User login
Prasugrel for acute coronary syndromes: Faster, more potent, but higher bleeding risk
Prasugrel (Effient) is more potent and consistent in its effects than clopidogrel (Plavix), thus preventing more thrombotic events—but at a price of more bleeding. Therefore, the drugs must be appropriately selected for the individual patient.
Over the last 9 years, the thienopyridines—ticlopidine (Ticlid), clopidogrel, and now prasugrel—have become essential tools for treating acute coronary syndromes.
The usual underlying mechanism of acute coronary syndromes is thrombosis, caused by rupture of atherosclerotic plaque.1 Accordingly, antithrombotic agents—aspirin, heparin, lowmolecular-weight heparin, glycoprotein IIb/IIIa inhibitors, the direct thrombin inhibitor bivalirudin (Angiomax), and thienopyridines—have all been shown to reduce the risk of major adverse cardiac outcomes in this setting.
In this article, we review the pharmacology and evidence of effectiveness of the thienopyridine drugs, focusing on prasugrel, the latest thienopyridine to be approved by the US Food and Drug Administration (FDA).
THIENOPYRIDINES INHIBIT PLATELET ACTIVATION AND AGGREGATION
Thienopyridines are prodrugs that require conversion by hepatic cytochrome P450 enzymes. The active metabolites bind irreversibly to platelet P2Y12 receptors. Consequently, they permanently block signalling mediated by platelet adenosine diphosphate-P2Y12 receptors, thereby inhibiting glycoprotein IIb/IIIa receptor activation and platelet aggregation.
Aspirin, in contrast, inhibits platelets by blocking the thromboxane-mediated pathway. Therefore, the combination of aspirin plus a thienopyridine has an additive effect.2
The effect of thienopyridines on platelets is irreversible. Therefore, although the half-life of prasugrel’s active metabolite is 3.7 hours, its inhibitory effects last for 96 hours, essentially the time for half the body’s circulating platelets to be replaced.
TICLOPIDINE, THE FIRST THIENOPYRIDINE
Ticlopidine was the first thienopyridine to be approved by the FDA. Its initial studies in unstable angina were small, their designs did not call for patients to concurrently receive aspirin, and all they showed was that ticlopidine was about as beneficial as aspirin. Consequently, the studies had little impact on clinical practice.3
In a pivotal trial,4 patients who received coronary stents were randomized to afterward receive either the combination of ticlopidine plus aspirin or anticoagulation therapy with heparin, phenprocoumon (a coumarin derivative available in Europe), and aspirin. At 30 days, an ischemic complication (death, myocardial infarction [MI], repeat intervention) had occurred in 6.2% of the anticoagulation therapy group vs 1.6% of the ticlopidine group, a risk reduction of 75%. Rates of stent occlusion, MI, and revascularization were 80% to 85% lower in the ticlodipine group. This study paved the way for widespread use of thienopyridines.
Ticlopidine’s use was limited, however, by a 2.4% incidence of serious granulocytopenia and rare cases of thrombocytopenic purpura.
BENEFIT OF CLOPIDOGREL
Although prasugrel is the focus of this review, the trials of prasugrel all compared its efficacy with that of clopidogrel. Furthermore, many patients should still receive clopidogrel and not prasugrel, so it is important to be familiar with the evidence of clopidogrel’s benefit.
Once approved for clinical use, clopidogrel was substituted for ticlopidine in patients undergoing coronary stenting on the basis of studies showing it to be at least as effective as ticlopidine and more tolerable. A series of trials of clopidogrel were done in patients across a spectrum of risk groups, from those at high risk of coronary heart disease to those presenting with ST-elevation MI. The time of pretreatment in the studies ranged from 3 hours to 6 days before percutaneous coronary intervention, and the duration of treatment following intervention ranged from 30 days to 1 year.
Clopidogrel in non-ST-elevation acute coronary syndromes
The CURE trial2 (Clopidogrel in Unstable Angina to Prevent Recurrent Events), published in 2001, established clopidogrel as a therapy for unstable ischemic syndromes, whether treated medically or with revascularization. In that trial, 12,562 patients with acute coronary syndromes without ST elevation (ie, unstable angina or non-ST-elevation MI), as defined by electrocardiographic changes or positive cardiac markers, were randomized to receive clopidogrel (a 300-mg loading dose followed by 75-mg maintenance doses) or placebo for a mean duration of 9 months. All patients also received aspirin 75 mg to 325 mg daily.
The composite outcome of death from cardiovascular causes, nonfatal MI, or stroke occurred in 20% fewer patients treated with clopidogrel than with placebo (9.3% vs 11.4%). The benefit was similar in patients undergoing revascularization compared with those treated medically.
Although there were significantly more cases of major bleeding in the clopidogrel group than in the placebo group (3.7% vs 2.7%), the number of episodes of life-threatening bleeding or hemorrhagic strokes was the same.
PCI-CURE5 was a substudy of the CURE trial in patients who underwent a percutaneous coronary intervention. Patients were pretreated with clopidogrel or placebo for a mean of 6 days before the procedure. Afterward, they all received clopidogrel plus aspirin in an unblinded fashion for 2 to 4 weeks, and then the randomized study drug was resumed for a mean of 8 months.
Significantly fewer adverse events occurred in the clopidogrel group as tallied at the time of the intervention, 1 month later, and 8 months later.
Clopidogrel in ST-elevation acute MI
The CLARITY-TIMI 28 trial6 (Clopidogrel as Adjunctive Reperfusion Therapy—Thrombolysis in Myocardial Infarction 28) showed that adding clopidogrel (a 300-mg loading dose, then 75 mg daily) to aspirin benefitted patients with ST-elevation MI receiving fibrinolytic therapy. At 30 days, cardiovascular death, recurrent MI, or urgent revascularization had occurred in 11.6% of the clopidogrel group vs 14.1% of the placebo group, a statistically significant difference. The rates of major or minor bleeding were no higher in the clopidogrel group than in the placebo group, an especially remarkable finding in patients receiving thrombolytic therapy.
PCI-CLARITY.7 About half of the patients in the CLARITY trial ultimately underwent a percutaneous coronary intervention after fibrinolytic therapy, with results reported as the PCI-CLARITY substudy. Like those in PCI-CURE, these patients were randomized to receive pretreatment with either clopidogrel or placebo before the procedure, in this study for a median of 3 days. Both groups received clopidogrel afterward. At 30 days from randomization, the outcome of cardiovascular death, MI, or stroke had occurred in 7.5% of the clopidogrel group compared with 12.0% of the placebo group, which was statistically significant, without any significant excess in the rates of major or minor bleeding.
COMMIT8 (the Clopidogrel and Metoprolol in Myocardial Infarction Trial) also showed clopidogrel to be beneficial in patients with acute MI. This trial included more than 45,000 patients in China with acute MI, 93% of whom had ST-segment elevation. In contrast to CLARITY, in COMMIT barely more than half of the patients received fibrinolysis, fewer than 5% proceeded to percutaneous interventions, and no loading dose was given: patients in the clopidogrel group received 75 mg/day from the outset.
At 15 days, the incidence of death, reinfarction, or stroke was 9.2% with clopidogrel compared with 10.1% with placebo, a small but statistically significant difference. Again, the rate of major bleeding was not significantly higher, either overall or in patients over age 70.
Of note, patients over age 75 were excluded from CLARITY, and as mentioned, no loading dose was used in COMMIT. Thus, for patients receiving fibrinolysis who are over age 75, there is no evidence to support the safety of a loading dose, and clopidogrel should be started at 75 mg daily.
Clopidogrel in elective percutaneous coronary intervention
The CREDO trial9 (Clopidogrel for the Reduction of Events During Observation) was in patients referred for elective percutaneous coronary intervention. Three to 24 hours before the procedure, the patients received either a 300-mg loading dose of clopidogrel or placebo; afterward, all patients received clopidogrel 75 mg/day for 28 days. All patients also received aspirin.
A clopidogrel loading dose 3 to 24 hours before the intervention did not produce a statistically significant reduction in ischemic events, although a post hoc subgroup analysis suggested that patients who received the loading dose between 6 and 24 hours before did benefit, with a relative risk reduction of 38.6% in the composite end point (P = .051).
After 28 days, the patients who had received the clopidogrel loading dose were continued on clopidogrel, while those in the placebo group were switched back to placebo. At 1 year, the investigators found a significantly lower rate of the composite end point with the prolonged course of clopidogrel (8.5% vs 11.5%).
In summary, these studies found clopidogrel to be beneficial in a broad spectrum of coronary diseases. Subgroup analyses suggest that pretreatment before percutaneous coronary intervention provides additional benefit, particularly if clopidogrel is given at least 6 hours in advance (the time necessary for clopidogrel to cause substantial platelet inhibition).
SOME PATIENTS RESPOND LESS TO CLOPIDOGREL
The level of platelet inhibition induced by clopidogrel varies. In different studies, the frequency of clopidogrel “nonresponsiveness” ranged from 5% to 56% of patients, depending on which test and which cutoff values were used. The distribution of responses to clopidogrel is wide and fits a normal gaussian curve.10
A large fraction of the population carries a gene that may account for some of the interpatient variation in platelet inhibition with clopidogrel. Carriers of a reduced-function CYP2C19 allele—approximately 30% of people in one study—have significantly lower levels of the active metabolite of clopidogrel, less platelet inhibition from clopidogrel therapy, and a 53% higher rate of death from cardiovascular causes, MI, or stroke.11
PRASUGREL, THE NEWEST THIENOPYRIDINE
Prasugrel, FDA-approved in July 2009 for the treatment of acute coronary syndromes, is given in an oral loading dose of 60 mg followed by an oral maintenance dose of 10 mg daily.
Pharmacology of prasugrel vs clopidogrel
As noted previously, the thienopyridines are prodrugs that require hepatic conversion to exert antiplatelet effects.
Metabolism. Prasugrel’s hepatic activation involves a single step, in contrast to the multiple-step process required for activation of clopidogrel. Clopidogrel is primarily hydrolyzed by intestinal and plasma esterases to an inactive terminal metabolite, with the residual unhydrolized drug undergoing a two-step metabolism that depends on cytochrome P450 enzymes. Prasugrel is also extensively hydrolyzed by these esterases, but the intermediate product is then metabolized in a single step to the active sulfhydryl compound, mainly by CYP3A4 and CYP2B6.
Thus, about 80% of an orally absorbed dose of prasugrel is converted to active drug, compared with only 10% to 20% of absorbed clopidogrel.
Time to peak effect. With clopidogrel, maximal inhibition of platelet aggregation occurs 3 to 5 days after starting therapy with 75 mg daily without a loading dose, but within 4 to 6 hours if a loading dose of 300 to 600 mg is given. In contrast, a prasugrel loading dose produces more than 80% of its platelet inhibitory effects by 30 minutes, and peak activity is observed within 4 hours.12 The platelet inhibition induced by prasugrel at 30 minutes after administration is comparable to the peak effect of clopidogrel at 6 hours.13
Dose-response. Prasugrel’s inhibition of platelet aggregation is dose-related.
Prasugrel is about 10 times more potent than clopidogrel and 100 times more potent than ticlopidine. Thus, treatment with 5 mg of prasugrel results in inhibition of platelet activity (distributed in a gaussian curve) very similar to that produced by 75 mg of clopidogrel. On the other hand, even a maintenance dose of 150 mg of clopidogrel inhibits platelet activity to a lesser degree than 10 mg of prasugrel (46% vs 61%),14 so clopidogrel appears to reach a plateau of platelet inhibition that prasugrel can overcome.
At the approved dose of prasugrel, inhibition of platelet aggregation is significantly greater and there are fewer “nonresponders” than with clopidogrel.
Interactions. Drugs that inhibit CYP3A4 do not inhibit the efficacy of prasugrel, but they can inhibit that of clopidogrel. Some commonly used drugs that have this effect are the statins (eg, atorvastain [Lipitor]) and the macrolide antibiotics (eg, erythromycin). Furthermore, whereas proton pump inhibitors have been shown to diminish the effect of clopidogrel by reducing the formation of its active metabolite, no such effect has been noted with prasugrel.
Prasugrel in phase 2 trials: Finding the optimal dosage
A phase 2 trial compared three prasugrel regimens (loading dose/daily maintenance dose of 40 mg/7.5 mg, 60 mg/10 mg, and 60 mg/15 mg) and standard clopidogrel therapy (300 mg/75 mg) in patients undergoing elective or urgent percutaneous coronary intervention.15 No significant difference in outcomes was seen in the groups receiving the three prasugrel regimens. However, more “minimal bleeding events” (defined by the criteria of the TIMI trial16) occurred with high-dose prasugrel than with lower-dose prasugrel or with clopidogrel, leading to use of the intermediate-dose prasugrel regimen (60-mg loading dose, 10-mg daily maintenance) for later trials.
Another phase 2 trial randomized 201 patients undergoing elective percutaneous coronary intervention to receive prasugrel 60 mg/10 mg or clopidogrel 600 mg/150 mg.14 In all patients, the loading dose was given about 1 hour before cardiac catheterization. As soon as 30 minutes after the loading dose, platelet inhibition was superior with prasugrel (31% vs 5% inhibition of platelet aggregation), and it remained significantly higher at 6 hours (75% vs 32%) and during the maintenance phase (61% vs 46%).
Phase 3 trial of prasugrel vs clopidogrel: TRITON-TIMI 38
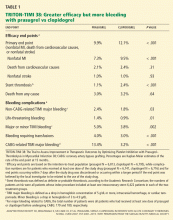
Only one large phase 3 trial of prasugrel has been completed: TRITON-TIMI 38 (the Trial to Assess Improvement in Therapeutic Outcomes by Optimizing Platelet Inhibition With Prasugrel—Thrombolysis in Myocardial Infarction),17 which enrolled adults with moderate-risk to high-risk acute coronary syndromes scheduled to undergo a percutaneous coronary intervention. In this trial, 10,074 patients were enrolled who had moderate-to high-risk unstable angina or non-ST-elevation MI, and 3,534 patients were enrolled who had ST-elevation MI.
Patients were randomized to receive prasugrel (a 60-mg loading dose, then 10 mg daily) or clopidogrel (a 300-mg loading dose, then 75 mg daily) and were treated for 6 to 15 months. All patients also received aspirin.
These benefits came at a price of more bleeding. Of those patients who did not undergo coronary artery bypass grafting, more experienced bleeding in the prasugrel group than in the clopidogrel group (2.4% vs 1.8%, P = .03), including a higher rate of life-threatening bleeding (1.4% vs 0.89%, P = .01) and fatal bleeding (0.4% vs 0.1%, P = .002). More patients discontinued prasugrel because of hemorrhage (2.5% vs 1.4%, P < .001). In patients who proceeded to coronary artery bypass grafting, the rate of major bleeding was more than four times higher in those who received prasugrel than in those who received clopidogrel (13.4% vs 3.2%, P < .001).
A higher rate of adverse events related to colon cancer was also noted in patients treated with prasugrel, although the authors suggest this may have resulted from the stronger antiplatelet effects of prasugrel bringing more tumors to medical attention due to bleeding.
Overall death rates did not differ significantly between the treatment groups.
In a post hoc analysis,18 prasugrel was superior to clopidogrel in preventing ischemic events both during the first 3 days following randomization (the “loading phase”) and for the remainder of the trial (the “maintenance phase”). Whereas bleeding risk was similar with the two drugs during the loading phase, prasugrel was subsequently associated with more bleeding during the maintenance phase.
Certain patient subgroups had no net benefit or even suffered harm from prasugrel compared with clopidogrel.17 Patients with previous stroke or transient ischemic attack had net harm from prasugrel (hazard ratio 1.54, P = .04) and showed a strong trend toward a greater rate of major bleeding (P = .06). Patients age 75 and older and those weighing less than 60 kg had no net benefit from prasugrel.
Cost of prasugrel
Prasugrel is currently priced at 18% more than clopidogrel, with average wholesale prices per pill of $6.65 for prasugrel 10 mg compared with $5.63 for clopidogrel 75 mg. (Prasugrel 10-mg pills cost $6.33 at drugstore.com or $7.60 at CVS; clopidogrel 75-mg pills cost $5.33 at drugstore.com or $6.43 at CVS.) The patent on clopidogrel expires in November 2011, after which the price differential is expected to become significantly greater.
TICAGRELOR, A REVERSIBLE ORAL AGENT
Ticagrelor, the first reversible oral P2Y12 receptor antagonist, is an alternative to thienopyridine therapy for acute coronary syndromes.
Ticagrelor is quickly absorbed, does not require metabolic activation, and has a rapid antiplatelet effect and offset of effect, which closely follow drug-exposure levels. In a large randomized controlled trial in patients with acute coronary syndromes with or without STsegment elevation, treatment with ticagrelor compared with clopidogrel resulted in a significant reduction in death from vascular causes, MI, or stroke (9.8% vs 11.7%).19
Given its reversible effect on platelet inhibition, ticagrelor may be preferred in patients whose coronary anatomy is unknown and for whom coronary artery bypass grafting is deemed probable. It is still undergoing trials and is not yet approved.
TAKE-HOME POINTS
Prasugrel is more potent, more rapid in onset, and more consistent in inhibiting platelet aggregation than clopidogrel. A large clinical trial17 found prasugrel to be superior to clopidogrel for patients with moderate-to high-risk acute coronary syndromes with high probability of undergoing a percutaneous coronary intervention.
Who should receive prasugrel, and how?
Prasugrel should be given after angiography to patients with non-ST-elevation acute coronary syndromes or at presentation to patients with ST-elevation MI. When used for planned percutaneous coronary intervention, prasugrel should be given at least 30 minutes before the intervention, as was done in phase 2 trials (although its routine use in this situation is not recommended—see below).
It is given in a one-time loading dose of 60 mg by mouth and then maintained with 10 mg by mouth once daily for at least 1 year. (At least 9 months of treatment with a thienopyridine is indicated for patients with acute coronary syndromes who are medically treated, and at least 1 year is indicated following urgent or elective percutaneous coronary intervention, including balloon angioplasty and placement of a bare-metal or drug-eluting stent.)
Who should not receive prasugrel?
For now, prasugrel should be avoided in favor of clopidogrel in patients at higher risk of bleeding. It is clearly contraindicated in patients with prior transient ischemic attack or stroke, for whom the risk of serious bleeding seems to be prohibitive. It should generally be avoided in patients age 75 and older, although it might be considered in those at particularly high risk of stent thrombosis, such as those with diabetes or prior MI. In patients weighing less than 60 kg, the package insert advises a reduced dose (5 mg), although clinical evidence for this practice is lacking.
As yet, we have no data assuring that prasugrel is safe to use in combination with fibrinolytic agents, so patients on thrombolytic therapy for acute MI should continue to receive clopidogrel starting immediately after lysis. Furthermore, in patients who proceeded to coronary artery bypass grafting, the rate of major bleeding was more than four times higher in the prasugrel group than in the clopidogrel group in the TRITON-TIMI 38 trial.17 No thienopyridine should be given to patients likely to proceed to coronary artery bypass grafting.
Only clopidogrel has evidence supporting its use as an alternative to aspirin for patients with atherosclerotic disease who cannot tolerate aspirin. Neither drug has evidence for use for primary prevention.
Other areas of uncertainty
Prior to angiography. Indications for prasugrel are currently limited by the narrow scope of the trial data. TRITON-TIMI 38,17 the only large trial completed to date, randomized patients to receive prasugrel only after their coronary anatomy was known, except for ST-elevation MI patients. It is unknown whether the benefits of prasugrel will outweigh the higher risk of bleeding in patients with acute coronary syndromes who do not proceed to percutaneous coronary interventions.
A clinical trial is currently under way comparing prasugrel with clopidogrel in 10,000 patients with acute coronary syndromes who will be medically managed without planned revascularization: A Comparison of Prasugrel and Clopidogrel in Acute Coronary Syndrome Subjects (TRILOGY ACS), ClinicalTrials.gov Identifier: NCT00699998. The trial has an estimated completion date of March 2011.
In cases of non-ST-elevation acute coronary syndrome, it is reasonable to wait to give a thienopyridine until after the coronary anatomy has been defined, if angiography will be completed soon after presentation. For example, a 1-hour delay before giving prasugrel still delivers antiplatelet therapy more quickly than giving clopidogrel on presentation. If longer delays are expected before angiography, however, the patient should be given a loading dose of clopidogrel “up front,” in accordance with guidelines published by the American College of Cardiology, American Heart Association, and European Society of Cardiology,20 which recommend starting a thienopyridine early during hospitalization based on trial data with clopidogrel.
Patients undergoing elective percutaneous coronary intervention are at lower risk of stent thrombosis and other ischemic complications, so it is possible that the benefits of prasugrel would not outweigh the risks in these patients. Thus, prasugrel cannot yet be recommended for routine elective percutaneous coronary intervention except in individual cases in which the interventionalist feels that the patient may be at higher risk of thrombosis.
- Yeghiazarians Y, Braunstein JB, Askari A, Stone PH. Unstable angina pectoris. N Engl J Med 2000; 342:101–114.
- Yusuf S, Zhao F, Mehta SR, Chrolavicius S, Tognoni G, Fox KK; Clopidogrel in Unstable Angina to Prevent Recurrent Events Trial Investigators. Effects of clopidogrel in addition to aspirin in patients with acute coronary syndromes without ST-segment elevation. N Engl J Med 2001; 345:494–502.
- Balsano F, Rizzon P, Violi F, et al. Antiplatelet treatment with ticlopidine in unstable angina. A controlled multicenter clinical trial. The Studio della Ticlopidina nell'Angina Instabile Group. Circulation 1990; 82:17–26.
- Schömig A, Neumann FJ, Kastrati A, et al. A randomized comparison of antiplatelet and anticoagulant therapy after the placement of coronary-artery stents. N Engl J Med 1996; 334:1084–1089.
- Mehta SR, Yusuf S, Peters RJG, et al; Clopidogrel in Unstable Angina to Prevent Recurrent Events Trial (CURE) Investigators. Effects of pretreatment with clopidogrel and aspirin followed by long-term therapy in patients undergoing percutaneous coronary intervention: the PCI-CURE study. Lancet 2001; 358:527–533.
- Sabatine MS, Cannon CP, Gibson CM, et al; CLA RITY-TIMI 28 Investigators. Addition of clopidogrel to aspirin and fibrinolytic therapy for myocardial infarction with STsegment elevation. N Engl J Med 2005; 352:1179–1189.
- Sabatine MS, Cannon CP, Gibson CM, et al; Clopidogrel as Adjunctive Reperfusion Therapy (CLARITY)-Thrombolysis in Myocardial Infarction (TIMI) 28 Investigators. Effect of clopidogrel pretreatment before percutaneous coronary intervention in patients with ST-elevation myocardial infarction treated with fibrinolytics: the PCI-CLARITY study. JAMA 2005: 294:1224–1232.
- Chen ZM, Jiang LX, Chen YP, et al; COMMIT (ClOpidogrel and Metoprolol in Myocardial Infarction Trial) collaborative group. Addition of clopidogrel to aspirin in 45,852 patients with acute myocardial infarction: randomised placebo-controlled trial. Lancet 2005; 366:1607–1621.
- Steinhubl SR, Berger PB, Mann JT, et al; CREDO Investigators. Clopidogrel for the reduction of events during observation. Early and sustained dual oral antiplatelet therapy following percutaneous coronary intervention: a randomized controlled trial. JAMA 2002; 288:2411–2420.
- Serebruany VL, Steinhubl SR, Berger PB, Malinin AI, Bhatt DL, Topol EJ. Variability in platelet responsiveness to clopidogrel among 544 individuals. J Am Coll Cardiol 2005; 45:246–251.
- Mega JL, Close SL, Wiviott SD, et al. Cytochrome P-450 polymorphisms and response to clopidogrel. N Engl J Med 2009; 360:354–362.
- Helft G, Osende JI, Worthley SG, et al. Acute antithrombotic effect of a front-loaded regimen of clopidogrel in patients with atherosclerosis on aspirin. Arterioscler Thromb Vasc Biol 2000; 20:2316–2321.
- Weerakkody GJ, Jakubowski JA, Brandt JT, et al. Comparison of speed of onset of platelet inhibition after loading doses of clopidogrel versus prasugrel in healthy volunteers and correlation with responder status. Am J Cardiol 2007; 100:331–336.
- Wiviott SD, Trenk D, Frelinger AL, et al; PRINCIPLETIMI 44 Investigators. Prasugrel compared with high loading-and maintenance-dose clopidogrel in patients with planned percutaneous coronary intervention: the Prasugrel in Comparison to Clopidogrel for Inhibition of Platelet Activation and Aggregation-Thrombolysis in Myocardial Infarction 44 trial. Circulation 2007; 116:2923–2932.
- Wiviott SD, Antman EM, Winters KJ, et al; JUMBO-TIMI 26 Investigators. Randomized comparison of prasugrel (CS-747, LY640315), a novel thienopyridine P2Y12 antagonist, with clopidogrel in percutaneous coronary intervention: results of the Joint Utilization of Medications to Block Platelets Optimally (JUMBO)-TIMI 26 Trial. Circulation 2005; 111:3366–3373.
- Bovill EG, Terrin ML, Stump DC, et al. Hemorrhagic events during therapy with recombinant tissue-type plasminogen activator, heparin, and aspirin for acute myocardial infarction. Results of the Thrombolysis in Myocardial Infarction (TIMI) Phase II Trial. Ann Intern Med 1991; 115:256–265.
- Wiviott SD, Braunwald E, McCabe CH, et al; TRITONTIMI 38 Investigators. Prasugrel versus clopidogrel in patients with acute coronary syndromes. N Engl J Med 2007; 357:2001–2015.
- Antman EM, Wiviott SD, Murphy SA, et al. Early and late benefits of prasugrel in patients with acute coronary syndromes undergoing percutaneous coronary intervention: a TRITON-TIMI 38 (TRial to Assess Improvement in Therapeutic Outcomes by Optimizing Platelet InhibitioN with Prasugrel-Thrombolysis In Myocardial Infarction) analysis. J Am Coll Cardiol 2008; 51:2028–2033.
- Wallentin L, Becker RC, Budaj A, Freij A, Thorsén M, et al; PLATO Investigators. Ticagrelor versus clopidogrel in patients with acute coronary syndromes. N Engl J Med 2009; 361:1045–1057.
- Braunwald E, Antman EM, Beasley JW, et al. ACC/AHA 2002 guideline update for the management of patients with unstable angina and non–ST-segment elevation myocardial infarction—summary article*1: A report of the American College of Cardiology/American Heart Association task force on practice guidelines (Committee on the Management of Patients With Unstable Angina). J Am Coll Cardiol 2002; 40:1366–1374.
Prasugrel (Effient) is more potent and consistent in its effects than clopidogrel (Plavix), thus preventing more thrombotic events—but at a price of more bleeding. Therefore, the drugs must be appropriately selected for the individual patient.
Over the last 9 years, the thienopyridines—ticlopidine (Ticlid), clopidogrel, and now prasugrel—have become essential tools for treating acute coronary syndromes.
The usual underlying mechanism of acute coronary syndromes is thrombosis, caused by rupture of atherosclerotic plaque.1 Accordingly, antithrombotic agents—aspirin, heparin, lowmolecular-weight heparin, glycoprotein IIb/IIIa inhibitors, the direct thrombin inhibitor bivalirudin (Angiomax), and thienopyridines—have all been shown to reduce the risk of major adverse cardiac outcomes in this setting.
In this article, we review the pharmacology and evidence of effectiveness of the thienopyridine drugs, focusing on prasugrel, the latest thienopyridine to be approved by the US Food and Drug Administration (FDA).
THIENOPYRIDINES INHIBIT PLATELET ACTIVATION AND AGGREGATION
Thienopyridines are prodrugs that require conversion by hepatic cytochrome P450 enzymes. The active metabolites bind irreversibly to platelet P2Y12 receptors. Consequently, they permanently block signalling mediated by platelet adenosine diphosphate-P2Y12 receptors, thereby inhibiting glycoprotein IIb/IIIa receptor activation and platelet aggregation.
Aspirin, in contrast, inhibits platelets by blocking the thromboxane-mediated pathway. Therefore, the combination of aspirin plus a thienopyridine has an additive effect.2
The effect of thienopyridines on platelets is irreversible. Therefore, although the half-life of prasugrel’s active metabolite is 3.7 hours, its inhibitory effects last for 96 hours, essentially the time for half the body’s circulating platelets to be replaced.
TICLOPIDINE, THE FIRST THIENOPYRIDINE
Ticlopidine was the first thienopyridine to be approved by the FDA. Its initial studies in unstable angina were small, their designs did not call for patients to concurrently receive aspirin, and all they showed was that ticlopidine was about as beneficial as aspirin. Consequently, the studies had little impact on clinical practice.3
In a pivotal trial,4 patients who received coronary stents were randomized to afterward receive either the combination of ticlopidine plus aspirin or anticoagulation therapy with heparin, phenprocoumon (a coumarin derivative available in Europe), and aspirin. At 30 days, an ischemic complication (death, myocardial infarction [MI], repeat intervention) had occurred in 6.2% of the anticoagulation therapy group vs 1.6% of the ticlopidine group, a risk reduction of 75%. Rates of stent occlusion, MI, and revascularization were 80% to 85% lower in the ticlodipine group. This study paved the way for widespread use of thienopyridines.
Ticlopidine’s use was limited, however, by a 2.4% incidence of serious granulocytopenia and rare cases of thrombocytopenic purpura.
BENEFIT OF CLOPIDOGREL
Although prasugrel is the focus of this review, the trials of prasugrel all compared its efficacy with that of clopidogrel. Furthermore, many patients should still receive clopidogrel and not prasugrel, so it is important to be familiar with the evidence of clopidogrel’s benefit.
Once approved for clinical use, clopidogrel was substituted for ticlopidine in patients undergoing coronary stenting on the basis of studies showing it to be at least as effective as ticlopidine and more tolerable. A series of trials of clopidogrel were done in patients across a spectrum of risk groups, from those at high risk of coronary heart disease to those presenting with ST-elevation MI. The time of pretreatment in the studies ranged from 3 hours to 6 days before percutaneous coronary intervention, and the duration of treatment following intervention ranged from 30 days to 1 year.
Clopidogrel in non-ST-elevation acute coronary syndromes
The CURE trial2 (Clopidogrel in Unstable Angina to Prevent Recurrent Events), published in 2001, established clopidogrel as a therapy for unstable ischemic syndromes, whether treated medically or with revascularization. In that trial, 12,562 patients with acute coronary syndromes without ST elevation (ie, unstable angina or non-ST-elevation MI), as defined by electrocardiographic changes or positive cardiac markers, were randomized to receive clopidogrel (a 300-mg loading dose followed by 75-mg maintenance doses) or placebo for a mean duration of 9 months. All patients also received aspirin 75 mg to 325 mg daily.
The composite outcome of death from cardiovascular causes, nonfatal MI, or stroke occurred in 20% fewer patients treated with clopidogrel than with placebo (9.3% vs 11.4%). The benefit was similar in patients undergoing revascularization compared with those treated medically.
Although there were significantly more cases of major bleeding in the clopidogrel group than in the placebo group (3.7% vs 2.7%), the number of episodes of life-threatening bleeding or hemorrhagic strokes was the same.
PCI-CURE5 was a substudy of the CURE trial in patients who underwent a percutaneous coronary intervention. Patients were pretreated with clopidogrel or placebo for a mean of 6 days before the procedure. Afterward, they all received clopidogrel plus aspirin in an unblinded fashion for 2 to 4 weeks, and then the randomized study drug was resumed for a mean of 8 months.
Significantly fewer adverse events occurred in the clopidogrel group as tallied at the time of the intervention, 1 month later, and 8 months later.
Clopidogrel in ST-elevation acute MI
The CLARITY-TIMI 28 trial6 (Clopidogrel as Adjunctive Reperfusion Therapy—Thrombolysis in Myocardial Infarction 28) showed that adding clopidogrel (a 300-mg loading dose, then 75 mg daily) to aspirin benefitted patients with ST-elevation MI receiving fibrinolytic therapy. At 30 days, cardiovascular death, recurrent MI, or urgent revascularization had occurred in 11.6% of the clopidogrel group vs 14.1% of the placebo group, a statistically significant difference. The rates of major or minor bleeding were no higher in the clopidogrel group than in the placebo group, an especially remarkable finding in patients receiving thrombolytic therapy.
PCI-CLARITY.7 About half of the patients in the CLARITY trial ultimately underwent a percutaneous coronary intervention after fibrinolytic therapy, with results reported as the PCI-CLARITY substudy. Like those in PCI-CURE, these patients were randomized to receive pretreatment with either clopidogrel or placebo before the procedure, in this study for a median of 3 days. Both groups received clopidogrel afterward. At 30 days from randomization, the outcome of cardiovascular death, MI, or stroke had occurred in 7.5% of the clopidogrel group compared with 12.0% of the placebo group, which was statistically significant, without any significant excess in the rates of major or minor bleeding.
COMMIT8 (the Clopidogrel and Metoprolol in Myocardial Infarction Trial) also showed clopidogrel to be beneficial in patients with acute MI. This trial included more than 45,000 patients in China with acute MI, 93% of whom had ST-segment elevation. In contrast to CLARITY, in COMMIT barely more than half of the patients received fibrinolysis, fewer than 5% proceeded to percutaneous interventions, and no loading dose was given: patients in the clopidogrel group received 75 mg/day from the outset.
At 15 days, the incidence of death, reinfarction, or stroke was 9.2% with clopidogrel compared with 10.1% with placebo, a small but statistically significant difference. Again, the rate of major bleeding was not significantly higher, either overall or in patients over age 70.
Of note, patients over age 75 were excluded from CLARITY, and as mentioned, no loading dose was used in COMMIT. Thus, for patients receiving fibrinolysis who are over age 75, there is no evidence to support the safety of a loading dose, and clopidogrel should be started at 75 mg daily.
Clopidogrel in elective percutaneous coronary intervention
The CREDO trial9 (Clopidogrel for the Reduction of Events During Observation) was in patients referred for elective percutaneous coronary intervention. Three to 24 hours before the procedure, the patients received either a 300-mg loading dose of clopidogrel or placebo; afterward, all patients received clopidogrel 75 mg/day for 28 days. All patients also received aspirin.
A clopidogrel loading dose 3 to 24 hours before the intervention did not produce a statistically significant reduction in ischemic events, although a post hoc subgroup analysis suggested that patients who received the loading dose between 6 and 24 hours before did benefit, with a relative risk reduction of 38.6% in the composite end point (P = .051).
After 28 days, the patients who had received the clopidogrel loading dose were continued on clopidogrel, while those in the placebo group were switched back to placebo. At 1 year, the investigators found a significantly lower rate of the composite end point with the prolonged course of clopidogrel (8.5% vs 11.5%).
In summary, these studies found clopidogrel to be beneficial in a broad spectrum of coronary diseases. Subgroup analyses suggest that pretreatment before percutaneous coronary intervention provides additional benefit, particularly if clopidogrel is given at least 6 hours in advance (the time necessary for clopidogrel to cause substantial platelet inhibition).
SOME PATIENTS RESPOND LESS TO CLOPIDOGREL
The level of platelet inhibition induced by clopidogrel varies. In different studies, the frequency of clopidogrel “nonresponsiveness” ranged from 5% to 56% of patients, depending on which test and which cutoff values were used. The distribution of responses to clopidogrel is wide and fits a normal gaussian curve.10
A large fraction of the population carries a gene that may account for some of the interpatient variation in platelet inhibition with clopidogrel. Carriers of a reduced-function CYP2C19 allele—approximately 30% of people in one study—have significantly lower levels of the active metabolite of clopidogrel, less platelet inhibition from clopidogrel therapy, and a 53% higher rate of death from cardiovascular causes, MI, or stroke.11
PRASUGREL, THE NEWEST THIENOPYRIDINE
Prasugrel, FDA-approved in July 2009 for the treatment of acute coronary syndromes, is given in an oral loading dose of 60 mg followed by an oral maintenance dose of 10 mg daily.
Pharmacology of prasugrel vs clopidogrel
As noted previously, the thienopyridines are prodrugs that require hepatic conversion to exert antiplatelet effects.
Metabolism. Prasugrel’s hepatic activation involves a single step, in contrast to the multiple-step process required for activation of clopidogrel. Clopidogrel is primarily hydrolyzed by intestinal and plasma esterases to an inactive terminal metabolite, with the residual unhydrolized drug undergoing a two-step metabolism that depends on cytochrome P450 enzymes. Prasugrel is also extensively hydrolyzed by these esterases, but the intermediate product is then metabolized in a single step to the active sulfhydryl compound, mainly by CYP3A4 and CYP2B6.
Thus, about 80% of an orally absorbed dose of prasugrel is converted to active drug, compared with only 10% to 20% of absorbed clopidogrel.
Time to peak effect. With clopidogrel, maximal inhibition of platelet aggregation occurs 3 to 5 days after starting therapy with 75 mg daily without a loading dose, but within 4 to 6 hours if a loading dose of 300 to 600 mg is given. In contrast, a prasugrel loading dose produces more than 80% of its platelet inhibitory effects by 30 minutes, and peak activity is observed within 4 hours.12 The platelet inhibition induced by prasugrel at 30 minutes after administration is comparable to the peak effect of clopidogrel at 6 hours.13
Dose-response. Prasugrel’s inhibition of platelet aggregation is dose-related.
Prasugrel is about 10 times more potent than clopidogrel and 100 times more potent than ticlopidine. Thus, treatment with 5 mg of prasugrel results in inhibition of platelet activity (distributed in a gaussian curve) very similar to that produced by 75 mg of clopidogrel. On the other hand, even a maintenance dose of 150 mg of clopidogrel inhibits platelet activity to a lesser degree than 10 mg of prasugrel (46% vs 61%),14 so clopidogrel appears to reach a plateau of platelet inhibition that prasugrel can overcome.
At the approved dose of prasugrel, inhibition of platelet aggregation is significantly greater and there are fewer “nonresponders” than with clopidogrel.
Interactions. Drugs that inhibit CYP3A4 do not inhibit the efficacy of prasugrel, but they can inhibit that of clopidogrel. Some commonly used drugs that have this effect are the statins (eg, atorvastain [Lipitor]) and the macrolide antibiotics (eg, erythromycin). Furthermore, whereas proton pump inhibitors have been shown to diminish the effect of clopidogrel by reducing the formation of its active metabolite, no such effect has been noted with prasugrel.
Prasugrel in phase 2 trials: Finding the optimal dosage
A phase 2 trial compared three prasugrel regimens (loading dose/daily maintenance dose of 40 mg/7.5 mg, 60 mg/10 mg, and 60 mg/15 mg) and standard clopidogrel therapy (300 mg/75 mg) in patients undergoing elective or urgent percutaneous coronary intervention.15 No significant difference in outcomes was seen in the groups receiving the three prasugrel regimens. However, more “minimal bleeding events” (defined by the criteria of the TIMI trial16) occurred with high-dose prasugrel than with lower-dose prasugrel or with clopidogrel, leading to use of the intermediate-dose prasugrel regimen (60-mg loading dose, 10-mg daily maintenance) for later trials.
Another phase 2 trial randomized 201 patients undergoing elective percutaneous coronary intervention to receive prasugrel 60 mg/10 mg or clopidogrel 600 mg/150 mg.14 In all patients, the loading dose was given about 1 hour before cardiac catheterization. As soon as 30 minutes after the loading dose, platelet inhibition was superior with prasugrel (31% vs 5% inhibition of platelet aggregation), and it remained significantly higher at 6 hours (75% vs 32%) and during the maintenance phase (61% vs 46%).
Phase 3 trial of prasugrel vs clopidogrel: TRITON-TIMI 38
Only one large phase 3 trial of prasugrel has been completed: TRITON-TIMI 38 (the Trial to Assess Improvement in Therapeutic Outcomes by Optimizing Platelet Inhibition With Prasugrel—Thrombolysis in Myocardial Infarction),17 which enrolled adults with moderate-risk to high-risk acute coronary syndromes scheduled to undergo a percutaneous coronary intervention. In this trial, 10,074 patients were enrolled who had moderate-to high-risk unstable angina or non-ST-elevation MI, and 3,534 patients were enrolled who had ST-elevation MI.
Patients were randomized to receive prasugrel (a 60-mg loading dose, then 10 mg daily) or clopidogrel (a 300-mg loading dose, then 75 mg daily) and were treated for 6 to 15 months. All patients also received aspirin.
These benefits came at a price of more bleeding. Of those patients who did not undergo coronary artery bypass grafting, more experienced bleeding in the prasugrel group than in the clopidogrel group (2.4% vs 1.8%, P = .03), including a higher rate of life-threatening bleeding (1.4% vs 0.89%, P = .01) and fatal bleeding (0.4% vs 0.1%, P = .002). More patients discontinued prasugrel because of hemorrhage (2.5% vs 1.4%, P < .001). In patients who proceeded to coronary artery bypass grafting, the rate of major bleeding was more than four times higher in those who received prasugrel than in those who received clopidogrel (13.4% vs 3.2%, P < .001).
A higher rate of adverse events related to colon cancer was also noted in patients treated with prasugrel, although the authors suggest this may have resulted from the stronger antiplatelet effects of prasugrel bringing more tumors to medical attention due to bleeding.
Overall death rates did not differ significantly between the treatment groups.
In a post hoc analysis,18 prasugrel was superior to clopidogrel in preventing ischemic events both during the first 3 days following randomization (the “loading phase”) and for the remainder of the trial (the “maintenance phase”). Whereas bleeding risk was similar with the two drugs during the loading phase, prasugrel was subsequently associated with more bleeding during the maintenance phase.
Certain patient subgroups had no net benefit or even suffered harm from prasugrel compared with clopidogrel.17 Patients with previous stroke or transient ischemic attack had net harm from prasugrel (hazard ratio 1.54, P = .04) and showed a strong trend toward a greater rate of major bleeding (P = .06). Patients age 75 and older and those weighing less than 60 kg had no net benefit from prasugrel.
Cost of prasugrel
Prasugrel is currently priced at 18% more than clopidogrel, with average wholesale prices per pill of $6.65 for prasugrel 10 mg compared with $5.63 for clopidogrel 75 mg. (Prasugrel 10-mg pills cost $6.33 at drugstore.com or $7.60 at CVS; clopidogrel 75-mg pills cost $5.33 at drugstore.com or $6.43 at CVS.) The patent on clopidogrel expires in November 2011, after which the price differential is expected to become significantly greater.
TICAGRELOR, A REVERSIBLE ORAL AGENT
Ticagrelor, the first reversible oral P2Y12 receptor antagonist, is an alternative to thienopyridine therapy for acute coronary syndromes.
Ticagrelor is quickly absorbed, does not require metabolic activation, and has a rapid antiplatelet effect and offset of effect, which closely follow drug-exposure levels. In a large randomized controlled trial in patients with acute coronary syndromes with or without STsegment elevation, treatment with ticagrelor compared with clopidogrel resulted in a significant reduction in death from vascular causes, MI, or stroke (9.8% vs 11.7%).19
Given its reversible effect on platelet inhibition, ticagrelor may be preferred in patients whose coronary anatomy is unknown and for whom coronary artery bypass grafting is deemed probable. It is still undergoing trials and is not yet approved.
TAKE-HOME POINTS
Prasugrel is more potent, more rapid in onset, and more consistent in inhibiting platelet aggregation than clopidogrel. A large clinical trial17 found prasugrel to be superior to clopidogrel for patients with moderate-to high-risk acute coronary syndromes with high probability of undergoing a percutaneous coronary intervention.
Who should receive prasugrel, and how?
Prasugrel should be given after angiography to patients with non-ST-elevation acute coronary syndromes or at presentation to patients with ST-elevation MI. When used for planned percutaneous coronary intervention, prasugrel should be given at least 30 minutes before the intervention, as was done in phase 2 trials (although its routine use in this situation is not recommended—see below).
It is given in a one-time loading dose of 60 mg by mouth and then maintained with 10 mg by mouth once daily for at least 1 year. (At least 9 months of treatment with a thienopyridine is indicated for patients with acute coronary syndromes who are medically treated, and at least 1 year is indicated following urgent or elective percutaneous coronary intervention, including balloon angioplasty and placement of a bare-metal or drug-eluting stent.)
Who should not receive prasugrel?
For now, prasugrel should be avoided in favor of clopidogrel in patients at higher risk of bleeding. It is clearly contraindicated in patients with prior transient ischemic attack or stroke, for whom the risk of serious bleeding seems to be prohibitive. It should generally be avoided in patients age 75 and older, although it might be considered in those at particularly high risk of stent thrombosis, such as those with diabetes or prior MI. In patients weighing less than 60 kg, the package insert advises a reduced dose (5 mg), although clinical evidence for this practice is lacking.
As yet, we have no data assuring that prasugrel is safe to use in combination with fibrinolytic agents, so patients on thrombolytic therapy for acute MI should continue to receive clopidogrel starting immediately after lysis. Furthermore, in patients who proceeded to coronary artery bypass grafting, the rate of major bleeding was more than four times higher in the prasugrel group than in the clopidogrel group in the TRITON-TIMI 38 trial.17 No thienopyridine should be given to patients likely to proceed to coronary artery bypass grafting.
Only clopidogrel has evidence supporting its use as an alternative to aspirin for patients with atherosclerotic disease who cannot tolerate aspirin. Neither drug has evidence for use for primary prevention.
Other areas of uncertainty
Prior to angiography. Indications for prasugrel are currently limited by the narrow scope of the trial data. TRITON-TIMI 38,17 the only large trial completed to date, randomized patients to receive prasugrel only after their coronary anatomy was known, except for ST-elevation MI patients. It is unknown whether the benefits of prasugrel will outweigh the higher risk of bleeding in patients with acute coronary syndromes who do not proceed to percutaneous coronary interventions.
A clinical trial is currently under way comparing prasugrel with clopidogrel in 10,000 patients with acute coronary syndromes who will be medically managed without planned revascularization: A Comparison of Prasugrel and Clopidogrel in Acute Coronary Syndrome Subjects (TRILOGY ACS), ClinicalTrials.gov Identifier: NCT00699998. The trial has an estimated completion date of March 2011.
In cases of non-ST-elevation acute coronary syndrome, it is reasonable to wait to give a thienopyridine until after the coronary anatomy has been defined, if angiography will be completed soon after presentation. For example, a 1-hour delay before giving prasugrel still delivers antiplatelet therapy more quickly than giving clopidogrel on presentation. If longer delays are expected before angiography, however, the patient should be given a loading dose of clopidogrel “up front,” in accordance with guidelines published by the American College of Cardiology, American Heart Association, and European Society of Cardiology,20 which recommend starting a thienopyridine early during hospitalization based on trial data with clopidogrel.
Patients undergoing elective percutaneous coronary intervention are at lower risk of stent thrombosis and other ischemic complications, so it is possible that the benefits of prasugrel would not outweigh the risks in these patients. Thus, prasugrel cannot yet be recommended for routine elective percutaneous coronary intervention except in individual cases in which the interventionalist feels that the patient may be at higher risk of thrombosis.
Prasugrel (Effient) is more potent and consistent in its effects than clopidogrel (Plavix), thus preventing more thrombotic events—but at a price of more bleeding. Therefore, the drugs must be appropriately selected for the individual patient.
Over the last 9 years, the thienopyridines—ticlopidine (Ticlid), clopidogrel, and now prasugrel—have become essential tools for treating acute coronary syndromes.
The usual underlying mechanism of acute coronary syndromes is thrombosis, caused by rupture of atherosclerotic plaque.1 Accordingly, antithrombotic agents—aspirin, heparin, lowmolecular-weight heparin, glycoprotein IIb/IIIa inhibitors, the direct thrombin inhibitor bivalirudin (Angiomax), and thienopyridines—have all been shown to reduce the risk of major adverse cardiac outcomes in this setting.
In this article, we review the pharmacology and evidence of effectiveness of the thienopyridine drugs, focusing on prasugrel, the latest thienopyridine to be approved by the US Food and Drug Administration (FDA).
THIENOPYRIDINES INHIBIT PLATELET ACTIVATION AND AGGREGATION
Thienopyridines are prodrugs that require conversion by hepatic cytochrome P450 enzymes. The active metabolites bind irreversibly to platelet P2Y12 receptors. Consequently, they permanently block signalling mediated by platelet adenosine diphosphate-P2Y12 receptors, thereby inhibiting glycoprotein IIb/IIIa receptor activation and platelet aggregation.
Aspirin, in contrast, inhibits platelets by blocking the thromboxane-mediated pathway. Therefore, the combination of aspirin plus a thienopyridine has an additive effect.2
The effect of thienopyridines on platelets is irreversible. Therefore, although the half-life of prasugrel’s active metabolite is 3.7 hours, its inhibitory effects last for 96 hours, essentially the time for half the body’s circulating platelets to be replaced.
TICLOPIDINE, THE FIRST THIENOPYRIDINE
Ticlopidine was the first thienopyridine to be approved by the FDA. Its initial studies in unstable angina were small, their designs did not call for patients to concurrently receive aspirin, and all they showed was that ticlopidine was about as beneficial as aspirin. Consequently, the studies had little impact on clinical practice.3
In a pivotal trial,4 patients who received coronary stents were randomized to afterward receive either the combination of ticlopidine plus aspirin or anticoagulation therapy with heparin, phenprocoumon (a coumarin derivative available in Europe), and aspirin. At 30 days, an ischemic complication (death, myocardial infarction [MI], repeat intervention) had occurred in 6.2% of the anticoagulation therapy group vs 1.6% of the ticlopidine group, a risk reduction of 75%. Rates of stent occlusion, MI, and revascularization were 80% to 85% lower in the ticlodipine group. This study paved the way for widespread use of thienopyridines.
Ticlopidine’s use was limited, however, by a 2.4% incidence of serious granulocytopenia and rare cases of thrombocytopenic purpura.
BENEFIT OF CLOPIDOGREL
Although prasugrel is the focus of this review, the trials of prasugrel all compared its efficacy with that of clopidogrel. Furthermore, many patients should still receive clopidogrel and not prasugrel, so it is important to be familiar with the evidence of clopidogrel’s benefit.
Once approved for clinical use, clopidogrel was substituted for ticlopidine in patients undergoing coronary stenting on the basis of studies showing it to be at least as effective as ticlopidine and more tolerable. A series of trials of clopidogrel were done in patients across a spectrum of risk groups, from those at high risk of coronary heart disease to those presenting with ST-elevation MI. The time of pretreatment in the studies ranged from 3 hours to 6 days before percutaneous coronary intervention, and the duration of treatment following intervention ranged from 30 days to 1 year.
Clopidogrel in non-ST-elevation acute coronary syndromes
The CURE trial2 (Clopidogrel in Unstable Angina to Prevent Recurrent Events), published in 2001, established clopidogrel as a therapy for unstable ischemic syndromes, whether treated medically or with revascularization. In that trial, 12,562 patients with acute coronary syndromes without ST elevation (ie, unstable angina or non-ST-elevation MI), as defined by electrocardiographic changes or positive cardiac markers, were randomized to receive clopidogrel (a 300-mg loading dose followed by 75-mg maintenance doses) or placebo for a mean duration of 9 months. All patients also received aspirin 75 mg to 325 mg daily.
The composite outcome of death from cardiovascular causes, nonfatal MI, or stroke occurred in 20% fewer patients treated with clopidogrel than with placebo (9.3% vs 11.4%). The benefit was similar in patients undergoing revascularization compared with those treated medically.
Although there were significantly more cases of major bleeding in the clopidogrel group than in the placebo group (3.7% vs 2.7%), the number of episodes of life-threatening bleeding or hemorrhagic strokes was the same.
PCI-CURE5 was a substudy of the CURE trial in patients who underwent a percutaneous coronary intervention. Patients were pretreated with clopidogrel or placebo for a mean of 6 days before the procedure. Afterward, they all received clopidogrel plus aspirin in an unblinded fashion for 2 to 4 weeks, and then the randomized study drug was resumed for a mean of 8 months.
Significantly fewer adverse events occurred in the clopidogrel group as tallied at the time of the intervention, 1 month later, and 8 months later.
Clopidogrel in ST-elevation acute MI
The CLARITY-TIMI 28 trial6 (Clopidogrel as Adjunctive Reperfusion Therapy—Thrombolysis in Myocardial Infarction 28) showed that adding clopidogrel (a 300-mg loading dose, then 75 mg daily) to aspirin benefitted patients with ST-elevation MI receiving fibrinolytic therapy. At 30 days, cardiovascular death, recurrent MI, or urgent revascularization had occurred in 11.6% of the clopidogrel group vs 14.1% of the placebo group, a statistically significant difference. The rates of major or minor bleeding were no higher in the clopidogrel group than in the placebo group, an especially remarkable finding in patients receiving thrombolytic therapy.
PCI-CLARITY.7 About half of the patients in the CLARITY trial ultimately underwent a percutaneous coronary intervention after fibrinolytic therapy, with results reported as the PCI-CLARITY substudy. Like those in PCI-CURE, these patients were randomized to receive pretreatment with either clopidogrel or placebo before the procedure, in this study for a median of 3 days. Both groups received clopidogrel afterward. At 30 days from randomization, the outcome of cardiovascular death, MI, or stroke had occurred in 7.5% of the clopidogrel group compared with 12.0% of the placebo group, which was statistically significant, without any significant excess in the rates of major or minor bleeding.
COMMIT8 (the Clopidogrel and Metoprolol in Myocardial Infarction Trial) also showed clopidogrel to be beneficial in patients with acute MI. This trial included more than 45,000 patients in China with acute MI, 93% of whom had ST-segment elevation. In contrast to CLARITY, in COMMIT barely more than half of the patients received fibrinolysis, fewer than 5% proceeded to percutaneous interventions, and no loading dose was given: patients in the clopidogrel group received 75 mg/day from the outset.
At 15 days, the incidence of death, reinfarction, or stroke was 9.2% with clopidogrel compared with 10.1% with placebo, a small but statistically significant difference. Again, the rate of major bleeding was not significantly higher, either overall or in patients over age 70.
Of note, patients over age 75 were excluded from CLARITY, and as mentioned, no loading dose was used in COMMIT. Thus, for patients receiving fibrinolysis who are over age 75, there is no evidence to support the safety of a loading dose, and clopidogrel should be started at 75 mg daily.
Clopidogrel in elective percutaneous coronary intervention
The CREDO trial9 (Clopidogrel for the Reduction of Events During Observation) was in patients referred for elective percutaneous coronary intervention. Three to 24 hours before the procedure, the patients received either a 300-mg loading dose of clopidogrel or placebo; afterward, all patients received clopidogrel 75 mg/day for 28 days. All patients also received aspirin.
A clopidogrel loading dose 3 to 24 hours before the intervention did not produce a statistically significant reduction in ischemic events, although a post hoc subgroup analysis suggested that patients who received the loading dose between 6 and 24 hours before did benefit, with a relative risk reduction of 38.6% in the composite end point (P = .051).
After 28 days, the patients who had received the clopidogrel loading dose were continued on clopidogrel, while those in the placebo group were switched back to placebo. At 1 year, the investigators found a significantly lower rate of the composite end point with the prolonged course of clopidogrel (8.5% vs 11.5%).
In summary, these studies found clopidogrel to be beneficial in a broad spectrum of coronary diseases. Subgroup analyses suggest that pretreatment before percutaneous coronary intervention provides additional benefit, particularly if clopidogrel is given at least 6 hours in advance (the time necessary for clopidogrel to cause substantial platelet inhibition).
SOME PATIENTS RESPOND LESS TO CLOPIDOGREL
The level of platelet inhibition induced by clopidogrel varies. In different studies, the frequency of clopidogrel “nonresponsiveness” ranged from 5% to 56% of patients, depending on which test and which cutoff values were used. The distribution of responses to clopidogrel is wide and fits a normal gaussian curve.10
A large fraction of the population carries a gene that may account for some of the interpatient variation in platelet inhibition with clopidogrel. Carriers of a reduced-function CYP2C19 allele—approximately 30% of people in one study—have significantly lower levels of the active metabolite of clopidogrel, less platelet inhibition from clopidogrel therapy, and a 53% higher rate of death from cardiovascular causes, MI, or stroke.11
PRASUGREL, THE NEWEST THIENOPYRIDINE
Prasugrel, FDA-approved in July 2009 for the treatment of acute coronary syndromes, is given in an oral loading dose of 60 mg followed by an oral maintenance dose of 10 mg daily.
Pharmacology of prasugrel vs clopidogrel
As noted previously, the thienopyridines are prodrugs that require hepatic conversion to exert antiplatelet effects.
Metabolism. Prasugrel’s hepatic activation involves a single step, in contrast to the multiple-step process required for activation of clopidogrel. Clopidogrel is primarily hydrolyzed by intestinal and plasma esterases to an inactive terminal metabolite, with the residual unhydrolized drug undergoing a two-step metabolism that depends on cytochrome P450 enzymes. Prasugrel is also extensively hydrolyzed by these esterases, but the intermediate product is then metabolized in a single step to the active sulfhydryl compound, mainly by CYP3A4 and CYP2B6.
Thus, about 80% of an orally absorbed dose of prasugrel is converted to active drug, compared with only 10% to 20% of absorbed clopidogrel.
Time to peak effect. With clopidogrel, maximal inhibition of platelet aggregation occurs 3 to 5 days after starting therapy with 75 mg daily without a loading dose, but within 4 to 6 hours if a loading dose of 300 to 600 mg is given. In contrast, a prasugrel loading dose produces more than 80% of its platelet inhibitory effects by 30 minutes, and peak activity is observed within 4 hours.12 The platelet inhibition induced by prasugrel at 30 minutes after administration is comparable to the peak effect of clopidogrel at 6 hours.13
Dose-response. Prasugrel’s inhibition of platelet aggregation is dose-related.
Prasugrel is about 10 times more potent than clopidogrel and 100 times more potent than ticlopidine. Thus, treatment with 5 mg of prasugrel results in inhibition of platelet activity (distributed in a gaussian curve) very similar to that produced by 75 mg of clopidogrel. On the other hand, even a maintenance dose of 150 mg of clopidogrel inhibits platelet activity to a lesser degree than 10 mg of prasugrel (46% vs 61%),14 so clopidogrel appears to reach a plateau of platelet inhibition that prasugrel can overcome.
At the approved dose of prasugrel, inhibition of platelet aggregation is significantly greater and there are fewer “nonresponders” than with clopidogrel.
Interactions. Drugs that inhibit CYP3A4 do not inhibit the efficacy of prasugrel, but they can inhibit that of clopidogrel. Some commonly used drugs that have this effect are the statins (eg, atorvastain [Lipitor]) and the macrolide antibiotics (eg, erythromycin). Furthermore, whereas proton pump inhibitors have been shown to diminish the effect of clopidogrel by reducing the formation of its active metabolite, no such effect has been noted with prasugrel.
Prasugrel in phase 2 trials: Finding the optimal dosage
A phase 2 trial compared three prasugrel regimens (loading dose/daily maintenance dose of 40 mg/7.5 mg, 60 mg/10 mg, and 60 mg/15 mg) and standard clopidogrel therapy (300 mg/75 mg) in patients undergoing elective or urgent percutaneous coronary intervention.15 No significant difference in outcomes was seen in the groups receiving the three prasugrel regimens. However, more “minimal bleeding events” (defined by the criteria of the TIMI trial16) occurred with high-dose prasugrel than with lower-dose prasugrel or with clopidogrel, leading to use of the intermediate-dose prasugrel regimen (60-mg loading dose, 10-mg daily maintenance) for later trials.
Another phase 2 trial randomized 201 patients undergoing elective percutaneous coronary intervention to receive prasugrel 60 mg/10 mg or clopidogrel 600 mg/150 mg.14 In all patients, the loading dose was given about 1 hour before cardiac catheterization. As soon as 30 minutes after the loading dose, platelet inhibition was superior with prasugrel (31% vs 5% inhibition of platelet aggregation), and it remained significantly higher at 6 hours (75% vs 32%) and during the maintenance phase (61% vs 46%).
Phase 3 trial of prasugrel vs clopidogrel: TRITON-TIMI 38
Only one large phase 3 trial of prasugrel has been completed: TRITON-TIMI 38 (the Trial to Assess Improvement in Therapeutic Outcomes by Optimizing Platelet Inhibition With Prasugrel—Thrombolysis in Myocardial Infarction),17 which enrolled adults with moderate-risk to high-risk acute coronary syndromes scheduled to undergo a percutaneous coronary intervention. In this trial, 10,074 patients were enrolled who had moderate-to high-risk unstable angina or non-ST-elevation MI, and 3,534 patients were enrolled who had ST-elevation MI.
Patients were randomized to receive prasugrel (a 60-mg loading dose, then 10 mg daily) or clopidogrel (a 300-mg loading dose, then 75 mg daily) and were treated for 6 to 15 months. All patients also received aspirin.
These benefits came at a price of more bleeding. Of those patients who did not undergo coronary artery bypass grafting, more experienced bleeding in the prasugrel group than in the clopidogrel group (2.4% vs 1.8%, P = .03), including a higher rate of life-threatening bleeding (1.4% vs 0.89%, P = .01) and fatal bleeding (0.4% vs 0.1%, P = .002). More patients discontinued prasugrel because of hemorrhage (2.5% vs 1.4%, P < .001). In patients who proceeded to coronary artery bypass grafting, the rate of major bleeding was more than four times higher in those who received prasugrel than in those who received clopidogrel (13.4% vs 3.2%, P < .001).
A higher rate of adverse events related to colon cancer was also noted in patients treated with prasugrel, although the authors suggest this may have resulted from the stronger antiplatelet effects of prasugrel bringing more tumors to medical attention due to bleeding.
Overall death rates did not differ significantly between the treatment groups.
In a post hoc analysis,18 prasugrel was superior to clopidogrel in preventing ischemic events both during the first 3 days following randomization (the “loading phase”) and for the remainder of the trial (the “maintenance phase”). Whereas bleeding risk was similar with the two drugs during the loading phase, prasugrel was subsequently associated with more bleeding during the maintenance phase.
Certain patient subgroups had no net benefit or even suffered harm from prasugrel compared with clopidogrel.17 Patients with previous stroke or transient ischemic attack had net harm from prasugrel (hazard ratio 1.54, P = .04) and showed a strong trend toward a greater rate of major bleeding (P = .06). Patients age 75 and older and those weighing less than 60 kg had no net benefit from prasugrel.
Cost of prasugrel
Prasugrel is currently priced at 18% more than clopidogrel, with average wholesale prices per pill of $6.65 for prasugrel 10 mg compared with $5.63 for clopidogrel 75 mg. (Prasugrel 10-mg pills cost $6.33 at drugstore.com or $7.60 at CVS; clopidogrel 75-mg pills cost $5.33 at drugstore.com or $6.43 at CVS.) The patent on clopidogrel expires in November 2011, after which the price differential is expected to become significantly greater.
TICAGRELOR, A REVERSIBLE ORAL AGENT
Ticagrelor, the first reversible oral P2Y12 receptor antagonist, is an alternative to thienopyridine therapy for acute coronary syndromes.
Ticagrelor is quickly absorbed, does not require metabolic activation, and has a rapid antiplatelet effect and offset of effect, which closely follow drug-exposure levels. In a large randomized controlled trial in patients with acute coronary syndromes with or without STsegment elevation, treatment with ticagrelor compared with clopidogrel resulted in a significant reduction in death from vascular causes, MI, or stroke (9.8% vs 11.7%).19
Given its reversible effect on platelet inhibition, ticagrelor may be preferred in patients whose coronary anatomy is unknown and for whom coronary artery bypass grafting is deemed probable. It is still undergoing trials and is not yet approved.
TAKE-HOME POINTS
Prasugrel is more potent, more rapid in onset, and more consistent in inhibiting platelet aggregation than clopidogrel. A large clinical trial17 found prasugrel to be superior to clopidogrel for patients with moderate-to high-risk acute coronary syndromes with high probability of undergoing a percutaneous coronary intervention.
Who should receive prasugrel, and how?
Prasugrel should be given after angiography to patients with non-ST-elevation acute coronary syndromes or at presentation to patients with ST-elevation MI. When used for planned percutaneous coronary intervention, prasugrel should be given at least 30 minutes before the intervention, as was done in phase 2 trials (although its routine use in this situation is not recommended—see below).
It is given in a one-time loading dose of 60 mg by mouth and then maintained with 10 mg by mouth once daily for at least 1 year. (At least 9 months of treatment with a thienopyridine is indicated for patients with acute coronary syndromes who are medically treated, and at least 1 year is indicated following urgent or elective percutaneous coronary intervention, including balloon angioplasty and placement of a bare-metal or drug-eluting stent.)
Who should not receive prasugrel?
For now, prasugrel should be avoided in favor of clopidogrel in patients at higher risk of bleeding. It is clearly contraindicated in patients with prior transient ischemic attack or stroke, for whom the risk of serious bleeding seems to be prohibitive. It should generally be avoided in patients age 75 and older, although it might be considered in those at particularly high risk of stent thrombosis, such as those with diabetes or prior MI. In patients weighing less than 60 kg, the package insert advises a reduced dose (5 mg), although clinical evidence for this practice is lacking.
As yet, we have no data assuring that prasugrel is safe to use in combination with fibrinolytic agents, so patients on thrombolytic therapy for acute MI should continue to receive clopidogrel starting immediately after lysis. Furthermore, in patients who proceeded to coronary artery bypass grafting, the rate of major bleeding was more than four times higher in the prasugrel group than in the clopidogrel group in the TRITON-TIMI 38 trial.17 No thienopyridine should be given to patients likely to proceed to coronary artery bypass grafting.
Only clopidogrel has evidence supporting its use as an alternative to aspirin for patients with atherosclerotic disease who cannot tolerate aspirin. Neither drug has evidence for use for primary prevention.
Other areas of uncertainty
Prior to angiography. Indications for prasugrel are currently limited by the narrow scope of the trial data. TRITON-TIMI 38,17 the only large trial completed to date, randomized patients to receive prasugrel only after their coronary anatomy was known, except for ST-elevation MI patients. It is unknown whether the benefits of prasugrel will outweigh the higher risk of bleeding in patients with acute coronary syndromes who do not proceed to percutaneous coronary interventions.
A clinical trial is currently under way comparing prasugrel with clopidogrel in 10,000 patients with acute coronary syndromes who will be medically managed without planned revascularization: A Comparison of Prasugrel and Clopidogrel in Acute Coronary Syndrome Subjects (TRILOGY ACS), ClinicalTrials.gov Identifier: NCT00699998. The trial has an estimated completion date of March 2011.
In cases of non-ST-elevation acute coronary syndrome, it is reasonable to wait to give a thienopyridine until after the coronary anatomy has been defined, if angiography will be completed soon after presentation. For example, a 1-hour delay before giving prasugrel still delivers antiplatelet therapy more quickly than giving clopidogrel on presentation. If longer delays are expected before angiography, however, the patient should be given a loading dose of clopidogrel “up front,” in accordance with guidelines published by the American College of Cardiology, American Heart Association, and European Society of Cardiology,20 which recommend starting a thienopyridine early during hospitalization based on trial data with clopidogrel.
Patients undergoing elective percutaneous coronary intervention are at lower risk of stent thrombosis and other ischemic complications, so it is possible that the benefits of prasugrel would not outweigh the risks in these patients. Thus, prasugrel cannot yet be recommended for routine elective percutaneous coronary intervention except in individual cases in which the interventionalist feels that the patient may be at higher risk of thrombosis.
- Yeghiazarians Y, Braunstein JB, Askari A, Stone PH. Unstable angina pectoris. N Engl J Med 2000; 342:101–114.
- Yusuf S, Zhao F, Mehta SR, Chrolavicius S, Tognoni G, Fox KK; Clopidogrel in Unstable Angina to Prevent Recurrent Events Trial Investigators. Effects of clopidogrel in addition to aspirin in patients with acute coronary syndromes without ST-segment elevation. N Engl J Med 2001; 345:494–502.
- Balsano F, Rizzon P, Violi F, et al. Antiplatelet treatment with ticlopidine in unstable angina. A controlled multicenter clinical trial. The Studio della Ticlopidina nell'Angina Instabile Group. Circulation 1990; 82:17–26.
- Schömig A, Neumann FJ, Kastrati A, et al. A randomized comparison of antiplatelet and anticoagulant therapy after the placement of coronary-artery stents. N Engl J Med 1996; 334:1084–1089.
- Mehta SR, Yusuf S, Peters RJG, et al; Clopidogrel in Unstable Angina to Prevent Recurrent Events Trial (CURE) Investigators. Effects of pretreatment with clopidogrel and aspirin followed by long-term therapy in patients undergoing percutaneous coronary intervention: the PCI-CURE study. Lancet 2001; 358:527–533.
- Sabatine MS, Cannon CP, Gibson CM, et al; CLA RITY-TIMI 28 Investigators. Addition of clopidogrel to aspirin and fibrinolytic therapy for myocardial infarction with STsegment elevation. N Engl J Med 2005; 352:1179–1189.
- Sabatine MS, Cannon CP, Gibson CM, et al; Clopidogrel as Adjunctive Reperfusion Therapy (CLARITY)-Thrombolysis in Myocardial Infarction (TIMI) 28 Investigators. Effect of clopidogrel pretreatment before percutaneous coronary intervention in patients with ST-elevation myocardial infarction treated with fibrinolytics: the PCI-CLARITY study. JAMA 2005: 294:1224–1232.
- Chen ZM, Jiang LX, Chen YP, et al; COMMIT (ClOpidogrel and Metoprolol in Myocardial Infarction Trial) collaborative group. Addition of clopidogrel to aspirin in 45,852 patients with acute myocardial infarction: randomised placebo-controlled trial. Lancet 2005; 366:1607–1621.
- Steinhubl SR, Berger PB, Mann JT, et al; CREDO Investigators. Clopidogrel for the reduction of events during observation. Early and sustained dual oral antiplatelet therapy following percutaneous coronary intervention: a randomized controlled trial. JAMA 2002; 288:2411–2420.
- Serebruany VL, Steinhubl SR, Berger PB, Malinin AI, Bhatt DL, Topol EJ. Variability in platelet responsiveness to clopidogrel among 544 individuals. J Am Coll Cardiol 2005; 45:246–251.
- Mega JL, Close SL, Wiviott SD, et al. Cytochrome P-450 polymorphisms and response to clopidogrel. N Engl J Med 2009; 360:354–362.
- Helft G, Osende JI, Worthley SG, et al. Acute antithrombotic effect of a front-loaded regimen of clopidogrel in patients with atherosclerosis on aspirin. Arterioscler Thromb Vasc Biol 2000; 20:2316–2321.
- Weerakkody GJ, Jakubowski JA, Brandt JT, et al. Comparison of speed of onset of platelet inhibition after loading doses of clopidogrel versus prasugrel in healthy volunteers and correlation with responder status. Am J Cardiol 2007; 100:331–336.
- Wiviott SD, Trenk D, Frelinger AL, et al; PRINCIPLETIMI 44 Investigators. Prasugrel compared with high loading-and maintenance-dose clopidogrel in patients with planned percutaneous coronary intervention: the Prasugrel in Comparison to Clopidogrel for Inhibition of Platelet Activation and Aggregation-Thrombolysis in Myocardial Infarction 44 trial. Circulation 2007; 116:2923–2932.
- Wiviott SD, Antman EM, Winters KJ, et al; JUMBO-TIMI 26 Investigators. Randomized comparison of prasugrel (CS-747, LY640315), a novel thienopyridine P2Y12 antagonist, with clopidogrel in percutaneous coronary intervention: results of the Joint Utilization of Medications to Block Platelets Optimally (JUMBO)-TIMI 26 Trial. Circulation 2005; 111:3366–3373.
- Bovill EG, Terrin ML, Stump DC, et al. Hemorrhagic events during therapy with recombinant tissue-type plasminogen activator, heparin, and aspirin for acute myocardial infarction. Results of the Thrombolysis in Myocardial Infarction (TIMI) Phase II Trial. Ann Intern Med 1991; 115:256–265.
- Wiviott SD, Braunwald E, McCabe CH, et al; TRITONTIMI 38 Investigators. Prasugrel versus clopidogrel in patients with acute coronary syndromes. N Engl J Med 2007; 357:2001–2015.
- Antman EM, Wiviott SD, Murphy SA, et al. Early and late benefits of prasugrel in patients with acute coronary syndromes undergoing percutaneous coronary intervention: a TRITON-TIMI 38 (TRial to Assess Improvement in Therapeutic Outcomes by Optimizing Platelet InhibitioN with Prasugrel-Thrombolysis In Myocardial Infarction) analysis. J Am Coll Cardiol 2008; 51:2028–2033.
- Wallentin L, Becker RC, Budaj A, Freij A, Thorsén M, et al; PLATO Investigators. Ticagrelor versus clopidogrel in patients with acute coronary syndromes. N Engl J Med 2009; 361:1045–1057.
- Braunwald E, Antman EM, Beasley JW, et al. ACC/AHA 2002 guideline update for the management of patients with unstable angina and non–ST-segment elevation myocardial infarction—summary article*1: A report of the American College of Cardiology/American Heart Association task force on practice guidelines (Committee on the Management of Patients With Unstable Angina). J Am Coll Cardiol 2002; 40:1366–1374.
- Yeghiazarians Y, Braunstein JB, Askari A, Stone PH. Unstable angina pectoris. N Engl J Med 2000; 342:101–114.
- Yusuf S, Zhao F, Mehta SR, Chrolavicius S, Tognoni G, Fox KK; Clopidogrel in Unstable Angina to Prevent Recurrent Events Trial Investigators. Effects of clopidogrel in addition to aspirin in patients with acute coronary syndromes without ST-segment elevation. N Engl J Med 2001; 345:494–502.
- Balsano F, Rizzon P, Violi F, et al. Antiplatelet treatment with ticlopidine in unstable angina. A controlled multicenter clinical trial. The Studio della Ticlopidina nell'Angina Instabile Group. Circulation 1990; 82:17–26.
- Schömig A, Neumann FJ, Kastrati A, et al. A randomized comparison of antiplatelet and anticoagulant therapy after the placement of coronary-artery stents. N Engl J Med 1996; 334:1084–1089.
- Mehta SR, Yusuf S, Peters RJG, et al; Clopidogrel in Unstable Angina to Prevent Recurrent Events Trial (CURE) Investigators. Effects of pretreatment with clopidogrel and aspirin followed by long-term therapy in patients undergoing percutaneous coronary intervention: the PCI-CURE study. Lancet 2001; 358:527–533.
- Sabatine MS, Cannon CP, Gibson CM, et al; CLA RITY-TIMI 28 Investigators. Addition of clopidogrel to aspirin and fibrinolytic therapy for myocardial infarction with STsegment elevation. N Engl J Med 2005; 352:1179–1189.
- Sabatine MS, Cannon CP, Gibson CM, et al; Clopidogrel as Adjunctive Reperfusion Therapy (CLARITY)-Thrombolysis in Myocardial Infarction (TIMI) 28 Investigators. Effect of clopidogrel pretreatment before percutaneous coronary intervention in patients with ST-elevation myocardial infarction treated with fibrinolytics: the PCI-CLARITY study. JAMA 2005: 294:1224–1232.
- Chen ZM, Jiang LX, Chen YP, et al; COMMIT (ClOpidogrel and Metoprolol in Myocardial Infarction Trial) collaborative group. Addition of clopidogrel to aspirin in 45,852 patients with acute myocardial infarction: randomised placebo-controlled trial. Lancet 2005; 366:1607–1621.
- Steinhubl SR, Berger PB, Mann JT, et al; CREDO Investigators. Clopidogrel for the reduction of events during observation. Early and sustained dual oral antiplatelet therapy following percutaneous coronary intervention: a randomized controlled trial. JAMA 2002; 288:2411–2420.
- Serebruany VL, Steinhubl SR, Berger PB, Malinin AI, Bhatt DL, Topol EJ. Variability in platelet responsiveness to clopidogrel among 544 individuals. J Am Coll Cardiol 2005; 45:246–251.
- Mega JL, Close SL, Wiviott SD, et al. Cytochrome P-450 polymorphisms and response to clopidogrel. N Engl J Med 2009; 360:354–362.
- Helft G, Osende JI, Worthley SG, et al. Acute antithrombotic effect of a front-loaded regimen of clopidogrel in patients with atherosclerosis on aspirin. Arterioscler Thromb Vasc Biol 2000; 20:2316–2321.
- Weerakkody GJ, Jakubowski JA, Brandt JT, et al. Comparison of speed of onset of platelet inhibition after loading doses of clopidogrel versus prasugrel in healthy volunteers and correlation with responder status. Am J Cardiol 2007; 100:331–336.
- Wiviott SD, Trenk D, Frelinger AL, et al; PRINCIPLETIMI 44 Investigators. Prasugrel compared with high loading-and maintenance-dose clopidogrel in patients with planned percutaneous coronary intervention: the Prasugrel in Comparison to Clopidogrel for Inhibition of Platelet Activation and Aggregation-Thrombolysis in Myocardial Infarction 44 trial. Circulation 2007; 116:2923–2932.
- Wiviott SD, Antman EM, Winters KJ, et al; JUMBO-TIMI 26 Investigators. Randomized comparison of prasugrel (CS-747, LY640315), a novel thienopyridine P2Y12 antagonist, with clopidogrel in percutaneous coronary intervention: results of the Joint Utilization of Medications to Block Platelets Optimally (JUMBO)-TIMI 26 Trial. Circulation 2005; 111:3366–3373.
- Bovill EG, Terrin ML, Stump DC, et al. Hemorrhagic events during therapy with recombinant tissue-type plasminogen activator, heparin, and aspirin for acute myocardial infarction. Results of the Thrombolysis in Myocardial Infarction (TIMI) Phase II Trial. Ann Intern Med 1991; 115:256–265.
- Wiviott SD, Braunwald E, McCabe CH, et al; TRITONTIMI 38 Investigators. Prasugrel versus clopidogrel in patients with acute coronary syndromes. N Engl J Med 2007; 357:2001–2015.
- Antman EM, Wiviott SD, Murphy SA, et al. Early and late benefits of prasugrel in patients with acute coronary syndromes undergoing percutaneous coronary intervention: a TRITON-TIMI 38 (TRial to Assess Improvement in Therapeutic Outcomes by Optimizing Platelet InhibitioN with Prasugrel-Thrombolysis In Myocardial Infarction) analysis. J Am Coll Cardiol 2008; 51:2028–2033.
- Wallentin L, Becker RC, Budaj A, Freij A, Thorsén M, et al; PLATO Investigators. Ticagrelor versus clopidogrel in patients with acute coronary syndromes. N Engl J Med 2009; 361:1045–1057.
- Braunwald E, Antman EM, Beasley JW, et al. ACC/AHA 2002 guideline update for the management of patients with unstable angina and non–ST-segment elevation myocardial infarction—summary article*1: A report of the American College of Cardiology/American Heart Association task force on practice guidelines (Committee on the Management of Patients With Unstable Angina). J Am Coll Cardiol 2002; 40:1366–1374.
KEY POINTS
- The thienopyridines—ticlopidine (Ticlid), clopidogrel (Plavix), and now prasugrel—reduce the risk of death from and serious complications of acute coronary syndromes by inhibiting platelet aggregation.
- Compared with clopidogrel, prasugrel is more potent, faster in onset, and more consistent in inhibiting platelets.
- Prasugrel should be avoided in patients at higher risk of bleeding, including those with a history of stroke or transient ischemic attack, those age 75 or older, or those who weigh less than 60 kg.
Cytogenetic Array Testing Reveals Genome-Wide Abnormalities in Children With Autism
Oligonucleotide microarray analysis reveals a higher yield of abnormalities in children with autism than previously seen with other techniques.
LOUISVILLE—Microarray analysis and testing for fragile X syndrome revealed genetic abnormalities in 10% of children with autism, according to research presented at the 38th National Meeting of the Child Neurology Society. Christa Lese Martin, PhD, and colleagues studied 93 children (79 males) with autism spectrum disorders (ASD) between ages 2 and 7 who were enrolled in an ongoing, comprehensive project aimed at finding meaningful subtypes of autism.
The children underwent a panel of genetic testing, including high-resolution karyotype and fluorescence in situ hybridization (FISH) for 15q, fragile X testing, and array comparative genomic hybridization using the EmArray Cyto array, a custom array of 44,000 oligos designed by Emory Genetics Laboratory in Atlanta, to detect copy number imbalances across the genome.
“Our study demonstrates that clinical microarray—ie, cytogenetic array—testing to look for genome-wide deletions or duplications and fragile X testing is strongly indicated in individuals with ASD,” Dr. Martin, Associate Professor of Human Genetics, Emory University School of Medicine in Atlanta, told Neurology Reviews. “The combination of these two tests identifies a cause for ASD in approximately 10% of individuals. The identification of a cause for ASD not only provides a clinical diagnosis, but also provides the opportunity for accurate genetic counseling for the families.”
New ASD Loci Identified
The pathogenic abnormalities identified included fragile X syndrome, a 17p deletion of the Smith-Magenis region, an unbalanced translocation resulting in duplication of 2p and deletion of 9p, and 16p11.2 duplication syndrome. The children with imbalances had similar cognitive and behavioral profiles as the remainder of the sample and had no obvious clues suggesting any underlying genetic abnormalities, the investigators noted.
“Karyotype alone is not sensitive, and multiple FISH probes would be needed to identify the most common autism loci, greatly increasing cost while still missing the important opportunity to examine the rest of the genome,” reported Dr. Martin’s group. “Our results also demonstrate that genome-wide microarray technology has increased yield and provides more cost-effective testing.”
In addition, four subjects had fragile X mutations; one was a full mutation, one was a premutation, and two were grey zone mutations. “Full mutation fragile X is a relatively rare finding in autism samples,” the researchers noted. “But premutation and grey zone mutations are more common than would be expected in a general population sample.”
Array Analysis in Diagnosing ASD
“Cytogenetic array analysis and fragile X testing are currently offered in many clinical genetic laboratories and should be offered as part of the diagnostic workup in the evaluation of ASD,” asserted Dr. Martin, who co-leads the array services at Emory Genetics Laboratory. “In addition, since all of the cases examined in our study had normal G-banded chromosome analysis, but several clinically significant abnormalities were identified by array analysis, these data provide further support that the array should be used as the first-line cytogenetic test since this analysis can identify imbalances that are below the resolution of a routine karyotype.
“The yield of cytogenetic array testing in individuals with ASD is quite high—8% to 10% has now been reported from various studies,” she added. “This information is invaluable to families to alleviate their search for a cause in their children and counsel them appropriately on recurrence risks in their family.”
—Rebecca K. Abma
Suggested Reading
Li MM, Andersson HC. Clinical application of microarray-based molecular cytogenetics: an emerging new era of genomic medicine. J Pediatr. 2009;155(3):311-317
Sebat J, Lakshmi B, Malhotra D, et al. Strong association of de novo copy number mutations with autism. Science. 2007;316(5823):445-449.
Oligonucleotide microarray analysis reveals a higher yield of abnormalities in children with autism than previously seen with other techniques.
LOUISVILLE—Microarray analysis and testing for fragile X syndrome revealed genetic abnormalities in 10% of children with autism, according to research presented at the 38th National Meeting of the Child Neurology Society. Christa Lese Martin, PhD, and colleagues studied 93 children (79 males) with autism spectrum disorders (ASD) between ages 2 and 7 who were enrolled in an ongoing, comprehensive project aimed at finding meaningful subtypes of autism.
The children underwent a panel of genetic testing, including high-resolution karyotype and fluorescence in situ hybridization (FISH) for 15q, fragile X testing, and array comparative genomic hybridization using the EmArray Cyto array, a custom array of 44,000 oligos designed by Emory Genetics Laboratory in Atlanta, to detect copy number imbalances across the genome.
“Our study demonstrates that clinical microarray—ie, cytogenetic array—testing to look for genome-wide deletions or duplications and fragile X testing is strongly indicated in individuals with ASD,” Dr. Martin, Associate Professor of Human Genetics, Emory University School of Medicine in Atlanta, told Neurology Reviews. “The combination of these two tests identifies a cause for ASD in approximately 10% of individuals. The identification of a cause for ASD not only provides a clinical diagnosis, but also provides the opportunity for accurate genetic counseling for the families.”
New ASD Loci Identified
The pathogenic abnormalities identified included fragile X syndrome, a 17p deletion of the Smith-Magenis region, an unbalanced translocation resulting in duplication of 2p and deletion of 9p, and 16p11.2 duplication syndrome. The children with imbalances had similar cognitive and behavioral profiles as the remainder of the sample and had no obvious clues suggesting any underlying genetic abnormalities, the investigators noted.
“Karyotype alone is not sensitive, and multiple FISH probes would be needed to identify the most common autism loci, greatly increasing cost while still missing the important opportunity to examine the rest of the genome,” reported Dr. Martin’s group. “Our results also demonstrate that genome-wide microarray technology has increased yield and provides more cost-effective testing.”
In addition, four subjects had fragile X mutations; one was a full mutation, one was a premutation, and two were grey zone mutations. “Full mutation fragile X is a relatively rare finding in autism samples,” the researchers noted. “But premutation and grey zone mutations are more common than would be expected in a general population sample.”
Array Analysis in Diagnosing ASD
“Cytogenetic array analysis and fragile X testing are currently offered in many clinical genetic laboratories and should be offered as part of the diagnostic workup in the evaluation of ASD,” asserted Dr. Martin, who co-leads the array services at Emory Genetics Laboratory. “In addition, since all of the cases examined in our study had normal G-banded chromosome analysis, but several clinically significant abnormalities were identified by array analysis, these data provide further support that the array should be used as the first-line cytogenetic test since this analysis can identify imbalances that are below the resolution of a routine karyotype.
“The yield of cytogenetic array testing in individuals with ASD is quite high—8% to 10% has now been reported from various studies,” she added. “This information is invaluable to families to alleviate their search for a cause in their children and counsel them appropriately on recurrence risks in their family.”
—Rebecca K. Abma
Oligonucleotide microarray analysis reveals a higher yield of abnormalities in children with autism than previously seen with other techniques.
LOUISVILLE—Microarray analysis and testing for fragile X syndrome revealed genetic abnormalities in 10% of children with autism, according to research presented at the 38th National Meeting of the Child Neurology Society. Christa Lese Martin, PhD, and colleagues studied 93 children (79 males) with autism spectrum disorders (ASD) between ages 2 and 7 who were enrolled in an ongoing, comprehensive project aimed at finding meaningful subtypes of autism.
The children underwent a panel of genetic testing, including high-resolution karyotype and fluorescence in situ hybridization (FISH) for 15q, fragile X testing, and array comparative genomic hybridization using the EmArray Cyto array, a custom array of 44,000 oligos designed by Emory Genetics Laboratory in Atlanta, to detect copy number imbalances across the genome.
“Our study demonstrates that clinical microarray—ie, cytogenetic array—testing to look for genome-wide deletions or duplications and fragile X testing is strongly indicated in individuals with ASD,” Dr. Martin, Associate Professor of Human Genetics, Emory University School of Medicine in Atlanta, told Neurology Reviews. “The combination of these two tests identifies a cause for ASD in approximately 10% of individuals. The identification of a cause for ASD not only provides a clinical diagnosis, but also provides the opportunity for accurate genetic counseling for the families.”
New ASD Loci Identified
The pathogenic abnormalities identified included fragile X syndrome, a 17p deletion of the Smith-Magenis region, an unbalanced translocation resulting in duplication of 2p and deletion of 9p, and 16p11.2 duplication syndrome. The children with imbalances had similar cognitive and behavioral profiles as the remainder of the sample and had no obvious clues suggesting any underlying genetic abnormalities, the investigators noted.
“Karyotype alone is not sensitive, and multiple FISH probes would be needed to identify the most common autism loci, greatly increasing cost while still missing the important opportunity to examine the rest of the genome,” reported Dr. Martin’s group. “Our results also demonstrate that genome-wide microarray technology has increased yield and provides more cost-effective testing.”
In addition, four subjects had fragile X mutations; one was a full mutation, one was a premutation, and two were grey zone mutations. “Full mutation fragile X is a relatively rare finding in autism samples,” the researchers noted. “But premutation and grey zone mutations are more common than would be expected in a general population sample.”
Array Analysis in Diagnosing ASD
“Cytogenetic array analysis and fragile X testing are currently offered in many clinical genetic laboratories and should be offered as part of the diagnostic workup in the evaluation of ASD,” asserted Dr. Martin, who co-leads the array services at Emory Genetics Laboratory. “In addition, since all of the cases examined in our study had normal G-banded chromosome analysis, but several clinically significant abnormalities were identified by array analysis, these data provide further support that the array should be used as the first-line cytogenetic test since this analysis can identify imbalances that are below the resolution of a routine karyotype.
“The yield of cytogenetic array testing in individuals with ASD is quite high—8% to 10% has now been reported from various studies,” she added. “This information is invaluable to families to alleviate their search for a cause in their children and counsel them appropriately on recurrence risks in their family.”
—Rebecca K. Abma
Suggested Reading
Li MM, Andersson HC. Clinical application of microarray-based molecular cytogenetics: an emerging new era of genomic medicine. J Pediatr. 2009;155(3):311-317
Sebat J, Lakshmi B, Malhotra D, et al. Strong association of de novo copy number mutations with autism. Science. 2007;316(5823):445-449.
Suggested Reading
Li MM, Andersson HC. Clinical application of microarray-based molecular cytogenetics: an emerging new era of genomic medicine. J Pediatr. 2009;155(3):311-317
Sebat J, Lakshmi B, Malhotra D, et al. Strong association of de novo copy number mutations with autism. Science. 2007;316(5823):445-449.
Children With Autism Rely on Proprioception During Motor Learning
LOUISVILLE—The autistic brain builds a stronger-than-normal association between motor commands and proprioceptive feedback, which may account for why children with autism have difficulty forming the models necessary to engage not only in motor behavior, but in social and communicative behaviors, according to research presented at the 38th National Meeting of the Child Neurology Society.
Stewart H. Mostofsky, MD, Associate Professor of Neurology at Kennedy Krieger Institute and the Johns Hopkins University School of Medicine in Baltimore, and colleagues, observed 14 children with autism spectrum disorders and 13 typically developing children as they learned to control a robotic arm. Subjects attempted to reach a target of interest while the robotic arm produced a force perpendicular to that location.
To test this hypothesis, children engaged in a second experiment in which Dr. Mostofsky’s group observed and assessed generalization, the signature of activation fields of neurons. “Generalization can tell you about how [children] learn, because you can look at the way they are able to transfer what they learn in one particular state to another,” Dr. Mostofsky said.
“The generalization patterns were strikingly different,” Dr. Mostofsky and colleagues reported. Typically developing children generalized in both proprioceptive and visual coordinates when generating models of behavior; whereas, children with autism spectrum disorders only generalized in proprioceptive coordinates, and approximately twice as strong as the typically developing children. Furthermore, the tendency to generalize in proprioceptive coordinates was highly predictive of autism-associated impairments in performance in skilled motor gestures to imitation, as well as performance of these gestures to command, and with actual tool use (often referred to as “dyspraxia”).
“[Moreover], notions of feed-forward hypotheses would suggest that these same internal models that are the basis of learning skilled movements might also be the basis for which our brain learns to understand and recognize the actions of others,” Dr. Mostofsky stated. Therefore, impaired acquisition of skill movements may contribute to the social deficits associated with autism.
Serum IL-6 Levels
Consistent with this hypothesis, generalization in intrinsic proprioceptive coordinates was highly predictive of higher (more impaired) Autism Diagnostic Observation Schedule scores for children with autism, and predictive of higher (more impaired) Social Responsiveness Scale scores for children with autism and in typically developing children, according to Dr. Mostofsky.
Dr. Mostofsky and colleagues are now examining whether these findings are specific to autism. In addition, they want to determine whether the formation of internal models of action are associated with abnormal patterns of neural connectivity. “Our preliminary diffusion tensor imaging findings do suggest that disorganization of white matter in the primary sensorimotor cortex may be associated with this increased proprioceptive bias,” Dr. Mostofsky commented.
The researchers also want to determine whether these observations can be used to modify the learning patterns in autism, either on a behavioral level, or as cortical stimulation methods used to upregulate visual-premotor connections.
—Laura Sassano
LOUISVILLE—The autistic brain builds a stronger-than-normal association between motor commands and proprioceptive feedback, which may account for why children with autism have difficulty forming the models necessary to engage not only in motor behavior, but in social and communicative behaviors, according to research presented at the 38th National Meeting of the Child Neurology Society.
Stewart H. Mostofsky, MD, Associate Professor of Neurology at Kennedy Krieger Institute and the Johns Hopkins University School of Medicine in Baltimore, and colleagues, observed 14 children with autism spectrum disorders and 13 typically developing children as they learned to control a robotic arm. Subjects attempted to reach a target of interest while the robotic arm produced a force perpendicular to that location.
To test this hypothesis, children engaged in a second experiment in which Dr. Mostofsky’s group observed and assessed generalization, the signature of activation fields of neurons. “Generalization can tell you about how [children] learn, because you can look at the way they are able to transfer what they learn in one particular state to another,” Dr. Mostofsky said.
“The generalization patterns were strikingly different,” Dr. Mostofsky and colleagues reported. Typically developing children generalized in both proprioceptive and visual coordinates when generating models of behavior; whereas, children with autism spectrum disorders only generalized in proprioceptive coordinates, and approximately twice as strong as the typically developing children. Furthermore, the tendency to generalize in proprioceptive coordinates was highly predictive of autism-associated impairments in performance in skilled motor gestures to imitation, as well as performance of these gestures to command, and with actual tool use (often referred to as “dyspraxia”).
“[Moreover], notions of feed-forward hypotheses would suggest that these same internal models that are the basis of learning skilled movements might also be the basis for which our brain learns to understand and recognize the actions of others,” Dr. Mostofsky stated. Therefore, impaired acquisition of skill movements may contribute to the social deficits associated with autism.
Serum IL-6 Levels
Consistent with this hypothesis, generalization in intrinsic proprioceptive coordinates was highly predictive of higher (more impaired) Autism Diagnostic Observation Schedule scores for children with autism, and predictive of higher (more impaired) Social Responsiveness Scale scores for children with autism and in typically developing children, according to Dr. Mostofsky.
Dr. Mostofsky and colleagues are now examining whether these findings are specific to autism. In addition, they want to determine whether the formation of internal models of action are associated with abnormal patterns of neural connectivity. “Our preliminary diffusion tensor imaging findings do suggest that disorganization of white matter in the primary sensorimotor cortex may be associated with this increased proprioceptive bias,” Dr. Mostofsky commented.
The researchers also want to determine whether these observations can be used to modify the learning patterns in autism, either on a behavioral level, or as cortical stimulation methods used to upregulate visual-premotor connections.
—Laura Sassano
LOUISVILLE—The autistic brain builds a stronger-than-normal association between motor commands and proprioceptive feedback, which may account for why children with autism have difficulty forming the models necessary to engage not only in motor behavior, but in social and communicative behaviors, according to research presented at the 38th National Meeting of the Child Neurology Society.
Stewart H. Mostofsky, MD, Associate Professor of Neurology at Kennedy Krieger Institute and the Johns Hopkins University School of Medicine in Baltimore, and colleagues, observed 14 children with autism spectrum disorders and 13 typically developing children as they learned to control a robotic arm. Subjects attempted to reach a target of interest while the robotic arm produced a force perpendicular to that location.
To test this hypothesis, children engaged in a second experiment in which Dr. Mostofsky’s group observed and assessed generalization, the signature of activation fields of neurons. “Generalization can tell you about how [children] learn, because you can look at the way they are able to transfer what they learn in one particular state to another,” Dr. Mostofsky said.
“The generalization patterns were strikingly different,” Dr. Mostofsky and colleagues reported. Typically developing children generalized in both proprioceptive and visual coordinates when generating models of behavior; whereas, children with autism spectrum disorders only generalized in proprioceptive coordinates, and approximately twice as strong as the typically developing children. Furthermore, the tendency to generalize in proprioceptive coordinates was highly predictive of autism-associated impairments in performance in skilled motor gestures to imitation, as well as performance of these gestures to command, and with actual tool use (often referred to as “dyspraxia”).
“[Moreover], notions of feed-forward hypotheses would suggest that these same internal models that are the basis of learning skilled movements might also be the basis for which our brain learns to understand and recognize the actions of others,” Dr. Mostofsky stated. Therefore, impaired acquisition of skill movements may contribute to the social deficits associated with autism.
Serum IL-6 Levels
Consistent with this hypothesis, generalization in intrinsic proprioceptive coordinates was highly predictive of higher (more impaired) Autism Diagnostic Observation Schedule scores for children with autism, and predictive of higher (more impaired) Social Responsiveness Scale scores for children with autism and in typically developing children, according to Dr. Mostofsky.
Dr. Mostofsky and colleagues are now examining whether these findings are specific to autism. In addition, they want to determine whether the formation of internal models of action are associated with abnormal patterns of neural connectivity. “Our preliminary diffusion tensor imaging findings do suggest that disorganization of white matter in the primary sensorimotor cortex may be associated with this increased proprioceptive bias,” Dr. Mostofsky commented.
The researchers also want to determine whether these observations can be used to modify the learning patterns in autism, either on a behavioral level, or as cortical stimulation methods used to upregulate visual-premotor connections.
—Laura Sassano
Eruptive Vellus Hair Cysts: Report of a Pediatric Case With Partial Response to Calcipotriene Therapy
Defensive medicine: Can it increase your malpractice risk?
In his June 2009 address to the American Medical Association, President Obama commented that “doctors feel like they are constantly looking over their shoulder for fear of lawsuits. Some doctors may feel the need to order more tests and treatments to avoid being legally vulnerable.”1 By practicing what the President called “excessive defensive medicine,” doctors provide “more treatment rather than better care” and drive up the cost of health care ( Box ).2-7
This column takes a look at how defensive practices can make psychiatric care more costly and less effective, by answering these questions:
- What is defensive medicine?
- How much medical practice is “defensive,” and what does it cost?
- Do psychiatrists practice defensive medicine?
- Does defensive psychiatric practice lead to suboptimal care?
- Are some defensive practices justified?
- Can you balance good defense with good care?
- Submit your malpractice-related questions to Dr. Mossman at douglas.mossman@dowdenhealth.com.
- Include your name, address, and practice location. If your question is chosen for publication, your name can be withheld by request.
- All readers who submit questions will be included in quarterly drawings for a $50 gift certificate for Professional Risk Management Services, Inc’s online marketplace of risk management publications and resources (www.prms.com).
What is defensive medicine?
In a 1994 study, the U.S. Office of Technology Assessment (OTA) said that defensive medicine occurs “when doctors order tests, procedures, or visits, or avoid high-risk patients or procedures, primarily (but not necessarily or solely) to reduce their exposure to malpractice liability.” This definition does not require that defensive clinical practices provide no benefit to patients, only that the expected benefits are small relative to their costs.8
Preventing the worst outcome
Studies suggest that doctors develop and maintain practice habits—consciously or not—that aim to reduce their risk of getting sued for malpractice. For example, when patients presenting with tick bites express concern about Lyme disease, doctors overuse tests and needlessly prescribe antibiotics.9 Although these practices are not evidence-based, they reduce doctors’ anxiety by “preventing the worst outcome at relatively little risk and cost and avoiding a potential lawsuit at the same time.”10
The OTA estimated that up to 8% of diagnostic procedures were ordered primarily because of conscious concern about malpractice liability, based on physicians’ responses to a set of written scenarios.8 In a recent study, 83% of Massachusetts physicians reported practicing defensive medicine and estimated that defensive reasons accounted for why they ordered:
- 18% of lab tests
- up to 30% of procedures and consultations
- 13% of hospitalizations.11
Almost all high-liability specialists (such as emergency room physicians, surgeons, and obstetrician/gynecologists) report practicing defensive medicine, often gaging in “assurance behavior”—ordering tests, doing diagnostic procedures, and referring patients to consultants.12
Defensive psychiatry
Compared with other specialists, psychiatrists are at lower risk for being sued, but we engage in defensive practices nonetheless. A survey of British psychiatrists found that during the previous month, 75% made clinical decisions—such as “overcautiously” admitting patients or ordering special observation—because of worries about possible legal claims, complaints, or disciplinary action.13
Younger psychiatrists and psychiatrists who have experienced complaints and critical incidents are more likely to practice defensive medicine. This is hardly surprising—a malpractice suit can be very stressful.14 But an amorphous dread of lawsuits affects many psychiatrists, including residents who never have been sued. The result: many needless, countertherapeutic, defensive practices.15,16
1996 study concluded that Medicare hospital costs for coronary care were 5% to 9% lower in states where effective tort reform has made malpractice suits less lucrative for plaintiffs and lawyers.2 A recent study estimated that laws limiting malpractice payments lower health care expenditures by up to 4%.3 Extrapolating these numbers to overall health care costs suggests that defensive medicine generates >$100 billion a year in expenditures.4
Defensive medicine has nonmonetary costs as well. In the United States, the rate of additional mammograms after initial screening is twice that in the United Kingdom, although breast cancer detection rates are similar.5 These differences—which may reflect relative liability fears in the 2 countries5,6 —mean that more American than British women receive false-positive biopsies and experience needless anxiety, surgery, scarring, and infection.6,7
Unintended consequences
Defensive medicine is not just expensive and wasteful. It could increase your risk of litigation if practices result in harm.17 Simon and Shuman16 give examples of how attempts to avoid litigation can compromise clinical care when treating patients at risk for suicide:
- not prescribing clozapine—a treatment known to lower the risk of suicide18 —to a chronically suicidal patient with schizophrenia because of fears of agranulocytosis (see “Clozapine for schizophrenia: Life-threatening or life-saving treatment?” Current Psychiatry, December 2009)
- not recommending electroconvulsive therapy—and possibly prolonging the period when a severely depressed patient is at high risk for suicide—to avoid a lawsuit related to memory loss
- hospitalizing a patient at chronic risk for suicide who could be managed as an outpatient with appropriate safeguards, a practice that could undermine a valuable treatment alliance.
Good clinical care lowers the likelihood of harm to patients, making it a sound risk management practice, though not a complete strategy. Even the best doctors can start to think defensively when confronted with awkward, troubling, or life-threatening situations that could have medicolegal implications.16 For example, when an outpatient threatens to hurt someone else, it may be tempting to just confine him in a hospital (which reduces the doctor’s anxiety) even when other less coercive and more therapeutic options might better resolve the patient’s problems and the risk of violence.
Recognizing that you’re making clinical decisions out of fear of getting sued is the first step toward curtailing needlessly defensive practice. See Table 19 for more strategies.
Table
3 strategies for avoiding needless defensive medicine
| Ask yourself, “If I weren’t worried about getting sued, what would I do?” or “If I were my patient, what would I want me to do?” These questions, which help you identify the best clinical response, also may help you to implement it without taking extraneous defensive measures. |
| “Never worry alone.” This recommendation from the Massachusetts General Hospital and McLean Hospital training programs19 means that if you’re concerned about a case, ask a colleague for a consultation. In addition to being helpful and reassuring, an outside perspective can support nondefensive, patient-oriented decision making. |
| If the treatment course you think is best involves a legal matter, make sure you understand the legal issues. For example, civil commitment is often the right intervention for a mentally ill person who poses a serious risk of harm, but some patients threaten to sue doctors who propose involuntary hospitalization. Your hospital’s attorney may provide explanation and legal guidance if you do not thoroughly understand legal mechanisms or whether you are properly invoking them |
Justifiable defensiveness
Of course, it’s perfectly appropriate for psychiatrists to recognize malpractice risks and take appropriate measures to avoid successful lawsuits. For example, thoughtful documentation of your data gathering, decision making, and informed consent is an appropriate protective practice. Usually, no one sees the documentation, and it contributes little to your patients’ well-being. Good documentation can be inexpensive, however, and if done creatively, can improve data recording that in turn contributes to better treatment.20
1. American Medical Association. Obama addresses physicians at AMA meeting: transcript of President Obama’s remarks. Available at: http://www.ama-assn.org/ama/pub/about-ama/our-people/house-delegates/2009-annual-meeting/speeches/president-obama-speech.shtml. Accessed July 30, 2009.
2. Kessler DP, McClellan M. Do doctors practice defensive medicine? Q J Econ. 1996;111:353-390.
3. Hellinger FJ, Encinosa WE. The impact of state laws limiting malpractice damage awards on health care expenditures. Am J Public Health. 2006;96:1375-1381.
4. McQuillan LJ, Abramyan H, Archie AP. Jackpot justice: the true cost of America’s tort system. San Francisco, CA: Pacific Research Institute; 2007. Available at: http://special.pacificresearch.org/pub/sab/entrep/2007/Jackpot_Justice/Jackpot_Justice.pdf. Accessed August 1, 2009.
5. Smith-Bindman R, Chu PW, Miglioretti DL, et al. Comparison of screening mammography in the United States and the United Kingdom. JAMA. 2003;290:2129-2137.
6. Elmore JG, Taplin SH, Barlow WE, et al. Does litigation influence medical practice? The influence of community radiologists’ medical malpractice perceptions and experience on screening mammography. Radiology. 2005;236:37-46.
7. Gigerenzer G. Calculated risks: how to know when numbers deceive you. New York, NY: Simon & Schuster; 2002.
8. U.S. Congress, Office of Technology Assessment. Defensive medicine and medical malpractice. Washington, DC: U.S. Government Printing Office; July 1994. OTA-H-602.
9. Fix AD, Strickland GT, Grant J. Tick bites and Lyme disease in an endemic setting: problematic use of serologic testing and prophylactic antibiotic therapy. JAMA. 1998;279:206-210.
10. Anderson RE. Billions for defense: the pervasive nature of defensive medicine. Arch Intern Med. 1999;159:2399-2402.
11. Massachusetts Medical Society Investigation of defensive medicine in Massachusetts. Waltham, MA: Massachusetts Medical Society; 2008. Available at: http://www.massmed.org/defensivemedicine. Accessed August 1, 2009.
12. Studdert DM, Mello MM, Sage WM, et al. Defensive medicine among high-risk specialist physicians in a volatile malpractice environment. JAMA. 2005;293:2609-2617.
13. Passmore K, Leung WC. Defensive practice among psychiatrists: a questionnaire survey. Postgrad Med J. 2002;78:671-673.
14. Charles SC. Malpractice distress: help yourself and others survive. Current Psychiatry. 2007;6(2):23-35.
15. Tellefsen C. Commentary: lawyer phobia. J Am Acad Psychiatry Law. 2009;37:162-164.
16. Simon RI, Shuman DW. Therapeutic risk management of clinical-legal dilemmas: should it be a core competency? J Am Acad Psychiatry Law. 2009;37:155-161.
17. Simon RI. Clinical psychiatry and the law. 2nd ed. Arlington, VA: American Psychiatric Publishing; 2003.
18. Meltzer HY, Alphs L, Green AI, et al. Clozapine treatment for suicidality in schizophrenia: International Suicide Intervention Trial (InterSePT). Arch Gen Psychiatry. 2003;60:82-91.
19. Donovan A. Challenges may be daunting, but APA helps meet them. Psychiatric News. 2007;42(12):13.-
20. Mossman D. Tips to make documentation easier, faster, and more satisfying. Current Psychiatry. 2008;7(2):80-86.
In his June 2009 address to the American Medical Association, President Obama commented that “doctors feel like they are constantly looking over their shoulder for fear of lawsuits. Some doctors may feel the need to order more tests and treatments to avoid being legally vulnerable.”1 By practicing what the President called “excessive defensive medicine,” doctors provide “more treatment rather than better care” and drive up the cost of health care ( Box ).2-7
This column takes a look at how defensive practices can make psychiatric care more costly and less effective, by answering these questions:
- What is defensive medicine?
- How much medical practice is “defensive,” and what does it cost?
- Do psychiatrists practice defensive medicine?
- Does defensive psychiatric practice lead to suboptimal care?
- Are some defensive practices justified?
- Can you balance good defense with good care?
- Submit your malpractice-related questions to Dr. Mossman at douglas.mossman@dowdenhealth.com.
- Include your name, address, and practice location. If your question is chosen for publication, your name can be withheld by request.
- All readers who submit questions will be included in quarterly drawings for a $50 gift certificate for Professional Risk Management Services, Inc’s online marketplace of risk management publications and resources (www.prms.com).
What is defensive medicine?
In a 1994 study, the U.S. Office of Technology Assessment (OTA) said that defensive medicine occurs “when doctors order tests, procedures, or visits, or avoid high-risk patients or procedures, primarily (but not necessarily or solely) to reduce their exposure to malpractice liability.” This definition does not require that defensive clinical practices provide no benefit to patients, only that the expected benefits are small relative to their costs.8
Preventing the worst outcome
Studies suggest that doctors develop and maintain practice habits—consciously or not—that aim to reduce their risk of getting sued for malpractice. For example, when patients presenting with tick bites express concern about Lyme disease, doctors overuse tests and needlessly prescribe antibiotics.9 Although these practices are not evidence-based, they reduce doctors’ anxiety by “preventing the worst outcome at relatively little risk and cost and avoiding a potential lawsuit at the same time.”10
The OTA estimated that up to 8% of diagnostic procedures were ordered primarily because of conscious concern about malpractice liability, based on physicians’ responses to a set of written scenarios.8 In a recent study, 83% of Massachusetts physicians reported practicing defensive medicine and estimated that defensive reasons accounted for why they ordered:
- 18% of lab tests
- up to 30% of procedures and consultations
- 13% of hospitalizations.11
Almost all high-liability specialists (such as emergency room physicians, surgeons, and obstetrician/gynecologists) report practicing defensive medicine, often gaging in “assurance behavior”—ordering tests, doing diagnostic procedures, and referring patients to consultants.12
Defensive psychiatry
Compared with other specialists, psychiatrists are at lower risk for being sued, but we engage in defensive practices nonetheless. A survey of British psychiatrists found that during the previous month, 75% made clinical decisions—such as “overcautiously” admitting patients or ordering special observation—because of worries about possible legal claims, complaints, or disciplinary action.13
Younger psychiatrists and psychiatrists who have experienced complaints and critical incidents are more likely to practice defensive medicine. This is hardly surprising—a malpractice suit can be very stressful.14 But an amorphous dread of lawsuits affects many psychiatrists, including residents who never have been sued. The result: many needless, countertherapeutic, defensive practices.15,16
1996 study concluded that Medicare hospital costs for coronary care were 5% to 9% lower in states where effective tort reform has made malpractice suits less lucrative for plaintiffs and lawyers.2 A recent study estimated that laws limiting malpractice payments lower health care expenditures by up to 4%.3 Extrapolating these numbers to overall health care costs suggests that defensive medicine generates >$100 billion a year in expenditures.4
Defensive medicine has nonmonetary costs as well. In the United States, the rate of additional mammograms after initial screening is twice that in the United Kingdom, although breast cancer detection rates are similar.5 These differences—which may reflect relative liability fears in the 2 countries5,6 —mean that more American than British women receive false-positive biopsies and experience needless anxiety, surgery, scarring, and infection.6,7
Unintended consequences
Defensive medicine is not just expensive and wasteful. It could increase your risk of litigation if practices result in harm.17 Simon and Shuman16 give examples of how attempts to avoid litigation can compromise clinical care when treating patients at risk for suicide:
- not prescribing clozapine—a treatment known to lower the risk of suicide18 —to a chronically suicidal patient with schizophrenia because of fears of agranulocytosis (see “Clozapine for schizophrenia: Life-threatening or life-saving treatment?” Current Psychiatry, December 2009)
- not recommending electroconvulsive therapy—and possibly prolonging the period when a severely depressed patient is at high risk for suicide—to avoid a lawsuit related to memory loss
- hospitalizing a patient at chronic risk for suicide who could be managed as an outpatient with appropriate safeguards, a practice that could undermine a valuable treatment alliance.
Good clinical care lowers the likelihood of harm to patients, making it a sound risk management practice, though not a complete strategy. Even the best doctors can start to think defensively when confronted with awkward, troubling, or life-threatening situations that could have medicolegal implications.16 For example, when an outpatient threatens to hurt someone else, it may be tempting to just confine him in a hospital (which reduces the doctor’s anxiety) even when other less coercive and more therapeutic options might better resolve the patient’s problems and the risk of violence.
Recognizing that you’re making clinical decisions out of fear of getting sued is the first step toward curtailing needlessly defensive practice. See Table 19 for more strategies.
Table
3 strategies for avoiding needless defensive medicine
| Ask yourself, “If I weren’t worried about getting sued, what would I do?” or “If I were my patient, what would I want me to do?” These questions, which help you identify the best clinical response, also may help you to implement it without taking extraneous defensive measures. |
| “Never worry alone.” This recommendation from the Massachusetts General Hospital and McLean Hospital training programs19 means that if you’re concerned about a case, ask a colleague for a consultation. In addition to being helpful and reassuring, an outside perspective can support nondefensive, patient-oriented decision making. |
| If the treatment course you think is best involves a legal matter, make sure you understand the legal issues. For example, civil commitment is often the right intervention for a mentally ill person who poses a serious risk of harm, but some patients threaten to sue doctors who propose involuntary hospitalization. Your hospital’s attorney may provide explanation and legal guidance if you do not thoroughly understand legal mechanisms or whether you are properly invoking them |
Justifiable defensiveness
Of course, it’s perfectly appropriate for psychiatrists to recognize malpractice risks and take appropriate measures to avoid successful lawsuits. For example, thoughtful documentation of your data gathering, decision making, and informed consent is an appropriate protective practice. Usually, no one sees the documentation, and it contributes little to your patients’ well-being. Good documentation can be inexpensive, however, and if done creatively, can improve data recording that in turn contributes to better treatment.20
In his June 2009 address to the American Medical Association, President Obama commented that “doctors feel like they are constantly looking over their shoulder for fear of lawsuits. Some doctors may feel the need to order more tests and treatments to avoid being legally vulnerable.”1 By practicing what the President called “excessive defensive medicine,” doctors provide “more treatment rather than better care” and drive up the cost of health care ( Box ).2-7
This column takes a look at how defensive practices can make psychiatric care more costly and less effective, by answering these questions:
- What is defensive medicine?
- How much medical practice is “defensive,” and what does it cost?
- Do psychiatrists practice defensive medicine?
- Does defensive psychiatric practice lead to suboptimal care?
- Are some defensive practices justified?
- Can you balance good defense with good care?
- Submit your malpractice-related questions to Dr. Mossman at douglas.mossman@dowdenhealth.com.
- Include your name, address, and practice location. If your question is chosen for publication, your name can be withheld by request.
- All readers who submit questions will be included in quarterly drawings for a $50 gift certificate for Professional Risk Management Services, Inc’s online marketplace of risk management publications and resources (www.prms.com).
What is defensive medicine?
In a 1994 study, the U.S. Office of Technology Assessment (OTA) said that defensive medicine occurs “when doctors order tests, procedures, or visits, or avoid high-risk patients or procedures, primarily (but not necessarily or solely) to reduce their exposure to malpractice liability.” This definition does not require that defensive clinical practices provide no benefit to patients, only that the expected benefits are small relative to their costs.8
Preventing the worst outcome
Studies suggest that doctors develop and maintain practice habits—consciously or not—that aim to reduce their risk of getting sued for malpractice. For example, when patients presenting with tick bites express concern about Lyme disease, doctors overuse tests and needlessly prescribe antibiotics.9 Although these practices are not evidence-based, they reduce doctors’ anxiety by “preventing the worst outcome at relatively little risk and cost and avoiding a potential lawsuit at the same time.”10
The OTA estimated that up to 8% of diagnostic procedures were ordered primarily because of conscious concern about malpractice liability, based on physicians’ responses to a set of written scenarios.8 In a recent study, 83% of Massachusetts physicians reported practicing defensive medicine and estimated that defensive reasons accounted for why they ordered:
- 18% of lab tests
- up to 30% of procedures and consultations
- 13% of hospitalizations.11
Almost all high-liability specialists (such as emergency room physicians, surgeons, and obstetrician/gynecologists) report practicing defensive medicine, often gaging in “assurance behavior”—ordering tests, doing diagnostic procedures, and referring patients to consultants.12
Defensive psychiatry
Compared with other specialists, psychiatrists are at lower risk for being sued, but we engage in defensive practices nonetheless. A survey of British psychiatrists found that during the previous month, 75% made clinical decisions—such as “overcautiously” admitting patients or ordering special observation—because of worries about possible legal claims, complaints, or disciplinary action.13
Younger psychiatrists and psychiatrists who have experienced complaints and critical incidents are more likely to practice defensive medicine. This is hardly surprising—a malpractice suit can be very stressful.14 But an amorphous dread of lawsuits affects many psychiatrists, including residents who never have been sued. The result: many needless, countertherapeutic, defensive practices.15,16
1996 study concluded that Medicare hospital costs for coronary care were 5% to 9% lower in states where effective tort reform has made malpractice suits less lucrative for plaintiffs and lawyers.2 A recent study estimated that laws limiting malpractice payments lower health care expenditures by up to 4%.3 Extrapolating these numbers to overall health care costs suggests that defensive medicine generates >$100 billion a year in expenditures.4
Defensive medicine has nonmonetary costs as well. In the United States, the rate of additional mammograms after initial screening is twice that in the United Kingdom, although breast cancer detection rates are similar.5 These differences—which may reflect relative liability fears in the 2 countries5,6 —mean that more American than British women receive false-positive biopsies and experience needless anxiety, surgery, scarring, and infection.6,7
Unintended consequences
Defensive medicine is not just expensive and wasteful. It could increase your risk of litigation if practices result in harm.17 Simon and Shuman16 give examples of how attempts to avoid litigation can compromise clinical care when treating patients at risk for suicide:
- not prescribing clozapine—a treatment known to lower the risk of suicide18 —to a chronically suicidal patient with schizophrenia because of fears of agranulocytosis (see “Clozapine for schizophrenia: Life-threatening or life-saving treatment?” Current Psychiatry, December 2009)
- not recommending electroconvulsive therapy—and possibly prolonging the period when a severely depressed patient is at high risk for suicide—to avoid a lawsuit related to memory loss
- hospitalizing a patient at chronic risk for suicide who could be managed as an outpatient with appropriate safeguards, a practice that could undermine a valuable treatment alliance.
Good clinical care lowers the likelihood of harm to patients, making it a sound risk management practice, though not a complete strategy. Even the best doctors can start to think defensively when confronted with awkward, troubling, or life-threatening situations that could have medicolegal implications.16 For example, when an outpatient threatens to hurt someone else, it may be tempting to just confine him in a hospital (which reduces the doctor’s anxiety) even when other less coercive and more therapeutic options might better resolve the patient’s problems and the risk of violence.
Recognizing that you’re making clinical decisions out of fear of getting sued is the first step toward curtailing needlessly defensive practice. See Table 19 for more strategies.
Table
3 strategies for avoiding needless defensive medicine
| Ask yourself, “If I weren’t worried about getting sued, what would I do?” or “If I were my patient, what would I want me to do?” These questions, which help you identify the best clinical response, also may help you to implement it without taking extraneous defensive measures. |
| “Never worry alone.” This recommendation from the Massachusetts General Hospital and McLean Hospital training programs19 means that if you’re concerned about a case, ask a colleague for a consultation. In addition to being helpful and reassuring, an outside perspective can support nondefensive, patient-oriented decision making. |
| If the treatment course you think is best involves a legal matter, make sure you understand the legal issues. For example, civil commitment is often the right intervention for a mentally ill person who poses a serious risk of harm, but some patients threaten to sue doctors who propose involuntary hospitalization. Your hospital’s attorney may provide explanation and legal guidance if you do not thoroughly understand legal mechanisms or whether you are properly invoking them |
Justifiable defensiveness
Of course, it’s perfectly appropriate for psychiatrists to recognize malpractice risks and take appropriate measures to avoid successful lawsuits. For example, thoughtful documentation of your data gathering, decision making, and informed consent is an appropriate protective practice. Usually, no one sees the documentation, and it contributes little to your patients’ well-being. Good documentation can be inexpensive, however, and if done creatively, can improve data recording that in turn contributes to better treatment.20
1. American Medical Association. Obama addresses physicians at AMA meeting: transcript of President Obama’s remarks. Available at: http://www.ama-assn.org/ama/pub/about-ama/our-people/house-delegates/2009-annual-meeting/speeches/president-obama-speech.shtml. Accessed July 30, 2009.
2. Kessler DP, McClellan M. Do doctors practice defensive medicine? Q J Econ. 1996;111:353-390.
3. Hellinger FJ, Encinosa WE. The impact of state laws limiting malpractice damage awards on health care expenditures. Am J Public Health. 2006;96:1375-1381.
4. McQuillan LJ, Abramyan H, Archie AP. Jackpot justice: the true cost of America’s tort system. San Francisco, CA: Pacific Research Institute; 2007. Available at: http://special.pacificresearch.org/pub/sab/entrep/2007/Jackpot_Justice/Jackpot_Justice.pdf. Accessed August 1, 2009.
5. Smith-Bindman R, Chu PW, Miglioretti DL, et al. Comparison of screening mammography in the United States and the United Kingdom. JAMA. 2003;290:2129-2137.
6. Elmore JG, Taplin SH, Barlow WE, et al. Does litigation influence medical practice? The influence of community radiologists’ medical malpractice perceptions and experience on screening mammography. Radiology. 2005;236:37-46.
7. Gigerenzer G. Calculated risks: how to know when numbers deceive you. New York, NY: Simon & Schuster; 2002.
8. U.S. Congress, Office of Technology Assessment. Defensive medicine and medical malpractice. Washington, DC: U.S. Government Printing Office; July 1994. OTA-H-602.
9. Fix AD, Strickland GT, Grant J. Tick bites and Lyme disease in an endemic setting: problematic use of serologic testing and prophylactic antibiotic therapy. JAMA. 1998;279:206-210.
10. Anderson RE. Billions for defense: the pervasive nature of defensive medicine. Arch Intern Med. 1999;159:2399-2402.
11. Massachusetts Medical Society Investigation of defensive medicine in Massachusetts. Waltham, MA: Massachusetts Medical Society; 2008. Available at: http://www.massmed.org/defensivemedicine. Accessed August 1, 2009.
12. Studdert DM, Mello MM, Sage WM, et al. Defensive medicine among high-risk specialist physicians in a volatile malpractice environment. JAMA. 2005;293:2609-2617.
13. Passmore K, Leung WC. Defensive practice among psychiatrists: a questionnaire survey. Postgrad Med J. 2002;78:671-673.
14. Charles SC. Malpractice distress: help yourself and others survive. Current Psychiatry. 2007;6(2):23-35.
15. Tellefsen C. Commentary: lawyer phobia. J Am Acad Psychiatry Law. 2009;37:162-164.
16. Simon RI, Shuman DW. Therapeutic risk management of clinical-legal dilemmas: should it be a core competency? J Am Acad Psychiatry Law. 2009;37:155-161.
17. Simon RI. Clinical psychiatry and the law. 2nd ed. Arlington, VA: American Psychiatric Publishing; 2003.
18. Meltzer HY, Alphs L, Green AI, et al. Clozapine treatment for suicidality in schizophrenia: International Suicide Intervention Trial (InterSePT). Arch Gen Psychiatry. 2003;60:82-91.
19. Donovan A. Challenges may be daunting, but APA helps meet them. Psychiatric News. 2007;42(12):13.-
20. Mossman D. Tips to make documentation easier, faster, and more satisfying. Current Psychiatry. 2008;7(2):80-86.
1. American Medical Association. Obama addresses physicians at AMA meeting: transcript of President Obama’s remarks. Available at: http://www.ama-assn.org/ama/pub/about-ama/our-people/house-delegates/2009-annual-meeting/speeches/president-obama-speech.shtml. Accessed July 30, 2009.
2. Kessler DP, McClellan M. Do doctors practice defensive medicine? Q J Econ. 1996;111:353-390.
3. Hellinger FJ, Encinosa WE. The impact of state laws limiting malpractice damage awards on health care expenditures. Am J Public Health. 2006;96:1375-1381.
4. McQuillan LJ, Abramyan H, Archie AP. Jackpot justice: the true cost of America’s tort system. San Francisco, CA: Pacific Research Institute; 2007. Available at: http://special.pacificresearch.org/pub/sab/entrep/2007/Jackpot_Justice/Jackpot_Justice.pdf. Accessed August 1, 2009.
5. Smith-Bindman R, Chu PW, Miglioretti DL, et al. Comparison of screening mammography in the United States and the United Kingdom. JAMA. 2003;290:2129-2137.
6. Elmore JG, Taplin SH, Barlow WE, et al. Does litigation influence medical practice? The influence of community radiologists’ medical malpractice perceptions and experience on screening mammography. Radiology. 2005;236:37-46.
7. Gigerenzer G. Calculated risks: how to know when numbers deceive you. New York, NY: Simon & Schuster; 2002.
8. U.S. Congress, Office of Technology Assessment. Defensive medicine and medical malpractice. Washington, DC: U.S. Government Printing Office; July 1994. OTA-H-602.
9. Fix AD, Strickland GT, Grant J. Tick bites and Lyme disease in an endemic setting: problematic use of serologic testing and prophylactic antibiotic therapy. JAMA. 1998;279:206-210.
10. Anderson RE. Billions for defense: the pervasive nature of defensive medicine. Arch Intern Med. 1999;159:2399-2402.
11. Massachusetts Medical Society Investigation of defensive medicine in Massachusetts. Waltham, MA: Massachusetts Medical Society; 2008. Available at: http://www.massmed.org/defensivemedicine. Accessed August 1, 2009.
12. Studdert DM, Mello MM, Sage WM, et al. Defensive medicine among high-risk specialist physicians in a volatile malpractice environment. JAMA. 2005;293:2609-2617.
13. Passmore K, Leung WC. Defensive practice among psychiatrists: a questionnaire survey. Postgrad Med J. 2002;78:671-673.
14. Charles SC. Malpractice distress: help yourself and others survive. Current Psychiatry. 2007;6(2):23-35.
15. Tellefsen C. Commentary: lawyer phobia. J Am Acad Psychiatry Law. 2009;37:162-164.
16. Simon RI, Shuman DW. Therapeutic risk management of clinical-legal dilemmas: should it be a core competency? J Am Acad Psychiatry Law. 2009;37:155-161.
17. Simon RI. Clinical psychiatry and the law. 2nd ed. Arlington, VA: American Psychiatric Publishing; 2003.
18. Meltzer HY, Alphs L, Green AI, et al. Clozapine treatment for suicidality in schizophrenia: International Suicide Intervention Trial (InterSePT). Arch Gen Psychiatry. 2003;60:82-91.
19. Donovan A. Challenges may be daunting, but APA helps meet them. Psychiatric News. 2007;42(12):13.-
20. Mossman D. Tips to make documentation easier, faster, and more satisfying. Current Psychiatry. 2008;7(2):80-86.
Mindfulness-based interventions: Effective for depression and anxiety
Mr. A, age 45, reports irritability, loss of interest, sleep disturbance, increased self-criticism, and decreased self care during the last month after a promotion at work. He has a history of 3 major depressive episodes, 1 of which required hospitalization. For the last 2 years his depressive symptoms had been successfully managed with escitalopram, 10 mg/d, plus bupropion, 150 mg/d. Mr. A wants to discontinue these medications because of sexual dysfunction. He asks if nonpharmacologic strategies might help.
One option to consider for Mr. A is mindfulness-based cognitive therapy (MBCT), which was originally developed to help prevent depressive relapse. MBCT also can reduce depression and anxiety symptoms. More recently, MBCT was shown to help individuals discontinue antidepressants after recovering from depression.
Regular mindfulness meditation has been shown to result in structural brain changes that may help explain how the practice effectively addresses psychiatric symptoms ( Box ). With appropriate training, psychiatrists can help patients reap the benefits of this cognitive treatment.
Regular mindfulness practice has been shown to increase cortical thickness in areas associated with attention, interoception, and sensory processing, such as the prefrontal cortex and right anterior insula.a This supports the hypothesis that mindfulness is a way of attuning the mind to one’s internal processes, and that this involves the same social neural circuits involved in interpersonal attunement—middle prefrontal regions, insula, superior temporal cortex, and the mirror neuron system.b
Amygdala responses. Mindfulness improves affect regulation by optimizing prefrontal cortex regulation of the amygdala. Recent developments in understanding the pathophysiology of depression have highlighted the lack of engagement of left lateral-ventromedial prefrontal circuitry important for the down-regulation of amygdala responses to negative stimuli.c Dispositional mindfulness is associated with greater prefrontal cortical activation and associated greater reduction in amygdala activity during affect labeling tasks, which results in enhanced affect regulation in individuals with higher levels of mindfulness.d
Left-sided anterior activation. Other researchers have examined mindfulness’ role in maintaining balanced prefrontal asymmetry. Relative left prefrontal activation is related to an affective style characterized by stronger tendencies toward positive emotional responses and approach/reward oriented behavior, whereas relative right-sided activation is associated with stronger tendencies toward negative emotional responses and avoidant/withdrawal oriented behavior.
One study found significant increases in left-sided anterior activation in mindfulness-based stress reduction participants compared with controls.e Similarly, in a study evaluating the effect of mindfulness-based cognitive therapy (MBCT) on frontal asymmetry in previously suicidal individuals, MBCT participants retained a balanced pattern of prefrontal activation, whereas the treatment-as-usual group showed significant deterioration toward decreased relative left frontal activation. These findings suggest a protective effect of the mindfulness intervention.f
Source: For references to studies described here see this article at CurrentPsychiatry.com
What is mindfulness meditation?
Meditation refers to a variety of practices that intentionally focus attention to help the practitioner disengage from unconscious absorption in thoughts and feelings. Unlike concentrative meditation—in which practitioners focus attention on a single object such as a word (mantra), body part, or external object—in mindfulness meditation participants bring their attention to a wide range of objects (such as breath, body, emotions, or thoughts) as they appear in moment-by-moment awareness.
Mindfulness is a nonjudgmental, present-centered awareness in which each thought, feeling, or sensation that arises in the attentional field is acknowledged and accepted as it is.1-3 Bishop et al4 defined a 2-component model of mindfulness:
- self-regulating attention of immediate experience, thereby allowing for increased recognition of mental events in the present moment
- adopting an orientation of curiosity, openness, and acceptance toward one’s experiences in each moment.
Mindfulness-based interventions
Buddhist and Western psychology inform the theoretical framework of most mindfulness-based clinical interventions, such as:
- acceptance and commitment therapy (ACT)
- dialectical behavioral therapy (DBT)
- mindfulness-based stress reduction (MBSR)
- MBCT.
Because mindfulness is only 1 of several components of ACT and DBT,5 this review focuses on MBCT and MBSR, in which teaching mindfulness skills is the central focus of treatment.
MBCT and MBSR. MBCT incorporates many aspects of the manualized MBSR treatment program developed for managing chronic pain.6,7 MBSR is devoted almost entirely to cultivating mindfulness through:
- formal mindfulness meditation practices such as body scan (intentionally bringing awareness to bodily sensations), mindful stretching, and mindfulness of breath/body/sounds/thoughts
- informal practices, including mindfulness of daily activities such as eating.1
MBSR typically involves 8 to 10 weekly group sessions of 2 to 2.5 hours with 10 to 40 participants with heterogeneous or homogenous clinical presentations. At each session, patients are taught mindfulness skills and practices. Typically, a full day of meditation practice on a weekend follows session 5 or 6. Participants also engage in a daily meditation practice and homework exercises directed at integrating awareness skills into daily life.
Meta-analytic and narrative reviews generally support MBSR’s efficacy for a wide range of clinical presentations, including improved quality of life for chronic pain and cancer patients.5,8-11 Variability in the methodologic rigor of clinical trials of mindfulness-based interventions—such as lack of active control groups and small sample sizes—limits the strength of these studies’ conclusions, however.8
MBCT integrates the mindfulness training of MBSR with cognitive therapy techniques ( Table 1 ) to prevent the consolidation of ruminative, negative thinking patterns that contribute to depressive relapse.2 These cognitive therapy techniques include:
- psychoeducation about depression symptoms and automatic thoughts
- exercises designed to demonstrate the cognitive model
- identifying activities that provide feelings of mastery and/or pleasure
- creating a specific relapse prevention plan.
In addition, MBCT introduces a new informal meditation—the 3-minute breathing space—to facilitate present-moment awareness in upsetting everyday situations.
Evidence supporting MBCT comes from randomized, controlled trials (RCTs) and uncontrolled trials ( Table 2 ).12-18 A systematic review of RCTs supported using MBCT in addition to usual care to prevent depressive relapse in individuals with a history of ≥3 depressive episodes.19 Since that review was published, a large RCT (123 patients) comparing antidepressant medication alone to antidepressants plus adjunctive MBCT with support to taper/discontinue antidepressant therapy found:
- MBCT comparable to maintenance antidepressant medication in preventing depressive relapse for individuals with ≥3 depressive episodes
- no difference in cost between these 2 treatments.12
In this study, MBCT was more effective than maintenance pharmacotherapy in reducing residual depressive symptoms and in improving quality of life; 75% in the MBCT group discontinued antidepressants. MBCT is included in the United Kingdom’s National Institute for Clinical Excellence Clinical Practice Guidelines for Depression20 for prevention of recurrent depression.
RCTs and uncontrolled studies have shown that MBCT reduces depressive and anxious symptoms in individuals suffering from mood disorders. In an open-label pilot study of MBCT’s efficacy in reducing depressive symptoms in patients with treatment-resistant depression and ≥3 depressive episodes, 61% of patients achieved a post-MBCT Beck Depression Inventory-II (BDI-II) score <14, which represents normal or near-normal mood (mean BDI-II scores decreased from 24.3 to 13.9; effect size 1.04).17
Mindfulness for other psychiatric conditions. A review by Toneatto and Nguyen21 of MBSR in the treatment of anxiety and depression symptoms in a range of clinical populations concluded that the evidence supporting a beneficial effect was equivocal. On the other hand, several uncontrolled studies and 1 RCT indicate that mindfulness-based treatments can reduce symptoms in other psychiatric conditions, including eating disorders,22 generalized anxiety disorder,23 bipolar disorder,24 and attention-deficit/hyperactivity disorder.25 Many of these studies were developed to target mood and anxiety symptoms by linking mindfulness and symptom management; this differs from MBSR, which focuses on stress reduction. Methodologically rigorous studies are necessary to evaluate mindfulness-based treatments in these and other psychiatric conditions.
Table 1
Skills and practices taught in mindfulness training
| MBCT session themes | Mindfulness skill | Associated practices |
|---|---|---|
| ‘Automatic pilot’ (acting without conscious awareness) | Awareness of automatic pilot Awareness of body | Mindful eating Body scan (intentionally bringing awareness to bodily sensations) |
| Dealing with barriers | Awareness of how the chatter of the mind influences feelings and behaviors | Body scan Short breathing meditation |
| Mindfulness of the breath | Awareness of breath and body | Breathing meditation 3-minute breathing space Mindful yoga |
| Staying present | Awareness of attachment and aversion | Breathing meditation Working with intense physical sensations |
| Acceptance | Acceptance of thoughts and emotions as fleeting events | Explicit instructions to practice acceptance are included in the breathing meditation and the 3-minute breathing space |
| Thoughts are not facts | Decentering or re-perceiving | Sitting meditation (awareness of thoughts) |
| How can I best take care of myself? | Awareness of signs of relapse; develop more flexible, deliberate responses at time of potential relapse | 3-minute coping breathing space |
| Dealing with future depression | Awareness of intention | Identifying coping strategies to address barriers to maintaining practice |
| MBCT: mindfulness-based cognitive therapy | ||
| Source: Reference 2 | ||
Table 2
Evidence of reduced depressive symptoms, anxiety with MBCT
| Study | Patients | Findings |
|---|---|---|
| Randomized controlled trials | ||
| Kuyken et al, 200812 | 123 patients with recurrent depression treated with antidepressants received maintenance antidepressants alone or adjunctive MBCT with support to taper/discontinue antidepressant therapy | Adjunctive MBCT was as effective as maintenance antidepressants in reducing relapse/recurrence rates but more effective in reducing residual depressive symptoms and improving quality of life; 75% in the MBCT group discontinued antidepressants |
| Kingston et al, 200713 | 19 outpatients with residual depressive symptoms following a depressive episode assigned to MBCT or treatment as usual | MBCT significantly reduced depressive symptoms, and these improvements were maintained over a 1-month follow-up period |
| Williams et al, 200814 | 14 patients with bipolar disorder who had no manic episodes in the last 6 months and ≤1 week of depressive symptoms in the last 8 weeks | MBCT resulted in a significant reduction in anxiety scores on the BAI compared with wait-list controls |
| Uncontrolled trials | ||
| Eisendrath et al, 200815 | 15 patients with treatment-resistant depression (failure to remit with ≥2 antidepressant trials) | MBCT significantly reduced anxiety and depression; increased mindfulness and decreased rumination and anxiety were associated with decreased depression |
| Finucane and Mercer, 200616 | 13 patients with recurrent depression or recurrent depression and anxiety | MBCT significantly reduced depression and anxiety scores on BDI-II and BAI |
| Kenny and Williams, 200717 | 46 depressed patients who had not fully responded to standard treatments | MBCT significantly reduced depression scores |
| Ree and Craigie, 200718 | 26 outpatients with mood and/or anxiety disorders | MBCT significantly improved symptoms of depression, anxiety, stress, and insomnia; improvements in insomnia were maintained at 3-month follow-up |
| BAI: Beck Anxiety Inventory; BDI-II: Beck Depression Inventory; MBCT: mindfulness-based cognitive therapy | ||
CASE CONTINUED: Explaining the potential benefits
You inform Mr. A that MBCT has been shown to improve acute mild-to-moderate depressive symptoms, may decrease his risk of depressive relapse by 50%26 and could help him discontinue his medications.12 He asks how mindfulness exercises will help his symptoms.
How mindfulness works
The assumption that increased mindfulness mediates treatment outcomes4 has been addressed systematically only recently, following the development of operational definitions of mindfulness and self-report mindfulness measures, including the:
- Mindful Attention Awareness Scale (MAAS)27
- Five Facet Mindfulness Questionnaire (FFMQ)12
- Toronto Mindfulness Scale (TMS).28
Uncontrolled studies using these measures demonstrated that self-reported mindfulness increased following MBSR28,29 and MBCT15,18 in individuals with general stress, anxiety disorder or primary depression, cancer, chronic pain disorder, diabetes, and multiple sclerosis. Accumulating evidence from 1 RCT30 and 2 other uncontrolled studies28,31 demonstrates that mindfulness is associated with symptom reduction following MBSR.
Researchers have begun to focus on how mindfulness skills reduce symptoms. Baer9 proposed several mechanisms, including:
- cognitive change
- improved self-management
- exposure to painful experiences leading to reduced emotional reactivity.
Cognitive change—also called meta-cognitive awareness—is the development of a “distanced “or “decentered” perspective in which patients experience their thoughts and feelings as “mental events” rather than as true, accurate versions of reality. This is thought to introduce a “space” between perception and response that enables patients to have a reflective—rather than a reflexive or reactive—response to situations, which in turn reduces vulnerability to psychological processes that contribute to emotional suffering. Some preliminary evidence suggests that MBCT-associated increases in metacognitive awareness reduce risk of depressive relapse.32
Teaching mindfulness
Guidelines for psychiatrists who wish to become MBCT instructors suggest undergoing formal teacher development training, attending a 7- to 10-day meditation retreat, and establishing your own daily mindfulness practice ( Table 3 ).33 Segal et al2 also recommend recognized training in counseling, psychotherapy, or as a mental health professional, as well as training in cognitive therapy and having experience leading psychotherapy groups.
The recommendation that a mindfulness teacher should practice meditation derives from the view that instructors teach from their own meditation experience and embody the attitudes they invite participants to practice. In an RCT, patients of psychotherapists in training (PiTs) who practiced meditation had greater symptom reductions than those of PiTs who did not engage in meditation.34
To cultivate your own mindfulness practice, consider enrolling in an MBSR group, participating in an MBCT training retreat (see Related Resources ), or attending a mindfulness meditation retreat.
Although patient access to MBCT and MBSR programs has been increasing, formal MBSR/MBCT group programs led by trained therapists are limited. Patients can go through an MBSR/MBCT book with a trained clinician or listen to audio recordings with guided meditation instructions. Alternately, they can join a meditation sitting group or an insight meditation correspondence course ( Table 4 ).
Table 3
Recommended process for becoming an MBCT instructor
| Complete a 5-day residential MBCT training program |
| Attend a 7- to 10-day residential mindfulness meditation retreat |
| Establish your own daily mindfulness meditation practice |
| Undergo professional training in cognitive therapy |
| Gain experience leading psychotherapy groups |
| MBCT: mindfulness-based cognitive therapy |
| Source: References 2,33 |
Table 4
Useful mindfulness resources for interested patients
| Insight Meditation Society: www.dharma.org |
| Kabat-Zinn J. MBSR meditation CDs/tapes: www.stressreductiontapes.com |
| Recordings of meditation (dharma) talks: www.dharmaseed.org |
| Salzberg S, Goldstein J. Insight meditation: an in-depth correspondence course. Louisville, CO: Sounds True, Inc; 2004 |
| Williams M, Teasdale J, Segal Z, et al. The mindful way through depression: freeing yourself from chronic unhappiness. New York, NY: Guilford Press; 2007 |
CASE CONTINUED: Daily mindfulness practice
Mr. A enrolls in and completes a group MBCT program. He rearranges his schedule to include 30 minutes of formal mindfulness practice daily. During an office visit after completing the MBCT course, he describes decreased irritability and self-criticism, newfound self-acceptance, an increased ability to tolerate previously distressing affect, and the ability to set realistic expectations of himself, particularly in light of increased responsibilities at work. He also reports an increased sense of engagement in and reward in his personal life.
Several months later he requests and successfully completes an antidepressant taper and has no recurrence of depressive episodes at 18-month follow-up. He participates in monthly meditation groups to support his home practice.
- Germer CK, Siegel R, Fulton PR, eds. Mindfulness and psychotherapy. New York, NY: Guilford Press; 2005.
- Mindfulness-based cognitive therapy. www.mbct.com; www.mbct.co.uk; www.bangor.ac.uk/mindfulness.
- Center for Mindfulness in Medicine, Health Care, and Society. www.umassmed.edu/cfm.
- Neurobiology of mindfulness. www.mindfulness-matters.org.
- Siegel DJ. The mindful brain: reflection and attunement in the cultivation of well-being. New York, NY: Norton; 2007.
- University of California, San Diego Center for Mindfulness. http://cme.ucsd.edu/mindfulness.
Drug brand names
- Bupropion • Wellbutrin
- Escitalopram • Lexapro
Disclosure
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Acknowledgment
The authors would like to thank Amanda Yu for her assistance in preparing the manuscript.
1. Kabat-Zinn J. Full catastrophe living: using the wisdom of your body and mind to face stress, pain and illness. New York, NY: Dell Publishing; 1990.
2. Segal ZV, Williams JMG, Teasdale JD. Mindfulness-based cognitive therapy for depression: a new approach for preventing relapse. New York, NY: Guilford Press; 2002.
3. Shapiro SL, Schwartz GE. Intentional systemic mindfulness: an integrative model for self-regulation and health. Adv Mind Body Med. 2000;15:128-134.
4. Bishop SR, Lau MA, Shapiro S, et al. Mindfulness: a proposed operational definition. Clin Psychol Sci Pr. 2004;11:230-241.
5. Brown KW, Ryan RM, Creswell JD. Mindfulness: theoretical foundations and evidence for its salutary effects. Psychol Inq. 2007;18(4):211-237.
6. Kabat-Zinn J. An outpatient program in behavioral medicine for chronic pain patients based on the practice of mindfulness meditation: theoretical considerations and preliminary results. Gen Hosp Psychiat. 1982;4(1):33-47.
7. Kabat-Zinn J, Lipworth L, Burney R. The clinical use of mindfulness meditation for the self-regulation of chronic pain. J Behav Med. 1985;8(2):163-190.
8. Bishop SR. What do we really know about mindfulness-based stress reduction? Am Psychosom Soc. 2002;64:71-83.
9. Baer RA. Mindfulness training as a clinical intervention: a conceptual and empirical review. Clin Psychol Sci Prac. 2003;10(2):125-143.
10. Grossman P, Nieman L, Schmidt S, et al. Mindfulness-based stress reduction and health benefits: a meta-analysis. J Psychosom Res. 2004;57(1):35-43.
11. Salmon P, Sephton S, Weissbecker I, et al. Mindfulness meditation in clinical practice. Cog Behav Ther. 2004;11(4):434-446.
12. Kuyken W, Byford S, Taylor RS, et al. Mindfulness-based cognitive therapy to prevent relapse in recurrent depression. J Consult Clin Psych. 2008;76(6):966-978.
13. Kingston T, Dooley B, Bates A, et al. Mindfulness-based cognitive therapy for residual depressive symptoms. Psychol Psychother. 2007;80:193-203.
14. Williams J, Alatiq Y, Crance C, et al. Mindfulness-based cognitive therapy (MBCT) in bipolar disorder: preliminary evaluation of immediate effects on between-episode functioning. J Affect Disord. 2008;107(2):275-279.
15. Eisendrath SJ, Delucchi K, Bitner R, et al. Mindfulness-based cognitive therapy for treatment resistant depression: a pilot study. Psychother Psychosom. 2008;77(5):319-320.
16. Finucane A, Mercer SW. An exploratory mixed methods study of the acceptability and effectiveness of mindfulness-based cognitive therapy for patients with active depression and anxiety in primary care. BMC Psychiatry. 2006;6:14.-
17. Kenny MA, Williams JGM. Treatment-resistant depressed patients show a good response to mindfulness-based cognitive therapy. Behav Res Ther. 2007;45(3):617-625.
18. Ree MJ, Craigie MA. Outcomes following mindfulness-based cognitive therapy in a heterogeneous sample of adult outpatients. Behav Cog Psychother. 2007;24(2):70-86.
19. Coelho HF, Canter PH, Ernst E. Mindfulness-based cognitive therapy: evaluating current evidence and informing future research. J Consult Clin Psych. 2007;75(6):1000-1005.
20. National Institute for Clinical Excellence. Depression: management of depression in primary and secondary care. Clinical guideline 23. 2004. Available at: http://www.nice.org.uk/CG023NICEguideline. Accessed September 30, 2009.
21. Toneatto T, Nguyen L. Does mindfulness meditation improve anxiety and mood symptoms? A review of the controlled research. Can J Psychiatry. 2007;52(4):260-266.
22. Kristeller JL, Hallett B. An exploratory study of a meditation-based intervention for binge eating disorder. J Health Psychol. 1999;4(3):357-363.
23. Evans S, Ferrando S, Findler M, et al. Mindfulness-based cognitive therapy for generalized anxiety disorder. J Anxiety Disord. 2008;22(4):716-721.
24. Williams J, Alatiq Y, Crane C, et al. Mindfulness-based cognitive therapy (MBCT) in bipolar disorder: preliminary evaluation of immediate effects on between-episode functioning. J Affect Disord. 2008;107(2):275-279.
25. Zylowska L, Ackerman DL, Yang MH, et al. Mindfulness meditation training in adults and adolescents with ADHD: a feasibility study. J Atten Disord. 2008;11(6):737-746.
26. Ma SH, Teasdale JD. Mindfulness-based cognitive therapy for depression: replication and exploration of differential relapse prevention effects. J Consult Clin Psychol. 2004;72:31-40.
27. Brown KW, Ryan RM. The benefits of being present: mindfulness and its role in psychological well-being. J Pers Soc Psychol. 2003;84:822-848.
28. Lau MA, Bishop SR, Segal ZV, et al. The Toronto Mindfulness Scale: development and validation. J Clin Psychol. 2006;62:1445-1467.
29. Carmody J, Reed G, Kristeller J, et al. Mindfulness, spirituality, and health-related symptoms. J Psychosom Res. 2008;64(4):393-403.
30. Shapiro SL, Oman D, Thoresen CE, et al. Cultivating mindfulness: effects on well-being. J Clin Psychol. 2008;64(7):840-862.
31. Carmody J, Baer RA. Relationships between mindfulness practice and levels of mindfulness, medical and psychological symptoms and well-being in a mindfulness-based stress reduction program. J Behav Med. 2008;31(1):23-33.
32. Teasdale JD, Moore RG, Hayhurst H, et al. Metacognitive awareness and prevention of relapse in depression: empirical evidence. J Consult Clin Psych. 2002;70:275-287.
33. Lau MA, Segal ZV. Mindfulness based cognitive therapy as a relapse prevention approach to depression. In: Witkiewitz K, Marlatt A, eds. Evidence-based relapse prevention. Oxford, UK: Elsevier Press; 2007:73–90.
34. Grepmair L, Mitterlehner F, Loew T, et al. Promoting mindfulness in psychotherapists in training influences the treatment results of their patients: a randomized, double-blind, controlled study. Psychother Psychosom. 2007;76:332-338.
Mr. A, age 45, reports irritability, loss of interest, sleep disturbance, increased self-criticism, and decreased self care during the last month after a promotion at work. He has a history of 3 major depressive episodes, 1 of which required hospitalization. For the last 2 years his depressive symptoms had been successfully managed with escitalopram, 10 mg/d, plus bupropion, 150 mg/d. Mr. A wants to discontinue these medications because of sexual dysfunction. He asks if nonpharmacologic strategies might help.
One option to consider for Mr. A is mindfulness-based cognitive therapy (MBCT), which was originally developed to help prevent depressive relapse. MBCT also can reduce depression and anxiety symptoms. More recently, MBCT was shown to help individuals discontinue antidepressants after recovering from depression.
Regular mindfulness meditation has been shown to result in structural brain changes that may help explain how the practice effectively addresses psychiatric symptoms ( Box ). With appropriate training, psychiatrists can help patients reap the benefits of this cognitive treatment.
Regular mindfulness practice has been shown to increase cortical thickness in areas associated with attention, interoception, and sensory processing, such as the prefrontal cortex and right anterior insula.a This supports the hypothesis that mindfulness is a way of attuning the mind to one’s internal processes, and that this involves the same social neural circuits involved in interpersonal attunement—middle prefrontal regions, insula, superior temporal cortex, and the mirror neuron system.b
Amygdala responses. Mindfulness improves affect regulation by optimizing prefrontal cortex regulation of the amygdala. Recent developments in understanding the pathophysiology of depression have highlighted the lack of engagement of left lateral-ventromedial prefrontal circuitry important for the down-regulation of amygdala responses to negative stimuli.c Dispositional mindfulness is associated with greater prefrontal cortical activation and associated greater reduction in amygdala activity during affect labeling tasks, which results in enhanced affect regulation in individuals with higher levels of mindfulness.d
Left-sided anterior activation. Other researchers have examined mindfulness’ role in maintaining balanced prefrontal asymmetry. Relative left prefrontal activation is related to an affective style characterized by stronger tendencies toward positive emotional responses and approach/reward oriented behavior, whereas relative right-sided activation is associated with stronger tendencies toward negative emotional responses and avoidant/withdrawal oriented behavior.
One study found significant increases in left-sided anterior activation in mindfulness-based stress reduction participants compared with controls.e Similarly, in a study evaluating the effect of mindfulness-based cognitive therapy (MBCT) on frontal asymmetry in previously suicidal individuals, MBCT participants retained a balanced pattern of prefrontal activation, whereas the treatment-as-usual group showed significant deterioration toward decreased relative left frontal activation. These findings suggest a protective effect of the mindfulness intervention.f
Source: For references to studies described here see this article at CurrentPsychiatry.com
What is mindfulness meditation?
Meditation refers to a variety of practices that intentionally focus attention to help the practitioner disengage from unconscious absorption in thoughts and feelings. Unlike concentrative meditation—in which practitioners focus attention on a single object such as a word (mantra), body part, or external object—in mindfulness meditation participants bring their attention to a wide range of objects (such as breath, body, emotions, or thoughts) as they appear in moment-by-moment awareness.
Mindfulness is a nonjudgmental, present-centered awareness in which each thought, feeling, or sensation that arises in the attentional field is acknowledged and accepted as it is.1-3 Bishop et al4 defined a 2-component model of mindfulness:
- self-regulating attention of immediate experience, thereby allowing for increased recognition of mental events in the present moment
- adopting an orientation of curiosity, openness, and acceptance toward one’s experiences in each moment.
Mindfulness-based interventions
Buddhist and Western psychology inform the theoretical framework of most mindfulness-based clinical interventions, such as:
- acceptance and commitment therapy (ACT)
- dialectical behavioral therapy (DBT)
- mindfulness-based stress reduction (MBSR)
- MBCT.
Because mindfulness is only 1 of several components of ACT and DBT,5 this review focuses on MBCT and MBSR, in which teaching mindfulness skills is the central focus of treatment.
MBCT and MBSR. MBCT incorporates many aspects of the manualized MBSR treatment program developed for managing chronic pain.6,7 MBSR is devoted almost entirely to cultivating mindfulness through:
- formal mindfulness meditation practices such as body scan (intentionally bringing awareness to bodily sensations), mindful stretching, and mindfulness of breath/body/sounds/thoughts
- informal practices, including mindfulness of daily activities such as eating.1
MBSR typically involves 8 to 10 weekly group sessions of 2 to 2.5 hours with 10 to 40 participants with heterogeneous or homogenous clinical presentations. At each session, patients are taught mindfulness skills and practices. Typically, a full day of meditation practice on a weekend follows session 5 or 6. Participants also engage in a daily meditation practice and homework exercises directed at integrating awareness skills into daily life.
Meta-analytic and narrative reviews generally support MBSR’s efficacy for a wide range of clinical presentations, including improved quality of life for chronic pain and cancer patients.5,8-11 Variability in the methodologic rigor of clinical trials of mindfulness-based interventions—such as lack of active control groups and small sample sizes—limits the strength of these studies’ conclusions, however.8
MBCT integrates the mindfulness training of MBSR with cognitive therapy techniques ( Table 1 ) to prevent the consolidation of ruminative, negative thinking patterns that contribute to depressive relapse.2 These cognitive therapy techniques include:
- psychoeducation about depression symptoms and automatic thoughts
- exercises designed to demonstrate the cognitive model
- identifying activities that provide feelings of mastery and/or pleasure
- creating a specific relapse prevention plan.
In addition, MBCT introduces a new informal meditation—the 3-minute breathing space—to facilitate present-moment awareness in upsetting everyday situations.
Evidence supporting MBCT comes from randomized, controlled trials (RCTs) and uncontrolled trials ( Table 2 ).12-18 A systematic review of RCTs supported using MBCT in addition to usual care to prevent depressive relapse in individuals with a history of ≥3 depressive episodes.19 Since that review was published, a large RCT (123 patients) comparing antidepressant medication alone to antidepressants plus adjunctive MBCT with support to taper/discontinue antidepressant therapy found:
- MBCT comparable to maintenance antidepressant medication in preventing depressive relapse for individuals with ≥3 depressive episodes
- no difference in cost between these 2 treatments.12
In this study, MBCT was more effective than maintenance pharmacotherapy in reducing residual depressive symptoms and in improving quality of life; 75% in the MBCT group discontinued antidepressants. MBCT is included in the United Kingdom’s National Institute for Clinical Excellence Clinical Practice Guidelines for Depression20 for prevention of recurrent depression.
RCTs and uncontrolled studies have shown that MBCT reduces depressive and anxious symptoms in individuals suffering from mood disorders. In an open-label pilot study of MBCT’s efficacy in reducing depressive symptoms in patients with treatment-resistant depression and ≥3 depressive episodes, 61% of patients achieved a post-MBCT Beck Depression Inventory-II (BDI-II) score <14, which represents normal or near-normal mood (mean BDI-II scores decreased from 24.3 to 13.9; effect size 1.04).17
Mindfulness for other psychiatric conditions. A review by Toneatto and Nguyen21 of MBSR in the treatment of anxiety and depression symptoms in a range of clinical populations concluded that the evidence supporting a beneficial effect was equivocal. On the other hand, several uncontrolled studies and 1 RCT indicate that mindfulness-based treatments can reduce symptoms in other psychiatric conditions, including eating disorders,22 generalized anxiety disorder,23 bipolar disorder,24 and attention-deficit/hyperactivity disorder.25 Many of these studies were developed to target mood and anxiety symptoms by linking mindfulness and symptom management; this differs from MBSR, which focuses on stress reduction. Methodologically rigorous studies are necessary to evaluate mindfulness-based treatments in these and other psychiatric conditions.
Table 1
Skills and practices taught in mindfulness training
| MBCT session themes | Mindfulness skill | Associated practices |
|---|---|---|
| ‘Automatic pilot’ (acting without conscious awareness) | Awareness of automatic pilot Awareness of body | Mindful eating Body scan (intentionally bringing awareness to bodily sensations) |
| Dealing with barriers | Awareness of how the chatter of the mind influences feelings and behaviors | Body scan Short breathing meditation |
| Mindfulness of the breath | Awareness of breath and body | Breathing meditation 3-minute breathing space Mindful yoga |
| Staying present | Awareness of attachment and aversion | Breathing meditation Working with intense physical sensations |
| Acceptance | Acceptance of thoughts and emotions as fleeting events | Explicit instructions to practice acceptance are included in the breathing meditation and the 3-minute breathing space |
| Thoughts are not facts | Decentering or re-perceiving | Sitting meditation (awareness of thoughts) |
| How can I best take care of myself? | Awareness of signs of relapse; develop more flexible, deliberate responses at time of potential relapse | 3-minute coping breathing space |
| Dealing with future depression | Awareness of intention | Identifying coping strategies to address barriers to maintaining practice |
| MBCT: mindfulness-based cognitive therapy | ||
| Source: Reference 2 | ||
Table 2
Evidence of reduced depressive symptoms, anxiety with MBCT
| Study | Patients | Findings |
|---|---|---|
| Randomized controlled trials | ||
| Kuyken et al, 200812 | 123 patients with recurrent depression treated with antidepressants received maintenance antidepressants alone or adjunctive MBCT with support to taper/discontinue antidepressant therapy | Adjunctive MBCT was as effective as maintenance antidepressants in reducing relapse/recurrence rates but more effective in reducing residual depressive symptoms and improving quality of life; 75% in the MBCT group discontinued antidepressants |
| Kingston et al, 200713 | 19 outpatients with residual depressive symptoms following a depressive episode assigned to MBCT or treatment as usual | MBCT significantly reduced depressive symptoms, and these improvements were maintained over a 1-month follow-up period |
| Williams et al, 200814 | 14 patients with bipolar disorder who had no manic episodes in the last 6 months and ≤1 week of depressive symptoms in the last 8 weeks | MBCT resulted in a significant reduction in anxiety scores on the BAI compared with wait-list controls |
| Uncontrolled trials | ||
| Eisendrath et al, 200815 | 15 patients with treatment-resistant depression (failure to remit with ≥2 antidepressant trials) | MBCT significantly reduced anxiety and depression; increased mindfulness and decreased rumination and anxiety were associated with decreased depression |
| Finucane and Mercer, 200616 | 13 patients with recurrent depression or recurrent depression and anxiety | MBCT significantly reduced depression and anxiety scores on BDI-II and BAI |
| Kenny and Williams, 200717 | 46 depressed patients who had not fully responded to standard treatments | MBCT significantly reduced depression scores |
| Ree and Craigie, 200718 | 26 outpatients with mood and/or anxiety disorders | MBCT significantly improved symptoms of depression, anxiety, stress, and insomnia; improvements in insomnia were maintained at 3-month follow-up |
| BAI: Beck Anxiety Inventory; BDI-II: Beck Depression Inventory; MBCT: mindfulness-based cognitive therapy | ||
CASE CONTINUED: Explaining the potential benefits
You inform Mr. A that MBCT has been shown to improve acute mild-to-moderate depressive symptoms, may decrease his risk of depressive relapse by 50%26 and could help him discontinue his medications.12 He asks how mindfulness exercises will help his symptoms.
How mindfulness works
The assumption that increased mindfulness mediates treatment outcomes4 has been addressed systematically only recently, following the development of operational definitions of mindfulness and self-report mindfulness measures, including the:
- Mindful Attention Awareness Scale (MAAS)27
- Five Facet Mindfulness Questionnaire (FFMQ)12
- Toronto Mindfulness Scale (TMS).28
Uncontrolled studies using these measures demonstrated that self-reported mindfulness increased following MBSR28,29 and MBCT15,18 in individuals with general stress, anxiety disorder or primary depression, cancer, chronic pain disorder, diabetes, and multiple sclerosis. Accumulating evidence from 1 RCT30 and 2 other uncontrolled studies28,31 demonstrates that mindfulness is associated with symptom reduction following MBSR.
Researchers have begun to focus on how mindfulness skills reduce symptoms. Baer9 proposed several mechanisms, including:
- cognitive change
- improved self-management
- exposure to painful experiences leading to reduced emotional reactivity.
Cognitive change—also called meta-cognitive awareness—is the development of a “distanced “or “decentered” perspective in which patients experience their thoughts and feelings as “mental events” rather than as true, accurate versions of reality. This is thought to introduce a “space” between perception and response that enables patients to have a reflective—rather than a reflexive or reactive—response to situations, which in turn reduces vulnerability to psychological processes that contribute to emotional suffering. Some preliminary evidence suggests that MBCT-associated increases in metacognitive awareness reduce risk of depressive relapse.32
Teaching mindfulness
Guidelines for psychiatrists who wish to become MBCT instructors suggest undergoing formal teacher development training, attending a 7- to 10-day meditation retreat, and establishing your own daily mindfulness practice ( Table 3 ).33 Segal et al2 also recommend recognized training in counseling, psychotherapy, or as a mental health professional, as well as training in cognitive therapy and having experience leading psychotherapy groups.
The recommendation that a mindfulness teacher should practice meditation derives from the view that instructors teach from their own meditation experience and embody the attitudes they invite participants to practice. In an RCT, patients of psychotherapists in training (PiTs) who practiced meditation had greater symptom reductions than those of PiTs who did not engage in meditation.34
To cultivate your own mindfulness practice, consider enrolling in an MBSR group, participating in an MBCT training retreat (see Related Resources ), or attending a mindfulness meditation retreat.
Although patient access to MBCT and MBSR programs has been increasing, formal MBSR/MBCT group programs led by trained therapists are limited. Patients can go through an MBSR/MBCT book with a trained clinician or listen to audio recordings with guided meditation instructions. Alternately, they can join a meditation sitting group or an insight meditation correspondence course ( Table 4 ).
Table 3
Recommended process for becoming an MBCT instructor
| Complete a 5-day residential MBCT training program |
| Attend a 7- to 10-day residential mindfulness meditation retreat |
| Establish your own daily mindfulness meditation practice |
| Undergo professional training in cognitive therapy |
| Gain experience leading psychotherapy groups |
| MBCT: mindfulness-based cognitive therapy |
| Source: References 2,33 |
Table 4
Useful mindfulness resources for interested patients
| Insight Meditation Society: www.dharma.org |
| Kabat-Zinn J. MBSR meditation CDs/tapes: www.stressreductiontapes.com |
| Recordings of meditation (dharma) talks: www.dharmaseed.org |
| Salzberg S, Goldstein J. Insight meditation: an in-depth correspondence course. Louisville, CO: Sounds True, Inc; 2004 |
| Williams M, Teasdale J, Segal Z, et al. The mindful way through depression: freeing yourself from chronic unhappiness. New York, NY: Guilford Press; 2007 |
CASE CONTINUED: Daily mindfulness practice
Mr. A enrolls in and completes a group MBCT program. He rearranges his schedule to include 30 minutes of formal mindfulness practice daily. During an office visit after completing the MBCT course, he describes decreased irritability and self-criticism, newfound self-acceptance, an increased ability to tolerate previously distressing affect, and the ability to set realistic expectations of himself, particularly in light of increased responsibilities at work. He also reports an increased sense of engagement in and reward in his personal life.
Several months later he requests and successfully completes an antidepressant taper and has no recurrence of depressive episodes at 18-month follow-up. He participates in monthly meditation groups to support his home practice.
- Germer CK, Siegel R, Fulton PR, eds. Mindfulness and psychotherapy. New York, NY: Guilford Press; 2005.
- Mindfulness-based cognitive therapy. www.mbct.com; www.mbct.co.uk; www.bangor.ac.uk/mindfulness.
- Center for Mindfulness in Medicine, Health Care, and Society. www.umassmed.edu/cfm.
- Neurobiology of mindfulness. www.mindfulness-matters.org.
- Siegel DJ. The mindful brain: reflection and attunement in the cultivation of well-being. New York, NY: Norton; 2007.
- University of California, San Diego Center for Mindfulness. http://cme.ucsd.edu/mindfulness.
Drug brand names
- Bupropion • Wellbutrin
- Escitalopram • Lexapro
Disclosure
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Acknowledgment
The authors would like to thank Amanda Yu for her assistance in preparing the manuscript.
Mr. A, age 45, reports irritability, loss of interest, sleep disturbance, increased self-criticism, and decreased self care during the last month after a promotion at work. He has a history of 3 major depressive episodes, 1 of which required hospitalization. For the last 2 years his depressive symptoms had been successfully managed with escitalopram, 10 mg/d, plus bupropion, 150 mg/d. Mr. A wants to discontinue these medications because of sexual dysfunction. He asks if nonpharmacologic strategies might help.
One option to consider for Mr. A is mindfulness-based cognitive therapy (MBCT), which was originally developed to help prevent depressive relapse. MBCT also can reduce depression and anxiety symptoms. More recently, MBCT was shown to help individuals discontinue antidepressants after recovering from depression.
Regular mindfulness meditation has been shown to result in structural brain changes that may help explain how the practice effectively addresses psychiatric symptoms ( Box ). With appropriate training, psychiatrists can help patients reap the benefits of this cognitive treatment.
Regular mindfulness practice has been shown to increase cortical thickness in areas associated with attention, interoception, and sensory processing, such as the prefrontal cortex and right anterior insula.a This supports the hypothesis that mindfulness is a way of attuning the mind to one’s internal processes, and that this involves the same social neural circuits involved in interpersonal attunement—middle prefrontal regions, insula, superior temporal cortex, and the mirror neuron system.b
Amygdala responses. Mindfulness improves affect regulation by optimizing prefrontal cortex regulation of the amygdala. Recent developments in understanding the pathophysiology of depression have highlighted the lack of engagement of left lateral-ventromedial prefrontal circuitry important for the down-regulation of amygdala responses to negative stimuli.c Dispositional mindfulness is associated with greater prefrontal cortical activation and associated greater reduction in amygdala activity during affect labeling tasks, which results in enhanced affect regulation in individuals with higher levels of mindfulness.d
Left-sided anterior activation. Other researchers have examined mindfulness’ role in maintaining balanced prefrontal asymmetry. Relative left prefrontal activation is related to an affective style characterized by stronger tendencies toward positive emotional responses and approach/reward oriented behavior, whereas relative right-sided activation is associated with stronger tendencies toward negative emotional responses and avoidant/withdrawal oriented behavior.
One study found significant increases in left-sided anterior activation in mindfulness-based stress reduction participants compared with controls.e Similarly, in a study evaluating the effect of mindfulness-based cognitive therapy (MBCT) on frontal asymmetry in previously suicidal individuals, MBCT participants retained a balanced pattern of prefrontal activation, whereas the treatment-as-usual group showed significant deterioration toward decreased relative left frontal activation. These findings suggest a protective effect of the mindfulness intervention.f
Source: For references to studies described here see this article at CurrentPsychiatry.com
What is mindfulness meditation?
Meditation refers to a variety of practices that intentionally focus attention to help the practitioner disengage from unconscious absorption in thoughts and feelings. Unlike concentrative meditation—in which practitioners focus attention on a single object such as a word (mantra), body part, or external object—in mindfulness meditation participants bring their attention to a wide range of objects (such as breath, body, emotions, or thoughts) as they appear in moment-by-moment awareness.
Mindfulness is a nonjudgmental, present-centered awareness in which each thought, feeling, or sensation that arises in the attentional field is acknowledged and accepted as it is.1-3 Bishop et al4 defined a 2-component model of mindfulness:
- self-regulating attention of immediate experience, thereby allowing for increased recognition of mental events in the present moment
- adopting an orientation of curiosity, openness, and acceptance toward one’s experiences in each moment.
Mindfulness-based interventions
Buddhist and Western psychology inform the theoretical framework of most mindfulness-based clinical interventions, such as:
- acceptance and commitment therapy (ACT)
- dialectical behavioral therapy (DBT)
- mindfulness-based stress reduction (MBSR)
- MBCT.
Because mindfulness is only 1 of several components of ACT and DBT,5 this review focuses on MBCT and MBSR, in which teaching mindfulness skills is the central focus of treatment.
MBCT and MBSR. MBCT incorporates many aspects of the manualized MBSR treatment program developed for managing chronic pain.6,7 MBSR is devoted almost entirely to cultivating mindfulness through:
- formal mindfulness meditation practices such as body scan (intentionally bringing awareness to bodily sensations), mindful stretching, and mindfulness of breath/body/sounds/thoughts
- informal practices, including mindfulness of daily activities such as eating.1
MBSR typically involves 8 to 10 weekly group sessions of 2 to 2.5 hours with 10 to 40 participants with heterogeneous or homogenous clinical presentations. At each session, patients are taught mindfulness skills and practices. Typically, a full day of meditation practice on a weekend follows session 5 or 6. Participants also engage in a daily meditation practice and homework exercises directed at integrating awareness skills into daily life.
Meta-analytic and narrative reviews generally support MBSR’s efficacy for a wide range of clinical presentations, including improved quality of life for chronic pain and cancer patients.5,8-11 Variability in the methodologic rigor of clinical trials of mindfulness-based interventions—such as lack of active control groups and small sample sizes—limits the strength of these studies’ conclusions, however.8
MBCT integrates the mindfulness training of MBSR with cognitive therapy techniques ( Table 1 ) to prevent the consolidation of ruminative, negative thinking patterns that contribute to depressive relapse.2 These cognitive therapy techniques include:
- psychoeducation about depression symptoms and automatic thoughts
- exercises designed to demonstrate the cognitive model
- identifying activities that provide feelings of mastery and/or pleasure
- creating a specific relapse prevention plan.
In addition, MBCT introduces a new informal meditation—the 3-minute breathing space—to facilitate present-moment awareness in upsetting everyday situations.
Evidence supporting MBCT comes from randomized, controlled trials (RCTs) and uncontrolled trials ( Table 2 ).12-18 A systematic review of RCTs supported using MBCT in addition to usual care to prevent depressive relapse in individuals with a history of ≥3 depressive episodes.19 Since that review was published, a large RCT (123 patients) comparing antidepressant medication alone to antidepressants plus adjunctive MBCT with support to taper/discontinue antidepressant therapy found:
- MBCT comparable to maintenance antidepressant medication in preventing depressive relapse for individuals with ≥3 depressive episodes
- no difference in cost between these 2 treatments.12
In this study, MBCT was more effective than maintenance pharmacotherapy in reducing residual depressive symptoms and in improving quality of life; 75% in the MBCT group discontinued antidepressants. MBCT is included in the United Kingdom’s National Institute for Clinical Excellence Clinical Practice Guidelines for Depression20 for prevention of recurrent depression.
RCTs and uncontrolled studies have shown that MBCT reduces depressive and anxious symptoms in individuals suffering from mood disorders. In an open-label pilot study of MBCT’s efficacy in reducing depressive symptoms in patients with treatment-resistant depression and ≥3 depressive episodes, 61% of patients achieved a post-MBCT Beck Depression Inventory-II (BDI-II) score <14, which represents normal or near-normal mood (mean BDI-II scores decreased from 24.3 to 13.9; effect size 1.04).17
Mindfulness for other psychiatric conditions. A review by Toneatto and Nguyen21 of MBSR in the treatment of anxiety and depression symptoms in a range of clinical populations concluded that the evidence supporting a beneficial effect was equivocal. On the other hand, several uncontrolled studies and 1 RCT indicate that mindfulness-based treatments can reduce symptoms in other psychiatric conditions, including eating disorders,22 generalized anxiety disorder,23 bipolar disorder,24 and attention-deficit/hyperactivity disorder.25 Many of these studies were developed to target mood and anxiety symptoms by linking mindfulness and symptom management; this differs from MBSR, which focuses on stress reduction. Methodologically rigorous studies are necessary to evaluate mindfulness-based treatments in these and other psychiatric conditions.
Table 1
Skills and practices taught in mindfulness training
| MBCT session themes | Mindfulness skill | Associated practices |
|---|---|---|
| ‘Automatic pilot’ (acting without conscious awareness) | Awareness of automatic pilot Awareness of body | Mindful eating Body scan (intentionally bringing awareness to bodily sensations) |
| Dealing with barriers | Awareness of how the chatter of the mind influences feelings and behaviors | Body scan Short breathing meditation |
| Mindfulness of the breath | Awareness of breath and body | Breathing meditation 3-minute breathing space Mindful yoga |
| Staying present | Awareness of attachment and aversion | Breathing meditation Working with intense physical sensations |
| Acceptance | Acceptance of thoughts and emotions as fleeting events | Explicit instructions to practice acceptance are included in the breathing meditation and the 3-minute breathing space |
| Thoughts are not facts | Decentering or re-perceiving | Sitting meditation (awareness of thoughts) |
| How can I best take care of myself? | Awareness of signs of relapse; develop more flexible, deliberate responses at time of potential relapse | 3-minute coping breathing space |
| Dealing with future depression | Awareness of intention | Identifying coping strategies to address barriers to maintaining practice |
| MBCT: mindfulness-based cognitive therapy | ||
| Source: Reference 2 | ||
Table 2
Evidence of reduced depressive symptoms, anxiety with MBCT
| Study | Patients | Findings |
|---|---|---|
| Randomized controlled trials | ||
| Kuyken et al, 200812 | 123 patients with recurrent depression treated with antidepressants received maintenance antidepressants alone or adjunctive MBCT with support to taper/discontinue antidepressant therapy | Adjunctive MBCT was as effective as maintenance antidepressants in reducing relapse/recurrence rates but more effective in reducing residual depressive symptoms and improving quality of life; 75% in the MBCT group discontinued antidepressants |
| Kingston et al, 200713 | 19 outpatients with residual depressive symptoms following a depressive episode assigned to MBCT or treatment as usual | MBCT significantly reduced depressive symptoms, and these improvements were maintained over a 1-month follow-up period |
| Williams et al, 200814 | 14 patients with bipolar disorder who had no manic episodes in the last 6 months and ≤1 week of depressive symptoms in the last 8 weeks | MBCT resulted in a significant reduction in anxiety scores on the BAI compared with wait-list controls |
| Uncontrolled trials | ||
| Eisendrath et al, 200815 | 15 patients with treatment-resistant depression (failure to remit with ≥2 antidepressant trials) | MBCT significantly reduced anxiety and depression; increased mindfulness and decreased rumination and anxiety were associated with decreased depression |
| Finucane and Mercer, 200616 | 13 patients with recurrent depression or recurrent depression and anxiety | MBCT significantly reduced depression and anxiety scores on BDI-II and BAI |
| Kenny and Williams, 200717 | 46 depressed patients who had not fully responded to standard treatments | MBCT significantly reduced depression scores |
| Ree and Craigie, 200718 | 26 outpatients with mood and/or anxiety disorders | MBCT significantly improved symptoms of depression, anxiety, stress, and insomnia; improvements in insomnia were maintained at 3-month follow-up |
| BAI: Beck Anxiety Inventory; BDI-II: Beck Depression Inventory; MBCT: mindfulness-based cognitive therapy | ||
CASE CONTINUED: Explaining the potential benefits
You inform Mr. A that MBCT has been shown to improve acute mild-to-moderate depressive symptoms, may decrease his risk of depressive relapse by 50%26 and could help him discontinue his medications.12 He asks how mindfulness exercises will help his symptoms.
How mindfulness works
The assumption that increased mindfulness mediates treatment outcomes4 has been addressed systematically only recently, following the development of operational definitions of mindfulness and self-report mindfulness measures, including the:
- Mindful Attention Awareness Scale (MAAS)27
- Five Facet Mindfulness Questionnaire (FFMQ)12
- Toronto Mindfulness Scale (TMS).28
Uncontrolled studies using these measures demonstrated that self-reported mindfulness increased following MBSR28,29 and MBCT15,18 in individuals with general stress, anxiety disorder or primary depression, cancer, chronic pain disorder, diabetes, and multiple sclerosis. Accumulating evidence from 1 RCT30 and 2 other uncontrolled studies28,31 demonstrates that mindfulness is associated with symptom reduction following MBSR.
Researchers have begun to focus on how mindfulness skills reduce symptoms. Baer9 proposed several mechanisms, including:
- cognitive change
- improved self-management
- exposure to painful experiences leading to reduced emotional reactivity.
Cognitive change—also called meta-cognitive awareness—is the development of a “distanced “or “decentered” perspective in which patients experience their thoughts and feelings as “mental events” rather than as true, accurate versions of reality. This is thought to introduce a “space” between perception and response that enables patients to have a reflective—rather than a reflexive or reactive—response to situations, which in turn reduces vulnerability to psychological processes that contribute to emotional suffering. Some preliminary evidence suggests that MBCT-associated increases in metacognitive awareness reduce risk of depressive relapse.32
Teaching mindfulness
Guidelines for psychiatrists who wish to become MBCT instructors suggest undergoing formal teacher development training, attending a 7- to 10-day meditation retreat, and establishing your own daily mindfulness practice ( Table 3 ).33 Segal et al2 also recommend recognized training in counseling, psychotherapy, or as a mental health professional, as well as training in cognitive therapy and having experience leading psychotherapy groups.
The recommendation that a mindfulness teacher should practice meditation derives from the view that instructors teach from their own meditation experience and embody the attitudes they invite participants to practice. In an RCT, patients of psychotherapists in training (PiTs) who practiced meditation had greater symptom reductions than those of PiTs who did not engage in meditation.34
To cultivate your own mindfulness practice, consider enrolling in an MBSR group, participating in an MBCT training retreat (see Related Resources ), or attending a mindfulness meditation retreat.
Although patient access to MBCT and MBSR programs has been increasing, formal MBSR/MBCT group programs led by trained therapists are limited. Patients can go through an MBSR/MBCT book with a trained clinician or listen to audio recordings with guided meditation instructions. Alternately, they can join a meditation sitting group or an insight meditation correspondence course ( Table 4 ).
Table 3
Recommended process for becoming an MBCT instructor
| Complete a 5-day residential MBCT training program |
| Attend a 7- to 10-day residential mindfulness meditation retreat |
| Establish your own daily mindfulness meditation practice |
| Undergo professional training in cognitive therapy |
| Gain experience leading psychotherapy groups |
| MBCT: mindfulness-based cognitive therapy |
| Source: References 2,33 |
Table 4
Useful mindfulness resources for interested patients
| Insight Meditation Society: www.dharma.org |
| Kabat-Zinn J. MBSR meditation CDs/tapes: www.stressreductiontapes.com |
| Recordings of meditation (dharma) talks: www.dharmaseed.org |
| Salzberg S, Goldstein J. Insight meditation: an in-depth correspondence course. Louisville, CO: Sounds True, Inc; 2004 |
| Williams M, Teasdale J, Segal Z, et al. The mindful way through depression: freeing yourself from chronic unhappiness. New York, NY: Guilford Press; 2007 |
CASE CONTINUED: Daily mindfulness practice
Mr. A enrolls in and completes a group MBCT program. He rearranges his schedule to include 30 minutes of formal mindfulness practice daily. During an office visit after completing the MBCT course, he describes decreased irritability and self-criticism, newfound self-acceptance, an increased ability to tolerate previously distressing affect, and the ability to set realistic expectations of himself, particularly in light of increased responsibilities at work. He also reports an increased sense of engagement in and reward in his personal life.
Several months later he requests and successfully completes an antidepressant taper and has no recurrence of depressive episodes at 18-month follow-up. He participates in monthly meditation groups to support his home practice.
- Germer CK, Siegel R, Fulton PR, eds. Mindfulness and psychotherapy. New York, NY: Guilford Press; 2005.
- Mindfulness-based cognitive therapy. www.mbct.com; www.mbct.co.uk; www.bangor.ac.uk/mindfulness.
- Center for Mindfulness in Medicine, Health Care, and Society. www.umassmed.edu/cfm.
- Neurobiology of mindfulness. www.mindfulness-matters.org.
- Siegel DJ. The mindful brain: reflection and attunement in the cultivation of well-being. New York, NY: Norton; 2007.
- University of California, San Diego Center for Mindfulness. http://cme.ucsd.edu/mindfulness.
Drug brand names
- Bupropion • Wellbutrin
- Escitalopram • Lexapro
Disclosure
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Acknowledgment
The authors would like to thank Amanda Yu for her assistance in preparing the manuscript.
1. Kabat-Zinn J. Full catastrophe living: using the wisdom of your body and mind to face stress, pain and illness. New York, NY: Dell Publishing; 1990.
2. Segal ZV, Williams JMG, Teasdale JD. Mindfulness-based cognitive therapy for depression: a new approach for preventing relapse. New York, NY: Guilford Press; 2002.
3. Shapiro SL, Schwartz GE. Intentional systemic mindfulness: an integrative model for self-regulation and health. Adv Mind Body Med. 2000;15:128-134.
4. Bishop SR, Lau MA, Shapiro S, et al. Mindfulness: a proposed operational definition. Clin Psychol Sci Pr. 2004;11:230-241.
5. Brown KW, Ryan RM, Creswell JD. Mindfulness: theoretical foundations and evidence for its salutary effects. Psychol Inq. 2007;18(4):211-237.
6. Kabat-Zinn J. An outpatient program in behavioral medicine for chronic pain patients based on the practice of mindfulness meditation: theoretical considerations and preliminary results. Gen Hosp Psychiat. 1982;4(1):33-47.
7. Kabat-Zinn J, Lipworth L, Burney R. The clinical use of mindfulness meditation for the self-regulation of chronic pain. J Behav Med. 1985;8(2):163-190.
8. Bishop SR. What do we really know about mindfulness-based stress reduction? Am Psychosom Soc. 2002;64:71-83.
9. Baer RA. Mindfulness training as a clinical intervention: a conceptual and empirical review. Clin Psychol Sci Prac. 2003;10(2):125-143.
10. Grossman P, Nieman L, Schmidt S, et al. Mindfulness-based stress reduction and health benefits: a meta-analysis. J Psychosom Res. 2004;57(1):35-43.
11. Salmon P, Sephton S, Weissbecker I, et al. Mindfulness meditation in clinical practice. Cog Behav Ther. 2004;11(4):434-446.
12. Kuyken W, Byford S, Taylor RS, et al. Mindfulness-based cognitive therapy to prevent relapse in recurrent depression. J Consult Clin Psych. 2008;76(6):966-978.
13. Kingston T, Dooley B, Bates A, et al. Mindfulness-based cognitive therapy for residual depressive symptoms. Psychol Psychother. 2007;80:193-203.
14. Williams J, Alatiq Y, Crance C, et al. Mindfulness-based cognitive therapy (MBCT) in bipolar disorder: preliminary evaluation of immediate effects on between-episode functioning. J Affect Disord. 2008;107(2):275-279.
15. Eisendrath SJ, Delucchi K, Bitner R, et al. Mindfulness-based cognitive therapy for treatment resistant depression: a pilot study. Psychother Psychosom. 2008;77(5):319-320.
16. Finucane A, Mercer SW. An exploratory mixed methods study of the acceptability and effectiveness of mindfulness-based cognitive therapy for patients with active depression and anxiety in primary care. BMC Psychiatry. 2006;6:14.-
17. Kenny MA, Williams JGM. Treatment-resistant depressed patients show a good response to mindfulness-based cognitive therapy. Behav Res Ther. 2007;45(3):617-625.
18. Ree MJ, Craigie MA. Outcomes following mindfulness-based cognitive therapy in a heterogeneous sample of adult outpatients. Behav Cog Psychother. 2007;24(2):70-86.
19. Coelho HF, Canter PH, Ernst E. Mindfulness-based cognitive therapy: evaluating current evidence and informing future research. J Consult Clin Psych. 2007;75(6):1000-1005.
20. National Institute for Clinical Excellence. Depression: management of depression in primary and secondary care. Clinical guideline 23. 2004. Available at: http://www.nice.org.uk/CG023NICEguideline. Accessed September 30, 2009.
21. Toneatto T, Nguyen L. Does mindfulness meditation improve anxiety and mood symptoms? A review of the controlled research. Can J Psychiatry. 2007;52(4):260-266.
22. Kristeller JL, Hallett B. An exploratory study of a meditation-based intervention for binge eating disorder. J Health Psychol. 1999;4(3):357-363.
23. Evans S, Ferrando S, Findler M, et al. Mindfulness-based cognitive therapy for generalized anxiety disorder. J Anxiety Disord. 2008;22(4):716-721.
24. Williams J, Alatiq Y, Crane C, et al. Mindfulness-based cognitive therapy (MBCT) in bipolar disorder: preliminary evaluation of immediate effects on between-episode functioning. J Affect Disord. 2008;107(2):275-279.
25. Zylowska L, Ackerman DL, Yang MH, et al. Mindfulness meditation training in adults and adolescents with ADHD: a feasibility study. J Atten Disord. 2008;11(6):737-746.
26. Ma SH, Teasdale JD. Mindfulness-based cognitive therapy for depression: replication and exploration of differential relapse prevention effects. J Consult Clin Psychol. 2004;72:31-40.
27. Brown KW, Ryan RM. The benefits of being present: mindfulness and its role in psychological well-being. J Pers Soc Psychol. 2003;84:822-848.
28. Lau MA, Bishop SR, Segal ZV, et al. The Toronto Mindfulness Scale: development and validation. J Clin Psychol. 2006;62:1445-1467.
29. Carmody J, Reed G, Kristeller J, et al. Mindfulness, spirituality, and health-related symptoms. J Psychosom Res. 2008;64(4):393-403.
30. Shapiro SL, Oman D, Thoresen CE, et al. Cultivating mindfulness: effects on well-being. J Clin Psychol. 2008;64(7):840-862.
31. Carmody J, Baer RA. Relationships between mindfulness practice and levels of mindfulness, medical and psychological symptoms and well-being in a mindfulness-based stress reduction program. J Behav Med. 2008;31(1):23-33.
32. Teasdale JD, Moore RG, Hayhurst H, et al. Metacognitive awareness and prevention of relapse in depression: empirical evidence. J Consult Clin Psych. 2002;70:275-287.
33. Lau MA, Segal ZV. Mindfulness based cognitive therapy as a relapse prevention approach to depression. In: Witkiewitz K, Marlatt A, eds. Evidence-based relapse prevention. Oxford, UK: Elsevier Press; 2007:73–90.
34. Grepmair L, Mitterlehner F, Loew T, et al. Promoting mindfulness in psychotherapists in training influences the treatment results of their patients: a randomized, double-blind, controlled study. Psychother Psychosom. 2007;76:332-338.
1. Kabat-Zinn J. Full catastrophe living: using the wisdom of your body and mind to face stress, pain and illness. New York, NY: Dell Publishing; 1990.
2. Segal ZV, Williams JMG, Teasdale JD. Mindfulness-based cognitive therapy for depression: a new approach for preventing relapse. New York, NY: Guilford Press; 2002.
3. Shapiro SL, Schwartz GE. Intentional systemic mindfulness: an integrative model for self-regulation and health. Adv Mind Body Med. 2000;15:128-134.
4. Bishop SR, Lau MA, Shapiro S, et al. Mindfulness: a proposed operational definition. Clin Psychol Sci Pr. 2004;11:230-241.
5. Brown KW, Ryan RM, Creswell JD. Mindfulness: theoretical foundations and evidence for its salutary effects. Psychol Inq. 2007;18(4):211-237.
6. Kabat-Zinn J. An outpatient program in behavioral medicine for chronic pain patients based on the practice of mindfulness meditation: theoretical considerations and preliminary results. Gen Hosp Psychiat. 1982;4(1):33-47.
7. Kabat-Zinn J, Lipworth L, Burney R. The clinical use of mindfulness meditation for the self-regulation of chronic pain. J Behav Med. 1985;8(2):163-190.
8. Bishop SR. What do we really know about mindfulness-based stress reduction? Am Psychosom Soc. 2002;64:71-83.
9. Baer RA. Mindfulness training as a clinical intervention: a conceptual and empirical review. Clin Psychol Sci Prac. 2003;10(2):125-143.
10. Grossman P, Nieman L, Schmidt S, et al. Mindfulness-based stress reduction and health benefits: a meta-analysis. J Psychosom Res. 2004;57(1):35-43.
11. Salmon P, Sephton S, Weissbecker I, et al. Mindfulness meditation in clinical practice. Cog Behav Ther. 2004;11(4):434-446.
12. Kuyken W, Byford S, Taylor RS, et al. Mindfulness-based cognitive therapy to prevent relapse in recurrent depression. J Consult Clin Psych. 2008;76(6):966-978.
13. Kingston T, Dooley B, Bates A, et al. Mindfulness-based cognitive therapy for residual depressive symptoms. Psychol Psychother. 2007;80:193-203.
14. Williams J, Alatiq Y, Crance C, et al. Mindfulness-based cognitive therapy (MBCT) in bipolar disorder: preliminary evaluation of immediate effects on between-episode functioning. J Affect Disord. 2008;107(2):275-279.
15. Eisendrath SJ, Delucchi K, Bitner R, et al. Mindfulness-based cognitive therapy for treatment resistant depression: a pilot study. Psychother Psychosom. 2008;77(5):319-320.
16. Finucane A, Mercer SW. An exploratory mixed methods study of the acceptability and effectiveness of mindfulness-based cognitive therapy for patients with active depression and anxiety in primary care. BMC Psychiatry. 2006;6:14.-
17. Kenny MA, Williams JGM. Treatment-resistant depressed patients show a good response to mindfulness-based cognitive therapy. Behav Res Ther. 2007;45(3):617-625.
18. Ree MJ, Craigie MA. Outcomes following mindfulness-based cognitive therapy in a heterogeneous sample of adult outpatients. Behav Cog Psychother. 2007;24(2):70-86.
19. Coelho HF, Canter PH, Ernst E. Mindfulness-based cognitive therapy: evaluating current evidence and informing future research. J Consult Clin Psych. 2007;75(6):1000-1005.
20. National Institute for Clinical Excellence. Depression: management of depression in primary and secondary care. Clinical guideline 23. 2004. Available at: http://www.nice.org.uk/CG023NICEguideline. Accessed September 30, 2009.
21. Toneatto T, Nguyen L. Does mindfulness meditation improve anxiety and mood symptoms? A review of the controlled research. Can J Psychiatry. 2007;52(4):260-266.
22. Kristeller JL, Hallett B. An exploratory study of a meditation-based intervention for binge eating disorder. J Health Psychol. 1999;4(3):357-363.
23. Evans S, Ferrando S, Findler M, et al. Mindfulness-based cognitive therapy for generalized anxiety disorder. J Anxiety Disord. 2008;22(4):716-721.
24. Williams J, Alatiq Y, Crane C, et al. Mindfulness-based cognitive therapy (MBCT) in bipolar disorder: preliminary evaluation of immediate effects on between-episode functioning. J Affect Disord. 2008;107(2):275-279.
25. Zylowska L, Ackerman DL, Yang MH, et al. Mindfulness meditation training in adults and adolescents with ADHD: a feasibility study. J Atten Disord. 2008;11(6):737-746.
26. Ma SH, Teasdale JD. Mindfulness-based cognitive therapy for depression: replication and exploration of differential relapse prevention effects. J Consult Clin Psychol. 2004;72:31-40.
27. Brown KW, Ryan RM. The benefits of being present: mindfulness and its role in psychological well-being. J Pers Soc Psychol. 2003;84:822-848.
28. Lau MA, Bishop SR, Segal ZV, et al. The Toronto Mindfulness Scale: development and validation. J Clin Psychol. 2006;62:1445-1467.
29. Carmody J, Reed G, Kristeller J, et al. Mindfulness, spirituality, and health-related symptoms. J Psychosom Res. 2008;64(4):393-403.
30. Shapiro SL, Oman D, Thoresen CE, et al. Cultivating mindfulness: effects on well-being. J Clin Psychol. 2008;64(7):840-862.
31. Carmody J, Baer RA. Relationships between mindfulness practice and levels of mindfulness, medical and psychological symptoms and well-being in a mindfulness-based stress reduction program. J Behav Med. 2008;31(1):23-33.
32. Teasdale JD, Moore RG, Hayhurst H, et al. Metacognitive awareness and prevention of relapse in depression: empirical evidence. J Consult Clin Psych. 2002;70:275-287.
33. Lau MA, Segal ZV. Mindfulness based cognitive therapy as a relapse prevention approach to depression. In: Witkiewitz K, Marlatt A, eds. Evidence-based relapse prevention. Oxford, UK: Elsevier Press; 2007:73–90.
34. Grepmair L, Mitterlehner F, Loew T, et al. Promoting mindfulness in psychotherapists in training influences the treatment results of their patients: a randomized, double-blind, controlled study. Psychother Psychosom. 2007;76:332-338.
The case for HPV immunization
The first quadrivalent human papillomavirus vaccine (HPV4) was licensed in the United States in 2006 (Gardasil, Merck & Co., Inc.).1 It contains viral proteins from HPV types 18, 16, 11, and 6, the types currently responsible for 70% of cervical cancers and 90% of anogenital warts.2 The vaccine is licensed for use in females ages 9 to 26 years for the prevention of cervical, vulvar, and vaginal precancerous lesions and cancer, and for the prevention of anogenital warts.1 It was recently licensed in the United States for the prevention of anogenital warts in males, as it has been in other countries.2,3
HPV and cancer: Quantifying the threat
Human papillomavirus (HPV) is responsible for cancers at several anatomical sites, including the cervix, anus, oral mucosa, vulva, vagina, and penis.1 The rate of cervical cancer in the United States has declined markedly since the introduction of screening programs using cervical cytology testing.1 This decline has been predominantly in squamous cell carcinomas, not adenocarcinomas, which are located in the endocervix and harder to detect.1
There are still around 12,000 cases of cervical cancer diagnosed each year in the United States, for an incidence of 8.1/100,000 women, and 3924 cervical cancer-related deaths.1 In addition, 7% to 10% of the 50 million cervical cytology tests done each year require some form of follow-up. Of these, 2 million to 3 million findings requiring follow-up are atypical squamous cells of undetermined significance (ASC-US) and 1.25 million are low-grade squamous intraepithelial lesions.1
There were more than 4000 cases of anal cancer recorded in 2003, a rate of 1.6/100,000 in women and 1.3/100,000 in men. In contrast to the trend in cervical cancer rates, anal cancer rates are increasing.4 It is not known how many incident cases of genital and anal warts there are annually, but some estimates place the number as high as 1 million. Lifetime cumulative risk has been estimated at 10%.5
Global morbidity and mortality from HPV is considerable, with 500,000 cases of cervical cancer and 260,000 cervical cancerrelated deaths reported worldwide in 2005.2 Rates are highest in developing countries in Latin America, Africa, and Asia.2
The vaccine is effective in women
HPV4 has proven to be highly effective in women ages 15 to 26 who have not been previously infected with the HPV types in the vaccine. Effectiveness has been 98% to 100% after 3 to 5 years in these women, using such end points as moderate and severe cervical intraepithelial neoplasia (CIN2 and CIN3), endocervical adenocarcinoma in situ (AIS), anogenital warts, and vulvar and vaginal intraepithelial neoplasia.1,2,6 These trials are ongoing.
Efficacy among women with current or past HPV infection is less certain. Studies of this question have included only small numbers and the confidence intervals have been large and included 0. In intention-to-treat studies, efficacy has been 39% to 46% for prevention of CIN2 or 3 and AIS caused by HPV 16 and 18, 69% for prevention of HPV 16/18-related vaginal intraepithelial neoplasia, and 68.5% for vaccine type-related warts.1
Who should be vaccinated?
According to the June 2006 recommendations of the Advisory Committee on Immunization Practices (ACIP) of the Centers for Disease Control and Prevention (CDC), immunization with 3 doses of HPV4 should be routine for girls between the ages of 11 and 12. Vaccination may be started in girls as young as age 9 and can also be done for females between the ages of 13 and 26.1 The ACIP recommendations are summarized in TABLE 1.
The World Health Organization (WHO) qualifies its recommendations a bit. “Routine HPV vaccination,” notes WHO, “should be included in national immunization programs provided that:
- prevention of cervical cancer or other HPV-related diseases, or both, constitutes a public health priority,
- vaccine introduction is programmatically feasible,
- sustainable financing can be secured, and
- the cost effectiveness of vaccination strategies in the country or region are considered.”2
WHO also says the vaccine is most effective prior to HPV infection and that, based on the age of initiation of sexual activity, the target population is most likely to be females 9 to 13 years of age. WHO does not recommend vaccination in males.2
In the United States, most professional organizations, including the American Academy of Family Physicians, have adopted recommendations in line with those of ACIP. One exception is the American Cancer Society (ACS), which takes issue with ACIP’s recommendations for the 19- to 26-year age group. The ACS position is that the evidence is insufficient to recommend for or against routine use of the HPV vaccine for this age group.7
TABLE 1
ACIP HPV4 recommendations1
|
| ACIP, Advisory Committee on Immunization Practices |
Some doubts among parents and physicians
Recent national vaccine survey data show that only 25% of females ages 13 to 17 had received 1 or more doses of HPV4 vaccine.8 Young women appear to be interested in the vaccine and in possibly receiving it, but they tend to underestimate their risk of contracting HPV.9,10 Some parents are concerned that the vaccine may encourage risk-taking behavior.11 Physicians report that some parents fear the vaccine is too new to be fully evaluated and are concerned that insurance may not cover the cost of the 3-shot series.12
Physician attitudes toward the vaccine are generally positive. Close to 90% of family physicians and 98% of pediatricians administer the vaccine in their practices. Eighty percent strongly recommend it to 13- to 15-year-olds, and 50% recommend it to 11- to 12-year-olds.
A small minority of family physicians has misconceptions regarding the vaccine:
- 15% believe an HPV test should be ordered before vaccination
- 19% believe the vaccine should not be given to those diagnosed with HPV
- 31% believe a pregnancy test should be ordered before administering the vaccine.12
Safety concerns, minor and major
Clinical trials conducted by the vaccine manufacturer demonstrated slightly higher rates of some systemic adverse reactions in the vaccinated group compared with placebo groups (TABLE 2). Data on adverse reactions at the injection site also showed somewhat higher percentages in the vaccine group. These trials were not large enough to detect severe, rare adverse reactions.
The CDC and the US Food and Drug Administration (FDA) collaboratively operate a passive reporting system, the Vaccine Adverse Events Reporting System (VAERS), as a way of conducting surveillance for these rare events. The manufacturer is required to report suspected adverse events to VAERS, but providers and consumers can also report any suspected adverse events.
There are problems with VAERS. Because it is a passive system, some adverse events may not be reported. At the same time, some events reported by consumers and physicians may be coincidental occurrences not caused by the vaccine. To complicate matters further, patients often receive more than 1 vaccine at the same time, so that attributing any particular adverse reaction to a single vaccine is problematic. These imperfections in VAERS should lead to caution in interpreting reports received on any 1 vaccine.
A recent article published in the Journal of the American Medical Association (JAMA) described the reports on the HPV4 vaccine received by the VAERS for the first 2½ years after licensure.13 Slightly more than 23 million doses had been distributed during this time, and 12,424 adverse events were reported. The most common were syncope (1847), dizziness (1763), nausea (1170), headache (957), and injection site reactions (926). Of all these reported events, 772 reactions were classified as serious, and 32 vaccine recipients died. Investigation of the deaths revealed that the mean time from vaccine to the death was 47 days, the deaths were caused by a variety of underlying conditions, and 4 deaths remained unexplained.
The only 2 serious adverse events that appeared to occur more frequently than background rates were venous thrombotic events, at 1 per 500,000 doses, and syncope, at a rate of 8.2 per 100,000 doses. The syncopal events were concentrated among the 11- to 18-year-olds and resulted in 293 falls and 200 head injuries. The authors of the JAMA article caution about attributing any cause and effect to the venous thromboembolism findings because of the high rates of oral contraceptive use in this age group, which increases the risk of this condition. Studies are ongoing to try to sort out these issues.
TABLE 2
HPV4 systemic adverse events in females, ages 9-23 years1
| Adverse events occurring 1 to 15 days post-vaccination | HPV4 recipients (N=5088) | Placebo recipients (N=3790) |
|---|---|---|
| Pyrexia | 13.0% | 11.2% |
| Nausea | 6.7% | 6.6% |
| Nasopharyngitis | 6.4% | 6.4% |
| Dizziness | 4.0% | 3.7% |
| Diarrhea | 3.6% | 3.5% |
| Vomiting | 2.4% | 1.9% |
| Myalgia | 2.0% | 2.0% |
| Cough | 2.0% | 1.5% |
| Toothache | 1.5% | 1.4% |
| Upper respiratory tract infection | 1.5% | 1.5% |
| Malaise | 1.4% | 1.2% |
| Arthralgia | 1.2% | 0.9% |
| Insomnia | 1.2% | 0.9% |
| Nasal congestion | 1.1% | 0.9% |
New developments: HPV4 for boys, licensing a bivalent vaccine
At its meeting in October 2009, ACIP decided to approve HPV4 for the prevention of anogenital warts in boys and young men ages 9 to 26.14 The potential benefits of using the HPV vaccine in males include reduced incidence of anogenital warts, possible reduction in HPV-related cancers, and reduced transmission of the HPV viruses in the vaccine to women and other men. The ACIP panel did not recommend routine immunization, however, leaving it up to physicians and patients to decide whether the vaccine is worthwhile. The advisory group said it would take up the question of the vaccine’s effectiveness in preventing HPV-related male cancers at future meetings.
At the same meeting, ACIP also voted to recommend Cervarix, the bivalent HPV vaccine from GlaxoSmithKline, for routine use in girls 11 and 12 years of age for the prevention of cancer and precancerous lesions.14 This vaccine contains antigens against HPV types 16 and 18 and does not provide protection against genital warts. Cervarix has been licensed in other countries and, to date, has demonstrated effectiveness comparable to that of the HPV4 against HPV 16- and 18-related outcomes.1,2,6
The availability of 2 HPV vaccines, 1 against both warts and cervical cancer and the other against cervical cancer only, will present some challenging ethical and practical issues for ACIP, as well as for states and physicians.
Unresolved issues
Some critics of the vaccine have pointed out that neither HPV vaccine has yet been proven to prevent cervical cancer. Because the amount of time it takes HPV infection to progress to cervical cancer is, on average, 10 to 20 years, vaccine trials will need to be continued for years to establish this point. However, high-grade cervical lesions and genital warts are outcomes important to patients on their own and are associated with considerable morbidity. It is unknown how continued use of the vaccine will affect the epidemiology of HPV infection and the incidence of HPV types not affected by the vaccine.
Safety monitoring of the vaccine continues. At this time it appears that syncopal episodes occur at increased rates shortly after administration of the HPV4 vaccine, and vaccine providers are encouraged to follow ACIP recommendations of a 15-minute waiting period after the administration of the vaccine.13 Ongoing studies will continue to look at potential rare adverse reactions and determine if the vaccine is truly a cause of venous thromboembolic events.
The approved age range for the use of HPV4 in women for the prevention of cancer, precancerous lesions, and warts may be expanded above 26 years. The benefit among women of this age will be less than for younger women, because of the higher probability of previous exposure to HPV. ACIP will need to decide on whether the vaccine should be routinely or selectively recommended above age 26.
1. Centers for Disease Control and Prevention. Quadrivalent human papillomavirus vaccine: Recommendations of the Advisory Committee on Immunization Practices. March 23, 2007. Available at: http://www.cdc.gov/mmwr/pdf/rr/rr5602.pdf. Accessed November 3, 2009.
2. World Health Organization. Human papillomavirus vaccines. WHO position paper. Weekly Epidemiological Record. 2009;84(15):118-131.Available at: http://www.who.int/wer/2009/wer8415.pdf. Accessed October 27, 2009.
3. U.S. Food and Drug Administration. October 16, 2009 Approval letter—Gardasil. Available at: www.fda.gov/BiologicsBloodVaccines/Vaccines/ApprovedProducts/ucm186991.htm. Accessed October 31, 2009.
4. Johnson LG, Madeleine MM, Newcomer LM, et al. Anal cancer incidence and survival; the surveillance, epidemiology and end results experience, 1973-2000. Cancer. 2004;101:281-288.
5. Trottier H, Franco EL. The epidemiology of genital human papillomavirus infection. Vaccine. 2006;24:1-15.
6. Rambout L, Hopkins L, Hutton B, et al. Prophylactic vaccination against human papillomavirus infection and disease in women: a systematic review of randomized controlled trials. CMAJ. 2007;177:469-479.
7. Saslow D, Castle PE, Cox JT, et al. American Cancer Society guideline for human papillomavirus vaccine use to prevent cervical cancer and its precursors. CA Cancer J Clin. 2007;57:7-28.
8. Centers for Disease Control and Prevention. Vaccination coverage among adolescents aged 13-17 years, United States 2007. MMWR Morb Mortal Wkly Rep. 2008;57:1100-1103.
9. Fisher R, Darrow DH, Tranter M, et al. Human papillomavirus vaccine: recommendations, issues and controversies. Curr Opin Pediatr. 2008;20:441-445.
10. Gerend MA, Magloire ZF. Awareness, knowledge and beliefs about human papillomavirus in a racially diverse sample of young adults. J Adolesc Health. 2008;42:237-242.
11. Advisory Committee on Immunization Practices: summary report, October 22-23, 2008, Atlanta, Ga. Available at: http://www.cdc.gov/vaccines/recs/ACIP/downloads/min-oct08.pdf. Accessed April 27, 2009.
12. Daley M. HPV vaccination practices. A national survey of physicians 18 months post licensure. Presentation at the October 2008 ACIP meeting. Available at: http://cdc.confex.com/cdc/nic2009/webprogram/Paper18003.html. Accessed November 3, 2009.
13. Slade BA, Leidel L, Vellozzi C, et al. Postlicensure safety surveillance for quadrivalent human papillomavirus recombinant vaccine. JAMA. 2009;302:750-757.
14. Meeting of the Advisory Committee on Immunization Practices; October 21-22, 2009; Atlanta, Ga.
The first quadrivalent human papillomavirus vaccine (HPV4) was licensed in the United States in 2006 (Gardasil, Merck & Co., Inc.).1 It contains viral proteins from HPV types 18, 16, 11, and 6, the types currently responsible for 70% of cervical cancers and 90% of anogenital warts.2 The vaccine is licensed for use in females ages 9 to 26 years for the prevention of cervical, vulvar, and vaginal precancerous lesions and cancer, and for the prevention of anogenital warts.1 It was recently licensed in the United States for the prevention of anogenital warts in males, as it has been in other countries.2,3
HPV and cancer: Quantifying the threat
Human papillomavirus (HPV) is responsible for cancers at several anatomical sites, including the cervix, anus, oral mucosa, vulva, vagina, and penis.1 The rate of cervical cancer in the United States has declined markedly since the introduction of screening programs using cervical cytology testing.1 This decline has been predominantly in squamous cell carcinomas, not adenocarcinomas, which are located in the endocervix and harder to detect.1
There are still around 12,000 cases of cervical cancer diagnosed each year in the United States, for an incidence of 8.1/100,000 women, and 3924 cervical cancer-related deaths.1 In addition, 7% to 10% of the 50 million cervical cytology tests done each year require some form of follow-up. Of these, 2 million to 3 million findings requiring follow-up are atypical squamous cells of undetermined significance (ASC-US) and 1.25 million are low-grade squamous intraepithelial lesions.1
There were more than 4000 cases of anal cancer recorded in 2003, a rate of 1.6/100,000 in women and 1.3/100,000 in men. In contrast to the trend in cervical cancer rates, anal cancer rates are increasing.4 It is not known how many incident cases of genital and anal warts there are annually, but some estimates place the number as high as 1 million. Lifetime cumulative risk has been estimated at 10%.5
Global morbidity and mortality from HPV is considerable, with 500,000 cases of cervical cancer and 260,000 cervical cancerrelated deaths reported worldwide in 2005.2 Rates are highest in developing countries in Latin America, Africa, and Asia.2
The vaccine is effective in women
HPV4 has proven to be highly effective in women ages 15 to 26 who have not been previously infected with the HPV types in the vaccine. Effectiveness has been 98% to 100% after 3 to 5 years in these women, using such end points as moderate and severe cervical intraepithelial neoplasia (CIN2 and CIN3), endocervical adenocarcinoma in situ (AIS), anogenital warts, and vulvar and vaginal intraepithelial neoplasia.1,2,6 These trials are ongoing.
Efficacy among women with current or past HPV infection is less certain. Studies of this question have included only small numbers and the confidence intervals have been large and included 0. In intention-to-treat studies, efficacy has been 39% to 46% for prevention of CIN2 or 3 and AIS caused by HPV 16 and 18, 69% for prevention of HPV 16/18-related vaginal intraepithelial neoplasia, and 68.5% for vaccine type-related warts.1
Who should be vaccinated?
According to the June 2006 recommendations of the Advisory Committee on Immunization Practices (ACIP) of the Centers for Disease Control and Prevention (CDC), immunization with 3 doses of HPV4 should be routine for girls between the ages of 11 and 12. Vaccination may be started in girls as young as age 9 and can also be done for females between the ages of 13 and 26.1 The ACIP recommendations are summarized in TABLE 1.
The World Health Organization (WHO) qualifies its recommendations a bit. “Routine HPV vaccination,” notes WHO, “should be included in national immunization programs provided that:
- prevention of cervical cancer or other HPV-related diseases, or both, constitutes a public health priority,
- vaccine introduction is programmatically feasible,
- sustainable financing can be secured, and
- the cost effectiveness of vaccination strategies in the country or region are considered.”2
WHO also says the vaccine is most effective prior to HPV infection and that, based on the age of initiation of sexual activity, the target population is most likely to be females 9 to 13 years of age. WHO does not recommend vaccination in males.2
In the United States, most professional organizations, including the American Academy of Family Physicians, have adopted recommendations in line with those of ACIP. One exception is the American Cancer Society (ACS), which takes issue with ACIP’s recommendations for the 19- to 26-year age group. The ACS position is that the evidence is insufficient to recommend for or against routine use of the HPV vaccine for this age group.7
TABLE 1
ACIP HPV4 recommendations1
|
| ACIP, Advisory Committee on Immunization Practices |
Some doubts among parents and physicians
Recent national vaccine survey data show that only 25% of females ages 13 to 17 had received 1 or more doses of HPV4 vaccine.8 Young women appear to be interested in the vaccine and in possibly receiving it, but they tend to underestimate their risk of contracting HPV.9,10 Some parents are concerned that the vaccine may encourage risk-taking behavior.11 Physicians report that some parents fear the vaccine is too new to be fully evaluated and are concerned that insurance may not cover the cost of the 3-shot series.12
Physician attitudes toward the vaccine are generally positive. Close to 90% of family physicians and 98% of pediatricians administer the vaccine in their practices. Eighty percent strongly recommend it to 13- to 15-year-olds, and 50% recommend it to 11- to 12-year-olds.
A small minority of family physicians has misconceptions regarding the vaccine:
- 15% believe an HPV test should be ordered before vaccination
- 19% believe the vaccine should not be given to those diagnosed with HPV
- 31% believe a pregnancy test should be ordered before administering the vaccine.12
Safety concerns, minor and major
Clinical trials conducted by the vaccine manufacturer demonstrated slightly higher rates of some systemic adverse reactions in the vaccinated group compared with placebo groups (TABLE 2). Data on adverse reactions at the injection site also showed somewhat higher percentages in the vaccine group. These trials were not large enough to detect severe, rare adverse reactions.
The CDC and the US Food and Drug Administration (FDA) collaboratively operate a passive reporting system, the Vaccine Adverse Events Reporting System (VAERS), as a way of conducting surveillance for these rare events. The manufacturer is required to report suspected adverse events to VAERS, but providers and consumers can also report any suspected adverse events.
There are problems with VAERS. Because it is a passive system, some adverse events may not be reported. At the same time, some events reported by consumers and physicians may be coincidental occurrences not caused by the vaccine. To complicate matters further, patients often receive more than 1 vaccine at the same time, so that attributing any particular adverse reaction to a single vaccine is problematic. These imperfections in VAERS should lead to caution in interpreting reports received on any 1 vaccine.
A recent article published in the Journal of the American Medical Association (JAMA) described the reports on the HPV4 vaccine received by the VAERS for the first 2½ years after licensure.13 Slightly more than 23 million doses had been distributed during this time, and 12,424 adverse events were reported. The most common were syncope (1847), dizziness (1763), nausea (1170), headache (957), and injection site reactions (926). Of all these reported events, 772 reactions were classified as serious, and 32 vaccine recipients died. Investigation of the deaths revealed that the mean time from vaccine to the death was 47 days, the deaths were caused by a variety of underlying conditions, and 4 deaths remained unexplained.
The only 2 serious adverse events that appeared to occur more frequently than background rates were venous thrombotic events, at 1 per 500,000 doses, and syncope, at a rate of 8.2 per 100,000 doses. The syncopal events were concentrated among the 11- to 18-year-olds and resulted in 293 falls and 200 head injuries. The authors of the JAMA article caution about attributing any cause and effect to the venous thromboembolism findings because of the high rates of oral contraceptive use in this age group, which increases the risk of this condition. Studies are ongoing to try to sort out these issues.
TABLE 2
HPV4 systemic adverse events in females, ages 9-23 years1
| Adverse events occurring 1 to 15 days post-vaccination | HPV4 recipients (N=5088) | Placebo recipients (N=3790) |
|---|---|---|
| Pyrexia | 13.0% | 11.2% |
| Nausea | 6.7% | 6.6% |
| Nasopharyngitis | 6.4% | 6.4% |
| Dizziness | 4.0% | 3.7% |
| Diarrhea | 3.6% | 3.5% |
| Vomiting | 2.4% | 1.9% |
| Myalgia | 2.0% | 2.0% |
| Cough | 2.0% | 1.5% |
| Toothache | 1.5% | 1.4% |
| Upper respiratory tract infection | 1.5% | 1.5% |
| Malaise | 1.4% | 1.2% |
| Arthralgia | 1.2% | 0.9% |
| Insomnia | 1.2% | 0.9% |
| Nasal congestion | 1.1% | 0.9% |
New developments: HPV4 for boys, licensing a bivalent vaccine
At its meeting in October 2009, ACIP decided to approve HPV4 for the prevention of anogenital warts in boys and young men ages 9 to 26.14 The potential benefits of using the HPV vaccine in males include reduced incidence of anogenital warts, possible reduction in HPV-related cancers, and reduced transmission of the HPV viruses in the vaccine to women and other men. The ACIP panel did not recommend routine immunization, however, leaving it up to physicians and patients to decide whether the vaccine is worthwhile. The advisory group said it would take up the question of the vaccine’s effectiveness in preventing HPV-related male cancers at future meetings.
At the same meeting, ACIP also voted to recommend Cervarix, the bivalent HPV vaccine from GlaxoSmithKline, for routine use in girls 11 and 12 years of age for the prevention of cancer and precancerous lesions.14 This vaccine contains antigens against HPV types 16 and 18 and does not provide protection against genital warts. Cervarix has been licensed in other countries and, to date, has demonstrated effectiveness comparable to that of the HPV4 against HPV 16- and 18-related outcomes.1,2,6
The availability of 2 HPV vaccines, 1 against both warts and cervical cancer and the other against cervical cancer only, will present some challenging ethical and practical issues for ACIP, as well as for states and physicians.
Unresolved issues
Some critics of the vaccine have pointed out that neither HPV vaccine has yet been proven to prevent cervical cancer. Because the amount of time it takes HPV infection to progress to cervical cancer is, on average, 10 to 20 years, vaccine trials will need to be continued for years to establish this point. However, high-grade cervical lesions and genital warts are outcomes important to patients on their own and are associated with considerable morbidity. It is unknown how continued use of the vaccine will affect the epidemiology of HPV infection and the incidence of HPV types not affected by the vaccine.
Safety monitoring of the vaccine continues. At this time it appears that syncopal episodes occur at increased rates shortly after administration of the HPV4 vaccine, and vaccine providers are encouraged to follow ACIP recommendations of a 15-minute waiting period after the administration of the vaccine.13 Ongoing studies will continue to look at potential rare adverse reactions and determine if the vaccine is truly a cause of venous thromboembolic events.
The approved age range for the use of HPV4 in women for the prevention of cancer, precancerous lesions, and warts may be expanded above 26 years. The benefit among women of this age will be less than for younger women, because of the higher probability of previous exposure to HPV. ACIP will need to decide on whether the vaccine should be routinely or selectively recommended above age 26.
The first quadrivalent human papillomavirus vaccine (HPV4) was licensed in the United States in 2006 (Gardasil, Merck & Co., Inc.).1 It contains viral proteins from HPV types 18, 16, 11, and 6, the types currently responsible for 70% of cervical cancers and 90% of anogenital warts.2 The vaccine is licensed for use in females ages 9 to 26 years for the prevention of cervical, vulvar, and vaginal precancerous lesions and cancer, and for the prevention of anogenital warts.1 It was recently licensed in the United States for the prevention of anogenital warts in males, as it has been in other countries.2,3
HPV and cancer: Quantifying the threat
Human papillomavirus (HPV) is responsible for cancers at several anatomical sites, including the cervix, anus, oral mucosa, vulva, vagina, and penis.1 The rate of cervical cancer in the United States has declined markedly since the introduction of screening programs using cervical cytology testing.1 This decline has been predominantly in squamous cell carcinomas, not adenocarcinomas, which are located in the endocervix and harder to detect.1
There are still around 12,000 cases of cervical cancer diagnosed each year in the United States, for an incidence of 8.1/100,000 women, and 3924 cervical cancer-related deaths.1 In addition, 7% to 10% of the 50 million cervical cytology tests done each year require some form of follow-up. Of these, 2 million to 3 million findings requiring follow-up are atypical squamous cells of undetermined significance (ASC-US) and 1.25 million are low-grade squamous intraepithelial lesions.1
There were more than 4000 cases of anal cancer recorded in 2003, a rate of 1.6/100,000 in women and 1.3/100,000 in men. In contrast to the trend in cervical cancer rates, anal cancer rates are increasing.4 It is not known how many incident cases of genital and anal warts there are annually, but some estimates place the number as high as 1 million. Lifetime cumulative risk has been estimated at 10%.5
Global morbidity and mortality from HPV is considerable, with 500,000 cases of cervical cancer and 260,000 cervical cancerrelated deaths reported worldwide in 2005.2 Rates are highest in developing countries in Latin America, Africa, and Asia.2
The vaccine is effective in women
HPV4 has proven to be highly effective in women ages 15 to 26 who have not been previously infected with the HPV types in the vaccine. Effectiveness has been 98% to 100% after 3 to 5 years in these women, using such end points as moderate and severe cervical intraepithelial neoplasia (CIN2 and CIN3), endocervical adenocarcinoma in situ (AIS), anogenital warts, and vulvar and vaginal intraepithelial neoplasia.1,2,6 These trials are ongoing.
Efficacy among women with current or past HPV infection is less certain. Studies of this question have included only small numbers and the confidence intervals have been large and included 0. In intention-to-treat studies, efficacy has been 39% to 46% for prevention of CIN2 or 3 and AIS caused by HPV 16 and 18, 69% for prevention of HPV 16/18-related vaginal intraepithelial neoplasia, and 68.5% for vaccine type-related warts.1
Who should be vaccinated?
According to the June 2006 recommendations of the Advisory Committee on Immunization Practices (ACIP) of the Centers for Disease Control and Prevention (CDC), immunization with 3 doses of HPV4 should be routine for girls between the ages of 11 and 12. Vaccination may be started in girls as young as age 9 and can also be done for females between the ages of 13 and 26.1 The ACIP recommendations are summarized in TABLE 1.
The World Health Organization (WHO) qualifies its recommendations a bit. “Routine HPV vaccination,” notes WHO, “should be included in national immunization programs provided that:
- prevention of cervical cancer or other HPV-related diseases, or both, constitutes a public health priority,
- vaccine introduction is programmatically feasible,
- sustainable financing can be secured, and
- the cost effectiveness of vaccination strategies in the country or region are considered.”2
WHO also says the vaccine is most effective prior to HPV infection and that, based on the age of initiation of sexual activity, the target population is most likely to be females 9 to 13 years of age. WHO does not recommend vaccination in males.2
In the United States, most professional organizations, including the American Academy of Family Physicians, have adopted recommendations in line with those of ACIP. One exception is the American Cancer Society (ACS), which takes issue with ACIP’s recommendations for the 19- to 26-year age group. The ACS position is that the evidence is insufficient to recommend for or against routine use of the HPV vaccine for this age group.7
TABLE 1
ACIP HPV4 recommendations1
|
| ACIP, Advisory Committee on Immunization Practices |
Some doubts among parents and physicians
Recent national vaccine survey data show that only 25% of females ages 13 to 17 had received 1 or more doses of HPV4 vaccine.8 Young women appear to be interested in the vaccine and in possibly receiving it, but they tend to underestimate their risk of contracting HPV.9,10 Some parents are concerned that the vaccine may encourage risk-taking behavior.11 Physicians report that some parents fear the vaccine is too new to be fully evaluated and are concerned that insurance may not cover the cost of the 3-shot series.12
Physician attitudes toward the vaccine are generally positive. Close to 90% of family physicians and 98% of pediatricians administer the vaccine in their practices. Eighty percent strongly recommend it to 13- to 15-year-olds, and 50% recommend it to 11- to 12-year-olds.
A small minority of family physicians has misconceptions regarding the vaccine:
- 15% believe an HPV test should be ordered before vaccination
- 19% believe the vaccine should not be given to those diagnosed with HPV
- 31% believe a pregnancy test should be ordered before administering the vaccine.12
Safety concerns, minor and major
Clinical trials conducted by the vaccine manufacturer demonstrated slightly higher rates of some systemic adverse reactions in the vaccinated group compared with placebo groups (TABLE 2). Data on adverse reactions at the injection site also showed somewhat higher percentages in the vaccine group. These trials were not large enough to detect severe, rare adverse reactions.
The CDC and the US Food and Drug Administration (FDA) collaboratively operate a passive reporting system, the Vaccine Adverse Events Reporting System (VAERS), as a way of conducting surveillance for these rare events. The manufacturer is required to report suspected adverse events to VAERS, but providers and consumers can also report any suspected adverse events.
There are problems with VAERS. Because it is a passive system, some adverse events may not be reported. At the same time, some events reported by consumers and physicians may be coincidental occurrences not caused by the vaccine. To complicate matters further, patients often receive more than 1 vaccine at the same time, so that attributing any particular adverse reaction to a single vaccine is problematic. These imperfections in VAERS should lead to caution in interpreting reports received on any 1 vaccine.
A recent article published in the Journal of the American Medical Association (JAMA) described the reports on the HPV4 vaccine received by the VAERS for the first 2½ years after licensure.13 Slightly more than 23 million doses had been distributed during this time, and 12,424 adverse events were reported. The most common were syncope (1847), dizziness (1763), nausea (1170), headache (957), and injection site reactions (926). Of all these reported events, 772 reactions were classified as serious, and 32 vaccine recipients died. Investigation of the deaths revealed that the mean time from vaccine to the death was 47 days, the deaths were caused by a variety of underlying conditions, and 4 deaths remained unexplained.
The only 2 serious adverse events that appeared to occur more frequently than background rates were venous thrombotic events, at 1 per 500,000 doses, and syncope, at a rate of 8.2 per 100,000 doses. The syncopal events were concentrated among the 11- to 18-year-olds and resulted in 293 falls and 200 head injuries. The authors of the JAMA article caution about attributing any cause and effect to the venous thromboembolism findings because of the high rates of oral contraceptive use in this age group, which increases the risk of this condition. Studies are ongoing to try to sort out these issues.
TABLE 2
HPV4 systemic adverse events in females, ages 9-23 years1
| Adverse events occurring 1 to 15 days post-vaccination | HPV4 recipients (N=5088) | Placebo recipients (N=3790) |
|---|---|---|
| Pyrexia | 13.0% | 11.2% |
| Nausea | 6.7% | 6.6% |
| Nasopharyngitis | 6.4% | 6.4% |
| Dizziness | 4.0% | 3.7% |
| Diarrhea | 3.6% | 3.5% |
| Vomiting | 2.4% | 1.9% |
| Myalgia | 2.0% | 2.0% |
| Cough | 2.0% | 1.5% |
| Toothache | 1.5% | 1.4% |
| Upper respiratory tract infection | 1.5% | 1.5% |
| Malaise | 1.4% | 1.2% |
| Arthralgia | 1.2% | 0.9% |
| Insomnia | 1.2% | 0.9% |
| Nasal congestion | 1.1% | 0.9% |
New developments: HPV4 for boys, licensing a bivalent vaccine
At its meeting in October 2009, ACIP decided to approve HPV4 for the prevention of anogenital warts in boys and young men ages 9 to 26.14 The potential benefits of using the HPV vaccine in males include reduced incidence of anogenital warts, possible reduction in HPV-related cancers, and reduced transmission of the HPV viruses in the vaccine to women and other men. The ACIP panel did not recommend routine immunization, however, leaving it up to physicians and patients to decide whether the vaccine is worthwhile. The advisory group said it would take up the question of the vaccine’s effectiveness in preventing HPV-related male cancers at future meetings.
At the same meeting, ACIP also voted to recommend Cervarix, the bivalent HPV vaccine from GlaxoSmithKline, for routine use in girls 11 and 12 years of age for the prevention of cancer and precancerous lesions.14 This vaccine contains antigens against HPV types 16 and 18 and does not provide protection against genital warts. Cervarix has been licensed in other countries and, to date, has demonstrated effectiveness comparable to that of the HPV4 against HPV 16- and 18-related outcomes.1,2,6
The availability of 2 HPV vaccines, 1 against both warts and cervical cancer and the other against cervical cancer only, will present some challenging ethical and practical issues for ACIP, as well as for states and physicians.
Unresolved issues
Some critics of the vaccine have pointed out that neither HPV vaccine has yet been proven to prevent cervical cancer. Because the amount of time it takes HPV infection to progress to cervical cancer is, on average, 10 to 20 years, vaccine trials will need to be continued for years to establish this point. However, high-grade cervical lesions and genital warts are outcomes important to patients on their own and are associated with considerable morbidity. It is unknown how continued use of the vaccine will affect the epidemiology of HPV infection and the incidence of HPV types not affected by the vaccine.
Safety monitoring of the vaccine continues. At this time it appears that syncopal episodes occur at increased rates shortly after administration of the HPV4 vaccine, and vaccine providers are encouraged to follow ACIP recommendations of a 15-minute waiting period after the administration of the vaccine.13 Ongoing studies will continue to look at potential rare adverse reactions and determine if the vaccine is truly a cause of venous thromboembolic events.
The approved age range for the use of HPV4 in women for the prevention of cancer, precancerous lesions, and warts may be expanded above 26 years. The benefit among women of this age will be less than for younger women, because of the higher probability of previous exposure to HPV. ACIP will need to decide on whether the vaccine should be routinely or selectively recommended above age 26.
1. Centers for Disease Control and Prevention. Quadrivalent human papillomavirus vaccine: Recommendations of the Advisory Committee on Immunization Practices. March 23, 2007. Available at: http://www.cdc.gov/mmwr/pdf/rr/rr5602.pdf. Accessed November 3, 2009.
2. World Health Organization. Human papillomavirus vaccines. WHO position paper. Weekly Epidemiological Record. 2009;84(15):118-131.Available at: http://www.who.int/wer/2009/wer8415.pdf. Accessed October 27, 2009.
3. U.S. Food and Drug Administration. October 16, 2009 Approval letter—Gardasil. Available at: www.fda.gov/BiologicsBloodVaccines/Vaccines/ApprovedProducts/ucm186991.htm. Accessed October 31, 2009.
4. Johnson LG, Madeleine MM, Newcomer LM, et al. Anal cancer incidence and survival; the surveillance, epidemiology and end results experience, 1973-2000. Cancer. 2004;101:281-288.
5. Trottier H, Franco EL. The epidemiology of genital human papillomavirus infection. Vaccine. 2006;24:1-15.
6. Rambout L, Hopkins L, Hutton B, et al. Prophylactic vaccination against human papillomavirus infection and disease in women: a systematic review of randomized controlled trials. CMAJ. 2007;177:469-479.
7. Saslow D, Castle PE, Cox JT, et al. American Cancer Society guideline for human papillomavirus vaccine use to prevent cervical cancer and its precursors. CA Cancer J Clin. 2007;57:7-28.
8. Centers for Disease Control and Prevention. Vaccination coverage among adolescents aged 13-17 years, United States 2007. MMWR Morb Mortal Wkly Rep. 2008;57:1100-1103.
9. Fisher R, Darrow DH, Tranter M, et al. Human papillomavirus vaccine: recommendations, issues and controversies. Curr Opin Pediatr. 2008;20:441-445.
10. Gerend MA, Magloire ZF. Awareness, knowledge and beliefs about human papillomavirus in a racially diverse sample of young adults. J Adolesc Health. 2008;42:237-242.
11. Advisory Committee on Immunization Practices: summary report, October 22-23, 2008, Atlanta, Ga. Available at: http://www.cdc.gov/vaccines/recs/ACIP/downloads/min-oct08.pdf. Accessed April 27, 2009.
12. Daley M. HPV vaccination practices. A national survey of physicians 18 months post licensure. Presentation at the October 2008 ACIP meeting. Available at: http://cdc.confex.com/cdc/nic2009/webprogram/Paper18003.html. Accessed November 3, 2009.
13. Slade BA, Leidel L, Vellozzi C, et al. Postlicensure safety surveillance for quadrivalent human papillomavirus recombinant vaccine. JAMA. 2009;302:750-757.
14. Meeting of the Advisory Committee on Immunization Practices; October 21-22, 2009; Atlanta, Ga.
1. Centers for Disease Control and Prevention. Quadrivalent human papillomavirus vaccine: Recommendations of the Advisory Committee on Immunization Practices. March 23, 2007. Available at: http://www.cdc.gov/mmwr/pdf/rr/rr5602.pdf. Accessed November 3, 2009.
2. World Health Organization. Human papillomavirus vaccines. WHO position paper. Weekly Epidemiological Record. 2009;84(15):118-131.Available at: http://www.who.int/wer/2009/wer8415.pdf. Accessed October 27, 2009.
3. U.S. Food and Drug Administration. October 16, 2009 Approval letter—Gardasil. Available at: www.fda.gov/BiologicsBloodVaccines/Vaccines/ApprovedProducts/ucm186991.htm. Accessed October 31, 2009.
4. Johnson LG, Madeleine MM, Newcomer LM, et al. Anal cancer incidence and survival; the surveillance, epidemiology and end results experience, 1973-2000. Cancer. 2004;101:281-288.
5. Trottier H, Franco EL. The epidemiology of genital human papillomavirus infection. Vaccine. 2006;24:1-15.
6. Rambout L, Hopkins L, Hutton B, et al. Prophylactic vaccination against human papillomavirus infection and disease in women: a systematic review of randomized controlled trials. CMAJ. 2007;177:469-479.
7. Saslow D, Castle PE, Cox JT, et al. American Cancer Society guideline for human papillomavirus vaccine use to prevent cervical cancer and its precursors. CA Cancer J Clin. 2007;57:7-28.
8. Centers for Disease Control and Prevention. Vaccination coverage among adolescents aged 13-17 years, United States 2007. MMWR Morb Mortal Wkly Rep. 2008;57:1100-1103.
9. Fisher R, Darrow DH, Tranter M, et al. Human papillomavirus vaccine: recommendations, issues and controversies. Curr Opin Pediatr. 2008;20:441-445.
10. Gerend MA, Magloire ZF. Awareness, knowledge and beliefs about human papillomavirus in a racially diverse sample of young adults. J Adolesc Health. 2008;42:237-242.
11. Advisory Committee on Immunization Practices: summary report, October 22-23, 2008, Atlanta, Ga. Available at: http://www.cdc.gov/vaccines/recs/ACIP/downloads/min-oct08.pdf. Accessed April 27, 2009.
12. Daley M. HPV vaccination practices. A national survey of physicians 18 months post licensure. Presentation at the October 2008 ACIP meeting. Available at: http://cdc.confex.com/cdc/nic2009/webprogram/Paper18003.html. Accessed November 3, 2009.
13. Slade BA, Leidel L, Vellozzi C, et al. Postlicensure safety surveillance for quadrivalent human papillomavirus recombinant vaccine. JAMA. 2009;302:750-757.
14. Meeting of the Advisory Committee on Immunization Practices; October 21-22, 2009; Atlanta, Ga.
The bedtime solution
CASE: Refractory depression
Ms. W, age 38, is brought to the emergency department after her son finds her unresponsive and calls 911. Suffering from worsening depression, she wrote a note telling her children goodbye, and overdosed on zolpidem from an old prescription and her daughter’s opioids. After being evaluated and medically cleared in the emergency department, Ms. W was admitted to the psychiatric unit.
Ms. W has a history of recurrent major depressive disorder that developed after she was sexually abused by a relative as a teen. She also has bulimia nervosa, alcohol dependence, and posttraumatic stress disorder. She was hospitalized twice for depression and suicidality but had not previously attempted suicide. In the mid-to-late 1990s, she had trials of paroxetine, clomipramine, lithium, and bupropion.
She was seen regularly in our outpatient psychiatry clinic for medication management and supportive psychotherapy. Since being followed in our clinic starting in early 2005, she has had the following medication trials:
- fluoxetine, citalopram, venlafaxine XR, and duloxetine for depression
- atomoxetine, buspirone, liothyronine, risperidone, and aripiprazole for antidepressant augmentation
- lorazepam, clonazepam, and gabapentin for anxiety
- zolpidem and trazodone for insomnia
- nortriptyline for migraine headache prophylaxis.
Some medications were not tolerated, primarily because of increased anxiety. Those that were tolerated were adequate trials in terms of dose titration and length. High-dose fluoxetine (80 mg/d) augmented by risperidone (0.375 to 0.5 mg/d) produced the most reliable and significant improvement.
Ms. W had 2 courses of electroconvulsive therapy (ECT) totaling 30 treatments—most recently in 2007—that resulted in significant memory loss with limited benefit. Premenstrual worsening of depression and suicidality were noted. In collaboration with her gynecologist, Ms. W was treated with a 3-month trial of leuprolide to suppress her ovarian axis, which was helpful. In 2008 she underwent bilateral oophorectomy. She has not had symptoms of mood elevation or psychosis. Family history includes schizophrenia, depression, anxiety, and alcoholism.
In the months before hospitalization, Ms. W had been increasingly depressed and intermittently suicidal, although she did not endorse a specific plan or intention to harm herself because she was concerned about the impact suicide would have on her children. Weight gain with risperidone had reactivated body image issues, so Ms. W stopped taking this medication 2 weeks before hospitalization. Her depression became worse, and she began using her husband’s hydrocodone/acetaminophen prescription.
The authors’ observations
Approximately 40% of patients with major depression fail to respond to an initial antidepressant trial.1 An additional 50% of these patients will be treatment-resistant to a subsequent antidepressant.1 Patients may be progressively less likely to respond to additional medication trials.2
One of the most rapid-acting and effective treatments for unipolar and bipolar depression is sleep deprivation. Wirz-Justice et al3 found total or partial sleep deprivation during the second half of the night induced rapid depression remission. Response rates range from 40% to 60% over hours to days.4 Sleep deprivation also can reduce suicidality in patients with seasonal depression.5 This treatment has not been widely employed, however, because up to 80% of patients who undergo sleep deprivation experience rapid and significant depressive relapse.4
Sleep deprivation usually is well tolerated. Potential side effects include:
- headache
- gastrointestinal upset
- fatigue
- cognitive impairment.
Less often, patients report worsening of depressive symptoms and, rarely, suicidal ideation or psychosis.4 Mania or hypomania are potential complications of sleep loss for patients with bipolar or unipolar depression. In a review, Oliwenstein6 suggested that rates of total sleep deprivation-induced mania are likely to be similar to or less than those reported for antidepressants. Because sleep deprivation can induce seizures, this therapy is contraindicated for patients with epilepsy or those at risk for seizures.4
Researchers have successfully explored strategies to reduce the rate of depressive relapse after sleep deprivation, including coadministering light therapy, antidepressants, lithium (particularly for bipolar depression), and sleep-phase advance.4 Sleep-phase advance involves shifting the sleep-wake schedule to a very early sleep time and wake-up time (such as 5 PM to midnight) for 1 day, and then pushing back this schedule by 1 or 2 hours each day until the patient is returned to a “normal” sleep schedule (such as 10 PM to 5 AM). Researchers have demonstrated that sleep-phase advance can have antidepressant effects.7
TREATMENT: Sleep manipulation
Ms. W is continued on fluoxetine, 80 mg/d. We opt for a trial of partial sleep deprivation and sleep-phase advance for Ms. W because of the severity of her depression, her multiple ineffective or poorly tolerated medication trials, and limited benefit from ECT. This treatment involves instituting partial sleep deprivation the first night and subsequently advancing her sleep phase over the next several days (Table 1).
Although she is sleepy the morning after partial sleep deprivation, Ms. W reports a marked improvement in her mood, decline in hopelessness, and absence of suicidal ideation. She continues the sleep-phase advance protocol for the next 3 nights and participates in cognitive-behavioral therapy groups and ward activities. Psychiatric unit staff support her continued wakefulness during sleep manipulation. Because Ms. W had previously responded to antidepressant augmentation with an atypical antipsychotic we add aripiprazole and titrate the dosage to 7.5 mg/d. We also continue fluoxetine, 80 mg/d, and add trazodone, 100 mg at bedtime, and hydroxyzine, 25 mg as needed.
Table 1
Ms. W’s chronotherapy protocol: Hours permitted for sleep*
| Day number | |||||
|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | |
| Sleep deprivation | 9 PM to2 AM | ||||
| Sleep-phase advance | 5 PM to midnight | 7 PM to 2 AM | 9 PM to 4 AM | 10 PM to 5 AM | |
| *Treatment was implemented while Ms. W was hospitalized | |||||
The authors’ observations
Chronotherapy incorporates manipulations of the sleep/wake cycle such as sleep deprivation and dark or light therapy. It may use combinations of interventions to generate and sustain a response in patients with depression. In a 4-week pilot study, Moscovici et al8 employed a regimen of late partial sleep deprivation, light, and sleep-phase advance to generate and maintain an anti depressant response in 12 patients. Benedetti et al9 used a similar regimen plus lithium to successfully treat bipolar depression and sleep-phase advance to continue that response in 50% of patients for 3 months.
Circadian rhythms affect the function of serotonin (5-HT), norepinephrine, and dopamine.9,10 In a manner similar to antidepressant medications, sleep deprivation may up-regulate or otherwise alter these neurotransmitters’ function. In animals, sleep deprivation increases serotonin function.11 Several hypothetical mechanisms of action for sleep deprivation and other types of chronotherapies have been suggested (Table 2).11-14
Chronotherapies may affect function in brain pathways, as demonstrated by neuroimaging with positron emission tomography (PET) and functional magnetic resonance imaging (fMRI). Depression has been associated with increased or decreased brain activity measured by PET or fMRI in regions of the limbic cortex (cingulate and anterior cingulate) and frontal cortex.12
Wu et al13 examined patients treated for depression with medication and total sleep deprivation therapy. Response to treatment was associated with increased function in the cingulate, anterior cingulate, and medial prefrontal cortex as measured by PET. In contrast, mood improvement was associated with reduced baseline activity in the left medial prefrontal cortex, left frontal pole, and right lateral prefrontal cortex.
Researchers have noted the convergence of sleep-wake rhythms and abnormalities seen in depression and the subsequent link with improved sleep-wake cycles related to depression remission. Bunney and Potkin14 note the powerful effect of zeitgebers—environmental agents that reset the body’s internal clock. They suggested that sleep deprivation may affect the function of “master clock” genes involved in controlling the biological clock. These effects on the suprachiasmatic nucleus hypothalamic pacemaker may improve mood by altering control of genetic expression through chromatin remodeling of this master clock circuit.
Certain factors may increase the likelihood that a patient may respond to chronotherapy (Table 3).9,15-17
Table 2
Sleep deprivation for depression: Possible mechanisms
| Mechanism | Components |
|---|---|
| Alterations to neurotransmitter function | Serotonin, norepinephrine, dopamine11 |
| Alterations to endogenous circadian pacemaker function | Increased gene expression14 |
| Changes in perfusion/activity of brain regions | Anterior cingulate, frontal cortex regions12,13 |
Table 3
Factors that suggest a patient might respond to chronotherapy
| Diurnal mood variation15 |
| Endogenous depression including insomnia and anorexia16 |
| Abnormal dexamethasone suppression17 |
| High motivation for treatment |
| Bipolar depression (possibly)9 |
OUTCOME: Lasting improvement
Ms. W’s mood improvement is sustained during her week-long hospitalization. At discharge she is hopeful about the future and does not have thoughts of suicide.
At subsequent outpatient visits up to 4 months after discharge, her depressive symptoms remain improved. Patient Health Questionnaire scores indicate mild depression, but Ms. W is not suicidal. She maintains a sleep schedule of 10 PM to 6:30 AM and undergoes 10,000 lux bright light therapy, which she began shortly after discharge, for 30 minutes every morning. She works more productively in psychotherapy, focusing on her eating disorder and anxiety.
Related resource
- Wu JC, Kelsoe JR, Schachat C, et al. Rapid and sustained antidepressant response with sleep deprivation and chronotherapy in bipolar disorder. Biol Psychiatry. 2009; 66(3): 298-301.
Drug brand names
- Aripiprazole • Abilify
- Atomoxetine • Strattera
- Bupropion • Wellbutrin
- Buspirone • BuSpar
- Citalopram • Celexa
- Clomipramine • Anafranil
- Clonazepam • Klonopin
- Duloxetine • Cymbalta
- Fluoxetine • Prozac
- Gabapentin • Neurontin
- Hydrocodone/APAP • Vicodin
- Hydroxyzine • Atarax, Vistaril
- Leuprolide • Lupron
- Liothyronine • Cytomel
- Lithium • Eskalith, Lithobid
- Lorazepam • Ativan
- Nortriptyline • Aventyl
- Paroxetine • Paxil
- Risperidone • Risperdal, Risperdal Consta
- Trazodone • Desyrel
- Venlafaxine XR • Effexor XR
- Zolpidem • Ambien
Disclosures
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. AHCPR Depression Guideline Panel. Clinical practice guideline number 5. Depression in primary care. Volume 2: Treatment of major depression. Rockville, MD: Agency for Health Care Policy and Research, Public Health Services, U.S. Department of Health and Human Services; 1993. AHCPR publication 93-0550.
2. Fava M, Rush JA, Wisniewski SR, et al. A comparison of mirtazapine and nortriptyline following two consecutive failed medication treatments for depressed outpatients: a STAR*D report. Am J Psychiatry. 2006;163(7):1161-1172.
3. Wirz-Justice A, Benedetti F, Berger M. Chronotherapeutics (light and wake therapy) in affective disorders. Psychol Med. 2005;35(7):939-944.
4. Giedke H, Schwärzler F. Therapeutic use of sleep deprivation in depression. Sleep Med Rev. 2002;6(5):361-377.
5. Lam RW, Tam EM, Shiah IS, et al. Effects of light therapy on suicidal ideation in patients with winter depression. J Clin Psychiatry. 2000;61(1):30-32.
6. Oliwenstein L. Lifting moods by losing sleep: an adjunct therapy for treating depression. Alternative and Complementary Therapies. 2006;12(2):66-70.
7. Wehr TA, Wirz-Justice A, Goodwin FK, et al. Phase advance of the circadian sleep-wake cycle as an antidepressant. Science. 1979;206(4419):710-713.
8. Moscovici L, Kotler M. A multistage chronobiologic intervention for the treatment of depression: a pilot study. J Affect Disord. 2009;116(3):201-217.
9. Benedetti F, Colombo C, Barbini B, et al. Morning sunlight reduces length of hospitalization in bipolar depression. J Affect Disord. 2001;62(3):221-223.
10. Benedetti F, Barbini B, Colombo C, et al. Chronotherapeutics in a psychiatric ward. Sleep Med Rev. 2007;11(6):509-522.
11. Lopez-Rodriguez F, Wilson CL, Maidment NT, et al. Total sleep deprivation increases extracellular serotonin in the rat hippocampus. Neuroscience. 2003;121(2):523-530.
12. Mayberg HS. Defining the neural circuitry of depression: toward a new nosology with therapeutic implications. Biol Psychiatry. 2007;61(6):729-730.
13. Wu JC, Gillin JC, Buchsbaum MS, et al. Sleep deprivation PET correlations of Hamilton symptom improvement ratings with changes in relative glucose metabolism in patients with depression. J Affect Disord. 2008;107(1-3):181-186.
14. Bunney JN, Potkin SG. Circadian abnormalities, molecular clock genes and chronobiological treatments in depression. Br Med Bull. 2008;86:23-32.
15. Benedetti F, Barbini B, Lucca A, et al. Sleep deprivation hastens the antidepressant action of fluoxetine. Eur Arch Psychiatry Clin Neurosci. 1997;247(2):100-103.
16. Vogel GW, Thurmond A, Gibbons P, et al. REM sleep reduction effects on depression syndromes. Arch Gen Psychiatry. 1975;32(6):765-777.
17. King D, Dowdy S, Jack R, et al. The dexamethasone suppression test as a predictor of sleep deprivation antidepressant effect. Psychiatry Res. 1982;7(1):93-99.
CASE: Refractory depression
Ms. W, age 38, is brought to the emergency department after her son finds her unresponsive and calls 911. Suffering from worsening depression, she wrote a note telling her children goodbye, and overdosed on zolpidem from an old prescription and her daughter’s opioids. After being evaluated and medically cleared in the emergency department, Ms. W was admitted to the psychiatric unit.
Ms. W has a history of recurrent major depressive disorder that developed after she was sexually abused by a relative as a teen. She also has bulimia nervosa, alcohol dependence, and posttraumatic stress disorder. She was hospitalized twice for depression and suicidality but had not previously attempted suicide. In the mid-to-late 1990s, she had trials of paroxetine, clomipramine, lithium, and bupropion.
She was seen regularly in our outpatient psychiatry clinic for medication management and supportive psychotherapy. Since being followed in our clinic starting in early 2005, she has had the following medication trials:
- fluoxetine, citalopram, venlafaxine XR, and duloxetine for depression
- atomoxetine, buspirone, liothyronine, risperidone, and aripiprazole for antidepressant augmentation
- lorazepam, clonazepam, and gabapentin for anxiety
- zolpidem and trazodone for insomnia
- nortriptyline for migraine headache prophylaxis.
Some medications were not tolerated, primarily because of increased anxiety. Those that were tolerated were adequate trials in terms of dose titration and length. High-dose fluoxetine (80 mg/d) augmented by risperidone (0.375 to 0.5 mg/d) produced the most reliable and significant improvement.
Ms. W had 2 courses of electroconvulsive therapy (ECT) totaling 30 treatments—most recently in 2007—that resulted in significant memory loss with limited benefit. Premenstrual worsening of depression and suicidality were noted. In collaboration with her gynecologist, Ms. W was treated with a 3-month trial of leuprolide to suppress her ovarian axis, which was helpful. In 2008 she underwent bilateral oophorectomy. She has not had symptoms of mood elevation or psychosis. Family history includes schizophrenia, depression, anxiety, and alcoholism.
In the months before hospitalization, Ms. W had been increasingly depressed and intermittently suicidal, although she did not endorse a specific plan or intention to harm herself because she was concerned about the impact suicide would have on her children. Weight gain with risperidone had reactivated body image issues, so Ms. W stopped taking this medication 2 weeks before hospitalization. Her depression became worse, and she began using her husband’s hydrocodone/acetaminophen prescription.
The authors’ observations
Approximately 40% of patients with major depression fail to respond to an initial antidepressant trial.1 An additional 50% of these patients will be treatment-resistant to a subsequent antidepressant.1 Patients may be progressively less likely to respond to additional medication trials.2
One of the most rapid-acting and effective treatments for unipolar and bipolar depression is sleep deprivation. Wirz-Justice et al3 found total or partial sleep deprivation during the second half of the night induced rapid depression remission. Response rates range from 40% to 60% over hours to days.4 Sleep deprivation also can reduce suicidality in patients with seasonal depression.5 This treatment has not been widely employed, however, because up to 80% of patients who undergo sleep deprivation experience rapid and significant depressive relapse.4
Sleep deprivation usually is well tolerated. Potential side effects include:
- headache
- gastrointestinal upset
- fatigue
- cognitive impairment.
Less often, patients report worsening of depressive symptoms and, rarely, suicidal ideation or psychosis.4 Mania or hypomania are potential complications of sleep loss for patients with bipolar or unipolar depression. In a review, Oliwenstein6 suggested that rates of total sleep deprivation-induced mania are likely to be similar to or less than those reported for antidepressants. Because sleep deprivation can induce seizures, this therapy is contraindicated for patients with epilepsy or those at risk for seizures.4
Researchers have successfully explored strategies to reduce the rate of depressive relapse after sleep deprivation, including coadministering light therapy, antidepressants, lithium (particularly for bipolar depression), and sleep-phase advance.4 Sleep-phase advance involves shifting the sleep-wake schedule to a very early sleep time and wake-up time (such as 5 PM to midnight) for 1 day, and then pushing back this schedule by 1 or 2 hours each day until the patient is returned to a “normal” sleep schedule (such as 10 PM to 5 AM). Researchers have demonstrated that sleep-phase advance can have antidepressant effects.7
TREATMENT: Sleep manipulation
Ms. W is continued on fluoxetine, 80 mg/d. We opt for a trial of partial sleep deprivation and sleep-phase advance for Ms. W because of the severity of her depression, her multiple ineffective or poorly tolerated medication trials, and limited benefit from ECT. This treatment involves instituting partial sleep deprivation the first night and subsequently advancing her sleep phase over the next several days (Table 1).
Although she is sleepy the morning after partial sleep deprivation, Ms. W reports a marked improvement in her mood, decline in hopelessness, and absence of suicidal ideation. She continues the sleep-phase advance protocol for the next 3 nights and participates in cognitive-behavioral therapy groups and ward activities. Psychiatric unit staff support her continued wakefulness during sleep manipulation. Because Ms. W had previously responded to antidepressant augmentation with an atypical antipsychotic we add aripiprazole and titrate the dosage to 7.5 mg/d. We also continue fluoxetine, 80 mg/d, and add trazodone, 100 mg at bedtime, and hydroxyzine, 25 mg as needed.
Table 1
Ms. W’s chronotherapy protocol: Hours permitted for sleep*
| Day number | |||||
|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | |
| Sleep deprivation | 9 PM to2 AM | ||||
| Sleep-phase advance | 5 PM to midnight | 7 PM to 2 AM | 9 PM to 4 AM | 10 PM to 5 AM | |
| *Treatment was implemented while Ms. W was hospitalized | |||||
The authors’ observations
Chronotherapy incorporates manipulations of the sleep/wake cycle such as sleep deprivation and dark or light therapy. It may use combinations of interventions to generate and sustain a response in patients with depression. In a 4-week pilot study, Moscovici et al8 employed a regimen of late partial sleep deprivation, light, and sleep-phase advance to generate and maintain an anti depressant response in 12 patients. Benedetti et al9 used a similar regimen plus lithium to successfully treat bipolar depression and sleep-phase advance to continue that response in 50% of patients for 3 months.
Circadian rhythms affect the function of serotonin (5-HT), norepinephrine, and dopamine.9,10 In a manner similar to antidepressant medications, sleep deprivation may up-regulate or otherwise alter these neurotransmitters’ function. In animals, sleep deprivation increases serotonin function.11 Several hypothetical mechanisms of action for sleep deprivation and other types of chronotherapies have been suggested (Table 2).11-14
Chronotherapies may affect function in brain pathways, as demonstrated by neuroimaging with positron emission tomography (PET) and functional magnetic resonance imaging (fMRI). Depression has been associated with increased or decreased brain activity measured by PET or fMRI in regions of the limbic cortex (cingulate and anterior cingulate) and frontal cortex.12
Wu et al13 examined patients treated for depression with medication and total sleep deprivation therapy. Response to treatment was associated with increased function in the cingulate, anterior cingulate, and medial prefrontal cortex as measured by PET. In contrast, mood improvement was associated with reduced baseline activity in the left medial prefrontal cortex, left frontal pole, and right lateral prefrontal cortex.
Researchers have noted the convergence of sleep-wake rhythms and abnormalities seen in depression and the subsequent link with improved sleep-wake cycles related to depression remission. Bunney and Potkin14 note the powerful effect of zeitgebers—environmental agents that reset the body’s internal clock. They suggested that sleep deprivation may affect the function of “master clock” genes involved in controlling the biological clock. These effects on the suprachiasmatic nucleus hypothalamic pacemaker may improve mood by altering control of genetic expression through chromatin remodeling of this master clock circuit.
Certain factors may increase the likelihood that a patient may respond to chronotherapy (Table 3).9,15-17
Table 2
Sleep deprivation for depression: Possible mechanisms
| Mechanism | Components |
|---|---|
| Alterations to neurotransmitter function | Serotonin, norepinephrine, dopamine11 |
| Alterations to endogenous circadian pacemaker function | Increased gene expression14 |
| Changes in perfusion/activity of brain regions | Anterior cingulate, frontal cortex regions12,13 |
Table 3
Factors that suggest a patient might respond to chronotherapy
| Diurnal mood variation15 |
| Endogenous depression including insomnia and anorexia16 |
| Abnormal dexamethasone suppression17 |
| High motivation for treatment |
| Bipolar depression (possibly)9 |
OUTCOME: Lasting improvement
Ms. W’s mood improvement is sustained during her week-long hospitalization. At discharge she is hopeful about the future and does not have thoughts of suicide.
At subsequent outpatient visits up to 4 months after discharge, her depressive symptoms remain improved. Patient Health Questionnaire scores indicate mild depression, but Ms. W is not suicidal. She maintains a sleep schedule of 10 PM to 6:30 AM and undergoes 10,000 lux bright light therapy, which she began shortly after discharge, for 30 minutes every morning. She works more productively in psychotherapy, focusing on her eating disorder and anxiety.
Related resource
- Wu JC, Kelsoe JR, Schachat C, et al. Rapid and sustained antidepressant response with sleep deprivation and chronotherapy in bipolar disorder. Biol Psychiatry. 2009; 66(3): 298-301.
Drug brand names
- Aripiprazole • Abilify
- Atomoxetine • Strattera
- Bupropion • Wellbutrin
- Buspirone • BuSpar
- Citalopram • Celexa
- Clomipramine • Anafranil
- Clonazepam • Klonopin
- Duloxetine • Cymbalta
- Fluoxetine • Prozac
- Gabapentin • Neurontin
- Hydrocodone/APAP • Vicodin
- Hydroxyzine • Atarax, Vistaril
- Leuprolide • Lupron
- Liothyronine • Cytomel
- Lithium • Eskalith, Lithobid
- Lorazepam • Ativan
- Nortriptyline • Aventyl
- Paroxetine • Paxil
- Risperidone • Risperdal, Risperdal Consta
- Trazodone • Desyrel
- Venlafaxine XR • Effexor XR
- Zolpidem • Ambien
Disclosures
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
CASE: Refractory depression
Ms. W, age 38, is brought to the emergency department after her son finds her unresponsive and calls 911. Suffering from worsening depression, she wrote a note telling her children goodbye, and overdosed on zolpidem from an old prescription and her daughter’s opioids. After being evaluated and medically cleared in the emergency department, Ms. W was admitted to the psychiatric unit.
Ms. W has a history of recurrent major depressive disorder that developed after she was sexually abused by a relative as a teen. She also has bulimia nervosa, alcohol dependence, and posttraumatic stress disorder. She was hospitalized twice for depression and suicidality but had not previously attempted suicide. In the mid-to-late 1990s, she had trials of paroxetine, clomipramine, lithium, and bupropion.
She was seen regularly in our outpatient psychiatry clinic for medication management and supportive psychotherapy. Since being followed in our clinic starting in early 2005, she has had the following medication trials:
- fluoxetine, citalopram, venlafaxine XR, and duloxetine for depression
- atomoxetine, buspirone, liothyronine, risperidone, and aripiprazole for antidepressant augmentation
- lorazepam, clonazepam, and gabapentin for anxiety
- zolpidem and trazodone for insomnia
- nortriptyline for migraine headache prophylaxis.
Some medications were not tolerated, primarily because of increased anxiety. Those that were tolerated were adequate trials in terms of dose titration and length. High-dose fluoxetine (80 mg/d) augmented by risperidone (0.375 to 0.5 mg/d) produced the most reliable and significant improvement.
Ms. W had 2 courses of electroconvulsive therapy (ECT) totaling 30 treatments—most recently in 2007—that resulted in significant memory loss with limited benefit. Premenstrual worsening of depression and suicidality were noted. In collaboration with her gynecologist, Ms. W was treated with a 3-month trial of leuprolide to suppress her ovarian axis, which was helpful. In 2008 she underwent bilateral oophorectomy. She has not had symptoms of mood elevation or psychosis. Family history includes schizophrenia, depression, anxiety, and alcoholism.
In the months before hospitalization, Ms. W had been increasingly depressed and intermittently suicidal, although she did not endorse a specific plan or intention to harm herself because she was concerned about the impact suicide would have on her children. Weight gain with risperidone had reactivated body image issues, so Ms. W stopped taking this medication 2 weeks before hospitalization. Her depression became worse, and she began using her husband’s hydrocodone/acetaminophen prescription.
The authors’ observations
Approximately 40% of patients with major depression fail to respond to an initial antidepressant trial.1 An additional 50% of these patients will be treatment-resistant to a subsequent antidepressant.1 Patients may be progressively less likely to respond to additional medication trials.2
One of the most rapid-acting and effective treatments for unipolar and bipolar depression is sleep deprivation. Wirz-Justice et al3 found total or partial sleep deprivation during the second half of the night induced rapid depression remission. Response rates range from 40% to 60% over hours to days.4 Sleep deprivation also can reduce suicidality in patients with seasonal depression.5 This treatment has not been widely employed, however, because up to 80% of patients who undergo sleep deprivation experience rapid and significant depressive relapse.4
Sleep deprivation usually is well tolerated. Potential side effects include:
- headache
- gastrointestinal upset
- fatigue
- cognitive impairment.
Less often, patients report worsening of depressive symptoms and, rarely, suicidal ideation or psychosis.4 Mania or hypomania are potential complications of sleep loss for patients with bipolar or unipolar depression. In a review, Oliwenstein6 suggested that rates of total sleep deprivation-induced mania are likely to be similar to or less than those reported for antidepressants. Because sleep deprivation can induce seizures, this therapy is contraindicated for patients with epilepsy or those at risk for seizures.4
Researchers have successfully explored strategies to reduce the rate of depressive relapse after sleep deprivation, including coadministering light therapy, antidepressants, lithium (particularly for bipolar depression), and sleep-phase advance.4 Sleep-phase advance involves shifting the sleep-wake schedule to a very early sleep time and wake-up time (such as 5 PM to midnight) for 1 day, and then pushing back this schedule by 1 or 2 hours each day until the patient is returned to a “normal” sleep schedule (such as 10 PM to 5 AM). Researchers have demonstrated that sleep-phase advance can have antidepressant effects.7
TREATMENT: Sleep manipulation
Ms. W is continued on fluoxetine, 80 mg/d. We opt for a trial of partial sleep deprivation and sleep-phase advance for Ms. W because of the severity of her depression, her multiple ineffective or poorly tolerated medication trials, and limited benefit from ECT. This treatment involves instituting partial sleep deprivation the first night and subsequently advancing her sleep phase over the next several days (Table 1).
Although she is sleepy the morning after partial sleep deprivation, Ms. W reports a marked improvement in her mood, decline in hopelessness, and absence of suicidal ideation. She continues the sleep-phase advance protocol for the next 3 nights and participates in cognitive-behavioral therapy groups and ward activities. Psychiatric unit staff support her continued wakefulness during sleep manipulation. Because Ms. W had previously responded to antidepressant augmentation with an atypical antipsychotic we add aripiprazole and titrate the dosage to 7.5 mg/d. We also continue fluoxetine, 80 mg/d, and add trazodone, 100 mg at bedtime, and hydroxyzine, 25 mg as needed.
Table 1
Ms. W’s chronotherapy protocol: Hours permitted for sleep*
| Day number | |||||
|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | |
| Sleep deprivation | 9 PM to2 AM | ||||
| Sleep-phase advance | 5 PM to midnight | 7 PM to 2 AM | 9 PM to 4 AM | 10 PM to 5 AM | |
| *Treatment was implemented while Ms. W was hospitalized | |||||
The authors’ observations
Chronotherapy incorporates manipulations of the sleep/wake cycle such as sleep deprivation and dark or light therapy. It may use combinations of interventions to generate and sustain a response in patients with depression. In a 4-week pilot study, Moscovici et al8 employed a regimen of late partial sleep deprivation, light, and sleep-phase advance to generate and maintain an anti depressant response in 12 patients. Benedetti et al9 used a similar regimen plus lithium to successfully treat bipolar depression and sleep-phase advance to continue that response in 50% of patients for 3 months.
Circadian rhythms affect the function of serotonin (5-HT), norepinephrine, and dopamine.9,10 In a manner similar to antidepressant medications, sleep deprivation may up-regulate or otherwise alter these neurotransmitters’ function. In animals, sleep deprivation increases serotonin function.11 Several hypothetical mechanisms of action for sleep deprivation and other types of chronotherapies have been suggested (Table 2).11-14
Chronotherapies may affect function in brain pathways, as demonstrated by neuroimaging with positron emission tomography (PET) and functional magnetic resonance imaging (fMRI). Depression has been associated with increased or decreased brain activity measured by PET or fMRI in regions of the limbic cortex (cingulate and anterior cingulate) and frontal cortex.12
Wu et al13 examined patients treated for depression with medication and total sleep deprivation therapy. Response to treatment was associated with increased function in the cingulate, anterior cingulate, and medial prefrontal cortex as measured by PET. In contrast, mood improvement was associated with reduced baseline activity in the left medial prefrontal cortex, left frontal pole, and right lateral prefrontal cortex.
Researchers have noted the convergence of sleep-wake rhythms and abnormalities seen in depression and the subsequent link with improved sleep-wake cycles related to depression remission. Bunney and Potkin14 note the powerful effect of zeitgebers—environmental agents that reset the body’s internal clock. They suggested that sleep deprivation may affect the function of “master clock” genes involved in controlling the biological clock. These effects on the suprachiasmatic nucleus hypothalamic pacemaker may improve mood by altering control of genetic expression through chromatin remodeling of this master clock circuit.
Certain factors may increase the likelihood that a patient may respond to chronotherapy (Table 3).9,15-17
Table 2
Sleep deprivation for depression: Possible mechanisms
| Mechanism | Components |
|---|---|
| Alterations to neurotransmitter function | Serotonin, norepinephrine, dopamine11 |
| Alterations to endogenous circadian pacemaker function | Increased gene expression14 |
| Changes in perfusion/activity of brain regions | Anterior cingulate, frontal cortex regions12,13 |
Table 3
Factors that suggest a patient might respond to chronotherapy
| Diurnal mood variation15 |
| Endogenous depression including insomnia and anorexia16 |
| Abnormal dexamethasone suppression17 |
| High motivation for treatment |
| Bipolar depression (possibly)9 |
OUTCOME: Lasting improvement
Ms. W’s mood improvement is sustained during her week-long hospitalization. At discharge she is hopeful about the future and does not have thoughts of suicide.
At subsequent outpatient visits up to 4 months after discharge, her depressive symptoms remain improved. Patient Health Questionnaire scores indicate mild depression, but Ms. W is not suicidal. She maintains a sleep schedule of 10 PM to 6:30 AM and undergoes 10,000 lux bright light therapy, which she began shortly after discharge, for 30 minutes every morning. She works more productively in psychotherapy, focusing on her eating disorder and anxiety.
Related resource
- Wu JC, Kelsoe JR, Schachat C, et al. Rapid and sustained antidepressant response with sleep deprivation and chronotherapy in bipolar disorder. Biol Psychiatry. 2009; 66(3): 298-301.
Drug brand names
- Aripiprazole • Abilify
- Atomoxetine • Strattera
- Bupropion • Wellbutrin
- Buspirone • BuSpar
- Citalopram • Celexa
- Clomipramine • Anafranil
- Clonazepam • Klonopin
- Duloxetine • Cymbalta
- Fluoxetine • Prozac
- Gabapentin • Neurontin
- Hydrocodone/APAP • Vicodin
- Hydroxyzine • Atarax, Vistaril
- Leuprolide • Lupron
- Liothyronine • Cytomel
- Lithium • Eskalith, Lithobid
- Lorazepam • Ativan
- Nortriptyline • Aventyl
- Paroxetine • Paxil
- Risperidone • Risperdal, Risperdal Consta
- Trazodone • Desyrel
- Venlafaxine XR • Effexor XR
- Zolpidem • Ambien
Disclosures
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. AHCPR Depression Guideline Panel. Clinical practice guideline number 5. Depression in primary care. Volume 2: Treatment of major depression. Rockville, MD: Agency for Health Care Policy and Research, Public Health Services, U.S. Department of Health and Human Services; 1993. AHCPR publication 93-0550.
2. Fava M, Rush JA, Wisniewski SR, et al. A comparison of mirtazapine and nortriptyline following two consecutive failed medication treatments for depressed outpatients: a STAR*D report. Am J Psychiatry. 2006;163(7):1161-1172.
3. Wirz-Justice A, Benedetti F, Berger M. Chronotherapeutics (light and wake therapy) in affective disorders. Psychol Med. 2005;35(7):939-944.
4. Giedke H, Schwärzler F. Therapeutic use of sleep deprivation in depression. Sleep Med Rev. 2002;6(5):361-377.
5. Lam RW, Tam EM, Shiah IS, et al. Effects of light therapy on suicidal ideation in patients with winter depression. J Clin Psychiatry. 2000;61(1):30-32.
6. Oliwenstein L. Lifting moods by losing sleep: an adjunct therapy for treating depression. Alternative and Complementary Therapies. 2006;12(2):66-70.
7. Wehr TA, Wirz-Justice A, Goodwin FK, et al. Phase advance of the circadian sleep-wake cycle as an antidepressant. Science. 1979;206(4419):710-713.
8. Moscovici L, Kotler M. A multistage chronobiologic intervention for the treatment of depression: a pilot study. J Affect Disord. 2009;116(3):201-217.
9. Benedetti F, Colombo C, Barbini B, et al. Morning sunlight reduces length of hospitalization in bipolar depression. J Affect Disord. 2001;62(3):221-223.
10. Benedetti F, Barbini B, Colombo C, et al. Chronotherapeutics in a psychiatric ward. Sleep Med Rev. 2007;11(6):509-522.
11. Lopez-Rodriguez F, Wilson CL, Maidment NT, et al. Total sleep deprivation increases extracellular serotonin in the rat hippocampus. Neuroscience. 2003;121(2):523-530.
12. Mayberg HS. Defining the neural circuitry of depression: toward a new nosology with therapeutic implications. Biol Psychiatry. 2007;61(6):729-730.
13. Wu JC, Gillin JC, Buchsbaum MS, et al. Sleep deprivation PET correlations of Hamilton symptom improvement ratings with changes in relative glucose metabolism in patients with depression. J Affect Disord. 2008;107(1-3):181-186.
14. Bunney JN, Potkin SG. Circadian abnormalities, molecular clock genes and chronobiological treatments in depression. Br Med Bull. 2008;86:23-32.
15. Benedetti F, Barbini B, Lucca A, et al. Sleep deprivation hastens the antidepressant action of fluoxetine. Eur Arch Psychiatry Clin Neurosci. 1997;247(2):100-103.
16. Vogel GW, Thurmond A, Gibbons P, et al. REM sleep reduction effects on depression syndromes. Arch Gen Psychiatry. 1975;32(6):765-777.
17. King D, Dowdy S, Jack R, et al. The dexamethasone suppression test as a predictor of sleep deprivation antidepressant effect. Psychiatry Res. 1982;7(1):93-99.
1. AHCPR Depression Guideline Panel. Clinical practice guideline number 5. Depression in primary care. Volume 2: Treatment of major depression. Rockville, MD: Agency for Health Care Policy and Research, Public Health Services, U.S. Department of Health and Human Services; 1993. AHCPR publication 93-0550.
2. Fava M, Rush JA, Wisniewski SR, et al. A comparison of mirtazapine and nortriptyline following two consecutive failed medication treatments for depressed outpatients: a STAR*D report. Am J Psychiatry. 2006;163(7):1161-1172.
3. Wirz-Justice A, Benedetti F, Berger M. Chronotherapeutics (light and wake therapy) in affective disorders. Psychol Med. 2005;35(7):939-944.
4. Giedke H, Schwärzler F. Therapeutic use of sleep deprivation in depression. Sleep Med Rev. 2002;6(5):361-377.
5. Lam RW, Tam EM, Shiah IS, et al. Effects of light therapy on suicidal ideation in patients with winter depression. J Clin Psychiatry. 2000;61(1):30-32.
6. Oliwenstein L. Lifting moods by losing sleep: an adjunct therapy for treating depression. Alternative and Complementary Therapies. 2006;12(2):66-70.
7. Wehr TA, Wirz-Justice A, Goodwin FK, et al. Phase advance of the circadian sleep-wake cycle as an antidepressant. Science. 1979;206(4419):710-713.
8. Moscovici L, Kotler M. A multistage chronobiologic intervention for the treatment of depression: a pilot study. J Affect Disord. 2009;116(3):201-217.
9. Benedetti F, Colombo C, Barbini B, et al. Morning sunlight reduces length of hospitalization in bipolar depression. J Affect Disord. 2001;62(3):221-223.
10. Benedetti F, Barbini B, Colombo C, et al. Chronotherapeutics in a psychiatric ward. Sleep Med Rev. 2007;11(6):509-522.
11. Lopez-Rodriguez F, Wilson CL, Maidment NT, et al. Total sleep deprivation increases extracellular serotonin in the rat hippocampus. Neuroscience. 2003;121(2):523-530.
12. Mayberg HS. Defining the neural circuitry of depression: toward a new nosology with therapeutic implications. Biol Psychiatry. 2007;61(6):729-730.
13. Wu JC, Gillin JC, Buchsbaum MS, et al. Sleep deprivation PET correlations of Hamilton symptom improvement ratings with changes in relative glucose metabolism in patients with depression. J Affect Disord. 2008;107(1-3):181-186.
14. Bunney JN, Potkin SG. Circadian abnormalities, molecular clock genes and chronobiological treatments in depression. Br Med Bull. 2008;86:23-32.
15. Benedetti F, Barbini B, Lucca A, et al. Sleep deprivation hastens the antidepressant action of fluoxetine. Eur Arch Psychiatry Clin Neurosci. 1997;247(2):100-103.
16. Vogel GW, Thurmond A, Gibbons P, et al. REM sleep reduction effects on depression syndromes. Arch Gen Psychiatry. 1975;32(6):765-777.
17. King D, Dowdy S, Jack R, et al. The dexamethasone suppression test as a predictor of sleep deprivation antidepressant effect. Psychiatry Res. 1982;7(1):93-99.
The Overweight Child With Hypertension
When presented with an overweight child who has hypertension, collect a detailed history, including a 24-hour food-intake history.
Also assess the child's nutritional habits, such as number of fast-food items typically eaten per week and number of family dinners.
Ask about the fluids these children generally consume. For instance, do they drink any caloric beverages other than low-fat milk?
Take an exercise history. Inquire how many hours per day the child is exposed to television, video games, and other media.
Social interaction can be particularly important with an overweight child. Ask if the child has been teased or bullied at home, in school, or elsewhere in the community.
Next ask the parent(s) and patient what they know about high blood pressure. Also inquire about a family history of hypertension.
Confirm any elevation in the child's blood pressure during a physical examination. If the patient has severe hypertension, it is usually time to refer the child to a specialist.
If the child has hypertension for three consecutive monthly visits, further evaluation with blood work is appropriate. Order a complete metabolic panel, urinalysis, and fasting lipid panel. Urinalysis, for example, is useful as a screen for type 2 diabetes.
On a full review of systems, identify other morbidities associated with obesity and perform appropriate tests.
For instance, the child with daytime sleepiness and snoring may require a sleep study to identify obstructive sleep apnea.
In addition, if liver function tests are elevated, a pediatric ultrasound exam can identify a fatty liver.
You can also order an electrocardiogram to identify heart pathology and refer the child if the findings are abnormal.
Many families request thyroid testing for an overweight child. Full thyroid function tests are not cost effective and need not be done. A thyroid-stimulating hormone test should suffice.
As for behavioral counseling, at the Cleveland Clinic Children's Hospital, we recommend our “5 to GO!” messaging, in which children are told to eat 5-a-day fruits and veggies; give 4 compliments a day to anyone they encounter, including other kids, and get 4 compliments a day from anyone; consume 3 dairy products a day; engage in no more than 2 hours of media/TV time a day; drink 0 sugar-sweetened beverages, and go!
For teenagers, we aim for 4 dairy/calcium servings and 3 compliments a day (not that they need fewer compliments, but they do need more calcium than the under age 10 crowd).
The key is to follow patients monthly. Slow, steady change—with positive motivation tailored to each family—works better than trying to do everything at once.
Follow up, follow up, and follow up—with a lot of cheerleading!
Patient education is also essential. Help patients and their families figure out how to cook a no-added-salt diet, how to shop the periphery of a grocery store where the fresh produce is located, and how to build physical activity and exercise into the family's daily plan.
Consider a weight management program such as our Fit Youth Program. Patients and families who participate in this 12-week program at the Cleveland Clinic receive group counseling sessions led by a psychologist in combination with a pediatrician, a dietitian, and an exercise physiologist.
Multidisciplinary interventions such as this one can accomplish modest weight loss versus progression toward 30 pounds of weight gain per year, as occurs in many of our children who do not receive effective treatment.
When presented with an overweight child who has hypertension, collect a detailed history, including a 24-hour food-intake history.
Also assess the child's nutritional habits, such as number of fast-food items typically eaten per week and number of family dinners.
Ask about the fluids these children generally consume. For instance, do they drink any caloric beverages other than low-fat milk?
Take an exercise history. Inquire how many hours per day the child is exposed to television, video games, and other media.
Social interaction can be particularly important with an overweight child. Ask if the child has been teased or bullied at home, in school, or elsewhere in the community.
Next ask the parent(s) and patient what they know about high blood pressure. Also inquire about a family history of hypertension.
Confirm any elevation in the child's blood pressure during a physical examination. If the patient has severe hypertension, it is usually time to refer the child to a specialist.
If the child has hypertension for three consecutive monthly visits, further evaluation with blood work is appropriate. Order a complete metabolic panel, urinalysis, and fasting lipid panel. Urinalysis, for example, is useful as a screen for type 2 diabetes.
On a full review of systems, identify other morbidities associated with obesity and perform appropriate tests.
For instance, the child with daytime sleepiness and snoring may require a sleep study to identify obstructive sleep apnea.
In addition, if liver function tests are elevated, a pediatric ultrasound exam can identify a fatty liver.
You can also order an electrocardiogram to identify heart pathology and refer the child if the findings are abnormal.
Many families request thyroid testing for an overweight child. Full thyroid function tests are not cost effective and need not be done. A thyroid-stimulating hormone test should suffice.
As for behavioral counseling, at the Cleveland Clinic Children's Hospital, we recommend our “5 to GO!” messaging, in which children are told to eat 5-a-day fruits and veggies; give 4 compliments a day to anyone they encounter, including other kids, and get 4 compliments a day from anyone; consume 3 dairy products a day; engage in no more than 2 hours of media/TV time a day; drink 0 sugar-sweetened beverages, and go!
For teenagers, we aim for 4 dairy/calcium servings and 3 compliments a day (not that they need fewer compliments, but they do need more calcium than the under age 10 crowd).
The key is to follow patients monthly. Slow, steady change—with positive motivation tailored to each family—works better than trying to do everything at once.
Follow up, follow up, and follow up—with a lot of cheerleading!
Patient education is also essential. Help patients and their families figure out how to cook a no-added-salt diet, how to shop the periphery of a grocery store where the fresh produce is located, and how to build physical activity and exercise into the family's daily plan.
Consider a weight management program such as our Fit Youth Program. Patients and families who participate in this 12-week program at the Cleveland Clinic receive group counseling sessions led by a psychologist in combination with a pediatrician, a dietitian, and an exercise physiologist.
Multidisciplinary interventions such as this one can accomplish modest weight loss versus progression toward 30 pounds of weight gain per year, as occurs in many of our children who do not receive effective treatment.
When presented with an overweight child who has hypertension, collect a detailed history, including a 24-hour food-intake history.
Also assess the child's nutritional habits, such as number of fast-food items typically eaten per week and number of family dinners.
Ask about the fluids these children generally consume. For instance, do they drink any caloric beverages other than low-fat milk?
Take an exercise history. Inquire how many hours per day the child is exposed to television, video games, and other media.
Social interaction can be particularly important with an overweight child. Ask if the child has been teased or bullied at home, in school, or elsewhere in the community.
Next ask the parent(s) and patient what they know about high blood pressure. Also inquire about a family history of hypertension.
Confirm any elevation in the child's blood pressure during a physical examination. If the patient has severe hypertension, it is usually time to refer the child to a specialist.
If the child has hypertension for three consecutive monthly visits, further evaluation with blood work is appropriate. Order a complete metabolic panel, urinalysis, and fasting lipid panel. Urinalysis, for example, is useful as a screen for type 2 diabetes.
On a full review of systems, identify other morbidities associated with obesity and perform appropriate tests.
For instance, the child with daytime sleepiness and snoring may require a sleep study to identify obstructive sleep apnea.
In addition, if liver function tests are elevated, a pediatric ultrasound exam can identify a fatty liver.
You can also order an electrocardiogram to identify heart pathology and refer the child if the findings are abnormal.
Many families request thyroid testing for an overweight child. Full thyroid function tests are not cost effective and need not be done. A thyroid-stimulating hormone test should suffice.
As for behavioral counseling, at the Cleveland Clinic Children's Hospital, we recommend our “5 to GO!” messaging, in which children are told to eat 5-a-day fruits and veggies; give 4 compliments a day to anyone they encounter, including other kids, and get 4 compliments a day from anyone; consume 3 dairy products a day; engage in no more than 2 hours of media/TV time a day; drink 0 sugar-sweetened beverages, and go!
For teenagers, we aim for 4 dairy/calcium servings and 3 compliments a day (not that they need fewer compliments, but they do need more calcium than the under age 10 crowd).
The key is to follow patients monthly. Slow, steady change—with positive motivation tailored to each family—works better than trying to do everything at once.
Follow up, follow up, and follow up—with a lot of cheerleading!
Patient education is also essential. Help patients and their families figure out how to cook a no-added-salt diet, how to shop the periphery of a grocery store where the fresh produce is located, and how to build physical activity and exercise into the family's daily plan.
Consider a weight management program such as our Fit Youth Program. Patients and families who participate in this 12-week program at the Cleveland Clinic receive group counseling sessions led by a psychologist in combination with a pediatrician, a dietitian, and an exercise physiologist.
Multidisciplinary interventions such as this one can accomplish modest weight loss versus progression toward 30 pounds of weight gain per year, as occurs in many of our children who do not receive effective treatment.
Mentorship Matters
A hospitalist-led initiative to boost the implementation of glycemic controls has exceeded initial goals at an Alabama hospital, an early sign of success for the SHM-sponsored pilot program.
Steven C. Smith, MD, FHM, medical director of hospitalist services at Healthcare Authority for Medical West in Bessemer, Ala., says that after the Glycemic Control Mentored Implementation (GCMI) program was put in place earlier this year, his group set a two-week goal of 5% utilization of the program's evidence-based order set. He also set a three-month goal of 25% compliance with the order set.
"Much to my surprise, we achieved 16% utilization at two weeks," Dr. Smith says, adding that three-month data are still being tabulated. "The involvement by SHM is what made the difference. Being able to tell people on the medical staff that this is part of a national-level QI project, we're participating in something bigger—that made a big difference in getting people interested in the order set."
Representatives from several of the 30 pilot sites have reported similar success in the early stages of the yearlong project. Among other issues, the GCMI program tackles subcutaneous insulin protocols, transition from subcutaneous to infusion, care coordination, improving follow-up care, and hypoglycemia management.
Although institutions entered the program in April, Dr. Smith and his colleagues didn't begin their formal mentoring relationship until July. Since then, his HM group has stayed in touch with program mentors through teleconferences and direct e-mail exchanges. The service also has access to a data-aggregation system through the Yale Center for Medical Informatics, which encourages more attention and utilization of newly created order sets.
"We are in the process of collecting data from a period of about one year prior to our project and comparing that to data since the implementation of our order set," Dr. Smith says. The analysis "will allow us, and our mentor, to tailor our efforts to our particular institution in an ongoing fashion. The ongoing measures of our success with this project will include outcomes measures like length of stay, cost of stay, mortality and morbidity, ICU length of stay, ventilator days, and others."
A hospitalist-led initiative to boost the implementation of glycemic controls has exceeded initial goals at an Alabama hospital, an early sign of success for the SHM-sponsored pilot program.
Steven C. Smith, MD, FHM, medical director of hospitalist services at Healthcare Authority for Medical West in Bessemer, Ala., says that after the Glycemic Control Mentored Implementation (GCMI) program was put in place earlier this year, his group set a two-week goal of 5% utilization of the program's evidence-based order set. He also set a three-month goal of 25% compliance with the order set.
"Much to my surprise, we achieved 16% utilization at two weeks," Dr. Smith says, adding that three-month data are still being tabulated. "The involvement by SHM is what made the difference. Being able to tell people on the medical staff that this is part of a national-level QI project, we're participating in something bigger—that made a big difference in getting people interested in the order set."
Representatives from several of the 30 pilot sites have reported similar success in the early stages of the yearlong project. Among other issues, the GCMI program tackles subcutaneous insulin protocols, transition from subcutaneous to infusion, care coordination, improving follow-up care, and hypoglycemia management.
Although institutions entered the program in April, Dr. Smith and his colleagues didn't begin their formal mentoring relationship until July. Since then, his HM group has stayed in touch with program mentors through teleconferences and direct e-mail exchanges. The service also has access to a data-aggregation system through the Yale Center for Medical Informatics, which encourages more attention and utilization of newly created order sets.
"We are in the process of collecting data from a period of about one year prior to our project and comparing that to data since the implementation of our order set," Dr. Smith says. The analysis "will allow us, and our mentor, to tailor our efforts to our particular institution in an ongoing fashion. The ongoing measures of our success with this project will include outcomes measures like length of stay, cost of stay, mortality and morbidity, ICU length of stay, ventilator days, and others."
A hospitalist-led initiative to boost the implementation of glycemic controls has exceeded initial goals at an Alabama hospital, an early sign of success for the SHM-sponsored pilot program.
Steven C. Smith, MD, FHM, medical director of hospitalist services at Healthcare Authority for Medical West in Bessemer, Ala., says that after the Glycemic Control Mentored Implementation (GCMI) program was put in place earlier this year, his group set a two-week goal of 5% utilization of the program's evidence-based order set. He also set a three-month goal of 25% compliance with the order set.
"Much to my surprise, we achieved 16% utilization at two weeks," Dr. Smith says, adding that three-month data are still being tabulated. "The involvement by SHM is what made the difference. Being able to tell people on the medical staff that this is part of a national-level QI project, we're participating in something bigger—that made a big difference in getting people interested in the order set."
Representatives from several of the 30 pilot sites have reported similar success in the early stages of the yearlong project. Among other issues, the GCMI program tackles subcutaneous insulin protocols, transition from subcutaneous to infusion, care coordination, improving follow-up care, and hypoglycemia management.
Although institutions entered the program in April, Dr. Smith and his colleagues didn't begin their formal mentoring relationship until July. Since then, his HM group has stayed in touch with program mentors through teleconferences and direct e-mail exchanges. The service also has access to a data-aggregation system through the Yale Center for Medical Informatics, which encourages more attention and utilization of newly created order sets.
"We are in the process of collecting data from a period of about one year prior to our project and comparing that to data since the implementation of our order set," Dr. Smith says. The analysis "will allow us, and our mentor, to tailor our efforts to our particular institution in an ongoing fashion. The ongoing measures of our success with this project will include outcomes measures like length of stay, cost of stay, mortality and morbidity, ICU length of stay, ventilator days, and others."