User login
When is a conservative approach best for proximal biceps tendon rupture?
CASE Mr. A, a 59-year-old high school science teacher, came into our medical clinic with severe pain (7/10) in his left shoulder and arm and weakness on flexion of his left elbow. A week earlier, he felt a “pop” and experienced sharp pain and immediate “swelling” of the left biceps after throwing a heavy trash bag away while at work. He went to the school nurse for evaluation and was referred to a physician.
Mr. A was healthy, had no chronic diseases, and reported no previous injuries or trauma. He denied smoking, drinking alcohol, using illegal drugs, or taking steroids or other medications. He had worked as a high school teacher for the last 10 years at the time of his clinic visit.
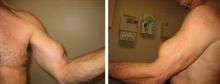
Imaging, physical exam tell the tale. The patient’s physical exam was normal, with one outstanding exception: a “Popeye” deformity in his left biceps (FIGURE), accompanied by severe pain and tenderness to palpation over the proximal aspect of the left biceps. Both active and passive range of motion of the elbow were full and symmetrical, but the patient had prominent pain and weakness on elbow flexion and supination. However, he had good rotator cuff strength without pain and no impingement signs or acromioclavicular joint pain. He had no atrophy or scapular dyskinesia. Similarly, a neurovascular exam of the distal aspect of the extremity was normal.
FIGURE
“Popeye” deformity in left biceps
With long head tendon rupture, the muscle belly retracts, causing “Popeye” biceps. Since only the long head tendon—and not the short head tendon—is involved, the biceps still functions.
The magnetic resonance imaging report revealed a complete tendon rupture of the long head of the biceps brachii muscle. The long head muscle was intact and there was a posttraumatic hemorrhage in the region of the tear in the upper arm. The remaining muscle, ligaments, and tendon were intact. There was no evidence of a fracture.
A 3-pronged approach. Once the diagnosis of acute complete rupture of the left long head tendon biceps brachii was reached, we laid out a 3-pronged treatment approach:
- nonsteroidal anti-inflammatory agents and muscle relaxants such as cyclobenzaprine, tizanidine, or metaxalone
- physical therapy (2-3 times per week) and daily home exercise
- modified activities—specifically, no overhead work or lifting of anything >10 lb with the affected arm.
Before we proceeded with this plan, we referred the patient to a specialist for evaluation and a second opinion.
Biceps tendon rupture usually follows a traumatic event
Long head biceps tendon ruptures often involve people between 40 and 60 years of age, with men affected significantly more often than women.1,2 Tennis players and ballplayers are also affected, as a result of frequent swinging motions.3 As you might expect, a person’s dominant arm is more often affected.3
Excessive weightlifting or rapid stress upon the tendon can cause an acute tendon rupture. As a rule, biceps tendon ruptures are caused by a single traumatic event that typically involves lifting a heavy object while the elbow is bent at a 90-degree angle. Weight lifters who use anabolic steroids are at an increased risk of sustaining a rupture at the tendon, and clinicians may also see such ruptures among patients who have fallen forcefully onto an outstretched arm.2,3
Keep in mind, however, that rupture can also occur in the absence of a traumatic event. This usually happens in elderly individuals with advanced tendon degeneration.4 Smoking, rheumatoid arthritis, steroid medications,2,5 fluoroquinolones,6 and statin therapy7 can affect this tendon and increase the risk of spontaneous rupture, as well.
“Popeye” biceps—a telltale sign. Understanding the function of the biceps brachii helps explain at least one of the telltale signs of long head tendon rupture. The biceps muscle enables supination of the forearm and flexion of the elbow. With long head tendon rupture, however, the muscle belly retracts, causing prominent fullness and bulging of the upper arm—what’s called “Popeye” biceps. Because the rupture involves only the long head tendon of the biceps and not the short head tendon, the biceps still functions.8
Surgical repair vs conservative management
Whether to pursue surgery or conservative management when caring for a patient with a biceps rupture remains a subject of debate in the medical literature. There are no studies that demonstrate the superiority of one approach over the other.2,5,9,10
Surgery. The serious complications associated with surgery have led some experts to question whether the risks of surgery outweigh the benefits.11 Equally important is the patient’s individual circumstances. Clinicians need to consider each patient’s occupation, lifestyle, and age when recommending a course of action.
Published clinical guidelines usually recommend surgical repair for young athletes who require maximum supination strength in daily activities. Although the size of the Popeye deformity does diminish after conservative treatment, surgery is often recommended for patients who are unwilling to accept the cosmetic defect seen after the tendon ruptures. And finally, operative treatment is indicated for middle-aged carpenters and manual laborers whose occupations require full supination and arm strength.2,12-14
The surgical procedure, called tenodesis, involves reattaching the torn section of the tendon to the bone.5,15 A recent study involving 5 professional wrestlers injured while performing noted that tenodesis restored full biceps function, gave excellent cosmetic results, and allowed all of the young men to return to wrestling.15
Conservative treatment. A conservative approach is appropriate for older patients when their profession and lifestyle do not demand a high degree of supination and upper arm strength.5,8,13,14 In addition, the more conservative approach is very well tolerated, which reduces the risk of serious complications and the cost of surgery.11 Avoiding surgery also permits patients to return to work much sooner.
Patients may, however, lose up to 20% of their supination strength with conservative treatment.14 But this approach does not cause weakness in grip, pronation, or elbow extension. Nor does it affect patients’ activities of daily living,14 which may explain why more patients are treated conservatively than with surgery.5,11 Additionally, some experts recommend nonoperative treatment of distal biceps tendon ruptures for people who are wary of surgery or present late with the injury.11
CASE Two orthopedic surgeons examined our patient and both supported our recommendation to pursue conservative treatment for Mr. A.
Over the next 4 to 6 weeks, he received physical therapy 2 to 3 times per week. With the help of the physiotherapist, Mr. A performed joint mobilization and flexibility exercises to improve the range of motion in his shoulder. The therapist also helped him with strengthening and stretching exercises to restore the strength of his biceps and elbow muscle.
At home, our patient’s regimen included elbow bend and straighten movements, elbow supination and pronation movements, and static biceps contractions.
Over time, his pain diminished and the strength in his left arm improved. Mr. A was able to return to work with modified duty, 2 to 3 weeks after his injury. By Week 8, he had full range of motion in his left arm and normal strength. He was able to do his job as a high school science teacher without any restrictions, but continued to have the Popeye deformity.
Our experience treating Mr. A serves as a reminder to physicians that complete long head biceps tendon rupture can be successfully treated conservatively. Patients working in sedentary occupations usually do not need a high degree of supination or physical strength in their upper extremities, making this a worthwhile treatment option for them.
CORRESPONDENCE
Sofya Pugach, MD, PhD, MPH, Complete Med Care, 8989 Forest Lane, Dallas, TX 75243; Drpugach@yahoo.com
1. Carter AN, Erikson SM. Proximal biceps tendon rupture: primarily an injury of middle age. Phys Sportsmed. 1999;27:95-101.
2. Miller R, Dlabach J. Sports medicine. In: Canale ST Beaty JH, eds. Campbell’s Operative Orthopaedics. 11th ed. Philadelphia, Pa: Mosby Elsevier; 2007: 2601–2775.
3. Brunelli MP, Gill TJ. Fractures and tendon injuries of the athletic shoulder. Orthop Clinic N Am. 2002;33:497-508.
4. Kannus P, Jozsa L. Histopathological changes preceding spontaneous rupture of the tendon. A controlled study of 891 patients. J Bone Joint Surg Am. 1991;73:1507-1525.
5. Branch GL, Wieting JM. Biceps rupture. Web MD web site. Updated 2012. Available at: http://emedicine.medscape.com/article/327119-overview. Accessed January 28 2013.
6. Gold L, Igra H. Levofloxacin-induced tendon rupture: a case report and review of the literature. J Am Board Fam Pract. 2003;16:458-460.
7. Pullatt RC, Gadarla MR, Karas RH, et al. Tendon rupture associated with simvastatin/ezetimibe therapy. Am J Cardiol. 2007;100:152-153.
8. Dvorkin ML. Office Orthopedics. Norwalk Conn: Appleton & Lange, 1993;1–35.
9. Gaskin CM, Anderson MW, Choudhri A, et al. Focal partial tears of the long head of the biceps brachii tendon at the entrance to the bicipital groove: MR imaging findings, surgical correction and clinical significance. Skeletal Radiol. 2009;38:959-965.
10. Busconi BB, DeAngelis N, Guerrero PE. The proximal biceps tendon: trick and pearls. Sports Med Arthrosc Rev. 2008;16:187-194.
11. Freeman CR, McCormick KR, Mahoney D, et al. Nonoperative treatment of distal biceps tendon ruptures compared with a historical control group. J Bone Joint Surg Am. 2009;91:2329-2334.
12. Roukoz S, Naccache N, Sleilaty G. The role of the musculocutaneous and radial nerves in elbow flexion and forearm supination: a biomechanical study. J Hand Surg Eur. 2008;33:201-204.
13. Curtis AS, Snyder SJ. Evaluation and treatment of biceps tendon pathology. Orthop Clin North Am. 1993;24:33-43.
14. Mariani EM, Cofield RH, Askew LJ, et al. Rupture of the tendon of the long head of the biceps brachii: Surgical versus nonsurgical treatment. Clin Orthop Relat Res. 1988;228:233-239.
15. Tangari M, Carbone S, Callo M, et al. Long head of the biceps tendon rupture in professional wrestlers: treatment with a mini-open tenodesis. J Shoulder Elbow Surg. 2011;20:409-413.
CASE Mr. A, a 59-year-old high school science teacher, came into our medical clinic with severe pain (7/10) in his left shoulder and arm and weakness on flexion of his left elbow. A week earlier, he felt a “pop” and experienced sharp pain and immediate “swelling” of the left biceps after throwing a heavy trash bag away while at work. He went to the school nurse for evaluation and was referred to a physician.
Mr. A was healthy, had no chronic diseases, and reported no previous injuries or trauma. He denied smoking, drinking alcohol, using illegal drugs, or taking steroids or other medications. He had worked as a high school teacher for the last 10 years at the time of his clinic visit.
Imaging, physical exam tell the tale. The patient’s physical exam was normal, with one outstanding exception: a “Popeye” deformity in his left biceps (FIGURE), accompanied by severe pain and tenderness to palpation over the proximal aspect of the left biceps. Both active and passive range of motion of the elbow were full and symmetrical, but the patient had prominent pain and weakness on elbow flexion and supination. However, he had good rotator cuff strength without pain and no impingement signs or acromioclavicular joint pain. He had no atrophy or scapular dyskinesia. Similarly, a neurovascular exam of the distal aspect of the extremity was normal.
FIGURE
“Popeye” deformity in left biceps
With long head tendon rupture, the muscle belly retracts, causing “Popeye” biceps. Since only the long head tendon—and not the short head tendon—is involved, the biceps still functions.
The magnetic resonance imaging report revealed a complete tendon rupture of the long head of the biceps brachii muscle. The long head muscle was intact and there was a posttraumatic hemorrhage in the region of the tear in the upper arm. The remaining muscle, ligaments, and tendon were intact. There was no evidence of a fracture.
A 3-pronged approach. Once the diagnosis of acute complete rupture of the left long head tendon biceps brachii was reached, we laid out a 3-pronged treatment approach:
- nonsteroidal anti-inflammatory agents and muscle relaxants such as cyclobenzaprine, tizanidine, or metaxalone
- physical therapy (2-3 times per week) and daily home exercise
- modified activities—specifically, no overhead work or lifting of anything >10 lb with the affected arm.
Before we proceeded with this plan, we referred the patient to a specialist for evaluation and a second opinion.
Biceps tendon rupture usually follows a traumatic event
Long head biceps tendon ruptures often involve people between 40 and 60 years of age, with men affected significantly more often than women.1,2 Tennis players and ballplayers are also affected, as a result of frequent swinging motions.3 As you might expect, a person’s dominant arm is more often affected.3
Excessive weightlifting or rapid stress upon the tendon can cause an acute tendon rupture. As a rule, biceps tendon ruptures are caused by a single traumatic event that typically involves lifting a heavy object while the elbow is bent at a 90-degree angle. Weight lifters who use anabolic steroids are at an increased risk of sustaining a rupture at the tendon, and clinicians may also see such ruptures among patients who have fallen forcefully onto an outstretched arm.2,3
Keep in mind, however, that rupture can also occur in the absence of a traumatic event. This usually happens in elderly individuals with advanced tendon degeneration.4 Smoking, rheumatoid arthritis, steroid medications,2,5 fluoroquinolones,6 and statin therapy7 can affect this tendon and increase the risk of spontaneous rupture, as well.
“Popeye” biceps—a telltale sign. Understanding the function of the biceps brachii helps explain at least one of the telltale signs of long head tendon rupture. The biceps muscle enables supination of the forearm and flexion of the elbow. With long head tendon rupture, however, the muscle belly retracts, causing prominent fullness and bulging of the upper arm—what’s called “Popeye” biceps. Because the rupture involves only the long head tendon of the biceps and not the short head tendon, the biceps still functions.8
Surgical repair vs conservative management
Whether to pursue surgery or conservative management when caring for a patient with a biceps rupture remains a subject of debate in the medical literature. There are no studies that demonstrate the superiority of one approach over the other.2,5,9,10
Surgery. The serious complications associated with surgery have led some experts to question whether the risks of surgery outweigh the benefits.11 Equally important is the patient’s individual circumstances. Clinicians need to consider each patient’s occupation, lifestyle, and age when recommending a course of action.
Published clinical guidelines usually recommend surgical repair for young athletes who require maximum supination strength in daily activities. Although the size of the Popeye deformity does diminish after conservative treatment, surgery is often recommended for patients who are unwilling to accept the cosmetic defect seen after the tendon ruptures. And finally, operative treatment is indicated for middle-aged carpenters and manual laborers whose occupations require full supination and arm strength.2,12-14
The surgical procedure, called tenodesis, involves reattaching the torn section of the tendon to the bone.5,15 A recent study involving 5 professional wrestlers injured while performing noted that tenodesis restored full biceps function, gave excellent cosmetic results, and allowed all of the young men to return to wrestling.15
Conservative treatment. A conservative approach is appropriate for older patients when their profession and lifestyle do not demand a high degree of supination and upper arm strength.5,8,13,14 In addition, the more conservative approach is very well tolerated, which reduces the risk of serious complications and the cost of surgery.11 Avoiding surgery also permits patients to return to work much sooner.
Patients may, however, lose up to 20% of their supination strength with conservative treatment.14 But this approach does not cause weakness in grip, pronation, or elbow extension. Nor does it affect patients’ activities of daily living,14 which may explain why more patients are treated conservatively than with surgery.5,11 Additionally, some experts recommend nonoperative treatment of distal biceps tendon ruptures for people who are wary of surgery or present late with the injury.11
CASE Two orthopedic surgeons examined our patient and both supported our recommendation to pursue conservative treatment for Mr. A.
Over the next 4 to 6 weeks, he received physical therapy 2 to 3 times per week. With the help of the physiotherapist, Mr. A performed joint mobilization and flexibility exercises to improve the range of motion in his shoulder. The therapist also helped him with strengthening and stretching exercises to restore the strength of his biceps and elbow muscle.
At home, our patient’s regimen included elbow bend and straighten movements, elbow supination and pronation movements, and static biceps contractions.
Over time, his pain diminished and the strength in his left arm improved. Mr. A was able to return to work with modified duty, 2 to 3 weeks after his injury. By Week 8, he had full range of motion in his left arm and normal strength. He was able to do his job as a high school science teacher without any restrictions, but continued to have the Popeye deformity.
Our experience treating Mr. A serves as a reminder to physicians that complete long head biceps tendon rupture can be successfully treated conservatively. Patients working in sedentary occupations usually do not need a high degree of supination or physical strength in their upper extremities, making this a worthwhile treatment option for them.
CORRESPONDENCE
Sofya Pugach, MD, PhD, MPH, Complete Med Care, 8989 Forest Lane, Dallas, TX 75243; Drpugach@yahoo.com
CASE Mr. A, a 59-year-old high school science teacher, came into our medical clinic with severe pain (7/10) in his left shoulder and arm and weakness on flexion of his left elbow. A week earlier, he felt a “pop” and experienced sharp pain and immediate “swelling” of the left biceps after throwing a heavy trash bag away while at work. He went to the school nurse for evaluation and was referred to a physician.
Mr. A was healthy, had no chronic diseases, and reported no previous injuries or trauma. He denied smoking, drinking alcohol, using illegal drugs, or taking steroids or other medications. He had worked as a high school teacher for the last 10 years at the time of his clinic visit.
Imaging, physical exam tell the tale. The patient’s physical exam was normal, with one outstanding exception: a “Popeye” deformity in his left biceps (FIGURE), accompanied by severe pain and tenderness to palpation over the proximal aspect of the left biceps. Both active and passive range of motion of the elbow were full and symmetrical, but the patient had prominent pain and weakness on elbow flexion and supination. However, he had good rotator cuff strength without pain and no impingement signs or acromioclavicular joint pain. He had no atrophy or scapular dyskinesia. Similarly, a neurovascular exam of the distal aspect of the extremity was normal.
FIGURE
“Popeye” deformity in left biceps
With long head tendon rupture, the muscle belly retracts, causing “Popeye” biceps. Since only the long head tendon—and not the short head tendon—is involved, the biceps still functions.
The magnetic resonance imaging report revealed a complete tendon rupture of the long head of the biceps brachii muscle. The long head muscle was intact and there was a posttraumatic hemorrhage in the region of the tear in the upper arm. The remaining muscle, ligaments, and tendon were intact. There was no evidence of a fracture.
A 3-pronged approach. Once the diagnosis of acute complete rupture of the left long head tendon biceps brachii was reached, we laid out a 3-pronged treatment approach:
- nonsteroidal anti-inflammatory agents and muscle relaxants such as cyclobenzaprine, tizanidine, or metaxalone
- physical therapy (2-3 times per week) and daily home exercise
- modified activities—specifically, no overhead work or lifting of anything >10 lb with the affected arm.
Before we proceeded with this plan, we referred the patient to a specialist for evaluation and a second opinion.
Biceps tendon rupture usually follows a traumatic event
Long head biceps tendon ruptures often involve people between 40 and 60 years of age, with men affected significantly more often than women.1,2 Tennis players and ballplayers are also affected, as a result of frequent swinging motions.3 As you might expect, a person’s dominant arm is more often affected.3
Excessive weightlifting or rapid stress upon the tendon can cause an acute tendon rupture. As a rule, biceps tendon ruptures are caused by a single traumatic event that typically involves lifting a heavy object while the elbow is bent at a 90-degree angle. Weight lifters who use anabolic steroids are at an increased risk of sustaining a rupture at the tendon, and clinicians may also see such ruptures among patients who have fallen forcefully onto an outstretched arm.2,3
Keep in mind, however, that rupture can also occur in the absence of a traumatic event. This usually happens in elderly individuals with advanced tendon degeneration.4 Smoking, rheumatoid arthritis, steroid medications,2,5 fluoroquinolones,6 and statin therapy7 can affect this tendon and increase the risk of spontaneous rupture, as well.
“Popeye” biceps—a telltale sign. Understanding the function of the biceps brachii helps explain at least one of the telltale signs of long head tendon rupture. The biceps muscle enables supination of the forearm and flexion of the elbow. With long head tendon rupture, however, the muscle belly retracts, causing prominent fullness and bulging of the upper arm—what’s called “Popeye” biceps. Because the rupture involves only the long head tendon of the biceps and not the short head tendon, the biceps still functions.8
Surgical repair vs conservative management
Whether to pursue surgery or conservative management when caring for a patient with a biceps rupture remains a subject of debate in the medical literature. There are no studies that demonstrate the superiority of one approach over the other.2,5,9,10
Surgery. The serious complications associated with surgery have led some experts to question whether the risks of surgery outweigh the benefits.11 Equally important is the patient’s individual circumstances. Clinicians need to consider each patient’s occupation, lifestyle, and age when recommending a course of action.
Published clinical guidelines usually recommend surgical repair for young athletes who require maximum supination strength in daily activities. Although the size of the Popeye deformity does diminish after conservative treatment, surgery is often recommended for patients who are unwilling to accept the cosmetic defect seen after the tendon ruptures. And finally, operative treatment is indicated for middle-aged carpenters and manual laborers whose occupations require full supination and arm strength.2,12-14
The surgical procedure, called tenodesis, involves reattaching the torn section of the tendon to the bone.5,15 A recent study involving 5 professional wrestlers injured while performing noted that tenodesis restored full biceps function, gave excellent cosmetic results, and allowed all of the young men to return to wrestling.15
Conservative treatment. A conservative approach is appropriate for older patients when their profession and lifestyle do not demand a high degree of supination and upper arm strength.5,8,13,14 In addition, the more conservative approach is very well tolerated, which reduces the risk of serious complications and the cost of surgery.11 Avoiding surgery also permits patients to return to work much sooner.
Patients may, however, lose up to 20% of their supination strength with conservative treatment.14 But this approach does not cause weakness in grip, pronation, or elbow extension. Nor does it affect patients’ activities of daily living,14 which may explain why more patients are treated conservatively than with surgery.5,11 Additionally, some experts recommend nonoperative treatment of distal biceps tendon ruptures for people who are wary of surgery or present late with the injury.11
CASE Two orthopedic surgeons examined our patient and both supported our recommendation to pursue conservative treatment for Mr. A.
Over the next 4 to 6 weeks, he received physical therapy 2 to 3 times per week. With the help of the physiotherapist, Mr. A performed joint mobilization and flexibility exercises to improve the range of motion in his shoulder. The therapist also helped him with strengthening and stretching exercises to restore the strength of his biceps and elbow muscle.
At home, our patient’s regimen included elbow bend and straighten movements, elbow supination and pronation movements, and static biceps contractions.
Over time, his pain diminished and the strength in his left arm improved. Mr. A was able to return to work with modified duty, 2 to 3 weeks after his injury. By Week 8, he had full range of motion in his left arm and normal strength. He was able to do his job as a high school science teacher without any restrictions, but continued to have the Popeye deformity.
Our experience treating Mr. A serves as a reminder to physicians that complete long head biceps tendon rupture can be successfully treated conservatively. Patients working in sedentary occupations usually do not need a high degree of supination or physical strength in their upper extremities, making this a worthwhile treatment option for them.
CORRESPONDENCE
Sofya Pugach, MD, PhD, MPH, Complete Med Care, 8989 Forest Lane, Dallas, TX 75243; Drpugach@yahoo.com
1. Carter AN, Erikson SM. Proximal biceps tendon rupture: primarily an injury of middle age. Phys Sportsmed. 1999;27:95-101.
2. Miller R, Dlabach J. Sports medicine. In: Canale ST Beaty JH, eds. Campbell’s Operative Orthopaedics. 11th ed. Philadelphia, Pa: Mosby Elsevier; 2007: 2601–2775.
3. Brunelli MP, Gill TJ. Fractures and tendon injuries of the athletic shoulder. Orthop Clinic N Am. 2002;33:497-508.
4. Kannus P, Jozsa L. Histopathological changes preceding spontaneous rupture of the tendon. A controlled study of 891 patients. J Bone Joint Surg Am. 1991;73:1507-1525.
5. Branch GL, Wieting JM. Biceps rupture. Web MD web site. Updated 2012. Available at: http://emedicine.medscape.com/article/327119-overview. Accessed January 28 2013.
6. Gold L, Igra H. Levofloxacin-induced tendon rupture: a case report and review of the literature. J Am Board Fam Pract. 2003;16:458-460.
7. Pullatt RC, Gadarla MR, Karas RH, et al. Tendon rupture associated with simvastatin/ezetimibe therapy. Am J Cardiol. 2007;100:152-153.
8. Dvorkin ML. Office Orthopedics. Norwalk Conn: Appleton & Lange, 1993;1–35.
9. Gaskin CM, Anderson MW, Choudhri A, et al. Focal partial tears of the long head of the biceps brachii tendon at the entrance to the bicipital groove: MR imaging findings, surgical correction and clinical significance. Skeletal Radiol. 2009;38:959-965.
10. Busconi BB, DeAngelis N, Guerrero PE. The proximal biceps tendon: trick and pearls. Sports Med Arthrosc Rev. 2008;16:187-194.
11. Freeman CR, McCormick KR, Mahoney D, et al. Nonoperative treatment of distal biceps tendon ruptures compared with a historical control group. J Bone Joint Surg Am. 2009;91:2329-2334.
12. Roukoz S, Naccache N, Sleilaty G. The role of the musculocutaneous and radial nerves in elbow flexion and forearm supination: a biomechanical study. J Hand Surg Eur. 2008;33:201-204.
13. Curtis AS, Snyder SJ. Evaluation and treatment of biceps tendon pathology. Orthop Clin North Am. 1993;24:33-43.
14. Mariani EM, Cofield RH, Askew LJ, et al. Rupture of the tendon of the long head of the biceps brachii: Surgical versus nonsurgical treatment. Clin Orthop Relat Res. 1988;228:233-239.
15. Tangari M, Carbone S, Callo M, et al. Long head of the biceps tendon rupture in professional wrestlers: treatment with a mini-open tenodesis. J Shoulder Elbow Surg. 2011;20:409-413.
1. Carter AN, Erikson SM. Proximal biceps tendon rupture: primarily an injury of middle age. Phys Sportsmed. 1999;27:95-101.
2. Miller R, Dlabach J. Sports medicine. In: Canale ST Beaty JH, eds. Campbell’s Operative Orthopaedics. 11th ed. Philadelphia, Pa: Mosby Elsevier; 2007: 2601–2775.
3. Brunelli MP, Gill TJ. Fractures and tendon injuries of the athletic shoulder. Orthop Clinic N Am. 2002;33:497-508.
4. Kannus P, Jozsa L. Histopathological changes preceding spontaneous rupture of the tendon. A controlled study of 891 patients. J Bone Joint Surg Am. 1991;73:1507-1525.
5. Branch GL, Wieting JM. Biceps rupture. Web MD web site. Updated 2012. Available at: http://emedicine.medscape.com/article/327119-overview. Accessed January 28 2013.
6. Gold L, Igra H. Levofloxacin-induced tendon rupture: a case report and review of the literature. J Am Board Fam Pract. 2003;16:458-460.
7. Pullatt RC, Gadarla MR, Karas RH, et al. Tendon rupture associated with simvastatin/ezetimibe therapy. Am J Cardiol. 2007;100:152-153.
8. Dvorkin ML. Office Orthopedics. Norwalk Conn: Appleton & Lange, 1993;1–35.
9. Gaskin CM, Anderson MW, Choudhri A, et al. Focal partial tears of the long head of the biceps brachii tendon at the entrance to the bicipital groove: MR imaging findings, surgical correction and clinical significance. Skeletal Radiol. 2009;38:959-965.
10. Busconi BB, DeAngelis N, Guerrero PE. The proximal biceps tendon: trick and pearls. Sports Med Arthrosc Rev. 2008;16:187-194.
11. Freeman CR, McCormick KR, Mahoney D, et al. Nonoperative treatment of distal biceps tendon ruptures compared with a historical control group. J Bone Joint Surg Am. 2009;91:2329-2334.
12. Roukoz S, Naccache N, Sleilaty G. The role of the musculocutaneous and radial nerves in elbow flexion and forearm supination: a biomechanical study. J Hand Surg Eur. 2008;33:201-204.
13. Curtis AS, Snyder SJ. Evaluation and treatment of biceps tendon pathology. Orthop Clin North Am. 1993;24:33-43.
14. Mariani EM, Cofield RH, Askew LJ, et al. Rupture of the tendon of the long head of the biceps brachii: Surgical versus nonsurgical treatment. Clin Orthop Relat Res. 1988;228:233-239.
15. Tangari M, Carbone S, Callo M, et al. Long head of the biceps tendon rupture in professional wrestlers: treatment with a mini-open tenodesis. J Shoulder Elbow Surg. 2011;20:409-413.
Chronic headache: Stop the pain before it starts
• Treat medication overuse headache by withdrawing abortive therapy and initiating prophylactic therapy. C
• Select prophylactic medications that are first-line therapy for chronic migraine or tension-type headache and are appropriate for the patient’s comorbidities. C
• Advise patients to limit intake of abortive headache medications to ≤9 per month. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
CASE Eric K, age 25, is in your office, seeking help for chronic headache—which he’s had nearly every day for the past 9 months. He says that the headaches vary in quality and intensity. Sometimes the pain is in the right temporal area; has a throbbing, pulsating quality; and is accompanied by nausea and photophobia. These headaches are incapacitating, with an intensity of 10 on a scale of one to 10. When they occur, the patient reports, all he can do is take migraine medication and lie down in a darkened room for several hours, until the pain goes away. He cannot identify any triggers.
He also gets headaches that are not incapacitating, but occur almost daily, the patient says, describing a dull bilateral pressure that usually begins in the afternoon and worsens until he takes headache medication. He denies any fever, chills, weight loss, visual changes, or tinnitus. His medical history is significant only for obesity, but a system review is positive for depression and insomnia. Physical examination reveals normal vital signs; normal head, eyes, ears, nose, and throat (HEENT); and normal fundoscopic and neurological exams.
Patients like Mr. K can be challenging for primary care physicians, but referral to a neurologist is indicated only in the most intractable cases. For the vast majority of patients with frequent headaches, family physicians can perform the diagnostic work-up and oversee treatment. This review will help with both.
What kind of headache?
While most headaches are sporadic in nature, the prevalence of “chronic daily headache” ranges from 3% to 5% worldwide.1-3
Chronic daily headache is not a diagnosis, however, nor is it an indication that a patient has a headache every day. According to the International Classification of Headache Disorders (ICHD-II), chronic daily headache encompasses several distinct primary headache disorders that have a frequency of ≥15 times per month for at least 3 months. These disorders are also classified by duration, as long (>4 hours) or short.4
The text and tables that follow focus on the diagnosis and treatment of the 4 primary headache disorders of long duration—chronic migraine (CM), chronic tension-type headache (CTTH), hemicrania continua (HC), and new daily persistent headache (NDPH) (TABLE 1).4,5 Although medication overuse headache (MOH) is not a primary headache, it is included in this review because it often contributes to and complicates treatment of primary headache disorders. What’s more, most chronic daily headache syndromes are inextricably linked to medication overuse.6,7
Which individuals are at risk?
Risk factors for chronic headache include female sex, older age, obesity, heavy caffeine consumption, tobacco use, low educational level, overuse of abortive headache medications, and a history of head and neck trauma.8 Episodic migraine (EM)—that is, migraines that occur ≤14 times a month—is also a risk factor for chronic headache.
ICHD-II classifies EM as a progressive disease that transforms to CM at a rate of 3% per year.9 Transformation of EM to CM has been found to occur after as few as 5 days of barbiturates or 8 days of opiates per month.10
Patients with EM should be warned about the potential for migraines to become chronic and have their acute headache medications replaced with a prophylactic drug if the frequency approaches 2 per week.
TABLE 1
Diagnosing and treating chronic headache4,5
| Type of headache | ICHD-II diagnostic criteria | First-line treatment |
|---|---|---|
| Chronic migraine |
| Prophylactic therapy:
|
| Chronic tension-type headache |
| Prophylactic therapy:
|
| Medication overuse headache |
| Discontinue overused acute meds; provide headache education; bridge with NSAIDs, prednisone, or botulinum toxin A; begin prophylactic medication |
| Hemicrania continua |
| Indomethacin |
| New daily persistent headache |
| Rule out secondary causes; treat according to migrainous or tension features of headache |
| ICHD-II, International Classification of Headache Disorders; NSAIDs, nonsteroidal anti-inflammatory drugs; SNRIs, serotonin-norepinephrine reuptake inhibitors; TCAs, tricyclic antidepressants. | ||
Pinpointing the type of headache
An accurate diagnosis requires a thorough headache history and a HEENT and neurological examination. The history should include questions about the characteristics of the headache, including the location, intensity, frequency, timing, associated symptoms, previous headache diagnoses, and triggers, and address comorbidities, medication use, caffeine intake, and family history.8 In the absence of red flags—age >50 years, history of headache or systemic illness, sudden onset, or papilledema, among other findings that may indicate more serious conditions (TABLE 2)7—advanced imaging and further work-up are not needed.
TABLE 2
Beyond headache: Red flags warrant additional testing7
| Red flag | Condition(s) to rule out |
|---|---|
| Age of onset >50 y | Giant cell arteritis, mass lesion, stroke |
| No prior history of headache OR change in characteristic from prior headaches | Cancer, aneurysm, stroke, cerebral sinus thrombosis, infection |
| “Thunderclap” headache | Ruptured aneurysm |
| Signs or symptoms of systemic illness (eg, fever, chills, weight loss) | Meningitis, encephalitis, cancer |
| History of systemic illness, such as cancer, autoimmune disease, or HIV | Brain metastasis, mass lesion, autoimmune meningitis, thrombosis |
| Headache brought on by change in head position or Valsalva maneuver | Spontaneous CSF leak or Chiari malformation |
| Occipital location of headache (in children) | Brain tumor |
| Neurological symptoms | Mass lesion, encephalitis |
| Papilledema | Idiopathic intracranial hypertension, cerebral sinus thrombosis |
| CSF, cerebrospinal fluid; HIV, human immunodeficiency virus. | |
Migraine or tension headache?
Chronic migraine. To be classified as CM, the headache must have occurred ≥15 days a month for 3 months or more and have features of migraine, such as unilateral location, pulsating quality, and moderate to severe intensity. Migraines are aggravated by physical activity and associated with nausea and/or vomiting, photophobia, and phonophobia, and may or may not be preceded by aura. Common triggers include stress, menstruation, alcohol, skipped meals, dehydration, and chocolate. Migraines typically respond to ergots and triptans.4,5
Partial treatment. Patients with CM often take medication early in the course of a headache. This sometimes results in a partially treated migraine that develops into a headache with tension-type features, such as a bilateral location, a pressing quality, and mild-to-moderate intensity, as well as a possible transformation to MOH. This is most likely to occur in patients who have migraines without an aura.
To avoid partial treatment, medications for acute migraine should be taken within 30 minutes of an attack, in a dose that’s sufficient to relieve the pain within 2 hours, with no need for a second dose—a protocol known as “one and done.” Efficacy of a triptan can be improved by adding a nonsteroidal anti-inflammatory drug (NSAID).10
A definitive diagnosis of CM is only possible in the absence of medication overuse.4,5 A patient who is overusing abortive headache medication and whose headache meets the criteria for CM should be given a diagnosis of probable CM instead.
Chronic tension-type headache. In addition to traits common to tension headaches, CTTH may be associated with mild nausea, photophobia, or phonophobia (but typically only one such feature at a time). There may also be tenderness to palpation of the pericranial muscles. Unlike migraine, CTTH is not affected by physical activity.
Here, too, overuse of headache medication is often a factor and should be stopped, if possible, before a definitive diagnosis of CTTH can be made.
Headache with overlapping features. It is possible for a patient to have chronic headache with features of both migraine and tension headache. Advise patients whose headaches have varying characteristics to keep a headache journal to determine which features are more prominent. Patients with smart phones can download a free app, such as iHeadache or My Headache Log Pro, to be used for this purpose.11,12
When to suspect medication overuse headache
MOH is sometimes referred to as a rebound headache or drug-induced headache. Headaches associated with medication overuse have variable intensity. Patients with MOH often awaken from sleep with a headache, and neck pain is highly prevalent.10
Quantifying overuse. According to ICHD-II, overuse is defined as using a single abortive headache medication ≥10 times a month or using 2 or more such drugs ≥15 times a month.5
Triptans have the potential to cause MOH more quickly and in lower doses compared with other acute headache medications. However, analgesics—especially combination products such as butalbital/acetaminophen/caffeine (Fioricet)—are most frequently associated with the development of MOH.13,14 NSAIDs have less potential to cause MOH and are sometimes given as bridge therapy for patients who are discontinuing their acute headache medication.
Less common primary headache disorders
Hemicrania continua, a rare cause of chronic daily headache, is unilateral, without shifting sides, and the intensity is moderate to severe—and unrelenting. HC is associated with autonomic features such as lacrimation, ptosis, and nasal congestion.
New daily persistent headache is characterized by an out-of-the-blue onset of a headache that becomes unremitting soon after it develops. To receive a diagnosis of NDPH, the patient must have a headache that started suddenly and has continued for 3 months or more.
Most patients diagnosed with NDPH are able to recall, to the day, when the headache started. More than 50% report a precipitating event, such as a viral illness, a stressful experience, or surgery.15 ICHD-II defines NDPH as having the characteristics of a tension headache. Notably, however, migrainous features are also common, and neurologists often diagnose NDPH with either migrainous or tension-type features.16
The sudden onset of NDPH is a red flag and, like other red flags, always warrants further work-up. Magnetic resonance imaging with gadolinium is preferred to computed tomography. Magnetic resonance venography or lumbar puncture may also be considered.15,16
Review comorbidities, rule out secondary causes
Patients who suffer from frequent headaches have a high prevalence of depression, anxiety,17,18 sleep disorders,19 obesity,20 irritable bowel disease, fibromyalgia,21 temporomandibular joint disorder,22 and chronic fatigue syndrome. Treatment of these disorders may increase the efficacy of headache treatment. Conversely, overuse of headache medications can make comorbidities harder to treat.
Treating chronic headache: Which drugs are best?
A multimodal approach combining pharmacologic and nonpharmacologic therapies is usually required for patients with chronic headache. The particular therapy and prognosis depend on the type of headache a patient has and the presence of comorbidities (TABLE 3).6,7,23,24
TABLE 3
Consider comorbidities in prophylaxis selection6,7,23,24
| Comorbidity | What to choose | What to avoid |
|---|---|---|
| Depression | Venlafaxine | — |
| Bipolar disorder | Valproic acid | Venlafaxine, amitriptyline, mirtazapine |
| Insomnia (CM) | Amitriptyline | — |
| Insomnia (CTTH) | Mirtazapine | — |
| Obesity | Topiramate | Amitriptyline |
| Hypertension | Metoprolol, propranolol | — |
| Cardiac conduction abnormalities | — | Amitriptyline |
| Fibromyalgia | Amitriptyline, tizanidine | — |
| CM, chronic migraine; CTTH, chronic tension-type headache. | ||
Choice for migraine prophylaxis? Here’s what the evidence tells us
Although most studies of the benefits of prophylaxis have involved patients with episodic or frequent migraine rather than CM, extrapolation of the findings to patients with CM is not unreasonable. And, although dozens of pharmacologic and complementary therapies have been studied for migraine prophylaxis and certain classes of drugs have been identified as effective, there are very few head-to-head trials comparing agents.
The American Academy of Neurology and the American Headache Society published a summary of the evidence in 2012.23 Key findings: The types of medication with the most evidence to support their use as first-line agents for CM are antidepressants, anticonvulsants, and beta-blockers.
Antidepressants, especially tricyclics (TCAs) and serotonin-norepinephrine reuptake inhibitors (SNRIs), have been found to be effective. Chief among them are amitriptyline, a TCA, which is inexpensive and may be beneficial for patients with coexisting insomnia due to its sedating effect, and venlafaxine, an SNRI, which may help treat comorbid depression.23 Amitriptyline is associated with weight gain and can prolong the QT interval at higher doses. There is insufficient or conflicting evidence of the value of selective serotonin reuptake inhibitors for migraine prophylaxis.
Anticonvulsants that have been studied most extensively for migraine are topiramate and sodium valproate. Both have level A ratings for established efficacy.23 Topiramate has also been shown to be noninferior to amitriptyline in reducing migraine frequency and is associated with weight loss, rather than weight gain.25 (Topiramate and valproic acid should be avoided in women who are hoping to become pregnant.) Gabapentin has conflicting evidence and is not recommended for migraine.23
Beta-blockers that appear to be most effective as prophylaxis for CM are propranolol, metoprolol, and timolol.23,26 Any of these would be the obvious choice for a patient with comorbid hypertension. Beta-blockers can take several months to have an effect on migraines, however. Their use as CM prophylaxis may be limited by their adverse effect profile, which includes erectile dysfunction, bradycardia, and hypotension, although the lower dosage needed for migraine prophylaxis may be a mitigating factor. Calcium channel blockers are commonly prescribed for migraine, but there is little evidence to support their use for CM.23
Other medications that are likely effective for migraine prophylaxis include naproxen24 and tizanidine27 (a muscle relaxant). Complementary and alternative treatments that appear to be effective include magnesium, feverfew, butterbur, and riboflavin, although the benefits may not be noticeable for several months.24
Botulinum toxin A is the only medication approved by the US Food and Drug Administration for prevention of CM. It is generally considered to be a second-line agent because of its high cost and the need for training and expertise to administer it. Botulinum toxin A is not effective as prophylaxis for EM.28
Treating other headache syndromes
Chronic tension-type headache. Treatment of CTTH applies similar principles to those of CM, and amitriptyline and venlafaxine—as well as mirtazapine, a sedating SNRI—have evidence to support their use for this type of headache.29 Overall, however, CTTH therapies have not been studied as extensively as those for migraine. There is conflicting evidence of the value of anticonvulsants for prophylaxis of CTTH, and botulinum toxin A has been shown to be no better than placebo.30
Medication overuse headache. Prophylactic medications are not effective in patients who are overusing acute headache medications, and patients with MOH should be instructed to stop the offending drugs. Withdrawal of triptans, simple analgesics, and ergots—either cold turkey or with a slow wean over 4 to 6 weeks—is fairly safe and can be done in an outpatient setting. Concomitant use of prednisone, long-acting NSAIDs, or botulinum toxin A can be used as “bridge therapy” to relieve acute pain. Start the patient on a prophylactic medication based on the best estimate of his or her baseline headache and comorbidities.31,32 For patients who have been overusing opiates or barbiturates, most experts recommend inpatient treatment to manage withdrawal symptoms and prevent complications.10
Most patients with MOH will improve with drug withdrawal, but some will be left with the same disabling headaches that caused them to overuse medication in the first place. For such patients, weekly office visits during the withdrawal period may be helpful. After completion of the bridge therapy, they will likely require abortive headache treatment, but its use must be limited to no more than twice a week. Referral to a specialty headache clinic may be appropriate for such patients.
Hemicrania continua. The treatment for HC is indomethacin. A 2- to 5-day course typically results in complete recovery.
New daily persistent headache. For patients with NDPH, the first step is ruling out secondary causes. Once that has been done, most experts recommend trying to characterize the headache as having features of either migraine or tension and treating accordingly with preventive therapy. If acute headache medication is still needed, limit the quantity you prescribe and stress the importance of taking it no more than twice a week.
CASE Mr. K receives a diagnosis of MOH and probable CM. You explain the way MOH develops and how his medication use has contributed to the escalation of his headaches, and ask him to stop all the headache medications he has been using and to keep a headache journal. You prescribe meloxicam as a short-term bridge therapy and low-dose venlafaxine, which is increased to 150 mg/d over the next 4 weeks; recommend riboflavin 400 mg/d; and refer Mr. K to a neurologist for botulinum toxin A.
You ask him to return in 4 weeks and explain that because he has successfully stopped the overuse of acute headache medications, he can begin taking them again—provided he limits their use to no more than twice a week.
Nonpharmacologic measures can help, too
Lifestyle modification can play an important role in the treatment of chronic daily headache. Advise patients of the importance of proper sleep hygiene, regular exercise, stress reduction, and a healthy diet, as well as avoiding known triggers and minimizing intake of caffeine. Tell patients that biofeedback, cognitive behavioral therapy, and physical therapy may play a role in conjunction with pharmacotherapy, especially for CTTH,26,29,33 but that hypnosis, acupuncture, chiropractic manipulation, transcutaneous electrical nerve stimulation, and hyperbaric oxygen have too little evidence to recommend for or against their use.26,34
In discussing treatment for chronic headache and the goals of therapy with a patient with chronic headache, it is important to be frank. Explain that while a complete cure is not always possible, a decrease in both the frequency and severity of headaches and an improvement in the quality of life and the patient’s ability to function are realistic goals.
CASE At the 3-month follow-up, Mr. K reports that his headaches are down to less than twice a week, and that he is undergoing cognitive behavioral therapy for depression. For acute headache pain, he takes sumatriptan 100 mg with ibuprofen 800 mg, and is careful not to do so more than twice a week.
CORRESPONDENCE
Kelly M. Latimer, MD, MPH, FAAFP, Naval Hospital, Camp Lejeune, NC 28542; kelly.latimer@med.navy.mil
1. Wiendels NJ, Neven AK, Rosendaal FR, et al. Chronic frequent headache in the general population: prevalence and associated factors. Cephalalgia. 2006;26:1434-1442.
2. Scher AI, Stewart WF, Liberman J, et al. Prevalence of frequent headache in a population sample. Headache. 1998;38:497-506.
3. Castillo J, Munoz P, Guitera V, et al. Kaplan Award 1998. Epidemiology of chronic daily headache in the general population. Headache. 1999;39:190-196.
4. Headache Classification Subcommittee of the International Headache Society. The International Classification of Headache Disorders: 2nd ed. Cephalalgia. 2004;24(suppl):S1-S9.
5. Headache Classification Committee, Olesen J, Bousser MG, Diener HC, et al. New appendix criteria open for a broader concept of chronic migraine. Cephalalgia. 2006;26:742-746.
6. Dodick DW. Clinical practice. Chronic daily headache. N Engl J Med. 2006;354:158-165.
7. Maizels M. The patient with daily headaches. Am Fam Physician. 2004;70:2299-2306.
8. Scher AI, Lipton RB, Stewart WF. Risk factors for chronic daily headache. Curr Pain Headache Rep. 2002;6:486-491.
9. Lipton RB. Tracing transformation: chronic migraine classification progression, and epidemiology. Neurology. 2009;72 (5 suppl):S3-S7.
10. Tepper SJ. Medication-overuse headache. Continuum. 2012;18:807-822.
11. iHeadache. Available at: https://itunes.apple.com/us/app/iheadache-free-headache-migraine/id374213833?mt=8. Accessed February 10, 2013.
12. My Headache Log Pro. Available at: https://play.google.com/store/apps/details?id=com.dontek.myheadachelog&hl=en. Accessed February 10 2013.
13. Bigal ME, Serrano D, Buse D, et al. Acute migraine medications and evolution from episodic to chronic migraine: a longitudinal population-based study. Headache. 2008;48:1157-1168.
14. Colas R, Munoz P, Temprano R, et al. Chronic daily headache with analgesic overuse: epidemiology and impact on quality of life. Neurology. 2004;62:1338-1342.
15. Li D, Rozen TD. The clinical characteristics of new daily persistent headache. Cephalalgia. 2002;22:66-69.
16. Young WB, Swanson JW. New daily-persistent headache: the switched-on headache. Neurology. 2010;74:1338-1339.
17. Verri AP, Proietti Cecchini A, Galli C, et al. Psychiatric comorbidity in chronic daily headache. Cephalalgia. 1998;18 (suppl 21):S45-S49.
18. Tietjen GE, Brandes JL, Digre KB, et al. High prevalence of somatic symptoms and depression in women with disabling chronic headache. Neurology. 2007;68:134.-
19. Kelman L, Rains JC. Headache and sleep: examination of sleep patterns and complaints in a large clinical sample of migraineurs. Headache. 2005;45:904-910.
20. Bigal ME, Lipton RB. Obesity is a risk factor for transformed migraine but not chronic tension-type headache. Neurology. 2006;67:252-257.
21. Peres MF, Young WB, Kaup AO, et al. Fibromyalgia is common in patients with transformed migraine. Neurology. 2001;57:1326-1328.
22. Ciancaglini R, Radaelli G. The relationship between headache and symptoms of temporomandibular disorders in the general population. J Dent. 2001;29:93-98.
23. Silberstein SD, Holland S, Freitag F, et al. Evidence-based guideline update: pharmacologic treatment for episodic migraine prevention in adults: report of the Quality Standards Subcommittee of the American Academy of Neurology and the American Headache Society. Neurology. 2012;78:1337-1345.
24. Holland S, Silberstein SD, Freitag F, et al. Evidence-based guideline update: NSAIDs and other complementary treatments for episodic migraine prevention in adults: report of the Quality Standards Subcommittee of the American Academy of Neurology and the American Headache Society. Neurology. 2012;78:1346-1353.
25. Dodick DW, Freitag F, Banks J, et al. Topiramate versus amitriptyline in migraine prevention: a 26-week, multicenter, randomized, double-blind, double-dummy, parallel-group noninferiority trial in adult migrainers. Clin Ther. 2009;31:542-559.
26. Linde K, Rossnagel K. Propranolol for migraine prophylaxis. Cochrane Database Syst Rev. 2004;(2):CD003225.-
27. Saper JR, Lake AE, Cantrell DT, et al. Chronic daily headache prophylaxis with tizanidine: a double-blind, placebo-controlled, multicenter outcome study. Headache. 2002;42:470-482.
28. Dodick DW, Turkel CC, DeGryse RE, et al. Onabotulinumtoxin A for treatment of chronic migraine: pooled results from the double-blind, randomized, placebo-controlled phases of the PREEMPT clinical program. Headache. 2010;50:921-936.
29. Bendsten L, Evers S, Linde M, et al. EFNS guideline on the treatment of tension-type headache – report of an EFNS task force. Eur J Neurol. 2010;17:1318-1325.
30. Silberstein SD, Gobel H, Jensen R, et al. Botulinum toxin type A in the prophylactic treatment of chronic tension-type headache: a multicenter, double-blind, randomized, placebo-controlled, parallel-group study. Cephalalgia. 2006;26:790-800.
31. Katsavara Z, Jensen R. Medication-overuse headache: where are we now? Curr Opin Neurol. 2007;20:326-330.
32. Zeeberg P, Olesen J, Jensen R. Discontinuation of medication overuse in headache patients: recovery of therapeutic responsiveness. Cephalalgia. 2006;26:1192-1198.
33. Garza I, Schwedt TJ. Diagnosis and management of chronic daily headache. Semin Neurol. 2010;30:154-166.
34. Li Y, Zheng H, Witt CM, et al. Acupuncture for migraine prophylaxis: a randomized controlled trial. CMAJ. 2012;184:401-410.
• Treat medication overuse headache by withdrawing abortive therapy and initiating prophylactic therapy. C
• Select prophylactic medications that are first-line therapy for chronic migraine or tension-type headache and are appropriate for the patient’s comorbidities. C
• Advise patients to limit intake of abortive headache medications to ≤9 per month. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
CASE Eric K, age 25, is in your office, seeking help for chronic headache—which he’s had nearly every day for the past 9 months. He says that the headaches vary in quality and intensity. Sometimes the pain is in the right temporal area; has a throbbing, pulsating quality; and is accompanied by nausea and photophobia. These headaches are incapacitating, with an intensity of 10 on a scale of one to 10. When they occur, the patient reports, all he can do is take migraine medication and lie down in a darkened room for several hours, until the pain goes away. He cannot identify any triggers.
He also gets headaches that are not incapacitating, but occur almost daily, the patient says, describing a dull bilateral pressure that usually begins in the afternoon and worsens until he takes headache medication. He denies any fever, chills, weight loss, visual changes, or tinnitus. His medical history is significant only for obesity, but a system review is positive for depression and insomnia. Physical examination reveals normal vital signs; normal head, eyes, ears, nose, and throat (HEENT); and normal fundoscopic and neurological exams.
Patients like Mr. K can be challenging for primary care physicians, but referral to a neurologist is indicated only in the most intractable cases. For the vast majority of patients with frequent headaches, family physicians can perform the diagnostic work-up and oversee treatment. This review will help with both.
What kind of headache?
While most headaches are sporadic in nature, the prevalence of “chronic daily headache” ranges from 3% to 5% worldwide.1-3
Chronic daily headache is not a diagnosis, however, nor is it an indication that a patient has a headache every day. According to the International Classification of Headache Disorders (ICHD-II), chronic daily headache encompasses several distinct primary headache disorders that have a frequency of ≥15 times per month for at least 3 months. These disorders are also classified by duration, as long (>4 hours) or short.4
The text and tables that follow focus on the diagnosis and treatment of the 4 primary headache disorders of long duration—chronic migraine (CM), chronic tension-type headache (CTTH), hemicrania continua (HC), and new daily persistent headache (NDPH) (TABLE 1).4,5 Although medication overuse headache (MOH) is not a primary headache, it is included in this review because it often contributes to and complicates treatment of primary headache disorders. What’s more, most chronic daily headache syndromes are inextricably linked to medication overuse.6,7
Which individuals are at risk?
Risk factors for chronic headache include female sex, older age, obesity, heavy caffeine consumption, tobacco use, low educational level, overuse of abortive headache medications, and a history of head and neck trauma.8 Episodic migraine (EM)—that is, migraines that occur ≤14 times a month—is also a risk factor for chronic headache.
ICHD-II classifies EM as a progressive disease that transforms to CM at a rate of 3% per year.9 Transformation of EM to CM has been found to occur after as few as 5 days of barbiturates or 8 days of opiates per month.10
Patients with EM should be warned about the potential for migraines to become chronic and have their acute headache medications replaced with a prophylactic drug if the frequency approaches 2 per week.
TABLE 1
Diagnosing and treating chronic headache4,5
| Type of headache | ICHD-II diagnostic criteria | First-line treatment |
|---|---|---|
| Chronic migraine |
| Prophylactic therapy:
|
| Chronic tension-type headache |
| Prophylactic therapy:
|
| Medication overuse headache |
| Discontinue overused acute meds; provide headache education; bridge with NSAIDs, prednisone, or botulinum toxin A; begin prophylactic medication |
| Hemicrania continua |
| Indomethacin |
| New daily persistent headache |
| Rule out secondary causes; treat according to migrainous or tension features of headache |
| ICHD-II, International Classification of Headache Disorders; NSAIDs, nonsteroidal anti-inflammatory drugs; SNRIs, serotonin-norepinephrine reuptake inhibitors; TCAs, tricyclic antidepressants. | ||
Pinpointing the type of headache
An accurate diagnosis requires a thorough headache history and a HEENT and neurological examination. The history should include questions about the characteristics of the headache, including the location, intensity, frequency, timing, associated symptoms, previous headache diagnoses, and triggers, and address comorbidities, medication use, caffeine intake, and family history.8 In the absence of red flags—age >50 years, history of headache or systemic illness, sudden onset, or papilledema, among other findings that may indicate more serious conditions (TABLE 2)7—advanced imaging and further work-up are not needed.
TABLE 2
Beyond headache: Red flags warrant additional testing7
| Red flag | Condition(s) to rule out |
|---|---|
| Age of onset >50 y | Giant cell arteritis, mass lesion, stroke |
| No prior history of headache OR change in characteristic from prior headaches | Cancer, aneurysm, stroke, cerebral sinus thrombosis, infection |
| “Thunderclap” headache | Ruptured aneurysm |
| Signs or symptoms of systemic illness (eg, fever, chills, weight loss) | Meningitis, encephalitis, cancer |
| History of systemic illness, such as cancer, autoimmune disease, or HIV | Brain metastasis, mass lesion, autoimmune meningitis, thrombosis |
| Headache brought on by change in head position or Valsalva maneuver | Spontaneous CSF leak or Chiari malformation |
| Occipital location of headache (in children) | Brain tumor |
| Neurological symptoms | Mass lesion, encephalitis |
| Papilledema | Idiopathic intracranial hypertension, cerebral sinus thrombosis |
| CSF, cerebrospinal fluid; HIV, human immunodeficiency virus. | |
Migraine or tension headache?
Chronic migraine. To be classified as CM, the headache must have occurred ≥15 days a month for 3 months or more and have features of migraine, such as unilateral location, pulsating quality, and moderate to severe intensity. Migraines are aggravated by physical activity and associated with nausea and/or vomiting, photophobia, and phonophobia, and may or may not be preceded by aura. Common triggers include stress, menstruation, alcohol, skipped meals, dehydration, and chocolate. Migraines typically respond to ergots and triptans.4,5
Partial treatment. Patients with CM often take medication early in the course of a headache. This sometimes results in a partially treated migraine that develops into a headache with tension-type features, such as a bilateral location, a pressing quality, and mild-to-moderate intensity, as well as a possible transformation to MOH. This is most likely to occur in patients who have migraines without an aura.
To avoid partial treatment, medications for acute migraine should be taken within 30 minutes of an attack, in a dose that’s sufficient to relieve the pain within 2 hours, with no need for a second dose—a protocol known as “one and done.” Efficacy of a triptan can be improved by adding a nonsteroidal anti-inflammatory drug (NSAID).10
A definitive diagnosis of CM is only possible in the absence of medication overuse.4,5 A patient who is overusing abortive headache medication and whose headache meets the criteria for CM should be given a diagnosis of probable CM instead.
Chronic tension-type headache. In addition to traits common to tension headaches, CTTH may be associated with mild nausea, photophobia, or phonophobia (but typically only one such feature at a time). There may also be tenderness to palpation of the pericranial muscles. Unlike migraine, CTTH is not affected by physical activity.
Here, too, overuse of headache medication is often a factor and should be stopped, if possible, before a definitive diagnosis of CTTH can be made.
Headache with overlapping features. It is possible for a patient to have chronic headache with features of both migraine and tension headache. Advise patients whose headaches have varying characteristics to keep a headache journal to determine which features are more prominent. Patients with smart phones can download a free app, such as iHeadache or My Headache Log Pro, to be used for this purpose.11,12
When to suspect medication overuse headache
MOH is sometimes referred to as a rebound headache or drug-induced headache. Headaches associated with medication overuse have variable intensity. Patients with MOH often awaken from sleep with a headache, and neck pain is highly prevalent.10
Quantifying overuse. According to ICHD-II, overuse is defined as using a single abortive headache medication ≥10 times a month or using 2 or more such drugs ≥15 times a month.5
Triptans have the potential to cause MOH more quickly and in lower doses compared with other acute headache medications. However, analgesics—especially combination products such as butalbital/acetaminophen/caffeine (Fioricet)—are most frequently associated with the development of MOH.13,14 NSAIDs have less potential to cause MOH and are sometimes given as bridge therapy for patients who are discontinuing their acute headache medication.
Less common primary headache disorders
Hemicrania continua, a rare cause of chronic daily headache, is unilateral, without shifting sides, and the intensity is moderate to severe—and unrelenting. HC is associated with autonomic features such as lacrimation, ptosis, and nasal congestion.
New daily persistent headache is characterized by an out-of-the-blue onset of a headache that becomes unremitting soon after it develops. To receive a diagnosis of NDPH, the patient must have a headache that started suddenly and has continued for 3 months or more.
Most patients diagnosed with NDPH are able to recall, to the day, when the headache started. More than 50% report a precipitating event, such as a viral illness, a stressful experience, or surgery.15 ICHD-II defines NDPH as having the characteristics of a tension headache. Notably, however, migrainous features are also common, and neurologists often diagnose NDPH with either migrainous or tension-type features.16
The sudden onset of NDPH is a red flag and, like other red flags, always warrants further work-up. Magnetic resonance imaging with gadolinium is preferred to computed tomography. Magnetic resonance venography or lumbar puncture may also be considered.15,16
Review comorbidities, rule out secondary causes
Patients who suffer from frequent headaches have a high prevalence of depression, anxiety,17,18 sleep disorders,19 obesity,20 irritable bowel disease, fibromyalgia,21 temporomandibular joint disorder,22 and chronic fatigue syndrome. Treatment of these disorders may increase the efficacy of headache treatment. Conversely, overuse of headache medications can make comorbidities harder to treat.
Treating chronic headache: Which drugs are best?
A multimodal approach combining pharmacologic and nonpharmacologic therapies is usually required for patients with chronic headache. The particular therapy and prognosis depend on the type of headache a patient has and the presence of comorbidities (TABLE 3).6,7,23,24
TABLE 3
Consider comorbidities in prophylaxis selection6,7,23,24
| Comorbidity | What to choose | What to avoid |
|---|---|---|
| Depression | Venlafaxine | — |
| Bipolar disorder | Valproic acid | Venlafaxine, amitriptyline, mirtazapine |
| Insomnia (CM) | Amitriptyline | — |
| Insomnia (CTTH) | Mirtazapine | — |
| Obesity | Topiramate | Amitriptyline |
| Hypertension | Metoprolol, propranolol | — |
| Cardiac conduction abnormalities | — | Amitriptyline |
| Fibromyalgia | Amitriptyline, tizanidine | — |
| CM, chronic migraine; CTTH, chronic tension-type headache. | ||
Choice for migraine prophylaxis? Here’s what the evidence tells us
Although most studies of the benefits of prophylaxis have involved patients with episodic or frequent migraine rather than CM, extrapolation of the findings to patients with CM is not unreasonable. And, although dozens of pharmacologic and complementary therapies have been studied for migraine prophylaxis and certain classes of drugs have been identified as effective, there are very few head-to-head trials comparing agents.
The American Academy of Neurology and the American Headache Society published a summary of the evidence in 2012.23 Key findings: The types of medication with the most evidence to support their use as first-line agents for CM are antidepressants, anticonvulsants, and beta-blockers.
Antidepressants, especially tricyclics (TCAs) and serotonin-norepinephrine reuptake inhibitors (SNRIs), have been found to be effective. Chief among them are amitriptyline, a TCA, which is inexpensive and may be beneficial for patients with coexisting insomnia due to its sedating effect, and venlafaxine, an SNRI, which may help treat comorbid depression.23 Amitriptyline is associated with weight gain and can prolong the QT interval at higher doses. There is insufficient or conflicting evidence of the value of selective serotonin reuptake inhibitors for migraine prophylaxis.
Anticonvulsants that have been studied most extensively for migraine are topiramate and sodium valproate. Both have level A ratings for established efficacy.23 Topiramate has also been shown to be noninferior to amitriptyline in reducing migraine frequency and is associated with weight loss, rather than weight gain.25 (Topiramate and valproic acid should be avoided in women who are hoping to become pregnant.) Gabapentin has conflicting evidence and is not recommended for migraine.23
Beta-blockers that appear to be most effective as prophylaxis for CM are propranolol, metoprolol, and timolol.23,26 Any of these would be the obvious choice for a patient with comorbid hypertension. Beta-blockers can take several months to have an effect on migraines, however. Their use as CM prophylaxis may be limited by their adverse effect profile, which includes erectile dysfunction, bradycardia, and hypotension, although the lower dosage needed for migraine prophylaxis may be a mitigating factor. Calcium channel blockers are commonly prescribed for migraine, but there is little evidence to support their use for CM.23
Other medications that are likely effective for migraine prophylaxis include naproxen24 and tizanidine27 (a muscle relaxant). Complementary and alternative treatments that appear to be effective include magnesium, feverfew, butterbur, and riboflavin, although the benefits may not be noticeable for several months.24
Botulinum toxin A is the only medication approved by the US Food and Drug Administration for prevention of CM. It is generally considered to be a second-line agent because of its high cost and the need for training and expertise to administer it. Botulinum toxin A is not effective as prophylaxis for EM.28
Treating other headache syndromes
Chronic tension-type headache. Treatment of CTTH applies similar principles to those of CM, and amitriptyline and venlafaxine—as well as mirtazapine, a sedating SNRI—have evidence to support their use for this type of headache.29 Overall, however, CTTH therapies have not been studied as extensively as those for migraine. There is conflicting evidence of the value of anticonvulsants for prophylaxis of CTTH, and botulinum toxin A has been shown to be no better than placebo.30
Medication overuse headache. Prophylactic medications are not effective in patients who are overusing acute headache medications, and patients with MOH should be instructed to stop the offending drugs. Withdrawal of triptans, simple analgesics, and ergots—either cold turkey or with a slow wean over 4 to 6 weeks—is fairly safe and can be done in an outpatient setting. Concomitant use of prednisone, long-acting NSAIDs, or botulinum toxin A can be used as “bridge therapy” to relieve acute pain. Start the patient on a prophylactic medication based on the best estimate of his or her baseline headache and comorbidities.31,32 For patients who have been overusing opiates or barbiturates, most experts recommend inpatient treatment to manage withdrawal symptoms and prevent complications.10
Most patients with MOH will improve with drug withdrawal, but some will be left with the same disabling headaches that caused them to overuse medication in the first place. For such patients, weekly office visits during the withdrawal period may be helpful. After completion of the bridge therapy, they will likely require abortive headache treatment, but its use must be limited to no more than twice a week. Referral to a specialty headache clinic may be appropriate for such patients.
Hemicrania continua. The treatment for HC is indomethacin. A 2- to 5-day course typically results in complete recovery.
New daily persistent headache. For patients with NDPH, the first step is ruling out secondary causes. Once that has been done, most experts recommend trying to characterize the headache as having features of either migraine or tension and treating accordingly with preventive therapy. If acute headache medication is still needed, limit the quantity you prescribe and stress the importance of taking it no more than twice a week.
CASE Mr. K receives a diagnosis of MOH and probable CM. You explain the way MOH develops and how his medication use has contributed to the escalation of his headaches, and ask him to stop all the headache medications he has been using and to keep a headache journal. You prescribe meloxicam as a short-term bridge therapy and low-dose venlafaxine, which is increased to 150 mg/d over the next 4 weeks; recommend riboflavin 400 mg/d; and refer Mr. K to a neurologist for botulinum toxin A.
You ask him to return in 4 weeks and explain that because he has successfully stopped the overuse of acute headache medications, he can begin taking them again—provided he limits their use to no more than twice a week.
Nonpharmacologic measures can help, too
Lifestyle modification can play an important role in the treatment of chronic daily headache. Advise patients of the importance of proper sleep hygiene, regular exercise, stress reduction, and a healthy diet, as well as avoiding known triggers and minimizing intake of caffeine. Tell patients that biofeedback, cognitive behavioral therapy, and physical therapy may play a role in conjunction with pharmacotherapy, especially for CTTH,26,29,33 but that hypnosis, acupuncture, chiropractic manipulation, transcutaneous electrical nerve stimulation, and hyperbaric oxygen have too little evidence to recommend for or against their use.26,34
In discussing treatment for chronic headache and the goals of therapy with a patient with chronic headache, it is important to be frank. Explain that while a complete cure is not always possible, a decrease in both the frequency and severity of headaches and an improvement in the quality of life and the patient’s ability to function are realistic goals.
CASE At the 3-month follow-up, Mr. K reports that his headaches are down to less than twice a week, and that he is undergoing cognitive behavioral therapy for depression. For acute headache pain, he takes sumatriptan 100 mg with ibuprofen 800 mg, and is careful not to do so more than twice a week.
CORRESPONDENCE
Kelly M. Latimer, MD, MPH, FAAFP, Naval Hospital, Camp Lejeune, NC 28542; kelly.latimer@med.navy.mil
• Treat medication overuse headache by withdrawing abortive therapy and initiating prophylactic therapy. C
• Select prophylactic medications that are first-line therapy for chronic migraine or tension-type headache and are appropriate for the patient’s comorbidities. C
• Advise patients to limit intake of abortive headache medications to ≤9 per month. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
CASE Eric K, age 25, is in your office, seeking help for chronic headache—which he’s had nearly every day for the past 9 months. He says that the headaches vary in quality and intensity. Sometimes the pain is in the right temporal area; has a throbbing, pulsating quality; and is accompanied by nausea and photophobia. These headaches are incapacitating, with an intensity of 10 on a scale of one to 10. When they occur, the patient reports, all he can do is take migraine medication and lie down in a darkened room for several hours, until the pain goes away. He cannot identify any triggers.
He also gets headaches that are not incapacitating, but occur almost daily, the patient says, describing a dull bilateral pressure that usually begins in the afternoon and worsens until he takes headache medication. He denies any fever, chills, weight loss, visual changes, or tinnitus. His medical history is significant only for obesity, but a system review is positive for depression and insomnia. Physical examination reveals normal vital signs; normal head, eyes, ears, nose, and throat (HEENT); and normal fundoscopic and neurological exams.
Patients like Mr. K can be challenging for primary care physicians, but referral to a neurologist is indicated only in the most intractable cases. For the vast majority of patients with frequent headaches, family physicians can perform the diagnostic work-up and oversee treatment. This review will help with both.
What kind of headache?
While most headaches are sporadic in nature, the prevalence of “chronic daily headache” ranges from 3% to 5% worldwide.1-3
Chronic daily headache is not a diagnosis, however, nor is it an indication that a patient has a headache every day. According to the International Classification of Headache Disorders (ICHD-II), chronic daily headache encompasses several distinct primary headache disorders that have a frequency of ≥15 times per month for at least 3 months. These disorders are also classified by duration, as long (>4 hours) or short.4
The text and tables that follow focus on the diagnosis and treatment of the 4 primary headache disorders of long duration—chronic migraine (CM), chronic tension-type headache (CTTH), hemicrania continua (HC), and new daily persistent headache (NDPH) (TABLE 1).4,5 Although medication overuse headache (MOH) is not a primary headache, it is included in this review because it often contributes to and complicates treatment of primary headache disorders. What’s more, most chronic daily headache syndromes are inextricably linked to medication overuse.6,7
Which individuals are at risk?
Risk factors for chronic headache include female sex, older age, obesity, heavy caffeine consumption, tobacco use, low educational level, overuse of abortive headache medications, and a history of head and neck trauma.8 Episodic migraine (EM)—that is, migraines that occur ≤14 times a month—is also a risk factor for chronic headache.
ICHD-II classifies EM as a progressive disease that transforms to CM at a rate of 3% per year.9 Transformation of EM to CM has been found to occur after as few as 5 days of barbiturates or 8 days of opiates per month.10
Patients with EM should be warned about the potential for migraines to become chronic and have their acute headache medications replaced with a prophylactic drug if the frequency approaches 2 per week.
TABLE 1
Diagnosing and treating chronic headache4,5
| Type of headache | ICHD-II diagnostic criteria | First-line treatment |
|---|---|---|
| Chronic migraine |
| Prophylactic therapy:
|
| Chronic tension-type headache |
| Prophylactic therapy:
|
| Medication overuse headache |
| Discontinue overused acute meds; provide headache education; bridge with NSAIDs, prednisone, or botulinum toxin A; begin prophylactic medication |
| Hemicrania continua |
| Indomethacin |
| New daily persistent headache |
| Rule out secondary causes; treat according to migrainous or tension features of headache |
| ICHD-II, International Classification of Headache Disorders; NSAIDs, nonsteroidal anti-inflammatory drugs; SNRIs, serotonin-norepinephrine reuptake inhibitors; TCAs, tricyclic antidepressants. | ||
Pinpointing the type of headache
An accurate diagnosis requires a thorough headache history and a HEENT and neurological examination. The history should include questions about the characteristics of the headache, including the location, intensity, frequency, timing, associated symptoms, previous headache diagnoses, and triggers, and address comorbidities, medication use, caffeine intake, and family history.8 In the absence of red flags—age >50 years, history of headache or systemic illness, sudden onset, or papilledema, among other findings that may indicate more serious conditions (TABLE 2)7—advanced imaging and further work-up are not needed.
TABLE 2
Beyond headache: Red flags warrant additional testing7
| Red flag | Condition(s) to rule out |
|---|---|
| Age of onset >50 y | Giant cell arteritis, mass lesion, stroke |
| No prior history of headache OR change in characteristic from prior headaches | Cancer, aneurysm, stroke, cerebral sinus thrombosis, infection |
| “Thunderclap” headache | Ruptured aneurysm |
| Signs or symptoms of systemic illness (eg, fever, chills, weight loss) | Meningitis, encephalitis, cancer |
| History of systemic illness, such as cancer, autoimmune disease, or HIV | Brain metastasis, mass lesion, autoimmune meningitis, thrombosis |
| Headache brought on by change in head position or Valsalva maneuver | Spontaneous CSF leak or Chiari malformation |
| Occipital location of headache (in children) | Brain tumor |
| Neurological symptoms | Mass lesion, encephalitis |
| Papilledema | Idiopathic intracranial hypertension, cerebral sinus thrombosis |
| CSF, cerebrospinal fluid; HIV, human immunodeficiency virus. | |
Migraine or tension headache?
Chronic migraine. To be classified as CM, the headache must have occurred ≥15 days a month for 3 months or more and have features of migraine, such as unilateral location, pulsating quality, and moderate to severe intensity. Migraines are aggravated by physical activity and associated with nausea and/or vomiting, photophobia, and phonophobia, and may or may not be preceded by aura. Common triggers include stress, menstruation, alcohol, skipped meals, dehydration, and chocolate. Migraines typically respond to ergots and triptans.4,5
Partial treatment. Patients with CM often take medication early in the course of a headache. This sometimes results in a partially treated migraine that develops into a headache with tension-type features, such as a bilateral location, a pressing quality, and mild-to-moderate intensity, as well as a possible transformation to MOH. This is most likely to occur in patients who have migraines without an aura.
To avoid partial treatment, medications for acute migraine should be taken within 30 minutes of an attack, in a dose that’s sufficient to relieve the pain within 2 hours, with no need for a second dose—a protocol known as “one and done.” Efficacy of a triptan can be improved by adding a nonsteroidal anti-inflammatory drug (NSAID).10
A definitive diagnosis of CM is only possible in the absence of medication overuse.4,5 A patient who is overusing abortive headache medication and whose headache meets the criteria for CM should be given a diagnosis of probable CM instead.
Chronic tension-type headache. In addition to traits common to tension headaches, CTTH may be associated with mild nausea, photophobia, or phonophobia (but typically only one such feature at a time). There may also be tenderness to palpation of the pericranial muscles. Unlike migraine, CTTH is not affected by physical activity.
Here, too, overuse of headache medication is often a factor and should be stopped, if possible, before a definitive diagnosis of CTTH can be made.
Headache with overlapping features. It is possible for a patient to have chronic headache with features of both migraine and tension headache. Advise patients whose headaches have varying characteristics to keep a headache journal to determine which features are more prominent. Patients with smart phones can download a free app, such as iHeadache or My Headache Log Pro, to be used for this purpose.11,12
When to suspect medication overuse headache
MOH is sometimes referred to as a rebound headache or drug-induced headache. Headaches associated with medication overuse have variable intensity. Patients with MOH often awaken from sleep with a headache, and neck pain is highly prevalent.10
Quantifying overuse. According to ICHD-II, overuse is defined as using a single abortive headache medication ≥10 times a month or using 2 or more such drugs ≥15 times a month.5
Triptans have the potential to cause MOH more quickly and in lower doses compared with other acute headache medications. However, analgesics—especially combination products such as butalbital/acetaminophen/caffeine (Fioricet)—are most frequently associated with the development of MOH.13,14 NSAIDs have less potential to cause MOH and are sometimes given as bridge therapy for patients who are discontinuing their acute headache medication.
Less common primary headache disorders
Hemicrania continua, a rare cause of chronic daily headache, is unilateral, without shifting sides, and the intensity is moderate to severe—and unrelenting. HC is associated with autonomic features such as lacrimation, ptosis, and nasal congestion.
New daily persistent headache is characterized by an out-of-the-blue onset of a headache that becomes unremitting soon after it develops. To receive a diagnosis of NDPH, the patient must have a headache that started suddenly and has continued for 3 months or more.
Most patients diagnosed with NDPH are able to recall, to the day, when the headache started. More than 50% report a precipitating event, such as a viral illness, a stressful experience, or surgery.15 ICHD-II defines NDPH as having the characteristics of a tension headache. Notably, however, migrainous features are also common, and neurologists often diagnose NDPH with either migrainous or tension-type features.16
The sudden onset of NDPH is a red flag and, like other red flags, always warrants further work-up. Magnetic resonance imaging with gadolinium is preferred to computed tomography. Magnetic resonance venography or lumbar puncture may also be considered.15,16
Review comorbidities, rule out secondary causes
Patients who suffer from frequent headaches have a high prevalence of depression, anxiety,17,18 sleep disorders,19 obesity,20 irritable bowel disease, fibromyalgia,21 temporomandibular joint disorder,22 and chronic fatigue syndrome. Treatment of these disorders may increase the efficacy of headache treatment. Conversely, overuse of headache medications can make comorbidities harder to treat.
Treating chronic headache: Which drugs are best?
A multimodal approach combining pharmacologic and nonpharmacologic therapies is usually required for patients with chronic headache. The particular therapy and prognosis depend on the type of headache a patient has and the presence of comorbidities (TABLE 3).6,7,23,24
TABLE 3
Consider comorbidities in prophylaxis selection6,7,23,24
| Comorbidity | What to choose | What to avoid |
|---|---|---|
| Depression | Venlafaxine | — |
| Bipolar disorder | Valproic acid | Venlafaxine, amitriptyline, mirtazapine |
| Insomnia (CM) | Amitriptyline | — |
| Insomnia (CTTH) | Mirtazapine | — |
| Obesity | Topiramate | Amitriptyline |
| Hypertension | Metoprolol, propranolol | — |
| Cardiac conduction abnormalities | — | Amitriptyline |
| Fibromyalgia | Amitriptyline, tizanidine | — |
| CM, chronic migraine; CTTH, chronic tension-type headache. | ||
Choice for migraine prophylaxis? Here’s what the evidence tells us
Although most studies of the benefits of prophylaxis have involved patients with episodic or frequent migraine rather than CM, extrapolation of the findings to patients with CM is not unreasonable. And, although dozens of pharmacologic and complementary therapies have been studied for migraine prophylaxis and certain classes of drugs have been identified as effective, there are very few head-to-head trials comparing agents.
The American Academy of Neurology and the American Headache Society published a summary of the evidence in 2012.23 Key findings: The types of medication with the most evidence to support their use as first-line agents for CM are antidepressants, anticonvulsants, and beta-blockers.
Antidepressants, especially tricyclics (TCAs) and serotonin-norepinephrine reuptake inhibitors (SNRIs), have been found to be effective. Chief among them are amitriptyline, a TCA, which is inexpensive and may be beneficial for patients with coexisting insomnia due to its sedating effect, and venlafaxine, an SNRI, which may help treat comorbid depression.23 Amitriptyline is associated with weight gain and can prolong the QT interval at higher doses. There is insufficient or conflicting evidence of the value of selective serotonin reuptake inhibitors for migraine prophylaxis.
Anticonvulsants that have been studied most extensively for migraine are topiramate and sodium valproate. Both have level A ratings for established efficacy.23 Topiramate has also been shown to be noninferior to amitriptyline in reducing migraine frequency and is associated with weight loss, rather than weight gain.25 (Topiramate and valproic acid should be avoided in women who are hoping to become pregnant.) Gabapentin has conflicting evidence and is not recommended for migraine.23
Beta-blockers that appear to be most effective as prophylaxis for CM are propranolol, metoprolol, and timolol.23,26 Any of these would be the obvious choice for a patient with comorbid hypertension. Beta-blockers can take several months to have an effect on migraines, however. Their use as CM prophylaxis may be limited by their adverse effect profile, which includes erectile dysfunction, bradycardia, and hypotension, although the lower dosage needed for migraine prophylaxis may be a mitigating factor. Calcium channel blockers are commonly prescribed for migraine, but there is little evidence to support their use for CM.23
Other medications that are likely effective for migraine prophylaxis include naproxen24 and tizanidine27 (a muscle relaxant). Complementary and alternative treatments that appear to be effective include magnesium, feverfew, butterbur, and riboflavin, although the benefits may not be noticeable for several months.24
Botulinum toxin A is the only medication approved by the US Food and Drug Administration for prevention of CM. It is generally considered to be a second-line agent because of its high cost and the need for training and expertise to administer it. Botulinum toxin A is not effective as prophylaxis for EM.28
Treating other headache syndromes
Chronic tension-type headache. Treatment of CTTH applies similar principles to those of CM, and amitriptyline and venlafaxine—as well as mirtazapine, a sedating SNRI—have evidence to support their use for this type of headache.29 Overall, however, CTTH therapies have not been studied as extensively as those for migraine. There is conflicting evidence of the value of anticonvulsants for prophylaxis of CTTH, and botulinum toxin A has been shown to be no better than placebo.30
Medication overuse headache. Prophylactic medications are not effective in patients who are overusing acute headache medications, and patients with MOH should be instructed to stop the offending drugs. Withdrawal of triptans, simple analgesics, and ergots—either cold turkey or with a slow wean over 4 to 6 weeks—is fairly safe and can be done in an outpatient setting. Concomitant use of prednisone, long-acting NSAIDs, or botulinum toxin A can be used as “bridge therapy” to relieve acute pain. Start the patient on a prophylactic medication based on the best estimate of his or her baseline headache and comorbidities.31,32 For patients who have been overusing opiates or barbiturates, most experts recommend inpatient treatment to manage withdrawal symptoms and prevent complications.10
Most patients with MOH will improve with drug withdrawal, but some will be left with the same disabling headaches that caused them to overuse medication in the first place. For such patients, weekly office visits during the withdrawal period may be helpful. After completion of the bridge therapy, they will likely require abortive headache treatment, but its use must be limited to no more than twice a week. Referral to a specialty headache clinic may be appropriate for such patients.
Hemicrania continua. The treatment for HC is indomethacin. A 2- to 5-day course typically results in complete recovery.
New daily persistent headache. For patients with NDPH, the first step is ruling out secondary causes. Once that has been done, most experts recommend trying to characterize the headache as having features of either migraine or tension and treating accordingly with preventive therapy. If acute headache medication is still needed, limit the quantity you prescribe and stress the importance of taking it no more than twice a week.
CASE Mr. K receives a diagnosis of MOH and probable CM. You explain the way MOH develops and how his medication use has contributed to the escalation of his headaches, and ask him to stop all the headache medications he has been using and to keep a headache journal. You prescribe meloxicam as a short-term bridge therapy and low-dose venlafaxine, which is increased to 150 mg/d over the next 4 weeks; recommend riboflavin 400 mg/d; and refer Mr. K to a neurologist for botulinum toxin A.
You ask him to return in 4 weeks and explain that because he has successfully stopped the overuse of acute headache medications, he can begin taking them again—provided he limits their use to no more than twice a week.
Nonpharmacologic measures can help, too
Lifestyle modification can play an important role in the treatment of chronic daily headache. Advise patients of the importance of proper sleep hygiene, regular exercise, stress reduction, and a healthy diet, as well as avoiding known triggers and minimizing intake of caffeine. Tell patients that biofeedback, cognitive behavioral therapy, and physical therapy may play a role in conjunction with pharmacotherapy, especially for CTTH,26,29,33 but that hypnosis, acupuncture, chiropractic manipulation, transcutaneous electrical nerve stimulation, and hyperbaric oxygen have too little evidence to recommend for or against their use.26,34
In discussing treatment for chronic headache and the goals of therapy with a patient with chronic headache, it is important to be frank. Explain that while a complete cure is not always possible, a decrease in both the frequency and severity of headaches and an improvement in the quality of life and the patient’s ability to function are realistic goals.
CASE At the 3-month follow-up, Mr. K reports that his headaches are down to less than twice a week, and that he is undergoing cognitive behavioral therapy for depression. For acute headache pain, he takes sumatriptan 100 mg with ibuprofen 800 mg, and is careful not to do so more than twice a week.
CORRESPONDENCE
Kelly M. Latimer, MD, MPH, FAAFP, Naval Hospital, Camp Lejeune, NC 28542; kelly.latimer@med.navy.mil
1. Wiendels NJ, Neven AK, Rosendaal FR, et al. Chronic frequent headache in the general population: prevalence and associated factors. Cephalalgia. 2006;26:1434-1442.
2. Scher AI, Stewart WF, Liberman J, et al. Prevalence of frequent headache in a population sample. Headache. 1998;38:497-506.
3. Castillo J, Munoz P, Guitera V, et al. Kaplan Award 1998. Epidemiology of chronic daily headache in the general population. Headache. 1999;39:190-196.
4. Headache Classification Subcommittee of the International Headache Society. The International Classification of Headache Disorders: 2nd ed. Cephalalgia. 2004;24(suppl):S1-S9.
5. Headache Classification Committee, Olesen J, Bousser MG, Diener HC, et al. New appendix criteria open for a broader concept of chronic migraine. Cephalalgia. 2006;26:742-746.
6. Dodick DW. Clinical practice. Chronic daily headache. N Engl J Med. 2006;354:158-165.
7. Maizels M. The patient with daily headaches. Am Fam Physician. 2004;70:2299-2306.
8. Scher AI, Lipton RB, Stewart WF. Risk factors for chronic daily headache. Curr Pain Headache Rep. 2002;6:486-491.
9. Lipton RB. Tracing transformation: chronic migraine classification progression, and epidemiology. Neurology. 2009;72 (5 suppl):S3-S7.
10. Tepper SJ. Medication-overuse headache. Continuum. 2012;18:807-822.
11. iHeadache. Available at: https://itunes.apple.com/us/app/iheadache-free-headache-migraine/id374213833?mt=8. Accessed February 10, 2013.
12. My Headache Log Pro. Available at: https://play.google.com/store/apps/details?id=com.dontek.myheadachelog&hl=en. Accessed February 10 2013.
13. Bigal ME, Serrano D, Buse D, et al. Acute migraine medications and evolution from episodic to chronic migraine: a longitudinal population-based study. Headache. 2008;48:1157-1168.
14. Colas R, Munoz P, Temprano R, et al. Chronic daily headache with analgesic overuse: epidemiology and impact on quality of life. Neurology. 2004;62:1338-1342.
15. Li D, Rozen TD. The clinical characteristics of new daily persistent headache. Cephalalgia. 2002;22:66-69.
16. Young WB, Swanson JW. New daily-persistent headache: the switched-on headache. Neurology. 2010;74:1338-1339.
17. Verri AP, Proietti Cecchini A, Galli C, et al. Psychiatric comorbidity in chronic daily headache. Cephalalgia. 1998;18 (suppl 21):S45-S49.
18. Tietjen GE, Brandes JL, Digre KB, et al. High prevalence of somatic symptoms and depression in women with disabling chronic headache. Neurology. 2007;68:134.-
19. Kelman L, Rains JC. Headache and sleep: examination of sleep patterns and complaints in a large clinical sample of migraineurs. Headache. 2005;45:904-910.
20. Bigal ME, Lipton RB. Obesity is a risk factor for transformed migraine but not chronic tension-type headache. Neurology. 2006;67:252-257.
21. Peres MF, Young WB, Kaup AO, et al. Fibromyalgia is common in patients with transformed migraine. Neurology. 2001;57:1326-1328.
22. Ciancaglini R, Radaelli G. The relationship between headache and symptoms of temporomandibular disorders in the general population. J Dent. 2001;29:93-98.
23. Silberstein SD, Holland S, Freitag F, et al. Evidence-based guideline update: pharmacologic treatment for episodic migraine prevention in adults: report of the Quality Standards Subcommittee of the American Academy of Neurology and the American Headache Society. Neurology. 2012;78:1337-1345.
24. Holland S, Silberstein SD, Freitag F, et al. Evidence-based guideline update: NSAIDs and other complementary treatments for episodic migraine prevention in adults: report of the Quality Standards Subcommittee of the American Academy of Neurology and the American Headache Society. Neurology. 2012;78:1346-1353.
25. Dodick DW, Freitag F, Banks J, et al. Topiramate versus amitriptyline in migraine prevention: a 26-week, multicenter, randomized, double-blind, double-dummy, parallel-group noninferiority trial in adult migrainers. Clin Ther. 2009;31:542-559.
26. Linde K, Rossnagel K. Propranolol for migraine prophylaxis. Cochrane Database Syst Rev. 2004;(2):CD003225.-
27. Saper JR, Lake AE, Cantrell DT, et al. Chronic daily headache prophylaxis with tizanidine: a double-blind, placebo-controlled, multicenter outcome study. Headache. 2002;42:470-482.
28. Dodick DW, Turkel CC, DeGryse RE, et al. Onabotulinumtoxin A for treatment of chronic migraine: pooled results from the double-blind, randomized, placebo-controlled phases of the PREEMPT clinical program. Headache. 2010;50:921-936.
29. Bendsten L, Evers S, Linde M, et al. EFNS guideline on the treatment of tension-type headache – report of an EFNS task force. Eur J Neurol. 2010;17:1318-1325.
30. Silberstein SD, Gobel H, Jensen R, et al. Botulinum toxin type A in the prophylactic treatment of chronic tension-type headache: a multicenter, double-blind, randomized, placebo-controlled, parallel-group study. Cephalalgia. 2006;26:790-800.
31. Katsavara Z, Jensen R. Medication-overuse headache: where are we now? Curr Opin Neurol. 2007;20:326-330.
32. Zeeberg P, Olesen J, Jensen R. Discontinuation of medication overuse in headache patients: recovery of therapeutic responsiveness. Cephalalgia. 2006;26:1192-1198.
33. Garza I, Schwedt TJ. Diagnosis and management of chronic daily headache. Semin Neurol. 2010;30:154-166.
34. Li Y, Zheng H, Witt CM, et al. Acupuncture for migraine prophylaxis: a randomized controlled trial. CMAJ. 2012;184:401-410.
1. Wiendels NJ, Neven AK, Rosendaal FR, et al. Chronic frequent headache in the general population: prevalence and associated factors. Cephalalgia. 2006;26:1434-1442.
2. Scher AI, Stewart WF, Liberman J, et al. Prevalence of frequent headache in a population sample. Headache. 1998;38:497-506.
3. Castillo J, Munoz P, Guitera V, et al. Kaplan Award 1998. Epidemiology of chronic daily headache in the general population. Headache. 1999;39:190-196.
4. Headache Classification Subcommittee of the International Headache Society. The International Classification of Headache Disorders: 2nd ed. Cephalalgia. 2004;24(suppl):S1-S9.
5. Headache Classification Committee, Olesen J, Bousser MG, Diener HC, et al. New appendix criteria open for a broader concept of chronic migraine. Cephalalgia. 2006;26:742-746.
6. Dodick DW. Clinical practice. Chronic daily headache. N Engl J Med. 2006;354:158-165.
7. Maizels M. The patient with daily headaches. Am Fam Physician. 2004;70:2299-2306.
8. Scher AI, Lipton RB, Stewart WF. Risk factors for chronic daily headache. Curr Pain Headache Rep. 2002;6:486-491.
9. Lipton RB. Tracing transformation: chronic migraine classification progression, and epidemiology. Neurology. 2009;72 (5 suppl):S3-S7.
10. Tepper SJ. Medication-overuse headache. Continuum. 2012;18:807-822.
11. iHeadache. Available at: https://itunes.apple.com/us/app/iheadache-free-headache-migraine/id374213833?mt=8. Accessed February 10, 2013.
12. My Headache Log Pro. Available at: https://play.google.com/store/apps/details?id=com.dontek.myheadachelog&hl=en. Accessed February 10 2013.
13. Bigal ME, Serrano D, Buse D, et al. Acute migraine medications and evolution from episodic to chronic migraine: a longitudinal population-based study. Headache. 2008;48:1157-1168.
14. Colas R, Munoz P, Temprano R, et al. Chronic daily headache with analgesic overuse: epidemiology and impact on quality of life. Neurology. 2004;62:1338-1342.
15. Li D, Rozen TD. The clinical characteristics of new daily persistent headache. Cephalalgia. 2002;22:66-69.
16. Young WB, Swanson JW. New daily-persistent headache: the switched-on headache. Neurology. 2010;74:1338-1339.
17. Verri AP, Proietti Cecchini A, Galli C, et al. Psychiatric comorbidity in chronic daily headache. Cephalalgia. 1998;18 (suppl 21):S45-S49.
18. Tietjen GE, Brandes JL, Digre KB, et al. High prevalence of somatic symptoms and depression in women with disabling chronic headache. Neurology. 2007;68:134.-
19. Kelman L, Rains JC. Headache and sleep: examination of sleep patterns and complaints in a large clinical sample of migraineurs. Headache. 2005;45:904-910.
20. Bigal ME, Lipton RB. Obesity is a risk factor for transformed migraine but not chronic tension-type headache. Neurology. 2006;67:252-257.
21. Peres MF, Young WB, Kaup AO, et al. Fibromyalgia is common in patients with transformed migraine. Neurology. 2001;57:1326-1328.
22. Ciancaglini R, Radaelli G. The relationship between headache and symptoms of temporomandibular disorders in the general population. J Dent. 2001;29:93-98.
23. Silberstein SD, Holland S, Freitag F, et al. Evidence-based guideline update: pharmacologic treatment for episodic migraine prevention in adults: report of the Quality Standards Subcommittee of the American Academy of Neurology and the American Headache Society. Neurology. 2012;78:1337-1345.
24. Holland S, Silberstein SD, Freitag F, et al. Evidence-based guideline update: NSAIDs and other complementary treatments for episodic migraine prevention in adults: report of the Quality Standards Subcommittee of the American Academy of Neurology and the American Headache Society. Neurology. 2012;78:1346-1353.
25. Dodick DW, Freitag F, Banks J, et al. Topiramate versus amitriptyline in migraine prevention: a 26-week, multicenter, randomized, double-blind, double-dummy, parallel-group noninferiority trial in adult migrainers. Clin Ther. 2009;31:542-559.
26. Linde K, Rossnagel K. Propranolol for migraine prophylaxis. Cochrane Database Syst Rev. 2004;(2):CD003225.-
27. Saper JR, Lake AE, Cantrell DT, et al. Chronic daily headache prophylaxis with tizanidine: a double-blind, placebo-controlled, multicenter outcome study. Headache. 2002;42:470-482.
28. Dodick DW, Turkel CC, DeGryse RE, et al. Onabotulinumtoxin A for treatment of chronic migraine: pooled results from the double-blind, randomized, placebo-controlled phases of the PREEMPT clinical program. Headache. 2010;50:921-936.
29. Bendsten L, Evers S, Linde M, et al. EFNS guideline on the treatment of tension-type headache – report of an EFNS task force. Eur J Neurol. 2010;17:1318-1325.
30. Silberstein SD, Gobel H, Jensen R, et al. Botulinum toxin type A in the prophylactic treatment of chronic tension-type headache: a multicenter, double-blind, randomized, placebo-controlled, parallel-group study. Cephalalgia. 2006;26:790-800.
31. Katsavara Z, Jensen R. Medication-overuse headache: where are we now? Curr Opin Neurol. 2007;20:326-330.
32. Zeeberg P, Olesen J, Jensen R. Discontinuation of medication overuse in headache patients: recovery of therapeutic responsiveness. Cephalalgia. 2006;26:1192-1198.
33. Garza I, Schwedt TJ. Diagnosis and management of chronic daily headache. Semin Neurol. 2010;30:154-166.
34. Li Y, Zheng H, Witt CM, et al. Acupuncture for migraine prophylaxis: a randomized controlled trial. CMAJ. 2012;184:401-410.
Victims of military sexual trauma—you see them, too
• Routinely question veterans about physical and sexual assault. C
• Suspect a history of military sexual trauma (MST) in veterans who present with multiple physical symptoms. B
• Screen patients with a history of MST for posttraumatic stress disorder and other psychiatric comorbidities. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
CASE A 29-year-old veteran (whom we’ll call Jane Doe) served as a medical corpsman in Iraq and has been pursuing a nursing degree since her honorable discharge a year ago. She comes in for a visit and reports a 3-month history of depression without suicidal ideation. In addition, Ms. Doe says, she has had abdominal pain that waxes and wanes for the past month. The pain is diffuse and nonfocal and appears to be unaffected by eating or bowel movements. She is unable to identify a particular pattern.
The patient has no significant medical or psychiatric history, and a physical examination is unremarkable. You advise her to follow a simplified dietary regimen, avoiding spicy foods and limiting dairy intake, and schedule a follow-up visit in 2 weeks.
Since 2002, some 2.4 million US troops have served in Iraq and Afghanistan,1 creating a new generation of veterans who need broad-based support to recover from the physical and psychological wounds of war. All too often, those wounds include sexual assault or harassment, collectively known as military sexual trauma (MST).
MST is a growing concern for the Veterans Administration (VA) for a number of reasons—an increase in women on the front lines and greater media coverage of patterns of sexual assault in the military among them.2 The official lifting of the ban on women in combat announced by the Pentagon in January brought the issue to the forefront, as well.3
In fact, MST should be a concern not only for clinicians within the VA, but also for civilian physicians. There are nearly 22 million American veterans, and the vast majority (>95%) get at least some of their medical care outside of the VA system4—often in outpatient facilities like yours.5 Family physicians need to be aware of the problem and able to give veterans who have suffered from sexual trauma the sensitive care they require.
The scope of the problem? No one is sure
How widespread is MST? That question is not easily answered. The prevalence rate among female service members is 20% to 43%,6 according to internal reports, while studies outside the military have reported rates that range from 3% to as high as 71%.5 In a recent anonymous survey of women in combat zones, led by a VA researcher—widely reported but still undergoing final review—half of those surveyed reported sexual harassment and nearly one in 4 reported sexual assault.7
There are far less data on rates of MST among male service members. The documented prevalence rate for men is 1.1%, with a range of 0.03% to 12.4%, but these figures are based on internal reports of sexual harassment and assault.8
Military culture and personal history are key factors
While the rate at which MST is reported has increased over the past 30 years,8 many reasons for not reporting it—stigma, fear of blame, accusations of homosexuality or promiscuity, and the threat of charges of fraternization among them—still remain.8,9 Military culture is still male-dominated, with an emphasis on self-sufficiency that often leaves victims of MST feeling as though they have nowhere to turn.
There are also circumstances military members face that can aggravate the effects of sexual trauma. Soldiers on deployment are typically isolated from their normal support systems, under significant pressure, and unable to leave their post, which often means they have ongoing exposure to the abuser.
A history of childhood sexual abuse (CSA). As many as 50% of female service members (and about 17% of military men) have reported CSA,10 compared with 25% to 27% of women and 16% of men outside of the military.5,11 That finding may be partially explained by data showing that nearly half of women in the military cited escaping from their home environment as a primary reason for enlisting.12
Women in the military who have a history of CSA, however, face a significantly higher risk for MST than servicewomen who were not sexually assaulted as children.8 Among female Navy recruits, for example, those who reported CSA were 4.8 times more likely to be raped than those who had no history of CSA.13
Combat-related trauma further complicates the picture. Evidence suggests that exposure to childhood physical and sexual abuse was associated with increased risk for combat-related posttraumatic stress disorder (PTSD) among men who served in Vietnam14 and women who served in Operation Desert Storm.15
Broaching the subject should be routine
Primary care physicians can play an important role in helping veterans transition back to their civilian lives and local communities, starting with a holistic medical assessment. When you see a patient whose return is relatively recent, inquire about his or her experiences during deployment. It is important to ask specifically about traumatic experiences, and to routinely screen for MST.
CASE When Ms. Doe returns. you begin by asking about her mood, using open-ended, nondirective questions. She responds by admitting that she had left important information off of the intake form she filled out on her last visit—most notably, a history of CSA. You gently ask about her experiences in the military, particularly during the year she spent in Iraq—and whether anything happened there that you should know.
Haltingly and with much emotion, the patient tells of her experience with another soldier. She worked with him every day, she says, and had grown close to him. One evening things went further than she expected. At first, it was only kissing, but then he forced himself on her sexually. She has not told anyone else about this event, Ms. Doe confides, because she wasn’t sure whether she precipitated it and felt embarrassed and humiliated by her choice to trust this man.
She did not feel that her supervising officers would listen or understand, as romantic attachments are best avoided in a combat zone and daily injuries are the norm. She says that her role as a medic kept her focused on the pain of others and enabled her to avoid looking at her own situation.
Evidence has shown that, like Ms. Doe, most survivors of trauma do not volunteer such information, but will often respond to direct and empathic questions from their physician.16 Routine screening of all veterans for MST, which the VA recommends, has been shown to increase their use of mental health resources.17,18 This can be easily incorporated into a medical history or an intake questionnaire, using this simple 2-question tool:17,18
While you were in the military:
- Did you receive uninvited and unwanted sexual attention, such as touching, cornering, pressure for sexual favors, or verbal remarks?
- Did anyone ever use force or the threat of force to have sexual contact with you against your will?
Screen for PTSD, and consider other psychiatric disorders
MST has been found to confer a 9-fold risk for PTSD. Indeed, more than 4 in 10 (42%) women with a history of MST have a PTSD diagnosis.19 Thus, if the screen for MST is positive—as indicated by a Yes answer to either question—follow up with the 4-question Primary Care PTSD screen (TABLE 1) is recommended.20
Veterans with a history of MST are twice as likely as other veterans to receive a mental health diagnosis;17 they’re also more likely to have 3 or more comorbid psychiatric conditions.21 Women appear to be more likely than men to suffer from depression, eating disorders, substance abuse,22 anxiety disorders,21 dissociative disorders, and personality disorders.17
Research on the mental health consequences of sexual assault in men (in any setting) is limited, however, and data on male survivors of MST are particularly sparse. What is known is that men who have experienced sexual trauma have higher rates of alcohol abuse23 and self-harm24 than women with a history of sexual trauma, and that MST has a greater association with bipolar disorder, schizophrenia, and psychosis in men.17
TABLE 1
Primary care PTSD screen (PC-PTSD)
In your life, have you ever had any experience that was so frightening, horrible, or upsetting that, in the past month, you:
| |||||||||
| A Yes response to any 3 questions is a positive screen, indicating a need for further investigation and possible referral to a mental health professional. PTSD, posttraumatic stress disorder. Source: National Center for PTSD. http://www.ptsd.va.gov/professional/pages/assessments/pc-ptsd.asp. | |||||||||
Multiple physical symptoms are often trauma-related
Veterans with a history of MST are also more likely to report physical symptoms25 and to have a lower health-related quality of life,26 poorer health status, and more outpatient visits12 than vets who were not exposed to MST. And, while pelvic pain is widely believed to be associated with female sexual abuse, survivors often present with a wide range of physical problems. The most common symptoms, similar to those affecting civilian rape survivors, include headache, gastrointestinal (GI) problems, chronic fatigue, severe menopause symptoms, and urological problems, as well as pelvic pain and sexual problems.27 Cardiac and respiratory disorders are also common (TABLE 2).17,25
Compared with their unaffected counterparts, women with a history of MST are more likely to be obese and sedentary, to smoke and drink, and to have had a hysterectomy before the age of 40 years.28 They are also more than twice as likely as other female veterans to say that they were treated for a heart attack within the past year.25 Data on the physical symptoms of male survivors of MST are extremely limited, but one study found an association with pulmonary and liver disease and human immunodeficiency virus and acquired immune deficiency syndrome.17
TABLE 2
Common physical symptoms reported by female MST survivors*17,25
Reproductive/gynecological
| Pulmonary
|
GI
| Neurologic/rheumatologic
|
Other
| CVD/CVD risk factors
|
| *This is a selection of the symptoms and risk factors MST survivors present with; it is not an exhaustive list. CVD, cardiovascular disease; GI, gastrointestinal; HTN, hypertension; MST, military sexual trauma. | |
A cluster of nonspecific findings?
Patients with a history of MST often present with complex and nonspecific signs and symptoms, making it difficult for a primary care physician to arrive at a diagnosis. MST and combat-related trauma should be considered in such cases, as well as in veterans who present with complaints involving multiple organ systems.21,25
Refer, treat—or do both
Once you have evidence that a patient is a survivor of MST, you need to consider a mental health referral or consultation and address physical symptoms. All honorably discharged veterans are eligible to receive VA treatment for MST, regardless of their disability rating or eligibility for other services. If a veteran indicates that he or she would like to seek psychotherapy or see a specialist outside of the VA system, it will fall to you to help the patient find the most appropriate treatment. (You’ll find links to VA and nonmilitary resources in the box.) Either way, patient acuity is a guide to the optimal approach.
Department of Veterans Affairs
Military sexual trauma
www.mentalhealth.va.gov/msthome.asp
National Center for PTSD
www.ptsd.va.gov
Vet center
www.vetcenter.va.gov
Women Veterans Health Care
www.womenshealth.va.gov/womenshealth/trauma.asp
Other resources:
American Psychiatric Association
www.psych.org
American Psychological Association
www.apa.org
Give an Hour
www.giveanhour.org
National Alliance on Mental Illness Veterans Resource Center
www.nami.org/veterans
Inpatient treatment will likely be needed for a patient who reveals thoughts of self-harm or harming others. If the patient is safe and stable enough for outpatient treatment, a therapist or psychiatrist with experience in treating sexual trauma is a good first step. Cognitive behavioral therapy and trauma-focused therapy have both been shown to have good outcomes in patients with sexual trauma and PTSD.29 Depending on the individual’s key presenting issues, a consultation with a substance abuse specialist, gynecologist, or other specialist may be helpful, as well.
As a family physician, you are in a position to build a long-term, trusting relationship with such a patient, which may be therapeutic in itself.9 In building such a relationship, keep in mind that the experience of serving in the military could make a patient particularly sensitive, or resistant, to your advice; you’ll need to strive for a collaborative approach.
CASE You tell Ms. Doe that the incident she described was indeed sexual violence—and specifically known as military sexual trauma. Her feelings about it are likely surfacing now due to the time away from the military—and by the fact that she’s beginning to date. In addition to spending some time listening to her story, you advise Ms. Doe to start seeing a therapist. You suggest she consider VA treatment services, and direct her to its MST web site (www.mentalhealth.va.gov/msthome.asp). Before she leaves, you make it clear that you will continue to see and support her through this difficult time, and you schedule a follow-up visit.
CORRESPONDENCE
Niranjan S. Karnik, MD, PhD, FAPA, University of Chicago, Pritzker School of Medicine, 5841 South Maryland, MC 3077, Chicago, IL 60637; nkarnik@bsd.uchicago.edu
1. US Department of Veterans Affairs. Analysis of VA health care utilization among Operation Enduring Freedom (OEF) Operation Iraqi Freedom (OIF), and Operation New Dawn (OND) Veterans. Cumulative from 1st Qtr FY 2002 through 1st Qtr FY 2012 (October 1, 2001 – December 31, 2011). Released March 2012. Available at: http://www.publichealth.va.gov/docs/epidemiology/healthcare-utilization-report-fy2012-qtr1.pdf. Accessed February 14, 2013.
2. Kaplan S. Military sexual trauma: a little-known veteran Issue. National Public Radio Web site. May 13 2010. Available at: http://www.npr.org/templates/story/story.php?storyId=126783956. Accessed February 14, 2013.
3. Pellerin C. Dempsey: Allowing women in combat strengthens joint force. US Department of Defense Web site. January 24 2013. Available at: http://www.defense.gov/news/newsarticle.aspx?id=119100. Accessed February 14, 2013.
4. National Center for Veterans Analysis and Statistics. Profile of veterans: 2009 data from the American Community Survey. January 2011. Available at: http://www.va.gov/vetdata/docs/SpecialReports/Profile_of_Veterans_2009_FINAL.pdf. Accessed February 14 2013.
5. Zinzow HM, Grubaugh AL, Monnier J, et al. Trauma among female veterans: a critical review. Trauma Violence Abuse. 2007;8:384-400.
6. Suris A, Lind L. Military sexual trauma: a review of prevalence and associated health consequences in veterans. Trauma Violence Abuse. 2008;9:250-269.
7. Zoroya G. Study: sex assault more common than DoD says. Army Times. December 27 2012. Available at: http://www.armytimes.com/news/2012/12/gannett-va-study-says-sex-assault-more-common-than-pentagon-reports-122712. Accessed February 12, 2013.
8. Hoyt T, Klosterman Rielage J, Williams LF. Military sexual trauma in men: a review of reported rates. J Trauma Dissociation. 2011;12:244-260.
9. Bell ME, Reardon A. Experiences of sexual harassment and sexual assault in the military among OEF/OIF veterans: implications for health care providers. Social Work Health Care. 2011;50:34-50.
10. Rosen LN, Martin L. The measurement of childhood trauma among male and female soldiers in the US Army. Mil Med. 1996;161:342-345.
11. Perez-Fuentes G, Olfson M, Villegas L, et al. Prevalence and correlates of child sex abuse: a national study. Comprehensive Psychiatry. 2013;54:16-27.
12. Sadler AG, Booth BM, Mengeling MA, et al. Life span and repeated violence against women during military service: effects on health status and outpatient utilization. J Womens Health (Larchmt). 2004;13:799-811.
13. Merrill LL, Newell CE, Thomsen CJ, et al. Childhood abuse and sexual revictimization in a female Navy recruit sample. J Trauma Stress. 1999;12:211-225.
14. Bremner JD, Southwick SM, Johnson DR, et al. Childhood physical abuse and combat-related posttraumatic stress disorder in Vietnam veterans. Am J Psychiatry. 1993;150:235-239.
15. Engel CC, Jr, Engel AL, Campbell SJ, et al. Posttraumatic stress disorder symptoms and precombat sexual and physical abuse in Desert Storm veterans. J Nerv Ment Dis. 1993;181:683-688.
16. Friedman LS, Samet JH, Roberts MS, et al. Inquiry about victimization experiences. A survey of patient p and physician practices. Arch Intern Med. 1992;152:1186-1190.
17. Kimerling R, Gima K, Smith MW, et al. The Veterans Health Administration and military sexual trauma. Am J Public Health. 2007;97:2160-2166.
18. Kimerling R, Street AE, Gima K, et al. Evaluation of universal screening for military-related sexual trauma. Psychiatr Serv. 2008;59:635-640.
19. Surís A, Lind L, Kashner TM, et al. Sexual assault in women veterans: an examination of PTSD risk, health care utilization, and cost of care. Psychosom Med. 2004;66:749-756.
20. Ouimette P, Wade M, Prins A, et al. Identifying PTSD in primary care: comparison of the Primary Care-PTSD screen (PC-PTSD) and the General Health Questionnaire-12 (GHQ). J Anxiety Disord. 2008;22:337-343.
21. Maguen S, Cohen B, Ren L, et al. Gender differences in military sexual trauma and mental health diagnoses among Iraq and Afghanistan veterans with posttraumatic stress disorder. Womens Health Issues. 2012;22:e61-e66.
22. Skinner KM, Kressin N, Frayne S, et al. The prevalence of military sexual assault among female Veterans’ Administration outpatients. J Interpers Violence. 2000;15:291-310.
23. Cucciare MA, Ghaus S, Weingardt KR, et al. Sexual assault and substance use in male veterans receiving a brief alcohol intervention. J Stud Alcohol Drugs. 2011;72:693-700.
24. Coxell A, King M, Mezey G, et al. Lifetime prevalence, characteristics, and associated problems of non-consensual sex in men: cross sectional survey. BMJ. 1999;318:846-850.
25. Frayne SM, Skinner KM, Sullivan LM, Tripp TJ, Hankin CS, Kressin NR, Miller DR. Medical profile of women Veterans Administration outpatients who report a history of sexual assault occurring while in the military. J Womens Health Gend Based Med. 1999;8:835-845.
26. Sadler AG, Booth BM, Nielson D, et al. Health-related consequences of physical and sexual violence: women in the military. Obstet Gynecol. 2000;96:473-480.
27. Petter LM, Whitehill DL. Management of female sexual assault. Am Fam Physician. 1998;58:920-926, 929–930.
28. Frayne SM, Skinner KM, Sullivan LM, et al. Sexual assault while in the military: violence as a predictor of cardiac risk? Violence Vict 2003;18:219-225.
29. Nemeroff C, Heim C, Thas ME, et al. Differential responses to psychotherapy versus pharmacotherapy in patients with chronic forms of major depression and childhood trauma. P Natl Acad Sci Usa. 2003;100:14293-14296.
• Routinely question veterans about physical and sexual assault. C
• Suspect a history of military sexual trauma (MST) in veterans who present with multiple physical symptoms. B
• Screen patients with a history of MST for posttraumatic stress disorder and other psychiatric comorbidities. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
CASE A 29-year-old veteran (whom we’ll call Jane Doe) served as a medical corpsman in Iraq and has been pursuing a nursing degree since her honorable discharge a year ago. She comes in for a visit and reports a 3-month history of depression without suicidal ideation. In addition, Ms. Doe says, she has had abdominal pain that waxes and wanes for the past month. The pain is diffuse and nonfocal and appears to be unaffected by eating or bowel movements. She is unable to identify a particular pattern.
The patient has no significant medical or psychiatric history, and a physical examination is unremarkable. You advise her to follow a simplified dietary regimen, avoiding spicy foods and limiting dairy intake, and schedule a follow-up visit in 2 weeks.
Since 2002, some 2.4 million US troops have served in Iraq and Afghanistan,1 creating a new generation of veterans who need broad-based support to recover from the physical and psychological wounds of war. All too often, those wounds include sexual assault or harassment, collectively known as military sexual trauma (MST).
MST is a growing concern for the Veterans Administration (VA) for a number of reasons—an increase in women on the front lines and greater media coverage of patterns of sexual assault in the military among them.2 The official lifting of the ban on women in combat announced by the Pentagon in January brought the issue to the forefront, as well.3
In fact, MST should be a concern not only for clinicians within the VA, but also for civilian physicians. There are nearly 22 million American veterans, and the vast majority (>95%) get at least some of their medical care outside of the VA system4—often in outpatient facilities like yours.5 Family physicians need to be aware of the problem and able to give veterans who have suffered from sexual trauma the sensitive care they require.
The scope of the problem? No one is sure
How widespread is MST? That question is not easily answered. The prevalence rate among female service members is 20% to 43%,6 according to internal reports, while studies outside the military have reported rates that range from 3% to as high as 71%.5 In a recent anonymous survey of women in combat zones, led by a VA researcher—widely reported but still undergoing final review—half of those surveyed reported sexual harassment and nearly one in 4 reported sexual assault.7
There are far less data on rates of MST among male service members. The documented prevalence rate for men is 1.1%, with a range of 0.03% to 12.4%, but these figures are based on internal reports of sexual harassment and assault.8
Military culture and personal history are key factors
While the rate at which MST is reported has increased over the past 30 years,8 many reasons for not reporting it—stigma, fear of blame, accusations of homosexuality or promiscuity, and the threat of charges of fraternization among them—still remain.8,9 Military culture is still male-dominated, with an emphasis on self-sufficiency that often leaves victims of MST feeling as though they have nowhere to turn.
There are also circumstances military members face that can aggravate the effects of sexual trauma. Soldiers on deployment are typically isolated from their normal support systems, under significant pressure, and unable to leave their post, which often means they have ongoing exposure to the abuser.
A history of childhood sexual abuse (CSA). As many as 50% of female service members (and about 17% of military men) have reported CSA,10 compared with 25% to 27% of women and 16% of men outside of the military.5,11 That finding may be partially explained by data showing that nearly half of women in the military cited escaping from their home environment as a primary reason for enlisting.12
Women in the military who have a history of CSA, however, face a significantly higher risk for MST than servicewomen who were not sexually assaulted as children.8 Among female Navy recruits, for example, those who reported CSA were 4.8 times more likely to be raped than those who had no history of CSA.13
Combat-related trauma further complicates the picture. Evidence suggests that exposure to childhood physical and sexual abuse was associated with increased risk for combat-related posttraumatic stress disorder (PTSD) among men who served in Vietnam14 and women who served in Operation Desert Storm.15
Broaching the subject should be routine
Primary care physicians can play an important role in helping veterans transition back to their civilian lives and local communities, starting with a holistic medical assessment. When you see a patient whose return is relatively recent, inquire about his or her experiences during deployment. It is important to ask specifically about traumatic experiences, and to routinely screen for MST.
CASE When Ms. Doe returns. you begin by asking about her mood, using open-ended, nondirective questions. She responds by admitting that she had left important information off of the intake form she filled out on her last visit—most notably, a history of CSA. You gently ask about her experiences in the military, particularly during the year she spent in Iraq—and whether anything happened there that you should know.
Haltingly and with much emotion, the patient tells of her experience with another soldier. She worked with him every day, she says, and had grown close to him. One evening things went further than she expected. At first, it was only kissing, but then he forced himself on her sexually. She has not told anyone else about this event, Ms. Doe confides, because she wasn’t sure whether she precipitated it and felt embarrassed and humiliated by her choice to trust this man.
She did not feel that her supervising officers would listen or understand, as romantic attachments are best avoided in a combat zone and daily injuries are the norm. She says that her role as a medic kept her focused on the pain of others and enabled her to avoid looking at her own situation.
Evidence has shown that, like Ms. Doe, most survivors of trauma do not volunteer such information, but will often respond to direct and empathic questions from their physician.16 Routine screening of all veterans for MST, which the VA recommends, has been shown to increase their use of mental health resources.17,18 This can be easily incorporated into a medical history or an intake questionnaire, using this simple 2-question tool:17,18
While you were in the military:
- Did you receive uninvited and unwanted sexual attention, such as touching, cornering, pressure for sexual favors, or verbal remarks?
- Did anyone ever use force or the threat of force to have sexual contact with you against your will?
Screen for PTSD, and consider other psychiatric disorders
MST has been found to confer a 9-fold risk for PTSD. Indeed, more than 4 in 10 (42%) women with a history of MST have a PTSD diagnosis.19 Thus, if the screen for MST is positive—as indicated by a Yes answer to either question—follow up with the 4-question Primary Care PTSD screen (TABLE 1) is recommended.20
Veterans with a history of MST are twice as likely as other veterans to receive a mental health diagnosis;17 they’re also more likely to have 3 or more comorbid psychiatric conditions.21 Women appear to be more likely than men to suffer from depression, eating disorders, substance abuse,22 anxiety disorders,21 dissociative disorders, and personality disorders.17
Research on the mental health consequences of sexual assault in men (in any setting) is limited, however, and data on male survivors of MST are particularly sparse. What is known is that men who have experienced sexual trauma have higher rates of alcohol abuse23 and self-harm24 than women with a history of sexual trauma, and that MST has a greater association with bipolar disorder, schizophrenia, and psychosis in men.17
TABLE 1
Primary care PTSD screen (PC-PTSD)
In your life, have you ever had any experience that was so frightening, horrible, or upsetting that, in the past month, you:
| |||||||||
| A Yes response to any 3 questions is a positive screen, indicating a need for further investigation and possible referral to a mental health professional. PTSD, posttraumatic stress disorder. Source: National Center for PTSD. http://www.ptsd.va.gov/professional/pages/assessments/pc-ptsd.asp. | |||||||||
Multiple physical symptoms are often trauma-related
Veterans with a history of MST are also more likely to report physical symptoms25 and to have a lower health-related quality of life,26 poorer health status, and more outpatient visits12 than vets who were not exposed to MST. And, while pelvic pain is widely believed to be associated with female sexual abuse, survivors often present with a wide range of physical problems. The most common symptoms, similar to those affecting civilian rape survivors, include headache, gastrointestinal (GI) problems, chronic fatigue, severe menopause symptoms, and urological problems, as well as pelvic pain and sexual problems.27 Cardiac and respiratory disorders are also common (TABLE 2).17,25
Compared with their unaffected counterparts, women with a history of MST are more likely to be obese and sedentary, to smoke and drink, and to have had a hysterectomy before the age of 40 years.28 They are also more than twice as likely as other female veterans to say that they were treated for a heart attack within the past year.25 Data on the physical symptoms of male survivors of MST are extremely limited, but one study found an association with pulmonary and liver disease and human immunodeficiency virus and acquired immune deficiency syndrome.17
TABLE 2
Common physical symptoms reported by female MST survivors*17,25
Reproductive/gynecological
| Pulmonary
|
GI
| Neurologic/rheumatologic
|
Other
| CVD/CVD risk factors
|
| *This is a selection of the symptoms and risk factors MST survivors present with; it is not an exhaustive list. CVD, cardiovascular disease; GI, gastrointestinal; HTN, hypertension; MST, military sexual trauma. | |
A cluster of nonspecific findings?
Patients with a history of MST often present with complex and nonspecific signs and symptoms, making it difficult for a primary care physician to arrive at a diagnosis. MST and combat-related trauma should be considered in such cases, as well as in veterans who present with complaints involving multiple organ systems.21,25
Refer, treat—or do both
Once you have evidence that a patient is a survivor of MST, you need to consider a mental health referral or consultation and address physical symptoms. All honorably discharged veterans are eligible to receive VA treatment for MST, regardless of their disability rating or eligibility for other services. If a veteran indicates that he or she would like to seek psychotherapy or see a specialist outside of the VA system, it will fall to you to help the patient find the most appropriate treatment. (You’ll find links to VA and nonmilitary resources in the box.) Either way, patient acuity is a guide to the optimal approach.
Department of Veterans Affairs
Military sexual trauma
www.mentalhealth.va.gov/msthome.asp
National Center for PTSD
www.ptsd.va.gov
Vet center
www.vetcenter.va.gov
Women Veterans Health Care
www.womenshealth.va.gov/womenshealth/trauma.asp
Other resources:
American Psychiatric Association
www.psych.org
American Psychological Association
www.apa.org
Give an Hour
www.giveanhour.org
National Alliance on Mental Illness Veterans Resource Center
www.nami.org/veterans
Inpatient treatment will likely be needed for a patient who reveals thoughts of self-harm or harming others. If the patient is safe and stable enough for outpatient treatment, a therapist or psychiatrist with experience in treating sexual trauma is a good first step. Cognitive behavioral therapy and trauma-focused therapy have both been shown to have good outcomes in patients with sexual trauma and PTSD.29 Depending on the individual’s key presenting issues, a consultation with a substance abuse specialist, gynecologist, or other specialist may be helpful, as well.
As a family physician, you are in a position to build a long-term, trusting relationship with such a patient, which may be therapeutic in itself.9 In building such a relationship, keep in mind that the experience of serving in the military could make a patient particularly sensitive, or resistant, to your advice; you’ll need to strive for a collaborative approach.
CASE You tell Ms. Doe that the incident she described was indeed sexual violence—and specifically known as military sexual trauma. Her feelings about it are likely surfacing now due to the time away from the military—and by the fact that she’s beginning to date. In addition to spending some time listening to her story, you advise Ms. Doe to start seeing a therapist. You suggest she consider VA treatment services, and direct her to its MST web site (www.mentalhealth.va.gov/msthome.asp). Before she leaves, you make it clear that you will continue to see and support her through this difficult time, and you schedule a follow-up visit.
CORRESPONDENCE
Niranjan S. Karnik, MD, PhD, FAPA, University of Chicago, Pritzker School of Medicine, 5841 South Maryland, MC 3077, Chicago, IL 60637; nkarnik@bsd.uchicago.edu
• Routinely question veterans about physical and sexual assault. C
• Suspect a history of military sexual trauma (MST) in veterans who present with multiple physical symptoms. B
• Screen patients with a history of MST for posttraumatic stress disorder and other psychiatric comorbidities. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
CASE A 29-year-old veteran (whom we’ll call Jane Doe) served as a medical corpsman in Iraq and has been pursuing a nursing degree since her honorable discharge a year ago. She comes in for a visit and reports a 3-month history of depression without suicidal ideation. In addition, Ms. Doe says, she has had abdominal pain that waxes and wanes for the past month. The pain is diffuse and nonfocal and appears to be unaffected by eating or bowel movements. She is unable to identify a particular pattern.
The patient has no significant medical or psychiatric history, and a physical examination is unremarkable. You advise her to follow a simplified dietary regimen, avoiding spicy foods and limiting dairy intake, and schedule a follow-up visit in 2 weeks.
Since 2002, some 2.4 million US troops have served in Iraq and Afghanistan,1 creating a new generation of veterans who need broad-based support to recover from the physical and psychological wounds of war. All too often, those wounds include sexual assault or harassment, collectively known as military sexual trauma (MST).
MST is a growing concern for the Veterans Administration (VA) for a number of reasons—an increase in women on the front lines and greater media coverage of patterns of sexual assault in the military among them.2 The official lifting of the ban on women in combat announced by the Pentagon in January brought the issue to the forefront, as well.3
In fact, MST should be a concern not only for clinicians within the VA, but also for civilian physicians. There are nearly 22 million American veterans, and the vast majority (>95%) get at least some of their medical care outside of the VA system4—often in outpatient facilities like yours.5 Family physicians need to be aware of the problem and able to give veterans who have suffered from sexual trauma the sensitive care they require.
The scope of the problem? No one is sure
How widespread is MST? That question is not easily answered. The prevalence rate among female service members is 20% to 43%,6 according to internal reports, while studies outside the military have reported rates that range from 3% to as high as 71%.5 In a recent anonymous survey of women in combat zones, led by a VA researcher—widely reported but still undergoing final review—half of those surveyed reported sexual harassment and nearly one in 4 reported sexual assault.7
There are far less data on rates of MST among male service members. The documented prevalence rate for men is 1.1%, with a range of 0.03% to 12.4%, but these figures are based on internal reports of sexual harassment and assault.8
Military culture and personal history are key factors
While the rate at which MST is reported has increased over the past 30 years,8 many reasons for not reporting it—stigma, fear of blame, accusations of homosexuality or promiscuity, and the threat of charges of fraternization among them—still remain.8,9 Military culture is still male-dominated, with an emphasis on self-sufficiency that often leaves victims of MST feeling as though they have nowhere to turn.
There are also circumstances military members face that can aggravate the effects of sexual trauma. Soldiers on deployment are typically isolated from their normal support systems, under significant pressure, and unable to leave their post, which often means they have ongoing exposure to the abuser.
A history of childhood sexual abuse (CSA). As many as 50% of female service members (and about 17% of military men) have reported CSA,10 compared with 25% to 27% of women and 16% of men outside of the military.5,11 That finding may be partially explained by data showing that nearly half of women in the military cited escaping from their home environment as a primary reason for enlisting.12
Women in the military who have a history of CSA, however, face a significantly higher risk for MST than servicewomen who were not sexually assaulted as children.8 Among female Navy recruits, for example, those who reported CSA were 4.8 times more likely to be raped than those who had no history of CSA.13
Combat-related trauma further complicates the picture. Evidence suggests that exposure to childhood physical and sexual abuse was associated with increased risk for combat-related posttraumatic stress disorder (PTSD) among men who served in Vietnam14 and women who served in Operation Desert Storm.15
Broaching the subject should be routine
Primary care physicians can play an important role in helping veterans transition back to their civilian lives and local communities, starting with a holistic medical assessment. When you see a patient whose return is relatively recent, inquire about his or her experiences during deployment. It is important to ask specifically about traumatic experiences, and to routinely screen for MST.
CASE When Ms. Doe returns. you begin by asking about her mood, using open-ended, nondirective questions. She responds by admitting that she had left important information off of the intake form she filled out on her last visit—most notably, a history of CSA. You gently ask about her experiences in the military, particularly during the year she spent in Iraq—and whether anything happened there that you should know.
Haltingly and with much emotion, the patient tells of her experience with another soldier. She worked with him every day, she says, and had grown close to him. One evening things went further than she expected. At first, it was only kissing, but then he forced himself on her sexually. She has not told anyone else about this event, Ms. Doe confides, because she wasn’t sure whether she precipitated it and felt embarrassed and humiliated by her choice to trust this man.
She did not feel that her supervising officers would listen or understand, as romantic attachments are best avoided in a combat zone and daily injuries are the norm. She says that her role as a medic kept her focused on the pain of others and enabled her to avoid looking at her own situation.
Evidence has shown that, like Ms. Doe, most survivors of trauma do not volunteer such information, but will often respond to direct and empathic questions from their physician.16 Routine screening of all veterans for MST, which the VA recommends, has been shown to increase their use of mental health resources.17,18 This can be easily incorporated into a medical history or an intake questionnaire, using this simple 2-question tool:17,18
While you were in the military:
- Did you receive uninvited and unwanted sexual attention, such as touching, cornering, pressure for sexual favors, or verbal remarks?
- Did anyone ever use force or the threat of force to have sexual contact with you against your will?
Screen for PTSD, and consider other psychiatric disorders
MST has been found to confer a 9-fold risk for PTSD. Indeed, more than 4 in 10 (42%) women with a history of MST have a PTSD diagnosis.19 Thus, if the screen for MST is positive—as indicated by a Yes answer to either question—follow up with the 4-question Primary Care PTSD screen (TABLE 1) is recommended.20
Veterans with a history of MST are twice as likely as other veterans to receive a mental health diagnosis;17 they’re also more likely to have 3 or more comorbid psychiatric conditions.21 Women appear to be more likely than men to suffer from depression, eating disorders, substance abuse,22 anxiety disorders,21 dissociative disorders, and personality disorders.17
Research on the mental health consequences of sexual assault in men (in any setting) is limited, however, and data on male survivors of MST are particularly sparse. What is known is that men who have experienced sexual trauma have higher rates of alcohol abuse23 and self-harm24 than women with a history of sexual trauma, and that MST has a greater association with bipolar disorder, schizophrenia, and psychosis in men.17
TABLE 1
Primary care PTSD screen (PC-PTSD)
In your life, have you ever had any experience that was so frightening, horrible, or upsetting that, in the past month, you:
| |||||||||
| A Yes response to any 3 questions is a positive screen, indicating a need for further investigation and possible referral to a mental health professional. PTSD, posttraumatic stress disorder. Source: National Center for PTSD. http://www.ptsd.va.gov/professional/pages/assessments/pc-ptsd.asp. | |||||||||
Multiple physical symptoms are often trauma-related
Veterans with a history of MST are also more likely to report physical symptoms25 and to have a lower health-related quality of life,26 poorer health status, and more outpatient visits12 than vets who were not exposed to MST. And, while pelvic pain is widely believed to be associated with female sexual abuse, survivors often present with a wide range of physical problems. The most common symptoms, similar to those affecting civilian rape survivors, include headache, gastrointestinal (GI) problems, chronic fatigue, severe menopause symptoms, and urological problems, as well as pelvic pain and sexual problems.27 Cardiac and respiratory disorders are also common (TABLE 2).17,25
Compared with their unaffected counterparts, women with a history of MST are more likely to be obese and sedentary, to smoke and drink, and to have had a hysterectomy before the age of 40 years.28 They are also more than twice as likely as other female veterans to say that they were treated for a heart attack within the past year.25 Data on the physical symptoms of male survivors of MST are extremely limited, but one study found an association with pulmonary and liver disease and human immunodeficiency virus and acquired immune deficiency syndrome.17
TABLE 2
Common physical symptoms reported by female MST survivors*17,25
Reproductive/gynecological
| Pulmonary
|
GI
| Neurologic/rheumatologic
|
Other
| CVD/CVD risk factors
|
| *This is a selection of the symptoms and risk factors MST survivors present with; it is not an exhaustive list. CVD, cardiovascular disease; GI, gastrointestinal; HTN, hypertension; MST, military sexual trauma. | |
A cluster of nonspecific findings?
Patients with a history of MST often present with complex and nonspecific signs and symptoms, making it difficult for a primary care physician to arrive at a diagnosis. MST and combat-related trauma should be considered in such cases, as well as in veterans who present with complaints involving multiple organ systems.21,25
Refer, treat—or do both
Once you have evidence that a patient is a survivor of MST, you need to consider a mental health referral or consultation and address physical symptoms. All honorably discharged veterans are eligible to receive VA treatment for MST, regardless of their disability rating or eligibility for other services. If a veteran indicates that he or she would like to seek psychotherapy or see a specialist outside of the VA system, it will fall to you to help the patient find the most appropriate treatment. (You’ll find links to VA and nonmilitary resources in the box.) Either way, patient acuity is a guide to the optimal approach.
Department of Veterans Affairs
Military sexual trauma
www.mentalhealth.va.gov/msthome.asp
National Center for PTSD
www.ptsd.va.gov
Vet center
www.vetcenter.va.gov
Women Veterans Health Care
www.womenshealth.va.gov/womenshealth/trauma.asp
Other resources:
American Psychiatric Association
www.psych.org
American Psychological Association
www.apa.org
Give an Hour
www.giveanhour.org
National Alliance on Mental Illness Veterans Resource Center
www.nami.org/veterans
Inpatient treatment will likely be needed for a patient who reveals thoughts of self-harm or harming others. If the patient is safe and stable enough for outpatient treatment, a therapist or psychiatrist with experience in treating sexual trauma is a good first step. Cognitive behavioral therapy and trauma-focused therapy have both been shown to have good outcomes in patients with sexual trauma and PTSD.29 Depending on the individual’s key presenting issues, a consultation with a substance abuse specialist, gynecologist, or other specialist may be helpful, as well.
As a family physician, you are in a position to build a long-term, trusting relationship with such a patient, which may be therapeutic in itself.9 In building such a relationship, keep in mind that the experience of serving in the military could make a patient particularly sensitive, or resistant, to your advice; you’ll need to strive for a collaborative approach.
CASE You tell Ms. Doe that the incident she described was indeed sexual violence—and specifically known as military sexual trauma. Her feelings about it are likely surfacing now due to the time away from the military—and by the fact that she’s beginning to date. In addition to spending some time listening to her story, you advise Ms. Doe to start seeing a therapist. You suggest she consider VA treatment services, and direct her to its MST web site (www.mentalhealth.va.gov/msthome.asp). Before she leaves, you make it clear that you will continue to see and support her through this difficult time, and you schedule a follow-up visit.
CORRESPONDENCE
Niranjan S. Karnik, MD, PhD, FAPA, University of Chicago, Pritzker School of Medicine, 5841 South Maryland, MC 3077, Chicago, IL 60637; nkarnik@bsd.uchicago.edu
1. US Department of Veterans Affairs. Analysis of VA health care utilization among Operation Enduring Freedom (OEF) Operation Iraqi Freedom (OIF), and Operation New Dawn (OND) Veterans. Cumulative from 1st Qtr FY 2002 through 1st Qtr FY 2012 (October 1, 2001 – December 31, 2011). Released March 2012. Available at: http://www.publichealth.va.gov/docs/epidemiology/healthcare-utilization-report-fy2012-qtr1.pdf. Accessed February 14, 2013.
2. Kaplan S. Military sexual trauma: a little-known veteran Issue. National Public Radio Web site. May 13 2010. Available at: http://www.npr.org/templates/story/story.php?storyId=126783956. Accessed February 14, 2013.
3. Pellerin C. Dempsey: Allowing women in combat strengthens joint force. US Department of Defense Web site. January 24 2013. Available at: http://www.defense.gov/news/newsarticle.aspx?id=119100. Accessed February 14, 2013.
4. National Center for Veterans Analysis and Statistics. Profile of veterans: 2009 data from the American Community Survey. January 2011. Available at: http://www.va.gov/vetdata/docs/SpecialReports/Profile_of_Veterans_2009_FINAL.pdf. Accessed February 14 2013.
5. Zinzow HM, Grubaugh AL, Monnier J, et al. Trauma among female veterans: a critical review. Trauma Violence Abuse. 2007;8:384-400.
6. Suris A, Lind L. Military sexual trauma: a review of prevalence and associated health consequences in veterans. Trauma Violence Abuse. 2008;9:250-269.
7. Zoroya G. Study: sex assault more common than DoD says. Army Times. December 27 2012. Available at: http://www.armytimes.com/news/2012/12/gannett-va-study-says-sex-assault-more-common-than-pentagon-reports-122712. Accessed February 12, 2013.
8. Hoyt T, Klosterman Rielage J, Williams LF. Military sexual trauma in men: a review of reported rates. J Trauma Dissociation. 2011;12:244-260.
9. Bell ME, Reardon A. Experiences of sexual harassment and sexual assault in the military among OEF/OIF veterans: implications for health care providers. Social Work Health Care. 2011;50:34-50.
10. Rosen LN, Martin L. The measurement of childhood trauma among male and female soldiers in the US Army. Mil Med. 1996;161:342-345.
11. Perez-Fuentes G, Olfson M, Villegas L, et al. Prevalence and correlates of child sex abuse: a national study. Comprehensive Psychiatry. 2013;54:16-27.
12. Sadler AG, Booth BM, Mengeling MA, et al. Life span and repeated violence against women during military service: effects on health status and outpatient utilization. J Womens Health (Larchmt). 2004;13:799-811.
13. Merrill LL, Newell CE, Thomsen CJ, et al. Childhood abuse and sexual revictimization in a female Navy recruit sample. J Trauma Stress. 1999;12:211-225.
14. Bremner JD, Southwick SM, Johnson DR, et al. Childhood physical abuse and combat-related posttraumatic stress disorder in Vietnam veterans. Am J Psychiatry. 1993;150:235-239.
15. Engel CC, Jr, Engel AL, Campbell SJ, et al. Posttraumatic stress disorder symptoms and precombat sexual and physical abuse in Desert Storm veterans. J Nerv Ment Dis. 1993;181:683-688.
16. Friedman LS, Samet JH, Roberts MS, et al. Inquiry about victimization experiences. A survey of patient p and physician practices. Arch Intern Med. 1992;152:1186-1190.
17. Kimerling R, Gima K, Smith MW, et al. The Veterans Health Administration and military sexual trauma. Am J Public Health. 2007;97:2160-2166.
18. Kimerling R, Street AE, Gima K, et al. Evaluation of universal screening for military-related sexual trauma. Psychiatr Serv. 2008;59:635-640.
19. Surís A, Lind L, Kashner TM, et al. Sexual assault in women veterans: an examination of PTSD risk, health care utilization, and cost of care. Psychosom Med. 2004;66:749-756.
20. Ouimette P, Wade M, Prins A, et al. Identifying PTSD in primary care: comparison of the Primary Care-PTSD screen (PC-PTSD) and the General Health Questionnaire-12 (GHQ). J Anxiety Disord. 2008;22:337-343.
21. Maguen S, Cohen B, Ren L, et al. Gender differences in military sexual trauma and mental health diagnoses among Iraq and Afghanistan veterans with posttraumatic stress disorder. Womens Health Issues. 2012;22:e61-e66.
22. Skinner KM, Kressin N, Frayne S, et al. The prevalence of military sexual assault among female Veterans’ Administration outpatients. J Interpers Violence. 2000;15:291-310.
23. Cucciare MA, Ghaus S, Weingardt KR, et al. Sexual assault and substance use in male veterans receiving a brief alcohol intervention. J Stud Alcohol Drugs. 2011;72:693-700.
24. Coxell A, King M, Mezey G, et al. Lifetime prevalence, characteristics, and associated problems of non-consensual sex in men: cross sectional survey. BMJ. 1999;318:846-850.
25. Frayne SM, Skinner KM, Sullivan LM, Tripp TJ, Hankin CS, Kressin NR, Miller DR. Medical profile of women Veterans Administration outpatients who report a history of sexual assault occurring while in the military. J Womens Health Gend Based Med. 1999;8:835-845.
26. Sadler AG, Booth BM, Nielson D, et al. Health-related consequences of physical and sexual violence: women in the military. Obstet Gynecol. 2000;96:473-480.
27. Petter LM, Whitehill DL. Management of female sexual assault. Am Fam Physician. 1998;58:920-926, 929–930.
28. Frayne SM, Skinner KM, Sullivan LM, et al. Sexual assault while in the military: violence as a predictor of cardiac risk? Violence Vict 2003;18:219-225.
29. Nemeroff C, Heim C, Thas ME, et al. Differential responses to psychotherapy versus pharmacotherapy in patients with chronic forms of major depression and childhood trauma. P Natl Acad Sci Usa. 2003;100:14293-14296.
1. US Department of Veterans Affairs. Analysis of VA health care utilization among Operation Enduring Freedom (OEF) Operation Iraqi Freedom (OIF), and Operation New Dawn (OND) Veterans. Cumulative from 1st Qtr FY 2002 through 1st Qtr FY 2012 (October 1, 2001 – December 31, 2011). Released March 2012. Available at: http://www.publichealth.va.gov/docs/epidemiology/healthcare-utilization-report-fy2012-qtr1.pdf. Accessed February 14, 2013.
2. Kaplan S. Military sexual trauma: a little-known veteran Issue. National Public Radio Web site. May 13 2010. Available at: http://www.npr.org/templates/story/story.php?storyId=126783956. Accessed February 14, 2013.
3. Pellerin C. Dempsey: Allowing women in combat strengthens joint force. US Department of Defense Web site. January 24 2013. Available at: http://www.defense.gov/news/newsarticle.aspx?id=119100. Accessed February 14, 2013.
4. National Center for Veterans Analysis and Statistics. Profile of veterans: 2009 data from the American Community Survey. January 2011. Available at: http://www.va.gov/vetdata/docs/SpecialReports/Profile_of_Veterans_2009_FINAL.pdf. Accessed February 14 2013.
5. Zinzow HM, Grubaugh AL, Monnier J, et al. Trauma among female veterans: a critical review. Trauma Violence Abuse. 2007;8:384-400.
6. Suris A, Lind L. Military sexual trauma: a review of prevalence and associated health consequences in veterans. Trauma Violence Abuse. 2008;9:250-269.
7. Zoroya G. Study: sex assault more common than DoD says. Army Times. December 27 2012. Available at: http://www.armytimes.com/news/2012/12/gannett-va-study-says-sex-assault-more-common-than-pentagon-reports-122712. Accessed February 12, 2013.
8. Hoyt T, Klosterman Rielage J, Williams LF. Military sexual trauma in men: a review of reported rates. J Trauma Dissociation. 2011;12:244-260.
9. Bell ME, Reardon A. Experiences of sexual harassment and sexual assault in the military among OEF/OIF veterans: implications for health care providers. Social Work Health Care. 2011;50:34-50.
10. Rosen LN, Martin L. The measurement of childhood trauma among male and female soldiers in the US Army. Mil Med. 1996;161:342-345.
11. Perez-Fuentes G, Olfson M, Villegas L, et al. Prevalence and correlates of child sex abuse: a national study. Comprehensive Psychiatry. 2013;54:16-27.
12. Sadler AG, Booth BM, Mengeling MA, et al. Life span and repeated violence against women during military service: effects on health status and outpatient utilization. J Womens Health (Larchmt). 2004;13:799-811.
13. Merrill LL, Newell CE, Thomsen CJ, et al. Childhood abuse and sexual revictimization in a female Navy recruit sample. J Trauma Stress. 1999;12:211-225.
14. Bremner JD, Southwick SM, Johnson DR, et al. Childhood physical abuse and combat-related posttraumatic stress disorder in Vietnam veterans. Am J Psychiatry. 1993;150:235-239.
15. Engel CC, Jr, Engel AL, Campbell SJ, et al. Posttraumatic stress disorder symptoms and precombat sexual and physical abuse in Desert Storm veterans. J Nerv Ment Dis. 1993;181:683-688.
16. Friedman LS, Samet JH, Roberts MS, et al. Inquiry about victimization experiences. A survey of patient p and physician practices. Arch Intern Med. 1992;152:1186-1190.
17. Kimerling R, Gima K, Smith MW, et al. The Veterans Health Administration and military sexual trauma. Am J Public Health. 2007;97:2160-2166.
18. Kimerling R, Street AE, Gima K, et al. Evaluation of universal screening for military-related sexual trauma. Psychiatr Serv. 2008;59:635-640.
19. Surís A, Lind L, Kashner TM, et al. Sexual assault in women veterans: an examination of PTSD risk, health care utilization, and cost of care. Psychosom Med. 2004;66:749-756.
20. Ouimette P, Wade M, Prins A, et al. Identifying PTSD in primary care: comparison of the Primary Care-PTSD screen (PC-PTSD) and the General Health Questionnaire-12 (GHQ). J Anxiety Disord. 2008;22:337-343.
21. Maguen S, Cohen B, Ren L, et al. Gender differences in military sexual trauma and mental health diagnoses among Iraq and Afghanistan veterans with posttraumatic stress disorder. Womens Health Issues. 2012;22:e61-e66.
22. Skinner KM, Kressin N, Frayne S, et al. The prevalence of military sexual assault among female Veterans’ Administration outpatients. J Interpers Violence. 2000;15:291-310.
23. Cucciare MA, Ghaus S, Weingardt KR, et al. Sexual assault and substance use in male veterans receiving a brief alcohol intervention. J Stud Alcohol Drugs. 2011;72:693-700.
24. Coxell A, King M, Mezey G, et al. Lifetime prevalence, characteristics, and associated problems of non-consensual sex in men: cross sectional survey. BMJ. 1999;318:846-850.
25. Frayne SM, Skinner KM, Sullivan LM, Tripp TJ, Hankin CS, Kressin NR, Miller DR. Medical profile of women Veterans Administration outpatients who report a history of sexual assault occurring while in the military. J Womens Health Gend Based Med. 1999;8:835-845.
26. Sadler AG, Booth BM, Nielson D, et al. Health-related consequences of physical and sexual violence: women in the military. Obstet Gynecol. 2000;96:473-480.
27. Petter LM, Whitehill DL. Management of female sexual assault. Am Fam Physician. 1998;58:920-926, 929–930.
28. Frayne SM, Skinner KM, Sullivan LM, et al. Sexual assault while in the military: violence as a predictor of cardiac risk? Violence Vict 2003;18:219-225.
29. Nemeroff C, Heim C, Thas ME, et al. Differential responses to psychotherapy versus pharmacotherapy in patients with chronic forms of major depression and childhood trauma. P Natl Acad Sci Usa. 2003;100:14293-14296.
Acne treatment: Easy ways to improve your care
• Use a classification system, such as that of the American Academy of Dermatology, to assess the severity of acne vulgaris. A
• Treat inflammatory lesions aggressively to prevent scarring. A
• When isotretinoin is indicated, consider prescribing a lower dosage (but longer duration) than the traditional regimen. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
CASE Janis S, an otherwise healthy 19-year-old, is in your office, seeking treatment for acne. She reports she has tried various over-the-counter (OTC) creams in recent months, but has seen little improvement. The acne first appeared about 5 years ago, and her pediatrician prescribed topical adapalene and doxycycline. The treatment helped, but she says her face never fully cleared up; over the past year, the acne has gotten worse.
On examination, you find several nodules and comedones on the patient’s face, chest, and back. Ms. S confides in you that the acne—particularly on her face—kept her from going to the senior prom.
More than 80% of adolescents and adults develop acne vulgaris at some point in their lives, and in at least 15% to 20% of cases, the acne is moderate to severe.1 Although acne typically starts in early puberty, it can continue well into adulthood.2 Females typically develop acne at an earlier age than males. There are no other sex or racial differences.3
Regardless of the age at which acne develops, it has substantial psychological effects, including embarrassment, shame, depression, anxiety, social isolation—and in extreme cases, suicidal ideation.4 This evidence-based update will better prepare you to provide optimal medical therapy—and alleviate patients’ emotional distress—without delay.
The pathophysiology of acne vulgaris
The American Academy of Dermatology (AAD) defines acne as a “chronic inflammatory dermatosis which is notable for open and/or closed comedones (blackheads and whiteheads) and inflammatory lesions, including papules, pustules, and nodules….”5 The underlying etiology is best described as a cascade of events involving the pilosebaceous unit.
Normally, single keratinocytes are shed into the follicular lumen for excretion. In acne, this process is disrupted and the keratinocytes accumulate, becoming interwoven with monofilaments and lipid droplets. The lipids, cellular debris, and excessive sebum, as well as the overgrowth of Propionibacterium acnes, block the follicles;6 the bacterial overgrowth can generate inflammation, as well.7 Areas rich in sebaceous glands, such as the face, neck, chest, upper arms, and back, are the sites at which acne is most likely to develop.
Clockwise, from top: closed comedones; open comedones; pustules; and scarring.
Androgen receptors play a role
For many years, the underlying pathophysiology of acne vulgaris was thought to be lesion progression, with microcomedone formation leading to both closed and open comedones. Emerging evidence has led to a deeper understanding of acne development. Sebum is now known to have androgen receptors (nuclear transcription factor Fox O1), which are modulated by insulinlike-growth factor 1 (IGF-1) and insulin.8,9 Research to determine whether these receptors can be influenced by diet and melanocortins is ongoing.8,10
Evidence has also shown that inflammation around the follicles and follicular differentiation precede bacterial overgrowth,7 and that P acnes overgrowth exacerbates the blockage and inflammation by creating a biofilm that plugs the follicles. Inflammation is one of the main complications of acne, causing hyperpigmentation and scarring.
These factors increase the risk
There are numerous risk factors for acne, ranging from genetics to stress to certain medications (TABLE 1).11 Although the exact genetic penetrance is unknown, acne often affects multiple family members;1,12 genetics is also associated with an increase in androgens, such as that found in patients with Cushing syndrome, polycystic ovary syndrome (PCOS), and congenital adrenal hyperplasia.13
Emotional and physical stress can increase the risk for acne,14 with the latter often related to excessive friction on the skin caused by sweat bands or helmet strips. Cosmetics that plug the follicles are a risk factor for acne, as well.
TABLE 1
Drugs that are potential acne triggers
| Common drugs/drug classes |
| Anabolic steroids (eg, danazol and testosterone) Bromides and iodides Corticosteroids (eg, prednisone) Corticotropin Isoniazid and ethionamide Lithium and barbiturates Phenytoin and trimethadione |
| Less common drugs |
| Azathioprine Cyclosporine Disulfiram Phenobarbital Quinidine |
| Adapted from: Sterry W, et al. Dermatology. Thieme Clinical Companions. 2006.11 |
The patient has acne, but how severe?
Because acne is often diagnosed clinically, there is often no need for routine testing. Nor is a bacterial culture for P acnes necessary.
If the patient has signs and symptoms suggestive of an endocrine disorder, however—eg, infertility, PCOS, or hirsutism—consider checking free testosterone, dehydroepiandrosterone sulfate (DHEA), luteinizing/follicle-stimulating hormones (LH/FSH), 17-alpha-progesterone, adrenocorticotropic hormone (ACTH), and/or dexamethasone suppression. Other indicators of a need for endocrine testing include male or female pattern balding, an abnormal menstrual cycle, acanthosis nigricans, and truncal obesity.5,6
Numerous acne classification systems have been developed; some are based on the type of lesions (ie, comedonal, papulopustular, nodulocystic), while others also consider the number of each type of lesion and areas affected.15 In 2002, the US Food and Drug Administration (FDA) defined the components of a Global Acne Severity Scale as having 6 grades (0-5), with 0 for normal skin and 5 representing a predominance of highly inflammatory lesions with a variable number of papules/pustules and nodulocystic lesions.16
The AAD’S classification system has only 3 grades—mild, moderate, and severe—and is one of the easiest to use:
- Mild cases have few to several papules and pustules, but no nodules
- Moderate cases have more papules and pustules, with a few nodules
- Severe cases have numerous papules, pustules, and nodules.5
CASE Ms. S is in obvious emotional distress, and her acne needs to be treated aggressively. Because of the emotional impact and the fact that she has lesions on several body parts, her case is classified as severe (and would be even if her face had only a few lesions).
Treatment: Prevention of new lesions is paramount
Preventing new formations is a key focus of acne therapy, and patients should be advised that it may take weeks for results to be seen. Nonetheless, aggressive treatment of inflammatory lesions is necessary to prevent scarring. Because most patients have both inflammatory and bacterial lesions, it is important to use combined therapies, including topical or oral antibiotics, to treat P acnes and inflammation (TABLE 2).13,17-23
TABLE 2
Acne classification helps guide treatment decisions13,17-23
| Treatment | Severity of acne | ||
|---|---|---|---|
| Mild | Moderate* | Severe* | |
| Dietary/lifestyle modifications (eg, reduce dairy intake, minimize use of cosmetics, reduce stress) PLUS benzoyl peroxide (2%-10%) PLUS retinoid (tretinoin, adapalene, or tazarotene) OR azelaic or salicylic acid | √ | √ | √ |
| Combined OCPs PLUS oral antibiotics OR topical antibiotics (for males and females who are not candidates for OCPs) | √ | √ | |
| Isotretinoin† | √ | ||
| Other therapies, as needed (eg, intralesional injections, chemical peels, or laser therapy)‡ | √ | √ | √ |
| *Treatments for moderate or severe acne are also appropriate for acne that extends to other parts of the body and/or does not respond to topical therapy. †Monitoring and counseling on adverse effects and teratogenic potential are required. ‡Should not be used concurrently or within 6-12 months of isotretinoin due to increased risk of keloid formation. OCPs, oral contraceptive pills. | |||
Topicals are the cornerstone of treatment
Retinoids and benzoyl peroxide topicals are the foundation of therapy for both comedonal and inflammatory acne,17 regardless of severity. Both are recommended by the AAD. But evidence suggests that only 55% of dermatologists and 10% of primary care providers recommend them.19,20
Retinoids inhibit microcomedone formation and regulate follicular keratinocytes, which have anti-inflammatory properties and help to prevent the formation of new lesions. Patients should be warned that topical retinoids can cause irritation, erythema, desquamation, pruritus, and burning. To reduce the adverse effects, advise patients to start retinoid therapy slowly, at a reduced frequency (eg, every other day or every third day) and shorter contact (washing it off after one to 4 hours for a week, then increasing the contact time). When it is clear that the medication is well tolerated, the frequency and amount can be increased. Use of the topical, as tolerated, should continue as long as the potential acne problem remains.
There are 3 retinoid formulations on the market—adapalene, tretinoin, and tazarotene—all of which have been shown to be effective. Adapalene is the least irritating and the most stable, and can be safely combined with benzoyl peroxide and topical antibiotics. If tretinoin and benzoyl peroxide are used concurrently, tretinoin should be applied at night and benzoyl peroxide during the day. To reduce the risk of inactivating the topical agents, advise patients not to use other skin products in conjunction with topical therapy.
Benzoyl peroxide, which is available as a cleanser, gel, or wash, affects keratinocyte dysmaturation, P acnes, and inflammation.11 The antibacterial activity is due to its oxidation. Benzoyl peroxide is available both OTC and by prescription, with concentrations ranging from 2% to 10%. Salicylic acid (2%-3%), a well-tolerated keratolytic agent, is often used with benzoyl peroxide, as well. Azelaic acid, sodium sulfacetamide, and dapsone are other topicals that have been found to be effective in treating acne.
Topical antibiotics, most commonly clindamycin 1% or sodium sulfacetamide, also affect both P acnes and inflammation,24 although the exact mechanism is unknown. Available in solution or as a gel or lotion, topical antibiotics can be combined with benzoyl peroxide. Use of topical erythromycin has declined in recent years because it has a higher rate of bacterial resistance.9,21
When to add oral antibiotics
When topical treatment does not produce the desired result or cannot be tolerated, oral antibiotics may be introduced, either as an addition or replacement. Like topicals, oral antibiotics have both antimicrobial and anti-inflammatory properties.
Tetracycline antibiotics (ie, doxycycline and minocycline) are first-line oral therapy.21 Minocycline has been found to be the most potent agent in this drug class; tetracycline is the least.22 Tetracyclines can cause tooth discoloration and inhibit skeletal growth, and are contraindicated for children younger than 10 years and pregnant women.
Photosensitivity is an adverse effect of tetracycline antibiotics, so patients should be advised to cover up and avoid sun exposure. Other adverse effects, particularly of minocycline, include dizziness, lupus-like syndrome, pseudotumor cerebri, skin and mucosal pigmentation, serum sickness, and hepatitis. If the patient is taking an oral contraceptive pill (OCP) concurrently for family planning, she should be advised that oral antibiotics have the potential to reduce the efficacy of the OCP.
Other oral antibiotics sometimes used to treat acne include erythromycin, trimethoprim-sulfamethoxazole, amoxicillin, and azithromycin, but data on their efficacy are limited. Erythromycin has similar potency to tetracycline, but may need to be taken 2 to 4 times a day and may cause more gastrointestinal disturbances. Cephalosporins, fluoroquinolones, aminoglycosides, chloramphenicol, sulfonamides/sulfur, and gyrase inhibitors should not be used for acne because of a lack of efficacy.6
No, it isn’t. Pustules on the face, like those on the patient pictured here, are a common manifestation of acne. But facial lesions alone are not sufficient for a definitive diagnosis. In fact, the pustules that this 59-year-old woman sought treatment for were correctly diagnosed as perioral dermatitis. The tip-off? The lack of comedones and the distribution of the lesions, which were concentrated around the mouth.
Regardless of the type of oral antibiotic prescribed, it should be tried for about 3 months (8-16 weeks) and discontinued once improvement occurs. If no improvement is seen within 3 months, consider changing antibiotics due to resistance or adding antifungal therapy for Pityrosporum and Malassezia species.6
Initiating isotretinoin therapy: An evidence-based approach
Oral isotretinoin is the only potential cure for acne vulgaris. The cure rate is about 30% to 40% (with about 20% of patients developing a recurrence that requires retreatment within one to 3 years).25
Isotretinoin is FDA approved for severe nodulocystic acne, but several organizations, including the AAD and the Global Alliance to Improve Outcomes in Acne, recommend its use for milder cases.25,26 It is also an excellent treatment for other forms of severe acne, such as acne fulminans and acne conglobata. Accutane is no longer available, but 5 other formulations of isotretinoin are on the market.
Because isotretinoin is a category X teratogen, all providers and patients must register with iPLEDGE (www.ipledgeprogram.com), an FDA-approved mandatory risk management program. Before starting to take isotretinoin, females of childbearing age are required to undergo 2 pregnancy tests; they must also agree to use 2 forms of program-approved birth control and submit to monthly pregnancy tests.
Patients on isotretinoin also need to be monitored for depression.27 Other potential adverse effects include hepatitis, hypertriglyceridemia, arthralgia, myalgias, and inflammatory bowel disease.28,29 Dry skin and mucosa are the most common adverse effects, and patients should be advised to use moisturizers regularly.
A better dosing regimen?
The standard starting dose of isotretinoin is 0.25 to 1 mg/kg/d, divided and taken twice a day, then titrated upward monthly to a maximum daily dose of 2 mg/kg. The goal is for the total intake of isotretinoin to be 120 to 150 mg/kg. So, for example, the goal for a patient weighing 60 kg might be a cumulative intake of 7200 mg (120 mg/kg × 60 kg), taken in doses of 20 mg BID (40 mg/d) for 180 days.
The medication should be taken with food (especially with fatty food) for better absorption. Treatment duration has typically been 16 to 32 weeks, with an average of 20 weeks, with the daily dose lowered in patients requiring treatment for a longer period of time. Continuous use of isotretinoin is more effective than taking it intermittently.26
Lower dosages? While that standard regimen has been adequate in the management of acne vulgaris, emerging evidence suggests that dosages of isotretinoin as low as 5 mg/d are equally effective and have significantly fewer adverse effects.30 Relapse continues to be a problem. Risk factors for relapse include a macrocomedonal pattern of acne, smoking, and age, with patients <14 years and >25 years at higher risk.30 While lower dosing was previously thought to be associated with greater risk of relapse, this appears to be related less to the cumulative dose of 120 to 150 mg/kg and more to the duration of sebaceous gland suppression.30
Based on the latest evidence, important changes in isotretinoin administration are called for—specifically, using a much lower dose (0.25-0.5 mg/kg, divided into 2 daily doses) for a longer period of time.30 While the traditional dosing generally requires a 3- to 5-month course of treatment, the lower dosing can take 6 to 8 months.
Who's a candidate for hormonal therapy?
Any hormone that has antiandrogenic properties can have a beneficial effect on acne.
The most common hormonal therapy is an estrogen-progestin combination OCP.23,31 Progesterone-only OCPs should not be used as they can worsen acne.
In theory, any OCP that contains estrogen can work because of its androgenic properties. The estrogen appears to suppress sebaceous gland activity. OCPs with FDA approval for the treatment of acne include Estrostep Fe (norethindrone/ethinyl estradiol [EE]), Ortho Tricyclen (norgestimate/EE), and Yasmin and Beyaz (drospirenone/EE). With any OCP, the effect is gradual, and it can take 3 to 4 months for patients to see an improvement. OCPs are an excellent choice for women with moderate-to-severe acne or those suffering from hirsutism and seborrhea.
Other hormonal therapies—which are not FDA approved for acne treatment—include spironolactone, cyproterone, and flutamide.24 There is no evidence to support the use of finasteride or cyproterone.
Spironolactone is the most studied and has modest benefits at 100 to 150 mg/d.22 Caution is needed when using spironolactone, as gynecomastia, hyperkalemia, and agranulocytosis are potential adverse effects. It is important to closely monitor the blood pressure, chemistry, and cell count of patients taking spironolactone.
CASE Because Ms. S is sexually active and does not wish to become pregnant, she is a candidate for an OCP. You prescribe a pill containing norgestimate and EE, add a topical retinoid to her regimen, and schedule a return visit in 3 months to evaluate the effectiveness of therapy. If there is little improvement, you will recommend isotretinoin at that time.
Talk to patients about lifestyle modifications
Although the role of lifestyle changes in acne treatment is controversial, there is some evidence to suggest that these modifications are worth considering:
Glycemic load. In Western society, where the typical diet includes foods with a high glycemic index, there appears to be a higher prevalence of acne compared with regions where foods with a low glycemic index (≤55-60) are the mainstay. A low glycemic load appears to reduce both the occurrence and severity of acne.17 Thus, patients who are willing to make dietary changes should be advised to consume foods with a lower glycemic index, such as peanuts and green vegetables.
Dairy. Milk is believed to have an androgenic effect, and dairy products in general have a positive correlation with acne. Thus, a reduction in milk intake has been found to improve acne.18,32 Stress the importance of calcium supplementation for patients whose dairy consumption is reduced or eliminated.
Fish oil. Omega-6 fatty acid, found in fish oil, has anti-inflammatory properties, and an increase in foods rich in omega-3 fatty acid (eg, salmon, sardines, walnuts) has been associated with improvement of acne.17
Probiotics. There is limited evidence for probiotics as a therapy for acne. They do appear to regulate inflammatory cytokines within the skin and to upregulate the IGF-1, both of which influence the formation of acne.10,33
Other treatment options to consider
Injections, chemical peels, and/or laser treatments may be considered as adjunctive therapy or when standard therapies fail.
Steroid injections. This treatment regimen centers around a midpotency steroid that is diluted with normal saline and is introduced into each lesion until the lesion is distended and/or blanched. There are limited data on the use of corticosteroid injections for acne, however, and these injections are reserved for severe cases to reduce inflammation. Potential adverse effects include hyperglycemia, obesity, and Cushing traits.
Chemical peels are used to decrease both inflammatory and noninflammatory lesions, and are typically well tolerated. In one study, more than 95% of patients were satisfied with the results.11,34
Various chemicals have been used, including alpha-hydroxyl acid (glycolic acid), beta-hydroxyl acid (salicylic acid), and Jessner’s solution, with equal efficacy.35-38 Chemical peels can be used on patients with darker skin, but caution is required to avoid dyschromia.39 Other adverse effects include dry skin, crusting, and facial erythema. More adverse effects have been reported with glycolic acid vs salicylic acid.37
Laser therapies include photodynamic therapy—blue light with amino-luvanic acid—and phototherapy (blue light alone).40-42P acnes accumulate photosensitizing porphyrins in the comedones; when the laser therapy is applied, the porphyrins absorb the light source and destroy the bacteria.
Laser treatment can also be used for scarring. Ablative laser resurfacing significantly improves acne scars; nonablative and fractional CO2 laser modalities can also be used, with minimal downtime and no serious complications.43
Other complementary therapies, including aloe vera, pyridoxine, kampo, tea tree extract, and fruit-based acids, have little or no data regarding their efficacy.
The importance of maintenance therapy
With the exception of patients whose acne was cured or who achieved remission with isotretinoin, maintenance is required once the desired appearance is reached. Without it, recurrence is likely—possibly within as little as 4 weeks.
For most patients, a topical retinoid is the only medication that should be continued. Tell patients to apply it nightly and to call for an appointment if an acne flare-up occurs.
CASE When Ms. S comes in for a follow-up visit, her acne is cleared except for a couple of lesions on her back and she is happy with the results. You advise her to continue on the OCP to avoid a recurrence but caution her that in a small percentage of cases, the acne may worsen even in women who continue to take OCPs and topicals. You agree to initiate isotretinoin if this occurs.
CORRESPONDENCE
Tam T. Nguyen, MD, San Joaquin General Hospital, 500 West Hospital Road, Suite 1103, French Camp, CA 95231; ttnguyen@sjgh.org
1. Ghodsi SZ, Orawa H, Zouboulis CC. Prevalence severity, and severity risk factors of acne in high school pupils: a community-based study. J Invest Dermatol. 2009;129:2136-2141.
2. Collier CN, Harper JC, Cafardi JA, et al. The prevalence of acne in adults 20 years and older. J Am Acad Dermatol. 2008;58:56-59.
3. Lucky AW, Biro FM, Huster GA, et al. Acne vulgaris in premenarchal girls. An early sign of puberty associated with rising levels of dehydroepiandrosterone. Arch Dermatol. 1994;130:308-314.
4. Kubota Y, Shirahige Y, Nakai K, et al. Community-based epidemiological study of psychosocial effects of acne in Japanese adolescents. J Dermatol. 2010;37:617-622.
5. trauss JS, Krowchuk DP, Leyden JJ, et al. Guidelines of care for acne vulgaris management. J Am Acad Dermatol. 2007;56:651-663.
6. Thiboutot D, Gollnick H, Bettoli V, et al. New insights into the management of acne: an update from the Global Alliance to Improve Outcomes in Acne group. J Am Acad Dermatol. 2009;60(suppl):S1-S50.
7. Jeremy AH, Holland DB, Roberts SG, et al. Inflammatory events are involved in acne lesion initiation. J Invest Dermatol. 2003;121:20-27.
8. Kurokawa I, Danby FW, Ju Q, et al. New developments in our understanding of acne pathogenesis and treatment. Exp Dermatol. 2009;18:821-832.
9. Zouboulis CC, Baron JM, Bohm M, et al. Frontiers in sebaceous gland biology and pathology. Exp Dermatol. 2008;17:542-551.
10. Melnik BC, Schmitz G. Role of insulin, insulin-like growth factor-1, hyperglycaemic food and milk consumption in the pathogenesis of acne vulgaris. Exp Dermatol. 2009;18:833-841.
11. Sterry W, Paus R. Burgdorf WHC. Dermatology. Thieme Clinical Companions. Stuttgart Germany: Thieme; 2006;530-535.
12. Ballanger F, Baudry P, Nguyen JM, et al. Heredity: a prognostic factor for acne. Dermatology. 2006;212:145-149.
13. Chen MJ, Chen CD, Yang JH, et al. High serum dehydroepiandrosterone sulfate is associated with phenotypic acne and a reduced risk of abdominal obesity in women with polycystic ovary syndrome. Hum Reprod. 2011;26:227-234.
14. Yosipovitch G, Tang M, Dawn AG, et al. Study of psychological stress, sebum production and acne vulgaris in adolescents. Acta Derm Venereol. 2007;87:135-139.
15. Adityan B, Kumari R, Thappa DM. Scoring systems in acne vulgaris. Indian J Dermatol Venereol Leprol. 2009;75:323-326.
16. US Food and Drug Administration. Global acne severity scale. Available at: http://www.fda.gov/ohrms/dockets/ac/02/briefing/3904B1_03_%20Acne%20Global%20Severity%20Scale.pdf. Accessed January 16 2013.
17. Jung JY, Yoon MY, Min SU, et al. The influence of dietary patterns on acne vulgaris in Koreans. Eur J Dermatol. 2010;20:768-772.
18. Adebamowo CA, Spiegelman D, Berkey CS, et al. Milk consumption and acne in teenaged boys. J Am Acad Dermatol. 2008;58:787-793.
19. Hsu P, Litman GI, Brodell RT. Overview of the treatment of acne vulgaris with topical retinoids. Postgrad Med. 2011;123:153-161.
20. Kim RH, Armstrong AW. Current state of acne treatment: highlighting lasers photodynamic therapy, and chemical peels. Dermatol Online J. 2011;17:2.-
21. Simonart T, Dramaix M, De Maertelaer V. Efficacy of tetracyclines in the treatment of acne vulgaris: a review. Br J Dermatol. 2008;158:208-216.
22. Ingram JR, Grindlay DJ, Williams HC. Management of acne vulgaris: an evidence-based update. Clin Exp Dermatol. 2010;35:351-354.
23. Rosen MP, Breitkopf DM, Nagamani M. A randomized controlled trial of second- versus third-generation oral contraceptives in the treatment of acne vulgaris. Am J Obstet Gynecol. 2003;188:1158-1160.
24. Simpson RC, Grindlay DJ, Williams HC. What’s new in acne? An analysis of systematic reviews and clinically significant trials published in 2010-11. Clin Exp Dermatol. 2011;36:840-844.
25. Borghi A, Mantovani L, Minghetti S, et al. Low-cumulative dose isotretinoin treatment in mild-to-moderate acne: efficacy in achieving stable remission. J Eur Acad Dermatol Venereol. 2011;25:1094-1098.
26. Agarwal US, Besarwal RK, Bhola K. Oral isotretinoin in different dose regimens for acne vulgaris: a randomized comparative trial. Indian J Dermatol Venereol Leprol. 2011;77:688-694.
27. Kaymak Y, Taner E, Taner Y. Comparison of depression anxiety and life quality in acne vulgaris patients who were treated with either isotretinoin or topical agents. Int J Dermatol. 2009;48:41-46.
28. Crockett SD, Porter CQ, Martin CF, et al. Isotretinoin use and the risk of inflammatory bowel disease: a case-control study. Am J Gastroenterol. 2010;105:1986-1993.
29. Crockett SD, Gulati A, Sandler RS, et al. Causal association between isotretinoin and inflammatory bowel disease has yet to be established. Am J Gastroenterol. 2009;104:2387-2393.
30. Rademaker M. Isotretinoin: dose duration and relapse. What does 30 years of usage tell us? Australas J Dermatol. 2012 September 26 [Epub ahead of print].
31. Worret I, Arp W, Zahradnik HP, et al. Acne resolution rates: results of a single-blind, randomized, controlled, parallel phase III trial with EE/CMA (Belara) and EE/LNG (Microgynon). Dermatology. 2001;203:38-44.
32. Adebamowo CA, Spiegelman D, Danby FW, et al. High school dietary dairy intake and teenage acne. J Am Acad Dermatol. 2005;52:207-214.
33. Bowe WP, Logan AC. Acne vulgaris, probiotics and the gut-brain-skin axis - back to the future? Gut Pathog. 2011;3:1.-
34. Atzori L, Brundu MA, Orru A, et al. Glycolic acid peeling in the treatment of acne. J Eur Acad Dermatol Venereol. 1999;12:119-122.
35. Levesque A, Hamzavi I, Seite S, et al. Randomized trial comparing a chemical peel containing a lipophilic hydroxy acid derivative of salicylic acid with a salicylic acid peel in subjects with comedonal acne. J Cosmet Dermatol. 2011;10:174-178.
36. Garg VK, Sinha S, Sarkar R. Glycolic acid peels versus salicylic-mandelic acid peels in active acne vulgaris and post-acne scarring and hyperpigmentation: a comparative study. Dermatol Surg. 2009;35:59-65.
37. Kessler E, Flanagan K, Chia C, et al. Comparison of alpha- and beta-hydroxy acid chemical peels in the treatment of mild to moderately severe facial acne vulgaris. Dermatol Surg. 2008;34:45-51.
38. Lee SH, Huh CH, Park KC, et al. Effects of repetitive superficial chemical peels on facial sebum secretion in acne patients. J Eur Acad Dermatol Venereol. 2006;20:964-968.
39. Grimes PE. The safety and efficacy of salicylic acid chemical peels in darker racial-ethnic groups. Dermatol Surg. 1999;25:18-22.
40. Gold MH. Acne and PDT: new techniques with lasers and light sources. Lasers Med Sci. 2007;22:67-72.
41. Gold MH. Acne vulgaris: lasers light sources and photodynamic therapy—an update 2007. Expert Rev Anti Infect Ther. 2007;5:1059-1069.
42. Orringer JS, Sachs DL, Bailey E, et al. Photodynamic therapy for acne vulgaris: a randomized, controlled, split-face clinical trial of topical aminolevulinic acid and pulsed dye laser therapy. J Cosmet Dermatol. 2010;9:28-34.
43. Chapas AM, Brightman L, Sukal S, et al. Successful treatment of acneiform scarring with CO2 ablative fractional resurfacing. Lasers Surg Med. 2008;40:381-386.
• Use a classification system, such as that of the American Academy of Dermatology, to assess the severity of acne vulgaris. A
• Treat inflammatory lesions aggressively to prevent scarring. A
• When isotretinoin is indicated, consider prescribing a lower dosage (but longer duration) than the traditional regimen. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
CASE Janis S, an otherwise healthy 19-year-old, is in your office, seeking treatment for acne. She reports she has tried various over-the-counter (OTC) creams in recent months, but has seen little improvement. The acne first appeared about 5 years ago, and her pediatrician prescribed topical adapalene and doxycycline. The treatment helped, but she says her face never fully cleared up; over the past year, the acne has gotten worse.
On examination, you find several nodules and comedones on the patient’s face, chest, and back. Ms. S confides in you that the acne—particularly on her face—kept her from going to the senior prom.
More than 80% of adolescents and adults develop acne vulgaris at some point in their lives, and in at least 15% to 20% of cases, the acne is moderate to severe.1 Although acne typically starts in early puberty, it can continue well into adulthood.2 Females typically develop acne at an earlier age than males. There are no other sex or racial differences.3
Regardless of the age at which acne develops, it has substantial psychological effects, including embarrassment, shame, depression, anxiety, social isolation—and in extreme cases, suicidal ideation.4 This evidence-based update will better prepare you to provide optimal medical therapy—and alleviate patients’ emotional distress—without delay.
The pathophysiology of acne vulgaris
The American Academy of Dermatology (AAD) defines acne as a “chronic inflammatory dermatosis which is notable for open and/or closed comedones (blackheads and whiteheads) and inflammatory lesions, including papules, pustules, and nodules….”5 The underlying etiology is best described as a cascade of events involving the pilosebaceous unit.
Normally, single keratinocytes are shed into the follicular lumen for excretion. In acne, this process is disrupted and the keratinocytes accumulate, becoming interwoven with monofilaments and lipid droplets. The lipids, cellular debris, and excessive sebum, as well as the overgrowth of Propionibacterium acnes, block the follicles;6 the bacterial overgrowth can generate inflammation, as well.7 Areas rich in sebaceous glands, such as the face, neck, chest, upper arms, and back, are the sites at which acne is most likely to develop.

Clockwise, from top: closed comedones; open comedones; pustules; and scarring.
Androgen receptors play a role
For many years, the underlying pathophysiology of acne vulgaris was thought to be lesion progression, with microcomedone formation leading to both closed and open comedones. Emerging evidence has led to a deeper understanding of acne development. Sebum is now known to have androgen receptors (nuclear transcription factor Fox O1), which are modulated by insulinlike-growth factor 1 (IGF-1) and insulin.8,9 Research to determine whether these receptors can be influenced by diet and melanocortins is ongoing.8,10
Evidence has also shown that inflammation around the follicles and follicular differentiation precede bacterial overgrowth,7 and that P acnes overgrowth exacerbates the blockage and inflammation by creating a biofilm that plugs the follicles. Inflammation is one of the main complications of acne, causing hyperpigmentation and scarring.
These factors increase the risk
There are numerous risk factors for acne, ranging from genetics to stress to certain medications (TABLE 1).11 Although the exact genetic penetrance is unknown, acne often affects multiple family members;1,12 genetics is also associated with an increase in androgens, such as that found in patients with Cushing syndrome, polycystic ovary syndrome (PCOS), and congenital adrenal hyperplasia.13
Emotional and physical stress can increase the risk for acne,14 with the latter often related to excessive friction on the skin caused by sweat bands or helmet strips. Cosmetics that plug the follicles are a risk factor for acne, as well.
TABLE 1
Drugs that are potential acne triggers
| Common drugs/drug classes |
| Anabolic steroids (eg, danazol and testosterone) Bromides and iodides Corticosteroids (eg, prednisone) Corticotropin Isoniazid and ethionamide Lithium and barbiturates Phenytoin and trimethadione |
| Less common drugs |
| Azathioprine Cyclosporine Disulfiram Phenobarbital Quinidine |
| Adapted from: Sterry W, et al. Dermatology. Thieme Clinical Companions. 2006.11 |
The patient has acne, but how severe?
Because acne is often diagnosed clinically, there is often no need for routine testing. Nor is a bacterial culture for P acnes necessary.
If the patient has signs and symptoms suggestive of an endocrine disorder, however—eg, infertility, PCOS, or hirsutism—consider checking free testosterone, dehydroepiandrosterone sulfate (DHEA), luteinizing/follicle-stimulating hormones (LH/FSH), 17-alpha-progesterone, adrenocorticotropic hormone (ACTH), and/or dexamethasone suppression. Other indicators of a need for endocrine testing include male or female pattern balding, an abnormal menstrual cycle, acanthosis nigricans, and truncal obesity.5,6
Numerous acne classification systems have been developed; some are based on the type of lesions (ie, comedonal, papulopustular, nodulocystic), while others also consider the number of each type of lesion and areas affected.15 In 2002, the US Food and Drug Administration (FDA) defined the components of a Global Acne Severity Scale as having 6 grades (0-5), with 0 for normal skin and 5 representing a predominance of highly inflammatory lesions with a variable number of papules/pustules and nodulocystic lesions.16
The AAD’S classification system has only 3 grades—mild, moderate, and severe—and is one of the easiest to use:
- Mild cases have few to several papules and pustules, but no nodules
- Moderate cases have more papules and pustules, with a few nodules
- Severe cases have numerous papules, pustules, and nodules.5
CASE Ms. S is in obvious emotional distress, and her acne needs to be treated aggressively. Because of the emotional impact and the fact that she has lesions on several body parts, her case is classified as severe (and would be even if her face had only a few lesions).
Treatment: Prevention of new lesions is paramount
Preventing new formations is a key focus of acne therapy, and patients should be advised that it may take weeks for results to be seen. Nonetheless, aggressive treatment of inflammatory lesions is necessary to prevent scarring. Because most patients have both inflammatory and bacterial lesions, it is important to use combined therapies, including topical or oral antibiotics, to treat P acnes and inflammation (TABLE 2).13,17-23
TABLE 2
Acne classification helps guide treatment decisions13,17-23
| Treatment | Severity of acne | ||
|---|---|---|---|
| Mild | Moderate* | Severe* | |
| Dietary/lifestyle modifications (eg, reduce dairy intake, minimize use of cosmetics, reduce stress) PLUS benzoyl peroxide (2%-10%) PLUS retinoid (tretinoin, adapalene, or tazarotene) OR azelaic or salicylic acid | √ | √ | √ |
| Combined OCPs PLUS oral antibiotics OR topical antibiotics (for males and females who are not candidates for OCPs) | √ | √ | |
| Isotretinoin† | √ | ||
| Other therapies, as needed (eg, intralesional injections, chemical peels, or laser therapy)‡ | √ | √ | √ |
| *Treatments for moderate or severe acne are also appropriate for acne that extends to other parts of the body and/or does not respond to topical therapy. †Monitoring and counseling on adverse effects and teratogenic potential are required. ‡Should not be used concurrently or within 6-12 months of isotretinoin due to increased risk of keloid formation. OCPs, oral contraceptive pills. | |||
Topicals are the cornerstone of treatment
Retinoids and benzoyl peroxide topicals are the foundation of therapy for both comedonal and inflammatory acne,17 regardless of severity. Both are recommended by the AAD. But evidence suggests that only 55% of dermatologists and 10% of primary care providers recommend them.19,20
Retinoids inhibit microcomedone formation and regulate follicular keratinocytes, which have anti-inflammatory properties and help to prevent the formation of new lesions. Patients should be warned that topical retinoids can cause irritation, erythema, desquamation, pruritus, and burning. To reduce the adverse effects, advise patients to start retinoid therapy slowly, at a reduced frequency (eg, every other day or every third day) and shorter contact (washing it off after one to 4 hours for a week, then increasing the contact time). When it is clear that the medication is well tolerated, the frequency and amount can be increased. Use of the topical, as tolerated, should continue as long as the potential acne problem remains.
There are 3 retinoid formulations on the market—adapalene, tretinoin, and tazarotene—all of which have been shown to be effective. Adapalene is the least irritating and the most stable, and can be safely combined with benzoyl peroxide and topical antibiotics. If tretinoin and benzoyl peroxide are used concurrently, tretinoin should be applied at night and benzoyl peroxide during the day. To reduce the risk of inactivating the topical agents, advise patients not to use other skin products in conjunction with topical therapy.
Benzoyl peroxide, which is available as a cleanser, gel, or wash, affects keratinocyte dysmaturation, P acnes, and inflammation.11 The antibacterial activity is due to its oxidation. Benzoyl peroxide is available both OTC and by prescription, with concentrations ranging from 2% to 10%. Salicylic acid (2%-3%), a well-tolerated keratolytic agent, is often used with benzoyl peroxide, as well. Azelaic acid, sodium sulfacetamide, and dapsone are other topicals that have been found to be effective in treating acne.
Topical antibiotics, most commonly clindamycin 1% or sodium sulfacetamide, also affect both P acnes and inflammation,24 although the exact mechanism is unknown. Available in solution or as a gel or lotion, topical antibiotics can be combined with benzoyl peroxide. Use of topical erythromycin has declined in recent years because it has a higher rate of bacterial resistance.9,21
When to add oral antibiotics
When topical treatment does not produce the desired result or cannot be tolerated, oral antibiotics may be introduced, either as an addition or replacement. Like topicals, oral antibiotics have both antimicrobial and anti-inflammatory properties.
Tetracycline antibiotics (ie, doxycycline and minocycline) are first-line oral therapy.21 Minocycline has been found to be the most potent agent in this drug class; tetracycline is the least.22 Tetracyclines can cause tooth discoloration and inhibit skeletal growth, and are contraindicated for children younger than 10 years and pregnant women.
Photosensitivity is an adverse effect of tetracycline antibiotics, so patients should be advised to cover up and avoid sun exposure. Other adverse effects, particularly of minocycline, include dizziness, lupus-like syndrome, pseudotumor cerebri, skin and mucosal pigmentation, serum sickness, and hepatitis. If the patient is taking an oral contraceptive pill (OCP) concurrently for family planning, she should be advised that oral antibiotics have the potential to reduce the efficacy of the OCP.
Other oral antibiotics sometimes used to treat acne include erythromycin, trimethoprim-sulfamethoxazole, amoxicillin, and azithromycin, but data on their efficacy are limited. Erythromycin has similar potency to tetracycline, but may need to be taken 2 to 4 times a day and may cause more gastrointestinal disturbances. Cephalosporins, fluoroquinolones, aminoglycosides, chloramphenicol, sulfonamides/sulfur, and gyrase inhibitors should not be used for acne because of a lack of efficacy.6

No, it isn’t. Pustules on the face, like those on the patient pictured here, are a common manifestation of acne. But facial lesions alone are not sufficient for a definitive diagnosis. In fact, the pustules that this 59-year-old woman sought treatment for were correctly diagnosed as perioral dermatitis. The tip-off? The lack of comedones and the distribution of the lesions, which were concentrated around the mouth.
Regardless of the type of oral antibiotic prescribed, it should be tried for about 3 months (8-16 weeks) and discontinued once improvement occurs. If no improvement is seen within 3 months, consider changing antibiotics due to resistance or adding antifungal therapy for Pityrosporum and Malassezia species.6
Initiating isotretinoin therapy: An evidence-based approach
Oral isotretinoin is the only potential cure for acne vulgaris. The cure rate is about 30% to 40% (with about 20% of patients developing a recurrence that requires retreatment within one to 3 years).25
Isotretinoin is FDA approved for severe nodulocystic acne, but several organizations, including the AAD and the Global Alliance to Improve Outcomes in Acne, recommend its use for milder cases.25,26 It is also an excellent treatment for other forms of severe acne, such as acne fulminans and acne conglobata. Accutane is no longer available, but 5 other formulations of isotretinoin are on the market.
Because isotretinoin is a category X teratogen, all providers and patients must register with iPLEDGE (www.ipledgeprogram.com), an FDA-approved mandatory risk management program. Before starting to take isotretinoin, females of childbearing age are required to undergo 2 pregnancy tests; they must also agree to use 2 forms of program-approved birth control and submit to monthly pregnancy tests.
Patients on isotretinoin also need to be monitored for depression.27 Other potential adverse effects include hepatitis, hypertriglyceridemia, arthralgia, myalgias, and inflammatory bowel disease.28,29 Dry skin and mucosa are the most common adverse effects, and patients should be advised to use moisturizers regularly.
A better dosing regimen?
The standard starting dose of isotretinoin is 0.25 to 1 mg/kg/d, divided and taken twice a day, then titrated upward monthly to a maximum daily dose of 2 mg/kg. The goal is for the total intake of isotretinoin to be 120 to 150 mg/kg. So, for example, the goal for a patient weighing 60 kg might be a cumulative intake of 7200 mg (120 mg/kg × 60 kg), taken in doses of 20 mg BID (40 mg/d) for 180 days.
The medication should be taken with food (especially with fatty food) for better absorption. Treatment duration has typically been 16 to 32 weeks, with an average of 20 weeks, with the daily dose lowered in patients requiring treatment for a longer period of time. Continuous use of isotretinoin is more effective than taking it intermittently.26
Lower dosages? While that standard regimen has been adequate in the management of acne vulgaris, emerging evidence suggests that dosages of isotretinoin as low as 5 mg/d are equally effective and have significantly fewer adverse effects.30 Relapse continues to be a problem. Risk factors for relapse include a macrocomedonal pattern of acne, smoking, and age, with patients <14 years and >25 years at higher risk.30 While lower dosing was previously thought to be associated with greater risk of relapse, this appears to be related less to the cumulative dose of 120 to 150 mg/kg and more to the duration of sebaceous gland suppression.30
Based on the latest evidence, important changes in isotretinoin administration are called for—specifically, using a much lower dose (0.25-0.5 mg/kg, divided into 2 daily doses) for a longer period of time.30 While the traditional dosing generally requires a 3- to 5-month course of treatment, the lower dosing can take 6 to 8 months.
Who's a candidate for hormonal therapy?
Any hormone that has antiandrogenic properties can have a beneficial effect on acne.
The most common hormonal therapy is an estrogen-progestin combination OCP.23,31 Progesterone-only OCPs should not be used as they can worsen acne.
In theory, any OCP that contains estrogen can work because of its androgenic properties. The estrogen appears to suppress sebaceous gland activity. OCPs with FDA approval for the treatment of acne include Estrostep Fe (norethindrone/ethinyl estradiol [EE]), Ortho Tricyclen (norgestimate/EE), and Yasmin and Beyaz (drospirenone/EE). With any OCP, the effect is gradual, and it can take 3 to 4 months for patients to see an improvement. OCPs are an excellent choice for women with moderate-to-severe acne or those suffering from hirsutism and seborrhea.
Other hormonal therapies—which are not FDA approved for acne treatment—include spironolactone, cyproterone, and flutamide.24 There is no evidence to support the use of finasteride or cyproterone.
Spironolactone is the most studied and has modest benefits at 100 to 150 mg/d.22 Caution is needed when using spironolactone, as gynecomastia, hyperkalemia, and agranulocytosis are potential adverse effects. It is important to closely monitor the blood pressure, chemistry, and cell count of patients taking spironolactone.
CASE Because Ms. S is sexually active and does not wish to become pregnant, she is a candidate for an OCP. You prescribe a pill containing norgestimate and EE, add a topical retinoid to her regimen, and schedule a return visit in 3 months to evaluate the effectiveness of therapy. If there is little improvement, you will recommend isotretinoin at that time.
Talk to patients about lifestyle modifications
Although the role of lifestyle changes in acne treatment is controversial, there is some evidence to suggest that these modifications are worth considering:
Glycemic load. In Western society, where the typical diet includes foods with a high glycemic index, there appears to be a higher prevalence of acne compared with regions where foods with a low glycemic index (≤55-60) are the mainstay. A low glycemic load appears to reduce both the occurrence and severity of acne.17 Thus, patients who are willing to make dietary changes should be advised to consume foods with a lower glycemic index, such as peanuts and green vegetables.
Dairy. Milk is believed to have an androgenic effect, and dairy products in general have a positive correlation with acne. Thus, a reduction in milk intake has been found to improve acne.18,32 Stress the importance of calcium supplementation for patients whose dairy consumption is reduced or eliminated.
Fish oil. Omega-6 fatty acid, found in fish oil, has anti-inflammatory properties, and an increase in foods rich in omega-3 fatty acid (eg, salmon, sardines, walnuts) has been associated with improvement of acne.17
Probiotics. There is limited evidence for probiotics as a therapy for acne. They do appear to regulate inflammatory cytokines within the skin and to upregulate the IGF-1, both of which influence the formation of acne.10,33
Other treatment options to consider
Injections, chemical peels, and/or laser treatments may be considered as adjunctive therapy or when standard therapies fail.
Steroid injections. This treatment regimen centers around a midpotency steroid that is diluted with normal saline and is introduced into each lesion until the lesion is distended and/or blanched. There are limited data on the use of corticosteroid injections for acne, however, and these injections are reserved for severe cases to reduce inflammation. Potential adverse effects include hyperglycemia, obesity, and Cushing traits.
Chemical peels are used to decrease both inflammatory and noninflammatory lesions, and are typically well tolerated. In one study, more than 95% of patients were satisfied with the results.11,34
Various chemicals have been used, including alpha-hydroxyl acid (glycolic acid), beta-hydroxyl acid (salicylic acid), and Jessner’s solution, with equal efficacy.35-38 Chemical peels can be used on patients with darker skin, but caution is required to avoid dyschromia.39 Other adverse effects include dry skin, crusting, and facial erythema. More adverse effects have been reported with glycolic acid vs salicylic acid.37
Laser therapies include photodynamic therapy—blue light with amino-luvanic acid—and phototherapy (blue light alone).40-42P acnes accumulate photosensitizing porphyrins in the comedones; when the laser therapy is applied, the porphyrins absorb the light source and destroy the bacteria.
Laser treatment can also be used for scarring. Ablative laser resurfacing significantly improves acne scars; nonablative and fractional CO2 laser modalities can also be used, with minimal downtime and no serious complications.43
Other complementary therapies, including aloe vera, pyridoxine, kampo, tea tree extract, and fruit-based acids, have little or no data regarding their efficacy.
The importance of maintenance therapy
With the exception of patients whose acne was cured or who achieved remission with isotretinoin, maintenance is required once the desired appearance is reached. Without it, recurrence is likely—possibly within as little as 4 weeks.
For most patients, a topical retinoid is the only medication that should be continued. Tell patients to apply it nightly and to call for an appointment if an acne flare-up occurs.
CASE When Ms. S comes in for a follow-up visit, her acne is cleared except for a couple of lesions on her back and she is happy with the results. You advise her to continue on the OCP to avoid a recurrence but caution her that in a small percentage of cases, the acne may worsen even in women who continue to take OCPs and topicals. You agree to initiate isotretinoin if this occurs.
CORRESPONDENCE
Tam T. Nguyen, MD, San Joaquin General Hospital, 500 West Hospital Road, Suite 1103, French Camp, CA 95231; ttnguyen@sjgh.org
• Use a classification system, such as that of the American Academy of Dermatology, to assess the severity of acne vulgaris. A
• Treat inflammatory lesions aggressively to prevent scarring. A
• When isotretinoin is indicated, consider prescribing a lower dosage (but longer duration) than the traditional regimen. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
CASE Janis S, an otherwise healthy 19-year-old, is in your office, seeking treatment for acne. She reports she has tried various over-the-counter (OTC) creams in recent months, but has seen little improvement. The acne first appeared about 5 years ago, and her pediatrician prescribed topical adapalene and doxycycline. The treatment helped, but she says her face never fully cleared up; over the past year, the acne has gotten worse.
On examination, you find several nodules and comedones on the patient’s face, chest, and back. Ms. S confides in you that the acne—particularly on her face—kept her from going to the senior prom.
More than 80% of adolescents and adults develop acne vulgaris at some point in their lives, and in at least 15% to 20% of cases, the acne is moderate to severe.1 Although acne typically starts in early puberty, it can continue well into adulthood.2 Females typically develop acne at an earlier age than males. There are no other sex or racial differences.3
Regardless of the age at which acne develops, it has substantial psychological effects, including embarrassment, shame, depression, anxiety, social isolation—and in extreme cases, suicidal ideation.4 This evidence-based update will better prepare you to provide optimal medical therapy—and alleviate patients’ emotional distress—without delay.
The pathophysiology of acne vulgaris
The American Academy of Dermatology (AAD) defines acne as a “chronic inflammatory dermatosis which is notable for open and/or closed comedones (blackheads and whiteheads) and inflammatory lesions, including papules, pustules, and nodules….”5 The underlying etiology is best described as a cascade of events involving the pilosebaceous unit.
Normally, single keratinocytes are shed into the follicular lumen for excretion. In acne, this process is disrupted and the keratinocytes accumulate, becoming interwoven with monofilaments and lipid droplets. The lipids, cellular debris, and excessive sebum, as well as the overgrowth of Propionibacterium acnes, block the follicles;6 the bacterial overgrowth can generate inflammation, as well.7 Areas rich in sebaceous glands, such as the face, neck, chest, upper arms, and back, are the sites at which acne is most likely to develop.

Clockwise, from top: closed comedones; open comedones; pustules; and scarring.
Androgen receptors play a role
For many years, the underlying pathophysiology of acne vulgaris was thought to be lesion progression, with microcomedone formation leading to both closed and open comedones. Emerging evidence has led to a deeper understanding of acne development. Sebum is now known to have androgen receptors (nuclear transcription factor Fox O1), which are modulated by insulinlike-growth factor 1 (IGF-1) and insulin.8,9 Research to determine whether these receptors can be influenced by diet and melanocortins is ongoing.8,10
Evidence has also shown that inflammation around the follicles and follicular differentiation precede bacterial overgrowth,7 and that P acnes overgrowth exacerbates the blockage and inflammation by creating a biofilm that plugs the follicles. Inflammation is one of the main complications of acne, causing hyperpigmentation and scarring.
These factors increase the risk
There are numerous risk factors for acne, ranging from genetics to stress to certain medications (TABLE 1).11 Although the exact genetic penetrance is unknown, acne often affects multiple family members;1,12 genetics is also associated with an increase in androgens, such as that found in patients with Cushing syndrome, polycystic ovary syndrome (PCOS), and congenital adrenal hyperplasia.13
Emotional and physical stress can increase the risk for acne,14 with the latter often related to excessive friction on the skin caused by sweat bands or helmet strips. Cosmetics that plug the follicles are a risk factor for acne, as well.
TABLE 1
Drugs that are potential acne triggers
| Common drugs/drug classes |
| Anabolic steroids (eg, danazol and testosterone) Bromides and iodides Corticosteroids (eg, prednisone) Corticotropin Isoniazid and ethionamide Lithium and barbiturates Phenytoin and trimethadione |
| Less common drugs |
| Azathioprine Cyclosporine Disulfiram Phenobarbital Quinidine |
| Adapted from: Sterry W, et al. Dermatology. Thieme Clinical Companions. 2006.11 |
The patient has acne, but how severe?
Because acne is often diagnosed clinically, there is often no need for routine testing. Nor is a bacterial culture for P acnes necessary.
If the patient has signs and symptoms suggestive of an endocrine disorder, however—eg, infertility, PCOS, or hirsutism—consider checking free testosterone, dehydroepiandrosterone sulfate (DHEA), luteinizing/follicle-stimulating hormones (LH/FSH), 17-alpha-progesterone, adrenocorticotropic hormone (ACTH), and/or dexamethasone suppression. Other indicators of a need for endocrine testing include male or female pattern balding, an abnormal menstrual cycle, acanthosis nigricans, and truncal obesity.5,6
Numerous acne classification systems have been developed; some are based on the type of lesions (ie, comedonal, papulopustular, nodulocystic), while others also consider the number of each type of lesion and areas affected.15 In 2002, the US Food and Drug Administration (FDA) defined the components of a Global Acne Severity Scale as having 6 grades (0-5), with 0 for normal skin and 5 representing a predominance of highly inflammatory lesions with a variable number of papules/pustules and nodulocystic lesions.16
The AAD’S classification system has only 3 grades—mild, moderate, and severe—and is one of the easiest to use:
- Mild cases have few to several papules and pustules, but no nodules
- Moderate cases have more papules and pustules, with a few nodules
- Severe cases have numerous papules, pustules, and nodules.5
CASE Ms. S is in obvious emotional distress, and her acne needs to be treated aggressively. Because of the emotional impact and the fact that she has lesions on several body parts, her case is classified as severe (and would be even if her face had only a few lesions).
Treatment: Prevention of new lesions is paramount
Preventing new formations is a key focus of acne therapy, and patients should be advised that it may take weeks for results to be seen. Nonetheless, aggressive treatment of inflammatory lesions is necessary to prevent scarring. Because most patients have both inflammatory and bacterial lesions, it is important to use combined therapies, including topical or oral antibiotics, to treat P acnes and inflammation (TABLE 2).13,17-23
TABLE 2
Acne classification helps guide treatment decisions13,17-23
| Treatment | Severity of acne | ||
|---|---|---|---|
| Mild | Moderate* | Severe* | |
| Dietary/lifestyle modifications (eg, reduce dairy intake, minimize use of cosmetics, reduce stress) PLUS benzoyl peroxide (2%-10%) PLUS retinoid (tretinoin, adapalene, or tazarotene) OR azelaic or salicylic acid | √ | √ | √ |
| Combined OCPs PLUS oral antibiotics OR topical antibiotics (for males and females who are not candidates for OCPs) | √ | √ | |
| Isotretinoin† | √ | ||
| Other therapies, as needed (eg, intralesional injections, chemical peels, or laser therapy)‡ | √ | √ | √ |
| *Treatments for moderate or severe acne are also appropriate for acne that extends to other parts of the body and/or does not respond to topical therapy. †Monitoring and counseling on adverse effects and teratogenic potential are required. ‡Should not be used concurrently or within 6-12 months of isotretinoin due to increased risk of keloid formation. OCPs, oral contraceptive pills. | |||
Topicals are the cornerstone of treatment
Retinoids and benzoyl peroxide topicals are the foundation of therapy for both comedonal and inflammatory acne,17 regardless of severity. Both are recommended by the AAD. But evidence suggests that only 55% of dermatologists and 10% of primary care providers recommend them.19,20
Retinoids inhibit microcomedone formation and regulate follicular keratinocytes, which have anti-inflammatory properties and help to prevent the formation of new lesions. Patients should be warned that topical retinoids can cause irritation, erythema, desquamation, pruritus, and burning. To reduce the adverse effects, advise patients to start retinoid therapy slowly, at a reduced frequency (eg, every other day or every third day) and shorter contact (washing it off after one to 4 hours for a week, then increasing the contact time). When it is clear that the medication is well tolerated, the frequency and amount can be increased. Use of the topical, as tolerated, should continue as long as the potential acne problem remains.
There are 3 retinoid formulations on the market—adapalene, tretinoin, and tazarotene—all of which have been shown to be effective. Adapalene is the least irritating and the most stable, and can be safely combined with benzoyl peroxide and topical antibiotics. If tretinoin and benzoyl peroxide are used concurrently, tretinoin should be applied at night and benzoyl peroxide during the day. To reduce the risk of inactivating the topical agents, advise patients not to use other skin products in conjunction with topical therapy.
Benzoyl peroxide, which is available as a cleanser, gel, or wash, affects keratinocyte dysmaturation, P acnes, and inflammation.11 The antibacterial activity is due to its oxidation. Benzoyl peroxide is available both OTC and by prescription, with concentrations ranging from 2% to 10%. Salicylic acid (2%-3%), a well-tolerated keratolytic agent, is often used with benzoyl peroxide, as well. Azelaic acid, sodium sulfacetamide, and dapsone are other topicals that have been found to be effective in treating acne.
Topical antibiotics, most commonly clindamycin 1% or sodium sulfacetamide, also affect both P acnes and inflammation,24 although the exact mechanism is unknown. Available in solution or as a gel or lotion, topical antibiotics can be combined with benzoyl peroxide. Use of topical erythromycin has declined in recent years because it has a higher rate of bacterial resistance.9,21
When to add oral antibiotics
When topical treatment does not produce the desired result or cannot be tolerated, oral antibiotics may be introduced, either as an addition or replacement. Like topicals, oral antibiotics have both antimicrobial and anti-inflammatory properties.
Tetracycline antibiotics (ie, doxycycline and minocycline) are first-line oral therapy.21 Minocycline has been found to be the most potent agent in this drug class; tetracycline is the least.22 Tetracyclines can cause tooth discoloration and inhibit skeletal growth, and are contraindicated for children younger than 10 years and pregnant women.
Photosensitivity is an adverse effect of tetracycline antibiotics, so patients should be advised to cover up and avoid sun exposure. Other adverse effects, particularly of minocycline, include dizziness, lupus-like syndrome, pseudotumor cerebri, skin and mucosal pigmentation, serum sickness, and hepatitis. If the patient is taking an oral contraceptive pill (OCP) concurrently for family planning, she should be advised that oral antibiotics have the potential to reduce the efficacy of the OCP.
Other oral antibiotics sometimes used to treat acne include erythromycin, trimethoprim-sulfamethoxazole, amoxicillin, and azithromycin, but data on their efficacy are limited. Erythromycin has similar potency to tetracycline, but may need to be taken 2 to 4 times a day and may cause more gastrointestinal disturbances. Cephalosporins, fluoroquinolones, aminoglycosides, chloramphenicol, sulfonamides/sulfur, and gyrase inhibitors should not be used for acne because of a lack of efficacy.6

No, it isn’t. Pustules on the face, like those on the patient pictured here, are a common manifestation of acne. But facial lesions alone are not sufficient for a definitive diagnosis. In fact, the pustules that this 59-year-old woman sought treatment for were correctly diagnosed as perioral dermatitis. The tip-off? The lack of comedones and the distribution of the lesions, which were concentrated around the mouth.
Regardless of the type of oral antibiotic prescribed, it should be tried for about 3 months (8-16 weeks) and discontinued once improvement occurs. If no improvement is seen within 3 months, consider changing antibiotics due to resistance or adding antifungal therapy for Pityrosporum and Malassezia species.6
Initiating isotretinoin therapy: An evidence-based approach
Oral isotretinoin is the only potential cure for acne vulgaris. The cure rate is about 30% to 40% (with about 20% of patients developing a recurrence that requires retreatment within one to 3 years).25
Isotretinoin is FDA approved for severe nodulocystic acne, but several organizations, including the AAD and the Global Alliance to Improve Outcomes in Acne, recommend its use for milder cases.25,26 It is also an excellent treatment for other forms of severe acne, such as acne fulminans and acne conglobata. Accutane is no longer available, but 5 other formulations of isotretinoin are on the market.
Because isotretinoin is a category X teratogen, all providers and patients must register with iPLEDGE (www.ipledgeprogram.com), an FDA-approved mandatory risk management program. Before starting to take isotretinoin, females of childbearing age are required to undergo 2 pregnancy tests; they must also agree to use 2 forms of program-approved birth control and submit to monthly pregnancy tests.
Patients on isotretinoin also need to be monitored for depression.27 Other potential adverse effects include hepatitis, hypertriglyceridemia, arthralgia, myalgias, and inflammatory bowel disease.28,29 Dry skin and mucosa are the most common adverse effects, and patients should be advised to use moisturizers regularly.
A better dosing regimen?
The standard starting dose of isotretinoin is 0.25 to 1 mg/kg/d, divided and taken twice a day, then titrated upward monthly to a maximum daily dose of 2 mg/kg. The goal is for the total intake of isotretinoin to be 120 to 150 mg/kg. So, for example, the goal for a patient weighing 60 kg might be a cumulative intake of 7200 mg (120 mg/kg × 60 kg), taken in doses of 20 mg BID (40 mg/d) for 180 days.
The medication should be taken with food (especially with fatty food) for better absorption. Treatment duration has typically been 16 to 32 weeks, with an average of 20 weeks, with the daily dose lowered in patients requiring treatment for a longer period of time. Continuous use of isotretinoin is more effective than taking it intermittently.26
Lower dosages? While that standard regimen has been adequate in the management of acne vulgaris, emerging evidence suggests that dosages of isotretinoin as low as 5 mg/d are equally effective and have significantly fewer adverse effects.30 Relapse continues to be a problem. Risk factors for relapse include a macrocomedonal pattern of acne, smoking, and age, with patients <14 years and >25 years at higher risk.30 While lower dosing was previously thought to be associated with greater risk of relapse, this appears to be related less to the cumulative dose of 120 to 150 mg/kg and more to the duration of sebaceous gland suppression.30
Based on the latest evidence, important changes in isotretinoin administration are called for—specifically, using a much lower dose (0.25-0.5 mg/kg, divided into 2 daily doses) for a longer period of time.30 While the traditional dosing generally requires a 3- to 5-month course of treatment, the lower dosing can take 6 to 8 months.
Who's a candidate for hormonal therapy?
Any hormone that has antiandrogenic properties can have a beneficial effect on acne.
The most common hormonal therapy is an estrogen-progestin combination OCP.23,31 Progesterone-only OCPs should not be used as they can worsen acne.
In theory, any OCP that contains estrogen can work because of its androgenic properties. The estrogen appears to suppress sebaceous gland activity. OCPs with FDA approval for the treatment of acne include Estrostep Fe (norethindrone/ethinyl estradiol [EE]), Ortho Tricyclen (norgestimate/EE), and Yasmin and Beyaz (drospirenone/EE). With any OCP, the effect is gradual, and it can take 3 to 4 months for patients to see an improvement. OCPs are an excellent choice for women with moderate-to-severe acne or those suffering from hirsutism and seborrhea.
Other hormonal therapies—which are not FDA approved for acne treatment—include spironolactone, cyproterone, and flutamide.24 There is no evidence to support the use of finasteride or cyproterone.
Spironolactone is the most studied and has modest benefits at 100 to 150 mg/d.22 Caution is needed when using spironolactone, as gynecomastia, hyperkalemia, and agranulocytosis are potential adverse effects. It is important to closely monitor the blood pressure, chemistry, and cell count of patients taking spironolactone.
CASE Because Ms. S is sexually active and does not wish to become pregnant, she is a candidate for an OCP. You prescribe a pill containing norgestimate and EE, add a topical retinoid to her regimen, and schedule a return visit in 3 months to evaluate the effectiveness of therapy. If there is little improvement, you will recommend isotretinoin at that time.
Talk to patients about lifestyle modifications
Although the role of lifestyle changes in acne treatment is controversial, there is some evidence to suggest that these modifications are worth considering:
Glycemic load. In Western society, where the typical diet includes foods with a high glycemic index, there appears to be a higher prevalence of acne compared with regions where foods with a low glycemic index (≤55-60) are the mainstay. A low glycemic load appears to reduce both the occurrence and severity of acne.17 Thus, patients who are willing to make dietary changes should be advised to consume foods with a lower glycemic index, such as peanuts and green vegetables.
Dairy. Milk is believed to have an androgenic effect, and dairy products in general have a positive correlation with acne. Thus, a reduction in milk intake has been found to improve acne.18,32 Stress the importance of calcium supplementation for patients whose dairy consumption is reduced or eliminated.
Fish oil. Omega-6 fatty acid, found in fish oil, has anti-inflammatory properties, and an increase in foods rich in omega-3 fatty acid (eg, salmon, sardines, walnuts) has been associated with improvement of acne.17
Probiotics. There is limited evidence for probiotics as a therapy for acne. They do appear to regulate inflammatory cytokines within the skin and to upregulate the IGF-1, both of which influence the formation of acne.10,33
Other treatment options to consider
Injections, chemical peels, and/or laser treatments may be considered as adjunctive therapy or when standard therapies fail.
Steroid injections. This treatment regimen centers around a midpotency steroid that is diluted with normal saline and is introduced into each lesion until the lesion is distended and/or blanched. There are limited data on the use of corticosteroid injections for acne, however, and these injections are reserved for severe cases to reduce inflammation. Potential adverse effects include hyperglycemia, obesity, and Cushing traits.
Chemical peels are used to decrease both inflammatory and noninflammatory lesions, and are typically well tolerated. In one study, more than 95% of patients were satisfied with the results.11,34
Various chemicals have been used, including alpha-hydroxyl acid (glycolic acid), beta-hydroxyl acid (salicylic acid), and Jessner’s solution, with equal efficacy.35-38 Chemical peels can be used on patients with darker skin, but caution is required to avoid dyschromia.39 Other adverse effects include dry skin, crusting, and facial erythema. More adverse effects have been reported with glycolic acid vs salicylic acid.37
Laser therapies include photodynamic therapy—blue light with amino-luvanic acid—and phototherapy (blue light alone).40-42P acnes accumulate photosensitizing porphyrins in the comedones; when the laser therapy is applied, the porphyrins absorb the light source and destroy the bacteria.
Laser treatment can also be used for scarring. Ablative laser resurfacing significantly improves acne scars; nonablative and fractional CO2 laser modalities can also be used, with minimal downtime and no serious complications.43
Other complementary therapies, including aloe vera, pyridoxine, kampo, tea tree extract, and fruit-based acids, have little or no data regarding their efficacy.
The importance of maintenance therapy
With the exception of patients whose acne was cured or who achieved remission with isotretinoin, maintenance is required once the desired appearance is reached. Without it, recurrence is likely—possibly within as little as 4 weeks.
For most patients, a topical retinoid is the only medication that should be continued. Tell patients to apply it nightly and to call for an appointment if an acne flare-up occurs.
CASE When Ms. S comes in for a follow-up visit, her acne is cleared except for a couple of lesions on her back and she is happy with the results. You advise her to continue on the OCP to avoid a recurrence but caution her that in a small percentage of cases, the acne may worsen even in women who continue to take OCPs and topicals. You agree to initiate isotretinoin if this occurs.
CORRESPONDENCE
Tam T. Nguyen, MD, San Joaquin General Hospital, 500 West Hospital Road, Suite 1103, French Camp, CA 95231; ttnguyen@sjgh.org
1. Ghodsi SZ, Orawa H, Zouboulis CC. Prevalence severity, and severity risk factors of acne in high school pupils: a community-based study. J Invest Dermatol. 2009;129:2136-2141.
2. Collier CN, Harper JC, Cafardi JA, et al. The prevalence of acne in adults 20 years and older. J Am Acad Dermatol. 2008;58:56-59.
3. Lucky AW, Biro FM, Huster GA, et al. Acne vulgaris in premenarchal girls. An early sign of puberty associated with rising levels of dehydroepiandrosterone. Arch Dermatol. 1994;130:308-314.
4. Kubota Y, Shirahige Y, Nakai K, et al. Community-based epidemiological study of psychosocial effects of acne in Japanese adolescents. J Dermatol. 2010;37:617-622.
5. trauss JS, Krowchuk DP, Leyden JJ, et al. Guidelines of care for acne vulgaris management. J Am Acad Dermatol. 2007;56:651-663.
6. Thiboutot D, Gollnick H, Bettoli V, et al. New insights into the management of acne: an update from the Global Alliance to Improve Outcomes in Acne group. J Am Acad Dermatol. 2009;60(suppl):S1-S50.
7. Jeremy AH, Holland DB, Roberts SG, et al. Inflammatory events are involved in acne lesion initiation. J Invest Dermatol. 2003;121:20-27.
8. Kurokawa I, Danby FW, Ju Q, et al. New developments in our understanding of acne pathogenesis and treatment. Exp Dermatol. 2009;18:821-832.
9. Zouboulis CC, Baron JM, Bohm M, et al. Frontiers in sebaceous gland biology and pathology. Exp Dermatol. 2008;17:542-551.
10. Melnik BC, Schmitz G. Role of insulin, insulin-like growth factor-1, hyperglycaemic food and milk consumption in the pathogenesis of acne vulgaris. Exp Dermatol. 2009;18:833-841.
11. Sterry W, Paus R. Burgdorf WHC. Dermatology. Thieme Clinical Companions. Stuttgart Germany: Thieme; 2006;530-535.
12. Ballanger F, Baudry P, Nguyen JM, et al. Heredity: a prognostic factor for acne. Dermatology. 2006;212:145-149.
13. Chen MJ, Chen CD, Yang JH, et al. High serum dehydroepiandrosterone sulfate is associated with phenotypic acne and a reduced risk of abdominal obesity in women with polycystic ovary syndrome. Hum Reprod. 2011;26:227-234.
14. Yosipovitch G, Tang M, Dawn AG, et al. Study of psychological stress, sebum production and acne vulgaris in adolescents. Acta Derm Venereol. 2007;87:135-139.
15. Adityan B, Kumari R, Thappa DM. Scoring systems in acne vulgaris. Indian J Dermatol Venereol Leprol. 2009;75:323-326.
16. US Food and Drug Administration. Global acne severity scale. Available at: http://www.fda.gov/ohrms/dockets/ac/02/briefing/3904B1_03_%20Acne%20Global%20Severity%20Scale.pdf. Accessed January 16 2013.
17. Jung JY, Yoon MY, Min SU, et al. The influence of dietary patterns on acne vulgaris in Koreans. Eur J Dermatol. 2010;20:768-772.
18. Adebamowo CA, Spiegelman D, Berkey CS, et al. Milk consumption and acne in teenaged boys. J Am Acad Dermatol. 2008;58:787-793.
19. Hsu P, Litman GI, Brodell RT. Overview of the treatment of acne vulgaris with topical retinoids. Postgrad Med. 2011;123:153-161.
20. Kim RH, Armstrong AW. Current state of acne treatment: highlighting lasers photodynamic therapy, and chemical peels. Dermatol Online J. 2011;17:2.-
21. Simonart T, Dramaix M, De Maertelaer V. Efficacy of tetracyclines in the treatment of acne vulgaris: a review. Br J Dermatol. 2008;158:208-216.
22. Ingram JR, Grindlay DJ, Williams HC. Management of acne vulgaris: an evidence-based update. Clin Exp Dermatol. 2010;35:351-354.
23. Rosen MP, Breitkopf DM, Nagamani M. A randomized controlled trial of second- versus third-generation oral contraceptives in the treatment of acne vulgaris. Am J Obstet Gynecol. 2003;188:1158-1160.
24. Simpson RC, Grindlay DJ, Williams HC. What’s new in acne? An analysis of systematic reviews and clinically significant trials published in 2010-11. Clin Exp Dermatol. 2011;36:840-844.
25. Borghi A, Mantovani L, Minghetti S, et al. Low-cumulative dose isotretinoin treatment in mild-to-moderate acne: efficacy in achieving stable remission. J Eur Acad Dermatol Venereol. 2011;25:1094-1098.
26. Agarwal US, Besarwal RK, Bhola K. Oral isotretinoin in different dose regimens for acne vulgaris: a randomized comparative trial. Indian J Dermatol Venereol Leprol. 2011;77:688-694.
27. Kaymak Y, Taner E, Taner Y. Comparison of depression anxiety and life quality in acne vulgaris patients who were treated with either isotretinoin or topical agents. Int J Dermatol. 2009;48:41-46.
28. Crockett SD, Porter CQ, Martin CF, et al. Isotretinoin use and the risk of inflammatory bowel disease: a case-control study. Am J Gastroenterol. 2010;105:1986-1993.
29. Crockett SD, Gulati A, Sandler RS, et al. Causal association between isotretinoin and inflammatory bowel disease has yet to be established. Am J Gastroenterol. 2009;104:2387-2393.
30. Rademaker M. Isotretinoin: dose duration and relapse. What does 30 years of usage tell us? Australas J Dermatol. 2012 September 26 [Epub ahead of print].
31. Worret I, Arp W, Zahradnik HP, et al. Acne resolution rates: results of a single-blind, randomized, controlled, parallel phase III trial with EE/CMA (Belara) and EE/LNG (Microgynon). Dermatology. 2001;203:38-44.
32. Adebamowo CA, Spiegelman D, Danby FW, et al. High school dietary dairy intake and teenage acne. J Am Acad Dermatol. 2005;52:207-214.
33. Bowe WP, Logan AC. Acne vulgaris, probiotics and the gut-brain-skin axis - back to the future? Gut Pathog. 2011;3:1.-
34. Atzori L, Brundu MA, Orru A, et al. Glycolic acid peeling in the treatment of acne. J Eur Acad Dermatol Venereol. 1999;12:119-122.
35. Levesque A, Hamzavi I, Seite S, et al. Randomized trial comparing a chemical peel containing a lipophilic hydroxy acid derivative of salicylic acid with a salicylic acid peel in subjects with comedonal acne. J Cosmet Dermatol. 2011;10:174-178.
36. Garg VK, Sinha S, Sarkar R. Glycolic acid peels versus salicylic-mandelic acid peels in active acne vulgaris and post-acne scarring and hyperpigmentation: a comparative study. Dermatol Surg. 2009;35:59-65.
37. Kessler E, Flanagan K, Chia C, et al. Comparison of alpha- and beta-hydroxy acid chemical peels in the treatment of mild to moderately severe facial acne vulgaris. Dermatol Surg. 2008;34:45-51.
38. Lee SH, Huh CH, Park KC, et al. Effects of repetitive superficial chemical peels on facial sebum secretion in acne patients. J Eur Acad Dermatol Venereol. 2006;20:964-968.
39. Grimes PE. The safety and efficacy of salicylic acid chemical peels in darker racial-ethnic groups. Dermatol Surg. 1999;25:18-22.
40. Gold MH. Acne and PDT: new techniques with lasers and light sources. Lasers Med Sci. 2007;22:67-72.
41. Gold MH. Acne vulgaris: lasers light sources and photodynamic therapy—an update 2007. Expert Rev Anti Infect Ther. 2007;5:1059-1069.
42. Orringer JS, Sachs DL, Bailey E, et al. Photodynamic therapy for acne vulgaris: a randomized, controlled, split-face clinical trial of topical aminolevulinic acid and pulsed dye laser therapy. J Cosmet Dermatol. 2010;9:28-34.
43. Chapas AM, Brightman L, Sukal S, et al. Successful treatment of acneiform scarring with CO2 ablative fractional resurfacing. Lasers Surg Med. 2008;40:381-386.
1. Ghodsi SZ, Orawa H, Zouboulis CC. Prevalence severity, and severity risk factors of acne in high school pupils: a community-based study. J Invest Dermatol. 2009;129:2136-2141.
2. Collier CN, Harper JC, Cafardi JA, et al. The prevalence of acne in adults 20 years and older. J Am Acad Dermatol. 2008;58:56-59.
3. Lucky AW, Biro FM, Huster GA, et al. Acne vulgaris in premenarchal girls. An early sign of puberty associated with rising levels of dehydroepiandrosterone. Arch Dermatol. 1994;130:308-314.
4. Kubota Y, Shirahige Y, Nakai K, et al. Community-based epidemiological study of psychosocial effects of acne in Japanese adolescents. J Dermatol. 2010;37:617-622.
5. trauss JS, Krowchuk DP, Leyden JJ, et al. Guidelines of care for acne vulgaris management. J Am Acad Dermatol. 2007;56:651-663.
6. Thiboutot D, Gollnick H, Bettoli V, et al. New insights into the management of acne: an update from the Global Alliance to Improve Outcomes in Acne group. J Am Acad Dermatol. 2009;60(suppl):S1-S50.
7. Jeremy AH, Holland DB, Roberts SG, et al. Inflammatory events are involved in acne lesion initiation. J Invest Dermatol. 2003;121:20-27.
8. Kurokawa I, Danby FW, Ju Q, et al. New developments in our understanding of acne pathogenesis and treatment. Exp Dermatol. 2009;18:821-832.
9. Zouboulis CC, Baron JM, Bohm M, et al. Frontiers in sebaceous gland biology and pathology. Exp Dermatol. 2008;17:542-551.
10. Melnik BC, Schmitz G. Role of insulin, insulin-like growth factor-1, hyperglycaemic food and milk consumption in the pathogenesis of acne vulgaris. Exp Dermatol. 2009;18:833-841.
11. Sterry W, Paus R. Burgdorf WHC. Dermatology. Thieme Clinical Companions. Stuttgart Germany: Thieme; 2006;530-535.
12. Ballanger F, Baudry P, Nguyen JM, et al. Heredity: a prognostic factor for acne. Dermatology. 2006;212:145-149.
13. Chen MJ, Chen CD, Yang JH, et al. High serum dehydroepiandrosterone sulfate is associated with phenotypic acne and a reduced risk of abdominal obesity in women with polycystic ovary syndrome. Hum Reprod. 2011;26:227-234.
14. Yosipovitch G, Tang M, Dawn AG, et al. Study of psychological stress, sebum production and acne vulgaris in adolescents. Acta Derm Venereol. 2007;87:135-139.
15. Adityan B, Kumari R, Thappa DM. Scoring systems in acne vulgaris. Indian J Dermatol Venereol Leprol. 2009;75:323-326.
16. US Food and Drug Administration. Global acne severity scale. Available at: http://www.fda.gov/ohrms/dockets/ac/02/briefing/3904B1_03_%20Acne%20Global%20Severity%20Scale.pdf. Accessed January 16 2013.
17. Jung JY, Yoon MY, Min SU, et al. The influence of dietary patterns on acne vulgaris in Koreans. Eur J Dermatol. 2010;20:768-772.
18. Adebamowo CA, Spiegelman D, Berkey CS, et al. Milk consumption and acne in teenaged boys. J Am Acad Dermatol. 2008;58:787-793.
19. Hsu P, Litman GI, Brodell RT. Overview of the treatment of acne vulgaris with topical retinoids. Postgrad Med. 2011;123:153-161.
20. Kim RH, Armstrong AW. Current state of acne treatment: highlighting lasers photodynamic therapy, and chemical peels. Dermatol Online J. 2011;17:2.-
21. Simonart T, Dramaix M, De Maertelaer V. Efficacy of tetracyclines in the treatment of acne vulgaris: a review. Br J Dermatol. 2008;158:208-216.
22. Ingram JR, Grindlay DJ, Williams HC. Management of acne vulgaris: an evidence-based update. Clin Exp Dermatol. 2010;35:351-354.
23. Rosen MP, Breitkopf DM, Nagamani M. A randomized controlled trial of second- versus third-generation oral contraceptives in the treatment of acne vulgaris. Am J Obstet Gynecol. 2003;188:1158-1160.
24. Simpson RC, Grindlay DJ, Williams HC. What’s new in acne? An analysis of systematic reviews and clinically significant trials published in 2010-11. Clin Exp Dermatol. 2011;36:840-844.
25. Borghi A, Mantovani L, Minghetti S, et al. Low-cumulative dose isotretinoin treatment in mild-to-moderate acne: efficacy in achieving stable remission. J Eur Acad Dermatol Venereol. 2011;25:1094-1098.
26. Agarwal US, Besarwal RK, Bhola K. Oral isotretinoin in different dose regimens for acne vulgaris: a randomized comparative trial. Indian J Dermatol Venereol Leprol. 2011;77:688-694.
27. Kaymak Y, Taner E, Taner Y. Comparison of depression anxiety and life quality in acne vulgaris patients who were treated with either isotretinoin or topical agents. Int J Dermatol. 2009;48:41-46.
28. Crockett SD, Porter CQ, Martin CF, et al. Isotretinoin use and the risk of inflammatory bowel disease: a case-control study. Am J Gastroenterol. 2010;105:1986-1993.
29. Crockett SD, Gulati A, Sandler RS, et al. Causal association between isotretinoin and inflammatory bowel disease has yet to be established. Am J Gastroenterol. 2009;104:2387-2393.
30. Rademaker M. Isotretinoin: dose duration and relapse. What does 30 years of usage tell us? Australas J Dermatol. 2012 September 26 [Epub ahead of print].
31. Worret I, Arp W, Zahradnik HP, et al. Acne resolution rates: results of a single-blind, randomized, controlled, parallel phase III trial with EE/CMA (Belara) and EE/LNG (Microgynon). Dermatology. 2001;203:38-44.
32. Adebamowo CA, Spiegelman D, Danby FW, et al. High school dietary dairy intake and teenage acne. J Am Acad Dermatol. 2005;52:207-214.
33. Bowe WP, Logan AC. Acne vulgaris, probiotics and the gut-brain-skin axis - back to the future? Gut Pathog. 2011;3:1.-
34. Atzori L, Brundu MA, Orru A, et al. Glycolic acid peeling in the treatment of acne. J Eur Acad Dermatol Venereol. 1999;12:119-122.
35. Levesque A, Hamzavi I, Seite S, et al. Randomized trial comparing a chemical peel containing a lipophilic hydroxy acid derivative of salicylic acid with a salicylic acid peel in subjects with comedonal acne. J Cosmet Dermatol. 2011;10:174-178.
36. Garg VK, Sinha S, Sarkar R. Glycolic acid peels versus salicylic-mandelic acid peels in active acne vulgaris and post-acne scarring and hyperpigmentation: a comparative study. Dermatol Surg. 2009;35:59-65.
37. Kessler E, Flanagan K, Chia C, et al. Comparison of alpha- and beta-hydroxy acid chemical peels in the treatment of mild to moderately severe facial acne vulgaris. Dermatol Surg. 2008;34:45-51.
38. Lee SH, Huh CH, Park KC, et al. Effects of repetitive superficial chemical peels on facial sebum secretion in acne patients. J Eur Acad Dermatol Venereol. 2006;20:964-968.
39. Grimes PE. The safety and efficacy of salicylic acid chemical peels in darker racial-ethnic groups. Dermatol Surg. 1999;25:18-22.
40. Gold MH. Acne and PDT: new techniques with lasers and light sources. Lasers Med Sci. 2007;22:67-72.
41. Gold MH. Acne vulgaris: lasers light sources and photodynamic therapy—an update 2007. Expert Rev Anti Infect Ther. 2007;5:1059-1069.
42. Orringer JS, Sachs DL, Bailey E, et al. Photodynamic therapy for acne vulgaris: a randomized, controlled, split-face clinical trial of topical aminolevulinic acid and pulsed dye laser therapy. J Cosmet Dermatol. 2010;9:28-34.
43. Chapas AM, Brightman L, Sukal S, et al. Successful treatment of acneiform scarring with CO2 ablative fractional resurfacing. Lasers Surg Med. 2008;40:381-386.
Patient abusing alcohol or drugs? Help starts with a single question
• Screen patients for substance use disorders, with a single (validated) question for alcohol and another for drugs. A
• Follow a positive screen for alcohol with an assessment to distinguish between hazardous drinking and drinking that is indicative of alcohol dependence. C
• Approach a substance use disorder as you would any chronic medical condition, seeking to engage the patient to encourage behavior change. Motivational interviewing is a useful tool. C
• Consider pharmacotherapeutic options for patients with alcohol or drug dependence. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Episodic heavy drinking, like alcoholism and drug addiction, is increasingly recognized as a medical problem that primary care physicians can, and should, address.1 But it is rarely the chief reason for an office visit. Nor is it a subject patients are likely to bring up.
However, patients are generally willing to talk to a trusted doctor who asks about their use (or misuse) of alcohol or other substances. And primary care physicians can do much to help—with brief interventions, a growing armamentarium of pharmacotherapy, and referrals as needed. In the pages that follow, you’ll find easy-to-use screening tools and effective intervention strategies.
SCREENING NEEDN'T BE TIME-CONSUMING
Screening for substance use isn’t difficult. In fact, it can usually be accomplished with 2 targeted questions—one for alcohol use and one for drugs.
Alcohol. Two single-question screens to detect hazardous drinking have been validated, despite having different parameters. Ask either:
Q: When was the last time you had more than ____ drinks (4 for women and 5 for men) in one day?
Q: How many times in the past year have you had ___ or more drinks (4 for women and 5 for men) in one day?
For the first question, any answer within the past 3 months is a positive screen for hazardous drinking.2 For the second, anything other than zero is positive.3,4
Initial screening can also be done with the AUDIT-C (TABLE), a validated short (3-question) version of the Alcohol Use Disorders Identification Test that can be self-administered.5,6
Drugs. Only one single-question screen for drug use has been validated:
Q: How many times in the past year have you used an illegal drug or taken a prescription medication for nonmedical reasons?
Any answer other than never is a positive screen for hazardous drug use.7
CASE Jason F, a healthy and fit 28-year-old, has been your patient, along with his family, for years. He’s in your office because of a knee injury he incurred while running, and you take a moment to ask him, for the first time, how much he drinks and whether he takes drugs. His answer—that he drinks 3 or 4 times a week and often has multiple drinks at parties or nights out with the guys—takes you a bit by surprise.
Now what?
TABLE
3-question AUDIT-C screen for alcohol dependence5,6
| 1. How often do you have a drink containing alcohol? | ||
| 0 Never | 3 2 to 3 times a week | |
| 1 Monthly or less | 4 4 or more times a week | |
| 2 2 to 4 times a month | ||
| 2. How many drinks containing alcohol do you have on a typical day when you are drinking? | ||
| 0 I don’t drink. | 2 5 or 6 | |
| 0 1 or 2 | 3 7 to 9 | |
| 1 3 or 4 | 4 10 or more | |
| 3. How often do you have 6 or more drinks on one occasion? | ||
| 0 Never | 3 Weekly | |
| 1 Less than monthly | 4 Daily or almost daily | |
| 2 Monthly | ||
| Scoring the AUDIT-C | ||
| Alcohol dependence | ||
| Men | Women | |
| Threshold score | 5 | 4 |
| Sensitivity (%) | 80 | 76 |
| Specificity (%) | 74 | 78 |
| AROC | 0.769 | 0.767 |
| AROC, area under the receiver operating characteristic curve; AUDIT, Alcohol Use Disorders Identification Test. | ||
TELL ME MORE ABOUT IT
It is important to respond to a positive screen by requesting more information. In the conversation that ensues, the patient may provide the details you need to determine whether the drinking or drug use is indicative of a diagnosable substance use disorder, an umbrella term for alcohol or drug abuse and alcohol or drug dependence.
Alcohol or drug abuse vs dependence. Criteria for alcohol or drug abuse in the Diagnostic and Statistical Manual of Mental Disorders, 4th ed, text revision (DSM-IV-TR) include risky behavior, such as drinking and driving; problems with work and/or close relationships; or run-ins with the law, such as an arrest for driving while intoxicated. Criteria for alcohol or drug dependence include the inability to cut down or stop using the substance; evidence of tolerance and withdrawal; spending more time ingesting the substance; increasing attention to substance use while interest in other activities diminishes; and continued use despite recurrent problems.8
Tools zero in on extent of problem
To learn more about your patient’s situation, consider using the following criteria and tools:
DSM criteria. Ask the following 2 questions, which are among DSM-IV-TR criteria for alcohol dependence:
- How many times in the last year have you had a lot more to drink than you intended?
- How many times in the last year have you been drinking in situations where it could have been hazardous, where you could have caused an accident or gotten hurt?
Any answer other than zero to either question is suggestive of a substance use disorder. In exploratory analyses, this approach had positive likelihood ratios of 4.7 to 16 and negative likelihood ratios of 0.05 to 0.30.9,10
Although the above questions refer to alcohol use, they could be revised to learn more about a patient’s use of marijuana or other drugs, as well. (There are few tools for the assessment of drug use, because any illegal or nonmedical use of controlled substances has clear risks of major harm.)
CAGE. Another tool that is effective in assessing alcohol use is the 4-question CAGE—an acronym for Cut down, Annoyed, Guilty, and Eye opener:
- Have you ever felt that you should cut down on your drinking?
- Have people annoyed you by criticizing your drinking?
- Have you ever felt bad or guilty about your drinking?
- Have you ever had a drink in the morning to get rid of a hangover?
One meta-analysis found that a positive CAGE test—ie, a positive response to one or more of the questions—had a sensitivity of 0.85 and a specificity of 0.78 in identifying alcohol dependence in a primary care setting (using DSM criteria as the gold standard).11
AUDIT. This 10-item tool, a longer version of the AUDIT-C (available at http://www.medstudentlearning.com/node/6556), can also be used to determine the extent of alcohol use. This test provides detailed information about the quantity and frequency of alcohol use; however, it does not clearly distinguish between hazardous drinking and alcohol use disorders.12
If the patient is a teen
Assessment methods can be adjusted without difficulty to fit the age of the patient. The National Institute on Alcohol Abuse and Alcoholism has published the Alcohol Screening and Brief Intervention for Youth: A Practitioner’s Guide, available at http://www.niaaa.nih.gov/Publications/EducationTrainingMaterials/Pages/YouthGuide.aspx. The 6-question CRAFFT (for Car, Relax, Alone, Forget, Friends, Trouble) is a validated tool designed to assess adolescents’ use of both alcohol and drugs (http://www.ceasar-boston.org/clinicians/crafft.php).13,14
CASE You give Mr. F the CAGE test, and he answers No to all 4 questions. You conclude that while his drinking may be hazardous, he does not appear to have alcohol abuse or dependence.
FOLLOW UP WITH A BRIEF INTERVENTION
For decades, evidence has shown that brief interventions are often effective in helping hazardous drinkers like Mr. F cut back to safer levels.15-18 In some cases, the impact has been great enough to reduce health care and societal costs for up to 4 years19 and to cut the risk of alcohol-related death by about half.20 As a result, the US Preventive Services Task Force has given a B rating to counseling to reduce alcohol misuse by primary care providers.21 (There is less evidence that brief interventions are effective for drug problems,22 or in settings other than primary care.23)
Treat drug/alcohol problems
If you determine that your patient is engaging in hazardous alcohol or drug use or has a diagnosable substance use disorder, you do not have to drop everything else or treat it as an acute event. What matters is long-term success, which is best achieved by partnering with the patient.
Start by approaching drug and alcohol problems as you would a case of newly elevated blood pressure. Bring up the problem, seeking to engage the patient in addres- sing it.
If he or she does not agree to quit or cut back on drinking the first time you broach the subject, don’t be surprised or discouraged. Keep in mind that patients do not always respond positively to advice about handling chronic medical conditions either, particularly at first, and that you’ll be working together over time. What’s important, in the jargon of the Stages of Change model,24 is to help the patient move from precontemplation to contemplation, and perhaps beyond that to planning or action.
Use motivational interviewing to partner with patients
Motivational interviewing is useful in helping patients change health-related behaviors. The technique, which is not hard to learn or apply, is based on the recognition that a simple shift in style toward a guiding (rather than directive) approach can often reap benefits that are immediately apparent. 25-28
Motivational Interviewing: Helping People Change (3rd ed, by William R. Miller and Stephen Rollnick; Guilford Press, 2013) is an excellent resource for clinicians who wish to master this technique. An online tutorial in screening and brief intervention for alcohol or drug misuse is available free at https://adept.missouri.edu. Video demonstrations of motivational interviewing to address these issues are also available here ; to access them, click on “Training”, then on “Go to SBIRT videos”).
CASE Before Mr. F’s visit is concluded, you initiate a conversation about alcohol use, stating: “As your doctor, I’m concerned that the amount of alcohol you’re drinking could be hazardous to your health. I recommend that you cut down to no more than 4 drinks in any one day and to no more than 14 drinks a week.” You make it clear that change is up to him, and ask what he thinks about what you’ve said.
You also schedule a return visit in one month, at which time you will continue the conversation.
PHARMACOTHERAPY IS A USEFUL TOOL
Increasingly, alcohol and drug dependence—like other chronic conditions—can be effectively addressed with medication.
Drugs to treat alcohol dependence
Naltrexone. A daily dose of naltrexone, starting at 25 mg daily for a few days and going as high as 100 mg/d, can help patients with alcohol dependence limit their drinking to safe levels (number needed to treat [NNT]=9).29 This will reduce the risk of alcohol-related harm while the patient considers quitting.
The most common adverse effect is nausea, but a low starting dose may alleviate it. Naltrexone, also available as a 380-mg intramuscular (IM) depot injection once every 4 weeks, is an opioid antagonist and should not be given to any patient who’s taking opioids.
A 2010 Cochrane review found only 4 trials of naltrexone IM, and failed to show significant reductions in drinking.29 But post hoc analyses of trials of both oral and IM naltrexone found that those in which compliance was assured (either by direct observation or IM administration) had better outcomes than those in which it was not.30 Another post hoc analysis found that patients whose alcohol dependence was more severe derived greater benefits from the drug than those who were less severely affected.31
Acamprosate. Two 333-mg pills tid can help newly abstinent drinkers remain alcohol-free (NNT=9).32,33 The most common adverse effect is diarrhea, which may subside with continued use.
Combining acamprosate and naltrexone does not appear to be more effective than either drug alone. In a recently published meta-analysis comparing the 2 drugs, those taking acamprosate had slightly better rates of abstinence from alcohol, while naltrexone was slightly better in reducing heavy drinking.34
Disulfiram Unlike naltrexone and acamprosate, which work by altering the brain’s reward circuits, disulfiram blocks metabolism of ethanol, leading to the accumulation of a toxic metabolite and its punishing syndrome. The major problem with the drug is noncompliance, which can be addressed by enlisting the help of a caregiver or partner to ensure that it is taken daily. 35-37
Other medications that have been tested (though not approved by the US Food and Drug Administration [FDA]) as a treatment for alcohol dependence include:
- topiramate, which has been found to have modest efficacy in increasing the number of abstinent days and decreasing heavy-drinking days;38
- baclofen, which has shown efficacy in small clinical trials;39 and
- ondansetron, which has been shown to effectively treat early-onset alcohol dependence.40,41
For patients with depression and alcohol dependence, the combination of naltrexone and sertraline has been found to be superior to either drug by itself—and to have fewer adverse effects. 42 Gabapentin and lorazepam have been compared in treating alcohol withdrawal, with gabapentin resulting in greater efficacy and fewer adverse effects than lorazepam.43,44
Pharmacotherapy for drug abuse, dependence
For methamphetamine abuse and dependence. Two randomized clinical trials have studied medications for methamphetamine abuse and dependence. In one small study, topiramate did not increase the proportion of patients who achieved abstinence, but in a post hoc subgroup analysis, it did appear to help newly abstinent patients avoid relapse.45
In another study, mirtazapine significantly decreased the proportion of patients whose weekly urine tests were positive for methamphetamine (from 73% to 44%); no significant change was found among those on placebo.46
Both drugs were well tolerated, but compliance was low in both trials despite weekly counseling. Each has only one clinical trial to support its use, and neither has FDA approval for addiction treatment.
For marijuana dependence. In a study of 50 people seeking treatment for marijuana dependence, gabapentin 400 mg 3 times a day significantly improved the proportion reporting no cannabis use and whose urine tested negative for the drug.47
Another recent trial randomized adolescents dependent on cannabis to placebo or N-acetylcysteine 1200 mg twice a day. Those on the active drug were 2.4 times more likely than those on placebo to have negative urine tests with a number needed to treat of 7.48 Both trials ran for about 3 months. Neither drug is FDA approved to treat marijuana dependence.
For opioid dependence. As maintenance medication for patients dependent on opioids, both methadone and buprenorphine have been shown to reduce the use of illicit opioids, lower mortality, and improve retention compared with treatment without medication.49 Methadone would be a better choice than buprenorphine, which is a partial agonist with a ceiling on both its good (eg, stopping craving) and bad (eg, overdose risk) effects. In an open-label observational study of patients’ preferences, those who chose methadone maintenance over buprenorphine were twice as likely to remain in treatment.50 Both drugs are FDA-approved for treating opioid dependence.
Methadone is a full agonist that can be given for opioid dependence only in a federally licensed methadone maintenance clinic. It has been shown to reduce the use of other opioids, reduce criminal behaviors,51 improve function in many areas,52 and reduce mortality.53 In a cohort study of Massachusetts Medicaid data, methadone reduced mortality, which was 75% higher among those receiving abstinence-based treatment.52,54
Buprenorphine (alone and in combination with naloxone), effectively reduces the use of illicit opioids and improves functional status.55-57
Naltrexone, an opioid antagonist, may be effective in the treatment of opioid dependence. As with disulfiram for alcohol dependence, a major limitation of naltrexone for opioid dependence is noncompliance. But once a patient has been on oral naltrexone, he or she can be switched to naltrexone IM, which can be administered every 4 weeks. A Cochrane review published in 2011 found no evidence that naltrexone was superior to placebo, 58 but since then another randomized clinical trial has been published that found naltrexone 380 mg IM every 4 weeks was superior to IM placebo. Patients on naltrexone were more likely to remain in treatment and have opioid-free urine tests, and reported less craving for opioids.59 Both oral and depot injection naltrexone are FDA approved for treatment of opioid dependence. No comparisons of naltrexone vs either methadone or buprenorphine have been published.
To learn more about pharmacotherapy for opioid dependence, see “Diagnosing and treating opioid dependence” (J Fam Pract. 2012;61:588-596).
DEALING WITH THE CHALLENGES
As noted earlier, some patients with substance use disorders, like some patients with depression or hypertension, respond well to care and counseling, and some do not. Just as with other conditions, consultation with a specialist often helps.
A major difference in arranging consultations for patients with substance use disorders, however, is that clinicians who specialize in substance abuse and dependence often work in health care systems that are largely, or entirely, separate from those in which primary care physicians typically work. This, plus the stigma that surrounds problems with substance use, presents barriers to patients, who may shy away from going across town or to another city to see a provider they don’t know for a problem they’re either resistant to “owning” or ashamed of.
Yet it is possible to reach across this divide and make it easier for patients. One way to do that might be to partner with a local alcohol- and drug-treatment program so that your patients are referred, not to a faceless agency, but rather to a specific clinician; you might even call the provider while the patient is in your office so they can “meet.” Another approach, taken by some multispecialty practices, is to add psychotherapists to the staff so that patients can simply walk down the hall to obtain the mental health care they need.
Reaching across this divide is also a useful strategy for primary care physicians, who may welcome opportunities to meet with someone from a local treatment agency, not just for referrals but to learn more about treating patients with substance use problems. The Patient Protection and Affordable Care Act, which cites substance use disorders as one of 6 chronic health conditions that primary care medical homes are expected to address, may lead to better integration of health care systems that address physical health, as well as mental health and substance use disorders.
CORRESPONDENCE
Daniel C. Vinson, MD, MSPH, MA306E Health Sciences, Family and Community Medicine, University of Missouri, Columbia, MO 652312; vinsond@health.missouri.edu
- Friedman PD. Alcohol use in adults. N Engl J Med. 2013;368:365-373.
- Williams RH, Vinson DC. Validation of a single question screen for problem drinking. J Fam Pract. 2001;50:307-312.
- Smith PC, Schmidt SM, Allensworth-Davies D, et al. Primary care validation of a single-question alcohol screening test. J Gen Intern Med. 2009;74:783-788.
- Dawson DA, Pulay AJ, Grant BF. A comparison of two single-item screeners for hazardous drinking and alcohol use disorder. Alcohol Clin Exp Res. 2010;34:364-374.
- Bush K, Kivlahan DR, Mcdonell MB, et al. The AUDIT alcohol consumption questions (AUDIT-C): an effective brief screening test for problem drinking. Arch Intern Med. 1998;158:1789-1795.
- Dawson DA, Grant BF, Stinson FS, et al. Effectiveness of the derived Alcohol Use Disorders Identification Test (AUDIT-C) in screening for alcohol use disorders and risk drinking in the US general population. Alcohol Clin Exp Res. 2005;29:844-854.
- Smith PC, Schmidt SM, Allensworth-Davies D, et al. A single-question screening test for drug use in primary care. Arch Intern Med. 2010;170:1155-1160.
- American Psychiatric Asociation. Diagnostic and Statistical Manual of Mental Disorders. 4th ed text rev. (DSM-IV-TR). Arlington, Va: American Psychiatric Association; 2000.
- Saha TD, Stinson FS, Grant BF. The role of alcohol consumption in future classifications of alcohol use disorders. Drug Alcohol Depend. 2007;89:82-92.
- Vinson DC, Kruse RL, Seale JP. Simplifying alcohol assessment:two questions to identify alcohol use disorders. Alcohol Clin Exp Res. 2007;31:1392-139.
- Aertgeerts B, Buntinx F, Kester A. The value of the CAGE in screening for alcohol abuse and alcohol dependence in general clinical populations: a diagnostic meta-analysis. J Clin Epidemiol. 2004;57:30-39.
- Johnson JA, Lee A, Vinson DC, et al. Use of AUDIT-based measures to identify unhealthy alcohol use and alcohol dependence in primary care: a validation study. Alcohol Clin Exp Res. 2012;July 26 [Epub ahead of print].
- Center for Adolescent Substance Abuse Research. The CRAFFT Screening Tool. Boston MA:2009 [updated 2012). Available at: http://www.ceasar-boston.org/clinicians/crafft.php. Accessed January 15, 2013.
- Knight JR, Sherritt L, Harris SK, et al. Validity of brief alcohol screening tests among adolescents: a comparison of the AUDIT, POSIT, CAGE, and CRAFFT. Alcohol Clin Exp Res. 2003;27:67-734.
- Wallace P, Cutler S, Haines A. Randomised controlled trial of general practitioner intervention in patients with excessive alcohol consumption. BMJ. 1988;297:663-668.
- Fleming MF, Barry KL, Manwell LB, et al. Brief physician advice for problem alcohol drinkers: a randomized controlled trial in community-based primary care practices. JAMA. 1997;277:1039-1044.
- Bertholet N, Daeppen JB, Wietlisbach V, et al. Brief alcohol intervention in primary care reduces alcohol consumption: Systematic review and meta-analysis. Arch Intern Med. 2005;165:986-995.
- Kaner EFS, Dickinson HO, Beyer F, et al. Effectiveness of brief alcohol interventions in primary care populations. Cochrane Database Syst Rev. 2007(2);CD004148.-
- Fleming MF, Mundt MP, French MT, et al. Brief physician advice for problem drinkers: long-term efficacy and benefit-cost analysis. Alcohol Clin Exp Res. 2002;26:36-43.
- Cuijpers P, Riper H, Lemmers L. The effects on mortality of brief interventions for problem drinking: a meta-analysis. Addiction. 2004;99:839-845.
- U.S.Preventive Services Task Force. Screening and behavioral counseling interventions in primary care to reduce alcohol misuse: recommendation statement. Ann Intern Med. 2004;140:554-556.
- Saitz R, Alford DP, Bernstein J, et al. Screening and brief intervention ffor unhealthy drug use in primary care settings: randomized clinical trials are needed. J Addict Med. 2010;4:131-136.
- Field CA, Baird J, Saitz R, et al. The mixed evidence for brief intervention in emergency departments, trauma care centers, and inpatient hospital settings: what should we do? Alcohol Clin Exp Res. 2010;34:2004-2010.
- DiClemente CC, Prochaska JO. Toward a comprehensive transtheoretical model of change: stages of change and addictive behaviors. In: Miller WR, Heather N, eds. Treating Addictive Behaviors. New York, NY:Plenum Press;1998:3–24.
- Mason P, Butler CC. Health Behavior Change: A Guide for Practitioners. 2nd ed. London UK:Elsevier; 2010.
- Miller WR, Rollnick S. Motivational Interviewing: Preparing People for Change. 2nd ed. New York NY: Guilford Press;2002.
- Hettema J, Steele J, Miller WR. Motivational interviewing. Ann Rev Clin Psych. 2005;1:91-111.
- Rollnick S, Butler CC, Kinnersley P, et al. Motivational interviewing. BMJ. 2010;340:c1900.-
- Rosner S, Hackl-Herrwerth A, Leucht S, et al. Opioid antagonists for alcohol dependence. Cochrane Database Syst Rev. 2010;(12):CD001867.-
- Swift R, Oslin DW, Alexander M, et al. Adherence monitoring in naltrexone pharmacotherapy trials: a systematic review. J Stud Alcohol Drugs. 2011;72:1012-1018.
- Pettinati HM, Silverman BL, Battisti JJ, et al. Efficacy of extended-release naltrexone in patients with relatively higher severity of alcohol dependence. Alcohol Clin Exp Res. 2011;35:1804-1811.
- Mann K, Lehert P, Morgan MY. The efficacy of acamprosate in the maintenance of abstinence in alcohol-dependent individuals: results of a meta-analysis. Alcohol Clin Exp Res. 2004;28:51-63.
- Rosner S, Hackl-Herrwerth A, Leucht S, et al. Acamprosate for alcohol dependence. Cochrane Database Syst Rev. 2010(9);CD004332.-
- Maisel NC, Blodgett JC, Wilbourne PL, et al. Meta-analysis of naltrexone and acamprosate for treating alcohol use disorders: when are these medications most helpful? Addiction. 2012;October 17 [Epub ahead of print].
- Azrin NH. Improvements in the community-reinforcement approach to alcoholism. Behav Res Ther. 1976;14:339-348.
- Azrin NH, Sisson RW, Meyers R, Godley M. Alcoholism treatment by disulfiram and community reinforcement therapy. J Behav Ther Exp Psychiatr. 1982;13:105-112.
- Keane TM, Foy DW, Nunn B, et al. Spouse contracting to increase antabuse compliance in alcoholic veterans. J Clin Psychol. 1984;40:340-344.
- Arbaizar B, Diersen-Sotos T, Gomez-Acebo I, et al. Topiramate in the treatment of alcohol dependence: a meta-analysis. Actas Espanolas de Psiquiatria. 2010;38:8-12.
- Johnson BA. Update on neuropharmacological treatments for alcoholism: scientific basis and clinical findings. Biochem Pharmacol. 2008;75:34-56.
- Johnson BA, Roache JD, Javors MA, et al. Ondansetron for reduction of drinking among biologically predisposed alcoholic patients: a randomized controlled trial. JAMA. 2000;284:963-971.
- Johnson BA, Ait-Daoud N, Seneviratne C, et al. Pharmacogenetic approach at the serotonin transporter gene as a method of reducing the severity of alcohol drinking. Am J Psychiatr. 2011;168:265-275.
- Pettatini HM, Oslin D, Lampman KM, et al. A double-blind, placebo-controlled trial combining sertraline and naltrexone for treating co-occurring depression and alcohol dependence. Am J Psychiatr. 2010;167:668-675.
- Anton RF, Myrick H, Wright TM, et al. Gabapentin combined with naltrexone for the treatment of alcohol dependence. Am J Psychiatr. 2011;168:709-717.
- Myrick H, Malcolm R, Randall PK, et al. A double-blind trial of gabapentin versus lorazepam in the treatment of alcohol withdrawal. Alcohol Clin Exp Res. 2009;33:1582-1588.
- Elkashef A, Kahn R, Yu E, et al. Topiramate for the treatment of methamphetamine addiction: a multi-center placebo-controlled trial. Addiction. 2012;107:1297-1306.
- Colfax GN, Santos GM, Das M, et al. Mirtazapine to reduce methamphetamine use: a randomized controlled trial. Arch Gen Psychiatr. 2011;68:1168-1175.
- Mason BJ, Crean R, Goodell V, et al. A proof-of-concept randomized controlled study of gabapentin: effects on cannabis use, withdrawal and executive function deficits in cannabis-dependent adults. Neuropsychopharmacology. 2012;37:1689-1698.
- Gray KM, Carpenter MJ, Baker NL, et al. A double-blind randomized controlled trial of N-acetylcysteine in cannabis-dependent adolescents. Am J Psychiatr. 2012;169:805-812.
- Farrell M, Wodak A, Gowing L. Maintenance drugs to treat opioid dependence. BMJ. 2012;344.-
- Pinto H, Maskrey V, Swift L, et al. The SUMMIT trial: a field comparison of buprenorphine versus methadone maintenance treatment. J Subst Abuse Treat. 2010;39:340-352.
- Marsch LA. The efficacy of methadone maintenance interventions in reducing illicit opiate use HIV risk behavior and criminality: a meta-analysis. Addiction. 1998;93:515-532.
- Sees KL, Delucchi KL, Masson C, et al. Methadone maintenance vs 180-day psychosocially enriched detoxification for treatment of opioid dependence: a randomized controlled trial. JAMA. 2000;283:1303-1310.
- Clausen T, Anchersen K, Waal H. Mortality prior to during and after opioid maintenance treatment (OMT): a national prospective cross-registry study. Drug Alcohol Depend. 2008;94:151-157.
- Clark RE, Samnaliev M, Baxter JD, et al. The evidence doesn’t justify steps by state Medicaid programs to restrict opioid addiction treatment with buprenorphine. Health Affairs. 2011;30:1425-1433.
- Center for Substance Abuse Treatment. Clinical Guidelines for the Use of Buprenorphine in the Treatment of Opioid Addiction: Treatment Improvement Protocol (TIP) Series 40. Rockville Md: Substance Abuse and Mental Health Services Administration; 2004.
- Fudala PJ, Bridge TP, Herbert S, et al. Office-based treatment of opiate addiction with a sublingual-tablet formulation of buprenorphine and naloxone. N Engl J Med. 2003;349:949-958.
- Fiellin DA. Buprenorphine: effective treatment of opioid addiction starts in the office. Am Fam Physician. 2006;73:1513.-
- Minozzi S, Amato L, Vecchi S, et al. Oral naltrexone maintainance treatment for opioid dependence. Cochrane Database Syst Rev. 2011;(4):CD001333.-
- Krupitsky E, Nunes EV, Ling W, et al. Injectable extended-release naltrexone for opioid dependence: a double-blind, placebo-controlled, multicentre randomised trial. Lancet. 2011;377:1506-1513.
• Screen patients for substance use disorders, with a single (validated) question for alcohol and another for drugs. A
• Follow a positive screen for alcohol with an assessment to distinguish between hazardous drinking and drinking that is indicative of alcohol dependence. C
• Approach a substance use disorder as you would any chronic medical condition, seeking to engage the patient to encourage behavior change. Motivational interviewing is a useful tool. C
• Consider pharmacotherapeutic options for patients with alcohol or drug dependence. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Episodic heavy drinking, like alcoholism and drug addiction, is increasingly recognized as a medical problem that primary care physicians can, and should, address.1 But it is rarely the chief reason for an office visit. Nor is it a subject patients are likely to bring up.
However, patients are generally willing to talk to a trusted doctor who asks about their use (or misuse) of alcohol or other substances. And primary care physicians can do much to help—with brief interventions, a growing armamentarium of pharmacotherapy, and referrals as needed. In the pages that follow, you’ll find easy-to-use screening tools and effective intervention strategies.
SCREENING NEEDN'T BE TIME-CONSUMING
Screening for substance use isn’t difficult. In fact, it can usually be accomplished with 2 targeted questions—one for alcohol use and one for drugs.
Alcohol. Two single-question screens to detect hazardous drinking have been validated, despite having different parameters. Ask either:
Q: When was the last time you had more than ____ drinks (4 for women and 5 for men) in one day?
Q: How many times in the past year have you had ___ or more drinks (4 for women and 5 for men) in one day?
For the first question, any answer within the past 3 months is a positive screen for hazardous drinking.2 For the second, anything other than zero is positive.3,4
Initial screening can also be done with the AUDIT-C (TABLE), a validated short (3-question) version of the Alcohol Use Disorders Identification Test that can be self-administered.5,6
Drugs. Only one single-question screen for drug use has been validated:
Q: How many times in the past year have you used an illegal drug or taken a prescription medication for nonmedical reasons?
Any answer other than never is a positive screen for hazardous drug use.7
CASE Jason F, a healthy and fit 28-year-old, has been your patient, along with his family, for years. He’s in your office because of a knee injury he incurred while running, and you take a moment to ask him, for the first time, how much he drinks and whether he takes drugs. His answer—that he drinks 3 or 4 times a week and often has multiple drinks at parties or nights out with the guys—takes you a bit by surprise.
Now what?
TABLE
3-question AUDIT-C screen for alcohol dependence5,6
| 1. How often do you have a drink containing alcohol? | ||
| 0 Never | 3 2 to 3 times a week | |
| 1 Monthly or less | 4 4 or more times a week | |
| 2 2 to 4 times a month | ||
| 2. How many drinks containing alcohol do you have on a typical day when you are drinking? | ||
| 0 I don’t drink. | 2 5 or 6 | |
| 0 1 or 2 | 3 7 to 9 | |
| 1 3 or 4 | 4 10 or more | |
| 3. How often do you have 6 or more drinks on one occasion? | ||
| 0 Never | 3 Weekly | |
| 1 Less than monthly | 4 Daily or almost daily | |
| 2 Monthly | ||
| Scoring the AUDIT-C | ||
| Alcohol dependence | ||
| Men | Women | |
| Threshold score | 5 | 4 |
| Sensitivity (%) | 80 | 76 |
| Specificity (%) | 74 | 78 |
| AROC | 0.769 | 0.767 |
| AROC, area under the receiver operating characteristic curve; AUDIT, Alcohol Use Disorders Identification Test. | ||
TELL ME MORE ABOUT IT
It is important to respond to a positive screen by requesting more information. In the conversation that ensues, the patient may provide the details you need to determine whether the drinking or drug use is indicative of a diagnosable substance use disorder, an umbrella term for alcohol or drug abuse and alcohol or drug dependence.
Alcohol or drug abuse vs dependence. Criteria for alcohol or drug abuse in the Diagnostic and Statistical Manual of Mental Disorders, 4th ed, text revision (DSM-IV-TR) include risky behavior, such as drinking and driving; problems with work and/or close relationships; or run-ins with the law, such as an arrest for driving while intoxicated. Criteria for alcohol or drug dependence include the inability to cut down or stop using the substance; evidence of tolerance and withdrawal; spending more time ingesting the substance; increasing attention to substance use while interest in other activities diminishes; and continued use despite recurrent problems.8
Tools zero in on extent of problem
To learn more about your patient’s situation, consider using the following criteria and tools:
DSM criteria. Ask the following 2 questions, which are among DSM-IV-TR criteria for alcohol dependence:
- How many times in the last year have you had a lot more to drink than you intended?
- How many times in the last year have you been drinking in situations where it could have been hazardous, where you could have caused an accident or gotten hurt?
Any answer other than zero to either question is suggestive of a substance use disorder. In exploratory analyses, this approach had positive likelihood ratios of 4.7 to 16 and negative likelihood ratios of 0.05 to 0.30.9,10
Although the above questions refer to alcohol use, they could be revised to learn more about a patient’s use of marijuana or other drugs, as well. (There are few tools for the assessment of drug use, because any illegal or nonmedical use of controlled substances has clear risks of major harm.)
CAGE. Another tool that is effective in assessing alcohol use is the 4-question CAGE—an acronym for Cut down, Annoyed, Guilty, and Eye opener:
- Have you ever felt that you should cut down on your drinking?
- Have people annoyed you by criticizing your drinking?
- Have you ever felt bad or guilty about your drinking?
- Have you ever had a drink in the morning to get rid of a hangover?
One meta-analysis found that a positive CAGE test—ie, a positive response to one or more of the questions—had a sensitivity of 0.85 and a specificity of 0.78 in identifying alcohol dependence in a primary care setting (using DSM criteria as the gold standard).11
AUDIT. This 10-item tool, a longer version of the AUDIT-C (available at http://www.medstudentlearning.com/node/6556), can also be used to determine the extent of alcohol use. This test provides detailed information about the quantity and frequency of alcohol use; however, it does not clearly distinguish between hazardous drinking and alcohol use disorders.12
If the patient is a teen
Assessment methods can be adjusted without difficulty to fit the age of the patient. The National Institute on Alcohol Abuse and Alcoholism has published the Alcohol Screening and Brief Intervention for Youth: A Practitioner’s Guide, available at http://www.niaaa.nih.gov/Publications/EducationTrainingMaterials/Pages/YouthGuide.aspx. The 6-question CRAFFT (for Car, Relax, Alone, Forget, Friends, Trouble) is a validated tool designed to assess adolescents’ use of both alcohol and drugs (http://www.ceasar-boston.org/clinicians/crafft.php).13,14
CASE You give Mr. F the CAGE test, and he answers No to all 4 questions. You conclude that while his drinking may be hazardous, he does not appear to have alcohol abuse or dependence.
FOLLOW UP WITH A BRIEF INTERVENTION
For decades, evidence has shown that brief interventions are often effective in helping hazardous drinkers like Mr. F cut back to safer levels.15-18 In some cases, the impact has been great enough to reduce health care and societal costs for up to 4 years19 and to cut the risk of alcohol-related death by about half.20 As a result, the US Preventive Services Task Force has given a B rating to counseling to reduce alcohol misuse by primary care providers.21 (There is less evidence that brief interventions are effective for drug problems,22 or in settings other than primary care.23)
Treat drug/alcohol problems
If you determine that your patient is engaging in hazardous alcohol or drug use or has a diagnosable substance use disorder, you do not have to drop everything else or treat it as an acute event. What matters is long-term success, which is best achieved by partnering with the patient.
Start by approaching drug and alcohol problems as you would a case of newly elevated blood pressure. Bring up the problem, seeking to engage the patient in addres- sing it.
If he or she does not agree to quit or cut back on drinking the first time you broach the subject, don’t be surprised or discouraged. Keep in mind that patients do not always respond positively to advice about handling chronic medical conditions either, particularly at first, and that you’ll be working together over time. What’s important, in the jargon of the Stages of Change model,24 is to help the patient move from precontemplation to contemplation, and perhaps beyond that to planning or action.
Use motivational interviewing to partner with patients
Motivational interviewing is useful in helping patients change health-related behaviors. The technique, which is not hard to learn or apply, is based on the recognition that a simple shift in style toward a guiding (rather than directive) approach can often reap benefits that are immediately apparent. 25-28
Motivational Interviewing: Helping People Change (3rd ed, by William R. Miller and Stephen Rollnick; Guilford Press, 2013) is an excellent resource for clinicians who wish to master this technique. An online tutorial in screening and brief intervention for alcohol or drug misuse is available free at https://adept.missouri.edu. Video demonstrations of motivational interviewing to address these issues are also available here ; to access them, click on “Training”, then on “Go to SBIRT videos”).
CASE Before Mr. F’s visit is concluded, you initiate a conversation about alcohol use, stating: “As your doctor, I’m concerned that the amount of alcohol you’re drinking could be hazardous to your health. I recommend that you cut down to no more than 4 drinks in any one day and to no more than 14 drinks a week.” You make it clear that change is up to him, and ask what he thinks about what you’ve said.
You also schedule a return visit in one month, at which time you will continue the conversation.
PHARMACOTHERAPY IS A USEFUL TOOL
Increasingly, alcohol and drug dependence—like other chronic conditions—can be effectively addressed with medication.
Drugs to treat alcohol dependence
Naltrexone. A daily dose of naltrexone, starting at 25 mg daily for a few days and going as high as 100 mg/d, can help patients with alcohol dependence limit their drinking to safe levels (number needed to treat [NNT]=9).29 This will reduce the risk of alcohol-related harm while the patient considers quitting.
The most common adverse effect is nausea, but a low starting dose may alleviate it. Naltrexone, also available as a 380-mg intramuscular (IM) depot injection once every 4 weeks, is an opioid antagonist and should not be given to any patient who’s taking opioids.
A 2010 Cochrane review found only 4 trials of naltrexone IM, and failed to show significant reductions in drinking.29 But post hoc analyses of trials of both oral and IM naltrexone found that those in which compliance was assured (either by direct observation or IM administration) had better outcomes than those in which it was not.30 Another post hoc analysis found that patients whose alcohol dependence was more severe derived greater benefits from the drug than those who were less severely affected.31
Acamprosate. Two 333-mg pills tid can help newly abstinent drinkers remain alcohol-free (NNT=9).32,33 The most common adverse effect is diarrhea, which may subside with continued use.
Combining acamprosate and naltrexone does not appear to be more effective than either drug alone. In a recently published meta-analysis comparing the 2 drugs, those taking acamprosate had slightly better rates of abstinence from alcohol, while naltrexone was slightly better in reducing heavy drinking.34
Disulfiram Unlike naltrexone and acamprosate, which work by altering the brain’s reward circuits, disulfiram blocks metabolism of ethanol, leading to the accumulation of a toxic metabolite and its punishing syndrome. The major problem with the drug is noncompliance, which can be addressed by enlisting the help of a caregiver or partner to ensure that it is taken daily. 35-37
Other medications that have been tested (though not approved by the US Food and Drug Administration [FDA]) as a treatment for alcohol dependence include:
- topiramate, which has been found to have modest efficacy in increasing the number of abstinent days and decreasing heavy-drinking days;38
- baclofen, which has shown efficacy in small clinical trials;39 and
- ondansetron, which has been shown to effectively treat early-onset alcohol dependence.40,41
For patients with depression and alcohol dependence, the combination of naltrexone and sertraline has been found to be superior to either drug by itself—and to have fewer adverse effects. 42 Gabapentin and lorazepam have been compared in treating alcohol withdrawal, with gabapentin resulting in greater efficacy and fewer adverse effects than lorazepam.43,44
Pharmacotherapy for drug abuse, dependence
For methamphetamine abuse and dependence. Two randomized clinical trials have studied medications for methamphetamine abuse and dependence. In one small study, topiramate did not increase the proportion of patients who achieved abstinence, but in a post hoc subgroup analysis, it did appear to help newly abstinent patients avoid relapse.45
In another study, mirtazapine significantly decreased the proportion of patients whose weekly urine tests were positive for methamphetamine (from 73% to 44%); no significant change was found among those on placebo.46
Both drugs were well tolerated, but compliance was low in both trials despite weekly counseling. Each has only one clinical trial to support its use, and neither has FDA approval for addiction treatment.
For marijuana dependence. In a study of 50 people seeking treatment for marijuana dependence, gabapentin 400 mg 3 times a day significantly improved the proportion reporting no cannabis use and whose urine tested negative for the drug.47
Another recent trial randomized adolescents dependent on cannabis to placebo or N-acetylcysteine 1200 mg twice a day. Those on the active drug were 2.4 times more likely than those on placebo to have negative urine tests with a number needed to treat of 7.48 Both trials ran for about 3 months. Neither drug is FDA approved to treat marijuana dependence.
For opioid dependence. As maintenance medication for patients dependent on opioids, both methadone and buprenorphine have been shown to reduce the use of illicit opioids, lower mortality, and improve retention compared with treatment without medication.49 Methadone would be a better choice than buprenorphine, which is a partial agonist with a ceiling on both its good (eg, stopping craving) and bad (eg, overdose risk) effects. In an open-label observational study of patients’ preferences, those who chose methadone maintenance over buprenorphine were twice as likely to remain in treatment.50 Both drugs are FDA-approved for treating opioid dependence.
Methadone is a full agonist that can be given for opioid dependence only in a federally licensed methadone maintenance clinic. It has been shown to reduce the use of other opioids, reduce criminal behaviors,51 improve function in many areas,52 and reduce mortality.53 In a cohort study of Massachusetts Medicaid data, methadone reduced mortality, which was 75% higher among those receiving abstinence-based treatment.52,54
Buprenorphine (alone and in combination with naloxone), effectively reduces the use of illicit opioids and improves functional status.55-57
Naltrexone, an opioid antagonist, may be effective in the treatment of opioid dependence. As with disulfiram for alcohol dependence, a major limitation of naltrexone for opioid dependence is noncompliance. But once a patient has been on oral naltrexone, he or she can be switched to naltrexone IM, which can be administered every 4 weeks. A Cochrane review published in 2011 found no evidence that naltrexone was superior to placebo, 58 but since then another randomized clinical trial has been published that found naltrexone 380 mg IM every 4 weeks was superior to IM placebo. Patients on naltrexone were more likely to remain in treatment and have opioid-free urine tests, and reported less craving for opioids.59 Both oral and depot injection naltrexone are FDA approved for treatment of opioid dependence. No comparisons of naltrexone vs either methadone or buprenorphine have been published.
To learn more about pharmacotherapy for opioid dependence, see “Diagnosing and treating opioid dependence” (J Fam Pract. 2012;61:588-596).
DEALING WITH THE CHALLENGES
As noted earlier, some patients with substance use disorders, like some patients with depression or hypertension, respond well to care and counseling, and some do not. Just as with other conditions, consultation with a specialist often helps.
A major difference in arranging consultations for patients with substance use disorders, however, is that clinicians who specialize in substance abuse and dependence often work in health care systems that are largely, or entirely, separate from those in which primary care physicians typically work. This, plus the stigma that surrounds problems with substance use, presents barriers to patients, who may shy away from going across town or to another city to see a provider they don’t know for a problem they’re either resistant to “owning” or ashamed of.
Yet it is possible to reach across this divide and make it easier for patients. One way to do that might be to partner with a local alcohol- and drug-treatment program so that your patients are referred, not to a faceless agency, but rather to a specific clinician; you might even call the provider while the patient is in your office so they can “meet.” Another approach, taken by some multispecialty practices, is to add psychotherapists to the staff so that patients can simply walk down the hall to obtain the mental health care they need.
Reaching across this divide is also a useful strategy for primary care physicians, who may welcome opportunities to meet with someone from a local treatment agency, not just for referrals but to learn more about treating patients with substance use problems. The Patient Protection and Affordable Care Act, which cites substance use disorders as one of 6 chronic health conditions that primary care medical homes are expected to address, may lead to better integration of health care systems that address physical health, as well as mental health and substance use disorders.
CORRESPONDENCE
Daniel C. Vinson, MD, MSPH, MA306E Health Sciences, Family and Community Medicine, University of Missouri, Columbia, MO 652312; vinsond@health.missouri.edu
• Screen patients for substance use disorders, with a single (validated) question for alcohol and another for drugs. A
• Follow a positive screen for alcohol with an assessment to distinguish between hazardous drinking and drinking that is indicative of alcohol dependence. C
• Approach a substance use disorder as you would any chronic medical condition, seeking to engage the patient to encourage behavior change. Motivational interviewing is a useful tool. C
• Consider pharmacotherapeutic options for patients with alcohol or drug dependence. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Episodic heavy drinking, like alcoholism and drug addiction, is increasingly recognized as a medical problem that primary care physicians can, and should, address.1 But it is rarely the chief reason for an office visit. Nor is it a subject patients are likely to bring up.
However, patients are generally willing to talk to a trusted doctor who asks about their use (or misuse) of alcohol or other substances. And primary care physicians can do much to help—with brief interventions, a growing armamentarium of pharmacotherapy, and referrals as needed. In the pages that follow, you’ll find easy-to-use screening tools and effective intervention strategies.
SCREENING NEEDN'T BE TIME-CONSUMING
Screening for substance use isn’t difficult. In fact, it can usually be accomplished with 2 targeted questions—one for alcohol use and one for drugs.
Alcohol. Two single-question screens to detect hazardous drinking have been validated, despite having different parameters. Ask either:
Q: When was the last time you had more than ____ drinks (4 for women and 5 for men) in one day?
Q: How many times in the past year have you had ___ or more drinks (4 for women and 5 for men) in one day?
For the first question, any answer within the past 3 months is a positive screen for hazardous drinking.2 For the second, anything other than zero is positive.3,4
Initial screening can also be done with the AUDIT-C (TABLE), a validated short (3-question) version of the Alcohol Use Disorders Identification Test that can be self-administered.5,6
Drugs. Only one single-question screen for drug use has been validated:
Q: How many times in the past year have you used an illegal drug or taken a prescription medication for nonmedical reasons?
Any answer other than never is a positive screen for hazardous drug use.7
CASE Jason F, a healthy and fit 28-year-old, has been your patient, along with his family, for years. He’s in your office because of a knee injury he incurred while running, and you take a moment to ask him, for the first time, how much he drinks and whether he takes drugs. His answer—that he drinks 3 or 4 times a week and often has multiple drinks at parties or nights out with the guys—takes you a bit by surprise.
Now what?
TABLE
3-question AUDIT-C screen for alcohol dependence5,6
| 1. How often do you have a drink containing alcohol? | ||
| 0 Never | 3 2 to 3 times a week | |
| 1 Monthly or less | 4 4 or more times a week | |
| 2 2 to 4 times a month | ||
| 2. How many drinks containing alcohol do you have on a typical day when you are drinking? | ||
| 0 I don’t drink. | 2 5 or 6 | |
| 0 1 or 2 | 3 7 to 9 | |
| 1 3 or 4 | 4 10 or more | |
| 3. How often do you have 6 or more drinks on one occasion? | ||
| 0 Never | 3 Weekly | |
| 1 Less than monthly | 4 Daily or almost daily | |
| 2 Monthly | ||
| Scoring the AUDIT-C | ||
| Alcohol dependence | ||
| Men | Women | |
| Threshold score | 5 | 4 |
| Sensitivity (%) | 80 | 76 |
| Specificity (%) | 74 | 78 |
| AROC | 0.769 | 0.767 |
| AROC, area under the receiver operating characteristic curve; AUDIT, Alcohol Use Disorders Identification Test. | ||
TELL ME MORE ABOUT IT
It is important to respond to a positive screen by requesting more information. In the conversation that ensues, the patient may provide the details you need to determine whether the drinking or drug use is indicative of a diagnosable substance use disorder, an umbrella term for alcohol or drug abuse and alcohol or drug dependence.
Alcohol or drug abuse vs dependence. Criteria for alcohol or drug abuse in the Diagnostic and Statistical Manual of Mental Disorders, 4th ed, text revision (DSM-IV-TR) include risky behavior, such as drinking and driving; problems with work and/or close relationships; or run-ins with the law, such as an arrest for driving while intoxicated. Criteria for alcohol or drug dependence include the inability to cut down or stop using the substance; evidence of tolerance and withdrawal; spending more time ingesting the substance; increasing attention to substance use while interest in other activities diminishes; and continued use despite recurrent problems.8
Tools zero in on extent of problem
To learn more about your patient’s situation, consider using the following criteria and tools:
DSM criteria. Ask the following 2 questions, which are among DSM-IV-TR criteria for alcohol dependence:
- How many times in the last year have you had a lot more to drink than you intended?
- How many times in the last year have you been drinking in situations where it could have been hazardous, where you could have caused an accident or gotten hurt?
Any answer other than zero to either question is suggestive of a substance use disorder. In exploratory analyses, this approach had positive likelihood ratios of 4.7 to 16 and negative likelihood ratios of 0.05 to 0.30.9,10
Although the above questions refer to alcohol use, they could be revised to learn more about a patient’s use of marijuana or other drugs, as well. (There are few tools for the assessment of drug use, because any illegal or nonmedical use of controlled substances has clear risks of major harm.)
CAGE. Another tool that is effective in assessing alcohol use is the 4-question CAGE—an acronym for Cut down, Annoyed, Guilty, and Eye opener:
- Have you ever felt that you should cut down on your drinking?
- Have people annoyed you by criticizing your drinking?
- Have you ever felt bad or guilty about your drinking?
- Have you ever had a drink in the morning to get rid of a hangover?
One meta-analysis found that a positive CAGE test—ie, a positive response to one or more of the questions—had a sensitivity of 0.85 and a specificity of 0.78 in identifying alcohol dependence in a primary care setting (using DSM criteria as the gold standard).11
AUDIT. This 10-item tool, a longer version of the AUDIT-C (available at http://www.medstudentlearning.com/node/6556), can also be used to determine the extent of alcohol use. This test provides detailed information about the quantity and frequency of alcohol use; however, it does not clearly distinguish between hazardous drinking and alcohol use disorders.12
If the patient is a teen
Assessment methods can be adjusted without difficulty to fit the age of the patient. The National Institute on Alcohol Abuse and Alcoholism has published the Alcohol Screening and Brief Intervention for Youth: A Practitioner’s Guide, available at http://www.niaaa.nih.gov/Publications/EducationTrainingMaterials/Pages/YouthGuide.aspx. The 6-question CRAFFT (for Car, Relax, Alone, Forget, Friends, Trouble) is a validated tool designed to assess adolescents’ use of both alcohol and drugs (http://www.ceasar-boston.org/clinicians/crafft.php).13,14
CASE You give Mr. F the CAGE test, and he answers No to all 4 questions. You conclude that while his drinking may be hazardous, he does not appear to have alcohol abuse or dependence.
FOLLOW UP WITH A BRIEF INTERVENTION
For decades, evidence has shown that brief interventions are often effective in helping hazardous drinkers like Mr. F cut back to safer levels.15-18 In some cases, the impact has been great enough to reduce health care and societal costs for up to 4 years19 and to cut the risk of alcohol-related death by about half.20 As a result, the US Preventive Services Task Force has given a B rating to counseling to reduce alcohol misuse by primary care providers.21 (There is less evidence that brief interventions are effective for drug problems,22 or in settings other than primary care.23)
Treat drug/alcohol problems
If you determine that your patient is engaging in hazardous alcohol or drug use or has a diagnosable substance use disorder, you do not have to drop everything else or treat it as an acute event. What matters is long-term success, which is best achieved by partnering with the patient.
Start by approaching drug and alcohol problems as you would a case of newly elevated blood pressure. Bring up the problem, seeking to engage the patient in addres- sing it.
If he or she does not agree to quit or cut back on drinking the first time you broach the subject, don’t be surprised or discouraged. Keep in mind that patients do not always respond positively to advice about handling chronic medical conditions either, particularly at first, and that you’ll be working together over time. What’s important, in the jargon of the Stages of Change model,24 is to help the patient move from precontemplation to contemplation, and perhaps beyond that to planning or action.
Use motivational interviewing to partner with patients
Motivational interviewing is useful in helping patients change health-related behaviors. The technique, which is not hard to learn or apply, is based on the recognition that a simple shift in style toward a guiding (rather than directive) approach can often reap benefits that are immediately apparent. 25-28
Motivational Interviewing: Helping People Change (3rd ed, by William R. Miller and Stephen Rollnick; Guilford Press, 2013) is an excellent resource for clinicians who wish to master this technique. An online tutorial in screening and brief intervention for alcohol or drug misuse is available free at https://adept.missouri.edu. Video demonstrations of motivational interviewing to address these issues are also available here ; to access them, click on “Training”, then on “Go to SBIRT videos”).
CASE Before Mr. F’s visit is concluded, you initiate a conversation about alcohol use, stating: “As your doctor, I’m concerned that the amount of alcohol you’re drinking could be hazardous to your health. I recommend that you cut down to no more than 4 drinks in any one day and to no more than 14 drinks a week.” You make it clear that change is up to him, and ask what he thinks about what you’ve said.
You also schedule a return visit in one month, at which time you will continue the conversation.
PHARMACOTHERAPY IS A USEFUL TOOL
Increasingly, alcohol and drug dependence—like other chronic conditions—can be effectively addressed with medication.
Drugs to treat alcohol dependence
Naltrexone. A daily dose of naltrexone, starting at 25 mg daily for a few days and going as high as 100 mg/d, can help patients with alcohol dependence limit their drinking to safe levels (number needed to treat [NNT]=9).29 This will reduce the risk of alcohol-related harm while the patient considers quitting.
The most common adverse effect is nausea, but a low starting dose may alleviate it. Naltrexone, also available as a 380-mg intramuscular (IM) depot injection once every 4 weeks, is an opioid antagonist and should not be given to any patient who’s taking opioids.
A 2010 Cochrane review found only 4 trials of naltrexone IM, and failed to show significant reductions in drinking.29 But post hoc analyses of trials of both oral and IM naltrexone found that those in which compliance was assured (either by direct observation or IM administration) had better outcomes than those in which it was not.30 Another post hoc analysis found that patients whose alcohol dependence was more severe derived greater benefits from the drug than those who were less severely affected.31
Acamprosate. Two 333-mg pills tid can help newly abstinent drinkers remain alcohol-free (NNT=9).32,33 The most common adverse effect is diarrhea, which may subside with continued use.
Combining acamprosate and naltrexone does not appear to be more effective than either drug alone. In a recently published meta-analysis comparing the 2 drugs, those taking acamprosate had slightly better rates of abstinence from alcohol, while naltrexone was slightly better in reducing heavy drinking.34
Disulfiram Unlike naltrexone and acamprosate, which work by altering the brain’s reward circuits, disulfiram blocks metabolism of ethanol, leading to the accumulation of a toxic metabolite and its punishing syndrome. The major problem with the drug is noncompliance, which can be addressed by enlisting the help of a caregiver or partner to ensure that it is taken daily. 35-37
Other medications that have been tested (though not approved by the US Food and Drug Administration [FDA]) as a treatment for alcohol dependence include:
- topiramate, which has been found to have modest efficacy in increasing the number of abstinent days and decreasing heavy-drinking days;38
- baclofen, which has shown efficacy in small clinical trials;39 and
- ondansetron, which has been shown to effectively treat early-onset alcohol dependence.40,41
For patients with depression and alcohol dependence, the combination of naltrexone and sertraline has been found to be superior to either drug by itself—and to have fewer adverse effects. 42 Gabapentin and lorazepam have been compared in treating alcohol withdrawal, with gabapentin resulting in greater efficacy and fewer adverse effects than lorazepam.43,44
Pharmacotherapy for drug abuse, dependence
For methamphetamine abuse and dependence. Two randomized clinical trials have studied medications for methamphetamine abuse and dependence. In one small study, topiramate did not increase the proportion of patients who achieved abstinence, but in a post hoc subgroup analysis, it did appear to help newly abstinent patients avoid relapse.45
In another study, mirtazapine significantly decreased the proportion of patients whose weekly urine tests were positive for methamphetamine (from 73% to 44%); no significant change was found among those on placebo.46
Both drugs were well tolerated, but compliance was low in both trials despite weekly counseling. Each has only one clinical trial to support its use, and neither has FDA approval for addiction treatment.
For marijuana dependence. In a study of 50 people seeking treatment for marijuana dependence, gabapentin 400 mg 3 times a day significantly improved the proportion reporting no cannabis use and whose urine tested negative for the drug.47
Another recent trial randomized adolescents dependent on cannabis to placebo or N-acetylcysteine 1200 mg twice a day. Those on the active drug were 2.4 times more likely than those on placebo to have negative urine tests with a number needed to treat of 7.48 Both trials ran for about 3 months. Neither drug is FDA approved to treat marijuana dependence.
For opioid dependence. As maintenance medication for patients dependent on opioids, both methadone and buprenorphine have been shown to reduce the use of illicit opioids, lower mortality, and improve retention compared with treatment without medication.49 Methadone would be a better choice than buprenorphine, which is a partial agonist with a ceiling on both its good (eg, stopping craving) and bad (eg, overdose risk) effects. In an open-label observational study of patients’ preferences, those who chose methadone maintenance over buprenorphine were twice as likely to remain in treatment.50 Both drugs are FDA-approved for treating opioid dependence.
Methadone is a full agonist that can be given for opioid dependence only in a federally licensed methadone maintenance clinic. It has been shown to reduce the use of other opioids, reduce criminal behaviors,51 improve function in many areas,52 and reduce mortality.53 In a cohort study of Massachusetts Medicaid data, methadone reduced mortality, which was 75% higher among those receiving abstinence-based treatment.52,54
Buprenorphine (alone and in combination with naloxone), effectively reduces the use of illicit opioids and improves functional status.55-57
Naltrexone, an opioid antagonist, may be effective in the treatment of opioid dependence. As with disulfiram for alcohol dependence, a major limitation of naltrexone for opioid dependence is noncompliance. But once a patient has been on oral naltrexone, he or she can be switched to naltrexone IM, which can be administered every 4 weeks. A Cochrane review published in 2011 found no evidence that naltrexone was superior to placebo, 58 but since then another randomized clinical trial has been published that found naltrexone 380 mg IM every 4 weeks was superior to IM placebo. Patients on naltrexone were more likely to remain in treatment and have opioid-free urine tests, and reported less craving for opioids.59 Both oral and depot injection naltrexone are FDA approved for treatment of opioid dependence. No comparisons of naltrexone vs either methadone or buprenorphine have been published.
To learn more about pharmacotherapy for opioid dependence, see “Diagnosing and treating opioid dependence” (J Fam Pract. 2012;61:588-596).
DEALING WITH THE CHALLENGES
As noted earlier, some patients with substance use disorders, like some patients with depression or hypertension, respond well to care and counseling, and some do not. Just as with other conditions, consultation with a specialist often helps.
A major difference in arranging consultations for patients with substance use disorders, however, is that clinicians who specialize in substance abuse and dependence often work in health care systems that are largely, or entirely, separate from those in which primary care physicians typically work. This, plus the stigma that surrounds problems with substance use, presents barriers to patients, who may shy away from going across town or to another city to see a provider they don’t know for a problem they’re either resistant to “owning” or ashamed of.
Yet it is possible to reach across this divide and make it easier for patients. One way to do that might be to partner with a local alcohol- and drug-treatment program so that your patients are referred, not to a faceless agency, but rather to a specific clinician; you might even call the provider while the patient is in your office so they can “meet.” Another approach, taken by some multispecialty practices, is to add psychotherapists to the staff so that patients can simply walk down the hall to obtain the mental health care they need.
Reaching across this divide is also a useful strategy for primary care physicians, who may welcome opportunities to meet with someone from a local treatment agency, not just for referrals but to learn more about treating patients with substance use problems. The Patient Protection and Affordable Care Act, which cites substance use disorders as one of 6 chronic health conditions that primary care medical homes are expected to address, may lead to better integration of health care systems that address physical health, as well as mental health and substance use disorders.
CORRESPONDENCE
Daniel C. Vinson, MD, MSPH, MA306E Health Sciences, Family and Community Medicine, University of Missouri, Columbia, MO 652312; vinsond@health.missouri.edu
- Friedman PD. Alcohol use in adults. N Engl J Med. 2013;368:365-373.
- Williams RH, Vinson DC. Validation of a single question screen for problem drinking. J Fam Pract. 2001;50:307-312.
- Smith PC, Schmidt SM, Allensworth-Davies D, et al. Primary care validation of a single-question alcohol screening test. J Gen Intern Med. 2009;74:783-788.
- Dawson DA, Pulay AJ, Grant BF. A comparison of two single-item screeners for hazardous drinking and alcohol use disorder. Alcohol Clin Exp Res. 2010;34:364-374.
- Bush K, Kivlahan DR, Mcdonell MB, et al. The AUDIT alcohol consumption questions (AUDIT-C): an effective brief screening test for problem drinking. Arch Intern Med. 1998;158:1789-1795.
- Dawson DA, Grant BF, Stinson FS, et al. Effectiveness of the derived Alcohol Use Disorders Identification Test (AUDIT-C) in screening for alcohol use disorders and risk drinking in the US general population. Alcohol Clin Exp Res. 2005;29:844-854.
- Smith PC, Schmidt SM, Allensworth-Davies D, et al. A single-question screening test for drug use in primary care. Arch Intern Med. 2010;170:1155-1160.
- American Psychiatric Asociation. Diagnostic and Statistical Manual of Mental Disorders. 4th ed text rev. (DSM-IV-TR). Arlington, Va: American Psychiatric Association; 2000.
- Saha TD, Stinson FS, Grant BF. The role of alcohol consumption in future classifications of alcohol use disorders. Drug Alcohol Depend. 2007;89:82-92.
- Vinson DC, Kruse RL, Seale JP. Simplifying alcohol assessment:two questions to identify alcohol use disorders. Alcohol Clin Exp Res. 2007;31:1392-139.
- Aertgeerts B, Buntinx F, Kester A. The value of the CAGE in screening for alcohol abuse and alcohol dependence in general clinical populations: a diagnostic meta-analysis. J Clin Epidemiol. 2004;57:30-39.
- Johnson JA, Lee A, Vinson DC, et al. Use of AUDIT-based measures to identify unhealthy alcohol use and alcohol dependence in primary care: a validation study. Alcohol Clin Exp Res. 2012;July 26 [Epub ahead of print].
- Center for Adolescent Substance Abuse Research. The CRAFFT Screening Tool. Boston MA:2009 [updated 2012). Available at: http://www.ceasar-boston.org/clinicians/crafft.php. Accessed January 15, 2013.
- Knight JR, Sherritt L, Harris SK, et al. Validity of brief alcohol screening tests among adolescents: a comparison of the AUDIT, POSIT, CAGE, and CRAFFT. Alcohol Clin Exp Res. 2003;27:67-734.
- Wallace P, Cutler S, Haines A. Randomised controlled trial of general practitioner intervention in patients with excessive alcohol consumption. BMJ. 1988;297:663-668.
- Fleming MF, Barry KL, Manwell LB, et al. Brief physician advice for problem alcohol drinkers: a randomized controlled trial in community-based primary care practices. JAMA. 1997;277:1039-1044.
- Bertholet N, Daeppen JB, Wietlisbach V, et al. Brief alcohol intervention in primary care reduces alcohol consumption: Systematic review and meta-analysis. Arch Intern Med. 2005;165:986-995.
- Kaner EFS, Dickinson HO, Beyer F, et al. Effectiveness of brief alcohol interventions in primary care populations. Cochrane Database Syst Rev. 2007(2);CD004148.-
- Fleming MF, Mundt MP, French MT, et al. Brief physician advice for problem drinkers: long-term efficacy and benefit-cost analysis. Alcohol Clin Exp Res. 2002;26:36-43.
- Cuijpers P, Riper H, Lemmers L. The effects on mortality of brief interventions for problem drinking: a meta-analysis. Addiction. 2004;99:839-845.
- U.S.Preventive Services Task Force. Screening and behavioral counseling interventions in primary care to reduce alcohol misuse: recommendation statement. Ann Intern Med. 2004;140:554-556.
- Saitz R, Alford DP, Bernstein J, et al. Screening and brief intervention ffor unhealthy drug use in primary care settings: randomized clinical trials are needed. J Addict Med. 2010;4:131-136.
- Field CA, Baird J, Saitz R, et al. The mixed evidence for brief intervention in emergency departments, trauma care centers, and inpatient hospital settings: what should we do? Alcohol Clin Exp Res. 2010;34:2004-2010.
- DiClemente CC, Prochaska JO. Toward a comprehensive transtheoretical model of change: stages of change and addictive behaviors. In: Miller WR, Heather N, eds. Treating Addictive Behaviors. New York, NY:Plenum Press;1998:3–24.
- Mason P, Butler CC. Health Behavior Change: A Guide for Practitioners. 2nd ed. London UK:Elsevier; 2010.
- Miller WR, Rollnick S. Motivational Interviewing: Preparing People for Change. 2nd ed. New York NY: Guilford Press;2002.
- Hettema J, Steele J, Miller WR. Motivational interviewing. Ann Rev Clin Psych. 2005;1:91-111.
- Rollnick S, Butler CC, Kinnersley P, et al. Motivational interviewing. BMJ. 2010;340:c1900.-
- Rosner S, Hackl-Herrwerth A, Leucht S, et al. Opioid antagonists for alcohol dependence. Cochrane Database Syst Rev. 2010;(12):CD001867.-
- Swift R, Oslin DW, Alexander M, et al. Adherence monitoring in naltrexone pharmacotherapy trials: a systematic review. J Stud Alcohol Drugs. 2011;72:1012-1018.
- Pettinati HM, Silverman BL, Battisti JJ, et al. Efficacy of extended-release naltrexone in patients with relatively higher severity of alcohol dependence. Alcohol Clin Exp Res. 2011;35:1804-1811.
- Mann K, Lehert P, Morgan MY. The efficacy of acamprosate in the maintenance of abstinence in alcohol-dependent individuals: results of a meta-analysis. Alcohol Clin Exp Res. 2004;28:51-63.
- Rosner S, Hackl-Herrwerth A, Leucht S, et al. Acamprosate for alcohol dependence. Cochrane Database Syst Rev. 2010(9);CD004332.-
- Maisel NC, Blodgett JC, Wilbourne PL, et al. Meta-analysis of naltrexone and acamprosate for treating alcohol use disorders: when are these medications most helpful? Addiction. 2012;October 17 [Epub ahead of print].
- Azrin NH. Improvements in the community-reinforcement approach to alcoholism. Behav Res Ther. 1976;14:339-348.
- Azrin NH, Sisson RW, Meyers R, Godley M. Alcoholism treatment by disulfiram and community reinforcement therapy. J Behav Ther Exp Psychiatr. 1982;13:105-112.
- Keane TM, Foy DW, Nunn B, et al. Spouse contracting to increase antabuse compliance in alcoholic veterans. J Clin Psychol. 1984;40:340-344.
- Arbaizar B, Diersen-Sotos T, Gomez-Acebo I, et al. Topiramate in the treatment of alcohol dependence: a meta-analysis. Actas Espanolas de Psiquiatria. 2010;38:8-12.
- Johnson BA. Update on neuropharmacological treatments for alcoholism: scientific basis and clinical findings. Biochem Pharmacol. 2008;75:34-56.
- Johnson BA, Roache JD, Javors MA, et al. Ondansetron for reduction of drinking among biologically predisposed alcoholic patients: a randomized controlled trial. JAMA. 2000;284:963-971.
- Johnson BA, Ait-Daoud N, Seneviratne C, et al. Pharmacogenetic approach at the serotonin transporter gene as a method of reducing the severity of alcohol drinking. Am J Psychiatr. 2011;168:265-275.
- Pettatini HM, Oslin D, Lampman KM, et al. A double-blind, placebo-controlled trial combining sertraline and naltrexone for treating co-occurring depression and alcohol dependence. Am J Psychiatr. 2010;167:668-675.
- Anton RF, Myrick H, Wright TM, et al. Gabapentin combined with naltrexone for the treatment of alcohol dependence. Am J Psychiatr. 2011;168:709-717.
- Myrick H, Malcolm R, Randall PK, et al. A double-blind trial of gabapentin versus lorazepam in the treatment of alcohol withdrawal. Alcohol Clin Exp Res. 2009;33:1582-1588.
- Elkashef A, Kahn R, Yu E, et al. Topiramate for the treatment of methamphetamine addiction: a multi-center placebo-controlled trial. Addiction. 2012;107:1297-1306.
- Colfax GN, Santos GM, Das M, et al. Mirtazapine to reduce methamphetamine use: a randomized controlled trial. Arch Gen Psychiatr. 2011;68:1168-1175.
- Mason BJ, Crean R, Goodell V, et al. A proof-of-concept randomized controlled study of gabapentin: effects on cannabis use, withdrawal and executive function deficits in cannabis-dependent adults. Neuropsychopharmacology. 2012;37:1689-1698.
- Gray KM, Carpenter MJ, Baker NL, et al. A double-blind randomized controlled trial of N-acetylcysteine in cannabis-dependent adolescents. Am J Psychiatr. 2012;169:805-812.
- Farrell M, Wodak A, Gowing L. Maintenance drugs to treat opioid dependence. BMJ. 2012;344.-
- Pinto H, Maskrey V, Swift L, et al. The SUMMIT trial: a field comparison of buprenorphine versus methadone maintenance treatment. J Subst Abuse Treat. 2010;39:340-352.
- Marsch LA. The efficacy of methadone maintenance interventions in reducing illicit opiate use HIV risk behavior and criminality: a meta-analysis. Addiction. 1998;93:515-532.
- Sees KL, Delucchi KL, Masson C, et al. Methadone maintenance vs 180-day psychosocially enriched detoxification for treatment of opioid dependence: a randomized controlled trial. JAMA. 2000;283:1303-1310.
- Clausen T, Anchersen K, Waal H. Mortality prior to during and after opioid maintenance treatment (OMT): a national prospective cross-registry study. Drug Alcohol Depend. 2008;94:151-157.
- Clark RE, Samnaliev M, Baxter JD, et al. The evidence doesn’t justify steps by state Medicaid programs to restrict opioid addiction treatment with buprenorphine. Health Affairs. 2011;30:1425-1433.
- Center for Substance Abuse Treatment. Clinical Guidelines for the Use of Buprenorphine in the Treatment of Opioid Addiction: Treatment Improvement Protocol (TIP) Series 40. Rockville Md: Substance Abuse and Mental Health Services Administration; 2004.
- Fudala PJ, Bridge TP, Herbert S, et al. Office-based treatment of opiate addiction with a sublingual-tablet formulation of buprenorphine and naloxone. N Engl J Med. 2003;349:949-958.
- Fiellin DA. Buprenorphine: effective treatment of opioid addiction starts in the office. Am Fam Physician. 2006;73:1513.-
- Minozzi S, Amato L, Vecchi S, et al. Oral naltrexone maintainance treatment for opioid dependence. Cochrane Database Syst Rev. 2011;(4):CD001333.-
- Krupitsky E, Nunes EV, Ling W, et al. Injectable extended-release naltrexone for opioid dependence: a double-blind, placebo-controlled, multicentre randomised trial. Lancet. 2011;377:1506-1513.
- Friedman PD. Alcohol use in adults. N Engl J Med. 2013;368:365-373.
- Williams RH, Vinson DC. Validation of a single question screen for problem drinking. J Fam Pract. 2001;50:307-312.
- Smith PC, Schmidt SM, Allensworth-Davies D, et al. Primary care validation of a single-question alcohol screening test. J Gen Intern Med. 2009;74:783-788.
- Dawson DA, Pulay AJ, Grant BF. A comparison of two single-item screeners for hazardous drinking and alcohol use disorder. Alcohol Clin Exp Res. 2010;34:364-374.
- Bush K, Kivlahan DR, Mcdonell MB, et al. The AUDIT alcohol consumption questions (AUDIT-C): an effective brief screening test for problem drinking. Arch Intern Med. 1998;158:1789-1795.
- Dawson DA, Grant BF, Stinson FS, et al. Effectiveness of the derived Alcohol Use Disorders Identification Test (AUDIT-C) in screening for alcohol use disorders and risk drinking in the US general population. Alcohol Clin Exp Res. 2005;29:844-854.
- Smith PC, Schmidt SM, Allensworth-Davies D, et al. A single-question screening test for drug use in primary care. Arch Intern Med. 2010;170:1155-1160.
- American Psychiatric Asociation. Diagnostic and Statistical Manual of Mental Disorders. 4th ed text rev. (DSM-IV-TR). Arlington, Va: American Psychiatric Association; 2000.
- Saha TD, Stinson FS, Grant BF. The role of alcohol consumption in future classifications of alcohol use disorders. Drug Alcohol Depend. 2007;89:82-92.
- Vinson DC, Kruse RL, Seale JP. Simplifying alcohol assessment:two questions to identify alcohol use disorders. Alcohol Clin Exp Res. 2007;31:1392-139.
- Aertgeerts B, Buntinx F, Kester A. The value of the CAGE in screening for alcohol abuse and alcohol dependence in general clinical populations: a diagnostic meta-analysis. J Clin Epidemiol. 2004;57:30-39.
- Johnson JA, Lee A, Vinson DC, et al. Use of AUDIT-based measures to identify unhealthy alcohol use and alcohol dependence in primary care: a validation study. Alcohol Clin Exp Res. 2012;July 26 [Epub ahead of print].
- Center for Adolescent Substance Abuse Research. The CRAFFT Screening Tool. Boston MA:2009 [updated 2012). Available at: http://www.ceasar-boston.org/clinicians/crafft.php. Accessed January 15, 2013.
- Knight JR, Sherritt L, Harris SK, et al. Validity of brief alcohol screening tests among adolescents: a comparison of the AUDIT, POSIT, CAGE, and CRAFFT. Alcohol Clin Exp Res. 2003;27:67-734.
- Wallace P, Cutler S, Haines A. Randomised controlled trial of general practitioner intervention in patients with excessive alcohol consumption. BMJ. 1988;297:663-668.
- Fleming MF, Barry KL, Manwell LB, et al. Brief physician advice for problem alcohol drinkers: a randomized controlled trial in community-based primary care practices. JAMA. 1997;277:1039-1044.
- Bertholet N, Daeppen JB, Wietlisbach V, et al. Brief alcohol intervention in primary care reduces alcohol consumption: Systematic review and meta-analysis. Arch Intern Med. 2005;165:986-995.
- Kaner EFS, Dickinson HO, Beyer F, et al. Effectiveness of brief alcohol interventions in primary care populations. Cochrane Database Syst Rev. 2007(2);CD004148.-
- Fleming MF, Mundt MP, French MT, et al. Brief physician advice for problem drinkers: long-term efficacy and benefit-cost analysis. Alcohol Clin Exp Res. 2002;26:36-43.
- Cuijpers P, Riper H, Lemmers L. The effects on mortality of brief interventions for problem drinking: a meta-analysis. Addiction. 2004;99:839-845.
- U.S.Preventive Services Task Force. Screening and behavioral counseling interventions in primary care to reduce alcohol misuse: recommendation statement. Ann Intern Med. 2004;140:554-556.
- Saitz R, Alford DP, Bernstein J, et al. Screening and brief intervention ffor unhealthy drug use in primary care settings: randomized clinical trials are needed. J Addict Med. 2010;4:131-136.
- Field CA, Baird J, Saitz R, et al. The mixed evidence for brief intervention in emergency departments, trauma care centers, and inpatient hospital settings: what should we do? Alcohol Clin Exp Res. 2010;34:2004-2010.
- DiClemente CC, Prochaska JO. Toward a comprehensive transtheoretical model of change: stages of change and addictive behaviors. In: Miller WR, Heather N, eds. Treating Addictive Behaviors. New York, NY:Plenum Press;1998:3–24.
- Mason P, Butler CC. Health Behavior Change: A Guide for Practitioners. 2nd ed. London UK:Elsevier; 2010.
- Miller WR, Rollnick S. Motivational Interviewing: Preparing People for Change. 2nd ed. New York NY: Guilford Press;2002.
- Hettema J, Steele J, Miller WR. Motivational interviewing. Ann Rev Clin Psych. 2005;1:91-111.
- Rollnick S, Butler CC, Kinnersley P, et al. Motivational interviewing. BMJ. 2010;340:c1900.-
- Rosner S, Hackl-Herrwerth A, Leucht S, et al. Opioid antagonists for alcohol dependence. Cochrane Database Syst Rev. 2010;(12):CD001867.-
- Swift R, Oslin DW, Alexander M, et al. Adherence monitoring in naltrexone pharmacotherapy trials: a systematic review. J Stud Alcohol Drugs. 2011;72:1012-1018.
- Pettinati HM, Silverman BL, Battisti JJ, et al. Efficacy of extended-release naltrexone in patients with relatively higher severity of alcohol dependence. Alcohol Clin Exp Res. 2011;35:1804-1811.
- Mann K, Lehert P, Morgan MY. The efficacy of acamprosate in the maintenance of abstinence in alcohol-dependent individuals: results of a meta-analysis. Alcohol Clin Exp Res. 2004;28:51-63.
- Rosner S, Hackl-Herrwerth A, Leucht S, et al. Acamprosate for alcohol dependence. Cochrane Database Syst Rev. 2010(9);CD004332.-
- Maisel NC, Blodgett JC, Wilbourne PL, et al. Meta-analysis of naltrexone and acamprosate for treating alcohol use disorders: when are these medications most helpful? Addiction. 2012;October 17 [Epub ahead of print].
- Azrin NH. Improvements in the community-reinforcement approach to alcoholism. Behav Res Ther. 1976;14:339-348.
- Azrin NH, Sisson RW, Meyers R, Godley M. Alcoholism treatment by disulfiram and community reinforcement therapy. J Behav Ther Exp Psychiatr. 1982;13:105-112.
- Keane TM, Foy DW, Nunn B, et al. Spouse contracting to increase antabuse compliance in alcoholic veterans. J Clin Psychol. 1984;40:340-344.
- Arbaizar B, Diersen-Sotos T, Gomez-Acebo I, et al. Topiramate in the treatment of alcohol dependence: a meta-analysis. Actas Espanolas de Psiquiatria. 2010;38:8-12.
- Johnson BA. Update on neuropharmacological treatments for alcoholism: scientific basis and clinical findings. Biochem Pharmacol. 2008;75:34-56.
- Johnson BA, Roache JD, Javors MA, et al. Ondansetron for reduction of drinking among biologically predisposed alcoholic patients: a randomized controlled trial. JAMA. 2000;284:963-971.
- Johnson BA, Ait-Daoud N, Seneviratne C, et al. Pharmacogenetic approach at the serotonin transporter gene as a method of reducing the severity of alcohol drinking. Am J Psychiatr. 2011;168:265-275.
- Pettatini HM, Oslin D, Lampman KM, et al. A double-blind, placebo-controlled trial combining sertraline and naltrexone for treating co-occurring depression and alcohol dependence. Am J Psychiatr. 2010;167:668-675.
- Anton RF, Myrick H, Wright TM, et al. Gabapentin combined with naltrexone for the treatment of alcohol dependence. Am J Psychiatr. 2011;168:709-717.
- Myrick H, Malcolm R, Randall PK, et al. A double-blind trial of gabapentin versus lorazepam in the treatment of alcohol withdrawal. Alcohol Clin Exp Res. 2009;33:1582-1588.
- Elkashef A, Kahn R, Yu E, et al. Topiramate for the treatment of methamphetamine addiction: a multi-center placebo-controlled trial. Addiction. 2012;107:1297-1306.
- Colfax GN, Santos GM, Das M, et al. Mirtazapine to reduce methamphetamine use: a randomized controlled trial. Arch Gen Psychiatr. 2011;68:1168-1175.
- Mason BJ, Crean R, Goodell V, et al. A proof-of-concept randomized controlled study of gabapentin: effects on cannabis use, withdrawal and executive function deficits in cannabis-dependent adults. Neuropsychopharmacology. 2012;37:1689-1698.
- Gray KM, Carpenter MJ, Baker NL, et al. A double-blind randomized controlled trial of N-acetylcysteine in cannabis-dependent adolescents. Am J Psychiatr. 2012;169:805-812.
- Farrell M, Wodak A, Gowing L. Maintenance drugs to treat opioid dependence. BMJ. 2012;344.-
- Pinto H, Maskrey V, Swift L, et al. The SUMMIT trial: a field comparison of buprenorphine versus methadone maintenance treatment. J Subst Abuse Treat. 2010;39:340-352.
- Marsch LA. The efficacy of methadone maintenance interventions in reducing illicit opiate use HIV risk behavior and criminality: a meta-analysis. Addiction. 1998;93:515-532.
- Sees KL, Delucchi KL, Masson C, et al. Methadone maintenance vs 180-day psychosocially enriched detoxification for treatment of opioid dependence: a randomized controlled trial. JAMA. 2000;283:1303-1310.
- Clausen T, Anchersen K, Waal H. Mortality prior to during and after opioid maintenance treatment (OMT): a national prospective cross-registry study. Drug Alcohol Depend. 2008;94:151-157.
- Clark RE, Samnaliev M, Baxter JD, et al. The evidence doesn’t justify steps by state Medicaid programs to restrict opioid addiction treatment with buprenorphine. Health Affairs. 2011;30:1425-1433.
- Center for Substance Abuse Treatment. Clinical Guidelines for the Use of Buprenorphine in the Treatment of Opioid Addiction: Treatment Improvement Protocol (TIP) Series 40. Rockville Md: Substance Abuse and Mental Health Services Administration; 2004.
- Fudala PJ, Bridge TP, Herbert S, et al. Office-based treatment of opiate addiction with a sublingual-tablet formulation of buprenorphine and naloxone. N Engl J Med. 2003;349:949-958.
- Fiellin DA. Buprenorphine: effective treatment of opioid addiction starts in the office. Am Fam Physician. 2006;73:1513.-
- Minozzi S, Amato L, Vecchi S, et al. Oral naltrexone maintainance treatment for opioid dependence. Cochrane Database Syst Rev. 2011;(4):CD001333.-
- Krupitsky E, Nunes EV, Ling W, et al. Injectable extended-release naltrexone for opioid dependence: a double-blind, placebo-controlled, multicentre randomised trial. Lancet. 2011;377:1506-1513.
Easing the burden of premenstrual dysphoric disorder
• Consider low doses of selective serotonin reuptake inhibitors such as fluoxetine, sertraline, or paroxetine as first-line therapy for premenstrual dysphoric disorder. A
• Consider other treatment options, including diet and lifestyle changes and hormonal therapy. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
CASE Carol J, 25, comes to your office and tells you that for the past year, she has been suffering from severe discomfort during her menstrual periods. She says that shortly before her period begins, she experiences headaches, breast tenderness, pain in her joints, and a “bloated feeling.” She has trouble sleeping and feels irritable and “on edge.” She is often too depressed or tired to go to work or go out with her friends. Although the symptoms go away within a few days after her period starts, Ms. J is miserable while they last and worried about the toll they are taking on her job and her relationships. How would you address Ms. J’s complaints?
Premenstrual dysphoric disorder (PMDD), the most severe type of premenstrual syndrome (PMS), is a chronic debilitating condition characterized by a constellation of somatic and behavioral symptoms that cause significant functional impairment and greatly diminish quality of life.1 Although PMDD has a prevalence of only 3% to 8%, compared with a prevalence as high as 75% for PMS, it carries a substantial health and economic burden.1,2 Diagnosing and managing it can present a clinical challenge.1
The diagnostic criteria for PMDD outlined in the Diagnostic and Statistical Manual of Mental Disorders (DSM-IV-TR), which classifies the condition as a depressive disorder not otherwise specified, help to differentiate PMDD from PMS.3 The diagnosis requires regular occurrence of at least 5 of 11 mood and physical symptoms during the last week of the luteal phase of the menstrual cycle and remission of symptoms within 4 days of the onset of menses (TABLE 1).3 One of the 5 symptoms must be a mood symptom: depression, anxiety, mood lability, or irritability.4
TABLE 1
Research criteria for premenstrual dysphoric disorder
|
| Source: American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders DSM-IV-TR. 4th ed. text revision. 2000.3 |
What causes PMDD?
Although the etiology of PMDD is unknown, neuroendocrine and psychological factors have been implicated. Because PMDD is cyclical, the cyclic fluctuations of normal ovarian function, not hormonal imbalance, are thought to trigger PMDD-related biochemical events within the central nervous system and other target tissues.5,6
Serotonergic dysregulation. Women with PMDD have increased sensitivity to central nervous system neurotransmitters, including serotonin, which is down-regulated by the cyclical change of the ovarian hormones estradiol and progesterone.7,8 Several clinical studies have shown strong correlation between serotonergic dysfunction and PMDD based on the proven efficacy of selective serotonin reuptake inhibitors (SSRIs) in controlling symptoms. A randomized, double-blind, placebo-controlled trial conducted at 47 outpatient centers in the United States and Canada is one of many studies that implicate serotonergic down-regulation by demonstrating that PMDD responds to the SSRI paroxetine.9
Gamma aminobutyric acid (GABA), the inhibitory neurotransmitter, also plays a role in PMDD. Low levels of GABA diminish its inhibitory effect, resulting in mood disorders. A small clinical trial comparing plasma GABA levels in healthy women and women with PMDD with and without major depression demonstrated this effect.10 The study found that plasma GABA levels decreased from the midfollicular to the late luteal phase in women with PMDD, whereas they increased in healthy women; in women with PMDD and depression, GABA levels were low during both phases.
Allopregnanolone, a metabolite of progesterone, produces anxiolytic, antiseizure, anesthetic, sedative, and hypnotic activity by enhancing GABA type A receptor-meditated inhibitory responses.11,12 Lower levels of allopregnanolone during periods of altered central nervous system excitability, such as stress or ovulation, reduce the inhibitory effect of GABA and lead to irritability, insomnia, tension, and depression. Clinical studies have shown a positive correlation between the severity of PMDD and lower levels of allopregnanolone during the luteal phase.13
Hypothalamic-pituitary-adrenal (HPA) axis. PMDD is associated with the dysregulation of the HPA axis, as demonstrated by a comparative study that found a blunted response to adrenocorticotropic hormone and cortisol in the luteal phase after treadmill exercise stress testing in women with PMDD compared with non-PMDD women.14 The study provides strong evidence for dysregulation of the HPA axis in response to stress in women with PMDD.14,15
Psychological factors. Although insufficient data are available to explain the role and impact of psychological stress in the pathogenesis of PMDD, several studies show that stress exacerbates PMDD.16-18 Girdler and colleagues demonstrated that stressful life events such as sexual and physical abuse may be important determinants of PMDD with their finding that women with PMDD who had a history of abuse scored higher than controls on the Beck Depression Inventory and State-Trait Anxiety Inventory during the luteal phase.19
Do the symptoms interfere with relationships and work?
PMDD is diagnosed using the DSM-IV-TR criteria described previously.3 The symptoms must be severe enough to cause psychosocial impairment that interferes with relationships and social functioning at work, school, or other activities, and they should not be merely an exacerbation of another disorder.3
No objective diagnostic tests are available. Diagnosis depends on a thorough history and physical examination and exclusion of other conditions, including thyroid disorders, migraines, chronic fatigue syndrome, irritable bowel syndrome, seizures, anemia, endometriosis, perimenopause, and drug and alcohol abuse.2 Laboratory tests should be ordered as clinically indicated and may include chemistry studies to assess electrolyte disturbances, a complete blood count to rule out anemia, and measurement of thyroid-stimulating hormone to rule out thyroid disease.
PMDD may coexist with psychiatric illness, particularly depression and anxiety disorders, and also dysthymia, panic disorders, bipolar disorders, and personality disorders.4,20 The lifetime incidence of psychiatric conditions in women diagnosed with PMDD is 50% to 75%.21 Take care to differentiate PMDD from premenstrual exacerbations of chronic psychiatric illness.20
Patients must prospectively record daily symptoms for at least 2 menstrual cycles to provide information about the severity and timing of symptoms.3 Standardized daily symptom calendars, such as the Calendar of Premenstrual Experiences and the Prospective Record of the Impact and Severity of Menstruation, are available to help differentiate luteal from nonluteal phase symptoms.22,23 The Premenstrual Symptoms Screening Tool, or PSST, a simpler, more user-friendly tool developed by researchers at McMaster University in Canada, has been validated against prospective daily charting in some countries (TABLE 2).24
TABLE 2
Premenstrual Symptoms Screening Tool
| Please mark an “X” in the appropriate box. | |||||
| Do you experience some or any of the following premenstrual symptoms which start before your period and stop within a few days of bleeding? | |||||
| Symptom | Not at all | Mild | Moderate | Severe | |
| 1. | Anger/irritability | ||||
| 2. | Anxiety/tension | ||||
| 3. | Tearful/increased sensitivity to rejection | ||||
| 4. | Depressed mood/hopelessness | ||||
| 5. | Decreased interest in work activities | ||||
| 6. | Decreased interest in home activities | ||||
| 7. | Decreased interest in social activities | ||||
| 8. | Difficulty concentrating | ||||
| 9. | Fatigue/lack of energy | ||||
| 10. | Overeating/food cravings | ||||
| 11. | Insomnia | ||||
| 12. | Hypersomnia (needing more sleep) | ||||
| 13. | Feeling overwhelmed or out of control | ||||
| 14. | Physical sypmptoms: breast tenderness, headaches, joint/muscle pain, bloating, weight gain | ||||
| Have your symptoms, as listed above, interfered with: | |||||
| Not at all | Mild | Moderate | Severe | ||
| A. | Your work efficiency or productivity | ||||
| B. | Your relationships with co-workers | ||||
| C. | Your relationships with your family | ||||
| D. | Your social life activities | ||||
| E. | Your home responsibilities | ||||
| Scoring: The following criteria must be present for a diagnosis of premenstrual dysphoric disorder: At least 1 of items 1-4 must be severe At least 4 of items 1-14 must be moderate to severe At least 1 of items A-E must be severe. Reproduced with permission from Springer Science+Business Media. Steiner M, Macdougall M, Brown E. The premenstrual symptoms screening tool (PSST) for clinicians. Arch Womens Ment Health. 2003;6:203-209, Appendix 1. | |||||
What are the treatment options?
Therapy for PMDD is highly individualized and should target well-defined symptoms.11,25 Treatment should begin with conservative management.2,26 Conservative measures, such as diet and lifestyle modification, are considered first-line treatment, although supporting evidence is scarce.11 Carbohydrate-rich foods such as brown rice and pasta and protein-poor diets have been found to alleviate symptoms, as has exercise.26
Consider antidepressants and hormonal therapy
SSRIs are the only class of antidepressants approved by the US Food and Drug Administration (FDA) for treatment of PMDD and are considered first-line pharmacologic therapy, although they have only about a 60% response rate.9,11,27,28 Within this class, fluoxetine, sertraline, and paroxetine are FDA-approved for PMDD.6,11,21,27
SSRIs have proven effective at low doses and produce a faster response time in treating PMDD—within 1 or 2 days of onset—than depression (2-4 weeks).6 Continuous and intermittent treatment are both effective.29,30 However, continuous dosing yields a higher response rate, with 85% to 90% of patients experiencing improvement.22,31 The symptoms that respond best to continuous dosing are irritability, mood swings, and affect lability.22 Lack of response to an SSRI after 2 menstrual cycles constitutes treatment failure.8
Another antidepressant that has been found effective compared with placebo is venlafaxine, a serotonin and noradrenaline reuptake inhibitor with a quick response time.32 TABLE 3 lists antidepressants used to treat PMDD.32-34
TABLE 3
SSRIs used to treat PMDD32-34
| Fluoxetine* | 10-20 mg |
| Sertraline* | 25-50 mg |
| Paroxetine CR* | 12.5-25 mg |
| Escitalopram | 20 mg |
| Venlafaxine | 50-200 mg |
| PMDD, premenstrual dysphoric disorder; SSRI, selective serotonin reuptake inhibitor. *FDA-approved for premenstrual dysphoric disorder. | |
The anxiolytic alprazolam, a GABA agonist,35 is also effective for PMDD but is considered second-line therapy because of its adverse effects (drowsiness and confusion) and potential for dependence.2,26
Hormonal therapy. Low-dose progestin oral contraceptives provide some relief of PMDD, although definitive evidence is lacking to support using progesterone to manage the disorder.36 A combination oral contraceptive pill (OCP) containing 3 mg of the progestin drospirenone and 20 mcg of ethinyl estradiol has proved beneficial in treating the bloating, food cravings, breast tenderness, and mood swings of PMDD because of drospirenone’s antimineralocorticoid and antiandrogenic activity.36-38 The OCP is taken in a 24/4 regimen (24 days of active medication with a 4-day hormone-free interval). It provides adequate blood levels of estrogen and progesterone to suppress gonadotropins at the beginning of the active medication cycle while the shortened hormone-free interval helps reduce symptoms.36-38
Gonadotropin-releasing hormone (GnRH) agonists such as leuprolide relieve symptoms of PMDD by decreasing secretion of both follicle-stimulating hormone and luteinizing hormone, inducing anovulation and amenorrhea.27 GnRH agonists also induce menopausal symptoms such as hot flashes, fatigue, irritability, vaginal dryness, osteopenia, and cardiac problems and are not recommended for prolonged use.27,38
Like GnRH, danazol, a derivative of the synthetic modified testosterone ethisterone (pregneninolone), relieves PMDD symptoms by suppressing the HPA axis and causing anovulation. Its adverse effects may include acne, increased facial hair, weight gain, and depression, however.38 GnRH and danazol are usually used as a last resort because of their adverse effects and significant cost.
Consider complementary therapy, as well
Nutritional and herbal supplements have been shown to be useful for relieving PMDD, but more evidence is needed to support their benefit.39 TABLE 4 describes nutritional and herbal supplements used to treat PMDD.27, 39-44
TABLE 4
How strong is the evidence for nutritional and herbal supplements for premenstrual dysphoric disorder?
| Nutritional supplements | Daily dose | Symptom(s) | Efficacy (SOR) | Adverse effects |
|---|---|---|---|---|
| Calcium27, 41 | 1200 mg | Pain, fatigue, depression, insomnia, bloating, food cravings | Yes (A) | Kidney stones with doses >2500 mg20 |
| Magnesium40,42 | 200-400 mg | Bloating, mood changes, pain | Possibly yes (B) | Diarrhea |
| Vitamin B640,43 | 50-100 mg | Depression, overall symptoms | Possibly yes (B) | Peripheral neuropathy >100 mg/d |
| Vitamin E40 | 400 IU | Mastalgia | Possibly yes (B) | Bleeding (hemorrhagic stroke), nausea, fatigue |
| Herbal supplements | ||||
| Chasteberry (Vitex agnus-castus)39,40,44 | 20 mg | Overall symptoms | Possibly yes (B) | Mild skin rash, acne, headache, gastrointestinal symptoms26 |
| Ginkgo biloba40,42 | 160 mg in 2 80-mg doses | Mastalgia, bloating, mood changes | Possibly yes (B) | Increased risk of bleeding |
| St. John’s wort40,42 | 900 mg in 3 300-mg doses | Mood changes | Possibly yes (B) | Photosensitivity |
| Evening primrose oil40 | 2-3 g | Overall symptoms | Possibly no (B) | None |
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Acupuncture, which is widely used in the United States according to the National Institutes of Health Consensus Conference,45 also shows promise for alleviating PMDD symptoms. A review article discussing the use of acupuncture in a woman who met 8 of the 11 DMV-IV-TR diagnostic criteria for PMDD reported that during menstrual cycles in which the woman was treated with acupuncture the number of symptoms, as recorded by the patient on the Menstrual Distress Questionnaire, decreased from 8 to between 3 and 5.46 When treatment was stopped, the number of symptoms returned to 8, then decreased to between 2 and 5 when acupuncture resumed.
CASE A thorough history and physical examination suggest that Ms. J’s symptoms are caused by PMDD uncomplicated by psychiatric illness. You ask her to record her symptoms for 2 menstrual cycles. You also suggest that she walk or engage in other regular exercise and eat more carbohydrates, such as brown rice and pasta, and less meat and other protein because doing these things may help her feel better in the meantime. When these measures fail to provide significant relief, you prescribe fluoxetine, 10 mg per day. Ms. J reports feeling markedly better at her next menstrual period.
CORRESPONDENCE
Folashade Omole, MD, 1513 East Cleveland Avenue, Building 100, Suite 300-A, East Point, GA 30344; fomole@msm.edu
1. Mishell DR, Jr. Premenstrual disorders: epidemiology and disease burden. Am J Manag Care. 2005;11(suppl 16):S473-S479.
2. Kaur G, Gonsalves L, Thacker HL. Premenstrual dysphoric disorder: a review for the treating practitioner. Cleve Clin J Med. 2004;71:303-305,312–313, 317–318.
3. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders DSM-IV-TR. 4th ed. text revision. Washington, DC: American Psychiatric Association; 2000.
4. Kim DR, Gyulai L, Freeman EW, et al. Premenstrual dysphoric disorder and psychiatric co-morbidity. Arch Womens Ment Health. 2004;7:37-47.
5. Ling FW. Recognizing and treating premenstrual dysphoric disorder in the obstetric, gynecologic, and primary care practices. J Clin Psychiatry. 2000;61(suppl 12):S9-S16.
6. Steiner M, Pearlstein T. Premenstrual dysphoria and the serotonin system: pathophysiology and treatment. J Clin Psychiatry. 2000;61(suppl 12):S17-S21.
7. Freeman EW, Sondheimer SJ. Premenstrual dysphoric disorder: recognition and treatment. Prim Care Companion J Clin Psychiatry. 2003;5:30-39.
8. Silber TJ, Valadez-Meltzer A. Premenstrual dysphoric disorder in adolescents: case reports of treatment with fluoxetine and review of the literature. J Adolesc Health. 2005;37:518-525.
9. Pearlstein TB, Bellew KM, Endicott J, et al. Paroxetine controlled release for premenstrual dysphoric disorder: remission analysis following a randomized, double-blind, placebo-controlled trial. Prim Care Companion J Clin Psychiatry. 2005;7:53-60.
10. Halbreich U, Petty F, Yonkers K, et al. Low plasma gamma-aminobutyric acid levels during the late luteal phase of women with premenstrual dysphoric disorder. Am J Psychiatry. 1996;153:718-720.
11. Jarvis CI, Lynch AM, Morin AK. Management strategies for premenstrual syndrome/premenstrual dysphoric disorder. Ann Pharmacother. 2008;42:967-978.
12. Kaura V, Ingram CD, Gartside SE, et al. The progesterone metabolite allopregnanolone potentiates GABA(A) receptor-mediated inhibition of 5-HT neuronal activity. Eur Neuropsychopharmacol. 2007;17:108-115.
13. Girdler SS, Straneva PA, Light KC, et al. Allopregnanolone levels and reactivity to mental stress in premenstrual dysphoric disorder. Biol Psychiatry. 2001;49:788-797.
14. Klatzkin RR, Lindgren ME, Forneris CA, et al. Histories of major depression and premenstrual dysphoric disorder: Evidence for phenotypic differences. Biol Psychol. 2010;84:235-247.
15. Girdler SS, Klatzkin R. Neurosteroids in the context of stress: implications for depressive disorders. Pharmacol Ther. 2007;116:125-139.
16. Perkonigg A, Yonkers KA, Pfister H, et al. Risk factors for premenstrual dysphoric disorder in a community sample of young women: the role of traumatic events and posttraumatic stress disorder. J Clin Psychiatry. 2004;65:1314-1322.
17. Tschudin S, Bertea PC, Zemp E. Prevalence and predictors of premenstrual syndrome and premenstrual dysphoric disorder in a population-based sample. Arch Womens Ment Health. 2010;13:485-494.
18. Girdler SS, Leserman J, Bunevicius R, et al. Persistent alterations in biological profiles in women with abuse histories: influence of premenstrual dysphoric disorder. Health Psychol. 2007;26:201-213.
19. Girdler SS, Sherwood A, Hinderliter AL, et al. Biological correlates of abuse in women with premenstrual dysphoric disorder and healthy controls. Psychosom Med. 2003;65:849-856.
20. Miyaoka Y, Akimoto Y, Ueda K, et al. Fulfillment of the premenstrual dysphoric disorder criteria confirmed using a self-rating questionnaire among Japanese women with depressive disorders. Biopsychosoc Med. 2011;5:5.-
21. Rapkin A, Winer S. Premenstrual syndrome and premenstrual dysphoric disorder: quality of life and burden of illness. Expert Rev Pharmacoecon Outcomes Res. 2009;9:157-170.
22. Cunningham J, Yonkers KA, O’Brien S, et al. Update on research and treatment of premenstrual dysphoric disorder. Harv Rev Psychiatry. 2009;17:120-137.
23. Dickerson LM, Hunter MH. Premenstrual syndrome. Am Fam Physician. 2003;67:1743-1752.
24. Steiner M, Macdougall M, Brown E. The premenstrual symptoms screening tool (PSST) for clinicians. Arch Womens Ment Health. 2003;6:203-209.
25. Halbreich U, O’Brien PM, Eriksson E, et al. Are there differential symptom profiles that improve in response to different pharmacological treatments of premenstrual syndrome/premenstrual dysphoric disorder? CNS Drugs. 2006;20:523-547.
26. Bianchi-Demicheli F. Premenstrual dysphoric disorder: diagnosis and therapeutic strategy. Rev Med Suisse. 2006;2:393-394,397–399.
27. Htay TT, Carrick J, Papiaca R. Premenstrual dysphoric disorder 2009. Available at: http://emedicine.medscape.com/article/293257-clinical. Accessed May 25, 2011.
28. Halbreich U. Selective serotonin reuptake inhibitors and initial oral contraceptives for the treatment of PMDD: effective but not enough. CNS Spectr. 2008;13:566-572.
29. Brown J, Marjoribanks J, Wyatt K. Selective serotonin reuptake inhibitors for premenstrual syndrome. Cochrane Database Syst Rev. 2009;(2):CD001396.-
30. Freeman EW. Effects of antidepressants on quality of life in women with premenstrual dysphoric disorder. Pharmacoeconomics. 2005;23:433-444.
31. Landen M, Nissbrandt H, Allgulander C, et al. Placebo-controlled trial comparing intermittent and continuous paroxetine in premenstrual dysphoric disorder. Neuropsychopharmacology. 2007;32:153-161.
32. Freeman EW, Rickels K, Yonkers KA, et al. Venlafaxine in the treatment of premenstrual dysphoric disorder. Obstet Gynecol. 2001;98:737-744.
33. Kroll R, Rapkin AJ. Treatment of premenstrual disorders. J Reprod Med. 2006;51(suppl 4):S359-S370.
34. Eriksson E, Ekman A, Sinclair S, et al. Escitalopram administered in the luteal phase exerts a marked and dose-dependent effect in premenstrual dysphoric disorder. J Clin Psychopharmacol. 2008;28:195-202.
35. Halbreich U. The pathophysiologic background for current treatments of premenstrual syndromes. Curr Psychiatry Rep. 2002;4:429-434.
36. Wyatt KM, Dimmock PW, Frischer M, et al. Prescribing patterns in premenstrual syndrome. BMC Womens Health. 2002;2:4.-
37. Rapkin AJ. YAZ in the treatment of premenstrual dysphoric disorder. J Reprod Med. 2008;53(suppl 9):S729-S741.
38. Elliott H. Premenstrual dysphoric disorder. A guide for the treating clinician. NC Med J. 2002;63:72-75.
39. Dante G, Facchinetti F. Herbal treatments for alleviating premenstrual symptoms: a systematic review. J Psychosom Obstet Gynaecol. 2011;32:42-51.
40. Whelan AM, Jurgens TM, Naylor H. Herbs, vitamins and minerals in the treatment of premenstrual syndrome: a systematic review. Can J Clin Pharmacol. 2009;16:e407-e429.
41. Pearlstein T, Steiner M. Non-antidepressant treatment of premenstrual syndrome. J Clin Psychiatry. 2000;61(suppl 12):22-27.
42. Girman A, Lee R, Kligler B. An integrative medicine approach to premenstrual syndrome. Am J Obstet Gynecol. 2003;188(suppl 5):S56-S65.
43. Wyatt KM, Dimmock PW, Jones PW, et al. Efficacy of vitamin B6 in the treatment of premenstrual syndrome: systematic review. BMJ. 1999;318:1375-1381.
44. Freeman EW. Therapeutic management of premenstrual syndrome. Expert Opin Pharmacother. 2010;11:2879-2889.
45. NIH Consensus Conference. Acupuncture. JAMA. 1998;280:1518-1524.
46. Taguchi R, Yoshimoto S, Imai K, et al. Acupuncture for premenstrual dysphoric disorder. Arch Gynecol Obstet. 2009;280:877-881.
• Consider low doses of selective serotonin reuptake inhibitors such as fluoxetine, sertraline, or paroxetine as first-line therapy for premenstrual dysphoric disorder. A
• Consider other treatment options, including diet and lifestyle changes and hormonal therapy. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
CASE Carol J, 25, comes to your office and tells you that for the past year, she has been suffering from severe discomfort during her menstrual periods. She says that shortly before her period begins, she experiences headaches, breast tenderness, pain in her joints, and a “bloated feeling.” She has trouble sleeping and feels irritable and “on edge.” She is often too depressed or tired to go to work or go out with her friends. Although the symptoms go away within a few days after her period starts, Ms. J is miserable while they last and worried about the toll they are taking on her job and her relationships. How would you address Ms. J’s complaints?
Premenstrual dysphoric disorder (PMDD), the most severe type of premenstrual syndrome (PMS), is a chronic debilitating condition characterized by a constellation of somatic and behavioral symptoms that cause significant functional impairment and greatly diminish quality of life.1 Although PMDD has a prevalence of only 3% to 8%, compared with a prevalence as high as 75% for PMS, it carries a substantial health and economic burden.1,2 Diagnosing and managing it can present a clinical challenge.1
The diagnostic criteria for PMDD outlined in the Diagnostic and Statistical Manual of Mental Disorders (DSM-IV-TR), which classifies the condition as a depressive disorder not otherwise specified, help to differentiate PMDD from PMS.3 The diagnosis requires regular occurrence of at least 5 of 11 mood and physical symptoms during the last week of the luteal phase of the menstrual cycle and remission of symptoms within 4 days of the onset of menses (TABLE 1).3 One of the 5 symptoms must be a mood symptom: depression, anxiety, mood lability, or irritability.4
TABLE 1
Research criteria for premenstrual dysphoric disorder
|
| Source: American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders DSM-IV-TR. 4th ed. text revision. 2000.3 |
What causes PMDD?
Although the etiology of PMDD is unknown, neuroendocrine and psychological factors have been implicated. Because PMDD is cyclical, the cyclic fluctuations of normal ovarian function, not hormonal imbalance, are thought to trigger PMDD-related biochemical events within the central nervous system and other target tissues.5,6
Serotonergic dysregulation. Women with PMDD have increased sensitivity to central nervous system neurotransmitters, including serotonin, which is down-regulated by the cyclical change of the ovarian hormones estradiol and progesterone.7,8 Several clinical studies have shown strong correlation between serotonergic dysfunction and PMDD based on the proven efficacy of selective serotonin reuptake inhibitors (SSRIs) in controlling symptoms. A randomized, double-blind, placebo-controlled trial conducted at 47 outpatient centers in the United States and Canada is one of many studies that implicate serotonergic down-regulation by demonstrating that PMDD responds to the SSRI paroxetine.9
Gamma aminobutyric acid (GABA), the inhibitory neurotransmitter, also plays a role in PMDD. Low levels of GABA diminish its inhibitory effect, resulting in mood disorders. A small clinical trial comparing plasma GABA levels in healthy women and women with PMDD with and without major depression demonstrated this effect.10 The study found that plasma GABA levels decreased from the midfollicular to the late luteal phase in women with PMDD, whereas they increased in healthy women; in women with PMDD and depression, GABA levels were low during both phases.
Allopregnanolone, a metabolite of progesterone, produces anxiolytic, antiseizure, anesthetic, sedative, and hypnotic activity by enhancing GABA type A receptor-meditated inhibitory responses.11,12 Lower levels of allopregnanolone during periods of altered central nervous system excitability, such as stress or ovulation, reduce the inhibitory effect of GABA and lead to irritability, insomnia, tension, and depression. Clinical studies have shown a positive correlation between the severity of PMDD and lower levels of allopregnanolone during the luteal phase.13
Hypothalamic-pituitary-adrenal (HPA) axis. PMDD is associated with the dysregulation of the HPA axis, as demonstrated by a comparative study that found a blunted response to adrenocorticotropic hormone and cortisol in the luteal phase after treadmill exercise stress testing in women with PMDD compared with non-PMDD women.14 The study provides strong evidence for dysregulation of the HPA axis in response to stress in women with PMDD.14,15
Psychological factors. Although insufficient data are available to explain the role and impact of psychological stress in the pathogenesis of PMDD, several studies show that stress exacerbates PMDD.16-18 Girdler and colleagues demonstrated that stressful life events such as sexual and physical abuse may be important determinants of PMDD with their finding that women with PMDD who had a history of abuse scored higher than controls on the Beck Depression Inventory and State-Trait Anxiety Inventory during the luteal phase.19
Do the symptoms interfere with relationships and work?
PMDD is diagnosed using the DSM-IV-TR criteria described previously.3 The symptoms must be severe enough to cause psychosocial impairment that interferes with relationships and social functioning at work, school, or other activities, and they should not be merely an exacerbation of another disorder.3
No objective diagnostic tests are available. Diagnosis depends on a thorough history and physical examination and exclusion of other conditions, including thyroid disorders, migraines, chronic fatigue syndrome, irritable bowel syndrome, seizures, anemia, endometriosis, perimenopause, and drug and alcohol abuse.2 Laboratory tests should be ordered as clinically indicated and may include chemistry studies to assess electrolyte disturbances, a complete blood count to rule out anemia, and measurement of thyroid-stimulating hormone to rule out thyroid disease.
PMDD may coexist with psychiatric illness, particularly depression and anxiety disorders, and also dysthymia, panic disorders, bipolar disorders, and personality disorders.4,20 The lifetime incidence of psychiatric conditions in women diagnosed with PMDD is 50% to 75%.21 Take care to differentiate PMDD from premenstrual exacerbations of chronic psychiatric illness.20
Patients must prospectively record daily symptoms for at least 2 menstrual cycles to provide information about the severity and timing of symptoms.3 Standardized daily symptom calendars, such as the Calendar of Premenstrual Experiences and the Prospective Record of the Impact and Severity of Menstruation, are available to help differentiate luteal from nonluteal phase symptoms.22,23 The Premenstrual Symptoms Screening Tool, or PSST, a simpler, more user-friendly tool developed by researchers at McMaster University in Canada, has been validated against prospective daily charting in some countries (TABLE 2).24
TABLE 2
Premenstrual Symptoms Screening Tool
| Please mark an “X” in the appropriate box. | |||||
| Do you experience some or any of the following premenstrual symptoms which start before your period and stop within a few days of bleeding? | |||||
| Symptom | Not at all | Mild | Moderate | Severe | |
| 1. | Anger/irritability | ||||
| 2. | Anxiety/tension | ||||
| 3. | Tearful/increased sensitivity to rejection | ||||
| 4. | Depressed mood/hopelessness | ||||
| 5. | Decreased interest in work activities | ||||
| 6. | Decreased interest in home activities | ||||
| 7. | Decreased interest in social activities | ||||
| 8. | Difficulty concentrating | ||||
| 9. | Fatigue/lack of energy | ||||
| 10. | Overeating/food cravings | ||||
| 11. | Insomnia | ||||
| 12. | Hypersomnia (needing more sleep) | ||||
| 13. | Feeling overwhelmed or out of control | ||||
| 14. | Physical sypmptoms: breast tenderness, headaches, joint/muscle pain, bloating, weight gain | ||||
| Have your symptoms, as listed above, interfered with: | |||||
| Not at all | Mild | Moderate | Severe | ||
| A. | Your work efficiency or productivity | ||||
| B. | Your relationships with co-workers | ||||
| C. | Your relationships with your family | ||||
| D. | Your social life activities | ||||
| E. | Your home responsibilities | ||||
| Scoring: The following criteria must be present for a diagnosis of premenstrual dysphoric disorder: At least 1 of items 1-4 must be severe At least 4 of items 1-14 must be moderate to severe At least 1 of items A-E must be severe. Reproduced with permission from Springer Science+Business Media. Steiner M, Macdougall M, Brown E. The premenstrual symptoms screening tool (PSST) for clinicians. Arch Womens Ment Health. 2003;6:203-209, Appendix 1. | |||||
What are the treatment options?
Therapy for PMDD is highly individualized and should target well-defined symptoms.11,25 Treatment should begin with conservative management.2,26 Conservative measures, such as diet and lifestyle modification, are considered first-line treatment, although supporting evidence is scarce.11 Carbohydrate-rich foods such as brown rice and pasta and protein-poor diets have been found to alleviate symptoms, as has exercise.26
Consider antidepressants and hormonal therapy
SSRIs are the only class of antidepressants approved by the US Food and Drug Administration (FDA) for treatment of PMDD and are considered first-line pharmacologic therapy, although they have only about a 60% response rate.9,11,27,28 Within this class, fluoxetine, sertraline, and paroxetine are FDA-approved for PMDD.6,11,21,27
SSRIs have proven effective at low doses and produce a faster response time in treating PMDD—within 1 or 2 days of onset—than depression (2-4 weeks).6 Continuous and intermittent treatment are both effective.29,30 However, continuous dosing yields a higher response rate, with 85% to 90% of patients experiencing improvement.22,31 The symptoms that respond best to continuous dosing are irritability, mood swings, and affect lability.22 Lack of response to an SSRI after 2 menstrual cycles constitutes treatment failure.8
Another antidepressant that has been found effective compared with placebo is venlafaxine, a serotonin and noradrenaline reuptake inhibitor with a quick response time.32 TABLE 3 lists antidepressants used to treat PMDD.32-34
TABLE 3
SSRIs used to treat PMDD32-34
| Fluoxetine* | 10-20 mg |
| Sertraline* | 25-50 mg |
| Paroxetine CR* | 12.5-25 mg |
| Escitalopram | 20 mg |
| Venlafaxine | 50-200 mg |
| PMDD, premenstrual dysphoric disorder; SSRI, selective serotonin reuptake inhibitor. *FDA-approved for premenstrual dysphoric disorder. | |
The anxiolytic alprazolam, a GABA agonist,35 is also effective for PMDD but is considered second-line therapy because of its adverse effects (drowsiness and confusion) and potential for dependence.2,26
Hormonal therapy. Low-dose progestin oral contraceptives provide some relief of PMDD, although definitive evidence is lacking to support using progesterone to manage the disorder.36 A combination oral contraceptive pill (OCP) containing 3 mg of the progestin drospirenone and 20 mcg of ethinyl estradiol has proved beneficial in treating the bloating, food cravings, breast tenderness, and mood swings of PMDD because of drospirenone’s antimineralocorticoid and antiandrogenic activity.36-38 The OCP is taken in a 24/4 regimen (24 days of active medication with a 4-day hormone-free interval). It provides adequate blood levels of estrogen and progesterone to suppress gonadotropins at the beginning of the active medication cycle while the shortened hormone-free interval helps reduce symptoms.36-38
Gonadotropin-releasing hormone (GnRH) agonists such as leuprolide relieve symptoms of PMDD by decreasing secretion of both follicle-stimulating hormone and luteinizing hormone, inducing anovulation and amenorrhea.27 GnRH agonists also induce menopausal symptoms such as hot flashes, fatigue, irritability, vaginal dryness, osteopenia, and cardiac problems and are not recommended for prolonged use.27,38
Like GnRH, danazol, a derivative of the synthetic modified testosterone ethisterone (pregneninolone), relieves PMDD symptoms by suppressing the HPA axis and causing anovulation. Its adverse effects may include acne, increased facial hair, weight gain, and depression, however.38 GnRH and danazol are usually used as a last resort because of their adverse effects and significant cost.
Consider complementary therapy, as well
Nutritional and herbal supplements have been shown to be useful for relieving PMDD, but more evidence is needed to support their benefit.39 TABLE 4 describes nutritional and herbal supplements used to treat PMDD.27, 39-44
TABLE 4
How strong is the evidence for nutritional and herbal supplements for premenstrual dysphoric disorder?
| Nutritional supplements | Daily dose | Symptom(s) | Efficacy (SOR) | Adverse effects |
|---|---|---|---|---|
| Calcium27, 41 | 1200 mg | Pain, fatigue, depression, insomnia, bloating, food cravings | Yes (A) | Kidney stones with doses >2500 mg20 |
| Magnesium40,42 | 200-400 mg | Bloating, mood changes, pain | Possibly yes (B) | Diarrhea |
| Vitamin B640,43 | 50-100 mg | Depression, overall symptoms | Possibly yes (B) | Peripheral neuropathy >100 mg/d |
| Vitamin E40 | 400 IU | Mastalgia | Possibly yes (B) | Bleeding (hemorrhagic stroke), nausea, fatigue |
| Herbal supplements | ||||
| Chasteberry (Vitex agnus-castus)39,40,44 | 20 mg | Overall symptoms | Possibly yes (B) | Mild skin rash, acne, headache, gastrointestinal symptoms26 |
| Ginkgo biloba40,42 | 160 mg in 2 80-mg doses | Mastalgia, bloating, mood changes | Possibly yes (B) | Increased risk of bleeding |
| St. John’s wort40,42 | 900 mg in 3 300-mg doses | Mood changes | Possibly yes (B) | Photosensitivity |
| Evening primrose oil40 | 2-3 g | Overall symptoms | Possibly no (B) | None |
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Acupuncture, which is widely used in the United States according to the National Institutes of Health Consensus Conference,45 also shows promise for alleviating PMDD symptoms. A review article discussing the use of acupuncture in a woman who met 8 of the 11 DMV-IV-TR diagnostic criteria for PMDD reported that during menstrual cycles in which the woman was treated with acupuncture the number of symptoms, as recorded by the patient on the Menstrual Distress Questionnaire, decreased from 8 to between 3 and 5.46 When treatment was stopped, the number of symptoms returned to 8, then decreased to between 2 and 5 when acupuncture resumed.
CASE A thorough history and physical examination suggest that Ms. J’s symptoms are caused by PMDD uncomplicated by psychiatric illness. You ask her to record her symptoms for 2 menstrual cycles. You also suggest that she walk or engage in other regular exercise and eat more carbohydrates, such as brown rice and pasta, and less meat and other protein because doing these things may help her feel better in the meantime. When these measures fail to provide significant relief, you prescribe fluoxetine, 10 mg per day. Ms. J reports feeling markedly better at her next menstrual period.
CORRESPONDENCE
Folashade Omole, MD, 1513 East Cleveland Avenue, Building 100, Suite 300-A, East Point, GA 30344; fomole@msm.edu
• Consider low doses of selective serotonin reuptake inhibitors such as fluoxetine, sertraline, or paroxetine as first-line therapy for premenstrual dysphoric disorder. A
• Consider other treatment options, including diet and lifestyle changes and hormonal therapy. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
CASE Carol J, 25, comes to your office and tells you that for the past year, she has been suffering from severe discomfort during her menstrual periods. She says that shortly before her period begins, she experiences headaches, breast tenderness, pain in her joints, and a “bloated feeling.” She has trouble sleeping and feels irritable and “on edge.” She is often too depressed or tired to go to work or go out with her friends. Although the symptoms go away within a few days after her period starts, Ms. J is miserable while they last and worried about the toll they are taking on her job and her relationships. How would you address Ms. J’s complaints?
Premenstrual dysphoric disorder (PMDD), the most severe type of premenstrual syndrome (PMS), is a chronic debilitating condition characterized by a constellation of somatic and behavioral symptoms that cause significant functional impairment and greatly diminish quality of life.1 Although PMDD has a prevalence of only 3% to 8%, compared with a prevalence as high as 75% for PMS, it carries a substantial health and economic burden.1,2 Diagnosing and managing it can present a clinical challenge.1
The diagnostic criteria for PMDD outlined in the Diagnostic and Statistical Manual of Mental Disorders (DSM-IV-TR), which classifies the condition as a depressive disorder not otherwise specified, help to differentiate PMDD from PMS.3 The diagnosis requires regular occurrence of at least 5 of 11 mood and physical symptoms during the last week of the luteal phase of the menstrual cycle and remission of symptoms within 4 days of the onset of menses (TABLE 1).3 One of the 5 symptoms must be a mood symptom: depression, anxiety, mood lability, or irritability.4
TABLE 1
Research criteria for premenstrual dysphoric disorder
|
| Source: American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders DSM-IV-TR. 4th ed. text revision. 2000.3 |
What causes PMDD?
Although the etiology of PMDD is unknown, neuroendocrine and psychological factors have been implicated. Because PMDD is cyclical, the cyclic fluctuations of normal ovarian function, not hormonal imbalance, are thought to trigger PMDD-related biochemical events within the central nervous system and other target tissues.5,6
Serotonergic dysregulation. Women with PMDD have increased sensitivity to central nervous system neurotransmitters, including serotonin, which is down-regulated by the cyclical change of the ovarian hormones estradiol and progesterone.7,8 Several clinical studies have shown strong correlation between serotonergic dysfunction and PMDD based on the proven efficacy of selective serotonin reuptake inhibitors (SSRIs) in controlling symptoms. A randomized, double-blind, placebo-controlled trial conducted at 47 outpatient centers in the United States and Canada is one of many studies that implicate serotonergic down-regulation by demonstrating that PMDD responds to the SSRI paroxetine.9
Gamma aminobutyric acid (GABA), the inhibitory neurotransmitter, also plays a role in PMDD. Low levels of GABA diminish its inhibitory effect, resulting in mood disorders. A small clinical trial comparing plasma GABA levels in healthy women and women with PMDD with and without major depression demonstrated this effect.10 The study found that plasma GABA levels decreased from the midfollicular to the late luteal phase in women with PMDD, whereas they increased in healthy women; in women with PMDD and depression, GABA levels were low during both phases.
Allopregnanolone, a metabolite of progesterone, produces anxiolytic, antiseizure, anesthetic, sedative, and hypnotic activity by enhancing GABA type A receptor-meditated inhibitory responses.11,12 Lower levels of allopregnanolone during periods of altered central nervous system excitability, such as stress or ovulation, reduce the inhibitory effect of GABA and lead to irritability, insomnia, tension, and depression. Clinical studies have shown a positive correlation between the severity of PMDD and lower levels of allopregnanolone during the luteal phase.13
Hypothalamic-pituitary-adrenal (HPA) axis. PMDD is associated with the dysregulation of the HPA axis, as demonstrated by a comparative study that found a blunted response to adrenocorticotropic hormone and cortisol in the luteal phase after treadmill exercise stress testing in women with PMDD compared with non-PMDD women.14 The study provides strong evidence for dysregulation of the HPA axis in response to stress in women with PMDD.14,15
Psychological factors. Although insufficient data are available to explain the role and impact of psychological stress in the pathogenesis of PMDD, several studies show that stress exacerbates PMDD.16-18 Girdler and colleagues demonstrated that stressful life events such as sexual and physical abuse may be important determinants of PMDD with their finding that women with PMDD who had a history of abuse scored higher than controls on the Beck Depression Inventory and State-Trait Anxiety Inventory during the luteal phase.19
Do the symptoms interfere with relationships and work?
PMDD is diagnosed using the DSM-IV-TR criteria described previously.3 The symptoms must be severe enough to cause psychosocial impairment that interferes with relationships and social functioning at work, school, or other activities, and they should not be merely an exacerbation of another disorder.3
No objective diagnostic tests are available. Diagnosis depends on a thorough history and physical examination and exclusion of other conditions, including thyroid disorders, migraines, chronic fatigue syndrome, irritable bowel syndrome, seizures, anemia, endometriosis, perimenopause, and drug and alcohol abuse.2 Laboratory tests should be ordered as clinically indicated and may include chemistry studies to assess electrolyte disturbances, a complete blood count to rule out anemia, and measurement of thyroid-stimulating hormone to rule out thyroid disease.
PMDD may coexist with psychiatric illness, particularly depression and anxiety disorders, and also dysthymia, panic disorders, bipolar disorders, and personality disorders.4,20 The lifetime incidence of psychiatric conditions in women diagnosed with PMDD is 50% to 75%.21 Take care to differentiate PMDD from premenstrual exacerbations of chronic psychiatric illness.20
Patients must prospectively record daily symptoms for at least 2 menstrual cycles to provide information about the severity and timing of symptoms.3 Standardized daily symptom calendars, such as the Calendar of Premenstrual Experiences and the Prospective Record of the Impact and Severity of Menstruation, are available to help differentiate luteal from nonluteal phase symptoms.22,23 The Premenstrual Symptoms Screening Tool, or PSST, a simpler, more user-friendly tool developed by researchers at McMaster University in Canada, has been validated against prospective daily charting in some countries (TABLE 2).24
TABLE 2
Premenstrual Symptoms Screening Tool
| Please mark an “X” in the appropriate box. | |||||
| Do you experience some or any of the following premenstrual symptoms which start before your period and stop within a few days of bleeding? | |||||
| Symptom | Not at all | Mild | Moderate | Severe | |
| 1. | Anger/irritability | ||||
| 2. | Anxiety/tension | ||||
| 3. | Tearful/increased sensitivity to rejection | ||||
| 4. | Depressed mood/hopelessness | ||||
| 5. | Decreased interest in work activities | ||||
| 6. | Decreased interest in home activities | ||||
| 7. | Decreased interest in social activities | ||||
| 8. | Difficulty concentrating | ||||
| 9. | Fatigue/lack of energy | ||||
| 10. | Overeating/food cravings | ||||
| 11. | Insomnia | ||||
| 12. | Hypersomnia (needing more sleep) | ||||
| 13. | Feeling overwhelmed or out of control | ||||
| 14. | Physical sypmptoms: breast tenderness, headaches, joint/muscle pain, bloating, weight gain | ||||
| Have your symptoms, as listed above, interfered with: | |||||
| Not at all | Mild | Moderate | Severe | ||
| A. | Your work efficiency or productivity | ||||
| B. | Your relationships with co-workers | ||||
| C. | Your relationships with your family | ||||
| D. | Your social life activities | ||||
| E. | Your home responsibilities | ||||
| Scoring: The following criteria must be present for a diagnosis of premenstrual dysphoric disorder: At least 1 of items 1-4 must be severe At least 4 of items 1-14 must be moderate to severe At least 1 of items A-E must be severe. Reproduced with permission from Springer Science+Business Media. Steiner M, Macdougall M, Brown E. The premenstrual symptoms screening tool (PSST) for clinicians. Arch Womens Ment Health. 2003;6:203-209, Appendix 1. | |||||
What are the treatment options?
Therapy for PMDD is highly individualized and should target well-defined symptoms.11,25 Treatment should begin with conservative management.2,26 Conservative measures, such as diet and lifestyle modification, are considered first-line treatment, although supporting evidence is scarce.11 Carbohydrate-rich foods such as brown rice and pasta and protein-poor diets have been found to alleviate symptoms, as has exercise.26
Consider antidepressants and hormonal therapy
SSRIs are the only class of antidepressants approved by the US Food and Drug Administration (FDA) for treatment of PMDD and are considered first-line pharmacologic therapy, although they have only about a 60% response rate.9,11,27,28 Within this class, fluoxetine, sertraline, and paroxetine are FDA-approved for PMDD.6,11,21,27
SSRIs have proven effective at low doses and produce a faster response time in treating PMDD—within 1 or 2 days of onset—than depression (2-4 weeks).6 Continuous and intermittent treatment are both effective.29,30 However, continuous dosing yields a higher response rate, with 85% to 90% of patients experiencing improvement.22,31 The symptoms that respond best to continuous dosing are irritability, mood swings, and affect lability.22 Lack of response to an SSRI after 2 menstrual cycles constitutes treatment failure.8
Another antidepressant that has been found effective compared with placebo is venlafaxine, a serotonin and noradrenaline reuptake inhibitor with a quick response time.32 TABLE 3 lists antidepressants used to treat PMDD.32-34
TABLE 3
SSRIs used to treat PMDD32-34
| Fluoxetine* | 10-20 mg |
| Sertraline* | 25-50 mg |
| Paroxetine CR* | 12.5-25 mg |
| Escitalopram | 20 mg |
| Venlafaxine | 50-200 mg |
| PMDD, premenstrual dysphoric disorder; SSRI, selective serotonin reuptake inhibitor. *FDA-approved for premenstrual dysphoric disorder. | |
The anxiolytic alprazolam, a GABA agonist,35 is also effective for PMDD but is considered second-line therapy because of its adverse effects (drowsiness and confusion) and potential for dependence.2,26
Hormonal therapy. Low-dose progestin oral contraceptives provide some relief of PMDD, although definitive evidence is lacking to support using progesterone to manage the disorder.36 A combination oral contraceptive pill (OCP) containing 3 mg of the progestin drospirenone and 20 mcg of ethinyl estradiol has proved beneficial in treating the bloating, food cravings, breast tenderness, and mood swings of PMDD because of drospirenone’s antimineralocorticoid and antiandrogenic activity.36-38 The OCP is taken in a 24/4 regimen (24 days of active medication with a 4-day hormone-free interval). It provides adequate blood levels of estrogen and progesterone to suppress gonadotropins at the beginning of the active medication cycle while the shortened hormone-free interval helps reduce symptoms.36-38
Gonadotropin-releasing hormone (GnRH) agonists such as leuprolide relieve symptoms of PMDD by decreasing secretion of both follicle-stimulating hormone and luteinizing hormone, inducing anovulation and amenorrhea.27 GnRH agonists also induce menopausal symptoms such as hot flashes, fatigue, irritability, vaginal dryness, osteopenia, and cardiac problems and are not recommended for prolonged use.27,38
Like GnRH, danazol, a derivative of the synthetic modified testosterone ethisterone (pregneninolone), relieves PMDD symptoms by suppressing the HPA axis and causing anovulation. Its adverse effects may include acne, increased facial hair, weight gain, and depression, however.38 GnRH and danazol are usually used as a last resort because of their adverse effects and significant cost.
Consider complementary therapy, as well
Nutritional and herbal supplements have been shown to be useful for relieving PMDD, but more evidence is needed to support their benefit.39 TABLE 4 describes nutritional and herbal supplements used to treat PMDD.27, 39-44
TABLE 4
How strong is the evidence for nutritional and herbal supplements for premenstrual dysphoric disorder?
| Nutritional supplements | Daily dose | Symptom(s) | Efficacy (SOR) | Adverse effects |
|---|---|---|---|---|
| Calcium27, 41 | 1200 mg | Pain, fatigue, depression, insomnia, bloating, food cravings | Yes (A) | Kidney stones with doses >2500 mg20 |
| Magnesium40,42 | 200-400 mg | Bloating, mood changes, pain | Possibly yes (B) | Diarrhea |
| Vitamin B640,43 | 50-100 mg | Depression, overall symptoms | Possibly yes (B) | Peripheral neuropathy >100 mg/d |
| Vitamin E40 | 400 IU | Mastalgia | Possibly yes (B) | Bleeding (hemorrhagic stroke), nausea, fatigue |
| Herbal supplements | ||||
| Chasteberry (Vitex agnus-castus)39,40,44 | 20 mg | Overall symptoms | Possibly yes (B) | Mild skin rash, acne, headache, gastrointestinal symptoms26 |
| Ginkgo biloba40,42 | 160 mg in 2 80-mg doses | Mastalgia, bloating, mood changes | Possibly yes (B) | Increased risk of bleeding |
| St. John’s wort40,42 | 900 mg in 3 300-mg doses | Mood changes | Possibly yes (B) | Photosensitivity |
| Evening primrose oil40 | 2-3 g | Overall symptoms | Possibly no (B) | None |
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Acupuncture, which is widely used in the United States according to the National Institutes of Health Consensus Conference,45 also shows promise for alleviating PMDD symptoms. A review article discussing the use of acupuncture in a woman who met 8 of the 11 DMV-IV-TR diagnostic criteria for PMDD reported that during menstrual cycles in which the woman was treated with acupuncture the number of symptoms, as recorded by the patient on the Menstrual Distress Questionnaire, decreased from 8 to between 3 and 5.46 When treatment was stopped, the number of symptoms returned to 8, then decreased to between 2 and 5 when acupuncture resumed.
CASE A thorough history and physical examination suggest that Ms. J’s symptoms are caused by PMDD uncomplicated by psychiatric illness. You ask her to record her symptoms for 2 menstrual cycles. You also suggest that she walk or engage in other regular exercise and eat more carbohydrates, such as brown rice and pasta, and less meat and other protein because doing these things may help her feel better in the meantime. When these measures fail to provide significant relief, you prescribe fluoxetine, 10 mg per day. Ms. J reports feeling markedly better at her next menstrual period.
CORRESPONDENCE
Folashade Omole, MD, 1513 East Cleveland Avenue, Building 100, Suite 300-A, East Point, GA 30344; fomole@msm.edu
1. Mishell DR, Jr. Premenstrual disorders: epidemiology and disease burden. Am J Manag Care. 2005;11(suppl 16):S473-S479.
2. Kaur G, Gonsalves L, Thacker HL. Premenstrual dysphoric disorder: a review for the treating practitioner. Cleve Clin J Med. 2004;71:303-305,312–313, 317–318.
3. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders DSM-IV-TR. 4th ed. text revision. Washington, DC: American Psychiatric Association; 2000.
4. Kim DR, Gyulai L, Freeman EW, et al. Premenstrual dysphoric disorder and psychiatric co-morbidity. Arch Womens Ment Health. 2004;7:37-47.
5. Ling FW. Recognizing and treating premenstrual dysphoric disorder in the obstetric, gynecologic, and primary care practices. J Clin Psychiatry. 2000;61(suppl 12):S9-S16.
6. Steiner M, Pearlstein T. Premenstrual dysphoria and the serotonin system: pathophysiology and treatment. J Clin Psychiatry. 2000;61(suppl 12):S17-S21.
7. Freeman EW, Sondheimer SJ. Premenstrual dysphoric disorder: recognition and treatment. Prim Care Companion J Clin Psychiatry. 2003;5:30-39.
8. Silber TJ, Valadez-Meltzer A. Premenstrual dysphoric disorder in adolescents: case reports of treatment with fluoxetine and review of the literature. J Adolesc Health. 2005;37:518-525.
9. Pearlstein TB, Bellew KM, Endicott J, et al. Paroxetine controlled release for premenstrual dysphoric disorder: remission analysis following a randomized, double-blind, placebo-controlled trial. Prim Care Companion J Clin Psychiatry. 2005;7:53-60.
10. Halbreich U, Petty F, Yonkers K, et al. Low plasma gamma-aminobutyric acid levels during the late luteal phase of women with premenstrual dysphoric disorder. Am J Psychiatry. 1996;153:718-720.
11. Jarvis CI, Lynch AM, Morin AK. Management strategies for premenstrual syndrome/premenstrual dysphoric disorder. Ann Pharmacother. 2008;42:967-978.
12. Kaura V, Ingram CD, Gartside SE, et al. The progesterone metabolite allopregnanolone potentiates GABA(A) receptor-mediated inhibition of 5-HT neuronal activity. Eur Neuropsychopharmacol. 2007;17:108-115.
13. Girdler SS, Straneva PA, Light KC, et al. Allopregnanolone levels and reactivity to mental stress in premenstrual dysphoric disorder. Biol Psychiatry. 2001;49:788-797.
14. Klatzkin RR, Lindgren ME, Forneris CA, et al. Histories of major depression and premenstrual dysphoric disorder: Evidence for phenotypic differences. Biol Psychol. 2010;84:235-247.
15. Girdler SS, Klatzkin R. Neurosteroids in the context of stress: implications for depressive disorders. Pharmacol Ther. 2007;116:125-139.
16. Perkonigg A, Yonkers KA, Pfister H, et al. Risk factors for premenstrual dysphoric disorder in a community sample of young women: the role of traumatic events and posttraumatic stress disorder. J Clin Psychiatry. 2004;65:1314-1322.
17. Tschudin S, Bertea PC, Zemp E. Prevalence and predictors of premenstrual syndrome and premenstrual dysphoric disorder in a population-based sample. Arch Womens Ment Health. 2010;13:485-494.
18. Girdler SS, Leserman J, Bunevicius R, et al. Persistent alterations in biological profiles in women with abuse histories: influence of premenstrual dysphoric disorder. Health Psychol. 2007;26:201-213.
19. Girdler SS, Sherwood A, Hinderliter AL, et al. Biological correlates of abuse in women with premenstrual dysphoric disorder and healthy controls. Psychosom Med. 2003;65:849-856.
20. Miyaoka Y, Akimoto Y, Ueda K, et al. Fulfillment of the premenstrual dysphoric disorder criteria confirmed using a self-rating questionnaire among Japanese women with depressive disorders. Biopsychosoc Med. 2011;5:5.-
21. Rapkin A, Winer S. Premenstrual syndrome and premenstrual dysphoric disorder: quality of life and burden of illness. Expert Rev Pharmacoecon Outcomes Res. 2009;9:157-170.
22. Cunningham J, Yonkers KA, O’Brien S, et al. Update on research and treatment of premenstrual dysphoric disorder. Harv Rev Psychiatry. 2009;17:120-137.
23. Dickerson LM, Hunter MH. Premenstrual syndrome. Am Fam Physician. 2003;67:1743-1752.
24. Steiner M, Macdougall M, Brown E. The premenstrual symptoms screening tool (PSST) for clinicians. Arch Womens Ment Health. 2003;6:203-209.
25. Halbreich U, O’Brien PM, Eriksson E, et al. Are there differential symptom profiles that improve in response to different pharmacological treatments of premenstrual syndrome/premenstrual dysphoric disorder? CNS Drugs. 2006;20:523-547.
26. Bianchi-Demicheli F. Premenstrual dysphoric disorder: diagnosis and therapeutic strategy. Rev Med Suisse. 2006;2:393-394,397–399.
27. Htay TT, Carrick J, Papiaca R. Premenstrual dysphoric disorder 2009. Available at: http://emedicine.medscape.com/article/293257-clinical. Accessed May 25, 2011.
28. Halbreich U. Selective serotonin reuptake inhibitors and initial oral contraceptives for the treatment of PMDD: effective but not enough. CNS Spectr. 2008;13:566-572.
29. Brown J, Marjoribanks J, Wyatt K. Selective serotonin reuptake inhibitors for premenstrual syndrome. Cochrane Database Syst Rev. 2009;(2):CD001396.-
30. Freeman EW. Effects of antidepressants on quality of life in women with premenstrual dysphoric disorder. Pharmacoeconomics. 2005;23:433-444.
31. Landen M, Nissbrandt H, Allgulander C, et al. Placebo-controlled trial comparing intermittent and continuous paroxetine in premenstrual dysphoric disorder. Neuropsychopharmacology. 2007;32:153-161.
32. Freeman EW, Rickels K, Yonkers KA, et al. Venlafaxine in the treatment of premenstrual dysphoric disorder. Obstet Gynecol. 2001;98:737-744.
33. Kroll R, Rapkin AJ. Treatment of premenstrual disorders. J Reprod Med. 2006;51(suppl 4):S359-S370.
34. Eriksson E, Ekman A, Sinclair S, et al. Escitalopram administered in the luteal phase exerts a marked and dose-dependent effect in premenstrual dysphoric disorder. J Clin Psychopharmacol. 2008;28:195-202.
35. Halbreich U. The pathophysiologic background for current treatments of premenstrual syndromes. Curr Psychiatry Rep. 2002;4:429-434.
36. Wyatt KM, Dimmock PW, Frischer M, et al. Prescribing patterns in premenstrual syndrome. BMC Womens Health. 2002;2:4.-
37. Rapkin AJ. YAZ in the treatment of premenstrual dysphoric disorder. J Reprod Med. 2008;53(suppl 9):S729-S741.
38. Elliott H. Premenstrual dysphoric disorder. A guide for the treating clinician. NC Med J. 2002;63:72-75.
39. Dante G, Facchinetti F. Herbal treatments for alleviating premenstrual symptoms: a systematic review. J Psychosom Obstet Gynaecol. 2011;32:42-51.
40. Whelan AM, Jurgens TM, Naylor H. Herbs, vitamins and minerals in the treatment of premenstrual syndrome: a systematic review. Can J Clin Pharmacol. 2009;16:e407-e429.
41. Pearlstein T, Steiner M. Non-antidepressant treatment of premenstrual syndrome. J Clin Psychiatry. 2000;61(suppl 12):22-27.
42. Girman A, Lee R, Kligler B. An integrative medicine approach to premenstrual syndrome. Am J Obstet Gynecol. 2003;188(suppl 5):S56-S65.
43. Wyatt KM, Dimmock PW, Jones PW, et al. Efficacy of vitamin B6 in the treatment of premenstrual syndrome: systematic review. BMJ. 1999;318:1375-1381.
44. Freeman EW. Therapeutic management of premenstrual syndrome. Expert Opin Pharmacother. 2010;11:2879-2889.
45. NIH Consensus Conference. Acupuncture. JAMA. 1998;280:1518-1524.
46. Taguchi R, Yoshimoto S, Imai K, et al. Acupuncture for premenstrual dysphoric disorder. Arch Gynecol Obstet. 2009;280:877-881.
1. Mishell DR, Jr. Premenstrual disorders: epidemiology and disease burden. Am J Manag Care. 2005;11(suppl 16):S473-S479.
2. Kaur G, Gonsalves L, Thacker HL. Premenstrual dysphoric disorder: a review for the treating practitioner. Cleve Clin J Med. 2004;71:303-305,312–313, 317–318.
3. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders DSM-IV-TR. 4th ed. text revision. Washington, DC: American Psychiatric Association; 2000.
4. Kim DR, Gyulai L, Freeman EW, et al. Premenstrual dysphoric disorder and psychiatric co-morbidity. Arch Womens Ment Health. 2004;7:37-47.
5. Ling FW. Recognizing and treating premenstrual dysphoric disorder in the obstetric, gynecologic, and primary care practices. J Clin Psychiatry. 2000;61(suppl 12):S9-S16.
6. Steiner M, Pearlstein T. Premenstrual dysphoria and the serotonin system: pathophysiology and treatment. J Clin Psychiatry. 2000;61(suppl 12):S17-S21.
7. Freeman EW, Sondheimer SJ. Premenstrual dysphoric disorder: recognition and treatment. Prim Care Companion J Clin Psychiatry. 2003;5:30-39.
8. Silber TJ, Valadez-Meltzer A. Premenstrual dysphoric disorder in adolescents: case reports of treatment with fluoxetine and review of the literature. J Adolesc Health. 2005;37:518-525.
9. Pearlstein TB, Bellew KM, Endicott J, et al. Paroxetine controlled release for premenstrual dysphoric disorder: remission analysis following a randomized, double-blind, placebo-controlled trial. Prim Care Companion J Clin Psychiatry. 2005;7:53-60.
10. Halbreich U, Petty F, Yonkers K, et al. Low plasma gamma-aminobutyric acid levels during the late luteal phase of women with premenstrual dysphoric disorder. Am J Psychiatry. 1996;153:718-720.
11. Jarvis CI, Lynch AM, Morin AK. Management strategies for premenstrual syndrome/premenstrual dysphoric disorder. Ann Pharmacother. 2008;42:967-978.
12. Kaura V, Ingram CD, Gartside SE, et al. The progesterone metabolite allopregnanolone potentiates GABA(A) receptor-mediated inhibition of 5-HT neuronal activity. Eur Neuropsychopharmacol. 2007;17:108-115.
13. Girdler SS, Straneva PA, Light KC, et al. Allopregnanolone levels and reactivity to mental stress in premenstrual dysphoric disorder. Biol Psychiatry. 2001;49:788-797.
14. Klatzkin RR, Lindgren ME, Forneris CA, et al. Histories of major depression and premenstrual dysphoric disorder: Evidence for phenotypic differences. Biol Psychol. 2010;84:235-247.
15. Girdler SS, Klatzkin R. Neurosteroids in the context of stress: implications for depressive disorders. Pharmacol Ther. 2007;116:125-139.
16. Perkonigg A, Yonkers KA, Pfister H, et al. Risk factors for premenstrual dysphoric disorder in a community sample of young women: the role of traumatic events and posttraumatic stress disorder. J Clin Psychiatry. 2004;65:1314-1322.
17. Tschudin S, Bertea PC, Zemp E. Prevalence and predictors of premenstrual syndrome and premenstrual dysphoric disorder in a population-based sample. Arch Womens Ment Health. 2010;13:485-494.
18. Girdler SS, Leserman J, Bunevicius R, et al. Persistent alterations in biological profiles in women with abuse histories: influence of premenstrual dysphoric disorder. Health Psychol. 2007;26:201-213.
19. Girdler SS, Sherwood A, Hinderliter AL, et al. Biological correlates of abuse in women with premenstrual dysphoric disorder and healthy controls. Psychosom Med. 2003;65:849-856.
20. Miyaoka Y, Akimoto Y, Ueda K, et al. Fulfillment of the premenstrual dysphoric disorder criteria confirmed using a self-rating questionnaire among Japanese women with depressive disorders. Biopsychosoc Med. 2011;5:5.-
21. Rapkin A, Winer S. Premenstrual syndrome and premenstrual dysphoric disorder: quality of life and burden of illness. Expert Rev Pharmacoecon Outcomes Res. 2009;9:157-170.
22. Cunningham J, Yonkers KA, O’Brien S, et al. Update on research and treatment of premenstrual dysphoric disorder. Harv Rev Psychiatry. 2009;17:120-137.
23. Dickerson LM, Hunter MH. Premenstrual syndrome. Am Fam Physician. 2003;67:1743-1752.
24. Steiner M, Macdougall M, Brown E. The premenstrual symptoms screening tool (PSST) for clinicians. Arch Womens Ment Health. 2003;6:203-209.
25. Halbreich U, O’Brien PM, Eriksson E, et al. Are there differential symptom profiles that improve in response to different pharmacological treatments of premenstrual syndrome/premenstrual dysphoric disorder? CNS Drugs. 2006;20:523-547.
26. Bianchi-Demicheli F. Premenstrual dysphoric disorder: diagnosis and therapeutic strategy. Rev Med Suisse. 2006;2:393-394,397–399.
27. Htay TT, Carrick J, Papiaca R. Premenstrual dysphoric disorder 2009. Available at: http://emedicine.medscape.com/article/293257-clinical. Accessed May 25, 2011.
28. Halbreich U. Selective serotonin reuptake inhibitors and initial oral contraceptives for the treatment of PMDD: effective but not enough. CNS Spectr. 2008;13:566-572.
29. Brown J, Marjoribanks J, Wyatt K. Selective serotonin reuptake inhibitors for premenstrual syndrome. Cochrane Database Syst Rev. 2009;(2):CD001396.-
30. Freeman EW. Effects of antidepressants on quality of life in women with premenstrual dysphoric disorder. Pharmacoeconomics. 2005;23:433-444.
31. Landen M, Nissbrandt H, Allgulander C, et al. Placebo-controlled trial comparing intermittent and continuous paroxetine in premenstrual dysphoric disorder. Neuropsychopharmacology. 2007;32:153-161.
32. Freeman EW, Rickels K, Yonkers KA, et al. Venlafaxine in the treatment of premenstrual dysphoric disorder. Obstet Gynecol. 2001;98:737-744.
33. Kroll R, Rapkin AJ. Treatment of premenstrual disorders. J Reprod Med. 2006;51(suppl 4):S359-S370.
34. Eriksson E, Ekman A, Sinclair S, et al. Escitalopram administered in the luteal phase exerts a marked and dose-dependent effect in premenstrual dysphoric disorder. J Clin Psychopharmacol. 2008;28:195-202.
35. Halbreich U. The pathophysiologic background for current treatments of premenstrual syndromes. Curr Psychiatry Rep. 2002;4:429-434.
36. Wyatt KM, Dimmock PW, Frischer M, et al. Prescribing patterns in premenstrual syndrome. BMC Womens Health. 2002;2:4.-
37. Rapkin AJ. YAZ in the treatment of premenstrual dysphoric disorder. J Reprod Med. 2008;53(suppl 9):S729-S741.
38. Elliott H. Premenstrual dysphoric disorder. A guide for the treating clinician. NC Med J. 2002;63:72-75.
39. Dante G, Facchinetti F. Herbal treatments for alleviating premenstrual symptoms: a systematic review. J Psychosom Obstet Gynaecol. 2011;32:42-51.
40. Whelan AM, Jurgens TM, Naylor H. Herbs, vitamins and minerals in the treatment of premenstrual syndrome: a systematic review. Can J Clin Pharmacol. 2009;16:e407-e429.
41. Pearlstein T, Steiner M. Non-antidepressant treatment of premenstrual syndrome. J Clin Psychiatry. 2000;61(suppl 12):22-27.
42. Girman A, Lee R, Kligler B. An integrative medicine approach to premenstrual syndrome. Am J Obstet Gynecol. 2003;188(suppl 5):S56-S65.
43. Wyatt KM, Dimmock PW, Jones PW, et al. Efficacy of vitamin B6 in the treatment of premenstrual syndrome: systematic review. BMJ. 1999;318:1375-1381.
44. Freeman EW. Therapeutic management of premenstrual syndrome. Expert Opin Pharmacother. 2010;11:2879-2889.
45. NIH Consensus Conference. Acupuncture. JAMA. 1998;280:1518-1524.
46. Taguchi R, Yoshimoto S, Imai K, et al. Acupuncture for premenstrual dysphoric disorder. Arch Gynecol Obstet. 2009;280:877-881.
Keeping older patients healthy and safe as they travel
• Advise older adults to prepare a health travel kit containing all their medications and medical supplies, a list of chronic conditions, and emergency contact information, and to pack it in their carry-on luggage. C
• Instruct patients who will be airborne for ≥4 hours to stay hydrated, avoid alcohol and sedating drugs, and either do seated calf exercises or get up and move about the cabin periodically. B
• Remind patients who will spend time in developing countries to drink only bottled beverages, eat only hot food and fruit that can be peeled, and avoid ice cubes and food from street vendors. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
CASE Larry R, a 77-year-old retired college professor, comes in for a checkup because he is planning a trip to Kenya—on a safari he describes excitedly as “the trip of a lifetime.” He’ll be going with a group, but before he signs on he wants to be sure you think he can manage the tour’s “moderate pace.” He also thinks that he’ll “need to get some shots.”
The patient is overweight (BMI 29) and smokes a pipe daily. He has a history of hypertension, hyperlipidemia, and mild osteoarthritis in both knees and hips, all of which are well controlled.
What would you advise Professor R about the health care preparations needed for his big trip?
Chances are you have patients like Professor R—retired and relatively healthy, and endowed with a sense of adventure and the financial resources that make it possible to visit distant lands. With the nation’s 78 million baby boomers starting to reach retirement age—the oldest cohort turned 65 in 2011—you’re likely to see increasing numbers of older patients with plans for international travel in the years ahead.1
Like their younger counterparts, older people travel for a variety of reasons: Some have planned for decades to take the “trip of a lifetime” when they retire. Others plan longer excursions, sometimes referred to as an adult “gap year,” to relive a long-ago experience, volunteer in an underdeveloped country, or hike and bird watch in a rainforest. Many more are immigrants who travel to visit relatives or friends in their country of origin, usually a lower-income, environmentally depressed locale with a higher incidence of infectious diseases like malaria, typhoid, and hepatitis A. 2
And while the older traveler will have to take many of the same steps to stay healthy as his or her younger counterpart, it is the older traveler who is more likely to have chronic conditions and special needs that require additional preparation. With careful planning, however, even those with decreased faculties, ranging from impaired vision or hearing to mild cognitive impairment, can safely travel abroad.3
A pretravel visit is your opportunity to assess the patient’s fitness to make the trip being planned, ensure optimal management of chronic conditions while traveling, and identify (and recommend steps to mitigate) travel-related risks.
Morbidity and mortality abroad: A review of the risks
Although much pretravel advice centers on the prevention of tropical infectious diseases, such infections account for a very small percentage of deaths of Americans outside of the United States.1 In fact, the major health risks facing older adults traveling abroad are similar to those they face at home: Cardiovascular events are responsible for the preponderance of deaths and for half of all travel-related illnesses.1
International travel can be physically demanding for older individuals and injuries are common, accounting for a large proportion of deaths of Americans overseas4 and an estimated 25% to 38% of travel-related incidents.1,5 A third of injury-related deaths of US citizens traveling abroad involve traffic accidents, followed by homicide (17% of cases) and drowning (13%).1,5 Thus, injury prevention and management of chronic conditions are key issues to address in a pretravel consult.
Even small steps help safeguard older travelers
Older patients planning to travel abroad should schedule an appointment at least 4 to 6 weeks before their departure.2 Ask about the locale, political and environmental climate, length of stay, location and type of accommodations, accessibility to health care, and activities planned,6 which will enable you to offer both general and destination-specific health and safety tips. When advising older adults with complex comorbidities and/or particularly high-risk itineraries, referral to a travel medicine specialist should be strongly considered.
Exercise. Encourage older patients to initiate a graduated exercise program, starting several months before the trip.3 Even a modest improvement in endurance, strength, and flexibility can reduce the likelihood of injury.
Luggage. The right luggage can benefit your patients. Recommend that older patients purchase lightweight suitcases with wheels, which are easier to maneuver in and out of airports6 and less likely to cause muscle strain or musculoskeletal injury.
Insurance. If an individual becomes ill or sustains an injury overseas, the right insurance can be crucial. Advise older adults to review their health insurance policy to see whether it provides overseas coverage. If not, suggest they consider a short-term supplemental policy to cover medical care and evacuation, if needed. Recommend trip cancellation insurance, as well.
Patients should pack pills, medical supplies in a carry-on kit
Encourage all older travelers to compile a personalized travel health kit equipped with common over-the-counter (OTC) medications, prescription drugs, and any personal medical supplies they’ll need, such as a continuous positive airway pressure (CPAP) machine.1,3 Remind patients to take an ample supply of both prescription and OTC drugs, each in its original labeled container.3,6 Buying medications outside the United States is not advisable, given the variation in international regulatory standards. Stress the importance of keeping the kit in a carry-on bag.
The health kit should also include descriptions of the patient’s preexisting medical conditions, which you or a nurse or medical assistant in the practice can help to prepare; a list of prescription drugs he or she takes (using both the generic and brand names); and a copy of a recent electrocardiogram, if available, along with contact information in case of an emergency.1,3,6 A patient who uses injectable medication, such as insulin, should obtain a letter (on the practice’s letterhead) from the prescribing physician and be prepared to show the letter to airport security personnel.3,6
Staying safe in the air
The lower barometric and oxygen partial pressures found in aircraft cabins, which are pressurized at 5000 to 8000 feet, can affect both the respiratory and cardiovascular systems of older adults—particularly those with pulmonary or cardiac disorders.6,7 Individuals who do not routinely require oxygen and are able to walk the equivalent of one city block or climb one flight of stairs without shortness of breath should have little trouble compensating for the reduced oxygen in the cabin.3 Patients with stable heart failure, including New York Heart Association grades III and IV, can tolerate flights of up to one hour without additional oxygen.7
Advise older adults who will require oxygen that they are not permitted to bring their own oxygen canisters onboard an airplane.1 In-flight oxygen needs to be ordered at least 7 days before departure, and there may be a charge.7 Most airlines have medical consultants available to help patients who will need oxygen or other medical provisions.7 In addition, tour companies or travel consultants can help older patients with special needs ensure that they have access to oxygen or other medical supplies at their destination.
Thrombosis—the other in-flight risk
Sometimes referred to as “economy class syndrome” or “traveler’s thrombosis,” the venous stasis of air travel is responsible for a 3-fold increase in the risk of venous thromboembolism (VTE).8 While fatal pulmonary embolism is rare, duration of travel and risk of VTE follow a dose-response relationship, with each 2-hour increase in flight time conferring an additional 18% risk.8 Other risk factors for VTE include varicose veins, metastatic cancer, major surgery within the past 2 weeks, prior VTE, and BMI >40. Advanced age increases travelers’ risk of VTE, as well.7,8 The absolute risk, however, is low.
Among travelers older than 50 years, symptomatic VTE occurs at an estimated rate of one in 600 for flights >4 hours and one in 500 for flights >12 hours.9,10 While there is no evidence that first-class seating lowers the risk, there are preventive measures that patients can take.11
Tell patients to stay hydrated, drinking plenty of fluids but avoiding alcohol during flights of ≥4 hours’ duration. Sedating drugs should be avoided, as well. Advise anyone planning a long flight to either do seated exercises (intermittent calf contractions) or to periodically get up and walk about the cabin.9 You may also want to recommend that patients purchase below-the-knee elastic compression stockings to help decrease venous stasis.12
There is no evidence to recommend the use of aspirin to prevent VTE.12,13 But you may consider prescribing a single 40-mg dose of enoxaparin for a patient who has multiple risk factors and will be airborne for >6 hours.13
Promote safety and comfort on the ground
It is crucial to remind all travelers about the risks associated with traveling in motor vehicles in other countries. Remind patients to wear seat belts whenever they’re available; exercise caution regarding public transportation, which may be overcrowded and have an increased risk of pickpockets and robbery; and avoid riding on motorcycles and scooters. If they do opt to ride on a scooter, tell them that it’s imperative that they wear a helmet.
Minimize the effects of jet lag
Travelers of any age may experience jet lag, which occurs when the individual’s circadian clock cannot keep pace with travel across time zones.14 Notably, however, older people appear to suffer less than their younger counterparts.3 Patients traveling great distances are not likely to avoid jet lag completely, of course. Recommend the following strategies:
Start adjusting your schedule in the week before you depart, gradually shifting 2 hours toward congruence with the time zone at your destination.14
Help reset your circadian rhythm through exposure to bright light, in the morning after eastward travel and in the evening after westward travel.14
Take it easy at first. An itinerary that accounts for initial fatigue is an important nonpharmacologic management strategy.14
Avoid sedating medications, including antihistamines, tranquilizers, anti-motion sickness agents, and benzodiazepines, as these can increase falls and confusion in older adults and make jet lag worse.3
Take melatonin. A dose of 0.5 to 5 mg, taken at bedtime, may promote sleep and decrease jet lag symptoms in travelers crossing multiple time zones.14
Prepare patients to cope with heat …
Unusually hot, humid weather increases morbidity and mortality in the elderly,3,15 and older patients traveling to such climates will need to take extra precautions. Strenuous exercise in the heat should be avoided, because both thirst and the capacity to conserve salt and water decrease with age.16 Acclimatization is helped by rest, air-conditioning, loose cotton clothing, brimmed hats, and cool baths or showers.3 Diuretics may have to be adjusted for fluids lost by increased perspiration, and a discussion about a dose reduction should be included in the pretravel consult for patients who take diuretics and will be traveling to a hot, humid climate.
… and increases in altitude
For older adults, exposure to a moderate altitude (<2500 meters) is initially associated with hypoxemia and a reduced exercise capacity, until acclimatization occurs by Day 5.17,18 Although older adults generally acclimatize well, advise them to limit their activities for the first few days at a higher altitude. This is especially important for patients with coronary artery disease (CAD).
To further ease the effects of a higher altitude, advise patients to drink plenty of fluids, but little or no alcohol.19 Review the medications of an older patient who will be spending time at very high altitude. Rarely, antihypertensive medication may need to be adjusted. The body compensates for lower oxygen with a faster heart rate, and some antihypertensives may interfere with this compensatory mechanism.3
Precautions (and prophylaxis) may prevent travelers’ diarrhea
Diarrhea—among the most common travel-related conditions20—affects an estimated 30% to 70% of international travelers.2 The incidence is highest among visitors to developing countries. Most (80%-90%) of travelers’ diarrhea is due to bacterial infection,21 10% of cases are caused by parasites, and 5% to 8% by viral infection.2,22
Although increasing age lowers the risk of travelers’ diarrhea,1 older patients traveling to developing areas should be cautioned to only eat food that is served hot or fruit they can peel themselves; drink only bottled water and sealed liquids; and avoid salad, ice, and food from street vendors.1 Studies have shown, however, that tourists often get diarrhea despite these safety measures.2
Treatment and prophylaxis. Prophylactic antibiotics can prevent travelers’ diarrhea. But the increased sun sensitivity, drug-drug interactions, and gastrointestinal (GI) adverse effects associated with antibiotics limit their usefulness. Prophylaxis is indicated, however, for older adults for whom the complications of dehydration would likely be so severe that the benefits of using antibiotics to prevent diarrhea clearly outweigh the risks.23
Fluoroquinolones are a first-line treatment for travelers’ diarrhea. But increasing microbial resistance to this class of drugs, especially among Campylobacter isolates,24 may limit their usefulness in some destinations.25 Azithromycin is recommended in such cases, and has been shown to be equally effective.26,27 Single-dose therapy is well established with fluoroquinolones, but the best regimen for azithromycin (1 vs 3 days) is still under evaluation.28,29 Along with instructions on when to take an antibiotic, travelers should be given prescriptions for treatment of travelers’ diarrhea before the start of their trip. Suggest that patients purchase oral rehydration packets to take on their trip, and stress the importance of using them and staying hydrated if diarrhea develops.
Decreased upper GI acidity due to acid-blocking medications such as proton-pump inhibitors can increase the risk for many infections, including salmonella and cholera. Patients taking such medications should be made aware of the risk, and the risks vs benefits of temporarily stopping them should be discussed. Vaccination against cholera should not routinely be recommended.29,30
Vaccines and pills protect against preventable diseases
An impending trip abroad also presents an opportunity to review the patient’s immunization status, catch up on recommended vaccines, and determine whether any additional vaccinations are needed.
Herpes zoster (HZ). Patients older than 60 years should receive a single dose of the HZ vaccine, whether or not they have a history of this condition. Because this is a live virus-containing vaccine, however, it should not be given to anyone who is immunocompromised.31
MMR booster. Adults born before 1957 can be considered immune from both measles and mumps, but not rubella. There is no data on immunization to rubella, but guidelines do not recommend MMR vaccination in the elderly.1
Pneumococcal polyvalent-23 (PPV-23). One dose of the PPV-23 vaccine is indicated for all adults at age 65. This is especially important for travelers, as the prevalence of pneumococcal disease is likely higher in crowded, urban environments within less developed countries.31
Tetanus. Although tetanus is mainly a disease of the elderly, only 45% of men ages 70 years or older and 21% of women in this age group were found to have protective antibodies.1,32 In 2011, the Advisory Committee on Immunization Practices (ACIP) recommended one dose of tetanus and diphtheria toxoid (Td) every 10 years, with a single dose of tetanus toxoid, diphtheria toxoid, and acellular pertussis (Tdap) vaccine given in place of Td for adults older than 65.33 Despite ACIP’s recommendation, the vaccine’s use in adults 65 years and older is an off-label indication, as Tdap is only approved for use in those 11 to 64 years of age.33
Additional vaccines are recommended for travelers, with some indicated for all travelers and others that are destination-specific (TABLE).
TABLE
Which travel-related vaccines does your patient need?
| Disease | Type of vaccine | Primary course | Booster/ follow-up | Route | For which destinations? |
|---|---|---|---|---|---|
| Vaccines for all travelers | |||||
| Hepatitis A* | Killed virus | 2 doses (6-18 mo apart)† | None | IM | All |
| Hepatitis B* | Recombinant viral antigen | 3 doses (0, 1, 6 mo) | None | IM | All |
| Influenza | Inactivated viral | Single dose | Annually | IM | All |
| Typhoid | Capsular polysaccharide Live attenuated bacteria | Single dose 4 doses (0, 2, 4, 6 mo) | 2-3 y 5 y | IM Oral | All |
| Vaccines for travelers to select destinations | |||||
| Japanese encephalitis | Inactivated viral | 2 doses (28 d apart) | Unknown | IM | Rural Asia‡ |
| Meningococcus | Quadrivalent conjugated polysaccharide | Single dose | >10 y | IM | Sub-Saharan Africa; Saudi Arabia |
| Polio | Inactivated viral | Single dose if patient had childhood series | None | SC; IM | Anyplace where polio still occurs |
| Rabies | Inactivated cell culture viral | 3 doses (0, 7, 21-28 d) | None unless exposure occurs | IM | |
| Yellow fever | Live attenuated virus | Single dose | 10 y | SC | Sub-Saharan Africa; tropical South America |
| IM, intramuscular; SC, subcutaneous. *A combined hepatitis A/B vaccine is approved for use in older adults. †Second dose may be delayed up to 8 years without diminished efficacy. ‡Required only for prolonged stays in rural areas of Asia. Adapted from: Mandell GL, Bennett JE, Dolin R, eds. Principles and Practice of Infectious Diseases. 7th ed. Philadelphia, Pa: Elsevier; 2010. | |||||
Meds and safety measures can minimize malaria risk
The risk of acquiring malaria differs significantly among travelers, based on destination, duration and type of travel, and season. Choice of antimalarial agents (eg, atovaquone/proguanil, chloroquine, doxycycline, mefloquine, and primaquine) should be made on an individual basis after considering these factors, as well as the resistance patterns of the countries on the patient’s itinerary, his or her medical history, and the adverse effects profile of potential agents. Because many older adults take multiple medications, the possibility of drug-drug interactions must be considered.1 You’ll find destination-specific recommendations on malaria prevention on the Centers for Disease Control and Prevention’s Travelers’ Health Web site, listed in “Travel and health: Resources for patients and physicians”. For guidance on the best drug to prescribe, you can also consult a travel medicine specialist.2
Access-able Travel Source Web site provides information for older adult travelers with special needs who need help traveling with oxygen or getting around despite decreased mobility (www.access-able.com/tips/).
American Diabetes Association publishes detailed information about traveling with diabetes (http://www.diabetes.org/living-with-diabetes/know-your-rights/discrimination/public-accommodations/air-travel-and-diabetes/).
Bureau of Consular Affairs publishes information regarding VISA and security requirements at various destinations and travel warnings (http://travel.state.gov/about/about_304.html).
Centers for Disease Control and Prevention publishes “The Yellow Book”—a reference for clinicians who advise international travelers about health risks. There is also a range of other travel-related information on its Travelers’ Health Web site (www.cdc.gov/travel/).
International Travel Medicine Society provides a global travel clinic directory (http://www.istm.org/Webforms/Searchclinics/Default.aspx?SearchType=advanced).
Transportation Security Administration Web site provides information on what can be brought on-board a plane. See “Can I bring my … through the checkpoint?” (http://apps.tsa.dhs.gov/mytsa/cib_home.aspx).
Travel Health Online offers a list of medical providers around the world (www.tripprep.com/scripts).
World Health Organization’s Travel and Health Web site provides free access to selected chapters of its book, “International Travel and Health 2012,” as well as interactive maps, information about infectious diseases and food safety, and more (www.who.int/ith/en/).
Patients should be mindful of mosquitos. Stress the importance of preventing mosquito bites (as much as possible). Advise patients traveling to mosquito-infested areas to use insect repellents containing 30% N-diethyl-meta-toluamide (DEET) and permethrin-treated clothing.34 Tell them, too, to wear long sleeves, pants, and footwear that provides full coverage.35 Ensuring that sleeping areas are properly screened or air-conditioned will further reduce the likelihood of mosquito bites.36
CASE After seeing the chief complaint listed as “Traveling to Kenya” on Professor R’s chart, you quickly review the CDC’s Travelers’ Health Web site. You encourage him to stay with his tour group and to wear a seatbelt whenever possible. You also review how to make a personalized travel health kit, and encourage him to register with the Smart Traveler Enrollment Program (STEP) (detailed at https://step.state.gov/rep) before leaving for the safari. You strongly suggest that he consider purchasing additional medical evacuation insurance, as well.
Given the prevalence of travelers’ diarrhea, along with dengue and malaria, in Kenya, you review food and water safety and avoidance of insect-transmitted diseases with the patient, and write a prescription for ciprofloxacin to be taken if he develops diarrhea. Professor R is not at high risk for VTE, but you encourage him to stay hydrated, avoid sedating medications, and be diligent about mobilization during lengthy flights. You recommend melatonin for jet lag.
To adjust to the heat, you recommend that he avoid strenuous exercise in the first few days and drink sufficient fluids throughout the trip. You administer the Tdap vaccine, an adult polio booster, and the hepatitis A vaccine, verify that he has received his pneumococcal and influenza vaccines, and prescribe an antimalarial medication.
And as you walk him toward the door, you offer him one final piece of advice: Take plenty of pictures.
CORRESPONDENCE
Jeffrey D. Schlaudecker, MD, The Christ Hospital/University of Cincinnati Family Medicine Residency Program, 2123 Auburn Avenue #340, Cincinnati, OH 45219; Jeffrey.schlaudecker@uc.edu
1. Reed CM. Travel recommendations for older adults. Clin Geriatr Med. 2007;23:687-713, ix.
2. Centers for Disease Control and Prevention. Health Information for International Travel 2012. New York, NY:Oxford University Press; 2012. Available at http://wwwnc.cdc.gov/travel/page/yellowboth-2012-home.htm. Accessed December 18, 2012.
3. Cooper MC. The elderly travellers. Travel Med Infect Dis. 2006;4:218-222.
4. Guse CE, Cortes LM, Hargarten SW, et al. Fatal injuries of US citizens abroad. J Travel Med. 2007;14:279-287
5. Tonellato DJ, Guse CE, Hargarten SW. Injury deaths of US citizens abroad: new data source, old travel problem. J Travel Med. 2009;16:304-310.
6. Fenner P. Fitness to travel - assessment in the elderly and medically impaired. Aust Fam Physician. 2007;36:312-315.
7. Smith D, Toff W, Joy M, et al. Fitness to fly for passengers with cardiovascular disease. Heart. 2010;96(suppl 2):Sii1-S16.
8. Chandra D, Parisini E, Mozaffarian D. Meta-analysis: travel and risk for venous thromboembolism. Ann Intern Med. 2009;151:180-190.
9. Gavish I, Brenner B. Air travel and the risk of thromboembolism. Intern Emerg Med. 2011;6:113-116.
10. Ansari MT, Cheung BM, Qing Huang J, et al. Traveler’s thrombosis: a systematic review. J Travel Med. 2005;12:142-154.
11. Schwarz T, Siegert G, Oettler W, et al. Venous thrombosis after long-haul flights. Arch Intern Med. 2003;163:2759-2764.
12. Cesarone MR, Belcaro G, Errichi BM, et al. The LONFLIT4—Concorde Deep Venous Thrombosis and Edema Study: prevention with travel stockings. Angiology. 2003;54:143-154.
13. Cesarone MR, Belcaro G, Nicolaides AN, et al. Venous thrombosis from air travel: the LONFLIT3 study—prevention with aspirin vs low-molecular-weight heparin (LMWH) in high-risk subjects: a randomized trial. Angiology. 2002;53:1-6.
14. Sack RL. Clinical practice. Jet lag. N Engl J Med. 2010: 440-447.
15. Davies I, O’Neill PA, McLean KA, et al. Age-associated alterations in thirst and arginine vasopressin in response to a water or sodium load. Age Ageing. 1995;24:151-159.
16. Rikkert MG, Melis RJ, Claassen JA. Heat waves and dehydration in the elderly. BMJ. 2009;339:b2663.-
17. Levine BD, Zuckerman JH, deFilippi CR. Effect of high-altitude exposure in the elderly: the Tenth Mountain Division study. Circulation. 1997;96:1224-1232.
18. Agostoni P, Cattadori G, Guazzi M, et al. Effects of simulated altitude-induced hypoxia on exercise capacity in patients with chronic heart failure. Am J Med. 2000;109:450-455.
19. Higgins JP, Tuttle T, Higgins JA. Altitude and the heart: is going high safe for your cardiac patient? Am Heart J. 2010;159:25-32.
20. Gautret P, Schlagenhauf P, Gaudart J, et al. Multicenter EuroTravNet/GeoSentinel study of travel-related infectious diseases in Europe. Emerg Infect Dis. 2009;15:1783-1790.
21. Adachi JA, Jiang ZD, Mathewson JJ, et al. Enteroaggregative Escherichia coli as a major etiologic agent in traveler’s diarrhea in 3 regions of the world. Clin Infect Dis. 2001;32:1706-1709.
22. Black RE. Epidemiology of travelers’ diarrhea and relative importance of various pathogens. Rev Infect Dis. 1990;12 (suppl 1):S73-S79.
23. Rendi-Wagner P, Kollaritsch H. Drug prophylaxis for travelers’ diarrhea. Clin Infect Dis. 2002;34:628-633.
24. Butzler JP. Campylobacter, from obscurity to celebrity. Clin Microbiol Infect. 2004;10:868-876.
25. Hoge CW, Gambel JM, Srijan A, et al. Trends in antibiotic resistance among diarrheal pathogens isolated in Thailand over 15 years. Clin Infect Dis. 1998;26:341-345.
26. Adachi JA, Ericsson CD, Jiang ZD, et al. Azithromycin found to be comparable to levofloxacin for the treatment of US travelers with acute diarrhea acquired in Mexico. Clin Infect Dis. 2003;37:1165-1171.
27. Tribble DR, Sanders JW, Pang LW, et al. Traveler’s diarrhea in Thailand: randomized, double-blind trial comparing single-dose and 3-day azithromycin-based regimens with a 3-day levofloxacin regimen. Clin Infect Dis. 2007;44:338-346.
28. Shanks GD, Smoak BL, Aleman GM, et al. Single dose of azithromycin or three-day course of ciprofloxacin as therapy for epidemic dysentery in Kenya. Acute Dysentery Study Group. Clin Infect Dis. 1999;29:942-943.
29. Heatley RV, Sobala GM. Acid suppression and the gastric flora. Baillieres Clin Gastroenterol. 1993;7:167-181.
30. Hill DR, Ford L, Lalloo DG. Oral cholera vaccines: use in clinical practice. Lancet Infect Dis. 2006;6:361-373.
31. Vaughn JA, Miller RA. Update on immunizations in adults. Am Fam Physician. 2011;84:1015-1020.
32. Gergen PJ, McQuillan GM, Kiely M, et al. A population-based serologic survey of immunity to tetanus in the United States. N Engl J Med. 1995;332:761-766.
33. World Health Organization. Typhoid vaccines: WHO position paper. Wkly Epidemiol Rec. 2008;83:49-59.
34. Fradin MS, Day JF. Comparative efficacy of insect repellents against mosquito bites. N Engl J Med. 2002;347:13-18.
35. Soto J, Medina F, Dember N, et al. Efficacy of permethrin-impregnated uniforms in the prevention of malaria and leishmaniasis in Colombian soldiers. Clin Infect Dis. 1995;21:599-602.
36. Freedman DO. Clinical practice. Malaria prevention in short-term travelers. N Engl J Med. 2008;359:603-612.
• Advise older adults to prepare a health travel kit containing all their medications and medical supplies, a list of chronic conditions, and emergency contact information, and to pack it in their carry-on luggage. C
• Instruct patients who will be airborne for ≥4 hours to stay hydrated, avoid alcohol and sedating drugs, and either do seated calf exercises or get up and move about the cabin periodically. B
• Remind patients who will spend time in developing countries to drink only bottled beverages, eat only hot food and fruit that can be peeled, and avoid ice cubes and food from street vendors. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
CASE Larry R, a 77-year-old retired college professor, comes in for a checkup because he is planning a trip to Kenya—on a safari he describes excitedly as “the trip of a lifetime.” He’ll be going with a group, but before he signs on he wants to be sure you think he can manage the tour’s “moderate pace.” He also thinks that he’ll “need to get some shots.”
The patient is overweight (BMI 29) and smokes a pipe daily. He has a history of hypertension, hyperlipidemia, and mild osteoarthritis in both knees and hips, all of which are well controlled.
What would you advise Professor R about the health care preparations needed for his big trip?
Chances are you have patients like Professor R—retired and relatively healthy, and endowed with a sense of adventure and the financial resources that make it possible to visit distant lands. With the nation’s 78 million baby boomers starting to reach retirement age—the oldest cohort turned 65 in 2011—you’re likely to see increasing numbers of older patients with plans for international travel in the years ahead.1
Like their younger counterparts, older people travel for a variety of reasons: Some have planned for decades to take the “trip of a lifetime” when they retire. Others plan longer excursions, sometimes referred to as an adult “gap year,” to relive a long-ago experience, volunteer in an underdeveloped country, or hike and bird watch in a rainforest. Many more are immigrants who travel to visit relatives or friends in their country of origin, usually a lower-income, environmentally depressed locale with a higher incidence of infectious diseases like malaria, typhoid, and hepatitis A. 2
And while the older traveler will have to take many of the same steps to stay healthy as his or her younger counterpart, it is the older traveler who is more likely to have chronic conditions and special needs that require additional preparation. With careful planning, however, even those with decreased faculties, ranging from impaired vision or hearing to mild cognitive impairment, can safely travel abroad.3
A pretravel visit is your opportunity to assess the patient’s fitness to make the trip being planned, ensure optimal management of chronic conditions while traveling, and identify (and recommend steps to mitigate) travel-related risks.
Morbidity and mortality abroad: A review of the risks
Although much pretravel advice centers on the prevention of tropical infectious diseases, such infections account for a very small percentage of deaths of Americans outside of the United States.1 In fact, the major health risks facing older adults traveling abroad are similar to those they face at home: Cardiovascular events are responsible for the preponderance of deaths and for half of all travel-related illnesses.1
International travel can be physically demanding for older individuals and injuries are common, accounting for a large proportion of deaths of Americans overseas4 and an estimated 25% to 38% of travel-related incidents.1,5 A third of injury-related deaths of US citizens traveling abroad involve traffic accidents, followed by homicide (17% of cases) and drowning (13%).1,5 Thus, injury prevention and management of chronic conditions are key issues to address in a pretravel consult.
Even small steps help safeguard older travelers
Older patients planning to travel abroad should schedule an appointment at least 4 to 6 weeks before their departure.2 Ask about the locale, political and environmental climate, length of stay, location and type of accommodations, accessibility to health care, and activities planned,6 which will enable you to offer both general and destination-specific health and safety tips. When advising older adults with complex comorbidities and/or particularly high-risk itineraries, referral to a travel medicine specialist should be strongly considered.
Exercise. Encourage older patients to initiate a graduated exercise program, starting several months before the trip.3 Even a modest improvement in endurance, strength, and flexibility can reduce the likelihood of injury.
Luggage. The right luggage can benefit your patients. Recommend that older patients purchase lightweight suitcases with wheels, which are easier to maneuver in and out of airports6 and less likely to cause muscle strain or musculoskeletal injury.
Insurance. If an individual becomes ill or sustains an injury overseas, the right insurance can be crucial. Advise older adults to review their health insurance policy to see whether it provides overseas coverage. If not, suggest they consider a short-term supplemental policy to cover medical care and evacuation, if needed. Recommend trip cancellation insurance, as well.
Patients should pack pills, medical supplies in a carry-on kit
Encourage all older travelers to compile a personalized travel health kit equipped with common over-the-counter (OTC) medications, prescription drugs, and any personal medical supplies they’ll need, such as a continuous positive airway pressure (CPAP) machine.1,3 Remind patients to take an ample supply of both prescription and OTC drugs, each in its original labeled container.3,6 Buying medications outside the United States is not advisable, given the variation in international regulatory standards. Stress the importance of keeping the kit in a carry-on bag.
The health kit should also include descriptions of the patient’s preexisting medical conditions, which you or a nurse or medical assistant in the practice can help to prepare; a list of prescription drugs he or she takes (using both the generic and brand names); and a copy of a recent electrocardiogram, if available, along with contact information in case of an emergency.1,3,6 A patient who uses injectable medication, such as insulin, should obtain a letter (on the practice’s letterhead) from the prescribing physician and be prepared to show the letter to airport security personnel.3,6
Staying safe in the air
The lower barometric and oxygen partial pressures found in aircraft cabins, which are pressurized at 5000 to 8000 feet, can affect both the respiratory and cardiovascular systems of older adults—particularly those with pulmonary or cardiac disorders.6,7 Individuals who do not routinely require oxygen and are able to walk the equivalent of one city block or climb one flight of stairs without shortness of breath should have little trouble compensating for the reduced oxygen in the cabin.3 Patients with stable heart failure, including New York Heart Association grades III and IV, can tolerate flights of up to one hour without additional oxygen.7
Advise older adults who will require oxygen that they are not permitted to bring their own oxygen canisters onboard an airplane.1 In-flight oxygen needs to be ordered at least 7 days before departure, and there may be a charge.7 Most airlines have medical consultants available to help patients who will need oxygen or other medical provisions.7 In addition, tour companies or travel consultants can help older patients with special needs ensure that they have access to oxygen or other medical supplies at their destination.
Thrombosis—the other in-flight risk
Sometimes referred to as “economy class syndrome” or “traveler’s thrombosis,” the venous stasis of air travel is responsible for a 3-fold increase in the risk of venous thromboembolism (VTE).8 While fatal pulmonary embolism is rare, duration of travel and risk of VTE follow a dose-response relationship, with each 2-hour increase in flight time conferring an additional 18% risk.8 Other risk factors for VTE include varicose veins, metastatic cancer, major surgery within the past 2 weeks, prior VTE, and BMI >40. Advanced age increases travelers’ risk of VTE, as well.7,8 The absolute risk, however, is low.
Among travelers older than 50 years, symptomatic VTE occurs at an estimated rate of one in 600 for flights >4 hours and one in 500 for flights >12 hours.9,10 While there is no evidence that first-class seating lowers the risk, there are preventive measures that patients can take.11
Tell patients to stay hydrated, drinking plenty of fluids but avoiding alcohol during flights of ≥4 hours’ duration. Sedating drugs should be avoided, as well. Advise anyone planning a long flight to either do seated exercises (intermittent calf contractions) or to periodically get up and walk about the cabin.9 You may also want to recommend that patients purchase below-the-knee elastic compression stockings to help decrease venous stasis.12
There is no evidence to recommend the use of aspirin to prevent VTE.12,13 But you may consider prescribing a single 40-mg dose of enoxaparin for a patient who has multiple risk factors and will be airborne for >6 hours.13
Promote safety and comfort on the ground
It is crucial to remind all travelers about the risks associated with traveling in motor vehicles in other countries. Remind patients to wear seat belts whenever they’re available; exercise caution regarding public transportation, which may be overcrowded and have an increased risk of pickpockets and robbery; and avoid riding on motorcycles and scooters. If they do opt to ride on a scooter, tell them that it’s imperative that they wear a helmet.
Minimize the effects of jet lag
Travelers of any age may experience jet lag, which occurs when the individual’s circadian clock cannot keep pace with travel across time zones.14 Notably, however, older people appear to suffer less than their younger counterparts.3 Patients traveling great distances are not likely to avoid jet lag completely, of course. Recommend the following strategies:
Start adjusting your schedule in the week before you depart, gradually shifting 2 hours toward congruence with the time zone at your destination.14
Help reset your circadian rhythm through exposure to bright light, in the morning after eastward travel and in the evening after westward travel.14
Take it easy at first. An itinerary that accounts for initial fatigue is an important nonpharmacologic management strategy.14
Avoid sedating medications, including antihistamines, tranquilizers, anti-motion sickness agents, and benzodiazepines, as these can increase falls and confusion in older adults and make jet lag worse.3
Take melatonin. A dose of 0.5 to 5 mg, taken at bedtime, may promote sleep and decrease jet lag symptoms in travelers crossing multiple time zones.14
Prepare patients to cope with heat …
Unusually hot, humid weather increases morbidity and mortality in the elderly,3,15 and older patients traveling to such climates will need to take extra precautions. Strenuous exercise in the heat should be avoided, because both thirst and the capacity to conserve salt and water decrease with age.16 Acclimatization is helped by rest, air-conditioning, loose cotton clothing, brimmed hats, and cool baths or showers.3 Diuretics may have to be adjusted for fluids lost by increased perspiration, and a discussion about a dose reduction should be included in the pretravel consult for patients who take diuretics and will be traveling to a hot, humid climate.
… and increases in altitude
For older adults, exposure to a moderate altitude (<2500 meters) is initially associated with hypoxemia and a reduced exercise capacity, until acclimatization occurs by Day 5.17,18 Although older adults generally acclimatize well, advise them to limit their activities for the first few days at a higher altitude. This is especially important for patients with coronary artery disease (CAD).
To further ease the effects of a higher altitude, advise patients to drink plenty of fluids, but little or no alcohol.19 Review the medications of an older patient who will be spending time at very high altitude. Rarely, antihypertensive medication may need to be adjusted. The body compensates for lower oxygen with a faster heart rate, and some antihypertensives may interfere with this compensatory mechanism.3
Precautions (and prophylaxis) may prevent travelers’ diarrhea
Diarrhea—among the most common travel-related conditions20—affects an estimated 30% to 70% of international travelers.2 The incidence is highest among visitors to developing countries. Most (80%-90%) of travelers’ diarrhea is due to bacterial infection,21 10% of cases are caused by parasites, and 5% to 8% by viral infection.2,22
Although increasing age lowers the risk of travelers’ diarrhea,1 older patients traveling to developing areas should be cautioned to only eat food that is served hot or fruit they can peel themselves; drink only bottled water and sealed liquids; and avoid salad, ice, and food from street vendors.1 Studies have shown, however, that tourists often get diarrhea despite these safety measures.2
Treatment and prophylaxis. Prophylactic antibiotics can prevent travelers’ diarrhea. But the increased sun sensitivity, drug-drug interactions, and gastrointestinal (GI) adverse effects associated with antibiotics limit their usefulness. Prophylaxis is indicated, however, for older adults for whom the complications of dehydration would likely be so severe that the benefits of using antibiotics to prevent diarrhea clearly outweigh the risks.23
Fluoroquinolones are a first-line treatment for travelers’ diarrhea. But increasing microbial resistance to this class of drugs, especially among Campylobacter isolates,24 may limit their usefulness in some destinations.25 Azithromycin is recommended in such cases, and has been shown to be equally effective.26,27 Single-dose therapy is well established with fluoroquinolones, but the best regimen for azithromycin (1 vs 3 days) is still under evaluation.28,29 Along with instructions on when to take an antibiotic, travelers should be given prescriptions for treatment of travelers’ diarrhea before the start of their trip. Suggest that patients purchase oral rehydration packets to take on their trip, and stress the importance of using them and staying hydrated if diarrhea develops.
Decreased upper GI acidity due to acid-blocking medications such as proton-pump inhibitors can increase the risk for many infections, including salmonella and cholera. Patients taking such medications should be made aware of the risk, and the risks vs benefits of temporarily stopping them should be discussed. Vaccination against cholera should not routinely be recommended.29,30
Vaccines and pills protect against preventable diseases
An impending trip abroad also presents an opportunity to review the patient’s immunization status, catch up on recommended vaccines, and determine whether any additional vaccinations are needed.
Herpes zoster (HZ). Patients older than 60 years should receive a single dose of the HZ vaccine, whether or not they have a history of this condition. Because this is a live virus-containing vaccine, however, it should not be given to anyone who is immunocompromised.31
MMR booster. Adults born before 1957 can be considered immune from both measles and mumps, but not rubella. There is no data on immunization to rubella, but guidelines do not recommend MMR vaccination in the elderly.1
Pneumococcal polyvalent-23 (PPV-23). One dose of the PPV-23 vaccine is indicated for all adults at age 65. This is especially important for travelers, as the prevalence of pneumococcal disease is likely higher in crowded, urban environments within less developed countries.31
Tetanus. Although tetanus is mainly a disease of the elderly, only 45% of men ages 70 years or older and 21% of women in this age group were found to have protective antibodies.1,32 In 2011, the Advisory Committee on Immunization Practices (ACIP) recommended one dose of tetanus and diphtheria toxoid (Td) every 10 years, with a single dose of tetanus toxoid, diphtheria toxoid, and acellular pertussis (Tdap) vaccine given in place of Td for adults older than 65.33 Despite ACIP’s recommendation, the vaccine’s use in adults 65 years and older is an off-label indication, as Tdap is only approved for use in those 11 to 64 years of age.33
Additional vaccines are recommended for travelers, with some indicated for all travelers and others that are destination-specific (TABLE).
TABLE
Which travel-related vaccines does your patient need?
| Disease | Type of vaccine | Primary course | Booster/ follow-up | Route | For which destinations? |
|---|---|---|---|---|---|
| Vaccines for all travelers | |||||
| Hepatitis A* | Killed virus | 2 doses (6-18 mo apart)† | None | IM | All |
| Hepatitis B* | Recombinant viral antigen | 3 doses (0, 1, 6 mo) | None | IM | All |
| Influenza | Inactivated viral | Single dose | Annually | IM | All |
| Typhoid | Capsular polysaccharide Live attenuated bacteria | Single dose 4 doses (0, 2, 4, 6 mo) | 2-3 y 5 y | IM Oral | All |
| Vaccines for travelers to select destinations | |||||
| Japanese encephalitis | Inactivated viral | 2 doses (28 d apart) | Unknown | IM | Rural Asia‡ |
| Meningococcus | Quadrivalent conjugated polysaccharide | Single dose | >10 y | IM | Sub-Saharan Africa; Saudi Arabia |
| Polio | Inactivated viral | Single dose if patient had childhood series | None | SC; IM | Anyplace where polio still occurs |
| Rabies | Inactivated cell culture viral | 3 doses (0, 7, 21-28 d) | None unless exposure occurs | IM | |
| Yellow fever | Live attenuated virus | Single dose | 10 y | SC | Sub-Saharan Africa; tropical South America |
| IM, intramuscular; SC, subcutaneous. *A combined hepatitis A/B vaccine is approved for use in older adults. †Second dose may be delayed up to 8 years without diminished efficacy. ‡Required only for prolonged stays in rural areas of Asia. Adapted from: Mandell GL, Bennett JE, Dolin R, eds. Principles and Practice of Infectious Diseases. 7th ed. Philadelphia, Pa: Elsevier; 2010. | |||||
Meds and safety measures can minimize malaria risk
The risk of acquiring malaria differs significantly among travelers, based on destination, duration and type of travel, and season. Choice of antimalarial agents (eg, atovaquone/proguanil, chloroquine, doxycycline, mefloquine, and primaquine) should be made on an individual basis after considering these factors, as well as the resistance patterns of the countries on the patient’s itinerary, his or her medical history, and the adverse effects profile of potential agents. Because many older adults take multiple medications, the possibility of drug-drug interactions must be considered.1 You’ll find destination-specific recommendations on malaria prevention on the Centers for Disease Control and Prevention’s Travelers’ Health Web site, listed in “Travel and health: Resources for patients and physicians”. For guidance on the best drug to prescribe, you can also consult a travel medicine specialist.2
Access-able Travel Source Web site provides information for older adult travelers with special needs who need help traveling with oxygen or getting around despite decreased mobility (www.access-able.com/tips/).
American Diabetes Association publishes detailed information about traveling with diabetes (http://www.diabetes.org/living-with-diabetes/know-your-rights/discrimination/public-accommodations/air-travel-and-diabetes/).
Bureau of Consular Affairs publishes information regarding VISA and security requirements at various destinations and travel warnings (http://travel.state.gov/about/about_304.html).
Centers for Disease Control and Prevention publishes “The Yellow Book”—a reference for clinicians who advise international travelers about health risks. There is also a range of other travel-related information on its Travelers’ Health Web site (www.cdc.gov/travel/).
International Travel Medicine Society provides a global travel clinic directory (http://www.istm.org/Webforms/Searchclinics/Default.aspx?SearchType=advanced).
Transportation Security Administration Web site provides information on what can be brought on-board a plane. See “Can I bring my … through the checkpoint?” (http://apps.tsa.dhs.gov/mytsa/cib_home.aspx).
Travel Health Online offers a list of medical providers around the world (www.tripprep.com/scripts).
World Health Organization’s Travel and Health Web site provides free access to selected chapters of its book, “International Travel and Health 2012,” as well as interactive maps, information about infectious diseases and food safety, and more (www.who.int/ith/en/).
Patients should be mindful of mosquitos. Stress the importance of preventing mosquito bites (as much as possible). Advise patients traveling to mosquito-infested areas to use insect repellents containing 30% N-diethyl-meta-toluamide (DEET) and permethrin-treated clothing.34 Tell them, too, to wear long sleeves, pants, and footwear that provides full coverage.35 Ensuring that sleeping areas are properly screened or air-conditioned will further reduce the likelihood of mosquito bites.36
CASE After seeing the chief complaint listed as “Traveling to Kenya” on Professor R’s chart, you quickly review the CDC’s Travelers’ Health Web site. You encourage him to stay with his tour group and to wear a seatbelt whenever possible. You also review how to make a personalized travel health kit, and encourage him to register with the Smart Traveler Enrollment Program (STEP) (detailed at https://step.state.gov/rep) before leaving for the safari. You strongly suggest that he consider purchasing additional medical evacuation insurance, as well.
Given the prevalence of travelers’ diarrhea, along with dengue and malaria, in Kenya, you review food and water safety and avoidance of insect-transmitted diseases with the patient, and write a prescription for ciprofloxacin to be taken if he develops diarrhea. Professor R is not at high risk for VTE, but you encourage him to stay hydrated, avoid sedating medications, and be diligent about mobilization during lengthy flights. You recommend melatonin for jet lag.
To adjust to the heat, you recommend that he avoid strenuous exercise in the first few days and drink sufficient fluids throughout the trip. You administer the Tdap vaccine, an adult polio booster, and the hepatitis A vaccine, verify that he has received his pneumococcal and influenza vaccines, and prescribe an antimalarial medication.
And as you walk him toward the door, you offer him one final piece of advice: Take plenty of pictures.
CORRESPONDENCE
Jeffrey D. Schlaudecker, MD, The Christ Hospital/University of Cincinnati Family Medicine Residency Program, 2123 Auburn Avenue #340, Cincinnati, OH 45219; Jeffrey.schlaudecker@uc.edu
• Advise older adults to prepare a health travel kit containing all their medications and medical supplies, a list of chronic conditions, and emergency contact information, and to pack it in their carry-on luggage. C
• Instruct patients who will be airborne for ≥4 hours to stay hydrated, avoid alcohol and sedating drugs, and either do seated calf exercises or get up and move about the cabin periodically. B
• Remind patients who will spend time in developing countries to drink only bottled beverages, eat only hot food and fruit that can be peeled, and avoid ice cubes and food from street vendors. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
CASE Larry R, a 77-year-old retired college professor, comes in for a checkup because he is planning a trip to Kenya—on a safari he describes excitedly as “the trip of a lifetime.” He’ll be going with a group, but before he signs on he wants to be sure you think he can manage the tour’s “moderate pace.” He also thinks that he’ll “need to get some shots.”
The patient is overweight (BMI 29) and smokes a pipe daily. He has a history of hypertension, hyperlipidemia, and mild osteoarthritis in both knees and hips, all of which are well controlled.
What would you advise Professor R about the health care preparations needed for his big trip?
Chances are you have patients like Professor R—retired and relatively healthy, and endowed with a sense of adventure and the financial resources that make it possible to visit distant lands. With the nation’s 78 million baby boomers starting to reach retirement age—the oldest cohort turned 65 in 2011—you’re likely to see increasing numbers of older patients with plans for international travel in the years ahead.1
Like their younger counterparts, older people travel for a variety of reasons: Some have planned for decades to take the “trip of a lifetime” when they retire. Others plan longer excursions, sometimes referred to as an adult “gap year,” to relive a long-ago experience, volunteer in an underdeveloped country, or hike and bird watch in a rainforest. Many more are immigrants who travel to visit relatives or friends in their country of origin, usually a lower-income, environmentally depressed locale with a higher incidence of infectious diseases like malaria, typhoid, and hepatitis A. 2
And while the older traveler will have to take many of the same steps to stay healthy as his or her younger counterpart, it is the older traveler who is more likely to have chronic conditions and special needs that require additional preparation. With careful planning, however, even those with decreased faculties, ranging from impaired vision or hearing to mild cognitive impairment, can safely travel abroad.3
A pretravel visit is your opportunity to assess the patient’s fitness to make the trip being planned, ensure optimal management of chronic conditions while traveling, and identify (and recommend steps to mitigate) travel-related risks.
Morbidity and mortality abroad: A review of the risks
Although much pretravel advice centers on the prevention of tropical infectious diseases, such infections account for a very small percentage of deaths of Americans outside of the United States.1 In fact, the major health risks facing older adults traveling abroad are similar to those they face at home: Cardiovascular events are responsible for the preponderance of deaths and for half of all travel-related illnesses.1
International travel can be physically demanding for older individuals and injuries are common, accounting for a large proportion of deaths of Americans overseas4 and an estimated 25% to 38% of travel-related incidents.1,5 A third of injury-related deaths of US citizens traveling abroad involve traffic accidents, followed by homicide (17% of cases) and drowning (13%).1,5 Thus, injury prevention and management of chronic conditions are key issues to address in a pretravel consult.
Even small steps help safeguard older travelers
Older patients planning to travel abroad should schedule an appointment at least 4 to 6 weeks before their departure.2 Ask about the locale, political and environmental climate, length of stay, location and type of accommodations, accessibility to health care, and activities planned,6 which will enable you to offer both general and destination-specific health and safety tips. When advising older adults with complex comorbidities and/or particularly high-risk itineraries, referral to a travel medicine specialist should be strongly considered.
Exercise. Encourage older patients to initiate a graduated exercise program, starting several months before the trip.3 Even a modest improvement in endurance, strength, and flexibility can reduce the likelihood of injury.
Luggage. The right luggage can benefit your patients. Recommend that older patients purchase lightweight suitcases with wheels, which are easier to maneuver in and out of airports6 and less likely to cause muscle strain or musculoskeletal injury.
Insurance. If an individual becomes ill or sustains an injury overseas, the right insurance can be crucial. Advise older adults to review their health insurance policy to see whether it provides overseas coverage. If not, suggest they consider a short-term supplemental policy to cover medical care and evacuation, if needed. Recommend trip cancellation insurance, as well.
Patients should pack pills, medical supplies in a carry-on kit
Encourage all older travelers to compile a personalized travel health kit equipped with common over-the-counter (OTC) medications, prescription drugs, and any personal medical supplies they’ll need, such as a continuous positive airway pressure (CPAP) machine.1,3 Remind patients to take an ample supply of both prescription and OTC drugs, each in its original labeled container.3,6 Buying medications outside the United States is not advisable, given the variation in international regulatory standards. Stress the importance of keeping the kit in a carry-on bag.
The health kit should also include descriptions of the patient’s preexisting medical conditions, which you or a nurse or medical assistant in the practice can help to prepare; a list of prescription drugs he or she takes (using both the generic and brand names); and a copy of a recent electrocardiogram, if available, along with contact information in case of an emergency.1,3,6 A patient who uses injectable medication, such as insulin, should obtain a letter (on the practice’s letterhead) from the prescribing physician and be prepared to show the letter to airport security personnel.3,6
Staying safe in the air
The lower barometric and oxygen partial pressures found in aircraft cabins, which are pressurized at 5000 to 8000 feet, can affect both the respiratory and cardiovascular systems of older adults—particularly those with pulmonary or cardiac disorders.6,7 Individuals who do not routinely require oxygen and are able to walk the equivalent of one city block or climb one flight of stairs without shortness of breath should have little trouble compensating for the reduced oxygen in the cabin.3 Patients with stable heart failure, including New York Heart Association grades III and IV, can tolerate flights of up to one hour without additional oxygen.7
Advise older adults who will require oxygen that they are not permitted to bring their own oxygen canisters onboard an airplane.1 In-flight oxygen needs to be ordered at least 7 days before departure, and there may be a charge.7 Most airlines have medical consultants available to help patients who will need oxygen or other medical provisions.7 In addition, tour companies or travel consultants can help older patients with special needs ensure that they have access to oxygen or other medical supplies at their destination.
Thrombosis—the other in-flight risk
Sometimes referred to as “economy class syndrome” or “traveler’s thrombosis,” the venous stasis of air travel is responsible for a 3-fold increase in the risk of venous thromboembolism (VTE).8 While fatal pulmonary embolism is rare, duration of travel and risk of VTE follow a dose-response relationship, with each 2-hour increase in flight time conferring an additional 18% risk.8 Other risk factors for VTE include varicose veins, metastatic cancer, major surgery within the past 2 weeks, prior VTE, and BMI >40. Advanced age increases travelers’ risk of VTE, as well.7,8 The absolute risk, however, is low.
Among travelers older than 50 years, symptomatic VTE occurs at an estimated rate of one in 600 for flights >4 hours and one in 500 for flights >12 hours.9,10 While there is no evidence that first-class seating lowers the risk, there are preventive measures that patients can take.11
Tell patients to stay hydrated, drinking plenty of fluids but avoiding alcohol during flights of ≥4 hours’ duration. Sedating drugs should be avoided, as well. Advise anyone planning a long flight to either do seated exercises (intermittent calf contractions) or to periodically get up and walk about the cabin.9 You may also want to recommend that patients purchase below-the-knee elastic compression stockings to help decrease venous stasis.12
There is no evidence to recommend the use of aspirin to prevent VTE.12,13 But you may consider prescribing a single 40-mg dose of enoxaparin for a patient who has multiple risk factors and will be airborne for >6 hours.13
Promote safety and comfort on the ground
It is crucial to remind all travelers about the risks associated with traveling in motor vehicles in other countries. Remind patients to wear seat belts whenever they’re available; exercise caution regarding public transportation, which may be overcrowded and have an increased risk of pickpockets and robbery; and avoid riding on motorcycles and scooters. If they do opt to ride on a scooter, tell them that it’s imperative that they wear a helmet.
Minimize the effects of jet lag
Travelers of any age may experience jet lag, which occurs when the individual’s circadian clock cannot keep pace with travel across time zones.14 Notably, however, older people appear to suffer less than their younger counterparts.3 Patients traveling great distances are not likely to avoid jet lag completely, of course. Recommend the following strategies:
Start adjusting your schedule in the week before you depart, gradually shifting 2 hours toward congruence with the time zone at your destination.14
Help reset your circadian rhythm through exposure to bright light, in the morning after eastward travel and in the evening after westward travel.14
Take it easy at first. An itinerary that accounts for initial fatigue is an important nonpharmacologic management strategy.14
Avoid sedating medications, including antihistamines, tranquilizers, anti-motion sickness agents, and benzodiazepines, as these can increase falls and confusion in older adults and make jet lag worse.3
Take melatonin. A dose of 0.5 to 5 mg, taken at bedtime, may promote sleep and decrease jet lag symptoms in travelers crossing multiple time zones.14
Prepare patients to cope with heat …
Unusually hot, humid weather increases morbidity and mortality in the elderly,3,15 and older patients traveling to such climates will need to take extra precautions. Strenuous exercise in the heat should be avoided, because both thirst and the capacity to conserve salt and water decrease with age.16 Acclimatization is helped by rest, air-conditioning, loose cotton clothing, brimmed hats, and cool baths or showers.3 Diuretics may have to be adjusted for fluids lost by increased perspiration, and a discussion about a dose reduction should be included in the pretravel consult for patients who take diuretics and will be traveling to a hot, humid climate.
… and increases in altitude
For older adults, exposure to a moderate altitude (<2500 meters) is initially associated with hypoxemia and a reduced exercise capacity, until acclimatization occurs by Day 5.17,18 Although older adults generally acclimatize well, advise them to limit their activities for the first few days at a higher altitude. This is especially important for patients with coronary artery disease (CAD).
To further ease the effects of a higher altitude, advise patients to drink plenty of fluids, but little or no alcohol.19 Review the medications of an older patient who will be spending time at very high altitude. Rarely, antihypertensive medication may need to be adjusted. The body compensates for lower oxygen with a faster heart rate, and some antihypertensives may interfere with this compensatory mechanism.3
Precautions (and prophylaxis) may prevent travelers’ diarrhea
Diarrhea—among the most common travel-related conditions20—affects an estimated 30% to 70% of international travelers.2 The incidence is highest among visitors to developing countries. Most (80%-90%) of travelers’ diarrhea is due to bacterial infection,21 10% of cases are caused by parasites, and 5% to 8% by viral infection.2,22
Although increasing age lowers the risk of travelers’ diarrhea,1 older patients traveling to developing areas should be cautioned to only eat food that is served hot or fruit they can peel themselves; drink only bottled water and sealed liquids; and avoid salad, ice, and food from street vendors.1 Studies have shown, however, that tourists often get diarrhea despite these safety measures.2
Treatment and prophylaxis. Prophylactic antibiotics can prevent travelers’ diarrhea. But the increased sun sensitivity, drug-drug interactions, and gastrointestinal (GI) adverse effects associated with antibiotics limit their usefulness. Prophylaxis is indicated, however, for older adults for whom the complications of dehydration would likely be so severe that the benefits of using antibiotics to prevent diarrhea clearly outweigh the risks.23
Fluoroquinolones are a first-line treatment for travelers’ diarrhea. But increasing microbial resistance to this class of drugs, especially among Campylobacter isolates,24 may limit their usefulness in some destinations.25 Azithromycin is recommended in such cases, and has been shown to be equally effective.26,27 Single-dose therapy is well established with fluoroquinolones, but the best regimen for azithromycin (1 vs 3 days) is still under evaluation.28,29 Along with instructions on when to take an antibiotic, travelers should be given prescriptions for treatment of travelers’ diarrhea before the start of their trip. Suggest that patients purchase oral rehydration packets to take on their trip, and stress the importance of using them and staying hydrated if diarrhea develops.
Decreased upper GI acidity due to acid-blocking medications such as proton-pump inhibitors can increase the risk for many infections, including salmonella and cholera. Patients taking such medications should be made aware of the risk, and the risks vs benefits of temporarily stopping them should be discussed. Vaccination against cholera should not routinely be recommended.29,30
Vaccines and pills protect against preventable diseases
An impending trip abroad also presents an opportunity to review the patient’s immunization status, catch up on recommended vaccines, and determine whether any additional vaccinations are needed.
Herpes zoster (HZ). Patients older than 60 years should receive a single dose of the HZ vaccine, whether or not they have a history of this condition. Because this is a live virus-containing vaccine, however, it should not be given to anyone who is immunocompromised.31
MMR booster. Adults born before 1957 can be considered immune from both measles and mumps, but not rubella. There is no data on immunization to rubella, but guidelines do not recommend MMR vaccination in the elderly.1
Pneumococcal polyvalent-23 (PPV-23). One dose of the PPV-23 vaccine is indicated for all adults at age 65. This is especially important for travelers, as the prevalence of pneumococcal disease is likely higher in crowded, urban environments within less developed countries.31
Tetanus. Although tetanus is mainly a disease of the elderly, only 45% of men ages 70 years or older and 21% of women in this age group were found to have protective antibodies.1,32 In 2011, the Advisory Committee on Immunization Practices (ACIP) recommended one dose of tetanus and diphtheria toxoid (Td) every 10 years, with a single dose of tetanus toxoid, diphtheria toxoid, and acellular pertussis (Tdap) vaccine given in place of Td for adults older than 65.33 Despite ACIP’s recommendation, the vaccine’s use in adults 65 years and older is an off-label indication, as Tdap is only approved for use in those 11 to 64 years of age.33
Additional vaccines are recommended for travelers, with some indicated for all travelers and others that are destination-specific (TABLE).
TABLE
Which travel-related vaccines does your patient need?
| Disease | Type of vaccine | Primary course | Booster/ follow-up | Route | For which destinations? |
|---|---|---|---|---|---|
| Vaccines for all travelers | |||||
| Hepatitis A* | Killed virus | 2 doses (6-18 mo apart)† | None | IM | All |
| Hepatitis B* | Recombinant viral antigen | 3 doses (0, 1, 6 mo) | None | IM | All |
| Influenza | Inactivated viral | Single dose | Annually | IM | All |
| Typhoid | Capsular polysaccharide Live attenuated bacteria | Single dose 4 doses (0, 2, 4, 6 mo) | 2-3 y 5 y | IM Oral | All |
| Vaccines for travelers to select destinations | |||||
| Japanese encephalitis | Inactivated viral | 2 doses (28 d apart) | Unknown | IM | Rural Asia‡ |
| Meningococcus | Quadrivalent conjugated polysaccharide | Single dose | >10 y | IM | Sub-Saharan Africa; Saudi Arabia |
| Polio | Inactivated viral | Single dose if patient had childhood series | None | SC; IM | Anyplace where polio still occurs |
| Rabies | Inactivated cell culture viral | 3 doses (0, 7, 21-28 d) | None unless exposure occurs | IM | |
| Yellow fever | Live attenuated virus | Single dose | 10 y | SC | Sub-Saharan Africa; tropical South America |
| IM, intramuscular; SC, subcutaneous. *A combined hepatitis A/B vaccine is approved for use in older adults. †Second dose may be delayed up to 8 years without diminished efficacy. ‡Required only for prolonged stays in rural areas of Asia. Adapted from: Mandell GL, Bennett JE, Dolin R, eds. Principles and Practice of Infectious Diseases. 7th ed. Philadelphia, Pa: Elsevier; 2010. | |||||
Meds and safety measures can minimize malaria risk
The risk of acquiring malaria differs significantly among travelers, based on destination, duration and type of travel, and season. Choice of antimalarial agents (eg, atovaquone/proguanil, chloroquine, doxycycline, mefloquine, and primaquine) should be made on an individual basis after considering these factors, as well as the resistance patterns of the countries on the patient’s itinerary, his or her medical history, and the adverse effects profile of potential agents. Because many older adults take multiple medications, the possibility of drug-drug interactions must be considered.1 You’ll find destination-specific recommendations on malaria prevention on the Centers for Disease Control and Prevention’s Travelers’ Health Web site, listed in “Travel and health: Resources for patients and physicians”. For guidance on the best drug to prescribe, you can also consult a travel medicine specialist.2
Access-able Travel Source Web site provides information for older adult travelers with special needs who need help traveling with oxygen or getting around despite decreased mobility (www.access-able.com/tips/).
American Diabetes Association publishes detailed information about traveling with diabetes (http://www.diabetes.org/living-with-diabetes/know-your-rights/discrimination/public-accommodations/air-travel-and-diabetes/).
Bureau of Consular Affairs publishes information regarding VISA and security requirements at various destinations and travel warnings (http://travel.state.gov/about/about_304.html).
Centers for Disease Control and Prevention publishes “The Yellow Book”—a reference for clinicians who advise international travelers about health risks. There is also a range of other travel-related information on its Travelers’ Health Web site (www.cdc.gov/travel/).
International Travel Medicine Society provides a global travel clinic directory (http://www.istm.org/Webforms/Searchclinics/Default.aspx?SearchType=advanced).
Transportation Security Administration Web site provides information on what can be brought on-board a plane. See “Can I bring my … through the checkpoint?” (http://apps.tsa.dhs.gov/mytsa/cib_home.aspx).
Travel Health Online offers a list of medical providers around the world (www.tripprep.com/scripts).
World Health Organization’s Travel and Health Web site provides free access to selected chapters of its book, “International Travel and Health 2012,” as well as interactive maps, information about infectious diseases and food safety, and more (www.who.int/ith/en/).
Patients should be mindful of mosquitos. Stress the importance of preventing mosquito bites (as much as possible). Advise patients traveling to mosquito-infested areas to use insect repellents containing 30% N-diethyl-meta-toluamide (DEET) and permethrin-treated clothing.34 Tell them, too, to wear long sleeves, pants, and footwear that provides full coverage.35 Ensuring that sleeping areas are properly screened or air-conditioned will further reduce the likelihood of mosquito bites.36
CASE After seeing the chief complaint listed as “Traveling to Kenya” on Professor R’s chart, you quickly review the CDC’s Travelers’ Health Web site. You encourage him to stay with his tour group and to wear a seatbelt whenever possible. You also review how to make a personalized travel health kit, and encourage him to register with the Smart Traveler Enrollment Program (STEP) (detailed at https://step.state.gov/rep) before leaving for the safari. You strongly suggest that he consider purchasing additional medical evacuation insurance, as well.
Given the prevalence of travelers’ diarrhea, along with dengue and malaria, in Kenya, you review food and water safety and avoidance of insect-transmitted diseases with the patient, and write a prescription for ciprofloxacin to be taken if he develops diarrhea. Professor R is not at high risk for VTE, but you encourage him to stay hydrated, avoid sedating medications, and be diligent about mobilization during lengthy flights. You recommend melatonin for jet lag.
To adjust to the heat, you recommend that he avoid strenuous exercise in the first few days and drink sufficient fluids throughout the trip. You administer the Tdap vaccine, an adult polio booster, and the hepatitis A vaccine, verify that he has received his pneumococcal and influenza vaccines, and prescribe an antimalarial medication.
And as you walk him toward the door, you offer him one final piece of advice: Take plenty of pictures.
CORRESPONDENCE
Jeffrey D. Schlaudecker, MD, The Christ Hospital/University of Cincinnati Family Medicine Residency Program, 2123 Auburn Avenue #340, Cincinnati, OH 45219; Jeffrey.schlaudecker@uc.edu
1. Reed CM. Travel recommendations for older adults. Clin Geriatr Med. 2007;23:687-713, ix.
2. Centers for Disease Control and Prevention. Health Information for International Travel 2012. New York, NY:Oxford University Press; 2012. Available at http://wwwnc.cdc.gov/travel/page/yellowboth-2012-home.htm. Accessed December 18, 2012.
3. Cooper MC. The elderly travellers. Travel Med Infect Dis. 2006;4:218-222.
4. Guse CE, Cortes LM, Hargarten SW, et al. Fatal injuries of US citizens abroad. J Travel Med. 2007;14:279-287
5. Tonellato DJ, Guse CE, Hargarten SW. Injury deaths of US citizens abroad: new data source, old travel problem. J Travel Med. 2009;16:304-310.
6. Fenner P. Fitness to travel - assessment in the elderly and medically impaired. Aust Fam Physician. 2007;36:312-315.
7. Smith D, Toff W, Joy M, et al. Fitness to fly for passengers with cardiovascular disease. Heart. 2010;96(suppl 2):Sii1-S16.
8. Chandra D, Parisini E, Mozaffarian D. Meta-analysis: travel and risk for venous thromboembolism. Ann Intern Med. 2009;151:180-190.
9. Gavish I, Brenner B. Air travel and the risk of thromboembolism. Intern Emerg Med. 2011;6:113-116.
10. Ansari MT, Cheung BM, Qing Huang J, et al. Traveler’s thrombosis: a systematic review. J Travel Med. 2005;12:142-154.
11. Schwarz T, Siegert G, Oettler W, et al. Venous thrombosis after long-haul flights. Arch Intern Med. 2003;163:2759-2764.
12. Cesarone MR, Belcaro G, Errichi BM, et al. The LONFLIT4—Concorde Deep Venous Thrombosis and Edema Study: prevention with travel stockings. Angiology. 2003;54:143-154.
13. Cesarone MR, Belcaro G, Nicolaides AN, et al. Venous thrombosis from air travel: the LONFLIT3 study—prevention with aspirin vs low-molecular-weight heparin (LMWH) in high-risk subjects: a randomized trial. Angiology. 2002;53:1-6.
14. Sack RL. Clinical practice. Jet lag. N Engl J Med. 2010: 440-447.
15. Davies I, O’Neill PA, McLean KA, et al. Age-associated alterations in thirst and arginine vasopressin in response to a water or sodium load. Age Ageing. 1995;24:151-159.
16. Rikkert MG, Melis RJ, Claassen JA. Heat waves and dehydration in the elderly. BMJ. 2009;339:b2663.-
17. Levine BD, Zuckerman JH, deFilippi CR. Effect of high-altitude exposure in the elderly: the Tenth Mountain Division study. Circulation. 1997;96:1224-1232.
18. Agostoni P, Cattadori G, Guazzi M, et al. Effects of simulated altitude-induced hypoxia on exercise capacity in patients with chronic heart failure. Am J Med. 2000;109:450-455.
19. Higgins JP, Tuttle T, Higgins JA. Altitude and the heart: is going high safe for your cardiac patient? Am Heart J. 2010;159:25-32.
20. Gautret P, Schlagenhauf P, Gaudart J, et al. Multicenter EuroTravNet/GeoSentinel study of travel-related infectious diseases in Europe. Emerg Infect Dis. 2009;15:1783-1790.
21. Adachi JA, Jiang ZD, Mathewson JJ, et al. Enteroaggregative Escherichia coli as a major etiologic agent in traveler’s diarrhea in 3 regions of the world. Clin Infect Dis. 2001;32:1706-1709.
22. Black RE. Epidemiology of travelers’ diarrhea and relative importance of various pathogens. Rev Infect Dis. 1990;12 (suppl 1):S73-S79.
23. Rendi-Wagner P, Kollaritsch H. Drug prophylaxis for travelers’ diarrhea. Clin Infect Dis. 2002;34:628-633.
24. Butzler JP. Campylobacter, from obscurity to celebrity. Clin Microbiol Infect. 2004;10:868-876.
25. Hoge CW, Gambel JM, Srijan A, et al. Trends in antibiotic resistance among diarrheal pathogens isolated in Thailand over 15 years. Clin Infect Dis. 1998;26:341-345.
26. Adachi JA, Ericsson CD, Jiang ZD, et al. Azithromycin found to be comparable to levofloxacin for the treatment of US travelers with acute diarrhea acquired in Mexico. Clin Infect Dis. 2003;37:1165-1171.
27. Tribble DR, Sanders JW, Pang LW, et al. Traveler’s diarrhea in Thailand: randomized, double-blind trial comparing single-dose and 3-day azithromycin-based regimens with a 3-day levofloxacin regimen. Clin Infect Dis. 2007;44:338-346.
28. Shanks GD, Smoak BL, Aleman GM, et al. Single dose of azithromycin or three-day course of ciprofloxacin as therapy for epidemic dysentery in Kenya. Acute Dysentery Study Group. Clin Infect Dis. 1999;29:942-943.
29. Heatley RV, Sobala GM. Acid suppression and the gastric flora. Baillieres Clin Gastroenterol. 1993;7:167-181.
30. Hill DR, Ford L, Lalloo DG. Oral cholera vaccines: use in clinical practice. Lancet Infect Dis. 2006;6:361-373.
31. Vaughn JA, Miller RA. Update on immunizations in adults. Am Fam Physician. 2011;84:1015-1020.
32. Gergen PJ, McQuillan GM, Kiely M, et al. A population-based serologic survey of immunity to tetanus in the United States. N Engl J Med. 1995;332:761-766.
33. World Health Organization. Typhoid vaccines: WHO position paper. Wkly Epidemiol Rec. 2008;83:49-59.
34. Fradin MS, Day JF. Comparative efficacy of insect repellents against mosquito bites. N Engl J Med. 2002;347:13-18.
35. Soto J, Medina F, Dember N, et al. Efficacy of permethrin-impregnated uniforms in the prevention of malaria and leishmaniasis in Colombian soldiers. Clin Infect Dis. 1995;21:599-602.
36. Freedman DO. Clinical practice. Malaria prevention in short-term travelers. N Engl J Med. 2008;359:603-612.
1. Reed CM. Travel recommendations for older adults. Clin Geriatr Med. 2007;23:687-713, ix.
2. Centers for Disease Control and Prevention. Health Information for International Travel 2012. New York, NY:Oxford University Press; 2012. Available at http://wwwnc.cdc.gov/travel/page/yellowboth-2012-home.htm. Accessed December 18, 2012.
3. Cooper MC. The elderly travellers. Travel Med Infect Dis. 2006;4:218-222.
4. Guse CE, Cortes LM, Hargarten SW, et al. Fatal injuries of US citizens abroad. J Travel Med. 2007;14:279-287
5. Tonellato DJ, Guse CE, Hargarten SW. Injury deaths of US citizens abroad: new data source, old travel problem. J Travel Med. 2009;16:304-310.
6. Fenner P. Fitness to travel - assessment in the elderly and medically impaired. Aust Fam Physician. 2007;36:312-315.
7. Smith D, Toff W, Joy M, et al. Fitness to fly for passengers with cardiovascular disease. Heart. 2010;96(suppl 2):Sii1-S16.
8. Chandra D, Parisini E, Mozaffarian D. Meta-analysis: travel and risk for venous thromboembolism. Ann Intern Med. 2009;151:180-190.
9. Gavish I, Brenner B. Air travel and the risk of thromboembolism. Intern Emerg Med. 2011;6:113-116.
10. Ansari MT, Cheung BM, Qing Huang J, et al. Traveler’s thrombosis: a systematic review. J Travel Med. 2005;12:142-154.
11. Schwarz T, Siegert G, Oettler W, et al. Venous thrombosis after long-haul flights. Arch Intern Med. 2003;163:2759-2764.
12. Cesarone MR, Belcaro G, Errichi BM, et al. The LONFLIT4—Concorde Deep Venous Thrombosis and Edema Study: prevention with travel stockings. Angiology. 2003;54:143-154.
13. Cesarone MR, Belcaro G, Nicolaides AN, et al. Venous thrombosis from air travel: the LONFLIT3 study—prevention with aspirin vs low-molecular-weight heparin (LMWH) in high-risk subjects: a randomized trial. Angiology. 2002;53:1-6.
14. Sack RL. Clinical practice. Jet lag. N Engl J Med. 2010: 440-447.
15. Davies I, O’Neill PA, McLean KA, et al. Age-associated alterations in thirst and arginine vasopressin in response to a water or sodium load. Age Ageing. 1995;24:151-159.
16. Rikkert MG, Melis RJ, Claassen JA. Heat waves and dehydration in the elderly. BMJ. 2009;339:b2663.-
17. Levine BD, Zuckerman JH, deFilippi CR. Effect of high-altitude exposure in the elderly: the Tenth Mountain Division study. Circulation. 1997;96:1224-1232.
18. Agostoni P, Cattadori G, Guazzi M, et al. Effects of simulated altitude-induced hypoxia on exercise capacity in patients with chronic heart failure. Am J Med. 2000;109:450-455.
19. Higgins JP, Tuttle T, Higgins JA. Altitude and the heart: is going high safe for your cardiac patient? Am Heart J. 2010;159:25-32.
20. Gautret P, Schlagenhauf P, Gaudart J, et al. Multicenter EuroTravNet/GeoSentinel study of travel-related infectious diseases in Europe. Emerg Infect Dis. 2009;15:1783-1790.
21. Adachi JA, Jiang ZD, Mathewson JJ, et al. Enteroaggregative Escherichia coli as a major etiologic agent in traveler’s diarrhea in 3 regions of the world. Clin Infect Dis. 2001;32:1706-1709.
22. Black RE. Epidemiology of travelers’ diarrhea and relative importance of various pathogens. Rev Infect Dis. 1990;12 (suppl 1):S73-S79.
23. Rendi-Wagner P, Kollaritsch H. Drug prophylaxis for travelers’ diarrhea. Clin Infect Dis. 2002;34:628-633.
24. Butzler JP. Campylobacter, from obscurity to celebrity. Clin Microbiol Infect. 2004;10:868-876.
25. Hoge CW, Gambel JM, Srijan A, et al. Trends in antibiotic resistance among diarrheal pathogens isolated in Thailand over 15 years. Clin Infect Dis. 1998;26:341-345.
26. Adachi JA, Ericsson CD, Jiang ZD, et al. Azithromycin found to be comparable to levofloxacin for the treatment of US travelers with acute diarrhea acquired in Mexico. Clin Infect Dis. 2003;37:1165-1171.
27. Tribble DR, Sanders JW, Pang LW, et al. Traveler’s diarrhea in Thailand: randomized, double-blind trial comparing single-dose and 3-day azithromycin-based regimens with a 3-day levofloxacin regimen. Clin Infect Dis. 2007;44:338-346.
28. Shanks GD, Smoak BL, Aleman GM, et al. Single dose of azithromycin or three-day course of ciprofloxacin as therapy for epidemic dysentery in Kenya. Acute Dysentery Study Group. Clin Infect Dis. 1999;29:942-943.
29. Heatley RV, Sobala GM. Acid suppression and the gastric flora. Baillieres Clin Gastroenterol. 1993;7:167-181.
30. Hill DR, Ford L, Lalloo DG. Oral cholera vaccines: use in clinical practice. Lancet Infect Dis. 2006;6:361-373.
31. Vaughn JA, Miller RA. Update on immunizations in adults. Am Fam Physician. 2011;84:1015-1020.
32. Gergen PJ, McQuillan GM, Kiely M, et al. A population-based serologic survey of immunity to tetanus in the United States. N Engl J Med. 1995;332:761-766.
33. World Health Organization. Typhoid vaccines: WHO position paper. Wkly Epidemiol Rec. 2008;83:49-59.
34. Fradin MS, Day JF. Comparative efficacy of insect repellents against mosquito bites. N Engl J Med. 2002;347:13-18.
35. Soto J, Medina F, Dember N, et al. Efficacy of permethrin-impregnated uniforms in the prevention of malaria and leishmaniasis in Colombian soldiers. Clin Infect Dis. 1995;21:599-602.
36. Freedman DO. Clinical practice. Malaria prevention in short-term travelers. N Engl J Med. 2008;359:603-612.
Community-acquired pneumonia in children: A look at the IDSA guidelines
• Chest x-rays and lab testing may be optional for children with community-acquired pneumonia (CAP) who are not seriously ill. A
• Start amoxicillin empirically for any child with mild-to-moderate CAP. B
• If an atypical bacterial pneumonia is suspected, azithromycin is the first-line treatment. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
What are the recommended antibiotic choices for children with mild-to-moderate bacterial community-acquired pneumonia (CAP) in the outpatient setting? How much diagnostic testing is required? When might hospitalization and combination antibiotic therapy be warranted?
Evidence-based answers to these and other questions relevant to the management of CAP in infants and children older than 3 months are provided in a set of guidelines jointly published by the Infectious Diseases Society of America (IDSA) and the Pediatric Infectious Diseases Society (PIDS) in 2011.1 We summarize them here.
What the guidelines do, and don’t, address
The IDSA/PIDS guidelines, which focus on the care of otherwise healthy children with CAP in both outpatient and inpatient settings, seek to decrease morbidity and mortality rates associated with this respiratory infection. The guidelines do not apply to children younger than 3 months, immunocompromised patients, children receiving home mechanical ventilation, or children with chronic conditions or underlying lung disease, such as cystic fibrosis.
The need for evidence-based guidance. Globally each year, 1.5 million children 5 years of age and younger suffer a pneumonia-related death, particularly in developing countries.2-5 This is more than the number of deaths associated with any other disease in the world, including acquired immune deficiency syndrome (AIDS), tuberculosis (TB), or malaria.2 In 2010, pneumonia was ranked in the United States as the sixth leading cause of death for children one to 4 years of age and the 10th leading cause of death in adolescents.5 It is estimated that out of every 1000 infants and children in North America and Europe, 35 to 40 will be affected by CAP.2
How the guidelines define CAP. Pneumonia can be broadly defined as a lower respiratory tract infection, but definitions vary depending on the organization, institution, or health care setting. For instance, the World Health Organization (WHO) defines pneumonia solely on the basis of clinical findings obtained by visual inspection and timing of the respiratory rate.6 Another definition published by Bone and colleagues states that pneumonia is the “inflammation of the pulmonary parenchyma brought about by the presence of virulent pathogens; usually differentiated from isolated infections of the major airways.”7 The new pediatric guidelines define CAP as “the presence of signs and symptoms of pneumonia in a previously healthy child caused by an infection that has been acquired outside the hospital.”1
CAP pathogens vary with the child’s age
Typically, diagnostic testing of children will reveal several microbes, viral and bacterial, making it difficult to determine which might be the pathogen.1 Viral pathogens are more common causes of CAP in children younger than 2 years, accounting for 80% of cases1; bacterial pathogens are more common in older children.1
The virus detected most often among children younger than 2 years is respiratory syncytial virus (RSV).1,8-12 Less common viruses include adenovirus, influenza types A and B, parainfluenza 1, 2, and 3, and rhinovirus. Streptococcus pneumoniae is the most common bacterial pathogen identified in older children.1,13 The overall incidence of pneumonia decreases with age, but it has been reported that the proportion of cases from atypical bacterial pathogens—Chlamydia pneumoniae and Mycoplasma pneumoniae—may increase among older children.1,13
Signs and symptoms also vary
Signs and symptoms of CAP differ depending on the severity of the infection and the age of the child. In general, respiratory distress (tachypnea, nasal flaring, decreased breath sounds, cough, and rales) with fever are the prominent symptoms associated with pneumonia.1,13,14
Infants and children with mild to moderate infection most commonly exhibit a temperature <38°C and a respiratory rate <50 breaths per minute (bpm).
Children with severe CAP commonly present with a temperature >38°C, flaring of nostrils, grunting with breathing, tachypnea, tachycardia, and cyanosis. Tachypnea is defined as >60 bpm in infants younger than 2 months, >50 bpm in infants 2 to 12 months, and >40 bpm in children ages 1 to 5 years.8 Although respiratory rate is a valuable clinical sign, the work of breathing (as evidenced by nasal flaring, breathlessness, cough, or wheeze) required by the infant or child may be more indicative of pneumonia.15
Utilize diagnostic testing judiciously
Not all patients with suspected CAP require the same amount of diagnostic testing. In fact, IDSA/PIDS recommendations vary for hospitalized patients and for outpatients.1 In all cases, conduct testing quickly to expedite diagnosis and minimize the need for additional testing, to help validate treatment choices, and to reduce time spent in the hospital.1
Blood and sputum cultures not always indicated. The IDSA/PIDS guidelines strongly recommend obtaining blood cultures for hospitalized patients with moderate-to-severe pneumonia, particularly those with complications.1
The guidelines strongly recommend against blood cultures for fully immunized children with CAP who are treated as outpatients. However, blood cultures are strongly recommended for any child who fails to improve after initiation of antibiotic therapy.1 These recommendations are consistent with clinical data, expert opinion, and other treatment guidelines.1,8,13-18
A weak recommendation from the new guidelines states that if a hospitalized child with CAP can produce sputum, gram staining of the specimen may be warranted.1,8,13,15
Use pulse oximetry. The guidelines strongly recommend using pulse oximetry with all children who have pneumonia or suspected hypoxemia.1,18
When chest radiography can help. Routine chest radiography may not be warranted for suspected CAP treated in the outpatient setting. Order chest films for patients with suspected or confirmed hypoxemia or respiratory distress (who tend to have worse outcomes), and for patients who do not respond to initial antibiotic treatment.1,18 Follow-up radiographs are recommended for patients with advancing symptoms 2 to 3 days after starting antibiotics, complicated pneumonia with worsening respiratory distress, or clinical symptoms without improvement.1
Other diagnostic tests mentioned in the guidelines include complete blood cell counts, which are recommended in severe cases of pneumonia.1
Acute-phase reactants such as erythrocyte sedimentation rate (ESR), serum procalcitonin, and C-reactive protein concentrations cannot distinguish between viral and bacterial causes of CAP, and are not routinely recommended for patients treated in the outpatient setting.1,13
For patients requiring endotracheal intubation, gram staining and cultures of aspirates of the trachea and virus testing are recommended.1
Immunocompetent patients hospitalized with severe CAP may be candidates for percutaneous lung aspiration, open lung biopsy, bronchoalveolar lavage (BAL), or bronchoscopic or blind protected brush specimen collection if prior diagnostic tests are negative.1
CAP treatment and prevention
The guidelines provide recommendations for treating bacterial and viral CAP in either inpatient or outpatient settings, and discuss appropriate preventive techniques.
Antiviral therapy. As mentioned earlier, children less than 2 years of age are commonly infected with viral pathogens. Those with mild cases of viral CAP do not require anti-microbial therapy. For children with moderate-to-severe CAP consistent with influenza infection, administer influenza antiviral therapy as soon as possible, especially during a widespread local circulation of influenza viruses. Some influenza A strains will be susceptible to antiviral therapy, even though genetic variability is high each year. The guidelines’ recommended agents for treating influenza in pediatric patients are listed in TABLE 1.1
TABLE 1
Influenza antiviral therapy in pediatric patients*1
| Drug (brand name) | Formulation | Dosing |
|---|---|---|
| Oseltamivir (Tamiflu) | 75 mg capsule; 60 mg/5 mL suspension | 4-8 mo: 6 mg/kg/d in 2 doses 9-23 mo: 7 mg/kg/d in 2 doses ≥24 mo: ~4 mg/kg/d in 2 doses, for 5 days ≤15 kg: 60 mg/d in 2 divided doses >15-23 kg: 90 mg/d in 2 divided doses >23-40 kg: 120 mg/d in 2 divided doses >40 kg: 150 mg/d in 2 divided doses |
| Zanamivir (Relenza) | 5 mg per inhalation, using a Diskhaler | ≥7 y: 2 inhalations (10 mg total per dose), twice daily for 5 days |
| Amantadine (Symmetrel)† | 100 mg tablet; 50 mg/5 mL suspension | 1-9 y: 5-8 mg/kg/d as single daily dose or in 2 doses; not to exceed 150 mg/d 9-12 y: 200 mg/d in 2 doses (not studied as a single dose) |
| Rimantadine (Flumadine)† | 100 mg tablet; 50 mg/5 mL suspension | Not FDA approved for treatment in children, but published data exist on safety and efficacy in children Suspension: 1-9 y: 6.6 mg/kg/d (max 150 mg/kg/d) in 2 doses ≥10 y: 200 mg/d, as single daily dose or in 2 doses |
| *In children for whom prophylaxis is indicated, antiviral drugs should be continued for the duration of known influenza activity in the community (because of the potential for repeated exposures) or until immunity can be achieved as a result of immunization. †Amantadine and rimantadine should be used for treatment and prophylaxis only in the winter, when most isolated influenza A virus strains are susceptible to adamantine; the adamantines should not be used for primary therapy because of the rapid emergence of resistance. However, for patients requiring adamantine therapy, a treatment course of about 7 days is suggested, or one that runs until a day or 2 after the signs and symptoms have disappeared. | ||
Antibacterial therapy. For patients with a suspected bacterial pathogen, start empiric antibiotic therapy as soon as possible. Preferred and alternative agents for specific age groups, immunization status, and specific pathogen(s) appear in TABLE 2.1,19
TABLE 2
Empiric outpatient antibiotic therapy for pediatric CAP1,19
Duration of treatment is 10 days unless otherwise noted
| Patient age | Presumed bacterial pneumonia | Presumed atypical pneumonia |
|---|---|---|
| 3 mo to <5 y, regardless of immunization status | Preferred: amoxicillin 90 mg/kg/d PO in 2 divided doses Alternative: amoxicillin clavulanate 90 mg/kg/d PO in 2 divided doses | For all children regardless of age and immunization status: Preferred: azithromycin 10 mg/kg PO on Day 1, followed by 5 mg/kg PO once daily on Days 2-5 Alternative: clarithromycin 15 mg/kg/d PO in 2 divided doses OR In children >7 y: erythromycin 40 mg/kg/d PO in 4 divided doses; or doxycycline 2-4 mg/kg/d PO in 2 divided doses |
| ≥5 y and fully immunized against Streptococcus pneumoniae and Haemophilus influenzae | Preferred:* amoxicillin 90 mg/kg/d PO in 2 divided doses to a maximum 4 g/d, with or without a macrolide antibiotic Alternatives: Second- or third-generation cephalosporins such as oral cefpodoxime, cefuroxime, or cefprozil OR levofloxacin (5-16 y) 8-10 mg/kg PO once daily (max 750 mg/d)†OR linezolid (<12 y) 30 mg/kg/d PO (max 1200 mg/d) in 3 divided doses; or (≥12 y) 20 mg/kg/d (max 1200 mg/d) in 2 divided doses | |
| ≥5 y and NOT fully immunized against S pneumoniae and H influenzae | Preferred:* amoxicillin 90 mg/kg/d PO in 2 divided doses to a max of 4 g/d; or amoxicillin clavulanate 90 mg/kg/d PO in 2 divided doses Alternatives: Second- or third-generation cephalosporins such as oral cefpodoxime, cefuroxime, or cefprozil OR levofloxacin (5-16 y) 8-10 mg/kg PO once daily (max 750 mg/d)† | |
| CAP, community-acquired pneumonia. *Preferred treatments of choice change in areas of high S pneumoniae resistance. Refer to the complete guidelines for specific recommendations. †The guidelines do not fully address the controversy concerning the use of quinolones in children. The use of quinolones in infants and children is considered a risk vs benefit decision. | ||
Patients with mild or moderate CAP may be treated first in the outpatient setting with amoxicillin. This antibiotic has been the agent of choice for many years and continues to be the empiric therapy recommended in the guidelines.1 Appropriate dosing depends on the age of the patient.
TABLE 2 also includes treatment alternatives to amoxicillin for patients with drug allergies, treatment failures, or suspected atypical pathogens. Amoxicillin and the alternative treatments provide coverage for S pneumoniae, the most common invasive bacterial pathogen in older children.1,20 When atypical pathogens are suspected, macrolide antibiotics become the antibiotic drug class of choice, with azithromycin being the preferred first-line agent.1,21-23
Bacterial CAP necessitating hospitalization. The guidelines strongly recommend hospitalization for infants and children with respiratory distress or hypoxemia (oxygen saturation <90%); for suspicion of infection caused by community-acquired methicillin-resistant Staphylococcus aureus (MRSA) or any pathogen with high virulence; or for infants 3 to 6 months old.1
Treat with parenteral antibiotics to provide reliable blood and tissue concentrations (TABLE 3).1,19 Ampicillin or penicillin G may be given to fully immunized children; however, take into account the local resistance pattern of S pneumoniae to drugs within the penicillin class. For hospitalized children who are not yet fully immunized, who have life-threatening infections, or who are in a facility with a documented high rate of penicillin resistance, administer a third-generation parenteral cephalosporin such as ceftriaxone or cefotaxime empirically.1,24 In monotherapy treatment of pneumococcal pneumonia, non–beta-lactam agents such as vancomycin have not been shown to be more effective than the third-generation cephalosporins.1
TABLE 3
Empiric antibiotic therapy for hospitalized patients with CAP1,19
Duration of treatment is 10 days unless otherwise noted
| Patient age | Presumed bacterial pneumonia | Presumed atypical pneumonia |
|---|---|---|
| 3 mo to <5 y and fully immunized against Streptococcus pneumoniae and Haemophilus influenzae | Preferred:* ampicillin 150-200 mg/kg/d IV divided every 6 h; or penicillin G 200,000-250,000 units/kg/d IV divided every 4-6 h Alternatives: ceftriaxone 50-100 mg/kg/d IV/IM divided every 12-24 h; or cefotaxime 150 mg/kg/d IV divided every 8 h | For all children regardless of age and immunization status: Preferred: azithromycin, 10 mg/kg IV (max of 500 mg) on Days 1 and 2, then transition to oral therapy 10 mg/kg/d for remaining 7-10 days of therapy Alternatives: erythromycin lactobionate 20 mg/kg/d IV divided every 6 h; or levofloxacin 16-20 mg/kg/d IV divided every 12 h to a max of 750 mg/d† |
| <5 y and NOT fully immunized against S pneumoniae and H influenzae | Preferred:* ceftriaxone 50-100 mg/kg/d IV/IM divided every 12-24 h; or cefotaxime 150 mg/kg/d IV divided every 8 h Alternative: levofloxacin (6 mo–<5 y) 16-20 mg/kg/d IV divided every 12 h† | |
| ≥5 y and fully immunized against S pneumoniae and H influenzae | Preferred:* ampicillin 150-200 mg/kg/d IV divided every 6 h; or penicillin G 200,000-250,000 units/kg/d IV divided every 4-6 h Alternatives: ceftriaxone 50-100 mg/kg/d IV/IM divided every 12-24 h; or cefotaxime 150 mg/kg/d IV divided every 8 h | |
| ≥5 y and NOT fully immunized against S pneumoniae and H influenzae | Preferred:* ceftriaxone 50-100 mg/kg/d IV/IM divided every 12-24 h; or cefotaxime 150 mg/kg/d IV divided every 8 h Alternatives: ampicillin 150-200 mg/kg/d IV divided every 6 h; or levofloxacin 8-10 mg/kg IV once daily (max of 750 mg/d)† | |
| CAP, community-acquired pneumonia. *The addition of clindamycin 40 mg/kg/d IV divided every 6-8 hours or vancomycin 40-60 mg/kg/day IV divided every 6-8 hours is recommended for suspected or confirmed community-acquired methicillin-resistant Staphylococcus aureus. †The guidelines do not fully address the controversy concerning the use of quinolones in children. Use of quinolones in infants and children is considered a risk vs benefit decision. | ||
If S aureus is the suspected microorganism or is confirmed with clinical, laboratory, or imaging characteristics, give vancomycin or clindamycin with a beta-lactam agent.1,25-26 If you suspect an atypical pathogen such as M pneumoniae or C pneumoniae, start empiric therapy with an oral or parenteral macrolide in combination with a beta-lactam.1
Once a pathogen has been identified, adjust antimicrobial therapy as needed to target the specific microbe, to limit empiric antibiotic exposure, and to help limit the potential for antibiotic resistance.
Duration of treatment. The recommended duration of treatment for CAP is 10 days, supported by clinical data and the practice guidelines.1,27-29 Shorter treatment courses may be effective, especially in mild cases or outpatient treatment.1 Specific pathogens, such as MRSA, may need to be treated longer.30
If a patient is receiving intravenous antibiotics, switch to an oral agent as soon as clinically feasible to decrease risks from parenteral administration, and plan for the earliest possible discharge from the hospital to limit exposure to nosocomial pathogens. Hospital discharge may be considered when a child is clinically stable (improved appetite and activity level, afebrile for 24 hours), mental status is back to baseline or stable, and the pulse oximetry level is >90% on room air for at least 24 hours.1
Children receiving adequate therapy regimens should demonstrate both clinical and laboratory signs of improvement within 48 to 72 hours.1 If improvement does not occur, further your investigation with additional cultures, laboratory tests, and imaging evaluation.
For preventive measures, the guidelines recommend properly immunizing children with vaccines for bacterial pathogens such as S pneumoniae, Haemophilus influenzae, and Bordetella pertussis.1 Influenza vaccine should also be offered to prevent CAP in infants and children 6 months of age and older. Offer influenza and pertussis vaccines to adults and those caring for infants and children, to help prevent the spread of disease. Also consider immune prophylaxis with RSV-specific monoclonal antibody for premature infants or those with bronchopulmonary dysplasia, congenital heart disease, or immunodeficiency, to decrease the risk of severe pneumonia and hospitalization. For detailed recommendations on the use of prophylaxis against RSV, refer to the 2003 American Academy of Pediatrics statement.31
CORRESPONDENCE
Stephanie Schauner, PharmD, BCPS, University of Missouri-Kansas City, Health Science Building, Room 2241, 2464 Charlotte Street, Kansas City, MO 64108-2792; schauners@umkc.edu
1. Bradley JS, Byington CL, Shah SS, et al. The management of community-acquired pneumonia in infants and children older than 3 months of age: clinical practice guidelines by the Pediatric Infectious Diseases Society and the Infectious Diseases Society of America. Clin Infect Dis. 2011;53:e25-e76.Available at: http://cid.oxfordjournals.org/content/53/7/e25.long. Accessed December 17, 2012.
2. Centers for Disease Control and Prevention Pneumonia Can Be Prevented–Vaccines Can Help. Available at: http://www.cdc.gov/features/pneumonia. Accessed January 17, 2012.
3. Bulla A, Hitze KL. Acute respiratory infections: a review. Bull World Health Organ. 1978;56:481-498.
4. Baqui AH, Black RE, Arifeen SE, et al. Causes of childhood deaths in Bangladesh: results of a nationwide verbal autopsy study. Bull World Health Organ. 1998;76:161-171.
5. Murphy SL, Xu JQ, Kochanek KD. Deaths: Preliminary data for 2010. National vital statistics reports; vol 60 no 4. Hyattsville, Md: National Center for Health Statistics. 2012. Available at: http://www.cdc.gov/nchs/data/nvsr/nvsr60/nvsr60_04.pdf. Accessed May 12, 2012.
6. Clinical management of acute respiratory infections in children: a WHO memorandum. Bull World Health Organ. 1981;59:707-716.
7. Feldman C, Anderson R. Community-acquired pneumonia. In; Bone RC, Dantzker DR, George RB, et al, eds. Pulmonary and Critical Care Medicine. Vol 2. St. Louis, Mo: Mosby-Year Book, Inc; 1997:719–733.
8. Davies HD. Community-acquired pneumonia in children. Paediatr Child Health. 2003;8:616-619.
9. Alexander ER, Foy HM, Kenny GE, et al. Pneumonia due to Mycoplasma pneumoniae. Its incidence in the membership of a co-operative medical group. N Engl J Med. 1966;275:131-136.
10. Foy HM, Cooney MK, Maletzky AJ, et al. Incidence and etiology of pneumonia, croup and bronchiolitis in preschool children belonging to a prepaid medical group over a four-year period. Am J Epidemiol. 1973;97:80-92.
11. Murphy TF, Henderson FW, Clyde WA, Jr, et al. Pneumonia: An eleven-year study in a pediatric practice. Am J Epidemiol. 1981;113:12-21.
12. Denny FW, Clyde WA. Acute lower respiratory tract infections in non-hospitalized children. J Pediatr. 1986;108:635-646.
13. Ostapchuk M, Roberts DM, Haddy R. Community-acquired pneumonia in infants and children. Am Fam Phys. 2004;70:899-908.
14. Margolis P, Gadomski A. The rational clinical examination. Does this infant have pneumonia? JAMA. 1998;279:308-313.
15. Harris M, Clark J, Coote N, et al. British Thoracic Society guidelines for the management of community acquired pneumonia in children: update 2011. Thorax. 2011;66 (suppl 2):ii1-ii23.
16. Gaston B. Pneumonia. Pediatr Rev. 2002;23:132-140.
17. McIntosh K. Community-acquired pneumonia in children. N Engl J Med. 2002;346:429-437.
18. Skolnik N, Tien P. Managing community-acquired pneumonia in infants and children. Fam Pract News. November 10, 2011. Available at: http://www.familypracticenews.com/views/clinical-guidelines-for-family-physicians-by-dr-skolnik/blog/managing-community-acquired-pneumonia-in-infants-and-children/3a77ebb81a.html. Accessed January 17, 2012.
19. O’Mara N. Empiric treatment for pediatric community-acquired pneumonia. Pharmacist’s Letter. November 2011. Available at: http://www.pharmacistletter.com. Accessed February 25, 2012.
20. Klein JO. Bacterial pneumonias. In: Cherry J, Kaplan S, Demmler-Harrison G, eds. Feigin & Cherry’s Textbook of Pediatric Infectious Diseases. 6th ed. Vol 1. Philadelphia, Pa: Saunders/Elsevier; 2009:302–314.
21. Morita JY, Kahn E, Thompson T, et al. Impact of azithromycin on oropharyngeal carriage of group A Streptococcus and nasopharyngeal carriage of macrolide-resistant Streptococcus pneumoniae. Pediatr Infect Dis J. 2000;19:41-46.
22. Block S, Hedrick J, Hammerschlag MR, et al. Mycoplasma pneumoniae and Chlamydia pneumoniae in pediatric community-acquired pneumonia: comparative efficacy and safety of clarithromycin vs. erythromycin ethylsuccinate. Pediatr Infect Dis J. 1995;14:471-477.
23. Harris JA, Kolokathis A, Campbell M, et al. Safety and efficacy of azithromycin in the treatment of community-acquired pneumonia in children. Pediatr Infect Dis J. 1998;17:865-871.
24. Pallares R, Capdevila O, Linares J, et al. The effect of cephalosporin resistance on mortality in adult patients with nonmeningeal systemic pneumococcal infections. Am J Med. 2002;113:120-126.
25. Roson B, Carratala J, Tubau F, et al. Usefulness of betalactam therapy for community-acquired pneumonia in the era of drug-resistant Streptococcus pneumoniae: a randomized study of amoxicillin-clavulanate and ceftriaxone. Microb Drug Resist. 2001;7:85-96.
26. Miller LG, Kaplan SL. Staphylococcus aureus: a community pathogen. Infect Dis Clin North Am. 2009;23:35-52.
27. Haider BA, Saeed MA, Bhutta ZA. Short-course versus long-course antibiotic therapy for non-severe community-acquired pneumonia in children aged 2 months to 59 months. Cochrane Database Syst Rev. 2008;(2):CD005976.-
28. Tice AD, Rehm SJ, Dalovisio JR, et al. Practice guidelines for outpatient parenteral antimicrobial therapy. IDSA guidelines. Clin Infect Dis. 2004;38:1651-1672.
29. Bradley JS, Ching DK, Hart CL. Invasive bacterial disease in childhood: efficacy of oral antibiotic therapy following short course parenteral therapy in non-central nervous system infections. Pediatr Infect Dis J. 1987;6:821-825.
30. Blaschke AJ, Heyrend C, Byington CL, et al. Molecular analysis improves pathogen identification and epidemiologic study of pediatric parapneumonic empyema. Pediatr Infect Dis J. 2011;30:289-294.
31. American Academy of Pediatrics Committee on Infectious Diseases and Committee on Fetus and Newborn. Revised indications for the use of palivizumab and RSV immune globulin intravenous for the prevention of respiratory syncytial virus infection. Pediatrics. 2003;112:1442-1446.
• Chest x-rays and lab testing may be optional for children with community-acquired pneumonia (CAP) who are not seriously ill. A
• Start amoxicillin empirically for any child with mild-to-moderate CAP. B
• If an atypical bacterial pneumonia is suspected, azithromycin is the first-line treatment. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
What are the recommended antibiotic choices for children with mild-to-moderate bacterial community-acquired pneumonia (CAP) in the outpatient setting? How much diagnostic testing is required? When might hospitalization and combination antibiotic therapy be warranted?
Evidence-based answers to these and other questions relevant to the management of CAP in infants and children older than 3 months are provided in a set of guidelines jointly published by the Infectious Diseases Society of America (IDSA) and the Pediatric Infectious Diseases Society (PIDS) in 2011.1 We summarize them here.
What the guidelines do, and don’t, address
The IDSA/PIDS guidelines, which focus on the care of otherwise healthy children with CAP in both outpatient and inpatient settings, seek to decrease morbidity and mortality rates associated with this respiratory infection. The guidelines do not apply to children younger than 3 months, immunocompromised patients, children receiving home mechanical ventilation, or children with chronic conditions or underlying lung disease, such as cystic fibrosis.
The need for evidence-based guidance. Globally each year, 1.5 million children 5 years of age and younger suffer a pneumonia-related death, particularly in developing countries.2-5 This is more than the number of deaths associated with any other disease in the world, including acquired immune deficiency syndrome (AIDS), tuberculosis (TB), or malaria.2 In 2010, pneumonia was ranked in the United States as the sixth leading cause of death for children one to 4 years of age and the 10th leading cause of death in adolescents.5 It is estimated that out of every 1000 infants and children in North America and Europe, 35 to 40 will be affected by CAP.2
How the guidelines define CAP. Pneumonia can be broadly defined as a lower respiratory tract infection, but definitions vary depending on the organization, institution, or health care setting. For instance, the World Health Organization (WHO) defines pneumonia solely on the basis of clinical findings obtained by visual inspection and timing of the respiratory rate.6 Another definition published by Bone and colleagues states that pneumonia is the “inflammation of the pulmonary parenchyma brought about by the presence of virulent pathogens; usually differentiated from isolated infections of the major airways.”7 The new pediatric guidelines define CAP as “the presence of signs and symptoms of pneumonia in a previously healthy child caused by an infection that has been acquired outside the hospital.”1
CAP pathogens vary with the child’s age
Typically, diagnostic testing of children will reveal several microbes, viral and bacterial, making it difficult to determine which might be the pathogen.1 Viral pathogens are more common causes of CAP in children younger than 2 years, accounting for 80% of cases1; bacterial pathogens are more common in older children.1
The virus detected most often among children younger than 2 years is respiratory syncytial virus (RSV).1,8-12 Less common viruses include adenovirus, influenza types A and B, parainfluenza 1, 2, and 3, and rhinovirus. Streptococcus pneumoniae is the most common bacterial pathogen identified in older children.1,13 The overall incidence of pneumonia decreases with age, but it has been reported that the proportion of cases from atypical bacterial pathogens—Chlamydia pneumoniae and Mycoplasma pneumoniae—may increase among older children.1,13
Signs and symptoms also vary
Signs and symptoms of CAP differ depending on the severity of the infection and the age of the child. In general, respiratory distress (tachypnea, nasal flaring, decreased breath sounds, cough, and rales) with fever are the prominent symptoms associated with pneumonia.1,13,14
Infants and children with mild to moderate infection most commonly exhibit a temperature <38°C and a respiratory rate <50 breaths per minute (bpm).
Children with severe CAP commonly present with a temperature >38°C, flaring of nostrils, grunting with breathing, tachypnea, tachycardia, and cyanosis. Tachypnea is defined as >60 bpm in infants younger than 2 months, >50 bpm in infants 2 to 12 months, and >40 bpm in children ages 1 to 5 years.8 Although respiratory rate is a valuable clinical sign, the work of breathing (as evidenced by nasal flaring, breathlessness, cough, or wheeze) required by the infant or child may be more indicative of pneumonia.15
Utilize diagnostic testing judiciously
Not all patients with suspected CAP require the same amount of diagnostic testing. In fact, IDSA/PIDS recommendations vary for hospitalized patients and for outpatients.1 In all cases, conduct testing quickly to expedite diagnosis and minimize the need for additional testing, to help validate treatment choices, and to reduce time spent in the hospital.1
Blood and sputum cultures not always indicated. The IDSA/PIDS guidelines strongly recommend obtaining blood cultures for hospitalized patients with moderate-to-severe pneumonia, particularly those with complications.1
The guidelines strongly recommend against blood cultures for fully immunized children with CAP who are treated as outpatients. However, blood cultures are strongly recommended for any child who fails to improve after initiation of antibiotic therapy.1 These recommendations are consistent with clinical data, expert opinion, and other treatment guidelines.1,8,13-18
A weak recommendation from the new guidelines states that if a hospitalized child with CAP can produce sputum, gram staining of the specimen may be warranted.1,8,13,15
Use pulse oximetry. The guidelines strongly recommend using pulse oximetry with all children who have pneumonia or suspected hypoxemia.1,18
When chest radiography can help. Routine chest radiography may not be warranted for suspected CAP treated in the outpatient setting. Order chest films for patients with suspected or confirmed hypoxemia or respiratory distress (who tend to have worse outcomes), and for patients who do not respond to initial antibiotic treatment.1,18 Follow-up radiographs are recommended for patients with advancing symptoms 2 to 3 days after starting antibiotics, complicated pneumonia with worsening respiratory distress, or clinical symptoms without improvement.1
Other diagnostic tests mentioned in the guidelines include complete blood cell counts, which are recommended in severe cases of pneumonia.1
Acute-phase reactants such as erythrocyte sedimentation rate (ESR), serum procalcitonin, and C-reactive protein concentrations cannot distinguish between viral and bacterial causes of CAP, and are not routinely recommended for patients treated in the outpatient setting.1,13
For patients requiring endotracheal intubation, gram staining and cultures of aspirates of the trachea and virus testing are recommended.1
Immunocompetent patients hospitalized with severe CAP may be candidates for percutaneous lung aspiration, open lung biopsy, bronchoalveolar lavage (BAL), or bronchoscopic or blind protected brush specimen collection if prior diagnostic tests are negative.1
CAP treatment and prevention
The guidelines provide recommendations for treating bacterial and viral CAP in either inpatient or outpatient settings, and discuss appropriate preventive techniques.
Antiviral therapy. As mentioned earlier, children less than 2 years of age are commonly infected with viral pathogens. Those with mild cases of viral CAP do not require anti-microbial therapy. For children with moderate-to-severe CAP consistent with influenza infection, administer influenza antiviral therapy as soon as possible, especially during a widespread local circulation of influenza viruses. Some influenza A strains will be susceptible to antiviral therapy, even though genetic variability is high each year. The guidelines’ recommended agents for treating influenza in pediatric patients are listed in TABLE 1.1
TABLE 1
Influenza antiviral therapy in pediatric patients*1
| Drug (brand name) | Formulation | Dosing |
|---|---|---|
| Oseltamivir (Tamiflu) | 75 mg capsule; 60 mg/5 mL suspension | 4-8 mo: 6 mg/kg/d in 2 doses 9-23 mo: 7 mg/kg/d in 2 doses ≥24 mo: ~4 mg/kg/d in 2 doses, for 5 days ≤15 kg: 60 mg/d in 2 divided doses >15-23 kg: 90 mg/d in 2 divided doses >23-40 kg: 120 mg/d in 2 divided doses >40 kg: 150 mg/d in 2 divided doses |
| Zanamivir (Relenza) | 5 mg per inhalation, using a Diskhaler | ≥7 y: 2 inhalations (10 mg total per dose), twice daily for 5 days |
| Amantadine (Symmetrel)† | 100 mg tablet; 50 mg/5 mL suspension | 1-9 y: 5-8 mg/kg/d as single daily dose or in 2 doses; not to exceed 150 mg/d 9-12 y: 200 mg/d in 2 doses (not studied as a single dose) |
| Rimantadine (Flumadine)† | 100 mg tablet; 50 mg/5 mL suspension | Not FDA approved for treatment in children, but published data exist on safety and efficacy in children Suspension: 1-9 y: 6.6 mg/kg/d (max 150 mg/kg/d) in 2 doses ≥10 y: 200 mg/d, as single daily dose or in 2 doses |
| *In children for whom prophylaxis is indicated, antiviral drugs should be continued for the duration of known influenza activity in the community (because of the potential for repeated exposures) or until immunity can be achieved as a result of immunization. †Amantadine and rimantadine should be used for treatment and prophylaxis only in the winter, when most isolated influenza A virus strains are susceptible to adamantine; the adamantines should not be used for primary therapy because of the rapid emergence of resistance. However, for patients requiring adamantine therapy, a treatment course of about 7 days is suggested, or one that runs until a day or 2 after the signs and symptoms have disappeared. | ||
Antibacterial therapy. For patients with a suspected bacterial pathogen, start empiric antibiotic therapy as soon as possible. Preferred and alternative agents for specific age groups, immunization status, and specific pathogen(s) appear in TABLE 2.1,19
TABLE 2
Empiric outpatient antibiotic therapy for pediatric CAP1,19
Duration of treatment is 10 days unless otherwise noted
| Patient age | Presumed bacterial pneumonia | Presumed atypical pneumonia |
|---|---|---|
| 3 mo to <5 y, regardless of immunization status | Preferred: amoxicillin 90 mg/kg/d PO in 2 divided doses Alternative: amoxicillin clavulanate 90 mg/kg/d PO in 2 divided doses | For all children regardless of age and immunization status: Preferred: azithromycin 10 mg/kg PO on Day 1, followed by 5 mg/kg PO once daily on Days 2-5 Alternative: clarithromycin 15 mg/kg/d PO in 2 divided doses OR In children >7 y: erythromycin 40 mg/kg/d PO in 4 divided doses; or doxycycline 2-4 mg/kg/d PO in 2 divided doses |
| ≥5 y and fully immunized against Streptococcus pneumoniae and Haemophilus influenzae | Preferred:* amoxicillin 90 mg/kg/d PO in 2 divided doses to a maximum 4 g/d, with or without a macrolide antibiotic Alternatives: Second- or third-generation cephalosporins such as oral cefpodoxime, cefuroxime, or cefprozil OR levofloxacin (5-16 y) 8-10 mg/kg PO once daily (max 750 mg/d)†OR linezolid (<12 y) 30 mg/kg/d PO (max 1200 mg/d) in 3 divided doses; or (≥12 y) 20 mg/kg/d (max 1200 mg/d) in 2 divided doses | |
| ≥5 y and NOT fully immunized against S pneumoniae and H influenzae | Preferred:* amoxicillin 90 mg/kg/d PO in 2 divided doses to a max of 4 g/d; or amoxicillin clavulanate 90 mg/kg/d PO in 2 divided doses Alternatives: Second- or third-generation cephalosporins such as oral cefpodoxime, cefuroxime, or cefprozil OR levofloxacin (5-16 y) 8-10 mg/kg PO once daily (max 750 mg/d)† | |
| CAP, community-acquired pneumonia. *Preferred treatments of choice change in areas of high S pneumoniae resistance. Refer to the complete guidelines for specific recommendations. †The guidelines do not fully address the controversy concerning the use of quinolones in children. The use of quinolones in infants and children is considered a risk vs benefit decision. | ||
Patients with mild or moderate CAP may be treated first in the outpatient setting with amoxicillin. This antibiotic has been the agent of choice for many years and continues to be the empiric therapy recommended in the guidelines.1 Appropriate dosing depends on the age of the patient.
TABLE 2 also includes treatment alternatives to amoxicillin for patients with drug allergies, treatment failures, or suspected atypical pathogens. Amoxicillin and the alternative treatments provide coverage for S pneumoniae, the most common invasive bacterial pathogen in older children.1,20 When atypical pathogens are suspected, macrolide antibiotics become the antibiotic drug class of choice, with azithromycin being the preferred first-line agent.1,21-23
Bacterial CAP necessitating hospitalization. The guidelines strongly recommend hospitalization for infants and children with respiratory distress or hypoxemia (oxygen saturation <90%); for suspicion of infection caused by community-acquired methicillin-resistant Staphylococcus aureus (MRSA) or any pathogen with high virulence; or for infants 3 to 6 months old.1
Treat with parenteral antibiotics to provide reliable blood and tissue concentrations (TABLE 3).1,19 Ampicillin or penicillin G may be given to fully immunized children; however, take into account the local resistance pattern of S pneumoniae to drugs within the penicillin class. For hospitalized children who are not yet fully immunized, who have life-threatening infections, or who are in a facility with a documented high rate of penicillin resistance, administer a third-generation parenteral cephalosporin such as ceftriaxone or cefotaxime empirically.1,24 In monotherapy treatment of pneumococcal pneumonia, non–beta-lactam agents such as vancomycin have not been shown to be more effective than the third-generation cephalosporins.1
TABLE 3
Empiric antibiotic therapy for hospitalized patients with CAP1,19
Duration of treatment is 10 days unless otherwise noted
| Patient age | Presumed bacterial pneumonia | Presumed atypical pneumonia |
|---|---|---|
| 3 mo to <5 y and fully immunized against Streptococcus pneumoniae and Haemophilus influenzae | Preferred:* ampicillin 150-200 mg/kg/d IV divided every 6 h; or penicillin G 200,000-250,000 units/kg/d IV divided every 4-6 h Alternatives: ceftriaxone 50-100 mg/kg/d IV/IM divided every 12-24 h; or cefotaxime 150 mg/kg/d IV divided every 8 h | For all children regardless of age and immunization status: Preferred: azithromycin, 10 mg/kg IV (max of 500 mg) on Days 1 and 2, then transition to oral therapy 10 mg/kg/d for remaining 7-10 days of therapy Alternatives: erythromycin lactobionate 20 mg/kg/d IV divided every 6 h; or levofloxacin 16-20 mg/kg/d IV divided every 12 h to a max of 750 mg/d† |
| <5 y and NOT fully immunized against S pneumoniae and H influenzae | Preferred:* ceftriaxone 50-100 mg/kg/d IV/IM divided every 12-24 h; or cefotaxime 150 mg/kg/d IV divided every 8 h Alternative: levofloxacin (6 mo–<5 y) 16-20 mg/kg/d IV divided every 12 h† | |
| ≥5 y and fully immunized against S pneumoniae and H influenzae | Preferred:* ampicillin 150-200 mg/kg/d IV divided every 6 h; or penicillin G 200,000-250,000 units/kg/d IV divided every 4-6 h Alternatives: ceftriaxone 50-100 mg/kg/d IV/IM divided every 12-24 h; or cefotaxime 150 mg/kg/d IV divided every 8 h | |
| ≥5 y and NOT fully immunized against S pneumoniae and H influenzae | Preferred:* ceftriaxone 50-100 mg/kg/d IV/IM divided every 12-24 h; or cefotaxime 150 mg/kg/d IV divided every 8 h Alternatives: ampicillin 150-200 mg/kg/d IV divided every 6 h; or levofloxacin 8-10 mg/kg IV once daily (max of 750 mg/d)† | |
| CAP, community-acquired pneumonia. *The addition of clindamycin 40 mg/kg/d IV divided every 6-8 hours or vancomycin 40-60 mg/kg/day IV divided every 6-8 hours is recommended for suspected or confirmed community-acquired methicillin-resistant Staphylococcus aureus. †The guidelines do not fully address the controversy concerning the use of quinolones in children. Use of quinolones in infants and children is considered a risk vs benefit decision. | ||
If S aureus is the suspected microorganism or is confirmed with clinical, laboratory, or imaging characteristics, give vancomycin or clindamycin with a beta-lactam agent.1,25-26 If you suspect an atypical pathogen such as M pneumoniae or C pneumoniae, start empiric therapy with an oral or parenteral macrolide in combination with a beta-lactam.1
Once a pathogen has been identified, adjust antimicrobial therapy as needed to target the specific microbe, to limit empiric antibiotic exposure, and to help limit the potential for antibiotic resistance.
Duration of treatment. The recommended duration of treatment for CAP is 10 days, supported by clinical data and the practice guidelines.1,27-29 Shorter treatment courses may be effective, especially in mild cases or outpatient treatment.1 Specific pathogens, such as MRSA, may need to be treated longer.30
If a patient is receiving intravenous antibiotics, switch to an oral agent as soon as clinically feasible to decrease risks from parenteral administration, and plan for the earliest possible discharge from the hospital to limit exposure to nosocomial pathogens. Hospital discharge may be considered when a child is clinically stable (improved appetite and activity level, afebrile for 24 hours), mental status is back to baseline or stable, and the pulse oximetry level is >90% on room air for at least 24 hours.1
Children receiving adequate therapy regimens should demonstrate both clinical and laboratory signs of improvement within 48 to 72 hours.1 If improvement does not occur, further your investigation with additional cultures, laboratory tests, and imaging evaluation.
For preventive measures, the guidelines recommend properly immunizing children with vaccines for bacterial pathogens such as S pneumoniae, Haemophilus influenzae, and Bordetella pertussis.1 Influenza vaccine should also be offered to prevent CAP in infants and children 6 months of age and older. Offer influenza and pertussis vaccines to adults and those caring for infants and children, to help prevent the spread of disease. Also consider immune prophylaxis with RSV-specific monoclonal antibody for premature infants or those with bronchopulmonary dysplasia, congenital heart disease, or immunodeficiency, to decrease the risk of severe pneumonia and hospitalization. For detailed recommendations on the use of prophylaxis against RSV, refer to the 2003 American Academy of Pediatrics statement.31
CORRESPONDENCE
Stephanie Schauner, PharmD, BCPS, University of Missouri-Kansas City, Health Science Building, Room 2241, 2464 Charlotte Street, Kansas City, MO 64108-2792; schauners@umkc.edu
• Chest x-rays and lab testing may be optional for children with community-acquired pneumonia (CAP) who are not seriously ill. A
• Start amoxicillin empirically for any child with mild-to-moderate CAP. B
• If an atypical bacterial pneumonia is suspected, azithromycin is the first-line treatment. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
What are the recommended antibiotic choices for children with mild-to-moderate bacterial community-acquired pneumonia (CAP) in the outpatient setting? How much diagnostic testing is required? When might hospitalization and combination antibiotic therapy be warranted?
Evidence-based answers to these and other questions relevant to the management of CAP in infants and children older than 3 months are provided in a set of guidelines jointly published by the Infectious Diseases Society of America (IDSA) and the Pediatric Infectious Diseases Society (PIDS) in 2011.1 We summarize them here.
What the guidelines do, and don’t, address
The IDSA/PIDS guidelines, which focus on the care of otherwise healthy children with CAP in both outpatient and inpatient settings, seek to decrease morbidity and mortality rates associated with this respiratory infection. The guidelines do not apply to children younger than 3 months, immunocompromised patients, children receiving home mechanical ventilation, or children with chronic conditions or underlying lung disease, such as cystic fibrosis.
The need for evidence-based guidance. Globally each year, 1.5 million children 5 years of age and younger suffer a pneumonia-related death, particularly in developing countries.2-5 This is more than the number of deaths associated with any other disease in the world, including acquired immune deficiency syndrome (AIDS), tuberculosis (TB), or malaria.2 In 2010, pneumonia was ranked in the United States as the sixth leading cause of death for children one to 4 years of age and the 10th leading cause of death in adolescents.5 It is estimated that out of every 1000 infants and children in North America and Europe, 35 to 40 will be affected by CAP.2
How the guidelines define CAP. Pneumonia can be broadly defined as a lower respiratory tract infection, but definitions vary depending on the organization, institution, or health care setting. For instance, the World Health Organization (WHO) defines pneumonia solely on the basis of clinical findings obtained by visual inspection and timing of the respiratory rate.6 Another definition published by Bone and colleagues states that pneumonia is the “inflammation of the pulmonary parenchyma brought about by the presence of virulent pathogens; usually differentiated from isolated infections of the major airways.”7 The new pediatric guidelines define CAP as “the presence of signs and symptoms of pneumonia in a previously healthy child caused by an infection that has been acquired outside the hospital.”1
CAP pathogens vary with the child’s age
Typically, diagnostic testing of children will reveal several microbes, viral and bacterial, making it difficult to determine which might be the pathogen.1 Viral pathogens are more common causes of CAP in children younger than 2 years, accounting for 80% of cases1; bacterial pathogens are more common in older children.1
The virus detected most often among children younger than 2 years is respiratory syncytial virus (RSV).1,8-12 Less common viruses include adenovirus, influenza types A and B, parainfluenza 1, 2, and 3, and rhinovirus. Streptococcus pneumoniae is the most common bacterial pathogen identified in older children.1,13 The overall incidence of pneumonia decreases with age, but it has been reported that the proportion of cases from atypical bacterial pathogens—Chlamydia pneumoniae and Mycoplasma pneumoniae—may increase among older children.1,13
Signs and symptoms also vary
Signs and symptoms of CAP differ depending on the severity of the infection and the age of the child. In general, respiratory distress (tachypnea, nasal flaring, decreased breath sounds, cough, and rales) with fever are the prominent symptoms associated with pneumonia.1,13,14
Infants and children with mild to moderate infection most commonly exhibit a temperature <38°C and a respiratory rate <50 breaths per minute (bpm).
Children with severe CAP commonly present with a temperature >38°C, flaring of nostrils, grunting with breathing, tachypnea, tachycardia, and cyanosis. Tachypnea is defined as >60 bpm in infants younger than 2 months, >50 bpm in infants 2 to 12 months, and >40 bpm in children ages 1 to 5 years.8 Although respiratory rate is a valuable clinical sign, the work of breathing (as evidenced by nasal flaring, breathlessness, cough, or wheeze) required by the infant or child may be more indicative of pneumonia.15
Utilize diagnostic testing judiciously
Not all patients with suspected CAP require the same amount of diagnostic testing. In fact, IDSA/PIDS recommendations vary for hospitalized patients and for outpatients.1 In all cases, conduct testing quickly to expedite diagnosis and minimize the need for additional testing, to help validate treatment choices, and to reduce time spent in the hospital.1
Blood and sputum cultures not always indicated. The IDSA/PIDS guidelines strongly recommend obtaining blood cultures for hospitalized patients with moderate-to-severe pneumonia, particularly those with complications.1
The guidelines strongly recommend against blood cultures for fully immunized children with CAP who are treated as outpatients. However, blood cultures are strongly recommended for any child who fails to improve after initiation of antibiotic therapy.1 These recommendations are consistent with clinical data, expert opinion, and other treatment guidelines.1,8,13-18
A weak recommendation from the new guidelines states that if a hospitalized child with CAP can produce sputum, gram staining of the specimen may be warranted.1,8,13,15
Use pulse oximetry. The guidelines strongly recommend using pulse oximetry with all children who have pneumonia or suspected hypoxemia.1,18
When chest radiography can help. Routine chest radiography may not be warranted for suspected CAP treated in the outpatient setting. Order chest films for patients with suspected or confirmed hypoxemia or respiratory distress (who tend to have worse outcomes), and for patients who do not respond to initial antibiotic treatment.1,18 Follow-up radiographs are recommended for patients with advancing symptoms 2 to 3 days after starting antibiotics, complicated pneumonia with worsening respiratory distress, or clinical symptoms without improvement.1
Other diagnostic tests mentioned in the guidelines include complete blood cell counts, which are recommended in severe cases of pneumonia.1
Acute-phase reactants such as erythrocyte sedimentation rate (ESR), serum procalcitonin, and C-reactive protein concentrations cannot distinguish between viral and bacterial causes of CAP, and are not routinely recommended for patients treated in the outpatient setting.1,13
For patients requiring endotracheal intubation, gram staining and cultures of aspirates of the trachea and virus testing are recommended.1
Immunocompetent patients hospitalized with severe CAP may be candidates for percutaneous lung aspiration, open lung biopsy, bronchoalveolar lavage (BAL), or bronchoscopic or blind protected brush specimen collection if prior diagnostic tests are negative.1
CAP treatment and prevention
The guidelines provide recommendations for treating bacterial and viral CAP in either inpatient or outpatient settings, and discuss appropriate preventive techniques.
Antiviral therapy. As mentioned earlier, children less than 2 years of age are commonly infected with viral pathogens. Those with mild cases of viral CAP do not require anti-microbial therapy. For children with moderate-to-severe CAP consistent with influenza infection, administer influenza antiviral therapy as soon as possible, especially during a widespread local circulation of influenza viruses. Some influenza A strains will be susceptible to antiviral therapy, even though genetic variability is high each year. The guidelines’ recommended agents for treating influenza in pediatric patients are listed in TABLE 1.1
TABLE 1
Influenza antiviral therapy in pediatric patients*1
| Drug (brand name) | Formulation | Dosing |
|---|---|---|
| Oseltamivir (Tamiflu) | 75 mg capsule; 60 mg/5 mL suspension | 4-8 mo: 6 mg/kg/d in 2 doses 9-23 mo: 7 mg/kg/d in 2 doses ≥24 mo: ~4 mg/kg/d in 2 doses, for 5 days ≤15 kg: 60 mg/d in 2 divided doses >15-23 kg: 90 mg/d in 2 divided doses >23-40 kg: 120 mg/d in 2 divided doses >40 kg: 150 mg/d in 2 divided doses |
| Zanamivir (Relenza) | 5 mg per inhalation, using a Diskhaler | ≥7 y: 2 inhalations (10 mg total per dose), twice daily for 5 days |
| Amantadine (Symmetrel)† | 100 mg tablet; 50 mg/5 mL suspension | 1-9 y: 5-8 mg/kg/d as single daily dose or in 2 doses; not to exceed 150 mg/d 9-12 y: 200 mg/d in 2 doses (not studied as a single dose) |
| Rimantadine (Flumadine)† | 100 mg tablet; 50 mg/5 mL suspension | Not FDA approved for treatment in children, but published data exist on safety and efficacy in children Suspension: 1-9 y: 6.6 mg/kg/d (max 150 mg/kg/d) in 2 doses ≥10 y: 200 mg/d, as single daily dose or in 2 doses |
| *In children for whom prophylaxis is indicated, antiviral drugs should be continued for the duration of known influenza activity in the community (because of the potential for repeated exposures) or until immunity can be achieved as a result of immunization. †Amantadine and rimantadine should be used for treatment and prophylaxis only in the winter, when most isolated influenza A virus strains are susceptible to adamantine; the adamantines should not be used for primary therapy because of the rapid emergence of resistance. However, for patients requiring adamantine therapy, a treatment course of about 7 days is suggested, or one that runs until a day or 2 after the signs and symptoms have disappeared. | ||
Antibacterial therapy. For patients with a suspected bacterial pathogen, start empiric antibiotic therapy as soon as possible. Preferred and alternative agents for specific age groups, immunization status, and specific pathogen(s) appear in TABLE 2.1,19
TABLE 2
Empiric outpatient antibiotic therapy for pediatric CAP1,19
Duration of treatment is 10 days unless otherwise noted
| Patient age | Presumed bacterial pneumonia | Presumed atypical pneumonia |
|---|---|---|
| 3 mo to <5 y, regardless of immunization status | Preferred: amoxicillin 90 mg/kg/d PO in 2 divided doses Alternative: amoxicillin clavulanate 90 mg/kg/d PO in 2 divided doses | For all children regardless of age and immunization status: Preferred: azithromycin 10 mg/kg PO on Day 1, followed by 5 mg/kg PO once daily on Days 2-5 Alternative: clarithromycin 15 mg/kg/d PO in 2 divided doses OR In children >7 y: erythromycin 40 mg/kg/d PO in 4 divided doses; or doxycycline 2-4 mg/kg/d PO in 2 divided doses |
| ≥5 y and fully immunized against Streptococcus pneumoniae and Haemophilus influenzae | Preferred:* amoxicillin 90 mg/kg/d PO in 2 divided doses to a maximum 4 g/d, with or without a macrolide antibiotic Alternatives: Second- or third-generation cephalosporins such as oral cefpodoxime, cefuroxime, or cefprozil OR levofloxacin (5-16 y) 8-10 mg/kg PO once daily (max 750 mg/d)†OR linezolid (<12 y) 30 mg/kg/d PO (max 1200 mg/d) in 3 divided doses; or (≥12 y) 20 mg/kg/d (max 1200 mg/d) in 2 divided doses | |
| ≥5 y and NOT fully immunized against S pneumoniae and H influenzae | Preferred:* amoxicillin 90 mg/kg/d PO in 2 divided doses to a max of 4 g/d; or amoxicillin clavulanate 90 mg/kg/d PO in 2 divided doses Alternatives: Second- or third-generation cephalosporins such as oral cefpodoxime, cefuroxime, or cefprozil OR levofloxacin (5-16 y) 8-10 mg/kg PO once daily (max 750 mg/d)† | |
| CAP, community-acquired pneumonia. *Preferred treatments of choice change in areas of high S pneumoniae resistance. Refer to the complete guidelines for specific recommendations. †The guidelines do not fully address the controversy concerning the use of quinolones in children. The use of quinolones in infants and children is considered a risk vs benefit decision. | ||
Patients with mild or moderate CAP may be treated first in the outpatient setting with amoxicillin. This antibiotic has been the agent of choice for many years and continues to be the empiric therapy recommended in the guidelines.1 Appropriate dosing depends on the age of the patient.
TABLE 2 also includes treatment alternatives to amoxicillin for patients with drug allergies, treatment failures, or suspected atypical pathogens. Amoxicillin and the alternative treatments provide coverage for S pneumoniae, the most common invasive bacterial pathogen in older children.1,20 When atypical pathogens are suspected, macrolide antibiotics become the antibiotic drug class of choice, with azithromycin being the preferred first-line agent.1,21-23
Bacterial CAP necessitating hospitalization. The guidelines strongly recommend hospitalization for infants and children with respiratory distress or hypoxemia (oxygen saturation <90%); for suspicion of infection caused by community-acquired methicillin-resistant Staphylococcus aureus (MRSA) or any pathogen with high virulence; or for infants 3 to 6 months old.1
Treat with parenteral antibiotics to provide reliable blood and tissue concentrations (TABLE 3).1,19 Ampicillin or penicillin G may be given to fully immunized children; however, take into account the local resistance pattern of S pneumoniae to drugs within the penicillin class. For hospitalized children who are not yet fully immunized, who have life-threatening infections, or who are in a facility with a documented high rate of penicillin resistance, administer a third-generation parenteral cephalosporin such as ceftriaxone or cefotaxime empirically.1,24 In monotherapy treatment of pneumococcal pneumonia, non–beta-lactam agents such as vancomycin have not been shown to be more effective than the third-generation cephalosporins.1
TABLE 3
Empiric antibiotic therapy for hospitalized patients with CAP1,19
Duration of treatment is 10 days unless otherwise noted
| Patient age | Presumed bacterial pneumonia | Presumed atypical pneumonia |
|---|---|---|
| 3 mo to <5 y and fully immunized against Streptococcus pneumoniae and Haemophilus influenzae | Preferred:* ampicillin 150-200 mg/kg/d IV divided every 6 h; or penicillin G 200,000-250,000 units/kg/d IV divided every 4-6 h Alternatives: ceftriaxone 50-100 mg/kg/d IV/IM divided every 12-24 h; or cefotaxime 150 mg/kg/d IV divided every 8 h | For all children regardless of age and immunization status: Preferred: azithromycin, 10 mg/kg IV (max of 500 mg) on Days 1 and 2, then transition to oral therapy 10 mg/kg/d for remaining 7-10 days of therapy Alternatives: erythromycin lactobionate 20 mg/kg/d IV divided every 6 h; or levofloxacin 16-20 mg/kg/d IV divided every 12 h to a max of 750 mg/d† |
| <5 y and NOT fully immunized against S pneumoniae and H influenzae | Preferred:* ceftriaxone 50-100 mg/kg/d IV/IM divided every 12-24 h; or cefotaxime 150 mg/kg/d IV divided every 8 h Alternative: levofloxacin (6 mo–<5 y) 16-20 mg/kg/d IV divided every 12 h† | |
| ≥5 y and fully immunized against S pneumoniae and H influenzae | Preferred:* ampicillin 150-200 mg/kg/d IV divided every 6 h; or penicillin G 200,000-250,000 units/kg/d IV divided every 4-6 h Alternatives: ceftriaxone 50-100 mg/kg/d IV/IM divided every 12-24 h; or cefotaxime 150 mg/kg/d IV divided every 8 h | |
| ≥5 y and NOT fully immunized against S pneumoniae and H influenzae | Preferred:* ceftriaxone 50-100 mg/kg/d IV/IM divided every 12-24 h; or cefotaxime 150 mg/kg/d IV divided every 8 h Alternatives: ampicillin 150-200 mg/kg/d IV divided every 6 h; or levofloxacin 8-10 mg/kg IV once daily (max of 750 mg/d)† | |
| CAP, community-acquired pneumonia. *The addition of clindamycin 40 mg/kg/d IV divided every 6-8 hours or vancomycin 40-60 mg/kg/day IV divided every 6-8 hours is recommended for suspected or confirmed community-acquired methicillin-resistant Staphylococcus aureus. †The guidelines do not fully address the controversy concerning the use of quinolones in children. Use of quinolones in infants and children is considered a risk vs benefit decision. | ||
If S aureus is the suspected microorganism or is confirmed with clinical, laboratory, or imaging characteristics, give vancomycin or clindamycin with a beta-lactam agent.1,25-26 If you suspect an atypical pathogen such as M pneumoniae or C pneumoniae, start empiric therapy with an oral or parenteral macrolide in combination with a beta-lactam.1
Once a pathogen has been identified, adjust antimicrobial therapy as needed to target the specific microbe, to limit empiric antibiotic exposure, and to help limit the potential for antibiotic resistance.
Duration of treatment. The recommended duration of treatment for CAP is 10 days, supported by clinical data and the practice guidelines.1,27-29 Shorter treatment courses may be effective, especially in mild cases or outpatient treatment.1 Specific pathogens, such as MRSA, may need to be treated longer.30
If a patient is receiving intravenous antibiotics, switch to an oral agent as soon as clinically feasible to decrease risks from parenteral administration, and plan for the earliest possible discharge from the hospital to limit exposure to nosocomial pathogens. Hospital discharge may be considered when a child is clinically stable (improved appetite and activity level, afebrile for 24 hours), mental status is back to baseline or stable, and the pulse oximetry level is >90% on room air for at least 24 hours.1
Children receiving adequate therapy regimens should demonstrate both clinical and laboratory signs of improvement within 48 to 72 hours.1 If improvement does not occur, further your investigation with additional cultures, laboratory tests, and imaging evaluation.
For preventive measures, the guidelines recommend properly immunizing children with vaccines for bacterial pathogens such as S pneumoniae, Haemophilus influenzae, and Bordetella pertussis.1 Influenza vaccine should also be offered to prevent CAP in infants and children 6 months of age and older. Offer influenza and pertussis vaccines to adults and those caring for infants and children, to help prevent the spread of disease. Also consider immune prophylaxis with RSV-specific monoclonal antibody for premature infants or those with bronchopulmonary dysplasia, congenital heart disease, or immunodeficiency, to decrease the risk of severe pneumonia and hospitalization. For detailed recommendations on the use of prophylaxis against RSV, refer to the 2003 American Academy of Pediatrics statement.31
CORRESPONDENCE
Stephanie Schauner, PharmD, BCPS, University of Missouri-Kansas City, Health Science Building, Room 2241, 2464 Charlotte Street, Kansas City, MO 64108-2792; schauners@umkc.edu
1. Bradley JS, Byington CL, Shah SS, et al. The management of community-acquired pneumonia in infants and children older than 3 months of age: clinical practice guidelines by the Pediatric Infectious Diseases Society and the Infectious Diseases Society of America. Clin Infect Dis. 2011;53:e25-e76.Available at: http://cid.oxfordjournals.org/content/53/7/e25.long. Accessed December 17, 2012.
2. Centers for Disease Control and Prevention Pneumonia Can Be Prevented–Vaccines Can Help. Available at: http://www.cdc.gov/features/pneumonia. Accessed January 17, 2012.
3. Bulla A, Hitze KL. Acute respiratory infections: a review. Bull World Health Organ. 1978;56:481-498.
4. Baqui AH, Black RE, Arifeen SE, et al. Causes of childhood deaths in Bangladesh: results of a nationwide verbal autopsy study. Bull World Health Organ. 1998;76:161-171.
5. Murphy SL, Xu JQ, Kochanek KD. Deaths: Preliminary data for 2010. National vital statistics reports; vol 60 no 4. Hyattsville, Md: National Center for Health Statistics. 2012. Available at: http://www.cdc.gov/nchs/data/nvsr/nvsr60/nvsr60_04.pdf. Accessed May 12, 2012.
6. Clinical management of acute respiratory infections in children: a WHO memorandum. Bull World Health Organ. 1981;59:707-716.
7. Feldman C, Anderson R. Community-acquired pneumonia. In; Bone RC, Dantzker DR, George RB, et al, eds. Pulmonary and Critical Care Medicine. Vol 2. St. Louis, Mo: Mosby-Year Book, Inc; 1997:719–733.
8. Davies HD. Community-acquired pneumonia in children. Paediatr Child Health. 2003;8:616-619.
9. Alexander ER, Foy HM, Kenny GE, et al. Pneumonia due to Mycoplasma pneumoniae. Its incidence in the membership of a co-operative medical group. N Engl J Med. 1966;275:131-136.
10. Foy HM, Cooney MK, Maletzky AJ, et al. Incidence and etiology of pneumonia, croup and bronchiolitis in preschool children belonging to a prepaid medical group over a four-year period. Am J Epidemiol. 1973;97:80-92.
11. Murphy TF, Henderson FW, Clyde WA, Jr, et al. Pneumonia: An eleven-year study in a pediatric practice. Am J Epidemiol. 1981;113:12-21.
12. Denny FW, Clyde WA. Acute lower respiratory tract infections in non-hospitalized children. J Pediatr. 1986;108:635-646.
13. Ostapchuk M, Roberts DM, Haddy R. Community-acquired pneumonia in infants and children. Am Fam Phys. 2004;70:899-908.
14. Margolis P, Gadomski A. The rational clinical examination. Does this infant have pneumonia? JAMA. 1998;279:308-313.
15. Harris M, Clark J, Coote N, et al. British Thoracic Society guidelines for the management of community acquired pneumonia in children: update 2011. Thorax. 2011;66 (suppl 2):ii1-ii23.
16. Gaston B. Pneumonia. Pediatr Rev. 2002;23:132-140.
17. McIntosh K. Community-acquired pneumonia in children. N Engl J Med. 2002;346:429-437.
18. Skolnik N, Tien P. Managing community-acquired pneumonia in infants and children. Fam Pract News. November 10, 2011. Available at: http://www.familypracticenews.com/views/clinical-guidelines-for-family-physicians-by-dr-skolnik/blog/managing-community-acquired-pneumonia-in-infants-and-children/3a77ebb81a.html. Accessed January 17, 2012.
19. O’Mara N. Empiric treatment for pediatric community-acquired pneumonia. Pharmacist’s Letter. November 2011. Available at: http://www.pharmacistletter.com. Accessed February 25, 2012.
20. Klein JO. Bacterial pneumonias. In: Cherry J, Kaplan S, Demmler-Harrison G, eds. Feigin & Cherry’s Textbook of Pediatric Infectious Diseases. 6th ed. Vol 1. Philadelphia, Pa: Saunders/Elsevier; 2009:302–314.
21. Morita JY, Kahn E, Thompson T, et al. Impact of azithromycin on oropharyngeal carriage of group A Streptococcus and nasopharyngeal carriage of macrolide-resistant Streptococcus pneumoniae. Pediatr Infect Dis J. 2000;19:41-46.
22. Block S, Hedrick J, Hammerschlag MR, et al. Mycoplasma pneumoniae and Chlamydia pneumoniae in pediatric community-acquired pneumonia: comparative efficacy and safety of clarithromycin vs. erythromycin ethylsuccinate. Pediatr Infect Dis J. 1995;14:471-477.
23. Harris JA, Kolokathis A, Campbell M, et al. Safety and efficacy of azithromycin in the treatment of community-acquired pneumonia in children. Pediatr Infect Dis J. 1998;17:865-871.
24. Pallares R, Capdevila O, Linares J, et al. The effect of cephalosporin resistance on mortality in adult patients with nonmeningeal systemic pneumococcal infections. Am J Med. 2002;113:120-126.
25. Roson B, Carratala J, Tubau F, et al. Usefulness of betalactam therapy for community-acquired pneumonia in the era of drug-resistant Streptococcus pneumoniae: a randomized study of amoxicillin-clavulanate and ceftriaxone. Microb Drug Resist. 2001;7:85-96.
26. Miller LG, Kaplan SL. Staphylococcus aureus: a community pathogen. Infect Dis Clin North Am. 2009;23:35-52.
27. Haider BA, Saeed MA, Bhutta ZA. Short-course versus long-course antibiotic therapy for non-severe community-acquired pneumonia in children aged 2 months to 59 months. Cochrane Database Syst Rev. 2008;(2):CD005976.-
28. Tice AD, Rehm SJ, Dalovisio JR, et al. Practice guidelines for outpatient parenteral antimicrobial therapy. IDSA guidelines. Clin Infect Dis. 2004;38:1651-1672.
29. Bradley JS, Ching DK, Hart CL. Invasive bacterial disease in childhood: efficacy of oral antibiotic therapy following short course parenteral therapy in non-central nervous system infections. Pediatr Infect Dis J. 1987;6:821-825.
30. Blaschke AJ, Heyrend C, Byington CL, et al. Molecular analysis improves pathogen identification and epidemiologic study of pediatric parapneumonic empyema. Pediatr Infect Dis J. 2011;30:289-294.
31. American Academy of Pediatrics Committee on Infectious Diseases and Committee on Fetus and Newborn. Revised indications for the use of palivizumab and RSV immune globulin intravenous for the prevention of respiratory syncytial virus infection. Pediatrics. 2003;112:1442-1446.
1. Bradley JS, Byington CL, Shah SS, et al. The management of community-acquired pneumonia in infants and children older than 3 months of age: clinical practice guidelines by the Pediatric Infectious Diseases Society and the Infectious Diseases Society of America. Clin Infect Dis. 2011;53:e25-e76.Available at: http://cid.oxfordjournals.org/content/53/7/e25.long. Accessed December 17, 2012.
2. Centers for Disease Control and Prevention Pneumonia Can Be Prevented–Vaccines Can Help. Available at: http://www.cdc.gov/features/pneumonia. Accessed January 17, 2012.
3. Bulla A, Hitze KL. Acute respiratory infections: a review. Bull World Health Organ. 1978;56:481-498.
4. Baqui AH, Black RE, Arifeen SE, et al. Causes of childhood deaths in Bangladesh: results of a nationwide verbal autopsy study. Bull World Health Organ. 1998;76:161-171.
5. Murphy SL, Xu JQ, Kochanek KD. Deaths: Preliminary data for 2010. National vital statistics reports; vol 60 no 4. Hyattsville, Md: National Center for Health Statistics. 2012. Available at: http://www.cdc.gov/nchs/data/nvsr/nvsr60/nvsr60_04.pdf. Accessed May 12, 2012.
6. Clinical management of acute respiratory infections in children: a WHO memorandum. Bull World Health Organ. 1981;59:707-716.
7. Feldman C, Anderson R. Community-acquired pneumonia. In; Bone RC, Dantzker DR, George RB, et al, eds. Pulmonary and Critical Care Medicine. Vol 2. St. Louis, Mo: Mosby-Year Book, Inc; 1997:719–733.
8. Davies HD. Community-acquired pneumonia in children. Paediatr Child Health. 2003;8:616-619.
9. Alexander ER, Foy HM, Kenny GE, et al. Pneumonia due to Mycoplasma pneumoniae. Its incidence in the membership of a co-operative medical group. N Engl J Med. 1966;275:131-136.
10. Foy HM, Cooney MK, Maletzky AJ, et al. Incidence and etiology of pneumonia, croup and bronchiolitis in preschool children belonging to a prepaid medical group over a four-year period. Am J Epidemiol. 1973;97:80-92.
11. Murphy TF, Henderson FW, Clyde WA, Jr, et al. Pneumonia: An eleven-year study in a pediatric practice. Am J Epidemiol. 1981;113:12-21.
12. Denny FW, Clyde WA. Acute lower respiratory tract infections in non-hospitalized children. J Pediatr. 1986;108:635-646.
13. Ostapchuk M, Roberts DM, Haddy R. Community-acquired pneumonia in infants and children. Am Fam Phys. 2004;70:899-908.
14. Margolis P, Gadomski A. The rational clinical examination. Does this infant have pneumonia? JAMA. 1998;279:308-313.
15. Harris M, Clark J, Coote N, et al. British Thoracic Society guidelines for the management of community acquired pneumonia in children: update 2011. Thorax. 2011;66 (suppl 2):ii1-ii23.
16. Gaston B. Pneumonia. Pediatr Rev. 2002;23:132-140.
17. McIntosh K. Community-acquired pneumonia in children. N Engl J Med. 2002;346:429-437.
18. Skolnik N, Tien P. Managing community-acquired pneumonia in infants and children. Fam Pract News. November 10, 2011. Available at: http://www.familypracticenews.com/views/clinical-guidelines-for-family-physicians-by-dr-skolnik/blog/managing-community-acquired-pneumonia-in-infants-and-children/3a77ebb81a.html. Accessed January 17, 2012.
19. O’Mara N. Empiric treatment for pediatric community-acquired pneumonia. Pharmacist’s Letter. November 2011. Available at: http://www.pharmacistletter.com. Accessed February 25, 2012.
20. Klein JO. Bacterial pneumonias. In: Cherry J, Kaplan S, Demmler-Harrison G, eds. Feigin & Cherry’s Textbook of Pediatric Infectious Diseases. 6th ed. Vol 1. Philadelphia, Pa: Saunders/Elsevier; 2009:302–314.
21. Morita JY, Kahn E, Thompson T, et al. Impact of azithromycin on oropharyngeal carriage of group A Streptococcus and nasopharyngeal carriage of macrolide-resistant Streptococcus pneumoniae. Pediatr Infect Dis J. 2000;19:41-46.
22. Block S, Hedrick J, Hammerschlag MR, et al. Mycoplasma pneumoniae and Chlamydia pneumoniae in pediatric community-acquired pneumonia: comparative efficacy and safety of clarithromycin vs. erythromycin ethylsuccinate. Pediatr Infect Dis J. 1995;14:471-477.
23. Harris JA, Kolokathis A, Campbell M, et al. Safety and efficacy of azithromycin in the treatment of community-acquired pneumonia in children. Pediatr Infect Dis J. 1998;17:865-871.
24. Pallares R, Capdevila O, Linares J, et al. The effect of cephalosporin resistance on mortality in adult patients with nonmeningeal systemic pneumococcal infections. Am J Med. 2002;113:120-126.
25. Roson B, Carratala J, Tubau F, et al. Usefulness of betalactam therapy for community-acquired pneumonia in the era of drug-resistant Streptococcus pneumoniae: a randomized study of amoxicillin-clavulanate and ceftriaxone. Microb Drug Resist. 2001;7:85-96.
26. Miller LG, Kaplan SL. Staphylococcus aureus: a community pathogen. Infect Dis Clin North Am. 2009;23:35-52.
27. Haider BA, Saeed MA, Bhutta ZA. Short-course versus long-course antibiotic therapy for non-severe community-acquired pneumonia in children aged 2 months to 59 months. Cochrane Database Syst Rev. 2008;(2):CD005976.-
28. Tice AD, Rehm SJ, Dalovisio JR, et al. Practice guidelines for outpatient parenteral antimicrobial therapy. IDSA guidelines. Clin Infect Dis. 2004;38:1651-1672.
29. Bradley JS, Ching DK, Hart CL. Invasive bacterial disease in childhood: efficacy of oral antibiotic therapy following short course parenteral therapy in non-central nervous system infections. Pediatr Infect Dis J. 1987;6:821-825.
30. Blaschke AJ, Heyrend C, Byington CL, et al. Molecular analysis improves pathogen identification and epidemiologic study of pediatric parapneumonic empyema. Pediatr Infect Dis J. 2011;30:289-294.
31. American Academy of Pediatrics Committee on Infectious Diseases and Committee on Fetus and Newborn. Revised indications for the use of palivizumab and RSV immune globulin intravenous for the prevention of respiratory syncytial virus infection. Pediatrics. 2003;112:1442-1446.
Speed your diagnosis of this gallbladder disorder
• Use the Rome III guidelines to diagnose and treat functional gallbladder disorder; when this benchmark is followed, cholecystectomy results in ~90% resolution rate. B
• Keep in mind that classic biliary symptoms, particularly right upper quadrant pain, pain after eating, and reproduction of pain with cholecystokinin injection, are highly predictive of a successful postoperative outcome. C
• Offer cholecystectomy to patients who present with classic biliary symptoms and an abnormal hepatobiliary iminodiacetic acid (HIDA) scan. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
CASE Dionne J, a 38-year-old woman with a BMI of 32, presents with a 2-month history of right upper abdominal pain. The pain is intermittent and often begins after eating, she reports, particularly when a meal includes fatty foods. She has no nausea, vomiting, diarrhea, constipation, or fever, and the pain is not getting progressively worse.
When the pain comes on, Ms. J says, it lasts about an hour, sometimes less. It is colicky in nature, and not relieved with bowel movements or position change. The patient tried ranitidine 150 mg twice a day for 2 weeks, with no relief. You suspect functional gallbladder disorder. But is Ms. J a candidate for a cholecystectomy? What would you do next?
Over the past 2 decades, the incidence of cholecystectomies due to functional gallbladder disorder (FGBD) has multiplied, going from about 5% to 20% to 25%.1 But definitive information about the etiology of FGBD has not kept pace.
Although the Rome III diagnostic guidelines for FGBD, published in 2006,2 remain the standard of care, a number of more recent studies have added to our understanding of this disorder. This review of the diagnosis and treatment of FGBD incorporates both the Rome III guidelines and the latest findings. The text and tables that follow can help you recognize this clinical entity earlier, minimize the number of tests needed to arrive at a definitive diagnosis, and establish a plan of care that is consistent with both the guidelines and the evidence.
As obesity rates rise, so does gallbladder dysfunction
Obesity has been shown to produce a chronic proinflammatory state throughout the body,3-6 which has been linked to fatty infiltration of the gallbladder (among other organs) and impaired contractility.3,6-9
A study by Al-Azzawi et al highlighted the importance of increased fat in the gallbladder wall as a key cause of dysmotility.10 The researchers compared wall thickness, inflammation, and the amount of fat in the walls of gallbladders that had been removed for both acalculous and calculous disease with the characteristics of gallbladders removed for reasons unrelated to organ dysfunction (the controls). Those with dysmotility, they found, had more fat in the wall but the same wall thickness as the controls. The amount of fat in the walls was similar for the acalculous and the calculous groups, but the gallbladders in which stones were found had more inflammation and increased wall thickness.10
Several other studies have found evidence of both inflammation and fatty deposits in the walls of gallbladders removed for acalculous disease.2,4,11-13
In one study, researchers found chronic inflammation in 99% of gallbladders removed from patients who had classic biliary symptoms but no gallstones.11
FGBD appears to be initiated by fatty infiltration of the gallbladder wall, causing increasing levels of inflammation and steatocholecystitis that lead to poor motility.3,4,6-10 This in turn alters bile composition, which can lead to sludge and stone formation.2,6,10 The finding by Al-Azzawi et al of greater thickness and inflammation in the walls of gallbladders with calculi suggests that gallstones result from progressively worsening inflammation and dysmotility.10
Steps to take for a definitive diagnosis
A diagnosis of FGBD requires a history of classic gallbladder symptoms, many but not all of which are specified in the Rome III diagnostic criteria (TABLE). Classic symptoms include nausea, vomiting, right upper quadrant pain, pain after eating, and reproduction of pain with cholecystokinin (CCK) injection. Cramping, bloating, reflux, diarrhea, fullness, and epigastric pain are atypical symptoms.2,11
TABLE
Rome III diagnostic criteria for functional gallbladder disorder
Must include episodes of pain located in the epigastrium and/or right upper quadrant and all of the following findings:
|
| Supportive criteria |
The pain may present with one or more of the following findings:
|
| Source: Behar et al. Gastroenterology. 2006;130:1498-1509.2 Used with permission from Elsevier. |
Rule out structural causes
There is no single test for FGBD, and a definitive diagnosis can be made only after structural causes of the symptoms (eg, gallstones, tumor, sclerosis, and cirrhosis) have been ruled out (ALGORITHM).2 Initial tests include liver and pancreatic enzyme laboratory screening and an ultrasound of the upper right quadrant. In patients with FGBD, both the lab tests and the ultrasound will be normal.
ALGORITHM
Diagnostic workup and management of functional gallbladder disorder (Rome III)
CCK, cholecystokinin; EGD, esophagogastroduodenoscopy; GBEF, gallbladder ejection fraction; HIDA, hepatobiliary iminodiacetic acid; LFTs, liver function tests; US, ultrasound.
Source: Behar J et al. Gastroenterology. 2006;130:1498-1509.2 Used with permission from Elsevier.
The Rome III guidelines also call for an esophagogastroduodenoscopy (EGD) to rule out esophagitis, gastritis, and duodenitis, although some researchers have held that if the other tests are normal, this test need not be done.12 If the EGD is also normal—or not done—a hepatobiliary iminodiacetic acid (HIDA) scan is the next step in the diagnostic pathway. The scan tests the gallbladder’s ejection fraction (EF), revealing the percentage of radioactive dye ejected from the organ after CCK is injected (FIGURE).2 The injection of CCK should be done over a minimum of 30 minutes, the guidelines specify. The shorter the time frame used for the injection, the less likely that the pain will be reproduced or that the EF findings will be reliable.14
FIGURE
Abnormal vs normal HIDA scans: What you’ll see
The larger amount of contrast dye retained in the abnormal scan (A) compared with the normal scan (B) is evidence of a poor ejection fraction.
Most researchers define a normal EF as >35%,10,11,13 but the Rome III criteria use a cutoff of 40%. A patient who has an EF <40% and meets the other guideline criteria is diagnosed with FGBD.
CASE On physical examination, Ms. J has pain in the right upper quadrant, with no guarding or rebound, and normal bowel sounds. Her liver and pancreatic enzyme tests are normal, and an ultrasound shows no sludge, no stones, and mild edema of the gallbladder wall. The patient declines an EGD because of the cost but undergoes a HIDA scan—which reveals that she has an EF of 25%.
Will cholecystectomy bring long-term relief?
There are 2 options for a patient diagnosed with FGBD—medical management, consisting of lifestyle modifications such as dietary change and weight loss and medication for symptom relief—or cholecystectomy. Surgery should be offered to any individual who, like Ms. J, meets the Rome III diagnostic criteria and has an abnormal HIDA scan. Recent studies have raised questions about the correlation between HIDA results and postoperative relief,11,12 however, and indicate that patients who have classic biliary symptoms and a normal HIDA scan often have good postoperative outcomes, as well.11,15
A careful workup is key to ensuring maximal benefit from surgery. The resolution of symptoms with a cholecystectomy when the Rome III criteria are followed for patient selection has been found to be close to 90%.11,15-20 Two recent studies have examined the resolution rate for FGBD, with conflicting results.11,12 Both studies were based on long-term postoperative follow-up, ranging from 6 to 24 months. The main difference was the selection bias used in determining eligibility for the study.
The initial selection criteria for the study by Carr et al (N=93) were presenting symptoms (either classic or atypical), followed by a typical workup. The long-term resolution rate for those with classic gallbladder symptoms was 88%11—close to the 90% associated with the Rome III guidelines. The study by Singhal et al (N=141)12 was done retrospectively, using objective data from tests (ie, normal ultrasound and liver biochemistries and abnormal HIDA) rather than patient history as the criteria for inclusion. Among participants in the Singhal study, the long-term resolution rate was just 57%.
Ironically, the patients in the Carr study who had atypical symptoms had mixed postoperative results. The rate of long-term resolution for this cohort was 57%—the same as the overall resolution rate found by Singhal et al.11,12 The fact that a group of patients who presented atypically had the same postoperative resolution rate as those for whom tests (rather than symptoms) were used as the selection criteria illustrates the importance of presenting symptoms as a prognostic indicator.
CASE Ms. J opts for a cholecystectomy and you refer her to a general surgeon. At her annual exam the following year, she reports that she has been symptom free since the surgery.
CORRESPONDENCE
David I. Croteau, MD, FAAFP, LRMC Family Medical Center, 300 Parkview Place, Lakeland, FL 33805; David.Croteau@lrmc.com
1. Majeski J. Gallbladder ejection fraction: an accurate evaluation of symptomatic acalculous gallbladder disease. Int Surg. 2003;88:95-99.
2. Behar J, Corazziari E, Guelrud M, et al. Functional gallbladder and sphincter of Oddi disorders. Gastroenterology. 2006;130:1498-1509.
3. Goldblatt MI, Swartz-Basile DA, Al-Azzawi HH, et al. Nonalcoholic fatty gallbladder disease: the Influence of diet in lean and obese mice. J Gastrointest Surg. 2006;10:193-201.
4. Chung-Jyi. Steatocholecystitis and fatty gallbladder disease. Dig Dis Sci. 2009;54:1857-1863.
5. Bastard JP, Maachi M, Lagathu C, et al. Recent advances in the relationship between obesity, inflammation, and insulin resistance. Eur Cytokine Netw. 2006;17:4-12.
6. Pitt HA. Hepato-pancreato-biliary fat: the good, the bad and the ugly. HPB (Oxford). 2007;9:92-97.
7. Merg AR, Kalinowski SE, Hinkhouse MM, et al. Mechanisms of impaired gallbladder contractile response in chronic acalculous cholecystitis. J Gastrointest Surg. 2002;6:432-437.
8. Amaral J, Xiao ZL, Chen Q, et al. Gallbladder muscle dysfunction in patients with chronic acalculous disease. Gastroenterology. 2001;120:506-511.
9. Portincasa P, Ciaula AD, Baldassarre G, et al. Gallbladder motor function in gallstone patients: sonographic and in vitro studies on the role of gallstones, smooth muscle function, and gallbladder wall inflammation. J Hepatol. 1994;21:430-440.
10. Al-Azzawi HH, Nakeeb A, Saxena R, et al. Cholecystosteatosis: an explanation for increased cholecystectomy rates. J Gastrointest Surg. 2007;11:835-843.
11. Carr JA, Walls J, Bryan LJ, et al. The treatment of gallbladder dyskinesia based upon symptoms: results of a 2-year, prospective, nonrandomized, concurrent cohort study. Surg Laparosc Endosc Percutan Tech. 2009;19:222-226.
12. Singhal V, Szeto P, Norman H, et al. Biliary dyskinesia: how effective is cholecystectomy? J Gastrointest Surg. 2012;16:135-141.
13. Francis G, Baillie J. Gallbladder dyskinesia: fact or fiction? Curr Gastroenterol Rep. 2011;13:188-192.
14. Ziessman HA. Nuclear medicine hepatobiliary imaging. Clin Gastroenterol Hepatol. 2010;8:111-116.
15. Delgado-Aros S, Cremonini R, Bredenoord AJ, et al. Systemic review and meta-analysis: does gallbladder ejection fraction on cholecystokinin cholescintigraphy predict outcome after cholecystectomy in suspected functional biliary pain? Aliment Pharmacol Ther. 2003;18:167-174.
16. Patel PA, Lamb JJ, Hogle NJ, et al. Therapeutic efficacy of laparoscopic cholecystectomy in the treatment of biliary dyskinesia. Am J Surg. 2004;187:209-212.
17. Mahid SS, Jafri NS, Brangers BC, et al. Meta-analysis of cholecystectomy in symptomatic patients with positive hepatoiminodiacetic acid scan results without gallstones. Arch Surg. 2009;144:180-187.
18. Hansel SL, DiBaise JK. Functional gallbladder disorder: gallbladder dyskinesia. Gastroenterol Clin North Am. 2010;39:369-379.
19. Canfield AJ, Hetz SP, Shriver JP, et al. Biliary dyskinesia: a study of more than 200 patients and review of the literature. J Gastrointest Surg. 1998;2:443-448.
20. Jagannath SB, Singh VK, Cruz-Correa M, et al. A long-term cohort study of outcome after cholecystectomy for chronic acalculous cholecystitis. Am J Surg. 2003;185:91-95.
• Use the Rome III guidelines to diagnose and treat functional gallbladder disorder; when this benchmark is followed, cholecystectomy results in ~90% resolution rate. B
• Keep in mind that classic biliary symptoms, particularly right upper quadrant pain, pain after eating, and reproduction of pain with cholecystokinin injection, are highly predictive of a successful postoperative outcome. C
• Offer cholecystectomy to patients who present with classic biliary symptoms and an abnormal hepatobiliary iminodiacetic acid (HIDA) scan. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
CASE Dionne J, a 38-year-old woman with a BMI of 32, presents with a 2-month history of right upper abdominal pain. The pain is intermittent and often begins after eating, she reports, particularly when a meal includes fatty foods. She has no nausea, vomiting, diarrhea, constipation, or fever, and the pain is not getting progressively worse.
When the pain comes on, Ms. J says, it lasts about an hour, sometimes less. It is colicky in nature, and not relieved with bowel movements or position change. The patient tried ranitidine 150 mg twice a day for 2 weeks, with no relief. You suspect functional gallbladder disorder. But is Ms. J a candidate for a cholecystectomy? What would you do next?
Over the past 2 decades, the incidence of cholecystectomies due to functional gallbladder disorder (FGBD) has multiplied, going from about 5% to 20% to 25%.1 But definitive information about the etiology of FGBD has not kept pace.
Although the Rome III diagnostic guidelines for FGBD, published in 2006,2 remain the standard of care, a number of more recent studies have added to our understanding of this disorder. This review of the diagnosis and treatment of FGBD incorporates both the Rome III guidelines and the latest findings. The text and tables that follow can help you recognize this clinical entity earlier, minimize the number of tests needed to arrive at a definitive diagnosis, and establish a plan of care that is consistent with both the guidelines and the evidence.
As obesity rates rise, so does gallbladder dysfunction
Obesity has been shown to produce a chronic proinflammatory state throughout the body,3-6 which has been linked to fatty infiltration of the gallbladder (among other organs) and impaired contractility.3,6-9
A study by Al-Azzawi et al highlighted the importance of increased fat in the gallbladder wall as a key cause of dysmotility.10 The researchers compared wall thickness, inflammation, and the amount of fat in the walls of gallbladders that had been removed for both acalculous and calculous disease with the characteristics of gallbladders removed for reasons unrelated to organ dysfunction (the controls). Those with dysmotility, they found, had more fat in the wall but the same wall thickness as the controls. The amount of fat in the walls was similar for the acalculous and the calculous groups, but the gallbladders in which stones were found had more inflammation and increased wall thickness.10
Several other studies have found evidence of both inflammation and fatty deposits in the walls of gallbladders removed for acalculous disease.2,4,11-13
In one study, researchers found chronic inflammation in 99% of gallbladders removed from patients who had classic biliary symptoms but no gallstones.11
FGBD appears to be initiated by fatty infiltration of the gallbladder wall, causing increasing levels of inflammation and steatocholecystitis that lead to poor motility.3,4,6-10 This in turn alters bile composition, which can lead to sludge and stone formation.2,6,10 The finding by Al-Azzawi et al of greater thickness and inflammation in the walls of gallbladders with calculi suggests that gallstones result from progressively worsening inflammation and dysmotility.10
Steps to take for a definitive diagnosis
A diagnosis of FGBD requires a history of classic gallbladder symptoms, many but not all of which are specified in the Rome III diagnostic criteria (TABLE). Classic symptoms include nausea, vomiting, right upper quadrant pain, pain after eating, and reproduction of pain with cholecystokinin (CCK) injection. Cramping, bloating, reflux, diarrhea, fullness, and epigastric pain are atypical symptoms.2,11
TABLE
Rome III diagnostic criteria for functional gallbladder disorder
Must include episodes of pain located in the epigastrium and/or right upper quadrant and all of the following findings:
|
| Supportive criteria |
The pain may present with one or more of the following findings:
|
| Source: Behar et al. Gastroenterology. 2006;130:1498-1509.2 Used with permission from Elsevier. |
Rule out structural causes
There is no single test for FGBD, and a definitive diagnosis can be made only after structural causes of the symptoms (eg, gallstones, tumor, sclerosis, and cirrhosis) have been ruled out (ALGORITHM).2 Initial tests include liver and pancreatic enzyme laboratory screening and an ultrasound of the upper right quadrant. In patients with FGBD, both the lab tests and the ultrasound will be normal.
ALGORITHM
Diagnostic workup and management of functional gallbladder disorder (Rome III)
CCK, cholecystokinin; EGD, esophagogastroduodenoscopy; GBEF, gallbladder ejection fraction; HIDA, hepatobiliary iminodiacetic acid; LFTs, liver function tests; US, ultrasound.
Source: Behar J et al. Gastroenterology. 2006;130:1498-1509.2 Used with permission from Elsevier.
The Rome III guidelines also call for an esophagogastroduodenoscopy (EGD) to rule out esophagitis, gastritis, and duodenitis, although some researchers have held that if the other tests are normal, this test need not be done.12 If the EGD is also normal—or not done—a hepatobiliary iminodiacetic acid (HIDA) scan is the next step in the diagnostic pathway. The scan tests the gallbladder’s ejection fraction (EF), revealing the percentage of radioactive dye ejected from the organ after CCK is injected (FIGURE).2 The injection of CCK should be done over a minimum of 30 minutes, the guidelines specify. The shorter the time frame used for the injection, the less likely that the pain will be reproduced or that the EF findings will be reliable.14
FIGURE
Abnormal vs normal HIDA scans: What you’ll see
The larger amount of contrast dye retained in the abnormal scan (A) compared with the normal scan (B) is evidence of a poor ejection fraction.
Most researchers define a normal EF as >35%,10,11,13 but the Rome III criteria use a cutoff of 40%. A patient who has an EF <40% and meets the other guideline criteria is diagnosed with FGBD.
CASE On physical examination, Ms. J has pain in the right upper quadrant, with no guarding or rebound, and normal bowel sounds. Her liver and pancreatic enzyme tests are normal, and an ultrasound shows no sludge, no stones, and mild edema of the gallbladder wall. The patient declines an EGD because of the cost but undergoes a HIDA scan—which reveals that she has an EF of 25%.
Will cholecystectomy bring long-term relief?
There are 2 options for a patient diagnosed with FGBD—medical management, consisting of lifestyle modifications such as dietary change and weight loss and medication for symptom relief—or cholecystectomy. Surgery should be offered to any individual who, like Ms. J, meets the Rome III diagnostic criteria and has an abnormal HIDA scan. Recent studies have raised questions about the correlation between HIDA results and postoperative relief,11,12 however, and indicate that patients who have classic biliary symptoms and a normal HIDA scan often have good postoperative outcomes, as well.11,15
A careful workup is key to ensuring maximal benefit from surgery. The resolution of symptoms with a cholecystectomy when the Rome III criteria are followed for patient selection has been found to be close to 90%.11,15-20 Two recent studies have examined the resolution rate for FGBD, with conflicting results.11,12 Both studies were based on long-term postoperative follow-up, ranging from 6 to 24 months. The main difference was the selection bias used in determining eligibility for the study.
The initial selection criteria for the study by Carr et al (N=93) were presenting symptoms (either classic or atypical), followed by a typical workup. The long-term resolution rate for those with classic gallbladder symptoms was 88%11—close to the 90% associated with the Rome III guidelines. The study by Singhal et al (N=141)12 was done retrospectively, using objective data from tests (ie, normal ultrasound and liver biochemistries and abnormal HIDA) rather than patient history as the criteria for inclusion. Among participants in the Singhal study, the long-term resolution rate was just 57%.
Ironically, the patients in the Carr study who had atypical symptoms had mixed postoperative results. The rate of long-term resolution for this cohort was 57%—the same as the overall resolution rate found by Singhal et al.11,12 The fact that a group of patients who presented atypically had the same postoperative resolution rate as those for whom tests (rather than symptoms) were used as the selection criteria illustrates the importance of presenting symptoms as a prognostic indicator.
CASE Ms. J opts for a cholecystectomy and you refer her to a general surgeon. At her annual exam the following year, she reports that she has been symptom free since the surgery.
CORRESPONDENCE
David I. Croteau, MD, FAAFP, LRMC Family Medical Center, 300 Parkview Place, Lakeland, FL 33805; David.Croteau@lrmc.com
• Use the Rome III guidelines to diagnose and treat functional gallbladder disorder; when this benchmark is followed, cholecystectomy results in ~90% resolution rate. B
• Keep in mind that classic biliary symptoms, particularly right upper quadrant pain, pain after eating, and reproduction of pain with cholecystokinin injection, are highly predictive of a successful postoperative outcome. C
• Offer cholecystectomy to patients who present with classic biliary symptoms and an abnormal hepatobiliary iminodiacetic acid (HIDA) scan. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
CASE Dionne J, a 38-year-old woman with a BMI of 32, presents with a 2-month history of right upper abdominal pain. The pain is intermittent and often begins after eating, she reports, particularly when a meal includes fatty foods. She has no nausea, vomiting, diarrhea, constipation, or fever, and the pain is not getting progressively worse.
When the pain comes on, Ms. J says, it lasts about an hour, sometimes less. It is colicky in nature, and not relieved with bowel movements or position change. The patient tried ranitidine 150 mg twice a day for 2 weeks, with no relief. You suspect functional gallbladder disorder. But is Ms. J a candidate for a cholecystectomy? What would you do next?
Over the past 2 decades, the incidence of cholecystectomies due to functional gallbladder disorder (FGBD) has multiplied, going from about 5% to 20% to 25%.1 But definitive information about the etiology of FGBD has not kept pace.
Although the Rome III diagnostic guidelines for FGBD, published in 2006,2 remain the standard of care, a number of more recent studies have added to our understanding of this disorder. This review of the diagnosis and treatment of FGBD incorporates both the Rome III guidelines and the latest findings. The text and tables that follow can help you recognize this clinical entity earlier, minimize the number of tests needed to arrive at a definitive diagnosis, and establish a plan of care that is consistent with both the guidelines and the evidence.
As obesity rates rise, so does gallbladder dysfunction
Obesity has been shown to produce a chronic proinflammatory state throughout the body,3-6 which has been linked to fatty infiltration of the gallbladder (among other organs) and impaired contractility.3,6-9
A study by Al-Azzawi et al highlighted the importance of increased fat in the gallbladder wall as a key cause of dysmotility.10 The researchers compared wall thickness, inflammation, and the amount of fat in the walls of gallbladders that had been removed for both acalculous and calculous disease with the characteristics of gallbladders removed for reasons unrelated to organ dysfunction (the controls). Those with dysmotility, they found, had more fat in the wall but the same wall thickness as the controls. The amount of fat in the walls was similar for the acalculous and the calculous groups, but the gallbladders in which stones were found had more inflammation and increased wall thickness.10
Several other studies have found evidence of both inflammation and fatty deposits in the walls of gallbladders removed for acalculous disease.2,4,11-13
In one study, researchers found chronic inflammation in 99% of gallbladders removed from patients who had classic biliary symptoms but no gallstones.11
FGBD appears to be initiated by fatty infiltration of the gallbladder wall, causing increasing levels of inflammation and steatocholecystitis that lead to poor motility.3,4,6-10 This in turn alters bile composition, which can lead to sludge and stone formation.2,6,10 The finding by Al-Azzawi et al of greater thickness and inflammation in the walls of gallbladders with calculi suggests that gallstones result from progressively worsening inflammation and dysmotility.10
Steps to take for a definitive diagnosis
A diagnosis of FGBD requires a history of classic gallbladder symptoms, many but not all of which are specified in the Rome III diagnostic criteria (TABLE). Classic symptoms include nausea, vomiting, right upper quadrant pain, pain after eating, and reproduction of pain with cholecystokinin (CCK) injection. Cramping, bloating, reflux, diarrhea, fullness, and epigastric pain are atypical symptoms.2,11
TABLE
Rome III diagnostic criteria for functional gallbladder disorder
Must include episodes of pain located in the epigastrium and/or right upper quadrant and all of the following findings:
|
| Supportive criteria |
The pain may present with one or more of the following findings:
|
| Source: Behar et al. Gastroenterology. 2006;130:1498-1509.2 Used with permission from Elsevier. |
Rule out structural causes
There is no single test for FGBD, and a definitive diagnosis can be made only after structural causes of the symptoms (eg, gallstones, tumor, sclerosis, and cirrhosis) have been ruled out (ALGORITHM).2 Initial tests include liver and pancreatic enzyme laboratory screening and an ultrasound of the upper right quadrant. In patients with FGBD, both the lab tests and the ultrasound will be normal.
ALGORITHM
Diagnostic workup and management of functional gallbladder disorder (Rome III)
CCK, cholecystokinin; EGD, esophagogastroduodenoscopy; GBEF, gallbladder ejection fraction; HIDA, hepatobiliary iminodiacetic acid; LFTs, liver function tests; US, ultrasound.
Source: Behar J et al. Gastroenterology. 2006;130:1498-1509.2 Used with permission from Elsevier.
The Rome III guidelines also call for an esophagogastroduodenoscopy (EGD) to rule out esophagitis, gastritis, and duodenitis, although some researchers have held that if the other tests are normal, this test need not be done.12 If the EGD is also normal—or not done—a hepatobiliary iminodiacetic acid (HIDA) scan is the next step in the diagnostic pathway. The scan tests the gallbladder’s ejection fraction (EF), revealing the percentage of radioactive dye ejected from the organ after CCK is injected (FIGURE).2 The injection of CCK should be done over a minimum of 30 minutes, the guidelines specify. The shorter the time frame used for the injection, the less likely that the pain will be reproduced or that the EF findings will be reliable.14
FIGURE
Abnormal vs normal HIDA scans: What you’ll see
The larger amount of contrast dye retained in the abnormal scan (A) compared with the normal scan (B) is evidence of a poor ejection fraction.
Most researchers define a normal EF as >35%,10,11,13 but the Rome III criteria use a cutoff of 40%. A patient who has an EF <40% and meets the other guideline criteria is diagnosed with FGBD.
CASE On physical examination, Ms. J has pain in the right upper quadrant, with no guarding or rebound, and normal bowel sounds. Her liver and pancreatic enzyme tests are normal, and an ultrasound shows no sludge, no stones, and mild edema of the gallbladder wall. The patient declines an EGD because of the cost but undergoes a HIDA scan—which reveals that she has an EF of 25%.
Will cholecystectomy bring long-term relief?
There are 2 options for a patient diagnosed with FGBD—medical management, consisting of lifestyle modifications such as dietary change and weight loss and medication for symptom relief—or cholecystectomy. Surgery should be offered to any individual who, like Ms. J, meets the Rome III diagnostic criteria and has an abnormal HIDA scan. Recent studies have raised questions about the correlation between HIDA results and postoperative relief,11,12 however, and indicate that patients who have classic biliary symptoms and a normal HIDA scan often have good postoperative outcomes, as well.11,15
A careful workup is key to ensuring maximal benefit from surgery. The resolution of symptoms with a cholecystectomy when the Rome III criteria are followed for patient selection has been found to be close to 90%.11,15-20 Two recent studies have examined the resolution rate for FGBD, with conflicting results.11,12 Both studies were based on long-term postoperative follow-up, ranging from 6 to 24 months. The main difference was the selection bias used in determining eligibility for the study.
The initial selection criteria for the study by Carr et al (N=93) were presenting symptoms (either classic or atypical), followed by a typical workup. The long-term resolution rate for those with classic gallbladder symptoms was 88%11—close to the 90% associated with the Rome III guidelines. The study by Singhal et al (N=141)12 was done retrospectively, using objective data from tests (ie, normal ultrasound and liver biochemistries and abnormal HIDA) rather than patient history as the criteria for inclusion. Among participants in the Singhal study, the long-term resolution rate was just 57%.
Ironically, the patients in the Carr study who had atypical symptoms had mixed postoperative results. The rate of long-term resolution for this cohort was 57%—the same as the overall resolution rate found by Singhal et al.11,12 The fact that a group of patients who presented atypically had the same postoperative resolution rate as those for whom tests (rather than symptoms) were used as the selection criteria illustrates the importance of presenting symptoms as a prognostic indicator.
CASE Ms. J opts for a cholecystectomy and you refer her to a general surgeon. At her annual exam the following year, she reports that she has been symptom free since the surgery.
CORRESPONDENCE
David I. Croteau, MD, FAAFP, LRMC Family Medical Center, 300 Parkview Place, Lakeland, FL 33805; David.Croteau@lrmc.com
1. Majeski J. Gallbladder ejection fraction: an accurate evaluation of symptomatic acalculous gallbladder disease. Int Surg. 2003;88:95-99.
2. Behar J, Corazziari E, Guelrud M, et al. Functional gallbladder and sphincter of Oddi disorders. Gastroenterology. 2006;130:1498-1509.
3. Goldblatt MI, Swartz-Basile DA, Al-Azzawi HH, et al. Nonalcoholic fatty gallbladder disease: the Influence of diet in lean and obese mice. J Gastrointest Surg. 2006;10:193-201.
4. Chung-Jyi. Steatocholecystitis and fatty gallbladder disease. Dig Dis Sci. 2009;54:1857-1863.
5. Bastard JP, Maachi M, Lagathu C, et al. Recent advances in the relationship between obesity, inflammation, and insulin resistance. Eur Cytokine Netw. 2006;17:4-12.
6. Pitt HA. Hepato-pancreato-biliary fat: the good, the bad and the ugly. HPB (Oxford). 2007;9:92-97.
7. Merg AR, Kalinowski SE, Hinkhouse MM, et al. Mechanisms of impaired gallbladder contractile response in chronic acalculous cholecystitis. J Gastrointest Surg. 2002;6:432-437.
8. Amaral J, Xiao ZL, Chen Q, et al. Gallbladder muscle dysfunction in patients with chronic acalculous disease. Gastroenterology. 2001;120:506-511.
9. Portincasa P, Ciaula AD, Baldassarre G, et al. Gallbladder motor function in gallstone patients: sonographic and in vitro studies on the role of gallstones, smooth muscle function, and gallbladder wall inflammation. J Hepatol. 1994;21:430-440.
10. Al-Azzawi HH, Nakeeb A, Saxena R, et al. Cholecystosteatosis: an explanation for increased cholecystectomy rates. J Gastrointest Surg. 2007;11:835-843.
11. Carr JA, Walls J, Bryan LJ, et al. The treatment of gallbladder dyskinesia based upon symptoms: results of a 2-year, prospective, nonrandomized, concurrent cohort study. Surg Laparosc Endosc Percutan Tech. 2009;19:222-226.
12. Singhal V, Szeto P, Norman H, et al. Biliary dyskinesia: how effective is cholecystectomy? J Gastrointest Surg. 2012;16:135-141.
13. Francis G, Baillie J. Gallbladder dyskinesia: fact or fiction? Curr Gastroenterol Rep. 2011;13:188-192.
14. Ziessman HA. Nuclear medicine hepatobiliary imaging. Clin Gastroenterol Hepatol. 2010;8:111-116.
15. Delgado-Aros S, Cremonini R, Bredenoord AJ, et al. Systemic review and meta-analysis: does gallbladder ejection fraction on cholecystokinin cholescintigraphy predict outcome after cholecystectomy in suspected functional biliary pain? Aliment Pharmacol Ther. 2003;18:167-174.
16. Patel PA, Lamb JJ, Hogle NJ, et al. Therapeutic efficacy of laparoscopic cholecystectomy in the treatment of biliary dyskinesia. Am J Surg. 2004;187:209-212.
17. Mahid SS, Jafri NS, Brangers BC, et al. Meta-analysis of cholecystectomy in symptomatic patients with positive hepatoiminodiacetic acid scan results without gallstones. Arch Surg. 2009;144:180-187.
18. Hansel SL, DiBaise JK. Functional gallbladder disorder: gallbladder dyskinesia. Gastroenterol Clin North Am. 2010;39:369-379.
19. Canfield AJ, Hetz SP, Shriver JP, et al. Biliary dyskinesia: a study of more than 200 patients and review of the literature. J Gastrointest Surg. 1998;2:443-448.
20. Jagannath SB, Singh VK, Cruz-Correa M, et al. A long-term cohort study of outcome after cholecystectomy for chronic acalculous cholecystitis. Am J Surg. 2003;185:91-95.
1. Majeski J. Gallbladder ejection fraction: an accurate evaluation of symptomatic acalculous gallbladder disease. Int Surg. 2003;88:95-99.
2. Behar J, Corazziari E, Guelrud M, et al. Functional gallbladder and sphincter of Oddi disorders. Gastroenterology. 2006;130:1498-1509.
3. Goldblatt MI, Swartz-Basile DA, Al-Azzawi HH, et al. Nonalcoholic fatty gallbladder disease: the Influence of diet in lean and obese mice. J Gastrointest Surg. 2006;10:193-201.
4. Chung-Jyi. Steatocholecystitis and fatty gallbladder disease. Dig Dis Sci. 2009;54:1857-1863.
5. Bastard JP, Maachi M, Lagathu C, et al. Recent advances in the relationship between obesity, inflammation, and insulin resistance. Eur Cytokine Netw. 2006;17:4-12.
6. Pitt HA. Hepato-pancreato-biliary fat: the good, the bad and the ugly. HPB (Oxford). 2007;9:92-97.
7. Merg AR, Kalinowski SE, Hinkhouse MM, et al. Mechanisms of impaired gallbladder contractile response in chronic acalculous cholecystitis. J Gastrointest Surg. 2002;6:432-437.
8. Amaral J, Xiao ZL, Chen Q, et al. Gallbladder muscle dysfunction in patients with chronic acalculous disease. Gastroenterology. 2001;120:506-511.
9. Portincasa P, Ciaula AD, Baldassarre G, et al. Gallbladder motor function in gallstone patients: sonographic and in vitro studies on the role of gallstones, smooth muscle function, and gallbladder wall inflammation. J Hepatol. 1994;21:430-440.
10. Al-Azzawi HH, Nakeeb A, Saxena R, et al. Cholecystosteatosis: an explanation for increased cholecystectomy rates. J Gastrointest Surg. 2007;11:835-843.
11. Carr JA, Walls J, Bryan LJ, et al. The treatment of gallbladder dyskinesia based upon symptoms: results of a 2-year, prospective, nonrandomized, concurrent cohort study. Surg Laparosc Endosc Percutan Tech. 2009;19:222-226.
12. Singhal V, Szeto P, Norman H, et al. Biliary dyskinesia: how effective is cholecystectomy? J Gastrointest Surg. 2012;16:135-141.
13. Francis G, Baillie J. Gallbladder dyskinesia: fact or fiction? Curr Gastroenterol Rep. 2011;13:188-192.
14. Ziessman HA. Nuclear medicine hepatobiliary imaging. Clin Gastroenterol Hepatol. 2010;8:111-116.
15. Delgado-Aros S, Cremonini R, Bredenoord AJ, et al. Systemic review and meta-analysis: does gallbladder ejection fraction on cholecystokinin cholescintigraphy predict outcome after cholecystectomy in suspected functional biliary pain? Aliment Pharmacol Ther. 2003;18:167-174.
16. Patel PA, Lamb JJ, Hogle NJ, et al. Therapeutic efficacy of laparoscopic cholecystectomy in the treatment of biliary dyskinesia. Am J Surg. 2004;187:209-212.
17. Mahid SS, Jafri NS, Brangers BC, et al. Meta-analysis of cholecystectomy in symptomatic patients with positive hepatoiminodiacetic acid scan results without gallstones. Arch Surg. 2009;144:180-187.
18. Hansel SL, DiBaise JK. Functional gallbladder disorder: gallbladder dyskinesia. Gastroenterol Clin North Am. 2010;39:369-379.
19. Canfield AJ, Hetz SP, Shriver JP, et al. Biliary dyskinesia: a study of more than 200 patients and review of the literature. J Gastrointest Surg. 1998;2:443-448.
20. Jagannath SB, Singh VK, Cruz-Correa M, et al. A long-term cohort study of outcome after cholecystectomy for chronic acalculous cholecystitis. Am J Surg. 2003;185:91-95.
The refugee medical exam: What you need to do
• Use tuberculin skin testing alone or in conjunction with interferon-gamma release assay to screen children younger than 5 years for tuberculosis. A
• Include 2 evaluations for ova and parasites plus a complete blood count with differential when screening refugees for parasitic infections. B
• Screen all adolescent and adult refugees for human immunodeficiency virus infection. A
• Check blood lead levels in all children 6 months to 16 years of age on arrival in the United States B and 6 months later. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
In 2011, 56,384 refugees fleeing persecution in their native countries were admitted to the United States. The largest numbers came from Burma (30.1%), Bhutan (26.6%), and Iraq (16.7%).1 They joined the more than 3 million refugees from all over the world who have resettled in this country since 1975. 1
Refugees arrive in the United States with complex medical issues, including illnesses rarely seen here, mental health concerns, and chronic conditions such as diabetes and hypertension. After arrival, they undergo a domestic refugee medical examination (DRME). This DRME, along with well-planned follow-up, can go a long way toward helping refugees show the proof of vaccination and control of chronic health conditions that are required when they apply for lawful permanent resident status.
The Centers for Disease Control and Prevention (CDC) has published guidelines to help with medical decision making and screening of refugees, but limited information is available on the necessary strategies to address chronic health conditions within the context of the DRME.2 Moreover, differences in refugee experience and health status based on country of origin may demand more detailed, region-specific guidelines.3-9 No standard recommendations address the importance of providing not just initial screening, but comprehensive longitudinal care, as well.
Since 2007, our outpatient practice (MA, KS, GM, PM) has performed the DRME and provided ongoing care for more than 900 refugees resettled in Philadelphia. The practice, which is associated with an urban academic medical center and closely coordinates refugee care with a local resettlement agency, has earned recognition as a Level 3 (top-level certification) patient-centered medical home by the National Committee on Quality Assurance. We offer here a framework for providing comprehensive care to refugees, based on CDC guidelines, available evidence, and our experience.
Prelude: The overseas medical exam
All refugees must undergo an overseas medical examination (OME) no longer than 12 months before resettlement in the United States. Physicians selected by US Department of State consular officials perform the examinations.
The OME includes a medical history, physical examination, and testing to screen for mental illness, drug abuse, syphilis, leprosy, and tuberculosis (TB). Some vaccinations and empiric treatment for parasites also may be provided at the time of the examination.10-12
The OME screens for Class A disorders, which render a refugee ineligible for admission to the United States until treated or stabilized, and Class B conditions, which require close follow-up on arrival (TABLE 1).12 Despite recent steps toward standardization, the quality and thoroughness of OMEs completed at different examination sites still vary substantially.
TABLE 1
Overseas medical examination: Class A and B conditions12
| Class A* | Class B† |
|---|---|
| Active or infectious tuberculosis Untreated STI: syphilis, gonorrhea, chancroid, granuloma inguinale, or lymphogranuloma venereum Hansen’s disease (leprosy) Drug or alcohol addiction/abuse Mental illness with harmful behavior | Inactive or noninfectious tuberculosis Treated STI Treated or paucibacillary Hansen’s disease Sustained remission from drug or alcohol addiction or abuse Well-controlled mental illness Pregnancy |
| STI, sexually transmitted infection. *Class A disorders render a refugee ineligible for admission to the United States until he or she is treated or stabilized. †Class B disorders require close follow-up upon the refugee’s arrival in the United States | |
Arrival in United States is followed by DRME
When refugees arrive in the United States, they are advised to undergo a DRME, which any licensed practitioner may perform, preferably within 90 days. More rapid evaluation is encouraged for medically complex refugees or refugees arriving with Class A or B conditions. Because refugees are eligible for only 8 months of medical assistance, we strongly recommend that the DRME be done promptly.
The CDC publishes guidelines for components of the initial DRME, but state requirements and individual examinations vary widely.2,10,13,14 We outline here the elements of the exam identified by the CDC, supplemented with recommendations based on published evidence and our experiences in caring for refugees.
Screen for tuberculosis
Refugees have a higher prevalence of latent tuberculosis infection (LTBI) and active TB than the general US population. An estimated one-third of the world’s population has LTBI.15 Since 2002, more than 50% of all people diagnosed with TB in the United States have been born outside the country.16
Although otherwise healthy adults with LTBI have a lifetime risk of approximately 10% that it will progress to active TB,17 infants, young children, and people coinfected with HIV have a rate of progression of around 10% per year. It is imperative, therefore, that all refugees be screened for TB and treated appropriately.8,18,19
Refugees are screened for active TB with a chest radiograph and possibly a sputum analysis during the OME. Because screening may take place as long as 12 months before arrival in the United States, refugees may be re-exposed to TB in the refugee camp before departure. They are not screened for LTBI before coming to the United States.11,12
Domestic screening for LTBI is complicated by routine use in many foreign countries of the Bacille Calmette-Guérin (BCG) vaccine, which can reduce the incidence of TB meningitis and disseminated TB in children, but does not protect adults against primary infection or reactivation of TB. Tuberculin skin testing using purified protein derivative, which has typically been used for screening, can render false-positive results, particularly in the context of previous BCG vaccination.
Interferon-gamma release assay (IGRA) is an alternative screening option that has been approved for use in the United States.15,20 Because the IGRA is a blood test, it eliminates interpretation errors associated with tuberculin skin testing and is not affected by BCG vaccination. IGRA testing also does not require an additional office visit.
For these reasons, we recommend screening all refugees older than 5 years with IGRAs, where available. In light of scant data and apparent differences in immune response in young children, the CDC recommends using tuberculin skin testing either alone or in conjunction with IGRA testing for all children younger than 5 years.20,21
Positive screening tests must be followed up with a chest radiograph. Perform serial sputum evaluation whenever the chest radiograph indicates potential active TB.
Everyone with latent or active TB must be treated according to CDC recommendations adapted from guidelines established by the American Thoracic Society and Infectious Diseases Society of America.22,23 For latent TB, the CDC calls for treatment with isoniazid for 9 months or rifampin for 4 months.
- Patients older than 18 years should receive the adult dose of isoniazid: 5 mg/kg per day orally to a maximum daily dose of 300 mg. Children should receive 10 to 20 mg/kg per day orally to a maximum daily dose of 300 mg. Twice weekly therapy schedules are also available and commonly used for children who receive directly observed treatment in school.
- The adult dosage of rifampin (for patients >15 years) is 10 mg/kg per day orally to a maximum daily dose of 600 mg; the pediatric dose is 10 to 20 mg/kg per day orally, also to a maximum daily dose of 600 mg.
Patients taking isoniazid who are pregnant or breastfeeding or have diabetes, renal failure, alcoholism, malnutrition, HIV, or a seizure disorder should receive pyridoxine (vitamin B6) supplementation to aid in preventing peripheral neuropathy, in an adult oral dose of 25 to 50 mg/d or a pediatric oral dose of 6.25 mg/d. Additional information on treating latent TB is available at http://www.cdc.gov/tb/topic/treatment/ltbi.htm.
For patients with active TB, treatment is more complex, based on the patient’s overall health. Please refer to the CDC recommendation for the treatment of active TB (http://www.cdc.gov/tb/topic/treatment/tbdisease.htm) or contact your local TB control division.
Patients may receive TB treatment from either individual medical providers or city or state health departments, depending on local capacity. In our practice, we treat LTBI in adults. The Philadelphia Department of Public Health’s TB Control Program manages LTBI in children and all suspected cases of active TB. We recommend providing everyone treated for latent or active TB with documentation of treatment completion.
Diagnose and treat problematic parasites
Intestinal parasites are among the infections most often found in refugee populations.7,8,24-29 Common pathogens in untreated refugees are Ascaris lumbricoides, hookworm (Ancylostoma duodenale and Necator americanus), Schistosoma species, Strongyloides stercoralis, Trichuris trichiura, and Giardia lamblia.
Although sustained domestic transmission is unlikely, these parasites may cause growth delay, anemia, hyperinfestation syndrome and disseminated infection (A lumbricoides and S stercoralis), and increased cancer risk (Schistosoma hematobium).7 In the late 1990s, the CDC initiated empiric treatment before departure for the United States for A lumbricoides (albendazole), S stercoralis (ivermectin), Schistosoma species (praziquantel), and other parasites in certain refugee populations, which has decreased but not eliminated the threat.7
All refugees should be receiving appropriate predeparture treatment for parasitic infections. For newly arrived refugees who have received no predeparture therapy or incomplete therapy, the CDC recommends screening for parasites or providing presumptive treatment (TABLE 2).
TABLE 2
Empiric treatment of parasites
| Refugee region of origin | Organism | Adult therapy |
|---|---|---|
| Middle East, South Asia, Southeast Asia | Strongyloides stercoralis Other roundworms | Ivermectin 200 μg/kg/d orally for 2 days Albendazole 400 mg orally, 1 dose |
| Africa | Schistosoma species S stercoralis Other roundworms | Praziquantel 20 mg/kg orally, 2 doses Ivermectin 200 μg/kg/d orally for 2 days Albendazole 400 mg orally, 1 dose |
| Source: CDC. Immigrant and Refugee Health: Domestic Intestinal Parasite Guidelines. Available at: http://www.cdc.gov/immigrantrefugeehealth/guidelines/domestic/intestinal-parasites-domestic.html. Accessed November 19, 2012. | ||
The optimal screening regimen for parasites in refugee populations is controversial. Although most screening programs rely on one or more microscopic examinations of stool for ova and parasites, this test is expensive, requires special handling, depends on the reviewer’s expertise, and remains relatively insensitive. A comprehensive review of stool ova and parasites in high-risk populations concluded that the use of 2 independently collected stool samples improved sensitivity at acceptable cost.30
New, more sensitive and specific assays have been developed for many parasites, including Cryptosporidium parvum, Entamoeba histolytica, G lamblia, S stercoralis, and Schistosoma species, but we do not recommend these specialized tests unless the provider strongly suspects a specific parasite based on history and physical exam or persistent eosinophilia.
All refugees should have a complete blood count with differential to help identify occult parasitemia. Although a finding of eosinophilia may result from successful empiric therapy for an already-treated parasite, it must be followed up with more specific testing for S stercoralis, even in otherwise asymptomatic patients. African refugees with eosinophilia also should be tested for Schistosoma, and Somali Bantu should be treated empirically for both S stercoralis and Schistosoma.31 In line with CDC guidelines, ongoing failure to identify the cause of eosinophilia in a refugee should prompt referral to an infectious disease specialist and further work-up.
Three to 6 months after antibiotic treatment of any parasite, immunocompromised patients and those with suspected treatment failure should undergo a test of cure comprised of 2 stool ova and parasite studies and a follow-up CBC with differential.32
Screen for HIV
Since January 4, 2010, after HIV was removed from the Class A diagnosis list, refugees are no longer tested for HIV before arrival in the United States.11 Nevertheless, we recommend screening all refugees on arrival, regardless of age, for HIV types 1 and 2, unless they opt out, for the following reasons:
- approximately 14% of incoming refugees arrive from countries with an HIV prevalence of more than 5%33
- the increasing use of rape as a tool of torture and repression puts refugees at particular risk for HIV
- current CDC guidelines recommend HIV screening at the time of first encounter in all health care settings for everyone from 13 to 64 years of age and any patient who requests it.34
We also strongly recommend repeat screening 3 to 6 months after resettlement for refugees with recent potential exposure or who engage in high-risk activity.
Watch for ubiquitous hepatitis infection
In accordance with CDC vaccination guidelines and American Association of Pediatrics (AAP) Bright Futures recommendations, we endorse hepatitis A serology testing with reflex vaccination unless immunity is documented for refugees 1 to 18 years of age.35,36
A third of the world’s population shows serologic evidence of past infection with hepatitis B virus (HBV); high rates occur in Southeast Asia and sub-Saharan Africa, where most infections are transmitted perinatally.37,38 A study of Minnesota refugees found 7% to be positive for hepatitis B surface antigen (HBsAg), with a higher prevalence among refugees from sub-Saharan Africa.8
Most screening protocols for refugees test for HBsAg and antibody to hepatitis B surface antigen (HBsAb); it is reasonable to add a screen for antibody to hepatitis B core antigen (HBcAb). We recommend screening for HBV infection using HBsAg, HBsAb, and HBcAb to minimize underdiagnosis in this high-risk population. Refugees without immunity to HBV should be offered vaccination.18 Encourage immunization, especially for patients with hepatitis or cirrhosis from any cause.
Hepatitis C screening should follow CDC guidelines for the general population, focusing on high-risk groups such as injection drug users, victims of sexual violence, people with multiple sexual partners, recipients of blood transfusions, people with any other type of hepatitis, and one-time screening for individuals born between 1945 and 1965.39,40
Monitor for malaria
Many refugees come to the United States from areas where malaria is endemic.41 In 2007, the CDC instituted empiric treatment before arrival in the United States for all refugees from sub-Saharan Africa because the rapid test for malaria approved by the US Food and Drug Administration has low sensitivity and specificity,2 malarial vectors are present throughout much of the United States, and malaria (specifically Plasmodium falciparum) causes significant morbidity and mortality. If written confirmation of predeparture treatment is not available, refugees from sub-Saharan Africa should receive presumptive treatment, outlined in TABLE 3,42 as part of the initial DRME.
TABLE 3
Presumptive postarrival malaria treatment for refugees from sub-Saharan Africa42
| Directly observed treatment received in country of origin? | Recommended treatment* | |
|---|---|---|
| Children | Adults | |
| Yes | None | None |
| No | Atovaquone-proguanil (62.5/25 mg): 5-8 kg: 2 tablets per day for 3 days 9-10 kg: 3 tablets per day for 3 days Atovaquone-proguanil (250/100 mg): 11-20 kg: 1 tablet per day for 3 days 21-30 kg: 2 tablets per day for 3 days 31-40 kg: 3 tablets per day for 3 days >40 kg: 4 tablets per day for 3 days | Atovaquone-proguanil (250/100 mg): 4 tablets per day for 3 days |
| *Do not presumptively treat pregnant or lactating women or children weighing <5 kg. An infectious disease consult is recommended for these patients. | ||
Based on our experience and expert opinion, we recommend routinely monitoring all refugees from endemic areas for symptoms of malarial disease during the initial 3 months after resettlement. Relapsing fevers, unexplained malaise or fatigue, pallor, thrombocytopenia, or splenomegaly should trigger additional testing with thick- and thin-blood smears for trophozoites (3 separate samples drawn at 12- to 24-hour intervals).
Be alert for malnutrition
Acute and chronic malnutrition, as well as micronutrient deficiencies, have been noted in refugees coming from refugee camps. A survey of Bhutanese refugees in a camp in Nepal found that 25.1% of children were underweight and 4.8% of them were severely underweight. Moreover, 43.3% of children had anemia.43 Recognizing that refugees may be at high risk for iron deficiency, we recommend evaluating children and adolescents for this deficit according to AAP guidelines.44
We also recommend screening body mass index (BMI) to identify refugees at risk. Height, weight, and BMI must be followed over time to ensure appropriate acclimation to the US diet.
Also consider vitamin D deficiency and rickets in refugee populations, particularly people with darker skin and women who wear veils.45,46 Based on our experiences and CDC guidelines, we recommend a multivitamin with iron for children 6 to 59 months of age.12
Check lead levels in children
Refugee children are at risk of elevated blood lead levels (>10 ’g/dL) resulting from pre-departure environmental exposure and iron deficiency anemia, which can enhance absorption of lead. Refugees also are more likely to resettle in poor neighborhoods with substandard housing, increasing their risk of domestic lead exposure.
Studies of refugee children at initial screening have shown prevalences of elevated blood lead levels of 6.3% in a Cuban refugee population in Miami and higher rates (11%-22%) in mixed refugee populations in Massachusetts.6,47 A study in New Hampshire found that approximately 30% of refugee children with normal lead levels on initial screen had elevated levels when checked several months later.48
Consistent with CDC guidelines,49 our experience, and the findings of the State of Minnesota,50 we recommend checking blood lead levels in all children 6 months to 16 years of age upon arrival in the United States and repeat lead testing 3 to 6 months after placement in a permanent residence.
Bring vaccinations up to date
US law requires anyone seeking an immigrant visa to show proof of vaccination against vaccine-preventable diseases, as recommended by the US Advisory Committee on Immunization Practices.51 Vaccination requirements that apply to other immigrant groups do not apply to refugees at the time of their initial admission to the United States, but refugees must be vaccinated when they seek a green card or permanent US residence.
All refugees are eligible for adjustment of status after they have lived in the United States for a year and need proof of vaccination to apply.51 Moreover, schools may bar refugee children from attending if their vaccinations are not up-to-date, which, in turn, may hinder their parents’ ability to find employment. CDC guidelines for vaccinating immigrants and refugees applying for permanent residence are available at http://www.cdc.gov/immigrantrefugeehealth/pdf/2009-vaccination-technical-instructions.pdf (see the table on page 12).52 Because of the large number of vaccinations required for children and even many adults, health care providers should be familiar with the CDC’s recommended immunization and catch-up schedules.35
Vaccinations given in other countries are acceptable if appropriately recorded in Institute of Medicine documentation, or if original vaccination records are available and the vaccinations conform to appropriate intervals and age guidelines. Refugees must bring their records with them to medical appointments. Laboratory evidence of immunity is acceptable for measles, mumps, rubella (MMR), hepatitis A, hepatitis B, polio, and varicella, but there is debate about whether such testing should be performed before immunization.18,53 Health care providers need to assess each patient based on age and risk factors to decide whether immunity testing is appropriate.
In our practice, we routinely test all adults for immunity to varicella, hepatitis A, hepatitis B, and MMR. For children, we rely on documented immunization records, not antibody titers, for evidence of previous vaccination.
Pay attention to mental health issues
Many refugees have been exposed to trauma, often including war and torture, increasing their risk for mental illness. A large 2005 review found that serious mental disorders, including post-traumatic stress disorder (PTSD), major depressive disorder, and generalized anxiety disorder are significantly more prevalent among refugees than the general population.5 Many screening tests for PTSD have been proposed54 but have not been validated in all immigrant or refugee populations.55
Mental health care for refugees is complicated by language and cultural barriers, adjustment disorders, access to psychiatric services, and uncertainty about effective treatments in refugee populations. Despite the higher prevalence of mental illness among refugees, many in the mental health field have raised concerns about the applicability of Western concepts of mental health, including PTSD, in this group.56
Refugees who are victims of torture should be referred to experienced mental health practitioners. After ruling out acute psychosis and destructive behaviors, we recommend postponing an exhaustive mental health screening until several months after arrival. In our medical home model, we evaluate patients on an ongoing basis, giving us an opportunity to identify emerging or worsening mental health conditions.
Evaluate dental health
The incidence of dental caries and periodontal disease among refugees varies widely among different groups of refugees. Data on pediatric refugees in the United States have shown dental caries to be common, with prevalences between 16.7% and 42%, with marked differences based on region of origin.3,57,58 In our practice, we also have noted heavy use of betel nut in the Southeast Asian community, leading to significant dental disease.
All refugees should have their dentition evaluated at the initial DRME. We recommend subsequent formal dental examination for all patients, giving priority to those with clear evidence of active disease.
Identify and address chronic disease
Refugees carry a substantial burden of chronic disease, although marked regional variation has been noted.4 A study of Massachusetts refugees from 2001 through 2005 demonstrated that 46.8% were overweight or obese, 22.6% had hypertension, and 3.1% had diabetes. Smoking is also highly prevalent in refugee populations.59
Our findings confirm high rates of chronic disease, particularly among Iraqi and geriatric refugees. These patients require close follow-up after the DRME to minimize sequelae from chronic conditions. Multi-disciplinary teams in the patient-centered medical home may provide an opportunity to promptly address chronic health conditions that can have severe short-term consequences if not adequately managed (eg, insulin dosage adjustment based on diet in patients with diabetes).
We recommend a comprehensive medical history and evaluation for chronic disease, including diabetes and hypertension, at the DRME and on an ongoing basis. Although many refugees have never had any health screening and substantial cultural barriers may exist, especially with regard to women’s health and age-based cancer screening, refugees generally should receive the same preventive care as the rest of the US population until further research has been done in this area.
We recommend introducing age-based cancer screening and other preventive care for refugees within 2 months of their initial visit. This model of care has already been endorsed by the Minnesota Department of Health’s Refugee Health Program, one of the leading health care providers for refugees in the United States.60
Toward better care models
The medical care of refugees is complex, but the prepared primary care provider can manage it effectively. TABLE 4 summarizes our recommendations for the DRME based on our experiences and the available literature. Standardized screening guidelines and comprehensive programs, perhaps incorporating the concept of the patient-centered medical home, will likely improve both the initial and continuing care of this population.
TABLE 4
Summary recommendations for the domestic refugee medical exam
History
|
| Physical exam In addition to the essential components of the physical exam, pay attention to:
|
Initial laboratory evaluation
|
| Ongoing care Include:
|
| CBC, complete blood count; HIV, human immunode"ciency virus; IGRA, interferon-gamma release assay; MMR, measles, mumps, rubella; TST, tuberculin skin testing. Adapted from: Centers for Disease Control and Prevention. Immigrant and Refugee Health: Guidelines for the US Domestic Medical Examination for Newly Arriving Refugees. Available at: http://www.cdc.gov/immigrantrefugeehealth/guidelines/domestic/domestic-guidelines.html. Accessed November 19, 2012. |
Ongoing study is essential to better address the health care needs of refugees. Although they comprise only a small segment of immigrants living in the United States, the experience of caring for them may help develop models to provide better care to other foreign-born patients.
CORRESPONDENCE Marc Altshuler, MD, Department of Family and Community Medicine, Jefferson Medical College, Thomas Jefferson University, 833 Chestnut Street, Suite 301, Philadelphia, PA 19107; marc.altshuler@jefferson.edu
1. Martin D, Yankay J. Refugees and Asylees: 2011. Annual Flow Report. Offce of Immigration Statistics, US Department of Home-land Security. May 2012. Available at: http://www.dhs.gov/xlibrary/assets/statistics/publications/ois_rfa_fr_2011.pdf. Accessed October 29, 2012.
2. Stauffer WM, Kamat D, Walker PF. Screening of international immigrants, refugees and adoptees. Prim Care. 2002;29:879-905.
3. Cote S, Geltman P, Nunn M, et al. Dental caries of refugee children compared with US children. Pediatrics. 2004;114:e733-e740.
4. Geltman PL, Dookeran NM, Battaglia T, et al. Chronic disease and its risk factors among refugees and asylees in Massachusetts, 2001-2005. Prev Chronic Dis. 2010;7:A51.-
5. Fazel M, Wheeler J, Danesh J. Prevalence of serious mental disorder in 7000 refugees resettled in western countries: a systematic review. Lancet. 2005;365:1309-1314.
6. Geltman PL, Brown MJ, Cochran J. Lead poisoning among refugee children resettled in Massachusetts, 1995 to 1999. Pediatrics. 2001;108:158-162.
7. Geltman PL, Cochran J, Hedgecock C. Intestinal parasites among African refugees resettled in Massachusetts and the impact of an overseas pre-departure treatment program. Am J Trop Med Hyg. 2003;69:657.-
8. Lifson AR, Thai D, O’Fallon A, et al. Prevalence of tuberculosis, hepatitis B virus, and intestinal parasitic infections among refugees to Minnesota. Public Health Rep. 2002;117:69-77.
9. Power DV, Moody E, Trussell K, et al. Caring for the Karen. A newly arrived refugee group. Minn Med. 2010;93:49-53.
10. Centers for Disease Control and Prevention. Health considerations of newly arrived immigrants and refugees. In: Centers for Disease Control and Prevention. Travelers’ Health—Yellow Book. Chapt. 9. Available at: http://wwwnc.cdc.gov/travel/yellow-book/2010/table-of-contents.aspx#20. Accessed November 15, 2012.
11. Centers for Disease Control and Prevention. Medical Examination of Immigrants and Refugees. Available at: http://www.cdc.gov/immigrantrefugeehealth/exams/medical-examination.html. Accessed November 15, 2012.
12. Centers for Disease Control and Prevention. Technical Instructions For The Medical History and Physical Examination of Aliens in the United States. Available at: http://www.cdc.gov/immigrantrefugeehealth/exams/ti/civil/technical-instructions/civil-surgeons/medical-history-physical-examination.html. Accessed November 15, 2010.
13. Seybolt L, Barnett E, Stauffer W. US medical screening for immigrants and refugees: clinical issues. In: Walker P, Barnett E, eds. Immigrant Medicine. Phildelphia, PA: Saunders Elsevier; 2007:135-150.
14. United States Department of Health and Human Services, Offce of Refugee Resettlement. ORR State Letter: Revised Medical Screening Guidelines for Newly Arrived Refugees. Available at: http://www.acf.hhs.gov/sites/default/files/orr/state_letter_12_09_revised_medical_screening_guidelines_for_newly.pdf. Accessed October 29, 2012.
15. Centers for Disease Control and Prevention. Guidelines for the investigation of contacts of persons with infectious tuberculosis— guidelines for using the QuantiFERON-TB gold test for detecting Mycobacterium tuberculosis infection, United States. MMWR Recomm Rep. 2005;54(RR-15):1-55.
16. Centers for Disease Control and Prevention. Executive Commentary: Highlights of 2011 Report. Available at: http://www.cdc.gov/tb/statistics/reports/2011/pdf/ExecutiveCommentary.pdf. Accessed November 19, 2012.
17. Kuma V, Abbas AK, Fausto N, et al. Robbins Basic Pathology. 8th ed. Philadelphia, Pa: Saunders Elsevier; 2007:516–522.
18. Barnett ED. Infectious disease screening for refugees resettled in the United States. Clin Infect Dis. 2004;39:833-841.
19. DeRiemer K, Chin DP, Schecter DF, et al. Tuberculosis among immigrants and refugees. Arch Intern Med. 1998;158:753-760.
20. Centers for Disease Control and Prevention. Updated guidelines for using interferon gamma release assays to detect Mycobacterium tuberculosis infection—United States, 2010. MMWR Recomm Rec. 2010;59(RR-5):1-25.
21. Bright Futures at Georgetown University. Health Supervision— Laboratory Tests: Tuberculosis (TB) Screening. Available at: http://www.brightfutures.org/pocket/pdf/30_37.pdf. Accessed November 19, 2012.
22. Centers for Disease Control and Prevention. Treatment of tuberculosis. American Thoracic Society, CDC, Infectious Diseases Society of America. MMWR Recomm Rep 2003;52(RR-11):1-77.
23. Centers for Disease Control and Prevention. Update: adverse event data and revised American Thoracic Society/CDC recommendations against the use of rifampin and pyrazinamide for treament of latent tuberculosis infection—United States. MMWR Morb Mortal Wkly Rep. 2003;52:735-739.
24. Caruana SR, Kelly HA, Ngeow JY, et al. Undiagnosed and potentially lethal parasite infections among immigrants and refugees in Australia. J Travel Med. 2006;13:233-239.
25. Dawson-Hahn EE, Greenberg SL, Domachowske JB, et al. Eosinophilia and the seroprevalence of schistosomiasis and strongyloidiasis in newly arrived pediatric refugees: an examination of Centers for Disease Control and Prevention screening guidelines. J Pediatr. 2010;156:1016-1018.
26. Garg PK, Perry S, Dorn M, et al. Risk of intestinal helminth and protozoan infection in a refugee population. Am J Trop Med Hyg. 2005;73:386-391.
27. Parenti DM, Lucas D, Lee A, et al. Health status of Ethiopian refugees in the United States. Am J Public Health. 1987;77:1542-1543.
28. Parish R. Intestinal parasites in Southeast Asian refugee children. West J Med. 1985;143:47-49.
29. Sutherland JE, Avant RF, Franz WB, 3rd, et al. Indochinese refugee health assessment and treatment. J Fam Pract. 1983;16:61-67.
30. Cartwright C. Utility of multiple-stool-specimen ova and parasite examinations in a high-prevalence setting. J Clin Microbiol. 1999;37:2408-2411.
31. Centers for Disease Control and Prevention. Recommendations for Presumptive Treatment of Schistosomiasis and Strongyloidiasis Among the Somali Bantu Refugees. June 13, 2005. Available at: http://archive.acf.hhs.gov/programs/orr/policy/sl05-18attach-ment2.pdf. Accessed November 19, 2012.
32. Centers for Disease Control and Prevention. Division of Global Migration and Quarantine. Guidelines for Evaluation of Refugees for Intestinal and Tissue-Invasive Parasitic Infections during Domestic Medical Examination. Available at: http://www.cdc.gov/immigrantrefugeehealth/guidelines/domestic/intestinal-parasites-domestic.html. Accessed October 30, 2012.
33. Centers for Disease Control and Prevention. Screening for HIV Infection During the Refugee Domestic Medical Examination. Available at: http://www.cdc.gov/immigrantrefugeehealth/guidelines/domestic/screening-hiv-infection-domestic.html. Accessed November 15, 2012.
34. Centers for Disease Control and Prevention. Revised recommendations for HIV testing of adults, adolescents and pregnant women in healthcare settings. MMWR Recomm Rep. 2006;55(RR-14):1-17.
35. Centers for Disease Control and Prevention National Immunization Program. Available at: http://www.cdc.gov/vaccines/vpdvac/hepa/default.htm. Accessed November 19, 2012.
36. American Academy of Pediatrics. Red Book. Available at: http://www2.aap.org/immunization/illnesses/hepb/hepa.html. Accessed November 19, 2012.
37. Lai CL, Ratziu V, Yuen MF, et al. Viral hepatitis B. Lancet. 2003;362:2089-2094.
38. Lin K, Kirchner J. Hepatitis B. Am Fam Phyician. 2004;69:75-82.
39. Ghany MG, Strader DB, Thomas DL, et al. American Association for the Study of Liver Diseases. Diagnosis, management, and treatment of hepatitis C: an update. Hepatology. 2009;49:1335-1374.
40. Centers for Disease Control and Prevention. Recommendations for the identification of chronic hepatitis C virus infection among persons born during 1945-1965. MMWR Morb Mortal Wkly Rep. 2012;61(RR-4):1-31.
41. Rowland M, Nosten F. Malaria epidemiology and control in refugee camps and complex emergencies. Ann Trop Med Parasitol. 2001;95:741-754.
42. Centers for Disease Control and Prevention, Malaria Branch, Division of Parasitic Diseases, Division of Global Migration and Quarantine and Malaria Branch. Presumptive Treatment of P falciparum Malaria in Refugees Relocating from sub-Saharan Africa to the United States. Available at: http://www.cdc.gov/immigrantrefugeehealth/guidelines/domestic/malaria-guidelines-domestic.html. Accessed November 15, 2012.
43. Centers for Disease Control and Prevention. Malnutrition and micronutrient deficiencies among Bhutanese refugee children— Nepal, 2007. MMWR Morb Mortal Wkly Rep. 2008;57:370-373.
44. American Academy of Pediatrics Bright Futures. Guidelines for Health Supervision of Infants, Children, and Adolescents— Theme 5: Promoting Healthy Nutrition. Available at: http://brightfutures.aap.org/pdfs/Guidelines_PDF/6-Promoting_Healthy_Nutrition.pdf. Accessed November 15, 2012.
45. Benson J, Skull S. Hiding from the sun: vitamin D deficiency in refugees. Aust Fam Physician. 2007;36:355-357.
46. Stellinga-Boelen A. Vitamin D levels in children of asylum seekers in The Netherlands in relation to season and dietary intake. Eur J Pediatr. 2007;166:201-206.
47. Trepka MJ, Pekovic V, Santana JC, et al. Risk factors for lead poisoning among Cuban refugee children. Public Health Rep. 2005;120:179-185.
48. Centers for Disease Control and Prevention. Elevated blood lead levels in refugee children, New Hampshire, 2003-2004. MMWR Morb Mortal Wkly Rep. 2005;54:42-46.
49. Centers for Disease Control and Prevention. Screening for Lead at the Domestic Refugee Medical Exam. Available at: http://www.cdc.gov/immigrantrefugeehealth/pdf/lead.pdf. Accessed November 15, 2012.
50. Zabel EW, Smith ME, O’Fallon A. Implementation of CDC refugee blood testing guidelines in Minnesota. Public Health Rep. 2008;123:111-125.
51. United States Citizenship and Immigration Services. Available at: http://www.uscis.gov/portal/site/uscis/menuitem.5af9bb95919f35e66f614176543f6d1a/?vgnextoid=3384cc5222$5210VgnVCM100000082ca60aRCRD&vgnextchannel=6abe6d26d17df110VgnVCM1000004718190aRCRD. Accessed November 15, 2012.
52. Centers for Disease Control and Prevention. Vaccination Requirements for Adjustment of Status for US Permanent Residence: Technical Instructions for Civil Surgeons. December 14, 2009. Available at: http://www.cdc.gov/immigrantrefugeehealth/pdf/2009-vaccination-technical-instructions.pdf. Accessed November 15, 2012
53. Phillips C. Better primary healthcare for refugees: catch up immunisation. Aust Fam Physician. 2007;36:440-443.
54. Brewin C. Systematic review of screening instruments for adults at risk of PTSD. J Trauma Stress. 2005;18:53-62.
55. Crumlish N, O’Rourke K. A systematic review of treatments for post-traumatic stress disorder among refugees and asylum-seekers. J Nerv Ment Dis. 2010;198:237-251.
56. Watters C. Emerging paradigms in the mental health care of refugees. Soc Sci Med. 2001;53:1709-1718.
57. Hayes EB, Talbot SB, Matheson ES, et al. Health status of pediatric refugees in Portland, ME. Arch Pediatr Adolesc Med. 1998;152:564-568.
58. Meropol S. Health status of pediatric refugees in Buffalo, NY. Arch Pediatr Adolesc Med. 1995;149:887-892.
59. Barnes DM, Harrison C, Heneghan R. Health risk and promotion behaviors in refugee populations. J Health Care Poor Underserved. 2004;15:347-356.
60. Dicker S, Stauffer WM, Mamo B, et al. Initial refugee health assessments: new recommendations for Minnesota. Minn Med. 2010;93:45-48.
• Use tuberculin skin testing alone or in conjunction with interferon-gamma release assay to screen children younger than 5 years for tuberculosis. A
• Include 2 evaluations for ova and parasites plus a complete blood count with differential when screening refugees for parasitic infections. B
• Screen all adolescent and adult refugees for human immunodeficiency virus infection. A
• Check blood lead levels in all children 6 months to 16 years of age on arrival in the United States B and 6 months later. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
In 2011, 56,384 refugees fleeing persecution in their native countries were admitted to the United States. The largest numbers came from Burma (30.1%), Bhutan (26.6%), and Iraq (16.7%).1 They joined the more than 3 million refugees from all over the world who have resettled in this country since 1975. 1
Refugees arrive in the United States with complex medical issues, including illnesses rarely seen here, mental health concerns, and chronic conditions such as diabetes and hypertension. After arrival, they undergo a domestic refugee medical examination (DRME). This DRME, along with well-planned follow-up, can go a long way toward helping refugees show the proof of vaccination and control of chronic health conditions that are required when they apply for lawful permanent resident status.
The Centers for Disease Control and Prevention (CDC) has published guidelines to help with medical decision making and screening of refugees, but limited information is available on the necessary strategies to address chronic health conditions within the context of the DRME.2 Moreover, differences in refugee experience and health status based on country of origin may demand more detailed, region-specific guidelines.3-9 No standard recommendations address the importance of providing not just initial screening, but comprehensive longitudinal care, as well.
Since 2007, our outpatient practice (MA, KS, GM, PM) has performed the DRME and provided ongoing care for more than 900 refugees resettled in Philadelphia. The practice, which is associated with an urban academic medical center and closely coordinates refugee care with a local resettlement agency, has earned recognition as a Level 3 (top-level certification) patient-centered medical home by the National Committee on Quality Assurance. We offer here a framework for providing comprehensive care to refugees, based on CDC guidelines, available evidence, and our experience.
Prelude: The overseas medical exam
All refugees must undergo an overseas medical examination (OME) no longer than 12 months before resettlement in the United States. Physicians selected by US Department of State consular officials perform the examinations.
The OME includes a medical history, physical examination, and testing to screen for mental illness, drug abuse, syphilis, leprosy, and tuberculosis (TB). Some vaccinations and empiric treatment for parasites also may be provided at the time of the examination.10-12
The OME screens for Class A disorders, which render a refugee ineligible for admission to the United States until treated or stabilized, and Class B conditions, which require close follow-up on arrival (TABLE 1).12 Despite recent steps toward standardization, the quality and thoroughness of OMEs completed at different examination sites still vary substantially.
TABLE 1
Overseas medical examination: Class A and B conditions12
| Class A* | Class B† |
|---|---|
| Active or infectious tuberculosis Untreated STI: syphilis, gonorrhea, chancroid, granuloma inguinale, or lymphogranuloma venereum Hansen’s disease (leprosy) Drug or alcohol addiction/abuse Mental illness with harmful behavior | Inactive or noninfectious tuberculosis Treated STI Treated or paucibacillary Hansen’s disease Sustained remission from drug or alcohol addiction or abuse Well-controlled mental illness Pregnancy |
| STI, sexually transmitted infection. *Class A disorders render a refugee ineligible for admission to the United States until he or she is treated or stabilized. †Class B disorders require close follow-up upon the refugee’s arrival in the United States | |
Arrival in United States is followed by DRME
When refugees arrive in the United States, they are advised to undergo a DRME, which any licensed practitioner may perform, preferably within 90 days. More rapid evaluation is encouraged for medically complex refugees or refugees arriving with Class A or B conditions. Because refugees are eligible for only 8 months of medical assistance, we strongly recommend that the DRME be done promptly.
The CDC publishes guidelines for components of the initial DRME, but state requirements and individual examinations vary widely.2,10,13,14 We outline here the elements of the exam identified by the CDC, supplemented with recommendations based on published evidence and our experiences in caring for refugees.
Screen for tuberculosis
Refugees have a higher prevalence of latent tuberculosis infection (LTBI) and active TB than the general US population. An estimated one-third of the world’s population has LTBI.15 Since 2002, more than 50% of all people diagnosed with TB in the United States have been born outside the country.16
Although otherwise healthy adults with LTBI have a lifetime risk of approximately 10% that it will progress to active TB,17 infants, young children, and people coinfected with HIV have a rate of progression of around 10% per year. It is imperative, therefore, that all refugees be screened for TB and treated appropriately.8,18,19
Refugees are screened for active TB with a chest radiograph and possibly a sputum analysis during the OME. Because screening may take place as long as 12 months before arrival in the United States, refugees may be re-exposed to TB in the refugee camp before departure. They are not screened for LTBI before coming to the United States.11,12
Domestic screening for LTBI is complicated by routine use in many foreign countries of the Bacille Calmette-Guérin (BCG) vaccine, which can reduce the incidence of TB meningitis and disseminated TB in children, but does not protect adults against primary infection or reactivation of TB. Tuberculin skin testing using purified protein derivative, which has typically been used for screening, can render false-positive results, particularly in the context of previous BCG vaccination.
Interferon-gamma release assay (IGRA) is an alternative screening option that has been approved for use in the United States.15,20 Because the IGRA is a blood test, it eliminates interpretation errors associated with tuberculin skin testing and is not affected by BCG vaccination. IGRA testing also does not require an additional office visit.
For these reasons, we recommend screening all refugees older than 5 years with IGRAs, where available. In light of scant data and apparent differences in immune response in young children, the CDC recommends using tuberculin skin testing either alone or in conjunction with IGRA testing for all children younger than 5 years.20,21
Positive screening tests must be followed up with a chest radiograph. Perform serial sputum evaluation whenever the chest radiograph indicates potential active TB.
Everyone with latent or active TB must be treated according to CDC recommendations adapted from guidelines established by the American Thoracic Society and Infectious Diseases Society of America.22,23 For latent TB, the CDC calls for treatment with isoniazid for 9 months or rifampin for 4 months.
- Patients older than 18 years should receive the adult dose of isoniazid: 5 mg/kg per day orally to a maximum daily dose of 300 mg. Children should receive 10 to 20 mg/kg per day orally to a maximum daily dose of 300 mg. Twice weekly therapy schedules are also available and commonly used for children who receive directly observed treatment in school.
- The adult dosage of rifampin (for patients >15 years) is 10 mg/kg per day orally to a maximum daily dose of 600 mg; the pediatric dose is 10 to 20 mg/kg per day orally, also to a maximum daily dose of 600 mg.
Patients taking isoniazid who are pregnant or breastfeeding or have diabetes, renal failure, alcoholism, malnutrition, HIV, or a seizure disorder should receive pyridoxine (vitamin B6) supplementation to aid in preventing peripheral neuropathy, in an adult oral dose of 25 to 50 mg/d or a pediatric oral dose of 6.25 mg/d. Additional information on treating latent TB is available at http://www.cdc.gov/tb/topic/treatment/ltbi.htm.
For patients with active TB, treatment is more complex, based on the patient’s overall health. Please refer to the CDC recommendation for the treatment of active TB (http://www.cdc.gov/tb/topic/treatment/tbdisease.htm) or contact your local TB control division.
Patients may receive TB treatment from either individual medical providers or city or state health departments, depending on local capacity. In our practice, we treat LTBI in adults. The Philadelphia Department of Public Health’s TB Control Program manages LTBI in children and all suspected cases of active TB. We recommend providing everyone treated for latent or active TB with documentation of treatment completion.
Diagnose and treat problematic parasites
Intestinal parasites are among the infections most often found in refugee populations.7,8,24-29 Common pathogens in untreated refugees are Ascaris lumbricoides, hookworm (Ancylostoma duodenale and Necator americanus), Schistosoma species, Strongyloides stercoralis, Trichuris trichiura, and Giardia lamblia.
Although sustained domestic transmission is unlikely, these parasites may cause growth delay, anemia, hyperinfestation syndrome and disseminated infection (A lumbricoides and S stercoralis), and increased cancer risk (Schistosoma hematobium).7 In the late 1990s, the CDC initiated empiric treatment before departure for the United States for A lumbricoides (albendazole), S stercoralis (ivermectin), Schistosoma species (praziquantel), and other parasites in certain refugee populations, which has decreased but not eliminated the threat.7
All refugees should be receiving appropriate predeparture treatment for parasitic infections. For newly arrived refugees who have received no predeparture therapy or incomplete therapy, the CDC recommends screening for parasites or providing presumptive treatment (TABLE 2).
TABLE 2
Empiric treatment of parasites
| Refugee region of origin | Organism | Adult therapy |
|---|---|---|
| Middle East, South Asia, Southeast Asia | Strongyloides stercoralis Other roundworms | Ivermectin 200 μg/kg/d orally for 2 days Albendazole 400 mg orally, 1 dose |
| Africa | Schistosoma species S stercoralis Other roundworms | Praziquantel 20 mg/kg orally, 2 doses Ivermectin 200 μg/kg/d orally for 2 days Albendazole 400 mg orally, 1 dose |
| Source: CDC. Immigrant and Refugee Health: Domestic Intestinal Parasite Guidelines. Available at: http://www.cdc.gov/immigrantrefugeehealth/guidelines/domestic/intestinal-parasites-domestic.html. Accessed November 19, 2012. | ||
The optimal screening regimen for parasites in refugee populations is controversial. Although most screening programs rely on one or more microscopic examinations of stool for ova and parasites, this test is expensive, requires special handling, depends on the reviewer’s expertise, and remains relatively insensitive. A comprehensive review of stool ova and parasites in high-risk populations concluded that the use of 2 independently collected stool samples improved sensitivity at acceptable cost.30
New, more sensitive and specific assays have been developed for many parasites, including Cryptosporidium parvum, Entamoeba histolytica, G lamblia, S stercoralis, and Schistosoma species, but we do not recommend these specialized tests unless the provider strongly suspects a specific parasite based on history and physical exam or persistent eosinophilia.
All refugees should have a complete blood count with differential to help identify occult parasitemia. Although a finding of eosinophilia may result from successful empiric therapy for an already-treated parasite, it must be followed up with more specific testing for S stercoralis, even in otherwise asymptomatic patients. African refugees with eosinophilia also should be tested for Schistosoma, and Somali Bantu should be treated empirically for both S stercoralis and Schistosoma.31 In line with CDC guidelines, ongoing failure to identify the cause of eosinophilia in a refugee should prompt referral to an infectious disease specialist and further work-up.
Three to 6 months after antibiotic treatment of any parasite, immunocompromised patients and those with suspected treatment failure should undergo a test of cure comprised of 2 stool ova and parasite studies and a follow-up CBC with differential.32
Screen for HIV
Since January 4, 2010, after HIV was removed from the Class A diagnosis list, refugees are no longer tested for HIV before arrival in the United States.11 Nevertheless, we recommend screening all refugees on arrival, regardless of age, for HIV types 1 and 2, unless they opt out, for the following reasons:
- approximately 14% of incoming refugees arrive from countries with an HIV prevalence of more than 5%33
- the increasing use of rape as a tool of torture and repression puts refugees at particular risk for HIV
- current CDC guidelines recommend HIV screening at the time of first encounter in all health care settings for everyone from 13 to 64 years of age and any patient who requests it.34
We also strongly recommend repeat screening 3 to 6 months after resettlement for refugees with recent potential exposure or who engage in high-risk activity.
Watch for ubiquitous hepatitis infection
In accordance with CDC vaccination guidelines and American Association of Pediatrics (AAP) Bright Futures recommendations, we endorse hepatitis A serology testing with reflex vaccination unless immunity is documented for refugees 1 to 18 years of age.35,36
A third of the world’s population shows serologic evidence of past infection with hepatitis B virus (HBV); high rates occur in Southeast Asia and sub-Saharan Africa, where most infections are transmitted perinatally.37,38 A study of Minnesota refugees found 7% to be positive for hepatitis B surface antigen (HBsAg), with a higher prevalence among refugees from sub-Saharan Africa.8
Most screening protocols for refugees test for HBsAg and antibody to hepatitis B surface antigen (HBsAb); it is reasonable to add a screen for antibody to hepatitis B core antigen (HBcAb). We recommend screening for HBV infection using HBsAg, HBsAb, and HBcAb to minimize underdiagnosis in this high-risk population. Refugees without immunity to HBV should be offered vaccination.18 Encourage immunization, especially for patients with hepatitis or cirrhosis from any cause.
Hepatitis C screening should follow CDC guidelines for the general population, focusing on high-risk groups such as injection drug users, victims of sexual violence, people with multiple sexual partners, recipients of blood transfusions, people with any other type of hepatitis, and one-time screening for individuals born between 1945 and 1965.39,40
Monitor for malaria
Many refugees come to the United States from areas where malaria is endemic.41 In 2007, the CDC instituted empiric treatment before arrival in the United States for all refugees from sub-Saharan Africa because the rapid test for malaria approved by the US Food and Drug Administration has low sensitivity and specificity,2 malarial vectors are present throughout much of the United States, and malaria (specifically Plasmodium falciparum) causes significant morbidity and mortality. If written confirmation of predeparture treatment is not available, refugees from sub-Saharan Africa should receive presumptive treatment, outlined in TABLE 3,42 as part of the initial DRME.
TABLE 3
Presumptive postarrival malaria treatment for refugees from sub-Saharan Africa42
| Directly observed treatment received in country of origin? | Recommended treatment* | |
|---|---|---|
| Children | Adults | |
| Yes | None | None |
| No | Atovaquone-proguanil (62.5/25 mg): 5-8 kg: 2 tablets per day for 3 days 9-10 kg: 3 tablets per day for 3 days Atovaquone-proguanil (250/100 mg): 11-20 kg: 1 tablet per day for 3 days 21-30 kg: 2 tablets per day for 3 days 31-40 kg: 3 tablets per day for 3 days >40 kg: 4 tablets per day for 3 days | Atovaquone-proguanil (250/100 mg): 4 tablets per day for 3 days |
| *Do not presumptively treat pregnant or lactating women or children weighing <5 kg. An infectious disease consult is recommended for these patients. | ||
Based on our experience and expert opinion, we recommend routinely monitoring all refugees from endemic areas for symptoms of malarial disease during the initial 3 months after resettlement. Relapsing fevers, unexplained malaise or fatigue, pallor, thrombocytopenia, or splenomegaly should trigger additional testing with thick- and thin-blood smears for trophozoites (3 separate samples drawn at 12- to 24-hour intervals).
Be alert for malnutrition
Acute and chronic malnutrition, as well as micronutrient deficiencies, have been noted in refugees coming from refugee camps. A survey of Bhutanese refugees in a camp in Nepal found that 25.1% of children were underweight and 4.8% of them were severely underweight. Moreover, 43.3% of children had anemia.43 Recognizing that refugees may be at high risk for iron deficiency, we recommend evaluating children and adolescents for this deficit according to AAP guidelines.44
We also recommend screening body mass index (BMI) to identify refugees at risk. Height, weight, and BMI must be followed over time to ensure appropriate acclimation to the US diet.
Also consider vitamin D deficiency and rickets in refugee populations, particularly people with darker skin and women who wear veils.45,46 Based on our experiences and CDC guidelines, we recommend a multivitamin with iron for children 6 to 59 months of age.12
Check lead levels in children
Refugee children are at risk of elevated blood lead levels (>10 ’g/dL) resulting from pre-departure environmental exposure and iron deficiency anemia, which can enhance absorption of lead. Refugees also are more likely to resettle in poor neighborhoods with substandard housing, increasing their risk of domestic lead exposure.
Studies of refugee children at initial screening have shown prevalences of elevated blood lead levels of 6.3% in a Cuban refugee population in Miami and higher rates (11%-22%) in mixed refugee populations in Massachusetts.6,47 A study in New Hampshire found that approximately 30% of refugee children with normal lead levels on initial screen had elevated levels when checked several months later.48
Consistent with CDC guidelines,49 our experience, and the findings of the State of Minnesota,50 we recommend checking blood lead levels in all children 6 months to 16 years of age upon arrival in the United States and repeat lead testing 3 to 6 months after placement in a permanent residence.
Bring vaccinations up to date
US law requires anyone seeking an immigrant visa to show proof of vaccination against vaccine-preventable diseases, as recommended by the US Advisory Committee on Immunization Practices.51 Vaccination requirements that apply to other immigrant groups do not apply to refugees at the time of their initial admission to the United States, but refugees must be vaccinated when they seek a green card or permanent US residence.
All refugees are eligible for adjustment of status after they have lived in the United States for a year and need proof of vaccination to apply.51 Moreover, schools may bar refugee children from attending if their vaccinations are not up-to-date, which, in turn, may hinder their parents’ ability to find employment. CDC guidelines for vaccinating immigrants and refugees applying for permanent residence are available at http://www.cdc.gov/immigrantrefugeehealth/pdf/2009-vaccination-technical-instructions.pdf (see the table on page 12).52 Because of the large number of vaccinations required for children and even many adults, health care providers should be familiar with the CDC’s recommended immunization and catch-up schedules.35
Vaccinations given in other countries are acceptable if appropriately recorded in Institute of Medicine documentation, or if original vaccination records are available and the vaccinations conform to appropriate intervals and age guidelines. Refugees must bring their records with them to medical appointments. Laboratory evidence of immunity is acceptable for measles, mumps, rubella (MMR), hepatitis A, hepatitis B, polio, and varicella, but there is debate about whether such testing should be performed before immunization.18,53 Health care providers need to assess each patient based on age and risk factors to decide whether immunity testing is appropriate.
In our practice, we routinely test all adults for immunity to varicella, hepatitis A, hepatitis B, and MMR. For children, we rely on documented immunization records, not antibody titers, for evidence of previous vaccination.
Pay attention to mental health issues
Many refugees have been exposed to trauma, often including war and torture, increasing their risk for mental illness. A large 2005 review found that serious mental disorders, including post-traumatic stress disorder (PTSD), major depressive disorder, and generalized anxiety disorder are significantly more prevalent among refugees than the general population.5 Many screening tests for PTSD have been proposed54 but have not been validated in all immigrant or refugee populations.55
Mental health care for refugees is complicated by language and cultural barriers, adjustment disorders, access to psychiatric services, and uncertainty about effective treatments in refugee populations. Despite the higher prevalence of mental illness among refugees, many in the mental health field have raised concerns about the applicability of Western concepts of mental health, including PTSD, in this group.56
Refugees who are victims of torture should be referred to experienced mental health practitioners. After ruling out acute psychosis and destructive behaviors, we recommend postponing an exhaustive mental health screening until several months after arrival. In our medical home model, we evaluate patients on an ongoing basis, giving us an opportunity to identify emerging or worsening mental health conditions.
Evaluate dental health
The incidence of dental caries and periodontal disease among refugees varies widely among different groups of refugees. Data on pediatric refugees in the United States have shown dental caries to be common, with prevalences between 16.7% and 42%, with marked differences based on region of origin.3,57,58 In our practice, we also have noted heavy use of betel nut in the Southeast Asian community, leading to significant dental disease.
All refugees should have their dentition evaluated at the initial DRME. We recommend subsequent formal dental examination for all patients, giving priority to those with clear evidence of active disease.
Identify and address chronic disease
Refugees carry a substantial burden of chronic disease, although marked regional variation has been noted.4 A study of Massachusetts refugees from 2001 through 2005 demonstrated that 46.8% were overweight or obese, 22.6% had hypertension, and 3.1% had diabetes. Smoking is also highly prevalent in refugee populations.59
Our findings confirm high rates of chronic disease, particularly among Iraqi and geriatric refugees. These patients require close follow-up after the DRME to minimize sequelae from chronic conditions. Multi-disciplinary teams in the patient-centered medical home may provide an opportunity to promptly address chronic health conditions that can have severe short-term consequences if not adequately managed (eg, insulin dosage adjustment based on diet in patients with diabetes).
We recommend a comprehensive medical history and evaluation for chronic disease, including diabetes and hypertension, at the DRME and on an ongoing basis. Although many refugees have never had any health screening and substantial cultural barriers may exist, especially with regard to women’s health and age-based cancer screening, refugees generally should receive the same preventive care as the rest of the US population until further research has been done in this area.
We recommend introducing age-based cancer screening and other preventive care for refugees within 2 months of their initial visit. This model of care has already been endorsed by the Minnesota Department of Health’s Refugee Health Program, one of the leading health care providers for refugees in the United States.60
Toward better care models
The medical care of refugees is complex, but the prepared primary care provider can manage it effectively. TABLE 4 summarizes our recommendations for the DRME based on our experiences and the available literature. Standardized screening guidelines and comprehensive programs, perhaps incorporating the concept of the patient-centered medical home, will likely improve both the initial and continuing care of this population.
TABLE 4
Summary recommendations for the domestic refugee medical exam
History
|
| Physical exam In addition to the essential components of the physical exam, pay attention to:
|
Initial laboratory evaluation
|
| Ongoing care Include:
|
| CBC, complete blood count; HIV, human immunode"ciency virus; IGRA, interferon-gamma release assay; MMR, measles, mumps, rubella; TST, tuberculin skin testing. Adapted from: Centers for Disease Control and Prevention. Immigrant and Refugee Health: Guidelines for the US Domestic Medical Examination for Newly Arriving Refugees. Available at: http://www.cdc.gov/immigrantrefugeehealth/guidelines/domestic/domestic-guidelines.html. Accessed November 19, 2012. |
Ongoing study is essential to better address the health care needs of refugees. Although they comprise only a small segment of immigrants living in the United States, the experience of caring for them may help develop models to provide better care to other foreign-born patients.
CORRESPONDENCE Marc Altshuler, MD, Department of Family and Community Medicine, Jefferson Medical College, Thomas Jefferson University, 833 Chestnut Street, Suite 301, Philadelphia, PA 19107; marc.altshuler@jefferson.edu
• Use tuberculin skin testing alone or in conjunction with interferon-gamma release assay to screen children younger than 5 years for tuberculosis. A
• Include 2 evaluations for ova and parasites plus a complete blood count with differential when screening refugees for parasitic infections. B
• Screen all adolescent and adult refugees for human immunodeficiency virus infection. A
• Check blood lead levels in all children 6 months to 16 years of age on arrival in the United States B and 6 months later. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
In 2011, 56,384 refugees fleeing persecution in their native countries were admitted to the United States. The largest numbers came from Burma (30.1%), Bhutan (26.6%), and Iraq (16.7%).1 They joined the more than 3 million refugees from all over the world who have resettled in this country since 1975. 1
Refugees arrive in the United States with complex medical issues, including illnesses rarely seen here, mental health concerns, and chronic conditions such as diabetes and hypertension. After arrival, they undergo a domestic refugee medical examination (DRME). This DRME, along with well-planned follow-up, can go a long way toward helping refugees show the proof of vaccination and control of chronic health conditions that are required when they apply for lawful permanent resident status.
The Centers for Disease Control and Prevention (CDC) has published guidelines to help with medical decision making and screening of refugees, but limited information is available on the necessary strategies to address chronic health conditions within the context of the DRME.2 Moreover, differences in refugee experience and health status based on country of origin may demand more detailed, region-specific guidelines.3-9 No standard recommendations address the importance of providing not just initial screening, but comprehensive longitudinal care, as well.
Since 2007, our outpatient practice (MA, KS, GM, PM) has performed the DRME and provided ongoing care for more than 900 refugees resettled in Philadelphia. The practice, which is associated with an urban academic medical center and closely coordinates refugee care with a local resettlement agency, has earned recognition as a Level 3 (top-level certification) patient-centered medical home by the National Committee on Quality Assurance. We offer here a framework for providing comprehensive care to refugees, based on CDC guidelines, available evidence, and our experience.
Prelude: The overseas medical exam
All refugees must undergo an overseas medical examination (OME) no longer than 12 months before resettlement in the United States. Physicians selected by US Department of State consular officials perform the examinations.
The OME includes a medical history, physical examination, and testing to screen for mental illness, drug abuse, syphilis, leprosy, and tuberculosis (TB). Some vaccinations and empiric treatment for parasites also may be provided at the time of the examination.10-12
The OME screens for Class A disorders, which render a refugee ineligible for admission to the United States until treated or stabilized, and Class B conditions, which require close follow-up on arrival (TABLE 1).12 Despite recent steps toward standardization, the quality and thoroughness of OMEs completed at different examination sites still vary substantially.
TABLE 1
Overseas medical examination: Class A and B conditions12
| Class A* | Class B† |
|---|---|
| Active or infectious tuberculosis Untreated STI: syphilis, gonorrhea, chancroid, granuloma inguinale, or lymphogranuloma venereum Hansen’s disease (leprosy) Drug or alcohol addiction/abuse Mental illness with harmful behavior | Inactive or noninfectious tuberculosis Treated STI Treated or paucibacillary Hansen’s disease Sustained remission from drug or alcohol addiction or abuse Well-controlled mental illness Pregnancy |
| STI, sexually transmitted infection. *Class A disorders render a refugee ineligible for admission to the United States until he or she is treated or stabilized. †Class B disorders require close follow-up upon the refugee’s arrival in the United States | |
Arrival in United States is followed by DRME
When refugees arrive in the United States, they are advised to undergo a DRME, which any licensed practitioner may perform, preferably within 90 days. More rapid evaluation is encouraged for medically complex refugees or refugees arriving with Class A or B conditions. Because refugees are eligible for only 8 months of medical assistance, we strongly recommend that the DRME be done promptly.
The CDC publishes guidelines for components of the initial DRME, but state requirements and individual examinations vary widely.2,10,13,14 We outline here the elements of the exam identified by the CDC, supplemented with recommendations based on published evidence and our experiences in caring for refugees.
Screen for tuberculosis
Refugees have a higher prevalence of latent tuberculosis infection (LTBI) and active TB than the general US population. An estimated one-third of the world’s population has LTBI.15 Since 2002, more than 50% of all people diagnosed with TB in the United States have been born outside the country.16
Although otherwise healthy adults with LTBI have a lifetime risk of approximately 10% that it will progress to active TB,17 infants, young children, and people coinfected with HIV have a rate of progression of around 10% per year. It is imperative, therefore, that all refugees be screened for TB and treated appropriately.8,18,19
Refugees are screened for active TB with a chest radiograph and possibly a sputum analysis during the OME. Because screening may take place as long as 12 months before arrival in the United States, refugees may be re-exposed to TB in the refugee camp before departure. They are not screened for LTBI before coming to the United States.11,12
Domestic screening for LTBI is complicated by routine use in many foreign countries of the Bacille Calmette-Guérin (BCG) vaccine, which can reduce the incidence of TB meningitis and disseminated TB in children, but does not protect adults against primary infection or reactivation of TB. Tuberculin skin testing using purified protein derivative, which has typically been used for screening, can render false-positive results, particularly in the context of previous BCG vaccination.
Interferon-gamma release assay (IGRA) is an alternative screening option that has been approved for use in the United States.15,20 Because the IGRA is a blood test, it eliminates interpretation errors associated with tuberculin skin testing and is not affected by BCG vaccination. IGRA testing also does not require an additional office visit.
For these reasons, we recommend screening all refugees older than 5 years with IGRAs, where available. In light of scant data and apparent differences in immune response in young children, the CDC recommends using tuberculin skin testing either alone or in conjunction with IGRA testing for all children younger than 5 years.20,21
Positive screening tests must be followed up with a chest radiograph. Perform serial sputum evaluation whenever the chest radiograph indicates potential active TB.
Everyone with latent or active TB must be treated according to CDC recommendations adapted from guidelines established by the American Thoracic Society and Infectious Diseases Society of America.22,23 For latent TB, the CDC calls for treatment with isoniazid for 9 months or rifampin for 4 months.
- Patients older than 18 years should receive the adult dose of isoniazid: 5 mg/kg per day orally to a maximum daily dose of 300 mg. Children should receive 10 to 20 mg/kg per day orally to a maximum daily dose of 300 mg. Twice weekly therapy schedules are also available and commonly used for children who receive directly observed treatment in school.
- The adult dosage of rifampin (for patients >15 years) is 10 mg/kg per day orally to a maximum daily dose of 600 mg; the pediatric dose is 10 to 20 mg/kg per day orally, also to a maximum daily dose of 600 mg.
Patients taking isoniazid who are pregnant or breastfeeding or have diabetes, renal failure, alcoholism, malnutrition, HIV, or a seizure disorder should receive pyridoxine (vitamin B6) supplementation to aid in preventing peripheral neuropathy, in an adult oral dose of 25 to 50 mg/d or a pediatric oral dose of 6.25 mg/d. Additional information on treating latent TB is available at http://www.cdc.gov/tb/topic/treatment/ltbi.htm.
For patients with active TB, treatment is more complex, based on the patient’s overall health. Please refer to the CDC recommendation for the treatment of active TB (http://www.cdc.gov/tb/topic/treatment/tbdisease.htm) or contact your local TB control division.
Patients may receive TB treatment from either individual medical providers or city or state health departments, depending on local capacity. In our practice, we treat LTBI in adults. The Philadelphia Department of Public Health’s TB Control Program manages LTBI in children and all suspected cases of active TB. We recommend providing everyone treated for latent or active TB with documentation of treatment completion.
Diagnose and treat problematic parasites
Intestinal parasites are among the infections most often found in refugee populations.7,8,24-29 Common pathogens in untreated refugees are Ascaris lumbricoides, hookworm (Ancylostoma duodenale and Necator americanus), Schistosoma species, Strongyloides stercoralis, Trichuris trichiura, and Giardia lamblia.
Although sustained domestic transmission is unlikely, these parasites may cause growth delay, anemia, hyperinfestation syndrome and disseminated infection (A lumbricoides and S stercoralis), and increased cancer risk (Schistosoma hematobium).7 In the late 1990s, the CDC initiated empiric treatment before departure for the United States for A lumbricoides (albendazole), S stercoralis (ivermectin), Schistosoma species (praziquantel), and other parasites in certain refugee populations, which has decreased but not eliminated the threat.7
All refugees should be receiving appropriate predeparture treatment for parasitic infections. For newly arrived refugees who have received no predeparture therapy or incomplete therapy, the CDC recommends screening for parasites or providing presumptive treatment (TABLE 2).
TABLE 2
Empiric treatment of parasites
| Refugee region of origin | Organism | Adult therapy |
|---|---|---|
| Middle East, South Asia, Southeast Asia | Strongyloides stercoralis Other roundworms | Ivermectin 200 μg/kg/d orally for 2 days Albendazole 400 mg orally, 1 dose |
| Africa | Schistosoma species S stercoralis Other roundworms | Praziquantel 20 mg/kg orally, 2 doses Ivermectin 200 μg/kg/d orally for 2 days Albendazole 400 mg orally, 1 dose |
| Source: CDC. Immigrant and Refugee Health: Domestic Intestinal Parasite Guidelines. Available at: http://www.cdc.gov/immigrantrefugeehealth/guidelines/domestic/intestinal-parasites-domestic.html. Accessed November 19, 2012. | ||
The optimal screening regimen for parasites in refugee populations is controversial. Although most screening programs rely on one or more microscopic examinations of stool for ova and parasites, this test is expensive, requires special handling, depends on the reviewer’s expertise, and remains relatively insensitive. A comprehensive review of stool ova and parasites in high-risk populations concluded that the use of 2 independently collected stool samples improved sensitivity at acceptable cost.30
New, more sensitive and specific assays have been developed for many parasites, including Cryptosporidium parvum, Entamoeba histolytica, G lamblia, S stercoralis, and Schistosoma species, but we do not recommend these specialized tests unless the provider strongly suspects a specific parasite based on history and physical exam or persistent eosinophilia.
All refugees should have a complete blood count with differential to help identify occult parasitemia. Although a finding of eosinophilia may result from successful empiric therapy for an already-treated parasite, it must be followed up with more specific testing for S stercoralis, even in otherwise asymptomatic patients. African refugees with eosinophilia also should be tested for Schistosoma, and Somali Bantu should be treated empirically for both S stercoralis and Schistosoma.31 In line with CDC guidelines, ongoing failure to identify the cause of eosinophilia in a refugee should prompt referral to an infectious disease specialist and further work-up.
Three to 6 months after antibiotic treatment of any parasite, immunocompromised patients and those with suspected treatment failure should undergo a test of cure comprised of 2 stool ova and parasite studies and a follow-up CBC with differential.32
Screen for HIV
Since January 4, 2010, after HIV was removed from the Class A diagnosis list, refugees are no longer tested for HIV before arrival in the United States.11 Nevertheless, we recommend screening all refugees on arrival, regardless of age, for HIV types 1 and 2, unless they opt out, for the following reasons:
- approximately 14% of incoming refugees arrive from countries with an HIV prevalence of more than 5%33
- the increasing use of rape as a tool of torture and repression puts refugees at particular risk for HIV
- current CDC guidelines recommend HIV screening at the time of first encounter in all health care settings for everyone from 13 to 64 years of age and any patient who requests it.34
We also strongly recommend repeat screening 3 to 6 months after resettlement for refugees with recent potential exposure or who engage in high-risk activity.
Watch for ubiquitous hepatitis infection
In accordance with CDC vaccination guidelines and American Association of Pediatrics (AAP) Bright Futures recommendations, we endorse hepatitis A serology testing with reflex vaccination unless immunity is documented for refugees 1 to 18 years of age.35,36
A third of the world’s population shows serologic evidence of past infection with hepatitis B virus (HBV); high rates occur in Southeast Asia and sub-Saharan Africa, where most infections are transmitted perinatally.37,38 A study of Minnesota refugees found 7% to be positive for hepatitis B surface antigen (HBsAg), with a higher prevalence among refugees from sub-Saharan Africa.8
Most screening protocols for refugees test for HBsAg and antibody to hepatitis B surface antigen (HBsAb); it is reasonable to add a screen for antibody to hepatitis B core antigen (HBcAb). We recommend screening for HBV infection using HBsAg, HBsAb, and HBcAb to minimize underdiagnosis in this high-risk population. Refugees without immunity to HBV should be offered vaccination.18 Encourage immunization, especially for patients with hepatitis or cirrhosis from any cause.
Hepatitis C screening should follow CDC guidelines for the general population, focusing on high-risk groups such as injection drug users, victims of sexual violence, people with multiple sexual partners, recipients of blood transfusions, people with any other type of hepatitis, and one-time screening for individuals born between 1945 and 1965.39,40
Monitor for malaria
Many refugees come to the United States from areas where malaria is endemic.41 In 2007, the CDC instituted empiric treatment before arrival in the United States for all refugees from sub-Saharan Africa because the rapid test for malaria approved by the US Food and Drug Administration has low sensitivity and specificity,2 malarial vectors are present throughout much of the United States, and malaria (specifically Plasmodium falciparum) causes significant morbidity and mortality. If written confirmation of predeparture treatment is not available, refugees from sub-Saharan Africa should receive presumptive treatment, outlined in TABLE 3,42 as part of the initial DRME.
TABLE 3
Presumptive postarrival malaria treatment for refugees from sub-Saharan Africa42
| Directly observed treatment received in country of origin? | Recommended treatment* | |
|---|---|---|
| Children | Adults | |
| Yes | None | None |
| No | Atovaquone-proguanil (62.5/25 mg): 5-8 kg: 2 tablets per day for 3 days 9-10 kg: 3 tablets per day for 3 days Atovaquone-proguanil (250/100 mg): 11-20 kg: 1 tablet per day for 3 days 21-30 kg: 2 tablets per day for 3 days 31-40 kg: 3 tablets per day for 3 days >40 kg: 4 tablets per day for 3 days | Atovaquone-proguanil (250/100 mg): 4 tablets per day for 3 days |
| *Do not presumptively treat pregnant or lactating women or children weighing <5 kg. An infectious disease consult is recommended for these patients. | ||
Based on our experience and expert opinion, we recommend routinely monitoring all refugees from endemic areas for symptoms of malarial disease during the initial 3 months after resettlement. Relapsing fevers, unexplained malaise or fatigue, pallor, thrombocytopenia, or splenomegaly should trigger additional testing with thick- and thin-blood smears for trophozoites (3 separate samples drawn at 12- to 24-hour intervals).
Be alert for malnutrition
Acute and chronic malnutrition, as well as micronutrient deficiencies, have been noted in refugees coming from refugee camps. A survey of Bhutanese refugees in a camp in Nepal found that 25.1% of children were underweight and 4.8% of them were severely underweight. Moreover, 43.3% of children had anemia.43 Recognizing that refugees may be at high risk for iron deficiency, we recommend evaluating children and adolescents for this deficit according to AAP guidelines.44
We also recommend screening body mass index (BMI) to identify refugees at risk. Height, weight, and BMI must be followed over time to ensure appropriate acclimation to the US diet.
Also consider vitamin D deficiency and rickets in refugee populations, particularly people with darker skin and women who wear veils.45,46 Based on our experiences and CDC guidelines, we recommend a multivitamin with iron for children 6 to 59 months of age.12
Check lead levels in children
Refugee children are at risk of elevated blood lead levels (>10 ’g/dL) resulting from pre-departure environmental exposure and iron deficiency anemia, which can enhance absorption of lead. Refugees also are more likely to resettle in poor neighborhoods with substandard housing, increasing their risk of domestic lead exposure.
Studies of refugee children at initial screening have shown prevalences of elevated blood lead levels of 6.3% in a Cuban refugee population in Miami and higher rates (11%-22%) in mixed refugee populations in Massachusetts.6,47 A study in New Hampshire found that approximately 30% of refugee children with normal lead levels on initial screen had elevated levels when checked several months later.48
Consistent with CDC guidelines,49 our experience, and the findings of the State of Minnesota,50 we recommend checking blood lead levels in all children 6 months to 16 years of age upon arrival in the United States and repeat lead testing 3 to 6 months after placement in a permanent residence.
Bring vaccinations up to date
US law requires anyone seeking an immigrant visa to show proof of vaccination against vaccine-preventable diseases, as recommended by the US Advisory Committee on Immunization Practices.51 Vaccination requirements that apply to other immigrant groups do not apply to refugees at the time of their initial admission to the United States, but refugees must be vaccinated when they seek a green card or permanent US residence.
All refugees are eligible for adjustment of status after they have lived in the United States for a year and need proof of vaccination to apply.51 Moreover, schools may bar refugee children from attending if their vaccinations are not up-to-date, which, in turn, may hinder their parents’ ability to find employment. CDC guidelines for vaccinating immigrants and refugees applying for permanent residence are available at http://www.cdc.gov/immigrantrefugeehealth/pdf/2009-vaccination-technical-instructions.pdf (see the table on page 12).52 Because of the large number of vaccinations required for children and even many adults, health care providers should be familiar with the CDC’s recommended immunization and catch-up schedules.35
Vaccinations given in other countries are acceptable if appropriately recorded in Institute of Medicine documentation, or if original vaccination records are available and the vaccinations conform to appropriate intervals and age guidelines. Refugees must bring their records with them to medical appointments. Laboratory evidence of immunity is acceptable for measles, mumps, rubella (MMR), hepatitis A, hepatitis B, polio, and varicella, but there is debate about whether such testing should be performed before immunization.18,53 Health care providers need to assess each patient based on age and risk factors to decide whether immunity testing is appropriate.
In our practice, we routinely test all adults for immunity to varicella, hepatitis A, hepatitis B, and MMR. For children, we rely on documented immunization records, not antibody titers, for evidence of previous vaccination.
Pay attention to mental health issues
Many refugees have been exposed to trauma, often including war and torture, increasing their risk for mental illness. A large 2005 review found that serious mental disorders, including post-traumatic stress disorder (PTSD), major depressive disorder, and generalized anxiety disorder are significantly more prevalent among refugees than the general population.5 Many screening tests for PTSD have been proposed54 but have not been validated in all immigrant or refugee populations.55
Mental health care for refugees is complicated by language and cultural barriers, adjustment disorders, access to psychiatric services, and uncertainty about effective treatments in refugee populations. Despite the higher prevalence of mental illness among refugees, many in the mental health field have raised concerns about the applicability of Western concepts of mental health, including PTSD, in this group.56
Refugees who are victims of torture should be referred to experienced mental health practitioners. After ruling out acute psychosis and destructive behaviors, we recommend postponing an exhaustive mental health screening until several months after arrival. In our medical home model, we evaluate patients on an ongoing basis, giving us an opportunity to identify emerging or worsening mental health conditions.
Evaluate dental health
The incidence of dental caries and periodontal disease among refugees varies widely among different groups of refugees. Data on pediatric refugees in the United States have shown dental caries to be common, with prevalences between 16.7% and 42%, with marked differences based on region of origin.3,57,58 In our practice, we also have noted heavy use of betel nut in the Southeast Asian community, leading to significant dental disease.
All refugees should have their dentition evaluated at the initial DRME. We recommend subsequent formal dental examination for all patients, giving priority to those with clear evidence of active disease.
Identify and address chronic disease
Refugees carry a substantial burden of chronic disease, although marked regional variation has been noted.4 A study of Massachusetts refugees from 2001 through 2005 demonstrated that 46.8% were overweight or obese, 22.6% had hypertension, and 3.1% had diabetes. Smoking is also highly prevalent in refugee populations.59
Our findings confirm high rates of chronic disease, particularly among Iraqi and geriatric refugees. These patients require close follow-up after the DRME to minimize sequelae from chronic conditions. Multi-disciplinary teams in the patient-centered medical home may provide an opportunity to promptly address chronic health conditions that can have severe short-term consequences if not adequately managed (eg, insulin dosage adjustment based on diet in patients with diabetes).
We recommend a comprehensive medical history and evaluation for chronic disease, including diabetes and hypertension, at the DRME and on an ongoing basis. Although many refugees have never had any health screening and substantial cultural barriers may exist, especially with regard to women’s health and age-based cancer screening, refugees generally should receive the same preventive care as the rest of the US population until further research has been done in this area.
We recommend introducing age-based cancer screening and other preventive care for refugees within 2 months of their initial visit. This model of care has already been endorsed by the Minnesota Department of Health’s Refugee Health Program, one of the leading health care providers for refugees in the United States.60
Toward better care models
The medical care of refugees is complex, but the prepared primary care provider can manage it effectively. TABLE 4 summarizes our recommendations for the DRME based on our experiences and the available literature. Standardized screening guidelines and comprehensive programs, perhaps incorporating the concept of the patient-centered medical home, will likely improve both the initial and continuing care of this population.
TABLE 4
Summary recommendations for the domestic refugee medical exam
History
|
| Physical exam In addition to the essential components of the physical exam, pay attention to:
|
Initial laboratory evaluation
|
| Ongoing care Include:
|
| CBC, complete blood count; HIV, human immunode"ciency virus; IGRA, interferon-gamma release assay; MMR, measles, mumps, rubella; TST, tuberculin skin testing. Adapted from: Centers for Disease Control and Prevention. Immigrant and Refugee Health: Guidelines for the US Domestic Medical Examination for Newly Arriving Refugees. Available at: http://www.cdc.gov/immigrantrefugeehealth/guidelines/domestic/domestic-guidelines.html. Accessed November 19, 2012. |
Ongoing study is essential to better address the health care needs of refugees. Although they comprise only a small segment of immigrants living in the United States, the experience of caring for them may help develop models to provide better care to other foreign-born patients.
CORRESPONDENCE Marc Altshuler, MD, Department of Family and Community Medicine, Jefferson Medical College, Thomas Jefferson University, 833 Chestnut Street, Suite 301, Philadelphia, PA 19107; marc.altshuler@jefferson.edu
1. Martin D, Yankay J. Refugees and Asylees: 2011. Annual Flow Report. Offce of Immigration Statistics, US Department of Home-land Security. May 2012. Available at: http://www.dhs.gov/xlibrary/assets/statistics/publications/ois_rfa_fr_2011.pdf. Accessed October 29, 2012.
2. Stauffer WM, Kamat D, Walker PF. Screening of international immigrants, refugees and adoptees. Prim Care. 2002;29:879-905.
3. Cote S, Geltman P, Nunn M, et al. Dental caries of refugee children compared with US children. Pediatrics. 2004;114:e733-e740.
4. Geltman PL, Dookeran NM, Battaglia T, et al. Chronic disease and its risk factors among refugees and asylees in Massachusetts, 2001-2005. Prev Chronic Dis. 2010;7:A51.-
5. Fazel M, Wheeler J, Danesh J. Prevalence of serious mental disorder in 7000 refugees resettled in western countries: a systematic review. Lancet. 2005;365:1309-1314.
6. Geltman PL, Brown MJ, Cochran J. Lead poisoning among refugee children resettled in Massachusetts, 1995 to 1999. Pediatrics. 2001;108:158-162.
7. Geltman PL, Cochran J, Hedgecock C. Intestinal parasites among African refugees resettled in Massachusetts and the impact of an overseas pre-departure treatment program. Am J Trop Med Hyg. 2003;69:657.-
8. Lifson AR, Thai D, O’Fallon A, et al. Prevalence of tuberculosis, hepatitis B virus, and intestinal parasitic infections among refugees to Minnesota. Public Health Rep. 2002;117:69-77.
9. Power DV, Moody E, Trussell K, et al. Caring for the Karen. A newly arrived refugee group. Minn Med. 2010;93:49-53.
10. Centers for Disease Control and Prevention. Health considerations of newly arrived immigrants and refugees. In: Centers for Disease Control and Prevention. Travelers’ Health—Yellow Book. Chapt. 9. Available at: http://wwwnc.cdc.gov/travel/yellow-book/2010/table-of-contents.aspx#20. Accessed November 15, 2012.
11. Centers for Disease Control and Prevention. Medical Examination of Immigrants and Refugees. Available at: http://www.cdc.gov/immigrantrefugeehealth/exams/medical-examination.html. Accessed November 15, 2012.
12. Centers for Disease Control and Prevention. Technical Instructions For The Medical History and Physical Examination of Aliens in the United States. Available at: http://www.cdc.gov/immigrantrefugeehealth/exams/ti/civil/technical-instructions/civil-surgeons/medical-history-physical-examination.html. Accessed November 15, 2010.
13. Seybolt L, Barnett E, Stauffer W. US medical screening for immigrants and refugees: clinical issues. In: Walker P, Barnett E, eds. Immigrant Medicine. Phildelphia, PA: Saunders Elsevier; 2007:135-150.
14. United States Department of Health and Human Services, Offce of Refugee Resettlement. ORR State Letter: Revised Medical Screening Guidelines for Newly Arrived Refugees. Available at: http://www.acf.hhs.gov/sites/default/files/orr/state_letter_12_09_revised_medical_screening_guidelines_for_newly.pdf. Accessed October 29, 2012.
15. Centers for Disease Control and Prevention. Guidelines for the investigation of contacts of persons with infectious tuberculosis— guidelines for using the QuantiFERON-TB gold test for detecting Mycobacterium tuberculosis infection, United States. MMWR Recomm Rep. 2005;54(RR-15):1-55.
16. Centers for Disease Control and Prevention. Executive Commentary: Highlights of 2011 Report. Available at: http://www.cdc.gov/tb/statistics/reports/2011/pdf/ExecutiveCommentary.pdf. Accessed November 19, 2012.
17. Kuma V, Abbas AK, Fausto N, et al. Robbins Basic Pathology. 8th ed. Philadelphia, Pa: Saunders Elsevier; 2007:516–522.
18. Barnett ED. Infectious disease screening for refugees resettled in the United States. Clin Infect Dis. 2004;39:833-841.
19. DeRiemer K, Chin DP, Schecter DF, et al. Tuberculosis among immigrants and refugees. Arch Intern Med. 1998;158:753-760.
20. Centers for Disease Control and Prevention. Updated guidelines for using interferon gamma release assays to detect Mycobacterium tuberculosis infection—United States, 2010. MMWR Recomm Rec. 2010;59(RR-5):1-25.
21. Bright Futures at Georgetown University. Health Supervision— Laboratory Tests: Tuberculosis (TB) Screening. Available at: http://www.brightfutures.org/pocket/pdf/30_37.pdf. Accessed November 19, 2012.
22. Centers for Disease Control and Prevention. Treatment of tuberculosis. American Thoracic Society, CDC, Infectious Diseases Society of America. MMWR Recomm Rep 2003;52(RR-11):1-77.
23. Centers for Disease Control and Prevention. Update: adverse event data and revised American Thoracic Society/CDC recommendations against the use of rifampin and pyrazinamide for treament of latent tuberculosis infection—United States. MMWR Morb Mortal Wkly Rep. 2003;52:735-739.
24. Caruana SR, Kelly HA, Ngeow JY, et al. Undiagnosed and potentially lethal parasite infections among immigrants and refugees in Australia. J Travel Med. 2006;13:233-239.
25. Dawson-Hahn EE, Greenberg SL, Domachowske JB, et al. Eosinophilia and the seroprevalence of schistosomiasis and strongyloidiasis in newly arrived pediatric refugees: an examination of Centers for Disease Control and Prevention screening guidelines. J Pediatr. 2010;156:1016-1018.
26. Garg PK, Perry S, Dorn M, et al. Risk of intestinal helminth and protozoan infection in a refugee population. Am J Trop Med Hyg. 2005;73:386-391.
27. Parenti DM, Lucas D, Lee A, et al. Health status of Ethiopian refugees in the United States. Am J Public Health. 1987;77:1542-1543.
28. Parish R. Intestinal parasites in Southeast Asian refugee children. West J Med. 1985;143:47-49.
29. Sutherland JE, Avant RF, Franz WB, 3rd, et al. Indochinese refugee health assessment and treatment. J Fam Pract. 1983;16:61-67.
30. Cartwright C. Utility of multiple-stool-specimen ova and parasite examinations in a high-prevalence setting. J Clin Microbiol. 1999;37:2408-2411.
31. Centers for Disease Control and Prevention. Recommendations for Presumptive Treatment of Schistosomiasis and Strongyloidiasis Among the Somali Bantu Refugees. June 13, 2005. Available at: http://archive.acf.hhs.gov/programs/orr/policy/sl05-18attach-ment2.pdf. Accessed November 19, 2012.
32. Centers for Disease Control and Prevention. Division of Global Migration and Quarantine. Guidelines for Evaluation of Refugees for Intestinal and Tissue-Invasive Parasitic Infections during Domestic Medical Examination. Available at: http://www.cdc.gov/immigrantrefugeehealth/guidelines/domestic/intestinal-parasites-domestic.html. Accessed October 30, 2012.
33. Centers for Disease Control and Prevention. Screening for HIV Infection During the Refugee Domestic Medical Examination. Available at: http://www.cdc.gov/immigrantrefugeehealth/guidelines/domestic/screening-hiv-infection-domestic.html. Accessed November 15, 2012.
34. Centers for Disease Control and Prevention. Revised recommendations for HIV testing of adults, adolescents and pregnant women in healthcare settings. MMWR Recomm Rep. 2006;55(RR-14):1-17.
35. Centers for Disease Control and Prevention National Immunization Program. Available at: http://www.cdc.gov/vaccines/vpdvac/hepa/default.htm. Accessed November 19, 2012.
36. American Academy of Pediatrics. Red Book. Available at: http://www2.aap.org/immunization/illnesses/hepb/hepa.html. Accessed November 19, 2012.
37. Lai CL, Ratziu V, Yuen MF, et al. Viral hepatitis B. Lancet. 2003;362:2089-2094.
38. Lin K, Kirchner J. Hepatitis B. Am Fam Phyician. 2004;69:75-82.
39. Ghany MG, Strader DB, Thomas DL, et al. American Association for the Study of Liver Diseases. Diagnosis, management, and treatment of hepatitis C: an update. Hepatology. 2009;49:1335-1374.
40. Centers for Disease Control and Prevention. Recommendations for the identification of chronic hepatitis C virus infection among persons born during 1945-1965. MMWR Morb Mortal Wkly Rep. 2012;61(RR-4):1-31.
41. Rowland M, Nosten F. Malaria epidemiology and control in refugee camps and complex emergencies. Ann Trop Med Parasitol. 2001;95:741-754.
42. Centers for Disease Control and Prevention, Malaria Branch, Division of Parasitic Diseases, Division of Global Migration and Quarantine and Malaria Branch. Presumptive Treatment of P falciparum Malaria in Refugees Relocating from sub-Saharan Africa to the United States. Available at: http://www.cdc.gov/immigrantrefugeehealth/guidelines/domestic/malaria-guidelines-domestic.html. Accessed November 15, 2012.
43. Centers for Disease Control and Prevention. Malnutrition and micronutrient deficiencies among Bhutanese refugee children— Nepal, 2007. MMWR Morb Mortal Wkly Rep. 2008;57:370-373.
44. American Academy of Pediatrics Bright Futures. Guidelines for Health Supervision of Infants, Children, and Adolescents— Theme 5: Promoting Healthy Nutrition. Available at: http://brightfutures.aap.org/pdfs/Guidelines_PDF/6-Promoting_Healthy_Nutrition.pdf. Accessed November 15, 2012.
45. Benson J, Skull S. Hiding from the sun: vitamin D deficiency in refugees. Aust Fam Physician. 2007;36:355-357.
46. Stellinga-Boelen A. Vitamin D levels in children of asylum seekers in The Netherlands in relation to season and dietary intake. Eur J Pediatr. 2007;166:201-206.
47. Trepka MJ, Pekovic V, Santana JC, et al. Risk factors for lead poisoning among Cuban refugee children. Public Health Rep. 2005;120:179-185.
48. Centers for Disease Control and Prevention. Elevated blood lead levels in refugee children, New Hampshire, 2003-2004. MMWR Morb Mortal Wkly Rep. 2005;54:42-46.
49. Centers for Disease Control and Prevention. Screening for Lead at the Domestic Refugee Medical Exam. Available at: http://www.cdc.gov/immigrantrefugeehealth/pdf/lead.pdf. Accessed November 15, 2012.
50. Zabel EW, Smith ME, O’Fallon A. Implementation of CDC refugee blood testing guidelines in Minnesota. Public Health Rep. 2008;123:111-125.
51. United States Citizenship and Immigration Services. Available at: http://www.uscis.gov/portal/site/uscis/menuitem.5af9bb95919f35e66f614176543f6d1a/?vgnextoid=3384cc5222$5210VgnVCM100000082ca60aRCRD&vgnextchannel=6abe6d26d17df110VgnVCM1000004718190aRCRD. Accessed November 15, 2012.
52. Centers for Disease Control and Prevention. Vaccination Requirements for Adjustment of Status for US Permanent Residence: Technical Instructions for Civil Surgeons. December 14, 2009. Available at: http://www.cdc.gov/immigrantrefugeehealth/pdf/2009-vaccination-technical-instructions.pdf. Accessed November 15, 2012
53. Phillips C. Better primary healthcare for refugees: catch up immunisation. Aust Fam Physician. 2007;36:440-443.
54. Brewin C. Systematic review of screening instruments for adults at risk of PTSD. J Trauma Stress. 2005;18:53-62.
55. Crumlish N, O’Rourke K. A systematic review of treatments for post-traumatic stress disorder among refugees and asylum-seekers. J Nerv Ment Dis. 2010;198:237-251.
56. Watters C. Emerging paradigms in the mental health care of refugees. Soc Sci Med. 2001;53:1709-1718.
57. Hayes EB, Talbot SB, Matheson ES, et al. Health status of pediatric refugees in Portland, ME. Arch Pediatr Adolesc Med. 1998;152:564-568.
58. Meropol S. Health status of pediatric refugees in Buffalo, NY. Arch Pediatr Adolesc Med. 1995;149:887-892.
59. Barnes DM, Harrison C, Heneghan R. Health risk and promotion behaviors in refugee populations. J Health Care Poor Underserved. 2004;15:347-356.
60. Dicker S, Stauffer WM, Mamo B, et al. Initial refugee health assessments: new recommendations for Minnesota. Minn Med. 2010;93:45-48.
1. Martin D, Yankay J. Refugees and Asylees: 2011. Annual Flow Report. Offce of Immigration Statistics, US Department of Home-land Security. May 2012. Available at: http://www.dhs.gov/xlibrary/assets/statistics/publications/ois_rfa_fr_2011.pdf. Accessed October 29, 2012.
2. Stauffer WM, Kamat D, Walker PF. Screening of international immigrants, refugees and adoptees. Prim Care. 2002;29:879-905.
3. Cote S, Geltman P, Nunn M, et al. Dental caries of refugee children compared with US children. Pediatrics. 2004;114:e733-e740.
4. Geltman PL, Dookeran NM, Battaglia T, et al. Chronic disease and its risk factors among refugees and asylees in Massachusetts, 2001-2005. Prev Chronic Dis. 2010;7:A51.-
5. Fazel M, Wheeler J, Danesh J. Prevalence of serious mental disorder in 7000 refugees resettled in western countries: a systematic review. Lancet. 2005;365:1309-1314.
6. Geltman PL, Brown MJ, Cochran J. Lead poisoning among refugee children resettled in Massachusetts, 1995 to 1999. Pediatrics. 2001;108:158-162.
7. Geltman PL, Cochran J, Hedgecock C. Intestinal parasites among African refugees resettled in Massachusetts and the impact of an overseas pre-departure treatment program. Am J Trop Med Hyg. 2003;69:657.-
8. Lifson AR, Thai D, O’Fallon A, et al. Prevalence of tuberculosis, hepatitis B virus, and intestinal parasitic infections among refugees to Minnesota. Public Health Rep. 2002;117:69-77.
9. Power DV, Moody E, Trussell K, et al. Caring for the Karen. A newly arrived refugee group. Minn Med. 2010;93:49-53.
10. Centers for Disease Control and Prevention. Health considerations of newly arrived immigrants and refugees. In: Centers for Disease Control and Prevention. Travelers’ Health—Yellow Book. Chapt. 9. Available at: http://wwwnc.cdc.gov/travel/yellow-book/2010/table-of-contents.aspx#20. Accessed November 15, 2012.
11. Centers for Disease Control and Prevention. Medical Examination of Immigrants and Refugees. Available at: http://www.cdc.gov/immigrantrefugeehealth/exams/medical-examination.html. Accessed November 15, 2012.
12. Centers for Disease Control and Prevention. Technical Instructions For The Medical History and Physical Examination of Aliens in the United States. Available at: http://www.cdc.gov/immigrantrefugeehealth/exams/ti/civil/technical-instructions/civil-surgeons/medical-history-physical-examination.html. Accessed November 15, 2010.
13. Seybolt L, Barnett E, Stauffer W. US medical screening for immigrants and refugees: clinical issues. In: Walker P, Barnett E, eds. Immigrant Medicine. Phildelphia, PA: Saunders Elsevier; 2007:135-150.
14. United States Department of Health and Human Services, Offce of Refugee Resettlement. ORR State Letter: Revised Medical Screening Guidelines for Newly Arrived Refugees. Available at: http://www.acf.hhs.gov/sites/default/files/orr/state_letter_12_09_revised_medical_screening_guidelines_for_newly.pdf. Accessed October 29, 2012.
15. Centers for Disease Control and Prevention. Guidelines for the investigation of contacts of persons with infectious tuberculosis— guidelines for using the QuantiFERON-TB gold test for detecting Mycobacterium tuberculosis infection, United States. MMWR Recomm Rep. 2005;54(RR-15):1-55.
16. Centers for Disease Control and Prevention. Executive Commentary: Highlights of 2011 Report. Available at: http://www.cdc.gov/tb/statistics/reports/2011/pdf/ExecutiveCommentary.pdf. Accessed November 19, 2012.
17. Kuma V, Abbas AK, Fausto N, et al. Robbins Basic Pathology. 8th ed. Philadelphia, Pa: Saunders Elsevier; 2007:516–522.
18. Barnett ED. Infectious disease screening for refugees resettled in the United States. Clin Infect Dis. 2004;39:833-841.
19. DeRiemer K, Chin DP, Schecter DF, et al. Tuberculosis among immigrants and refugees. Arch Intern Med. 1998;158:753-760.
20. Centers for Disease Control and Prevention. Updated guidelines for using interferon gamma release assays to detect Mycobacterium tuberculosis infection—United States, 2010. MMWR Recomm Rec. 2010;59(RR-5):1-25.
21. Bright Futures at Georgetown University. Health Supervision— Laboratory Tests: Tuberculosis (TB) Screening. Available at: http://www.brightfutures.org/pocket/pdf/30_37.pdf. Accessed November 19, 2012.
22. Centers for Disease Control and Prevention. Treatment of tuberculosis. American Thoracic Society, CDC, Infectious Diseases Society of America. MMWR Recomm Rep 2003;52(RR-11):1-77.
23. Centers for Disease Control and Prevention. Update: adverse event data and revised American Thoracic Society/CDC recommendations against the use of rifampin and pyrazinamide for treament of latent tuberculosis infection—United States. MMWR Morb Mortal Wkly Rep. 2003;52:735-739.
24. Caruana SR, Kelly HA, Ngeow JY, et al. Undiagnosed and potentially lethal parasite infections among immigrants and refugees in Australia. J Travel Med. 2006;13:233-239.
25. Dawson-Hahn EE, Greenberg SL, Domachowske JB, et al. Eosinophilia and the seroprevalence of schistosomiasis and strongyloidiasis in newly arrived pediatric refugees: an examination of Centers for Disease Control and Prevention screening guidelines. J Pediatr. 2010;156:1016-1018.
26. Garg PK, Perry S, Dorn M, et al. Risk of intestinal helminth and protozoan infection in a refugee population. Am J Trop Med Hyg. 2005;73:386-391.
27. Parenti DM, Lucas D, Lee A, et al. Health status of Ethiopian refugees in the United States. Am J Public Health. 1987;77:1542-1543.
28. Parish R. Intestinal parasites in Southeast Asian refugee children. West J Med. 1985;143:47-49.
29. Sutherland JE, Avant RF, Franz WB, 3rd, et al. Indochinese refugee health assessment and treatment. J Fam Pract. 1983;16:61-67.
30. Cartwright C. Utility of multiple-stool-specimen ova and parasite examinations in a high-prevalence setting. J Clin Microbiol. 1999;37:2408-2411.
31. Centers for Disease Control and Prevention. Recommendations for Presumptive Treatment of Schistosomiasis and Strongyloidiasis Among the Somali Bantu Refugees. June 13, 2005. Available at: http://archive.acf.hhs.gov/programs/orr/policy/sl05-18attach-ment2.pdf. Accessed November 19, 2012.
32. Centers for Disease Control and Prevention. Division of Global Migration and Quarantine. Guidelines for Evaluation of Refugees for Intestinal and Tissue-Invasive Parasitic Infections during Domestic Medical Examination. Available at: http://www.cdc.gov/immigrantrefugeehealth/guidelines/domestic/intestinal-parasites-domestic.html. Accessed October 30, 2012.
33. Centers for Disease Control and Prevention. Screening for HIV Infection During the Refugee Domestic Medical Examination. Available at: http://www.cdc.gov/immigrantrefugeehealth/guidelines/domestic/screening-hiv-infection-domestic.html. Accessed November 15, 2012.
34. Centers for Disease Control and Prevention. Revised recommendations for HIV testing of adults, adolescents and pregnant women in healthcare settings. MMWR Recomm Rep. 2006;55(RR-14):1-17.
35. Centers for Disease Control and Prevention National Immunization Program. Available at: http://www.cdc.gov/vaccines/vpdvac/hepa/default.htm. Accessed November 19, 2012.
36. American Academy of Pediatrics. Red Book. Available at: http://www2.aap.org/immunization/illnesses/hepb/hepa.html. Accessed November 19, 2012.
37. Lai CL, Ratziu V, Yuen MF, et al. Viral hepatitis B. Lancet. 2003;362:2089-2094.
38. Lin K, Kirchner J. Hepatitis B. Am Fam Phyician. 2004;69:75-82.
39. Ghany MG, Strader DB, Thomas DL, et al. American Association for the Study of Liver Diseases. Diagnosis, management, and treatment of hepatitis C: an update. Hepatology. 2009;49:1335-1374.
40. Centers for Disease Control and Prevention. Recommendations for the identification of chronic hepatitis C virus infection among persons born during 1945-1965. MMWR Morb Mortal Wkly Rep. 2012;61(RR-4):1-31.
41. Rowland M, Nosten F. Malaria epidemiology and control in refugee camps and complex emergencies. Ann Trop Med Parasitol. 2001;95:741-754.
42. Centers for Disease Control and Prevention, Malaria Branch, Division of Parasitic Diseases, Division of Global Migration and Quarantine and Malaria Branch. Presumptive Treatment of P falciparum Malaria in Refugees Relocating from sub-Saharan Africa to the United States. Available at: http://www.cdc.gov/immigrantrefugeehealth/guidelines/domestic/malaria-guidelines-domestic.html. Accessed November 15, 2012.
43. Centers for Disease Control and Prevention. Malnutrition and micronutrient deficiencies among Bhutanese refugee children— Nepal, 2007. MMWR Morb Mortal Wkly Rep. 2008;57:370-373.
44. American Academy of Pediatrics Bright Futures. Guidelines for Health Supervision of Infants, Children, and Adolescents— Theme 5: Promoting Healthy Nutrition. Available at: http://brightfutures.aap.org/pdfs/Guidelines_PDF/6-Promoting_Healthy_Nutrition.pdf. Accessed November 15, 2012.
45. Benson J, Skull S. Hiding from the sun: vitamin D deficiency in refugees. Aust Fam Physician. 2007;36:355-357.
46. Stellinga-Boelen A. Vitamin D levels in children of asylum seekers in The Netherlands in relation to season and dietary intake. Eur J Pediatr. 2007;166:201-206.
47. Trepka MJ, Pekovic V, Santana JC, et al. Risk factors for lead poisoning among Cuban refugee children. Public Health Rep. 2005;120:179-185.
48. Centers for Disease Control and Prevention. Elevated blood lead levels in refugee children, New Hampshire, 2003-2004. MMWR Morb Mortal Wkly Rep. 2005;54:42-46.
49. Centers for Disease Control and Prevention. Screening for Lead at the Domestic Refugee Medical Exam. Available at: http://www.cdc.gov/immigrantrefugeehealth/pdf/lead.pdf. Accessed November 15, 2012.
50. Zabel EW, Smith ME, O’Fallon A. Implementation of CDC refugee blood testing guidelines in Minnesota. Public Health Rep. 2008;123:111-125.
51. United States Citizenship and Immigration Services. Available at: http://www.uscis.gov/portal/site/uscis/menuitem.5af9bb95919f35e66f614176543f6d1a/?vgnextoid=3384cc5222$5210VgnVCM100000082ca60aRCRD&vgnextchannel=6abe6d26d17df110VgnVCM1000004718190aRCRD. Accessed November 15, 2012.
52. Centers for Disease Control and Prevention. Vaccination Requirements for Adjustment of Status for US Permanent Residence: Technical Instructions for Civil Surgeons. December 14, 2009. Available at: http://www.cdc.gov/immigrantrefugeehealth/pdf/2009-vaccination-technical-instructions.pdf. Accessed November 15, 2012
53. Phillips C. Better primary healthcare for refugees: catch up immunisation. Aust Fam Physician. 2007;36:440-443.
54. Brewin C. Systematic review of screening instruments for adults at risk of PTSD. J Trauma Stress. 2005;18:53-62.
55. Crumlish N, O’Rourke K. A systematic review of treatments for post-traumatic stress disorder among refugees and asylum-seekers. J Nerv Ment Dis. 2010;198:237-251.
56. Watters C. Emerging paradigms in the mental health care of refugees. Soc Sci Med. 2001;53:1709-1718.
57. Hayes EB, Talbot SB, Matheson ES, et al. Health status of pediatric refugees in Portland, ME. Arch Pediatr Adolesc Med. 1998;152:564-568.
58. Meropol S. Health status of pediatric refugees in Buffalo, NY. Arch Pediatr Adolesc Med. 1995;149:887-892.
59. Barnes DM, Harrison C, Heneghan R. Health risk and promotion behaviors in refugee populations. J Health Care Poor Underserved. 2004;15:347-356.
60. Dicker S, Stauffer WM, Mamo B, et al. Initial refugee health assessments: new recommendations for Minnesota. Minn Med. 2010;93:45-48.