of QTc prolongation involve amitriptyline and maprotiline.9 Risk factors include demographics (eg, female sex, age), personal or family history (congenital long QT syndrome, cardiovascular disease), and concurrent conditions or drug use, particularly those associated with QTc prolongation.3 Desipramine and nortriptyline also have been identified as high-risk agents.10
QTc prolongation has been reported with all selective serotonin reuptake inhibitors at plasma concentrations above the therapeutic level.11 Fluoxetine-associated QTc prolongation was limited to cases of overdose or when additional risk factors were reported.4 QTc prolongation from psychotropics could increase the risk of torsades de pointes, according to an analysis of the FDA Adverse Event Reporting System.12 In 2011, the FDA reported an increased risk of abnormal heart rhythms—including QTc prolongation—with citalopram doses >40 mg/d.13 Although cases of QTc prolongation with paroxetine have not been reported,11 the Arizona Center for Education and Research on Therapeutics lists paroxetine with other agents that may increase the risk for QTc prolongation with concurrent use of medications that may prolong QTc interval.14 Venlafaxine doses >300 mg/d may require additional cardiac monitoring.5,12 Data from venlafaxine poisoning case reports found a positive correlation between dose and QTc prolongation.15 In a review of toxicology database information, Wenzel-Seifert et al4 found extended QT interval with citalopram, fluoxetine, and venlafaxine at toxic doses or in the presence of additional risk factors such as sex, older age, or personal or family history of congenital long QT syndrome or cardiovascular disease.
Antipsychotics
Case reports, case series, and research trials have evaluated the risk of QTc prolongation with antipsychotics (Table).1,2,4,16,17 The first-generation antipsychotics thioridazine,4,16,18 mesoridazine,16,18 chlorpromazine,19 and haloperidol3 warrant cardiac monitoring. The QTc prolongation effects of thioridazine and its active metabolite mesoridazine are well-documented and thioridazine-mediated QTc prolongation increases are dose-dependent.4,18 ECG monitoring is recommended with IV haloperidol, which is used for delirium in adults.20 QTc prolongation has been associated with long-term ziprasidone use more often than with risperidone, olanzapine, or quetiapine.19 Ziprasidone prolongs the QTc interval an average of 20 milliseconds,21 which could represent a clinically significant change. QTc prolongation for iloperidone is comparable to ziprasidone and haloperidol.22 There is some evidence that aripiprazole may shorten, rather than prolong, the QTc interval.4,17
Cardiovascular adverse effects associated with clozapine—including QTc prolongation—are dose-dependent.3 Olanzapine prolongs QTc interval, although the mean change is less than with other agents unless other variables were present, such as:
- concomitant use of medications that may prolong QTc interval (ie, amantadine, hydroxyzine, or tamoxifen2)
- preexisting cardiovascular conduction disorders
- higher doses (>40 mg/d).3,23
In 17 case reports of cardiac changes associated with quetiapine use, doses ranged from 100 mg/d24 to an overdose of 36 g/d.25 Only 1 patient death was reported secondary to overdose and preexisting dysrhythmia and hypertension.26 QTc prolongation associated with risperidone was minor1 based on oral doses in the normal therapeutic range and incidences of overdose.10 Paliperidone27 and lurasidone28 are associated with clinically insignificant QTc prolongation. Changes in QTc interval were positively correlated with asenapine dose, although at the highest dose of 40 mg/d, the increase was <5 milliseconds.29
Mrs. A presents with a number of risk factors for QTc prolongation, including older age, female sex, and psychiatric and medical comorbidities that require medication. A pill count revealed that she was taking more than the prescribed daily doses of her medications. During the interview, Mrs. A said that if she missed her medication time, she would take them when she remembered. If she could not remember if she took her pills, she would take them again. Her physicians will explore strategies to increase medication adherence.
Table
Examples of QTc prolongation associated with select antipsychoticsa
| Antipsychotic | Approximate QTc interval prolongation in millisecondsb |
|---|---|
| Aripiprazole4,17 | -1 to -4 |
| Clozapine4 | 10 |
| Haloperidol1,2 | 7 to 15 |
| Mesoridazine16 | 39 to 53 |
| Olanzapine1 | 2 to 6.5 |
| Paliperidone4 | 2 to 4 |
| Pimozide2 | 19 |
| Quetiapine1,2 | 6 to 15 |
| Risperidone1,2 | 3.5 to 10 |
| Sertindole1 | 30 |
| Thioridazine2,16 | 33 to 41 |
| Ziprasidone1,2 | 16 to 21 |
| aList is not comprehensive. Other antipsychotics may be associated with QTc prolongation bQTc prolongation interval may depend on the route of administration | |
Related Resources
- De Hert M, Detraux J, van Winkel R, et al. Metabolic and cardiovascular adverse effects associated with antipsychotic drugs. Nat Rev Endocrinol. 2011;8(2):114-126.
- Vieweg WV, Wood MA, Fernandez A, et al. Proarrhythmic risk with antipsychotic and antidepressant drugs: implications in the elderly. Drugs Aging. 2009;26(12):997-1012.
- Sandson NB, Armstrong SC, Cozza KL. An overview of psychotropic drug-drug interactions. Psychosomatics. 2005;46(5):464-494.
Drug Brand Names
- Amantadine • Symmetrel
- Amitriptyline • Elavil
- Aripiprazole • Abilify
- Asenapine • Saphris
- Chlorpromazine • Thorazine
- Citalopram • Celexa
- Clozapine • Clozaril
- Desipramine • Norpramin
- Fluoxetine • Prozac
- Furosemide • Lasix
- Haloperidol • Haldol
- Hydroxyzine • Atarax, Vistaril
- Iloperidone • Fanapt
- Lisinopril • Prinivil, Zestril
- Lorazepam • Ativan
- Lurasidone • Latuda
- Maprotiline • Ludiomil
- Mesoridazine • Serentil
- Metformin • Glucophage
- Nortriptyline • Pamelor
- Olanzapine • Zyprexa
- Paliperidone • Invega
- Paroxetine • Paxil
- Pimozide • Orap
- Quetiapine • Seroquel
- Risperidone • Risperdal
- Tamoxifen • Nolvadex, Soltamox
- Thioridazine • Mellaril
- Venlafaxine • Effexor
- Ziprasidone • Geodon
Disclosures
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products. No similar work by the authors is under review or in press. No funding was requested or received in conjunction with this manuscript.
- Screen patients for risk factors for prolonged QTc interval, such as congenital long QT syndrome, family history of cardiac conduction abnormalities, and previous occurrences of medication-mediated QTc prolongation.
- Obtain baseline and steady state ECG when initiating high-risk agents, particularly when administering combination therapy.
- Use the lowest effective dose of antidepressants and antipsychotics and monitor symptoms closely.
Mrs. A, age 68, has a 40-year history of schizoaffective disorder with comorbid anxiety disorder not otherwise specified, type 2 diabetes mellitus, and hypertension. She takes furosemide, 40 mg/d, lisinopril, 20 mg/d, and metformin, 2,000 mg/d, for hypertension and diabetes; lorazepam, 1.5 mg/d, and paroxetine, 40 mg/d, for anxiety; and quetiapine extended release, 800 mg/d, for psychotic features and mood dysregulation with schizoaffective disorder. Mrs. A’s husband died 5 years ago and she lives alone in a senior care facility. Mrs. A uses a weekly pill reminder box because her residential facility does not monitor medication adherence. She sees her psychiatrist once a month and her primary care provider every 3 months. She has no history of illicit drug, alcohol, or tobacco use.
Two weeks ago, Mrs. A was found leaning against the wall in a hallway, complaining of dizziness and disorientation, and unable to find her way back to her apartment. In the emergency department, her serum potassium is low (3.0 mEq/L; normal range: 3.5 to 5.0), fasting glucose is elevated (110 mg/dL; range: 65 to 99), and ECG reveals a prolonged QTc interval of 530 milliseconds. Before this episode, Mrs. A had been medically stable without mood or psychotic symptoms, although her daughter reported medication self-administration was becoming difficult.
Exposure to psychotropics carries a risk of QTc prolongation. The QT interval is an ECG measure of ventricular depolarization and repolarization. The QTc designation indicates a correction for heart rate with increasing heart rate correlating with a shorter QT interval. Readings of 440 milliseconds are considered normal.1 QTc prolongation is defined as >450 milliseconds for men and >470 milliseconds for women.2 An increase in the QT interval is a predictor of serious cardiac events.3
Antidepressants and antipsychotics have been associated with QTc prolongation. When identifying agents that could disrupt cardiac conduction, clinicians need to consider whether the drug’s molecular structure, receptor affinity, or pharmacologic effects are most critical.2 Although these may be important, patient-specific variables that increase the risk of QTc prolongation may have greater impact. These include:
- age >65
- female sex
- electrolyte imbalances (specifically low serum potassium and magnesium levels)
- high or toxic serum levels of the suspected drug
- preexisting cardiovascular impairment, such as bradycardia.4,5
Other risk factors include concurrent use of an agent with similar cardiovascular effects or one that competes for metabolism (either enzymatic or at the binding site), physiologic limitations such as renal insufficiency, and medication changes that may increase or decrease psychotropic clearance.4,6 Geriatric patients with dementia have an increased risk for cardiovascular-related death.7,8
Antidepressants
Among tricyclic antidepressants, most reports of QTc prolongation involve amitriptyline and maprotiline.9 Risk factors include demographics (eg, female sex, age), personal or family history (congenital long QT syndrome, cardiovascular disease), and concurrent conditions or drug use, particularly those associated with QTc prolongation.3 Desipramine and nortriptyline also have been identified as high-risk agents.10
QTc prolongation has been reported with all selective serotonin reuptake inhibitors at plasma concentrations above the therapeutic level.11 Fluoxetine-associated QTc prolongation was limited to cases of overdose or when additional risk factors were reported.4 QTc prolongation from psychotropics could increase the risk of torsades de pointes, according to an analysis of the FDA Adverse Event Reporting System.12 In 2011, the FDA reported an increased risk of abnormal heart rhythms—including QTc prolongation—with citalopram doses >40 mg/d.13 Although cases of QTc prolongation with paroxetine have not been reported,11 the Arizona Center for Education and Research on Therapeutics lists paroxetine with other agents that may increase the risk for QTc prolongation with concurrent use of medications that may prolong QTc interval.14 Venlafaxine doses >300 mg/d may require additional cardiac monitoring.5,12 Data from venlafaxine poisoning case reports found a positive correlation between dose and QTc prolongation.15 In a review of toxicology database information, Wenzel-Seifert et al4 found extended QT interval with citalopram, fluoxetine, and venlafaxine at toxic doses or in the presence of additional risk factors such as sex, older age, or personal or family history of congenital long QT syndrome or cardiovascular disease.
Antipsychotics
Case reports, case series, and research trials have evaluated the risk of QTc prolongation with antipsychotics (Table).1,2,4,16,17 The first-generation antipsychotics thioridazine,4,16,18 mesoridazine,16,18 chlorpromazine,19 and haloperidol3 warrant cardiac monitoring. The QTc prolongation effects of thioridazine and its active metabolite mesoridazine are well-documented and thioridazine-mediated QTc prolongation increases are dose-dependent.4,18 ECG monitoring is recommended with IV haloperidol, which is used for delirium in adults.20 QTc prolongation has been associated with long-term ziprasidone use more often than with risperidone, olanzapine, or quetiapine.19 Ziprasidone prolongs the QTc interval an average of 20 milliseconds,21 which could represent a clinically significant change. QTc prolongation for iloperidone is comparable to ziprasidone and haloperidol.22 There is some evidence that aripiprazole may shorten, rather than prolong, the QTc interval.4,17
Cardiovascular adverse effects associated with clozapine—including QTc prolongation—are dose-dependent.3 Olanzapine prolongs QTc interval, although the mean change is less than with other agents unless other variables were present, such as:
- concomitant use of medications that may prolong QTc interval (ie, amantadine, hydroxyzine, or tamoxifen2)
- preexisting cardiovascular conduction disorders
- higher doses (>40 mg/d).3,23
In 17 case reports of cardiac changes associated with quetiapine use, doses ranged from 100 mg/d24 to an overdose of 36 g/d.25 Only 1 patient death was reported secondary to overdose and preexisting dysrhythmia and hypertension.26 QTc prolongation associated with risperidone was minor1 based on oral doses in the normal therapeutic range and incidences of overdose.10 Paliperidone27 and lurasidone28 are associated with clinically insignificant QTc prolongation. Changes in QTc interval were positively correlated with asenapine dose, although at the highest dose of 40 mg/d, the increase was <5 milliseconds.29
Mrs. A presents with a number of risk factors for QTc prolongation, including older age, female sex, and psychiatric and medical comorbidities that require medication. A pill count revealed that she was taking more than the prescribed daily doses of her medications. During the interview, Mrs. A said that if she missed her medication time, she would take them when she remembered. If she could not remember if she took her pills, she would take them again. Her physicians will explore strategies to increase medication adherence.
Table
Examples of QTc prolongation associated with select antipsychoticsa
| Antipsychotic | Approximate QTc interval prolongation in millisecondsb |
|---|---|
| Aripiprazole4,17 | -1 to -4 |
| Clozapine4 | 10 |
| Haloperidol1,2 | 7 to 15 |
| Mesoridazine16 | 39 to 53 |
| Olanzapine1 | 2 to 6.5 |
| Paliperidone4 | 2 to 4 |
| Pimozide2 | 19 |
| Quetiapine1,2 | 6 to 15 |
| Risperidone1,2 | 3.5 to 10 |
| Sertindole1 | 30 |
| Thioridazine2,16 | 33 to 41 |
| Ziprasidone1,2 | 16 to 21 |
| aList is not comprehensive. Other antipsychotics may be associated with QTc prolongation bQTc prolongation interval may depend on the route of administration | |
Related Resources
- De Hert M, Detraux J, van Winkel R, et al. Metabolic and cardiovascular adverse effects associated with antipsychotic drugs. Nat Rev Endocrinol. 2011;8(2):114-126.
- Vieweg WV, Wood MA, Fernandez A, et al. Proarrhythmic risk with antipsychotic and antidepressant drugs: implications in the elderly. Drugs Aging. 2009;26(12):997-1012.
- Sandson NB, Armstrong SC, Cozza KL. An overview of psychotropic drug-drug interactions. Psychosomatics. 2005;46(5):464-494.
Drug Brand Names
- Amantadine • Symmetrel
- Amitriptyline • Elavil
- Aripiprazole • Abilify
- Asenapine • Saphris
- Chlorpromazine • Thorazine
- Citalopram • Celexa
- Clozapine • Clozaril
- Desipramine • Norpramin
- Fluoxetine • Prozac
- Furosemide • Lasix
- Haloperidol • Haldol
- Hydroxyzine • Atarax, Vistaril
- Iloperidone • Fanapt
- Lisinopril • Prinivil, Zestril
- Lorazepam • Ativan
- Lurasidone • Latuda
- Maprotiline • Ludiomil
- Mesoridazine • Serentil
- Metformin • Glucophage
- Nortriptyline • Pamelor
- Olanzapine • Zyprexa
- Paliperidone • Invega
- Paroxetine • Paxil
- Pimozide • Orap
- Quetiapine • Seroquel
- Risperidone • Risperdal
- Tamoxifen • Nolvadex, Soltamox
- Thioridazine • Mellaril
- Venlafaxine • Effexor
- Ziprasidone • Geodon
Disclosures
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products. No similar work by the authors is under review or in press. No funding was requested or received in conjunction with this manuscript.
1. Muscatello MR, Bruno A, Pandolfo G, et al. Emerging treatments in the management of schizophrenia - focus on sertindole. Drug Des Devel Ther. 2010;4:187-201.
2. Taylor DM. Antipsychotics and QT prolongation. Acta Psychiatr Scand. 2003;107(2):85-95.
3. Alvarez PA, Pahissa J. QT alterations in psychopharmacology: proven candidates and suspects. Curr Drug Saf. 2010;5(1):97-104.
4. Wenzel-Seifert K, Wittmann M, Haen E. QTc prolongation by psychotropic drugs and the risk of torsade de pointes. Dtsch Arztebl Int. 2011;108(41):687-693.
5. Vieweg WV. New generation antipsychotic drugs and QTc interval prolongation. Prim Care Companion J Clin Psychiatry. 2003;5(5):205-215.
6. Nielsen J, Graff C, Kanters JK, et al. Assessing QT interval prolongation and its associated risks with antipsychotics. CNS Drugs. 2011;25(6):473-490.
7. Gill SS, Bronskill SE, Normand SL, et al. Antipsychotic drug use and mortality in older adults with dementia. Ann Intern Med. 2007;146(11):775-786.
8. Schneeweiss S, Setoguchi S, Brookhart A, et al. Risk of death associated with the use of conventional versus atypical antipsychotic drugs among elderly patients. CMAJ. 2007;176(5):627-632.
9. Vieweg WV, Wood MA. Tricyclic antidepressants QT interval prolongation, and torsade de pointes. Psychosomatics. 2004;45(5):371-377.
10. Jeon SH, Jaekal J, Lee SH, et al. Effects of nortriptyline on QT prolongation: a safety pharmacology study. Hum Exp Toxicol. 2011;30(10):1649-1656.
11. Wenzel-Seifert K, Wittmann M, Haen E. Torsade de pointes episodes under treatment with selective serotonin reuptake inhibitors. Pharmacopsychiatry. 2010;43(7):279-281.
12. Poluzzi E, Raschi E, Moretti U, et al. Drug-induced torsades de pointes: data mining of the public version of the FDA Adverse Event Reporting System (AERS). Pharmacoepidemiol Drug Saf. 2009;18(6):512-518.
13. U.S. Food and Drug Administration. FDA drug safety communication: revised recommendations for Celexa (citalopram hydrobromide) related to a potential risk of abnormal heart rhythms with high doses. http://www.fda.gov/Drugs/DrugSafety/ucm297391.htm. Published March 28, 2012. Accessed June 26, 2012.
14. Arizona CERT-QT Center for Education and Research on Therapeutics. QT drug lists by risk groups. http://www.azcert.org/medical-pros/drug-lists/drug-lists.cfm. Accessed June 26 2012.
15. Howell C, Wilson AD, Waring WS. Cardiovascular toxicity due to venlafaxine poisoning in adults: a review of 235 consecutive cases. Br J Clin Pharmacol. 2007;64(2):192-197.
16. Salih IS, Thanacoody RH, McKay GA, et al. Comparison of the effects of thioridazine and mesoridazine on the QT interval in healthy adults after single oral doses. Clin Pharmacol Ther. 2007;82(5):548-554.
17. Goodnick PJ, Jerry J, Parra F. Psychotropic drugs and the ECG: focus on the QTc interval. Expert Opin Pharmacother. 2002;3(5):479-498.
18. Dallaire S. Thioridazine (Mellaril) and mesoridazine (Serentil): prolongation of the QTc interval. CMAJ. 2001;164(1):91,95.-
19. Haddad PM, Anderson IM. Antipsychotic-related QTc prolongation torsade de pointes and sudden death. Drugs. 2002;62(11):1649-1671.
20. Shapiro BA, Warren J, Egol AB, et al. Practice parameters for intravenous analgesia and sedation for adult patients in the intensive care unit: an executive summary. Crit Care Med. 1995;23(9):1596-1600.
21. Vieweg WV, Hasnain M. Question regarding ziprasidone and QTc interval prolongation in the ZODIAC Study. Am J Psychiatry. 2011;168(6):650-651.
22. Caccia S, Pasina L, Nobili A. New atypical antipsychotics for schizophrenia: iloperidone. Drug Des Devel Ther. 2010;4:33-48.
23. Dineen S, Withrow K, Voronovitch L, et al. QTc prolongation and high-dose olanzapine. Psychosomatics. 2003;44(2):174-175.
24. Vieweg WV, Schneider RK, Wood MA. Torsade de pointes in a patient with complex medical and psychiatric conditions receiving low-dose quetiapine. Acta Psychiatr Scand. 2005;112(4):318-322.
25. Capuano A, Ruggiero S, Vestini F, et al. Survival from coma induced by an intentional 36-g overdose of extended-release quetiapine. Drug Chem Toxicol. 2011;34(4):475-477.
26. Fernandes PP, Marcil WA. Death associated with quetiapine overdose. Am J Psychiatry. 2002;159(12):2114.-
27. Sedky K, Nazir R, Lindenmayer JP, et al. Paliperidone palmitate: once-monthly treatment option for schizophrenia. Current Psychiatry. 2010;9(3):48-50.
28. Citrome L. Lurasidone for schizophrenia: a review of the efficacy and safety profile for this newly approved second-generation antipsychotic. Int J Clin Pract. 2011;65(2):189-210.
29. Chapel S, Hutmacher MM, Haig G, et al. Exposure-response analysis in patients with schizophrenia to assess the effect of asenapine on QTc prolongation. J Clin Pharmacol. 2009;49(11):1297-1308.
1. Muscatello MR, Bruno A, Pandolfo G, et al. Emerging treatments in the management of schizophrenia - focus on sertindole. Drug Des Devel Ther. 2010;4:187-201.
2. Taylor DM. Antipsychotics and QT prolongation. Acta Psychiatr Scand. 2003;107(2):85-95.
3. Alvarez PA, Pahissa J. QT alterations in psychopharmacology: proven candidates and suspects. Curr Drug Saf. 2010;5(1):97-104.
4. Wenzel-Seifert K, Wittmann M, Haen E. QTc prolongation by psychotropic drugs and the risk of torsade de pointes. Dtsch Arztebl Int. 2011;108(41):687-693.
5. Vieweg WV. New generation antipsychotic drugs and QTc interval prolongation. Prim Care Companion J Clin Psychiatry. 2003;5(5):205-215.
6. Nielsen J, Graff C, Kanters JK, et al. Assessing QT interval prolongation and its associated risks with antipsychotics. CNS Drugs. 2011;25(6):473-490.
7. Gill SS, Bronskill SE, Normand SL, et al. Antipsychotic drug use and mortality in older adults with dementia. Ann Intern Med. 2007;146(11):775-786.
8. Schneeweiss S, Setoguchi S, Brookhart A, et al. Risk of death associated with the use of conventional versus atypical antipsychotic drugs among elderly patients. CMAJ. 2007;176(5):627-632.
9. Vieweg WV, Wood MA. Tricyclic antidepressants QT interval prolongation, and torsade de pointes. Psychosomatics. 2004;45(5):371-377.
10. Jeon SH, Jaekal J, Lee SH, et al. Effects of nortriptyline on QT prolongation: a safety pharmacology study. Hum Exp Toxicol. 2011;30(10):1649-1656.
11. Wenzel-Seifert K, Wittmann M, Haen E. Torsade de pointes episodes under treatment with selective serotonin reuptake inhibitors. Pharmacopsychiatry. 2010;43(7):279-281.
12. Poluzzi E, Raschi E, Moretti U, et al. Drug-induced torsades de pointes: data mining of the public version of the FDA Adverse Event Reporting System (AERS). Pharmacoepidemiol Drug Saf. 2009;18(6):512-518.
13. U.S. Food and Drug Administration. FDA drug safety communication: revised recommendations for Celexa (citalopram hydrobromide) related to a potential risk of abnormal heart rhythms with high doses. http://www.fda.gov/Drugs/DrugSafety/ucm297391.htm. Published March 28, 2012. Accessed June 26, 2012.
14. Arizona CERT-QT Center for Education and Research on Therapeutics. QT drug lists by risk groups. http://www.azcert.org/medical-pros/drug-lists/drug-lists.cfm. Accessed June 26 2012.
15. Howell C, Wilson AD, Waring WS. Cardiovascular toxicity due to venlafaxine poisoning in adults: a review of 235 consecutive cases. Br J Clin Pharmacol. 2007;64(2):192-197.
16. Salih IS, Thanacoody RH, McKay GA, et al. Comparison of the effects of thioridazine and mesoridazine on the QT interval in healthy adults after single oral doses. Clin Pharmacol Ther. 2007;82(5):548-554.
17. Goodnick PJ, Jerry J, Parra F. Psychotropic drugs and the ECG: focus on the QTc interval. Expert Opin Pharmacother. 2002;3(5):479-498.
18. Dallaire S. Thioridazine (Mellaril) and mesoridazine (Serentil): prolongation of the QTc interval. CMAJ. 2001;164(1):91,95.-
19. Haddad PM, Anderson IM. Antipsychotic-related QTc prolongation torsade de pointes and sudden death. Drugs. 2002;62(11):1649-1671.
20. Shapiro BA, Warren J, Egol AB, et al. Practice parameters for intravenous analgesia and sedation for adult patients in the intensive care unit: an executive summary. Crit Care Med. 1995;23(9):1596-1600.
21. Vieweg WV, Hasnain M. Question regarding ziprasidone and QTc interval prolongation in the ZODIAC Study. Am J Psychiatry. 2011;168(6):650-651.
22. Caccia S, Pasina L, Nobili A. New atypical antipsychotics for schizophrenia: iloperidone. Drug Des Devel Ther. 2010;4:33-48.
23. Dineen S, Withrow K, Voronovitch L, et al. QTc prolongation and high-dose olanzapine. Psychosomatics. 2003;44(2):174-175.
24. Vieweg WV, Schneider RK, Wood MA. Torsade de pointes in a patient with complex medical and psychiatric conditions receiving low-dose quetiapine. Acta Psychiatr Scand. 2005;112(4):318-322.
25. Capuano A, Ruggiero S, Vestini F, et al. Survival from coma induced by an intentional 36-g overdose of extended-release quetiapine. Drug Chem Toxicol. 2011;34(4):475-477.
26. Fernandes PP, Marcil WA. Death associated with quetiapine overdose. Am J Psychiatry. 2002;159(12):2114.-
27. Sedky K, Nazir R, Lindenmayer JP, et al. Paliperidone palmitate: once-monthly treatment option for schizophrenia. Current Psychiatry. 2010;9(3):48-50.
28. Citrome L. Lurasidone for schizophrenia: a review of the efficacy and safety profile for this newly approved second-generation antipsychotic. Int J Clin Pract. 2011;65(2):189-210.
29. Chapel S, Hutmacher MM, Haig G, et al. Exposure-response analysis in patients with schizophrenia to assess the effect of asenapine on QTc prolongation. J Clin Pharmacol. 2009;49(11):1297-1308.
Can combining triptans with SSRIs or SNRIs cause serotonin syndrome?
In 2006, the FDA issued a warning of the risk of potentially fatal serotonin syndrome when 5-hydroxytryptamine receptor agonist antimigraine medications (triptans) and selective serotonin reuptake inhibitors (SSRIs) or serotonin-norepinephrine reuptake inhibitors (SNRI) are coprescribed.1 As a result, most drug interaction programs trigger a serotonin syndrome warning when triptans are prescribed with an SSRI or SNRI.2 However, many patients with depression or anxiety also suffer from migraines and require treatment with both triptans and an SSRI or SNRI.3,4 Kalaydjian et al4 found the incidence of major depression and generalized anxiety disorder were approximately 3 times greater in patients with migraines than in those without migraines. Should we avoid coprescribing triptans and SSRIs or SNRIs?
What is serotonin syndrome?
Serotonin syndrome is an adverse drug reaction that results from excessive serotonin stimulation. There are 2 sets of validated diagnostic criteria: the Sternbach Criteria and the Hunter Serotonin Toxicity Criteria; the latter is considered more stringent.3,5-7 Symptoms of serotonin syndrome include mental status changes, autonomic hyperactivity, and neuromuscular changes such as muscle rigidity.5-7 Typical manifestations of serotonin syndrome on physical exam include spontaneous and/or inducible clonus, agitation, diaphoresis, tremor, hyperreflexia, hypertonia, and temperature >38°C.6 In severe cases, serotonin syndrome can lead to seizures, coma, and death. Management includes supportive treatment, discontinuing the offending agents, controlling agitation with medications such as benzodiazepines, and possibly administering cyproheptadine, a 5HT2A antagonist.8 Most cases resolve within 24 hours of discontinuing the offending agents or appropriate treatment.5
What did the FDA say?
The 2006 FDA warning initially was based on 27 reports of serotonin syndrome in patients receiving triptans and SSRIs or SNRIs; this was later expanded to include 29 patients.1,9 No patients died but 13 required hospitalization and 2 had life-threatening symptoms. However, most cases lacked data necessary to diagnose serotonin syndrome.9 Further, reviews of the available clinical information have suggested that in some cases, clinicians did not rule out other disorders as required by diagnostic criteria, while others were viral in nature or resolved despite ongoing treatment with the presumed offending agents.9-11
Some clinicians met the FDA’s assessment with skepticism. Only 10 of the 29 cases met the Sternbach criteria of serotonin syndrome and none met the more rigorous Hunter criteria. Additionally, the theoretical basis has been questioned.9-11 Available evidence indicates that serotonin syndrome requires activation of 5HT2A receptors and a possible limited role of 5HT1A.9-12 However, triptans are agonists at the 5HT1B/1D/1F receptor subtypes, with weak affinity for 5HT1A receptors and no activity at the 5HT2 receptors.13,14 Additionally, triptan medications are used as needed, not as standing treatments, with parameters limiting the maximum dose, dosing interval, and frequency of use. In clinical practice, it appears that these dosing guidelines are being followed: Tepper et al15 found the typical female patient experiences 1 to 2 migraines per month; on average, patients use 1.2 to 1.8 triptan tablets per month.
Our opinion
We believe it is reasonable to coprescribe SSRIs or SNRIs with triptans because:
- data indicate that many patients are treated with a combination of triptans and SSRIs or SNRIs but the number of reported cases of serotonin syndrome is extremely limited
- the nature of serotonin syndrome cases reported in the literature is questionable
- the interaction is biologically implausible
- triptans remain in the body for a limited time
- triptans are used infrequently.5-11
This view is supported by the most recent American Headache Society position paper,11 which states that inadequate data are available to assess the risk but current evidence does not support limiting use of triptans with SSRIs and SNRIs.
How we deal with the warning in clinical practice. In practice we are alerted to this interaction by notification in our e-prescribing systems, by pharmacists calling with concerns about dispensing an SSRI or SNRI for a patient already receiving a triptan, and during patient visits that involve prescribing an SSRI or SNRI.
Although it is relatively easy to override a drug interaction warning in our e-prescribing system, we discuss the issue with pharmacists and patients. We provide information about the signs and symptoms of serotonin syndrome and its potential dangerousness. We note that serotonin syndrome is a theoretical concern, but highly unlikely with this combination of medications because of their pharmacologic properties. We explain the parameters of triptan use, recommend that our patients use triptans for migraines when needed, and reassure patients we are available to answer questions. When a patient uses triptans more than twice monthly, we consider discussing this usage with the patient and the treating physician.
Related Resource
- Sclar DA, Robison LM, Castillo LV, et al. Concomitant use of triptan, and SSRI or SNRI after the US Food and Drug Administration alert on serotonin syndrome. Headache. 2012. www.headachejournal.org/SpringboardWebApp/userfiles/headache/file/sclar.pdf.
Drug Brand Name
- Cyproheptadine • Perinctin
Disclosure
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. U.S. Food and Drug Administration. Public health advisory—combined use of 5-hydroxytryptamine receptor agonists (triptans), selective serotonin reuptake inhibitors (SSRIs) or selective serotonin/norepinephrine reuptake inhibitors (SNRIs) may result in life-threatening serotonin syndrome. http://1.usa.gov/U0A0V4. Published July 19, 2006. Accessed September 18, 2012.
2. Kogut SJ. Do triptan antimigraine medications interact with SSRI/SNRI antidepressants? What does your decision support system say? J Manag Care Pharm. 2011;17(7):547-551.
3. Tepper SJ. Serotonin syndrome: SSRIs SNRIs, triptans, and current clinical practice. Headache. 2012;52(2):195-197.
4. Kalaydjian A, Merikangas K. Physical and mental comorbidity of headache in a nationally representative sample of US adults. Psychosom Med. 2008;70(7):773-780.
5. Boyer EW, Shannon M. The serotonin syndrome. N Engl J Med. 2005;352(11):1112-1120.
6. Sternbach H. The serotonin syndrome. Am J Psychiatry. 1991;148(6):705-713.
7. Dunkley EJ, Isbister GK, Sibbritt D, et al. The Hunter Serotonin Toxicity Criteria: simple and accurate diagnostic decision rules for serotonin toxicity. QJM. 2003;96(9):635-642.
8. Ables AZ, Nagubilli R. Prevention recognition, and management of serotonin syndrome. Am Fam Physician. 2010;81(9):1139-1142.
9. Evans RW. The FDA alert on serotonin syndrome with combined use of SSRIs or SNRIs and triptans: an analysis of the 29 case reports. MedGenMed. 2007;9(3):48.-
10. Gillman PK. Triptans serotonin agonists, and serotonin syndrome (serotonin toxicity): a review. Headache. 2010;50(2):264-272.
11. Evans RW, Tepper SJ, Shapiro RE, et al. The FDA alert on serotonin syndrome with use of triptans combined with selective serotonin reuptake inhibitors or selective serotonin-norepinephrine reuptake inhibitors: American Headache Society position paper. Headache. 2010;50(6):1089-1099.
12. Ahn AH, Basbaum AI. Where do triptans act in the treatment of migraine? Pain. 2005;115(1-2):1-4.
13. Pediatric & Neonatal Lexi-Drugs. Hudson, OH: Lexi-Comp, Inc.; 2011.
14. Sclar DA, Robison LM, Castillo LV, et al. Concomitant use of triptan, and SSRI or SNRI after the US Food and Drug Administration alert on serotonin syndrome. Headache. 2012;52(2):198-203.
15. Tepper S, Allen C, Sanders D, et al. Coprescription of triptans with potentially interacting medications: a cohort study involving 240,268 patients. Headache. 2003;43(1):44-48.
In 2006, the FDA issued a warning of the risk of potentially fatal serotonin syndrome when 5-hydroxytryptamine receptor agonist antimigraine medications (triptans) and selective serotonin reuptake inhibitors (SSRIs) or serotonin-norepinephrine reuptake inhibitors (SNRI) are coprescribed.1 As a result, most drug interaction programs trigger a serotonin syndrome warning when triptans are prescribed with an SSRI or SNRI.2 However, many patients with depression or anxiety also suffer from migraines and require treatment with both triptans and an SSRI or SNRI.3,4 Kalaydjian et al4 found the incidence of major depression and generalized anxiety disorder were approximately 3 times greater in patients with migraines than in those without migraines. Should we avoid coprescribing triptans and SSRIs or SNRIs?
What is serotonin syndrome?
Serotonin syndrome is an adverse drug reaction that results from excessive serotonin stimulation. There are 2 sets of validated diagnostic criteria: the Sternbach Criteria and the Hunter Serotonin Toxicity Criteria; the latter is considered more stringent.3,5-7 Symptoms of serotonin syndrome include mental status changes, autonomic hyperactivity, and neuromuscular changes such as muscle rigidity.5-7 Typical manifestations of serotonin syndrome on physical exam include spontaneous and/or inducible clonus, agitation, diaphoresis, tremor, hyperreflexia, hypertonia, and temperature >38°C.6 In severe cases, serotonin syndrome can lead to seizures, coma, and death. Management includes supportive treatment, discontinuing the offending agents, controlling agitation with medications such as benzodiazepines, and possibly administering cyproheptadine, a 5HT2A antagonist.8 Most cases resolve within 24 hours of discontinuing the offending agents or appropriate treatment.5
What did the FDA say?
The 2006 FDA warning initially was based on 27 reports of serotonin syndrome in patients receiving triptans and SSRIs or SNRIs; this was later expanded to include 29 patients.1,9 No patients died but 13 required hospitalization and 2 had life-threatening symptoms. However, most cases lacked data necessary to diagnose serotonin syndrome.9 Further, reviews of the available clinical information have suggested that in some cases, clinicians did not rule out other disorders as required by diagnostic criteria, while others were viral in nature or resolved despite ongoing treatment with the presumed offending agents.9-11
Some clinicians met the FDA’s assessment with skepticism. Only 10 of the 29 cases met the Sternbach criteria of serotonin syndrome and none met the more rigorous Hunter criteria. Additionally, the theoretical basis has been questioned.9-11 Available evidence indicates that serotonin syndrome requires activation of 5HT2A receptors and a possible limited role of 5HT1A.9-12 However, triptans are agonists at the 5HT1B/1D/1F receptor subtypes, with weak affinity for 5HT1A receptors and no activity at the 5HT2 receptors.13,14 Additionally, triptan medications are used as needed, not as standing treatments, with parameters limiting the maximum dose, dosing interval, and frequency of use. In clinical practice, it appears that these dosing guidelines are being followed: Tepper et al15 found the typical female patient experiences 1 to 2 migraines per month; on average, patients use 1.2 to 1.8 triptan tablets per month.
Our opinion
We believe it is reasonable to coprescribe SSRIs or SNRIs with triptans because:
- data indicate that many patients are treated with a combination of triptans and SSRIs or SNRIs but the number of reported cases of serotonin syndrome is extremely limited
- the nature of serotonin syndrome cases reported in the literature is questionable
- the interaction is biologically implausible
- triptans remain in the body for a limited time
- triptans are used infrequently.5-11
This view is supported by the most recent American Headache Society position paper,11 which states that inadequate data are available to assess the risk but current evidence does not support limiting use of triptans with SSRIs and SNRIs.
How we deal with the warning in clinical practice. In practice we are alerted to this interaction by notification in our e-prescribing systems, by pharmacists calling with concerns about dispensing an SSRI or SNRI for a patient already receiving a triptan, and during patient visits that involve prescribing an SSRI or SNRI.
Although it is relatively easy to override a drug interaction warning in our e-prescribing system, we discuss the issue with pharmacists and patients. We provide information about the signs and symptoms of serotonin syndrome and its potential dangerousness. We note that serotonin syndrome is a theoretical concern, but highly unlikely with this combination of medications because of their pharmacologic properties. We explain the parameters of triptan use, recommend that our patients use triptans for migraines when needed, and reassure patients we are available to answer questions. When a patient uses triptans more than twice monthly, we consider discussing this usage with the patient and the treating physician.
Related Resource
- Sclar DA, Robison LM, Castillo LV, et al. Concomitant use of triptan, and SSRI or SNRI after the US Food and Drug Administration alert on serotonin syndrome. Headache. 2012. www.headachejournal.org/SpringboardWebApp/userfiles/headache/file/sclar.pdf.
Drug Brand Name
- Cyproheptadine • Perinctin
Disclosure
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
In 2006, the FDA issued a warning of the risk of potentially fatal serotonin syndrome when 5-hydroxytryptamine receptor agonist antimigraine medications (triptans) and selective serotonin reuptake inhibitors (SSRIs) or serotonin-norepinephrine reuptake inhibitors (SNRI) are coprescribed.1 As a result, most drug interaction programs trigger a serotonin syndrome warning when triptans are prescribed with an SSRI or SNRI.2 However, many patients with depression or anxiety also suffer from migraines and require treatment with both triptans and an SSRI or SNRI.3,4 Kalaydjian et al4 found the incidence of major depression and generalized anxiety disorder were approximately 3 times greater in patients with migraines than in those without migraines. Should we avoid coprescribing triptans and SSRIs or SNRIs?
What is serotonin syndrome?
Serotonin syndrome is an adverse drug reaction that results from excessive serotonin stimulation. There are 2 sets of validated diagnostic criteria: the Sternbach Criteria and the Hunter Serotonin Toxicity Criteria; the latter is considered more stringent.3,5-7 Symptoms of serotonin syndrome include mental status changes, autonomic hyperactivity, and neuromuscular changes such as muscle rigidity.5-7 Typical manifestations of serotonin syndrome on physical exam include spontaneous and/or inducible clonus, agitation, diaphoresis, tremor, hyperreflexia, hypertonia, and temperature >38°C.6 In severe cases, serotonin syndrome can lead to seizures, coma, and death. Management includes supportive treatment, discontinuing the offending agents, controlling agitation with medications such as benzodiazepines, and possibly administering cyproheptadine, a 5HT2A antagonist.8 Most cases resolve within 24 hours of discontinuing the offending agents or appropriate treatment.5
What did the FDA say?
The 2006 FDA warning initially was based on 27 reports of serotonin syndrome in patients receiving triptans and SSRIs or SNRIs; this was later expanded to include 29 patients.1,9 No patients died but 13 required hospitalization and 2 had life-threatening symptoms. However, most cases lacked data necessary to diagnose serotonin syndrome.9 Further, reviews of the available clinical information have suggested that in some cases, clinicians did not rule out other disorders as required by diagnostic criteria, while others were viral in nature or resolved despite ongoing treatment with the presumed offending agents.9-11
Some clinicians met the FDA’s assessment with skepticism. Only 10 of the 29 cases met the Sternbach criteria of serotonin syndrome and none met the more rigorous Hunter criteria. Additionally, the theoretical basis has been questioned.9-11 Available evidence indicates that serotonin syndrome requires activation of 5HT2A receptors and a possible limited role of 5HT1A.9-12 However, triptans are agonists at the 5HT1B/1D/1F receptor subtypes, with weak affinity for 5HT1A receptors and no activity at the 5HT2 receptors.13,14 Additionally, triptan medications are used as needed, not as standing treatments, with parameters limiting the maximum dose, dosing interval, and frequency of use. In clinical practice, it appears that these dosing guidelines are being followed: Tepper et al15 found the typical female patient experiences 1 to 2 migraines per month; on average, patients use 1.2 to 1.8 triptan tablets per month.
Our opinion
We believe it is reasonable to coprescribe SSRIs or SNRIs with triptans because:
- data indicate that many patients are treated with a combination of triptans and SSRIs or SNRIs but the number of reported cases of serotonin syndrome is extremely limited
- the nature of serotonin syndrome cases reported in the literature is questionable
- the interaction is biologically implausible
- triptans remain in the body for a limited time
- triptans are used infrequently.5-11
This view is supported by the most recent American Headache Society position paper,11 which states that inadequate data are available to assess the risk but current evidence does not support limiting use of triptans with SSRIs and SNRIs.
How we deal with the warning in clinical practice. In practice we are alerted to this interaction by notification in our e-prescribing systems, by pharmacists calling with concerns about dispensing an SSRI or SNRI for a patient already receiving a triptan, and during patient visits that involve prescribing an SSRI or SNRI.
Although it is relatively easy to override a drug interaction warning in our e-prescribing system, we discuss the issue with pharmacists and patients. We provide information about the signs and symptoms of serotonin syndrome and its potential dangerousness. We note that serotonin syndrome is a theoretical concern, but highly unlikely with this combination of medications because of their pharmacologic properties. We explain the parameters of triptan use, recommend that our patients use triptans for migraines when needed, and reassure patients we are available to answer questions. When a patient uses triptans more than twice monthly, we consider discussing this usage with the patient and the treating physician.
Related Resource
- Sclar DA, Robison LM, Castillo LV, et al. Concomitant use of triptan, and SSRI or SNRI after the US Food and Drug Administration alert on serotonin syndrome. Headache. 2012. www.headachejournal.org/SpringboardWebApp/userfiles/headache/file/sclar.pdf.
Drug Brand Name
- Cyproheptadine • Perinctin
Disclosure
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. U.S. Food and Drug Administration. Public health advisory—combined use of 5-hydroxytryptamine receptor agonists (triptans), selective serotonin reuptake inhibitors (SSRIs) or selective serotonin/norepinephrine reuptake inhibitors (SNRIs) may result in life-threatening serotonin syndrome. http://1.usa.gov/U0A0V4. Published July 19, 2006. Accessed September 18, 2012.
2. Kogut SJ. Do triptan antimigraine medications interact with SSRI/SNRI antidepressants? What does your decision support system say? J Manag Care Pharm. 2011;17(7):547-551.
3. Tepper SJ. Serotonin syndrome: SSRIs SNRIs, triptans, and current clinical practice. Headache. 2012;52(2):195-197.
4. Kalaydjian A, Merikangas K. Physical and mental comorbidity of headache in a nationally representative sample of US adults. Psychosom Med. 2008;70(7):773-780.
5. Boyer EW, Shannon M. The serotonin syndrome. N Engl J Med. 2005;352(11):1112-1120.
6. Sternbach H. The serotonin syndrome. Am J Psychiatry. 1991;148(6):705-713.
7. Dunkley EJ, Isbister GK, Sibbritt D, et al. The Hunter Serotonin Toxicity Criteria: simple and accurate diagnostic decision rules for serotonin toxicity. QJM. 2003;96(9):635-642.
8. Ables AZ, Nagubilli R. Prevention recognition, and management of serotonin syndrome. Am Fam Physician. 2010;81(9):1139-1142.
9. Evans RW. The FDA alert on serotonin syndrome with combined use of SSRIs or SNRIs and triptans: an analysis of the 29 case reports. MedGenMed. 2007;9(3):48.-
10. Gillman PK. Triptans serotonin agonists, and serotonin syndrome (serotonin toxicity): a review. Headache. 2010;50(2):264-272.
11. Evans RW, Tepper SJ, Shapiro RE, et al. The FDA alert on serotonin syndrome with use of triptans combined with selective serotonin reuptake inhibitors or selective serotonin-norepinephrine reuptake inhibitors: American Headache Society position paper. Headache. 2010;50(6):1089-1099.
12. Ahn AH, Basbaum AI. Where do triptans act in the treatment of migraine? Pain. 2005;115(1-2):1-4.
13. Pediatric & Neonatal Lexi-Drugs. Hudson, OH: Lexi-Comp, Inc.; 2011.
14. Sclar DA, Robison LM, Castillo LV, et al. Concomitant use of triptan, and SSRI or SNRI after the US Food and Drug Administration alert on serotonin syndrome. Headache. 2012;52(2):198-203.
15. Tepper S, Allen C, Sanders D, et al. Coprescription of triptans with potentially interacting medications: a cohort study involving 240,268 patients. Headache. 2003;43(1):44-48.
1. U.S. Food and Drug Administration. Public health advisory—combined use of 5-hydroxytryptamine receptor agonists (triptans), selective serotonin reuptake inhibitors (SSRIs) or selective serotonin/norepinephrine reuptake inhibitors (SNRIs) may result in life-threatening serotonin syndrome. http://1.usa.gov/U0A0V4. Published July 19, 2006. Accessed September 18, 2012.
2. Kogut SJ. Do triptan antimigraine medications interact with SSRI/SNRI antidepressants? What does your decision support system say? J Manag Care Pharm. 2011;17(7):547-551.
3. Tepper SJ. Serotonin syndrome: SSRIs SNRIs, triptans, and current clinical practice. Headache. 2012;52(2):195-197.
4. Kalaydjian A, Merikangas K. Physical and mental comorbidity of headache in a nationally representative sample of US adults. Psychosom Med. 2008;70(7):773-780.
5. Boyer EW, Shannon M. The serotonin syndrome. N Engl J Med. 2005;352(11):1112-1120.
6. Sternbach H. The serotonin syndrome. Am J Psychiatry. 1991;148(6):705-713.
7. Dunkley EJ, Isbister GK, Sibbritt D, et al. The Hunter Serotonin Toxicity Criteria: simple and accurate diagnostic decision rules for serotonin toxicity. QJM. 2003;96(9):635-642.
8. Ables AZ, Nagubilli R. Prevention recognition, and management of serotonin syndrome. Am Fam Physician. 2010;81(9):1139-1142.
9. Evans RW. The FDA alert on serotonin syndrome with combined use of SSRIs or SNRIs and triptans: an analysis of the 29 case reports. MedGenMed. 2007;9(3):48.-
10. Gillman PK. Triptans serotonin agonists, and serotonin syndrome (serotonin toxicity): a review. Headache. 2010;50(2):264-272.
11. Evans RW, Tepper SJ, Shapiro RE, et al. The FDA alert on serotonin syndrome with use of triptans combined with selective serotonin reuptake inhibitors or selective serotonin-norepinephrine reuptake inhibitors: American Headache Society position paper. Headache. 2010;50(6):1089-1099.
12. Ahn AH, Basbaum AI. Where do triptans act in the treatment of migraine? Pain. 2005;115(1-2):1-4.
13. Pediatric & Neonatal Lexi-Drugs. Hudson, OH: Lexi-Comp, Inc.; 2011.
14. Sclar DA, Robison LM, Castillo LV, et al. Concomitant use of triptan, and SSRI or SNRI after the US Food and Drug Administration alert on serotonin syndrome. Headache. 2012;52(2):198-203.
15. Tepper S, Allen C, Sanders D, et al. Coprescription of triptans with potentially interacting medications: a cohort study involving 240,268 patients. Headache. 2003;43(1):44-48.
Management of dermatological toxicities in patients receiving EGFR inhibitors
Patients receiving treatment with epidermal growth factor receptor inhibitors often experience dermatological toxicities. The majority of patients develop skin rash, and may also experience adverse nail and periungual alterations. EGFR inhibitors have become part of the standard of care for several solid tumors, including metastatic colorectal cancer, cancers of the head and neck, and non small-cell lung cancer, thus adequate management of these side effects is necessary to ensure patient compliance to therapy, as well as to maximize patient comfort and quality of life. This review presents a protocol our center optimized to successfully manage cetuximab-associated acneiform rash and nail toxicities.
Click on the PDF icon at the top of this introduction to read the full article.
Patients receiving treatment with epidermal growth factor receptor inhibitors often experience dermatological toxicities. The majority of patients develop skin rash, and may also experience adverse nail and periungual alterations. EGFR inhibitors have become part of the standard of care for several solid tumors, including metastatic colorectal cancer, cancers of the head and neck, and non small-cell lung cancer, thus adequate management of these side effects is necessary to ensure patient compliance to therapy, as well as to maximize patient comfort and quality of life. This review presents a protocol our center optimized to successfully manage cetuximab-associated acneiform rash and nail toxicities.
Click on the PDF icon at the top of this introduction to read the full article.
Patients receiving treatment with epidermal growth factor receptor inhibitors often experience dermatological toxicities. The majority of patients develop skin rash, and may also experience adverse nail and periungual alterations. EGFR inhibitors have become part of the standard of care for several solid tumors, including metastatic colorectal cancer, cancers of the head and neck, and non small-cell lung cancer, thus adequate management of these side effects is necessary to ensure patient compliance to therapy, as well as to maximize patient comfort and quality of life. This review presents a protocol our center optimized to successfully manage cetuximab-associated acneiform rash and nail toxicities.
Click on the PDF icon at the top of this introduction to read the full article.
Hypertension in cancer patients
Hypertension is the force of blood pushing against the walls of the arteries. It is measured as systolic pressure when the heart beats and pumps blood and as diastolic pressure in the arteries when the heart rests between beats. There are 4 stages in blood pressure classification—normal, prehypertension, stage 1, and stage 2. Hypertension affects approximately 50 million people in the United States and 1 billion people worldwide. People who are normotensive at age 55 years have a 90% chance of developing hypertension in their lifetime. Starting with a blood pressure of 115/75 mmHg, the risk of cardiovascular death doubles with each 20/10 mmHg increment...
*For PDFs of the full article and related Commentary, click on the links to the left of this introduction.
Hypertension is the force of blood pushing against the walls of the arteries. It is measured as systolic pressure when the heart beats and pumps blood and as diastolic pressure in the arteries when the heart rests between beats. There are 4 stages in blood pressure classification—normal, prehypertension, stage 1, and stage 2. Hypertension affects approximately 50 million people in the United States and 1 billion people worldwide. People who are normotensive at age 55 years have a 90% chance of developing hypertension in their lifetime. Starting with a blood pressure of 115/75 mmHg, the risk of cardiovascular death doubles with each 20/10 mmHg increment...
*For PDFs of the full article and related Commentary, click on the links to the left of this introduction.
Hypertension is the force of blood pushing against the walls of the arteries. It is measured as systolic pressure when the heart beats and pumps blood and as diastolic pressure in the arteries when the heart rests between beats. There are 4 stages in blood pressure classification—normal, prehypertension, stage 1, and stage 2. Hypertension affects approximately 50 million people in the United States and 1 billion people worldwide. People who are normotensive at age 55 years have a 90% chance of developing hypertension in their lifetime. Starting with a blood pressure of 115/75 mmHg, the risk of cardiovascular death doubles with each 20/10 mmHg increment...
*For PDFs of the full article and related Commentary, click on the links to the left of this introduction.
Recent developments in the treatment of high-grade gliomas
Patients with glioblastoma and other high-grade gliomas have poor outcomes and are challenging to treat. The relative rarity of these tumors has made large-scale, practice-changing trials difficult to accomplish and has led to the formation of large multinational organizations that focus on neuro-oncology. This has resulted in the rapid completion of several large trials that in some cases have set new standards of care that can offer increased progression-free and overall survivals for some patients. The incorporation of correlative tissue studies in these trials has led to the identification of prognostic and predictive genetic markers that demonstrate the heterogeneity of these tumors and will assist in developing individualized treatment strategies as research continues to uncover new therapeutic targets. This review of recently completed and in-progress phase 3 trials in high-grade gliomas highlights the developments and future directions in the treatment of these tumors...
*For PDFs of the full article and related Commentary, click on the links to the left of this introduction.
Patients with glioblastoma and other high-grade gliomas have poor outcomes and are challenging to treat. The relative rarity of these tumors has made large-scale, practice-changing trials difficult to accomplish and has led to the formation of large multinational organizations that focus on neuro-oncology. This has resulted in the rapid completion of several large trials that in some cases have set new standards of care that can offer increased progression-free and overall survivals for some patients. The incorporation of correlative tissue studies in these trials has led to the identification of prognostic and predictive genetic markers that demonstrate the heterogeneity of these tumors and will assist in developing individualized treatment strategies as research continues to uncover new therapeutic targets. This review of recently completed and in-progress phase 3 trials in high-grade gliomas highlights the developments and future directions in the treatment of these tumors...
*For PDFs of the full article and related Commentary, click on the links to the left of this introduction.
Patients with glioblastoma and other high-grade gliomas have poor outcomes and are challenging to treat. The relative rarity of these tumors has made large-scale, practice-changing trials difficult to accomplish and has led to the formation of large multinational organizations that focus on neuro-oncology. This has resulted in the rapid completion of several large trials that in some cases have set new standards of care that can offer increased progression-free and overall survivals for some patients. The incorporation of correlative tissue studies in these trials has led to the identification of prognostic and predictive genetic markers that demonstrate the heterogeneity of these tumors and will assist in developing individualized treatment strategies as research continues to uncover new therapeutic targets. This review of recently completed and in-progress phase 3 trials in high-grade gliomas highlights the developments and future directions in the treatment of these tumors...
*For PDFs of the full article and related Commentary, click on the links to the left of this introduction.
Second TNF-Blocker Approved for Refractory Ulcerative Colitis
Adalimumab, a subcutaneously administered tumor necrosis factor blocker, has been approved for treating adults with moderately to severely active ulcerative colitis who have not had an adequate response with conventional treatments, the Food and Drug Administration announced on Sept. 28.
The safety and effectiveness of adalimumab for this patient population was established in two clinical studies of 908 patients with moderately to severely active ulcerative colitis (UC).
Adalimumab, marketed as Humira by Abbott Laboratories, was first approved for treating rheumatoid arthritis in 2002, followed by psoriatic arthritis in 2005, ankylosing spondylitis in 2006, Crohn’s disease in 2007, and plaque psoriasis and juvenile idiopathic arthritis in 2008.
Adalimumab is the second TNF blocker to be approved for ulcerative colitis; infliximab (Remicade), an intravenous TNF blocker, was previously approved for treating UC.
Clinical remission rates in the two studies were significantly greater among patients treated with infliximab than among those who received placebo: In an 8-week study, which did not include patients who had previously been treated with a TNF blocker, the clinical remission rate at 8 weeks was 18.5% among those on adalimumab vs. 9.2% in those on placebo, a 9.3% difference. In the second study, which followed patients for 1 year and included some who had been treated with infliximab, the clinical remission rate at 8 weeks was 16.5% among those on adalimumab, vs. 9.3% among those on placebo, a 7.2% difference.
At a meeting on Aug. 28 held to review these data, the majority of the FDA’s Gastrointestinal Drugs Advisory Committee agreed that these differences represented clinically meaningful benefits and supported approval of adalimumab for this indication. Panelists cited the need for more treatments for UC and for a subcutaneous TNF blocker for these patients, as well as its potential steroid-sparing effects.
In the studies, no new side effects were identified, the agency said. The FDA statement points out that the effectiveness of adalimumab "has not been established in patients with ulcerative colitis who have lost response to or were intolerant to TNF blockers."
The approved dosing regimen for adalimumab is a starting dose of 160 mg, followed by a second dose of 80 mg 2 weeks later and then a maintenance dose of 40 mg every other week. "The drug should only continue to be used in patients who have shown evidence of clinical remission by 8 weeks of therapy," according to the FDA statement.
Adalimumab is the first self-administered biologic treatment for ulcerative colitis to be approved.
Adalimumab, a subcutaneously administered tumor necrosis factor blocker, has been approved for treating adults with moderately to severely active ulcerative colitis who have not had an adequate response with conventional treatments, the Food and Drug Administration announced on Sept. 28.
The safety and effectiveness of adalimumab for this patient population was established in two clinical studies of 908 patients with moderately to severely active ulcerative colitis (UC).
Adalimumab, marketed as Humira by Abbott Laboratories, was first approved for treating rheumatoid arthritis in 2002, followed by psoriatic arthritis in 2005, ankylosing spondylitis in 2006, Crohn’s disease in 2007, and plaque psoriasis and juvenile idiopathic arthritis in 2008.
Adalimumab is the second TNF blocker to be approved for ulcerative colitis; infliximab (Remicade), an intravenous TNF blocker, was previously approved for treating UC.
Clinical remission rates in the two studies were significantly greater among patients treated with infliximab than among those who received placebo: In an 8-week study, which did not include patients who had previously been treated with a TNF blocker, the clinical remission rate at 8 weeks was 18.5% among those on adalimumab vs. 9.2% in those on placebo, a 9.3% difference. In the second study, which followed patients for 1 year and included some who had been treated with infliximab, the clinical remission rate at 8 weeks was 16.5% among those on adalimumab, vs. 9.3% among those on placebo, a 7.2% difference.
At a meeting on Aug. 28 held to review these data, the majority of the FDA’s Gastrointestinal Drugs Advisory Committee agreed that these differences represented clinically meaningful benefits and supported approval of adalimumab for this indication. Panelists cited the need for more treatments for UC and for a subcutaneous TNF blocker for these patients, as well as its potential steroid-sparing effects.
In the studies, no new side effects were identified, the agency said. The FDA statement points out that the effectiveness of adalimumab "has not been established in patients with ulcerative colitis who have lost response to or were intolerant to TNF blockers."
The approved dosing regimen for adalimumab is a starting dose of 160 mg, followed by a second dose of 80 mg 2 weeks later and then a maintenance dose of 40 mg every other week. "The drug should only continue to be used in patients who have shown evidence of clinical remission by 8 weeks of therapy," according to the FDA statement.
Adalimumab is the first self-administered biologic treatment for ulcerative colitis to be approved.
Adalimumab, a subcutaneously administered tumor necrosis factor blocker, has been approved for treating adults with moderately to severely active ulcerative colitis who have not had an adequate response with conventional treatments, the Food and Drug Administration announced on Sept. 28.
The safety and effectiveness of adalimumab for this patient population was established in two clinical studies of 908 patients with moderately to severely active ulcerative colitis (UC).
Adalimumab, marketed as Humira by Abbott Laboratories, was first approved for treating rheumatoid arthritis in 2002, followed by psoriatic arthritis in 2005, ankylosing spondylitis in 2006, Crohn’s disease in 2007, and plaque psoriasis and juvenile idiopathic arthritis in 2008.
Adalimumab is the second TNF blocker to be approved for ulcerative colitis; infliximab (Remicade), an intravenous TNF blocker, was previously approved for treating UC.
Clinical remission rates in the two studies were significantly greater among patients treated with infliximab than among those who received placebo: In an 8-week study, which did not include patients who had previously been treated with a TNF blocker, the clinical remission rate at 8 weeks was 18.5% among those on adalimumab vs. 9.2% in those on placebo, a 9.3% difference. In the second study, which followed patients for 1 year and included some who had been treated with infliximab, the clinical remission rate at 8 weeks was 16.5% among those on adalimumab, vs. 9.3% among those on placebo, a 7.2% difference.
At a meeting on Aug. 28 held to review these data, the majority of the FDA’s Gastrointestinal Drugs Advisory Committee agreed that these differences represented clinically meaningful benefits and supported approval of adalimumab for this indication. Panelists cited the need for more treatments for UC and for a subcutaneous TNF blocker for these patients, as well as its potential steroid-sparing effects.
In the studies, no new side effects were identified, the agency said. The FDA statement points out that the effectiveness of adalimumab "has not been established in patients with ulcerative colitis who have lost response to or were intolerant to TNF blockers."
The approved dosing regimen for adalimumab is a starting dose of 160 mg, followed by a second dose of 80 mg 2 weeks later and then a maintenance dose of 40 mg every other week. "The drug should only continue to be used in patients who have shown evidence of clinical remission by 8 weeks of therapy," according to the FDA statement.
Adalimumab is the first self-administered biologic treatment for ulcerative colitis to be approved.
New Gout Guidelines Inspired by Recent Data
The first guidelines on the management of gout from the American College of Rheumatology recommend new ways of using old drugs and changes in prophylaxis strategies, among other things.
The two-part guidelines, published online Sept. 28, should help speed up effective treatment of gout and get physicians to treat patients to a target urate level of less than 6 mg/dL in order to improve symptoms, Dr. John D. FitzGerald said in an interview.
"There has been a fair amount of recent movement on gout medications" including new alternatives to allopurinol and colchicine and new data on how to use those traditional drugs in safer ways, said Dr. FitzGerald, acting chief of the rheumatology division at the University of California, Los Angeles. "It’s a fair number of changes for medications that people had been using for decades."
The documents update previous guidelines from medical organizations in Europe, the Netherlands, and Japan. The new guidelines will be published in October 2012 by the journal Arthritis Care & Research.
Part 1 of the American College of Rheumatology (ACR) guidelines covers nonpharmacologic and pharmacologic approaches to managing hyperuricemia (Arthritis Care Res. 2012;64:1431-46 [doi:10.1002/acr.21772]).
Part 2 addresses prophylaxis and treatment for acute gouty arthritis (Arthritis Care Res. 2012;64:1447-61 [doi.wiley.com/10.1002/acr.21773]).
Dr. FitzGerald and two other co-leaders of the project, Dr. Dinesh Khanna of the University of Michigan, Ann Arbor and Dr. Robert Terkeltaub of the University of California, San Diego, reviewed the medical literature on gout from the 1950s to the present and drew up nine clinical case scenarios commonly seen in practice. A task force panel comprising seven rheumatologists, two primary care physicians, a nephrologist, and a patient representative used the scenarios to create consensus recommendations.
Among the recommendations, for example, on the use of allopurinol is to start at a low dose of 100 mg/day (instead of the common practice of starting with 300 mg/day), or even lower for patients with chronic kidney disease, and then gradually titrate upward every 2-5 weeks. That recommendation supports previous statements from the Food and Drug Administration and the European League Against Rheumatism.
Also, allopurinol therapy should be actively managed and patients followed to make sure the uric acid target is achieved. "You can’t just give a prescription and say your job is done," though some recent studies suggest that many physicians do just that, Dr. FitzGerald said. "The corollary would be if someone gave blood pressure medication and then didn’t follow the patient’s blood pressure. That wouldn’t be seen as good medicine."
Maintenance doses of allopurinol to prevent acute gout attacks can exceed 300 mg even in patients with chronic kidney disease provided there is adequate patient education and monitoring.
A new recommendation drops the starting dose of oral colchicine for acute gout attacks to a loading dose of 1.2 mg, followed by 0.6 mg an hour later, and then starting prophylaxis 12 hours later at dosing of 0.6 mg once or twice daily.
"We used to give up to eight tablets a day," Dr. FitzGerald said. "That is dropped down to three to four tablets at the start of an attacks, because of findings that more colchicine didn’t really help outcomes" and that smaller doses are safer. The authors called this recommendation from ACR "a paradigm shift" that’s in accordance with Food and Drug Administration-approved label language.
Other highlights of the new ACR recommendations include sections on screening for HLA-B*5801 in patients at high risk of severe adverse reaction to allopurinol, combination therapy when target urate levels are not achieved, medication options including new drugs, and more.
Although the reports are titled "Guidelines," the text makes clear that they are expert recommendations and that clinicians are expected to take active roles in choosing the best management strategies for their particular patients. The authors were "very concerned" that the guidelines not be used by third-party payers to restrict access to medications or to promote one drug over another if there isn’t clear evidence to support it, Dr. FitzGerald said.
The methodology of the project precluded evaluations of costs and cost effectiveness, instead focusing on efficacy. So, for example, the guidelines say that allopurinol and febuxostat can be used equivalently in some circumstances, but clinicians need to consider all other aspects of these options including cost, patient preference, and more.
The ACR plans to update the guidelines as new data become available. The task force panel did create specific indications for use of imaging studies because results should be available in the next few years from studies on the use of high-resolution ultrasound and dual-energy CT for patients with gout.
In the United States, gout affects an estimated 4% of adults – more than 8 million people.
"I’m most excited and hopeful about trying to get this out to internal medicine and family practice doctors," Dr. FitzGerald said. "They see more gout than rheumatologists."
Dr. FitzGerald reported having no financial disclosures. Some members of the task force reported financial associations with multiple pharmaceutical companies but, by design, a majority of task force members had no perceived potential conflicts of interest.
Writing guidelines on gout is a difficult task. I think they made a very good effort to cover as many treatment issues as they could.
Most patients with gout in the United States are cared for by primary care physicians. The guidelines will be helpful to both primary practitioners and rheumatologists, but the subtleties may be lost on the general practitioner, whereas the rheumatologist would pick these up right away. The devil is often in the details when it comes to treating gout. If physicians use the guidelines employing a cookbook approach, they might run into some problems.
For instance, the guidelines cover the use of colchicine as a first-line agent for an acute attack: It’s a good choice, but even the randomized controlled trials that have been published on this, especially using the low-dose approach, show that a significant proportion of patients will not respond to this regimen. The guidelines recommend a dosage higher than what has been advised previously for the low-dose colchicine approach. This may actually be a better method, so I hope this will allow primary practitioners to be able identify more people using this approach. But there are definitely going to be people who do not respond to the colchicine.
Another example of where the guidelines may mislead primary care physicians is the recommendation on when to start urate-lowering therapy (ULT). Their indications for starting pharmacologic ULT include an established diagnosis of gouty arthritis and at least two attacks per year. My colleagues and I think that may exclude too many people. Theoretically, you could have a patient with one attack per year who is having gout-related joint damage and, with this criteria, wouldn’t qualify for ULT. A rheumatologist would pick that up right away, but general practitioners who adhere to these guidelines might end up undertreating some patients.
Also, they recommend using adrenocorticotropic hormone (ACTH) for people who cannot take oral medications. Not only is ACTH is extremely expensive, but the Food and Drug Administration has taken gout off the list of indications for ACTH, so I doubt it would be readily available in a real clinical situation.
When the recommendations discuss using prednisone as a prophylactic against gout attacks, they suggest using 10 mg or less. I think that the authors are trying for the best of both worlds and ending up not having either. We generally try to avoid using steroids long term, so the authors suggest using low-dose prednisone; the problem is that 10 mg would probably be ineffective. There are data suggesting that gout prophylaxis requires higher doses, maybe as much as 20 mg/day. You could try 10 mg but I anticipate that it is not going to work very well.
In their defense, were the authors to go into the subtleties and side effects, what to do with a patient with liver or coronary disease, or issues of cost effectiveness, the guidelines would have become an unmanageable length. But the devil is in the details.
That said, it’s a major effort here. It’s good work. They tried to answer a lot of questions.
Dr. Christopher M. Burns is a rheumatologist at the Geisel School of Medicine at Dartmouth, Hanover, N.H. He reported having no financial disclosures.
Writing guidelines on gout is a difficult task. I think they made a very good effort to cover as many treatment issues as they could.
Most patients with gout in the United States are cared for by primary care physicians. The guidelines will be helpful to both primary practitioners and rheumatologists, but the subtleties may be lost on the general practitioner, whereas the rheumatologist would pick these up right away. The devil is often in the details when it comes to treating gout. If physicians use the guidelines employing a cookbook approach, they might run into some problems.
For instance, the guidelines cover the use of colchicine as a first-line agent for an acute attack: It’s a good choice, but even the randomized controlled trials that have been published on this, especially using the low-dose approach, show that a significant proportion of patients will not respond to this regimen. The guidelines recommend a dosage higher than what has been advised previously for the low-dose colchicine approach. This may actually be a better method, so I hope this will allow primary practitioners to be able identify more people using this approach. But there are definitely going to be people who do not respond to the colchicine.
Another example of where the guidelines may mislead primary care physicians is the recommendation on when to start urate-lowering therapy (ULT). Their indications for starting pharmacologic ULT include an established diagnosis of gouty arthritis and at least two attacks per year. My colleagues and I think that may exclude too many people. Theoretically, you could have a patient with one attack per year who is having gout-related joint damage and, with this criteria, wouldn’t qualify for ULT. A rheumatologist would pick that up right away, but general practitioners who adhere to these guidelines might end up undertreating some patients.
Also, they recommend using adrenocorticotropic hormone (ACTH) for people who cannot take oral medications. Not only is ACTH is extremely expensive, but the Food and Drug Administration has taken gout off the list of indications for ACTH, so I doubt it would be readily available in a real clinical situation.
When the recommendations discuss using prednisone as a prophylactic against gout attacks, they suggest using 10 mg or less. I think that the authors are trying for the best of both worlds and ending up not having either. We generally try to avoid using steroids long term, so the authors suggest using low-dose prednisone; the problem is that 10 mg would probably be ineffective. There are data suggesting that gout prophylaxis requires higher doses, maybe as much as 20 mg/day. You could try 10 mg but I anticipate that it is not going to work very well.
In their defense, were the authors to go into the subtleties and side effects, what to do with a patient with liver or coronary disease, or issues of cost effectiveness, the guidelines would have become an unmanageable length. But the devil is in the details.
That said, it’s a major effort here. It’s good work. They tried to answer a lot of questions.
Dr. Christopher M. Burns is a rheumatologist at the Geisel School of Medicine at Dartmouth, Hanover, N.H. He reported having no financial disclosures.
Writing guidelines on gout is a difficult task. I think they made a very good effort to cover as many treatment issues as they could.
Most patients with gout in the United States are cared for by primary care physicians. The guidelines will be helpful to both primary practitioners and rheumatologists, but the subtleties may be lost on the general practitioner, whereas the rheumatologist would pick these up right away. The devil is often in the details when it comes to treating gout. If physicians use the guidelines employing a cookbook approach, they might run into some problems.
For instance, the guidelines cover the use of colchicine as a first-line agent for an acute attack: It’s a good choice, but even the randomized controlled trials that have been published on this, especially using the low-dose approach, show that a significant proportion of patients will not respond to this regimen. The guidelines recommend a dosage higher than what has been advised previously for the low-dose colchicine approach. This may actually be a better method, so I hope this will allow primary practitioners to be able identify more people using this approach. But there are definitely going to be people who do not respond to the colchicine.
Another example of where the guidelines may mislead primary care physicians is the recommendation on when to start urate-lowering therapy (ULT). Their indications for starting pharmacologic ULT include an established diagnosis of gouty arthritis and at least two attacks per year. My colleagues and I think that may exclude too many people. Theoretically, you could have a patient with one attack per year who is having gout-related joint damage and, with this criteria, wouldn’t qualify for ULT. A rheumatologist would pick that up right away, but general practitioners who adhere to these guidelines might end up undertreating some patients.
Also, they recommend using adrenocorticotropic hormone (ACTH) for people who cannot take oral medications. Not only is ACTH is extremely expensive, but the Food and Drug Administration has taken gout off the list of indications for ACTH, so I doubt it would be readily available in a real clinical situation.
When the recommendations discuss using prednisone as a prophylactic against gout attacks, they suggest using 10 mg or less. I think that the authors are trying for the best of both worlds and ending up not having either. We generally try to avoid using steroids long term, so the authors suggest using low-dose prednisone; the problem is that 10 mg would probably be ineffective. There are data suggesting that gout prophylaxis requires higher doses, maybe as much as 20 mg/day. You could try 10 mg but I anticipate that it is not going to work very well.
In their defense, were the authors to go into the subtleties and side effects, what to do with a patient with liver or coronary disease, or issues of cost effectiveness, the guidelines would have become an unmanageable length. But the devil is in the details.
That said, it’s a major effort here. It’s good work. They tried to answer a lot of questions.
Dr. Christopher M. Burns is a rheumatologist at the Geisel School of Medicine at Dartmouth, Hanover, N.H. He reported having no financial disclosures.
The first guidelines on the management of gout from the American College of Rheumatology recommend new ways of using old drugs and changes in prophylaxis strategies, among other things.
The two-part guidelines, published online Sept. 28, should help speed up effective treatment of gout and get physicians to treat patients to a target urate level of less than 6 mg/dL in order to improve symptoms, Dr. John D. FitzGerald said in an interview.
"There has been a fair amount of recent movement on gout medications" including new alternatives to allopurinol and colchicine and new data on how to use those traditional drugs in safer ways, said Dr. FitzGerald, acting chief of the rheumatology division at the University of California, Los Angeles. "It’s a fair number of changes for medications that people had been using for decades."
The documents update previous guidelines from medical organizations in Europe, the Netherlands, and Japan. The new guidelines will be published in October 2012 by the journal Arthritis Care & Research.
Part 1 of the American College of Rheumatology (ACR) guidelines covers nonpharmacologic and pharmacologic approaches to managing hyperuricemia (Arthritis Care Res. 2012;64:1431-46 [doi:10.1002/acr.21772]).
Part 2 addresses prophylaxis and treatment for acute gouty arthritis (Arthritis Care Res. 2012;64:1447-61 [doi.wiley.com/10.1002/acr.21773]).
Dr. FitzGerald and two other co-leaders of the project, Dr. Dinesh Khanna of the University of Michigan, Ann Arbor and Dr. Robert Terkeltaub of the University of California, San Diego, reviewed the medical literature on gout from the 1950s to the present and drew up nine clinical case scenarios commonly seen in practice. A task force panel comprising seven rheumatologists, two primary care physicians, a nephrologist, and a patient representative used the scenarios to create consensus recommendations.
Among the recommendations, for example, on the use of allopurinol is to start at a low dose of 100 mg/day (instead of the common practice of starting with 300 mg/day), or even lower for patients with chronic kidney disease, and then gradually titrate upward every 2-5 weeks. That recommendation supports previous statements from the Food and Drug Administration and the European League Against Rheumatism.
Also, allopurinol therapy should be actively managed and patients followed to make sure the uric acid target is achieved. "You can’t just give a prescription and say your job is done," though some recent studies suggest that many physicians do just that, Dr. FitzGerald said. "The corollary would be if someone gave blood pressure medication and then didn’t follow the patient’s blood pressure. That wouldn’t be seen as good medicine."
Maintenance doses of allopurinol to prevent acute gout attacks can exceed 300 mg even in patients with chronic kidney disease provided there is adequate patient education and monitoring.
A new recommendation drops the starting dose of oral colchicine for acute gout attacks to a loading dose of 1.2 mg, followed by 0.6 mg an hour later, and then starting prophylaxis 12 hours later at dosing of 0.6 mg once or twice daily.
"We used to give up to eight tablets a day," Dr. FitzGerald said. "That is dropped down to three to four tablets at the start of an attacks, because of findings that more colchicine didn’t really help outcomes" and that smaller doses are safer. The authors called this recommendation from ACR "a paradigm shift" that’s in accordance with Food and Drug Administration-approved label language.
Other highlights of the new ACR recommendations include sections on screening for HLA-B*5801 in patients at high risk of severe adverse reaction to allopurinol, combination therapy when target urate levels are not achieved, medication options including new drugs, and more.
Although the reports are titled "Guidelines," the text makes clear that they are expert recommendations and that clinicians are expected to take active roles in choosing the best management strategies for their particular patients. The authors were "very concerned" that the guidelines not be used by third-party payers to restrict access to medications or to promote one drug over another if there isn’t clear evidence to support it, Dr. FitzGerald said.
The methodology of the project precluded evaluations of costs and cost effectiveness, instead focusing on efficacy. So, for example, the guidelines say that allopurinol and febuxostat can be used equivalently in some circumstances, but clinicians need to consider all other aspects of these options including cost, patient preference, and more.
The ACR plans to update the guidelines as new data become available. The task force panel did create specific indications for use of imaging studies because results should be available in the next few years from studies on the use of high-resolution ultrasound and dual-energy CT for patients with gout.
In the United States, gout affects an estimated 4% of adults – more than 8 million people.
"I’m most excited and hopeful about trying to get this out to internal medicine and family practice doctors," Dr. FitzGerald said. "They see more gout than rheumatologists."
Dr. FitzGerald reported having no financial disclosures. Some members of the task force reported financial associations with multiple pharmaceutical companies but, by design, a majority of task force members had no perceived potential conflicts of interest.
The first guidelines on the management of gout from the American College of Rheumatology recommend new ways of using old drugs and changes in prophylaxis strategies, among other things.
The two-part guidelines, published online Sept. 28, should help speed up effective treatment of gout and get physicians to treat patients to a target urate level of less than 6 mg/dL in order to improve symptoms, Dr. John D. FitzGerald said in an interview.
"There has been a fair amount of recent movement on gout medications" including new alternatives to allopurinol and colchicine and new data on how to use those traditional drugs in safer ways, said Dr. FitzGerald, acting chief of the rheumatology division at the University of California, Los Angeles. "It’s a fair number of changes for medications that people had been using for decades."
The documents update previous guidelines from medical organizations in Europe, the Netherlands, and Japan. The new guidelines will be published in October 2012 by the journal Arthritis Care & Research.
Part 1 of the American College of Rheumatology (ACR) guidelines covers nonpharmacologic and pharmacologic approaches to managing hyperuricemia (Arthritis Care Res. 2012;64:1431-46 [doi:10.1002/acr.21772]).
Part 2 addresses prophylaxis and treatment for acute gouty arthritis (Arthritis Care Res. 2012;64:1447-61 [doi.wiley.com/10.1002/acr.21773]).
Dr. FitzGerald and two other co-leaders of the project, Dr. Dinesh Khanna of the University of Michigan, Ann Arbor and Dr. Robert Terkeltaub of the University of California, San Diego, reviewed the medical literature on gout from the 1950s to the present and drew up nine clinical case scenarios commonly seen in practice. A task force panel comprising seven rheumatologists, two primary care physicians, a nephrologist, and a patient representative used the scenarios to create consensus recommendations.
Among the recommendations, for example, on the use of allopurinol is to start at a low dose of 100 mg/day (instead of the common practice of starting with 300 mg/day), or even lower for patients with chronic kidney disease, and then gradually titrate upward every 2-5 weeks. That recommendation supports previous statements from the Food and Drug Administration and the European League Against Rheumatism.
Also, allopurinol therapy should be actively managed and patients followed to make sure the uric acid target is achieved. "You can’t just give a prescription and say your job is done," though some recent studies suggest that many physicians do just that, Dr. FitzGerald said. "The corollary would be if someone gave blood pressure medication and then didn’t follow the patient’s blood pressure. That wouldn’t be seen as good medicine."
Maintenance doses of allopurinol to prevent acute gout attacks can exceed 300 mg even in patients with chronic kidney disease provided there is adequate patient education and monitoring.
A new recommendation drops the starting dose of oral colchicine for acute gout attacks to a loading dose of 1.2 mg, followed by 0.6 mg an hour later, and then starting prophylaxis 12 hours later at dosing of 0.6 mg once or twice daily.
"We used to give up to eight tablets a day," Dr. FitzGerald said. "That is dropped down to three to four tablets at the start of an attacks, because of findings that more colchicine didn’t really help outcomes" and that smaller doses are safer. The authors called this recommendation from ACR "a paradigm shift" that’s in accordance with Food and Drug Administration-approved label language.
Other highlights of the new ACR recommendations include sections on screening for HLA-B*5801 in patients at high risk of severe adverse reaction to allopurinol, combination therapy when target urate levels are not achieved, medication options including new drugs, and more.
Although the reports are titled "Guidelines," the text makes clear that they are expert recommendations and that clinicians are expected to take active roles in choosing the best management strategies for their particular patients. The authors were "very concerned" that the guidelines not be used by third-party payers to restrict access to medications or to promote one drug over another if there isn’t clear evidence to support it, Dr. FitzGerald said.
The methodology of the project precluded evaluations of costs and cost effectiveness, instead focusing on efficacy. So, for example, the guidelines say that allopurinol and febuxostat can be used equivalently in some circumstances, but clinicians need to consider all other aspects of these options including cost, patient preference, and more.
The ACR plans to update the guidelines as new data become available. The task force panel did create specific indications for use of imaging studies because results should be available in the next few years from studies on the use of high-resolution ultrasound and dual-energy CT for patients with gout.
In the United States, gout affects an estimated 4% of adults – more than 8 million people.
"I’m most excited and hopeful about trying to get this out to internal medicine and family practice doctors," Dr. FitzGerald said. "They see more gout than rheumatologists."
Dr. FitzGerald reported having no financial disclosures. Some members of the task force reported financial associations with multiple pharmaceutical companies but, by design, a majority of task force members had no perceived potential conflicts of interest.
Childhood Problem Flares at Age 50
ANSWER
The correct interpretation includes normal sinus rhythm, right bundle branch block, and left anterior fascicular block. Normal sinus rhythm is evidenced by a rate between 60 and 100 beats/min, with a corresponding P for every QRS and a QRS for every P.
Right bundle branch block is evidenced by a QRS duration > 120 ms, a terminal broad S wave in lead I, and an RSR’ complex in lead V1. Left anterior fascicular block is evident from the finding that the S waves are greater than the R waves in leads II, III, and aVF.
The presence of a right ventricular block and left anterior fascicular block (bifascicular block) is consistent with a history of a VSD and/or surgical repair. The right and left bundles proceed from the atrioventricular node and bundle of His down the ventricular septum to the Purkinje fibers in the distal ventricular myocardium. Therefore, congenital anomalies of the ventricular septum, and/or surgical intervention within it, often affect conduction of the right and/or left bundle.
This patient’s symptoms were a result of his dilated aorta, and he underwent successful repair, with resolution of his symptoms.
ANSWER
The correct interpretation includes normal sinus rhythm, right bundle branch block, and left anterior fascicular block. Normal sinus rhythm is evidenced by a rate between 60 and 100 beats/min, with a corresponding P for every QRS and a QRS for every P.
Right bundle branch block is evidenced by a QRS duration > 120 ms, a terminal broad S wave in lead I, and an RSR’ complex in lead V1. Left anterior fascicular block is evident from the finding that the S waves are greater than the R waves in leads II, III, and aVF.
The presence of a right ventricular block and left anterior fascicular block (bifascicular block) is consistent with a history of a VSD and/or surgical repair. The right and left bundles proceed from the atrioventricular node and bundle of His down the ventricular septum to the Purkinje fibers in the distal ventricular myocardium. Therefore, congenital anomalies of the ventricular septum, and/or surgical intervention within it, often affect conduction of the right and/or left bundle.
This patient’s symptoms were a result of his dilated aorta, and he underwent successful repair, with resolution of his symptoms.
ANSWER
The correct interpretation includes normal sinus rhythm, right bundle branch block, and left anterior fascicular block. Normal sinus rhythm is evidenced by a rate between 60 and 100 beats/min, with a corresponding P for every QRS and a QRS for every P.
Right bundle branch block is evidenced by a QRS duration > 120 ms, a terminal broad S wave in lead I, and an RSR’ complex in lead V1. Left anterior fascicular block is evident from the finding that the S waves are greater than the R waves in leads II, III, and aVF.
The presence of a right ventricular block and left anterior fascicular block (bifascicular block) is consistent with a history of a VSD and/or surgical repair. The right and left bundles proceed from the atrioventricular node and bundle of His down the ventricular septum to the Purkinje fibers in the distal ventricular myocardium. Therefore, congenital anomalies of the ventricular septum, and/or surgical intervention within it, often affect conduction of the right and/or left bundle.
This patient’s symptoms were a result of his dilated aorta, and he underwent successful repair, with resolution of his symptoms.
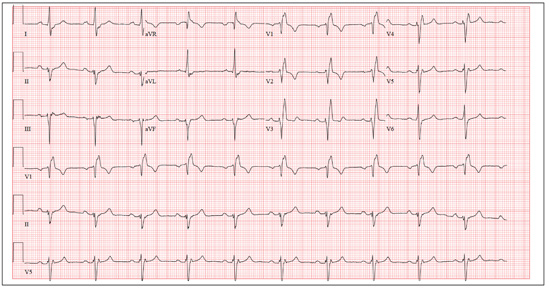
A man, 50, has a history of tetralogy of Fallot (ventricular septal defect [VSD], pulmonary stenosis, right ventricular hypertrophy, and overriding aorta). He underwent surgical correction at age 4, with placement of a Blalock-Taussig shunt and closure of his VSD, and was asymptomatic until one year ago. In the past year, he has developed progressive shortness of breath and dyspnea on exertion. In the past three months, he has developed chest pain that he describes as sharp, nonradiating, and occurring most often with dyspnea on exertion. He denies syncope, near-syncope, palpitations, or tachycardia. He cannot walk more than one-and-a-half blocks before stopping to rest, and he avoids hills and stairs if at all possible. A review of his most recent cardiac work-up (performed six months ago) reveals no significant coronary artery disease or evidence of aortic stenosis; it shows moderate aortic regurgitation, normal systolic aortic pressures, and normal left ventricular end diastolic pressures. The right ventricular pressures were elevated due to pulmonic stenosis; however, the estimated pulmonary artery pressures were normal. A cardiac MRI performed one month ago shows a significantly dilated aortic root with aneurysmal dilatation extending to the aortic arch, with effacement at the sinotubular junction and moderate aortic regurgitation. Additional findings include a markedly dilated right ventricular outflow tract with no pulmonic stenosis, evi-dence of a previous right Blalock-Taussig shunt, and moderate right atrial enlargement. Medical history is remarkable for hypertension. Family history is remarkable for hypertension, diabetes, and coronary artery disease, but not congenital heart disease. The patient does not smoke and drinks socially on the weekends. His medications include amlodipine, aspirin, and lisinopril. He is allergic to penicillin and amox-icillin. A review of systems reveals that he has had flulike symptoms for the past four days, with a dry, nonproductive cough. Physical exam reveals a well-developed, obese male in no distress. His height is 67”and his weight, 208 lb. Blood pressure is 102/70 mm Hg; pulse, 70 beats/min; respiratory rate, 16 breaths/min-1; and temperature, 98.4°F. His oxygen saturation is 98% on room air. Pertinent physical findings include a grade II/VI holosystolic murmur and a grade III/VI diastolic murmur, with a prominent S2 best heard at the left lower sternal border. There is no jugular venous distention, no peripheral edema, and no abnormal pulmonary finding. An ECG previously ordered for today’s visit reveals the following: a ventricular rate of 63 beats/min; PR interval, 196 ms; QRS duration, 174 ms; QT/QTc inter-val, 460/470 ms; P axis, 34°; R axis, –67°; and T axis, 56°. What is your interpretation of this ECG? How does the patient’s history predict the findings?
Wife is Worried That Her Husband's Condition is Contagious
ANSWER
The correct answer is petaloid seborrheic dermatitis (choice “d”), named for the flowerlike appearance of its polycyclic borders. Psoriasis (choice “a”) can present in this area, but tends to be scalier and usually involves multiple areas (eg, elbows, knees, and nails).
Rashes like this patient’s are often termed yeast infection (choice “b”). However, while a commensal yeast (Pityrosporum) can play a role in its formation, it appears that seborrhea represents an idiosyncratic reaction to increased numbers of this organism, rather than an actual infection.
Bowen’s disease (choice “c”) is a superficial squamous cell carcinoma, usually caused by overexposure to sunlight. Its lesions will be fixed, slowly growing larger with time, while seborrheic dermatitis will typically come and go. Biopsy is sometimes necessary to distinguish one from the other.
DISCUSSION
Seborrheic dermatitis (SD, aka seborrhea) is common, affecting up to 5% of the population. Dandruff is its usual manifestation, but it affects numerous other areas (as in this case), including the axillae, groin, beard, and genitals.
Presenting with scaling on an erythematous base, SD often flares and remits with the season (especially winter), with stress, and with increases in alcohol intake. Although it is usually mild, some cases can be severe. SD is associated with or accentuated by several other conditions, including Parkinson’s, stroke, and HIV. Severe SD in infants raises the possibility of Langerhans cell histiocytosis, especially when the presentation is atypical.
The diagnosis of SD can be difficult when it appears elsewhere than the scalp and face (eg, as an axillary or genital rash). Likewise, sternal petaloid SD is mystifying, unless other corroboratory manifestations are sought and found.
A few patients show signs of SD and psoriasis such that a definitive diagnosis cannot be made. Such overlap cases are sometimes termed sebopsoriasis. But psoriasis will usually exhibit signs not seen with SD, such as pitting of the nails, involvement of extensor surfaces of elbows and knees, and characteristic signs of psoriatic arthropathy in about 20% of cases. Pinpoint bleeding caused by peeling away scale, called the Auspitz sign, is seen with psoriasis and not with SD.
TREATMENT
This patient’s chest involvement responded rapidly to topical betamethasone foam, quickly tapered to avoid thinning the skin. Less powerful steroid creams, lotions, or gels (eg, triamcinolone 0.025%) can be used on other areas, such as ears and face. The daily use of an OTC dandruff shampoo (containing selenium sulfide, zinc pyrithione, tar, or ketoconazole) is an effective approach to controlling scalp involvement, but the product should be changed weekly.
Once the initial inflammation is controlled, topical antiyeast/antifungal preparations (eg, ketoconazole cream or any of the imidazoles, such as clotrimazole or oxiconazole) can be useful.
Finally, emphasis must be placed on educating the patient to expect control of the condition but not a cure.
ANSWER
The correct answer is petaloid seborrheic dermatitis (choice “d”), named for the flowerlike appearance of its polycyclic borders. Psoriasis (choice “a”) can present in this area, but tends to be scalier and usually involves multiple areas (eg, elbows, knees, and nails).
Rashes like this patient’s are often termed yeast infection (choice “b”). However, while a commensal yeast (Pityrosporum) can play a role in its formation, it appears that seborrhea represents an idiosyncratic reaction to increased numbers of this organism, rather than an actual infection.
Bowen’s disease (choice “c”) is a superficial squamous cell carcinoma, usually caused by overexposure to sunlight. Its lesions will be fixed, slowly growing larger with time, while seborrheic dermatitis will typically come and go. Biopsy is sometimes necessary to distinguish one from the other.
DISCUSSION
Seborrheic dermatitis (SD, aka seborrhea) is common, affecting up to 5% of the population. Dandruff is its usual manifestation, but it affects numerous other areas (as in this case), including the axillae, groin, beard, and genitals.
Presenting with scaling on an erythematous base, SD often flares and remits with the season (especially winter), with stress, and with increases in alcohol intake. Although it is usually mild, some cases can be severe. SD is associated with or accentuated by several other conditions, including Parkinson’s, stroke, and HIV. Severe SD in infants raises the possibility of Langerhans cell histiocytosis, especially when the presentation is atypical.
The diagnosis of SD can be difficult when it appears elsewhere than the scalp and face (eg, as an axillary or genital rash). Likewise, sternal petaloid SD is mystifying, unless other corroboratory manifestations are sought and found.
A few patients show signs of SD and psoriasis such that a definitive diagnosis cannot be made. Such overlap cases are sometimes termed sebopsoriasis. But psoriasis will usually exhibit signs not seen with SD, such as pitting of the nails, involvement of extensor surfaces of elbows and knees, and characteristic signs of psoriatic arthropathy in about 20% of cases. Pinpoint bleeding caused by peeling away scale, called the Auspitz sign, is seen with psoriasis and not with SD.
TREATMENT
This patient’s chest involvement responded rapidly to topical betamethasone foam, quickly tapered to avoid thinning the skin. Less powerful steroid creams, lotions, or gels (eg, triamcinolone 0.025%) can be used on other areas, such as ears and face. The daily use of an OTC dandruff shampoo (containing selenium sulfide, zinc pyrithione, tar, or ketoconazole) is an effective approach to controlling scalp involvement, but the product should be changed weekly.
Once the initial inflammation is controlled, topical antiyeast/antifungal preparations (eg, ketoconazole cream or any of the imidazoles, such as clotrimazole or oxiconazole) can be useful.
Finally, emphasis must be placed on educating the patient to expect control of the condition but not a cure.
ANSWER
The correct answer is petaloid seborrheic dermatitis (choice “d”), named for the flowerlike appearance of its polycyclic borders. Psoriasis (choice “a”) can present in this area, but tends to be scalier and usually involves multiple areas (eg, elbows, knees, and nails).
Rashes like this patient’s are often termed yeast infection (choice “b”). However, while a commensal yeast (Pityrosporum) can play a role in its formation, it appears that seborrhea represents an idiosyncratic reaction to increased numbers of this organism, rather than an actual infection.
Bowen’s disease (choice “c”) is a superficial squamous cell carcinoma, usually caused by overexposure to sunlight. Its lesions will be fixed, slowly growing larger with time, while seborrheic dermatitis will typically come and go. Biopsy is sometimes necessary to distinguish one from the other.
DISCUSSION
Seborrheic dermatitis (SD, aka seborrhea) is common, affecting up to 5% of the population. Dandruff is its usual manifestation, but it affects numerous other areas (as in this case), including the axillae, groin, beard, and genitals.
Presenting with scaling on an erythematous base, SD often flares and remits with the season (especially winter), with stress, and with increases in alcohol intake. Although it is usually mild, some cases can be severe. SD is associated with or accentuated by several other conditions, including Parkinson’s, stroke, and HIV. Severe SD in infants raises the possibility of Langerhans cell histiocytosis, especially when the presentation is atypical.
The diagnosis of SD can be difficult when it appears elsewhere than the scalp and face (eg, as an axillary or genital rash). Likewise, sternal petaloid SD is mystifying, unless other corroboratory manifestations are sought and found.
A few patients show signs of SD and psoriasis such that a definitive diagnosis cannot be made. Such overlap cases are sometimes termed sebopsoriasis. But psoriasis will usually exhibit signs not seen with SD, such as pitting of the nails, involvement of extensor surfaces of elbows and knees, and characteristic signs of psoriatic arthropathy in about 20% of cases. Pinpoint bleeding caused by peeling away scale, called the Auspitz sign, is seen with psoriasis and not with SD.
TREATMENT
This patient’s chest involvement responded rapidly to topical betamethasone foam, quickly tapered to avoid thinning the skin. Less powerful steroid creams, lotions, or gels (eg, triamcinolone 0.025%) can be used on other areas, such as ears and face. The daily use of an OTC dandruff shampoo (containing selenium sulfide, zinc pyrithione, tar, or ketoconazole) is an effective approach to controlling scalp involvement, but the product should be changed weekly.
Once the initial inflammation is controlled, topical antiyeast/antifungal preparations (eg, ketoconazole cream or any of the imidazoles, such as clotrimazole or oxiconazole) can be useful.
Finally, emphasis must be placed on educating the patient to expect control of the condition but not a cure.
A 70-year-old man presents with a slightly itchy rash on his sternum that has appeared intermittently for years. Told it is “ringworm” by his primary care provider, the patient tried tolnaftate cream, to no avail. He is seeking additional consultation primarily because his wife is concerned she will catch the “infection.” The patient denies other skin problems, but then remembers that he has dandruff that flares from time to time, as well as a curious scaly red rash that “comes and goes” between his eyes, in his nasolabial folds, and behind his ears, especially in the winter. His father had similar problems. The patient is otherwise healthy, except for mild hypertension. The rash, located on the lower right sternum, measures about 6 cm at its largest dimension. Faintly pink, it has a papulosquamous surface, especially on its pol-ycyclic borders. Results of a KOH prep are negative for fungal elements. Elsewhere, a faintly scaly, orange-red rash is seen in the glabellar area and behind both ears. The man’s knees, elbows, and nails are free of any changes.
Proactive Rounding by RRT
Rapid response teams (RRT) have been promoted by numerous patient safety organizations to reduce preventable in‐hospital deaths.14 Initial studies of RRTs were promising,57 but recent literature,811 including systematic reviews and meta‐analyses, has called these findings into question. Nevertheless, RRTs remain popular in academic and community hospitals worldwide, and many have expanded their roles beyond solely responding to the deteriorating patient.12
Some RRTs, for example, proactively round on seriously ill ward patients and patients recently discharged from the intensive care unit (ICU) in an effort to prevent transitions to higher levels of care. Priestley and colleagues demonstrated that institution of such a team, referred to as a critical care outreach team (CCOT), decreased in‐hospital mortality while possibly increasing hospital length of stay (LOS).13 Three additional single‐center studies from the United Kingdom, where CCOTs are common, specifically examined proactive rounding by CCOTs on the ICU readmission rate: 2 observed no improvement,14, 15 while the third, limited by a small sample size, demonstrated a modest reduction in ICU readmissions.16
We sought to determine the impact of proactive rounding by an RRT on patients discharged from intensive care on the ICU readmission rate, ICU LOS, and in‐hospital mortality of patients discharged from the ICU. We hypothesized that proactive rounding by an RRT would decrease the ICU readmission rate, ICU LOS, and the in‐hospital mortality of patients discharged from the ICU.
MATERIALS AND METHODS
Site and Subjects
We carried out a retrospective, observational study of adult patients discharged from the ICU at University of California San Francisco (UCSF) Medical Center between January 2006 and June 2009. UCSF is a 790‐bed quaternary care academic hospital that admits approximately 17,000 patients annually and has 5 adult ICUs, with 62 beds and 3500 to 4000 ICU admissions annually. Our study was approved by the UCSF Medical Center Committee on Human Research; need for informed consent was waived.
Description of the RRT Before June 1, 2007
Throughout the study, the goal of the RRT was unchanged: to assess, triage, and institute early treatment in patients who experienced an acute decline in their clinical status. From November 2005 to October 2006, the RRT was staffed by an attending hospitalist and medicine resident during daytime, and by a critical care fellow at nighttime and on weekends. The RRT could be activated by any concerned staff member in response to a set of predetermined vital sign abnormalities, decreased urine output, or altered mental status, or simply if the staff member was concerned about the patient's clinical status. Despite extensive educational efforts, utilization of the team was low (2.7 calls per 1000 admissions) and, accordingly, it was discontinued in October 2006. After this time, staff would contact the primary team caring for the patient, should concerns regarding the patient's condition arise.
Description of the RRT After June 1, 2007
In an effort to expand its scope and utility, the RRT was reinstated on June 1, 2007 with a new composition and increased responsibilities. After this date, physician roles were eliminated, and the team composition changed to a dedicated critical care nurse and respiratory therapist, available 24 hours a day. Criteria for calling the team remained unchanged. In addition to responding to acute deteriorations in patients' clinical courses, the RRT began to proactively assess all patients within 12 hours of discharge from the ICU and would continue to round on these patients daily until it was felt that they were clinically stable. During these rounds, the RRT would provide consultation expertise to the bedside nurse and contact the patient's clinicians if concern existed about a patient's clinical trajectory; decisions to transfer a patient back to the ICU ultimately rested with the patient's primary team. During this time period, the RRT received an average of 110.6 calls per 1000 admissions.
Data Sources
Data collected included: demographics, clinical information (all patient refined [APR] severity of illness, APR risk of mortality, and the presence of 29 comorbidities), whether there was a readmission to the ICU, the total ICU LOS, and the vital status at the time of hospital discharge.
Outcomes
Outcomes included: readmission to the ICU, defined as 2 noncontiguous ICU stays during a single hospitalization; ICU LOS, defined as the total number of ICU days accrued during hospitalization; and in‐hospital mortality of patients discharged from the ICU.
Adjustment Variables
Patient age, gender, race, and ethnicity were available from administrative data. We used admission diagnosis code data to classify comorbidities using the method of Elixhauser et al.17
Statistical Analysis
For each of the 3 study outcomes, we assessed the effects of the intervention using multivariable models adjusting for patient‐ and service‐level factors, including a gamma model for ICU LOS and logistic models for ICU readmission and in‐hospital mortality of patients discharged from the ICU. We first compared unadjusted outcome levels before and after implementation. We then used an interrupted time series (ITS) framework to assess the effects of the intervention in terms of 5 measures: 1) the secular trend in the mean of the outcome before the intervention; 2) the change in the mean at the start of the implementation, or immediate effects; 3) the secular trend in the mean after implementation; 4) the change in secular trend, reflecting cumulative intervention effects; and 5) the net effect of the intervention, estimated as the adjusted difference between the fitted mean at the end of the postintervention period and the expected mean if the preintervention trend had continued without interruption or change.
Secondary Analyses
Given the heterogeneity of the RRT in the preintervention period, we assessed potential changes in trend at October 2006, the month in which the RRT was discontinued. We also examined changes in trend midway through the postimplementation period to evaluate for increased efficacy of the RRT with time.
Selection of Covariates
Age, race, and admitting service were included in both the prepost and ITS models by default for face validity. Additional covariates were selected for each outcome using backwards deletion with a retention criterion of P < 0.05, based on models that allowed the outcome rate to vary freely month to month. Because these data were obtained from administrative billing datasets, and the presence of comorbidities could not be definitively linked with time points during hospitalization, only those comorbidities that were likely present prior at ICU discharge were included. For similar reasons, APR severity of illness and risk of mortality scores, which were calculated from billing diagnoses at the end of hospitalization, were excluded from the models.
RESULTS
Patient Characteristics
During the study period, 11,687 patients were admitted to the ICU; 10,288 were discharged from the ICU alive and included in the analysis. In the 17 months prior to the introduction of proactive rounding by the RRT, 4902 (41.9%) patients were admitted, and during the 25 months afterwards, 6785 (58.1%) patients. Patients admitted in the 2 time periods were similar, although there were clinically small but statistically significant differences in race, APR severity of illness, APR risk of mortality, and certain comorbidities between the 2 groups (Table 1).
| Pre‐RRT (n = 4305) N (%) | Post‐RRT (n = 5983) N (%) | P Value | |
|---|---|---|---|
| |||
| Age, mean (y [SD]) | 57.7 [16.6] | 57.9 [16.5] | 0.50 |
| Female gender | 2,005 (46.6) | 2,824 (47.2) | 0.53 |
| Race | 0.0013 | ||
| White | 2,538 (59.0) | 3,520 (58.8) | |
| Black | 327 (7.6) | 436 (7.3) | |
| Asian | 642 (14.9) | 842 (14.1) | |
| Other | 719 (16.7) | 1,121 (18.7) | |
| Unknown | 79 (1.8) | 64 (1.1) | |
| Ethnicity | 0.87 | ||
| Hispanic | 480 (11.2) | 677 (11.3%) | |
| Non‐Hispanic | 3,547 (82.4) | 4,907 (82.0%) | |
| Unknown | 278 (6.5) | 399 (6.7) | |
| Insurance | 0.50 | ||
| Medicare | 1,788 (41.5) | 2,415 (40.4) | |
| Medicaid/Medi‐Cal | 699 (16.2) | 968 (16.2) | |
| Private | 1,642 (38.1) | 2,329 (38.9) | |
| Other | 176 (4.1) | 271 (4.5) | |
| Admission source | 0.41 | ||
| ED | 1,621 (37.7) | 2,244 (37.5) | |
| Outside hospital | 652 (15.2) | 855 (14.3) | |
| Direct admit | 2,032 (47.2) | 2,884 (48.2) | |
| Major surgery | 0.99 | ||
| Yes | 3,107 (72.2) | 4,319 (72.2) | |
| APR severity of illness | 0.0001 | ||
| Mild | 622 (14.5) | 828 (13.8) | |
| Moderate | 1,328 (30.9) | 1,626 (27.2) | |
| Major | 1,292 (30.0) | 1,908 (31.9) | |
| Extreme | 1,063 (24.7) | 1,621 (27.1) | |
| APR risk of mortality | 0.0109 | ||
| Mild | 1,422 (33.0) | 1,821 (30.4) | |
| Moderate | 1,074 (25.0) | 1,467 (24.5) | |
| Major | 947 (22.0) | 1,437 (24.0) | |
| Extreme | 862 (20.0) | 1,258 (21.0) | |
| Admitting service | 0.11 | ||
| Adult general surgery | 190 (4.4) | 260 (4.4) | |
| Cardiology | 347 (8.1) | 424 (7.1) | |
| Cardiothoracic surgery | 671 (15.6) | 930 (15.5) | |
| Kidney transplant surgery | 105 (2.4) | 112 (1.9) | |
| Liver transplant surgery | 298 (6.9) | 379 (6.3) | |
| Medicine | 683 (15.9) | 958 (16.0) | |
| Neurology | 420 (9.8) | 609 (10.2) | |
| Neurosurgery | 1,345 (31.2) | 1,995 (33.3) | |
| Vascular surgery | 246 (5.7) | 316 (5.3) | |
| Comorbidities | |||
| Hypertension | 2,054 (47.7) | 2,886 (48.2) | 0.60 |
| Fluid and electrolyte disorders | 998 (23.2) | 1,723 (28.8) | <0.0001 |
| Diabetes | 708 (16.5) | 880 (14.7) | 0.02 |
| Chronic obstructive pulmonary disease | 632 (14.7) | 849 (14.2) | 0.48 |
| Iron deficiency anemia | 582 (13.5) | 929 (15.5) | 0.005 |
| Renal failure | 541 (12.6) | 744 (12.4) | 0.84 |
| Coagulopathy | 418 (9.7) | 712 (11.9) | 0.0005 |
| Liver disease | 400 (9.3) | 553 (9.2) | 0.93 |
| Hypothyroidism | 330 (7.7) | 500 (8.4) | 0.20 |
| Depression | 306 (7.1) | 508 (8.5) | 0.01 |
| Peripheral vascular disease | 304 (7.1) | 422 (7.1) | 0.99 |
| Congestive heart failure | 263 (6.1) | 360 (6.0) | 0.85 |
| Weight loss | 236 (5.5) | 425 (7.1) | 0.0009 |
| Paralysis | 225 (5.2) | 328 (5.5) | 0.57 |
| Neurological disorders | 229 (5.3) | 276 (4.6) | 0.10 |
| Valvular disease | 210 (4.9) | 329 (5.5) | 0.16 |
| Drug abuse | 198 (4.6) | 268 (4.5) | 0.77 |
| Metastatic cancer | 198 (4.6) | 296 (5.0) | 0.42 |
| Obesity | 201 (4.7) | 306 (5.1) | 0.30 |
| Alcohol abuse | 178 (4.1) | 216 (3.6) | 0.17 |
| Diabetes with complications | 175 (4.1) | 218 (3.6) | 0.27 |
| Solid tumor without metastasis | 146 (3.4) | 245 (4.1) | 0.07 |
| Psychoses | 115 (2.7) | 183 (3.1) | 0.25 |
| Rheumatoid arthritis/collagen vascular disease | 96 (2.2) | 166 (2.8) | 0.08 |
| Pulmonary circulation disease | 83 (1.9) | 181 (3.0) | 0.0005 |
| Outcomes | |||
| Readmission to ICU | 288 (6.7) | 433 (7.3) | 0.24 |
| ICU length of stay, mean [SD] | 5.1 [9.7] | 4.9 [8.3] | 0.24 |
| In‐hospital mortality of patients discharged from the ICU | 260 (6.0) | 326 (5.5) | 0.24 |
ICU Readmission Rate
Introduction of proactive rounding by the RRT was not associated with unadjusted differences in the ICU readmission rate (6.7% preintervention vs 7.3% postintervention, P = 0.24; Table 1). In the adjusted ITS model, the intervention had no net effect on the odds of ICU readmission (adjusted odds ratio [AOR] for net intervention effect 0.98, 95% confidence interval [CI] 0.42, 2.28), with similar secular trends both preintervention (AOR 1.00 per year, 95% CI 0.97, 1.03), and afterwards (AOR 0.99 per year, 95% CI 0.98, 01.00), and a nonsignificant increase at implementation (Table 2). Figure 1 uses solid lines to show the fitted readmission rates, a hatched line to show the projection of the preintervention secular trend into the postintervention period, and circles to represent adjusted monthly means. The lack of a net intervention effect is indicated by the convergence of the solid and hatched lines 24 months postintervention.
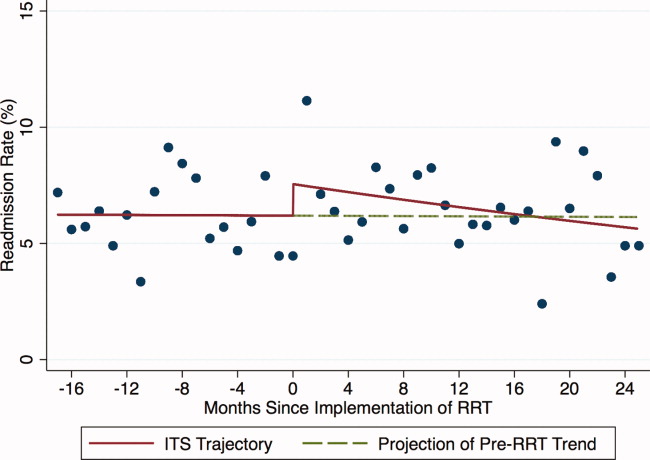
| Outcome: Summary Effect Measure | Value (95% CI) | P Value |
|---|---|---|
| ||
| ICU readmission rateadjusted odds ratio | ||
| Pre‐RRT trend | 1.00 (0.97, 1.03) | 0.98 |
| Change at RRT implementation | 1.24 (0.94, 1.63) | 0.13 |
| Post‐RRT trend | 0.98 (0.97, 1.00) | 0.06 |
| Change in trend | 0.98 (0.96, 1.02) | 0.39 |
| Net intervention effect | 0.92 (0.40, 2.12) | 0.85 |
| ICU average length of stayadjusted ratio of means | ||
| Trend at 9 mo pre‐RRT | 0.98 (0.96, 1.00) | 0.05 |
| Trend at 3 mo pre‐RRT | 1.02 (0.99, 1.04) | 0.19 |
| Change in trend at 3 mo pre‐RRT | 1.03 (1.00, 1.07) | 0.07 |
| Change at RRT implementation | 0.92 (0.80, 1.06) | 0.27 |
| Post‐RRT trend | 1.00 (0.99, 1.00) | 0.35 |
| Change in trend at RRT implementation | 0.98 (0.96, 1.01) | 0.14 |
| Net intervention effect | 0.60 (0.31, 1.18) | 0.14 |
| In‐hospital mortality of patients discharged from the ICUadjusted odds ratio | ||
| Pre‐RRT trend | 1.02 (0.99, 1.06) | 0.15 |
| Change at RRT implementation | 0.74 (0.51, 1.08) | 0.12 |
| Post‐RRT trend | 1.00 (0.98, 1.01) | 0.68 |
| Change in trend | 0.97 (0.94, 1.01) | 0.14 |
| Net intervention effect | 0.39 (0.14, 1.10) | 0.08 |
ICU Average LOS
Introduction of proactive rounding by the RRT was not associated with unadjusted differences in ICU average LOS (5.1 days preintervention vs 4.9 days postintervention, P = 0.24; Table 1). Trends in ICU LOS may have changed in October 2006 (P = 0.07), decreasing in the first half of the study period (adjusted rate ratio [ARR] 0.98 per year, 95% CI 0.961.00), but did not change significantly thereafter. As with the ICU readmission rate, neither the change in estimated secular trend after implementation (ARR 0.98, 95% CI 0.961.01), nor the net effect of the intervention (ARR 0.62, 95% CI 0.321.22) was statistically significant (Table 2); these results are depicted graphically in Figure 2.
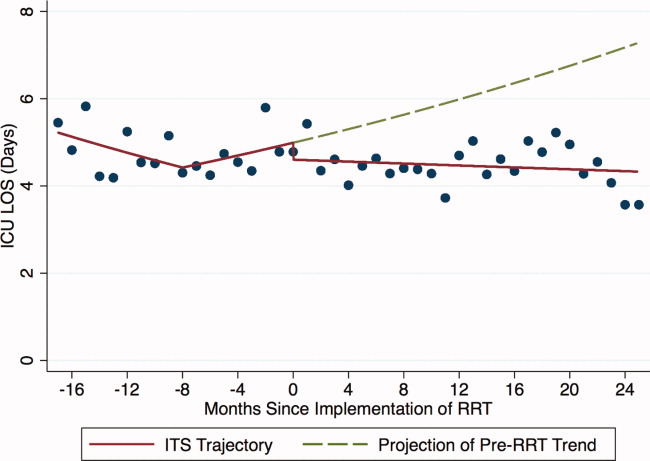
In‐Hospital Mortality of Patients Discharged From the ICU
Introduction of proactive rounding by the RRT was not associated with unadjusted differences in the mortality of patients discharged from the ICU (6.0% preintervention vs 5.5% postintervention, P = 0.24; Table 1). Similarly, in the adjusted ITS model, the intervention had no statistically significant net effect on the mortality outcome (Table 2 and Figure 3).
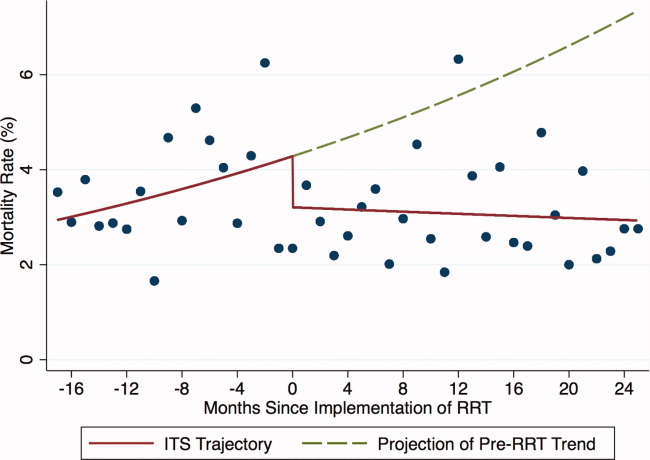
Secondary Analyses
Apart from weak evidence for a change in trend in ICU LOS in October 2006, no other changes in trend were found within the preintervention or postintervention periods (data not shown). This suggests that the heterogeneity of the preintervention RRT had no significant impact on the 3 outcomes examined, and that the RRT intervention failed to gain efficacy with time in the postintervention period. Additionally, we saw no outcome benefit in sensitivity analyses among all ICU patients or in service‐defined analyses (eg, surgical services), where ability to control for illness severity was improved.
DISCUSSION
In this single center study, introduction of an RRT that proactively rounded on patients discharged from the ICU did not reduce the ICU readmission rate, ICU LOS, or mortality of patients discharged from the ICU, after accounting for secular trends using robust ITS methods and adjusting for patient level factors.
Our study is consistent with 2 smaller studies that assessed the impact of proactive rounding by a CCOT on ICU readmission rate. Leary and Ridley14 found that proactively rounding by a CCOT did not reduce ICU readmissions or shorten the ICU LOS, although this study was limited by a surprisingly low ICU readmission rate and short ICU LOS prior to the intervention. Another study15 also observed no change in the ICU readmission rate following introduction of a proactively rounding CCOT but noted small reductions in both ICU and hospital mortality. The sole study showing an effect16 observed a lower ICU readmission rate and increased survival to hospital discharge (after excluding do not resuscitate [DNR] patients) with implementation of a CCOT, although some of their findings may be explained by their CCOT's use of palliative care services, a function not featured in our model.
Our study adds to the meta‐analyses and systematic reviews810 that have questioned the hypothesis that a trained and proactive team of caregivers should be able to prevent patients from returning to the ICU. Perhaps one reason why this is not true is that proactive rounding by RRTs may have minimal effect in systems where step‐down beds are readily available. At UCSF, nearly every patient transferred out of the ICU is triaged to a step‐down unit, where telemetry and pulse oximetry are continuously monitored. Despite this, however, our institution's 2 step‐down units generate more calls to our RRT than any other units in the hospital.
We were surprised to see that proactive rounding failed to shorten ICU LOS, hypothesizing that clinicians would be more comfortable discharging patients from the ICU knowing that the RRT would be closely monitoring them afterwards. Although we have no data to support this hypothesis, increased use of the RRT may have also increased step‐down bed use, as patients on the general medicalsurgical floors were transferred to a higher level of care upon recommendation of the RRT, thereby delaying transfers out of the ICU. Moreover, the opening of an additional 16‐bed ICU in October 2008 might have encouraged clinicians to transfer patients back to the ICU simply because beds were more easily accessible than before.
Introduction of proactive rounding by the RRT was also not associated with differences in the mortality rate of patients discharged from the ICU. This finding conflicts with the results of Garcea et al,15 Ball et al,16 and Priestley et al13, all of which found that implementation of a CCOT led to small but statistically significant reductions in in‐hospital mortality. All 3 of these studies, however, examined smaller patient populations (1380, 470, and 2903 patients, respectively), and both the Priestley and Ball studies13, 16 had significantly shorter periods of data collection (24 months and 32 weeks, respectively). Our results are based on models with confidence intervals and P values that account for variability in all 3 underlying effect estimates but assume a linear extrapolation of the preintervention trend. This approach allowed us to flexibly deal with changes related to the intervention, while relying on our large sample size to define time trends not dealt with adequately (or at all) in previous research.
The lack of improvement in outcomes cannot be attributed to immaturity of the RRT or failure of the clinical staff to use the RRT adequately. A prespecified secondary data analysis midway through the postintervention time period demonstrated that the RRT failed to gain efficacy with time with respect to all 3 outcomes. The postintervention RRT was also utilized far more frequently than its predecessor (110.6 vs 2.7 calls per 1000 admissions, respectively), and this degree of RRT utilization far surpasses the dose considered to be indicative of a mature RRT system.12
Our study has several limitations. First, we relied on administrative rather than chart‐collected data to determine the reason for ICU admission, and the APR severity of illness and risk of mortality scores. It seems unlikely, however, that coding deficiencies or biases affected the preintervention and postintervention patient populations differently. Even though we adjusted for all available measures, it is possible that we were not able to account for time trends in all potential confounders. Second, we did not have detailed clinical information on reasons for ICU readmission and whether readmissions occurred before or after the RRT proactively rounded on the patient. Therefore, potential readmissions to the ICU that might have been planned or which would have happened regardless of the presence of the RRT, such as for antibiotic desensitization, could not be accounted for. Third, introduction of proactive rounding by the RRT in June 2007 was accompanied by a change in the RRT's composition, from a physician‐led model to a nurse‐led model. Therefore, inherent differences in the way that physicians and nurses might assess and triage patients could not have been adjusted for. Lastly, this was a retrospective study conducted at a single academic medical center with a specific RRT model, and our results may not be directly applicable to nonteaching settings or to different RRT models.
Our findings raise further questions about the benefits of RRTs as they assume additional roles, such as proactive rounding on patients recently discharged from the ICU. The failure of our RRT to reduce the ICU readmission rate, the ICU average LOS, and the mortality of patients discharged from the ICU raises concerns that the benefits of our RRT are not commensurate with its cost. While defining the degree of impact and underlying mechanisms are worthy of prospective study, hospitals seeking to improve their RRT models should consider how to develop systems that achieve the RRT's promise in measurable ways.
Acknowledgements
The authors acknowledge Heather Leicester, MSPH, Senior Performance Improvement Analyst for Patient Safety and Quality Services at the University of California San Francisco for her work in data acquisition.
Disclosures: Dr Vittinghoff received salary support from an NIH grant during the time of this work for statistical consulting. He receives textbook royalties from Springer Verlag. Dr Auerbach was supported by 5K24HL098372‐02 from the National Heart Lung and Blood Institute during the period of this study although not specifically for this study; they had no role in the design or conduct of the study; the collection, analysis, or interpretation of the data; or the preparation, review, or approval of the manuscript. The other authors have no financial conflicts of interest.
- , , , . The 100,000 lives campaign: setting a goal and a deadline for improving health care quality. JAMA. 2006;295(3):324–327.
- Clinical Governance Unit, Quality and Safety Branch, Rural and Regional Health and Aged Care Services Division Safer Systems, Department of Human Services, State Government of Victoria. Safer Systems—Saving Lives Campaign. Available at: http://www.health.vic.gov.au/sssl. Accessed April 5, 2012.
- Canadian Patient Safety Institute. Safer Healthcare Now! Campaign. Available at: http://www.saferhealthcarenow.ca. Accessed April 5, 2012.
- , . The growth of rapid response systems. Jt Comm J Qual Patient Saf. 2008;34:489–495.
- , , , . The medical emergency team. Anaesth Intensive Care. 1995;23(2):183–186.
- , , , et al. Rates of in‐hospital arrests, deaths, and intensive care admission: the effect of a medical emergency team. Med J Aust. 2000;173:236–240.
- , , , , . The patient‐at‐risk team: identifying and managing seriously ill ward patients. Anaesthesia. 1999;54:853–860.
- , , , , . Effects of rapid response systems on clinical outcomes: systemic review and meta‐analysis. J Hosp Med. 2007;2:422–432.
- , , , , . Rapid response teams: a systemic review and meta‐analysis. Arch Intern Med. 2010;170(1):18–26.
- , , , , , . Rapid response systems: a systematic review. Crit Care Med. 2007;35(5):1238–1243.
- , , , et al. Introduction of the medical emergency team (MET) system: a cluster‐randomised controlled trial. Lancet. 2005;365:2091–2097.
- , , . Rapid response teams. N Engl J Med. 2011;365:139–146.
- , , , et al. Introducing critical care outreach: a ward‐randomised trial of phased introduction in a general hospital. Intensive Care Med. 2004;30(7):1398–1404.
- , . Impact of an outreach team on re‐admissions to a critical care unit. Anaesthesia. 2003;58:328–332.
- , , , , . Impact of a critical care outreach team on critical care readmissions and mortality. Acta Anaesthesiol Scand. 2004;48:1096–1100.
- , , . Effect of the critical care outreach team on patient survival to discharge from hospital and readmission to critical care: non‐randomised population based study. BMJ. 2003;327:1014–1017.
- , , , . Comorbidity measures for use with administrative data. Med Care. 1998;36(1):8–27.
Rapid response teams (RRT) have been promoted by numerous patient safety organizations to reduce preventable in‐hospital deaths.14 Initial studies of RRTs were promising,57 but recent literature,811 including systematic reviews and meta‐analyses, has called these findings into question. Nevertheless, RRTs remain popular in academic and community hospitals worldwide, and many have expanded their roles beyond solely responding to the deteriorating patient.12
Some RRTs, for example, proactively round on seriously ill ward patients and patients recently discharged from the intensive care unit (ICU) in an effort to prevent transitions to higher levels of care. Priestley and colleagues demonstrated that institution of such a team, referred to as a critical care outreach team (CCOT), decreased in‐hospital mortality while possibly increasing hospital length of stay (LOS).13 Three additional single‐center studies from the United Kingdom, where CCOTs are common, specifically examined proactive rounding by CCOTs on the ICU readmission rate: 2 observed no improvement,14, 15 while the third, limited by a small sample size, demonstrated a modest reduction in ICU readmissions.16
We sought to determine the impact of proactive rounding by an RRT on patients discharged from intensive care on the ICU readmission rate, ICU LOS, and in‐hospital mortality of patients discharged from the ICU. We hypothesized that proactive rounding by an RRT would decrease the ICU readmission rate, ICU LOS, and the in‐hospital mortality of patients discharged from the ICU.
MATERIALS AND METHODS
Site and Subjects
We carried out a retrospective, observational study of adult patients discharged from the ICU at University of California San Francisco (UCSF) Medical Center between January 2006 and June 2009. UCSF is a 790‐bed quaternary care academic hospital that admits approximately 17,000 patients annually and has 5 adult ICUs, with 62 beds and 3500 to 4000 ICU admissions annually. Our study was approved by the UCSF Medical Center Committee on Human Research; need for informed consent was waived.
Description of the RRT Before June 1, 2007
Throughout the study, the goal of the RRT was unchanged: to assess, triage, and institute early treatment in patients who experienced an acute decline in their clinical status. From November 2005 to October 2006, the RRT was staffed by an attending hospitalist and medicine resident during daytime, and by a critical care fellow at nighttime and on weekends. The RRT could be activated by any concerned staff member in response to a set of predetermined vital sign abnormalities, decreased urine output, or altered mental status, or simply if the staff member was concerned about the patient's clinical status. Despite extensive educational efforts, utilization of the team was low (2.7 calls per 1000 admissions) and, accordingly, it was discontinued in October 2006. After this time, staff would contact the primary team caring for the patient, should concerns regarding the patient's condition arise.
Description of the RRT After June 1, 2007
In an effort to expand its scope and utility, the RRT was reinstated on June 1, 2007 with a new composition and increased responsibilities. After this date, physician roles were eliminated, and the team composition changed to a dedicated critical care nurse and respiratory therapist, available 24 hours a day. Criteria for calling the team remained unchanged. In addition to responding to acute deteriorations in patients' clinical courses, the RRT began to proactively assess all patients within 12 hours of discharge from the ICU and would continue to round on these patients daily until it was felt that they were clinically stable. During these rounds, the RRT would provide consultation expertise to the bedside nurse and contact the patient's clinicians if concern existed about a patient's clinical trajectory; decisions to transfer a patient back to the ICU ultimately rested with the patient's primary team. During this time period, the RRT received an average of 110.6 calls per 1000 admissions.
Data Sources
Data collected included: demographics, clinical information (all patient refined [APR] severity of illness, APR risk of mortality, and the presence of 29 comorbidities), whether there was a readmission to the ICU, the total ICU LOS, and the vital status at the time of hospital discharge.
Outcomes
Outcomes included: readmission to the ICU, defined as 2 noncontiguous ICU stays during a single hospitalization; ICU LOS, defined as the total number of ICU days accrued during hospitalization; and in‐hospital mortality of patients discharged from the ICU.
Adjustment Variables
Patient age, gender, race, and ethnicity were available from administrative data. We used admission diagnosis code data to classify comorbidities using the method of Elixhauser et al.17
Statistical Analysis
For each of the 3 study outcomes, we assessed the effects of the intervention using multivariable models adjusting for patient‐ and service‐level factors, including a gamma model for ICU LOS and logistic models for ICU readmission and in‐hospital mortality of patients discharged from the ICU. We first compared unadjusted outcome levels before and after implementation. We then used an interrupted time series (ITS) framework to assess the effects of the intervention in terms of 5 measures: 1) the secular trend in the mean of the outcome before the intervention; 2) the change in the mean at the start of the implementation, or immediate effects; 3) the secular trend in the mean after implementation; 4) the change in secular trend, reflecting cumulative intervention effects; and 5) the net effect of the intervention, estimated as the adjusted difference between the fitted mean at the end of the postintervention period and the expected mean if the preintervention trend had continued without interruption or change.
Secondary Analyses
Given the heterogeneity of the RRT in the preintervention period, we assessed potential changes in trend at October 2006, the month in which the RRT was discontinued. We also examined changes in trend midway through the postimplementation period to evaluate for increased efficacy of the RRT with time.
Selection of Covariates
Age, race, and admitting service were included in both the prepost and ITS models by default for face validity. Additional covariates were selected for each outcome using backwards deletion with a retention criterion of P < 0.05, based on models that allowed the outcome rate to vary freely month to month. Because these data were obtained from administrative billing datasets, and the presence of comorbidities could not be definitively linked with time points during hospitalization, only those comorbidities that were likely present prior at ICU discharge were included. For similar reasons, APR severity of illness and risk of mortality scores, which were calculated from billing diagnoses at the end of hospitalization, were excluded from the models.
RESULTS
Patient Characteristics
During the study period, 11,687 patients were admitted to the ICU; 10,288 were discharged from the ICU alive and included in the analysis. In the 17 months prior to the introduction of proactive rounding by the RRT, 4902 (41.9%) patients were admitted, and during the 25 months afterwards, 6785 (58.1%) patients. Patients admitted in the 2 time periods were similar, although there were clinically small but statistically significant differences in race, APR severity of illness, APR risk of mortality, and certain comorbidities between the 2 groups (Table 1).
| Pre‐RRT (n = 4305) N (%) | Post‐RRT (n = 5983) N (%) | P Value | |
|---|---|---|---|
| |||
| Age, mean (y [SD]) | 57.7 [16.6] | 57.9 [16.5] | 0.50 |
| Female gender | 2,005 (46.6) | 2,824 (47.2) | 0.53 |
| Race | 0.0013 | ||
| White | 2,538 (59.0) | 3,520 (58.8) | |
| Black | 327 (7.6) | 436 (7.3) | |
| Asian | 642 (14.9) | 842 (14.1) | |
| Other | 719 (16.7) | 1,121 (18.7) | |
| Unknown | 79 (1.8) | 64 (1.1) | |
| Ethnicity | 0.87 | ||
| Hispanic | 480 (11.2) | 677 (11.3%) | |
| Non‐Hispanic | 3,547 (82.4) | 4,907 (82.0%) | |
| Unknown | 278 (6.5) | 399 (6.7) | |
| Insurance | 0.50 | ||
| Medicare | 1,788 (41.5) | 2,415 (40.4) | |
| Medicaid/Medi‐Cal | 699 (16.2) | 968 (16.2) | |
| Private | 1,642 (38.1) | 2,329 (38.9) | |
| Other | 176 (4.1) | 271 (4.5) | |
| Admission source | 0.41 | ||
| ED | 1,621 (37.7) | 2,244 (37.5) | |
| Outside hospital | 652 (15.2) | 855 (14.3) | |
| Direct admit | 2,032 (47.2) | 2,884 (48.2) | |
| Major surgery | 0.99 | ||
| Yes | 3,107 (72.2) | 4,319 (72.2) | |
| APR severity of illness | 0.0001 | ||
| Mild | 622 (14.5) | 828 (13.8) | |
| Moderate | 1,328 (30.9) | 1,626 (27.2) | |
| Major | 1,292 (30.0) | 1,908 (31.9) | |
| Extreme | 1,063 (24.7) | 1,621 (27.1) | |
| APR risk of mortality | 0.0109 | ||
| Mild | 1,422 (33.0) | 1,821 (30.4) | |
| Moderate | 1,074 (25.0) | 1,467 (24.5) | |
| Major | 947 (22.0) | 1,437 (24.0) | |
| Extreme | 862 (20.0) | 1,258 (21.0) | |
| Admitting service | 0.11 | ||
| Adult general surgery | 190 (4.4) | 260 (4.4) | |
| Cardiology | 347 (8.1) | 424 (7.1) | |
| Cardiothoracic surgery | 671 (15.6) | 930 (15.5) | |
| Kidney transplant surgery | 105 (2.4) | 112 (1.9) | |
| Liver transplant surgery | 298 (6.9) | 379 (6.3) | |
| Medicine | 683 (15.9) | 958 (16.0) | |
| Neurology | 420 (9.8) | 609 (10.2) | |
| Neurosurgery | 1,345 (31.2) | 1,995 (33.3) | |
| Vascular surgery | 246 (5.7) | 316 (5.3) | |
| Comorbidities | |||
| Hypertension | 2,054 (47.7) | 2,886 (48.2) | 0.60 |
| Fluid and electrolyte disorders | 998 (23.2) | 1,723 (28.8) | <0.0001 |
| Diabetes | 708 (16.5) | 880 (14.7) | 0.02 |
| Chronic obstructive pulmonary disease | 632 (14.7) | 849 (14.2) | 0.48 |
| Iron deficiency anemia | 582 (13.5) | 929 (15.5) | 0.005 |
| Renal failure | 541 (12.6) | 744 (12.4) | 0.84 |
| Coagulopathy | 418 (9.7) | 712 (11.9) | 0.0005 |
| Liver disease | 400 (9.3) | 553 (9.2) | 0.93 |
| Hypothyroidism | 330 (7.7) | 500 (8.4) | 0.20 |
| Depression | 306 (7.1) | 508 (8.5) | 0.01 |
| Peripheral vascular disease | 304 (7.1) | 422 (7.1) | 0.99 |
| Congestive heart failure | 263 (6.1) | 360 (6.0) | 0.85 |
| Weight loss | 236 (5.5) | 425 (7.1) | 0.0009 |
| Paralysis | 225 (5.2) | 328 (5.5) | 0.57 |
| Neurological disorders | 229 (5.3) | 276 (4.6) | 0.10 |
| Valvular disease | 210 (4.9) | 329 (5.5) | 0.16 |
| Drug abuse | 198 (4.6) | 268 (4.5) | 0.77 |
| Metastatic cancer | 198 (4.6) | 296 (5.0) | 0.42 |
| Obesity | 201 (4.7) | 306 (5.1) | 0.30 |
| Alcohol abuse | 178 (4.1) | 216 (3.6) | 0.17 |
| Diabetes with complications | 175 (4.1) | 218 (3.6) | 0.27 |
| Solid tumor without metastasis | 146 (3.4) | 245 (4.1) | 0.07 |
| Psychoses | 115 (2.7) | 183 (3.1) | 0.25 |
| Rheumatoid arthritis/collagen vascular disease | 96 (2.2) | 166 (2.8) | 0.08 |
| Pulmonary circulation disease | 83 (1.9) | 181 (3.0) | 0.0005 |
| Outcomes | |||
| Readmission to ICU | 288 (6.7) | 433 (7.3) | 0.24 |
| ICU length of stay, mean [SD] | 5.1 [9.7] | 4.9 [8.3] | 0.24 |
| In‐hospital mortality of patients discharged from the ICU | 260 (6.0) | 326 (5.5) | 0.24 |
ICU Readmission Rate
Introduction of proactive rounding by the RRT was not associated with unadjusted differences in the ICU readmission rate (6.7% preintervention vs 7.3% postintervention, P = 0.24; Table 1). In the adjusted ITS model, the intervention had no net effect on the odds of ICU readmission (adjusted odds ratio [AOR] for net intervention effect 0.98, 95% confidence interval [CI] 0.42, 2.28), with similar secular trends both preintervention (AOR 1.00 per year, 95% CI 0.97, 1.03), and afterwards (AOR 0.99 per year, 95% CI 0.98, 01.00), and a nonsignificant increase at implementation (Table 2). Figure 1 uses solid lines to show the fitted readmission rates, a hatched line to show the projection of the preintervention secular trend into the postintervention period, and circles to represent adjusted monthly means. The lack of a net intervention effect is indicated by the convergence of the solid and hatched lines 24 months postintervention.

| Outcome: Summary Effect Measure | Value (95% CI) | P Value |
|---|---|---|
| ||
| ICU readmission rateadjusted odds ratio | ||
| Pre‐RRT trend | 1.00 (0.97, 1.03) | 0.98 |
| Change at RRT implementation | 1.24 (0.94, 1.63) | 0.13 |
| Post‐RRT trend | 0.98 (0.97, 1.00) | 0.06 |
| Change in trend | 0.98 (0.96, 1.02) | 0.39 |
| Net intervention effect | 0.92 (0.40, 2.12) | 0.85 |
| ICU average length of stayadjusted ratio of means | ||
| Trend at 9 mo pre‐RRT | 0.98 (0.96, 1.00) | 0.05 |
| Trend at 3 mo pre‐RRT | 1.02 (0.99, 1.04) | 0.19 |
| Change in trend at 3 mo pre‐RRT | 1.03 (1.00, 1.07) | 0.07 |
| Change at RRT implementation | 0.92 (0.80, 1.06) | 0.27 |
| Post‐RRT trend | 1.00 (0.99, 1.00) | 0.35 |
| Change in trend at RRT implementation | 0.98 (0.96, 1.01) | 0.14 |
| Net intervention effect | 0.60 (0.31, 1.18) | 0.14 |
| In‐hospital mortality of patients discharged from the ICUadjusted odds ratio | ||
| Pre‐RRT trend | 1.02 (0.99, 1.06) | 0.15 |
| Change at RRT implementation | 0.74 (0.51, 1.08) | 0.12 |
| Post‐RRT trend | 1.00 (0.98, 1.01) | 0.68 |
| Change in trend | 0.97 (0.94, 1.01) | 0.14 |
| Net intervention effect | 0.39 (0.14, 1.10) | 0.08 |
ICU Average LOS
Introduction of proactive rounding by the RRT was not associated with unadjusted differences in ICU average LOS (5.1 days preintervention vs 4.9 days postintervention, P = 0.24; Table 1). Trends in ICU LOS may have changed in October 2006 (P = 0.07), decreasing in the first half of the study period (adjusted rate ratio [ARR] 0.98 per year, 95% CI 0.961.00), but did not change significantly thereafter. As with the ICU readmission rate, neither the change in estimated secular trend after implementation (ARR 0.98, 95% CI 0.961.01), nor the net effect of the intervention (ARR 0.62, 95% CI 0.321.22) was statistically significant (Table 2); these results are depicted graphically in Figure 2.

In‐Hospital Mortality of Patients Discharged From the ICU
Introduction of proactive rounding by the RRT was not associated with unadjusted differences in the mortality of patients discharged from the ICU (6.0% preintervention vs 5.5% postintervention, P = 0.24; Table 1). Similarly, in the adjusted ITS model, the intervention had no statistically significant net effect on the mortality outcome (Table 2 and Figure 3).

Secondary Analyses
Apart from weak evidence for a change in trend in ICU LOS in October 2006, no other changes in trend were found within the preintervention or postintervention periods (data not shown). This suggests that the heterogeneity of the preintervention RRT had no significant impact on the 3 outcomes examined, and that the RRT intervention failed to gain efficacy with time in the postintervention period. Additionally, we saw no outcome benefit in sensitivity analyses among all ICU patients or in service‐defined analyses (eg, surgical services), where ability to control for illness severity was improved.
DISCUSSION
In this single center study, introduction of an RRT that proactively rounded on patients discharged from the ICU did not reduce the ICU readmission rate, ICU LOS, or mortality of patients discharged from the ICU, after accounting for secular trends using robust ITS methods and adjusting for patient level factors.
Our study is consistent with 2 smaller studies that assessed the impact of proactive rounding by a CCOT on ICU readmission rate. Leary and Ridley14 found that proactively rounding by a CCOT did not reduce ICU readmissions or shorten the ICU LOS, although this study was limited by a surprisingly low ICU readmission rate and short ICU LOS prior to the intervention. Another study15 also observed no change in the ICU readmission rate following introduction of a proactively rounding CCOT but noted small reductions in both ICU and hospital mortality. The sole study showing an effect16 observed a lower ICU readmission rate and increased survival to hospital discharge (after excluding do not resuscitate [DNR] patients) with implementation of a CCOT, although some of their findings may be explained by their CCOT's use of palliative care services, a function not featured in our model.
Our study adds to the meta‐analyses and systematic reviews810 that have questioned the hypothesis that a trained and proactive team of caregivers should be able to prevent patients from returning to the ICU. Perhaps one reason why this is not true is that proactive rounding by RRTs may have minimal effect in systems where step‐down beds are readily available. At UCSF, nearly every patient transferred out of the ICU is triaged to a step‐down unit, where telemetry and pulse oximetry are continuously monitored. Despite this, however, our institution's 2 step‐down units generate more calls to our RRT than any other units in the hospital.
We were surprised to see that proactive rounding failed to shorten ICU LOS, hypothesizing that clinicians would be more comfortable discharging patients from the ICU knowing that the RRT would be closely monitoring them afterwards. Although we have no data to support this hypothesis, increased use of the RRT may have also increased step‐down bed use, as patients on the general medicalsurgical floors were transferred to a higher level of care upon recommendation of the RRT, thereby delaying transfers out of the ICU. Moreover, the opening of an additional 16‐bed ICU in October 2008 might have encouraged clinicians to transfer patients back to the ICU simply because beds were more easily accessible than before.
Introduction of proactive rounding by the RRT was also not associated with differences in the mortality rate of patients discharged from the ICU. This finding conflicts with the results of Garcea et al,15 Ball et al,16 and Priestley et al13, all of which found that implementation of a CCOT led to small but statistically significant reductions in in‐hospital mortality. All 3 of these studies, however, examined smaller patient populations (1380, 470, and 2903 patients, respectively), and both the Priestley and Ball studies13, 16 had significantly shorter periods of data collection (24 months and 32 weeks, respectively). Our results are based on models with confidence intervals and P values that account for variability in all 3 underlying effect estimates but assume a linear extrapolation of the preintervention trend. This approach allowed us to flexibly deal with changes related to the intervention, while relying on our large sample size to define time trends not dealt with adequately (or at all) in previous research.
The lack of improvement in outcomes cannot be attributed to immaturity of the RRT or failure of the clinical staff to use the RRT adequately. A prespecified secondary data analysis midway through the postintervention time period demonstrated that the RRT failed to gain efficacy with time with respect to all 3 outcomes. The postintervention RRT was also utilized far more frequently than its predecessor (110.6 vs 2.7 calls per 1000 admissions, respectively), and this degree of RRT utilization far surpasses the dose considered to be indicative of a mature RRT system.12
Our study has several limitations. First, we relied on administrative rather than chart‐collected data to determine the reason for ICU admission, and the APR severity of illness and risk of mortality scores. It seems unlikely, however, that coding deficiencies or biases affected the preintervention and postintervention patient populations differently. Even though we adjusted for all available measures, it is possible that we were not able to account for time trends in all potential confounders. Second, we did not have detailed clinical information on reasons for ICU readmission and whether readmissions occurred before or after the RRT proactively rounded on the patient. Therefore, potential readmissions to the ICU that might have been planned or which would have happened regardless of the presence of the RRT, such as for antibiotic desensitization, could not be accounted for. Third, introduction of proactive rounding by the RRT in June 2007 was accompanied by a change in the RRT's composition, from a physician‐led model to a nurse‐led model. Therefore, inherent differences in the way that physicians and nurses might assess and triage patients could not have been adjusted for. Lastly, this was a retrospective study conducted at a single academic medical center with a specific RRT model, and our results may not be directly applicable to nonteaching settings or to different RRT models.
Our findings raise further questions about the benefits of RRTs as they assume additional roles, such as proactive rounding on patients recently discharged from the ICU. The failure of our RRT to reduce the ICU readmission rate, the ICU average LOS, and the mortality of patients discharged from the ICU raises concerns that the benefits of our RRT are not commensurate with its cost. While defining the degree of impact and underlying mechanisms are worthy of prospective study, hospitals seeking to improve their RRT models should consider how to develop systems that achieve the RRT's promise in measurable ways.
Acknowledgements
The authors acknowledge Heather Leicester, MSPH, Senior Performance Improvement Analyst for Patient Safety and Quality Services at the University of California San Francisco for her work in data acquisition.
Disclosures: Dr Vittinghoff received salary support from an NIH grant during the time of this work for statistical consulting. He receives textbook royalties from Springer Verlag. Dr Auerbach was supported by 5K24HL098372‐02 from the National Heart Lung and Blood Institute during the period of this study although not specifically for this study; they had no role in the design or conduct of the study; the collection, analysis, or interpretation of the data; or the preparation, review, or approval of the manuscript. The other authors have no financial conflicts of interest.
Rapid response teams (RRT) have been promoted by numerous patient safety organizations to reduce preventable in‐hospital deaths.14 Initial studies of RRTs were promising,57 but recent literature,811 including systematic reviews and meta‐analyses, has called these findings into question. Nevertheless, RRTs remain popular in academic and community hospitals worldwide, and many have expanded their roles beyond solely responding to the deteriorating patient.12
Some RRTs, for example, proactively round on seriously ill ward patients and patients recently discharged from the intensive care unit (ICU) in an effort to prevent transitions to higher levels of care. Priestley and colleagues demonstrated that institution of such a team, referred to as a critical care outreach team (CCOT), decreased in‐hospital mortality while possibly increasing hospital length of stay (LOS).13 Three additional single‐center studies from the United Kingdom, where CCOTs are common, specifically examined proactive rounding by CCOTs on the ICU readmission rate: 2 observed no improvement,14, 15 while the third, limited by a small sample size, demonstrated a modest reduction in ICU readmissions.16
We sought to determine the impact of proactive rounding by an RRT on patients discharged from intensive care on the ICU readmission rate, ICU LOS, and in‐hospital mortality of patients discharged from the ICU. We hypothesized that proactive rounding by an RRT would decrease the ICU readmission rate, ICU LOS, and the in‐hospital mortality of patients discharged from the ICU.
MATERIALS AND METHODS
Site and Subjects
We carried out a retrospective, observational study of adult patients discharged from the ICU at University of California San Francisco (UCSF) Medical Center between January 2006 and June 2009. UCSF is a 790‐bed quaternary care academic hospital that admits approximately 17,000 patients annually and has 5 adult ICUs, with 62 beds and 3500 to 4000 ICU admissions annually. Our study was approved by the UCSF Medical Center Committee on Human Research; need for informed consent was waived.
Description of the RRT Before June 1, 2007
Throughout the study, the goal of the RRT was unchanged: to assess, triage, and institute early treatment in patients who experienced an acute decline in their clinical status. From November 2005 to October 2006, the RRT was staffed by an attending hospitalist and medicine resident during daytime, and by a critical care fellow at nighttime and on weekends. The RRT could be activated by any concerned staff member in response to a set of predetermined vital sign abnormalities, decreased urine output, or altered mental status, or simply if the staff member was concerned about the patient's clinical status. Despite extensive educational efforts, utilization of the team was low (2.7 calls per 1000 admissions) and, accordingly, it was discontinued in October 2006. After this time, staff would contact the primary team caring for the patient, should concerns regarding the patient's condition arise.
Description of the RRT After June 1, 2007
In an effort to expand its scope and utility, the RRT was reinstated on June 1, 2007 with a new composition and increased responsibilities. After this date, physician roles were eliminated, and the team composition changed to a dedicated critical care nurse and respiratory therapist, available 24 hours a day. Criteria for calling the team remained unchanged. In addition to responding to acute deteriorations in patients' clinical courses, the RRT began to proactively assess all patients within 12 hours of discharge from the ICU and would continue to round on these patients daily until it was felt that they were clinically stable. During these rounds, the RRT would provide consultation expertise to the bedside nurse and contact the patient's clinicians if concern existed about a patient's clinical trajectory; decisions to transfer a patient back to the ICU ultimately rested with the patient's primary team. During this time period, the RRT received an average of 110.6 calls per 1000 admissions.
Data Sources
Data collected included: demographics, clinical information (all patient refined [APR] severity of illness, APR risk of mortality, and the presence of 29 comorbidities), whether there was a readmission to the ICU, the total ICU LOS, and the vital status at the time of hospital discharge.
Outcomes
Outcomes included: readmission to the ICU, defined as 2 noncontiguous ICU stays during a single hospitalization; ICU LOS, defined as the total number of ICU days accrued during hospitalization; and in‐hospital mortality of patients discharged from the ICU.
Adjustment Variables
Patient age, gender, race, and ethnicity were available from administrative data. We used admission diagnosis code data to classify comorbidities using the method of Elixhauser et al.17
Statistical Analysis
For each of the 3 study outcomes, we assessed the effects of the intervention using multivariable models adjusting for patient‐ and service‐level factors, including a gamma model for ICU LOS and logistic models for ICU readmission and in‐hospital mortality of patients discharged from the ICU. We first compared unadjusted outcome levels before and after implementation. We then used an interrupted time series (ITS) framework to assess the effects of the intervention in terms of 5 measures: 1) the secular trend in the mean of the outcome before the intervention; 2) the change in the mean at the start of the implementation, or immediate effects; 3) the secular trend in the mean after implementation; 4) the change in secular trend, reflecting cumulative intervention effects; and 5) the net effect of the intervention, estimated as the adjusted difference between the fitted mean at the end of the postintervention period and the expected mean if the preintervention trend had continued without interruption or change.
Secondary Analyses
Given the heterogeneity of the RRT in the preintervention period, we assessed potential changes in trend at October 2006, the month in which the RRT was discontinued. We also examined changes in trend midway through the postimplementation period to evaluate for increased efficacy of the RRT with time.
Selection of Covariates
Age, race, and admitting service were included in both the prepost and ITS models by default for face validity. Additional covariates were selected for each outcome using backwards deletion with a retention criterion of P < 0.05, based on models that allowed the outcome rate to vary freely month to month. Because these data were obtained from administrative billing datasets, and the presence of comorbidities could not be definitively linked with time points during hospitalization, only those comorbidities that were likely present prior at ICU discharge were included. For similar reasons, APR severity of illness and risk of mortality scores, which were calculated from billing diagnoses at the end of hospitalization, were excluded from the models.
RESULTS
Patient Characteristics
During the study period, 11,687 patients were admitted to the ICU; 10,288 were discharged from the ICU alive and included in the analysis. In the 17 months prior to the introduction of proactive rounding by the RRT, 4902 (41.9%) patients were admitted, and during the 25 months afterwards, 6785 (58.1%) patients. Patients admitted in the 2 time periods were similar, although there were clinically small but statistically significant differences in race, APR severity of illness, APR risk of mortality, and certain comorbidities between the 2 groups (Table 1).
| Pre‐RRT (n = 4305) N (%) | Post‐RRT (n = 5983) N (%) | P Value | |
|---|---|---|---|
| |||
| Age, mean (y [SD]) | 57.7 [16.6] | 57.9 [16.5] | 0.50 |
| Female gender | 2,005 (46.6) | 2,824 (47.2) | 0.53 |
| Race | 0.0013 | ||
| White | 2,538 (59.0) | 3,520 (58.8) | |
| Black | 327 (7.6) | 436 (7.3) | |
| Asian | 642 (14.9) | 842 (14.1) | |
| Other | 719 (16.7) | 1,121 (18.7) | |
| Unknown | 79 (1.8) | 64 (1.1) | |
| Ethnicity | 0.87 | ||
| Hispanic | 480 (11.2) | 677 (11.3%) | |
| Non‐Hispanic | 3,547 (82.4) | 4,907 (82.0%) | |
| Unknown | 278 (6.5) | 399 (6.7) | |
| Insurance | 0.50 | ||
| Medicare | 1,788 (41.5) | 2,415 (40.4) | |
| Medicaid/Medi‐Cal | 699 (16.2) | 968 (16.2) | |
| Private | 1,642 (38.1) | 2,329 (38.9) | |
| Other | 176 (4.1) | 271 (4.5) | |
| Admission source | 0.41 | ||
| ED | 1,621 (37.7) | 2,244 (37.5) | |
| Outside hospital | 652 (15.2) | 855 (14.3) | |
| Direct admit | 2,032 (47.2) | 2,884 (48.2) | |
| Major surgery | 0.99 | ||
| Yes | 3,107 (72.2) | 4,319 (72.2) | |
| APR severity of illness | 0.0001 | ||
| Mild | 622 (14.5) | 828 (13.8) | |
| Moderate | 1,328 (30.9) | 1,626 (27.2) | |
| Major | 1,292 (30.0) | 1,908 (31.9) | |
| Extreme | 1,063 (24.7) | 1,621 (27.1) | |
| APR risk of mortality | 0.0109 | ||
| Mild | 1,422 (33.0) | 1,821 (30.4) | |
| Moderate | 1,074 (25.0) | 1,467 (24.5) | |
| Major | 947 (22.0) | 1,437 (24.0) | |
| Extreme | 862 (20.0) | 1,258 (21.0) | |
| Admitting service | 0.11 | ||
| Adult general surgery | 190 (4.4) | 260 (4.4) | |
| Cardiology | 347 (8.1) | 424 (7.1) | |
| Cardiothoracic surgery | 671 (15.6) | 930 (15.5) | |
| Kidney transplant surgery | 105 (2.4) | 112 (1.9) | |
| Liver transplant surgery | 298 (6.9) | 379 (6.3) | |
| Medicine | 683 (15.9) | 958 (16.0) | |
| Neurology | 420 (9.8) | 609 (10.2) | |
| Neurosurgery | 1,345 (31.2) | 1,995 (33.3) | |
| Vascular surgery | 246 (5.7) | 316 (5.3) | |
| Comorbidities | |||
| Hypertension | 2,054 (47.7) | 2,886 (48.2) | 0.60 |
| Fluid and electrolyte disorders | 998 (23.2) | 1,723 (28.8) | <0.0001 |
| Diabetes | 708 (16.5) | 880 (14.7) | 0.02 |
| Chronic obstructive pulmonary disease | 632 (14.7) | 849 (14.2) | 0.48 |
| Iron deficiency anemia | 582 (13.5) | 929 (15.5) | 0.005 |
| Renal failure | 541 (12.6) | 744 (12.4) | 0.84 |
| Coagulopathy | 418 (9.7) | 712 (11.9) | 0.0005 |
| Liver disease | 400 (9.3) | 553 (9.2) | 0.93 |
| Hypothyroidism | 330 (7.7) | 500 (8.4) | 0.20 |
| Depression | 306 (7.1) | 508 (8.5) | 0.01 |
| Peripheral vascular disease | 304 (7.1) | 422 (7.1) | 0.99 |
| Congestive heart failure | 263 (6.1) | 360 (6.0) | 0.85 |
| Weight loss | 236 (5.5) | 425 (7.1) | 0.0009 |
| Paralysis | 225 (5.2) | 328 (5.5) | 0.57 |
| Neurological disorders | 229 (5.3) | 276 (4.6) | 0.10 |
| Valvular disease | 210 (4.9) | 329 (5.5) | 0.16 |
| Drug abuse | 198 (4.6) | 268 (4.5) | 0.77 |
| Metastatic cancer | 198 (4.6) | 296 (5.0) | 0.42 |
| Obesity | 201 (4.7) | 306 (5.1) | 0.30 |
| Alcohol abuse | 178 (4.1) | 216 (3.6) | 0.17 |
| Diabetes with complications | 175 (4.1) | 218 (3.6) | 0.27 |
| Solid tumor without metastasis | 146 (3.4) | 245 (4.1) | 0.07 |
| Psychoses | 115 (2.7) | 183 (3.1) | 0.25 |
| Rheumatoid arthritis/collagen vascular disease | 96 (2.2) | 166 (2.8) | 0.08 |
| Pulmonary circulation disease | 83 (1.9) | 181 (3.0) | 0.0005 |
| Outcomes | |||
| Readmission to ICU | 288 (6.7) | 433 (7.3) | 0.24 |
| ICU length of stay, mean [SD] | 5.1 [9.7] | 4.9 [8.3] | 0.24 |
| In‐hospital mortality of patients discharged from the ICU | 260 (6.0) | 326 (5.5) | 0.24 |
ICU Readmission Rate
Introduction of proactive rounding by the RRT was not associated with unadjusted differences in the ICU readmission rate (6.7% preintervention vs 7.3% postintervention, P = 0.24; Table 1). In the adjusted ITS model, the intervention had no net effect on the odds of ICU readmission (adjusted odds ratio [AOR] for net intervention effect 0.98, 95% confidence interval [CI] 0.42, 2.28), with similar secular trends both preintervention (AOR 1.00 per year, 95% CI 0.97, 1.03), and afterwards (AOR 0.99 per year, 95% CI 0.98, 01.00), and a nonsignificant increase at implementation (Table 2). Figure 1 uses solid lines to show the fitted readmission rates, a hatched line to show the projection of the preintervention secular trend into the postintervention period, and circles to represent adjusted monthly means. The lack of a net intervention effect is indicated by the convergence of the solid and hatched lines 24 months postintervention.

| Outcome: Summary Effect Measure | Value (95% CI) | P Value |
|---|---|---|
| ||
| ICU readmission rateadjusted odds ratio | ||
| Pre‐RRT trend | 1.00 (0.97, 1.03) | 0.98 |
| Change at RRT implementation | 1.24 (0.94, 1.63) | 0.13 |
| Post‐RRT trend | 0.98 (0.97, 1.00) | 0.06 |
| Change in trend | 0.98 (0.96, 1.02) | 0.39 |
| Net intervention effect | 0.92 (0.40, 2.12) | 0.85 |
| ICU average length of stayadjusted ratio of means | ||
| Trend at 9 mo pre‐RRT | 0.98 (0.96, 1.00) | 0.05 |
| Trend at 3 mo pre‐RRT | 1.02 (0.99, 1.04) | 0.19 |
| Change in trend at 3 mo pre‐RRT | 1.03 (1.00, 1.07) | 0.07 |
| Change at RRT implementation | 0.92 (0.80, 1.06) | 0.27 |
| Post‐RRT trend | 1.00 (0.99, 1.00) | 0.35 |
| Change in trend at RRT implementation | 0.98 (0.96, 1.01) | 0.14 |
| Net intervention effect | 0.60 (0.31, 1.18) | 0.14 |
| In‐hospital mortality of patients discharged from the ICUadjusted odds ratio | ||
| Pre‐RRT trend | 1.02 (0.99, 1.06) | 0.15 |
| Change at RRT implementation | 0.74 (0.51, 1.08) | 0.12 |
| Post‐RRT trend | 1.00 (0.98, 1.01) | 0.68 |
| Change in trend | 0.97 (0.94, 1.01) | 0.14 |
| Net intervention effect | 0.39 (0.14, 1.10) | 0.08 |
ICU Average LOS
Introduction of proactive rounding by the RRT was not associated with unadjusted differences in ICU average LOS (5.1 days preintervention vs 4.9 days postintervention, P = 0.24; Table 1). Trends in ICU LOS may have changed in October 2006 (P = 0.07), decreasing in the first half of the study period (adjusted rate ratio [ARR] 0.98 per year, 95% CI 0.961.00), but did not change significantly thereafter. As with the ICU readmission rate, neither the change in estimated secular trend after implementation (ARR 0.98, 95% CI 0.961.01), nor the net effect of the intervention (ARR 0.62, 95% CI 0.321.22) was statistically significant (Table 2); these results are depicted graphically in Figure 2.

In‐Hospital Mortality of Patients Discharged From the ICU
Introduction of proactive rounding by the RRT was not associated with unadjusted differences in the mortality of patients discharged from the ICU (6.0% preintervention vs 5.5% postintervention, P = 0.24; Table 1). Similarly, in the adjusted ITS model, the intervention had no statistically significant net effect on the mortality outcome (Table 2 and Figure 3).

Secondary Analyses
Apart from weak evidence for a change in trend in ICU LOS in October 2006, no other changes in trend were found within the preintervention or postintervention periods (data not shown). This suggests that the heterogeneity of the preintervention RRT had no significant impact on the 3 outcomes examined, and that the RRT intervention failed to gain efficacy with time in the postintervention period. Additionally, we saw no outcome benefit in sensitivity analyses among all ICU patients or in service‐defined analyses (eg, surgical services), where ability to control for illness severity was improved.
DISCUSSION
In this single center study, introduction of an RRT that proactively rounded on patients discharged from the ICU did not reduce the ICU readmission rate, ICU LOS, or mortality of patients discharged from the ICU, after accounting for secular trends using robust ITS methods and adjusting for patient level factors.
Our study is consistent with 2 smaller studies that assessed the impact of proactive rounding by a CCOT on ICU readmission rate. Leary and Ridley14 found that proactively rounding by a CCOT did not reduce ICU readmissions or shorten the ICU LOS, although this study was limited by a surprisingly low ICU readmission rate and short ICU LOS prior to the intervention. Another study15 also observed no change in the ICU readmission rate following introduction of a proactively rounding CCOT but noted small reductions in both ICU and hospital mortality. The sole study showing an effect16 observed a lower ICU readmission rate and increased survival to hospital discharge (after excluding do not resuscitate [DNR] patients) with implementation of a CCOT, although some of their findings may be explained by their CCOT's use of palliative care services, a function not featured in our model.
Our study adds to the meta‐analyses and systematic reviews810 that have questioned the hypothesis that a trained and proactive team of caregivers should be able to prevent patients from returning to the ICU. Perhaps one reason why this is not true is that proactive rounding by RRTs may have minimal effect in systems where step‐down beds are readily available. At UCSF, nearly every patient transferred out of the ICU is triaged to a step‐down unit, where telemetry and pulse oximetry are continuously monitored. Despite this, however, our institution's 2 step‐down units generate more calls to our RRT than any other units in the hospital.
We were surprised to see that proactive rounding failed to shorten ICU LOS, hypothesizing that clinicians would be more comfortable discharging patients from the ICU knowing that the RRT would be closely monitoring them afterwards. Although we have no data to support this hypothesis, increased use of the RRT may have also increased step‐down bed use, as patients on the general medicalsurgical floors were transferred to a higher level of care upon recommendation of the RRT, thereby delaying transfers out of the ICU. Moreover, the opening of an additional 16‐bed ICU in October 2008 might have encouraged clinicians to transfer patients back to the ICU simply because beds were more easily accessible than before.
Introduction of proactive rounding by the RRT was also not associated with differences in the mortality rate of patients discharged from the ICU. This finding conflicts with the results of Garcea et al,15 Ball et al,16 and Priestley et al13, all of which found that implementation of a CCOT led to small but statistically significant reductions in in‐hospital mortality. All 3 of these studies, however, examined smaller patient populations (1380, 470, and 2903 patients, respectively), and both the Priestley and Ball studies13, 16 had significantly shorter periods of data collection (24 months and 32 weeks, respectively). Our results are based on models with confidence intervals and P values that account for variability in all 3 underlying effect estimates but assume a linear extrapolation of the preintervention trend. This approach allowed us to flexibly deal with changes related to the intervention, while relying on our large sample size to define time trends not dealt with adequately (or at all) in previous research.
The lack of improvement in outcomes cannot be attributed to immaturity of the RRT or failure of the clinical staff to use the RRT adequately. A prespecified secondary data analysis midway through the postintervention time period demonstrated that the RRT failed to gain efficacy with time with respect to all 3 outcomes. The postintervention RRT was also utilized far more frequently than its predecessor (110.6 vs 2.7 calls per 1000 admissions, respectively), and this degree of RRT utilization far surpasses the dose considered to be indicative of a mature RRT system.12
Our study has several limitations. First, we relied on administrative rather than chart‐collected data to determine the reason for ICU admission, and the APR severity of illness and risk of mortality scores. It seems unlikely, however, that coding deficiencies or biases affected the preintervention and postintervention patient populations differently. Even though we adjusted for all available measures, it is possible that we were not able to account for time trends in all potential confounders. Second, we did not have detailed clinical information on reasons for ICU readmission and whether readmissions occurred before or after the RRT proactively rounded on the patient. Therefore, potential readmissions to the ICU that might have been planned or which would have happened regardless of the presence of the RRT, such as for antibiotic desensitization, could not be accounted for. Third, introduction of proactive rounding by the RRT in June 2007 was accompanied by a change in the RRT's composition, from a physician‐led model to a nurse‐led model. Therefore, inherent differences in the way that physicians and nurses might assess and triage patients could not have been adjusted for. Lastly, this was a retrospective study conducted at a single academic medical center with a specific RRT model, and our results may not be directly applicable to nonteaching settings or to different RRT models.
Our findings raise further questions about the benefits of RRTs as they assume additional roles, such as proactive rounding on patients recently discharged from the ICU. The failure of our RRT to reduce the ICU readmission rate, the ICU average LOS, and the mortality of patients discharged from the ICU raises concerns that the benefits of our RRT are not commensurate with its cost. While defining the degree of impact and underlying mechanisms are worthy of prospective study, hospitals seeking to improve their RRT models should consider how to develop systems that achieve the RRT's promise in measurable ways.
Acknowledgements
The authors acknowledge Heather Leicester, MSPH, Senior Performance Improvement Analyst for Patient Safety and Quality Services at the University of California San Francisco for her work in data acquisition.
Disclosures: Dr Vittinghoff received salary support from an NIH grant during the time of this work for statistical consulting. He receives textbook royalties from Springer Verlag. Dr Auerbach was supported by 5K24HL098372‐02 from the National Heart Lung and Blood Institute during the period of this study although not specifically for this study; they had no role in the design or conduct of the study; the collection, analysis, or interpretation of the data; or the preparation, review, or approval of the manuscript. The other authors have no financial conflicts of interest.
- , , , . The 100,000 lives campaign: setting a goal and a deadline for improving health care quality. JAMA. 2006;295(3):324–327.
- Clinical Governance Unit, Quality and Safety Branch, Rural and Regional Health and Aged Care Services Division Safer Systems, Department of Human Services, State Government of Victoria. Safer Systems—Saving Lives Campaign. Available at: http://www.health.vic.gov.au/sssl. Accessed April 5, 2012.
- Canadian Patient Safety Institute. Safer Healthcare Now! Campaign. Available at: http://www.saferhealthcarenow.ca. Accessed April 5, 2012.
- , . The growth of rapid response systems. Jt Comm J Qual Patient Saf. 2008;34:489–495.
- , , , . The medical emergency team. Anaesth Intensive Care. 1995;23(2):183–186.
- , , , et al. Rates of in‐hospital arrests, deaths, and intensive care admission: the effect of a medical emergency team. Med J Aust. 2000;173:236–240.
- , , , , . The patient‐at‐risk team: identifying and managing seriously ill ward patients. Anaesthesia. 1999;54:853–860.
- , , , , . Effects of rapid response systems on clinical outcomes: systemic review and meta‐analysis. J Hosp Med. 2007;2:422–432.
- , , , , . Rapid response teams: a systemic review and meta‐analysis. Arch Intern Med. 2010;170(1):18–26.
- , , , , , . Rapid response systems: a systematic review. Crit Care Med. 2007;35(5):1238–1243.
- , , , et al. Introduction of the medical emergency team (MET) system: a cluster‐randomised controlled trial. Lancet. 2005;365:2091–2097.
- , , . Rapid response teams. N Engl J Med. 2011;365:139–146.
- , , , et al. Introducing critical care outreach: a ward‐randomised trial of phased introduction in a general hospital. Intensive Care Med. 2004;30(7):1398–1404.
- , . Impact of an outreach team on re‐admissions to a critical care unit. Anaesthesia. 2003;58:328–332.
- , , , , . Impact of a critical care outreach team on critical care readmissions and mortality. Acta Anaesthesiol Scand. 2004;48:1096–1100.
- , , . Effect of the critical care outreach team on patient survival to discharge from hospital and readmission to critical care: non‐randomised population based study. BMJ. 2003;327:1014–1017.
- , , , . Comorbidity measures for use with administrative data. Med Care. 1998;36(1):8–27.
- , , , . The 100,000 lives campaign: setting a goal and a deadline for improving health care quality. JAMA. 2006;295(3):324–327.
- Clinical Governance Unit, Quality and Safety Branch, Rural and Regional Health and Aged Care Services Division Safer Systems, Department of Human Services, State Government of Victoria. Safer Systems—Saving Lives Campaign. Available at: http://www.health.vic.gov.au/sssl. Accessed April 5, 2012.
- Canadian Patient Safety Institute. Safer Healthcare Now! Campaign. Available at: http://www.saferhealthcarenow.ca. Accessed April 5, 2012.
- , . The growth of rapid response systems. Jt Comm J Qual Patient Saf. 2008;34:489–495.
- , , , . The medical emergency team. Anaesth Intensive Care. 1995;23(2):183–186.
- , , , et al. Rates of in‐hospital arrests, deaths, and intensive care admission: the effect of a medical emergency team. Med J Aust. 2000;173:236–240.
- , , , , . The patient‐at‐risk team: identifying and managing seriously ill ward patients. Anaesthesia. 1999;54:853–860.
- , , , , . Effects of rapid response systems on clinical outcomes: systemic review and meta‐analysis. J Hosp Med. 2007;2:422–432.
- , , , , . Rapid response teams: a systemic review and meta‐analysis. Arch Intern Med. 2010;170(1):18–26.
- , , , , , . Rapid response systems: a systematic review. Crit Care Med. 2007;35(5):1238–1243.
- , , , et al. Introduction of the medical emergency team (MET) system: a cluster‐randomised controlled trial. Lancet. 2005;365:2091–2097.
- , , . Rapid response teams. N Engl J Med. 2011;365:139–146.
- , , , et al. Introducing critical care outreach: a ward‐randomised trial of phased introduction in a general hospital. Intensive Care Med. 2004;30(7):1398–1404.
- , . Impact of an outreach team on re‐admissions to a critical care unit. Anaesthesia. 2003;58:328–332.
- , , , , . Impact of a critical care outreach team on critical care readmissions and mortality. Acta Anaesthesiol Scand. 2004;48:1096–1100.
- , , . Effect of the critical care outreach team on patient survival to discharge from hospital and readmission to critical care: non‐randomised population based study. BMJ. 2003;327:1014–1017.
- , , , . Comorbidity measures for use with administrative data. Med Care. 1998;36(1):8–27.
Copyright © 2012 Society of Hospital Medicine