User login
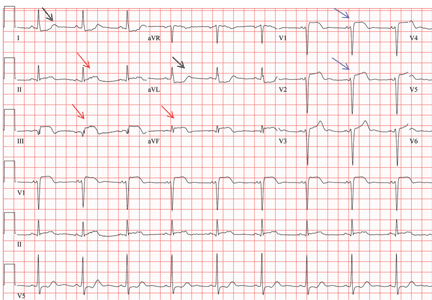
V1: The most important lead in inferior STEMI
Q: Which would be the most appropriate diagnosis?
- Pericarditis
- Acute inferior and right ventricular myocardial infarction
- Anterior and inferior myocardial infarction
- None of the above
A: The correct answer is acute inferior and right ventricular myocardial infarction.
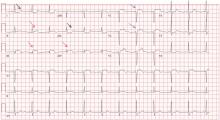
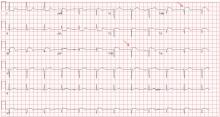
Her electrocardiogram showed sinus rhythm and inferior ST-segment elevation myocardial infarction (STEMI) evidenced by ST-segment elevation in leads II, III, and aVF. Hemodynamic instability or ST-segment elevation of more than 1 mm in lead V1 raises the suspicion of right ventricular myocardial infarction. In such patients, the American Heart Association guidelines recommend electrocardiography with right-sided precordial leads.1
A 1-mm ST-segment elevation in the right precordial lead V4R is one of the most predictive electrocardiographic findings in right ventricular infarction.2 The electrocardiographic changes in this type of myocardial infarction may be transient and resolve within 10 hours in up to 48% of cases.3
Echocardiography can also be used to confirm the possibility of right ventricular infarction.
Q: Which clinical condition can occur as a complication of right ventricular myocardial infarction?
- Profound hypotension after nitrate administration
- High-degree heart block
- Atrial fibrillation
- All of the above
A: All of the conditions can occur.
Right ventricular involvement is very common, noted in up to 50% of patients with acute inferior STEMI in postmortem studies.4 However, hemodynamically significant right ventricular dysfunction is much less common.
Intravenous volume loading with normal saline is one of the first steps in the management of hypotension associated with right ventricular infarction. Patients with significant bradycardia or a high degree of atrioventricular block may require pacing. Early reperfusion should be achieved, if possible. Heightened suspicion is critical to the early diagnosis of this condition, since the prognosis is much worse than for isolated inferior STEMI.4
Our patient was found to have right coronary artery disease requiring percutaneous coronary intervention.
- Antman EM, Anbe DT, Armstrong PW, et al; American College of Cardiology. ACC/AHA guidelines for the management of patients with ST-elevation myocardial infarction: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee to Revise the 1999 Guidelines for the Management of Patients with Acute Myocardial Infarction). Circulation 2004; 110:e82–e292.
- Robalino BD, Whitlow PL, Underwood DA, Salcedo EE. Electrocardiographic manifestations of right ventricular infarction. Am Heart J 1989; 118:138–144.
- Braat SH, Brugada P, de Zwaan C, Coenegracht JM, Wellens HJ. Value of electrocardiogram in diagnosing right ventricular involvement in patients with an acute inferior wall myocardial infarction. Br Heart J 1983; 49:368–372.
- Zehender M, Kasper W, Kauder E, et al. Right ventricular infarction as an independent predictor of prognosis after acute inferior myocardial infarction. N Engl J Med 1993; 328:981–988.
Q: Which would be the most appropriate diagnosis?
- Pericarditis
- Acute inferior and right ventricular myocardial infarction
- Anterior and inferior myocardial infarction
- None of the above
A: The correct answer is acute inferior and right ventricular myocardial infarction.
Her electrocardiogram showed sinus rhythm and inferior ST-segment elevation myocardial infarction (STEMI) evidenced by ST-segment elevation in leads II, III, and aVF. Hemodynamic instability or ST-segment elevation of more than 1 mm in lead V1 raises the suspicion of right ventricular myocardial infarction. In such patients, the American Heart Association guidelines recommend electrocardiography with right-sided precordial leads.1
A 1-mm ST-segment elevation in the right precordial lead V4R is one of the most predictive electrocardiographic findings in right ventricular infarction.2 The electrocardiographic changes in this type of myocardial infarction may be transient and resolve within 10 hours in up to 48% of cases.3
Echocardiography can also be used to confirm the possibility of right ventricular infarction.
Q: Which clinical condition can occur as a complication of right ventricular myocardial infarction?
- Profound hypotension after nitrate administration
- High-degree heart block
- Atrial fibrillation
- All of the above
A: All of the conditions can occur.
Right ventricular involvement is very common, noted in up to 50% of patients with acute inferior STEMI in postmortem studies.4 However, hemodynamically significant right ventricular dysfunction is much less common.
Intravenous volume loading with normal saline is one of the first steps in the management of hypotension associated with right ventricular infarction. Patients with significant bradycardia or a high degree of atrioventricular block may require pacing. Early reperfusion should be achieved, if possible. Heightened suspicion is critical to the early diagnosis of this condition, since the prognosis is much worse than for isolated inferior STEMI.4
Our patient was found to have right coronary artery disease requiring percutaneous coronary intervention.
Q: Which would be the most appropriate diagnosis?
- Pericarditis
- Acute inferior and right ventricular myocardial infarction
- Anterior and inferior myocardial infarction
- None of the above
A: The correct answer is acute inferior and right ventricular myocardial infarction.
Her electrocardiogram showed sinus rhythm and inferior ST-segment elevation myocardial infarction (STEMI) evidenced by ST-segment elevation in leads II, III, and aVF. Hemodynamic instability or ST-segment elevation of more than 1 mm in lead V1 raises the suspicion of right ventricular myocardial infarction. In such patients, the American Heart Association guidelines recommend electrocardiography with right-sided precordial leads.1
A 1-mm ST-segment elevation in the right precordial lead V4R is one of the most predictive electrocardiographic findings in right ventricular infarction.2 The electrocardiographic changes in this type of myocardial infarction may be transient and resolve within 10 hours in up to 48% of cases.3
Echocardiography can also be used to confirm the possibility of right ventricular infarction.
Q: Which clinical condition can occur as a complication of right ventricular myocardial infarction?
- Profound hypotension after nitrate administration
- High-degree heart block
- Atrial fibrillation
- All of the above
A: All of the conditions can occur.
Right ventricular involvement is very common, noted in up to 50% of patients with acute inferior STEMI in postmortem studies.4 However, hemodynamically significant right ventricular dysfunction is much less common.
Intravenous volume loading with normal saline is one of the first steps in the management of hypotension associated with right ventricular infarction. Patients with significant bradycardia or a high degree of atrioventricular block may require pacing. Early reperfusion should be achieved, if possible. Heightened suspicion is critical to the early diagnosis of this condition, since the prognosis is much worse than for isolated inferior STEMI.4
Our patient was found to have right coronary artery disease requiring percutaneous coronary intervention.
- Antman EM, Anbe DT, Armstrong PW, et al; American College of Cardiology. ACC/AHA guidelines for the management of patients with ST-elevation myocardial infarction: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee to Revise the 1999 Guidelines for the Management of Patients with Acute Myocardial Infarction). Circulation 2004; 110:e82–e292.
- Robalino BD, Whitlow PL, Underwood DA, Salcedo EE. Electrocardiographic manifestations of right ventricular infarction. Am Heart J 1989; 118:138–144.
- Braat SH, Brugada P, de Zwaan C, Coenegracht JM, Wellens HJ. Value of electrocardiogram in diagnosing right ventricular involvement in patients with an acute inferior wall myocardial infarction. Br Heart J 1983; 49:368–372.
- Zehender M, Kasper W, Kauder E, et al. Right ventricular infarction as an independent predictor of prognosis after acute inferior myocardial infarction. N Engl J Med 1993; 328:981–988.
- Antman EM, Anbe DT, Armstrong PW, et al; American College of Cardiology. ACC/AHA guidelines for the management of patients with ST-elevation myocardial infarction: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee to Revise the 1999 Guidelines for the Management of Patients with Acute Myocardial Infarction). Circulation 2004; 110:e82–e292.
- Robalino BD, Whitlow PL, Underwood DA, Salcedo EE. Electrocardiographic manifestations of right ventricular infarction. Am Heart J 1989; 118:138–144.
- Braat SH, Brugada P, de Zwaan C, Coenegracht JM, Wellens HJ. Value of electrocardiogram in diagnosing right ventricular involvement in patients with an acute inferior wall myocardial infarction. Br Heart J 1983; 49:368–372.
- Zehender M, Kasper W, Kauder E, et al. Right ventricular infarction as an independent predictor of prognosis after acute inferior myocardial infarction. N Engl J Med 1993; 328:981–988.
Home testing: The metamorphosis of attitudes about HIV infection
Most of us have not spent the past 25 years on the front line continuously managing HIV-infected patients, but I am sure that at various points in our lives we all have been touched by the AIDS epidemic. Whether comforting a woman with knee pain in the office who is crying over the impending death of her son who lives in a group home for men with AIDS, diagnosing immune thrombocytopenia in a college student only to realize it is the seminal manifestation of his HIV infection, pleading unsuccessfully with several neurosurgeons to get one to perform a brain biopsy on an “enhancing ring lesion” in a young gay opera singer, or being part of a team caring for a gouty patient with AIDS and hepatitis C who had just undergone a successful liver transplantation, we all have our stories with resultant reflections on the era of medicine in which we practice.
In July 2012, the New York Times described the new home test for HIV infection as part of “the normalization of a disease once seen as a mark of shame.”1 As with home pregnancy testing, people can now self-manage their need to know about what is going on in their body. But HIV goes so much deeper than this: it has been and remains a metaphor for and a reflection of many of the social issues that permeate our current political and social environment.
The politics and the social reactions to testing for HIV over the years since the virus was recognized in 1983–1984 is stuff for sociopsychologic treatises. Antibody testing was available in 1985, but in the absence of treatment, to test was simply to deliver a death sentence. Plus, with a diagnosis of AIDS, there would be no dental care, no insurance, no renting of an apartment, and perhaps no job. For some, family ties would be broken as closet doors would be thrown open, revealing a now unrecognized visage wearing the “mark of shame.” Some gay advocates rallied hard against testing, since anonymity and social protection for the infected could not be assured, a pragmatic response to blatant discrimination. In 1987, the first home test for HIV was in development, but—no surprise—there was no need for it.
As early treatments such as zidovudine (AZT) appeared and the value of specific antibiotic prophylaxis was demonstrated, there was some initial hope for treatment, and thus testing made medical sense. The size of the population infected (we were looking at the tip of the iceberg) was also being realized, so testing appealed to the social consciousness—try to limit infection. But discrimination wasn’t gone, and the politics of the time couldn’t quite handle all of the implications of a rapidly growing epidemic. America wasn’t ready for clean-needle-exchange programs, promotion of condom use, or open discussion of gay lifestyles. The Reagan White House was initially dead silent, except for proposing to limit entrance of potentially infected immigrants and promoting abstinence as the ideal protective approach.
Social righteousness took some hold, and protection of patient anonymity and autonomy became of paramount importance. But unintended consequences turned out to include limitation of testing: laws were written to require that HIV testing be accompanied by “appropriate,” stringently defined counseling, something that wasn’t always feasible. Patients needed to sign a release to be tested (“opt in”); many just said no. This tied the hands of physicians, so we developed work-arounds: we checked lymphocyte counts and CD4 counts to help us take care of patients too afraid to let us test for HIV directly.
Finally, in 2006, as therapies began to become increasingly effective and more data started to accumulate regarding the benefits of early antiretroviral therapy, the US Centers for Disease Control and Prevention recommended routine testing for all patients entering most acute health care facilities, unless they would actively decline (“opt out”). We have still not hit full stride in implementing universal testing for HIV. Nor have we hit our stride on fully accepting all demographic segments of the population. In some communities, HIV infection is still equivalent to the scarlet letter of Hester Prynne, not just because of the disease itself but because of the lifestyle it implies. Legislating laboratory testing practices cannot change all social attitudes. But maybe, hopefully, it is another step.
Dr. Christine Koval in this issue of the Journal discusses the practical use of the newly approved home HIV test. It is a short article, but it took a very, very long time for social and political forces to be modestly aligned sufficiently for there to be anything to write about. Since perhaps 18% of HIV-infected Americans are unaware of their infection, maybe some TV ads for this test, wedged between the ads for treating erectile dysfunction, can indeed bring (as the New York Times described) further “normalization” to the approach to managing HIV-infected patients.
- McNeil DG. Rapid HIV home test wins federal approval. The New York Times 2012 July 3. www.nytimes.com/2012/07/04/health/oraquick-at-home-hiv-test-wins-fda-approval.html. Accessed September 12, 2012.
Most of us have not spent the past 25 years on the front line continuously managing HIV-infected patients, but I am sure that at various points in our lives we all have been touched by the AIDS epidemic. Whether comforting a woman with knee pain in the office who is crying over the impending death of her son who lives in a group home for men with AIDS, diagnosing immune thrombocytopenia in a college student only to realize it is the seminal manifestation of his HIV infection, pleading unsuccessfully with several neurosurgeons to get one to perform a brain biopsy on an “enhancing ring lesion” in a young gay opera singer, or being part of a team caring for a gouty patient with AIDS and hepatitis C who had just undergone a successful liver transplantation, we all have our stories with resultant reflections on the era of medicine in which we practice.
In July 2012, the New York Times described the new home test for HIV infection as part of “the normalization of a disease once seen as a mark of shame.”1 As with home pregnancy testing, people can now self-manage their need to know about what is going on in their body. But HIV goes so much deeper than this: it has been and remains a metaphor for and a reflection of many of the social issues that permeate our current political and social environment.
The politics and the social reactions to testing for HIV over the years since the virus was recognized in 1983–1984 is stuff for sociopsychologic treatises. Antibody testing was available in 1985, but in the absence of treatment, to test was simply to deliver a death sentence. Plus, with a diagnosis of AIDS, there would be no dental care, no insurance, no renting of an apartment, and perhaps no job. For some, family ties would be broken as closet doors would be thrown open, revealing a now unrecognized visage wearing the “mark of shame.” Some gay advocates rallied hard against testing, since anonymity and social protection for the infected could not be assured, a pragmatic response to blatant discrimination. In 1987, the first home test for HIV was in development, but—no surprise—there was no need for it.
As early treatments such as zidovudine (AZT) appeared and the value of specific antibiotic prophylaxis was demonstrated, there was some initial hope for treatment, and thus testing made medical sense. The size of the population infected (we were looking at the tip of the iceberg) was also being realized, so testing appealed to the social consciousness—try to limit infection. But discrimination wasn’t gone, and the politics of the time couldn’t quite handle all of the implications of a rapidly growing epidemic. America wasn’t ready for clean-needle-exchange programs, promotion of condom use, or open discussion of gay lifestyles. The Reagan White House was initially dead silent, except for proposing to limit entrance of potentially infected immigrants and promoting abstinence as the ideal protective approach.
Social righteousness took some hold, and protection of patient anonymity and autonomy became of paramount importance. But unintended consequences turned out to include limitation of testing: laws were written to require that HIV testing be accompanied by “appropriate,” stringently defined counseling, something that wasn’t always feasible. Patients needed to sign a release to be tested (“opt in”); many just said no. This tied the hands of physicians, so we developed work-arounds: we checked lymphocyte counts and CD4 counts to help us take care of patients too afraid to let us test for HIV directly.
Finally, in 2006, as therapies began to become increasingly effective and more data started to accumulate regarding the benefits of early antiretroviral therapy, the US Centers for Disease Control and Prevention recommended routine testing for all patients entering most acute health care facilities, unless they would actively decline (“opt out”). We have still not hit full stride in implementing universal testing for HIV. Nor have we hit our stride on fully accepting all demographic segments of the population. In some communities, HIV infection is still equivalent to the scarlet letter of Hester Prynne, not just because of the disease itself but because of the lifestyle it implies. Legislating laboratory testing practices cannot change all social attitudes. But maybe, hopefully, it is another step.
Dr. Christine Koval in this issue of the Journal discusses the practical use of the newly approved home HIV test. It is a short article, but it took a very, very long time for social and political forces to be modestly aligned sufficiently for there to be anything to write about. Since perhaps 18% of HIV-infected Americans are unaware of their infection, maybe some TV ads for this test, wedged between the ads for treating erectile dysfunction, can indeed bring (as the New York Times described) further “normalization” to the approach to managing HIV-infected patients.
Most of us have not spent the past 25 years on the front line continuously managing HIV-infected patients, but I am sure that at various points in our lives we all have been touched by the AIDS epidemic. Whether comforting a woman with knee pain in the office who is crying over the impending death of her son who lives in a group home for men with AIDS, diagnosing immune thrombocytopenia in a college student only to realize it is the seminal manifestation of his HIV infection, pleading unsuccessfully with several neurosurgeons to get one to perform a brain biopsy on an “enhancing ring lesion” in a young gay opera singer, or being part of a team caring for a gouty patient with AIDS and hepatitis C who had just undergone a successful liver transplantation, we all have our stories with resultant reflections on the era of medicine in which we practice.
In July 2012, the New York Times described the new home test for HIV infection as part of “the normalization of a disease once seen as a mark of shame.”1 As with home pregnancy testing, people can now self-manage their need to know about what is going on in their body. But HIV goes so much deeper than this: it has been and remains a metaphor for and a reflection of many of the social issues that permeate our current political and social environment.
The politics and the social reactions to testing for HIV over the years since the virus was recognized in 1983–1984 is stuff for sociopsychologic treatises. Antibody testing was available in 1985, but in the absence of treatment, to test was simply to deliver a death sentence. Plus, with a diagnosis of AIDS, there would be no dental care, no insurance, no renting of an apartment, and perhaps no job. For some, family ties would be broken as closet doors would be thrown open, revealing a now unrecognized visage wearing the “mark of shame.” Some gay advocates rallied hard against testing, since anonymity and social protection for the infected could not be assured, a pragmatic response to blatant discrimination. In 1987, the first home test for HIV was in development, but—no surprise—there was no need for it.
As early treatments such as zidovudine (AZT) appeared and the value of specific antibiotic prophylaxis was demonstrated, there was some initial hope for treatment, and thus testing made medical sense. The size of the population infected (we were looking at the tip of the iceberg) was also being realized, so testing appealed to the social consciousness—try to limit infection. But discrimination wasn’t gone, and the politics of the time couldn’t quite handle all of the implications of a rapidly growing epidemic. America wasn’t ready for clean-needle-exchange programs, promotion of condom use, or open discussion of gay lifestyles. The Reagan White House was initially dead silent, except for proposing to limit entrance of potentially infected immigrants and promoting abstinence as the ideal protective approach.
Social righteousness took some hold, and protection of patient anonymity and autonomy became of paramount importance. But unintended consequences turned out to include limitation of testing: laws were written to require that HIV testing be accompanied by “appropriate,” stringently defined counseling, something that wasn’t always feasible. Patients needed to sign a release to be tested (“opt in”); many just said no. This tied the hands of physicians, so we developed work-arounds: we checked lymphocyte counts and CD4 counts to help us take care of patients too afraid to let us test for HIV directly.
Finally, in 2006, as therapies began to become increasingly effective and more data started to accumulate regarding the benefits of early antiretroviral therapy, the US Centers for Disease Control and Prevention recommended routine testing for all patients entering most acute health care facilities, unless they would actively decline (“opt out”). We have still not hit full stride in implementing universal testing for HIV. Nor have we hit our stride on fully accepting all demographic segments of the population. In some communities, HIV infection is still equivalent to the scarlet letter of Hester Prynne, not just because of the disease itself but because of the lifestyle it implies. Legislating laboratory testing practices cannot change all social attitudes. But maybe, hopefully, it is another step.
Dr. Christine Koval in this issue of the Journal discusses the practical use of the newly approved home HIV test. It is a short article, but it took a very, very long time for social and political forces to be modestly aligned sufficiently for there to be anything to write about. Since perhaps 18% of HIV-infected Americans are unaware of their infection, maybe some TV ads for this test, wedged between the ads for treating erectile dysfunction, can indeed bring (as the New York Times described) further “normalization” to the approach to managing HIV-infected patients.
- McNeil DG. Rapid HIV home test wins federal approval. The New York Times 2012 July 3. www.nytimes.com/2012/07/04/health/oraquick-at-home-hiv-test-wins-fda-approval.html. Accessed September 12, 2012.
- McNeil DG. Rapid HIV home test wins federal approval. The New York Times 2012 July 3. www.nytimes.com/2012/07/04/health/oraquick-at-home-hiv-test-wins-fda-approval.html. Accessed September 12, 2012.
Home testing for HIV: Hopefully, a step forward
In July 2012, the US Food and Drug Administration approved the first over-the-counter test kit for human immunodeficiency virus (HIV) infection, the OraQuick In-Home HIV Test (OraSure Technologies, Bethlehem, PA). This test is a variation of the currently available OraQuick ADVANCE Rapid HIV-1/2 Antibody Test used in clinical settings by trained personnel for rapid detection of HIV.
The home HIV test is expected to become available in the fall of 2012 from the company’s Web site and at retail drugstores. This will put the power of HIV testing into the hands of anyone able to afford the estimated $60 price and willing to purchase the item online or in stores.
GOAL: TO REDUCE THE NUMBER OF INFECTED PEOPLE WHO ARE UNAWARE
How home testing will change the demographics of HIV testing is not clear, but the intention is to reduce the number of HIV-infected people who are unaware of their infection and to get them in for care. Anthony Fauci, MD, the director of the National Institutes of Allergy and Infectious Diseases, has called the new test a “positive step forward” in bringing the HIV epidemic under control.1
Recent figures from the US Centers for Disease Control and Prevention (CDC) indicate that, of the 1.2 million HIV-infected people in the United States, up to 220,000 are unaware of their infection.2,3 Since antiretroviral therapy is now considered beneficial even in the early stages of HIV infection, those who are unaware of their infection are missing an opportunity for the most effective therapies.
They may also be unknowingly transmitting the virus, thus perpetuating the HIV epidemic. Awareness of one’s HIV infection may lead to behavioral changes that can reduce the risk of transmission. It has also become clear that antiretroviral therapy can dramatically reduce transmission rates, a concept known as “treatment as prevention.” 4 Thus, access to care and initiation of antiretroviral therapy have the potential to prevent progression to acquired immunodeficiency syndrome (AIDS) in the individual and to interrupt the spread of the virus in the community.
There are several steps between awareness of HIV infection and full engagement in HIV care that require attention from the health care community.5 Only a quarter of those with known HIV infection are in care and adherent to antiretroviral therapy, leaving much work to be done on removing barriers to effective treatment.5 The first step is still to identify those infected. The effort to increase the percentage of HIV-infected individuals who know their HIV status is one of the goals of the National HIV/AIDS Strategy and HealthyPeople2020.6
HOW THE TEST IS USED
The OraQuick In-Home Test consists of the device and reagents, instructional materials, information on interpreting the results, and contact information for the OraQuick Answer Center for information, support, and local medical referral.7 The overall time needed for testing is 20 to 40 minutes.
To perform the test, an oral fluid specimen is collected by swabbing the upper and lower buccal mucosa along the gum line. Once inserted into the developer solution the swabbed sample is carried onto a membrane strip containing HIV-1/2 antigens.
The device has two windows, one labelled “T” (for test) and the other labelled “C” (for control). If the patient has sufficient antibodies to HIV proteins, the “T” window indicates a positive result if a band is visible. The “C” (control) window displays a band to indicate if the device and reagents are working. If the control window does not show a band, then the kit has not functioned properly and the test result is not reliable.
SOME PEOPLE MAY STILL NEED HELP
For the test to succeed in informing people of their HIV status, it must be used effectively and the results must be interpretable. Of 5,662 participants in phase III investigational-device studies, 99% were able to use the kit and determine a result.7 While the test’s simplicity is similar to that of pregnancy test kits, it is possible that some people (at least 1% of those using the kit) may seek guidance from medical practitioners because they are unable to understand the test results.
For a test result to have the desired outcome of leading to HIV care, individuals must act on a positive result. When home test results are positive, the instructions indicate that “you may have HIV” and provide contact information for the OraQuick Answer Center. It is unclear how reliable the counseling, information, and referral process from OraSure will be and if people will use the service.
Individuals may access medical care at a variety of levels for further assistance if they have a positive test result. These may include primary care offices, emergency and urgent care settings, health departments, and HIV clinics.
LESS SENSITIVE THAN BLOOD TESTS
To provide additional care, clinicians must understand the performance of the home HIV test. Most importantly, the test result must be confirmed.
The In-Home test is less sensitive than currently available HIV blood tests used in the clinical setting, particularly the HIV-1/2 enzyme immunoassay (EIA) with confirmatory Western blot testing. The In-Home test is less likely to detect HIV infection during the 90-day “window period” when seroconversion is occurring, and so it should not be relied on to rule out HIV during this early period after infection.
The sensitivity and specificity of the OraQuick In-Home HIV test were determined in a phase III trial in 5,662 people (80% at risk of HIV), who were tested concurrently with the “gold standard” blood tests (EIA and Western blot). The sensitivity was 93% (giving a positive result in 106 of 114 patients who had a positive result on blood testing), and the specificity was 99.9% (giving a negative result in 5,384 of 5,385 patients who had a negative result on blood testing).7
Therefore, a positive In-Home test result is likely to be truly positive, but a negative result is not as reliably truly negative. False-negative results may occur particularly in the window period early after HIV infection, so the test should not be relied on within 90 days of high-risk behavior. In contrast, with the fourth-generation blood HIV tests, the window period is approximately 16 days.
The predictive value of the test will depend on the population using it and on the patient’s pretest probability of disease at the time of testing. In the population tested by OraQuick, the positive predictive value was 99.1% and the negative predictive value was 99.9%.7 Mathematical modeling has been done to examine the potential outcomes for use in subpopulations at lower risk and at higher risk.
As clinicians, we will have to address the potential for both false-positive and false-negative test results. False-positive results may be more likely in low-risk populations and may occur in the setting of cross-reactive antibodies from pregnancy, autoimmune diseases, or previous receipt of an experimental HIV vaccination. False-negative results may occur in the setting of acute HIV infection and in those with severely impaired immunity (eg, from agammaglobulinemia or immunosuppressive drugs) and will be more likely in higher-risk populations, such as men who have sex with men, intravenous drug users, blacks, and Hispanics ages 18 to 35 with multiple sexual partners. A positive In-Home HIV test should be followed up with a blood EIA and confirmed with Western blot in all patients.
WHO WILL USE THIS TEST?
It is unclear who will use this new test. In OraSure’s clinical trial, the percentages of people who indicated they would “definitely or probably buy” the test were:
- 20% of the general population
- 27% of those ages 18 to 35
- 49% of blacks ages 18 to 35
- 47% of homosexual men
- 43% of people who said they had more than two sexual partners per year
- 32% who said they use condoms inconsistently.
If this is true, the test may appropriately target several populations that are not currently being tested, either because they lack access to care or because they do not see themselves as being at high risk. Of those with newly diagnosed HIV infection from 2006 to 2009, 40% had had no prior testing, and the groups with the highest percentages of people in this category were black, men with injection drug use as their sole risk factor, those older than 50 years, and those with heterosexual contact as their sole risk factor.8 Because of difficulties in identifying some of these groups as “at risk,” the current CDC guidelines recommend that HIV testing be offered to all patients ages 13 to 64, regardless of their risk factors.9
The home HIV test may fill a gap in testing, extending it to those still not tested in the health care setting or to those who have not sought health care. For the home test to fill that gap, people still have to perceive themselves as at risk and then purchase the test. Through public health strategies and at clinical points of care, we must continue to inform our patients about HIV risk and work to identify new or ongoing risk factors that would prompt additional testing.
MANY QUESTIONS REMAIN
- Will those who need testing want to use this test? People will buy the test only if they perceive themselves to be at risk.
- Is this test affordable for the target populations? $60 will be unaffordable to some.
- Will the directions be followed effectively?
- Will home testing reduce opportunities to counsel patients on their HIV risk factors?
- Will there be situations in which individuals are socially pressured to take the test?
- Can users of the test expect the appropriate amount of privacy? Availability on the Internet and in drug stores is not a guarantee of privacy when purchasing the test, although the result presumably will not be known.
- Will those with positive results seek medical care?
- Will those with negative results who are still at high risk forgo more sensitive testing and continue to engage in high-risk activities?
Nevertheless, since early and continued treatment prevents disease progression and reduces HIV transmission, testing is the first step toward access to effective HIV care. The home HIV test is a step forward in providing high-quality HIV testing to the wider population.
- McNeil DG. Rapid H.I.V. Home Test Wins Federal Approval. New York Times, July 3, 2012. http://www.nytimes.com/2012/07/04/health/oraquick-at-home-hiv-test-wins-fda-approval.html. Accessed August 27, 2012.
- Centers for Disease Control and Prevention (CDC). Monitoring Selected National HIV Prevention and Care Objectives by Using HIV Surveillance Data—United States and 6 US Dependent Areas—2010 HIV Surveillance Supplemental Report, Volume 17, Number 3 (Part A). http://www.cdc.gov/hiv/surveillance/resources/reports/2010supp_vol-17no3/index.htm. Accessed August 27, 2012.
- Centers for Disease Control and Prevention (CDC). Diagnoses of HIV Infection and AIDS in the United States and Dependent Areas, 2010 HIV Surveillance Report, Volume 22. http://www.cdc.gov/hiv/surveillance/resources/reports/2010report/index.htm. Accessed August 27, 2012.
- Attia S, Egger M, Müller M, Zwahlen M, Low N. Sexual transmission of HIV according to viral load and antiretroviral therapy: systematic review and meta-analysis. AIDS 2009; 23:1397–1404.
- Gardner EM, McLees MP, Steiner JF, Del Rio C, Burman WJ. The spectrum of engagement in HIV care and its relevance to test-and-treat strategies for prevention of HIV infection. Clin Infect Dis 2011; 52:793–800.
- Centers for Disease Control and Prevention (CDC). Healthy People 2020 Summary of Objectives. http://healthypeople.gov/2020/topicsobjectives2020/pdfs/HIV.pdf. Accessed August 27, 2012.
- Food and Drug Administration (FDA). 102nd Meeting of The Blood Product Advisory Committee (BPAC). Evaluation of the Safety and Effectiveness of the OraQuick In-Home HIV Test. May 15, 2012.
- Centers for Disease Control and Prevention (CDC). Previous HIV testing among adults and adolescents newly diagnosed with HIV infection—National HIV Surveillance System, 18 jurisdictions, United States, 2006–2009. MMWR Morb Mortal Wkly Rep 2012; 61:441–445.
- Branson BM, Handsfield HH, Lampe MA, et al; Centers for Disease Control and Prevention (CDC). Revised recommendations for HIV testing of adults, adolescents, and pregnant women in health-care settings. MMWR Recomm Rep 2006; 55:1–17.
In July 2012, the US Food and Drug Administration approved the first over-the-counter test kit for human immunodeficiency virus (HIV) infection, the OraQuick In-Home HIV Test (OraSure Technologies, Bethlehem, PA). This test is a variation of the currently available OraQuick ADVANCE Rapid HIV-1/2 Antibody Test used in clinical settings by trained personnel for rapid detection of HIV.
The home HIV test is expected to become available in the fall of 2012 from the company’s Web site and at retail drugstores. This will put the power of HIV testing into the hands of anyone able to afford the estimated $60 price and willing to purchase the item online or in stores.
GOAL: TO REDUCE THE NUMBER OF INFECTED PEOPLE WHO ARE UNAWARE
How home testing will change the demographics of HIV testing is not clear, but the intention is to reduce the number of HIV-infected people who are unaware of their infection and to get them in for care. Anthony Fauci, MD, the director of the National Institutes of Allergy and Infectious Diseases, has called the new test a “positive step forward” in bringing the HIV epidemic under control.1
Recent figures from the US Centers for Disease Control and Prevention (CDC) indicate that, of the 1.2 million HIV-infected people in the United States, up to 220,000 are unaware of their infection.2,3 Since antiretroviral therapy is now considered beneficial even in the early stages of HIV infection, those who are unaware of their infection are missing an opportunity for the most effective therapies.
They may also be unknowingly transmitting the virus, thus perpetuating the HIV epidemic. Awareness of one’s HIV infection may lead to behavioral changes that can reduce the risk of transmission. It has also become clear that antiretroviral therapy can dramatically reduce transmission rates, a concept known as “treatment as prevention.” 4 Thus, access to care and initiation of antiretroviral therapy have the potential to prevent progression to acquired immunodeficiency syndrome (AIDS) in the individual and to interrupt the spread of the virus in the community.
There are several steps between awareness of HIV infection and full engagement in HIV care that require attention from the health care community.5 Only a quarter of those with known HIV infection are in care and adherent to antiretroviral therapy, leaving much work to be done on removing barriers to effective treatment.5 The first step is still to identify those infected. The effort to increase the percentage of HIV-infected individuals who know their HIV status is one of the goals of the National HIV/AIDS Strategy and HealthyPeople2020.6
HOW THE TEST IS USED
The OraQuick In-Home Test consists of the device and reagents, instructional materials, information on interpreting the results, and contact information for the OraQuick Answer Center for information, support, and local medical referral.7 The overall time needed for testing is 20 to 40 minutes.
To perform the test, an oral fluid specimen is collected by swabbing the upper and lower buccal mucosa along the gum line. Once inserted into the developer solution the swabbed sample is carried onto a membrane strip containing HIV-1/2 antigens.
The device has two windows, one labelled “T” (for test) and the other labelled “C” (for control). If the patient has sufficient antibodies to HIV proteins, the “T” window indicates a positive result if a band is visible. The “C” (control) window displays a band to indicate if the device and reagents are working. If the control window does not show a band, then the kit has not functioned properly and the test result is not reliable.
SOME PEOPLE MAY STILL NEED HELP
For the test to succeed in informing people of their HIV status, it must be used effectively and the results must be interpretable. Of 5,662 participants in phase III investigational-device studies, 99% were able to use the kit and determine a result.7 While the test’s simplicity is similar to that of pregnancy test kits, it is possible that some people (at least 1% of those using the kit) may seek guidance from medical practitioners because they are unable to understand the test results.
For a test result to have the desired outcome of leading to HIV care, individuals must act on a positive result. When home test results are positive, the instructions indicate that “you may have HIV” and provide contact information for the OraQuick Answer Center. It is unclear how reliable the counseling, information, and referral process from OraSure will be and if people will use the service.
Individuals may access medical care at a variety of levels for further assistance if they have a positive test result. These may include primary care offices, emergency and urgent care settings, health departments, and HIV clinics.
LESS SENSITIVE THAN BLOOD TESTS
To provide additional care, clinicians must understand the performance of the home HIV test. Most importantly, the test result must be confirmed.
The In-Home test is less sensitive than currently available HIV blood tests used in the clinical setting, particularly the HIV-1/2 enzyme immunoassay (EIA) with confirmatory Western blot testing. The In-Home test is less likely to detect HIV infection during the 90-day “window period” when seroconversion is occurring, and so it should not be relied on to rule out HIV during this early period after infection.
The sensitivity and specificity of the OraQuick In-Home HIV test were determined in a phase III trial in 5,662 people (80% at risk of HIV), who were tested concurrently with the “gold standard” blood tests (EIA and Western blot). The sensitivity was 93% (giving a positive result in 106 of 114 patients who had a positive result on blood testing), and the specificity was 99.9% (giving a negative result in 5,384 of 5,385 patients who had a negative result on blood testing).7
Therefore, a positive In-Home test result is likely to be truly positive, but a negative result is not as reliably truly negative. False-negative results may occur particularly in the window period early after HIV infection, so the test should not be relied on within 90 days of high-risk behavior. In contrast, with the fourth-generation blood HIV tests, the window period is approximately 16 days.
The predictive value of the test will depend on the population using it and on the patient’s pretest probability of disease at the time of testing. In the population tested by OraQuick, the positive predictive value was 99.1% and the negative predictive value was 99.9%.7 Mathematical modeling has been done to examine the potential outcomes for use in subpopulations at lower risk and at higher risk.
As clinicians, we will have to address the potential for both false-positive and false-negative test results. False-positive results may be more likely in low-risk populations and may occur in the setting of cross-reactive antibodies from pregnancy, autoimmune diseases, or previous receipt of an experimental HIV vaccination. False-negative results may occur in the setting of acute HIV infection and in those with severely impaired immunity (eg, from agammaglobulinemia or immunosuppressive drugs) and will be more likely in higher-risk populations, such as men who have sex with men, intravenous drug users, blacks, and Hispanics ages 18 to 35 with multiple sexual partners. A positive In-Home HIV test should be followed up with a blood EIA and confirmed with Western blot in all patients.
WHO WILL USE THIS TEST?
It is unclear who will use this new test. In OraSure’s clinical trial, the percentages of people who indicated they would “definitely or probably buy” the test were:
- 20% of the general population
- 27% of those ages 18 to 35
- 49% of blacks ages 18 to 35
- 47% of homosexual men
- 43% of people who said they had more than two sexual partners per year
- 32% who said they use condoms inconsistently.
If this is true, the test may appropriately target several populations that are not currently being tested, either because they lack access to care or because they do not see themselves as being at high risk. Of those with newly diagnosed HIV infection from 2006 to 2009, 40% had had no prior testing, and the groups with the highest percentages of people in this category were black, men with injection drug use as their sole risk factor, those older than 50 years, and those with heterosexual contact as their sole risk factor.8 Because of difficulties in identifying some of these groups as “at risk,” the current CDC guidelines recommend that HIV testing be offered to all patients ages 13 to 64, regardless of their risk factors.9
The home HIV test may fill a gap in testing, extending it to those still not tested in the health care setting or to those who have not sought health care. For the home test to fill that gap, people still have to perceive themselves as at risk and then purchase the test. Through public health strategies and at clinical points of care, we must continue to inform our patients about HIV risk and work to identify new or ongoing risk factors that would prompt additional testing.
MANY QUESTIONS REMAIN
- Will those who need testing want to use this test? People will buy the test only if they perceive themselves to be at risk.
- Is this test affordable for the target populations? $60 will be unaffordable to some.
- Will the directions be followed effectively?
- Will home testing reduce opportunities to counsel patients on their HIV risk factors?
- Will there be situations in which individuals are socially pressured to take the test?
- Can users of the test expect the appropriate amount of privacy? Availability on the Internet and in drug stores is not a guarantee of privacy when purchasing the test, although the result presumably will not be known.
- Will those with positive results seek medical care?
- Will those with negative results who are still at high risk forgo more sensitive testing and continue to engage in high-risk activities?
Nevertheless, since early and continued treatment prevents disease progression and reduces HIV transmission, testing is the first step toward access to effective HIV care. The home HIV test is a step forward in providing high-quality HIV testing to the wider population.
In July 2012, the US Food and Drug Administration approved the first over-the-counter test kit for human immunodeficiency virus (HIV) infection, the OraQuick In-Home HIV Test (OraSure Technologies, Bethlehem, PA). This test is a variation of the currently available OraQuick ADVANCE Rapid HIV-1/2 Antibody Test used in clinical settings by trained personnel for rapid detection of HIV.
The home HIV test is expected to become available in the fall of 2012 from the company’s Web site and at retail drugstores. This will put the power of HIV testing into the hands of anyone able to afford the estimated $60 price and willing to purchase the item online or in stores.
GOAL: TO REDUCE THE NUMBER OF INFECTED PEOPLE WHO ARE UNAWARE
How home testing will change the demographics of HIV testing is not clear, but the intention is to reduce the number of HIV-infected people who are unaware of their infection and to get them in for care. Anthony Fauci, MD, the director of the National Institutes of Allergy and Infectious Diseases, has called the new test a “positive step forward” in bringing the HIV epidemic under control.1
Recent figures from the US Centers for Disease Control and Prevention (CDC) indicate that, of the 1.2 million HIV-infected people in the United States, up to 220,000 are unaware of their infection.2,3 Since antiretroviral therapy is now considered beneficial even in the early stages of HIV infection, those who are unaware of their infection are missing an opportunity for the most effective therapies.
They may also be unknowingly transmitting the virus, thus perpetuating the HIV epidemic. Awareness of one’s HIV infection may lead to behavioral changes that can reduce the risk of transmission. It has also become clear that antiretroviral therapy can dramatically reduce transmission rates, a concept known as “treatment as prevention.” 4 Thus, access to care and initiation of antiretroviral therapy have the potential to prevent progression to acquired immunodeficiency syndrome (AIDS) in the individual and to interrupt the spread of the virus in the community.
There are several steps between awareness of HIV infection and full engagement in HIV care that require attention from the health care community.5 Only a quarter of those with known HIV infection are in care and adherent to antiretroviral therapy, leaving much work to be done on removing barriers to effective treatment.5 The first step is still to identify those infected. The effort to increase the percentage of HIV-infected individuals who know their HIV status is one of the goals of the National HIV/AIDS Strategy and HealthyPeople2020.6
HOW THE TEST IS USED
The OraQuick In-Home Test consists of the device and reagents, instructional materials, information on interpreting the results, and contact information for the OraQuick Answer Center for information, support, and local medical referral.7 The overall time needed for testing is 20 to 40 minutes.
To perform the test, an oral fluid specimen is collected by swabbing the upper and lower buccal mucosa along the gum line. Once inserted into the developer solution the swabbed sample is carried onto a membrane strip containing HIV-1/2 antigens.
The device has two windows, one labelled “T” (for test) and the other labelled “C” (for control). If the patient has sufficient antibodies to HIV proteins, the “T” window indicates a positive result if a band is visible. The “C” (control) window displays a band to indicate if the device and reagents are working. If the control window does not show a band, then the kit has not functioned properly and the test result is not reliable.
SOME PEOPLE MAY STILL NEED HELP
For the test to succeed in informing people of their HIV status, it must be used effectively and the results must be interpretable. Of 5,662 participants in phase III investigational-device studies, 99% were able to use the kit and determine a result.7 While the test’s simplicity is similar to that of pregnancy test kits, it is possible that some people (at least 1% of those using the kit) may seek guidance from medical practitioners because they are unable to understand the test results.
For a test result to have the desired outcome of leading to HIV care, individuals must act on a positive result. When home test results are positive, the instructions indicate that “you may have HIV” and provide contact information for the OraQuick Answer Center. It is unclear how reliable the counseling, information, and referral process from OraSure will be and if people will use the service.
Individuals may access medical care at a variety of levels for further assistance if they have a positive test result. These may include primary care offices, emergency and urgent care settings, health departments, and HIV clinics.
LESS SENSITIVE THAN BLOOD TESTS
To provide additional care, clinicians must understand the performance of the home HIV test. Most importantly, the test result must be confirmed.
The In-Home test is less sensitive than currently available HIV blood tests used in the clinical setting, particularly the HIV-1/2 enzyme immunoassay (EIA) with confirmatory Western blot testing. The In-Home test is less likely to detect HIV infection during the 90-day “window period” when seroconversion is occurring, and so it should not be relied on to rule out HIV during this early period after infection.
The sensitivity and specificity of the OraQuick In-Home HIV test were determined in a phase III trial in 5,662 people (80% at risk of HIV), who were tested concurrently with the “gold standard” blood tests (EIA and Western blot). The sensitivity was 93% (giving a positive result in 106 of 114 patients who had a positive result on blood testing), and the specificity was 99.9% (giving a negative result in 5,384 of 5,385 patients who had a negative result on blood testing).7
Therefore, a positive In-Home test result is likely to be truly positive, but a negative result is not as reliably truly negative. False-negative results may occur particularly in the window period early after HIV infection, so the test should not be relied on within 90 days of high-risk behavior. In contrast, with the fourth-generation blood HIV tests, the window period is approximately 16 days.
The predictive value of the test will depend on the population using it and on the patient’s pretest probability of disease at the time of testing. In the population tested by OraQuick, the positive predictive value was 99.1% and the negative predictive value was 99.9%.7 Mathematical modeling has been done to examine the potential outcomes for use in subpopulations at lower risk and at higher risk.
As clinicians, we will have to address the potential for both false-positive and false-negative test results. False-positive results may be more likely in low-risk populations and may occur in the setting of cross-reactive antibodies from pregnancy, autoimmune diseases, or previous receipt of an experimental HIV vaccination. False-negative results may occur in the setting of acute HIV infection and in those with severely impaired immunity (eg, from agammaglobulinemia or immunosuppressive drugs) and will be more likely in higher-risk populations, such as men who have sex with men, intravenous drug users, blacks, and Hispanics ages 18 to 35 with multiple sexual partners. A positive In-Home HIV test should be followed up with a blood EIA and confirmed with Western blot in all patients.
WHO WILL USE THIS TEST?
It is unclear who will use this new test. In OraSure’s clinical trial, the percentages of people who indicated they would “definitely or probably buy” the test were:
- 20% of the general population
- 27% of those ages 18 to 35
- 49% of blacks ages 18 to 35
- 47% of homosexual men
- 43% of people who said they had more than two sexual partners per year
- 32% who said they use condoms inconsistently.
If this is true, the test may appropriately target several populations that are not currently being tested, either because they lack access to care or because they do not see themselves as being at high risk. Of those with newly diagnosed HIV infection from 2006 to 2009, 40% had had no prior testing, and the groups with the highest percentages of people in this category were black, men with injection drug use as their sole risk factor, those older than 50 years, and those with heterosexual contact as their sole risk factor.8 Because of difficulties in identifying some of these groups as “at risk,” the current CDC guidelines recommend that HIV testing be offered to all patients ages 13 to 64, regardless of their risk factors.9
The home HIV test may fill a gap in testing, extending it to those still not tested in the health care setting or to those who have not sought health care. For the home test to fill that gap, people still have to perceive themselves as at risk and then purchase the test. Through public health strategies and at clinical points of care, we must continue to inform our patients about HIV risk and work to identify new or ongoing risk factors that would prompt additional testing.
MANY QUESTIONS REMAIN
- Will those who need testing want to use this test? People will buy the test only if they perceive themselves to be at risk.
- Is this test affordable for the target populations? $60 will be unaffordable to some.
- Will the directions be followed effectively?
- Will home testing reduce opportunities to counsel patients on their HIV risk factors?
- Will there be situations in which individuals are socially pressured to take the test?
- Can users of the test expect the appropriate amount of privacy? Availability on the Internet and in drug stores is not a guarantee of privacy when purchasing the test, although the result presumably will not be known.
- Will those with positive results seek medical care?
- Will those with negative results who are still at high risk forgo more sensitive testing and continue to engage in high-risk activities?
Nevertheless, since early and continued treatment prevents disease progression and reduces HIV transmission, testing is the first step toward access to effective HIV care. The home HIV test is a step forward in providing high-quality HIV testing to the wider population.
- McNeil DG. Rapid H.I.V. Home Test Wins Federal Approval. New York Times, July 3, 2012. http://www.nytimes.com/2012/07/04/health/oraquick-at-home-hiv-test-wins-fda-approval.html. Accessed August 27, 2012.
- Centers for Disease Control and Prevention (CDC). Monitoring Selected National HIV Prevention and Care Objectives by Using HIV Surveillance Data—United States and 6 US Dependent Areas—2010 HIV Surveillance Supplemental Report, Volume 17, Number 3 (Part A). http://www.cdc.gov/hiv/surveillance/resources/reports/2010supp_vol-17no3/index.htm. Accessed August 27, 2012.
- Centers for Disease Control and Prevention (CDC). Diagnoses of HIV Infection and AIDS in the United States and Dependent Areas, 2010 HIV Surveillance Report, Volume 22. http://www.cdc.gov/hiv/surveillance/resources/reports/2010report/index.htm. Accessed August 27, 2012.
- Attia S, Egger M, Müller M, Zwahlen M, Low N. Sexual transmission of HIV according to viral load and antiretroviral therapy: systematic review and meta-analysis. AIDS 2009; 23:1397–1404.
- Gardner EM, McLees MP, Steiner JF, Del Rio C, Burman WJ. The spectrum of engagement in HIV care and its relevance to test-and-treat strategies for prevention of HIV infection. Clin Infect Dis 2011; 52:793–800.
- Centers for Disease Control and Prevention (CDC). Healthy People 2020 Summary of Objectives. http://healthypeople.gov/2020/topicsobjectives2020/pdfs/HIV.pdf. Accessed August 27, 2012.
- Food and Drug Administration (FDA). 102nd Meeting of The Blood Product Advisory Committee (BPAC). Evaluation of the Safety and Effectiveness of the OraQuick In-Home HIV Test. May 15, 2012.
- Centers for Disease Control and Prevention (CDC). Previous HIV testing among adults and adolescents newly diagnosed with HIV infection—National HIV Surveillance System, 18 jurisdictions, United States, 2006–2009. MMWR Morb Mortal Wkly Rep 2012; 61:441–445.
- Branson BM, Handsfield HH, Lampe MA, et al; Centers for Disease Control and Prevention (CDC). Revised recommendations for HIV testing of adults, adolescents, and pregnant women in health-care settings. MMWR Recomm Rep 2006; 55:1–17.
- McNeil DG. Rapid H.I.V. Home Test Wins Federal Approval. New York Times, July 3, 2012. http://www.nytimes.com/2012/07/04/health/oraquick-at-home-hiv-test-wins-fda-approval.html. Accessed August 27, 2012.
- Centers for Disease Control and Prevention (CDC). Monitoring Selected National HIV Prevention and Care Objectives by Using HIV Surveillance Data—United States and 6 US Dependent Areas—2010 HIV Surveillance Supplemental Report, Volume 17, Number 3 (Part A). http://www.cdc.gov/hiv/surveillance/resources/reports/2010supp_vol-17no3/index.htm. Accessed August 27, 2012.
- Centers for Disease Control and Prevention (CDC). Diagnoses of HIV Infection and AIDS in the United States and Dependent Areas, 2010 HIV Surveillance Report, Volume 22. http://www.cdc.gov/hiv/surveillance/resources/reports/2010report/index.htm. Accessed August 27, 2012.
- Attia S, Egger M, Müller M, Zwahlen M, Low N. Sexual transmission of HIV according to viral load and antiretroviral therapy: systematic review and meta-analysis. AIDS 2009; 23:1397–1404.
- Gardner EM, McLees MP, Steiner JF, Del Rio C, Burman WJ. The spectrum of engagement in HIV care and its relevance to test-and-treat strategies for prevention of HIV infection. Clin Infect Dis 2011; 52:793–800.
- Centers for Disease Control and Prevention (CDC). Healthy People 2020 Summary of Objectives. http://healthypeople.gov/2020/topicsobjectives2020/pdfs/HIV.pdf. Accessed August 27, 2012.
- Food and Drug Administration (FDA). 102nd Meeting of The Blood Product Advisory Committee (BPAC). Evaluation of the Safety and Effectiveness of the OraQuick In-Home HIV Test. May 15, 2012.
- Centers for Disease Control and Prevention (CDC). Previous HIV testing among adults and adolescents newly diagnosed with HIV infection—National HIV Surveillance System, 18 jurisdictions, United States, 2006–2009. MMWR Morb Mortal Wkly Rep 2012; 61:441–445.
- Branson BM, Handsfield HH, Lampe MA, et al; Centers for Disease Control and Prevention (CDC). Revised recommendations for HIV testing of adults, adolescents, and pregnant women in health-care settings. MMWR Recomm Rep 2006; 55:1–17.
KEY POINTS
- The new test is highly (99.9%) specific for HIV but is not quite as reliable at ruling out infection (93% sensitivity). Therefore, it may miss some cases of HIV, especially during the 90-day window after initial infection.
- False-positive test results may occur, especially in people at low risk. A positive result must be confirmed with a laboratory-based third- or fourth-generation blood test.
- It is important to continue to assess and counsel patients on how to modify their risk of HIV infection.
- Providers are urged to offer HIV testing to all patients ages 13 to 64 at least once, regardless of their risk.
- At least once a year, patients at high risk should get one of the more sensitive laboratory blood tests.
- People who choose to test themselves at home should seek medical care for verification of the test result and for HIV counseling, and, if the result is confirmed positive, access to HIV care.
Novel strategy to prevent recurrent UTI in premenopausal women
Related article Update on Pelvic Floor Dysfunction (October 2012)
Related article Update on Pelvic Floor Dysfunction (October 2012)
Related article Update on Pelvic Floor Dysfunction (October 2012)
Venous Thromboembolism and Weight Changes in Veteran Patients Using Megestrol Acetate as an Appetite Stimulant
New and Noteworthy Information—October
Transient ischemic attack (TIA) is linked with a substantial risk of disability, researchers reported in the September 13 online Stroke. The 510 consecutive patients prospectively enrolled in the study had minor stroke or TIA, were not previously disabled, and had a CT or CT angiography completed within 24 hours of symptom onset. After assessing disability 90 days following the event, the investigators found that 15% of patients had a disabled outcome. Those who experienced recurrent strokes were more likely to be disabled—53% of patients with recurrent strokes were disabled, compared with 12% of those who did not have a recurrent stroke. “In terms of absolute numbers, most patients have disability as a result of their presenting event; however, recurrent events have the largest relative impact on outcome,” the study authors concluded.
Persons with high plasma glucose levels that are still within the normal range are more likely to have atrophy of brain structures associated with neurodegenerative processes, according to a study published in the September 4 Neurology. Investigators used MRI scans to assess hippocampal and amygdalar volumes in a sample of 266 cognitively healthy persons ages 60 to 64 who did not have type 2 diabetes. Results showed that plasma glucose levels were significantly linked with hippocampal and amygdalar atrophy. After controlling for age, sex, BMI, hypertension, alcohol, and smoking, the researchers found that plasma glucose levels accounted for a 6% to 10% change in volume. “These findings suggest that even in the subclinical range and in the absence of diabetes, monitoring and management of plasma glucose levels could have an impact on cerebral health,” the study authors wrote.
The FDA has approved once-a-day tablet Aubagio (teriflunomide) for treatment of adults with relapsing forms of multiple sclerosis (MS). During a clinical trial, patients taking teriflunomide had a relapse rate that was 30% lower than that of patients taking placebo. The most common side effects observed during clinical trials were diarrhea, abnormal liver tests, nausea, and hair loss, and physicians should conduct blood tests to check patients’ liver function before the drug is prescribed as well as periodically during treatment, researchers said. In addition, because of a risk of fetal harm, women of childbearing age must have a negative pregnancy test before beginning teriflunomide and should use birth control throughout treatment. Teriflunomide is the second oral treatment therapy for MS to be approved in the United States.
Patients who appear likely to have sporadic Creutzfeldt-Jakob disease may benefit from CSF 14-3-3 assays to clarify the diagnosis, researchers reported in the online September 19 Neurology. In a systematic literature review, the investigators identified articles from 1995 to January 1, 2011, that involved patients who had CSF analysis for protein 14-3-3. Based on data from 1,849 patients, the researchers determined that assays for CSF 14-3-3 are probably moderately accurate in diagnosing Creutzfeldt-Jakob—the assays had a sensitivity of 92%, specificity of 80%, likelihood ratio of 4.7, and negative likelihood ratio of 0.10. The study authors recommend CSF 14-3-3 assays “for patients who have rapidly progressive dementia and are strongly suspected of having sporadic Creutzfeldt-Jakob and for whom diagnosis remains uncertain (pretest probably of between 20% and 90%).”
Children with migraine and children with tension-type headaches are significantly more likely to have behavioral and emotional symptoms, and the frequency of headaches affects the likelihood of these symptoms, according to a study published in the online September 17 Cephalagia. After examining a sample of 1,856 children ages 5 to 11, investigators found that those with migraine were significantly more likely to experience abnormalities in somatic, anxiety-depressive, social, attention, internalizing, and total score domains of the Child Behavior Checklist. Children with tension-type headaches had a lower rate of abnormalities than children with migraine, but those with tension-type headaches still had significantly more abnormalities than controls. Children with headaches are more likely to have internalizing symptoms than externalizing symptoms such as rule breaking and aggressivity, the researchers found.
Heavy alcohol intake is associated with experiencing intracerebral hemorrhage at a younger age, according to a study published in the September 11 Neurology. Researchers prospectively followed 562 adults with spontaneous intracerebral hemorrhage and recorded information about their alcohol intake. A total of 137 patients were heavy alcohol drinkers, and these patients were more likely to be younger (median age, 60), to have a history of ischemic heart disease, and to be smokers. Furthermore, heavy alcohol drinkers had significantly lower platelet counts and prothrombin ratio. The investigators noted that although heavy alcohol intake is associated with intracerebral hemorrhage at a younger age, “the underlying vasculopathy remains unexplored in these patients. Indirect markers suggest small-vessel disease at an early stage that might be enhanced by moderate hemostatic disorders,” the authors concluded.
Long-term use of ginkgo biloba extract does not prevent the onset of Alzheimer’s disease in older patients, according to a study published in the online September 5 Lancet Neurology. Researchers enrolled 2,854 participants in a parallel-group, double-blind clinical trial in which 1,406 persons were randomized to receive ginkgo biloba extract and 1,414 persons were randomized to placebo. After five years of follow-up, 61 participants taking ginkgo biloba were diagnosed with probable Alzheimer’s disease, while 73 participants in the placebo group received a diagnosis of probable Alzheimer’s disease, though the risk was not proportional over time. The incidence of adverse events, as well as hemorrhagic or cardiovascular events, did not differ between groups. “Long-term use of standardized ginkgo biloba extract in this trial did not reduce the risk of progression to Alzheimer’s disease compared with placebo,” the researchers concluded.
The 13.3–mg/24 h dosage strength of the Exelon Patch (rivastigmine transdermal system) has been approved by the FDA for treatment of mild to moderate Alzheimer’s disease. Approval was based on the performance of the 13.3–mg/24 h dosage in the 48-week, double-blind phase of the OPTIMA study, which analyzed patients with mild to moderate Alzheimer’s disease who met predefined functional and cognitive decline criteria for the 9.5–mg/24 h dose. Compared with patients taking the 9.5–mg/24 h dose, patients taking the 13.3–mg/24 h dose showed statistically significant improvement in overall function. In addition, the overall safety profile of the 13.3–mg/24 h dose was the same as that of the lower dose, and fewer patients on the 13.3–mg/24 h dose needed to discontinue treatment than patients on the 9.5–mg/24 h dose.
The FDA has approved Nucynta (tapentadol) for management of neuropathic pain associated with diabetic peripheral neuropathy when a continuous, around-the-clock opioid analgesic is needed for an extended period of time. According to preclinical studies, the drug is a centrally acting synthetic analgesic, though the exact mechanism of action is unknown. In two randomized-withdrawal, placebo-controlled phase III trials, researchers studied patients who had at least a one-point reduction in pain intensity during three weeks of treatment and then continued for an additional 12 weeks on the same dose, which was titrated to balance individual tolerability and efficacy. These patients had significantly better pain control than those who switched to placebo. The most common adverse events associated with the drug were nausea, constipation, vomiting, dizziness, headache, and somnolence, but tapentadol was generally well tolerated.
A mouse model of abnormal adult-generated granule cells (DGCs) has provided the first direct evidence that abnormal DGCs are linked to seizures, researchers reported in the September 20 Neuron. To isolate the effects of the abnormal cells, investigators used a transgenic mouse model to selectively delete PTEN from DGCs generated after birth. As a result of PTEN deletion, the mammalian target of rapamycin pathway was hyperactivated, which produced abnormal DGCs that resembled those in epilepsy. “Strikingly, animals in which PTEN was deleted from 9% or more of the DGC population developed spontaneous seizures in about four weeks, confirming that abnormal DGCs, which are present in both animals and humans with epilepsy, are capable of causing the disease,” the researchers stated.
Patients with multiple sclerosis (MS) who receive gingko biloba 120 mg twice a day do not show improved cognitive performance, researchers reported in the September 18 Neurology. The investigators compared the performance of two groups of patients with MS who scored 1 SD or more below the mean on one of four neuropsychologic tests. Sixty-one patients received 120 mg of ginkgo biloba twice a day for 12 weeks, and 59 patients received placebo. The researchers evaluated participants’ cognitive performance following treatment and found no statistically significant difference in scores between the two groups. Furthermore, no significant adverse events related to gingko biloba treatment occurred, according to the study authors. Overall, the investigators concluded that gingko biloba does not improve cognitive function in patients with MS.
The Solitaire Flow Restoration device performs substantially better than the Merci Retrieval System in treating acute ischemic stroke, according to a study published in the online August 24 Lancet. In a randomized, parallel-group, noninferiority trial, the efficacy and safety of the Solitaire device, a self-expanding stent retriever designed to quickly restore blood flow, was compared with the efficacy and safety of the standard Merci Retrieval system. The 58 patients in the Solitaire group achieved the primary efficacy outcome 61% of the time, compared with 24% of patients in the Merci group, investigators said. Furthermore, patients in the Solitaire group had lower 90-day mortality than patients in the Merci group (17 versus 38). “The Solitaire device might be a future treatment of choice for endovascular recanalization in acute ischemic stroke,” the researchers concluded.
—Lauren LeBano
Transient ischemic attack (TIA) is linked with a substantial risk of disability, researchers reported in the September 13 online Stroke. The 510 consecutive patients prospectively enrolled in the study had minor stroke or TIA, were not previously disabled, and had a CT or CT angiography completed within 24 hours of symptom onset. After assessing disability 90 days following the event, the investigators found that 15% of patients had a disabled outcome. Those who experienced recurrent strokes were more likely to be disabled—53% of patients with recurrent strokes were disabled, compared with 12% of those who did not have a recurrent stroke. “In terms of absolute numbers, most patients have disability as a result of their presenting event; however, recurrent events have the largest relative impact on outcome,” the study authors concluded.
Persons with high plasma glucose levels that are still within the normal range are more likely to have atrophy of brain structures associated with neurodegenerative processes, according to a study published in the September 4 Neurology. Investigators used MRI scans to assess hippocampal and amygdalar volumes in a sample of 266 cognitively healthy persons ages 60 to 64 who did not have type 2 diabetes. Results showed that plasma glucose levels were significantly linked with hippocampal and amygdalar atrophy. After controlling for age, sex, BMI, hypertension, alcohol, and smoking, the researchers found that plasma glucose levels accounted for a 6% to 10% change in volume. “These findings suggest that even in the subclinical range and in the absence of diabetes, monitoring and management of plasma glucose levels could have an impact on cerebral health,” the study authors wrote.
The FDA has approved once-a-day tablet Aubagio (teriflunomide) for treatment of adults with relapsing forms of multiple sclerosis (MS). During a clinical trial, patients taking teriflunomide had a relapse rate that was 30% lower than that of patients taking placebo. The most common side effects observed during clinical trials were diarrhea, abnormal liver tests, nausea, and hair loss, and physicians should conduct blood tests to check patients’ liver function before the drug is prescribed as well as periodically during treatment, researchers said. In addition, because of a risk of fetal harm, women of childbearing age must have a negative pregnancy test before beginning teriflunomide and should use birth control throughout treatment. Teriflunomide is the second oral treatment therapy for MS to be approved in the United States.
Patients who appear likely to have sporadic Creutzfeldt-Jakob disease may benefit from CSF 14-3-3 assays to clarify the diagnosis, researchers reported in the online September 19 Neurology. In a systematic literature review, the investigators identified articles from 1995 to January 1, 2011, that involved patients who had CSF analysis for protein 14-3-3. Based on data from 1,849 patients, the researchers determined that assays for CSF 14-3-3 are probably moderately accurate in diagnosing Creutzfeldt-Jakob—the assays had a sensitivity of 92%, specificity of 80%, likelihood ratio of 4.7, and negative likelihood ratio of 0.10. The study authors recommend CSF 14-3-3 assays “for patients who have rapidly progressive dementia and are strongly suspected of having sporadic Creutzfeldt-Jakob and for whom diagnosis remains uncertain (pretest probably of between 20% and 90%).”
Children with migraine and children with tension-type headaches are significantly more likely to have behavioral and emotional symptoms, and the frequency of headaches affects the likelihood of these symptoms, according to a study published in the online September 17 Cephalagia. After examining a sample of 1,856 children ages 5 to 11, investigators found that those with migraine were significantly more likely to experience abnormalities in somatic, anxiety-depressive, social, attention, internalizing, and total score domains of the Child Behavior Checklist. Children with tension-type headaches had a lower rate of abnormalities than children with migraine, but those with tension-type headaches still had significantly more abnormalities than controls. Children with headaches are more likely to have internalizing symptoms than externalizing symptoms such as rule breaking and aggressivity, the researchers found.
Heavy alcohol intake is associated with experiencing intracerebral hemorrhage at a younger age, according to a study published in the September 11 Neurology. Researchers prospectively followed 562 adults with spontaneous intracerebral hemorrhage and recorded information about their alcohol intake. A total of 137 patients were heavy alcohol drinkers, and these patients were more likely to be younger (median age, 60), to have a history of ischemic heart disease, and to be smokers. Furthermore, heavy alcohol drinkers had significantly lower platelet counts and prothrombin ratio. The investigators noted that although heavy alcohol intake is associated with intracerebral hemorrhage at a younger age, “the underlying vasculopathy remains unexplored in these patients. Indirect markers suggest small-vessel disease at an early stage that might be enhanced by moderate hemostatic disorders,” the authors concluded.
Long-term use of ginkgo biloba extract does not prevent the onset of Alzheimer’s disease in older patients, according to a study published in the online September 5 Lancet Neurology. Researchers enrolled 2,854 participants in a parallel-group, double-blind clinical trial in which 1,406 persons were randomized to receive ginkgo biloba extract and 1,414 persons were randomized to placebo. After five years of follow-up, 61 participants taking ginkgo biloba were diagnosed with probable Alzheimer’s disease, while 73 participants in the placebo group received a diagnosis of probable Alzheimer’s disease, though the risk was not proportional over time. The incidence of adverse events, as well as hemorrhagic or cardiovascular events, did not differ between groups. “Long-term use of standardized ginkgo biloba extract in this trial did not reduce the risk of progression to Alzheimer’s disease compared with placebo,” the researchers concluded.
The 13.3–mg/24 h dosage strength of the Exelon Patch (rivastigmine transdermal system) has been approved by the FDA for treatment of mild to moderate Alzheimer’s disease. Approval was based on the performance of the 13.3–mg/24 h dosage in the 48-week, double-blind phase of the OPTIMA study, which analyzed patients with mild to moderate Alzheimer’s disease who met predefined functional and cognitive decline criteria for the 9.5–mg/24 h dose. Compared with patients taking the 9.5–mg/24 h dose, patients taking the 13.3–mg/24 h dose showed statistically significant improvement in overall function. In addition, the overall safety profile of the 13.3–mg/24 h dose was the same as that of the lower dose, and fewer patients on the 13.3–mg/24 h dose needed to discontinue treatment than patients on the 9.5–mg/24 h dose.
The FDA has approved Nucynta (tapentadol) for management of neuropathic pain associated with diabetic peripheral neuropathy when a continuous, around-the-clock opioid analgesic is needed for an extended period of time. According to preclinical studies, the drug is a centrally acting synthetic analgesic, though the exact mechanism of action is unknown. In two randomized-withdrawal, placebo-controlled phase III trials, researchers studied patients who had at least a one-point reduction in pain intensity during three weeks of treatment and then continued for an additional 12 weeks on the same dose, which was titrated to balance individual tolerability and efficacy. These patients had significantly better pain control than those who switched to placebo. The most common adverse events associated with the drug were nausea, constipation, vomiting, dizziness, headache, and somnolence, but tapentadol was generally well tolerated.
A mouse model of abnormal adult-generated granule cells (DGCs) has provided the first direct evidence that abnormal DGCs are linked to seizures, researchers reported in the September 20 Neuron. To isolate the effects of the abnormal cells, investigators used a transgenic mouse model to selectively delete PTEN from DGCs generated after birth. As a result of PTEN deletion, the mammalian target of rapamycin pathway was hyperactivated, which produced abnormal DGCs that resembled those in epilepsy. “Strikingly, animals in which PTEN was deleted from 9% or more of the DGC population developed spontaneous seizures in about four weeks, confirming that abnormal DGCs, which are present in both animals and humans with epilepsy, are capable of causing the disease,” the researchers stated.
Patients with multiple sclerosis (MS) who receive gingko biloba 120 mg twice a day do not show improved cognitive performance, researchers reported in the September 18 Neurology. The investigators compared the performance of two groups of patients with MS who scored 1 SD or more below the mean on one of four neuropsychologic tests. Sixty-one patients received 120 mg of ginkgo biloba twice a day for 12 weeks, and 59 patients received placebo. The researchers evaluated participants’ cognitive performance following treatment and found no statistically significant difference in scores between the two groups. Furthermore, no significant adverse events related to gingko biloba treatment occurred, according to the study authors. Overall, the investigators concluded that gingko biloba does not improve cognitive function in patients with MS.
The Solitaire Flow Restoration device performs substantially better than the Merci Retrieval System in treating acute ischemic stroke, according to a study published in the online August 24 Lancet. In a randomized, parallel-group, noninferiority trial, the efficacy and safety of the Solitaire device, a self-expanding stent retriever designed to quickly restore blood flow, was compared with the efficacy and safety of the standard Merci Retrieval system. The 58 patients in the Solitaire group achieved the primary efficacy outcome 61% of the time, compared with 24% of patients in the Merci group, investigators said. Furthermore, patients in the Solitaire group had lower 90-day mortality than patients in the Merci group (17 versus 38). “The Solitaire device might be a future treatment of choice for endovascular recanalization in acute ischemic stroke,” the researchers concluded.
—Lauren LeBano
Transient ischemic attack (TIA) is linked with a substantial risk of disability, researchers reported in the September 13 online Stroke. The 510 consecutive patients prospectively enrolled in the study had minor stroke or TIA, were not previously disabled, and had a CT or CT angiography completed within 24 hours of symptom onset. After assessing disability 90 days following the event, the investigators found that 15% of patients had a disabled outcome. Those who experienced recurrent strokes were more likely to be disabled—53% of patients with recurrent strokes were disabled, compared with 12% of those who did not have a recurrent stroke. “In terms of absolute numbers, most patients have disability as a result of their presenting event; however, recurrent events have the largest relative impact on outcome,” the study authors concluded.
Persons with high plasma glucose levels that are still within the normal range are more likely to have atrophy of brain structures associated with neurodegenerative processes, according to a study published in the September 4 Neurology. Investigators used MRI scans to assess hippocampal and amygdalar volumes in a sample of 266 cognitively healthy persons ages 60 to 64 who did not have type 2 diabetes. Results showed that plasma glucose levels were significantly linked with hippocampal and amygdalar atrophy. After controlling for age, sex, BMI, hypertension, alcohol, and smoking, the researchers found that plasma glucose levels accounted for a 6% to 10% change in volume. “These findings suggest that even in the subclinical range and in the absence of diabetes, monitoring and management of plasma glucose levels could have an impact on cerebral health,” the study authors wrote.
The FDA has approved once-a-day tablet Aubagio (teriflunomide) for treatment of adults with relapsing forms of multiple sclerosis (MS). During a clinical trial, patients taking teriflunomide had a relapse rate that was 30% lower than that of patients taking placebo. The most common side effects observed during clinical trials were diarrhea, abnormal liver tests, nausea, and hair loss, and physicians should conduct blood tests to check patients’ liver function before the drug is prescribed as well as periodically during treatment, researchers said. In addition, because of a risk of fetal harm, women of childbearing age must have a negative pregnancy test before beginning teriflunomide and should use birth control throughout treatment. Teriflunomide is the second oral treatment therapy for MS to be approved in the United States.
Patients who appear likely to have sporadic Creutzfeldt-Jakob disease may benefit from CSF 14-3-3 assays to clarify the diagnosis, researchers reported in the online September 19 Neurology. In a systematic literature review, the investigators identified articles from 1995 to January 1, 2011, that involved patients who had CSF analysis for protein 14-3-3. Based on data from 1,849 patients, the researchers determined that assays for CSF 14-3-3 are probably moderately accurate in diagnosing Creutzfeldt-Jakob—the assays had a sensitivity of 92%, specificity of 80%, likelihood ratio of 4.7, and negative likelihood ratio of 0.10. The study authors recommend CSF 14-3-3 assays “for patients who have rapidly progressive dementia and are strongly suspected of having sporadic Creutzfeldt-Jakob and for whom diagnosis remains uncertain (pretest probably of between 20% and 90%).”
Children with migraine and children with tension-type headaches are significantly more likely to have behavioral and emotional symptoms, and the frequency of headaches affects the likelihood of these symptoms, according to a study published in the online September 17 Cephalagia. After examining a sample of 1,856 children ages 5 to 11, investigators found that those with migraine were significantly more likely to experience abnormalities in somatic, anxiety-depressive, social, attention, internalizing, and total score domains of the Child Behavior Checklist. Children with tension-type headaches had a lower rate of abnormalities than children with migraine, but those with tension-type headaches still had significantly more abnormalities than controls. Children with headaches are more likely to have internalizing symptoms than externalizing symptoms such as rule breaking and aggressivity, the researchers found.
Heavy alcohol intake is associated with experiencing intracerebral hemorrhage at a younger age, according to a study published in the September 11 Neurology. Researchers prospectively followed 562 adults with spontaneous intracerebral hemorrhage and recorded information about their alcohol intake. A total of 137 patients were heavy alcohol drinkers, and these patients were more likely to be younger (median age, 60), to have a history of ischemic heart disease, and to be smokers. Furthermore, heavy alcohol drinkers had significantly lower platelet counts and prothrombin ratio. The investigators noted that although heavy alcohol intake is associated with intracerebral hemorrhage at a younger age, “the underlying vasculopathy remains unexplored in these patients. Indirect markers suggest small-vessel disease at an early stage that might be enhanced by moderate hemostatic disorders,” the authors concluded.
Long-term use of ginkgo biloba extract does not prevent the onset of Alzheimer’s disease in older patients, according to a study published in the online September 5 Lancet Neurology. Researchers enrolled 2,854 participants in a parallel-group, double-blind clinical trial in which 1,406 persons were randomized to receive ginkgo biloba extract and 1,414 persons were randomized to placebo. After five years of follow-up, 61 participants taking ginkgo biloba were diagnosed with probable Alzheimer’s disease, while 73 participants in the placebo group received a diagnosis of probable Alzheimer’s disease, though the risk was not proportional over time. The incidence of adverse events, as well as hemorrhagic or cardiovascular events, did not differ between groups. “Long-term use of standardized ginkgo biloba extract in this trial did not reduce the risk of progression to Alzheimer’s disease compared with placebo,” the researchers concluded.
The 13.3–mg/24 h dosage strength of the Exelon Patch (rivastigmine transdermal system) has been approved by the FDA for treatment of mild to moderate Alzheimer’s disease. Approval was based on the performance of the 13.3–mg/24 h dosage in the 48-week, double-blind phase of the OPTIMA study, which analyzed patients with mild to moderate Alzheimer’s disease who met predefined functional and cognitive decline criteria for the 9.5–mg/24 h dose. Compared with patients taking the 9.5–mg/24 h dose, patients taking the 13.3–mg/24 h dose showed statistically significant improvement in overall function. In addition, the overall safety profile of the 13.3–mg/24 h dose was the same as that of the lower dose, and fewer patients on the 13.3–mg/24 h dose needed to discontinue treatment than patients on the 9.5–mg/24 h dose.
The FDA has approved Nucynta (tapentadol) for management of neuropathic pain associated with diabetic peripheral neuropathy when a continuous, around-the-clock opioid analgesic is needed for an extended period of time. According to preclinical studies, the drug is a centrally acting synthetic analgesic, though the exact mechanism of action is unknown. In two randomized-withdrawal, placebo-controlled phase III trials, researchers studied patients who had at least a one-point reduction in pain intensity during three weeks of treatment and then continued for an additional 12 weeks on the same dose, which was titrated to balance individual tolerability and efficacy. These patients had significantly better pain control than those who switched to placebo. The most common adverse events associated with the drug were nausea, constipation, vomiting, dizziness, headache, and somnolence, but tapentadol was generally well tolerated.
A mouse model of abnormal adult-generated granule cells (DGCs) has provided the first direct evidence that abnormal DGCs are linked to seizures, researchers reported in the September 20 Neuron. To isolate the effects of the abnormal cells, investigators used a transgenic mouse model to selectively delete PTEN from DGCs generated after birth. As a result of PTEN deletion, the mammalian target of rapamycin pathway was hyperactivated, which produced abnormal DGCs that resembled those in epilepsy. “Strikingly, animals in which PTEN was deleted from 9% or more of the DGC population developed spontaneous seizures in about four weeks, confirming that abnormal DGCs, which are present in both animals and humans with epilepsy, are capable of causing the disease,” the researchers stated.
Patients with multiple sclerosis (MS) who receive gingko biloba 120 mg twice a day do not show improved cognitive performance, researchers reported in the September 18 Neurology. The investigators compared the performance of two groups of patients with MS who scored 1 SD or more below the mean on one of four neuropsychologic tests. Sixty-one patients received 120 mg of ginkgo biloba twice a day for 12 weeks, and 59 patients received placebo. The researchers evaluated participants’ cognitive performance following treatment and found no statistically significant difference in scores between the two groups. Furthermore, no significant adverse events related to gingko biloba treatment occurred, according to the study authors. Overall, the investigators concluded that gingko biloba does not improve cognitive function in patients with MS.
The Solitaire Flow Restoration device performs substantially better than the Merci Retrieval System in treating acute ischemic stroke, according to a study published in the online August 24 Lancet. In a randomized, parallel-group, noninferiority trial, the efficacy and safety of the Solitaire device, a self-expanding stent retriever designed to quickly restore blood flow, was compared with the efficacy and safety of the standard Merci Retrieval system. The 58 patients in the Solitaire group achieved the primary efficacy outcome 61% of the time, compared with 24% of patients in the Merci group, investigators said. Furthermore, patients in the Solitaire group had lower 90-day mortality than patients in the Merci group (17 versus 38). “The Solitaire device might be a future treatment of choice for endovascular recanalization in acute ischemic stroke,” the researchers concluded.
—Lauren LeBano
Livedoid Vasculopathy
What's Eating You? Bedbugs Revisited (Cimex lectularius)
Menkes Syndrome Presenting as Possible Child Abuse
Stalked by a ‘patient’
CASE: Delusions and threats
For over 20 months, Ms. I, age 48, sends a psychiatric resident letters and postcards that total approximately 3,000 pages and come from dozens of return addresses. Ms. I expresses romantic feelings toward the resident and believes that he was her physician and prescribed medications, including “mood stabilizers.” The resident never treated Ms. I; to his knowledge, he has never interacted with her.
Ms. I describes the resident’s refusal to continue treating her as “abandonment” and states that she is contemplating self-harm because of this rejection. In her letters, Ms. I admits that she was a long-term patient in a state psychiatric hospital in her home state and suffers from persistent auditory hallucinations. She also wants a romantic relationship with the resident and repeatedly threatens the resident’s female acquaintances and former romantic partners whose relationships she had surmised from news articles available on the Internet. Ms. I also threatens to strangle the resident. The resident sends her multiple written requests that she cease contact, but they are not acknowledged.
The authors’ observations
Stalking—repeated, unwanted attention or communication that would cause a reasonable person fear—is a serious threat for many psychiatric clinicians.1 Prevalence rates among mental health care providers range from 3% to 21%.2,3 Most stalkers have engaged in previous stalking behavior.3
Being stalked is highly distressing,4 and mental health professionals often do not reveal such experiences to colleagues.5 Irrational feelings of guilt or embarrassment, such as being thought to have poorly managed interactions with the stalker, often motivate a self-imposed silence (Table 1).6 This isolation may foster anxiety, interfere with receiving problem-solving advice, and increase physical vulnerability. In the case involving Ms. I, the psychiatric resident’s primary responsibility is safeguarding his own physical and psychological welfare.
Clinicians who work in a hospital or other institutional setting who are being stalked should inform their supervisors and the facility’s security personnel. Security personnel may be able to gather data about the stalker, decrease the stalker’s ability to communicate with the victim, and reduce unwanted physical access to the victim by distributing a photo of the stalker or installing a camera or receptionist-controlled door lock in patient entryways. Security personnel also may collaborate with local law enforcement. Having a third party respond to a stalker’s aggressive behavior—rather than the victim responding directly—avoids rewarding the stalker, which may generate further unwanted contact.7 Any intervention by the victim may increase the risk of violence, creating an “intervention dilemma.” Resnick8 argues that before deciding how best to address the stalker’s behavior, a stalking victim must “first separate the risk of continued stalking from the risk that the stalker will commit a violent act.”
Mental health professionals in private practice who are being stalked should consider retaining an attorney. An attorney often can maintain privacy of communications regarding the stalker via the attorney-client and attorney-work product privileges, which may help during legal proceedings.
Table 1
Factors that can impede psychiatrists from reporting stalking
| Fear of being perceived as a failure |
| Embarrassment |
| High professional tolerance for antisocial and threatening behavior |
| Misplaced sense of duty |
| Source: Reference 6 |
RESPONSE: Involving police
Over 2 months, Ms. I phones the resident’s home 105 times (the resident screens the calls). During 1 call, she states that she is hidden in a closet in her home and will hurt herself unless the resident “resumes” her psychiatric care. The resident contacts police in his city and Ms. I’s community, but authorities are reluctant to act when he acknowledges that he is not Ms. I’s psychiatrist and does not know her. Police officers in Ms. I’s hometown tell the resident no one answered the door when they visited her home. They state that they would enter the residence forcibly only if Ms. I’s physician or a family member asked them to do so, and because the resident admits that he is not her psychiatrist, they cannot take further action. Ms. I leaves the resident a phone message several hours later to inform him she is safe.
The authors’ observations
Stalking-induced countertransference responses may lead a psychiatrist to unwittingly place himself in harm’s way. For example, intense rage at a stalker’s request for treatment may generate guilt that motivates the psychiatrist to agree to treat the stalker. Feelings of helplessness may produce a frantic desire to do something even when such activity is ill-advised. Psychiatrists may develop a tolerance for antisocial or threatening behavior—which is common in mental health settings—and could accept unnecessary risks.
A psychiatrist who is being stalked may be able to assist a mentally ill stalker in a way that does not create a duty to treat and does not expose the psychiatrist to harm, such as contacting a mobile crisis intervention team, a mental health professional who recently treated the stalker, a family member of the stalker, or law enforcement personnel. A psychiatrist who is thrust from the role of helper to victim and must protect his or her own well-being instead of attending to a patient’s welfare is prone to suffer substantial countertransference distress.
The situation with Ms. I was particularly challenging because the resident did not know her complete history and therefore had little information to gauge how likely she was to act on her aggressive threats. Factors that predict future violence include:
- a history of violence
- significant prior criminality
- young age at first arrest
- concomitant substance abuse
- male sex.9
Unfortunately, other than sex, this data regarding Ms. I could not be readily obtained.
A psychiatrist’s duty
Although sympathetic to his stalker’s distress, the resident did not want to treat this woman, nor was he ethically or legally obligated to do so. An individual’s wish to be treated by a particular psychiatrist does not create a duty for the psychiatrist to satisfy this wish.10 State-based “Good Samaritan” laws encourage physicians to assist those in acute need by shielding them from liability, as long as they reasonably act within the scope of their expertise.11 However, they do not require a physician to care for an individual in acute need. A delusional wish for treatment or a false belief of already being in treatment does not create a duty to care for a person.
OUTCOME: Seeking help
Ms. I’s phone calls and letters continue. The resident discusses the situation with his associate residency director, who refers him to the hospital’s legal and investigative staffs. Based on advice from the hospital’s private investigator, the resident sends Ms. I a formal “cease and desist” letter that threatens her with legal action and possible jail time. The staff at the front desk of the clinic where the resident works and the hospital’s security department are instructed to watch for a visitor with Ms. I’s name and description, although the hospital’s investigator is unable to obtain a photograph of her. Shortly after the resident sends the letter, Ms. I ceases communication.
The authors’ observations
This case is unusual because most stalking victims know their stalkers. Identifying a stalker’s motivation can be helpful in formulating a risk assessment. One classification system recognizes 5 categories of stalkers: rejected, intimacy seeking, incompetent, resentful, and predatory (Table 2).1 Rejected stalkers appear to pose the greatest risk of violence and homicide.8 However, all stalkers may pose a risk of violence and therefore all stalking behavior should be treated seriously.
Table 2
Classification of stalkers
| Category | Common features |
|---|---|
| Rejected | Most have a personality disorder; often seeking reconciliation and revenge; most frequent victims are ex-romantic partners, but also target estranged relatives, former friends |
| Intimacy seeking | Erotomania; “morbid infatuation” |
| Incompetent | Lacking social skills; often have stalked others |
| Resentful | Pursuing a vendetta; generally feeling aggrieved |
| Predatory | Often comorbid with paraphilias; may have past convictions for sex offenses |
| Source: Adapted from reference 1 | |
Responding to a stalker
The approach should be tailored to the stalker’s characteristics.12 Silence—ie, lack of acknowledgement of a stalker’s intrusions—is one tactic.13 Consistent and persistent lack of engagement may bore the stalker, but also may provoke frustration or narcissistic or paranoia-fueled rage, and increased efforts to interact with the mental health professional. Other responses include:
- obtaining a protection or restraining order
- promoting the stalker’s participation in adversarial civil litigation, such as a lawsuit
- issuing verbal counterthreats.
Restraining orders are controversial and assessments of their effectiveness vary.14 How well a restraining order works may depend on the stalker’s:
- ability to appreciate reality, and how likely he or she is to experience anxiety when confronted with adverse consequences of his or her actions
- how consistently, rapidly, and harshly the criminal justice system responds to violations of restraining orders.
Restraining orders also may provide the victim a false sense of security.15 One of her letters revealed that Ms. I violated a criminal plea arrangement years earlier, which suggests she was capable of violating a restraining order.
Litigation. A stalker may initiate civil litigation against the victim to feel that he or she has an impact on the victim, which may reduce the stalker’s risk of violence if he or she is emotionally engaged in the litigation. Based on the authors’ experience, as long as the stalker is talking, he or she generally is less likely to act out violently and terminate a satisfying process. Adversarial civil litigation could give a stalker the opportunity to be “close” to the victim and a means of expressing aggressive wishes. The benefit of litigation lasts only as long as the case persists and the stalker believes he or she may prevail. In one of her letters, Ms. I bragged that she had represented herself as a pro se litigant in a complex civil matter, suggesting that she might be constructively channeled into litigation.
Promoting litigation carries significant risk.16 Being a defendant in pro se litigation may be emotionally and financially stressful. This approach may be desirable if the psychiatrist’s institution is willing to offer substantial support. For example, an institution may provide legal assistance—including helping to defray the cost of litigation—and litigation-related scheduling flexibility. An attorney may serve as a boundary between the victim and the pro se litigant’s sometimes ceaseless, time-devouring, anxiety-inducing legal maneuvers.
Counterthreats. Warning a stalker that he or she will face severe civil and criminal consequences if his or her behavior continues can make clear that his or her conduct is unacceptable.17 Such warnings may be delivered verbally or in writing by a legal representative, law enforcement personnel, a private security agent, or the victim.
Issuing a counterthreat can be risky. Stalkers with antisocial or narcissistic personality features may perceive a counterthreat as narcissistically diminishing, and to save face will escalate their stalking in retaliation. Avoid counterthreats if you believe the stalker might be psychotic because destabilizing such an individual—such as by precipitating a short psychotic episode—may increase unpredictability and diminish their responsive to interventions.
Ms. I’s contact with the resident lasted approximately 20 months, slightly less than the average 26 months reported in a survey of mental health professionals.3 Because stalkers are unpredictable, the psychiatric resident remains cautious.
Related Resources
- National Center for Victims of Crime. Stalking resource center. www.victimsofcrime.org/our-programs/stalking-resource-center.
- Mullen PE, Pathé M, Purcell R. Stalkers and their victims. New York, NY: Cambridge University Press; 2009.
Disclosure
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Mullen PE, Pathé M, Purcell R, et al. Study of stalkers. Am J Psychiatry. 1999;156(8):1244-1249.
2. Sandberg DA, McNiel DE, Binder RL. Stalking threatening, and harassing behavior by psychiatric patients toward clinicians. J Am Acad Psychiatry Law. 2002;30(2):221-229.
3. McIvor R, Potter L, Davies L. Stalking behavior by patients towards psychiatrists in a large mental health organization. Int J Soc Psychiatry. 2008;54(4):350-357.
4. Mullen PE, Pathé M. Stalking. Crime and Justice. 2002;29:273-318.
5. Bird S. Strategies for managing and minimizing the impact of harassment and stalking by patients. ANZ J Surg. 2009;79(7-8):537-538.
6. Sinwelski SA, Vinton L. Stalking: the constant threat of violence. Affilia. 2001;16(1):46-65.
7. Meloy JR. Commentary: stalking threatening, and harassing behavior by patients—the risk-management response. J Am Acad Psychiatry Law. 2002;30(2):230-231.
8. Resnick PJ. Stalking risk assessment. In: Pinals DA, ed. Stalking: psychiatric perspectives and practical approaches. New York, NY: Oxford University Press; 2007:61–84.
9. Dietz PE. Defenses against dangerous people when arrest and commitment fail. In: Simon RI, ed. American Psychiatric Press review of clinical psychiatry and the law. 1st ed. Washington, DC: American Psychiatric Press; 1989:205–219.
10. Hilliard J. Termination of treatment with troublesome patients. In: Lifson LE, Simon RI, eds. The mental health practitioner and the law: a comprehensive handbook. Cambridge, MA: Harvard University Press; 1998:216–224.
11. Paterick TJ, Paterick BB, Paterick TE. Implications of Good Samaritan laws for physicians. J Med Pract Manage. 2008;23(6):372-375.
12. MacKenzie RD, James DV. Management and treatment of stalkers: problems options, and solutions. Behav Sci Law. 2011;29(2):220-239.
13. Fremouw WJ, Westrup D, Pennypacker J. Stalking on campus: the prevalence and strategies for coping with stalking. J Forensic Sci. 1997;42(4):666-669.
14. Nicastro AM, Cousins AV, Spitzberg BH. The tactical face of stalking. Journal of Criminal Justice. 2000;28(1):69-82.
15. Spitzberg BH. The tactical topography of stalking victimization and management. Trauma Violence Abuse. 2002;3(4):261-288.
16. Pathé M, MacKenzie R, Mullen PE. Stalking by law: damaging victims and rewarding offenders. J Law Med. 2004;12(1):103-111.
17. Lion JR, Herschler JA. The stalking of physicians by their patients. In: Meloy JR. The psychology of stalking: clinical and forensic perspectives. San Diego, CA: Academic Press; 1998:163–173.
CASE: Delusions and threats
For over 20 months, Ms. I, age 48, sends a psychiatric resident letters and postcards that total approximately 3,000 pages and come from dozens of return addresses. Ms. I expresses romantic feelings toward the resident and believes that he was her physician and prescribed medications, including “mood stabilizers.” The resident never treated Ms. I; to his knowledge, he has never interacted with her.
Ms. I describes the resident’s refusal to continue treating her as “abandonment” and states that she is contemplating self-harm because of this rejection. In her letters, Ms. I admits that she was a long-term patient in a state psychiatric hospital in her home state and suffers from persistent auditory hallucinations. She also wants a romantic relationship with the resident and repeatedly threatens the resident’s female acquaintances and former romantic partners whose relationships she had surmised from news articles available on the Internet. Ms. I also threatens to strangle the resident. The resident sends her multiple written requests that she cease contact, but they are not acknowledged.
The authors’ observations
Stalking—repeated, unwanted attention or communication that would cause a reasonable person fear—is a serious threat for many psychiatric clinicians.1 Prevalence rates among mental health care providers range from 3% to 21%.2,3 Most stalkers have engaged in previous stalking behavior.3
Being stalked is highly distressing,4 and mental health professionals often do not reveal such experiences to colleagues.5 Irrational feelings of guilt or embarrassment, such as being thought to have poorly managed interactions with the stalker, often motivate a self-imposed silence (Table 1).6 This isolation may foster anxiety, interfere with receiving problem-solving advice, and increase physical vulnerability. In the case involving Ms. I, the psychiatric resident’s primary responsibility is safeguarding his own physical and psychological welfare.
Clinicians who work in a hospital or other institutional setting who are being stalked should inform their supervisors and the facility’s security personnel. Security personnel may be able to gather data about the stalker, decrease the stalker’s ability to communicate with the victim, and reduce unwanted physical access to the victim by distributing a photo of the stalker or installing a camera or receptionist-controlled door lock in patient entryways. Security personnel also may collaborate with local law enforcement. Having a third party respond to a stalker’s aggressive behavior—rather than the victim responding directly—avoids rewarding the stalker, which may generate further unwanted contact.7 Any intervention by the victim may increase the risk of violence, creating an “intervention dilemma.” Resnick8 argues that before deciding how best to address the stalker’s behavior, a stalking victim must “first separate the risk of continued stalking from the risk that the stalker will commit a violent act.”
Mental health professionals in private practice who are being stalked should consider retaining an attorney. An attorney often can maintain privacy of communications regarding the stalker via the attorney-client and attorney-work product privileges, which may help during legal proceedings.
Table 1
Factors that can impede psychiatrists from reporting stalking
| Fear of being perceived as a failure |
| Embarrassment |
| High professional tolerance for antisocial and threatening behavior |
| Misplaced sense of duty |
| Source: Reference 6 |
RESPONSE: Involving police
Over 2 months, Ms. I phones the resident’s home 105 times (the resident screens the calls). During 1 call, she states that she is hidden in a closet in her home and will hurt herself unless the resident “resumes” her psychiatric care. The resident contacts police in his city and Ms. I’s community, but authorities are reluctant to act when he acknowledges that he is not Ms. I’s psychiatrist and does not know her. Police officers in Ms. I’s hometown tell the resident no one answered the door when they visited her home. They state that they would enter the residence forcibly only if Ms. I’s physician or a family member asked them to do so, and because the resident admits that he is not her psychiatrist, they cannot take further action. Ms. I leaves the resident a phone message several hours later to inform him she is safe.
The authors’ observations
Stalking-induced countertransference responses may lead a psychiatrist to unwittingly place himself in harm’s way. For example, intense rage at a stalker’s request for treatment may generate guilt that motivates the psychiatrist to agree to treat the stalker. Feelings of helplessness may produce a frantic desire to do something even when such activity is ill-advised. Psychiatrists may develop a tolerance for antisocial or threatening behavior—which is common in mental health settings—and could accept unnecessary risks.
A psychiatrist who is being stalked may be able to assist a mentally ill stalker in a way that does not create a duty to treat and does not expose the psychiatrist to harm, such as contacting a mobile crisis intervention team, a mental health professional who recently treated the stalker, a family member of the stalker, or law enforcement personnel. A psychiatrist who is thrust from the role of helper to victim and must protect his or her own well-being instead of attending to a patient’s welfare is prone to suffer substantial countertransference distress.
The situation with Ms. I was particularly challenging because the resident did not know her complete history and therefore had little information to gauge how likely she was to act on her aggressive threats. Factors that predict future violence include:
- a history of violence
- significant prior criminality
- young age at first arrest
- concomitant substance abuse
- male sex.9
Unfortunately, other than sex, this data regarding Ms. I could not be readily obtained.
A psychiatrist’s duty
Although sympathetic to his stalker’s distress, the resident did not want to treat this woman, nor was he ethically or legally obligated to do so. An individual’s wish to be treated by a particular psychiatrist does not create a duty for the psychiatrist to satisfy this wish.10 State-based “Good Samaritan” laws encourage physicians to assist those in acute need by shielding them from liability, as long as they reasonably act within the scope of their expertise.11 However, they do not require a physician to care for an individual in acute need. A delusional wish for treatment or a false belief of already being in treatment does not create a duty to care for a person.
OUTCOME: Seeking help
Ms. I’s phone calls and letters continue. The resident discusses the situation with his associate residency director, who refers him to the hospital’s legal and investigative staffs. Based on advice from the hospital’s private investigator, the resident sends Ms. I a formal “cease and desist” letter that threatens her with legal action and possible jail time. The staff at the front desk of the clinic where the resident works and the hospital’s security department are instructed to watch for a visitor with Ms. I’s name and description, although the hospital’s investigator is unable to obtain a photograph of her. Shortly after the resident sends the letter, Ms. I ceases communication.
The authors’ observations
This case is unusual because most stalking victims know their stalkers. Identifying a stalker’s motivation can be helpful in formulating a risk assessment. One classification system recognizes 5 categories of stalkers: rejected, intimacy seeking, incompetent, resentful, and predatory (Table 2).1 Rejected stalkers appear to pose the greatest risk of violence and homicide.8 However, all stalkers may pose a risk of violence and therefore all stalking behavior should be treated seriously.
Table 2
Classification of stalkers
| Category | Common features |
|---|---|
| Rejected | Most have a personality disorder; often seeking reconciliation and revenge; most frequent victims are ex-romantic partners, but also target estranged relatives, former friends |
| Intimacy seeking | Erotomania; “morbid infatuation” |
| Incompetent | Lacking social skills; often have stalked others |
| Resentful | Pursuing a vendetta; generally feeling aggrieved |
| Predatory | Often comorbid with paraphilias; may have past convictions for sex offenses |
| Source: Adapted from reference 1 | |
Responding to a stalker
The approach should be tailored to the stalker’s characteristics.12 Silence—ie, lack of acknowledgement of a stalker’s intrusions—is one tactic.13 Consistent and persistent lack of engagement may bore the stalker, but also may provoke frustration or narcissistic or paranoia-fueled rage, and increased efforts to interact with the mental health professional. Other responses include:
- obtaining a protection or restraining order
- promoting the stalker’s participation in adversarial civil litigation, such as a lawsuit
- issuing verbal counterthreats.
Restraining orders are controversial and assessments of their effectiveness vary.14 How well a restraining order works may depend on the stalker’s:
- ability to appreciate reality, and how likely he or she is to experience anxiety when confronted with adverse consequences of his or her actions
- how consistently, rapidly, and harshly the criminal justice system responds to violations of restraining orders.
Restraining orders also may provide the victim a false sense of security.15 One of her letters revealed that Ms. I violated a criminal plea arrangement years earlier, which suggests she was capable of violating a restraining order.
Litigation. A stalker may initiate civil litigation against the victim to feel that he or she has an impact on the victim, which may reduce the stalker’s risk of violence if he or she is emotionally engaged in the litigation. Based on the authors’ experience, as long as the stalker is talking, he or she generally is less likely to act out violently and terminate a satisfying process. Adversarial civil litigation could give a stalker the opportunity to be “close” to the victim and a means of expressing aggressive wishes. The benefit of litigation lasts only as long as the case persists and the stalker believes he or she may prevail. In one of her letters, Ms. I bragged that she had represented herself as a pro se litigant in a complex civil matter, suggesting that she might be constructively channeled into litigation.
Promoting litigation carries significant risk.16 Being a defendant in pro se litigation may be emotionally and financially stressful. This approach may be desirable if the psychiatrist’s institution is willing to offer substantial support. For example, an institution may provide legal assistance—including helping to defray the cost of litigation—and litigation-related scheduling flexibility. An attorney may serve as a boundary between the victim and the pro se litigant’s sometimes ceaseless, time-devouring, anxiety-inducing legal maneuvers.
Counterthreats. Warning a stalker that he or she will face severe civil and criminal consequences if his or her behavior continues can make clear that his or her conduct is unacceptable.17 Such warnings may be delivered verbally or in writing by a legal representative, law enforcement personnel, a private security agent, or the victim.
Issuing a counterthreat can be risky. Stalkers with antisocial or narcissistic personality features may perceive a counterthreat as narcissistically diminishing, and to save face will escalate their stalking in retaliation. Avoid counterthreats if you believe the stalker might be psychotic because destabilizing such an individual—such as by precipitating a short psychotic episode—may increase unpredictability and diminish their responsive to interventions.
Ms. I’s contact with the resident lasted approximately 20 months, slightly less than the average 26 months reported in a survey of mental health professionals.3 Because stalkers are unpredictable, the psychiatric resident remains cautious.
Related Resources
- National Center for Victims of Crime. Stalking resource center. www.victimsofcrime.org/our-programs/stalking-resource-center.
- Mullen PE, Pathé M, Purcell R. Stalkers and their victims. New York, NY: Cambridge University Press; 2009.
Disclosure
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
CASE: Delusions and threats
For over 20 months, Ms. I, age 48, sends a psychiatric resident letters and postcards that total approximately 3,000 pages and come from dozens of return addresses. Ms. I expresses romantic feelings toward the resident and believes that he was her physician and prescribed medications, including “mood stabilizers.” The resident never treated Ms. I; to his knowledge, he has never interacted with her.
Ms. I describes the resident’s refusal to continue treating her as “abandonment” and states that she is contemplating self-harm because of this rejection. In her letters, Ms. I admits that she was a long-term patient in a state psychiatric hospital in her home state and suffers from persistent auditory hallucinations. She also wants a romantic relationship with the resident and repeatedly threatens the resident’s female acquaintances and former romantic partners whose relationships she had surmised from news articles available on the Internet. Ms. I also threatens to strangle the resident. The resident sends her multiple written requests that she cease contact, but they are not acknowledged.
The authors’ observations
Stalking—repeated, unwanted attention or communication that would cause a reasonable person fear—is a serious threat for many psychiatric clinicians.1 Prevalence rates among mental health care providers range from 3% to 21%.2,3 Most stalkers have engaged in previous stalking behavior.3
Being stalked is highly distressing,4 and mental health professionals often do not reveal such experiences to colleagues.5 Irrational feelings of guilt or embarrassment, such as being thought to have poorly managed interactions with the stalker, often motivate a self-imposed silence (Table 1).6 This isolation may foster anxiety, interfere with receiving problem-solving advice, and increase physical vulnerability. In the case involving Ms. I, the psychiatric resident’s primary responsibility is safeguarding his own physical and psychological welfare.
Clinicians who work in a hospital or other institutional setting who are being stalked should inform their supervisors and the facility’s security personnel. Security personnel may be able to gather data about the stalker, decrease the stalker’s ability to communicate with the victim, and reduce unwanted physical access to the victim by distributing a photo of the stalker or installing a camera or receptionist-controlled door lock in patient entryways. Security personnel also may collaborate with local law enforcement. Having a third party respond to a stalker’s aggressive behavior—rather than the victim responding directly—avoids rewarding the stalker, which may generate further unwanted contact.7 Any intervention by the victim may increase the risk of violence, creating an “intervention dilemma.” Resnick8 argues that before deciding how best to address the stalker’s behavior, a stalking victim must “first separate the risk of continued stalking from the risk that the stalker will commit a violent act.”
Mental health professionals in private practice who are being stalked should consider retaining an attorney. An attorney often can maintain privacy of communications regarding the stalker via the attorney-client and attorney-work product privileges, which may help during legal proceedings.
Table 1
Factors that can impede psychiatrists from reporting stalking
| Fear of being perceived as a failure |
| Embarrassment |
| High professional tolerance for antisocial and threatening behavior |
| Misplaced sense of duty |
| Source: Reference 6 |
RESPONSE: Involving police
Over 2 months, Ms. I phones the resident’s home 105 times (the resident screens the calls). During 1 call, she states that she is hidden in a closet in her home and will hurt herself unless the resident “resumes” her psychiatric care. The resident contacts police in his city and Ms. I’s community, but authorities are reluctant to act when he acknowledges that he is not Ms. I’s psychiatrist and does not know her. Police officers in Ms. I’s hometown tell the resident no one answered the door when they visited her home. They state that they would enter the residence forcibly only if Ms. I’s physician or a family member asked them to do so, and because the resident admits that he is not her psychiatrist, they cannot take further action. Ms. I leaves the resident a phone message several hours later to inform him she is safe.
The authors’ observations
Stalking-induced countertransference responses may lead a psychiatrist to unwittingly place himself in harm’s way. For example, intense rage at a stalker’s request for treatment may generate guilt that motivates the psychiatrist to agree to treat the stalker. Feelings of helplessness may produce a frantic desire to do something even when such activity is ill-advised. Psychiatrists may develop a tolerance for antisocial or threatening behavior—which is common in mental health settings—and could accept unnecessary risks.
A psychiatrist who is being stalked may be able to assist a mentally ill stalker in a way that does not create a duty to treat and does not expose the psychiatrist to harm, such as contacting a mobile crisis intervention team, a mental health professional who recently treated the stalker, a family member of the stalker, or law enforcement personnel. A psychiatrist who is thrust from the role of helper to victim and must protect his or her own well-being instead of attending to a patient’s welfare is prone to suffer substantial countertransference distress.
The situation with Ms. I was particularly challenging because the resident did not know her complete history and therefore had little information to gauge how likely she was to act on her aggressive threats. Factors that predict future violence include:
- a history of violence
- significant prior criminality
- young age at first arrest
- concomitant substance abuse
- male sex.9
Unfortunately, other than sex, this data regarding Ms. I could not be readily obtained.
A psychiatrist’s duty
Although sympathetic to his stalker’s distress, the resident did not want to treat this woman, nor was he ethically or legally obligated to do so. An individual’s wish to be treated by a particular psychiatrist does not create a duty for the psychiatrist to satisfy this wish.10 State-based “Good Samaritan” laws encourage physicians to assist those in acute need by shielding them from liability, as long as they reasonably act within the scope of their expertise.11 However, they do not require a physician to care for an individual in acute need. A delusional wish for treatment or a false belief of already being in treatment does not create a duty to care for a person.
OUTCOME: Seeking help
Ms. I’s phone calls and letters continue. The resident discusses the situation with his associate residency director, who refers him to the hospital’s legal and investigative staffs. Based on advice from the hospital’s private investigator, the resident sends Ms. I a formal “cease and desist” letter that threatens her with legal action and possible jail time. The staff at the front desk of the clinic where the resident works and the hospital’s security department are instructed to watch for a visitor with Ms. I’s name and description, although the hospital’s investigator is unable to obtain a photograph of her. Shortly after the resident sends the letter, Ms. I ceases communication.
The authors’ observations
This case is unusual because most stalking victims know their stalkers. Identifying a stalker’s motivation can be helpful in formulating a risk assessment. One classification system recognizes 5 categories of stalkers: rejected, intimacy seeking, incompetent, resentful, and predatory (Table 2).1 Rejected stalkers appear to pose the greatest risk of violence and homicide.8 However, all stalkers may pose a risk of violence and therefore all stalking behavior should be treated seriously.
Table 2
Classification of stalkers
| Category | Common features |
|---|---|
| Rejected | Most have a personality disorder; often seeking reconciliation and revenge; most frequent victims are ex-romantic partners, but also target estranged relatives, former friends |
| Intimacy seeking | Erotomania; “morbid infatuation” |
| Incompetent | Lacking social skills; often have stalked others |
| Resentful | Pursuing a vendetta; generally feeling aggrieved |
| Predatory | Often comorbid with paraphilias; may have past convictions for sex offenses |
| Source: Adapted from reference 1 | |
Responding to a stalker
The approach should be tailored to the stalker’s characteristics.12 Silence—ie, lack of acknowledgement of a stalker’s intrusions—is one tactic.13 Consistent and persistent lack of engagement may bore the stalker, but also may provoke frustration or narcissistic or paranoia-fueled rage, and increased efforts to interact with the mental health professional. Other responses include:
- obtaining a protection or restraining order
- promoting the stalker’s participation in adversarial civil litigation, such as a lawsuit
- issuing verbal counterthreats.
Restraining orders are controversial and assessments of their effectiveness vary.14 How well a restraining order works may depend on the stalker’s:
- ability to appreciate reality, and how likely he or she is to experience anxiety when confronted with adverse consequences of his or her actions
- how consistently, rapidly, and harshly the criminal justice system responds to violations of restraining orders.
Restraining orders also may provide the victim a false sense of security.15 One of her letters revealed that Ms. I violated a criminal plea arrangement years earlier, which suggests she was capable of violating a restraining order.
Litigation. A stalker may initiate civil litigation against the victim to feel that he or she has an impact on the victim, which may reduce the stalker’s risk of violence if he or she is emotionally engaged in the litigation. Based on the authors’ experience, as long as the stalker is talking, he or she generally is less likely to act out violently and terminate a satisfying process. Adversarial civil litigation could give a stalker the opportunity to be “close” to the victim and a means of expressing aggressive wishes. The benefit of litigation lasts only as long as the case persists and the stalker believes he or she may prevail. In one of her letters, Ms. I bragged that she had represented herself as a pro se litigant in a complex civil matter, suggesting that she might be constructively channeled into litigation.
Promoting litigation carries significant risk.16 Being a defendant in pro se litigation may be emotionally and financially stressful. This approach may be desirable if the psychiatrist’s institution is willing to offer substantial support. For example, an institution may provide legal assistance—including helping to defray the cost of litigation—and litigation-related scheduling flexibility. An attorney may serve as a boundary between the victim and the pro se litigant’s sometimes ceaseless, time-devouring, anxiety-inducing legal maneuvers.
Counterthreats. Warning a stalker that he or she will face severe civil and criminal consequences if his or her behavior continues can make clear that his or her conduct is unacceptable.17 Such warnings may be delivered verbally or in writing by a legal representative, law enforcement personnel, a private security agent, or the victim.
Issuing a counterthreat can be risky. Stalkers with antisocial or narcissistic personality features may perceive a counterthreat as narcissistically diminishing, and to save face will escalate their stalking in retaliation. Avoid counterthreats if you believe the stalker might be psychotic because destabilizing such an individual—such as by precipitating a short psychotic episode—may increase unpredictability and diminish their responsive to interventions.
Ms. I’s contact with the resident lasted approximately 20 months, slightly less than the average 26 months reported in a survey of mental health professionals.3 Because stalkers are unpredictable, the psychiatric resident remains cautious.
Related Resources
- National Center for Victims of Crime. Stalking resource center. www.victimsofcrime.org/our-programs/stalking-resource-center.
- Mullen PE, Pathé M, Purcell R. Stalkers and their victims. New York, NY: Cambridge University Press; 2009.
Disclosure
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Mullen PE, Pathé M, Purcell R, et al. Study of stalkers. Am J Psychiatry. 1999;156(8):1244-1249.
2. Sandberg DA, McNiel DE, Binder RL. Stalking threatening, and harassing behavior by psychiatric patients toward clinicians. J Am Acad Psychiatry Law. 2002;30(2):221-229.
3. McIvor R, Potter L, Davies L. Stalking behavior by patients towards psychiatrists in a large mental health organization. Int J Soc Psychiatry. 2008;54(4):350-357.
4. Mullen PE, Pathé M. Stalking. Crime and Justice. 2002;29:273-318.
5. Bird S. Strategies for managing and minimizing the impact of harassment and stalking by patients. ANZ J Surg. 2009;79(7-8):537-538.
6. Sinwelski SA, Vinton L. Stalking: the constant threat of violence. Affilia. 2001;16(1):46-65.
7. Meloy JR. Commentary: stalking threatening, and harassing behavior by patients—the risk-management response. J Am Acad Psychiatry Law. 2002;30(2):230-231.
8. Resnick PJ. Stalking risk assessment. In: Pinals DA, ed. Stalking: psychiatric perspectives and practical approaches. New York, NY: Oxford University Press; 2007:61–84.
9. Dietz PE. Defenses against dangerous people when arrest and commitment fail. In: Simon RI, ed. American Psychiatric Press review of clinical psychiatry and the law. 1st ed. Washington, DC: American Psychiatric Press; 1989:205–219.
10. Hilliard J. Termination of treatment with troublesome patients. In: Lifson LE, Simon RI, eds. The mental health practitioner and the law: a comprehensive handbook. Cambridge, MA: Harvard University Press; 1998:216–224.
11. Paterick TJ, Paterick BB, Paterick TE. Implications of Good Samaritan laws for physicians. J Med Pract Manage. 2008;23(6):372-375.
12. MacKenzie RD, James DV. Management and treatment of stalkers: problems options, and solutions. Behav Sci Law. 2011;29(2):220-239.
13. Fremouw WJ, Westrup D, Pennypacker J. Stalking on campus: the prevalence and strategies for coping with stalking. J Forensic Sci. 1997;42(4):666-669.
14. Nicastro AM, Cousins AV, Spitzberg BH. The tactical face of stalking. Journal of Criminal Justice. 2000;28(1):69-82.
15. Spitzberg BH. The tactical topography of stalking victimization and management. Trauma Violence Abuse. 2002;3(4):261-288.
16. Pathé M, MacKenzie R, Mullen PE. Stalking by law: damaging victims and rewarding offenders. J Law Med. 2004;12(1):103-111.
17. Lion JR, Herschler JA. The stalking of physicians by their patients. In: Meloy JR. The psychology of stalking: clinical and forensic perspectives. San Diego, CA: Academic Press; 1998:163–173.
1. Mullen PE, Pathé M, Purcell R, et al. Study of stalkers. Am J Psychiatry. 1999;156(8):1244-1249.
2. Sandberg DA, McNiel DE, Binder RL. Stalking threatening, and harassing behavior by psychiatric patients toward clinicians. J Am Acad Psychiatry Law. 2002;30(2):221-229.
3. McIvor R, Potter L, Davies L. Stalking behavior by patients towards psychiatrists in a large mental health organization. Int J Soc Psychiatry. 2008;54(4):350-357.
4. Mullen PE, Pathé M. Stalking. Crime and Justice. 2002;29:273-318.
5. Bird S. Strategies for managing and minimizing the impact of harassment and stalking by patients. ANZ J Surg. 2009;79(7-8):537-538.
6. Sinwelski SA, Vinton L. Stalking: the constant threat of violence. Affilia. 2001;16(1):46-65.
7. Meloy JR. Commentary: stalking threatening, and harassing behavior by patients—the risk-management response. J Am Acad Psychiatry Law. 2002;30(2):230-231.
8. Resnick PJ. Stalking risk assessment. In: Pinals DA, ed. Stalking: psychiatric perspectives and practical approaches. New York, NY: Oxford University Press; 2007:61–84.
9. Dietz PE. Defenses against dangerous people when arrest and commitment fail. In: Simon RI, ed. American Psychiatric Press review of clinical psychiatry and the law. 1st ed. Washington, DC: American Psychiatric Press; 1989:205–219.
10. Hilliard J. Termination of treatment with troublesome patients. In: Lifson LE, Simon RI, eds. The mental health practitioner and the law: a comprehensive handbook. Cambridge, MA: Harvard University Press; 1998:216–224.
11. Paterick TJ, Paterick BB, Paterick TE. Implications of Good Samaritan laws for physicians. J Med Pract Manage. 2008;23(6):372-375.
12. MacKenzie RD, James DV. Management and treatment of stalkers: problems options, and solutions. Behav Sci Law. 2011;29(2):220-239.
13. Fremouw WJ, Westrup D, Pennypacker J. Stalking on campus: the prevalence and strategies for coping with stalking. J Forensic Sci. 1997;42(4):666-669.
14. Nicastro AM, Cousins AV, Spitzberg BH. The tactical face of stalking. Journal of Criminal Justice. 2000;28(1):69-82.
15. Spitzberg BH. The tactical topography of stalking victimization and management. Trauma Violence Abuse. 2002;3(4):261-288.
16. Pathé M, MacKenzie R, Mullen PE. Stalking by law: damaging victims and rewarding offenders. J Law Med. 2004;12(1):103-111.
17. Lion JR, Herschler JA. The stalking of physicians by their patients. In: Meloy JR. The psychology of stalking: clinical and forensic perspectives. San Diego, CA: Academic Press; 1998:163–173.